User login
Study Finds No Significant Effect of Low-Dose Oral Minoxidil on BP
TOPLINE:
but is associated with a slight increase in heart rate and a 5% incidence of hypotensive symptoms.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis of 16 studies, which involved 2387 patients with alopecia (60.7% women) who received minoxidil, a vasodilator originally developed as an antihypertensive, at doses of 5 mg or less per day.
- Outcomes included changes in mean arterial pressure, systolic BP, diastolic BP, and heart rate.
- Mean differences were calculated between pretreatment and posttreatment values.
TAKEAWAY:
- Hypotensive symptoms were reported in 5% patients, with no significant hypotensive episodes. About 1.8% patients experienced lightheadedness or syncope, 1.2% experienced dizziness, 0.9% had tachycardia, and 0.8% had palpitations.
- LDOM did not significantly alter systolic BP (mean difference, –0.13; 95% CI, –2.67 to 2.41), diastolic BP (mean difference, –1.25; 95% CI, –3.21 to 0.71), and mean arterial pressure (mean difference, –1.92; 95% CI, –4.00 to 0.17).
- LDOM led to a significant increase in heart rate (mean difference, 2.67 beats/min; 95% CI, 0.34-5.01), a difference the authors wrote would “likely not be clinically significant for most patients.”
- Hypertrichosis was the most common side effect (59.6%) and reason for stopping treatment (accounting for nearly 35% of discontinuations).
IN PRACTICE:
“LDOM appears to be a safe treatment for alopecia with no significant impact on blood pressure,” the authors wrote, noting that the study “addresses gaps in clinical knowledge involving LDOM.” Based on their results, they recommended that BP and heart rate “do not need to be closely monitored in patients without prior cardiovascular risk history.”
SOURCE:
The study was led by Matthew Chen, BS, Stony Brook Dermatology in New York. It was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The studies included had small sample sizes and retrospective designs, which may limit the reliability of the findings. Additional limitations include the absence of control groups, a potential recall bias in adverse effect reporting, and variability in dosing regimens and BP monitoring.
DISCLOSURES:
The authors reported no external funding or conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
but is associated with a slight increase in heart rate and a 5% incidence of hypotensive symptoms.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis of 16 studies, which involved 2387 patients with alopecia (60.7% women) who received minoxidil, a vasodilator originally developed as an antihypertensive, at doses of 5 mg or less per day.
- Outcomes included changes in mean arterial pressure, systolic BP, diastolic BP, and heart rate.
- Mean differences were calculated between pretreatment and posttreatment values.
TAKEAWAY:
- Hypotensive symptoms were reported in 5% patients, with no significant hypotensive episodes. About 1.8% patients experienced lightheadedness or syncope, 1.2% experienced dizziness, 0.9% had tachycardia, and 0.8% had palpitations.
- LDOM did not significantly alter systolic BP (mean difference, –0.13; 95% CI, –2.67 to 2.41), diastolic BP (mean difference, –1.25; 95% CI, –3.21 to 0.71), and mean arterial pressure (mean difference, –1.92; 95% CI, –4.00 to 0.17).
- LDOM led to a significant increase in heart rate (mean difference, 2.67 beats/min; 95% CI, 0.34-5.01), a difference the authors wrote would “likely not be clinically significant for most patients.”
- Hypertrichosis was the most common side effect (59.6%) and reason for stopping treatment (accounting for nearly 35% of discontinuations).
IN PRACTICE:
“LDOM appears to be a safe treatment for alopecia with no significant impact on blood pressure,” the authors wrote, noting that the study “addresses gaps in clinical knowledge involving LDOM.” Based on their results, they recommended that BP and heart rate “do not need to be closely monitored in patients without prior cardiovascular risk history.”
SOURCE:
The study was led by Matthew Chen, BS, Stony Brook Dermatology in New York. It was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The studies included had small sample sizes and retrospective designs, which may limit the reliability of the findings. Additional limitations include the absence of control groups, a potential recall bias in adverse effect reporting, and variability in dosing regimens and BP monitoring.
DISCLOSURES:
The authors reported no external funding or conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
but is associated with a slight increase in heart rate and a 5% incidence of hypotensive symptoms.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis of 16 studies, which involved 2387 patients with alopecia (60.7% women) who received minoxidil, a vasodilator originally developed as an antihypertensive, at doses of 5 mg or less per day.
- Outcomes included changes in mean arterial pressure, systolic BP, diastolic BP, and heart rate.
- Mean differences were calculated between pretreatment and posttreatment values.
TAKEAWAY:
- Hypotensive symptoms were reported in 5% patients, with no significant hypotensive episodes. About 1.8% patients experienced lightheadedness or syncope, 1.2% experienced dizziness, 0.9% had tachycardia, and 0.8% had palpitations.
- LDOM did not significantly alter systolic BP (mean difference, –0.13; 95% CI, –2.67 to 2.41), diastolic BP (mean difference, –1.25; 95% CI, –3.21 to 0.71), and mean arterial pressure (mean difference, –1.92; 95% CI, –4.00 to 0.17).
- LDOM led to a significant increase in heart rate (mean difference, 2.67 beats/min; 95% CI, 0.34-5.01), a difference the authors wrote would “likely not be clinically significant for most patients.”
- Hypertrichosis was the most common side effect (59.6%) and reason for stopping treatment (accounting for nearly 35% of discontinuations).
IN PRACTICE:
“LDOM appears to be a safe treatment for alopecia with no significant impact on blood pressure,” the authors wrote, noting that the study “addresses gaps in clinical knowledge involving LDOM.” Based on their results, they recommended that BP and heart rate “do not need to be closely monitored in patients without prior cardiovascular risk history.”
SOURCE:
The study was led by Matthew Chen, BS, Stony Brook Dermatology in New York. It was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The studies included had small sample sizes and retrospective designs, which may limit the reliability of the findings. Additional limitations include the absence of control groups, a potential recall bias in adverse effect reporting, and variability in dosing regimens and BP monitoring.
DISCLOSURES:
The authors reported no external funding or conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Lichen Planus Responds to Treatment with Topical Ruxolitinib in Phase 2 Study
both when given twice daily and as needed, according to data from a phase 2 trial.
The research, presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress, involved 64 patients older than 18 years. Ruxolitinib cream (Opzelura) is a topical formulation of a Janus kinase (JAK)1/JAK2 inhibitor, approved by the Food and Drug Administration (FDA) for treating mild to moderate atopic dermatitis and for nonsegmental vitiligo in adults and children aged 12 years or older.
Ruxolitinib cream twice daily resulted in “significant improvements in cutaneous lichen planus disease severity vs vehicle” after 16 weeks of treatment, said the study presenter, Aaron R. Mangold, MD, a dermatologist at Mayo Clinic, Scottsdale, Arizona.
Further improvements were seen during another 16 weeks of additional open-label, as-needed application, he added, and the topical treatment was “generally well tolerated.”
Consequently, “ruxolitinib cream represents a promising potential treatment for cutaneous lichen planus,” Mangold concluded.
Asked to comment on the results, Adam Friedman, MD, Professor and Chair of Dermatology, George Washington University, Washington, DC, who was not involved with the study, said that in keeping with the characterization of lichen planus using the four Ps — purple, polygonal, pruritic, papules — it is “Pretty common, Predictably disabling and disfiguring, and Passed over again and again in the drug development world.”
He said in an interview that this chronic inflammatory skin condition, which affects roughly 2% of the population, also “lacks consensus on work-up and management, likely in part owing to the absence of sizable clinical trial data.”
A recent survey conducted at a meeting indicated that dermatologists “heavily lean on topical therapies for the management of all severity levels,” noted Friedman, one of the survey authors. “Therefore, the phase 2 data presented at EADV is a welcome addition to the mix.”
Phase 2 Study Results
At the meeting, Mangold said that a previous proof-of-concept single-arm study in 12 patients suggested that topical ruxolitinib was highly effective in treating cutaneous lichen planus.
The current phase 2 trial enrolled 64 patients with predominantly cutaneous disease who had an Investigator’s Global Assessment (IGA) score of 3 or 4 and an Itch Numeric Rating Scale (NRS) score of ≥ 4. Their median age was 57 years, and 71.9% were women. Nearly 63% were White, 28.1% were Black, and 6.3% were Asian. The median duration of disease was 4.9 years, and 90.6% had received prior treatment for their lichen planus.
They were randomized to receive 1.5% ruxolitinib cream or a vehicle cream twice daily for 16 weeks, and following a primary endpoint assessment, they were transferred to an open-label extension period, during which they used ruxolitinib cream as needed for another 16 weeks. There was an additional 30-day safety follow-up period.
At week 16, significantly more patients treated with the ruxolitinib cream (50.0%) vs vehicle cream (21.9%) achieved IGA treatment success (the primary endpoint), defined as an IGA score of 0 or 1 with ≥ 2-grade improvement from baseline (odds ratio, 4.04; P = .0129).
In the open-label extension, when all patients used the active cream as needed, the proportion achieving IGA treatment success increased to 60% among the patients originally treated with ruxolitinib cream and 60.9% among those who switched from the vehicle cream.
A similar pattern was seen with Itch NRS scores. At 16 weeks, 57.7% of those treated with the ruxolitinib cream and 19.2% of those given the vehicle cream achieved an Itch NRS score of ≥ 4 (P < .01), rising to 84.2% and 73.3%, respectively, during the open-label extension.
The time to achievement of an Itch NRS of ≥ 4 was also significantly shorter with the ruxolitinib cream than with the vehicle cream (median days, 17 vs 97; hazard ratio, 2.85; P = .0008).
In both treatment groups, Skin Pain NRS scores decreased by a mean of 3.0 with ruxolitinib cream and 1.3 with the vehicle cream at week 16. By the end of the open-label extension, scores dropped by 4.3 among those who continued on active treatment and by 3.5 among those who switched from vehicle to topical ruxolitinib.
There were few treatment-emergent adverse events, with just three ruxolitinib patients affected during the randomized phase of the trial. There was one grade ≥ 3 event considered unrelated to the study drug, and no serious treatment-emergent adverse events were reported.
The most common adverse events during the randomized period were nasopharyngitis, hypertension, and contusion, all experienced by fewer than 10% of patients, whereas sinusitis, increased blood cholesterol levels, and increased blood creatine phosphokinase were most common in the open-label extension, experienced by no more than 5% of patients.
In the interview, Friedman commented that “these data provide hope that one day soon, there will be an FDA-approved, effective, and well-tolerated approach for this condition, validating the patient and supporting the dermatologist with an evidence-based option.”
The study was funded by Incyte. Mangold declared relationships with Argenx, Boehringer Ingelheim, Bristol-Myers Squibb, Clarivate, Incyte Corporation, Janssen, Nuvig Therapeutics, Pfizer, Regeneron Pharmaceuticals, Soligenix, Tourmaline Bio, AbbVie, Corbus, Eli Lilly, Kyowa, Merck, miRagen Therapeutics, Palvella Therapeutics, Priovant Therapeutics, and Adelphi Values. Friedman declared a relationship with Incyte, but it is not related to this topic.
A version of this article first appeared on Medscape.com.
both when given twice daily and as needed, according to data from a phase 2 trial.
The research, presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress, involved 64 patients older than 18 years. Ruxolitinib cream (Opzelura) is a topical formulation of a Janus kinase (JAK)1/JAK2 inhibitor, approved by the Food and Drug Administration (FDA) for treating mild to moderate atopic dermatitis and for nonsegmental vitiligo in adults and children aged 12 years or older.
Ruxolitinib cream twice daily resulted in “significant improvements in cutaneous lichen planus disease severity vs vehicle” after 16 weeks of treatment, said the study presenter, Aaron R. Mangold, MD, a dermatologist at Mayo Clinic, Scottsdale, Arizona.
Further improvements were seen during another 16 weeks of additional open-label, as-needed application, he added, and the topical treatment was “generally well tolerated.”
Consequently, “ruxolitinib cream represents a promising potential treatment for cutaneous lichen planus,” Mangold concluded.
Asked to comment on the results, Adam Friedman, MD, Professor and Chair of Dermatology, George Washington University, Washington, DC, who was not involved with the study, said that in keeping with the characterization of lichen planus using the four Ps — purple, polygonal, pruritic, papules — it is “Pretty common, Predictably disabling and disfiguring, and Passed over again and again in the drug development world.”
He said in an interview that this chronic inflammatory skin condition, which affects roughly 2% of the population, also “lacks consensus on work-up and management, likely in part owing to the absence of sizable clinical trial data.”
A recent survey conducted at a meeting indicated that dermatologists “heavily lean on topical therapies for the management of all severity levels,” noted Friedman, one of the survey authors. “Therefore, the phase 2 data presented at EADV is a welcome addition to the mix.”
Phase 2 Study Results
At the meeting, Mangold said that a previous proof-of-concept single-arm study in 12 patients suggested that topical ruxolitinib was highly effective in treating cutaneous lichen planus.
The current phase 2 trial enrolled 64 patients with predominantly cutaneous disease who had an Investigator’s Global Assessment (IGA) score of 3 or 4 and an Itch Numeric Rating Scale (NRS) score of ≥ 4. Their median age was 57 years, and 71.9% were women. Nearly 63% were White, 28.1% were Black, and 6.3% were Asian. The median duration of disease was 4.9 years, and 90.6% had received prior treatment for their lichen planus.
They were randomized to receive 1.5% ruxolitinib cream or a vehicle cream twice daily for 16 weeks, and following a primary endpoint assessment, they were transferred to an open-label extension period, during which they used ruxolitinib cream as needed for another 16 weeks. There was an additional 30-day safety follow-up period.
At week 16, significantly more patients treated with the ruxolitinib cream (50.0%) vs vehicle cream (21.9%) achieved IGA treatment success (the primary endpoint), defined as an IGA score of 0 or 1 with ≥ 2-grade improvement from baseline (odds ratio, 4.04; P = .0129).
In the open-label extension, when all patients used the active cream as needed, the proportion achieving IGA treatment success increased to 60% among the patients originally treated with ruxolitinib cream and 60.9% among those who switched from the vehicle cream.
A similar pattern was seen with Itch NRS scores. At 16 weeks, 57.7% of those treated with the ruxolitinib cream and 19.2% of those given the vehicle cream achieved an Itch NRS score of ≥ 4 (P < .01), rising to 84.2% and 73.3%, respectively, during the open-label extension.
The time to achievement of an Itch NRS of ≥ 4 was also significantly shorter with the ruxolitinib cream than with the vehicle cream (median days, 17 vs 97; hazard ratio, 2.85; P = .0008).
In both treatment groups, Skin Pain NRS scores decreased by a mean of 3.0 with ruxolitinib cream and 1.3 with the vehicle cream at week 16. By the end of the open-label extension, scores dropped by 4.3 among those who continued on active treatment and by 3.5 among those who switched from vehicle to topical ruxolitinib.
There were few treatment-emergent adverse events, with just three ruxolitinib patients affected during the randomized phase of the trial. There was one grade ≥ 3 event considered unrelated to the study drug, and no serious treatment-emergent adverse events were reported.
The most common adverse events during the randomized period were nasopharyngitis, hypertension, and contusion, all experienced by fewer than 10% of patients, whereas sinusitis, increased blood cholesterol levels, and increased blood creatine phosphokinase were most common in the open-label extension, experienced by no more than 5% of patients.
In the interview, Friedman commented that “these data provide hope that one day soon, there will be an FDA-approved, effective, and well-tolerated approach for this condition, validating the patient and supporting the dermatologist with an evidence-based option.”
The study was funded by Incyte. Mangold declared relationships with Argenx, Boehringer Ingelheim, Bristol-Myers Squibb, Clarivate, Incyte Corporation, Janssen, Nuvig Therapeutics, Pfizer, Regeneron Pharmaceuticals, Soligenix, Tourmaline Bio, AbbVie, Corbus, Eli Lilly, Kyowa, Merck, miRagen Therapeutics, Palvella Therapeutics, Priovant Therapeutics, and Adelphi Values. Friedman declared a relationship with Incyte, but it is not related to this topic.
A version of this article first appeared on Medscape.com.
both when given twice daily and as needed, according to data from a phase 2 trial.
The research, presented at the European Academy of Dermatology and Venereology (EADV) 2024 Congress, involved 64 patients older than 18 years. Ruxolitinib cream (Opzelura) is a topical formulation of a Janus kinase (JAK)1/JAK2 inhibitor, approved by the Food and Drug Administration (FDA) for treating mild to moderate atopic dermatitis and for nonsegmental vitiligo in adults and children aged 12 years or older.
Ruxolitinib cream twice daily resulted in “significant improvements in cutaneous lichen planus disease severity vs vehicle” after 16 weeks of treatment, said the study presenter, Aaron R. Mangold, MD, a dermatologist at Mayo Clinic, Scottsdale, Arizona.
Further improvements were seen during another 16 weeks of additional open-label, as-needed application, he added, and the topical treatment was “generally well tolerated.”
Consequently, “ruxolitinib cream represents a promising potential treatment for cutaneous lichen planus,” Mangold concluded.
Asked to comment on the results, Adam Friedman, MD, Professor and Chair of Dermatology, George Washington University, Washington, DC, who was not involved with the study, said that in keeping with the characterization of lichen planus using the four Ps — purple, polygonal, pruritic, papules — it is “Pretty common, Predictably disabling and disfiguring, and Passed over again and again in the drug development world.”
He said in an interview that this chronic inflammatory skin condition, which affects roughly 2% of the population, also “lacks consensus on work-up and management, likely in part owing to the absence of sizable clinical trial data.”
A recent survey conducted at a meeting indicated that dermatologists “heavily lean on topical therapies for the management of all severity levels,” noted Friedman, one of the survey authors. “Therefore, the phase 2 data presented at EADV is a welcome addition to the mix.”
Phase 2 Study Results
At the meeting, Mangold said that a previous proof-of-concept single-arm study in 12 patients suggested that topical ruxolitinib was highly effective in treating cutaneous lichen planus.
The current phase 2 trial enrolled 64 patients with predominantly cutaneous disease who had an Investigator’s Global Assessment (IGA) score of 3 or 4 and an Itch Numeric Rating Scale (NRS) score of ≥ 4. Their median age was 57 years, and 71.9% were women. Nearly 63% were White, 28.1% were Black, and 6.3% were Asian. The median duration of disease was 4.9 years, and 90.6% had received prior treatment for their lichen planus.
They were randomized to receive 1.5% ruxolitinib cream or a vehicle cream twice daily for 16 weeks, and following a primary endpoint assessment, they were transferred to an open-label extension period, during which they used ruxolitinib cream as needed for another 16 weeks. There was an additional 30-day safety follow-up period.
At week 16, significantly more patients treated with the ruxolitinib cream (50.0%) vs vehicle cream (21.9%) achieved IGA treatment success (the primary endpoint), defined as an IGA score of 0 or 1 with ≥ 2-grade improvement from baseline (odds ratio, 4.04; P = .0129).
In the open-label extension, when all patients used the active cream as needed, the proportion achieving IGA treatment success increased to 60% among the patients originally treated with ruxolitinib cream and 60.9% among those who switched from the vehicle cream.
