User login
Vaccine hope now for leading cause of U.S. infant hospitalizations: RSV
Respiratory syncytial virus (RSV) is the leading cause of U.S. infant hospitalizations overall and across population subgroups, new data published in the Journal of Infectious Diseases confirm.
Acute bronchiolitis caused by RSV accounted for 9.6% (95% confidence interval, 9.4%-9.9%) and 9.3% (95% CI, 9.0%-9.6%) of total infant hospitalizations from January 2009 to September 2015 and October 2015 to December 2019, respectively.
Journal issue includes 14 RSV studies
The latest issue of the journal includes a special section with results from 14 studies related to the widespread, easy-to-catch virus, highlighting the urgency of finding a solution for all infants.
In one study, authors led by Mina Suh, MPH, with EpidStrategies, a division of ToxStrategies in Rockville, Md., reported that, in children under the age of 5 years in the United States, RSV caused 58,000 annual hospitalizations and from 100 to 500 annual deaths from 2009 to 2019 (the latest year data were available).
Globally, in 2015, among infants younger than 6 months, an estimated 1.4 million hospital admissions and 27,300 in-hospital deaths were attributed to RSV lower respiratory tract infection (LRTI).
The researchers used the largest publicly available, all-payer database in the United States – the National (Nationwide) Inpatient Sample – to describe the leading causes of infant hospitalizations.
The authors noted that, because clinicians don’t routinely perform lab tests for RSV, the true health care burden is likely higher and its public health impact greater than these numbers show.
Immunization candidates advance
There are no preventative options currently available to substantially cut RSV infections in all infants, though immunization candidates are advancing, showing safety and efficacy in clinical trials.
Palivizumab is currently the only available option in the United States to prevent RSV and is recommended only for a small group of infants with particular forms of heart or lung disease and those born prematurely at 29 weeks’ gestational age. Further, palivizumab has to be given monthly throughout the RSV season.
Another of the studies in the journal supplement concluded that a universal immunization strategy with one of the candidates, nirsevimab (Sanofi, AstraZeneca), an investigational long-acting monoclonal antibody, could substantially reduce the health burden and economic burden for U.S. infants in their first RSV season.
The researchers, led by Alexia Kieffer, MSc, MPH, with Sanofi, used static decision-analytic modeling for the estimates. Modeled RSV-related outcomes included primary care and ED visits, hospitalizations, including ICU admission and mechanical ventilations, and RSV-related deaths.
“The results of this model suggested that the use of nirsevimab in all infants could reduce health events by 55% and the overall costs to the payer by 49%,” the authors of the study wrote.
According to the study, universal immunization of all infants with nirsevimab is expected to reduce 290,174 RSV-related medically attended LRTI (MALRTI), 24,986 hospitalizations, and cut $612 million in costs to the health care system.
The authors wrote: “While this reduction would be driven by term infants, who account for most of the RSV-MALRTI burden; all infants, including palivizumab-eligible and preterm infants who suffer from significantly higher rates of disease, would benefit from this immunization strategy.”
Excitement for another option
Jörn-Hendrik Weitkamp, MD, professor of pediatrics and director for patient-oriented research at Monroe Carell Jr. Children’s Hospital at Vanderbilt University, Nashville, Tenn., said in an interview there is much excitement in the field for nirsevimab as it has significant advantages over palivizumab.
RSV “is a huge burden to the children, the families, the hospitals, and the medical system,” he said.
Ideally there would be a vaccine to offer the best protection, he noted.
“People have spent their lives, their careers trying to develop a vaccine for RSV,” he said, but that has been elusive for more than 60 years. Therefore, passive immunization is the best of the current options, he says, and nirsevimab “seems to be very effective.”
What’s not clear, Dr. Weitkamp said, is how much nirsevimab will cost as it is not yet approved by the Food and Drug Administration. However, it has the great advantage of being given only once before the season starts instead of monthly (as required for palivizumab) through the season, “which is painful, inconvenient, and traumatizing. We limit that one to the children at highest risk.”
Rolling out an infant nirsevimab program would likely vary by geographic region, Ms. Kieffer and colleagues said, to help ensure infants are protected during the peak of their region’s RSV season.
The journal’s RSV supplement was supported by Sanofi and AstraZeneca. The studies by Ms. Suh and colleagues and Ms. Kieffer and colleagues were supported by AstraZeneca and Sanofi. Ms. Suh and several coauthors are employees of EpidStrategies. One coauthor is an employee of Sanofi and may hold shares and/or stock options in the company. Ms. Kieffer and several coauthors are employees of Sanofi and may hold shares and/or stock options in the company. Dr. Weitkamp reported no relevant financial relationships.
Respiratory syncytial virus (RSV) is the leading cause of U.S. infant hospitalizations overall and across population subgroups, new data published in the Journal of Infectious Diseases confirm.
Acute bronchiolitis caused by RSV accounted for 9.6% (95% confidence interval, 9.4%-9.9%) and 9.3% (95% CI, 9.0%-9.6%) of total infant hospitalizations from January 2009 to September 2015 and October 2015 to December 2019, respectively.
Journal issue includes 14 RSV studies
The latest issue of the journal includes a special section with results from 14 studies related to the widespread, easy-to-catch virus, highlighting the urgency of finding a solution for all infants.
In one study, authors led by Mina Suh, MPH, with EpidStrategies, a division of ToxStrategies in Rockville, Md., reported that, in children under the age of 5 years in the United States, RSV caused 58,000 annual hospitalizations and from 100 to 500 annual deaths from 2009 to 2019 (the latest year data were available).
Globally, in 2015, among infants younger than 6 months, an estimated 1.4 million hospital admissions and 27,300 in-hospital deaths were attributed to RSV lower respiratory tract infection (LRTI).
The researchers used the largest publicly available, all-payer database in the United States – the National (Nationwide) Inpatient Sample – to describe the leading causes of infant hospitalizations.
The authors noted that, because clinicians don’t routinely perform lab tests for RSV, the true health care burden is likely higher and its public health impact greater than these numbers show.
Immunization candidates advance
There are no preventative options currently available to substantially cut RSV infections in all infants, though immunization candidates are advancing, showing safety and efficacy in clinical trials.
Palivizumab is currently the only available option in the United States to prevent RSV and is recommended only for a small group of infants with particular forms of heart or lung disease and those born prematurely at 29 weeks’ gestational age. Further, palivizumab has to be given monthly throughout the RSV season.
Another of the studies in the journal supplement concluded that a universal immunization strategy with one of the candidates, nirsevimab (Sanofi, AstraZeneca), an investigational long-acting monoclonal antibody, could substantially reduce the health burden and economic burden for U.S. infants in their first RSV season.
The researchers, led by Alexia Kieffer, MSc, MPH, with Sanofi, used static decision-analytic modeling for the estimates. Modeled RSV-related outcomes included primary care and ED visits, hospitalizations, including ICU admission and mechanical ventilations, and RSV-related deaths.
“The results of this model suggested that the use of nirsevimab in all infants could reduce health events by 55% and the overall costs to the payer by 49%,” the authors of the study wrote.
According to the study, universal immunization of all infants with nirsevimab is expected to reduce 290,174 RSV-related medically attended LRTI (MALRTI), 24,986 hospitalizations, and cut $612 million in costs to the health care system.
The authors wrote: “While this reduction would be driven by term infants, who account for most of the RSV-MALRTI burden; all infants, including palivizumab-eligible and preterm infants who suffer from significantly higher rates of disease, would benefit from this immunization strategy.”
Excitement for another option
Jörn-Hendrik Weitkamp, MD, professor of pediatrics and director for patient-oriented research at Monroe Carell Jr. Children’s Hospital at Vanderbilt University, Nashville, Tenn., said in an interview there is much excitement in the field for nirsevimab as it has significant advantages over palivizumab.
RSV “is a huge burden to the children, the families, the hospitals, and the medical system,” he said.
Ideally there would be a vaccine to offer the best protection, he noted.
“People have spent their lives, their careers trying to develop a vaccine for RSV,” he said, but that has been elusive for more than 60 years. Therefore, passive immunization is the best of the current options, he says, and nirsevimab “seems to be very effective.”
What’s not clear, Dr. Weitkamp said, is how much nirsevimab will cost as it is not yet approved by the Food and Drug Administration. However, it has the great advantage of being given only once before the season starts instead of monthly (as required for palivizumab) through the season, “which is painful, inconvenient, and traumatizing. We limit that one to the children at highest risk.”
Rolling out an infant nirsevimab program would likely vary by geographic region, Ms. Kieffer and colleagues said, to help ensure infants are protected during the peak of their region’s RSV season.
The journal’s RSV supplement was supported by Sanofi and AstraZeneca. The studies by Ms. Suh and colleagues and Ms. Kieffer and colleagues were supported by AstraZeneca and Sanofi. Ms. Suh and several coauthors are employees of EpidStrategies. One coauthor is an employee of Sanofi and may hold shares and/or stock options in the company. Ms. Kieffer and several coauthors are employees of Sanofi and may hold shares and/or stock options in the company. Dr. Weitkamp reported no relevant financial relationships.
Respiratory syncytial virus (RSV) is the leading cause of U.S. infant hospitalizations overall and across population subgroups, new data published in the Journal of Infectious Diseases confirm.
Acute bronchiolitis caused by RSV accounted for 9.6% (95% confidence interval, 9.4%-9.9%) and 9.3% (95% CI, 9.0%-9.6%) of total infant hospitalizations from January 2009 to September 2015 and October 2015 to December 2019, respectively.
Journal issue includes 14 RSV studies
The latest issue of the journal includes a special section with results from 14 studies related to the widespread, easy-to-catch virus, highlighting the urgency of finding a solution for all infants.
In one study, authors led by Mina Suh, MPH, with EpidStrategies, a division of ToxStrategies in Rockville, Md., reported that, in children under the age of 5 years in the United States, RSV caused 58,000 annual hospitalizations and from 100 to 500 annual deaths from 2009 to 2019 (the latest year data were available).
Globally, in 2015, among infants younger than 6 months, an estimated 1.4 million hospital admissions and 27,300 in-hospital deaths were attributed to RSV lower respiratory tract infection (LRTI).
The researchers used the largest publicly available, all-payer database in the United States – the National (Nationwide) Inpatient Sample – to describe the leading causes of infant hospitalizations.
The authors noted that, because clinicians don’t routinely perform lab tests for RSV, the true health care burden is likely higher and its public health impact greater than these numbers show.
Immunization candidates advance
There are no preventative options currently available to substantially cut RSV infections in all infants, though immunization candidates are advancing, showing safety and efficacy in clinical trials.
Palivizumab is currently the only available option in the United States to prevent RSV and is recommended only for a small group of infants with particular forms of heart or lung disease and those born prematurely at 29 weeks’ gestational age. Further, palivizumab has to be given monthly throughout the RSV season.
Another of the studies in the journal supplement concluded that a universal immunization strategy with one of the candidates, nirsevimab (Sanofi, AstraZeneca), an investigational long-acting monoclonal antibody, could substantially reduce the health burden and economic burden for U.S. infants in their first RSV season.
The researchers, led by Alexia Kieffer, MSc, MPH, with Sanofi, used static decision-analytic modeling for the estimates. Modeled RSV-related outcomes included primary care and ED visits, hospitalizations, including ICU admission and mechanical ventilations, and RSV-related deaths.
“The results of this model suggested that the use of nirsevimab in all infants could reduce health events by 55% and the overall costs to the payer by 49%,” the authors of the study wrote.
According to the study, universal immunization of all infants with nirsevimab is expected to reduce 290,174 RSV-related medically attended LRTI (MALRTI), 24,986 hospitalizations, and cut $612 million in costs to the health care system.
The authors wrote: “While this reduction would be driven by term infants, who account for most of the RSV-MALRTI burden; all infants, including palivizumab-eligible and preterm infants who suffer from significantly higher rates of disease, would benefit from this immunization strategy.”
Excitement for another option
Jörn-Hendrik Weitkamp, MD, professor of pediatrics and director for patient-oriented research at Monroe Carell Jr. Children’s Hospital at Vanderbilt University, Nashville, Tenn., said in an interview there is much excitement in the field for nirsevimab as it has significant advantages over palivizumab.
RSV “is a huge burden to the children, the families, the hospitals, and the medical system,” he said.
Ideally there would be a vaccine to offer the best protection, he noted.
“People have spent their lives, their careers trying to develop a vaccine for RSV,” he said, but that has been elusive for more than 60 years. Therefore, passive immunization is the best of the current options, he says, and nirsevimab “seems to be very effective.”
What’s not clear, Dr. Weitkamp said, is how much nirsevimab will cost as it is not yet approved by the Food and Drug Administration. However, it has the great advantage of being given only once before the season starts instead of monthly (as required for palivizumab) through the season, “which is painful, inconvenient, and traumatizing. We limit that one to the children at highest risk.”
Rolling out an infant nirsevimab program would likely vary by geographic region, Ms. Kieffer and colleagues said, to help ensure infants are protected during the peak of their region’s RSV season.
The journal’s RSV supplement was supported by Sanofi and AstraZeneca. The studies by Ms. Suh and colleagues and Ms. Kieffer and colleagues were supported by AstraZeneca and Sanofi. Ms. Suh and several coauthors are employees of EpidStrategies. One coauthor is an employee of Sanofi and may hold shares and/or stock options in the company. Ms. Kieffer and several coauthors are employees of Sanofi and may hold shares and/or stock options in the company. Dr. Weitkamp reported no relevant financial relationships.
FROM THE JOURNAL OF INFECTIOUS DISEASES
Langya, a new zoonotic virus, detected in China
Between 2018 and August 2022, These cases were reported in The New England Journal of Medicine. When asked by Nature about this emerging virus that has until now flown under the radar, scientists said that they were not overly concerned because the virus doesn’t seem to spread easily between people nor is it fatal.
Researchers think that the virus is carried by shrews. It might have infected people directly or through an intermediate animal.
First identified in Langya
The authors describe 35 cases of infection with a virus called Langya henipavirus (LayV) since 2018. It is closely related to two other henipaviruses known to infect people – Hendra virus and Nipah virus. The virus was named Langya after the town in Shandong province in China where the first patient identified with the disease was from, explained coauthor Linfa Wang, PhD, a virologist at Duke-NUS Medical School, Singapore.
Langya can cause respiratory symptoms such as fever, cough, and fatigue. Hendra virus and Nipah virus also cause respiratory infections and can be fatal, the article in Nature reports.
Hendra and Nipah
According to the World Health Organization, Nipah virus, which was discovered in 1999, is a new virus responsible for a zoonosis that causes the disease in animals and humans who have had contact with infected animals. Its name comes from the location where it was first identified in Malaysia. Patients may have asymptomatic infection or symptoms such as acute respiratory infection and severe encephalitis. The case fatality rate is between 40% and 75%.
Nipah virus is closely related to another recently discovered (1994) zoonotic virus called Hendra virus, which is named after the Australian city in which it first appeared. On that day in July 2016, 53 cases were identified involving 70 horses. These incidents remained confined to the northeastern coast of Australia.
Nipah virus and Hendra virus belong to the Paramyxoviridae family. “While the members of this group of viruses are only responsible for a few limited outbreaks, the ability of these viruses to infect a wide range of hosts and cause a disease leading to high fatalities in humans has made them a public health concern,” stated the WHO.
Related to measles
The research team identified LayV while monitoring patients at three hospitals in the eastern Chinese provinces of Shandong and Henan between April 2018 and August 2021. Throughout the study period, the researchers found 35 people infected with LayV, mostly farmers, with symptoms ranging from a cough to severe pneumonia. Participants were recruited into the study if they had a fever. The team sequenced the LayV genome from a throat swab taken from the first patient identified with the disease, a 53-year-old woman.
The LayV genome showed that the virus is most closely related to Mojiang henipavirus, which was first isolated in rats in an abandoned mine in the southern Chinese province of Yunnan in 2012. Henipaviruses belong to the Paramyxoviridae family of viruses, which includes measles, mumps, and many respiratory viruses that infect humans. Several other henipaviruses have been discovered in bats, rats, and shrews from Australia to South Korea and China, but only Hendra, Nipah, and now LayV are known to infect people, according to Nature.
Animal origin likely
Because most patients stated in a questionnaire that they had been exposed to an animal during the month preceding the onset of their symptoms, the researchers tested goats, dogs, pigs, and cattle living in the villages of infected patients for antibodies against LayV. They found LayV antibodies in a handful of goats and dogs and identified LayV viral RNA in 27% of the 262 sampled shrews. These findings suggest that the shrew may be a natural reservoir of LayV, passing it between themselves “and somehow infecting people here and there by chance,” Emily Gurley, PhD, MPH, an infectious diseases epidemiologist at Johns Hopkins University, Baltimore, told Nature.
