User login
Helping Patients With Intellectual Disabilities Make Informed Decisions
BOSTON — Primary care clinicians caring for patients with intellectual and developmental disabilities often recommend guardianship, a responsibility with life-altering implications.
But only approximately 30% of primary care residency programs in the United States provide training on how to assess the ability of patients with disabilities to make decisions for themselves, and much of this training is optional, according to a recent study cited during a workshop at the 2024 annual meeting of the Society of General Internal Medicine.
Assessing the capacity of patients with disabilities involves navigating a maze of legal, ethical, and clinical considerations, according to Mary Thomas, MD, MPH, a clinical fellow in geriatrics at Yale University School of Medicine in New Haven, Connecticut, who co-moderated the workshop.
Guardianship, while sometimes necessary, can be overly restrictive and diminish patient autonomy, she said. The legal process — ultimately decided through the courts — gives a guardian permission to manage medical care and make decisions for someone who cannot make or communicate those decisions themselves.
Clinicians can assess patients through an evaluation of functional capacity, which allows them to observe a patient’s demeanor and administer a cognition test. Alternatives such as supported decision-making may be less restrictive and can better serve patients, she said. Supported decision-making allows for a person with disabilities to receive assistance from a supporter who can help a patient process medical conditions and treatment needs. The supporter helps empower capable patients to decide on their own.
Some states have introduced legislation that would legally recognize supported decision-making as a less restrictive alternative to guardianship or conservatorship, in which a court-appointed individual manages all aspects of a person’s life.
Sara Mixter, MD, MPH, an assistant professor of medicine and pediatrics at the Johns Hopkins University School of Medicine in Baltimore and a co-moderator of the workshop, called the use of inclusive language in patient communication the “first step toward fostering an environment where patients feel respected and understood.”
Inclusive conversations can include person-first language and using words such as “caregiver” rather than “caretaker.”
Dr. Thomas and Dr. Mixter also called for the directors of residency programs to provide more training on disabilities. They cited a 2023 survey of directors, many of whom said that educational boards do not require training in disability-specific care and that experts in the care of people with disabilities are few and far between.
“Education and awareness are key to overcoming the challenges we face,” Dr. Thomas said. “Improving our training programs means we can ensure that all patients receive the care and respect they deserve.”
Dr. Thomas and Dr. Mixter report no relevant disclosures.
A version of this article first appeared on Medscape.com.
BOSTON — Primary care clinicians caring for patients with intellectual and developmental disabilities often recommend guardianship, a responsibility with life-altering implications.
But only approximately 30% of primary care residency programs in the United States provide training on how to assess the ability of patients with disabilities to make decisions for themselves, and much of this training is optional, according to a recent study cited during a workshop at the 2024 annual meeting of the Society of General Internal Medicine.
Assessing the capacity of patients with disabilities involves navigating a maze of legal, ethical, and clinical considerations, according to Mary Thomas, MD, MPH, a clinical fellow in geriatrics at Yale University School of Medicine in New Haven, Connecticut, who co-moderated the workshop.
Guardianship, while sometimes necessary, can be overly restrictive and diminish patient autonomy, she said. The legal process — ultimately decided through the courts — gives a guardian permission to manage medical care and make decisions for someone who cannot make or communicate those decisions themselves.
Clinicians can assess patients through an evaluation of functional capacity, which allows them to observe a patient’s demeanor and administer a cognition test. Alternatives such as supported decision-making may be less restrictive and can better serve patients, she said. Supported decision-making allows for a person with disabilities to receive assistance from a supporter who can help a patient process medical conditions and treatment needs. The supporter helps empower capable patients to decide on their own.
Some states have introduced legislation that would legally recognize supported decision-making as a less restrictive alternative to guardianship or conservatorship, in which a court-appointed individual manages all aspects of a person’s life.
Sara Mixter, MD, MPH, an assistant professor of medicine and pediatrics at the Johns Hopkins University School of Medicine in Baltimore and a co-moderator of the workshop, called the use of inclusive language in patient communication the “first step toward fostering an environment where patients feel respected and understood.”
Inclusive conversations can include person-first language and using words such as “caregiver” rather than “caretaker.”
Dr. Thomas and Dr. Mixter also called for the directors of residency programs to provide more training on disabilities. They cited a 2023 survey of directors, many of whom said that educational boards do not require training in disability-specific care and that experts in the care of people with disabilities are few and far between.
“Education and awareness are key to overcoming the challenges we face,” Dr. Thomas said. “Improving our training programs means we can ensure that all patients receive the care and respect they deserve.”
Dr. Thomas and Dr. Mixter report no relevant disclosures.
A version of this article first appeared on Medscape.com.
BOSTON — Primary care clinicians caring for patients with intellectual and developmental disabilities often recommend guardianship, a responsibility with life-altering implications.
But only approximately 30% of primary care residency programs in the United States provide training on how to assess the ability of patients with disabilities to make decisions for themselves, and much of this training is optional, according to a recent study cited during a workshop at the 2024 annual meeting of the Society of General Internal Medicine.
Assessing the capacity of patients with disabilities involves navigating a maze of legal, ethical, and clinical considerations, according to Mary Thomas, MD, MPH, a clinical fellow in geriatrics at Yale University School of Medicine in New Haven, Connecticut, who co-moderated the workshop.
Guardianship, while sometimes necessary, can be overly restrictive and diminish patient autonomy, she said. The legal process — ultimately decided through the courts — gives a guardian permission to manage medical care and make decisions for someone who cannot make or communicate those decisions themselves.
Clinicians can assess patients through an evaluation of functional capacity, which allows them to observe a patient’s demeanor and administer a cognition test. Alternatives such as supported decision-making may be less restrictive and can better serve patients, she said. Supported decision-making allows for a person with disabilities to receive assistance from a supporter who can help a patient process medical conditions and treatment needs. The supporter helps empower capable patients to decide on their own.
Some states have introduced legislation that would legally recognize supported decision-making as a less restrictive alternative to guardianship or conservatorship, in which a court-appointed individual manages all aspects of a person’s life.
Sara Mixter, MD, MPH, an assistant professor of medicine and pediatrics at the Johns Hopkins University School of Medicine in Baltimore and a co-moderator of the workshop, called the use of inclusive language in patient communication the “first step toward fostering an environment where patients feel respected and understood.”
Inclusive conversations can include person-first language and using words such as “caregiver” rather than “caretaker.”
Dr. Thomas and Dr. Mixter also called for the directors of residency programs to provide more training on disabilities. They cited a 2023 survey of directors, many of whom said that educational boards do not require training in disability-specific care and that experts in the care of people with disabilities are few and far between.
“Education and awareness are key to overcoming the challenges we face,” Dr. Thomas said. “Improving our training programs means we can ensure that all patients receive the care and respect they deserve.”
Dr. Thomas and Dr. Mixter report no relevant disclosures.
A version of this article first appeared on Medscape.com.
Former UCLA Doctor Receives $14 Million in Gender Discrimination Retrial
A California jury has awarded $14 million to a former University of California, Los Angeles (UCLA) oncologist who claimed she was paid thousands less than her male colleagues and wrongfully terminated after her complaints of gender-based harassment and intimidation were ignored by program leadership.
The decision comes after a lengthy 8-year legal battle in which an appellate judge reversed a previous jury decision in her favor.
Lauren Pinter-Brown, MD, a hematologic oncologist, was hired in 2005 by the University of California, Los Angeles School of Medicine — now called UCLA’s David Geffen School of Medicine. As the school’s lymphoma program director, she conducted clinical research alongside other oncology doctors, including Sven de Vos, MD.
She claimed that her professional relationship with Dr. de Vos became contentious after he demonstrated “oppositional” and “disrespectful” behavior at team meetings, such as talking over her and turning his chair so Dr. Pinter-Brown faced his back. Court documents indicated that Dr. de Vos refused to use Dr. Pinter-Brown’s title in front of colleagues despite doing so for male counterparts.
Dr. Pinter-Brown argued that she was treated as the “butt of a joke” by Dr. de Vos and other male colleagues. In 2016, she sued Dr. de Vos, the university, and its governing body, the Board of Regents, for wrongful termination.
She was awarded a $13 million verdict in 2018. However, the California Court of Appeals overturned it in 2020 after concluding that several mistakes during the court proceedings impeded the school’s right to a fair and impartial trial. The case was retried, culminating in the even higher award of $14 million issued on May 9.
“Two juries have come to virtually identical findings showing multiple problems at UCLA involving gender discrimination,” Dr. Pinter-Brown’s attorney, Carney R. Shegerian, JD, told this news organization.
A spokesperson from UCLA’s David Geffen School of Medicine said administrators are carefully reviewing the new decision.
The spokesperson told this news organization that the medical school and its health system remain “deeply committed to maintaining a workplace free from discrimination, intimidation, retaliation, or harassment of any kind” and fostering a “respectful and inclusive environment ... in research, medical education, and patient care.”
Gender Pay Disparities Persist in Medicine
The gender pay gap in medicine is well documented. The 2024 Medscape Physician Compensation Report found that male doctors earn about 29% more than their female counterparts, with the disparity growing larger among specialists. In addition, a recent JAMA Health Forum study found that male physicians earned 21%-24% more per hour than female physicians.
Dr. Pinter-Brown, who now works at the University of California, Irvine, alleged that she was paid $200,000 less annually, on average, than her male colleagues.
That’s not surprising, says Martha Gulati, MD, professor and director of preventive cardiology at Cedars-Sinai Smidt Heart Institute, Los Angeles. She coauthored a commentary about gender disparities in JAMA Network Open. Dr. Gulati told this news organization that even a “small” pay disparity of $100,000 annually adds up.
“Let’s say the [male physician] invests it at 3% and adds to it yearly. Even without a raise, in 20 years, that is approximately $3 million,” Dr. Gulati explained. “Once you find out you are paid less than your male colleagues, you are upset. Your sense of value and self-worth disappears.”
Eileen Barrett, MD, MPH, president-elect of the American Medical Women’s Association, said that gender discrimination is likely more prevalent than research indicates. She told this news organization that self-doubt and fear of retaliation keep many from exposing the mistreatment.
Although more women are entering medicine, too few rise to the highest positions, Dr. Barrett said.
“Unfortunately, many are pulled and pushed into specialties and subspecialties that have lower compensation and are not promoted to leadership, so just having numbers isn’t enough to achieve equity,” Dr. Barrett said.
Dr. Pinter-Brown claimed she was repeatedly harassed and intimidated by Dr. de Vos from 2008 to 2015. Despite voicing concerns multiple times about the discriminatory behavior, the only resolutions offered by the male-dominated program leadership were for her to separate from the group and conduct lymphoma research independently or to avoid interacting with Dr. de Vos, court records said.
Even the school’s male Title IX officer, Jan Tillisch, MD, who handled gender-based discrimination complaints, reportedly made sexist comments. When Dr. Pinter-Brown sought his help, he allegedly told her that she had a reputation as an “angry woman” and “diva,” court records showed.
According to court documents, Dr. Pinter-Brown endured nitpicking and research audits as retaliation for speaking out, temporarily suspending her research privileges. She said she was subsequently removed from the director position and replaced by Dr. de Vos.
Female physicians who report discriminatory behavior often have unfavorable outcomes and risk future career prospects, Dr. Gulati said.
To shift this dynamic, she said institutions must increase transparency and practices that support female doctors receiving “equal pay for equal work.”
A version of this article appeared on Medscape.com.
A California jury has awarded $14 million to a former University of California, Los Angeles (UCLA) oncologist who claimed she was paid thousands less than her male colleagues and wrongfully terminated after her complaints of gender-based harassment and intimidation were ignored by program leadership.
The decision comes after a lengthy 8-year legal battle in which an appellate judge reversed a previous jury decision in her favor.
Lauren Pinter-Brown, MD, a hematologic oncologist, was hired in 2005 by the University of California, Los Angeles School of Medicine — now called UCLA’s David Geffen School of Medicine. As the school’s lymphoma program director, she conducted clinical research alongside other oncology doctors, including Sven de Vos, MD.
She claimed that her professional relationship with Dr. de Vos became contentious after he demonstrated “oppositional” and “disrespectful” behavior at team meetings, such as talking over her and turning his chair so Dr. Pinter-Brown faced his back. Court documents indicated that Dr. de Vos refused to use Dr. Pinter-Brown’s title in front of colleagues despite doing so for male counterparts.
Dr. Pinter-Brown argued that she was treated as the “butt of a joke” by Dr. de Vos and other male colleagues. In 2016, she sued Dr. de Vos, the university, and its governing body, the Board of Regents, for wrongful termination.
She was awarded a $13 million verdict in 2018. However, the California Court of Appeals overturned it in 2020 after concluding that several mistakes during the court proceedings impeded the school’s right to a fair and impartial trial. The case was retried, culminating in the even higher award of $14 million issued on May 9.
“Two juries have come to virtually identical findings showing multiple problems at UCLA involving gender discrimination,” Dr. Pinter-Brown’s attorney, Carney R. Shegerian, JD, told this news organization.
A spokesperson from UCLA’s David Geffen School of Medicine said administrators are carefully reviewing the new decision.
The spokesperson told this news organization that the medical school and its health system remain “deeply committed to maintaining a workplace free from discrimination, intimidation, retaliation, or harassment of any kind” and fostering a “respectful and inclusive environment ... in research, medical education, and patient care.”
Gender Pay Disparities Persist in Medicine
The gender pay gap in medicine is well documented. The 2024 Medscape Physician Compensation Report found that male doctors earn about 29% more than their female counterparts, with the disparity growing larger among specialists. In addition, a recent JAMA Health Forum study found that male physicians earned 21%-24% more per hour than female physicians.
Dr. Pinter-Brown, who now works at the University of California, Irvine, alleged that she was paid $200,000 less annually, on average, than her male colleagues.
That’s not surprising, says Martha Gulati, MD, professor and director of preventive cardiology at Cedars-Sinai Smidt Heart Institute, Los Angeles. She coauthored a commentary about gender disparities in JAMA Network Open. Dr. Gulati told this news organization that even a “small” pay disparity of $100,000 annually adds up.
“Let’s say the [male physician] invests it at 3% and adds to it yearly. Even without a raise, in 20 years, that is approximately $3 million,” Dr. Gulati explained. “Once you find out you are paid less than your male colleagues, you are upset. Your sense of value and self-worth disappears.”
Eileen Barrett, MD, MPH, president-elect of the American Medical Women’s Association, said that gender discrimination is likely more prevalent than research indicates. She told this news organization that self-doubt and fear of retaliation keep many from exposing the mistreatment.
Although more women are entering medicine, too few rise to the highest positions, Dr. Barrett said.
“Unfortunately, many are pulled and pushed into specialties and subspecialties that have lower compensation and are not promoted to leadership, so just having numbers isn’t enough to achieve equity,” Dr. Barrett said.
Dr. Pinter-Brown claimed she was repeatedly harassed and intimidated by Dr. de Vos from 2008 to 2015. Despite voicing concerns multiple times about the discriminatory behavior, the only resolutions offered by the male-dominated program leadership were for her to separate from the group and conduct lymphoma research independently or to avoid interacting with Dr. de Vos, court records said.
Even the school’s male Title IX officer, Jan Tillisch, MD, who handled gender-based discrimination complaints, reportedly made sexist comments. When Dr. Pinter-Brown sought his help, he allegedly told her that she had a reputation as an “angry woman” and “diva,” court records showed.
According to court documents, Dr. Pinter-Brown endured nitpicking and research audits as retaliation for speaking out, temporarily suspending her research privileges. She said she was subsequently removed from the director position and replaced by Dr. de Vos.
Female physicians who report discriminatory behavior often have unfavorable outcomes and risk future career prospects, Dr. Gulati said.
To shift this dynamic, she said institutions must increase transparency and practices that support female doctors receiving “equal pay for equal work.”
A version of this article appeared on Medscape.com.
A California jury has awarded $14 million to a former University of California, Los Angeles (UCLA) oncologist who claimed she was paid thousands less than her male colleagues and wrongfully terminated after her complaints of gender-based harassment and intimidation were ignored by program leadership.
The decision comes after a lengthy 8-year legal battle in which an appellate judge reversed a previous jury decision in her favor.
Lauren Pinter-Brown, MD, a hematologic oncologist, was hired in 2005 by the University of California, Los Angeles School of Medicine — now called UCLA’s David Geffen School of Medicine. As the school’s lymphoma program director, she conducted clinical research alongside other oncology doctors, including Sven de Vos, MD.
She claimed that her professional relationship with Dr. de Vos became contentious after he demonstrated “oppositional” and “disrespectful” behavior at team meetings, such as talking over her and turning his chair so Dr. Pinter-Brown faced his back. Court documents indicated that Dr. de Vos refused to use Dr. Pinter-Brown’s title in front of colleagues despite doing so for male counterparts.
Dr. Pinter-Brown argued that she was treated as the “butt of a joke” by Dr. de Vos and other male colleagues. In 2016, she sued Dr. de Vos, the university, and its governing body, the Board of Regents, for wrongful termination.
She was awarded a $13 million verdict in 2018. However, the California Court of Appeals overturned it in 2020 after concluding that several mistakes during the court proceedings impeded the school’s right to a fair and impartial trial. The case was retried, culminating in the even higher award of $14 million issued on May 9.
“Two juries have come to virtually identical findings showing multiple problems at UCLA involving gender discrimination,” Dr. Pinter-Brown’s attorney, Carney R. Shegerian, JD, told this news organization.
A spokesperson from UCLA’s David Geffen School of Medicine said administrators are carefully reviewing the new decision.
The spokesperson told this news organization that the medical school and its health system remain “deeply committed to maintaining a workplace free from discrimination, intimidation, retaliation, or harassment of any kind” and fostering a “respectful and inclusive environment ... in research, medical education, and patient care.”
Gender Pay Disparities Persist in Medicine
The gender pay gap in medicine is well documented. The 2024 Medscape Physician Compensation Report found that male doctors earn about 29% more than their female counterparts, with the disparity growing larger among specialists. In addition, a recent JAMA Health Forum study found that male physicians earned 21%-24% more per hour than female physicians.
Dr. Pinter-Brown, who now works at the University of California, Irvine, alleged that she was paid $200,000 less annually, on average, than her male colleagues.
That’s not surprising, says Martha Gulati, MD, professor and director of preventive cardiology at Cedars-Sinai Smidt Heart Institute, Los Angeles. She coauthored a commentary about gender disparities in JAMA Network Open. Dr. Gulati told this news organization that even a “small” pay disparity of $100,000 annually adds up.
“Let’s say the [male physician] invests it at 3% and adds to it yearly. Even without a raise, in 20 years, that is approximately $3 million,” Dr. Gulati explained. “Once you find out you are paid less than your male colleagues, you are upset. Your sense of value and self-worth disappears.”
Eileen Barrett, MD, MPH, president-elect of the American Medical Women’s Association, said that gender discrimination is likely more prevalent than research indicates. She told this news organization that self-doubt and fear of retaliation keep many from exposing the mistreatment.
Although more women are entering medicine, too few rise to the highest positions, Dr. Barrett said.
“Unfortunately, many are pulled and pushed into specialties and subspecialties that have lower compensation and are not promoted to leadership, so just having numbers isn’t enough to achieve equity,” Dr. Barrett said.
Dr. Pinter-Brown claimed she was repeatedly harassed and intimidated by Dr. de Vos from 2008 to 2015. Despite voicing concerns multiple times about the discriminatory behavior, the only resolutions offered by the male-dominated program leadership were for her to separate from the group and conduct lymphoma research independently or to avoid interacting with Dr. de Vos, court records said.
Even the school’s male Title IX officer, Jan Tillisch, MD, who handled gender-based discrimination complaints, reportedly made sexist comments. When Dr. Pinter-Brown sought his help, he allegedly told her that she had a reputation as an “angry woman” and “diva,” court records showed.
According to court documents, Dr. Pinter-Brown endured nitpicking and research audits as retaliation for speaking out, temporarily suspending her research privileges. She said she was subsequently removed from the director position and replaced by Dr. de Vos.
Female physicians who report discriminatory behavior often have unfavorable outcomes and risk future career prospects, Dr. Gulati said.
To shift this dynamic, she said institutions must increase transparency and practices that support female doctors receiving “equal pay for equal work.”
A version of this article appeared on Medscape.com.
Resource Menu Gives Choice to Caregivers Struggling to Meet Basic Needs
Screenings may not be the way to get needed resources to children and their caregivers, according to new research presented at the annual meeting of the Pediatric Academic Societies (PAS).
Caregivers and parents who were asked if they wanted assistance in several areas of need, including transportation and childcare, were nearly twice as likely to say they wanted such help than those who received a screening on current hardships. Generally, each questionnaire is administered in front of their children in primary care or pediatric hospital settings.
“Families have a lot of concern about being seen a different way by their healthcare team, being seen as unfit, and having child protective services involved in their childcare for issues related to poverty,” said Danielle Cullen, MD, a pediatric emergency medicine specialist at Children’s Hospital of Philadelphia (CHOP) and assistant professor of pediatrics at the University of Pennsylvania in Philadelphia.
Dr. Cullen and her colleagues analyzed data from nearly 4000 caregivers of children up to age 21 at emergency departments or primary care clinics at CHOP between 2021 and 2023.
Caregivers were randomly assigned to one of three arms — screening with a version of WE CARE (Well Child Care, Evaluation, Community Resources, Advocacy, Referral, Education), use of an online menu of options for help in areas like housing, or neither approach.
Caregivers in all three arms received a map of resources and a follow-up text from a resource navigator to assist them as needed.
Nearly 40% of caregivers who presented with the digital menu said they wanted resources compared with 29% of those who were screened (P < .001). Non-native English speakers given the menu were 2.5 times more likely to say yes to resources compared with those who were screened.
“We need to be thoughtful about these mandates to screen for social determinants of health: It’s not that straightforward,” said Esther K. Chung, MD, a pediatrician and professor of pediatrics at the University of Washington Medicine in Seattle, who was not involved in the study. “What we’re getting from this study is that patients want choice, and the menu provides them choice.”
Dr. Cullen said the menu option allows caregivers to make choices based on their priorities and not on whether they meet the screening thresholds for need.
While some health clinics utilize tablet forms for screenings to offer more privacy with questions, asking direct questions about income, food insecurity, and housing stability can be stigmatizing, Dr. Cullen said.
“Screening positive for social risk doesn’t mean that you actually want resources, and on the flip side, the literature shows that about half of the people who screen negative want resources,” she said.
Dr. Cullen and her team also conducted follow-up interviews with caregivers and found many feared that their clinician would assume a medical condition was connected to living conditions. They also had concerns about insurance companies gaining access to the data and using it to deny coverage or raise costs.
Spanish-speaking caregivers cited fears about their immigration status, experiences of discrimination, and language barriers when trying to access resources.
Participants said a few key strategies could make screening less intimidating, such as abstaining from screening during a serious medical visit, asking for consent to record answers in medical records, and communicating in an empathetic manner.
“Some families are a bit surprised when we ask about things like housing and food insecurity, but I think as long as we contextualize it, we can minimize the stigma associated with it,” Dr. Chung said. “That takes quite a bit of nuance and skill.”
The study was funded by the William T. Grant Foundation and the Emergency Medicine Foundation. The authors reported no disclosures.
A version of this article appeared on Medscape.com.
Screenings may not be the way to get needed resources to children and their caregivers, according to new research presented at the annual meeting of the Pediatric Academic Societies (PAS).
Caregivers and parents who were asked if they wanted assistance in several areas of need, including transportation and childcare, were nearly twice as likely to say they wanted such help than those who received a screening on current hardships. Generally, each questionnaire is administered in front of their children in primary care or pediatric hospital settings.
“Families have a lot of concern about being seen a different way by their healthcare team, being seen as unfit, and having child protective services involved in their childcare for issues related to poverty,” said Danielle Cullen, MD, a pediatric emergency medicine specialist at Children’s Hospital of Philadelphia (CHOP) and assistant professor of pediatrics at the University of Pennsylvania in Philadelphia.
Dr. Cullen and her colleagues analyzed data from nearly 4000 caregivers of children up to age 21 at emergency departments or primary care clinics at CHOP between 2021 and 2023.
Caregivers were randomly assigned to one of three arms — screening with a version of WE CARE (Well Child Care, Evaluation, Community Resources, Advocacy, Referral, Education), use of an online menu of options for help in areas like housing, or neither approach.
Caregivers in all three arms received a map of resources and a follow-up text from a resource navigator to assist them as needed.
