User login
Transcervical fibroid radiofrequency ablation: A look inside
Uterine leiomyomas affect 70% to 80% of reproductive-age women. Interventions for symptomatic patients include myomectomy, hysterectomy, uterine artery embolization (UAE), and radiofrequency ablation (RFA). Several RFA devices exist on the market. One such device is the sonography-guided transcervical ablation of uterine fibroids (Sonata), which is unique in its transcervical approach that allows for incisionless treatment.1 It can be used to treat fibroids classified as FIGO 1-6 with a radius up to 5 cm.1 Postablative therapy outcomes at 1 and 2 years have been promising for total volume reduction (mean maximal volume reduction, 63.8%) and improvement in symptoms, including quality-of-life measures and amount of bleeding (95% reported reduction).2,3
In our practice, we find this tool most helpful for medium-sized (3–5 cm) intramural fibroids and large type 2 fibroids.
In the accompanying video, we illustrate the steps for use of transcervical ultrasonographic RFA with Sonata treatment and demonstrate its impact on the uterus during simultaneous laparoscopy. We present a patient who underwent Sonata treatment for a 4-cm intramural fibroid and simultaneous laparoscopic myomectomy for a 4-cm pedunculated fibroid. This allowed for the unique ability to view the external effect on the uterus during Sonata use. We review the key surgical steps with this approach, including:
- cervical dilation
- introduction of the Sonata system
- sonographic identification of the target fibroid
- adjust size and shape of Smart Guide overlays
- deploy the introducer
- safety rotation check
- deploy the needle electrodes
- initiate RFA
- withdraw needle electrodes and introducer.
RFA with Sonata treatment is a simple, minimally invasive therapeutic option for fibroids.
We hope that you find this video useful to your clinical practice.
>>DR. ARNOLD P. ADVINCULA AND COLLEAGUES

- Toub DB. A new paradigm for uterine fibroid treatment: transcervical, intrauterine sonography-guided radiofrequency ablation of uterine fibroids with the Sonata system. Curr Obstet Gynecol Rep. 2017;6:67-73.
- Hudgens J, Johns DA, Lukes AS, et al. 12-month outcomes of the US patient cohort in the Sonata pivotal IDE trial of transcervical ablation of uterine fibroids. Int J Womens Health. 2019;11:387-394.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
Uterine leiomyomas affect 70% to 80% of reproductive-age women. Interventions for symptomatic patients include myomectomy, hysterectomy, uterine artery embolization (UAE), and radiofrequency ablation (RFA). Several RFA devices exist on the market. One such device is the sonography-guided transcervical ablation of uterine fibroids (Sonata), which is unique in its transcervical approach that allows for incisionless treatment.1 It can be used to treat fibroids classified as FIGO 1-6 with a radius up to 5 cm.1 Postablative therapy outcomes at 1 and 2 years have been promising for total volume reduction (mean maximal volume reduction, 63.8%) and improvement in symptoms, including quality-of-life measures and amount of bleeding (95% reported reduction).2,3
In our practice, we find this tool most helpful for medium-sized (3–5 cm) intramural fibroids and large type 2 fibroids.
In the accompanying video, we illustrate the steps for use of transcervical ultrasonographic RFA with Sonata treatment and demonstrate its impact on the uterus during simultaneous laparoscopy. We present a patient who underwent Sonata treatment for a 4-cm intramural fibroid and simultaneous laparoscopic myomectomy for a 4-cm pedunculated fibroid. This allowed for the unique ability to view the external effect on the uterus during Sonata use. We review the key surgical steps with this approach, including:
- cervical dilation
- introduction of the Sonata system
- sonographic identification of the target fibroid
- adjust size and shape of Smart Guide overlays
- deploy the introducer
- safety rotation check
- deploy the needle electrodes
- initiate RFA
- withdraw needle electrodes and introducer.
RFA with Sonata treatment is a simple, minimally invasive therapeutic option for fibroids.
We hope that you find this video useful to your clinical practice.
>>DR. ARNOLD P. ADVINCULA AND COLLEAGUES

Uterine leiomyomas affect 70% to 80% of reproductive-age women. Interventions for symptomatic patients include myomectomy, hysterectomy, uterine artery embolization (UAE), and radiofrequency ablation (RFA). Several RFA devices exist on the market. One such device is the sonography-guided transcervical ablation of uterine fibroids (Sonata), which is unique in its transcervical approach that allows for incisionless treatment.1 It can be used to treat fibroids classified as FIGO 1-6 with a radius up to 5 cm.1 Postablative therapy outcomes at 1 and 2 years have been promising for total volume reduction (mean maximal volume reduction, 63.8%) and improvement in symptoms, including quality-of-life measures and amount of bleeding (95% reported reduction).2,3
In our practice, we find this tool most helpful for medium-sized (3–5 cm) intramural fibroids and large type 2 fibroids.
In the accompanying video, we illustrate the steps for use of transcervical ultrasonographic RFA with Sonata treatment and demonstrate its impact on the uterus during simultaneous laparoscopy. We present a patient who underwent Sonata treatment for a 4-cm intramural fibroid and simultaneous laparoscopic myomectomy for a 4-cm pedunculated fibroid. This allowed for the unique ability to view the external effect on the uterus during Sonata use. We review the key surgical steps with this approach, including:
- cervical dilation
- introduction of the Sonata system
- sonographic identification of the target fibroid
- adjust size and shape of Smart Guide overlays
- deploy the introducer
- safety rotation check
- deploy the needle electrodes
- initiate RFA
- withdraw needle electrodes and introducer.
RFA with Sonata treatment is a simple, minimally invasive therapeutic option for fibroids.
We hope that you find this video useful to your clinical practice.
>>DR. ARNOLD P. ADVINCULA AND COLLEAGUES