A similar pattern was seen with Itch NRS scores. At 16 weeks, 57.7% of those treated with the ruxolitinib cream and 19.2% of those given the vehicle cream achieved an Itch NRS score of ≥ 4 (P < .01), rising to 84.2% and 73.3%, respectively, during the open-label extension.
The time to achievement of an Itch NRS of ≥ 4 was also significantly shorter with the ruxolitinib cream than with the vehicle cream (median days, 17 vs 97; hazard ratio, 2.85; P = .0008).
In both treatment groups, Skin Pain NRS scores decreased by a mean of 3.0 with ruxolitinib cream and 1.3 with the vehicle cream at week 16. By the end of the open-label extension, scores dropped by 4.3 among those who continued on active treatment and by 3.5 among those who switched from vehicle to topical ruxolitinib.
There were few treatment-emergent adverse events, with just three ruxolitinib patients affected during the randomized phase of the trial. There was one grade ≥ 3 event considered unrelated to the study drug, and no serious treatment-emergent adverse events were reported.
The most common adverse events during the randomized period were nasopharyngitis, hypertension, and contusion, all experienced by fewer than 10% of patients, whereas sinusitis, increased blood cholesterol levels, and increased blood creatine phosphokinase were most common in the open-label extension, experienced by no more than 5% of patients.
In the interview, Friedman commented that “these data provide hope that one day soon, there will be an FDA-approved, effective, and well-tolerated approach for this condition, validating the patient and supporting the dermatologist with an evidence-based option.”
The study was funded by Incyte. Mangold declared relationships with Argenx, Boehringer Ingelheim, Bristol-Myers Squibb, Clarivate, Incyte Corporation, Janssen, Nuvig Therapeutics, Pfizer, Regeneron Pharmaceuticals, Soligenix, Tourmaline Bio, AbbVie, Corbus, Eli Lilly, Kyowa, Merck, miRagen Therapeutics, Palvella Therapeutics, Priovant Therapeutics, and Adelphi Values. Friedman declared a relationship with Incyte, but it is not related to this topic.
A version of this article first appeared on Medscape.com.
FROM EADV 2024
Alopecia Induced by Poly-L-Lactic Acid Injection
Cosmetic procedures carry inherent risks of adverse events. Transient and permanent alopecia are rare complications of these procedures. Although they have not been fully elucidated, several pathologic mechanisms for hair loss following cosmetic procedures have been proposed, including extravascular compression (a phenomenon that has been well documented in bedridden patients) as well as intravascular occlusion leading to inflammation and necrosis, which has been associated with hyaluronic acid (HA) fillers.¹ Cases of alopecia also have been reported following mesotherapy and calcium hydroxyapatite, deoxycholic acid, and botulinum toxin injections.² We report a case of alopecia resulting from poly-L-lactic acid (PLLA) injection in a 35-year-old woman with the intent to raise awareness of this rare adverse event.
Case Report
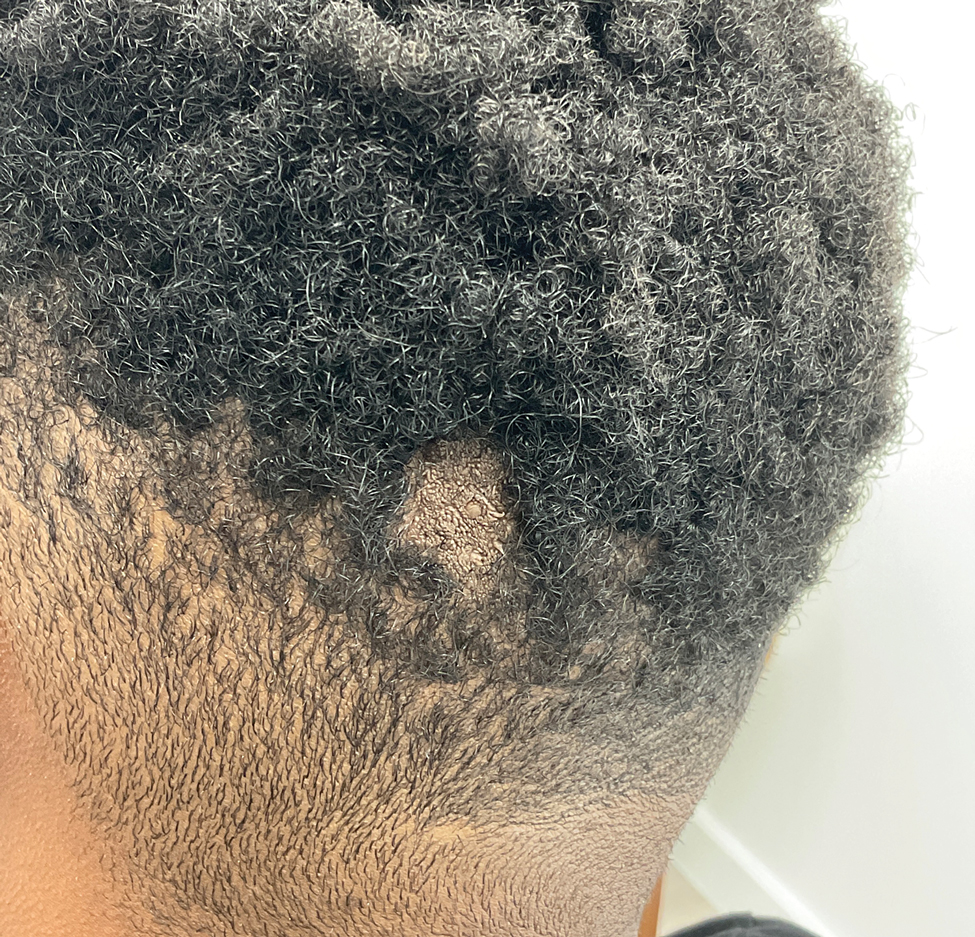
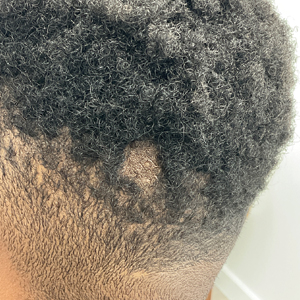
A healthy 35-year-old woman received aesthetic PLLA injections on the face and frontal hairline performed by an outside dermatologist using the vector technique. During the procedure, the patient experienced intense itchiness at the right temporal artery vascular territory and reported a substantial headache the next day. She also presented with erythema and edema of the frontal and right parietal scalp with a well-delimited livedoid vascular area along the temporal artery territory on the right side of the head 1 day after the procedure (Figure 1). These signs were reported to the outside dermatologist who performed the procedure, but they were not assumed to be adverse events at that time.
The condition persisted for 4 days followed by the development of an irregular 3×2-cm patch of alopecia on the right parietal scalp. A 3-day course of self-administered oral prednisolone 0.2 mg/kg/d was prescribed.
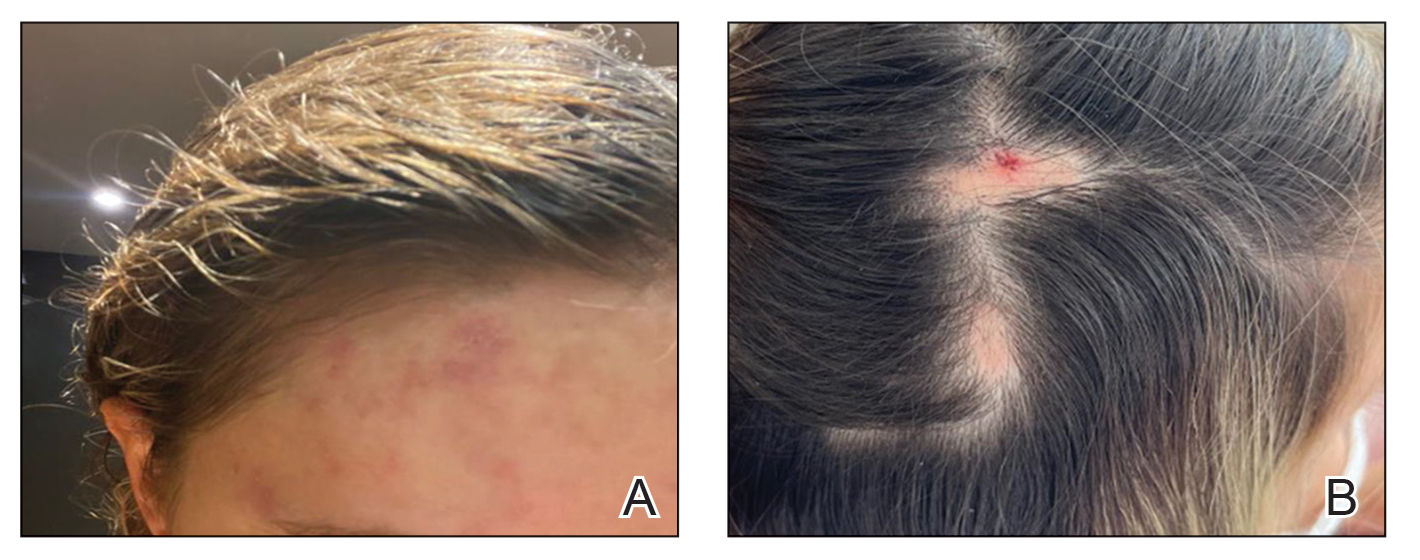
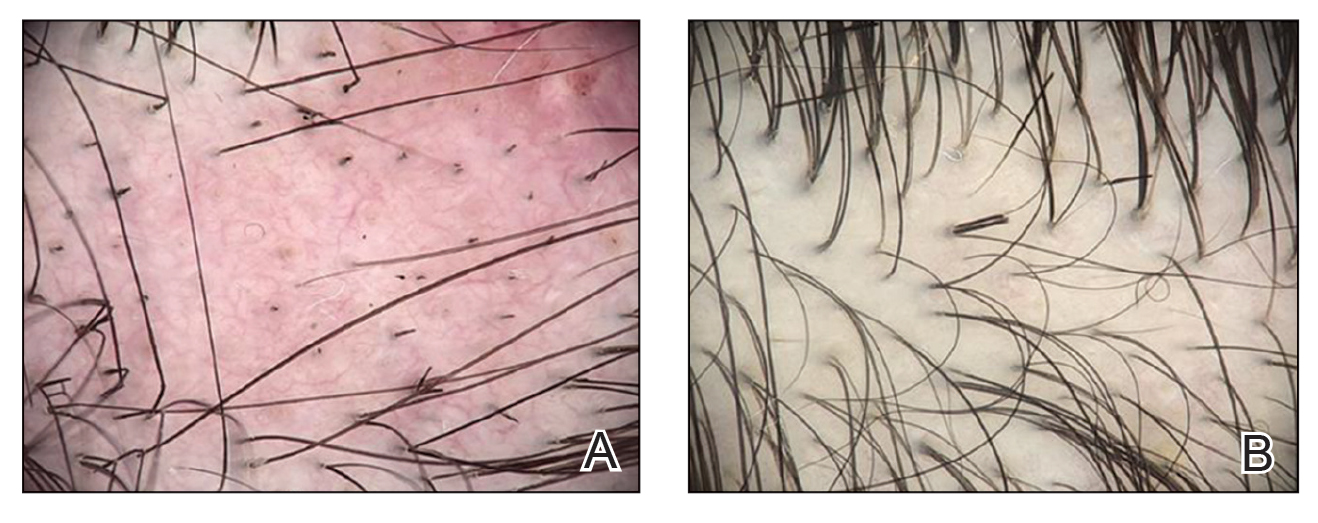
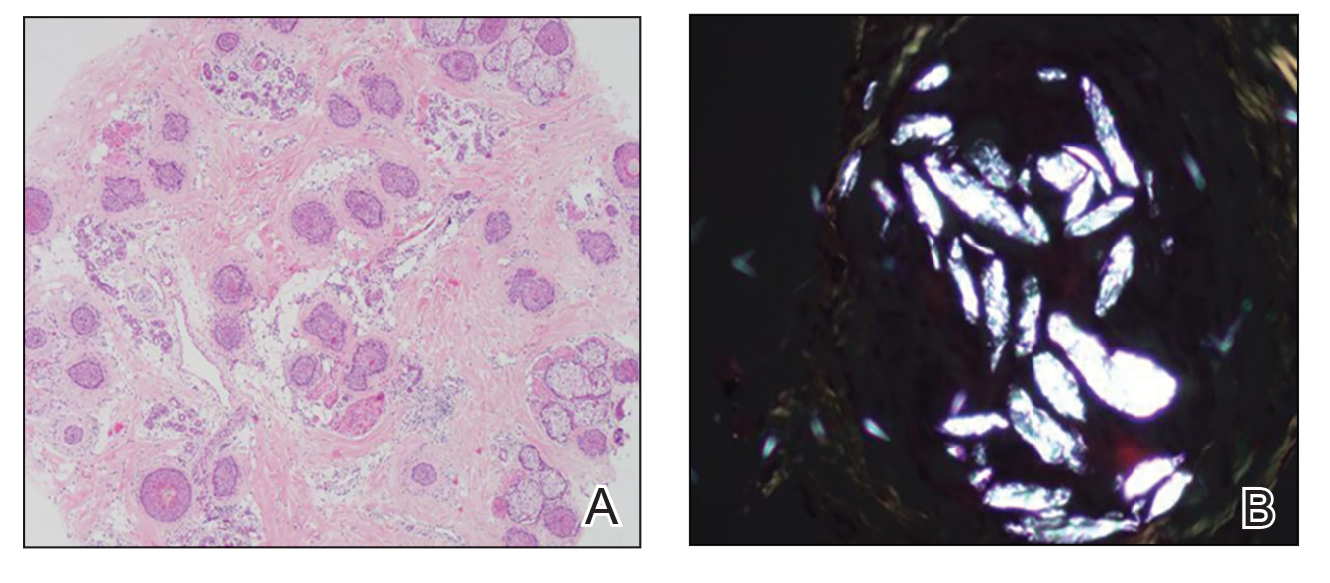
Twenty-seven days after the procedure, the patient presented to our trichology clinic for evaluation of a single patch of nonscarring alopecia on the right parietal scalp. Trichoscopy showed multiple yellow and black dots, broken hairs, pigment deposits, and an erythematous background mainly composed of linear telangiectatic vessels (Figure 2). Histopathologic analysis revealed a lymphocytic inflammatory infiltrate surrounding the follicular units that was compatible with an alopecia areata–like pattern as well as PLLA deposits in the subcutaneous tissue forming foreign body granulomas (Figure 3). The diagnosis of PLLA-induced alopecia was made based on the detection of PLLA at the biopsy site within the patchy alopecia.
Intralesional triamcinolone acetonide 5 mg/mL was administered at 1-cm intervals in the subdermal space (0.1 mL/puncture site). After 14 days, the patient developed an additional patch of alopecia in the same vascular territory as the right temporal artery, positioned just beneath the initial patch, with similar trichoscopy findings. The patches were treated with intralesional triamcinolone acetonide for 3 additional sessions, administered every 4 weeks. Long-term monitoring of the patient revealed regrowth with comparable hair count to the unaffected contralateral scalp, indicative of a nonscarring alopecia.
Comment
Poly-L-lactic acid is a biostimulator synthesized from the α-hydroxy acid family in 1954 that has been safely used in suture materials, resorbable plates, and orthopedic screws.4 Alopecia has been reported as a systemic allergic reaction to biodegradable screws following an orthopedic procedure.5 Prior reports of embolization and retinal ischemia with PLLA have raised concerns regarding its occlusive potential.6-9
Approved by the US Food and Drug Administration in 2004 for soft tissue restoration in HIV-related lipoatrophy, PLLA was expanded to cosmetic applications in 2009. As previously reported with HA fillers, we hypothesize that extravascular compression resulting from the placement of the filler material (due to the volume injected in the scalp area) contributes to the development of alopecia plus PLLA embolism–induced ischemic alopecia in the affected areas.10 In our case, the diagnosis of PLLA-induced alopecia was confirmed based on the finding of the filler material in the subcutaneous tissue on histopathology, probably due to embolization. Moreover, trichoscopic findings were all similar to those described after HA embolization.11 The features found in our patient due to the PLLA local reaction were similar to those seen in other conditions such as alopecia areata, pressure alopecia, and chemotherapy-induced alopecia; therefore, histopathology confirmation is mandatory in cases of hair loss associated with PLLA.
The emergence of a secondary patch of alopecia prompts consideration of an intrinsic late inflammatory propensity of PLLA. Immune cells recognize PLLA as a foreign body, and subclinical inflammatory foreign body reactions can cause PLLA-induced collagen synthesis.12 This phenomenon underscores the need for further investigation into the immunologic implications of PLLA in alopecia pathogenesis.
The angiogenic properties of the anagen phase require an adequate blood supply for effective hair growth; therefore, the lack of blood and nutrient supply to the hair bulb triggers miniaturization, a possible explanation for the hair thinning found in the alopecic patch.13
Conclusion
Alopecia as an adverse effect of cosmetic procedures can be distressing for patients, even when reversible. A detailed understanding of scalp anatomy is critical for satisfactory outcomes with aesthetic procedures. Physicians must pay attention to the amount and area of material injected in order to avoid possible mechanisms of ischemia—embolization and/or extravascular compression—especially in highly vascularized areas.
We present a rare report of alopecia as an adverse event of PLLA injection. Dermatologists must be aware of this rare condition, and trichoscopy combined with histopathologic analysis are encouraged for early recognition and proper management.
- Issa NT, Kaiser M, Martinez-Velasco A, et al. Alopecia after cosmetic injection procedures: a review. Dermatol Surg. 2022;48:855-861.
- Alopecia with foreign body granulomas induced by Radiesse injection: a case report. J Cosmet Laser Ther. 2018;20:462-464.
- Munia C, Parada M, de Alvarenga Morais MH. Changes in facial morphology using poly-L-lactic acid application according to vector technique: a case series. J Clin Aesthet Dermatol. 2022;15:38-42.
- Attenello NH, Maas CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31:29-34.
- Mastrokalos DS, Paessler HH. Allergic reaction to biodegradable interference poly-L-lactic acid screws after anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft. Arthroscopy. 2008;24:732-733.
- Wu CW, Wu HJ. Retinal artery occlusion following cosmetic injection of poly-L-lactic acid. Taiwan J Ophthalmol. 2021;11:317-320.
- Yuan JT, Chang TW, Yu SS, et al. Mental artery occlusion from poly-L-lactic acid injection at the lateral chin. Dermatol Surg. 2017;43:1402-1405.
- Ragam A, Agemy SA, Dave SB, et al. Ipsilateral ophthalmic and cerebral infarctions after cosmetic polylactic acid injection into the forehead. J Neuroophthalmol. 2017;37:77-80.
- Witmanowski H, Błochowiak K. Another face of dermal fillers. Postepy Dermatol Alergol. 2020;37:651-659.
- Yang Q, Qiu L, Yi C, et al. Reversible alopecia with localized scalp necrosis after accidental embolization of the parietal artery with hyaluronic acid. Aesthetic Plast Surg. 2017;41:695-699.
- Asz-Sigall D, Iñigo-Gomez K, Ortega-Springall MF, et al. Alopecia secondary to hyaluronic acid embolization: trichoscopic findings. Skin Appendage Disord. 2019;5:396-400.
