User login
Stop smoking and reduce death risk from pneumonia?
; the risk decreased even more with added years of not smoking, according to data from nearly 95,000 individuals.
Smoking is associated with an increased risk for pneumonia, but the extent to which smoking cessation reduces this risk long-term has not been explored, wrote Tomomi Kihara, MD, PhD, of the University of Tsukuba, Japan, and colleagues on behalf of the Japan Collaborative Cohort.
In the Japan Collaborative Cohort Study for Evaluation of Cancer Risk, known as the JACC Study, a community-based cohort of 110,585 individuals aged 40-79 years participated in health screening exams and self-administered questionnaires that included information about smoking. Other findings from the study have been previously published.
In the current study published in Preventive Medicine, the researchers reviewed data from 94,972 JACC participants who provided data about smoking status, including 59,514 never-smokers, 10,554 former smokers, and 24,904 current smokers. The mean age of the participants was 57 years; 57% were women.
The respondents were divided into groups based on years of smoking cessation: 0-1 year, 2-4 years, 5-9 years, 10-14 years, and 15 or more years. The primary endpoint was an underlying cause of death from pneumonia.
Over a median follow-up period of 19 years, 1,806 participants (1,115 men and 691 women) died of pneumonia.
In a multivariate analysis, the hazard ratio for those who quit smoking, compared with current smokers, was 1.02 for 0-1 year of smoking cessation, 0.92 for 2-4 years, 0.95 for 5-9 years, 0.71 for 10-14 years, and 0.63 (0.48-0.83) for 15 or more years. The HR for never smokers was 0.50. The analysis adjusted for competing risk for death without pneumonia in the study population.
Most of the benefits of smoking cessation occurred after 10-14 years, the researchers wrote in their discussion of the findings, and smoking cessation of 10 years or more resulted in risk for death from pneumonia similar to that of never-smokers.
“To our knowledge, no previous studies have examined the association between years of smoking cessation and pneumonia in a general population,” they added.
The study findings were limited by several factors, including the use of data on smoking and smoking cessation at baseline as well as a lack of data on the use of tobacco products other than cigarettes, although alternative tobacco products are rarely used in Japan, the researchers noted. Other limitations include the use of pneumonia mortality as an endpoint, which could have ignored the impact of smoking cessation on less severe pneumonia, and the inability to clarify the association between smoking cessation and pneumonia mortality by sex because of the small number of female former smokers. However, the results were strengthened by the large sample size and long observation period, they said.
“The present study provides empirical evidence that smoking cessation may lead to a decline in the risk of mortality from pneumonia,” and supports smoking cessation as a preventive measure, the researchers concluded.
The study was supported by the Japanese Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labour and Welfare, Health and Labor Sciences; and an Intramural Research Fund for Cardiovascular Diseases of National Cerebral and Cardiovascular Center. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
; the risk decreased even more with added years of not smoking, according to data from nearly 95,000 individuals.
Smoking is associated with an increased risk for pneumonia, but the extent to which smoking cessation reduces this risk long-term has not been explored, wrote Tomomi Kihara, MD, PhD, of the University of Tsukuba, Japan, and colleagues on behalf of the Japan Collaborative Cohort.
In the Japan Collaborative Cohort Study for Evaluation of Cancer Risk, known as the JACC Study, a community-based cohort of 110,585 individuals aged 40-79 years participated in health screening exams and self-administered questionnaires that included information about smoking. Other findings from the study have been previously published.
In the current study published in Preventive Medicine, the researchers reviewed data from 94,972 JACC participants who provided data about smoking status, including 59,514 never-smokers, 10,554 former smokers, and 24,904 current smokers. The mean age of the participants was 57 years; 57% were women.
The respondents were divided into groups based on years of smoking cessation: 0-1 year, 2-4 years, 5-9 years, 10-14 years, and 15 or more years. The primary endpoint was an underlying cause of death from pneumonia.
Over a median follow-up period of 19 years, 1,806 participants (1,115 men and 691 women) died of pneumonia.
In a multivariate analysis, the hazard ratio for those who quit smoking, compared with current smokers, was 1.02 for 0-1 year of smoking cessation, 0.92 for 2-4 years, 0.95 for 5-9 years, 0.71 for 10-14 years, and 0.63 (0.48-0.83) for 15 or more years. The HR for never smokers was 0.50. The analysis adjusted for competing risk for death without pneumonia in the study population.
Most of the benefits of smoking cessation occurred after 10-14 years, the researchers wrote in their discussion of the findings, and smoking cessation of 10 years or more resulted in risk for death from pneumonia similar to that of never-smokers.
“To our knowledge, no previous studies have examined the association between years of smoking cessation and pneumonia in a general population,” they added.
The study findings were limited by several factors, including the use of data on smoking and smoking cessation at baseline as well as a lack of data on the use of tobacco products other than cigarettes, although alternative tobacco products are rarely used in Japan, the researchers noted. Other limitations include the use of pneumonia mortality as an endpoint, which could have ignored the impact of smoking cessation on less severe pneumonia, and the inability to clarify the association between smoking cessation and pneumonia mortality by sex because of the small number of female former smokers. However, the results were strengthened by the large sample size and long observation period, they said.
“The present study provides empirical evidence that smoking cessation may lead to a decline in the risk of mortality from pneumonia,” and supports smoking cessation as a preventive measure, the researchers concluded.
The study was supported by the Japanese Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labour and Welfare, Health and Labor Sciences; and an Intramural Research Fund for Cardiovascular Diseases of National Cerebral and Cardiovascular Center. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
; the risk decreased even more with added years of not smoking, according to data from nearly 95,000 individuals.
Smoking is associated with an increased risk for pneumonia, but the extent to which smoking cessation reduces this risk long-term has not been explored, wrote Tomomi Kihara, MD, PhD, of the University of Tsukuba, Japan, and colleagues on behalf of the Japan Collaborative Cohort.
In the Japan Collaborative Cohort Study for Evaluation of Cancer Risk, known as the JACC Study, a community-based cohort of 110,585 individuals aged 40-79 years participated in health screening exams and self-administered questionnaires that included information about smoking. Other findings from the study have been previously published.
In the current study published in Preventive Medicine, the researchers reviewed data from 94,972 JACC participants who provided data about smoking status, including 59,514 never-smokers, 10,554 former smokers, and 24,904 current smokers. The mean age of the participants was 57 years; 57% were women.
The respondents were divided into groups based on years of smoking cessation: 0-1 year, 2-4 years, 5-9 years, 10-14 years, and 15 or more years. The primary endpoint was an underlying cause of death from pneumonia.
Over a median follow-up period of 19 years, 1,806 participants (1,115 men and 691 women) died of pneumonia.
In a multivariate analysis, the hazard ratio for those who quit smoking, compared with current smokers, was 1.02 for 0-1 year of smoking cessation, 0.92 for 2-4 years, 0.95 for 5-9 years, 0.71 for 10-14 years, and 0.63 (0.48-0.83) for 15 or more years. The HR for never smokers was 0.50. The analysis adjusted for competing risk for death without pneumonia in the study population.
Most of the benefits of smoking cessation occurred after 10-14 years, the researchers wrote in their discussion of the findings, and smoking cessation of 10 years or more resulted in risk for death from pneumonia similar to that of never-smokers.
“To our knowledge, no previous studies have examined the association between years of smoking cessation and pneumonia in a general population,” they added.
The study findings were limited by several factors, including the use of data on smoking and smoking cessation at baseline as well as a lack of data on the use of tobacco products other than cigarettes, although alternative tobacco products are rarely used in Japan, the researchers noted. Other limitations include the use of pneumonia mortality as an endpoint, which could have ignored the impact of smoking cessation on less severe pneumonia, and the inability to clarify the association between smoking cessation and pneumonia mortality by sex because of the small number of female former smokers. However, the results were strengthened by the large sample size and long observation period, they said.
“The present study provides empirical evidence that smoking cessation may lead to a decline in the risk of mortality from pneumonia,” and supports smoking cessation as a preventive measure, the researchers concluded.
The study was supported by the Japanese Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labour and Welfare, Health and Labor Sciences; and an Intramural Research Fund for Cardiovascular Diseases of National Cerebral and Cardiovascular Center. The researchers had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Large study amplifies evidence of COVID vaccine safety in pregnancy
The research team wrote in the BMJ that their reassuring findings – drawn from a registry of all births in Ontario over an 8-month period – “can inform evidence-based decision-making” about COVID vaccination during pregnancy.
Previous research has found that pregnant patients are at higher risk of severe complications and death if they become infected with COVID and that vaccination before or during pregnancy prevents such outcomes and reduces the risk of newborn infection, noted Jeffrey Ecker, chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
This new study “adds to a growing body of information arguing clearly and reassuringly that vaccination during pregnancy is not associated with complications during pregnancy,” said Dr. Ecker, who was not involved in the new study.
He added that it “should help obstetric providers further reassure those who are hesitant that vaccination is safe and best both for the pregnant patient and their pregnancy.”
Methods and results
For the new study, researchers tapped a provincial registry of all live and stillborn infants with a gestational age of at least 20 weeks or birth weight of at least 500 g. Unique health card numbers were used to link birth records to a database of COVID vaccinations.
Of 85,162 infants born from May through December of 2021, 43,099 (50.6%) were born to individuals who received at least one vaccine dose during pregnancy. Among those, 99.7% received an mRNA vaccine such as Pfizer-BioNTech or Moderna.
Vaccination during pregnancy was not associated with greater risk of overall preterm birth (6.5% among vaccinated individuals versus 6.9% among unvaccinated; hazard ratio, 1.02; 95% confidence interval, 0.96-1.08), spontaneous preterm birth (3.7% versus 4.4%; hazard ratio, 0.96; 95% CI, 0.90-1.03) or very preterm birth (0.59% versus 0.89%; hazard ratio, 0.80; 95% CI, 0.67-0.95).
Likewise, no increase was observed in the risk of an infant being small for gestational age at birth (9.1% versus 9.2%; hazard ratio, 0.98; 95% CI, 0.93-1.03).
The researchers observed a reduction in the risk of stillbirth, even after adjusting for potential confounders. Stillbirths occurred in 0.25% of vaccinated individuals, compared with 0.44% of unvaccinated individuals (hazard ratio, 0.65; 95% CI, 0.51-0.84).
A reduced risk of stillbirth – albeit to a smaller degree – was also found in a Scandinavian registry study that included 28,506 babies born to individuals who were vaccinated during pregnancy.
“Collectively, the findings from these two studies are reassuring and are consistent with no increased risk of stillbirth after COVID-19 vaccination during pregnancy. In contrast, COVID-19 disease during pregnancy has been associated with an increased risk of stillbirth,” the researchers wrote.
Findings did not vary by which mRNA vaccine a mother received, the number of doses she received, or the trimester in which a vaccine was given, the researchers reported.
Stillbirth findings will be ‘very reassuring’ for patients
The lead investigator, Deshayne Fell, PhD, said in an interview, the fact that the study comprised the entire population of pregnant people in Ontario during the study period “increases our confidence” about the validity and relevance of the findings for other geographic settings.
Dr. Fell, an associate professor in epidemiology and public health at the University of Ottawa and a scientist at the Children’s Hospital of Eastern Ontario Research Institute, Ottawa, said the evaluation of stillbirth in particular, “a rare but devastating outcome,” will be “very reassuring and useful for clinical counseling.”
A limitation cited by the research team included a lack of data on vaccination prior to pregnancy.
In the new study, Dr, Ecker said, “Though the investigators were able to adjust for many variables they cannot be certain that some unmeasured variable that, accordingly, was not adjusted for does not hide a small risk. This seems very unlikely, however.”
The Canadian research team said similar studies of non-mRNA COVID vaccines “should be a research priority.” However, such studies are not underway in Canada, where only mRNA vaccines are used in pregnancy, Dr. Fell said.
This study was supported by the Public Health Agency of Canada.
Dr. Fell and Dr. Ecker reported no competing financial interests.
The research team wrote in the BMJ that their reassuring findings – drawn from a registry of all births in Ontario over an 8-month period – “can inform evidence-based decision-making” about COVID vaccination during pregnancy.
Previous research has found that pregnant patients are at higher risk of severe complications and death if they become infected with COVID and that vaccination before or during pregnancy prevents such outcomes and reduces the risk of newborn infection, noted Jeffrey Ecker, chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
This new study “adds to a growing body of information arguing clearly and reassuringly that vaccination during pregnancy is not associated with complications during pregnancy,” said Dr. Ecker, who was not involved in the new study.
He added that it “should help obstetric providers further reassure those who are hesitant that vaccination is safe and best both for the pregnant patient and their pregnancy.”
Methods and results
For the new study, researchers tapped a provincial registry of all live and stillborn infants with a gestational age of at least 20 weeks or birth weight of at least 500 g. Unique health card numbers were used to link birth records to a database of COVID vaccinations.
Of 85,162 infants born from May through December of 2021, 43,099 (50.6%) were born to individuals who received at least one vaccine dose during pregnancy. Among those, 99.7% received an mRNA vaccine such as Pfizer-BioNTech or Moderna.
Vaccination during pregnancy was not associated with greater risk of overall preterm birth (6.5% among vaccinated individuals versus 6.9% among unvaccinated; hazard ratio, 1.02; 95% confidence interval, 0.96-1.08), spontaneous preterm birth (3.7% versus 4.4%; hazard ratio, 0.96; 95% CI, 0.90-1.03) or very preterm birth (0.59% versus 0.89%; hazard ratio, 0.80; 95% CI, 0.67-0.95).
Likewise, no increase was observed in the risk of an infant being small for gestational age at birth (9.1% versus 9.2%; hazard ratio, 0.98; 95% CI, 0.93-1.03).
The researchers observed a reduction in the risk of stillbirth, even after adjusting for potential confounders. Stillbirths occurred in 0.25% of vaccinated individuals, compared with 0.44% of unvaccinated individuals (hazard ratio, 0.65; 95% CI, 0.51-0.84).
A reduced risk of stillbirth – albeit to a smaller degree – was also found in a Scandinavian registry study that included 28,506 babies born to individuals who were vaccinated during pregnancy.
“Collectively, the findings from these two studies are reassuring and are consistent with no increased risk of stillbirth after COVID-19 vaccination during pregnancy. In contrast, COVID-19 disease during pregnancy has been associated with an increased risk of stillbirth,” the researchers wrote.
Findings did not vary by which mRNA vaccine a mother received, the number of doses she received, or the trimester in which a vaccine was given, the researchers reported.
Stillbirth findings will be ‘very reassuring’ for patients
The lead investigator, Deshayne Fell, PhD, said in an interview, the fact that the study comprised the entire population of pregnant people in Ontario during the study period “increases our confidence” about the validity and relevance of the findings for other geographic settings.
Dr. Fell, an associate professor in epidemiology and public health at the University of Ottawa and a scientist at the Children’s Hospital of Eastern Ontario Research Institute, Ottawa, said the evaluation of stillbirth in particular, “a rare but devastating outcome,” will be “very reassuring and useful for clinical counseling.”
A limitation cited by the research team included a lack of data on vaccination prior to pregnancy.
In the new study, Dr, Ecker said, “Though the investigators were able to adjust for many variables they cannot be certain that some unmeasured variable that, accordingly, was not adjusted for does not hide a small risk. This seems very unlikely, however.”
The Canadian research team said similar studies of non-mRNA COVID vaccines “should be a research priority.” However, such studies are not underway in Canada, where only mRNA vaccines are used in pregnancy, Dr. Fell said.
This study was supported by the Public Health Agency of Canada.
Dr. Fell and Dr. Ecker reported no competing financial interests.
The research team wrote in the BMJ that their reassuring findings – drawn from a registry of all births in Ontario over an 8-month period – “can inform evidence-based decision-making” about COVID vaccination during pregnancy.
