User login
Subungual Nodule in a Pediatric Patient
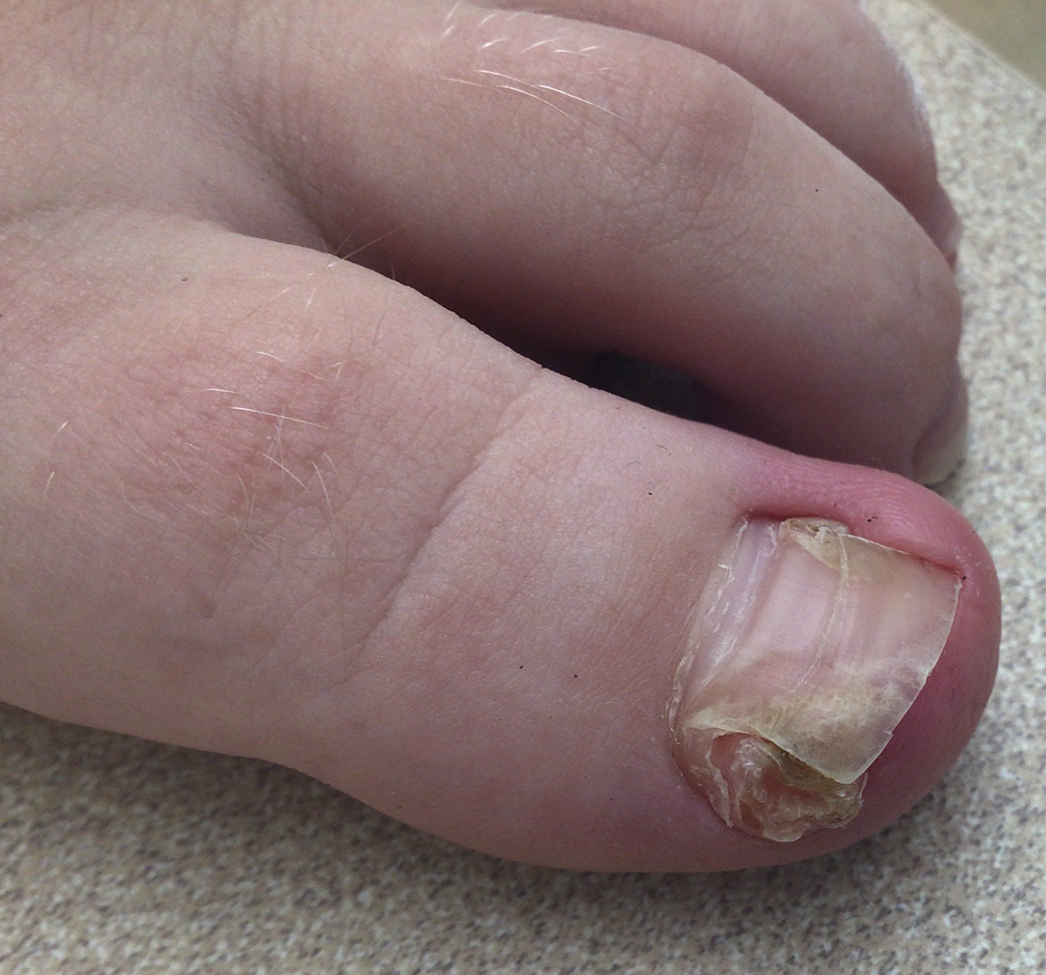
The Diagnosis: Subungual Exostosis
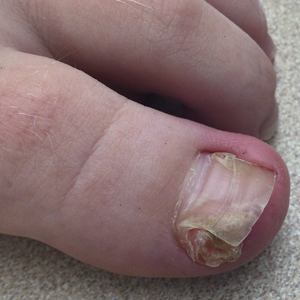
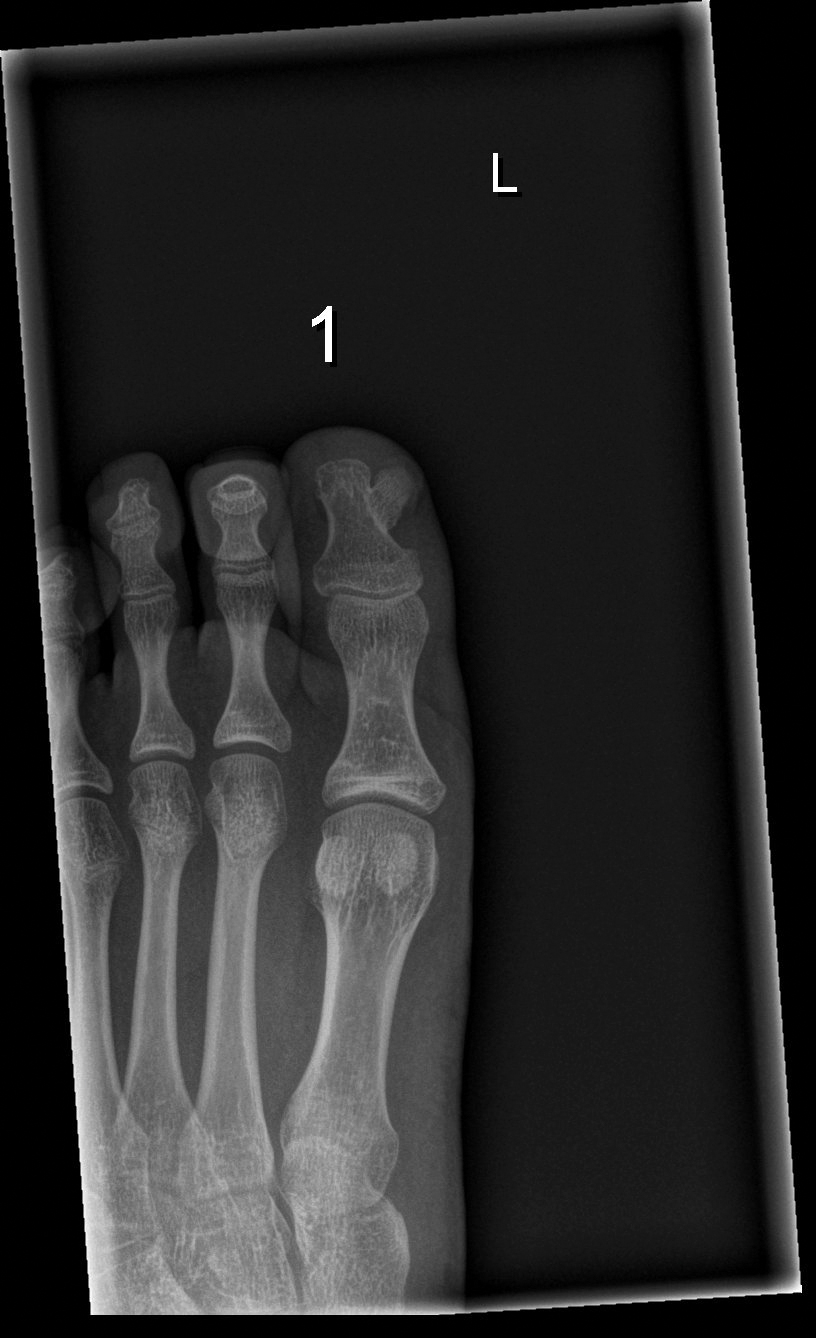
Subungual exostosis should be considered as a possible cause of an exophytic subungual nodule in a young active female. In our patient, the involvement of the great toe was a clue, as the hallux is the most common location of subungual exostosis. The patient’s age and sex also were supportive, as subungual exostosis is most common in female children and adolescents— particularly those who are active, as trauma is thought to play a possible role in development of this benign tumor.1-3 Radiography is the preferred modality for diagnosis; in our case, it showed a trabecular bony overgrowth (Figure 1), which confirmed the diagnosis. Subungual exostosis is a rare, benign, osteocartilaginous tumor of trabecular bone. The etiology is unknown but is hypothesized to be related to trauma, infection, or activation of a cartilaginous cyst.1,3 The subungual nodule may be asymptomatic or painful. Disruption and elevation of the nail plate is common.4 The differential diagnosis includes amelanotic melanoma, fibroma, fibrokeratoma, osteochondroma, pyogenic granuloma, squamous cell carcinoma, glomus tumor, and verruca vulgaris, among others.5
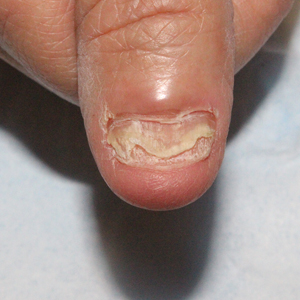
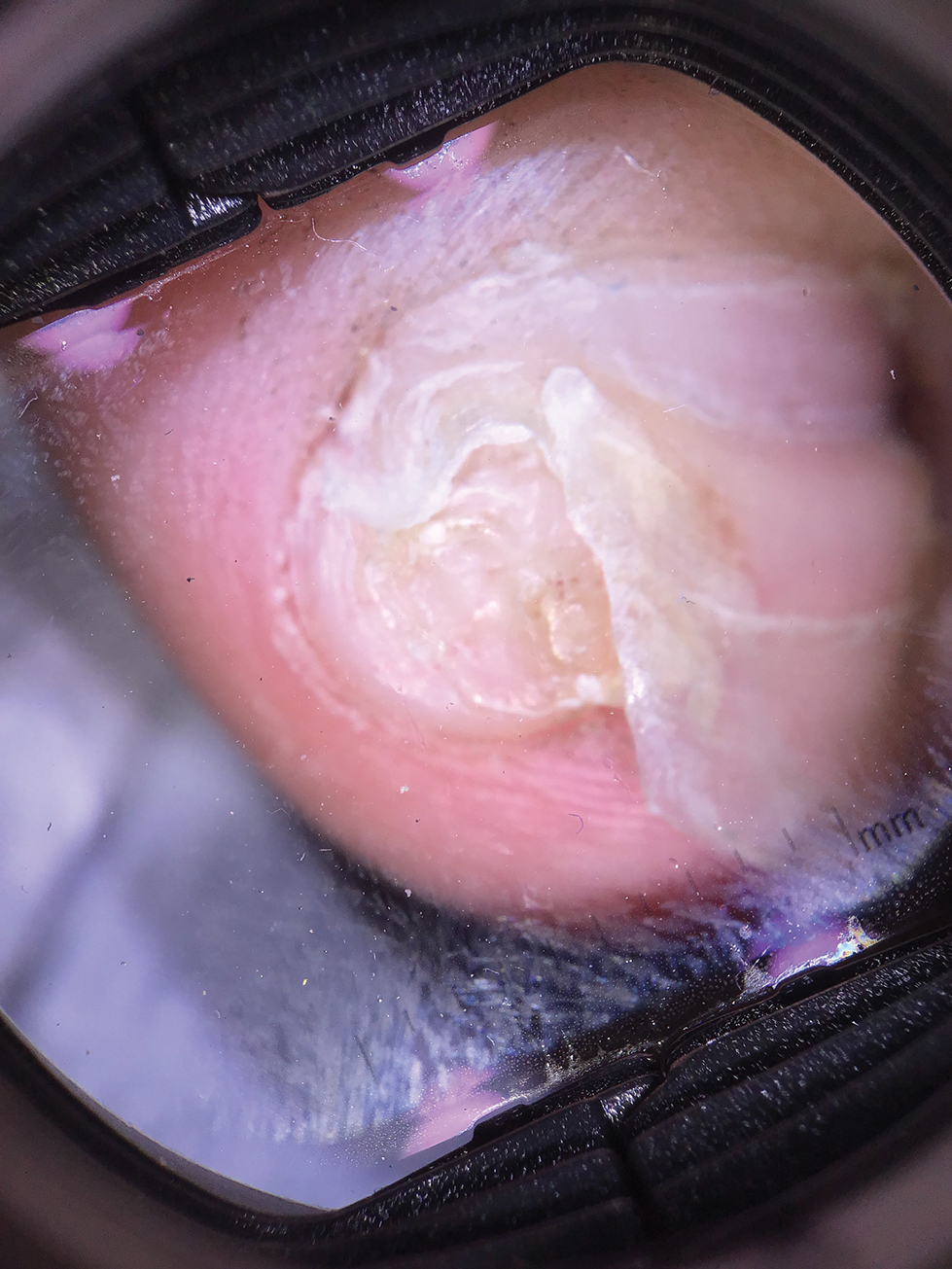
Physical examination demonstrates a firm, fixed, subungual nodule, often with an accompanying nail deformity. Further workup is required to confirm the benign nature of the lesion and exclude nail tumors such as melanoma or squamous cell carcinoma. Radiography is the gold standard for diagnosis, demonstrating a trabecular bony overgrowth.6 Performing a radiograph as the initial diagnostic test spares the patient from unnecessary procedures such as biopsy or expensive imaging techniques such as magnetic resonance imaging. Early lesions may not demonstrate sufficient bone formation shown on radiography. In these situations, a combination of dermoscopy and histopathologic examination may aid in diagnosis (Figure 2).4 Vascular ectasia, hyperkeratosis, onycholysis, and ulceration are the most common findings on dermoscopy (in ascending order).7 Histopathology typically demonstrates a base or stalk of normal-appearing trabecular bone with a fibrocartilage cap.8 However, initial clinical workup via radiography allows for the least-invasive and highest-yield intervention. Clinical suspicion for this condition is important, as it can be diagnosed with noninvasive inexpensive imaging rather than biopsy or more specialized imaging modalities. Appropriate recognition can save young patients from unnecessary and expensive procedures. Treatment typically involves surgical excision; to prevent regrowth, removal of the lesion at the base of the bone is recommended.2
Although amelanotic melanoma also can manifest as a subungual nail tumor, it would be unusual in a young child and would not be expected to show characteristic changes on radiography. A glomus tumor would be painful, is more common on the fingers than on the toes, and typically has a bluish hue.9 Verruca vulgaris can occur subungually but is more common around the nailfold and often has the characteristic dermoscopic finding of thrombosed capillaries. It also would not be expected to show characteristic radiographic findings. Osteochondroma can occur in young patients and can appear clinically similar to subungual exostosis; however, it typically is painful.10
- Pascoal D, Balaco I, Alves C, et al. Subungual exostosis—treatment results with preservation of the nail bed. J Pediatr Orthop B. 2020;29:382-386.
- Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257.
- Chiheb S, Slimani Y, Karam R, et al. Subungual exostosis: a case series of 48 patients. Skin Appendage Disord. 2021;7:475-479.
- Zhang W, Gu L, Fan H, et al. Subungual exostosis with an unusual dermoscopic feature. JAAD Case Rep. 2020;6:725-726.
- Demirdag HG, Tugrul Ayanoglu B, Akay BN. Dermoscopic features of subungual exostosis. Australas J Dermatol. 2019;60:E138-E141.
- Tritto M, Mirkin G, Hao X. Subungual exostosis on the right hallux. J Am Podiatr Med Assoc. 2021;111.
- Piccolo V, Argenziano G, Alessandrini AM, et al. Dermoscopy of subungual exostosis: a retrospective study of 10 patients. Dermatology. 2017;233:80-85.
- Lee SK, Jung MS, Lee YH, et al. Two distinctive subungual pathologies: subungual exostosis and subungual osteochondroma. Foot Ankle Int. 2007;28:595-601. doi:10.3113/FAI.2007.0595
- Samaniego E, Crespo A, Sanz A. Key diagnostic features and treatment of subungual glomus tumor. Actas Dermosifiliogr. 2009;100:875-882.
- Glick S. Subungual osteochondroma of the third toe. Consult.360. 2013;12.
The Diagnosis: Subungual Exostosis
Subungual exostosis should be considered as a possible cause of an exophytic subungual nodule in a young active female. In our patient, the involvement of the great toe was a clue, as the hallux is the most common location of subungual exostosis. The patient’s age and sex also were supportive, as subungual exostosis is most common in female children and adolescents— particularly those who are active, as trauma is thought to play a possible role in development of this benign tumor.1-3 Radiography is the preferred modality for diagnosis; in our case, it showed a trabecular bony overgrowth (Figure 1), which confirmed the diagnosis. Subungual exostosis is a rare, benign, osteocartilaginous tumor of trabecular bone. The etiology is unknown but is hypothesized to be related to trauma, infection, or activation of a cartilaginous cyst.1,3 The subungual nodule may be asymptomatic or painful. Disruption and elevation of the nail plate is common.4 The differential diagnosis includes amelanotic melanoma, fibroma, fibrokeratoma, osteochondroma, pyogenic granuloma, squamous cell carcinoma, glomus tumor, and verruca vulgaris, among others.5

Physical examination demonstrates a firm, fixed, subungual nodule, often with an accompanying nail deformity. Further workup is required to confirm the benign nature of the lesion and exclude nail tumors such as melanoma or squamous cell carcinoma. Radiography is the gold standard for diagnosis, demonstrating a trabecular bony overgrowth.6 Performing a radiograph as the initial diagnostic test spares the patient from unnecessary procedures such as biopsy or expensive imaging techniques such as magnetic resonance imaging. Early lesions may not demonstrate sufficient bone formation shown on radiography. In these situations, a combination of dermoscopy and histopathologic examination may aid in diagnosis (Figure 2).4 Vascular ectasia, hyperkeratosis, onycholysis, and ulceration are the most common findings on dermoscopy (in ascending order).7 Histopathology typically demonstrates a base or stalk of normal-appearing trabecular bone with a fibrocartilage cap.8 However, initial clinical workup via radiography allows for the least-invasive and highest-yield intervention. Clinical suspicion for this condition is important, as it can be diagnosed with noninvasive inexpensive imaging rather than biopsy or more specialized imaging modalities. Appropriate recognition can save young patients from unnecessary and expensive procedures. Treatment typically involves surgical excision; to prevent regrowth, removal of the lesion at the base of the bone is recommended.2

Although amelanotic melanoma also can manifest as a subungual nail tumor, it would be unusual in a young child and would not be expected to show characteristic changes on radiography. A glomus tumor would be painful, is more common on the fingers than on the toes, and typically has a bluish hue.9 Verruca vulgaris can occur subungually but is more common around the nailfold and often has the characteristic dermoscopic finding of thrombosed capillaries. It also would not be expected to show characteristic radiographic findings. Osteochondroma can occur in young patients and can appear clinically similar to subungual exostosis; however, it typically is painful.10
The Diagnosis: Subungual Exostosis
Subungual exostosis should be considered as a possible cause of an exophytic subungual nodule in a young active female. In our patient, the involvement of the great toe was a clue, as the hallux is the most common location of subungual exostosis. The patient’s age and sex also were supportive, as subungual exostosis is most common in female children and adolescents— particularly those who are active, as trauma is thought to play a possible role in development of this benign tumor.1-3 Radiography is the preferred modality for diagnosis; in our case, it showed a trabecular bony overgrowth (Figure 1), which confirmed the diagnosis. Subungual exostosis is a rare, benign, osteocartilaginous tumor of trabecular bone. The etiology is unknown but is hypothesized to be related to trauma, infection, or activation of a cartilaginous cyst.1,3 The subungual nodule may be asymptomatic or painful. Disruption and elevation of the nail plate is common.4 The differential diagnosis includes amelanotic melanoma, fibroma, fibrokeratoma, osteochondroma, pyogenic granuloma, squamous cell carcinoma, glomus tumor, and verruca vulgaris, among others.5

Physical examination demonstrates a firm, fixed, subungual nodule, often with an accompanying nail deformity. Further workup is required to confirm the benign nature of the lesion and exclude nail tumors such as melanoma or squamous cell carcinoma. Radiography is the gold standard for diagnosis, demonstrating a trabecular bony overgrowth.6 Performing a radiograph as the initial diagnostic test spares the patient from unnecessary procedures such as biopsy or expensive imaging techniques such as magnetic resonance imaging. Early lesions may not demonstrate sufficient bone formation shown on radiography. In these situations, a combination of dermoscopy and histopathologic examination may aid in diagnosis (Figure 2).4 Vascular ectasia, hyperkeratosis, onycholysis, and ulceration are the most common findings on dermoscopy (in ascending order).7 Histopathology typically demonstrates a base or stalk of normal-appearing trabecular bone with a fibrocartilage cap.8 However, initial clinical workup via radiography allows for the least-invasive and highest-yield intervention. Clinical suspicion for this condition is important, as it can be diagnosed with noninvasive inexpensive imaging rather than biopsy or more specialized imaging modalities. Appropriate recognition can save young patients from unnecessary and expensive procedures. Treatment typically involves surgical excision; to prevent regrowth, removal of the lesion at the base of the bone is recommended.2

