User login
Perioperative infliximab does not increase serious infection risk
Administration of infliximab within 4 weeks of elective knee or hip arthroplasty did not have any significant effect on patients’ risk of serious infection after surgery, whereas the use of glucocorticoids increased that risk, in an analysis of a Medicare claims database.
“This increased risk with glucocorticoids has been suggested by previous studies [and] although this risk may be related in part to increased disease severity among glucocorticoid treated patients, a direct medication effect is likely. [These data suggest] that prolonged interruptions in infliximab therapy prior to surgery may be counterproductive if higher dose glucocorticoid therapy is used in substitution,” wrote the authors of the new study, led by Michael D. George, MD, of the University of Pennsylvania in Philadelphia.
Dr. George and his colleagues examined data from the U.S. Medicare claims system on 4,288 elective knee or hip arthroplasties in individuals with rheumatoid arthritis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or ankylosing spondylitis who received infliximab within 6 months prior to the operation during 2007-2013 (Arthritis Care Res. 2017 Jan 27. doi: 10.1002/acr.23209).
The patients had to have received infliximab at least three times within a year of their procedure to establish that they were receiving stable therapy over a long-term period. The investigators also looked at oral prednisone, prednisolone, and methylprednisolone prescriptions and used data on average dosing to determine how much was administered to each subject.
“Although previous studies have treated TNF stopping vs. not stopping as a dichotomous exposure based on an arbitrary (and variable) stopping definition, in this study the primary analysis evaluated stop timing as a more general categorical exposure using 4-week intervals (half the standard rheumatoid arthritis dosing interval) to allow better assessment of the optimal stop timing,” the authors explained.
Stopping infliximab within 4 weeks of the operation did not significantly influence the rate of serious infection within 30 days (adjusted odds ratio, 0.90; 95% CI, 0.60-1.34) and neither did stopping within 4-8 weeks (OR, 0.95; 95% CI, 0.62-1.36) when compared against stopping 8-12 weeks before surgery. Of the 4,288 arthroplasties, 270 serious infections (6.3%) occurred within 30 days of the operation.
There also was no significant difference between stopping within 4 weeks and 8-12 weeks in the rate of prosthetic joint infection within 1 year of the operation (hazard ratio, 0.98; 95% CI, 0.52-1.87). Overall, prosthetic joint infection occurred 2.9 times per 100 person-years.
However, glucocorticoid doses of more than 10 mg per day were risky. The odds for a serious infection within 30 days after surgery more than doubled with that level of use (OR, 2.11; 95% CI, 1.30-3.40), while the risk for a prosthetic joint infection within 1 year of the surgery also rose significantly (HR, 2.70; 95% CI, 1.30-5.60).
“This is a very well done paper that adds important observational data to our understanding of perioperative medication risk,” Dr. Goodman said.
But the study results will not, at least initially, bring about any changes to the proposed guidelines for perioperative management of patients taking antirheumatic drugs that were described at the 2016 annual meeting of the American College of Rheumatology, she said.
“We were aware of the abstract, which was also presented at the ACR last fall at the time the current perioperative medication management guidelines were presented, and it won’t change guidelines at this point,” said Dr. Goodman, who is one of the lead authors of the proposed guidelines. “[But] I think [the study] could provide important background information to use in a randomized clinical trial to compare infection on [and] not on TNF inhibitors.”
The proposed guidelines conditionally recommend that all biologics should be withheld prior to surgery in patients with inflammatory arthritis, that surgery should be planned for the end of the dosing cycle, and that current daily doses of glucocorticoids, rather than supraphysiologic doses, should be continued in adults with rheumatoid arthritis, lupus, or inflammatory arthritis.
The National Institutes of Health, the Rheumatology Research Foundation, and the Department of Veterans Affairs funded the study. Dr. George did not report any relevant financial disclosures. Two coauthors disclosed receiving research grants or consulting fees from pharmaceutical companies for unrelated work.
Administration of infliximab within 4 weeks of elective knee or hip arthroplasty did not have any significant effect on patients’ risk of serious infection after surgery, whereas the use of glucocorticoids increased that risk, in an analysis of a Medicare claims database.
“This increased risk with glucocorticoids has been suggested by previous studies [and] although this risk may be related in part to increased disease severity among glucocorticoid treated patients, a direct medication effect is likely. [These data suggest] that prolonged interruptions in infliximab therapy prior to surgery may be counterproductive if higher dose glucocorticoid therapy is used in substitution,” wrote the authors of the new study, led by Michael D. George, MD, of the University of Pennsylvania in Philadelphia.
Dr. George and his colleagues examined data from the U.S. Medicare claims system on 4,288 elective knee or hip arthroplasties in individuals with rheumatoid arthritis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or ankylosing spondylitis who received infliximab within 6 months prior to the operation during 2007-2013 (Arthritis Care Res. 2017 Jan 27. doi: 10.1002/acr.23209).
The patients had to have received infliximab at least three times within a year of their procedure to establish that they were receiving stable therapy over a long-term period. The investigators also looked at oral prednisone, prednisolone, and methylprednisolone prescriptions and used data on average dosing to determine how much was administered to each subject.
“Although previous studies have treated TNF stopping vs. not stopping as a dichotomous exposure based on an arbitrary (and variable) stopping definition, in this study the primary analysis evaluated stop timing as a more general categorical exposure using 4-week intervals (half the standard rheumatoid arthritis dosing interval) to allow better assessment of the optimal stop timing,” the authors explained.
Stopping infliximab within 4 weeks of the operation did not significantly influence the rate of serious infection within 30 days (adjusted odds ratio, 0.90; 95% CI, 0.60-1.34) and neither did stopping within 4-8 weeks (OR, 0.95; 95% CI, 0.62-1.36) when compared against stopping 8-12 weeks before surgery. Of the 4,288 arthroplasties, 270 serious infections (6.3%) occurred within 30 days of the operation.
There also was no significant difference between stopping within 4 weeks and 8-12 weeks in the rate of prosthetic joint infection within 1 year of the operation (hazard ratio, 0.98; 95% CI, 0.52-1.87). Overall, prosthetic joint infection occurred 2.9 times per 100 person-years.
However, glucocorticoid doses of more than 10 mg per day were risky. The odds for a serious infection within 30 days after surgery more than doubled with that level of use (OR, 2.11; 95% CI, 1.30-3.40), while the risk for a prosthetic joint infection within 1 year of the surgery also rose significantly (HR, 2.70; 95% CI, 1.30-5.60).
“This is a very well done paper that adds important observational data to our understanding of perioperative medication risk,” Dr. Goodman said.
But the study results will not, at least initially, bring about any changes to the proposed guidelines for perioperative management of patients taking antirheumatic drugs that were described at the 2016 annual meeting of the American College of Rheumatology, she said.
“We were aware of the abstract, which was also presented at the ACR last fall at the time the current perioperative medication management guidelines were presented, and it won’t change guidelines at this point,” said Dr. Goodman, who is one of the lead authors of the proposed guidelines. “[But] I think [the study] could provide important background information to use in a randomized clinical trial to compare infection on [and] not on TNF inhibitors.”
The proposed guidelines conditionally recommend that all biologics should be withheld prior to surgery in patients with inflammatory arthritis, that surgery should be planned for the end of the dosing cycle, and that current daily doses of glucocorticoids, rather than supraphysiologic doses, should be continued in adults with rheumatoid arthritis, lupus, or inflammatory arthritis.
The National Institutes of Health, the Rheumatology Research Foundation, and the Department of Veterans Affairs funded the study. Dr. George did not report any relevant financial disclosures. Two coauthors disclosed receiving research grants or consulting fees from pharmaceutical companies for unrelated work.
Administration of infliximab within 4 weeks of elective knee or hip arthroplasty did not have any significant effect on patients’ risk of serious infection after surgery, whereas the use of glucocorticoids increased that risk, in an analysis of a Medicare claims database.
“This increased risk with glucocorticoids has been suggested by previous studies [and] although this risk may be related in part to increased disease severity among glucocorticoid treated patients, a direct medication effect is likely. [These data suggest] that prolonged interruptions in infliximab therapy prior to surgery may be counterproductive if higher dose glucocorticoid therapy is used in substitution,” wrote the authors of the new study, led by Michael D. George, MD, of the University of Pennsylvania in Philadelphia.
Dr. George and his colleagues examined data from the U.S. Medicare claims system on 4,288 elective knee or hip arthroplasties in individuals with rheumatoid arthritis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or ankylosing spondylitis who received infliximab within 6 months prior to the operation during 2007-2013 (Arthritis Care Res. 2017 Jan 27. doi: 10.1002/acr.23209).
The patients had to have received infliximab at least three times within a year of their procedure to establish that they were receiving stable therapy over a long-term period. The investigators also looked at oral prednisone, prednisolone, and methylprednisolone prescriptions and used data on average dosing to determine how much was administered to each subject.
“Although previous studies have treated TNF stopping vs. not stopping as a dichotomous exposure based on an arbitrary (and variable) stopping definition, in this study the primary analysis evaluated stop timing as a more general categorical exposure using 4-week intervals (half the standard rheumatoid arthritis dosing interval) to allow better assessment of the optimal stop timing,” the authors explained.
Stopping infliximab within 4 weeks of the operation did not significantly influence the rate of serious infection within 30 days (adjusted odds ratio, 0.90; 95% CI, 0.60-1.34) and neither did stopping within 4-8 weeks (OR, 0.95; 95% CI, 0.62-1.36) when compared against stopping 8-12 weeks before surgery. Of the 4,288 arthroplasties, 270 serious infections (6.3%) occurred within 30 days of the operation.
There also was no significant difference between stopping within 4 weeks and 8-12 weeks in the rate of prosthetic joint infection within 1 year of the operation (hazard ratio, 0.98; 95% CI, 0.52-1.87). Overall, prosthetic joint infection occurred 2.9 times per 100 person-years.
However, glucocorticoid doses of more than 10 mg per day were risky. The odds for a serious infection within 30 days after surgery more than doubled with that level of use (OR, 2.11; 95% CI, 1.30-3.40), while the risk for a prosthetic joint infection within 1 year of the surgery also rose significantly (HR, 2.70; 95% CI, 1.30-5.60).
“This is a very well done paper that adds important observational data to our understanding of perioperative medication risk,” Dr. Goodman said.
But the study results will not, at least initially, bring about any changes to the proposed guidelines for perioperative management of patients taking antirheumatic drugs that were described at the 2016 annual meeting of the American College of Rheumatology, she said.
“We were aware of the abstract, which was also presented at the ACR last fall at the time the current perioperative medication management guidelines were presented, and it won’t change guidelines at this point,” said Dr. Goodman, who is one of the lead authors of the proposed guidelines. “[But] I think [the study] could provide important background information to use in a randomized clinical trial to compare infection on [and] not on TNF inhibitors.”
The proposed guidelines conditionally recommend that all biologics should be withheld prior to surgery in patients with inflammatory arthritis, that surgery should be planned for the end of the dosing cycle, and that current daily doses of glucocorticoids, rather than supraphysiologic doses, should be continued in adults with rheumatoid arthritis, lupus, or inflammatory arthritis.
The National Institutes of Health, the Rheumatology Research Foundation, and the Department of Veterans Affairs funded the study. Dr. George did not report any relevant financial disclosures. Two coauthors disclosed receiving research grants or consulting fees from pharmaceutical companies for unrelated work.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: Subjects on glucocorticoids had an OR of 2.11 (95% CI 1.30-3.40) for serious infection within 30 days and an HR of 2.70 (95% CI 1.30-5.60) for prosthetic joint infection within 1 year.
Data source: Retrospective cohort study of 4,288 elective knee and hip arthroplasties in Medicare patients with rheumatoid arthritis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or ankylosing spondylitis during 2007-2013.
Disclosures: The National Institutes of Health, the Rheumatology Research Foundation, and the Department of Veterans Affairs funded the study. Dr. George did not report any relevant financial disclosures. Two coauthors disclosed receiving research grants or consulting fees from pharmaceutical companies for unrelated work.
Poorer Arthroscopic Outcomes of Mild Dysplasia With Cam Femoroacetabular Impingement Versus Mixed Femoroacetabular Impingement in Absence of Capsular Repair
Take-Home Points
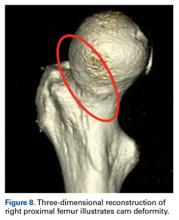
- Cam deformity often occurs with dysplasia.
- Borderline or mild dysplasia has been treated with isolated hip arthroscopy.
- Avoid rim trimming that can make mild dysplasia more severe.
- Labral preservation, cam decompression, and capsular repair or plication are currently suggested.
- Poorer outcomes occurred in borderline or mild dysplasia with cam impingement relative to controls following hip arthroscopy without capsular repair.
- Initial clinical improvement may be followed by clinical deterioration suggesting close long-term follow-up with prompt addition of reorientation acetabular osteotomy if indicated.
- It is unknown whether small capsulotomies may yield comparable outcomes with larger capsulotomies plus repair.
It is unknown whether small capsulotomies may yield comparable outcomes with larger capsulotomies plus repair. There is growing interest in hip preservation surgery in general and arthroscopic hip preservation in particular. Chondrolabral pathology leading to symptoms and degenerative progression typically is caused by structural abnormalities, mainly femoroacetabular impingement (FAI) and developmental dysplasia of the hip. Unlike the bony overcoverage of pincer FAI, developmental dysplasia of the hip typically exhibits insufficient anterolateral coverage of the femoral head.
The role of hip arthroscopy in the treatment of dysplasia remains undefined. Emerging evidence shows a high incidence of dysplasia with associated cam deformity,1,2 but there is a paucity of evidence-based information for this specific patient population. Clinical outcomes of hip arthroscopy in the setting of dysplasia are conflicting: some poor3-5 and others successful.1,6-9 Although reorientation periacetabular osteotomy (PAO) is considered a mainstay in the treatment of dysplasia—providing improvement in symptoms, deficient anterolateral acetabular coverage, and hip biomechanics—midterm failure rates approaching 24% have been reported.10-12 Many young patients with symptomatic dysplasia want a surgical option that is less invasive than open PAO.4 Intra-articular central compartment pathology and cam FAI commonly occur with dysplasia and are amenable to arthroscopic treatment.1,13,14 Moreover, staged PAO may be successful in cases in which arthroscopic intervention fails to provide clinical improvement.5,15
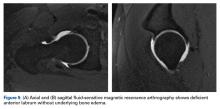
Emerging evidence suggests beneficial effects of arthroscopic capsular repair or plication in the setting of borderline or mild dysplasia.7,9 However, the literature provides little information on arthroscopic outcomes without capsular repair. One study found poor outcomes of arthroscopic surgery for dysplasia, but its patients underwent labral débridement, not repair.3 Two patients in a case report demonstrated rapidly progressive osteoarthritis after arthroscopic labral repairs and concurrent femoroplasties for cam FAI, but each had marked dysplasia with a lateral center-edge angle (LCEA) of <15°.4
Arthroscopy with capsular repair has been assumed to provide better outcomes than arthroscopy without repair, but to our knowledge there are no studies that have compared outcomes of mild dysplasia with cam FAI and outcomes of mixed FAI treated without capsular repair. Clinical equipoise makes it ethically challenging to perform a prospective study comparing dysplasia treated with and without capsular repair. We conducted a study to compare outcomes of mild dysplasia with cam FAI and outcomes of mixed FAI treated with arthroscopic surgery and to fill the knowledge gap regarding outcomes of mild dysplasia treated without capsular repair.
Methods
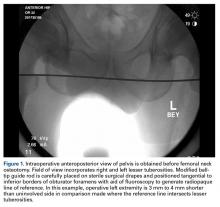
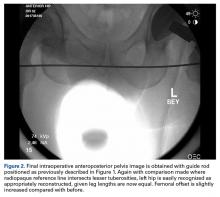
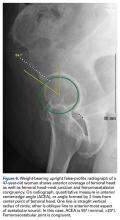
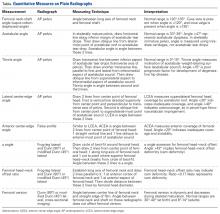
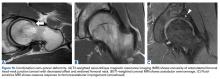
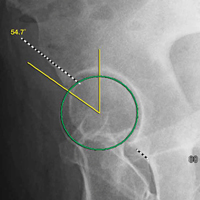
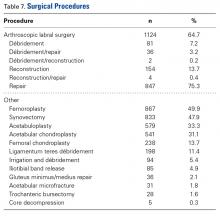
In this study, which received Institutional Review Board approval, we retrospectively reviewed radiographs and data from a prospective 3-center study of arthroscopic outcomes of FAI in 150 patients (159 hips) who underwent arthroscopic surgery by 1 of 3 surgeons between March 2009 and June 2010. In all cases, digital images of anteroposterior pelvic radiographs were used for radiographic measurements. On these images, the LCEA is formed by the intersection of the vertical line (corrected for obliquity using a horizontal reference line connecting the inferior extents of both radiographic teardrops) through the center of the femoral head (determined with a digital centering tool) with the line extending to the lateral edge of the sourcil (radiographic eyebrow of the weight-bearing region or roof of the acetabulum). Measurements were made in blinded fashion (by a nonsurgeon coauthor, Dr. Nikhil Gupta, who completed training modules) and were confirmed without alteration by the principal investigator Dr. Dean K. Matsuda. Inclusion criteria were mild acetabular dysplasia (LCEA, 15°-24°) and mixed FAI including focal pincer component (LCEA, 25°-39°), radiographic crossover sign, and successful completion of patient-reported outcome (PRO) measures at minimum 2-year follow-up. Exclusion criteria were severe dysplasia (LCEA, <15°), hip subluxation, broken Shenton line, global pincer FAI (LCEA, ≥40°), Tönnis grade 3 osteoarthritis, Legg-Calvé-Perthes disease, osteonecrosis, prior hip surgery, and unsuccessful completion of PRO measures. Outcome measures included investigator-blinded preoperative and postoperative Nonarthritic Hip Score (NAHS) and 5-point Likert satisfaction score. Complications, revision surgeries, and conversion arthroplasties were recorded.
Statistical Analysis
We examined outcomes with descriptive statistics for each of the candidate covariates in the model classified by femoroacetabular subtype: focal pincer and cam (mixed FAI) and dysplasia with cam. We examined the variables of sex, age, weight, height, body mass index, preoperative NAHS, presence of dysplasia (yes/no), presence of osteoarthritis (yes/no), Tönnis osteoarthritis grade, Outerbridge class, American Society of Anesthesiologists (ASA) score, months of pain, bilateral procedure (yes/no), and pincer involvement with cam FAI (yes/no). Before beginning linear regression modeling, we screened the candidate variables for strong correlations with other variables and looked for those variables with minimal missing data. For all these covariates, we then performed linear regression with a selection process—both a stepwise selection method and a backward elimination method—to verify we determined the same model for 24-month NAHS, or to understand why we could not. Finally, we ran the model we found from the linear regression as a linear mixed model of 24-month NAHS with the dichotomous variables taken as fixed effects and the other variables taken as random effects, using variance-components representation for the random effects. We then examined 3-month and 12-month NAHS with the same variables selected for the 24-month model.
To further examine and verify the effects of dysplasia on outcomes found in our linear mixed model, we performed a nested case–control analysis matching each member of cohort D (cases) with 2 members of cohort M (controls). We used an optimal-matching algorithm to match focal patients in the linear regression dataset with dysplasia patients in the linear regression dataset in such a way as to minimize the overall differences between the datasets. We matched cases and controls on preoperative NAHS, age, sex, presence of osteoarthritis, months of pain, ASA score, and body mass index. The differences between the matched cases and controls (control value minus case value) were compared using Wilcoxon rank sum tests for statistical significance of differences from 0 (with differences generated for each control group member, 2 differences per case) to examine the quality of the match. Finally, we examined the statistical significance of the difference of the outcome variables (3-, 12-, and 24-month NAHS) from 0, again using Wilcoxon rank sum tests. Statistical significance was set at P < .05 using SAS Version 9.3 (SAS Institute).
Surgical Procedure
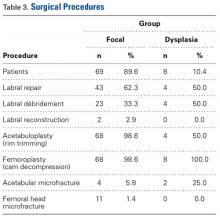
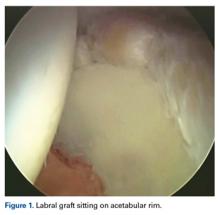
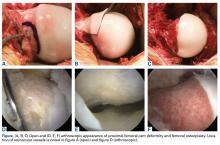
In all cases, supine outpatient hip arthroscopy was performed under general anesthesia. Anterolateral and modified midanterior portals16 were used. T-capsulotomies were performed in both cohorts. Cohort M underwent anterosuperior acetabuloplasty with a motorized burr. Labral refixation or selective débridement was performed in cohort M, whereas labral repair (with limited freshening of acetabular rim attachment site) or selective débridement (but no segmental resection) was performed in cohort D. Arthroscopic femoroplasty was performed with similar endpoints of 120° minimum hip flexion and 30° minimum flexed hip internal rotation with retention of the labral fluid seal. Capsular repair or plication was not performed for either cohort during the study period.
The cohorts underwent similar postoperative protocols: 2 weeks of protected ambulation using 2 crutches, exercise cycling without resistance beginning postoperative day 1, swimming at 2 weeks, elliptical machine workouts at 6 weeks, jogging at 12 weeks, and return to unrestricted athletics at 5 months.
Results
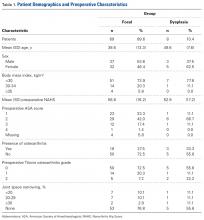
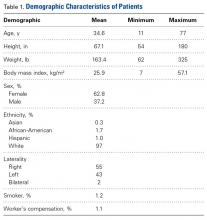
In cohort D, which consisted of 8 patients (5 female), mean age was 49.6 years, and mean LCEA was 19° (range, 16°-24°).
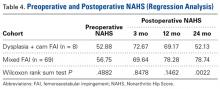
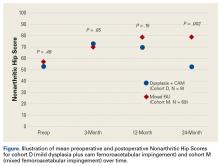
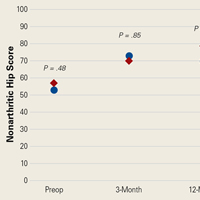
In cohort D, mean (SD) change in NAHS was +20.00 (6.24) (P = .25) at 3 months (n = 3), +14.33 (9.77) (P = .03) at 12 months (n = 6), and –0.75 (19.86) (P = .74) at 24 months (n = 8).
In cohort M, mean (SD) change in NAHS was +12.09 (18.98) (P < .0001) at 3 months (n = 45), +20.39 (16.49) (P < .0001) at 12 months (n = 57), and +21.99 (17.32) (P < .0001) at 24 months (n = 69).
In a pairwise case–control comparison, the mean (SD) change-from-baseline difference between cohorts D and M was +8.2 (12.85) (P = .31) at 3 months (n = 5), –8.7 (11.52) (P = .03) at 12 months (n = 10), and –31.06 (23.55) (P = .0002) at 24 months (n = 16). Dysplasia had an impact of –23.4 points on 24-month NAHS (standard error = 5.35 points; P < .0001), which corresponds to a 95% confidence interval of –12.9 to –33.9 points on NAHS.
Compared with cohort M, cohort D had significantly less NAHS improvement (P = .002), less satisfaction (P = .15) and more hip arthroplasty conversions (P = .22, not statistically significant).
There were no statistically significant differences between cohorts in demographics, preoperative variables, intraoperative findings, or surgical procedures in the regression analysis. Of the investigated variables, only group membership (cohort D) was a statistically significant predictor of poorer outcomes in the model of change from preoperative to 24 months. However, older age was associated with cohort D (older patients with dysplasia, P = .07), and therefore in the nested case–control analysis we were able to match on all variables except age (8.74 years older in cohort D, P = .0013) to a level of statistical nonsignificance.
Discussion
The principal finding of this study is the significantly poorer outcomes of mild dysplasia and cam FAI relative to mixed FAI after hip arthroscopy without capsular repair. Study group (cohort D) and control group (cohort M) had associated cam deformities treated with femoroplasty with similar decompression endpoints and labral preservation in the form of selective débridement or labral repair (no labral resections in either cohort) with similar rehabilitation protocols.
Our study findings suggest short-term improvement may be followed by midterm worsening in patients with mild dysplasia and sustained improvement in patients with mixed FAI. These findings have practical clinical applications. Jackson and colleagues5 reported on a patient who, after undergoing “successful” arthroscopic surgery for mild dysplasia, clinically deteriorated after 13 months and eventually required PAO. Patients undergoing isolated hip arthroscopy for mild dysplasia with cam FAI should be informed of the possible need for secondary PAO or even hip arthroplasty, be followed up more often and longer than comparable patients with FAI, and have follow-up supplemented with interval radiographs.4 If even subtle subluxation or joint narrowing occurs, we suggest resumption of protected weight-bearing and prompt progression to PAO in younger patients with joint congruency or eventual conversion arthroplasty in older ones.
Although mean preoperative NAHS (52.88) and mean 24-month postoperative NAHS (52.13) suggest essentially no change in PROs for cohort D, all patients with dysplasia either worsened or improved, though those who improved did so at a lesser relative magnitude than those with mixed FAI (cohort M). This finding may help explain the divergent outcomes reported in the literature on dysplasia treated with hip arthroscopy.
Cohort D was older than cohort M, but the difference was not statistically significant. Age may still be a confounding variable, and it may have contributed in part to the poorer outcomes for the patients with dysplasia. However, emerging studies demonstrate select older patients with FAI and/or labral tears may have successful outcomes with arthroscopic intervention.17,18 Our findings support mild dysplasia as the main contributor to the poor outcomes observed in this study.
With identical postoperative rehabilitation protocols, patients in both cohorts typically were ambulating without crutches by the end of postoperative week 2. Delayed weight-bearing has been suggested as contributing to successful outcomes in the setting of dysplasia7,19,20 but has not been shown to adversely affect nondysplastic hips.21 Whether delayed weight-bearing contributed to the poor outcomes in our dysplasia cohort is unknown, but the early successful outcomes may discount its influence.
Our findings support successful outcomes of arthroscopic treatment of mixed FAI (specifically focal pincer plus cam FAI) without capsular repair. Perhaps more important, we found inferior outcomes of arthroscopic treatment of mild dysplasia plus cam FAI without capsular repair—filling the knowledge gap regarding the need for arthroscopic capsular repair for mild dysplasia. Although a recent study demonstrated no significant difference in outcomes between hip arthroscopy with and without capsular repair,22 2 studies specific to mild dysplasia demonstrated successful outcomes of capsular repair.7,9 One found that mild dysplasia treated with arthroscopy, including capsular plication, resulted in 77% good/excellent outcomes and LCEA as low as 18° at minimum 2-year follow-up.7 The other found clinical improvement in mild dysplasia (LCEA, 15°-19°) when capsular repair was performed as part of arthroscopic treatment.9 In the present study, we retrospectively reviewed outcomes from a prospective study performed in 2009 to 2010, before the era of common capsular repair. It appears that capsular repair9 or plication7 in the setting of mild dysplasia may yield improved outcomes approaching those of arthroscopic FAI surgery. Our study results showed that, despite labral preservation and cam decompression, mild dysplasia without the closure of T-capsulotomy had inferior outcomes at 2 years. However, we do not know if outcomes would have been better with capsular repair or plication and/or smaller capsulotomies, perhaps with minimal violation of the iliofemoral ligament in this specific subset of patients. Furthermore, we do not know if optimal outcomes can best be achieved with arthroscopic and/or open surgery, with or without acetabular reorientation, in patients with mild dysplasia and cam FAI.
Dysplasia with cam FAI is an emerging common condition for which patients may seek less invasive treatment in the form of hip arthroscopy. The findings of this study suggest caution in using hip arthroscopy without capsular repair in the treatment of mild dysplasia with cam FAI, even in the presence of cam decompression and labral and acetabular rim preservation.
Study Strengths and Limitations
One strength was the relative lack of surgeon bias. When the surgeries were performed (2009-2010), we recognized cam and pincer FAI but did not discriminate for mild dysplasia, because at that time it was not known to be a potential predictor of poorer outcomes. Another strength was the strict methodology, with blinding of all investigator surgeons to PROs and stringent retention of all PROs, including “failures” (eg, total hip arthroplasty conversions and complications), in both cohorts. Moreover, the crucial case-control analysis matched on multiple variables verified statistically significant results demonstrating poorer outcomes at minimum 2-year follow-up, despite more improvement in the dysplasia cohort at 3 months. The latter, we think, is also valuable new information; it emphasizes the need for close and prolonged follow-up of patients with mild dysplasia despite early improvement.
Limitations include the small number of study patients, the retrospective study design (using prospectively collected data), and the isolated use of LCEA to define dysplasia. Pereira and colleagues23 recommended using LCEA with Tönnis angle to define minor dysplasia. Although dysplasia cannot be precisely defined with only this radiographic measurement, LCEA has been shown to be a reliable, clinically relevant measure.24 In addition, LCEA has been used in most reports on arthroscopic management of dysplastic hips and thus allows for comparison. Furthermore, other studies have used LCEA of <15° as a threshold between mild and severe dysplasia, and we did as well. This broad inclusion criterion allowed for heterogeneity in our mild dysplasia cohort and was a study limitation. Interobserver reliability of measured LCEA was not assessed and is another limitation.
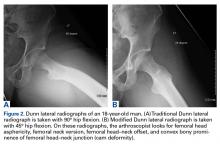
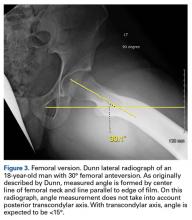
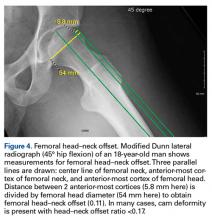
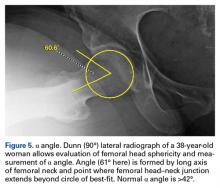
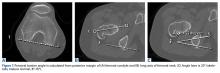
The initial prospective study (2009) did not record α angles to quantify cam FAI. This is a study limitation. However, the surgical range-of-motion endpoints considered sufficient for cam decompression were the same in both cohorts. In addition, femoral version was not assessed in the original database (2009-2010), as this aspect of hip anatomy was not thought significant during initial data collection. These areas of interest merit further investigation.
Use of a focal pincer cohort may be challenged as a suboptimal control group. However, there were very few completely normal acetabulae with pure cam FAI in the original prospective study, and the focal pincer cohort was used as a control cohort in previous studies.25
Conclusion
The common combination of mild dysplasia and cam FAI has poorer outcomes than mixed FAI after arthroscopic surgery without capsular repair.
Am J Orthop. 2017;46(1):E47-E53. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Paliobeis CP, Villar RN. The prevalence of dysplasia in femoroacetabular impingement. Hip Int. 2011;21(2):141-145.
2. Clohisy JC, Nunley RM, Carlisle JC, Schoenecker PL. Incidence and characteristics of femoral deformities in the dysplastic hip. Clin Orthop Relat Res. 2009;467(1):128-134.
3. Parvizi J, Bican O, Bender B, et al. Arthroscopy for labral tears in patients with developmental dysplasia of the hip: a cautionary note. J Arthroplasty. 2009;24(6 suppl):110-113.
4. Matsuda DK, Khatod M. Rapidly progressive osteoarthritis after arthroscopic labral repair in patients with hip dysplasia. Arthroscopy. 2012;28(11):1738-1743.
5. Jackson TJ, Watson J, LaReau JM, Domb BG. Periacetabular osteotomy and arthroscopic labral repair after failed hip arthroscopy due to iatrogenic aggravation of hip dysplasia. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):911-914.
6. Byrd JW, Jones KS. Hip arthroscopy in the presence of dysplasia. Arthroscopy. 2003;19(10):1055-1060.
7. Domb BG, Stake CE, Lindner D, El-Bitar Y, Jackson TJ. Arthroscopic capsular plication and labral preservation in borderline hip dysplasia: two-year clinical outcomes of a surgical approach to a challenging problem. Am J Sports Med. 2013;41(11):2591-2598.
8. Jayasekera N, Aprato A, Villar RN. Hip arthroscopy in the presence of acetabular dysplasia. Open Orthop J. 2015;9:185-187.
9. Fukui K, Briggs KK, Trindade CA, Philippon MJ. Outcomes after labral repair in patients with femoroacetabular impingement and borderline dysplasia. Arthroscopy. 2015;31(12):2371-2379.
10. Siebenrock KA, Leunig M, Ganz R. Periacetabular osteotomy: the Bernese experience. Instr Course Lect. 2001;50:239-245.
11. Garras DN, Crowder TT, Olson SA. Medium-term results of the Bernese periacetabular osteotomy in the treatment of symptomatic developmental dysplasia of the hip. J Bone Joint Surg Br. 2007;89(6):721-724.
12. Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H. Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop. 2008;32(5):611-617.
13. Ross JR, Zaltz I, Nepple JJ, Schoenecker PL, Clohisy JC. Arthroscopic disease classification and interventions as an adjunct in the treatment of acetabular dysplasia. Am J Sports Med. 2011;39(suppl):72S-78S.
14. Domb BG, LaReau JM, Baydoun H, Botser I, Millis MB, Yen YM. Is intraarticular pathology common in patients with hip dysplasia undergoing periacetabular osteotomy? Clin Orthop Relat Res. 2014;472(2):674-680.
15. Kain MS, Novais EN, Vallim C, Millis MB, Kim YJ. Periacetabular osteotomy after failed hip arthroscopy for labral tears in patients with acetabular dysplasia. J Bone Joint Surg Am. 2011;93(suppl 2):57-61.
16. Matsuda DK, Villamor A. The modified mid-anterior portal for hip arthroscopy. Arthrosc Tech. 2014;3(4):e469-e474.
17. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
18. Redmond JM, Gupta A, Cregar WM, Hammarstedt JE, Gui C, Domb BG. Arthroscopic treatment of labral tears in patients aged 60 years or older. Arthroscopy. 2015;31(10):1921-1927.
19. Mei-Dan O, McConkey MO, Brick M. Catastrophic failure of hip arthroscopy due to iatrogenic instability: can partial division of the ligamentum teres and iliofemoral ligament cause subluxation? Arthroscopy. 2012;28(3):440-445.
20. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
21. Jayasekera N, Aprato A, Villar RN. Are crutches required after hip arthroscopy? A case–control study. Hip Int. 2013;23(3):269-273.
22. Domb BG, Stake CE, Finley ZJ, Chen T, Giordano BD. Influence of capsular repair versus unrepaired capsulotomy on 2-year clinical outcomes after arthroscopic hip preservation surgery. Arthroscopy. 2015;31(4):643-650.
23. Pereira F, Giles A, Wood G, Board TN. Recognition of minor adult hip dysplasia: which anatomical indices are important? Hip Int. 2014;24(2):175-179.
24. Murphy SB, Ganz R, Müller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am. 1995;77(7):985-989.
25. Matsuda DK, Gupta N, Burchette R, Sehgal B. Arthroscopic surgery for global versus focal pincer femoroacetabular impingement: are the outcomes different? J Hip Preserv Surg. 2015;2(1):42-50.
Take-Home Points
- Cam deformity often occurs with dysplasia.
- Borderline or mild dysplasia has been treated with isolated hip arthroscopy.
- Avoid rim trimming that can make mild dysplasia more severe.
- Labral preservation, cam decompression, and capsular repair or plication are currently suggested.
- Poorer outcomes occurred in borderline or mild dysplasia with cam impingement relative to controls following hip arthroscopy without capsular repair.
- Initial clinical improvement may be followed by clinical deterioration suggesting close long-term follow-up with prompt addition of reorientation acetabular osteotomy if indicated.
- It is unknown whether small capsulotomies may yield comparable outcomes with larger capsulotomies plus repair.
It is unknown whether small capsulotomies may yield comparable outcomes with larger capsulotomies plus repair. There is growing interest in hip preservation surgery in general and arthroscopic hip preservation in particular. Chondrolabral pathology leading to symptoms and degenerative progression typically is caused by structural abnormalities, mainly femoroacetabular impingement (FAI) and developmental dysplasia of the hip. Unlike the bony overcoverage of pincer FAI, developmental dysplasia of the hip typically exhibits insufficient anterolateral coverage of the femoral head.
The role of hip arthroscopy in the treatment of dysplasia remains undefined. Emerging evidence shows a high incidence of dysplasia with associated cam deformity,1,2 but there is a paucity of evidence-based information for this specific patient population. Clinical outcomes of hip arthroscopy in the setting of dysplasia are conflicting: some poor3-5 and others successful.1,6-9 Although reorientation periacetabular osteotomy (PAO) is considered a mainstay in the treatment of dysplasia—providing improvement in symptoms, deficient anterolateral acetabular coverage, and hip biomechanics—midterm failure rates approaching 24% have been reported.10-12 Many young patients with symptomatic dysplasia want a surgical option that is less invasive than open PAO.4 Intra-articular central compartment pathology and cam FAI commonly occur with dysplasia and are amenable to arthroscopic treatment.1,13,14 Moreover, staged PAO may be successful in cases in which arthroscopic intervention fails to provide clinical improvement.5,15
Emerging evidence suggests beneficial effects of arthroscopic capsular repair or plication in the setting of borderline or mild dysplasia.7,9 However, the literature provides little information on arthroscopic outcomes without capsular repair. One study found poor outcomes of arthroscopic surgery for dysplasia, but its patients underwent labral débridement, not repair.3 Two patients in a case report demonstrated rapidly progressive osteoarthritis after arthroscopic labral repairs and concurrent femoroplasties for cam FAI, but each had marked dysplasia with a lateral center-edge angle (LCEA) of <15°.4
Arthroscopy with capsular repair has been assumed to provide better outcomes than arthroscopy without repair, but to our knowledge there are no studies that have compared outcomes of mild dysplasia with cam FAI and outcomes of mixed FAI treated without capsular repair. Clinical equipoise makes it ethically challenging to perform a prospective study comparing dysplasia treated with and without capsular repair. We conducted a study to compare outcomes of mild dysplasia with cam FAI and outcomes of mixed FAI treated with arthroscopic surgery and to fill the knowledge gap regarding outcomes of mild dysplasia treated without capsular repair.
Methods
In this study, which received Institutional Review Board approval, we retrospectively reviewed radiographs and data from a prospective 3-center study of arthroscopic outcomes of FAI in 150 patients (159 hips) who underwent arthroscopic surgery by 1 of 3 surgeons between March 2009 and June 2010. In all cases, digital images of anteroposterior pelvic radiographs were used for radiographic measurements. On these images, the LCEA is formed by the intersection of the vertical line (corrected for obliquity using a horizontal reference line connecting the inferior extents of both radiographic teardrops) through the center of the femoral head (determined with a digital centering tool) with the line extending to the lateral edge of the sourcil (radiographic eyebrow of the weight-bearing region or roof of the acetabulum). Measurements were made in blinded fashion (by a nonsurgeon coauthor, Dr. Nikhil Gupta, who completed training modules) and were confirmed without alteration by the principal investigator Dr. Dean K. Matsuda. Inclusion criteria were mild acetabular dysplasia (LCEA, 15°-24°) and mixed FAI including focal pincer component (LCEA, 25°-39°), radiographic crossover sign, and successful completion of patient-reported outcome (PRO) measures at minimum 2-year follow-up. Exclusion criteria were severe dysplasia (LCEA, <15°), hip subluxation, broken Shenton line, global pincer FAI (LCEA, ≥40°), Tönnis grade 3 osteoarthritis, Legg-Calvé-Perthes disease, osteonecrosis, prior hip surgery, and unsuccessful completion of PRO measures. Outcome measures included investigator-blinded preoperative and postoperative Nonarthritic Hip Score (NAHS) and 5-point Likert satisfaction score. Complications, revision surgeries, and conversion arthroplasties were recorded.
Statistical Analysis
We examined outcomes with descriptive statistics for each of the candidate covariates in the model classified by femoroacetabular subtype: focal pincer and cam (mixed FAI) and dysplasia with cam. We examined the variables of sex, age, weight, height, body mass index, preoperative NAHS, presence of dysplasia (yes/no), presence of osteoarthritis (yes/no), Tönnis osteoarthritis grade, Outerbridge class, American Society of Anesthesiologists (ASA) score, months of pain, bilateral procedure (yes/no), and pincer involvement with cam FAI (yes/no). Before beginning linear regression modeling, we screened the candidate variables for strong correlations with other variables and looked for those variables with minimal missing data. For all these covariates, we then performed linear regression with a selection process—both a stepwise selection method and a backward elimination method—to verify we determined the same model for 24-month NAHS, or to understand why we could not. Finally, we ran the model we found from the linear regression as a linear mixed model of 24-month NAHS with the dichotomous variables taken as fixed effects and the other variables taken as random effects, using variance-components representation for the random effects. We then examined 3-month and 12-month NAHS with the same variables selected for the 24-month model.
To further examine and verify the effects of dysplasia on outcomes found in our linear mixed model, we performed a nested case–control analysis matching each member of cohort D (cases) with 2 members of cohort M (controls). We used an optimal-matching algorithm to match focal patients in the linear regression dataset with dysplasia patients in the linear regression dataset in such a way as to minimize the overall differences between the datasets. We matched cases and controls on preoperative NAHS, age, sex, presence of osteoarthritis, months of pain, ASA score, and body mass index. The differences between the matched cases and controls (control value minus case value) were compared using Wilcoxon rank sum tests for statistical significance of differences from 0 (with differences generated for each control group member, 2 differences per case) to examine the quality of the match. Finally, we examined the statistical significance of the difference of the outcome variables (3-, 12-, and 24-month NAHS) from 0, again using Wilcoxon rank sum tests. Statistical significance was set at P < .05 using SAS Version 9.3 (SAS Institute).
Surgical Procedure
In all cases, supine outpatient hip arthroscopy was performed under general anesthesia. Anterolateral and modified midanterior portals16 were used. T-capsulotomies were performed in both cohorts. Cohort M underwent anterosuperior acetabuloplasty with a motorized burr. Labral refixation or selective débridement was performed in cohort M, whereas labral repair (with limited freshening of acetabular rim attachment site) or selective débridement (but no segmental resection) was performed in cohort D. Arthroscopic femoroplasty was performed with similar endpoints of 120° minimum hip flexion and 30° minimum flexed hip internal rotation with retention of the labral fluid seal. Capsular repair or plication was not performed for either cohort during the study period.
The cohorts underwent similar postoperative protocols: 2 weeks of protected ambulation using 2 crutches, exercise cycling without resistance beginning postoperative day 1, swimming at 2 weeks, elliptical machine workouts at 6 weeks, jogging at 12 weeks, and return to unrestricted athletics at 5 months.
Results
In cohort D, which consisted of 8 patients (5 female), mean age was 49.6 years, and mean LCEA was 19° (range, 16°-24°).
In cohort D, mean (SD) change in NAHS was +20.00 (6.24) (P = .25) at 3 months (n = 3), +14.33 (9.77) (P = .03) at 12 months (n = 6), and –0.75 (19.86) (P = .74) at 24 months (n = 8).
In cohort M, mean (SD) change in NAHS was +12.09 (18.98) (P < .0001) at 3 months (n = 45), +20.39 (16.49) (P < .0001) at 12 months (n = 57), and +21.99 (17.32) (P < .0001) at 24 months (n = 69).
In a pairwise case–control comparison, the mean (SD) change-from-baseline difference between cohorts D and M was +8.2 (12.85) (P = .31) at 3 months (n = 5), –8.7 (11.52) (P = .03) at 12 months (n = 10), and –31.06 (23.55) (P = .0002) at 24 months (n = 16). Dysplasia had an impact of –23.4 points on 24-month NAHS (standard error = 5.35 points; P < .0001), which corresponds to a 95% confidence interval of –12.9 to –33.9 points on NAHS.
Compared with cohort M, cohort D had significantly less NAHS improvement (P = .002), less satisfaction (P = .15) and more hip arthroplasty conversions (P = .22, not statistically significant).
There were no statistically significant differences between cohorts in demographics, preoperative variables, intraoperative findings, or surgical procedures in the regression analysis. Of the investigated variables, only group membership (cohort D) was a statistically significant predictor of poorer outcomes in the model of change from preoperative to 24 months. However, older age was associated with cohort D (older patients with dysplasia, P = .07), and therefore in the nested case–control analysis we were able to match on all variables except age (8.74 years older in cohort D, P = .0013) to a level of statistical nonsignificance.
Discussion
The principal finding of this study is the significantly poorer outcomes of mild dysplasia and cam FAI relative to mixed FAI after hip arthroscopy without capsular repair. Study group (cohort D) and control group (cohort M) had associated cam deformities treated with femoroplasty with similar decompression endpoints and labral preservation in the form of selective débridement or labral repair (no labral resections in either cohort) with similar rehabilitation protocols.
Our study findings suggest short-term improvement may be followed by midterm worsening in patients with mild dysplasia and sustained improvement in patients with mixed FAI. These findings have practical clinical applications. Jackson and colleagues5 reported on a patient who, after undergoing “successful” arthroscopic surgery for mild dysplasia, clinically deteriorated after 13 months and eventually required PAO. Patients undergoing isolated hip arthroscopy for mild dysplasia with cam FAI should be informed of the possible need for secondary PAO or even hip arthroplasty, be followed up more often and longer than comparable patients with FAI, and have follow-up supplemented with interval radiographs.4 If even subtle subluxation or joint narrowing occurs, we suggest resumption of protected weight-bearing and prompt progression to PAO in younger patients with joint congruency or eventual conversion arthroplasty in older ones.
Although mean preoperative NAHS (52.88) and mean 24-month postoperative NAHS (52.13) suggest essentially no change in PROs for cohort D, all patients with dysplasia either worsened or improved, though those who improved did so at a lesser relative magnitude than those with mixed FAI (cohort M). This finding may help explain the divergent outcomes reported in the literature on dysplasia treated with hip arthroscopy.
Cohort D was older than cohort M, but the difference was not statistically significant. Age may still be a confounding variable, and it may have contributed in part to the poorer outcomes for the patients with dysplasia. However, emerging studies demonstrate select older patients with FAI and/or labral tears may have successful outcomes with arthroscopic intervention.17,18 Our findings support mild dysplasia as the main contributor to the poor outcomes observed in this study.
With identical postoperative rehabilitation protocols, patients in both cohorts typically were ambulating without crutches by the end of postoperative week 2. Delayed weight-bearing has been suggested as contributing to successful outcomes in the setting of dysplasia7,19,20 but has not been shown to adversely affect nondysplastic hips.21 Whether delayed weight-bearing contributed to the poor outcomes in our dysplasia cohort is unknown, but the early successful outcomes may discount its influence.
