User login
Hair-Straightening Products Entail Acute Kidney Failure Risk
The observation was made by a team of French researchers who tested the suspected straightening product on animals. The product is believed to be the cause of several episodes of renal damage in a young woman.
“The results on mice are striking,” said study author Emmanuel Letavernier, MD, a nephrologist at Tenon Hospital in Paris. “They develop extremely severe acute kidney failure within 24 hours of applying the straightening cream. Samples show the presence of calcium oxalate crystals in all renal tubules.”
Given the potential nephrotoxicity of glyoxylic acid through topical application, products containing this compound should be avoided and ideally withdrawn from the market, the researchers suggested in a letter published in The New England Journal of Medicine. The appropriate departments of the French Agency for Food, Environmental, and Occupational Health and Safety have been alerted, Dr. Letavernier added.
Replacing Formaldehyde
Glyoxylic acid has recently been introduced into certain cosmetic products (such as shampoo, styling lotion, and straightening products), often as a replacement for formaldehyde, which is irritating and possibly carcinogenic. Glyoxylic acid is praised for its smoothing qualities. However, it is recommended to avoid contact with the scalp.
Cases of renal complications could be underdiagnosed, according to the researchers, who are preparing a nationwide survey. Renal failure can be silent. Among the signs that should raise concern are “scalp irritation accompanied by nausea or vomiting after a hair salon visit,” said Dr. Letavernier.
Similar cases have already been reported in the literature. An Israeli team recently described 26 patients treated for acute renal injuries after hair straightening in hair salons. Biopsies revealed calcium oxalate crystals in the kidneys.
The Israeli researchers suspected an effect of glycolic acid, another substance found in many cosmetic products, including straightening products. However, they could not provide evidence.
Glycolic Acid Safe?
By conducting a second animal study, which should be published soon, Dr. Letavernier and his team were able to rule out this hypothesis. “Glycolic acid does not pose a problem. Unlike glyoxylic acid, the application of glycolic acid on the skin of mice does not induce the formation of oxalate crystals in the kidneys, nor acute kidney failure.”
The French clinical case reported in the correspondence concerns a 26-year-old woman with no prior health history who had three episodes of acute renal damage 1 year apart. It turned out that each episode occurred shortly after hair straightening at a hair salon in Marseille.
The patient reported feeling a burning sensation during the hair treatment. Scalp irritations appeared. She then experienced vomiting, diarrhea, fever, and back pain. Analyses revealed high levels of plasma creatinine during each episode, indicating renal failure.
A CT scan showed no signs of urinary tract obstruction. However, the patient had a small kidney stone. Further analysis revealed the presence of blood and leukocytes in the urine. But there was no proteinuria or urinary infection.
Chronic Renal Failure
After each episode, renal function rapidly improved. “The repetition of episodes of acute renal failure is, however, a major risk factor for developing chronic renal failure in the long term,” said Dr. Letavernier.
The cream used in the hair salon to straighten hair was retrieved by the researchers. It contained a significant amount of glyoxylic acid but no glycolic acid.
To explore its potential nephrotoxic effect, they conducted a study on 10 mice. The animals were divided into two groups to test on one side topical application of the product and a gel without active product (control group) on the other.
Mice exposed to the product had oxalate crystals in their urine, unlike mice in the control group. A scan confirmed calcium oxalate deposits in the kidneys. Plasma creatinine levels increased significantly after exposure to glyoxylic acid.
“After passing through the epidermis, glyoxylic acid is rapidly converted in the blood to glyoxylate. In the liver and probably in other organs, glyoxylate is metabolized to become oxalate, which upon contact with calcium in the urine forms calcium oxalate crystals,” explained the specialist.
Excess calcium oxalate crystals causing renal failure are observed in rare conditions such as primary hyperoxaluria, a genetic disease affecting liver metabolism, or enteric hyperoxaluria, which is linked to increased intestinal permeability to oxalate: an anion naturally found in certain plants.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The observation was made by a team of French researchers who tested the suspected straightening product on animals. The product is believed to be the cause of several episodes of renal damage in a young woman.
“The results on mice are striking,” said study author Emmanuel Letavernier, MD, a nephrologist at Tenon Hospital in Paris. “They develop extremely severe acute kidney failure within 24 hours of applying the straightening cream. Samples show the presence of calcium oxalate crystals in all renal tubules.”
Given the potential nephrotoxicity of glyoxylic acid through topical application, products containing this compound should be avoided and ideally withdrawn from the market, the researchers suggested in a letter published in The New England Journal of Medicine. The appropriate departments of the French Agency for Food, Environmental, and Occupational Health and Safety have been alerted, Dr. Letavernier added.
Replacing Formaldehyde
Glyoxylic acid has recently been introduced into certain cosmetic products (such as shampoo, styling lotion, and straightening products), often as a replacement for formaldehyde, which is irritating and possibly carcinogenic. Glyoxylic acid is praised for its smoothing qualities. However, it is recommended to avoid contact with the scalp.
Cases of renal complications could be underdiagnosed, according to the researchers, who are preparing a nationwide survey. Renal failure can be silent. Among the signs that should raise concern are “scalp irritation accompanied by nausea or vomiting after a hair salon visit,” said Dr. Letavernier.
Similar cases have already been reported in the literature. An Israeli team recently described 26 patients treated for acute renal injuries after hair straightening in hair salons. Biopsies revealed calcium oxalate crystals in the kidneys.
The Israeli researchers suspected an effect of glycolic acid, another substance found in many cosmetic products, including straightening products. However, they could not provide evidence.
Glycolic Acid Safe?
By conducting a second animal study, which should be published soon, Dr. Letavernier and his team were able to rule out this hypothesis. “Glycolic acid does not pose a problem. Unlike glyoxylic acid, the application of glycolic acid on the skin of mice does not induce the formation of oxalate crystals in the kidneys, nor acute kidney failure.”
The French clinical case reported in the correspondence concerns a 26-year-old woman with no prior health history who had three episodes of acute renal damage 1 year apart. It turned out that each episode occurred shortly after hair straightening at a hair salon in Marseille.
The patient reported feeling a burning sensation during the hair treatment. Scalp irritations appeared. She then experienced vomiting, diarrhea, fever, and back pain. Analyses revealed high levels of plasma creatinine during each episode, indicating renal failure.
A CT scan showed no signs of urinary tract obstruction. However, the patient had a small kidney stone. Further analysis revealed the presence of blood and leukocytes in the urine. But there was no proteinuria or urinary infection.
Chronic Renal Failure
After each episode, renal function rapidly improved. “The repetition of episodes of acute renal failure is, however, a major risk factor for developing chronic renal failure in the long term,” said Dr. Letavernier.
The cream used in the hair salon to straighten hair was retrieved by the researchers. It contained a significant amount of glyoxylic acid but no glycolic acid.
To explore its potential nephrotoxic effect, they conducted a study on 10 mice. The animals were divided into two groups to test on one side topical application of the product and a gel without active product (control group) on the other.
Mice exposed to the product had oxalate crystals in their urine, unlike mice in the control group. A scan confirmed calcium oxalate deposits in the kidneys. Plasma creatinine levels increased significantly after exposure to glyoxylic acid.
“After passing through the epidermis, glyoxylic acid is rapidly converted in the blood to glyoxylate. In the liver and probably in other organs, glyoxylate is metabolized to become oxalate, which upon contact with calcium in the urine forms calcium oxalate crystals,” explained the specialist.
Excess calcium oxalate crystals causing renal failure are observed in rare conditions such as primary hyperoxaluria, a genetic disease affecting liver metabolism, or enteric hyperoxaluria, which is linked to increased intestinal permeability to oxalate: an anion naturally found in certain plants.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The observation was made by a team of French researchers who tested the suspected straightening product on animals. The product is believed to be the cause of several episodes of renal damage in a young woman.
“The results on mice are striking,” said study author Emmanuel Letavernier, MD, a nephrologist at Tenon Hospital in Paris. “They develop extremely severe acute kidney failure within 24 hours of applying the straightening cream. Samples show the presence of calcium oxalate crystals in all renal tubules.”
Given the potential nephrotoxicity of glyoxylic acid through topical application, products containing this compound should be avoided and ideally withdrawn from the market, the researchers suggested in a letter published in The New England Journal of Medicine. The appropriate departments of the French Agency for Food, Environmental, and Occupational Health and Safety have been alerted, Dr. Letavernier added.
Replacing Formaldehyde
Glyoxylic acid has recently been introduced into certain cosmetic products (such as shampoo, styling lotion, and straightening products), often as a replacement for formaldehyde, which is irritating and possibly carcinogenic. Glyoxylic acid is praised for its smoothing qualities. However, it is recommended to avoid contact with the scalp.
Cases of renal complications could be underdiagnosed, according to the researchers, who are preparing a nationwide survey. Renal failure can be silent. Among the signs that should raise concern are “scalp irritation accompanied by nausea or vomiting after a hair salon visit,” said Dr. Letavernier.
Similar cases have already been reported in the literature. An Israeli team recently described 26 patients treated for acute renal injuries after hair straightening in hair salons. Biopsies revealed calcium oxalate crystals in the kidneys.
The Israeli researchers suspected an effect of glycolic acid, another substance found in many cosmetic products, including straightening products. However, they could not provide evidence.
Glycolic Acid Safe?
By conducting a second animal study, which should be published soon, Dr. Letavernier and his team were able to rule out this hypothesis. “Glycolic acid does not pose a problem. Unlike glyoxylic acid, the application of glycolic acid on the skin of mice does not induce the formation of oxalate crystals in the kidneys, nor acute kidney failure.”
The French clinical case reported in the correspondence concerns a 26-year-old woman with no prior health history who had three episodes of acute renal damage 1 year apart. It turned out that each episode occurred shortly after hair straightening at a hair salon in Marseille.
The patient reported feeling a burning sensation during the hair treatment. Scalp irritations appeared. She then experienced vomiting, diarrhea, fever, and back pain. Analyses revealed high levels of plasma creatinine during each episode, indicating renal failure.
A CT scan showed no signs of urinary tract obstruction. However, the patient had a small kidney stone. Further analysis revealed the presence of blood and leukocytes in the urine. But there was no proteinuria or urinary infection.
Chronic Renal Failure
After each episode, renal function rapidly improved. “The repetition of episodes of acute renal failure is, however, a major risk factor for developing chronic renal failure in the long term,” said Dr. Letavernier.
The cream used in the hair salon to straighten hair was retrieved by the researchers. It contained a significant amount of glyoxylic acid but no glycolic acid.
To explore its potential nephrotoxic effect, they conducted a study on 10 mice. The animals were divided into two groups to test on one side topical application of the product and a gel without active product (control group) on the other.
Mice exposed to the product had oxalate crystals in their urine, unlike mice in the control group. A scan confirmed calcium oxalate deposits in the kidneys. Plasma creatinine levels increased significantly after exposure to glyoxylic acid.
“After passing through the epidermis, glyoxylic acid is rapidly converted in the blood to glyoxylate. In the liver and probably in other organs, glyoxylate is metabolized to become oxalate, which upon contact with calcium in the urine forms calcium oxalate crystals,” explained the specialist.
Excess calcium oxalate crystals causing renal failure are observed in rare conditions such as primary hyperoxaluria, a genetic disease affecting liver metabolism, or enteric hyperoxaluria, which is linked to increased intestinal permeability to oxalate: an anion naturally found in certain plants.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Studies Reinforce JAK Inhibitor Efficacy for Most Challenging Alopecia Types
FROM AAD 2024
SAN DIEGO — , according to late-breaking data presented at the annual meeting of the American Academy of Dermatology.
In one study of brepocitinib, the target was cicatricial alopecia (CA), a form of hair loss for which there are no approved therapies. In the other, a subanalysis from phase 3 trials of ritlecitinib for alopecia areata (AA), hair regrowth was shown in the subset of patients who entered the study with alopecia totalis or alopecia universalis (AT/AU).
Reflecting comments from several experts, including one of the late-breaking session moderators, April W. Armstrong, MD, MPH, professor and chief of dermatology, University of California, Los Angeles, said that the CA study, which matched clinical response to changes in CA biomarkers, suggested that the results are a potential breakthrough.
“This is the first placebo-controlled study with an oral JAK inhibitor that not only shows that scarring alopecia can be reversible but also gives insights to the mechanism of action and which patients might respond best,” Emma Guttman-Yassky, MD, PhD, said in an interview. Dr. Guttman-Yassky, professor of Dermatology and Immunology, and director of the Laboratory of Inflammatory Skin Diseases, Icahn School of Medicine at Mount Sinai, New York City, was the study’s senior investigator.
Scarring Alopecia and Brepocitinib
For the study of scarring alopecia, 49 patients with CA were randomized in a 3:1 ratio to brepocitinib, a first-in-class inhibitor that targets both JAK1 and TYK2, or placebo. Because of the small size of the study, the primary endpoint was the change in CA biomarkers. The secondary outcome was clinical response, but because of a correlation between the two, these were mutually reinforcing.
Of the subtypes, nine patients enrolled in the study had frontal fibrosing alopecia (FFA), 16 had lichen planopilaris (LPP) alopecia, and 24 had central centrifugal cicatricial alopecia (CCCA). All of the forms of CA are more common in women overall and women of color specifically, particularly CCCA. For this analysis, the FFA and LPP subtypes were considered similar for assessing response and were combined.
The data included a comparison of response and safety during the 24-week randomization phase, as well as an additional follow-up conducted after another 24 weeks of open-label treatment. During the second phase, all patients on placebo were switched to active treatment.
Overall, there was a reduction in all four of the key scalp inflammatory biomarkers measured among those in the combined FFA/LLP group. In the placebo group, each of these markers — interferon gamma (IFN-gamma), CCLS, CXCL10, and STAT1 — increased over the same time period. In almost all cases, the differences were statistically significant.
In the CCCA subgroup, the same pattern (an increase among those on placebo but a decrease among those on brepocitinib) was observed for CCLS and CXCL10. For IFN-gamma and STAT1, a rise was observed among those on placebo and those on active treatment, although the rise was greater for placebo.
For clinical response, improvement on brepocitinib was observed on disease activity indexes, particularly among those in the FFA/LLP group, according to Marguerite Meariman, MD, a dermatology resident at Mount Sinai, who presented the results. She called the improvement in clinical activity scores at 48 weeks “dramatic.” Moreover, improvement was apparent within 4 weeks of starting therapy.
For CCCA, a more challenging condition to treat, Dr. Meariman said that no further progression might represent an acceptable response for many patients, but there were also cases of hair regrowth in this subset. Although improvement was not generally on the order seen among those with FFA/LLP, she suggested that there is promise even in these more difficult patients.
Further studies are planned, but Dr. Meariman said that it might be important to focus on early treatment regardless of CA subtype. She noted that patients with less than 5 years disease duration typically did better than those with longer durations.
Ritlecitinib for AT/AU
The analysis of patients with AT/AU was based on a subset analysis from the ALLEGRO phase 2b/3 study of ritlecitinib, which targets JAK3 and TEC kinases. The full results of the ALLEGRO trial were published last year in The Lancet. In the new late-breaker analysis, Severity of Alopecia Tool (SALT) scores were evaluated on an observed or last-observation-carried-forward basis. Generally, responses in the subgroup of patients with AT/AU, who had a median SALT score of 80.3 (signifying 80.3% hair loss) at baseline, were only modestly lower than those in the overall trial.
At 24 months, about 50% of patients achieved a SALT score of 20, according to Melissa Piliang, MD, chair of Dermatology at the Cleveland Clinic, Cleveland, Ohio, who presented the data. In this group, as in the non-AT/AU population, responses climbed over time, and these responses have been maintained for as long as patients have remained on therapy.
At the more rigorous threshold of SALT < 10, the proportion of responders was only slightly lower, meaning a substantial proportion of patients with AT/AU “are achieving 90% or more of hair regrowth, so really an excellent response,” Dr. Piliang said.
For the subgroup with AU, specifically, regrowth of eyebrows and eyelashes was also observed in a substantial proportion, according to Dr. Piliang. Attributed to the often-devastating psychological burden of hair loss, patient-reported assessments of these responses global were generally “even better” than those reported by the investigators.
However, Dr. Piliang advised clinicians to treat AA as early as possible. Despite the benefits seen in the AT/AU subgroup, she pointed out that starting treatment before total hair loss is associated with a higher likelihood of complete or nearly complete hair regrowth.
There are no data from the ALLEGRO trial to determine how long hair regrowth persists after discontinuation of ritlecitinib, which has been approved for the treatment of AA, but Dr. Piliang said that patients should be told that lifelong therapy should be expected in the vast majority of individuals, whether or not AA has advanced to AT/AU.
“In my experience with JAK inhibitors, you lose response when you come off these drugs,” she said.
Dr. Meariman reported a financial relationship with AbbVie. Dr. Piliang reported financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble. Dr. Armstrong reported financial relationships with more than 30 pharmaceutical companies, including those that manufacture JAK inhibitors. Dr. Guttman-Yassky reported financial relationships with more than 30 companies, including those that manufacture JAK inhibitors.
A version of this article appeared on Medscape.com.
FROM AAD 2024
SAN DIEGO — , according to late-breaking data presented at the annual meeting of the American Academy of Dermatology.
In one study of brepocitinib, the target was cicatricial alopecia (CA), a form of hair loss for which there are no approved therapies. In the other, a subanalysis from phase 3 trials of ritlecitinib for alopecia areata (AA), hair regrowth was shown in the subset of patients who entered the study with alopecia totalis or alopecia universalis (AT/AU).
Reflecting comments from several experts, including one of the late-breaking session moderators, April W. Armstrong, MD, MPH, professor and chief of dermatology, University of California, Los Angeles, said that the CA study, which matched clinical response to changes in CA biomarkers, suggested that the results are a potential breakthrough.
“This is the first placebo-controlled study with an oral JAK inhibitor that not only shows that scarring alopecia can be reversible but also gives insights to the mechanism of action and which patients might respond best,” Emma Guttman-Yassky, MD, PhD, said in an interview. Dr. Guttman-Yassky, professor of Dermatology and Immunology, and director of the Laboratory of Inflammatory Skin Diseases, Icahn School of Medicine at Mount Sinai, New York City, was the study’s senior investigator.
Scarring Alopecia and Brepocitinib
For the study of scarring alopecia, 49 patients with CA were randomized in a 3:1 ratio to brepocitinib, a first-in-class inhibitor that targets both JAK1 and TYK2, or placebo. Because of the small size of the study, the primary endpoint was the change in CA biomarkers. The secondary outcome was clinical response, but because of a correlation between the two, these were mutually reinforcing.
Of the subtypes, nine patients enrolled in the study had frontal fibrosing alopecia (FFA), 16 had lichen planopilaris (LPP) alopecia, and 24 had central centrifugal cicatricial alopecia (CCCA). All of the forms of CA are more common in women overall and women of color specifically, particularly CCCA. For this analysis, the FFA and LPP subtypes were considered similar for assessing response and were combined.
The data included a comparison of response and safety during the 24-week randomization phase, as well as an additional follow-up conducted after another 24 weeks of open-label treatment. During the second phase, all patients on placebo were switched to active treatment.
Overall, there was a reduction in all four of the key scalp inflammatory biomarkers measured among those in the combined FFA/LLP group. In the placebo group, each of these markers — interferon gamma (IFN-gamma), CCLS, CXCL10, and STAT1 — increased over the same time period. In almost all cases, the differences were statistically significant.
In the CCCA subgroup, the same pattern (an increase among those on placebo but a decrease among those on brepocitinib) was observed for CCLS and CXCL10. For IFN-gamma and STAT1, a rise was observed among those on placebo and those on active treatment, although the rise was greater for placebo.
For clinical response, improvement on brepocitinib was observed on disease activity indexes, particularly among those in the FFA/LLP group, according to Marguerite Meariman, MD, a dermatology resident at Mount Sinai, who presented the results. She called the improvement in clinical activity scores at 48 weeks “dramatic.” Moreover, improvement was apparent within 4 weeks of starting therapy.
For CCCA, a more challenging condition to treat, Dr. Meariman said that no further progression might represent an acceptable response for many patients, but there were also cases of hair regrowth in this subset. Although improvement was not generally on the order seen among those with FFA/LLP, she suggested that there is promise even in these more difficult patients.
Further studies are planned, but Dr. Meariman said that it might be important to focus on early treatment regardless of CA subtype. She noted that patients with less than 5 years disease duration typically did better than those with longer durations.