- Oh S, Lee JH, Kim HM, et al. Poly-L-lactic acid fillers improved dermal collagen synthesis by modulating M2 macrophage polarization in aged animal skin. Cells. 2023;12:1320. doi:10.3390/cells12091320
- Natarelli N, Gahoonia N, Sivamani RK. Integrative and mechanistic approach to the hair growth cycle and hair loss. J Clin Med. 2023;12:893.2. Liu RF, Kuo TT, Chao YY, et al.
Cosmetic procedures carry inherent risks of adverse events. Transient and permanent alopecia are rare complications of these procedures. Although they have not been fully elucidated, several pathologic mechanisms for hair loss following cosmetic procedures have been proposed, including extravascular compression (a phenomenon that has been well documented in bedridden patients) as well as intravascular occlusion leading to inflammation and necrosis, which has been associated with hyaluronic acid (HA) fillers.¹ Cases of alopecia also have been reported following mesotherapy and calcium hydroxyapatite, deoxycholic acid, and botulinum toxin injections.² We report a case of alopecia resulting from poly-L-lactic acid (PLLA) injection in a 35-year-old woman with the intent to raise awareness of this rare adverse event.
Case Report
A healthy 35-year-old woman received aesthetic PLLA injections on the face and frontal hairline performed by an outside dermatologist using the vector technique. During the procedure, the patient experienced intense itchiness at the right temporal artery vascular territory and reported a substantial headache the next day. She also presented with erythema and edema of the frontal and right parietal scalp with a well-delimited livedoid vascular area along the temporal artery territory on the right side of the head 1 day after the procedure (Figure 1). These signs were reported to the outside dermatologist who performed the procedure, but they were not assumed to be adverse events at that time.

The condition persisted for 4 days followed by the development of an irregular 3×2-cm patch of alopecia on the right parietal scalp. A 3-day course of self-administered oral prednisolone 0.2 mg/kg/d was prescribed.
Twenty-seven days after the procedure, the patient presented to our trichology clinic for evaluation of a single patch of nonscarring alopecia on the right parietal scalp. Trichoscopy showed multiple yellow and black dots, broken hairs, pigment deposits, and an erythematous background mainly composed of linear telangiectatic vessels (Figure 2). Histopathologic analysis revealed a lymphocytic inflammatory infiltrate surrounding the follicular units that was compatible with an alopecia areata–like pattern as well as PLLA deposits in the subcutaneous tissue forming foreign body granulomas (Figure 3). The diagnosis of PLLA-induced alopecia was made based on the detection of PLLA at the biopsy site within the patchy alopecia.


Intralesional triamcinolone acetonide 5 mg/mL was administered at 1-cm intervals in the subdermal space (0.1 mL/puncture site). After 14 days, the patient developed an additional patch of alopecia in the same vascular territory as the right temporal artery, positioned just beneath the initial patch, with similar trichoscopy findings. The patches were treated with intralesional triamcinolone acetonide for 3 additional sessions, administered every 4 weeks. Long-term monitoring of the patient revealed regrowth with comparable hair count to the unaffected contralateral scalp, indicative of a nonscarring alopecia.
Comment
Poly-L-lactic acid is a biostimulator synthesized from the α-hydroxy acid family in 1954 that has been safely used in suture materials, resorbable plates, and orthopedic screws.4 Alopecia has been reported as a systemic allergic reaction to biodegradable screws following an orthopedic procedure.5 Prior reports of embolization and retinal ischemia with PLLA have raised concerns regarding its occlusive potential.6-9
Approved by the US Food and Drug Administration in 2004 for soft tissue restoration in HIV-related lipoatrophy, PLLA was expanded to cosmetic applications in 2009. As previously reported with HA fillers, we hypothesize that extravascular compression resulting from the placement of the filler material (due to the volume injected in the scalp area) contributes to the development of alopecia plus PLLA embolism–induced ischemic alopecia in the affected areas.10 In our case, the diagnosis of PLLA-induced alopecia was confirmed based on the finding of the filler material in the subcutaneous tissue on histopathology, probably due to embolization. Moreover, trichoscopic findings were all similar to those described after HA embolization.11 The features found in our patient due to the PLLA local reaction were similar to those seen in other conditions such as alopecia areata, pressure alopecia, and chemotherapy-induced alopecia; therefore, histopathology confirmation is mandatory in cases of hair loss associated with PLLA.
The emergence of a secondary patch of alopecia prompts consideration of an intrinsic late inflammatory propensity of PLLA. Immune cells recognize PLLA as a foreign body, and subclinical inflammatory foreign body reactions can cause PLLA-induced collagen synthesis.12 This phenomenon underscores the need for further investigation into the immunologic implications of PLLA in alopecia pathogenesis.
The angiogenic properties of the anagen phase require an adequate blood supply for effective hair growth; therefore, the lack of blood and nutrient supply to the hair bulb triggers miniaturization, a possible explanation for the hair thinning found in the alopecic patch.13
Conclusion
Alopecia as an adverse effect of cosmetic procedures can be distressing for patients, even when reversible. A detailed understanding of scalp anatomy is critical for satisfactory outcomes with aesthetic procedures. Physicians must pay attention to the amount and area of material injected in order to avoid possible mechanisms of ischemia—embolization and/or extravascular compression—especially in highly vascularized areas.
We present a rare report of alopecia as an adverse event of PLLA injection. Dermatologists must be aware of this rare condition, and trichoscopy combined with histopathologic analysis are encouraged for early recognition and proper management.
Cosmetic procedures carry inherent risks of adverse events. Transient and permanent alopecia are rare complications of these procedures. Although they have not been fully elucidated, several pathologic mechanisms for hair loss following cosmetic procedures have been proposed, including extravascular compression (a phenomenon that has been well documented in bedridden patients) as well as intravascular occlusion leading to inflammation and necrosis, which has been associated with hyaluronic acid (HA) fillers.¹ Cases of alopecia also have been reported following mesotherapy and calcium hydroxyapatite, deoxycholic acid, and botulinum toxin injections.² We report a case of alopecia resulting from poly-L-lactic acid (PLLA) injection in a 35-year-old woman with the intent to raise awareness of this rare adverse event.
Case Report
A healthy 35-year-old woman received aesthetic PLLA injections on the face and frontal hairline performed by an outside dermatologist using the vector technique. During the procedure, the patient experienced intense itchiness at the right temporal artery vascular territory and reported a substantial headache the next day. She also presented with erythema and edema of the frontal and right parietal scalp with a well-delimited livedoid vascular area along the temporal artery territory on the right side of the head 1 day after the procedure (Figure 1). These signs were reported to the outside dermatologist who performed the procedure, but they were not assumed to be adverse events at that time.

The condition persisted for 4 days followed by the development of an irregular 3×2-cm patch of alopecia on the right parietal scalp. A 3-day course of self-administered oral prednisolone 0.2 mg/kg/d was prescribed.
Twenty-seven days after the procedure, the patient presented to our trichology clinic for evaluation of a single patch of nonscarring alopecia on the right parietal scalp. Trichoscopy showed multiple yellow and black dots, broken hairs, pigment deposits, and an erythematous background mainly composed of linear telangiectatic vessels (Figure 2). Histopathologic analysis revealed a lymphocytic inflammatory infiltrate surrounding the follicular units that was compatible with an alopecia areata–like pattern as well as PLLA deposits in the subcutaneous tissue forming foreign body granulomas (Figure 3). The diagnosis of PLLA-induced alopecia was made based on the detection of PLLA at the biopsy site within the patchy alopecia.


Intralesional triamcinolone acetonide 5 mg/mL was administered at 1-cm intervals in the subdermal space (0.1 mL/puncture site). After 14 days, the patient developed an additional patch of alopecia in the same vascular territory as the right temporal artery, positioned just beneath the initial patch, with similar trichoscopy findings. The patches were treated with intralesional triamcinolone acetonide for 3 additional sessions, administered every 4 weeks. Long-term monitoring of the patient revealed regrowth with comparable hair count to the unaffected contralateral scalp, indicative of a nonscarring alopecia.
Comment
Poly-L-lactic acid is a biostimulator synthesized from the α-hydroxy acid family in 1954 that has been safely used in suture materials, resorbable plates, and orthopedic screws.4 Alopecia has been reported as a systemic allergic reaction to biodegradable screws following an orthopedic procedure.5 Prior reports of embolization and retinal ischemia with PLLA have raised concerns regarding its occlusive potential.6-9
Approved by the US Food and Drug Administration in 2004 for soft tissue restoration in HIV-related lipoatrophy, PLLA was expanded to cosmetic applications in 2009. As previously reported with HA fillers, we hypothesize that extravascular compression resulting from the placement of the filler material (due to the volume injected in the scalp area) contributes to the development of alopecia plus PLLA embolism–induced ischemic alopecia in the affected areas.10 In our case, the diagnosis of PLLA-induced alopecia was confirmed based on the finding of the filler material in the subcutaneous tissue on histopathology, probably due to embolization. Moreover, trichoscopic findings were all similar to those described after HA embolization.11 The features found in our patient due to the PLLA local reaction were similar to those seen in other conditions such as alopecia areata, pressure alopecia, and chemotherapy-induced alopecia; therefore, histopathology confirmation is mandatory in cases of hair loss associated with PLLA.
The emergence of a secondary patch of alopecia prompts consideration of an intrinsic late inflammatory propensity of PLLA. Immune cells recognize PLLA as a foreign body, and subclinical inflammatory foreign body reactions can cause PLLA-induced collagen synthesis.12 This phenomenon underscores the need for further investigation into the immunologic implications of PLLA in alopecia pathogenesis.
The angiogenic properties of the anagen phase require an adequate blood supply for effective hair growth; therefore, the lack of blood and nutrient supply to the hair bulb triggers miniaturization, a possible explanation for the hair thinning found in the alopecic patch.13
Conclusion
Alopecia as an adverse effect of cosmetic procedures can be distressing for patients, even when reversible. A detailed understanding of scalp anatomy is critical for satisfactory outcomes with aesthetic procedures. Physicians must pay attention to the amount and area of material injected in order to avoid possible mechanisms of ischemia—embolization and/or extravascular compression—especially in highly vascularized areas.
We present a rare report of alopecia as an adverse event of PLLA injection. Dermatologists must be aware of this rare condition, and trichoscopy combined with histopathologic analysis are encouraged for early recognition and proper management.
- Issa NT, Kaiser M, Martinez-Velasco A, et al. Alopecia after cosmetic injection procedures: a review. Dermatol Surg. 2022;48:855-861.
- Alopecia with foreign body granulomas induced by Radiesse injection: a case report. J Cosmet Laser Ther. 2018;20:462-464.
- Munia C, Parada M, de Alvarenga Morais MH. Changes in facial morphology using poly-L-lactic acid application according to vector technique: a case series. J Clin Aesthet Dermatol. 2022;15:38-42.
- Attenello NH, Maas CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31:29-34.
- Mastrokalos DS, Paessler HH. Allergic reaction to biodegradable interference poly-L-lactic acid screws after anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft. Arthroscopy. 2008;24:732-733.
- Wu CW, Wu HJ. Retinal artery occlusion following cosmetic injection of poly-L-lactic acid. Taiwan J Ophthalmol. 2021;11:317-320.
- Yuan JT, Chang TW, Yu SS, et al. Mental artery occlusion from poly-L-lactic acid injection at the lateral chin. Dermatol Surg. 2017;43:1402-1405.
- Ragam A, Agemy SA, Dave SB, et al. Ipsilateral ophthalmic and cerebral infarctions after cosmetic polylactic acid injection into the forehead. J Neuroophthalmol. 2017;37:77-80.
- Witmanowski H, Błochowiak K. Another face of dermal fillers. Postepy Dermatol Alergol. 2020;37:651-659.
- Yang Q, Qiu L, Yi C, et al. Reversible alopecia with localized scalp necrosis after accidental embolization of the parietal artery with hyaluronic acid. Aesthetic Plast Surg. 2017;41:695-699.
- Asz-Sigall D, Iñigo-Gomez K, Ortega-Springall MF, et al. Alopecia secondary to hyaluronic acid embolization: trichoscopic findings. Skin Appendage Disord. 2019;5:396-400.
- Oh S, Lee JH, Kim HM, et al. Poly-L-lactic acid fillers improved dermal collagen synthesis by modulating M2 macrophage polarization in aged animal skin. Cells. 2023;12:1320. doi:10.3390/cells12091320
- Natarelli N, Gahoonia N, Sivamani RK. Integrative and mechanistic approach to the hair growth cycle and hair loss. J Clin Med. 2023;12:893.2. Liu RF, Kuo TT, Chao YY, et al.
- Issa NT, Kaiser M, Martinez-Velasco A, et al. Alopecia after cosmetic injection procedures: a review. Dermatol Surg. 2022;48:855-861.
- Alopecia with foreign body granulomas induced by Radiesse injection: a case report. J Cosmet Laser Ther. 2018;20:462-464.
- Munia C, Parada M, de Alvarenga Morais MH. Changes in facial morphology using poly-L-lactic acid application according to vector technique: a case series. J Clin Aesthet Dermatol. 2022;15:38-42.
- Attenello NH, Maas CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31:29-34.
- Mastrokalos DS, Paessler HH. Allergic reaction to biodegradable interference poly-L-lactic acid screws after anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft. Arthroscopy. 2008;24:732-733.
- Wu CW, Wu HJ. Retinal artery occlusion following cosmetic injection of poly-L-lactic acid. Taiwan J Ophthalmol. 2021;11:317-320.
- Yuan JT, Chang TW, Yu SS, et al. Mental artery occlusion from poly-L-lactic acid injection at the lateral chin. Dermatol Surg. 2017;43:1402-1405.
- Ragam A, Agemy SA, Dave SB, et al. Ipsilateral ophthalmic and cerebral infarctions after cosmetic polylactic acid injection into the forehead. J Neuroophthalmol. 2017;37:77-80.
- Witmanowski H, Błochowiak K. Another face of dermal fillers. Postepy Dermatol Alergol. 2020;37:651-659.
- Yang Q, Qiu L, Yi C, et al. Reversible alopecia with localized scalp necrosis after accidental embolization of the parietal artery with hyaluronic acid. Aesthetic Plast Surg. 2017;41:695-699.
- Asz-Sigall D, Iñigo-Gomez K, Ortega-Springall MF, et al. Alopecia secondary to hyaluronic acid embolization: trichoscopic findings. Skin Appendage Disord. 2019;5:396-400.
- Oh S, Lee JH, Kim HM, et al. Poly-L-lactic acid fillers improved dermal collagen synthesis by modulating M2 macrophage polarization in aged animal skin. Cells. 2023;12:1320. doi:10.3390/cells12091320
- Natarelli N, Gahoonia N, Sivamani RK. Integrative and mechanistic approach to the hair growth cycle and hair loss. J Clin Med. 2023;12:893.2. Liu RF, Kuo TT, Chao YY, et al.
Practice Points
- Alopecia is a potential adverse event of poly-L-lactic acid (PLLA) injection, and prior reports of embolization and retinal ischemia with PLLA use raise the concern of its occlusive potential.
- The combination of extravascular compression due to the presence of the filler material in the subcutaneous tissue as well as intravascular PLLA embolism may contribute to tissue ischemia–induced alopecia in the affected areas.
- Poly-L-lactic acid also may cause a local inflammatory reaction that is alopecia areata–like, which would explain its similar trichoscopy findings.
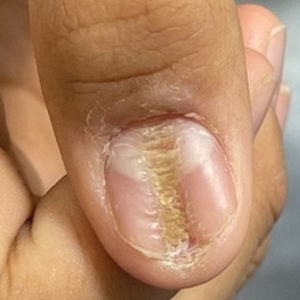
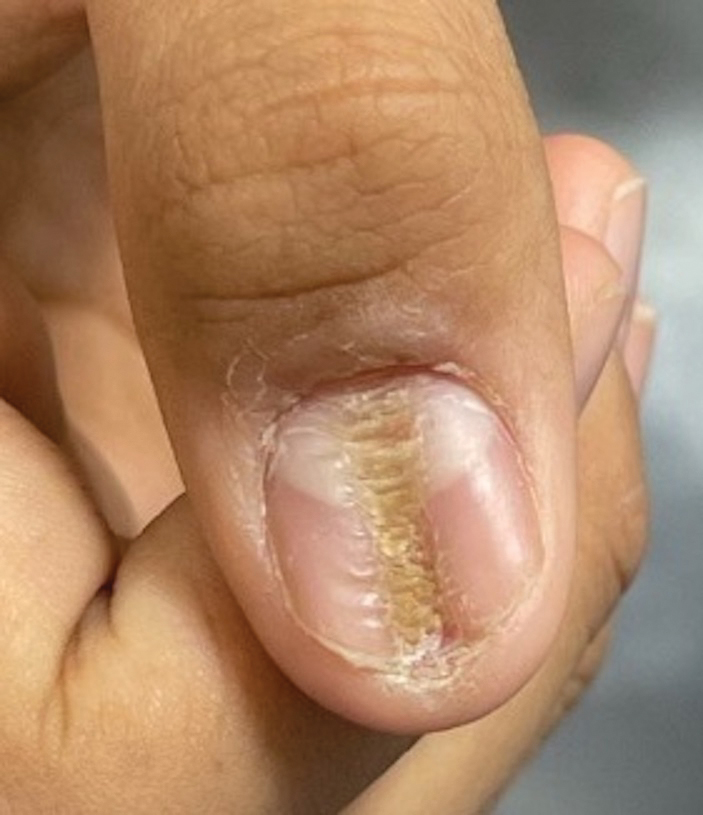
Longitudinal Depression on the Right Thumbnail
THE DIAGNOSIS: Habit-Tic Deformity
Habit-tic deformity is a cause of nail dystrophy that commonly arises in children and adults due to subconscious repetitive and self-injurious manipulation of the nail bed or cuticle, which ultimately damages the nail matrix.1,2 It can be considered a variant of onychotillomania.1
Characteristic features of habit-tic deformity include a longitudinal depression on the central nail plate with transverse ridges,1 which can be more prominent on the dominant hand.3 Patients typically note a long duration of nail deformity, often without insight into its etiology.2 Diagnosis relies on careful assessment of the clinical presentation and the patient’s history to rule out other differential diagnoses. Based on our patient’s clinical presentation and history, we excluded wart, squamous cell carcinoma, eczema, psoriasis, lichen planus, autoimmune connective tissue disease, onychomycosis, paronychia, pincer nail deformity, and Beau line as potential diagnoses. Biopsy also can be performed to exclude these diagnoses from the differential if the cause is unclear following clinical examination.
Treatment for habit-tic deformity involves identifying and addressing the underlying habit. Barrier methods such as bandages and cyanoacrylate adhesives that prevent further manipulation of the nail matrix are effective treatments for habit-tic deformity.2 A multidisciplinary approach with psychiatry may be optimal to identify underlying psychological comorbidities and break the habit through behavior interventions and medications.4 Nail dystrophy generally improves once the habit is disrupted; however, a younger age of onset may carry a worse prognosis.3 Patients should be counseled that the affected nail may never grow normally.
Our patient was advised to use fluocinonide ointment 0.05% to reduce inflammation of the proximal nail fold and to cover the thumbnail with a bandage to prevent picking. He also was counseled that the nail may show ongoing abnormal growth. Minimal improvement was noted after 6 months.