Our findings support successful outcomes of arthroscopic treatment of mixed FAI (specifically focal pincer plus cam FAI) without capsular repair. Perhaps more important, we found inferior outcomes of arthroscopic treatment of mild dysplasia plus cam FAI without capsular repair—filling the knowledge gap regarding the need for arthroscopic capsular repair for mild dysplasia. Although a recent study demonstrated no significant difference in outcomes between hip arthroscopy with and without capsular repair,22 2 studies specific to mild dysplasia demonstrated successful outcomes of capsular repair.7,9 One found that mild dysplasia treated with arthroscopy, including capsular plication, resulted in 77% good/excellent outcomes and LCEA as low as 18° at minimum 2-year follow-up.7 The other found clinical improvement in mild dysplasia (LCEA, 15°-19°) when capsular repair was performed as part of arthroscopic treatment.9 In the present study, we retrospectively reviewed outcomes from a prospective study performed in 2009 to 2010, before the era of common capsular repair. It appears that capsular repair9 or plication7 in the setting of mild dysplasia may yield improved outcomes approaching those of arthroscopic FAI surgery. Our study results showed that, despite labral preservation and cam decompression, mild dysplasia without the closure of T-capsulotomy had inferior outcomes at 2 years. However, we do not know if outcomes would have been better with capsular repair or plication and/or smaller capsulotomies, perhaps with minimal violation of the iliofemoral ligament in this specific subset of patients. Furthermore, we do not know if optimal outcomes can best be achieved with arthroscopic and/or open surgery, with or without acetabular reorientation, in patients with mild dysplasia and cam FAI.
Dysplasia with cam FAI is an emerging common condition for which patients may seek less invasive treatment in the form of hip arthroscopy. The findings of this study suggest caution in using hip arthroscopy without capsular repair in the treatment of mild dysplasia with cam FAI, even in the presence of cam decompression and labral and acetabular rim preservation.
Study Strengths and Limitations
One strength was the relative lack of surgeon bias. When the surgeries were performed (2009-2010), we recognized cam and pincer FAI but did not discriminate for mild dysplasia, because at that time it was not known to be a potential predictor of poorer outcomes. Another strength was the strict methodology, with blinding of all investigator surgeons to PROs and stringent retention of all PROs, including “failures” (eg, total hip arthroplasty conversions and complications), in both cohorts. Moreover, the crucial case-control analysis matched on multiple variables verified statistically significant results demonstrating poorer outcomes at minimum 2-year follow-up, despite more improvement in the dysplasia cohort at 3 months. The latter, we think, is also valuable new information; it emphasizes the need for close and prolonged follow-up of patients with mild dysplasia despite early improvement.
Limitations include the small number of study patients, the retrospective study design (using prospectively collected data), and the isolated use of LCEA to define dysplasia. Pereira and colleagues23 recommended using LCEA with Tönnis angle to define minor dysplasia. Although dysplasia cannot be precisely defined with only this radiographic measurement, LCEA has been shown to be a reliable, clinically relevant measure.24 In addition, LCEA has been used in most reports on arthroscopic management of dysplastic hips and thus allows for comparison. Furthermore, other studies have used LCEA of <15° as a threshold between mild and severe dysplasia, and we did as well. This broad inclusion criterion allowed for heterogeneity in our mild dysplasia cohort and was a study limitation. Interobserver reliability of measured LCEA was not assessed and is another limitation.
The initial prospective study (2009) did not record α angles to quantify cam FAI. This is a study limitation. However, the surgical range-of-motion endpoints considered sufficient for cam decompression were the same in both cohorts. In addition, femoral version was not assessed in the original database (2009-2010), as this aspect of hip anatomy was not thought significant during initial data collection. These areas of interest merit further investigation.
Use of a focal pincer cohort may be challenged as a suboptimal control group. However, there were very few completely normal acetabulae with pure cam FAI in the original prospective study, and the focal pincer cohort was used as a control cohort in previous studies.25
Conclusion
The common combination of mild dysplasia and cam FAI has poorer outcomes than mixed FAI after arthroscopic surgery without capsular repair.
Am J Orthop. 2017;46(1):E47-E53. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Cam deformity often occurs with dysplasia.
- Borderline or mild dysplasia has been treated with isolated hip arthroscopy.
- Avoid rim trimming that can make mild dysplasia more severe.
- Labral preservation, cam decompression, and capsular repair or plication are currently suggested.
- Poorer outcomes occurred in borderline or mild dysplasia with cam impingement relative to controls following hip arthroscopy without capsular repair.
- Initial clinical improvement may be followed by clinical deterioration suggesting close long-term follow-up with prompt addition of reorientation acetabular osteotomy if indicated.
- It is unknown whether small capsulotomies may yield comparable outcomes with larger capsulotomies plus repair.
It is unknown whether small capsulotomies may yield comparable outcomes with larger capsulotomies plus repair. There is growing interest in hip preservation surgery in general and arthroscopic hip preservation in particular. Chondrolabral pathology leading to symptoms and degenerative progression typically is caused by structural abnormalities, mainly femoroacetabular impingement (FAI) and developmental dysplasia of the hip. Unlike the bony overcoverage of pincer FAI, developmental dysplasia of the hip typically exhibits insufficient anterolateral coverage of the femoral head.
The role of hip arthroscopy in the treatment of dysplasia remains undefined. Emerging evidence shows a high incidence of dysplasia with associated cam deformity,1,2 but there is a paucity of evidence-based information for this specific patient population. Clinical outcomes of hip arthroscopy in the setting of dysplasia are conflicting: some poor3-5 and others successful.1,6-9 Although reorientation periacetabular osteotomy (PAO) is considered a mainstay in the treatment of dysplasia—providing improvement in symptoms, deficient anterolateral acetabular coverage, and hip biomechanics—midterm failure rates approaching 24% have been reported.10-12 Many young patients with symptomatic dysplasia want a surgical option that is less invasive than open PAO.4 Intra-articular central compartment pathology and cam FAI commonly occur with dysplasia and are amenable to arthroscopic treatment.1,13,14 Moreover, staged PAO may be successful in cases in which arthroscopic intervention fails to provide clinical improvement.5,15
Emerging evidence suggests beneficial effects of arthroscopic capsular repair or plication in the setting of borderline or mild dysplasia.7,9 However, the literature provides little information on arthroscopic outcomes without capsular repair. One study found poor outcomes of arthroscopic surgery for dysplasia, but its patients underwent labral débridement, not repair.3 Two patients in a case report demonstrated rapidly progressive osteoarthritis after arthroscopic labral repairs and concurrent femoroplasties for cam FAI, but each had marked dysplasia with a lateral center-edge angle (LCEA) of <15°.4
Arthroscopy with capsular repair has been assumed to provide better outcomes than arthroscopy without repair, but to our knowledge there are no studies that have compared outcomes of mild dysplasia with cam FAI and outcomes of mixed FAI treated without capsular repair. Clinical equipoise makes it ethically challenging to perform a prospective study comparing dysplasia treated with and without capsular repair. We conducted a study to compare outcomes of mild dysplasia with cam FAI and outcomes of mixed FAI treated with arthroscopic surgery and to fill the knowledge gap regarding outcomes of mild dysplasia treated without capsular repair.
Methods
In this study, which received Institutional Review Board approval, we retrospectively reviewed radiographs and data from a prospective 3-center study of arthroscopic outcomes of FAI in 150 patients (159 hips) who underwent arthroscopic surgery by 1 of 3 surgeons between March 2009 and June 2010. In all cases, digital images of anteroposterior pelvic radiographs were used for radiographic measurements. On these images, the LCEA is formed by the intersection of the vertical line (corrected for obliquity using a horizontal reference line connecting the inferior extents of both radiographic teardrops) through the center of the femoral head (determined with a digital centering tool) with the line extending to the lateral edge of the sourcil (radiographic eyebrow of the weight-bearing region or roof of the acetabulum). Measurements were made in blinded fashion (by a nonsurgeon coauthor, Dr. Nikhil Gupta, who completed training modules) and were confirmed without alteration by the principal investigator Dr. Dean K. Matsuda. Inclusion criteria were mild acetabular dysplasia (LCEA, 15°-24°) and mixed FAI including focal pincer component (LCEA, 25°-39°), radiographic crossover sign, and successful completion of patient-reported outcome (PRO) measures at minimum 2-year follow-up. Exclusion criteria were severe dysplasia (LCEA, <15°), hip subluxation, broken Shenton line, global pincer FAI (LCEA, ≥40°), Tönnis grade 3 osteoarthritis, Legg-Calvé-Perthes disease, osteonecrosis, prior hip surgery, and unsuccessful completion of PRO measures. Outcome measures included investigator-blinded preoperative and postoperative Nonarthritic Hip Score (NAHS) and 5-point Likert satisfaction score. Complications, revision surgeries, and conversion arthroplasties were recorded.
Statistical Analysis
We examined outcomes with descriptive statistics for each of the candidate covariates in the model classified by femoroacetabular subtype: focal pincer and cam (mixed FAI) and dysplasia with cam. We examined the variables of sex, age, weight, height, body mass index, preoperative NAHS, presence of dysplasia (yes/no), presence of osteoarthritis (yes/no), Tönnis osteoarthritis grade, Outerbridge class, American Society of Anesthesiologists (ASA) score, months of pain, bilateral procedure (yes/no), and pincer involvement with cam FAI (yes/no). Before beginning linear regression modeling, we screened the candidate variables for strong correlations with other variables and looked for those variables with minimal missing data. For all these covariates, we then performed linear regression with a selection process—both a stepwise selection method and a backward elimination method—to verify we determined the same model for 24-month NAHS, or to understand why we could not. Finally, we ran the model we found from the linear regression as a linear mixed model of 24-month NAHS with the dichotomous variables taken as fixed effects and the other variables taken as random effects, using variance-components representation for the random effects. We then examined 3-month and 12-month NAHS with the same variables selected for the 24-month model.
To further examine and verify the effects of dysplasia on outcomes found in our linear mixed model, we performed a nested case–control analysis matching each member of cohort D (cases) with 2 members of cohort M (controls). We used an optimal-matching algorithm to match focal patients in the linear regression dataset with dysplasia patients in the linear regression dataset in such a way as to minimize the overall differences between the datasets. We matched cases and controls on preoperative NAHS, age, sex, presence of osteoarthritis, months of pain, ASA score, and body mass index. The differences between the matched cases and controls (control value minus case value) were compared using Wilcoxon rank sum tests for statistical significance of differences from 0 (with differences generated for each control group member, 2 differences per case) to examine the quality of the match. Finally, we examined the statistical significance of the difference of the outcome variables (3-, 12-, and 24-month NAHS) from 0, again using Wilcoxon rank sum tests. Statistical significance was set at P < .05 using SAS Version 9.3 (SAS Institute).
Surgical Procedure
In all cases, supine outpatient hip arthroscopy was performed under general anesthesia. Anterolateral and modified midanterior portals16 were used. T-capsulotomies were performed in both cohorts. Cohort M underwent anterosuperior acetabuloplasty with a motorized burr. Labral refixation or selective débridement was performed in cohort M, whereas labral repair (with limited freshening of acetabular rim attachment site) or selective débridement (but no segmental resection) was performed in cohort D. Arthroscopic femoroplasty was performed with similar endpoints of 120° minimum hip flexion and 30° minimum flexed hip internal rotation with retention of the labral fluid seal. Capsular repair or plication was not performed for either cohort during the study period.
The cohorts underwent similar postoperative protocols: 2 weeks of protected ambulation using 2 crutches, exercise cycling without resistance beginning postoperative day 1, swimming at 2 weeks, elliptical machine workouts at 6 weeks, jogging at 12 weeks, and return to unrestricted athletics at 5 months.
Results
In cohort D, which consisted of 8 patients (5 female), mean age was 49.6 years, and mean LCEA was 19° (range, 16°-24°).
In cohort D, mean (SD) change in NAHS was +20.00 (6.24) (P = .25) at 3 months (n = 3), +14.33 (9.77) (P = .03) at 12 months (n = 6), and –0.75 (19.86) (P = .74) at 24 months (n = 8).
In cohort M, mean (SD) change in NAHS was +12.09 (18.98) (P < .0001) at 3 months (n = 45), +20.39 (16.49) (P < .0001) at 12 months (n = 57), and +21.99 (17.32) (P < .0001) at 24 months (n = 69).
In a pairwise case–control comparison, the mean (SD) change-from-baseline difference between cohorts D and M was +8.2 (12.85) (P = .31) at 3 months (n = 5), –8.7 (11.52) (P = .03) at 12 months (n = 10), and –31.06 (23.55) (P = .0002) at 24 months (n = 16). Dysplasia had an impact of –23.4 points on 24-month NAHS (standard error = 5.35 points; P < .0001), which corresponds to a 95% confidence interval of –12.9 to –33.9 points on NAHS.
Compared with cohort M, cohort D had significantly less NAHS improvement (P = .002), less satisfaction (P = .15) and more hip arthroplasty conversions (P = .22, not statistically significant).
There were no statistically significant differences between cohorts in demographics, preoperative variables, intraoperative findings, or surgical procedures in the regression analysis. Of the investigated variables, only group membership (cohort D) was a statistically significant predictor of poorer outcomes in the model of change from preoperative to 24 months. However, older age was associated with cohort D (older patients with dysplasia, P = .07), and therefore in the nested case–control analysis we were able to match on all variables except age (8.74 years older in cohort D, P = .0013) to a level of statistical nonsignificance.
Discussion
The principal finding of this study is the significantly poorer outcomes of mild dysplasia and cam FAI relative to mixed FAI after hip arthroscopy without capsular repair. Study group (cohort D) and control group (cohort M) had associated cam deformities treated with femoroplasty with similar decompression endpoints and labral preservation in the form of selective débridement or labral repair (no labral resections in either cohort) with similar rehabilitation protocols.
Our study findings suggest short-term improvement may be followed by midterm worsening in patients with mild dysplasia and sustained improvement in patients with mixed FAI. These findings have practical clinical applications. Jackson and colleagues5 reported on a patient who, after undergoing “successful” arthroscopic surgery for mild dysplasia, clinically deteriorated after 13 months and eventually required PAO. Patients undergoing isolated hip arthroscopy for mild dysplasia with cam FAI should be informed of the possible need for secondary PAO or even hip arthroplasty, be followed up more often and longer than comparable patients with FAI, and have follow-up supplemented with interval radiographs.4 If even subtle subluxation or joint narrowing occurs, we suggest resumption of protected weight-bearing and prompt progression to PAO in younger patients with joint congruency or eventual conversion arthroplasty in older ones.
Although mean preoperative NAHS (52.88) and mean 24-month postoperative NAHS (52.13) suggest essentially no change in PROs for cohort D, all patients with dysplasia either worsened or improved, though those who improved did so at a lesser relative magnitude than those with mixed FAI (cohort M). This finding may help explain the divergent outcomes reported in the literature on dysplasia treated with hip arthroscopy.
Cohort D was older than cohort M, but the difference was not statistically significant. Age may still be a confounding variable, and it may have contributed in part to the poorer outcomes for the patients with dysplasia. However, emerging studies demonstrate select older patients with FAI and/or labral tears may have successful outcomes with arthroscopic intervention.17,18 Our findings support mild dysplasia as the main contributor to the poor outcomes observed in this study.
With identical postoperative rehabilitation protocols, patients in both cohorts typically were ambulating without crutches by the end of postoperative week 2. Delayed weight-bearing has been suggested as contributing to successful outcomes in the setting of dysplasia7,19,20 but has not been shown to adversely affect nondysplastic hips.21 Whether delayed weight-bearing contributed to the poor outcomes in our dysplasia cohort is unknown, but the early successful outcomes may discount its influence.
Our findings support successful outcomes of arthroscopic treatment of mixed FAI (specifically focal pincer plus cam FAI) without capsular repair. Perhaps more important, we found inferior outcomes of arthroscopic treatment of mild dysplasia plus cam FAI without capsular repair—filling the knowledge gap regarding the need for arthroscopic capsular repair for mild dysplasia. Although a recent study demonstrated no significant difference in outcomes between hip arthroscopy with and without capsular repair,22 2 studies specific to mild dysplasia demonstrated successful outcomes of capsular repair.7,9 One found that mild dysplasia treated with arthroscopy, including capsular plication, resulted in 77% good/excellent outcomes and LCEA as low as 18° at minimum 2-year follow-up.7 The other found clinical improvement in mild dysplasia (LCEA, 15°-19°) when capsular repair was performed as part of arthroscopic treatment.9 In the present study, we retrospectively reviewed outcomes from a prospective study performed in 2009 to 2010, before the era of common capsular repair. It appears that capsular repair9 or plication7 in the setting of mild dysplasia may yield improved outcomes approaching those of arthroscopic FAI surgery. Our study results showed that, despite labral preservation and cam decompression, mild dysplasia without the closure of T-capsulotomy had inferior outcomes at 2 years. However, we do not know if outcomes would have been better with capsular repair or plication and/or smaller capsulotomies, perhaps with minimal violation of the iliofemoral ligament in this specific subset of patients. Furthermore, we do not know if optimal outcomes can best be achieved with arthroscopic and/or open surgery, with or without acetabular reorientation, in patients with mild dysplasia and cam FAI.
Dysplasia with cam FAI is an emerging common condition for which patients may seek less invasive treatment in the form of hip arthroscopy. The findings of this study suggest caution in using hip arthroscopy without capsular repair in the treatment of mild dysplasia with cam FAI, even in the presence of cam decompression and labral and acetabular rim preservation.
Study Strengths and Limitations
One strength was the relative lack of surgeon bias. When the surgeries were performed (2009-2010), we recognized cam and pincer FAI but did not discriminate for mild dysplasia, because at that time it was not known to be a potential predictor of poorer outcomes. Another strength was the strict methodology, with blinding of all investigator surgeons to PROs and stringent retention of all PROs, including “failures” (eg, total hip arthroplasty conversions and complications), in both cohorts. Moreover, the crucial case-control analysis matched on multiple variables verified statistically significant results demonstrating poorer outcomes at minimum 2-year follow-up, despite more improvement in the dysplasia cohort at 3 months. The latter, we think, is also valuable new information; it emphasizes the need for close and prolonged follow-up of patients with mild dysplasia despite early improvement.
Limitations include the small number of study patients, the retrospective study design (using prospectively collected data), and the isolated use of LCEA to define dysplasia. Pereira and colleagues23 recommended using LCEA with Tönnis angle to define minor dysplasia. Although dysplasia cannot be precisely defined with only this radiographic measurement, LCEA has been shown to be a reliable, clinically relevant measure.24 In addition, LCEA has been used in most reports on arthroscopic management of dysplastic hips and thus allows for comparison. Furthermore, other studies have used LCEA of <15° as a threshold between mild and severe dysplasia, and we did as well. This broad inclusion criterion allowed for heterogeneity in our mild dysplasia cohort and was a study limitation. Interobserver reliability of measured LCEA was not assessed and is another limitation.
The initial prospective study (2009) did not record α angles to quantify cam FAI. This is a study limitation. However, the surgical range-of-motion endpoints considered sufficient for cam decompression were the same in both cohorts. In addition, femoral version was not assessed in the original database (2009-2010), as this aspect of hip anatomy was not thought significant during initial data collection. These areas of interest merit further investigation.
Use of a focal pincer cohort may be challenged as a suboptimal control group. However, there were very few completely normal acetabulae with pure cam FAI in the original prospective study, and the focal pincer cohort was used as a control cohort in previous studies.25
Conclusion
The common combination of mild dysplasia and cam FAI has poorer outcomes than mixed FAI after arthroscopic surgery without capsular repair.
Am J Orthop. 2017;46(1):E47-E53. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Paliobeis CP, Villar RN. The prevalence of dysplasia in femoroacetabular impingement. Hip Int. 2011;21(2):141-145.
2. Clohisy JC, Nunley RM, Carlisle JC, Schoenecker PL. Incidence and characteristics of femoral deformities in the dysplastic hip. Clin Orthop Relat Res. 2009;467(1):128-134.
3. Parvizi J, Bican O, Bender B, et al. Arthroscopy for labral tears in patients with developmental dysplasia of the hip: a cautionary note. J Arthroplasty. 2009;24(6 suppl):110-113.
4. Matsuda DK, Khatod M. Rapidly progressive osteoarthritis after arthroscopic labral repair in patients with hip dysplasia. Arthroscopy. 2012;28(11):1738-1743.
5. Jackson TJ, Watson J, LaReau JM, Domb BG. Periacetabular osteotomy and arthroscopic labral repair after failed hip arthroscopy due to iatrogenic aggravation of hip dysplasia. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):911-914.
6. Byrd JW, Jones KS. Hip arthroscopy in the presence of dysplasia. Arthroscopy. 2003;19(10):1055-1060.
7. Domb BG, Stake CE, Lindner D, El-Bitar Y, Jackson TJ. Arthroscopic capsular plication and labral preservation in borderline hip dysplasia: two-year clinical outcomes of a surgical approach to a challenging problem. Am J Sports Med. 2013;41(11):2591-2598.
8. Jayasekera N, Aprato A, Villar RN. Hip arthroscopy in the presence of acetabular dysplasia. Open Orthop J. 2015;9:185-187.
9. Fukui K, Briggs KK, Trindade CA, Philippon MJ. Outcomes after labral repair in patients with femoroacetabular impingement and borderline dysplasia. Arthroscopy. 2015;31(12):2371-2379.
10. Siebenrock KA, Leunig M, Ganz R. Periacetabular osteotomy: the Bernese experience. Instr Course Lect. 2001;50:239-245.
11. Garras DN, Crowder TT, Olson SA. Medium-term results of the Bernese periacetabular osteotomy in the treatment of symptomatic developmental dysplasia of the hip. J Bone Joint Surg Br. 2007;89(6):721-724.
12. Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H. Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop. 2008;32(5):611-617.
13. Ross JR, Zaltz I, Nepple JJ, Schoenecker PL, Clohisy JC. Arthroscopic disease classification and interventions as an adjunct in the treatment of acetabular dysplasia. Am J Sports Med. 2011;39(suppl):72S-78S.
14. Domb BG, LaReau JM, Baydoun H, Botser I, Millis MB, Yen YM. Is intraarticular pathology common in patients with hip dysplasia undergoing periacetabular osteotomy? Clin Orthop Relat Res. 2014;472(2):674-680.
15. Kain MS, Novais EN, Vallim C, Millis MB, Kim YJ. Periacetabular osteotomy after failed hip arthroscopy for labral tears in patients with acetabular dysplasia. J Bone Joint Surg Am. 2011;93(suppl 2):57-61.
16. Matsuda DK, Villamor A. The modified mid-anterior portal for hip arthroscopy. Arthrosc Tech. 2014;3(4):e469-e474.
17. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
18. Redmond JM, Gupta A, Cregar WM, Hammarstedt JE, Gui C, Domb BG. Arthroscopic treatment of labral tears in patients aged 60 years or older. Arthroscopy. 2015;31(10):1921-1927.
19. Mei-Dan O, McConkey MO, Brick M. Catastrophic failure of hip arthroscopy due to iatrogenic instability: can partial division of the ligamentum teres and iliofemoral ligament cause subluxation? Arthroscopy. 2012;28(3):440-445.
20. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
21. Jayasekera N, Aprato A, Villar RN. Are crutches required after hip arthroscopy? A case–control study. Hip Int. 2013;23(3):269-273.
22. Domb BG, Stake CE, Finley ZJ, Chen T, Giordano BD. Influence of capsular repair versus unrepaired capsulotomy on 2-year clinical outcomes after arthroscopic hip preservation surgery. Arthroscopy. 2015;31(4):643-650.
23. Pereira F, Giles A, Wood G, Board TN. Recognition of minor adult hip dysplasia: which anatomical indices are important? Hip Int. 2014;24(2):175-179.
24. Murphy SB, Ganz R, Müller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am. 1995;77(7):985-989.
25. Matsuda DK, Gupta N, Burchette R, Sehgal B. Arthroscopic surgery for global versus focal pincer femoroacetabular impingement: are the outcomes different? J Hip Preserv Surg. 2015;2(1):42-50.
1. Paliobeis CP, Villar RN. The prevalence of dysplasia in femoroacetabular impingement. Hip Int. 2011;21(2):141-145.
2. Clohisy JC, Nunley RM, Carlisle JC, Schoenecker PL. Incidence and characteristics of femoral deformities in the dysplastic hip. Clin Orthop Relat Res. 2009;467(1):128-134.
3. Parvizi J, Bican O, Bender B, et al. Arthroscopy for labral tears in patients with developmental dysplasia of the hip: a cautionary note. J Arthroplasty. 2009;24(6 suppl):110-113.
4. Matsuda DK, Khatod M. Rapidly progressive osteoarthritis after arthroscopic labral repair in patients with hip dysplasia. Arthroscopy. 2012;28(11):1738-1743.
5. Jackson TJ, Watson J, LaReau JM, Domb BG. Periacetabular osteotomy and arthroscopic labral repair after failed hip arthroscopy due to iatrogenic aggravation of hip dysplasia. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):911-914.
6. Byrd JW, Jones KS. Hip arthroscopy in the presence of dysplasia. Arthroscopy. 2003;19(10):1055-1060.
7. Domb BG, Stake CE, Lindner D, El-Bitar Y, Jackson TJ. Arthroscopic capsular plication and labral preservation in borderline hip dysplasia: two-year clinical outcomes of a surgical approach to a challenging problem. Am J Sports Med. 2013;41(11):2591-2598.
8. Jayasekera N, Aprato A, Villar RN. Hip arthroscopy in the presence of acetabular dysplasia. Open Orthop J. 2015;9:185-187.
9. Fukui K, Briggs KK, Trindade CA, Philippon MJ. Outcomes after labral repair in patients with femoroacetabular impingement and borderline dysplasia. Arthroscopy. 2015;31(12):2371-2379.
10. Siebenrock KA, Leunig M, Ganz R. Periacetabular osteotomy: the Bernese experience. Instr Course Lect. 2001;50:239-245.
11. Garras DN, Crowder TT, Olson SA. Medium-term results of the Bernese periacetabular osteotomy in the treatment of symptomatic developmental dysplasia of the hip. J Bone Joint Surg Br. 2007;89(6):721-724.
12. Biedermann R, Donnan L, Gabriel A, Wachter R, Krismer M, Behensky H. Complications and patient satisfaction after periacetabular pelvic osteotomy. Int Orthop. 2008;32(5):611-617.
13. Ross JR, Zaltz I, Nepple JJ, Schoenecker PL, Clohisy JC. Arthroscopic disease classification and interventions as an adjunct in the treatment of acetabular dysplasia. Am J Sports Med. 2011;39(suppl):72S-78S.
14. Domb BG, LaReau JM, Baydoun H, Botser I, Millis MB, Yen YM. Is intraarticular pathology common in patients with hip dysplasia undergoing periacetabular osteotomy? Clin Orthop Relat Res. 2014;472(2):674-680.
15. Kain MS, Novais EN, Vallim C, Millis MB, Kim YJ. Periacetabular osteotomy after failed hip arthroscopy for labral tears in patients with acetabular dysplasia. J Bone Joint Surg Am. 2011;93(suppl 2):57-61.
16. Matsuda DK, Villamor A. The modified mid-anterior portal for hip arthroscopy. Arthrosc Tech. 2014;3(4):e469-e474.
17. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
18. Redmond JM, Gupta A, Cregar WM, Hammarstedt JE, Gui C, Domb BG. Arthroscopic treatment of labral tears in patients aged 60 years or older. Arthroscopy. 2015;31(10):1921-1927.
19. Mei-Dan O, McConkey MO, Brick M. Catastrophic failure of hip arthroscopy due to iatrogenic instability: can partial division of the ligamentum teres and iliofemoral ligament cause subluxation? Arthroscopy. 2012;28(3):440-445.
20. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
21. Jayasekera N, Aprato A, Villar RN. Are crutches required after hip arthroscopy? A case–control study. Hip Int. 2013;23(3):269-273.
22. Domb BG, Stake CE, Finley ZJ, Chen T, Giordano BD. Influence of capsular repair versus unrepaired capsulotomy on 2-year clinical outcomes after arthroscopic hip preservation surgery. Arthroscopy. 2015;31(4):643-650.
23. Pereira F, Giles A, Wood G, Board TN. Recognition of minor adult hip dysplasia: which anatomical indices are important? Hip Int. 2014;24(2):175-179.
24. Murphy SB, Ganz R, Müller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am. 1995;77(7):985-989.
25. Matsuda DK, Gupta N, Burchette R, Sehgal B. Arthroscopic surgery for global versus focal pincer femoroacetabular impingement: are the outcomes different? J Hip Preserv Surg. 2015;2(1):42-50.
Using a Modified Ball-Tip Guide Rod to Equalize Leg Length and Restore Femoral Offset
Take-Home Points
- Preoperative radiographic templating alerts surgeons to certain intraoperative issues that may arise during surgery.
- Intraoperative fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip arthroplasty components.
- Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.
- A radiopaque line generated by the guide rod serves as a reference point that permits immediate objective comparison of femoral leg length and offset intraoperatively.
- The modified ball-tip guide rod is relatively inexpensive and has several practical purposes in total joint surgery.
Patient satisfaction scores after total hip arthroplasty (THA) approach 100%.1 Goals of this surgery include pain alleviation, motion restoration, and normalization of leg-length inequality. Asymmetric leg lengths are associated with nerve traction injuries, lower extremity joint pain, sacroiliac discomfort, low back pain, and patient dissatisfaction.1-3 For these reasons, postoperative leg-length discrepancy has become the most common reason for THA-related litigation.1,4
With preoperative education, patients and surgeons can discuss realistic THA goals and expectations. Besides ensuring that the correct tools and implants are available for the procedure, radiographic templating alerts surgeons to certain intraoperative issues that may arise during cases. For instance, an extremity may need to be lengthened during the surgery in order to generate the amount of soft-tissue tension needed to convey adequate stability to the hip joint.
In asymptomatic populations, lower extremity leg lengths inherently vary by an average of 5 mm.5 Studies have found normal populations are unable to accurately perceive a leg-length inequality of <1 cm.3,6,7 Lengthening an extremity >2.5 cm causes sciatic nerve symptoms.2 Patients may notice a leg-length discrepancy during the first few months after hip replacement, but this perception often subsides as gait normalizes and soft tissues acclimatize.
Our hospital uses a special arthroplasty table and intraoperative fluoroscopy for direct anterior (DA) THA cases. The table permits the operative extremity to undergo traction and the necessary mobility for proximal femur exposure. Fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip components.8We have developed an innovative use for a ball-tip guide rod (3.0 mm × 1000 mm; Smith & Nephew) to help accurately restore leg length and femoral offset after DA-THA. The ball-tip guide rod was modified to a length of 500 mm and rough edges were smoothed.
Technique
After the patient is prepared and draped in standard fashion on the operating table, a 10-cm skin incision is made directly over the proximal aspect of the tensor fascia lata muscle. Soft tissues are dissected down to the hip capsule, which is then incised and tagged for closure at the end of the case.
The fluoroscopic C-arm is sterilely draped and positioned from the nonoperative side. The image intensifier is centered over the pubic symphysis and lowered within 1 inch of the perineal post and surgical drapes. The C-arm unit is then aimed 10° to 15° cephalad until the size and orientation of the obturator foramens on fluoroscopic imaging coincide with the preoperative template.
Next, the modified guide rod, ball tip first, is carefully advanced toward the nonoperative side and over the surgical drapes between the pelvis and the C-arm image intensifier. Care is taken to avoid violating the sterile field by inadvertently puncturing the surgical drapes with the guide rod. The lower extremities are externally rotated 20° to bring the lesser tuberosities into profile view. With use of several fluoroscopic views, the guide rod is aligned with the inferior borders of the ischial tuberosities or the obturator foramens, whichever are more readily identified on the intraoperative images. A skin marker is then used to illustrate the position of the guide rod on the operative drapes for future reference.
At this point, the relationship between the radiopaque guide rod and the lesser trochanters is noted to gain a sense of native femoral leg length and offset, and the image (Figure 1) is saved in the C-arm computer for later recall and comparison views.
Next, the femoral neck osteotomy is performed according to the preoperative template. Acetabular preparation and component insertion are completed under fluoroscopic guidance.
After appropriate soft-tissue releases, the operating table is used to position the operative leg in extension, external rotation, and adduction. The femur is then sequentially broached until the template size is reached or until there is an audible change in pitch. At this point, a trial neck with head ball is fixed to the broach, and the hip is reduced.
The fluoroscopic C-arm is then repositioned over the pelvis, as previously described, with the guide rod over the pelvis and tangential to the ischial tuberosities. A new image (Figure 2) is obtained with the trial components in place.
The radiopaque line generated by the guide rod represents a reference point that permits objective comparison of femoral leg length and offset based on distance to the lesser trochanters. Different modular components can be trialed until the correct combination of variables accurately restores the desired parameters.
Once parameters are restored, trial femoral components are removed, and a corresponding monolithic femoral stem is gently impacted into the proximal femur and fitted with the appropriate head ball. A final image is obtained with the guide rod and implants in place and is saved as proof of restoration of leg length.
Discussion
Various techniques of assessing intraoperative leg length have been described, and each has its advantages and disadvantages. Relying on abductor tension or comparing leg lengths on the operating table is not always accurate and is strongly dependent on patient position.2,6
Referencing the tip of the greater trochanter to a Steinmann pin inserted into the ilium provides a precise reference point, but this invasive technique has the potential for fracture propagation through the drill hole.2,7Superimposing a trial femoral component over the proximal femur to determine the appropriate femoral neck osteotomy has been described, but this process can be difficult through a tight DA approach.9Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.2 Gililland and colleagues10 developed a reusable fluoroscopic transparent grid system that significantly improves component positioning during DA-THA.
The modified ball-tip guide rod is relatively inexpensive (<$100) and has several practical purposes in total joint surgery. The guide rod historically has been used to sound the center of the femoral canal before broaching. In revision cases and in cases of poor bone stock, the tool can be used to verify that cortical perforation has not occurred during canal preparation. In this article, we describe another realistic use for the guide rod: to create, during DA-THA, a radiographic reference line that can be used to help restore leg length and femoral offset.
Several authors have mentioned surgeons’ drawing the reference line on paper printouts of intraoperative images.11 Not only is this practice fraught with potential contamination of the operative field, but valuable time is lost waiting for paper copies and putting on a new gown and gloves before reentering the sterile field.
We used to train a radiologic technician or operating room nurse to draw a computerized reference line connecting the lesser trochanters on the fluoroscopic image. Problems arose in working with revolving nursing staff and in distinguishing the thin black line on computer monitors. In contrast, the radiopaque line from the guide rod is easily differentiated on fluoroscopic images, the technique poses less of a risk to the sterile field, and proper orientation of the guide rod to obtain the appropriate reference line is entirely surgeon-dependent.
A drawback of this technique is the additional radiation exposure that occurs when extra images are obtained to ensure satisfactory alignment of the guide rod. Another issue is fluoroscopic parallax. Some machines in the operating department generate a magnetic field that can interfere with the fluoroscopy beam and thereby slightly distort the intraoperative images.8 Therefore, it is imperative that the guide rod remain perfectly straight to avoid confounding measurements.
Our modified guide rod technique is a reliable, quick, and inexpensive intraoperative tool that helps in accurately restoring leg length and femoral offset during DA-THA.
Am J Orthop. 2017;46(1):E10-E12. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Whitehouse MR, Stefanovich-Lawbuary NS, Brunton LR, Blom AW. The impact of leg length discrepancy on patient satisfaction and functional outcome following total hip arthroplasty. J Arthroplasty. 2013;28(8):1408-1414.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
4. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
5. Knutson GA. Anatomic and functional leg-length inequality: a review and recommendation for clinical decision-making. Part I, anatomic leg-length inequality: prevalence, magnitude, effects and clinical significance. Chiropr Osteopat. 2005;13:11.
6. Iagulli ND, Mallory TH, Berend KR, et al. A simple and accurate method for determining leg length in primary total hip arthroplasty. Am J Orthop. 2006;35(10):455-457.
7. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
8. Weber M, Woerner M, Springorum R, et al. Fluoroscopy and imageless navigation enable an equivalent reconstruction of leg length and global and femoral offset in THA. Clin Orthop Relat Res. 2014;472(10):3150-3158.
9. Alazzawi S, Douglas SL, Haddad FS. A novel intra-operative technique to achieve accurate leg length and femoral offset during total hip replacement. Ann R Coll Surg Engl. 2012;94(4):281-282.
10. Gililland JM, Anderson LA, Boffeli SL, Pelt CE, Peters CL, Kubiak EN. A fluoroscopic grid in supine total hip arthroplasty: improving cup position, limb length, and hip offset. J Arthroplasty. 2012;27(8 suppl):111-116.
11. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
Take-Home Points
- Preoperative radiographic templating alerts surgeons to certain intraoperative issues that may arise during surgery.
- Intraoperative fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip arthroplasty components.
- Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.
- A radiopaque line generated by the guide rod serves as a reference point that permits immediate objective comparison of femoral leg length and offset intraoperatively.
- The modified ball-tip guide rod is relatively inexpensive and has several practical purposes in total joint surgery.
Patient satisfaction scores after total hip arthroplasty (THA) approach 100%.1 Goals of this surgery include pain alleviation, motion restoration, and normalization of leg-length inequality. Asymmetric leg lengths are associated with nerve traction injuries, lower extremity joint pain, sacroiliac discomfort, low back pain, and patient dissatisfaction.1-3 For these reasons, postoperative leg-length discrepancy has become the most common reason for THA-related litigation.1,4
With preoperative education, patients and surgeons can discuss realistic THA goals and expectations. Besides ensuring that the correct tools and implants are available for the procedure, radiographic templating alerts surgeons to certain intraoperative issues that may arise during cases. For instance, an extremity may need to be lengthened during the surgery in order to generate the amount of soft-tissue tension needed to convey adequate stability to the hip joint.
In asymptomatic populations, lower extremity leg lengths inherently vary by an average of 5 mm.5 Studies have found normal populations are unable to accurately perceive a leg-length inequality of <1 cm.3,6,7 Lengthening an extremity >2.5 cm causes sciatic nerve symptoms.2 Patients may notice a leg-length discrepancy during the first few months after hip replacement, but this perception often subsides as gait normalizes and soft tissues acclimatize.
Our hospital uses a special arthroplasty table and intraoperative fluoroscopy for direct anterior (DA) THA cases. The table permits the operative extremity to undergo traction and the necessary mobility for proximal femur exposure. Fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip components.8We have developed an innovative use for a ball-tip guide rod (3.0 mm × 1000 mm; Smith & Nephew) to help accurately restore leg length and femoral offset after DA-THA. The ball-tip guide rod was modified to a length of 500 mm and rough edges were smoothed.
Technique
After the patient is prepared and draped in standard fashion on the operating table, a 10-cm skin incision is made directly over the proximal aspect of the tensor fascia lata muscle. Soft tissues are dissected down to the hip capsule, which is then incised and tagged for closure at the end of the case.
The fluoroscopic C-arm is sterilely draped and positioned from the nonoperative side. The image intensifier is centered over the pubic symphysis and lowered within 1 inch of the perineal post and surgical drapes. The C-arm unit is then aimed 10° to 15° cephalad until the size and orientation of the obturator foramens on fluoroscopic imaging coincide with the preoperative template.
Next, the modified guide rod, ball tip first, is carefully advanced toward the nonoperative side and over the surgical drapes between the pelvis and the C-arm image intensifier. Care is taken to avoid violating the sterile field by inadvertently puncturing the surgical drapes with the guide rod. The lower extremities are externally rotated 20° to bring the lesser tuberosities into profile view. With use of several fluoroscopic views, the guide rod is aligned with the inferior borders of the ischial tuberosities or the obturator foramens, whichever are more readily identified on the intraoperative images. A skin marker is then used to illustrate the position of the guide rod on the operative drapes for future reference.
At this point, the relationship between the radiopaque guide rod and the lesser trochanters is noted to gain a sense of native femoral leg length and offset, and the image (Figure 1) is saved in the C-arm computer for later recall and comparison views.
Next, the femoral neck osteotomy is performed according to the preoperative template. Acetabular preparation and component insertion are completed under fluoroscopic guidance.
After appropriate soft-tissue releases, the operating table is used to position the operative leg in extension, external rotation, and adduction. The femur is then sequentially broached until the template size is reached or until there is an audible change in pitch. At this point, a trial neck with head ball is fixed to the broach, and the hip is reduced.
The fluoroscopic C-arm is then repositioned over the pelvis, as previously described, with the guide rod over the pelvis and tangential to the ischial tuberosities. A new image (Figure 2) is obtained with the trial components in place.
The radiopaque line generated by the guide rod represents a reference point that permits objective comparison of femoral leg length and offset based on distance to the lesser trochanters. Different modular components can be trialed until the correct combination of variables accurately restores the desired parameters.
Once parameters are restored, trial femoral components are removed, and a corresponding monolithic femoral stem is gently impacted into the proximal femur and fitted with the appropriate head ball. A final image is obtained with the guide rod and implants in place and is saved as proof of restoration of leg length.
Discussion
Various techniques of assessing intraoperative leg length have been described, and each has its advantages and disadvantages. Relying on abductor tension or comparing leg lengths on the operating table is not always accurate and is strongly dependent on patient position.2,6
Referencing the tip of the greater trochanter to a Steinmann pin inserted into the ilium provides a precise reference point, but this invasive technique has the potential for fracture propagation through the drill hole.2,7Superimposing a trial femoral component over the proximal femur to determine the appropriate femoral neck osteotomy has been described, but this process can be difficult through a tight DA approach.9Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.2 Gililland and colleagues10 developed a reusable fluoroscopic transparent grid system that significantly improves component positioning during DA-THA.
The modified ball-tip guide rod is relatively inexpensive (<$100) and has several practical purposes in total joint surgery. The guide rod historically has been used to sound the center of the femoral canal before broaching. In revision cases and in cases of poor bone stock, the tool can be used to verify that cortical perforation has not occurred during canal preparation. In this article, we describe another realistic use for the guide rod: to create, during DA-THA, a radiographic reference line that can be used to help restore leg length and femoral offset.
Several authors have mentioned surgeons’ drawing the reference line on paper printouts of intraoperative images.11 Not only is this practice fraught with potential contamination of the operative field, but valuable time is lost waiting for paper copies and putting on a new gown and gloves before reentering the sterile field.
We used to train a radiologic technician or operating room nurse to draw a computerized reference line connecting the lesser trochanters on the fluoroscopic image. Problems arose in working with revolving nursing staff and in distinguishing the thin black line on computer monitors. In contrast, the radiopaque line from the guide rod is easily differentiated on fluoroscopic images, the technique poses less of a risk to the sterile field, and proper orientation of the guide rod to obtain the appropriate reference line is entirely surgeon-dependent.
A drawback of this technique is the additional radiation exposure that occurs when extra images are obtained to ensure satisfactory alignment of the guide rod. Another issue is fluoroscopic parallax. Some machines in the operating department generate a magnetic field that can interfere with the fluoroscopy beam and thereby slightly distort the intraoperative images.8 Therefore, it is imperative that the guide rod remain perfectly straight to avoid confounding measurements.
Our modified guide rod technique is a reliable, quick, and inexpensive intraoperative tool that helps in accurately restoring leg length and femoral offset during DA-THA.
Am J Orthop. 2017;46(1):E10-E12. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Preoperative radiographic templating alerts surgeons to certain intraoperative issues that may arise during surgery.
- Intraoperative fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip arthroplasty components.
- Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.
- A radiopaque line generated by the guide rod serves as a reference point that permits immediate objective comparison of femoral leg length and offset intraoperatively.
- The modified ball-tip guide rod is relatively inexpensive and has several practical purposes in total joint surgery.
Patient satisfaction scores after total hip arthroplasty (THA) approach 100%.1 Goals of this surgery include pain alleviation, motion restoration, and normalization of leg-length inequality. Asymmetric leg lengths are associated with nerve traction injuries, lower extremity joint pain, sacroiliac discomfort, low back pain, and patient dissatisfaction.1-3 For these reasons, postoperative leg-length discrepancy has become the most common reason for THA-related litigation.1,4
With preoperative education, patients and surgeons can discuss realistic THA goals and expectations. Besides ensuring that the correct tools and implants are available for the procedure, radiographic templating alerts surgeons to certain intraoperative issues that may arise during cases. For instance, an extremity may need to be lengthened during the surgery in order to generate the amount of soft-tissue tension needed to convey adequate stability to the hip joint.
In asymptomatic populations, lower extremity leg lengths inherently vary by an average of 5 mm.5 Studies have found normal populations are unable to accurately perceive a leg-length inequality of <1 cm.3,6,7 Lengthening an extremity >2.5 cm causes sciatic nerve symptoms.2 Patients may notice a leg-length discrepancy during the first few months after hip replacement, but this perception often subsides as gait normalizes and soft tissues acclimatize.
Our hospital uses a special arthroplasty table and intraoperative fluoroscopy for direct anterior (DA) THA cases. The table permits the operative extremity to undergo traction and the necessary mobility for proximal femur exposure. Fluoroscopy has been shown to significantly improve the position and orientation of the implanted hip components.8We have developed an innovative use for a ball-tip guide rod (3.0 mm × 1000 mm; Smith & Nephew) to help accurately restore leg length and femoral offset after DA-THA. The ball-tip guide rod was modified to a length of 500 mm and rough edges were smoothed.
Technique
After the patient is prepared and draped in standard fashion on the operating table, a 10-cm skin incision is made directly over the proximal aspect of the tensor fascia lata muscle. Soft tissues are dissected down to the hip capsule, which is then incised and tagged for closure at the end of the case.
The fluoroscopic C-arm is sterilely draped and positioned from the nonoperative side. The image intensifier is centered over the pubic symphysis and lowered within 1 inch of the perineal post and surgical drapes. The C-arm unit is then aimed 10° to 15° cephalad until the size and orientation of the obturator foramens on fluoroscopic imaging coincide with the preoperative template.
Next, the modified guide rod, ball tip first, is carefully advanced toward the nonoperative side and over the surgical drapes between the pelvis and the C-arm image intensifier. Care is taken to avoid violating the sterile field by inadvertently puncturing the surgical drapes with the guide rod. The lower extremities are externally rotated 20° to bring the lesser tuberosities into profile view. With use of several fluoroscopic views, the guide rod is aligned with the inferior borders of the ischial tuberosities or the obturator foramens, whichever are more readily identified on the intraoperative images. A skin marker is then used to illustrate the position of the guide rod on the operative drapes for future reference.
At this point, the relationship between the radiopaque guide rod and the lesser trochanters is noted to gain a sense of native femoral leg length and offset, and the image (Figure 1) is saved in the C-arm computer for later recall and comparison views.
Next, the femoral neck osteotomy is performed according to the preoperative template. Acetabular preparation and component insertion are completed under fluoroscopic guidance.
After appropriate soft-tissue releases, the operating table is used to position the operative leg in extension, external rotation, and adduction. The femur is then sequentially broached until the template size is reached or until there is an audible change in pitch. At this point, a trial neck with head ball is fixed to the broach, and the hip is reduced.
The fluoroscopic C-arm is then repositioned over the pelvis, as previously described, with the guide rod over the pelvis and tangential to the ischial tuberosities. A new image (Figure 2) is obtained with the trial components in place.
