User login
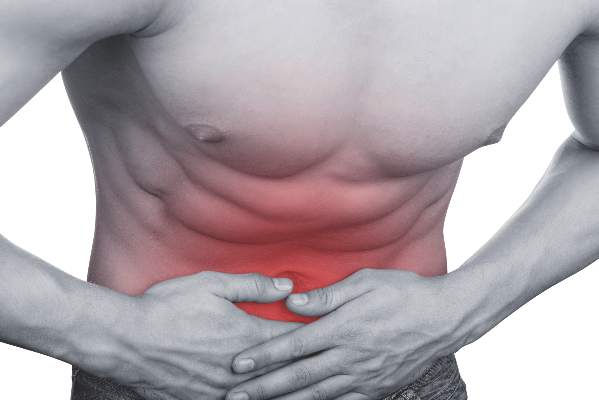
Risk of IBD doubled in hidradenitis suppurativa
WASHINGTON – Patients with hidradenitis suppurativa (HS) face an increased risk of also developing inflammatory bowel disease (IBD), Ahuva Cices said at the annual meeting of the American Academy of Dermatology.
In a case-control study, Ms. Cices found that those with a new diagnosis of HS were twice as likely as controls to also be diagnosed with inflammatory bowel disease, either concurrently or shortly afterward.
“Our findings support a comorbid relationship between these two disorders, and suggest that hidradenitis suppurativa should be considered a risk factor for IBD and impart increased clinical suspicion for undiagnosed IBD,” said Ms. Cices of Northwestern University, Chicago.
She and her coinvestigators at Northwestern searched a large academic medical center database for patients with a diagnosis of HS from 2005 to 2015. These 1,332 patients were matched with about 2,600 control subjects. Of the individuals with HS, 20 were also concurrently or subsequently diagnosed with IBD. The mean lag time to an IBD diagnosis was 9 months.
The mean age of the patients with both conditions was almost 40 years. Most (75%) were female – significantly more than in the control group (42%). About half of the patients (45%) were black – also significantly more than in the control group (10%).
In a multivariate analysis, a diagnosis of HS conferred a doubling in the risk of a concurrent or subsequent diagnosis of IBD (odds ratio, 2.11), which was statistically significant.
The pathophysiologic link between the two diseases has been theorized but not confirmed, Ms. Cices said.
“The multifactorial pathways of HS and IBD are complicated, but they both involve inflammatory responses to normal flora, immune dysfunction, and dysregulation of Notch, the sulfotransferase enzyme, [tumor necrosis factor–]alpha, and T-helper 17 cells.”
She had no relevant financial disclosures.
WASHINGTON – Patients with hidradenitis suppurativa (HS) face an increased risk of also developing inflammatory bowel disease (IBD), Ahuva Cices said at the annual meeting of the American Academy of Dermatology.
In a case-control study, Ms. Cices found that those with a new diagnosis of HS were twice as likely as controls to also be diagnosed with inflammatory bowel disease, either concurrently or shortly afterward.
“Our findings support a comorbid relationship between these two disorders, and suggest that hidradenitis suppurativa should be considered a risk factor for IBD and impart increased clinical suspicion for undiagnosed IBD,” said Ms. Cices of Northwestern University, Chicago.
She and her coinvestigators at Northwestern searched a large academic medical center database for patients with a diagnosis of HS from 2005 to 2015. These 1,332 patients were matched with about 2,600 control subjects. Of the individuals with HS, 20 were also concurrently or subsequently diagnosed with IBD. The mean lag time to an IBD diagnosis was 9 months.
The mean age of the patients with both conditions was almost 40 years. Most (75%) were female – significantly more than in the control group (42%). About half of the patients (45%) were black – also significantly more than in the control group (10%).
In a multivariate analysis, a diagnosis of HS conferred a doubling in the risk of a concurrent or subsequent diagnosis of IBD (odds ratio, 2.11), which was statistically significant.
The pathophysiologic link between the two diseases has been theorized but not confirmed, Ms. Cices said.
“The multifactorial pathways of HS and IBD are complicated, but they both involve inflammatory responses to normal flora, immune dysfunction, and dysregulation of Notch, the sulfotransferase enzyme, [tumor necrosis factor–]alpha, and T-helper 17 cells.”
She had no relevant financial disclosures.
WASHINGTON – Patients with hidradenitis suppurativa (HS) face an increased risk of also developing inflammatory bowel disease (IBD), Ahuva Cices said at the annual meeting of the American Academy of Dermatology.
In a case-control study, Ms. Cices found that those with a new diagnosis of HS were twice as likely as controls to also be diagnosed with inflammatory bowel disease, either concurrently or shortly afterward.
“Our findings support a comorbid relationship between these two disorders, and suggest that hidradenitis suppurativa should be considered a risk factor for IBD and impart increased clinical suspicion for undiagnosed IBD,” said Ms. Cices of Northwestern University, Chicago.
She and her coinvestigators at Northwestern searched a large academic medical center database for patients with a diagnosis of HS from 2005 to 2015. These 1,332 patients were matched with about 2,600 control subjects. Of the individuals with HS, 20 were also concurrently or subsequently diagnosed with IBD. The mean lag time to an IBD diagnosis was 9 months.
The mean age of the patients with both conditions was almost 40 years. Most (75%) were female – significantly more than in the control group (42%). About half of the patients (45%) were black – also significantly more than in the control group (10%).
In a multivariate analysis, a diagnosis of HS conferred a doubling in the risk of a concurrent or subsequent diagnosis of IBD (odds ratio, 2.11), which was statistically significant.
The pathophysiologic link between the two diseases has been theorized but not confirmed, Ms. Cices said.
“The multifactorial pathways of HS and IBD are complicated, but they both involve inflammatory responses to normal flora, immune dysfunction, and dysregulation of Notch, the sulfotransferase enzyme, [tumor necrosis factor–]alpha, and T-helper 17 cells.”
She had no relevant financial disclosures.
AT AAD 16
Key clinical point: Hidradenitis suppurativa may be considered a risk factor for inflammatory bowel disease.
Major finding: Patients with hidradenitis suppurativa were twice as likely as controls to have a concurrent or subsequent diagnosis of inflammatory bowel disease.
Data source: A case-control study of 1,332 patients and about 2,600 age-matched controls.
Disclosures: Ms. Cices had no relevant financial disclosures.
Clostridium difficile management guidance updated by AHRQ
Clinical trial data support the use of vancomycin over metronidazole for those with initial Clostridium difficile infection (CDI), and fidaxomicin over vancomycin for the prevention of recurrent infection, according to a newly-released update of the 2011 Agency for Healthcare Research and Quality Comparative Effectiveness Review.
In contrast, data were considered to be weak though consistent in their pattern of efficacy regarding the use of fecal microbiota transplantation and lactobacillus probiotics to restore the biodiversity of the gut microbiota and improve patient resistance to CDI or its recurrence, according to Mary Butler, Ph.D., of the division of health policy and management, University of Minnesota School of Public Health, and her associates.
Since publication of the original review, data have been reported supporting the effectiveness of new diagnostic tests, as well as the comparative efficacy of particular agents over others in different stages of the illness. Dr. Butler and six additional experts in the fields of health policy and management and infectious disease reviewed published literature from 2010 through 2015 focusing on a limited number of quality improvement opportunities identified as most important to improve patient care, adding seven new findings and updating six findings from the original review.
To update the 2011 review, the authors conducted a literature review covering the years 2010 through 2015. Of the 7,416 individual citations identified using this search methodology, 252 were selected for further scrutiny. Of these, 37 diagnostic studies and 56 studies evaluating prevention or treatment interventions were identified and assessed from a pool of 252 eligible studies selected from 7,416 citations regarding early diagnosis, prevention, and treatment of Clostridium difficile.
Dr. Butler and her colleagues noted that the four years between the original review and the update were marked by an overall increase in research regarding both diagnosis and interventions. Aside from the aforementioned interventional updates, four diagnostic methodologies without recommendations in the original review (nucleic acid amplification tests, enzyme tests for toxins A/B, assay tests for glutamate dehydrogenase, and test algorithms) are all included in the update, with high, moderate, and low levels of evidence, respectively.
Regarding preventive strategies, the design and reporting of new studies published since the release of the original review were considered to have shown improvement; however, only weak evidence was found for effectiveness in reducing the incidence of CDI.
The updated management review has been published online at www.effectivehealthcare.ahrq.gov/reports/final.cfm (AHRQ Publication No. 16-EHC012-EF. March 2016).
The Agency for Healthcare Research and Quality funded the update. None of the investigators reported any affiliations or financial involvements that would have conflicted with the material presented in this report.
Clinical trial data support the use of vancomycin over metronidazole for those with initial Clostridium difficile infection (CDI), and fidaxomicin over vancomycin for the prevention of recurrent infection, according to a newly-released update of the 2011 Agency for Healthcare Research and Quality Comparative Effectiveness Review.
In contrast, data were considered to be weak though consistent in their pattern of efficacy regarding the use of fecal microbiota transplantation and lactobacillus probiotics to restore the biodiversity of the gut microbiota and improve patient resistance to CDI or its recurrence, according to Mary Butler, Ph.D., of the division of health policy and management, University of Minnesota School of Public Health, and her associates.
Since publication of the original review, data have been reported supporting the effectiveness of new diagnostic tests, as well as the comparative efficacy of particular agents over others in different stages of the illness. Dr. Butler and six additional experts in the fields of health policy and management and infectious disease reviewed published literature from 2010 through 2015 focusing on a limited number of quality improvement opportunities identified as most important to improve patient care, adding seven new findings and updating six findings from the original review.
To update the 2011 review, the authors conducted a literature review covering the years 2010 through 2015. Of the 7,416 individual citations identified using this search methodology, 252 were selected for further scrutiny. Of these, 37 diagnostic studies and 56 studies evaluating prevention or treatment interventions were identified and assessed from a pool of 252 eligible studies selected from 7,416 citations regarding early diagnosis, prevention, and treatment of Clostridium difficile.
Dr. Butler and her colleagues noted that the four years between the original review and the update were marked by an overall increase in research regarding both diagnosis and interventions. Aside from the aforementioned interventional updates, four diagnostic methodologies without recommendations in the original review (nucleic acid amplification tests, enzyme tests for toxins A/B, assay tests for glutamate dehydrogenase, and test algorithms) are all included in the update, with high, moderate, and low levels of evidence, respectively.
Regarding preventive strategies, the design and reporting of new studies published since the release of the original review were considered to have shown improvement; however, only weak evidence was found for effectiveness in reducing the incidence of CDI.
The updated management review has been published online at www.effectivehealthcare.ahrq.gov/reports/final.cfm (AHRQ Publication No. 16-EHC012-EF. March 2016).
The Agency for Healthcare Research and Quality funded the update. None of the investigators reported any affiliations or financial involvements that would have conflicted with the material presented in this report.
Clinical trial data support the use of vancomycin over metronidazole for those with initial Clostridium difficile infection (CDI), and fidaxomicin over vancomycin for the prevention of recurrent infection, according to a newly-released update of the 2011 Agency for Healthcare Research and Quality Comparative Effectiveness Review.
In contrast, data were considered to be weak though consistent in their pattern of efficacy regarding the use of fecal microbiota transplantation and lactobacillus probiotics to restore the biodiversity of the gut microbiota and improve patient resistance to CDI or its recurrence, according to Mary Butler, Ph.D., of the division of health policy and management, University of Minnesota School of Public Health, and her associates.
Since publication of the original review, data have been reported supporting the effectiveness of new diagnostic tests, as well as the comparative efficacy of particular agents over others in different stages of the illness. Dr. Butler and six additional experts in the fields of health policy and management and infectious disease reviewed published literature from 2010 through 2015 focusing on a limited number of quality improvement opportunities identified as most important to improve patient care, adding seven new findings and updating six findings from the original review.
To update the 2011 review, the authors conducted a literature review covering the years 2010 through 2015. Of the 7,416 individual citations identified using this search methodology, 252 were selected for further scrutiny. Of these, 37 diagnostic studies and 56 studies evaluating prevention or treatment interventions were identified and assessed from a pool of 252 eligible studies selected from 7,416 citations regarding early diagnosis, prevention, and treatment of Clostridium difficile.
Dr. Butler and her colleagues noted that the four years between the original review and the update were marked by an overall increase in research regarding both diagnosis and interventions. Aside from the aforementioned interventional updates, four diagnostic methodologies without recommendations in the original review (nucleic acid amplification tests, enzyme tests for toxins A/B, assay tests for glutamate dehydrogenase, and test algorithms) are all included in the update, with high, moderate, and low levels of evidence, respectively.
Regarding preventive strategies, the design and reporting of new studies published since the release of the original review were considered to have shown improvement; however, only weak evidence was found for effectiveness in reducing the incidence of CDI.
The updated management review has been published online at www.effectivehealthcare.ahrq.gov/reports/final.cfm (AHRQ Publication No. 16-EHC012-EF. March 2016).
The Agency for Healthcare Research and Quality funded the update. None of the investigators reported any affiliations or financial involvements that would have conflicted with the material presented in this report.
Statins inversely linked to colorectal cancer in patients with IBD
Patients with inflammatory bowel disease who were prescribed statins had 65% lower odds of subsequent colorectal cancer, compared with other IBD patients, even after controlling for multiple potential confounders, researchers reported in Clinical Gastroenterology and Hepatology.
“Further confirmation from other cohorts may provide support for the use of statins as a chemopreventive in patients with IBD,” said Dr. Ashwin Ananthakrishnan of Massachusetts General Hospital in Boston, and his associates.
Patients with long-standing ulcerative colitis or colonic Crohn’s disease have about twice the risk of colorectal cancer (CRC), compared with the general population, and up to an 18% lifetime risk of CRC by 30 years after diagnosis, the researchers noted. Early results supporting mesalamine as chemoprophylaxis did not hold up in later trials. Although several studies suggested that statins might help prevent sporadic colon cancer, the only such study in IBD patients was small and did not control for key covariates such as smoking, the investigators added. Therefore, they collected data from 11,001 patients with IBD who were seen at Boston area hospitals between 1998 and 2010. They identified CRC diagnoses based on ICD-9 codes, and analyzed electronic prescriptions to see whether and when patients had used statins (Clin Gastroenterol Hepatol. 2016 Feb 21. doi: 10.1016/j.cgh.2016.02.017).
A total of 1,376 patients (12.5%) were prescribed at least one statin. Over 9 years of follow-up, 2% of statin users developed CRC, compared with 3% of nonusers (age-adjusted odds ratio, 0.35; 95% confidence interval, 0.24-0.53). Statin users were more likely to be older, male, white, smokers, and had more comorbidities than nonusers. Nonetheless, the protective effect of statins remained significant after controlling for demographic factors, smoking status, number of colonoscopies, use of steroids and immunomodulators, the presence of primary sclerosing cholangitis, and increases in inflammatory biomarkers (OR, 0.42; 95% CI, 0.28-0.62). The effect occurred for both Crohn’s disease and ulcerative colitis. Notably, the inverse association was even stronger among patients who had been prescribed at least two statins or who had at least a 2-year interval between statin use and CRC diagnosis.
Statins might help prevent CRC through HMG-CoA reductase inhibition and other mechanisms, according to the researchers. By inhibiting HMG-CoA reductase, statins lower production of farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which are needed for post-translational activation of Ras, Rho, and other proteins that are overexpressed in CRC and that have been linked to tumor invasion. Statins also might help prevent CRC through antioxidant effects or by inhibiting inflammation, cell adhesion, and angiogenesis, the investigators added. “Although we did not see a difference in median C-reactive protein levels between statin users and nonusers, statin users were less likely to require immunomodulator or biologic therapy for their IBD, supporting a potential anti-inflammatory role for statins.”