The researchers did not find strong evidence of LayV spreading between the people included in the study. There were no clusters of cases in the same family, within a short time span, or in close geographical proximity. “Of the 35 cases, not a single one is linked,” said Dr. Wang, which Dr. Gurley considered good news. It should be noted, however ,”that the study did retrospective contact tracing on only 15 family members of nine infected individuals, which makes it difficult to determine how exactly the individuals were exposed,” reported Nature.
Vigilance is needed
Should we be worried about a potential new epidemic? The replies from two experts interviewed by Nature were reassuring. “There is no particular need to worry about this virus, but ongoing surveillance is critical,” said Professor Edward Holmes, an evolutionary virologist at the University of Sydney. Regularly testing people and animals for emerging viruses is important to understand the risk for zoonotic diseases – those that can be transmitted from other animals to humans, he said.
It is still not clear how people were infected in the first place – whether directly from shrews or an intermediate animal, said Dr. Gurley. That’s why a lot of research still needs to be done to work out how the virus is spreading in shrews and how people are getting infected, she added.
Nevertheless, Dr. Gurley finds that large outbreaks of infectious diseases typically take off after a lot of false starts. “If we are actively looking for those sparks, then we are in a much better position to stop or to find something early.” Still, she noted that she didn’t see anything in the data to “cause alarm from a pandemic-threat perspective.”
Though there is not currently any cause for worry of a new pandemic, vigilance is crucial. Professor Holmes says there is an urgent need for a global surveillance system to detect virus spillovers and rapidly communicate those results to avoid more pandemics, such as the one sparked by COVID-19. “These sorts of zoonotic spillover events happen all the time,” he said. “The world needs to wake up.”
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
Between 2018 and August 2022, These cases were reported in The New England Journal of Medicine. When asked by Nature about this emerging virus that has until now flown under the radar, scientists said that they were not overly concerned because the virus doesn’t seem to spread easily between people nor is it fatal.
Researchers think that the virus is carried by shrews. It might have infected people directly or through an intermediate animal.
First identified in Langya
The authors describe 35 cases of infection with a virus called Langya henipavirus (LayV) since 2018. It is closely related to two other henipaviruses known to infect people – Hendra virus and Nipah virus. The virus was named Langya after the town in Shandong province in China where the first patient identified with the disease was from, explained coauthor Linfa Wang, PhD, a virologist at Duke-NUS Medical School, Singapore.
Langya can cause respiratory symptoms such as fever, cough, and fatigue. Hendra virus and Nipah virus also cause respiratory infections and can be fatal, the article in Nature reports.
Hendra and Nipah
According to the World Health Organization, Nipah virus, which was discovered in 1999, is a new virus responsible for a zoonosis that causes the disease in animals and humans who have had contact with infected animals. Its name comes from the location where it was first identified in Malaysia. Patients may have asymptomatic infection or symptoms such as acute respiratory infection and severe encephalitis. The case fatality rate is between 40% and 75%.
Nipah virus is closely related to another recently discovered (1994) zoonotic virus called Hendra virus, which is named after the Australian city in which it first appeared. On that day in July 2016, 53 cases were identified involving 70 horses. These incidents remained confined to the northeastern coast of Australia.
Nipah virus and Hendra virus belong to the Paramyxoviridae family. “While the members of this group of viruses are only responsible for a few limited outbreaks, the ability of these viruses to infect a wide range of hosts and cause a disease leading to high fatalities in humans has made them a public health concern,” stated the WHO.
Related to measles
The research team identified LayV while monitoring patients at three hospitals in the eastern Chinese provinces of Shandong and Henan between April 2018 and August 2021. Throughout the study period, the researchers found 35 people infected with LayV, mostly farmers, with symptoms ranging from a cough to severe pneumonia. Participants were recruited into the study if they had a fever. The team sequenced the LayV genome from a throat swab taken from the first patient identified with the disease, a 53-year-old woman.
The LayV genome showed that the virus is most closely related to Mojiang henipavirus, which was first isolated in rats in an abandoned mine in the southern Chinese province of Yunnan in 2012. Henipaviruses belong to the Paramyxoviridae family of viruses, which includes measles, mumps, and many respiratory viruses that infect humans. Several other henipaviruses have been discovered in bats, rats, and shrews from Australia to South Korea and China, but only Hendra, Nipah, and now LayV are known to infect people, according to Nature.
Animal origin likely
Because most patients stated in a questionnaire that they had been exposed to an animal during the month preceding the onset of their symptoms, the researchers tested goats, dogs, pigs, and cattle living in the villages of infected patients for antibodies against LayV. They found LayV antibodies in a handful of goats and dogs and identified LayV viral RNA in 27% of the 262 sampled shrews. These findings suggest that the shrew may be a natural reservoir of LayV, passing it between themselves “and somehow infecting people here and there by chance,” Emily Gurley, PhD, MPH, an infectious diseases epidemiologist at Johns Hopkins University, Baltimore, told Nature.
The researchers did not find strong evidence of LayV spreading between the people included in the study. There were no clusters of cases in the same family, within a short time span, or in close geographical proximity. “Of the 35 cases, not a single one is linked,” said Dr. Wang, which Dr. Gurley considered good news. It should be noted, however ,”that the study did retrospective contact tracing on only 15 family members of nine infected individuals, which makes it difficult to determine how exactly the individuals were exposed,” reported Nature.
Vigilance is needed
Should we be worried about a potential new epidemic? The replies from two experts interviewed by Nature were reassuring. “There is no particular need to worry about this virus, but ongoing surveillance is critical,” said Professor Edward Holmes, an evolutionary virologist at the University of Sydney. Regularly testing people and animals for emerging viruses is important to understand the risk for zoonotic diseases – those that can be transmitted from other animals to humans, he said.
It is still not clear how people were infected in the first place – whether directly from shrews or an intermediate animal, said Dr. Gurley. That’s why a lot of research still needs to be done to work out how the virus is spreading in shrews and how people are getting infected, she added.
Nevertheless, Dr. Gurley finds that large outbreaks of infectious diseases typically take off after a lot of false starts. “If we are actively looking for those sparks, then we are in a much better position to stop or to find something early.” Still, she noted that she didn’t see anything in the data to “cause alarm from a pandemic-threat perspective.”
Though there is not currently any cause for worry of a new pandemic, vigilance is crucial. Professor Holmes says there is an urgent need for a global surveillance system to detect virus spillovers and rapidly communicate those results to avoid more pandemics, such as the one sparked by COVID-19. “These sorts of zoonotic spillover events happen all the time,” he said. “The world needs to wake up.”
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
Between 2018 and August 2022, These cases were reported in The New England Journal of Medicine. When asked by Nature about this emerging virus that has until now flown under the radar, scientists said that they were not overly concerned because the virus doesn’t seem to spread easily between people nor is it fatal.
Researchers think that the virus is carried by shrews. It might have infected people directly or through an intermediate animal.
First identified in Langya
The authors describe 35 cases of infection with a virus called Langya henipavirus (LayV) since 2018. It is closely related to two other henipaviruses known to infect people – Hendra virus and Nipah virus. The virus was named Langya after the town in Shandong province in China where the first patient identified with the disease was from, explained coauthor Linfa Wang, PhD, a virologist at Duke-NUS Medical School, Singapore.
Langya can cause respiratory symptoms such as fever, cough, and fatigue. Hendra virus and Nipah virus also cause respiratory infections and can be fatal, the article in Nature reports.
Hendra and Nipah
According to the World Health Organization, Nipah virus, which was discovered in 1999, is a new virus responsible for a zoonosis that causes the disease in animals and humans who have had contact with infected animals. Its name comes from the location where it was first identified in Malaysia. Patients may have asymptomatic infection or symptoms such as acute respiratory infection and severe encephalitis. The case fatality rate is between 40% and 75%.
Nipah virus is closely related to another recently discovered (1994) zoonotic virus called Hendra virus, which is named after the Australian city in which it first appeared. On that day in July 2016, 53 cases were identified involving 70 horses. These incidents remained confined to the northeastern coast of Australia.
Nipah virus and Hendra virus belong to the Paramyxoviridae family. “While the members of this group of viruses are only responsible for a few limited outbreaks, the ability of these viruses to infect a wide range of hosts and cause a disease leading to high fatalities in humans has made them a public health concern,” stated the WHO.
Related to measles
The research team identified LayV while monitoring patients at three hospitals in the eastern Chinese provinces of Shandong and Henan between April 2018 and August 2021. Throughout the study period, the researchers found 35 people infected with LayV, mostly farmers, with symptoms ranging from a cough to severe pneumonia. Participants were recruited into the study if they had a fever. The team sequenced the LayV genome from a throat swab taken from the first patient identified with the disease, a 53-year-old woman.
The LayV genome showed that the virus is most closely related to Mojiang henipavirus, which was first isolated in rats in an abandoned mine in the southern Chinese province of Yunnan in 2012. Henipaviruses belong to the Paramyxoviridae family of viruses, which includes measles, mumps, and many respiratory viruses that infect humans. Several other henipaviruses have been discovered in bats, rats, and shrews from Australia to South Korea and China, but only Hendra, Nipah, and now LayV are known to infect people, according to Nature.
Animal origin likely
Because most patients stated in a questionnaire that they had been exposed to an animal during the month preceding the onset of their symptoms, the researchers tested goats, dogs, pigs, and cattle living in the villages of infected patients for antibodies against LayV. They found LayV antibodies in a handful of goats and dogs and identified LayV viral RNA in 27% of the 262 sampled shrews. These findings suggest that the shrew may be a natural reservoir of LayV, passing it between themselves “and somehow infecting people here and there by chance,” Emily Gurley, PhD, MPH, an infectious diseases epidemiologist at Johns Hopkins University, Baltimore, told Nature.
The researchers did not find strong evidence of LayV spreading between the people included in the study. There were no clusters of cases in the same family, within a short time span, or in close geographical proximity. “Of the 35 cases, not a single one is linked,” said Dr. Wang, which Dr. Gurley considered good news. It should be noted, however ,”that the study did retrospective contact tracing on only 15 family members of nine infected individuals, which makes it difficult to determine how exactly the individuals were exposed,” reported Nature.
Vigilance is needed
Should we be worried about a potential new epidemic? The replies from two experts interviewed by Nature were reassuring. “There is no particular need to worry about this virus, but ongoing surveillance is critical,” said Professor Edward Holmes, an evolutionary virologist at the University of Sydney. Regularly testing people and animals for emerging viruses is important to understand the risk for zoonotic diseases – those that can be transmitted from other animals to humans, he said.
It is still not clear how people were infected in the first place – whether directly from shrews or an intermediate animal, said Dr. Gurley. That’s why a lot of research still needs to be done to work out how the virus is spreading in shrews and how people are getting infected, she added.
Nevertheless, Dr. Gurley finds that large outbreaks of infectious diseases typically take off after a lot of false starts. “If we are actively looking for those sparks, then we are in a much better position to stop or to find something early.” Still, she noted that she didn’t see anything in the data to “cause alarm from a pandemic-threat perspective.”
Though there is not currently any cause for worry of a new pandemic, vigilance is crucial. Professor Holmes says there is an urgent need for a global surveillance system to detect virus spillovers and rapidly communicate those results to avoid more pandemics, such as the one sparked by COVID-19. “These sorts of zoonotic spillover events happen all the time,” he said. “The world needs to wake up.”
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Monkeypox in children and women remains rare, CDC data show
Monkeypox cases in the United States continue to be rare in children younger than 15, women, and in individuals older than 60, according to new data released by the Centers for Disease Control and Prevention. Men aged 26-40 make up the highest proportion of cases.
The age distribution of cases is similar to those of sexually transmitted infections, said Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco. It is most common in younger to middle-aged age groups, and less common in children and older individuals. As of Aug. 21, only 17 children younger than 15 have been diagnosed with monkeypox in the United States, and women make up fewer than 1.5% of cases.
“This data should be very reassuring to parents and to children going to back to school,” Dr. Gandhi said in an interview. After 3 months of monitoring the virus, the data suggest that monkeypox is primarily spreading in networks of men who have sex with men (MSM) through sexual activity, “and that isn’t something we worry about with school-spread illness.”
In addition to the reassuring data about children and monkeypox, the CDC released laboratory testing data, a behavioral survey of MSM, patient data on the antiviral medication tecovirimat (TPOXX), and other case demographics and symptoms.
Though the number of positive monkeypox tests have continued to rise, the test-positivity rates have declined over the past month, data show. Since July 16, the positivity rate has dipped from 54% to 23%. This trend is likely because of an increase in testing availability, said Randolph Hubach, PhD, MPH, the director of the Sexual Health Research Lab at Purdue University, West Lafayette, Ind.
“We also saw this with COVID early on with testing: it was really limited to folks who were symptomatic,” he said in an interview . “As testing ramped up in accessibility, you had a lot more negative results, but because testing was more widely available, you were able to capture more positive results.”
The data also show that case numbers continue to grow in the United States, whereas in other countries that identified cases before the United States – Spain, the United Kingdom, and France, for example – cases have been leveling off, noted Dr. Gandhi.
The CDC also shared responses from a survey of gay, bisexual, and other MSM conducted from Aug. 5-15, about how they have changed their sexual behaviors in response to the monkeypox outbreak. Half of respondents reported reduced one-time sexual encounters, 49% reported reducing sex with partners met on dating apps or at sex venues, and 48% reported reducing their number of sex partners. These responses are “heartening to see,” Dr. Gandhi said, and shows that individuals are taking proactive steps to reduce their potential exposure risk to monkeypox.
More detailed demographic data showed that Black, Hispanic, or Latinx individuals make up an increasing proportion of cases in the United States. In May, 71% of people with reported monkeypox infection were White and 29% were Black. For the week of August 8-14, about a third (31%) of monkeypox cases were in White people, 32% were in Hispanic or Latinx people, and 33% were in Black people.
The most common symptoms of monkeypox were rash (98.6%), malaise (72.7%), fever (72.1%), and chills (68.9%). Rectal pain was reported in 43.9% of patients, and 25% had rectal bleeding.
The CDC also released information on 288 patients with monkeypox treated with TPOXX under compassionate use. The median age of patients was 37 and 98.9% were male. About 40% of recipients were White, 35% were Hispanic, and about 16% were Black. This information does not include every patient treated with TPOXX, the agency said, as providers can begin treatment before submitting paperwork. As of Aug. 18, the CDC had received 400 patient intake forms for TPOXX, according to its website.
The agency has yet to release data on vaccination rates, which Dr. Hubach is eager to see. Demographic information on who is receiving vaccinations, and where, can illuminate issues with access as vaccine eligibility continues to expand. “Vaccination is probably going to be the largest tool within our toolbox to try to inhibit disease acquisition and spread,” he said.
A version of this article first appeared on Medscape.com.
Monkeypox cases in the United States continue to be rare in children younger than 15, women, and in individuals older than 60, according to new data released by the Centers for Disease Control and Prevention. Men aged 26-40 make up the highest proportion of cases.
The age distribution of cases is similar to those of sexually transmitted infections, said Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco. It is most common in younger to middle-aged age groups, and less common in children and older individuals. As of Aug. 21, only 17 children younger than 15 have been diagnosed with monkeypox in the United States, and women make up fewer than 1.5% of cases.
“This data should be very reassuring to parents and to children going to back to school,” Dr. Gandhi said in an interview. After 3 months of monitoring the virus, the data suggest that monkeypox is primarily spreading in networks of men who have sex with men (MSM) through sexual activity, “and that isn’t something we worry about with school-spread illness.”
In addition to the reassuring data about children and monkeypox, the CDC released laboratory testing data, a behavioral survey of MSM, patient data on the antiviral medication tecovirimat (TPOXX), and other case demographics and symptoms.
Though the number of positive monkeypox tests have continued to rise, the test-positivity rates have declined over the past month, data show. Since July 16, the positivity rate has dipped from 54% to 23%. This trend is likely because of an increase in testing availability, said Randolph Hubach, PhD, MPH, the director of the Sexual Health Research Lab at Purdue University, West Lafayette, Ind.
“We also saw this with COVID early on with testing: it was really limited to folks who were symptomatic,” he said in an interview . “As testing ramped up in accessibility, you had a lot more negative results, but because testing was more widely available, you were able to capture more positive results.”
The data also show that case numbers continue to grow in the United States, whereas in other countries that identified cases before the United States – Spain, the United Kingdom, and France, for example – cases have been leveling off, noted Dr. Gandhi.
The CDC also shared responses from a survey of gay, bisexual, and other MSM conducted from Aug. 5-15, about how they have changed their sexual behaviors in response to the monkeypox outbreak. Half of respondents reported reduced one-time sexual encounters, 49% reported reducing sex with partners met on dating apps or at sex venues, and 48% reported reducing their number of sex partners. These responses are “heartening to see,” Dr. Gandhi said, and shows that individuals are taking proactive steps to reduce their potential exposure risk to monkeypox.