Previous research has found that pregnant patients are at higher risk of severe complications and death if they become infected with COVID and that vaccination before or during pregnancy prevents such outcomes and reduces the risk of newborn infection, noted Jeffrey Ecker, chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
This new study “adds to a growing body of information arguing clearly and reassuringly that vaccination during pregnancy is not associated with complications during pregnancy,” said Dr. Ecker, who was not involved in the new study.
He added that it “should help obstetric providers further reassure those who are hesitant that vaccination is safe and best both for the pregnant patient and their pregnancy.”
Methods and results
For the new study, researchers tapped a provincial registry of all live and stillborn infants with a gestational age of at least 20 weeks or birth weight of at least 500 g. Unique health card numbers were used to link birth records to a database of COVID vaccinations.
Of 85,162 infants born from May through December of 2021, 43,099 (50.6%) were born to individuals who received at least one vaccine dose during pregnancy. Among those, 99.7% received an mRNA vaccine such as Pfizer-BioNTech or Moderna.
Vaccination during pregnancy was not associated with greater risk of overall preterm birth (6.5% among vaccinated individuals versus 6.9% among unvaccinated; hazard ratio, 1.02; 95% confidence interval, 0.96-1.08), spontaneous preterm birth (3.7% versus 4.4%; hazard ratio, 0.96; 95% CI, 0.90-1.03) or very preterm birth (0.59% versus 0.89%; hazard ratio, 0.80; 95% CI, 0.67-0.95).
Likewise, no increase was observed in the risk of an infant being small for gestational age at birth (9.1% versus 9.2%; hazard ratio, 0.98; 95% CI, 0.93-1.03).
The researchers observed a reduction in the risk of stillbirth, even after adjusting for potential confounders. Stillbirths occurred in 0.25% of vaccinated individuals, compared with 0.44% of unvaccinated individuals (hazard ratio, 0.65; 95% CI, 0.51-0.84).
A reduced risk of stillbirth – albeit to a smaller degree – was also found in a Scandinavian registry study that included 28,506 babies born to individuals who were vaccinated during pregnancy.
“Collectively, the findings from these two studies are reassuring and are consistent with no increased risk of stillbirth after COVID-19 vaccination during pregnancy. In contrast, COVID-19 disease during pregnancy has been associated with an increased risk of stillbirth,” the researchers wrote.
Findings did not vary by which mRNA vaccine a mother received, the number of doses she received, or the trimester in which a vaccine was given, the researchers reported.
Stillbirth findings will be ‘very reassuring’ for patients
The lead investigator, Deshayne Fell, PhD, said in an interview, the fact that the study comprised the entire population of pregnant people in Ontario during the study period “increases our confidence” about the validity and relevance of the findings for other geographic settings.
Dr. Fell, an associate professor in epidemiology and public health at the University of Ottawa and a scientist at the Children’s Hospital of Eastern Ontario Research Institute, Ottawa, said the evaluation of stillbirth in particular, “a rare but devastating outcome,” will be “very reassuring and useful for clinical counseling.”
A limitation cited by the research team included a lack of data on vaccination prior to pregnancy.
In the new study, Dr, Ecker said, “Though the investigators were able to adjust for many variables they cannot be certain that some unmeasured variable that, accordingly, was not adjusted for does not hide a small risk. This seems very unlikely, however.”
The Canadian research team said similar studies of non-mRNA COVID vaccines “should be a research priority.” However, such studies are not underway in Canada, where only mRNA vaccines are used in pregnancy, Dr. Fell said.
This study was supported by the Public Health Agency of Canada.
Dr. Fell and Dr. Ecker reported no competing financial interests.
FROM BMJ
Intralesional Human Papillomavirus Vaccine Therapy for Recalcitrant Plantar Wart Triggers Gout Flare
To the Editor:
There is increasing evidence supporting the use of the human papillomavirus (HPV) vaccine in the treatment of recalcitrant common warts.1 We describe a potential complication associated with HPV vaccine treatment of warts that would be of interest to dermatologists.
A 70-year-old woman presented with a plantar wart measuring 6 mm in diameter at the base of the right hallux of 5 years’ duration. Prior failed therapies for wart removal included multiple paring treatments, cryotherapy, and topical salicylic acid 40% to 60%. The patient had no notable comorbidities; no history of gout; and no known risk factors for gout, such as hypertension, renal insufficiency, diuretic use, obesity, family history, or trauma.
Prior reports cited effective treatment of recalcitrant warts with recombinant HPV vaccines, both intralesionally1 and intramuscularly.2,3 With this knowledge in mind, we administered an intralesional injection with 0.1-mL recombinant HPV 9-valent vaccine to the patient’s plantar wart. Gradual erythema and swelling of the right first metatarsophalangeal joint developed over the next 7 days. Synovial fluid analysis demonstrated negatively birefringent crystals. The patient commenced treatment with colchicine and indomethacin and improved over the next 5 days. The wart resolved 3 months later and required no further treatment.
Prophylactic quadrivalent HPV vaccines have shown efficacy in treating HPV-associated precancerous and cancerous lesions.4 Case reports have suggested that HPV vaccines may be an effective treatment option for recalcitrant warts,1-3,5 especially in cases that do not respond to traditional treatment. It is possible that the mechanism of wart treatment involves overlap in the antigenic epitopes of the HPV types targeted by the vaccine vs the HPV types responsible for causing warts.2 Papillomaviruslike particles, based on the L1 capsid protein, can induce a specific CD8+ activation signal, leading to a vaccine-induced cytotoxic T-cell response that targets the wart cells with HPV-like antigens.6 The HPV vaccine contains aluminium, which has been shown to activate NLRP3 inflammasome,5 which may trigger gout by increasing monosodium urate crystal deposition via IL-1β production.7 This may lead to an increased risk for gout flares, an adverse effect of the HPV vaccine. This finding is supported by other studies of aluminium-containing vaccines that show an association with gout.6 It is noted that these vaccines are mostly delivered intramuscularly or subcutaneously in some cases.
We reported a case of gout triggered by intralesional HPV vaccine treatment of warts. It is unclear whether the gout was induced by the vaccine itself or whether it was due to trauma caused by the intralesional injection near the joint space. Based on our findings, we recommend that patients receiving intralesional injections for wart treatment be advised of this potential adverse effect, especially if they have risk factors for gout or have a history of gout.
- Nofal A, Marei A, Ibrahim AM et al. Intralesional versus intramuscular bivalent human papillomavirus vaccine in the treatment of recalcitrant common warts. J Am Acad Dermatol. 2020;82:94-100.
- Venugopal SS, Murrell DF. Recalcitrant cutaneous warts treated with recombinant quadrivalent human papillomavirus vaccine (types 6, 11, 16, and 18) in a developmentally delayed, 31-year-old white man. Arch Dermatol. 2010;146:475-477.
- Daniel BS, Murrell DF. Complete resolution of chronic multiple verruca vulgaris treated with quadrivalent human papillomavirus vaccine. JAMA Dermatol. 2013;149:370-372.
- Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838-1847.
- Eisenbarth SC, Colegio OR, O’Connor W, et al. Crucial role for the NALP3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122-1166.
- Bellone S, El-Sahwi K, Cocco E, et al. Human papillomavirus type 16 (HPV-16) virus-like particle L1-specific CD8+ cytotoxic T lymphocytes (CTLs) are equally effective as E7-specific CD8+ CTLs in killing autologous HPV-16-positive tumor cells in cervical cancer patients: implications for L1 dendritic cell-based therapeutic vaccines. J Virol. 2009;83:6779-6789.
- Yokose C, McCormick N, Chen C, et al. Risk of gout flares after vaccination: a prospective case cross-over study. Ann Rheum Dis. 2019;78:1601-1604.
To the Editor:
There is increasing evidence supporting the use of the human papillomavirus (HPV) vaccine in the treatment of recalcitrant common warts.1 We describe a potential complication associated with HPV vaccine treatment of warts that would be of interest to dermatologists.
A 70-year-old woman presented with a plantar wart measuring 6 mm in diameter at the base of the right hallux of 5 years’ duration. Prior failed therapies for wart removal included multiple paring treatments, cryotherapy, and topical salicylic acid 40% to 60%. The patient had no notable comorbidities; no history of gout; and no known risk factors for gout, such as hypertension, renal insufficiency, diuretic use, obesity, family history, or trauma.
Prior reports cited effective treatment of recalcitrant warts with recombinant HPV vaccines, both intralesionally1 and intramuscularly.2,3 With this knowledge in mind, we administered an intralesional injection with 0.1-mL recombinant HPV 9-valent vaccine to the patient’s plantar wart. Gradual erythema and swelling of the right first metatarsophalangeal joint developed over the next 7 days. Synovial fluid analysis demonstrated negatively birefringent crystals. The patient commenced treatment with colchicine and indomethacin and improved over the next 5 days. The wart resolved 3 months later and required no further treatment.
Prophylactic quadrivalent HPV vaccines have shown efficacy in treating HPV-associated precancerous and cancerous lesions.4 Case reports have suggested that HPV vaccines may be an effective treatment option for recalcitrant warts,1-3,5 especially in cases that do not respond to traditional treatment. It is possible that the mechanism of wart treatment involves overlap in the antigenic epitopes of the HPV types targeted by the vaccine vs the HPV types responsible for causing warts.2 Papillomaviruslike particles, based on the L1 capsid protein, can induce a specific CD8+ activation signal, leading to a vaccine-induced cytotoxic T-cell response that targets the wart cells with HPV-like antigens.6 The HPV vaccine contains aluminium, which has been shown to activate NLRP3 inflammasome,5 which may trigger gout by increasing monosodium urate crystal deposition via IL-1β production.7 This may lead to an increased risk for gout flares, an adverse effect of the HPV vaccine. This finding is supported by other studies of aluminium-containing vaccines that show an association with gout.6 It is noted that these vaccines are mostly delivered intramuscularly or subcutaneously in some cases.
We reported a case of gout triggered by intralesional HPV vaccine treatment of warts. It is unclear whether the gout was induced by the vaccine itself or whether it was due to trauma caused by the intralesional injection near the joint space. Based on our findings, we recommend that patients receiving intralesional injections for wart treatment be advised of this potential adverse effect, especially if they have risk factors for gout or have a history of gout.
To the Editor:
There is increasing evidence supporting the use of the human papillomavirus (HPV) vaccine in the treatment of recalcitrant common warts.1 We describe a potential complication associated with HPV vaccine treatment of warts that would be of interest to dermatologists.
A 70-year-old woman presented with a plantar wart measuring 6 mm in diameter at the base of the right hallux of 5 years’ duration. Prior failed therapies for wart removal included multiple paring treatments, cryotherapy, and topical salicylic acid 40% to 60%. The patient had no notable comorbidities; no history of gout; and no known risk factors for gout, such as hypertension, renal insufficiency, diuretic use, obesity, family history, or trauma.
Prior reports cited effective treatment of recalcitrant warts with recombinant HPV vaccines, both intralesionally1 and intramuscularly.2,3 With this knowledge in mind, we administered an intralesional injection with 0.1-mL recombinant HPV 9-valent vaccine to the patient’s plantar wart. Gradual erythema and swelling of the right first metatarsophalangeal joint developed over the next 7 days. Synovial fluid analysis demonstrated negatively birefringent crystals. The patient commenced treatment with colchicine and indomethacin and improved over the next 5 days. The wart resolved 3 months later and required no further treatment.
Prophylactic quadrivalent HPV vaccines have shown efficacy in treating HPV-associated precancerous and cancerous lesions.4 Case reports have suggested that HPV vaccines may be an effective treatment option for recalcitrant warts,1-3,5 especially in cases that do not respond to traditional treatment. It is possible that the mechanism of wart treatment involves overlap in the antigenic epitopes of the HPV types targeted by the vaccine vs the HPV types responsible for causing warts.2 Papillomaviruslike particles, based on the L1 capsid protein, can induce a specific CD8+ activation signal, leading to a vaccine-induced cytotoxic T-cell response that targets the wart cells with HPV-like antigens.6 The HPV vaccine contains aluminium, which has been shown to activate NLRP3 inflammasome,5 which may trigger gout by increasing monosodium urate crystal deposition via IL-1β production.7 This may lead to an increased risk for gout flares, an adverse effect of the HPV vaccine. This finding is supported by other studies of aluminium-containing vaccines that show an association with gout.6 It is noted that these vaccines are mostly delivered intramuscularly or subcutaneously in some cases.
We reported a case of gout triggered by intralesional HPV vaccine treatment of warts. It is unclear whether the gout was induced by the vaccine itself or whether it was due to trauma caused by the intralesional injection near the joint space. Based on our findings, we recommend that patients receiving intralesional injections for wart treatment be advised of this potential adverse effect, especially if they have risk factors for gout or have a history of gout.
- Nofal A, Marei A, Ibrahim AM et al. Intralesional versus intramuscular bivalent human papillomavirus vaccine in the treatment of recalcitrant common warts. J Am Acad Dermatol. 2020;82:94-100.
- Venugopal SS, Murrell DF. Recalcitrant cutaneous warts treated with recombinant quadrivalent human papillomavirus vaccine (types 6, 11, 16, and 18) in a developmentally delayed, 31-year-old white man. Arch Dermatol. 2010;146:475-477.
- Daniel BS, Murrell DF. Complete resolution of chronic multiple verruca vulgaris treated with quadrivalent human papillomavirus vaccine. JAMA Dermatol. 2013;149:370-372.
- Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838-1847.
- Eisenbarth SC, Colegio OR, O’Connor W, et al. Crucial role for the NALP3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122-1166.
- Bellone S, El-Sahwi K, Cocco E, et al. Human papillomavirus type 16 (HPV-16) virus-like particle L1-specific CD8+ cytotoxic T lymphocytes (CTLs) are equally effective as E7-specific CD8+ CTLs in killing autologous HPV-16-positive tumor cells in cervical cancer patients: implications for L1 dendritic cell-based therapeutic vaccines. J Virol. 2009;83:6779-6789.
- Yokose C, McCormick N, Chen C, et al. Risk of gout flares after vaccination: a prospective case cross-over study. Ann Rheum Dis. 2019;78:1601-1604.
- Nofal A, Marei A, Ibrahim AM et al. Intralesional versus intramuscular bivalent human papillomavirus vaccine in the treatment of recalcitrant common warts. J Am Acad Dermatol. 2020;82:94-100.
- Venugopal SS, Murrell DF. Recalcitrant cutaneous warts treated with recombinant quadrivalent human papillomavirus vaccine (types 6, 11, 16, and 18) in a developmentally delayed, 31-year-old white man. Arch Dermatol. 2010;146:475-477.
- Daniel BS, Murrell DF. Complete resolution of chronic multiple verruca vulgaris treated with quadrivalent human papillomavirus vaccine. JAMA Dermatol. 2013;149:370-372.
- Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838-1847.
- Eisenbarth SC, Colegio OR, O’Connor W, et al. Crucial role for the NALP3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122-1166.
- Bellone S, El-Sahwi K, Cocco E, et al. Human papillomavirus type 16 (HPV-16) virus-like particle L1-specific CD8+ cytotoxic T lymphocytes (CTLs) are equally effective as E7-specific CD8+ CTLs in killing autologous HPV-16-positive tumor cells in cervical cancer patients: implications for L1 dendritic cell-based therapeutic vaccines. J Virol. 2009;83:6779-6789.
- Yokose C, McCormick N, Chen C, et al. Risk of gout flares after vaccination: a prospective case cross-over study. Ann Rheum Dis. 2019;78:1601-1604.
Practice Points
- Human papillomavirus (HPV) vaccines are increasingly used for recalcitrant warts.
- We describe an unreported adverse effect of gout flare following HPV vaccine treatment of plantar wart.