Nearly 40% of caregivers who presented with the digital menu said they wanted resources compared with 29% of those who were screened (P < .001). Non-native English speakers given the menu were 2.5 times more likely to say yes to resources compared with those who were screened.
“We need to be thoughtful about these mandates to screen for social determinants of health: It’s not that straightforward,” said Esther K. Chung, MD, a pediatrician and professor of pediatrics at the University of Washington Medicine in Seattle, who was not involved in the study. “What we’re getting from this study is that patients want choice, and the menu provides them choice.”
Dr. Cullen said the menu option allows caregivers to make choices based on their priorities and not on whether they meet the screening thresholds for need.
While some health clinics utilize tablet forms for screenings to offer more privacy with questions, asking direct questions about income, food insecurity, and housing stability can be stigmatizing, Dr. Cullen said.
“Screening positive for social risk doesn’t mean that you actually want resources, and on the flip side, the literature shows that about half of the people who screen negative want resources,” she said.
Dr. Cullen and her team also conducted follow-up interviews with caregivers and found many feared that their clinician would assume a medical condition was connected to living conditions. They also had concerns about insurance companies gaining access to the data and using it to deny coverage or raise costs.
Spanish-speaking caregivers cited fears about their immigration status, experiences of discrimination, and language barriers when trying to access resources.
Participants said a few key strategies could make screening less intimidating, such as abstaining from screening during a serious medical visit, asking for consent to record answers in medical records, and communicating in an empathetic manner.
“Some families are a bit surprised when we ask about things like housing and food insecurity, but I think as long as we contextualize it, we can minimize the stigma associated with it,” Dr. Chung said. “That takes quite a bit of nuance and skill.”
The study was funded by the William T. Grant Foundation and the Emergency Medicine Foundation. The authors reported no disclosures.
A version of this article appeared on Medscape.com.
Screenings may not be the way to get needed resources to children and their caregivers, according to new research presented at the annual meeting of the Pediatric Academic Societies (PAS).
Caregivers and parents who were asked if they wanted assistance in several areas of need, including transportation and childcare, were nearly twice as likely to say they wanted such help than those who received a screening on current hardships. Generally, each questionnaire is administered in front of their children in primary care or pediatric hospital settings.
“Families have a lot of concern about being seen a different way by their healthcare team, being seen as unfit, and having child protective services involved in their childcare for issues related to poverty,” said Danielle Cullen, MD, a pediatric emergency medicine specialist at Children’s Hospital of Philadelphia (CHOP) and assistant professor of pediatrics at the University of Pennsylvania in Philadelphia.
Dr. Cullen and her colleagues analyzed data from nearly 4000 caregivers of children up to age 21 at emergency departments or primary care clinics at CHOP between 2021 and 2023.
Caregivers were randomly assigned to one of three arms — screening with a version of WE CARE (Well Child Care, Evaluation, Community Resources, Advocacy, Referral, Education), use of an online menu of options for help in areas like housing, or neither approach.
Caregivers in all three arms received a map of resources and a follow-up text from a resource navigator to assist them as needed.
Nearly 40% of caregivers who presented with the digital menu said they wanted resources compared with 29% of those who were screened (P < .001). Non-native English speakers given the menu were 2.5 times more likely to say yes to resources compared with those who were screened.
“We need to be thoughtful about these mandates to screen for social determinants of health: It’s not that straightforward,” said Esther K. Chung, MD, a pediatrician and professor of pediatrics at the University of Washington Medicine in Seattle, who was not involved in the study. “What we’re getting from this study is that patients want choice, and the menu provides them choice.”
Dr. Cullen said the menu option allows caregivers to make choices based on their priorities and not on whether they meet the screening thresholds for need.
While some health clinics utilize tablet forms for screenings to offer more privacy with questions, asking direct questions about income, food insecurity, and housing stability can be stigmatizing, Dr. Cullen said.
“Screening positive for social risk doesn’t mean that you actually want resources, and on the flip side, the literature shows that about half of the people who screen negative want resources,” she said.
Dr. Cullen and her team also conducted follow-up interviews with caregivers and found many feared that their clinician would assume a medical condition was connected to living conditions. They also had concerns about insurance companies gaining access to the data and using it to deny coverage or raise costs.
Spanish-speaking caregivers cited fears about their immigration status, experiences of discrimination, and language barriers when trying to access resources.
Participants said a few key strategies could make screening less intimidating, such as abstaining from screening during a serious medical visit, asking for consent to record answers in medical records, and communicating in an empathetic manner.
“Some families are a bit surprised when we ask about things like housing and food insecurity, but I think as long as we contextualize it, we can minimize the stigma associated with it,” Dr. Chung said. “That takes quite a bit of nuance and skill.”
The study was funded by the William T. Grant Foundation and the Emergency Medicine Foundation. The authors reported no disclosures.
A version of this article appeared on Medscape.com.
FROM PAS 2024
Exploring Skin Pigmentation Adaptation: A Systematic Review on the Vitamin D Adaptation Hypothesis
The risk for developing skin cancer can be somewhat attributed to variations in skin pigmentation. Historically, lighter skin pigmentation has been observed in populations living in higher latitudes and darker pigmentation in populations near the equator. Although skin pigmentation is a conglomeration of genetic and environmental factors, anthropologic studies have demonstrated an association of human skin lightening with historic human migratory patterns.1 It is postulated that migration to latitudes with less UVB light penetration has resulted in a compensatory natural selection of lighter skin types. Furthermore, the driving force behind this migration-associated skin lightening has remained unclear.1
The need for folate metabolism, vitamin D synthesis, and barrier protection, as well as cultural practices, has been postulated as driving factors for skin pigmentation variation. Synthesis of vitamin D is a UV radiation (UVR)–dependent process and has remained a prominent theoretical driver for the basis of evolutionary skin lightening. Vitamin D can be acquired both exogenously or endogenously via dietary supplementation or sunlight; however, historically it has been obtained through UVB exposure primarily. Once UVB is absorbed by the skin, it catalyzes conversion of 7-dehydrocholesterol to previtamin D3, which is converted to vitamin D in the kidneys.2,3 It is suggested that lighter skin tones have an advantage over darker skin tones in synthesizing vitamin D at higher latitudes where there is less UVB, thus leading to the adaptation process.1 In this systematic review, we analyzed the evolutionary vitamin D adaptation hypothesis and assessed the validity of evidence supporting this theory in the literature.
Methods
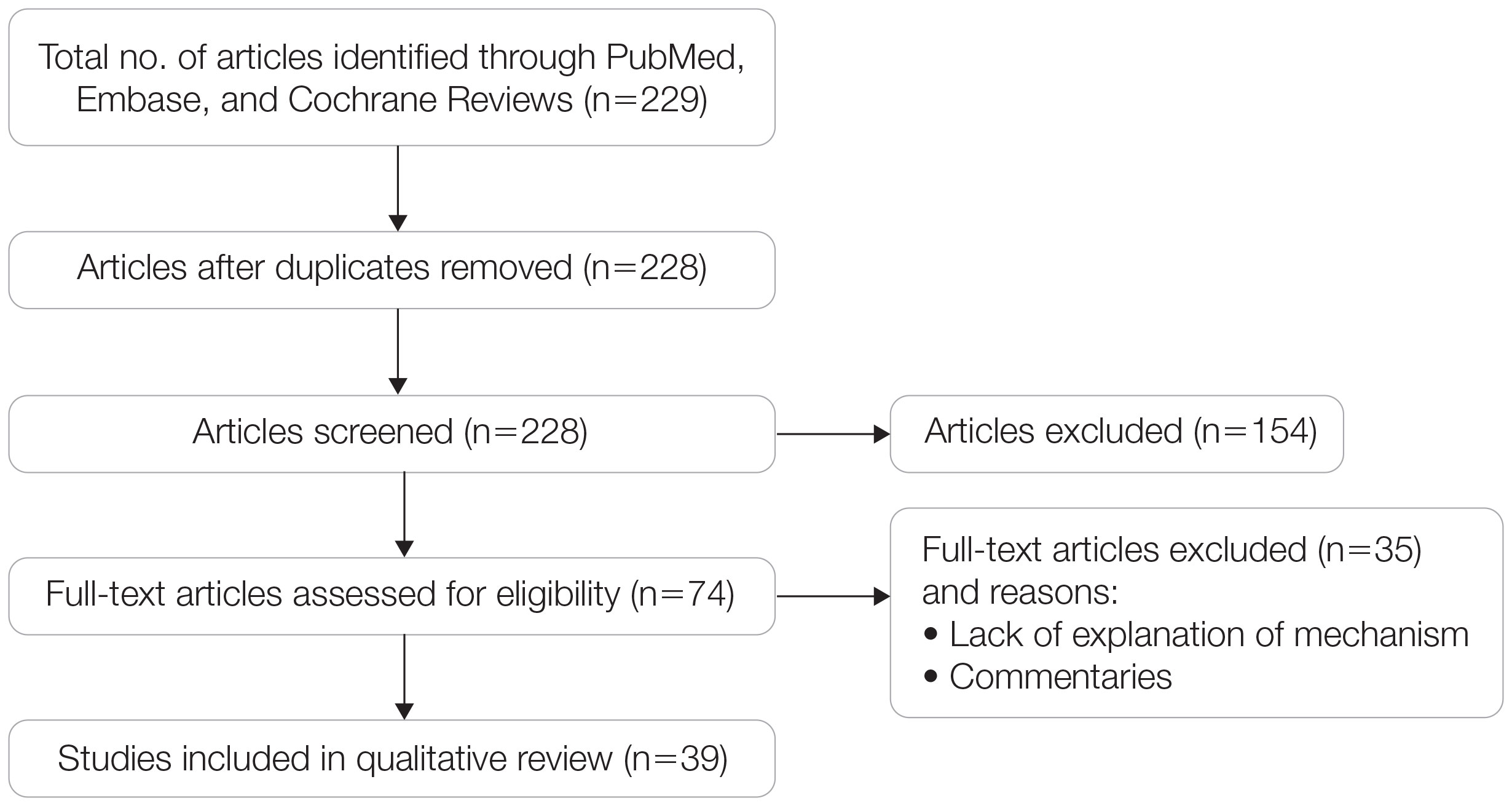
A search of PubMed, Embase, and the Cochrane Reviews database was conducted using the terms evolution, vitamin D, and skin to generate articles published from 2010 to 2022 that evaluated the influence of UVR-dependent production of vitamin D on skin pigmentation through historical migration patterns (Figure). Studies were excluded during an initial screening of abstracts followed by full-text assessment if they only had abstracts and if articles were inaccessible for review or in the form of case reports and commentaries.
The following data were extracted from each included study: reference citation, affiliated institutions of authors, author specialties, journal name, year of publication, study period, type of article, type of study, mechanism of adaptation, data concluding or supporting vitamin D as the driver, and data concluding or suggesting against vitamin D as the driver. Data concluding or supporting vitamin D as the driver were recorded from statistically significant results, study conclusions, and direct quotations. Data concluding or suggesting against vitamin D as the driver also were recorded from significant results, study conclusions, and direct quotes. The mechanism of adaptation was based on vitamin D synthesis modulation, melanin upregulation, genetic selections, genetic drift, mating patterns, increased vitamin D sensitivity, interbreeding, and diet.
Studies included in the analysis were placed into 1 of 3 categories: supporting, neutral, and against. Strength of Recommendation Taxonomy (SORT) criteria were used to classify the level of evidence of each article.4 Each article’s level of evidence was then graded (Table 1). The SORT grading levels were based on quality and evidence type: level 1 signified good-quality, patient-oriented evidence; level 2 signified limited-quality, patient-oriented evidence; and level 3 signified other evidence.4
Results
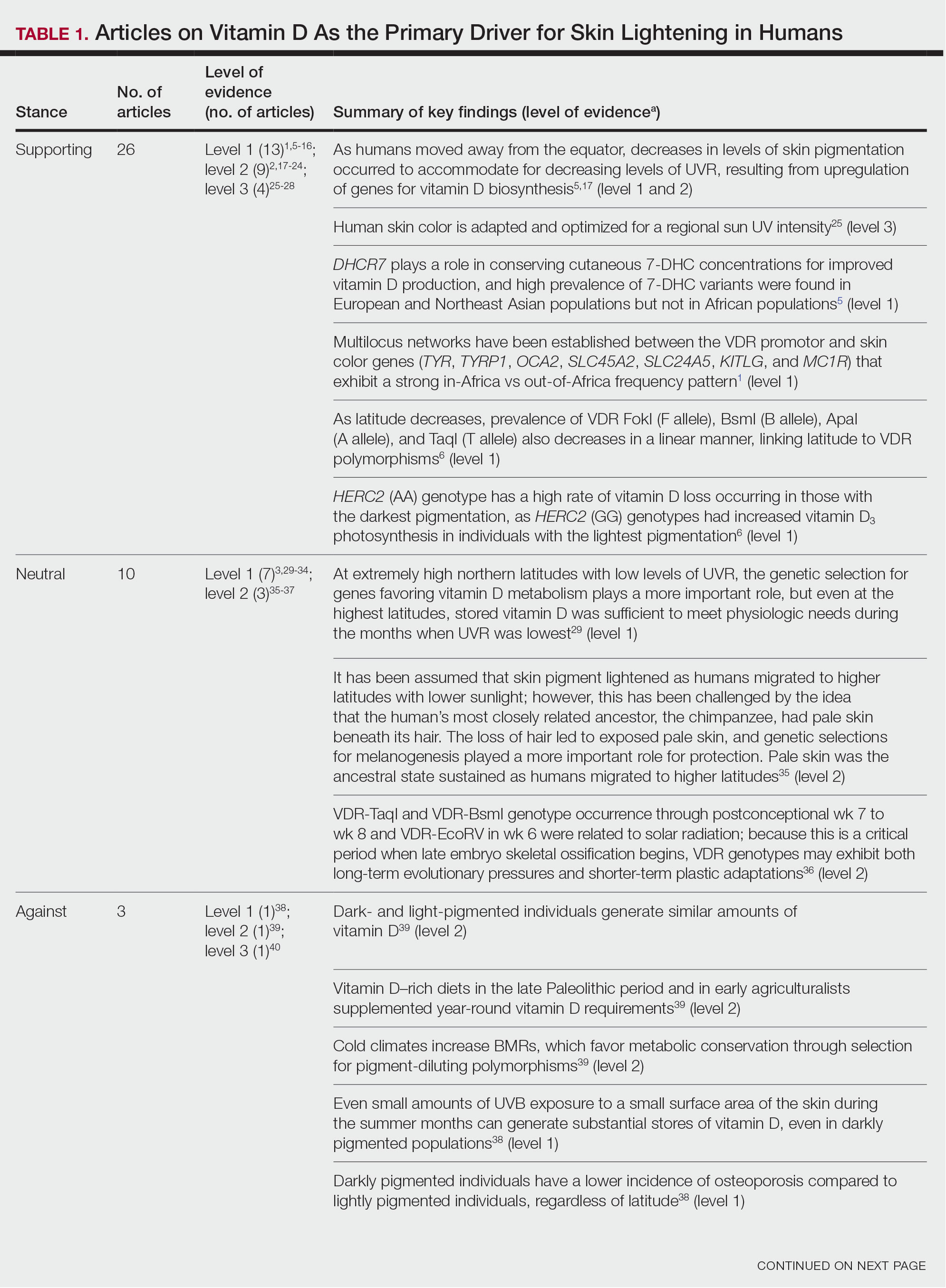
Article Selection—A total of 229 articles were identified for screening, and 39 studies met inclusion criteria.1-3,5-40 Systematic and retrospective reviews were the most common types of studies. Genomic analysis/sequencing/genome-wide association studies (GWAS) were the most common methods of analysis. Of these 39 articles, 26 were classified as supporting the evolutionary vitamin D adaptation hypothesis, 10 were classified as neutral, and 3 were classified as against (Table 1).
Of the articles classified as supporting the vitamin D hypothesis, 13 articles were level 1 evidence, 9 were level 2, and 4 were level 3. Key findings supporting the vitamin D hypothesis included genetic natural selection favoring vitamin D synthesis genes at higher latitudes with lower UVR and the skin lightening that occurred to protect against vitamin D deficiency (Table 1). Specific genes supporting these findings included 7-dehydrocholesterol reductase (DHCR7), vitamin D receptor (VDR), tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), oculocutaneous albinism type 2 melanosomal transmembrane protein (OCA2), solute carrier family 45 member 2 (SLC45A2), solute carrier family 4 member 5 (SLC24A5), Kit ligand (KITLG), melanocortin 1 receptor (MC1R), and HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2)(Table 2).
Of the articles classified as being against the vitamin D hypothesis, 1 article was level 1 evidence, 1 was level 2, and 1 was level 3. Key findings refuting the vitamin D hypothesis included similar amounts of vitamin D synthesis in contemporary dark- and light-pigmented individuals, vitamin D–rich diets in the late Paleolithic period and in early agriculturalists, and metabolic conservation being the primary driver (Table 1).
Of the articles classified as neutral to the hypothesis, 7 articles were level 1 evidence and 3 were level 2. Key findings of these articles included genetic selection favoring vitamin D synthesis only for populations at extremely northern latitudes, skin lightening that was sustained in northern latitudes from the neighboring human ancestor the chimpanzee, and evidence for long-term evolutionary pressures and short-term plastic adaptations in vitamin D genes (Table 1).
Comment
The importance of appropriate vitamin D levels is hypothesized as a potent driver in skin lightening because the vitamin is essential for many biochemical processes within the human body. Proper calcification of bones requires activated vitamin D to prevent rickets in childhood. Pelvic deformation in women with rickets can obstruct childbirth in primitive medical environments.15 This direct reproductive impairment suggests a strong selective pressure for skin lightening in populations that migrated northward to enhance vitamin D synthesis.
Of the 39 articles that we reviewed, the majority (n=26 [66.7%]) supported the hypothesis that vitamin D synthesis was the main driver behind skin lightening, whereas 3 (7.7%) did not support the hypothesis and 10 (25.6%) were neutral. Other leading theories explaining skin lightening included the idea that enhanced melanogenesis protected against folate degradation; genetic selection for light-skin alleles due to genetic drift; skin lightening being the result of sexual selection; and a combination of factors, including dietary choices, clothing preferences, and skin permeability barriers.
Articles With Supporting Evidence for the Vitamin D Theory—As Homo sapiens migrated out of Africa, migration patterns demonstrated the correlation between distance from the equator and skin pigmentation from natural selection. Individuals with darker skin pigment required higher levels of UVR to synthesize vitamin D. According to Beleza et al,1 as humans migrated to areas of higher latitudes with lower levels of UVR, natural selection favored the development of lighter skin to maximize vitamin D production. Vitamin D is linked to calcium metabolism, and its deficiency can lead to bone malformations and poor immune function.35 Several genes affecting melanogenesis and skin pigment have been found to have geospatial patterns that map to different geographic locations of various populations, indicating how human migration patterns out of Africa created this natural selection for skin lightening. The gene KITLG—associated with lighter skin pigmentation—has been found in high frequencies in both European and East Asian populations and is proposed to have increased in frequency after the migration out of Africa. However, the genes TYRP1, SLC24A5, and SLC45A2 were found at high frequencies only in European populations, and this selection occurred 11,000 to 19,000 years ago during the Last Glacial Maximum (15,000–20,000 years ago), demonstrating the selection for European over East Asian characteristics. During this period, seasonal changes increased the risk for vitamin D deficiency and provided an urgency for selection to a lighter skin pigment.1
The migration of H sapiens to northern latitudes prompted the selection of alleles that would increasevitamin D synthesis to counteract the reduced UV exposure. Genetic analysis studies have found key associations between genes encoding for the metabolism of vitamin D and pigmentation. Among this complex network are the essential downstream enzymes in the melanocortin receptor 1 pathway, including TYR and TYRP1. Forty-six of 960 single-nucleotide polymorphisms located in 29 different genes involved in skin pigmentation that were analyzed in a cohort of 2970 individuals were significantly associated with serum vitamin D levels (P<.05). The exocyst complex component 2 (EXOC2), TYR, and TYRP1 gene variants were shown to have the greatest influence on vitamin D status.9 These data reveal how pigment genotypes are predictive of vitamin D levels and the epistatic potential among many genes in this complex network.
Gene variation plays an important role in vitamin D status when comparing genetic polymorphisms in populations in northern latitudes to African populations. Vitamin D3 precursor availability is decreased by 7-DHCR catalyzing the precursors substrate to cholesterol. In a study using GWAS, it was found that “variations in DHCR7 may aid vitamin D production by conserving cutaneous 7-DHC levels. A high prevalence of DHCR7 variants were found in European and Northeast Asian populations but not in African populations, suggesting that selection occurred for these DHCR7 mutations in populations who migrated to more northern latitudes.5 Multilocus networks have been established between the VDR promotor and skin color genes (Table 2) that exhibit a strong in-Africa vs out-of-Africa frequency pattern. It also has been shown that genetic variation (suggesting a long-term evolutionary inclination) and epigenetic modification (indicative of short-term exposure) of VDR lends support to the vitamin D hypothesis. As latitude decreases, prevalence of VDR FokI (F allele), BsmI (B allele), ApaI (A allele), and TaqI (T allele) also decreases in a linear manner, linking latitude to VDR polymorphisms. Plasma vitamin D levels and photoperiod of conception—UV exposure during the periconceptional period—also were extrapolative of VDR methylation in a study involving 80 participants, where these 2 factors accounted for 17% of variance in methylation.6
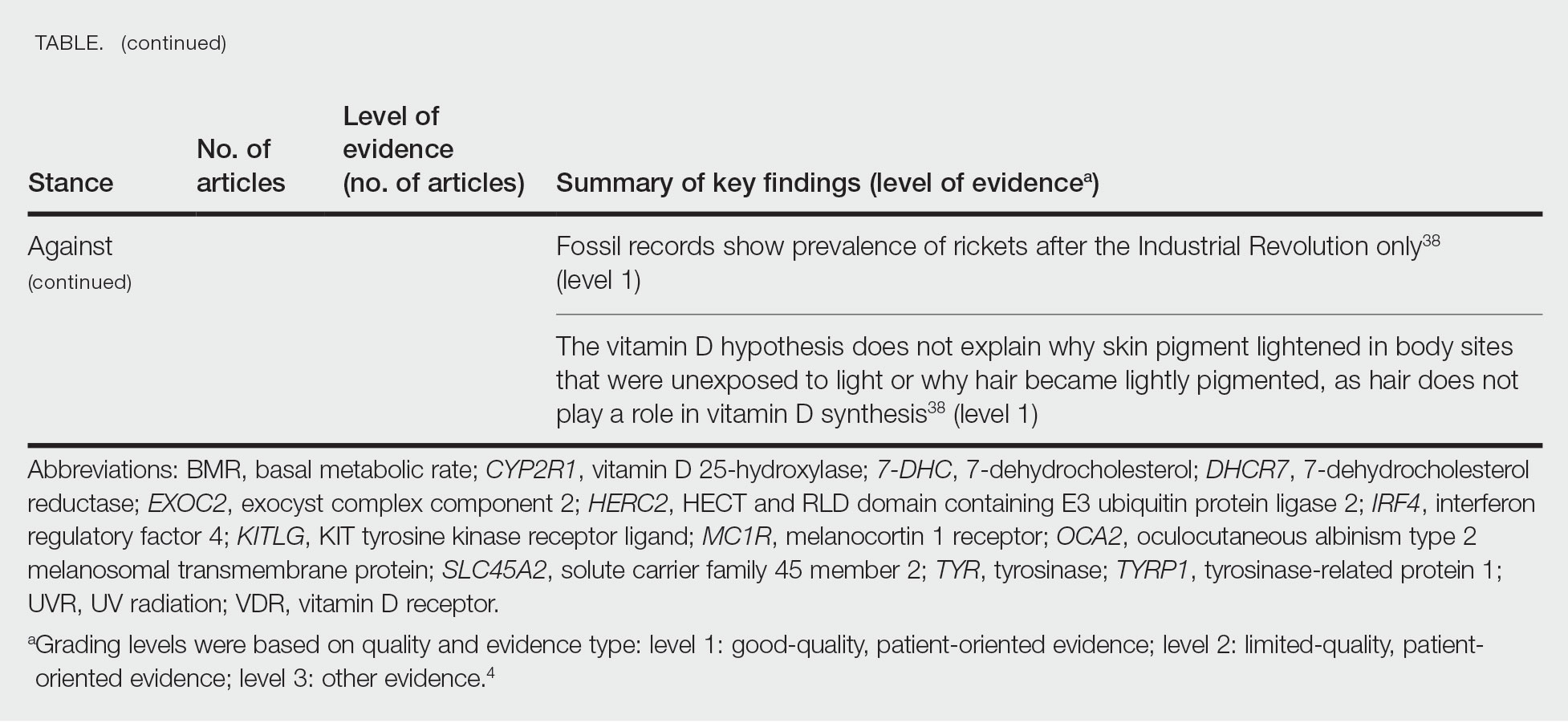
Other noteworthy genes included HERC2, which has implications in the expression of OCA2 (melanocyte-specific transporter protein), and IRF4, which encodes for an important enzyme in folate-dependent melanin production. In an Australian cross-sectional study that analyzed vitamin D and pigmentation gene polymorphisms in conjunction with plasma vitamin D levels, the most notable rate of vitamin D loss occurred in individuals with the darkest pigmentation HERC2 (AA) genotype.31 In contrast, the lightest pigmentation HERC2 (GG) genotypes had increased vitamin D3 photosynthesis. Interestingly, the lightest interferon regulatory factor 4 (IRF4) TT genotype and the darkest HERC2 AA genotype, rendering the greatest folate loss and largest synthesis of vitamin D3, were not seen in combination in any of the participants.30 In addition to HERC2, derived alleles from pigment-associated genes SLC24A5*A and SLC45A2*G demonstrated greater frequencies in Europeans (>90%) compared to Africans and East Asians, where the allelic frequencies were either rare or absent.1 This evidence delineates not only the complexity but also the strong relationship between skin pigmentation, latitude, and vitamin D status. The GWAS also have supported this concept. In comparing European populations to African populations, there was a 4-fold increase in the frequencies of “derived alleles of the vitamin D transport protein (GC, rs3755967), the 25(OH)D3 synthesizing enzyme (CYP2R1, rs10741657), VDR (rs2228570 (commonly known as FokI polymorphism), rs1544410 (Bsm1), and rs731236 (Taq1) and the VDR target genes CYP24A1 (rs17216707), CD14 (rs2569190), and CARD9 (rs4077515).”32
Articles With Evidence Against the Vitamin D Theory—This review analyzed the level of support for the theory that vitamin D was the main driver for skin lightening. Although most articles supported this theory, there were articles that listed other plausible counterarguments. Jablonski and Chaplin3 suggested that humans living in higher latitudes compensated for increased demand of vitamin D by placing cultural importance on a diet of vitamin D–rich foods and thus would not have experienced decreased vitamin D levels, which we hypothesize were the driver for skin lightening. Elias et al39 argued that initial pigment dilution may have instead served to improve metabolic conservation, as the authors found no evidence of rickets—the sequelae of vitamin D deficiency—in pre–industrial age human fossils. Elias and Williams38 proposed that differences in skin pigment are due to a more intact skin permeability barrier as “a requirement for life in a desiccating terrestrial environment,” which is seen in darker skin tones compared to lighter skin tones and thus can survive better in warmer climates with less risk of infections or dehydration.