More detailed demographic data showed that Black, Hispanic, or Latinx individuals make up an increasing proportion of cases in the United States. In May, 71% of people with reported monkeypox infection were White and 29% were Black. For the week of August 8-14, about a third (31%) of monkeypox cases were in White people, 32% were in Hispanic or Latinx people, and 33% were in Black people.
The most common symptoms of monkeypox were rash (98.6%), malaise (72.7%), fever (72.1%), and chills (68.9%). Rectal pain was reported in 43.9% of patients, and 25% had rectal bleeding.
The CDC also released information on 288 patients with monkeypox treated with TPOXX under compassionate use. The median age of patients was 37 and 98.9% were male. About 40% of recipients were White, 35% were Hispanic, and about 16% were Black. This information does not include every patient treated with TPOXX, the agency said, as providers can begin treatment before submitting paperwork. As of Aug. 18, the CDC had received 400 patient intake forms for TPOXX, according to its website.
The agency has yet to release data on vaccination rates, which Dr. Hubach is eager to see. Demographic information on who is receiving vaccinations, and where, can illuminate issues with access as vaccine eligibility continues to expand. “Vaccination is probably going to be the largest tool within our toolbox to try to inhibit disease acquisition and spread,” he said.
A version of this article first appeared on Medscape.com.
Monkeypox cases in the United States continue to be rare in children younger than 15, women, and in individuals older than 60, according to new data released by the Centers for Disease Control and Prevention. Men aged 26-40 make up the highest proportion of cases.
The age distribution of cases is similar to those of sexually transmitted infections, said Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco. It is most common in younger to middle-aged age groups, and less common in children and older individuals. As of Aug. 21, only 17 children younger than 15 have been diagnosed with monkeypox in the United States, and women make up fewer than 1.5% of cases.
“This data should be very reassuring to parents and to children going to back to school,” Dr. Gandhi said in an interview. After 3 months of monitoring the virus, the data suggest that monkeypox is primarily spreading in networks of men who have sex with men (MSM) through sexual activity, “and that isn’t something we worry about with school-spread illness.”
In addition to the reassuring data about children and monkeypox, the CDC released laboratory testing data, a behavioral survey of MSM, patient data on the antiviral medication tecovirimat (TPOXX), and other case demographics and symptoms.
Though the number of positive monkeypox tests have continued to rise, the test-positivity rates have declined over the past month, data show. Since July 16, the positivity rate has dipped from 54% to 23%. This trend is likely because of an increase in testing availability, said Randolph Hubach, PhD, MPH, the director of the Sexual Health Research Lab at Purdue University, West Lafayette, Ind.
“We also saw this with COVID early on with testing: it was really limited to folks who were symptomatic,” he said in an interview . “As testing ramped up in accessibility, you had a lot more negative results, but because testing was more widely available, you were able to capture more positive results.”
The data also show that case numbers continue to grow in the United States, whereas in other countries that identified cases before the United States – Spain, the United Kingdom, and France, for example – cases have been leveling off, noted Dr. Gandhi.
The CDC also shared responses from a survey of gay, bisexual, and other MSM conducted from Aug. 5-15, about how they have changed their sexual behaviors in response to the monkeypox outbreak. Half of respondents reported reduced one-time sexual encounters, 49% reported reducing sex with partners met on dating apps or at sex venues, and 48% reported reducing their number of sex partners. These responses are “heartening to see,” Dr. Gandhi said, and shows that individuals are taking proactive steps to reduce their potential exposure risk to monkeypox.
More detailed demographic data showed that Black, Hispanic, or Latinx individuals make up an increasing proportion of cases in the United States. In May, 71% of people with reported monkeypox infection were White and 29% were Black. For the week of August 8-14, about a third (31%) of monkeypox cases were in White people, 32% were in Hispanic or Latinx people, and 33% were in Black people.
The most common symptoms of monkeypox were rash (98.6%), malaise (72.7%), fever (72.1%), and chills (68.9%). Rectal pain was reported in 43.9% of patients, and 25% had rectal bleeding.
The CDC also released information on 288 patients with monkeypox treated with TPOXX under compassionate use. The median age of patients was 37 and 98.9% were male. About 40% of recipients were White, 35% were Hispanic, and about 16% were Black. This information does not include every patient treated with TPOXX, the agency said, as providers can begin treatment before submitting paperwork. As of Aug. 18, the CDC had received 400 patient intake forms for TPOXX, according to its website.
The agency has yet to release data on vaccination rates, which Dr. Hubach is eager to see. Demographic information on who is receiving vaccinations, and where, can illuminate issues with access as vaccine eligibility continues to expand. “Vaccination is probably going to be the largest tool within our toolbox to try to inhibit disease acquisition and spread,” he said.
A version of this article first appeared on Medscape.com.
Preparing for back to school amid monkeypox outbreak and ever-changing COVID landscape
Unlike last school year, there are now vaccines available for all over the age of 6 months, and home rapid antigen tests are more readily available. Additionally, many have now been exposed either by infection or vaccination to the virus.
The CDC has removed the recommendations for maintaining cohorts in the K-12 population. This changing landscape along with differing levels of personal risk make it challenging to counsel families about what to expect in terms of COVID this year.
The best defense that we currently have against COVID is the vaccine. Although it seems that many are susceptible to the virus despite the vaccine, those who have been vaccinated are less susceptible to serious disease, including young children.
As older children may be heading to college, it is important
to encourage them to isolate when they have symptoms, even when they test negative for COVID as we would all like to avoid being sick in general.
Additionally, they should pay attention to the COVID risk level in their area and wear masks, particularly when indoors, as the levels increase. College students should have a plan for where they can isolate when not feeling well. If anyone does test positive for COVID, they should follow the most recent quarantine guidelines, including wearing a well fitted mask when they do begin returning to activities.
Monkeypox
We now have a new health concern for this school year.
Monkeypox has come onto the scene with information changing as rapidly as information previously did for COVID. With this virus, we must particularly counsel those heading away to college to be careful to limit their exposure to this disease.
Dormitories and other congregate settings are high-risk locations for the spread of monkeypox. Particularly, students headed to stay in dormitories should be counseled about avoiding:
- sexual activity with those with lesions consistent with monkeypox;
- sharing eating and drinking utensils; and
- sleeping in the same bed as or sharing bedding or towels with anyone with a diagnosis of or lesions consistent with monkeypox.
Additionally, as with prevention of all infections, it is important to frequently wash hands or use alcohol-based sanitizer before eating, and avoid touching the face after using the restroom.
Guidance for those eligible for vaccines against monkeypox seems to be quickly changing as well.
At the time of this article, CDC guidance recommends the vaccine against monkeypox for:
- those considered to be at high risk for it, including those identified by public health officials as a contact of someone with monkeypox;
- those who are aware that a sexual partner had a diagnosis of monkeypox within the past 2 weeks;
- those with multiple sex partners in the past 2 weeks in an area with known monkeypox; and
- those whose jobs may expose them to monkeypox.
Currently, the CDC recommends the vaccine JYNNEOS, a two-dose vaccine that reaches maximum protection after fourteen days. Ultimately, guidance is likely to continue to quickly change for both COVID-19 and Monkeypox throughout the fall. It is possible that new vaccinations will become available, and families and physicians alike will have many questions.
Primary care offices should ensure that someone is keeping up to date with the latest guidance to share with the office so that physicians may share accurate information with their patients.
Families should be counseled that we anticipate information about monkeypox, particularly related to vaccinations, to continue to change, as it has during all stages of the COVID pandemic.
As always, patients should be reminded to continue regular routine vaccinations, including the annual influenza vaccine.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
Unlike last school year, there are now vaccines available for all over the age of 6 months, and home rapid antigen tests are more readily available. Additionally, many have now been exposed either by infection or vaccination to the virus.
The CDC has removed the recommendations for maintaining cohorts in the K-12 population. This changing landscape along with differing levels of personal risk make it challenging to counsel families about what to expect in terms of COVID this year.
The best defense that we currently have against COVID is the vaccine. Although it seems that many are susceptible to the virus despite the vaccine, those who have been vaccinated are less susceptible to serious disease, including young children.
As older children may be heading to college, it is important
to encourage them to isolate when they have symptoms, even when they test negative for COVID as we would all like to avoid being sick in general.
Additionally, they should pay attention to the COVID risk level in their area and wear masks, particularly when indoors, as the levels increase. College students should have a plan for where they can isolate when not feeling well. If anyone does test positive for COVID, they should follow the most recent quarantine guidelines, including wearing a well fitted mask when they do begin returning to activities.
Monkeypox
We now have a new health concern for this school year.
Monkeypox has come onto the scene with information changing as rapidly as information previously did for COVID. With this virus, we must particularly counsel those heading away to college to be careful to limit their exposure to this disease.
Dormitories and other congregate settings are high-risk locations for the spread of monkeypox. Particularly, students headed to stay in dormitories should be counseled about avoiding:
- sexual activity with those with lesions consistent with monkeypox;
- sharing eating and drinking utensils; and
- sleeping in the same bed as or sharing bedding or towels with anyone with a diagnosis of or lesions consistent with monkeypox.
Additionally, as with prevention of all infections, it is important to frequently wash hands or use alcohol-based sanitizer before eating, and avoid touching the face after using the restroom.
Guidance for those eligible for vaccines against monkeypox seems to be quickly changing as well.
At the time of this article, CDC guidance recommends the vaccine against monkeypox for:
- those considered to be at high risk for it, including those identified by public health officials as a contact of someone with monkeypox;
- those who are aware that a sexual partner had a diagnosis of monkeypox within the past 2 weeks;
- those with multiple sex partners in the past 2 weeks in an area with known monkeypox; and
- those whose jobs may expose them to monkeypox.
Currently, the CDC recommends the vaccine JYNNEOS, a two-dose vaccine that reaches maximum protection after fourteen days. Ultimately, guidance is likely to continue to quickly change for both COVID-19 and Monkeypox throughout the fall. It is possible that new vaccinations will become available, and families and physicians alike will have many questions.
Primary care offices should ensure that someone is keeping up to date with the latest guidance to share with the office so that physicians may share accurate information with their patients.
Families should be counseled that we anticipate information about monkeypox, particularly related to vaccinations, to continue to change, as it has during all stages of the COVID pandemic.
As always, patients should be reminded to continue regular routine vaccinations, including the annual influenza vaccine.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
Unlike last school year, there are now vaccines available for all over the age of 6 months, and home rapid antigen tests are more readily available. Additionally, many have now been exposed either by infection or vaccination to the virus.
The CDC has removed the recommendations for maintaining cohorts in the K-12 population. This changing landscape along with differing levels of personal risk make it challenging to counsel families about what to expect in terms of COVID this year.
The best defense that we currently have against COVID is the vaccine. Although it seems that many are susceptible to the virus despite the vaccine, those who have been vaccinated are less susceptible to serious disease, including young children.
As older children may be heading to college, it is important
to encourage them to isolate when they have symptoms, even when they test negative for COVID as we would all like to avoid being sick in general.
Additionally, they should pay attention to the COVID risk level in their area and wear masks, particularly when indoors, as the levels increase. College students should have a plan for where they can isolate when not feeling well. If anyone does test positive for COVID, they should follow the most recent quarantine guidelines, including wearing a well fitted mask when they do begin returning to activities.
Monkeypox
We now have a new health concern for this school year.
Monkeypox has come onto the scene with information changing as rapidly as information previously did for COVID. With this virus, we must particularly counsel those heading away to college to be careful to limit their exposure to this disease.
Dormitories and other congregate settings are high-risk locations for the spread of monkeypox. Particularly, students headed to stay in dormitories should be counseled about avoiding:
- sexual activity with those with lesions consistent with monkeypox;
- sharing eating and drinking utensils; and
- sleeping in the same bed as or sharing bedding or towels with anyone with a diagnosis of or lesions consistent with monkeypox.
Additionally, as with prevention of all infections, it is important to frequently wash hands or use alcohol-based sanitizer before eating, and avoid touching the face after using the restroom.
Guidance for those eligible for vaccines against monkeypox seems to be quickly changing as well.
At the time of this article, CDC guidance recommends the vaccine against monkeypox for:
- those considered to be at high risk for it, including those identified by public health officials as a contact of someone with monkeypox;
- those who are aware that a sexual partner had a diagnosis of monkeypox within the past 2 weeks;
- those with multiple sex partners in the past 2 weeks in an area with known monkeypox; and
- those whose jobs may expose them to monkeypox.
Currently, the CDC recommends the vaccine JYNNEOS, a two-dose vaccine that reaches maximum protection after fourteen days. Ultimately, guidance is likely to continue to quickly change for both COVID-19 and Monkeypox throughout the fall. It is possible that new vaccinations will become available, and families and physicians alike will have many questions.
Primary care offices should ensure that someone is keeping up to date with the latest guidance to share with the office so that physicians may share accurate information with their patients.
Families should be counseled that we anticipate information about monkeypox, particularly related to vaccinations, to continue to change, as it has during all stages of the COVID pandemic.
As always, patients should be reminded to continue regular routine vaccinations, including the annual influenza vaccine.
Dr. Wheat is a family physician at Erie Family Health Center and program director of Northwestern University’s McGaw Family Medicine residency program, both in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at fpnews@mdedge.com.
Pfizer seeks approval for updated COVID booster
Pfizer has sent an application to the Food and Drug Administration for emergency use authorization of its updated COVID-19 booster vaccine for the fall of 2022, the company announced on Aug. 22.
The vaccine, which is adapted for the BA.4 and BA.5 Omicron variants, would be meant for ages 12 and older. If authorized by the FDA, the doses could ship as soon as September.
“Having rapidly scaled up production, we are positioned to immediately begin distribution of the bivalent Omicron BA.4/BA.5 boosters, if authorized, to help protect individuals and families as we prepare for potential fall and winter surges,” Albert Bourla, PhD, Pfizer’s chairman and CEO, said in the statement.
Earlier this year, the FDA ordered vaccine makers such as Pfizer and Moderna to update their shots to target BA.4 and BA.5, which are better at escaping immunity from earlier vaccines and previous infections.
The United States has a contract to buy 105 million of the Pfizer doses and 66 million of the Moderna doses, according to The Associated Press. Moderna is expected to file its FDA application soon as well.
The new shots target both the original spike protein on the coronavirus and the spike mutations carried by BA.4 and BA.5. For now, BA.5 is causing 89% of new infections in the United States, followed by BA.4.6 with 6.3% and BA.4 with 4.3%, according to the latest Centers for Disease Control and Prevention data.
There’s no way to tell if BA.5 will still be the dominant strain this winter or if new variant will replace it, the AP reported. But public health officials have supported the updated boosters as a way to target the most recent strains and increase immunity again.
On Aug. 15, Great Britain became the first country to authorize another one of Moderna’s updated vaccines, which adds protection against BA.1, or the original Omicron strain that became dominant in the winter of 2021-2022. European regulators are considering this shot, the AP reported, but the United States opted not to use this version since new Omicron variants have become dominant.
To approve the latest Pfizer shot, the FDA will rely on scientific testing of prior updates to the vaccine, rather than the newest boosters, to decide whether to fast-track the updated shots for fall, the AP reported. This method is like how flu vaccines are updated each year without large studies that take months.
Previously, Pfizer announced results from a study that found the earlier Omicron update significantly boosted antibodies capable of fighting the BA.1 variant and provided some protection against BA.4 and BA.5. The company’s latest FDA application contains that data and animal testing on the newest booster, the AP reported.
Pfizer will start a trial using the BA.4/BA.5 booster in coming weeks to get more data on how well the latest shot works. Moderna has begun a similar study.
The full results from these studies won’t be available before a fall booster campaign, which is why the FDA and public health officials have called for an updated shot to be ready for distribution in September.
“It’s clear that none of these vaccines are going to completely prevent infection,” Rachel Presti, MD, a researcher with the Moderna trial and an infectious diseases specialist at Washington University in St. Louis, told the AP.
But previous studies of variant booster candidates have shown that “you still get a broader immune response giving a variant booster than giving the same booster,” she said.
A version of this article first appeared on WebMD.com.
Pfizer has sent an application to the Food and Drug Administration for emergency use authorization of its updated COVID-19 booster vaccine for the fall of 2022, the company announced on Aug. 22.
The vaccine, which is adapted for the BA.4 and BA.5 Omicron variants, would be meant for ages 12 and older. If authorized by the FDA, the doses could ship as soon as September.
“Having rapidly scaled up production, we are positioned to immediately begin distribution of the bivalent Omicron BA.4/BA.5 boosters, if authorized, to help protect individuals and families as we prepare for potential fall and winter surges,” Albert Bourla, PhD, Pfizer’s chairman and CEO, said in the statement.