Although amelanotic melanoma also can manifest as a subungual nail tumor, it would be unusual in a young child and would not be expected to show characteristic changes on radiography. A glomus tumor would be painful, is more common on the fingers than on the toes, and typically has a bluish hue.9 Verruca vulgaris can occur subungually but is more common around the nailfold and often has the characteristic dermoscopic finding of thrombosed capillaries. It also would not be expected to show characteristic radiographic findings. Osteochondroma can occur in young patients and can appear clinically similar to subungual exostosis; however, it typically is painful.10
- Pascoal D, Balaco I, Alves C, et al. Subungual exostosis—treatment results with preservation of the nail bed. J Pediatr Orthop B. 2020;29:382-386.
- Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257.
- Chiheb S, Slimani Y, Karam R, et al. Subungual exostosis: a case series of 48 patients. Skin Appendage Disord. 2021;7:475-479.
- Zhang W, Gu L, Fan H, et al. Subungual exostosis with an unusual dermoscopic feature. JAAD Case Rep. 2020;6:725-726.
- Demirdag HG, Tugrul Ayanoglu B, Akay BN. Dermoscopic features of subungual exostosis. Australas J Dermatol. 2019;60:E138-E141.
- Tritto M, Mirkin G, Hao X. Subungual exostosis on the right hallux. J Am Podiatr Med Assoc. 2021;111.
- Piccolo V, Argenziano G, Alessandrini AM, et al. Dermoscopy of subungual exostosis: a retrospective study of 10 patients. Dermatology. 2017;233:80-85.
- Lee SK, Jung MS, Lee YH, et al. Two distinctive subungual pathologies: subungual exostosis and subungual osteochondroma. Foot Ankle Int. 2007;28:595-601. doi:10.3113/FAI.2007.0595
- Samaniego E, Crespo A, Sanz A. Key diagnostic features and treatment of subungual glomus tumor. Actas Dermosifiliogr. 2009;100:875-882.
- Glick S. Subungual osteochondroma of the third toe. Consult.360. 2013;12.
- Pascoal D, Balaco I, Alves C, et al. Subungual exostosis—treatment results with preservation of the nail bed. J Pediatr Orthop B. 2020;29:382-386.
- Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257.
- Chiheb S, Slimani Y, Karam R, et al. Subungual exostosis: a case series of 48 patients. Skin Appendage Disord. 2021;7:475-479.
- Zhang W, Gu L, Fan H, et al. Subungual exostosis with an unusual dermoscopic feature. JAAD Case Rep. 2020;6:725-726.
- Demirdag HG, Tugrul Ayanoglu B, Akay BN. Dermoscopic features of subungual exostosis. Australas J Dermatol. 2019;60:E138-E141.
- Tritto M, Mirkin G, Hao X. Subungual exostosis on the right hallux. J Am Podiatr Med Assoc. 2021;111.
- Piccolo V, Argenziano G, Alessandrini AM, et al. Dermoscopy of subungual exostosis: a retrospective study of 10 patients. Dermatology. 2017;233:80-85.
- Lee SK, Jung MS, Lee YH, et al. Two distinctive subungual pathologies: subungual exostosis and subungual osteochondroma. Foot Ankle Int. 2007;28:595-601. doi:10.3113/FAI.2007.0595
- Samaniego E, Crespo A, Sanz A. Key diagnostic features and treatment of subungual glomus tumor. Actas Dermosifiliogr. 2009;100:875-882.
- Glick S. Subungual osteochondroma of the third toe. Consult.360. 2013;12.
A 13-year-old girl presented to her pediatrician with a small pink bump under the left great toenail of 8 months’ duration that was slowly growing. Months later, she developed an ingrown nail on the same toe, which was treated with partial nail avulsion by the pediatrician. Given continued nail dystrophy and a visible bump under the nail, the patient was referred to dermatology. Physical examination revealed a subungual, flesh-colored, sessile nodule causing distortion of the nail plate on the left great toe with associated intermittent redness and swelling. She denied wearing new shoes or experiencing any pain, pruritus, or purulent drainage or bleeding from the lesion. She reported being physically active and playing tennis.
Dupilumab Evaluated as Treatment for Pediatric Alopecia Areata
showed.
“We might be opening a new avenue for a safe, long-term treatment for our children with AA,” the study’s lead investigator, Emma Guttman-Yassky, MD, PhD, professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai, New York City, said in an interview during the annual meeting of the Society for Investigative Dermatology (SID), where the results were presented during a poster session. “I think AA is likely joining the atopic march, which may allow us to adapt some treatments from the atopy world to AA.”
When the original phase 2 and phase 3 trials of dupilumab for patients with moderate to severe AD were being conducted, Dr. Guttman-Yassky, one of the investigators, recalled observing that some patients who also had patch alopecia experienced hair regrowth. “I was scratching my head because, at the time, AA was considered to be only a Th1-driven disease,” she said. “I asked myself, ‘How can this happen?’ I looked in the literature and found many publications linking atopy in general to alopecia areata. The largest of the dermatologic publications showed that eczema and atopy in general are the highest comorbidities in alopecia areata.”
“This and other findings such as IL [interleukin]-13 genetic linkage with AA and high IgE in patients with AA link AA with Th2 immune skewing, particularly in the setting of atopy,” she continued. In addition, she said, in a large biomarker study involving the scalp and blood of patients with AA, “we found increases in Th2 biomarkers that were associated with alopecia severity.”
Case Series of 20 Pediatric Patients
As part of a case series of children with both AD and AA, Dr. Guttman-Yassky and colleagues evaluated hair regrowth using the Severity of Alopecia Tool (SALT) in 20 pediatric patients (mean age, 10.8 years) who were being treated at Mount Sinai. They collected patient demographics, atopic history, immunoglobulin E (IgE) levels, and SALT scores at follow-up visits every 12-16 weeks for more than 72 weeks and performed Spearman correlations between clinical scores, demographics, and IgE levels.
At baseline, the mean SALT score was 54.4, the mean IgE level was 1567.7 IU/mL, and 75% of patients also had a family history of atopy. The mean follow-up was 67.6 weeks. The researchers observed a significant reduction in SALT scores at week 48 compared with baseline (a mean score of 20.4; P < .01) and continued improvement up to at least 72 weeks (P < .01 vs baseline). They also noted that patients who achieved a treatment response at week 24 had baseline IgE levels > 200 IU/mL.
In other findings, baseline IgE positively correlated with improvement in SALT scores at week 36 (P < .05), while baseline SALT scores positively correlated with disease duration (P < .01) and negatively correlated with improvement in SALT scores at weeks 24, 36, and 48 (P < .005). “The robustness of the response surprised me,” Dr. Guttman-Yassky said in the interview. “Dupilumab for AA takes time to work, but once it kicks in, it kicks in. It takes anywhere from 6 to 12 months to see hair regrowth.”
She acknowledged certain limitations of the analysis, including its small sample size and the fact that it was not a standardized trial. “But, based on our data and the adult data, we are very encouraged about the potential of using dupilumab for children with AA,” she said.
Mount Sinai recently announced that the National Institutes of Health awarded a $6.6 million, 5-year grant to Dr. Guttman-Yassky to further investigate dupilumab as a treatment for children with AA. She will lead a multicenter controlled trial of 76 children with alopecia affecting at least 30% of the scalp, who will be randomized 2:1 (dupilumab:placebo) for 48 weeks, followed by 48 weeks of open-label dupilumab for all participants, with 16 weeks of follow-up, for a total of 112 weeks. Participating sites include Mount Sinai, Yale University, Northwestern University, and the University of California, Irvine.
Dr. Guttman-Yassky disclosed that she is a consultant to many pharmaceutical companies, including dupilumab manufacturers Sanofi and Regeneron.
A version of this article appeared on Medscape.com.
showed.
“We might be opening a new avenue for a safe, long-term treatment for our children with AA,” the study’s lead investigator, Emma Guttman-Yassky, MD, PhD, professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai, New York City, said in an interview during the annual meeting of the Society for Investigative Dermatology (SID), where the results were presented during a poster session. “I think AA is likely joining the atopic march, which may allow us to adapt some treatments from the atopy world to AA.”
When the original phase 2 and phase 3 trials of dupilumab for patients with moderate to severe AD were being conducted, Dr. Guttman-Yassky, one of the investigators, recalled observing that some patients who also had patch alopecia experienced hair regrowth. “I was scratching my head because, at the time, AA was considered to be only a Th1-driven disease,” she said. “I asked myself, ‘How can this happen?’ I looked in the literature and found many publications linking atopy in general to alopecia areata. The largest of the dermatologic publications showed that eczema and atopy in general are the highest comorbidities in alopecia areata.”
“This and other findings such as IL [interleukin]-13 genetic linkage with AA and high IgE in patients with AA link AA with Th2 immune skewing, particularly in the setting of atopy,” she continued. In addition, she said, in a large biomarker study involving the scalp and blood of patients with AA, “we found increases in Th2 biomarkers that were associated with alopecia severity.”
Case Series of 20 Pediatric Patients
As part of a case series of children with both AD and AA, Dr. Guttman-Yassky and colleagues evaluated hair regrowth using the Severity of Alopecia Tool (SALT) in 20 pediatric patients (mean age, 10.8 years) who were being treated at Mount Sinai. They collected patient demographics, atopic history, immunoglobulin E (IgE) levels, and SALT scores at follow-up visits every 12-16 weeks for more than 72 weeks and performed Spearman correlations between clinical scores, demographics, and IgE levels.
At baseline, the mean SALT score was 54.4, the mean IgE level was 1567.7 IU/mL, and 75% of patients also had a family history of atopy. The mean follow-up was 67.6 weeks. The researchers observed a significant reduction in SALT scores at week 48 compared with baseline (a mean score of 20.4; P < .01) and continued improvement up to at least 72 weeks (P < .01 vs baseline). They also noted that patients who achieved a treatment response at week 24 had baseline IgE levels > 200 IU/mL.
In other findings, baseline IgE positively correlated with improvement in SALT scores at week 36 (P < .05), while baseline SALT scores positively correlated with disease duration (P < .01) and negatively correlated with improvement in SALT scores at weeks 24, 36, and 48 (P < .005). “The robustness of the response surprised me,” Dr. Guttman-Yassky said in the interview. “Dupilumab for AA takes time to work, but once it kicks in, it kicks in. It takes anywhere from 6 to 12 months to see hair regrowth.”
She acknowledged certain limitations of the analysis, including its small sample size and the fact that it was not a standardized trial. “But, based on our data and the adult data, we are very encouraged about the potential of using dupilumab for children with AA,” she said.
Mount Sinai recently announced that the National Institutes of Health awarded a $6.6 million, 5-year grant to Dr. Guttman-Yassky to further investigate dupilumab as a treatment for children with AA. She will lead a multicenter controlled trial of 76 children with alopecia affecting at least 30% of the scalp, who will be randomized 2:1 (dupilumab:placebo) for 48 weeks, followed by 48 weeks of open-label dupilumab for all participants, with 16 weeks of follow-up, for a total of 112 weeks. Participating sites include Mount Sinai, Yale University, Northwestern University, and the University of California, Irvine.
Dr. Guttman-Yassky disclosed that she is a consultant to many pharmaceutical companies, including dupilumab manufacturers Sanofi and Regeneron.
A version of this article appeared on Medscape.com.
showed.
“We might be opening a new avenue for a safe, long-term treatment for our children with AA,” the study’s lead investigator, Emma Guttman-Yassky, MD, PhD, professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai, New York City, said in an interview during the annual meeting of the Society for Investigative Dermatology (SID), where the results were presented during a poster session. “I think AA is likely joining the atopic march, which may allow us to adapt some treatments from the atopy world to AA.”
When the original phase 2 and phase 3 trials of dupilumab for patients with moderate to severe AD were being conducted, Dr. Guttman-Yassky, one of the investigators, recalled observing that some patients who also had patch alopecia experienced hair regrowth. “I was scratching my head because, at the time, AA was considered to be only a Th1-driven disease,” she said. “I asked myself, ‘How can this happen?’ I looked in the literature and found many publications linking atopy in general to alopecia areata. The largest of the dermatologic publications showed that eczema and atopy in general are the highest comorbidities in alopecia areata.”
“This and other findings such as IL [interleukin]-13 genetic linkage with AA and high IgE in patients with AA link AA with Th2 immune skewing, particularly in the setting of atopy,” she continued. In addition, she said, in a large biomarker study involving the scalp and blood of patients with AA, “we found increases in Th2 biomarkers that were associated with alopecia severity.”
Case Series of 20 Pediatric Patients
As part of a case series of children with both AD and AA, Dr. Guttman-Yassky and colleagues evaluated hair regrowth using the Severity of Alopecia Tool (SALT) in 20 pediatric patients (mean age, 10.8 years) who were being treated at Mount Sinai. They collected patient demographics, atopic history, immunoglobulin E (IgE) levels, and SALT scores at follow-up visits every 12-16 weeks for more than 72 weeks and performed Spearman correlations between clinical scores, demographics, and IgE levels.
At baseline, the mean SALT score was 54.4, the mean IgE level was 1567.7 IU/mL, and 75% of patients also had a family history of atopy. The mean follow-up was 67.6 weeks. The researchers observed a significant reduction in SALT scores at week 48 compared with baseline (a mean score of 20.4; P < .01) and continued improvement up to at least 72 weeks (P < .01 vs baseline). They also noted that patients who achieved a treatment response at week 24 had baseline IgE levels > 200 IU/mL.
In other findings, baseline IgE positively correlated with improvement in SALT scores at week 36 (P < .05), while baseline SALT scores positively correlated with disease duration (P < .01) and negatively correlated with improvement in SALT scores at weeks 24, 36, and 48 (P < .005). “The robustness of the response surprised me,” Dr. Guttman-Yassky said in the interview. “Dupilumab for AA takes time to work, but once it kicks in, it kicks in. It takes anywhere from 6 to 12 months to see hair regrowth.”
She acknowledged certain limitations of the analysis, including its small sample size and the fact that it was not a standardized trial. “But, based on our data and the adult data, we are very encouraged about the potential of using dupilumab for children with AA,” she said.
Mount Sinai recently announced that the National Institutes of Health awarded a $6.6 million, 5-year grant to Dr. Guttman-Yassky to further investigate dupilumab as a treatment for children with AA. She will lead a multicenter controlled trial of 76 children with alopecia affecting at least 30% of the scalp, who will be randomized 2:1 (dupilumab:placebo) for 48 weeks, followed by 48 weeks of open-label dupilumab for all participants, with 16 weeks of follow-up, for a total of 112 weeks. Participating sites include Mount Sinai, Yale University, Northwestern University, and the University of California, Irvine.
Dr. Guttman-Yassky disclosed that she is a consultant to many pharmaceutical companies, including dupilumab manufacturers Sanofi and Regeneron.
A version of this article appeared on Medscape.com.
FROM SID 2024
Frontal Fibrosing Alopecia: Study Finds Oral Contraceptive Use Modulates Risk In Women with Genetic Variant
TOPLINE:
Investigators found that .
METHODOLOGY:
- OC use has been considered a possible factor behind the increased incidence of FFA because it was first documented in 1994, and a recent genome-wide association study of FFA identified a signal for an association with a variant in CYP1B1.
- The same researchers conducted a gene-environment interaction study with a case-control design involving 489 White female patients (mean age, 65.8 years) with FFA and 34,254 controls, matched for age and genetic ancestry.
- Data were collected from July 2015 to September 2017 and analyzed from October 2022 to December 2023.
- The study aimed to investigate the modulatory effect of OC use on the CYP1B1 variant’s impact on FFA risk, using logistic regression models for analysis.
TAKEAWAY:
- The use of OCs was associated with a 1.9 times greater risk for FFA in individuals with the specific CYP1B1 genetic variant, but there was no association among those with no history of OC use.
- The study suggests a significant gene-environment interaction, indicating that OC use may influence FFA risk in genetically predisposed individuals.
IN PRACTICE:
“This gene-environment interaction analysis suggests that the protective effect of the CYPIB1 missense variant on FFA risk might be mediated by exposure” to OCs, the authors wrote. The study, they added, “underscores the importance of considering genetic predispositions and environmental factors, such as oral contraceptive use, in understanding and managing frontal fibrosing alopecia.”
SOURCE:
Tuntas Rayinda, MD, MSc, PhD, of St. John’s Institute of Dermatology, King’s College London, led the study, which was published online May 29, 2024, in JAMA Dermatology.
LIMITATIONS:
The study’s reliance on self-reported OC use may have introduced recall and differences in ascertainment of OC use between patient and control groups and could have affected the study’s findings. The study also did not collect information on the type of OC used, which could have influenced the observed interaction.
DISCLOSURES:
The study was supported by the British Skin Foundation Young Investigator Award. One investigator reported being a subinvestigator on an alopecia areata study funded by Pfizer. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
Investigators found that .
METHODOLOGY:
- OC use has been considered a possible factor behind the increased incidence of FFA because it was first documented in 1994, and a recent genome-wide association study of FFA identified a signal for an association with a variant in CYP1B1.
- The same researchers conducted a gene-environment interaction study with a case-control design involving 489 White female patients (mean age, 65.8 years) with FFA and 34,254 controls, matched for age and genetic ancestry.
- Data were collected from July 2015 to September 2017 and analyzed from October 2022 to December 2023.
- The study aimed to investigate the modulatory effect of OC use on the CYP1B1 variant’s impact on FFA risk, using logistic regression models for analysis.
TAKEAWAY:
- The use of OCs was associated with a 1.9 times greater risk for FFA in individuals with the specific CYP1B1 genetic variant, but there was no association among those with no history of OC use.
- The study suggests a significant gene-environment interaction, indicating that OC use may influence FFA risk in genetically predisposed individuals.
IN PRACTICE:
“This gene-environment interaction analysis suggests that the protective effect of the CYPIB1 missense variant on FFA risk might be mediated by exposure” to OCs, the authors wrote. The study, they added, “underscores the importance of considering genetic predispositions and environmental factors, such as oral contraceptive use, in understanding and managing frontal fibrosing alopecia.”
SOURCE:
Tuntas Rayinda, MD, MSc, PhD, of St. John’s Institute of Dermatology, King’s College London, led the study, which was published online May 29, 2024, in JAMA Dermatology.
LIMITATIONS:
The study’s reliance on self-reported OC use may have introduced recall and differences in ascertainment of OC use between patient and control groups and could have affected the study’s findings. The study also did not collect information on the type of OC used, which could have influenced the observed interaction.
DISCLOSURES:
The study was supported by the British Skin Foundation Young Investigator Award. One investigator reported being a subinvestigator on an alopecia areata study funded by Pfizer. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
Investigators found that .
METHODOLOGY:
- OC use has been considered a possible factor behind the increased incidence of FFA because it was first documented in 1994, and a recent genome-wide association study of FFA identified a signal for an association with a variant in CYP1B1.
- The same researchers conducted a gene-environment interaction study with a case-control design involving 489 White female patients (mean age, 65.8 years) with FFA and 34,254 controls, matched for age and genetic ancestry.
- Data were collected from July 2015 to September 2017 and analyzed from October 2022 to December 2023.
- The study aimed to investigate the modulatory effect of OC use on the CYP1B1 variant’s impact on FFA risk, using logistic regression models for analysis.
TAKEAWAY:
- The use of OCs was associated with a 1.9 times greater risk for FFA in individuals with the specific CYP1B1 genetic variant, but there was no association among those with no history of OC use.
- The study suggests a significant gene-environment interaction, indicating that OC use may influence FFA risk in genetically predisposed individuals.
IN PRACTICE:
“This gene-environment interaction analysis suggests that the protective effect of the CYPIB1 missense variant on FFA risk might be mediated by exposure” to OCs, the authors wrote. The study, they added, “underscores the importance of considering genetic predispositions and environmental factors, such as oral contraceptive use, in understanding and managing frontal fibrosing alopecia.”
SOURCE:
Tuntas Rayinda, MD, MSc, PhD, of St. John’s Institute of Dermatology, King’s College London, led the study, which was published online May 29, 2024, in JAMA Dermatology.
LIMITATIONS:
The study’s reliance on self-reported OC use may have introduced recall and differences in ascertainment of OC use between patient and control groups and could have affected the study’s findings. The study also did not collect information on the type of OC used, which could have influenced the observed interaction.
DISCLOSURES:
The study was supported by the British Skin Foundation Young Investigator Award. One investigator reported being a subinvestigator on an alopecia areata study funded by Pfizer. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Recalcitrant Folliculitis Decalvans Treatment Outcomes With Biologics and Small Molecule Inhibitors
Folliculitis decalvans (FD) is classified as a rare primary neutrophilic cicatricial alopecia occurring predominantly in middle-aged adults. Although the true etiology is still unknown, the pathogenesis behind the inflammatory follicular lesions stems from possible Staphylococcus aureus infection and an impaired host immune system in response to released superantigens. 1 The clinical severity of this inflammatory scalp disorder can range from mild to severe and debilitating. Multiple treatment regimens have been developed with the goal of maintaining full remission. We provide a summary of tumor necrosis factor (TNF) inhibitors, Janus kinase (JAK) inhibitors, phosphodiesterase 4 (PDE4) inhibitors, and monoclonal antibodies being utilized for patients with therapy-recalcitrant FD.
Methods
We conducted a PubMed, Medline, and Google Scholar search for the terms refractory FD, recalcitrant FD, or therapy-resistant FD to identify articles published in English from 1998 to 2022. Articles that reported recalcitrant cases and subsequent therapy with TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies were included. Articles were excluded if recalcitrant cases were not clearly defined. Remission was defined as no recurrence in lesions or pustules or as a reduction in the inflammatory process with stabilization upon continuation or discontinuation of the therapy regimen. Two reviewers (T.F. and K.U.) independently searched for and screened each report.
Results
Treatment of recalcitrant FD with biologics or small molecule inhibitors was discussed in 9 studies with a combined total of 35 patients.2-10 The treatment regimens included TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies (Table).
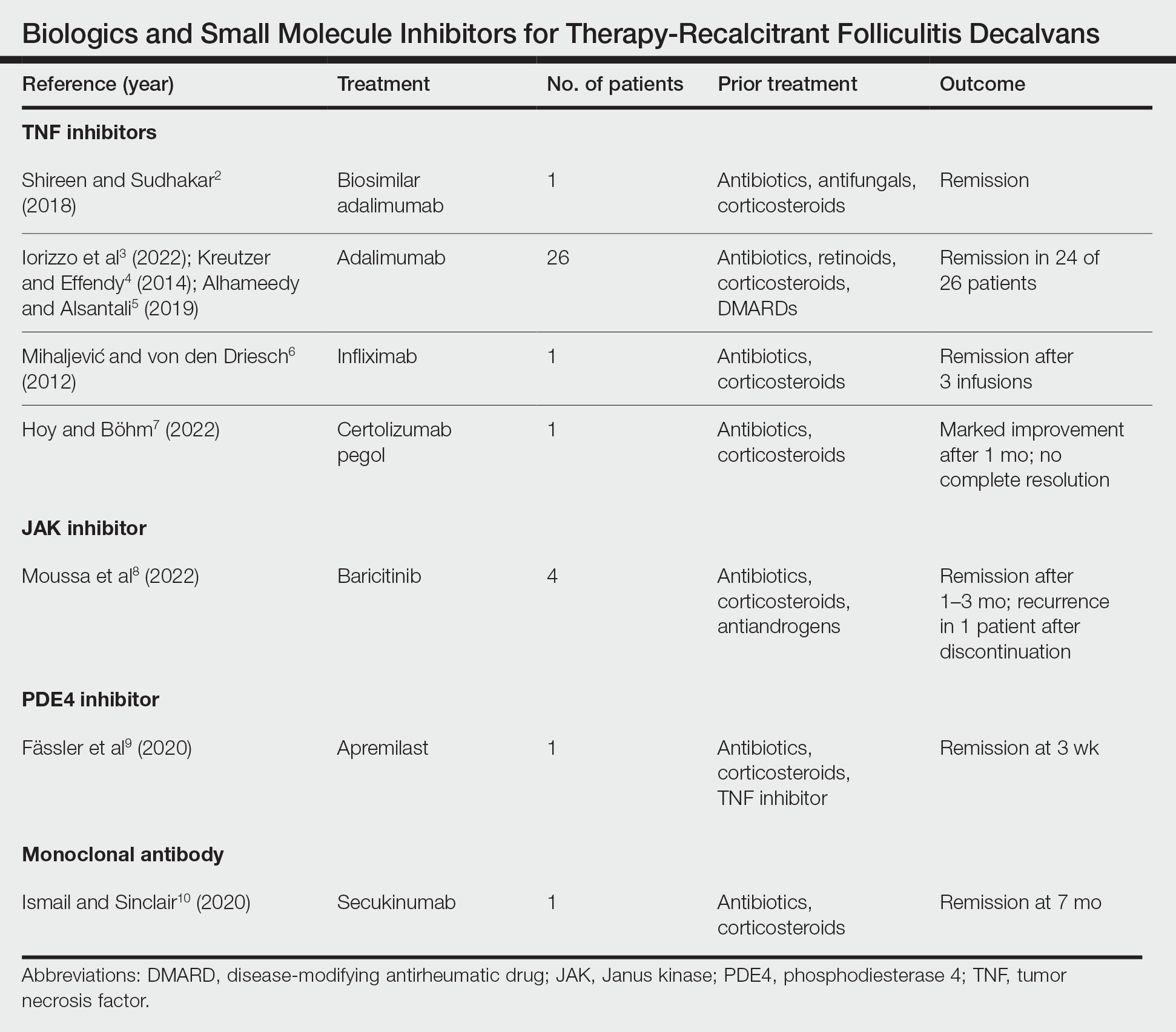
The TNF inhibitors were utilized in 6 reports with a combined total of 29 patients. Treatments included adalimumab or biosimilar adalimumab (27/29 patients), infliximab (1/29 patients), and certolizumab pegol (1/29 patients). Remission was reported in 26 of 29 cases. There were 2 nonresponders to adalimumab and marked improvement with certolizumab pegol without complete resolution. The use of the JAK inhibitor baricitinib in 4 patients resulted in remission. In all 4 patients, baricitinib was used with concurrent treatments, and remission was achieved in an average of 2.25 months. The use of a PDE4 inhibitor, apremilast, was reported in 1 case; remission was achieved in 3 weeks. Secukinumab, a monoclonal antibody that targets IL-17, was utilized in 1 patient. Marked improvement was seen after 2 months, with complete remission in 7 months.
Comment
Traditional treatment regimens for FD most often include a combination of topical and oral antibiotics; isotretinoin; and oral, topical, or intralesional corticosteroids. In the past, interventions typically were suppressive as opposed to curative; however, recent treatment advancements have shown promise in achieving lasting remission.
Most reports targeting treatment-resistant FD involved the use of TNF inhibitors, including adalimumab, biosimilar adalimumab, infliximab, and certolizumab pegol. Adalimumab was the most frequently used TNF inhibitor, with 24 of 26 treated patients achieving remission. Adalimumab may have been used the most in the treatment of FD because TNF is pronounced in other neutrophilic dermatoses that have been successfully treated with TNF inhibitors. It has been reported that adalimumab needs to be continued, as stoppage or interruption led to relapse.3
Although there are few reports of the use of JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies for FD, these treatment modalities show promise, as their use led to marked improvement or lasting remission with ongoing treatment. The use of the PDE4 inhibitor apremilast displayed the most rapid improvement of any of the reviewed treatments, with remission achieved in just 3 weeks.9 The rapid success of apremilast may be attributed to the inhibitory effect on neutrophils.
Miguel-Gómez et al11 provided a therapeutic protocol for FD based on the severity of disease (N=60). The protocol included rifampicin plus clindamycin for the treatment of severe disease, as 90.5% (19/21) of resistant cases showed clinical response, with remission of 5 months’ duration. Although this may be acceptable for some patients, others may require an alternative approach. Tietze et al12 showed that rifampicin and clindamycin had the lowest success rate for long-term remission, with 8 of 10 patients relapsing within 2 to 4 months. In addition, the emergence of antimicrobial resistance remains a major concern in the treatment of FD. Upon the review of the most recent reports of successful treatment of therapy-resistant FD, biologics and small molecule inhibitors have shown remission extending through a 12-month follow-up period. We suggest considering the addition of biologics and small molecule inhibitors to the treatment protocol for severe or resistant disease.
Limitations—In the articles reviewed, the definition of remission was inconsistent among authors—some characterized it as no recurrence in lesions or pustules and some as a reduction in the inflammatory process. True duration of remission was difficult to assess from case reports, as follow-up periods varied prior to publication. The studies included in this review consisted mainly of small sample sizes owing to the rarity of FD, and consequently, strength of evidence is lacking. Inherent to the nature of systematic reviews, publication bias may have occurred. Lastly, several studies were impacted by difficulty in obtaining optimal treatment due to financial hardship, and regimens were adjusted accordingly.
Conclusion
The relapsing nature of FD leads to frustration and poor quality of life for patients. There is a paucity of data to guide treatment when FD remains recalcitrant to traditional therapy. Therapies such as TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies have shown success in the treatment of this often difficult-to-treat disease. Small sample sizes in reports discussing treatment for resistant cases as well as conflicting results make it challenging to draw conclusions about treatment efficacy. Larger studies are needed to understand the long-term outcomes of treatment options. Regardless, disease severity, patient history, patient preferences, and treatment goals can guide the selection of therapeutic options.
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Shireen F, Sudhakar A. A case of isotretinoin therapy-refractory folliculitis decalvans treated successfully with biosimilar adalimumab (Exemptia). Int J Trichology. 2018;10:240-241.
- Iorizzo M, Starace M, Vano-Galvan S, et al. Refractory folliculitis decalvans treated with adalimumab: a case series of 23 patients. J Am Acad Dermatol. 2022;87:666-669. doi:10.1016/j.jaad.2022.02.044
- Kreutzer K, Effendy I. Therapy-resistant folliculitis decalvans and lichen planopilaris successfully treated with adalimumab. J Dtsch Dermatol Ges. 2014;12:74-76. doi:10.1111/ddg.12224
- Alhameedy MM, Alsantali AM. Therapy-recalcitrant folliculitis decalvans controlled successfully with adalimumab. Int J Trichology. 2019;11:241-243. doi:10.4103/ijt.ijt_92_19
- Mihaljevic´ N, von den Driesch P. Successful use of infliximab in a patient with recalcitrant folliculitis decalvans. J Dtsch Dermatol Ges. 2012;10:589-590. doi:10.1111/j.1610-0387.2012.07972.x
- Hoy M, Böhm M. Therapy-refractory folliculitis decalvans treated with certolizumab pegol. Int J Dermatol. 2022;61:e26-e28. doi:10.1111/ijd.15914
- Moussa A, Asfour L, Eisman S, et al. Successful treatment of folliculitis decalvans with baricitinib: a case series. Australas J Dermatol. 2022;63:279-281. doi:10.1111/ajd.13786
- Fässler M, Radonjic-Hoesli S, Feldmeyer L, et al. Successful treatment of refractory folliculitis decalvans with apremilast. JAAD Case Rep. 2020;6:1079-1081. doi:10.1016/j.jdcr.2020.08.019
- Ismail FF, Sinclair R. Successful treatment of refractory folliculitis decalvans with secukinumab. Australas J Dermatol. 2020;61:165-166. doi:10.1111/ajd.13190
- Miguel-Gómez L, Rodrigues-Barata AR, Molina-Ruiz A, et al. Folliculitis decalvans: effectiveness of therapies and prognostic factors in a multicenter series of 60 patients with long-term follow-up. J Am Acad Dermatol. 2018;79:878-883. doi:10.1016/j.jaad.2018.05.1240
- Tietze JK, Heppt MV, von Preußen A, et al. Oral isotretinoin as the most effective treatment in folliculitis decalvans: a retrospective comparison of different treatment regimens in 28 patients. J Eur Acad Dermatol Venereol. 2015;29:1816-1821. doi:10.1111/jdv.13052
Folliculitis decalvans (FD) is classified as a rare primary neutrophilic cicatricial alopecia occurring predominantly in middle-aged adults. Although the true etiology is still unknown, the pathogenesis behind the inflammatory follicular lesions stems from possible Staphylococcus aureus infection and an impaired host immune system in response to released superantigens. 1 The clinical severity of this inflammatory scalp disorder can range from mild to severe and debilitating. Multiple treatment regimens have been developed with the goal of maintaining full remission. We provide a summary of tumor necrosis factor (TNF) inhibitors, Janus kinase (JAK) inhibitors, phosphodiesterase 4 (PDE4) inhibitors, and monoclonal antibodies being utilized for patients with therapy-recalcitrant FD.
Methods
We conducted a PubMed, Medline, and Google Scholar search for the terms refractory FD, recalcitrant FD, or therapy-resistant FD to identify articles published in English from 1998 to 2022. Articles that reported recalcitrant cases and subsequent therapy with TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies were included. Articles were excluded if recalcitrant cases were not clearly defined. Remission was defined as no recurrence in lesions or pustules or as a reduction in the inflammatory process with stabilization upon continuation or discontinuation of the therapy regimen. Two reviewers (T.F. and K.U.) independently searched for and screened each report.
Results
Treatment of recalcitrant FD with biologics or small molecule inhibitors was discussed in 9 studies with a combined total of 35 patients.2-10 The treatment regimens included TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies (Table).