Articles With Neutral Evidence for the Vitamin D Theory—Greaves41 argued against the idea that skin evolved to become lighter to protect against vitamin D deficiency. They proposed that the chimpanzee, which is the human’s most closely related species, had light skin covered by hair, and the loss of this hair led to exposed pale skin that created a need for increased melanin production for protection from UVR. Greaves41 stated that the MC1R gene (associated with darker pigmentation) was selected for in African populations, and those with pale skin retained their original pigment as they migrated to higher latitudes. Further research has demonstrated that the genetic natural selection for skin pigment is a complex process that involves multiple gene variants found throughout cultures across the globe.
Conclusion
Skin pigmentation has continuously evolved alongside humans. Genetic selection for lighter skin coincides with a favorable selection for genes involved in vitamin D synthesis as humans migrated to northern latitudes, which enabled humans to produce adequate levels of exogenous vitamin D in low-UVR areas and in turn promoted survival. Early humans without access to supplementation or foods rich in vitamin D acquired vitamin D primarily through sunlight. In comparison to modern society, where vitamin D supplementation is accessible and human lifespans are prolonged, lighter skin tone is now a risk factor for malignant cancers of the skin rather than being a protective adaptation. Current sun behavior recommendations conclude that the body’s need for vitamin D is satisfied by UV exposure to the arms, legs, hands, and/or face for only 5 to 30 minutes between 10
The hypothesis that skin lightening primarily was driven by the need for vitamin D can only be partially supported by our review. Studies have shown that there is a corresponding complex network of genes that determines skin pigmentation as well as vitamin D synthesis and conservation. However, there is sufficient evidence that skin lightening is multifactorial in nature, and vitamin D alone may not be the sole driver. The information in this review can be used by health care providers to educate patients on sun protection, given the lesser threat of severe vitamin D deficiency in developed communities today that have access to adequate nutrition and supplementation.
Skin lightening and its coinciding evolutionary drivers are a rather neglected area of research. Due to heterogeneous cohorts and conservative data analysis, GWAS studies run the risk of type II error, yielding a limitation in our data analysis.9 Furthermore, the data regarding specific time frames in evolutionary skin lightening as well as the intensity of gene polymorphisms are limited.1 Further studies are needed to determine the interconnectedness of the current skin-lightening theories to identify other important factors that may play a role in the process. Determining the key event can help us better understand skin-adaptation mechanisms and create a framework for understanding the vital process involved in adaptation, survival, and disease manifestation in different patient populations.
- Beleza S, Santos AM, McEvoy B, et al. The timing of pigmentation lightening in Europeans. Mol Biol Evol. 2013;30:24-35. doi:10.1093/molbev/mss207
- Carlberg C. Nutrigenomics of vitamin D. Nutrients. 2019;11:676. doi:10.3390/nu11030676
- Jablonski NG, Chaplin G. The roles of vitamin D and cutaneous vitamin D production in human evolution and health. Int J Paleopathol. 2018;23:54-59. doi:10.1016/j.ijpp.2018.01.005
- Weiss BD. SORT: strength of recommendation taxonomy. Fam Med. 2004;36:141-143.
- Wolf ST, Kenney WL. The vitamin D–folate hypothesis in human vascular health. Am J Physiol Regul Integr Comp Physiology. 2019;317:R491-R501. doi:10.1152/ajpregu.00136.2019
- Lucock M, Jones P, Martin C, et al. Photobiology of vitamins. Nutr Rev. 2018;76:512-525. doi:10.1093/nutrit/nuy013
- Hochberg Z, Hochberg I. Evolutionary perspective in rickets and vitamin D. Front Endocrinol (Lausanne). 2019;10:306. doi:10.3389/fendo.2019.00306
- Rossberg W, Saternus R, Wagenpfeil S, et al. Human pigmentation, cutaneous vitamin D synthesis and evolution: variants of genes (SNPs) involved in skin pigmentation are associated with 25(OH)D serum concentration. Anticancer Res. 2016;36:1429-1437.
- Saternus R, Pilz S, Gräber S, et al. A closer look at evolution: variants (SNPs) of genes involved in skin pigmentation, including EXOC2, TYR, TYRP1, and DCT, are associated with 25(OH)D serum concentration. Endocrinology. 2015;156:39-47. doi:10.1210/en.2014-1238
- López S, García Ó, Yurrebaso I, et al. The interplay between natural selection and susceptibility to melanoma on allele 374F of SLC45A2 gene in a south European population. PloS One. 2014;9:E104367. doi:1371/journal.pone.0104367
- Lucock M, Yates Z, Martin C, et al. Vitamin D, folate, and potential early lifecycle environmental origin of significant adult phenotypes. Evol Med Public Health. 2014;2014:69-91. doi:10.1093/emph/eou013
- Hudjashov G, Villems R, Kivisild T. Global patterns of diversity and selection in human tyrosinase gene. PloS One. 2013;8:E74307. doi:10.1371/journal.pone.0074307
- Khan R, Khan BSR. Diet, disease and pigment variation in humans. Med Hypotheses. 2010;75:363-367. doi:10.1016/j.mehy.2010.03.033
- Kuan V, Martineau AR, Griffiths CJ, et al. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evol Biol. 2013;13:144. doi:10.1186/1471-2148-13-144
- Omenn GS. Evolution and public health. Proc National Acad Sci. 2010;107(suppl 1):1702-1709. doi:10.1073/pnas.0906198106
- Yuen AWC, Jablonski NG. Vitamin D: in the evolution of human skin colour. Med Hypotheses. 2010;74:39-44. doi:10.1016/j.mehy.2009.08.007
- Vieth R. Weaker bones and white skin as adaptions to improve anthropological “fitness” for northern environments. Osteoporosis Int. 2020;31:617-624. doi:10.1007/s00198-019-05167-4
- Carlberg C. Vitamin D: a micronutrient regulating genes. Curr Pharm Des. 2019;25:1740-1746. doi:10.2174/1381612825666190705193227
- Haddadeen C, Lai C, Cho SY, et al. Variants of the melanocortin‐1 receptor: do they matter clinically? Exp Dermatol. 2015;1:5-9. doi:10.1111/exd.12540
- Yao S, Ambrosone CB. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J Steroid Biochem Mol Biol. 2013;136:337-341. doi:10.1016/j.jsbmb.2012.09.010
- Jablonski N. The evolution of human skin colouration and its relevance to health in the modern world. J Royal Coll Physicians Edinb. 2012;42:58-63. doi:10.4997/jrcpe.2012.114
- Jablonski NG, Chaplin G. Human skin pigmentation as an adaptation to UV radiation. Proc National Acad Sci. 2010;107(suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Hochberg Z, Templeton AR. Evolutionary perspective in skin color, vitamin D and its receptor. Hormones. 2010;9:307-311. doi:10.14310/horm.2002.1281
- Jones P, Lucock M, Veysey M, et al. The vitamin D–folate hypothesis as an evolutionary model for skin pigmentation: an update and integration of current ideas. Nutrients. 2018;10:554. doi:10.3390/nu10050554
- Lindqvist PG, Epstein E, Landin-Olsson M, et al. Women with fair phenotypes seem to confer a survival advantage in a low UV milieu. a nested matched case control study. PloS One. 2020;15:E0228582. doi:10.1371/journal.pone.0228582
- Holick MF. Shedding new light on the role of the sunshine vitamin D for skin health: the lncRNA–skin cancer connection. Exp Dermatol. 2014;23:391-392. doi:10.1111/exd.12386
- Jablonski NG, Chaplin G. Epidermal pigmentation in the human lineage is an adaptation to ultraviolet radiation. J Hum Evol. 2013;65:671-675. doi:10.1016/j.jhevol.2013.06.004
- Jablonski NG, Chaplin G. The evolution of skin pigmentation and hair texture in people of African ancestry. Dermatol Clin. 2014;32:113-121. doi:10.1016/j.det.2013.11.003
- Jablonski NG. The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell Melanoma Res. 2021;34:707-7 doi:10.1111/pcmr.12976
- Lucock MD, Jones PR, Veysey M, et al. Biophysical evidence to support and extend the vitamin D‐folate hypothesis as a paradigm for the evolution of human skin pigmentation. Am J Hum Biol. 2022;34:E23667. doi:10.1002/ajhb.23667
- Missaggia BO, Reales G, Cybis GB, et al. Adaptation and co‐adaptation of skin pigmentation and vitamin D genes in native Americans. Am J Med Genet C Semin Med Genet. 2020;184:1060-1077. doi:10.1002/ajmg.c.31873
- Hanel A, Carlberg C. Skin colour and vitamin D: an update. Exp Dermatol. 2020;29:864-875. doi:10.1111/exd.14142
- Hanel A, Carlberg C. Vitamin D and evolution: pharmacologic implications. Biochem Pharmacol. 2020;173:113595. doi:10.1016/j.bcp.2019.07.024
- Flegr J, Sýkorová K, Fiala V, et al. Increased 25(OH)D3 level in redheaded people: could redheadedness be an adaptation to temperate climate? Exp Dermatol. 2020;29:598-609. doi:10.1111/exd.14119
- James WPT, Johnson RJ, Speakman JR, et al. Nutrition and its role in human evolution. J Intern Med. 2019;285:533-549. doi:10.1111/joim.12878
- Lucock M, Jones P, Martin C, et al. Vitamin D: beyond metabolism. J Evid Based Complementary Altern Med. 2015;20:310-322. doi:10.1177/2156587215580491
- Jarrett P, Scragg R. Evolution, prehistory and vitamin D. Int J Environ Res Public Health. 2020;17:646. doi:10.3390/ijerph17020646
- Elias PM, Williams ML. Re-appraisal of current theories for thedevelopment and loss of epidermal pigmentation in hominins and modern humans. J Hum Evol. 2013;64:687-692. doi:10.1016/j.jhevol.2013.02.003
- Elias PM, Williams ML. Basis for the gain and subsequent dilution of epidermal pigmentation during human evolution: the barrier and metabolic conservation hypotheses revisited. Am J Phys Anthropol. 2016;161:189-207. doi:10.1002/ajpa.23030
- Williams JD, Jacobson EL, Kim H, et al. Water soluble vitamins, clinical research and future application. Subcell Biochem. 2011;56:181-197. doi:10.1007/978-94-007-2199-9_10
- Greaves M. Was skin cancer a selective force for black pigmentation in early hominin evolution [published online February 26, 2014]? Proc Biol Sci. 2014;281:20132955. doi:10.1098/rspb.2013.2955
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. doi:10.1056/nejmra070553
- Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466-479. doi:10.1038/nrendo.2017.31
- US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. US Dept of Health and Human Services, Office of the Surgeon General; 2014. Accessed April 29, 2024. https://www.hhs.gov/sites/default/files/call-to-action-prevent-skin-cancer.pdf
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; 2011. https://www.ncbi.nlm.nih.gov/books/NBK56070/
The risk for developing skin cancer can be somewhat attributed to variations in skin pigmentation. Historically, lighter skin pigmentation has been observed in populations living in higher latitudes and darker pigmentation in populations near the equator. Although skin pigmentation is a conglomeration of genetic and environmental factors, anthropologic studies have demonstrated an association of human skin lightening with historic human migratory patterns.1 It is postulated that migration to latitudes with less UVB light penetration has resulted in a compensatory natural selection of lighter skin types. Furthermore, the driving force behind this migration-associated skin lightening has remained unclear.1
The need for folate metabolism, vitamin D synthesis, and barrier protection, as well as cultural practices, has been postulated as driving factors for skin pigmentation variation. Synthesis of vitamin D is a UV radiation (UVR)–dependent process and has remained a prominent theoretical driver for the basis of evolutionary skin lightening. Vitamin D can be acquired both exogenously or endogenously via dietary supplementation or sunlight; however, historically it has been obtained through UVB exposure primarily. Once UVB is absorbed by the skin, it catalyzes conversion of 7-dehydrocholesterol to previtamin D3, which is converted to vitamin D in the kidneys.2,3 It is suggested that lighter skin tones have an advantage over darker skin tones in synthesizing vitamin D at higher latitudes where there is less UVB, thus leading to the adaptation process.1 In this systematic review, we analyzed the evolutionary vitamin D adaptation hypothesis and assessed the validity of evidence supporting this theory in the literature.
Methods
A search of PubMed, Embase, and the Cochrane Reviews database was conducted using the terms evolution, vitamin D, and skin to generate articles published from 2010 to 2022 that evaluated the influence of UVR-dependent production of vitamin D on skin pigmentation through historical migration patterns (Figure). Studies were excluded during an initial screening of abstracts followed by full-text assessment if they only had abstracts and if articles were inaccessible for review or in the form of case reports and commentaries.
The following data were extracted from each included study: reference citation, affiliated institutions of authors, author specialties, journal name, year of publication, study period, type of article, type of study, mechanism of adaptation, data concluding or supporting vitamin D as the driver, and data concluding or suggesting against vitamin D as the driver. Data concluding or supporting vitamin D as the driver were recorded from statistically significant results, study conclusions, and direct quotations. Data concluding or suggesting against vitamin D as the driver also were recorded from significant results, study conclusions, and direct quotes. The mechanism of adaptation was based on vitamin D synthesis modulation, melanin upregulation, genetic selections, genetic drift, mating patterns, increased vitamin D sensitivity, interbreeding, and diet.
Studies included in the analysis were placed into 1 of 3 categories: supporting, neutral, and against. Strength of Recommendation Taxonomy (SORT) criteria were used to classify the level of evidence of each article.4 Each article’s level of evidence was then graded (Table 1). The SORT grading levels were based on quality and evidence type: level 1 signified good-quality, patient-oriented evidence; level 2 signified limited-quality, patient-oriented evidence; and level 3 signified other evidence.4
Results
Article Selection—A total of 229 articles were identified for screening, and 39 studies met inclusion criteria.1-3,5-40 Systematic and retrospective reviews were the most common types of studies. Genomic analysis/sequencing/genome-wide association studies (GWAS) were the most common methods of analysis. Of these 39 articles, 26 were classified as supporting the evolutionary vitamin D adaptation hypothesis, 10 were classified as neutral, and 3 were classified as against (Table 1).
Of the articles classified as supporting the vitamin D hypothesis, 13 articles were level 1 evidence, 9 were level 2, and 4 were level 3. Key findings supporting the vitamin D hypothesis included genetic natural selection favoring vitamin D synthesis genes at higher latitudes with lower UVR and the skin lightening that occurred to protect against vitamin D deficiency (Table 1). Specific genes supporting these findings included 7-dehydrocholesterol reductase (DHCR7), vitamin D receptor (VDR), tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), oculocutaneous albinism type 2 melanosomal transmembrane protein (OCA2), solute carrier family 45 member 2 (SLC45A2), solute carrier family 4 member 5 (SLC24A5), Kit ligand (KITLG), melanocortin 1 receptor (MC1R), and HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2)(Table 2).

Of the articles classified as being against the vitamin D hypothesis, 1 article was level 1 evidence, 1 was level 2, and 1 was level 3. Key findings refuting the vitamin D hypothesis included similar amounts of vitamin D synthesis in contemporary dark- and light-pigmented individuals, vitamin D–rich diets in the late Paleolithic period and in early agriculturalists, and metabolic conservation being the primary driver (Table 1).
Of the articles classified as neutral to the hypothesis, 7 articles were level 1 evidence and 3 were level 2. Key findings of these articles included genetic selection favoring vitamin D synthesis only for populations at extremely northern latitudes, skin lightening that was sustained in northern latitudes from the neighboring human ancestor the chimpanzee, and evidence for long-term evolutionary pressures and short-term plastic adaptations in vitamin D genes (Table 1).
Comment
The importance of appropriate vitamin D levels is hypothesized as a potent driver in skin lightening because the vitamin is essential for many biochemical processes within the human body. Proper calcification of bones requires activated vitamin D to prevent rickets in childhood. Pelvic deformation in women with rickets can obstruct childbirth in primitive medical environments.15 This direct reproductive impairment suggests a strong selective pressure for skin lightening in populations that migrated northward to enhance vitamin D synthesis.
Of the 39 articles that we reviewed, the majority (n=26 [66.7%]) supported the hypothesis that vitamin D synthesis was the main driver behind skin lightening, whereas 3 (7.7%) did not support the hypothesis and 10 (25.6%) were neutral. Other leading theories explaining skin lightening included the idea that enhanced melanogenesis protected against folate degradation; genetic selection for light-skin alleles due to genetic drift; skin lightening being the result of sexual selection; and a combination of factors, including dietary choices, clothing preferences, and skin permeability barriers.
Articles With Supporting Evidence for the Vitamin D Theory—As Homo sapiens migrated out of Africa, migration patterns demonstrated the correlation between distance from the equator and skin pigmentation from natural selection. Individuals with darker skin pigment required higher levels of UVR to synthesize vitamin D. According to Beleza et al,1 as humans migrated to areas of higher latitudes with lower levels of UVR, natural selection favored the development of lighter skin to maximize vitamin D production. Vitamin D is linked to calcium metabolism, and its deficiency can lead to bone malformations and poor immune function.35 Several genes affecting melanogenesis and skin pigment have been found to have geospatial patterns that map to different geographic locations of various populations, indicating how human migration patterns out of Africa created this natural selection for skin lightening. The gene KITLG—associated with lighter skin pigmentation—has been found in high frequencies in both European and East Asian populations and is proposed to have increased in frequency after the migration out of Africa. However, the genes TYRP1, SLC24A5, and SLC45A2 were found at high frequencies only in European populations, and this selection occurred 11,000 to 19,000 years ago during the Last Glacial Maximum (15,000–20,000 years ago), demonstrating the selection for European over East Asian characteristics. During this period, seasonal changes increased the risk for vitamin D deficiency and provided an urgency for selection to a lighter skin pigment.1
The migration of H sapiens to northern latitudes prompted the selection of alleles that would increasevitamin D synthesis to counteract the reduced UV exposure. Genetic analysis studies have found key associations between genes encoding for the metabolism of vitamin D and pigmentation. Among this complex network are the essential downstream enzymes in the melanocortin receptor 1 pathway, including TYR and TYRP1. Forty-six of 960 single-nucleotide polymorphisms located in 29 different genes involved in skin pigmentation that were analyzed in a cohort of 2970 individuals were significantly associated with serum vitamin D levels (P<.05). The exocyst complex component 2 (EXOC2), TYR, and TYRP1 gene variants were shown to have the greatest influence on vitamin D status.9 These data reveal how pigment genotypes are predictive of vitamin D levels and the epistatic potential among many genes in this complex network.
Gene variation plays an important role in vitamin D status when comparing genetic polymorphisms in populations in northern latitudes to African populations. Vitamin D3 precursor availability is decreased by 7-DHCR catalyzing the precursors substrate to cholesterol. In a study using GWAS, it was found that “variations in DHCR7 may aid vitamin D production by conserving cutaneous 7-DHC levels. A high prevalence of DHCR7 variants were found in European and Northeast Asian populations but not in African populations, suggesting that selection occurred for these DHCR7 mutations in populations who migrated to more northern latitudes.5 Multilocus networks have been established between the VDR promotor and skin color genes (Table 2) that exhibit a strong in-Africa vs out-of-Africa frequency pattern. It also has been shown that genetic variation (suggesting a long-term evolutionary inclination) and epigenetic modification (indicative of short-term exposure) of VDR lends support to the vitamin D hypothesis. As latitude decreases, prevalence of VDR FokI (F allele), BsmI (B allele), ApaI (A allele), and TaqI (T allele) also decreases in a linear manner, linking latitude to VDR polymorphisms. Plasma vitamin D levels and photoperiod of conception—UV exposure during the periconceptional period—also were extrapolative of VDR methylation in a study involving 80 participants, where these 2 factors accounted for 17% of variance in methylation.6


Other noteworthy genes included HERC2, which has implications in the expression of OCA2 (melanocyte-specific transporter protein), and IRF4, which encodes for an important enzyme in folate-dependent melanin production. In an Australian cross-sectional study that analyzed vitamin D and pigmentation gene polymorphisms in conjunction with plasma vitamin D levels, the most notable rate of vitamin D loss occurred in individuals with the darkest pigmentation HERC2 (AA) genotype.31 In contrast, the lightest pigmentation HERC2 (GG) genotypes had increased vitamin D3 photosynthesis. Interestingly, the lightest interferon regulatory factor 4 (IRF4) TT genotype and the darkest HERC2 AA genotype, rendering the greatest folate loss and largest synthesis of vitamin D3, were not seen in combination in any of the participants.30 In addition to HERC2, derived alleles from pigment-associated genes SLC24A5*A and SLC45A2*G demonstrated greater frequencies in Europeans (>90%) compared to Africans and East Asians, where the allelic frequencies were either rare or absent.1 This evidence delineates not only the complexity but also the strong relationship between skin pigmentation, latitude, and vitamin D status. The GWAS also have supported this concept. In comparing European populations to African populations, there was a 4-fold increase in the frequencies of “derived alleles of the vitamin D transport protein (GC, rs3755967), the 25(OH)D3 synthesizing enzyme (CYP2R1, rs10741657), VDR (rs2228570 (commonly known as FokI polymorphism), rs1544410 (Bsm1), and rs731236 (Taq1) and the VDR target genes CYP24A1 (rs17216707), CD14 (rs2569190), and CARD9 (rs4077515).”32
Articles With Evidence Against the Vitamin D Theory—This review analyzed the level of support for the theory that vitamin D was the main driver for skin lightening. Although most articles supported this theory, there were articles that listed other plausible counterarguments. Jablonski and Chaplin3 suggested that humans living in higher latitudes compensated for increased demand of vitamin D by placing cultural importance on a diet of vitamin D–rich foods and thus would not have experienced decreased vitamin D levels, which we hypothesize were the driver for skin lightening. Elias et al39 argued that initial pigment dilution may have instead served to improve metabolic conservation, as the authors found no evidence of rickets—the sequelae of vitamin D deficiency—in pre–industrial age human fossils. Elias and Williams38 proposed that differences in skin pigment are due to a more intact skin permeability barrier as “a requirement for life in a desiccating terrestrial environment,” which is seen in darker skin tones compared to lighter skin tones and thus can survive better in warmer climates with less risk of infections or dehydration.