Ritlecitinib for AT/AU
The analysis of patients with AT/AU was based on a subset analysis from the ALLEGRO phase 2b/3 study of ritlecitinib, which targets JAK3 and TEC kinases. The full results of the ALLEGRO trial were published last year in The Lancet. In the new late-breaker analysis, Severity of Alopecia Tool (SALT) scores were evaluated on an observed or last-observation-carried-forward basis. Generally, responses in the subgroup of patients with AT/AU, who had a median SALT score of 80.3 (signifying 80.3% hair loss) at baseline, were only modestly lower than those in the overall trial.
At 24 months, about 50% of patients achieved a SALT score of 20, according to Melissa Piliang, MD, chair of Dermatology at the Cleveland Clinic, Cleveland, Ohio, who presented the data. In this group, as in the non-AT/AU population, responses climbed over time, and these responses have been maintained for as long as patients have remained on therapy.
At the more rigorous threshold of SALT < 10, the proportion of responders was only slightly lower, meaning a substantial proportion of patients with AT/AU “are achieving 90% or more of hair regrowth, so really an excellent response,” Dr. Piliang said.
For the subgroup with AU, specifically, regrowth of eyebrows and eyelashes was also observed in a substantial proportion, according to Dr. Piliang. Attributed to the often-devastating psychological burden of hair loss, patient-reported assessments of these responses global were generally “even better” than those reported by the investigators.
However, Dr. Piliang advised clinicians to treat AA as early as possible. Despite the benefits seen in the AT/AU subgroup, she pointed out that starting treatment before total hair loss is associated with a higher likelihood of complete or nearly complete hair regrowth.
There are no data from the ALLEGRO trial to determine how long hair regrowth persists after discontinuation of ritlecitinib, which has been approved for the treatment of AA, but Dr. Piliang said that patients should be told that lifelong therapy should be expected in the vast majority of individuals, whether or not AA has advanced to AT/AU.
“In my experience with JAK inhibitors, you lose response when you come off these drugs,” she said.
Dr. Meariman reported a financial relationship with AbbVie. Dr. Piliang reported financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble. Dr. Armstrong reported financial relationships with more than 30 pharmaceutical companies, including those that manufacture JAK inhibitors. Dr. Guttman-Yassky reported financial relationships with more than 30 companies, including those that manufacture JAK inhibitors.
A version of this article appeared on Medscape.com.
FROM AAD 2024
SAN DIEGO — , according to late-breaking data presented at the annual meeting of the American Academy of Dermatology.
In one study of brepocitinib, the target was cicatricial alopecia (CA), a form of hair loss for which there are no approved therapies. In the other, a subanalysis from phase 3 trials of ritlecitinib for alopecia areata (AA), hair regrowth was shown in the subset of patients who entered the study with alopecia totalis or alopecia universalis (AT/AU).
Reflecting comments from several experts, including one of the late-breaking session moderators, April W. Armstrong, MD, MPH, professor and chief of dermatology, University of California, Los Angeles, said that the CA study, which matched clinical response to changes in CA biomarkers, suggested that the results are a potential breakthrough.
“This is the first placebo-controlled study with an oral JAK inhibitor that not only shows that scarring alopecia can be reversible but also gives insights to the mechanism of action and which patients might respond best,” Emma Guttman-Yassky, MD, PhD, said in an interview. Dr. Guttman-Yassky, professor of Dermatology and Immunology, and director of the Laboratory of Inflammatory Skin Diseases, Icahn School of Medicine at Mount Sinai, New York City, was the study’s senior investigator.
Scarring Alopecia and Brepocitinib
For the study of scarring alopecia, 49 patients with CA were randomized in a 3:1 ratio to brepocitinib, a first-in-class inhibitor that targets both JAK1 and TYK2, or placebo. Because of the small size of the study, the primary endpoint was the change in CA biomarkers. The secondary outcome was clinical response, but because of a correlation between the two, these were mutually reinforcing.
Of the subtypes, nine patients enrolled in the study had frontal fibrosing alopecia (FFA), 16 had lichen planopilaris (LPP) alopecia, and 24 had central centrifugal cicatricial alopecia (CCCA). All of the forms of CA are more common in women overall and women of color specifically, particularly CCCA. For this analysis, the FFA and LPP subtypes were considered similar for assessing response and were combined.
The data included a comparison of response and safety during the 24-week randomization phase, as well as an additional follow-up conducted after another 24 weeks of open-label treatment. During the second phase, all patients on placebo were switched to active treatment.
Overall, there was a reduction in all four of the key scalp inflammatory biomarkers measured among those in the combined FFA/LLP group. In the placebo group, each of these markers — interferon gamma (IFN-gamma), CCLS, CXCL10, and STAT1 — increased over the same time period. In almost all cases, the differences were statistically significant.
In the CCCA subgroup, the same pattern (an increase among those on placebo but a decrease among those on brepocitinib) was observed for CCLS and CXCL10. For IFN-gamma and STAT1, a rise was observed among those on placebo and those on active treatment, although the rise was greater for placebo.
For clinical response, improvement on brepocitinib was observed on disease activity indexes, particularly among those in the FFA/LLP group, according to Marguerite Meariman, MD, a dermatology resident at Mount Sinai, who presented the results. She called the improvement in clinical activity scores at 48 weeks “dramatic.” Moreover, improvement was apparent within 4 weeks of starting therapy.
For CCCA, a more challenging condition to treat, Dr. Meariman said that no further progression might represent an acceptable response for many patients, but there were also cases of hair regrowth in this subset. Although improvement was not generally on the order seen among those with FFA/LLP, she suggested that there is promise even in these more difficult patients.
Further studies are planned, but Dr. Meariman said that it might be important to focus on early treatment regardless of CA subtype. She noted that patients with less than 5 years disease duration typically did better than those with longer durations.
Ritlecitinib for AT/AU
The analysis of patients with AT/AU was based on a subset analysis from the ALLEGRO phase 2b/3 study of ritlecitinib, which targets JAK3 and TEC kinases. The full results of the ALLEGRO trial were published last year in The Lancet. In the new late-breaker analysis, Severity of Alopecia Tool (SALT) scores were evaluated on an observed or last-observation-carried-forward basis. Generally, responses in the subgroup of patients with AT/AU, who had a median SALT score of 80.3 (signifying 80.3% hair loss) at baseline, were only modestly lower than those in the overall trial.
At 24 months, about 50% of patients achieved a SALT score of 20, according to Melissa Piliang, MD, chair of Dermatology at the Cleveland Clinic, Cleveland, Ohio, who presented the data. In this group, as in the non-AT/AU population, responses climbed over time, and these responses have been maintained for as long as patients have remained on therapy.
At the more rigorous threshold of SALT < 10, the proportion of responders was only slightly lower, meaning a substantial proportion of patients with AT/AU “are achieving 90% or more of hair regrowth, so really an excellent response,” Dr. Piliang said.
For the subgroup with AU, specifically, regrowth of eyebrows and eyelashes was also observed in a substantial proportion, according to Dr. Piliang. Attributed to the often-devastating psychological burden of hair loss, patient-reported assessments of these responses global were generally “even better” than those reported by the investigators.
However, Dr. Piliang advised clinicians to treat AA as early as possible. Despite the benefits seen in the AT/AU subgroup, she pointed out that starting treatment before total hair loss is associated with a higher likelihood of complete or nearly complete hair regrowth.
There are no data from the ALLEGRO trial to determine how long hair regrowth persists after discontinuation of ritlecitinib, which has been approved for the treatment of AA, but Dr. Piliang said that patients should be told that lifelong therapy should be expected in the vast majority of individuals, whether or not AA has advanced to AT/AU.
“In my experience with JAK inhibitors, you lose response when you come off these drugs,” she said.
Dr. Meariman reported a financial relationship with AbbVie. Dr. Piliang reported financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble. Dr. Armstrong reported financial relationships with more than 30 pharmaceutical companies, including those that manufacture JAK inhibitors. Dr. Guttman-Yassky reported financial relationships with more than 30 companies, including those that manufacture JAK inhibitors.
A version of this article appeared on Medscape.com.
Study Finds No Increased Cancer Risk With Spironolactone
TOPLINE:
than that of unexposed women.
METHODOLOGY:
- Spironolactone, used off-label for several skin conditions in women, carries a warning about an increased tumor risk associated with high doses in rat models, and its antiandrogen properties have prompted hypotheses about a possible increased risk for breast or gynecologic cancers.
- The researchers reviewed data on 420 women with a history of spironolactone use for acne, hair loss, and hirsutism and 3272 women with no spironolactone use at the authors› institution. Their mean age ranged from 42 to 63 years; the majority were White, and 38% were non-White.
- Median spironolactone doses ranged from 25 mg to 225 mg; chart reviews included 5-year follow-up data from the first spironolactone exposure to allow time for tumor development.
TAKEAWAY:
- A total of 37 of the 420 women exposed to spironolactone developed any tumors, as did 546 of the 3272 with no spironolactone exposure.
- After the researchers controlled for age and race, women exposed to spironolactone were no more likely to develop a malignant tumor than a benign tumor, compared with unexposed women (odds ratio [OR], 0.48, P = .2).
- The risk for breast or uterine cancer was not significantly different in the spironolactone and non-spironolactone groups (OR, 0.95, P > .9).
IN PRACTICE:
“Women taking spironolactone for acne, hair loss, and hirsutism and who are at low risk of breast or gynecologic cancers may be counseled to have regular gynecology follow-up, but no more frequently than the general population,” but more studies are needed to evaluate risk over longer periods of time, the researchers wrote.
SOURCE:
The lead author of the study was Rachel C. Hill, BS, a student at Weill Cornell Medical College, New York City, and Shari R. Lipner, MD, PhD, of the department of dermatology at Weill Cornell Medical College, was the corresponding author. The study was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The findings were limited by the retrospective design, as well as the small number of spironolactone patients analyzed, the short follow-up period, the lack of information about spironolactone courses, and the inability to control for family history of malignancy.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences and a grant from the Clinical and Translational Science Center at Weill Cornell Medical College awarded to Ms. Hill. None of the authors had relevant disclosures; Dr. Lipner disclosed serving as a consultant for Ortho-Dermatologics, Eli Lilly, Moberg Pharmaceuticals, and BelleTorus Corporation.
A version of this article appeared on Medscape.com.
TOPLINE:
than that of unexposed women.
METHODOLOGY:
- Spironolactone, used off-label for several skin conditions in women, carries a warning about an increased tumor risk associated with high doses in rat models, and its antiandrogen properties have prompted hypotheses about a possible increased risk for breast or gynecologic cancers.
- The researchers reviewed data on 420 women with a history of spironolactone use for acne, hair loss, and hirsutism and 3272 women with no spironolactone use at the authors› institution. Their mean age ranged from 42 to 63 years; the majority were White, and 38% were non-White.
- Median spironolactone doses ranged from 25 mg to 225 mg; chart reviews included 5-year follow-up data from the first spironolactone exposure to allow time for tumor development.
TAKEAWAY:
- A total of 37 of the 420 women exposed to spironolactone developed any tumors, as did 546 of the 3272 with no spironolactone exposure.
- After the researchers controlled for age and race, women exposed to spironolactone were no more likely to develop a malignant tumor than a benign tumor, compared with unexposed women (odds ratio [OR], 0.48, P = .2).
- The risk for breast or uterine cancer was not significantly different in the spironolactone and non-spironolactone groups (OR, 0.95, P > .9).
IN PRACTICE:
“Women taking spironolactone for acne, hair loss, and hirsutism and who are at low risk of breast or gynecologic cancers may be counseled to have regular gynecology follow-up, but no more frequently than the general population,” but more studies are needed to evaluate risk over longer periods of time, the researchers wrote.
SOURCE:
The lead author of the study was Rachel C. Hill, BS, a student at Weill Cornell Medical College, New York City, and Shari R. Lipner, MD, PhD, of the department of dermatology at Weill Cornell Medical College, was the corresponding author. The study was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The findings were limited by the retrospective design, as well as the small number of spironolactone patients analyzed, the short follow-up period, the lack of information about spironolactone courses, and the inability to control for family history of malignancy.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences and a grant from the Clinical and Translational Science Center at Weill Cornell Medical College awarded to Ms. Hill. None of the authors had relevant disclosures; Dr. Lipner disclosed serving as a consultant for Ortho-Dermatologics, Eli Lilly, Moberg Pharmaceuticals, and BelleTorus Corporation.
A version of this article appeared on Medscape.com.
TOPLINE:
than that of unexposed women.
METHODOLOGY:
- Spironolactone, used off-label for several skin conditions in women, carries a warning about an increased tumor risk associated with high doses in rat models, and its antiandrogen properties have prompted hypotheses about a possible increased risk for breast or gynecologic cancers.
- The researchers reviewed data on 420 women with a history of spironolactone use for acne, hair loss, and hirsutism and 3272 women with no spironolactone use at the authors› institution. Their mean age ranged from 42 to 63 years; the majority were White, and 38% were non-White.
- Median spironolactone doses ranged from 25 mg to 225 mg; chart reviews included 5-year follow-up data from the first spironolactone exposure to allow time for tumor development.
TAKEAWAY:
- A total of 37 of the 420 women exposed to spironolactone developed any tumors, as did 546 of the 3272 with no spironolactone exposure.
- After the researchers controlled for age and race, women exposed to spironolactone were no more likely to develop a malignant tumor than a benign tumor, compared with unexposed women (odds ratio [OR], 0.48, P = .2).
- The risk for breast or uterine cancer was not significantly different in the spironolactone and non-spironolactone groups (OR, 0.95, P > .9).
IN PRACTICE:
“Women taking spironolactone for acne, hair loss, and hirsutism and who are at low risk of breast or gynecologic cancers may be counseled to have regular gynecology follow-up, but no more frequently than the general population,” but more studies are needed to evaluate risk over longer periods of time, the researchers wrote.
SOURCE:
The lead author of the study was Rachel C. Hill, BS, a student at Weill Cornell Medical College, New York City, and Shari R. Lipner, MD, PhD, of the department of dermatology at Weill Cornell Medical College, was the corresponding author. The study was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The findings were limited by the retrospective design, as well as the small number of spironolactone patients analyzed, the short follow-up period, the lack of information about spironolactone courses, and the inability to control for family history of malignancy.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences and a grant from the Clinical and Translational Science Center at Weill Cornell Medical College awarded to Ms. Hill. None of the authors had relevant disclosures; Dr. Lipner disclosed serving as a consultant for Ortho-Dermatologics, Eli Lilly, Moberg Pharmaceuticals, and BelleTorus Corporation.
A version of this article appeared on Medscape.com.
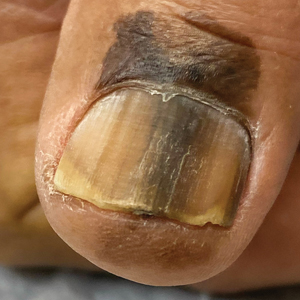
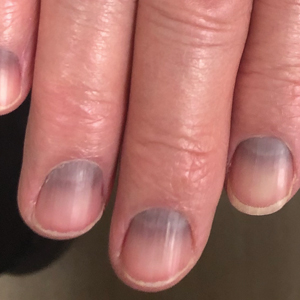
Longitudinal Melanonychia
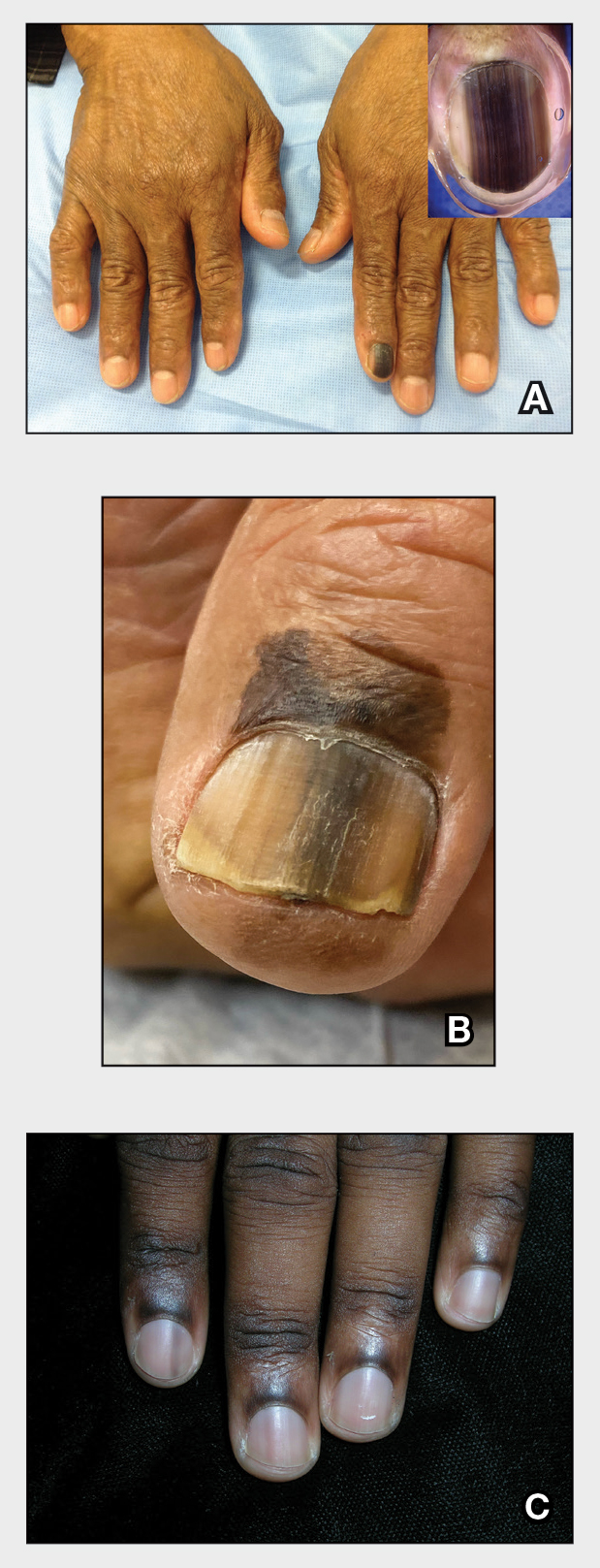
THE COMPARISON
A Melanoma in situ manifesting as longitudinal melanonychia (LM) in a single digit in a Black man. Dermoscopy showed irregular dark bands of brown pigmentation and micro-Hutchinson sign on the cuticle (inset).
B Melanoma manifesting as LM with a prominent Hutchinson sign in a Hispanic man, with variable shades of brown covering more than 50% of the nail width.
C Longitudinal melanonychia of at least 2 nails with a pseudo-Hutchinson sign (pigment on the nail folds in a benign case of LM) in a young Black man demonstrating ethnic/racial melanosis. The longitudinal bands, which were caused by benign melanocytic activation, are more gray than brown and are less than 3 mm wide.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P=.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥18 years) with subungual melanoma, with no reported cases in childhood (aged <18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
- In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
- Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
- Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
- Dermoscopic findings of LM in patients with skin of color include wider bands (P=.0125), lower band brightness (P<.032), and higher frequency of changing appearance of bands (P=.0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
- Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
- Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
- Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
- Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
- Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017; 31:732-736. doi:10.1111/jdv.13991
- Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
- Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019; 9:38-43. doi:10.5826/dpc.0901a10
- Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
- Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
- Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016 /j.jaad.2016.11.053
- LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390 /medsci9030057
- Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
- Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.1365-2133.2005.06668.x
- Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009

THE COMPARISON
A Melanoma in situ manifesting as longitudinal melanonychia (LM) in a single digit in a Black man. Dermoscopy showed irregular dark bands of brown pigmentation and micro-Hutchinson sign on the cuticle (inset).
B Melanoma manifesting as LM with a prominent Hutchinson sign in a Hispanic man, with variable shades of brown covering more than 50% of the nail width.
C Longitudinal melanonychia of at least 2 nails with a pseudo-Hutchinson sign (pigment on the nail folds in a benign case of LM) in a young Black man demonstrating ethnic/racial melanosis. The longitudinal bands, which were caused by benign melanocytic activation, are more gray than brown and are less than 3 mm wide.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P=.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥18 years) with subungual melanoma, with no reported cases in childhood (aged <18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
- In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
- Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
- Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
- Dermoscopic findings of LM in patients with skin of color include wider bands (P=.0125), lower band brightness (P<.032), and higher frequency of changing appearance of bands (P=.0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.

THE COMPARISON
A Melanoma in situ manifesting as longitudinal melanonychia (LM) in a single digit in a Black man. Dermoscopy showed irregular dark bands of brown pigmentation and micro-Hutchinson sign on the cuticle (inset).