- Toub DB. A new paradigm for uterine fibroid treatment: transcervical, intrauterine sonography-guided radiofrequency ablation of uterine fibroids with the Sonata system. Curr Obstet Gynecol Rep. 2017;6:67-73.
- Hudgens J, Johns DA, Lukes AS, et al. 12-month outcomes of the US patient cohort in the Sonata pivotal IDE trial of transcervical ablation of uterine fibroids. Int J Womens Health. 2019;11:387-394.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Toub DB. A new paradigm for uterine fibroid treatment: transcervical, intrauterine sonography-guided radiofrequency ablation of uterine fibroids with the Sonata system. Curr Obstet Gynecol Rep. 2017;6:67-73.
- Hudgens J, Johns DA, Lukes AS, et al. 12-month outcomes of the US patient cohort in the Sonata pivotal IDE trial of transcervical ablation of uterine fibroids. Int J Womens Health. 2019;11:387-394.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
The Supreme Court 2020‒2021: What will affect ObGyns?
The Supreme Court’s usual processes were disrupted this term. The COVID-19 pandemic required audio hearings rather than in-person, and it resulted in a number of emergency legal appeals. As the Court began its regular sessions on October 5, 2020, there were only 8 justices—Justice Ruth Bader Ginsburg had passed away and Amy Coney Barrett had not yet been confirmed by the Senate. The Court decided many important cases this term, including dealing with the delivery of drugs to induce abortions, a Centers for Disease Control and Prevention (CDC) moratorium on housing evictions, yet another case on the Affordable Care Act, state laws concerning pharmacy benefit managers, and the Hologic and Minerva endometrial ablation systems patents. After considering these cases, we also will briefly look at other cases of general interest.
Abortion
Patient access to mifepristone
In May 2020, the American College of Obstetricians and Gynecologists (ACOG) was the named plaintiff in a lawsuit against the US Food and Drug Administration (FDA) regarding the drugs mifepristone and misoprostol that are used to induce medical abortions.1 The case was filed by the American Civil Liberties Union on behalf of ACOG and others2,3 and raised the issue of patients’ access to these medications. The basic claim of the case was that during the pandemic, the FDA’s regulation of mifepristone was unconstitutional in that they imposed an undue burden on the decision of women to have an abortion.4 (Although misoprostol is a part of the medical abortion regimen, it is not subject to special regulation and was not part of the litigation.)
The FDA regulation of mifepristone, begun in 2000 but modified since then, includes 3 elements to assure safe use:
- prescribers must have special training or certification
- the drug can be dispensed to patients only in a hospital, clinic, or medical office under the supervision of a certified health care provider (known as the “in-person dispensing requirement” because retail pharmacy or mail distribution are prohibited)
- the health care provider must review a “patient agreement form” with the patient and have the patient sign the consent form in the provider’s presence.5
The pandemic made fulfilling these requirements substantially more burdensome and difficult. The question was whether the FDA was constitutionally required to modify its regulations during a pandemic to take account of the undue burden of the regulation created by the pandemic. That is, the question was not whether the FDA could have or should have chosen to make the modification, but whether it was required to do so.
In July 2020, a federal district court in Maryland held that the FDA regulation was an unconstitutional burden on the abortion rights of women during the pandemic and issued a preliminary injunction to stop the FDA from enforcing the in-person dispensing and signature rules. The district judge applied the injunction to Maryland, but also made it a nationwide injunction. (The issue of district court nationwide injunctions is considered in, “District court ‘nationwide injunctions’”).
The FDA asked the Fourth Circuit Court of Appeals to stay the enforcement of the injunction, which the appeals court denied. The FDA then appealed to the Supreme Court, asking it to stay the injunction. In October 2020, the Court announced that it was holding the FDA’s request “in abeyance” to allow the district court to consider a motion by the FDA to dissolve or change the injunction. It gave the district court 40 days in which to act. That decision by the Court was in the “Shadow Docket” (see sidebar on page XX), so the exact vote of the Court in October is not clear, but 2 Justices (Alito and Thomas) dissented and would have stayed the injunction.6 Over the next 40 days, the district court did not withdraw its nationwide injunction.
Thus, on January 12, 2021, the case was again before the Supreme Court, which let the FDA’s regulations regarding mifepristone remain in place by lifting the district court’s injunction. Most of the justices supporting the stay did not write to explain their decision, although their dissent in the earlier cases may have served that purpose. (Maryland was permitting many kinds of activity that were more risky than visiting a clinic—indoor dining, with open hair salons, gyms, and casinos.)7 Chief Justice Roberts wrote a concurrence to indicate that, in his view, the issue was not whether the FDA’s regulations placed an undue burden on a right to an abortion generally, but that “My view is that courts owe significant deference” to the public health authorities (here meaning the FDA). Justices Sotomayor and Kagan dissented, saying that the issue was the undue burden on women, given the difficulties of the pandemic, particularly going to medical facilities during the COVID-19 pandemic.8
The injunction, sought by ACOG and others, was issued by the district court and was in effect for several months before it was dissolved by the Supreme Court. Following the change in presidential administrations, in April 2021 the FDA announced that it was going to “exercise enforcement discretion with respect to the in-person dispensing requirement…during the COVID-19 public health emergency.”9
Continue to: The Texas abortion case...
The Texas abortion case
The Court, on September 1, 2021, declined to block a Texas abortion statute from taking effect.10 This law precludes abortions after a fetal heartbeat is present at about 6 weeks of gestation. The Fifth Circuit declined to grant an injunction delaying implementation of the Texas law, and the Court did not reverse that decision.
Over the years, a variety of states have placed limitations on abortion, and those almost always have been enjoined by federal courts before they went into effect. However, the Texas statute, which undoubtedly is unconstitutional, was creatively constructed to avoid an early injunction.11 The statute does not allow state officials to enforce the new law, but rather it allows almost any private citizen to seek monetary damages from anyone performing an abortion or who “aids and abets” an abortion. Thus, it is difficult to tailor a lawsuit before this law is enforced. First, courts do not enjoin laws; they usually enjoin individuals from enforcing the law, and in this case it is difficult to know which individuals will be enforcing the laws and what their decisions might be. There also are some questions about the degree to which federal courts can enjoin state courts from deciding lawsuits under state law. For these procedural reasons, the majority of the Court found that those attacking the Texas law had not met their burden of showing that that they would win their case.
Even 3 of the dissenting justices said the defendants may be right that “existing doctrines preclude judicial intervention,” but that the consequences are such that the Court should delay the law until there is time for briefing and argument. The other 3 dissenting justices thought there would be ways of getting around the clever roadblock Texas had erected for the federal courts.
There has been some commentary that this case portends the abandonment of Roe v Wade and Casey,12 but that conclusion does not seem warranted by this case. The Court has accepted a Mississippi abortion law to be heard next term.13 In addition, the Texas statute is likely to be back in federal court once a private individual has filed a claim for money from an abortion provider (and likely even before that).
COVID-19 cases
The Supreme Court decided several cases related to COVID-19, including adjustments to election procedures, church services, and CDC eviction moratoria. As a general matter early in the pandemic, the Court deferred to government authorities, generally upholding government actions. Chief Justice Roberts emphasized the importance of the Court deferring to government officials in emergencies. As the pandemic progressed into 2021, however, the Court became less and less sympathetic to government actions that were not consistent, permitted by existing law, or reasonably necessary. For example, regulations of churches that were inconsistent with the regulation of similar organizations were struck down.14
Among the most interesting of the summer 2021 cases was the CDC eviction moratorium that essentially prohibited landlords nationwide from evicting tenants for nonpayment of rent. When the challenges to these CDC regulations first reached the Court, the moratorium was about to expire; in a 5-4 decision, the Court did not enjoin the CDC from continuing that policy. Justice Kavanaugh (the fifth vote) warned that “clear and specific congressional authorization…would be necessary to extend the moratorium past July 31.”15 Despite telling the Court that the moratorium would expire on July 31, just 3 days after the expiration and without any congressional authorization, the CDC reinstated what was practically the same moratorium.16 On August 26, the Court struck down the reinstated regulation, probably by a 6-3 margin. (Because this case arose in the “Shadow Docket,” the vote of some justices is not certain).17
Continue to: The Affordable Care Act...
The Affordable Care Act
The Affordable Care Act was challenged in the Court for the third time.18 In this term’s case, several states argued that when Congress essentially eliminated the penalty/tax for not purchasing insurance coverage, there was no longer a constitutional basis for the individual mandate. With that centerpiece gone, they claimed, the whole statute should be declared unconstitutional.
Along with many other specialty groups, ACOG joined an amicus curiae brief sponsored by the American Medical Association (AMA).19 An amicus brief is one not filed by the parties to the case, but by organizations or individuals who have information that may be of use to the Court in considering the case. Among other things, the filing of an amicus brief indicates the interest of the organization in the outcome of the case. In this case, the crux of the amicus was that even if the individual mandate currently is not constitutional, the Court should sever that provision and retain the rest of the ACA.
Despite some wild predictions about what the Court might do, it did not decide any substantive issue. Rather, it found that none of the parties to the case had “standing” to challenge the constitutionality of the ACA. Therefore, in effect, the Court dismissed the case without deciding the substantive legal issues.
Pharmacy Benefit Managers
The powerful Pharmacy Benefit Managers (PBMs) are a hidden part of the health care system; however, in recent years there has been increasing regulatory attention paid to them. Some states have begun regulating aspects of PBMs. In this term, the Court considered an Arkansas law that sought to protect local pharmacies from PBM pricing practices.20 The AMA filed an amicus brief in the case which made legal arguments, most of which had been made by the parties to the litigation.21
PBMs generally tell pharmacies how much they will reimburse the pharmacy for filling a prescription for a particular drug. In some instances, PBMs will set a reimbursement price that is lower than the wholesale price at which local pharmacies can purchase the drug. The Arkansas law prohibited PBMs in the state from reimbursing pharmacies for less than the wholesale cost the pharmacy paid for the drug.
The claim of the PBMs was that the Arkansas law violated the Employee Retirement Income Security Act (ERISA). In part, this act preempts state law that relates to fringe benefit plans. States have the authority to regulate insurance, but ERISA limits what they can do when the insurance relates to fringe benefits. The Court held that ERISA does not preempt the Arkansas law or similar state laws in other states. Because the state law was not preempted by the state law, the Arkansas regulation was upheld. The fact that this was a unanimous decision (8-0, because Justice Barrett was not on the Court when the case was heard) suggests that states may have leeway in additional regulations of PBMs, and it would not be surprising to see more of that state regulation in the future.
Continue to: Patent uncertainty...
Patent uncertainty
Csaba Truckai invented and patented the NovaSure System ablation device with a “moisture permeable” head. He sold his company and the related patents, which eventually were purchased by Hologic. Over time, Hologic added claims to the original patent. In the meantime, Truckai went on to invent another device, the Minerva Endometrial Ablation System (MEAS), which had a “moisture impermeable” head. (Note that the “Minerva Surgical, Inc.” involved in this case is not related to the company “Minerva Industries,” which some identified as a “patent troll.”)22
Hologic sued Minerva, claiming that Truckai’s second device (MEAS) infringed on its patent for the first device (NovaSure). Truckai’s defense was that the patent on NovaSure was invalid. Hologic felt that since Truckai had obtained that patent and then sold it, it was improper for him now to claim it was invalid. There is a doctrine for that: assignor estoppel—the person who sold (assigned) the patent is prevented from later claiming it was invalid. The question in this case was whether assignor estoppel is part of the patent law of the United States. It is not in the patent statutes, so it is a court-determined part of the law.
In a 5-4 decision this Term, the Court held that assignor estoppel is recognized, but that it is narrow.23 The Court identified several exceptions to assignor estoppel, notably for this case, including the situation in which the purchaser of the patent, after the purchase, returns to the Patent and Trademark Office to expand (amend) the patent’s claims. In that case, the seller could not be estopped by the amended terms of the patent. Minerva claimed that it was attacking the expanded patent that included changes made after it sold the patent. The Court, therefore, returned the case to the Federal Circuit to apply the principles it laid out about assignor estoppel.
Biotech and other fast-moving fields frequently have new technology building on slightly earlier technology. The current patent system often leaves uncertainty about who owns which part of a valid patent. This uncertainty is a drag on innovation, and the patent system is supposed to spur innovation. Assignor estoppel is likely to create additional complexity and uncertainty in some patents, which is regrettable.
Review of the Term
In addition to the other disruptions of the Term, during the first part of the Term, Amy Coney Barrett was not yet confirmed by the Senate, so there were only 8 justices until October 27. She did not participate in those cases that were heard before she joined the Court. The consensus is that the Court heard 67 cases: 57 were formally briefed and argued along with 8 summary reversals and 2 religious cases in the Shadow Docket. In my opinion, this undercounts both the number and the importance of the Shadow Docket cases, but the following data use the 67 case convention.24
The Court was unanimous in 43% of the cases, including some of the most divisive issues. That unanimity reflects very narrow decisions. There were (by conventional count) only eight 5-4 opinions (12%), an unusually low number. Justice Kavanaugh is viewed as the “median” justice. He was in the majority in 97% of all cases. Chief Justice Roberts and Justice Barrett were in the majority 91%, and Justice Gorsuch 90%. As for the other justices, they were in the majority (all cases) most of the time: Justice Alito, 83%; Justice Thomas, 81%; Justice Breyer, 76%; Justice Kagan, 75%; and Justice Sotomayor, 69%. In “divided cases” (when unanimous cases are removed), the percentages are: Justice Kavanaugh, 95%; Chief Justice Roberts and Justice Barrett, 84%; Justice Gorsuch, 82%; Justice Alito, 70%; Justice Thomas, 66%; Justice Breyer, 58%; Justice Kagan, 55%; and Justice Sotomayor, 45%.
When the term began, many Court watchers expected a relatively uninteresting term, dealing with many technical legal details. In fact, it turned out to be more interesting and important than expected, even with narrow holdings in important cases. Part of the secret of the term was that a lot of the real action was in the Shadow Docket. The end of the term is sometimes the moment when a justice announces a plan to retire. Many commentators expected Justice Breyer might announce—he has been under pressure to do so, to allow President Biden to nominate and a Democratic Senate to confirm a progressive justice. However, he did not do so. It is possible that he will announce his retirement to be effective when his successor is confirmed, but that is pure speculation.
Continue to: Next Term...
Next Term
The next term began on Monday, October 4, 2021. With the considerable current activity in the Shadow Docket, there was not much of a summer break. The coming term looks extraordinary. The headline case is an abortion case from Mississippi, Dobbs v Jackson Women’s Health Organization.25 The legal question is the constitutionality of Mississippi law that prohibits most abortions after 15 weeks of gestation. The Texas abortion law will also be back before the Court. As we saw this term, big cases may produce very narrow results, but this case has the potential for being a notable abortion decision.
In a different case the Court will decide whether a state attorney general can step in to defend an abortion law when the state health secretary does not do so.26
The Court also has accepted 3 cases dealing with reimbursement for health services. One deals with whether or not the Department of Health and Human Services can set reimbursement rates without good survey data regarding costs,27 another involves the calculation of additional payments for hospitals that serve a “disproportionate number of low-income patients,”28 and the third whether state Medicaid programs can take funds from an injured beneficiary’s tort recovery to cover future Medicaid costs.29
In other cases, the Court will review a gun control law from New York. The Court’s earlier Second Amendment cases involved guns in the home used for self-defense, but this case raises the question of whether a state can practically preclude “concealed-carry licenses.”30 Many experts believe the Court will accept a case dealing with racial preferences in college admissions, perhaps the Harvard case in which the claim is discrimination against Asian Americans.31
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum.
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum. Reference Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
Reference
1. Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
The “Shadow Docket”
The ACOG mifepristone decisions do not appear on the Supreme Court’s “Court Opinions” website.1 They appear in what has become known in recent years as “The Shadow Docket,” an informal term that includes many orders of the Court and statements of individual justices regarding some cases.2 There are hundreds of orders by the Court each Term, there is nothing particularly shadowy about any of these items—they are all publicly available on the Court’s website and later in paper format. It is, however, a little harder to find and much harder to sort through than the major opinions. In some cases, it is not possible to tell what the vote was, how each justice voted, and what the reasoning of the Court was. In a few cases it is difficult to know exactly what the Court was holding or otherwise leaves some confusion about what the law actually is.3
The part of the Shadow Docket that is most intriguing for commentators, and where the ACOG cases appear, is the “Opinions Relating to Orders.”4 These are a variety of opinions, some written by the Court and many by individual justices. It also includes the action of the Court in some cases in which there was not full briefing or oral argument. The statements by justices often are to dissent from the denial of cert of decisions of the Court. These opinions have become much more common over the years. In this past term, there were approximately 60 such opinions related to about 50 cases. In part, this relates to the number of pandemic cases that could not wait for a Court decision going through the extended ordinary process. Although the Shadow Docket has been of interest to academic observers and Court watchers for years, this year it has attracted the attention of Congress.5
References
1. Opinions of the Court. Supreme Court website. https://www.supremecourt.gov/opinions/slipopinion/20#list. Accessed October 10, 2021.
2. Baude W. Foreword: the Supreme Court’s Shadow Docket, 9 N.Y.U. J.L. & Liberty 1 (2015).
3. Vladeck SI. The Solicitor General and the Shadow Docket, 133 Harvard Law Review. 123 (2019).
4. Opinions relating to orders. Supreme Court website. https://www.supremecourt.gov/opinions/relatingtoorders/20#list. Accessed October 10, 2021.
5. The Supreme Court’s Shadow Docket: Hearing Before the Subcommittee on Courts, Intellectual Property and the Internet of the H. Committee on the Judiciary, 117th Congress (2021).
- American College of Obstetricians & Gynecologists v. United States FDA, 472 F. Supp. 3d 183 (D. Md. 2020).
- Michael Kunzelman, Doctors Sue to Block FDA Abortion Pill Rule During Pandemic, (May 29, 2020).
- ACLU, American College Of Obstetricians And Gynecologists V. U.S. Food And Drug Administration, https://www.aclu.org/cases/american-college-obstetricians-and-gynecologists-v-us-food-and-drug-administration. Updated February 12, 2021. Accessed August 27, 2021.
- Whole Woman’s Health v Hellerstedt, 579 US ___ (2016), 136 S Ct 2292.
- 2016 Clinical Review at 39, 47, 49, Opp’n Mot. PI Ex. 19, ECF No. 62-11.
- American College of Obstetricians and Gynecologists v FDA (I), decided October 8, 2020.
- October 8, 2020, dissenting opinion by Justice Alito.
- January 12, 2021, dissenting opinion by Justice Sotomayor.
- Questions and answers on Mifeprex. U.S. Food and Drug Administration website. Published April 13, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-mifeprex. Accessed October 9, 2021.
- Whole Woman’s Health v Jackson, decided September 1, 2021.
- Texas Senate Bill 8, relating to abortion, including abortions after detection of unborn child’s heartbeat; authorizing a private civil right of action. LegiScan website. https://legiscan.com/TX/text/SB8/id/2395961. Accessed October 9, 2021.
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U. S. 833 (1992); Roe v Wade, 410 U. S. 113 (1973).
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Roman Catholic Diocese of Brooklyn v Cuomo, decided November 25, 2020.
- Alabama Association of Realtors v Department of Health and Human Services, decided June 29, 2021.
- Temporary halt in residential evictions in communities with substantial or high levels of community transmission of COVID-19 to prevent the further spread of COVID-19. August 6, 2021. https://www.federalregister.gov/documents/2021/08/06/2021-16945/temporary-halt-in-residential-evictions-in-communities-with-substantial-or-high-transmission-of.
- Alabama Association of Realtors v Department of Health and Human Services, decided August 26, 2021.
- California v Texas, decided June 17, 2021.
- Brief of Amici Curiae American Medical Association, American Academy of Allergy, Asthma and Immunology, Aerospace Medical Association, American Academy of Family Physicians, American Academy of Pediatrics, American College of Cardiology, American College of Emergency Physicians, American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, American College of Physicians, American College of Radiation Oncology, American College of Radiology, American Psychiatric Association, American Society of Gastrointestinal Endoscopy, American Society of Hematology, American Society of Metabolic and Bariatric Surgery, Endocrine Society, GLMA: Health Professionals Advancing LGBTQ Equality, Renal Physicians Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology in Support of Petitioners, in California v. Texas. May 13, 2020. https://www.supremecourt.gov/DocketPDF/19/19-840/143469/20200513150051995_19-840%20Amici%20Brief%20AMA.pdf. Accessed October 9, 2021.
- Rutledge v Pharmaceutical Care Management Association, decided December 10, 2020.
- Brief of the American Medical Association, The Arkansas Medical Society, and The Litigation Center of the American Medical Association and the State Medical Societies as Amici Curiae in Support of Petitioner in Rutledge v Pharmaceutical Care Management Association. March 2, 2020. https://www.supremecourt.gov/DocketPDF/18/18-540/134670/20200302163622018_Rutledge%20v.%20PCMA%20Amicus%20Brief%20of%20AMA%20et%20al.pdf. Accessed October 9, 2021.
- Apple quietly settles patent lawsuit, promptly gets hit with another one. TechCrunch website. Published July 30, 2010. https://techcrunch.com/2010/07/30/apple-minerva-emblaze/. Accessed October 9, 2021.
- Minerva Surgical, Inc. v Hologic, Inc., decided June 29, 2021.
- Stat pack. SCOTUS Blog website. Published July 6, 2021. https://www.scotusblog.com/wp-content/uploads/2021/07/Final-Stat-Pack-7.6.21.pdf. Accessed October 9, 2021.
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Cameron v. EMW Women’s Surgical Center, https://www.scotusblog.com/case-files/cases/cameron-v-emw-womens-surgical-center-p-s-c/. Accessed August 28, 2021.
- American Hospital Association v Becerra, No. 20-1114.
- Becerra v Empire Health Foundation, No. 20-1312.
- Gallardo v Marstiller, No. 20-1263.
- New York State Rifle & Pistol Association Inc. v Corlett, No. 20-843.
- Students for Fair Admissions v President & Fellows of Harvard College, No. 20-1199.
The Supreme Court’s usual processes were disrupted this term. The COVID-19 pandemic required audio hearings rather than in-person, and it resulted in a number of emergency legal appeals. As the Court began its regular sessions on October 5, 2020, there were only 8 justices—Justice Ruth Bader Ginsburg had passed away and Amy Coney Barrett had not yet been confirmed by the Senate. The Court decided many important cases this term, including dealing with the delivery of drugs to induce abortions, a Centers for Disease Control and Prevention (CDC) moratorium on housing evictions, yet another case on the Affordable Care Act, state laws concerning pharmacy benefit managers, and the Hologic and Minerva endometrial ablation systems patents. After considering these cases, we also will briefly look at other cases of general interest.
Abortion
Patient access to mifepristone
In May 2020, the American College of Obstetricians and Gynecologists (ACOG) was the named plaintiff in a lawsuit against the US Food and Drug Administration (FDA) regarding the drugs mifepristone and misoprostol that are used to induce medical abortions.1 The case was filed by the American Civil Liberties Union on behalf of ACOG and others2,3 and raised the issue of patients’ access to these medications. The basic claim of the case was that during the pandemic, the FDA’s regulation of mifepristone was unconstitutional in that they imposed an undue burden on the decision of women to have an abortion.4 (Although misoprostol is a part of the medical abortion regimen, it is not subject to special regulation and was not part of the litigation.)
The FDA regulation of mifepristone, begun in 2000 but modified since then, includes 3 elements to assure safe use:
- prescribers must have special training or certification
- the drug can be dispensed to patients only in a hospital, clinic, or medical office under the supervision of a certified health care provider (known as the “in-person dispensing requirement” because retail pharmacy or mail distribution are prohibited)
- the health care provider must review a “patient agreement form” with the patient and have the patient sign the consent form in the provider’s presence.5
The pandemic made fulfilling these requirements substantially more burdensome and difficult. The question was whether the FDA was constitutionally required to modify its regulations during a pandemic to take account of the undue burden of the regulation created by the pandemic. That is, the question was not whether the FDA could have or should have chosen to make the modification, but whether it was required to do so.
In July 2020, a federal district court in Maryland held that the FDA regulation was an unconstitutional burden on the abortion rights of women during the pandemic and issued a preliminary injunction to stop the FDA from enforcing the in-person dispensing and signature rules. The district judge applied the injunction to Maryland, but also made it a nationwide injunction. (The issue of district court nationwide injunctions is considered in, “District court ‘nationwide injunctions’”).
The FDA asked the Fourth Circuit Court of Appeals to stay the enforcement of the injunction, which the appeals court denied. The FDA then appealed to the Supreme Court, asking it to stay the injunction. In October 2020, the Court announced that it was holding the FDA’s request “in abeyance” to allow the district court to consider a motion by the FDA to dissolve or change the injunction. It gave the district court 40 days in which to act. That decision by the Court was in the “Shadow Docket” (see sidebar on page XX), so the exact vote of the Court in October is not clear, but 2 Justices (Alito and Thomas) dissented and would have stayed the injunction.6 Over the next 40 days, the district court did not withdraw its nationwide injunction.
Thus, on January 12, 2021, the case was again before the Supreme Court, which let the FDA’s regulations regarding mifepristone remain in place by lifting the district court’s injunction. Most of the justices supporting the stay did not write to explain their decision, although their dissent in the earlier cases may have served that purpose. (Maryland was permitting many kinds of activity that were more risky than visiting a clinic—indoor dining, with open hair salons, gyms, and casinos.)7 Chief Justice Roberts wrote a concurrence to indicate that, in his view, the issue was not whether the FDA’s regulations placed an undue burden on a right to an abortion generally, but that “My view is that courts owe significant deference” to the public health authorities (here meaning the FDA). Justices Sotomayor and Kagan dissented, saying that the issue was the undue burden on women, given the difficulties of the pandemic, particularly going to medical facilities during the COVID-19 pandemic.8
The injunction, sought by ACOG and others, was issued by the district court and was in effect for several months before it was dissolved by the Supreme Court. Following the change in presidential administrations, in April 2021 the FDA announced that it was going to “exercise enforcement discretion with respect to the in-person dispensing requirement…during the COVID-19 public health emergency.”9
Continue to: The Texas abortion case...
The Texas abortion case
The Court, on September 1, 2021, declined to block a Texas abortion statute from taking effect.10 This law precludes abortions after a fetal heartbeat is present at about 6 weeks of gestation. The Fifth Circuit declined to grant an injunction delaying implementation of the Texas law, and the Court did not reverse that decision.
Over the years, a variety of states have placed limitations on abortion, and those almost always have been enjoined by federal courts before they went into effect. However, the Texas statute, which undoubtedly is unconstitutional, was creatively constructed to avoid an early injunction.11 The statute does not allow state officials to enforce the new law, but rather it allows almost any private citizen to seek monetary damages from anyone performing an abortion or who “aids and abets” an abortion. Thus, it is difficult to tailor a lawsuit before this law is enforced. First, courts do not enjoin laws; they usually enjoin individuals from enforcing the law, and in this case it is difficult to know which individuals will be enforcing the laws and what their decisions might be. There also are some questions about the degree to which federal courts can enjoin state courts from deciding lawsuits under state law. For these procedural reasons, the majority of the Court found that those attacking the Texas law had not met their burden of showing that that they would win their case.
Even 3 of the dissenting justices said the defendants may be right that “existing doctrines preclude judicial intervention,” but that the consequences are such that the Court should delay the law until there is time for briefing and argument. The other 3 dissenting justices thought there would be ways of getting around the clever roadblock Texas had erected for the federal courts.
There has been some commentary that this case portends the abandonment of Roe v Wade and Casey,12 but that conclusion does not seem warranted by this case. The Court has accepted a Mississippi abortion law to be heard next term.13 In addition, the Texas statute is likely to be back in federal court once a private individual has filed a claim for money from an abortion provider (and likely even before that).
COVID-19 cases
The Supreme Court decided several cases related to COVID-19, including adjustments to election procedures, church services, and CDC eviction moratoria. As a general matter early in the pandemic, the Court deferred to government authorities, generally upholding government actions. Chief Justice Roberts emphasized the importance of the Court deferring to government officials in emergencies. As the pandemic progressed into 2021, however, the Court became less and less sympathetic to government actions that were not consistent, permitted by existing law, or reasonably necessary. For example, regulations of churches that were inconsistent with the regulation of similar organizations were struck down.14
Among the most interesting of the summer 2021 cases was the CDC eviction moratorium that essentially prohibited landlords nationwide from evicting tenants for nonpayment of rent. When the challenges to these CDC regulations first reached the Court, the moratorium was about to expire; in a 5-4 decision, the Court did not enjoin the CDC from continuing that policy. Justice Kavanaugh (the fifth vote) warned that “clear and specific congressional authorization…would be necessary to extend the moratorium past July 31.”15 Despite telling the Court that the moratorium would expire on July 31, just 3 days after the expiration and without any congressional authorization, the CDC reinstated what was practically the same moratorium.16 On August 26, the Court struck down the reinstated regulation, probably by a 6-3 margin. (Because this case arose in the “Shadow Docket,” the vote of some justices is not certain).17
Continue to: The Affordable Care Act...
The Affordable Care Act
The Affordable Care Act was challenged in the Court for the third time.18 In this term’s case, several states argued that when Congress essentially eliminated the penalty/tax for not purchasing insurance coverage, there was no longer a constitutional basis for the individual mandate. With that centerpiece gone, they claimed, the whole statute should be declared unconstitutional.
Along with many other specialty groups, ACOG joined an amicus curiae brief sponsored by the American Medical Association (AMA).19 An amicus brief is one not filed by the parties to the case, but by organizations or individuals who have information that may be of use to the Court in considering the case. Among other things, the filing of an amicus brief indicates the interest of the organization in the outcome of the case. In this case, the crux of the amicus was that even if the individual mandate currently is not constitutional, the Court should sever that provision and retain the rest of the ACA.
Despite some wild predictions about what the Court might do, it did not decide any substantive issue. Rather, it found that none of the parties to the case had “standing” to challenge the constitutionality of the ACA. Therefore, in effect, the Court dismissed the case without deciding the substantive legal issues.
Pharmacy Benefit Managers
The powerful Pharmacy Benefit Managers (PBMs) are a hidden part of the health care system; however, in recent years there has been increasing regulatory attention paid to them. Some states have begun regulating aspects of PBMs. In this term, the Court considered an Arkansas law that sought to protect local pharmacies from PBM pricing practices.20 The AMA filed an amicus brief in the case which made legal arguments, most of which had been made by the parties to the litigation.21
PBMs generally tell pharmacies how much they will reimburse the pharmacy for filling a prescription for a particular drug. In some instances, PBMs will set a reimbursement price that is lower than the wholesale price at which local pharmacies can purchase the drug. The Arkansas law prohibited PBMs in the state from reimbursing pharmacies for less than the wholesale cost the pharmacy paid for the drug.
The claim of the PBMs was that the Arkansas law violated the Employee Retirement Income Security Act (ERISA). In part, this act preempts state law that relates to fringe benefit plans. States have the authority to regulate insurance, but ERISA limits what they can do when the insurance relates to fringe benefits. The Court held that ERISA does not preempt the Arkansas law or similar state laws in other states. Because the state law was not preempted by the state law, the Arkansas regulation was upheld. The fact that this was a unanimous decision (8-0, because Justice Barrett was not on the Court when the case was heard) suggests that states may have leeway in additional regulations of PBMs, and it would not be surprising to see more of that state regulation in the future.
Continue to: Patent uncertainty...
Patent uncertainty
Csaba Truckai invented and patented the NovaSure System ablation device with a “moisture permeable” head. He sold his company and the related patents, which eventually were purchased by Hologic. Over time, Hologic added claims to the original patent. In the meantime, Truckai went on to invent another device, the Minerva Endometrial Ablation System (MEAS), which had a “moisture impermeable” head. (Note that the “Minerva Surgical, Inc.” involved in this case is not related to the company “Minerva Industries,” which some identified as a “patent troll.”)22
Hologic sued Minerva, claiming that Truckai’s second device (MEAS) infringed on its patent for the first device (NovaSure). Truckai’s defense was that the patent on NovaSure was invalid. Hologic felt that since Truckai had obtained that patent and then sold it, it was improper for him now to claim it was invalid. There is a doctrine for that: assignor estoppel—the person who sold (assigned) the patent is prevented from later claiming it was invalid. The question in this case was whether assignor estoppel is part of the patent law of the United States. It is not in the patent statutes, so it is a court-determined part of the law.
In a 5-4 decision this Term, the Court held that assignor estoppel is recognized, but that it is narrow.23 The Court identified several exceptions to assignor estoppel, notably for this case, including the situation in which the purchaser of the patent, after the purchase, returns to the Patent and Trademark Office to expand (amend) the patent’s claims. In that case, the seller could not be estopped by the amended terms of the patent. Minerva claimed that it was attacking the expanded patent that included changes made after it sold the patent. The Court, therefore, returned the case to the Federal Circuit to apply the principles it laid out about assignor estoppel.
Biotech and other fast-moving fields frequently have new technology building on slightly earlier technology. The current patent system often leaves uncertainty about who owns which part of a valid patent. This uncertainty is a drag on innovation, and the patent system is supposed to spur innovation. Assignor estoppel is likely to create additional complexity and uncertainty in some patents, which is regrettable.
Review of the Term
In addition to the other disruptions of the Term, during the first part of the Term, Amy Coney Barrett was not yet confirmed by the Senate, so there were only 8 justices until October 27. She did not participate in those cases that were heard before she joined the Court. The consensus is that the Court heard 67 cases: 57 were formally briefed and argued along with 8 summary reversals and 2 religious cases in the Shadow Docket. In my opinion, this undercounts both the number and the importance of the Shadow Docket cases, but the following data use the 67 case convention.24
The Court was unanimous in 43% of the cases, including some of the most divisive issues. That unanimity reflects very narrow decisions. There were (by conventional count) only eight 5-4 opinions (12%), an unusually low number. Justice Kavanaugh is viewed as the “median” justice. He was in the majority in 97% of all cases. Chief Justice Roberts and Justice Barrett were in the majority 91%, and Justice Gorsuch 90%. As for the other justices, they were in the majority (all cases) most of the time: Justice Alito, 83%; Justice Thomas, 81%; Justice Breyer, 76%; Justice Kagan, 75%; and Justice Sotomayor, 69%. In “divided cases” (when unanimous cases are removed), the percentages are: Justice Kavanaugh, 95%; Chief Justice Roberts and Justice Barrett, 84%; Justice Gorsuch, 82%; Justice Alito, 70%; Justice Thomas, 66%; Justice Breyer, 58%; Justice Kagan, 55%; and Justice Sotomayor, 45%.
When the term began, many Court watchers expected a relatively uninteresting term, dealing with many technical legal details. In fact, it turned out to be more interesting and important than expected, even with narrow holdings in important cases. Part of the secret of the term was that a lot of the real action was in the Shadow Docket. The end of the term is sometimes the moment when a justice announces a plan to retire. Many commentators expected Justice Breyer might announce—he has been under pressure to do so, to allow President Biden to nominate and a Democratic Senate to confirm a progressive justice. However, he did not do so. It is possible that he will announce his retirement to be effective when his successor is confirmed, but that is pure speculation.
Continue to: Next Term...
Next Term
The next term began on Monday, October 4, 2021. With the considerable current activity in the Shadow Docket, there was not much of a summer break. The coming term looks extraordinary. The headline case is an abortion case from Mississippi, Dobbs v Jackson Women’s Health Organization.25 The legal question is the constitutionality of Mississippi law that prohibits most abortions after 15 weeks of gestation. The Texas abortion law will also be back before the Court. As we saw this term, big cases may produce very narrow results, but this case has the potential for being a notable abortion decision.
In a different case the Court will decide whether a state attorney general can step in to defend an abortion law when the state health secretary does not do so.26
The Court also has accepted 3 cases dealing with reimbursement for health services. One deals with whether or not the Department of Health and Human Services can set reimbursement rates without good survey data regarding costs,27 another involves the calculation of additional payments for hospitals that serve a “disproportionate number of low-income patients,”28 and the third whether state Medicaid programs can take funds from an injured beneficiary’s tort recovery to cover future Medicaid costs.29
In other cases, the Court will review a gun control law from New York. The Court’s earlier Second Amendment cases involved guns in the home used for self-defense, but this case raises the question of whether a state can practically preclude “concealed-carry licenses.”30 Many experts believe the Court will accept a case dealing with racial preferences in college admissions, perhaps the Harvard case in which the claim is discrimination against Asian Americans.31
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum.
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum. Reference Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
Reference
1. Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
The “Shadow Docket”
The ACOG mifepristone decisions do not appear on the Supreme Court’s “Court Opinions” website.1 They appear in what has become known in recent years as “The Shadow Docket,” an informal term that includes many orders of the Court and statements of individual justices regarding some cases.2 There are hundreds of orders by the Court each Term, there is nothing particularly shadowy about any of these items—they are all publicly available on the Court’s website and later in paper format. It is, however, a little harder to find and much harder to sort through than the major opinions. In some cases, it is not possible to tell what the vote was, how each justice voted, and what the reasoning of the Court was. In a few cases it is difficult to know exactly what the Court was holding or otherwise leaves some confusion about what the law actually is.3
The part of the Shadow Docket that is most intriguing for commentators, and where the ACOG cases appear, is the “Opinions Relating to Orders.”4 These are a variety of opinions, some written by the Court and many by individual justices. It also includes the action of the Court in some cases in which there was not full briefing or oral argument. The statements by justices often are to dissent from the denial of cert of decisions of the Court. These opinions have become much more common over the years. In this past term, there were approximately 60 such opinions related to about 50 cases. In part, this relates to the number of pandemic cases that could not wait for a Court decision going through the extended ordinary process. Although the Shadow Docket has been of interest to academic observers and Court watchers for years, this year it has attracted the attention of Congress.5
References
1. Opinions of the Court. Supreme Court website. https://www.supremecourt.gov/opinions/slipopinion/20#list. Accessed October 10, 2021.
2. Baude W. Foreword: the Supreme Court’s Shadow Docket, 9 N.Y.U. J.L. & Liberty 1 (2015).
3. Vladeck SI. The Solicitor General and the Shadow Docket, 133 Harvard Law Review. 123 (2019).
4. Opinions relating to orders. Supreme Court website. https://www.supremecourt.gov/opinions/relatingtoorders/20#list. Accessed October 10, 2021.
5. The Supreme Court’s Shadow Docket: Hearing Before the Subcommittee on Courts, Intellectual Property and the Internet of the H. Committee on the Judiciary, 117th Congress (2021).
The Supreme Court’s usual processes were disrupted this term. The COVID-19 pandemic required audio hearings rather than in-person, and it resulted in a number of emergency legal appeals. As the Court began its regular sessions on October 5, 2020, there were only 8 justices—Justice Ruth Bader Ginsburg had passed away and Amy Coney Barrett had not yet been confirmed by the Senate. The Court decided many important cases this term, including dealing with the delivery of drugs to induce abortions, a Centers for Disease Control and Prevention (CDC) moratorium on housing evictions, yet another case on the Affordable Care Act, state laws concerning pharmacy benefit managers, and the Hologic and Minerva endometrial ablation systems patents. After considering these cases, we also will briefly look at other cases of general interest.
Abortion
Patient access to mifepristone
In May 2020, the American College of Obstetricians and Gynecologists (ACOG) was the named plaintiff in a lawsuit against the US Food and Drug Administration (FDA) regarding the drugs mifepristone and misoprostol that are used to induce medical abortions.1 The case was filed by the American Civil Liberties Union on behalf of ACOG and others2,3 and raised the issue of patients’ access to these medications. The basic claim of the case was that during the pandemic, the FDA’s regulation of mifepristone was unconstitutional in that they imposed an undue burden on the decision of women to have an abortion.4 (Although misoprostol is a part of the medical abortion regimen, it is not subject to special regulation and was not part of the litigation.)
The FDA regulation of mifepristone, begun in 2000 but modified since then, includes 3 elements to assure safe use:
- prescribers must have special training or certification
- the drug can be dispensed to patients only in a hospital, clinic, or medical office under the supervision of a certified health care provider (known as the “in-person dispensing requirement” because retail pharmacy or mail distribution are prohibited)
- the health care provider must review a “patient agreement form” with the patient and have the patient sign the consent form in the provider’s presence.5
The pandemic made fulfilling these requirements substantially more burdensome and difficult. The question was whether the FDA was constitutionally required to modify its regulations during a pandemic to take account of the undue burden of the regulation created by the pandemic. That is, the question was not whether the FDA could have or should have chosen to make the modification, but whether it was required to do so.
In July 2020, a federal district court in Maryland held that the FDA regulation was an unconstitutional burden on the abortion rights of women during the pandemic and issued a preliminary injunction to stop the FDA from enforcing the in-person dispensing and signature rules. The district judge applied the injunction to Maryland, but also made it a nationwide injunction. (The issue of district court nationwide injunctions is considered in, “District court ‘nationwide injunctions’”).
The FDA asked the Fourth Circuit Court of Appeals to stay the enforcement of the injunction, which the appeals court denied. The FDA then appealed to the Supreme Court, asking it to stay the injunction. In October 2020, the Court announced that it was holding the FDA’s request “in abeyance” to allow the district court to consider a motion by the FDA to dissolve or change the injunction. It gave the district court 40 days in which to act. That decision by the Court was in the “Shadow Docket” (see sidebar on page XX), so the exact vote of the Court in October is not clear, but 2 Justices (Alito and Thomas) dissented and would have stayed the injunction.6 Over the next 40 days, the district court did not withdraw its nationwide injunction.
Thus, on January 12, 2021, the case was again before the Supreme Court, which let the FDA’s regulations regarding mifepristone remain in place by lifting the district court’s injunction. Most of the justices supporting the stay did not write to explain their decision, although their dissent in the earlier cases may have served that purpose. (Maryland was permitting many kinds of activity that were more risky than visiting a clinic—indoor dining, with open hair salons, gyms, and casinos.)7 Chief Justice Roberts wrote a concurrence to indicate that, in his view, the issue was not whether the FDA’s regulations placed an undue burden on a right to an abortion generally, but that “My view is that courts owe significant deference” to the public health authorities (here meaning the FDA). Justices Sotomayor and Kagan dissented, saying that the issue was the undue burden on women, given the difficulties of the pandemic, particularly going to medical facilities during the COVID-19 pandemic.8
The injunction, sought by ACOG and others, was issued by the district court and was in effect for several months before it was dissolved by the Supreme Court. Following the change in presidential administrations, in April 2021 the FDA announced that it was going to “exercise enforcement discretion with respect to the in-person dispensing requirement…during the COVID-19 public health emergency.”9
Continue to: The Texas abortion case...
The Texas abortion case
The Court, on September 1, 2021, declined to block a Texas abortion statute from taking effect.10 This law precludes abortions after a fetal heartbeat is present at about 6 weeks of gestation. The Fifth Circuit declined to grant an injunction delaying implementation of the Texas law, and the Court did not reverse that decision.
Over the years, a variety of states have placed limitations on abortion, and those almost always have been enjoined by federal courts before they went into effect. However, the Texas statute, which undoubtedly is unconstitutional, was creatively constructed to avoid an early injunction.11 The statute does not allow state officials to enforce the new law, but rather it allows almost any private citizen to seek monetary damages from anyone performing an abortion or who “aids and abets” an abortion. Thus, it is difficult to tailor a lawsuit before this law is enforced. First, courts do not enjoin laws; they usually enjoin individuals from enforcing the law, and in this case it is difficult to know which individuals will be enforcing the laws and what their decisions might be. There also are some questions about the degree to which federal courts can enjoin state courts from deciding lawsuits under state law. For these procedural reasons, the majority of the Court found that those attacking the Texas law had not met their burden of showing that that they would win their case.
Even 3 of the dissenting justices said the defendants may be right that “existing doctrines preclude judicial intervention,” but that the consequences are such that the Court should delay the law until there is time for briefing and argument. The other 3 dissenting justices thought there would be ways of getting around the clever roadblock Texas had erected for the federal courts.
There has been some commentary that this case portends the abandonment of Roe v Wade and Casey,12 but that conclusion does not seem warranted by this case. The Court has accepted a Mississippi abortion law to be heard next term.13 In addition, the Texas statute is likely to be back in federal court once a private individual has filed a claim for money from an abortion provider (and likely even before that).
COVID-19 cases
The Supreme Court decided several cases related to COVID-19, including adjustments to election procedures, church services, and CDC eviction moratoria. As a general matter early in the pandemic, the Court deferred to government authorities, generally upholding government actions. Chief Justice Roberts emphasized the importance of the Court deferring to government officials in emergencies. As the pandemic progressed into 2021, however, the Court became less and less sympathetic to government actions that were not consistent, permitted by existing law, or reasonably necessary. For example, regulations of churches that were inconsistent with the regulation of similar organizations were struck down.14
Among the most interesting of the summer 2021 cases was the CDC eviction moratorium that essentially prohibited landlords nationwide from evicting tenants for nonpayment of rent. When the challenges to these CDC regulations first reached the Court, the moratorium was about to expire; in a 5-4 decision, the Court did not enjoin the CDC from continuing that policy. Justice Kavanaugh (the fifth vote) warned that “clear and specific congressional authorization…would be necessary to extend the moratorium past July 31.”15 Despite telling the Court that the moratorium would expire on July 31, just 3 days after the expiration and without any congressional authorization, the CDC reinstated what was practically the same moratorium.16 On August 26, the Court struck down the reinstated regulation, probably by a 6-3 margin. (Because this case arose in the “Shadow Docket,” the vote of some justices is not certain).17
Continue to: The Affordable Care Act...
The Affordable Care Act
The Affordable Care Act was challenged in the Court for the third time.18 In this term’s case, several states argued that when Congress essentially eliminated the penalty/tax for not purchasing insurance coverage, there was no longer a constitutional basis for the individual mandate. With that centerpiece gone, they claimed, the whole statute should be declared unconstitutional.
Along with many other specialty groups, ACOG joined an amicus curiae brief sponsored by the American Medical Association (AMA).19 An amicus brief is one not filed by the parties to the case, but by organizations or individuals who have information that may be of use to the Court in considering the case. Among other things, the filing of an amicus brief indicates the interest of the organization in the outcome of the case. In this case, the crux of the amicus was that even if the individual mandate currently is not constitutional, the Court should sever that provision and retain the rest of the ACA.
Despite some wild predictions about what the Court might do, it did not decide any substantive issue. Rather, it found that none of the parties to the case had “standing” to challenge the constitutionality of the ACA. Therefore, in effect, the Court dismissed the case without deciding the substantive legal issues.
Pharmacy Benefit Managers
The powerful Pharmacy Benefit Managers (PBMs) are a hidden part of the health care system; however, in recent years there has been increasing regulatory attention paid to them. Some states have begun regulating aspects of PBMs. In this term, the Court considered an Arkansas law that sought to protect local pharmacies from PBM pricing practices.20 The AMA filed an amicus brief in the case which made legal arguments, most of which had been made by the parties to the litigation.21
PBMs generally tell pharmacies how much they will reimburse the pharmacy for filling a prescription for a particular drug. In some instances, PBMs will set a reimbursement price that is lower than the wholesale price at which local pharmacies can purchase the drug. The Arkansas law prohibited PBMs in the state from reimbursing pharmacies for less than the wholesale cost the pharmacy paid for the drug.
The claim of the PBMs was that the Arkansas law violated the Employee Retirement Income Security Act (ERISA). In part, this act preempts state law that relates to fringe benefit plans. States have the authority to regulate insurance, but ERISA limits what they can do when the insurance relates to fringe benefits. The Court held that ERISA does not preempt the Arkansas law or similar state laws in other states. Because the state law was not preempted by the state law, the Arkansas regulation was upheld. The fact that this was a unanimous decision (8-0, because Justice Barrett was not on the Court when the case was heard) suggests that states may have leeway in additional regulations of PBMs, and it would not be surprising to see more of that state regulation in the future.
Continue to: Patent uncertainty...
Patent uncertainty
Csaba Truckai invented and patented the NovaSure System ablation device with a “moisture permeable” head. He sold his company and the related patents, which eventually were purchased by Hologic. Over time, Hologic added claims to the original patent. In the meantime, Truckai went on to invent another device, the Minerva Endometrial Ablation System (MEAS), which had a “moisture impermeable” head. (Note that the “Minerva Surgical, Inc.” involved in this case is not related to the company “Minerva Industries,” which some identified as a “patent troll.”)22
Hologic sued Minerva, claiming that Truckai’s second device (MEAS) infringed on its patent for the first device (NovaSure). Truckai’s defense was that the patent on NovaSure was invalid. Hologic felt that since Truckai had obtained that patent and then sold it, it was improper for him now to claim it was invalid. There is a doctrine for that: assignor estoppel—the person who sold (assigned) the patent is prevented from later claiming it was invalid. The question in this case was whether assignor estoppel is part of the patent law of the United States. It is not in the patent statutes, so it is a court-determined part of the law.
In a 5-4 decision this Term, the Court held that assignor estoppel is recognized, but that it is narrow.23 The Court identified several exceptions to assignor estoppel, notably for this case, including the situation in which the purchaser of the patent, after the purchase, returns to the Patent and Trademark Office to expand (amend) the patent’s claims. In that case, the seller could not be estopped by the amended terms of the patent. Minerva claimed that it was attacking the expanded patent that included changes made after it sold the patent. The Court, therefore, returned the case to the Federal Circuit to apply the principles it laid out about assignor estoppel.
Biotech and other fast-moving fields frequently have new technology building on slightly earlier technology. The current patent system often leaves uncertainty about who owns which part of a valid patent. This uncertainty is a drag on innovation, and the patent system is supposed to spur innovation. Assignor estoppel is likely to create additional complexity and uncertainty in some patents, which is regrettable.
Review of the Term
In addition to the other disruptions of the Term, during the first part of the Term, Amy Coney Barrett was not yet confirmed by the Senate, so there were only 8 justices until October 27. She did not participate in those cases that were heard before she joined the Court. The consensus is that the Court heard 67 cases: 57 were formally briefed and argued along with 8 summary reversals and 2 religious cases in the Shadow Docket. In my opinion, this undercounts both the number and the importance of the Shadow Docket cases, but the following data use the 67 case convention.24
The Court was unanimous in 43% of the cases, including some of the most divisive issues. That unanimity reflects very narrow decisions. There were (by conventional count) only eight 5-4 opinions (12%), an unusually low number. Justice Kavanaugh is viewed as the “median” justice. He was in the majority in 97% of all cases. Chief Justice Roberts and Justice Barrett were in the majority 91%, and Justice Gorsuch 90%. As for the other justices, they were in the majority (all cases) most of the time: Justice Alito, 83%; Justice Thomas, 81%; Justice Breyer, 76%; Justice Kagan, 75%; and Justice Sotomayor, 69%. In “divided cases” (when unanimous cases are removed), the percentages are: Justice Kavanaugh, 95%; Chief Justice Roberts and Justice Barrett, 84%; Justice Gorsuch, 82%; Justice Alito, 70%; Justice Thomas, 66%; Justice Breyer, 58%; Justice Kagan, 55%; and Justice Sotomayor, 45%.
When the term began, many Court watchers expected a relatively uninteresting term, dealing with many technical legal details. In fact, it turned out to be more interesting and important than expected, even with narrow holdings in important cases. Part of the secret of the term was that a lot of the real action was in the Shadow Docket. The end of the term is sometimes the moment when a justice announces a plan to retire. Many commentators expected Justice Breyer might announce—he has been under pressure to do so, to allow President Biden to nominate and a Democratic Senate to confirm a progressive justice. However, he did not do so. It is possible that he will announce his retirement to be effective when his successor is confirmed, but that is pure speculation.
Continue to: Next Term...
Next Term
The next term began on Monday, October 4, 2021. With the considerable current activity in the Shadow Docket, there was not much of a summer break. The coming term looks extraordinary. The headline case is an abortion case from Mississippi, Dobbs v Jackson Women’s Health Organization.25 The legal question is the constitutionality of Mississippi law that prohibits most abortions after 15 weeks of gestation. The Texas abortion law will also be back before the Court. As we saw this term, big cases may produce very narrow results, but this case has the potential for being a notable abortion decision.
In a different case the Court will decide whether a state attorney general can step in to defend an abortion law when the state health secretary does not do so.26
The Court also has accepted 3 cases dealing with reimbursement for health services. One deals with whether or not the Department of Health and Human Services can set reimbursement rates without good survey data regarding costs,27 another involves the calculation of additional payments for hospitals that serve a “disproportionate number of low-income patients,”28 and the third whether state Medicaid programs can take funds from an injured beneficiary’s tort recovery to cover future Medicaid costs.29
In other cases, the Court will review a gun control law from New York. The Court’s earlier Second Amendment cases involved guns in the home used for self-defense, but this case raises the question of whether a state can practically preclude “concealed-carry licenses.”30 Many experts believe the Court will accept a case dealing with racial preferences in college admissions, perhaps the Harvard case in which the claim is discrimination against Asian Americans.31
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum.
The ACOG mifepristone case was interesting, in part because the federal district court issued a nationwide injunction against the Americans with Disabilities Act, enforcing its rules anywhere in the country. The effect of these orders is for a single district judge to create the “law of the land,” at least until that is reviewed—which can take months. The advantage of the nationwide injunction is that it avoids having to repeatedly litigate the same issues in multiple courts around the country. The downside is that plaintiffs can seek out a nonrepresentative judge or circuit and receive an injunction that would be granted by few other circuits. In addition, a nationwide injunction can apply to specific circumstances that are not before the court issuing the injunction. In the mifepristone case, for example, 10 states requested to intervene in the ACOG case. The court rejected the request, but the nationwide injunction applied to those states.1
Although federal judges have had the authority to issue nationwide injunctions for years, they are becoming much more common. One reason is the ease of forum shopping noted earlier—organizations can cherry-pick district courts and circuits sympathetic to their views. Both left- and right-leaning organizations have learned this lesson, so left-leaning groups are likely to file in specific districts in the Ninth Circuit, and right-leaning groups to districts in the Fifth Circuit.
If the current trend of increasing nationwide injunctions continues, either the rules for the federal courts or congressional action may be required to reduce some of the abuses by both sides of the political spectrum. Reference Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
Reference
1. Am. Coll. of Obstetricians & Gynecologists v. United States FDA, 467 F. Supp. 3d 282, 284 (D. Md. 2020).
The “Shadow Docket”
The ACOG mifepristone decisions do not appear on the Supreme Court’s “Court Opinions” website.1 They appear in what has become known in recent years as “The Shadow Docket,” an informal term that includes many orders of the Court and statements of individual justices regarding some cases.2 There are hundreds of orders by the Court each Term, there is nothing particularly shadowy about any of these items—they are all publicly available on the Court’s website and later in paper format. It is, however, a little harder to find and much harder to sort through than the major opinions. In some cases, it is not possible to tell what the vote was, how each justice voted, and what the reasoning of the Court was. In a few cases it is difficult to know exactly what the Court was holding or otherwise leaves some confusion about what the law actually is.3
The part of the Shadow Docket that is most intriguing for commentators, and where the ACOG cases appear, is the “Opinions Relating to Orders.”4 These are a variety of opinions, some written by the Court and many by individual justices. It also includes the action of the Court in some cases in which there was not full briefing or oral argument. The statements by justices often are to dissent from the denial of cert of decisions of the Court. These opinions have become much more common over the years. In this past term, there were approximately 60 such opinions related to about 50 cases. In part, this relates to the number of pandemic cases that could not wait for a Court decision going through the extended ordinary process. Although the Shadow Docket has been of interest to academic observers and Court watchers for years, this year it has attracted the attention of Congress.5
References
1. Opinions of the Court. Supreme Court website. https://www.supremecourt.gov/opinions/slipopinion/20#list. Accessed October 10, 2021.
2. Baude W. Foreword: the Supreme Court’s Shadow Docket, 9 N.Y.U. J.L. & Liberty 1 (2015).
3. Vladeck SI. The Solicitor General and the Shadow Docket, 133 Harvard Law Review. 123 (2019).
4. Opinions relating to orders. Supreme Court website. https://www.supremecourt.gov/opinions/relatingtoorders/20#list. Accessed October 10, 2021.
5. The Supreme Court’s Shadow Docket: Hearing Before the Subcommittee on Courts, Intellectual Property and the Internet of the H. Committee on the Judiciary, 117th Congress (2021).
- American College of Obstetricians & Gynecologists v. United States FDA, 472 F. Supp. 3d 183 (D. Md. 2020).
- Michael Kunzelman, Doctors Sue to Block FDA Abortion Pill Rule During Pandemic, (May 29, 2020).
- ACLU, American College Of Obstetricians And Gynecologists V. U.S. Food And Drug Administration, https://www.aclu.org/cases/american-college-obstetricians-and-gynecologists-v-us-food-and-drug-administration. Updated February 12, 2021. Accessed August 27, 2021.
- Whole Woman’s Health v Hellerstedt, 579 US ___ (2016), 136 S Ct 2292.
- 2016 Clinical Review at 39, 47, 49, Opp’n Mot. PI Ex. 19, ECF No. 62-11.
- American College of Obstetricians and Gynecologists v FDA (I), decided October 8, 2020.
- October 8, 2020, dissenting opinion by Justice Alito.
- January 12, 2021, dissenting opinion by Justice Sotomayor.
- Questions and answers on Mifeprex. U.S. Food and Drug Administration website. Published April 13, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-mifeprex. Accessed October 9, 2021.
- Whole Woman’s Health v Jackson, decided September 1, 2021.
- Texas Senate Bill 8, relating to abortion, including abortions after detection of unborn child’s heartbeat; authorizing a private civil right of action. LegiScan website. https://legiscan.com/TX/text/SB8/id/2395961. Accessed October 9, 2021.
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U. S. 833 (1992); Roe v Wade, 410 U. S. 113 (1973).
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Roman Catholic Diocese of Brooklyn v Cuomo, decided November 25, 2020.
- Alabama Association of Realtors v Department of Health and Human Services, decided June 29, 2021.
- Temporary halt in residential evictions in communities with substantial or high levels of community transmission of COVID-19 to prevent the further spread of COVID-19. August 6, 2021. https://www.federalregister.gov/documents/2021/08/06/2021-16945/temporary-halt-in-residential-evictions-in-communities-with-substantial-or-high-transmission-of.
- Alabama Association of Realtors v Department of Health and Human Services, decided August 26, 2021.
- California v Texas, decided June 17, 2021.
- Brief of Amici Curiae American Medical Association, American Academy of Allergy, Asthma and Immunology, Aerospace Medical Association, American Academy of Family Physicians, American Academy of Pediatrics, American College of Cardiology, American College of Emergency Physicians, American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, American College of Physicians, American College of Radiation Oncology, American College of Radiology, American Psychiatric Association, American Society of Gastrointestinal Endoscopy, American Society of Hematology, American Society of Metabolic and Bariatric Surgery, Endocrine Society, GLMA: Health Professionals Advancing LGBTQ Equality, Renal Physicians Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology in Support of Petitioners, in California v. Texas. May 13, 2020. https://www.supremecourt.gov/DocketPDF/19/19-840/143469/20200513150051995_19-840%20Amici%20Brief%20AMA.pdf. Accessed October 9, 2021.
- Rutledge v Pharmaceutical Care Management Association, decided December 10, 2020.
- Brief of the American Medical Association, The Arkansas Medical Society, and The Litigation Center of the American Medical Association and the State Medical Societies as Amici Curiae in Support of Petitioner in Rutledge v Pharmaceutical Care Management Association. March 2, 2020. https://www.supremecourt.gov/DocketPDF/18/18-540/134670/20200302163622018_Rutledge%20v.%20PCMA%20Amicus%20Brief%20of%20AMA%20et%20al.pdf. Accessed October 9, 2021.
- Apple quietly settles patent lawsuit, promptly gets hit with another one. TechCrunch website. Published July 30, 2010. https://techcrunch.com/2010/07/30/apple-minerva-emblaze/. Accessed October 9, 2021.
- Minerva Surgical, Inc. v Hologic, Inc., decided June 29, 2021.
- Stat pack. SCOTUS Blog website. Published July 6, 2021. https://www.scotusblog.com/wp-content/uploads/2021/07/Final-Stat-Pack-7.6.21.pdf. Accessed October 9, 2021.
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Cameron v. EMW Women’s Surgical Center, https://www.scotusblog.com/case-files/cases/cameron-v-emw-womens-surgical-center-p-s-c/. Accessed August 28, 2021.
- American Hospital Association v Becerra, No. 20-1114.
- Becerra v Empire Health Foundation, No. 20-1312.
- Gallardo v Marstiller, No. 20-1263.
- New York State Rifle & Pistol Association Inc. v Corlett, No. 20-843.
- Students for Fair Admissions v President & Fellows of Harvard College, No. 20-1199.
- American College of Obstetricians & Gynecologists v. United States FDA, 472 F. Supp. 3d 183 (D. Md. 2020).
- Michael Kunzelman, Doctors Sue to Block FDA Abortion Pill Rule During Pandemic, (May 29, 2020).
- ACLU, American College Of Obstetricians And Gynecologists V. U.S. Food And Drug Administration, https://www.aclu.org/cases/american-college-obstetricians-and-gynecologists-v-us-food-and-drug-administration. Updated February 12, 2021. Accessed August 27, 2021.
- Whole Woman’s Health v Hellerstedt, 579 US ___ (2016), 136 S Ct 2292.
- 2016 Clinical Review at 39, 47, 49, Opp’n Mot. PI Ex. 19, ECF No. 62-11.
- American College of Obstetricians and Gynecologists v FDA (I), decided October 8, 2020.
- October 8, 2020, dissenting opinion by Justice Alito.
- January 12, 2021, dissenting opinion by Justice Sotomayor.
- Questions and answers on Mifeprex. U.S. Food and Drug Administration website. Published April 13, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-mifeprex. Accessed October 9, 2021.
- Whole Woman’s Health v Jackson, decided September 1, 2021.
- Texas Senate Bill 8, relating to abortion, including abortions after detection of unborn child’s heartbeat; authorizing a private civil right of action. LegiScan website. https://legiscan.com/TX/text/SB8/id/2395961. Accessed October 9, 2021.
- Planned Parenthood of Southeastern Pennsylvania v Casey, 505 U. S. 833 (1992); Roe v Wade, 410 U. S. 113 (1973).
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Roman Catholic Diocese of Brooklyn v Cuomo, decided November 25, 2020.
- Alabama Association of Realtors v Department of Health and Human Services, decided June 29, 2021.
- Temporary halt in residential evictions in communities with substantial or high levels of community transmission of COVID-19 to prevent the further spread of COVID-19. August 6, 2021. https://www.federalregister.gov/documents/2021/08/06/2021-16945/temporary-halt-in-residential-evictions-in-communities-with-substantial-or-high-transmission-of.
- Alabama Association of Realtors v Department of Health and Human Services, decided August 26, 2021.
- California v Texas, decided June 17, 2021.
- Brief of Amici Curiae American Medical Association, American Academy of Allergy, Asthma and Immunology, Aerospace Medical Association, American Academy of Family Physicians, American Academy of Pediatrics, American College of Cardiology, American College of Emergency Physicians, American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, American College of Physicians, American College of Radiation Oncology, American College of Radiology, American Psychiatric Association, American Society of Gastrointestinal Endoscopy, American Society of Hematology, American Society of Metabolic and Bariatric Surgery, Endocrine Society, GLMA: Health Professionals Advancing LGBTQ Equality, Renal Physicians Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology in Support of Petitioners, in California v. Texas. May 13, 2020. https://www.supremecourt.gov/DocketPDF/19/19-840/143469/20200513150051995_19-840%20Amici%20Brief%20AMA.pdf. Accessed October 9, 2021.
- Rutledge v Pharmaceutical Care Management Association, decided December 10, 2020.
- Brief of the American Medical Association, The Arkansas Medical Society, and The Litigation Center of the American Medical Association and the State Medical Societies as Amici Curiae in Support of Petitioner in Rutledge v Pharmaceutical Care Management Association. March 2, 2020. https://www.supremecourt.gov/DocketPDF/18/18-540/134670/20200302163622018_Rutledge%20v.%20PCMA%20Amicus%20Brief%20of%20AMA%20et%20al.pdf. Accessed October 9, 2021.
- Apple quietly settles patent lawsuit, promptly gets hit with another one. TechCrunch website. Published July 30, 2010. https://techcrunch.com/2010/07/30/apple-minerva-emblaze/. Accessed October 9, 2021.
- Minerva Surgical, Inc. v Hologic, Inc., decided June 29, 2021.
- Stat pack. SCOTUS Blog website. Published July 6, 2021. https://www.scotusblog.com/wp-content/uploads/2021/07/Final-Stat-Pack-7.6.21.pdf. Accessed October 9, 2021.
- Dobbs v Jackson Women’s Health Organization, No. 19-1392.
- Cameron v. EMW Women’s Surgical Center, https://www.scotusblog.com/case-files/cases/cameron-v-emw-womens-surgical-center-p-s-c/. Accessed August 28, 2021.
- American Hospital Association v Becerra, No. 20-1114.
- Becerra v Empire Health Foundation, No. 20-1312.
- Gallardo v Marstiller, No. 20-1263.
- New York State Rifle & Pistol Association Inc. v Corlett, No. 20-843.
- Students for Fair Admissions v President & Fellows of Harvard College, No. 20-1199.
Success of HPV vaccination: ‘Dramatic’ reduction in cervical cancer
Among young women who received the HPV vaccine when they were 12-13 years old (before their sexual debut), cervical cancer rates are 87% lower than among previous nonvaccinated generations.
“It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding.”
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, U.K. Health Security Agency, London, commented in a statement.
Vanessa Saliba, MD, a consultant epidemiologist for the U.K. Health Security Agency, agreed, saying that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she added.
The study was published online Nov. 3, 2021, in The Lancet.
Approached for comment on the new study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, noted that the results of the English study are very similar to those of a Swedish study of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. He said that, as an oncologist who has been treating cervical cancer for 40 years, particularly patients with advanced cervical cancer, “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful.
“I can only emphasize the critical importance of all parents to see that their children who are eligible for the vaccine receive it. This is a cancer prevention strategy that is unbelievably, remarkably effective and safe,” Dr. Markman added.
National vaccination program
The national HPV vaccination program in England began in 2008. Initially, the bivalent Cervarix vaccine against HPV 16 and 18 was used. HPV 16 and 18 are responsible for 70% to 80% of all cervical cancers in England, the researchers note in their article.
In 2012, the program switched to the quadrivalent HPV vaccine (Gardasil), which is effective against two additional HPV types, HPV 6 and 11. Those strains cause genital warts.
The prevention program originally recommended a three-dose regimen in which both HPV vaccines were used. Currently, two doses are given to girls younger than 15 years. In addition, a single dose of the HPV vaccine provides good protection against persistent infection. The efficacy rate of a single dose is similar to that of three doses, the authors comment.
Population-based registry
The new data come from a population-based cancer registry that shows the incidence of cervical cancer and noninvasive cervical carcinoma (CIN3) in England between January 2006 and June 2019.
The study included seven cohorts of women who were aged 20-64 years at the end of 2019. Three of these cohorts composed the vaccinated population.
The team reports that overall, from January 2006 to June 2019, there were 27,946 cases of cervical cancer and 318,058 cases of CIN3.
In the three vaccinated cohorts, there were around 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would be expected in a nonvaccinated population.
The three vaccinated cohorts had been eligible to receive Cervarix when they were aged 12-13 years. A catch-up scheme aimed at 14- to 16-year-olds and 16- to 18-year-olds. Most of these persons were vaccinated through a school vaccination program.
The team analyzed the data for each of these cohorts.
Among the cohort eligible for vaccination at 12-13 years of age, 89% received at least one dose of the HPV vaccine; 85% received three shots and were fully vaccinated. Among these persons, the rate of cervical cancer was 87% lower than expected in a nonvaccinated population, and the rate of CIN3 was 97% lower than expected.
For the cohort that was eligible to be vaccinated between the ages of 14 and 16 years, the corresponding reductions were 62% for cervical cancer and 75% for CIN3.
For the cohort eligible for vaccination between the ages of 16 and 18 years (of whom 60% had received at least one dose and 45% were fully vaccinated), the corresponding reduction were 34% for cervical cancer and 39% for CIN3.
The authors acknowledge some limitations with the study, principally that cervical cancer is rare in young women, and these vaccinated populations are still young. The youngest would have been vaccinated at age 12 in 2008 and so would be only 23 years old in 2019, when the follow-up in this current study ended. The authors emphasize that because the vaccinated populations are still young, it is too early to assess the full impact of HPV vaccination on cervical cancer rates.
Editorial commentary
“The relative reductions in cervical cancer, expected as a result of the HPV vaccination program, support the anticipated vaccine effectiveness,” commented two authors of an accompanying editorial, Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania.
“The scale of the HPV vaccination effect reported by this study should also stimulate vaccination programs in low-income and middle-income countries where the problem of cervical cancer is a far greater public health issue than in those with well established systems of vaccination and screening,” they comment.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept the vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country, such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by WHO [World Health Organization].”
The authors and editorialists disclosed no relevant financial relationships. Dr. Markman is a regular contributor to Medscape Oncology. He has received income of $250 or more from Genentech, AstraZeneca, Celgene, Clovis, and Amgen.
A version of this article first appeared on Medscape.com.
Among young women who received the HPV vaccine when they were 12-13 years old (before their sexual debut), cervical cancer rates are 87% lower than among previous nonvaccinated generations.
“It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding.”
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, U.K. Health Security Agency, London, commented in a statement.
Vanessa Saliba, MD, a consultant epidemiologist for the U.K. Health Security Agency, agreed, saying that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she added.
The study was published online Nov. 3, 2021, in The Lancet.
Approached for comment on the new study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, noted that the results of the English study are very similar to those of a Swedish study of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. He said that, as an oncologist who has been treating cervical cancer for 40 years, particularly patients with advanced cervical cancer, “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful.
“I can only emphasize the critical importance of all parents to see that their children who are eligible for the vaccine receive it. This is a cancer prevention strategy that is unbelievably, remarkably effective and safe,” Dr. Markman added.
National vaccination program
The national HPV vaccination program in England began in 2008. Initially, the bivalent Cervarix vaccine against HPV 16 and 18 was used. HPV 16 and 18 are responsible for 70% to 80% of all cervical cancers in England, the researchers note in their article.
In 2012, the program switched to the quadrivalent HPV vaccine (Gardasil), which is effective against two additional HPV types, HPV 6 and 11. Those strains cause genital warts.
The prevention program originally recommended a three-dose regimen in which both HPV vaccines were used. Currently, two doses are given to girls younger than 15 years. In addition, a single dose of the HPV vaccine provides good protection against persistent infection. The efficacy rate of a single dose is similar to that of three doses, the authors comment.
Population-based registry
The new data come from a population-based cancer registry that shows the incidence of cervical cancer and noninvasive cervical carcinoma (CIN3) in England between January 2006 and June 2019.
The study included seven cohorts of women who were aged 20-64 years at the end of 2019. Three of these cohorts composed the vaccinated population.
The team reports that overall, from January 2006 to June 2019, there were 27,946 cases of cervical cancer and 318,058 cases of CIN3.
In the three vaccinated cohorts, there were around 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would be expected in a nonvaccinated population.
The three vaccinated cohorts had been eligible to receive Cervarix when they were aged 12-13 years. A catch-up scheme aimed at 14- to 16-year-olds and 16- to 18-year-olds. Most of these persons were vaccinated through a school vaccination program.
The team analyzed the data for each of these cohorts.
Among the cohort eligible for vaccination at 12-13 years of age, 89% received at least one dose of the HPV vaccine; 85% received three shots and were fully vaccinated. Among these persons, the rate of cervical cancer was 87% lower than expected in a nonvaccinated population, and the rate of CIN3 was 97% lower than expected.
For the cohort that was eligible to be vaccinated between the ages of 14 and 16 years, the corresponding reductions were 62% for cervical cancer and 75% for CIN3.
For the cohort eligible for vaccination between the ages of 16 and 18 years (of whom 60% had received at least one dose and 45% were fully vaccinated), the corresponding reduction were 34% for cervical cancer and 39% for CIN3.
The authors acknowledge some limitations with the study, principally that cervical cancer is rare in young women, and these vaccinated populations are still young. The youngest would have been vaccinated at age 12 in 2008 and so would be only 23 years old in 2019, when the follow-up in this current study ended. The authors emphasize that because the vaccinated populations are still young, it is too early to assess the full impact of HPV vaccination on cervical cancer rates.
Editorial commentary
“The relative reductions in cervical cancer, expected as a result of the HPV vaccination program, support the anticipated vaccine effectiveness,” commented two authors of an accompanying editorial, Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania.
“The scale of the HPV vaccination effect reported by this study should also stimulate vaccination programs in low-income and middle-income countries where the problem of cervical cancer is a far greater public health issue than in those with well established systems of vaccination and screening,” they comment.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept the vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country, such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by WHO [World Health Organization].”
The authors and editorialists disclosed no relevant financial relationships. Dr. Markman is a regular contributor to Medscape Oncology. He has received income of $250 or more from Genentech, AstraZeneca, Celgene, Clovis, and Amgen.
A version of this article first appeared on Medscape.com.
Among young women who received the HPV vaccine when they were 12-13 years old (before their sexual debut), cervical cancer rates are 87% lower than among previous nonvaccinated generations.
“It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding.”
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, U.K. Health Security Agency, London, commented in a statement.
Vanessa Saliba, MD, a consultant epidemiologist for the U.K. Health Security Agency, agreed, saying that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she added.
The study was published online Nov. 3, 2021, in The Lancet.
Approached for comment on the new study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, noted that the results of the English study are very similar to those of a Swedish study of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. He said that, as an oncologist who has been treating cervical cancer for 40 years, particularly patients with advanced cervical cancer, “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful.
“I can only emphasize the critical importance of all parents to see that their children who are eligible for the vaccine receive it. This is a cancer prevention strategy that is unbelievably, remarkably effective and safe,” Dr. Markman added.
National vaccination program
The national HPV vaccination program in England began in 2008. Initially, the bivalent Cervarix vaccine against HPV 16 and 18 was used. HPV 16 and 18 are responsible for 70% to 80% of all cervical cancers in England, the researchers note in their article.
In 2012, the program switched to the quadrivalent HPV vaccine (Gardasil), which is effective against two additional HPV types, HPV 6 and 11. Those strains cause genital warts.
The prevention program originally recommended a three-dose regimen in which both HPV vaccines were used. Currently, two doses are given to girls younger than 15 years. In addition, a single dose of the HPV vaccine provides good protection against persistent infection. The efficacy rate of a single dose is similar to that of three doses, the authors comment.
Population-based registry
The new data come from a population-based cancer registry that shows the incidence of cervical cancer and noninvasive cervical carcinoma (CIN3) in England between January 2006 and June 2019.
The study included seven cohorts of women who were aged 20-64 years at the end of 2019. Three of these cohorts composed the vaccinated population.
The team reports that overall, from January 2006 to June 2019, there were 27,946 cases of cervical cancer and 318,058 cases of CIN3.
In the three vaccinated cohorts, there were around 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would be expected in a nonvaccinated population.
The three vaccinated cohorts had been eligible to receive Cervarix when they were aged 12-13 years. A catch-up scheme aimed at 14- to 16-year-olds and 16- to 18-year-olds. Most of these persons were vaccinated through a school vaccination program.
The team analyzed the data for each of these cohorts.
Among the cohort eligible for vaccination at 12-13 years of age, 89% received at least one dose of the HPV vaccine; 85% received three shots and were fully vaccinated. Among these persons, the rate of cervical cancer was 87% lower than expected in a nonvaccinated population, and the rate of CIN3 was 97% lower than expected.
For the cohort that was eligible to be vaccinated between the ages of 14 and 16 years, the corresponding reductions were 62% for cervical cancer and 75% for CIN3.
For the cohort eligible for vaccination between the ages of 16 and 18 years (of whom 60% had received at least one dose and 45% were fully vaccinated), the corresponding reduction were 34% for cervical cancer and 39% for CIN3.
The authors acknowledge some limitations with the study, principally that cervical cancer is rare in young women, and these vaccinated populations are still young. The youngest would have been vaccinated at age 12 in 2008 and so would be only 23 years old in 2019, when the follow-up in this current study ended. The authors emphasize that because the vaccinated populations are still young, it is too early to assess the full impact of HPV vaccination on cervical cancer rates.
Editorial commentary
“The relative reductions in cervical cancer, expected as a result of the HPV vaccination program, support the anticipated vaccine effectiveness,” commented two authors of an accompanying editorial, Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania.
“The scale of the HPV vaccination effect reported by this study should also stimulate vaccination programs in low-income and middle-income countries where the problem of cervical cancer is a far greater public health issue than in those with well established systems of vaccination and screening,” they comment.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept the vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country, such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by WHO [World Health Organization].”
The authors and editorialists disclosed no relevant financial relationships. Dr. Markman is a regular contributor to Medscape Oncology. He has received income of $250 or more from Genentech, AstraZeneca, Celgene, Clovis, and Amgen.
A version of this article first appeared on Medscape.com.
How to screen for prediabetes and type 2 diabetes in an ObGyn practice
The prevalence of T2DM is on the rise in the United States, and T2DM is currently the 7th leading cause of death.1 In a study of 28,143 participants in the US National Health and Nutrition Examination Survey (NHANES) who were 18 years or older, the prevalence of diabetes increased from 9.8% to 14.3% between 2000 and 2008.2 About 24% of the participants had undiagnosed diabetes prior to the testing they received as a study participant.2 People from minority groups have a higher rate of T2DM than non-Hispanic White people. Using data from 2018, the Centers for Disease Control and Prevention reported that the prevalence of diagnosed diabetes was highest among American Indians/Alaska Natives (14.7%), people of Hispanic origin (12.5%), and non-Hispanic Blacks (11.7%), followed by non-Hispanic Asians (9.2%) and non-Hispanic Whites (7.5%).1 Diabetes is a major risk factor for myocardial infarction, stroke, renal failure, retinopathy, peripheral vascular disease, and neuropathy.1 Early detection and treatment of both prediabetes and diabetes may improve health and reduce these preventable complications, saving lives, preventing heart and renal failure and blindness.
T2DM is caused by a combination of insulin resistance and insufficient pancreatic secretion of insulin to overcome the insulin resistance.3 In young adults with insulin resistance, pancreatic secretion of insulin is often sufficient to overcome the insulin resistance resulting in normal glucose levels and persistently increased insulin concentration. As individuals with insulin resistance age, pancreatic secretion of insulin may decline, resulting in insufficient production of insulin and rising glucose levels. Many individuals experience a prolonged stage of prediabetes that may be present for decades prior to transitioning to T2DM. In 2020, 35% of US adults were reported to have prediabetes.1
Screening for diabetes mellitus
The US Preventive Services Task Force (USPSTF) recently recommended that all adults aged 35 to 70 years who are overweight or obese be screened for T2DM (B recommendation).4 Screening for diabetes will also result in detecting many people with prediabetes. The criteria for diagnosing diabetes and prediabetes are presented in the TABLE. Based on cohort studies, the USPSTF noted that screening every 3 years is a reasonable approach.4 They also recommended that people diagnosed with prediabetes should initiate preventive measures, including optimizing diet, weight loss, exercise, and in some cases, medication treatment such as metformin.5
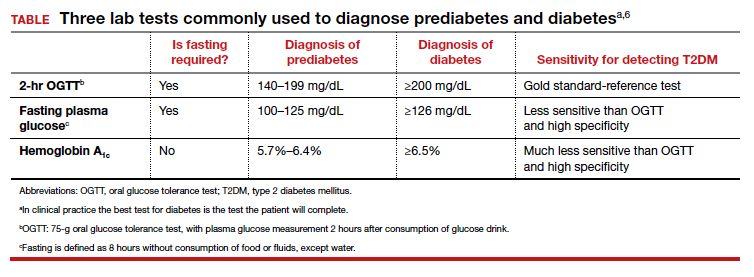
Approaches to the diagnosis of diabetes and prediabetes
Three laboratory tests are widely utilized for the diagnosis of prediabetes and diabetes: measurement of a plasma glucose 2 hours following consumption of oral glucose 75 g (2-hr oral glucose tolerance test [OGTT]), measurement of a fasting plasma glucose, and measurement of hemoglobin A1c (see Table).6In clinical practice, the best diabetes screening test is the test the patient will complete. Most evidence indicates that, compared with the 2-hr OGTT, a hemoglobin A1c measurement is specific for diagnosing T2DM, but not sensitive. In other words, if the hemoglobin A1c is ≥6.5%, the glucose measurement 2 hours following an OGTT will very likely be ≥200 mg/dL. But if the hemoglobin A1c is between 5.7% and 6.5%, the person might be diagnosed with T2DM if they had a 2-hr OGTT.6
In one study, 1,241 nondiabetic, overweight, or obese participants had all 3 tests to diagnose T2DM.7 The 2-hr OGTT diagnosed T2DM in 148 participants (12%). However, the hemoglobin A1c test only diagnosed T2DM in 78 of the 148 participants who were diagnosed with T2DM based on the 2-hr OGTT, missing 47% of the cases of T2DM. In this study, using the 2-hr OGTT as the “gold standard” reference test, the hemoglobin A1c test had a sensitivity of 53% and specificity of 97%.7
In clinical practice one approach is to explain to the patient the pros and cons of the 3 tests for T2DM and ask them to select the test they prefer to complete. In a high-risk population, including people with obesity, completing any of the 3 tests is better than not testing for diabetes. It also should be noted that, among people who have a normal body mass index (BMI), a “prediabetes” diagnosis is controversial. Compared with obese persons with prediabetes, people with a normal BMI and prediabetes diagnosed by a blood test progress to diabetes at a much lower rate. The value of diagnosing prediabetes after 70 years of age is also controversial because few people in this situation progress to diabetes.8 Clinicians should be cautious about diagnosing prediabetes in lean or elderly people.
The reliability of the hemoglobin A1c test is reduced in conditions associated with increased red blood cell turnover, including sickle cell disease, pregnancy (second and third trimesters), hemodialysis, recent blood transfusions or erythropoietin therapy. In these clinical situations, only blood glucose measurements should be used to diagnose prediabetes and T2DM.6 It should be noted that concordance among any of the 3 tests is not perfect.6
Continue to: A 2-step approach to diagnosing T2DM...
A 2-step approach to diagnosing T2DM
An alternative to relying on a single test for T2DM is to use a 2-step approach for screening. The first step is a hemoglobin A1c measurement, which neither requires fasting nor waiting for 2 hours for post–glucose load blood draw. If the hemoglobin A1c result is ≥6.5%, a T2DM diagnosis can be made, with no additional testing. If the hemoglobin A1c result is 5.7% to 6.4%, the person probably has either prediabetes or diabetes and can be offered a 2-hr OGTT to definitively determine if T2DM is the proper diagnosis. If the hemoglobin A1c test is <5.7%, it is unlikely that the person has T2DM or prediabetes at the time of the test. In this situation, the testing could be repeated in 3 years. Using a 2-step approach reduces the number of people who are tested with a 2-hr OGTT and detects more cases of T2DM than a 1-step approach that relies on a hemoglobin A1c measurement alone.
Treatment of prediabetes is warranted in people at high risk for developing diabetes
It is better to prevent diabetes among people with a high risk of diabetes than to treat diabetes once it is established. People with prediabetes who are overweight or obese are at high risk for developing diabetes. Prediabetes is diagnosed by a fasting plasma glucose level of 100 to 125 mg/dL or a hemoglobin A1c measurement of 5.7% to 6.4%.
High-quality randomized clinical trials have definitively demonstrated that, among people at high risk for developing diabetes, lifestyle modification and metformin treatment reduce the risk of developing diabetes. In the Diabetes Prevention Program (DPP) 3,234 people with a high risk of diabetes, mean BMI 34 kg/m2, were randomly assigned to 1 of 3 groups9:
- a control group
- metformin (850 mg twice daily) or
- lifestyle modification that included exercise (moderate intensity exercise for 150 minutes per week and weight loss (7% of body weight using a low-calorie, low-fat diet).
At 2.8 years of follow-up the incidence of diabetes was 11%, 7.8%, and 4.8% per 100 person-years in the people assigned to the control, metformin, and lifestyle modification groups, respectively.9 In the DPP study, compared with the control group, metformin was most effective in decreasing the risk of transitioning to diabetes in people who had a BMI ≥35 kg/m2 (53% reduction in risk) or a BMI from 30 to 35 kg/m2 (16% reduction in risk).9 Metformin was not as effective at preventing the transition to diabetes in people who had a normal BMI or who were overweight (3% reduction).9
In the Finnish Diabetes Prevention Study, 522 obese people with impaired glucose tolerance were randomly assigned to lifestyle modification or a control group. After 4 years, the cumulative incidence of diabetes was 11% and 23% in the lifestyle modification and control groups, respectively.10 A meta-analysis of 23 randomized clinical trials reported that, among people with a high risk of developing diabetes, compared with no intervention (control group), lifestyle modification, including dieting, exercising, and weight loss significantly reduced the risk of developing diabetes (pooled relative risk [RR], 0.78; 95% confidence interval [CI], 0.69‒0.88).5
In clinical practice, offering a patient at high risk for diabetes a suite of options, including5,9,10:
- a formal nutrition consult with the goal of targeting a 7% reduction in weight
- recommending moderate intensity exercise, 150 minutes weekly
- metformin treatment, if the patient is obese
would reduce the patient’s risk of developing diabetes.
Treatment of T2DM is complex
For people with T2DM, a widely recommended treatment goal is to reduce the hemoglobin A1c measurement to ≤7%. Initial treatment includes a comprehensive diabetes self-management education program, weight loss using diet and exercise, and metformin treatment. Metformin may be associated with an increased risk of lactic acidosis, especially in people with renal insufficiency. The US Food and Drug Administration (FDA) recommends against initiating metformin therapy for people with an estimated glomerular filtration rate (eGFR) of 30 to 45 mL/min/1.73 m2. The FDA determined that metformin is contraindicated in people with an eGFR of <30 mL/min/1.73 m2.11 Many people with T2DM will require treatment with multiple pharmacologic agents to achieve a hemoglobin A1c ≤7%. In addition to metformin, pharmacologic agents used to treat T2DM include insulin, sulfonylureas, glucagon-like peptide-1(GLP-1) receptor agonists, a sodium glucose cotransporter (SGLT2) inhibitor, dipeptidyl peptidase-4 (DPP-4) inhibitors, or an alpha-glucosidase inhibitor. Given the complexity of managing T2DM over a lifetime, most individuals with T2DM receive their diabetes care from a primary care clinician or subspecialist in endocrinology.
Experts predict that, within the next 8 years, the prevalence of obesity among adults in the United States will be approximately 50%.12 The US health care system has not been effective in controlling the obesity epidemic. Our failure to control the obesity epidemic will result in an increase in the prevalence of prediabetes and T2DM, leading to a rise in cardiovascular, renal, and eye disease. The diagnosis of prediabetes and diabetes is within the scope of practice of obstetrics and gynecology. The treatment of prediabetes is also within the scope of ObGyns, who have both expertise and familiarity in the diagnosis of gestational diabetes, a form of prediabetes. ●
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 26, 2021.
- Wang L, Li X, Wang Z, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among U.S. adults, 1999-2018. JAMA. 2021;326:1-13. doi: 10.1001/jama.2021.9883.
- Type 2 diabetes. Centers for Disease Control and Prevention website. . Last reviewed August 10, 2021 Accessed October 27, 2021.
- US Preventive Services Task Force. Screening for prediabetes and diabetes. US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531.
- Jonas D, Crotty K, Yun JD, et al. Screening for prediabetes and type 2 diabetes mellitus: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326:744-760. doi: 10.1001/jama.2021.10403.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‒2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi: 10.2337/dc20-S002.
- Meijnikman AS, De Block CE, Dirinck E, et al. Not performing an OGTT results in significant under diagnosis of (pre)diabetes in a high-risk adult Caucasian population. Int J Obes. 2017;41:1615-1620. doi: 10.1038/ijo.2017.165.
- Rooney MR, Rawlings AM, Pankow JS, et al. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med. 2021;181:511-519. doi: 10.1001/jamainternmed.2020.8774.
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. doi: 10.1056/NEJMoa012512.
- Tuomilehto J, Lindström J, Eriksson JG, et al; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. doi: 10.1056/NEJM200105033441801.
- Glucophage [package insert]. Princeton, NJ: Bristol Meyers Squibb; April 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017020357s037s039,021202s021s023lbl.pdf. Accessed October 27, 2021.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381;2440-2450. doi: 10.1056/NEJMc1917339.
The prevalence of T2DM is on the rise in the United States, and T2DM is currently the 7th leading cause of death.1 In a study of 28,143 participants in the US National Health and Nutrition Examination Survey (NHANES) who were 18 years or older, the prevalence of diabetes increased from 9.8% to 14.3% between 2000 and 2008.2 About 24% of the participants had undiagnosed diabetes prior to the testing they received as a study participant.2 People from minority groups have a higher rate of T2DM than non-Hispanic White people. Using data from 2018, the Centers for Disease Control and Prevention reported that the prevalence of diagnosed diabetes was highest among American Indians/Alaska Natives (14.7%), people of Hispanic origin (12.5%), and non-Hispanic Blacks (11.7%), followed by non-Hispanic Asians (9.2%) and non-Hispanic Whites (7.5%).1 Diabetes is a major risk factor for myocardial infarction, stroke, renal failure, retinopathy, peripheral vascular disease, and neuropathy.1 Early detection and treatment of both prediabetes and diabetes may improve health and reduce these preventable complications, saving lives, preventing heart and renal failure and blindness.
T2DM is caused by a combination of insulin resistance and insufficient pancreatic secretion of insulin to overcome the insulin resistance.3 In young adults with insulin resistance, pancreatic secretion of insulin is often sufficient to overcome the insulin resistance resulting in normal glucose levels and persistently increased insulin concentration. As individuals with insulin resistance age, pancreatic secretion of insulin may decline, resulting in insufficient production of insulin and rising glucose levels. Many individuals experience a prolonged stage of prediabetes that may be present for decades prior to transitioning to T2DM. In 2020, 35% of US adults were reported to have prediabetes.1
Screening for diabetes mellitus
The US Preventive Services Task Force (USPSTF) recently recommended that all adults aged 35 to 70 years who are overweight or obese be screened for T2DM (B recommendation).4 Screening for diabetes will also result in detecting many people with prediabetes. The criteria for diagnosing diabetes and prediabetes are presented in the TABLE. Based on cohort studies, the USPSTF noted that screening every 3 years is a reasonable approach.4 They also recommended that people diagnosed with prediabetes should initiate preventive measures, including optimizing diet, weight loss, exercise, and in some cases, medication treatment such as metformin.5