Earlier this year, the FDA ordered vaccine makers such as Pfizer and Moderna to update their shots to target BA.4 and BA.5, which are better at escaping immunity from earlier vaccines and previous infections.
The United States has a contract to buy 105 million of the Pfizer doses and 66 million of the Moderna doses, according to The Associated Press. Moderna is expected to file its FDA application soon as well.
The new shots target both the original spike protein on the coronavirus and the spike mutations carried by BA.4 and BA.5. For now, BA.5 is causing 89% of new infections in the United States, followed by BA.4.6 with 6.3% and BA.4 with 4.3%, according to the latest Centers for Disease Control and Prevention data.
There’s no way to tell if BA.5 will still be the dominant strain this winter or if new variant will replace it, the AP reported. But public health officials have supported the updated boosters as a way to target the most recent strains and increase immunity again.
On Aug. 15, Great Britain became the first country to authorize another one of Moderna’s updated vaccines, which adds protection against BA.1, or the original Omicron strain that became dominant in the winter of 2021-2022. European regulators are considering this shot, the AP reported, but the United States opted not to use this version since new Omicron variants have become dominant.
To approve the latest Pfizer shot, the FDA will rely on scientific testing of prior updates to the vaccine, rather than the newest boosters, to decide whether to fast-track the updated shots for fall, the AP reported. This method is like how flu vaccines are updated each year without large studies that take months.
Previously, Pfizer announced results from a study that found the earlier Omicron update significantly boosted antibodies capable of fighting the BA.1 variant and provided some protection against BA.4 and BA.5. The company’s latest FDA application contains that data and animal testing on the newest booster, the AP reported.
Pfizer will start a trial using the BA.4/BA.5 booster in coming weeks to get more data on how well the latest shot works. Moderna has begun a similar study.
The full results from these studies won’t be available before a fall booster campaign, which is why the FDA and public health officials have called for an updated shot to be ready for distribution in September.
“It’s clear that none of these vaccines are going to completely prevent infection,” Rachel Presti, MD, a researcher with the Moderna trial and an infectious diseases specialist at Washington University in St. Louis, told the AP.
But previous studies of variant booster candidates have shown that “you still get a broader immune response giving a variant booster than giving the same booster,” she said.
A version of this article first appeared on WebMD.com.
Pfizer has sent an application to the Food and Drug Administration for emergency use authorization of its updated COVID-19 booster vaccine for the fall of 2022, the company announced on Aug. 22.
The vaccine, which is adapted for the BA.4 and BA.5 Omicron variants, would be meant for ages 12 and older. If authorized by the FDA, the doses could ship as soon as September.
“Having rapidly scaled up production, we are positioned to immediately begin distribution of the bivalent Omicron BA.4/BA.5 boosters, if authorized, to help protect individuals and families as we prepare for potential fall and winter surges,” Albert Bourla, PhD, Pfizer’s chairman and CEO, said in the statement.
Earlier this year, the FDA ordered vaccine makers such as Pfizer and Moderna to update their shots to target BA.4 and BA.5, which are better at escaping immunity from earlier vaccines and previous infections.
The United States has a contract to buy 105 million of the Pfizer doses and 66 million of the Moderna doses, according to The Associated Press. Moderna is expected to file its FDA application soon as well.
The new shots target both the original spike protein on the coronavirus and the spike mutations carried by BA.4 and BA.5. For now, BA.5 is causing 89% of new infections in the United States, followed by BA.4.6 with 6.3% and BA.4 with 4.3%, according to the latest Centers for Disease Control and Prevention data.
There’s no way to tell if BA.5 will still be the dominant strain this winter or if new variant will replace it, the AP reported. But public health officials have supported the updated boosters as a way to target the most recent strains and increase immunity again.
On Aug. 15, Great Britain became the first country to authorize another one of Moderna’s updated vaccines, which adds protection against BA.1, or the original Omicron strain that became dominant in the winter of 2021-2022. European regulators are considering this shot, the AP reported, but the United States opted not to use this version since new Omicron variants have become dominant.
To approve the latest Pfizer shot, the FDA will rely on scientific testing of prior updates to the vaccine, rather than the newest boosters, to decide whether to fast-track the updated shots for fall, the AP reported. This method is like how flu vaccines are updated each year without large studies that take months.
Previously, Pfizer announced results from a study that found the earlier Omicron update significantly boosted antibodies capable of fighting the BA.1 variant and provided some protection against BA.4 and BA.5. The company’s latest FDA application contains that data and animal testing on the newest booster, the AP reported.
Pfizer will start a trial using the BA.4/BA.5 booster in coming weeks to get more data on how well the latest shot works. Moderna has begun a similar study.
The full results from these studies won’t be available before a fall booster campaign, which is why the FDA and public health officials have called for an updated shot to be ready for distribution in September.
“It’s clear that none of these vaccines are going to completely prevent infection,” Rachel Presti, MD, a researcher with the Moderna trial and an infectious diseases specialist at Washington University in St. Louis, told the AP.
But previous studies of variant booster candidates have shown that “you still get a broader immune response giving a variant booster than giving the same booster,” she said.
A version of this article first appeared on WebMD.com.
Are we up the creek without a paddle? What COVID, monkeypox, and nature are trying to tell us
Monkeypox. Polio. Covid. A quick glance at the news on any given day seems to indicate that outbreaks, epidemics, and perhaps even pandemics are increasing in frequency.
Granted, these types of events are hardly new; from the plagues of the 5th and 13th centuries to the Spanish flu in the 20th century and SARS-CoV-2 today, they’ve been with us from time immemorial.
What appears to be different, however, is not their frequency, but their intensity, with research reinforcing that we may be facing unique challenges and smaller windows to intervene as we move forward.
Findings from a modeling study, published in 2021 in Proceedings of the National Academy of Sciences, underscore that without effective intervention, the probability of extreme events like COVID-19 will likely increase threefold in the coming decades.
Amesh Adalja, MD, senior scholar, Johns Hopkins Center for Health Security, Baltimore, told this news organization.
“It’s all been based on some unusual cluster of cases that were causing severe disease and overwhelming local authorities. So often, like Indiana Jones, somebody got dispatched to deal with an outbreak,” Dr. Adalja said.
In a perfect post-COVID world, government bodies, scientists, clinicians, and others would cross silos to coordinate pandemic prevention, not just preparedness. The public would trust those who carry the title “public health” in their daily responsibilities, and in turn, public health experts would get back to their core responsibility – infectious disease preparedness – the role they were initially assigned following Europe’s Black Death during the 14th century. Instead, the world finds itself at a crossroads, with emerging and reemerging infectious disease outbreaks that on the surface appear to arise haphazardly but in reality are the result of decades of reaction and containment policies aimed at putting out fires, not addressing their cause.
Dr. Adalja noted that only when the threat of biological weapons became a reality in the mid-2000s was there a realization that economies of scale could be exploited by merging interests and efforts to develop health security medical countermeasures. For example, it encouraged governments to more closely integrate agencies like the Biomedical Advanced Research and Development Authority and infectious disease research organizations and individuals.
Still, while significant strides have been made in certain areas, the ongoing COVID-19 pandemic has revealed substantial weaknesses remaining in public and private health systems, as well as major gaps in infectious disease preparedness.
The role of spillover events
No matter whom you ask, scientists, public health and conservation experts, and infectious disease clinicians all point to one of the most important threats to human health. As Walt Kelly’s Pogo famously put it, “We have met the enemy, and he is us.”
“The reason why these outbreaks of novel infectious diseases are increasingly occurring is because of human-driven environmental change, particularly land use, unsafe practices when raising farmed animals, and commercial wildlife markets,” Neil M. Vora, MD, a physician specializing in pandemic prevention at Conservation International and a former Centers for Disease Control and Prevention epidemic intelligence officer, said in an interview.
In fact, more than 60% of emerging infections and diseases are due to these “spillover events” (zoonotic spillover) that occur when pathogens that commonly circulate in wildlife jump over to new, human hosts.
Several examples come to mind.
COVID-19 may have begun as an enzootic virus from two undetermined animals, using the Huanan Seafood Market as a possible intermediate reservoir, according to a July 26 preprint in the journal Science.
Likewise, while the Ebola virus was originally attributed to deforestation efforts to create palm oil (which allowed fruit bat carriers to transfer the virus to humans), recent research suggests that bats dwelling in the walls of human dwellings and hospitals are responsible for the 2018 outbreak in the Democratic Republic of Congo.
(Incidentally, just this week, a new Ebola case was confirmed in Eastern Congo, and it has been genetically linked to the previous outbreak, despite that outbreak having been declared over in early July.)
“When we clear forests, we create opportunities for humans to live alongside the forest edge and displace wildlife. There’s evidence that shows when [these] biodiverse areas are cleared, specialist species that evolved to live in the forests first start to disappear, whereas generalist species – rodents and bats – continue to survive and are able to carry pathogens that can be passed on to humans,” Dr. Vora explained.
So far, China’s outbreak of the novel Langya henipavirus is believed to have spread (either directly or indirectly) by rodents and shrews, according to reports from public health authorities like the European Centre for Disease Prevention and Control, which is currently monitoring the situation.
Yet, an overreliance on surveillance and containment only perpetuates what Dr. Vora says are cycles of panic and neglect.
“We saw it with Ebola in 2015, in 2016 to 2017 with Zika, you see it with tuberculosis, with sexually transmitted infections, and with COVID. You have policymakers working on solutions, and once they think that they’ve fixed the problem, they’re going to move on to the next crisis.”
It’s also a question of equity.
Reports detailing the reemergence of monkeypox in Nigeria in 2017 were largely ignored, despite the fact that the United States assisted in diagnosing an early case in an 11-year-old boy. At the time, it was clear that the virus was spreading by human-to-human transmission versus animal-to-human transmission, something that had not been seen previously.
“The current model [is] waiting for pathogens to spill over and then [continuing] to spread signals that rich countries are tolerant of these outbreaks so long as they don’t grow into epidemics or pandemics,” Dr. Vora said.
This model is clearly broken; roughly 5 years after Nigeria reported the resurgence of monkeypox, the United States has more than 14,000 confirmed cases, which represents more than a quarter of the total number of cases reported worldwide.
Public health on the brink
I’s difficult to imagine a future without outbreaks and more pandemics, and if experts are to be believed, we are ill-prepared.
“I think that we are in a situation where this is a major threat, and people have become complacent about it,” said Dr. Adalja, who noted that we should be asking ourselves if the “government is actually in a position to be able to respond in a way that we need them to or is [that response] tied up in bureaucracy and inefficiency?”
COVID-19 should have been seen as a wake-up call, and many of those deaths were preventable. “With monkeypox, they’re faltering; it should have been a layup, not a disaster,” he emphasized.
Ellen Eaton, MD, associate professor of infectious diseases at the University of Alabama at Birmingham, also pointed to the reality that by the time COVID-19 reached North America, the United States had already moved away from the model of the public health department as the epicenter of knowledge, education, awareness, and, ironically, public health.
“Thinking about my community, very few people knew the face and name of our local and state health officers,” she told this news organization.
“There was just this inherent mistrust of these people. If you add in a lot of talking heads, a lot of politicians and messaging from non-experts that countered what was coming out of our public health agencies early, you had this huge disconnect; in the South, it was the perfect storm for vaccine hesitancy.”
At last count, this perfect storm has led to 1.46 million COVID cases and just over 20,000 deaths – many of which were preventable – in Alabama alone.
“In certain parts of America, we were starting with a broken system with limited resources and few providers,” Dr. Eaton explained.
Dr. Eaton said that a lot of fields, not just medicine and public health, have finite resources that have been stretched to capacity by COVID, and now monkeypox, and wondered what was next as we’re headed into autumn and influenza season. But she also mentioned the tremendous implications of climate change on infectious diseases and community health and wellness.
“There’s a tremendous need to have the ability to survey not just humans but also how the disease burden in our environment that is fluctuating with climate change is going to impact communities in really important ways,” Dr. Eaton said.
Upstream prevention
Dr. Vora said he could not agree more and believes that upstream prevention holds the key.
“We have to make sure while there’s tension on this issue that the right solutions are implemented,” he said.
In coming years, postspillover containment strategies – vaccine research and development and strengthening health care surveillance, for example – are likely to become inadequate.
“We saw it with COVID and we are seeing it again with monkeypox,” Dr. Vora said. “We also have to invest further upstream to prevent spillovers in the first place, for example, by addressing deforestation, commercial wildlife markets and trade, [and] infection control when raising farm animals.”
“The thing is, when you invest in those upstream solutions, you are also mitigating climate change and loss of biodiversity. I’m not saying that we should not invest in postspillover containment efforts; we’re never going to contain every spillover. But we also have to invest in prevention,” he added.
In a piece published in Nature, Dr. Vora and his coauthors acknowledge that several international bodies such as the World Health Organization and G7 have invested in initiatives to facilitate coordinated, global responses to climate change, pandemic preparedness, and response. But they point out that these efforts fail to “explicitly address the negative feedback cycle between environmental degradation, wildlife exploitation, and the emergence of pathogens.”
“Environmental conservation is no longer a left-wing fringe issue, it’s moving into public consciousness, and ... it is public health,” Dr. Vora said. “When we destroy nature, we’re destroying our own ability to survive.”
Dr. Adalja, Dr. Vora, and Dr. Eaton report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox. Polio. Covid. A quick glance at the news on any given day seems to indicate that outbreaks, epidemics, and perhaps even pandemics are increasing in frequency.
Granted, these types of events are hardly new; from the plagues of the 5th and 13th centuries to the Spanish flu in the 20th century and SARS-CoV-2 today, they’ve been with us from time immemorial.
What appears to be different, however, is not their frequency, but their intensity, with research reinforcing that we may be facing unique challenges and smaller windows to intervene as we move forward.
Findings from a modeling study, published in 2021 in Proceedings of the National Academy of Sciences, underscore that without effective intervention, the probability of extreme events like COVID-19 will likely increase threefold in the coming decades.
Amesh Adalja, MD, senior scholar, Johns Hopkins Center for Health Security, Baltimore, told this news organization.
“It’s all been based on some unusual cluster of cases that were causing severe disease and overwhelming local authorities. So often, like Indiana Jones, somebody got dispatched to deal with an outbreak,” Dr. Adalja said.
In a perfect post-COVID world, government bodies, scientists, clinicians, and others would cross silos to coordinate pandemic prevention, not just preparedness. The public would trust those who carry the title “public health” in their daily responsibilities, and in turn, public health experts would get back to their core responsibility – infectious disease preparedness – the role they were initially assigned following Europe’s Black Death during the 14th century. Instead, the world finds itself at a crossroads, with emerging and reemerging infectious disease outbreaks that on the surface appear to arise haphazardly but in reality are the result of decades of reaction and containment policies aimed at putting out fires, not addressing their cause.
Dr. Adalja noted that only when the threat of biological weapons became a reality in the mid-2000s was there a realization that economies of scale could be exploited by merging interests and efforts to develop health security medical countermeasures. For example, it encouraged governments to more closely integrate agencies like the Biomedical Advanced Research and Development Authority and infectious disease research organizations and individuals.
Still, while significant strides have been made in certain areas, the ongoing COVID-19 pandemic has revealed substantial weaknesses remaining in public and private health systems, as well as major gaps in infectious disease preparedness.
The role of spillover events
No matter whom you ask, scientists, public health and conservation experts, and infectious disease clinicians all point to one of the most important threats to human health. As Walt Kelly’s Pogo famously put it, “We have met the enemy, and he is us.”
“The reason why these outbreaks of novel infectious diseases are increasingly occurring is because of human-driven environmental change, particularly land use, unsafe practices when raising farmed animals, and commercial wildlife markets,” Neil M. Vora, MD, a physician specializing in pandemic prevention at Conservation International and a former Centers for Disease Control and Prevention epidemic intelligence officer, said in an interview.
In fact, more than 60% of emerging infections and diseases are due to these “spillover events” (zoonotic spillover) that occur when pathogens that commonly circulate in wildlife jump over to new, human hosts.
Several examples come to mind.
COVID-19 may have begun as an enzootic virus from two undetermined animals, using the Huanan Seafood Market as a possible intermediate reservoir, according to a July 26 preprint in the journal Science.
Likewise, while the Ebola virus was originally attributed to deforestation efforts to create palm oil (which allowed fruit bat carriers to transfer the virus to humans), recent research suggests that bats dwelling in the walls of human dwellings and hospitals are responsible for the 2018 outbreak in the Democratic Republic of Congo.
(Incidentally, just this week, a new Ebola case was confirmed in Eastern Congo, and it has been genetically linked to the previous outbreak, despite that outbreak having been declared over in early July.)