Because patients mainly were treated at two tertiary referral hospitals, they may have had more severe disease than the general population of patients with IBD, the investigators acknowledged. They noted that in some meta-analyses, referral center studies yielded chemopreventive effects that did not hold up in population-based cohorts.
The study was funded by the National Institutes of Health, the American Gastroenterological Association, and the Harold and Duval Bowen Fund. The researchers had no disclosures.
Patients with inflammatory bowel disease who were prescribed statins had 65% lower odds of subsequent colorectal cancer, compared with other IBD patients, even after controlling for multiple potential confounders, researchers reported in Clinical Gastroenterology and Hepatology.
“Further confirmation from other cohorts may provide support for the use of statins as a chemopreventive in patients with IBD,” said Dr. Ashwin Ananthakrishnan of Massachusetts General Hospital in Boston, and his associates.
Patients with long-standing ulcerative colitis or colonic Crohn’s disease have about twice the risk of colorectal cancer (CRC), compared with the general population, and up to an 18% lifetime risk of CRC by 30 years after diagnosis, the researchers noted. Early results supporting mesalamine as chemoprophylaxis did not hold up in later trials. Although several studies suggested that statins might help prevent sporadic colon cancer, the only such study in IBD patients was small and did not control for key covariates such as smoking, the investigators added. Therefore, they collected data from 11,001 patients with IBD who were seen at Boston area hospitals between 1998 and 2010. They identified CRC diagnoses based on ICD-9 codes, and analyzed electronic prescriptions to see whether and when patients had used statins (Clin Gastroenterol Hepatol. 2016 Feb 21. doi: 10.1016/j.cgh.2016.02.017).
A total of 1,376 patients (12.5%) were prescribed at least one statin. Over 9 years of follow-up, 2% of statin users developed CRC, compared with 3% of nonusers (age-adjusted odds ratio, 0.35; 95% confidence interval, 0.24-0.53). Statin users were more likely to be older, male, white, smokers, and had more comorbidities than nonusers. Nonetheless, the protective effect of statins remained significant after controlling for demographic factors, smoking status, number of colonoscopies, use of steroids and immunomodulators, the presence of primary sclerosing cholangitis, and increases in inflammatory biomarkers (OR, 0.42; 95% CI, 0.28-0.62). The effect occurred for both Crohn’s disease and ulcerative colitis. Notably, the inverse association was even stronger among patients who had been prescribed at least two statins or who had at least a 2-year interval between statin use and CRC diagnosis.
Statins might help prevent CRC through HMG-CoA reductase inhibition and other mechanisms, according to the researchers. By inhibiting HMG-CoA reductase, statins lower production of farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which are needed for post-translational activation of Ras, Rho, and other proteins that are overexpressed in CRC and that have been linked to tumor invasion. Statins also might help prevent CRC through antioxidant effects or by inhibiting inflammation, cell adhesion, and angiogenesis, the investigators added. “Although we did not see a difference in median C-reactive protein levels between statin users and nonusers, statin users were less likely to require immunomodulator or biologic therapy for their IBD, supporting a potential anti-inflammatory role for statins.”
Because patients mainly were treated at two tertiary referral hospitals, they may have had more severe disease than the general population of patients with IBD, the investigators acknowledged. They noted that in some meta-analyses, referral center studies yielded chemopreventive effects that did not hold up in population-based cohorts.
The study was funded by the National Institutes of Health, the American Gastroenterological Association, and the Harold and Duval Bowen Fund. The researchers had no disclosures.
Patients with inflammatory bowel disease who were prescribed statins had 65% lower odds of subsequent colorectal cancer, compared with other IBD patients, even after controlling for multiple potential confounders, researchers reported in Clinical Gastroenterology and Hepatology.
“Further confirmation from other cohorts may provide support for the use of statins as a chemopreventive in patients with IBD,” said Dr. Ashwin Ananthakrishnan of Massachusetts General Hospital in Boston, and his associates.
Patients with long-standing ulcerative colitis or colonic Crohn’s disease have about twice the risk of colorectal cancer (CRC), compared with the general population, and up to an 18% lifetime risk of CRC by 30 years after diagnosis, the researchers noted. Early results supporting mesalamine as chemoprophylaxis did not hold up in later trials. Although several studies suggested that statins might help prevent sporadic colon cancer, the only such study in IBD patients was small and did not control for key covariates such as smoking, the investigators added. Therefore, they collected data from 11,001 patients with IBD who were seen at Boston area hospitals between 1998 and 2010. They identified CRC diagnoses based on ICD-9 codes, and analyzed electronic prescriptions to see whether and when patients had used statins (Clin Gastroenterol Hepatol. 2016 Feb 21. doi: 10.1016/j.cgh.2016.02.017).
A total of 1,376 patients (12.5%) were prescribed at least one statin. Over 9 years of follow-up, 2% of statin users developed CRC, compared with 3% of nonusers (age-adjusted odds ratio, 0.35; 95% confidence interval, 0.24-0.53). Statin users were more likely to be older, male, white, smokers, and had more comorbidities than nonusers. Nonetheless, the protective effect of statins remained significant after controlling for demographic factors, smoking status, number of colonoscopies, use of steroids and immunomodulators, the presence of primary sclerosing cholangitis, and increases in inflammatory biomarkers (OR, 0.42; 95% CI, 0.28-0.62). The effect occurred for both Crohn’s disease and ulcerative colitis. Notably, the inverse association was even stronger among patients who had been prescribed at least two statins or who had at least a 2-year interval between statin use and CRC diagnosis.
Statins might help prevent CRC through HMG-CoA reductase inhibition and other mechanisms, according to the researchers. By inhibiting HMG-CoA reductase, statins lower production of farnesyl pyrophosphate and geranylgeranyl pyrophosphate, which are needed for post-translational activation of Ras, Rho, and other proteins that are overexpressed in CRC and that have been linked to tumor invasion. Statins also might help prevent CRC through antioxidant effects or by inhibiting inflammation, cell adhesion, and angiogenesis, the investigators added. “Although we did not see a difference in median C-reactive protein levels between statin users and nonusers, statin users were less likely to require immunomodulator or biologic therapy for their IBD, supporting a potential anti-inflammatory role for statins.”
Because patients mainly were treated at two tertiary referral hospitals, they may have had more severe disease than the general population of patients with IBD, the investigators acknowledged. They noted that in some meta-analyses, referral center studies yielded chemopreventive effects that did not hold up in population-based cohorts.
The study was funded by the National Institutes of Health, the American Gastroenterological Association, and the Harold and Duval Bowen Fund. The researchers had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Preliminary evidence suggests that statins might help prevent colorectal cancer in patients with inflammatory bowel disease.
Major finding: A total of 2% of statin users developed CRC over 9 years of follow-up, compared with 3% of nonusers (age-adjusted odds ratio, 0.35).
Data source: An analysis of ICD-9 codes and electronic prescription data for 11,001 patients with IBD.
Disclosures: The study was funded by the National Institutes of Health, the American Gastroenterological Association, and the Harold and Duval Bowen Fund. The researchers had no disclosures.
Mongersen could be an impressive new treatment for inflammatory bowel disease
MAUI, HAWAII – Oral mongersen shows promise of being an ideal treatment for inflammatory bowel disease (IBD) if the promising phase II results are confirmed in phase III clinical trials, Dr. Uma Mahadevan, AGAF, said at the 2016 Rheumatology Winter Clinical Symposium.*
This perfect drug for IBD would be something that’s effective, safe, and well tolerated; orally administered; shows long-term efficacy; and can be dosed as needed. Preliminary indications are that mongersen may check off those boxes, according to Dr. Mahadevan, professor of medicine and joint medical director of the Center for Colitis and Crohn’s Disease at the University of California, San Francisco.
An Italian double-blind, placebo-controlled, phase II study of mongersen in Crohn’s disease generated enormous attention among IBD patients and gastroenterologists, with reported day 15 clinical remission rates of 55% and 65% at 40 and 160 mg/day, respectively (N Engl J Med. 2015 Mar 19;372:1104-13).
“We haven’t seen numbers like that in a Crohn’s trial since the very first Remicade [infliximab] trial. So there’s a lot of excitement about it. The study mechanics are a little suspect, but if the data in the current phase III trials are half as good, mongersen may be what we’re looking for,” Dr. Mahadevan said.
Mongersen is an oligonucleotide that inhibits ileal and colonic SMAD7, a protein that prevents transforming growth factor beta1–mediated suppression of inflammatory genes.
Why was a gastroenterologist providing an IBD update at a rheumatology conference? Dr. Mahadevan was invited in order to provide the conference’s annual “Outside the Box” feature, in which a nonrheumatologist expert shares the latest thinking about a disease relevant to rheumatology.
IBD fills the bill for several reasons. For one, many of the extraintestinal manifestations of IBD overlap with rheumatologic conditions. These include uveitis, arthritis, and arthralgias; osteopenia and osteoporosis; psoriasiform skin lesions; and thromboembolism. It’s not unusual for these extraintestinal manifestations to bring a patient to a nongastroenterologist before the diagnosis of IBD has been made.
Also, gastroenterologists and rheumatologists use a lot of the same drugs.
“The only drug gastroenterologists use for IBD that didn’t come from rheumatology first is vedolizumab [Entyvio],” she observed.
Ustekinumab (Stelara), which Dr. Mahadevan and other IBD experts have been using off-label for refractory Crohn’s disease with what she termed “very good” results, is expected to receive an Food and Drug Administration indication for Crohn’s disease by year’s end on the strength of impressive phase III data reported last fall.
“When approved, ustekinumab should be a first-line therapy, parallel to the TNF [tumor necrosis factor] inhibitors,” she predicted.
Another reason it’s worthwhile for rheumatologists to be up to date on IBD is that in many parts of the country, some gastroenterologists in community practice are uncomfortable using TNF inhibitors, which are now standard therapy for IBD. They may refer those patients to their local rheumatologist. But be advised: the dosing is very different from in rheumatology.
“For example, you start adalimumab [Humira] at 40 mg every other week for rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis, but we [gastroenterologists] do a loading dose of four 40-mg injections on day 1, then 80 mg 2 weeks later, and then maintenance dosing at 40 mg every 2 weeks. Sometimes in our patient population you’re not going to get a response unless you use a high dose like that,” the gastroenterologist explained.
The incidence of IBD is climbing. It was estimated at 5-7 cases per 100,000 population in the late 1990s, but it’s now believed to be 7-12 per 100,000. The peak age of onset is still in 15- to 30-year-olds, but now physicians are seeing a strong second peak later in life, among individuals in their 60s and 70s. And while Caucasians and Ashkenazi Jews have traditionally been thought of as the groups at increased risk for IBD, treatment centers are now springing up in India, China, and other countries where IBD was formerly thought to be less common.
“What is happening? Is it that because of better medical care we’re now picking up more cases outside of North America and Europe? Or is it a change in the environment – the microbiome, the Westernized diet, pollution? We really don’t know. But this is where a lot of study is going,” according to Dr. Mahadevan.
Something nongastroenterologists need to know about the clinical care of IBD patients is that even though TNF inhibitors are now considered standard therapy for patients with more severe disease and these biologic agents are routinely used in IBD centers of excellence, their penetration into community practice has been slow. Although high-dose anti-TNF therapy is supposed to be employed in ulcerative colitis patients who are refractory to corticosteroid induction therapy or who are steroid dependent, it’s fairly common in some parts of the country for a patient to undergo colectomy without ever having received an anti-TNF biologic.
“If you’re in a surgery-dominated area, the surgeons were very influenced by a Cleveland Clinic paper that said patients who received infliximab had more complications after surgery. There were a lot of caveats to that paper, but colorectal surgeons don’t want you to use a TNF inhibitor if they feel the patient is going to surgery,” she said.
In addition to mongersen, other novel agents targeting new pathways in IBD include the oral Janus-associated kinase inhibitor tofacitinib (Xeljanz), under study for both ulcerative colitis and Crohn’s disease; ozanimod, an oral sphingosine 1–phosphate 1 and 5 receptor modulator in clinical trials for ulcerative colitis; and the humanized monoclonal antibody etrolizumab, a selective anti-integrin agent.
Dr. Mahadevan reported serving as a consultant to and/or research grant recipient from more than half a dozen pharmaceutical companies.
*Changes were made to this story on March 24, 2016.
MAUI, HAWAII – Oral mongersen shows promise of being an ideal treatment for inflammatory bowel disease (IBD) if the promising phase II results are confirmed in phase III clinical trials, Dr. Uma Mahadevan, AGAF, said at the 2016 Rheumatology Winter Clinical Symposium.*
This perfect drug for IBD would be something that’s effective, safe, and well tolerated; orally administered; shows long-term efficacy; and can be dosed as needed. Preliminary indications are that mongersen may check off those boxes, according to Dr. Mahadevan, professor of medicine and joint medical director of the Center for Colitis and Crohn’s Disease at the University of California, San Francisco.
An Italian double-blind, placebo-controlled, phase II study of mongersen in Crohn’s disease generated enormous attention among IBD patients and gastroenterologists, with reported day 15 clinical remission rates of 55% and 65% at 40 and 160 mg/day, respectively (N Engl J Med. 2015 Mar 19;372:1104-13).
“We haven’t seen numbers like that in a Crohn’s trial since the very first Remicade [infliximab] trial. So there’s a lot of excitement about it. The study mechanics are a little suspect, but if the data in the current phase III trials are half as good, mongersen may be what we’re looking for,” Dr. Mahadevan said.
Mongersen is an oligonucleotide that inhibits ileal and colonic SMAD7, a protein that prevents transforming growth factor beta1–mediated suppression of inflammatory genes.
Why was a gastroenterologist providing an IBD update at a rheumatology conference? Dr. Mahadevan was invited in order to provide the conference’s annual “Outside the Box” feature, in which a nonrheumatologist expert shares the latest thinking about a disease relevant to rheumatology.
IBD fills the bill for several reasons. For one, many of the extraintestinal manifestations of IBD overlap with rheumatologic conditions. These include uveitis, arthritis, and arthralgias; osteopenia and osteoporosis; psoriasiform skin lesions; and thromboembolism. It’s not unusual for these extraintestinal manifestations to bring a patient to a nongastroenterologist before the diagnosis of IBD has been made.
Also, gastroenterologists and rheumatologists use a lot of the same drugs.
“The only drug gastroenterologists use for IBD that didn’t come from rheumatology first is vedolizumab [Entyvio],” she observed.
Ustekinumab (Stelara), which Dr. Mahadevan and other IBD experts have been using off-label for refractory Crohn’s disease with what she termed “very good” results, is expected to receive an Food and Drug Administration indication for Crohn’s disease by year’s end on the strength of impressive phase III data reported last fall.
“When approved, ustekinumab should be a first-line therapy, parallel to the TNF [tumor necrosis factor] inhibitors,” she predicted.
Another reason it’s worthwhile for rheumatologists to be up to date on IBD is that in many parts of the country, some gastroenterologists in community practice are uncomfortable using TNF inhibitors, which are now standard therapy for IBD. They may refer those patients to their local rheumatologist. But be advised: the dosing is very different from in rheumatology.
