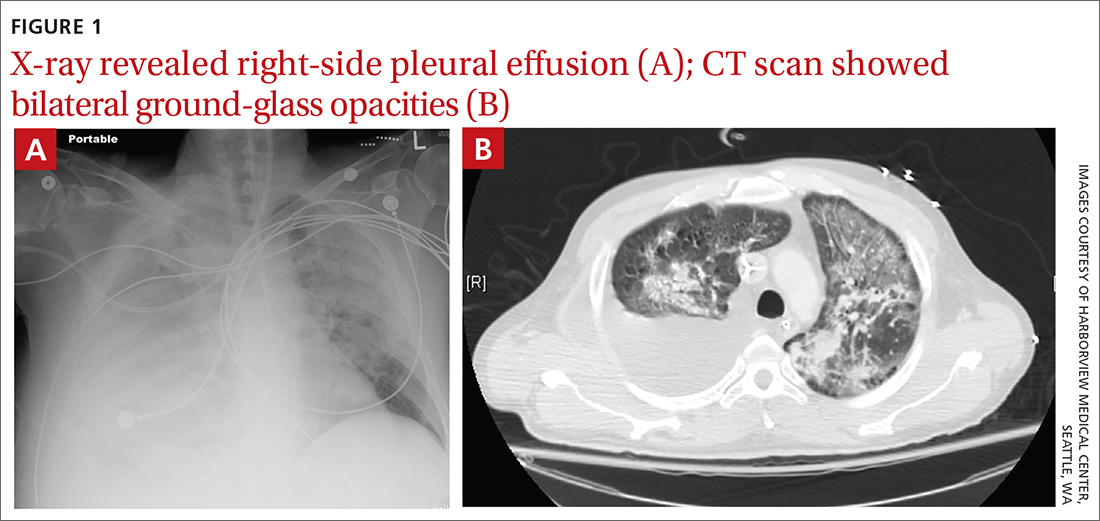
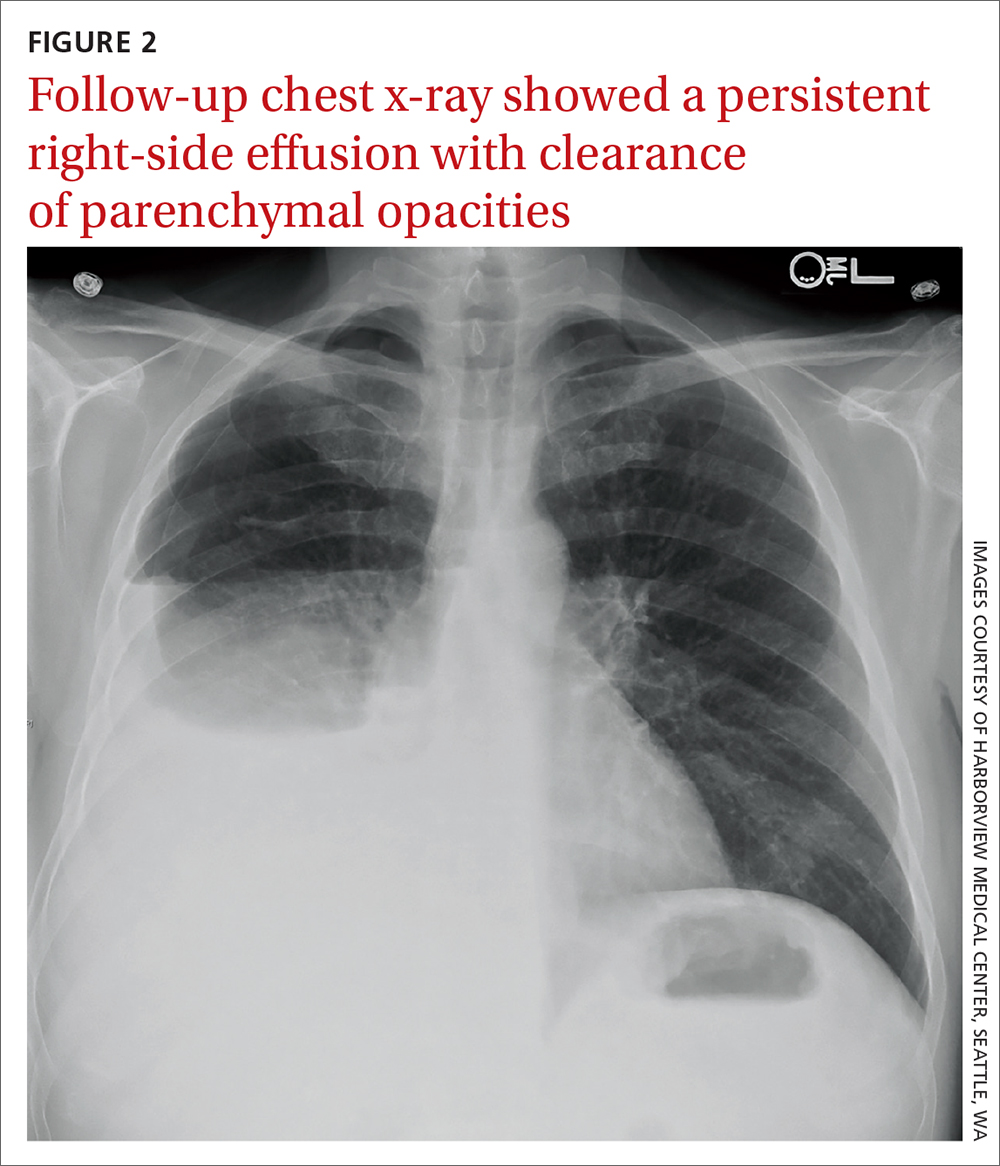
User login
Pediatric hepatitis cases may be linked to adenovirus, CDC says
Internationally, 108 cases have been reported in the United Kingdom, with 79 cases occurring in England. There are three documented cases in Spain, and similar cases are being reported in Denmark and the Netherlands, according to an article in Science. In the United Kingdom, cases have been reported in children up to 16 years old, but most affected children are between 2 and 5 years old. Eight children in the United Kingdom have required liver transplants.
On April 14, the CDC said that nine cases have been recorded in Alabama since the fall of 2021. All of these cases have been in children between 1 and 6 years old, and two children have needed liver transplants. Two additional cases have been reported in North Carolina, according to Stat News, and both children have since recovered.
Hepatitis A, B, C, D, and E viruses—common causes of hepatitis—have been ruled out in the U.K. and Spanish cases. More than three-fourths (77%) of the children sickened in the United Kingdom and all nine cases in Alabama have tested positive for a form of the adenovirus. While adenovirus can cause hepatitis in children, it is usually in those who are immunocompromised.
The CDC health alert advises clinicians who have cases of unexplained hepatitis in children to test for adenovirus and report these cases to the CDC as well as state public health authorities. The agency recommends nucleic acid amplification testing to detect adenovirus using respiratory swabs, stool samples or rectal swabs, or blood.
Officials are exploring whether these cases are linked to a version of the virus called adenovirus 41, which is associated with gut inflammation. The most recent case in Alabama was reported in February, and five of the nine children in the state with these puzzling cases of hepatitis have tested positive for adenovirus 41.
There have yet to be any links among the cases in Alabama or North Carolina, and investigators in the United Kingdom have also not found any connections in their cases, STAT News reports.
“CDC is working with state health departments to see if there are additional U.S. cases and what may be causing these cases,” said Kristen Nordlund, a CDC spokesperson, in a statement to STAT News. “At this time, adenovirus may be the cause for these, but investigators are still learning more – including ruling out the more common causes of hepatitis.”
Looking for other explanations
None of the children in the United States with hepatitis had COVID-19, but a few children in the United Kingdom have tested positive for the virus; none of these children have received the COVID-19 vaccine.
While the U.K. Health Security Agency says their investigation “continues to point toward a link to adenovirus infection,” they are also considering other contributing factors such as an environmental cause or COVID-19.
“COVID has been consistently shown to increase liver test numbers,” Nancy Reau, MD, the section chief of hepatology at Rush University in Chicago, said in an interview with this news organization. “It has been shown to cause other organ involvement besides just pulmonary symptoms and respiratory failure. As this virus evolves, it might be that in children, it is more able to present as hepatitis.”
A version of this article first appeared on Medscape.com.
This article was updated 4/22/22.
Internationally, 108 cases have been reported in the United Kingdom, with 79 cases occurring in England. There are three documented cases in Spain, and similar cases are being reported in Denmark and the Netherlands, according to an article in Science. In the United Kingdom, cases have been reported in children up to 16 years old, but most affected children are between 2 and 5 years old. Eight children in the United Kingdom have required liver transplants.
On April 14, the CDC said that nine cases have been recorded in Alabama since the fall of 2021. All of these cases have been in children between 1 and 6 years old, and two children have needed liver transplants. Two additional cases have been reported in North Carolina, according to Stat News, and both children have since recovered.
Hepatitis A, B, C, D, and E viruses—common causes of hepatitis—have been ruled out in the U.K. and Spanish cases. More than three-fourths (77%) of the children sickened in the United Kingdom and all nine cases in Alabama have tested positive for a form of the adenovirus. While adenovirus can cause hepatitis in children, it is usually in those who are immunocompromised.
The CDC health alert advises clinicians who have cases of unexplained hepatitis in children to test for adenovirus and report these cases to the CDC as well as state public health authorities. The agency recommends nucleic acid amplification testing to detect adenovirus using respiratory swabs, stool samples or rectal swabs, or blood.
Officials are exploring whether these cases are linked to a version of the virus called adenovirus 41, which is associated with gut inflammation. The most recent case in Alabama was reported in February, and five of the nine children in the state with these puzzling cases of hepatitis have tested positive for adenovirus 41.
There have yet to be any links among the cases in Alabama or North Carolina, and investigators in the United Kingdom have also not found any connections in their cases, STAT News reports.
“CDC is working with state health departments to see if there are additional U.S. cases and what may be causing these cases,” said Kristen Nordlund, a CDC spokesperson, in a statement to STAT News. “At this time, adenovirus may be the cause for these, but investigators are still learning more – including ruling out the more common causes of hepatitis.”
Looking for other explanations
None of the children in the United States with hepatitis had COVID-19, but a few children in the United Kingdom have tested positive for the virus; none of these children have received the COVID-19 vaccine.
While the U.K. Health Security Agency says their investigation “continues to point toward a link to adenovirus infection,” they are also considering other contributing factors such as an environmental cause or COVID-19.
“COVID has been consistently shown to increase liver test numbers,” Nancy Reau, MD, the section chief of hepatology at Rush University in Chicago, said in an interview with this news organization. “It has been shown to cause other organ involvement besides just pulmonary symptoms and respiratory failure. As this virus evolves, it might be that in children, it is more able to present as hepatitis.”
A version of this article first appeared on Medscape.com.
This article was updated 4/22/22.
Internationally, 108 cases have been reported in the United Kingdom, with 79 cases occurring in England. There are three documented cases in Spain, and similar cases are being reported in Denmark and the Netherlands, according to an article in Science. In the United Kingdom, cases have been reported in children up to 16 years old, but most affected children are between 2 and 5 years old. Eight children in the United Kingdom have required liver transplants.
On April 14, the CDC said that nine cases have been recorded in Alabama since the fall of 2021. All of these cases have been in children between 1 and 6 years old, and two children have needed liver transplants. Two additional cases have been reported in North Carolina, according to Stat News, and both children have since recovered.
Hepatitis A, B, C, D, and E viruses—common causes of hepatitis—have been ruled out in the U.K. and Spanish cases. More than three-fourths (77%) of the children sickened in the United Kingdom and all nine cases in Alabama have tested positive for a form of the adenovirus. While adenovirus can cause hepatitis in children, it is usually in those who are immunocompromised.
The CDC health alert advises clinicians who have cases of unexplained hepatitis in children to test for adenovirus and report these cases to the CDC as well as state public health authorities. The agency recommends nucleic acid amplification testing to detect adenovirus using respiratory swabs, stool samples or rectal swabs, or blood.
Officials are exploring whether these cases are linked to a version of the virus called adenovirus 41, which is associated with gut inflammation. The most recent case in Alabama was reported in February, and five of the nine children in the state with these puzzling cases of hepatitis have tested positive for adenovirus 41.
There have yet to be any links among the cases in Alabama or North Carolina, and investigators in the United Kingdom have also not found any connections in their cases, STAT News reports.
“CDC is working with state health departments to see if there are additional U.S. cases and what may be causing these cases,” said Kristen Nordlund, a CDC spokesperson, in a statement to STAT News. “At this time, adenovirus may be the cause for these, but investigators are still learning more – including ruling out the more common causes of hepatitis.”
Looking for other explanations
None of the children in the United States with hepatitis had COVID-19, but a few children in the United Kingdom have tested positive for the virus; none of these children have received the COVID-19 vaccine.
While the U.K. Health Security Agency says their investigation “continues to point toward a link to adenovirus infection,” they are also considering other contributing factors such as an environmental cause or COVID-19.
“COVID has been consistently shown to increase liver test numbers,” Nancy Reau, MD, the section chief of hepatology at Rush University in Chicago, said in an interview with this news organization. “It has been shown to cause other organ involvement besides just pulmonary symptoms and respiratory failure. As this virus evolves, it might be that in children, it is more able to present as hepatitis.”
A version of this article first appeared on Medscape.com.
This article was updated 4/22/22.
Omicron BA.2: What do we know so far?
Omicron has 30 mutations of the spike protein, compared with the original Wuhan-Hu-1 variant, with 15 mutations of the receptor-binding domain (which are linked to a decrease in antibody binding), mutations at the furin S1/S2 site (which improves furin binding and increases infectiousness), and mutations of the amino terminal domain (which is the main binding site for some of the therapeutic antibodies used to treat COVID-19 infections).
Omicron’s functional characteristics
Non–peer-reviewed studies have shown a replication of Omicron in pulmonary epithelial cells, which was shown to be less efficient, when compared with Delta and Wuhan-Hu-1. The number of viral copies from an Omicron infection in pulmonary epithelial cells was significantly lower, compared with infection with the Delta or Wuhan-Hu-1 variants. The association of these characteristics found an increase in the number of viral copies in human epithelial cells (taken from the nasal airways) infected with Omicron. This supports the understanding that Omicron is more transmissible but results in a less severe manifestation of the disease.
As for the phenotypic expression of the infection, attention has been focused on Omicron’s reduced capacity to cause syncytia in pulmonary tissue cultures, information which is relevant to its clinical significance, if we consider that the formation of syncytia has been associated with a more severe manifestation of the disease. Furthermore, it has emerged that Omicron can use different cellular entry routes, with a preference for endosomal fusion over superficial cellular fusion. This characteristic allows Omicron to significantly increase the number of types of cells it can infect.
Omicron BA.2 evolves
Between November and December 2021, Omicron progressed, evolving into a variant with characteristics similar to those of its predecessors (that is, it underwent a gradual and progressive increase in transmissibility). Early studies on the Omicron variant were mainly based on the BA.1 subvariant. Since the start of January 2022, there has been an unexpected increase in BA.2 in Europe and Asia. Since then, continued surveillance on the evolution of Omicron has shown an increased prevalence of two subvariants: BA.1 with a R346K mutation (BA.1 + R346K) and B.1.1.529.2 (BA.2), with the latter containing eight unique spike mutations and 13 missing spike mutations, compared with those found in BA.1.
From these differences, we cannot presume that their antigenic properties are similar or different, but they seem to be antigenically equidistant from wild-type SARS-CoV-2, likely jeopardizing in equal measures the efficacy of current COVID-19 vaccines. Furthermore, BA.2 shows significant resistance to 17 out of 19 neutralizing monoclonal antibodies tested in this study, demonstrating that current monoclonal antibody therapy may have significant limitations in terms of adequate coverage for all subvariants of the Omicron variant.
Omicron BA.2 and reinfection
BA.2 initially represented only 13% of Omicron sequences at a global level, quickly becoming the dominant form in some countries, such as Denmark. At the end of 2021, BA.2 represented around 20% of all Danish cases of SARS-CoV-2. Halfway through January 2022, this had increased to around 45%, data that indicate that BA.2 carries an advantage over BA.1 within the highly vaccinated population of Denmark.
BA.2 is associated with an increased susceptibility of infection for unvaccinated individuals (odds ratio, 2.19; 95% confidence interval, 1.58-3.04), fully vaccinated individuals (OR, 2.45; 95% CI, 1.77-3.40), and booster-vaccinated individuals (OR, 2.99; 95% CI, 2.11-4.24), compared with BA.1. The pattern of increased transmissibility in BA.2 households was not observed for fully vaccinated and booster-vaccinated primary cases, where the OR of transmission was below 1 for BA.2, compared with BA.1. These data confirm the immune-evasive properties of BA.2 that further reduce the protective effect of vaccination against infection, but do not increase its transmissibility from vaccinated individuals with breakthrough infections.