The radiopaque line generated by the guide rod represents a reference point that permits objective comparison of femoral leg length and offset based on distance to the lesser trochanters. Different modular components can be trialed until the correct combination of variables accurately restores the desired parameters.
Once parameters are restored, trial femoral components are removed, and a corresponding monolithic femoral stem is gently impacted into the proximal femur and fitted with the appropriate head ball. A final image is obtained with the guide rod and implants in place and is saved as proof of restoration of leg length.
Discussion
Various techniques of assessing intraoperative leg length have been described, and each has its advantages and disadvantages. Relying on abductor tension or comparing leg lengths on the operating table is not always accurate and is strongly dependent on patient position.2,6
Referencing the tip of the greater trochanter to a Steinmann pin inserted into the ilium provides a precise reference point, but this invasive technique has the potential for fracture propagation through the drill hole.2,7Superimposing a trial femoral component over the proximal femur to determine the appropriate femoral neck osteotomy has been described, but this process can be difficult through a tight DA approach.9Numerous measuring devices have been designed to help restore leg length, but in many cases the purchase cost and required maintenance outweigh their utility.2 Gililland and colleagues10 developed a reusable fluoroscopic transparent grid system that significantly improves component positioning during DA-THA.
The modified ball-tip guide rod is relatively inexpensive (<$100) and has several practical purposes in total joint surgery. The guide rod historically has been used to sound the center of the femoral canal before broaching. In revision cases and in cases of poor bone stock, the tool can be used to verify that cortical perforation has not occurred during canal preparation. In this article, we describe another realistic use for the guide rod: to create, during DA-THA, a radiographic reference line that can be used to help restore leg length and femoral offset.
Several authors have mentioned surgeons’ drawing the reference line on paper printouts of intraoperative images.11 Not only is this practice fraught with potential contamination of the operative field, but valuable time is lost waiting for paper copies and putting on a new gown and gloves before reentering the sterile field.
We used to train a radiologic technician or operating room nurse to draw a computerized reference line connecting the lesser trochanters on the fluoroscopic image. Problems arose in working with revolving nursing staff and in distinguishing the thin black line on computer monitors. In contrast, the radiopaque line from the guide rod is easily differentiated on fluoroscopic images, the technique poses less of a risk to the sterile field, and proper orientation of the guide rod to obtain the appropriate reference line is entirely surgeon-dependent.
A drawback of this technique is the additional radiation exposure that occurs when extra images are obtained to ensure satisfactory alignment of the guide rod. Another issue is fluoroscopic parallax. Some machines in the operating department generate a magnetic field that can interfere with the fluoroscopy beam and thereby slightly distort the intraoperative images.8 Therefore, it is imperative that the guide rod remain perfectly straight to avoid confounding measurements.
Our modified guide rod technique is a reliable, quick, and inexpensive intraoperative tool that helps in accurately restoring leg length and femoral offset during DA-THA.
Am J Orthop. 2017;46(1):E10-E12. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Whitehouse MR, Stefanovich-Lawbuary NS, Brunton LR, Blom AW. The impact of leg length discrepancy on patient satisfaction and functional outcome following total hip arthroplasty. J Arthroplasty. 2013;28(8):1408-1414.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
4. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
5. Knutson GA. Anatomic and functional leg-length inequality: a review and recommendation for clinical decision-making. Part I, anatomic leg-length inequality: prevalence, magnitude, effects and clinical significance. Chiropr Osteopat. 2005;13:11.
6. Iagulli ND, Mallory TH, Berend KR, et al. A simple and accurate method for determining leg length in primary total hip arthroplasty. Am J Orthop. 2006;35(10):455-457.
7. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
8. Weber M, Woerner M, Springorum R, et al. Fluoroscopy and imageless navigation enable an equivalent reconstruction of leg length and global and femoral offset in THA. Clin Orthop Relat Res. 2014;472(10):3150-3158.
9. Alazzawi S, Douglas SL, Haddad FS. A novel intra-operative technique to achieve accurate leg length and femoral offset during total hip replacement. Ann R Coll Surg Engl. 2012;94(4):281-282.
10. Gililland JM, Anderson LA, Boffeli SL, Pelt CE, Peters CL, Kubiak EN. A fluoroscopic grid in supine total hip arthroplasty: improving cup position, limb length, and hip offset. J Arthroplasty. 2012;27(8 suppl):111-116.
11. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
1. Whitehouse MR, Stefanovich-Lawbuary NS, Brunton LR, Blom AW. The impact of leg length discrepancy on patient satisfaction and functional outcome following total hip arthroplasty. J Arthroplasty. 2013;28(8):1408-1414.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
4. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
5. Knutson GA. Anatomic and functional leg-length inequality: a review and recommendation for clinical decision-making. Part I, anatomic leg-length inequality: prevalence, magnitude, effects and clinical significance. Chiropr Osteopat. 2005;13:11.
6. Iagulli ND, Mallory TH, Berend KR, et al. A simple and accurate method for determining leg length in primary total hip arthroplasty. Am J Orthop. 2006;35(10):455-457.
7. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
8. Weber M, Woerner M, Springorum R, et al. Fluoroscopy and imageless navigation enable an equivalent reconstruction of leg length and global and femoral offset in THA. Clin Orthop Relat Res. 2014;472(10):3150-3158.
9. Alazzawi S, Douglas SL, Haddad FS. A novel intra-operative technique to achieve accurate leg length and femoral offset during total hip replacement. Ann R Coll Surg Engl. 2012;94(4):281-282.
10. Gililland JM, Anderson LA, Boffeli SL, Pelt CE, Peters CL, Kubiak EN. A fluoroscopic grid in supine total hip arthroplasty: improving cup position, limb length, and hip offset. J Arthroplasty. 2012;27(8 suppl):111-116.
11. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
Complications and Risk Factors for Morbidity in Elective Hip Arthroscopy: A Review of 1325 Cases
Take-Home Points
- Using the NSQIP database, the authors report that the overall complication rate was 1.21% after hip arthroscopy.
- The most common complications cited were bleeding requiring transfusion (0.45%), return to OR (0.23%), superficial infection (0.23%), and thrombophlebitis (0.15).
- Most common 10CPT code was arthroscopic débridement in 50% of cases, reflecting the types of cases being performed in the time period.
- FAI codes were less common in this database–labral repair in 24%, femoral osteochondroplasty in 16%, and acetabuloplasty in 9%.
- Use caution in patients over age 65 years as this appears to be a risk factor for morbidity.
Hip arthroscopy is a well-described method for treating a number of pathologies.1-3 Surgical indications are wide-ranging and include femoral acetabular impingement (FAI), labral tears, loose bodies, osteochondral injuries, ruptured ligamentum teres, and synovitis, as well as extra-articular injuries, including hip abductor tears and sciatic nerve entrapment.2,4-6 Authors have suggested that the advantages of hip arthroscopy over open procedures include less traumatic access to the hip joint and faster recovery,7,8 and hip arthroscopy has been found cost-effective in select groups of patients.9
Overall complications have been reported in 1% to 20% of hip arthroscopy patients,6,8,10,11 and a meta-analysis identified an overall complication rate of 4%.8 Complications include iatrogenic chondrolabral injury, nerve injury, superficial surgical-site infection, deep vein thrombosis (DVT), instrument failure, portal wound bleeding, soft-tissue injury, and intra-abdominal fluid extravasation.6,8,10-13 Rates of major complications are relatively low, 0.3% to 0.58%, according to several recent systematic reviews.8,12 Given the lack of universally accepted definitions, reports of minor complications (eg, iatrogenic chondrolabral injury, neuropraxia) in hip arthroscopy vary widely.8 Furthermore, many of the series with high complication rates represent early experience with the technique, and later authors suggested that complications should decrease with improvements in technique and technology.12,14,15The literature is lacking in reports of risk factors for patient morbidity and large multi-institutional cohorts in the setting of hip arthroscopy. We conducted a study of elective hip arthroscopy patients to determine type and incidence of complications and rates of and risk factors for minor and major morbidity.
Materials and Methods
This retrospective study was deemed compliant with HIPAA (Health Insurance Portability and Accountability Act of 1996) and exempt from the need for Institutional Review Board approval. In the National Surgical Quality Improvement Program (NSQIP), academic and private medical institutions prospectively collect patient preoperative and operative data as well as 30-day outcome data from more than 500 hospitals throughout the United States.16-21 Surgical clinical reviewers, who are responsible for data acquisition, prospectively collect morbidity data for 30 days after surgery through a chart review of patient progress notes, operative notes, and follow-up clinic visits. Patients may be contacted by a surgical clinical reviewer if they have not had a clinic visit within 30 days after a procedure to verify the presence or absence of complications or admissions at outside institutions, and in this way even outpatient complications should be captured. If the medical record is unclear, the reviewer may also contact the surgeon directly. In addition, NSQIP data are routinely audited; the interobserver disagreement rate is 1.56%.22
We used Current Procedural Terminology (CPT) billing codes to retrospectively survey the NSQIP database for hip arthroscopies performed between 2006 and 2013. Excluding cases of compromised surgical wounds, emergent surgeries, surgeries involving fracture, hip dislocations, preoperative sepsis, septic joints, and osteomyelitis, we identified 1325 cases with CPT codes 29861 (hip arthroscopy), 29862 (arthroscopic hip débridement, shaving), 29914 (arthroscopic femoroplasty), 29915 (arthroscopic acetabuloplasty), and 29916 (arthroscopic labral repair). Postoperative outcomes were categorized as major morbidity or mortality, minor morbidity, and any complication. A major complication was a systemic life-threatening event or a substantial threat to a vital organ, whereas a minor complication did not pose a major systemic threat and was localized to the operative extremity (previously used definitions23,24). We have used similar methods to report the rates of and risk factors for complications of knee arthroscopy, shoulder arthroscopy, and total shoulder arthroplasty.16,20,21 For any-complication outcomes, we included both major and minor morbidities, and mortality. NSQIP applies strict definitions (listed in its user file17) to patient comorbidities and complications. Data points collected included patient demographics, medical comorbidities, laboratory values, and surgical characteristics.
Initially, we performed a univariate analysis that considered age, sex, race, body mass index, current alcohol abuse, current smoking status, recent weight loss, dyspnea, chronic obstructive pulmonary disease, CPT code, congestive heart failure, hypertension, diabetes, peripheral vascular disease, esophageal varices, disseminated cancer, steroid use, bleeding disorder, dialysis, chemotherapy within previous 30 days, radiation therapy within previous 90 days, operation within previous 30 days, American Society of Anesthesiologists class, operative time, resident involvement, and patient functional status. We also included mean preoperative sodium, blood urea nitrogen, and albumin levels; white blood cell count; hematocrit; platelet count; and international normalized ratio. The analysis revealed unadjusted differences between patients with and without complications (t test was used for continuous variables, χ2 test for categorical variables). Any variable with P < .2 in the univariate analysis and more than 80% complete data was considered fit for our multivariate model. We controlled for confounders by performing a multivariate logistic regression analysis. Three separate analyses were performed; the outcome variables were major morbidity or mortality, minor morbidity, and any complication. P < .05 was used for statistical significance across all models. We used SAS Version 9.3 (SAS Institute) for statistical analysis. Model quality was evaluated for calibration (Hosmer-Lemeshow test) and discrimination (C statistics). The calibration test yielded a modified χ2 statistic, and P > .05 indicated the model was appropriate and fit the data well. Good discrimination is commonly reported to be between 0.65 and 0.85.
Results
Of the 1325 patients who underwent hip arthroscopy, 60% were female. Regarding age, 52% were younger than 40 years, and 45% were between 45 years and 60 years. The most common diagnoses were articular cartilage disorder involving the pelvic region (15%), enthesopathy of the hip (12%), and joint pain involving the pelvic region or thigh (11%). The most common primary CPT code (50%) was for hip arthroscopic débridement (29862), followed by 24% for arthroscopic labral repair (29916), 16% for arthroscopic femoroplasty (29914), and 9% for arthroscopic acetabuloplasty (29915). Of the 16 complications found, 12 involved hip arthroscopic débridement, and 4 involved hip arthroscopic femoroplasty. There were no complications of arthroscopic acetabuloplasty (29915), arthroscopic labral repair (29916), or hip arthroscopy (29861).
Of the 1325 hip arthroscopy patients, 16 (1.21%) had at least 1 complication (Table 1).
Univariate analysis identified age (P = .014), CPT code (P = .036), hypertension (P = .128), and steroid use (P = .188) as risk factors for any complication (Table 2).
Discussion
Earlier reports on hip arthroscopy did not consider risk factors for systemic morbidity and were mainly single-institution case series.3,10,11,13,25 Given a renewed focus on outcomes measurement and quality assessment in orthopedic surgery, we wanted to describe short-term complications of and risk factors for morbidity in hip arthroscopy. In this article, we report baseline data from a large multicenter cohort. For hip arthroscopy, we found low rates of short-term complications (1.21%) and major morbidities (0.45%). We considered many modifiable and nonmodifiable risk factors for complications and found age over 65 years to be an independent risk factor for any complication and minor morbidity. Several of our findings merit further discussion.
Other authors have reported hip arthroscopy complication rates of 1% to 20%, citing both systemic and local complications,6,8,10-12 and major complication rates of 0.3% to 0.58%.8,12 Minor complications of hip arthroscopy vary, and depend on definition, with long-term consequences unknown in some cases.8 Sensory neuropraxia, a relatively common minor complication in hip arthroscopy, is thought to be affected by the amount of traction against a perineal post and by increased operative time, with operative time under 2 hours previously suggested.3,6,10,11,13,25,26
In the present study, the overall rate of any complication of hip arthroscopy was 1.21%, and the most common complications were bleeding resulting in transfusion, return to operating room, superficial surgical-site infection, and DVT/thrombophlebitis. When we excluded bleeding resulting in transfusion, the overall complication rate fell to 0.75%. Operative time was relatively short, <2 hours for 70% of patients. Last, there were no mortalities. As our data set did not include variables encompassing sensory neuropraxia or iatrogenic chondrolabral injury, we were unable to report on these data.
Surgeons and healthcare systems should be advised that rates of systemic complications in hip arthroscopy are low and that hip arthroscopy is a relatively safe procedure. Surgeons and healthcare systems can refer to our reported complication rates and risk factors when assessing quality and performing cost analysis in hip arthroscopy. For our 1325 patients, the major morbidity rate was 0.45%, within the range of previous reports.8,12 There were no nerve injuries in our patient cohort, likely because of the strict NSQIP definitions of nerve injury. We cannot report on sensory neuropraxia and iatrogenic chondrolabral injury. We speculate that lack of these variables may have artificially lowered our minor complication rate.
Some authors have reported clinical benefits of hip arthroscopy in older patients,27-29 whereas others have suggested age may be a negative prognostic factor.27,30 Suggested indications for hip arthroscopy in an elderly population include chondral defects, labral tears, and FAI in the absence of significant arthritic changes.28,29 Larson and colleagues,30 who reported a 52% failure rate for osteoarthritis patients who underwent hip arthroscopy for FAI, concluded that arthroscopy should not be offered to patients with evidence of advanced radiographic joint space narrowing. Others have noted that patients who were under age 55 years and had minimal osteoarthritic changes had a longer interval between hip arthroscopy and total hip arthroplasty in comparison with patients over age 55 years.31 Previous work in knee arthroscopy found older age (40-65 years vs <40 years) was an independent predictor of short-term complications (1.5 times increased risk).21 In the present study, 7.69% of patients who were over age 65 years when they underwent hip arthroscopy had a complication, and we report age over 65 years as an independent risk factor for any complication (OR, 6.52) and minor morbidity (OR, 7.97). Surgeons should be aware that advanced age is an independent risk factor for complications in hip arthroscopy. Potential benefits of hip arthroscopy should be carefully weighed against the increased risk in this patient cohort, and surgeons should ascertain the scope of an elderly patient’s disease to determine if hip arthroscopy is indicated and worth the potential risks.
To our knowledge, bleeding resulting in transfusion was not previously described as a complication of hip arthroscopy. In the present study, bleeding resulting in transfusion was the most common complication (6 patients, 0.45%), and all the affected patients had a primary CPT code for arthroscopic débridement (29862). The 6 primary diagnoses were hip osteoarthrosis (3), thigh/pelvis pain (1), unspecified injury (1), and congenital hip deformity (1). The 6 transfusion patients also tended to be older (ages 30, 53, 64, 67, 76, and 90 years). Although drawing firm conclusions from so few patients would be inappropriate, we acknowledge that the majority who received a transfusion were older, underwent arthroscopic débridement of a hip, and had a primary diagnosis of osteoarthrosis or pain. As transfusion practices can differ between surgeons and groups, we conclude that the risk for bleeding requiring transfusion is low in hip arthroscopy. Patients who are older and who undergo arthroscopic débridement of an osteoarthritic hip may be at elevated risk for transfusion.
This study had several limitations. First, with use of the NSQIP database, follow-up was limited to 30 days. We speculate that longer follow-up might yield higher complication rates and additional risk factors. Second, we could not distinguish individual surgeon or site data and acknowledge complications might differ between surgeons and sites that perform hip arthroscopy more frequently. Third, as data were limited to medical and broadly applicable surgical variables included in the NSQIP database, they might not be specific to hip arthroscopy, and we cannot report on iatrogenic chondrolabral injury and neuropraxia, 2 previously reported minor complications in hip arthroscopy. We speculate that data collection focused on problems specific to hip arthroscopy would yield more complications and risk factors.
Conclusion
According to the NSQIP data, the rate of short-term morbidity after elective hip arthroscopy was low, 1.21%. Surgeons may use our reported complications and risk factors when counseling patients, and healthcare systems may use our data when assessing quality and performance in hip arthroscopy. Surgeons who perform elective hip arthroscopy should be aware that age over 65 years is an independent predictor of complications. Careful attention should be given to this patient group when indicating hip arthroscopy procedures.
Am J Orthop. 2017;46(1):E1-E9. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Byrd JW. Hip arthroscopy utilizing the supine position. Arthroscopy. 1994;10(3):275-280.
2. Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 10-year followup. Clin Orthop Relat Res. 2010;468(3):741-746.
3. Griffin DR, Villar RN. Complications of arthroscopy of the hip. J Bone Joint Surg Br. 1999;81(4):604-606.
4. de Sa D, Alradwan H, Cargnelli S, et al. Extra-articular hip impingement: a systematic review examining operative treatment of psoas, subspine, ischiofemoral, and greater trochanteric/pelvic impingement. Arthroscopy. 2014;30(8):1026-1041.
5. de Sa D, Phillips M, Philippon MJ, Letkemann S, Simunovic N, Ayeni OR. Ligamentum teres injuries of the hip: a systematic review examining surgical indications, treatment options, and outcomes. Arthroscopy. 2014;30(12):1634-1641.
6. Oak N, Mendez-Zfass M, Lesniak BP, Larson CM, Kelly BT, Bedi A. Complications in hip arthroscopy. Sports Med Arthrosc. 2013;21(2):97-105.
7. Botser IB, Smith TW Jr, Nasser R, Domb BG. Open surgical dislocation versus arthroscopy for femoroacetabular impingement: a comparison of clinical outcomes. Arthroscopy. 2011;27(2):270-278.
8. Kowalczuk M, Bhandari M, Farrokhyar F, et al. Complications following hip arthroscopy: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1669-1675.
9. Shearer DW, Kramer J, Bozic KJ, Feeley BT. Is hip arthroscopy cost-effective for femoroacetabular impingement? Clin Orthop Relat Res. 2012;470(4):1079-1089.
10. Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;(406):84-88.
11. Pailhé R, Chiron P, Reina N, Cavaignac E, Lafontan V, Laffosse JM. Pudendal nerve neuralgia after hip arthroscopy: retrospective study and literature review. Orthop Traumatol Surg Res. 2013;99(7):785-790.
12. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
13. Sampson TG. Complications of hip arthroscopy. Clin Sports Med. 2001;20(4):831-835.
14. Konan S, Rhee SJ, Haddad FS. Hip arthroscopy: analysis of a single surgeon’s learning experience. J Bone Joint Surg Am. 2011;93(suppl 2):52-56.
15. Souza BG, Dani WS, Honda EK, et al. Do complications in hip arthroscopy change with experience? Arthroscopy. 2010;26(8):1053-1057.
16. Anthony CA, Westermann RW, Gao Y, Pugely AJ, Wolf BR, Hettrich CM. What are risk factors for 30-day morbidity and transfusion in total shoulder arthroplasty? A review of 1922 cases. Clin Orthop Relat Res. 2015;473(6):2099-2105.
17. Daley J, Khuri SF, Henderson W, et al. Risk adjustment of the postoperative morbidity rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185(4):328-340.
18. Fink AS, Campbell DA, Mentzer RM, et al. The National Surgical Quality Improvement Program in non-Veterans Administration hospitals: initial demonstration of feasibility. Ann Surg. 2002;236(3):344-353.
19. Khuri SF, Daley J, Henderson W, et al. The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180(5):519-531.
20. Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.
21. Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2013;95(14):e98.
22. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16.
23. Schoenfeld AJ, Ochoa LM, Bader JO, Belmont PJ Jr. Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program. J Bone Joint Surg Am. 2011;93(17):1577-1582.
24. Yadla S, Malone J, Campbell PG, et al. Obesity and spine surgery: reassessment based on a prospective evaluation of perioperative complications in elective degenerative thoracolumbar procedures. Spine J. 2010;10(7):581-587.
25. Lo YP, Chan YS, Lien LC, Lee MS, Hsu KY, Shih CH. Complications of hip arthroscopy: analysis of seventy three cases. Chang Gung Med J. 2006;29(1):86-92.
26. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop Relat Res. 2009;467(3):760-768.
27. Domb BG, Linder D, Finley Z, et al. Outcomes of hip arthroscopy in patients aged 50 years or older compared with a matched-pair control of patients aged 30 years or younger. Arthroscopy. 2015;31(2):231-238.
28. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
29. Philippon MJ, Schroder E Souza BG, Briggs KK. Hip arthroscopy for femoroacetabular impingement in patients aged 50 years or older. Arthroscopy. 2012;28(1):59-65.
30. Larson CM, Giveans MR, Taylor M. Does arthroscopic FAI correction improve function with radiographic arthritis? Clin Orthop Relat Res. 2011;469(6):1667-1676.
31. Haviv B, O’Donnell J. The incidence of total hip arthroplasty after hip arthroscopy in osteoarthritic patients. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:18.
Take-Home Points
- Using the NSQIP database, the authors report that the overall complication rate was 1.21% after hip arthroscopy.
- The most common complications cited were bleeding requiring transfusion (0.45%), return to OR (0.23%), superficial infection (0.23%), and thrombophlebitis (0.15).
- Most common 10CPT code was arthroscopic débridement in 50% of cases, reflecting the types of cases being performed in the time period.
- FAI codes were less common in this database–labral repair in 24%, femoral osteochondroplasty in 16%, and acetabuloplasty in 9%.
- Use caution in patients over age 65 years as this appears to be a risk factor for morbidity.
Hip arthroscopy is a well-described method for treating a number of pathologies.1-3 Surgical indications are wide-ranging and include femoral acetabular impingement (FAI), labral tears, loose bodies, osteochondral injuries, ruptured ligamentum teres, and synovitis, as well as extra-articular injuries, including hip abductor tears and sciatic nerve entrapment.2,4-6 Authors have suggested that the advantages of hip arthroscopy over open procedures include less traumatic access to the hip joint and faster recovery,7,8 and hip arthroscopy has been found cost-effective in select groups of patients.9
Overall complications have been reported in 1% to 20% of hip arthroscopy patients,6,8,10,11 and a meta-analysis identified an overall complication rate of 4%.8 Complications include iatrogenic chondrolabral injury, nerve injury, superficial surgical-site infection, deep vein thrombosis (DVT), instrument failure, portal wound bleeding, soft-tissue injury, and intra-abdominal fluid extravasation.6,8,10-13 Rates of major complications are relatively low, 0.3% to 0.58%, according to several recent systematic reviews.8,12 Given the lack of universally accepted definitions, reports of minor complications (eg, iatrogenic chondrolabral injury, neuropraxia) in hip arthroscopy vary widely.8 Furthermore, many of the series with high complication rates represent early experience with the technique, and later authors suggested that complications should decrease with improvements in technique and technology.12,14,15The literature is lacking in reports of risk factors for patient morbidity and large multi-institutional cohorts in the setting of hip arthroscopy. We conducted a study of elective hip arthroscopy patients to determine type and incidence of complications and rates of and risk factors for minor and major morbidity.
Materials and Methods
This retrospective study was deemed compliant with HIPAA (Health Insurance Portability and Accountability Act of 1996) and exempt from the need for Institutional Review Board approval. In the National Surgical Quality Improvement Program (NSQIP), academic and private medical institutions prospectively collect patient preoperative and operative data as well as 30-day outcome data from more than 500 hospitals throughout the United States.16-21 Surgical clinical reviewers, who are responsible for data acquisition, prospectively collect morbidity data for 30 days after surgery through a chart review of patient progress notes, operative notes, and follow-up clinic visits. Patients may be contacted by a surgical clinical reviewer if they have not had a clinic visit within 30 days after a procedure to verify the presence or absence of complications or admissions at outside institutions, and in this way even outpatient complications should be captured. If the medical record is unclear, the reviewer may also contact the surgeon directly. In addition, NSQIP data are routinely audited; the interobserver disagreement rate is 1.56%.22
We used Current Procedural Terminology (CPT) billing codes to retrospectively survey the NSQIP database for hip arthroscopies performed between 2006 and 2013. Excluding cases of compromised surgical wounds, emergent surgeries, surgeries involving fracture, hip dislocations, preoperative sepsis, septic joints, and osteomyelitis, we identified 1325 cases with CPT codes 29861 (hip arthroscopy), 29862 (arthroscopic hip débridement, shaving), 29914 (arthroscopic femoroplasty), 29915 (arthroscopic acetabuloplasty), and 29916 (arthroscopic labral repair). Postoperative outcomes were categorized as major morbidity or mortality, minor morbidity, and any complication. A major complication was a systemic life-threatening event or a substantial threat to a vital organ, whereas a minor complication did not pose a major systemic threat and was localized to the operative extremity (previously used definitions23,24). We have used similar methods to report the rates of and risk factors for complications of knee arthroscopy, shoulder arthroscopy, and total shoulder arthroplasty.16,20,21 For any-complication outcomes, we included both major and minor morbidities, and mortality. NSQIP applies strict definitions (listed in its user file17) to patient comorbidities and complications. Data points collected included patient demographics, medical comorbidities, laboratory values, and surgical characteristics.
Initially, we performed a univariate analysis that considered age, sex, race, body mass index, current alcohol abuse, current smoking status, recent weight loss, dyspnea, chronic obstructive pulmonary disease, CPT code, congestive heart failure, hypertension, diabetes, peripheral vascular disease, esophageal varices, disseminated cancer, steroid use, bleeding disorder, dialysis, chemotherapy within previous 30 days, radiation therapy within previous 90 days, operation within previous 30 days, American Society of Anesthesiologists class, operative time, resident involvement, and patient functional status. We also included mean preoperative sodium, blood urea nitrogen, and albumin levels; white blood cell count; hematocrit; platelet count; and international normalized ratio. The analysis revealed unadjusted differences between patients with and without complications (t test was used for continuous variables, χ2 test for categorical variables). Any variable with P < .2 in the univariate analysis and more than 80% complete data was considered fit for our multivariate model. We controlled for confounders by performing a multivariate logistic regression analysis. Three separate analyses were performed; the outcome variables were major morbidity or mortality, minor morbidity, and any complication. P < .05 was used for statistical significance across all models. We used SAS Version 9.3 (SAS Institute) for statistical analysis. Model quality was evaluated for calibration (Hosmer-Lemeshow test) and discrimination (C statistics). The calibration test yielded a modified χ2 statistic, and P > .05 indicated the model was appropriate and fit the data well. Good discrimination is commonly reported to be between 0.65 and 0.85.
Results
Of the 1325 patients who underwent hip arthroscopy, 60% were female. Regarding age, 52% were younger than 40 years, and 45% were between 45 years and 60 years. The most common diagnoses were articular cartilage disorder involving the pelvic region (15%), enthesopathy of the hip (12%), and joint pain involving the pelvic region or thigh (11%). The most common primary CPT code (50%) was for hip arthroscopic débridement (29862), followed by 24% for arthroscopic labral repair (29916), 16% for arthroscopic femoroplasty (29914), and 9% for arthroscopic acetabuloplasty (29915). Of the 16 complications found, 12 involved hip arthroscopic débridement, and 4 involved hip arthroscopic femoroplasty. There were no complications of arthroscopic acetabuloplasty (29915), arthroscopic labral repair (29916), or hip arthroscopy (29861).
Of the 1325 hip arthroscopy patients, 16 (1.21%) had at least 1 complication (Table 1).
Univariate analysis identified age (P = .014), CPT code (P = .036), hypertension (P = .128), and steroid use (P = .188) as risk factors for any complication (Table 2).
Discussion
Earlier reports on hip arthroscopy did not consider risk factors for systemic morbidity and were mainly single-institution case series.3,10,11,13,25 Given a renewed focus on outcomes measurement and quality assessment in orthopedic surgery, we wanted to describe short-term complications of and risk factors for morbidity in hip arthroscopy. In this article, we report baseline data from a large multicenter cohort. For hip arthroscopy, we found low rates of short-term complications (1.21%) and major morbidities (0.45%). We considered many modifiable and nonmodifiable risk factors for complications and found age over 65 years to be an independent risk factor for any complication and minor morbidity. Several of our findings merit further discussion.
Other authors have reported hip arthroscopy complication rates of 1% to 20%, citing both systemic and local complications,6,8,10-12 and major complication rates of 0.3% to 0.58%.8,12 Minor complications of hip arthroscopy vary, and depend on definition, with long-term consequences unknown in some cases.8 Sensory neuropraxia, a relatively common minor complication in hip arthroscopy, is thought to be affected by the amount of traction against a perineal post and by increased operative time, with operative time under 2 hours previously suggested.3,6,10,11,13,25,26
In the present study, the overall rate of any complication of hip arthroscopy was 1.21%, and the most common complications were bleeding resulting in transfusion, return to operating room, superficial surgical-site infection, and DVT/thrombophlebitis. When we excluded bleeding resulting in transfusion, the overall complication rate fell to 0.75%. Operative time was relatively short, <2 hours for 70% of patients. Last, there were no mortalities. As our data set did not include variables encompassing sensory neuropraxia or iatrogenic chondrolabral injury, we were unable to report on these data.
Surgeons and healthcare systems should be advised that rates of systemic complications in hip arthroscopy are low and that hip arthroscopy is a relatively safe procedure. Surgeons and healthcare systems can refer to our reported complication rates and risk factors when assessing quality and performing cost analysis in hip arthroscopy. For our 1325 patients, the major morbidity rate was 0.45%, within the range of previous reports.8,12 There were no nerve injuries in our patient cohort, likely because of the strict NSQIP definitions of nerve injury. We cannot report on sensory neuropraxia and iatrogenic chondrolabral injury. We speculate that lack of these variables may have artificially lowered our minor complication rate.
Some authors have reported clinical benefits of hip arthroscopy in older patients,27-29 whereas others have suggested age may be a negative prognostic factor.27,30 Suggested indications for hip arthroscopy in an elderly population include chondral defects, labral tears, and FAI in the absence of significant arthritic changes.28,29 Larson and colleagues,30 who reported a 52% failure rate for osteoarthritis patients who underwent hip arthroscopy for FAI, concluded that arthroscopy should not be offered to patients with evidence of advanced radiographic joint space narrowing. Others have noted that patients who were under age 55 years and had minimal osteoarthritic changes had a longer interval between hip arthroscopy and total hip arthroplasty in comparison with patients over age 55 years.31 Previous work in knee arthroscopy found older age (40-65 years vs <40 years) was an independent predictor of short-term complications (1.5 times increased risk).21 In the present study, 7.69% of patients who were over age 65 years when they underwent hip arthroscopy had a complication, and we report age over 65 years as an independent risk factor for any complication (OR, 6.52) and minor morbidity (OR, 7.97). Surgeons should be aware that advanced age is an independent risk factor for complications in hip arthroscopy. Potential benefits of hip arthroscopy should be carefully weighed against the increased risk in this patient cohort, and surgeons should ascertain the scope of an elderly patient’s disease to determine if hip arthroscopy is indicated and worth the potential risks.
To our knowledge, bleeding resulting in transfusion was not previously described as a complication of hip arthroscopy. In the present study, bleeding resulting in transfusion was the most common complication (6 patients, 0.45%), and all the affected patients had a primary CPT code for arthroscopic débridement (29862). The 6 primary diagnoses were hip osteoarthrosis (3), thigh/pelvis pain (1), unspecified injury (1), and congenital hip deformity (1). The 6 transfusion patients also tended to be older (ages 30, 53, 64, 67, 76, and 90 years). Although drawing firm conclusions from so few patients would be inappropriate, we acknowledge that the majority who received a transfusion were older, underwent arthroscopic débridement of a hip, and had a primary diagnosis of osteoarthrosis or pain. As transfusion practices can differ between surgeons and groups, we conclude that the risk for bleeding requiring transfusion is low in hip arthroscopy. Patients who are older and who undergo arthroscopic débridement of an osteoarthritic hip may be at elevated risk for transfusion.
This study had several limitations. First, with use of the NSQIP database, follow-up was limited to 30 days. We speculate that longer follow-up might yield higher complication rates and additional risk factors. Second, we could not distinguish individual surgeon or site data and acknowledge complications might differ between surgeons and sites that perform hip arthroscopy more frequently. Third, as data were limited to medical and broadly applicable surgical variables included in the NSQIP database, they might not be specific to hip arthroscopy, and we cannot report on iatrogenic chondrolabral injury and neuropraxia, 2 previously reported minor complications in hip arthroscopy. We speculate that data collection focused on problems specific to hip arthroscopy would yield more complications and risk factors.
Conclusion
According to the NSQIP data, the rate of short-term morbidity after elective hip arthroscopy was low, 1.21%. Surgeons may use our reported complications and risk factors when counseling patients, and healthcare systems may use our data when assessing quality and performance in hip arthroscopy. Surgeons who perform elective hip arthroscopy should be aware that age over 65 years is an independent predictor of complications. Careful attention should be given to this patient group when indicating hip arthroscopy procedures.
Am J Orthop. 2017;46(1):E1-E9. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Using the NSQIP database, the authors report that the overall complication rate was 1.21% after hip arthroscopy.
- The most common complications cited were bleeding requiring transfusion (0.45%), return to OR (0.23%), superficial infection (0.23%), and thrombophlebitis (0.15).
- Most common 10CPT code was arthroscopic débridement in 50% of cases, reflecting the types of cases being performed in the time period.
- FAI codes were less common in this database–labral repair in 24%, femoral osteochondroplasty in 16%, and acetabuloplasty in 9%.
- Use caution in patients over age 65 years as this appears to be a risk factor for morbidity.
Hip arthroscopy is a well-described method for treating a number of pathologies.1-3 Surgical indications are wide-ranging and include femoral acetabular impingement (FAI), labral tears, loose bodies, osteochondral injuries, ruptured ligamentum teres, and synovitis, as well as extra-articular injuries, including hip abductor tears and sciatic nerve entrapment.2,4-6 Authors have suggested that the advantages of hip arthroscopy over open procedures include less traumatic access to the hip joint and faster recovery,7,8 and hip arthroscopy has been found cost-effective in select groups of patients.9
Overall complications have been reported in 1% to 20% of hip arthroscopy patients,6,8,10,11 and a meta-analysis identified an overall complication rate of 4%.8 Complications include iatrogenic chondrolabral injury, nerve injury, superficial surgical-site infection, deep vein thrombosis (DVT), instrument failure, portal wound bleeding, soft-tissue injury, and intra-abdominal fluid extravasation.6,8,10-13 Rates of major complications are relatively low, 0.3% to 0.58%, according to several recent systematic reviews.8,12 Given the lack of universally accepted definitions, reports of minor complications (eg, iatrogenic chondrolabral injury, neuropraxia) in hip arthroscopy vary widely.8 Furthermore, many of the series with high complication rates represent early experience with the technique, and later authors suggested that complications should decrease with improvements in technique and technology.12,14,15The literature is lacking in reports of risk factors for patient morbidity and large multi-institutional cohorts in the setting of hip arthroscopy. We conducted a study of elective hip arthroscopy patients to determine type and incidence of complications and rates of and risk factors for minor and major morbidity.
Materials and Methods
This retrospective study was deemed compliant with HIPAA (Health Insurance Portability and Accountability Act of 1996) and exempt from the need for Institutional Review Board approval. In the National Surgical Quality Improvement Program (NSQIP), academic and private medical institutions prospectively collect patient preoperative and operative data as well as 30-day outcome data from more than 500 hospitals throughout the United States.16-21 Surgical clinical reviewers, who are responsible for data acquisition, prospectively collect morbidity data for 30 days after surgery through a chart review of patient progress notes, operative notes, and follow-up clinic visits. Patients may be contacted by a surgical clinical reviewer if they have not had a clinic visit within 30 days after a procedure to verify the presence or absence of complications or admissions at outside institutions, and in this way even outpatient complications should be captured. If the medical record is unclear, the reviewer may also contact the surgeon directly. In addition, NSQIP data are routinely audited; the interobserver disagreement rate is 1.56%.22
We used Current Procedural Terminology (CPT) billing codes to retrospectively survey the NSQIP database for hip arthroscopies performed between 2006 and 2013. Excluding cases of compromised surgical wounds, emergent surgeries, surgeries involving fracture, hip dislocations, preoperative sepsis, septic joints, and osteomyelitis, we identified 1325 cases with CPT codes 29861 (hip arthroscopy), 29862 (arthroscopic hip débridement, shaving), 29914 (arthroscopic femoroplasty), 29915 (arthroscopic acetabuloplasty), and 29916 (arthroscopic labral repair). Postoperative outcomes were categorized as major morbidity or mortality, minor morbidity, and any complication. A major complication was a systemic life-threatening event or a substantial threat to a vital organ, whereas a minor complication did not pose a major systemic threat and was localized to the operative extremity (previously used definitions23,24). We have used similar methods to report the rates of and risk factors for complications of knee arthroscopy, shoulder arthroscopy, and total shoulder arthroplasty.16,20,21 For any-complication outcomes, we included both major and minor morbidities, and mortality. NSQIP applies strict definitions (listed in its user file17) to patient comorbidities and complications. Data points collected included patient demographics, medical comorbidities, laboratory values, and surgical characteristics.
Initially, we performed a univariate analysis that considered age, sex, race, body mass index, current alcohol abuse, current smoking status, recent weight loss, dyspnea, chronic obstructive pulmonary disease, CPT code, congestive heart failure, hypertension, diabetes, peripheral vascular disease, esophageal varices, disseminated cancer, steroid use, bleeding disorder, dialysis, chemotherapy within previous 30 days, radiation therapy within previous 90 days, operation within previous 30 days, American Society of Anesthesiologists class, operative time, resident involvement, and patient functional status. We also included mean preoperative sodium, blood urea nitrogen, and albumin levels; white blood cell count; hematocrit; platelet count; and international normalized ratio. The analysis revealed unadjusted differences between patients with and without complications (t test was used for continuous variables, χ2 test for categorical variables). Any variable with P < .2 in the univariate analysis and more than 80% complete data was considered fit for our multivariate model. We controlled for confounders by performing a multivariate logistic regression analysis. Three separate analyses were performed; the outcome variables were major morbidity or mortality, minor morbidity, and any complication. P < .05 was used for statistical significance across all models. We used SAS Version 9.3 (SAS Institute) for statistical analysis. Model quality was evaluated for calibration (Hosmer-Lemeshow test) and discrimination (C statistics). The calibration test yielded a modified χ2 statistic, and P > .05 indicated the model was appropriate and fit the data well. Good discrimination is commonly reported to be between 0.65 and 0.85.
Results
Of the 1325 patients who underwent hip arthroscopy, 60% were female. Regarding age, 52% were younger than 40 years, and 45% were between 45 years and 60 years. The most common diagnoses were articular cartilage disorder involving the pelvic region (15%), enthesopathy of the hip (12%), and joint pain involving the pelvic region or thigh (11%). The most common primary CPT code (50%) was for hip arthroscopic débridement (29862), followed by 24% for arthroscopic labral repair (29916), 16% for arthroscopic femoroplasty (29914), and 9% for arthroscopic acetabuloplasty (29915). Of the 16 complications found, 12 involved hip arthroscopic débridement, and 4 involved hip arthroscopic femoroplasty. There were no complications of arthroscopic acetabuloplasty (29915), arthroscopic labral repair (29916), or hip arthroscopy (29861).
Of the 1325 hip arthroscopy patients, 16 (1.21%) had at least 1 complication (Table 1).
Univariate analysis identified age (P = .014), CPT code (P = .036), hypertension (P = .128), and steroid use (P = .188) as risk factors for any complication (Table 2).
Discussion
Earlier reports on hip arthroscopy did not consider risk factors for systemic morbidity and were mainly single-institution case series.3,10,11,13,25 Given a renewed focus on outcomes measurement and quality assessment in orthopedic surgery, we wanted to describe short-term complications of and risk factors for morbidity in hip arthroscopy. In this article, we report baseline data from a large multicenter cohort. For hip arthroscopy, we found low rates of short-term complications (1.21%) and major morbidities (0.45%). We considered many modifiable and nonmodifiable risk factors for complications and found age over 65 years to be an independent risk factor for any complication and minor morbidity. Several of our findings merit further discussion.
Other authors have reported hip arthroscopy complication rates of 1% to 20%, citing both systemic and local complications,6,8,10-12 and major complication rates of 0.3% to 0.58%.8,12 Minor complications of hip arthroscopy vary, and depend on definition, with long-term consequences unknown in some cases.8 Sensory neuropraxia, a relatively common minor complication in hip arthroscopy, is thought to be affected by the amount of traction against a perineal post and by increased operative time, with operative time under 2 hours previously suggested.3,6,10,11,13,25,26
In the present study, the overall rate of any complication of hip arthroscopy was 1.21%, and the most common complications were bleeding resulting in transfusion, return to operating room, superficial surgical-site infection, and DVT/thrombophlebitis. When we excluded bleeding resulting in transfusion, the overall complication rate fell to 0.75%. Operative time was relatively short, <2 hours for 70% of patients. Last, there were no mortalities. As our data set did not include variables encompassing sensory neuropraxia or iatrogenic chondrolabral injury, we were unable to report on these data.
Surgeons and healthcare systems should be advised that rates of systemic complications in hip arthroscopy are low and that hip arthroscopy is a relatively safe procedure. Surgeons and healthcare systems can refer to our reported complication rates and risk factors when assessing quality and performing cost analysis in hip arthroscopy. For our 1325 patients, the major morbidity rate was 0.45%, within the range of previous reports.8,12 There were no nerve injuries in our patient cohort, likely because of the strict NSQIP definitions of nerve injury. We cannot report on sensory neuropraxia and iatrogenic chondrolabral injury. We speculate that lack of these variables may have artificially lowered our minor complication rate.
Some authors have reported clinical benefits of hip arthroscopy in older patients,27-29 whereas others have suggested age may be a negative prognostic factor.27,30 Suggested indications for hip arthroscopy in an elderly population include chondral defects, labral tears, and FAI in the absence of significant arthritic changes.28,29 Larson and colleagues,30 who reported a 52% failure rate for osteoarthritis patients who underwent hip arthroscopy for FAI, concluded that arthroscopy should not be offered to patients with evidence of advanced radiographic joint space narrowing. Others have noted that patients who were under age 55 years and had minimal osteoarthritic changes had a longer interval between hip arthroscopy and total hip arthroplasty in comparison with patients over age 55 years.31 Previous work in knee arthroscopy found older age (40-65 years vs <40 years) was an independent predictor of short-term complications (1.5 times increased risk).21 In the present study, 7.69% of patients who were over age 65 years when they underwent hip arthroscopy had a complication, and we report age over 65 years as an independent risk factor for any complication (OR, 6.52) and minor morbidity (OR, 7.97). Surgeons should be aware that advanced age is an independent risk factor for complications in hip arthroscopy. Potential benefits of hip arthroscopy should be carefully weighed against the increased risk in this patient cohort, and surgeons should ascertain the scope of an elderly patient’s disease to determine if hip arthroscopy is indicated and worth the potential risks.
To our knowledge, bleeding resulting in transfusion was not previously described as a complication of hip arthroscopy. In the present study, bleeding resulting in transfusion was the most common complication (6 patients, 0.45%), and all the affected patients had a primary CPT code for arthroscopic débridement (29862). The 6 primary diagnoses were hip osteoarthrosis (3), thigh/pelvis pain (1), unspecified injury (1), and congenital hip deformity (1). The 6 transfusion patients also tended to be older (ages 30, 53, 64, 67, 76, and 90 years). Although drawing firm conclusions from so few patients would be inappropriate, we acknowledge that the majority who received a transfusion were older, underwent arthroscopic débridement of a hip, and had a primary diagnosis of osteoarthrosis or pain. As transfusion practices can differ between surgeons and groups, we conclude that the risk for bleeding requiring transfusion is low in hip arthroscopy. Patients who are older and who undergo arthroscopic débridement of an osteoarthritic hip may be at elevated risk for transfusion.
This study had several limitations. First, with use of the NSQIP database, follow-up was limited to 30 days. We speculate that longer follow-up might yield higher complication rates and additional risk factors. Second, we could not distinguish individual surgeon or site data and acknowledge complications might differ between surgeons and sites that perform hip arthroscopy more frequently. Third, as data were limited to medical and broadly applicable surgical variables included in the NSQIP database, they might not be specific to hip arthroscopy, and we cannot report on iatrogenic chondrolabral injury and neuropraxia, 2 previously reported minor complications in hip arthroscopy. We speculate that data collection focused on problems specific to hip arthroscopy would yield more complications and risk factors.
Conclusion
According to the NSQIP data, the rate of short-term morbidity after elective hip arthroscopy was low, 1.21%. Surgeons may use our reported complications and risk factors when counseling patients, and healthcare systems may use our data when assessing quality and performance in hip arthroscopy. Surgeons who perform elective hip arthroscopy should be aware that age over 65 years is an independent predictor of complications. Careful attention should be given to this patient group when indicating hip arthroscopy procedures.
Am J Orthop. 2017;46(1):E1-E9. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Byrd JW. Hip arthroscopy utilizing the supine position. Arthroscopy. 1994;10(3):275-280.
2. Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 10-year followup. Clin Orthop Relat Res. 2010;468(3):741-746.
3. Griffin DR, Villar RN. Complications of arthroscopy of the hip. J Bone Joint Surg Br. 1999;81(4):604-606.
4. de Sa D, Alradwan H, Cargnelli S, et al. Extra-articular hip impingement: a systematic review examining operative treatment of psoas, subspine, ischiofemoral, and greater trochanteric/pelvic impingement. Arthroscopy. 2014;30(8):1026-1041.