“For example, you start adalimumab [Humira] at 40 mg every other week for rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis, but we [gastroenterologists] do a loading dose of four 40-mg injections on day 1, then 80 mg 2 weeks later, and then maintenance dosing at 40 mg every 2 weeks. Sometimes in our patient population you’re not going to get a response unless you use a high dose like that,” the gastroenterologist explained.
The incidence of IBD is climbing. It was estimated at 5-7 cases per 100,000 population in the late 1990s, but it’s now believed to be 7-12 per 100,000. The peak age of onset is still in 15- to 30-year-olds, but now physicians are seeing a strong second peak later in life, among individuals in their 60s and 70s. And while Caucasians and Ashkenazi Jews have traditionally been thought of as the groups at increased risk for IBD, treatment centers are now springing up in India, China, and other countries where IBD was formerly thought to be less common.
“What is happening? Is it that because of better medical care we’re now picking up more cases outside of North America and Europe? Or is it a change in the environment – the microbiome, the Westernized diet, pollution? We really don’t know. But this is where a lot of study is going,” according to Dr. Mahadevan.
Something nongastroenterologists need to know about the clinical care of IBD patients is that even though TNF inhibitors are now considered standard therapy for patients with more severe disease and these biologic agents are routinely used in IBD centers of excellence, their penetration into community practice has been slow. Although high-dose anti-TNF therapy is supposed to be employed in ulcerative colitis patients who are refractory to corticosteroid induction therapy or who are steroid dependent, it’s fairly common in some parts of the country for a patient to undergo colectomy without ever having received an anti-TNF biologic.
“If you’re in a surgery-dominated area, the surgeons were very influenced by a Cleveland Clinic paper that said patients who received infliximab had more complications after surgery. There were a lot of caveats to that paper, but colorectal surgeons don’t want you to use a TNF inhibitor if they feel the patient is going to surgery,” she said.
In addition to mongersen, other novel agents targeting new pathways in IBD include the oral Janus-associated kinase inhibitor tofacitinib (Xeljanz), under study for both ulcerative colitis and Crohn’s disease; ozanimod, an oral sphingosine 1–phosphate 1 and 5 receptor modulator in clinical trials for ulcerative colitis; and the humanized monoclonal antibody etrolizumab, a selective anti-integrin agent.
Dr. Mahadevan reported serving as a consultant to and/or research grant recipient from more than half a dozen pharmaceutical companies.
*Changes were made to this story on March 24, 2016.
MAUI, HAWAII – Oral mongersen shows promise of being an ideal treatment for inflammatory bowel disease (IBD) if the promising phase II results are confirmed in phase III clinical trials, Dr. Uma Mahadevan, AGAF, said at the 2016 Rheumatology Winter Clinical Symposium.*
This perfect drug for IBD would be something that’s effective, safe, and well tolerated; orally administered; shows long-term efficacy; and can be dosed as needed. Preliminary indications are that mongersen may check off those boxes, according to Dr. Mahadevan, professor of medicine and joint medical director of the Center for Colitis and Crohn’s Disease at the University of California, San Francisco.
An Italian double-blind, placebo-controlled, phase II study of mongersen in Crohn’s disease generated enormous attention among IBD patients and gastroenterologists, with reported day 15 clinical remission rates of 55% and 65% at 40 and 160 mg/day, respectively (N Engl J Med. 2015 Mar 19;372:1104-13).
“We haven’t seen numbers like that in a Crohn’s trial since the very first Remicade [infliximab] trial. So there’s a lot of excitement about it. The study mechanics are a little suspect, but if the data in the current phase III trials are half as good, mongersen may be what we’re looking for,” Dr. Mahadevan said.
Mongersen is an oligonucleotide that inhibits ileal and colonic SMAD7, a protein that prevents transforming growth factor beta1–mediated suppression of inflammatory genes.
Why was a gastroenterologist providing an IBD update at a rheumatology conference? Dr. Mahadevan was invited in order to provide the conference’s annual “Outside the Box” feature, in which a nonrheumatologist expert shares the latest thinking about a disease relevant to rheumatology.
IBD fills the bill for several reasons. For one, many of the extraintestinal manifestations of IBD overlap with rheumatologic conditions. These include uveitis, arthritis, and arthralgias; osteopenia and osteoporosis; psoriasiform skin lesions; and thromboembolism. It’s not unusual for these extraintestinal manifestations to bring a patient to a nongastroenterologist before the diagnosis of IBD has been made.
Also, gastroenterologists and rheumatologists use a lot of the same drugs.
“The only drug gastroenterologists use for IBD that didn’t come from rheumatology first is vedolizumab [Entyvio],” she observed.
Ustekinumab (Stelara), which Dr. Mahadevan and other IBD experts have been using off-label for refractory Crohn’s disease with what she termed “very good” results, is expected to receive an Food and Drug Administration indication for Crohn’s disease by year’s end on the strength of impressive phase III data reported last fall.
“When approved, ustekinumab should be a first-line therapy, parallel to the TNF [tumor necrosis factor] inhibitors,” she predicted.
Another reason it’s worthwhile for rheumatologists to be up to date on IBD is that in many parts of the country, some gastroenterologists in community practice are uncomfortable using TNF inhibitors, which are now standard therapy for IBD. They may refer those patients to their local rheumatologist. But be advised: the dosing is very different from in rheumatology.
“For example, you start adalimumab [Humira] at 40 mg every other week for rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis, but we [gastroenterologists] do a loading dose of four 40-mg injections on day 1, then 80 mg 2 weeks later, and then maintenance dosing at 40 mg every 2 weeks. Sometimes in our patient population you’re not going to get a response unless you use a high dose like that,” the gastroenterologist explained.
The incidence of IBD is climbing. It was estimated at 5-7 cases per 100,000 population in the late 1990s, but it’s now believed to be 7-12 per 100,000. The peak age of onset is still in 15- to 30-year-olds, but now physicians are seeing a strong second peak later in life, among individuals in their 60s and 70s. And while Caucasians and Ashkenazi Jews have traditionally been thought of as the groups at increased risk for IBD, treatment centers are now springing up in India, China, and other countries where IBD was formerly thought to be less common.
“What is happening? Is it that because of better medical care we’re now picking up more cases outside of North America and Europe? Or is it a change in the environment – the microbiome, the Westernized diet, pollution? We really don’t know. But this is where a lot of study is going,” according to Dr. Mahadevan.
Something nongastroenterologists need to know about the clinical care of IBD patients is that even though TNF inhibitors are now considered standard therapy for patients with more severe disease and these biologic agents are routinely used in IBD centers of excellence, their penetration into community practice has been slow. Although high-dose anti-TNF therapy is supposed to be employed in ulcerative colitis patients who are refractory to corticosteroid induction therapy or who are steroid dependent, it’s fairly common in some parts of the country for a patient to undergo colectomy without ever having received an anti-TNF biologic.
“If you’re in a surgery-dominated area, the surgeons were very influenced by a Cleveland Clinic paper that said patients who received infliximab had more complications after surgery. There were a lot of caveats to that paper, but colorectal surgeons don’t want you to use a TNF inhibitor if they feel the patient is going to surgery,” she said.
In addition to mongersen, other novel agents targeting new pathways in IBD include the oral Janus-associated kinase inhibitor tofacitinib (Xeljanz), under study for both ulcerative colitis and Crohn’s disease; ozanimod, an oral sphingosine 1–phosphate 1 and 5 receptor modulator in clinical trials for ulcerative colitis; and the humanized monoclonal antibody etrolizumab, a selective anti-integrin agent.
Dr. Mahadevan reported serving as a consultant to and/or research grant recipient from more than half a dozen pharmaceutical companies.
*Changes were made to this story on March 24, 2016.
EXPERT ANALYSIS FROM RWCS 2016
High gluten consumption early in life upped risk of celiac disease
Children who were genetically susceptible to celiac disease and consumed high amounts of gluten at 12 months of age were at least twice as likely to develop the autoimmune disorder as genetically predisposed children who consumed less gluten, researchers reported in the March issue of Clinical Gastroenterology and Hepatology.
The association was similar among children who carried any of the major human leukocyte antigen (HLA) risk genotypes for celiac disease, said Dr. Carin Aronsson at Lund University in Sweden and her associates. “Because these HLA risk genotypes are widely distributed in the general population, these findings may have consequence for future infant feeding recommendations,” they said. They recommended repeating the study in other countries to confirm the link.
In order to develop celiac disease, patients must consume gluten and carry at least one of the relevant DR3-DQ2 and DR4-DQ8 HLA risk haplotypes. But because gluten is widely consumed in products containing wheat, rye, and barley, and because about half of whites have at least one of the two haplotypes, gluten intolerance probably depends on other environmental factors, the researchers said. To further study these factors, they compared 3-day food diaries collected at ages 9, 12, 18, and 24 months for 146 children with positive tissue transglutaminase autoantibody (tTGA) assays and biopsy-confirmed celiac disease (cases) and 436 tTGA-negative children (controls). Cases and controls were matched by age, sex, and HLA genotype (Clin Gastroenterol Hepatol. 2015 Oct 7. doi: 10.1016/j.cgh.2015.09.030).
The food diaries revealed higher gluten intake among cases, compared with controls, beginning at the age of 12 months, said the researchers. Notably, cases consumed a median of 4.9 g of gluten a day before tTGA seroconversion, 1 g more than the median amount for controls of the same age (odds ratio, 1.3; 95% confidence interval, 1.1-1.5; P = .0002). Furthermore, significantly more cases than controls consumed the highest tertile of gluten, more than 5 g per day, before seroconversion (OR, 2.7; 95% CI, 1.7-4.1; P less than .0001). These associations were similar among children of all haplotype profiles and trended in the same direction among children with and without first-degree relatives with celiac disease.
Cases and controls resembled each other in terms of breastfeeding duration, age at first introduction to gluten, and total daily caloric intake, the investigators noted. “The prospective design of this birth cohort study enabled us to obtain the diet information before seroconversion of tTGA as a marker of celiac disease,” they said. “This eliminated the risk of reporting biases or a change in feeding habits because of the knowledge of serology results or disease status.” But they did not analyze the number of daily servings of foods that contained gluten. “We cannot exclude the possibility that the number of portions given frequently during the course of the day may have different effects on disease risk,” they said.
The National Institutes of Health, Juvenile Diabetes Research Foundation, and the Centers for Disease Control and Prevention funded the study. The investigators had no disclosures.
Source: American Gastroenterological Association
Long-suffering Swedish children probably have the highest rate of celiac disease in the world. This rate has dramatically increased. Why and why not? Previous studies have shown that it is not breastfeeding. It is not age or timing of introduction of gluten. It is not likely to be infections. This study shows that it is the amount of gluten that drives children with the highest genetic risk for celiac disease to develop the disease early in life. This conversion is preceded by a high intake of gluten. While these results alone should not determine general infant feeding practices, it suggests that if you are a Swedish child who carries these high-risk genes, high quantities of gluten early in life are not for you.
This study also raises the question of the effect high-dose gluten in adults at risk. Previously, studies have shown that the prevalence of celiac disease in adults in Sweden is not much different from the pediatric population. This study needs to be expanded to other Western populations where the rate of celiac disease is not so high. While nutritional engineering on a grand scale should not be undertaken lightly given the possibility of unexpected consequences, it behooves at least the Swedish population to perhaps reexamine their cultural practices of incorporating high gluten-containing cereals early in the lives of children, most especially those at particular risk for celiac disease.
Dr. Joseph A. Murray, AGAF, is professor of medicine, consultant, division of gastroenterology and hepatology, and department of immunology, and director of the Celiac Disease Program at the Mayo Clinic, Rochester, Minn.
Long-suffering Swedish children probably have the highest rate of celiac disease in the world. This rate has dramatically increased. Why and why not? Previous studies have shown that it is not breastfeeding. It is not age or timing of introduction of gluten. It is not likely to be infections. This study shows that it is the amount of gluten that drives children with the highest genetic risk for celiac disease to develop the disease early in life. This conversion is preceded by a high intake of gluten. While these results alone should not determine general infant feeding practices, it suggests that if you are a Swedish child who carries these high-risk genes, high quantities of gluten early in life are not for you.
This study also raises the question of the effect high-dose gluten in adults at risk. Previously, studies have shown that the prevalence of celiac disease in adults in Sweden is not much different from the pediatric population. This study needs to be expanded to other Western populations where the rate of celiac disease is not so high. While nutritional engineering on a grand scale should not be undertaken lightly given the possibility of unexpected consequences, it behooves at least the Swedish population to perhaps reexamine their cultural practices of incorporating high gluten-containing cereals early in the lives of children, most especially those at particular risk for celiac disease.
Dr. Joseph A. Murray, AGAF, is professor of medicine, consultant, division of gastroenterology and hepatology, and department of immunology, and director of the Celiac Disease Program at the Mayo Clinic, Rochester, Minn.
Long-suffering Swedish children probably have the highest rate of celiac disease in the world. This rate has dramatically increased. Why and why not? Previous studies have shown that it is not breastfeeding. It is not age or timing of introduction of gluten. It is not likely to be infections. This study shows that it is the amount of gluten that drives children with the highest genetic risk for celiac disease to develop the disease early in life. This conversion is preceded by a high intake of gluten. While these results alone should not determine general infant feeding practices, it suggests that if you are a Swedish child who carries these high-risk genes, high quantities of gluten early in life are not for you.
This study also raises the question of the effect high-dose gluten in adults at risk. Previously, studies have shown that the prevalence of celiac disease in adults in Sweden is not much different from the pediatric population. This study needs to be expanded to other Western populations where the rate of celiac disease is not so high. While nutritional engineering on a grand scale should not be undertaken lightly given the possibility of unexpected consequences, it behooves at least the Swedish population to perhaps reexamine their cultural practices of incorporating high gluten-containing cereals early in the lives of children, most especially those at particular risk for celiac disease.
Dr. Joseph A. Murray, AGAF, is professor of medicine, consultant, division of gastroenterology and hepatology, and department of immunology, and director of the Celiac Disease Program at the Mayo Clinic, Rochester, Minn.
Children who were genetically susceptible to celiac disease and consumed high amounts of gluten at 12 months of age were at least twice as likely to develop the autoimmune disorder as genetically predisposed children who consumed less gluten, researchers reported in the March issue of Clinical Gastroenterology and Hepatology.
The association was similar among children who carried any of the major human leukocyte antigen (HLA) risk genotypes for celiac disease, said Dr. Carin Aronsson at Lund University in Sweden and her associates. “Because these HLA risk genotypes are widely distributed in the general population, these findings may have consequence for future infant feeding recommendations,” they said. They recommended repeating the study in other countries to confirm the link.
In order to develop celiac disease, patients must consume gluten and carry at least one of the relevant DR3-DQ2 and DR4-DQ8 HLA risk haplotypes. But because gluten is widely consumed in products containing wheat, rye, and barley, and because about half of whites have at least one of the two haplotypes, gluten intolerance probably depends on other environmental factors, the researchers said. To further study these factors, they compared 3-day food diaries collected at ages 9, 12, 18, and 24 months for 146 children with positive tissue transglutaminase autoantibody (tTGA) assays and biopsy-confirmed celiac disease (cases) and 436 tTGA-negative children (controls). Cases and controls were matched by age, sex, and HLA genotype (Clin Gastroenterol Hepatol. 2015 Oct 7. doi: 10.1016/j.cgh.2015.09.030).