Omicron, BA.2, and vaccination
The understanding of serum neutralizing activity, in correlation to the efficacy of a vaccine, is a priority of research because of the growing epidemiological significance of BA.2. There is evidence to support the claim that the immune-evasive nature of BA.2 doesn›t seem to be as severe as that of BA.1, and it is possible that there are other viral or host factors that are enabling the rapid diffusion of BA.2. A study published in Science Immunology investigated humoral and cellular immune responses to Omicron and other variants of concern (VOCs), looking to understand how, and to what degree, vaccinated individuals are protected against Omicron. From the results, a very low level of antibody cross-neutralization of Omicron, or a lack thereof, was seen when compared with wild type, Beta, and Delta variants, which could be partially restored by a third booster vaccination. Furthermore, T lymphocytes were shown to recognize Omicron with the same efficacy as seen for the other VOCs, suggesting that vaccinated individuals maintain T lymphocyte immunity, an element that is capable of providing protection in the absence of neutralizing antibodies, limiting the chance of serious disease.
These results are consistent with those available from a study performed in a population from Qatar made up of 2,239,193 people who had received at least two doses of a BNT162b2 or mRNA-1273 vaccine. The efficacy of the booster against a symptomatic Omicron infection, compared with that from the primary series, was 49.4% (95% CI, 47.1-51.6). The efficacy of the booster against hospitalization for COVID-19 and the death rate from Omicron infection, compared with the primary series, was 76.5% (95% CI, 55.9-87.5). The efficacy of the BNT162b2 booster against a symptomatic Delta variant infection (or B.1.617.2), compared with the primary series, was 86.1% (95% CI, 67.3-94.1).
To summarize, the constant increase in the prevalence of BA.2 in more countries over the world has confirmed the growth advantage that this variant has compared with others. BA.2 reduces the protective effect of vaccination against infection. Omicron antibody cross-neutralization can be partially restored by a third booster vaccination, an aspect that becomes problematic in the context of a low vaccination rate, where peaks of Omicron may increase the likelihood of infection in the elderly and in other groups at a higher risk of severe disease. Omicron BA.2 opens up new evolution channels, but what do the experts think will happen?
A version of this article was originally published in Italian on Univadis.
Omicron has 30 mutations of the spike protein, compared with the original Wuhan-Hu-1 variant, with 15 mutations of the receptor-binding domain (which are linked to a decrease in antibody binding), mutations at the furin S1/S2 site (which improves furin binding and increases infectiousness), and mutations of the amino terminal domain (which is the main binding site for some of the therapeutic antibodies used to treat COVID-19 infections).
Omicron’s functional characteristics
Non–peer-reviewed studies have shown a replication of Omicron in pulmonary epithelial cells, which was shown to be less efficient, when compared with Delta and Wuhan-Hu-1. The number of viral copies from an Omicron infection in pulmonary epithelial cells was significantly lower, compared with infection with the Delta or Wuhan-Hu-1 variants. The association of these characteristics found an increase in the number of viral copies in human epithelial cells (taken from the nasal airways) infected with Omicron. This supports the understanding that Omicron is more transmissible but results in a less severe manifestation of the disease.
As for the phenotypic expression of the infection, attention has been focused on Omicron’s reduced capacity to cause syncytia in pulmonary tissue cultures, information which is relevant to its clinical significance, if we consider that the formation of syncytia has been associated with a more severe manifestation of the disease. Furthermore, it has emerged that Omicron can use different cellular entry routes, with a preference for endosomal fusion over superficial cellular fusion. This characteristic allows Omicron to significantly increase the number of types of cells it can infect.
Omicron BA.2 evolves
Between November and December 2021, Omicron progressed, evolving into a variant with characteristics similar to those of its predecessors (that is, it underwent a gradual and progressive increase in transmissibility). Early studies on the Omicron variant were mainly based on the BA.1 subvariant. Since the start of January 2022, there has been an unexpected increase in BA.2 in Europe and Asia. Since then, continued surveillance on the evolution of Omicron has shown an increased prevalence of two subvariants: BA.1 with a R346K mutation (BA.1 + R346K) and B.1.1.529.2 (BA.2), with the latter containing eight unique spike mutations and 13 missing spike mutations, compared with those found in BA.1.
From these differences, we cannot presume that their antigenic properties are similar or different, but they seem to be antigenically equidistant from wild-type SARS-CoV-2, likely jeopardizing in equal measures the efficacy of current COVID-19 vaccines. Furthermore, BA.2 shows significant resistance to 17 out of 19 neutralizing monoclonal antibodies tested in this study, demonstrating that current monoclonal antibody therapy may have significant limitations in terms of adequate coverage for all subvariants of the Omicron variant.
Omicron BA.2 and reinfection
BA.2 initially represented only 13% of Omicron sequences at a global level, quickly becoming the dominant form in some countries, such as Denmark. At the end of 2021, BA.2 represented around 20% of all Danish cases of SARS-CoV-2. Halfway through January 2022, this had increased to around 45%, data that indicate that BA.2 carries an advantage over BA.1 within the highly vaccinated population of Denmark.
BA.2 is associated with an increased susceptibility of infection for unvaccinated individuals (odds ratio, 2.19; 95% confidence interval, 1.58-3.04), fully vaccinated individuals (OR, 2.45; 95% CI, 1.77-3.40), and booster-vaccinated individuals (OR, 2.99; 95% CI, 2.11-4.24), compared with BA.1. The pattern of increased transmissibility in BA.2 households was not observed for fully vaccinated and booster-vaccinated primary cases, where the OR of transmission was below 1 for BA.2, compared with BA.1. These data confirm the immune-evasive properties of BA.2 that further reduce the protective effect of vaccination against infection, but do not increase its transmissibility from vaccinated individuals with breakthrough infections.
Omicron, BA.2, and vaccination
The understanding of serum neutralizing activity, in correlation to the efficacy of a vaccine, is a priority of research because of the growing epidemiological significance of BA.2. There is evidence to support the claim that the immune-evasive nature of BA.2 doesn›t seem to be as severe as that of BA.1, and it is possible that there are other viral or host factors that are enabling the rapid diffusion of BA.2. A study published in Science Immunology investigated humoral and cellular immune responses to Omicron and other variants of concern (VOCs), looking to understand how, and to what degree, vaccinated individuals are protected against Omicron. From the results, a very low level of antibody cross-neutralization of Omicron, or a lack thereof, was seen when compared with wild type, Beta, and Delta variants, which could be partially restored by a third booster vaccination. Furthermore, T lymphocytes were shown to recognize Omicron with the same efficacy as seen for the other VOCs, suggesting that vaccinated individuals maintain T lymphocyte immunity, an element that is capable of providing protection in the absence of neutralizing antibodies, limiting the chance of serious disease.
These results are consistent with those available from a study performed in a population from Qatar made up of 2,239,193 people who had received at least two doses of a BNT162b2 or mRNA-1273 vaccine. The efficacy of the booster against a symptomatic Omicron infection, compared with that from the primary series, was 49.4% (95% CI, 47.1-51.6). The efficacy of the booster against hospitalization for COVID-19 and the death rate from Omicron infection, compared with the primary series, was 76.5% (95% CI, 55.9-87.5). The efficacy of the BNT162b2 booster against a symptomatic Delta variant infection (or B.1.617.2), compared with the primary series, was 86.1% (95% CI, 67.3-94.1).
To summarize, the constant increase in the prevalence of BA.2 in more countries over the world has confirmed the growth advantage that this variant has compared with others. BA.2 reduces the protective effect of vaccination against infection. Omicron antibody cross-neutralization can be partially restored by a third booster vaccination, an aspect that becomes problematic in the context of a low vaccination rate, where peaks of Omicron may increase the likelihood of infection in the elderly and in other groups at a higher risk of severe disease. Omicron BA.2 opens up new evolution channels, but what do the experts think will happen?
A version of this article was originally published in Italian on Univadis.
Omicron has 30 mutations of the spike protein, compared with the original Wuhan-Hu-1 variant, with 15 mutations of the receptor-binding domain (which are linked to a decrease in antibody binding), mutations at the furin S1/S2 site (which improves furin binding and increases infectiousness), and mutations of the amino terminal domain (which is the main binding site for some of the therapeutic antibodies used to treat COVID-19 infections).
Omicron’s functional characteristics
Non–peer-reviewed studies have shown a replication of Omicron in pulmonary epithelial cells, which was shown to be less efficient, when compared with Delta and Wuhan-Hu-1. The number of viral copies from an Omicron infection in pulmonary epithelial cells was significantly lower, compared with infection with the Delta or Wuhan-Hu-1 variants. The association of these characteristics found an increase in the number of viral copies in human epithelial cells (taken from the nasal airways) infected with Omicron. This supports the understanding that Omicron is more transmissible but results in a less severe manifestation of the disease.
As for the phenotypic expression of the infection, attention has been focused on Omicron’s reduced capacity to cause syncytia in pulmonary tissue cultures, information which is relevant to its clinical significance, if we consider that the formation of syncytia has been associated with a more severe manifestation of the disease. Furthermore, it has emerged that Omicron can use different cellular entry routes, with a preference for endosomal fusion over superficial cellular fusion. This characteristic allows Omicron to significantly increase the number of types of cells it can infect.
Omicron BA.2 evolves
Between November and December 2021, Omicron progressed, evolving into a variant with characteristics similar to those of its predecessors (that is, it underwent a gradual and progressive increase in transmissibility). Early studies on the Omicron variant were mainly based on the BA.1 subvariant. Since the start of January 2022, there has been an unexpected increase in BA.2 in Europe and Asia. Since then, continued surveillance on the evolution of Omicron has shown an increased prevalence of two subvariants: BA.1 with a R346K mutation (BA.1 + R346K) and B.1.1.529.2 (BA.2), with the latter containing eight unique spike mutations and 13 missing spike mutations, compared with those found in BA.1.
From these differences, we cannot presume that their antigenic properties are similar or different, but they seem to be antigenically equidistant from wild-type SARS-CoV-2, likely jeopardizing in equal measures the efficacy of current COVID-19 vaccines. Furthermore, BA.2 shows significant resistance to 17 out of 19 neutralizing monoclonal antibodies tested in this study, demonstrating that current monoclonal antibody therapy may have significant limitations in terms of adequate coverage for all subvariants of the Omicron variant.
Omicron BA.2 and reinfection
BA.2 initially represented only 13% of Omicron sequences at a global level, quickly becoming the dominant form in some countries, such as Denmark. At the end of 2021, BA.2 represented around 20% of all Danish cases of SARS-CoV-2. Halfway through January 2022, this had increased to around 45%, data that indicate that BA.2 carries an advantage over BA.1 within the highly vaccinated population of Denmark.
BA.2 is associated with an increased susceptibility of infection for unvaccinated individuals (odds ratio, 2.19; 95% confidence interval, 1.58-3.04), fully vaccinated individuals (OR, 2.45; 95% CI, 1.77-3.40), and booster-vaccinated individuals (OR, 2.99; 95% CI, 2.11-4.24), compared with BA.1. The pattern of increased transmissibility in BA.2 households was not observed for fully vaccinated and booster-vaccinated primary cases, where the OR of transmission was below 1 for BA.2, compared with BA.1. These data confirm the immune-evasive properties of BA.2 that further reduce the protective effect of vaccination against infection, but do not increase its transmissibility from vaccinated individuals with breakthrough infections.
Omicron, BA.2, and vaccination
The understanding of serum neutralizing activity, in correlation to the efficacy of a vaccine, is a priority of research because of the growing epidemiological significance of BA.2. There is evidence to support the claim that the immune-evasive nature of BA.2 doesn›t seem to be as severe as that of BA.1, and it is possible that there are other viral or host factors that are enabling the rapid diffusion of BA.2. A study published in Science Immunology investigated humoral and cellular immune responses to Omicron and other variants of concern (VOCs), looking to understand how, and to what degree, vaccinated individuals are protected against Omicron. From the results, a very low level of antibody cross-neutralization of Omicron, or a lack thereof, was seen when compared with wild type, Beta, and Delta variants, which could be partially restored by a third booster vaccination. Furthermore, T lymphocytes were shown to recognize Omicron with the same efficacy as seen for the other VOCs, suggesting that vaccinated individuals maintain T lymphocyte immunity, an element that is capable of providing protection in the absence of neutralizing antibodies, limiting the chance of serious disease.
These results are consistent with those available from a study performed in a population from Qatar made up of 2,239,193 people who had received at least two doses of a BNT162b2 or mRNA-1273 vaccine. The efficacy of the booster against a symptomatic Omicron infection, compared with that from the primary series, was 49.4% (95% CI, 47.1-51.6). The efficacy of the booster against hospitalization for COVID-19 and the death rate from Omicron infection, compared with the primary series, was 76.5% (95% CI, 55.9-87.5). The efficacy of the BNT162b2 booster against a symptomatic Delta variant infection (or B.1.617.2), compared with the primary series, was 86.1% (95% CI, 67.3-94.1).
To summarize, the constant increase in the prevalence of BA.2 in more countries over the world has confirmed the growth advantage that this variant has compared with others. BA.2 reduces the protective effect of vaccination against infection. Omicron antibody cross-neutralization can be partially restored by a third booster vaccination, an aspect that becomes problematic in the context of a low vaccination rate, where peaks of Omicron may increase the likelihood of infection in the elderly and in other groups at a higher risk of severe disease. Omicron BA.2 opens up new evolution channels, but what do the experts think will happen?
A version of this article was originally published in Italian on Univadis.
Babies die as congenital syphilis continues a decade-long surge across the U.S.
For a decade, the number of babies born with syphilis in the United States has surged, undeterred. Data released Apr. 12 by the Centers for Disease Control and Prevention shows just how dire the outbreak has become.
In 2012, 332 babies were born infected with the disease. In 2021, that number had climbed nearly sevenfold, to at least 2,268, according to preliminary estimates. And 166 of those babies died.
About 7% of babies diagnosed with syphilis in recent years have died; thousands of others born with the disease have faced problems that include brain and bone malformations, blindness, and organ damage.
For public health officials, the situation is all the more heartbreaking, considering that congenital syphilis rates reached near-historic modern lows from 2000 to 2012 amid ambitious prevention and education efforts. By 2020, following a sharp erosion in funding and attention, the nationwide case rate was more than seven times that of 2012.
“The really depressing thing about it is we had this thing virtually eradicated back in the year 2000,” said William Andrews, a public information officer for Oklahoma’s sexual health and harm reduction service. “Now it’s back with a vengeance. We are really trying to get the message out that sexual health is health. It’s nothing to be ashamed of.”
Even as caseloads soar, the CDC budget for STD prevention – the primary funding source for most public health departments – has been largely stagnant for two decades, its purchasing power dragged even lower by inflation.
The CDC report on STD trends provides official data on congenital syphilis cases for 2020, as well as preliminary case counts for 2021 that are expected to increase. CDC data shows that congenital syphilis rates in 2020 continued to climb in already overwhelmed states like Texas, California, and Nevada and that the disease is now present in almost every state in the nation. All but three states – Maine, New Hampshire, and Vermont – reported congenital syphilis cases in 2020.
From 2011 to 2020, congenital syphilis resulted in 633 documented stillbirths and infant deaths, according to the new CDC data.
Preventing congenital syphilis – the term used when syphilis is transferred to a fetus in utero – is from a medical standpoint exceedingly simple: If a pregnant woman is diagnosed at least a month before giving birth, just a few shots of penicillin have a near-perfect cure rate for mother and baby. But funding cuts and competing priorities in the nation’s fragmented public health care system have vastly narrowed access to such services.
The reasons pregnant people with syphilis go undiagnosed or untreated vary geographically, according to data collected by states and analyzed by the CDC.
In Western states, the largest share of cases involve women who have received little to no prenatal care and aren’t tested for syphilis until they give birth. Many have substance use disorders, primarily related to methamphetamines. “They’ve felt a lot of judgment and stigma by the medical community,” said Stephanie Pierce, MD, a maternal fetal medicine specialist at the University of Oklahoma, Oklahoma City, who runs a clinic for women with high-risk pregnancies.
In Southern states, a CDC study of 2018 data found that the largest share of congenital syphilis cases were among women who had been tested and diagnosed but hadn’t received treatment. That year, among Black moms who gave birth to a baby with syphilis, 37% had not been treated adequately even though they’d received a timely diagnosis. Among white moms, that number was 24%. Longstanding racism in medical care, poverty, transportation issues, poorly funded public health departments, and crowded clinics whose employees are too overworked to follow up with patients all contribute to the problem, according to infectious disease experts.
Doctors are also noticing a growing number of women who are treated for syphilis but reinfected during pregnancy. Amid rising cases and stagnant resources, some states have focused disease investigations on pregnant women of childbearing age; they can no longer prioritize treating sexual partners who are also infected.
Eric McGrath, MD, a pediatric infectious disease specialist at Wayne State University, Detroit, said that he’d seen several newborns in recent years whose mothers had been treated for syphilis but then were re-exposed during pregnancy by partners who hadn’t been treated.
Treating a newborn baby for syphilis isn’t trivial. Penicillin carries little risk, but delivering it to a baby often involves a lumbar puncture and other painful procedures. And treatment typically means keeping the baby in the hospital for 10 days, interrupting an important time for family bonding.