Approaches to the diagnosis of diabetes and prediabetes
Three laboratory tests are widely utilized for the diagnosis of prediabetes and diabetes: measurement of a plasma glucose 2 hours following consumption of oral glucose 75 g (2-hr oral glucose tolerance test [OGTT]), measurement of a fasting plasma glucose, and measurement of hemoglobin A1c (see Table).6In clinical practice, the best diabetes screening test is the test the patient will complete. Most evidence indicates that, compared with the 2-hr OGTT, a hemoglobin A1c measurement is specific for diagnosing T2DM, but not sensitive. In other words, if the hemoglobin A1c is ≥6.5%, the glucose measurement 2 hours following an OGTT will very likely be ≥200 mg/dL. But if the hemoglobin A1c is between 5.7% and 6.5%, the person might be diagnosed with T2DM if they had a 2-hr OGTT.6
In one study, 1,241 nondiabetic, overweight, or obese participants had all 3 tests to diagnose T2DM.7 The 2-hr OGTT diagnosed T2DM in 148 participants (12%). However, the hemoglobin A1c test only diagnosed T2DM in 78 of the 148 participants who were diagnosed with T2DM based on the 2-hr OGTT, missing 47% of the cases of T2DM. In this study, using the 2-hr OGTT as the “gold standard” reference test, the hemoglobin A1c test had a sensitivity of 53% and specificity of 97%.7
In clinical practice one approach is to explain to the patient the pros and cons of the 3 tests for T2DM and ask them to select the test they prefer to complete. In a high-risk population, including people with obesity, completing any of the 3 tests is better than not testing for diabetes. It also should be noted that, among people who have a normal body mass index (BMI), a “prediabetes” diagnosis is controversial. Compared with obese persons with prediabetes, people with a normal BMI and prediabetes diagnosed by a blood test progress to diabetes at a much lower rate. The value of diagnosing prediabetes after 70 years of age is also controversial because few people in this situation progress to diabetes.8 Clinicians should be cautious about diagnosing prediabetes in lean or elderly people.
The reliability of the hemoglobin A1c test is reduced in conditions associated with increased red blood cell turnover, including sickle cell disease, pregnancy (second and third trimesters), hemodialysis, recent blood transfusions or erythropoietin therapy. In these clinical situations, only blood glucose measurements should be used to diagnose prediabetes and T2DM.6 It should be noted that concordance among any of the 3 tests is not perfect.6
Continue to: A 2-step approach to diagnosing T2DM...
A 2-step approach to diagnosing T2DM
An alternative to relying on a single test for T2DM is to use a 2-step approach for screening. The first step is a hemoglobin A1c measurement, which neither requires fasting nor waiting for 2 hours for post–glucose load blood draw. If the hemoglobin A1c result is ≥6.5%, a T2DM diagnosis can be made, with no additional testing. If the hemoglobin A1c result is 5.7% to 6.4%, the person probably has either prediabetes or diabetes and can be offered a 2-hr OGTT to definitively determine if T2DM is the proper diagnosis. If the hemoglobin A1c test is <5.7%, it is unlikely that the person has T2DM or prediabetes at the time of the test. In this situation, the testing could be repeated in 3 years. Using a 2-step approach reduces the number of people who are tested with a 2-hr OGTT and detects more cases of T2DM than a 1-step approach that relies on a hemoglobin A1c measurement alone.
Treatment of prediabetes is warranted in people at high risk for developing diabetes
It is better to prevent diabetes among people with a high risk of diabetes than to treat diabetes once it is established. People with prediabetes who are overweight or obese are at high risk for developing diabetes. Prediabetes is diagnosed by a fasting plasma glucose level of 100 to 125 mg/dL or a hemoglobin A1c measurement of 5.7% to 6.4%.
High-quality randomized clinical trials have definitively demonstrated that, among people at high risk for developing diabetes, lifestyle modification and metformin treatment reduce the risk of developing diabetes. In the Diabetes Prevention Program (DPP) 3,234 people with a high risk of diabetes, mean BMI 34 kg/m2, were randomly assigned to 1 of 3 groups9:
- a control group
- metformin (850 mg twice daily) or
- lifestyle modification that included exercise (moderate intensity exercise for 150 minutes per week and weight loss (7% of body weight using a low-calorie, low-fat diet).
At 2.8 years of follow-up the incidence of diabetes was 11%, 7.8%, and 4.8% per 100 person-years in the people assigned to the control, metformin, and lifestyle modification groups, respectively.9 In the DPP study, compared with the control group, metformin was most effective in decreasing the risk of transitioning to diabetes in people who had a BMI ≥35 kg/m2 (53% reduction in risk) or a BMI from 30 to 35 kg/m2 (16% reduction in risk).9 Metformin was not as effective at preventing the transition to diabetes in people who had a normal BMI or who were overweight (3% reduction).9
In the Finnish Diabetes Prevention Study, 522 obese people with impaired glucose tolerance were randomly assigned to lifestyle modification or a control group. After 4 years, the cumulative incidence of diabetes was 11% and 23% in the lifestyle modification and control groups, respectively.10 A meta-analysis of 23 randomized clinical trials reported that, among people with a high risk of developing diabetes, compared with no intervention (control group), lifestyle modification, including dieting, exercising, and weight loss significantly reduced the risk of developing diabetes (pooled relative risk [RR], 0.78; 95% confidence interval [CI], 0.69‒0.88).5
In clinical practice, offering a patient at high risk for diabetes a suite of options, including5,9,10:
- a formal nutrition consult with the goal of targeting a 7% reduction in weight
- recommending moderate intensity exercise, 150 minutes weekly
- metformin treatment, if the patient is obese
would reduce the patient’s risk of developing diabetes.
Treatment of T2DM is complex
For people with T2DM, a widely recommended treatment goal is to reduce the hemoglobin A1c measurement to ≤7%. Initial treatment includes a comprehensive diabetes self-management education program, weight loss using diet and exercise, and metformin treatment. Metformin may be associated with an increased risk of lactic acidosis, especially in people with renal insufficiency. The US Food and Drug Administration (FDA) recommends against initiating metformin therapy for people with an estimated glomerular filtration rate (eGFR) of 30 to 45 mL/min/1.73 m2. The FDA determined that metformin is contraindicated in people with an eGFR of <30 mL/min/1.73 m2.11 Many people with T2DM will require treatment with multiple pharmacologic agents to achieve a hemoglobin A1c ≤7%. In addition to metformin, pharmacologic agents used to treat T2DM include insulin, sulfonylureas, glucagon-like peptide-1(GLP-1) receptor agonists, a sodium glucose cotransporter (SGLT2) inhibitor, dipeptidyl peptidase-4 (DPP-4) inhibitors, or an alpha-glucosidase inhibitor. Given the complexity of managing T2DM over a lifetime, most individuals with T2DM receive their diabetes care from a primary care clinician or subspecialist in endocrinology.
Experts predict that, within the next 8 years, the prevalence of obesity among adults in the United States will be approximately 50%.12 The US health care system has not been effective in controlling the obesity epidemic. Our failure to control the obesity epidemic will result in an increase in the prevalence of prediabetes and T2DM, leading to a rise in cardiovascular, renal, and eye disease. The diagnosis of prediabetes and diabetes is within the scope of practice of obstetrics and gynecology. The treatment of prediabetes is also within the scope of ObGyns, who have both expertise and familiarity in the diagnosis of gestational diabetes, a form of prediabetes. ●
The prevalence of T2DM is on the rise in the United States, and T2DM is currently the 7th leading cause of death.1 In a study of 28,143 participants in the US National Health and Nutrition Examination Survey (NHANES) who were 18 years or older, the prevalence of diabetes increased from 9.8% to 14.3% between 2000 and 2008.2 About 24% of the participants had undiagnosed diabetes prior to the testing they received as a study participant.2 People from minority groups have a higher rate of T2DM than non-Hispanic White people. Using data from 2018, the Centers for Disease Control and Prevention reported that the prevalence of diagnosed diabetes was highest among American Indians/Alaska Natives (14.7%), people of Hispanic origin (12.5%), and non-Hispanic Blacks (11.7%), followed by non-Hispanic Asians (9.2%) and non-Hispanic Whites (7.5%).1 Diabetes is a major risk factor for myocardial infarction, stroke, renal failure, retinopathy, peripheral vascular disease, and neuropathy.1 Early detection and treatment of both prediabetes and diabetes may improve health and reduce these preventable complications, saving lives, preventing heart and renal failure and blindness.
T2DM is caused by a combination of insulin resistance and insufficient pancreatic secretion of insulin to overcome the insulin resistance.3 In young adults with insulin resistance, pancreatic secretion of insulin is often sufficient to overcome the insulin resistance resulting in normal glucose levels and persistently increased insulin concentration. As individuals with insulin resistance age, pancreatic secretion of insulin may decline, resulting in insufficient production of insulin and rising glucose levels. Many individuals experience a prolonged stage of prediabetes that may be present for decades prior to transitioning to T2DM. In 2020, 35% of US adults were reported to have prediabetes.1
Screening for diabetes mellitus
The US Preventive Services Task Force (USPSTF) recently recommended that all adults aged 35 to 70 years who are overweight or obese be screened for T2DM (B recommendation).4 Screening for diabetes will also result in detecting many people with prediabetes. The criteria for diagnosing diabetes and prediabetes are presented in the TABLE. Based on cohort studies, the USPSTF noted that screening every 3 years is a reasonable approach.4 They also recommended that people diagnosed with prediabetes should initiate preventive measures, including optimizing diet, weight loss, exercise, and in some cases, medication treatment such as metformin.5