The food diaries revealed higher gluten intake among cases, compared with controls, beginning at the age of 12 months, said the researchers. Notably, cases consumed a median of 4.9 g of gluten a day before tTGA seroconversion, 1 g more than the median amount for controls of the same age (odds ratio, 1.3; 95% confidence interval, 1.1-1.5; P = .0002). Furthermore, significantly more cases than controls consumed the highest tertile of gluten, more than 5 g per day, before seroconversion (OR, 2.7; 95% CI, 1.7-4.1; P less than .0001). These associations were similar among children of all haplotype profiles and trended in the same direction among children with and without first-degree relatives with celiac disease.
Cases and controls resembled each other in terms of breastfeeding duration, age at first introduction to gluten, and total daily caloric intake, the investigators noted. “The prospective design of this birth cohort study enabled us to obtain the diet information before seroconversion of tTGA as a marker of celiac disease,” they said. “This eliminated the risk of reporting biases or a change in feeding habits because of the knowledge of serology results or disease status.” But they did not analyze the number of daily servings of foods that contained gluten. “We cannot exclude the possibility that the number of portions given frequently during the course of the day may have different effects on disease risk,” they said.
The National Institutes of Health, Juvenile Diabetes Research Foundation, and the Centers for Disease Control and Prevention funded the study. The investigators had no disclosures.
Source: American Gastroenterological Association
Children who were genetically susceptible to celiac disease and consumed high amounts of gluten at 12 months of age were at least twice as likely to develop the autoimmune disorder as genetically predisposed children who consumed less gluten, researchers reported in the March issue of Clinical Gastroenterology and Hepatology.
The association was similar among children who carried any of the major human leukocyte antigen (HLA) risk genotypes for celiac disease, said Dr. Carin Aronsson at Lund University in Sweden and her associates. “Because these HLA risk genotypes are widely distributed in the general population, these findings may have consequence for future infant feeding recommendations,” they said. They recommended repeating the study in other countries to confirm the link.
In order to develop celiac disease, patients must consume gluten and carry at least one of the relevant DR3-DQ2 and DR4-DQ8 HLA risk haplotypes. But because gluten is widely consumed in products containing wheat, rye, and barley, and because about half of whites have at least one of the two haplotypes, gluten intolerance probably depends on other environmental factors, the researchers said. To further study these factors, they compared 3-day food diaries collected at ages 9, 12, 18, and 24 months for 146 children with positive tissue transglutaminase autoantibody (tTGA) assays and biopsy-confirmed celiac disease (cases) and 436 tTGA-negative children (controls). Cases and controls were matched by age, sex, and HLA genotype (Clin Gastroenterol Hepatol. 2015 Oct 7. doi: 10.1016/j.cgh.2015.09.030).
The food diaries revealed higher gluten intake among cases, compared with controls, beginning at the age of 12 months, said the researchers. Notably, cases consumed a median of 4.9 g of gluten a day before tTGA seroconversion, 1 g more than the median amount for controls of the same age (odds ratio, 1.3; 95% confidence interval, 1.1-1.5; P = .0002). Furthermore, significantly more cases than controls consumed the highest tertile of gluten, more than 5 g per day, before seroconversion (OR, 2.7; 95% CI, 1.7-4.1; P less than .0001). These associations were similar among children of all haplotype profiles and trended in the same direction among children with and without first-degree relatives with celiac disease.
Cases and controls resembled each other in terms of breastfeeding duration, age at first introduction to gluten, and total daily caloric intake, the investigators noted. “The prospective design of this birth cohort study enabled us to obtain the diet information before seroconversion of tTGA as a marker of celiac disease,” they said. “This eliminated the risk of reporting biases or a change in feeding habits because of the knowledge of serology results or disease status.” But they did not analyze the number of daily servings of foods that contained gluten. “We cannot exclude the possibility that the number of portions given frequently during the course of the day may have different effects on disease risk,” they said.
The National Institutes of Health, Juvenile Diabetes Research Foundation, and the Centers for Disease Control and Prevention funded the study. The investigators had no disclosures.
Source: American Gastroenterological Association
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: High levels of gluten consumption in early life significantly increased the risk of celiac disease.
Major finding: The odds of celiac disease were more than twice as high among children who consumed more than 5 g of gluten a day, compared with those who consumed less gluten (OR, 2.65; P less than .0001).
Data source: A 1 to 3 matched nested case-control study of 146 children with biopsy-confirmed celiac disease (cases) and 436 tissue transglutaminase (tTGA)-negative controls.
Disclosures: The National Institutes of Health, Juvenile Diabetes Research Foundation, and the Centers for Disease Control and Prevention funded the study. The investigators had no disclosures.
Mirtazapine improves functional dyspepsia in small study
The antidepressant mirtazapine improved weight loss, early satiation, nausea, and other signs and symptoms in patients with functional dyspepsia, said the authors of a placebo-controlled pilot study published in the March issue of Clinical Gastroenterology and Hepatology.
The findings suggest that mirtazapine “has the potential to become the treatment of choice for functional dyspepsia in patients with weight loss, and evaluation in larger multicenter studies is warranted,” said Dr. Jan Tack and his associates at the University of Leuven, Belgium.
Functional dyspepsia, one of the most prevalent gastrointestinal disorders, is characterized by early satiation, postprandial fullness, and epigastric pain and burning in the absence of underlying systemic or metabolic disease. Up to 40% of affected patients lose weight, an “alarm symptom” that until now has lacked effective treatment, the researchers said.
Mirtazapine, an antagonist of the H1, alpha2, 5-hydroxytryptamine (5-HT)2c, and 5-HT3 receptors, often causes weight gain when used to treat depression. Therefore, the investigators designed a double-blind single-center pilot trial of 34 patients with functional dyspepsia who had lost more than 10% of their original body weight. After a 2-week run-in period, half the patients were randomized to 15 mg of mirtazapine every evening and the other half to placebo (Clin Gastroenterol Hepatol. 2016 Jan 9. doi: 10.1016/j.cgh.2015.09.043).
The average weight of placebo patients remained almost unchanged throughout the trial, while patients on mirtazapine gained an average of 2.5 + 0.6 kg by week 4 (P = .003 for between-group comparison) and 3.9 + 0.7 kg, or 6.4% of their original body weight, by week 8 (P less than .0001). Mean scores on a validated dyspepsia symptom severity (DSS) questionnaire improved significantly between baseline and weeks 4 (P = .003) and 8 (P = .017) for mirtazapine but not placebo. Directly comparing the two groups in terms of the DSS revealed a large effect size that trended toward significance (P = .06) at week 4 but not at week 8 (P = .55). However, mirtazapine significantly outperformed placebo in measures of early satiety, quality of life, gastrointestinal-specific anxiety, and nutrient tolerance, “mostly with large effect sizes,” the investigators said.
Mirtazapine did not affect epigastric pain or gastric emptying, and had little effect on postprandial fullness. Moreover, 2 of 17 patients in the mirtazapine group dropped out of the study because of unacceptable levels of drowsiness, which is a common side effect of the medication.
Many patients with functional dyspepsia respond inadequately to first-line treatment with acid-suppressive or prokinetic drugs, the investigators noted. While tegaserod, buspirone, and acotiamide can improve gastric accommodation, it is unknown if they promote weight gain. The results for mirtazapine are promising, but the pilot trial included only tertiary care patients, and the small sample size precluded separate analyses of patients with postprandial distress syndrome as opposed to epigastric pain syndrome, the researchers said.
The study was funded by Leuven University, the FWO, and the KU Leuven Special Research Fund. Mirtazapine and placebo were supplied by MSD Belgium. The investigators had no disclosures.
The antidepressant mirtazapine improved weight loss, early satiation, nausea, and other signs and symptoms in patients with functional dyspepsia, said the authors of a placebo-controlled pilot study published in the March issue of Clinical Gastroenterology and Hepatology.
The findings suggest that mirtazapine “has the potential to become the treatment of choice for functional dyspepsia in patients with weight loss, and evaluation in larger multicenter studies is warranted,” said Dr. Jan Tack and his associates at the University of Leuven, Belgium.
Functional dyspepsia, one of the most prevalent gastrointestinal disorders, is characterized by early satiation, postprandial fullness, and epigastric pain and burning in the absence of underlying systemic or metabolic disease. Up to 40% of affected patients lose weight, an “alarm symptom” that until now has lacked effective treatment, the researchers said.
Mirtazapine, an antagonist of the H1, alpha2, 5-hydroxytryptamine (5-HT)2c, and 5-HT3 receptors, often causes weight gain when used to treat depression. Therefore, the investigators designed a double-blind single-center pilot trial of 34 patients with functional dyspepsia who had lost more than 10% of their original body weight. After a 2-week run-in period, half the patients were randomized to 15 mg of mirtazapine every evening and the other half to placebo (Clin Gastroenterol Hepatol. 2016 Jan 9. doi: 10.1016/j.cgh.2015.09.043).
The average weight of placebo patients remained almost unchanged throughout the trial, while patients on mirtazapine gained an average of 2.5 + 0.6 kg by week 4 (P = .003 for between-group comparison) and 3.9 + 0.7 kg, or 6.4% of their original body weight, by week 8 (P less than .0001). Mean scores on a validated dyspepsia symptom severity (DSS) questionnaire improved significantly between baseline and weeks 4 (P = .003) and 8 (P = .017) for mirtazapine but not placebo. Directly comparing the two groups in terms of the DSS revealed a large effect size that trended toward significance (P = .06) at week 4 but not at week 8 (P = .55). However, mirtazapine significantly outperformed placebo in measures of early satiety, quality of life, gastrointestinal-specific anxiety, and nutrient tolerance, “mostly with large effect sizes,” the investigators said.
Mirtazapine did not affect epigastric pain or gastric emptying, and had little effect on postprandial fullness. Moreover, 2 of 17 patients in the mirtazapine group dropped out of the study because of unacceptable levels of drowsiness, which is a common side effect of the medication.
Many patients with functional dyspepsia respond inadequately to first-line treatment with acid-suppressive or prokinetic drugs, the investigators noted. While tegaserod, buspirone, and acotiamide can improve gastric accommodation, it is unknown if they promote weight gain. The results for mirtazapine are promising, but the pilot trial included only tertiary care patients, and the small sample size precluded separate analyses of patients with postprandial distress syndrome as opposed to epigastric pain syndrome, the researchers said.
The study was funded by Leuven University, the FWO, and the KU Leuven Special Research Fund. Mirtazapine and placebo were supplied by MSD Belgium. The investigators had no disclosures.
The antidepressant mirtazapine improved weight loss, early satiation, nausea, and other signs and symptoms in patients with functional dyspepsia, said the authors of a placebo-controlled pilot study published in the March issue of Clinical Gastroenterology and Hepatology.
The findings suggest that mirtazapine “has the potential to become the treatment of choice for functional dyspepsia in patients with weight loss, and evaluation in larger multicenter studies is warranted,” said Dr. Jan Tack and his associates at the University of Leuven, Belgium.
Functional dyspepsia, one of the most prevalent gastrointestinal disorders, is characterized by early satiation, postprandial fullness, and epigastric pain and burning in the absence of underlying systemic or metabolic disease. Up to 40% of affected patients lose weight, an “alarm symptom” that until now has lacked effective treatment, the researchers said.
Mirtazapine, an antagonist of the H1, alpha2, 5-hydroxytryptamine (5-HT)2c, and 5-HT3 receptors, often causes weight gain when used to treat depression. Therefore, the investigators designed a double-blind single-center pilot trial of 34 patients with functional dyspepsia who had lost more than 10% of their original body weight. After a 2-week run-in period, half the patients were randomized to 15 mg of mirtazapine every evening and the other half to placebo (Clin Gastroenterol Hepatol. 2016 Jan 9. doi: 10.1016/j.cgh.2015.09.043).
The average weight of placebo patients remained almost unchanged throughout the trial, while patients on mirtazapine gained an average of 2.5 + 0.6 kg by week 4 (P = .003 for between-group comparison) and 3.9 + 0.7 kg, or 6.4% of their original body weight, by week 8 (P less than .0001). Mean scores on a validated dyspepsia symptom severity (DSS) questionnaire improved significantly between baseline and weeks 4 (P = .003) and 8 (P = .017) for mirtazapine but not placebo. Directly comparing the two groups in terms of the DSS revealed a large effect size that trended toward significance (P = .06) at week 4 but not at week 8 (P = .55). However, mirtazapine significantly outperformed placebo in measures of early satiety, quality of life, gastrointestinal-specific anxiety, and nutrient tolerance, “mostly with large effect sizes,” the investigators said.
Mirtazapine did not affect epigastric pain or gastric emptying, and had little effect on postprandial fullness. Moreover, 2 of 17 patients in the mirtazapine group dropped out of the study because of unacceptable levels of drowsiness, which is a common side effect of the medication.
Many patients with functional dyspepsia respond inadequately to first-line treatment with acid-suppressive or prokinetic drugs, the investigators noted. While tegaserod, buspirone, and acotiamide can improve gastric accommodation, it is unknown if they promote weight gain. The results for mirtazapine are promising, but the pilot trial included only tertiary care patients, and the small sample size precluded separate analyses of patients with postprandial distress syndrome as opposed to epigastric pain syndrome, the researchers said.
The study was funded by Leuven University, the FWO, and the KU Leuven Special Research Fund. Mirtazapine and placebo were supplied by MSD Belgium. The investigators had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Mirtazapine treatment led to weight gain and a number of other improvements among patients with functional dyspepsia and weight loss.
Major finding: Patients regained an average of 6.5% of their original body weight on mirtazapine, and did not regain weight on placebo.
Data source: A single-center randomized double-blind study of 34 patients with functional dyspepsia.
Disclosures: Leuven University, the FWO, and the KU Leuven Special Research Fund helped fund the study. Mirtazapine and placebo were supplied by MSD Belgium. The investigators had no disclosures.
Biosimilar infliximab gains FDA Advisory Committee endorsement
A biosimilar agent to Remicade, the brand-name and reference form of infliximab, stayed on track to become the second biosimilar drug to enter the U.S. market when the Arthritis Advisory Committee of the Food and Drug Administration voted overwhelmingly in favor of licensure of the biosimilar at a meeting on Feb. 9.
The vote was 21 in favor and 3 against, with no abstentions.
Because of the way the FDA staff worded the question that the Advisory Committee voted on, the panel not only was in favor of approving biosimilar licensure but also recommended that license for six of the seven diverse indications that Remicade currently has: treatment of rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis, adult and pediatric Crohn’s disease, and adult ulcerative colitis. The panel did not vote on licensing the biosimilar for treatment of pediatric ulcerative colitis because that specific indication for Remicade remains on patent for a few more years.
The broad range of indications for which the Committee recommended approval was notable because the formulation of biosimilar infliximab under review, manufactured by Celltrion and known in the United States as CT-P13, had been clinically studied only in patients with rheumatoid arthritis or ankylosing spondylitis. The other four recommended indications represented extrapolations, based on the totality of biosimilar evidence presented at the meeting by both Celltrion staffers and consultants as well as analyses presented by FDA staff members.
The overall thrust of the extrapolation issue was that if biosimilarity to Remicade was proven by a range of preclinical and clinical testing, and if safety and efficacy similar to Remicade was shown in trials that enrolled only patients with rheumatoid arthritis or ankylosing spondylitis, then the safety and efficacy previously proven for Remicade for the other indications could be reasonably extrapolated to apply to CT-P13 also, even though CT-P13 was never tested on patients with those conditions. This turned out to often be the key issue that panel members grappled with as they decided whether to vote in favor of the question the FDA asked them to address.
“Many of us are uncomfortable with this new pathway” of extrapolation, said panel member Dr. Beth L. Jonas, a rheumatologist at the University of North Carolina at Chapel Hill.