Dr. McGrath has seen a couple of babies in his career who weren’t diagnosed or treated at birth and later came to him with full-blown syphilis complications, including full-body rashes and inflamed livers. It was an awful experience he doesn’t want to repeat. The preferred course, he said, is to spare the baby the ordeal and treat parents early in the pregnancy.
But in some places, providers aren’t routinely testing for syphilis. Although most states mandate testing at some point during pregnancy, as of last year just 14 required it for everyone in the third trimester. The CDC recommends third-trimester testing in areas with high rates of syphilis, a growing share of the United States.
After Arizona declared a statewide outbreak in 2018, state health officials wanted to know whether widespread testing in the third trimester could have prevented infections. Looking at 18 months of data, analysts found that nearly three-quarters of the more than 200 pregnant women diagnosed with syphilis in 2017 and the first half of 2018 got treatment. That left 57 babies born with syphilis, nine of whom died. The analysts estimated that a third of the infections could have been prevented with testing in the third trimester.
Based on the numbers they saw in those 18 months, officials estimated that screening all women on Medicaid in the third trimester would cost the state $113,300 annually, and that treating all cases of syphilis that screening would catch could be done for just $113. Factoring in the hospitalization costs for infected infants, the officials concluded the additional testing would save the state money.
And yet prevention money has been hard to come by. Taking inflation into account, CDC prevention funding for STDs has fallen 41% since 2003, according to an analysis by the National Coalition of STD Directors. That’s even as cases have risen, leaving public health departments saddled with more work and far less money.
Janine Waters, STD program manager for the state of New Mexico, has watched the unraveling. When Ms. Waters started her career more than 20 years ago, she and her colleagues followed up on every case of chlamydia, gonorrhea, and syphilis reported, not only making sure that people got treatment but also getting in touch with their sexual partners, with the aim of stopping the spread of infection. In a 2019 interview with Kaiser Health News, she said her team was struggling to keep up with syphilis alone, even as they registered with dread congenital syphilis cases surging in neighboring Texas and Arizona.
By 2020, New Mexico had the highest rate of congenital syphilis in the country.
The COVID-19 pandemic drained the remaining resources. Half of health departments across the country discontinued STD fieldwork altogether, diverting their resources to COVID. In California, which for years has struggled with high rates of congenital syphilis, three-quarters of local health departments dispatched more than half of their STD staffers to work on COVID.
As the pandemic ebbs – at least in the short term – many public health departments are turning their attention back to syphilis and other diseases. And they are doing it with reinforcements. Although the Biden administration’s proposed STD prevention budget for 2023 remains flat, the American Rescue Plan Act included $200 million to help health departments boost contact tracing and surveillance for covid and other infectious diseases. Many departments are funneling that money toward STDs.
The money is an infusion that state health officials say will make a difference. But when taking inflation into account, it essentially brings STD prevention funding back to what it was in 2003, said Stephanie Arnold Pang of the National Coalition of STD Directors. And the American Rescue Plan money doesn’t cover some aspects of STD prevention, including clinical services.
The coalition wants to revive dedicated STD clinics, where people can drop in for testing and treatment at little to no cost. Advocates say that would fill a void that has plagued treatment efforts since public clinics closed en masse in the wake of the 2008 recession.
Texas, battling its own pervasive outbreak, will use its share of American Rescue Plan money to fill 94 new positions focused on various aspects of STD prevention. Those hires will bolster a range of measures the state put in place before the pandemic, including an updated data system to track infections, review boards in major cities that examine what went wrong for every case of congenital syphilis, and a requirement that providers test for syphilis during the third trimester of pregnancy. The suite of interventions seems to be working, but it could be a while before cases go down, said Amy Carter, the state’s congenital syphilis coordinator.
“The growth didn’t happen overnight,” Ms. Carter said. “So our prevention efforts aren’t going to have a direct impact overnight either.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation
For a decade, the number of babies born with syphilis in the United States has surged, undeterred. Data released Apr. 12 by the Centers for Disease Control and Prevention shows just how dire the outbreak has become.
In 2012, 332 babies were born infected with the disease. In 2021, that number had climbed nearly sevenfold, to at least 2,268, according to preliminary estimates. And 166 of those babies died.
About 7% of babies diagnosed with syphilis in recent years have died; thousands of others born with the disease have faced problems that include brain and bone malformations, blindness, and organ damage.
For public health officials, the situation is all the more heartbreaking, considering that congenital syphilis rates reached near-historic modern lows from 2000 to 2012 amid ambitious prevention and education efforts. By 2020, following a sharp erosion in funding and attention, the nationwide case rate was more than seven times that of 2012.
“The really depressing thing about it is we had this thing virtually eradicated back in the year 2000,” said William Andrews, a public information officer for Oklahoma’s sexual health and harm reduction service. “Now it’s back with a vengeance. We are really trying to get the message out that sexual health is health. It’s nothing to be ashamed of.”
Even as caseloads soar, the CDC budget for STD prevention – the primary funding source for most public health departments – has been largely stagnant for two decades, its purchasing power dragged even lower by inflation.
The CDC report on STD trends provides official data on congenital syphilis cases for 2020, as well as preliminary case counts for 2021 that are expected to increase. CDC data shows that congenital syphilis rates in 2020 continued to climb in already overwhelmed states like Texas, California, and Nevada and that the disease is now present in almost every state in the nation. All but three states – Maine, New Hampshire, and Vermont – reported congenital syphilis cases in 2020.
From 2011 to 2020, congenital syphilis resulted in 633 documented stillbirths and infant deaths, according to the new CDC data.
Preventing congenital syphilis – the term used when syphilis is transferred to a fetus in utero – is from a medical standpoint exceedingly simple: If a pregnant woman is diagnosed at least a month before giving birth, just a few shots of penicillin have a near-perfect cure rate for mother and baby. But funding cuts and competing priorities in the nation’s fragmented public health care system have vastly narrowed access to such services.
The reasons pregnant people with syphilis go undiagnosed or untreated vary geographically, according to data collected by states and analyzed by the CDC.
In Western states, the largest share of cases involve women who have received little to no prenatal care and aren’t tested for syphilis until they give birth. Many have substance use disorders, primarily related to methamphetamines. “They’ve felt a lot of judgment and stigma by the medical community,” said Stephanie Pierce, MD, a maternal fetal medicine specialist at the University of Oklahoma, Oklahoma City, who runs a clinic for women with high-risk pregnancies.
In Southern states, a CDC study of 2018 data found that the largest share of congenital syphilis cases were among women who had been tested and diagnosed but hadn’t received treatment. That year, among Black moms who gave birth to a baby with syphilis, 37% had not been treated adequately even though they’d received a timely diagnosis. Among white moms, that number was 24%. Longstanding racism in medical care, poverty, transportation issues, poorly funded public health departments, and crowded clinics whose employees are too overworked to follow up with patients all contribute to the problem, according to infectious disease experts.
Doctors are also noticing a growing number of women who are treated for syphilis but reinfected during pregnancy. Amid rising cases and stagnant resources, some states have focused disease investigations on pregnant women of childbearing age; they can no longer prioritize treating sexual partners who are also infected.
Eric McGrath, MD, a pediatric infectious disease specialist at Wayne State University, Detroit, said that he’d seen several newborns in recent years whose mothers had been treated for syphilis but then were re-exposed during pregnancy by partners who hadn’t been treated.
Treating a newborn baby for syphilis isn’t trivial. Penicillin carries little risk, but delivering it to a baby often involves a lumbar puncture and other painful procedures. And treatment typically means keeping the baby in the hospital for 10 days, interrupting an important time for family bonding.
Dr. McGrath has seen a couple of babies in his career who weren’t diagnosed or treated at birth and later came to him with full-blown syphilis complications, including full-body rashes and inflamed livers. It was an awful experience he doesn’t want to repeat. The preferred course, he said, is to spare the baby the ordeal and treat parents early in the pregnancy.
But in some places, providers aren’t routinely testing for syphilis. Although most states mandate testing at some point during pregnancy, as of last year just 14 required it for everyone in the third trimester. The CDC recommends third-trimester testing in areas with high rates of syphilis, a growing share of the United States.
After Arizona declared a statewide outbreak in 2018, state health officials wanted to know whether widespread testing in the third trimester could have prevented infections. Looking at 18 months of data, analysts found that nearly three-quarters of the more than 200 pregnant women diagnosed with syphilis in 2017 and the first half of 2018 got treatment. That left 57 babies born with syphilis, nine of whom died. The analysts estimated that a third of the infections could have been prevented with testing in the third trimester.
Based on the numbers they saw in those 18 months, officials estimated that screening all women on Medicaid in the third trimester would cost the state $113,300 annually, and that treating all cases of syphilis that screening would catch could be done for just $113. Factoring in the hospitalization costs for infected infants, the officials concluded the additional testing would save the state money.
And yet prevention money has been hard to come by. Taking inflation into account, CDC prevention funding for STDs has fallen 41% since 2003, according to an analysis by the National Coalition of STD Directors. That’s even as cases have risen, leaving public health departments saddled with more work and far less money.
Janine Waters, STD program manager for the state of New Mexico, has watched the unraveling. When Ms. Waters started her career more than 20 years ago, she and her colleagues followed up on every case of chlamydia, gonorrhea, and syphilis reported, not only making sure that people got treatment but also getting in touch with their sexual partners, with the aim of stopping the spread of infection. In a 2019 interview with Kaiser Health News, she said her team was struggling to keep up with syphilis alone, even as they registered with dread congenital syphilis cases surging in neighboring Texas and Arizona.
By 2020, New Mexico had the highest rate of congenital syphilis in the country.
The COVID-19 pandemic drained the remaining resources. Half of health departments across the country discontinued STD fieldwork altogether, diverting their resources to COVID. In California, which for years has struggled with high rates of congenital syphilis, three-quarters of local health departments dispatched more than half of their STD staffers to work on COVID.
As the pandemic ebbs – at least in the short term – many public health departments are turning their attention back to syphilis and other diseases. And they are doing it with reinforcements. Although the Biden administration’s proposed STD prevention budget for 2023 remains flat, the American Rescue Plan Act included $200 million to help health departments boost contact tracing and surveillance for covid and other infectious diseases. Many departments are funneling that money toward STDs.
The money is an infusion that state health officials say will make a difference. But when taking inflation into account, it essentially brings STD prevention funding back to what it was in 2003, said Stephanie Arnold Pang of the National Coalition of STD Directors. And the American Rescue Plan money doesn’t cover some aspects of STD prevention, including clinical services.
The coalition wants to revive dedicated STD clinics, where people can drop in for testing and treatment at little to no cost. Advocates say that would fill a void that has plagued treatment efforts since public clinics closed en masse in the wake of the 2008 recession.
Texas, battling its own pervasive outbreak, will use its share of American Rescue Plan money to fill 94 new positions focused on various aspects of STD prevention. Those hires will bolster a range of measures the state put in place before the pandemic, including an updated data system to track infections, review boards in major cities that examine what went wrong for every case of congenital syphilis, and a requirement that providers test for syphilis during the third trimester of pregnancy. The suite of interventions seems to be working, but it could be a while before cases go down, said Amy Carter, the state’s congenital syphilis coordinator.
“The growth didn’t happen overnight,” Ms. Carter said. “So our prevention efforts aren’t going to have a direct impact overnight either.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation
For a decade, the number of babies born with syphilis in the United States has surged, undeterred. Data released Apr. 12 by the Centers for Disease Control and Prevention shows just how dire the outbreak has become.
In 2012, 332 babies were born infected with the disease. In 2021, that number had climbed nearly sevenfold, to at least 2,268, according to preliminary estimates. And 166 of those babies died.
About 7% of babies diagnosed with syphilis in recent years have died; thousands of others born with the disease have faced problems that include brain and bone malformations, blindness, and organ damage.
For public health officials, the situation is all the more heartbreaking, considering that congenital syphilis rates reached near-historic modern lows from 2000 to 2012 amid ambitious prevention and education efforts. By 2020, following a sharp erosion in funding and attention, the nationwide case rate was more than seven times that of 2012.
“The really depressing thing about it is we had this thing virtually eradicated back in the year 2000,” said William Andrews, a public information officer for Oklahoma’s sexual health and harm reduction service. “Now it’s back with a vengeance. We are really trying to get the message out that sexual health is health. It’s nothing to be ashamed of.”
Even as caseloads soar, the CDC budget for STD prevention – the primary funding source for most public health departments – has been largely stagnant for two decades, its purchasing power dragged even lower by inflation.
The CDC report on STD trends provides official data on congenital syphilis cases for 2020, as well as preliminary case counts for 2021 that are expected to increase. CDC data shows that congenital syphilis rates in 2020 continued to climb in already overwhelmed states like Texas, California, and Nevada and that the disease is now present in almost every state in the nation. All but three states – Maine, New Hampshire, and Vermont – reported congenital syphilis cases in 2020.
From 2011 to 2020, congenital syphilis resulted in 633 documented stillbirths and infant deaths, according to the new CDC data.
Preventing congenital syphilis – the term used when syphilis is transferred to a fetus in utero – is from a medical standpoint exceedingly simple: If a pregnant woman is diagnosed at least a month before giving birth, just a few shots of penicillin have a near-perfect cure rate for mother and baby. But funding cuts and competing priorities in the nation’s fragmented public health care system have vastly narrowed access to such services.
The reasons pregnant people with syphilis go undiagnosed or untreated vary geographically, according to data collected by states and analyzed by the CDC.
In Western states, the largest share of cases involve women who have received little to no prenatal care and aren’t tested for syphilis until they give birth. Many have substance use disorders, primarily related to methamphetamines. “They’ve felt a lot of judgment and stigma by the medical community,” said Stephanie Pierce, MD, a maternal fetal medicine specialist at the University of Oklahoma, Oklahoma City, who runs a clinic for women with high-risk pregnancies.
In Southern states, a CDC study of 2018 data found that the largest share of congenital syphilis cases were among women who had been tested and diagnosed but hadn’t received treatment. That year, among Black moms who gave birth to a baby with syphilis, 37% had not been treated adequately even though they’d received a timely diagnosis. Among white moms, that number was 24%. Longstanding racism in medical care, poverty, transportation issues, poorly funded public health departments, and crowded clinics whose employees are too overworked to follow up with patients all contribute to the problem, according to infectious disease experts.
Doctors are also noticing a growing number of women who are treated for syphilis but reinfected during pregnancy. Amid rising cases and stagnant resources, some states have focused disease investigations on pregnant women of childbearing age; they can no longer prioritize treating sexual partners who are also infected.
Eric McGrath, MD, a pediatric infectious disease specialist at Wayne State University, Detroit, said that he’d seen several newborns in recent years whose mothers had been treated for syphilis but then were re-exposed during pregnancy by partners who hadn’t been treated.
Treating a newborn baby for syphilis isn’t trivial. Penicillin carries little risk, but delivering it to a baby often involves a lumbar puncture and other painful procedures. And treatment typically means keeping the baby in the hospital for 10 days, interrupting an important time for family bonding.
Dr. McGrath has seen a couple of babies in his career who weren’t diagnosed or treated at birth and later came to him with full-blown syphilis complications, including full-body rashes and inflamed livers. It was an awful experience he doesn’t want to repeat. The preferred course, he said, is to spare the baby the ordeal and treat parents early in the pregnancy.
But in some places, providers aren’t routinely testing for syphilis. Although most states mandate testing at some point during pregnancy, as of last year just 14 required it for everyone in the third trimester. The CDC recommends third-trimester testing in areas with high rates of syphilis, a growing share of the United States.
After Arizona declared a statewide outbreak in 2018, state health officials wanted to know whether widespread testing in the third trimester could have prevented infections. Looking at 18 months of data, analysts found that nearly three-quarters of the more than 200 pregnant women diagnosed with syphilis in 2017 and the first half of 2018 got treatment. That left 57 babies born with syphilis, nine of whom died. The analysts estimated that a third of the infections could have been prevented with testing in the third trimester.
Based on the numbers they saw in those 18 months, officials estimated that screening all women on Medicaid in the third trimester would cost the state $113,300 annually, and that treating all cases of syphilis that screening would catch could be done for just $113. Factoring in the hospitalization costs for infected infants, the officials concluded the additional testing would save the state money.
And yet prevention money has been hard to come by. Taking inflation into account, CDC prevention funding for STDs has fallen 41% since 2003, according to an analysis by the National Coalition of STD Directors. That’s even as cases have risen, leaving public health departments saddled with more work and far less money.
Janine Waters, STD program manager for the state of New Mexico, has watched the unraveling. When Ms. Waters started her career more than 20 years ago, she and her colleagues followed up on every case of chlamydia, gonorrhea, and syphilis reported, not only making sure that people got treatment but also getting in touch with their sexual partners, with the aim of stopping the spread of infection. In a 2019 interview with Kaiser Health News, she said her team was struggling to keep up with syphilis alone, even as they registered with dread congenital syphilis cases surging in neighboring Texas and Arizona.