“When we clear forests, we create opportunities for humans to live alongside the forest edge and displace wildlife. There’s evidence that shows when [these] biodiverse areas are cleared, specialist species that evolved to live in the forests first start to disappear, whereas generalist species – rodents and bats – continue to survive and are able to carry pathogens that can be passed on to humans,” Dr. Vora explained.
So far, China’s outbreak of the novel Langya henipavirus is believed to have spread (either directly or indirectly) by rodents and shrews, according to reports from public health authorities like the European Centre for Disease Prevention and Control, which is currently monitoring the situation.
Yet, an overreliance on surveillance and containment only perpetuates what Dr. Vora says are cycles of panic and neglect.
“We saw it with Ebola in 2015, in 2016 to 2017 with Zika, you see it with tuberculosis, with sexually transmitted infections, and with COVID. You have policymakers working on solutions, and once they think that they’ve fixed the problem, they’re going to move on to the next crisis.”
It’s also a question of equity.
Reports detailing the reemergence of monkeypox in Nigeria in 2017 were largely ignored, despite the fact that the United States assisted in diagnosing an early case in an 11-year-old boy. At the time, it was clear that the virus was spreading by human-to-human transmission versus animal-to-human transmission, something that had not been seen previously.
“The current model [is] waiting for pathogens to spill over and then [continuing] to spread signals that rich countries are tolerant of these outbreaks so long as they don’t grow into epidemics or pandemics,” Dr. Vora said.
This model is clearly broken; roughly 5 years after Nigeria reported the resurgence of monkeypox, the United States has more than 14,000 confirmed cases, which represents more than a quarter of the total number of cases reported worldwide.
Public health on the brink
I’s difficult to imagine a future without outbreaks and more pandemics, and if experts are to be believed, we are ill-prepared.
“I think that we are in a situation where this is a major threat, and people have become complacent about it,” said Dr. Adalja, who noted that we should be asking ourselves if the “government is actually in a position to be able to respond in a way that we need them to or is [that response] tied up in bureaucracy and inefficiency?”
COVID-19 should have been seen as a wake-up call, and many of those deaths were preventable. “With monkeypox, they’re faltering; it should have been a layup, not a disaster,” he emphasized.
Ellen Eaton, MD, associate professor of infectious diseases at the University of Alabama at Birmingham, also pointed to the reality that by the time COVID-19 reached North America, the United States had already moved away from the model of the public health department as the epicenter of knowledge, education, awareness, and, ironically, public health.
“Thinking about my community, very few people knew the face and name of our local and state health officers,” she told this news organization.
“There was just this inherent mistrust of these people. If you add in a lot of talking heads, a lot of politicians and messaging from non-experts that countered what was coming out of our public health agencies early, you had this huge disconnect; in the South, it was the perfect storm for vaccine hesitancy.”
At last count, this perfect storm has led to 1.46 million COVID cases and just over 20,000 deaths – many of which were preventable – in Alabama alone.
“In certain parts of America, we were starting with a broken system with limited resources and few providers,” Dr. Eaton explained.
Dr. Eaton said that a lot of fields, not just medicine and public health, have finite resources that have been stretched to capacity by COVID, and now monkeypox, and wondered what was next as we’re headed into autumn and influenza season. But she also mentioned the tremendous implications of climate change on infectious diseases and community health and wellness.
“There’s a tremendous need to have the ability to survey not just humans but also how the disease burden in our environment that is fluctuating with climate change is going to impact communities in really important ways,” Dr. Eaton said.
Upstream prevention
Dr. Vora said he could not agree more and believes that upstream prevention holds the key.
“We have to make sure while there’s tension on this issue that the right solutions are implemented,” he said.
In coming years, postspillover containment strategies – vaccine research and development and strengthening health care surveillance, for example – are likely to become inadequate.
“We saw it with COVID and we are seeing it again with monkeypox,” Dr. Vora said. “We also have to invest further upstream to prevent spillovers in the first place, for example, by addressing deforestation, commercial wildlife markets and trade, [and] infection control when raising farm animals.”
“The thing is, when you invest in those upstream solutions, you are also mitigating climate change and loss of biodiversity. I’m not saying that we should not invest in postspillover containment efforts; we’re never going to contain every spillover. But we also have to invest in prevention,” he added.
In a piece published in Nature, Dr. Vora and his coauthors acknowledge that several international bodies such as the World Health Organization and G7 have invested in initiatives to facilitate coordinated, global responses to climate change, pandemic preparedness, and response. But they point out that these efforts fail to “explicitly address the negative feedback cycle between environmental degradation, wildlife exploitation, and the emergence of pathogens.”
“Environmental conservation is no longer a left-wing fringe issue, it’s moving into public consciousness, and ... it is public health,” Dr. Vora said. “When we destroy nature, we’re destroying our own ability to survive.”
Dr. Adalja, Dr. Vora, and Dr. Eaton report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Monkeypox. Polio. Covid. A quick glance at the news on any given day seems to indicate that outbreaks, epidemics, and perhaps even pandemics are increasing in frequency.
Granted, these types of events are hardly new; from the plagues of the 5th and 13th centuries to the Spanish flu in the 20th century and SARS-CoV-2 today, they’ve been with us from time immemorial.
What appears to be different, however, is not their frequency, but their intensity, with research reinforcing that we may be facing unique challenges and smaller windows to intervene as we move forward.
Findings from a modeling study, published in 2021 in Proceedings of the National Academy of Sciences, underscore that without effective intervention, the probability of extreme events like COVID-19 will likely increase threefold in the coming decades.
Amesh Adalja, MD, senior scholar, Johns Hopkins Center for Health Security, Baltimore, told this news organization.
“It’s all been based on some unusual cluster of cases that were causing severe disease and overwhelming local authorities. So often, like Indiana Jones, somebody got dispatched to deal with an outbreak,” Dr. Adalja said.
In a perfect post-COVID world, government bodies, scientists, clinicians, and others would cross silos to coordinate pandemic prevention, not just preparedness. The public would trust those who carry the title “public health” in their daily responsibilities, and in turn, public health experts would get back to their core responsibility – infectious disease preparedness – the role they were initially assigned following Europe’s Black Death during the 14th century. Instead, the world finds itself at a crossroads, with emerging and reemerging infectious disease outbreaks that on the surface appear to arise haphazardly but in reality are the result of decades of reaction and containment policies aimed at putting out fires, not addressing their cause.
Dr. Adalja noted that only when the threat of biological weapons became a reality in the mid-2000s was there a realization that economies of scale could be exploited by merging interests and efforts to develop health security medical countermeasures. For example, it encouraged governments to more closely integrate agencies like the Biomedical Advanced Research and Development Authority and infectious disease research organizations and individuals.
Still, while significant strides have been made in certain areas, the ongoing COVID-19 pandemic has revealed substantial weaknesses remaining in public and private health systems, as well as major gaps in infectious disease preparedness.
The role of spillover events
No matter whom you ask, scientists, public health and conservation experts, and infectious disease clinicians all point to one of the most important threats to human health. As Walt Kelly’s Pogo famously put it, “We have met the enemy, and he is us.”
“The reason why these outbreaks of novel infectious diseases are increasingly occurring is because of human-driven environmental change, particularly land use, unsafe practices when raising farmed animals, and commercial wildlife markets,” Neil M. Vora, MD, a physician specializing in pandemic prevention at Conservation International and a former Centers for Disease Control and Prevention epidemic intelligence officer, said in an interview.
In fact, more than 60% of emerging infections and diseases are due to these “spillover events” (zoonotic spillover) that occur when pathogens that commonly circulate in wildlife jump over to new, human hosts.
Several examples come to mind.
COVID-19 may have begun as an enzootic virus from two undetermined animals, using the Huanan Seafood Market as a possible intermediate reservoir, according to a July 26 preprint in the journal Science.
Likewise, while the Ebola virus was originally attributed to deforestation efforts to create palm oil (which allowed fruit bat carriers to transfer the virus to humans), recent research suggests that bats dwelling in the walls of human dwellings and hospitals are responsible for the 2018 outbreak in the Democratic Republic of Congo.
(Incidentally, just this week, a new Ebola case was confirmed in Eastern Congo, and it has been genetically linked to the previous outbreak, despite that outbreak having been declared over in early July.)
“When we clear forests, we create opportunities for humans to live alongside the forest edge and displace wildlife. There’s evidence that shows when [these] biodiverse areas are cleared, specialist species that evolved to live in the forests first start to disappear, whereas generalist species – rodents and bats – continue to survive and are able to carry pathogens that can be passed on to humans,” Dr. Vora explained.
So far, China’s outbreak of the novel Langya henipavirus is believed to have spread (either directly or indirectly) by rodents and shrews, according to reports from public health authorities like the European Centre for Disease Prevention and Control, which is currently monitoring the situation.
Yet, an overreliance on surveillance and containment only perpetuates what Dr. Vora says are cycles of panic and neglect.
“We saw it with Ebola in 2015, in 2016 to 2017 with Zika, you see it with tuberculosis, with sexually transmitted infections, and with COVID. You have policymakers working on solutions, and once they think that they’ve fixed the problem, they’re going to move on to the next crisis.”
It’s also a question of equity.
Reports detailing the reemergence of monkeypox in Nigeria in 2017 were largely ignored, despite the fact that the United States assisted in diagnosing an early case in an 11-year-old boy. At the time, it was clear that the virus was spreading by human-to-human transmission versus animal-to-human transmission, something that had not been seen previously.
“The current model [is] waiting for pathogens to spill over and then [continuing] to spread signals that rich countries are tolerant of these outbreaks so long as they don’t grow into epidemics or pandemics,” Dr. Vora said.
This model is clearly broken; roughly 5 years after Nigeria reported the resurgence of monkeypox, the United States has more than 14,000 confirmed cases, which represents more than a quarter of the total number of cases reported worldwide.
Public health on the brink
I’s difficult to imagine a future without outbreaks and more pandemics, and if experts are to be believed, we are ill-prepared.
“I think that we are in a situation where this is a major threat, and people have become complacent about it,” said Dr. Adalja, who noted that we should be asking ourselves if the “government is actually in a position to be able to respond in a way that we need them to or is [that response] tied up in bureaucracy and inefficiency?”
COVID-19 should have been seen as a wake-up call, and many of those deaths were preventable. “With monkeypox, they’re faltering; it should have been a layup, not a disaster,” he emphasized.
Ellen Eaton, MD, associate professor of infectious diseases at the University of Alabama at Birmingham, also pointed to the reality that by the time COVID-19 reached North America, the United States had already moved away from the model of the public health department as the epicenter of knowledge, education, awareness, and, ironically, public health.
“Thinking about my community, very few people knew the face and name of our local and state health officers,” she told this news organization.
“There was just this inherent mistrust of these people. If you add in a lot of talking heads, a lot of politicians and messaging from non-experts that countered what was coming out of our public health agencies early, you had this huge disconnect; in the South, it was the perfect storm for vaccine hesitancy.”
At last count, this perfect storm has led to 1.46 million COVID cases and just over 20,000 deaths – many of which were preventable – in Alabama alone.
“In certain parts of America, we were starting with a broken system with limited resources and few providers,” Dr. Eaton explained.
Dr. Eaton said that a lot of fields, not just medicine and public health, have finite resources that have been stretched to capacity by COVID, and now monkeypox, and wondered what was next as we’re headed into autumn and influenza season. But she also mentioned the tremendous implications of climate change on infectious diseases and community health and wellness.
“There’s a tremendous need to have the ability to survey not just humans but also how the disease burden in our environment that is fluctuating with climate change is going to impact communities in really important ways,” Dr. Eaton said.
Upstream prevention
Dr. Vora said he could not agree more and believes that upstream prevention holds the key.
“We have to make sure while there’s tension on this issue that the right solutions are implemented,” he said.
In coming years, postspillover containment strategies – vaccine research and development and strengthening health care surveillance, for example – are likely to become inadequate.
“We saw it with COVID and we are seeing it again with monkeypox,” Dr. Vora said. “We also have to invest further upstream to prevent spillovers in the first place, for example, by addressing deforestation, commercial wildlife markets and trade, [and] infection control when raising farm animals.”
“The thing is, when you invest in those upstream solutions, you are also mitigating climate change and loss of biodiversity. I’m not saying that we should not invest in postspillover containment efforts; we’re never going to contain every spillover. But we also have to invest in prevention,” he added.
In a piece published in Nature, Dr. Vora and his coauthors acknowledge that several international bodies such as the World Health Organization and G7 have invested in initiatives to facilitate coordinated, global responses to climate change, pandemic preparedness, and response. But they point out that these efforts fail to “explicitly address the negative feedback cycle between environmental degradation, wildlife exploitation, and the emergence of pathogens.”
“Environmental conservation is no longer a left-wing fringe issue, it’s moving into public consciousness, and ... it is public health,” Dr. Vora said. “When we destroy nature, we’re destroying our own ability to survive.”
Dr. Adalja, Dr. Vora, and Dr. Eaton report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and COVID: New cases fall again, ED rates rebound for some
The 7-day average percentage of ED visits with diagnosed COVID, which had reached a post-Omicron high of 3.5% in late July for those aged 12-15, began to fall and was down to 3.0% on Aug. 12. That trend reversed, however, and the rate was up to 3.6% on Aug. 19, the last date for which data are available from the Centers for Disease Control and Prevention.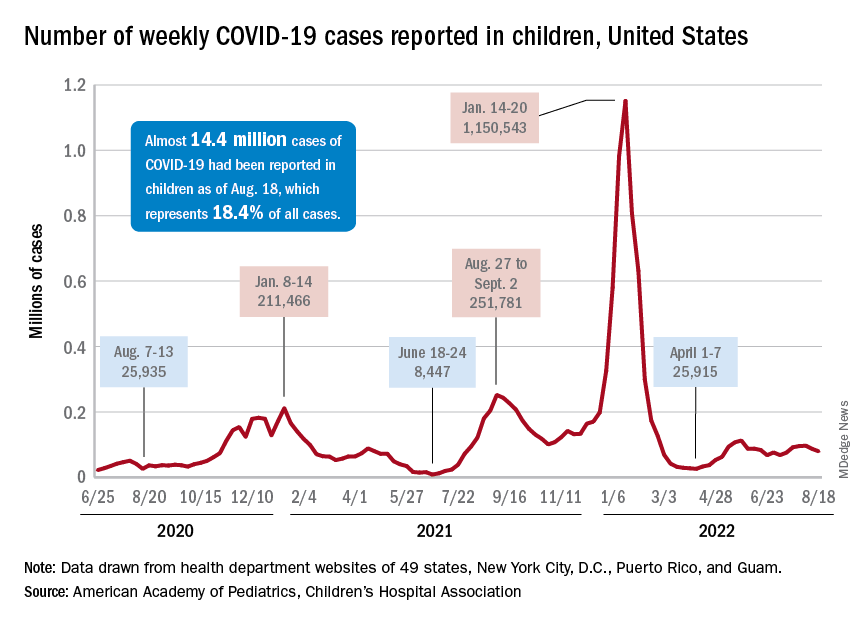
That change of COVID fortunes cannot yet be seen for all children. The 7-day average ED visit rate for those aged 0-11 years peaked at 6.8% during the last week of July and has continued to fall, dropping from 5.7% on Aug. 12 to 5.1% on Aug. 19. Children aged 16-17 years seem to be taking a middle path: Their ED-visit rate declined from late July into mid-August but held steady over the last week, according to the CDC’s COVID Data Tracker.
There is a hint of the same trend regarding new admissions among children aged 0-17 years. The national rate, which had declined in recent weeks, ticked up from 0.42 to 0.43 new admissions per 100,000 population over the last week of available data, the CDC said.
Weekly cases fall below 80,000
New cases in general were down by 8.5% from the previous week, dropping from 87,902 for the week of Aug. 5-11 to 79,525 for Aug. 12-18. That marked the second straight week with fewer cases after a 4-week period that saw weekly totals increase from almost 68,000 to nearly 97,000, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP and CHA put the cumulative number of child COVID-19 cases at just under 14.4 million since the pandemic began, which represents 18.4% of cases among all ages. The CDC estimates that there have been almost 14.7 million cases in children aged 0-17 years, as well as 1,750 deaths, of which 14 were reported in the last week (Aug. 16-22).
The CDC age subgroups indicate that children aged 0-4 years have experienced fewer cases (2.9 million) than children aged 5-11 years (5.6 million cases) and 12-15 (3.0 million cases) but more deaths: 548 so far, versus 432 for 5- to 11-year-olds and 437 for 12- to 15-year-olds, the COVID Data Tracker shows. Those aged 0-4 make up 6% of the total U.S. population, compared with 8.7% and 5.1%, respectively, for the older children.