The TNF inhibitors were utilized in 6 reports with a combined total of 29 patients. Treatments included adalimumab or biosimilar adalimumab (27/29 patients), infliximab (1/29 patients), and certolizumab pegol (1/29 patients). Remission was reported in 26 of 29 cases. There were 2 nonresponders to adalimumab and marked improvement with certolizumab pegol without complete resolution. The use of the JAK inhibitor baricitinib in 4 patients resulted in remission. In all 4 patients, baricitinib was used with concurrent treatments, and remission was achieved in an average of 2.25 months. The use of a PDE4 inhibitor, apremilast, was reported in 1 case; remission was achieved in 3 weeks. Secukinumab, a monoclonal antibody that targets IL-17, was utilized in 1 patient. Marked improvement was seen after 2 months, with complete remission in 7 months.
Comment
Traditional treatment regimens for FD most often include a combination of topical and oral antibiotics; isotretinoin; and oral, topical, or intralesional corticosteroids. In the past, interventions typically were suppressive as opposed to curative; however, recent treatment advancements have shown promise in achieving lasting remission.
Most reports targeting treatment-resistant FD involved the use of TNF inhibitors, including adalimumab, biosimilar adalimumab, infliximab, and certolizumab pegol. Adalimumab was the most frequently used TNF inhibitor, with 24 of 26 treated patients achieving remission. Adalimumab may have been used the most in the treatment of FD because TNF is pronounced in other neutrophilic dermatoses that have been successfully treated with TNF inhibitors. It has been reported that adalimumab needs to be continued, as stoppage or interruption led to relapse.3
Although there are few reports of the use of JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies for FD, these treatment modalities show promise, as their use led to marked improvement or lasting remission with ongoing treatment. The use of the PDE4 inhibitor apremilast displayed the most rapid improvement of any of the reviewed treatments, with remission achieved in just 3 weeks.9 The rapid success of apremilast may be attributed to the inhibitory effect on neutrophils.
Miguel-Gómez et al11 provided a therapeutic protocol for FD based on the severity of disease (N=60). The protocol included rifampicin plus clindamycin for the treatment of severe disease, as 90.5% (19/21) of resistant cases showed clinical response, with remission of 5 months’ duration. Although this may be acceptable for some patients, others may require an alternative approach. Tietze et al12 showed that rifampicin and clindamycin had the lowest success rate for long-term remission, with 8 of 10 patients relapsing within 2 to 4 months. In addition, the emergence of antimicrobial resistance remains a major concern in the treatment of FD. Upon the review of the most recent reports of successful treatment of therapy-resistant FD, biologics and small molecule inhibitors have shown remission extending through a 12-month follow-up period. We suggest considering the addition of biologics and small molecule inhibitors to the treatment protocol for severe or resistant disease.
Limitations—In the articles reviewed, the definition of remission was inconsistent among authors—some characterized it as no recurrence in lesions or pustules and some as a reduction in the inflammatory process. True duration of remission was difficult to assess from case reports, as follow-up periods varied prior to publication. The studies included in this review consisted mainly of small sample sizes owing to the rarity of FD, and consequently, strength of evidence is lacking. Inherent to the nature of systematic reviews, publication bias may have occurred. Lastly, several studies were impacted by difficulty in obtaining optimal treatment due to financial hardship, and regimens were adjusted accordingly.
Conclusion
The relapsing nature of FD leads to frustration and poor quality of life for patients. There is a paucity of data to guide treatment when FD remains recalcitrant to traditional therapy. Therapies such as TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies have shown success in the treatment of this often difficult-to-treat disease. Small sample sizes in reports discussing treatment for resistant cases as well as conflicting results make it challenging to draw conclusions about treatment efficacy. Larger studies are needed to understand the long-term outcomes of treatment options. Regardless, disease severity, patient history, patient preferences, and treatment goals can guide the selection of therapeutic options.
Folliculitis decalvans (FD) is classified as a rare primary neutrophilic cicatricial alopecia occurring predominantly in middle-aged adults. Although the true etiology is still unknown, the pathogenesis behind the inflammatory follicular lesions stems from possible Staphylococcus aureus infection and an impaired host immune system in response to released superantigens. 1 The clinical severity of this inflammatory scalp disorder can range from mild to severe and debilitating. Multiple treatment regimens have been developed with the goal of maintaining full remission. We provide a summary of tumor necrosis factor (TNF) inhibitors, Janus kinase (JAK) inhibitors, phosphodiesterase 4 (PDE4) inhibitors, and monoclonal antibodies being utilized for patients with therapy-recalcitrant FD.
Methods
We conducted a PubMed, Medline, and Google Scholar search for the terms refractory FD, recalcitrant FD, or therapy-resistant FD to identify articles published in English from 1998 to 2022. Articles that reported recalcitrant cases and subsequent therapy with TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies were included. Articles were excluded if recalcitrant cases were not clearly defined. Remission was defined as no recurrence in lesions or pustules or as a reduction in the inflammatory process with stabilization upon continuation or discontinuation of the therapy regimen. Two reviewers (T.F. and K.U.) independently searched for and screened each report.
Results
Treatment of recalcitrant FD with biologics or small molecule inhibitors was discussed in 9 studies with a combined total of 35 patients.2-10 The treatment regimens included TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies (Table).