By 2020, New Mexico had the highest rate of congenital syphilis in the country.
The COVID-19 pandemic drained the remaining resources. Half of health departments across the country discontinued STD fieldwork altogether, diverting their resources to COVID. In California, which for years has struggled with high rates of congenital syphilis, three-quarters of local health departments dispatched more than half of their STD staffers to work on COVID.
As the pandemic ebbs – at least in the short term – many public health departments are turning their attention back to syphilis and other diseases. And they are doing it with reinforcements. Although the Biden administration’s proposed STD prevention budget for 2023 remains flat, the American Rescue Plan Act included $200 million to help health departments boost contact tracing and surveillance for covid and other infectious diseases. Many departments are funneling that money toward STDs.
The money is an infusion that state health officials say will make a difference. But when taking inflation into account, it essentially brings STD prevention funding back to what it was in 2003, said Stephanie Arnold Pang of the National Coalition of STD Directors. And the American Rescue Plan money doesn’t cover some aspects of STD prevention, including clinical services.
The coalition wants to revive dedicated STD clinics, where people can drop in for testing and treatment at little to no cost. Advocates say that would fill a void that has plagued treatment efforts since public clinics closed en masse in the wake of the 2008 recession.
Texas, battling its own pervasive outbreak, will use its share of American Rescue Plan money to fill 94 new positions focused on various aspects of STD prevention. Those hires will bolster a range of measures the state put in place before the pandemic, including an updated data system to track infections, review boards in major cities that examine what went wrong for every case of congenital syphilis, and a requirement that providers test for syphilis during the third trimester of pregnancy. The suite of interventions seems to be working, but it could be a while before cases go down, said Amy Carter, the state’s congenital syphilis coordinator.
“The growth didn’t happen overnight,” Ms. Carter said. “So our prevention efforts aren’t going to have a direct impact overnight either.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation
To engage injection drug users in HCV care, go to where they are
For injection drug users with hepatitis C virus (HCV) infection, providing treatment opportunities within a local needle exchange program can provide care to more patients and eventually cure more patients, a new study suggests.
The study’s findings help “counteract the implicit belief within the medical community that people who inject drugs can’t or don’t want to engage in health care,” lead author Benjamin Eckhardt, MD, with NYU Grossman School of Medicine, told this news organization.
“By simply focusing on patient accompaniment, limiting stigma, and removing the punitive response for missed appointments, we can effectively engage people who inject drugs in health care and more specifically cure their infection, making significant inroads to HCV elimination,” Dr. Eckhardt said.
The study was published online in JAMA Internal Medicine.
Nonjudgmental, patient-centered approach
Researchers included 165 injection drug users with HCV (mean age, 42 years; 78% men); 82 were randomly allocated to the accessible care intervention and 83 to a usual care control group.
The accessible care model provides HCV treatment within a community-based needle exchange program in a comfortable, nonjudgmental atmosphere, “without fear of shame or stigma that people who inject drugs often experience in mainstream institutions,” the investigators explain.
Control participants were connected to a patient navigator who facilitated referrals to community direct antigen antiviral therapy programs that were not at a syringe service program.
In an intent-to-treat analysis, those enrolled in the accessible care group achieved sustained viral eradication at 12 months at significantly higher rates than those in the control group (67% vs. 23%; P < .001).
Once patients initiated treatment, cure rates were the same in both groups (86%), indicating that the major benefit of the accessible care program was in facilitating treatment, rather than increasing adherence to or response to treatment, the researchers noted.
This is reflected in the fact that the percentage of participants who advanced along the care cascade was significantly higher at each step for the accessible care group than the control group, from referral to an HCV clinician (93% vs. 45%), attendance of the initial HCV clinical visit (87% vs. 37%), completion of baseline laboratory testing (87% vs. 31%), and treatment initiation (78% vs. 27%).
Getting to the population in need
“The most surprising aspect of the study was how successful we were at recruiting, engaging, and treating people who inject drugs who lived outside the immediate community where the syringe exchange program was located and had no prior connection to the program,” Dr. Eckhardt said.
“We had numerous individuals travel 45-plus minutes on the subway from the South Bronx, passing four major medical centers with robust hepatitis C treatment programs, to seek care for hepatitis C in a small, dark office – but also an office they’d heard can be trusted – without fear of stigma or preconditions,” Dr. Eckhardt said.
Commenting on the study’s findings, Nancy Reau, MD, section chief of hepatology at Rush Medical College, Chicago, said, “This is another successful example of making therapy accessible to the population who is in need versus trying to move them into a tertiary care model.”
Dr. Reau noted that similar care models exist in the United States but are not always accessible to the population in need.
“The safety and efficacy of current therapy and the simplified care cascade make HCV an appropriate disease for this delivery,” she said, adding that this study “highlights not just the importance of these programs but also the necessity of engaging the medical community, changing policy, and using patient navigators and monetary support/prioritization to provide appropriate HCV management to those who are at high risk for the disease and for transmission.”
Accessible care beyond HCV
The coauthors of an accompanying editor’s note point out that the treatment for HCV has improved substantially, but it can be a real challenge to provide treatment to injection drug users because the U.S. health care system is not oriented toward the needs of this population.
“It is not surprising that the accessible care arm achieved a higher rate of viral eradication, as it created a patient-focused experience,” write Asha Choudhury, MD, MPH, with the University of California, San Francisco, and Mitchell Katz, MD, with NYC Health and Hospitals. “Creating inviting and engaging environments is particularly important when caring for patients from stigmatized groups. Having more sites that are accessible and inclusive like this for treating patients will likely increase treatment of hepatitis C.”
In their view, the study raises “two dueling questions: Is this model replicable across the U.S.? And, conversely, why isn’t all medical care offered in friendly, nonjudgmental settings with the intention of meeting patient goals?”
They conclude that the study’s lessons extend beyond this particular population and have implications for the field at large.
“The model is replicable to the extent that health care systems are prepared to provide nonjudgmental supportive care for persons who inject drugs,” they write. “However, all patients would benefit from a health care system that provided more patient-centered environments.”
The study was funded by the National Institute on Drug Abuse. Dr. Eckhardt reports receiving grants from the National Institutes of Health and Gilead during the conduct of the study. Dr. Choudhury, Dr. Katz, and Dr. Reau report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For injection drug users with hepatitis C virus (HCV) infection, providing treatment opportunities within a local needle exchange program can provide care to more patients and eventually cure more patients, a new study suggests.
The study’s findings help “counteract the implicit belief within the medical community that people who inject drugs can’t or don’t want to engage in health care,” lead author Benjamin Eckhardt, MD, with NYU Grossman School of Medicine, told this news organization.
“By simply focusing on patient accompaniment, limiting stigma, and removing the punitive response for missed appointments, we can effectively engage people who inject drugs in health care and more specifically cure their infection, making significant inroads to HCV elimination,” Dr. Eckhardt said.
The study was published online in JAMA Internal Medicine.
Nonjudgmental, patient-centered approach
Researchers included 165 injection drug users with HCV (mean age, 42 years; 78% men); 82 were randomly allocated to the accessible care intervention and 83 to a usual care control group.
The accessible care model provides HCV treatment within a community-based needle exchange program in a comfortable, nonjudgmental atmosphere, “without fear of shame or stigma that people who inject drugs often experience in mainstream institutions,” the investigators explain.
Control participants were connected to a patient navigator who facilitated referrals to community direct antigen antiviral therapy programs that were not at a syringe service program.
In an intent-to-treat analysis, those enrolled in the accessible care group achieved sustained viral eradication at 12 months at significantly higher rates than those in the control group (67% vs. 23%; P < .001).
Once patients initiated treatment, cure rates were the same in both groups (86%), indicating that the major benefit of the accessible care program was in facilitating treatment, rather than increasing adherence to or response to treatment, the researchers noted.
This is reflected in the fact that the percentage of participants who advanced along the care cascade was significantly higher at each step for the accessible care group than the control group, from referral to an HCV clinician (93% vs. 45%), attendance of the initial HCV clinical visit (87% vs. 37%), completion of baseline laboratory testing (87% vs. 31%), and treatment initiation (78% vs. 27%).
Getting to the population in need
“The most surprising aspect of the study was how successful we were at recruiting, engaging, and treating people who inject drugs who lived outside the immediate community where the syringe exchange program was located and had no prior connection to the program,” Dr. Eckhardt said.
“We had numerous individuals travel 45-plus minutes on the subway from the South Bronx, passing four major medical centers with robust hepatitis C treatment programs, to seek care for hepatitis C in a small, dark office – but also an office they’d heard can be trusted – without fear of stigma or preconditions,” Dr. Eckhardt said.
Commenting on the study’s findings, Nancy Reau, MD, section chief of hepatology at Rush Medical College, Chicago, said, “This is another successful example of making therapy accessible to the population who is in need versus trying to move them into a tertiary care model.”
Dr. Reau noted that similar care models exist in the United States but are not always accessible to the population in need.
“The safety and efficacy of current therapy and the simplified care cascade make HCV an appropriate disease for this delivery,” she said, adding that this study “highlights not just the importance of these programs but also the necessity of engaging the medical community, changing policy, and using patient navigators and monetary support/prioritization to provide appropriate HCV management to those who are at high risk for the disease and for transmission.”
Accessible care beyond HCV
The coauthors of an accompanying editor’s note point out that the treatment for HCV has improved substantially, but it can be a real challenge to provide treatment to injection drug users because the U.S. health care system is not oriented toward the needs of this population.
“It is not surprising that the accessible care arm achieved a higher rate of viral eradication, as it created a patient-focused experience,” write Asha Choudhury, MD, MPH, with the University of California, San Francisco, and Mitchell Katz, MD, with NYC Health and Hospitals. “Creating inviting and engaging environments is particularly important when caring for patients from stigmatized groups. Having more sites that are accessible and inclusive like this for treating patients will likely increase treatment of hepatitis C.”
In their view, the study raises “two dueling questions: Is this model replicable across the U.S.? And, conversely, why isn’t all medical care offered in friendly, nonjudgmental settings with the intention of meeting patient goals?”
They conclude that the study’s lessons extend beyond this particular population and have implications for the field at large.
“The model is replicable to the extent that health care systems are prepared to provide nonjudgmental supportive care for persons who inject drugs,” they write. “However, all patients would benefit from a health care system that provided more patient-centered environments.”
The study was funded by the National Institute on Drug Abuse. Dr. Eckhardt reports receiving grants from the National Institutes of Health and Gilead during the conduct of the study. Dr. Choudhury, Dr. Katz, and Dr. Reau report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For injection drug users with hepatitis C virus (HCV) infection, providing treatment opportunities within a local needle exchange program can provide care to more patients and eventually cure more patients, a new study suggests.
The study’s findings help “counteract the implicit belief within the medical community that people who inject drugs can’t or don’t want to engage in health care,” lead author Benjamin Eckhardt, MD, with NYU Grossman School of Medicine, told this news organization.
“By simply focusing on patient accompaniment, limiting stigma, and removing the punitive response for missed appointments, we can effectively engage people who inject drugs in health care and more specifically cure their infection, making significant inroads to HCV elimination,” Dr. Eckhardt said.
The study was published online in JAMA Internal Medicine.
Nonjudgmental, patient-centered approach
Researchers included 165 injection drug users with HCV (mean age, 42 years; 78% men); 82 were randomly allocated to the accessible care intervention and 83 to a usual care control group.
The accessible care model provides HCV treatment within a community-based needle exchange program in a comfortable, nonjudgmental atmosphere, “without fear of shame or stigma that people who inject drugs often experience in mainstream institutions,” the investigators explain.
Control participants were connected to a patient navigator who facilitated referrals to community direct antigen antiviral therapy programs that were not at a syringe service program.
In an intent-to-treat analysis, those enrolled in the accessible care group achieved sustained viral eradication at 12 months at significantly higher rates than those in the control group (67% vs. 23%; P < .001).
Once patients initiated treatment, cure rates were the same in both groups (86%), indicating that the major benefit of the accessible care program was in facilitating treatment, rather than increasing adherence to or response to treatment, the researchers noted.
This is reflected in the fact that the percentage of participants who advanced along the care cascade was significantly higher at each step for the accessible care group than the control group, from referral to an HCV clinician (93% vs. 45%), attendance of the initial HCV clinical visit (87% vs. 37%), completion of baseline laboratory testing (87% vs. 31%), and treatment initiation (78% vs. 27%).
Getting to the population in need
“The most surprising aspect of the study was how successful we were at recruiting, engaging, and treating people who inject drugs who lived outside the immediate community where the syringe exchange program was located and had no prior connection to the program,” Dr. Eckhardt said.
“We had numerous individuals travel 45-plus minutes on the subway from the South Bronx, passing four major medical centers with robust hepatitis C treatment programs, to seek care for hepatitis C in a small, dark office – but also an office they’d heard can be trusted – without fear of stigma or preconditions,” Dr. Eckhardt said.
Commenting on the study’s findings, Nancy Reau, MD, section chief of hepatology at Rush Medical College, Chicago, said, “This is another successful example of making therapy accessible to the population who is in need versus trying to move them into a tertiary care model.”
Dr. Reau noted that similar care models exist in the United States but are not always accessible to the population in need.
“The safety and efficacy of current therapy and the simplified care cascade make HCV an appropriate disease for this delivery,” she said, adding that this study “highlights not just the importance of these programs but also the necessity of engaging the medical community, changing policy, and using patient navigators and monetary support/prioritization to provide appropriate HCV management to those who are at high risk for the disease and for transmission.”
Accessible care beyond HCV
The coauthors of an accompanying editor’s note point out that the treatment for HCV has improved substantially, but it can be a real challenge to provide treatment to injection drug users because the U.S. health care system is not oriented toward the needs of this population.
“It is not surprising that the accessible care arm achieved a higher rate of viral eradication, as it created a patient-focused experience,” write Asha Choudhury, MD, MPH, with the University of California, San Francisco, and Mitchell Katz, MD, with NYC Health and Hospitals. “Creating inviting and engaging environments is particularly important when caring for patients from stigmatized groups. Having more sites that are accessible and inclusive like this for treating patients will likely increase treatment of hepatitis C.”
In their view, the study raises “two dueling questions: Is this model replicable across the U.S.? And, conversely, why isn’t all medical care offered in friendly, nonjudgmental settings with the intention of meeting patient goals?”
They conclude that the study’s lessons extend beyond this particular population and have implications for the field at large.
“The model is replicable to the extent that health care systems are prepared to provide nonjudgmental supportive care for persons who inject drugs,” they write. “However, all patients would benefit from a health care system that provided more patient-centered environments.”
The study was funded by the National Institute on Drug Abuse. Dr. Eckhardt reports receiving grants from the National Institutes of Health and Gilead during the conduct of the study. Dr. Choudhury, Dr. Katz, and Dr. Reau report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Asymptomatic C. difficile carriers may infect the people they live with after hospitalization
Hospitalized patients who are asymptomatic Clostridioides difficile carriers may infect people they live with after they return home, a study based on U.S. insurance claim data suggests.
Although C. difficile infection (CDI) is considered to be a common hospital-acquired infection, reports of community-associated CDI in patients who have not been hospitalized are increasing, the authors wrote in Emerging Infectious Diseases.
“Individuals in households where another family member was recently hospitalized but not diagnosed with a CDI appear to be at increased risk for CDI,” said lead author Aaron C. Miller, PhD, a research assistant professor in the department of internal medicine at the University of Iowa, Iowa City. “When individuals are hospitalized, they may become colonized with C. difficile without developing symptoms and subsequently transmit the pathogen to other family members after they return home,” he said by email.
Dr. Miller and colleagues analyzed insurance claims data from 2001 through 2017 using the U.S. Commercial Claims and Medicare Supplemental datasets of IBM MarketScan Research Databases. Over that period, they searched employer-sponsored commercial insurance claims and Medicare supplemental claims of 194,424 enrollees, and they linked claims from multiple family members in the same enrollment plan.
They identified 224,818 CDI cases, and 3,871 of them were considered potential asymptomatic C. difficile transmissions from a recently hospitalized family member.
The researchers gathered monthly C. difficile incidence data from households with a family member who had been hospitalized within the past 60 days and compared them with data from households without a hospitalized family member.
Enrollees exposed to a recently hospitalized family member had a 73% greater incidence of CDI compared with enrollees who were not exposed. The longer the family member’s hospital stay, the greater the risk that someone in the household became infected.
Compared with people whose family members were hospitalized less than 1 day, people whose family members were hospitalized from 1 to 3 days had an incidence rate ratio (IRR) of 1.30 (95% confidence interval [CI], 1.19-1.41), and those whose family members were hospitalized for more than 30 days had an IRR of 2.45 (95% CI, 1.66-3.60).
CDI incidence increased with age. Compared with people 17 years of age or younger, the IRR increased to 9.32 (95% CI, 8.92-9.73) for those over 65.
Females had higher CDI incidence than males (IRR 1.30; 95% CI, 1.28-1.33).
Households with an infant also had higher CDI incidence than those without (IRR 1.5; 95% CI, 1.44-1.58).