Articles With Neutral Evidence for the Vitamin D Theory—Greaves41 argued against the idea that skin evolved to become lighter to protect against vitamin D deficiency. They proposed that the chimpanzee, which is the human’s most closely related species, had light skin covered by hair, and the loss of this hair led to exposed pale skin that created a need for increased melanin production for protection from UVR. Greaves41 stated that the MC1R gene (associated with darker pigmentation) was selected for in African populations, and those with pale skin retained their original pigment as they migrated to higher latitudes. Further research has demonstrated that the genetic natural selection for skin pigment is a complex process that involves multiple gene variants found throughout cultures across the globe.

Conclusion
Skin pigmentation has continuously evolved alongside humans. Genetic selection for lighter skin coincides with a favorable selection for genes involved in vitamin D synthesis as humans migrated to northern latitudes, which enabled humans to produce adequate levels of exogenous vitamin D in low-UVR areas and in turn promoted survival. Early humans without access to supplementation or foods rich in vitamin D acquired vitamin D primarily through sunlight. In comparison to modern society, where vitamin D supplementation is accessible and human lifespans are prolonged, lighter skin tone is now a risk factor for malignant cancers of the skin rather than being a protective adaptation. Current sun behavior recommendations conclude that the body’s need for vitamin D is satisfied by UV exposure to the arms, legs, hands, and/or face for only 5 to 30 minutes between 10
The hypothesis that skin lightening primarily was driven by the need for vitamin D can only be partially supported by our review. Studies have shown that there is a corresponding complex network of genes that determines skin pigmentation as well as vitamin D synthesis and conservation. However, there is sufficient evidence that skin lightening is multifactorial in nature, and vitamin D alone may not be the sole driver. The information in this review can be used by health care providers to educate patients on sun protection, given the lesser threat of severe vitamin D deficiency in developed communities today that have access to adequate nutrition and supplementation.
Skin lightening and its coinciding evolutionary drivers are a rather neglected area of research. Due to heterogeneous cohorts and conservative data analysis, GWAS studies run the risk of type II error, yielding a limitation in our data analysis.9 Furthermore, the data regarding specific time frames in evolutionary skin lightening as well as the intensity of gene polymorphisms are limited.1 Further studies are needed to determine the interconnectedness of the current skin-lightening theories to identify other important factors that may play a role in the process. Determining the key event can help us better understand skin-adaptation mechanisms and create a framework for understanding the vital process involved in adaptation, survival, and disease manifestation in different patient populations.
The risk for developing skin cancer can be somewhat attributed to variations in skin pigmentation. Historically, lighter skin pigmentation has been observed in populations living in higher latitudes and darker pigmentation in populations near the equator. Although skin pigmentation is a conglomeration of genetic and environmental factors, anthropologic studies have demonstrated an association of human skin lightening with historic human migratory patterns.1 It is postulated that migration to latitudes with less UVB light penetration has resulted in a compensatory natural selection of lighter skin types. Furthermore, the driving force behind this migration-associated skin lightening has remained unclear.1
The need for folate metabolism, vitamin D synthesis, and barrier protection, as well as cultural practices, has been postulated as driving factors for skin pigmentation variation. Synthesis of vitamin D is a UV radiation (UVR)–dependent process and has remained a prominent theoretical driver for the basis of evolutionary skin lightening. Vitamin D can be acquired both exogenously or endogenously via dietary supplementation or sunlight; however, historically it has been obtained through UVB exposure primarily. Once UVB is absorbed by the skin, it catalyzes conversion of 7-dehydrocholesterol to previtamin D3, which is converted to vitamin D in the kidneys.2,3 It is suggested that lighter skin tones have an advantage over darker skin tones in synthesizing vitamin D at higher latitudes where there is less UVB, thus leading to the adaptation process.1 In this systematic review, we analyzed the evolutionary vitamin D adaptation hypothesis and assessed the validity of evidence supporting this theory in the literature.
Methods
A search of PubMed, Embase, and the Cochrane Reviews database was conducted using the terms evolution, vitamin D, and skin to generate articles published from 2010 to 2022 that evaluated the influence of UVR-dependent production of vitamin D on skin pigmentation through historical migration patterns (Figure). Studies were excluded during an initial screening of abstracts followed by full-text assessment if they only had abstracts and if articles were inaccessible for review or in the form of case reports and commentaries.
The following data were extracted from each included study: reference citation, affiliated institutions of authors, author specialties, journal name, year of publication, study period, type of article, type of study, mechanism of adaptation, data concluding or supporting vitamin D as the driver, and data concluding or suggesting against vitamin D as the driver. Data concluding or supporting vitamin D as the driver were recorded from statistically significant results, study conclusions, and direct quotations. Data concluding or suggesting against vitamin D as the driver also were recorded from significant results, study conclusions, and direct quotes. The mechanism of adaptation was based on vitamin D synthesis modulation, melanin upregulation, genetic selections, genetic drift, mating patterns, increased vitamin D sensitivity, interbreeding, and diet.
Studies included in the analysis were placed into 1 of 3 categories: supporting, neutral, and against. Strength of Recommendation Taxonomy (SORT) criteria were used to classify the level of evidence of each article.4 Each article’s level of evidence was then graded (Table 1). The SORT grading levels were based on quality and evidence type: level 1 signified good-quality, patient-oriented evidence; level 2 signified limited-quality, patient-oriented evidence; and level 3 signified other evidence.4
Results
Article Selection—A total of 229 articles were identified for screening, and 39 studies met inclusion criteria.1-3,5-40 Systematic and retrospective reviews were the most common types of studies. Genomic analysis/sequencing/genome-wide association studies (GWAS) were the most common methods of analysis. Of these 39 articles, 26 were classified as supporting the evolutionary vitamin D adaptation hypothesis, 10 were classified as neutral, and 3 were classified as against (Table 1).
Of the articles classified as supporting the vitamin D hypothesis, 13 articles were level 1 evidence, 9 were level 2, and 4 were level 3. Key findings supporting the vitamin D hypothesis included genetic natural selection favoring vitamin D synthesis genes at higher latitudes with lower UVR and the skin lightening that occurred to protect against vitamin D deficiency (Table 1). Specific genes supporting these findings included 7-dehydrocholesterol reductase (DHCR7), vitamin D receptor (VDR), tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), oculocutaneous albinism type 2 melanosomal transmembrane protein (OCA2), solute carrier family 45 member 2 (SLC45A2), solute carrier family 4 member 5 (SLC24A5), Kit ligand (KITLG), melanocortin 1 receptor (MC1R), and HECT and RLD domain containing E3 ubiquitin protein ligase 2 (HERC2)(Table 2).

Of the articles classified as being against the vitamin D hypothesis, 1 article was level 1 evidence, 1 was level 2, and 1 was level 3. Key findings refuting the vitamin D hypothesis included similar amounts of vitamin D synthesis in contemporary dark- and light-pigmented individuals, vitamin D–rich diets in the late Paleolithic period and in early agriculturalists, and metabolic conservation being the primary driver (Table 1).
Of the articles classified as neutral to the hypothesis, 7 articles were level 1 evidence and 3 were level 2. Key findings of these articles included genetic selection favoring vitamin D synthesis only for populations at extremely northern latitudes, skin lightening that was sustained in northern latitudes from the neighboring human ancestor the chimpanzee, and evidence for long-term evolutionary pressures and short-term plastic adaptations in vitamin D genes (Table 1).
Comment
The importance of appropriate vitamin D levels is hypothesized as a potent driver in skin lightening because the vitamin is essential for many biochemical processes within the human body. Proper calcification of bones requires activated vitamin D to prevent rickets in childhood. Pelvic deformation in women with rickets can obstruct childbirth in primitive medical environments.15 This direct reproductive impairment suggests a strong selective pressure for skin lightening in populations that migrated northward to enhance vitamin D synthesis.
Of the 39 articles that we reviewed, the majority (n=26 [66.7%]) supported the hypothesis that vitamin D synthesis was the main driver behind skin lightening, whereas 3 (7.7%) did not support the hypothesis and 10 (25.6%) were neutral. Other leading theories explaining skin lightening included the idea that enhanced melanogenesis protected against folate degradation; genetic selection for light-skin alleles due to genetic drift; skin lightening being the result of sexual selection; and a combination of factors, including dietary choices, clothing preferences, and skin permeability barriers.
Articles With Supporting Evidence for the Vitamin D Theory—As Homo sapiens migrated out of Africa, migration patterns demonstrated the correlation between distance from the equator and skin pigmentation from natural selection. Individuals with darker skin pigment required higher levels of UVR to synthesize vitamin D. According to Beleza et al,1 as humans migrated to areas of higher latitudes with lower levels of UVR, natural selection favored the development of lighter skin to maximize vitamin D production. Vitamin D is linked to calcium metabolism, and its deficiency can lead to bone malformations and poor immune function.35 Several genes affecting melanogenesis and skin pigment have been found to have geospatial patterns that map to different geographic locations of various populations, indicating how human migration patterns out of Africa created this natural selection for skin lightening. The gene KITLG—associated with lighter skin pigmentation—has been found in high frequencies in both European and East Asian populations and is proposed to have increased in frequency after the migration out of Africa. However, the genes TYRP1, SLC24A5, and SLC45A2 were found at high frequencies only in European populations, and this selection occurred 11,000 to 19,000 years ago during the Last Glacial Maximum (15,000–20,000 years ago), demonstrating the selection for European over East Asian characteristics. During this period, seasonal changes increased the risk for vitamin D deficiency and provided an urgency for selection to a lighter skin pigment.1
The migration of H sapiens to northern latitudes prompted the selection of alleles that would increasevitamin D synthesis to counteract the reduced UV exposure. Genetic analysis studies have found key associations between genes encoding for the metabolism of vitamin D and pigmentation. Among this complex network are the essential downstream enzymes in the melanocortin receptor 1 pathway, including TYR and TYRP1. Forty-six of 960 single-nucleotide polymorphisms located in 29 different genes involved in skin pigmentation that were analyzed in a cohort of 2970 individuals were significantly associated with serum vitamin D levels (P<.05). The exocyst complex component 2 (EXOC2), TYR, and TYRP1 gene variants were shown to have the greatest influence on vitamin D status.9 These data reveal how pigment genotypes are predictive of vitamin D levels and the epistatic potential among many genes in this complex network.
Gene variation plays an important role in vitamin D status when comparing genetic polymorphisms in populations in northern latitudes to African populations. Vitamin D3 precursor availability is decreased by 7-DHCR catalyzing the precursors substrate to cholesterol. In a study using GWAS, it was found that “variations in DHCR7 may aid vitamin D production by conserving cutaneous 7-DHC levels. A high prevalence of DHCR7 variants were found in European and Northeast Asian populations but not in African populations, suggesting that selection occurred for these DHCR7 mutations in populations who migrated to more northern latitudes.5 Multilocus networks have been established between the VDR promotor and skin color genes (Table 2) that exhibit a strong in-Africa vs out-of-Africa frequency pattern. It also has been shown that genetic variation (suggesting a long-term evolutionary inclination) and epigenetic modification (indicative of short-term exposure) of VDR lends support to the vitamin D hypothesis. As latitude decreases, prevalence of VDR FokI (F allele), BsmI (B allele), ApaI (A allele), and TaqI (T allele) also decreases in a linear manner, linking latitude to VDR polymorphisms. Plasma vitamin D levels and photoperiod of conception—UV exposure during the periconceptional period—also were extrapolative of VDR methylation in a study involving 80 participants, where these 2 factors accounted for 17% of variance in methylation.6


Other noteworthy genes included HERC2, which has implications in the expression of OCA2 (melanocyte-specific transporter protein), and IRF4, which encodes for an important enzyme in folate-dependent melanin production. In an Australian cross-sectional study that analyzed vitamin D and pigmentation gene polymorphisms in conjunction with plasma vitamin D levels, the most notable rate of vitamin D loss occurred in individuals with the darkest pigmentation HERC2 (AA) genotype.31 In contrast, the lightest pigmentation HERC2 (GG) genotypes had increased vitamin D3 photosynthesis. Interestingly, the lightest interferon regulatory factor 4 (IRF4) TT genotype and the darkest HERC2 AA genotype, rendering the greatest folate loss and largest synthesis of vitamin D3, were not seen in combination in any of the participants.30 In addition to HERC2, derived alleles from pigment-associated genes SLC24A5*A and SLC45A2*G demonstrated greater frequencies in Europeans (>90%) compared to Africans and East Asians, where the allelic frequencies were either rare or absent.1 This evidence delineates not only the complexity but also the strong relationship between skin pigmentation, latitude, and vitamin D status. The GWAS also have supported this concept. In comparing European populations to African populations, there was a 4-fold increase in the frequencies of “derived alleles of the vitamin D transport protein (GC, rs3755967), the 25(OH)D3 synthesizing enzyme (CYP2R1, rs10741657), VDR (rs2228570 (commonly known as FokI polymorphism), rs1544410 (Bsm1), and rs731236 (Taq1) and the VDR target genes CYP24A1 (rs17216707), CD14 (rs2569190), and CARD9 (rs4077515).”32
Articles With Evidence Against the Vitamin D Theory—This review analyzed the level of support for the theory that vitamin D was the main driver for skin lightening. Although most articles supported this theory, there were articles that listed other plausible counterarguments. Jablonski and Chaplin3 suggested that humans living in higher latitudes compensated for increased demand of vitamin D by placing cultural importance on a diet of vitamin D–rich foods and thus would not have experienced decreased vitamin D levels, which we hypothesize were the driver for skin lightening. Elias et al39 argued that initial pigment dilution may have instead served to improve metabolic conservation, as the authors found no evidence of rickets—the sequelae of vitamin D deficiency—in pre–industrial age human fossils. Elias and Williams38 proposed that differences in skin pigment are due to a more intact skin permeability barrier as “a requirement for life in a desiccating terrestrial environment,” which is seen in darker skin tones compared to lighter skin tones and thus can survive better in warmer climates with less risk of infections or dehydration.
Articles With Neutral Evidence for the Vitamin D Theory—Greaves41 argued against the idea that skin evolved to become lighter to protect against vitamin D deficiency. They proposed that the chimpanzee, which is the human’s most closely related species, had light skin covered by hair, and the loss of this hair led to exposed pale skin that created a need for increased melanin production for protection from UVR. Greaves41 stated that the MC1R gene (associated with darker pigmentation) was selected for in African populations, and those with pale skin retained their original pigment as they migrated to higher latitudes. Further research has demonstrated that the genetic natural selection for skin pigment is a complex process that involves multiple gene variants found throughout cultures across the globe.

Conclusion
Skin pigmentation has continuously evolved alongside humans. Genetic selection for lighter skin coincides with a favorable selection for genes involved in vitamin D synthesis as humans migrated to northern latitudes, which enabled humans to produce adequate levels of exogenous vitamin D in low-UVR areas and in turn promoted survival. Early humans without access to supplementation or foods rich in vitamin D acquired vitamin D primarily through sunlight. In comparison to modern society, where vitamin D supplementation is accessible and human lifespans are prolonged, lighter skin tone is now a risk factor for malignant cancers of the skin rather than being a protective adaptation. Current sun behavior recommendations conclude that the body’s need for vitamin D is satisfied by UV exposure to the arms, legs, hands, and/or face for only 5 to 30 minutes between 10
The hypothesis that skin lightening primarily was driven by the need for vitamin D can only be partially supported by our review. Studies have shown that there is a corresponding complex network of genes that determines skin pigmentation as well as vitamin D synthesis and conservation. However, there is sufficient evidence that skin lightening is multifactorial in nature, and vitamin D alone may not be the sole driver. The information in this review can be used by health care providers to educate patients on sun protection, given the lesser threat of severe vitamin D deficiency in developed communities today that have access to adequate nutrition and supplementation.
Skin lightening and its coinciding evolutionary drivers are a rather neglected area of research. Due to heterogeneous cohorts and conservative data analysis, GWAS studies run the risk of type II error, yielding a limitation in our data analysis.9 Furthermore, the data regarding specific time frames in evolutionary skin lightening as well as the intensity of gene polymorphisms are limited.1 Further studies are needed to determine the interconnectedness of the current skin-lightening theories to identify other important factors that may play a role in the process. Determining the key event can help us better understand skin-adaptation mechanisms and create a framework for understanding the vital process involved in adaptation, survival, and disease manifestation in different patient populations.
- Beleza S, Santos AM, McEvoy B, et al. The timing of pigmentation lightening in Europeans. Mol Biol Evol. 2013;30:24-35. doi:10.1093/molbev/mss207
- Carlberg C. Nutrigenomics of vitamin D. Nutrients. 2019;11:676. doi:10.3390/nu11030676
- Jablonski NG, Chaplin G. The roles of vitamin D and cutaneous vitamin D production in human evolution and health. Int J Paleopathol. 2018;23:54-59. doi:10.1016/j.ijpp.2018.01.005
- Weiss BD. SORT: strength of recommendation taxonomy. Fam Med. 2004;36:141-143.
- Wolf ST, Kenney WL. The vitamin D–folate hypothesis in human vascular health. Am J Physiol Regul Integr Comp Physiology. 2019;317:R491-R501. doi:10.1152/ajpregu.00136.2019
- Lucock M, Jones P, Martin C, et al. Photobiology of vitamins. Nutr Rev. 2018;76:512-525. doi:10.1093/nutrit/nuy013
- Hochberg Z, Hochberg I. Evolutionary perspective in rickets and vitamin D. Front Endocrinol (Lausanne). 2019;10:306. doi:10.3389/fendo.2019.00306
- Rossberg W, Saternus R, Wagenpfeil S, et al. Human pigmentation, cutaneous vitamin D synthesis and evolution: variants of genes (SNPs) involved in skin pigmentation are associated with 25(OH)D serum concentration. Anticancer Res. 2016;36:1429-1437.
- Saternus R, Pilz S, Gräber S, et al. A closer look at evolution: variants (SNPs) of genes involved in skin pigmentation, including EXOC2, TYR, TYRP1, and DCT, are associated with 25(OH)D serum concentration. Endocrinology. 2015;156:39-47. doi:10.1210/en.2014-1238
- López S, García Ó, Yurrebaso I, et al. The interplay between natural selection and susceptibility to melanoma on allele 374F of SLC45A2 gene in a south European population. PloS One. 2014;9:E104367. doi:1371/journal.pone.0104367
- Lucock M, Yates Z, Martin C, et al. Vitamin D, folate, and potential early lifecycle environmental origin of significant adult phenotypes. Evol Med Public Health. 2014;2014:69-91. doi:10.1093/emph/eou013
- Hudjashov G, Villems R, Kivisild T. Global patterns of diversity and selection in human tyrosinase gene. PloS One. 2013;8:E74307. doi:10.1371/journal.pone.0074307
- Khan R, Khan BSR. Diet, disease and pigment variation in humans. Med Hypotheses. 2010;75:363-367. doi:10.1016/j.mehy.2010.03.033
- Kuan V, Martineau AR, Griffiths CJ, et al. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evol Biol. 2013;13:144. doi:10.1186/1471-2148-13-144
- Omenn GS. Evolution and public health. Proc National Acad Sci. 2010;107(suppl 1):1702-1709. doi:10.1073/pnas.0906198106
- Yuen AWC, Jablonski NG. Vitamin D: in the evolution of human skin colour. Med Hypotheses. 2010;74:39-44. doi:10.1016/j.mehy.2009.08.007
- Vieth R. Weaker bones and white skin as adaptions to improve anthropological “fitness” for northern environments. Osteoporosis Int. 2020;31:617-624. doi:10.1007/s00198-019-05167-4
- Carlberg C. Vitamin D: a micronutrient regulating genes. Curr Pharm Des. 2019;25:1740-1746. doi:10.2174/1381612825666190705193227
- Haddadeen C, Lai C, Cho SY, et al. Variants of the melanocortin‐1 receptor: do they matter clinically? Exp Dermatol. 2015;1:5-9. doi:10.1111/exd.12540
- Yao S, Ambrosone CB. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J Steroid Biochem Mol Biol. 2013;136:337-341. doi:10.1016/j.jsbmb.2012.09.010
- Jablonski N. The evolution of human skin colouration and its relevance to health in the modern world. J Royal Coll Physicians Edinb. 2012;42:58-63. doi:10.4997/jrcpe.2012.114
- Jablonski NG, Chaplin G. Human skin pigmentation as an adaptation to UV radiation. Proc National Acad Sci. 2010;107(suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Hochberg Z, Templeton AR. Evolutionary perspective in skin color, vitamin D and its receptor. Hormones. 2010;9:307-311. doi:10.14310/horm.2002.1281
- Jones P, Lucock M, Veysey M, et al. The vitamin D–folate hypothesis as an evolutionary model for skin pigmentation: an update and integration of current ideas. Nutrients. 2018;10:554. doi:10.3390/nu10050554
- Lindqvist PG, Epstein E, Landin-Olsson M, et al. Women with fair phenotypes seem to confer a survival advantage in a low UV milieu. a nested matched case control study. PloS One. 2020;15:E0228582. doi:10.1371/journal.pone.0228582
- Holick MF. Shedding new light on the role of the sunshine vitamin D for skin health: the lncRNA–skin cancer connection. Exp Dermatol. 2014;23:391-392. doi:10.1111/exd.12386
- Jablonski NG, Chaplin G. Epidermal pigmentation in the human lineage is an adaptation to ultraviolet radiation. J Hum Evol. 2013;65:671-675. doi:10.1016/j.jhevol.2013.06.004
- Jablonski NG, Chaplin G. The evolution of skin pigmentation and hair texture in people of African ancestry. Dermatol Clin. 2014;32:113-121. doi:10.1016/j.det.2013.11.003
- Jablonski NG. The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell Melanoma Res. 2021;34:707-7 doi:10.1111/pcmr.12976
- Lucock MD, Jones PR, Veysey M, et al. Biophysical evidence to support and extend the vitamin D‐folate hypothesis as a paradigm for the evolution of human skin pigmentation. Am J Hum Biol. 2022;34:E23667. doi:10.1002/ajhb.23667
- Missaggia BO, Reales G, Cybis GB, et al. Adaptation and co‐adaptation of skin pigmentation and vitamin D genes in native Americans. Am J Med Genet C Semin Med Genet. 2020;184:1060-1077. doi:10.1002/ajmg.c.31873
- Hanel A, Carlberg C. Skin colour and vitamin D: an update. Exp Dermatol. 2020;29:864-875. doi:10.1111/exd.14142
- Hanel A, Carlberg C. Vitamin D and evolution: pharmacologic implications. Biochem Pharmacol. 2020;173:113595. doi:10.1016/j.bcp.2019.07.024
- Flegr J, Sýkorová K, Fiala V, et al. Increased 25(OH)D3 level in redheaded people: could redheadedness be an adaptation to temperate climate? Exp Dermatol. 2020;29:598-609. doi:10.1111/exd.14119
- James WPT, Johnson RJ, Speakman JR, et al. Nutrition and its role in human evolution. J Intern Med. 2019;285:533-549. doi:10.1111/joim.12878
- Lucock M, Jones P, Martin C, et al. Vitamin D: beyond metabolism. J Evid Based Complementary Altern Med. 2015;20:310-322. doi:10.1177/2156587215580491
- Jarrett P, Scragg R. Evolution, prehistory and vitamin D. Int J Environ Res Public Health. 2020;17:646. doi:10.3390/ijerph17020646
- Elias PM, Williams ML. Re-appraisal of current theories for thedevelopment and loss of epidermal pigmentation in hominins and modern humans. J Hum Evol. 2013;64:687-692. doi:10.1016/j.jhevol.2013.02.003
- Elias PM, Williams ML. Basis for the gain and subsequent dilution of epidermal pigmentation during human evolution: the barrier and metabolic conservation hypotheses revisited. Am J Phys Anthropol. 2016;161:189-207. doi:10.1002/ajpa.23030
- Williams JD, Jacobson EL, Kim H, et al. Water soluble vitamins, clinical research and future application. Subcell Biochem. 2011;56:181-197. doi:10.1007/978-94-007-2199-9_10
- Greaves M. Was skin cancer a selective force for black pigmentation in early hominin evolution [published online February 26, 2014]? Proc Biol Sci. 2014;281:20132955. doi:10.1098/rspb.2013.2955
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. doi:10.1056/nejmra070553
- Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466-479. doi:10.1038/nrendo.2017.31
- US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. US Dept of Health and Human Services, Office of the Surgeon General; 2014. Accessed April 29, 2024. https://www.hhs.gov/sites/default/files/call-to-action-prevent-skin-cancer.pdf
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; 2011. https://www.ncbi.nlm.nih.gov/books/NBK56070/
- Beleza S, Santos AM, McEvoy B, et al. The timing of pigmentation lightening in Europeans. Mol Biol Evol. 2013;30:24-35. doi:10.1093/molbev/mss207
- Carlberg C. Nutrigenomics of vitamin D. Nutrients. 2019;11:676. doi:10.3390/nu11030676
- Jablonski NG, Chaplin G. The roles of vitamin D and cutaneous vitamin D production in human evolution and health. Int J Paleopathol. 2018;23:54-59. doi:10.1016/j.ijpp.2018.01.005
- Weiss BD. SORT: strength of recommendation taxonomy. Fam Med. 2004;36:141-143.