Approaches to the diagnosis of diabetes and prediabetes
Three laboratory tests are widely utilized for the diagnosis of prediabetes and diabetes: measurement of a plasma glucose 2 hours following consumption of oral glucose 75 g (2-hr oral glucose tolerance test [OGTT]), measurement of a fasting plasma glucose, and measurement of hemoglobin A1c (see Table).6In clinical practice, the best diabetes screening test is the test the patient will complete. Most evidence indicates that, compared with the 2-hr OGTT, a hemoglobin A1c measurement is specific for diagnosing T2DM, but not sensitive. In other words, if the hemoglobin A1c is ≥6.5%, the glucose measurement 2 hours following an OGTT will very likely be ≥200 mg/dL. But if the hemoglobin A1c is between 5.7% and 6.5%, the person might be diagnosed with T2DM if they had a 2-hr OGTT.6
In one study, 1,241 nondiabetic, overweight, or obese participants had all 3 tests to diagnose T2DM.7 The 2-hr OGTT diagnosed T2DM in 148 participants (12%). However, the hemoglobin A1c test only diagnosed T2DM in 78 of the 148 participants who were diagnosed with T2DM based on the 2-hr OGTT, missing 47% of the cases of T2DM. In this study, using the 2-hr OGTT as the “gold standard” reference test, the hemoglobin A1c test had a sensitivity of 53% and specificity of 97%.7
In clinical practice one approach is to explain to the patient the pros and cons of the 3 tests for T2DM and ask them to select the test they prefer to complete. In a high-risk population, including people with obesity, completing any of the 3 tests is better than not testing for diabetes. It also should be noted that, among people who have a normal body mass index (BMI), a “prediabetes” diagnosis is controversial. Compared with obese persons with prediabetes, people with a normal BMI and prediabetes diagnosed by a blood test progress to diabetes at a much lower rate. The value of diagnosing prediabetes after 70 years of age is also controversial because few people in this situation progress to diabetes.8 Clinicians should be cautious about diagnosing prediabetes in lean or elderly people.
The reliability of the hemoglobin A1c test is reduced in conditions associated with increased red blood cell turnover, including sickle cell disease, pregnancy (second and third trimesters), hemodialysis, recent blood transfusions or erythropoietin therapy. In these clinical situations, only blood glucose measurements should be used to diagnose prediabetes and T2DM.6 It should be noted that concordance among any of the 3 tests is not perfect.6
Continue to: A 2-step approach to diagnosing T2DM...
A 2-step approach to diagnosing T2DM
An alternative to relying on a single test for T2DM is to use a 2-step approach for screening. The first step is a hemoglobin A1c measurement, which neither requires fasting nor waiting for 2 hours for post–glucose load blood draw. If the hemoglobin A1c result is ≥6.5%, a T2DM diagnosis can be made, with no additional testing. If the hemoglobin A1c result is 5.7% to 6.4%, the person probably has either prediabetes or diabetes and can be offered a 2-hr OGTT to definitively determine if T2DM is the proper diagnosis. If the hemoglobin A1c test is <5.7%, it is unlikely that the person has T2DM or prediabetes at the time of the test. In this situation, the testing could be repeated in 3 years. Using a 2-step approach reduces the number of people who are tested with a 2-hr OGTT and detects more cases of T2DM than a 1-step approach that relies on a hemoglobin A1c measurement alone.
Treatment of prediabetes is warranted in people at high risk for developing diabetes
It is better to prevent diabetes among people with a high risk of diabetes than to treat diabetes once it is established. People with prediabetes who are overweight or obese are at high risk for developing diabetes. Prediabetes is diagnosed by a fasting plasma glucose level of 100 to 125 mg/dL or a hemoglobin A1c measurement of 5.7% to 6.4%.
High-quality randomized clinical trials have definitively demonstrated that, among people at high risk for developing diabetes, lifestyle modification and metformin treatment reduce the risk of developing diabetes. In the Diabetes Prevention Program (DPP) 3,234 people with a high risk of diabetes, mean BMI 34 kg/m2, were randomly assigned to 1 of 3 groups9:
- a control group
- metformin (850 mg twice daily) or
- lifestyle modification that included exercise (moderate intensity exercise for 150 minutes per week and weight loss (7% of body weight using a low-calorie, low-fat diet).
At 2.8 years of follow-up the incidence of diabetes was 11%, 7.8%, and 4.8% per 100 person-years in the people assigned to the control, metformin, and lifestyle modification groups, respectively.9 In the DPP study, compared with the control group, metformin was most effective in decreasing the risk of transitioning to diabetes in people who had a BMI ≥35 kg/m2 (53% reduction in risk) or a BMI from 30 to 35 kg/m2 (16% reduction in risk).9 Metformin was not as effective at preventing the transition to diabetes in people who had a normal BMI or who were overweight (3% reduction).9
In the Finnish Diabetes Prevention Study, 522 obese people with impaired glucose tolerance were randomly assigned to lifestyle modification or a control group. After 4 years, the cumulative incidence of diabetes was 11% and 23% in the lifestyle modification and control groups, respectively.10 A meta-analysis of 23 randomized clinical trials reported that, among people with a high risk of developing diabetes, compared with no intervention (control group), lifestyle modification, including dieting, exercising, and weight loss significantly reduced the risk of developing diabetes (pooled relative risk [RR], 0.78; 95% confidence interval [CI], 0.69‒0.88).5
In clinical practice, offering a patient at high risk for diabetes a suite of options, including5,9,10:
- a formal nutrition consult with the goal of targeting a 7% reduction in weight
- recommending moderate intensity exercise, 150 minutes weekly
- metformin treatment, if the patient is obese
would reduce the patient’s risk of developing diabetes.
Treatment of T2DM is complex
For people with T2DM, a widely recommended treatment goal is to reduce the hemoglobin A1c measurement to ≤7%. Initial treatment includes a comprehensive diabetes self-management education program, weight loss using diet and exercise, and metformin treatment. Metformin may be associated with an increased risk of lactic acidosis, especially in people with renal insufficiency. The US Food and Drug Administration (FDA) recommends against initiating metformin therapy for people with an estimated glomerular filtration rate (eGFR) of 30 to 45 mL/min/1.73 m2. The FDA determined that metformin is contraindicated in people with an eGFR of <30 mL/min/1.73 m2.11 Many people with T2DM will require treatment with multiple pharmacologic agents to achieve a hemoglobin A1c ≤7%. In addition to metformin, pharmacologic agents used to treat T2DM include insulin, sulfonylureas, glucagon-like peptide-1(GLP-1) receptor agonists, a sodium glucose cotransporter (SGLT2) inhibitor, dipeptidyl peptidase-4 (DPP-4) inhibitors, or an alpha-glucosidase inhibitor. Given the complexity of managing T2DM over a lifetime, most individuals with T2DM receive their diabetes care from a primary care clinician or subspecialist in endocrinology.
Experts predict that, within the next 8 years, the prevalence of obesity among adults in the United States will be approximately 50%.12 The US health care system has not been effective in controlling the obesity epidemic. Our failure to control the obesity epidemic will result in an increase in the prevalence of prediabetes and T2DM, leading to a rise in cardiovascular, renal, and eye disease. The diagnosis of prediabetes and diabetes is within the scope of practice of obstetrics and gynecology. The treatment of prediabetes is also within the scope of ObGyns, who have both expertise and familiarity in the diagnosis of gestational diabetes, a form of prediabetes. ●
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 26, 2021.
- Wang L, Li X, Wang Z, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among U.S. adults, 1999-2018. JAMA. 2021;326:1-13. doi: 10.1001/jama.2021.9883.
- Type 2 diabetes. Centers for Disease Control and Prevention website. . Last reviewed August 10, 2021 Accessed October 27, 2021.
- US Preventive Services Task Force. Screening for prediabetes and diabetes. US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531.
- Jonas D, Crotty K, Yun JD, et al. Screening for prediabetes and type 2 diabetes mellitus: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326:744-760. doi: 10.1001/jama.2021.10403.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‒2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi: 10.2337/dc20-S002.
- Meijnikman AS, De Block CE, Dirinck E, et al. Not performing an OGTT results in significant under diagnosis of (pre)diabetes in a high-risk adult Caucasian population. Int J Obes. 2017;41:1615-1620. doi: 10.1038/ijo.2017.165.
- Rooney MR, Rawlings AM, Pankow JS, et al. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med. 2021;181:511-519. doi: 10.1001/jamainternmed.2020.8774.
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. doi: 10.1056/NEJMoa012512.
- Tuomilehto J, Lindström J, Eriksson JG, et al; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. doi: 10.1056/NEJM200105033441801.
- Glucophage [package insert]. Princeton, NJ: Bristol Meyers Squibb; April 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017020357s037s039,021202s021s023lbl.pdf. Accessed October 27, 2021.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381;2440-2450. doi: 10.1056/NEJMc1917339.
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed October 26, 2021.
- Wang L, Li X, Wang Z, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among U.S. adults, 1999-2018. JAMA. 2021;326:1-13. doi: 10.1001/jama.2021.9883.
- Type 2 diabetes. Centers for Disease Control and Prevention website. . Last reviewed August 10, 2021 Accessed October 27, 2021.
- US Preventive Services Task Force. Screening for prediabetes and diabetes. US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326:736-743. doi: 10.1001/jama.2021.12531.
- Jonas D, Crotty K, Yun JD, et al. Screening for prediabetes and type 2 diabetes mellitus: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326:744-760. doi: 10.1001/jama.2021.10403.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‒2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi: 10.2337/dc20-S002.
- Meijnikman AS, De Block CE, Dirinck E, et al. Not performing an OGTT results in significant under diagnosis of (pre)diabetes in a high-risk adult Caucasian population. Int J Obes. 2017;41:1615-1620. doi: 10.1038/ijo.2017.165.
- Rooney MR, Rawlings AM, Pankow JS, et al. Risk of progression to diabetes among older adults with prediabetes. JAMA Intern Med. 2021;181:511-519. doi: 10.1001/jamainternmed.2020.8774.
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. doi: 10.1056/NEJMoa012512.
- Tuomilehto J, Lindström J, Eriksson JG, et al; Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350. doi: 10.1056/NEJM200105033441801.
- Glucophage [package insert]. Princeton, NJ: Bristol Meyers Squibb; April 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017020357s037s039,021202s021s023lbl.pdf. Accessed October 27, 2021.
- Ward ZJ, Bleich SN, Cradock AL, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381;2440-2450. doi: 10.1056/NEJMc1917339.
Remote and in-home prenatal care: Safe, inclusive, and here to stay