- Rieder EA, Tosti A. Onychotillomania: an underrecognized disorder. J Am Acad Dermatol. 2016;75:1245-1250.doi:10.1016/j.jaad.2016
- Ring DS. Inexpensive solution for habit-tic deformity. Arch Dermatol. 2010;146:1222-1223. doi:10.1001/archdermatol.2010.287
- Horne MI, Utzig JB, Rieder EA, et al. Alopecia areata and habit tic deformities. Skin Appendage Disord. 2018;4:323-325. doi:10.1159/000486540
- Sonthalia S, Sharma P, Kapoor J, et al. Habit tic deformity: need fora comprehensive approach. Skin Appendage Disord. 2019;5:117-118.doi:10.1159/000489320 .05.036
THE DIAGNOSIS: Habit-Tic Deformity
Habit-tic deformity is a cause of nail dystrophy that commonly arises in children and adults due to subconscious repetitive and self-injurious manipulation of the nail bed or cuticle, which ultimately damages the nail matrix.1,2 It can be considered a variant of onychotillomania.1
Characteristic features of habit-tic deformity include a longitudinal depression on the central nail plate with transverse ridges,1 which can be more prominent on the dominant hand.3 Patients typically note a long duration of nail deformity, often without insight into its etiology.2 Diagnosis relies on careful assessment of the clinical presentation and the patient’s history to rule out other differential diagnoses. Based on our patient’s clinical presentation and history, we excluded wart, squamous cell carcinoma, eczema, psoriasis, lichen planus, autoimmune connective tissue disease, onychomycosis, paronychia, pincer nail deformity, and Beau line as potential diagnoses. Biopsy also can be performed to exclude these diagnoses from the differential if the cause is unclear following clinical examination.
Treatment for habit-tic deformity involves identifying and addressing the underlying habit. Barrier methods such as bandages and cyanoacrylate adhesives that prevent further manipulation of the nail matrix are effective treatments for habit-tic deformity.2 A multidisciplinary approach with psychiatry may be optimal to identify underlying psychological comorbidities and break the habit through behavior interventions and medications.4 Nail dystrophy generally improves once the habit is disrupted; however, a younger age of onset may carry a worse prognosis.3 Patients should be counseled that the affected nail may never grow normally.
Our patient was advised to use fluocinonide ointment 0.05% to reduce inflammation of the proximal nail fold and to cover the thumbnail with a bandage to prevent picking. He also was counseled that the nail may show ongoing abnormal growth. Minimal improvement was noted after 6 months.
THE DIAGNOSIS: Habit-Tic Deformity
Habit-tic deformity is a cause of nail dystrophy that commonly arises in children and adults due to subconscious repetitive and self-injurious manipulation of the nail bed or cuticle, which ultimately damages the nail matrix.1,2 It can be considered a variant of onychotillomania.1
Characteristic features of habit-tic deformity include a longitudinal depression on the central nail plate with transverse ridges,1 which can be more prominent on the dominant hand.3 Patients typically note a long duration of nail deformity, often without insight into its etiology.2 Diagnosis relies on careful assessment of the clinical presentation and the patient’s history to rule out other differential diagnoses. Based on our patient’s clinical presentation and history, we excluded wart, squamous cell carcinoma, eczema, psoriasis, lichen planus, autoimmune connective tissue disease, onychomycosis, paronychia, pincer nail deformity, and Beau line as potential diagnoses. Biopsy also can be performed to exclude these diagnoses from the differential if the cause is unclear following clinical examination.
Treatment for habit-tic deformity involves identifying and addressing the underlying habit. Barrier methods such as bandages and cyanoacrylate adhesives that prevent further manipulation of the nail matrix are effective treatments for habit-tic deformity.2 A multidisciplinary approach with psychiatry may be optimal to identify underlying psychological comorbidities and break the habit through behavior interventions and medications.4 Nail dystrophy generally improves once the habit is disrupted; however, a younger age of onset may carry a worse prognosis.3 Patients should be counseled that the affected nail may never grow normally.
Our patient was advised to use fluocinonide ointment 0.05% to reduce inflammation of the proximal nail fold and to cover the thumbnail with a bandage to prevent picking. He also was counseled that the nail may show ongoing abnormal growth. Minimal improvement was noted after 6 months.
- Rieder EA, Tosti A. Onychotillomania: an underrecognized disorder. J Am Acad Dermatol. 2016;75:1245-1250.doi:10.1016/j.jaad.2016
- Ring DS. Inexpensive solution for habit-tic deformity. Arch Dermatol. 2010;146:1222-1223. doi:10.1001/archdermatol.2010.287
- Horne MI, Utzig JB, Rieder EA, et al. Alopecia areata and habit tic deformities. Skin Appendage Disord. 2018;4:323-325. doi:10.1159/000486540
- Sonthalia S, Sharma P, Kapoor J, et al. Habit tic deformity: need fora comprehensive approach. Skin Appendage Disord. 2019;5:117-118.doi:10.1159/000489320 .05.036
- Rieder EA, Tosti A. Onychotillomania: an underrecognized disorder. J Am Acad Dermatol. 2016;75:1245-1250.doi:10.1016/j.jaad.2016
- Ring DS. Inexpensive solution for habit-tic deformity. Arch Dermatol. 2010;146:1222-1223. doi:10.1001/archdermatol.2010.287
- Horne MI, Utzig JB, Rieder EA, et al. Alopecia areata and habit tic deformities. Skin Appendage Disord. 2018;4:323-325. doi:10.1159/000486540
- Sonthalia S, Sharma P, Kapoor J, et al. Habit tic deformity: need fora comprehensive approach. Skin Appendage Disord. 2019;5:117-118.doi:10.1159/000489320 .05.036
A healthy 13-year-old boy presented to the dermatology department with dystrophy of the right thumbnail of 3 to 4 years’ duration. A 5-mm-wide, depressed median longitudinal groove with a fir tree pattern was noted on the central nail plate. The patient noted that the groove had been gradually deepening. There was erythema, edema, and lichenification of the proximal nailfold without vascular changes, and the lunula was enlarged. No hyperkeratosis, subungual debris, erythematous nail folds, or inward curvature of the lateral aspects of the nail were noted. The patient denied any pruritus, pain, discomfort, or bleeding; he also denied any recent illness or trauma to the nail. None of the other nails were affected, and no other lesions or rashes were observed elsewhere on the body. The patient was unsure if he picked at the nail but acknowledged that he may have done so subconsciously. He had no history of eczema, psoriasis, or autoimmune connective tissue disorders.
Sea Buckthorn
A member of the Elaeagnaceae family, Hippophae rhamnoides, better known as sea buckthorn, is a high-altitude wild shrub endemic to Europe and Asia with edible fruits and a lengthy record of use in traditional Chinese medicine.1-6 Used as a health supplement and consumed in the diet throughout the world,5 sea buckthorn berries, seeds, and leaves have been used in traditional medicine to treat burns/injuries, edema, hypertension, inflammation, skin grafts, ulcers, and wounds.4,7
This hardy plant is associated with a wide range of biologic activities, including anti-atherogenic, anti-atopic dermatitis, antibacterial, anticancer, antifungal, anti-inflammatory, antimicrobial, antioxidant, anti-psoriasis, anti-sebum, anti-stress, anti-tumor, cytoprotective, hepatoprotective, immunomodulatory, neuroprotective, radioprotective, and tissue regenerative functions.4,5,8-11
Key Constituents
Functional constituents identified in sea buckthorn include alkaloids, carotenoids, flavonoids, lignans, organic acids, phenolic acids, proanthocyanidins, polyunsaturated acids (including omega-3, -6, -7, and -9), steroids, tannins, terpenoids, and volatile oils, as well as nutritional compounds such as minerals, proteins, and vitamins.4,5,11 Sea buckthorn pericarp oil contains copious amounts of saturated palmitic acid (29%-36%) and omega-7 unsaturated palmitoleic acid (36%-48%), which fosters cutaneous and mucosal epithelialization, as well as linoleic (10%-12%) and oleic (4%-6%) acids.12,6 Significant amounts of carotenoids as well as alpha‐linolenic fatty acid (38%), linoleic (36%), oleic (13%), and palmitic (7%) acids are present in sea buckthorn seed oil.6
Polysaccharides
In an expansive review on the pharmacological activities of sea buckthorn polysaccharides, Teng and colleagues reported in April 2024 that 20 diverse polysaccharides have been culled from sea buckthorn and exhibited various healthy activities, including antioxidant, anti-fatigue, anti-inflammatory, anti-obesity, anti-tumor, hepatoprotective, hypoglycemic, and immunoregulation, and regulation of intestinal flora activities.1
Proanthocyanidins and Anti-Aging
In 2023, Liu and colleagues investigated the anti–skin aging impact of sea buckthorn proanthocyanidins in D-galactose-induced aging in mice given the known free radical scavenging activity of these compounds. They found the proanthocyanidins mitigated D-galactose-induced aging and can augment the total antioxidant capacity of the body. Sea buckthorn proanthocyanidins can further attenuate the effects of skin aging by regulating the TGF-beta1/Smads pathway and MMPs/TIMP system, thus amplifying collagen I and tropoelastin content.13
A year earlier, many of the same investigators assessed the possible protective activity of sea buckthorn proanthocyanidins against cutaneous aging engendered by oxidative stress from hydrogen peroxide. The compounds amplified superoxide dismutase and glutathione antioxidant functions. The extracts also fostered collagen I production in aging human skin fibroblasts via the TGF-beta1/Smads pathway and hindered collagen I degradation by regulating the MMPs/TIMPs system, which maintained extracellular matrix integrity. Senescent cell migration was also promoted with 100 mcg/mL of sea buckthorn proanthocyanidins. The researchers concluded that this sets the stage for investigating how sea buckthorn proanthocyanidins can be incorporated in cosmetic formulations.14 In a separate study, Liu and colleagues demonstrated that sea buckthorn proanthocyanidins can attenuate oxidative damage and protect mitochondrial function.9
Acne and Barrier Functions
The extracts of H rhamnoides and Cassia fistula in a combined formulation were found to be effective in lowering skin sebum content in humans with grade I and grade II acne vulgaris in a 2014 single-blind, randomized, placebo-controlled, split-face study with two groups of 25 patients each (aged 18-37 years).15 Khan and colleagues have also reported that a sea buckthorn oil-in-water emulsion improved barrier function in human skin as tested by a tewameter and corneometer (noninvasive probes) in 13 healthy males with a mean age of 27 ± 4.8 years.16
Anti-Aging, Antioxidant, Antibacterial, Skin-Whitening Activity
Zaman and colleagues reported in 2011 that results from an in vivo study of the effects of a sea buckthorn fruit extract topical cream on stratum corneum water content and transepidermal water loss indicated that the formulation enhanced cell surface integrin expression thus facilitating collagen contraction.17
In 2012, Khan and colleagues reported amelioration in skin elasticity, thus achieving an anti-aging result, from the use of a water-in-oil–based hydroalcoholic cream loaded with fruit extract of H rhamnoides, as measured with a Cutometer.18 The previous year, some of the same researchers reported that the antioxidants and flavonoids found in a topical sea buckthorn formulation could decrease cutaneous melanin and erythema levels.
More recently, Gęgotek and colleagues found that sea buckthorn seed oil prevented redox balance and lipid metabolism disturbances in skin fibroblasts and keratinocytes caused by UVA or UVB. They suggested that such findings point to the potential of this natural agent to confer anti-inflammatory properties and photoprotection to the skin.19
In 2020, Ivanišová and colleagues investigated the antioxidant and antimicrobial activities of H rhamnoides 100% oil, 100% juice, dry berries, and tea (dry berries, leaves, and twigs). They found that all of the studied sea buckthorn products displayed high antioxidant activity (identified through DPPH radical scavenging and molybdenum reducing antioxidant power tests). Sea buckthorn juice contained the highest total content of polyphenols, flavonoids, and carotenoids. All of the tested products also exhibited substantial antibacterial activity against the tested microbes.20
Burns and Wound Healing
In a preclinical study of the effects of sea buckthorn leaf extracts on wound healing in albino rats using an excision-punch wound model in 2005, Gupta and colleagues found that twice daily topical application of the aqueous leaf extract fostered wound healing. This was indicated by higher hydroxyproline and protein levels, a diminished wound area, and lower lipid peroxide levels. The investigators suggested that sea buckthorn may facilitate wound healing at least in part because of elevated antioxidant activity in the granulation tissue.3
A year later, Wang and colleagues reported on observations of using H rhamnoides oil, a traditional Chinese herbal medicine derived from sea buckthorn fruit, as a burn treatment. In the study, 151 burn patients received an H rhamnoides oil dressing (changed every other day until wound healing) that was covered with a disinfecting dressing. The dressing reduced swelling and effusion, and alleviated pain, with patients receiving the sea buckthorn dressing experiencing greater apparent exudation reduction, pain reduction, and more rapid epithelial cell growth and wound healing than controls (treated only with Vaseline gauze). The difference between the two groups was statistically significant.21
Conclusion
Sea buckthorn has been used for hundreds if not thousands of years in traditional medical applications, including for dermatologic purposes. Emerging data appear to support the use of this dynamic plant for consideration in dermatologic applications. As is often the case, much more work is necessary in the form of randomized controlled trials to determine the effectiveness of sea buckthorn formulations as well as the most appropriate avenues of research or uses for dermatologic application of this traditionally used botanical agent.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at dermnews@mdedge.com.
References
1. Teng H et al. J Ethnopharmacol. 2024 Apr 24;324:117809. doi: 10.1016/j.jep.2024.117809.
2. Wang Z et al. Int J Biol Macromol. 2024 Apr;263(Pt 1):130206. doi: 10.1016/j.ijbiomac.2024.130206.
3. Gupta A et al. Int J Low Extrem Wounds. 2005 Jun;4(2):88-92. doi: 10.1177/1534734605277401.
4. Pundir S et al. J Ethnopharmacol. 2021 Feb 10;266:113434. doi: 10.1016/j.jep.2020.113434.
5. Ma QG et al. J Agric Food Chem. 2023 Mar 29;71(12):4769-4788. doi: 10.1021/acs.jafc.2c06916.
6. Poljšak N et al. Phytother Res. 2020 Feb;34(2):254-269. doi: 10.1002/ptr.6524. doi: 10.1002/ptr.6524.
7. Upadhyay NK et al. Evid Based Complement Alternat Med. 2011;2011:659705. doi: 10.1093/ecam/nep189.
8. Suryakumar G, Gupta A. J Ethnopharmacol. 2011 Nov 18;138(2):268-78. doi: 10.1016/j.jep.2011.09.024.
9. Liu K et al. Front Pharmacol. 2022 Jul 8;13:914146. doi: 10.3389/fphar.2022.914146.
10. Akhtar N et al. J Pharm Bioallied Sci. 2010 Jan;2(1):13-7. doi: 10.4103/0975-7406.62698.
11. Ren R et al. RSC Adv. 2020 Dec 17;10(73):44654-44671. doi: 10.1039/d0ra06488b.
12. Ito H et al. Burns. 2014 May;40(3):511-9. doi: 10.1016/j.burns.2013.08.011.
13. Liu X et al. Food Sci Nutr. 2023 Dec 7;12(2):1082-1094. doi: 10.1002/fsn3.3823.
14. Liu X at al. Antioxidants (Basel). 2022 Sep 25;11(10):1900. doi: 10.3390/antiox11101900.
15. Khan BA, Akhtar N. Postepy Dermatol Alergol. 2014 Aug;31(4):229-234. doi: 10.5114/pdia.2014.40934.
16. Khan BA, Akhtar N. Pak J Pharm Sci. 2014 Nov;27(6):1919-22.
17. Khan AB et al. African J Pharm Pharmacol. 2011 Aug;5(8):1092-5.
18. Khan BA, Akhtar N, Braga VA. Trop J Pharm Res. 2012;11(6):955-62.
19. Gęgotek A et al. Antioxidants (Basel). 2018 Aug 23;7(9):110. doi: 10.3390/antiox7090110.
20. Ivanišová E et al. Acta Sci Pol Technol Aliment. 2020 Apr-Jun;19(2):195-205. doi: 10.17306/J.AFS.0809.
21. Wang ZY, Luo XL, He CP. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Jan;26(1):124-5.
A member of the Elaeagnaceae family, Hippophae rhamnoides, better known as sea buckthorn, is a high-altitude wild shrub endemic to Europe and Asia with edible fruits and a lengthy record of use in traditional Chinese medicine.1-6 Used as a health supplement and consumed in the diet throughout the world,5 sea buckthorn berries, seeds, and leaves have been used in traditional medicine to treat burns/injuries, edema, hypertension, inflammation, skin grafts, ulcers, and wounds.4,7
This hardy plant is associated with a wide range of biologic activities, including anti-atherogenic, anti-atopic dermatitis, antibacterial, anticancer, antifungal, anti-inflammatory, antimicrobial, antioxidant, anti-psoriasis, anti-sebum, anti-stress, anti-tumor, cytoprotective, hepatoprotective, immunomodulatory, neuroprotective, radioprotective, and tissue regenerative functions.4,5,8-11
Key Constituents
Functional constituents identified in sea buckthorn include alkaloids, carotenoids, flavonoids, lignans, organic acids, phenolic acids, proanthocyanidins, polyunsaturated acids (including omega-3, -6, -7, and -9), steroids, tannins, terpenoids, and volatile oils, as well as nutritional compounds such as minerals, proteins, and vitamins.4,5,11 Sea buckthorn pericarp oil contains copious amounts of saturated palmitic acid (29%-36%) and omega-7 unsaturated palmitoleic acid (36%-48%), which fosters cutaneous and mucosal epithelialization, as well as linoleic (10%-12%) and oleic (4%-6%) acids.12,6 Significant amounts of carotenoids as well as alpha‐linolenic fatty acid (38%), linoleic (36%), oleic (13%), and palmitic (7%) acids are present in sea buckthorn seed oil.6
Polysaccharides
In an expansive review on the pharmacological activities of sea buckthorn polysaccharides, Teng and colleagues reported in April 2024 that 20 diverse polysaccharides have been culled from sea buckthorn and exhibited various healthy activities, including antioxidant, anti-fatigue, anti-inflammatory, anti-obesity, anti-tumor, hepatoprotective, hypoglycemic, and immunoregulation, and regulation of intestinal flora activities.1
Proanthocyanidins and Anti-Aging
In 2023, Liu and colleagues investigated the anti–skin aging impact of sea buckthorn proanthocyanidins in D-galactose-induced aging in mice given the known free radical scavenging activity of these compounds. They found the proanthocyanidins mitigated D-galactose-induced aging and can augment the total antioxidant capacity of the body. Sea buckthorn proanthocyanidins can further attenuate the effects of skin aging by regulating the TGF-beta1/Smads pathway and MMPs/TIMP system, thus amplifying collagen I and tropoelastin content.13
A year earlier, many of the same investigators assessed the possible protective activity of sea buckthorn proanthocyanidins against cutaneous aging engendered by oxidative stress from hydrogen peroxide. The compounds amplified superoxide dismutase and glutathione antioxidant functions. The extracts also fostered collagen I production in aging human skin fibroblasts via the TGF-beta1/Smads pathway and hindered collagen I degradation by regulating the MMPs/TIMPs system, which maintained extracellular matrix integrity. Senescent cell migration was also promoted with 100 mcg/mL of sea buckthorn proanthocyanidins. The researchers concluded that this sets the stage for investigating how sea buckthorn proanthocyanidins can be incorporated in cosmetic formulations.14 In a separate study, Liu and colleagues demonstrated that sea buckthorn proanthocyanidins can attenuate oxidative damage and protect mitochondrial function.9
Acne and Barrier Functions
The extracts of H rhamnoides and Cassia fistula in a combined formulation were found to be effective in lowering skin sebum content in humans with grade I and grade II acne vulgaris in a 2014 single-blind, randomized, placebo-controlled, split-face study with two groups of 25 patients each (aged 18-37 years).15 Khan and colleagues have also reported that a sea buckthorn oil-in-water emulsion improved barrier function in human skin as tested by a tewameter and corneometer (noninvasive probes) in 13 healthy males with a mean age of 27 ± 4.8 years.16
Anti-Aging, Antioxidant, Antibacterial, Skin-Whitening Activity
Zaman and colleagues reported in 2011 that results from an in vivo study of the effects of a sea buckthorn fruit extract topical cream on stratum corneum water content and transepidermal water loss indicated that the formulation enhanced cell surface integrin expression thus facilitating collagen contraction.17
In 2012, Khan and colleagues reported amelioration in skin elasticity, thus achieving an anti-aging result, from the use of a water-in-oil–based hydroalcoholic cream loaded with fruit extract of H rhamnoides, as measured with a Cutometer.18 The previous year, some of the same researchers reported that the antioxidants and flavonoids found in a topical sea buckthorn formulation could decrease cutaneous melanin and erythema levels.