B Melanoma manifesting as LM with a prominent Hutchinson sign in a Hispanic man, with variable shades of brown covering more than 50% of the nail width.
C Longitudinal melanonychia of at least 2 nails with a pseudo-Hutchinson sign (pigment on the nail folds in a benign case of LM) in a young Black man demonstrating ethnic/racial melanosis. The longitudinal bands, which were caused by benign melanocytic activation, are more gray than brown and are less than 3 mm wide.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P=.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥18 years) with subungual melanoma, with no reported cases in childhood (aged <18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
- In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
- Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
- Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
- Dermoscopic findings of LM in patients with skin of color include wider bands (P=.0125), lower band brightness (P<.032), and higher frequency of changing appearance of bands (P=.0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
- Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
- Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
- Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
- Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
- Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017; 31:732-736. doi:10.1111/jdv.13991
- Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
- Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019; 9:38-43. doi:10.5826/dpc.0901a10
- Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
- Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
- Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016 /j.jaad.2016.11.053
- LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390 /medsci9030057
- Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
- Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.1365-2133.2005.06668.x
- Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
- Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
- Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
- Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
- Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017; 31:732-736. doi:10.1111/jdv.13991
- Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
- Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019; 9:38-43. doi:10.5826/dpc.0901a10
- Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
- Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
- Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016 /j.jaad.2016.11.053
- LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390 /medsci9030057
- Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
- Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.1365-2133.2005.06668.x
- Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
Tangled Truths: Unraveling the Link Between Frontal Fibrosing Alopecia and Allergic Contact Dermatitis
Frontal fibrosing alopecia (FFA) is an increasingly common diagnosis, especially in middle-aged women, and was first described by Kossard1 in 1994. It is a variant of lichen planopilaris (LPP), a progressive scarring cicatricial alopecia that affects the frontotemporal area of the scalp, eyebrows, and sometimes even body hair.1 Although its etiology remains unclear, genetic causes, drugs, hormones, and environmental exposures—including certain chemicals found in sunscreens—have been implicated in its pathogenesis.2,3 An association between contact allergy to ingredients in personal care products and FFA diagnosis has been suggested; however, there is no evidence of causality to date. In this article, we highlight the potential relationship between contact allergy and FFA as well as clinical considerations for management.
Clinical Features and Diagnosis
Frontal fibrosing alopecia typically manifests with gradual symmetric recession of the frontal hairline leading to bandlike hair loss along the forehead, sometimes extending to the temporal region.4 Some patients may experience symptoms of scalp itching, burning, or tenderness that may precede or accompany the hair loss. Perifollicular erythema may be visible during the early stages and can be visualized on trichoscopy. The affected skin may appear pale and shiny and may have a smooth texture with a distinct lack of follicular openings. Aside from scalp involvement, other manifestations may include lichen planus pigmentosus, facial papules, body hair involvement, hypochromic lesions, diffuse redness on the face and neck, and prominent frontal veins.5 Although most FFA cases have characteristic clinical features and trichoscopic findings, biopsy for histopathologic examination is still recommended to confirm the diagnosis and ensure appropriate treatment.4 Classic histopathologic features include perifollicular lymphocytic inflammation, follicular destruction, and scarring.
Pathophysiology of FFA
The pathogenesis of FFA is thought to involve a variety of triggers, including immune-mediated inflammation, stress, genetics, hormones, and possibly environmental factors.6 Frontal fibrosing alopecia demonstrates considerable upregulation in cytotoxic helper T cells (TH1) and IFN-γ activity resulting in epithelial hair follicle stem cell apoptosis and replacement of normal epithelial tissue with fibrous tissue.7 There is some suspicion of genetic susceptibility in the onset of FFA as suggested by familial reports and genome-wide association studies.8-10 Hormonal and autoimmune factors also have been linked to FFA, including an increased risk for thyroid disease and the postmenopausal rise of androgen levels.6
Allergic Contact Dermatitis and FFA
Although they are 2 distinct conditions with differing etiologies, allergic contact dermatitis (ACD) and FFA may share environmental triggers, especially in susceptible individuals. This may support the coexistence and potential association between ACD and FFA.
In one case report, a woman who developed facial eczema followed by FFA showed positive patch tests to the UV filters drometrizole trisiloxane and ethylhexyl salicylate, which were listed as ingredients in her sunscreens. Avoidance of these allergens reportedly led to notable improvement of the symptoms.11 Case-control studies have found an association between the use of facial sunscreen and risk for FFA.12 A 2016 questionnaire that assessed a wide range of lifestyle, social, and medical factors related to FFA found that the use of sunscreens was significantly higher in patients with FFA than controls (P<.001), pointing to sunscreens as a potential contributing factor, but further research has been inconclusive. A higher frequency of positive patch tests to hydroperoxides of linalool and balsam of Peru (BoP) in patients with FFA have been documented; however, a direct cause cannot be established.2
In a 2020 prospective study conducted at multiple international centers, 65% (13/20) of FFA patients and 37.5% (9/24) of the control group had a positive patch test reaction to one or more allergens (P=.003). The most common allergens that were identified included cobalt chloride (positive in 35% [7/20] of patients with FFA), nickel sulfate (25% [5/20]), and potassium dichromate (15% [3/20]).13 In a recent 2-year cohort study of 42 patients with FFA who were referred for patch testing, the most common allergens included gallates, hydroperoxides of linalool, and other fragrances.14 After a 3-month period of allergen avoidance, 70% (29/42) of patients had decreased scalp erythema on examination, indicating that avoiding relevant allergens may reduce local inflammation. Furthermore, 76.2% (32/42) of patients with FFA showed delayed-type hypersensitivity to allergens found in daily personal care products such as shampoos, sunscreens, and moisturizers, among others.14 Notably, the study lacked a control group. A case-control study of 36 Hispanic women conducted in Mexico also resulted in 83.3% (15/18) of patients with FFA and 55.5% (10/18) of controls having at least 1 positive patch test; in the FFA group, these included iodopropynyl butylcarbamate (16.7% [3/18]) and propolis (16.7% [3/18]).15
Most recently, a retrospective study conducted by Shtaynberger et al16 included 12 patients with LPP or FFA diagnosed via clinical findings or biopsy. It also included an age- and temporally matched control group tested with identical allergens. Among the 12 patients who had FFA/LPP, all had at least 1 allergen identified on patch testing. The most common allergens identified were propolis (positive in 50% [6/12] of patients with FFA/LPP), fragrance mix I (16%), and methylisothiazolinone (16% [2/12]). Follow-up data were available for 9 of these patients, of whom 6 (66.7%) experienced symptom improvement after 6 months of allergen avoidance. Four (44.4%) patients experienced decreased follicular redness or scaling, 2 (22.2%) patients experienced improved scalp pain/itch, 2 (22.2%) patients had stable/improved hair density, and 1 (1.1%) patient had decreased hair shedding. Although this suggests an environmental trigger for FFA/LPP, the authors stated that changes in patient treatment plans could have contributed to their improvement. The study also was limited by its small size and its overall generalizability.16
These studies have underscored the significance of patch testing in individuals diagnosed with FFA and have identified common allergens prevalent in this patient population. They have suggested that patients with FFA are more likely to have positive patch tests, and in some cases patients could experience improvements in scalp pruritus and erythema with allergen avoidance; however, we emphasize that a causal association between contact allergy and FFA remains unproven to date.
Most Common Allergens Pertinent to FFA
Preservatives—In some studies, patients with FFA have had positive patch tests to preservatives such as gallates and methylchloroisothiazolinone/methylisothiazolinone (MCI/MI).14 Gallates are antioxidants that are used in food preservation, pharmaceuticals, and cosmetics due to their ability to inhibit oxidation and rancidity of fats and oils.17 The most common gallates include propyl gallate, octyl gallate, and dodecyl gallate. Propyl gallate is utilized in some waxy or oily cosmetics and personal care items including sunscreens, shampoos, conditioners, bar soaps, facial cleansers, and moisturizers.18 Typically, if patients have a positive patch test to one gallate, they should be advised to avoid all gallate compounds, as they can cross-react.
Similarly, MCI/MI can prevent product degradation through their antibacterial and antifungal properties. This combination of MCI and MI is used as an effective method of prolonging the shelf life of cosmetic products and commonly is found in sunscreens, facial moisturizing creams, shampoos, and conditioners19; it is banned from use in leave-on products in the European Union and Canada due to increased rates of contact allergy.20 In patients with FFA who commonly use facial sunscreen, preservatives can be a potential allergen exposure to consider.
Iodopropynyl butylcarbamate also is a preservative used in cosmetic formulations. Similar to MCI/MI, it is a potent fungicide and bactericide. This allergen can be found in hair care products, bodywashes, and other personal products.21
UV Light–Absorbing Agents—A systematic review and meta-analysis conducted in 2022 showed a significant (P<.001) association between sunscreen use and FFA.22 A majority of allergens identified on patch testing included UVA- and UVB-absorbing agents found in sunscreens and other products including cosmetics,11,12 such as drometrizole trisiloxane, ethylhexyl salicylate, avobenzone, and benzophenone-4. Drometrizole trisiloxane is a photostabilizer and a broad-spectrum UV filter that is not approved for use in sunscreens in the United States.23 It also is effective in stabilizing and preventing the degradation of avobenzone, a commonly used UVA filter.24
Fragrances—Fragrances are present in nearly every personal and cosmetic product, sometimes even in those advertised as being “fragrance free.” Hydroperoxides of linalool, BoP, and fragrance mix are common allergens that are found in a variety of personal care products including perfumes, cosmetics, and even household cleaning supplies.25 Simultaneous positive patch tests to BoP and fragrance mix are common due to shared components. Linalool can be found in various plants such as lavender, rose, bergamot, and jasmine.26 Upon air exposure, linalool auto-oxidizes to form allergenic hydroperoxides of linalool. Among patients with FFA, positive patch test reactions to fragrance chemicals are common and could be attributed to the use of fragranced hair products and facial cosmetics.
Hair Dyes and Bleaches—Allergic reactions to hair dyes and bleaches can result in severe ACD of the head/neck and, in rare cases, scarring alopecia.27 Chemicals found in these products include paraphenylenediamine (PPD) and ammonium persulfate. The most common hair dye allergen, PPD also is used in some rubbers and plastics. Ammonium persulfate is a chemical used in hair bleaches and to deodorize oils. One case study reported a patient with FFA who developed chemically induced vitiligo immediately after the use of a hair color product that contained PPD.28 However, without patch testing to confirm the presence of contact allergy, other patient-specific and environmental risk factors could have contributed to FFA in this case.
A Knot in the Truth
In this endeavor to untangle the truth, it should be remembered that at the time of writing, the purported association between FFA and ACD remains debatable. Contact dermatitis specialists have voiced that the association between FFA and ACD, especially with regard to sunscreen, cannot be supported due to the lack of sufficient evidence.29 A large majority of the research conducted on FFA and ACD is based on case reports and studies limited to a small sample size, and most of these patch test studies lack a control group. Felmingham et al30 noted that the recent epidemiology of FFA aligns with increased sunscreen use. They also highlighted the limitations of the aforementioned studies, which include misclassification of exposures in the control group2 and recall bias in questionnaire participants.2,12 The most pressing limitation that permeates through most of these studies is the temporal ambiguity associated with sunscreen use. A study by Dhana et al31 failed to specify whether increased sunscreen use preceded the diagnosis of FFA or if it stems from the need to protect more exposed skin as a consequence of disease. Broad sunscreen avoidance due to concern for a possible association with hair loss could have detrimental health implications by increasing the risk for photodamage and skin cancer.
FFA Patch Testing
The avoidance of pertinent allergens could be effective in reducing local inflammation, pruritus, and erythema in FFA.9,14,32 At our institution, we selectively patch test patients with FFA when there is a suspected contact allergy. Clinical features that may allude to a potential contact allergy include an erythematous or eczematous dermatitis or symptoms of pruritus along the scalp or eyebrows. If patients recall hair loss or symptoms after using a hair or facial product, then a potential contact allergy to these products could be considered. Patch testing in patients with FFA includes the North American 80 Comprehensive Series and the cosmetic and hairdresser supplemental series, as well as an additional customized panel of 8 allergens as determined by patch testing experts at the University of Massachusetts, Brigham and Women’s Hospital, and Massachusetts General Hospital (private email communication, November 2017). Patch test readings are performed at 48 and 96 or 120 hours. Using the American Contact Dermatitis Society’s Contact Allergen Management Program, patients are provided personalized safe product lists and avoidance strategies are discussed.
Final Interpretation
In a world where cosmetic products are ubiquitous, it is hard to define the potential role of contact allergens in the entangled pathogenesis of FFA and ACD. As evidenced by emerging literature that correlates the 2 conditions and their exacerbating factors, it is important for physicians to have a comprehensive diagnostic approach and heightened awareness for potential allergens at play in FFA (Table). The identification of certain chemicals and preservatives as potential triggers for FFA should emphasize the importance of patch testing in these patients; however, whether the positive reactions are relevant to the pathogenesis or disease course of FFA still is unknown. While these findings begin to unravel the intertwined causes of FFA and ACD, further research encompassing larger cohorts and prospective studies is imperative to solidify these associations, define concrete guidelines, and improve patient outcomes.
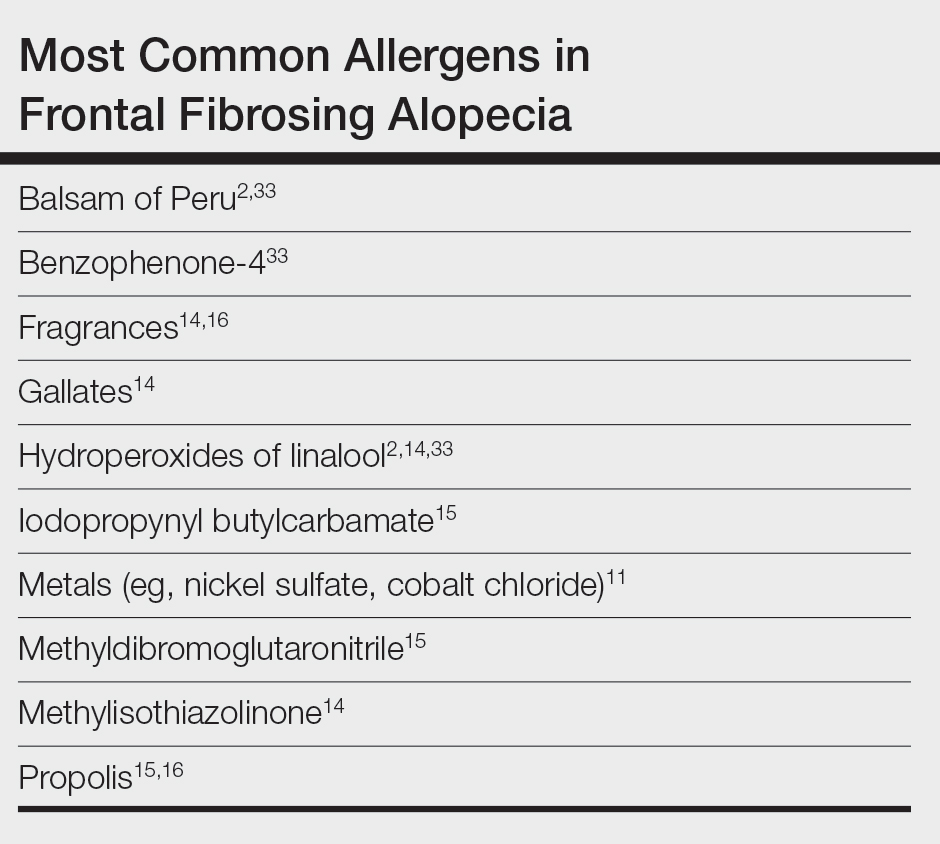
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016;175:762-767. doi:10.1111/bjd.14535
- Debroy Kidambi A, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with facial moisturizers and sunscreens. Br J Dermatol. 2017;177:260-261. doi:10.1111/bjd.15311
- Starace M, Orlando G, Iorizzo M, et al. Clinical and dermoscopic approaches to diagnosis of frontal fibrosing alopecia: results from a multicenter study of the International Dermoscopy Society. Dermatol Pract Concept. 2022;12:E2022080. doi:10.5826/dpc.1201a80
- Fechine COC, Valente NYS, Romiti R. Lichen planopilaris and frontal fibrosing alopecia: review and update of diagnostic and therapeutic features. An Bras Dermatol. 2022;97:348-357. doi:10.1016/j.abd.2021.08.008
- Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Del Duca E, Ruano Ruiz J, Pavel AB, et al. Frontal fibrosing alopecia shows robust T helper 1 and Janus kinase 3 skewing. Br J Dermatol. 2020;183:1083-1093. doi:10.1111/bjd.19040
- Tziotzios C, Petridis C, Dand N, et al. Genome-wide association study in frontal fibrosing alopecia identifies four susceptibility loci including HLA-B*07:02. Nat Commun. 2019;10:1150. doi:10.1038/s41467-019-09117-w
- Navarro‐Belmonte MR, Navarro‐López V, Ramírez‐Boscà A, et al. Case series of familial frontal fibrosing alopecia and a review of the literature. J Cosmet Dermatol. 2015;14:64-69. doi:10.1111/jocd.12125
- Cuenca-Barrales C, Ruiz-Villaverde R, Molina-Leyva A. Familial frontal fibrosing alopecia. Sultan Qaboos Univ Med J. 2021;21:E320-E323. doi:10.18295/squmj.2021.21.02.025
- Pastor-Nieto MA, Gatica-Ortega ME. Allergic contact dermatitis to drometrizole trisiloxane in a woman thereafter diagnosed with frontal fibrosing alopecia. Contact Dermatitis. 2023;89:215-217. doi:10.1111/cod.14370
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case–control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Rudnicka L, Rokni GR, Lotti T, et al. Allergic contact dermatitis in patients with frontal fibrosing alopecia: an international multi-center study. Dermatol Ther. 2020;33:E13560. doi:10.1111/dth.13560
- Prasad S, Marks DH, Burns LJ, et al. Patch testing and contact allergen avoidance in patients with lichen planopilaris and/or frontal fibrosing alopecia: a cohort study. J Am Acad Dermatol. 2020;83:659-661. doi:10.1016/j.jaad.2020.01.026
- Ocampo-Garza SS, Herz-Ruelas ME, Chavez-Alvarez S, et al. Association of frontal fibrosing alopecia and contact allergens in everyday skincare products in Hispanic females: a case-control study. An Bras Dermatol. 2021;96:776-778. doi:10.1016/j.abd.2020.09.013
- Shtaynberger B, Bruder P, Zippin JH. The prevalence of type iv hypersensitivity in patients with lichen planopilaris and frontal fibrosing alopecia. Dermatitis. 2023;34:351-352. doi:10.1097/DER.0000000000000965
- Kahkeshani N, Farzaei F, Fotouhi M, et al. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci. 2019;22:225-237. doi:10.22038/ijbms.2019.32806.7897
- Holcomb ZE, Van Noord MG, Atwater AR. Gallate contact dermatitis: product update and systematic review. Dermatitis. 2017;28:115-127. doi:10.1097/DER.0000000000000263
- Gorris A, Valencak J, Schremser V, et al. Contact allergy to methylisothiazolinone with three clinical presentations in one patient. Contact Dermatitis. 2020;82:162-164. doi:10.1111/cod.13384
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures. J Eur Acad Dermatol Venereol. 2020;34:333-339. doi:10.1111/jdv.15875
- Batista M, Morgado F, Gonçalo M. Patch test reactivity to iodopropynyl butylcarbamate in consecutive patients during a period of 7 years. Contact Dermatitis. 2019;81:54-55. doi:10.1111/cod.13213
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396. doi:10.1016/j.jaad.2021.12.058
- Drometrizole trisiloxane. PubChem website. Accessed February 21, 2024. https://pubchem.ncbi.nlm.nih.gov/compound/9848888
- Hughes TM, Martin JA, Lewis VJ, et al. Allergic contact dermatitis to drometrizole trisiloxane in a sunscreen with concomitant sensitivities to other sunscreens. Contact Dermatitis. 2005;52:226-227. doi:10.1111/j.0105-1873.2005.0566a.x
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Sköld M, Börje A, Matura M, et al. Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermatitis. 2002;46:267-272. doi:10.1034/j.1600-0536.2002.460504.x
- Dev T, Khan E, Patel U, et al. Cicatricial alopecia following allergic contact dermatitis from hair dyes: a rare clinical presentation. Contact Dermatitis. 2022;86:59-61. doi:10.1111/cod.13974
- De Souza B, Burns L, Senna MM. Frontal fibrosing alopecia preceding the development of vitiligo: a case report. JAAD Case Rep. 2020;6:154-155. doi:10.1016/j.jdcr.2019.12.011
- Abuav R, Shon W. Are sunscreen particles involved in frontal fibrosing alopecia?—a TEM-EDXS analysis on formalin-fixed paraffin-embedded alopecia biopsies (pilot study). Am J Dermatopathol. 2022;44:E135. doi:10.1097/DAD.0000000000002317
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482. doi:10.1111/bjd.18380
- Dhana A, Gumedze F, Khumalo N. Regarding “frontal fibrosing alopecia: possible association with leave-on facial skincare products and sunscreens; a questionnaire study.” Br J Dermatol. 2016;176:836-837. doi:10.1111/bjd.15197
- Pastor-Nieto MA, Gatica-Ortega ME, Sánchez-Herreros C, et al. Sensitization to benzyl salicylate and other allergens in patients with frontal fibrosing alopecia. Contact Dermatitis. 2021;84:423-430. doi:10.1111/cod.13763
- Rocha VB, Donati A, Contin LA, et al. Photopatch and patch testing in 63 patients with frontal fibrosing alopecia: a case series. Br J Dermatol. 2018;179:1402-1403. doi:10.1111/bjd.16933
Frontal fibrosing alopecia (FFA) is an increasingly common diagnosis, especially in middle-aged women, and was first described by Kossard1 in 1994. It is a variant of lichen planopilaris (LPP), a progressive scarring cicatricial alopecia that affects the frontotemporal area of the scalp, eyebrows, and sometimes even body hair.1 Although its etiology remains unclear, genetic causes, drugs, hormones, and environmental exposures—including certain chemicals found in sunscreens—have been implicated in its pathogenesis.2,3 An association between contact allergy to ingredients in personal care products and FFA diagnosis has been suggested; however, there is no evidence of causality to date. In this article, we highlight the potential relationship between contact allergy and FFA as well as clinical considerations for management.