For much of the general public, in-home care from a physician is akin to the rotary telephone: a feature of a bygone age, long since replaced by vastly different systems. While approximately 40% of physician-patient interactions in 1930 were house calls, by the early 1980s this had dwindled to less than 1%,1 with almost all physician-patient encounters taking place in a clinical setting, whether in a hospital or in a free-standing clinic. In the last 2 decades, a smattering of primary care and medical subspecialty clinicians started to incorporate some in-home care into their practices in the form of telemedicine, using video and telephone technology to facilitate care outside of the clinical setting, and by 2016, approximately 15% of physicians reported using some form of telemedicine in their interactions with patients.2
Despite these advances, prior to the COVID-19 pandemic, obstetricians lagged significantly behind in their use of at-home or remote care. Although there were some efforts to promote a hybrid care model that incorporated prenatal telemedicine,3 pre-pandemic ObGyn was one of the least likely fields to offer telemedicine to their patients, with only 9% of practices offering such services.2 In this article, we discuss how the COVID-19 pandemic resulted in a shift from traditional, in-person care to a hybrid remote model and how this may benefit obstetrics patients as well as clinicians.
Pre-pandemic patient management
The traditional model of prenatal care presents a particularly intense time period for patients in terms of its demands. Women who are pregnant and start care in their first trimester typically have 12 to 14 visits during the subsequent 6 to 7 months, with additional visits for those with high-risk pregnancies. Although some of these visits coincide with the need for in-person laboratory work or imaging, many are chiefly oriented around assessment of vital signs or counseling. These frequent prenatal visits represent a significant commitment from patients in terms of transportation, time off work, and childcare resources—all of which may be exacerbated for patients who need to receive their care from overbooked, high-risk specialists.
After delivery, attending an in-person postpartum visit with a newborn can be even more daunting. Despite the increased recognition from professional groups of the importance of postpartum care to support breastfeeding, physical recovery, and mental health, as many as 40% of recently delivered patients do not attend their scheduled postpartum visit(s).4 Still, before 2020, few obstetricians had revised their workflows to “meet patients where they are,” with many continuing to only offer in-person care and assessments.
COVID-19: An impetus for change
As with so many things, the COVID-19 pandemic has challenged our ideas of what is normal. In a sense, the pandemic has catalyzed a revolution in the prenatal care model. The very real risks of exposure and contagion during the pandemic—for clinicians and patients alike—has forced ObGyns to reexamine the actual risks and benefits of in-person and in-clinic prenatal care. As a result, many ObGyns have rapidly adopted telemedicine into practices that were strictly in-person. For example, a national survey of 172 clinicians who offered contraception counseling during the pandemic found that 91% of them were now offering telemedicine services, with 78% of those clinicians new to telemedicine.5 Similarly, although a minority of surveyed obstetricians in New York City reported using telemedicine pre-pandemic, 89% planned to continue using such technology in the future.6
Continue to: Incorporating mobile technology...
Incorporating mobile technology
Obstetricians, forced to consolidate and maximize their in-person care to protect their patients’ safety, have started to realize that many of the conversations and counseling offered to patients can be managed equally effectively with telemedicine. Furthermore, basic home monitoring devices, such as blood pressure machines, can be safely and accurately used by patients without requiring them to come to the office.
More recent research into mobile medical devices suggests that patients can safely and appropriately manage more complex tools. One such example is a mobile, self-operated, ultrasound transducer that is controlled through a smartphone (Instinct, Pulsenmore Ltd). This device was evaluated in an observational, noninterventional trial of 100 women carrying a singleton fetus at 14/0 weeks’ to 39/6 weeks’ gestation. Patients performed 1,360 self-scans, which were reviewed by a clinician in real time online or subsequently off-line. Results showed successful detection rates of 95.3% for fetal heart activity, 88.3% for body movements, 69.4% for tone, 23.8% for breathing movements, and 92.2% for normal amniotic fluid volume.7 The authors concluded that this represents a feasible solution for remote sonographic fetal assessment.
Coordinating care with health care extenders
Remote monitoring options allow patients to be safely monitored during their pregnancies while remaining at home more often, especially when used in conjunction with trained health care extenders such as registered nurses, primary care associates, or “maternity navigators” who can facilitate off-site care. In fact, many aspects of prenatal care are particularly amenable to remote medicine or non–physician-based home care. Different variations of this model of “hybrid” prenatal care may be appropriate depending upon the needs of the patient population served by a given obstetrics practice. Ideally, a prenatal care model personalizes care based on the known risk factors that are identified at the beginning of prenatal care, the anticipated barriers to care, and the patient’s own preferences. As a result, alternatives to the traditional model may be to alternate in-person and telemedicine visits,3,8 to incorporate in-person or remote group prenatal visits,9,10 or to incorporate staff with basic health care skills to serve as health care extenders in the community and provide home visits for basic monitoring, laboratory work, and patient education.11
Benefits of hybrid prenatal models
As we look ahead to the end of the pandemic, how should obstetricians view these hybrid prenatal care models? Are these models safe for patients? Were they only worthwhile to minimize infection risk, or do they have potential benefits for patients going forward?
In fact, data on the use of telemedicine in prenatal care indicate that these models may be equally as safe as the traditional model in terms of clinical outcomes and may have important additional benefits with regard to patient convenience and access to and satisfaction with care. Even audio-only prenatal televisits have been found to be equivalent to in-person visits in terms of serious perinatal outcomes.12 Common pregnancy diagnoses are also well-served by telemedicine. For example, several recent investigations of patients with gestational diabetes have found that telemedicine was as effective as standard care for glucose control.13,14 Management of hypertension during pregnancy, another antenatal condition that is commonly managed with frequent in-person check-ups, also was found to be adequately feasible with telemedicine using home monitors and symptom checklists, with high rates of patient satisfaction.15
With good evidence for safety, the added potential for patients to benefit in such hybrid models is multifactorial. For one, despite our collective hopes, the COVID-19 pandemic may have a long tail. Vaccine hesitancy and COVID-19 variants may mean that clinicians will have to consider the real threat of infection risk in the clinic setting for years to come. In-home prenatal care also provides a wide variety of social, economic, and psychological benefits for pregnant women across various patient populations. The pandemic has introduced many patients to the full potential of working and meeting remotely; pregnant patients are becoming more familiar with these technology platforms and appreciate its incorporation into their busy lives.5 Furthermore, hybrid models actually can provide otherwise “nonadherent” patients with better access to care. From the patient perspective, an in-person 15-minute health care provider visit actually represents a significant commitment of time and resources (ie, hours spent on public transportation, lost wages for those with inflexible work schedules, and childcare costs for patients discouraged from bringing their children to prenatal visits). Especially for patients with fewer socioeconomic resources, these barriers to in-person clinic visits may be daunting, if not insurmountable; the option of remote visits or house calls reduces these barriers and facilitates care.16
Such hybrid models benefit prenatal clinicians as well. In addition to a decreased risk of infection, clinicians may be able to attract a wider potential prenatal patient population with telemedicine by appealing to younger and potentially more technology-savvy patients.17 Importantly, telemedicine is increasingly recognized as on par with in-person visits in many billing algorithms. Changes during the pandemic led Medicare to cover telemedicine visits as well as in-person visits18,19; among other groundbreaking changes, new patients can have an initial billable visit via telemedicine. Although the billing landscape will likely continue to evolve, such changes allow clinicians to focus on patient safety and convenience without financial risk to their practices.
The future of prenatal appointment scheduling
The future of prenatal care certainly doesn’t look like a dozen 15-minute visits in a private physician’s office. While these emerging hybrid models of prenatal care certainly can benefit patients with low-risk uncomplicated pregnancies, they are already being adopted by clinicians who care for patients with antenatal complications that require specialist consultation; for those with conditions that require frequent, low-complexity check-ins (gestational diabetes, chronic hypertension, history of pre-term birth, etc.); and for patients who struggle with financial or logistical barriers to in-person care. Although obstetrics may have lagged behind other subspecialties in revising its traditional health care models, the pandemic has opened up a new world of possibilities of remote and in-home care for this field. ●
- Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34. doi:10.1016/j.cger.2008.10.005.
- Kane CK, Gillis K. The use of telemedicine by physicians: still the exception rather than the rule. Health Aff (Millwood). 2018;37:1923-1930. doi:10.1377/hlthaff.2018.05077.
- Weigel G, Frederiksen B, Ranji U. Telemedicine and pregnancy care. Kaiser Family Foundation website. https://www.kff.org/womens-health-policy/issue-brief/telemedicine-and-pregnancy-care. Accessed August 23, 2021.
- ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi:10.1097/AOG.0000000000002633.
- Stifani BM, Avila K, Levi EE. Telemedicine for contraceptive counseling: an exploratory survey of US family planning providers following rapid adoption of services during the COVID-19 pandemic. Contraception. 2021;103:157-162. doi:10.1016/j.contraception.2020.11.006.
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during the COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939.
- Hadar E, Wolff L, Tenenbaum-Gavish K, et al. Mobile self-operated home ultrasound system for remote fetal assessment during pregnancy. Telemed J E Health. 2021. doi:10.1089/tmj.2020.0541.
- Thomas Jefferson University Division of Maternal Fetal Medicine. Jefferson Maternal Fetal Medicine COVID19 Preparedness. Version 2.1. March 19, 2020. https://communities.smfm.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=a109df77-74fe-462b-87fb-895d6ee7d0e6. Accessed August 23, 2021.
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.AOG.0000275284.24298.23.
- Wicklund M. Oakland launches telehealth program for Black prenatal, postpartum care. Telehealth News. https://mhealthintelligence.com/news/oakland-launches-telehealth-program-for-black-prenatal-postpartum-care. Accessed August 23, 2021.
- Home-based pregnancy care. CayabaCare website. https://www.cayabacare.com. Accessed August 23, 2021.
- Duryea EL, Adhikari EH, Ambia A, et al. Comparison between in-person and audio-only virtual prenatal visits and perinatal outcomes. JAMA Netw Open. 2021;4:e215854. doi:10.1001/jamanetworkopen.2021.5854.
- Ming WK, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e290. doi:10.2196/jmir.6556.
- Tian Y, Zhang S, Huang F, et al. Comparing the efficacies of telemedicine and standard prenatal care on blood glucose control in women with gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9:e22881. doi:10.2196/22881.
- van den Heuvel JFM, Kariman SS, van Solinge WW, et al. SAFE@HOME – feasibility study of a telemonitoring platform combining blood pressure and preeclampsia symptoms in pregnancy care. Eur J Obstet Gynecol Reprod Biol. 2019;240:226-231. doi:10.1016/j.ejogrb.2019.07.012.
- Dixon-Shambley K, Gabbe PT. Using telehealth approaches to address social determinants of health and improve pregnancy and postpartum outcomes. Clin Obstet Gynecol. 2021;64:333-344. doi:10.1097/GRF.0000000000000611.
- Eruchalu CN, Pichardo MS, Bharadwaj M, et al. The expanding digital divide: digital health access inequities during the COVID-19 pandemic in New York City. J Urban Health. 2021;98:183-186. doi:10.1007/s11524-020-00508-9.
- COVID-19 FAQs for obstetrician-gynecologists, telehealth. The American College of Obstetricians and Gynecologists website. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-telehealth. Accessed August 23, 2021.
- Managing patients remotely: billing for digital and telehealth services. The American College of Obstetricians and Gynecologists website. Updated October 19, 2020. https://www.acog.org/practice-management/coding/coding-library/managing-patients-remotely-billing-for-digital-and-telehealth-services. Accessed August 23, 2021.

For much of the general public, in-home care from a physician is akin to the rotary telephone: a feature of a bygone age, long since replaced by vastly different systems. While approximately 40% of physician-patient interactions in 1930 were house calls, by the early 1980s this had dwindled to less than 1%,1 with almost all physician-patient encounters taking place in a clinical setting, whether in a hospital or in a free-standing clinic. In the last 2 decades, a smattering of primary care and medical subspecialty clinicians started to incorporate some in-home care into their practices in the form of telemedicine, using video and telephone technology to facilitate care outside of the clinical setting, and by 2016, approximately 15% of physicians reported using some form of telemedicine in their interactions with patients.2
Despite these advances, prior to the COVID-19 pandemic, obstetricians lagged significantly behind in their use of at-home or remote care. Although there were some efforts to promote a hybrid care model that incorporated prenatal telemedicine,3 pre-pandemic ObGyn was one of the least likely fields to offer telemedicine to their patients, with only 9% of practices offering such services.2 In this article, we discuss how the COVID-19 pandemic resulted in a shift from traditional, in-person care to a hybrid remote model and how this may benefit obstetrics patients as well as clinicians.
Pre-pandemic patient management
The traditional model of prenatal care presents a particularly intense time period for patients in terms of its demands. Women who are pregnant and start care in their first trimester typically have 12 to 14 visits during the subsequent 6 to 7 months, with additional visits for those with high-risk pregnancies. Although some of these visits coincide with the need for in-person laboratory work or imaging, many are chiefly oriented around assessment of vital signs or counseling. These frequent prenatal visits represent a significant commitment from patients in terms of transportation, time off work, and childcare resources—all of which may be exacerbated for patients who need to receive their care from overbooked, high-risk specialists.
After delivery, attending an in-person postpartum visit with a newborn can be even more daunting. Despite the increased recognition from professional groups of the importance of postpartum care to support breastfeeding, physical recovery, and mental health, as many as 40% of recently delivered patients do not attend their scheduled postpartum visit(s).4 Still, before 2020, few obstetricians had revised their workflows to “meet patients where they are,” with many continuing to only offer in-person care and assessments.
COVID-19: An impetus for change
As with so many things, the COVID-19 pandemic has challenged our ideas of what is normal. In a sense, the pandemic has catalyzed a revolution in the prenatal care model. The very real risks of exposure and contagion during the pandemic—for clinicians and patients alike—has forced ObGyns to reexamine the actual risks and benefits of in-person and in-clinic prenatal care. As a result, many ObGyns have rapidly adopted telemedicine into practices that were strictly in-person. For example, a national survey of 172 clinicians who offered contraception counseling during the pandemic found that 91% of them were now offering telemedicine services, with 78% of those clinicians new to telemedicine.5 Similarly, although a minority of surveyed obstetricians in New York City reported using telemedicine pre-pandemic, 89% planned to continue using such technology in the future.6
Continue to: Incorporating mobile technology...
Incorporating mobile technology
Obstetricians, forced to consolidate and maximize their in-person care to protect their patients’ safety, have started to realize that many of the conversations and counseling offered to patients can be managed equally effectively with telemedicine. Furthermore, basic home monitoring devices, such as blood pressure machines, can be safely and accurately used by patients without requiring them to come to the office.
More recent research into mobile medical devices suggests that patients can safely and appropriately manage more complex tools. One such example is a mobile, self-operated, ultrasound transducer that is controlled through a smartphone (Instinct, Pulsenmore Ltd). This device was evaluated in an observational, noninterventional trial of 100 women carrying a singleton fetus at 14/0 weeks’ to 39/6 weeks’ gestation. Patients performed 1,360 self-scans, which were reviewed by a clinician in real time online or subsequently off-line. Results showed successful detection rates of 95.3% for fetal heart activity, 88.3% for body movements, 69.4% for tone, 23.8% for breathing movements, and 92.2% for normal amniotic fluid volume.7 The authors concluded that this represents a feasible solution for remote sonographic fetal assessment.
Coordinating care with health care extenders
Remote monitoring options allow patients to be safely monitored during their pregnancies while remaining at home more often, especially when used in conjunction with trained health care extenders such as registered nurses, primary care associates, or “maternity navigators” who can facilitate off-site care. In fact, many aspects of prenatal care are particularly amenable to remote medicine or non–physician-based home care. Different variations of this model of “hybrid” prenatal care may be appropriate depending upon the needs of the patient population served by a given obstetrics practice. Ideally, a prenatal care model personalizes care based on the known risk factors that are identified at the beginning of prenatal care, the anticipated barriers to care, and the patient’s own preferences. As a result, alternatives to the traditional model may be to alternate in-person and telemedicine visits,3,8 to incorporate in-person or remote group prenatal visits,9,10 or to incorporate staff with basic health care skills to serve as health care extenders in the community and provide home visits for basic monitoring, laboratory work, and patient education.11
Benefits of hybrid prenatal models
As we look ahead to the end of the pandemic, how should obstetricians view these hybrid prenatal care models? Are these models safe for patients? Were they only worthwhile to minimize infection risk, or do they have potential benefits for patients going forward?
In fact, data on the use of telemedicine in prenatal care indicate that these models may be equally as safe as the traditional model in terms of clinical outcomes and may have important additional benefits with regard to patient convenience and access to and satisfaction with care. Even audio-only prenatal televisits have been found to be equivalent to in-person visits in terms of serious perinatal outcomes.12 Common pregnancy diagnoses are also well-served by telemedicine. For example, several recent investigations of patients with gestational diabetes have found that telemedicine was as effective as standard care for glucose control.13,14 Management of hypertension during pregnancy, another antenatal condition that is commonly managed with frequent in-person check-ups, also was found to be adequately feasible with telemedicine using home monitors and symptom checklists, with high rates of patient satisfaction.15
With good evidence for safety, the added potential for patients to benefit in such hybrid models is multifactorial. For one, despite our collective hopes, the COVID-19 pandemic may have a long tail. Vaccine hesitancy and COVID-19 variants may mean that clinicians will have to consider the real threat of infection risk in the clinic setting for years to come. In-home prenatal care also provides a wide variety of social, economic, and psychological benefits for pregnant women across various patient populations. The pandemic has introduced many patients to the full potential of working and meeting remotely; pregnant patients are becoming more familiar with these technology platforms and appreciate its incorporation into their busy lives.5 Furthermore, hybrid models actually can provide otherwise “nonadherent” patients with better access to care. From the patient perspective, an in-person 15-minute health care provider visit actually represents a significant commitment of time and resources (ie, hours spent on public transportation, lost wages for those with inflexible work schedules, and childcare costs for patients discouraged from bringing their children to prenatal visits). Especially for patients with fewer socioeconomic resources, these barriers to in-person clinic visits may be daunting, if not insurmountable; the option of remote visits or house calls reduces these barriers and facilitates care.16
Such hybrid models benefit prenatal clinicians as well. In addition to a decreased risk of infection, clinicians may be able to attract a wider potential prenatal patient population with telemedicine by appealing to younger and potentially more technology-savvy patients.17 Importantly, telemedicine is increasingly recognized as on par with in-person visits in many billing algorithms. Changes during the pandemic led Medicare to cover telemedicine visits as well as in-person visits18,19; among other groundbreaking changes, new patients can have an initial billable visit via telemedicine. Although the billing landscape will likely continue to evolve, such changes allow clinicians to focus on patient safety and convenience without financial risk to their practices.
The future of prenatal appointment scheduling
The future of prenatal care certainly doesn’t look like a dozen 15-minute visits in a private physician’s office. While these emerging hybrid models of prenatal care certainly can benefit patients with low-risk uncomplicated pregnancies, they are already being adopted by clinicians who care for patients with antenatal complications that require specialist consultation; for those with conditions that require frequent, low-complexity check-ins (gestational diabetes, chronic hypertension, history of pre-term birth, etc.); and for patients who struggle with financial or logistical barriers to in-person care. Although obstetrics may have lagged behind other subspecialties in revising its traditional health care models, the pandemic has opened up a new world of possibilities of remote and in-home care for this field. ●