People taking antimicrobials had higher CDI IRRs: 2.69 (95% CI, 2.59-2.79) for low-CDI-risk antibiotics and 8.83 (95% CI, 8.63-9.03) for high-CDI-risk antibiotics.
People taking proton-pump inhibitors had an IRR of 2.23 (95% CI, 2.15-2.30).
Reactions from four experts
Douglas S. Paauw MD, MACP, professor of medicine and the chair for patient-centered clinical education at the University of Washington, Seattle, was not surprised by the findings. “We have wondered for a while how community-acquired CDI occurs,” he said in an email. “This important study offers a plausible explanation for some cases.”
Dr. Paauw advises doctors to consider CDI in their patients who have been exposed to hospitalized people.
David M. Aronoff, MD, FIDSA, FAAM, professor of medicine and the chair of the department of medicine at Indiana University, Indianapolis, advises providers to educate hospital patients being discharged about how CDI is spread and how they can practice good hand hygiene at home.
“An open question of this strong study is whether we should be testing certain hospital patients for asymptomatic C. difficile carriage before they are discharged,” he added in an email.
In a phone interview, Paul G. Auwaerter, MD, MBA, professor of medicine and clinical director of the division of infectious diseases at Johns Hopkins University, Baltimore, noted that community-acquired CDI is frequent enough that his institution performs routine C. difficile testing on all patients with unexplained severe diarrhea.
“This intriguing study bears additional research and follow-up because clearly these spores are hardy,” he said. “But a key point in this billings- and claims-based study is that no one knows where household members acquired CDI, whether it was actually through household transmission.”
Ramin Asgary, MD, MPH, FASTMH, associate professor of global health in the Milken Institute School of Public Health at George Washington University, Washington, cautioned about “an increasing issue with drug-resistant CDI.
“This important, timely study provides another step in the right direction to better understanding and addressing CDI and other hospital-based infections that have become increasing threats to the safety of our patients, their families, and health care in general,” he said in an email.
Dr. Miller said that the scale and scope of the data are strengths of the study, and he acknowledged that its basis in claims and billing data is a limitation. He and his group plan to explore genetic relationships involved in CDI transmission.
The study was funded by the Centers for Disease Control and Prevention. All authors and independent experts have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hospitalized patients who are asymptomatic Clostridioides difficile carriers may infect people they live with after they return home, a study based on U.S. insurance claim data suggests.
Although C. difficile infection (CDI) is considered to be a common hospital-acquired infection, reports of community-associated CDI in patients who have not been hospitalized are increasing, the authors wrote in Emerging Infectious Diseases.
“Individuals in households where another family member was recently hospitalized but not diagnosed with a CDI appear to be at increased risk for CDI,” said lead author Aaron C. Miller, PhD, a research assistant professor in the department of internal medicine at the University of Iowa, Iowa City. “When individuals are hospitalized, they may become colonized with C. difficile without developing symptoms and subsequently transmit the pathogen to other family members after they return home,” he said by email.
Dr. Miller and colleagues analyzed insurance claims data from 2001 through 2017 using the U.S. Commercial Claims and Medicare Supplemental datasets of IBM MarketScan Research Databases. Over that period, they searched employer-sponsored commercial insurance claims and Medicare supplemental claims of 194,424 enrollees, and they linked claims from multiple family members in the same enrollment plan.
They identified 224,818 CDI cases, and 3,871 of them were considered potential asymptomatic C. difficile transmissions from a recently hospitalized family member.
The researchers gathered monthly C. difficile incidence data from households with a family member who had been hospitalized within the past 60 days and compared them with data from households without a hospitalized family member.
Enrollees exposed to a recently hospitalized family member had a 73% greater incidence of CDI compared with enrollees who were not exposed. The longer the family member’s hospital stay, the greater the risk that someone in the household became infected.
Compared with people whose family members were hospitalized less than 1 day, people whose family members were hospitalized from 1 to 3 days had an incidence rate ratio (IRR) of 1.30 (95% confidence interval [CI], 1.19-1.41), and those whose family members were hospitalized for more than 30 days had an IRR of 2.45 (95% CI, 1.66-3.60).
CDI incidence increased with age. Compared with people 17 years of age or younger, the IRR increased to 9.32 (95% CI, 8.92-9.73) for those over 65.
Females had higher CDI incidence than males (IRR 1.30; 95% CI, 1.28-1.33).
Households with an infant also had higher CDI incidence than those without (IRR 1.5; 95% CI, 1.44-1.58).
People taking antimicrobials had higher CDI IRRs: 2.69 (95% CI, 2.59-2.79) for low-CDI-risk antibiotics and 8.83 (95% CI, 8.63-9.03) for high-CDI-risk antibiotics.
People taking proton-pump inhibitors had an IRR of 2.23 (95% CI, 2.15-2.30).
Reactions from four experts
Douglas S. Paauw MD, MACP, professor of medicine and the chair for patient-centered clinical education at the University of Washington, Seattle, was not surprised by the findings. “We have wondered for a while how community-acquired CDI occurs,” he said in an email. “This important study offers a plausible explanation for some cases.”
Dr. Paauw advises doctors to consider CDI in their patients who have been exposed to hospitalized people.
David M. Aronoff, MD, FIDSA, FAAM, professor of medicine and the chair of the department of medicine at Indiana University, Indianapolis, advises providers to educate hospital patients being discharged about how CDI is spread and how they can practice good hand hygiene at home.
“An open question of this strong study is whether we should be testing certain hospital patients for asymptomatic C. difficile carriage before they are discharged,” he added in an email.
In a phone interview, Paul G. Auwaerter, MD, MBA, professor of medicine and clinical director of the division of infectious diseases at Johns Hopkins University, Baltimore, noted that community-acquired CDI is frequent enough that his institution performs routine C. difficile testing on all patients with unexplained severe diarrhea.
“This intriguing study bears additional research and follow-up because clearly these spores are hardy,” he said. “But a key point in this billings- and claims-based study is that no one knows where household members acquired CDI, whether it was actually through household transmission.”
Ramin Asgary, MD, MPH, FASTMH, associate professor of global health in the Milken Institute School of Public Health at George Washington University, Washington, cautioned about “an increasing issue with drug-resistant CDI.
“This important, timely study provides another step in the right direction to better understanding and addressing CDI and other hospital-based infections that have become increasing threats to the safety of our patients, their families, and health care in general,” he said in an email.
Dr. Miller said that the scale and scope of the data are strengths of the study, and he acknowledged that its basis in claims and billing data is a limitation. He and his group plan to explore genetic relationships involved in CDI transmission.
The study was funded by the Centers for Disease Control and Prevention. All authors and independent experts have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hospitalized patients who are asymptomatic Clostridioides difficile carriers may infect people they live with after they return home, a study based on U.S. insurance claim data suggests.
Although C. difficile infection (CDI) is considered to be a common hospital-acquired infection, reports of community-associated CDI in patients who have not been hospitalized are increasing, the authors wrote in Emerging Infectious Diseases.
“Individuals in households where another family member was recently hospitalized but not diagnosed with a CDI appear to be at increased risk for CDI,” said lead author Aaron C. Miller, PhD, a research assistant professor in the department of internal medicine at the University of Iowa, Iowa City. “When individuals are hospitalized, they may become colonized with C. difficile without developing symptoms and subsequently transmit the pathogen to other family members after they return home,” he said by email.
Dr. Miller and colleagues analyzed insurance claims data from 2001 through 2017 using the U.S. Commercial Claims and Medicare Supplemental datasets of IBM MarketScan Research Databases. Over that period, they searched employer-sponsored commercial insurance claims and Medicare supplemental claims of 194,424 enrollees, and they linked claims from multiple family members in the same enrollment plan.
They identified 224,818 CDI cases, and 3,871 of them were considered potential asymptomatic C. difficile transmissions from a recently hospitalized family member.
The researchers gathered monthly C. difficile incidence data from households with a family member who had been hospitalized within the past 60 days and compared them with data from households without a hospitalized family member.
Enrollees exposed to a recently hospitalized family member had a 73% greater incidence of CDI compared with enrollees who were not exposed. The longer the family member’s hospital stay, the greater the risk that someone in the household became infected.
Compared with people whose family members were hospitalized less than 1 day, people whose family members were hospitalized from 1 to 3 days had an incidence rate ratio (IRR) of 1.30 (95% confidence interval [CI], 1.19-1.41), and those whose family members were hospitalized for more than 30 days had an IRR of 2.45 (95% CI, 1.66-3.60).
CDI incidence increased with age. Compared with people 17 years of age or younger, the IRR increased to 9.32 (95% CI, 8.92-9.73) for those over 65.
Females had higher CDI incidence than males (IRR 1.30; 95% CI, 1.28-1.33).
Households with an infant also had higher CDI incidence than those without (IRR 1.5; 95% CI, 1.44-1.58).
People taking antimicrobials had higher CDI IRRs: 2.69 (95% CI, 2.59-2.79) for low-CDI-risk antibiotics and 8.83 (95% CI, 8.63-9.03) for high-CDI-risk antibiotics.
People taking proton-pump inhibitors had an IRR of 2.23 (95% CI, 2.15-2.30).
Reactions from four experts
Douglas S. Paauw MD, MACP, professor of medicine and the chair for patient-centered clinical education at the University of Washington, Seattle, was not surprised by the findings. “We have wondered for a while how community-acquired CDI occurs,” he said in an email. “This important study offers a plausible explanation for some cases.”
Dr. Paauw advises doctors to consider CDI in their patients who have been exposed to hospitalized people.
David M. Aronoff, MD, FIDSA, FAAM, professor of medicine and the chair of the department of medicine at Indiana University, Indianapolis, advises providers to educate hospital patients being discharged about how CDI is spread and how they can practice good hand hygiene at home.
“An open question of this strong study is whether we should be testing certain hospital patients for asymptomatic C. difficile carriage before they are discharged,” he added in an email.
In a phone interview, Paul G. Auwaerter, MD, MBA, professor of medicine and clinical director of the division of infectious diseases at Johns Hopkins University, Baltimore, noted that community-acquired CDI is frequent enough that his institution performs routine C. difficile testing on all patients with unexplained severe diarrhea.
“This intriguing study bears additional research and follow-up because clearly these spores are hardy,” he said. “But a key point in this billings- and claims-based study is that no one knows where household members acquired CDI, whether it was actually through household transmission.”
Ramin Asgary, MD, MPH, FASTMH, associate professor of global health in the Milken Institute School of Public Health at George Washington University, Washington, cautioned about “an increasing issue with drug-resistant CDI.
“This important, timely study provides another step in the right direction to better understanding and addressing CDI and other hospital-based infections that have become increasing threats to the safety of our patients, their families, and health care in general,” he said in an email.
Dr. Miller said that the scale and scope of the data are strengths of the study, and he acknowledged that its basis in claims and billing data is a limitation. He and his group plan to explore genetic relationships involved in CDI transmission.
The study was funded by the Centers for Disease Control and Prevention. All authors and independent experts have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nontuberculous mycobacterial lung disease can be challenging to treat
Living in coastal areas of Florida and California has great appeal for many, with the warm, sunny climate and nearby fresh water and salt water.
But, unknown to many, those balmy coasts also carry the risk of infection from nontuberculous (atypical) mycobacteria (NTM). Unlike its relative, tuberculosis, NTM is not transmitted from person to person, with one exception: patients with cystic fibrosis.
It is estimated that there were 181,000 people with NTM lung disease in the U.S. in 2015, and according to one study, the incidence is increasing by 8.2% annually among those aged 65 years and older. But NTM doesn’t only affect the elderly; it’s estimated that 31% of all NTM patients are younger than 65 years.
With the warm, moist soil and water, NTM is most commonly found in Florida, California, Hawaii, and the Gulf Coast states. The incidence is somewhat lower in states along the Great Lakes. Other states are not without risk – but NTM is perhaps even more likely to be overlooked in these states by physicians because of a lack of awareness of the disease.
Rebecca Prevots, PhD, MPH, chief of the epidemiology and population studies unit of the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, told this news organization that “why NTM is increasing is one of the most common questions” she gets, followed by whether it is due to climate change. “The short answer is, we don’t know.”
She suggests that the increase in diagnoses is due to a combination of increased awareness, host susceptibility, and perhaps environmental changes. One problem is that NTM is not a reportable disease. Also, public health resources have been decimated, both through funding cuts and loss of personnel. Dr. Prevots said, “It’s not just NTM surveillance that is important, but you can’t just make a certain condition reportable and expect to have good data without putting resources to it. ... Diseases are made reportable at the state level. There’s no mandated reporting up to CDC. So CDC is piloting reporting events through their emerging infectious program.”
Anthony Cannella, MD, assistant professor of infectious diseases at the University of South Florida (USF), is in the midst of NTM. He told this news organization that “there’s a huge circle with big old dots right over the center of the state.” He is adamant that “a soil-water survey has to occur. We need to know what the devil is happening.”
Florida legislators agreed to allocate $519,000 for NTM testing and surveillance in 2019. But Florida Governor Ron DeSantis vetoed that line item in the budget. WUSF (a National Public Radio affiliate on the USF campus) was unable to get a response to their query about this from the governor’s office.
Who gets NTM?
Mycobacterium avium complex primarily causes lung disease, which presents as two clinical syndromes.
“These infections don’t affect everyone,” Kenneth Olivier, MD, MPH, chief of pulmonary clinical medicine, Cardiovascular Pulmonary Branch of the National Heart, Lung, and Blood Institute, said in an interview. They affect “patients that have underlying genetic conditions that cause abnormalities in the airway clearance mechanisms, particularly cystic fibrosis and primary ciliary dyskinesia [and], to some extent, patients with COPD.”
The second group is “comprised mainly of postmenopausal women, many of whom have had no predisposing medical problems prior to onset of generally frequent throat clearing or chronic cough, which is what brings them to medical attention.” Dr. Olivier added that “many of these patients have a fairly unique appearance. They tend to have a high prevalence of curvature of the spine, scoliosis, indentation of the chest wall (pectus excavatum), and physical characteristics that overlap heritable connective tissue disorders like Marfan syndrome or Ehlers-Danlos syndrome.”
Dr. Olivier pointed out a major problem in NTM diagnosis and treatment: “The guidelines-based approach to chronic cough generally calls for treating postnasal drip, airway reactivity, asthma type symptoms first empirically, before doing different diagnostic studies. That generally causes a delay in obtaining things like CT scan, where you can see the characteristic changes.”
Dr. Cannella added, “People are starting to become more aware of it. It’s kind of like pneumocystis back in the 80s. ... We’ve had patients who have had long periods of febrile neutropenia, and NTM wasn’t on the radar. Now we’ve picked up at least seven or eight.”
In addition to pulmonary infections, nosocomial outbreaks have occurred, owing to contaminated heater-cooler units, catheter infections, nail salons, or to medical tourism. These more commonly involve rapidly growing species, such as M abscessus, M chelonae, and M fortuitum. Clinicians should also be aware of skin infections from M marinum, which come from wounds from aquariums, fish, or shellfish. Incubation can occur over months, highlighting the importance of a detailed history and special cultures.
Diagnostics
The diagnosis of NTM is delayed for several reasons. One is the lack of awareness among clinicians about NTM and its risk factors, including hobbies such as gardening or working in places where dirt is aerosolized, such as on road crews, or even from hot tubs. A thorough history is critical.
Another is not recognizing the need for an acid-fast bacilli (AFB) culture, which requires specialized media. Fortunately, NTM can be picked up on fungal cultures, Dr. Cannella noted. Clinicians are sometimes discouraged from culturing AFB because doing so may not be cost-effective. And many hospital laboratories are increasingly sending cultures to outside labs, and it can take days – sometimes even more than a week – to receive a report of results.
Charles Daley, MD, chief of the Division of Mycobacterial and Respiratory Infections at National Jewish Health, expressed his frustration about labs in an interview, saying diagnostics is “an important hole in the U.S., as our laboratories do not provide clinicians with the results that they need to make good decisions. Most laboratories in the U.S. just don’t speciate the organisms or subspeciate in the setting of abscesses. They don’t tell the clinician enough about the susceptibility, particularly whether there’s inducible resistance. As a clinician, you just don’t have the information to make the right decisions. ... We need to improve diagnostics in NTM. Everything is there and available. They just don’t want to do it because it increases the costs.”
Men tend to have fibrocavitary disease, which shows on ordinary chest x-rays, but CT scans are essential for women because women tend to have either nodular disease or bronchiectasis, which does not show on a plain film.
Treatment
A standard treatment for NTM lung disease includes three or four medications – clarithromycin or azithromycin, rifampin or rifabutin, ethambutol, and streptomycin or amikacin. In vitro resistance is important in predicting the clinical response to a macrolide or amikacin.
For bronchiectatic disease, National Jewish Hospital recommends treatment three times per week rather than daily therapy, as it is better tolerated. Azithromycin is preferred over clarithromycin. Amikacin should be added if there is cavitary or severe disease, and the macrolide is then given daily.
Dr. Olivier suggested that physicians stagger the initiation of those drugs to improve the tolerability of the difficult regimen. Generally, treatment is for 18 months – a year after sputum cultures become negative.