Mechanistic link between herpes virus, Alzheimer’s revealed?
, new research suggests.
“Our results suggest one pathway to Alzheimer’s disease, caused by a VZV infection which creates inflammatory triggers that awaken HSV in the brain,” lead author Dana Cairns, PhD, research associate, department of biomedical engineering at Tufts University, Boston, said in a news release.
The findings were published online in Journal of Alzheimer’s Disease.
‘One-two punch’
Previous research has suggested a correlation between HSV-1 and AD and involvement of VZV. However, the sequence of events that the viruses create to set the disease in motion has been unclear.
“We think we now have evidence of those events,” co–senior author David Kaplan, PhD, chair of the department of biomedical engineering at Tufts, said in the release.
Working with co–senior author Ruth Itzhaki, PhD, University of Oxford, United Kingdom, the researchers infected human-induced neural stem cells (hiNSCs) and 3D brain tissue models with HSV-1 and/or VZV. Dr. Itzhaki was one of the first to hypothesize a connection between herpes virus and AD.
The investigators found that HSV-1 infection of hiNSCs induces amyloid-beta and P-tau accumulation: the main components of AD plaques and neurofibrillary tangles, respectively.
On the other hand, VZV infection of cultured hiNSCs did not lead to amyloid-beta and P-tau accumulation but instead resulted in gliosis and increased levels of proinflammatory cytokines.
“Strikingly,” VZV infection of cells quiescently infected with HSV-1 caused reactivation of HSV-1, leading to AD-like changes, including amyloid-beta and P-tau accumulation, the investigators report.
This suggests that VZV is unlikely to be a direct cause of AD but rather acts indirectly via reactivation of HSV-1, they add.
Similar findings emerged in similar experiments using 3D human brain tissue models.
“It’s a one-two punch of two viruses that are very common and usually harmless, but the lab studies suggest that if a new exposure to VZV wakes up dormant HSV-1, they could cause trouble,” Dr. Cairns said.
The researchers note that vaccination against VZV has been shown previously to reduce risk for dementia. It is possible, they add, that the vaccine is helping to stop the cycle of viral reactivation, inflammation, and neuronal damage.
‘A first step’
Heather M. Snyder, PhD, vice president of Medical & Scientific Relations at the Alzheimer’s Association, said that the study “is using artificial systems with the goal of more clearly and more deeply understanding” the assessed associations.
She added that although it is a first step, it may provide valuable direction for follow-up research.
“This is preliminary work that first needs replication, validation, and further development to understand if any association that is uncovered between viruses and Alzheimer’s/dementia has a mechanistic link,” said Dr. Snyder.
She noted that several past studies have sought to help the research field better understand the links between different viruses and Alzheimer’s and other forms of dementia.
“There have been some challenges in evaluating these associations in our current model systems or in individuals for a number of reasons,” said Dr. Snyder.
However, “the COVID-19 pandemic has created an opportunity to examine and investigate the relationships between different viruses and Alzheimer’s and other dementias by following individuals in more common and well-established ways,” she added.
She reported that her organization is “leading and working with a large global network of studies and investigators to address some of these questions” from during and after the COVID pandemic.
“The lessons we learn and share may inform our understanding of how other viruses are, or are not, connected to Alzheimer’s and other dementia,” Dr. Snyder said.
More information on the Alzheimer’s Association International Cohort Study of Chronic Neurological Sequelae of SARS-CoV-2 is available online.
The study was funded by the National Institutes of Health. Dr. Cairns, Dr. Kaplan, Dr. Itzhaki, and Dr. Snyder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
“Our results suggest one pathway to Alzheimer’s disease, caused by a VZV infection which creates inflammatory triggers that awaken HSV in the brain,” lead author Dana Cairns, PhD, research associate, department of biomedical engineering at Tufts University, Boston, said in a news release.
The findings were published online in Journal of Alzheimer’s Disease.
‘One-two punch’
Previous research has suggested a correlation between HSV-1 and AD and involvement of VZV. However, the sequence of events that the viruses create to set the disease in motion has been unclear.
“We think we now have evidence of those events,” co–senior author David Kaplan, PhD, chair of the department of biomedical engineering at Tufts, said in the release.
Working with co–senior author Ruth Itzhaki, PhD, University of Oxford, United Kingdom, the researchers infected human-induced neural stem cells (hiNSCs) and 3D brain tissue models with HSV-1 and/or VZV. Dr. Itzhaki was one of the first to hypothesize a connection between herpes virus and AD.
The investigators found that HSV-1 infection of hiNSCs induces amyloid-beta and P-tau accumulation: the main components of AD plaques and neurofibrillary tangles, respectively.
On the other hand, VZV infection of cultured hiNSCs did not lead to amyloid-beta and P-tau accumulation but instead resulted in gliosis and increased levels of proinflammatory cytokines.
“Strikingly,” VZV infection of cells quiescently infected with HSV-1 caused reactivation of HSV-1, leading to AD-like changes, including amyloid-beta and P-tau accumulation, the investigators report.
This suggests that VZV is unlikely to be a direct cause of AD but rather acts indirectly via reactivation of HSV-1, they add.
Similar findings emerged in similar experiments using 3D human brain tissue models.
“It’s a one-two punch of two viruses that are very common and usually harmless, but the lab studies suggest that if a new exposure to VZV wakes up dormant HSV-1, they could cause trouble,” Dr. Cairns said.
The researchers note that vaccination against VZV has been shown previously to reduce risk for dementia. It is possible, they add, that the vaccine is helping to stop the cycle of viral reactivation, inflammation, and neuronal damage.
‘A first step’
Heather M. Snyder, PhD, vice president of Medical & Scientific Relations at the Alzheimer’s Association, said that the study “is using artificial systems with the goal of more clearly and more deeply understanding” the assessed associations.
She added that although it is a first step, it may provide valuable direction for follow-up research.
“This is preliminary work that first needs replication, validation, and further development to understand if any association that is uncovered between viruses and Alzheimer’s/dementia has a mechanistic link,” said Dr. Snyder.
She noted that several past studies have sought to help the research field better understand the links between different viruses and Alzheimer’s and other forms of dementia.
“There have been some challenges in evaluating these associations in our current model systems or in individuals for a number of reasons,” said Dr. Snyder.
However, “the COVID-19 pandemic has created an opportunity to examine and investigate the relationships between different viruses and Alzheimer’s and other dementias by following individuals in more common and well-established ways,” she added.
She reported that her organization is “leading and working with a large global network of studies and investigators to address some of these questions” from during and after the COVID pandemic.
“The lessons we learn and share may inform our understanding of how other viruses are, or are not, connected to Alzheimer’s and other dementia,” Dr. Snyder said.
More information on the Alzheimer’s Association International Cohort Study of Chronic Neurological Sequelae of SARS-CoV-2 is available online.
The study was funded by the National Institutes of Health. Dr. Cairns, Dr. Kaplan, Dr. Itzhaki, and Dr. Snyder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
“Our results suggest one pathway to Alzheimer’s disease, caused by a VZV infection which creates inflammatory triggers that awaken HSV in the brain,” lead author Dana Cairns, PhD, research associate, department of biomedical engineering at Tufts University, Boston, said in a news release.
The findings were published online in Journal of Alzheimer’s Disease.
‘One-two punch’
Previous research has suggested a correlation between HSV-1 and AD and involvement of VZV. However, the sequence of events that the viruses create to set the disease in motion has been unclear.
“We think we now have evidence of those events,” co–senior author David Kaplan, PhD, chair of the department of biomedical engineering at Tufts, said in the release.
Working with co–senior author Ruth Itzhaki, PhD, University of Oxford, United Kingdom, the researchers infected human-induced neural stem cells (hiNSCs) and 3D brain tissue models with HSV-1 and/or VZV. Dr. Itzhaki was one of the first to hypothesize a connection between herpes virus and AD.
The investigators found that HSV-1 infection of hiNSCs induces amyloid-beta and P-tau accumulation: the main components of AD plaques and neurofibrillary tangles, respectively.
On the other hand, VZV infection of cultured hiNSCs did not lead to amyloid-beta and P-tau accumulation but instead resulted in gliosis and increased levels of proinflammatory cytokines.
“Strikingly,” VZV infection of cells quiescently infected with HSV-1 caused reactivation of HSV-1, leading to AD-like changes, including amyloid-beta and P-tau accumulation, the investigators report.
This suggests that VZV is unlikely to be a direct cause of AD but rather acts indirectly via reactivation of HSV-1, they add.
Similar findings emerged in similar experiments using 3D human brain tissue models.
“It’s a one-two punch of two viruses that are very common and usually harmless, but the lab studies suggest that if a new exposure to VZV wakes up dormant HSV-1, they could cause trouble,” Dr. Cairns said.
The researchers note that vaccination against VZV has been shown previously to reduce risk for dementia. It is possible, they add, that the vaccine is helping to stop the cycle of viral reactivation, inflammation, and neuronal damage.
‘A first step’
Heather M. Snyder, PhD, vice president of Medical & Scientific Relations at the Alzheimer’s Association, said that the study “is using artificial systems with the goal of more clearly and more deeply understanding” the assessed associations.
She added that although it is a first step, it may provide valuable direction for follow-up research.
“This is preliminary work that first needs replication, validation, and further development to understand if any association that is uncovered between viruses and Alzheimer’s/dementia has a mechanistic link,” said Dr. Snyder.
She noted that several past studies have sought to help the research field better understand the links between different viruses and Alzheimer’s and other forms of dementia.
“There have been some challenges in evaluating these associations in our current model systems or in individuals for a number of reasons,” said Dr. Snyder.
However, “the COVID-19 pandemic has created an opportunity to examine and investigate the relationships between different viruses and Alzheimer’s and other dementias by following individuals in more common and well-established ways,” she added.
She reported that her organization is “leading and working with a large global network of studies and investigators to address some of these questions” from during and after the COVID pandemic.
“The lessons we learn and share may inform our understanding of how other viruses are, or are not, connected to Alzheimer’s and other dementia,” Dr. Snyder said.
More information on the Alzheimer’s Association International Cohort Study of Chronic Neurological Sequelae of SARS-CoV-2 is available online.
The study was funded by the National Institutes of Health. Dr. Cairns, Dr. Kaplan, Dr. Itzhaki, and Dr. Snyder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE
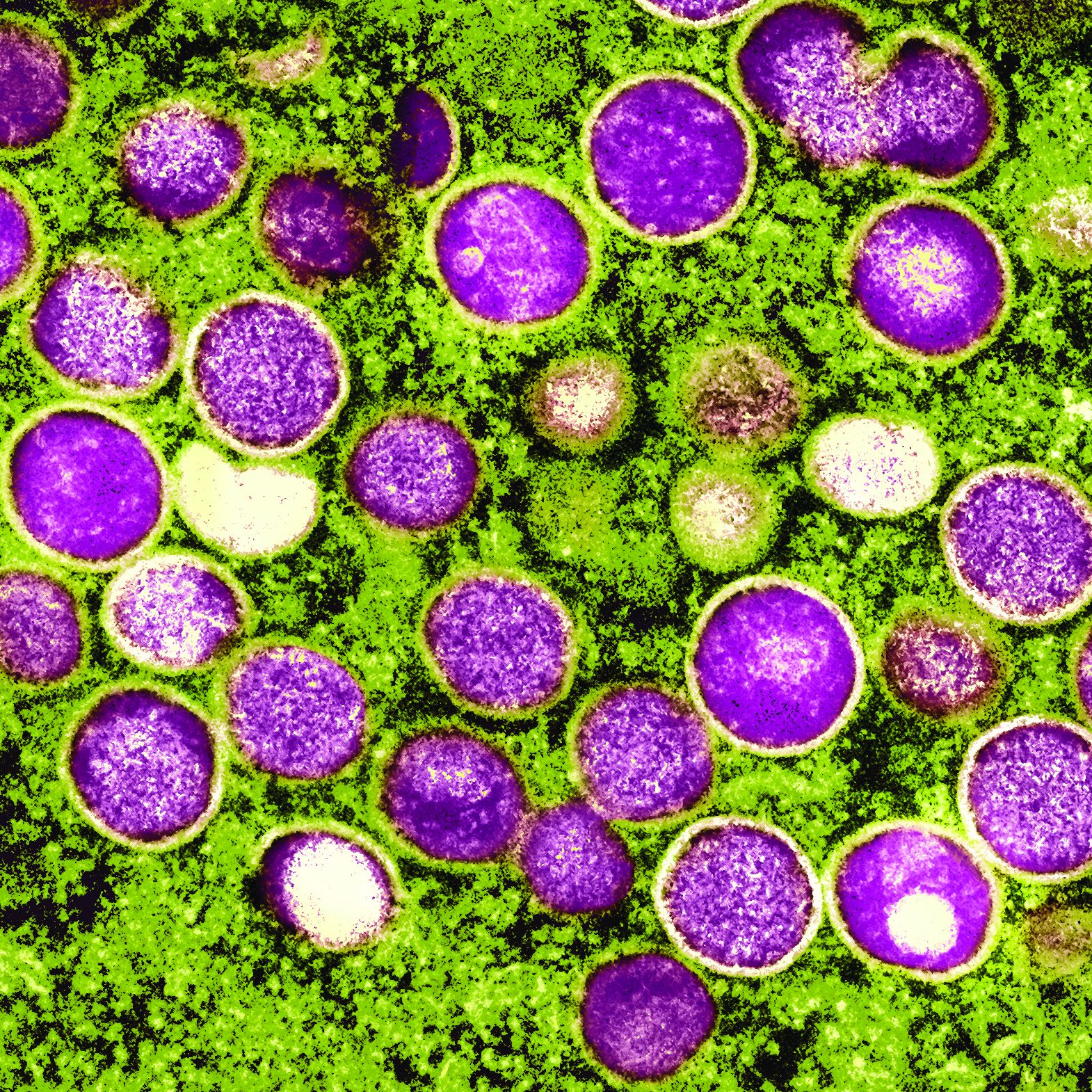
New international dermatology registry tracks monkeypox cases
The American Academy of Dermatology and the International League of Dermatological Societies (ILDS) have created a new registry that now accepts reports from health care providers worldwide about monkeypox cases and monkeypox vaccine reactions.
Patient data such as names and dates of birth will not be collected.
“As with our joint COVID-19 registry, we will be doing real-time data analysis during the outbreak,” dermatologist Esther Freeman, MD, PhD, director of MGH Global Health Dermatology at Massachusetts General Hospital, Boston, and a member of the AAD’s monkeypox task force, said in an interview. “We will to try to feed information back to our front line in terms of clinical characteristics of cases, morphology, and any unexpected findings.”
According to Dr. Freeman, the principal investigator for the COVID-19 registry, this registry has allowed the quick gathering of information about dermatologic findings of COVID-19 from over 53 countries. “We have published over 15 papers, and we share data with outside investigators wishing to do their own analysis of registry-related data,” she said. “Our most-cited paper on COVID vaccine skin reactions has been cited almost 500 times since 2021. It has been used to educate the public on vaccine side effects and to combat vaccine hesitancy.”
The monkeypox registry “doesn’t belong to any one group or person,” Dr. Freeman said. “The idea with rapid data analysis is to be able to give back to the dermatologic community what is hard for us to see with any single case: Patterns and new findings that can be helpful to share with dermatologists and other physicians worldwide, all working together to stop an outbreak.”
The American Academy of Dermatology and the International League of Dermatological Societies (ILDS) have created a new registry that now accepts reports from health care providers worldwide about monkeypox cases and monkeypox vaccine reactions.
Patient data such as names and dates of birth will not be collected.
“As with our joint COVID-19 registry, we will be doing real-time data analysis during the outbreak,” dermatologist Esther Freeman, MD, PhD, director of MGH Global Health Dermatology at Massachusetts General Hospital, Boston, and a member of the AAD’s monkeypox task force, said in an interview. “We will to try to feed information back to our front line in terms of clinical characteristics of cases, morphology, and any unexpected findings.”