For much of the general public, in-home care from a physician is akin to the rotary telephone: a feature of a bygone age, long since replaced by vastly different systems. While approximately 40% of physician-patient interactions in 1930 were house calls, by the early 1980s this had dwindled to less than 1%,1 with almost all physician-patient encounters taking place in a clinical setting, whether in a hospital or in a free-standing clinic. In the last 2 decades, a smattering of primary care and medical subspecialty clinicians started to incorporate some in-home care into their practices in the form of telemedicine, using video and telephone technology to facilitate care outside of the clinical setting, and by 2016, approximately 15% of physicians reported using some form of telemedicine in their interactions with patients.2
Despite these advances, prior to the COVID-19 pandemic, obstetricians lagged significantly behind in their use of at-home or remote care. Although there were some efforts to promote a hybrid care model that incorporated prenatal telemedicine,3 pre-pandemic ObGyn was one of the least likely fields to offer telemedicine to their patients, with only 9% of practices offering such services.2 In this article, we discuss how the COVID-19 pandemic resulted in a shift from traditional, in-person care to a hybrid remote model and how this may benefit obstetrics patients as well as clinicians.
Pre-pandemic patient management
The traditional model of prenatal care presents a particularly intense time period for patients in terms of its demands. Women who are pregnant and start care in their first trimester typically have 12 to 14 visits during the subsequent 6 to 7 months, with additional visits for those with high-risk pregnancies. Although some of these visits coincide with the need for in-person laboratory work or imaging, many are chiefly oriented around assessment of vital signs or counseling. These frequent prenatal visits represent a significant commitment from patients in terms of transportation, time off work, and childcare resources—all of which may be exacerbated for patients who need to receive their care from overbooked, high-risk specialists.
After delivery, attending an in-person postpartum visit with a newborn can be even more daunting. Despite the increased recognition from professional groups of the importance of postpartum care to support breastfeeding, physical recovery, and mental health, as many as 40% of recently delivered patients do not attend their scheduled postpartum visit(s).4 Still, before 2020, few obstetricians had revised their workflows to “meet patients where they are,” with many continuing to only offer in-person care and assessments.
COVID-19: An impetus for change
As with so many things, the COVID-19 pandemic has challenged our ideas of what is normal. In a sense, the pandemic has catalyzed a revolution in the prenatal care model. The very real risks of exposure and contagion during the pandemic—for clinicians and patients alike—has forced ObGyns to reexamine the actual risks and benefits of in-person and in-clinic prenatal care. As a result, many ObGyns have rapidly adopted telemedicine into practices that were strictly in-person. For example, a national survey of 172 clinicians who offered contraception counseling during the pandemic found that 91% of them were now offering telemedicine services, with 78% of those clinicians new to telemedicine.5 Similarly, although a minority of surveyed obstetricians in New York City reported using telemedicine pre-pandemic, 89% planned to continue using such technology in the future.6
Continue to: Incorporating mobile technology...
Incorporating mobile technology
Obstetricians, forced to consolidate and maximize their in-person care to protect their patients’ safety, have started to realize that many of the conversations and counseling offered to patients can be managed equally effectively with telemedicine. Furthermore, basic home monitoring devices, such as blood pressure machines, can be safely and accurately used by patients without requiring them to come to the office.
More recent research into mobile medical devices suggests that patients can safely and appropriately manage more complex tools. One such example is a mobile, self-operated, ultrasound transducer that is controlled through a smartphone (Instinct, Pulsenmore Ltd). This device was evaluated in an observational, noninterventional trial of 100 women carrying a singleton fetus at 14/0 weeks’ to 39/6 weeks’ gestation. Patients performed 1,360 self-scans, which were reviewed by a clinician in real time online or subsequently off-line. Results showed successful detection rates of 95.3% for fetal heart activity, 88.3% for body movements, 69.4% for tone, 23.8% for breathing movements, and 92.2% for normal amniotic fluid volume.7 The authors concluded that this represents a feasible solution for remote sonographic fetal assessment.
Coordinating care with health care extenders
Remote monitoring options allow patients to be safely monitored during their pregnancies while remaining at home more often, especially when used in conjunction with trained health care extenders such as registered nurses, primary care associates, or “maternity navigators” who can facilitate off-site care. In fact, many aspects of prenatal care are particularly amenable to remote medicine or non–physician-based home care. Different variations of this model of “hybrid” prenatal care may be appropriate depending upon the needs of the patient population served by a given obstetrics practice. Ideally, a prenatal care model personalizes care based on the known risk factors that are identified at the beginning of prenatal care, the anticipated barriers to care, and the patient’s own preferences. As a result, alternatives to the traditional model may be to alternate in-person and telemedicine visits,3,8 to incorporate in-person or remote group prenatal visits,9,10 or to incorporate staff with basic health care skills to serve as health care extenders in the community and provide home visits for basic monitoring, laboratory work, and patient education.11
Benefits of hybrid prenatal models
As we look ahead to the end of the pandemic, how should obstetricians view these hybrid prenatal care models? Are these models safe for patients? Were they only worthwhile to minimize infection risk, or do they have potential benefits for patients going forward?
In fact, data on the use of telemedicine in prenatal care indicate that these models may be equally as safe as the traditional model in terms of clinical outcomes and may have important additional benefits with regard to patient convenience and access to and satisfaction with care. Even audio-only prenatal televisits have been found to be equivalent to in-person visits in terms of serious perinatal outcomes.12 Common pregnancy diagnoses are also well-served by telemedicine. For example, several recent investigations of patients with gestational diabetes have found that telemedicine was as effective as standard care for glucose control.13,14 Management of hypertension during pregnancy, another antenatal condition that is commonly managed with frequent in-person check-ups, also was found to be adequately feasible with telemedicine using home monitors and symptom checklists, with high rates of patient satisfaction.15
With good evidence for safety, the added potential for patients to benefit in such hybrid models is multifactorial. For one, despite our collective hopes, the COVID-19 pandemic may have a long tail. Vaccine hesitancy and COVID-19 variants may mean that clinicians will have to consider the real threat of infection risk in the clinic setting for years to come. In-home prenatal care also provides a wide variety of social, economic, and psychological benefits for pregnant women across various patient populations. The pandemic has introduced many patients to the full potential of working and meeting remotely; pregnant patients are becoming more familiar with these technology platforms and appreciate its incorporation into their busy lives.5 Furthermore, hybrid models actually can provide otherwise “nonadherent” patients with better access to care. From the patient perspective, an in-person 15-minute health care provider visit actually represents a significant commitment of time and resources (ie, hours spent on public transportation, lost wages for those with inflexible work schedules, and childcare costs for patients discouraged from bringing their children to prenatal visits). Especially for patients with fewer socioeconomic resources, these barriers to in-person clinic visits may be daunting, if not insurmountable; the option of remote visits or house calls reduces these barriers and facilitates care.16
Such hybrid models benefit prenatal clinicians as well. In addition to a decreased risk of infection, clinicians may be able to attract a wider potential prenatal patient population with telemedicine by appealing to younger and potentially more technology-savvy patients.17 Importantly, telemedicine is increasingly recognized as on par with in-person visits in many billing algorithms. Changes during the pandemic led Medicare to cover telemedicine visits as well as in-person visits18,19; among other groundbreaking changes, new patients can have an initial billable visit via telemedicine. Although the billing landscape will likely continue to evolve, such changes allow clinicians to focus on patient safety and convenience without financial risk to their practices.
The future of prenatal appointment scheduling
The future of prenatal care certainly doesn’t look like a dozen 15-minute visits in a private physician’s office. While these emerging hybrid models of prenatal care certainly can benefit patients with low-risk uncomplicated pregnancies, they are already being adopted by clinicians who care for patients with antenatal complications that require specialist consultation; for those with conditions that require frequent, low-complexity check-ins (gestational diabetes, chronic hypertension, history of pre-term birth, etc.); and for patients who struggle with financial or logistical barriers to in-person care. Although obstetrics may have lagged behind other subspecialties in revising its traditional health care models, the pandemic has opened up a new world of possibilities of remote and in-home care for this field. ●
- Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34. doi:10.1016/j.cger.2008.10.005.
- Kane CK, Gillis K. The use of telemedicine by physicians: still the exception rather than the rule. Health Aff (Millwood). 2018;37:1923-1930. doi:10.1377/hlthaff.2018.05077.
- Weigel G, Frederiksen B, Ranji U. Telemedicine and pregnancy care. Kaiser Family Foundation website. https://www.kff.org/womens-health-policy/issue-brief/telemedicine-and-pregnancy-care. Accessed August 23, 2021.
- ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi:10.1097/AOG.0000000000002633.
- Stifani BM, Avila K, Levi EE. Telemedicine for contraceptive counseling: an exploratory survey of US family planning providers following rapid adoption of services during the COVID-19 pandemic. Contraception. 2021;103:157-162. doi:10.1016/j.contraception.2020.11.006.
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during the COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939.
- Hadar E, Wolff L, Tenenbaum-Gavish K, et al. Mobile self-operated home ultrasound system for remote fetal assessment during pregnancy. Telemed J E Health. 2021. doi:10.1089/tmj.2020.0541.
- Thomas Jefferson University Division of Maternal Fetal Medicine. Jefferson Maternal Fetal Medicine COVID19 Preparedness. Version 2.1. March 19, 2020. https://communities.smfm.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=a109df77-74fe-462b-87fb-895d6ee7d0e6. Accessed August 23, 2021.
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.AOG.0000275284.24298.23.
- Wicklund M. Oakland launches telehealth program for Black prenatal, postpartum care. Telehealth News. https://mhealthintelligence.com/news/oakland-launches-telehealth-program-for-black-prenatal-postpartum-care. Accessed August 23, 2021.
- Home-based pregnancy care. CayabaCare website. https://www.cayabacare.com. Accessed August 23, 2021.
- Duryea EL, Adhikari EH, Ambia A, et al. Comparison between in-person and audio-only virtual prenatal visits and perinatal outcomes. JAMA Netw Open. 2021;4:e215854. doi:10.1001/jamanetworkopen.2021.5854.
- Ming WK, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e290. doi:10.2196/jmir.6556.
- Tian Y, Zhang S, Huang F, et al. Comparing the efficacies of telemedicine and standard prenatal care on blood glucose control in women with gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9:e22881. doi:10.2196/22881.
- van den Heuvel JFM, Kariman SS, van Solinge WW, et al. SAFE@HOME – feasibility study of a telemonitoring platform combining blood pressure and preeclampsia symptoms in pregnancy care. Eur J Obstet Gynecol Reprod Biol. 2019;240:226-231. doi:10.1016/j.ejogrb.2019.07.012.
- Dixon-Shambley K, Gabbe PT. Using telehealth approaches to address social determinants of health and improve pregnancy and postpartum outcomes. Clin Obstet Gynecol. 2021;64:333-344. doi:10.1097/GRF.0000000000000611.
- Eruchalu CN, Pichardo MS, Bharadwaj M, et al. The expanding digital divide: digital health access inequities during the COVID-19 pandemic in New York City. J Urban Health. 2021;98:183-186. doi:10.1007/s11524-020-00508-9.
- COVID-19 FAQs for obstetrician-gynecologists, telehealth. The American College of Obstetricians and Gynecologists website. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-telehealth. Accessed August 23, 2021.
- Managing patients remotely: billing for digital and telehealth services. The American College of Obstetricians and Gynecologists website. Updated October 19, 2020. https://www.acog.org/practice-management/coding/coding-library/managing-patients-remotely-billing-for-digital-and-telehealth-services. Accessed August 23, 2021.
- Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34. doi:10.1016/j.cger.2008.10.005.
- Kane CK, Gillis K. The use of telemedicine by physicians: still the exception rather than the rule. Health Aff (Millwood). 2018;37:1923-1930. doi:10.1377/hlthaff.2018.05077.
- Weigel G, Frederiksen B, Ranji U. Telemedicine and pregnancy care. Kaiser Family Foundation website. https://www.kff.org/womens-health-policy/issue-brief/telemedicine-and-pregnancy-care. Accessed August 23, 2021.
- ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi:10.1097/AOG.0000000000002633.
- Stifani BM, Avila K, Levi EE. Telemedicine for contraceptive counseling: an exploratory survey of US family planning providers following rapid adoption of services during the COVID-19 pandemic. Contraception. 2021;103:157-162. doi:10.1016/j.contraception.2020.11.006.
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during the COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939.
- Hadar E, Wolff L, Tenenbaum-Gavish K, et al. Mobile self-operated home ultrasound system for remote fetal assessment during pregnancy. Telemed J E Health. 2021. doi:10.1089/tmj.2020.0541.
- Thomas Jefferson University Division of Maternal Fetal Medicine. Jefferson Maternal Fetal Medicine COVID19 Preparedness. Version 2.1. March 19, 2020. https://communities.smfm.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=a109df77-74fe-462b-87fb-895d6ee7d0e6. Accessed August 23, 2021.
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.AOG.0000275284.24298.23.
- Wicklund M. Oakland launches telehealth program for Black prenatal, postpartum care. Telehealth News. https://mhealthintelligence.com/news/oakland-launches-telehealth-program-for-black-prenatal-postpartum-care. Accessed August 23, 2021.
- Home-based pregnancy care. CayabaCare website. https://www.cayabacare.com. Accessed August 23, 2021.
- Duryea EL, Adhikari EH, Ambia A, et al. Comparison between in-person and audio-only virtual prenatal visits and perinatal outcomes. JAMA Netw Open. 2021;4:e215854. doi:10.1001/jamanetworkopen.2021.5854.
- Ming WK, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e290. doi:10.2196/jmir.6556.
- Tian Y, Zhang S, Huang F, et al. Comparing the efficacies of telemedicine and standard prenatal care on blood glucose control in women with gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9:e22881. doi:10.2196/22881.
- van den Heuvel JFM, Kariman SS, van Solinge WW, et al. SAFE@HOME – feasibility study of a telemonitoring platform combining blood pressure and preeclampsia symptoms in pregnancy care. Eur J Obstet Gynecol Reprod Biol. 2019;240:226-231. doi:10.1016/j.ejogrb.2019.07.012.
- Dixon-Shambley K, Gabbe PT. Using telehealth approaches to address social determinants of health and improve pregnancy and postpartum outcomes. Clin Obstet Gynecol. 2021;64:333-344. doi:10.1097/GRF.0000000000000611.
- Eruchalu CN, Pichardo MS, Bharadwaj M, et al. The expanding digital divide: digital health access inequities during the COVID-19 pandemic in New York City. J Urban Health. 2021;98:183-186. doi:10.1007/s11524-020-00508-9.
- COVID-19 FAQs for obstetrician-gynecologists, telehealth. The American College of Obstetricians and Gynecologists website. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-telehealth. Accessed August 23, 2021.
- Managing patients remotely: billing for digital and telehealth services. The American College of Obstetricians and Gynecologists website. Updated October 19, 2020. https://www.acog.org/practice-management/coding/coding-library/managing-patients-remotely-billing-for-digital-and-telehealth-services. Accessed August 23, 2021.
Time to retire race- and ethnicity-based carrier screening
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.
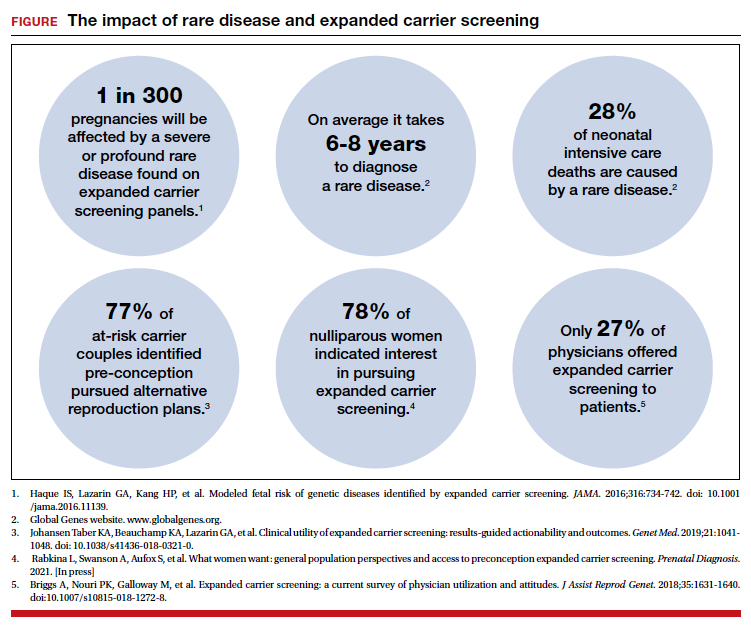
Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.

Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
The social reckoning of 2020 has led to many discussions and conversations around equity and disparities. With the COVID-19 pandemic, there has been a particular spotlight on health care disparities and race-based medicine. Racism in medicine is pervasive; little has been done over the years to dismantle and unlearn practices that continue to contribute to existing gaps and disparities. Race and ethnicity are both social constructs that have long been used within medical practice and in dictating the type of care an individual receives. Without a universal definition, race, ethnicity, and ancestry have long been used interchangeably within medicine and society. Appreciating that race and ethnicity-based constructs can have other social implications in health care, with their impact on structural racism beyond health care settings, these constructs may still be part of assessments and key modifiers to understanding health differences. It is imperative that medical providers examine the use of race and ethnicity within the care that they provide.
While racial determinants of health cannot be removed from historical access, utilization, and barriers related to reproductive care, guidelines structured around historical ethnicity and race further restrict universal access to carrier screening and informed reproductive testing decisions.
Carrier screening
The goal of preconception and prenatal carrier screening is to provide individuals and reproductive partners with information to optimize pregnancy outcomes based on personal values and preferences.1 The practice of carrier screening began almost half a century ago with screening for individual conditions seen more frequently in certain populations, such as Tay-Sachs disease in those of Ashkenazi Jewish descent and sickle cell disease in those of African descent. Cystic fibrosis carrier screening was first recommended for individuals of Northern European descent in 2001 before being recommended for pan ethnic screening a decade later. Other individual conditions are also recommended for screening based on race/ethnicity (eg, Canavan disease in the Ashkenazi Jewish population, Tay-Sachs disease in individuals of Cajun or French-Canadian descent).2-4 Practice guidelines from professional societies recommend offering carrier screening for individual conditions based on condition severity, race or ethnicity, prevalence, carrier frequency, detection rates, and residual risk.1 However, this process can be problematic, as the data frequently used in updating guidelines and recommendations come primarily from studies and databases where much of the cohort is White.5,6 Failing to identify genetic associations in diverse populations limits the ability to illuminate new discoveries that inform risk management and treatment, especially for populations that are disproportionately underserved in medicine.7
Need for expanded carrier screening
The evolution of genomics and technology within the realm of carrier screening has enabled the simultaneous screening for many serious Mendelian diseases, known as expanded carrier screening (ECS). A 2016 study illustrated that, in most racial/ethnic categories, the cumulative risk of severe and profound conditions found on ECS panels outside the guideline recommendations are greater than the risk identified by guideline-based panels.8 Additionally, a 2020 study showed that self-reported ethnicity was an imperfect indicator of genetic ancestry, with 9% of those in the cohort having a >50% genetic ancestry from a lineage inconsistent with their self-reported ethnicity.9 Data over the past decade have established the clinical utility,10 clinical validity,11 analytical validity,12 and cost-effectiveness13 of pan-ethnic ECS. In 2021, American College of Medical Genetics and Genomics (ACMG) recommended a panel of pan-ethnic conditions that should be offered to all patients due to smaller ethnicity-based panels failing to provide equitable evaluation of all racial and ethnic groups.14 The guidelines from the American College of Obstetricians and Gynecologists (ACOG) fall short of recommending that ECS be offered to all individuals in lieu of screening based on self-reported ethnicity.3,4
Phasing out ethnicity-based carrier screening
This begs the question: Do race, ethnicity, or ancestry have a role in carrier screening? While each may have had a role at the inception of offering carrier screening due to high costs of technology, recent studies have shown the limitations of using self-reported ethnicity in screening. Guideline-based carrier screenings miss a significant percentage of pregnancies (13% to 94%) affected by serious conditions on expanded carrier screening panels.8 Additionally, 40% of Americans cannot identify the ethnicity of all 4 grandparents.15
Founder mutations due to ancestry patterns are still present; however, stratification of care should only be pursued when the presence or absence of these markers would alter clinical management. While the reproductive risk an individual may receive varies based on their self-reported ethnicity, the clinically indicated follow-up testing is the same: offering carrier screening for the reproductive partner or gamete donor. With increased detection rates via sequencing for most autosomal recessive conditions, if the reproductive partner or gamete donor is not identified as a carrier, no further testing is generally indicated regardless of ancestry. Genotyping platforms should not be used for partner carrier screening as they primarily target common pathogenic variants based on dominant ancestry groups and do not provide the same risk reduction.

Continue to: Variant reporting...
Variant reporting
We have long known that databases and registries in the United States have an increased representation of individuals from European ancestries.5,6 However, there have been limited conversations about how the lack of representation within our databases and registries leads to inequities in guidelines and the care that we provide to patients. As a result, studies have shown higher rates of variants of uncertain significance (VUS) identified during genetic testing in non-White individuals than in Whites.16 When it comes to reporting of variants, carrier screening laboratories follow guidelines set forth by the ACMG, and most laboratories only report likely pathogenic or pathogenic variants.17 It is unknown how the higher rate of VUSs in the non-White population, and lack of data and representation in databases and software used to calculate predicted phenotype, impacts identification of at-risk carrier couples in these underrepresented populations. It is imperative that we increase knowledge and representation of variants across ethnicities to improve sensitivity and specificity across the population and not just for those of European descent.
Moving forward
Being aware of social- and race-based biases in carrier screening is important, but modifying structural systems to increase representation, access, and utility of carrier screening is a critical next step. Organizations like ACOG and ACMG have committed not only to understanding but also to addressing factors that have led to disparities and inequities in health care delivery and access.18,19 Actionable steps include offering a universal carrier screening program to all preconception and prenatal patients that addresses conditions with increased carrier frequency, in any population, defined as severe and moderate phenotype with established natural history.3,4 Educational materials should be provided to detail risks, benefits, and limitations of carrier screening, as well as shared decision making between patient and provider to align the patient’s wishes for the information provided by carrier screening.
A broader number of conditions offered through carrier screening will increase the likelihood of positive carrier results. The increase in carriers identified should be viewed as more accurate reproductive risk assessment in the context of equitable care, rather than justification for panels to be limited to specific ancestries. Simultaneous or tandem reproductive partner or donor testing can be considered to reduce clinical workload and time for results return.
In addition, increased representation of individuals who are from diverse ancestries in promotional and educational resources can reinforce that risk for Mendelian conditions is not specific to single ancestries or for targeted conditions. Future research should be conducted to examine the role of racial disparities related to carrier screening and greater inclusion and recruitment of diverse populations in data sets and research studies.
Learned biases toward race, religion, gender identity, sexual orientation, and economic status in the context of carrier screening should be examined and challenged to increase access for all patients who may benefit from this testing. For example, the use of gendered language within carrier screening guidelines and policies and how such screening is offered to patients should be examined. Guidelines do not specify what to do when someone is adopted, for instance, or does not know their ethnicity. It is important that, as genomic testing becomes more available, individuals and groups are not left behind and existing gaps are not further widened. Assessing for genetic variation that modifies for disease or treatment will be more powerful than stratifying based on race. Carrier screening panels should be comprehensive regardless of ancestry to ensure coverage for global genetic variation and to increase access for all patients to risk assessments that promote informed reproductive decision making.
Health equity requires unlearning certain behaviors
As clinicians we all have a commitment to educate and empower one another to offer care that helps promote health equity. Equitable care requires us to look at the current gaps and figure out what programs and initiatives need to be designed to address those gaps. Carrier screening is one such area in which we can work together to improve the overall care that our patients receive, but it is imperative that we examine our practices and unlearn behaviors that contribute to existing disparities. ●
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
- Edwards JG, Feldman G, Goldberg J, et al. Expanded carrier screening in reproductive medicine—points to consider: a joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet Gynecol. 2015;125:653-662. doi: 10.1097 /AOG.0000000000000666.
- Grody WW, Thompson BH, Gregg AR, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15:482-483. doi: 10.1038/gim.2013.47.
- Committee Opinion No. 690. Summary: carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129: 595-596. doi: 10.1097/AOG.0000000000001947.
- Committee Opinion No. 691. Carrier screening for genetic conditions. Obstet Gynecol. 2017;129:e41-e55. doi: 10.1097 /AOG.0000000000001952.
- Need AC, Goldstein DB. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489-494. doi: 10.1016/j.tig.2009.09.012.
- Popejoy A, Fullerton S. Genomics is failing on diversity. Nature. 2016;538;161-164. doi: 10.1038/538161a.
- Ewing A. Reimagining health equity in genetic testing. Medpage Today. June 17, 2021. https://www.medpagetoday.com /opinion/second-opinions/93173. Accessed October 27, 2021.
- Haque IS, Lazarin GA, Kang HP, et al. Modeled fetal risk of genetic diseases identified by expanded carrier screening. JAMA. 2016;316:734-742. doi: 10.1001/jama.2016.11139.
- Kaseniit KE, Haque IS, Goldberg JD, et al. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet Med. 2020;22:1694-1702. doi: 10 .1038/s41436-020-0869-3.
- Johansen Taber KA, Beauchamp KA, Lazarin GA, et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet Med. 2019;21:1041-1048. doi: 10.1038/s41436-018-0321-0.
- Balzotti M, Meng L, Muzzey D, et al. Clinical validity of expanded carrier screening: Evaluating the gene-disease relationship in more than 200 conditions. Hum Mutat. 2020;41:1365-1371. doi: 10.1002/humu.24033.
- Hogan GJ, Vysotskaia VS, Beauchamp KA, et al. Validation of an expanded carrier screen that optimizes sensitivity via full-exon sequencing and panel-wide copy number variant identification. Clin Chem. 2018;64:1063-1073. doi: 10.1373 /clinchem.2018.286823.
- Beauchamp KA, Johansen Taber KA, Muzzey D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet Med. 2019;21:1948-1957. doi: 10.1038/s41436-019-0455-8.
- Gregg AR, Aarabi M, Klugman S, et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: a practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1793-1806. doi: 10.1038/s41436-021-01203-z.
- Condit C, Templeton A, Bates BR, et al. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet Med. 2003;5:385-392. doi: 10 .1097/01.gim.0000087990.30961.72.
- Caswell-Jin J, Gupta T, Hall E, et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet Med. 2018;20:234-239.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. doi:10.1038/gim.2015.30.
- Gregg AR. Message from ACMG President: overcoming disparities. Genet Med. 2020;22:1758.
3D vs 2D mammography for detecting cancer in dense breasts
Text copyright DenseBreast-info.org.
Answer
C. Overall, tomosynthesis depicts an additional 1 to 2 cancers per thousand women screened in the first round of screening when added to standard digital mammography;1-3 however, this improvement in cancer detection is only observed in women with fatty breasts (category A), scattered fibroglandular tissue (category B), and heterogeneously dense breasts (category C). Importantly, tomosynthesis does not significantly improve breast cancer detection in women with extremely dense breasts (category D).2,4
Digital breast tomosynthesis, also referred to as “3-dimensional mammography” (3D mammography) or tomosynthesis, uses a dedicated electronic detector system to obtain multiple projection images that are reconstructed by the computer to create thin slices or slabs of multiple slices of the breast. These slices can be individually “scrolled through” by the radiologist to reduce tissue overlap that may obscure breast cancers on a standard mammogram. While tomosynthesis improves breast cancer detection in women with fatty, scattered fibroglandular density, and heterogeneously dense breasts, there is very little soft tissue contrast in extremely dense breasts due to insufficient fat, and some cancers will remain hidden by dense tissue even on sliced images through the breast.
FIGURE 2 shows an example of cancer that was missed on tomosynthesis in a 51-year-old woman with extremely dense breasts and right breast pain. The cancer was masked by extremely dense tissue on standard digital mammography and tomosynthesis; no abnormalities were detected. Ultrasonography showed a 1.6-cm, irregular, hypoechoic mass at the site of pain, and biopsy revealed a grade 3 triple-receptor negative invasive ductal carcinoma.
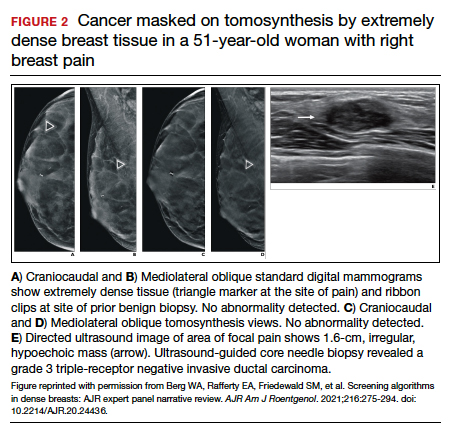
In women with dense breasts, especially extremely dense breasts, supplemental screening beyond tomosynthesis should be considered. Although tomosynthesis doesn’t improve cancer detection in extremely dense breasts, it does reduce callbacks for additional testing in all breast densities compared with standard digital mammography. Callbacks are reduced from approximately 100‒120 per 1,000 women screened with standard digital mammography alone1,5 to an average of 80 per 1,000 women when tomosynthesis and standard mammography are interpreted together.1-3 ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Conant EF, Zuckerman SP, McDonald ES, et al. Five consecutive years of screening with digital breast tomosynthesis: outcomes by screening year and round. Radiology. 2020;295:285-293.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786.
- Skaane P, Bandos AI, Niklason LT, et al. Digital mammography versus digital mammography plus tomosynthesis in breast cancer screening: the Oslo Tomosynthesis Screening Trial. Radiology. 2019;291:23-30.
- Lowry KP, Coley RY, Miglioretti DL, et al. Screening performance of digital breast tomosynthesis vs digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw Open. 2020;3:e2011792.
- Lee CS, Sengupta D, Bhargavan-Chatfield M, et al. Association of patient age with outcomes of current-era, large-scale screening mammography: analysis of data from the National Mammography Database. JAMA Oncol. 2017;3:1134-1136.
Text copyright DenseBreast-info.org.
Answer
C. Overall, tomosynthesis depicts an additional 1 to 2 cancers per thousand women screened in the first round of screening when added to standard digital mammography;1-3 however, this improvement in cancer detection is only observed in women with fatty breasts (category A), scattered fibroglandular tissue (category B), and heterogeneously dense breasts (category C). Importantly, tomosynthesis does not significantly improve breast cancer detection in women with extremely dense breasts (category D).2,4
Digital breast tomosynthesis, also referred to as “3-dimensional mammography” (3D mammography) or tomosynthesis, uses a dedicated electronic detector system to obtain multiple projection images that are reconstructed by the computer to create thin slices or slabs of multiple slices of the breast. These slices can be individually “scrolled through” by the radiologist to reduce tissue overlap that may obscure breast cancers on a standard mammogram. While tomosynthesis improves breast cancer detection in women with fatty, scattered fibroglandular density, and heterogeneously dense breasts, there is very little soft tissue contrast in extremely dense breasts due to insufficient fat, and some cancers will remain hidden by dense tissue even on sliced images through the breast.
FIGURE 2 shows an example of cancer that was missed on tomosynthesis in a 51-year-old woman with extremely dense breasts and right breast pain. The cancer was masked by extremely dense tissue on standard digital mammography and tomosynthesis; no abnormalities were detected. Ultrasonography showed a 1.6-cm, irregular, hypoechoic mass at the site of pain, and biopsy revealed a grade 3 triple-receptor negative invasive ductal carcinoma.