The TNF inhibitors were utilized in 6 reports with a combined total of 29 patients. Treatments included adalimumab or biosimilar adalimumab (27/29 patients), infliximab (1/29 patients), and certolizumab pegol (1/29 patients). Remission was reported in 26 of 29 cases. There were 2 nonresponders to adalimumab and marked improvement with certolizumab pegol without complete resolution. The use of the JAK inhibitor baricitinib in 4 patients resulted in remission. In all 4 patients, baricitinib was used with concurrent treatments, and remission was achieved in an average of 2.25 months. The use of a PDE4 inhibitor, apremilast, was reported in 1 case; remission was achieved in 3 weeks. Secukinumab, a monoclonal antibody that targets IL-17, was utilized in 1 patient. Marked improvement was seen after 2 months, with complete remission in 7 months.
Comment
Traditional treatment regimens for FD most often include a combination of topical and oral antibiotics; isotretinoin; and oral, topical, or intralesional corticosteroids. In the past, interventions typically were suppressive as opposed to curative; however, recent treatment advancements have shown promise in achieving lasting remission.
Most reports targeting treatment-resistant FD involved the use of TNF inhibitors, including adalimumab, biosimilar adalimumab, infliximab, and certolizumab pegol. Adalimumab was the most frequently used TNF inhibitor, with 24 of 26 treated patients achieving remission. Adalimumab may have been used the most in the treatment of FD because TNF is pronounced in other neutrophilic dermatoses that have been successfully treated with TNF inhibitors. It has been reported that adalimumab needs to be continued, as stoppage or interruption led to relapse.3
Although there are few reports of the use of JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies for FD, these treatment modalities show promise, as their use led to marked improvement or lasting remission with ongoing treatment. The use of the PDE4 inhibitor apremilast displayed the most rapid improvement of any of the reviewed treatments, with remission achieved in just 3 weeks.9 The rapid success of apremilast may be attributed to the inhibitory effect on neutrophils.
Miguel-Gómez et al11 provided a therapeutic protocol for FD based on the severity of disease (N=60). The protocol included rifampicin plus clindamycin for the treatment of severe disease, as 90.5% (19/21) of resistant cases showed clinical response, with remission of 5 months’ duration. Although this may be acceptable for some patients, others may require an alternative approach. Tietze et al12 showed that rifampicin and clindamycin had the lowest success rate for long-term remission, with 8 of 10 patients relapsing within 2 to 4 months. In addition, the emergence of antimicrobial resistance remains a major concern in the treatment of FD. Upon the review of the most recent reports of successful treatment of therapy-resistant FD, biologics and small molecule inhibitors have shown remission extending through a 12-month follow-up period. We suggest considering the addition of biologics and small molecule inhibitors to the treatment protocol for severe or resistant disease.
Limitations—In the articles reviewed, the definition of remission was inconsistent among authors—some characterized it as no recurrence in lesions or pustules and some as a reduction in the inflammatory process. True duration of remission was difficult to assess from case reports, as follow-up periods varied prior to publication. The studies included in this review consisted mainly of small sample sizes owing to the rarity of FD, and consequently, strength of evidence is lacking. Inherent to the nature of systematic reviews, publication bias may have occurred. Lastly, several studies were impacted by difficulty in obtaining optimal treatment due to financial hardship, and regimens were adjusted accordingly.
Conclusion
The relapsing nature of FD leads to frustration and poor quality of life for patients. There is a paucity of data to guide treatment when FD remains recalcitrant to traditional therapy. Therapies such as TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies have shown success in the treatment of this often difficult-to-treat disease. Small sample sizes in reports discussing treatment for resistant cases as well as conflicting results make it challenging to draw conclusions about treatment efficacy. Larger studies are needed to understand the long-term outcomes of treatment options. Regardless, disease severity, patient history, patient preferences, and treatment goals can guide the selection of therapeutic options.
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Shireen F, Sudhakar A. A case of isotretinoin therapy-refractory folliculitis decalvans treated successfully with biosimilar adalimumab (Exemptia). Int J Trichology. 2018;10:240-241.
- Iorizzo M, Starace M, Vano-Galvan S, et al. Refractory folliculitis decalvans treated with adalimumab: a case series of 23 patients. J Am Acad Dermatol. 2022;87:666-669. doi:10.1016/j.jaad.2022.02.044
- Kreutzer K, Effendy I. Therapy-resistant folliculitis decalvans and lichen planopilaris successfully treated with adalimumab. J Dtsch Dermatol Ges. 2014;12:74-76. doi:10.1111/ddg.12224
- Alhameedy MM, Alsantali AM. Therapy-recalcitrant folliculitis decalvans controlled successfully with adalimumab. Int J Trichology. 2019;11:241-243. doi:10.4103/ijt.ijt_92_19
- Mihaljevic´ N, von den Driesch P. Successful use of infliximab in a patient with recalcitrant folliculitis decalvans. J Dtsch Dermatol Ges. 2012;10:589-590. doi:10.1111/j.1610-0387.2012.07972.x
- Hoy M, Böhm M. Therapy-refractory folliculitis decalvans treated with certolizumab pegol. Int J Dermatol. 2022;61:e26-e28. doi:10.1111/ijd.15914
- Moussa A, Asfour L, Eisman S, et al. Successful treatment of folliculitis decalvans with baricitinib: a case series. Australas J Dermatol. 2022;63:279-281. doi:10.1111/ajd.13786
- Fässler M, Radonjic-Hoesli S, Feldmeyer L, et al. Successful treatment of refractory folliculitis decalvans with apremilast. JAAD Case Rep. 2020;6:1079-1081. doi:10.1016/j.jdcr.2020.08.019
- Ismail FF, Sinclair R. Successful treatment of refractory folliculitis decalvans with secukinumab. Australas J Dermatol. 2020;61:165-166. doi:10.1111/ajd.13190
- Miguel-Gómez L, Rodrigues-Barata AR, Molina-Ruiz A, et al. Folliculitis decalvans: effectiveness of therapies and prognostic factors in a multicenter series of 60 patients with long-term follow-up. J Am Acad Dermatol. 2018;79:878-883. doi:10.1016/j.jaad.2018.05.1240
- Tietze JK, Heppt MV, von Preußen A, et al. Oral isotretinoin as the most effective treatment in folliculitis decalvans: a retrospective comparison of different treatment regimens in 28 patients. J Eur Acad Dermatol Venereol. 2015;29:1816-1821. doi:10.1111/jdv.13052
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Shireen F, Sudhakar A. A case of isotretinoin therapy-refractory folliculitis decalvans treated successfully with biosimilar adalimumab (Exemptia). Int J Trichology. 2018;10:240-241.
- Iorizzo M, Starace M, Vano-Galvan S, et al. Refractory folliculitis decalvans treated with adalimumab: a case series of 23 patients. J Am Acad Dermatol. 2022;87:666-669. doi:10.1016/j.jaad.2022.02.044
- Kreutzer K, Effendy I. Therapy-resistant folliculitis decalvans and lichen planopilaris successfully treated with adalimumab. J Dtsch Dermatol Ges. 2014;12:74-76. doi:10.1111/ddg.12224
- Alhameedy MM, Alsantali AM. Therapy-recalcitrant folliculitis decalvans controlled successfully with adalimumab. Int J Trichology. 2019;11:241-243. doi:10.4103/ijt.ijt_92_19
- Mihaljevic´ N, von den Driesch P. Successful use of infliximab in a patient with recalcitrant folliculitis decalvans. J Dtsch Dermatol Ges. 2012;10:589-590. doi:10.1111/j.1610-0387.2012.07972.x
- Hoy M, Böhm M. Therapy-refractory folliculitis decalvans treated with certolizumab pegol. Int J Dermatol. 2022;61:e26-e28. doi:10.1111/ijd.15914
- Moussa A, Asfour L, Eisman S, et al. Successful treatment of folliculitis decalvans with baricitinib: a case series. Australas J Dermatol. 2022;63:279-281. doi:10.1111/ajd.13786
- Fässler M, Radonjic-Hoesli S, Feldmeyer L, et al. Successful treatment of refractory folliculitis decalvans with apremilast. JAAD Case Rep. 2020;6:1079-1081. doi:10.1016/j.jdcr.2020.08.019
- Ismail FF, Sinclair R. Successful treatment of refractory folliculitis decalvans with secukinumab. Australas J Dermatol. 2020;61:165-166. doi:10.1111/ajd.13190
- Miguel-Gómez L, Rodrigues-Barata AR, Molina-Ruiz A, et al. Folliculitis decalvans: effectiveness of therapies and prognostic factors in a multicenter series of 60 patients with long-term follow-up. J Am Acad Dermatol. 2018;79:878-883. doi:10.1016/j.jaad.2018.05.1240
- Tietze JK, Heppt MV, von Preußen A, et al. Oral isotretinoin as the most effective treatment in folliculitis decalvans: a retrospective comparison of different treatment regimens in 28 patients. J Eur Acad Dermatol Venereol. 2015;29:1816-1821. doi:10.1111/jdv.13052
Practice Points
- Tumor necrosis factor inhibitors, Janus kinase inhibitors, phosphodiesterase 4 inhibitors, and monoclonal antibodies have shown success in the treatment of folliculitis decalvans resistant to traditional therapies.
- The true etiology of folliculitis decalvans is still unknown, but possible factors include Staphylococcus aureus infection and an impaired host immune system, which may benefit from treatment with biologics and small molecule inhibitors.
PCOS: Laser, Light Therapy Helpful for Hirsutism
BY DEEPA VARMA
TOPLINE:
, according to the results of a systematic review.
METHODOLOGY:
- Hirsutism, which affects 70%-80% of women with PCOS, is frequently marginalized as a cosmetic issue by healthcare providers, despite its significant psychological repercussions, including diminished self-esteem, reduced quality of life, and heightened depression.
- The 2023 international evidence-based PCOS guideline considers managing hirsutism a priority in women with PCOS.
- Researchers reviewed six studies (four randomized controlled trials and two cohort studies), which included 423 patients with PCOS who underwent laser or light-based hair reduction therapies, published through 2022.
- The studies evaluated the alexandrite laser, diode laser, and intense pulsed light (IPL) therapy, with and without pharmacological treatments. The main outcomes were hirsutism severity, psychological outcome, and adverse events.
TAKEAWAY:
- Alexandrite laser (wavelength, 755 nm) showed effective hair reduction and improved patient satisfaction (one study); high-fluence treatment yielded better outcomes than low-fluence treatment (one study). Alexandrite laser 755 nm also showed longer hair-free intervals and greater hair reduction than IPL therapy at 650-1000 nm (one study).
- Combined IPL (600 nm) and metformin therapy improved hirsutism and hair count reduction compared with IPL alone, but with more side effects (one study).
- Diode laser treatments (810 nm) with combined oral contraceptives improved hirsutism and related quality of life measures compared with diode laser alone or with metformin (one study).
- Comparing two diode lasers (wavelengths, 810 nm), low-fluence, high repetition laser showed superior hair width reduction and lower pain scores than high fluence, low-repetition laser (one study).
IN PRACTICE:
Laser and light treatments alone or combined with other treatments have demonstrated “encouraging results in reducing hirsutism severity, enhancing psychological well-being, and improving overall quality of life for affected individuals,” the authors wrote, noting that additional high-quality trials evaluating these treatments, which include more patients with different skin tones, are needed.
SOURCE:
The first author of the review is Katrina Tan, MD, Monash Health, Department of Dermatology, Melbourne, Victoria, Australia, and it was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include low certainty of evidence because of the observational nature of some of the studies, the small number of studies, and underrepresentation of darker skin types, limiting generalizability.
DISCLOSURES:
The review is part of an update to the PCOS guideline, which was funded by the Australian National Health and Medical Research Council through various organizations. Several authors reported receiving grants and personal fees outside this work. Dr. Tan was a member of the 2023 PCOS guideline evidence team. Other authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
BY DEEPA VARMA
TOPLINE:
, according to the results of a systematic review.
METHODOLOGY:
- Hirsutism, which affects 70%-80% of women with PCOS, is frequently marginalized as a cosmetic issue by healthcare providers, despite its significant psychological repercussions, including diminished self-esteem, reduced quality of life, and heightened depression.
- The 2023 international evidence-based PCOS guideline considers managing hirsutism a priority in women with PCOS.
- Researchers reviewed six studies (four randomized controlled trials and two cohort studies), which included 423 patients with PCOS who underwent laser or light-based hair reduction therapies, published through 2022.
- The studies evaluated the alexandrite laser, diode laser, and intense pulsed light (IPL) therapy, with and without pharmacological treatments. The main outcomes were hirsutism severity, psychological outcome, and adverse events.
TAKEAWAY:
- Alexandrite laser (wavelength, 755 nm) showed effective hair reduction and improved patient satisfaction (one study); high-fluence treatment yielded better outcomes than low-fluence treatment (one study). Alexandrite laser 755 nm also showed longer hair-free intervals and greater hair reduction than IPL therapy at 650-1000 nm (one study).
- Combined IPL (600 nm) and metformin therapy improved hirsutism and hair count reduction compared with IPL alone, but with more side effects (one study).
- Diode laser treatments (810 nm) with combined oral contraceptives improved hirsutism and related quality of life measures compared with diode laser alone or with metformin (one study).
- Comparing two diode lasers (wavelengths, 810 nm), low-fluence, high repetition laser showed superior hair width reduction and lower pain scores than high fluence, low-repetition laser (one study).
IN PRACTICE:
Laser and light treatments alone or combined with other treatments have demonstrated “encouraging results in reducing hirsutism severity, enhancing psychological well-being, and improving overall quality of life for affected individuals,” the authors wrote, noting that additional high-quality trials evaluating these treatments, which include more patients with different skin tones, are needed.
SOURCE:
The first author of the review is Katrina Tan, MD, Monash Health, Department of Dermatology, Melbourne, Victoria, Australia, and it was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include low certainty of evidence because of the observational nature of some of the studies, the small number of studies, and underrepresentation of darker skin types, limiting generalizability.
DISCLOSURES:
The review is part of an update to the PCOS guideline, which was funded by the Australian National Health and Medical Research Council through various organizations. Several authors reported receiving grants and personal fees outside this work. Dr. Tan was a member of the 2023 PCOS guideline evidence team. Other authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
BY DEEPA VARMA
TOPLINE:
, according to the results of a systematic review.
METHODOLOGY:
- Hirsutism, which affects 70%-80% of women with PCOS, is frequently marginalized as a cosmetic issue by healthcare providers, despite its significant psychological repercussions, including diminished self-esteem, reduced quality of life, and heightened depression.
- The 2023 international evidence-based PCOS guideline considers managing hirsutism a priority in women with PCOS.
- Researchers reviewed six studies (four randomized controlled trials and two cohort studies), which included 423 patients with PCOS who underwent laser or light-based hair reduction therapies, published through 2022.
- The studies evaluated the alexandrite laser, diode laser, and intense pulsed light (IPL) therapy, with and without pharmacological treatments. The main outcomes were hirsutism severity, psychological outcome, and adverse events.
TAKEAWAY:
- Alexandrite laser (wavelength, 755 nm) showed effective hair reduction and improved patient satisfaction (one study); high-fluence treatment yielded better outcomes than low-fluence treatment (one study). Alexandrite laser 755 nm also showed longer hair-free intervals and greater hair reduction than IPL therapy at 650-1000 nm (one study).
- Combined IPL (600 nm) and metformin therapy improved hirsutism and hair count reduction compared with IPL alone, but with more side effects (one study).
- Diode laser treatments (810 nm) with combined oral contraceptives improved hirsutism and related quality of life measures compared with diode laser alone or with metformin (one study).
- Comparing two diode lasers (wavelengths, 810 nm), low-fluence, high repetition laser showed superior hair width reduction and lower pain scores than high fluence, low-repetition laser (one study).
IN PRACTICE:
Laser and light treatments alone or combined with other treatments have demonstrated “encouraging results in reducing hirsutism severity, enhancing psychological well-being, and improving overall quality of life for affected individuals,” the authors wrote, noting that additional high-quality trials evaluating these treatments, which include more patients with different skin tones, are needed.
SOURCE:
The first author of the review is Katrina Tan, MD, Monash Health, Department of Dermatology, Melbourne, Victoria, Australia, and it was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include low certainty of evidence because of the observational nature of some of the studies, the small number of studies, and underrepresentation of darker skin types, limiting generalizability.
DISCLOSURES:
The review is part of an update to the PCOS guideline, which was funded by the Australian National Health and Medical Research Council through various organizations. Several authors reported receiving grants and personal fees outside this work. Dr. Tan was a member of the 2023 PCOS guideline evidence team. Other authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Consensus Statement Aims to Guide Use of Low-Dose Oral Minoxidil for Hair Loss
SAN DIEGO — .
Those are among the key recommendations that resulted from a modified eDelphi consensus of experts who convened to develop guidelines for LDOM prescribing and monitoring.
“Topical minoxidil is safe, effective, over-the-counter, and FDA-approved to treat the most common form of hair loss, androgenetic alopecia,” one of the study authors, Jennifer Fu, MD, a dermatologist who directs the Hair Disorders Clinic at the University of California, San Francisco, told this news organization following the annual meeting of the American Academy of Dermatology. The results of the expert consensus were presented during a poster session at the meeting. “It is often used off label for other types of hair loss, yet clinicians who treat hair loss know that patient compliance with topical minoxidil can be poor for a variety of reasons,” she said. “Patients report that it can be difficult to apply and complicate hair styling. For many patients, topical minoxidil can be drying or cause irritant or allergic contact reactions.”
LDOM has become a popular alternative for patients for whom topical minoxidil is logistically challenging, irritating, or ineffective, she continued. Although oral minoxidil is no longer a first-line antihypertensive agent given the risk of cardiovascular adverse effects at higher antihypertensive dosing (10-40 mg daily), a growing number of small studies have documented the use of LDOM at doses ranging from 0.25 mg to 5 mg daily as a safe, effective option for various types of hair loss.
“Given the current absence of larger trials on this topic, our research group identified a need for expert-based guidelines for prescribing and monitoring LDOM use in hair loss patients,” Dr. Fu said. “Our goal was to provide clinicians who treat hair loss patients a road map for using LDOM effectively, maximizing hair growth, and minimizing potential cardiovascular adverse effects.”
Arriving at a Consensus
The process involved 43 hair loss specialists from 12 countries with an average of 6.29 years of experience with LDOM for hair loss, who participated in a multi-round modified Delphi process. They considered questions that addressed LDOM safety, efficacy, dosing, and monitoring for hair loss, and consensus was reached if at least 70% of participants indicated “agree” or “strongly agree” on a five-point Likert scale. Round 1 consisted of 180 open-ended, multiple-choice, or Likert-scale questions, while round 2 involved 121 Likert-scale questions, round 3 consisted of 16 Likert-scale questions, and round 4 included 11 Likert-scale questions. In all, 94 items achieved Likert-scale consensus.
Specifically, experts on the panel found a direct benefit of LDOM for androgenetic alopecia, age-related patterned thinning, alopecia areata, telogen effluvium, traction alopecia, persistent chemotherapy-induced alopecia, and endocrine therapy-induced alopecia. They found a supportive benefit of LDOM for lichen planopilaris, frontal fibrosing alopecia, central centrifugal alopecia, and fibrosing alopecia in a patterned distribution.
“LDOM can be considered when topical minoxidil is more expensive, logistically challenging, has plateaued in efficacy, results in undesirable product residue/skin irritation,” or exacerbates inflammatory processes (ie eczema, psoriasis), they added.
Contraindications to LDOM listed in the consensus recommendations include hypersensitivity to minoxidil, significant drug-drug interactions with LDOM, a history of pericardial effusion/tamponade, pericarditis, heart failure, pulmonary hypertension associated with mitral stenosis, pheochromocytoma, and pregnancy/breastfeeding. Cited precautions of LDOM use include a history of tachycardia or arrhythmia, hypotension, renal impairment, and being on dialysis.
Dr. Fu and colleagues noted that the earliest time point at which LDOM should be expected to demonstrate efficacy is 3-6 months. “Baseline testing is not routine but may be considered in case of identified precautions,” they wrote. They also noted that LDOM can possibly be co-administered with beta-blockers with a specialty consultation, and with spironolactone in biologic female or transgender female patients with hirsutism, acne, polycystic ovary syndrome (PCOS), and with lower extremity and facial edema.
According to the consensus statement, the most frequently prescribed LDOM dosing regimen in adult females aged 18 years and older includes a starting dose of 1.25 mg daily, with a dosing range between 0.625 mg and 5 mg daily. For adult males, the most frequently prescribed dosing regimen is a starting dose of 2.5 daily, with a dosing range between 1.25 mg and 5 mg daily. The most frequently prescribed LDOM dosing regimen in adolescent females aged 12-17 years is a starting dose of 0.625 mg daily, with a dosing range of 0.625 to 2.5 mg daily. For adolescent males, the recommended regimen is a starting dose of 1.25 mg daily, with a dosing range of 1.25 mg to 5 mg daily.
“We hope that this consensus statement will guide our colleagues who would like to use LDOM to treat hair loss in their adult and adolescent patients,” Dr. Fu told this news organization. “These recommendations may be used to inform clinical practice until additional evidence-based data becomes available.”
She acknowledged certain limitations of the effort, including the fact that the expert panel was underrepresented in treating hair loss in pediatric patients, “and therefore failed to reach consensus on LDOM pediatric use and dosing,” she said. “We encourage our pediatric dermatology colleagues to further research LDOM in pediatric patients.”
In an interview, Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, who was asked to comment, but was not involved with the work, characterized the consensus as a “helpful, concise reference guide for dermatologists.”
The advantages of the study are the standardized methods used, “and the experience of the panel,” she said. “Study limitations include the response rate, which was less than 60%, and the risk of potential side effects are not stratified by age, sex, or comorbidities,” she added.
Dr. Fu disclosed that she is a consultant to Pfizer. Dr. Lipner reported having no relevant disclosures.
SAN DIEGO — .
Those are among the key recommendations that resulted from a modified eDelphi consensus of experts who convened to develop guidelines for LDOM prescribing and monitoring.
“Topical minoxidil is safe, effective, over-the-counter, and FDA-approved to treat the most common form of hair loss, androgenetic alopecia,” one of the study authors, Jennifer Fu, MD, a dermatologist who directs the Hair Disorders Clinic at the University of California, San Francisco, told this news organization following the annual meeting of the American Academy of Dermatology. The results of the expert consensus were presented during a poster session at the meeting. “It is often used off label for other types of hair loss, yet clinicians who treat hair loss know that patient compliance with topical minoxidil can be poor for a variety of reasons,” she said. “Patients report that it can be difficult to apply and complicate hair styling. For many patients, topical minoxidil can be drying or cause irritant or allergic contact reactions.”
LDOM has become a popular alternative for patients for whom topical minoxidil is logistically challenging, irritating, or ineffective, she continued. Although oral minoxidil is no longer a first-line antihypertensive agent given the risk of cardiovascular adverse effects at higher antihypertensive dosing (10-40 mg daily), a growing number of small studies have documented the use of LDOM at doses ranging from 0.25 mg to 5 mg daily as a safe, effective option for various types of hair loss.
“Given the current absence of larger trials on this topic, our research group identified a need for expert-based guidelines for prescribing and monitoring LDOM use in hair loss patients,” Dr. Fu said. “Our goal was to provide clinicians who treat hair loss patients a road map for using LDOM effectively, maximizing hair growth, and minimizing potential cardiovascular adverse effects.”
Arriving at a Consensus
The process involved 43 hair loss specialists from 12 countries with an average of 6.29 years of experience with LDOM for hair loss, who participated in a multi-round modified Delphi process. They considered questions that addressed LDOM safety, efficacy, dosing, and monitoring for hair loss, and consensus was reached if at least 70% of participants indicated “agree” or “strongly agree” on a five-point Likert scale. Round 1 consisted of 180 open-ended, multiple-choice, or Likert-scale questions, while round 2 involved 121 Likert-scale questions, round 3 consisted of 16 Likert-scale questions, and round 4 included 11 Likert-scale questions. In all, 94 items achieved Likert-scale consensus.
Specifically, experts on the panel found a direct benefit of LDOM for androgenetic alopecia, age-related patterned thinning, alopecia areata, telogen effluvium, traction alopecia, persistent chemotherapy-induced alopecia, and endocrine therapy-induced alopecia. They found a supportive benefit of LDOM for lichen planopilaris, frontal fibrosing alopecia, central centrifugal alopecia, and fibrosing alopecia in a patterned distribution.
“LDOM can be considered when topical minoxidil is more expensive, logistically challenging, has plateaued in efficacy, results in undesirable product residue/skin irritation,” or exacerbates inflammatory processes (ie eczema, psoriasis), they added.
Contraindications to LDOM listed in the consensus recommendations include hypersensitivity to minoxidil, significant drug-drug interactions with LDOM, a history of pericardial effusion/tamponade, pericarditis, heart failure, pulmonary hypertension associated with mitral stenosis, pheochromocytoma, and pregnancy/breastfeeding. Cited precautions of LDOM use include a history of tachycardia or arrhythmia, hypotension, renal impairment, and being on dialysis.
Dr. Fu and colleagues noted that the earliest time point at which LDOM should be expected to demonstrate efficacy is 3-6 months. “Baseline testing is not routine but may be considered in case of identified precautions,” they wrote. They also noted that LDOM can possibly be co-administered with beta-blockers with a specialty consultation, and with spironolactone in biologic female or transgender female patients with hirsutism, acne, polycystic ovary syndrome (PCOS), and with lower extremity and facial edema.
According to the consensus statement, the most frequently prescribed LDOM dosing regimen in adult females aged 18 years and older includes a starting dose of 1.25 mg daily, with a dosing range between 0.625 mg and 5 mg daily. For adult males, the most frequently prescribed dosing regimen is a starting dose of 2.5 daily, with a dosing range between 1.25 mg and 5 mg daily. The most frequently prescribed LDOM dosing regimen in adolescent females aged 12-17 years is a starting dose of 0.625 mg daily, with a dosing range of 0.625 to 2.5 mg daily. For adolescent males, the recommended regimen is a starting dose of 1.25 mg daily, with a dosing range of 1.25 mg to 5 mg daily.
“We hope that this consensus statement will guide our colleagues who would like to use LDOM to treat hair loss in their adult and adolescent patients,” Dr. Fu told this news organization. “These recommendations may be used to inform clinical practice until additional evidence-based data becomes available.”
She acknowledged certain limitations of the effort, including the fact that the expert panel was underrepresented in treating hair loss in pediatric patients, “and therefore failed to reach consensus on LDOM pediatric use and dosing,” she said. “We encourage our pediatric dermatology colleagues to further research LDOM in pediatric patients.”
In an interview, Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, who was asked to comment, but was not involved with the work, characterized the consensus as a “helpful, concise reference guide for dermatologists.”
The advantages of the study are the standardized methods used, “and the experience of the panel,” she said. “Study limitations include the response rate, which was less than 60%, and the risk of potential side effects are not stratified by age, sex, or comorbidities,” she added.
Dr. Fu disclosed that she is a consultant to Pfizer. Dr. Lipner reported having no relevant disclosures.
SAN DIEGO — .
Those are among the key recommendations that resulted from a modified eDelphi consensus of experts who convened to develop guidelines for LDOM prescribing and monitoring.
“Topical minoxidil is safe, effective, over-the-counter, and FDA-approved to treat the most common form of hair loss, androgenetic alopecia,” one of the study authors, Jennifer Fu, MD, a dermatologist who directs the Hair Disorders Clinic at the University of California, San Francisco, told this news organization following the annual meeting of the American Academy of Dermatology. The results of the expert consensus were presented during a poster session at the meeting. “It is often used off label for other types of hair loss, yet clinicians who treat hair loss know that patient compliance with topical minoxidil can be poor for a variety of reasons,” she said. “Patients report that it can be difficult to apply and complicate hair styling. For many patients, topical minoxidil can be drying or cause irritant or allergic contact reactions.”
LDOM has become a popular alternative for patients for whom topical minoxidil is logistically challenging, irritating, or ineffective, she continued. Although oral minoxidil is no longer a first-line antihypertensive agent given the risk of cardiovascular adverse effects at higher antihypertensive dosing (10-40 mg daily), a growing number of small studies have documented the use of LDOM at doses ranging from 0.25 mg to 5 mg daily as a safe, effective option for various types of hair loss.
“Given the current absence of larger trials on this topic, our research group identified a need for expert-based guidelines for prescribing and monitoring LDOM use in hair loss patients,” Dr. Fu said. “Our goal was to provide clinicians who treat hair loss patients a road map for using LDOM effectively, maximizing hair growth, and minimizing potential cardiovascular adverse effects.”
Arriving at a Consensus
The process involved 43 hair loss specialists from 12 countries with an average of 6.29 years of experience with LDOM for hair loss, who participated in a multi-round modified Delphi process. They considered questions that addressed LDOM safety, efficacy, dosing, and monitoring for hair loss, and consensus was reached if at least 70% of participants indicated “agree” or “strongly agree” on a five-point Likert scale. Round 1 consisted of 180 open-ended, multiple-choice, or Likert-scale questions, while round 2 involved 121 Likert-scale questions, round 3 consisted of 16 Likert-scale questions, and round 4 included 11 Likert-scale questions. In all, 94 items achieved Likert-scale consensus.
Specifically, experts on the panel found a direct benefit of LDOM for androgenetic alopecia, age-related patterned thinning, alopecia areata, telogen effluvium, traction alopecia, persistent chemotherapy-induced alopecia, and endocrine therapy-induced alopecia. They found a supportive benefit of LDOM for lichen planopilaris, frontal fibrosing alopecia, central centrifugal alopecia, and fibrosing alopecia in a patterned distribution.
“LDOM can be considered when topical minoxidil is more expensive, logistically challenging, has plateaued in efficacy, results in undesirable product residue/skin irritation,” or exacerbates inflammatory processes (ie eczema, psoriasis), they added.
Contraindications to LDOM listed in the consensus recommendations include hypersensitivity to minoxidil, significant drug-drug interactions with LDOM, a history of pericardial effusion/tamponade, pericarditis, heart failure, pulmonary hypertension associated with mitral stenosis, pheochromocytoma, and pregnancy/breastfeeding. Cited precautions of LDOM use include a history of tachycardia or arrhythmia, hypotension, renal impairment, and being on dialysis.
Dr. Fu and colleagues noted that the earliest time point at which LDOM should be expected to demonstrate efficacy is 3-6 months. “Baseline testing is not routine but may be considered in case of identified precautions,” they wrote. They also noted that LDOM can possibly be co-administered with beta-blockers with a specialty consultation, and with spironolactone in biologic female or transgender female patients with hirsutism, acne, polycystic ovary syndrome (PCOS), and with lower extremity and facial edema.
According to the consensus statement, the most frequently prescribed LDOM dosing regimen in adult females aged 18 years and older includes a starting dose of 1.25 mg daily, with a dosing range between 0.625 mg and 5 mg daily. For adult males, the most frequently prescribed dosing regimen is a starting dose of 2.5 daily, with a dosing range between 1.25 mg and 5 mg daily. The most frequently prescribed LDOM dosing regimen in adolescent females aged 12-17 years is a starting dose of 0.625 mg daily, with a dosing range of 0.625 to 2.5 mg daily. For adolescent males, the recommended regimen is a starting dose of 1.25 mg daily, with a dosing range of 1.25 mg to 5 mg daily.
“We hope that this consensus statement will guide our colleagues who would like to use LDOM to treat hair loss in their adult and adolescent patients,” Dr. Fu told this news organization. “These recommendations may be used to inform clinical practice until additional evidence-based data becomes available.”
She acknowledged certain limitations of the effort, including the fact that the expert panel was underrepresented in treating hair loss in pediatric patients, “and therefore failed to reach consensus on LDOM pediatric use and dosing,” she said. “We encourage our pediatric dermatology colleagues to further research LDOM in pediatric patients.”
In an interview, Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, who was asked to comment, but was not involved with the work, characterized the consensus as a “helpful, concise reference guide for dermatologists.”
The advantages of the study are the standardized methods used, “and the experience of the panel,” she said. “Study limitations include the response rate, which was less than 60%, and the risk of potential side effects are not stratified by age, sex, or comorbidities,” she added.
Dr. Fu disclosed that she is a consultant to Pfizer. Dr. Lipner reported having no relevant disclosures.
FROM AAD 2024
Alopecia Areata: Late Responses Complicate Definition of JAK Inhibitor Failure
SAN DIEGO — , according to late breaker data presented at the annual meeting of the American Academy of Dermatology.
Although the majority respond within months, response curves have so far climbed for as long as patients are followed, allowing many with disappointing early results to catch up, according to Rodney D. Sinclair, MD, professor of dermatology at the University of Melbourne, Australia.
His remarks were derived specifically from new long-term follow-up with baricitinib, the first JAK inhibitor approved for AA, but the pattern appears to be similar with ritlecitinib, the only other JAK inhibitor approved for AA, and for several if not all JAK inhibitors in phase 3 AA trials.
“We have had patients on baricitinib where not much was happening at 18 months, but now, at 4 years, they have a SALT score of zero,” Dr. Sinclair reported
A Severity of Alopecia Tool (SALT) score of 0 signifies complete hair regrowth. On a scale with a maximum score of 100 (complete hair loss), a SALT score of 20 or less, signaling clinical success, has been a primary endpoint in many JAK inhibitor trials, including those conducted with baricitinib.
Providing the most recent analysis in patients with severe AA participating in the phase 3 BRAVE-AA1 and BRAVE-AA2 trials of baricitinib, which were published together in 2022, Dr. Sinclair broke the data down into responders, mixed responders, and nonresponders at 52 weeks. The proportion of patients who responded with even longer follow-up were then tallied.
In the as-observed responses over time, the trajectory of response continued to climb through 76 weeks of follow-up in all three groups.
Relative to the 44.5% rate of overall response (SALT ≤ 20 ) at 52 weeks, there was some further growth in every group maintained on JAK inhibitor therapy over longer follow-up. In Dr. Sinclair’s late breaking analysis, this did not include nonresponders, who stopped therapy by week 52, but 78.4% of the combined responders and mixed responders who remained on treatment had reached treatment success at 76 weeks.
Response Curves Climb More Slowly With Severe Alopecia
While improvement in SALT scores was even seen in nonresponders over time as long as they remained on therapy, Dr. Sinclair reported that response curves tended to climb more slowly in those with more severe alopecia at baseline. Yet, they still climbed. For example, 28.1% of those with a baseline SALT score of 95 to 100 had reached treatment success at week 52, but the proportion had climbed to 35.4% by week 76.
The response curves climbed more quickly among those with a SALT score between 50 and 95 at baseline than among those with more severe alopecia, but the differences in SALT scores at 52 weeks and 76 weeks among patients in this range of baseline SALT scores were small.
Basically, “those with a SALT score of 94 did just as well as those with a SALT score of 51 when followed long-term,” he said, noting that this was among several findings that confounded expectations.
Duration of AA was found to be an important prognostic factor, with 4 years emerging as a general threshold separating those with a diminished likelihood of benefit relative to those with a shorter AA duration.
“When the duration of AA is more than 4 years, the response to any JAK inhibitor seems to fall off a cliff,” Dr. Sinclair said.
To clarify this observation, Dr. Sinclair made an analogy between acute and chronic urticaria. Chronicity appears to change the pathophysiology of both urticaria and AA, making durable remissions more difficult to achieve if the inflammatory response was persistently upregulated, he said.
The delayed responses in some patients “suggests that it is not enough to control inflammation for the hair to regrow. You actually have to activate the hair to grow as well as treat the inflammation,” Dr. Sinclair said.
This heterogeneity that has been observed in the speed of AA response to JAK inhibitors might be explained at least in part by the individual differences in hair growth activation. For ritlecitinib, the only other JAK inhibitor approved for AA to date, 62% were categorized as responders in the registration ALLEGRO trials, but only 44% were early responders, meaning SALT scores of ≤ 20 by week 24, according to a summary published last year. Of the remaining 16%, 11% were middle responders, meaning a SALT score of ≤ 20 reached at week 48, and 6% were late responders, meaning a SALT score of ≤ 20 reached at week 96.
In the context of late breaking 68-week data with deuruxolitinib, an oral JAK inhibitor currently under FDA review for treating moderate to severe AA, presented in the same AAD session as Dr. Sinclair’s baricitinib data, Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Connecticut, described similar long-term response curves. At 24 weeks, the SALT ≤ 20 response was achieved in 34.9% of patients, but climbed to 62.8% with continuous therapy over 68 weeks.
The difference between AA and most other inflammatory conditions treated with a JAK inhibitor is that “it takes time to treat,” Dr. King said.
Time Factor Is Important for Response
“What we are learning is that patients keep getting better over time,” Dr. Sinclair said. Asked specifically how long he would treat a patient before giving up, he acknowledged that he used to consider 6 months adequate, but that he has now changed his mind.
“It might be that even 2 years is too short,” he said, although he conceded that a trial of therapy for this long “might be an issue for third-part payers.”
Asked to comment, Melissa Piliang, MD, chair of the department of dermatology at the Cleveland Clinic, agreed with the principle that early responses are not necessarily predictive of complete response.
“In my clinical experience, 6 months is not long enough to assess response,” she told this news organization. “Some patients have hair growth after 18 months to 2 years” of treatment. Additional studies to identify the characteristics and predictors of late response, she said, “would be very helpful, as would trials allowing multiple therapies to simulate real-world practice.”
Like Dr. Sinclair, Dr. Piliang is interested in the possibility of combining a JAK inhibitor with another therapy aimed specially at promoting hair regrowth.
“Using a secondary therapy to stimulate regrowth as an addition to an anti-inflammatory medicine like a JAK inhibitor might speed up response in some patients,” she speculated. Dr. Sinclair reports financial relationships with more than 30 pharmaceutical companies, including Eli Lilly, the manufacturer of baricitinib. Dr. King reports financial relationships with multiple companies, including Concert Pharmaceuticals (consultant and investigator), the manufacturer of deuruxolitinib. Dr. Piliang reports financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble.
SAN DIEGO — , according to late breaker data presented at the annual meeting of the American Academy of Dermatology.
Although the majority respond within months, response curves have so far climbed for as long as patients are followed, allowing many with disappointing early results to catch up, according to Rodney D. Sinclair, MD, professor of dermatology at the University of Melbourne, Australia.
His remarks were derived specifically from new long-term follow-up with baricitinib, the first JAK inhibitor approved for AA, but the pattern appears to be similar with ritlecitinib, the only other JAK inhibitor approved for AA, and for several if not all JAK inhibitors in phase 3 AA trials.
“We have had patients on baricitinib where not much was happening at 18 months, but now, at 4 years, they have a SALT score of zero,” Dr. Sinclair reported
A Severity of Alopecia Tool (SALT) score of 0 signifies complete hair regrowth. On a scale with a maximum score of 100 (complete hair loss), a SALT score of 20 or less, signaling clinical success, has been a primary endpoint in many JAK inhibitor trials, including those conducted with baricitinib.
Providing the most recent analysis in patients with severe AA participating in the phase 3 BRAVE-AA1 and BRAVE-AA2 trials of baricitinib, which were published together in 2022, Dr. Sinclair broke the data down into responders, mixed responders, and nonresponders at 52 weeks. The proportion of patients who responded with even longer follow-up were then tallied.
In the as-observed responses over time, the trajectory of response continued to climb through 76 weeks of follow-up in all three groups.
Relative to the 44.5% rate of overall response (SALT ≤ 20 ) at 52 weeks, there was some further growth in every group maintained on JAK inhibitor therapy over longer follow-up. In Dr. Sinclair’s late breaking analysis, this did not include nonresponders, who stopped therapy by week 52, but 78.4% of the combined responders and mixed responders who remained on treatment had reached treatment success at 76 weeks.
Response Curves Climb More Slowly With Severe Alopecia
While improvement in SALT scores was even seen in nonresponders over time as long as they remained on therapy, Dr. Sinclair reported that response curves tended to climb more slowly in those with more severe alopecia at baseline. Yet, they still climbed. For example, 28.1% of those with a baseline SALT score of 95 to 100 had reached treatment success at week 52, but the proportion had climbed to 35.4% by week 76.
The response curves climbed more quickly among those with a SALT score between 50 and 95 at baseline than among those with more severe alopecia, but the differences in SALT scores at 52 weeks and 76 weeks among patients in this range of baseline SALT scores were small.
Basically, “those with a SALT score of 94 did just as well as those with a SALT score of 51 when followed long-term,” he said, noting that this was among several findings that confounded expectations.
Duration of AA was found to be an important prognostic factor, with 4 years emerging as a general threshold separating those with a diminished likelihood of benefit relative to those with a shorter AA duration.
“When the duration of AA is more than 4 years, the response to any JAK inhibitor seems to fall off a cliff,” Dr. Sinclair said.
To clarify this observation, Dr. Sinclair made an analogy between acute and chronic urticaria. Chronicity appears to change the pathophysiology of both urticaria and AA, making durable remissions more difficult to achieve if the inflammatory response was persistently upregulated, he said.
The delayed responses in some patients “suggests that it is not enough to control inflammation for the hair to regrow. You actually have to activate the hair to grow as well as treat the inflammation,” Dr. Sinclair said.
This heterogeneity that has been observed in the speed of AA response to JAK inhibitors might be explained at least in part by the individual differences in hair growth activation. For ritlecitinib, the only other JAK inhibitor approved for AA to date, 62% were categorized as responders in the registration ALLEGRO trials, but only 44% were early responders, meaning SALT scores of ≤ 20 by week 24, according to a summary published last year. Of the remaining 16%, 11% were middle responders, meaning a SALT score of ≤ 20 reached at week 48, and 6% were late responders, meaning a SALT score of ≤ 20 reached at week 96.
In the context of late breaking 68-week data with deuruxolitinib, an oral JAK inhibitor currently under FDA review for treating moderate to severe AA, presented in the same AAD session as Dr. Sinclair’s baricitinib data, Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Connecticut, described similar long-term response curves. At 24 weeks, the SALT ≤ 20 response was achieved in 34.9% of patients, but climbed to 62.8% with continuous therapy over 68 weeks.
The difference between AA and most other inflammatory conditions treated with a JAK inhibitor is that “it takes time to treat,” Dr. King said.
Time Factor Is Important for Response
“What we are learning is that patients keep getting better over time,” Dr. Sinclair said. Asked specifically how long he would treat a patient before giving up, he acknowledged that he used to consider 6 months adequate, but that he has now changed his mind.
“It might be that even 2 years is too short,” he said, although he conceded that a trial of therapy for this long “might be an issue for third-part payers.”
Asked to comment, Melissa Piliang, MD, chair of the department of dermatology at the Cleveland Clinic, agreed with the principle that early responses are not necessarily predictive of complete response.
“In my clinical experience, 6 months is not long enough to assess response,” she told this news organization. “Some patients have hair growth after 18 months to 2 years” of treatment. Additional studies to identify the characteristics and predictors of late response, she said, “would be very helpful, as would trials allowing multiple therapies to simulate real-world practice.”
Like Dr. Sinclair, Dr. Piliang is interested in the possibility of combining a JAK inhibitor with another therapy aimed specially at promoting hair regrowth.
“Using a secondary therapy to stimulate regrowth as an addition to an anti-inflammatory medicine like a JAK inhibitor might speed up response in some patients,” she speculated. Dr. Sinclair reports financial relationships with more than 30 pharmaceutical companies, including Eli Lilly, the manufacturer of baricitinib. Dr. King reports financial relationships with multiple companies, including Concert Pharmaceuticals (consultant and investigator), the manufacturer of deuruxolitinib. Dr. Piliang reports financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble.
SAN DIEGO — , according to late breaker data presented at the annual meeting of the American Academy of Dermatology.
Although the majority respond within months, response curves have so far climbed for as long as patients are followed, allowing many with disappointing early results to catch up, according to Rodney D. Sinclair, MD, professor of dermatology at the University of Melbourne, Australia.
His remarks were derived specifically from new long-term follow-up with baricitinib, the first JAK inhibitor approved for AA, but the pattern appears to be similar with ritlecitinib, the only other JAK inhibitor approved for AA, and for several if not all JAK inhibitors in phase 3 AA trials.
“We have had patients on baricitinib where not much was happening at 18 months, but now, at 4 years, they have a SALT score of zero,” Dr. Sinclair reported
A Severity of Alopecia Tool (SALT) score of 0 signifies complete hair regrowth. On a scale with a maximum score of 100 (complete hair loss), a SALT score of 20 or less, signaling clinical success, has been a primary endpoint in many JAK inhibitor trials, including those conducted with baricitinib.
Providing the most recent analysis in patients with severe AA participating in the phase 3 BRAVE-AA1 and BRAVE-AA2 trials of baricitinib, which were published together in 2022, Dr. Sinclair broke the data down into responders, mixed responders, and nonresponders at 52 weeks. The proportion of patients who responded with even longer follow-up were then tallied.
In the as-observed responses over time, the trajectory of response continued to climb through 76 weeks of follow-up in all three groups.
Relative to the 44.5% rate of overall response (SALT ≤ 20 ) at 52 weeks, there was some further growth in every group maintained on JAK inhibitor therapy over longer follow-up. In Dr. Sinclair’s late breaking analysis, this did not include nonresponders, who stopped therapy by week 52, but 78.4% of the combined responders and mixed responders who remained on treatment had reached treatment success at 76 weeks.
Response Curves Climb More Slowly With Severe Alopecia
While improvement in SALT scores was even seen in nonresponders over time as long as they remained on therapy, Dr. Sinclair reported that response curves tended to climb more slowly in those with more severe alopecia at baseline. Yet, they still climbed. For example, 28.1% of those with a baseline SALT score of 95 to 100 had reached treatment success at week 52, but the proportion had climbed to 35.4% by week 76.
The response curves climbed more quickly among those with a SALT score between 50 and 95 at baseline than among those with more severe alopecia, but the differences in SALT scores at 52 weeks and 76 weeks among patients in this range of baseline SALT scores were small.
Basically, “those with a SALT score of 94 did just as well as those with a SALT score of 51 when followed long-term,” he said, noting that this was among several findings that confounded expectations.
Duration of AA was found to be an important prognostic factor, with 4 years emerging as a general threshold separating those with a diminished likelihood of benefit relative to those with a shorter AA duration.
“When the duration of AA is more than 4 years, the response to any JAK inhibitor seems to fall off a cliff,” Dr. Sinclair said.
To clarify this observation, Dr. Sinclair made an analogy between acute and chronic urticaria. Chronicity appears to change the pathophysiology of both urticaria and AA, making durable remissions more difficult to achieve if the inflammatory response was persistently upregulated, he said.
The delayed responses in some patients “suggests that it is not enough to control inflammation for the hair to regrow. You actually have to activate the hair to grow as well as treat the inflammation,” Dr. Sinclair said.
This heterogeneity that has been observed in the speed of AA response to JAK inhibitors might be explained at least in part by the individual differences in hair growth activation. For ritlecitinib, the only other JAK inhibitor approved for AA to date, 62% were categorized as responders in the registration ALLEGRO trials, but only 44% were early responders, meaning SALT scores of ≤ 20 by week 24, according to a summary published last year. Of the remaining 16%, 11% were middle responders, meaning a SALT score of ≤ 20 reached at week 48, and 6% were late responders, meaning a SALT score of ≤ 20 reached at week 96.
In the context of late breaking 68-week data with deuruxolitinib, an oral JAK inhibitor currently under FDA review for treating moderate to severe AA, presented in the same AAD session as Dr. Sinclair’s baricitinib data, Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Connecticut, described similar long-term response curves. At 24 weeks, the SALT ≤ 20 response was achieved in 34.9% of patients, but climbed to 62.8% with continuous therapy over 68 weeks.
The difference between AA and most other inflammatory conditions treated with a JAK inhibitor is that “it takes time to treat,” Dr. King said.
Time Factor Is Important for Response
“What we are learning is that patients keep getting better over time,” Dr. Sinclair said. Asked specifically how long he would treat a patient before giving up, he acknowledged that he used to consider 6 months adequate, but that he has now changed his mind.
“It might be that even 2 years is too short,” he said, although he conceded that a trial of therapy for this long “might be an issue for third-part payers.”
Asked to comment, Melissa Piliang, MD, chair of the department of dermatology at the Cleveland Clinic, agreed with the principle that early responses are not necessarily predictive of complete response.
“In my clinical experience, 6 months is not long enough to assess response,” she told this news organization. “Some patients have hair growth after 18 months to 2 years” of treatment. Additional studies to identify the characteristics and predictors of late response, she said, “would be very helpful, as would trials allowing multiple therapies to simulate real-world practice.”
Like Dr. Sinclair, Dr. Piliang is interested in the possibility of combining a JAK inhibitor with another therapy aimed specially at promoting hair regrowth.
“Using a secondary therapy to stimulate regrowth as an addition to an anti-inflammatory medicine like a JAK inhibitor might speed up response in some patients,” she speculated. Dr. Sinclair reports financial relationships with more than 30 pharmaceutical companies, including Eli Lilly, the manufacturer of baricitinib. Dr. King reports financial relationships with multiple companies, including Concert Pharmaceuticals (consultant and investigator), the manufacturer of deuruxolitinib. Dr. Piliang reports financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble.
FROM AAD 2024
Androgenetic Alopecia: Study Finds Efficacy of Topical and Oral Minoxidil Similar
Oral minoxidil, 5 mg once a day, “did not demonstrate superiority” over topical minoxidil, 5%, applied twice a day, after 24 weeks, reported Mariana Alvares Penha, MD, of the department of dermatology at São Paulo State University, in Botucatu, Brazil, and coauthors. Their randomized, controlled, double-blind study was published online in JAMA Dermatology.
Topical minoxidil is approved by the US Food and Drug Administration (FDA) for androgenetic alopecia (AGA), but there has been increasing interest worldwide in the use of low-dose oral minoxidil, a vasodilator approved as an antihypertensive, as an alternative treatment.
The trial “is important information that’s never been elucidated before,” Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said in an interview. The data, he added, can be used to reassure patients who do not want to take the oral form of the drug that a topical is just as effective.
“This study does let us counsel patients better and really give them the evidence,” said Shari Lipner, MD, PhD, associate professor of clinical dermatology at Weill Cornell Medicine, New York, who was also asked to comment on the results.
Both Dr. Lipner and Dr. Friedman said the study was well-designed.
The investigators enrolled 90 men aged 18-55; 68 completed the trial. Most had mild to moderate AGA. Men were excluded if they had received treatment for alopecia in the previous 6 months, a history of hair transplant, cardiopathy, nephropathy, dermatoses involving the scalp, any clinical conditions causing hair loss, or hypersensitivity to minoxidil.
They were randomized to receive either 5 mg of oral minoxidil a day, plus a placebo solution to apply to the scalp, or topical minoxidil solution (5%) applied twice a day plus placebo capsules. They were told to take a capsule at bedtime and to apply 1 mL of the solution to dry hair in the morning and at night.
The final analysis included 35 men in the topical group and 33 in the oral group (mean age, 36.6 years). Seven people in the topical group and 11 in the oral group were not able to attend the final appointment at 24 weeks. Three additional patients in the topical group dropped out for insomnia, hair shedding, and scalp eczema, while one dropped out of the oral group because of headache.
At 24 weeks, the percentage increase in terminal hair density in the oral minoxidil group was 27% higher (P = .005) in the vertex and 13% higher (P = .15) in the frontal scalp, compared with the topical-treated group.
Total hair density increased by 2% in the oral group compared with topical treatment in the vertex and decreased by 0.2% in the frontal area compared with topical treatment. None of these differences were statistically significant.
Three dermatologists blinded to the treatments, who analyzed photographs, determined that 60% of the men in the oral group and 48% in the topical group had clinical improvement in the frontal area, which was not statistically significant. More orally-treated patients had improvement in the vertex area: 70% compared with 46% of those on topical treatment (P = .04).
Hypertrichosis, Headache
Of the original 90 patients in the trial, more men taking oral minoxidil had hypertrichosis: 49% compared with 25% in the topical formulation group. Headache was also more common among those on oral minoxidil: six cases (14%) vs. one case (2%) among those on topical minoxidil. There was no difference in mean arterial blood pressure or resting heart rate between the two groups. Transient hair loss was more common with topical treatment, but it was not significant.
Dr. Friedman said that the study results would not change how he practices, but that it would give him data to use to inform patients who do not want to take oral minoxidil. He generally prescribes the oral form, unless patients do not want to take it or there is a medical contraindication, which he said is rare.
“I personally think oral is superior to topical,” mainly “because the patient’s actually using it,” said Dr. Friedman. “They’re more likely to take a pill a day versus apply something topically twice a day,” he added.
Both Dr. Lipner and Dr. Friedman said that they doubted that individuals could — or would want to — follow the twice-daily topical regimen used in the trial.
“In real life, not in the clinical trial scenario, it may be very hard for patients to comply with putting on the topical minoxidil twice a day or even once a day,” Dr. Lipner said.
However, she continues to prescribe more topical minoxidil than oral, because she believes “there’s less potential for side effects.” For patients who can adhere to the topical regimen, the study shows that they will get results, said Dr. Lipner.
Dr. Friedman, however, said that for patients who are looking at a lifetime of medication, “an oral will always win out on a topical to the scalp from an adherence perspective.”
The study was supported by the Brazilian Dermatology Society Support Fund. Dr. Penha reported receiving grants from the fund; no other disclosures were reported. Dr. Friedman and Dr. Lipner reported no conflicts related to minoxidil.
Oral minoxidil, 5 mg once a day, “did not demonstrate superiority” over topical minoxidil, 5%, applied twice a day, after 24 weeks, reported Mariana Alvares Penha, MD, of the department of dermatology at São Paulo State University, in Botucatu, Brazil, and coauthors. Their randomized, controlled, double-blind study was published online in JAMA Dermatology.
Topical minoxidil is approved by the US Food and Drug Administration (FDA) for androgenetic alopecia (AGA), but there has been increasing interest worldwide in the use of low-dose oral minoxidil, a vasodilator approved as an antihypertensive, as an alternative treatment.
The trial “is important information that’s never been elucidated before,” Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said in an interview. The data, he added, can be used to reassure patients who do not want to take the oral form of the drug that a topical is just as effective.
“This study does let us counsel patients better and really give them the evidence,” said Shari Lipner, MD, PhD, associate professor of clinical dermatology at Weill Cornell Medicine, New York, who was also asked to comment on the results.
Both Dr. Lipner and Dr. Friedman said the study was well-designed.
The investigators enrolled 90 men aged 18-55; 68 completed the trial. Most had mild to moderate AGA. Men were excluded if they had received treatment for alopecia in the previous 6 months, a history of hair transplant, cardiopathy, nephropathy, dermatoses involving the scalp, any clinical conditions causing hair loss, or hypersensitivity to minoxidil.
They were randomized to receive either 5 mg of oral minoxidil a day, plus a placebo solution to apply to the scalp, or topical minoxidil solution (5%) applied twice a day plus placebo capsules. They were told to take a capsule at bedtime and to apply 1 mL of the solution to dry hair in the morning and at night.
The final analysis included 35 men in the topical group and 33 in the oral group (mean age, 36.6 years). Seven people in the topical group and 11 in the oral group were not able to attend the final appointment at 24 weeks. Three additional patients in the topical group dropped out for insomnia, hair shedding, and scalp eczema, while one dropped out of the oral group because of headache.
At 24 weeks, the percentage increase in terminal hair density in the oral minoxidil group was 27% higher (P = .005) in the vertex and 13% higher (P = .15) in the frontal scalp, compared with the topical-treated group.
Total hair density increased by 2% in the oral group compared with topical treatment in the vertex and decreased by 0.2% in the frontal area compared with topical treatment. None of these differences were statistically significant.
Three dermatologists blinded to the treatments, who analyzed photographs, determined that 60% of the men in the oral group and 48% in the topical group had clinical improvement in the frontal area, which was not statistically significant. More orally-treated patients had improvement in the vertex area: 70% compared with 46% of those on topical treatment (P = .04).
Hypertrichosis, Headache
Of the original 90 patients in the trial, more men taking oral minoxidil had hypertrichosis: 49% compared with 25% in the topical formulation group. Headache was also more common among those on oral minoxidil: six cases (14%) vs. one case (2%) among those on topical minoxidil. There was no difference in mean arterial blood pressure or resting heart rate between the two groups. Transient hair loss was more common with topical treatment, but it was not significant.
Dr. Friedman said that the study results would not change how he practices, but that it would give him data to use to inform patients who do not want to take oral minoxidil. He generally prescribes the oral form, unless patients do not want to take it or there is a medical contraindication, which he said is rare.
“I personally think oral is superior to topical,” mainly “because the patient’s actually using it,” said Dr. Friedman. “They’re more likely to take a pill a day versus apply something topically twice a day,” he added.
Both Dr. Lipner and Dr. Friedman said that they doubted that individuals could — or would want to — follow the twice-daily topical regimen used in the trial.
“In real life, not in the clinical trial scenario, it may be very hard for patients to comply with putting on the topical minoxidil twice a day or even once a day,” Dr. Lipner said.
However, she continues to prescribe more topical minoxidil than oral, because she believes “there’s less potential for side effects.” For patients who can adhere to the topical regimen, the study shows that they will get results, said Dr. Lipner.
Dr. Friedman, however, said that for patients who are looking at a lifetime of medication, “an oral will always win out on a topical to the scalp from an adherence perspective.”
The study was supported by the Brazilian Dermatology Society Support Fund. Dr. Penha reported receiving grants from the fund; no other disclosures were reported. Dr. Friedman and Dr. Lipner reported no conflicts related to minoxidil.
Oral minoxidil, 5 mg once a day, “did not demonstrate superiority” over topical minoxidil, 5%, applied twice a day, after 24 weeks, reported Mariana Alvares Penha, MD, of the department of dermatology at São Paulo State University, in Botucatu, Brazil, and coauthors. Their randomized, controlled, double-blind study was published online in JAMA Dermatology.
Topical minoxidil is approved by the US Food and Drug Administration (FDA) for androgenetic alopecia (AGA), but there has been increasing interest worldwide in the use of low-dose oral minoxidil, a vasodilator approved as an antihypertensive, as an alternative treatment.
The trial “is important information that’s never been elucidated before,” Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said in an interview. The data, he added, can be used to reassure patients who do not want to take the oral form of the drug that a topical is just as effective.
“This study does let us counsel patients better and really give them the evidence,” said Shari Lipner, MD, PhD, associate professor of clinical dermatology at Weill Cornell Medicine, New York, who was also asked to comment on the results.
Both Dr. Lipner and Dr. Friedman said the study was well-designed.
The investigators enrolled 90 men aged 18-55; 68 completed the trial. Most had mild to moderate AGA. Men were excluded if they had received treatment for alopecia in the previous 6 months, a history of hair transplant, cardiopathy, nephropathy, dermatoses involving the scalp, any clinical conditions causing hair loss, or hypersensitivity to minoxidil.
They were randomized to receive either 5 mg of oral minoxidil a day, plus a placebo solution to apply to the scalp, or topical minoxidil solution (5%) applied twice a day plus placebo capsules. They were told to take a capsule at bedtime and to apply 1 mL of the solution to dry hair in the morning and at night.
The final analysis included 35 men in the topical group and 33 in the oral group (mean age, 36.6 years). Seven people in the topical group and 11 in the oral group were not able to attend the final appointment at 24 weeks. Three additional patients in the topical group dropped out for insomnia, hair shedding, and scalp eczema, while one dropped out of the oral group because of headache.
At 24 weeks, the percentage increase in terminal hair density in the oral minoxidil group was 27% higher (P = .005) in the vertex and 13% higher (P = .15) in the frontal scalp, compared with the topical-treated group.
Total hair density increased by 2% in the oral group compared with topical treatment in the vertex and decreased by 0.2% in the frontal area compared with topical treatment. None of these differences were statistically significant.
Three dermatologists blinded to the treatments, who analyzed photographs, determined that 60% of the men in the oral group and 48% in the topical group had clinical improvement in the frontal area, which was not statistically significant. More orally-treated patients had improvement in the vertex area: 70% compared with 46% of those on topical treatment (P = .04).
Hypertrichosis, Headache
Of the original 90 patients in the trial, more men taking oral minoxidil had hypertrichosis: 49% compared with 25% in the topical formulation group. Headache was also more common among those on oral minoxidil: six cases (14%) vs. one case (2%) among those on topical minoxidil. There was no difference in mean arterial blood pressure or resting heart rate between the two groups. Transient hair loss was more common with topical treatment, but it was not significant.
Dr. Friedman said that the study results would not change how he practices, but that it would give him data to use to inform patients who do not want to take oral minoxidil. He generally prescribes the oral form, unless patients do not want to take it or there is a medical contraindication, which he said is rare.
“I personally think oral is superior to topical,” mainly “because the patient’s actually using it,” said Dr. Friedman. “They’re more likely to take a pill a day versus apply something topically twice a day,” he added.
Both Dr. Lipner and Dr. Friedman said that they doubted that individuals could — or would want to — follow the twice-daily topical regimen used in the trial.
“In real life, not in the clinical trial scenario, it may be very hard for patients to comply with putting on the topical minoxidil twice a day or even once a day,” Dr. Lipner said.
However, she continues to prescribe more topical minoxidil than oral, because she believes “there’s less potential for side effects.” For patients who can adhere to the topical regimen, the study shows that they will get results, said Dr. Lipner.
Dr. Friedman, however, said that for patients who are looking at a lifetime of medication, “an oral will always win out on a topical to the scalp from an adherence perspective.”
The study was supported by the Brazilian Dermatology Society Support Fund. Dr. Penha reported receiving grants from the fund; no other disclosures were reported. Dr. Friedman and Dr. Lipner reported no conflicts related to minoxidil.
FROM JAMA DERMATOLOGY
Enhancing Cosmetic and Functional Improvement of Recalcitrant Nail Lichen Planus With Resin Nail
Practice Gap
Lichen planus (LP)—a chronic inflammatory disorder affecting the nails—is prevalent in 10% to 15% of patients and is more common in the fingernails than toenails. Clinical manifestation includes longitudinal ridges, nail plate atrophy, and splitting, which all contribute to cosmetic disfigurement and difficulty with functionality. Quality of life and daily activities may be impacted profoundly.1 First-line therapies include intralesional and systemic corticosteroids; however, efficacy is limited and recurrence is common.1,2 Lichen planus is one of the few conditions that may cause permanent and debilitating nail loss.
Tools
A resin nail can be used to improve cosmetic appearance and functionality in patients with recalcitrant nail LP. The composite resin creates a flexible nonporous nail and allows the underlying natural nail to grow. Application of resin nails has been used for toenail onychodystrophies to improve cosmesis and functionality but has not been reported for fingernails. The resin typically lasts 6 to 8 weeks on toenails.
The Technique
Application of a resin nail involves several steps (see video online). First, the affected nail should be debrided and a bonding agent applied. Next, multiple layers of resin are applied until the patient’s desired thickness is achieved (typically 2 layers), followed by a sealing agent. Finally, the nail is cured with UV light. We recommend applying sunscreen to the hand(s) prior to curing with UV light. The liquid resin allows the nail to be customized to the patient’s desired length and shape. The overall procedure takes approximately 20 minutes for a single nail.