According to Dr. Freeman, the principal investigator for the COVID-19 registry, this registry has allowed the quick gathering of information about dermatologic findings of COVID-19 from over 53 countries. “We have published over 15 papers, and we share data with outside investigators wishing to do their own analysis of registry-related data,” she said. “Our most-cited paper on COVID vaccine skin reactions has been cited almost 500 times since 2021. It has been used to educate the public on vaccine side effects and to combat vaccine hesitancy.”
The monkeypox registry “doesn’t belong to any one group or person,” Dr. Freeman said. “The idea with rapid data analysis is to be able to give back to the dermatologic community what is hard for us to see with any single case: Patterns and new findings that can be helpful to share with dermatologists and other physicians worldwide, all working together to stop an outbreak.”
The American Academy of Dermatology and the International League of Dermatological Societies (ILDS) have created a new registry that now accepts reports from health care providers worldwide about monkeypox cases and monkeypox vaccine reactions.
Patient data such as names and dates of birth will not be collected.
“As with our joint COVID-19 registry, we will be doing real-time data analysis during the outbreak,” dermatologist Esther Freeman, MD, PhD, director of MGH Global Health Dermatology at Massachusetts General Hospital, Boston, and a member of the AAD’s monkeypox task force, said in an interview. “We will to try to feed information back to our front line in terms of clinical characteristics of cases, morphology, and any unexpected findings.”
According to Dr. Freeman, the principal investigator for the COVID-19 registry, this registry has allowed the quick gathering of information about dermatologic findings of COVID-19 from over 53 countries. “We have published over 15 papers, and we share data with outside investigators wishing to do their own analysis of registry-related data,” she said. “Our most-cited paper on COVID vaccine skin reactions has been cited almost 500 times since 2021. It has been used to educate the public on vaccine side effects and to combat vaccine hesitancy.”
The monkeypox registry “doesn’t belong to any one group or person,” Dr. Freeman said. “The idea with rapid data analysis is to be able to give back to the dermatologic community what is hard for us to see with any single case: Patterns and new findings that can be helpful to share with dermatologists and other physicians worldwide, all working together to stop an outbreak.”
Dermatology and monkeypox: What you need to know
.
Diagnosing cases “can be hard and folks should keep a very open mind and consider monkeypox virus,” said Misha Rosenbach, MD, a University of Pennsylvania dermatologist and member of the American Academy of Dermatology’s ad hoc task force to develop monkeypox content.
Although it’s named after a primate, it turns out that monkeypox is quite the copycat. As dermatologists have learned, its lesions can look like those caused by a long list of other diseases including herpes, varicella, and syphilis. In small numbers, they can even appear to be insect bites.
To make things more complicated, a patient can have one or two lesions – or dozens. They often cluster in the anogenital area, likely reflecting transmission via sexual intercourse, unlike previous outbreaks in which lesions appeared all over the body. “We have to let go of some of our conceptions about what monkeypox might look like,” said dermatologist Esther Freeman, MD, PhD, associate professor of dermatology, Harvard University, Boston, and a member of the AAD task force.
To make things even more complicated, “the spectrum of illness that we are seeing has ranged from limited, subtle lesions to dramatic, widespread, ulcerative/necrotic lesions,” said Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
But monkeypox has unique traits that can set it apart and pave the way toward a diagnosis, dermatologists say. And important patient data can help dermatologists gauge the likelihood of a case: Almost 99% of cases with data available have been in men, and among men with available information, 94% reported male-to-male sexual or close intimate contact during the 3 weeks before developing symptoms, according to a CDC report tracking cases from May through late July. So far, cases in women and children are extremely rare, although there have been some reported in the United States.
Are dermatologists likely to see monkeypox in the clinic? It’s unclear so far. Of four dermatologists interviewed for this article, only one has seen patients with monkeypox in person. But others say they’ve been sought for consultations. “I have been asked by infectious disease colleagues for advice remotely but have not seen it,” said dermatologist Howa Yeung, MD, MSc, assistant professor of dermatology, Emory University, Atlanta. “Most of the time, they’re catching all the symptomatic cases before any need for dermatology in-person referrals.”
Still, the rapid rate of growth of the outbreak – up from 3,487 in the United States on July 25 to 12,689 as of Aug.16 – suggests that more dermatologists will see cases, and consultations may become more common too.
Know your lesions
Lesions are the telltale signs of symptomatic monkeypox. According to a recent New England Journal of Medicine study of 528 monkeypox cases from 16 nations, diagnosed between April 27 and June 24, 2022, 95% had skin lesions (58% were vesiculopustular), most commonly in the anogenital area (73%), and on the trunk/arms/or legs (55%) and face (25%), and the palms/soles (10%).
However, “the current monkeypox outbreak often presents differently from the multiple classic vesiculopustules on the skin we see in textbooks,” Dr. Yeung said. “Sometimes people can present with throat pain or rectal pain, with isolated pharyngitis or proctitis. Sometimes there are so few lesions on the skin that it can be easily confused with a bug bite, folliculitis, herpes, dyshidrotic eczema, or other skin problems. This is where dermatologists will get consulted to clarify the diagnosis while the monkeypox PCR test is pending.”
Dr. Rosenbach, who has provided consultation services to other physicians about cases, said the lesions often appear to be vesicles or pustules, “but if you go to ‘pop’ it – e.g., for testing – it’s firm and without fluid. This is likely due to pox virus inclusion, similar to other diseases such as molluscum,” caused by another pox virus, he said. Molluscum lesions are “characteristically umbilicated, with a dimple in the center, and monkeypox lesions seem to be showing a roughly similar morphology with many bowl- or caldera-shaped lesions that are donut-like in appearance,” he added.
Over time, Dr. Rosenbach said, “lesions tend to evolve slowly from smaller flesh-colored or vaguely white firm papules to broader more umbilicated/donut-shaped lesions which may erode, ulcerate, develop a crust or scab, and then heal. The amount of scarring is not yet clear, but we anticipate it to be significant, especially in patients with more widespread or severe disease.”
Jon Peebles, MD, a dermatologist at Kaiser Permanente in Largo, Md., who has treated a few in-person monkeypox cases, said the lesions can be “exquisitely painful,” although he’s also seen patients with asymptomatic lesions. “Lesions are showing a predilection for the anogenital skin, though they can occur anywhere and not uncommonly involve the oral mucosa,” said Dr. Peebles, also a member of the AAD monkeypox task force.
Dr. Yeung said it’s important to ask patients about their sexual orientation, gender identity, and sexual behaviors. “That is the only way to know who your patients are and the only way to understand who else may be at risks and can benefit from contact tracing and additional prevention measures, such as vaccination for asymptomatic sex partners.” (The Jynneos smallpox vaccine is Food and Drug Administration–approved to prevent monkeypox, although its efficacy is not entirely clear, and there’s controversy over expanding its limited availability by administering the vaccine intradermally.)
It’s also important to keep in mind that sexually transmitted infections (STIs) are common in gay and bisexual men. “Just because the patient is diagnosed with gonorrhea or syphilis does not mean the patient cannot also have monkeypox,” Dr. Rosenbach said. Indeed, the NEJM study reported that of 377 patients screened, 29% had an STI other than HIV, mostly syphilis (9%) and gonorrhea (8%). Of all 528 patients in the study (all male or transgender/nonbinary), 41% were HIV-positive, and the median number of sex partners in the last 3 months was 5 (range, 3-15).
Testing is crucial to rule monkeypox in – or out
While monkeypox lesions can be confused for other diseases, Dr. Rosenbach said that a diagnosis can be confirmed through various tests. Varicella zoster virus (VZV) and herpes simplex virus (HSV) have distinct findings on Tzanck smears (nuclear molding, multinucleated cells), and have widely available fairly rapid tests (PCR, or in some places, DFA). “Staph and bacterial folliculitis can usually be cultured quickly,” he said. “If you have someone with no risk factors/exposure, and you test for VZV, HSV, folliculitis, and it’s negative – you should know within 24 hours in most places – then you can broaden your differential diagnosis and consider alternate explanations, including monkeypox.”
Quest Diagnostics and Labcorp, two of the largest commercial labs in the United States, are now offering monkeypox tests. Labcorp says its test has a 2- to 3-day turnaround time.
As for treatment, some physicians are prescribing off-label use of tecovirimat (also known as TPOXX or ST-246), a smallpox antiviral treatment. The CDC offers guidelines about its use. “It seems to work very fast, with patients improving in 24-72 hours,” Dr. Rosenbach said. However, “it is still very challenging to give and get. There’s a cumbersome system to prescribe it, and it needs to be shipped from the national stockpile. Dermatologists should be working with their state health department, infection control, and infectious disease doctors.”
It’s likely that dermatologists are not comfortable with the process to access the drug, he said, “but if we do not act quickly to control the current outbreak, we will all – unfortunately – need to learn to be comfortable prescribing it.”
In regard to pain control, an over-the-counter painkiller approach may be appropriate depending on comorbidities, Dr. Rosenbach said. “Some patients with very severe disease, such as perianal involvement and proctitis, have such severe pain they need to be hospitalized. This is less common.”
Recommendations pending on scarring prevention
There’s limited high-quality evidence about the prevention of scarring in diseases like monkeypox, Dr. Rosenbach noted. “Any recommendations are usually based on very small, limited, uncontrolled studies. In the case of monkeypox, truly we are off the edge of the map.”
He advises cleaning lesions with gentle soap and water – keeping in mind that contaminated towels may spread disease – and potentially using a topical ointment-based dressing such as a Vaseline/nonstick dressing or Vaseline-impregnated gauze. If there’s concern about superinfection, as can occur with staph infections, topical antibiotics such as mupirocin 2% ointment may be appropriate, he said.
“Some folks like to try silica gel sheets to prevent scarring,” Dr. Rosenbach said. “There’s not a lot of evidence to support that, but they’re unlikely to be harmful. I would personally consider them, but it really depends on the extent of disease, anatomic sites involved, and access to care.”
Emory University’s Dr. Yeung also suggested using silicone gel or sheets to optimize the scar appearance once the lesions have crusted over. “People have used lasers, microneedling, etc., to improve smallpox scar appearance,” he added, “and I’m sure dermatologists will be the ones to study what works best for treating monkeypox scars.”
As for the big picture, Dr. Yeung said that dermatologists are critical in the fight to control monkeypox: “We can help our colleagues and patients manage symptoms and wound care, advocate for vaccination and treatment, treat long-term scarring sequelae, and destigmatize LGBTQ health care.”
The dermatologists interviewed for this article report no disclosures.
.
Diagnosing cases “can be hard and folks should keep a very open mind and consider monkeypox virus,” said Misha Rosenbach, MD, a University of Pennsylvania dermatologist and member of the American Academy of Dermatology’s ad hoc task force to develop monkeypox content.
Although it’s named after a primate, it turns out that monkeypox is quite the copycat. As dermatologists have learned, its lesions can look like those caused by a long list of other diseases including herpes, varicella, and syphilis. In small numbers, they can even appear to be insect bites.
To make things more complicated, a patient can have one or two lesions – or dozens. They often cluster in the anogenital area, likely reflecting transmission via sexual intercourse, unlike previous outbreaks in which lesions appeared all over the body. “We have to let go of some of our conceptions about what monkeypox might look like,” said dermatologist Esther Freeman, MD, PhD, associate professor of dermatology, Harvard University, Boston, and a member of the AAD task force.
To make things even more complicated, “the spectrum of illness that we are seeing has ranged from limited, subtle lesions to dramatic, widespread, ulcerative/necrotic lesions,” said Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
But monkeypox has unique traits that can set it apart and pave the way toward a diagnosis, dermatologists say. And important patient data can help dermatologists gauge the likelihood of a case: Almost 99% of cases with data available have been in men, and among men with available information, 94% reported male-to-male sexual or close intimate contact during the 3 weeks before developing symptoms, according to a CDC report tracking cases from May through late July. So far, cases in women and children are extremely rare, although there have been some reported in the United States.
Are dermatologists likely to see monkeypox in the clinic? It’s unclear so far. Of four dermatologists interviewed for this article, only one has seen patients with monkeypox in person. But others say they’ve been sought for consultations. “I have been asked by infectious disease colleagues for advice remotely but have not seen it,” said dermatologist Howa Yeung, MD, MSc, assistant professor of dermatology, Emory University, Atlanta. “Most of the time, they’re catching all the symptomatic cases before any need for dermatology in-person referrals.”
Still, the rapid rate of growth of the outbreak – up from 3,487 in the United States on July 25 to 12,689 as of Aug.16 – suggests that more dermatologists will see cases, and consultations may become more common too.
Know your lesions
Lesions are the telltale signs of symptomatic monkeypox. According to a recent New England Journal of Medicine study of 528 monkeypox cases from 16 nations, diagnosed between April 27 and June 24, 2022, 95% had skin lesions (58% were vesiculopustular), most commonly in the anogenital area (73%), and on the trunk/arms/or legs (55%) and face (25%), and the palms/soles (10%).
However, “the current monkeypox outbreak often presents differently from the multiple classic vesiculopustules on the skin we see in textbooks,” Dr. Yeung said. “Sometimes people can present with throat pain or rectal pain, with isolated pharyngitis or proctitis. Sometimes there are so few lesions on the skin that it can be easily confused with a bug bite, folliculitis, herpes, dyshidrotic eczema, or other skin problems. This is where dermatologists will get consulted to clarify the diagnosis while the monkeypox PCR test is pending.”
Dr. Rosenbach, who has provided consultation services to other physicians about cases, said the lesions often appear to be vesicles or pustules, “but if you go to ‘pop’ it – e.g., for testing – it’s firm and without fluid. This is likely due to pox virus inclusion, similar to other diseases such as molluscum,” caused by another pox virus, he said. Molluscum lesions are “characteristically umbilicated, with a dimple in the center, and monkeypox lesions seem to be showing a roughly similar morphology with many bowl- or caldera-shaped lesions that are donut-like in appearance,” he added.
Over time, Dr. Rosenbach said, “lesions tend to evolve slowly from smaller flesh-colored or vaguely white firm papules to broader more umbilicated/donut-shaped lesions which may erode, ulcerate, develop a crust or scab, and then heal. The amount of scarring is not yet clear, but we anticipate it to be significant, especially in patients with more widespread or severe disease.”
Jon Peebles, MD, a dermatologist at Kaiser Permanente in Largo, Md., who has treated a few in-person monkeypox cases, said the lesions can be “exquisitely painful,” although he’s also seen patients with asymptomatic lesions. “Lesions are showing a predilection for the anogenital skin, though they can occur anywhere and not uncommonly involve the oral mucosa,” said Dr. Peebles, also a member of the AAD monkeypox task force.
Dr. Yeung said it’s important to ask patients about their sexual orientation, gender identity, and sexual behaviors. “That is the only way to know who your patients are and the only way to understand who else may be at risks and can benefit from contact tracing and additional prevention measures, such as vaccination for asymptomatic sex partners.” (The Jynneos smallpox vaccine is Food and Drug Administration–approved to prevent monkeypox, although its efficacy is not entirely clear, and there’s controversy over expanding its limited availability by administering the vaccine intradermally.)
It’s also important to keep in mind that sexually transmitted infections (STIs) are common in gay and bisexual men. “Just because the patient is diagnosed with gonorrhea or syphilis does not mean the patient cannot also have monkeypox,” Dr. Rosenbach said. Indeed, the NEJM study reported that of 377 patients screened, 29% had an STI other than HIV, mostly syphilis (9%) and gonorrhea (8%). Of all 528 patients in the study (all male or transgender/nonbinary), 41% were HIV-positive, and the median number of sex partners in the last 3 months was 5 (range, 3-15).