In women with dense breasts, especially extremely dense breasts, supplemental screening beyond tomosynthesis should be considered. Although tomosynthesis doesn’t improve cancer detection in extremely dense breasts, it does reduce callbacks for additional testing in all breast densities compared with standard digital mammography. Callbacks are reduced from approximately 100‒120 per 1,000 women screened with standard digital mammography alone1,5 to an average of 80 per 1,000 women when tomosynthesis and standard mammography are interpreted together.1-3 ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Text copyright DenseBreast-info.org.
Answer
C. Overall, tomosynthesis depicts an additional 1 to 2 cancers per thousand women screened in the first round of screening when added to standard digital mammography;1-3 however, this improvement in cancer detection is only observed in women with fatty breasts (category A), scattered fibroglandular tissue (category B), and heterogeneously dense breasts (category C). Importantly, tomosynthesis does not significantly improve breast cancer detection in women with extremely dense breasts (category D).2,4
Digital breast tomosynthesis, also referred to as “3-dimensional mammography” (3D mammography) or tomosynthesis, uses a dedicated electronic detector system to obtain multiple projection images that are reconstructed by the computer to create thin slices or slabs of multiple slices of the breast. These slices can be individually “scrolled through” by the radiologist to reduce tissue overlap that may obscure breast cancers on a standard mammogram. While tomosynthesis improves breast cancer detection in women with fatty, scattered fibroglandular density, and heterogeneously dense breasts, there is very little soft tissue contrast in extremely dense breasts due to insufficient fat, and some cancers will remain hidden by dense tissue even on sliced images through the breast.
FIGURE 2 shows an example of cancer that was missed on tomosynthesis in a 51-year-old woman with extremely dense breasts and right breast pain. The cancer was masked by extremely dense tissue on standard digital mammography and tomosynthesis; no abnormalities were detected. Ultrasonography showed a 1.6-cm, irregular, hypoechoic mass at the site of pain, and biopsy revealed a grade 3 triple-receptor negative invasive ductal carcinoma.

In women with dense breasts, especially extremely dense breasts, supplemental screening beyond tomosynthesis should be considered. Although tomosynthesis doesn’t improve cancer detection in extremely dense breasts, it does reduce callbacks for additional testing in all breast densities compared with standard digital mammography. Callbacks are reduced from approximately 100‒120 per 1,000 women screened with standard digital mammography alone1,5 to an average of 80 per 1,000 women when tomosynthesis and standard mammography are interpreted together.1-3 ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Conant EF, Zuckerman SP, McDonald ES, et al. Five consecutive years of screening with digital breast tomosynthesis: outcomes by screening year and round. Radiology. 2020;295:285-293.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786.
- Skaane P, Bandos AI, Niklason LT, et al. Digital mammography versus digital mammography plus tomosynthesis in breast cancer screening: the Oslo Tomosynthesis Screening Trial. Radiology. 2019;291:23-30.
- Lowry KP, Coley RY, Miglioretti DL, et al. Screening performance of digital breast tomosynthesis vs digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw Open. 2020;3:e2011792.
- Lee CS, Sengupta D, Bhargavan-Chatfield M, et al. Association of patient age with outcomes of current-era, large-scale screening mammography: analysis of data from the National Mammography Database. JAMA Oncol. 2017;3:1134-1136.
- Conant EF, Zuckerman SP, McDonald ES, et al. Five consecutive years of screening with digital breast tomosynthesis: outcomes by screening year and round. Radiology. 2020;295:285-293.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786.
- Skaane P, Bandos AI, Niklason LT, et al. Digital mammography versus digital mammography plus tomosynthesis in breast cancer screening: the Oslo Tomosynthesis Screening Trial. Radiology. 2019;291:23-30.
- Lowry KP, Coley RY, Miglioretti DL, et al. Screening performance of digital breast tomosynthesis vs digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw Open. 2020;3:e2011792.
- Lee CS, Sengupta D, Bhargavan-Chatfield M, et al. Association of patient age with outcomes of current-era, large-scale screening mammography: analysis of data from the National Mammography Database. JAMA Oncol. 2017;3:1134-1136.
Quiz developed in collaboration with 
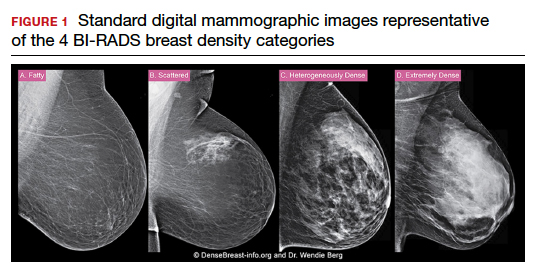
My patient is having an affair and has an STI. I’m treating both partners. What would you do?
A psychiatrist was treating a couple individually, one of whom was HIV-positive. During a session, the infected partner revealed he was having sex with other men outside the relationship and not using safe sex practices.
“He was being treated for major depression and anxiety at the time,” explained the anonymous psychiatrist. “I strongly encouraged him to tell his partner, but he was scared of doing so. He stated that they had not been using safe sex practices between the two of them, but he was willing to start at that point.”
At a session with the HIV-negative partner, the psychiatrist inquired about the couple’s current sex practices. The HIV-negative partner reported no changes and said the two continued to have sex without condoms, said the psychiatrist, who shared the experience in Medscape’s Ethics 2020 Survey open-ended questions.
“My dilemma now was whether or not to inform him about his partner’s ‘extracurricular sex behavior,’ the psychiatrist said. “Since he was now at greater risk of contracting HIV, I felt compelled to do something to intervene.”
What would you do in this situation?
according to responses from the Ethics 2020 Report. When asked to share their toughest ethical dilemma, one internist for example, wrote, “I have couples as patients, and it is very challenging if they reveal infidelity or separate/divorce; I cannot reveal info to the spouse, but it makes me very uncomfortable caring for both.” Similarly, an obstetrician-gynecologist wrote about her experience counseling patients who reveal extramarital affairs.
“Women confide deeply with their gynecologist, and although I was not successful in rescuing 100% of them, the majority accepted my counseling and saved their marriages,” the anonymous ob/gyn wrote. “In every case in which my patient was willing to resume her marital relationship, I always ensured that she advised her spouse of the infidelity, and the couple was referred to a qualified provider for marriage counseling.”
When a sexually transmitted infection (STI) comes into play however, physicians describe a deeper level of internal conflict. A family physician wrote her top ethical dilemma was “Cheating spouses and STIs: how do you get the other spouse treated?” An ob-gyn stated that, “disclosure of STI status in couples when this may indicate infidelity,” was a frequent ethical issue in her specialty. Commenters on Medscape’s recent story, “The Secret I’ll Take to my Grave: Doc Reveals,” also raised the uncomfortable topic. One physician recalled a deaf female patient who requested in writing not to test for syphilis and not to discuss the issue with her husband. “Patient knew that she had syphilis, but she did not want her husband to know,” the physician wrote.
It’s not uncommon for physicians to encounter such scenarios when treating long-term couples, especially in the digital era, said Shannon Dowler, MD, chief medical officer for North Carolina Medicaid and a family physician at the Buncombe County STI Clinic.
“This is definitely something I think we see more of in our age of ‘hookup apps’ and easier access to casual sexual connections than we did before,” said Dr. Dowler, who serves on the CDC Advisory Committee on HIV, Viral Hepatitis, and STD Prevention and Treatment.
The topic is particularly timely because of the pandemic’s impact on STI testing and the expected rise in sexually transmitted infection rates over the next year, Dr. Dowler notes.
“People weren’t necessarily coming in for routine screening or testing during the pandemic because they didn’t want to take a chance on being exposed to COVID,” she said. “But also, the reagent used for testing for certain types of transmitted infections was in short supply because they use that same reagent for the COVID test. We had shortages of STI testing in many parts of the country. I expect what we’re going to see over the next year are a lot of diagnoses that were missed during the pandemic and a lot of asymptomatic spread.”
What do the experts suggest?
Caring for spouses or two partners when an STI is discovered can be challenging for physicians, particularly in small towns where many people know each other, said Kenneth Goodman, PhD, founder and director of the Institute for Bioethics and Health Policy at the University of Miami.
“This can be a real challenge for family physicians and others in a small town,” he said. “If you discover one partner is positive for a sexually transmitted infection and the other is negative, then you’ve got a challenge to manage. The way to do that is to start with moral persuasion, namely you tell your patient, ‘You really need to disclose this. Because when he or she gets it, chances are, you’re going to be the prime suspect.’ “
Dr. Dowler, who practices in an STI clinic, said she once diagnosed a sexually transmitted infection in a patient who was married to one of Dowler’s coworkers. The patient would not allow the partner to be notified, she said. In this case, Dr. Dowler practiced expedited partner therapy (EPT), the clinical practice of treating sex partners of patients diagnosed with chlamydia or gonorrhea by giving the patient prescriptions or medications to take to the partner without having first examined the partner. The practice is legal to some extent in all states, Dr. Dowler said, but some states have different rules about how the practice can be utilized.
Physicians are obligated to report communicable diseases to their local health department, Dr. Goodman said. The health department would then do contract tracing and be responsible for conveying the STI diagnosis to any relevant parties. Even so, Dr. Goodman said physicians have a moral obligation to strongly encourage patients to divulge the infection to their partner.
“Doctors should work on being persuasive to change behavior,” he said. “Tell your patients to do the right thing and follow up with them. You should tell patients they have a responsibility to disclose a sexually transmitted infection to any of their partners and a responsibility not to have unprotected sex. Doctors can be very powerful advocates for that.”
Dr. Dowler said if she is treating two partners, and one is diagnosed with a sexually transmitted infection, she generally asks the patient for their consent to disclose the diagnosis to the partner. She ensures a witness, usually a nurse, is present when she asks. If consent is refused, Dr. Dowler guides her treatment to be as protective as possible, she said. A helpful resource for patients is Tellyourpartner.org, a website that sends an anonymous text or email about infection exposure and provides guidance on treatment locations and options.
Of course, if the sexually transmitted infection is HIV, another set of rules apply. As of 2021, 35 states have laws that criminalize HIV exposure. Laws vary, but many hold patients criminally liable if they knowingly expose another party to HIV. Many states and some cities also have ‘partner notification’ laws that require health providers to disclose an HIV diagnosis to the patient’s sex partners or to report the names of sex partners to the health department, if known.
However, case law on a physician’s duty to warn is mixed, and doctors’ responsibility for STI reporting and partner notification is determined by individual states. Making matters more complex is the fact that some states have recently changed their HIV control requirements, Dr. Dowler said. In North Carolina for example, patients living with HIV who have been virally suppressed for 6 months and who are adherent to medications, are no longer in violation of the control measure if they do not disclose their HIV diagnosis to sex partners or if they don’t wear a condom.
“This means physicians would not have to report a virally suppressed, adequately treated HIV-positive patient who is having unprotected sex or take measures to inform any known sex partners of the diagnosis,” she said. “The landscape is constantly changing so physicians have to be vigilant about their state public health statutes. It’s a tricky area. It takes an already complicated topic and makes it just a little more complicated.”
Consider drafting a policy
It’s a good idea to have a policy in place at your practice that addresses such ethical dilemmas before they occur, says Michael Heitt, PsyD, a clinical psychologist on the faculty of Loyola University Maryland in Baltimore, and a member of the Maryland Psychological Association’s Ethics Committee. Dr. Heitt developed a model of ethical reasoning called CLEAR Lenses, which stands for Clinical, Legal, Ethical, Administrative, and Risk management. The approach encourages clinicians to identify often competing factors in the decision-making process before choosing a course of action to take.
In the situation of an unfaithful spouse who contracted an STI for example, the physician should consider clinical issues such as the medical likelihood the unaware partner has the STI, and legal issues such as maintaining the confidentiality of all patient information and possible mandated reporting of STI data, Dr. Heitt said. The lenses overlap since confidentiality is also a key ethical issue, and other ethical issues involve the balance of helping the unaware spouse and not harming the infected spouse, he explained. Administrative issues might include how medical records are maintained and whether the physician documents information about patients’ family members in the medical record, while risk management elements may include informed consent, documentation, and consultation.
“So, if the physician has a policy about how such matters are dealt with, and patients are informed about this when they come to the practice, this can guide the physician much more easily through this sticky situation,” Dr. Heitt said. “Documentation of the decision-making process in the medical record demonstrates the physician’s thought process should it ever be challenged in the future, and consultation with peers (while disguising the identity of the patients, of course) sets a foundation of what a ‘reasonable standard’ might be in such situations.”
There is also the conflict-avoidant approach, Dr. Heitt said, in which the physician could perform “routine” STI testing if the unaware spouse was due for an appointment soon.
“But of course, this is far from avoiding any conflict; it just kicks the can down the road as there will surely be conflict — and plenty of confusion — if the wife tests positive for an STI,” he said. “In most situations, it is usually best to be brave, do the hard work upfront, and deal with the tough situation then, rather than trying to avoid the probable inevitable difficult conversation.”
As for the psychiatrist who was treating the cheating HIV-positive partner, the physician ultimately convinced both patients to come in for a couple’s session. The doctor allowed for a 2-hour timeframe to encourage discussion of any conflicts and unresolved issues, the psychiatrist said. After several more couple’s sessions, it was apparent the HIV-positive partner wanted out of the relationship, according to the psychiatrist’s account. The physician referred them to a couples’ therapist for ongoing treatment.
“During that same session, the HIV positive partner disclosed his recent behaviors and, as a result, they decided not to have further sexual contact until they could explore this further in therapy,” the psychiatrist wrote. “At last communication the couple decided to end the relationship, and the HIV negative partner remained negative.”
A version of this article first appeared on Medscape.com.
A psychiatrist was treating a couple individually, one of whom was HIV-positive. During a session, the infected partner revealed he was having sex with other men outside the relationship and not using safe sex practices.
“He was being treated for major depression and anxiety at the time,” explained the anonymous psychiatrist. “I strongly encouraged him to tell his partner, but he was scared of doing so. He stated that they had not been using safe sex practices between the two of them, but he was willing to start at that point.”
At a session with the HIV-negative partner, the psychiatrist inquired about the couple’s current sex practices. The HIV-negative partner reported no changes and said the two continued to have sex without condoms, said the psychiatrist, who shared the experience in Medscape’s Ethics 2020 Survey open-ended questions.
“My dilemma now was whether or not to inform him about his partner’s ‘extracurricular sex behavior,’ the psychiatrist said. “Since he was now at greater risk of contracting HIV, I felt compelled to do something to intervene.”
What would you do in this situation?
according to responses from the Ethics 2020 Report. When asked to share their toughest ethical dilemma, one internist for example, wrote, “I have couples as patients, and it is very challenging if they reveal infidelity or separate/divorce; I cannot reveal info to the spouse, but it makes me very uncomfortable caring for both.” Similarly, an obstetrician-gynecologist wrote about her experience counseling patients who reveal extramarital affairs.
“Women confide deeply with their gynecologist, and although I was not successful in rescuing 100% of them, the majority accepted my counseling and saved their marriages,” the anonymous ob/gyn wrote. “In every case in which my patient was willing to resume her marital relationship, I always ensured that she advised her spouse of the infidelity, and the couple was referred to a qualified provider for marriage counseling.”
When a sexually transmitted infection (STI) comes into play however, physicians describe a deeper level of internal conflict. A family physician wrote her top ethical dilemma was “Cheating spouses and STIs: how do you get the other spouse treated?” An ob-gyn stated that, “disclosure of STI status in couples when this may indicate infidelity,” was a frequent ethical issue in her specialty. Commenters on Medscape’s recent story, “The Secret I’ll Take to my Grave: Doc Reveals,” also raised the uncomfortable topic. One physician recalled a deaf female patient who requested in writing not to test for syphilis and not to discuss the issue with her husband. “Patient knew that she had syphilis, but she did not want her husband to know,” the physician wrote.
It’s not uncommon for physicians to encounter such scenarios when treating long-term couples, especially in the digital era, said Shannon Dowler, MD, chief medical officer for North Carolina Medicaid and a family physician at the Buncombe County STI Clinic.
“This is definitely something I think we see more of in our age of ‘hookup apps’ and easier access to casual sexual connections than we did before,” said Dr. Dowler, who serves on the CDC Advisory Committee on HIV, Viral Hepatitis, and STD Prevention and Treatment.
The topic is particularly timely because of the pandemic’s impact on STI testing and the expected rise in sexually transmitted infection rates over the next year, Dr. Dowler notes.
“People weren’t necessarily coming in for routine screening or testing during the pandemic because they didn’t want to take a chance on being exposed to COVID,” she said. “But also, the reagent used for testing for certain types of transmitted infections was in short supply because they use that same reagent for the COVID test. We had shortages of STI testing in many parts of the country. I expect what we’re going to see over the next year are a lot of diagnoses that were missed during the pandemic and a lot of asymptomatic spread.”
What do the experts suggest?
Caring for spouses or two partners when an STI is discovered can be challenging for physicians, particularly in small towns where many people know each other, said Kenneth Goodman, PhD, founder and director of the Institute for Bioethics and Health Policy at the University of Miami.
“This can be a real challenge for family physicians and others in a small town,” he said. “If you discover one partner is positive for a sexually transmitted infection and the other is negative, then you’ve got a challenge to manage. The way to do that is to start with moral persuasion, namely you tell your patient, ‘You really need to disclose this. Because when he or she gets it, chances are, you’re going to be the prime suspect.’ “
Dr. Dowler, who practices in an STI clinic, said she once diagnosed a sexually transmitted infection in a patient who was married to one of Dowler’s coworkers. The patient would not allow the partner to be notified, she said. In this case, Dr. Dowler practiced expedited partner therapy (EPT), the clinical practice of treating sex partners of patients diagnosed with chlamydia or gonorrhea by giving the patient prescriptions or medications to take to the partner without having first examined the partner. The practice is legal to some extent in all states, Dr. Dowler said, but some states have different rules about how the practice can be utilized.
Physicians are obligated to report communicable diseases to their local health department, Dr. Goodman said. The health department would then do contract tracing and be responsible for conveying the STI diagnosis to any relevant parties. Even so, Dr. Goodman said physicians have a moral obligation to strongly encourage patients to divulge the infection to their partner.
“Doctors should work on being persuasive to change behavior,” he said. “Tell your patients to do the right thing and follow up with them. You should tell patients they have a responsibility to disclose a sexually transmitted infection to any of their partners and a responsibility not to have unprotected sex. Doctors can be very powerful advocates for that.”
Dr. Dowler said if she is treating two partners, and one is diagnosed with a sexually transmitted infection, she generally asks the patient for their consent to disclose the diagnosis to the partner. She ensures a witness, usually a nurse, is present when she asks. If consent is refused, Dr. Dowler guides her treatment to be as protective as possible, she said. A helpful resource for patients is Tellyourpartner.org, a website that sends an anonymous text or email about infection exposure and provides guidance on treatment locations and options.
Of course, if the sexually transmitted infection is HIV, another set of rules apply. As of 2021, 35 states have laws that criminalize HIV exposure. Laws vary, but many hold patients criminally liable if they knowingly expose another party to HIV. Many states and some cities also have ‘partner notification’ laws that require health providers to disclose an HIV diagnosis to the patient’s sex partners or to report the names of sex partners to the health department, if known.
However, case law on a physician’s duty to warn is mixed, and doctors’ responsibility for STI reporting and partner notification is determined by individual states. Making matters more complex is the fact that some states have recently changed their HIV control requirements, Dr. Dowler said. In North Carolina for example, patients living with HIV who have been virally suppressed for 6 months and who are adherent to medications, are no longer in violation of the control measure if they do not disclose their HIV diagnosis to sex partners or if they don’t wear a condom.
“This means physicians would not have to report a virally suppressed, adequately treated HIV-positive patient who is having unprotected sex or take measures to inform any known sex partners of the diagnosis,” she said. “The landscape is constantly changing so physicians have to be vigilant about their state public health statutes. It’s a tricky area. It takes an already complicated topic and makes it just a little more complicated.”
Consider drafting a policy
It’s a good idea to have a policy in place at your practice that addresses such ethical dilemmas before they occur, says Michael Heitt, PsyD, a clinical psychologist on the faculty of Loyola University Maryland in Baltimore, and a member of the Maryland Psychological Association’s Ethics Committee. Dr. Heitt developed a model of ethical reasoning called CLEAR Lenses, which stands for Clinical, Legal, Ethical, Administrative, and Risk management. The approach encourages clinicians to identify often competing factors in the decision-making process before choosing a course of action to take.
In the situation of an unfaithful spouse who contracted an STI for example, the physician should consider clinical issues such as the medical likelihood the unaware partner has the STI, and legal issues such as maintaining the confidentiality of all patient information and possible mandated reporting of STI data, Dr. Heitt said. The lenses overlap since confidentiality is also a key ethical issue, and other ethical issues involve the balance of helping the unaware spouse and not harming the infected spouse, he explained. Administrative issues might include how medical records are maintained and whether the physician documents information about patients’ family members in the medical record, while risk management elements may include informed consent, documentation, and consultation.
“So, if the physician has a policy about how such matters are dealt with, and patients are informed about this when they come to the practice, this can guide the physician much more easily through this sticky situation,” Dr. Heitt said. “Documentation of the decision-making process in the medical record demonstrates the physician’s thought process should it ever be challenged in the future, and consultation with peers (while disguising the identity of the patients, of course) sets a foundation of what a ‘reasonable standard’ might be in such situations.”
There is also the conflict-avoidant approach, Dr. Heitt said, in which the physician could perform “routine” STI testing if the unaware spouse was due for an appointment soon.
“But of course, this is far from avoiding any conflict; it just kicks the can down the road as there will surely be conflict — and plenty of confusion — if the wife tests positive for an STI,” he said. “In most situations, it is usually best to be brave, do the hard work upfront, and deal with the tough situation then, rather than trying to avoid the probable inevitable difficult conversation.”
As for the psychiatrist who was treating the cheating HIV-positive partner, the physician ultimately convinced both patients to come in for a couple’s session. The doctor allowed for a 2-hour timeframe to encourage discussion of any conflicts and unresolved issues, the psychiatrist said. After several more couple’s sessions, it was apparent the HIV-positive partner wanted out of the relationship, according to the psychiatrist’s account. The physician referred them to a couples’ therapist for ongoing treatment.
“During that same session, the HIV positive partner disclosed his recent behaviors and, as a result, they decided not to have further sexual contact until they could explore this further in therapy,” the psychiatrist wrote. “At last communication the couple decided to end the relationship, and the HIV negative partner remained negative.”
A version of this article first appeared on Medscape.com.
A psychiatrist was treating a couple individually, one of whom was HIV-positive. During a session, the infected partner revealed he was having sex with other men outside the relationship and not using safe sex practices.
“He was being treated for major depression and anxiety at the time,” explained the anonymous psychiatrist. “I strongly encouraged him to tell his partner, but he was scared of doing so. He stated that they had not been using safe sex practices between the two of them, but he was willing to start at that point.”
At a session with the HIV-negative partner, the psychiatrist inquired about the couple’s current sex practices. The HIV-negative partner reported no changes and said the two continued to have sex without condoms, said the psychiatrist, who shared the experience in Medscape’s Ethics 2020 Survey open-ended questions.
“My dilemma now was whether or not to inform him about his partner’s ‘extracurricular sex behavior,’ the psychiatrist said. “Since he was now at greater risk of contracting HIV, I felt compelled to do something to intervene.”
What would you do in this situation?
according to responses from the Ethics 2020 Report. When asked to share their toughest ethical dilemma, one internist for example, wrote, “I have couples as patients, and it is very challenging if they reveal infidelity or separate/divorce; I cannot reveal info to the spouse, but it makes me very uncomfortable caring for both.” Similarly, an obstetrician-gynecologist wrote about her experience counseling patients who reveal extramarital affairs.
“Women confide deeply with their gynecologist, and although I was not successful in rescuing 100% of them, the majority accepted my counseling and saved their marriages,” the anonymous ob/gyn wrote. “In every case in which my patient was willing to resume her marital relationship, I always ensured that she advised her spouse of the infidelity, and the couple was referred to a qualified provider for marriage counseling.”
When a sexually transmitted infection (STI) comes into play however, physicians describe a deeper level of internal conflict. A family physician wrote her top ethical dilemma was “Cheating spouses and STIs: how do you get the other spouse treated?” An ob-gyn stated that, “disclosure of STI status in couples when this may indicate infidelity,” was a frequent ethical issue in her specialty. Commenters on Medscape’s recent story, “The Secret I’ll Take to my Grave: Doc Reveals,” also raised the uncomfortable topic. One physician recalled a deaf female patient who requested in writing not to test for syphilis and not to discuss the issue with her husband. “Patient knew that she had syphilis, but she did not want her husband to know,” the physician wrote.
It’s not uncommon for physicians to encounter such scenarios when treating long-term couples, especially in the digital era, said Shannon Dowler, MD, chief medical officer for North Carolina Medicaid and a family physician at the Buncombe County STI Clinic.
“This is definitely something I think we see more of in our age of ‘hookup apps’ and easier access to casual sexual connections than we did before,” said Dr. Dowler, who serves on the CDC Advisory Committee on HIV, Viral Hepatitis, and STD Prevention and Treatment.
The topic is particularly timely because of the pandemic’s impact on STI testing and the expected rise in sexually transmitted infection rates over the next year, Dr. Dowler notes.
“People weren’t necessarily coming in for routine screening or testing during the pandemic because they didn’t want to take a chance on being exposed to COVID,” she said. “But also, the reagent used for testing for certain types of transmitted infections was in short supply because they use that same reagent for the COVID test. We had shortages of STI testing in many parts of the country. I expect what we’re going to see over the next year are a lot of diagnoses that were missed during the pandemic and a lot of asymptomatic spread.”
What do the experts suggest?
Caring for spouses or two partners when an STI is discovered can be challenging for physicians, particularly in small towns where many people know each other, said Kenneth Goodman, PhD, founder and director of the Institute for Bioethics and Health Policy at the University of Miami.
“This can be a real challenge for family physicians and others in a small town,” he said. “If you discover one partner is positive for a sexually transmitted infection and the other is negative, then you’ve got a challenge to manage. The way to do that is to start with moral persuasion, namely you tell your patient, ‘You really need to disclose this. Because when he or she gets it, chances are, you’re going to be the prime suspect.’ “
Dr. Dowler, who practices in an STI clinic, said she once diagnosed a sexually transmitted infection in a patient who was married to one of Dowler’s coworkers. The patient would not allow the partner to be notified, she said. In this case, Dr. Dowler practiced expedited partner therapy (EPT), the clinical practice of treating sex partners of patients diagnosed with chlamydia or gonorrhea by giving the patient prescriptions or medications to take to the partner without having first examined the partner. The practice is legal to some extent in all states, Dr. Dowler said, but some states have different rules about how the practice can be utilized.
Physicians are obligated to report communicable diseases to their local health department, Dr. Goodman said. The health department would then do contract tracing and be responsible for conveying the STI diagnosis to any relevant parties. Even so, Dr. Goodman said physicians have a moral obligation to strongly encourage patients to divulge the infection to their partner.
“Doctors should work on being persuasive to change behavior,” he said. “Tell your patients to do the right thing and follow up with them. You should tell patients they have a responsibility to disclose a sexually transmitted infection to any of their partners and a responsibility not to have unprotected sex. Doctors can be very powerful advocates for that.”
Dr. Dowler said if she is treating two partners, and one is diagnosed with a sexually transmitted infection, she generally asks the patient for their consent to disclose the diagnosis to the partner. She ensures a witness, usually a nurse, is present when she asks. If consent is refused, Dr. Dowler guides her treatment to be as protective as possible, she said. A helpful resource for patients is Tellyourpartner.org, a website that sends an anonymous text or email about infection exposure and provides guidance on treatment locations and options.
Of course, if the sexually transmitted infection is HIV, another set of rules apply. As of 2021, 35 states have laws that criminalize HIV exposure. Laws vary, but many hold patients criminally liable if they knowingly expose another party to HIV. Many states and some cities also have ‘partner notification’ laws that require health providers to disclose an HIV diagnosis to the patient’s sex partners or to report the names of sex partners to the health department, if known.
However, case law on a physician’s duty to warn is mixed, and doctors’ responsibility for STI reporting and partner notification is determined by individual states. Making matters more complex is the fact that some states have recently changed their HIV control requirements, Dr. Dowler said. In North Carolina for example, patients living with HIV who have been virally suppressed for 6 months and who are adherent to medications, are no longer in violation of the control measure if they do not disclose their HIV diagnosis to sex partners or if they don’t wear a condom.
“This means physicians would not have to report a virally suppressed, adequately treated HIV-positive patient who is having unprotected sex or take measures to inform any known sex partners of the diagnosis,” she said. “The landscape is constantly changing so physicians have to be vigilant about their state public health statutes. It’s a tricky area. It takes an already complicated topic and makes it just a little more complicated.”
Consider drafting a policy
It’s a good idea to have a policy in place at your practice that addresses such ethical dilemmas before they occur, says Michael Heitt, PsyD, a clinical psychologist on the faculty of Loyola University Maryland in Baltimore, and a member of the Maryland Psychological Association’s Ethics Committee. Dr. Heitt developed a model of ethical reasoning called CLEAR Lenses, which stands for Clinical, Legal, Ethical, Administrative, and Risk management. The approach encourages clinicians to identify often competing factors in the decision-making process before choosing a course of action to take.
In the situation of an unfaithful spouse who contracted an STI for example, the physician should consider clinical issues such as the medical likelihood the unaware partner has the STI, and legal issues such as maintaining the confidentiality of all patient information and possible mandated reporting of STI data, Dr. Heitt said. The lenses overlap since confidentiality is also a key ethical issue, and other ethical issues involve the balance of helping the unaware spouse and not harming the infected spouse, he explained. Administrative issues might include how medical records are maintained and whether the physician documents information about patients’ family members in the medical record, while risk management elements may include informed consent, documentation, and consultation.
“So, if the physician has a policy about how such matters are dealt with, and patients are informed about this when they come to the practice, this can guide the physician much more easily through this sticky situation,” Dr. Heitt said. “Documentation of the decision-making process in the medical record demonstrates the physician’s thought process should it ever be challenged in the future, and consultation with peers (while disguising the identity of the patients, of course) sets a foundation of what a ‘reasonable standard’ might be in such situations.”
There is also the conflict-avoidant approach, Dr. Heitt said, in which the physician could perform “routine” STI testing if the unaware spouse was due for an appointment soon.
“But of course, this is far from avoiding any conflict; it just kicks the can down the road as there will surely be conflict — and plenty of confusion — if the wife tests positive for an STI,” he said. “In most situations, it is usually best to be brave, do the hard work upfront, and deal with the tough situation then, rather than trying to avoid the probable inevitable difficult conversation.”
As for the psychiatrist who was treating the cheating HIV-positive partner, the physician ultimately convinced both patients to come in for a couple’s session. The doctor allowed for a 2-hour timeframe to encourage discussion of any conflicts and unresolved issues, the psychiatrist said. After several more couple’s sessions, it was apparent the HIV-positive partner wanted out of the relationship, according to the psychiatrist’s account. The physician referred them to a couples’ therapist for ongoing treatment.
“During that same session, the HIV positive partner disclosed his recent behaviors and, as a result, they decided not to have further sexual contact until they could explore this further in therapy,” the psychiatrist wrote. “At last communication the couple decided to end the relationship, and the HIV negative partner remained negative.”
A version of this article first appeared on Medscape.com.
No increased risk of relugolix side effects in fibroid, endometriosis patients
Side effects from relugolix combination therapy (Myfembree) in premenopausal women treated for uterine fibroids and endometriosis are minimal, according to research presented at the American Society for Reproductive Medicine’s 2021 meeting.
The Food and Drug Administration approved relugolix, a daily oral gonadotropin-releasing hormone antagonist medication, earlier this year to treat heavy menstrual bleeding associated with uterine fibroids. It has not received Food and Drug Administration approval to treat endometriosis yet.
“It was a good kind of vindication about the safety of relugolix combination therapy,” Ayman Al-Hendy, MD, PhD, gynecologist and endoscopic surgeon at the University of Chicago, said in an interview.
Researchers led by Dr. Al-Hendy analyzed the results from two 24-week clinical trials that examined the effects of relugolix on premenopausal women between the ages of 18 and 50 suffering from uterine fibroids and endometriosis, both of which found that the treatment was well tolerated. With 1,344 patients in total, researchers found that the most common side effects of the treatment were headache, which occurred in 24.3% of participants, and hot flush, which affected 10.6%.
However, the prevalence of adverse reactions was similar to that of the placebo group in which 21.4% of participants experienced headaches and 6.4% experienced hot flushes, which, according to Dr. Al-Hendy, means that there is “really no increased risk” of experiencing an adverse event while taking relugolix.
“If we follow a large number of patients [with uterine fibroids or endometriosis], they will have some of these symptoms like headache or hot flushes or fatigue and so on. Either because it just happens in women for no known reason or because maybe the disease itself is causing some of these symptoms. The question is does the treatment in this case increase the frequency of these events?” Dr. Al-Hendy said.
“As long as it’s similar, fairly similar, or close between the [treatment and placebo group], then we know it’s not because of the medication,” Dr. Al-Hendy added.
Other adverse reactions that occurred while taking relugolix were “relatively rare” Dr. Al-Hendy said during his presentation. About 5.5% of those who took relugolix had uterine bleeding, 3.4% had decreased libido, 1.9% suffered from hyperhidrosis, 1.2% experienced night sweats, and 1.3% suffered from vaginal dryness.
The study shows that the risk profile of relugolix combination therapy is favorable and the side effects are relatively mild compared with past treatment options used to treat fibroids or endometriosis, said J. Ricardo Loret de Mola, MD, FACOG, FACS, who was not involved in the study.
However, Dr. Loret de Mola emphasized that this treatment isn’t for women who are seeking fertility or to get pregnant so it’s important for physicians to ask patients about their goals for treatment. Relugolix treatment could be a way for fibroid patients in their reproductive age to buy time and reduce the number of surgeries needed to get them to “the point where they would be ready to become mothers.”
He said surgery could be the right option for endometriosis patients who want to have children in the near future.
“This is an additional tool that we have available now that’s effective,” Dr. Loret de Mola said. “It is not going to cure either one of the two conditions, but could buy enough time for patients to be able to reach their goals, which is not having symptoms of endometriosis and fibroids after menopause or for people who just want to buy time.”
Dr. Al-Hendy said he hopes his findings reassure and encourage health care providers to discuss with patients different options for treating fibroids, and not just counsel them about surgery.
“So more awareness of these nonsurgical options hopefully will offer our patients a wide range of options when they seek help with fibroids and then against endometriosis [if or when] it’s [FDA]-approved,” Dr. Al-Hendy said.
None of the experts interviewed had conflicts of interest.
Side effects from relugolix combination therapy (Myfembree) in premenopausal women treated for uterine fibroids and endometriosis are minimal, according to research presented at the American Society for Reproductive Medicine’s 2021 meeting.
The Food and Drug Administration approved relugolix, a daily oral gonadotropin-releasing hormone antagonist medication, earlier this year to treat heavy menstrual bleeding associated with uterine fibroids. It has not received Food and Drug Administration approval to treat endometriosis yet.
“It was a good kind of vindication about the safety of relugolix combination therapy,” Ayman Al-Hendy, MD, PhD, gynecologist and endoscopic surgeon at the University of Chicago, said in an interview.
Researchers led by Dr. Al-Hendy analyzed the results from two 24-week clinical trials that examined the effects of relugolix on premenopausal women between the ages of 18 and 50 suffering from uterine fibroids and endometriosis, both of which found that the treatment was well tolerated. With 1,344 patients in total, researchers found that the most common side effects of the treatment were headache, which occurred in 24.3% of participants, and hot flush, which affected 10.6%.
However, the prevalence of adverse reactions was similar to that of the placebo group in which 21.4% of participants experienced headaches and 6.4% experienced hot flushes, which, according to Dr. Al-Hendy, means that there is “really no increased risk” of experiencing an adverse event while taking relugolix.
“If we follow a large number of patients [with uterine fibroids or endometriosis], they will have some of these symptoms like headache or hot flushes or fatigue and so on. Either because it just happens in women for no known reason or because maybe the disease itself is causing some of these symptoms. The question is does the treatment in this case increase the frequency of these events?” Dr. Al-Hendy said.
“As long as it’s similar, fairly similar, or close between the [treatment and placebo group], then we know it’s not because of the medication,” Dr. Al-Hendy added.
Other adverse reactions that occurred while taking relugolix were “relatively rare” Dr. Al-Hendy said during his presentation. About 5.5% of those who took relugolix had uterine bleeding, 3.4% had decreased libido, 1.9% suffered from hyperhidrosis, 1.2% experienced night sweats, and 1.3% suffered from vaginal dryness.
The study shows that the risk profile of relugolix combination therapy is favorable and the side effects are relatively mild compared with past treatment options used to treat fibroids or endometriosis, said J. Ricardo Loret de Mola, MD, FACOG, FACS, who was not involved in the study.
However, Dr. Loret de Mola emphasized that this treatment isn’t for women who are seeking fertility or to get pregnant so it’s important for physicians to ask patients about their goals for treatment. Relugolix treatment could be a way for fibroid patients in their reproductive age to buy time and reduce the number of surgeries needed to get them to “the point where they would be ready to become mothers.”
He said surgery could be the right option for endometriosis patients who want to have children in the near future.
“This is an additional tool that we have available now that’s effective,” Dr. Loret de Mola said. “It is not going to cure either one of the two conditions, but could buy enough time for patients to be able to reach their goals, which is not having symptoms of endometriosis and fibroids after menopause or for people who just want to buy time.”
Dr. Al-Hendy said he hopes his findings reassure and encourage health care providers to discuss with patients different options for treating fibroids, and not just counsel them about surgery.
“So more awareness of these nonsurgical options hopefully will offer our patients a wide range of options when they seek help with fibroids and then against endometriosis [if or when] it’s [FDA]-approved,” Dr. Al-Hendy said.
None of the experts interviewed had conflicts of interest.
Side effects from relugolix combination therapy (Myfembree) in premenopausal women treated for uterine fibroids and endometriosis are minimal, according to research presented at the American Society for Reproductive Medicine’s 2021 meeting.
The Food and Drug Administration approved relugolix, a daily oral gonadotropin-releasing hormone antagonist medication, earlier this year to treat heavy menstrual bleeding associated with uterine fibroids. It has not received Food and Drug Administration approval to treat endometriosis yet.
“It was a good kind of vindication about the safety of relugolix combination therapy,” Ayman Al-Hendy, MD, PhD, gynecologist and endoscopic surgeon at the University of Chicago, said in an interview.
Researchers led by Dr. Al-Hendy analyzed the results from two 24-week clinical trials that examined the effects of relugolix on premenopausal women between the ages of 18 and 50 suffering from uterine fibroids and endometriosis, both of which found that the treatment was well tolerated. With 1,344 patients in total, researchers found that the most common side effects of the treatment were headache, which occurred in 24.3% of participants, and hot flush, which affected 10.6%.
However, the prevalence of adverse reactions was similar to that of the placebo group in which 21.4% of participants experienced headaches and 6.4% experienced hot flushes, which, according to Dr. Al-Hendy, means that there is “really no increased risk” of experiencing an adverse event while taking relugolix.
“If we follow a large number of patients [with uterine fibroids or endometriosis], they will have some of these symptoms like headache or hot flushes or fatigue and so on. Either because it just happens in women for no known reason or because maybe the disease itself is causing some of these symptoms. The question is does the treatment in this case increase the frequency of these events?” Dr. Al-Hendy said.
“As long as it’s similar, fairly similar, or close between the [treatment and placebo group], then we know it’s not because of the medication,” Dr. Al-Hendy added.
Other adverse reactions that occurred while taking relugolix were “relatively rare” Dr. Al-Hendy said during his presentation. About 5.5% of those who took relugolix had uterine bleeding, 3.4% had decreased libido, 1.9% suffered from hyperhidrosis, 1.2% experienced night sweats, and 1.3% suffered from vaginal dryness.
The study shows that the risk profile of relugolix combination therapy is favorable and the side effects are relatively mild compared with past treatment options used to treat fibroids or endometriosis, said J. Ricardo Loret de Mola, MD, FACOG, FACS, who was not involved in the study.
However, Dr. Loret de Mola emphasized that this treatment isn’t for women who are seeking fertility or to get pregnant so it’s important for physicians to ask patients about their goals for treatment. Relugolix treatment could be a way for fibroid patients in their reproductive age to buy time and reduce the number of surgeries needed to get them to “the point where they would be ready to become mothers.”
He said surgery could be the right option for endometriosis patients who want to have children in the near future.
“This is an additional tool that we have available now that’s effective,” Dr. Loret de Mola said. “It is not going to cure either one of the two conditions, but could buy enough time for patients to be able to reach their goals, which is not having symptoms of endometriosis and fibroids after menopause or for people who just want to buy time.”
Dr. Al-Hendy said he hopes his findings reassure and encourage health care providers to discuss with patients different options for treating fibroids, and not just counsel them about surgery.
“So more awareness of these nonsurgical options hopefully will offer our patients a wide range of options when they seek help with fibroids and then against endometriosis [if or when] it’s [FDA]-approved,” Dr. Al-Hendy said.
None of the experts interviewed had conflicts of interest.
FROM ASRM 2021
True or false: Breast density increases breast cancer risk
Which of the following statements about breast density is TRUE?
Text copyright DenseBreast-info.org.
Answer
D. The risks associated with dense breast tissue are 2-fold: Dense tissue can mask cancer on a mammogram, and having dense breasts also increases the risk of developing breast cancer. As breast density increases, the sensitivity of mammography decreases, and the risk of developing breast cancer increases.
A woman’s breast density is usually determined by a radiologist’s visual evaluation of the mammogram. Breast density also can be measured quantitatively by computer software or estimated on computed tomography scan or magnetic resonance imaging. Breast density cannot be determined by the way a breast looks or feels.
Breast density and mammographic sensitivity
Cancers can be hidden or “masked” by dense tissue. On a mammogram, cancer is white. Normal dense tissue also appears white. If a cancer develops in an area of normal dense tissue, it can be harder or sometimes impossible to see it on the mammogram, like trying to see a snowman in a blizzard. As breast density increases, the ability to see cancer on mammography decreases (FIGURE 1).
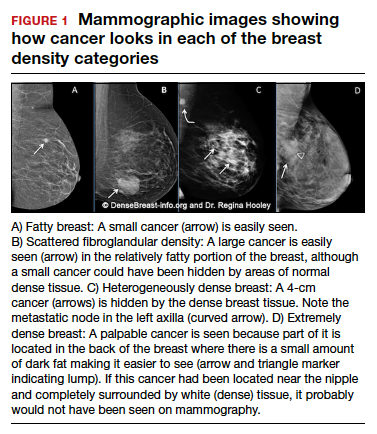
Standard 2D mammography has been shown to miss about 40% of cancers present in women with extremely dense breasts and 25% of cancers present in women with heterogeneously dense breasts.1-6 A cancer still can be masked on tomosynthesis (3D mammography) if it occurs in an area of dense tissue (where breast cancers more commonly occur), and tomosynthesis does not improve cancer detection appreciably in women with extremely dense breasts. To find cancer in a woman with dense breasts, additional screening beyond mammography should be considered.
Breast density and breast cancer risk
Dense breast tissue not only reduces mammography effectiveness, it also is a risk factor for the development of breast cancer: the denser the breast, the higher the risk.7 A meta-analysis across many studies concluded that magnitude of risk increases with each increase in density category, and women with extremely dense breasts (category D) have a 4-fold greater risk of developing breast cancer than do women with fatty breasts (category A), with upper limit of nearly 6-fold greater risk (FIGURE 2).8
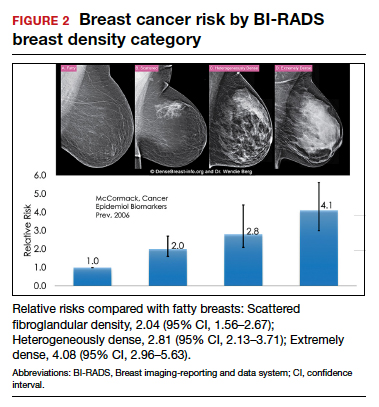
Most women do not have fatty breasts, however. More women have breasts with scattered fibroglandular density.9 Women with heterogeneously dense breasts (category C) have about a 1.5-fold greater risk of developing breast cancer than those with scattered fibroglandular density (category B), while women with extremely dense breasts (category D) have about a 2-fold greater risk.
There are probably several reasons that dense tissue increases breast cancer risk. One is that cancers arise microscopically in the glandular tissue. The more glandular tissue, the more susceptible tissue where cancer can develop. Glandular cells divide with hormonal stimulation throughout a woman’s lifetime, and each time a cell divides, “mistakes” can be made. An accumulation of mistakes can result in cancer. The more glandular the tissue, the greater the breast cancer risk. Women who have had breast reduction experience a reduced risk for breast cancer: thus, even a reduced absolute amount of glandular tissue reduces the risk for breast cancer. The second is that the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this is observed with fibrous breast tissue more than fatty breast tissue. ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404. doi: 10.1001 /jama.2012.388.
- Destounis S, Johnston L, Highnam R, et al. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR Am J Roentgenol. 2017;208:222-227. doi: 10.2214/AJR.16.16489.
- Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med. 2018;168:757-765. doi: 10.7326/M17-3008.
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175. doi: 10.1148/radiol.2251011667.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081-1087. doi: 10.1093/jnci/92.13.1081.
- Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017;19:67. doi: 10.1186/s13058-017-0859-9.
- Society AC. Breast Cancer Facts & Figures 2019-2020. American Cancer Society, Inc. https://www.cancer.org/content/dam/cancer-org/research/cancer -facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts -and-figures-2019-2020.pdf. Published 2019. Accessed September 23, 2021.
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-1169. doi: 10.1158/1055-9965.EPI-06-0034.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830-3837. doi: 10.1200/JCO.2009.26.4770.
Which of the following statements about breast density is TRUE?
Text copyright DenseBreast-info.org.
Answer
D. The risks associated with dense breast tissue are 2-fold: Dense tissue can mask cancer on a mammogram, and having dense breasts also increases the risk of developing breast cancer. As breast density increases, the sensitivity of mammography decreases, and the risk of developing breast cancer increases.
A woman’s breast density is usually determined by a radiologist’s visual evaluation of the mammogram. Breast density also can be measured quantitatively by computer software or estimated on computed tomography scan or magnetic resonance imaging. Breast density cannot be determined by the way a breast looks or feels.
Breast density and mammographic sensitivity
Cancers can be hidden or “masked” by dense tissue. On a mammogram, cancer is white. Normal dense tissue also appears white. If a cancer develops in an area of normal dense tissue, it can be harder or sometimes impossible to see it on the mammogram, like trying to see a snowman in a blizzard. As breast density increases, the ability to see cancer on mammography decreases (FIGURE 1).