We applied resin nail to the thumbnail of a 46-year-old woman with recalcitrant isolated nail LP of 7 years’ duration (Figure). She previously had difficulties performing everyday activities, and the resin improved her functionality. She also was pleased with the cosmetic appearance. After 2 weeks, the resin started falling off with corresponding natural nail growth. The patient denied any adverse events.
Practice Implications
Resin nail application may serve as a temporary solution to improve cosmesis and functionality in patients with recalcitrant nail LP. As shown in our patient, the resin may fall off faster on the fingernails than the toenails, likely because of the faster growth rate of fingernails and more frequent exposure from daily activities. Further studies of resin nail application for the fingernails are needed to establish duration in patients with varying levels of activity (eg, washing dishes, woodworking).
Because the resin nail may be removed easily at any time, resin nail application does not interfere with treatments such as intralesional steroid injections. For patients using a topical medication regimen, the resin nail may be applied slightly distal to the cuticle so that the medication can still be applied by the proximal nail fold of the underlying natural nail.
The resin nail should be kept short and removed after 2 to 4 weeks for the fingernails and 6 to 8 weeks for the toenails to examine the underlying natural nail. Patients may go about their daily activities with the resin nail, including applying nail polish to the resin nail, bathing, and swimming. Resin nail application may complement medical treatments and improve quality of life for patients with nail LP.
- Gupta MK, Lipner SR. Review of nail lichen planus: epidemiology, pathogenesis, diagnosis, and treatment. Dermatol Clin. 2021;39:221-230. doi:10.1016/j.det.2020.12.002
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
Practice Gap
Lichen planus (LP)—a chronic inflammatory disorder affecting the nails—is prevalent in 10% to 15% of patients and is more common in the fingernails than toenails. Clinical manifestation includes longitudinal ridges, nail plate atrophy, and splitting, which all contribute to cosmetic disfigurement and difficulty with functionality. Quality of life and daily activities may be impacted profoundly.1 First-line therapies include intralesional and systemic corticosteroids; however, efficacy is limited and recurrence is common.1,2 Lichen planus is one of the few conditions that may cause permanent and debilitating nail loss.
Tools
A resin nail can be used to improve cosmetic appearance and functionality in patients with recalcitrant nail LP. The composite resin creates a flexible nonporous nail and allows the underlying natural nail to grow. Application of resin nails has been used for toenail onychodystrophies to improve cosmesis and functionality but has not been reported for fingernails. The resin typically lasts 6 to 8 weeks on toenails.
The Technique
Application of a resin nail involves several steps (see video online). First, the affected nail should be debrided and a bonding agent applied. Next, multiple layers of resin are applied until the patient’s desired thickness is achieved (typically 2 layers), followed by a sealing agent. Finally, the nail is cured with UV light. We recommend applying sunscreen to the hand(s) prior to curing with UV light. The liquid resin allows the nail to be customized to the patient’s desired length and shape. The overall procedure takes approximately 20 minutes for a single nail.