Testing is crucial to rule monkeypox in – or out
While monkeypox lesions can be confused for other diseases, Dr. Rosenbach said that a diagnosis can be confirmed through various tests. Varicella zoster virus (VZV) and herpes simplex virus (HSV) have distinct findings on Tzanck smears (nuclear molding, multinucleated cells), and have widely available fairly rapid tests (PCR, or in some places, DFA). “Staph and bacterial folliculitis can usually be cultured quickly,” he said. “If you have someone with no risk factors/exposure, and you test for VZV, HSV, folliculitis, and it’s negative – you should know within 24 hours in most places – then you can broaden your differential diagnosis and consider alternate explanations, including monkeypox.”
Quest Diagnostics and Labcorp, two of the largest commercial labs in the United States, are now offering monkeypox tests. Labcorp says its test has a 2- to 3-day turnaround time.
As for treatment, some physicians are prescribing off-label use of tecovirimat (also known as TPOXX or ST-246), a smallpox antiviral treatment. The CDC offers guidelines about its use. “It seems to work very fast, with patients improving in 24-72 hours,” Dr. Rosenbach said. However, “it is still very challenging to give and get. There’s a cumbersome system to prescribe it, and it needs to be shipped from the national stockpile. Dermatologists should be working with their state health department, infection control, and infectious disease doctors.”
It’s likely that dermatologists are not comfortable with the process to access the drug, he said, “but if we do not act quickly to control the current outbreak, we will all – unfortunately – need to learn to be comfortable prescribing it.”
In regard to pain control, an over-the-counter painkiller approach may be appropriate depending on comorbidities, Dr. Rosenbach said. “Some patients with very severe disease, such as perianal involvement and proctitis, have such severe pain they need to be hospitalized. This is less common.”
Recommendations pending on scarring prevention
There’s limited high-quality evidence about the prevention of scarring in diseases like monkeypox, Dr. Rosenbach noted. “Any recommendations are usually based on very small, limited, uncontrolled studies. In the case of monkeypox, truly we are off the edge of the map.”
He advises cleaning lesions with gentle soap and water – keeping in mind that contaminated towels may spread disease – and potentially using a topical ointment-based dressing such as a Vaseline/nonstick dressing or Vaseline-impregnated gauze. If there’s concern about superinfection, as can occur with staph infections, topical antibiotics such as mupirocin 2% ointment may be appropriate, he said.
“Some folks like to try silica gel sheets to prevent scarring,” Dr. Rosenbach said. “There’s not a lot of evidence to support that, but they’re unlikely to be harmful. I would personally consider them, but it really depends on the extent of disease, anatomic sites involved, and access to care.”
Emory University’s Dr. Yeung also suggested using silicone gel or sheets to optimize the scar appearance once the lesions have crusted over. “People have used lasers, microneedling, etc., to improve smallpox scar appearance,” he added, “and I’m sure dermatologists will be the ones to study what works best for treating monkeypox scars.”
As for the big picture, Dr. Yeung said that dermatologists are critical in the fight to control monkeypox: “We can help our colleagues and patients manage symptoms and wound care, advocate for vaccination and treatment, treat long-term scarring sequelae, and destigmatize LGBTQ health care.”
The dermatologists interviewed for this article report no disclosures.
.
Diagnosing cases “can be hard and folks should keep a very open mind and consider monkeypox virus,” said Misha Rosenbach, MD, a University of Pennsylvania dermatologist and member of the American Academy of Dermatology’s ad hoc task force to develop monkeypox content.
Although it’s named after a primate, it turns out that monkeypox is quite the copycat. As dermatologists have learned, its lesions can look like those caused by a long list of other diseases including herpes, varicella, and syphilis. In small numbers, they can even appear to be insect bites.
To make things more complicated, a patient can have one or two lesions – or dozens. They often cluster in the anogenital area, likely reflecting transmission via sexual intercourse, unlike previous outbreaks in which lesions appeared all over the body. “We have to let go of some of our conceptions about what monkeypox might look like,” said dermatologist Esther Freeman, MD, PhD, associate professor of dermatology, Harvard University, Boston, and a member of the AAD task force.
To make things even more complicated, “the spectrum of illness that we are seeing has ranged from limited, subtle lesions to dramatic, widespread, ulcerative/necrotic lesions,” said Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia.
But monkeypox has unique traits that can set it apart and pave the way toward a diagnosis, dermatologists say. And important patient data can help dermatologists gauge the likelihood of a case: Almost 99% of cases with data available have been in men, and among men with available information, 94% reported male-to-male sexual or close intimate contact during the 3 weeks before developing symptoms, according to a CDC report tracking cases from May through late July. So far, cases in women and children are extremely rare, although there have been some reported in the United States.
Are dermatologists likely to see monkeypox in the clinic? It’s unclear so far. Of four dermatologists interviewed for this article, only one has seen patients with monkeypox in person. But others say they’ve been sought for consultations. “I have been asked by infectious disease colleagues for advice remotely but have not seen it,” said dermatologist Howa Yeung, MD, MSc, assistant professor of dermatology, Emory University, Atlanta. “Most of the time, they’re catching all the symptomatic cases before any need for dermatology in-person referrals.”
Still, the rapid rate of growth of the outbreak – up from 3,487 in the United States on July 25 to 12,689 as of Aug.16 – suggests that more dermatologists will see cases, and consultations may become more common too.
Know your lesions
Lesions are the telltale signs of symptomatic monkeypox. According to a recent New England Journal of Medicine study of 528 monkeypox cases from 16 nations, diagnosed between April 27 and June 24, 2022, 95% had skin lesions (58% were vesiculopustular), most commonly in the anogenital area (73%), and on the trunk/arms/or legs (55%) and face (25%), and the palms/soles (10%).
However, “the current monkeypox outbreak often presents differently from the multiple classic vesiculopustules on the skin we see in textbooks,” Dr. Yeung said. “Sometimes people can present with throat pain or rectal pain, with isolated pharyngitis or proctitis. Sometimes there are so few lesions on the skin that it can be easily confused with a bug bite, folliculitis, herpes, dyshidrotic eczema, or other skin problems. This is where dermatologists will get consulted to clarify the diagnosis while the monkeypox PCR test is pending.”
Dr. Rosenbach, who has provided consultation services to other physicians about cases, said the lesions often appear to be vesicles or pustules, “but if you go to ‘pop’ it – e.g., for testing – it’s firm and without fluid. This is likely due to pox virus inclusion, similar to other diseases such as molluscum,” caused by another pox virus, he said. Molluscum lesions are “characteristically umbilicated, with a dimple in the center, and monkeypox lesions seem to be showing a roughly similar morphology with many bowl- or caldera-shaped lesions that are donut-like in appearance,” he added.
Over time, Dr. Rosenbach said, “lesions tend to evolve slowly from smaller flesh-colored or vaguely white firm papules to broader more umbilicated/donut-shaped lesions which may erode, ulcerate, develop a crust or scab, and then heal. The amount of scarring is not yet clear, but we anticipate it to be significant, especially in patients with more widespread or severe disease.”
Jon Peebles, MD, a dermatologist at Kaiser Permanente in Largo, Md., who has treated a few in-person monkeypox cases, said the lesions can be “exquisitely painful,” although he’s also seen patients with asymptomatic lesions. “Lesions are showing a predilection for the anogenital skin, though they can occur anywhere and not uncommonly involve the oral mucosa,” said Dr. Peebles, also a member of the AAD monkeypox task force.
Dr. Yeung said it’s important to ask patients about their sexual orientation, gender identity, and sexual behaviors. “That is the only way to know who your patients are and the only way to understand who else may be at risks and can benefit from contact tracing and additional prevention measures, such as vaccination for asymptomatic sex partners.” (The Jynneos smallpox vaccine is Food and Drug Administration–approved to prevent monkeypox, although its efficacy is not entirely clear, and there’s controversy over expanding its limited availability by administering the vaccine intradermally.)
It’s also important to keep in mind that sexually transmitted infections (STIs) are common in gay and bisexual men. “Just because the patient is diagnosed with gonorrhea or syphilis does not mean the patient cannot also have monkeypox,” Dr. Rosenbach said. Indeed, the NEJM study reported that of 377 patients screened, 29% had an STI other than HIV, mostly syphilis (9%) and gonorrhea (8%). Of all 528 patients in the study (all male or transgender/nonbinary), 41% were HIV-positive, and the median number of sex partners in the last 3 months was 5 (range, 3-15).
Testing is crucial to rule monkeypox in – or out
While monkeypox lesions can be confused for other diseases, Dr. Rosenbach said that a diagnosis can be confirmed through various tests. Varicella zoster virus (VZV) and herpes simplex virus (HSV) have distinct findings on Tzanck smears (nuclear molding, multinucleated cells), and have widely available fairly rapid tests (PCR, or in some places, DFA). “Staph and bacterial folliculitis can usually be cultured quickly,” he said. “If you have someone with no risk factors/exposure, and you test for VZV, HSV, folliculitis, and it’s negative – you should know within 24 hours in most places – then you can broaden your differential diagnosis and consider alternate explanations, including monkeypox.”
Quest Diagnostics and Labcorp, two of the largest commercial labs in the United States, are now offering monkeypox tests. Labcorp says its test has a 2- to 3-day turnaround time.
As for treatment, some physicians are prescribing off-label use of tecovirimat (also known as TPOXX or ST-246), a smallpox antiviral treatment. The CDC offers guidelines about its use. “It seems to work very fast, with patients improving in 24-72 hours,” Dr. Rosenbach said. However, “it is still very challenging to give and get. There’s a cumbersome system to prescribe it, and it needs to be shipped from the national stockpile. Dermatologists should be working with their state health department, infection control, and infectious disease doctors.”
It’s likely that dermatologists are not comfortable with the process to access the drug, he said, “but if we do not act quickly to control the current outbreak, we will all – unfortunately – need to learn to be comfortable prescribing it.”
In regard to pain control, an over-the-counter painkiller approach may be appropriate depending on comorbidities, Dr. Rosenbach said. “Some patients with very severe disease, such as perianal involvement and proctitis, have such severe pain they need to be hospitalized. This is less common.”
Recommendations pending on scarring prevention
There’s limited high-quality evidence about the prevention of scarring in diseases like monkeypox, Dr. Rosenbach noted. “Any recommendations are usually based on very small, limited, uncontrolled studies. In the case of monkeypox, truly we are off the edge of the map.”
He advises cleaning lesions with gentle soap and water – keeping in mind that contaminated towels may spread disease – and potentially using a topical ointment-based dressing such as a Vaseline/nonstick dressing or Vaseline-impregnated gauze. If there’s concern about superinfection, as can occur with staph infections, topical antibiotics such as mupirocin 2% ointment may be appropriate, he said.
“Some folks like to try silica gel sheets to prevent scarring,” Dr. Rosenbach said. “There’s not a lot of evidence to support that, but they’re unlikely to be harmful. I would personally consider them, but it really depends on the extent of disease, anatomic sites involved, and access to care.”
Emory University’s Dr. Yeung also suggested using silicone gel or sheets to optimize the scar appearance once the lesions have crusted over. “People have used lasers, microneedling, etc., to improve smallpox scar appearance,” he added, “and I’m sure dermatologists will be the ones to study what works best for treating monkeypox scars.”
As for the big picture, Dr. Yeung said that dermatologists are critical in the fight to control monkeypox: “We can help our colleagues and patients manage symptoms and wound care, advocate for vaccination and treatment, treat long-term scarring sequelae, and destigmatize LGBTQ health care.”
The dermatologists interviewed for this article report no disclosures.
Children and COVID: ED visits and new admissions change course
New child cases of COVID-19 made at least a temporary transition from slow increase to decrease, and emergency department visits and new admissions seem to be following a downward trend.
, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. For some historical perspective, the latest weekly count falls below last year’s Delta surge figure of 121,000 (Aug. 6-12) but above the summer 2020 total of 26,000 (Aug. 7-13).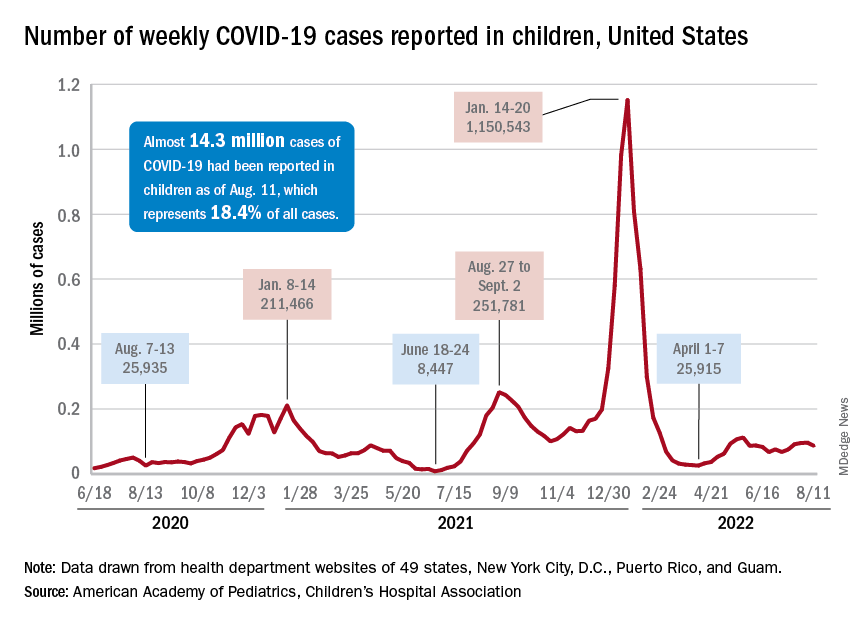
Measures of serious illness finally head downward
The prolonged rise in ED visits and new admissions over the last 5 months, which continued even through late spring when cases were declining, seems to have peaked, CDC data suggest.
That upward trend, driven largely by continued increases among younger children, peaked in late July, when 6.7% of all ED visits for children aged 0-11 years involved diagnosed COVID-19. The corresponding peaks for older children occurred around the same time but were only about half as high: 3.4% for 12- to 15-year-olds and 3.6% for those aged 16-17, the CDC reported.
The data for new admissions present a similar scenario: an increase starting in mid-April that continued unabated into late July despite the decline in new cases. By the time admissions among children aged 0-17 years peaked at 0.46 per 100,000 population in late July, they had reached the same level seen during the Delta surge. By Aug. 7, the rate of new hospitalizations was down to 0.42 per 100,000, the CDC said on its COVID Data Tracker.
The vaccine is ready for all students, but …
As children all over the country start or get ready to start a new school year, the only large-scale student vaccine mandate belongs to the District of Columbia. California has a mandate pending, but it will not go into effect until after July 1, 2023. There are, however, 20 states that have banned vaccine mandates for students, according to the National Academy for State Health Policy.
Nonmandated vaccination of the youngest children against COVID-19 continues to be slow. In the approximately 7 weeks (June 19 to Aug. 9) since the vaccine was approved for use in children younger than 5 years, just 4.4% of that age group has received at least one dose and 0.7% are fully vaccinated. Among those aged 5-11 years, who have been vaccine-eligible since early November of last year, 37.6% have received at least one dose and 30.2% are fully vaccinated, the CDC said.
New child cases of COVID-19 made at least a temporary transition from slow increase to decrease, and emergency department visits and new admissions seem to be following a downward trend.
, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. For some historical perspective, the latest weekly count falls below last year’s Delta surge figure of 121,000 (Aug. 6-12) but above the summer 2020 total of 26,000 (Aug. 7-13).
Measures of serious illness finally head downward
The prolonged rise in ED visits and new admissions over the last 5 months, which continued even through late spring when cases were declining, seems to have peaked, CDC data suggest.