- Wolf ST, Kenney WL. The vitamin D–folate hypothesis in human vascular health. Am J Physiol Regul Integr Comp Physiology. 2019;317:R491-R501. doi:10.1152/ajpregu.00136.2019
- Lucock M, Jones P, Martin C, et al. Photobiology of vitamins. Nutr Rev. 2018;76:512-525. doi:10.1093/nutrit/nuy013
- Hochberg Z, Hochberg I. Evolutionary perspective in rickets and vitamin D. Front Endocrinol (Lausanne). 2019;10:306. doi:10.3389/fendo.2019.00306
- Rossberg W, Saternus R, Wagenpfeil S, et al. Human pigmentation, cutaneous vitamin D synthesis and evolution: variants of genes (SNPs) involved in skin pigmentation are associated with 25(OH)D serum concentration. Anticancer Res. 2016;36:1429-1437.
- Saternus R, Pilz S, Gräber S, et al. A closer look at evolution: variants (SNPs) of genes involved in skin pigmentation, including EXOC2, TYR, TYRP1, and DCT, are associated with 25(OH)D serum concentration. Endocrinology. 2015;156:39-47. doi:10.1210/en.2014-1238
- López S, García Ó, Yurrebaso I, et al. The interplay between natural selection and susceptibility to melanoma on allele 374F of SLC45A2 gene in a south European population. PloS One. 2014;9:E104367. doi:1371/journal.pone.0104367
- Lucock M, Yates Z, Martin C, et al. Vitamin D, folate, and potential early lifecycle environmental origin of significant adult phenotypes. Evol Med Public Health. 2014;2014:69-91. doi:10.1093/emph/eou013
- Hudjashov G, Villems R, Kivisild T. Global patterns of diversity and selection in human tyrosinase gene. PloS One. 2013;8:E74307. doi:10.1371/journal.pone.0074307
- Khan R, Khan BSR. Diet, disease and pigment variation in humans. Med Hypotheses. 2010;75:363-367. doi:10.1016/j.mehy.2010.03.033
- Kuan V, Martineau AR, Griffiths CJ, et al. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evol Biol. 2013;13:144. doi:10.1186/1471-2148-13-144
- Omenn GS. Evolution and public health. Proc National Acad Sci. 2010;107(suppl 1):1702-1709. doi:10.1073/pnas.0906198106
- Yuen AWC, Jablonski NG. Vitamin D: in the evolution of human skin colour. Med Hypotheses. 2010;74:39-44. doi:10.1016/j.mehy.2009.08.007
- Vieth R. Weaker bones and white skin as adaptions to improve anthropological “fitness” for northern environments. Osteoporosis Int. 2020;31:617-624. doi:10.1007/s00198-019-05167-4
- Carlberg C. Vitamin D: a micronutrient regulating genes. Curr Pharm Des. 2019;25:1740-1746. doi:10.2174/1381612825666190705193227
- Haddadeen C, Lai C, Cho SY, et al. Variants of the melanocortin‐1 receptor: do they matter clinically? Exp Dermatol. 2015;1:5-9. doi:10.1111/exd.12540
- Yao S, Ambrosone CB. Associations between vitamin D deficiency and risk of aggressive breast cancer in African-American women. J Steroid Biochem Mol Biol. 2013;136:337-341. doi:10.1016/j.jsbmb.2012.09.010
- Jablonski N. The evolution of human skin colouration and its relevance to health in the modern world. J Royal Coll Physicians Edinb. 2012;42:58-63. doi:10.4997/jrcpe.2012.114
- Jablonski NG, Chaplin G. Human skin pigmentation as an adaptation to UV radiation. Proc National Acad Sci. 2010;107(suppl 2):8962-8968. doi:10.1073/pnas.0914628107
- Hochberg Z, Templeton AR. Evolutionary perspective in skin color, vitamin D and its receptor. Hormones. 2010;9:307-311. doi:10.14310/horm.2002.1281
- Jones P, Lucock M, Veysey M, et al. The vitamin D–folate hypothesis as an evolutionary model for skin pigmentation: an update and integration of current ideas. Nutrients. 2018;10:554. doi:10.3390/nu10050554
- Lindqvist PG, Epstein E, Landin-Olsson M, et al. Women with fair phenotypes seem to confer a survival advantage in a low UV milieu. a nested matched case control study. PloS One. 2020;15:E0228582. doi:10.1371/journal.pone.0228582
- Holick MF. Shedding new light on the role of the sunshine vitamin D for skin health: the lncRNA–skin cancer connection. Exp Dermatol. 2014;23:391-392. doi:10.1111/exd.12386
- Jablonski NG, Chaplin G. Epidermal pigmentation in the human lineage is an adaptation to ultraviolet radiation. J Hum Evol. 2013;65:671-675. doi:10.1016/j.jhevol.2013.06.004
- Jablonski NG, Chaplin G. The evolution of skin pigmentation and hair texture in people of African ancestry. Dermatol Clin. 2014;32:113-121. doi:10.1016/j.det.2013.11.003
- Jablonski NG. The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell Melanoma Res. 2021;34:707-7 doi:10.1111/pcmr.12976
- Lucock MD, Jones PR, Veysey M, et al. Biophysical evidence to support and extend the vitamin D‐folate hypothesis as a paradigm for the evolution of human skin pigmentation. Am J Hum Biol. 2022;34:E23667. doi:10.1002/ajhb.23667
- Missaggia BO, Reales G, Cybis GB, et al. Adaptation and co‐adaptation of skin pigmentation and vitamin D genes in native Americans. Am J Med Genet C Semin Med Genet. 2020;184:1060-1077. doi:10.1002/ajmg.c.31873
- Hanel A, Carlberg C. Skin colour and vitamin D: an update. Exp Dermatol. 2020;29:864-875. doi:10.1111/exd.14142
- Hanel A, Carlberg C. Vitamin D and evolution: pharmacologic implications. Biochem Pharmacol. 2020;173:113595. doi:10.1016/j.bcp.2019.07.024
- Flegr J, Sýkorová K, Fiala V, et al. Increased 25(OH)D3 level in redheaded people: could redheadedness be an adaptation to temperate climate? Exp Dermatol. 2020;29:598-609. doi:10.1111/exd.14119
- James WPT, Johnson RJ, Speakman JR, et al. Nutrition and its role in human evolution. J Intern Med. 2019;285:533-549. doi:10.1111/joim.12878
- Lucock M, Jones P, Martin C, et al. Vitamin D: beyond metabolism. J Evid Based Complementary Altern Med. 2015;20:310-322. doi:10.1177/2156587215580491
- Jarrett P, Scragg R. Evolution, prehistory and vitamin D. Int J Environ Res Public Health. 2020;17:646. doi:10.3390/ijerph17020646
- Elias PM, Williams ML. Re-appraisal of current theories for thedevelopment and loss of epidermal pigmentation in hominins and modern humans. J Hum Evol. 2013;64:687-692. doi:10.1016/j.jhevol.2013.02.003
- Elias PM, Williams ML. Basis for the gain and subsequent dilution of epidermal pigmentation during human evolution: the barrier and metabolic conservation hypotheses revisited. Am J Phys Anthropol. 2016;161:189-207. doi:10.1002/ajpa.23030
- Williams JD, Jacobson EL, Kim H, et al. Water soluble vitamins, clinical research and future application. Subcell Biochem. 2011;56:181-197. doi:10.1007/978-94-007-2199-9_10
- Greaves M. Was skin cancer a selective force for black pigmentation in early hominin evolution [published online February 26, 2014]? Proc Biol Sci. 2014;281:20132955. doi:10.1098/rspb.2013.2955
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-281. doi:10.1056/nejmra070553
- Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466-479. doi:10.1038/nrendo.2017.31
- US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. US Dept of Health and Human Services, Office of the Surgeon General; 2014. Accessed April 29, 2024. https://www.hhs.gov/sites/default/files/call-to-action-prevent-skin-cancer.pdf
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, et al, eds. Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; 2011. https://www.ncbi.nlm.nih.gov/books/NBK56070/
Practice Points
- Sufficient UV radiation exposure is required to synthesize vitamin D, but excess exposure increases skin cancer risk.
- Genes associated with vitamin D production and melanin synthesis form an interconnected network that explains skin tone polymorphisms and their influence on healthy sun behaviors.
- Adaptations in genetics of skin pigmentation and vitamin D metabolism due to anthropologic patterns of migration to northern latitudes may help explain predisposition to dermatologic diseases such as skin cancer.
Does Racism in Black Americans Boost Alzheimer’s Risk?
Racial discrimination in Black Americans is associated with an increased risk of developing Alzheimer’s disease (AD) in later life, new findings showed.
Researchers found that Black Americans who experience racism in their 40s and 50s are more likely to have increased serum levels of AD biomarkers p-tau181 and neurofilament light (NfL) more than a decade later.
“We know that Black Americans are at an elevated risk of Alzheimer’s disease and other dementias compared to non-Hispanic White Americans, but we don’t fully understand all the factors that contribute to this disproportionate risk,” Michelle Mielke, PhD, co-author and professor of epidemiology and prevention at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said in a press release.
Recent data show AD is twice as prevalent in Black Americans as in Whites, at 18.6% and 10%, respectively. Dr. Mielke said this level of disparity cannot be attributed solely to genetic differences, and evidence suggests that racism and its related stress may play a role.
The findings were published online in Alzheimer’s and Dementia.
AD Biomarker Testing
To further explore a possible link between exposure to racism and AD risk, investigators analyzed data from the Family and Community Health Study, a multisite, longitudinal investigation that included more than 800 families in the United States.
Blood samples and information on racial discrimination were collected from 255 middle-aged Black Americans between 2002 and 2005.
Blood samples were tested for serum phosphorylated tau181 (p-Tau181), a marker of AD pathology; NfL, a nonspecific marker of neurodegeneration; and glial fibrillary acidic protein (GFAP), a marker of brain inflammation.
Participants answered questions about racial discrimination, which included whether they have been subjected to disrespectful treatment including racial slurs, harassment from law enforcement, or if they had ever been excluded from social activities because of their race.
The sample included 212 females and 43 males with a mean age of 46. Most participants (70%) lived in urban areas.
Stress-Related?
Investigators found no correlation between racial discrimination and increased levels of AD blood biomarkers in 2008 when participants were a mean age of 46 years. However, 11 years later, when participants were roughly 57 years old, investigators found experiencing racism in middle age was significantly correlated with higher levels of both p-Tau181 (r = 0.158; P ≤ .012) and NfL (r = 0.143; P ≤ .023). There was no significant association between reported discrimination and GFAP.
“These findings support the hypothesis that unique life stressors encountered by Black Americans in midlife become biologically embedded and contribute to AD pathology and neurodegeneration later in life,” the authors wrote.
Investigators speculated based on previous research that the stress related to discrimination may be associated with reductions in hippocampal and prefrontal cortex volumes and neurodegeneration in general.
Dr. Mielke also said it’s clear that future studies should focus on racism experienced by Black Americans to further understand their risk for dementia.
“This research can help inform policies and interventions to reduce racial disparities and reduce dementia risk,” she said.
Study limitations include the absence of amyloid biomarkers. Investigators noted that participants had non-detectable levels of amyloid, likely due to the use of serum vs cerebrospinal fluid.
The study was funded by the National Institute on Aging and the National Heart, Lung, and Blood Institute. Mielke reported serving on scientific advisory boards and/or having consulted for Acadia, Biogen, Eisai, LabCorp, Lilly, Merck, PeerView Institute, Roche, Siemens Healthineers, and Sunbird Bio.
A version of this article appeared on Medscape.com.
Racial discrimination in Black Americans is associated with an increased risk of developing Alzheimer’s disease (AD) in later life, new findings showed.
Researchers found that Black Americans who experience racism in their 40s and 50s are more likely to have increased serum levels of AD biomarkers p-tau181 and neurofilament light (NfL) more than a decade later.
“We know that Black Americans are at an elevated risk of Alzheimer’s disease and other dementias compared to non-Hispanic White Americans, but we don’t fully understand all the factors that contribute to this disproportionate risk,” Michelle Mielke, PhD, co-author and professor of epidemiology and prevention at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said in a press release.
Recent data show AD is twice as prevalent in Black Americans as in Whites, at 18.6% and 10%, respectively. Dr. Mielke said this level of disparity cannot be attributed solely to genetic differences, and evidence suggests that racism and its related stress may play a role.
The findings were published online in Alzheimer’s and Dementia.
AD Biomarker Testing
To further explore a possible link between exposure to racism and AD risk, investigators analyzed data from the Family and Community Health Study, a multisite, longitudinal investigation that included more than 800 families in the United States.
Blood samples and information on racial discrimination were collected from 255 middle-aged Black Americans between 2002 and 2005.
Blood samples were tested for serum phosphorylated tau181 (p-Tau181), a marker of AD pathology; NfL, a nonspecific marker of neurodegeneration; and glial fibrillary acidic protein (GFAP), a marker of brain inflammation.
Participants answered questions about racial discrimination, which included whether they have been subjected to disrespectful treatment including racial slurs, harassment from law enforcement, or if they had ever been excluded from social activities because of their race.
The sample included 212 females and 43 males with a mean age of 46. Most participants (70%) lived in urban areas.
Stress-Related?
Investigators found no correlation between racial discrimination and increased levels of AD blood biomarkers in 2008 when participants were a mean age of 46 years. However, 11 years later, when participants were roughly 57 years old, investigators found experiencing racism in middle age was significantly correlated with higher levels of both p-Tau181 (r = 0.158; P ≤ .012) and NfL (r = 0.143; P ≤ .023). There was no significant association between reported discrimination and GFAP.
“These findings support the hypothesis that unique life stressors encountered by Black Americans in midlife become biologically embedded and contribute to AD pathology and neurodegeneration later in life,” the authors wrote.
Investigators speculated based on previous research that the stress related to discrimination may be associated with reductions in hippocampal and prefrontal cortex volumes and neurodegeneration in general.
Dr. Mielke also said it’s clear that future studies should focus on racism experienced by Black Americans to further understand their risk for dementia.
“This research can help inform policies and interventions to reduce racial disparities and reduce dementia risk,” she said.
Study limitations include the absence of amyloid biomarkers. Investigators noted that participants had non-detectable levels of amyloid, likely due to the use of serum vs cerebrospinal fluid.
The study was funded by the National Institute on Aging and the National Heart, Lung, and Blood Institute. Mielke reported serving on scientific advisory boards and/or having consulted for Acadia, Biogen, Eisai, LabCorp, Lilly, Merck, PeerView Institute, Roche, Siemens Healthineers, and Sunbird Bio.
A version of this article appeared on Medscape.com.
Racial discrimination in Black Americans is associated with an increased risk of developing Alzheimer’s disease (AD) in later life, new findings showed.
Researchers found that Black Americans who experience racism in their 40s and 50s are more likely to have increased serum levels of AD biomarkers p-tau181 and neurofilament light (NfL) more than a decade later.
“We know that Black Americans are at an elevated risk of Alzheimer’s disease and other dementias compared to non-Hispanic White Americans, but we don’t fully understand all the factors that contribute to this disproportionate risk,” Michelle Mielke, PhD, co-author and professor of epidemiology and prevention at Wake Forest University School of Medicine, Winston-Salem, North Carolina, said in a press release.
Recent data show AD is twice as prevalent in Black Americans as in Whites, at 18.6% and 10%, respectively. Dr. Mielke said this level of disparity cannot be attributed solely to genetic differences, and evidence suggests that racism and its related stress may play a role.
The findings were published online in Alzheimer’s and Dementia.
AD Biomarker Testing
To further explore a possible link between exposure to racism and AD risk, investigators analyzed data from the Family and Community Health Study, a multisite, longitudinal investigation that included more than 800 families in the United States.
Blood samples and information on racial discrimination were collected from 255 middle-aged Black Americans between 2002 and 2005.
Blood samples were tested for serum phosphorylated tau181 (p-Tau181), a marker of AD pathology; NfL, a nonspecific marker of neurodegeneration; and glial fibrillary acidic protein (GFAP), a marker of brain inflammation.
Participants answered questions about racial discrimination, which included whether they have been subjected to disrespectful treatment including racial slurs, harassment from law enforcement, or if they had ever been excluded from social activities because of their race.
The sample included 212 females and 43 males with a mean age of 46. Most participants (70%) lived in urban areas.
Stress-Related?
Investigators found no correlation between racial discrimination and increased levels of AD blood biomarkers in 2008 when participants were a mean age of 46 years. However, 11 years later, when participants were roughly 57 years old, investigators found experiencing racism in middle age was significantly correlated with higher levels of both p-Tau181 (r = 0.158; P ≤ .012) and NfL (r = 0.143; P ≤ .023). There was no significant association between reported discrimination and GFAP.
“These findings support the hypothesis that unique life stressors encountered by Black Americans in midlife become biologically embedded and contribute to AD pathology and neurodegeneration later in life,” the authors wrote.
Investigators speculated based on previous research that the stress related to discrimination may be associated with reductions in hippocampal and prefrontal cortex volumes and neurodegeneration in general.
Dr. Mielke also said it’s clear that future studies should focus on racism experienced by Black Americans to further understand their risk for dementia.
“This research can help inform policies and interventions to reduce racial disparities and reduce dementia risk,” she said.
Study limitations include the absence of amyloid biomarkers. Investigators noted that participants had non-detectable levels of amyloid, likely due to the use of serum vs cerebrospinal fluid.
The study was funded by the National Institute on Aging and the National Heart, Lung, and Blood Institute. Mielke reported serving on scientific advisory boards and/or having consulted for Acadia, Biogen, Eisai, LabCorp, Lilly, Merck, PeerView Institute, Roche, Siemens Healthineers, and Sunbird Bio.
A version of this article appeared on Medscape.com.
Darker Skin Tones Underrepresented on Skin Cancer Education Websites
“Given the known disparities patients with darker skin tones face in terms of increased skin cancer morbidity and mortality, this lack of representation further disadvantages those patients by not providing them with an adequate representation of how skin cancers manifest on their skin tones,” the study’s first author, Alana Sadur, who recently completed her third year at the George Washington School of Medicine and Health Sciences, Washington, said in an interview. “By not having images to refer to, patients are less likely to self-identify and seek treatment for concerning skin lesions.”
For the study, which was published in Journal of Drugs in Dermatology, Ms. Sadur and coauthors evaluated the inclusivity and representation of skin tones in photos of skin cancer on the following patient-facing websites: CDC.gov, NIH.gov, skincancer.org, americancancerfund.org, mayoclinic.org, and cancer.org. The researchers counted each individual person or image showing skin as a separate representation, and three independent reviewers used the 5-color Pantone swatch as described in a dermatology atlas to categorize representations as “lighter-toned skin” (Pantones A-B or lighter) or “darker-toned skin” (Pantones C-E or darker).
Of the 372 total representations identified on the websites, only 49 (13.2%) showed darker skin tones. Of these, 44.9% depicted Pantone C, 34.7% depicted Pantone D, and 20.4% depicted Pantone E. The researchers also found that only 11% of nonmelanoma skin cancers (NMSC) and 5.8% of melanoma skin cancers (MSC) were shown on darker skin tones, while no cartoon portrayals of NMSC or MSC included darker skin tones.
In findings related to nondisease representations on the websites, darker skin tones were depicted in just 22.7% of stock photos and 26.1% of website front pages.
The study’s senior author, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, emphasized the need for trusted sources like national organizations and federally funded agencies to be purposeful with their selection of images to “ensure all visitors to the site are represented,” he told this news organization.
“This is very important when dealing with skin cancer as a lack of representation could easily be misinterpreted as epidemiological data, meaning this gap could suggest certain individuals do not get skin cancer because photos in those skin tones are not present,” he added. “This doesn’t even begin to touch upon the diversity of individuals in the stock photos or lack thereof, which can perpetuate the lack of diversity in our specialty. We need to do better.”
The authors reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
“Given the known disparities patients with darker skin tones face in terms of increased skin cancer morbidity and mortality, this lack of representation further disadvantages those patients by not providing them with an adequate representation of how skin cancers manifest on their skin tones,” the study’s first author, Alana Sadur, who recently completed her third year at the George Washington School of Medicine and Health Sciences, Washington, said in an interview. “By not having images to refer to, patients are less likely to self-identify and seek treatment for concerning skin lesions.”
For the study, which was published in Journal of Drugs in Dermatology, Ms. Sadur and coauthors evaluated the inclusivity and representation of skin tones in photos of skin cancer on the following patient-facing websites: CDC.gov, NIH.gov, skincancer.org, americancancerfund.org, mayoclinic.org, and cancer.org. The researchers counted each individual person or image showing skin as a separate representation, and three independent reviewers used the 5-color Pantone swatch as described in a dermatology atlas to categorize representations as “lighter-toned skin” (Pantones A-B or lighter) or “darker-toned skin” (Pantones C-E or darker).
Of the 372 total representations identified on the websites, only 49 (13.2%) showed darker skin tones. Of these, 44.9% depicted Pantone C, 34.7% depicted Pantone D, and 20.4% depicted Pantone E. The researchers also found that only 11% of nonmelanoma skin cancers (NMSC) and 5.8% of melanoma skin cancers (MSC) were shown on darker skin tones, while no cartoon portrayals of NMSC or MSC included darker skin tones.
In findings related to nondisease representations on the websites, darker skin tones were depicted in just 22.7% of stock photos and 26.1% of website front pages.
The study’s senior author, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, emphasized the need for trusted sources like national organizations and federally funded agencies to be purposeful with their selection of images to “ensure all visitors to the site are represented,” he told this news organization.
“This is very important when dealing with skin cancer as a lack of representation could easily be misinterpreted as epidemiological data, meaning this gap could suggest certain individuals do not get skin cancer because photos in those skin tones are not present,” he added. “This doesn’t even begin to touch upon the diversity of individuals in the stock photos or lack thereof, which can perpetuate the lack of diversity in our specialty. We need to do better.”
The authors reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
“Given the known disparities patients with darker skin tones face in terms of increased skin cancer morbidity and mortality, this lack of representation further disadvantages those patients by not providing them with an adequate representation of how skin cancers manifest on their skin tones,” the study’s first author, Alana Sadur, who recently completed her third year at the George Washington School of Medicine and Health Sciences, Washington, said in an interview. “By not having images to refer to, patients are less likely to self-identify and seek treatment for concerning skin lesions.”
For the study, which was published in Journal of Drugs in Dermatology, Ms. Sadur and coauthors evaluated the inclusivity and representation of skin tones in photos of skin cancer on the following patient-facing websites: CDC.gov, NIH.gov, skincancer.org, americancancerfund.org, mayoclinic.org, and cancer.org. The researchers counted each individual person or image showing skin as a separate representation, and three independent reviewers used the 5-color Pantone swatch as described in a dermatology atlas to categorize representations as “lighter-toned skin” (Pantones A-B or lighter) or “darker-toned skin” (Pantones C-E or darker).
Of the 372 total representations identified on the websites, only 49 (13.2%) showed darker skin tones. Of these, 44.9% depicted Pantone C, 34.7% depicted Pantone D, and 20.4% depicted Pantone E. The researchers also found that only 11% of nonmelanoma skin cancers (NMSC) and 5.8% of melanoma skin cancers (MSC) were shown on darker skin tones, while no cartoon portrayals of NMSC or MSC included darker skin tones.