Most younger children still not vaccinated
Although it may not qualify as a big push to vaccinate children before the start of the new school year, first-time vaccinations did rise somewhat in late July and August for children aged 5-17 years. Among children younger than 5 years, though, initial doses of the vaccine fell during the second full week of August, especially in 2- to 4-year-olds, based on the CDC data.
Through almost 2 months of vaccine eligibility, 4.8% of children under age 5 have received at least one dose and 0.9% are fully vaccinated as of Aug. 17. The current rates are 37.8% (one dose) and 30.4% (completed) for those aged 5-11 and 70.5% and 60.3% for 12- to 17-year-olds.
The 7-day average percentage of ED visits with diagnosed COVID, which had reached a post-Omicron high of 3.5% in late July for those aged 12-15, began to fall and was down to 3.0% on Aug. 12. That trend reversed, however, and the rate was up to 3.6% on Aug. 19, the last date for which data are available from the Centers for Disease Control and Prevention.
That change of COVID fortunes cannot yet be seen for all children. The 7-day average ED visit rate for those aged 0-11 years peaked at 6.8% during the last week of July and has continued to fall, dropping from 5.7% on Aug. 12 to 5.1% on Aug. 19. Children aged 16-17 years seem to be taking a middle path: Their ED-visit rate declined from late July into mid-August but held steady over the last week, according to the CDC’s COVID Data Tracker.
There is a hint of the same trend regarding new admissions among children aged 0-17 years. The national rate, which had declined in recent weeks, ticked up from 0.42 to 0.43 new admissions per 100,000 population over the last week of available data, the CDC said.
Weekly cases fall below 80,000
New cases in general were down by 8.5% from the previous week, dropping from 87,902 for the week of Aug. 5-11 to 79,525 for Aug. 12-18. That marked the second straight week with fewer cases after a 4-week period that saw weekly totals increase from almost 68,000 to nearly 97,000, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP and CHA put the cumulative number of child COVID-19 cases at just under 14.4 million since the pandemic began, which represents 18.4% of cases among all ages. The CDC estimates that there have been almost 14.7 million cases in children aged 0-17 years, as well as 1,750 deaths, of which 14 were reported in the last week (Aug. 16-22).
The CDC age subgroups indicate that children aged 0-4 years have experienced fewer cases (2.9 million) than children aged 5-11 years (5.6 million cases) and 12-15 (3.0 million cases) but more deaths: 548 so far, versus 432 for 5- to 11-year-olds and 437 for 12- to 15-year-olds, the COVID Data Tracker shows. Those aged 0-4 make up 6% of the total U.S. population, compared with 8.7% and 5.1%, respectively, for the older children.
Most younger children still not vaccinated
Although it may not qualify as a big push to vaccinate children before the start of the new school year, first-time vaccinations did rise somewhat in late July and August for children aged 5-17 years. Among children younger than 5 years, though, initial doses of the vaccine fell during the second full week of August, especially in 2- to 4-year-olds, based on the CDC data.

Through almost 2 months of vaccine eligibility, 4.8% of children under age 5 have received at least one dose and 0.9% are fully vaccinated as of Aug. 17. The current rates are 37.8% (one dose) and 30.4% (completed) for those aged 5-11 and 70.5% and 60.3% for 12- to 17-year-olds.
The 7-day average percentage of ED visits with diagnosed COVID, which had reached a post-Omicron high of 3.5% in late July for those aged 12-15, began to fall and was down to 3.0% on Aug. 12. That trend reversed, however, and the rate was up to 3.6% on Aug. 19, the last date for which data are available from the Centers for Disease Control and Prevention.
That change of COVID fortunes cannot yet be seen for all children. The 7-day average ED visit rate for those aged 0-11 years peaked at 6.8% during the last week of July and has continued to fall, dropping from 5.7% on Aug. 12 to 5.1% on Aug. 19. Children aged 16-17 years seem to be taking a middle path: Their ED-visit rate declined from late July into mid-August but held steady over the last week, according to the CDC’s COVID Data Tracker.
There is a hint of the same trend regarding new admissions among children aged 0-17 years. The national rate, which had declined in recent weeks, ticked up from 0.42 to 0.43 new admissions per 100,000 population over the last week of available data, the CDC said.
Weekly cases fall below 80,000
New cases in general were down by 8.5% from the previous week, dropping from 87,902 for the week of Aug. 5-11 to 79,525 for Aug. 12-18. That marked the second straight week with fewer cases after a 4-week period that saw weekly totals increase from almost 68,000 to nearly 97,000, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The AAP and CHA put the cumulative number of child COVID-19 cases at just under 14.4 million since the pandemic began, which represents 18.4% of cases among all ages. The CDC estimates that there have been almost 14.7 million cases in children aged 0-17 years, as well as 1,750 deaths, of which 14 were reported in the last week (Aug. 16-22).
The CDC age subgroups indicate that children aged 0-4 years have experienced fewer cases (2.9 million) than children aged 5-11 years (5.6 million cases) and 12-15 (3.0 million cases) but more deaths: 548 so far, versus 432 for 5- to 11-year-olds and 437 for 12- to 15-year-olds, the COVID Data Tracker shows. Those aged 0-4 make up 6% of the total U.S. population, compared with 8.7% and 5.1%, respectively, for the older children.
Most younger children still not vaccinated
Although it may not qualify as a big push to vaccinate children before the start of the new school year, first-time vaccinations did rise somewhat in late July and August for children aged 5-17 years. Among children younger than 5 years, though, initial doses of the vaccine fell during the second full week of August, especially in 2- to 4-year-olds, based on the CDC data.

Through almost 2 months of vaccine eligibility, 4.8% of children under age 5 have received at least one dose and 0.9% are fully vaccinated as of Aug. 17. The current rates are 37.8% (one dose) and 30.4% (completed) for those aged 5-11 and 70.5% and 60.3% for 12- to 17-year-olds.
Will monkeypox be the ‘syphilis of the 21st century’?
PARIS – France is boosting its vaccination campaign in response to the increase in cases of monkeypox. After a sluggish start, newly appointed French health minister François Braun has announced the release of 42,000 vaccine doses. At the same time, medical students will be able to lend a helping hand at vaccination sites. However, some experts have criticized the measures taken as being too lax to combat what the World Health Organization has designated a global health emergency.
For Benjamin Davido, MD, MSc, PhD, an infectious disease specialist at the Raymond-Poincaré Hospital (Paris Public Hospital Trust, AP-HP, Garches region), the risks of this disease have been minimized and the measures taken are not adequate, despite the ready availability of the tools needed to manage the epidemic. We must remain alert to the risks posed by this monkeypox epidemic, which seems different from the sporadic outbreaks that usually crop up in Central and West Africa, he said. Dr. Davido recently shared his opinions in an interview.
Question: What do you think about the monkeypox vaccination campaign currently underway in France?
Dr. Davido: It doesn’t go far enough, and I am surprised by the lack of a concrete and specific objective. It seems we have to wait until the fire is out of control before we can call the fire department. We should have been more reactive and taken a more drastic approach from the get-go. In France, as in other countries affected by this epidemic, we are still, unfortunately, in a phase of observation, reassuring ourselves that this will surely not become another pandemic, as that would be really bad luck.
Yet we find ourselves in an unprecedented situation: We have known about the disease in question for a long time, the target population has been identified, and we have a vaccine immediately available. So, we have all the tools and knowledge acquired from the COVID-19 pandemic at our disposal, yet we are choosing to wait and see. We have clearly underestimated the risks of failing after a stalled start to the vaccination campaign.
Question: What exactly are the risks, in your opinion? Should we already be worried about how the epidemic is progressing?
Dr. Davido: The situation is definitely worrying. I personally am convinced that this disease will be the syphilis of the 21st century. Although the risk is low, it is not beyond the bounds of possibility that this could be the start of a new pandemic. For the time being, its spread is limited to at-risk populations, mainly men who have sex with other men and who have multiple partners, which accounts for around 300,000 people in France. However, the risk for heterosexuals must not be minimized; we must not forget that this disease can also be transmitted through contact with an infected person and by respiratory droplets from people living in the same household. There have been recent cases of women and children infected with monkeypox. If monkeypox starts to spread in the community, rather than being a sexually transmitted infection, the epidemic could spread to the rest of the population. With the rise in cases, scientists are also concerned about transmission to animals. Monkeypox could become endemic like it is in Africa, where rodents are the main reservoir of the virus.
Question: What do we know about the dynamics of this epidemic? What can be done to effectively improve the situation?
Dr. Davido: Experience gained from African countries affected by monkeypox, as well as from the spate of cases that occurred in the United States in 2003, has shown us that the epidemic can be controlled once the cases have been contained. It is hoped that further waves of the epidemic can be avoided, providing the monkeypox vaccine achieves its objectives.
But we need to give ourselves the means to do so. The expansion of the vaccination program to the most at-risk populations in early July was the right decision. We have seen that ring vaccination targeting close-contact cases does not work with monkeypox. The current problem is that this vaccine is nearly exclusively restricted to hospital settings. We are making the same mistakes as [we did] at the start of the COVID-19 epidemic. We don’t have the right infrastructure in place for this vaccination program. We need to get doctors, paramedics, pharmacists, etc., involved. And cut back on the red tape. After embracing digital procedures during COVID-19, we find ourselves having to complete paper copies of documents for every single person attending a vaccination site. It just doesn’t make sense!
Question: You highlighted the lack of a clear objective with this vaccination campaign. What should we be aiming for?
Dr. Davido: During the COVID-19 vaccination campaign, there was a set number of people to be vaccinated within a given time frame. The approach demanded a fast pace and a desired outcome. Yes, it was an ambitious target from the get-go, but it was one that we stuck to. Currently, no figure, no target, has been set for the monkeypox vaccination program. Ideally, we would have completed the vaccination campaign before the start of the new school year to limit new infections.
As it stands now, only 10% of the target population has received the vaccine. There is talk of the summer period not being favorable. Yet I remember that last year, the COVID-19 vaccination program was strengthened in the middle of August. If the monkeypox vaccination campaign is not given a boost by the end of the summer, we run the risk of encouraging transmission of the virus between close contacts when different groups mix after being on holiday at the start of the new school year. I think that, first and foremost, we must make general practitioners aware of the disease and train them in how to diagnose it so that patients can be isolated and vaccinated as quickly as possible.
Question: There has also been talk of increasing the set 28-day period between the two doses, or even getting rid of it entirely. Would this perhaps lead to better vaccine uptake?
Dr. Davido: The United Kingdom has chosen to give a single dose and recommends a second dose after exposure. I am not sure that this is the best strategy. Although the efficacy data are still limited, the results are not as good after a single dose. According to initial data from the French National Agency for the Safety of Medicines and Health Products (the ANSM), the rate of seroconversion after one dose rises from 10% to 56% on D28 in healthy volunteers, but is between 77% and 89% 2 weeks after the second dose administered on D28.
So, the second dose is needed, especially as immunological memory seems to drop 2 years after the first injection. The U.S. Centers for Disease Control and Prevention proposes leaving 35 days between the two doses. I think this is a reasonable time frame. So, delaying the second dose makes administration of the first dose even easier because the second often fell in the middle of the holiday period and so we also save precious doses. If the time between doses is longer, we risk vaccinated individuals becoming lax and possibly being tempted to skip the “optional” booster or simply forgetting about it.
Question: Are people who have already had the smallpox vaccine better protected against monkeypox?
Dr. Davido: The efficacy of this vaccine against monkeypox is not perfect on a very long-term basis and, to be honest, we don’t really know the level of protection afforded by first-generation vaccines after 20 years. We must not forget that 20% of people infected with monkeypox were vaccinated against smallpox before mandatory vaccination for this disease was abolished [Editor’s note: The requirement of an initial dose of smallpox vaccine was lifted in 1979, once smallpox had been eradicated].
It is hoped that, as a minimum, this vaccine protects against serious illness. Yet in my department, we regularly see severe cases of monkeypox with widespread lesions in the over 45s, who are said to be vaccinated against smallpox.
Question: By comparison, is it likely that a third-generation vaccine would afford better protection against severe illness?
Dr. Davido: We still don’t have enough data or hindsight to assess the real-world impact of third-generation vaccines. This vaccine has a better tolerance profile than its predecessors, but we currently don’t know if it protects against severe forms of monkeypox. We also need to learn more about the disease causing the current epidemic, since it seems different from the sporadic outbreaks that usually crop up in Central and West Africa. The lesions seen are notably milder. The WHO has given this vaccine an efficacy level of 85% against infection by the monkeypox virus, but we must remain cautious: This figure is based on data from Africa. The epidemic in which we find ourselves is not the same. Overall, we must be wary of overly optimistic rhetoric around this new epidemic.
A version of this article appeared on Medscape.com. The article was translated from the Medscape French edition.
PARIS – France is boosting its vaccination campaign in response to the increase in cases of monkeypox. After a sluggish start, newly appointed French health minister François Braun has announced the release of 42,000 vaccine doses. At the same time, medical students will be able to lend a helping hand at vaccination sites. However, some experts have criticized the measures taken as being too lax to combat what the World Health Organization has designated a global health emergency.
For Benjamin Davido, MD, MSc, PhD, an infectious disease specialist at the Raymond-Poincaré Hospital (Paris Public Hospital Trust, AP-HP, Garches region), the risks of this disease have been minimized and the measures taken are not adequate, despite the ready availability of the tools needed to manage the epidemic. We must remain alert to the risks posed by this monkeypox epidemic, which seems different from the sporadic outbreaks that usually crop up in Central and West Africa, he said. Dr. Davido recently shared his opinions in an interview.
Question: What do you think about the monkeypox vaccination campaign currently underway in France?
Dr. Davido: It doesn’t go far enough, and I am surprised by the lack of a concrete and specific objective. It seems we have to wait until the fire is out of control before we can call the fire department. We should have been more reactive and taken a more drastic approach from the get-go. In France, as in other countries affected by this epidemic, we are still, unfortunately, in a phase of observation, reassuring ourselves that this will surely not become another pandemic, as that would be really bad luck.
Yet we find ourselves in an unprecedented situation: We have known about the disease in question for a long time, the target population has been identified, and we have a vaccine immediately available. So, we have all the tools and knowledge acquired from the COVID-19 pandemic at our disposal, yet we are choosing to wait and see. We have clearly underestimated the risks of failing after a stalled start to the vaccination campaign.
Question: What exactly are the risks, in your opinion? Should we already be worried about how the epidemic is progressing?
Dr. Davido: The situation is definitely worrying. I personally am convinced that this disease will be the syphilis of the 21st century. Although the risk is low, it is not beyond the bounds of possibility that this could be the start of a new pandemic. For the time being, its spread is limited to at-risk populations, mainly men who have sex with other men and who have multiple partners, which accounts for around 300,000 people in France. However, the risk for heterosexuals must not be minimized; we must not forget that this disease can also be transmitted through contact with an infected person and by respiratory droplets from people living in the same household. There have been recent cases of women and children infected with monkeypox. If monkeypox starts to spread in the community, rather than being a sexually transmitted infection, the epidemic could spread to the rest of the population. With the rise in cases, scientists are also concerned about transmission to animals. Monkeypox could become endemic like it is in Africa, where rodents are the main reservoir of the virus.
Question: What do we know about the dynamics of this epidemic? What can be done to effectively improve the situation?
Dr. Davido: Experience gained from African countries affected by monkeypox, as well as from the spate of cases that occurred in the United States in 2003, has shown us that the epidemic can be controlled once the cases have been contained. It is hoped that further waves of the epidemic can be avoided, providing the monkeypox vaccine achieves its objectives.
But we need to give ourselves the means to do so. The expansion of the vaccination program to the most at-risk populations in early July was the right decision. We have seen that ring vaccination targeting close-contact cases does not work with monkeypox. The current problem is that this vaccine is nearly exclusively restricted to hospital settings. We are making the same mistakes as [we did] at the start of the COVID-19 epidemic. We don’t have the right infrastructure in place for this vaccination program. We need to get doctors, paramedics, pharmacists, etc., involved. And cut back on the red tape. After embracing digital procedures during COVID-19, we find ourselves having to complete paper copies of documents for every single person attending a vaccination site. It just doesn’t make sense!
Question: You highlighted the lack of a clear objective with this vaccination campaign. What should we be aiming for?
Dr. Davido: During the COVID-19 vaccination campaign, there was a set number of people to be vaccinated within a given time frame. The approach demanded a fast pace and a desired outcome. Yes, it was an ambitious target from the get-go, but it was one that we stuck to. Currently, no figure, no target, has been set for the monkeypox vaccination program. Ideally, we would have completed the vaccination campaign before the start of the new school year to limit new infections.