5. de Sa D, Phillips M, Philippon MJ, Letkemann S, Simunovic N, Ayeni OR. Ligamentum teres injuries of the hip: a systematic review examining surgical indications, treatment options, and outcomes. Arthroscopy. 2014;30(12):1634-1641.
6. Oak N, Mendez-Zfass M, Lesniak BP, Larson CM, Kelly BT, Bedi A. Complications in hip arthroscopy. Sports Med Arthrosc. 2013;21(2):97-105.
7. Botser IB, Smith TW Jr, Nasser R, Domb BG. Open surgical dislocation versus arthroscopy for femoroacetabular impingement: a comparison of clinical outcomes. Arthroscopy. 2011;27(2):270-278.
8. Kowalczuk M, Bhandari M, Farrokhyar F, et al. Complications following hip arthroscopy: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1669-1675.
9. Shearer DW, Kramer J, Bozic KJ, Feeley BT. Is hip arthroscopy cost-effective for femoroacetabular impingement? Clin Orthop Relat Res. 2012;470(4):1079-1089.
10. Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;(406):84-88.
11. Pailhé R, Chiron P, Reina N, Cavaignac E, Lafontan V, Laffosse JM. Pudendal nerve neuralgia after hip arthroscopy: retrospective study and literature review. Orthop Traumatol Surg Res. 2013;99(7):785-790.
12. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
13. Sampson TG. Complications of hip arthroscopy. Clin Sports Med. 2001;20(4):831-835.
14. Konan S, Rhee SJ, Haddad FS. Hip arthroscopy: analysis of a single surgeon’s learning experience. J Bone Joint Surg Am. 2011;93(suppl 2):52-56.
15. Souza BG, Dani WS, Honda EK, et al. Do complications in hip arthroscopy change with experience? Arthroscopy. 2010;26(8):1053-1057.
16. Anthony CA, Westermann RW, Gao Y, Pugely AJ, Wolf BR, Hettrich CM. What are risk factors for 30-day morbidity and transfusion in total shoulder arthroplasty? A review of 1922 cases. Clin Orthop Relat Res. 2015;473(6):2099-2105.
17. Daley J, Khuri SF, Henderson W, et al. Risk adjustment of the postoperative morbidity rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185(4):328-340.
18. Fink AS, Campbell DA, Mentzer RM, et al. The National Surgical Quality Improvement Program in non-Veterans Administration hospitals: initial demonstration of feasibility. Ann Surg. 2002;236(3):344-353.
19. Khuri SF, Daley J, Henderson W, et al. The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180(5):519-531.
20. Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.
21. Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2013;95(14):e98.
22. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16.
23. Schoenfeld AJ, Ochoa LM, Bader JO, Belmont PJ Jr. Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program. J Bone Joint Surg Am. 2011;93(17):1577-1582.
24. Yadla S, Malone J, Campbell PG, et al. Obesity and spine surgery: reassessment based on a prospective evaluation of perioperative complications in elective degenerative thoracolumbar procedures. Spine J. 2010;10(7):581-587.
25. Lo YP, Chan YS, Lien LC, Lee MS, Hsu KY, Shih CH. Complications of hip arthroscopy: analysis of seventy three cases. Chang Gung Med J. 2006;29(1):86-92.
26. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop Relat Res. 2009;467(3):760-768.
27. Domb BG, Linder D, Finley Z, et al. Outcomes of hip arthroscopy in patients aged 50 years or older compared with a matched-pair control of patients aged 30 years or younger. Arthroscopy. 2015;31(2):231-238.
28. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
29. Philippon MJ, Schroder E Souza BG, Briggs KK. Hip arthroscopy for femoroacetabular impingement in patients aged 50 years or older. Arthroscopy. 2012;28(1):59-65.
30. Larson CM, Giveans MR, Taylor M. Does arthroscopic FAI correction improve function with radiographic arthritis? Clin Orthop Relat Res. 2011;469(6):1667-1676.
31. Haviv B, O’Donnell J. The incidence of total hip arthroplasty after hip arthroscopy in osteoarthritic patients. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:18.
1. Byrd JW. Hip arthroscopy utilizing the supine position. Arthroscopy. 1994;10(3):275-280.
2. Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 10-year followup. Clin Orthop Relat Res. 2010;468(3):741-746.
3. Griffin DR, Villar RN. Complications of arthroscopy of the hip. J Bone Joint Surg Br. 1999;81(4):604-606.
4. de Sa D, Alradwan H, Cargnelli S, et al. Extra-articular hip impingement: a systematic review examining operative treatment of psoas, subspine, ischiofemoral, and greater trochanteric/pelvic impingement. Arthroscopy. 2014;30(8):1026-1041.
5. de Sa D, Phillips M, Philippon MJ, Letkemann S, Simunovic N, Ayeni OR. Ligamentum teres injuries of the hip: a systematic review examining surgical indications, treatment options, and outcomes. Arthroscopy. 2014;30(12):1634-1641.
6. Oak N, Mendez-Zfass M, Lesniak BP, Larson CM, Kelly BT, Bedi A. Complications in hip arthroscopy. Sports Med Arthrosc. 2013;21(2):97-105.
7. Botser IB, Smith TW Jr, Nasser R, Domb BG. Open surgical dislocation versus arthroscopy for femoroacetabular impingement: a comparison of clinical outcomes. Arthroscopy. 2011;27(2):270-278.
8. Kowalczuk M, Bhandari M, Farrokhyar F, et al. Complications following hip arthroscopy: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1669-1675.
9. Shearer DW, Kramer J, Bozic KJ, Feeley BT. Is hip arthroscopy cost-effective for femoroacetabular impingement? Clin Orthop Relat Res. 2012;470(4):1079-1089.
10. Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;(406):84-88.
11. Pailhé R, Chiron P, Reina N, Cavaignac E, Lafontan V, Laffosse JM. Pudendal nerve neuralgia after hip arthroscopy: retrospective study and literature review. Orthop Traumatol Surg Res. 2013;99(7):785-790.
12. Harris JD, McCormick FM, Abrams GD, et al. Complications and reoperations during and after hip arthroscopy: a systematic review of 92 studies and more than 6,000 patients. Arthroscopy. 2013;29(3):589-595.
13. Sampson TG. Complications of hip arthroscopy. Clin Sports Med. 2001;20(4):831-835.
14. Konan S, Rhee SJ, Haddad FS. Hip arthroscopy: analysis of a single surgeon’s learning experience. J Bone Joint Surg Am. 2011;93(suppl 2):52-56.
15. Souza BG, Dani WS, Honda EK, et al. Do complications in hip arthroscopy change with experience? Arthroscopy. 2010;26(8):1053-1057.
16. Anthony CA, Westermann RW, Gao Y, Pugely AJ, Wolf BR, Hettrich CM. What are risk factors for 30-day morbidity and transfusion in total shoulder arthroplasty? A review of 1922 cases. Clin Orthop Relat Res. 2015;473(6):2099-2105.
17. Daley J, Khuri SF, Henderson W, et al. Risk adjustment of the postoperative morbidity rate for the comparative assessment of the quality of surgical care: results of the National Veterans Affairs Surgical Risk Study. J Am Coll Surg. 1997;185(4):328-340.
18. Fink AS, Campbell DA, Mentzer RM, et al. The National Surgical Quality Improvement Program in non-Veterans Administration hospitals: initial demonstration of feasibility. Ann Surg. 2002;236(3):344-353.
19. Khuri SF, Daley J, Henderson W, et al. The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg. 1995;180(5):519-531.
20. Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.
21. Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the National Surgical Quality Improvement Program database. J Bone Joint Surg Am. 2013;95(14):e98.
22. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16.
23. Schoenfeld AJ, Ochoa LM, Bader JO, Belmont PJ Jr. Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program. J Bone Joint Surg Am. 2011;93(17):1577-1582.
24. Yadla S, Malone J, Campbell PG, et al. Obesity and spine surgery: reassessment based on a prospective evaluation of perioperative complications in elective degenerative thoracolumbar procedures. Spine J. 2010;10(7):581-587.
25. Lo YP, Chan YS, Lien LC, Lee MS, Hsu KY, Shih CH. Complications of hip arthroscopy: analysis of seventy three cases. Chang Gung Med J. 2006;29(1):86-92.
26. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop Relat Res. 2009;467(3):760-768.
27. Domb BG, Linder D, Finley Z, et al. Outcomes of hip arthroscopy in patients aged 50 years or older compared with a matched-pair control of patients aged 30 years or younger. Arthroscopy. 2015;31(2):231-238.
28. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
29. Philippon MJ, Schroder E Souza BG, Briggs KK. Hip arthroscopy for femoroacetabular impingement in patients aged 50 years or older. Arthroscopy. 2012;28(1):59-65.
30. Larson CM, Giveans MR, Taylor M. Does arthroscopic FAI correction improve function with radiographic arthritis? Clin Orthop Relat Res. 2011;469(6):1667-1676.
31. Haviv B, O’Donnell J. The incidence of total hip arthroplasty after hip arthroscopy in osteoarthritic patients. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:18.
Hip Arthroscopy
Editor’s Note: AJO is fortunate to have Shane Nho, one of the nation’s leading hip arthroscopists, as our Deputy Editor-in-Chief. He has compiled an outstanding update for all orthopedic surgeons who see hip patients. It’s my pleasure to turn this issue over to him. On a side note, we’ve added a new feature for our speed readers. From now on, all articles published in AJO will feature a “Take-Home Points” text box. These points represent the most important items that the authors wish to convey from their article. Please enjoy this month’s issue and keep the feedback coming. We are striving to continuously improve AJO and make it your go-to journal for practical information that you can apply directly to your practice.
—Bryan T. Hanypsiak, MD
Hip arthroscopy has been evolving over the past 2 decades as our techniques have been refined and our clinical outcomes have been reported. We have reached a point in our field to look back at the progress that has been made while also providing our readers with the most up-to-date information on diagnosis, imaging studies, and decision making for appropriate treatment.
Trofa and colleagues provide an excellent overview on intra- and extra-articular pathology of the hip and pelvis in their article, “Mastering the Physical Examination of the Athlete’s Hip”. The authors review common injuries in the athlete and provide physical examination tests to differentiate between adductor strain, athletic pubalgia, osteitis pubis, and femoroacetabular impingement (FAI). Also in this issue, Lewis and colleagues provide a comprehensive review of imaging studies in the “Imaging for Nonarthritic Hip Pathology”. The authors review the most common radiographic measurements to detect FAI as well as describe the role of computed tomography and magnetic resonance imaging.
The mastery of hip arthroscopy for the treatment of FAI has a steep learning curve and the techniques have evolved along with our understanding of the importance of the labrum and capsule. We are fortunate to have an article provided by one of the pioneers in the field, Dr. Marc J. Philippon, describing his role in advancing the field in the article “Treatment of FAI: Labrum, Cartilage, Osseous Deformity, and Capsule”. Kollmorgen and Mather provide the most up-to-date techniques for labrum repair and reconstruction. Friel and colleagues report on capsular repair and plication using the T-capsulotomy and the extensile interportal capsulotomy.
We also have the opportunity to read about a number of clinical studies describing the experiences of multi-center studies and epidemiologic studies on large volumes of data. The ANCHOR group provides a summary of the experiences of some of the most renowned hip surgeons in North America as the treatment of FAI evolved from an open approach to an all-arthroscopic approach. The MASH group is a large multi-center group of hip arthroscopists in the United States who describe their current indications for surgical treatment of FAI.
On AmJOrthopedics.com, Matsuda and colleagues describe the outcomes of borderline dysplasia patients compared to normal controls across multiple centers. Anthony and colleagues report on the complication rates using the National Surgical Quality Improvement Program database.
I believe that our Hip Arthroscopy issue will not disappoint you. It is a comprehensive review of the state-of-the-art in hip arthroscopy from physical examination to current surgical techniques to clinical outcomes from large databases for the treatment of FAI. After reviewing this issue, you will be equipped with the most up-to-date information on the treatment of nonarthritic hip disease.
Am J Orthop. 2017;46(1):8. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Editor’s Note: AJO is fortunate to have Shane Nho, one of the nation’s leading hip arthroscopists, as our Deputy Editor-in-Chief. He has compiled an outstanding update for all orthopedic surgeons who see hip patients. It’s my pleasure to turn this issue over to him. On a side note, we’ve added a new feature for our speed readers. From now on, all articles published in AJO will feature a “Take-Home Points” text box. These points represent the most important items that the authors wish to convey from their article. Please enjoy this month’s issue and keep the feedback coming. We are striving to continuously improve AJO and make it your go-to journal for practical information that you can apply directly to your practice.
—Bryan T. Hanypsiak, MD
Hip arthroscopy has been evolving over the past 2 decades as our techniques have been refined and our clinical outcomes have been reported. We have reached a point in our field to look back at the progress that has been made while also providing our readers with the most up-to-date information on diagnosis, imaging studies, and decision making for appropriate treatment.
Trofa and colleagues provide an excellent overview on intra- and extra-articular pathology of the hip and pelvis in their article, “Mastering the Physical Examination of the Athlete’s Hip”. The authors review common injuries in the athlete and provide physical examination tests to differentiate between adductor strain, athletic pubalgia, osteitis pubis, and femoroacetabular impingement (FAI). Also in this issue, Lewis and colleagues provide a comprehensive review of imaging studies in the “Imaging for Nonarthritic Hip Pathology”. The authors review the most common radiographic measurements to detect FAI as well as describe the role of computed tomography and magnetic resonance imaging.
The mastery of hip arthroscopy for the treatment of FAI has a steep learning curve and the techniques have evolved along with our understanding of the importance of the labrum and capsule. We are fortunate to have an article provided by one of the pioneers in the field, Dr. Marc J. Philippon, describing his role in advancing the field in the article “Treatment of FAI: Labrum, Cartilage, Osseous Deformity, and Capsule”. Kollmorgen and Mather provide the most up-to-date techniques for labrum repair and reconstruction. Friel and colleagues report on capsular repair and plication using the T-capsulotomy and the extensile interportal capsulotomy.
We also have the opportunity to read about a number of clinical studies describing the experiences of multi-center studies and epidemiologic studies on large volumes of data. The ANCHOR group provides a summary of the experiences of some of the most renowned hip surgeons in North America as the treatment of FAI evolved from an open approach to an all-arthroscopic approach. The MASH group is a large multi-center group of hip arthroscopists in the United States who describe their current indications for surgical treatment of FAI.
On AmJOrthopedics.com, Matsuda and colleagues describe the outcomes of borderline dysplasia patients compared to normal controls across multiple centers. Anthony and colleagues report on the complication rates using the National Surgical Quality Improvement Program database.
I believe that our Hip Arthroscopy issue will not disappoint you. It is a comprehensive review of the state-of-the-art in hip arthroscopy from physical examination to current surgical techniques to clinical outcomes from large databases for the treatment of FAI. After reviewing this issue, you will be equipped with the most up-to-date information on the treatment of nonarthritic hip disease.
Am J Orthop. 2017;46(1):8. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Editor’s Note: AJO is fortunate to have Shane Nho, one of the nation’s leading hip arthroscopists, as our Deputy Editor-in-Chief. He has compiled an outstanding update for all orthopedic surgeons who see hip patients. It’s my pleasure to turn this issue over to him. On a side note, we’ve added a new feature for our speed readers. From now on, all articles published in AJO will feature a “Take-Home Points” text box. These points represent the most important items that the authors wish to convey from their article. Please enjoy this month’s issue and keep the feedback coming. We are striving to continuously improve AJO and make it your go-to journal for practical information that you can apply directly to your practice.
—Bryan T. Hanypsiak, MD
Hip arthroscopy has been evolving over the past 2 decades as our techniques have been refined and our clinical outcomes have been reported. We have reached a point in our field to look back at the progress that has been made while also providing our readers with the most up-to-date information on diagnosis, imaging studies, and decision making for appropriate treatment.
Trofa and colleagues provide an excellent overview on intra- and extra-articular pathology of the hip and pelvis in their article, “Mastering the Physical Examination of the Athlete’s Hip”. The authors review common injuries in the athlete and provide physical examination tests to differentiate between adductor strain, athletic pubalgia, osteitis pubis, and femoroacetabular impingement (FAI). Also in this issue, Lewis and colleagues provide a comprehensive review of imaging studies in the “Imaging for Nonarthritic Hip Pathology”. The authors review the most common radiographic measurements to detect FAI as well as describe the role of computed tomography and magnetic resonance imaging.
The mastery of hip arthroscopy for the treatment of FAI has a steep learning curve and the techniques have evolved along with our understanding of the importance of the labrum and capsule. We are fortunate to have an article provided by one of the pioneers in the field, Dr. Marc J. Philippon, describing his role in advancing the field in the article “Treatment of FAI: Labrum, Cartilage, Osseous Deformity, and Capsule”. Kollmorgen and Mather provide the most up-to-date techniques for labrum repair and reconstruction. Friel and colleagues report on capsular repair and plication using the T-capsulotomy and the extensile interportal capsulotomy.
We also have the opportunity to read about a number of clinical studies describing the experiences of multi-center studies and epidemiologic studies on large volumes of data. The ANCHOR group provides a summary of the experiences of some of the most renowned hip surgeons in North America as the treatment of FAI evolved from an open approach to an all-arthroscopic approach. The MASH group is a large multi-center group of hip arthroscopists in the United States who describe their current indications for surgical treatment of FAI.
On AmJOrthopedics.com, Matsuda and colleagues describe the outcomes of borderline dysplasia patients compared to normal controls across multiple centers. Anthony and colleagues report on the complication rates using the National Surgical Quality Improvement Program database.
I believe that our Hip Arthroscopy issue will not disappoint you. It is a comprehensive review of the state-of-the-art in hip arthroscopy from physical examination to current surgical techniques to clinical outcomes from large databases for the treatment of FAI. After reviewing this issue, you will be equipped with the most up-to-date information on the treatment of nonarthritic hip disease.
Am J Orthop. 2017;46(1):8. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Mastering the Physical Examination of the Athlete’s Hip
Take-Home Points
- Perform a comprehensive examination to determine intra-articular pathology as well as potential extra-articular sources of hip and pelvic pain.
- Adductor strains can be prevented with adequate rehabilitation focused on correcting predisposing factors (ie, adductor weakness or tightness, limited range of motion, and core imbalance).
- Athletic pubalgia is diagnosed when tenderness can be elicited over the pubic tubercle.
- Osteitis pubis is diagnosed with pain over the pubic symphysis.
- FAI and labral injury classically present with a C-sign but can also present with lateral hip pain, buttock pain, low back pain, anterior thigh pain, and knee pain.
Hip and groin pain is a common finding among athletes of all ages and activity levels. Such pain most often occurs among athletes in sports such as football, hockey, rugby, soccer, and ballet, which demand frequent cutting, pivoting, and acceleration.1-4 Previously, pain about the hip and groin was attributed to muscular strains and soft-tissue contusions, but improvements in physical examination skills, imaging modalities, and disease-specific treatment options have led to increased recognition of hip injuries as a significant source of disability in the athletic population.5,6 These injuries make up 6% or more of all sports injuries, and the rate is increasing.7-9
In this review, we describe precise methods for evaluating the athlete’s hip or groin with an emphasis on recognizing the most common extra-articular and intra-articular pathologies, including adductor strains, athletic pubalgia, osteitis pubis, and femoroacetabular impingement (FAI) with labral tears.
Hip Pathoanatomy
The first step in determining the etiology of pain is to establish if there is true pathology of the hip joint and surrounding structures, or if the pain is referred from another source.
Patient History
The physical examination is guided by the patient’s history. Important patient-specific factors to be ascertained include age, sport(s) played, competition level, seasonal timing, and effect of the injury on performance. Regarding presenting symptoms, attention should be given to pain location, timing (acute vs chronic), onset, nature (clicking, catching, instability), and precipitating factors. Acute-onset pain with muscle contraction or stretching, possibly accompanied by an audible pop, is likely musculotendinous in origin. Insidious-onset dull aching pain that worsens with activity more commonly involves intra-articular processes. Most classically, this pain occurs deep in the groin and is demonstrated by the C sign: The patient cups a hand with its fingers pointing toward the anterior groin at the level of the greater trochanter (Figure 1).11
A comprehensive hip evaluation can be performed with the patient in the standing, seated, supine, lateral, and prone positions, as previously described (Table 2).6,12,13
Extra-Articular Hip Pathologies
Adductor Strains
The adductor muscle group includes the adductor magnus, adductor brevis, gracilis, obturator externus, pectineus, and adductor longus, which is the most commonly strained. Adductor strains are the most common cause of groin pain in athletes, and usually occur in sports that require forceful eccentric contraction of the adductors.14 Among professional soccer players, adductor strains represent almost one fourth of all muscle injuries and result in lost playing time averaging 2 weeks and an 18% reinjury rate.15 These injuries are particularly detrimental to performance because the adductor muscles help stabilize the pelvis during closed-chain activities.3 Diagnosis and adequate rehabilitation focused on correcting predisposing factors (eg, adductor weakness or tightness, loss of hip range of motion, core imbalance) are paramount in reinjury prevention.16,17
On presentation, athletes complain of aching groin or medial thigh pain. The examiner should assess for swelling or ecchymosis. There typically is tenderness to palpation at or near the origin on the pubic bones, with pain exacerbated with resisted adduction and passive stretch into abduction during examination. Palpation of adductors requires proper exposure and is most easily performed with the patient supine and the lower extremity in a figure-of-4 position (Figure 2A).
Athletic Pubalgia
Athletic pubalgia, also known as sports hernia or core muscle injury, is an injury to the soft tissues of the lower abdominal or posterior inguinal wall. Although not fully understood, the condition is considered the result of repetitive trunk hyperextension and thigh hyperabduction resulting in shearing at the pubic symphysis where there is a muscle imbalance between the strong proximal thigh muscles and weaker abdominals. This condition is more common in men and typically is insidious in onset with a prolonged course recalcitrant to nonoperative treatment.18 In studies of chronic groin pain in athletes, the rate of athletic pubalgia as the primary etiology ranges from 39% to 85%.9,19,20
Patients typically complain of increasing pain in the lower abdominal and proximal adductors during activity. Symptoms include unilateral or bilateral lower abdominal pain, which can radiate toward the perineum, rectus muscle, and proximal adductors during sport but usually abates with rest.18 Athletes endorse they are not capable of playing at their full athletic potential. Symptoms are initiated with sudden forceful movements, as in sit-ups, sprints, and valsalva maneuvers like coughs and sneezes. Valsalva maneuvers worsen pain in about 10% of patients.21-23On physical examination with the patient supine, tenderness can be elicited over the pubic tubercle, abdominal obliques, and/or rectus abdominis insertion (Figure 3A). Athletes may also have tenderness at the adductor longus tendon origin at or near the pubic symphysis, which may make the diagnosis difficult to distinguish from an adductor strain.
Osteitis Pubis
Osteitis pubis is a painful overuse injury that results in noninfectious inflammation of the pubic symphysis from increased motion at this normally stable immobile joint.3 As with athletic pubalgia, the exact mechanism is unclear, but likely it is similar to the repetitive stress placed on the pubic symphysis by unequal forces of the abdominal and adductor muscles.24 The disease can result in bony erosions and cartilage breakdown with irregularity of the pubic symphysis.
Athletes may complain of anterior and medial groin pain that can radiate to the lower abdominal muscles, perineum, inguinal region, and medial thigh. Walking, pelvic motion, adductor stretching, abdominal muscle exercises, and standing up can exacerbate pain.24 Some cases involve impaired internal or external rotation of the hip, sacroiliac joint dysfunction, or adductor and abductor muscle weakness.25The distinguishing feature of osteitis pubis is pain over the pubic symphysis with direct palpation (Figure 4A). Examination maneuvers that place stress on the pubic symphysis can aid in diagnosis.26
Intra-Articular Hip Pathology: Femoroacetabular Impingement
In athletes, FAI is a leading cause of intra-articular pathology, which can lead to labral tears.28,29 FAI lesions include cam-type impingement from an aspherical femoral head and pincer impingement from acetabular overcoverage, both of which limit internal rotation and cause acetabular rim abutment, which damages the labrum.
Athletes present with activity-related groin or hip pain that is exacerbated by hip flexion and internal rotation, with possible mechanical symptoms from labral tearing.30 However, the pain distribution varies. In a study by Clohisy and colleagues,31 of patients with symptomatic FAI that required surgical intervention, 88% had groin pain, 67% had lateral hip pain, 35% had anterior thigh pain, 29% had buttock pain, 27% had knee pain, and 23% had low back pain.
Careful attention should be given to range of motion in FAI patients, as they can usually flex their hip to 90° to 110°, and in this position there is limited internal rotation and asymmetric external rotation relative to the contralateral leg.32 The anterior impingement test is one of the most reliable tests for FAI (Figure 5A).32 With the patient supine, the hip is dynamically flexed to 90°, adducted, and internally rotated. A positive test elicits deep anterior groin pain that generally replicates the patient’s symptoms.29
Conclusion
Careful, directed history taking and physical examination are essential in narrowing the diagnostic possibilities before initiating a workup for the common intra-articular and extra-articular causes of hip and groin pain in athletes.
Am J Orthop. 2017;46(1):10-16. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Boyd KT, Peirce NS, Batt ME. Common hip injuries in sport. Sports Med. 1997;24(4):273-288.
2. Duthon VB, Charbonnier C, Kolo FC, et al. Correlation of clinical and magnetic resonance imaging findings in hips of elite female ballet dancers. Arthroscopy. 2013;29(3):411-419.
3. Prather H, Cheng A. Diagnosis and treatment of hip girdle pain in the athlete. PM R. 2016;8(3 suppl):S45-S60.
4. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
5. Bizzini M, Notzli HP, Maffiuletti NA. Femoroacetabular impingement in professional ice hockey players: a case series of 5 athletes after open surgical decompression of the hip. Am J Sports Med. 2007;35(11):1955-1959.
6. Lynch TS, Terry MA, Bedi A, Kelly BT. Hip arthroscopic surgery: patient evaluation, current indications, and outcomes. Am J Sports Med. 2013;41(5):1174-1189.
7. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
8. Fon LJ, Spence RA. Sportsman’s hernia. Br J Surg. 2000;87(5):545-552.
9. Kluin J, den Hoed PT, van Linschoten R, IJzerman JC, van Steensel CJ. Endoscopic evaluation and treatment of groin pain in the athlete. Am J Sports Med. 2004;32(4):944-949.
10. Ward D, Parvizi J. Management of hip pain in young adults. Orthop Clin North Am. 2016;47(3):485-496.
11. Byrd JW. Hip arthroscopy. J Am Acad Orthop Surg. 2006;14(7):433-444.
12. Martin HD, Palmer IJ. History and physical examination of the hip: the basics. Curr Rev Musculoskelet Med. 2013;6(3):219-225.
13. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
14. Morelli V, Smith V. Groin injuries in athletes. Am Fam Physician. 2001;64(8):1405-1414.
15. Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011;39(6):1226-1232.
16. Ekstrand J, Gillquist J. The avoidability of soccer injuries. Int J Sports Med. 1983;4(2):124-128.
17. Tyler TF, Nicholas SJ, Campbell RJ, McHugh MP. The association of hip strength and flexibility with the incidence of adductor muscle strains in professional ice hockey players. Am J Sports Med. 2001;29(2):124-128.
18. Farber AJ, Wilckens JH. Sports hernia: diagnosis and therapeutic approach. J Am Acad Orthop Surg. 2007;15(8):507-514.
19. De Paulis F, Cacchio A, Michelini O, Damiani A, Saggini R. Sports injuries in the pelvis and hip: diagnostic imaging. Eur J Radiol. 1998;27(suppl 1):S49-S59.
20. Lovell G. The diagnosis of chronic groin pain in athletes: a review of 189 cases. Aust J Sci Med Sport. 1995;27(suppl 1):76-79.
21. Strosberg DS, Ellis TJ, Renton DB. The role of femoroacetabular impingement in core muscle injury/athletic pubalgia: diagnosis and management. Front Surg. 2016;3:6.
22. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
23. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
24. Angoules AG. Osteitis pubis in elite athletes: diagnostic and therapeutic approach. World J Orthop. 2015;6(9):672-679.
25. Hiti CJ, Stevens KJ, Jamati MK, Garza D, Matheson GO. Athletic osteitis pubis. Sports Med. 2011;41(5):361-376.
26. Mehin R, Meek R, O’Brien P, Blachut P. Surgery for osteitis pubis. Can J Surg. 2006;49(3):170-176.
27. Grace JN, Sim FH, Shives TC, Coventry MB. Wedge resection of the symphysis pubis for the treatment of osteitis pubis. J Bone Joint Surg Am. 1989;71(3):358-364.
28. Amanatullah DF, Antkowiak T, Pillay K, et al. Femoroacetabular impingement: current concepts in diagnosis and treatment. Orthopedics. 2015;38(3):185-199.
29. Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):112-120.
30. Redmond JM, Gupta A, Hammarstedt JE, Stake CE, Dunne KF, Domb BG. Labral injury: radiographic predictors at the time of hip arthroscopy. Arthroscopy. 2015;31(1):51-56.
31. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644.
32. Klaue K, Durnin CW, Ganz R. The acetabular rim syndrome. A clinical presentation of dysplasia of the hip. J Bone Joint Surg Br. 1991;73(3):423-429.
33. Philippon MJ, Schenker ML. Arthroscopy for the treatment of femoroacetabular impingement in the athlete. Clin Sports Med. 2006;25(2):299-308.
34. McCarthy JC, Lee JA. Hip arthroscopy: indications, outcomes, and complications. Instr Course Lect. 2006;55:301-308.
Take-Home Points
- Perform a comprehensive examination to determine intra-articular pathology as well as potential extra-articular sources of hip and pelvic pain.
- Adductor strains can be prevented with adequate rehabilitation focused on correcting predisposing factors (ie, adductor weakness or tightness, limited range of motion, and core imbalance).
- Athletic pubalgia is diagnosed when tenderness can be elicited over the pubic tubercle.
- Osteitis pubis is diagnosed with pain over the pubic symphysis.
- FAI and labral injury classically present with a C-sign but can also present with lateral hip pain, buttock pain, low back pain, anterior thigh pain, and knee pain.
Hip and groin pain is a common finding among athletes of all ages and activity levels. Such pain most often occurs among athletes in sports such as football, hockey, rugby, soccer, and ballet, which demand frequent cutting, pivoting, and acceleration.1-4 Previously, pain about the hip and groin was attributed to muscular strains and soft-tissue contusions, but improvements in physical examination skills, imaging modalities, and disease-specific treatment options have led to increased recognition of hip injuries as a significant source of disability in the athletic population.5,6 These injuries make up 6% or more of all sports injuries, and the rate is increasing.7-9
In this review, we describe precise methods for evaluating the athlete’s hip or groin with an emphasis on recognizing the most common extra-articular and intra-articular pathologies, including adductor strains, athletic pubalgia, osteitis pubis, and femoroacetabular impingement (FAI) with labral tears.
Hip Pathoanatomy
The first step in determining the etiology of pain is to establish if there is true pathology of the hip joint and surrounding structures, or if the pain is referred from another source.
Patient History
The physical examination is guided by the patient’s history. Important patient-specific factors to be ascertained include age, sport(s) played, competition level, seasonal timing, and effect of the injury on performance. Regarding presenting symptoms, attention should be given to pain location, timing (acute vs chronic), onset, nature (clicking, catching, instability), and precipitating factors. Acute-onset pain with muscle contraction or stretching, possibly accompanied by an audible pop, is likely musculotendinous in origin. Insidious-onset dull aching pain that worsens with activity more commonly involves intra-articular processes. Most classically, this pain occurs deep in the groin and is demonstrated by the C sign: The patient cups a hand with its fingers pointing toward the anterior groin at the level of the greater trochanter (Figure 1).11
A comprehensive hip evaluation can be performed with the patient in the standing, seated, supine, lateral, and prone positions, as previously described (Table 2).6,12,13
Extra-Articular Hip Pathologies
Adductor Strains
The adductor muscle group includes the adductor magnus, adductor brevis, gracilis, obturator externus, pectineus, and adductor longus, which is the most commonly strained. Adductor strains are the most common cause of groin pain in athletes, and usually occur in sports that require forceful eccentric contraction of the adductors.14 Among professional soccer players, adductor strains represent almost one fourth of all muscle injuries and result in lost playing time averaging 2 weeks and an 18% reinjury rate.15 These injuries are particularly detrimental to performance because the adductor muscles help stabilize the pelvis during closed-chain activities.3 Diagnosis and adequate rehabilitation focused on correcting predisposing factors (eg, adductor weakness or tightness, loss of hip range of motion, core imbalance) are paramount in reinjury prevention.16,17
On presentation, athletes complain of aching groin or medial thigh pain. The examiner should assess for swelling or ecchymosis. There typically is tenderness to palpation at or near the origin on the pubic bones, with pain exacerbated with resisted adduction and passive stretch into abduction during examination. Palpation of adductors requires proper exposure and is most easily performed with the patient supine and the lower extremity in a figure-of-4 position (Figure 2A).
Athletic Pubalgia
Athletic pubalgia, also known as sports hernia or core muscle injury, is an injury to the soft tissues of the lower abdominal or posterior inguinal wall. Although not fully understood, the condition is considered the result of repetitive trunk hyperextension and thigh hyperabduction resulting in shearing at the pubic symphysis where there is a muscle imbalance between the strong proximal thigh muscles and weaker abdominals. This condition is more common in men and typically is insidious in onset with a prolonged course recalcitrant to nonoperative treatment.18 In studies of chronic groin pain in athletes, the rate of athletic pubalgia as the primary etiology ranges from 39% to 85%.9,19,20
Patients typically complain of increasing pain in the lower abdominal and proximal adductors during activity. Symptoms include unilateral or bilateral lower abdominal pain, which can radiate toward the perineum, rectus muscle, and proximal adductors during sport but usually abates with rest.18 Athletes endorse they are not capable of playing at their full athletic potential. Symptoms are initiated with sudden forceful movements, as in sit-ups, sprints, and valsalva maneuvers like coughs and sneezes. Valsalva maneuvers worsen pain in about 10% of patients.21-23On physical examination with the patient supine, tenderness can be elicited over the pubic tubercle, abdominal obliques, and/or rectus abdominis insertion (Figure 3A). Athletes may also have tenderness at the adductor longus tendon origin at or near the pubic symphysis, which may make the diagnosis difficult to distinguish from an adductor strain.
Osteitis Pubis
Osteitis pubis is a painful overuse injury that results in noninfectious inflammation of the pubic symphysis from increased motion at this normally stable immobile joint.3 As with athletic pubalgia, the exact mechanism is unclear, but likely it is similar to the repetitive stress placed on the pubic symphysis by unequal forces of the abdominal and adductor muscles.24 The disease can result in bony erosions and cartilage breakdown with irregularity of the pubic symphysis.
Athletes may complain of anterior and medial groin pain that can radiate to the lower abdominal muscles, perineum, inguinal region, and medial thigh. Walking, pelvic motion, adductor stretching, abdominal muscle exercises, and standing up can exacerbate pain.24 Some cases involve impaired internal or external rotation of the hip, sacroiliac joint dysfunction, or adductor and abductor muscle weakness.25The distinguishing feature of osteitis pubis is pain over the pubic symphysis with direct palpation (Figure 4A). Examination maneuvers that place stress on the pubic symphysis can aid in diagnosis.26
Intra-Articular Hip Pathology: Femoroacetabular Impingement
In athletes, FAI is a leading cause of intra-articular pathology, which can lead to labral tears.28,29 FAI lesions include cam-type impingement from an aspherical femoral head and pincer impingement from acetabular overcoverage, both of which limit internal rotation and cause acetabular rim abutment, which damages the labrum.
Athletes present with activity-related groin or hip pain that is exacerbated by hip flexion and internal rotation, with possible mechanical symptoms from labral tearing.30 However, the pain distribution varies. In a study by Clohisy and colleagues,31 of patients with symptomatic FAI that required surgical intervention, 88% had groin pain, 67% had lateral hip pain, 35% had anterior thigh pain, 29% had buttock pain, 27% had knee pain, and 23% had low back pain.
Careful attention should be given to range of motion in FAI patients, as they can usually flex their hip to 90° to 110°, and in this position there is limited internal rotation and asymmetric external rotation relative to the contralateral leg.32 The anterior impingement test is one of the most reliable tests for FAI (Figure 5A).32 With the patient supine, the hip is dynamically flexed to 90°, adducted, and internally rotated. A positive test elicits deep anterior groin pain that generally replicates the patient’s symptoms.29
Conclusion
Careful, directed history taking and physical examination are essential in narrowing the diagnostic possibilities before initiating a workup for the common intra-articular and extra-articular causes of hip and groin pain in athletes.
Am J Orthop. 2017;46(1):10-16. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Perform a comprehensive examination to determine intra-articular pathology as well as potential extra-articular sources of hip and pelvic pain.
- Adductor strains can be prevented with adequate rehabilitation focused on correcting predisposing factors (ie, adductor weakness or tightness, limited range of motion, and core imbalance).
- Athletic pubalgia is diagnosed when tenderness can be elicited over the pubic tubercle.
- Osteitis pubis is diagnosed with pain over the pubic symphysis.
- FAI and labral injury classically present with a C-sign but can also present with lateral hip pain, buttock pain, low back pain, anterior thigh pain, and knee pain.
Hip and groin pain is a common finding among athletes of all ages and activity levels. Such pain most often occurs among athletes in sports such as football, hockey, rugby, soccer, and ballet, which demand frequent cutting, pivoting, and acceleration.1-4 Previously, pain about the hip and groin was attributed to muscular strains and soft-tissue contusions, but improvements in physical examination skills, imaging modalities, and disease-specific treatment options have led to increased recognition of hip injuries as a significant source of disability in the athletic population.5,6 These injuries make up 6% or more of all sports injuries, and the rate is increasing.7-9
In this review, we describe precise methods for evaluating the athlete’s hip or groin with an emphasis on recognizing the most common extra-articular and intra-articular pathologies, including adductor strains, athletic pubalgia, osteitis pubis, and femoroacetabular impingement (FAI) with labral tears.
Hip Pathoanatomy
The first step in determining the etiology of pain is to establish if there is true pathology of the hip joint and surrounding structures, or if the pain is referred from another source.
Patient History
The physical examination is guided by the patient’s history. Important patient-specific factors to be ascertained include age, sport(s) played, competition level, seasonal timing, and effect of the injury on performance. Regarding presenting symptoms, attention should be given to pain location, timing (acute vs chronic), onset, nature (clicking, catching, instability), and precipitating factors. Acute-onset pain with muscle contraction or stretching, possibly accompanied by an audible pop, is likely musculotendinous in origin. Insidious-onset dull aching pain that worsens with activity more commonly involves intra-articular processes. Most classically, this pain occurs deep in the groin and is demonstrated by the C sign: The patient cups a hand with its fingers pointing toward the anterior groin at the level of the greater trochanter (Figure 1).11
A comprehensive hip evaluation can be performed with the patient in the standing, seated, supine, lateral, and prone positions, as previously described (Table 2).6,12,13
Extra-Articular Hip Pathologies
Adductor Strains
The adductor muscle group includes the adductor magnus, adductor brevis, gracilis, obturator externus, pectineus, and adductor longus, which is the most commonly strained. Adductor strains are the most common cause of groin pain in athletes, and usually occur in sports that require forceful eccentric contraction of the adductors.14 Among professional soccer players, adductor strains represent almost one fourth of all muscle injuries and result in lost playing time averaging 2 weeks and an 18% reinjury rate.15 These injuries are particularly detrimental to performance because the adductor muscles help stabilize the pelvis during closed-chain activities.3 Diagnosis and adequate rehabilitation focused on correcting predisposing factors (eg, adductor weakness or tightness, loss of hip range of motion, core imbalance) are paramount in reinjury prevention.16,17
On presentation, athletes complain of aching groin or medial thigh pain. The examiner should assess for swelling or ecchymosis. There typically is tenderness to palpation at or near the origin on the pubic bones, with pain exacerbated with resisted adduction and passive stretch into abduction during examination. Palpation of adductors requires proper exposure and is most easily performed with the patient supine and the lower extremity in a figure-of-4 position (Figure 2A).
Athletic Pubalgia
Athletic pubalgia, also known as sports hernia or core muscle injury, is an injury to the soft tissues of the lower abdominal or posterior inguinal wall. Although not fully understood, the condition is considered the result of repetitive trunk hyperextension and thigh hyperabduction resulting in shearing at the pubic symphysis where there is a muscle imbalance between the strong proximal thigh muscles and weaker abdominals. This condition is more common in men and typically is insidious in onset with a prolonged course recalcitrant to nonoperative treatment.18 In studies of chronic groin pain in athletes, the rate of athletic pubalgia as the primary etiology ranges from 39% to 85%.9,19,20
Patients typically complain of increasing pain in the lower abdominal and proximal adductors during activity. Symptoms include unilateral or bilateral lower abdominal pain, which can radiate toward the perineum, rectus muscle, and proximal adductors during sport but usually abates with rest.18 Athletes endorse they are not capable of playing at their full athletic potential. Symptoms are initiated with sudden forceful movements, as in sit-ups, sprints, and valsalva maneuvers like coughs and sneezes. Valsalva maneuvers worsen pain in about 10% of patients.21-23On physical examination with the patient supine, tenderness can be elicited over the pubic tubercle, abdominal obliques, and/or rectus abdominis insertion (Figure 3A). Athletes may also have tenderness at the adductor longus tendon origin at or near the pubic symphysis, which may make the diagnosis difficult to distinguish from an adductor strain.
Osteitis Pubis
Osteitis pubis is a painful overuse injury that results in noninfectious inflammation of the pubic symphysis from increased motion at this normally stable immobile joint.3 As with athletic pubalgia, the exact mechanism is unclear, but likely it is similar to the repetitive stress placed on the pubic symphysis by unequal forces of the abdominal and adductor muscles.24 The disease can result in bony erosions and cartilage breakdown with irregularity of the pubic symphysis.
Athletes may complain of anterior and medial groin pain that can radiate to the lower abdominal muscles, perineum, inguinal region, and medial thigh. Walking, pelvic motion, adductor stretching, abdominal muscle exercises, and standing up can exacerbate pain.24 Some cases involve impaired internal or external rotation of the hip, sacroiliac joint dysfunction, or adductor and abductor muscle weakness.25The distinguishing feature of osteitis pubis is pain over the pubic symphysis with direct palpation (Figure 4A). Examination maneuvers that place stress on the pubic symphysis can aid in diagnosis.26
Intra-Articular Hip Pathology: Femoroacetabular Impingement
In athletes, FAI is a leading cause of intra-articular pathology, which can lead to labral tears.28,29 FAI lesions include cam-type impingement from an aspherical femoral head and pincer impingement from acetabular overcoverage, both of which limit internal rotation and cause acetabular rim abutment, which damages the labrum.
Athletes present with activity-related groin or hip pain that is exacerbated by hip flexion and internal rotation, with possible mechanical symptoms from labral tearing.30 However, the pain distribution varies. In a study by Clohisy and colleagues,31 of patients with symptomatic FAI that required surgical intervention, 88% had groin pain, 67% had lateral hip pain, 35% had anterior thigh pain, 29% had buttock pain, 27% had knee pain, and 23% had low back pain.
Careful attention should be given to range of motion in FAI patients, as they can usually flex their hip to 90° to 110°, and in this position there is limited internal rotation and asymmetric external rotation relative to the contralateral leg.32 The anterior impingement test is one of the most reliable tests for FAI (Figure 5A).32 With the patient supine, the hip is dynamically flexed to 90°, adducted, and internally rotated. A positive test elicits deep anterior groin pain that generally replicates the patient’s symptoms.29
Conclusion
Careful, directed history taking and physical examination are essential in narrowing the diagnostic possibilities before initiating a workup for the common intra-articular and extra-articular causes of hip and groin pain in athletes.
Am J Orthop. 2017;46(1):10-16. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Boyd KT, Peirce NS, Batt ME. Common hip injuries in sport. Sports Med. 1997;24(4):273-288.
2. Duthon VB, Charbonnier C, Kolo FC, et al. Correlation of clinical and magnetic resonance imaging findings in hips of elite female ballet dancers. Arthroscopy. 2013;29(3):411-419.
3. Prather H, Cheng A. Diagnosis and treatment of hip girdle pain in the athlete. PM R. 2016;8(3 suppl):S45-S60.
4. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
5. Bizzini M, Notzli HP, Maffiuletti NA. Femoroacetabular impingement in professional ice hockey players: a case series of 5 athletes after open surgical decompression of the hip. Am J Sports Med. 2007;35(11):1955-1959.
6. Lynch TS, Terry MA, Bedi A, Kelly BT. Hip arthroscopic surgery: patient evaluation, current indications, and outcomes. Am J Sports Med. 2013;41(5):1174-1189.
7. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
8. Fon LJ, Spence RA. Sportsman’s hernia. Br J Surg. 2000;87(5):545-552.
9. Kluin J, den Hoed PT, van Linschoten R, IJzerman JC, van Steensel CJ. Endoscopic evaluation and treatment of groin pain in the athlete. Am J Sports Med. 2004;32(4):944-949.
10. Ward D, Parvizi J. Management of hip pain in young adults. Orthop Clin North Am. 2016;47(3):485-496.
11. Byrd JW. Hip arthroscopy. J Am Acad Orthop Surg. 2006;14(7):433-444.
12. Martin HD, Palmer IJ. History and physical examination of the hip: the basics. Curr Rev Musculoskelet Med. 2013;6(3):219-225.
13. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
14. Morelli V, Smith V. Groin injuries in athletes. Am Fam Physician. 2001;64(8):1405-1414.
15. Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011;39(6):1226-1232.
16. Ekstrand J, Gillquist J. The avoidability of soccer injuries. Int J Sports Med. 1983;4(2):124-128.
17. Tyler TF, Nicholas SJ, Campbell RJ, McHugh MP. The association of hip strength and flexibility with the incidence of adductor muscle strains in professional ice hockey players. Am J Sports Med. 2001;29(2):124-128.
18. Farber AJ, Wilckens JH. Sports hernia: diagnosis and therapeutic approach. J Am Acad Orthop Surg. 2007;15(8):507-514.
19. De Paulis F, Cacchio A, Michelini O, Damiani A, Saggini R. Sports injuries in the pelvis and hip: diagnostic imaging. Eur J Radiol. 1998;27(suppl 1):S49-S59.
20. Lovell G. The diagnosis of chronic groin pain in athletes: a review of 189 cases. Aust J Sci Med Sport. 1995;27(suppl 1):76-79.
21. Strosberg DS, Ellis TJ, Renton DB. The role of femoroacetabular impingement in core muscle injury/athletic pubalgia: diagnosis and management. Front Surg. 2016;3:6.
22. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
23. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
24. Angoules AG. Osteitis pubis in elite athletes: diagnostic and therapeutic approach. World J Orthop. 2015;6(9):672-679.