Standard 2D mammography has been shown to miss about 40% of cancers present in women with extremely dense breasts and 25% of cancers present in women with heterogeneously dense breasts.1-6 A cancer still can be masked on tomosynthesis (3D mammography) if it occurs in an area of dense tissue (where breast cancers more commonly occur), and tomosynthesis does not improve cancer detection appreciably in women with extremely dense breasts. To find cancer in a woman with dense breasts, additional screening beyond mammography should be considered.
Breast density and breast cancer risk
Dense breast tissue not only reduces mammography effectiveness, it also is a risk factor for the development of breast cancer: the denser the breast, the higher the risk.7 A meta-analysis across many studies concluded that magnitude of risk increases with each increase in density category, and women with extremely dense breasts (category D) have a 4-fold greater risk of developing breast cancer than do women with fatty breasts (category A), with upper limit of nearly 6-fold greater risk (FIGURE 2).8

Most women do not have fatty breasts, however. More women have breasts with scattered fibroglandular density.9 Women with heterogeneously dense breasts (category C) have about a 1.5-fold greater risk of developing breast cancer than those with scattered fibroglandular density (category B), while women with extremely dense breasts (category D) have about a 2-fold greater risk.
There are probably several reasons that dense tissue increases breast cancer risk. One is that cancers arise microscopically in the glandular tissue. The more glandular tissue, the more susceptible tissue where cancer can develop. Glandular cells divide with hormonal stimulation throughout a woman’s lifetime, and each time a cell divides, “mistakes” can be made. An accumulation of mistakes can result in cancer. The more glandular the tissue, the greater the breast cancer risk. Women who have had breast reduction experience a reduced risk for breast cancer: thus, even a reduced absolute amount of glandular tissue reduces the risk for breast cancer. The second is that the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this is observed with fibrous breast tissue more than fatty breast tissue. ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Which of the following statements about breast density is TRUE?
Text copyright DenseBreast-info.org.
Answer
D. The risks associated with dense breast tissue are 2-fold: Dense tissue can mask cancer on a mammogram, and having dense breasts also increases the risk of developing breast cancer. As breast density increases, the sensitivity of mammography decreases, and the risk of developing breast cancer increases.
A woman’s breast density is usually determined by a radiologist’s visual evaluation of the mammogram. Breast density also can be measured quantitatively by computer software or estimated on computed tomography scan or magnetic resonance imaging. Breast density cannot be determined by the way a breast looks or feels.
Breast density and mammographic sensitivity
Cancers can be hidden or “masked” by dense tissue. On a mammogram, cancer is white. Normal dense tissue also appears white. If a cancer develops in an area of normal dense tissue, it can be harder or sometimes impossible to see it on the mammogram, like trying to see a snowman in a blizzard. As breast density increases, the ability to see cancer on mammography decreases (FIGURE 1).

Standard 2D mammography has been shown to miss about 40% of cancers present in women with extremely dense breasts and 25% of cancers present in women with heterogeneously dense breasts.1-6 A cancer still can be masked on tomosynthesis (3D mammography) if it occurs in an area of dense tissue (where breast cancers more commonly occur), and tomosynthesis does not improve cancer detection appreciably in women with extremely dense breasts. To find cancer in a woman with dense breasts, additional screening beyond mammography should be considered.
Breast density and breast cancer risk
Dense breast tissue not only reduces mammography effectiveness, it also is a risk factor for the development of breast cancer: the denser the breast, the higher the risk.7 A meta-analysis across many studies concluded that magnitude of risk increases with each increase in density category, and women with extremely dense breasts (category D) have a 4-fold greater risk of developing breast cancer than do women with fatty breasts (category A), with upper limit of nearly 6-fold greater risk (FIGURE 2).8

Most women do not have fatty breasts, however. More women have breasts with scattered fibroglandular density.9 Women with heterogeneously dense breasts (category C) have about a 1.5-fold greater risk of developing breast cancer than those with scattered fibroglandular density (category B), while women with extremely dense breasts (category D) have about a 2-fold greater risk.
There are probably several reasons that dense tissue increases breast cancer risk. One is that cancers arise microscopically in the glandular tissue. The more glandular tissue, the more susceptible tissue where cancer can develop. Glandular cells divide with hormonal stimulation throughout a woman’s lifetime, and each time a cell divides, “mistakes” can be made. An accumulation of mistakes can result in cancer. The more glandular the tissue, the greater the breast cancer risk. Women who have had breast reduction experience a reduced risk for breast cancer: thus, even a reduced absolute amount of glandular tissue reduces the risk for breast cancer. The second is that the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this is observed with fibrous breast tissue more than fatty breast tissue. ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404. doi: 10.1001 /jama.2012.388.
- Destounis S, Johnston L, Highnam R, et al. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR Am J Roentgenol. 2017;208:222-227. doi: 10.2214/AJR.16.16489.
- Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med. 2018;168:757-765. doi: 10.7326/M17-3008.
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175. doi: 10.1148/radiol.2251011667.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081-1087. doi: 10.1093/jnci/92.13.1081.
- Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017;19:67. doi: 10.1186/s13058-017-0859-9.
- Society AC. Breast Cancer Facts & Figures 2019-2020. American Cancer Society, Inc. https://www.cancer.org/content/dam/cancer-org/research/cancer -facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts -and-figures-2019-2020.pdf. Published 2019. Accessed September 23, 2021.
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-1169. doi: 10.1158/1055-9965.EPI-06-0034.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830-3837. doi: 10.1200/JCO.2009.26.4770.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404. doi: 10.1001 /jama.2012.388.
- Destounis S, Johnston L, Highnam R, et al. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR Am J Roentgenol. 2017;208:222-227. doi: 10.2214/AJR.16.16489.
- Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med. 2018;168:757-765. doi: 10.7326/M17-3008.
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175. doi: 10.1148/radiol.2251011667.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081-1087. doi: 10.1093/jnci/92.13.1081.
- Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017;19:67. doi: 10.1186/s13058-017-0859-9.
- Society AC. Breast Cancer Facts & Figures 2019-2020. American Cancer Society, Inc. https://www.cancer.org/content/dam/cancer-org/research/cancer -facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts -and-figures-2019-2020.pdf. Published 2019. Accessed September 23, 2021.
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-1169. doi: 10.1158/1055-9965.EPI-06-0034.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830-3837. doi: 10.1200/JCO.2009.26.4770.
Quiz developed in collaboration with