Clinical Features and Diagnosis
Frontal fibrosing alopecia typically manifests with gradual symmetric recession of the frontal hairline leading to bandlike hair loss along the forehead, sometimes extending to the temporal region.4 Some patients may experience symptoms of scalp itching, burning, or tenderness that may precede or accompany the hair loss. Perifollicular erythema may be visible during the early stages and can be visualized on trichoscopy. The affected skin may appear pale and shiny and may have a smooth texture with a distinct lack of follicular openings. Aside from scalp involvement, other manifestations may include lichen planus pigmentosus, facial papules, body hair involvement, hypochromic lesions, diffuse redness on the face and neck, and prominent frontal veins.5 Although most FFA cases have characteristic clinical features and trichoscopic findings, biopsy for histopathologic examination is still recommended to confirm the diagnosis and ensure appropriate treatment.4 Classic histopathologic features include perifollicular lymphocytic inflammation, follicular destruction, and scarring.
Pathophysiology of FFA
The pathogenesis of FFA is thought to involve a variety of triggers, including immune-mediated inflammation, stress, genetics, hormones, and possibly environmental factors.6 Frontal fibrosing alopecia demonstrates considerable upregulation in cytotoxic helper T cells (TH1) and IFN-γ activity resulting in epithelial hair follicle stem cell apoptosis and replacement of normal epithelial tissue with fibrous tissue.7 There is some suspicion of genetic susceptibility in the onset of FFA as suggested by familial reports and genome-wide association studies.8-10 Hormonal and autoimmune factors also have been linked to FFA, including an increased risk for thyroid disease and the postmenopausal rise of androgen levels.6
Allergic Contact Dermatitis and FFA
Although they are 2 distinct conditions with differing etiologies, allergic contact dermatitis (ACD) and FFA may share environmental triggers, especially in susceptible individuals. This may support the coexistence and potential association between ACD and FFA.
In one case report, a woman who developed facial eczema followed by FFA showed positive patch tests to the UV filters drometrizole trisiloxane and ethylhexyl salicylate, which were listed as ingredients in her sunscreens. Avoidance of these allergens reportedly led to notable improvement of the symptoms.11 Case-control studies have found an association between the use of facial sunscreen and risk for FFA.12 A 2016 questionnaire that assessed a wide range of lifestyle, social, and medical factors related to FFA found that the use of sunscreens was significantly higher in patients with FFA than controls (P<.001), pointing to sunscreens as a potential contributing factor, but further research has been inconclusive. A higher frequency of positive patch tests to hydroperoxides of linalool and balsam of Peru (BoP) in patients with FFA have been documented; however, a direct cause cannot be established.2
In a 2020 prospective study conducted at multiple international centers, 65% (13/20) of FFA patients and 37.5% (9/24) of the control group had a positive patch test reaction to one or more allergens (P=.003). The most common allergens that were identified included cobalt chloride (positive in 35% [7/20] of patients with FFA), nickel sulfate (25% [5/20]), and potassium dichromate (15% [3/20]).13 In a recent 2-year cohort study of 42 patients with FFA who were referred for patch testing, the most common allergens included gallates, hydroperoxides of linalool, and other fragrances.14 After a 3-month period of allergen avoidance, 70% (29/42) of patients had decreased scalp erythema on examination, indicating that avoiding relevant allergens may reduce local inflammation. Furthermore, 76.2% (32/42) of patients with FFA showed delayed-type hypersensitivity to allergens found in daily personal care products such as shampoos, sunscreens, and moisturizers, among others.14 Notably, the study lacked a control group. A case-control study of 36 Hispanic women conducted in Mexico also resulted in 83.3% (15/18) of patients with FFA and 55.5% (10/18) of controls having at least 1 positive patch test; in the FFA group, these included iodopropynyl butylcarbamate (16.7% [3/18]) and propolis (16.7% [3/18]).15
Most recently, a retrospective study conducted by Shtaynberger et al16 included 12 patients with LPP or FFA diagnosed via clinical findings or biopsy. It also included an age- and temporally matched control group tested with identical allergens. Among the 12 patients who had FFA/LPP, all had at least 1 allergen identified on patch testing. The most common allergens identified were propolis (positive in 50% [6/12] of patients with FFA/LPP), fragrance mix I (16%), and methylisothiazolinone (16% [2/12]). Follow-up data were available for 9 of these patients, of whom 6 (66.7%) experienced symptom improvement after 6 months of allergen avoidance. Four (44.4%) patients experienced decreased follicular redness or scaling, 2 (22.2%) patients experienced improved scalp pain/itch, 2 (22.2%) patients had stable/improved hair density, and 1 (1.1%) patient had decreased hair shedding. Although this suggests an environmental trigger for FFA/LPP, the authors stated that changes in patient treatment plans could have contributed to their improvement. The study also was limited by its small size and its overall generalizability.16
These studies have underscored the significance of patch testing in individuals diagnosed with FFA and have identified common allergens prevalent in this patient population. They have suggested that patients with FFA are more likely to have positive patch tests, and in some cases patients could experience improvements in scalp pruritus and erythema with allergen avoidance; however, we emphasize that a causal association between contact allergy and FFA remains unproven to date.
Most Common Allergens Pertinent to FFA
Preservatives—In some studies, patients with FFA have had positive patch tests to preservatives such as gallates and methylchloroisothiazolinone/methylisothiazolinone (MCI/MI).14 Gallates are antioxidants that are used in food preservation, pharmaceuticals, and cosmetics due to their ability to inhibit oxidation and rancidity of fats and oils.17 The most common gallates include propyl gallate, octyl gallate, and dodecyl gallate. Propyl gallate is utilized in some waxy or oily cosmetics and personal care items including sunscreens, shampoos, conditioners, bar soaps, facial cleansers, and moisturizers.18 Typically, if patients have a positive patch test to one gallate, they should be advised to avoid all gallate compounds, as they can cross-react.
Similarly, MCI/MI can prevent product degradation through their antibacterial and antifungal properties. This combination of MCI and MI is used as an effective method of prolonging the shelf life of cosmetic products and commonly is found in sunscreens, facial moisturizing creams, shampoos, and conditioners19; it is banned from use in leave-on products in the European Union and Canada due to increased rates of contact allergy.20 In patients with FFA who commonly use facial sunscreen, preservatives can be a potential allergen exposure to consider.
Iodopropynyl butylcarbamate also is a preservative used in cosmetic formulations. Similar to MCI/MI, it is a potent fungicide and bactericide. This allergen can be found in hair care products, bodywashes, and other personal products.21
UV Light–Absorbing Agents—A systematic review and meta-analysis conducted in 2022 showed a significant (P<.001) association between sunscreen use and FFA.22 A majority of allergens identified on patch testing included UVA- and UVB-absorbing agents found in sunscreens and other products including cosmetics,11,12 such as drometrizole trisiloxane, ethylhexyl salicylate, avobenzone, and benzophenone-4. Drometrizole trisiloxane is a photostabilizer and a broad-spectrum UV filter that is not approved for use in sunscreens in the United States.23 It also is effective in stabilizing and preventing the degradation of avobenzone, a commonly used UVA filter.24
Fragrances—Fragrances are present in nearly every personal and cosmetic product, sometimes even in those advertised as being “fragrance free.” Hydroperoxides of linalool, BoP, and fragrance mix are common allergens that are found in a variety of personal care products including perfumes, cosmetics, and even household cleaning supplies.25 Simultaneous positive patch tests to BoP and fragrance mix are common due to shared components. Linalool can be found in various plants such as lavender, rose, bergamot, and jasmine.26 Upon air exposure, linalool auto-oxidizes to form allergenic hydroperoxides of linalool. Among patients with FFA, positive patch test reactions to fragrance chemicals are common and could be attributed to the use of fragranced hair products and facial cosmetics.
Hair Dyes and Bleaches—Allergic reactions to hair dyes and bleaches can result in severe ACD of the head/neck and, in rare cases, scarring alopecia.27 Chemicals found in these products include paraphenylenediamine (PPD) and ammonium persulfate. The most common hair dye allergen, PPD also is used in some rubbers and plastics. Ammonium persulfate is a chemical used in hair bleaches and to deodorize oils. One case study reported a patient with FFA who developed chemically induced vitiligo immediately after the use of a hair color product that contained PPD.28 However, without patch testing to confirm the presence of contact allergy, other patient-specific and environmental risk factors could have contributed to FFA in this case.
A Knot in the Truth
In this endeavor to untangle the truth, it should be remembered that at the time of writing, the purported association between FFA and ACD remains debatable. Contact dermatitis specialists have voiced that the association between FFA and ACD, especially with regard to sunscreen, cannot be supported due to the lack of sufficient evidence.29 A large majority of the research conducted on FFA and ACD is based on case reports and studies limited to a small sample size, and most of these patch test studies lack a control group. Felmingham et al30 noted that the recent epidemiology of FFA aligns with increased sunscreen use. They also highlighted the limitations of the aforementioned studies, which include misclassification of exposures in the control group2 and recall bias in questionnaire participants.2,12 The most pressing limitation that permeates through most of these studies is the temporal ambiguity associated with sunscreen use. A study by Dhana et al31 failed to specify whether increased sunscreen use preceded the diagnosis of FFA or if it stems from the need to protect more exposed skin as a consequence of disease. Broad sunscreen avoidance due to concern for a possible association with hair loss could have detrimental health implications by increasing the risk for photodamage and skin cancer.
FFA Patch Testing
The avoidance of pertinent allergens could be effective in reducing local inflammation, pruritus, and erythema in FFA.9,14,32 At our institution, we selectively patch test patients with FFA when there is a suspected contact allergy. Clinical features that may allude to a potential contact allergy include an erythematous or eczematous dermatitis or symptoms of pruritus along the scalp or eyebrows. If patients recall hair loss or symptoms after using a hair or facial product, then a potential contact allergy to these products could be considered. Patch testing in patients with FFA includes the North American 80 Comprehensive Series and the cosmetic and hairdresser supplemental series, as well as an additional customized panel of 8 allergens as determined by patch testing experts at the University of Massachusetts, Brigham and Women’s Hospital, and Massachusetts General Hospital (private email communication, November 2017). Patch test readings are performed at 48 and 96 or 120 hours. Using the American Contact Dermatitis Society’s Contact Allergen Management Program, patients are provided personalized safe product lists and avoidance strategies are discussed.
Final Interpretation
In a world where cosmetic products are ubiquitous, it is hard to define the potential role of contact allergens in the entangled pathogenesis of FFA and ACD. As evidenced by emerging literature that correlates the 2 conditions and their exacerbating factors, it is important for physicians to have a comprehensive diagnostic approach and heightened awareness for potential allergens at play in FFA (Table). The identification of certain chemicals and preservatives as potential triggers for FFA should emphasize the importance of patch testing in these patients; however, whether the positive reactions are relevant to the pathogenesis or disease course of FFA still is unknown. While these findings begin to unravel the intertwined causes of FFA and ACD, further research encompassing larger cohorts and prospective studies is imperative to solidify these associations, define concrete guidelines, and improve patient outcomes.

Frontal fibrosing alopecia (FFA) is an increasingly common diagnosis, especially in middle-aged women, and was first described by Kossard1 in 1994. It is a variant of lichen planopilaris (LPP), a progressive scarring cicatricial alopecia that affects the frontotemporal area of the scalp, eyebrows, and sometimes even body hair.1 Although its etiology remains unclear, genetic causes, drugs, hormones, and environmental exposures—including certain chemicals found in sunscreens—have been implicated in its pathogenesis.2,3 An association between contact allergy to ingredients in personal care products and FFA diagnosis has been suggested; however, there is no evidence of causality to date. In this article, we highlight the potential relationship between contact allergy and FFA as well as clinical considerations for management.
Clinical Features and Diagnosis
Frontal fibrosing alopecia typically manifests with gradual symmetric recession of the frontal hairline leading to bandlike hair loss along the forehead, sometimes extending to the temporal region.4 Some patients may experience symptoms of scalp itching, burning, or tenderness that may precede or accompany the hair loss. Perifollicular erythema may be visible during the early stages and can be visualized on trichoscopy. The affected skin may appear pale and shiny and may have a smooth texture with a distinct lack of follicular openings. Aside from scalp involvement, other manifestations may include lichen planus pigmentosus, facial papules, body hair involvement, hypochromic lesions, diffuse redness on the face and neck, and prominent frontal veins.5 Although most FFA cases have characteristic clinical features and trichoscopic findings, biopsy for histopathologic examination is still recommended to confirm the diagnosis and ensure appropriate treatment.4 Classic histopathologic features include perifollicular lymphocytic inflammation, follicular destruction, and scarring.
Pathophysiology of FFA
The pathogenesis of FFA is thought to involve a variety of triggers, including immune-mediated inflammation, stress, genetics, hormones, and possibly environmental factors.6 Frontal fibrosing alopecia demonstrates considerable upregulation in cytotoxic helper T cells (TH1) and IFN-γ activity resulting in epithelial hair follicle stem cell apoptosis and replacement of normal epithelial tissue with fibrous tissue.7 There is some suspicion of genetic susceptibility in the onset of FFA as suggested by familial reports and genome-wide association studies.8-10 Hormonal and autoimmune factors also have been linked to FFA, including an increased risk for thyroid disease and the postmenopausal rise of androgen levels.6
Allergic Contact Dermatitis and FFA
Although they are 2 distinct conditions with differing etiologies, allergic contact dermatitis (ACD) and FFA may share environmental triggers, especially in susceptible individuals. This may support the coexistence and potential association between ACD and FFA.
In one case report, a woman who developed facial eczema followed by FFA showed positive patch tests to the UV filters drometrizole trisiloxane and ethylhexyl salicylate, which were listed as ingredients in her sunscreens. Avoidance of these allergens reportedly led to notable improvement of the symptoms.11 Case-control studies have found an association between the use of facial sunscreen and risk for FFA.12 A 2016 questionnaire that assessed a wide range of lifestyle, social, and medical factors related to FFA found that the use of sunscreens was significantly higher in patients with FFA than controls (P<.001), pointing to sunscreens as a potential contributing factor, but further research has been inconclusive. A higher frequency of positive patch tests to hydroperoxides of linalool and balsam of Peru (BoP) in patients with FFA have been documented; however, a direct cause cannot be established.2
In a 2020 prospective study conducted at multiple international centers, 65% (13/20) of FFA patients and 37.5% (9/24) of the control group had a positive patch test reaction to one or more allergens (P=.003). The most common allergens that were identified included cobalt chloride (positive in 35% [7/20] of patients with FFA), nickel sulfate (25% [5/20]), and potassium dichromate (15% [3/20]).13 In a recent 2-year cohort study of 42 patients with FFA who were referred for patch testing, the most common allergens included gallates, hydroperoxides of linalool, and other fragrances.14 After a 3-month period of allergen avoidance, 70% (29/42) of patients had decreased scalp erythema on examination, indicating that avoiding relevant allergens may reduce local inflammation. Furthermore, 76.2% (32/42) of patients with FFA showed delayed-type hypersensitivity to allergens found in daily personal care products such as shampoos, sunscreens, and moisturizers, among others.14 Notably, the study lacked a control group. A case-control study of 36 Hispanic women conducted in Mexico also resulted in 83.3% (15/18) of patients with FFA and 55.5% (10/18) of controls having at least 1 positive patch test; in the FFA group, these included iodopropynyl butylcarbamate (16.7% [3/18]) and propolis (16.7% [3/18]).15
Most recently, a retrospective study conducted by Shtaynberger et al16 included 12 patients with LPP or FFA diagnosed via clinical findings or biopsy. It also included an age- and temporally matched control group tested with identical allergens. Among the 12 patients who had FFA/LPP, all had at least 1 allergen identified on patch testing. The most common allergens identified were propolis (positive in 50% [6/12] of patients with FFA/LPP), fragrance mix I (16%), and methylisothiazolinone (16% [2/12]). Follow-up data were available for 9 of these patients, of whom 6 (66.7%) experienced symptom improvement after 6 months of allergen avoidance. Four (44.4%) patients experienced decreased follicular redness or scaling, 2 (22.2%) patients experienced improved scalp pain/itch, 2 (22.2%) patients had stable/improved hair density, and 1 (1.1%) patient had decreased hair shedding. Although this suggests an environmental trigger for FFA/LPP, the authors stated that changes in patient treatment plans could have contributed to their improvement. The study also was limited by its small size and its overall generalizability.16
These studies have underscored the significance of patch testing in individuals diagnosed with FFA and have identified common allergens prevalent in this patient population. They have suggested that patients with FFA are more likely to have positive patch tests, and in some cases patients could experience improvements in scalp pruritus and erythema with allergen avoidance; however, we emphasize that a causal association between contact allergy and FFA remains unproven to date.
Most Common Allergens Pertinent to FFA
Preservatives—In some studies, patients with FFA have had positive patch tests to preservatives such as gallates and methylchloroisothiazolinone/methylisothiazolinone (MCI/MI).14 Gallates are antioxidants that are used in food preservation, pharmaceuticals, and cosmetics due to their ability to inhibit oxidation and rancidity of fats and oils.17 The most common gallates include propyl gallate, octyl gallate, and dodecyl gallate. Propyl gallate is utilized in some waxy or oily cosmetics and personal care items including sunscreens, shampoos, conditioners, bar soaps, facial cleansers, and moisturizers.18 Typically, if patients have a positive patch test to one gallate, they should be advised to avoid all gallate compounds, as they can cross-react.
Similarly, MCI/MI can prevent product degradation through their antibacterial and antifungal properties. This combination of MCI and MI is used as an effective method of prolonging the shelf life of cosmetic products and commonly is found in sunscreens, facial moisturizing creams, shampoos, and conditioners19; it is banned from use in leave-on products in the European Union and Canada due to increased rates of contact allergy.20 In patients with FFA who commonly use facial sunscreen, preservatives can be a potential allergen exposure to consider.