25. Hiti CJ, Stevens KJ, Jamati MK, Garza D, Matheson GO. Athletic osteitis pubis. Sports Med. 2011;41(5):361-376.
26. Mehin R, Meek R, O’Brien P, Blachut P. Surgery for osteitis pubis. Can J Surg. 2006;49(3):170-176.
27. Grace JN, Sim FH, Shives TC, Coventry MB. Wedge resection of the symphysis pubis for the treatment of osteitis pubis. J Bone Joint Surg Am. 1989;71(3):358-364.
28. Amanatullah DF, Antkowiak T, Pillay K, et al. Femoroacetabular impingement: current concepts in diagnosis and treatment. Orthopedics. 2015;38(3):185-199.
29. Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):112-120.
30. Redmond JM, Gupta A, Hammarstedt JE, Stake CE, Dunne KF, Domb BG. Labral injury: radiographic predictors at the time of hip arthroscopy. Arthroscopy. 2015;31(1):51-56.
31. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644.
32. Klaue K, Durnin CW, Ganz R. The acetabular rim syndrome. A clinical presentation of dysplasia of the hip. J Bone Joint Surg Br. 1991;73(3):423-429.
33. Philippon MJ, Schenker ML. Arthroscopy for the treatment of femoroacetabular impingement in the athlete. Clin Sports Med. 2006;25(2):299-308.
34. McCarthy JC, Lee JA. Hip arthroscopy: indications, outcomes, and complications. Instr Course Lect. 2006;55:301-308.
1. Boyd KT, Peirce NS, Batt ME. Common hip injuries in sport. Sports Med. 1997;24(4):273-288.
2. Duthon VB, Charbonnier C, Kolo FC, et al. Correlation of clinical and magnetic resonance imaging findings in hips of elite female ballet dancers. Arthroscopy. 2013;29(3):411-419.
3. Prather H, Cheng A. Diagnosis and treatment of hip girdle pain in the athlete. PM R. 2016;8(3 suppl):S45-S60.
4. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144.
5. Bizzini M, Notzli HP, Maffiuletti NA. Femoroacetabular impingement in professional ice hockey players: a case series of 5 athletes after open surgical decompression of the hip. Am J Sports Med. 2007;35(11):1955-1959.
6. Lynch TS, Terry MA, Bedi A, Kelly BT. Hip arthroscopic surgery: patient evaluation, current indications, and outcomes. Am J Sports Med. 2013;41(5):1174-1189.
7. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533.
8. Fon LJ, Spence RA. Sportsman’s hernia. Br J Surg. 2000;87(5):545-552.
9. Kluin J, den Hoed PT, van Linschoten R, IJzerman JC, van Steensel CJ. Endoscopic evaluation and treatment of groin pain in the athlete. Am J Sports Med. 2004;32(4):944-949.
10. Ward D, Parvizi J. Management of hip pain in young adults. Orthop Clin North Am. 2016;47(3):485-496.
11. Byrd JW. Hip arthroscopy. J Am Acad Orthop Surg. 2006;14(7):433-444.
12. Martin HD, Palmer IJ. History and physical examination of the hip: the basics. Curr Rev Musculoskelet Med. 2013;6(3):219-225.
13. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
14. Morelli V, Smith V. Groin injuries in athletes. Am Fam Physician. 2001;64(8):1405-1414.
15. Ekstrand J, Hagglund M, Walden M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011;39(6):1226-1232.
16. Ekstrand J, Gillquist J. The avoidability of soccer injuries. Int J Sports Med. 1983;4(2):124-128.
17. Tyler TF, Nicholas SJ, Campbell RJ, McHugh MP. The association of hip strength and flexibility with the incidence of adductor muscle strains in professional ice hockey players. Am J Sports Med. 2001;29(2):124-128.
18. Farber AJ, Wilckens JH. Sports hernia: diagnosis and therapeutic approach. J Am Acad Orthop Surg. 2007;15(8):507-514.
19. De Paulis F, Cacchio A, Michelini O, Damiani A, Saggini R. Sports injuries in the pelvis and hip: diagnostic imaging. Eur J Radiol. 1998;27(suppl 1):S49-S59.
20. Lovell G. The diagnosis of chronic groin pain in athletes: a review of 189 cases. Aust J Sci Med Sport. 1995;27(suppl 1):76-79.
21. Strosberg DS, Ellis TJ, Renton DB. The role of femoroacetabular impingement in core muscle injury/athletic pubalgia: diagnosis and management. Front Surg. 2016;3:6.
22. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. PAIN (Performing Athletes with Abdominal or Inguinal Neuromuscular Pain Study Group). Am J Sports Med. 2000;28(1):2-8.
23. Ahumada LA, Ashruf S, Espinosa-de-los-Monteros A, et al. Athletic pubalgia: definition and surgical treatment. Ann Plast Surg. 2005;55(4):393-396.
24. Angoules AG. Osteitis pubis in elite athletes: diagnostic and therapeutic approach. World J Orthop. 2015;6(9):672-679.
25. Hiti CJ, Stevens KJ, Jamati MK, Garza D, Matheson GO. Athletic osteitis pubis. Sports Med. 2011;41(5):361-376.
26. Mehin R, Meek R, O’Brien P, Blachut P. Surgery for osteitis pubis. Can J Surg. 2006;49(3):170-176.
27. Grace JN, Sim FH, Shives TC, Coventry MB. Wedge resection of the symphysis pubis for the treatment of osteitis pubis. J Bone Joint Surg Am. 1989;71(3):358-364.
28. Amanatullah DF, Antkowiak T, Pillay K, et al. Femoroacetabular impingement: current concepts in diagnosis and treatment. Orthopedics. 2015;38(3):185-199.
29. Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):112-120.
30. Redmond JM, Gupta A, Hammarstedt JE, Stake CE, Dunne KF, Domb BG. Labral injury: radiographic predictors at the time of hip arthroscopy. Arthroscopy. 2015;31(1):51-56.
31. Clohisy JC, Knaus ER, Hunt DM, Lesher JM, Harris-Hayes M, Prather H. Clinical presentation of patients with symptomatic anterior hip impingement. Clin Orthop Relat Res. 2009;467(3):638-644.
32. Klaue K, Durnin CW, Ganz R. The acetabular rim syndrome. A clinical presentation of dysplasia of the hip. J Bone Joint Surg Br. 1991;73(3):423-429.
33. Philippon MJ, Schenker ML. Arthroscopy for the treatment of femoroacetabular impingement in the athlete. Clin Sports Med. 2006;25(2):299-308.
34. McCarthy JC, Lee JA. Hip arthroscopy: indications, outcomes, and complications. Instr Course Lect. 2006;55:301-308.
Imaging for Nonarthritic Hip Pathology
Take-Home Points
- Be sure to have a well centered AP pelvis without rotation.
- Get at least 3 plain radiographs—AP pelvis, false profile, and lateral hip view.
- Ensure that there is sufficient acetabular coverage, LCEA >20° on AP pelvis and ACEA >20° on false profile view.
- CT scans are helpful for precise hip pathomorphology but must be weighed against risk of radiation exposure.
- MRI or MRA can be helpful to diagnose intra-articular as well as extra-articular hip and pelvis abnormalities.
In the work-up for nonarthritic hip pain, the value of diagnostic imaging is in objective findings, which can support or weaken the leading diagnoses based on subjective complaints, recalled history, and, in some cases, elusive physical examination findings. Morphologic changes alone, however, do not always indicate pathology.1,2 At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Radiography
The first step in diagnostic imaging is radiography. Although use of plain radiographs is routine, their value cannot be understated. Standard hip radiographs—an anteroposterior (AP) radiograph of the pelvis and AP and frog-leg (cross-table lateral) radiographs of the hip—provide a wealth of information.3-6
Evaluated first is the radiograph itself. For example, the ideal AP radiograph of the pelvis (Figure 1) is centered on the lower sacrum, and the patient is not rotated.
AP radiographs allow for evaluation of fractures, intraosseous sclerosis, acetabular depth, inclination and version, acetabular overcoverage, joint-space narrowing, femoroacetabular congruency, femoral head sphericity, and femoral head–neck offset.7,8,10 Inspection for labral calcification is important, as it can indicate repetitive damage at the extremes of range of motion.
On AP pelvis radiographs, it is important to distinguish coxa profunda from acetabular protrusion. These entities are on the same pathomorphologic spectrum and are similar but distinctively different. Coxa profunda refers to the depth of the acetabulum relative to the ilioischial line, and acetabular protrusion refers to the depth (or medial position) of the femoral head relative to the ilioischial line. Each condition suggests—but is not diagnostic for—pincer-type femoroacetabular impingement (FAI).11Acetabular rotation is another important entity that can be evaluated on well-centered, nontilted AP pelvic radiographs. Acetabular rotation refers to the opening direction of the acetabulum. It may be anterior (anteverted), neutral, or posterior (retroverted). Anteversion is present when the anterior acetabular rim does not traverse the posterior rim shadow4; in other words, the ring formed by the acetabulum is not twisted. When the walls overlap but do not intersect, the cup has neutral version. Retroversion is qualitatively determined by the crossover (figure-of-8) and posterior wall signs12 and is associated with pincer-type FAI and the development of hip osteoarthritis.12Dunn lateral radiographs (Figure 2A), taken with 90° hip flexion, were originally used to measure femoral neck anteversion.13
False-profile radiographs (Figure 6), valuable in evaluating anterior acetabular coverage and femoral head–neck junction morphology,14,15 allow characterization of both cam-type and pincer-type FAI.
Quantitative measures warrant specific consideration (Table). Femoroacetabular morphology is quantitatively measured by α angle, Tönnis angle (acetabular inclination angle), and lateral center-edge angle (LCEA).7,8,10 The α angle (Figure 4) detects the loss of normal anterosuperior femoral head–neck junction concavity caused by a convex osseous prominence. An α angle >50° represents a cam deformity.16 In a cohort study of 338 patients, Nepple and colleagues17 qualitatively associated increased α angle with severe intra-articular hip disease. Murphy and colleagues18 found a Tönnis angle >15° to be a poor prognostic factor in untreated hip dysplasia. LCEA quantifies superolateral femoral head coverage,19 and its normal range is 20° to 40°.20 LCEA <20° indicates dysplasia of the femoroacetabular joint, and LCEA >40° indicates overcoverage and pincer-type FAI. As with any quantitative radiographic measurement, results should be interpreted within the presenting clinical context.
Radiographic findings, even findings based on these special radiographs, may underestimate the pathologic process.
Computed Tomography
The benefits of computed tomography (CT) outweigh the risk of radiation exposure. CT is most useful in characterizing osseous morphology.21 In FAI cases, CT can distinguish acetabular version abnormalities from femoral torsion (Figures 7A-7C), entities with very different treatment approaches.21
Magnetic Resonance Imaging
MRI is becoming essential in the work-up for nonarthritic hip pain.11,22 It is used for assessment of osseous, chondral, and musculotendinous soft tissues. Further, it affords appreciation of outside-the-hip-joint pathology that may mimic joint-centered pathology.
MRI techniques range from noncontrast to indirect and direct magnetic resonance arthrography (MRA).22 Indirect MRA is performed with contrast medium administered through an intravenous line. Direct MRA has contrast administered intra-articularly and is more sensitive and specific for labral tears and ligamentous injury.23 Excellent detection of intra-articular pathology on noncontrast studies questions the need for MRA.24 Nevertheless, direct MRA can also be used as a therapeutic procedure when lidocaine is included in the injected gadolinium.
Labral tears, focal chondral defects, and stress or insufficiency fractures are important differentials in the work-up for nonarthritic hip pain. Over the dysplasia-to-FAI spectrum, MRI distinguishes symptomatic pathoanatomy from asymptomatic anatomical variants by revealing underlying bone edema. Capsule findings should also be considered.21The most practical classification of labral tears, proposed by Blankenbaker and colleagues,25 is based on tear type (frayed, unstable, flap), location, and extent. More than half of labral tears occur in the anterosuperior quadrant of the labrum.25
Chondral damage is identified much as labral tears are. With chondral injury, the normal intermediate signal is interrupted by a fluid-intense signal extending to the subchondral bone. A fat-saturated T2or short-tau inversion recovery (STIR) sequence is useful in emphasizing this finding.27
MRI detects osseous pathology from surrounding soft-tissue edema and bone remodeling to stress and fragility fractures. In athletes, the most common fractures are pubic rami, sacral, and apophyseal avulsion fractures.28 In all patients, attention should be given to the lower spine and the proximal femurs. Aside from MRI, nuclear medicine bone scan might also identify active osseous reaction representative of a fracture.
Conclusion
The work-up for nonarthritic hip pain substantiates differential diagnoses. A case’s complexity determines the course of diagnostic imaging. At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Am J Orthop . 2017;46(1):17-22. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. McCall DA, Safran MR. MRI and arthroscopy correlations of the hip: a case-based approach. Instr Course Lect . 2012;61:327-344.
2. Register B, Pennock AT, Ho CP, Strickland CD, Lawand A, Philippon MJ. Prevalence of abnormal hip findings in asymptomatic participants: a prospective, blinded study. Am J Sports Med . 2012;40(12):2720-2724.
3. Campbell SE. Radiography of the hip: lines, signs, and patterns of disease. Semin Roentgenol . 2005;40(3):290-319.
4. Clohisy JC, Carlisle JC, Beaulé PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am . 2008;90(suppl 4):47-66.
5. Malviya A, Raza A, Witt JD. Reliability in the diagnosis of femoroacetabular impingement and dysplasia among hip surgeons: role of surgeon volume and experience. Hip Int . 2016;26(3):284-289.
6. Nepple JJ, Martel JM, Kim YJ, Zaltz I, Clohisy JC, Group AS. Do plain radiographs correlate with CT for imaging of cam-type femoroacetabular impingement? Clin Orthop Relat Res . 2012;470(12):3313-3320.
7. Kosuge D, Cordier T, Solomon LB, Howie DW. Dilemmas in imaging for peri-acetabular osteotomy: the influence of patient position and imaging technique on the radiological features of hip dysplasia. Bone Joint J . 2014;96(9):1155-1160.
8. Tannast M, Fritsch S, Zheng G, Siebenrock KA, Steppacher SD. Which radiographic hip parameters do not have to be corrected for pelvic rotation and tilt? Clin Orthop Relat Res . 2015;473(4):1255-1266.
9. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res . 2003;(407):241-248.
10. Griffin JW, Weber AE, Kuhns B, Lewis P, Nho SJ. Imaging in hip arthroscopy for femoroacetabular impingement: a comprehensive approach. Clin Sports Med . 2016;35(3):331-344.
11. Nepple JJ, Lehmann CL, Ross JR, Schoenecker PL, Clohisy JC. Coxa profunda is not a useful radiographic parameter for diagnosing pincer-type femoroacetabular impingement. J Bone Joint Surg Am . 2013;95(5):417-423.
12. Reynolds D, Lucas J, Klaue K. Retroversion of the acetabulum. A cause of hip pain. J Bone Joint Surg Br . 1999;81(2):281-288.
13. Dunn DM. Anteversion of the neck of the femur; a method of measurement. J Bone Joint Surg Br . 1952;34(2):181-186.
14. Meyer DC, Beck M, Ellis T, Ganz R, Leunig M. Comparison of six radiographic projections to assess femoral head/neck asphericity. Clin Orthop Relat Res . 2006;(445):181-185.
15. Hellman MD, Mascarenhas R, Gupta A, et al. The false-profile view may be used to identify cam morphology. Arthroscopy . 2015;31(9):1728-1732.
16. Barton C, Salineros MJ, Rakhra KS, Beaulé PE. Validity of the alpha angle measurement on plain radiographs in the evaluation of cam-type femoroacetabular impingement. Clin Orthop Relat Res . 2011;469(2):464-469.
17. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med . 2011;39(2):296-303.
18. Murphy SB, Ganz R, Muller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am . 1995;77(7):985-989.
19. Mast NH, Impellizzeri F, Keller S, Leunig M. Reliability and agreement of measures used in radiographic evaluation of the adult hip. Clin Orthop Relat Res . 2011;469(1):188-199.
20. Monazzam S, Bomar JD, Cidambi K, Kruk P, Hosalkar H. Lateral center-edge angle on conventional radiography and computed tomography. Clin Orthop Relat Res . 2013;471(7):2233-2237.
21. Weber AE, Jacobson JA, Bedi A. A review of imaging modalities for the hip. Curr Rev Musculoskelet Med . 2013;6(3):226-234.
22. Bencardino JT, Palmer WE. Imaging of hip disorders in athletes. Radiol Clin North Am . 2002;40(2):267-287, vi-vii.
23. Byrd JW, Jones KS. Diagnostic accuracy of clinical assessment, magnetic resonance imaging, magnetic resonance arthrography, and intra-articular injection in hip arthroscopy patients. Am J Sports Med . 2004;32(7):1668-1674.
24. Mintz DN, Hooper T, Connell D, Buly R, Padgett DE, Potter HG. Magnetic resonance imaging of the hip: detection of labral and chondral abnormalities using noncontrast imaging. Arthroscopy . 2005;21(4):385-393.
25. Blankenbaker DG, De Smet AA, Keene JS, Fine JP. Classification and localization of acetabular labral tears. Skeletal Radiol . 2007;36(5):391-397.
26. Aydingöz U, Oztürk MH. MR imaging of the acetabular labrum: a comparative study of both hips in 180 asymptomatic volunteers. Eur Radiol . 2001;11(4):567-574.
27. Gold GE, Chen CA, Koo S, Hargreaves BA, Bangerter NK. Recent advances in MRI of articular cartilage. AJR Am J Roentgenol . 2009;193(3):628-638.
28. Liong SY, Whitehouse RW. Lower extremity and pelvic stress fractures in athletes. Br J Radiol . 2012;85(1016):1148-1156.
Take-Home Points
- Be sure to have a well centered AP pelvis without rotation.
- Get at least 3 plain radiographs—AP pelvis, false profile, and lateral hip view.
- Ensure that there is sufficient acetabular coverage, LCEA >20° on AP pelvis and ACEA >20° on false profile view.
- CT scans are helpful for precise hip pathomorphology but must be weighed against risk of radiation exposure.
- MRI or MRA can be helpful to diagnose intra-articular as well as extra-articular hip and pelvis abnormalities.
In the work-up for nonarthritic hip pain, the value of diagnostic imaging is in objective findings, which can support or weaken the leading diagnoses based on subjective complaints, recalled history, and, in some cases, elusive physical examination findings. Morphologic changes alone, however, do not always indicate pathology.1,2 At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Radiography
The first step in diagnostic imaging is radiography. Although use of plain radiographs is routine, their value cannot be understated. Standard hip radiographs—an anteroposterior (AP) radiograph of the pelvis and AP and frog-leg (cross-table lateral) radiographs of the hip—provide a wealth of information.3-6
Evaluated first is the radiograph itself. For example, the ideal AP radiograph of the pelvis (Figure 1) is centered on the lower sacrum, and the patient is not rotated.
AP radiographs allow for evaluation of fractures, intraosseous sclerosis, acetabular depth, inclination and version, acetabular overcoverage, joint-space narrowing, femoroacetabular congruency, femoral head sphericity, and femoral head–neck offset.7,8,10 Inspection for labral calcification is important, as it can indicate repetitive damage at the extremes of range of motion.
On AP pelvis radiographs, it is important to distinguish coxa profunda from acetabular protrusion. These entities are on the same pathomorphologic spectrum and are similar but distinctively different. Coxa profunda refers to the depth of the acetabulum relative to the ilioischial line, and acetabular protrusion refers to the depth (or medial position) of the femoral head relative to the ilioischial line. Each condition suggests—but is not diagnostic for—pincer-type femoroacetabular impingement (FAI).11Acetabular rotation is another important entity that can be evaluated on well-centered, nontilted AP pelvic radiographs. Acetabular rotation refers to the opening direction of the acetabulum. It may be anterior (anteverted), neutral, or posterior (retroverted). Anteversion is present when the anterior acetabular rim does not traverse the posterior rim shadow4; in other words, the ring formed by the acetabulum is not twisted. When the walls overlap but do not intersect, the cup has neutral version. Retroversion is qualitatively determined by the crossover (figure-of-8) and posterior wall signs12 and is associated with pincer-type FAI and the development of hip osteoarthritis.12Dunn lateral radiographs (Figure 2A), taken with 90° hip flexion, were originally used to measure femoral neck anteversion.13
False-profile radiographs (Figure 6), valuable in evaluating anterior acetabular coverage and femoral head–neck junction morphology,14,15 allow characterization of both cam-type and pincer-type FAI.
Quantitative measures warrant specific consideration (Table). Femoroacetabular morphology is quantitatively measured by α angle, Tönnis angle (acetabular inclination angle), and lateral center-edge angle (LCEA).7,8,10 The α angle (Figure 4) detects the loss of normal anterosuperior femoral head–neck junction concavity caused by a convex osseous prominence. An α angle >50° represents a cam deformity.16 In a cohort study of 338 patients, Nepple and colleagues17 qualitatively associated increased α angle with severe intra-articular hip disease. Murphy and colleagues18 found a Tönnis angle >15° to be a poor prognostic factor in untreated hip dysplasia. LCEA quantifies superolateral femoral head coverage,19 and its normal range is 20° to 40°.20 LCEA <20° indicates dysplasia of the femoroacetabular joint, and LCEA >40° indicates overcoverage and pincer-type FAI. As with any quantitative radiographic measurement, results should be interpreted within the presenting clinical context.
Radiographic findings, even findings based on these special radiographs, may underestimate the pathologic process.
Computed Tomography
The benefits of computed tomography (CT) outweigh the risk of radiation exposure. CT is most useful in characterizing osseous morphology.21 In FAI cases, CT can distinguish acetabular version abnormalities from femoral torsion (Figures 7A-7C), entities with very different treatment approaches.21
Magnetic Resonance Imaging
MRI is becoming essential in the work-up for nonarthritic hip pain.11,22 It is used for assessment of osseous, chondral, and musculotendinous soft tissues. Further, it affords appreciation of outside-the-hip-joint pathology that may mimic joint-centered pathology.
MRI techniques range from noncontrast to indirect and direct magnetic resonance arthrography (MRA).22 Indirect MRA is performed with contrast medium administered through an intravenous line. Direct MRA has contrast administered intra-articularly and is more sensitive and specific for labral tears and ligamentous injury.23 Excellent detection of intra-articular pathology on noncontrast studies questions the need for MRA.24 Nevertheless, direct MRA can also be used as a therapeutic procedure when lidocaine is included in the injected gadolinium.
Labral tears, focal chondral defects, and stress or insufficiency fractures are important differentials in the work-up for nonarthritic hip pain. Over the dysplasia-to-FAI spectrum, MRI distinguishes symptomatic pathoanatomy from asymptomatic anatomical variants by revealing underlying bone edema. Capsule findings should also be considered.21The most practical classification of labral tears, proposed by Blankenbaker and colleagues,25 is based on tear type (frayed, unstable, flap), location, and extent. More than half of labral tears occur in the anterosuperior quadrant of the labrum.25
Chondral damage is identified much as labral tears are. With chondral injury, the normal intermediate signal is interrupted by a fluid-intense signal extending to the subchondral bone. A fat-saturated T2or short-tau inversion recovery (STIR) sequence is useful in emphasizing this finding.27
MRI detects osseous pathology from surrounding soft-tissue edema and bone remodeling to stress and fragility fractures. In athletes, the most common fractures are pubic rami, sacral, and apophyseal avulsion fractures.28 In all patients, attention should be given to the lower spine and the proximal femurs. Aside from MRI, nuclear medicine bone scan might also identify active osseous reaction representative of a fracture.
Conclusion
The work-up for nonarthritic hip pain substantiates differential diagnoses. A case’s complexity determines the course of diagnostic imaging. At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Am J Orthop . 2017;46(1):17-22. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Be sure to have a well centered AP pelvis without rotation.
- Get at least 3 plain radiographs—AP pelvis, false profile, and lateral hip view.
- Ensure that there is sufficient acetabular coverage, LCEA >20° on AP pelvis and ACEA >20° on false profile view.
- CT scans are helpful for precise hip pathomorphology but must be weighed against risk of radiation exposure.
- MRI or MRA can be helpful to diagnose intra-articular as well as extra-articular hip and pelvis abnormalities.
In the work-up for nonarthritic hip pain, the value of diagnostic imaging is in objective findings, which can support or weaken the leading diagnoses based on subjective complaints, recalled history, and, in some cases, elusive physical examination findings. Morphologic changes alone, however, do not always indicate pathology.1,2 At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Radiography
The first step in diagnostic imaging is radiography. Although use of plain radiographs is routine, their value cannot be understated. Standard hip radiographs—an anteroposterior (AP) radiograph of the pelvis and AP and frog-leg (cross-table lateral) radiographs of the hip—provide a wealth of information.3-6
Evaluated first is the radiograph itself. For example, the ideal AP radiograph of the pelvis (Figure 1) is centered on the lower sacrum, and the patient is not rotated.
AP radiographs allow for evaluation of fractures, intraosseous sclerosis, acetabular depth, inclination and version, acetabular overcoverage, joint-space narrowing, femoroacetabular congruency, femoral head sphericity, and femoral head–neck offset.7,8,10 Inspection for labral calcification is important, as it can indicate repetitive damage at the extremes of range of motion.
On AP pelvis radiographs, it is important to distinguish coxa profunda from acetabular protrusion. These entities are on the same pathomorphologic spectrum and are similar but distinctively different. Coxa profunda refers to the depth of the acetabulum relative to the ilioischial line, and acetabular protrusion refers to the depth (or medial position) of the femoral head relative to the ilioischial line. Each condition suggests—but is not diagnostic for—pincer-type femoroacetabular impingement (FAI).11Acetabular rotation is another important entity that can be evaluated on well-centered, nontilted AP pelvic radiographs. Acetabular rotation refers to the opening direction of the acetabulum. It may be anterior (anteverted), neutral, or posterior (retroverted). Anteversion is present when the anterior acetabular rim does not traverse the posterior rim shadow4; in other words, the ring formed by the acetabulum is not twisted. When the walls overlap but do not intersect, the cup has neutral version. Retroversion is qualitatively determined by the crossover (figure-of-8) and posterior wall signs12 and is associated with pincer-type FAI and the development of hip osteoarthritis.12Dunn lateral radiographs (Figure 2A), taken with 90° hip flexion, were originally used to measure femoral neck anteversion.13
False-profile radiographs (Figure 6), valuable in evaluating anterior acetabular coverage and femoral head–neck junction morphology,14,15 allow characterization of both cam-type and pincer-type FAI.
Quantitative measures warrant specific consideration (Table). Femoroacetabular morphology is quantitatively measured by α angle, Tönnis angle (acetabular inclination angle), and lateral center-edge angle (LCEA).7,8,10 The α angle (Figure 4) detects the loss of normal anterosuperior femoral head–neck junction concavity caused by a convex osseous prominence. An α angle >50° represents a cam deformity.16 In a cohort study of 338 patients, Nepple and colleagues17 qualitatively associated increased α angle with severe intra-articular hip disease. Murphy and colleagues18 found a Tönnis angle >15° to be a poor prognostic factor in untreated hip dysplasia. LCEA quantifies superolateral femoral head coverage,19 and its normal range is 20° to 40°.20 LCEA <20° indicates dysplasia of the femoroacetabular joint, and LCEA >40° indicates overcoverage and pincer-type FAI. As with any quantitative radiographic measurement, results should be interpreted within the presenting clinical context.
Radiographic findings, even findings based on these special radiographs, may underestimate the pathologic process.
Computed Tomography
The benefits of computed tomography (CT) outweigh the risk of radiation exposure. CT is most useful in characterizing osseous morphology.21 In FAI cases, CT can distinguish acetabular version abnormalities from femoral torsion (Figures 7A-7C), entities with very different treatment approaches.21
Magnetic Resonance Imaging
MRI is becoming essential in the work-up for nonarthritic hip pain.11,22 It is used for assessment of osseous, chondral, and musculotendinous soft tissues. Further, it affords appreciation of outside-the-hip-joint pathology that may mimic joint-centered pathology.
MRI techniques range from noncontrast to indirect and direct magnetic resonance arthrography (MRA).22 Indirect MRA is performed with contrast medium administered through an intravenous line. Direct MRA has contrast administered intra-articularly and is more sensitive and specific for labral tears and ligamentous injury.23 Excellent detection of intra-articular pathology on noncontrast studies questions the need for MRA.24 Nevertheless, direct MRA can also be used as a therapeutic procedure when lidocaine is included in the injected gadolinium.
Labral tears, focal chondral defects, and stress or insufficiency fractures are important differentials in the work-up for nonarthritic hip pain. Over the dysplasia-to-FAI spectrum, MRI distinguishes symptomatic pathoanatomy from asymptomatic anatomical variants by revealing underlying bone edema. Capsule findings should also be considered.21The most practical classification of labral tears, proposed by Blankenbaker and colleagues,25 is based on tear type (frayed, unstable, flap), location, and extent. More than half of labral tears occur in the anterosuperior quadrant of the labrum.25
Chondral damage is identified much as labral tears are. With chondral injury, the normal intermediate signal is interrupted by a fluid-intense signal extending to the subchondral bone. A fat-saturated T2or short-tau inversion recovery (STIR) sequence is useful in emphasizing this finding.27
MRI detects osseous pathology from surrounding soft-tissue edema and bone remodeling to stress and fragility fractures. In athletes, the most common fractures are pubic rami, sacral, and apophyseal avulsion fractures.28 In all patients, attention should be given to the lower spine and the proximal femurs. Aside from MRI, nuclear medicine bone scan might also identify active osseous reaction representative of a fracture.
Conclusion
The work-up for nonarthritic hip pain substantiates differential diagnoses. A case’s complexity determines the course of diagnostic imaging. At presentation and at each step in the work-up, it is imperative to evaluate the entire clinical picture. The prudent clinician uses both clinical and radiographic findings to make the diagnosis and direct treatment.
Am J Orthop . 2017;46(1):17-22. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. McCall DA, Safran MR. MRI and arthroscopy correlations of the hip: a case-based approach. Instr Course Lect . 2012;61:327-344.
2. Register B, Pennock AT, Ho CP, Strickland CD, Lawand A, Philippon MJ. Prevalence of abnormal hip findings in asymptomatic participants: a prospective, blinded study. Am J Sports Med . 2012;40(12):2720-2724.
3. Campbell SE. Radiography of the hip: lines, signs, and patterns of disease. Semin Roentgenol . 2005;40(3):290-319.
4. Clohisy JC, Carlisle JC, Beaulé PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am . 2008;90(suppl 4):47-66.
5. Malviya A, Raza A, Witt JD. Reliability in the diagnosis of femoroacetabular impingement and dysplasia among hip surgeons: role of surgeon volume and experience. Hip Int . 2016;26(3):284-289.
6. Nepple JJ, Martel JM, Kim YJ, Zaltz I, Clohisy JC, Group AS. Do plain radiographs correlate with CT for imaging of cam-type femoroacetabular impingement? Clin Orthop Relat Res . 2012;470(12):3313-3320.
7. Kosuge D, Cordier T, Solomon LB, Howie DW. Dilemmas in imaging for peri-acetabular osteotomy: the influence of patient position and imaging technique on the radiological features of hip dysplasia. Bone Joint J . 2014;96(9):1155-1160.
8. Tannast M, Fritsch S, Zheng G, Siebenrock KA, Steppacher SD. Which radiographic hip parameters do not have to be corrected for pelvic rotation and tilt? Clin Orthop Relat Res . 2015;473(4):1255-1266.
9. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res . 2003;(407):241-248.
10. Griffin JW, Weber AE, Kuhns B, Lewis P, Nho SJ. Imaging in hip arthroscopy for femoroacetabular impingement: a comprehensive approach. Clin Sports Med . 2016;35(3):331-344.
11. Nepple JJ, Lehmann CL, Ross JR, Schoenecker PL, Clohisy JC. Coxa profunda is not a useful radiographic parameter for diagnosing pincer-type femoroacetabular impingement. J Bone Joint Surg Am . 2013;95(5):417-423.
12. Reynolds D, Lucas J, Klaue K. Retroversion of the acetabulum. A cause of hip pain. J Bone Joint Surg Br . 1999;81(2):281-288.
13. Dunn DM. Anteversion of the neck of the femur; a method of measurement. J Bone Joint Surg Br . 1952;34(2):181-186.
14. Meyer DC, Beck M, Ellis T, Ganz R, Leunig M. Comparison of six radiographic projections to assess femoral head/neck asphericity. Clin Orthop Relat Res . 2006;(445):181-185.
15. Hellman MD, Mascarenhas R, Gupta A, et al. The false-profile view may be used to identify cam morphology. Arthroscopy . 2015;31(9):1728-1732.
16. Barton C, Salineros MJ, Rakhra KS, Beaulé PE. Validity of the alpha angle measurement on plain radiographs in the evaluation of cam-type femoroacetabular impingement. Clin Orthop Relat Res . 2011;469(2):464-469.
17. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med . 2011;39(2):296-303.
18. Murphy SB, Ganz R, Muller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am . 1995;77(7):985-989.
19. Mast NH, Impellizzeri F, Keller S, Leunig M. Reliability and agreement of measures used in radiographic evaluation of the adult hip. Clin Orthop Relat Res . 2011;469(1):188-199.
20. Monazzam S, Bomar JD, Cidambi K, Kruk P, Hosalkar H. Lateral center-edge angle on conventional radiography and computed tomography. Clin Orthop Relat Res . 2013;471(7):2233-2237.
21. Weber AE, Jacobson JA, Bedi A. A review of imaging modalities for the hip. Curr Rev Musculoskelet Med . 2013;6(3):226-234.
22. Bencardino JT, Palmer WE. Imaging of hip disorders in athletes. Radiol Clin North Am . 2002;40(2):267-287, vi-vii.
23. Byrd JW, Jones KS. Diagnostic accuracy of clinical assessment, magnetic resonance imaging, magnetic resonance arthrography, and intra-articular injection in hip arthroscopy patients. Am J Sports Med . 2004;32(7):1668-1674.
24. Mintz DN, Hooper T, Connell D, Buly R, Padgett DE, Potter HG. Magnetic resonance imaging of the hip: detection of labral and chondral abnormalities using noncontrast imaging. Arthroscopy . 2005;21(4):385-393.
25. Blankenbaker DG, De Smet AA, Keene JS, Fine JP. Classification and localization of acetabular labral tears. Skeletal Radiol . 2007;36(5):391-397.
26. Aydingöz U, Oztürk MH. MR imaging of the acetabular labrum: a comparative study of both hips in 180 asymptomatic volunteers. Eur Radiol . 2001;11(4):567-574.
27. Gold GE, Chen CA, Koo S, Hargreaves BA, Bangerter NK. Recent advances in MRI of articular cartilage. AJR Am J Roentgenol . 2009;193(3):628-638.
28. Liong SY, Whitehouse RW. Lower extremity and pelvic stress fractures in athletes. Br J Radiol . 2012;85(1016):1148-1156.
1. McCall DA, Safran MR. MRI and arthroscopy correlations of the hip: a case-based approach. Instr Course Lect . 2012;61:327-344.
2. Register B, Pennock AT, Ho CP, Strickland CD, Lawand A, Philippon MJ. Prevalence of abnormal hip findings in asymptomatic participants: a prospective, blinded study. Am J Sports Med . 2012;40(12):2720-2724.
3. Campbell SE. Radiography of the hip: lines, signs, and patterns of disease. Semin Roentgenol . 2005;40(3):290-319.
4. Clohisy JC, Carlisle JC, Beaulé PE, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. J Bone Joint Surg Am . 2008;90(suppl 4):47-66.
5. Malviya A, Raza A, Witt JD. Reliability in the diagnosis of femoroacetabular impingement and dysplasia among hip surgeons: role of surgeon volume and experience. Hip Int . 2016;26(3):284-289.
6. Nepple JJ, Martel JM, Kim YJ, Zaltz I, Clohisy JC, Group AS. Do plain radiographs correlate with CT for imaging of cam-type femoroacetabular impingement? Clin Orthop Relat Res . 2012;470(12):3313-3320.
7. Kosuge D, Cordier T, Solomon LB, Howie DW. Dilemmas in imaging for peri-acetabular osteotomy: the influence of patient position and imaging technique on the radiological features of hip dysplasia. Bone Joint J . 2014;96(9):1155-1160.
8. Tannast M, Fritsch S, Zheng G, Siebenrock KA, Steppacher SD. Which radiographic hip parameters do not have to be corrected for pelvic rotation and tilt? Clin Orthop Relat Res . 2015;473(4):1255-1266.
9. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res . 2003;(407):241-248.
10. Griffin JW, Weber AE, Kuhns B, Lewis P, Nho SJ. Imaging in hip arthroscopy for femoroacetabular impingement: a comprehensive approach. Clin Sports Med . 2016;35(3):331-344.
11. Nepple JJ, Lehmann CL, Ross JR, Schoenecker PL, Clohisy JC. Coxa profunda is not a useful radiographic parameter for diagnosing pincer-type femoroacetabular impingement. J Bone Joint Surg Am . 2013;95(5):417-423.
12. Reynolds D, Lucas J, Klaue K. Retroversion of the acetabulum. A cause of hip pain. J Bone Joint Surg Br . 1999;81(2):281-288.
13. Dunn DM. Anteversion of the neck of the femur; a method of measurement. J Bone Joint Surg Br . 1952;34(2):181-186.
14. Meyer DC, Beck M, Ellis T, Ganz R, Leunig M. Comparison of six radiographic projections to assess femoral head/neck asphericity. Clin Orthop Relat Res . 2006;(445):181-185.
15. Hellman MD, Mascarenhas R, Gupta A, et al. The false-profile view may be used to identify cam morphology. Arthroscopy . 2015;31(9):1728-1732.
16. Barton C, Salineros MJ, Rakhra KS, Beaulé PE. Validity of the alpha angle measurement on plain radiographs in the evaluation of cam-type femoroacetabular impingement. Clin Orthop Relat Res . 2011;469(2):464-469.
17. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med . 2011;39(2):296-303.
18. Murphy SB, Ganz R, Muller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am . 1995;77(7):985-989.
19. Mast NH, Impellizzeri F, Keller S, Leunig M. Reliability and agreement of measures used in radiographic evaluation of the adult hip. Clin Orthop Relat Res . 2011;469(1):188-199.
20. Monazzam S, Bomar JD, Cidambi K, Kruk P, Hosalkar H. Lateral center-edge angle on conventional radiography and computed tomography. Clin Orthop Relat Res . 2013;471(7):2233-2237.
21. Weber AE, Jacobson JA, Bedi A. A review of imaging modalities for the hip. Curr Rev Musculoskelet Med . 2013;6(3):226-234.
22. Bencardino JT, Palmer WE. Imaging of hip disorders in athletes. Radiol Clin North Am . 2002;40(2):267-287, vi-vii.
23. Byrd JW, Jones KS. Diagnostic accuracy of clinical assessment, magnetic resonance imaging, magnetic resonance arthrography, and intra-articular injection in hip arthroscopy patients. Am J Sports Med . 2004;32(7):1668-1674.
24. Mintz DN, Hooper T, Connell D, Buly R, Padgett DE, Potter HG. Magnetic resonance imaging of the hip: detection of labral and chondral abnormalities using noncontrast imaging. Arthroscopy . 2005;21(4):385-393.
25. Blankenbaker DG, De Smet AA, Keene JS, Fine JP. Classification and localization of acetabular labral tears. Skeletal Radiol . 2007;36(5):391-397.
26. Aydingöz U, Oztürk MH. MR imaging of the acetabular labrum: a comparative study of both hips in 180 asymptomatic volunteers. Eur Radiol . 2001;11(4):567-574.
27. Gold GE, Chen CA, Koo S, Hargreaves BA, Bangerter NK. Recent advances in MRI of articular cartilage. AJR Am J Roentgenol . 2009;193(3):628-638.
28. Liong SY, Whitehouse RW. Lower extremity and pelvic stress fractures in athletes. Br J Radiol . 2012;85(1016):1148-1156.
Treatment of Femoroacetabular Impingement: Labrum, Cartilage, Osseous Deformity, and Capsule
Take-Home Points
- Repair the labrum when tissue quality is good.
- Avoid overcorrection of acetabulum by measuring center edge angle.
- Cam resection should be comprehensive and restore a smooth head-neck offset to restore the suction seal.
- Chondral débridement for Outerbridge grade 0-3 and microfracture for grade 4.
- Routine capsular closure to prevent postoperative instability.
The surgical approach of femoroacetabular impingement (FAI) pathology should cover the entire hip joint. Both bony and cartilaginous tissue pathology should be adequately addressed. However, treating soft-tissue abnormalities (acetabular labrum and joint capsule) is also crucial. Overall, any surgical intervention should focus on restoring the hip labrum seal mechanism to ensure successful clinical outcomes. This restoration, combined with the use of biological therapies and rehabilitation, will produce the maximum benefit for the patient.
Management of Acetabular Labrum
The final decision regarding how to surgically approach the acetabular labrum is made during the operation. We focus restoring the labrum seal mechanism, which is crucial for proper function and health of the hip joint.1 The intra-articular hydrostatic pressure loss caused by labral deficiency results in abnormal load distribution and joint microinstability, which have detrimental effects on cartilage and periarticular tissues. A biomechanical study highlighted the role of the hip labrum in maintaining intra-articular fluid pressurization and showed that labral reconstruction restores intra-articular fluid pressure to levels similar to those of the intact state.1
In cases in which the remaining labral tissue is adequate and of good quality (reparable), the labral repair technique is preferred.2 After diagnostic arthroscopy, the labral tear is identified, and a 4.5-mm burr is used to correct (rim-trim) any osseous deformity of the acetabulum to create a “new rim” for labrum reattachment. Suture anchors are placed on the rim about 2 mm to 3 mm below the cartilage surface. Considering the rim angle3 is helpful in avoiding acetabular cartilage damage. Labral sutures can be looped around or pierced through the labrum to secure it to the acetabulum. No difference in clinical outcomes was found between the 2 suture types,4 though biomechanically piercing sutures help restore the labrum seal better.1 When the labrum is deficient and longitudinal fibers remain but are insufficient for seal restoration, the repair can be augmented with adjacent iliotibial band (ITB) tissue. This technique is similar to labral reconstruction but involves placing a graft on top of the remaining labral tissue, and suture around both the native tissue and the graft. The additional tissue gives the labrum the volume it needs to recreate the seal.
The labral reconstruction technique is indicated when the remaining labrum is irreparable, absent, or severely hypotrophic or deficient, or when an irreparable complex tear or poor-quality tissue is present. Different types of grafts can be used to reconstruct the labrum. ITB, semitendinosus, gracilis, and anterior tibialis grafts and the human acetabular labrum exhibit similar cyclic elongation behavior in response to simulated physiologic forces, though there is variability in both elongation and geometry for all graft types.5 We prefer the ITB autograft technique.6 The graft should be about 30% to 40% longer than the labral defect as measured with arthroscopic probe. With the leg in traction, the graft is inserted through the mid-anterior portal, and a suture anchor is used to secure it against the acetabulum medially.
With proper patient selection, these techniques have excellent clinical outcomes.4,7 Severe osteoarthritis (joint space <2 mm) is a contraindication for these procedures.8
Osseous Deformity
On approaching the bony structures of the hip joint, the surgeon should examine the acetabular rim (pincer lesion), the femoral head and neck shape (cam lesion), and the anterior inferior iliac spine (AIIS). Preoperative imaging and physical examination are important for identifying severe bone deformities that can complicate the procedure.9
The acetabular rim can be directly viewed after labrum detachment, but usually complete detachment is not necessary. Pincer deformity causes focal or global overcoverage of the femoral head. Rim trimming is performed with a 4.5-mm round curved burr. Resection is usually performed to the end of rim chondrosis (about 3-5 mm). Using a simple formula, you can calculate how the lateral center edge will be reduced by the amount of rim resected, maintaining a safe margin.2 A new acetabular “bed” is created where the to-be-attached labral tissue will contribute to the suction seal mechanism of the joint.2Cam lesion correction is challenging, and the amount of bone that should be resected is a matter of disagreement. We perform cam osteochondroplasty2 with a 5.5-mm round burr inserted through the anterolateral portal while the hip is positioned in 45° of flexion, neutral rotation, and adduction/abduction. This position allows an osteoplasty from 6 to 10 o’clock on the head–neck junction. Osteoplasty performed between 10 and 12 o’clock requires hip extension and slight traction. The proximal limit of osteochondroplasty is about 15 mm from the labral edge, while distally the resection stops beneath the zona orbicularis. The lateral epiphyseal vessels and the Weitbrecht ligament constitute the lateral and medial borders, respectively.
The surgeon should create a smooth head–neck offset that prevents elevation of the labrum during flexion and achieves a nearly perfect anatomical relationship between the femoral head and the acetabular labrum, restoring the hip joint seal (Figure 2).
A hypertrophic AIIS can impinge the femur (extra-articular subspinal impingement). Patients present with limited range of motion (especially hip flexion), pain in the AIIS area, and, in some cases, a history of avulsion injury.11 Seeing a bruised labrum (Figure 3) during surgery is common with this pathology.
Treatment of Cartilage Lesions
The indications and contraindications for hip arthroscopy in patients with cartilage lesions are important. Our study’s 5-year outcomes of treating FAI with hip arthroscopy in patients with preserved joint space (>2 mm) were promising, though 86% of patients with limited joint space (≤2 mm) converted to total hip arthroplasty.8 We regard patients with severe osteoarthritis as not being candidates for hip arthroscopy.
As 3 Tesla magnetic resonance imaging has low positive predictive value in identifying severe cartilage damage,13 the cartilage should be examined during surgery to further define the diagnosis. Nearly half of the hip arthroscopy patients in our study had at least 1 Outerbridge grade 3 or 4 cartilage lesion.14 Compared with the femoral head, acetabular cartilage was damaged 3 times more often. More than 90% of acetabular cartilage lesions were in the anterosuperior region.
Grades 0 and 1 cartilage lesions are usually left untreated; no intervention is necessary. Grades 2 and 3 cartilage lesions are reduced by partial débridement and/or thermal shrinkage. With the improved joint microenvironment arising from simple correction of the underlying hip bony abnormalities, these lesions should not produce further symptoms.
Grade 4 hip cartilage defects are challenging. We prefer microfracture for grade 4 lesions (Figure 4).
A ring curette is used to prepare the defect, and perpendicular borders are created to hold the clot in place. Deep débridement removes the calcified layer while maintaining the integrity of the subchondral plate.15 As a recent study found microfracture performed with small-diameter awls improved cartilage repair more effectively than microfracture with large-diameter awls,16 we prefer making small-diameter holes when establishing the maximum number of holes possible. As it is important to make a perpendicular hole, not a scratch, we use an XL Microfracture Pick (Smith & Nephew) 90° curve, which is suitable for creating a vertical entry point. The 60° curved awl is then used to further deepen the hole. Creation and stability of the marrow clot are ensured by shutting down the infusion pump device and verifying that blood and marrow elements are released from the microfractures.