In findings related to nondisease representations on the websites, darker skin tones were depicted in just 22.7% of stock photos and 26.1% of website front pages.
The study’s senior author, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, emphasized the need for trusted sources like national organizations and federally funded agencies to be purposeful with their selection of images to “ensure all visitors to the site are represented,” he told this news organization.
“This is very important when dealing with skin cancer as a lack of representation could easily be misinterpreted as epidemiological data, meaning this gap could suggest certain individuals do not get skin cancer because photos in those skin tones are not present,” he added. “This doesn’t even begin to touch upon the diversity of individuals in the stock photos or lack thereof, which can perpetuate the lack of diversity in our specialty. We need to do better.”
The authors reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF DRUGS IN DERMATOLOGY
Lentigines: Study Finds Less PIH With Modified Laser Treatment
BALTIMORE — Laser treatment for solar lentigines in individuals with darker skin types has long been associated with a higher risk of postinflammatory hyperpigmentation (PIH), but.
The study enrolled 27 patients with solar lentigines and Fitzpatrick skin types (FSTs) III-IV, Woraphong Manuskiatti, MD, professor of dermatology at Siriraj Hospital, Mahidol University, Bangkok, reported at the annual meeting of the American Society for Laser Medicine and Surgery. They received the fractional beam treatment on one side of the face and the full-beam on the other side. At 6 months, the incidence of PIH was about 81% lower on the fractional-beam side, Dr. Manuskiatti said.
“In the past, when we used laser to treat pigmented lesions, we used the so-called full-beam technique on the pigmented area,” Dr. Manuskiatti told this news organization. “From the study, we found that you don’t need to treat it at 100%. You can fractionally treat the pigmented lesion and get a really comparable treatment outcome and, at that reduced beam, less incidence of postinflammatory hyperpigmentation.”
Study Design and Results
Of the 27 patients in the study, 12 were FST III (44%), 14 were FST IV (52%), and one was FST V (4%). On the fractional-beam side, the laser was delivered through a 9-mm spot size with an average fluence of 0.47 J/cm² at a frequency of 2 Hz for a total of two passes without pulse overlapping. On the full-beam side, the laser was operated with a 4.5-mm handpiece, with fluence ranging from 0.3 to 0.7 J/cm² (using an endpoint of slight darkening of the pigmented lesion) at 2 Hz.
The patients received a single treatment and had a clinical evaluation and color reading assessments at 2 weeks, 1 month, 3 months, and 6 months after the treatment. Twenty-five patients completed the study.
The researchers found no statistically significant differences in lesional clearance between the two techniques at any of the follow-up assessments, Dr. Manuskiatti said. “This might be one of the alternative treatments of treating solar lentigines in dark-skinned patients,” he said when presenting the study results.
He reported the rates of PIH on the full-beam and fractional-beam sides, respectively, at the following intervals were: 64% and 8% at 2 weeks, 80% and 32% at 1 month, 96% and 36% at 3 months, and 88% and 16% at 6 months.
“The incidence of PIH on the full-beam side was statistically higher than that on the fractional-beam side throughout the follow-up period,” he said. Transient and mild hypopigmentation was observed in one patient (4%) on the fractional-beam side and in five (20%) on the full-beam side. Dr. Manuskiatti added that no other adverse effects were documented during the study.
“ Normally when you use laser to treat skin type I or II, you don’t have … PIH or darkening of the skin,” Dr. Manuskiatti told this news organization, “but when you have skin type III and above, you run into a really high incidence of postinflammatory hyperpigmentation — and treating that with fractional beam can lead to a reduced incidence of darkening of the skin afterward.”
A Lower-Cost Option
This study showed that the 532-nm picosecond laser with fractional beam MLA is a useful option for patients with darker skin types, Kelly Stankiewicz, MD, a dermatologist who practices in Park City, Utah, and moderated the session where these results were presented, told this news organization.
“The most challenging thing about treating lentigines in darker skin types is preventing potential side effects, mainly dyspigmentation,” she said after the meeting. “These side effects are, for the most part, temporary, but they can take 6-18 months to resolve, so it’s important to prevent them in the first place.”
She noted that the 532-nm and 1064-nm wavelengths are the most commonly available for picosecond lasers and that they’re easier to produce and less expensive. “There are picosecond lasers with middle wavelengths in the red light to near-infrared range (650-785 nm) that are better for darker skin types because they are more gentle yet still effective at targeting pigment, but these lasers are more expensive and less widely available,” Dr. Stankiewicz said.
“The microlens array, used in this study with the 532-nm wavelength, is an inexpensive piece that fits at the end of the laser,” she added. “So, to have an option that turns a 532-nm laser into a safer device for the treatment of lentigines in darker skin types is very helpful.”
Dr. Manuskiatti and Dr. Stankiewicz had no relevant disclosures to report.
A version of this article first appeared on Medscape.com.
BALTIMORE — Laser treatment for solar lentigines in individuals with darker skin types has long been associated with a higher risk of postinflammatory hyperpigmentation (PIH), but.
The study enrolled 27 patients with solar lentigines and Fitzpatrick skin types (FSTs) III-IV, Woraphong Manuskiatti, MD, professor of dermatology at Siriraj Hospital, Mahidol University, Bangkok, reported at the annual meeting of the American Society for Laser Medicine and Surgery. They received the fractional beam treatment on one side of the face and the full-beam on the other side. At 6 months, the incidence of PIH was about 81% lower on the fractional-beam side, Dr. Manuskiatti said.
“In the past, when we used laser to treat pigmented lesions, we used the so-called full-beam technique on the pigmented area,” Dr. Manuskiatti told this news organization. “From the study, we found that you don’t need to treat it at 100%. You can fractionally treat the pigmented lesion and get a really comparable treatment outcome and, at that reduced beam, less incidence of postinflammatory hyperpigmentation.”
Study Design and Results
Of the 27 patients in the study, 12 were FST III (44%), 14 were FST IV (52%), and one was FST V (4%). On the fractional-beam side, the laser was delivered through a 9-mm spot size with an average fluence of 0.47 J/cm² at a frequency of 2 Hz for a total of two passes without pulse overlapping. On the full-beam side, the laser was operated with a 4.5-mm handpiece, with fluence ranging from 0.3 to 0.7 J/cm² (using an endpoint of slight darkening of the pigmented lesion) at 2 Hz.
The patients received a single treatment and had a clinical evaluation and color reading assessments at 2 weeks, 1 month, 3 months, and 6 months after the treatment. Twenty-five patients completed the study.
The researchers found no statistically significant differences in lesional clearance between the two techniques at any of the follow-up assessments, Dr. Manuskiatti said. “This might be one of the alternative treatments of treating solar lentigines in dark-skinned patients,” he said when presenting the study results.
He reported the rates of PIH on the full-beam and fractional-beam sides, respectively, at the following intervals were: 64% and 8% at 2 weeks, 80% and 32% at 1 month, 96% and 36% at 3 months, and 88% and 16% at 6 months.
“The incidence of PIH on the full-beam side was statistically higher than that on the fractional-beam side throughout the follow-up period,” he said. Transient and mild hypopigmentation was observed in one patient (4%) on the fractional-beam side and in five (20%) on the full-beam side. Dr. Manuskiatti added that no other adverse effects were documented during the study.
“ Normally when you use laser to treat skin type I or II, you don’t have … PIH or darkening of the skin,” Dr. Manuskiatti told this news organization, “but when you have skin type III and above, you run into a really high incidence of postinflammatory hyperpigmentation — and treating that with fractional beam can lead to a reduced incidence of darkening of the skin afterward.”
A Lower-Cost Option
This study showed that the 532-nm picosecond laser with fractional beam MLA is a useful option for patients with darker skin types, Kelly Stankiewicz, MD, a dermatologist who practices in Park City, Utah, and moderated the session where these results were presented, told this news organization.
“The most challenging thing about treating lentigines in darker skin types is preventing potential side effects, mainly dyspigmentation,” she said after the meeting. “These side effects are, for the most part, temporary, but they can take 6-18 months to resolve, so it’s important to prevent them in the first place.”
She noted that the 532-nm and 1064-nm wavelengths are the most commonly available for picosecond lasers and that they’re easier to produce and less expensive. “There are picosecond lasers with middle wavelengths in the red light to near-infrared range (650-785 nm) that are better for darker skin types because they are more gentle yet still effective at targeting pigment, but these lasers are more expensive and less widely available,” Dr. Stankiewicz said.
“The microlens array, used in this study with the 532-nm wavelength, is an inexpensive piece that fits at the end of the laser,” she added. “So, to have an option that turns a 532-nm laser into a safer device for the treatment of lentigines in darker skin types is very helpful.”
Dr. Manuskiatti and Dr. Stankiewicz had no relevant disclosures to report.
A version of this article first appeared on Medscape.com.
BALTIMORE — Laser treatment for solar lentigines in individuals with darker skin types has long been associated with a higher risk of postinflammatory hyperpigmentation (PIH), but.
The study enrolled 27 patients with solar lentigines and Fitzpatrick skin types (FSTs) III-IV, Woraphong Manuskiatti, MD, professor of dermatology at Siriraj Hospital, Mahidol University, Bangkok, reported at the annual meeting of the American Society for Laser Medicine and Surgery. They received the fractional beam treatment on one side of the face and the full-beam on the other side. At 6 months, the incidence of PIH was about 81% lower on the fractional-beam side, Dr. Manuskiatti said.
“In the past, when we used laser to treat pigmented lesions, we used the so-called full-beam technique on the pigmented area,” Dr. Manuskiatti told this news organization. “From the study, we found that you don’t need to treat it at 100%. You can fractionally treat the pigmented lesion and get a really comparable treatment outcome and, at that reduced beam, less incidence of postinflammatory hyperpigmentation.”
Study Design and Results
Of the 27 patients in the study, 12 were FST III (44%), 14 were FST IV (52%), and one was FST V (4%). On the fractional-beam side, the laser was delivered through a 9-mm spot size with an average fluence of 0.47 J/cm² at a frequency of 2 Hz for a total of two passes without pulse overlapping. On the full-beam side, the laser was operated with a 4.5-mm handpiece, with fluence ranging from 0.3 to 0.7 J/cm² (using an endpoint of slight darkening of the pigmented lesion) at 2 Hz.
The patients received a single treatment and had a clinical evaluation and color reading assessments at 2 weeks, 1 month, 3 months, and 6 months after the treatment. Twenty-five patients completed the study.
The researchers found no statistically significant differences in lesional clearance between the two techniques at any of the follow-up assessments, Dr. Manuskiatti said. “This might be one of the alternative treatments of treating solar lentigines in dark-skinned patients,” he said when presenting the study results.
He reported the rates of PIH on the full-beam and fractional-beam sides, respectively, at the following intervals were: 64% and 8% at 2 weeks, 80% and 32% at 1 month, 96% and 36% at 3 months, and 88% and 16% at 6 months.
“The incidence of PIH on the full-beam side was statistically higher than that on the fractional-beam side throughout the follow-up period,” he said. Transient and mild hypopigmentation was observed in one patient (4%) on the fractional-beam side and in five (20%) on the full-beam side. Dr. Manuskiatti added that no other adverse effects were documented during the study.
“ Normally when you use laser to treat skin type I or II, you don’t have … PIH or darkening of the skin,” Dr. Manuskiatti told this news organization, “but when you have skin type III and above, you run into a really high incidence of postinflammatory hyperpigmentation — and treating that with fractional beam can lead to a reduced incidence of darkening of the skin afterward.”
A Lower-Cost Option
This study showed that the 532-nm picosecond laser with fractional beam MLA is a useful option for patients with darker skin types, Kelly Stankiewicz, MD, a dermatologist who practices in Park City, Utah, and moderated the session where these results were presented, told this news organization.
“The most challenging thing about treating lentigines in darker skin types is preventing potential side effects, mainly dyspigmentation,” she said after the meeting. “These side effects are, for the most part, temporary, but they can take 6-18 months to resolve, so it’s important to prevent them in the first place.”
She noted that the 532-nm and 1064-nm wavelengths are the most commonly available for picosecond lasers and that they’re easier to produce and less expensive. “There are picosecond lasers with middle wavelengths in the red light to near-infrared range (650-785 nm) that are better for darker skin types because they are more gentle yet still effective at targeting pigment, but these lasers are more expensive and less widely available,” Dr. Stankiewicz said.
“The microlens array, used in this study with the 532-nm wavelength, is an inexpensive piece that fits at the end of the laser,” she added. “So, to have an option that turns a 532-nm laser into a safer device for the treatment of lentigines in darker skin types is very helpful.”
Dr. Manuskiatti and Dr. Stankiewicz had no relevant disclosures to report.
A version of this article first appeared on Medscape.com.
FROM ASLMS 2024
Botulinum Toxin, Dermal Fillers Safe in Skin of Color Patients, Review Finds
TOPLINE:
, and more data on Black and Latinx populations are needed, according to a literature review.
METHODOLOGY:
- Understanding the efficacy and safety of cosmetic injectables in diverse skin types is important because individuals identifying as racial and ethnic minorities accounted for 18% of neuromodulator procedures and 22% of soft tissue augmentation procedures in 2020 in the United States.
- Researchers reviewed available literature on the usability and efficacy of neuromodulators and soft tissue augmentation in individuals with SOC because of the limited data available in these populations, particularly non-Asian, SOC populations.
- Overall, 88 studies in English were included, which were either dedicated to discussing safety and/or efficacy of injectables in SOC populations or enrolled more than 20% of participants from SOC populations.
- High-quality level I and II evidence was found in 50 studies, and 9940 patients were analyzed in this review.
TAKEAWAY:
- Studies considered high quality indicated that botulinum toxin is safe and effective for treating glabellar lines in Asians; tailored guidelines recommended specific strategies; and adverse events, such as eyelid issues, were more common in Asians.
- Hyaluronic acid fillers showed significant improvement in moderate to severe cases of nasolabial folds in Asians, and adverse effects like swelling and pain were mild to moderate — some cases of granuloma formation and vascular compromise have been reported.
- In Black individuals, botulinum toxin was well tolerated; hyaluronic acid fillers showed favorable safety, with mild to moderate adverse events; and measures like slower injections and subdermal techniques minimized risks.
- In Latinx populations, there was a lack of robust study data on safety and efficacy of botulinum toxin, whereas hyaluronic acid and poly-L-lactic acid fillers were well tolerated.
IN PRACTICE:
“Neuromodulators and dermal fillers are useful and safe as cosmetic and antiaging treatments in SOC populations, with the greatest amount of data supporting its use in Asian populations,” although more data on Black and Latinx populations are needed, the authors concluded. “During cosmetic consultations, physicians should consider the impact of different cultural beauty norms on the aesthetic goals of diverse patient populations,” they added.
SOURCE:
This study led by Shanice McKenzie, MD, from the Department of Dermatology, University of Southern California, Los Angeles, California, was published online in the Journal of Cosmetic Dermatology.
LIMITATIONS:
Most of the recent data and formal consensus guidelines on injectables in the review came from Asian countries, and there was “a relative paucity of research on Black and Latinx populations,” the authors noted.
DISCLOSURES:
The study did not receive any funding. Two authors declared serving as a consultant, investigator, and/or speaker for various companies; the rest had no disclosures to report.
A version of this article appeared on Medscape.com.
TOPLINE:
, and more data on Black and Latinx populations are needed, according to a literature review.
METHODOLOGY:
- Understanding the efficacy and safety of cosmetic injectables in diverse skin types is important because individuals identifying as racial and ethnic minorities accounted for 18% of neuromodulator procedures and 22% of soft tissue augmentation procedures in 2020 in the United States.
- Researchers reviewed available literature on the usability and efficacy of neuromodulators and soft tissue augmentation in individuals with SOC because of the limited data available in these populations, particularly non-Asian, SOC populations.
- Overall, 88 studies in English were included, which were either dedicated to discussing safety and/or efficacy of injectables in SOC populations or enrolled more than 20% of participants from SOC populations.
- High-quality level I and II evidence was found in 50 studies, and 9940 patients were analyzed in this review.
TAKEAWAY:
- Studies considered high quality indicated that botulinum toxin is safe and effective for treating glabellar lines in Asians; tailored guidelines recommended specific strategies; and adverse events, such as eyelid issues, were more common in Asians.
- Hyaluronic acid fillers showed significant improvement in moderate to severe cases of nasolabial folds in Asians, and adverse effects like swelling and pain were mild to moderate — some cases of granuloma formation and vascular compromise have been reported.
- In Black individuals, botulinum toxin was well tolerated; hyaluronic acid fillers showed favorable safety, with mild to moderate adverse events; and measures like slower injections and subdermal techniques minimized risks.
- In Latinx populations, there was a lack of robust study data on safety and efficacy of botulinum toxin, whereas hyaluronic acid and poly-L-lactic acid fillers were well tolerated.
IN PRACTICE:
“Neuromodulators and dermal fillers are useful and safe as cosmetic and antiaging treatments in SOC populations, with the greatest amount of data supporting its use in Asian populations,” although more data on Black and Latinx populations are needed, the authors concluded. “During cosmetic consultations, physicians should consider the impact of different cultural beauty norms on the aesthetic goals of diverse patient populations,” they added.
SOURCE:
This study led by Shanice McKenzie, MD, from the Department of Dermatology, University of Southern California, Los Angeles, California, was published online in the Journal of Cosmetic Dermatology.
LIMITATIONS:
Most of the recent data and formal consensus guidelines on injectables in the review came from Asian countries, and there was “a relative paucity of research on Black and Latinx populations,” the authors noted.
DISCLOSURES:
The study did not receive any funding. Two authors declared serving as a consultant, investigator, and/or speaker for various companies; the rest had no disclosures to report.
A version of this article appeared on Medscape.com.
TOPLINE:
, and more data on Black and Latinx populations are needed, according to a literature review.
METHODOLOGY:
- Understanding the efficacy and safety of cosmetic injectables in diverse skin types is important because individuals identifying as racial and ethnic minorities accounted for 18% of neuromodulator procedures and 22% of soft tissue augmentation procedures in 2020 in the United States.
- Researchers reviewed available literature on the usability and efficacy of neuromodulators and soft tissue augmentation in individuals with SOC because of the limited data available in these populations, particularly non-Asian, SOC populations.
- Overall, 88 studies in English were included, which were either dedicated to discussing safety and/or efficacy of injectables in SOC populations or enrolled more than 20% of participants from SOC populations.
- High-quality level I and II evidence was found in 50 studies, and 9940 patients were analyzed in this review.
TAKEAWAY:
- Studies considered high quality indicated that botulinum toxin is safe and effective for treating glabellar lines in Asians; tailored guidelines recommended specific strategies; and adverse events, such as eyelid issues, were more common in Asians.
- Hyaluronic acid fillers showed significant improvement in moderate to severe cases of nasolabial folds in Asians, and adverse effects like swelling and pain were mild to moderate — some cases of granuloma formation and vascular compromise have been reported.
- In Black individuals, botulinum toxin was well tolerated; hyaluronic acid fillers showed favorable safety, with mild to moderate adverse events; and measures like slower injections and subdermal techniques minimized risks.
- In Latinx populations, there was a lack of robust study data on safety and efficacy of botulinum toxin, whereas hyaluronic acid and poly-L-lactic acid fillers were well tolerated.
IN PRACTICE:
“Neuromodulators and dermal fillers are useful and safe as cosmetic and antiaging treatments in SOC populations, with the greatest amount of data supporting its use in Asian populations,” although more data on Black and Latinx populations are needed, the authors concluded. “During cosmetic consultations, physicians should consider the impact of different cultural beauty norms on the aesthetic goals of diverse patient populations,” they added.
SOURCE:
This study led by Shanice McKenzie, MD, from the Department of Dermatology, University of Southern California, Los Angeles, California, was published online in the Journal of Cosmetic Dermatology.
LIMITATIONS:
Most of the recent data and formal consensus guidelines on injectables in the review came from Asian countries, and there was “a relative paucity of research on Black and Latinx populations,” the authors noted.
DISCLOSURES:
The study did not receive any funding. Two authors declared serving as a consultant, investigator, and/or speaker for various companies; the rest had no disclosures to report.
A version of this article appeared on Medscape.com.
What’s Driving the Higher Breast Cancer Death Rate in Black Women?
More women today are surviving breast cancer if it’s caught early, largely because of better screening and more effective and targeted treatments.
However, not everyone has benefited equitably from this progress. Critical gaps in breast cancer outcomes and survival remain for women in racial and ethnic minority groups.
Black women for instance, have a 41% higher death rate from breast cancer compared with White patients. They also have a greater incidence of aggressive disease like triple-negative breast cancer. Native American and Hispanic women, meanwhile, are more likely to be diagnosed with breast cancer at an earlier age than White women and experience more aggressive breast cancers.
In 2023, Farhad Islami, MD, PhD, and his team published an updated analysis of racial/ethnic and socioeconomic disparities in cancer trends based on data from 2014 to 2020. The analysis found that Black women in particular, were the least likely to have an early-stage diagnosis of breast cancer. Localized‐stage breast cancer was diagnosed in 57% of Black women versus 68% of White women.
“Despite substantial progress in cancer prevention, early detection, and treatments, the burden of cancer remains greater among populations that have been historically marginalized, including people of color, people with lower socioeconomic status, and people living in nonmetropolitan areas,” said Dr. Islami, who is senior scientific director of cancer disparity research in the Surveillance & Health Equity Science Department at the American Cancer Society.
The reasons behind outcomes disparities in breast cancer are complex, making solutions challenging, say experts researching racial differences in cancer outcomes.
Among the findings of this research is that breast cancer tests may be contributing to the disparities and misguiding care for some patients of color.
SDH and Screening Rates Differences By Race
A range of factors contribute to racial and ethnic disparities in breast cancer outcomes, said Pamela Ganschow, MD, an associate professor in the Department of Internal Medicine at the University of Illinois Cancer Center in Chicago and part of the university’s Cancer Prevention and Control research program. These include socioeconomic status, access to timely and high-quality care across the cancer control continuum, cultural beliefs, differences in genetic makeup and tumor biology, as well as system biases, such as implicit biases and systemic racism, Dr. Ganschow said.
Dr. Islami adds that gaps in access to cancer prevention, early detection, and treatment are largely rooted in fundamental inequities in social determinants of health (SDH), such as whether a patient has safe housing, transportation, education, job opportunities, income, access to nutritious foods, and language and literacy skills, among others.
Dr. Islami’s analysis, for example, shows that people of color are generally more likely to have lower educational attainment and to experience poverty, food insecurity, and housing insecurity compared with White people. Among people aged 18-64 years, the age-adjusted proportion of individuals with no health insurance in 2021 was also higher among Black (13.7%), American Indian/Alaskan Native (18.7%), and Hispanic (28.7%) patients than among White (7.8%) or Asian (5.9%) people, according to the report.
Competing needs can also get in the way of prioritizing cancer screenings, especially for patients in lower socio-economic populations, Dr. Ganschow said.
“You’ve got people who are working a job or three jobs, just to make ends meet for their family and can’t necessarily take time off to get that done,” she said. “Nor is it prioritized in their head because they’ve got to put a meal on the table.”
But the racial disparities between Black and White women, at least, are not clearly explained by differences between the screening rates..
Of patients who received mammograms 76% were White and 79% were Black, according to another recent study coauthored by Dr. Islami. While Black women appear to have the highest breast cancer screening rates, some data suggest such rates are being overreported.
Lower screening rates were seen in American Indian/Alaska Native (59%), Asian (67%), and Hispanic women (74%).
Biological Differences, Bad Testing Recommendations May Contribute to Poor Outcomes
Differences in biology may be one overlooked internal driver of lower breast cancer survival in Black women.
Researchers at Sanford Burnham Prebys in La Jolla, California, recently analyzed the breast cells of White and Black women, finding significant molecular differences that may be contributing to higher breast cancer mortality rates in Black women.
Investigators analyzed both healthy tissue and tumor tissue from 185 Black women and compared the samples to that of White women. They discovered differences among Black and White women in the way their DNA repair genes are expressed, both in healthy breast tissue and in tumors positive for estrogen receptor breast cancer. Molecular differences were also present in the cellular signals that control how fast cells, including cancer cells, grow.
DNA repair is part of normal cellular function and helps cells recover from damage that can occur during DNA replication or in response to external factors, such as stress.