More recently, Gęgotek and colleagues found that sea buckthorn seed oil prevented redox balance and lipid metabolism disturbances in skin fibroblasts and keratinocytes caused by UVA or UVB. They suggested that such findings point to the potential of this natural agent to confer anti-inflammatory properties and photoprotection to the skin.19
In 2020, Ivanišová and colleagues investigated the antioxidant and antimicrobial activities of H rhamnoides 100% oil, 100% juice, dry berries, and tea (dry berries, leaves, and twigs). They found that all of the studied sea buckthorn products displayed high antioxidant activity (identified through DPPH radical scavenging and molybdenum reducing antioxidant power tests). Sea buckthorn juice contained the highest total content of polyphenols, flavonoids, and carotenoids. All of the tested products also exhibited substantial antibacterial activity against the tested microbes.20
Burns and Wound Healing
In a preclinical study of the effects of sea buckthorn leaf extracts on wound healing in albino rats using an excision-punch wound model in 2005, Gupta and colleagues found that twice daily topical application of the aqueous leaf extract fostered wound healing. This was indicated by higher hydroxyproline and protein levels, a diminished wound area, and lower lipid peroxide levels. The investigators suggested that sea buckthorn may facilitate wound healing at least in part because of elevated antioxidant activity in the granulation tissue.3
A year later, Wang and colleagues reported on observations of using H rhamnoides oil, a traditional Chinese herbal medicine derived from sea buckthorn fruit, as a burn treatment. In the study, 151 burn patients received an H rhamnoides oil dressing (changed every other day until wound healing) that was covered with a disinfecting dressing. The dressing reduced swelling and effusion, and alleviated pain, with patients receiving the sea buckthorn dressing experiencing greater apparent exudation reduction, pain reduction, and more rapid epithelial cell growth and wound healing than controls (treated only with Vaseline gauze). The difference between the two groups was statistically significant.21
Conclusion
Sea buckthorn has been used for hundreds if not thousands of years in traditional medical applications, including for dermatologic purposes. Emerging data appear to support the use of this dynamic plant for consideration in dermatologic applications. As is often the case, much more work is necessary in the form of randomized controlled trials to determine the effectiveness of sea buckthorn formulations as well as the most appropriate avenues of research or uses for dermatologic application of this traditionally used botanical agent.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at dermnews@mdedge.com.
References
1. Teng H et al. J Ethnopharmacol. 2024 Apr 24;324:117809. doi: 10.1016/j.jep.2024.117809.
2. Wang Z et al. Int J Biol Macromol. 2024 Apr;263(Pt 1):130206. doi: 10.1016/j.ijbiomac.2024.130206.
3. Gupta A et al. Int J Low Extrem Wounds. 2005 Jun;4(2):88-92. doi: 10.1177/1534734605277401.
4. Pundir S et al. J Ethnopharmacol. 2021 Feb 10;266:113434. doi: 10.1016/j.jep.2020.113434.
5. Ma QG et al. J Agric Food Chem. 2023 Mar 29;71(12):4769-4788. doi: 10.1021/acs.jafc.2c06916.
6. Poljšak N et al. Phytother Res. 2020 Feb;34(2):254-269. doi: 10.1002/ptr.6524. doi: 10.1002/ptr.6524.
7. Upadhyay NK et al. Evid Based Complement Alternat Med. 2011;2011:659705. doi: 10.1093/ecam/nep189.
8. Suryakumar G, Gupta A. J Ethnopharmacol. 2011 Nov 18;138(2):268-78. doi: 10.1016/j.jep.2011.09.024.
9. Liu K et al. Front Pharmacol. 2022 Jul 8;13:914146. doi: 10.3389/fphar.2022.914146.
10. Akhtar N et al. J Pharm Bioallied Sci. 2010 Jan;2(1):13-7. doi: 10.4103/0975-7406.62698.
11. Ren R et al. RSC Adv. 2020 Dec 17;10(73):44654-44671. doi: 10.1039/d0ra06488b.
12. Ito H et al. Burns. 2014 May;40(3):511-9. doi: 10.1016/j.burns.2013.08.011.
13. Liu X et al. Food Sci Nutr. 2023 Dec 7;12(2):1082-1094. doi: 10.1002/fsn3.3823.
14. Liu X at al. Antioxidants (Basel). 2022 Sep 25;11(10):1900. doi: 10.3390/antiox11101900.
15. Khan BA, Akhtar N. Postepy Dermatol Alergol. 2014 Aug;31(4):229-234. doi: 10.5114/pdia.2014.40934.
16. Khan BA, Akhtar N. Pak J Pharm Sci. 2014 Nov;27(6):1919-22.
17. Khan AB et al. African J Pharm Pharmacol. 2011 Aug;5(8):1092-5.
18. Khan BA, Akhtar N, Braga VA. Trop J Pharm Res. 2012;11(6):955-62.
19. Gęgotek A et al. Antioxidants (Basel). 2018 Aug 23;7(9):110. doi: 10.3390/antiox7090110.
20. Ivanišová E et al. Acta Sci Pol Technol Aliment. 2020 Apr-Jun;19(2):195-205. doi: 10.17306/J.AFS.0809.
21. Wang ZY, Luo XL, He CP. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Jan;26(1):124-5.
A member of the Elaeagnaceae family, Hippophae rhamnoides, better known as sea buckthorn, is a high-altitude wild shrub endemic to Europe and Asia with edible fruits and a lengthy record of use in traditional Chinese medicine.1-6 Used as a health supplement and consumed in the diet throughout the world,5 sea buckthorn berries, seeds, and leaves have been used in traditional medicine to treat burns/injuries, edema, hypertension, inflammation, skin grafts, ulcers, and wounds.4,7
This hardy plant is associated with a wide range of biologic activities, including anti-atherogenic, anti-atopic dermatitis, antibacterial, anticancer, antifungal, anti-inflammatory, antimicrobial, antioxidant, anti-psoriasis, anti-sebum, anti-stress, anti-tumor, cytoprotective, hepatoprotective, immunomodulatory, neuroprotective, radioprotective, and tissue regenerative functions.4,5,8-11
Key Constituents
Functional constituents identified in sea buckthorn include alkaloids, carotenoids, flavonoids, lignans, organic acids, phenolic acids, proanthocyanidins, polyunsaturated acids (including omega-3, -6, -7, and -9), steroids, tannins, terpenoids, and volatile oils, as well as nutritional compounds such as minerals, proteins, and vitamins.4,5,11 Sea buckthorn pericarp oil contains copious amounts of saturated palmitic acid (29%-36%) and omega-7 unsaturated palmitoleic acid (36%-48%), which fosters cutaneous and mucosal epithelialization, as well as linoleic (10%-12%) and oleic (4%-6%) acids.12,6 Significant amounts of carotenoids as well as alpha‐linolenic fatty acid (38%), linoleic (36%), oleic (13%), and palmitic (7%) acids are present in sea buckthorn seed oil.6
Polysaccharides
In an expansive review on the pharmacological activities of sea buckthorn polysaccharides, Teng and colleagues reported in April 2024 that 20 diverse polysaccharides have been culled from sea buckthorn and exhibited various healthy activities, including antioxidant, anti-fatigue, anti-inflammatory, anti-obesity, anti-tumor, hepatoprotective, hypoglycemic, and immunoregulation, and regulation of intestinal flora activities.1
Proanthocyanidins and Anti-Aging
In 2023, Liu and colleagues investigated the anti–skin aging impact of sea buckthorn proanthocyanidins in D-galactose-induced aging in mice given the known free radical scavenging activity of these compounds. They found the proanthocyanidins mitigated D-galactose-induced aging and can augment the total antioxidant capacity of the body. Sea buckthorn proanthocyanidins can further attenuate the effects of skin aging by regulating the TGF-beta1/Smads pathway and MMPs/TIMP system, thus amplifying collagen I and tropoelastin content.13
A year earlier, many of the same investigators assessed the possible protective activity of sea buckthorn proanthocyanidins against cutaneous aging engendered by oxidative stress from hydrogen peroxide. The compounds amplified superoxide dismutase and glutathione antioxidant functions. The extracts also fostered collagen I production in aging human skin fibroblasts via the TGF-beta1/Smads pathway and hindered collagen I degradation by regulating the MMPs/TIMPs system, which maintained extracellular matrix integrity. Senescent cell migration was also promoted with 100 mcg/mL of sea buckthorn proanthocyanidins. The researchers concluded that this sets the stage for investigating how sea buckthorn proanthocyanidins can be incorporated in cosmetic formulations.14 In a separate study, Liu and colleagues demonstrated that sea buckthorn proanthocyanidins can attenuate oxidative damage and protect mitochondrial function.9
Acne and Barrier Functions
The extracts of H rhamnoides and Cassia fistula in a combined formulation were found to be effective in lowering skin sebum content in humans with grade I and grade II acne vulgaris in a 2014 single-blind, randomized, placebo-controlled, split-face study with two groups of 25 patients each (aged 18-37 years).15 Khan and colleagues have also reported that a sea buckthorn oil-in-water emulsion improved barrier function in human skin as tested by a tewameter and corneometer (noninvasive probes) in 13 healthy males with a mean age of 27 ± 4.8 years.16
Anti-Aging, Antioxidant, Antibacterial, Skin-Whitening Activity
Zaman and colleagues reported in 2011 that results from an in vivo study of the effects of a sea buckthorn fruit extract topical cream on stratum corneum water content and transepidermal water loss indicated that the formulation enhanced cell surface integrin expression thus facilitating collagen contraction.17
In 2012, Khan and colleagues reported amelioration in skin elasticity, thus achieving an anti-aging result, from the use of a water-in-oil–based hydroalcoholic cream loaded with fruit extract of H rhamnoides, as measured with a Cutometer.18 The previous year, some of the same researchers reported that the antioxidants and flavonoids found in a topical sea buckthorn formulation could decrease cutaneous melanin and erythema levels.
More recently, Gęgotek and colleagues found that sea buckthorn seed oil prevented redox balance and lipid metabolism disturbances in skin fibroblasts and keratinocytes caused by UVA or UVB. They suggested that such findings point to the potential of this natural agent to confer anti-inflammatory properties and photoprotection to the skin.19
In 2020, Ivanišová and colleagues investigated the antioxidant and antimicrobial activities of H rhamnoides 100% oil, 100% juice, dry berries, and tea (dry berries, leaves, and twigs). They found that all of the studied sea buckthorn products displayed high antioxidant activity (identified through DPPH radical scavenging and molybdenum reducing antioxidant power tests). Sea buckthorn juice contained the highest total content of polyphenols, flavonoids, and carotenoids. All of the tested products also exhibited substantial antibacterial activity against the tested microbes.20
Burns and Wound Healing
In a preclinical study of the effects of sea buckthorn leaf extracts on wound healing in albino rats using an excision-punch wound model in 2005, Gupta and colleagues found that twice daily topical application of the aqueous leaf extract fostered wound healing. This was indicated by higher hydroxyproline and protein levels, a diminished wound area, and lower lipid peroxide levels. The investigators suggested that sea buckthorn may facilitate wound healing at least in part because of elevated antioxidant activity in the granulation tissue.3
A year later, Wang and colleagues reported on observations of using H rhamnoides oil, a traditional Chinese herbal medicine derived from sea buckthorn fruit, as a burn treatment. In the study, 151 burn patients received an H rhamnoides oil dressing (changed every other day until wound healing) that was covered with a disinfecting dressing. The dressing reduced swelling and effusion, and alleviated pain, with patients receiving the sea buckthorn dressing experiencing greater apparent exudation reduction, pain reduction, and more rapid epithelial cell growth and wound healing than controls (treated only with Vaseline gauze). The difference between the two groups was statistically significant.21
Conclusion
Sea buckthorn has been used for hundreds if not thousands of years in traditional medical applications, including for dermatologic purposes. Emerging data appear to support the use of this dynamic plant for consideration in dermatologic applications. As is often the case, much more work is necessary in the form of randomized controlled trials to determine the effectiveness of sea buckthorn formulations as well as the most appropriate avenues of research or uses for dermatologic application of this traditionally used botanical agent.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as a e-commerce solution. Write to her at dermnews@mdedge.com.
References
1. Teng H et al. J Ethnopharmacol. 2024 Apr 24;324:117809. doi: 10.1016/j.jep.2024.117809.
2. Wang Z et al. Int J Biol Macromol. 2024 Apr;263(Pt 1):130206. doi: 10.1016/j.ijbiomac.2024.130206.
3. Gupta A et al. Int J Low Extrem Wounds. 2005 Jun;4(2):88-92. doi: 10.1177/1534734605277401.
4. Pundir S et al. J Ethnopharmacol. 2021 Feb 10;266:113434. doi: 10.1016/j.jep.2020.113434.
5. Ma QG et al. J Agric Food Chem. 2023 Mar 29;71(12):4769-4788. doi: 10.1021/acs.jafc.2c06916.
6. Poljšak N et al. Phytother Res. 2020 Feb;34(2):254-269. doi: 10.1002/ptr.6524. doi: 10.1002/ptr.6524.
7. Upadhyay NK et al. Evid Based Complement Alternat Med. 2011;2011:659705. doi: 10.1093/ecam/nep189.
8. Suryakumar G, Gupta A. J Ethnopharmacol. 2011 Nov 18;138(2):268-78. doi: 10.1016/j.jep.2011.09.024.
9. Liu K et al. Front Pharmacol. 2022 Jul 8;13:914146. doi: 10.3389/fphar.2022.914146.
10. Akhtar N et al. J Pharm Bioallied Sci. 2010 Jan;2(1):13-7. doi: 10.4103/0975-7406.62698.
11. Ren R et al. RSC Adv. 2020 Dec 17;10(73):44654-44671. doi: 10.1039/d0ra06488b.
12. Ito H et al. Burns. 2014 May;40(3):511-9. doi: 10.1016/j.burns.2013.08.011.
13. Liu X et al. Food Sci Nutr. 2023 Dec 7;12(2):1082-1094. doi: 10.1002/fsn3.3823.
14. Liu X at al. Antioxidants (Basel). 2022 Sep 25;11(10):1900. doi: 10.3390/antiox11101900.
15. Khan BA, Akhtar N. Postepy Dermatol Alergol. 2014 Aug;31(4):229-234. doi: 10.5114/pdia.2014.40934.
16. Khan BA, Akhtar N. Pak J Pharm Sci. 2014 Nov;27(6):1919-22.
17. Khan AB et al. African J Pharm Pharmacol. 2011 Aug;5(8):1092-5.
18. Khan BA, Akhtar N, Braga VA. Trop J Pharm Res. 2012;11(6):955-62.
19. Gęgotek A et al. Antioxidants (Basel). 2018 Aug 23;7(9):110. doi: 10.3390/antiox7090110.
20. Ivanišová E et al. Acta Sci Pol Technol Aliment. 2020 Apr-Jun;19(2):195-205. doi: 10.17306/J.AFS.0809.
21. Wang ZY, Luo XL, He CP. Nan Fang Yi Ke Da Xue Xue Bao. 2006 Jan;26(1):124-5.
ICD-10-CM Codes for CCCA, FFA Now Available
in the field of hair loss disorders.
“CCCA and FFA are conditions that require early diagnosis and intervention to prevent irreversible hair loss,” Maria Hordinsky, MD, professor of dermatology at the University of Minnesota, Minneapolis, and a member of the Board of Directors, Scarring Alopecia Foundation (SAF), said in an interview.
“The use of these new codes will make it easier for clinicians to identify affected patients and improve treatment outcomes. It also opens the door for more robust research efforts aimed at understanding the etiology and progression of CCCA and FFA, which could lead to new and more effective treatments in the future. Overall, this development represents a positive step toward improving care for individuals affected by these challenging conditions.”
The new codes — L66.81 for CCCA and L66.12 for FFA — were approved by the Centers for Disease Control and Prevention (CDC) on June 15, 2023, but not implemented until October 1, 2024.
Amy J. McMichael, MD, professor of dermatology at Wake Forest University School of Medicine, Winston-Salem, North Carolina, and a scientific advisor to SAF, told this news organization that Itisha Jefferson, a medical student at Loyola University Chicago’s Stritch School of Medicine, and her peers on the SAF’s Medical Student Executive Board, played a pivotal role in advocating for the codes.
In 2022, Jefferson, who has CCCA, and her fellow medical students helped create the proposals that were ultimately submitted to the CDC.
“They were critical in working with the CDC leaders to get the necessary information submitted and processed,” McMichael said. “They were also amazing at corralling our dermatologist group for the development of the necessary presentations and helped to shepherd us to the finish line for all logistic issues.”
On March 8, 2023, McMichael and Hordinsky made their pitch for the codes in person at the CDC’s ICD-10 Coordination and Maintenance Committee meeting, with McMichael discussing CCCA and Hordinsky discussing FFA.
“We also discussed the lack of standardized tracking, which has contributed to misdiagnoses and inadequate treatment options,” Hordinsky recalled. “We highlighted the importance of having distinct codes for these conditions to improve clinical outcomes, ensure that patients have access to appropriate care, better tracking of disease prevalence, and greater epidemiologic monitoring with access to electronic medical records and other large real-world evidence datasets and databases, the results of which could contribute to health policy decision-making.”
To spread the word about the new codes, McMichael, Hordinsky, and other members of the SAF are working with the original team of medical students, some of whom who are now dermatology residents, to develop an information guide to send to societies and organizations that were supportive of the codes. A publication in the dermatology literature is also planned.