“I feel we’re taking a risk” with the extrapolations, said Dr. Mary E. Maloney, professor of medicine and chief of dermatology at the University of Massachusetts in Worcester. “We have a responsibility to take a risk to provide biosimilars to patients and to reduce their cost” for needed treatments, she said during the Committee’s discussion of their votes.
“Biosimilar is a new concept, but it’s the future of how we will look at drugs,” explained panel member Dr. Wilma Bergfeld, professor of dermatology at the Cleveland Clinic.
CT-P13 is currently marketed in many other countries worldwide under the brand names Remsima or Inflectra.
The FDA’s staff was clearly behind this application. After summarizing the agency’s internal analysis of the data submitted by Celltrion, Dr. Nikolay Nikolov, clinical team leader for the FDA’s Division of Pulmonary, Allergy and Rheumatology Products, concluded that “the totality of evidence provided by the applicant supports a conclusion that CT-P13 is biosimilar to U.S.-licensed Remicade,” and that “scientific justification for extrapolating the clinical data supports a finding of biosimilarity for all indications for which U.S.-licensed Remicade is licensed.” The FDA’s position makes it seem very likely that the agency will accept the Advisory Committee’s vote and grant CT-P13 license for U.S. marketing in the near future.
CT-P13 also received support during the public comment period of the Committee’s deliberations. At that time, Dr. Gideon P. Smith, a dermatologist at Massachusetts General Hospital in Boston spoke on behalf of the American Academy of Dermatology Association. “Biologics are some of the most important recent developments in treating plaque psoriasis, but cost is an important issue. We hope that biosimilars will decrease the cost of this treatment,” Dr. Smith said. “Infliximab is a complex molecule with a complex production process. We are concerned about the safety and efficacy of treatment. The AADA supports approval based on reducing cost and improving patient access. However, we strongly recommend caution through long-term postmarketing surveillance and using registry data to identify issues of immunogenicity, efficacy, and safety that were not seen in the clinical trials.”
The drug also received support from Dr. Angus B. Worthing, who represented the American College of Rheumatology. “Biosimilars may be the only tool to keep prices of biologics within reason,” said Dr. Worthing, a rheumatologist in Washington. But he also stressed that “extrapolation should be done with caution and not routinely granted.”
CT-P13 has the potential to make a fairly widely used biologic significantly more affordable. In countries where it has come onto the market, it’s been priced at roughly 30% below the prevailing cost of Remicade prior to this competition.
“Infliximab is an extremely important tool in our armamentarium for treatment of both ulcerative colitis and Crohn’s disease,” commented Dr. Stephen B. Hanauer, professor of gastroenterology and hepatology at Northwestern University in Chicago. “Biologic therapies account for an increasing proportion of health care costs for chronic diseases such as inflammatory bowel disease and reducing these costs will be important as increasing numbers of patients are benefiting from long-term biologic therapies. Having reviewed the extensive preclinical and clinical data with CT-P13, I am comfortable with potential substitution or switching as long as physicians are aware of the change and can track any potential reactions to the administered product,” he said in an interview.
“Infliximab is currently used by U.S. rheumatologists to treat certain patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. It is not the most-widely used tumor necrosis factor inhibitor, which is adalimumab, but it is often used. After FDA approval, biosimilar infliximab is anticipated to be priced lower than Remicade and that would likely increase use of infliximab for rheumatologic conditions,” said Dr. Jonathan Kay, a rheumatologist and professor of medicine at the University of Massachusetts in Worcester. “The clinical experience with CT-P13 in trials and in routine use in other countries show no significant loss of efficacy or any other major problem when changing patients from Remicade to CT-P13. All the data suggest that CT-P13 is highly similar to the reference product. It’s almost akin to comparing one lot of Remicade to another lot of Remicade. I personally would not have a problem initiating a patient on CT-P13 if infliximab was the appropriate drug to use,” Dr. Kay said in an interview.
Dr. Hanauer has been a consultant to Celltrion. Dr. Kay has been a consultant to several drug companies.
On Twitter @mitchelzoler
A biosimilar agent to Remicade, the brand-name and reference form of infliximab, stayed on track to become the second biosimilar drug to enter the U.S. market when the Arthritis Advisory Committee of the Food and Drug Administration voted overwhelmingly in favor of licensure of the biosimilar at a meeting on Feb. 9.
The vote was 21 in favor and 3 against, with no abstentions.
Because of the way the FDA staff worded the question that the Advisory Committee voted on, the panel not only was in favor of approving biosimilar licensure but also recommended that license for six of the seven diverse indications that Remicade currently has: treatment of rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis, adult and pediatric Crohn’s disease, and adult ulcerative colitis. The panel did not vote on licensing the biosimilar for treatment of pediatric ulcerative colitis because that specific indication for Remicade remains on patent for a few more years.
The broad range of indications for which the Committee recommended approval was notable because the formulation of biosimilar infliximab under review, manufactured by Celltrion and known in the United States as CT-P13, had been clinically studied only in patients with rheumatoid arthritis or ankylosing spondylitis. The other four recommended indications represented extrapolations, based on the totality of biosimilar evidence presented at the meeting by both Celltrion staffers and consultants as well as analyses presented by FDA staff members.
The overall thrust of the extrapolation issue was that if biosimilarity to Remicade was proven by a range of preclinical and clinical testing, and if safety and efficacy similar to Remicade was shown in trials that enrolled only patients with rheumatoid arthritis or ankylosing spondylitis, then the safety and efficacy previously proven for Remicade for the other indications could be reasonably extrapolated to apply to CT-P13 also, even though CT-P13 was never tested on patients with those conditions. This turned out to often be the key issue that panel members grappled with as they decided whether to vote in favor of the question the FDA asked them to address.
“Many of us are uncomfortable with this new pathway” of extrapolation, said panel member Dr. Beth L. Jonas, a rheumatologist at the University of North Carolina at Chapel Hill.
“I feel we’re taking a risk” with the extrapolations, said Dr. Mary E. Maloney, professor of medicine and chief of dermatology at the University of Massachusetts in Worcester. “We have a responsibility to take a risk to provide biosimilars to patients and to reduce their cost” for needed treatments, she said during the Committee’s discussion of their votes.
“Biosimilar is a new concept, but it’s the future of how we will look at drugs,” explained panel member Dr. Wilma Bergfeld, professor of dermatology at the Cleveland Clinic.
CT-P13 is currently marketed in many other countries worldwide under the brand names Remsima or Inflectra.
The FDA’s staff was clearly behind this application. After summarizing the agency’s internal analysis of the data submitted by Celltrion, Dr. Nikolay Nikolov, clinical team leader for the FDA’s Division of Pulmonary, Allergy and Rheumatology Products, concluded that “the totality of evidence provided by the applicant supports a conclusion that CT-P13 is biosimilar to U.S.-licensed Remicade,” and that “scientific justification for extrapolating the clinical data supports a finding of biosimilarity for all indications for which U.S.-licensed Remicade is licensed.” The FDA’s position makes it seem very likely that the agency will accept the Advisory Committee’s vote and grant CT-P13 license for U.S. marketing in the near future.
CT-P13 also received support during the public comment period of the Committee’s deliberations. At that time, Dr. Gideon P. Smith, a dermatologist at Massachusetts General Hospital in Boston spoke on behalf of the American Academy of Dermatology Association. “Biologics are some of the most important recent developments in treating plaque psoriasis, but cost is an important issue. We hope that biosimilars will decrease the cost of this treatment,” Dr. Smith said. “Infliximab is a complex molecule with a complex production process. We are concerned about the safety and efficacy of treatment. The AADA supports approval based on reducing cost and improving patient access. However, we strongly recommend caution through long-term postmarketing surveillance and using registry data to identify issues of immunogenicity, efficacy, and safety that were not seen in the clinical trials.”
The drug also received support from Dr. Angus B. Worthing, who represented the American College of Rheumatology. “Biosimilars may be the only tool to keep prices of biologics within reason,” said Dr. Worthing, a rheumatologist in Washington. But he also stressed that “extrapolation should be done with caution and not routinely granted.”
CT-P13 has the potential to make a fairly widely used biologic significantly more affordable. In countries where it has come onto the market, it’s been priced at roughly 30% below the prevailing cost of Remicade prior to this competition.
“Infliximab is an extremely important tool in our armamentarium for treatment of both ulcerative colitis and Crohn’s disease,” commented Dr. Stephen B. Hanauer, professor of gastroenterology and hepatology at Northwestern University in Chicago. “Biologic therapies account for an increasing proportion of health care costs for chronic diseases such as inflammatory bowel disease and reducing these costs will be important as increasing numbers of patients are benefiting from long-term biologic therapies. Having reviewed the extensive preclinical and clinical data with CT-P13, I am comfortable with potential substitution or switching as long as physicians are aware of the change and can track any potential reactions to the administered product,” he said in an interview.
“Infliximab is currently used by U.S. rheumatologists to treat certain patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. It is not the most-widely used tumor necrosis factor inhibitor, which is adalimumab, but it is often used. After FDA approval, biosimilar infliximab is anticipated to be priced lower than Remicade and that would likely increase use of infliximab for rheumatologic conditions,” said Dr. Jonathan Kay, a rheumatologist and professor of medicine at the University of Massachusetts in Worcester. “The clinical experience with CT-P13 in trials and in routine use in other countries show no significant loss of efficacy or any other major problem when changing patients from Remicade to CT-P13. All the data suggest that CT-P13 is highly similar to the reference product. It’s almost akin to comparing one lot of Remicade to another lot of Remicade. I personally would not have a problem initiating a patient on CT-P13 if infliximab was the appropriate drug to use,” Dr. Kay said in an interview.
Dr. Hanauer has been a consultant to Celltrion. Dr. Kay has been a consultant to several drug companies.
On Twitter @mitchelzoler
A biosimilar agent to Remicade, the brand-name and reference form of infliximab, stayed on track to become the second biosimilar drug to enter the U.S. market when the Arthritis Advisory Committee of the Food and Drug Administration voted overwhelmingly in favor of licensure of the biosimilar at a meeting on Feb. 9.
The vote was 21 in favor and 3 against, with no abstentions.
Because of the way the FDA staff worded the question that the Advisory Committee voted on, the panel not only was in favor of approving biosimilar licensure but also recommended that license for six of the seven diverse indications that Remicade currently has: treatment of rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis, adult and pediatric Crohn’s disease, and adult ulcerative colitis. The panel did not vote on licensing the biosimilar for treatment of pediatric ulcerative colitis because that specific indication for Remicade remains on patent for a few more years.
The broad range of indications for which the Committee recommended approval was notable because the formulation of biosimilar infliximab under review, manufactured by Celltrion and known in the United States as CT-P13, had been clinically studied only in patients with rheumatoid arthritis or ankylosing spondylitis. The other four recommended indications represented extrapolations, based on the totality of biosimilar evidence presented at the meeting by both Celltrion staffers and consultants as well as analyses presented by FDA staff members.
The overall thrust of the extrapolation issue was that if biosimilarity to Remicade was proven by a range of preclinical and clinical testing, and if safety and efficacy similar to Remicade was shown in trials that enrolled only patients with rheumatoid arthritis or ankylosing spondylitis, then the safety and efficacy previously proven for Remicade for the other indications could be reasonably extrapolated to apply to CT-P13 also, even though CT-P13 was never tested on patients with those conditions. This turned out to often be the key issue that panel members grappled with as they decided whether to vote in favor of the question the FDA asked them to address.
“Many of us are uncomfortable with this new pathway” of extrapolation, said panel member Dr. Beth L. Jonas, a rheumatologist at the University of North Carolina at Chapel Hill.
“I feel we’re taking a risk” with the extrapolations, said Dr. Mary E. Maloney, professor of medicine and chief of dermatology at the University of Massachusetts in Worcester. “We have a responsibility to take a risk to provide biosimilars to patients and to reduce their cost” for needed treatments, she said during the Committee’s discussion of their votes.
“Biosimilar is a new concept, but it’s the future of how we will look at drugs,” explained panel member Dr. Wilma Bergfeld, professor of dermatology at the Cleveland Clinic.
CT-P13 is currently marketed in many other countries worldwide under the brand names Remsima or Inflectra.
The FDA’s staff was clearly behind this application. After summarizing the agency’s internal analysis of the data submitted by Celltrion, Dr. Nikolay Nikolov, clinical team leader for the FDA’s Division of Pulmonary, Allergy and Rheumatology Products, concluded that “the totality of evidence provided by the applicant supports a conclusion that CT-P13 is biosimilar to U.S.-licensed Remicade,” and that “scientific justification for extrapolating the clinical data supports a finding of biosimilarity for all indications for which U.S.-licensed Remicade is licensed.” The FDA’s position makes it seem very likely that the agency will accept the Advisory Committee’s vote and grant CT-P13 license for U.S. marketing in the near future.
CT-P13 also received support during the public comment period of the Committee’s deliberations. At that time, Dr. Gideon P. Smith, a dermatologist at Massachusetts General Hospital in Boston spoke on behalf of the American Academy of Dermatology Association. “Biologics are some of the most important recent developments in treating plaque psoriasis, but cost is an important issue. We hope that biosimilars will decrease the cost of this treatment,” Dr. Smith said. “Infliximab is a complex molecule with a complex production process. We are concerned about the safety and efficacy of treatment. The AADA supports approval based on reducing cost and improving patient access. However, we strongly recommend caution through long-term postmarketing surveillance and using registry data to identify issues of immunogenicity, efficacy, and safety that were not seen in the clinical trials.”
The drug also received support from Dr. Angus B. Worthing, who represented the American College of Rheumatology. “Biosimilars may be the only tool to keep prices of biologics within reason,” said Dr. Worthing, a rheumatologist in Washington. But he also stressed that “extrapolation should be done with caution and not routinely granted.”
CT-P13 has the potential to make a fairly widely used biologic significantly more affordable. In countries where it has come onto the market, it’s been priced at roughly 30% below the prevailing cost of Remicade prior to this competition.
“Infliximab is an extremely important tool in our armamentarium for treatment of both ulcerative colitis and Crohn’s disease,” commented Dr. Stephen B. Hanauer, professor of gastroenterology and hepatology at Northwestern University in Chicago. “Biologic therapies account for an increasing proportion of health care costs for chronic diseases such as inflammatory bowel disease and reducing these costs will be important as increasing numbers of patients are benefiting from long-term biologic therapies. Having reviewed the extensive preclinical and clinical data with CT-P13, I am comfortable with potential substitution or switching as long as physicians are aware of the change and can track any potential reactions to the administered product,” he said in an interview.
“Infliximab is currently used by U.S. rheumatologists to treat certain patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. It is not the most-widely used tumor necrosis factor inhibitor, which is adalimumab, but it is often used. After FDA approval, biosimilar infliximab is anticipated to be priced lower than Remicade and that would likely increase use of infliximab for rheumatologic conditions,” said Dr. Jonathan Kay, a rheumatologist and professor of medicine at the University of Massachusetts in Worcester. “The clinical experience with CT-P13 in trials and in routine use in other countries show no significant loss of efficacy or any other major problem when changing patients from Remicade to CT-P13. All the data suggest that CT-P13 is highly similar to the reference product. It’s almost akin to comparing one lot of Remicade to another lot of Remicade. I personally would not have a problem initiating a patient on CT-P13 if infliximab was the appropriate drug to use,” Dr. Kay said in an interview.
Dr. Hanauer has been a consultant to Celltrion. Dr. Kay has been a consultant to several drug companies.