If therapy fails – that is, sputum is persistently positive at 6 months – amikacin liposomal inhalation solution (Arikayce) is likely to be added. Patients should be monitored with monthly safety labs, sputum cultures, and an audiogram (if receiving amikacin). Every 3 months, vestibular tests, eye exams, and spirometry should be conducted, and every 6 months, physicians should order a CT, an audiogram, and an electrocardiogram.
Despite completing such a rigorous regimen, about half of patients experience reinfection because of their underlying host susceptibility. Genomic sequencing shows that these are new infections, not relapses, Dr. Prevots said. She also noted that gastroesophageal reflux disease is a significant risk factor because of chronic aspiration.
Dr. Daley outlined the newer treatments being studied. They include Arikayce, omadocycline, and bedaquiline. He added, “There’s a neutrophil elastase inhibitor trial that’s ongoing, a huge trial. There’s another one looking at basically eosinophilic inflammation.”
Other trials are in the offing, he said, all focusing on the inflammatory response – a development he described as exciting, because for the longest time, there were few if any NTM trials.
Dr. Cannella is also buoyed by the potential synergy of dual beta-lactam therapy with ceftaroline and a carbapenem for M abscessus infections, which are notoriously difficult to treat.
There are unique problems facing drug development for NTM because, for approval, the U.S. Food and Drug Administration requires the drug to “improve how a patient feels, functions, or survives.” NTM is associated with low mortality, so that “is off the table,” Dr. Daley explained. It’s hard to quantify improvement in function. The top two symptoms to measure are coughing and fatigue, he said. But both are difficult to measure, and some of the medicines worsen cough. Some research groups are now trying to validate patient-reported outcome instruments to satisfy the FDA’s requirements.
Tips for patients and physicians
The experts this news organization spoke to had very consistent recommendations for patients:
- NTM is resistant to chlorine and bromine, so tap water is a major source of infection. Patients should consider to greater than 130° F and using metal showerheads or bathing rather than showering.
- Good bathroom ventilation helps.
- Patients should consider using a water filter that filters entities less than 5 mcm in size – but not carbon filters, which concentrate the organisms.
- Humidifiers and hot tubs should be avoided.
- A good face mask, such as an N95, should be worn when gardening or repotting plants.
Dr. Olivier stressed that clinicians should familiarize themselves with the guidelines for diagnosing and treating NTM. In particular, clinicians should be aware that using azithromycin for bronchitis might cause resistance in NTM. “Macrolide resistance turns what may be a slowly progressive or bothersome infection into a lethal infection with a 1-year mortality of 35%.”
He concluded, “I would just urge that if the patient’s on their second or third Z-Pak within a year, it’s probably time to look for other causes of what might be happening.”
Dr. Cannella, Dr. Prevots, and Dr. Olivier reported no relevant financial relationships. Dr. Cannella adds, “My views are not those of my employers, the U.S. Dept of VA, or the University of South Florida Morsani College of Medicine.” Dr. Daley reports research grants/contracts with AN2, Beyond Air, Bugworks, Insmed, and Paratek and service on advisory boards or as a consultant for AN2, AstraZeneca, Genentech, Insmed, Matinas, Paratek, Pfizer, and Spero.
A version of this article first appeared on Medscape.com.
Living in coastal areas of Florida and California has great appeal for many, with the warm, sunny climate and nearby fresh water and salt water.
But, unknown to many, those balmy coasts also carry the risk of infection from nontuberculous (atypical) mycobacteria (NTM). Unlike its relative, tuberculosis, NTM is not transmitted from person to person, with one exception: patients with cystic fibrosis.
It is estimated that there were 181,000 people with NTM lung disease in the U.S. in 2015, and according to one study, the incidence is increasing by 8.2% annually among those aged 65 years and older. But NTM doesn’t only affect the elderly; it’s estimated that 31% of all NTM patients are younger than 65 years.
With the warm, moist soil and water, NTM is most commonly found in Florida, California, Hawaii, and the Gulf Coast states. The incidence is somewhat lower in states along the Great Lakes. Other states are not without risk – but NTM is perhaps even more likely to be overlooked in these states by physicians because of a lack of awareness of the disease.
Rebecca Prevots, PhD, MPH, chief of the epidemiology and population studies unit of the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, told this news organization that “why NTM is increasing is one of the most common questions” she gets, followed by whether it is due to climate change. “The short answer is, we don’t know.”
She suggests that the increase in diagnoses is due to a combination of increased awareness, host susceptibility, and perhaps environmental changes. One problem is that NTM is not a reportable disease. Also, public health resources have been decimated, both through funding cuts and loss of personnel. Dr. Prevots said, “It’s not just NTM surveillance that is important, but you can’t just make a certain condition reportable and expect to have good data without putting resources to it. ... Diseases are made reportable at the state level. There’s no mandated reporting up to CDC. So CDC is piloting reporting events through their emerging infectious program.”
Anthony Cannella, MD, assistant professor of infectious diseases at the University of South Florida (USF), is in the midst of NTM. He told this news organization that “there’s a huge circle with big old dots right over the center of the state.” He is adamant that “a soil-water survey has to occur. We need to know what the devil is happening.”
Florida legislators agreed to allocate $519,000 for NTM testing and surveillance in 2019. But Florida Governor Ron DeSantis vetoed that line item in the budget. WUSF (a National Public Radio affiliate on the USF campus) was unable to get a response to their query about this from the governor’s office.
Who gets NTM?
Mycobacterium avium complex primarily causes lung disease, which presents as two clinical syndromes.
“These infections don’t affect everyone,” Kenneth Olivier, MD, MPH, chief of pulmonary clinical medicine, Cardiovascular Pulmonary Branch of the National Heart, Lung, and Blood Institute, said in an interview. They affect “patients that have underlying genetic conditions that cause abnormalities in the airway clearance mechanisms, particularly cystic fibrosis and primary ciliary dyskinesia [and], to some extent, patients with COPD.”
The second group is “comprised mainly of postmenopausal women, many of whom have had no predisposing medical problems prior to onset of generally frequent throat clearing or chronic cough, which is what brings them to medical attention.” Dr. Olivier added that “many of these patients have a fairly unique appearance. They tend to have a high prevalence of curvature of the spine, scoliosis, indentation of the chest wall (pectus excavatum), and physical characteristics that overlap heritable connective tissue disorders like Marfan syndrome or Ehlers-Danlos syndrome.”
Dr. Olivier pointed out a major problem in NTM diagnosis and treatment: “The guidelines-based approach to chronic cough generally calls for treating postnasal drip, airway reactivity, asthma type symptoms first empirically, before doing different diagnostic studies. That generally causes a delay in obtaining things like CT scan, where you can see the characteristic changes.”
Dr. Cannella added, “People are starting to become more aware of it. It’s kind of like pneumocystis back in the 80s. ... We’ve had patients who have had long periods of febrile neutropenia, and NTM wasn’t on the radar. Now we’ve picked up at least seven or eight.”
In addition to pulmonary infections, nosocomial outbreaks have occurred, owing to contaminated heater-cooler units, catheter infections, nail salons, or to medical tourism. These more commonly involve rapidly growing species, such as M abscessus, M chelonae, and M fortuitum. Clinicians should also be aware of skin infections from M marinum, which come from wounds from aquariums, fish, or shellfish. Incubation can occur over months, highlighting the importance of a detailed history and special cultures.
Diagnostics
The diagnosis of NTM is delayed for several reasons. One is the lack of awareness among clinicians about NTM and its risk factors, including hobbies such as gardening or working in places where dirt is aerosolized, such as on road crews, or even from hot tubs. A thorough history is critical.
Another is not recognizing the need for an acid-fast bacilli (AFB) culture, which requires specialized media. Fortunately, NTM can be picked up on fungal cultures, Dr. Cannella noted. Clinicians are sometimes discouraged from culturing AFB because doing so may not be cost-effective. And many hospital laboratories are increasingly sending cultures to outside labs, and it can take days – sometimes even more than a week – to receive a report of results.
Charles Daley, MD, chief of the Division of Mycobacterial and Respiratory Infections at National Jewish Health, expressed his frustration about labs in an interview, saying diagnostics is “an important hole in the U.S., as our laboratories do not provide clinicians with the results that they need to make good decisions. Most laboratories in the U.S. just don’t speciate the organisms or subspeciate in the setting of abscesses. They don’t tell the clinician enough about the susceptibility, particularly whether there’s inducible resistance. As a clinician, you just don’t have the information to make the right decisions. ... We need to improve diagnostics in NTM. Everything is there and available. They just don’t want to do it because it increases the costs.”
Men tend to have fibrocavitary disease, which shows on ordinary chest x-rays, but CT scans are essential for women because women tend to have either nodular disease or bronchiectasis, which does not show on a plain film.
Treatment
A standard treatment for NTM lung disease includes three or four medications – clarithromycin or azithromycin, rifampin or rifabutin, ethambutol, and streptomycin or amikacin. In vitro resistance is important in predicting the clinical response to a macrolide or amikacin.
For bronchiectatic disease, National Jewish Hospital recommends treatment three times per week rather than daily therapy, as it is better tolerated. Azithromycin is preferred over clarithromycin. Amikacin should be added if there is cavitary or severe disease, and the macrolide is then given daily.
Dr. Olivier suggested that physicians stagger the initiation of those drugs to improve the tolerability of the difficult regimen. Generally, treatment is for 18 months – a year after sputum cultures become negative.
If therapy fails – that is, sputum is persistently positive at 6 months – amikacin liposomal inhalation solution (Arikayce) is likely to be added. Patients should be monitored with monthly safety labs, sputum cultures, and an audiogram (if receiving amikacin). Every 3 months, vestibular tests, eye exams, and spirometry should be conducted, and every 6 months, physicians should order a CT, an audiogram, and an electrocardiogram.
Despite completing such a rigorous regimen, about half of patients experience reinfection because of their underlying host susceptibility. Genomic sequencing shows that these are new infections, not relapses, Dr. Prevots said. She also noted that gastroesophageal reflux disease is a significant risk factor because of chronic aspiration.
Dr. Daley outlined the newer treatments being studied. They include Arikayce, omadocycline, and bedaquiline. He added, “There’s a neutrophil elastase inhibitor trial that’s ongoing, a huge trial. There’s another one looking at basically eosinophilic inflammation.”
Other trials are in the offing, he said, all focusing on the inflammatory response – a development he described as exciting, because for the longest time, there were few if any NTM trials.
Dr. Cannella is also buoyed by the potential synergy of dual beta-lactam therapy with ceftaroline and a carbapenem for M abscessus infections, which are notoriously difficult to treat.
There are unique problems facing drug development for NTM because, for approval, the U.S. Food and Drug Administration requires the drug to “improve how a patient feels, functions, or survives.” NTM is associated with low mortality, so that “is off the table,” Dr. Daley explained. It’s hard to quantify improvement in function. The top two symptoms to measure are coughing and fatigue, he said. But both are difficult to measure, and some of the medicines worsen cough. Some research groups are now trying to validate patient-reported outcome instruments to satisfy the FDA’s requirements.
Tips for patients and physicians
The experts this news organization spoke to had very consistent recommendations for patients:
- NTM is resistant to chlorine and bromine, so tap water is a major source of infection. Patients should consider to greater than 130° F and using metal showerheads or bathing rather than showering.
- Good bathroom ventilation helps.
- Patients should consider using a water filter that filters entities less than 5 mcm in size – but not carbon filters, which concentrate the organisms.
- Humidifiers and hot tubs should be avoided.
- A good face mask, such as an N95, should be worn when gardening or repotting plants.
Dr. Olivier stressed that clinicians should familiarize themselves with the guidelines for diagnosing and treating NTM. In particular, clinicians should be aware that using azithromycin for bronchitis might cause resistance in NTM. “Macrolide resistance turns what may be a slowly progressive or bothersome infection into a lethal infection with a 1-year mortality of 35%.”
He concluded, “I would just urge that if the patient’s on their second or third Z-Pak within a year, it’s probably time to look for other causes of what might be happening.”
Dr. Cannella, Dr. Prevots, and Dr. Olivier reported no relevant financial relationships. Dr. Cannella adds, “My views are not those of my employers, the U.S. Dept of VA, or the University of South Florida Morsani College of Medicine.” Dr. Daley reports research grants/contracts with AN2, Beyond Air, Bugworks, Insmed, and Paratek and service on advisory boards or as a consultant for AN2, AstraZeneca, Genentech, Insmed, Matinas, Paratek, Pfizer, and Spero.
A version of this article first appeared on Medscape.com.
Living in coastal areas of Florida and California has great appeal for many, with the warm, sunny climate and nearby fresh water and salt water.
But, unknown to many, those balmy coasts also carry the risk of infection from nontuberculous (atypical) mycobacteria (NTM). Unlike its relative, tuberculosis, NTM is not transmitted from person to person, with one exception: patients with cystic fibrosis.
It is estimated that there were 181,000 people with NTM lung disease in the U.S. in 2015, and according to one study, the incidence is increasing by 8.2% annually among those aged 65 years and older. But NTM doesn’t only affect the elderly; it’s estimated that 31% of all NTM patients are younger than 65 years.
With the warm, moist soil and water, NTM is most commonly found in Florida, California, Hawaii, and the Gulf Coast states. The incidence is somewhat lower in states along the Great Lakes. Other states are not without risk – but NTM is perhaps even more likely to be overlooked in these states by physicians because of a lack of awareness of the disease.
Rebecca Prevots, PhD, MPH, chief of the epidemiology and population studies unit of the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, told this news organization that “why NTM is increasing is one of the most common questions” she gets, followed by whether it is due to climate change. “The short answer is, we don’t know.”
She suggests that the increase in diagnoses is due to a combination of increased awareness, host susceptibility, and perhaps environmental changes. One problem is that NTM is not a reportable disease. Also, public health resources have been decimated, both through funding cuts and loss of personnel. Dr. Prevots said, “It’s not just NTM surveillance that is important, but you can’t just make a certain condition reportable and expect to have good data without putting resources to it. ... Diseases are made reportable at the state level. There’s no mandated reporting up to CDC. So CDC is piloting reporting events through their emerging infectious program.”
Anthony Cannella, MD, assistant professor of infectious diseases at the University of South Florida (USF), is in the midst of NTM. He told this news organization that “there’s a huge circle with big old dots right over the center of the state.” He is adamant that “a soil-water survey has to occur. We need to know what the devil is happening.”
Florida legislators agreed to allocate $519,000 for NTM testing and surveillance in 2019. But Florida Governor Ron DeSantis vetoed that line item in the budget. WUSF (a National Public Radio affiliate on the USF campus) was unable to get a response to their query about this from the governor’s office.
Who gets NTM?
Mycobacterium avium complex primarily causes lung disease, which presents as two clinical syndromes.
“These infections don’t affect everyone,” Kenneth Olivier, MD, MPH, chief of pulmonary clinical medicine, Cardiovascular Pulmonary Branch of the National Heart, Lung, and Blood Institute, said in an interview. They affect “patients that have underlying genetic conditions that cause abnormalities in the airway clearance mechanisms, particularly cystic fibrosis and primary ciliary dyskinesia [and], to some extent, patients with COPD.”
The second group is “comprised mainly of postmenopausal women, many of whom have had no predisposing medical problems prior to onset of generally frequent throat clearing or chronic cough, which is what brings them to medical attention.” Dr. Olivier added that “many of these patients have a fairly unique appearance. They tend to have a high prevalence of curvature of the spine, scoliosis, indentation of the chest wall (pectus excavatum), and physical characteristics that overlap heritable connective tissue disorders like Marfan syndrome or Ehlers-Danlos syndrome.”
Dr. Olivier pointed out a major problem in NTM diagnosis and treatment: “The guidelines-based approach to chronic cough generally calls for treating postnasal drip, airway reactivity, asthma type symptoms first empirically, before doing different diagnostic studies. That generally causes a delay in obtaining things like CT scan, where you can see the characteristic changes.”
Dr. Cannella added, “People are starting to become more aware of it. It’s kind of like pneumocystis back in the 80s. ... We’ve had patients who have had long periods of febrile neutropenia, and NTM wasn’t on the radar. Now we’ve picked up at least seven or eight.”
In addition to pulmonary infections, nosocomial outbreaks have occurred, owing to contaminated heater-cooler units, catheter infections, nail salons, or to medical tourism. These more commonly involve rapidly growing species, such as M abscessus, M chelonae, and M fortuitum. Clinicians should also be aware of skin infections from M marinum, which come from wounds from aquariums, fish, or shellfish. Incubation can occur over months, highlighting the importance of a detailed history and special cultures.
Diagnostics
The diagnosis of NTM is delayed for several reasons. One is the lack of awareness among clinicians about NTM and its risk factors, including hobbies such as gardening or working in places where dirt is aerosolized, such as on road crews, or even from hot tubs. A thorough history is critical.