Capsule Management
The increase in hip arthroscopies performed worldwide has generated interest in proper capsular management and development of iatrogenic microinstability.17 Hip capsulotomy is routinely performed for adequate visualization of the intra-articular compartment. Standard anterosuperior interportal capsulotomy for hip arthroscopic surgery (12 to 3 o’clock) sacrifices the integrity of the iliofemoral ligament (ligament of Bigelow),18 which provides rotational stability. Failure to restore the anatomical and biomechanical properties of the iliofemoral ligament after arthroscopic surgery increases the likelihood of postoperative microinstability or gross instability,19 which can lead to persistent pain and/or sense of an unstable joint, in addition to accelerated cartilage wear.
Capsulotomies are useful in obtaining adequate intraoperative exposure of the central and peripheral compartments. In the past, little attention was given to capsular closure on completion of the procedure. However, concern about postoperative instability from capsular laxity or deficiency made the introduction of capsular repair techniques necessary. Although deciding between capsular closure and plication remains debatable, we routinely perform capsular closure with a Quebec City slider knot.20 Mindful management of the capsule throughout the procedure is important in avoiding irreversible capsular damage, which would complicate capsular closure. Mindful management involves leaving a proximal leaflet of at least 1 cm during the capsulotomy, avoiding capsular thinning during shaver use, and using a cannula to prevent soft-tissue bridging.
Recent evidence suggests that capsule repair restores near native hip joint stability.17 In addition to capsular shift or capsulorrhaphy, 2 to 6 sutures have been used for capsular closure or plication after an interportal or T capsulotomy. Chahla and colleagues21 reported that 2- and 3-suture constructs produced comparable biomechanical failure torques when external rotation forces were applied to conventional hip capsulotomy on cadavers. Three-suture constructs were significantly stronger than 1-suture constructs, but there was no significant difference between 2- and 3-suture constructs. All constructs failed at about 36° of external rotation. Therefore, restricted external rotation is recommended for 3 weeks after surgery.
In one study, 35% of revision hip arthroscopy patients had undiagnosed hip instability from iatrogenic injury,22 which can lead to labral and chondral injury.17 Capsular reconstruction is recommended in cases of symptomatic capsular deficiency; capsular deficiency caused by adhesion removal; and pain and range-of-motion limitation caused by capsular adhesions. However, indications need to be further established. We have performed capsular reconstruction with ITB allograft23 (Figure 5).
Biologics
At the end of the procedure, we use platelet-rich plasma and/or bone marrow aspirate injections (individualized to the patient) to potentiate the biological healing of the tissues. Further research is planned to determine how to prepare these biological products to provide the best mix of biological factors for improved healing. Antifibrotic factors are useful in preventing adhesions, and angiotensin II receptor blockers are effective, but clinical studies are needed to establish their use.
Rehabilitation
Immediately after surgery, a postoperative hip brace and antirotational boots are applied to the patient to protect the operative site and reduce pain. The actual postoperative protocol is based on the procedure and individualized to the patient. During microfractures, the patient is kept 20 pounds touch-toe weight-bearing for 4 to 8 weeks. The capsular closure is brace-protected by limiting abduction to 0° to 45° and hip flexion to 0° to 90° while external rotation and extension are prohibited (first 3 weeks). Immediate mobilization with passive rotational movement is crucial in preventing adhesions. Stationary bike exercise and use of a continuous passive motion machine are helpful. Progressive functional and sport-specific rehabilitation help the patient return to full activity, though the decision to return to full activity is based on several factors, both objective (functional tests) and subjective (physician–patient co-decisions).
Conclusion
Although hip arthroscopic techniques have expanded significantly in recent years, our treatment approach is based on restoring the normal anatomy of the hip joint—combining the procedures with biological therapies and a postoperative rehabilitation program that is individualized to the patient’s special needs.
Am J Orthop. 2017;46(1):23-27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
3. Lertwanich P, Ejnisman L, Torry MR, Giphart JE, Philippon MJ. Defining a safety margin for labral suture anchor insertion using the acetabular rim angle. Am J Sports Med. 2011;39(suppl):111S-116S.
4. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
5. Ferro FP, Philippon MJ, Rasmussen MT, Smith SD, LaPrade RF, Wijdicks CA. Tensile properties of the human acetabular labrum and hip labral reconstruction grafts. Am J Sports Med. 2015;43(5):1222-1227.
6. Philippon MJ, Briggs KK, Boykin RE. Results of arthroscopic labral reconstruction of the hip in elite athletes: response. Am J Sports Med. 2014;42(10):NP48.
7. Geyer MR, Philippon MJ, Fagrelius TS, Briggs KK. Acetabular labral reconstruction with an iliotibial band autograft: outcome and survivorship analysis at minimum 3-year follow-up. Am J Sports Med. 2013;41(8):1750-1756.
8. Skendzel JG, Philippon MJ, Briggs KK, Goljan P. The effect of joint space on midterm outcomes after arthroscopic hip surgery for femoroacetabular impingement. Am J Sports Med. 2014;42(5):1127-1133.
9. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
10. Locks R, Chahla J, Mitchell JJ, Soares E, Philippon MJ. Dynamic hip examination for assesment of impingement during hip arthroscopy. Arthroscopy Tech. 2016 Nov 28. http://dx.doi.org/10.1016/j.eats.2016.08.011
11. Nabhan DC, Moreau WJ, McNamara SC, Briggs KK, Philippon MJ. Subspine hip impingement: an unusual cause of hip pain in an elite weightlifter. Curr Sports Med Rep. 2016;15(5):315-319.
12. Philippon MJ, Michalski MP, Campbell KJ, et al. An anatomical study of the acetabulum with clinical applications to hip arthroscopy. J Bone Joint Surg Am. 2014;96(20):1673-1682.
13. Ho CP, Ommen ND, Bhatia S, et al. Predictive value of 3-T magnetic resonance imaging in diagnosing grade 3 and 4 chondral lesions in the hip. Arthroscopy. 2016;32(9):1808-1813.
14. Bhatia S, Nowak DD, Briggs KK, Patterson DC, Philippon MJ. Outerbridge grade IV cartilage lesions in the hip identified at arthroscopy. Arthroscopy. 2016;32(5):814-819.
15. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34(11):1824-1831.
16. Orth P, Duffner J, Zurakowski D, Cucchiarini M, Madry H. Small-diameter awls improve articular cartilage repair after microfracture treatment in a translational animal model. Am J Sports Med. 2016;44(1):209-219.
17. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
18. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
19. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
20. Menge TJ, Chahla J, Soares E, Mitchell JJ, Philippon MJ. The Quebec City slider: a technique for capsular closure and plication in hip arthroscopy. Arthrosc Tech. 2016;5(5):e971-e974.
21. Chahla J, Mikula JD, Schon JM, et al. Hip capsular closure: a biomechanical analysis of failure torque. Am J Sports Med. doi:10.1177/0363546516666353.
22. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
23. Trindade CA, Sawyer GA, Fukui K, Briggs KK, Philippon MJ. Arthroscopic capsule reconstruction in the hip using iliotibial band allograft. Arthrosc Tech. 2015;4(1):e71-e74.
Take-Home Points
- Repair the labrum when tissue quality is good.
- Avoid overcorrection of acetabulum by measuring center edge angle.
- Cam resection should be comprehensive and restore a smooth head-neck offset to restore the suction seal.
- Chondral débridement for Outerbridge grade 0-3 and microfracture for grade 4.
- Routine capsular closure to prevent postoperative instability.
The surgical approach of femoroacetabular impingement (FAI) pathology should cover the entire hip joint. Both bony and cartilaginous tissue pathology should be adequately addressed. However, treating soft-tissue abnormalities (acetabular labrum and joint capsule) is also crucial. Overall, any surgical intervention should focus on restoring the hip labrum seal mechanism to ensure successful clinical outcomes. This restoration, combined with the use of biological therapies and rehabilitation, will produce the maximum benefit for the patient.
Management of Acetabular Labrum
The final decision regarding how to surgically approach the acetabular labrum is made during the operation. We focus restoring the labrum seal mechanism, which is crucial for proper function and health of the hip joint.1 The intra-articular hydrostatic pressure loss caused by labral deficiency results in abnormal load distribution and joint microinstability, which have detrimental effects on cartilage and periarticular tissues. A biomechanical study highlighted the role of the hip labrum in maintaining intra-articular fluid pressurization and showed that labral reconstruction restores intra-articular fluid pressure to levels similar to those of the intact state.1
In cases in which the remaining labral tissue is adequate and of good quality (reparable), the labral repair technique is preferred.2 After diagnostic arthroscopy, the labral tear is identified, and a 4.5-mm burr is used to correct (rim-trim) any osseous deformity of the acetabulum to create a “new rim” for labrum reattachment. Suture anchors are placed on the rim about 2 mm to 3 mm below the cartilage surface. Considering the rim angle3 is helpful in avoiding acetabular cartilage damage. Labral sutures can be looped around or pierced through the labrum to secure it to the acetabulum. No difference in clinical outcomes was found between the 2 suture types,4 though biomechanically piercing sutures help restore the labrum seal better.1 When the labrum is deficient and longitudinal fibers remain but are insufficient for seal restoration, the repair can be augmented with adjacent iliotibial band (ITB) tissue. This technique is similar to labral reconstruction but involves placing a graft on top of the remaining labral tissue, and suture around both the native tissue and the graft. The additional tissue gives the labrum the volume it needs to recreate the seal.
The labral reconstruction technique is indicated when the remaining labrum is irreparable, absent, or severely hypotrophic or deficient, or when an irreparable complex tear or poor-quality tissue is present. Different types of grafts can be used to reconstruct the labrum. ITB, semitendinosus, gracilis, and anterior tibialis grafts and the human acetabular labrum exhibit similar cyclic elongation behavior in response to simulated physiologic forces, though there is variability in both elongation and geometry for all graft types.5 We prefer the ITB autograft technique.6 The graft should be about 30% to 40% longer than the labral defect as measured with arthroscopic probe. With the leg in traction, the graft is inserted through the mid-anterior portal, and a suture anchor is used to secure it against the acetabulum medially.
With proper patient selection, these techniques have excellent clinical outcomes.4,7 Severe osteoarthritis (joint space <2 mm) is a contraindication for these procedures.8
Osseous Deformity
On approaching the bony structures of the hip joint, the surgeon should examine the acetabular rim (pincer lesion), the femoral head and neck shape (cam lesion), and the anterior inferior iliac spine (AIIS). Preoperative imaging and physical examination are important for identifying severe bone deformities that can complicate the procedure.9
The acetabular rim can be directly viewed after labrum detachment, but usually complete detachment is not necessary. Pincer deformity causes focal or global overcoverage of the femoral head. Rim trimming is performed with a 4.5-mm round curved burr. Resection is usually performed to the end of rim chondrosis (about 3-5 mm). Using a simple formula, you can calculate how the lateral center edge will be reduced by the amount of rim resected, maintaining a safe margin.2 A new acetabular “bed” is created where the to-be-attached labral tissue will contribute to the suction seal mechanism of the joint.2Cam lesion correction is challenging, and the amount of bone that should be resected is a matter of disagreement. We perform cam osteochondroplasty2 with a 5.5-mm round burr inserted through the anterolateral portal while the hip is positioned in 45° of flexion, neutral rotation, and adduction/abduction. This position allows an osteoplasty from 6 to 10 o’clock on the head–neck junction. Osteoplasty performed between 10 and 12 o’clock requires hip extension and slight traction. The proximal limit of osteochondroplasty is about 15 mm from the labral edge, while distally the resection stops beneath the zona orbicularis. The lateral epiphyseal vessels and the Weitbrecht ligament constitute the lateral and medial borders, respectively.
The surgeon should create a smooth head–neck offset that prevents elevation of the labrum during flexion and achieves a nearly perfect anatomical relationship between the femoral head and the acetabular labrum, restoring the hip joint seal (Figure 2).
A hypertrophic AIIS can impinge the femur (extra-articular subspinal impingement). Patients present with limited range of motion (especially hip flexion), pain in the AIIS area, and, in some cases, a history of avulsion injury.11 Seeing a bruised labrum (Figure 3) during surgery is common with this pathology.
Treatment of Cartilage Lesions
The indications and contraindications for hip arthroscopy in patients with cartilage lesions are important. Our study’s 5-year outcomes of treating FAI with hip arthroscopy in patients with preserved joint space (>2 mm) were promising, though 86% of patients with limited joint space (≤2 mm) converted to total hip arthroplasty.8 We regard patients with severe osteoarthritis as not being candidates for hip arthroscopy.
As 3 Tesla magnetic resonance imaging has low positive predictive value in identifying severe cartilage damage,13 the cartilage should be examined during surgery to further define the diagnosis. Nearly half of the hip arthroscopy patients in our study had at least 1 Outerbridge grade 3 or 4 cartilage lesion.14 Compared with the femoral head, acetabular cartilage was damaged 3 times more often. More than 90% of acetabular cartilage lesions were in the anterosuperior region.
Grades 0 and 1 cartilage lesions are usually left untreated; no intervention is necessary. Grades 2 and 3 cartilage lesions are reduced by partial débridement and/or thermal shrinkage. With the improved joint microenvironment arising from simple correction of the underlying hip bony abnormalities, these lesions should not produce further symptoms.
Grade 4 hip cartilage defects are challenging. We prefer microfracture for grade 4 lesions (Figure 4).
A ring curette is used to prepare the defect, and perpendicular borders are created to hold the clot in place. Deep débridement removes the calcified layer while maintaining the integrity of the subchondral plate.15 As a recent study found microfracture performed with small-diameter awls improved cartilage repair more effectively than microfracture with large-diameter awls,16 we prefer making small-diameter holes when establishing the maximum number of holes possible. As it is important to make a perpendicular hole, not a scratch, we use an XL Microfracture Pick (Smith & Nephew) 90° curve, which is suitable for creating a vertical entry point. The 60° curved awl is then used to further deepen the hole. Creation and stability of the marrow clot are ensured by shutting down the infusion pump device and verifying that blood and marrow elements are released from the microfractures.
Capsule Management
The increase in hip arthroscopies performed worldwide has generated interest in proper capsular management and development of iatrogenic microinstability.17 Hip capsulotomy is routinely performed for adequate visualization of the intra-articular compartment. Standard anterosuperior interportal capsulotomy for hip arthroscopic surgery (12 to 3 o’clock) sacrifices the integrity of the iliofemoral ligament (ligament of Bigelow),18 which provides rotational stability. Failure to restore the anatomical and biomechanical properties of the iliofemoral ligament after arthroscopic surgery increases the likelihood of postoperative microinstability or gross instability,19 which can lead to persistent pain and/or sense of an unstable joint, in addition to accelerated cartilage wear.
Capsulotomies are useful in obtaining adequate intraoperative exposure of the central and peripheral compartments. In the past, little attention was given to capsular closure on completion of the procedure. However, concern about postoperative instability from capsular laxity or deficiency made the introduction of capsular repair techniques necessary. Although deciding between capsular closure and plication remains debatable, we routinely perform capsular closure with a Quebec City slider knot.20 Mindful management of the capsule throughout the procedure is important in avoiding irreversible capsular damage, which would complicate capsular closure. Mindful management involves leaving a proximal leaflet of at least 1 cm during the capsulotomy, avoiding capsular thinning during shaver use, and using a cannula to prevent soft-tissue bridging.
Recent evidence suggests that capsule repair restores near native hip joint stability.17 In addition to capsular shift or capsulorrhaphy, 2 to 6 sutures have been used for capsular closure or plication after an interportal or T capsulotomy. Chahla and colleagues21 reported that 2- and 3-suture constructs produced comparable biomechanical failure torques when external rotation forces were applied to conventional hip capsulotomy on cadavers. Three-suture constructs were significantly stronger than 1-suture constructs, but there was no significant difference between 2- and 3-suture constructs. All constructs failed at about 36° of external rotation. Therefore, restricted external rotation is recommended for 3 weeks after surgery.
In one study, 35% of revision hip arthroscopy patients had undiagnosed hip instability from iatrogenic injury,22 which can lead to labral and chondral injury.17 Capsular reconstruction is recommended in cases of symptomatic capsular deficiency; capsular deficiency caused by adhesion removal; and pain and range-of-motion limitation caused by capsular adhesions. However, indications need to be further established. We have performed capsular reconstruction with ITB allograft23 (Figure 5).
Biologics
At the end of the procedure, we use platelet-rich plasma and/or bone marrow aspirate injections (individualized to the patient) to potentiate the biological healing of the tissues. Further research is planned to determine how to prepare these biological products to provide the best mix of biological factors for improved healing. Antifibrotic factors are useful in preventing adhesions, and angiotensin II receptor blockers are effective, but clinical studies are needed to establish their use.
Rehabilitation
Immediately after surgery, a postoperative hip brace and antirotational boots are applied to the patient to protect the operative site and reduce pain. The actual postoperative protocol is based on the procedure and individualized to the patient. During microfractures, the patient is kept 20 pounds touch-toe weight-bearing for 4 to 8 weeks. The capsular closure is brace-protected by limiting abduction to 0° to 45° and hip flexion to 0° to 90° while external rotation and extension are prohibited (first 3 weeks). Immediate mobilization with passive rotational movement is crucial in preventing adhesions. Stationary bike exercise and use of a continuous passive motion machine are helpful. Progressive functional and sport-specific rehabilitation help the patient return to full activity, though the decision to return to full activity is based on several factors, both objective (functional tests) and subjective (physician–patient co-decisions).
Conclusion
Although hip arthroscopic techniques have expanded significantly in recent years, our treatment approach is based on restoring the normal anatomy of the hip joint—combining the procedures with biological therapies and a postoperative rehabilitation program that is individualized to the patient’s special needs.
Am J Orthop. 2017;46(1):23-27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Repair the labrum when tissue quality is good.
- Avoid overcorrection of acetabulum by measuring center edge angle.
- Cam resection should be comprehensive and restore a smooth head-neck offset to restore the suction seal.
- Chondral débridement for Outerbridge grade 0-3 and microfracture for grade 4.
- Routine capsular closure to prevent postoperative instability.
The surgical approach of femoroacetabular impingement (FAI) pathology should cover the entire hip joint. Both bony and cartilaginous tissue pathology should be adequately addressed. However, treating soft-tissue abnormalities (acetabular labrum and joint capsule) is also crucial. Overall, any surgical intervention should focus on restoring the hip labrum seal mechanism to ensure successful clinical outcomes. This restoration, combined with the use of biological therapies and rehabilitation, will produce the maximum benefit for the patient.
Management of Acetabular Labrum
The final decision regarding how to surgically approach the acetabular labrum is made during the operation. We focus restoring the labrum seal mechanism, which is crucial for proper function and health of the hip joint.1 The intra-articular hydrostatic pressure loss caused by labral deficiency results in abnormal load distribution and joint microinstability, which have detrimental effects on cartilage and periarticular tissues. A biomechanical study highlighted the role of the hip labrum in maintaining intra-articular fluid pressurization and showed that labral reconstruction restores intra-articular fluid pressure to levels similar to those of the intact state.1
In cases in which the remaining labral tissue is adequate and of good quality (reparable), the labral repair technique is preferred.2 After diagnostic arthroscopy, the labral tear is identified, and a 4.5-mm burr is used to correct (rim-trim) any osseous deformity of the acetabulum to create a “new rim” for labrum reattachment. Suture anchors are placed on the rim about 2 mm to 3 mm below the cartilage surface. Considering the rim angle3 is helpful in avoiding acetabular cartilage damage. Labral sutures can be looped around or pierced through the labrum to secure it to the acetabulum. No difference in clinical outcomes was found between the 2 suture types,4 though biomechanically piercing sutures help restore the labrum seal better.1 When the labrum is deficient and longitudinal fibers remain but are insufficient for seal restoration, the repair can be augmented with adjacent iliotibial band (ITB) tissue. This technique is similar to labral reconstruction but involves placing a graft on top of the remaining labral tissue, and suture around both the native tissue and the graft. The additional tissue gives the labrum the volume it needs to recreate the seal.
The labral reconstruction technique is indicated when the remaining labrum is irreparable, absent, or severely hypotrophic or deficient, or when an irreparable complex tear or poor-quality tissue is present. Different types of grafts can be used to reconstruct the labrum. ITB, semitendinosus, gracilis, and anterior tibialis grafts and the human acetabular labrum exhibit similar cyclic elongation behavior in response to simulated physiologic forces, though there is variability in both elongation and geometry for all graft types.5 We prefer the ITB autograft technique.6 The graft should be about 30% to 40% longer than the labral defect as measured with arthroscopic probe. With the leg in traction, the graft is inserted through the mid-anterior portal, and a suture anchor is used to secure it against the acetabulum medially.
With proper patient selection, these techniques have excellent clinical outcomes.4,7 Severe osteoarthritis (joint space <2 mm) is a contraindication for these procedures.8
Osseous Deformity
On approaching the bony structures of the hip joint, the surgeon should examine the acetabular rim (pincer lesion), the femoral head and neck shape (cam lesion), and the anterior inferior iliac spine (AIIS). Preoperative imaging and physical examination are important for identifying severe bone deformities that can complicate the procedure.9
The acetabular rim can be directly viewed after labrum detachment, but usually complete detachment is not necessary. Pincer deformity causes focal or global overcoverage of the femoral head. Rim trimming is performed with a 4.5-mm round curved burr. Resection is usually performed to the end of rim chondrosis (about 3-5 mm). Using a simple formula, you can calculate how the lateral center edge will be reduced by the amount of rim resected, maintaining a safe margin.2 A new acetabular “bed” is created where the to-be-attached labral tissue will contribute to the suction seal mechanism of the joint.2Cam lesion correction is challenging, and the amount of bone that should be resected is a matter of disagreement. We perform cam osteochondroplasty2 with a 5.5-mm round burr inserted through the anterolateral portal while the hip is positioned in 45° of flexion, neutral rotation, and adduction/abduction. This position allows an osteoplasty from 6 to 10 o’clock on the head–neck junction. Osteoplasty performed between 10 and 12 o’clock requires hip extension and slight traction. The proximal limit of osteochondroplasty is about 15 mm from the labral edge, while distally the resection stops beneath the zona orbicularis. The lateral epiphyseal vessels and the Weitbrecht ligament constitute the lateral and medial borders, respectively.
The surgeon should create a smooth head–neck offset that prevents elevation of the labrum during flexion and achieves a nearly perfect anatomical relationship between the femoral head and the acetabular labrum, restoring the hip joint seal (Figure 2).
A hypertrophic AIIS can impinge the femur (extra-articular subspinal impingement). Patients present with limited range of motion (especially hip flexion), pain in the AIIS area, and, in some cases, a history of avulsion injury.11 Seeing a bruised labrum (Figure 3) during surgery is common with this pathology.
Treatment of Cartilage Lesions
The indications and contraindications for hip arthroscopy in patients with cartilage lesions are important. Our study’s 5-year outcomes of treating FAI with hip arthroscopy in patients with preserved joint space (>2 mm) were promising, though 86% of patients with limited joint space (≤2 mm) converted to total hip arthroplasty.8 We regard patients with severe osteoarthritis as not being candidates for hip arthroscopy.
As 3 Tesla magnetic resonance imaging has low positive predictive value in identifying severe cartilage damage,13 the cartilage should be examined during surgery to further define the diagnosis. Nearly half of the hip arthroscopy patients in our study had at least 1 Outerbridge grade 3 or 4 cartilage lesion.14 Compared with the femoral head, acetabular cartilage was damaged 3 times more often. More than 90% of acetabular cartilage lesions were in the anterosuperior region.
Grades 0 and 1 cartilage lesions are usually left untreated; no intervention is necessary. Grades 2 and 3 cartilage lesions are reduced by partial débridement and/or thermal shrinkage. With the improved joint microenvironment arising from simple correction of the underlying hip bony abnormalities, these lesions should not produce further symptoms.
Grade 4 hip cartilage defects are challenging. We prefer microfracture for grade 4 lesions (Figure 4).
A ring curette is used to prepare the defect, and perpendicular borders are created to hold the clot in place. Deep débridement removes the calcified layer while maintaining the integrity of the subchondral plate.15 As a recent study found microfracture performed with small-diameter awls improved cartilage repair more effectively than microfracture with large-diameter awls,16 we prefer making small-diameter holes when establishing the maximum number of holes possible. As it is important to make a perpendicular hole, not a scratch, we use an XL Microfracture Pick (Smith & Nephew) 90° curve, which is suitable for creating a vertical entry point. The 60° curved awl is then used to further deepen the hole. Creation and stability of the marrow clot are ensured by shutting down the infusion pump device and verifying that blood and marrow elements are released from the microfractures.
Capsule Management
The increase in hip arthroscopies performed worldwide has generated interest in proper capsular management and development of iatrogenic microinstability.17 Hip capsulotomy is routinely performed for adequate visualization of the intra-articular compartment. Standard anterosuperior interportal capsulotomy for hip arthroscopic surgery (12 to 3 o’clock) sacrifices the integrity of the iliofemoral ligament (ligament of Bigelow),18 which provides rotational stability. Failure to restore the anatomical and biomechanical properties of the iliofemoral ligament after arthroscopic surgery increases the likelihood of postoperative microinstability or gross instability,19 which can lead to persistent pain and/or sense of an unstable joint, in addition to accelerated cartilage wear.
Capsulotomies are useful in obtaining adequate intraoperative exposure of the central and peripheral compartments. In the past, little attention was given to capsular closure on completion of the procedure. However, concern about postoperative instability from capsular laxity or deficiency made the introduction of capsular repair techniques necessary. Although deciding between capsular closure and plication remains debatable, we routinely perform capsular closure with a Quebec City slider knot.20 Mindful management of the capsule throughout the procedure is important in avoiding irreversible capsular damage, which would complicate capsular closure. Mindful management involves leaving a proximal leaflet of at least 1 cm during the capsulotomy, avoiding capsular thinning during shaver use, and using a cannula to prevent soft-tissue bridging.
Recent evidence suggests that capsule repair restores near native hip joint stability.17 In addition to capsular shift or capsulorrhaphy, 2 to 6 sutures have been used for capsular closure or plication after an interportal or T capsulotomy. Chahla and colleagues21 reported that 2- and 3-suture constructs produced comparable biomechanical failure torques when external rotation forces were applied to conventional hip capsulotomy on cadavers. Three-suture constructs were significantly stronger than 1-suture constructs, but there was no significant difference between 2- and 3-suture constructs. All constructs failed at about 36° of external rotation. Therefore, restricted external rotation is recommended for 3 weeks after surgery.
In one study, 35% of revision hip arthroscopy patients had undiagnosed hip instability from iatrogenic injury,22 which can lead to labral and chondral injury.17 Capsular reconstruction is recommended in cases of symptomatic capsular deficiency; capsular deficiency caused by adhesion removal; and pain and range-of-motion limitation caused by capsular adhesions. However, indications need to be further established. We have performed capsular reconstruction with ITB allograft23 (Figure 5).
Biologics
At the end of the procedure, we use platelet-rich plasma and/or bone marrow aspirate injections (individualized to the patient) to potentiate the biological healing of the tissues. Further research is planned to determine how to prepare these biological products to provide the best mix of biological factors for improved healing. Antifibrotic factors are useful in preventing adhesions, and angiotensin II receptor blockers are effective, but clinical studies are needed to establish their use.
Rehabilitation
Immediately after surgery, a postoperative hip brace and antirotational boots are applied to the patient to protect the operative site and reduce pain. The actual postoperative protocol is based on the procedure and individualized to the patient. During microfractures, the patient is kept 20 pounds touch-toe weight-bearing for 4 to 8 weeks. The capsular closure is brace-protected by limiting abduction to 0° to 45° and hip flexion to 0° to 90° while external rotation and extension are prohibited (first 3 weeks). Immediate mobilization with passive rotational movement is crucial in preventing adhesions. Stationary bike exercise and use of a continuous passive motion machine are helpful. Progressive functional and sport-specific rehabilitation help the patient return to full activity, though the decision to return to full activity is based on several factors, both objective (functional tests) and subjective (physician–patient co-decisions).
Conclusion
Although hip arthroscopic techniques have expanded significantly in recent years, our treatment approach is based on restoring the normal anatomy of the hip joint—combining the procedures with biological therapies and a postoperative rehabilitation program that is individualized to the patient’s special needs.
Am J Orthop. 2017;46(1):23-27. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
3. Lertwanich P, Ejnisman L, Torry MR, Giphart JE, Philippon MJ. Defining a safety margin for labral suture anchor insertion using the acetabular rim angle. Am J Sports Med. 2011;39(suppl):111S-116S.
4. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
5. Ferro FP, Philippon MJ, Rasmussen MT, Smith SD, LaPrade RF, Wijdicks CA. Tensile properties of the human acetabular labrum and hip labral reconstruction grafts. Am J Sports Med. 2015;43(5):1222-1227.
6. Philippon MJ, Briggs KK, Boykin RE. Results of arthroscopic labral reconstruction of the hip in elite athletes: response. Am J Sports Med. 2014;42(10):NP48.
7. Geyer MR, Philippon MJ, Fagrelius TS, Briggs KK. Acetabular labral reconstruction with an iliotibial band autograft: outcome and survivorship analysis at minimum 3-year follow-up. Am J Sports Med. 2013;41(8):1750-1756.
8. Skendzel JG, Philippon MJ, Briggs KK, Goljan P. The effect of joint space on midterm outcomes after arthroscopic hip surgery for femoroacetabular impingement. Am J Sports Med. 2014;42(5):1127-1133.
9. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
10. Locks R, Chahla J, Mitchell JJ, Soares E, Philippon MJ. Dynamic hip examination for assesment of impingement during hip arthroscopy. Arthroscopy Tech. 2016 Nov 28. http://dx.doi.org/10.1016/j.eats.2016.08.011
11. Nabhan DC, Moreau WJ, McNamara SC, Briggs KK, Philippon MJ. Subspine hip impingement: an unusual cause of hip pain in an elite weightlifter. Curr Sports Med Rep. 2016;15(5):315-319.
12. Philippon MJ, Michalski MP, Campbell KJ, et al. An anatomical study of the acetabulum with clinical applications to hip arthroscopy. J Bone Joint Surg Am. 2014;96(20):1673-1682.
13. Ho CP, Ommen ND, Bhatia S, et al. Predictive value of 3-T magnetic resonance imaging in diagnosing grade 3 and 4 chondral lesions in the hip. Arthroscopy. 2016;32(9):1808-1813.
14. Bhatia S, Nowak DD, Briggs KK, Patterson DC, Philippon MJ. Outerbridge grade IV cartilage lesions in the hip identified at arthroscopy. Arthroscopy. 2016;32(5):814-819.
15. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34(11):1824-1831.
16. Orth P, Duffner J, Zurakowski D, Cucchiarini M, Madry H. Small-diameter awls improve articular cartilage repair after microfracture treatment in a translational animal model. Am J Sports Med. 2016;44(1):209-219.
17. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
18. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
19. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
20. Menge TJ, Chahla J, Soares E, Mitchell JJ, Philippon MJ. The Quebec City slider: a technique for capsular closure and plication in hip arthroscopy. Arthrosc Tech. 2016;5(5):e971-e974.
21. Chahla J, Mikula JD, Schon JM, et al. Hip capsular closure: a biomechanical analysis of failure torque. Am J Sports Med. doi:10.1177/0363546516666353.
22. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
23. Trindade CA, Sawyer GA, Fukui K, Briggs KK, Philippon MJ. Arthroscopic capsule reconstruction in the hip using iliotibial band allograft. Arthrosc Tech. 2015;4(1):e71-e74.
1. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
2. Philippon MJ, Faucet SC, Briggs KK. Arthroscopic hip labral repair. Arthrosc Tech. 2013;2(2):e73-e76.
3. Lertwanich P, Ejnisman L, Torry MR, Giphart JE, Philippon MJ. Defining a safety margin for labral suture anchor insertion using the acetabular rim angle. Am J Sports Med. 2011;39(suppl):111S-116S.
4. Sawyer GA, Briggs KK, Dornan GJ, Ommen ND, Philippon MJ. Clinical outcomes after arthroscopic hip labral repair using looped versus pierced suture techniques. Am J Sports Med. 2015;43(7):1683-1688.
5. Ferro FP, Philippon MJ, Rasmussen MT, Smith SD, LaPrade RF, Wijdicks CA. Tensile properties of the human acetabular labrum and hip labral reconstruction grafts. Am J Sports Med. 2015;43(5):1222-1227.
6. Philippon MJ, Briggs KK, Boykin RE. Results of arthroscopic labral reconstruction of the hip in elite athletes: response. Am J Sports Med. 2014;42(10):NP48.
7. Geyer MR, Philippon MJ, Fagrelius TS, Briggs KK. Acetabular labral reconstruction with an iliotibial band autograft: outcome and survivorship analysis at minimum 3-year follow-up. Am J Sports Med. 2013;41(8):1750-1756.
8. Skendzel JG, Philippon MJ, Briggs KK, Goljan P. The effect of joint space on midterm outcomes after arthroscopic hip surgery for femoroacetabular impingement. Am J Sports Med. 2014;42(5):1127-1133.
9. Yeung M, Kowalczuk M, Simunovic N, Ayeni OR. Hip arthroscopy in the setting of hip dysplasia: a systematic review. Bone Joint Res. 2016;5(6):225-231.
10. Locks R, Chahla J, Mitchell JJ, Soares E, Philippon MJ. Dynamic hip examination for assesment of impingement during hip arthroscopy. Arthroscopy Tech. 2016 Nov 28. http://dx.doi.org/10.1016/j.eats.2016.08.011
11. Nabhan DC, Moreau WJ, McNamara SC, Briggs KK, Philippon MJ. Subspine hip impingement: an unusual cause of hip pain in an elite weightlifter. Curr Sports Med Rep. 2016;15(5):315-319.
12. Philippon MJ, Michalski MP, Campbell KJ, et al. An anatomical study of the acetabulum with clinical applications to hip arthroscopy. J Bone Joint Surg Am. 2014;96(20):1673-1682.
13. Ho CP, Ommen ND, Bhatia S, et al. Predictive value of 3-T magnetic resonance imaging in diagnosing grade 3 and 4 chondral lesions in the hip. Arthroscopy. 2016;32(9):1808-1813.
14. Bhatia S, Nowak DD, Briggs KK, Patterson DC, Philippon MJ. Outerbridge grade IV cartilage lesions in the hip identified at arthroscopy. Arthroscopy. 2016;32(5):814-819.
15. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34(11):1824-1831.
16. Orth P, Duffner J, Zurakowski D, Cucchiarini M, Madry H. Small-diameter awls improve articular cartilage repair after microfracture treatment in a translational animal model. Am J Sports Med. 2016;44(1):209-219.
17. Domb BG, Philippon MJ, Giordano BD. Arthroscopic capsulotomy, capsular repair, and capsular plication of the hip: relation to atraumatic instability. Arthroscopy. 2013;29(1):162-173.
18. Asopa V, Singh PJ. The intracapsular atraumatic arthroscopic technique for closure of the hip capsule. Arthrosc Tech. 2014;3(2):e245-e247.
19. Frank RM, Lee S, Bush-Joseph CA, Kelly BT, Salata MJ, Nho SJ. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: a comparative matched-pair analysis. Am J Sports Med. 2014;42(11):2634-2642.
20. Menge TJ, Chahla J, Soares E, Mitchell JJ, Philippon MJ. The Quebec City slider: a technique for capsular closure and plication in hip arthroscopy. Arthrosc Tech. 2016;5(5):e971-e974.
21. Chahla J, Mikula JD, Schon JM, et al. Hip capsular closure: a biomechanical analysis of failure torque. Am J Sports Med. doi:10.1177/0363546516666353.
22. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
23. Trindade CA, Sawyer GA, Fukui K, Briggs KK, Philippon MJ. Arthroscopic capsule reconstruction in the hip using iliotibial band allograft. Arthrosc Tech. 2015;4(1):e71-e74.
Evolution of Femoroacetabular Impingement Treatment: The ANCHOR Experience
Take-Home Points
- Our understanding of FAI has evolved from cam-type and pincer-type impingement to much more complex disease patterns.
- Most surgeons are performing less aggressive acetabular rim trimming.
- Inadequate osseous correction is still the most common cause of the failed hip arthroscopy.
- Labral preservation is important to maintaining suction seal effect.
- Open surgical techniques have a role for more severe and complex FAI deformities.
Femoroacetabular impingement (FAI) was described by Ganz and colleagues1 in 2003 as a refinement of concepts introduced decades earlier. This description advanced our understanding of FAI as a mechanism for prearthritic hip pain and secondary hip osteoarthritis1 (OA) and allowed for treatment of FAI. The concept of proximal femoral and acetabular/pelvic deformity contributing to OA had been previously speculated by Smith-Petersen,2 Murray,3 Solomon,4 and Stulberg.5 Early cases of overcorrection of dysplasia using the periacetabular osteotomy created iatrogenic FAI, which further stimulated early development of the FAI concept.6 Improved anatomical characterization of the proximal femoral blood supply (medial femoral circumflex artery) allowed for development of the open surgical hip dislocation.7 Through open surgical hip dislocation, an improved understanding of hip pathomechanics by direct visualization helped pave the way for a better understanding of FAI. Open surgical hip dislocation allows for global treatment of labrochondral pathology and deformity of the proximal femoral head–neck junction and/or acetabular rim in FAI.
Hip arthroscopy has further developed and improved our understanding of FAI. Early hip arthroscopy was generally limited to débridement of labral and chondral pathology, and management of the soft-tissue structures. Advances in the understanding of FAI through open techniques allowed for application of similar techniques to hip arthroscopy. Improvements in arthroscopic instrumentation and techniques have allowed for treatment of labrochondral and acetabular-sided rim deformity in the central compartment and cam morphologies in the peripheral compartment through arthroscopic surgery. Appropriate bony correction by arthroscopic techniques has always been a concern, but improved techniques, dynamic assessment, and accurate use of intraoperative imaging have made this feasible and more predictable. Treatment of cam deformities extending adjacent and proximal to the retinacular vessels is possible but more technically demanding. Inadequate bony correction of FAI by arthroscopic means remains one of the most common causes of failure.8-10In 2013, the Academic Network of Conservational Hip Outcome Research (ANCHOR) Study Group reported the characteristics of a FAI cohort of 1130 hips (1076 patients) that underwent surgical treatment of FAI across 8 institutions and 12 surgeons.11 At that time, most ANCHOR surgeons (or surgeon groups) performed both open and arthroscopic surgeries and had significant referral volumes of complex cases that may have overrepresented the proportion of complex FAI cases in the cohort. During the 2008 to 2011 study period, FAI was treated with arthroscopy in 56% of these cases, open surgical hip dislocation in 34%, and reverse periacetabular osteotomy in 9%. FAI was characterized as isolated cam-type in 48%, combined cam–pincer type in 45%, and isolated pincer-type in 8%. Fifty-five percent of the patients were female. Patient-reported outcome studies in this cohort of patients are ongoing.
The FAI Concept
In 2003, after treating more than 600 open surgical hip dislocations over the previous decade, Ganz and colleagues1 coined the term femoroacetabular impingement to describe a “mechanism for the development of early osteoarthritis for most nondysplastic hips.” They reported surgical treatment focused on “improving the clearance for hip motion and alleviation of femoral abutment against the acetabular rim” with the goal of improving pain and possibly of halting progression of the degenerative process. FAI was defined as “abnormal contact between the proximal femur and acetabular rim that occurs during terminal motion of the hip” leading to “lesions of the acetabular labrum and/or the adjacent acetabular cartilage.” Subtle, previously overlooked deformities of the proximal femur and acetabulum were recognized as the cause of FAI, “including the presence of a bony prominence usually in the anterolateral head and neck junction that is seen best on the lateral radiographs, reduced offset of the femoral neck and head junction, and changes on the acetabular rim such as os acetabuli or a double line that is seen with rim ossification.” Ganz and colleagues1 recognized that “normal or near normal” hips could also experience FAI in the setting of excessive or supraphysiologic range of motion. Cam-type and pincer-type FAI deformities were introduced as 2 distinct mechanisms of FAI. By 2003, arthroscopic hip surgery was increasingly being used as a treatment for labral tears but not bony abnormalities. These FAI concepts seemed to explain the prevalence of labral tears at the anterosuperior rim, which had been noted during hip arthroscopy, and paved the way for major changes in arthroscopic hip surgery during the next decade. The ANCHOR group reported the descriptive epidemiology of a cohort of more than 1000 patients with FAI.11
Cam-Type FAI
Cam-type impingement results from femoral-sided deformities. The mechanism was described as inclusion-type impingement in which “jamming of an abnormal femoral head with increasing radius into the acetabulum during forceful motion, especially flexion.”1 This results in outside-in abrasion of the acetabular cartilage of the anterosuperior rim with detachment of the “principally uninvolved labrum”1 and potentially delamination of the adjacent cartilage from the subchondral bone. Ganz and colleagues1 recognized in their initial descriptions of FAI that cam-type FAI could involve decreased femoral version, femoral head–neck junction asphericity, and decreased head–neck offset. The complexity and variability in the topography and geography of the cam morphology have been increasingly recognized. Accurate understanding and characterization of the proximal femoral deformity are important in guiding surgical correction of the cam deformity.
Advances in understanding the prevalence of the cam morphology and the association with OA have been important to our understanding of the pathophysiology of FAI. Several studies12 have established that a cam morphology of the proximal femur (defined by a variety of different metrics) is common among asymptomatic individuals. In light of this fact, a description of the femoral anatomy as a “cam morphology” rather than a cam deformity is now favored. Similarly, FAI is better used to refer to symptomatic individuals and is not equivalent to a cam morphology. The cam morphology seems significantly more common among athletes. Siebenrock and colleagues13 demonstrated the correlation of high-level athletics during late stages of skeletal immaturity and development of a cam morphology. A recent systematic review of 9 studies found that elite male athletes in late skeletal immaturity were 2 to 8 times more likely to develop a cam morphology before skeletal maturity.14Several population-based studies15,16 have quantified the apparent association of the cam morphology with hip OA. However, the studies were limited in their ability to adequately define the presence of cam morphology based on anteroposterior (AP) pelvis radiographs.
In a prospective study, Agricola and colleagues15 found the risk of OA was increased 2.4 times in the setting of moderate cam morphology (α angle, >60°) over a 5-year period. Thomas and colleagues16 found increased risk in a female cohort when the α angle was >65°.
Treatment of cam-type FAI is focused on adequate correction of the abnormal bone morphology. Inadequate or inappropriate bony correction of FAI is a common cause of treatment failure and is more common with arthroscopic techniques.9,10,17 Inadequate bony resection may be the result of surgical inexperience, poor visualization, or lack of understanding of the underlying bony deformity. Modern osteoplasty techniques also focus on gradual bony contour correction that restores the normal concavity–convexity transition of the head–neck junction. Overresection of the cam deformity not only may increase the risk of femoral neck fracture but may result in early disruption of the hip fluid seal from loss of contact between the femoral head and the acetabular labrum earlier in the arc of motion. In addition, high range-of-motion impingement can be seen in various athletic populations (dance, gymnastics, martial arts, hockey goalies), and the regions of impingement tend to be farther away from classically described impingement. Impingement in these situations occurs at the distal femoral neck and subspine regions, adding a level of complexity and unpredictability from a surgical standpoint.
FAI can also occur in the setting of more complex deformities than the typical cam morphology. Complex cases of FAI caused by slipped capital femoral epiphysis (SCFE) and residual Legg-Calvé-Perthes disease are relatively common. Complex deformities may also result in extra-articular impingement of the proximal femur (greater/lesser trochanter, distal femoral neck) on the pelvis (ilium, ischium) in addition to typical FAI. Mild to moderate cases of residual SCFE may be adequately treated with osteoplasty by arthroscopic techniques. In the setting of more severe residual SCFE, presence of underlying femoral retroversion and retrotilt of the femoral epiphysis may prevent adequate deformity correction and motion improvement by arthroscopy. Surgical hip dislocation (with or without relative femoral neck lengthening) and/or proximal femoral flexion derotational osteotomy may be the best means of treatment in these more severe deformities but may be dependent on the chronicity of the deformity and associated compensatory changes occurring on the acetabular side. Similarly, in moderate to severe residual Legg-Calvé-Perthes disease, presence of coxa vara, high greater trochanter, short femoral neck, and ovoid femoral head may be better treated in open techniques to allow comprehensive deformity correction, including correction of acetabular dysplasia in some cases.
Pincer-Type FAI
Pincer-type FAI results from acetabular-sided deformities in which acetabular deformity leads to impaction-type impingement with “linear contact between the acetabular rim and the femoral head–neck junction.”1 Pincer FAI causes primarily labral damage with progressive degeneration and, in some cases, ossification of the acetabular labrum that further worsens the acetabular overcoverage and premature rim impaction. Chondral damage in pincer-type FAI is generally less significant and limited to the peripheral acetabular rim.
Pincer-type FAI may be caused by acetabular retroversion, coxa profunda, or protrusio acetabuli. Our understanding of what defines a pincer morphology has evolved significantly. Through efforts to better define structural features of the acetabular rim that represent abnormalities, we have improved our understanding of how these features may influence OA development. One example of improved understanding involves coxa profunda, classically defined as the medial acetabular fossa touching or projecting medial to the ilioischial line on an AP pelvis radiograph. Several studies have found that this classic definition poorly describes the “overcovered” hip, as it is present in 70% of females and commonly present (41%) in the setting of acetabular dysplasia.18,19 Acetabular retroversion was previously associated with hip OA. Although central acetabular retroversion is relatively uncommon, cranial acetabular retroversion is more common. Presence of a crossover sign on AP pelvis radiographs generally has been viewed as indicative of acetabular retroversion. However, alterations in pelvic tilt on supine or standing AP pelvis radiographs can result in apparent retroversion in the setting of normal acetabular anatomy20 and potentially influence the development of impingement.21 Zaltz and colleagues22 found that abnormal morphology of the anterior inferior iliac spine can also lead to the presence of a crossover sign in an otherwise anteverted acetabulum. Larson and colleagues23 recently found that a crossover sign is present in 11% of asymptomatic hips (19% of males) and may be considered a normal variant. A crossover sign can also be present in the setting of posterior acetabular deficiency with normal anterior acetabular coverage. Ultimately, acetabular retroversion might indicate pincer-type FAI or dysplasia or be a normal variant that does not require treatment. Global acetabular overcoverage, including coxa protrusio, may be associated with OA in population-based studies but is not uniformly demonstrated in all studies.16,24,25 A lateral center edge angle of >40° and a Tönnis angle (acetabular inclination) of <0° are commonly viewed as markers of global overcoverage.
FAI Treatment
Improvements in hip arthroscopy techniques and instrumentation have led to hip arthroscopy becoming the primary surgical technique for the treatment of most cases of FAI. Hip arthroscopy allows for precise visualization and treatment of labral and chondral disease in the central compartment by traction. Larson and colleagues26 reported complication rates for hip arthroscopy in a prospective series of >1600 cases. The overall complication rate was 8.3%, with higher rates noted in female patients and in the setting of traction time longer than 60 minutes. Nonetheless, major complications occurred in 1.1%, with only 0.1% having persistent disability. The most common complications were lateral femoral cutaneous nerve dysesthesias (1.6%), pudendal nerve neuropraxia (1.4%), and iatrogenic labral/chondral damage (2.1%).