On Twitter @mitchelzoler
Step therapy and biologics: An easier road ahead?
Laws recently passed or under consideration in state legislatures may offer some relief to physicians and patients dogged by the “step” or “fail-first” therapy protocols mandated by insurers, but until better clinical evidence is available to support treatment decisions and biosimilars reduce costs, clinicians must strategize to get patients through the step pathways as fast as possible.
Rheumatologists, gastroenterologists, and dermatologists all confront fail-first policies in their practices, particularly when prescribing the biologic agents that have been game changers in treating rheumatoid arthritis (RA), inflammatory bowel disease (IBD), and psoriasis, among other diseases.
In RA, for example, a patient might be required to fail a series of disease-modifying antirheumatic drugs (DMARDs), including methotrexate, before starting a biologic. In Crohn’s disease, patients might have to first fail on steroids and immunosuppressants.
Most clinicians consider cost concerns fair as a basis for insurance decisions. But they can also have strong rationales for making exceptions. This may mean starting patients on a biologic early, particularly those they deem unlikely to respond to first- or second-line treatments – which may be cheaper but are not necessarily safer.
In egregious cases, a patient already stable on a biologic who has changed insurance plans may be forced to go backwards in the treatment pathway, and fail first- and second-line therapies all over again before resuming – a process unlikely to be cost-effective in the long term, and also rife with ethical concerns, say clinicians.
“Making a patient fail to get a less toxic drug sort of violates our ‘do no harm’ principle,” Dr. Stephen B. Hanauer, medical director of the Digestive Health Center at Northwestern University, Chicago, said in an interview.
“I always say that if biologics cost a dollar, we’d be using them for everybody. If you take away the steroids and the immunosuppressants, these are very safe drugs for IBD, far safer than steroids – but steroids are cheap,” Dr. Hanauer said.
And with some debilitating disease presentations, such as severe Crohn’s, “being told that we have to try conventional therapies and the patient has to fail them can mean putting the patient through progression of their disease, and suffering,” Dr. David T. Rubin, codirector of the Digestive Diseases Center at the University of Chicago, said in an interview. “We really struggle with this.”
Dr. Joseph S. Eastern, a dermatologist practicing in Belleville, N.J., said his specialty faces similar challenges with step therapy. “Dermatologists as a group are pretty risk averse. When given the opportunity, we do an excellent job of prescribing conventional medications, ultraviolet therapy, and biologics in the most cost-efficient possible way,” he said in an email.
Yet “third-party payers tell us, for example, that a patient must fail methotrexate before we can use a biologic, when the whole advantage of biologic therapy for many of these patients is the avoidance of organotoxicity and other serious risks.”
As a result, Dr. Eastern said, “I write a lot more vehement letters to payers about the biologics these days.”
Choice vs. cost
Rheumatologists are among the clinicians most affected by fail-first and step therapy mandates, as the diseases they treat – particularly RA – are the most established indications for biologic therapies, and for which the largest number of these are approved.
Though Medicare and Medicaid allow physicians considerable leeway, private insurers often tightly circumscribe the timing and choice of biologics in RA. As insurers’ first-choice biologic drug changes frequently, and varies from plan to plan, a patient who is stable on one agent might be asked to switch to another, a phenomenon known as nonmedical switching.
Insurers are seldom transparent about their reasons for establishing certain biologic drugs as their go-to agents, clinicians say, while making it difficult for physicians to prescribe others. “There’s a tremendous amount of discounting going on that we are oblivious to as physicians. I’ve seen situations where drug A is the first one you have to use this month and the next month, drug C,” said Dr. Norman Gaylis, a rheumatologist practicing in Aventura, Fla.
Attempting to start a patient on a nonpreferential biologic will generate paperwork and delays, Dr. Gaylis said, which can cost patients valuable time. “There’s a window of opportunity to treat these diseases, and by creating a step therapy pathway we’re closing the window at least partially.”
“As an example, rituximab in most payer plans is not tiered as a first-line biologic treatment option despite the fact that there are frequent scenarios where the clinical and serological presentation of a patient would suggest it to be preferable as a first-line treatment choice over an anti-TNF [tumor necrosis factor],” Dr. Gaylis said.
“There is no room for clinical decision making based upon the unique presentation of each different patient when choosing the best treatment option,” he said.
Rheumatologists often become overwhelmed with authorization paperwork, “and still in many instances end up with a denial of their request.”
Dr. Karen Kolba, a rheumatologist in private practice in Santa Maria, Calif., said that she agreed in principle with the way step therapy protocols have been established, and that some of the frustration with step therapy amounts to a tendency among specialist clinicians to bristle at being told what to do.
“Physicians hate protocol,” Dr. Kolba said. “But comparing one protocol to another is the only way we are going to make advances.” It took the rheumatology community about 30 years to come to terms with the use of methotrexate in RA, she noted, and the stepped approach grew naturally from the treatment of methotrexate failures with biologic agents when these first emerged in the late 1990s.
A majority of RA patients started on a stepped approach using DMARDs will respond, Dr. Kolba said, and for those who must move into the biologic realm, the vast majority will succeed on the first anti-TNF agent prescribed. And there is little science to establish that one TNF inhibitor is superior – only that patients can sometimes succeed with one and fail another.
“As far as which biologic to initiate, my personal opinion is I don’t care, and I tell the patients that I don’t mind if the insurer picks out of this category because I’m flipping a coin as well,” she said.
Step mandates become objectionable, Dr. Kolba said, when they are purportedly based in science that doesn’t exist, or when they seem to exist only to wear down the provider.
“With private insurance not only do they have the drug of the year, they’re going to make me battle for every single prescription. When I say I have tried this patient on maximal tolerable doses of all these DMARDs, they ought to believe me. Yet I get six-page forms back saying, ‘Give me the start and stop dates of all the drugs you’ve used.’”
States constrain fail first
For many specialists treating patients with biologics, some of these hurdles are already getting lowered.
Concerns about physician choice, a lack of transparency in insurer decision making, and the ethics of forcing patients to fail have led advocacy groups to press hard in recent years for legislation limiting step therapy – with successes in a dozen states.
While the state legislation is not disease or drug specific, it has important implications for clinicians treating with biologics. “Step therapy in its genesis was a good idea – it’s OK to try to reduce costs in the health care system,” said Patrick Stone, state government relations manager at the National Psoriasis Foundation in Annapolis, Md., a group that works extensively on step therapy issues. “But when these protocols were first crafted, medications like biologics weren’t in use.”
Jeff Okazaki, associate director of the Coalition of State Rheumatology Organizations, a group based in Schaumberg, Ill., said lawmakers are starting to accept that in terms of cost of care, “somebody not being treated appropriately and down the line has organ damage or comorbidity because of incorrect treatment decisions due to step therapy is a higher burden.”
Moreover, he said, “we’d seen protocols requiring five or more steps, and for each step you have to try it at least 90 days.” For a patient with rheumatic or autoimmune disease, “getting through something like that can just be devastating.”
In 2011, Connecticut, Mississippi, and Arkansas became the first states to pass legislation limiting some aspect of step therapy. Since then, nine additional states have passed legislation varying in focus and scope.
In Kentucky, for example, patients cannot be forced by their insurer to remain on an ineffective therapy for more than 30 days, and insurers must respond to physician requests for an override within 2 days. Mississippi allows physicians to override insurer decisions with proof of clinical evidence. In California, legislation passed last year aims to reduce bureaucracy and speed up response to physician requests for overrides.
Mr. Stone and Mr. Okazaki are working in a coalition with other dermatology, rheumatology, and GI groups to push bills in seven more states, including New York, North Carolina, and Ohio.
While all the bills differ in what they attempt to limit, the model legislation has three basic objectives, Mr. Okazaki said. “We want a clear set of clinical guidelines, a quick review process, and overrides that allow for exceptions in cases where patients shouldn’t have to go through step therapy.”
Clinical strategies and research gaps
New legislation undoubtedly will help providers and patients get access to their choice of treatment agents. But so long as biologics are expensive – and it will be a while before the first biosimilar drugs, which will have efficacy and safety similar to their reference biologics, reduce prices in any meaningful way – step therapy will likely remain the norm.
One of the key difficulties providers face when pushing back on an insurer in favor of a biologic drug is insufficient clinical evidence.
With IBD, Dr. Rubin said, “we need a need more longitudinal understanding” and better prognostic indicators “in order to justify spending the extra money or going to one of these therapies.”
Dr. Hanauer said one of the limitations he faces in practice is insufficient clinical evidence for biologics early in the treatment pathway for IBD.
RA “is much more common than Crohn’s disease is. In trials, it’s much easier to recruit hundreds of patients [for an RA trial], while with Crohn’s it’s very hard to enroll more than a couple a year at most sites,” he said. “And as you move earlier in the treatment pathway that becomes somewhat more difficult as well.”
His solution for now, he said, is to follow established step pathways in an accelerated way, for “a rapid transition toward highly effective therapies” without having to face extensive pushback from insurers.
“The idea is to initiate immunosuppressants for any patients with sufficient disease activity to justify steroids,” Dr. Hanauer said. “Their steroids are then tapered, and while on immunosuppressants, patients are in a perfect setup to get combination therapy with an immunosuppressive and a biologic – and that’s a 2- to 3-month transition, not 2-3 years.”
Dr. Kolba said that despite the wide array of options for treating RA, the specialty suffers from a dearth of understanding as to why some patients fail drugs while others succeed, even within the same drug class.
Rheumatologists’ prescribing choices would be highly influenced by better biomarkers, were they to become available, she said. And they’d have far better arguments when confronted with payer pushback.
“We’re all looking for that magic biologic marker to tell me which drug to use,” Dr. Kolba said, “because God knows if I had a blood test that said ‘this is the drug,’ I would go to the mat with the insurer.”
AGA Resource
AGA offers an IBD Clinical Service line to provide GIs with the tools needed to be more efficient, understand quality standards, receive appropriate reimbursements and improve the process of care for their patients with IBD at www.gastro.org/practice/clinical-service-line/ibd-clinical-service-line.
Laws recently passed or under consideration in state legislatures may offer some relief to physicians and patients dogged by the “step” or “fail-first” therapy protocols mandated by insurers, but until better clinical evidence is available to support treatment decisions and biosimilars reduce costs, clinicians must strategize to get patients through the step pathways as fast as possible.
Rheumatologists, gastroenterologists, and dermatologists all confront fail-first policies in their practices, particularly when prescribing the biologic agents that have been game changers in treating rheumatoid arthritis (RA), inflammatory bowel disease (IBD), and psoriasis, among other diseases.
In RA, for example, a patient might be required to fail a series of disease-modifying antirheumatic drugs (DMARDs), including methotrexate, before starting a biologic. In Crohn’s disease, patients might have to first fail on steroids and immunosuppressants.
Most clinicians consider cost concerns fair as a basis for insurance decisions. But they can also have strong rationales for making exceptions. This may mean starting patients on a biologic early, particularly those they deem unlikely to respond to first- or second-line treatments – which may be cheaper but are not necessarily safer.
In egregious cases, a patient already stable on a biologic who has changed insurance plans may be forced to go backwards in the treatment pathway, and fail first- and second-line therapies all over again before resuming – a process unlikely to be cost-effective in the long term, and also rife with ethical concerns, say clinicians.
“Making a patient fail to get a less toxic drug sort of violates our ‘do no harm’ principle,” Dr. Stephen B. Hanauer, medical director of the Digestive Health Center at Northwestern University, Chicago, said in an interview.
“I always say that if biologics cost a dollar, we’d be using them for everybody. If you take away the steroids and the immunosuppressants, these are very safe drugs for IBD, far safer than steroids – but steroids are cheap,” Dr. Hanauer said.
And with some debilitating disease presentations, such as severe Crohn’s, “being told that we have to try conventional therapies and the patient has to fail them can mean putting the patient through progression of their disease, and suffering,” Dr. David T. Rubin, codirector of the Digestive Diseases Center at the University of Chicago, said in an interview. “We really struggle with this.”
Dr. Joseph S. Eastern, a dermatologist practicing in Belleville, N.J., said his specialty faces similar challenges with step therapy. “Dermatologists as a group are pretty risk averse. When given the opportunity, we do an excellent job of prescribing conventional medications, ultraviolet therapy, and biologics in the most cost-efficient possible way,” he said in an email.
Yet “third-party payers tell us, for example, that a patient must fail methotrexate before we can use a biologic, when the whole advantage of biologic therapy for many of these patients is the avoidance of organotoxicity and other serious risks.”
As a result, Dr. Eastern said, “I write a lot more vehement letters to payers about the biologics these days.”
Choice vs. cost
Rheumatologists are among the clinicians most affected by fail-first and step therapy mandates, as the diseases they treat – particularly RA – are the most established indications for biologic therapies, and for which the largest number of these are approved.
Though Medicare and Medicaid allow physicians considerable leeway, private insurers often tightly circumscribe the timing and choice of biologics in RA. As insurers’ first-choice biologic drug changes frequently, and varies from plan to plan, a patient who is stable on one agent might be asked to switch to another, a phenomenon known as nonmedical switching.
Insurers are seldom transparent about their reasons for establishing certain biologic drugs as their go-to agents, clinicians say, while making it difficult for physicians to prescribe others. “There’s a tremendous amount of discounting going on that we are oblivious to as physicians. I’ve seen situations where drug A is the first one you have to use this month and the next month, drug C,” said Dr. Norman Gaylis, a rheumatologist practicing in Aventura, Fla.
Attempting to start a patient on a nonpreferential biologic will generate paperwork and delays, Dr. Gaylis said, which can cost patients valuable time. “There’s a window of opportunity to treat these diseases, and by creating a step therapy pathway we’re closing the window at least partially.”
“As an example, rituximab in most payer plans is not tiered as a first-line biologic treatment option despite the fact that there are frequent scenarios where the clinical and serological presentation of a patient would suggest it to be preferable as a first-line treatment choice over an anti-TNF [tumor necrosis factor],” Dr. Gaylis said.
“There is no room for clinical decision making based upon the unique presentation of each different patient when choosing the best treatment option,” he said.
Rheumatologists often become overwhelmed with authorization paperwork, “and still in many instances end up with a denial of their request.”
Dr. Karen Kolba, a rheumatologist in private practice in Santa Maria, Calif., said that she agreed in principle with the way step therapy protocols have been established, and that some of the frustration with step therapy amounts to a tendency among specialist clinicians to bristle at being told what to do.
“Physicians hate protocol,” Dr. Kolba said. “But comparing one protocol to another is the only way we are going to make advances.” It took the rheumatology community about 30 years to come to terms with the use of methotrexate in RA, she noted, and the stepped approach grew naturally from the treatment of methotrexate failures with biologic agents when these first emerged in the late 1990s.
A majority of RA patients started on a stepped approach using DMARDs will respond, Dr. Kolba said, and for those who must move into the biologic realm, the vast majority will succeed on the first anti-TNF agent prescribed. And there is little science to establish that one TNF inhibitor is superior – only that patients can sometimes succeed with one and fail another.
“As far as which biologic to initiate, my personal opinion is I don’t care, and I tell the patients that I don’t mind if the insurer picks out of this category because I’m flipping a coin as well,” she said.
Step mandates become objectionable, Dr. Kolba said, when they are purportedly based in science that doesn’t exist, or when they seem to exist only to wear down the provider.
“With private insurance not only do they have the drug of the year, they’re going to make me battle for every single prescription. When I say I have tried this patient on maximal tolerable doses of all these DMARDs, they ought to believe me. Yet I get six-page forms back saying, ‘Give me the start and stop dates of all the drugs you’ve used.’”