For her part, Jefferson said that she will continue to advocate for patients with scarring alopecia as a medical student and when she becomes a physician. “I hope in the near future we will see an externally led FDA Patient-Focused Drug Development meeting for both CCCA and FFA, further advancing care and research for these conditions,” she said in an interview.
McMichael, Hordinsky, and Jefferson had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
in the field of hair loss disorders.
“CCCA and FFA are conditions that require early diagnosis and intervention to prevent irreversible hair loss,” Maria Hordinsky, MD, professor of dermatology at the University of Minnesota, Minneapolis, and a member of the Board of Directors, Scarring Alopecia Foundation (SAF), said in an interview.
“The use of these new codes will make it easier for clinicians to identify affected patients and improve treatment outcomes. It also opens the door for more robust research efforts aimed at understanding the etiology and progression of CCCA and FFA, which could lead to new and more effective treatments in the future. Overall, this development represents a positive step toward improving care for individuals affected by these challenging conditions.”
The new codes — L66.81 for CCCA and L66.12 for FFA — were approved by the Centers for Disease Control and Prevention (CDC) on June 15, 2023, but not implemented until October 1, 2024.
Amy J. McMichael, MD, professor of dermatology at Wake Forest University School of Medicine, Winston-Salem, North Carolina, and a scientific advisor to SAF, told this news organization that Itisha Jefferson, a medical student at Loyola University Chicago’s Stritch School of Medicine, and her peers on the SAF’s Medical Student Executive Board, played a pivotal role in advocating for the codes.
In 2022, Jefferson, who has CCCA, and her fellow medical students helped create the proposals that were ultimately submitted to the CDC.
“They were critical in working with the CDC leaders to get the necessary information submitted and processed,” McMichael said. “They were also amazing at corralling our dermatologist group for the development of the necessary presentations and helped to shepherd us to the finish line for all logistic issues.”
On March 8, 2023, McMichael and Hordinsky made their pitch for the codes in person at the CDC’s ICD-10 Coordination and Maintenance Committee meeting, with McMichael discussing CCCA and Hordinsky discussing FFA.
“We also discussed the lack of standardized tracking, which has contributed to misdiagnoses and inadequate treatment options,” Hordinsky recalled. “We highlighted the importance of having distinct codes for these conditions to improve clinical outcomes, ensure that patients have access to appropriate care, better tracking of disease prevalence, and greater epidemiologic monitoring with access to electronic medical records and other large real-world evidence datasets and databases, the results of which could contribute to health policy decision-making.”
To spread the word about the new codes, McMichael, Hordinsky, and other members of the SAF are working with the original team of medical students, some of whom who are now dermatology residents, to develop an information guide to send to societies and organizations that were supportive of the codes. A publication in the dermatology literature is also planned.
For her part, Jefferson said that she will continue to advocate for patients with scarring alopecia as a medical student and when she becomes a physician. “I hope in the near future we will see an externally led FDA Patient-Focused Drug Development meeting for both CCCA and FFA, further advancing care and research for these conditions,” she said in an interview.
McMichael, Hordinsky, and Jefferson had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
in the field of hair loss disorders.
“CCCA and FFA are conditions that require early diagnosis and intervention to prevent irreversible hair loss,” Maria Hordinsky, MD, professor of dermatology at the University of Minnesota, Minneapolis, and a member of the Board of Directors, Scarring Alopecia Foundation (SAF), said in an interview.
“The use of these new codes will make it easier for clinicians to identify affected patients and improve treatment outcomes. It also opens the door for more robust research efforts aimed at understanding the etiology and progression of CCCA and FFA, which could lead to new and more effective treatments in the future. Overall, this development represents a positive step toward improving care for individuals affected by these challenging conditions.”
The new codes — L66.81 for CCCA and L66.12 for FFA — were approved by the Centers for Disease Control and Prevention (CDC) on June 15, 2023, but not implemented until October 1, 2024.
Amy J. McMichael, MD, professor of dermatology at Wake Forest University School of Medicine, Winston-Salem, North Carolina, and a scientific advisor to SAF, told this news organization that Itisha Jefferson, a medical student at Loyola University Chicago’s Stritch School of Medicine, and her peers on the SAF’s Medical Student Executive Board, played a pivotal role in advocating for the codes.
In 2022, Jefferson, who has CCCA, and her fellow medical students helped create the proposals that were ultimately submitted to the CDC.
“They were critical in working with the CDC leaders to get the necessary information submitted and processed,” McMichael said. “They were also amazing at corralling our dermatologist group for the development of the necessary presentations and helped to shepherd us to the finish line for all logistic issues.”
On March 8, 2023, McMichael and Hordinsky made their pitch for the codes in person at the CDC’s ICD-10 Coordination and Maintenance Committee meeting, with McMichael discussing CCCA and Hordinsky discussing FFA.
“We also discussed the lack of standardized tracking, which has contributed to misdiagnoses and inadequate treatment options,” Hordinsky recalled. “We highlighted the importance of having distinct codes for these conditions to improve clinical outcomes, ensure that patients have access to appropriate care, better tracking of disease prevalence, and greater epidemiologic monitoring with access to electronic medical records and other large real-world evidence datasets and databases, the results of which could contribute to health policy decision-making.”
To spread the word about the new codes, McMichael, Hordinsky, and other members of the SAF are working with the original team of medical students, some of whom who are now dermatology residents, to develop an information guide to send to societies and organizations that were supportive of the codes. A publication in the dermatology literature is also planned.
For her part, Jefferson said that she will continue to advocate for patients with scarring alopecia as a medical student and when she becomes a physician. “I hope in the near future we will see an externally led FDA Patient-Focused Drug Development meeting for both CCCA and FFA, further advancing care and research for these conditions,” she said in an interview.
McMichael, Hordinsky, and Jefferson had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
Responses Sustained with Ritlecitinib in Patients with Alopecia Through 48 Weeks
TOPLINE:
, and up to one third of nonresponders at week 24 also achieved responses by week 48.
METHODOLOGY:
- Researchers conducted a post hoc analysis of an international, randomized, double-blind, placebo-controlled, phase 2b/3 trial (ALLEGRO) and included 718 adults and adolescents aged 12 or older with severe AA (Severity of Alopecia Tool [SALT] score ≥ 50).
- Patients received various doses of the oral Janus kinase inhibitor ritlecitinib, with or without a 4-week loading dose, including 200/50 mg, 200/30 mg, 50 mg, or 30 mg, with or without a 4-week loading dose for up to 24 weeks and continued to receive their assigned maintenance dose.
- Researchers assessed sustained clinical responses at week 48 for those who had achieved SALT scores ≤ 20 and ≤ 10 at 24 weeks, and nonresponders at week 24 were assessed for responses through week 48.
- Adverse events were also evaluated.
TAKEAWAY:
- Among patients on ritlecitinib who had responded at week 24, SALT responses ≤ 20 were sustained in 85.2%-100% of patients through week 48. Similar results were seen among patients who achieved a SALT score ≤ 10 (68.8%-91.7%) and improvements in eyebrow (70.4%-96.9%) or eyelash (52.4%-94.1%) assessment scores.
- Among those who were nonresponders at week 24, 22.2%-33.7% achieved a SALT score ≤ 20 and 19.8%-25.5% achieved a SALT score ≤ 10 by week 48. Similarly, among those with no eyebrow or eyelash responses at week 24, 19.7%-32.8% and 16.7%-30.2% had improved eyebrow or eyelash assessment scores, respectively, at week 48.
- Between weeks 24 and 48, adverse events were reported in 74%-93% of patients who achieved a SALT score ≤ 20, most were mild or moderate; two serious events were reported but deemed unrelated to treatment. The safety profile was similar across all subgroups.
- No deaths, malignancies, major cardiovascular events, opportunistic infections, or herpes zoster infections were observed.
IN PRACTICE:
“The majority of ritlecitinib-treated patients with AA who met target clinical response based on scalp, eyebrow, or eyelash regrowth at week 24 sustained their response through week 48 with continued treatment,” the authors wrote. “Some patients, including those with more extensive hair loss, may require ritlecitinib treatment beyond 6 months to achieve target clinical response,” they added.
SOURCE:
The study was led by Melissa Piliang, MD, of the Department of Dermatology, Cleveland Clinic, and was published online on October 17 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The analysis was limited by its post hoc nature, small sample size in each treatment group, and a follow-up period of only 48 weeks.
DISCLOSURES:
This study was funded by Pfizer. Piliang disclosed being a consultant or investigator for Pfizer, Eli Lilly, and Procter & Gamble. Six authors were employees or shareholders of or received salary from Pfizer. Other authors also reported financial relationships with pharmaceutical companies outside this work, including Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
, and up to one third of nonresponders at week 24 also achieved responses by week 48.
METHODOLOGY:
- Researchers conducted a post hoc analysis of an international, randomized, double-blind, placebo-controlled, phase 2b/3 trial (ALLEGRO) and included 718 adults and adolescents aged 12 or older with severe AA (Severity of Alopecia Tool [SALT] score ≥ 50).
- Patients received various doses of the oral Janus kinase inhibitor ritlecitinib, with or without a 4-week loading dose, including 200/50 mg, 200/30 mg, 50 mg, or 30 mg, with or without a 4-week loading dose for up to 24 weeks and continued to receive their assigned maintenance dose.
- Researchers assessed sustained clinical responses at week 48 for those who had achieved SALT scores ≤ 20 and ≤ 10 at 24 weeks, and nonresponders at week 24 were assessed for responses through week 48.
- Adverse events were also evaluated.
TAKEAWAY:
- Among patients on ritlecitinib who had responded at week 24, SALT responses ≤ 20 were sustained in 85.2%-100% of patients through week 48. Similar results were seen among patients who achieved a SALT score ≤ 10 (68.8%-91.7%) and improvements in eyebrow (70.4%-96.9%) or eyelash (52.4%-94.1%) assessment scores.
- Among those who were nonresponders at week 24, 22.2%-33.7% achieved a SALT score ≤ 20 and 19.8%-25.5% achieved a SALT score ≤ 10 by week 48. Similarly, among those with no eyebrow or eyelash responses at week 24, 19.7%-32.8% and 16.7%-30.2% had improved eyebrow or eyelash assessment scores, respectively, at week 48.
- Between weeks 24 and 48, adverse events were reported in 74%-93% of patients who achieved a SALT score ≤ 20, most were mild or moderate; two serious events were reported but deemed unrelated to treatment. The safety profile was similar across all subgroups.
- No deaths, malignancies, major cardiovascular events, opportunistic infections, or herpes zoster infections were observed.
IN PRACTICE:
“The majority of ritlecitinib-treated patients with AA who met target clinical response based on scalp, eyebrow, or eyelash regrowth at week 24 sustained their response through week 48 with continued treatment,” the authors wrote. “Some patients, including those with more extensive hair loss, may require ritlecitinib treatment beyond 6 months to achieve target clinical response,” they added.
SOURCE:
The study was led by Melissa Piliang, MD, of the Department of Dermatology, Cleveland Clinic, and was published online on October 17 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The analysis was limited by its post hoc nature, small sample size in each treatment group, and a follow-up period of only 48 weeks.
DISCLOSURES:
This study was funded by Pfizer. Piliang disclosed being a consultant or investigator for Pfizer, Eli Lilly, and Procter & Gamble. Six authors were employees or shareholders of or received salary from Pfizer. Other authors also reported financial relationships with pharmaceutical companies outside this work, including Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
, and up to one third of nonresponders at week 24 also achieved responses by week 48.
METHODOLOGY:
- Researchers conducted a post hoc analysis of an international, randomized, double-blind, placebo-controlled, phase 2b/3 trial (ALLEGRO) and included 718 adults and adolescents aged 12 or older with severe AA (Severity of Alopecia Tool [SALT] score ≥ 50).
- Patients received various doses of the oral Janus kinase inhibitor ritlecitinib, with or without a 4-week loading dose, including 200/50 mg, 200/30 mg, 50 mg, or 30 mg, with or without a 4-week loading dose for up to 24 weeks and continued to receive their assigned maintenance dose.
- Researchers assessed sustained clinical responses at week 48 for those who had achieved SALT scores ≤ 20 and ≤ 10 at 24 weeks, and nonresponders at week 24 were assessed for responses through week 48.
- Adverse events were also evaluated.
TAKEAWAY:
- Among patients on ritlecitinib who had responded at week 24, SALT responses ≤ 20 were sustained in 85.2%-100% of patients through week 48. Similar results were seen among patients who achieved a SALT score ≤ 10 (68.8%-91.7%) and improvements in eyebrow (70.4%-96.9%) or eyelash (52.4%-94.1%) assessment scores.
- Among those who were nonresponders at week 24, 22.2%-33.7% achieved a SALT score ≤ 20 and 19.8%-25.5% achieved a SALT score ≤ 10 by week 48. Similarly, among those with no eyebrow or eyelash responses at week 24, 19.7%-32.8% and 16.7%-30.2% had improved eyebrow or eyelash assessment scores, respectively, at week 48.
- Between weeks 24 and 48, adverse events were reported in 74%-93% of patients who achieved a SALT score ≤ 20, most were mild or moderate; two serious events were reported but deemed unrelated to treatment. The safety profile was similar across all subgroups.
- No deaths, malignancies, major cardiovascular events, opportunistic infections, or herpes zoster infections were observed.
IN PRACTICE:
“The majority of ritlecitinib-treated patients with AA who met target clinical response based on scalp, eyebrow, or eyelash regrowth at week 24 sustained their response through week 48 with continued treatment,” the authors wrote. “Some patients, including those with more extensive hair loss, may require ritlecitinib treatment beyond 6 months to achieve target clinical response,” they added.
SOURCE:
The study was led by Melissa Piliang, MD, of the Department of Dermatology, Cleveland Clinic, and was published online on October 17 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The analysis was limited by its post hoc nature, small sample size in each treatment group, and a follow-up period of only 48 weeks.
DISCLOSURES:
This study was funded by Pfizer. Piliang disclosed being a consultant or investigator for Pfizer, Eli Lilly, and Procter & Gamble. Six authors were employees or shareholders of or received salary from Pfizer. Other authors also reported financial relationships with pharmaceutical companies outside this work, including Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Topical JAK Inhibitor Shows Benefits in Small Frontal Fibrosing Alopecia Study
AMSTERDAM —
“This is an exciting avenue for FFA if the data are recapitulated in a larger population. It could be an important new treatment option,” said Maryanne Senna, MD, director at Lahey Hospital & Medical Center’s Hair Loss Center of Excellence, Burlington, Massachusetts, and assistant dermatology professor at Harvard Medical School, Boston, Massachusetts.
In a design characterized as “exploratory,” the trial had two parts: a randomized, double-blind, vehicle-controlled intervention for 12 weeks, followed by an open-label extension of topical delgocitinib for all participants for another 12 weeks.
The primary efficacy endpoint was change in the molecular signature of FFA inflammation at 12 weeks. Clinical improvement was monitored with both trichoscopic images capturing the numbers of hairs and follicular units at 12 weeks and clinical severity scores through week 24. In a topical cream formulation, the Janus kinase inhibitor (JAKi) delgocitinib was associated with favorable activity for both.
Some Hair Regrowth for All
“At 24 weeks, all patients achieved some degree of hair regrowth and a stabilization of disease based on hairline measurements,” Senna reported in a late-breaking news session at the 2024 European Academy of Dermatology and Venereology (EADV) Congress.
On the clinical endpoints, Senna noted an upward trajectory in clinical improvement at the completion of the study.
The 30 participants were randomly assigned in a 1:1 ratio to receive delgocitinib cream in a concentration of 20 mg/g or vehicle cream applied twice daily for 12 weeks. At the end of this double-blind period, patients on vehicle were crossed over to the active therapy, and all patients were monitored for another 12 weeks in an open-label extension.
The change from baseline in FFA biomarkers was selected as the primary endpoint based on previous work showing up-regulation in the expression of the Th1 biomarkers CXCL9, CXCL10, and interferon gamma in lesional vs nonlesional scalp in patients with FFA.
When biopsies at the end of 12 weeks in the double-blind phase of the study were compared with the baseline biopsies, researchers found a decrease in expression of the three local inflammation markers in all patients receiving the JAKi, but not in those receiving the vehicle cream. In this small patient sample, only the reduction in expression of CXCL9, a cytokine known for differentiation and promotion of leukocytes, reached statistical significance (P < .05).
But in an analysis involving the expression of multiple genes, “lesions treated with delgocitinib had a 4% improvement in normalization toward a nonlesional transcriptomic profile, while patients treated with vehicle had a 33% worsening,” Senna reported. The difference was highly significant (P < .001).
Furthermore, the decrease in total Lichen Planopilaris Activity Index and FFA severity scores were numerically and statistically greater (P = .023) in the active-treatment arm than in the vehicle arm by the end of the double-blind part of the trial, she said.
On trichoscopy, there was an increased number of hairs and follicular units at 12 weeks relative to baseline among those treated with topical delgocitinib but a reduction in those treated with vehicle.
JAKi Patients Gained Hair, Vehicle Patients Lost Hair
On the basis of hair count per square centimeter from baseline, delgocitinib-treated patients gained on average of seven hairs whereas vehicle recipients lost an average of 11 hairs at 24 weeks, Senna reported.
Patients originally treated with vehicle did improve in most outcome measures in the open-label extension of the experimental treatment after crossover, but they did not catch up to those initially randomized to delgocitinib because of further accrual of favorable changes in the active-treatment group over time.
“There were no adverse events associated with active therapy or vehicle, including application-site reactions,” Senna said. The one between-group difference was a higher rate of COVID-19, but this was greater in the control arm.
All 30 of the participants in this study were women, and all had moderate to severe disease at enrollment. The median age was 64 years. Because of the predominant population at the hair loss center, all but one of the participants were White, and one participant was Asian.
Characterizing FFA as “devastating and disfiguring,” Senna, who specializes in the care of alopecia, noted that this a difficult disease to control with the off-label strategies that are now used. The slow progress to identify treatments for FFA is illustrated by the fact that only one other double-blind and randomized trial has ever been conducted in FFA, she said.
Exploratory Study Supports Anecdotal Experience
On the basis of prior anecdotal experience with JAKi treatment for FFA, Senna said, “I do think that it is possible to get largely clear skin with this therapy.” However, she is now hoping for definitive trials to better characterize the efficacy and safety of oral and topical therapies, perhaps used sequentially to maintain clinical improvement.
In light of the limited current options, Menno de Rie, MD, PhD, professor of dermatology at the University of Amsterdam in the Netherlands, called these data “very inspiring and hopeful.” He suggested the promise of this therapy was reinforced by the upward trajectory of the biomarkers and clinical improvement over the study period.
“Any improvement in treatment options would be welcome, because we do not [have] any reliable therapies for this condition,” de Rie, who was not an investigator, said in an interview after the presentation.
Ultimately, Senna said, once effective therapy is established, the goal will be to start as early as possible in the disease process. She noted that there is evidence that prompt therapy can reverse the disorder, not just prevent progression.
“If you can get to the hair follicles before the point of no return, there is [a] chance [of] follicular rescue,” she said.
Delgocitinib cream (Anzupgo) was approved in Europe for treating chronic hand eczema in late September and is under review for the same indication in the United States.
Senna has financial relationships with Arena, Concert, Eli Lilly, Pfizer, and Leo Pharma, which provided funding for this study. de Rie reported no potential conflicts of interest.