Iodopropynyl butylcarbamate also is a preservative used in cosmetic formulations. Similar to MCI/MI, it is a potent fungicide and bactericide. This allergen can be found in hair care products, bodywashes, and other personal products.21
UV Light–Absorbing Agents—A systematic review and meta-analysis conducted in 2022 showed a significant (P<.001) association between sunscreen use and FFA.22 A majority of allergens identified on patch testing included UVA- and UVB-absorbing agents found in sunscreens and other products including cosmetics,11,12 such as drometrizole trisiloxane, ethylhexyl salicylate, avobenzone, and benzophenone-4. Drometrizole trisiloxane is a photostabilizer and a broad-spectrum UV filter that is not approved for use in sunscreens in the United States.23 It also is effective in stabilizing and preventing the degradation of avobenzone, a commonly used UVA filter.24
Fragrances—Fragrances are present in nearly every personal and cosmetic product, sometimes even in those advertised as being “fragrance free.” Hydroperoxides of linalool, BoP, and fragrance mix are common allergens that are found in a variety of personal care products including perfumes, cosmetics, and even household cleaning supplies.25 Simultaneous positive patch tests to BoP and fragrance mix are common due to shared components. Linalool can be found in various plants such as lavender, rose, bergamot, and jasmine.26 Upon air exposure, linalool auto-oxidizes to form allergenic hydroperoxides of linalool. Among patients with FFA, positive patch test reactions to fragrance chemicals are common and could be attributed to the use of fragranced hair products and facial cosmetics.
Hair Dyes and Bleaches—Allergic reactions to hair dyes and bleaches can result in severe ACD of the head/neck and, in rare cases, scarring alopecia.27 Chemicals found in these products include paraphenylenediamine (PPD) and ammonium persulfate. The most common hair dye allergen, PPD also is used in some rubbers and plastics. Ammonium persulfate is a chemical used in hair bleaches and to deodorize oils. One case study reported a patient with FFA who developed chemically induced vitiligo immediately after the use of a hair color product that contained PPD.28 However, without patch testing to confirm the presence of contact allergy, other patient-specific and environmental risk factors could have contributed to FFA in this case.
A Knot in the Truth
In this endeavor to untangle the truth, it should be remembered that at the time of writing, the purported association between FFA and ACD remains debatable. Contact dermatitis specialists have voiced that the association between FFA and ACD, especially with regard to sunscreen, cannot be supported due to the lack of sufficient evidence.29 A large majority of the research conducted on FFA and ACD is based on case reports and studies limited to a small sample size, and most of these patch test studies lack a control group. Felmingham et al30 noted that the recent epidemiology of FFA aligns with increased sunscreen use. They also highlighted the limitations of the aforementioned studies, which include misclassification of exposures in the control group2 and recall bias in questionnaire participants.2,12 The most pressing limitation that permeates through most of these studies is the temporal ambiguity associated with sunscreen use. A study by Dhana et al31 failed to specify whether increased sunscreen use preceded the diagnosis of FFA or if it stems from the need to protect more exposed skin as a consequence of disease. Broad sunscreen avoidance due to concern for a possible association with hair loss could have detrimental health implications by increasing the risk for photodamage and skin cancer.
FFA Patch Testing
The avoidance of pertinent allergens could be effective in reducing local inflammation, pruritus, and erythema in FFA.9,14,32 At our institution, we selectively patch test patients with FFA when there is a suspected contact allergy. Clinical features that may allude to a potential contact allergy include an erythematous or eczematous dermatitis or symptoms of pruritus along the scalp or eyebrows. If patients recall hair loss or symptoms after using a hair or facial product, then a potential contact allergy to these products could be considered. Patch testing in patients with FFA includes the North American 80 Comprehensive Series and the cosmetic and hairdresser supplemental series, as well as an additional customized panel of 8 allergens as determined by patch testing experts at the University of Massachusetts, Brigham and Women’s Hospital, and Massachusetts General Hospital (private email communication, November 2017). Patch test readings are performed at 48 and 96 or 120 hours. Using the American Contact Dermatitis Society’s Contact Allergen Management Program, patients are provided personalized safe product lists and avoidance strategies are discussed.
Final Interpretation
In a world where cosmetic products are ubiquitous, it is hard to define the potential role of contact allergens in the entangled pathogenesis of FFA and ACD. As evidenced by emerging literature that correlates the 2 conditions and their exacerbating factors, it is important for physicians to have a comprehensive diagnostic approach and heightened awareness for potential allergens at play in FFA (Table). The identification of certain chemicals and preservatives as potential triggers for FFA should emphasize the importance of patch testing in these patients; however, whether the positive reactions are relevant to the pathogenesis or disease course of FFA still is unknown. While these findings begin to unravel the intertwined causes of FFA and ACD, further research encompassing larger cohorts and prospective studies is imperative to solidify these associations, define concrete guidelines, and improve patient outcomes.

- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016;175:762-767. doi:10.1111/bjd.14535
- Debroy Kidambi A, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with facial moisturizers and sunscreens. Br J Dermatol. 2017;177:260-261. doi:10.1111/bjd.15311
- Starace M, Orlando G, Iorizzo M, et al. Clinical and dermoscopic approaches to diagnosis of frontal fibrosing alopecia: results from a multicenter study of the International Dermoscopy Society. Dermatol Pract Concept. 2022;12:E2022080. doi:10.5826/dpc.1201a80
- Fechine COC, Valente NYS, Romiti R. Lichen planopilaris and frontal fibrosing alopecia: review and update of diagnostic and therapeutic features. An Bras Dermatol. 2022;97:348-357. doi:10.1016/j.abd.2021.08.008
- Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Del Duca E, Ruano Ruiz J, Pavel AB, et al. Frontal fibrosing alopecia shows robust T helper 1 and Janus kinase 3 skewing. Br J Dermatol. 2020;183:1083-1093. doi:10.1111/bjd.19040
- Tziotzios C, Petridis C, Dand N, et al. Genome-wide association study in frontal fibrosing alopecia identifies four susceptibility loci including HLA-B*07:02. Nat Commun. 2019;10:1150. doi:10.1038/s41467-019-09117-w
- Navarro‐Belmonte MR, Navarro‐López V, Ramírez‐Boscà A, et al. Case series of familial frontal fibrosing alopecia and a review of the literature. J Cosmet Dermatol. 2015;14:64-69. doi:10.1111/jocd.12125
- Cuenca-Barrales C, Ruiz-Villaverde R, Molina-Leyva A. Familial frontal fibrosing alopecia. Sultan Qaboos Univ Med J. 2021;21:E320-E323. doi:10.18295/squmj.2021.21.02.025
- Pastor-Nieto MA, Gatica-Ortega ME. Allergic contact dermatitis to drometrizole trisiloxane in a woman thereafter diagnosed with frontal fibrosing alopecia. Contact Dermatitis. 2023;89:215-217. doi:10.1111/cod.14370
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case–control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Rudnicka L, Rokni GR, Lotti T, et al. Allergic contact dermatitis in patients with frontal fibrosing alopecia: an international multi-center study. Dermatol Ther. 2020;33:E13560. doi:10.1111/dth.13560
- Prasad S, Marks DH, Burns LJ, et al. Patch testing and contact allergen avoidance in patients with lichen planopilaris and/or frontal fibrosing alopecia: a cohort study. J Am Acad Dermatol. 2020;83:659-661. doi:10.1016/j.jaad.2020.01.026
- Ocampo-Garza SS, Herz-Ruelas ME, Chavez-Alvarez S, et al. Association of frontal fibrosing alopecia and contact allergens in everyday skincare products in Hispanic females: a case-control study. An Bras Dermatol. 2021;96:776-778. doi:10.1016/j.abd.2020.09.013
- Shtaynberger B, Bruder P, Zippin JH. The prevalence of type iv hypersensitivity in patients with lichen planopilaris and frontal fibrosing alopecia. Dermatitis. 2023;34:351-352. doi:10.1097/DER.0000000000000965
- Kahkeshani N, Farzaei F, Fotouhi M, et al. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci. 2019;22:225-237. doi:10.22038/ijbms.2019.32806.7897
- Holcomb ZE, Van Noord MG, Atwater AR. Gallate contact dermatitis: product update and systematic review. Dermatitis. 2017;28:115-127. doi:10.1097/DER.0000000000000263
- Gorris A, Valencak J, Schremser V, et al. Contact allergy to methylisothiazolinone with three clinical presentations in one patient. Contact Dermatitis. 2020;82:162-164. doi:10.1111/cod.13384
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures. J Eur Acad Dermatol Venereol. 2020;34:333-339. doi:10.1111/jdv.15875
- Batista M, Morgado F, Gonçalo M. Patch test reactivity to iodopropynyl butylcarbamate in consecutive patients during a period of 7 years. Contact Dermatitis. 2019;81:54-55. doi:10.1111/cod.13213
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396. doi:10.1016/j.jaad.2021.12.058
- Drometrizole trisiloxane. PubChem website. Accessed February 21, 2024. https://pubchem.ncbi.nlm.nih.gov/compound/9848888
- Hughes TM, Martin JA, Lewis VJ, et al. Allergic contact dermatitis to drometrizole trisiloxane in a sunscreen with concomitant sensitivities to other sunscreens. Contact Dermatitis. 2005;52:226-227. doi:10.1111/j.0105-1873.2005.0566a.x
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Sköld M, Börje A, Matura M, et al. Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermatitis. 2002;46:267-272. doi:10.1034/j.1600-0536.2002.460504.x
- Dev T, Khan E, Patel U, et al. Cicatricial alopecia following allergic contact dermatitis from hair dyes: a rare clinical presentation. Contact Dermatitis. 2022;86:59-61. doi:10.1111/cod.13974
- De Souza B, Burns L, Senna MM. Frontal fibrosing alopecia preceding the development of vitiligo: a case report. JAAD Case Rep. 2020;6:154-155. doi:10.1016/j.jdcr.2019.12.011
- Abuav R, Shon W. Are sunscreen particles involved in frontal fibrosing alopecia?—a TEM-EDXS analysis on formalin-fixed paraffin-embedded alopecia biopsies (pilot study). Am J Dermatopathol. 2022;44:E135. doi:10.1097/DAD.0000000000002317
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482. doi:10.1111/bjd.18380
- Dhana A, Gumedze F, Khumalo N. Regarding “frontal fibrosing alopecia: possible association with leave-on facial skincare products and sunscreens; a questionnaire study.” Br J Dermatol. 2016;176:836-837. doi:10.1111/bjd.15197
- Pastor-Nieto MA, Gatica-Ortega ME, Sánchez-Herreros C, et al. Sensitization to benzyl salicylate and other allergens in patients with frontal fibrosing alopecia. Contact Dermatitis. 2021;84:423-430. doi:10.1111/cod.13763
- Rocha VB, Donati A, Contin LA, et al. Photopatch and patch testing in 63 patients with frontal fibrosing alopecia: a case series. Br J Dermatol. 2018;179:1402-1403. doi:10.1111/bjd.16933
- Kossard S. Postmenopausal frontal fibrosing alopecia: scarring alopecia in a pattern distribution. Arch Dermatol. 1994;130:770-774. doi:10.1001/archderm.1994.01690060100013
- Aldoori N, Dobson K, Holden CR, et al. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016;175:762-767. doi:10.1111/bjd.14535
- Debroy Kidambi A, Dobson K, Holmes S, et al. Frontal fibrosing alopecia in men: an association with facial moisturizers and sunscreens. Br J Dermatol. 2017;177:260-261. doi:10.1111/bjd.15311
- Starace M, Orlando G, Iorizzo M, et al. Clinical and dermoscopic approaches to diagnosis of frontal fibrosing alopecia: results from a multicenter study of the International Dermoscopy Society. Dermatol Pract Concept. 2022;12:E2022080. doi:10.5826/dpc.1201a80
- Fechine COC, Valente NYS, Romiti R. Lichen planopilaris and frontal fibrosing alopecia: review and update of diagnostic and therapeutic features. An Bras Dermatol. 2022;97:348-357. doi:10.1016/j.abd.2021.08.008
- Frontal fibrosing alopecia: a review of disease pathogenesis. Front Med (Lausanne). 2022;9:911944. doi:10.3389/fmed.2022.911944
- Del Duca E, Ruano Ruiz J, Pavel AB, et al. Frontal fibrosing alopecia shows robust T helper 1 and Janus kinase 3 skewing. Br J Dermatol. 2020;183:1083-1093. doi:10.1111/bjd.19040
- Tziotzios C, Petridis C, Dand N, et al. Genome-wide association study in frontal fibrosing alopecia identifies four susceptibility loci including HLA-B*07:02. Nat Commun. 2019;10:1150. doi:10.1038/s41467-019-09117-w
- Navarro‐Belmonte MR, Navarro‐López V, Ramírez‐Boscà A, et al. Case series of familial frontal fibrosing alopecia and a review of the literature. J Cosmet Dermatol. 2015;14:64-69. doi:10.1111/jocd.12125
- Cuenca-Barrales C, Ruiz-Villaverde R, Molina-Leyva A. Familial frontal fibrosing alopecia. Sultan Qaboos Univ Med J. 2021;21:E320-E323. doi:10.18295/squmj.2021.21.02.025
- Pastor-Nieto MA, Gatica-Ortega ME. Allergic contact dermatitis to drometrizole trisiloxane in a woman thereafter diagnosed with frontal fibrosing alopecia. Contact Dermatitis. 2023;89:215-217. doi:10.1111/cod.14370
- Moreno-Arrones OM, Saceda-Corralo D, Rodrigues-Barata AR, et al. Risk factors associated with frontal fibrosing alopecia: a multicentre case–control study. Clin Exp Dermatol. 2019;44:404-410. doi:10.1111/ced.13785
- Rudnicka L, Rokni GR, Lotti T, et al. Allergic contact dermatitis in patients with frontal fibrosing alopecia: an international multi-center study. Dermatol Ther. 2020;33:E13560. doi:10.1111/dth.13560
- Prasad S, Marks DH, Burns LJ, et al. Patch testing and contact allergen avoidance in patients with lichen planopilaris and/or frontal fibrosing alopecia: a cohort study. J Am Acad Dermatol. 2020;83:659-661. doi:10.1016/j.jaad.2020.01.026
- Ocampo-Garza SS, Herz-Ruelas ME, Chavez-Alvarez S, et al. Association of frontal fibrosing alopecia and contact allergens in everyday skincare products in Hispanic females: a case-control study. An Bras Dermatol. 2021;96:776-778. doi:10.1016/j.abd.2020.09.013
- Shtaynberger B, Bruder P, Zippin JH. The prevalence of type iv hypersensitivity in patients with lichen planopilaris and frontal fibrosing alopecia. Dermatitis. 2023;34:351-352. doi:10.1097/DER.0000000000000965
- Kahkeshani N, Farzaei F, Fotouhi M, et al. Pharmacological effects of gallic acid in health and diseases: a mechanistic review. Iran J Basic Med Sci. 2019;22:225-237. doi:10.22038/ijbms.2019.32806.7897
- Holcomb ZE, Van Noord MG, Atwater AR. Gallate contact dermatitis: product update and systematic review. Dermatitis. 2017;28:115-127. doi:10.1097/DER.0000000000000263
- Gorris A, Valencak J, Schremser V, et al. Contact allergy to methylisothiazolinone with three clinical presentations in one patient. Contact Dermatitis. 2020;82:162-164. doi:10.1111/cod.13384
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures. J Eur Acad Dermatol Venereol. 2020;34:333-339. doi:10.1111/jdv.15875
- Batista M, Morgado F, Gonçalo M. Patch test reactivity to iodopropynyl butylcarbamate in consecutive patients during a period of 7 years. Contact Dermatitis. 2019;81:54-55. doi:10.1111/cod.13213
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396. doi:10.1016/j.jaad.2021.12.058
- Drometrizole trisiloxane. PubChem website. Accessed February 21, 2024. https://pubchem.ncbi.nlm.nih.gov/compound/9848888
- Hughes TM, Martin JA, Lewis VJ, et al. Allergic contact dermatitis to drometrizole trisiloxane in a sunscreen with concomitant sensitivities to other sunscreens. Contact Dermatitis. 2005;52:226-227. doi:10.1111/j.0105-1873.2005.0566a.x
- de Groot AC. Myroxylon pereirae resin (balsam of Peru)—a critical review of the literature and assessment of the significance of positive patch test reactions and the usefulness of restrictive diets. Contact Dermatitis. 2019;80:335-353. doi:10.1111/cod.13263
- Sköld M, Börje A, Matura M, et al. Studies on the autoxidation and sensitizing capacity of the fragrance chemical linalool, identifying a linalool hydroperoxide. Contact Dermatitis. 2002;46:267-272. doi:10.1034/j.1600-0536.2002.460504.x
- Dev T, Khan E, Patel U, et al. Cicatricial alopecia following allergic contact dermatitis from hair dyes: a rare clinical presentation. Contact Dermatitis. 2022;86:59-61. doi:10.1111/cod.13974
- De Souza B, Burns L, Senna MM. Frontal fibrosing alopecia preceding the development of vitiligo: a case report. JAAD Case Rep. 2020;6:154-155. doi:10.1016/j.jdcr.2019.12.011
- Abuav R, Shon W. Are sunscreen particles involved in frontal fibrosing alopecia?—a TEM-EDXS analysis on formalin-fixed paraffin-embedded alopecia biopsies (pilot study). Am J Dermatopathol. 2022;44:E135. doi:10.1097/DAD.0000000000002317
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482. doi:10.1111/bjd.18380
- Dhana A, Gumedze F, Khumalo N. Regarding “frontal fibrosing alopecia: possible association with leave-on facial skincare products and sunscreens; a questionnaire study.” Br J Dermatol. 2016;176:836-837. doi:10.1111/bjd.15197
- Pastor-Nieto MA, Gatica-Ortega ME, Sánchez-Herreros C, et al. Sensitization to benzyl salicylate and other allergens in patients with frontal fibrosing alopecia. Contact Dermatitis. 2021;84:423-430. doi:10.1111/cod.13763
- Rocha VB, Donati A, Contin LA, et al. Photopatch and patch testing in 63 patients with frontal fibrosing alopecia: a case series. Br J Dermatol. 2018;179:1402-1403. doi:10.1111/bjd.16933
Practice Points
- Frontal fibrosing alopecia (FFA), a variant of lichen planopilaris (LPP), is an increasingly prevalent type of scarring alopecia that may have a closer relationship to contact allergy than was previously understood. However, there is no evidence of a causal association to date.
- When evaluating for FFA/LPP, clinicians should assess for use of cosmetic products or sunscreens that may have a potential impact on the disease course.
Association Between LDL-C and Androgenetic Alopecia Among Female Patients in a Specialty Alopecia Clinic
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).
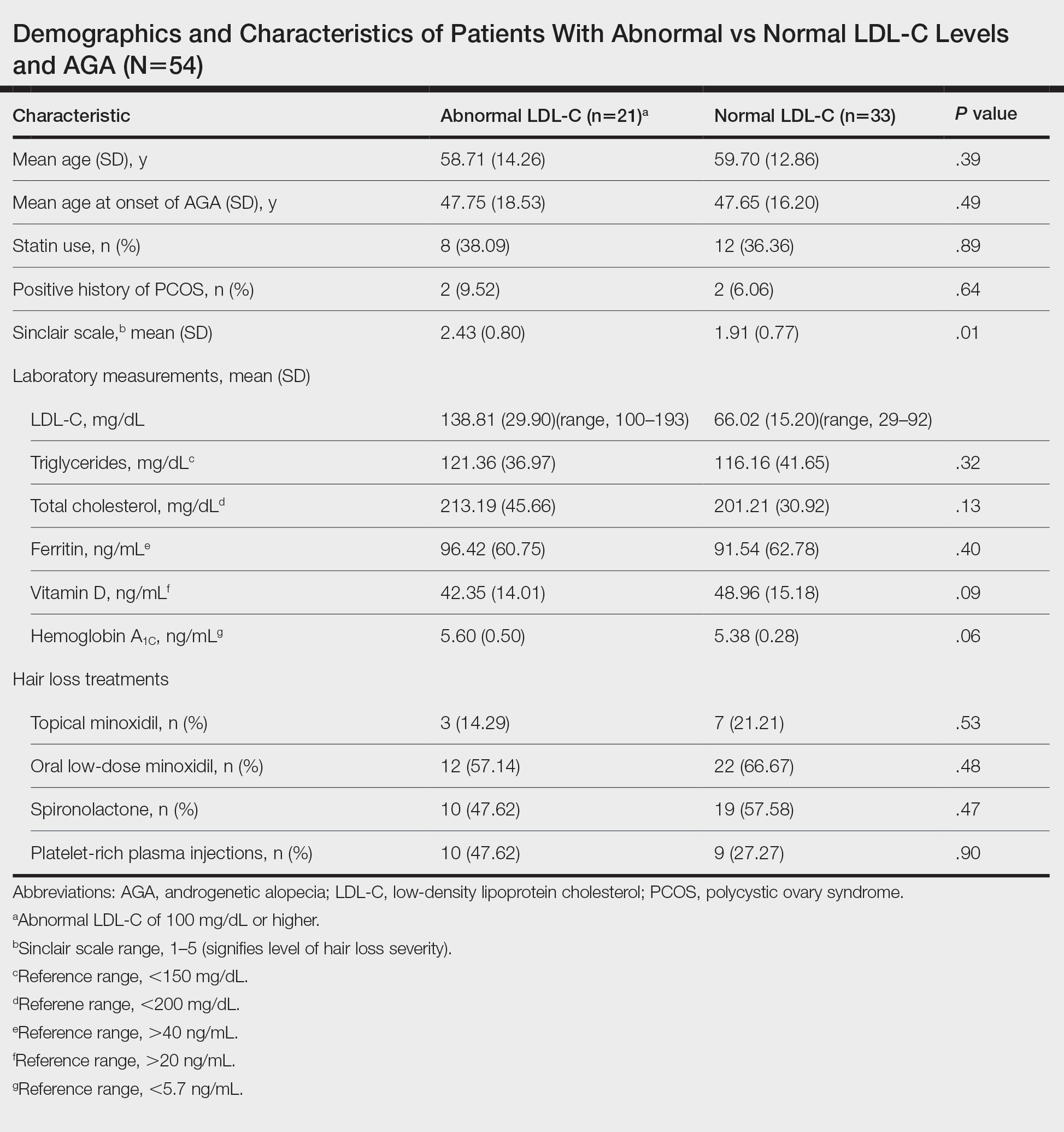
We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).