States constrain fail first
For many specialists treating patients with biologics, some of these hurdles are already getting lowered.
Concerns about physician choice, a lack of transparency in insurer decision making, and the ethics of forcing patients to fail have led advocacy groups to press hard in recent years for legislation limiting step therapy – with successes in a dozen states.
While the state legislation is not disease or drug specific, it has important implications for clinicians treating with biologics. “Step therapy in its genesis was a good idea – it’s OK to try to reduce costs in the health care system,” said Patrick Stone, state government relations manager at the National Psoriasis Foundation in Annapolis, Md., a group that works extensively on step therapy issues. “But when these protocols were first crafted, medications like biologics weren’t in use.”
Jeff Okazaki, associate director of the Coalition of State Rheumatology Organizations, a group based in Schaumberg, Ill., said lawmakers are starting to accept that in terms of cost of care, “somebody not being treated appropriately and down the line has organ damage or comorbidity because of incorrect treatment decisions due to step therapy is a higher burden.”
Moreover, he said, “we’d seen protocols requiring five or more steps, and for each step you have to try it at least 90 days.” For a patient with rheumatic or autoimmune disease, “getting through something like that can just be devastating.”
In 2011, Connecticut, Mississippi, and Arkansas became the first states to pass legislation limiting some aspect of step therapy. Since then, nine additional states have passed legislation varying in focus and scope.
In Kentucky, for example, patients cannot be forced by their insurer to remain on an ineffective therapy for more than 30 days, and insurers must respond to physician requests for an override within 2 days. Mississippi allows physicians to override insurer decisions with proof of clinical evidence. In California, legislation passed last year aims to reduce bureaucracy and speed up response to physician requests for overrides.
Mr. Stone and Mr. Okazaki are working in a coalition with other dermatology, rheumatology, and GI groups to push bills in seven more states, including New York, North Carolina, and Ohio.
While all the bills differ in what they attempt to limit, the model legislation has three basic objectives, Mr. Okazaki said. “We want a clear set of clinical guidelines, a quick review process, and overrides that allow for exceptions in cases where patients shouldn’t have to go through step therapy.”
Clinical strategies and research gaps
New legislation undoubtedly will help providers and patients get access to their choice of treatment agents. But so long as biologics are expensive – and it will be a while before the first biosimilar drugs, which will have efficacy and safety similar to their reference biologics, reduce prices in any meaningful way – step therapy will likely remain the norm.
One of the key difficulties providers face when pushing back on an insurer in favor of a biologic drug is insufficient clinical evidence.
With IBD, Dr. Rubin said, “we need a need more longitudinal understanding” and better prognostic indicators “in order to justify spending the extra money or going to one of these therapies.”
Dr. Hanauer said one of the limitations he faces in practice is insufficient clinical evidence for biologics early in the treatment pathway for IBD.
RA “is much more common than Crohn’s disease is. In trials, it’s much easier to recruit hundreds of patients [for an RA trial], while with Crohn’s it’s very hard to enroll more than a couple a year at most sites,” he said. “And as you move earlier in the treatment pathway that becomes somewhat more difficult as well.”
His solution for now, he said, is to follow established step pathways in an accelerated way, for “a rapid transition toward highly effective therapies” without having to face extensive pushback from insurers.
“The idea is to initiate immunosuppressants for any patients with sufficient disease activity to justify steroids,” Dr. Hanauer said. “Their steroids are then tapered, and while on immunosuppressants, patients are in a perfect setup to get combination therapy with an immunosuppressive and a biologic – and that’s a 2- to 3-month transition, not 2-3 years.”
Dr. Kolba said that despite the wide array of options for treating RA, the specialty suffers from a dearth of understanding as to why some patients fail drugs while others succeed, even within the same drug class.
Rheumatologists’ prescribing choices would be highly influenced by better biomarkers, were they to become available, she said. And they’d have far better arguments when confronted with payer pushback.
“We’re all looking for that magic biologic marker to tell me which drug to use,” Dr. Kolba said, “because God knows if I had a blood test that said ‘this is the drug,’ I would go to the mat with the insurer.”
AGA Resource
AGA offers an IBD Clinical Service line to provide GIs with the tools needed to be more efficient, understand quality standards, receive appropriate reimbursements and improve the process of care for their patients with IBD at www.gastro.org/practice/clinical-service-line/ibd-clinical-service-line.
Laws recently passed or under consideration in state legislatures may offer some relief to physicians and patients dogged by the “step” or “fail-first” therapy protocols mandated by insurers, but until better clinical evidence is available to support treatment decisions and biosimilars reduce costs, clinicians must strategize to get patients through the step pathways as fast as possible.
Rheumatologists, gastroenterologists, and dermatologists all confront fail-first policies in their practices, particularly when prescribing the biologic agents that have been game changers in treating rheumatoid arthritis (RA), inflammatory bowel disease (IBD), and psoriasis, among other diseases.
In RA, for example, a patient might be required to fail a series of disease-modifying antirheumatic drugs (DMARDs), including methotrexate, before starting a biologic. In Crohn’s disease, patients might have to first fail on steroids and immunosuppressants.
Most clinicians consider cost concerns fair as a basis for insurance decisions. But they can also have strong rationales for making exceptions. This may mean starting patients on a biologic early, particularly those they deem unlikely to respond to first- or second-line treatments – which may be cheaper but are not necessarily safer.
In egregious cases, a patient already stable on a biologic who has changed insurance plans may be forced to go backwards in the treatment pathway, and fail first- and second-line therapies all over again before resuming – a process unlikely to be cost-effective in the long term, and also rife with ethical concerns, say clinicians.
“Making a patient fail to get a less toxic drug sort of violates our ‘do no harm’ principle,” Dr. Stephen B. Hanauer, medical director of the Digestive Health Center at Northwestern University, Chicago, said in an interview.
“I always say that if biologics cost a dollar, we’d be using them for everybody. If you take away the steroids and the immunosuppressants, these are very safe drugs for IBD, far safer than steroids – but steroids are cheap,” Dr. Hanauer said.
And with some debilitating disease presentations, such as severe Crohn’s, “being told that we have to try conventional therapies and the patient has to fail them can mean putting the patient through progression of their disease, and suffering,” Dr. David T. Rubin, codirector of the Digestive Diseases Center at the University of Chicago, said in an interview. “We really struggle with this.”
Dr. Joseph S. Eastern, a dermatologist practicing in Belleville, N.J., said his specialty faces similar challenges with step therapy. “Dermatologists as a group are pretty risk averse. When given the opportunity, we do an excellent job of prescribing conventional medications, ultraviolet therapy, and biologics in the most cost-efficient possible way,” he said in an email.
Yet “third-party payers tell us, for example, that a patient must fail methotrexate before we can use a biologic, when the whole advantage of biologic therapy for many of these patients is the avoidance of organotoxicity and other serious risks.”
As a result, Dr. Eastern said, “I write a lot more vehement letters to payers about the biologics these days.”
Choice vs. cost
Rheumatologists are among the clinicians most affected by fail-first and step therapy mandates, as the diseases they treat – particularly RA – are the most established indications for biologic therapies, and for which the largest number of these are approved.
Though Medicare and Medicaid allow physicians considerable leeway, private insurers often tightly circumscribe the timing and choice of biologics in RA. As insurers’ first-choice biologic drug changes frequently, and varies from plan to plan, a patient who is stable on one agent might be asked to switch to another, a phenomenon known as nonmedical switching.
Insurers are seldom transparent about their reasons for establishing certain biologic drugs as their go-to agents, clinicians say, while making it difficult for physicians to prescribe others. “There’s a tremendous amount of discounting going on that we are oblivious to as physicians. I’ve seen situations where drug A is the first one you have to use this month and the next month, drug C,” said Dr. Norman Gaylis, a rheumatologist practicing in Aventura, Fla.
Attempting to start a patient on a nonpreferential biologic will generate paperwork and delays, Dr. Gaylis said, which can cost patients valuable time. “There’s a window of opportunity to treat these diseases, and by creating a step therapy pathway we’re closing the window at least partially.”
“As an example, rituximab in most payer plans is not tiered as a first-line biologic treatment option despite the fact that there are frequent scenarios where the clinical and serological presentation of a patient would suggest it to be preferable as a first-line treatment choice over an anti-TNF [tumor necrosis factor],” Dr. Gaylis said.
“There is no room for clinical decision making based upon the unique presentation of each different patient when choosing the best treatment option,” he said.
Rheumatologists often become overwhelmed with authorization paperwork, “and still in many instances end up with a denial of their request.”
Dr. Karen Kolba, a rheumatologist in private practice in Santa Maria, Calif., said that she agreed in principle with the way step therapy protocols have been established, and that some of the frustration with step therapy amounts to a tendency among specialist clinicians to bristle at being told what to do.
“Physicians hate protocol,” Dr. Kolba said. “But comparing one protocol to another is the only way we are going to make advances.” It took the rheumatology community about 30 years to come to terms with the use of methotrexate in RA, she noted, and the stepped approach grew naturally from the treatment of methotrexate failures with biologic agents when these first emerged in the late 1990s.
A majority of RA patients started on a stepped approach using DMARDs will respond, Dr. Kolba said, and for those who must move into the biologic realm, the vast majority will succeed on the first anti-TNF agent prescribed. And there is little science to establish that one TNF inhibitor is superior – only that patients can sometimes succeed with one and fail another.
“As far as which biologic to initiate, my personal opinion is I don’t care, and I tell the patients that I don’t mind if the insurer picks out of this category because I’m flipping a coin as well,” she said.
Step mandates become objectionable, Dr. Kolba said, when they are purportedly based in science that doesn’t exist, or when they seem to exist only to wear down the provider.
“With private insurance not only do they have the drug of the year, they’re going to make me battle for every single prescription. When I say I have tried this patient on maximal tolerable doses of all these DMARDs, they ought to believe me. Yet I get six-page forms back saying, ‘Give me the start and stop dates of all the drugs you’ve used.’”
States constrain fail first
For many specialists treating patients with biologics, some of these hurdles are already getting lowered.
Concerns about physician choice, a lack of transparency in insurer decision making, and the ethics of forcing patients to fail have led advocacy groups to press hard in recent years for legislation limiting step therapy – with successes in a dozen states.
While the state legislation is not disease or drug specific, it has important implications for clinicians treating with biologics. “Step therapy in its genesis was a good idea – it’s OK to try to reduce costs in the health care system,” said Patrick Stone, state government relations manager at the National Psoriasis Foundation in Annapolis, Md., a group that works extensively on step therapy issues. “But when these protocols were first crafted, medications like biologics weren’t in use.”
Jeff Okazaki, associate director of the Coalition of State Rheumatology Organizations, a group based in Schaumberg, Ill., said lawmakers are starting to accept that in terms of cost of care, “somebody not being treated appropriately and down the line has organ damage or comorbidity because of incorrect treatment decisions due to step therapy is a higher burden.”
Moreover, he said, “we’d seen protocols requiring five or more steps, and for each step you have to try it at least 90 days.” For a patient with rheumatic or autoimmune disease, “getting through something like that can just be devastating.”
In 2011, Connecticut, Mississippi, and Arkansas became the first states to pass legislation limiting some aspect of step therapy. Since then, nine additional states have passed legislation varying in focus and scope.
In Kentucky, for example, patients cannot be forced by their insurer to remain on an ineffective therapy for more than 30 days, and insurers must respond to physician requests for an override within 2 days. Mississippi allows physicians to override insurer decisions with proof of clinical evidence. In California, legislation passed last year aims to reduce bureaucracy and speed up response to physician requests for overrides.
Mr. Stone and Mr. Okazaki are working in a coalition with other dermatology, rheumatology, and GI groups to push bills in seven more states, including New York, North Carolina, and Ohio.
While all the bills differ in what they attempt to limit, the model legislation has three basic objectives, Mr. Okazaki said. “We want a clear set of clinical guidelines, a quick review process, and overrides that allow for exceptions in cases where patients shouldn’t have to go through step therapy.”
Clinical strategies and research gaps
New legislation undoubtedly will help providers and patients get access to their choice of treatment agents. But so long as biologics are expensive – and it will be a while before the first biosimilar drugs, which will have efficacy and safety similar to their reference biologics, reduce prices in any meaningful way – step therapy will likely remain the norm.
One of the key difficulties providers face when pushing back on an insurer in favor of a biologic drug is insufficient clinical evidence.
With IBD, Dr. Rubin said, “we need a need more longitudinal understanding” and better prognostic indicators “in order to justify spending the extra money or going to one of these therapies.”
Dr. Hanauer said one of the limitations he faces in practice is insufficient clinical evidence for biologics early in the treatment pathway for IBD.
RA “is much more common than Crohn’s disease is. In trials, it’s much easier to recruit hundreds of patients [for an RA trial], while with Crohn’s it’s very hard to enroll more than a couple a year at most sites,” he said. “And as you move earlier in the treatment pathway that becomes somewhat more difficult as well.”
His solution for now, he said, is to follow established step pathways in an accelerated way, for “a rapid transition toward highly effective therapies” without having to face extensive pushback from insurers.
“The idea is to initiate immunosuppressants for any patients with sufficient disease activity to justify steroids,” Dr. Hanauer said. “Their steroids are then tapered, and while on immunosuppressants, patients are in a perfect setup to get combination therapy with an immunosuppressive and a biologic – and that’s a 2- to 3-month transition, not 2-3 years.”
Dr. Kolba said that despite the wide array of options for treating RA, the specialty suffers from a dearth of understanding as to why some patients fail drugs while others succeed, even within the same drug class.
Rheumatologists’ prescribing choices would be highly influenced by better biomarkers, were they to become available, she said. And they’d have far better arguments when confronted with payer pushback.
“We’re all looking for that magic biologic marker to tell me which drug to use,” Dr. Kolba said, “because God knows if I had a blood test that said ‘this is the drug,’ I would go to the mat with the insurer.”
AGA Resource
AGA offers an IBD Clinical Service line to provide GIs with the tools needed to be more efficient, understand quality standards, receive appropriate reimbursements and improve the process of care for their patients with IBD at www.gastro.org/practice/clinical-service-line/ibd-clinical-service-line.
Tips for treating C. difficile in IBD
ORLANDO – You can confidently treat mild to severe Clostridium difficile infection in patients with inflammatory bowel disease (IBD) without disrupting their immunosuppression or other treatments, according to an expert.
“If your patient with IBD needs a fecal transplant for C. diff., you should not be concerned about withholding it,” Dr. Alan C. Moss, AGAF, said during a basic science presentation at a conference on IBD, sponsored by the Crohn’s and Colitis Foundation of America. Dr. Moss is associate professor of medicine and the director of translational research at Harvard Medical School, Boston.
The first step, after you’ve determined that your patient has a C. diff. infection, as opposed to having been only colonized by the bacterium, is choosing the best antibiotic. “Unfortunately, almost all IBD patients are excluded from controlled trials of antibiotics in C. diff. infection, so all we really have to go on are retrospective cohort data,” he said.One such study, uncontrolled for disease severity, showed that a third of 114 inpatients with IBD who had a co-occurring C. diff. infection had higher 30-day readmission rates when treated first with metronidazole, per current standards of care, compared with the remaining two-thirds of patients who were treated first with vancomycin. The metronidazole group also averaged double the length of stays of the vancomycin group (Antimicrob Agents Chemother. 2014 Sep;58:5054-9 [doi: 10.1128/AAC.02606-13]).“This suggests that in IBD patients, especially for those who meet criteria for a severe C. diff. infection, vancomycin is the way to go,” Dr. Moss said, noting a trend of metronidazole for mild infections in this cohort having ever less efficacy.