We applied resin nail to the thumbnail of a 46-year-old woman with recalcitrant isolated nail LP of 7 years’ duration (Figure). She previously had difficulties performing everyday activities, and the resin improved her functionality. She also was pleased with the cosmetic appearance. After 2 weeks, the resin started falling off with corresponding natural nail growth. The patient denied any adverse events.

Practice Implications
Resin nail application may serve as a temporary solution to improve cosmesis and functionality in patients with recalcitrant nail LP. As shown in our patient, the resin may fall off faster on the fingernails than the toenails, likely because of the faster growth rate of fingernails and more frequent exposure from daily activities. Further studies of resin nail application for the fingernails are needed to establish duration in patients with varying levels of activity (eg, washing dishes, woodworking).
Because the resin nail may be removed easily at any time, resin nail application does not interfere with treatments such as intralesional steroid injections. For patients using a topical medication regimen, the resin nail may be applied slightly distal to the cuticle so that the medication can still be applied by the proximal nail fold of the underlying natural nail.
The resin nail should be kept short and removed after 2 to 4 weeks for the fingernails and 6 to 8 weeks for the toenails to examine the underlying natural nail. Patients may go about their daily activities with the resin nail, including applying nail polish to the resin nail, bathing, and swimming. Resin nail application may complement medical treatments and improve quality of life for patients with nail LP.
Practice Gap
Lichen planus (LP)—a chronic inflammatory disorder affecting the nails—is prevalent in 10% to 15% of patients and is more common in the fingernails than toenails. Clinical manifestation includes longitudinal ridges, nail plate atrophy, and splitting, which all contribute to cosmetic disfigurement and difficulty with functionality. Quality of life and daily activities may be impacted profoundly.1 First-line therapies include intralesional and systemic corticosteroids; however, efficacy is limited and recurrence is common.1,2 Lichen planus is one of the few conditions that may cause permanent and debilitating nail loss.
Tools
A resin nail can be used to improve cosmetic appearance and functionality in patients with recalcitrant nail LP. The composite resin creates a flexible nonporous nail and allows the underlying natural nail to grow. Application of resin nails has been used for toenail onychodystrophies to improve cosmesis and functionality but has not been reported for fingernails. The resin typically lasts 6 to 8 weeks on toenails.
The Technique
Application of a resin nail involves several steps (see video online). First, the affected nail should be debrided and a bonding agent applied. Next, multiple layers of resin are applied until the patient’s desired thickness is achieved (typically 2 layers), followed by a sealing agent. Finally, the nail is cured with UV light. We recommend applying sunscreen to the hand(s) prior to curing with UV light. The liquid resin allows the nail to be customized to the patient’s desired length and shape. The overall procedure takes approximately 20 minutes for a single nail.

We applied resin nail to the thumbnail of a 46-year-old woman with recalcitrant isolated nail LP of 7 years’ duration (Figure). She previously had difficulties performing everyday activities, and the resin improved her functionality. She also was pleased with the cosmetic appearance. After 2 weeks, the resin started falling off with corresponding natural nail growth. The patient denied any adverse events.