We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).

We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
Practice Points
- Associations have been shown between hair loss and markers of bad health such as insulin resistance and high cholesterol. Research has not yet shown the relationship between hair loss severity and these markers, particularly cholesterol.
Analysis of Nail Excision Practice Patterns in the Medicare Provider Utilization and Payment Database 2012-2017
To the Editor:
Partial or total nail plate excisions commonly are used for the treatment of onychocryptosis and nail spicules. Procedures involving the nail unit require advanced technical skills to achieve optimal functional and aesthetic outcomes, avoid complications, and minimize health care costs. Data on the frequency of nail plate excisions performed by dermatologists and their relative frequency compared to other medical providers are limited. The objective of our study was to analyze trends in nail excision practice patterns among medical providers in the United States.
A retrospective analysis on nail excisions using the Current Procedural Terminology (CPT) code 11750 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail] for permanent removal), which is distinct from code 11755 (biopsy of nail unit [eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds][separate procedure]), was performed using data from the Medicare Provider Utilization and Payment Database 2012-2017.1,2 This file also is used by Peck et al3 in an article submitted to the Journal of the American Podiatric Medical Association and currently under consideration for publication. Procedures were recorded by year and provider type—dermatologist, podiatrist, physician assistant (PA)/nurse practitioner (NP), nondermatologist physician—and subcategorized by provider specialty (Table). Practice locations subcategorized by provider type were mapped using Tableau Software (Salesforce)(Figure). Descriptive statistics including number of providers, mean and median excisions per provider, and minimum/maximum nail excisions were calculated (Table). Practice types of PAs/NPs and specialization of nondermatologist physicians were determined using provider name, identification number, and practice address. This study did not require institutional review board review, as only publicly available data were utilized in our analysis.
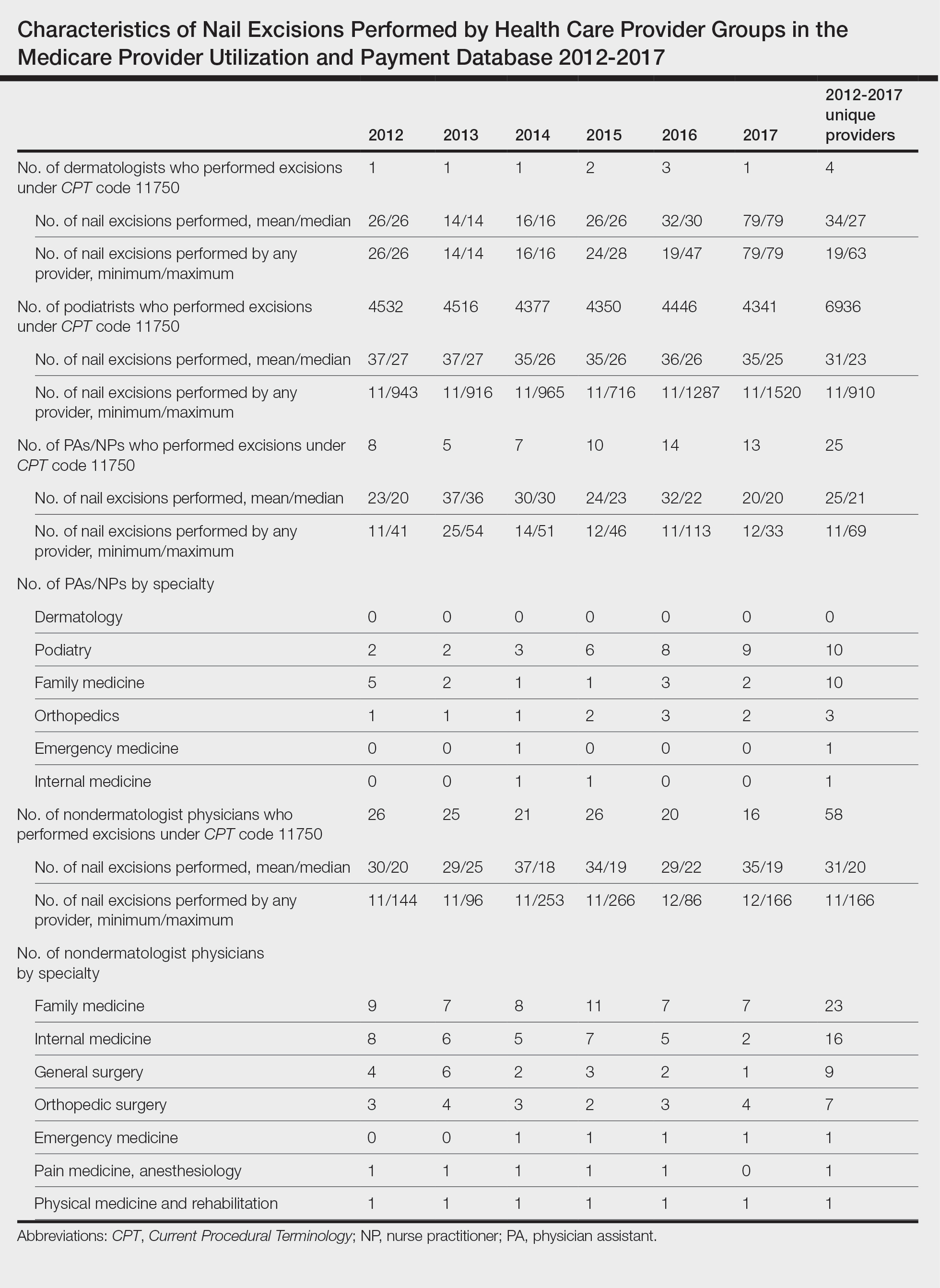
A total of 6936 podiatrists, 58 nondermatologist physicians, 25 PAs/NPs, and 4 dermatologists performed 10 or more nail excisions annually under CPT code 11750 from January 2012 to December 2017 with annual means of 31, 31, 25, and 34, respectively (Table). No PAs/NPs included in the dataset worked in dermatology practices during the study period. Physician assistants and NPs most often practiced in podiatry and family medicine (FM) settings (both 40% [10/25]). Nondermatologist physicians most often specialized in FM (40% [23/58])(Table). The greatest number of providers practiced in 3 of the 4 most-populous states: California, Texas, and Florida; the fewest number practiced in 3 of the 10 least-populous states: Alaska, Hawaii, and Vermont. Vermont, Wyoming, and North Dakota—3 of the 5 least-populous states—had the fewest practitioners among the contiguous United States (Figure).
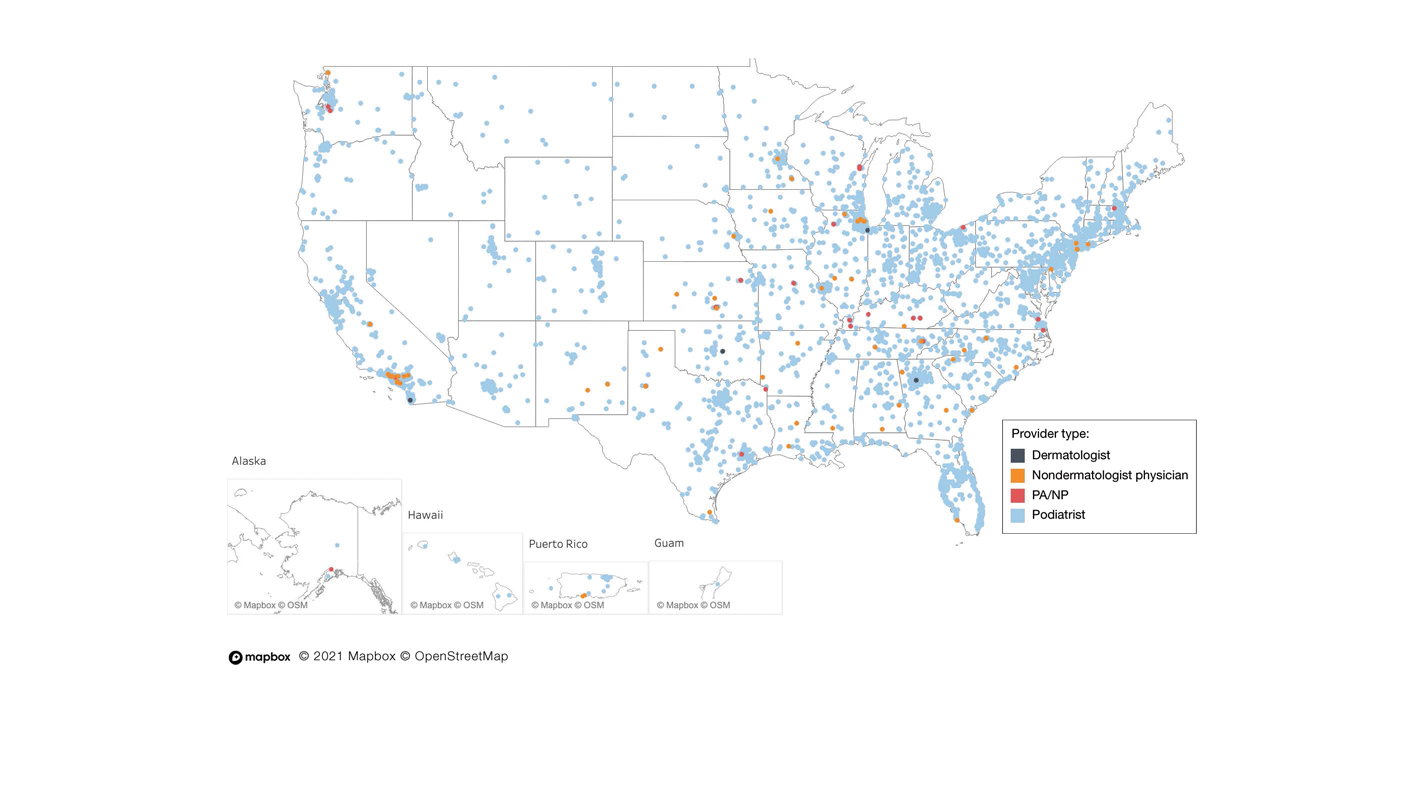
Our study showed that from January 2012 to December 2017, fewer dermatologists performed nail excisions than any other provider type (0.06%, 4 dermatologists of 7023 total providers), and dermatologists performed 1734-fold fewer nail excisions than podiatrists (99%, 6936 podiatrists of 7023 total providers). Only dermatologists practicing in California, Georgia, Indiana, and Oklahoma performed nail excisions. Conversely, podiatrists were more geographically distributed across the United States and other territories, with representation in all 50 states as well as the District of Columbia, Puerto Rico, and Guam.
Reasons for these large discrepancies in practice between dermatologists and other providers likely are multifactorial, encompassing a lack of emphasis on nail procedures in dermatology training, patient perception of the scope of dermatologic practice, and nail excision reimbursement patterns. Most dermatologists likely lack experience in performing nail procedures. The Accreditation Council for Graduate Medical Education requirements mandate that dermatology residents observe or perform 3 nail procedures over 3 years of residency, including 1 that may be performed on a human cadaver.4 In contrast, podiatry trainees must gain competency in toenail avulsion (both partial and complete), participate in anesthesia workshops, and become proficient in administering lower extremity blocks by the end of their training.5 Therefore, incorporating aspects of podiatric surgical training into dermatology residency requirements may increase the competency and comfort of dermatologists in performing nail excisions and practicing as nail experts as attending physicians.
It is likely that US patients do not perceive dermatologists as nail specialists and instead primarily consult podiatrists or FM and/or internal medicine physicians for treatment; for example, nail disease was one of the least common reasons for consulting a dermatologist (5%) in a German nationwide survey-based study (N=1015).6 Therefore, increased efforts are needed to educate the general public about the expertise of dermatologists in the diagnosis and management of nail conditions.
Reimbursement also may be a barrier to dermatologists performing nail procedures as part of their scope of practice; for example, in a retrospective study of nail biopsies using the Medicare Provider Utilization and Payment Database, there was no statistically significant difference in reimbursements for nail biopsies vs skin biopsies from 2012 to 2017 (P=0.69).7 Similar to nail biopsies, nail excisions typically are much more time consuming and technically demanding than skin biopsies, which may discourage dermatologists from routinely performing nail excision procedures.
Our study is subject to a number of limitations. The data reflected only US-based practice patterns and may not be applicable to nail procedures globally. There also is the potential for miscoding of procedures in the Medicare database. The data included only Part B Medicare fee-for-service and excludes non-Medicare insured, uninsured, and self-pay patients, as well as aggregated records from 10 or fewer Medicare beneficiaries.
Dermatologists rarely perform nail excisions and perform fewer nail excisions than any other provider type in the United States. There currently is an unmet need for comprehensive nail surgery education in US-based dermatology residency programs. We hope that our study fosters interdisciplinary collegiality and training between podiatrists and dermatologists and promotes expanded access to care across the United States to serve patients with nail disorders.
- Centers for Medicare & Medicaid Services. Medicare Fee-For-Service Provider Utilization & Payment Data Physician and Other Supplier Public Use File: A Methodological Overview . Updated September 22, 2020. Accessed January 5, 2024. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/downloads/medicare-physician-and-other-supplier-puf-methodology.pdf
- Centers for Medicare and Medicaid Services. Billing and Coding: Surgical Treatment of Nails. Updated November 9, 2023. Accessed January 8, 2024. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleID=52998#:~:text=The%20description%20of%20CPT%20codes,date%20of%20service%20(DOS).
- Peck GM, Vlahovic TC, Hill R, et al. Senior podiatrists in solo practice are high performers of nail excisions. JAPMA. In press.
- Accreditation Council for Graduate Medical Education. Case log minimums. review committee for dermatology. Published May 2019. Accessed January 5, 2024. https://www.acgme.org/Portals/0/PFAssets/ProgramResources/CaseLogMinimums.pdf?ver=2018-04-03-102751-650
- Council on Podiatric Medical Education. Standards and Requirements for Approval of Podiatric Medicine and Surgery Residencies. Published July 2023. Accessed January 17, 2024. https://www.cpme.org/files/320%20Council%20Approved%20October%202022%20-%20April%202023%20edits.pdf
- Augustin M, Eissing L, Elsner P, et al. Perception and image of dermatology in the German general population 2002-2014. J Eur Acad Dermatol Venereol. 2017;31:2124-2130.
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare provider utilization and payment database 2012 to 2017. Dermatol Ther. 2021;34:E14928.
To the Editor:
Partial or total nail plate excisions commonly are used for the treatment of onychocryptosis and nail spicules. Procedures involving the nail unit require advanced technical skills to achieve optimal functional and aesthetic outcomes, avoid complications, and minimize health care costs. Data on the frequency of nail plate excisions performed by dermatologists and their relative frequency compared to other medical providers are limited. The objective of our study was to analyze trends in nail excision practice patterns among medical providers in the United States.
A retrospective analysis on nail excisions using the Current Procedural Terminology (CPT) code 11750 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail] for permanent removal), which is distinct from code 11755 (biopsy of nail unit [eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds][separate procedure]), was performed using data from the Medicare Provider Utilization and Payment Database 2012-2017.1,2 This file also is used by Peck et al3 in an article submitted to the Journal of the American Podiatric Medical Association and currently under consideration for publication. Procedures were recorded by year and provider type—dermatologist, podiatrist, physician assistant (PA)/nurse practitioner (NP), nondermatologist physician—and subcategorized by provider specialty (Table). Practice locations subcategorized by provider type were mapped using Tableau Software (Salesforce)(Figure). Descriptive statistics including number of providers, mean and median excisions per provider, and minimum/maximum nail excisions were calculated (Table). Practice types of PAs/NPs and specialization of nondermatologist physicians were determined using provider name, identification number, and practice address. This study did not require institutional review board review, as only publicly available data were utilized in our analysis.

A total of 6936 podiatrists, 58 nondermatologist physicians, 25 PAs/NPs, and 4 dermatologists performed 10 or more nail excisions annually under CPT code 11750 from January 2012 to December 2017 with annual means of 31, 31, 25, and 34, respectively (Table). No PAs/NPs included in the dataset worked in dermatology practices during the study period. Physician assistants and NPs most often practiced in podiatry and family medicine (FM) settings (both 40% [10/25]). Nondermatologist physicians most often specialized in FM (40% [23/58])(Table). The greatest number of providers practiced in 3 of the 4 most-populous states: California, Texas, and Florida; the fewest number practiced in 3 of the 10 least-populous states: Alaska, Hawaii, and Vermont. Vermont, Wyoming, and North Dakota—3 of the 5 least-populous states—had the fewest practitioners among the contiguous United States (Figure).

Our study showed that from January 2012 to December 2017, fewer dermatologists performed nail excisions than any other provider type (0.06%, 4 dermatologists of 7023 total providers), and dermatologists performed 1734-fold fewer nail excisions than podiatrists (99%, 6936 podiatrists of 7023 total providers). Only dermatologists practicing in California, Georgia, Indiana, and Oklahoma performed nail excisions. Conversely, podiatrists were more geographically distributed across the United States and other territories, with representation in all 50 states as well as the District of Columbia, Puerto Rico, and Guam.
Reasons for these large discrepancies in practice between dermatologists and other providers likely are multifactorial, encompassing a lack of emphasis on nail procedures in dermatology training, patient perception of the scope of dermatologic practice, and nail excision reimbursement patterns. Most dermatologists likely lack experience in performing nail procedures. The Accreditation Council for Graduate Medical Education requirements mandate that dermatology residents observe or perform 3 nail procedures over 3 years of residency, including 1 that may be performed on a human cadaver.4 In contrast, podiatry trainees must gain competency in toenail avulsion (both partial and complete), participate in anesthesia workshops, and become proficient in administering lower extremity blocks by the end of their training.5 Therefore, incorporating aspects of podiatric surgical training into dermatology residency requirements may increase the competency and comfort of dermatologists in performing nail excisions and practicing as nail experts as attending physicians.
It is likely that US patients do not perceive dermatologists as nail specialists and instead primarily consult podiatrists or FM and/or internal medicine physicians for treatment; for example, nail disease was one of the least common reasons for consulting a dermatologist (5%) in a German nationwide survey-based study (N=1015).6 Therefore, increased efforts are needed to educate the general public about the expertise of dermatologists in the diagnosis and management of nail conditions.
Reimbursement also may be a barrier to dermatologists performing nail procedures as part of their scope of practice; for example, in a retrospective study of nail biopsies using the Medicare Provider Utilization and Payment Database, there was no statistically significant difference in reimbursements for nail biopsies vs skin biopsies from 2012 to 2017 (P=0.69).7 Similar to nail biopsies, nail excisions typically are much more time consuming and technically demanding than skin biopsies, which may discourage dermatologists from routinely performing nail excision procedures.
Our study is subject to a number of limitations. The data reflected only US-based practice patterns and may not be applicable to nail procedures globally. There also is the potential for miscoding of procedures in the Medicare database. The data included only Part B Medicare fee-for-service and excludes non-Medicare insured, uninsured, and self-pay patients, as well as aggregated records from 10 or fewer Medicare beneficiaries.