A version of this article appeared on Medscape.com.
AMSTERDAM —
“This is an exciting avenue for FFA if the data are recapitulated in a larger population. It could be an important new treatment option,” said Maryanne Senna, MD, director at Lahey Hospital & Medical Center’s Hair Loss Center of Excellence, Burlington, Massachusetts, and assistant dermatology professor at Harvard Medical School, Boston, Massachusetts.
In a design characterized as “exploratory,” the trial had two parts: a randomized, double-blind, vehicle-controlled intervention for 12 weeks, followed by an open-label extension of topical delgocitinib for all participants for another 12 weeks.
The primary efficacy endpoint was change in the molecular signature of FFA inflammation at 12 weeks. Clinical improvement was monitored with both trichoscopic images capturing the numbers of hairs and follicular units at 12 weeks and clinical severity scores through week 24. In a topical cream formulation, the Janus kinase inhibitor (JAKi) delgocitinib was associated with favorable activity for both.
Some Hair Regrowth for All
“At 24 weeks, all patients achieved some degree of hair regrowth and a stabilization of disease based on hairline measurements,” Senna reported in a late-breaking news session at the 2024 European Academy of Dermatology and Venereology (EADV) Congress.
On the clinical endpoints, Senna noted an upward trajectory in clinical improvement at the completion of the study.
The 30 participants were randomly assigned in a 1:1 ratio to receive delgocitinib cream in a concentration of 20 mg/g or vehicle cream applied twice daily for 12 weeks. At the end of this double-blind period, patients on vehicle were crossed over to the active therapy, and all patients were monitored for another 12 weeks in an open-label extension.
The change from baseline in FFA biomarkers was selected as the primary endpoint based on previous work showing up-regulation in the expression of the Th1 biomarkers CXCL9, CXCL10, and interferon gamma in lesional vs nonlesional scalp in patients with FFA.
When biopsies at the end of 12 weeks in the double-blind phase of the study were compared with the baseline biopsies, researchers found a decrease in expression of the three local inflammation markers in all patients receiving the JAKi, but not in those receiving the vehicle cream. In this small patient sample, only the reduction in expression of CXCL9, a cytokine known for differentiation and promotion of leukocytes, reached statistical significance (P < .05).
But in an analysis involving the expression of multiple genes, “lesions treated with delgocitinib had a 4% improvement in normalization toward a nonlesional transcriptomic profile, while patients treated with vehicle had a 33% worsening,” Senna reported. The difference was highly significant (P < .001).
Furthermore, the decrease in total Lichen Planopilaris Activity Index and FFA severity scores were numerically and statistically greater (P = .023) in the active-treatment arm than in the vehicle arm by the end of the double-blind part of the trial, she said.
On trichoscopy, there was an increased number of hairs and follicular units at 12 weeks relative to baseline among those treated with topical delgocitinib but a reduction in those treated with vehicle.
JAKi Patients Gained Hair, Vehicle Patients Lost Hair
On the basis of hair count per square centimeter from baseline, delgocitinib-treated patients gained on average of seven hairs whereas vehicle recipients lost an average of 11 hairs at 24 weeks, Senna reported.
Patients originally treated with vehicle did improve in most outcome measures in the open-label extension of the experimental treatment after crossover, but they did not catch up to those initially randomized to delgocitinib because of further accrual of favorable changes in the active-treatment group over time.
“There were no adverse events associated with active therapy or vehicle, including application-site reactions,” Senna said. The one between-group difference was a higher rate of COVID-19, but this was greater in the control arm.
All 30 of the participants in this study were women, and all had moderate to severe disease at enrollment. The median age was 64 years. Because of the predominant population at the hair loss center, all but one of the participants were White, and one participant was Asian.
Characterizing FFA as “devastating and disfiguring,” Senna, who specializes in the care of alopecia, noted that this a difficult disease to control with the off-label strategies that are now used. The slow progress to identify treatments for FFA is illustrated by the fact that only one other double-blind and randomized trial has ever been conducted in FFA, she said.
Exploratory Study Supports Anecdotal Experience
On the basis of prior anecdotal experience with JAKi treatment for FFA, Senna said, “I do think that it is possible to get largely clear skin with this therapy.” However, she is now hoping for definitive trials to better characterize the efficacy and safety of oral and topical therapies, perhaps used sequentially to maintain clinical improvement.
In light of the limited current options, Menno de Rie, MD, PhD, professor of dermatology at the University of Amsterdam in the Netherlands, called these data “very inspiring and hopeful.” He suggested the promise of this therapy was reinforced by the upward trajectory of the biomarkers and clinical improvement over the study period.
“Any improvement in treatment options would be welcome, because we do not [have] any reliable therapies for this condition,” de Rie, who was not an investigator, said in an interview after the presentation.
Ultimately, Senna said, once effective therapy is established, the goal will be to start as early as possible in the disease process. She noted that there is evidence that prompt therapy can reverse the disorder, not just prevent progression.
“If you can get to the hair follicles before the point of no return, there is [a] chance [of] follicular rescue,” she said.
Delgocitinib cream (Anzupgo) was approved in Europe for treating chronic hand eczema in late September and is under review for the same indication in the United States.
Senna has financial relationships with Arena, Concert, Eli Lilly, Pfizer, and Leo Pharma, which provided funding for this study. de Rie reported no potential conflicts of interest.
A version of this article appeared on Medscape.com.
AMSTERDAM —
“This is an exciting avenue for FFA if the data are recapitulated in a larger population. It could be an important new treatment option,” said Maryanne Senna, MD, director at Lahey Hospital & Medical Center’s Hair Loss Center of Excellence, Burlington, Massachusetts, and assistant dermatology professor at Harvard Medical School, Boston, Massachusetts.
In a design characterized as “exploratory,” the trial had two parts: a randomized, double-blind, vehicle-controlled intervention for 12 weeks, followed by an open-label extension of topical delgocitinib for all participants for another 12 weeks.
The primary efficacy endpoint was change in the molecular signature of FFA inflammation at 12 weeks. Clinical improvement was monitored with both trichoscopic images capturing the numbers of hairs and follicular units at 12 weeks and clinical severity scores through week 24. In a topical cream formulation, the Janus kinase inhibitor (JAKi) delgocitinib was associated with favorable activity for both.
Some Hair Regrowth for All
“At 24 weeks, all patients achieved some degree of hair regrowth and a stabilization of disease based on hairline measurements,” Senna reported in a late-breaking news session at the 2024 European Academy of Dermatology and Venereology (EADV) Congress.
On the clinical endpoints, Senna noted an upward trajectory in clinical improvement at the completion of the study.
The 30 participants were randomly assigned in a 1:1 ratio to receive delgocitinib cream in a concentration of 20 mg/g or vehicle cream applied twice daily for 12 weeks. At the end of this double-blind period, patients on vehicle were crossed over to the active therapy, and all patients were monitored for another 12 weeks in an open-label extension.
The change from baseline in FFA biomarkers was selected as the primary endpoint based on previous work showing up-regulation in the expression of the Th1 biomarkers CXCL9, CXCL10, and interferon gamma in lesional vs nonlesional scalp in patients with FFA.
When biopsies at the end of 12 weeks in the double-blind phase of the study were compared with the baseline biopsies, researchers found a decrease in expression of the three local inflammation markers in all patients receiving the JAKi, but not in those receiving the vehicle cream. In this small patient sample, only the reduction in expression of CXCL9, a cytokine known for differentiation and promotion of leukocytes, reached statistical significance (P < .05).
But in an analysis involving the expression of multiple genes, “lesions treated with delgocitinib had a 4% improvement in normalization toward a nonlesional transcriptomic profile, while patients treated with vehicle had a 33% worsening,” Senna reported. The difference was highly significant (P < .001).
Furthermore, the decrease in total Lichen Planopilaris Activity Index and FFA severity scores were numerically and statistically greater (P = .023) in the active-treatment arm than in the vehicle arm by the end of the double-blind part of the trial, she said.
On trichoscopy, there was an increased number of hairs and follicular units at 12 weeks relative to baseline among those treated with topical delgocitinib but a reduction in those treated with vehicle.
JAKi Patients Gained Hair, Vehicle Patients Lost Hair
On the basis of hair count per square centimeter from baseline, delgocitinib-treated patients gained on average of seven hairs whereas vehicle recipients lost an average of 11 hairs at 24 weeks, Senna reported.
Patients originally treated with vehicle did improve in most outcome measures in the open-label extension of the experimental treatment after crossover, but they did not catch up to those initially randomized to delgocitinib because of further accrual of favorable changes in the active-treatment group over time.
“There were no adverse events associated with active therapy or vehicle, including application-site reactions,” Senna said. The one between-group difference was a higher rate of COVID-19, but this was greater in the control arm.
All 30 of the participants in this study were women, and all had moderate to severe disease at enrollment. The median age was 64 years. Because of the predominant population at the hair loss center, all but one of the participants were White, and one participant was Asian.
Characterizing FFA as “devastating and disfiguring,” Senna, who specializes in the care of alopecia, noted that this a difficult disease to control with the off-label strategies that are now used. The slow progress to identify treatments for FFA is illustrated by the fact that only one other double-blind and randomized trial has ever been conducted in FFA, she said.
Exploratory Study Supports Anecdotal Experience
On the basis of prior anecdotal experience with JAKi treatment for FFA, Senna said, “I do think that it is possible to get largely clear skin with this therapy.” However, she is now hoping for definitive trials to better characterize the efficacy and safety of oral and topical therapies, perhaps used sequentially to maintain clinical improvement.
In light of the limited current options, Menno de Rie, MD, PhD, professor of dermatology at the University of Amsterdam in the Netherlands, called these data “very inspiring and hopeful.” He suggested the promise of this therapy was reinforced by the upward trajectory of the biomarkers and clinical improvement over the study period.
“Any improvement in treatment options would be welcome, because we do not [have] any reliable therapies for this condition,” de Rie, who was not an investigator, said in an interview after the presentation.
Ultimately, Senna said, once effective therapy is established, the goal will be to start as early as possible in the disease process. She noted that there is evidence that prompt therapy can reverse the disorder, not just prevent progression.
“If you can get to the hair follicles before the point of no return, there is [a] chance [of] follicular rescue,” she said.
Delgocitinib cream (Anzupgo) was approved in Europe for treating chronic hand eczema in late September and is under review for the same indication in the United States.
Senna has financial relationships with Arena, Concert, Eli Lilly, Pfizer, and Leo Pharma, which provided funding for this study. de Rie reported no potential conflicts of interest.
A version of this article appeared on Medscape.com.
FROM EADV 2024
Nailing the Nail Biopsy: Surgical Instruments and Their Function in Nail Biopsy Procedures
Practice Gap
The term nail biopsy (NB) may refer to a punch, excisional, shave, or longitudinal biopsy of the nail matrix and/or nail bed.1 Nail surgeries, including NBs, are performed relatively infrequently. In a study using data from the Medicare Provider Utilization and Payment Database 2012-2017, only 1.01% of Mohs surgeons and 0.28% of general dermatologists in the United States performed NBs. Thirty-one states had no dermatologist-performed NBs, while 3 states had no nail biopsies performed by any physician, podiatrist, nurse practitioner, or physician assistant, indicating that there is a shortage of dermatology clinicians performing nail surgeries.2
Dermatologists may not be performing NBs due to unfamiliarity with nail unit anatomy and lack of formal NB training during residency.3 In a survey of 240 dermatology residents in the United States, 58% reported performing fewer than 10 nail procedures during residency, with 25% observing only.4 Of those surveyed, 1% had no exposure to nail procedures during 3 years of residency. Furthermore, when asked to assess their competency in nail surgery on a scale of not competent, competent, and very competent, approximately 30% responded that they were not competent.4 Without sufficient education on procedures involving the nail unit, residents may be reluctant to incorporate nail surgery into their clinical practice.
Due to their complexity, NBs require the use of several specialized surgical instruments that are not used for other dermatologic procedures, and residents and attending physicians who have limited nail training may be unfamiliar with these tools. To address this educational gap, we sought to create a guide that details the surgical instruments used for the nail matrix tangential excision (shave) biopsy technique—the most common technique used in our nail specialty clinic. This guide is intended for educational use by dermatologists who wish to incorporate NB as part of their practice.
Tools and Technique
As a major referral center, our New York City–based nail specialty clinic performs a large volume of NBs, many of them performed for clinically concerning longitudinal melanonychias for which a nail matrix shave biopsy most often is performed. We utilize a standardized tray consisting of 12 surgical instruments that are needed to successfully perform a NB from start to finish (Figure). In addition to standard surgical tray items, such as sutures and tissue scissors, additional specialized instruments are necessary for NB procedures, including a nail elevator, an English nail splitter, and skin hook.
After the initial incisions are made at 45° angles to the proximal nail fold surrounding the longitudinal band, the nail elevator is used to separate the proximal nail plate from the underlying nail bed. The English nail splitter is used to create a transverse split separating the proximal from the distal nail plate, and the proximal nail plate then is retracted using a clamp. The skin hook is used to retract the proximal nail fold to expose the pigment in the nail matrix, which is biopsied using the #15 blade and sent for histopathology. The proximal nail fold and retracted nail plate then are put back in place, and absorbable sutures are used to repair the defect. In certain cases, a 3-mm punch biopsy may be used to sample the nail plate and/or the surrounding soft tissue.
Practice Implications
A guide to surgical tools used during NB procedures, including less commonly encountered tools such as a nail elevator and English nail splitter, helps to close the educational gap of NB procedures among dermatology trainees and attending physicians. In conjunction with practical training with cadavers and models, a guide to surgical tools can be reviewed by trainees before hands-on exposure to nail surgery in a clinical setting. By increasing awareness of the tools needed to complete the procedure from start to finish, dermatologists may feel more prepared and confident in their ability to perform NBs, ultimately allowing for more rapid diagnosis of nail malignancies.
- Grover C, Bansal S. Nail biopsy: a user’s manual. Indian Dermatol Online J. 2018;9:3-15. doi:10.4103/idoj.IDOJ_268_17
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare Provider Utilization and Payment Database 2012 to 2017. Dermatol Ther. 2021;34:e14928. doi:10.1111/dth.14928
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273. doi:10.1016/j.det.2016.02.002
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.e4835. doi:10.1016/j.jaad.2010.05.044
Practice Gap
The term nail biopsy (NB) may refer to a punch, excisional, shave, or longitudinal biopsy of the nail matrix and/or nail bed.1 Nail surgeries, including NBs, are performed relatively infrequently. In a study using data from the Medicare Provider Utilization and Payment Database 2012-2017, only 1.01% of Mohs surgeons and 0.28% of general dermatologists in the United States performed NBs. Thirty-one states had no dermatologist-performed NBs, while 3 states had no nail biopsies performed by any physician, podiatrist, nurse practitioner, or physician assistant, indicating that there is a shortage of dermatology clinicians performing nail surgeries.2
Dermatologists may not be performing NBs due to unfamiliarity with nail unit anatomy and lack of formal NB training during residency.3 In a survey of 240 dermatology residents in the United States, 58% reported performing fewer than 10 nail procedures during residency, with 25% observing only.4 Of those surveyed, 1% had no exposure to nail procedures during 3 years of residency. Furthermore, when asked to assess their competency in nail surgery on a scale of not competent, competent, and very competent, approximately 30% responded that they were not competent.4 Without sufficient education on procedures involving the nail unit, residents may be reluctant to incorporate nail surgery into their clinical practice.
Due to their complexity, NBs require the use of several specialized surgical instruments that are not used for other dermatologic procedures, and residents and attending physicians who have limited nail training may be unfamiliar with these tools. To address this educational gap, we sought to create a guide that details the surgical instruments used for the nail matrix tangential excision (shave) biopsy technique—the most common technique used in our nail specialty clinic. This guide is intended for educational use by dermatologists who wish to incorporate NB as part of their practice.
Tools and Technique
As a major referral center, our New York City–based nail specialty clinic performs a large volume of NBs, many of them performed for clinically concerning longitudinal melanonychias for which a nail matrix shave biopsy most often is performed. We utilize a standardized tray consisting of 12 surgical instruments that are needed to successfully perform a NB from start to finish (Figure). In addition to standard surgical tray items, such as sutures and tissue scissors, additional specialized instruments are necessary for NB procedures, including a nail elevator, an English nail splitter, and skin hook.

After the initial incisions are made at 45° angles to the proximal nail fold surrounding the longitudinal band, the nail elevator is used to separate the proximal nail plate from the underlying nail bed. The English nail splitter is used to create a transverse split separating the proximal from the distal nail plate, and the proximal nail plate then is retracted using a clamp. The skin hook is used to retract the proximal nail fold to expose the pigment in the nail matrix, which is biopsied using the #15 blade and sent for histopathology. The proximal nail fold and retracted nail plate then are put back in place, and absorbable sutures are used to repair the defect. In certain cases, a 3-mm punch biopsy may be used to sample the nail plate and/or the surrounding soft tissue.
Practice Implications
A guide to surgical tools used during NB procedures, including less commonly encountered tools such as a nail elevator and English nail splitter, helps to close the educational gap of NB procedures among dermatology trainees and attending physicians. In conjunction with practical training with cadavers and models, a guide to surgical tools can be reviewed by trainees before hands-on exposure to nail surgery in a clinical setting. By increasing awareness of the tools needed to complete the procedure from start to finish, dermatologists may feel more prepared and confident in their ability to perform NBs, ultimately allowing for more rapid diagnosis of nail malignancies.
Practice Gap
The term nail biopsy (NB) may refer to a punch, excisional, shave, or longitudinal biopsy of the nail matrix and/or nail bed.1 Nail surgeries, including NBs, are performed relatively infrequently. In a study using data from the Medicare Provider Utilization and Payment Database 2012-2017, only 1.01% of Mohs surgeons and 0.28% of general dermatologists in the United States performed NBs. Thirty-one states had no dermatologist-performed NBs, while 3 states had no nail biopsies performed by any physician, podiatrist, nurse practitioner, or physician assistant, indicating that there is a shortage of dermatology clinicians performing nail surgeries.2
Dermatologists may not be performing NBs due to unfamiliarity with nail unit anatomy and lack of formal NB training during residency.3 In a survey of 240 dermatology residents in the United States, 58% reported performing fewer than 10 nail procedures during residency, with 25% observing only.4 Of those surveyed, 1% had no exposure to nail procedures during 3 years of residency. Furthermore, when asked to assess their competency in nail surgery on a scale of not competent, competent, and very competent, approximately 30% responded that they were not competent.4 Without sufficient education on procedures involving the nail unit, residents may be reluctant to incorporate nail surgery into their clinical practice.
Due to their complexity, NBs require the use of several specialized surgical instruments that are not used for other dermatologic procedures, and residents and attending physicians who have limited nail training may be unfamiliar with these tools. To address this educational gap, we sought to create a guide that details the surgical instruments used for the nail matrix tangential excision (shave) biopsy technique—the most common technique used in our nail specialty clinic. This guide is intended for educational use by dermatologists who wish to incorporate NB as part of their practice.