As it stands now, only 10% of the target population has received the vaccine. There is talk of the summer period not being favorable. Yet I remember that last year, the COVID-19 vaccination program was strengthened in the middle of August. If the monkeypox vaccination campaign is not given a boost by the end of the summer, we run the risk of encouraging transmission of the virus between close contacts when different groups mix after being on holiday at the start of the new school year. I think that, first and foremost, we must make general practitioners aware of the disease and train them in how to diagnose it so that patients can be isolated and vaccinated as quickly as possible.
Question: There has also been talk of increasing the set 28-day period between the two doses, or even getting rid of it entirely. Would this perhaps lead to better vaccine uptake?
Dr. Davido: The United Kingdom has chosen to give a single dose and recommends a second dose after exposure. I am not sure that this is the best strategy. Although the efficacy data are still limited, the results are not as good after a single dose. According to initial data from the French National Agency for the Safety of Medicines and Health Products (the ANSM), the rate of seroconversion after one dose rises from 10% to 56% on D28 in healthy volunteers, but is between 77% and 89% 2 weeks after the second dose administered on D28.
So, the second dose is needed, especially as immunological memory seems to drop 2 years after the first injection. The U.S. Centers for Disease Control and Prevention proposes leaving 35 days between the two doses. I think this is a reasonable time frame. So, delaying the second dose makes administration of the first dose even easier because the second often fell in the middle of the holiday period and so we also save precious doses. If the time between doses is longer, we risk vaccinated individuals becoming lax and possibly being tempted to skip the “optional” booster or simply forgetting about it.
Question: Are people who have already had the smallpox vaccine better protected against monkeypox?
Dr. Davido: The efficacy of this vaccine against monkeypox is not perfect on a very long-term basis and, to be honest, we don’t really know the level of protection afforded by first-generation vaccines after 20 years. We must not forget that 20% of people infected with monkeypox were vaccinated against smallpox before mandatory vaccination for this disease was abolished [Editor’s note: The requirement of an initial dose of smallpox vaccine was lifted in 1979, once smallpox had been eradicated].
It is hoped that, as a minimum, this vaccine protects against serious illness. Yet in my department, we regularly see severe cases of monkeypox with widespread lesions in the over 45s, who are said to be vaccinated against smallpox.
Question: By comparison, is it likely that a third-generation vaccine would afford better protection against severe illness?
Dr. Davido: We still don’t have enough data or hindsight to assess the real-world impact of third-generation vaccines. This vaccine has a better tolerance profile than its predecessors, but we currently don’t know if it protects against severe forms of monkeypox. We also need to learn more about the disease causing the current epidemic, since it seems different from the sporadic outbreaks that usually crop up in Central and West Africa. The lesions seen are notably milder. The WHO has given this vaccine an efficacy level of 85% against infection by the monkeypox virus, but we must remain cautious: This figure is based on data from Africa. The epidemic in which we find ourselves is not the same. Overall, we must be wary of overly optimistic rhetoric around this new epidemic.
A version of this article appeared on Medscape.com. The article was translated from the Medscape French edition.
PARIS – France is boosting its vaccination campaign in response to the increase in cases of monkeypox. After a sluggish start, newly appointed French health minister François Braun has announced the release of 42,000 vaccine doses. At the same time, medical students will be able to lend a helping hand at vaccination sites. However, some experts have criticized the measures taken as being too lax to combat what the World Health Organization has designated a global health emergency.
For Benjamin Davido, MD, MSc, PhD, an infectious disease specialist at the Raymond-Poincaré Hospital (Paris Public Hospital Trust, AP-HP, Garches region), the risks of this disease have been minimized and the measures taken are not adequate, despite the ready availability of the tools needed to manage the epidemic. We must remain alert to the risks posed by this monkeypox epidemic, which seems different from the sporadic outbreaks that usually crop up in Central and West Africa, he said. Dr. Davido recently shared his opinions in an interview.
Question: What do you think about the monkeypox vaccination campaign currently underway in France?
Dr. Davido: It doesn’t go far enough, and I am surprised by the lack of a concrete and specific objective. It seems we have to wait until the fire is out of control before we can call the fire department. We should have been more reactive and taken a more drastic approach from the get-go. In France, as in other countries affected by this epidemic, we are still, unfortunately, in a phase of observation, reassuring ourselves that this will surely not become another pandemic, as that would be really bad luck.
Yet we find ourselves in an unprecedented situation: We have known about the disease in question for a long time, the target population has been identified, and we have a vaccine immediately available. So, we have all the tools and knowledge acquired from the COVID-19 pandemic at our disposal, yet we are choosing to wait and see. We have clearly underestimated the risks of failing after a stalled start to the vaccination campaign.
Question: What exactly are the risks, in your opinion? Should we already be worried about how the epidemic is progressing?
Dr. Davido: The situation is definitely worrying. I personally am convinced that this disease will be the syphilis of the 21st century. Although the risk is low, it is not beyond the bounds of possibility that this could be the start of a new pandemic. For the time being, its spread is limited to at-risk populations, mainly men who have sex with other men and who have multiple partners, which accounts for around 300,000 people in France. However, the risk for heterosexuals must not be minimized; we must not forget that this disease can also be transmitted through contact with an infected person and by respiratory droplets from people living in the same household. There have been recent cases of women and children infected with monkeypox. If monkeypox starts to spread in the community, rather than being a sexually transmitted infection, the epidemic could spread to the rest of the population. With the rise in cases, scientists are also concerned about transmission to animals. Monkeypox could become endemic like it is in Africa, where rodents are the main reservoir of the virus.
Question: What do we know about the dynamics of this epidemic? What can be done to effectively improve the situation?
Dr. Davido: Experience gained from African countries affected by monkeypox, as well as from the spate of cases that occurred in the United States in 2003, has shown us that the epidemic can be controlled once the cases have been contained. It is hoped that further waves of the epidemic can be avoided, providing the monkeypox vaccine achieves its objectives.
But we need to give ourselves the means to do so. The expansion of the vaccination program to the most at-risk populations in early July was the right decision. We have seen that ring vaccination targeting close-contact cases does not work with monkeypox. The current problem is that this vaccine is nearly exclusively restricted to hospital settings. We are making the same mistakes as [we did] at the start of the COVID-19 epidemic. We don’t have the right infrastructure in place for this vaccination program. We need to get doctors, paramedics, pharmacists, etc., involved. And cut back on the red tape. After embracing digital procedures during COVID-19, we find ourselves having to complete paper copies of documents for every single person attending a vaccination site. It just doesn’t make sense!
Question: You highlighted the lack of a clear objective with this vaccination campaign. What should we be aiming for?
Dr. Davido: During the COVID-19 vaccination campaign, there was a set number of people to be vaccinated within a given time frame. The approach demanded a fast pace and a desired outcome. Yes, it was an ambitious target from the get-go, but it was one that we stuck to. Currently, no figure, no target, has been set for the monkeypox vaccination program. Ideally, we would have completed the vaccination campaign before the start of the new school year to limit new infections.
As it stands now, only 10% of the target population has received the vaccine. There is talk of the summer period not being favorable. Yet I remember that last year, the COVID-19 vaccination program was strengthened in the middle of August. If the monkeypox vaccination campaign is not given a boost by the end of the summer, we run the risk of encouraging transmission of the virus between close contacts when different groups mix after being on holiday at the start of the new school year. I think that, first and foremost, we must make general practitioners aware of the disease and train them in how to diagnose it so that patients can be isolated and vaccinated as quickly as possible.
Question: There has also been talk of increasing the set 28-day period between the two doses, or even getting rid of it entirely. Would this perhaps lead to better vaccine uptake?
Dr. Davido: The United Kingdom has chosen to give a single dose and recommends a second dose after exposure. I am not sure that this is the best strategy. Although the efficacy data are still limited, the results are not as good after a single dose. According to initial data from the French National Agency for the Safety of Medicines and Health Products (the ANSM), the rate of seroconversion after one dose rises from 10% to 56% on D28 in healthy volunteers, but is between 77% and 89% 2 weeks after the second dose administered on D28.
So, the second dose is needed, especially as immunological memory seems to drop 2 years after the first injection. The U.S. Centers for Disease Control and Prevention proposes leaving 35 days between the two doses. I think this is a reasonable time frame. So, delaying the second dose makes administration of the first dose even easier because the second often fell in the middle of the holiday period and so we also save precious doses. If the time between doses is longer, we risk vaccinated individuals becoming lax and possibly being tempted to skip the “optional” booster or simply forgetting about it.
Question: Are people who have already had the smallpox vaccine better protected against monkeypox?
Dr. Davido: The efficacy of this vaccine against monkeypox is not perfect on a very long-term basis and, to be honest, we don’t really know the level of protection afforded by first-generation vaccines after 20 years. We must not forget that 20% of people infected with monkeypox were vaccinated against smallpox before mandatory vaccination for this disease was abolished [Editor’s note: The requirement of an initial dose of smallpox vaccine was lifted in 1979, once smallpox had been eradicated].
It is hoped that, as a minimum, this vaccine protects against serious illness. Yet in my department, we regularly see severe cases of monkeypox with widespread lesions in the over 45s, who are said to be vaccinated against smallpox.
Question: By comparison, is it likely that a third-generation vaccine would afford better protection against severe illness?
Dr. Davido: We still don’t have enough data or hindsight to assess the real-world impact of third-generation vaccines. This vaccine has a better tolerance profile than its predecessors, but we currently don’t know if it protects against severe forms of monkeypox. We also need to learn more about the disease causing the current epidemic, since it seems different from the sporadic outbreaks that usually crop up in Central and West Africa. The lesions seen are notably milder. The WHO has given this vaccine an efficacy level of 85% against infection by the monkeypox virus, but we must remain cautious: This figure is based on data from Africa. The epidemic in which we find ourselves is not the same. Overall, we must be wary of overly optimistic rhetoric around this new epidemic.
A version of this article appeared on Medscape.com. The article was translated from the Medscape French edition.
Monkeypox virus found in asymptomatic people
The findings, published in Annals of Internal Medicine, follow a similar, non–peer-reviewed report from Belgium. Researchers in both studies tested swabs for monkeypox in men who have sex with men. These swabs had been collected for routine STI screening.
It’s unclear whether asymptomatic individuals who test positive for monkeypox can spread the virus, the French team wrote. But if so, public health strategies to vaccinate those with known exposure “may not be sufficient to contain spread.”
In an editorial accompanying their paper, Stuart Isaacs, MD, associate professor at the University of Pennsylvania, Philadelphia, said it “raises the question of whether asymptomatic or subclinical infections are contributing to the current worldwide outbreak.”
Historically, transmission of monkeypox and its close relative, smallpox, was thought to be greatest when a rash was present, Dr. Isaacs wrote. “Long chains of human-to-human transmission were rare” with monkeypox.
That’s changed with the current outbreak, which was first detected in May. On Aug. 17, the World Health Organization reported more than 35,000 cases in 92 countries, with 12 deaths.
Research methods
For the French study, researchers conducted polymerase chain reaction tests on 200 anorectal swabs from asymptomatic individuals that had been collected from June 5 to July 11 in order to screen for gonorrhea and chlamydia. Of those, 13 (6.5%) were positive for monkeypox.
During the study period, STI testing had been suspended in individuals with monkeypox symptoms because of safety concerns, the researchers reported.
The research team contacted the 13 monkeypox-positive patients and advised them to limit sexual activity for 21 days following their test and notify recent sexual partners. None reported having developed symptoms, but two subsequently returned to the clinic with symptoms – one had an anal rash and the other a sore throat.
In the Belgian report, posted publicly on June 21 as a preprint, 3 of 224 anal samples collected for STI screening in May tested positive for monkeypox. All three of the men who tested positive said they did not have any symptoms in the weeks before and after the sample was taken.
At follow-up testing, 21-37 days after the initial samples were taken, all patients who had previously tested positive were negative. This was “likely as a consequence of spontaneous clearance of the infection,” the authors of that paper wrote.
Clinical implications of findings are uncertain
Monica Gandhi, MD, MPH, a professor of medicine at the University of California, San Francisco, said in an interview that the clinical implications of the findings are uncertain because it’s not known how much viral transmission results from asymptomatic individuals.
Nevertheless, Dr. Gandhi said that “vaccinating all gay men for monkeypox who will accept the vaccine is prudent,” compared with a less aggressive strategy of only vaccinating those with known exposure, which is called ring vaccination. That way, “we can be assured to provide immunity to large swaths of the at-risk population.”
Dr. Gandhi said that movement toward mass vaccination of gay men is occurring in the United States, Canada, Europe, and Australia, despite limited vaccine supply.
She added that, although monkeypox has been concentrated in communities of men who have sex with men, “anyone with multiple sexual partners should be vaccinated given the data.”
However, a WHO official recently cautioned that reports of breakthrough infections in individuals who were vaccinated against monkeypox constitute a reminder that “vaccine is not a silver bullet.”
Non-vaccine interventions are also needed
Other experts stressed the need for nonvaccine interventions.
In his editorial, Dr. Isaacs said an “expanded” ring vaccination strategy in communities of high risk is likely needed, but ultimately the outbreak will only be controlled if vaccination is accompanied by other measures such as identifying and isolating cases, making treatment available, and educating individuals about how to reduce their risk.
Aileen Marty, MD, a professor of infectious diseases at Florida International University, Miami, said in an interview that the new evidence makes it “incredibly important” to inform people that they might be infected by a sex partner even if that person does not have telltale lesions.
Dr. Marty said she has been advising men who have sex with men to “reduce or eliminate situations in which they find themselves with multiple anonymous individuals.”
Although most individuals recover from monkeypox, the disease can lead to hospitalization, disfigurement, blindness, and even death, Dr. Marty noted, adding that monkeypox is “absolutely a disease to avoid.”
Authors of the French study reported financial relationships with Gilead Sciences, Viiv Healthcare, MSD, AstraZeneca, Theratechnologies, Janssen Pharmaceuticals, Pfizer, GlaxoSmithKline, and bioMérieux. Dr. Isaacs reported grants from the Department of Veterans Affairs and the National Institutes of Health and royalties from UpToDate. Dr. Gandhi and Dr. Marty reported no relevant financial interests.
The findings, published in Annals of Internal Medicine, follow a similar, non–peer-reviewed report from Belgium. Researchers in both studies tested swabs for monkeypox in men who have sex with men. These swabs had been collected for routine STI screening.
It’s unclear whether asymptomatic individuals who test positive for monkeypox can spread the virus, the French team wrote. But if so, public health strategies to vaccinate those with known exposure “may not be sufficient to contain spread.”
In an editorial accompanying their paper, Stuart Isaacs, MD, associate professor at the University of Pennsylvania, Philadelphia, said it “raises the question of whether asymptomatic or subclinical infections are contributing to the current worldwide outbreak.”
Historically, transmission of monkeypox and its close relative, smallpox, was thought to be greatest when a rash was present, Dr. Isaacs wrote. “Long chains of human-to-human transmission were rare” with monkeypox.
That’s changed with the current outbreak, which was first detected in May. On Aug. 17, the World Health Organization reported more than 35,000 cases in 92 countries, with 12 deaths.
Research methods
For the French study, researchers conducted polymerase chain reaction tests on 200 anorectal swabs from asymptomatic individuals that had been collected from June 5 to July 11 in order to screen for gonorrhea and chlamydia. Of those, 13 (6.5%) were positive for monkeypox.
During the study period, STI testing had been suspended in individuals with monkeypox symptoms because of safety concerns, the researchers reported.
The research team contacted the 13 monkeypox-positive patients and advised them to limit sexual activity for 21 days following their test and notify recent sexual partners. None reported having developed symptoms, but two subsequently returned to the clinic with symptoms – one had an anal rash and the other a sore throat.
In the Belgian report, posted publicly on June 21 as a preprint, 3 of 224 anal samples collected for STI screening in May tested positive for monkeypox. All three of the men who tested positive said they did not have any symptoms in the weeks before and after the sample was taken.
At follow-up testing, 21-37 days after the initial samples were taken, all patients who had previously tested positive were negative. This was “likely as a consequence of spontaneous clearance of the infection,” the authors of that paper wrote.
Clinical implications of findings are uncertain
Monica Gandhi, MD, MPH, a professor of medicine at the University of California, San Francisco, said in an interview that the clinical implications of the findings are uncertain because it’s not known how much viral transmission results from asymptomatic individuals.
Nevertheless, Dr. Gandhi said that “vaccinating all gay men for monkeypox who will accept the vaccine is prudent,” compared with a less aggressive strategy of only vaccinating those with known exposure, which is called ring vaccination. That way, “we can be assured to provide immunity to large swaths of the at-risk population.”
Dr. Gandhi said that movement toward mass vaccination of gay men is occurring in the United States, Canada, Europe, and Australia, despite limited vaccine supply.