Another is not recognizing the need for an acid-fast bacilli (AFB) culture, which requires specialized media. Fortunately, NTM can be picked up on fungal cultures, Dr. Cannella noted. Clinicians are sometimes discouraged from culturing AFB because doing so may not be cost-effective. And many hospital laboratories are increasingly sending cultures to outside labs, and it can take days – sometimes even more than a week – to receive a report of results.
Charles Daley, MD, chief of the Division of Mycobacterial and Respiratory Infections at National Jewish Health, expressed his frustration about labs in an interview, saying diagnostics is “an important hole in the U.S., as our laboratories do not provide clinicians with the results that they need to make good decisions. Most laboratories in the U.S. just don’t speciate the organisms or subspeciate in the setting of abscesses. They don’t tell the clinician enough about the susceptibility, particularly whether there’s inducible resistance. As a clinician, you just don’t have the information to make the right decisions. ... We need to improve diagnostics in NTM. Everything is there and available. They just don’t want to do it because it increases the costs.”
Men tend to have fibrocavitary disease, which shows on ordinary chest x-rays, but CT scans are essential for women because women tend to have either nodular disease or bronchiectasis, which does not show on a plain film.
Treatment
A standard treatment for NTM lung disease includes three or four medications – clarithromycin or azithromycin, rifampin or rifabutin, ethambutol, and streptomycin or amikacin. In vitro resistance is important in predicting the clinical response to a macrolide or amikacin.
For bronchiectatic disease, National Jewish Hospital recommends treatment three times per week rather than daily therapy, as it is better tolerated. Azithromycin is preferred over clarithromycin. Amikacin should be added if there is cavitary or severe disease, and the macrolide is then given daily.
Dr. Olivier suggested that physicians stagger the initiation of those drugs to improve the tolerability of the difficult regimen. Generally, treatment is for 18 months – a year after sputum cultures become negative.
If therapy fails – that is, sputum is persistently positive at 6 months – amikacin liposomal inhalation solution (Arikayce) is likely to be added. Patients should be monitored with monthly safety labs, sputum cultures, and an audiogram (if receiving amikacin). Every 3 months, vestibular tests, eye exams, and spirometry should be conducted, and every 6 months, physicians should order a CT, an audiogram, and an electrocardiogram.
Despite completing such a rigorous regimen, about half of patients experience reinfection because of their underlying host susceptibility. Genomic sequencing shows that these are new infections, not relapses, Dr. Prevots said. She also noted that gastroesophageal reflux disease is a significant risk factor because of chronic aspiration.
Dr. Daley outlined the newer treatments being studied. They include Arikayce, omadocycline, and bedaquiline. He added, “There’s a neutrophil elastase inhibitor trial that’s ongoing, a huge trial. There’s another one looking at basically eosinophilic inflammation.”
Other trials are in the offing, he said, all focusing on the inflammatory response – a development he described as exciting, because for the longest time, there were few if any NTM trials.
Dr. Cannella is also buoyed by the potential synergy of dual beta-lactam therapy with ceftaroline and a carbapenem for M abscessus infections, which are notoriously difficult to treat.
There are unique problems facing drug development for NTM because, for approval, the U.S. Food and Drug Administration requires the drug to “improve how a patient feels, functions, or survives.” NTM is associated with low mortality, so that “is off the table,” Dr. Daley explained. It’s hard to quantify improvement in function. The top two symptoms to measure are coughing and fatigue, he said. But both are difficult to measure, and some of the medicines worsen cough. Some research groups are now trying to validate patient-reported outcome instruments to satisfy the FDA’s requirements.
Tips for patients and physicians
The experts this news organization spoke to had very consistent recommendations for patients:
- NTM is resistant to chlorine and bromine, so tap water is a major source of infection. Patients should consider to greater than 130° F and using metal showerheads or bathing rather than showering.
- Good bathroom ventilation helps.
- Patients should consider using a water filter that filters entities less than 5 mcm in size – but not carbon filters, which concentrate the organisms.
- Humidifiers and hot tubs should be avoided.
- A good face mask, such as an N95, should be worn when gardening or repotting plants.
Dr. Olivier stressed that clinicians should familiarize themselves with the guidelines for diagnosing and treating NTM. In particular, clinicians should be aware that using azithromycin for bronchitis might cause resistance in NTM. “Macrolide resistance turns what may be a slowly progressive or bothersome infection into a lethal infection with a 1-year mortality of 35%.”
He concluded, “I would just urge that if the patient’s on their second or third Z-Pak within a year, it’s probably time to look for other causes of what might be happening.”
Dr. Cannella, Dr. Prevots, and Dr. Olivier reported no relevant financial relationships. Dr. Cannella adds, “My views are not those of my employers, the U.S. Dept of VA, or the University of South Florida Morsani College of Medicine.” Dr. Daley reports research grants/contracts with AN2, Beyond Air, Bugworks, Insmed, and Paratek and service on advisory boards or as a consultant for AN2, AstraZeneca, Genentech, Insmed, Matinas, Paratek, Pfizer, and Spero.
A version of this article first appeared on Medscape.com.
Meningococcal vaccine shows moderate protective effect against gonorrhea
A widely approved vaccine for meningitis may provide up to 40% protection against gonorrhea in young adults and adolescents, according to new research. This moderate efficacy paired with a targeted risk-based approach could reduce cases as well as lead to health care savings over 10 years, an additional modeling study showed.
The results – in three linked papers – were published in The Lancet Infectious Diseases.
Gonorrhea, caused by the bacterium Neisseria gonorrhoeae, is the second most commonly reported sexually transmitted infection in the United States, according to the Centers for Disease Control and Prevention. Globally, the World Health Organization estimates that there were 82.4 million new cases in people aged 15-49 in 2020. At the same time, it is becoming more difficult to treat the infection because of the increasing prevalence of drug-resistant strains of N. gonorrhoeae.
“New approaches, such as vaccination, are needed as long-term strategies to prevent gonorrhea and address the emerging threat of antimicrobial resistance,” Winston Abara, MD, PhD, Division of STD Prevention, Centers for Disease Control and Prevention, and colleagues wrote.
While there is currently no vaccine for gonorrhea, observational studies have found an association between a meningococcal serogroup B vaccine and reduced gonorrhea cases. One study in New Zealand found that people vaccinated with the MeNZB vaccine, which was produced to control an outbreak of meningococcal disease in the country, were 31% less likely to contract gonorrhea.
This cross-reactivity comes about because Neisseria meningitidis, the bacterium that can cause meningitis, is closely related to N. gonorrhoeae, Joseph Alex Duncan, MD, PhD, associate professor of medicine, Division of Infectious Diseases, University of North Carolina School of Medicine, Chapel Hill, said in an interview. He was not involved with the research. The thought is that “a large proportion of the proteins that are in the vaccine also recognize proteins from Neisseria gonorrhea, because the bacteria are so similar at the genetic level,” he said.
To see if this association was still found for the four-component serogroup B meningococcal vaccine (MenB-4C), which is now widely available, Dr. Abara and colleagues looked through health records to identify laboratory-confirmed gonorrhea and chlamydia infections in adolescents and young adults in New York City and Philadelphia. All individuals included in the analysis were age 16-23 and all infections occurred between Jan. 1, 2016, and Dec. 31, 2018. These infections were then linked to vaccination records to determine individuals’ MenB-4C vaccination status. Complete vaccination was defined as two MenB-4C doses, delivered 30-180 days apart.
The research team identified over 167,700 infections, including 18,099 gonococcal infections, 124,876 chlamydial infections, and 24,731 coinfections, among 109,737 individuals. A total of 7,692 individuals had received at least one shot of the vaccine, and 3,660 people were fully vaccinated. Full MenB-4C vaccination was estimated to be 40% protective (APR 0.60; P < .0001) against gonorrhea, and partial vaccination was 26% protective (APR 0.74; P = .0012).
“The findings of our study add to the body of evidence that demonstrates that the MenB-4C may offer cross-protection against Neisseria gonorrhoeae, and it supports feasibility of an effective gonococcal vaccine with implications for gonorrhea prevention and control,” Dr. Abara told this news organization.
A second study conducted in South Australia looked at the effectiveness of the MenB-4C vaccine against meningitis and gonorrhea as part of a vaccination program. Using infection data from the Government of South Australia and vaccination records from the Australian Immunization Register, researchers identified individuals born between Feb. 1, 1998, and Feb. 1, 2005, with a documented gonorrhea or chlamydia infection between Feb. 1, 2019, and Jan. 31, 2021. Individuals with chlamydia served as the controls to account for similar sexual behavioral risks.
The analysis included 512 individuals with 575 cases of gonorrhea and 3,140 individuals with 3,847 episodes of chlamydia. In this group, the estimated vaccine effectiveness against gonorrhea was 32.7% (95% confidence interval, 8.3-50.6) in individuals who were fully vaccinated and 32.6% (95% CI, 10.6-49.1) in those who had received at least one dose of the MenB-4C.
While these findings are “confirmatory” because they showed results similar to those in previous observational studies, they are still exciting, Dr. Duncan said. “Up until now, we really haven’t had any real progress in knowing what type of immune responses could actually be protective from the disease,” he said. “These observational studies have really reinvigorated the Neisseria gonorrhea vaccine research community.”
A vaccine with moderate efficacy – like the protection demonstrated in both studies – could lead to a significant reduction in cases, he noted. A 2015 Australian modeling study estimated that a nonwaning vaccine with 20% efficacy could reduce cases by 40% over 20 years. Focusing on vaccinating higher-risk groups could also have an “outsize impact,” said Jeanne Marrazzo, MD, director, Division of Infectious Diseases, UAB Medicine, Birmingham, Alabama, in an interview. In the third study published in The Lancet, researchers estimated the possible reduction of cases and the potential health care cost savings in England in a vaccination effort focusing on men who have sex with men (MSM) at high risk for gonorrhea infection. They predicted that a vaccine with 31% efficacy could prevent 110,200 cases in MSM and save about £8 million ($10.4 million) over 10 years.
Both Dr. Duncan and Dr. Marrazzo agreed that clinical trials are needed to tease out whether the decrease in gonorrhea cases is due to the MenB-4C vaccine or the association is incidental. There are two ongoing clinical trials, one in Australia and one in the United States. Dr. Marrazzo leads the U.S. multicenter study, which also has two locations in Bangkok. The trial will also look at whether vaccination protection varies by the location of gonococcal infection: urethra, rectum, cervix, or pharynx. The two new observational studies did not distinguish the different sites of infection.
Dr. Marrazzo’s trial has enrolled almost 500 individuals so far, with the goal of enrolling over 2,000 participants in total. She hopes to see results by late 2023. “It’s a pretty ambitious effort, but I’m hoping it will give us not only a definitive answer in terms of reduction in infection by anatomic site,” she said, but “also give us a lot of information about how the immune response works to protect you from getting gonorrhea if you do get the vaccine.”
Dr. Duncan has received research grants from the National Institutes of Health. Dr. Marrazzo leads a clinical trial of the MenB-4C vaccine sponsored by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
A widely approved vaccine for meningitis may provide up to 40% protection against gonorrhea in young adults and adolescents, according to new research. This moderate efficacy paired with a targeted risk-based approach could reduce cases as well as lead to health care savings over 10 years, an additional modeling study showed.
The results – in three linked papers – were published in The Lancet Infectious Diseases.
Gonorrhea, caused by the bacterium Neisseria gonorrhoeae, is the second most commonly reported sexually transmitted infection in the United States, according to the Centers for Disease Control and Prevention. Globally, the World Health Organization estimates that there were 82.4 million new cases in people aged 15-49 in 2020. At the same time, it is becoming more difficult to treat the infection because of the increasing prevalence of drug-resistant strains of N. gonorrhoeae.
“New approaches, such as vaccination, are needed as long-term strategies to prevent gonorrhea and address the emerging threat of antimicrobial resistance,” Winston Abara, MD, PhD, Division of STD Prevention, Centers for Disease Control and Prevention, and colleagues wrote.
While there is currently no vaccine for gonorrhea, observational studies have found an association between a meningococcal serogroup B vaccine and reduced gonorrhea cases. One study in New Zealand found that people vaccinated with the MeNZB vaccine, which was produced to control an outbreak of meningococcal disease in the country, were 31% less likely to contract gonorrhea.
This cross-reactivity comes about because Neisseria meningitidis, the bacterium that can cause meningitis, is closely related to N. gonorrhoeae, Joseph Alex Duncan, MD, PhD, associate professor of medicine, Division of Infectious Diseases, University of North Carolina School of Medicine, Chapel Hill, said in an interview. He was not involved with the research. The thought is that “a large proportion of the proteins that are in the vaccine also recognize proteins from Neisseria gonorrhea, because the bacteria are so similar at the genetic level,” he said.
To see if this association was still found for the four-component serogroup B meningococcal vaccine (MenB-4C), which is now widely available, Dr. Abara and colleagues looked through health records to identify laboratory-confirmed gonorrhea and chlamydia infections in adolescents and young adults in New York City and Philadelphia. All individuals included in the analysis were age 16-23 and all infections occurred between Jan. 1, 2016, and Dec. 31, 2018. These infections were then linked to vaccination records to determine individuals’ MenB-4C vaccination status. Complete vaccination was defined as two MenB-4C doses, delivered 30-180 days apart.
The research team identified over 167,700 infections, including 18,099 gonococcal infections, 124,876 chlamydial infections, and 24,731 coinfections, among 109,737 individuals. A total of 7,692 individuals had received at least one shot of the vaccine, and 3,660 people were fully vaccinated. Full MenB-4C vaccination was estimated to be 40% protective (APR 0.60; P < .0001) against gonorrhea, and partial vaccination was 26% protective (APR 0.74; P = .0012).
“The findings of our study add to the body of evidence that demonstrates that the MenB-4C may offer cross-protection against Neisseria gonorrhoeae, and it supports feasibility of an effective gonococcal vaccine with implications for gonorrhea prevention and control,” Dr. Abara told this news organization.
A second study conducted in South Australia looked at the effectiveness of the MenB-4C vaccine against meningitis and gonorrhea as part of a vaccination program. Using infection data from the Government of South Australia and vaccination records from the Australian Immunization Register, researchers identified individuals born between Feb. 1, 1998, and Feb. 1, 2005, with a documented gonorrhea or chlamydia infection between Feb. 1, 2019, and Jan. 31, 2021. Individuals with chlamydia served as the controls to account for similar sexual behavioral risks.
The analysis included 512 individuals with 575 cases of gonorrhea and 3,140 individuals with 3,847 episodes of chlamydia. In this group, the estimated vaccine effectiveness against gonorrhea was 32.7% (95% confidence interval, 8.3-50.6) in individuals who were fully vaccinated and 32.6% (95% CI, 10.6-49.1) in those who had received at least one dose of the MenB-4C.
While these findings are “confirmatory” because they showed results similar to those in previous observational studies, they are still exciting, Dr. Duncan said. “Up until now, we really haven’t had any real progress in knowing what type of immune responses could actually be protective from the disease,” he said. “These observational studies have really reinvigorated the Neisseria gonorrhea vaccine research community.”
A vaccine with moderate efficacy – like the protection demonstrated in both studies – could lead to a significant reduction in cases, he noted. A 2015 Australian modeling study estimated that a nonwaning vaccine with 20% efficacy could reduce cases by 40% over 20 years. Focusing on vaccinating higher-risk groups could also have an “outsize impact,” said Jeanne Marrazzo, MD, director, Division of Infectious Diseases, UAB Medicine, Birmingham, Alabama, in an interview. In the third study published in The Lancet, researchers estimated the possible reduction of cases and the potential health care cost savings in England in a vaccination effort focusing on men who have sex with men (MSM) at high risk for gonorrhea infection. They predicted that a vaccine with 31% efficacy could prevent 110,200 cases in MSM and save about £8 million ($10.4 million) over 10 years.
Both Dr. Duncan and Dr. Marrazzo agreed that clinical trials are needed to tease out whether the decrease in gonorrhea cases is due to the MenB-4C vaccine or the association is incidental. There are two ongoing clinical trials, one in Australia and one in the United States. Dr. Marrazzo leads the U.S. multicenter study, which also has two locations in Bangkok. The trial will also look at whether vaccination protection varies by the location of gonococcal infection: urethra, rectum, cervix, or pharynx. The two new observational studies did not distinguish the different sites of infection.
Dr. Marrazzo’s trial has enrolled almost 500 individuals so far, with the goal of enrolling over 2,000 participants in total. She hopes to see results by late 2023. “It’s a pretty ambitious effort, but I’m hoping it will give us not only a definitive answer in terms of reduction in infection by anatomic site,” she said, but “also give us a lot of information about how the immune response works to protect you from getting gonorrhea if you do get the vaccine.”
Dr. Duncan has received research grants from the National Institutes of Health. Dr. Marrazzo leads a clinical trial of the MenB-4C vaccine sponsored by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
A widely approved vaccine for meningitis may provide up to 40% protection against gonorrhea in young adults and adolescents, according to new research. This moderate efficacy paired with a targeted risk-based approach could reduce cases as well as lead to health care savings over 10 years, an additional modeling study showed.
The results – in three linked papers – were published in The Lancet Infectious Diseases.