Dermatologists rarely perform nail excisions and perform fewer nail excisions than any other provider type in the United States. There currently is an unmet need for comprehensive nail surgery education in US-based dermatology residency programs. We hope that our study fosters interdisciplinary collegiality and training between podiatrists and dermatologists and promotes expanded access to care across the United States to serve patients with nail disorders.
To the Editor:
Partial or total nail plate excisions commonly are used for the treatment of onychocryptosis and nail spicules. Procedures involving the nail unit require advanced technical skills to achieve optimal functional and aesthetic outcomes, avoid complications, and minimize health care costs. Data on the frequency of nail plate excisions performed by dermatologists and their relative frequency compared to other medical providers are limited. The objective of our study was to analyze trends in nail excision practice patterns among medical providers in the United States.
A retrospective analysis on nail excisions using the Current Procedural Terminology (CPT) code 11750 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail] for permanent removal), which is distinct from code 11755 (biopsy of nail unit [eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds][separate procedure]), was performed using data from the Medicare Provider Utilization and Payment Database 2012-2017.1,2 This file also is used by Peck et al3 in an article submitted to the Journal of the American Podiatric Medical Association and currently under consideration for publication. Procedures were recorded by year and provider type—dermatologist, podiatrist, physician assistant (PA)/nurse practitioner (NP), nondermatologist physician—and subcategorized by provider specialty (Table). Practice locations subcategorized by provider type were mapped using Tableau Software (Salesforce)(Figure). Descriptive statistics including number of providers, mean and median excisions per provider, and minimum/maximum nail excisions were calculated (Table). Practice types of PAs/NPs and specialization of nondermatologist physicians were determined using provider name, identification number, and practice address. This study did not require institutional review board review, as only publicly available data were utilized in our analysis.

A total of 6936 podiatrists, 58 nondermatologist physicians, 25 PAs/NPs, and 4 dermatologists performed 10 or more nail excisions annually under CPT code 11750 from January 2012 to December 2017 with annual means of 31, 31, 25, and 34, respectively (Table). No PAs/NPs included in the dataset worked in dermatology practices during the study period. Physician assistants and NPs most often practiced in podiatry and family medicine (FM) settings (both 40% [10/25]). Nondermatologist physicians most often specialized in FM (40% [23/58])(Table). The greatest number of providers practiced in 3 of the 4 most-populous states: California, Texas, and Florida; the fewest number practiced in 3 of the 10 least-populous states: Alaska, Hawaii, and Vermont. Vermont, Wyoming, and North Dakota—3 of the 5 least-populous states—had the fewest practitioners among the contiguous United States (Figure).

Our study showed that from January 2012 to December 2017, fewer dermatologists performed nail excisions than any other provider type (0.06%, 4 dermatologists of 7023 total providers), and dermatologists performed 1734-fold fewer nail excisions than podiatrists (99%, 6936 podiatrists of 7023 total providers). Only dermatologists practicing in California, Georgia, Indiana, and Oklahoma performed nail excisions. Conversely, podiatrists were more geographically distributed across the United States and other territories, with representation in all 50 states as well as the District of Columbia, Puerto Rico, and Guam.
Reasons for these large discrepancies in practice between dermatologists and other providers likely are multifactorial, encompassing a lack of emphasis on nail procedures in dermatology training, patient perception of the scope of dermatologic practice, and nail excision reimbursement patterns. Most dermatologists likely lack experience in performing nail procedures. The Accreditation Council for Graduate Medical Education requirements mandate that dermatology residents observe or perform 3 nail procedures over 3 years of residency, including 1 that may be performed on a human cadaver.4 In contrast, podiatry trainees must gain competency in toenail avulsion (both partial and complete), participate in anesthesia workshops, and become proficient in administering lower extremity blocks by the end of their training.5 Therefore, incorporating aspects of podiatric surgical training into dermatology residency requirements may increase the competency and comfort of dermatologists in performing nail excisions and practicing as nail experts as attending physicians.
It is likely that US patients do not perceive dermatologists as nail specialists and instead primarily consult podiatrists or FM and/or internal medicine physicians for treatment; for example, nail disease was one of the least common reasons for consulting a dermatologist (5%) in a German nationwide survey-based study (N=1015).6 Therefore, increased efforts are needed to educate the general public about the expertise of dermatologists in the diagnosis and management of nail conditions.
Reimbursement also may be a barrier to dermatologists performing nail procedures as part of their scope of practice; for example, in a retrospective study of nail biopsies using the Medicare Provider Utilization and Payment Database, there was no statistically significant difference in reimbursements for nail biopsies vs skin biopsies from 2012 to 2017 (P=0.69).7 Similar to nail biopsies, nail excisions typically are much more time consuming and technically demanding than skin biopsies, which may discourage dermatologists from routinely performing nail excision procedures.
Our study is subject to a number of limitations. The data reflected only US-based practice patterns and may not be applicable to nail procedures globally. There also is the potential for miscoding of procedures in the Medicare database. The data included only Part B Medicare fee-for-service and excludes non-Medicare insured, uninsured, and self-pay patients, as well as aggregated records from 10 or fewer Medicare beneficiaries.
Dermatologists rarely perform nail excisions and perform fewer nail excisions than any other provider type in the United States. There currently is an unmet need for comprehensive nail surgery education in US-based dermatology residency programs. We hope that our study fosters interdisciplinary collegiality and training between podiatrists and dermatologists and promotes expanded access to care across the United States to serve patients with nail disorders.
- Centers for Medicare & Medicaid Services. Medicare Fee-For-Service Provider Utilization & Payment Data Physician and Other Supplier Public Use File: A Methodological Overview . Updated September 22, 2020. Accessed January 5, 2024. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/downloads/medicare-physician-and-other-supplier-puf-methodology.pdf
- Centers for Medicare and Medicaid Services. Billing and Coding: Surgical Treatment of Nails. Updated November 9, 2023. Accessed January 8, 2024. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleID=52998#:~:text=The%20description%20of%20CPT%20codes,date%20of%20service%20(DOS).
- Peck GM, Vlahovic TC, Hill R, et al. Senior podiatrists in solo practice are high performers of nail excisions. JAPMA. In press.
- Accreditation Council for Graduate Medical Education. Case log minimums. review committee for dermatology. Published May 2019. Accessed January 5, 2024. https://www.acgme.org/Portals/0/PFAssets/ProgramResources/CaseLogMinimums.pdf?ver=2018-04-03-102751-650
- Council on Podiatric Medical Education. Standards and Requirements for Approval of Podiatric Medicine and Surgery Residencies. Published July 2023. Accessed January 17, 2024. https://www.cpme.org/files/320%20Council%20Approved%20October%202022%20-%20April%202023%20edits.pdf
- Augustin M, Eissing L, Elsner P, et al. Perception and image of dermatology in the German general population 2002-2014. J Eur Acad Dermatol Venereol. 2017;31:2124-2130.
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare provider utilization and payment database 2012 to 2017. Dermatol Ther. 2021;34:E14928.
- Centers for Medicare & Medicaid Services. Medicare Fee-For-Service Provider Utilization & Payment Data Physician and Other Supplier Public Use File: A Methodological Overview . Updated September 22, 2020. Accessed January 5, 2024. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/downloads/medicare-physician-and-other-supplier-puf-methodology.pdf
- Centers for Medicare and Medicaid Services. Billing and Coding: Surgical Treatment of Nails. Updated November 9, 2023. Accessed January 8, 2024. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleID=52998#:~:text=The%20description%20of%20CPT%20codes,date%20of%20service%20(DOS).
- Peck GM, Vlahovic TC, Hill R, et al. Senior podiatrists in solo practice are high performers of nail excisions. JAPMA. In press.
- Accreditation Council for Graduate Medical Education. Case log minimums. review committee for dermatology. Published May 2019. Accessed January 5, 2024. https://www.acgme.org/Portals/0/PFAssets/ProgramResources/CaseLogMinimums.pdf?ver=2018-04-03-102751-650
- Council on Podiatric Medical Education. Standards and Requirements for Approval of Podiatric Medicine and Surgery Residencies. Published July 2023. Accessed January 17, 2024. https://www.cpme.org/files/320%20Council%20Approved%20October%202022%20-%20April%202023%20edits.pdf
- Augustin M, Eissing L, Elsner P, et al. Perception and image of dermatology in the German general population 2002-2014. J Eur Acad Dermatol Venereol. 2017;31:2124-2130.
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare provider utilization and payment database 2012 to 2017. Dermatol Ther. 2021;34:E14928.
Practice Points
- Dermatologists are considered nail experts but perform nail excisions less frequently than their podiatric counterparts and physicians in other specialties.
- Aspects of podiatric surgical training should be incorporated into dermatology residency to increase competency and comfort of dermatologists in nail excision procedures.
- Dermatologists may not be perceived as nail experts by the public, indicating a need for increased community education on the role of dermatologists in treating nail disease.
Blue to Slate Gray Discoloration of the Proximal Fingernails
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
An 88-year-old man presented with asymptomatic and unchanging discoloration of the proximal fingernails of both hands of 50 years’ duration. Physical examination revealed blue to slate gray, subungual pigmentary changes of the fingernails of both hands sparing the nail bed distal to the lunulae. There was no overlying plate dystrophy, toenail involvement, or additional mucocutaneous abnormalities. His medical history was notable for heart failure, obstructive sleep apnea, and type 2 diabetes mellitus. He had no history of hepatic, ophthalmologic, or neurologic dysfunction.
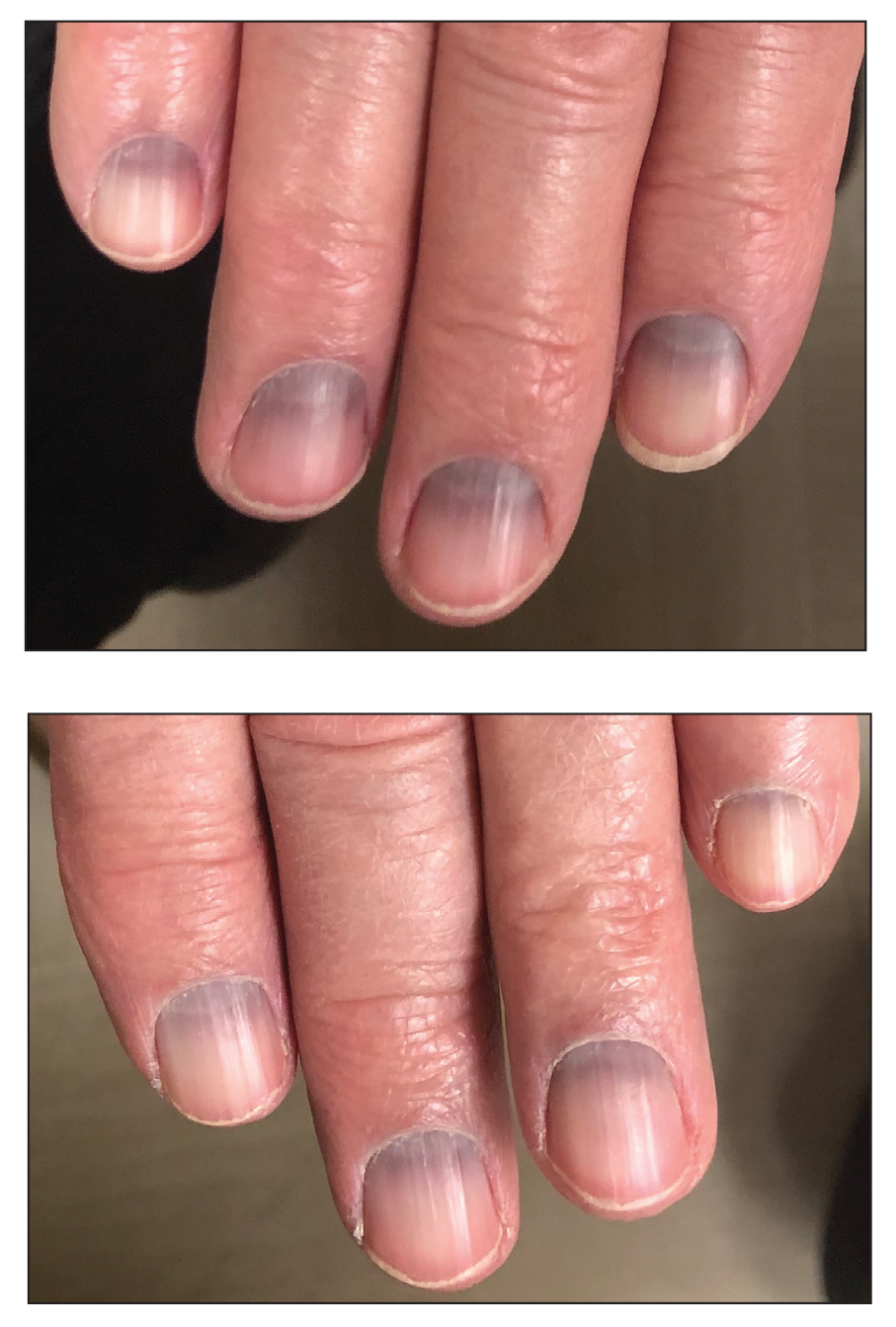
Hair Creams: Do You Know the Health Risks?
In late December 2023, Brazil’s National Health Surveillance Agency (ANVISA) suspended the commercialization of approximately 1200 hair creams because of reports of eye irritation and temporary blindness.
A similar measure encompassing all hair creams sold in the country had already been announced by the agency in March. However, after a few weeks, ANVISA issued a resolution with rules for the products’ commercialization, allowing them back on the shelves.
With the new resolution, the sale of products that do not comply with the standards has once again been suspended. The reason is that reports of adverse events have reemerged. These events include temporary vision loss, headaches, and burning, tearing, itching, redness, and swelling of the eyes. According to reports, these adverse effects occurred mainly in people who used the specific products before swimming in the sea or in pools, or even going out in the rain.
The banned products contain 20% or more ethoxylated alcohols in their formulations. , potentially causing allergies and burns to the eyes and skin. They also have a high pulmonary and neurological toxicity. All these substances are eye irritants and can cause chemical keratitis. In extreme cases, corneal ulcers may develop, leading to vision loss.
The Brazilian Council of Ophthalmology also issued a warning on these products. It emphasized that, in addition to the sales prohibition, consumers should check the labels of hair creams to make sure that these toxic substances are not present in the product formulation.
The ANVISA website contains a list of creams that are considered safe and have not had their commercialization suspended, along with links to adverse event notifications reported by healthcare professionals or consumers.
For consumers who have recently used hair creams, the agency advises careful hair washing, including tilting the head backward to prevent the product from coming into contact with the eye area. If there is accidental eye contact, the eyes should be washed with plenty of water.
If there are any undesired effects after using these products, users should immediately seek the nearest healthcare service. Treatment should be individualized, possibly including ocular occlusion and the use of eye drops containing antibiotics or corticosteroids, among other medications.
Not every patient has easy access to an ophthalmologist in an emergency, so it is crucial for general practitioners to be prepared for initial care. In this regard, one of the most important measures is eye washing with copious amounts of clean water or saline solution for 5-10 minutes.
Eye itching is a frequent manifestation of using hair creams, and scratching the area may worsen the condition. Ocular occlusion can protect the cornea until an evaluation can be performed by a specialist.
Although we prefer our patients to stay away from these creams, it is also important to disseminate this information and advise them to read labels and use safe cosmetics.
This article was translated from the Medscape Portuguese edition. A version of this article appeared on Medscape.com.
In late December 2023, Brazil’s National Health Surveillance Agency (ANVISA) suspended the commercialization of approximately 1200 hair creams because of reports of eye irritation and temporary blindness.
A similar measure encompassing all hair creams sold in the country had already been announced by the agency in March. However, after a few weeks, ANVISA issued a resolution with rules for the products’ commercialization, allowing them back on the shelves.
With the new resolution, the sale of products that do not comply with the standards has once again been suspended. The reason is that reports of adverse events have reemerged. These events include temporary vision loss, headaches, and burning, tearing, itching, redness, and swelling of the eyes. According to reports, these adverse effects occurred mainly in people who used the specific products before swimming in the sea or in pools, or even going out in the rain.
The banned products contain 20% or more ethoxylated alcohols in their formulations. , potentially causing allergies and burns to the eyes and skin. They also have a high pulmonary and neurological toxicity. All these substances are eye irritants and can cause chemical keratitis. In extreme cases, corneal ulcers may develop, leading to vision loss.
The Brazilian Council of Ophthalmology also issued a warning on these products. It emphasized that, in addition to the sales prohibition, consumers should check the labels of hair creams to make sure that these toxic substances are not present in the product formulation.
The ANVISA website contains a list of creams that are considered safe and have not had their commercialization suspended, along with links to adverse event notifications reported by healthcare professionals or consumers.
For consumers who have recently used hair creams, the agency advises careful hair washing, including tilting the head backward to prevent the product from coming into contact with the eye area. If there is accidental eye contact, the eyes should be washed with plenty of water.
If there are any undesired effects after using these products, users should immediately seek the nearest healthcare service. Treatment should be individualized, possibly including ocular occlusion and the use of eye drops containing antibiotics or corticosteroids, among other medications.
Not every patient has easy access to an ophthalmologist in an emergency, so it is crucial for general practitioners to be prepared for initial care. In this regard, one of the most important measures is eye washing with copious amounts of clean water or saline solution for 5-10 minutes.
Eye itching is a frequent manifestation of using hair creams, and scratching the area may worsen the condition. Ocular occlusion can protect the cornea until an evaluation can be performed by a specialist.
Although we prefer our patients to stay away from these creams, it is also important to disseminate this information and advise them to read labels and use safe cosmetics.
This article was translated from the Medscape Portuguese edition. A version of this article appeared on Medscape.com.
In late December 2023, Brazil’s National Health Surveillance Agency (ANVISA) suspended the commercialization of approximately 1200 hair creams because of reports of eye irritation and temporary blindness.
A similar measure encompassing all hair creams sold in the country had already been announced by the agency in March. However, after a few weeks, ANVISA issued a resolution with rules for the products’ commercialization, allowing them back on the shelves.
With the new resolution, the sale of products that do not comply with the standards has once again been suspended. The reason is that reports of adverse events have reemerged. These events include temporary vision loss, headaches, and burning, tearing, itching, redness, and swelling of the eyes. According to reports, these adverse effects occurred mainly in people who used the specific products before swimming in the sea or in pools, or even going out in the rain.
The banned products contain 20% or more ethoxylated alcohols in their formulations. , potentially causing allergies and burns to the eyes and skin. They also have a high pulmonary and neurological toxicity. All these substances are eye irritants and can cause chemical keratitis. In extreme cases, corneal ulcers may develop, leading to vision loss.
The Brazilian Council of Ophthalmology also issued a warning on these products. It emphasized that, in addition to the sales prohibition, consumers should check the labels of hair creams to make sure that these toxic substances are not present in the product formulation.
The ANVISA website contains a list of creams that are considered safe and have not had their commercialization suspended, along with links to adverse event notifications reported by healthcare professionals or consumers.
For consumers who have recently used hair creams, the agency advises careful hair washing, including tilting the head backward to prevent the product from coming into contact with the eye area. If there is accidental eye contact, the eyes should be washed with plenty of water.
If there are any undesired effects after using these products, users should immediately seek the nearest healthcare service. Treatment should be individualized, possibly including ocular occlusion and the use of eye drops containing antibiotics or corticosteroids, among other medications.
Not every patient has easy access to an ophthalmologist in an emergency, so it is crucial for general practitioners to be prepared for initial care. In this regard, one of the most important measures is eye washing with copious amounts of clean water or saline solution for 5-10 minutes.
Eye itching is a frequent manifestation of using hair creams, and scratching the area may worsen the condition. Ocular occlusion can protect the cornea until an evaluation can be performed by a specialist.
Although we prefer our patients to stay away from these creams, it is also important to disseminate this information and advise them to read labels and use safe cosmetics.
This article was translated from the Medscape Portuguese edition. A version of this article appeared on Medscape.com.
Hair Loss in Children: How to Spot and Treat Different Causes
ORLANDO, FLORIDA — There are subtleties and nuances to diagnosing, treating, and monitoring the progress of treatment of hair loss in children. Moreover, hair loss in children can be challenging because it can be caused by a range of conditions, some common and others relatively rare.
Michelle Oboite, MD, shared tips on how to distinguish types of hair loss, when to treat with medications such as topical corticosteroids or Janus kinase (JAK) inhibitors, and why shared decision-making is important, at the ODAC Dermatology, Aesthetic & Surgical Conference.