She added that, although monkeypox has been concentrated in communities of men who have sex with men, “anyone with multiple sexual partners should be vaccinated given the data.”
However, a WHO official recently cautioned that reports of breakthrough infections in individuals who were vaccinated against monkeypox constitute a reminder that “vaccine is not a silver bullet.”
Non-vaccine interventions are also needed
Other experts stressed the need for nonvaccine interventions.
In his editorial, Dr. Isaacs said an “expanded” ring vaccination strategy in communities of high risk is likely needed, but ultimately the outbreak will only be controlled if vaccination is accompanied by other measures such as identifying and isolating cases, making treatment available, and educating individuals about how to reduce their risk.
Aileen Marty, MD, a professor of infectious diseases at Florida International University, Miami, said in an interview that the new evidence makes it “incredibly important” to inform people that they might be infected by a sex partner even if that person does not have telltale lesions.
Dr. Marty said she has been advising men who have sex with men to “reduce or eliminate situations in which they find themselves with multiple anonymous individuals.”
Although most individuals recover from monkeypox, the disease can lead to hospitalization, disfigurement, blindness, and even death, Dr. Marty noted, adding that monkeypox is “absolutely a disease to avoid.”
Authors of the French study reported financial relationships with Gilead Sciences, Viiv Healthcare, MSD, AstraZeneca, Theratechnologies, Janssen Pharmaceuticals, Pfizer, GlaxoSmithKline, and bioMérieux. Dr. Isaacs reported grants from the Department of Veterans Affairs and the National Institutes of Health and royalties from UpToDate. Dr. Gandhi and Dr. Marty reported no relevant financial interests.
The findings, published in Annals of Internal Medicine, follow a similar, non–peer-reviewed report from Belgium. Researchers in both studies tested swabs for monkeypox in men who have sex with men. These swabs had been collected for routine STI screening.
It’s unclear whether asymptomatic individuals who test positive for monkeypox can spread the virus, the French team wrote. But if so, public health strategies to vaccinate those with known exposure “may not be sufficient to contain spread.”
In an editorial accompanying their paper, Stuart Isaacs, MD, associate professor at the University of Pennsylvania, Philadelphia, said it “raises the question of whether asymptomatic or subclinical infections are contributing to the current worldwide outbreak.”
Historically, transmission of monkeypox and its close relative, smallpox, was thought to be greatest when a rash was present, Dr. Isaacs wrote. “Long chains of human-to-human transmission were rare” with monkeypox.
That’s changed with the current outbreak, which was first detected in May. On Aug. 17, the World Health Organization reported more than 35,000 cases in 92 countries, with 12 deaths.
Research methods
For the French study, researchers conducted polymerase chain reaction tests on 200 anorectal swabs from asymptomatic individuals that had been collected from June 5 to July 11 in order to screen for gonorrhea and chlamydia. Of those, 13 (6.5%) were positive for monkeypox.
During the study period, STI testing had been suspended in individuals with monkeypox symptoms because of safety concerns, the researchers reported.
The research team contacted the 13 monkeypox-positive patients and advised them to limit sexual activity for 21 days following their test and notify recent sexual partners. None reported having developed symptoms, but two subsequently returned to the clinic with symptoms – one had an anal rash and the other a sore throat.
In the Belgian report, posted publicly on June 21 as a preprint, 3 of 224 anal samples collected for STI screening in May tested positive for monkeypox. All three of the men who tested positive said they did not have any symptoms in the weeks before and after the sample was taken.
At follow-up testing, 21-37 days after the initial samples were taken, all patients who had previously tested positive were negative. This was “likely as a consequence of spontaneous clearance of the infection,” the authors of that paper wrote.
Clinical implications of findings are uncertain
Monica Gandhi, MD, MPH, a professor of medicine at the University of California, San Francisco, said in an interview that the clinical implications of the findings are uncertain because it’s not known how much viral transmission results from asymptomatic individuals.
Nevertheless, Dr. Gandhi said that “vaccinating all gay men for monkeypox who will accept the vaccine is prudent,” compared with a less aggressive strategy of only vaccinating those with known exposure, which is called ring vaccination. That way, “we can be assured to provide immunity to large swaths of the at-risk population.”
Dr. Gandhi said that movement toward mass vaccination of gay men is occurring in the United States, Canada, Europe, and Australia, despite limited vaccine supply.
She added that, although monkeypox has been concentrated in communities of men who have sex with men, “anyone with multiple sexual partners should be vaccinated given the data.”
However, a WHO official recently cautioned that reports of breakthrough infections in individuals who were vaccinated against monkeypox constitute a reminder that “vaccine is not a silver bullet.”
Non-vaccine interventions are also needed
Other experts stressed the need for nonvaccine interventions.
In his editorial, Dr. Isaacs said an “expanded” ring vaccination strategy in communities of high risk is likely needed, but ultimately the outbreak will only be controlled if vaccination is accompanied by other measures such as identifying and isolating cases, making treatment available, and educating individuals about how to reduce their risk.
Aileen Marty, MD, a professor of infectious diseases at Florida International University, Miami, said in an interview that the new evidence makes it “incredibly important” to inform people that they might be infected by a sex partner even if that person does not have telltale lesions.
Dr. Marty said she has been advising men who have sex with men to “reduce or eliminate situations in which they find themselves with multiple anonymous individuals.”
Although most individuals recover from monkeypox, the disease can lead to hospitalization, disfigurement, blindness, and even death, Dr. Marty noted, adding that monkeypox is “absolutely a disease to avoid.”
Authors of the French study reported financial relationships with Gilead Sciences, Viiv Healthcare, MSD, AstraZeneca, Theratechnologies, Janssen Pharmaceuticals, Pfizer, GlaxoSmithKline, and bioMérieux. Dr. Isaacs reported grants from the Department of Veterans Affairs and the National Institutes of Health and royalties from UpToDate. Dr. Gandhi and Dr. Marty reported no relevant financial interests.
FROM ANNALS OF INTERNAL MEDICINE
Higher rates of group B strep disease found in Black and Asian newborns
Health charities called for action to address racial health disparities after population-wide analysis by the UK Health Security Agency found that Black and Asian neonates had a significantly higher risk of early-onset group B streptococcal disease (GBS), compared with White infants.
One support group said more research was now needed to identify the cause of the disparity, and called for pregnant women to be better informed about the disease and what it could mean for them and their baby.
The study, published in Pediatrics, used UKHSA data on laboratory-confirmed infant group B streptococcal (iGBS) disease cases between Jan. 1, 2016, and Dec. 31, 2020, and were linked to hospital ethnicity records.
Cases of iGBS were defined as isolation of Streptococcus agalactiae from a normally sterile site at 0-6 days of life for early-onset iGBS and 7-90 days for late-onset disease.
Hospital data and parent-reported ethnicity
Researchers found 2,512 iGBS cases in England during the study period, 65.3% were early onset and 34.8% late onset, equivalent to 0.52 and 0.28 cases per 1000 live births respectively.
Researchers were able to link 85.6% of those to ethnicity. Among those 2,149 cases, Black infants had a 48% higher risk, and Asian infants a 40% higher risk of early onset iGBS, compared with White infants. Among those from an Asian background, the risk was 87% higher for Bangladeshi and 38% higher for Pakistani neonates.
Rates of early onset iGBS per 1,000 live births were 0.43 for White infants, 0.63 for Black infants, and 0.60 for those of Asian ethnicity.
In contrast, Indian infants had an early-onset rate of 0.47 per 1,000 live births, which was similar to White infants.
Black infants had 57% higher rates of late-onset iGBS (0.37) than White infants (0.24), the researchers reported.
The study authors highlighted previous research which found higher prevalence of group B streptococcal colonization in mothers from Black and some Asian ethnic groups, but lower prevalence in mothers from the Indian subcontinent. More research was needed to establish causes, the researchers said, including whether higher preterm birth rates in minority ethnic groups led to increased iGBS risk in neonates, or whether maternal group B streptococcal disease led to higher preterm birth rates and subsequent neonatal iGBS.
The researchers concluded: “Understanding the factors underpinning differences in rates of early-onset iGBS within south Asian groups in England may lead to new opportunities for prevention such as prioritized antenatal screening. Strategies to prevent neonatal iGBS must be tailored from high-quality quantitative and qualitative data to reach all women and protect all infants, irrespective of racial or ethnic background.”
‘Shocking but not surprising’
Commenting on the study, Edward Morris, president of the Royal College of Obstetricians and Gynaecologists, said: “This research is striking reading, and is yet another example of how far we have to go to tackle health inequalities within women’s health care.”
Philip Steer, professor emeritus at Imperial College London, said that the results were “consistent with previous reports of higher GBS carriage and higher maternal and neonatal mortality rates in minority groups” and “emphasize the importance of studying not just whether, but why, these differences exist.” He added: “We need to understand the reasons for the differences before we can design much-needed intervention to eliminate them.”
Jane Plumb, chief executive of Group B Strep Support, called the findings “shocking, but unfortunately not surprising” and said that they offered “another example of racial disparities in maternal and neonatal health.” She said: “We’re calling for all pregnant women and birthing people to be informed about GBS and its risks, so they can make empowered choices for themselves and their baby. It is also critical that trusts sign up to take part in the internationally significant [National Institute for Health and Care Research]–funded GBS3 clinical trial, designed to improve the prevention of GBS infection.”
Baroness Shaista Gohir, chief executive of the Muslim Women’s Network, said: “With significantly higher rates of group B Strep infection in Black and Asian babies, greater efforts must be made to improve awareness among pregnant women within these communities.”
A version of this article first appeared on Medscape UK.
Health charities called for action to address racial health disparities after population-wide analysis by the UK Health Security Agency found that Black and Asian neonates had a significantly higher risk of early-onset group B streptococcal disease (GBS), compared with White infants.
One support group said more research was now needed to identify the cause of the disparity, and called for pregnant women to be better informed about the disease and what it could mean for them and their baby.
The study, published in Pediatrics, used UKHSA data on laboratory-confirmed infant group B streptococcal (iGBS) disease cases between Jan. 1, 2016, and Dec. 31, 2020, and were linked to hospital ethnicity records.
Cases of iGBS were defined as isolation of Streptococcus agalactiae from a normally sterile site at 0-6 days of life for early-onset iGBS and 7-90 days for late-onset disease.
Hospital data and parent-reported ethnicity
Researchers found 2,512 iGBS cases in England during the study period, 65.3% were early onset and 34.8% late onset, equivalent to 0.52 and 0.28 cases per 1000 live births respectively.
Researchers were able to link 85.6% of those to ethnicity. Among those 2,149 cases, Black infants had a 48% higher risk, and Asian infants a 40% higher risk of early onset iGBS, compared with White infants. Among those from an Asian background, the risk was 87% higher for Bangladeshi and 38% higher for Pakistani neonates.
Rates of early onset iGBS per 1,000 live births were 0.43 for White infants, 0.63 for Black infants, and 0.60 for those of Asian ethnicity.
In contrast, Indian infants had an early-onset rate of 0.47 per 1,000 live births, which was similar to White infants.
Black infants had 57% higher rates of late-onset iGBS (0.37) than White infants (0.24), the researchers reported.
The study authors highlighted previous research which found higher prevalence of group B streptococcal colonization in mothers from Black and some Asian ethnic groups, but lower prevalence in mothers from the Indian subcontinent. More research was needed to establish causes, the researchers said, including whether higher preterm birth rates in minority ethnic groups led to increased iGBS risk in neonates, or whether maternal group B streptococcal disease led to higher preterm birth rates and subsequent neonatal iGBS.
The researchers concluded: “Understanding the factors underpinning differences in rates of early-onset iGBS within south Asian groups in England may lead to new opportunities for prevention such as prioritized antenatal screening. Strategies to prevent neonatal iGBS must be tailored from high-quality quantitative and qualitative data to reach all women and protect all infants, irrespective of racial or ethnic background.”
‘Shocking but not surprising’
Commenting on the study, Edward Morris, president of the Royal College of Obstetricians and Gynaecologists, said: “This research is striking reading, and is yet another example of how far we have to go to tackle health inequalities within women’s health care.”
Philip Steer, professor emeritus at Imperial College London, said that the results were “consistent with previous reports of higher GBS carriage and higher maternal and neonatal mortality rates in minority groups” and “emphasize the importance of studying not just whether, but why, these differences exist.” He added: “We need to understand the reasons for the differences before we can design much-needed intervention to eliminate them.”
Jane Plumb, chief executive of Group B Strep Support, called the findings “shocking, but unfortunately not surprising” and said that they offered “another example of racial disparities in maternal and neonatal health.” She said: “We’re calling for all pregnant women and birthing people to be informed about GBS and its risks, so they can make empowered choices for themselves and their baby. It is also critical that trusts sign up to take part in the internationally significant [National Institute for Health and Care Research]–funded GBS3 clinical trial, designed to improve the prevention of GBS infection.”
Baroness Shaista Gohir, chief executive of the Muslim Women’s Network, said: “With significantly higher rates of group B Strep infection in Black and Asian babies, greater efforts must be made to improve awareness among pregnant women within these communities.”
A version of this article first appeared on Medscape UK.
Health charities called for action to address racial health disparities after population-wide analysis by the UK Health Security Agency found that Black and Asian neonates had a significantly higher risk of early-onset group B streptococcal disease (GBS), compared with White infants.
One support group said more research was now needed to identify the cause of the disparity, and called for pregnant women to be better informed about the disease and what it could mean for them and their baby.
The study, published in Pediatrics, used UKHSA data on laboratory-confirmed infant group B streptococcal (iGBS) disease cases between Jan. 1, 2016, and Dec. 31, 2020, and were linked to hospital ethnicity records.
Cases of iGBS were defined as isolation of Streptococcus agalactiae from a normally sterile site at 0-6 days of life for early-onset iGBS and 7-90 days for late-onset disease.
Hospital data and parent-reported ethnicity
Researchers found 2,512 iGBS cases in England during the study period, 65.3% were early onset and 34.8% late onset, equivalent to 0.52 and 0.28 cases per 1000 live births respectively.
Researchers were able to link 85.6% of those to ethnicity. Among those 2,149 cases, Black infants had a 48% higher risk, and Asian infants a 40% higher risk of early onset iGBS, compared with White infants. Among those from an Asian background, the risk was 87% higher for Bangladeshi and 38% higher for Pakistani neonates.
Rates of early onset iGBS per 1,000 live births were 0.43 for White infants, 0.63 for Black infants, and 0.60 for those of Asian ethnicity.
In contrast, Indian infants had an early-onset rate of 0.47 per 1,000 live births, which was similar to White infants.
Black infants had 57% higher rates of late-onset iGBS (0.37) than White infants (0.24), the researchers reported.
The study authors highlighted previous research which found higher prevalence of group B streptococcal colonization in mothers from Black and some Asian ethnic groups, but lower prevalence in mothers from the Indian subcontinent. More research was needed to establish causes, the researchers said, including whether higher preterm birth rates in minority ethnic groups led to increased iGBS risk in neonates, or whether maternal group B streptococcal disease led to higher preterm birth rates and subsequent neonatal iGBS.
The researchers concluded: “Understanding the factors underpinning differences in rates of early-onset iGBS within south Asian groups in England may lead to new opportunities for prevention such as prioritized antenatal screening. Strategies to prevent neonatal iGBS must be tailored from high-quality quantitative and qualitative data to reach all women and protect all infants, irrespective of racial or ethnic background.”
‘Shocking but not surprising’
Commenting on the study, Edward Morris, president of the Royal College of Obstetricians and Gynaecologists, said: “This research is striking reading, and is yet another example of how far we have to go to tackle health inequalities within women’s health care.”
Philip Steer, professor emeritus at Imperial College London, said that the results were “consistent with previous reports of higher GBS carriage and higher maternal and neonatal mortality rates in minority groups” and “emphasize the importance of studying not just whether, but why, these differences exist.” He added: “We need to understand the reasons for the differences before we can design much-needed intervention to eliminate them.”
Jane Plumb, chief executive of Group B Strep Support, called the findings “shocking, but unfortunately not surprising” and said that they offered “another example of racial disparities in maternal and neonatal health.” She said: “We’re calling for all pregnant women and birthing people to be informed about GBS and its risks, so they can make empowered choices for themselves and their baby. It is also critical that trusts sign up to take part in the internationally significant [National Institute for Health and Care Research]–funded GBS3 clinical trial, designed to improve the prevention of GBS infection.”
Baroness Shaista Gohir, chief executive of the Muslim Women’s Network, said: “With significantly higher rates of group B Strep infection in Black and Asian babies, greater efforts must be made to improve awareness among pregnant women within these communities.”
A version of this article first appeared on Medscape UK.
FROM PEDIATRICS