“One of the first lines of defense, to prevent the cell from becoming a tumor are DNA damage repair pathways,” said Svasti Haricharan, PhD, a coauthor of the study and an assistant professor at Sanford Burnham Prebys. “We know there are many different DNA damage repair pathways that respond to different types of DNA damage. What we didn’t know was that, even in our normal cells, based on your race and ethnicity, you have different levels of DNA repair proteins.”
The study found that many of the proteins associated with endocrine resistance and poor outcomes in breast cancer patients are differently regulated in Black women compared with White woman. These differences contribute to resistance to standard endocrine therapy, Dr. Haricharan said.
“Because we never studied the biology in Black woman, it was just assumed that across all demographics, it must be the same,” she said. “We are not even accounting for the possibility there are likely intrinsic differences for how you will respond to an endocrine treatment.”
Testing and treatment may also be playing a role in worse breast cancer outcomes for Black women.
In an analysis of 73,363 women with early-stage, estrogen receptor–positive breast cancer, investigators found that a common test used to decide the treatment course for patients may be leading to bad recommendations for Black women.
The test, known as the 21-gene breast recurrence score, is the most commonly ordered biomarker test used to guide doctor’s recommendations for patients with estrogen receptor–positive breast cancer, the most common form of cancer in Black women, representing about 70%-80% of cases.
The test helps physicians identify which patients are good candidates for chemo, but the test may underestimate the benefit of chemo for Black women. It ranks some Black women as unlikely to benefit from chemo, when they actually would have benefited, according to the January 2024 study, published in the Journal of the National Comprehensive Cancer Network.
The test gives a score of zero to 100, explains Kent Hoskins, MD, oncology service line medical director at the University of Illinois (UI) Health and director of the Familial Breast Cancer Clinic at UI Health, both in Chicago. The higher the score, the higher the risk and the greater the benefit of chemotherapy. A patient is either above the cut-off score and receives chemo, or is below the cut-off score and does not. In the analysis, investigators found that Black women start improving with chemo at a lower score than White women do.
Dr. Hoskins said the results raise questions about whether the biomarker test should be modified to be more applicable to Black women, whether other tests should be used, or if physicians should judge cut-off scores differently, depending on race.
How Neighborhood Impacts Breast Cancer, Death Rates
Living in a disadvantaged neighborhood also lowers breast cancer survival, according to new research. A disadvantaged neighborhood is generally defined as a location associated with higher concentrations of poverty, higher rates of unemployment, and less access to health care, quality housing, food, and community resources, according to the Centers for Disease Control and Prevention.
Authors of a study published in JAMA Network Open on April 18 identified 350,824 patients with breast cancer. Of these, 41,519 (11.8%) were Hispanic, 39,631 (11.3%) were non-Hispanic Black, and 234,698 (66.9%) were non-Hispanic White. Investigators divided the patients into five groups representing the lowest to highest neighborhood socioeconomic indices using the Yost Index. (The Yost Index is used by the National Cancer Institute for cancer surveillance and is based on variables such as household income, home value, median rent, percentage below 150% of the poverty line, education, and unemployment.)
Of the Black and Hispanic patients in the study, the highest proportions of both demographics lived in the most disadvantaged neighborhoods. (16,141 Black patients [30.9%]) and 10,168 Hispanic patients [19.5%]). Although 45% of White patients also fell into that same category, the highest proportion of White patients in the study lived in the most advantaged neighborhoods (66,529 patients [76.2%]).
Findings showed patients in the most disadvantaged neighborhoods had the highest proportion of triple-negative breast cancer. Patients in this group also had the lowest proportion of patients who completed surgery and radiation, and the highest proportion of patients who received chemotherapy, compared with all other neighborhood groups. The most advantaged neighborhoods group had higher proportions of localized-stage cancer, a higher proportion of patients who underwent surgery and radiation, and the lowest proportion of patients receiving chemotherapy treatment.
Patients in the most disadvantaged neighborhoods also had the highest risk of mortality (hazard ratio [HR,] 1.53; 95% CI, 1.48-1.59; P less than .001) compared with patients living in the most advantaged neighborhoods. Non-Hispanic Black patients in particular, had the highest risk of mortality, compared with non-Hispanic White patients (HR, 1.16; 95% CI, 1.13-1.20; P less than .001).
Authors wrote that the findings suggest neighborhood disadvantage is independently associated with shorter survival in patients with breast cancer, even after controlling for individual-level factors, tumor characteristics, and treatment.
“To address these residual disparities associated with neighborhood disadvantage, research must focus on which components of the built environment influence outcomes,” the authors said.
Another recent study also found correlations among where breast cancer patients lived and how they fared with the disease.
Jasmine M. Miller-Kleinhenz, PhD, an assistant professor at University of Mississippi Medical Center in Jackson, studied how historical redlining impacts breast cancer development and outcomes in her research published in JAMA Network Open, earlier this year. Redlining refers to the practice of denying people access to credit because of where they live. Historically, mortgage lenders widely redlined neighborhoods with predominantly Black residents. The 1968 Fair Housing Act outlawed racially motivated redlining, but consequences from historical redlining still exist.
Dr. Miller-Kleinhenz and her colleagues analyzed a cohort of 1764 women diagnosed with breast cancer between January 2010 and December 2017, who were followed up through December 2019. Investigators accessed the cohort based on three exposures: historic redlining (HRL), contemporary mortgage discrimination (CMD), and persistent mortgage discrimination (PMD). Contemporary mortgage discrimination refers to current-day discriminatory mortgage practices and persistent mortgage discrimination refers to neighborhoods that have experienced both HRL and CMD.
Findings showed that Black women living in historical redlined areas had increased odds of being diagnosed with aggressive forms of breast cancer, while White women in redlined areas had increased odds of late-stage diagnosis.
White women exposed to persistent mortgage discrimination were twice as likely to die of breast cancer, compared with their White counterparts living in areas without historical redlining or contemporary mortgage discrimination, the study found.
That is not to say that Black women did not have an increased risk of breast cancer mortality, Dr. Miller-Kleinhenz explained. Black women had a more than threefold elevated risk of breast cancer mortality compared with White women no matter where they lived, according to the findings.
“These results were surprising because it is showing that while neighborhood conditions might be a major driver of breast cancer mortality in White women, there are factors beyond the neighborhood that are additional drivers that are contributing to poor outcomes in Black women,” she said.
Hope for Improved Outcomes, Higher Survival Rates
Investigators hope the findings of all of this new research lead to better, more targeted treatments and, in turn, improved outcomes.
Dr. Haricharan is optimistic about the improvement of breast cancer outcomes as more is learned about the biology of Black patients and other non-White patients.
There is a growing effort to include more data from minoritized populations in breast cancer research studies, Dr. Haricharan said, and she foresees associated changes to clinical protocols in the future. Her own team is working on creating larger data sets that are more representative of non-White patients to further analyze the differences found in their prior study.
“I think there’s this understanding that, until we have data sets that are more representative, we really are catering to [only one] population in terms of our diagnostic and therapeutic technological advances,” she said.
The American Cancer Society meanwhile, is launching a new initiative in May that aims to collect more health data from Black women to ultimately develop more effective cancer interventions. VOICES of Black Women will focus on collecting and studying health data from Black women through online surveys. The society’s goal is to enroll at least 100,000 Black women in the United States between ages 25 and 55.
Dr. Miller-Kleinhenz called the initiative “an important step to starting to research and answer some of these lingering questions about why there continue to be breast cancer disparities.”
More women today are surviving breast cancer if it’s caught early, largely because of better screening and more effective and targeted treatments.
However, not everyone has benefited equitably from this progress. Critical gaps in breast cancer outcomes and survival remain for women in racial and ethnic minority groups.
Black women for instance, have a 41% higher death rate from breast cancer compared with White patients. They also have a greater incidence of aggressive disease like triple-negative breast cancer. Native American and Hispanic women, meanwhile, are more likely to be diagnosed with breast cancer at an earlier age than White women and experience more aggressive breast cancers.
In 2023, Farhad Islami, MD, PhD, and his team published an updated analysis of racial/ethnic and socioeconomic disparities in cancer trends based on data from 2014 to 2020. The analysis found that Black women in particular, were the least likely to have an early-stage diagnosis of breast cancer. Localized‐stage breast cancer was diagnosed in 57% of Black women versus 68% of White women.
“Despite substantial progress in cancer prevention, early detection, and treatments, the burden of cancer remains greater among populations that have been historically marginalized, including people of color, people with lower socioeconomic status, and people living in nonmetropolitan areas,” said Dr. Islami, who is senior scientific director of cancer disparity research in the Surveillance & Health Equity Science Department at the American Cancer Society.
The reasons behind outcomes disparities in breast cancer are complex, making solutions challenging, say experts researching racial differences in cancer outcomes.
Among the findings of this research is that breast cancer tests may be contributing to the disparities and misguiding care for some patients of color.
SDH and Screening Rates Differences By Race
A range of factors contribute to racial and ethnic disparities in breast cancer outcomes, said Pamela Ganschow, MD, an associate professor in the Department of Internal Medicine at the University of Illinois Cancer Center in Chicago and part of the university’s Cancer Prevention and Control research program. These include socioeconomic status, access to timely and high-quality care across the cancer control continuum, cultural beliefs, differences in genetic makeup and tumor biology, as well as system biases, such as implicit biases and systemic racism, Dr. Ganschow said.
Dr. Islami adds that gaps in access to cancer prevention, early detection, and treatment are largely rooted in fundamental inequities in social determinants of health (SDH), such as whether a patient has safe housing, transportation, education, job opportunities, income, access to nutritious foods, and language and literacy skills, among others.
Dr. Islami’s analysis, for example, shows that people of color are generally more likely to have lower educational attainment and to experience poverty, food insecurity, and housing insecurity compared with White people. Among people aged 18-64 years, the age-adjusted proportion of individuals with no health insurance in 2021 was also higher among Black (13.7%), American Indian/Alaskan Native (18.7%), and Hispanic (28.7%) patients than among White (7.8%) or Asian (5.9%) people, according to the report.
Competing needs can also get in the way of prioritizing cancer screenings, especially for patients in lower socio-economic populations, Dr. Ganschow said.
“You’ve got people who are working a job or three jobs, just to make ends meet for their family and can’t necessarily take time off to get that done,” she said. “Nor is it prioritized in their head because they’ve got to put a meal on the table.”
But the racial disparities between Black and White women, at least, are not clearly explained by differences between the screening rates..
Of patients who received mammograms 76% were White and 79% were Black, according to another recent study coauthored by Dr. Islami. While Black women appear to have the highest breast cancer screening rates, some data suggest such rates are being overreported.
Lower screening rates were seen in American Indian/Alaska Native (59%), Asian (67%), and Hispanic women (74%).
Biological Differences, Bad Testing Recommendations May Contribute to Poor Outcomes
Differences in biology may be one overlooked internal driver of lower breast cancer survival in Black women.
Researchers at Sanford Burnham Prebys in La Jolla, California, recently analyzed the breast cells of White and Black women, finding significant molecular differences that may be contributing to higher breast cancer mortality rates in Black women.
Investigators analyzed both healthy tissue and tumor tissue from 185 Black women and compared the samples to that of White women. They discovered differences among Black and White women in the way their DNA repair genes are expressed, both in healthy breast tissue and in tumors positive for estrogen receptor breast cancer. Molecular differences were also present in the cellular signals that control how fast cells, including cancer cells, grow.
DNA repair is part of normal cellular function and helps cells recover from damage that can occur during DNA replication or in response to external factors, such as stress.
“One of the first lines of defense, to prevent the cell from becoming a tumor are DNA damage repair pathways,” said Svasti Haricharan, PhD, a coauthor of the study and an assistant professor at Sanford Burnham Prebys. “We know there are many different DNA damage repair pathways that respond to different types of DNA damage. What we didn’t know was that, even in our normal cells, based on your race and ethnicity, you have different levels of DNA repair proteins.”
The study found that many of the proteins associated with endocrine resistance and poor outcomes in breast cancer patients are differently regulated in Black women compared with White woman. These differences contribute to resistance to standard endocrine therapy, Dr. Haricharan said.
“Because we never studied the biology in Black woman, it was just assumed that across all demographics, it must be the same,” she said. “We are not even accounting for the possibility there are likely intrinsic differences for how you will respond to an endocrine treatment.”
Testing and treatment may also be playing a role in worse breast cancer outcomes for Black women.
In an analysis of 73,363 women with early-stage, estrogen receptor–positive breast cancer, investigators found that a common test used to decide the treatment course for patients may be leading to bad recommendations for Black women.
The test, known as the 21-gene breast recurrence score, is the most commonly ordered biomarker test used to guide doctor’s recommendations for patients with estrogen receptor–positive breast cancer, the most common form of cancer in Black women, representing about 70%-80% of cases.
The test helps physicians identify which patients are good candidates for chemo, but the test may underestimate the benefit of chemo for Black women. It ranks some Black women as unlikely to benefit from chemo, when they actually would have benefited, according to the January 2024 study, published in the Journal of the National Comprehensive Cancer Network.
The test gives a score of zero to 100, explains Kent Hoskins, MD, oncology service line medical director at the University of Illinois (UI) Health and director of the Familial Breast Cancer Clinic at UI Health, both in Chicago. The higher the score, the higher the risk and the greater the benefit of chemotherapy. A patient is either above the cut-off score and receives chemo, or is below the cut-off score and does not. In the analysis, investigators found that Black women start improving with chemo at a lower score than White women do.
Dr. Hoskins said the results raise questions about whether the biomarker test should be modified to be more applicable to Black women, whether other tests should be used, or if physicians should judge cut-off scores differently, depending on race.
How Neighborhood Impacts Breast Cancer, Death Rates
Living in a disadvantaged neighborhood also lowers breast cancer survival, according to new research. A disadvantaged neighborhood is generally defined as a location associated with higher concentrations of poverty, higher rates of unemployment, and less access to health care, quality housing, food, and community resources, according to the Centers for Disease Control and Prevention.
Authors of a study published in JAMA Network Open on April 18 identified 350,824 patients with breast cancer. Of these, 41,519 (11.8%) were Hispanic, 39,631 (11.3%) were non-Hispanic Black, and 234,698 (66.9%) were non-Hispanic White. Investigators divided the patients into five groups representing the lowest to highest neighborhood socioeconomic indices using the Yost Index. (The Yost Index is used by the National Cancer Institute for cancer surveillance and is based on variables such as household income, home value, median rent, percentage below 150% of the poverty line, education, and unemployment.)
Of the Black and Hispanic patients in the study, the highest proportions of both demographics lived in the most disadvantaged neighborhoods. (16,141 Black patients [30.9%]) and 10,168 Hispanic patients [19.5%]). Although 45% of White patients also fell into that same category, the highest proportion of White patients in the study lived in the most advantaged neighborhoods (66,529 patients [76.2%]).
Findings showed patients in the most disadvantaged neighborhoods had the highest proportion of triple-negative breast cancer. Patients in this group also had the lowest proportion of patients who completed surgery and radiation, and the highest proportion of patients who received chemotherapy, compared with all other neighborhood groups. The most advantaged neighborhoods group had higher proportions of localized-stage cancer, a higher proportion of patients who underwent surgery and radiation, and the lowest proportion of patients receiving chemotherapy treatment.
Patients in the most disadvantaged neighborhoods also had the highest risk of mortality (hazard ratio [HR,] 1.53; 95% CI, 1.48-1.59; P less than .001) compared with patients living in the most advantaged neighborhoods. Non-Hispanic Black patients in particular, had the highest risk of mortality, compared with non-Hispanic White patients (HR, 1.16; 95% CI, 1.13-1.20; P less than .001).
Authors wrote that the findings suggest neighborhood disadvantage is independently associated with shorter survival in patients with breast cancer, even after controlling for individual-level factors, tumor characteristics, and treatment.
“To address these residual disparities associated with neighborhood disadvantage, research must focus on which components of the built environment influence outcomes,” the authors said.
Another recent study also found correlations among where breast cancer patients lived and how they fared with the disease.
Jasmine M. Miller-Kleinhenz, PhD, an assistant professor at University of Mississippi Medical Center in Jackson, studied how historical redlining impacts breast cancer development and outcomes in her research published in JAMA Network Open, earlier this year. Redlining refers to the practice of denying people access to credit because of where they live. Historically, mortgage lenders widely redlined neighborhoods with predominantly Black residents. The 1968 Fair Housing Act outlawed racially motivated redlining, but consequences from historical redlining still exist.
Dr. Miller-Kleinhenz and her colleagues analyzed a cohort of 1764 women diagnosed with breast cancer between January 2010 and December 2017, who were followed up through December 2019. Investigators accessed the cohort based on three exposures: historic redlining (HRL), contemporary mortgage discrimination (CMD), and persistent mortgage discrimination (PMD). Contemporary mortgage discrimination refers to current-day discriminatory mortgage practices and persistent mortgage discrimination refers to neighborhoods that have experienced both HRL and CMD.
Findings showed that Black women living in historical redlined areas had increased odds of being diagnosed with aggressive forms of breast cancer, while White women in redlined areas had increased odds of late-stage diagnosis.
White women exposed to persistent mortgage discrimination were twice as likely to die of breast cancer, compared with their White counterparts living in areas without historical redlining or contemporary mortgage discrimination, the study found.
That is not to say that Black women did not have an increased risk of breast cancer mortality, Dr. Miller-Kleinhenz explained. Black women had a more than threefold elevated risk of breast cancer mortality compared with White women no matter where they lived, according to the findings.
“These results were surprising because it is showing that while neighborhood conditions might be a major driver of breast cancer mortality in White women, there are factors beyond the neighborhood that are additional drivers that are contributing to poor outcomes in Black women,” she said.
Hope for Improved Outcomes, Higher Survival Rates
Investigators hope the findings of all of this new research lead to better, more targeted treatments and, in turn, improved outcomes.
Dr. Haricharan is optimistic about the improvement of breast cancer outcomes as more is learned about the biology of Black patients and other non-White patients.
There is a growing effort to include more data from minoritized populations in breast cancer research studies, Dr. Haricharan said, and she foresees associated changes to clinical protocols in the future. Her own team is working on creating larger data sets that are more representative of non-White patients to further analyze the differences found in their prior study.
“I think there’s this understanding that, until we have data sets that are more representative, we really are catering to [only one] population in terms of our diagnostic and therapeutic technological advances,” she said.
The American Cancer Society meanwhile, is launching a new initiative in May that aims to collect more health data from Black women to ultimately develop more effective cancer interventions. VOICES of Black Women will focus on collecting and studying health data from Black women through online surveys. The society’s goal is to enroll at least 100,000 Black women in the United States between ages 25 and 55.
Dr. Miller-Kleinhenz called the initiative “an important step to starting to research and answer some of these lingering questions about why there continue to be breast cancer disparities.”
More women today are surviving breast cancer if it’s caught early, largely because of better screening and more effective and targeted treatments.
However, not everyone has benefited equitably from this progress. Critical gaps in breast cancer outcomes and survival remain for women in racial and ethnic minority groups.
Black women for instance, have a 41% higher death rate from breast cancer compared with White patients. They also have a greater incidence of aggressive disease like triple-negative breast cancer. Native American and Hispanic women, meanwhile, are more likely to be diagnosed with breast cancer at an earlier age than White women and experience more aggressive breast cancers.
In 2023, Farhad Islami, MD, PhD, and his team published an updated analysis of racial/ethnic and socioeconomic disparities in cancer trends based on data from 2014 to 2020. The analysis found that Black women in particular, were the least likely to have an early-stage diagnosis of breast cancer. Localized‐stage breast cancer was diagnosed in 57% of Black women versus 68% of White women.
“Despite substantial progress in cancer prevention, early detection, and treatments, the burden of cancer remains greater among populations that have been historically marginalized, including people of color, people with lower socioeconomic status, and people living in nonmetropolitan areas,” said Dr. Islami, who is senior scientific director of cancer disparity research in the Surveillance & Health Equity Science Department at the American Cancer Society.
The reasons behind outcomes disparities in breast cancer are complex, making solutions challenging, say experts researching racial differences in cancer outcomes.
Among the findings of this research is that breast cancer tests may be contributing to the disparities and misguiding care for some patients of color.
SDH and Screening Rates Differences By Race
A range of factors contribute to racial and ethnic disparities in breast cancer outcomes, said Pamela Ganschow, MD, an associate professor in the Department of Internal Medicine at the University of Illinois Cancer Center in Chicago and part of the university’s Cancer Prevention and Control research program. These include socioeconomic status, access to timely and high-quality care across the cancer control continuum, cultural beliefs, differences in genetic makeup and tumor biology, as well as system biases, such as implicit biases and systemic racism, Dr. Ganschow said.
Dr. Islami adds that gaps in access to cancer prevention, early detection, and treatment are largely rooted in fundamental inequities in social determinants of health (SDH), such as whether a patient has safe housing, transportation, education, job opportunities, income, access to nutritious foods, and language and literacy skills, among others.
Dr. Islami’s analysis, for example, shows that people of color are generally more likely to have lower educational attainment and to experience poverty, food insecurity, and housing insecurity compared with White people. Among people aged 18-64 years, the age-adjusted proportion of individuals with no health insurance in 2021 was also higher among Black (13.7%), American Indian/Alaskan Native (18.7%), and Hispanic (28.7%) patients than among White (7.8%) or Asian (5.9%) people, according to the report.
Competing needs can also get in the way of prioritizing cancer screenings, especially for patients in lower socio-economic populations, Dr. Ganschow said.
“You’ve got people who are working a job or three jobs, just to make ends meet for their family and can’t necessarily take time off to get that done,” she said. “Nor is it prioritized in their head because they’ve got to put a meal on the table.”
But the racial disparities between Black and White women, at least, are not clearly explained by differences between the screening rates..
Of patients who received mammograms 76% were White and 79% were Black, according to another recent study coauthored by Dr. Islami. While Black women appear to have the highest breast cancer screening rates, some data suggest such rates are being overreported.
Lower screening rates were seen in American Indian/Alaska Native (59%), Asian (67%), and Hispanic women (74%).
Biological Differences, Bad Testing Recommendations May Contribute to Poor Outcomes
Differences in biology may be one overlooked internal driver of lower breast cancer survival in Black women.
Researchers at Sanford Burnham Prebys in La Jolla, California, recently analyzed the breast cells of White and Black women, finding significant molecular differences that may be contributing to higher breast cancer mortality rates in Black women.
Investigators analyzed both healthy tissue and tumor tissue from 185 Black women and compared the samples to that of White women. They discovered differences among Black and White women in the way their DNA repair genes are expressed, both in healthy breast tissue and in tumors positive for estrogen receptor breast cancer. Molecular differences were also present in the cellular signals that control how fast cells, including cancer cells, grow.
DNA repair is part of normal cellular function and helps cells recover from damage that can occur during DNA replication or in response to external factors, such as stress.
“One of the first lines of defense, to prevent the cell from becoming a tumor are DNA damage repair pathways,” said Svasti Haricharan, PhD, a coauthor of the study and an assistant professor at Sanford Burnham Prebys. “We know there are many different DNA damage repair pathways that respond to different types of DNA damage. What we didn’t know was that, even in our normal cells, based on your race and ethnicity, you have different levels of DNA repair proteins.”
The study found that many of the proteins associated with endocrine resistance and poor outcomes in breast cancer patients are differently regulated in Black women compared with White woman. These differences contribute to resistance to standard endocrine therapy, Dr. Haricharan said.
“Because we never studied the biology in Black woman, it was just assumed that across all demographics, it must be the same,” she said. “We are not even accounting for the possibility there are likely intrinsic differences for how you will respond to an endocrine treatment.”
Testing and treatment may also be playing a role in worse breast cancer outcomes for Black women.
In an analysis of 73,363 women with early-stage, estrogen receptor–positive breast cancer, investigators found that a common test used to decide the treatment course for patients may be leading to bad recommendations for Black women.
The test, known as the 21-gene breast recurrence score, is the most commonly ordered biomarker test used to guide doctor’s recommendations for patients with estrogen receptor–positive breast cancer, the most common form of cancer in Black women, representing about 70%-80% of cases.
The test helps physicians identify which patients are good candidates for chemo, but the test may underestimate the benefit of chemo for Black women. It ranks some Black women as unlikely to benefit from chemo, when they actually would have benefited, according to the January 2024 study, published in the Journal of the National Comprehensive Cancer Network.
The test gives a score of zero to 100, explains Kent Hoskins, MD, oncology service line medical director at the University of Illinois (UI) Health and director of the Familial Breast Cancer Clinic at UI Health, both in Chicago. The higher the score, the higher the risk and the greater the benefit of chemotherapy. A patient is either above the cut-off score and receives chemo, or is below the cut-off score and does not. In the analysis, investigators found that Black women start improving with chemo at a lower score than White women do.