What these conditions share is that they can negatively affect the quality of life for a child or teenager when the condition leads to anxiety, teasing, or bullying. “It is very isolating to have this condition that everyone in the world can see that you have and judge you for it,” said Dr. Oboite, an attending physician in the dermatology section of Children’s Hospital of Philadelphia.
Others are lichen planopilaris and genetic conditions, including loose anagen syndrome, uncombable hair syndrome, and “something so rare” — it has no acronym — autosomal recessive hypotrichosis with recurrent skin vesicles, Dr. Oboite said.
Alopecia Areata
Alopecia areata can differ from child to child and can appear in different stages: A localized patch stage, a diffuse patchy stage, or alopecia universalis. In this last stage, the child has already lost most or all the hair on the scalp and eyebrows, as well as the eyelashes.
The decision to treat or not to treat, particularly in younger children, should be on the basis of shared decision-making between a healthcare provider and caregiver, said Dr. Oboite, who is also an assistant professor of clinical dermatology at the University of Pennsylvania, Philadelphia.
Some younger children may not experience any negative impact from the condition, so waiting until they are older is an option.
Also, consider the impact of treatment on a child. Some therapies require frequent blood draws for monitoring, and some topical therapies that are applied multiple times a day “can be very overwhelming” for young children, Dr. Oboite said.
Most children with alopecia areata are healthy and do not need extensive screening laboratory testing. However, one exception is if thyroid dysfunction, commonly associated with alopecia areata, is suspected.
For alopecia areata, Dr. Oboite recommends starting with topical therapies, either topical corticosteroids (as first line) or topical JAK inhibitors (either topical ruxolitinib or compounded topical tofacitinib, both off-label for this indication).
Topical corticosteroids can be effective, but “you want to be thoughtful of the strength you’re using, the application frequency, and then the total amount of surface area that you’re treating,” Dr. Oboite said. Too potent or too much of a topical corticosteroid increases the risk for atrophy and systemic absorption, respectively. To reduce the risk, she reserves the use of ultrahigh-potency topical corticosteroids, such as clobetasol, for children ages 10 years or older. For children younger than 10 years, she recommends using mid-high-potency topical corticosteroids instead.
She recommends once-a-day application around bedtime 5 days a week, generally Monday through Friday to make it easier to remember.
“For children who have over 50% of the scalp involved, I do consider systemic therapy,” Dr. Oboite said. This can include oral steroids such as dexamethasone, prednisone, or prednisolone. For children with recalcitrant disease, she is more likely to use the oral JAK inhibitor ritlecitinib because it was recently approved by the Food and Drug Administration for treating severe alopecia areata in children 12 years and older and in adults.
Another strategy Dr. Oboite uses is to add low-dose oral minoxidil as an adjuvant to other systemic therapy. “I find that it helps with faster hair regrowth,” she said.
Tinea Capitis
Oral treatment is indicated for tinea capitis. “Topicals just don’t really clear this,” Dr. Oboite said. Also, talk to patients and families about preventing reinfection with the dermatophyte that causes this condition. “Make sure we’re cleaning hats, combs, brushes, and pillowcases. That is really important.”
Some patients can develop a widespread rash while on treatment. But in most cases, it’s not an adverse reaction to the medication but rather an indication that the body’s response is revving up, she noted.
Griseofulvin 20 mg/kg/d is one treatment option. Another is terbinafine (using weight-based dosing). A tip with terbinafine is that because the tablet needs to be crushed for a young child, “you can put it in anything, besides applesauce or yogurt with fruit on the bottom, which can be acidic and reduce the effectiveness of the medication,” Dr. Oboite said.
For cases of severe, inflammatory tinea capitis such as a kerion, “I will say you have to hold the hands of these patients, the journey can be long,” she added.
Trichotillomania
Trichotillomania occurs when someone cannot stop pulling their own hair, and in the early phases, it can be confused with alopecia areata. A thorough history and examination of the patient can help distinguish the two conditions. Sometimes a child or teen has a history of anxiety-related behaviors like nail biting that points to trichotillomania. Another tip is to use a dermatoscope to help distinguish hair loss conditions because it avoids having to do as many biopsies in children.
Redirection therapy can work for younger children, and cognitive behavioral therapy (CBT) can help older children with trichotillomania. In response to a question during the Q&A period, Dr. Oboite said psychiatrists or psychologists can perform CBT. If it takes time to get an appointment, there are some CBT apps that can help in the meantime, she said.
“One thing really important is to not blame the child,” Dr. Oboite said. “Most children don’t even know that they’re doing this. This is often not a behavior that is being done on purpose.”
Androgenetic Alopecia
Rarely, children and teenagers can also present with androgenetic alopecia, which Dr. Oboite has successfully treated with topical minoxidil, applied once a day before increasing to twice a day if tolerated. “I will tell them that when they pick it up, it will say ‘you should not use in children.’ But it actually can be used in children safely.”
Low-dose oral minoxidil is another option. Both treatments require a commitment by patients and parents because they are “taking this for a long time.”
Loose Anagen and Uncombable Hair Syndromes
A rare genetic form of hair loss is called loose anagen syndrome. Children with this disorder will have thin hair that is easily pulled out without a lot of force. Their hair appears to typically only grow to a certain length (such as to the nape of the neck) and then stops.
Another genetic hair loss condition is uncombable hair syndrome. It can cause hair to grow out of the scalp in all directions, and as the name suggests, it is almost impossible to comb or brush down. Along with loose anagen syndrome, uncombable hair syndrome tends to improve as the child gets older. “The key point here is telling parents that it can get better with time,” Dr. Oboite said.
A Condition With No Well-Known Acronym
She described a child she treated who had hair that never grew and was easily broken. The patient’s skin was prone to bruising, and her fingernails would easily fall off after trauma; her dentist noted that she had no buds for adult teeth on x-rays. These different presentations are important because hair, teeth, and nails all come from the same ectoderm germ line in embryo development, Dr. Oboite said.
Exome sequencing revealed the girl had a very rare diagnosis called autosomal recessive hypotrichosis with recurrent skin vesicles. “So, it is really important to recognize that children who are presenting with hair issues can have a genetic, underlying condition,” she said. Examining the skin, nails, and teeth, in addition to the hair, can be clues to these very rare diagnoses.
Some of these hair loss conditions in children can be challenging to diagnose and manage, Dr. Oboite said. “So don’t be afraid to ask for help on complex or rare cases.” Pediatric dermatologists “are always happy to help you. Hair loss is daunting, and hair loss in children can be even more daunting,” but the rewards of accurate diagnosis and successful treatment can be great, she said.
Dr. Oboite reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
ORLANDO, FLORIDA — There are subtleties and nuances to diagnosing, treating, and monitoring the progress of treatment of hair loss in children. Moreover, hair loss in children can be challenging because it can be caused by a range of conditions, some common and others relatively rare.
Michelle Oboite, MD, shared tips on how to distinguish types of hair loss, when to treat with medications such as topical corticosteroids or Janus kinase (JAK) inhibitors, and why shared decision-making is important, at the ODAC Dermatology, Aesthetic & Surgical Conference.
What these conditions share is that they can negatively affect the quality of life for a child or teenager when the condition leads to anxiety, teasing, or bullying. “It is very isolating to have this condition that everyone in the world can see that you have and judge you for it,” said Dr. Oboite, an attending physician in the dermatology section of Children’s Hospital of Philadelphia.
Others are lichen planopilaris and genetic conditions, including loose anagen syndrome, uncombable hair syndrome, and “something so rare” — it has no acronym — autosomal recessive hypotrichosis with recurrent skin vesicles, Dr. Oboite said.
Alopecia Areata
Alopecia areata can differ from child to child and can appear in different stages: A localized patch stage, a diffuse patchy stage, or alopecia universalis. In this last stage, the child has already lost most or all the hair on the scalp and eyebrows, as well as the eyelashes.
The decision to treat or not to treat, particularly in younger children, should be on the basis of shared decision-making between a healthcare provider and caregiver, said Dr. Oboite, who is also an assistant professor of clinical dermatology at the University of Pennsylvania, Philadelphia.
Some younger children may not experience any negative impact from the condition, so waiting until they are older is an option.
Also, consider the impact of treatment on a child. Some therapies require frequent blood draws for monitoring, and some topical therapies that are applied multiple times a day “can be very overwhelming” for young children, Dr. Oboite said.
Most children with alopecia areata are healthy and do not need extensive screening laboratory testing. However, one exception is if thyroid dysfunction, commonly associated with alopecia areata, is suspected.
For alopecia areata, Dr. Oboite recommends starting with topical therapies, either topical corticosteroids (as first line) or topical JAK inhibitors (either topical ruxolitinib or compounded topical tofacitinib, both off-label for this indication).
Topical corticosteroids can be effective, but “you want to be thoughtful of the strength you’re using, the application frequency, and then the total amount of surface area that you’re treating,” Dr. Oboite said. Too potent or too much of a topical corticosteroid increases the risk for atrophy and systemic absorption, respectively. To reduce the risk, she reserves the use of ultrahigh-potency topical corticosteroids, such as clobetasol, for children ages 10 years or older. For children younger than 10 years, she recommends using mid-high-potency topical corticosteroids instead.
She recommends once-a-day application around bedtime 5 days a week, generally Monday through Friday to make it easier to remember.
“For children who have over 50% of the scalp involved, I do consider systemic therapy,” Dr. Oboite said. This can include oral steroids such as dexamethasone, prednisone, or prednisolone. For children with recalcitrant disease, she is more likely to use the oral JAK inhibitor ritlecitinib because it was recently approved by the Food and Drug Administration for treating severe alopecia areata in children 12 years and older and in adults.
Another strategy Dr. Oboite uses is to add low-dose oral minoxidil as an adjuvant to other systemic therapy. “I find that it helps with faster hair regrowth,” she said.
Tinea Capitis
Oral treatment is indicated for tinea capitis. “Topicals just don’t really clear this,” Dr. Oboite said. Also, talk to patients and families about preventing reinfection with the dermatophyte that causes this condition. “Make sure we’re cleaning hats, combs, brushes, and pillowcases. That is really important.”
Some patients can develop a widespread rash while on treatment. But in most cases, it’s not an adverse reaction to the medication but rather an indication that the body’s response is revving up, she noted.
Griseofulvin 20 mg/kg/d is one treatment option. Another is terbinafine (using weight-based dosing). A tip with terbinafine is that because the tablet needs to be crushed for a young child, “you can put it in anything, besides applesauce or yogurt with fruit on the bottom, which can be acidic and reduce the effectiveness of the medication,” Dr. Oboite said.
For cases of severe, inflammatory tinea capitis such as a kerion, “I will say you have to hold the hands of these patients, the journey can be long,” she added.
Trichotillomania
Trichotillomania occurs when someone cannot stop pulling their own hair, and in the early phases, it can be confused with alopecia areata. A thorough history and examination of the patient can help distinguish the two conditions. Sometimes a child or teen has a history of anxiety-related behaviors like nail biting that points to trichotillomania. Another tip is to use a dermatoscope to help distinguish hair loss conditions because it avoids having to do as many biopsies in children.
Redirection therapy can work for younger children, and cognitive behavioral therapy (CBT) can help older children with trichotillomania. In response to a question during the Q&A period, Dr. Oboite said psychiatrists or psychologists can perform CBT. If it takes time to get an appointment, there are some CBT apps that can help in the meantime, she said.
“One thing really important is to not blame the child,” Dr. Oboite said. “Most children don’t even know that they’re doing this. This is often not a behavior that is being done on purpose.”
Androgenetic Alopecia
Rarely, children and teenagers can also present with androgenetic alopecia, which Dr. Oboite has successfully treated with topical minoxidil, applied once a day before increasing to twice a day if tolerated. “I will tell them that when they pick it up, it will say ‘you should not use in children.’ But it actually can be used in children safely.”
Low-dose oral minoxidil is another option. Both treatments require a commitment by patients and parents because they are “taking this for a long time.”
Loose Anagen and Uncombable Hair Syndromes
A rare genetic form of hair loss is called loose anagen syndrome. Children with this disorder will have thin hair that is easily pulled out without a lot of force. Their hair appears to typically only grow to a certain length (such as to the nape of the neck) and then stops.
Another genetic hair loss condition is uncombable hair syndrome. It can cause hair to grow out of the scalp in all directions, and as the name suggests, it is almost impossible to comb or brush down. Along with loose anagen syndrome, uncombable hair syndrome tends to improve as the child gets older. “The key point here is telling parents that it can get better with time,” Dr. Oboite said.
A Condition With No Well-Known Acronym
She described a child she treated who had hair that never grew and was easily broken. The patient’s skin was prone to bruising, and her fingernails would easily fall off after trauma; her dentist noted that she had no buds for adult teeth on x-rays. These different presentations are important because hair, teeth, and nails all come from the same ectoderm germ line in embryo development, Dr. Oboite said.
Exome sequencing revealed the girl had a very rare diagnosis called autosomal recessive hypotrichosis with recurrent skin vesicles. “So, it is really important to recognize that children who are presenting with hair issues can have a genetic, underlying condition,” she said. Examining the skin, nails, and teeth, in addition to the hair, can be clues to these very rare diagnoses.
Some of these hair loss conditions in children can be challenging to diagnose and manage, Dr. Oboite said. “So don’t be afraid to ask for help on complex or rare cases.” Pediatric dermatologists “are always happy to help you. Hair loss is daunting, and hair loss in children can be even more daunting,” but the rewards of accurate diagnosis and successful treatment can be great, she said.
Dr. Oboite reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
ORLANDO, FLORIDA — There are subtleties and nuances to diagnosing, treating, and monitoring the progress of treatment of hair loss in children. Moreover, hair loss in children can be challenging because it can be caused by a range of conditions, some common and others relatively rare.
Michelle Oboite, MD, shared tips on how to distinguish types of hair loss, when to treat with medications such as topical corticosteroids or Janus kinase (JAK) inhibitors, and why shared decision-making is important, at the ODAC Dermatology, Aesthetic & Surgical Conference.
What these conditions share is that they can negatively affect the quality of life for a child or teenager when the condition leads to anxiety, teasing, or bullying. “It is very isolating to have this condition that everyone in the world can see that you have and judge you for it,” said Dr. Oboite, an attending physician in the dermatology section of Children’s Hospital of Philadelphia.
Others are lichen planopilaris and genetic conditions, including loose anagen syndrome, uncombable hair syndrome, and “something so rare” — it has no acronym — autosomal recessive hypotrichosis with recurrent skin vesicles, Dr. Oboite said.
Alopecia Areata
Alopecia areata can differ from child to child and can appear in different stages: A localized patch stage, a diffuse patchy stage, or alopecia universalis. In this last stage, the child has already lost most or all the hair on the scalp and eyebrows, as well as the eyelashes.
The decision to treat or not to treat, particularly in younger children, should be on the basis of shared decision-making between a healthcare provider and caregiver, said Dr. Oboite, who is also an assistant professor of clinical dermatology at the University of Pennsylvania, Philadelphia.
Some younger children may not experience any negative impact from the condition, so waiting until they are older is an option.
Also, consider the impact of treatment on a child. Some therapies require frequent blood draws for monitoring, and some topical therapies that are applied multiple times a day “can be very overwhelming” for young children, Dr. Oboite said.
Most children with alopecia areata are healthy and do not need extensive screening laboratory testing. However, one exception is if thyroid dysfunction, commonly associated with alopecia areata, is suspected.
For alopecia areata, Dr. Oboite recommends starting with topical therapies, either topical corticosteroids (as first line) or topical JAK inhibitors (either topical ruxolitinib or compounded topical tofacitinib, both off-label for this indication).
Topical corticosteroids can be effective, but “you want to be thoughtful of the strength you’re using, the application frequency, and then the total amount of surface area that you’re treating,” Dr. Oboite said. Too potent or too much of a topical corticosteroid increases the risk for atrophy and systemic absorption, respectively. To reduce the risk, she reserves the use of ultrahigh-potency topical corticosteroids, such as clobetasol, for children ages 10 years or older. For children younger than 10 years, she recommends using mid-high-potency topical corticosteroids instead.
She recommends once-a-day application around bedtime 5 days a week, generally Monday through Friday to make it easier to remember.
“For children who have over 50% of the scalp involved, I do consider systemic therapy,” Dr. Oboite said. This can include oral steroids such as dexamethasone, prednisone, or prednisolone. For children with recalcitrant disease, she is more likely to use the oral JAK inhibitor ritlecitinib because it was recently approved by the Food and Drug Administration for treating severe alopecia areata in children 12 years and older and in adults.
Another strategy Dr. Oboite uses is to add low-dose oral minoxidil as an adjuvant to other systemic therapy. “I find that it helps with faster hair regrowth,” she said.
Tinea Capitis
Oral treatment is indicated for tinea capitis. “Topicals just don’t really clear this,” Dr. Oboite said. Also, talk to patients and families about preventing reinfection with the dermatophyte that causes this condition. “Make sure we’re cleaning hats, combs, brushes, and pillowcases. That is really important.”
Some patients can develop a widespread rash while on treatment. But in most cases, it’s not an adverse reaction to the medication but rather an indication that the body’s response is revving up, she noted.
Griseofulvin 20 mg/kg/d is one treatment option. Another is terbinafine (using weight-based dosing). A tip with terbinafine is that because the tablet needs to be crushed for a young child, “you can put it in anything, besides applesauce or yogurt with fruit on the bottom, which can be acidic and reduce the effectiveness of the medication,” Dr. Oboite said.
For cases of severe, inflammatory tinea capitis such as a kerion, “I will say you have to hold the hands of these patients, the journey can be long,” she added.
Trichotillomania
Trichotillomania occurs when someone cannot stop pulling their own hair, and in the early phases, it can be confused with alopecia areata. A thorough history and examination of the patient can help distinguish the two conditions. Sometimes a child or teen has a history of anxiety-related behaviors like nail biting that points to trichotillomania. Another tip is to use a dermatoscope to help distinguish hair loss conditions because it avoids having to do as many biopsies in children.
Redirection therapy can work for younger children, and cognitive behavioral therapy (CBT) can help older children with trichotillomania. In response to a question during the Q&A period, Dr. Oboite said psychiatrists or psychologists can perform CBT. If it takes time to get an appointment, there are some CBT apps that can help in the meantime, she said.
“One thing really important is to not blame the child,” Dr. Oboite said. “Most children don’t even know that they’re doing this. This is often not a behavior that is being done on purpose.”
Androgenetic Alopecia
Rarely, children and teenagers can also present with androgenetic alopecia, which Dr. Oboite has successfully treated with topical minoxidil, applied once a day before increasing to twice a day if tolerated. “I will tell them that when they pick it up, it will say ‘you should not use in children.’ But it actually can be used in children safely.”
Low-dose oral minoxidil is another option. Both treatments require a commitment by patients and parents because they are “taking this for a long time.”
Loose Anagen and Uncombable Hair Syndromes
A rare genetic form of hair loss is called loose anagen syndrome. Children with this disorder will have thin hair that is easily pulled out without a lot of force. Their hair appears to typically only grow to a certain length (such as to the nape of the neck) and then stops.
Another genetic hair loss condition is uncombable hair syndrome. It can cause hair to grow out of the scalp in all directions, and as the name suggests, it is almost impossible to comb or brush down. Along with loose anagen syndrome, uncombable hair syndrome tends to improve as the child gets older. “The key point here is telling parents that it can get better with time,” Dr. Oboite said.
A Condition With No Well-Known Acronym
She described a child she treated who had hair that never grew and was easily broken. The patient’s skin was prone to bruising, and her fingernails would easily fall off after trauma; her dentist noted that she had no buds for adult teeth on x-rays. These different presentations are important because hair, teeth, and nails all come from the same ectoderm germ line in embryo development, Dr. Oboite said.
Exome sequencing revealed the girl had a very rare diagnosis called autosomal recessive hypotrichosis with recurrent skin vesicles. “So, it is really important to recognize that children who are presenting with hair issues can have a genetic, underlying condition,” she said. Examining the skin, nails, and teeth, in addition to the hair, can be clues to these very rare diagnoses.
Some of these hair loss conditions in children can be challenging to diagnose and manage, Dr. Oboite said. “So don’t be afraid to ask for help on complex or rare cases.” Pediatric dermatologists “are always happy to help you. Hair loss is daunting, and hair loss in children can be even more daunting,” but the rewards of accurate diagnosis and successful treatment can be great, she said.
Dr. Oboite reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
AT ODAC 2024