The importance of preserving the acetabular labrum is now well accepted from clinical and biomechanical evidence.27-29 As in previous studies in surgical hip dislocation,30 arthroscopic labral repair (vs débridement) results in improved clinical outcomes.31,32 Labral repair techniques currently focus on stable fixation of the labrum while maintaining the normal position of the labrum relative to the femoral head and avoiding labral eversion, which may compromise the hip suction seal. With continued technical advancements and biomechanical support, arthroscopic labral reconstruction is possible in the setting of labral deficiency, often resulting from prior resection. However, the optimal indications, surgical techniques, and long-term outcomes continue to be better defined. Open and arthroscopic techniques have shown similar ability to correct the typical mild to moderate cam morphology in FAI.33 Yet, inadequate femoral bony correction of FAI seems to be the most common cause for revision hip preservation surgery.9,10,17Mild to moderate acetabular rim deformities are commonly treated with hip arthroscopy. As our understanding of pincer-type FAI continues to improve, many surgeons are performing less- aggressive bone resection along the anterior rim. On the other hand, subspinous impingement was recently recognized as a form of extra-articular pincer FAI variant.34 Subspine decompression without true acetabular rim resection has become a more common treatment for pincer lesions and may be a consideration even with restricted range of motion after periacetabular osteotomy. Severe acetabular deformities with global overcoverage or acetabular protrusion are particularly challenging by arthroscopy, even for the most experienced surgeons. Although some improvement in deformity is feasible with arthroscopy, even cases reported in the literature have demonstrated incomplete deformity correction. Open surgical hip dislocation may continue to be the ideal treatment technique for severe pincer impingement.
Cam-type FAI is commonly treated with hip arthroscopy (Figures A-F).
Open surgical techniques will continue to have an important role in the treatment of severe and complex FAI deformities in which arthroscopic techniques do not consistently achieve adequate bony correction (Figures A-F). Surgical hip dislocation remains a powerful surgical technique for deformity correction in FAI. Sink and colleagues37 reported rates of complications after open surgical hip dislocation in the ANCHOR study group. In a cohort of 334 hips (302 patients), trochanteric nonunion occurred in 1.8% of cases, and there were no cases of avascular necrosis. Overall major complications were observed in 4.8% of cases, with 0.3% having chronic disability. Excellent outcomes, including high rates of return to sports, have been reported after surgical hip dislocation for FAI.38 Midterm studies from the early phase of surgical treatment of FAI have helped identify factors that may play a major role in optimizing patient outcomes.
Conclusion
Our understanding and treatment of FAI continue to evolve. Both open and arthroscopic techniques have demonstrated excellent outcomes in the treatment of FAI. Most cases of FAI are now amenable to arthroscopic treatment. Inadequate resection and underlying acetabular dysplasia remain common causes of treatment failure. Open surgical hip dislocation continues to play a role in the treatment of severe deformities that are poorly accessible by arthroscopy—including cam lesions with posterior extension, severe global acetabular overcoverage, or extra-articular impingement. The association of FAI with OA is most apparent for cam-type FAI. Future research will define the optimal treatment strategies and determine if they modify disease progression.
Am J Orthop. 2017;46(1):28-34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):112-120.
2. Smith-Petersen MN. The classic: treatment of malum coxae senilis, old slipped upper femoral epiphysis, intrapelvic protrusion of the acetabulum, and coxa plana by means of acetabuloplasty. 1936. Clin Orthop Relat Res. 2009;467(3):608-615.
3. Murray RO. The aetiology of primary osteoarthritis of the hip. Br J Radiol. 1965;38(455):810-824.
4. Solomon L. Patterns of osteoarthritis of the hip. J Bone Joint Surg Br. 1976;58(2):176-183.
5. Stulberg SD. Unrecognized childhood hip disease: a major cause of idiopathic osteoarthritis of the hip. In: Cordell LD, Harris WH, Ramsey PL, MacEwen GD, eds. The Hip: Proceedings of the Third Open Scientific Meeting of the Hip Society. St. Louis, MO: Mosby; 1975:212-228.
6. Myers SR, Eijer H, Ganz R. Anterior femoroacetabular impingement after periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):93-99.
7. Ganz R, Gill TJ, Gautier E, Ganz K, Krügel N, Berlemann U. Surgical dislocation of the adult hip: a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg Br. 2001;83(8):1119-1124.
8. Ross JR, Larson CM, Adeoye O, Kelly BT, Bedi A. Residual deformity is the most common reason for revision hip arthroscopy: a three-dimensional CT study. Clin Orthop Relat Res. 2015;473(4):1388-1395.
9. Clohisy JC, Nepple JJ, Larson CM, Zaltz I, Millis M; Academic Network of Conservation Hip Outcome Research (ANCHOR) Members. Persistent structural disease is the most common cause of repeat hip preservation surgery. Clin Orthop Relat Res. 2013;471(12):3788-3794.
10. Heyworth BE, Shindle MK, Voos JE, Rudzki JR, Kelly BT. Radiologic and intraoperative findings in revision hip arthroscopy. Arthroscopy. 2007;23(12):1295-1302.
11. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
12. Frank JM, Harris JD, Erickson BJ, et al. Prevalence of femoroacetabular impingement imaging findings in asymptomatic volunteers: a systematic review. Arthroscopy. 2015;31(6):1199-11204.
13. Siebenrock KA, Ferner F, Noble PC, Santore RF, Werlen S, Mamisch TC. The cam-type deformity of the proximal femur arises in childhood in response to vigorous sporting activity. Clin Orthop Relat Res. 2011;469(11):3229-3240.
14. Nepple JJ, Vigdorchik JM, Clohisy JC. What is the association between sports participation and the development of proximal femoral cam deformity? A systematic review and meta-analysis. Am J Sports Med. 2015;43(11):2833-2840.
15. Agricola R, Waarsing JH, Arden NK, et al. Cam impingement of the hip: a risk factor for hip osteoarthritis. Nat Rev Rheumatol. 2013;9(10):630-634.
16. Thomas GE, Palmer AJ, Batra RN, et al. Subclinical deformities of the hip are significant predictors of radiographic osteoarthritis and joint replacement in women. A 20 year longitudinal cohort study. Osteoarthritis Cartilage. 2014;22(10):1504-1510.
17. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
18. Nepple JJ, Lehmann CL, Ross JR, Schoenecker PL, Clohisy JC. Coxa profunda is not a useful radiographic parameter for diagnosing pincer-type femoroacetabular impingement. J Bone Joint Surg Am. 2013;95(5):417-423.
19. Anderson LA, Kapron AL, Aoki SK, Peters CL. Coxa profunda: is the deep acetabulum overcovered? Clin Orthop Relat Res. 2012;470(12):3375-3382.
20. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res. 2003;(407):241-248.
21. Ross JR, Nepple JJ, Philippon MJ, Kelly BT, Larson CM, Bedi A. Effect of changes in pelvic tilt on range of motion to impingement and radiographic parameters of acetabular morphologic characteristics. Am J Sports Med. 2014;42(10):2402-2409.
22. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470.
23. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254.
24. Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am. 2010;92(5):1162-1169.
25. Agricola R, Heijboer MP, Roze RH, et al. Pincer deformity does not lead to osteoarthritis of the hip whereas acetabular dysplasia does: acetabular coverage and development of osteoarthritis in a nationwide prospective cohort study (CHECK). Osteoarthritis Cartilage. 2013;21(10):1514-1521.
26. Larson CM, Clohisy JC, Beaulé PE, et al; ANCHOR Study Group. Intraoperative and early postoperative complications after hip arthroscopic surgery: a prospective multicenter trial utilizing a validated grading scheme. Am J Sports Med. 2016;44(9):2292-2298.
27. Ferguson SJ, Bryant JT, Ganz R, Ito K. An in vitro investigation of the acetabular labral seal in hip joint mechanics. J Biomech. 2003;36(2):171-178.
28. Nepple JJ, Philippon MJ, Campbell KJ, et al. The hip fluid seal—part II: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip stability to distraction. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):730-736.
29. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
30. Espinosa N, Rothenfluh DA, Beck M, Ganz R, Leunig M. Treatment of femoro-acetabular impingement: preliminary results of labral refixation. J Bone Joint Surg Am. 2006;88(5):925-935.
31. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
32. Larson CM, Giveans MR. Arthroscopic debridement versus refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy. 2009;25(4):369-376.
33. Bedi A, Zaltz I, De La Torre K, Kelly BT. Radiographic comparison of surgical hip dislocation and hip arthroscopy for treatment of cam deformity in femoroacetabular impingement. Am J Sports Med. 2011;39(suppl):20S–28S.
34. Larson CM, Kelly BT, Stone RM. Making a case for anterior inferior iliac spine/subspine hip impingement: three representative case reports and proposed concept. Arthroscopy. 2011;27(12):1732-1737.
35. Ross JR, Bedi A, Stone RM, et al. Intraoperative fluoroscopic imaging to treat cam deformities: correlation with 3-dimensional computed tomography [published correction appears in Am J Sports Med. 2015;43(8):NP27]. Am J Sports Med. 2014;42(6):1370-1376.
36. Fabricant PD, Fields KG, Taylor SA, Magennis E, Bedi A, Kelly BT. The effect of femoral and acetabular version on clinical outcomes after arthroscopic femoroacetabular impingement surgery. J Bone Joint Surg Am. 2015;97(7):537-543.
37. Sink EL, Beaulé PE, Sucato D, et al. Multicenter study of complications following surgical dislocation of the hip. J Bone Joint Surg Am. 2011;93(12):1132-1136.
38. Naal FD, Miozzari HH, Wyss TF, Nötzli HP. Surgical hip dislocation for the treatment of femoroacetabular impingement in high-level athletes. Am J Sports Med. 2011;39(3):544-550.
Take-Home Points
- Our understanding of FAI has evolved from cam-type and pincer-type impingement to much more complex disease patterns.
- Most surgeons are performing less aggressive acetabular rim trimming.
- Inadequate osseous correction is still the most common cause of the failed hip arthroscopy.
- Labral preservation is important to maintaining suction seal effect.
- Open surgical techniques have a role for more severe and complex FAI deformities.
Femoroacetabular impingement (FAI) was described by Ganz and colleagues1 in 2003 as a refinement of concepts introduced decades earlier. This description advanced our understanding of FAI as a mechanism for prearthritic hip pain and secondary hip osteoarthritis1 (OA) and allowed for treatment of FAI. The concept of proximal femoral and acetabular/pelvic deformity contributing to OA had been previously speculated by Smith-Petersen,2 Murray,3 Solomon,4 and Stulberg.5 Early cases of overcorrection of dysplasia using the periacetabular osteotomy created iatrogenic FAI, which further stimulated early development of the FAI concept.6 Improved anatomical characterization of the proximal femoral blood supply (medial femoral circumflex artery) allowed for development of the open surgical hip dislocation.7 Through open surgical hip dislocation, an improved understanding of hip pathomechanics by direct visualization helped pave the way for a better understanding of FAI. Open surgical hip dislocation allows for global treatment of labrochondral pathology and deformity of the proximal femoral head–neck junction and/or acetabular rim in FAI.
Hip arthroscopy has further developed and improved our understanding of FAI. Early hip arthroscopy was generally limited to débridement of labral and chondral pathology, and management of the soft-tissue structures. Advances in the understanding of FAI through open techniques allowed for application of similar techniques to hip arthroscopy. Improvements in arthroscopic instrumentation and techniques have allowed for treatment of labrochondral and acetabular-sided rim deformity in the central compartment and cam morphologies in the peripheral compartment through arthroscopic surgery. Appropriate bony correction by arthroscopic techniques has always been a concern, but improved techniques, dynamic assessment, and accurate use of intraoperative imaging have made this feasible and more predictable. Treatment of cam deformities extending adjacent and proximal to the retinacular vessels is possible but more technically demanding. Inadequate bony correction of FAI by arthroscopic means remains one of the most common causes of failure.8-10In 2013, the Academic Network of Conservational Hip Outcome Research (ANCHOR) Study Group reported the characteristics of a FAI cohort of 1130 hips (1076 patients) that underwent surgical treatment of FAI across 8 institutions and 12 surgeons.11 At that time, most ANCHOR surgeons (or surgeon groups) performed both open and arthroscopic surgeries and had significant referral volumes of complex cases that may have overrepresented the proportion of complex FAI cases in the cohort. During the 2008 to 2011 study period, FAI was treated with arthroscopy in 56% of these cases, open surgical hip dislocation in 34%, and reverse periacetabular osteotomy in 9%. FAI was characterized as isolated cam-type in 48%, combined cam–pincer type in 45%, and isolated pincer-type in 8%. Fifty-five percent of the patients were female. Patient-reported outcome studies in this cohort of patients are ongoing.
The FAI Concept
In 2003, after treating more than 600 open surgical hip dislocations over the previous decade, Ganz and colleagues1 coined the term femoroacetabular impingement to describe a “mechanism for the development of early osteoarthritis for most nondysplastic hips.” They reported surgical treatment focused on “improving the clearance for hip motion and alleviation of femoral abutment against the acetabular rim” with the goal of improving pain and possibly of halting progression of the degenerative process. FAI was defined as “abnormal contact between the proximal femur and acetabular rim that occurs during terminal motion of the hip” leading to “lesions of the acetabular labrum and/or the adjacent acetabular cartilage.” Subtle, previously overlooked deformities of the proximal femur and acetabulum were recognized as the cause of FAI, “including the presence of a bony prominence usually in the anterolateral head and neck junction that is seen best on the lateral radiographs, reduced offset of the femoral neck and head junction, and changes on the acetabular rim such as os acetabuli or a double line that is seen with rim ossification.” Ganz and colleagues1 recognized that “normal or near normal” hips could also experience FAI in the setting of excessive or supraphysiologic range of motion. Cam-type and pincer-type FAI deformities were introduced as 2 distinct mechanisms of FAI. By 2003, arthroscopic hip surgery was increasingly being used as a treatment for labral tears but not bony abnormalities. These FAI concepts seemed to explain the prevalence of labral tears at the anterosuperior rim, which had been noted during hip arthroscopy, and paved the way for major changes in arthroscopic hip surgery during the next decade. The ANCHOR group reported the descriptive epidemiology of a cohort of more than 1000 patients with FAI.11
Cam-Type FAI
Cam-type impingement results from femoral-sided deformities. The mechanism was described as inclusion-type impingement in which “jamming of an abnormal femoral head with increasing radius into the acetabulum during forceful motion, especially flexion.”1 This results in outside-in abrasion of the acetabular cartilage of the anterosuperior rim with detachment of the “principally uninvolved labrum”1 and potentially delamination of the adjacent cartilage from the subchondral bone. Ganz and colleagues1 recognized in their initial descriptions of FAI that cam-type FAI could involve decreased femoral version, femoral head–neck junction asphericity, and decreased head–neck offset. The complexity and variability in the topography and geography of the cam morphology have been increasingly recognized. Accurate understanding and characterization of the proximal femoral deformity are important in guiding surgical correction of the cam deformity.
Advances in understanding the prevalence of the cam morphology and the association with OA have been important to our understanding of the pathophysiology of FAI. Several studies12 have established that a cam morphology of the proximal femur (defined by a variety of different metrics) is common among asymptomatic individuals. In light of this fact, a description of the femoral anatomy as a “cam morphology” rather than a cam deformity is now favored. Similarly, FAI is better used to refer to symptomatic individuals and is not equivalent to a cam morphology. The cam morphology seems significantly more common among athletes. Siebenrock and colleagues13 demonstrated the correlation of high-level athletics during late stages of skeletal immaturity and development of a cam morphology. A recent systematic review of 9 studies found that elite male athletes in late skeletal immaturity were 2 to 8 times more likely to develop a cam morphology before skeletal maturity.14Several population-based studies15,16 have quantified the apparent association of the cam morphology with hip OA. However, the studies were limited in their ability to adequately define the presence of cam morphology based on anteroposterior (AP) pelvis radiographs.
In a prospective study, Agricola and colleagues15 found the risk of OA was increased 2.4 times in the setting of moderate cam morphology (α angle, >60°) over a 5-year period. Thomas and colleagues16 found increased risk in a female cohort when the α angle was >65°.
Treatment of cam-type FAI is focused on adequate correction of the abnormal bone morphology. Inadequate or inappropriate bony correction of FAI is a common cause of treatment failure and is more common with arthroscopic techniques.9,10,17 Inadequate bony resection may be the result of surgical inexperience, poor visualization, or lack of understanding of the underlying bony deformity. Modern osteoplasty techniques also focus on gradual bony contour correction that restores the normal concavity–convexity transition of the head–neck junction. Overresection of the cam deformity not only may increase the risk of femoral neck fracture but may result in early disruption of the hip fluid seal from loss of contact between the femoral head and the acetabular labrum earlier in the arc of motion. In addition, high range-of-motion impingement can be seen in various athletic populations (dance, gymnastics, martial arts, hockey goalies), and the regions of impingement tend to be farther away from classically described impingement. Impingement in these situations occurs at the distal femoral neck and subspine regions, adding a level of complexity and unpredictability from a surgical standpoint.
FAI can also occur in the setting of more complex deformities than the typical cam morphology. Complex cases of FAI caused by slipped capital femoral epiphysis (SCFE) and residual Legg-Calvé-Perthes disease are relatively common. Complex deformities may also result in extra-articular impingement of the proximal femur (greater/lesser trochanter, distal femoral neck) on the pelvis (ilium, ischium) in addition to typical FAI. Mild to moderate cases of residual SCFE may be adequately treated with osteoplasty by arthroscopic techniques. In the setting of more severe residual SCFE, presence of underlying femoral retroversion and retrotilt of the femoral epiphysis may prevent adequate deformity correction and motion improvement by arthroscopy. Surgical hip dislocation (with or without relative femoral neck lengthening) and/or proximal femoral flexion derotational osteotomy may be the best means of treatment in these more severe deformities but may be dependent on the chronicity of the deformity and associated compensatory changes occurring on the acetabular side. Similarly, in moderate to severe residual Legg-Calvé-Perthes disease, presence of coxa vara, high greater trochanter, short femoral neck, and ovoid femoral head may be better treated in open techniques to allow comprehensive deformity correction, including correction of acetabular dysplasia in some cases.
Pincer-Type FAI
Pincer-type FAI results from acetabular-sided deformities in which acetabular deformity leads to impaction-type impingement with “linear contact between the acetabular rim and the femoral head–neck junction.”1 Pincer FAI causes primarily labral damage with progressive degeneration and, in some cases, ossification of the acetabular labrum that further worsens the acetabular overcoverage and premature rim impaction. Chondral damage in pincer-type FAI is generally less significant and limited to the peripheral acetabular rim.
Pincer-type FAI may be caused by acetabular retroversion, coxa profunda, or protrusio acetabuli. Our understanding of what defines a pincer morphology has evolved significantly. Through efforts to better define structural features of the acetabular rim that represent abnormalities, we have improved our understanding of how these features may influence OA development. One example of improved understanding involves coxa profunda, classically defined as the medial acetabular fossa touching or projecting medial to the ilioischial line on an AP pelvis radiograph. Several studies have found that this classic definition poorly describes the “overcovered” hip, as it is present in 70% of females and commonly present (41%) in the setting of acetabular dysplasia.18,19 Acetabular retroversion was previously associated with hip OA. Although central acetabular retroversion is relatively uncommon, cranial acetabular retroversion is more common. Presence of a crossover sign on AP pelvis radiographs generally has been viewed as indicative of acetabular retroversion. However, alterations in pelvic tilt on supine or standing AP pelvis radiographs can result in apparent retroversion in the setting of normal acetabular anatomy20 and potentially influence the development of impingement.21 Zaltz and colleagues22 found that abnormal morphology of the anterior inferior iliac spine can also lead to the presence of a crossover sign in an otherwise anteverted acetabulum. Larson and colleagues23 recently found that a crossover sign is present in 11% of asymptomatic hips (19% of males) and may be considered a normal variant. A crossover sign can also be present in the setting of posterior acetabular deficiency with normal anterior acetabular coverage. Ultimately, acetabular retroversion might indicate pincer-type FAI or dysplasia or be a normal variant that does not require treatment. Global acetabular overcoverage, including coxa protrusio, may be associated with OA in population-based studies but is not uniformly demonstrated in all studies.16,24,25 A lateral center edge angle of >40° and a Tönnis angle (acetabular inclination) of <0° are commonly viewed as markers of global overcoverage.
FAI Treatment
Improvements in hip arthroscopy techniques and instrumentation have led to hip arthroscopy becoming the primary surgical technique for the treatment of most cases of FAI. Hip arthroscopy allows for precise visualization and treatment of labral and chondral disease in the central compartment by traction. Larson and colleagues26 reported complication rates for hip arthroscopy in a prospective series of >1600 cases. The overall complication rate was 8.3%, with higher rates noted in female patients and in the setting of traction time longer than 60 minutes. Nonetheless, major complications occurred in 1.1%, with only 0.1% having persistent disability. The most common complications were lateral femoral cutaneous nerve dysesthesias (1.6%), pudendal nerve neuropraxia (1.4%), and iatrogenic labral/chondral damage (2.1%).
The importance of preserving the acetabular labrum is now well accepted from clinical and biomechanical evidence.27-29 As in previous studies in surgical hip dislocation,30 arthroscopic labral repair (vs débridement) results in improved clinical outcomes.31,32 Labral repair techniques currently focus on stable fixation of the labrum while maintaining the normal position of the labrum relative to the femoral head and avoiding labral eversion, which may compromise the hip suction seal. With continued technical advancements and biomechanical support, arthroscopic labral reconstruction is possible in the setting of labral deficiency, often resulting from prior resection. However, the optimal indications, surgical techniques, and long-term outcomes continue to be better defined. Open and arthroscopic techniques have shown similar ability to correct the typical mild to moderate cam morphology in FAI.33 Yet, inadequate femoral bony correction of FAI seems to be the most common cause for revision hip preservation surgery.9,10,17Mild to moderate acetabular rim deformities are commonly treated with hip arthroscopy. As our understanding of pincer-type FAI continues to improve, many surgeons are performing less- aggressive bone resection along the anterior rim. On the other hand, subspinous impingement was recently recognized as a form of extra-articular pincer FAI variant.34 Subspine decompression without true acetabular rim resection has become a more common treatment for pincer lesions and may be a consideration even with restricted range of motion after periacetabular osteotomy. Severe acetabular deformities with global overcoverage or acetabular protrusion are particularly challenging by arthroscopy, even for the most experienced surgeons. Although some improvement in deformity is feasible with arthroscopy, even cases reported in the literature have demonstrated incomplete deformity correction. Open surgical hip dislocation may continue to be the ideal treatment technique for severe pincer impingement.
Cam-type FAI is commonly treated with hip arthroscopy (Figures A-F).
Open surgical techniques will continue to have an important role in the treatment of severe and complex FAI deformities in which arthroscopic techniques do not consistently achieve adequate bony correction (Figures A-F). Surgical hip dislocation remains a powerful surgical technique for deformity correction in FAI. Sink and colleagues37 reported rates of complications after open surgical hip dislocation in the ANCHOR study group. In a cohort of 334 hips (302 patients), trochanteric nonunion occurred in 1.8% of cases, and there were no cases of avascular necrosis. Overall major complications were observed in 4.8% of cases, with 0.3% having chronic disability. Excellent outcomes, including high rates of return to sports, have been reported after surgical hip dislocation for FAI.38 Midterm studies from the early phase of surgical treatment of FAI have helped identify factors that may play a major role in optimizing patient outcomes.
Conclusion
Our understanding and treatment of FAI continue to evolve. Both open and arthroscopic techniques have demonstrated excellent outcomes in the treatment of FAI. Most cases of FAI are now amenable to arthroscopic treatment. Inadequate resection and underlying acetabular dysplasia remain common causes of treatment failure. Open surgical hip dislocation continues to play a role in the treatment of severe deformities that are poorly accessible by arthroscopy—including cam lesions with posterior extension, severe global acetabular overcoverage, or extra-articular impingement. The association of FAI with OA is most apparent for cam-type FAI. Future research will define the optimal treatment strategies and determine if they modify disease progression.
Am J Orthop. 2017;46(1):28-34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Our understanding of FAI has evolved from cam-type and pincer-type impingement to much more complex disease patterns.
- Most surgeons are performing less aggressive acetabular rim trimming.
- Inadequate osseous correction is still the most common cause of the failed hip arthroscopy.
- Labral preservation is important to maintaining suction seal effect.
- Open surgical techniques have a role for more severe and complex FAI deformities.
Femoroacetabular impingement (FAI) was described by Ganz and colleagues1 in 2003 as a refinement of concepts introduced decades earlier. This description advanced our understanding of FAI as a mechanism for prearthritic hip pain and secondary hip osteoarthritis1 (OA) and allowed for treatment of FAI. The concept of proximal femoral and acetabular/pelvic deformity contributing to OA had been previously speculated by Smith-Petersen,2 Murray,3 Solomon,4 and Stulberg.5 Early cases of overcorrection of dysplasia using the periacetabular osteotomy created iatrogenic FAI, which further stimulated early development of the FAI concept.6 Improved anatomical characterization of the proximal femoral blood supply (medial femoral circumflex artery) allowed for development of the open surgical hip dislocation.7 Through open surgical hip dislocation, an improved understanding of hip pathomechanics by direct visualization helped pave the way for a better understanding of FAI. Open surgical hip dislocation allows for global treatment of labrochondral pathology and deformity of the proximal femoral head–neck junction and/or acetabular rim in FAI.
Hip arthroscopy has further developed and improved our understanding of FAI. Early hip arthroscopy was generally limited to débridement of labral and chondral pathology, and management of the soft-tissue structures. Advances in the understanding of FAI through open techniques allowed for application of similar techniques to hip arthroscopy. Improvements in arthroscopic instrumentation and techniques have allowed for treatment of labrochondral and acetabular-sided rim deformity in the central compartment and cam morphologies in the peripheral compartment through arthroscopic surgery. Appropriate bony correction by arthroscopic techniques has always been a concern, but improved techniques, dynamic assessment, and accurate use of intraoperative imaging have made this feasible and more predictable. Treatment of cam deformities extending adjacent and proximal to the retinacular vessels is possible but more technically demanding. Inadequate bony correction of FAI by arthroscopic means remains one of the most common causes of failure.8-10In 2013, the Academic Network of Conservational Hip Outcome Research (ANCHOR) Study Group reported the characteristics of a FAI cohort of 1130 hips (1076 patients) that underwent surgical treatment of FAI across 8 institutions and 12 surgeons.11 At that time, most ANCHOR surgeons (or surgeon groups) performed both open and arthroscopic surgeries and had significant referral volumes of complex cases that may have overrepresented the proportion of complex FAI cases in the cohort. During the 2008 to 2011 study period, FAI was treated with arthroscopy in 56% of these cases, open surgical hip dislocation in 34%, and reverse periacetabular osteotomy in 9%. FAI was characterized as isolated cam-type in 48%, combined cam–pincer type in 45%, and isolated pincer-type in 8%. Fifty-five percent of the patients were female. Patient-reported outcome studies in this cohort of patients are ongoing.
The FAI Concept
In 2003, after treating more than 600 open surgical hip dislocations over the previous decade, Ganz and colleagues1 coined the term femoroacetabular impingement to describe a “mechanism for the development of early osteoarthritis for most nondysplastic hips.” They reported surgical treatment focused on “improving the clearance for hip motion and alleviation of femoral abutment against the acetabular rim” with the goal of improving pain and possibly of halting progression of the degenerative process. FAI was defined as “abnormal contact between the proximal femur and acetabular rim that occurs during terminal motion of the hip” leading to “lesions of the acetabular labrum and/or the adjacent acetabular cartilage.” Subtle, previously overlooked deformities of the proximal femur and acetabulum were recognized as the cause of FAI, “including the presence of a bony prominence usually in the anterolateral head and neck junction that is seen best on the lateral radiographs, reduced offset of the femoral neck and head junction, and changes on the acetabular rim such as os acetabuli or a double line that is seen with rim ossification.” Ganz and colleagues1 recognized that “normal or near normal” hips could also experience FAI in the setting of excessive or supraphysiologic range of motion. Cam-type and pincer-type FAI deformities were introduced as 2 distinct mechanisms of FAI. By 2003, arthroscopic hip surgery was increasingly being used as a treatment for labral tears but not bony abnormalities. These FAI concepts seemed to explain the prevalence of labral tears at the anterosuperior rim, which had been noted during hip arthroscopy, and paved the way for major changes in arthroscopic hip surgery during the next decade. The ANCHOR group reported the descriptive epidemiology of a cohort of more than 1000 patients with FAI.11
Cam-Type FAI
Cam-type impingement results from femoral-sided deformities. The mechanism was described as inclusion-type impingement in which “jamming of an abnormal femoral head with increasing radius into the acetabulum during forceful motion, especially flexion.”1 This results in outside-in abrasion of the acetabular cartilage of the anterosuperior rim with detachment of the “principally uninvolved labrum”1 and potentially delamination of the adjacent cartilage from the subchondral bone. Ganz and colleagues1 recognized in their initial descriptions of FAI that cam-type FAI could involve decreased femoral version, femoral head–neck junction asphericity, and decreased head–neck offset. The complexity and variability in the topography and geography of the cam morphology have been increasingly recognized. Accurate understanding and characterization of the proximal femoral deformity are important in guiding surgical correction of the cam deformity.
Advances in understanding the prevalence of the cam morphology and the association with OA have been important to our understanding of the pathophysiology of FAI. Several studies12 have established that a cam morphology of the proximal femur (defined by a variety of different metrics) is common among asymptomatic individuals. In light of this fact, a description of the femoral anatomy as a “cam morphology” rather than a cam deformity is now favored. Similarly, FAI is better used to refer to symptomatic individuals and is not equivalent to a cam morphology. The cam morphology seems significantly more common among athletes. Siebenrock and colleagues13 demonstrated the correlation of high-level athletics during late stages of skeletal immaturity and development of a cam morphology. A recent systematic review of 9 studies found that elite male athletes in late skeletal immaturity were 2 to 8 times more likely to develop a cam morphology before skeletal maturity.14Several population-based studies15,16 have quantified the apparent association of the cam morphology with hip OA. However, the studies were limited in their ability to adequately define the presence of cam morphology based on anteroposterior (AP) pelvis radiographs.
In a prospective study, Agricola and colleagues15 found the risk of OA was increased 2.4 times in the setting of moderate cam morphology (α angle, >60°) over a 5-year period. Thomas and colleagues16 found increased risk in a female cohort when the α angle was >65°.
Treatment of cam-type FAI is focused on adequate correction of the abnormal bone morphology. Inadequate or inappropriate bony correction of FAI is a common cause of treatment failure and is more common with arthroscopic techniques.9,10,17 Inadequate bony resection may be the result of surgical inexperience, poor visualization, or lack of understanding of the underlying bony deformity. Modern osteoplasty techniques also focus on gradual bony contour correction that restores the normal concavity–convexity transition of the head–neck junction. Overresection of the cam deformity not only may increase the risk of femoral neck fracture but may result in early disruption of the hip fluid seal from loss of contact between the femoral head and the acetabular labrum earlier in the arc of motion. In addition, high range-of-motion impingement can be seen in various athletic populations (dance, gymnastics, martial arts, hockey goalies), and the regions of impingement tend to be farther away from classically described impingement. Impingement in these situations occurs at the distal femoral neck and subspine regions, adding a level of complexity and unpredictability from a surgical standpoint.
FAI can also occur in the setting of more complex deformities than the typical cam morphology. Complex cases of FAI caused by slipped capital femoral epiphysis (SCFE) and residual Legg-Calvé-Perthes disease are relatively common. Complex deformities may also result in extra-articular impingement of the proximal femur (greater/lesser trochanter, distal femoral neck) on the pelvis (ilium, ischium) in addition to typical FAI. Mild to moderate cases of residual SCFE may be adequately treated with osteoplasty by arthroscopic techniques. In the setting of more severe residual SCFE, presence of underlying femoral retroversion and retrotilt of the femoral epiphysis may prevent adequate deformity correction and motion improvement by arthroscopy. Surgical hip dislocation (with or without relative femoral neck lengthening) and/or proximal femoral flexion derotational osteotomy may be the best means of treatment in these more severe deformities but may be dependent on the chronicity of the deformity and associated compensatory changes occurring on the acetabular side. Similarly, in moderate to severe residual Legg-Calvé-Perthes disease, presence of coxa vara, high greater trochanter, short femoral neck, and ovoid femoral head may be better treated in open techniques to allow comprehensive deformity correction, including correction of acetabular dysplasia in some cases.
Pincer-Type FAI
Pincer-type FAI results from acetabular-sided deformities in which acetabular deformity leads to impaction-type impingement with “linear contact between the acetabular rim and the femoral head–neck junction.”1 Pincer FAI causes primarily labral damage with progressive degeneration and, in some cases, ossification of the acetabular labrum that further worsens the acetabular overcoverage and premature rim impaction. Chondral damage in pincer-type FAI is generally less significant and limited to the peripheral acetabular rim.
Pincer-type FAI may be caused by acetabular retroversion, coxa profunda, or protrusio acetabuli. Our understanding of what defines a pincer morphology has evolved significantly. Through efforts to better define structural features of the acetabular rim that represent abnormalities, we have improved our understanding of how these features may influence OA development. One example of improved understanding involves coxa profunda, classically defined as the medial acetabular fossa touching or projecting medial to the ilioischial line on an AP pelvis radiograph. Several studies have found that this classic definition poorly describes the “overcovered” hip, as it is present in 70% of females and commonly present (41%) in the setting of acetabular dysplasia.18,19 Acetabular retroversion was previously associated with hip OA. Although central acetabular retroversion is relatively uncommon, cranial acetabular retroversion is more common. Presence of a crossover sign on AP pelvis radiographs generally has been viewed as indicative of acetabular retroversion. However, alterations in pelvic tilt on supine or standing AP pelvis radiographs can result in apparent retroversion in the setting of normal acetabular anatomy20 and potentially influence the development of impingement.21 Zaltz and colleagues22 found that abnormal morphology of the anterior inferior iliac spine can also lead to the presence of a crossover sign in an otherwise anteverted acetabulum. Larson and colleagues23 recently found that a crossover sign is present in 11% of asymptomatic hips (19% of males) and may be considered a normal variant. A crossover sign can also be present in the setting of posterior acetabular deficiency with normal anterior acetabular coverage. Ultimately, acetabular retroversion might indicate pincer-type FAI or dysplasia or be a normal variant that does not require treatment. Global acetabular overcoverage, including coxa protrusio, may be associated with OA in population-based studies but is not uniformly demonstrated in all studies.16,24,25 A lateral center edge angle of >40° and a Tönnis angle (acetabular inclination) of <0° are commonly viewed as markers of global overcoverage.
FAI Treatment
Improvements in hip arthroscopy techniques and instrumentation have led to hip arthroscopy becoming the primary surgical technique for the treatment of most cases of FAI. Hip arthroscopy allows for precise visualization and treatment of labral and chondral disease in the central compartment by traction. Larson and colleagues26 reported complication rates for hip arthroscopy in a prospective series of >1600 cases. The overall complication rate was 8.3%, with higher rates noted in female patients and in the setting of traction time longer than 60 minutes. Nonetheless, major complications occurred in 1.1%, with only 0.1% having persistent disability. The most common complications were lateral femoral cutaneous nerve dysesthesias (1.6%), pudendal nerve neuropraxia (1.4%), and iatrogenic labral/chondral damage (2.1%).
The importance of preserving the acetabular labrum is now well accepted from clinical and biomechanical evidence.27-29 As in previous studies in surgical hip dislocation,30 arthroscopic labral repair (vs débridement) results in improved clinical outcomes.31,32 Labral repair techniques currently focus on stable fixation of the labrum while maintaining the normal position of the labrum relative to the femoral head and avoiding labral eversion, which may compromise the hip suction seal. With continued technical advancements and biomechanical support, arthroscopic labral reconstruction is possible in the setting of labral deficiency, often resulting from prior resection. However, the optimal indications, surgical techniques, and long-term outcomes continue to be better defined. Open and arthroscopic techniques have shown similar ability to correct the typical mild to moderate cam morphology in FAI.33 Yet, inadequate femoral bony correction of FAI seems to be the most common cause for revision hip preservation surgery.9,10,17Mild to moderate acetabular rim deformities are commonly treated with hip arthroscopy. As our understanding of pincer-type FAI continues to improve, many surgeons are performing less- aggressive bone resection along the anterior rim. On the other hand, subspinous impingement was recently recognized as a form of extra-articular pincer FAI variant.34 Subspine decompression without true acetabular rim resection has become a more common treatment for pincer lesions and may be a consideration even with restricted range of motion after periacetabular osteotomy. Severe acetabular deformities with global overcoverage or acetabular protrusion are particularly challenging by arthroscopy, even for the most experienced surgeons. Although some improvement in deformity is feasible with arthroscopy, even cases reported in the literature have demonstrated incomplete deformity correction. Open surgical hip dislocation may continue to be the ideal treatment technique for severe pincer impingement.
Cam-type FAI is commonly treated with hip arthroscopy (Figures A-F).
Open surgical techniques will continue to have an important role in the treatment of severe and complex FAI deformities in which arthroscopic techniques do not consistently achieve adequate bony correction (Figures A-F). Surgical hip dislocation remains a powerful surgical technique for deformity correction in FAI. Sink and colleagues37 reported rates of complications after open surgical hip dislocation in the ANCHOR study group. In a cohort of 334 hips (302 patients), trochanteric nonunion occurred in 1.8% of cases, and there were no cases of avascular necrosis. Overall major complications were observed in 4.8% of cases, with 0.3% having chronic disability. Excellent outcomes, including high rates of return to sports, have been reported after surgical hip dislocation for FAI.38 Midterm studies from the early phase of surgical treatment of FAI have helped identify factors that may play a major role in optimizing patient outcomes.
Conclusion
Our understanding and treatment of FAI continue to evolve. Both open and arthroscopic techniques have demonstrated excellent outcomes in the treatment of FAI. Most cases of FAI are now amenable to arthroscopic treatment. Inadequate resection and underlying acetabular dysplasia remain common causes of treatment failure. Open surgical hip dislocation continues to play a role in the treatment of severe deformities that are poorly accessible by arthroscopy—including cam lesions with posterior extension, severe global acetabular overcoverage, or extra-articular impingement. The association of FAI with OA is most apparent for cam-type FAI. Future research will define the optimal treatment strategies and determine if they modify disease progression.
Am J Orthop. 2017;46(1):28-34. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):112-120.
2. Smith-Petersen MN. The classic: treatment of malum coxae senilis, old slipped upper femoral epiphysis, intrapelvic protrusion of the acetabulum, and coxa plana by means of acetabuloplasty. 1936. Clin Orthop Relat Res. 2009;467(3):608-615.
3. Murray RO. The aetiology of primary osteoarthritis of the hip. Br J Radiol. 1965;38(455):810-824.
4. Solomon L. Patterns of osteoarthritis of the hip. J Bone Joint Surg Br. 1976;58(2):176-183.
5. Stulberg SD. Unrecognized childhood hip disease: a major cause of idiopathic osteoarthritis of the hip. In: Cordell LD, Harris WH, Ramsey PL, MacEwen GD, eds. The Hip: Proceedings of the Third Open Scientific Meeting of the Hip Society. St. Louis, MO: Mosby; 1975:212-228.
6. Myers SR, Eijer H, Ganz R. Anterior femoroacetabular impingement after periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):93-99.
7. Ganz R, Gill TJ, Gautier E, Ganz K, Krügel N, Berlemann U. Surgical dislocation of the adult hip: a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg Br. 2001;83(8):1119-1124.
8. Ross JR, Larson CM, Adeoye O, Kelly BT, Bedi A. Residual deformity is the most common reason for revision hip arthroscopy: a three-dimensional CT study. Clin Orthop Relat Res. 2015;473(4):1388-1395.
9. Clohisy JC, Nepple JJ, Larson CM, Zaltz I, Millis M; Academic Network of Conservation Hip Outcome Research (ANCHOR) Members. Persistent structural disease is the most common cause of repeat hip preservation surgery. Clin Orthop Relat Res. 2013;471(12):3788-3794.
10. Heyworth BE, Shindle MK, Voos JE, Rudzki JR, Kelly BT. Radiologic and intraoperative findings in revision hip arthroscopy. Arthroscopy. 2007;23(12):1295-1302.
11. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
12. Frank JM, Harris JD, Erickson BJ, et al. Prevalence of femoroacetabular impingement imaging findings in asymptomatic volunteers: a systematic review. Arthroscopy. 2015;31(6):1199-11204.
13. Siebenrock KA, Ferner F, Noble PC, Santore RF, Werlen S, Mamisch TC. The cam-type deformity of the proximal femur arises in childhood in response to vigorous sporting activity. Clin Orthop Relat Res. 2011;469(11):3229-3240.
14. Nepple JJ, Vigdorchik JM, Clohisy JC. What is the association between sports participation and the development of proximal femoral cam deformity? A systematic review and meta-analysis. Am J Sports Med. 2015;43(11):2833-2840.
15. Agricola R, Waarsing JH, Arden NK, et al. Cam impingement of the hip: a risk factor for hip osteoarthritis. Nat Rev Rheumatol. 2013;9(10):630-634.
16. Thomas GE, Palmer AJ, Batra RN, et al. Subclinical deformities of the hip are significant predictors of radiographic osteoarthritis and joint replacement in women. A 20 year longitudinal cohort study. Osteoarthritis Cartilage. 2014;22(10):1504-1510.
17. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
18. Nepple JJ, Lehmann CL, Ross JR, Schoenecker PL, Clohisy JC. Coxa profunda is not a useful radiographic parameter for diagnosing pincer-type femoroacetabular impingement. J Bone Joint Surg Am. 2013;95(5):417-423.
19. Anderson LA, Kapron AL, Aoki SK, Peters CL. Coxa profunda: is the deep acetabulum overcovered? Clin Orthop Relat Res. 2012;470(12):3375-3382.
20. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res. 2003;(407):241-248.
21. Ross JR, Nepple JJ, Philippon MJ, Kelly BT, Larson CM, Bedi A. Effect of changes in pelvic tilt on range of motion to impingement and radiographic parameters of acetabular morphologic characteristics. Am J Sports Med. 2014;42(10):2402-2409.
22. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470.
23. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254.
24. Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am. 2010;92(5):1162-1169.
25. Agricola R, Heijboer MP, Roze RH, et al. Pincer deformity does not lead to osteoarthritis of the hip whereas acetabular dysplasia does: acetabular coverage and development of osteoarthritis in a nationwide prospective cohort study (CHECK). Osteoarthritis Cartilage. 2013;21(10):1514-1521.
26. Larson CM, Clohisy JC, Beaulé PE, et al; ANCHOR Study Group. Intraoperative and early postoperative complications after hip arthroscopic surgery: a prospective multicenter trial utilizing a validated grading scheme. Am J Sports Med. 2016;44(9):2292-2298.
27. Ferguson SJ, Bryant JT, Ganz R, Ito K. An in vitro investigation of the acetabular labral seal in hip joint mechanics. J Biomech. 2003;36(2):171-178.
28. Nepple JJ, Philippon MJ, Campbell KJ, et al. The hip fluid seal—part II: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip stability to distraction. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):730-736.
29. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
30. Espinosa N, Rothenfluh DA, Beck M, Ganz R, Leunig M. Treatment of femoro-acetabular impingement: preliminary results of labral refixation. J Bone Joint Surg Am. 2006;88(5):925-935.
31. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
32. Larson CM, Giveans MR. Arthroscopic debridement versus refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy. 2009;25(4):369-376.
33. Bedi A, Zaltz I, De La Torre K, Kelly BT. Radiographic comparison of surgical hip dislocation and hip arthroscopy for treatment of cam deformity in femoroacetabular impingement. Am J Sports Med. 2011;39(suppl):20S–28S.
34. Larson CM, Kelly BT, Stone RM. Making a case for anterior inferior iliac spine/subspine hip impingement: three representative case reports and proposed concept. Arthroscopy. 2011;27(12):1732-1737.
35. Ross JR, Bedi A, Stone RM, et al. Intraoperative fluoroscopic imaging to treat cam deformities: correlation with 3-dimensional computed tomography [published correction appears in Am J Sports Med. 2015;43(8):NP27]. Am J Sports Med. 2014;42(6):1370-1376.
36. Fabricant PD, Fields KG, Taylor SA, Magennis E, Bedi A, Kelly BT. The effect of femoral and acetabular version on clinical outcomes after arthroscopic femoroacetabular impingement surgery. J Bone Joint Surg Am. 2015;97(7):537-543.
37. Sink EL, Beaulé PE, Sucato D, et al. Multicenter study of complications following surgical dislocation of the hip. J Bone Joint Surg Am. 2011;93(12):1132-1136.
38. Naal FD, Miozzari HH, Wyss TF, Nötzli HP. Surgical hip dislocation for the treatment of femoroacetabular impingement in high-level athletes. Am J Sports Med. 2011;39(3):544-550.
1. Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;(417):112-120.
2. Smith-Petersen MN. The classic: treatment of malum coxae senilis, old slipped upper femoral epiphysis, intrapelvic protrusion of the acetabulum, and coxa plana by means of acetabuloplasty. 1936. Clin Orthop Relat Res. 2009;467(3):608-615.
3. Murray RO. The aetiology of primary osteoarthritis of the hip. Br J Radiol. 1965;38(455):810-824.
4. Solomon L. Patterns of osteoarthritis of the hip. J Bone Joint Surg Br. 1976;58(2):176-183.
5. Stulberg SD. Unrecognized childhood hip disease: a major cause of idiopathic osteoarthritis of the hip. In: Cordell LD, Harris WH, Ramsey PL, MacEwen GD, eds. The Hip: Proceedings of the Third Open Scientific Meeting of the Hip Society. St. Louis, MO: Mosby; 1975:212-228.
6. Myers SR, Eijer H, Ganz R. Anterior femoroacetabular impingement after periacetabular osteotomy. Clin Orthop Relat Res. 1999;(363):93-99.
7. Ganz R, Gill TJ, Gautier E, Ganz K, Krügel N, Berlemann U. Surgical dislocation of the adult hip: a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg Br. 2001;83(8):1119-1124.
8. Ross JR, Larson CM, Adeoye O, Kelly BT, Bedi A. Residual deformity is the most common reason for revision hip arthroscopy: a three-dimensional CT study. Clin Orthop Relat Res. 2015;473(4):1388-1395.
9. Clohisy JC, Nepple JJ, Larson CM, Zaltz I, Millis M; Academic Network of Conservation Hip Outcome Research (ANCHOR) Members. Persistent structural disease is the most common cause of repeat hip preservation surgery. Clin Orthop Relat Res. 2013;471(12):3788-3794.