That upward trend, driven largely by continued increases among younger children, peaked in late July, when 6.7% of all ED visits for children aged 0-11 years involved diagnosed COVID-19. The corresponding peaks for older children occurred around the same time but were only about half as high: 3.4% for 12- to 15-year-olds and 3.6% for those aged 16-17, the CDC reported.
The data for new admissions present a similar scenario: an increase starting in mid-April that continued unabated into late July despite the decline in new cases. By the time admissions among children aged 0-17 years peaked at 0.46 per 100,000 population in late July, they had reached the same level seen during the Delta surge. By Aug. 7, the rate of new hospitalizations was down to 0.42 per 100,000, the CDC said on its COVID Data Tracker.
The vaccine is ready for all students, but …
As children all over the country start or get ready to start a new school year, the only large-scale student vaccine mandate belongs to the District of Columbia. California has a mandate pending, but it will not go into effect until after July 1, 2023. There are, however, 20 states that have banned vaccine mandates for students, according to the National Academy for State Health Policy.
Nonmandated vaccination of the youngest children against COVID-19 continues to be slow. In the approximately 7 weeks (June 19 to Aug. 9) since the vaccine was approved for use in children younger than 5 years, just 4.4% of that age group has received at least one dose and 0.7% are fully vaccinated. Among those aged 5-11 years, who have been vaccine-eligible since early November of last year, 37.6% have received at least one dose and 30.2% are fully vaccinated, the CDC said.
New child cases of COVID-19 made at least a temporary transition from slow increase to decrease, and emergency department visits and new admissions seem to be following a downward trend.
, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association. For some historical perspective, the latest weekly count falls below last year’s Delta surge figure of 121,000 (Aug. 6-12) but above the summer 2020 total of 26,000 (Aug. 7-13).
Measures of serious illness finally head downward
The prolonged rise in ED visits and new admissions over the last 5 months, which continued even through late spring when cases were declining, seems to have peaked, CDC data suggest.
That upward trend, driven largely by continued increases among younger children, peaked in late July, when 6.7% of all ED visits for children aged 0-11 years involved diagnosed COVID-19. The corresponding peaks for older children occurred around the same time but were only about half as high: 3.4% for 12- to 15-year-olds and 3.6% for those aged 16-17, the CDC reported.
The data for new admissions present a similar scenario: an increase starting in mid-April that continued unabated into late July despite the decline in new cases. By the time admissions among children aged 0-17 years peaked at 0.46 per 100,000 population in late July, they had reached the same level seen during the Delta surge. By Aug. 7, the rate of new hospitalizations was down to 0.42 per 100,000, the CDC said on its COVID Data Tracker.
The vaccine is ready for all students, but …
As children all over the country start or get ready to start a new school year, the only large-scale student vaccine mandate belongs to the District of Columbia. California has a mandate pending, but it will not go into effect until after July 1, 2023. There are, however, 20 states that have banned vaccine mandates for students, according to the National Academy for State Health Policy.
Nonmandated vaccination of the youngest children against COVID-19 continues to be slow. In the approximately 7 weeks (June 19 to Aug. 9) since the vaccine was approved for use in children younger than 5 years, just 4.4% of that age group has received at least one dose and 0.7% are fully vaccinated. Among those aged 5-11 years, who have been vaccine-eligible since early November of last year, 37.6% have received at least one dose and 30.2% are fully vaccinated, the CDC said.
Acute otitis media pneumococcal disease burden in children due to serotypes not included in vaccines
My group in Rochester, N.Y., examined the current pneumococcal serotypes causing AOM in children. From our data, we can determine the PCV13 vaccine types that escape prevention and cause AOM and understand what effect to expect from the new pneumococcal conjugate vaccines (PCVs) that will be coming soon. There are limited data from middle ear fluid (MEF) cultures on which to base such analyses. Tympanocentesis is the preferred method for securing MEF for culture and our group is unique in providing such data to the Centers for Disease Control and publishing our results on a periodic basis to inform clinicians.
Pneumococci are the second most common cause of acute otitis media (AOM) since the introduction of pneumococcal conjugate vaccines (PCVs) more than 2 decades ago.1,2 Pneumococcal AOM causes more severe acute disease and more often causes suppurative complications than Haemophilus influenzae, which is the most common cause of AOM. Prevention of pneumococcal AOM will be a highly relevant contributor to cost-effectiveness analyses for the anticipated introduction of PCV15 (Merck) and PCV20 (Pfizer). Both PCV15 and PCV20 have been licensed for adult use; PCV15 licensure for infants and children occurred in June 2022 for invasive pneumococcal disease and is anticipated in the near future for PCV20. They are improvements over PCV13 because they add serotypes that cause invasive pneumococcal diseases, although less so for prevention of AOM, on the basis of our data.
Nasopharyngeal colonization is a necessary pathogenic step in progression to pneumococcal disease. However, not all strains of pneumococci expressing different capsular serotypes are equally virulent and likely to cause disease. In PCV-vaccinated populations, vaccine pressure and antibiotic resistance drive PCV serotype replacement with nonvaccine serotypes (NVTs), gradually reducing the net effectiveness of the vaccines. Therefore, knowledge of prevalent NVTs colonizing the nasopharynx identifies future pneumococcal serotypes most likely to emerge as pathogenic.
We published an effectiveness study of PCV13.3 A relative reduction of 86% in AOM caused by strains expressing PCV13 serotypes was observed in the first few years after PCV13 introduction. The greatest reduction in MEF samples was in serotype 19A, with a relative reduction of 91%. However, over time the vaccine type efficacy of PCV13 against MEF-positive pneumococcal AOM has eroded. There was no clear efficacy against serotype 3, and we still observed cases of serotype 19A and 19F. PCV13 vaccine failures have been even more frequent in Europe (nearly 30% of pneumococcal AOM in Europe is caused by vaccine serotypes) than our data indicate, where about 10% of AOM is caused by PCV13 serotypes.
In our most recent publication covering 2015-2019, we described results from 589 children, aged 6-36 months, from whom we collected 2,042 nasopharyngeal samples.2,4 During AOM, 495 MEF samples from 319 AOM-infected children were collected (during bilateral infections, tympanocentesis was performed in both ears). Whether bacteria were isolated was based per AOM case, not per tap. The average age of children with AOM was 15 months (range 6-31 months). The three most prevalent nasopharyngeal pneumococcal serotypes were 35B, 23B, and 15B/C. Serotype 35B was the most common at AOM visits in both the nasopharynx and MEF samples followed by serotype 15B/C. Nonsusceptibility among pneumococci to penicillin, azithromycin, and multiple other antibiotics was high. Increasing resistance to ceftriaxone was also observed.
Based on our results, if PCV15 (PCV13 + 22F and 33F) effectiveness is identical to PCV13 for the included serotypes and 100% efficacy for the added serotypes is presumed, PCV15 will reduce pneumococcal AOMs by 8%, pneumococcal nasopharyngeal colonization events at onset of AOM by 6%, and pneumococcal nasopharyngeal colonization events during health by 3%. As for the projected reductions brought about by PCV20 (PCV15 + 8, 10A, 11A, 12F, and 15B), presuming serotype 15B is efficacious against serotype 15C and 100% efficacy for the added serotypes, PCV20 will reduce pneumococcal AOMs by 22%, pneumococcal nasopharyngeal colonization events at onset of AOM by 20%, and pneumococcal nasopharyngeal colonization events during health by 3% (Figure).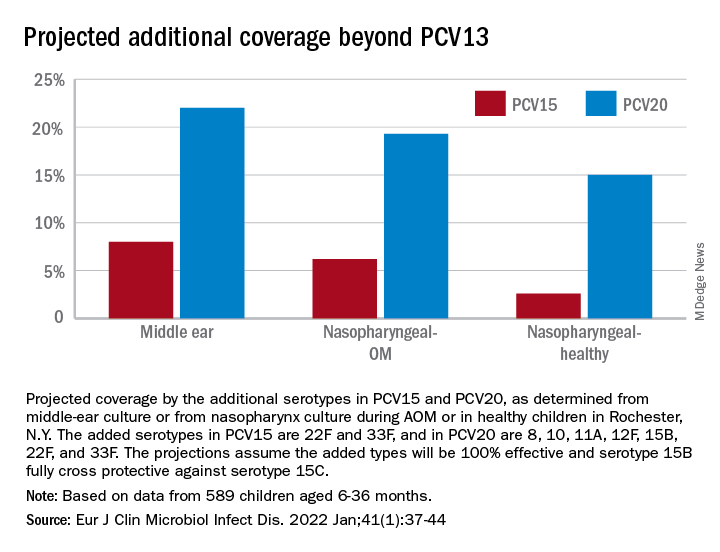
The CDC estimated that, in 2004, pneumococcal disease in the United States caused 4 million illness episodes, 22,000 deaths, 445,000 hospitalizations, 774,000 emergency department visits, 5 million outpatient visits, and 4.1 million outpatient antibiotic prescriptions. Direct medical costs totaled $3.5 billion. Pneumonia (866,000 cases) accounted for 22% of all cases and 72% of pneumococcal costs. AOM and sinusitis (1.5 million cases each) composed 75% of cases and 16% of direct medical costs.5 However, if indirect costs are taken into account, such as work loss by parents of young children, the cost of pneumococcal disease caused by AOM alone may exceed $6 billion annually6 and become dominant in the cost-effectiveness analysis in high-income countries.
Despite widespread use of PCV13, Pneumococcus has shown its resilience under vaccine pressure such that the organism remains a very common AOM pathogen. All-cause AOM has declined modestly and pneumococcal AOM caused by the specific serotypes in PCVs has declined dramatically since the introduction of PCVs. However, the burden of pneumococcal AOM disease is still considerable.
The notion that strains expressing serotypes that were not included in PCV7 were less virulent was proven wrong within a few years after introduction of PCV7, with the emergence of strains expressing serotype 19A, and others. The same cycle occurred after introduction of PCV13. It appears to take about 4 years after introduction of a PCV before peak effectiveness is achieved – which then begins to erode with emergence of NVTs. First, the NVTs are observed to colonize the nasopharynx as commensals and then from among those strains new disease-causing strains emerge.
At the most recent meeting of the International Society of Pneumococci and Pneumococcal Diseases in Toronto in June, many presentations focused on the fact that PCVs elicit highly effective protective serotype-specific antibodies to the capsular polysaccharides of included types. However, 100 serotypes are known. The limitations of PCVs are becoming increasingly apparent. They are costly and consume a large portion of the Vaccines for Children budget. Children in the developing world remain largely unvaccinated because of the high cost. NVTs that have emerged to cause disease vary by country, vary by adult vs. pediatric populations, and are dynamically changing year to year. Forthcoming PCVs of 15 and 20 serotypes will be even more costly than PCV13, will not include many newly emerged serotypes, and will probably likewise encounter “serotype replacement” because of high immune evasion by pneumococci.
When Merck and Pfizer made their decisions on serotype composition for PCV15 and PCV20, respectively, they were based on available data at the time regarding predominant serotypes causing invasive pneumococcal disease in countries that had the best data and would be the market for their products. However, from the time of the decision to licensure of vaccine is many years, and during that time the pneumococcal serotypes have changed, more so for AOM, and I predict more change will occur in the future.
In the past 3 years, Dr. Pichichero has received honoraria from Merck to attend 1-day consulting meetings and his institution has received investigator-initiated research grants to study aspects of PCV15. In the past 3 years, he was reimbursed for expenses to attend the ISPPD meeting in Toronto to present a poster on potential efficacy of PCV20 to prevent complicated AOM.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital.
References
1. Kaur R et al. Pediatrics. 2017;140(3).
2. Kaur R et al. Eur J Clin Microbiol Infect Dis. 2021;41:37-44..
3. Pichichero M et al. Lancet Child Adolesc Health. 2018;2(8):561-8.
4. Zhou F et al. Pediatrics. 2008;121(2):253-60.
5. Huang SS et al. Vaccine. 2011;29(18):3398-412.
6. Casey JR and Pichichero ME. Clin Pediatr (Phila). 2014;53(9):865-73. .
My group in Rochester, N.Y., examined the current pneumococcal serotypes causing AOM in children. From our data, we can determine the PCV13 vaccine types that escape prevention and cause AOM and understand what effect to expect from the new pneumococcal conjugate vaccines (PCVs) that will be coming soon. There are limited data from middle ear fluid (MEF) cultures on which to base such analyses. Tympanocentesis is the preferred method for securing MEF for culture and our group is unique in providing such data to the Centers for Disease Control and publishing our results on a periodic basis to inform clinicians.
Pneumococci are the second most common cause of acute otitis media (AOM) since the introduction of pneumococcal conjugate vaccines (PCVs) more than 2 decades ago.1,2 Pneumococcal AOM causes more severe acute disease and more often causes suppurative complications than Haemophilus influenzae, which is the most common cause of AOM. Prevention of pneumococcal AOM will be a highly relevant contributor to cost-effectiveness analyses for the anticipated introduction of PCV15 (Merck) and PCV20 (Pfizer). Both PCV15 and PCV20 have been licensed for adult use; PCV15 licensure for infants and children occurred in June 2022 for invasive pneumococcal disease and is anticipated in the near future for PCV20. They are improvements over PCV13 because they add serotypes that cause invasive pneumococcal diseases, although less so for prevention of AOM, on the basis of our data.
Nasopharyngeal colonization is a necessary pathogenic step in progression to pneumococcal disease. However, not all strains of pneumococci expressing different capsular serotypes are equally virulent and likely to cause disease. In PCV-vaccinated populations, vaccine pressure and antibiotic resistance drive PCV serotype replacement with nonvaccine serotypes (NVTs), gradually reducing the net effectiveness of the vaccines. Therefore, knowledge of prevalent NVTs colonizing the nasopharynx identifies future pneumococcal serotypes most likely to emerge as pathogenic.
We published an effectiveness study of PCV13.3 A relative reduction of 86% in AOM caused by strains expressing PCV13 serotypes was observed in the first few years after PCV13 introduction. The greatest reduction in MEF samples was in serotype 19A, with a relative reduction of 91%. However, over time the vaccine type efficacy of PCV13 against MEF-positive pneumococcal AOM has eroded. There was no clear efficacy against serotype 3, and we still observed cases of serotype 19A and 19F. PCV13 vaccine failures have been even more frequent in Europe (nearly 30% of pneumococcal AOM in Europe is caused by vaccine serotypes) than our data indicate, where about 10% of AOM is caused by PCV13 serotypes.
In our most recent publication covering 2015-2019, we described results from 589 children, aged 6-36 months, from whom we collected 2,042 nasopharyngeal samples.2,4 During AOM, 495 MEF samples from 319 AOM-infected children were collected (during bilateral infections, tympanocentesis was performed in both ears). Whether bacteria were isolated was based per AOM case, not per tap. The average age of children with AOM was 15 months (range 6-31 months). The three most prevalent nasopharyngeal pneumococcal serotypes were 35B, 23B, and 15B/C. Serotype 35B was the most common at AOM visits in both the nasopharynx and MEF samples followed by serotype 15B/C. Nonsusceptibility among pneumococci to penicillin, azithromycin, and multiple other antibiotics was high. Increasing resistance to ceftriaxone was also observed.
Based on our results, if PCV15 (PCV13 + 22F and 33F) effectiveness is identical to PCV13 for the included serotypes and 100% efficacy for the added serotypes is presumed, PCV15 will reduce pneumococcal AOMs by 8%, pneumococcal nasopharyngeal colonization events at onset of AOM by 6%, and pneumococcal nasopharyngeal colonization events during health by 3%. As for the projected reductions brought about by PCV20 (PCV15 + 8, 10A, 11A, 12F, and 15B), presuming serotype 15B is efficacious against serotype 15C and 100% efficacy for the added serotypes, PCV20 will reduce pneumococcal AOMs by 22%, pneumococcal nasopharyngeal colonization events at onset of AOM by 20%, and pneumococcal nasopharyngeal colonization events during health by 3% (Figure).