Dr. Hoskins said the results raise questions about whether the biomarker test should be modified to be more applicable to Black women, whether other tests should be used, or if physicians should judge cut-off scores differently, depending on race.
How Neighborhood Impacts Breast Cancer, Death Rates
Living in a disadvantaged neighborhood also lowers breast cancer survival, according to new research. A disadvantaged neighborhood is generally defined as a location associated with higher concentrations of poverty, higher rates of unemployment, and less access to health care, quality housing, food, and community resources, according to the Centers for Disease Control and Prevention.
Authors of a study published in JAMA Network Open on April 18 identified 350,824 patients with breast cancer. Of these, 41,519 (11.8%) were Hispanic, 39,631 (11.3%) were non-Hispanic Black, and 234,698 (66.9%) were non-Hispanic White. Investigators divided the patients into five groups representing the lowest to highest neighborhood socioeconomic indices using the Yost Index. (The Yost Index is used by the National Cancer Institute for cancer surveillance and is based on variables such as household income, home value, median rent, percentage below 150% of the poverty line, education, and unemployment.)
Of the Black and Hispanic patients in the study, the highest proportions of both demographics lived in the most disadvantaged neighborhoods. (16,141 Black patients [30.9%]) and 10,168 Hispanic patients [19.5%]). Although 45% of White patients also fell into that same category, the highest proportion of White patients in the study lived in the most advantaged neighborhoods (66,529 patients [76.2%]).
Findings showed patients in the most disadvantaged neighborhoods had the highest proportion of triple-negative breast cancer. Patients in this group also had the lowest proportion of patients who completed surgery and radiation, and the highest proportion of patients who received chemotherapy, compared with all other neighborhood groups. The most advantaged neighborhoods group had higher proportions of localized-stage cancer, a higher proportion of patients who underwent surgery and radiation, and the lowest proportion of patients receiving chemotherapy treatment.
Patients in the most disadvantaged neighborhoods also had the highest risk of mortality (hazard ratio [HR,] 1.53; 95% CI, 1.48-1.59; P less than .001) compared with patients living in the most advantaged neighborhoods. Non-Hispanic Black patients in particular, had the highest risk of mortality, compared with non-Hispanic White patients (HR, 1.16; 95% CI, 1.13-1.20; P less than .001).
Authors wrote that the findings suggest neighborhood disadvantage is independently associated with shorter survival in patients with breast cancer, even after controlling for individual-level factors, tumor characteristics, and treatment.
“To address these residual disparities associated with neighborhood disadvantage, research must focus on which components of the built environment influence outcomes,” the authors said.
Another recent study also found correlations among where breast cancer patients lived and how they fared with the disease.
Jasmine M. Miller-Kleinhenz, PhD, an assistant professor at University of Mississippi Medical Center in Jackson, studied how historical redlining impacts breast cancer development and outcomes in her research published in JAMA Network Open, earlier this year. Redlining refers to the practice of denying people access to credit because of where they live. Historically, mortgage lenders widely redlined neighborhoods with predominantly Black residents. The 1968 Fair Housing Act outlawed racially motivated redlining, but consequences from historical redlining still exist.
Dr. Miller-Kleinhenz and her colleagues analyzed a cohort of 1764 women diagnosed with breast cancer between January 2010 and December 2017, who were followed up through December 2019. Investigators accessed the cohort based on three exposures: historic redlining (HRL), contemporary mortgage discrimination (CMD), and persistent mortgage discrimination (PMD). Contemporary mortgage discrimination refers to current-day discriminatory mortgage practices and persistent mortgage discrimination refers to neighborhoods that have experienced both HRL and CMD.
Findings showed that Black women living in historical redlined areas had increased odds of being diagnosed with aggressive forms of breast cancer, while White women in redlined areas had increased odds of late-stage diagnosis.
White women exposed to persistent mortgage discrimination were twice as likely to die of breast cancer, compared with their White counterparts living in areas without historical redlining or contemporary mortgage discrimination, the study found.
That is not to say that Black women did not have an increased risk of breast cancer mortality, Dr. Miller-Kleinhenz explained. Black women had a more than threefold elevated risk of breast cancer mortality compared with White women no matter where they lived, according to the findings.
“These results were surprising because it is showing that while neighborhood conditions might be a major driver of breast cancer mortality in White women, there are factors beyond the neighborhood that are additional drivers that are contributing to poor outcomes in Black women,” she said.
Hope for Improved Outcomes, Higher Survival Rates
Investigators hope the findings of all of this new research lead to better, more targeted treatments and, in turn, improved outcomes.
Dr. Haricharan is optimistic about the improvement of breast cancer outcomes as more is learned about the biology of Black patients and other non-White patients.
There is a growing effort to include more data from minoritized populations in breast cancer research studies, Dr. Haricharan said, and she foresees associated changes to clinical protocols in the future. Her own team is working on creating larger data sets that are more representative of non-White patients to further analyze the differences found in their prior study.
“I think there’s this understanding that, until we have data sets that are more representative, we really are catering to [only one] population in terms of our diagnostic and therapeutic technological advances,” she said.
The American Cancer Society meanwhile, is launching a new initiative in May that aims to collect more health data from Black women to ultimately develop more effective cancer interventions. VOICES of Black Women will focus on collecting and studying health data from Black women through online surveys. The society’s goal is to enroll at least 100,000 Black women in the United States between ages 25 and 55.
Dr. Miller-Kleinhenz called the initiative “an important step to starting to research and answer some of these lingering questions about why there continue to be breast cancer disparities.”
Half-Truths Produce Whole Failures in Health Policy
On May 5, 2023, the director of the Centers for Disease Control and Prevention (CDC), Rochelle Walensky, in announcing her resignation after more than 2 years of dedicated service, wrote that she “took on this role … with the goal of leaving behind the dark days of the pandemic and moving the CDC — and public health — forward into a much better and more trusted place.”
Three times in the past 3 years I have written a Beyond the White Coat column emphasizing the importance of trust. Trust in the expertise of scientists. Trust in the integrity of medical research and public health institutions. Trust in the commitment of providers — doctors, nurses, therapists, and first responders — to shepherd us through the pandemic and other medical crises in our lives. This column is take four.
All human institutions have human imperfections. However, imperfect humans working together in community are more productive and more reliable than nihilism and political polarization. Underlying all of healthcare are compassion and honesty. Honesty means the truth, the whole truth, and nothing but the truth. Honesty is such a simple concept in the moral formation of children, but the concept has evolved aberrantly in the world of woke adults. There appear to be irresistible temptations to shade that truth for political gain. The dominant current mutation is the half-truth. One tells the part of the truth that appears to advance one’s own political aspirations and at the same time one omits or censors other viewpoints.
On April 17, 2023, the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the American Psychiatric Association wrote an open letter to Congressional leaders advocating for transgender female students’ participation in girl’s and women’s sports. The letter was written “On behalf of the more than 165,000” members of those organizations, though public opinion polls show a majority of those members likely oppose the opinion expressed. The letter goes on to extol the benefits that sports might bring to transgender students, but it contains not one word acknowledging the negative impact that participation has on others. That is a half-truth.
The same half-truth methodology distorts dialogue about various therapies for gender dysphoria in children and young adults.
In April 2022, U.S. Assistant Secretary for Health Rachel Levine in an NPR interview declared that, “There is no argument about the value and importance of gender-affirming care.” That might be a half-truth, since I could not locate U.S. specialists who dare to go on record questioning the party line of the World Professional Association for Transgender Health. However, Dr. Levine’s dismissal of any dissent is bizarre since in the prior 2 years multiple countries, including Australia, New Zealand, Sweden, Finland, and the United Kingdom had all issued reports questioning and even rescinding the practices that evolved since the 2012 WPATH guidance. Their main concerns included 1) the marked increase in incidence of gender dysphoria first manifesting in tween and early teenage girls, 2) the inadequate access to mental health screening before considering transitioning, 3) the long-term risks of puberty blockers particularly to bone density, and 4) the low quality of evidence supporting a measurable reduction in suicide rates. There may be reasonable counterarguments to each of those concerns, but a high ranking U.S. government official labeling all those international reports as “no argument” does not produce high quality decision making and does not foster the public’s trust.
Indeed, the public in many cases has decided its elected legislators are more trustworthy on these topics than the medical organizations. As I wrote the first draft of this column, the Missouri state legislators had passed a bill banning gender-affirming health care for transgender minors. They also passed a bill preventing participation of transgender females in women’s sports. Per reckoning by CBS News in the summer of 2023, 16 states had recently enacted laws restricting gender-affirming care and 22 states had restricted transgender participation in sports.
In 2022, I wrote a column claiming that suppressing viewpoints and debate leads to exploding spaceships. I believe the current legislative carnage is just such an explosion. It harms children.
The AAP has experts in advocacy. I am no expert in political advocacy. Perhaps politics has to be played by different rules where half-truths are normalized. Criminal law and advertising use those rules. But this explosion of vitriol and legislative intrusion into medicine should prompt everyone to reassess the use of one-sided advocacy in public and professional circles in healthcare. I want to be associated with a profession that uses evidence-based medicine that is not corrupted with political agendas. I want to be associated with a profession known for telling the whole truth.
In a society that is increasingly polarized, I want to embrace the advice of John Stuart Mill, a 19th century English philosopher best known for utilitarianism, which is often expressed as “the greatest good for the greatest number.” Mr. Mill also wrote on social theory, liberty, and even some early feminist theory. His 1859 work, On Liberty, chapter II, asserts: “He who knows only his own side of the case, knows little of that. His reasons may be good, and no one may have been able to refute them. But if he is equally unable to refute the reasons on the opposite side; if he does not so much as know what they are, he has no ground for preferring either opinion.”
Mr. Mill did not like half-truths.
It’s About Trust
My column is not the instrument to debate the use of hormones as puberty blockers or the fairness of transgender women participating in women’s sports. Those judgments will be rendered by others. I may report on those deliberations, but my column’s emphasis is on how professionals, and their organizations, go about making those determinations
For instance, the National Health Service in the United Kingdom spent 2 years reassessing transgender care for children and in October 2022 released a draft proposal to reduce and limit the aggressive therapies. On June 9, 2023, the NHS fully enacted those changes. Puberty blockers for gender dysphoria would be used only in experimental trials. In April 2024 the NHS began implementing those changes, joining other European countries that have imposed similar restrictions.
Similarly, the debate about transgender participation in women’s sports has continued to rage for years. On April 8, 2024, the National Association of Intercollegiate Athletics passed a resolution that bans almost all transgender participation in NAIA-regulated intercollegiate women’s sports. Dance and cheerleading are exceptions. Participation is still permissible at the intramural level. The NCAA has different rules.
Go to those sources to learn more substance for those debates. This column is about trust.
A major problem currently facing medicine is the public’s trust in expertise. That trust had been seriously weakened before the pandemic and was repeatedly wounded during the pandemic with arguments over masks, vaccines, and shutdowns. It needs repair.
This is especially true for pediatric hospitalists that do not have the opportunity that office-based pediatricians have to build rapport with a family over years. At a recent university conference on diversity, equity, and inclusivity, one female rabbi stated, “I cannot be rabbi to everybody.” I agreed, but as a medical professional, sometimes I must be.
Telling half-truths harms the public’s trust in their personal physicians and in the medical establishment. Once people suspect an organization is making decisions based on ideology rather than science, credibility is lost and difficult to recover.
Let us stop telling half-truths. Let us stop suppressing dialogue. Truth can never be completely captured by humans, but if one side of an issue is suppressed by cancel culture, censorship, accusations of homophobia, or threat of cultural war, the search for truth is severely impaired.
Let us, as medical professionals, adopt Stephen Covey’s habit number 5, “Seek first to understand, then to be understood.” Empower voices. Listen to all stakeholders. And when we finally do speak, remember John Stuart Mill and tell the whole truth.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
On May 5, 2023, the director of the Centers for Disease Control and Prevention (CDC), Rochelle Walensky, in announcing her resignation after more than 2 years of dedicated service, wrote that she “took on this role … with the goal of leaving behind the dark days of the pandemic and moving the CDC — and public health — forward into a much better and more trusted place.”
Three times in the past 3 years I have written a Beyond the White Coat column emphasizing the importance of trust. Trust in the expertise of scientists. Trust in the integrity of medical research and public health institutions. Trust in the commitment of providers — doctors, nurses, therapists, and first responders — to shepherd us through the pandemic and other medical crises in our lives. This column is take four.
All human institutions have human imperfections. However, imperfect humans working together in community are more productive and more reliable than nihilism and political polarization. Underlying all of healthcare are compassion and honesty. Honesty means the truth, the whole truth, and nothing but the truth. Honesty is such a simple concept in the moral formation of children, but the concept has evolved aberrantly in the world of woke adults. There appear to be irresistible temptations to shade that truth for political gain. The dominant current mutation is the half-truth. One tells the part of the truth that appears to advance one’s own political aspirations and at the same time one omits or censors other viewpoints.
On April 17, 2023, the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the American Psychiatric Association wrote an open letter to Congressional leaders advocating for transgender female students’ participation in girl’s and women’s sports. The letter was written “On behalf of the more than 165,000” members of those organizations, though public opinion polls show a majority of those members likely oppose the opinion expressed. The letter goes on to extol the benefits that sports might bring to transgender students, but it contains not one word acknowledging the negative impact that participation has on others. That is a half-truth.
The same half-truth methodology distorts dialogue about various therapies for gender dysphoria in children and young adults.
In April 2022, U.S. Assistant Secretary for Health Rachel Levine in an NPR interview declared that, “There is no argument about the value and importance of gender-affirming care.” That might be a half-truth, since I could not locate U.S. specialists who dare to go on record questioning the party line of the World Professional Association for Transgender Health. However, Dr. Levine’s dismissal of any dissent is bizarre since in the prior 2 years multiple countries, including Australia, New Zealand, Sweden, Finland, and the United Kingdom had all issued reports questioning and even rescinding the practices that evolved since the 2012 WPATH guidance. Their main concerns included 1) the marked increase in incidence of gender dysphoria first manifesting in tween and early teenage girls, 2) the inadequate access to mental health screening before considering transitioning, 3) the long-term risks of puberty blockers particularly to bone density, and 4) the low quality of evidence supporting a measurable reduction in suicide rates. There may be reasonable counterarguments to each of those concerns, but a high ranking U.S. government official labeling all those international reports as “no argument” does not produce high quality decision making and does not foster the public’s trust.
Indeed, the public in many cases has decided its elected legislators are more trustworthy on these topics than the medical organizations. As I wrote the first draft of this column, the Missouri state legislators had passed a bill banning gender-affirming health care for transgender minors. They also passed a bill preventing participation of transgender females in women’s sports. Per reckoning by CBS News in the summer of 2023, 16 states had recently enacted laws restricting gender-affirming care and 22 states had restricted transgender participation in sports.
In 2022, I wrote a column claiming that suppressing viewpoints and debate leads to exploding spaceships. I believe the current legislative carnage is just such an explosion. It harms children.
The AAP has experts in advocacy. I am no expert in political advocacy. Perhaps politics has to be played by different rules where half-truths are normalized. Criminal law and advertising use those rules. But this explosion of vitriol and legislative intrusion into medicine should prompt everyone to reassess the use of one-sided advocacy in public and professional circles in healthcare. I want to be associated with a profession that uses evidence-based medicine that is not corrupted with political agendas. I want to be associated with a profession known for telling the whole truth.
In a society that is increasingly polarized, I want to embrace the advice of John Stuart Mill, a 19th century English philosopher best known for utilitarianism, which is often expressed as “the greatest good for the greatest number.” Mr. Mill also wrote on social theory, liberty, and even some early feminist theory. His 1859 work, On Liberty, chapter II, asserts: “He who knows only his own side of the case, knows little of that. His reasons may be good, and no one may have been able to refute them. But if he is equally unable to refute the reasons on the opposite side; if he does not so much as know what they are, he has no ground for preferring either opinion.”
Mr. Mill did not like half-truths.
It’s About Trust
My column is not the instrument to debate the use of hormones as puberty blockers or the fairness of transgender women participating in women’s sports. Those judgments will be rendered by others. I may report on those deliberations, but my column’s emphasis is on how professionals, and their organizations, go about making those determinations
For instance, the National Health Service in the United Kingdom spent 2 years reassessing transgender care for children and in October 2022 released a draft proposal to reduce and limit the aggressive therapies. On June 9, 2023, the NHS fully enacted those changes. Puberty blockers for gender dysphoria would be used only in experimental trials. In April 2024 the NHS began implementing those changes, joining other European countries that have imposed similar restrictions.
Similarly, the debate about transgender participation in women’s sports has continued to rage for years. On April 8, 2024, the National Association of Intercollegiate Athletics passed a resolution that bans almost all transgender participation in NAIA-regulated intercollegiate women’s sports. Dance and cheerleading are exceptions. Participation is still permissible at the intramural level. The NCAA has different rules.
Go to those sources to learn more substance for those debates. This column is about trust.
A major problem currently facing medicine is the public’s trust in expertise. That trust had been seriously weakened before the pandemic and was repeatedly wounded during the pandemic with arguments over masks, vaccines, and shutdowns. It needs repair.
This is especially true for pediatric hospitalists that do not have the opportunity that office-based pediatricians have to build rapport with a family over years. At a recent university conference on diversity, equity, and inclusivity, one female rabbi stated, “I cannot be rabbi to everybody.” I agreed, but as a medical professional, sometimes I must be.
Telling half-truths harms the public’s trust in their personal physicians and in the medical establishment. Once people suspect an organization is making decisions based on ideology rather than science, credibility is lost and difficult to recover.
Let us stop telling half-truths. Let us stop suppressing dialogue. Truth can never be completely captured by humans, but if one side of an issue is suppressed by cancel culture, censorship, accusations of homophobia, or threat of cultural war, the search for truth is severely impaired.
Let us, as medical professionals, adopt Stephen Covey’s habit number 5, “Seek first to understand, then to be understood.” Empower voices. Listen to all stakeholders. And when we finally do speak, remember John Stuart Mill and tell the whole truth.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.
On May 5, 2023, the director of the Centers for Disease Control and Prevention (CDC), Rochelle Walensky, in announcing her resignation after more than 2 years of dedicated service, wrote that she “took on this role … with the goal of leaving behind the dark days of the pandemic and moving the CDC — and public health — forward into a much better and more trusted place.”
Three times in the past 3 years I have written a Beyond the White Coat column emphasizing the importance of trust. Trust in the expertise of scientists. Trust in the integrity of medical research and public health institutions. Trust in the commitment of providers — doctors, nurses, therapists, and first responders — to shepherd us through the pandemic and other medical crises in our lives. This column is take four.
All human institutions have human imperfections. However, imperfect humans working together in community are more productive and more reliable than nihilism and political polarization. Underlying all of healthcare are compassion and honesty. Honesty means the truth, the whole truth, and nothing but the truth. Honesty is such a simple concept in the moral formation of children, but the concept has evolved aberrantly in the world of woke adults. There appear to be irresistible temptations to shade that truth for political gain. The dominant current mutation is the half-truth. One tells the part of the truth that appears to advance one’s own political aspirations and at the same time one omits or censors other viewpoints.
On April 17, 2023, the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the American Psychiatric Association wrote an open letter to Congressional leaders advocating for transgender female students’ participation in girl’s and women’s sports. The letter was written “On behalf of the more than 165,000” members of those organizations, though public opinion polls show a majority of those members likely oppose the opinion expressed. The letter goes on to extol the benefits that sports might bring to transgender students, but it contains not one word acknowledging the negative impact that participation has on others. That is a half-truth.
The same half-truth methodology distorts dialogue about various therapies for gender dysphoria in children and young adults.
In April 2022, U.S. Assistant Secretary for Health Rachel Levine in an NPR interview declared that, “There is no argument about the value and importance of gender-affirming care.” That might be a half-truth, since I could not locate U.S. specialists who dare to go on record questioning the party line of the World Professional Association for Transgender Health. However, Dr. Levine’s dismissal of any dissent is bizarre since in the prior 2 years multiple countries, including Australia, New Zealand, Sweden, Finland, and the United Kingdom had all issued reports questioning and even rescinding the practices that evolved since the 2012 WPATH guidance. Their main concerns included 1) the marked increase in incidence of gender dysphoria first manifesting in tween and early teenage girls, 2) the inadequate access to mental health screening before considering transitioning, 3) the long-term risks of puberty blockers particularly to bone density, and 4) the low quality of evidence supporting a measurable reduction in suicide rates. There may be reasonable counterarguments to each of those concerns, but a high ranking U.S. government official labeling all those international reports as “no argument” does not produce high quality decision making and does not foster the public’s trust.
Indeed, the public in many cases has decided its elected legislators are more trustworthy on these topics than the medical organizations. As I wrote the first draft of this column, the Missouri state legislators had passed a bill banning gender-affirming health care for transgender minors. They also passed a bill preventing participation of transgender females in women’s sports. Per reckoning by CBS News in the summer of 2023, 16 states had recently enacted laws restricting gender-affirming care and 22 states had restricted transgender participation in sports.
In 2022, I wrote a column claiming that suppressing viewpoints and debate leads to exploding spaceships. I believe the current legislative carnage is just such an explosion. It harms children.
The AAP has experts in advocacy. I am no expert in political advocacy. Perhaps politics has to be played by different rules where half-truths are normalized. Criminal law and advertising use those rules. But this explosion of vitriol and legislative intrusion into medicine should prompt everyone to reassess the use of one-sided advocacy in public and professional circles in healthcare. I want to be associated with a profession that uses evidence-based medicine that is not corrupted with political agendas. I want to be associated with a profession known for telling the whole truth.
In a society that is increasingly polarized, I want to embrace the advice of John Stuart Mill, a 19th century English philosopher best known for utilitarianism, which is often expressed as “the greatest good for the greatest number.” Mr. Mill also wrote on social theory, liberty, and even some early feminist theory. His 1859 work, On Liberty, chapter II, asserts: “He who knows only his own side of the case, knows little of that. His reasons may be good, and no one may have been able to refute them. But if he is equally unable to refute the reasons on the opposite side; if he does not so much as know what they are, he has no ground for preferring either opinion.”
Mr. Mill did not like half-truths.
It’s About Trust
My column is not the instrument to debate the use of hormones as puberty blockers or the fairness of transgender women participating in women’s sports. Those judgments will be rendered by others. I may report on those deliberations, but my column’s emphasis is on how professionals, and their organizations, go about making those determinations
For instance, the National Health Service in the United Kingdom spent 2 years reassessing transgender care for children and in October 2022 released a draft proposal to reduce and limit the aggressive therapies. On June 9, 2023, the NHS fully enacted those changes. Puberty blockers for gender dysphoria would be used only in experimental trials. In April 2024 the NHS began implementing those changes, joining other European countries that have imposed similar restrictions.
Similarly, the debate about transgender participation in women’s sports has continued to rage for years. On April 8, 2024, the National Association of Intercollegiate Athletics passed a resolution that bans almost all transgender participation in NAIA-regulated intercollegiate women’s sports. Dance and cheerleading are exceptions. Participation is still permissible at the intramural level. The NCAA has different rules.
Go to those sources to learn more substance for those debates. This column is about trust.
A major problem currently facing medicine is the public’s trust in expertise. That trust had been seriously weakened before the pandemic and was repeatedly wounded during the pandemic with arguments over masks, vaccines, and shutdowns. It needs repair.
This is especially true for pediatric hospitalists that do not have the opportunity that office-based pediatricians have to build rapport with a family over years. At a recent university conference on diversity, equity, and inclusivity, one female rabbi stated, “I cannot be rabbi to everybody.” I agreed, but as a medical professional, sometimes I must be.
Telling half-truths harms the public’s trust in their personal physicians and in the medical establishment. Once people suspect an organization is making decisions based on ideology rather than science, credibility is lost and difficult to recover.
Let us stop telling half-truths. Let us stop suppressing dialogue. Truth can never be completely captured by humans, but if one side of an issue is suppressed by cancel culture, censorship, accusations of homophobia, or threat of cultural war, the search for truth is severely impaired.
Let us, as medical professionals, adopt Stephen Covey’s habit number 5, “Seek first to understand, then to be understood.” Empower voices. Listen to all stakeholders. And when we finally do speak, remember John Stuart Mill and tell the whole truth.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@mdedge.com.