10. Heyworth BE, Shindle MK, Voos JE, Rudzki JR, Kelly BT. Radiologic and intraoperative findings in revision hip arthroscopy. Arthroscopy. 2007;23(12):1295-1302.
11. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
12. Frank JM, Harris JD, Erickson BJ, et al. Prevalence of femoroacetabular impingement imaging findings in asymptomatic volunteers: a systematic review. Arthroscopy. 2015;31(6):1199-11204.
13. Siebenrock KA, Ferner F, Noble PC, Santore RF, Werlen S, Mamisch TC. The cam-type deformity of the proximal femur arises in childhood in response to vigorous sporting activity. Clin Orthop Relat Res. 2011;469(11):3229-3240.
14. Nepple JJ, Vigdorchik JM, Clohisy JC. What is the association between sports participation and the development of proximal femoral cam deformity? A systematic review and meta-analysis. Am J Sports Med. 2015;43(11):2833-2840.
15. Agricola R, Waarsing JH, Arden NK, et al. Cam impingement of the hip: a risk factor for hip osteoarthritis. Nat Rev Rheumatol. 2013;9(10):630-634.
16. Thomas GE, Palmer AJ, Batra RN, et al. Subclinical deformities of the hip are significant predictors of radiographic osteoarthritis and joint replacement in women. A 20 year longitudinal cohort study. Osteoarthritis Cartilage. 2014;22(10):1504-1510.
17. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-1921.
18. Nepple JJ, Lehmann CL, Ross JR, Schoenecker PL, Clohisy JC. Coxa profunda is not a useful radiographic parameter for diagnosing pincer-type femoroacetabular impingement. J Bone Joint Surg Am. 2013;95(5):417-423.
19. Anderson LA, Kapron AL, Aoki SK, Peters CL. Coxa profunda: is the deep acetabulum overcovered? Clin Orthop Relat Res. 2012;470(12):3375-3382.
20. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop Relat Res. 2003;(407):241-248.
21. Ross JR, Nepple JJ, Philippon MJ, Kelly BT, Larson CM, Bedi A. Effect of changes in pelvic tilt on range of motion to impingement and radiographic parameters of acetabular morphologic characteristics. Am J Sports Med. 2014;42(10):2402-2409.
22. Zaltz I, Kelly BT, Hetsroni I, Bedi A. The crossover sign overestimates acetabular retroversion. Clin Orthop Relat Res. 2013;471(8):2463-2470.
23. Larson CM, Moreau-Gaudry A, Kelly BT, et al. Are normal hips being labeled as pathologic? A CT-based method for defining normal acetabular coverage. Clin Orthop Relat Res. 2015;473(4):1247-1254.
24. Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am. 2010;92(5):1162-1169.
25. Agricola R, Heijboer MP, Roze RH, et al. Pincer deformity does not lead to osteoarthritis of the hip whereas acetabular dysplasia does: acetabular coverage and development of osteoarthritis in a nationwide prospective cohort study (CHECK). Osteoarthritis Cartilage. 2013;21(10):1514-1521.
26. Larson CM, Clohisy JC, Beaulé PE, et al; ANCHOR Study Group. Intraoperative and early postoperative complications after hip arthroscopic surgery: a prospective multicenter trial utilizing a validated grading scheme. Am J Sports Med. 2016;44(9):2292-2298.
27. Ferguson SJ, Bryant JT, Ganz R, Ito K. An in vitro investigation of the acetabular labral seal in hip joint mechanics. J Biomech. 2003;36(2):171-178.
28. Nepple JJ, Philippon MJ, Campbell KJ, et al. The hip fluid seal—part II: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip stability to distraction. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):730-736.
29. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
30. Espinosa N, Rothenfluh DA, Beck M, Ganz R, Leunig M. Treatment of femoro-acetabular impingement: preliminary results of labral refixation. J Bone Joint Surg Am. 2006;88(5):925-935.
31. Krych AJ, Thompson M, Knutson Z, Scoon J, Coleman SH. Arthroscopic labral repair versus selective labral debridement in female patients with femoroacetabular impingement: a prospective randomized study. Arthroscopy. 2013;29(1):46-53.
32. Larson CM, Giveans MR. Arthroscopic debridement versus refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy. 2009;25(4):369-376.
33. Bedi A, Zaltz I, De La Torre K, Kelly BT. Radiographic comparison of surgical hip dislocation and hip arthroscopy for treatment of cam deformity in femoroacetabular impingement. Am J Sports Med. 2011;39(suppl):20S–28S.
34. Larson CM, Kelly BT, Stone RM. Making a case for anterior inferior iliac spine/subspine hip impingement: three representative case reports and proposed concept. Arthroscopy. 2011;27(12):1732-1737.
35. Ross JR, Bedi A, Stone RM, et al. Intraoperative fluoroscopic imaging to treat cam deformities: correlation with 3-dimensional computed tomography [published correction appears in Am J Sports Med. 2015;43(8):NP27]. Am J Sports Med. 2014;42(6):1370-1376.
36. Fabricant PD, Fields KG, Taylor SA, Magennis E, Bedi A, Kelly BT. The effect of femoral and acetabular version on clinical outcomes after arthroscopic femoroacetabular impingement surgery. J Bone Joint Surg Am. 2015;97(7):537-543.
37. Sink EL, Beaulé PE, Sucato D, et al. Multicenter study of complications following surgical dislocation of the hip. J Bone Joint Surg Am. 2011;93(12):1132-1136.
38. Naal FD, Miozzari HH, Wyss TF, Nötzli HP. Surgical hip dislocation for the treatment of femoroacetabular impingement in high-level athletes. Am J Sports Med. 2011;39(3):544-550.
Multicenter Outcomes After Hip Arthroscopy: Epidemiology (MASH Study Group). What Are We Seeing in the Office, and Who Are We Choosing to Treat?
Take-Home Points
- MASH is a multicenter arthroscopic study of the hip that features a large prospective database of 10 separate institutions in the United States.
- The mean patient demographic was age 34.6 years, BMI 25.9 kg/m2, 62.8% females, and 97% white.
- Most patients had anterior or groin pain, but 17.6% had lateral hip pain, 13.8% had posterior hip pain, and 2.9% had low back or sacral pain.
- Patients typically had pain for about 1 year that was worsened with athletic activity as well as sitting.
- The most common surgical procedures that were performed included labral surgery in 64.7%, femoroplasty in 49.9%, acetabuloplasty in 33.3%, and chondroplasty in 31.1%
Arthroscopic surgery of the hip has been growing over the past decade, with drastically increasing rates of arthroscopic hip procedures and increased education and interest in orthopedic trainees.1-3 The rise of this minimally invasive surgical technique may be attributed to expanding knowledge of surgical management of morphologic hip disorders as a means of hip preservation. Many arthroscopic techniques have been developed to treat intra-articular hip joint pathologies, including femoroacetabular impingement (FAI), labral tears, and cartilage damage.4-11 These hip pathologies are widely recognized as painful limitations to activities of daily living and sports as well as early indicators of hip osteoarthritis.12,13 Limited evidence suggests that arthroscopic treatment of these intra-articular hip joint pathologies preserves the hip from osteoarthritis and progression to total hip arthroplasty.13-15
FAI is the most common etiology of pathologies related to arthroscopic surgery of the hip, including both labral tears and cartilage damage.4,7,14 FAI is a morphologic bone disorder characterized by impingement of the femur and the acetabulum on flexion or rotation. The etiology of FAI is not completely understood, but evidence suggests that stress to the proximal femoral physis during skeletal growth increases the risk of developing femoral head and neck deformations leading to cam-type FAI.15-17 Understanding the characteristics of the patient population in which FAI occurs may shed light on the processes of intra-articular damage, such as labral tears and cartilage damage.
In the present study, we collected epidemiologic data, including demographics, pathologic entities treated, patient-reported measures of disease, and surgical treatment preferences, on a hip pathology population that elected to undergo arthroscopic surgery. These data are important in gaining a better understanding of the population and environment in which hip arthroscopy is performed across multiple centers throughout the United States and may help guide clinical practice and research to advance hip arthroscopy.
Methods
The Multicenter Arthroscopic Study of the Hip (MASH) Study Group conducts multicenter clinical studies in arthroscopic hip preservation surgery. Patients are enrolled in this large prospective longitudinal study at 10 sites nationwide by 10 fellowship-trained hip arthroscopists. Institutional Review Board approval was obtained from all institutions before patient enrollment. After enrollment, we collected comprehensive patient data, including demographics, common symptoms and their duration, provocative activities, patient-reported outcome measures (modified Harris Hip Score, International Hip Outcome Tool, 12-item Short Form Health Survey, visual analog scale pain rating, Hip Outcome Score), physical examination findings, imaging findings, diagnoses, surgical findings, and surgical procedures.
All study participants were patients undergoing arthroscopic hip surgery by one of the members of the MASH Study Group. Patients with incomplete preoperative information (needed for data analysis) were excluded. Data analysis was performed with SPSS Statistics Version 21.0 (SPSS Inc.) to obtain descriptive statistics of the quantitative data and frequencies of the nominal data.
Results
Between January 2014 and November 2016, we enrolled 1738 patients (647 male, 1091 female) in the study. Table 1 lists the demographics of the population.
Regarding symptom location, 40.9% of patients described pain in the groin region, 24.2% in the anterior hip region, and 11.3% in a C-sign distribution (Table 2).
Table 3 lists the results of the patient-reported outcome measures.
Of the 1738 patients enrolled, 424 (24.4%) had prior surgery related to current symptoms, 252 (14.5%) had 1 previous surgery, 120 (6.9%) had 2 previous surgeries, and 52 (3%) had 3 previous surgeries. Twenty-six patients (1.5%) had a previous revision hip arthroscopy on the ipsilateral side, and 14 (0.8%) had a previous hip arthroscopy on the contralateral side. Before surgery, 80% of patients received an intra-articular injection of corticosteroid and lidocaine. The peritrochanteric region was injected in 11.5% of patients and the psoas bursa in 2.2% (Table 4).
Of the 1011 patients who had magnetic resonance imaging (MRI) performed, 943 (93.3%) had abnormal acetabular labrum findings, and 163 (17.1%) had acetabular articular damage. According to radiographic evaluation, 953 patients had abnormal hip joint morphology consistent with FAI. Figure 3 shows the FAI classification percentages.
On clinical examination, 1079 patients (62.1%) had a positive anterior impingement sign. The subspine impingement sign was positive in 447 patients (25.7%), and the trochanteric pain sign was positive in 400 (23%). Table 5 lists range-of-motion values for flexion and hip rotation from 90° of flexion.
As seen in Table 6, labral pathology was the most common diagnosis (1426/1738 patients, 82%).
As seen in Table 7, the most common procedure was femoroplasty (867/1738, 49.9%).
Discussion
In this study, we collected epidemiologic data (demographics, pathologic entities treated, patient-reported measures of disease, surgical treatment preferences) from a large multicenter population of hip pathology patients who elected to undergo arthroscopic surgery. Our results showed these patients were most commonly younger to middle-aged white females with pain primarily in the groin region. Most had pain for at least 1 year, and it was commonly exacerbated by sitting and athletics. Patients reported clinically significant pain and functional limitation, which showed evidence of affecting general physical and mental health. It was not uncommon for patients to have undergone another, related surgery and nonoperative treatments, including intra-articular injection and/or physical therapy, before surgery. There was a high incidence of abnormal hip morphology suggestive of a cam lesion, but the incidence of arthritic changes on radiographs was relatively low. Labral tear was the most common diagnosis, and most often it was addressed with repair. Many patients underwent femoroplasty, acetabuloplasty, and chondroplasty in addition to labral repair.
According to patient-reported outcome measures administered before surgery, 40% to 65% of patients seeking hip preservation surgery reported functional deficits and pain—which falls within the range of results from other multicenter studies on the epidemiology of FAI.18,19 There was, however, a high amount of variability in individual scores on the functional and pain measures; some patients rated their functional ability very high. These findings were supported by the general health forms measuring global physical and mental health. Mean Physical Health and Mental Health scores on the 12-item Short Form Health Survey indicated that patients seeking hip preservation surgery thought their hip condition affected their general well-being. This finding is consistent with research on FAI,18 hip arthritis,20 and total hip arthroplasty.19Our results further showed that hip arthroscopists commonly prescribed alternative treatment measures ahead of surgery. Before elective surgery, 80% of patients received an intra-articular injection, underwent physical therapy, or both. This could suggest a high failure rate for patients who chose conservative treatment approaches for hip-related pathology. However, our study was limited in that it may have included patients who had improved significantly with conservative measures and decided to forgo arthroscopic hip surgery. Although conservative treatment often is recommended in an effort to potentially avoid surgery, there is a lack of research evaluating the efficacy of nonoperative care.21,22Analysis of diagnostic imaging and clinical examination findings revealed some unique characteristics of patients undergoing elective hip preservation surgery. MRI showed labral pathology in an overwhelming majority of these patients, but few had evidence of articular damage. Previous research has found a 67% rate of arthritic changes on diagnostic imaging, but our rate was much lower (17%).23 Radiograph evaluation confirmed the pattern: More than 90% of our patients had Tönnis grade 0 osteoarthritis. Tönnis grade 1 or 2 osteoarthritis is a predictor of acetabular cartilage degeneration,23 and long-term studies have related these osteoarthritic changes to poorer hip arthroscopy outcomes.24 Thus, the lower incidence of osteoarthritis in our study population may reflect current evidence-based practice and a contemporary approach to patient selection.
Most of our patients had isolated cam-type FAI as opposed to pincer-type FAI or a combination of cam and pincer—contrary to research findings that combination cam–pincer FAI is most prevalent.25,26 Our results are more consistent with more recent research findings of a higher incidence of isolated cam lesion, particularly in female patients, and combination cam–pincer in male patients.18,27,28 Similar distributions of surgical procedures and diagnoses exist between the present study and other multicenter evaluations of the epidemiologic characteristics of patients with hip pathology.18Our study had several limitations. First, the population consisted entirely of patients who sought evaluation by a hip arthroscopy specialist and underwent elective surgery. Therefore, the data cannot be applied to a more general orthopedic population or to patients who consult other medical specialists. Second, the population, which was 97% white and had small percentages of African-American, Latino, and Asian patients, lacked ethnic diversity. This finding is consistent with recent epidemiologic research in which ethnicity was identified as a factor in patterns of hip disease.13,29,30 Access to specialists, however, was likely affected by multiple other factors. Fourth, the validity and the reliability of the imaging modalities used have been questioned.31-33 There is controversy regarding ideal imaging modalities for assessment of articular cartilage damage31,32 and FAI. However, the modalities that we used to determine diagnoses in this study are well supported26 and represent common practice patterns.
Am J Orthop. 2017;46(1):35-41. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016;32(7):1286-1292.
2. Peters CL, Aoki SK, Erickson JA, Anderson LA, Anderson AE. Early experience with a comprehensive hip preservation service intended to improve clinical care, education, and academic productivity. Clin Orthop Relat Res. 2012;470(12):3446-3452.
3. Siebenrock KA, Peters CL. ABJS Carl T. Brighton workshop on hip preservation surgery: editorial comment. Clin Orthop Relat Res. 2012;470(12):3281-3283.
4. Parvizi J, Leunig M, Ganz R. Femoroacetabular impingement. J Am Acad Orthop Surg. 2007;15(9):561-570.
5. Poh SY, Hube R, Dienst M. Arthroscopic treatment of femoroacetabular pincer impingement. Oper Orthop Traumatol. 2015;27(6):536-552.
6. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
7. Groh MM, Herrera J. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med. 2009;2(2):105-117.
8. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
9. White BJ, Herzog MM. Labral reconstruction: when to perform and how. Front Surg. 2015;2:27.
10. Yen YM, Kocher MS. Chondral lesions of the hip: microfracture and chondroplasty. Sports Med Arthrosc. 2010;18(2):83-89.
11. Jordan MA, Van Thiel GS, Chahal J, Nho SJ. Operative treatment of chondral defects in the hip joint: a systematic review. Curr Rev Musculoskelet Med. 2012;5(3):244-253.
12. Griffin DW, Kinnard MJ, Formby PM, McCabe MP, Anderson TD. Outcomes of hip arthroscopy in the older adult: a systematic review of the literature [published online October 18, 2016]. Am J Sports Med. doi:10.1177/0363546516667915.
13. Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 2008;466(2):264-272.
14. Kaya M, Suzuki T, Emori M, Yamashita T. Hip morphology influences the pattern of articular cartilage damage. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):2016-2023.
15. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
16. Byrd JT. Hip arthroscopy in athletes. Oper Tech Sports Med. 2005;13(1):24-36.
17. Werner BC, Gaudiani MA, Ranawat AS. The etiology and arthroscopic surgical management of cam lesions. Clin Sports Med. 2016;35(3):391-404.
18. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
19. Shia DS, Clohisy JC, Schinsky MF, Martell JM, Maloney WJ. THA with highly cross-linked polyethylene in patients 50 years or younger. Clin Orthop Relat Res. 2009;467(8):2059-2065.
20. Gandhi SK, Salmon JW, Zhao SZ, Lambert BL, Gore PR, Conrad K. Psychometric evaluation of the 12-item Short-Form Health Survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clin Ther. 2001;23(7):1080-1098.
21. Loudon JK, Reiman MP. Conservative management of femoroacetabular impingement (FAI) in the long distance runner. Phys Ther Sport. 2014;15(2):82-90.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med. 2011;39(2):296-303.
24. McCormick F, Nwachukwu BU, Alpaugh K, Martin SD. Predictors of hip arthroscopy outcomes for labral tears at minimum 2-year follow-up: the influence of age and arthritis. Arthroscopy. 2012;28(10):1359-1364.
25. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
26. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis—what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552.
27. Kapron AL, Peters CL, Aoki SK, et al. The prevalence of radiographic findings of structural hip deformities in female collegiate athletes. Am J Sports Med. 2015;43(6):1324-1330.
28. Lee WY, Kang C, Hwang DS, Jeon JH, Zheng L. Descriptive epidemiology of symptomatic femoroacetabular impingement in young athlete: single center study. Hip Pelvis. 2016;28(1):29-34.
29. Dudda M, Kim YJ, Zhang Y, et al. Morphologic differences between the hips of Chinese women and white women: could they account for the ethnic difference in the prevalence of hip osteoarthritis? Arthritis Rheum. 2011;63(10):2992-2999.
30. Solomon L, Beighton P. Osteoarthrosis of the hip and its relationship to pre-existing in an African population. J Bone Joint Surg Br. 1973;55(1):216-217.
31. Keeney JA, Peelle MW, Jackson J, Rubin D, Maloney WJ, Clohisy JC. Magnetic resonance arthrography versus arthroscopy in the evaluation of articular hip pathology. Clin Orthop Relat Res. 2004;(429):163-169.
32. Schmid MR, Nötzli HP, Zanetti M, Wyss TF, Hodler J. Cartilage lesions in the hip: diagnostic effectiveness of MR arthrography. Radiology. 2003;226(2):382-386.
33. Chevillotte CJ, Ali MH, Trousdale RT, Pagnano MW. Variability in hip range of motion on clinical examination. J Arthroplasty. 2009;24(5):693-697.
Take-Home Points
- MASH is a multicenter arthroscopic study of the hip that features a large prospective database of 10 separate institutions in the United States.
- The mean patient demographic was age 34.6 years, BMI 25.9 kg/m2, 62.8% females, and 97% white.
- Most patients had anterior or groin pain, but 17.6% had lateral hip pain, 13.8% had posterior hip pain, and 2.9% had low back or sacral pain.
- Patients typically had pain for about 1 year that was worsened with athletic activity as well as sitting.
- The most common surgical procedures that were performed included labral surgery in 64.7%, femoroplasty in 49.9%, acetabuloplasty in 33.3%, and chondroplasty in 31.1%
Arthroscopic surgery of the hip has been growing over the past decade, with drastically increasing rates of arthroscopic hip procedures and increased education and interest in orthopedic trainees.1-3 The rise of this minimally invasive surgical technique may be attributed to expanding knowledge of surgical management of morphologic hip disorders as a means of hip preservation. Many arthroscopic techniques have been developed to treat intra-articular hip joint pathologies, including femoroacetabular impingement (FAI), labral tears, and cartilage damage.4-11 These hip pathologies are widely recognized as painful limitations to activities of daily living and sports as well as early indicators of hip osteoarthritis.12,13 Limited evidence suggests that arthroscopic treatment of these intra-articular hip joint pathologies preserves the hip from osteoarthritis and progression to total hip arthroplasty.13-15
FAI is the most common etiology of pathologies related to arthroscopic surgery of the hip, including both labral tears and cartilage damage.4,7,14 FAI is a morphologic bone disorder characterized by impingement of the femur and the acetabulum on flexion or rotation. The etiology of FAI is not completely understood, but evidence suggests that stress to the proximal femoral physis during skeletal growth increases the risk of developing femoral head and neck deformations leading to cam-type FAI.15-17 Understanding the characteristics of the patient population in which FAI occurs may shed light on the processes of intra-articular damage, such as labral tears and cartilage damage.
In the present study, we collected epidemiologic data, including demographics, pathologic entities treated, patient-reported measures of disease, and surgical treatment preferences, on a hip pathology population that elected to undergo arthroscopic surgery. These data are important in gaining a better understanding of the population and environment in which hip arthroscopy is performed across multiple centers throughout the United States and may help guide clinical practice and research to advance hip arthroscopy.
Methods
The Multicenter Arthroscopic Study of the Hip (MASH) Study Group conducts multicenter clinical studies in arthroscopic hip preservation surgery. Patients are enrolled in this large prospective longitudinal study at 10 sites nationwide by 10 fellowship-trained hip arthroscopists. Institutional Review Board approval was obtained from all institutions before patient enrollment. After enrollment, we collected comprehensive patient data, including demographics, common symptoms and their duration, provocative activities, patient-reported outcome measures (modified Harris Hip Score, International Hip Outcome Tool, 12-item Short Form Health Survey, visual analog scale pain rating, Hip Outcome Score), physical examination findings, imaging findings, diagnoses, surgical findings, and surgical procedures.
All study participants were patients undergoing arthroscopic hip surgery by one of the members of the MASH Study Group. Patients with incomplete preoperative information (needed for data analysis) were excluded. Data analysis was performed with SPSS Statistics Version 21.0 (SPSS Inc.) to obtain descriptive statistics of the quantitative data and frequencies of the nominal data.
Results
Between January 2014 and November 2016, we enrolled 1738 patients (647 male, 1091 female) in the study. Table 1 lists the demographics of the population.
Regarding symptom location, 40.9% of patients described pain in the groin region, 24.2% in the anterior hip region, and 11.3% in a C-sign distribution (Table 2).
Table 3 lists the results of the patient-reported outcome measures.
Of the 1738 patients enrolled, 424 (24.4%) had prior surgery related to current symptoms, 252 (14.5%) had 1 previous surgery, 120 (6.9%) had 2 previous surgeries, and 52 (3%) had 3 previous surgeries. Twenty-six patients (1.5%) had a previous revision hip arthroscopy on the ipsilateral side, and 14 (0.8%) had a previous hip arthroscopy on the contralateral side. Before surgery, 80% of patients received an intra-articular injection of corticosteroid and lidocaine. The peritrochanteric region was injected in 11.5% of patients and the psoas bursa in 2.2% (Table 4).
Of the 1011 patients who had magnetic resonance imaging (MRI) performed, 943 (93.3%) had abnormal acetabular labrum findings, and 163 (17.1%) had acetabular articular damage. According to radiographic evaluation, 953 patients had abnormal hip joint morphology consistent with FAI. Figure 3 shows the FAI classification percentages.
On clinical examination, 1079 patients (62.1%) had a positive anterior impingement sign. The subspine impingement sign was positive in 447 patients (25.7%), and the trochanteric pain sign was positive in 400 (23%). Table 5 lists range-of-motion values for flexion and hip rotation from 90° of flexion.
As seen in Table 6, labral pathology was the most common diagnosis (1426/1738 patients, 82%).
As seen in Table 7, the most common procedure was femoroplasty (867/1738, 49.9%).
Discussion
In this study, we collected epidemiologic data (demographics, pathologic entities treated, patient-reported measures of disease, surgical treatment preferences) from a large multicenter population of hip pathology patients who elected to undergo arthroscopic surgery. Our results showed these patients were most commonly younger to middle-aged white females with pain primarily in the groin region. Most had pain for at least 1 year, and it was commonly exacerbated by sitting and athletics. Patients reported clinically significant pain and functional limitation, which showed evidence of affecting general physical and mental health. It was not uncommon for patients to have undergone another, related surgery and nonoperative treatments, including intra-articular injection and/or physical therapy, before surgery. There was a high incidence of abnormal hip morphology suggestive of a cam lesion, but the incidence of arthritic changes on radiographs was relatively low. Labral tear was the most common diagnosis, and most often it was addressed with repair. Many patients underwent femoroplasty, acetabuloplasty, and chondroplasty in addition to labral repair.
According to patient-reported outcome measures administered before surgery, 40% to 65% of patients seeking hip preservation surgery reported functional deficits and pain—which falls within the range of results from other multicenter studies on the epidemiology of FAI.18,19 There was, however, a high amount of variability in individual scores on the functional and pain measures; some patients rated their functional ability very high. These findings were supported by the general health forms measuring global physical and mental health. Mean Physical Health and Mental Health scores on the 12-item Short Form Health Survey indicated that patients seeking hip preservation surgery thought their hip condition affected their general well-being. This finding is consistent with research on FAI,18 hip arthritis,20 and total hip arthroplasty.19Our results further showed that hip arthroscopists commonly prescribed alternative treatment measures ahead of surgery. Before elective surgery, 80% of patients received an intra-articular injection, underwent physical therapy, or both. This could suggest a high failure rate for patients who chose conservative treatment approaches for hip-related pathology. However, our study was limited in that it may have included patients who had improved significantly with conservative measures and decided to forgo arthroscopic hip surgery. Although conservative treatment often is recommended in an effort to potentially avoid surgery, there is a lack of research evaluating the efficacy of nonoperative care.21,22Analysis of diagnostic imaging and clinical examination findings revealed some unique characteristics of patients undergoing elective hip preservation surgery. MRI showed labral pathology in an overwhelming majority of these patients, but few had evidence of articular damage. Previous research has found a 67% rate of arthritic changes on diagnostic imaging, but our rate was much lower (17%).23 Radiograph evaluation confirmed the pattern: More than 90% of our patients had Tönnis grade 0 osteoarthritis. Tönnis grade 1 or 2 osteoarthritis is a predictor of acetabular cartilage degeneration,23 and long-term studies have related these osteoarthritic changes to poorer hip arthroscopy outcomes.24 Thus, the lower incidence of osteoarthritis in our study population may reflect current evidence-based practice and a contemporary approach to patient selection.
Most of our patients had isolated cam-type FAI as opposed to pincer-type FAI or a combination of cam and pincer—contrary to research findings that combination cam–pincer FAI is most prevalent.25,26 Our results are more consistent with more recent research findings of a higher incidence of isolated cam lesion, particularly in female patients, and combination cam–pincer in male patients.18,27,28 Similar distributions of surgical procedures and diagnoses exist between the present study and other multicenter evaluations of the epidemiologic characteristics of patients with hip pathology.18Our study had several limitations. First, the population consisted entirely of patients who sought evaluation by a hip arthroscopy specialist and underwent elective surgery. Therefore, the data cannot be applied to a more general orthopedic population or to patients who consult other medical specialists. Second, the population, which was 97% white and had small percentages of African-American, Latino, and Asian patients, lacked ethnic diversity. This finding is consistent with recent epidemiologic research in which ethnicity was identified as a factor in patterns of hip disease.13,29,30 Access to specialists, however, was likely affected by multiple other factors. Fourth, the validity and the reliability of the imaging modalities used have been questioned.31-33 There is controversy regarding ideal imaging modalities for assessment of articular cartilage damage31,32 and FAI. However, the modalities that we used to determine diagnoses in this study are well supported26 and represent common practice patterns.
Am J Orthop. 2017;46(1):35-41. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- MASH is a multicenter arthroscopic study of the hip that features a large prospective database of 10 separate institutions in the United States.
- The mean patient demographic was age 34.6 years, BMI 25.9 kg/m2, 62.8% females, and 97% white.
- Most patients had anterior or groin pain, but 17.6% had lateral hip pain, 13.8% had posterior hip pain, and 2.9% had low back or sacral pain.
- Patients typically had pain for about 1 year that was worsened with athletic activity as well as sitting.
- The most common surgical procedures that were performed included labral surgery in 64.7%, femoroplasty in 49.9%, acetabuloplasty in 33.3%, and chondroplasty in 31.1%
Arthroscopic surgery of the hip has been growing over the past decade, with drastically increasing rates of arthroscopic hip procedures and increased education and interest in orthopedic trainees.1-3 The rise of this minimally invasive surgical technique may be attributed to expanding knowledge of surgical management of morphologic hip disorders as a means of hip preservation. Many arthroscopic techniques have been developed to treat intra-articular hip joint pathologies, including femoroacetabular impingement (FAI), labral tears, and cartilage damage.4-11 These hip pathologies are widely recognized as painful limitations to activities of daily living and sports as well as early indicators of hip osteoarthritis.12,13 Limited evidence suggests that arthroscopic treatment of these intra-articular hip joint pathologies preserves the hip from osteoarthritis and progression to total hip arthroplasty.13-15
FAI is the most common etiology of pathologies related to arthroscopic surgery of the hip, including both labral tears and cartilage damage.4,7,14 FAI is a morphologic bone disorder characterized by impingement of the femur and the acetabulum on flexion or rotation. The etiology of FAI is not completely understood, but evidence suggests that stress to the proximal femoral physis during skeletal growth increases the risk of developing femoral head and neck deformations leading to cam-type FAI.15-17 Understanding the characteristics of the patient population in which FAI occurs may shed light on the processes of intra-articular damage, such as labral tears and cartilage damage.
In the present study, we collected epidemiologic data, including demographics, pathologic entities treated, patient-reported measures of disease, and surgical treatment preferences, on a hip pathology population that elected to undergo arthroscopic surgery. These data are important in gaining a better understanding of the population and environment in which hip arthroscopy is performed across multiple centers throughout the United States and may help guide clinical practice and research to advance hip arthroscopy.
Methods
The Multicenter Arthroscopic Study of the Hip (MASH) Study Group conducts multicenter clinical studies in arthroscopic hip preservation surgery. Patients are enrolled in this large prospective longitudinal study at 10 sites nationwide by 10 fellowship-trained hip arthroscopists. Institutional Review Board approval was obtained from all institutions before patient enrollment. After enrollment, we collected comprehensive patient data, including demographics, common symptoms and their duration, provocative activities, patient-reported outcome measures (modified Harris Hip Score, International Hip Outcome Tool, 12-item Short Form Health Survey, visual analog scale pain rating, Hip Outcome Score), physical examination findings, imaging findings, diagnoses, surgical findings, and surgical procedures.
All study participants were patients undergoing arthroscopic hip surgery by one of the members of the MASH Study Group. Patients with incomplete preoperative information (needed for data analysis) were excluded. Data analysis was performed with SPSS Statistics Version 21.0 (SPSS Inc.) to obtain descriptive statistics of the quantitative data and frequencies of the nominal data.
Results
Between January 2014 and November 2016, we enrolled 1738 patients (647 male, 1091 female) in the study. Table 1 lists the demographics of the population.
Regarding symptom location, 40.9% of patients described pain in the groin region, 24.2% in the anterior hip region, and 11.3% in a C-sign distribution (Table 2).
Table 3 lists the results of the patient-reported outcome measures.
Of the 1738 patients enrolled, 424 (24.4%) had prior surgery related to current symptoms, 252 (14.5%) had 1 previous surgery, 120 (6.9%) had 2 previous surgeries, and 52 (3%) had 3 previous surgeries. Twenty-six patients (1.5%) had a previous revision hip arthroscopy on the ipsilateral side, and 14 (0.8%) had a previous hip arthroscopy on the contralateral side. Before surgery, 80% of patients received an intra-articular injection of corticosteroid and lidocaine. The peritrochanteric region was injected in 11.5% of patients and the psoas bursa in 2.2% (Table 4).
Of the 1011 patients who had magnetic resonance imaging (MRI) performed, 943 (93.3%) had abnormal acetabular labrum findings, and 163 (17.1%) had acetabular articular damage. According to radiographic evaluation, 953 patients had abnormal hip joint morphology consistent with FAI. Figure 3 shows the FAI classification percentages.
On clinical examination, 1079 patients (62.1%) had a positive anterior impingement sign. The subspine impingement sign was positive in 447 patients (25.7%), and the trochanteric pain sign was positive in 400 (23%). Table 5 lists range-of-motion values for flexion and hip rotation from 90° of flexion.
As seen in Table 6, labral pathology was the most common diagnosis (1426/1738 patients, 82%).
As seen in Table 7, the most common procedure was femoroplasty (867/1738, 49.9%).
Discussion
In this study, we collected epidemiologic data (demographics, pathologic entities treated, patient-reported measures of disease, surgical treatment preferences) from a large multicenter population of hip pathology patients who elected to undergo arthroscopic surgery. Our results showed these patients were most commonly younger to middle-aged white females with pain primarily in the groin region. Most had pain for at least 1 year, and it was commonly exacerbated by sitting and athletics. Patients reported clinically significant pain and functional limitation, which showed evidence of affecting general physical and mental health. It was not uncommon for patients to have undergone another, related surgery and nonoperative treatments, including intra-articular injection and/or physical therapy, before surgery. There was a high incidence of abnormal hip morphology suggestive of a cam lesion, but the incidence of arthritic changes on radiographs was relatively low. Labral tear was the most common diagnosis, and most often it was addressed with repair. Many patients underwent femoroplasty, acetabuloplasty, and chondroplasty in addition to labral repair.
According to patient-reported outcome measures administered before surgery, 40% to 65% of patients seeking hip preservation surgery reported functional deficits and pain—which falls within the range of results from other multicenter studies on the epidemiology of FAI.18,19 There was, however, a high amount of variability in individual scores on the functional and pain measures; some patients rated their functional ability very high. These findings were supported by the general health forms measuring global physical and mental health. Mean Physical Health and Mental Health scores on the 12-item Short Form Health Survey indicated that patients seeking hip preservation surgery thought their hip condition affected their general well-being. This finding is consistent with research on FAI,18 hip arthritis,20 and total hip arthroplasty.19Our results further showed that hip arthroscopists commonly prescribed alternative treatment measures ahead of surgery. Before elective surgery, 80% of patients received an intra-articular injection, underwent physical therapy, or both. This could suggest a high failure rate for patients who chose conservative treatment approaches for hip-related pathology. However, our study was limited in that it may have included patients who had improved significantly with conservative measures and decided to forgo arthroscopic hip surgery. Although conservative treatment often is recommended in an effort to potentially avoid surgery, there is a lack of research evaluating the efficacy of nonoperative care.21,22Analysis of diagnostic imaging and clinical examination findings revealed some unique characteristics of patients undergoing elective hip preservation surgery. MRI showed labral pathology in an overwhelming majority of these patients, but few had evidence of articular damage. Previous research has found a 67% rate of arthritic changes on diagnostic imaging, but our rate was much lower (17%).23 Radiograph evaluation confirmed the pattern: More than 90% of our patients had Tönnis grade 0 osteoarthritis. Tönnis grade 1 or 2 osteoarthritis is a predictor of acetabular cartilage degeneration,23 and long-term studies have related these osteoarthritic changes to poorer hip arthroscopy outcomes.24 Thus, the lower incidence of osteoarthritis in our study population may reflect current evidence-based practice and a contemporary approach to patient selection.
Most of our patients had isolated cam-type FAI as opposed to pincer-type FAI or a combination of cam and pincer—contrary to research findings that combination cam–pincer FAI is most prevalent.25,26 Our results are more consistent with more recent research findings of a higher incidence of isolated cam lesion, particularly in female patients, and combination cam–pincer in male patients.18,27,28 Similar distributions of surgical procedures and diagnoses exist between the present study and other multicenter evaluations of the epidemiologic characteristics of patients with hip pathology.18Our study had several limitations. First, the population consisted entirely of patients who sought evaluation by a hip arthroscopy specialist and underwent elective surgery. Therefore, the data cannot be applied to a more general orthopedic population or to patients who consult other medical specialists. Second, the population, which was 97% white and had small percentages of African-American, Latino, and Asian patients, lacked ethnic diversity. This finding is consistent with recent epidemiologic research in which ethnicity was identified as a factor in patterns of hip disease.13,29,30 Access to specialists, however, was likely affected by multiple other factors. Fourth, the validity and the reliability of the imaging modalities used have been questioned.31-33 There is controversy regarding ideal imaging modalities for assessment of articular cartilage damage31,32 and FAI. However, the modalities that we used to determine diagnoses in this study are well supported26 and represent common practice patterns.
Am J Orthop. 2017;46(1):35-41. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016;32(7):1286-1292.
2. Peters CL, Aoki SK, Erickson JA, Anderson LA, Anderson AE. Early experience with a comprehensive hip preservation service intended to improve clinical care, education, and academic productivity. Clin Orthop Relat Res. 2012;470(12):3446-3452.
3. Siebenrock KA, Peters CL. ABJS Carl T. Brighton workshop on hip preservation surgery: editorial comment. Clin Orthop Relat Res. 2012;470(12):3281-3283.
4. Parvizi J, Leunig M, Ganz R. Femoroacetabular impingement. J Am Acad Orthop Surg. 2007;15(9):561-570.
5. Poh SY, Hube R, Dienst M. Arthroscopic treatment of femoroacetabular pincer impingement. Oper Orthop Traumatol. 2015;27(6):536-552.
6. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
7. Groh MM, Herrera J. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med. 2009;2(2):105-117.
8. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
9. White BJ, Herzog MM. Labral reconstruction: when to perform and how. Front Surg. 2015;2:27.
10. Yen YM, Kocher MS. Chondral lesions of the hip: microfracture and chondroplasty. Sports Med Arthrosc. 2010;18(2):83-89.
11. Jordan MA, Van Thiel GS, Chahal J, Nho SJ. Operative treatment of chondral defects in the hip joint: a systematic review. Curr Rev Musculoskelet Med. 2012;5(3):244-253.
12. Griffin DW, Kinnard MJ, Formby PM, McCabe MP, Anderson TD. Outcomes of hip arthroscopy in the older adult: a systematic review of the literature [published online October 18, 2016]. Am J Sports Med. doi:10.1177/0363546516667915.
13. Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 2008;466(2):264-272.
14. Kaya M, Suzuki T, Emori M, Yamashita T. Hip morphology influences the pattern of articular cartilage damage. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):2016-2023.
15. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
16. Byrd JT. Hip arthroscopy in athletes. Oper Tech Sports Med. 2005;13(1):24-36.
17. Werner BC, Gaudiani MA, Ranawat AS. The etiology and arthroscopic surgical management of cam lesions. Clin Sports Med. 2016;35(3):391-404.
18. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
19. Shia DS, Clohisy JC, Schinsky MF, Martell JM, Maloney WJ. THA with highly cross-linked polyethylene in patients 50 years or younger. Clin Orthop Relat Res. 2009;467(8):2059-2065.
20. Gandhi SK, Salmon JW, Zhao SZ, Lambert BL, Gore PR, Conrad K. Psychometric evaluation of the 12-item Short-Form Health Survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clin Ther. 2001;23(7):1080-1098.
21. Loudon JK, Reiman MP. Conservative management of femoroacetabular impingement (FAI) in the long distance runner. Phys Ther Sport. 2014;15(2):82-90.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med. 2011;39(2):296-303.
24. McCormick F, Nwachukwu BU, Alpaugh K, Martin SD. Predictors of hip arthroscopy outcomes for labral tears at minimum 2-year follow-up: the influence of age and arthritis. Arthroscopy. 2012;28(10):1359-1364.
25. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
26. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis—what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552.
27. Kapron AL, Peters CL, Aoki SK, et al. The prevalence of radiographic findings of structural hip deformities in female collegiate athletes. Am J Sports Med. 2015;43(6):1324-1330.
28. Lee WY, Kang C, Hwang DS, Jeon JH, Zheng L. Descriptive epidemiology of symptomatic femoroacetabular impingement in young athlete: single center study. Hip Pelvis. 2016;28(1):29-34.
29. Dudda M, Kim YJ, Zhang Y, et al. Morphologic differences between the hips of Chinese women and white women: could they account for the ethnic difference in the prevalence of hip osteoarthritis? Arthritis Rheum. 2011;63(10):2992-2999.
30. Solomon L, Beighton P. Osteoarthrosis of the hip and its relationship to pre-existing in an African population. J Bone Joint Surg Br. 1973;55(1):216-217.
31. Keeney JA, Peelle MW, Jackson J, Rubin D, Maloney WJ, Clohisy JC. Magnetic resonance arthrography versus arthroscopy in the evaluation of articular hip pathology. Clin Orthop Relat Res. 2004;(429):163-169.
32. Schmid MR, Nötzli HP, Zanetti M, Wyss TF, Hodler J. Cartilage lesions in the hip: diagnostic effectiveness of MR arthrography. Radiology. 2003;226(2):382-386.
33. Chevillotte CJ, Ali MH, Trousdale RT, Pagnano MW. Variability in hip range of motion on clinical examination. J Arthroplasty. 2009;24(5):693-697.
1. Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016;32(7):1286-1292.
2. Peters CL, Aoki SK, Erickson JA, Anderson LA, Anderson AE. Early experience with a comprehensive hip preservation service intended to improve clinical care, education, and academic productivity. Clin Orthop Relat Res. 2012;470(12):3446-3452.
3. Siebenrock KA, Peters CL. ABJS Carl T. Brighton workshop on hip preservation surgery: editorial comment. Clin Orthop Relat Res. 2012;470(12):3281-3283.
4. Parvizi J, Leunig M, Ganz R. Femoroacetabular impingement. J Am Acad Orthop Surg. 2007;15(9):561-570.
5. Poh SY, Hube R, Dienst M. Arthroscopic treatment of femoroacetabular pincer impingement. Oper Orthop Traumatol. 2015;27(6):536-552.
6. Javed A, O’Donnell JM. Arthroscopic femoral osteochondroplasty for cam femoroacetabular impingement in patients over 60 years of age. J Bone Joint Surg Br. 2011;93(3):326-331.
7. Groh MM, Herrera J. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med. 2009;2(2):105-117.
8. Philippon MJ, Nepple JJ, Campbell KJ, et al. The hip fluid seal—part I: the effect of an acetabular labral tear, repair, resection, and reconstruction on hip fluid pressurization. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):722-729.
9. White BJ, Herzog MM. Labral reconstruction: when to perform and how. Front Surg. 2015;2:27.
10. Yen YM, Kocher MS. Chondral lesions of the hip: microfracture and chondroplasty. Sports Med Arthrosc. 2010;18(2):83-89.
11. Jordan MA, Van Thiel GS, Chahal J, Nho SJ. Operative treatment of chondral defects in the hip joint: a systematic review. Curr Rev Musculoskelet Med. 2012;5(3):244-253.
12. Griffin DW, Kinnard MJ, Formby PM, McCabe MP, Anderson TD. Outcomes of hip arthroscopy in the older adult: a systematic review of the literature [published online October 18, 2016]. Am J Sports Med. doi:10.1177/0363546516667915.
13. Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 2008;466(2):264-272.
14. Kaya M, Suzuki T, Emori M, Yamashita T. Hip morphology influences the pattern of articular cartilage damage. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):2016-2023.
15. Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;(418):67-73.
16. Byrd JT. Hip arthroscopy in athletes. Oper Tech Sports Med. 2005;13(1):24-36.
17. Werner BC, Gaudiani MA, Ranawat AS. The etiology and arthroscopic surgical management of cam lesions. Clin Sports Med. 2016;35(3):391-404.
18. Clohisy JC, Baca G, Beaulé PE, et al; ANCHOR Study Group. Descriptive epidemiology of femoroacetabular impingement: a North American cohort of patients undergoing surgery. Am J Sports Med. 2013;41(6):1348-1356.
19. Shia DS, Clohisy JC, Schinsky MF, Martell JM, Maloney WJ. THA with highly cross-linked polyethylene in patients 50 years or younger. Clin Orthop Relat Res. 2009;467(8):2059-2065.
20. Gandhi SK, Salmon JW, Zhao SZ, Lambert BL, Gore PR, Conrad K. Psychometric evaluation of the 12-item Short-Form Health Survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clin Ther. 2001;23(7):1080-1098.
21. Loudon JK, Reiman MP. Conservative management of femoroacetabular impingement (FAI) in the long distance runner. Phys Ther Sport. 2014;15(2):82-90.
22. Wall PD, Fernandez M, Griffin DR, Foster NE. Nonoperative treatment for femoroacetabular impingement: a systematic review of the literature. PM R. 2013;5(5):418-426.
23. Nepple JJ, Carlisle JC, Nunley RM, Clohisy JC. Clinical and radiographic predictors of intra-articular hip disease in arthroscopy. Am J Sports Med. 2011;39(2):296-303.
24. McCormick F, Nwachukwu BU, Alpaugh K, Martin SD. Predictors of hip arthroscopy outcomes for labral tears at minimum 2-year follow-up: the influence of age and arthritis. Arthroscopy. 2012;28(10):1359-1364.
25. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-1018.
26. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis—what the radiologist should know. AJR Am J Roentgenol. 2007;188(6):1540-1552.
27. Kapron AL, Peters CL, Aoki SK, et al. The prevalence of radiographic findings of structural hip deformities in female collegiate athletes. Am J Sports Med. 2015;43(6):1324-1330.
28. Lee WY, Kang C, Hwang DS, Jeon JH, Zheng L. Descriptive epidemiology of symptomatic femoroacetabular impingement in young athlete: single center study. Hip Pelvis. 2016;28(1):29-34.
29. Dudda M, Kim YJ, Zhang Y, et al. Morphologic differences between the hips of Chinese women and white women: could they account for the ethnic difference in the prevalence of hip osteoarthritis? Arthritis Rheum. 2011;63(10):2992-2999.
30. Solomon L, Beighton P. Osteoarthrosis of the hip and its relationship to pre-existing in an African population. J Bone Joint Surg Br. 1973;55(1):216-217.
31. Keeney JA, Peelle MW, Jackson J, Rubin D, Maloney WJ, Clohisy JC. Magnetic resonance arthrography versus arthroscopy in the evaluation of articular hip pathology. Clin Orthop Relat Res. 2004;(429):163-169.
32. Schmid MR, Nötzli HP, Zanetti M, Wyss TF, Hodler J. Cartilage lesions in the hip: diagnostic effectiveness of MR arthrography. Radiology. 2003;226(2):382-386.
33. Chevillotte CJ, Ali MH, Trousdale RT, Pagnano MW. Variability in hip range of motion on clinical examination. J Arthroplasty. 2009;24(5):693-697.