The CDC estimated that, in 2004, pneumococcal disease in the United States caused 4 million illness episodes, 22,000 deaths, 445,000 hospitalizations, 774,000 emergency department visits, 5 million outpatient visits, and 4.1 million outpatient antibiotic prescriptions. Direct medical costs totaled $3.5 billion. Pneumonia (866,000 cases) accounted for 22% of all cases and 72% of pneumococcal costs. AOM and sinusitis (1.5 million cases each) composed 75% of cases and 16% of direct medical costs.5 However, if indirect costs are taken into account, such as work loss by parents of young children, the cost of pneumococcal disease caused by AOM alone may exceed $6 billion annually6 and become dominant in the cost-effectiveness analysis in high-income countries.
Despite widespread use of PCV13, Pneumococcus has shown its resilience under vaccine pressure such that the organism remains a very common AOM pathogen. All-cause AOM has declined modestly and pneumococcal AOM caused by the specific serotypes in PCVs has declined dramatically since the introduction of PCVs. However, the burden of pneumococcal AOM disease is still considerable.
The notion that strains expressing serotypes that were not included in PCV7 were less virulent was proven wrong within a few years after introduction of PCV7, with the emergence of strains expressing serotype 19A, and others. The same cycle occurred after introduction of PCV13. It appears to take about 4 years after introduction of a PCV before peak effectiveness is achieved – which then begins to erode with emergence of NVTs. First, the NVTs are observed to colonize the nasopharynx as commensals and then from among those strains new disease-causing strains emerge.
At the most recent meeting of the International Society of Pneumococci and Pneumococcal Diseases in Toronto in June, many presentations focused on the fact that PCVs elicit highly effective protective serotype-specific antibodies to the capsular polysaccharides of included types. However, 100 serotypes are known. The limitations of PCVs are becoming increasingly apparent. They are costly and consume a large portion of the Vaccines for Children budget. Children in the developing world remain largely unvaccinated because of the high cost. NVTs that have emerged to cause disease vary by country, vary by adult vs. pediatric populations, and are dynamically changing year to year. Forthcoming PCVs of 15 and 20 serotypes will be even more costly than PCV13, will not include many newly emerged serotypes, and will probably likewise encounter “serotype replacement” because of high immune evasion by pneumococci.
When Merck and Pfizer made their decisions on serotype composition for PCV15 and PCV20, respectively, they were based on available data at the time regarding predominant serotypes causing invasive pneumococcal disease in countries that had the best data and would be the market for their products. However, from the time of the decision to licensure of vaccine is many years, and during that time the pneumococcal serotypes have changed, more so for AOM, and I predict more change will occur in the future.
In the past 3 years, Dr. Pichichero has received honoraria from Merck to attend 1-day consulting meetings and his institution has received investigator-initiated research grants to study aspects of PCV15. In the past 3 years, he was reimbursed for expenses to attend the ISPPD meeting in Toronto to present a poster on potential efficacy of PCV20 to prevent complicated AOM.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital.
References
1. Kaur R et al. Pediatrics. 2017;140(3).
2. Kaur R et al. Eur J Clin Microbiol Infect Dis. 2021;41:37-44..
3. Pichichero M et al. Lancet Child Adolesc Health. 2018;2(8):561-8.
4. Zhou F et al. Pediatrics. 2008;121(2):253-60.
5. Huang SS et al. Vaccine. 2011;29(18):3398-412.
6. Casey JR and Pichichero ME. Clin Pediatr (Phila). 2014;53(9):865-73. .
My group in Rochester, N.Y., examined the current pneumococcal serotypes causing AOM in children. From our data, we can determine the PCV13 vaccine types that escape prevention and cause AOM and understand what effect to expect from the new pneumococcal conjugate vaccines (PCVs) that will be coming soon. There are limited data from middle ear fluid (MEF) cultures on which to base such analyses. Tympanocentesis is the preferred method for securing MEF for culture and our group is unique in providing such data to the Centers for Disease Control and publishing our results on a periodic basis to inform clinicians.
Pneumococci are the second most common cause of acute otitis media (AOM) since the introduction of pneumococcal conjugate vaccines (PCVs) more than 2 decades ago.1,2 Pneumococcal AOM causes more severe acute disease and more often causes suppurative complications than Haemophilus influenzae, which is the most common cause of AOM. Prevention of pneumococcal AOM will be a highly relevant contributor to cost-effectiveness analyses for the anticipated introduction of PCV15 (Merck) and PCV20 (Pfizer). Both PCV15 and PCV20 have been licensed for adult use; PCV15 licensure for infants and children occurred in June 2022 for invasive pneumococcal disease and is anticipated in the near future for PCV20. They are improvements over PCV13 because they add serotypes that cause invasive pneumococcal diseases, although less so for prevention of AOM, on the basis of our data.
Nasopharyngeal colonization is a necessary pathogenic step in progression to pneumococcal disease. However, not all strains of pneumococci expressing different capsular serotypes are equally virulent and likely to cause disease. In PCV-vaccinated populations, vaccine pressure and antibiotic resistance drive PCV serotype replacement with nonvaccine serotypes (NVTs), gradually reducing the net effectiveness of the vaccines. Therefore, knowledge of prevalent NVTs colonizing the nasopharynx identifies future pneumococcal serotypes most likely to emerge as pathogenic.
We published an effectiveness study of PCV13.3 A relative reduction of 86% in AOM caused by strains expressing PCV13 serotypes was observed in the first few years after PCV13 introduction. The greatest reduction in MEF samples was in serotype 19A, with a relative reduction of 91%. However, over time the vaccine type efficacy of PCV13 against MEF-positive pneumococcal AOM has eroded. There was no clear efficacy against serotype 3, and we still observed cases of serotype 19A and 19F. PCV13 vaccine failures have been even more frequent in Europe (nearly 30% of pneumococcal AOM in Europe is caused by vaccine serotypes) than our data indicate, where about 10% of AOM is caused by PCV13 serotypes.
In our most recent publication covering 2015-2019, we described results from 589 children, aged 6-36 months, from whom we collected 2,042 nasopharyngeal samples.2,4 During AOM, 495 MEF samples from 319 AOM-infected children were collected (during bilateral infections, tympanocentesis was performed in both ears). Whether bacteria were isolated was based per AOM case, not per tap. The average age of children with AOM was 15 months (range 6-31 months). The three most prevalent nasopharyngeal pneumococcal serotypes were 35B, 23B, and 15B/C. Serotype 35B was the most common at AOM visits in both the nasopharynx and MEF samples followed by serotype 15B/C. Nonsusceptibility among pneumococci to penicillin, azithromycin, and multiple other antibiotics was high. Increasing resistance to ceftriaxone was also observed.
Based on our results, if PCV15 (PCV13 + 22F and 33F) effectiveness is identical to PCV13 for the included serotypes and 100% efficacy for the added serotypes is presumed, PCV15 will reduce pneumococcal AOMs by 8%, pneumococcal nasopharyngeal colonization events at onset of AOM by 6%, and pneumococcal nasopharyngeal colonization events during health by 3%. As for the projected reductions brought about by PCV20 (PCV15 + 8, 10A, 11A, 12F, and 15B), presuming serotype 15B is efficacious against serotype 15C and 100% efficacy for the added serotypes, PCV20 will reduce pneumococcal AOMs by 22%, pneumococcal nasopharyngeal colonization events at onset of AOM by 20%, and pneumococcal nasopharyngeal colonization events during health by 3% (Figure).
The CDC estimated that, in 2004, pneumococcal disease in the United States caused 4 million illness episodes, 22,000 deaths, 445,000 hospitalizations, 774,000 emergency department visits, 5 million outpatient visits, and 4.1 million outpatient antibiotic prescriptions. Direct medical costs totaled $3.5 billion. Pneumonia (866,000 cases) accounted for 22% of all cases and 72% of pneumococcal costs. AOM and sinusitis (1.5 million cases each) composed 75% of cases and 16% of direct medical costs.5 However, if indirect costs are taken into account, such as work loss by parents of young children, the cost of pneumococcal disease caused by AOM alone may exceed $6 billion annually6 and become dominant in the cost-effectiveness analysis in high-income countries.
Despite widespread use of PCV13, Pneumococcus has shown its resilience under vaccine pressure such that the organism remains a very common AOM pathogen. All-cause AOM has declined modestly and pneumococcal AOM caused by the specific serotypes in PCVs has declined dramatically since the introduction of PCVs. However, the burden of pneumococcal AOM disease is still considerable.
The notion that strains expressing serotypes that were not included in PCV7 were less virulent was proven wrong within a few years after introduction of PCV7, with the emergence of strains expressing serotype 19A, and others. The same cycle occurred after introduction of PCV13. It appears to take about 4 years after introduction of a PCV before peak effectiveness is achieved – which then begins to erode with emergence of NVTs. First, the NVTs are observed to colonize the nasopharynx as commensals and then from among those strains new disease-causing strains emerge.
At the most recent meeting of the International Society of Pneumococci and Pneumococcal Diseases in Toronto in June, many presentations focused on the fact that PCVs elicit highly effective protective serotype-specific antibodies to the capsular polysaccharides of included types. However, 100 serotypes are known. The limitations of PCVs are becoming increasingly apparent. They are costly and consume a large portion of the Vaccines for Children budget. Children in the developing world remain largely unvaccinated because of the high cost. NVTs that have emerged to cause disease vary by country, vary by adult vs. pediatric populations, and are dynamically changing year to year. Forthcoming PCVs of 15 and 20 serotypes will be even more costly than PCV13, will not include many newly emerged serotypes, and will probably likewise encounter “serotype replacement” because of high immune evasion by pneumococci.
When Merck and Pfizer made their decisions on serotype composition for PCV15 and PCV20, respectively, they were based on available data at the time regarding predominant serotypes causing invasive pneumococcal disease in countries that had the best data and would be the market for their products. However, from the time of the decision to licensure of vaccine is many years, and during that time the pneumococcal serotypes have changed, more so for AOM, and I predict more change will occur in the future.
In the past 3 years, Dr. Pichichero has received honoraria from Merck to attend 1-day consulting meetings and his institution has received investigator-initiated research grants to study aspects of PCV15. In the past 3 years, he was reimbursed for expenses to attend the ISPPD meeting in Toronto to present a poster on potential efficacy of PCV20 to prevent complicated AOM.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital.
References
1. Kaur R et al. Pediatrics. 2017;140(3).
2. Kaur R et al. Eur J Clin Microbiol Infect Dis. 2021;41:37-44..
3. Pichichero M et al. Lancet Child Adolesc Health. 2018;2(8):561-8.
4. Zhou F et al. Pediatrics. 2008;121(2):253-60.
5. Huang SS et al. Vaccine. 2011;29(18):3398-412.
6. Casey JR and Pichichero ME. Clin Pediatr (Phila). 2014;53(9):865-73. .
Few hepatitis C patients receive timely treatment: CDC
Fewer than 1 in 3 people infected with hepatitis C virus (HCV) begin receiving treatment within a year of their diagnosis, according to a new report by the Centers for Disease Control and Prevention.
Although HCV infection can be cured in more than 95% of patients with safe, oral medication, many barriers prevent people from receiving the care they need, experts say. These include insurance restrictions and the need for specialist visits.
to diagnosis and treatment,” said Carolyn Wester, MD, MPH, director of the CDC’s Division of Viral Hepatitis, during an Aug. 9 press call. “People shouldn’t have to jump over hurdles to access lifesaving treatments.”
The CDC report was published in Vital Signs.
An estimated 2.2 million Americans are living with HCV infection. The most recent data indicate that new infections increased more than threefold from 2011 to 2019. HCV transmission usually occurs through contact with the blood of an infected person. Today, most people become infected with the virus by sharing needles, syringes, and other equipment used to inject drugs, according to the CDC.
The researchers used a nationwide administrative claims database to identify more than 47,600 adults diagnosed with HCV infection from Jan. 30, 2019 through Oct. 31, 2020. Most patients (79%) were Medicaid recipients, 7% were Medicare patients, and 14% had private insurance. CDC researchers found that just 23% of Medicaid recipients, 28% of Medicare patients, and 35% of patients with private insurance began receiving direct-acting antiviral agents (DAAs) within 360 days of receiving a positive HCV test result. Of those who did receive treatment, most (from 75% to 84%) began receiving treatment within 180 days of their diagnosis.
Among people on Medicaid plans, patients who lived in states with treatment restrictions were 23% less likely to receive timely treatment (adjusted odds ratio, 0.77; 95% confidence interval, 0.74-0.81), compared with those living in states with no restrictions. Medicaid patients who were Black or of another race other than White were also less likely than White patients to be treated for HCV within the same year as their diagnosis. The lowest rates of treatment were among adults younger than 40 years, regardless of insurance type. This age group had the highest rates of new infections.
Actual treatment percentages may be even smaller than the number captured in this study, because the study included patients with continuous insurance coverage, Dr. Wester said, “so in many ways, [these] are the individuals who are set up to have the best access to care and treatment.”
Dr. Wester mentioned several steps that could improve access to DAAs for patients with HCV infection:
- Provide treatment outside of specialist offices, such as primary care and community clinics, substance use treatment centers, and syringe services programs.
- Increase the number of primary care providers offering hepatitis C treatment.
- Provide treatment in as few visits as possible.
- Eliminate restrictions by insurance providers on treatment.
A ‘health injustice’
While DAA treatments are effective, they are also expensive. Generic medications cost around $24,000 for a 12-week course, and some brand-name drugs are estimated to cost more than three times that amount. Many insurance companies, therefore, have treatment restrictions in place, including the following:
- There must be evidence of liver fibrosis for a patient to be treated.
- The doctor prescribing treatment must be a liver specialist or an infectious disease specialist.
- The patient must meet sobriety requirements.
- Treatment requires preauthorization approval from insurance carriers.
These criteria prevent patients from getting the care that they need, said Jonathan Mermin, MD, MPH, director of the CDC’s National Center for HIV, Viral Hepatitis, STD, and TB Prevention, during the press call. “Restricting access to hepatitis C treatment turns an infectious disease into a health injustice,” he added.
Oluwaseun Falade-Nwulia, MBBS, MPH, an infectious disease specialist and assistant professor of medicine at the Johns Hopkins University School of Medicine, Baltimore, emphasized the importance of removing barriers to HCV treatment and expanding HCV care out of specialist offices. She noted that treatment for HCV infection should begin immediately after a patient’s diagnosis. Previously, guidelines recommended waiting 6 months from the time a patient was diagnosed with HCV to begin treatment to see whether the patient’s body could clear the infection on its own. Now, guidelines recommend that after a diagnosis of acute HCV, “HCV treatment should be initiated without awaiting spontaneous resolution.” But some insurance companies still ask for evidence that a patient has been infected for at least 6 months before approving therapy, Dr. Falade-Nwulia noted.
“We have a system that has so many structural barriers for patients who we know already have so many social determinants of health working against them to access any health care,” she said. “I think it’s doubly devastating that patients that can actually get to a provider and get a prescription may still not have access to [the medication] because of structural barriers, such as restrictions based on a need to prove chronicity.”
A version of this article first appeared on Medscape.com.
Fewer than 1 in 3 people infected with hepatitis C virus (HCV) begin receiving treatment within a year of their diagnosis, according to a new report by the Centers for Disease Control and Prevention.
Although HCV infection can be cured in more than 95% of patients with safe, oral medication, many barriers prevent people from receiving the care they need, experts say. These include insurance restrictions and the need for specialist visits.
to diagnosis and treatment,” said Carolyn Wester, MD, MPH, director of the CDC’s Division of Viral Hepatitis, during an Aug. 9 press call. “People shouldn’t have to jump over hurdles to access lifesaving treatments.”