Beyond mild infection, the first line of treatment should be vancomycin 125 mg four times daily, or 500 mg four times daily if it is complicated disease, he said. If your patient has recurrent C. diff. infection, Dr. Moss recommends a prolonged taper of vancomycin, and vigilance to be sure it’s truly an infection and not a flare-up of colonized bacteria.
“My bar for doing fecal transplant in these patients has dropped considerably in the last few years, because if you really want to squeeze out the niche that C. diff. occupies in the microbiome, fecal transplant is really the most effective way we have of doing that,” Dr. Moss said.
While there is a division in the field over whether to continue immunosuppression during antibiotic treatment, Dr. Moss cited a small study indicating that patients who were on two or more immunosuppressants had a higher risk of death, megacolon, or shock during C. diff. treatment. “It’s hard to draw many conclusions from that,” he said. “It may just be a surrogate marker of severity of disease rather than infection, per se.”
The standard of care for recurrent and refractory C. diff. infection is fecal transplant, Dr. Moss said. A study showed an 89% cure rate of C. diff. infection after a single fecal transplant in IBD patients. Of the 36 IBD patients in the study, half of whom were on biologic and immunosuppressive therapies, 4 had disease flare-ups (Am J Gastroenterol. 2014 Jul;109:1065-71; N Engl J Med. 2013 Jan 31;368:474-5). As for determining if there is an actual infection rather than colonization of C. diff., Dr. Moss said switching from using ELISA (enzyme-linked immunoassay) to PCR (polymerase chain reaction) testing was helpful in first-time infections because the latter is more sensitive for determining actual infection. However, if a patient has recurrent infection, the higher clinical specificity of PCR makes it harder to tell if a positive result is infection or simply colonization. Some institutions have dropped ELISA testing altogether, but the use of single-molecule array testing is, with its exponential sensitivity, a “good half-way step” between ELISA and PCR, and is useful for determining who is colonized vs. who is actually producing the toxin, even at a very low level, Dr Moss said.
Dr. Moss disclosed he has consulted for Janssen, Theravance, and Seres, and has received research support from the National Institute for Diabetes, Digestive, and Kidney Diseases, and Helmsley.
wmcknight@frontlinemedcom.com
On Twitter @whitneymcknight
ORLANDO – You can confidently treat mild to severe Clostridium difficile infection in patients with inflammatory bowel disease (IBD) without disrupting their immunosuppression or other treatments, according to an expert.
“If your patient with IBD needs a fecal transplant for C. diff., you should not be concerned about withholding it,” Dr. Alan C. Moss, AGAF, said during a basic science presentation at a conference on IBD, sponsored by the Crohn’s and Colitis Foundation of America. Dr. Moss is associate professor of medicine and the director of translational research at Harvard Medical School, Boston.
The first step, after you’ve determined that your patient has a C. diff. infection, as opposed to having been only colonized by the bacterium, is choosing the best antibiotic. “Unfortunately, almost all IBD patients are excluded from controlled trials of antibiotics in C. diff. infection, so all we really have to go on are retrospective cohort data,” he said.One such study, uncontrolled for disease severity, showed that a third of 114 inpatients with IBD who had a co-occurring C. diff. infection had higher 30-day readmission rates when treated first with metronidazole, per current standards of care, compared with the remaining two-thirds of patients who were treated first with vancomycin. The metronidazole group also averaged double the length of stays of the vancomycin group (Antimicrob Agents Chemother. 2014 Sep;58:5054-9 [doi: 10.1128/AAC.02606-13]).“This suggests that in IBD patients, especially for those who meet criteria for a severe C. diff. infection, vancomycin is the way to go,” Dr. Moss said, noting a trend of metronidazole for mild infections in this cohort having ever less efficacy.
Beyond mild infection, the first line of treatment should be vancomycin 125 mg four times daily, or 500 mg four times daily if it is complicated disease, he said. If your patient has recurrent C. diff. infection, Dr. Moss recommends a prolonged taper of vancomycin, and vigilance to be sure it’s truly an infection and not a flare-up of colonized bacteria.
“My bar for doing fecal transplant in these patients has dropped considerably in the last few years, because if you really want to squeeze out the niche that C. diff. occupies in the microbiome, fecal transplant is really the most effective way we have of doing that,” Dr. Moss said.
While there is a division in the field over whether to continue immunosuppression during antibiotic treatment, Dr. Moss cited a small study indicating that patients who were on two or more immunosuppressants had a higher risk of death, megacolon, or shock during C. diff. treatment. “It’s hard to draw many conclusions from that,” he said. “It may just be a surrogate marker of severity of disease rather than infection, per se.”
The standard of care for recurrent and refractory C. diff. infection is fecal transplant, Dr. Moss said. A study showed an 89% cure rate of C. diff. infection after a single fecal transplant in IBD patients. Of the 36 IBD patients in the study, half of whom were on biologic and immunosuppressive therapies, 4 had disease flare-ups (Am J Gastroenterol. 2014 Jul;109:1065-71; N Engl J Med. 2013 Jan 31;368:474-5). As for determining if there is an actual infection rather than colonization of C. diff., Dr. Moss said switching from using ELISA (enzyme-linked immunoassay) to PCR (polymerase chain reaction) testing was helpful in first-time infections because the latter is more sensitive for determining actual infection. However, if a patient has recurrent infection, the higher clinical specificity of PCR makes it harder to tell if a positive result is infection or simply colonization. Some institutions have dropped ELISA testing altogether, but the use of single-molecule array testing is, with its exponential sensitivity, a “good half-way step” between ELISA and PCR, and is useful for determining who is colonized vs. who is actually producing the toxin, even at a very low level, Dr Moss said.
Dr. Moss disclosed he has consulted for Janssen, Theravance, and Seres, and has received research support from the National Institute for Diabetes, Digestive, and Kidney Diseases, and Helmsley.
wmcknight@frontlinemedcom.com
On Twitter @whitneymcknight
ORLANDO – You can confidently treat mild to severe Clostridium difficile infection in patients with inflammatory bowel disease (IBD) without disrupting their immunosuppression or other treatments, according to an expert.
“If your patient with IBD needs a fecal transplant for C. diff., you should not be concerned about withholding it,” Dr. Alan C. Moss, AGAF, said during a basic science presentation at a conference on IBD, sponsored by the Crohn’s and Colitis Foundation of America. Dr. Moss is associate professor of medicine and the director of translational research at Harvard Medical School, Boston.
The first step, after you’ve determined that your patient has a C. diff. infection, as opposed to having been only colonized by the bacterium, is choosing the best antibiotic. “Unfortunately, almost all IBD patients are excluded from controlled trials of antibiotics in C. diff. infection, so all we really have to go on are retrospective cohort data,” he said.One such study, uncontrolled for disease severity, showed that a third of 114 inpatients with IBD who had a co-occurring C. diff. infection had higher 30-day readmission rates when treated first with metronidazole, per current standards of care, compared with the remaining two-thirds of patients who were treated first with vancomycin. The metronidazole group also averaged double the length of stays of the vancomycin group (Antimicrob Agents Chemother. 2014 Sep;58:5054-9 [doi: 10.1128/AAC.02606-13]).“This suggests that in IBD patients, especially for those who meet criteria for a severe C. diff. infection, vancomycin is the way to go,” Dr. Moss said, noting a trend of metronidazole for mild infections in this cohort having ever less efficacy.
Beyond mild infection, the first line of treatment should be vancomycin 125 mg four times daily, or 500 mg four times daily if it is complicated disease, he said. If your patient has recurrent C. diff. infection, Dr. Moss recommends a prolonged taper of vancomycin, and vigilance to be sure it’s truly an infection and not a flare-up of colonized bacteria.
“My bar for doing fecal transplant in these patients has dropped considerably in the last few years, because if you really want to squeeze out the niche that C. diff. occupies in the microbiome, fecal transplant is really the most effective way we have of doing that,” Dr. Moss said.
While there is a division in the field over whether to continue immunosuppression during antibiotic treatment, Dr. Moss cited a small study indicating that patients who were on two or more immunosuppressants had a higher risk of death, megacolon, or shock during C. diff. treatment. “It’s hard to draw many conclusions from that,” he said. “It may just be a surrogate marker of severity of disease rather than infection, per se.”
The standard of care for recurrent and refractory C. diff. infection is fecal transplant, Dr. Moss said. A study showed an 89% cure rate of C. diff. infection after a single fecal transplant in IBD patients. Of the 36 IBD patients in the study, half of whom were on biologic and immunosuppressive therapies, 4 had disease flare-ups (Am J Gastroenterol. 2014 Jul;109:1065-71; N Engl J Med. 2013 Jan 31;368:474-5). As for determining if there is an actual infection rather than colonization of C. diff., Dr. Moss said switching from using ELISA (enzyme-linked immunoassay) to PCR (polymerase chain reaction) testing was helpful in first-time infections because the latter is more sensitive for determining actual infection. However, if a patient has recurrent infection, the higher clinical specificity of PCR makes it harder to tell if a positive result is infection or simply colonization. Some institutions have dropped ELISA testing altogether, but the use of single-molecule array testing is, with its exponential sensitivity, a “good half-way step” between ELISA and PCR, and is useful for determining who is colonized vs. who is actually producing the toxin, even at a very low level, Dr Moss said.
Dr. Moss disclosed he has consulted for Janssen, Theravance, and Seres, and has received research support from the National Institute for Diabetes, Digestive, and Kidney Diseases, and Helmsley.
wmcknight@frontlinemedcom.com
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM 2015 ADVANCES IN IBD
Physicians need to be more proactive in learning about patient IBS symptoms
Patients can be reluctant to talk about bowel issues and physicians may need to take more of an initiative to draw that information out.
This was a key takeaway from the recent American Gastroenterological Association’s “IBS In America” survey.
“We found that patients are reluctant to talk to physicians about their symptoms,” survey coauthor Dr. Anthony Lembo, associate professor of medicine at Harvard Medical School, Boston, said in an interview. He noted that the survey of patient and physician views on irritable bowel syndrome revealed that patients find it easier to talk about sexually transmitted diseases than they do about bowel functions.
In fact, according to the survey results, 67% of patients who are experiencing symptoms of IBS will go for more than 1 year before seeing a doctor about them, and 43% will go longer than 1 year after taking over-the-counter remedies before speaking to a physician about their symptoms.
However, patients will have conversations with family and friends, often taking advice from them, before consulting with a medical professional. Fifty-nine percent said they have received advice from family and friends and 90% of those receiving advice said they followed it.
Physicians “should recognize that and initiate the conversation so we can find out what the symptoms are,” Dr. Lembo said.
In fact, 71% of survey respondents who have never sought treatment for their GI symptoms said “no” when asked if a health care professional asked about gastrointestinal symptoms or regularity during an annual check-up or exam, with 40% saying they did not tell their doctor about their symptoms.
The other point that the survey drives home and that physicians should be aware of, Dr. Lembo added, is the impact IBS has on patient quality of life.
“We know that IBS can have a major impact on their quality of life, but this survey put more concrete numbers to it in terms of how patients deal with it on a day-in, day-out basis,” he said.
For example, he highlighted that those suffering from IBS report missing on average 2 work or school days per month and have between 8 and 9 days per month when their productivity is hampered due to IBS symptoms.
Indeed, the survey noted that patients were willing to go so far as to trade a month without sex if it meant a month of relief from their IBS symptoms.
“Those kind of numbers, I think they’re important for physicians to know. IBS symptoms can have an impact on [patients’] quality of life and their day-to-day function,” he said.
Patients can be reluctant to talk about bowel issues and physicians may need to take more of an initiative to draw that information out.
This was a key takeaway from the recent American Gastroenterological Association’s “IBS In America” survey.
“We found that patients are reluctant to talk to physicians about their symptoms,” survey coauthor Dr. Anthony Lembo, associate professor of medicine at Harvard Medical School, Boston, said in an interview. He noted that the survey of patient and physician views on irritable bowel syndrome revealed that patients find it easier to talk about sexually transmitted diseases than they do about bowel functions.
In fact, according to the survey results, 67% of patients who are experiencing symptoms of IBS will go for more than 1 year before seeing a doctor about them, and 43% will go longer than 1 year after taking over-the-counter remedies before speaking to a physician about their symptoms.
However, patients will have conversations with family and friends, often taking advice from them, before consulting with a medical professional. Fifty-nine percent said they have received advice from family and friends and 90% of those receiving advice said they followed it.
Physicians “should recognize that and initiate the conversation so we can find out what the symptoms are,” Dr. Lembo said.
In fact, 71% of survey respondents who have never sought treatment for their GI symptoms said “no” when asked if a health care professional asked about gastrointestinal symptoms or regularity during an annual check-up or exam, with 40% saying they did not tell their doctor about their symptoms.
The other point that the survey drives home and that physicians should be aware of, Dr. Lembo added, is the impact IBS has on patient quality of life.
“We know that IBS can have a major impact on their quality of life, but this survey put more concrete numbers to it in terms of how patients deal with it on a day-in, day-out basis,” he said.
For example, he highlighted that those suffering from IBS report missing on average 2 work or school days per month and have between 8 and 9 days per month when their productivity is hampered due to IBS symptoms.
Indeed, the survey noted that patients were willing to go so far as to trade a month without sex if it meant a month of relief from their IBS symptoms.
“Those kind of numbers, I think they’re important for physicians to know. IBS symptoms can have an impact on [patients’] quality of life and their day-to-day function,” he said.
Patients can be reluctant to talk about bowel issues and physicians may need to take more of an initiative to draw that information out.
This was a key takeaway from the recent American Gastroenterological Association’s “IBS In America” survey.
“We found that patients are reluctant to talk to physicians about their symptoms,” survey coauthor Dr. Anthony Lembo, associate professor of medicine at Harvard Medical School, Boston, said in an interview. He noted that the survey of patient and physician views on irritable bowel syndrome revealed that patients find it easier to talk about sexually transmitted diseases than they do about bowel functions.
In fact, according to the survey results, 67% of patients who are experiencing symptoms of IBS will go for more than 1 year before seeing a doctor about them, and 43% will go longer than 1 year after taking over-the-counter remedies before speaking to a physician about their symptoms.
However, patients will have conversations with family and friends, often taking advice from them, before consulting with a medical professional. Fifty-nine percent said they have received advice from family and friends and 90% of those receiving advice said they followed it.
Physicians “should recognize that and initiate the conversation so we can find out what the symptoms are,” Dr. Lembo said.
In fact, 71% of survey respondents who have never sought treatment for their GI symptoms said “no” when asked if a health care professional asked about gastrointestinal symptoms or regularity during an annual check-up or exam, with 40% saying they did not tell their doctor about their symptoms.
The other point that the survey drives home and that physicians should be aware of, Dr. Lembo added, is the impact IBS has on patient quality of life.
“We know that IBS can have a major impact on their quality of life, but this survey put more concrete numbers to it in terms of how patients deal with it on a day-in, day-out basis,” he said.
For example, he highlighted that those suffering from IBS report missing on average 2 work or school days per month and have between 8 and 9 days per month when their productivity is hampered due to IBS symptoms.
Indeed, the survey noted that patients were willing to go so far as to trade a month without sex if it meant a month of relief from their IBS symptoms.
“Those kind of numbers, I think they’re important for physicians to know. IBS symptoms can have an impact on [patients’] quality of life and their day-to-day function,” he said.