Practice Implications
Resin nail application may serve as a temporary solution to improve cosmesis and functionality in patients with recalcitrant nail LP. As shown in our patient, the resin may fall off faster on the fingernails than the toenails, likely because of the faster growth rate of fingernails and more frequent exposure from daily activities. Further studies of resin nail application for the fingernails are needed to establish duration in patients with varying levels of activity (eg, washing dishes, woodworking).
Because the resin nail may be removed easily at any time, resin nail application does not interfere with treatments such as intralesional steroid injections. For patients using a topical medication regimen, the resin nail may be applied slightly distal to the cuticle so that the medication can still be applied by the proximal nail fold of the underlying natural nail.
The resin nail should be kept short and removed after 2 to 4 weeks for the fingernails and 6 to 8 weeks for the toenails to examine the underlying natural nail. Patients may go about their daily activities with the resin nail, including applying nail polish to the resin nail, bathing, and swimming. Resin nail application may complement medical treatments and improve quality of life for patients with nail LP.
- Gupta MK, Lipner SR. Review of nail lichen planus: epidemiology, pathogenesis, diagnosis, and treatment. Dermatol Clin. 2021;39:221-230. doi:10.1016/j.det.2020.12.002
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Gupta MK, Lipner SR. Review of nail lichen planus: epidemiology, pathogenesis, diagnosis, and treatment. Dermatol Clin. 2021;39:221-230. doi:10.1016/j.det.2020.12.002
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
Evaluating the Cost Burden of Alopecia Areata Treatment: A Comprehensive Review for Dermatologists
Alopecia areata (AA) affects 4.5 million individuals in the United States, with 66% younger than 30 years.1,2 Inflammation causes hair loss in well-circumscribed, nonscarring patches on the body with a predilection for the scalp.3-6 The disease can devastate a patient’s self-esteem, in turn reducing quality of life.1,7 Alopecia areata is an autoimmune T-cell–mediated disease in which hair follicles lose their immune privilege.8-10 Several specific mechanisms in the cytokine interactions between T cells and the hair follicle have been discovered, revealing the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway as pivotal in the pathogenesis of the disease and leading to the use of JAK inhibitors for treatment.11
There is no cure for AA, and the condition is managed with prolonged medical treatments and cosmetic therapies.2 Although some patients may be able to manage the annual cost, the cumulative cost of AA treatment can be burdensome.12 This cumulative cost may increase if newer, potentially expensive treatments become the standard of care. Patients with AA report dipping into their savings (41.3%) and cutting back on food or clothing expenses (33.9%) to account for the cost of alopecia treatment. Although prior estimates of the annual out-of-pocket cost of AA treatments range from $1354 to $2685, the cost burden of individual therapies is poorly understood.12-14
Patients who must juggle expensive medical bills with basic living expenses may be lost to follow-up or fall into treatment nonadherence.15 Other patients’ out-of-pocket costs may be manageable, but the costs to the health care system may compromise care in other ways. We conducted a literature review of the recommended therapies for AA based on American Academy of Dermatology (AAD) guidelines to identify the costs of alopecia treatment and consolidate the available data for the practicing dermatologist.
Methods
We conducted a PubMed search of articles indexed for MEDLINE through September 15, 2022, using the terms alopecia and cost plus one of the treatments (n=21) identified by the AAD2 for the treatment of AA (Figure). The reference lists of included articles were reviewed to identify other potentially relevant studies. Forty-five articles were identified.
Given the dearth of cost research in alopecia and the paucity of large prospective studies, we excluded articles that were not available in their full-text form or were not in English (n=3), articles whose primary study topic was not AA or an expert-approved alopecia treatment (n=15), and articles with no concrete cost data (n=17), which yielded 10 relevant articles that we studied using qualitative analysis.
Due to substantial differences in study methods and outcome measures, we did not compare the costs of alopecia among studies and did not perform statistical analysis. The quality of each study was investigated and assigned a level of evidence per the 2009 criteria from the Centre for Evidence-Based Medicine.16
All cost data were converted into US dollars ($) using the conversion rate from the time of the original article’s publication.
Results
Total and Out-of-pocket Costs of AA—Li et al13 studied out-of-pocket health care costs for AA patients (N=675). Of these participants, 56.9% said their AA was moderately to seriously financially burdensome, and 41.3% reported using their savings to manage these expenses. Participants reported median out-of-pocket spending of $1354 (interquartile range, $537–$3300) annually. The most common categories of expenses were hair appointments (81.8%) and vitamins/supplements (67.7%).13
Mesinkovska et al14 studied the qualitative and quantitative financial burdens of moderate to severe AA (N=216). Fifty-seven percent of patients reported the financial impact of AA as moderately to severely burdensome with a willingness to borrow money or use savings to cover out-of-pocket costs. Patients without insurance cited cost as a major barrier to obtaining reatment. In addition to direct treatment-related expenses, AA patients spent a mean of $1961 per year on therapy to cope with the disease’s psychological burden. Lost work hours represented another source of financial burden; 61% of patients were employed, and 45% of them reported missing time from their job because of AA.14
Mostaghimi et al12 studied health care resource utilization and all-cause direct health care costs in privately insured AA patients with or without alopecia totalis (AT) or alopecia universalis (AU)(n=14,972) matched with non-AA controls (n=44,916)(1:3 ratio). Mean total all-cause medical and pharmacy costs were higher in both AA groups compared with controls (AT/AU, $18,988 vs $11,030; non-AT/AU, $13,686 vs $9336; P<.001 for both). Out-of-pocket costs were higher for AA vs controls (AT/AU, $2685 vs $1457; non-AT/AU, $2223 vs $1341; P<.001 for both). Medical costs in the AT/AU and non-AT/AU groups largely were driven by outpatient costs (AT/AU, $10,277 vs $5713; non-AT/AU, $8078 vs $4672; P<.001 for both).12
Costs of Concealment—When studying the out-of-pocket costs of AA (N=675), Li et al13 discovered that the median yearly spending was highest on headwear or cosmetic items such as hats, wigs, and makeup ($450; interquartile range, $50–$1500). Mesinkovska et al14 reported that 49% of patients had insurance that covered AA treatment. However, 75% of patients reported that their insurance would not cover costs of concealment (eg, weave, wig, hair piece). Patients (N=112) spent a mean of $2211 per year and 10.3 hours per week on concealment.14
Minoxidil—Minoxidil solution is available over-the-counter, and its ease of access makes it a popular treatment for AA.17 Because manufacturers can sell directly to the public, minoxidil is marketed with bold claims and convincing packaging. Shrank18 noted that the product can take 4 months to work, meaning customers must incur a substantial cost burden before realizing the treatment’s benefit, which is not always obvious when purchasing minoxidil products, leaving customers—who were marketed a miracle drug—disappointed. Per Shrank,18 patients who did not experience hair regrowth after 4 months were advised to continue treatment for a year, leading them to spend hundreds of dollars for uncertain results. Those who did experience hair regrowth were advised to continue using the product twice daily 7 days per week indefinitely.18
Wehner et al19 studied the association between gender and drug cost for over-the-counter minoxidil. The price that women paid for 2% regular-strength minoxidil solutions was similar to the price that men paid for 5% extra-strength minoxidil solutions (women’s 2%, $7.63/30 mL; men’s 5%, $7.61/30 mL; P=.67). Minoxidil 5% foams with identical ingredients were priced significantly more per volume of the same product when sold as a product directed at women vs a product directed at men (men’s 5%, $8.05/30 mL; women’s 5%, $11.27/30 mL; P<.001).19
Beach20 compared the cost of oral minoxidil to topical minoxidil. At $28.60 for a 3-month supply, oral minoxidil demonstrated cost savings compared to topical minoxidil ($48.30).20
Diphencyprone—Bhat et al21 studied the cost-efficiency of diphencyprone (DPC) in patients with AA resistant to at least 2 conventional treatments (N=29). After initial sensitization with 2% DPC, patients received weekly or fortnightly treatments. Most of the annual cost burden of DPC treatment was due to staff time and overhead rather than the cost of the DPC itself: $258 for the DPC, $978 in staff time and overhead for the department, and $1233 directly charged to the patient.21
Lekhavat et al22 studied the economic impact of home-use vs office-use DPC in extensive AA (N=82). Both groups received weekly treatments in the hospital until DPC concentrations had been adjusted. Afterward, the home group was given training on self-applying DPC at home. The home group had monthly office visits for DPC concentration evaluation and refills, while the office group had weekly appointments for DPC treatment at the hospital. Calculated costs included those to the health care provider (ie, material, labor, capital costs) and the patient’s final out-of-pocket expense. The total cost to the health care provider was higher for the office group than the home group at 48 weeks (office, $683.52; home, $303.67; P<.001). Median out-of-pocket costs did not vary significantly between groups, which may have been due to small sample size affecting the range (office, $418.07; home, $189.69; P=.101). There was no significant difference between groups in the proportion of patients who responded favorably to the DPC.22
JAK Inhibitors—Chen et al23 studied the efficacy of low-dose (5 mg) tofacitinib to treat severe AA (N=6). Compared to prior studies,24-27 this analysis reported the efficacy of low-dose tofacitinib was not inferior to higher doses (10–20 mg), and low-dose tofacitinib reduced treatment costs by more than 50%.23
Per the GlobalData Healthcare database, the estimated annual cost of therapy for JAK inhibitors following US Food and Drug Administration approval was $50,000. At the time of their reporting, the next most expensive immunomodulatory drug for AA was cyclosporine, with an annual cost of therapy of $1400.28 Dillon29 reviewed the use of JAK inhibitors for the treatment of AA. The cost estimates by Dillon29 prior to FDA approval aligned with the pricing of Eli Lilly and Company for the now-approved JAK inhibitor baricitinib.30 The list price of baricitinib is $2739.99 for a 30-day supply of 2-mg tablets or $5479.98 for a 30-day supply of 4-mg tablets. This amounts to $32,879.88 for an annual supply of 2-mg tablets and $65,759.76 for an annual supply for 4-mg tablets, though the out-of-pocket costs will vary.30
Comment
We reviewed the global and treatment-specific costs of AA, consolidating the available data for the practicing dermatologist. Ten studies of approximately 16,000 patients with AA across a range of levels of evidence (1a to 4) were included (Table). Three of 10 articles studied global costs of AA, 1 studied costs of concealment, 3 studied costs of minoxidil, 2 studied costs of DPC, and 2 studied costs of JAK inhibitors. Only 2 studies achieved level of evidence 1a: the first assessed the economic impact of home-use vs office-use DPC,22 and the second researched the efficacy and outcomes of JAK inhibitors.29
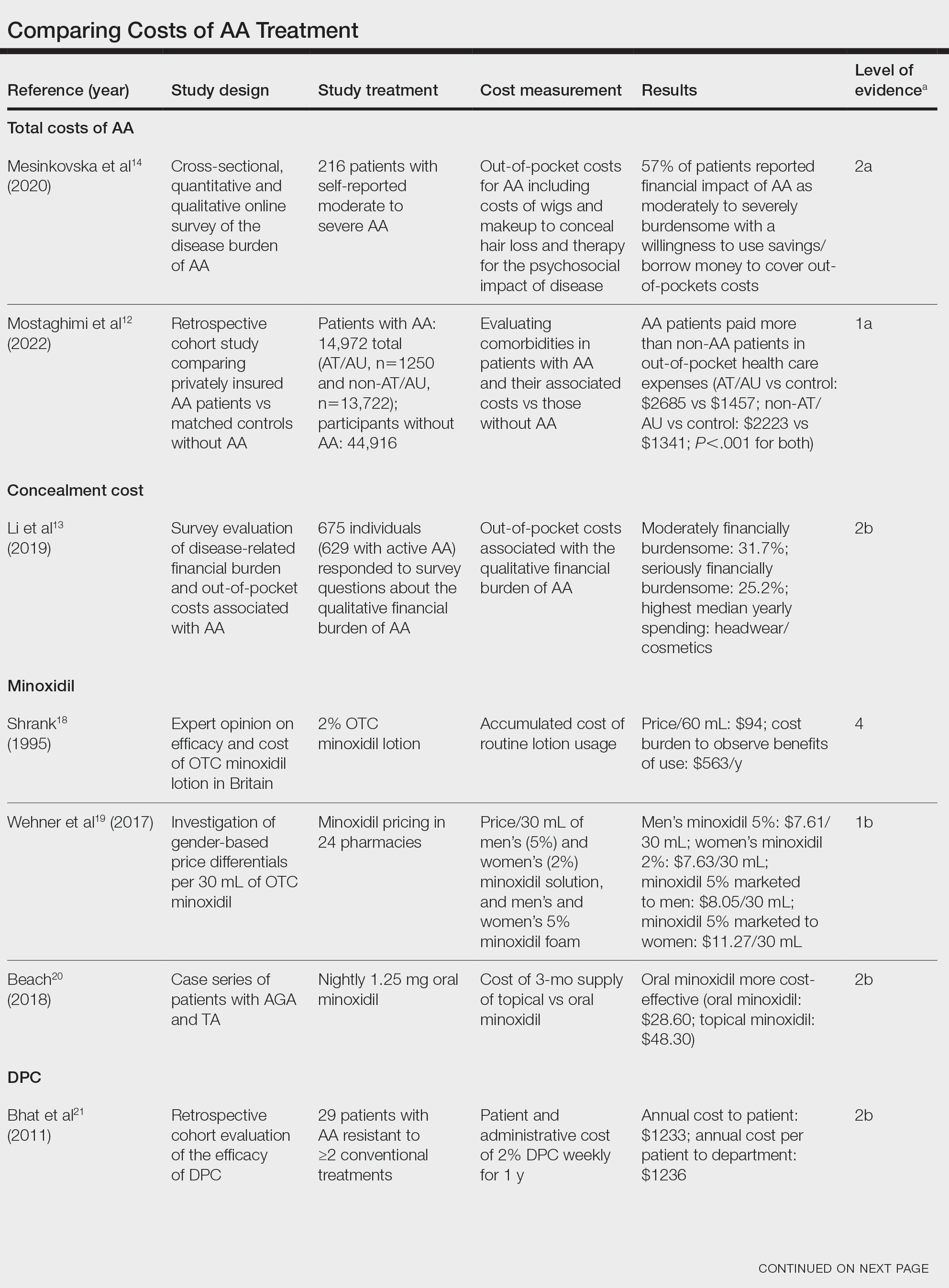
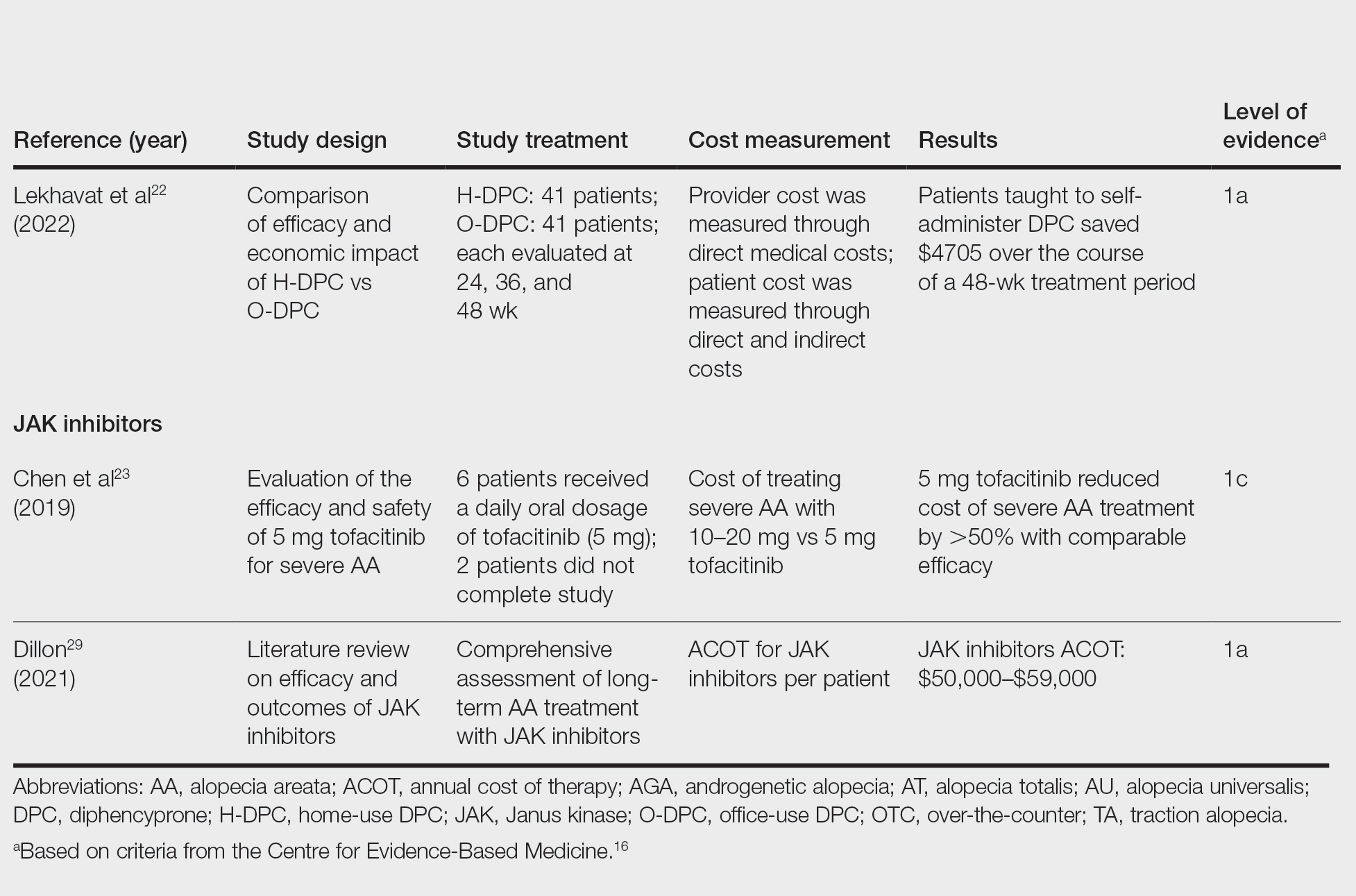
Hair-loss treatments and concealment techniques cost the average patient thousands of dollars. Spending was highest on headwear or cosmetic items, which were rarely covered by insurance.13 Psychosocial sequelae further increased cost via therapy charges and lost time at work.14 Patients with AA had greater all-cause medical costs than those without AA, with most of the cost driven by outpatient visits. Patients with AA also paid nearly twice as much as non-AA patients on out-of-pocket health care expenses.14 Despite the high costs and limited efficacy of many AA therapies, patients reported willingness to incur debt or use savings to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress.13
Minoxidil solution does not require physician office visits and is available over-the-counter.17 Despite identical ingredients, minoxidil is priced more per volume when marketed to women compared with men, which reflects the larger issue of gender-based pricing that does not exist for other AAD-approved alopecia therapies but may exist for cosmetic treatments and nonapproved therapies (eg, vitamins/supplements) that are popular in the treatment of AA.19 Oral minoxidil was more cost-effective than the topical form, and gender-based pricing was a nonissue.20 However, oral minoxidil requires a prescription, mandating patients incur the cost of an office visit. Patients should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective choice.
Diphencyprone is a relatively affordable drug for AA, but the regular office visits traditionally required for its administration increase associated cost.21 Self-administration of DPC at home was more cost- and time-effective than in-office DPC administration and did not decrease efficacy. A regimen combining office visits for initial DPC titration, at-home DPC administration, and periodic office follow-up could minimize costs while preserving outcomes and safety.22
Janus kinase inhibitors are cutting-edge and expensive therapies for AA. The annual cost of these medications poses a tremendous burden on the payer (list price of annual supply ritlecitinib is $49,000),31 be that the patient or the insurance company. Low-dose tofacitinib may be similarly efficacious and could substantially reduce treatment costs.23 The true utility of these medications, specifically considering their steep costs, remains to be determined.
Conclusion
Alopecia areata poses a substantial and recurring cost burden on patients that is multifactorial including treatment, office visits, concealment, alternative therapies, psychosocial costs, and missed time at work. Although several treatment options exist, none of them are definitive. Oral minoxidil and at-home DPC administration can be cost-effective, though the cumulative cost is still high. The cost utility of JAK inhibitors remains unclear. When JAK inhibitors are prescribed, low-dose therapy may be used as maintenance to curb treatment costs. Concealment and therapy costs pose an additional, largely out-of-pocket financial burden. Despite the limited efficacy of many AA therapies, patients incur substantial expenses to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress. There are no head-to-head studies comparing the cost-effectiveness of the different AA therapies; thus, it is unclear if one treatment is most efficacious. This topic remains an avenue for future investigation. Much of the cost burden of AA treatment falls directly on patients. Increasing coverage of AA-associated expenses, such as minoxidil therapy or wigs, could decrease the cost burden on patients. Providers also can inform patients about cost-saving tactics, such as purchasing minoxidil based on concentration and vehicle rather than marketing directed at men vs women. Finally, some patients may have insurance plans that at least partially cover the costs of wigs but may not be aware of this benefit. Querying a patient’s insurance provider can further minimize costs.
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96-98. doi:10.1067/mjd.2003.423
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78:15-24. doi:10.1016/j.jaad.2017.04.1142
- Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
- Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395-399. doi:10.1016/j.jaad.2015.06.045
- Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145. doi:10.1111/bjd.12266
- Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169:992-999. doi:10.1111/bjd.12517
- van der Steen PH, van Baar HM, Happle R, et al. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2, pt 1):227-230. doi:10.1016/0190-9622(91)70032-w
- Strazzulla LC, Avila L, Lo Sicco K, et al. Image gallery: treatment of refractory alopecia universalis with oral tofacitinib citrate and adjunct intralesional triamcinolone injections. Br J Dermatol. 2017;176:E125. doi:10.1111/bjd.15483
- Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549-566; quiz 567-570.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478-483. doi:10.1097/00055735-200012000-00016
- Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1:E1500973. doi:10.1126/sciadv.1500973
- Mostaghimi A, Gandhi K, Done N, et al. All-cause health care resource utilization and costs among adults with alopecia areata: a retrospective claims database study in the United States. J Manag Care Spec Pharm. 2022;28:426-434. doi:10.18553/jmcp.2022.28.4.426
- Li SJ, Mostaghimi A, Tkachenko E, et al. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155:493-494. doi:10.1001/jamadermatol.2018.5218
- Mesinkovska N, King B, Mirmirani P, et al. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20:S62-S68. doi:10.1016/j.jisp.2020.05.007
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44. doi:10.2147/rmhp.S19801
- Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). University of Oxford website. Accessed March 25, 2024. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- Klifto KM, Othman S, Kovach SJ. Minoxidil, platelet-rich plasma (PRP), or combined minoxidil and PRP for androgenetic alopecia in men: a cost-effectiveness Markov decision analysis of prospective studies. Cureus. 2021;13:E20839. doi:10.7759/cureus.20839
- Shrank AB. Minoxidil over the counter. BMJ. 1995;311:526. doi:10.1136/bmj.311.7004.526
- Wehner MR, Nead KT, Lipoff JB. Association between gender and drug cost for over-the-counter minoxidil. JAMA Dermatol. 2017;153:825-826.
- Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: tolerability & the five C’s of oral therapy. Dermatol Ther. 2018;31:E12707. doi:10.1111/dth.12707
- Bhat A, Sripathy K, Wahie S, et al. Efficacy and cost-efficiency of diphencyprone for alopecia areata. Br J Dermatol. 2011;165:43-44.
- Lekhavat C, Rattanaumpawan P, Juengsamranphong I. Economic impact of home-use versus office-use diphenylcyclopropenone in extensive alopecia areata. Skin Appendage Disord. 2022;8:108-117.
- Chen YY, Lin SY, Chen YC, et al. Low-dose tofacitinib for treating patients with severe alopecia areata: an efficient and cost-saving regimen. Eur J Dermatol. 2019;29:667-669. doi:10.1684/ejd.2019.3668
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28. doi:10.1016/j.jaad.2016.09.007
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776. doi:10.1172/jci.insight.89776
- Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138:1539-1545. doi:10.1016/j.jid.2018.01.032
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32. doi:10.1016/j.jaad.2016.09.006
- GlobalData Healthcare. Can JAK inhibitors penetrate the alopecia areata market effectively? Pharmaceutical Technology. July 15, 2019. Accessed February 8, 2024. https://www.pharmaceutical-technology.com/analyst-comment/alopecia-areata-treatment-2019/
- Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14:691-714. doi:10.2147/ccid.S309215
- How much should I expect to pay for Olumiant? Accessed March 20, 2024. https://www.lillypricinginfo.com/olumiant
- McNamee A. FDA approves first-ever adolescent alopecia treatment from Pfizer. Pharmaceutical Technology. June 26, 2023. Accessed March 20, 2024. https://www.pharmaceutical-technology.com/news/fda-approves-first-ever-adolescent-alopecia-treatment-from-pfizer/?cf-view
Alopecia areata (AA) affects 4.5 million individuals in the United States, with 66% younger than 30 years.1,2 Inflammation causes hair loss in well-circumscribed, nonscarring patches on the body with a predilection for the scalp.3-6 The disease can devastate a patient’s self-esteem, in turn reducing quality of life.1,7 Alopecia areata is an autoimmune T-cell–mediated disease in which hair follicles lose their immune privilege.8-10 Several specific mechanisms in the cytokine interactions between T cells and the hair follicle have been discovered, revealing the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway as pivotal in the pathogenesis of the disease and leading to the use of JAK inhibitors for treatment.11
There is no cure for AA, and the condition is managed with prolonged medical treatments and cosmetic therapies.2 Although some patients may be able to manage the annual cost, the cumulative cost of AA treatment can be burdensome.12 This cumulative cost may increase if newer, potentially expensive treatments become the standard of care. Patients with AA report dipping into their savings (41.3%) and cutting back on food or clothing expenses (33.9%) to account for the cost of alopecia treatment. Although prior estimates of the annual out-of-pocket cost of AA treatments range from $1354 to $2685, the cost burden of individual therapies is poorly understood.12-14
Patients who must juggle expensive medical bills with basic living expenses may be lost to follow-up or fall into treatment nonadherence.15 Other patients’ out-of-pocket costs may be manageable, but the costs to the health care system may compromise care in other ways. We conducted a literature review of the recommended therapies for AA based on American Academy of Dermatology (AAD) guidelines to identify the costs of alopecia treatment and consolidate the available data for the practicing dermatologist.
Methods
We conducted a PubMed search of articles indexed for MEDLINE through September 15, 2022, using the terms alopecia and cost plus one of the treatments (n=21) identified by the AAD2 for the treatment of AA (Figure). The reference lists of included articles were reviewed to identify other potentially relevant studies. Forty-five articles were identified.

Given the dearth of cost research in alopecia and the paucity of large prospective studies, we excluded articles that were not available in their full-text form or were not in English (n=3), articles whose primary study topic was not AA or an expert-approved alopecia treatment (n=15), and articles with no concrete cost data (n=17), which yielded 10 relevant articles that we studied using qualitative analysis.
Due to substantial differences in study methods and outcome measures, we did not compare the costs of alopecia among studies and did not perform statistical analysis. The quality of each study was investigated and assigned a level of evidence per the 2009 criteria from the Centre for Evidence-Based Medicine.16
All cost data were converted into US dollars ($) using the conversion rate from the time of the original article’s publication.
Results
Total and Out-of-pocket Costs of AA—Li et al13 studied out-of-pocket health care costs for AA patients (N=675). Of these participants, 56.9% said their AA was moderately to seriously financially burdensome, and 41.3% reported using their savings to manage these expenses. Participants reported median out-of-pocket spending of $1354 (interquartile range, $537–$3300) annually. The most common categories of expenses were hair appointments (81.8%) and vitamins/supplements (67.7%).13
Mesinkovska et al14 studied the qualitative and quantitative financial burdens of moderate to severe AA (N=216). Fifty-seven percent of patients reported the financial impact of AA as moderately to severely burdensome with a willingness to borrow money or use savings to cover out-of-pocket costs. Patients without insurance cited cost as a major barrier to obtaining reatment. In addition to direct treatment-related expenses, AA patients spent a mean of $1961 per year on therapy to cope with the disease’s psychological burden. Lost work hours represented another source of financial burden; 61% of patients were employed, and 45% of them reported missing time from their job because of AA.14
Mostaghimi et al12 studied health care resource utilization and all-cause direct health care costs in privately insured AA patients with or without alopecia totalis (AT) or alopecia universalis (AU)(n=14,972) matched with non-AA controls (n=44,916)(1:3 ratio). Mean total all-cause medical and pharmacy costs were higher in both AA groups compared with controls (AT/AU, $18,988 vs $11,030; non-AT/AU, $13,686 vs $9336; P<.001 for both). Out-of-pocket costs were higher for AA vs controls (AT/AU, $2685 vs $1457; non-AT/AU, $2223 vs $1341; P<.001 for both). Medical costs in the AT/AU and non-AT/AU groups largely were driven by outpatient costs (AT/AU, $10,277 vs $5713; non-AT/AU, $8078 vs $4672; P<.001 for both).12
Costs of Concealment—When studying the out-of-pocket costs of AA (N=675), Li et al13 discovered that the median yearly spending was highest on headwear or cosmetic items such as hats, wigs, and makeup ($450; interquartile range, $50–$1500). Mesinkovska et al14 reported that 49% of patients had insurance that covered AA treatment. However, 75% of patients reported that their insurance would not cover costs of concealment (eg, weave, wig, hair piece). Patients (N=112) spent a mean of $2211 per year and 10.3 hours per week on concealment.14
Minoxidil—Minoxidil solution is available over-the-counter, and its ease of access makes it a popular treatment for AA.17 Because manufacturers can sell directly to the public, minoxidil is marketed with bold claims and convincing packaging. Shrank18 noted that the product can take 4 months to work, meaning customers must incur a substantial cost burden before realizing the treatment’s benefit, which is not always obvious when purchasing minoxidil products, leaving customers—who were marketed a miracle drug—disappointed. Per Shrank,18 patients who did not experience hair regrowth after 4 months were advised to continue treatment for a year, leading them to spend hundreds of dollars for uncertain results. Those who did experience hair regrowth were advised to continue using the product twice daily 7 days per week indefinitely.18
Wehner et al19 studied the association between gender and drug cost for over-the-counter minoxidil. The price that women paid for 2% regular-strength minoxidil solutions was similar to the price that men paid for 5% extra-strength minoxidil solutions (women’s 2%, $7.63/30 mL; men’s 5%, $7.61/30 mL; P=.67). Minoxidil 5% foams with identical ingredients were priced significantly more per volume of the same product when sold as a product directed at women vs a product directed at men (men’s 5%, $8.05/30 mL; women’s 5%, $11.27/30 mL; P<.001).19
Beach20 compared the cost of oral minoxidil to topical minoxidil. At $28.60 for a 3-month supply, oral minoxidil demonstrated cost savings compared to topical minoxidil ($48.30).20
Diphencyprone—Bhat et al21 studied the cost-efficiency of diphencyprone (DPC) in patients with AA resistant to at least 2 conventional treatments (N=29). After initial sensitization with 2% DPC, patients received weekly or fortnightly treatments. Most of the annual cost burden of DPC treatment was due to staff time and overhead rather than the cost of the DPC itself: $258 for the DPC, $978 in staff time and overhead for the department, and $1233 directly charged to the patient.21
Lekhavat et al22 studied the economic impact of home-use vs office-use DPC in extensive AA (N=82). Both groups received weekly treatments in the hospital until DPC concentrations had been adjusted. Afterward, the home group was given training on self-applying DPC at home. The home group had monthly office visits for DPC concentration evaluation and refills, while the office group had weekly appointments for DPC treatment at the hospital. Calculated costs included those to the health care provider (ie, material, labor, capital costs) and the patient’s final out-of-pocket expense. The total cost to the health care provider was higher for the office group than the home group at 48 weeks (office, $683.52; home, $303.67; P<.001). Median out-of-pocket costs did not vary significantly between groups, which may have been due to small sample size affecting the range (office, $418.07; home, $189.69; P=.101). There was no significant difference between groups in the proportion of patients who responded favorably to the DPC.22
JAK Inhibitors—Chen et al23 studied the efficacy of low-dose (5 mg) tofacitinib to treat severe AA (N=6). Compared to prior studies,24-27 this analysis reported the efficacy of low-dose tofacitinib was not inferior to higher doses (10–20 mg), and low-dose tofacitinib reduced treatment costs by more than 50%.23
Per the GlobalData Healthcare database, the estimated annual cost of therapy for JAK inhibitors following US Food and Drug Administration approval was $50,000. At the time of their reporting, the next most expensive immunomodulatory drug for AA was cyclosporine, with an annual cost of therapy of $1400.28 Dillon29 reviewed the use of JAK inhibitors for the treatment of AA. The cost estimates by Dillon29 prior to FDA approval aligned with the pricing of Eli Lilly and Company for the now-approved JAK inhibitor baricitinib.30 The list price of baricitinib is $2739.99 for a 30-day supply of 2-mg tablets or $5479.98 for a 30-day supply of 4-mg tablets. This amounts to $32,879.88 for an annual supply of 2-mg tablets and $65,759.76 for an annual supply for 4-mg tablets, though the out-of-pocket costs will vary.30
Comment
We reviewed the global and treatment-specific costs of AA, consolidating the available data for the practicing dermatologist. Ten studies of approximately 16,000 patients with AA across a range of levels of evidence (1a to 4) were included (Table). Three of 10 articles studied global costs of AA, 1 studied costs of concealment, 3 studied costs of minoxidil, 2 studied costs of DPC, and 2 studied costs of JAK inhibitors. Only 2 studies achieved level of evidence 1a: the first assessed the economic impact of home-use vs office-use DPC,22 and the second researched the efficacy and outcomes of JAK inhibitors.29