Tools and Technique
As a major referral center, our New York City–based nail specialty clinic performs a large volume of NBs, many of them performed for clinically concerning longitudinal melanonychias for which a nail matrix shave biopsy most often is performed. We utilize a standardized tray consisting of 12 surgical instruments that are needed to successfully perform a NB from start to finish (Figure). In addition to standard surgical tray items, such as sutures and tissue scissors, additional specialized instruments are necessary for NB procedures, including a nail elevator, an English nail splitter, and skin hook.

After the initial incisions are made at 45° angles to the proximal nail fold surrounding the longitudinal band, the nail elevator is used to separate the proximal nail plate from the underlying nail bed. The English nail splitter is used to create a transverse split separating the proximal from the distal nail plate, and the proximal nail plate then is retracted using a clamp. The skin hook is used to retract the proximal nail fold to expose the pigment in the nail matrix, which is biopsied using the #15 blade and sent for histopathology. The proximal nail fold and retracted nail plate then are put back in place, and absorbable sutures are used to repair the defect. In certain cases, a 3-mm punch biopsy may be used to sample the nail plate and/or the surrounding soft tissue.
Practice Implications
A guide to surgical tools used during NB procedures, including less commonly encountered tools such as a nail elevator and English nail splitter, helps to close the educational gap of NB procedures among dermatology trainees and attending physicians. In conjunction with practical training with cadavers and models, a guide to surgical tools can be reviewed by trainees before hands-on exposure to nail surgery in a clinical setting. By increasing awareness of the tools needed to complete the procedure from start to finish, dermatologists may feel more prepared and confident in their ability to perform NBs, ultimately allowing for more rapid diagnosis of nail malignancies.
- Grover C, Bansal S. Nail biopsy: a user’s manual. Indian Dermatol Online J. 2018;9:3-15. doi:10.4103/idoj.IDOJ_268_17
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare Provider Utilization and Payment Database 2012 to 2017. Dermatol Ther. 2021;34:e14928. doi:10.1111/dth.14928
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273. doi:10.1016/j.det.2016.02.002
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.e4835. doi:10.1016/j.jaad.2010.05.044
- Grover C, Bansal S. Nail biopsy: a user’s manual. Indian Dermatol Online J. 2018;9:3-15. doi:10.4103/idoj.IDOJ_268_17
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare Provider Utilization and Payment Database 2012 to 2017. Dermatol Ther. 2021;34:e14928. doi:10.1111/dth.14928
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273. doi:10.1016/j.det.2016.02.002
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.e4835. doi:10.1016/j.jaad.2010.05.044
Hairless Scalp Lesion
The Diagnosis: Nevus Sebaceus of Jadassohn
The diagnosis of nevus sebaceus of Jadassohn was made clinically based on the lesion’s appearance and presence since birth as well as the absence of systemic symptoms. Clinically, nevus sebaceus of Jadassohn typically manifests as a well-demarcated, yellow- brown plaque often located on the scalp, as was seen in our patient. The lack of pruritus and pain further supported the diagnosis in our patient. No biopsy was performed, as the presentation was considered classic for this condition. Our patient opted to forgo surgery and will be routinely monitored for any changes, as nevus sebaceus has a potential risk, albeit low, for malignant transformation later in life. No changes have been observed since the initial presentation, and regular follow-ups are planned to monitor for future developments.
Nevus sebaceus of Jadassohn is a hamartomatous lesion involving the pilosebaceous follicle and adjacent adnexal structures.1-3 It most commonly forms on the scalp (59.3%) and is accompanied by partial or total alopecia. 3,4 It is seen less often on the face, periauricular area, or neck1,4; thorax or limbs5; and oral or genital mucosae.6 Nevus sebaceus of Jadassohn affects approximately 0.3% of newborns,1 usually as a solitary lesion that can form an extensive plaque. The male-to-female occurrence ratio has been reported as equal to slightly more predominant in females; all races and ethnicities are affected.1,5
Nevus sebaceus of Jadassohn follows 3 stages of clinical development: infantile, adolescent, and adulthood. It manifests at birth or shortly afterward as a smooth hairless patch or plaque that is yellowish and can be hyperpigmented in Black patients.5 It may have an oval or linear configuration, typically is asymptomatic, and often arises along the Blaschko lines when it occurs as multiple lesions (a rare manifestation).1 During puberty, hormonal changes cause accelerated growth, sebaceous gland maturation, and epidermal hyperplasia. 7 Nevus sebaceus of Jadassohn often is not identified until this stage, when its classic wartlike appearance has fully developed.1
Patients with nevus sebaceus of Jadassohn have a 10% to 20% risk for tumor development in adulthood.2,7 Trichoblastoma and syringocystadenoma papilliferum are the most frequently described neoplasms.8 Basal cell carcinoma is the most common malignant secondary neoplasm with an occurrence rate of 0.8%.6,9 However, basal cell carcinoma and trichoblastoma may share histopathologic features, which may lead to misdiagnosis and a higher reported incidence of basal cell carcinoma in adults than is accurate.2
Early prophylactic surgical removal of nevus sebaceus of Jadassohn has been recommended; however, surgical management is controversial because the risk for a benign secondary neoplasm remains relatively high while the risk for malignancy is much lower.2,7 Surgical excision remains an acceptable option once the patient is mature enough to tolerate the procedure.1 However, patient education regarding watchful waiting vs a surgical approach— and the risks of each—is critical to ensure shared decision-making and a management plan tailored to the individual.
The differential diagnosis includes hypertrophic lichen planus, Langerhans cell histiocytosis (Letterer-Siwe disease type), epidermal nevus, and seborrheic keratosis. Hypertrophic lichen planus often occurs symmetrically on the dorsal feet and shins with thick, scaly, and extremely pruritic plaques. The lesions often persist for an average of 6 years and may lead to multiple keratoacanthomas or follicular base squamous cell carcinomas. Langerhans cell histiocytosis (Letterer-Siwe disease type) manifests with acute, disseminated, visceral, and cutaneous lesions before 2 years of age. These lesions appear as 1- to 2-mm, pink, seborrheic papules, pustules, or vesicles on the scalp, flexural neck, axilla, perineum, and trunk; they often are associated with petechiae, purpura, scale, crust, erosion, impetiginization, and tender fissures. Epidermal nevus occurs within the first year of life and is a hamartoma of the epidermis and papillary dermis. It manifests as papillomatous pigmented linear lines along the Blaschko lines. Seborrheic keratosis manifests as well-demarcated, waxy/verrucous, brown papules with a “stuck on” appearance on hair-bearing skin sparing the mucosae. They are common benign lesions associated with sun exposure and often manifest in the fourth decade of life.10
- Baigrie D, Troxell T, Cook C. Nevus sebaceus. StatPearls [Internet]. Updated August 16, 2023. Accessed September 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482493/
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239. doi:10.1097/01 .scs.0000221531.56529.cc
- Kelati A, Baybay H, Gallouj S, et al. Dermoscopic analysis of nevus sebaceus of Jadassohn: a study of 13 cases. Skin Appendage Disord. 2017;3:83-91. doi:10.1159/000460258
- Ugras N, Ozgun G, Adim SB, et al. Nevus sebaceous at unusual location: a rare presentation. Indian J Pathol Microbiol. 2012;55:419-420. doi:10.4103/0377-4929.101768
- Serpas de Lopez RM, Hernandez-Perez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72. doi:10.1111/j.1524-4725 .1985.tb02893.x
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268. doi:10.1016/S0190-9622(00)90136-1
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660. doi:10.1097/00001665-200309000-00010
- Chahboun F, Eljazouly M, Elomari M, et al. Trichoblastoma arising from the nevus sebaceus of Jadassohn. Cureus. 2021;13:E15325. doi:10.7759/cureus.15325
- Cazzato G, Cimmino A, Colagrande A, et al. The multiple faces of nodular trichoblastoma: review of the literature with case presentation. Dermatopathology (Basel). 2021;8:265-270. doi:10.3390 /dermatopathology8030032
- Dandekar MN, Gandhi RK. Neoplastic dermatology. In: Alikhan A, Hocker TLH (eds). Review of Dermatology. Elsevier; 2016: 321-366.
The Diagnosis: Nevus Sebaceus of Jadassohn
The diagnosis of nevus sebaceus of Jadassohn was made clinically based on the lesion’s appearance and presence since birth as well as the absence of systemic symptoms. Clinically, nevus sebaceus of Jadassohn typically manifests as a well-demarcated, yellow- brown plaque often located on the scalp, as was seen in our patient. The lack of pruritus and pain further supported the diagnosis in our patient. No biopsy was performed, as the presentation was considered classic for this condition. Our patient opted to forgo surgery and will be routinely monitored for any changes, as nevus sebaceus has a potential risk, albeit low, for malignant transformation later in life. No changes have been observed since the initial presentation, and regular follow-ups are planned to monitor for future developments.
Nevus sebaceus of Jadassohn is a hamartomatous lesion involving the pilosebaceous follicle and adjacent adnexal structures.1-3 It most commonly forms on the scalp (59.3%) and is accompanied by partial or total alopecia. 3,4 It is seen less often on the face, periauricular area, or neck1,4; thorax or limbs5; and oral or genital mucosae.6 Nevus sebaceus of Jadassohn affects approximately 0.3% of newborns,1 usually as a solitary lesion that can form an extensive plaque. The male-to-female occurrence ratio has been reported as equal to slightly more predominant in females; all races and ethnicities are affected.1,5
Nevus sebaceus of Jadassohn follows 3 stages of clinical development: infantile, adolescent, and adulthood. It manifests at birth or shortly afterward as a smooth hairless patch or plaque that is yellowish and can be hyperpigmented in Black patients.5 It may have an oval or linear configuration, typically is asymptomatic, and often arises along the Blaschko lines when it occurs as multiple lesions (a rare manifestation).1 During puberty, hormonal changes cause accelerated growth, sebaceous gland maturation, and epidermal hyperplasia. 7 Nevus sebaceus of Jadassohn often is not identified until this stage, when its classic wartlike appearance has fully developed.1
Patients with nevus sebaceus of Jadassohn have a 10% to 20% risk for tumor development in adulthood.2,7 Trichoblastoma and syringocystadenoma papilliferum are the most frequently described neoplasms.8 Basal cell carcinoma is the most common malignant secondary neoplasm with an occurrence rate of 0.8%.6,9 However, basal cell carcinoma and trichoblastoma may share histopathologic features, which may lead to misdiagnosis and a higher reported incidence of basal cell carcinoma in adults than is accurate.2
Early prophylactic surgical removal of nevus sebaceus of Jadassohn has been recommended; however, surgical management is controversial because the risk for a benign secondary neoplasm remains relatively high while the risk for malignancy is much lower.2,7 Surgical excision remains an acceptable option once the patient is mature enough to tolerate the procedure.1 However, patient education regarding watchful waiting vs a surgical approach— and the risks of each—is critical to ensure shared decision-making and a management plan tailored to the individual.
The differential diagnosis includes hypertrophic lichen planus, Langerhans cell histiocytosis (Letterer-Siwe disease type), epidermal nevus, and seborrheic keratosis. Hypertrophic lichen planus often occurs symmetrically on the dorsal feet and shins with thick, scaly, and extremely pruritic plaques. The lesions often persist for an average of 6 years and may lead to multiple keratoacanthomas or follicular base squamous cell carcinomas. Langerhans cell histiocytosis (Letterer-Siwe disease type) manifests with acute, disseminated, visceral, and cutaneous lesions before 2 years of age. These lesions appear as 1- to 2-mm, pink, seborrheic papules, pustules, or vesicles on the scalp, flexural neck, axilla, perineum, and trunk; they often are associated with petechiae, purpura, scale, crust, erosion, impetiginization, and tender fissures. Epidermal nevus occurs within the first year of life and is a hamartoma of the epidermis and papillary dermis. It manifests as papillomatous pigmented linear lines along the Blaschko lines. Seborrheic keratosis manifests as well-demarcated, waxy/verrucous, brown papules with a “stuck on” appearance on hair-bearing skin sparing the mucosae. They are common benign lesions associated with sun exposure and often manifest in the fourth decade of life.10
The Diagnosis: Nevus Sebaceus of Jadassohn
The diagnosis of nevus sebaceus of Jadassohn was made clinically based on the lesion’s appearance and presence since birth as well as the absence of systemic symptoms. Clinically, nevus sebaceus of Jadassohn typically manifests as a well-demarcated, yellow- brown plaque often located on the scalp, as was seen in our patient. The lack of pruritus and pain further supported the diagnosis in our patient. No biopsy was performed, as the presentation was considered classic for this condition. Our patient opted to forgo surgery and will be routinely monitored for any changes, as nevus sebaceus has a potential risk, albeit low, for malignant transformation later in life. No changes have been observed since the initial presentation, and regular follow-ups are planned to monitor for future developments.
Nevus sebaceus of Jadassohn is a hamartomatous lesion involving the pilosebaceous follicle and adjacent adnexal structures.1-3 It most commonly forms on the scalp (59.3%) and is accompanied by partial or total alopecia. 3,4 It is seen less often on the face, periauricular area, or neck1,4; thorax or limbs5; and oral or genital mucosae.6 Nevus sebaceus of Jadassohn affects approximately 0.3% of newborns,1 usually as a solitary lesion that can form an extensive plaque. The male-to-female occurrence ratio has been reported as equal to slightly more predominant in females; all races and ethnicities are affected.1,5
Nevus sebaceus of Jadassohn follows 3 stages of clinical development: infantile, adolescent, and adulthood. It manifests at birth or shortly afterward as a smooth hairless patch or plaque that is yellowish and can be hyperpigmented in Black patients.5 It may have an oval or linear configuration, typically is asymptomatic, and often arises along the Blaschko lines when it occurs as multiple lesions (a rare manifestation).1 During puberty, hormonal changes cause accelerated growth, sebaceous gland maturation, and epidermal hyperplasia. 7 Nevus sebaceus of Jadassohn often is not identified until this stage, when its classic wartlike appearance has fully developed.1
Patients with nevus sebaceus of Jadassohn have a 10% to 20% risk for tumor development in adulthood.2,7 Trichoblastoma and syringocystadenoma papilliferum are the most frequently described neoplasms.8 Basal cell carcinoma is the most common malignant secondary neoplasm with an occurrence rate of 0.8%.6,9 However, basal cell carcinoma and trichoblastoma may share histopathologic features, which may lead to misdiagnosis and a higher reported incidence of basal cell carcinoma in adults than is accurate.2
Early prophylactic surgical removal of nevus sebaceus of Jadassohn has been recommended; however, surgical management is controversial because the risk for a benign secondary neoplasm remains relatively high while the risk for malignancy is much lower.2,7 Surgical excision remains an acceptable option once the patient is mature enough to tolerate the procedure.1 However, patient education regarding watchful waiting vs a surgical approach— and the risks of each—is critical to ensure shared decision-making and a management plan tailored to the individual.
The differential diagnosis includes hypertrophic lichen planus, Langerhans cell histiocytosis (Letterer-Siwe disease type), epidermal nevus, and seborrheic keratosis. Hypertrophic lichen planus often occurs symmetrically on the dorsal feet and shins with thick, scaly, and extremely pruritic plaques. The lesions often persist for an average of 6 years and may lead to multiple keratoacanthomas or follicular base squamous cell carcinomas. Langerhans cell histiocytosis (Letterer-Siwe disease type) manifests with acute, disseminated, visceral, and cutaneous lesions before 2 years of age. These lesions appear as 1- to 2-mm, pink, seborrheic papules, pustules, or vesicles on the scalp, flexural neck, axilla, perineum, and trunk; they often are associated with petechiae, purpura, scale, crust, erosion, impetiginization, and tender fissures. Epidermal nevus occurs within the first year of life and is a hamartoma of the epidermis and papillary dermis. It manifests as papillomatous pigmented linear lines along the Blaschko lines. Seborrheic keratosis manifests as well-demarcated, waxy/verrucous, brown papules with a “stuck on” appearance on hair-bearing skin sparing the mucosae. They are common benign lesions associated with sun exposure and often manifest in the fourth decade of life.10
- Baigrie D, Troxell T, Cook C. Nevus sebaceus. StatPearls [Internet]. Updated August 16, 2023. Accessed September 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482493/
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239. doi:10.1097/01 .scs.0000221531.56529.cc
- Kelati A, Baybay H, Gallouj S, et al. Dermoscopic analysis of nevus sebaceus of Jadassohn: a study of 13 cases. Skin Appendage Disord. 2017;3:83-91. doi:10.1159/000460258
- Ugras N, Ozgun G, Adim SB, et al. Nevus sebaceous at unusual location: a rare presentation. Indian J Pathol Microbiol. 2012;55:419-420. doi:10.4103/0377-4929.101768
- Serpas de Lopez RM, Hernandez-Perez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72. doi:10.1111/j.1524-4725 .1985.tb02893.x
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268. doi:10.1016/S0190-9622(00)90136-1
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660. doi:10.1097/00001665-200309000-00010
- Chahboun F, Eljazouly M, Elomari M, et al. Trichoblastoma arising from the nevus sebaceus of Jadassohn. Cureus. 2021;13:E15325. doi:10.7759/cureus.15325
- Cazzato G, Cimmino A, Colagrande A, et al. The multiple faces of nodular trichoblastoma: review of the literature with case presentation. Dermatopathology (Basel). 2021;8:265-270. doi:10.3390 /dermatopathology8030032
- Dandekar MN, Gandhi RK. Neoplastic dermatology. In: Alikhan A, Hocker TLH (eds). Review of Dermatology. Elsevier; 2016: 321-366.
- Baigrie D, Troxell T, Cook C. Nevus sebaceus. StatPearls [Internet]. Updated August 16, 2023. Accessed September 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482493/
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239. doi:10.1097/01 .scs.0000221531.56529.cc
- Kelati A, Baybay H, Gallouj S, et al. Dermoscopic analysis of nevus sebaceus of Jadassohn: a study of 13 cases. Skin Appendage Disord. 2017;3:83-91. doi:10.1159/000460258
- Ugras N, Ozgun G, Adim SB, et al. Nevus sebaceous at unusual location: a rare presentation. Indian J Pathol Microbiol. 2012;55:419-420. doi:10.4103/0377-4929.101768
- Serpas de Lopez RM, Hernandez-Perez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72. doi:10.1111/j.1524-4725 .1985.tb02893.x
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42(2 pt 1):263-268. doi:10.1016/S0190-9622(00)90136-1
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660. doi:10.1097/00001665-200309000-00010
- Chahboun F, Eljazouly M, Elomari M, et al. Trichoblastoma arising from the nevus sebaceus of Jadassohn. Cureus. 2021;13:E15325. doi:10.7759/cureus.15325
- Cazzato G, Cimmino A, Colagrande A, et al. The multiple faces of nodular trichoblastoma: review of the literature with case presentation. Dermatopathology (Basel). 2021;8:265-270. doi:10.3390 /dermatopathology8030032
- Dandekar MN, Gandhi RK. Neoplastic dermatology. In: Alikhan A, Hocker TLH (eds). Review of Dermatology. Elsevier; 2016: 321-366.
A 23-year-old man presented to the dermatology clinic with hair loss on the scalp of several years’ duration. The patient reported persistent pigmented bumps on the back of the scalp. He denied any pruritus or pain and had no systemic symptoms or comorbidities. Physical examination revealed a 1×1.5-cm, yellow-brown, hairless plaque on the left parietal scalp.