The CDC report was published in Vital Signs.
An estimated 2.2 million Americans are living with HCV infection. The most recent data indicate that new infections increased more than threefold from 2011 to 2019. HCV transmission usually occurs through contact with the blood of an infected person. Today, most people become infected with the virus by sharing needles, syringes, and other equipment used to inject drugs, according to the CDC.
The researchers used a nationwide administrative claims database to identify more than 47,600 adults diagnosed with HCV infection from Jan. 30, 2019 through Oct. 31, 2020. Most patients (79%) were Medicaid recipients, 7% were Medicare patients, and 14% had private insurance. CDC researchers found that just 23% of Medicaid recipients, 28% of Medicare patients, and 35% of patients with private insurance began receiving direct-acting antiviral agents (DAAs) within 360 days of receiving a positive HCV test result. Of those who did receive treatment, most (from 75% to 84%) began receiving treatment within 180 days of their diagnosis.
Among people on Medicaid plans, patients who lived in states with treatment restrictions were 23% less likely to receive timely treatment (adjusted odds ratio, 0.77; 95% confidence interval, 0.74-0.81), compared with those living in states with no restrictions. Medicaid patients who were Black or of another race other than White were also less likely than White patients to be treated for HCV within the same year as their diagnosis. The lowest rates of treatment were among adults younger than 40 years, regardless of insurance type. This age group had the highest rates of new infections.
Actual treatment percentages may be even smaller than the number captured in this study, because the study included patients with continuous insurance coverage, Dr. Wester said, “so in many ways, [these] are the individuals who are set up to have the best access to care and treatment.”
Dr. Wester mentioned several steps that could improve access to DAAs for patients with HCV infection:
- Provide treatment outside of specialist offices, such as primary care and community clinics, substance use treatment centers, and syringe services programs.
- Increase the number of primary care providers offering hepatitis C treatment.
- Provide treatment in as few visits as possible.
- Eliminate restrictions by insurance providers on treatment.
A ‘health injustice’
While DAA treatments are effective, they are also expensive. Generic medications cost around $24,000 for a 12-week course, and some brand-name drugs are estimated to cost more than three times that amount. Many insurance companies, therefore, have treatment restrictions in place, including the following:
- There must be evidence of liver fibrosis for a patient to be treated.
- The doctor prescribing treatment must be a liver specialist or an infectious disease specialist.
- The patient must meet sobriety requirements.
- Treatment requires preauthorization approval from insurance carriers.
These criteria prevent patients from getting the care that they need, said Jonathan Mermin, MD, MPH, director of the CDC’s National Center for HIV, Viral Hepatitis, STD, and TB Prevention, during the press call. “Restricting access to hepatitis C treatment turns an infectious disease into a health injustice,” he added.
Oluwaseun Falade-Nwulia, MBBS, MPH, an infectious disease specialist and assistant professor of medicine at the Johns Hopkins University School of Medicine, Baltimore, emphasized the importance of removing barriers to HCV treatment and expanding HCV care out of specialist offices. She noted that treatment for HCV infection should begin immediately after a patient’s diagnosis. Previously, guidelines recommended waiting 6 months from the time a patient was diagnosed with HCV to begin treatment to see whether the patient’s body could clear the infection on its own. Now, guidelines recommend that after a diagnosis of acute HCV, “HCV treatment should be initiated without awaiting spontaneous resolution.” But some insurance companies still ask for evidence that a patient has been infected for at least 6 months before approving therapy, Dr. Falade-Nwulia noted.
“We have a system that has so many structural barriers for patients who we know already have so many social determinants of health working against them to access any health care,” she said. “I think it’s doubly devastating that patients that can actually get to a provider and get a prescription may still not have access to [the medication] because of structural barriers, such as restrictions based on a need to prove chronicity.”
A version of this article first appeared on Medscape.com.
Fewer than 1 in 3 people infected with hepatitis C virus (HCV) begin receiving treatment within a year of their diagnosis, according to a new report by the Centers for Disease Control and Prevention.
Although HCV infection can be cured in more than 95% of patients with safe, oral medication, many barriers prevent people from receiving the care they need, experts say. These include insurance restrictions and the need for specialist visits.
to diagnosis and treatment,” said Carolyn Wester, MD, MPH, director of the CDC’s Division of Viral Hepatitis, during an Aug. 9 press call. “People shouldn’t have to jump over hurdles to access lifesaving treatments.”
The CDC report was published in Vital Signs.
An estimated 2.2 million Americans are living with HCV infection. The most recent data indicate that new infections increased more than threefold from 2011 to 2019. HCV transmission usually occurs through contact with the blood of an infected person. Today, most people become infected with the virus by sharing needles, syringes, and other equipment used to inject drugs, according to the CDC.
The researchers used a nationwide administrative claims database to identify more than 47,600 adults diagnosed with HCV infection from Jan. 30, 2019 through Oct. 31, 2020. Most patients (79%) were Medicaid recipients, 7% were Medicare patients, and 14% had private insurance. CDC researchers found that just 23% of Medicaid recipients, 28% of Medicare patients, and 35% of patients with private insurance began receiving direct-acting antiviral agents (DAAs) within 360 days of receiving a positive HCV test result. Of those who did receive treatment, most (from 75% to 84%) began receiving treatment within 180 days of their diagnosis.
Among people on Medicaid plans, patients who lived in states with treatment restrictions were 23% less likely to receive timely treatment (adjusted odds ratio, 0.77; 95% confidence interval, 0.74-0.81), compared with those living in states with no restrictions. Medicaid patients who were Black or of another race other than White were also less likely than White patients to be treated for HCV within the same year as their diagnosis. The lowest rates of treatment were among adults younger than 40 years, regardless of insurance type. This age group had the highest rates of new infections.
Actual treatment percentages may be even smaller than the number captured in this study, because the study included patients with continuous insurance coverage, Dr. Wester said, “so in many ways, [these] are the individuals who are set up to have the best access to care and treatment.”
Dr. Wester mentioned several steps that could improve access to DAAs for patients with HCV infection:
- Provide treatment outside of specialist offices, such as primary care and community clinics, substance use treatment centers, and syringe services programs.
- Increase the number of primary care providers offering hepatitis C treatment.
- Provide treatment in as few visits as possible.
- Eliminate restrictions by insurance providers on treatment.
A ‘health injustice’
While DAA treatments are effective, they are also expensive. Generic medications cost around $24,000 for a 12-week course, and some brand-name drugs are estimated to cost more than three times that amount. Many insurance companies, therefore, have treatment restrictions in place, including the following:
- There must be evidence of liver fibrosis for a patient to be treated.
- The doctor prescribing treatment must be a liver specialist or an infectious disease specialist.
- The patient must meet sobriety requirements.
- Treatment requires preauthorization approval from insurance carriers.
These criteria prevent patients from getting the care that they need, said Jonathan Mermin, MD, MPH, director of the CDC’s National Center for HIV, Viral Hepatitis, STD, and TB Prevention, during the press call. “Restricting access to hepatitis C treatment turns an infectious disease into a health injustice,” he added.
Oluwaseun Falade-Nwulia, MBBS, MPH, an infectious disease specialist and assistant professor of medicine at the Johns Hopkins University School of Medicine, Baltimore, emphasized the importance of removing barriers to HCV treatment and expanding HCV care out of specialist offices. She noted that treatment for HCV infection should begin immediately after a patient’s diagnosis. Previously, guidelines recommended waiting 6 months from the time a patient was diagnosed with HCV to begin treatment to see whether the patient’s body could clear the infection on its own. Now, guidelines recommend that after a diagnosis of acute HCV, “HCV treatment should be initiated without awaiting spontaneous resolution.” But some insurance companies still ask for evidence that a patient has been infected for at least 6 months before approving therapy, Dr. Falade-Nwulia noted.
“We have a system that has so many structural barriers for patients who we know already have so many social determinants of health working against them to access any health care,” she said. “I think it’s doubly devastating that patients that can actually get to a provider and get a prescription may still not have access to [the medication] because of structural barriers, such as restrictions based on a need to prove chronicity.”
A version of this article first appeared on Medscape.com.
Polio virus found in NYC sewer system
Polio virus has been discovered in New York City’s sewers, suggesting that the virus is circulating in the city, New York’s health authorities said Aug. 12.
“For every one case of paralytic polio identified, hundreds more may be undetected,” Dr. Bassett said. “The best way to keep adults and children polio-free is through safe and effective immunization.”
Polio can cause permanent paralysis of limbs and even death in some cases. Before this outbreak, the last case of polio in the United States was in 2013.
The announcement came after a man in Rockland County, New York, north of the city, was stricken with polio at the end of July and paralyzed.
Now, health officials fear that the detection of polio in NYC wastewater could bring other cases of paralytic polio.
“It is not surprising, since this is something already seen with Rockland County,” Amesh Adalja, MD, senior scholar at the Johns Hopkins Center for Health Security in Baltimore, told this news organization. “This is solely the result of under-vaccination in the area. I think it’s likely that we will see a few paralytic cases but not a high number.”
Vaccinations declined in pandemic
Among the worries is that vaccination rates across New York City dipped during the pandemic because pediatrician visits were postponed.
In New York City, the overall rate of polio vaccination among children aged 5 years or younger is 86%. Still, in some city ZIP codes, fewer than two-thirds of children in that age group have received the full dosage, which worries health officials.
However, most adults were vaccinated against polio as children.
Across New York state, nearly 80% of people have been vaccinated, according to data from the state public health department. Those who are unvaccinated are at risk, but the polio vaccine is nearly 100% effective in people who are fully immunized.
New York health authorities are calling on those who are unvaccinated to get their shots immediately.
“The risk to New Yorkers is real, but the defense is so simple – get vaccinated against polio,” New York City Health Commissioner Ashwin Vasan, MD, PhD, said in a statement. “Polio is entirely preventable, and its reappearance should be a call for all of us.”
Though many of those who are infected have no symptoms, about 4% will get viral meningitis “and about 1 in 200 will become paralyzed,” according to a news release.
Symptoms can be flu-like
Symptoms can include those similar to the flu, such as sore throat, fever, fatigue, nausea, and stomach ache. There is no cure for the disease.
The city’s health department has given no details about where exactly polio had been found in NYC’s wastewater nor did they give dates the virus was detected.
Health authorities urged parents of children who are not yet fully vaccinated to bring them to their pediatricians.
In 1916, polio killed 6,000 people in the United States and left at least another 21,000 – most of them children – permanently disabled.
An outbreak in 1952 caused paralysis in more than 20,000 people and left many children on iron lungs. The first effective vaccine emerged just a few years later and the virus began to wane.
A version of this article first appeared on Medscape.com.
Polio virus has been discovered in New York City’s sewers, suggesting that the virus is circulating in the city, New York’s health authorities said Aug. 12.
“For every one case of paralytic polio identified, hundreds more may be undetected,” Dr. Bassett said. “The best way to keep adults and children polio-free is through safe and effective immunization.”
Polio can cause permanent paralysis of limbs and even death in some cases. Before this outbreak, the last case of polio in the United States was in 2013.
The announcement came after a man in Rockland County, New York, north of the city, was stricken with polio at the end of July and paralyzed.
Now, health officials fear that the detection of polio in NYC wastewater could bring other cases of paralytic polio.
“It is not surprising, since this is something already seen with Rockland County,” Amesh Adalja, MD, senior scholar at the Johns Hopkins Center for Health Security in Baltimore, told this news organization. “This is solely the result of under-vaccination in the area. I think it’s likely that we will see a few paralytic cases but not a high number.”
Vaccinations declined in pandemic
Among the worries is that vaccination rates across New York City dipped during the pandemic because pediatrician visits were postponed.
In New York City, the overall rate of polio vaccination among children aged 5 years or younger is 86%. Still, in some city ZIP codes, fewer than two-thirds of children in that age group have received the full dosage, which worries health officials.
However, most adults were vaccinated against polio as children.
Across New York state, nearly 80% of people have been vaccinated, according to data from the state public health department. Those who are unvaccinated are at risk, but the polio vaccine is nearly 100% effective in people who are fully immunized.
New York health authorities are calling on those who are unvaccinated to get their shots immediately.
“The risk to New Yorkers is real, but the defense is so simple – get vaccinated against polio,” New York City Health Commissioner Ashwin Vasan, MD, PhD, said in a statement. “Polio is entirely preventable, and its reappearance should be a call for all of us.”
Though many of those who are infected have no symptoms, about 4% will get viral meningitis “and about 1 in 200 will become paralyzed,” according to a news release.
Symptoms can be flu-like
Symptoms can include those similar to the flu, such as sore throat, fever, fatigue, nausea, and stomach ache. There is no cure for the disease.
The city’s health department has given no details about where exactly polio had been found in NYC’s wastewater nor did they give dates the virus was detected.
Health authorities urged parents of children who are not yet fully vaccinated to bring them to their pediatricians.
In 1916, polio killed 6,000 people in the United States and left at least another 21,000 – most of them children – permanently disabled.
An outbreak in 1952 caused paralysis in more than 20,000 people and left many children on iron lungs. The first effective vaccine emerged just a few years later and the virus began to wane.
A version of this article first appeared on Medscape.com.
Polio virus has been discovered in New York City’s sewers, suggesting that the virus is circulating in the city, New York’s health authorities said Aug. 12.
“For every one case of paralytic polio identified, hundreds more may be undetected,” Dr. Bassett said. “The best way to keep adults and children polio-free is through safe and effective immunization.”
Polio can cause permanent paralysis of limbs and even death in some cases. Before this outbreak, the last case of polio in the United States was in 2013.
The announcement came after a man in Rockland County, New York, north of the city, was stricken with polio at the end of July and paralyzed.
Now, health officials fear that the detection of polio in NYC wastewater could bring other cases of paralytic polio.
“It is not surprising, since this is something already seen with Rockland County,” Amesh Adalja, MD, senior scholar at the Johns Hopkins Center for Health Security in Baltimore, told this news organization. “This is solely the result of under-vaccination in the area. I think it’s likely that we will see a few paralytic cases but not a high number.”
Vaccinations declined in pandemic
Among the worries is that vaccination rates across New York City dipped during the pandemic because pediatrician visits were postponed.
In New York City, the overall rate of polio vaccination among children aged 5 years or younger is 86%. Still, in some city ZIP codes, fewer than two-thirds of children in that age group have received the full dosage, which worries health officials.
However, most adults were vaccinated against polio as children.
Across New York state, nearly 80% of people have been vaccinated, according to data from the state public health department. Those who are unvaccinated are at risk, but the polio vaccine is nearly 100% effective in people who are fully immunized.
New York health authorities are calling on those who are unvaccinated to get their shots immediately.
“The risk to New Yorkers is real, but the defense is so simple – get vaccinated against polio,” New York City Health Commissioner Ashwin Vasan, MD, PhD, said in a statement. “Polio is entirely preventable, and its reappearance should be a call for all of us.”
Though many of those who are infected have no symptoms, about 4% will get viral meningitis “and about 1 in 200 will become paralyzed,” according to a news release.
Symptoms can be flu-like
Symptoms can include those similar to the flu, such as sore throat, fever, fatigue, nausea, and stomach ache. There is no cure for the disease.
The city’s health department has given no details about where exactly polio had been found in NYC’s wastewater nor did they give dates the virus was detected.
Health authorities urged parents of children who are not yet fully vaccinated to bring them to their pediatricians.
In 1916, polio killed 6,000 people in the United States and left at least another 21,000 – most of them children – permanently disabled.
An outbreak in 1952 caused paralysis in more than 20,000 people and left many children on iron lungs. The first effective vaccine emerged just a few years later and the virus began to wane.
A version of this article first appeared on Medscape.com.