Hair-loss treatments and concealment techniques cost the average patient thousands of dollars. Spending was highest on headwear or cosmetic items, which were rarely covered by insurance.13 Psychosocial sequelae further increased cost via therapy charges and lost time at work.14 Patients with AA had greater all-cause medical costs than those without AA, with most of the cost driven by outpatient visits. Patients with AA also paid nearly twice as much as non-AA patients on out-of-pocket health care expenses.14 Despite the high costs and limited efficacy of many AA therapies, patients reported willingness to incur debt or use savings to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress.13
Minoxidil solution does not require physician office visits and is available over-the-counter.17 Despite identical ingredients, minoxidil is priced more per volume when marketed to women compared with men, which reflects the larger issue of gender-based pricing that does not exist for other AAD-approved alopecia therapies but may exist for cosmetic treatments and nonapproved therapies (eg, vitamins/supplements) that are popular in the treatment of AA.19 Oral minoxidil was more cost-effective than the topical form, and gender-based pricing was a nonissue.20 However, oral minoxidil requires a prescription, mandating patients incur the cost of an office visit. Patients should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective choice.
Diphencyprone is a relatively affordable drug for AA, but the regular office visits traditionally required for its administration increase associated cost.21 Self-administration of DPC at home was more cost- and time-effective than in-office DPC administration and did not decrease efficacy. A regimen combining office visits for initial DPC titration, at-home DPC administration, and periodic office follow-up could minimize costs while preserving outcomes and safety.22
Janus kinase inhibitors are cutting-edge and expensive therapies for AA. The annual cost of these medications poses a tremendous burden on the payer (list price of annual supply ritlecitinib is $49,000),31 be that the patient or the insurance company. Low-dose tofacitinib may be similarly efficacious and could substantially reduce treatment costs.23 The true utility of these medications, specifically considering their steep costs, remains to be determined.
Conclusion
Alopecia areata poses a substantial and recurring cost burden on patients that is multifactorial including treatment, office visits, concealment, alternative therapies, psychosocial costs, and missed time at work. Although several treatment options exist, none of them are definitive. Oral minoxidil and at-home DPC administration can be cost-effective, though the cumulative cost is still high. The cost utility of JAK inhibitors remains unclear. When JAK inhibitors are prescribed, low-dose therapy may be used as maintenance to curb treatment costs. Concealment and therapy costs pose an additional, largely out-of-pocket financial burden. Despite the limited efficacy of many AA therapies, patients incur substantial expenses to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress. There are no head-to-head studies comparing the cost-effectiveness of the different AA therapies; thus, it is unclear if one treatment is most efficacious. This topic remains an avenue for future investigation. Much of the cost burden of AA treatment falls directly on patients. Increasing coverage of AA-associated expenses, such as minoxidil therapy or wigs, could decrease the cost burden on patients. Providers also can inform patients about cost-saving tactics, such as purchasing minoxidil based on concentration and vehicle rather than marketing directed at men vs women. Finally, some patients may have insurance plans that at least partially cover the costs of wigs but may not be aware of this benefit. Querying a patient’s insurance provider can further minimize costs.
Alopecia areata (AA) affects 4.5 million individuals in the United States, with 66% younger than 30 years.1,2 Inflammation causes hair loss in well-circumscribed, nonscarring patches on the body with a predilection for the scalp.3-6 The disease can devastate a patient’s self-esteem, in turn reducing quality of life.1,7 Alopecia areata is an autoimmune T-cell–mediated disease in which hair follicles lose their immune privilege.8-10 Several specific mechanisms in the cytokine interactions between T cells and the hair follicle have been discovered, revealing the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway as pivotal in the pathogenesis of the disease and leading to the use of JAK inhibitors for treatment.11
There is no cure for AA, and the condition is managed with prolonged medical treatments and cosmetic therapies.2 Although some patients may be able to manage the annual cost, the cumulative cost of AA treatment can be burdensome.12 This cumulative cost may increase if newer, potentially expensive treatments become the standard of care. Patients with AA report dipping into their savings (41.3%) and cutting back on food or clothing expenses (33.9%) to account for the cost of alopecia treatment. Although prior estimates of the annual out-of-pocket cost of AA treatments range from $1354 to $2685, the cost burden of individual therapies is poorly understood.12-14
Patients who must juggle expensive medical bills with basic living expenses may be lost to follow-up or fall into treatment nonadherence.15 Other patients’ out-of-pocket costs may be manageable, but the costs to the health care system may compromise care in other ways. We conducted a literature review of the recommended therapies for AA based on American Academy of Dermatology (AAD) guidelines to identify the costs of alopecia treatment and consolidate the available data for the practicing dermatologist.
Methods
We conducted a PubMed search of articles indexed for MEDLINE through September 15, 2022, using the terms alopecia and cost plus one of the treatments (n=21) identified by the AAD2 for the treatment of AA (Figure). The reference lists of included articles were reviewed to identify other potentially relevant studies. Forty-five articles were identified.

Given the dearth of cost research in alopecia and the paucity of large prospective studies, we excluded articles that were not available in their full-text form or were not in English (n=3), articles whose primary study topic was not AA or an expert-approved alopecia treatment (n=15), and articles with no concrete cost data (n=17), which yielded 10 relevant articles that we studied using qualitative analysis.
Due to substantial differences in study methods and outcome measures, we did not compare the costs of alopecia among studies and did not perform statistical analysis. The quality of each study was investigated and assigned a level of evidence per the 2009 criteria from the Centre for Evidence-Based Medicine.16
All cost data were converted into US dollars ($) using the conversion rate from the time of the original article’s publication.
Results
Total and Out-of-pocket Costs of AA—Li et al13 studied out-of-pocket health care costs for AA patients (N=675). Of these participants, 56.9% said their AA was moderately to seriously financially burdensome, and 41.3% reported using their savings to manage these expenses. Participants reported median out-of-pocket spending of $1354 (interquartile range, $537–$3300) annually. The most common categories of expenses were hair appointments (81.8%) and vitamins/supplements (67.7%).13
Mesinkovska et al14 studied the qualitative and quantitative financial burdens of moderate to severe AA (N=216). Fifty-seven percent of patients reported the financial impact of AA as moderately to severely burdensome with a willingness to borrow money or use savings to cover out-of-pocket costs. Patients without insurance cited cost as a major barrier to obtaining reatment. In addition to direct treatment-related expenses, AA patients spent a mean of $1961 per year on therapy to cope with the disease’s psychological burden. Lost work hours represented another source of financial burden; 61% of patients were employed, and 45% of them reported missing time from their job because of AA.14
Mostaghimi et al12 studied health care resource utilization and all-cause direct health care costs in privately insured AA patients with or without alopecia totalis (AT) or alopecia universalis (AU)(n=14,972) matched with non-AA controls (n=44,916)(1:3 ratio). Mean total all-cause medical and pharmacy costs were higher in both AA groups compared with controls (AT/AU, $18,988 vs $11,030; non-AT/AU, $13,686 vs $9336; P<.001 for both). Out-of-pocket costs were higher for AA vs controls (AT/AU, $2685 vs $1457; non-AT/AU, $2223 vs $1341; P<.001 for both). Medical costs in the AT/AU and non-AT/AU groups largely were driven by outpatient costs (AT/AU, $10,277 vs $5713; non-AT/AU, $8078 vs $4672; P<.001 for both).12
Costs of Concealment—When studying the out-of-pocket costs of AA (N=675), Li et al13 discovered that the median yearly spending was highest on headwear or cosmetic items such as hats, wigs, and makeup ($450; interquartile range, $50–$1500). Mesinkovska et al14 reported that 49% of patients had insurance that covered AA treatment. However, 75% of patients reported that their insurance would not cover costs of concealment (eg, weave, wig, hair piece). Patients (N=112) spent a mean of $2211 per year and 10.3 hours per week on concealment.14
Minoxidil—Minoxidil solution is available over-the-counter, and its ease of access makes it a popular treatment for AA.17 Because manufacturers can sell directly to the public, minoxidil is marketed with bold claims and convincing packaging. Shrank18 noted that the product can take 4 months to work, meaning customers must incur a substantial cost burden before realizing the treatment’s benefit, which is not always obvious when purchasing minoxidil products, leaving customers—who were marketed a miracle drug—disappointed. Per Shrank,18 patients who did not experience hair regrowth after 4 months were advised to continue treatment for a year, leading them to spend hundreds of dollars for uncertain results. Those who did experience hair regrowth were advised to continue using the product twice daily 7 days per week indefinitely.18
Wehner et al19 studied the association between gender and drug cost for over-the-counter minoxidil. The price that women paid for 2% regular-strength minoxidil solutions was similar to the price that men paid for 5% extra-strength minoxidil solutions (women’s 2%, $7.63/30 mL; men’s 5%, $7.61/30 mL; P=.67). Minoxidil 5% foams with identical ingredients were priced significantly more per volume of the same product when sold as a product directed at women vs a product directed at men (men’s 5%, $8.05/30 mL; women’s 5%, $11.27/30 mL; P<.001).19
Beach20 compared the cost of oral minoxidil to topical minoxidil. At $28.60 for a 3-month supply, oral minoxidil demonstrated cost savings compared to topical minoxidil ($48.30).20
Diphencyprone—Bhat et al21 studied the cost-efficiency of diphencyprone (DPC) in patients with AA resistant to at least 2 conventional treatments (N=29). After initial sensitization with 2% DPC, patients received weekly or fortnightly treatments. Most of the annual cost burden of DPC treatment was due to staff time and overhead rather than the cost of the DPC itself: $258 for the DPC, $978 in staff time and overhead for the department, and $1233 directly charged to the patient.21
Lekhavat et al22 studied the economic impact of home-use vs office-use DPC in extensive AA (N=82). Both groups received weekly treatments in the hospital until DPC concentrations had been adjusted. Afterward, the home group was given training on self-applying DPC at home. The home group had monthly office visits for DPC concentration evaluation and refills, while the office group had weekly appointments for DPC treatment at the hospital. Calculated costs included those to the health care provider (ie, material, labor, capital costs) and the patient’s final out-of-pocket expense. The total cost to the health care provider was higher for the office group than the home group at 48 weeks (office, $683.52; home, $303.67; P<.001). Median out-of-pocket costs did not vary significantly between groups, which may have been due to small sample size affecting the range (office, $418.07; home, $189.69; P=.101). There was no significant difference between groups in the proportion of patients who responded favorably to the DPC.22
JAK Inhibitors—Chen et al23 studied the efficacy of low-dose (5 mg) tofacitinib to treat severe AA (N=6). Compared to prior studies,24-27 this analysis reported the efficacy of low-dose tofacitinib was not inferior to higher doses (10–20 mg), and low-dose tofacitinib reduced treatment costs by more than 50%.23
Per the GlobalData Healthcare database, the estimated annual cost of therapy for JAK inhibitors following US Food and Drug Administration approval was $50,000. At the time of their reporting, the next most expensive immunomodulatory drug for AA was cyclosporine, with an annual cost of therapy of $1400.28 Dillon29 reviewed the use of JAK inhibitors for the treatment of AA. The cost estimates by Dillon29 prior to FDA approval aligned with the pricing of Eli Lilly and Company for the now-approved JAK inhibitor baricitinib.30 The list price of baricitinib is $2739.99 for a 30-day supply of 2-mg tablets or $5479.98 for a 30-day supply of 4-mg tablets. This amounts to $32,879.88 for an annual supply of 2-mg tablets and $65,759.76 for an annual supply for 4-mg tablets, though the out-of-pocket costs will vary.30
Comment
We reviewed the global and treatment-specific costs of AA, consolidating the available data for the practicing dermatologist. Ten studies of approximately 16,000 patients with AA across a range of levels of evidence (1a to 4) were included (Table). Three of 10 articles studied global costs of AA, 1 studied costs of concealment, 3 studied costs of minoxidil, 2 studied costs of DPC, and 2 studied costs of JAK inhibitors. Only 2 studies achieved level of evidence 1a: the first assessed the economic impact of home-use vs office-use DPC,22 and the second researched the efficacy and outcomes of JAK inhibitors.29


Hair-loss treatments and concealment techniques cost the average patient thousands of dollars. Spending was highest on headwear or cosmetic items, which were rarely covered by insurance.13 Psychosocial sequelae further increased cost via therapy charges and lost time at work.14 Patients with AA had greater all-cause medical costs than those without AA, with most of the cost driven by outpatient visits. Patients with AA also paid nearly twice as much as non-AA patients on out-of-pocket health care expenses.14 Despite the high costs and limited efficacy of many AA therapies, patients reported willingness to incur debt or use savings to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress.13
Minoxidil solution does not require physician office visits and is available over-the-counter.17 Despite identical ingredients, minoxidil is priced more per volume when marketed to women compared with men, which reflects the larger issue of gender-based pricing that does not exist for other AAD-approved alopecia therapies but may exist for cosmetic treatments and nonapproved therapies (eg, vitamins/supplements) that are popular in the treatment of AA.19 Oral minoxidil was more cost-effective than the topical form, and gender-based pricing was a nonissue.20 However, oral minoxidil requires a prescription, mandating patients incur the cost of an office visit. Patients should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective choice.
Diphencyprone is a relatively affordable drug for AA, but the regular office visits traditionally required for its administration increase associated cost.21 Self-administration of DPC at home was more cost- and time-effective than in-office DPC administration and did not decrease efficacy. A regimen combining office visits for initial DPC titration, at-home DPC administration, and periodic office follow-up could minimize costs while preserving outcomes and safety.22
Janus kinase inhibitors are cutting-edge and expensive therapies for AA. The annual cost of these medications poses a tremendous burden on the payer (list price of annual supply ritlecitinib is $49,000),31 be that the patient or the insurance company. Low-dose tofacitinib may be similarly efficacious and could substantially reduce treatment costs.23 The true utility of these medications, specifically considering their steep costs, remains to be determined.
Conclusion
Alopecia areata poses a substantial and recurring cost burden on patients that is multifactorial including treatment, office visits, concealment, alternative therapies, psychosocial costs, and missed time at work. Although several treatment options exist, none of them are definitive. Oral minoxidil and at-home DPC administration can be cost-effective, though the cumulative cost is still high. The cost utility of JAK inhibitors remains unclear. When JAK inhibitors are prescribed, low-dose therapy may be used as maintenance to curb treatment costs. Concealment and therapy costs pose an additional, largely out-of-pocket financial burden. Despite the limited efficacy of many AA therapies, patients incur substantial expenses to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress. There are no head-to-head studies comparing the cost-effectiveness of the different AA therapies; thus, it is unclear if one treatment is most efficacious. This topic remains an avenue for future investigation. Much of the cost burden of AA treatment falls directly on patients. Increasing coverage of AA-associated expenses, such as minoxidil therapy or wigs, could decrease the cost burden on patients. Providers also can inform patients about cost-saving tactics, such as purchasing minoxidil based on concentration and vehicle rather than marketing directed at men vs women. Finally, some patients may have insurance plans that at least partially cover the costs of wigs but may not be aware of this benefit. Querying a patient’s insurance provider can further minimize costs.
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96-98. doi:10.1067/mjd.2003.423
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78:15-24. doi:10.1016/j.jaad.2017.04.1142
- Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
- Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395-399. doi:10.1016/j.jaad.2015.06.045
- Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145. doi:10.1111/bjd.12266
- Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169:992-999. doi:10.1111/bjd.12517
- van der Steen PH, van Baar HM, Happle R, et al. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2, pt 1):227-230. doi:10.1016/0190-9622(91)70032-w
- Strazzulla LC, Avila L, Lo Sicco K, et al. Image gallery: treatment of refractory alopecia universalis with oral tofacitinib citrate and adjunct intralesional triamcinolone injections. Br J Dermatol. 2017;176:E125. doi:10.1111/bjd.15483
- Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549-566; quiz 567-570.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478-483. doi:10.1097/00055735-200012000-00016
- Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1:E1500973. doi:10.1126/sciadv.1500973
- Mostaghimi A, Gandhi K, Done N, et al. All-cause health care resource utilization and costs among adults with alopecia areata: a retrospective claims database study in the United States. J Manag Care Spec Pharm. 2022;28:426-434. doi:10.18553/jmcp.2022.28.4.426
- Li SJ, Mostaghimi A, Tkachenko E, et al. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155:493-494. doi:10.1001/jamadermatol.2018.5218
- Mesinkovska N, King B, Mirmirani P, et al. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20:S62-S68. doi:10.1016/j.jisp.2020.05.007
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44. doi:10.2147/rmhp.S19801
- Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). University of Oxford website. Accessed March 25, 2024. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- Klifto KM, Othman S, Kovach SJ. Minoxidil, platelet-rich plasma (PRP), or combined minoxidil and PRP for androgenetic alopecia in men: a cost-effectiveness Markov decision analysis of prospective studies. Cureus. 2021;13:E20839. doi:10.7759/cureus.20839
- Shrank AB. Minoxidil over the counter. BMJ. 1995;311:526. doi:10.1136/bmj.311.7004.526
- Wehner MR, Nead KT, Lipoff JB. Association between gender and drug cost for over-the-counter minoxidil. JAMA Dermatol. 2017;153:825-826.
- Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: tolerability & the five C’s of oral therapy. Dermatol Ther. 2018;31:E12707. doi:10.1111/dth.12707
- Bhat A, Sripathy K, Wahie S, et al. Efficacy and cost-efficiency of diphencyprone for alopecia areata. Br J Dermatol. 2011;165:43-44.
- Lekhavat C, Rattanaumpawan P, Juengsamranphong I. Economic impact of home-use versus office-use diphenylcyclopropenone in extensive alopecia areata. Skin Appendage Disord. 2022;8:108-117.
- Chen YY, Lin SY, Chen YC, et al. Low-dose tofacitinib for treating patients with severe alopecia areata: an efficient and cost-saving regimen. Eur J Dermatol. 2019;29:667-669. doi:10.1684/ejd.2019.3668
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28. doi:10.1016/j.jaad.2016.09.007
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776. doi:10.1172/jci.insight.89776
- Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138:1539-1545. doi:10.1016/j.jid.2018.01.032
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32. doi:10.1016/j.jaad.2016.09.006
- GlobalData Healthcare. Can JAK inhibitors penetrate the alopecia areata market effectively? Pharmaceutical Technology. July 15, 2019. Accessed February 8, 2024. https://www.pharmaceutical-technology.com/analyst-comment/alopecia-areata-treatment-2019/
- Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14:691-714. doi:10.2147/ccid.S309215
- How much should I expect to pay for Olumiant? Accessed March 20, 2024. https://www.lillypricinginfo.com/olumiant
- McNamee A. FDA approves first-ever adolescent alopecia treatment from Pfizer. Pharmaceutical Technology. June 26, 2023. Accessed March 20, 2024. https://www.pharmaceutical-technology.com/news/fda-approves-first-ever-adolescent-alopecia-treatment-from-pfizer/?cf-view
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96-98. doi:10.1067/mjd.2003.423
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78:15-24. doi:10.1016/j.jaad.2017.04.1142
- Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
- Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395-399. doi:10.1016/j.jaad.2015.06.045
- Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145. doi:10.1111/bjd.12266
- Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169:992-999. doi:10.1111/bjd.12517
- van der Steen PH, van Baar HM, Happle R, et al. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2, pt 1):227-230. doi:10.1016/0190-9622(91)70032-w
- Strazzulla LC, Avila L, Lo Sicco K, et al. Image gallery: treatment of refractory alopecia universalis with oral tofacitinib citrate and adjunct intralesional triamcinolone injections. Br J Dermatol. 2017;176:E125. doi:10.1111/bjd.15483
- Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549-566; quiz 567-570.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478-483. doi:10.1097/00055735-200012000-00016
- Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1:E1500973. doi:10.1126/sciadv.1500973
- Mostaghimi A, Gandhi K, Done N, et al. All-cause health care resource utilization and costs among adults with alopecia areata: a retrospective claims database study in the United States. J Manag Care Spec Pharm. 2022;28:426-434. doi:10.18553/jmcp.2022.28.4.426
- Li SJ, Mostaghimi A, Tkachenko E, et al. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155:493-494. doi:10.1001/jamadermatol.2018.5218
- Mesinkovska N, King B, Mirmirani P, et al. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20:S62-S68. doi:10.1016/j.jisp.2020.05.007
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44. doi:10.2147/rmhp.S19801
- Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). University of Oxford website. Accessed March 25, 2024. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- Klifto KM, Othman S, Kovach SJ. Minoxidil, platelet-rich plasma (PRP), or combined minoxidil and PRP for androgenetic alopecia in men: a cost-effectiveness Markov decision analysis of prospective studies. Cureus. 2021;13:E20839. doi:10.7759/cureus.20839
- Shrank AB. Minoxidil over the counter. BMJ. 1995;311:526. doi:10.1136/bmj.311.7004.526
- Wehner MR, Nead KT, Lipoff JB. Association between gender and drug cost for over-the-counter minoxidil. JAMA Dermatol. 2017;153:825-826.
- Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: tolerability & the five C’s of oral therapy. Dermatol Ther. 2018;31:E12707. doi:10.1111/dth.12707
- Bhat A, Sripathy K, Wahie S, et al. Efficacy and cost-efficiency of diphencyprone for alopecia areata. Br J Dermatol. 2011;165:43-44.
- Lekhavat C, Rattanaumpawan P, Juengsamranphong I. Economic impact of home-use versus office-use diphenylcyclopropenone in extensive alopecia areata. Skin Appendage Disord. 2022;8:108-117.
- Chen YY, Lin SY, Chen YC, et al. Low-dose tofacitinib for treating patients with severe alopecia areata: an efficient and cost-saving regimen. Eur J Dermatol. 2019;29:667-669. doi:10.1684/ejd.2019.3668
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28. doi:10.1016/j.jaad.2016.09.007
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776. doi:10.1172/jci.insight.89776
- Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138:1539-1545. doi:10.1016/j.jid.2018.01.032
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32. doi:10.1016/j.jaad.2016.09.006
- GlobalData Healthcare. Can JAK inhibitors penetrate the alopecia areata market effectively? Pharmaceutical Technology. July 15, 2019. Accessed February 8, 2024. https://www.pharmaceutical-technology.com/analyst-comment/alopecia-areata-treatment-2019/
- Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14:691-714. doi:10.2147/ccid.S309215
- How much should I expect to pay for Olumiant? Accessed March 20, 2024. https://www.lillypricinginfo.com/olumiant
- McNamee A. FDA approves first-ever adolescent alopecia treatment from Pfizer. Pharmaceutical Technology. June 26, 2023. Accessed March 20, 2024. https://www.pharmaceutical-technology.com/news/fda-approves-first-ever-adolescent-alopecia-treatment-from-pfizer/?cf-view
Practice Points
- Hair loss treatments and concealment techniques cost the average patient thousands of dollars. Much of this cost burden comes from items not covered by insurance.
- Providers should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective option.
- Self-administering diphencyprone at home is more cost- and time-effective than in-office diphencyprone administration and does not decrease efficacy.