Gonorrhea, caused by the bacterium Neisseria gonorrhoeae, is the second most commonly reported sexually transmitted infection in the United States, according to the Centers for Disease Control and Prevention. Globally, the World Health Organization estimates that there were 82.4 million new cases in people aged 15-49 in 2020. At the same time, it is becoming more difficult to treat the infection because of the increasing prevalence of drug-resistant strains of N. gonorrhoeae.
“New approaches, such as vaccination, are needed as long-term strategies to prevent gonorrhea and address the emerging threat of antimicrobial resistance,” Winston Abara, MD, PhD, Division of STD Prevention, Centers for Disease Control and Prevention, and colleagues wrote.
While there is currently no vaccine for gonorrhea, observational studies have found an association between a meningococcal serogroup B vaccine and reduced gonorrhea cases. One study in New Zealand found that people vaccinated with the MeNZB vaccine, which was produced to control an outbreak of meningococcal disease in the country, were 31% less likely to contract gonorrhea.
This cross-reactivity comes about because Neisseria meningitidis, the bacterium that can cause meningitis, is closely related to N. gonorrhoeae, Joseph Alex Duncan, MD, PhD, associate professor of medicine, Division of Infectious Diseases, University of North Carolina School of Medicine, Chapel Hill, said in an interview. He was not involved with the research. The thought is that “a large proportion of the proteins that are in the vaccine also recognize proteins from Neisseria gonorrhea, because the bacteria are so similar at the genetic level,” he said.
To see if this association was still found for the four-component serogroup B meningococcal vaccine (MenB-4C), which is now widely available, Dr. Abara and colleagues looked through health records to identify laboratory-confirmed gonorrhea and chlamydia infections in adolescents and young adults in New York City and Philadelphia. All individuals included in the analysis were age 16-23 and all infections occurred between Jan. 1, 2016, and Dec. 31, 2018. These infections were then linked to vaccination records to determine individuals’ MenB-4C vaccination status. Complete vaccination was defined as two MenB-4C doses, delivered 30-180 days apart.
The research team identified over 167,700 infections, including 18,099 gonococcal infections, 124,876 chlamydial infections, and 24,731 coinfections, among 109,737 individuals. A total of 7,692 individuals had received at least one shot of the vaccine, and 3,660 people were fully vaccinated. Full MenB-4C vaccination was estimated to be 40% protective (APR 0.60; P < .0001) against gonorrhea, and partial vaccination was 26% protective (APR 0.74; P = .0012).
“The findings of our study add to the body of evidence that demonstrates that the MenB-4C may offer cross-protection against Neisseria gonorrhoeae, and it supports feasibility of an effective gonococcal vaccine with implications for gonorrhea prevention and control,” Dr. Abara told this news organization.
A second study conducted in South Australia looked at the effectiveness of the MenB-4C vaccine against meningitis and gonorrhea as part of a vaccination program. Using infection data from the Government of South Australia and vaccination records from the Australian Immunization Register, researchers identified individuals born between Feb. 1, 1998, and Feb. 1, 2005, with a documented gonorrhea or chlamydia infection between Feb. 1, 2019, and Jan. 31, 2021. Individuals with chlamydia served as the controls to account for similar sexual behavioral risks.
The analysis included 512 individuals with 575 cases of gonorrhea and 3,140 individuals with 3,847 episodes of chlamydia. In this group, the estimated vaccine effectiveness against gonorrhea was 32.7% (95% confidence interval, 8.3-50.6) in individuals who were fully vaccinated and 32.6% (95% CI, 10.6-49.1) in those who had received at least one dose of the MenB-4C.
While these findings are “confirmatory” because they showed results similar to those in previous observational studies, they are still exciting, Dr. Duncan said. “Up until now, we really haven’t had any real progress in knowing what type of immune responses could actually be protective from the disease,” he said. “These observational studies have really reinvigorated the Neisseria gonorrhea vaccine research community.”
A vaccine with moderate efficacy – like the protection demonstrated in both studies – could lead to a significant reduction in cases, he noted. A 2015 Australian modeling study estimated that a nonwaning vaccine with 20% efficacy could reduce cases by 40% over 20 years. Focusing on vaccinating higher-risk groups could also have an “outsize impact,” said Jeanne Marrazzo, MD, director, Division of Infectious Diseases, UAB Medicine, Birmingham, Alabama, in an interview. In the third study published in The Lancet, researchers estimated the possible reduction of cases and the potential health care cost savings in England in a vaccination effort focusing on men who have sex with men (MSM) at high risk for gonorrhea infection. They predicted that a vaccine with 31% efficacy could prevent 110,200 cases in MSM and save about £8 million ($10.4 million) over 10 years.
Both Dr. Duncan and Dr. Marrazzo agreed that clinical trials are needed to tease out whether the decrease in gonorrhea cases is due to the MenB-4C vaccine or the association is incidental. There are two ongoing clinical trials, one in Australia and one in the United States. Dr. Marrazzo leads the U.S. multicenter study, which also has two locations in Bangkok. The trial will also look at whether vaccination protection varies by the location of gonococcal infection: urethra, rectum, cervix, or pharynx. The two new observational studies did not distinguish the different sites of infection.
Dr. Marrazzo’s trial has enrolled almost 500 individuals so far, with the goal of enrolling over 2,000 participants in total. She hopes to see results by late 2023. “It’s a pretty ambitious effort, but I’m hoping it will give us not only a definitive answer in terms of reduction in infection by anatomic site,” she said, but “also give us a lot of information about how the immune response works to protect you from getting gonorrhea if you do get the vaccine.”
Dr. Duncan has received research grants from the National Institutes of Health. Dr. Marrazzo leads a clinical trial of the MenB-4C vaccine sponsored by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
FROM THE LANCET INFECTIOUS DISEASES
Children with RMDs not at high risk for severe COVID-19, study finds
The of short-term COVID-19 outcomes in this patient group to date.
In the study, only 1 in 15 (7%) children and young people (younger than 19 years) with RMDs and COVID-19 were hospitalized, and even then, they experienced only mild symptoms; 4 of 5 of those hospitalized did not require supplemental oxygen or ventilatory support.
The study also found that those with severe systemic RMDs and obesity were more likely to be hospitalized than children with juvenile idiopathic arthritis (JIA).
Treatment with biologics, such as tumor necrosis factor inhibitors, did not appear to be associated with more severe COVID-19; however, the study found that children and young people with obesity (body mass index ≥ 30) were more likely to be hospitalized, although only 6% of patients in this study had a BMI in this category. Three patients died – two from areas of lower resources who were diagnosed with systemic lupus erythematosus (SLE) at approximately the same time they were diagnosed with COVID-19, and one with a preexisting autoinflammatory syndrome who was being treated with low-dose glucocorticoids and methotrexate.
Published in Annals of the Rheumatic Diseases, the study was led by Kimme L. Hyrich, MD, PhD, and Lianne Kearsley-Fleet, PhD, both from the University of Manchester (England). Dr. Hyrich is also a consultant rheumatologist at Manchester University Hospitals NHS Foundation Trust.
In an interview, Dr. Hyrich explained that overall these data are reassuring and show that the majority of children and young people with RMDs are not at high risk of severe COVID-19.
“Many parents and families with children who have RMDs have lived with great fear over the pandemic about whether or not their children are at an increased risk of severe COVID-19,” said Dr. Hyrich. “Many are immunosuppressed or take other immunomodulatory medications. This has also had a great impact on schooling and children’s well-being.”
In the study, children with SLE, mixed connective tissue disease (MCTD), or vasculitis were more likely to have severe COVID-19. “[This] is not surprising given the typically greater systemic involvement and need for more aggressive immunosuppressive therapy than the majority of individuals with JIA,” the researchers wrote.
Dr. Hyrich added: “There may be times when children are on particularly high doses of immunosuppression or their disease is particularly active, when they may need more protection, and rheumatology teams can advise parents and young people about this.”
Studies such as those by Zimmerman and Curtis and Viner and colleagues have found that generally, children with no underlying disease are less susceptible to symptomatic COVID-19 and that reports of death are rare. Findings show that the younger the child, the less likely they will be symptomatic.
Adult data suggest a higher risk of COVID-related death among patients with arthritis, lupus, or psoriasis. A recent systematic review of the literature suggested that increased risk of COVID-related death only applies to subgroups of people with RMDs.
However, whether children and young people with RMDs are likely to have more severe COVID-19 and whether there is additional risk attributable to either their underlying disease or its therapy remain unknown. The goal of the study by Dr. Hyrich and colleagues was to address these questions.
The global analysis aimed to describe characteristics of those children and young people (younger than 19 years) with preexisting RMDs who also had COVID-19; to describe outcomes following COVID-19; and to identify characteristics associated with more severe COVID-19 outcomes.
Data were drawn from the European Alliance of Associations for Rheumatology COVID-19 Registry, the Childhood Arthritis and Rheumatology Research Alliance Registry, and the CARRA-sponsored COVID-19 Global Paediatric Rheumatology Database.
Demographic information included primary RMD diagnosis; RMD disease activity (remission, low, moderate, high, or unknown); RMD treatments, including glucocorticoid use and which disease-modifying antirheumatic drug (DMARD) the patient was taking at the time of COVID-19; and comorbidities (none, ocular inflammation, interstitial lung disease, asthma, diabetes, obesity, hypertension, cerebrovascular accident, renal disease, inflammatory bowel disease, and heart disease).
With respect to COVID-19, information collected included diagnosis date, whether the case was presumptive or confirmed, clinical symptoms, hospitalization and/or death because of COVID-19, and whether the patient stopped receiving rheumatic therapies.
Rheumatology diagnoses were categorized into four groups: JIA; SLE, MCTD, vasculitis, or other RMD; autoinflammatory syndromes; and “other,” including chronic recurrent multifocal osteomyelitis, sarcoidosis, or ocular inflammation.
Of the 607 children and young people with reported SARS-CoV-2 infection from 25 different countries (464 from the EULAR COVID-19 Registry), 499 (82%) cases were polymerase chain reaction confirmed, and 399 (66%) patients were female (median age, 14 years). Most (62%) had JIA: 37%, polyarticular JIA; 30%, oligoarticular JIA; 12%, enthesitis-related JIA; 9%, systemic JIA; 4%, psoriatic JIA; and 9%, JIA of unknown subcategory. Furthermore, 13% of patients had autoinflammatory syndromes, 8% with SLE or MCTD, 3% with vasculitis, and 2% with inflammatory myopathy.
No associations were seen between DMARD treatment (conventional-synthetic, biologic/targeted-synthetic, or combination therapy), compared with no DMARD treatment, glucocorticoid use, and hospitalization.
Owing to substantial differences in reporting of race and ethnicity between data sources, the researchers were unable to analyze whether Black, Asian, and minority ethnic groups with pediatric RMDs are at higher risk of COVID-19–related death, compared with those of White ethnicity, as has been reported for the general population.
The study also did not account for variants of SARS-CoV-2 other than to note that data were collected prior to the spread of the Omicron variant. Also, the registries did not capture vaccination status (though very few children had received vaccines at the time of data collection) or information on long COVID or multisystem inflammatory syndrome in children.
Dr. Hyrich and Dr. Kearsley-Fleet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The of short-term COVID-19 outcomes in this patient group to date.
In the study, only 1 in 15 (7%) children and young people (younger than 19 years) with RMDs and COVID-19 were hospitalized, and even then, they experienced only mild symptoms; 4 of 5 of those hospitalized did not require supplemental oxygen or ventilatory support.
The study also found that those with severe systemic RMDs and obesity were more likely to be hospitalized than children with juvenile idiopathic arthritis (JIA).
Treatment with biologics, such as tumor necrosis factor inhibitors, did not appear to be associated with more severe COVID-19; however, the study found that children and young people with obesity (body mass index ≥ 30) were more likely to be hospitalized, although only 6% of patients in this study had a BMI in this category. Three patients died – two from areas of lower resources who were diagnosed with systemic lupus erythematosus (SLE) at approximately the same time they were diagnosed with COVID-19, and one with a preexisting autoinflammatory syndrome who was being treated with low-dose glucocorticoids and methotrexate.
Published in Annals of the Rheumatic Diseases, the study was led by Kimme L. Hyrich, MD, PhD, and Lianne Kearsley-Fleet, PhD, both from the University of Manchester (England). Dr. Hyrich is also a consultant rheumatologist at Manchester University Hospitals NHS Foundation Trust.
In an interview, Dr. Hyrich explained that overall these data are reassuring and show that the majority of children and young people with RMDs are not at high risk of severe COVID-19.
“Many parents and families with children who have RMDs have lived with great fear over the pandemic about whether or not their children are at an increased risk of severe COVID-19,” said Dr. Hyrich. “Many are immunosuppressed or take other immunomodulatory medications. This has also had a great impact on schooling and children’s well-being.”
In the study, children with SLE, mixed connective tissue disease (MCTD), or vasculitis were more likely to have severe COVID-19. “[This] is not surprising given the typically greater systemic involvement and need for more aggressive immunosuppressive therapy than the majority of individuals with JIA,” the researchers wrote.
Dr. Hyrich added: “There may be times when children are on particularly high doses of immunosuppression or their disease is particularly active, when they may need more protection, and rheumatology teams can advise parents and young people about this.”
Studies such as those by Zimmerman and Curtis and Viner and colleagues have found that generally, children with no underlying disease are less susceptible to symptomatic COVID-19 and that reports of death are rare. Findings show that the younger the child, the less likely they will be symptomatic.
Adult data suggest a higher risk of COVID-related death among patients with arthritis, lupus, or psoriasis. A recent systematic review of the literature suggested that increased risk of COVID-related death only applies to subgroups of people with RMDs.
However, whether children and young people with RMDs are likely to have more severe COVID-19 and whether there is additional risk attributable to either their underlying disease or its therapy remain unknown. The goal of the study by Dr. Hyrich and colleagues was to address these questions.
The global analysis aimed to describe characteristics of those children and young people (younger than 19 years) with preexisting RMDs who also had COVID-19; to describe outcomes following COVID-19; and to identify characteristics associated with more severe COVID-19 outcomes.
Data were drawn from the European Alliance of Associations for Rheumatology COVID-19 Registry, the Childhood Arthritis and Rheumatology Research Alliance Registry, and the CARRA-sponsored COVID-19 Global Paediatric Rheumatology Database.
Demographic information included primary RMD diagnosis; RMD disease activity (remission, low, moderate, high, or unknown); RMD treatments, including glucocorticoid use and which disease-modifying antirheumatic drug (DMARD) the patient was taking at the time of COVID-19; and comorbidities (none, ocular inflammation, interstitial lung disease, asthma, diabetes, obesity, hypertension, cerebrovascular accident, renal disease, inflammatory bowel disease, and heart disease).
With respect to COVID-19, information collected included diagnosis date, whether the case was presumptive or confirmed, clinical symptoms, hospitalization and/or death because of COVID-19, and whether the patient stopped receiving rheumatic therapies.
Rheumatology diagnoses were categorized into four groups: JIA; SLE, MCTD, vasculitis, or other RMD; autoinflammatory syndromes; and “other,” including chronic recurrent multifocal osteomyelitis, sarcoidosis, or ocular inflammation.
Of the 607 children and young people with reported SARS-CoV-2 infection from 25 different countries (464 from the EULAR COVID-19 Registry), 499 (82%) cases were polymerase chain reaction confirmed, and 399 (66%) patients were female (median age, 14 years). Most (62%) had JIA: 37%, polyarticular JIA; 30%, oligoarticular JIA; 12%, enthesitis-related JIA; 9%, systemic JIA; 4%, psoriatic JIA; and 9%, JIA of unknown subcategory. Furthermore, 13% of patients had autoinflammatory syndromes, 8% with SLE or MCTD, 3% with vasculitis, and 2% with inflammatory myopathy.
No associations were seen between DMARD treatment (conventional-synthetic, biologic/targeted-synthetic, or combination therapy), compared with no DMARD treatment, glucocorticoid use, and hospitalization.
Owing to substantial differences in reporting of race and ethnicity between data sources, the researchers were unable to analyze whether Black, Asian, and minority ethnic groups with pediatric RMDs are at higher risk of COVID-19–related death, compared with those of White ethnicity, as has been reported for the general population.
The study also did not account for variants of SARS-CoV-2 other than to note that data were collected prior to the spread of the Omicron variant. Also, the registries did not capture vaccination status (though very few children had received vaccines at the time of data collection) or information on long COVID or multisystem inflammatory syndrome in children.
Dr. Hyrich and Dr. Kearsley-Fleet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The of short-term COVID-19 outcomes in this patient group to date.
In the study, only 1 in 15 (7%) children and young people (younger than 19 years) with RMDs and COVID-19 were hospitalized, and even then, they experienced only mild symptoms; 4 of 5 of those hospitalized did not require supplemental oxygen or ventilatory support.
The study also found that those with severe systemic RMDs and obesity were more likely to be hospitalized than children with juvenile idiopathic arthritis (JIA).
Treatment with biologics, such as tumor necrosis factor inhibitors, did not appear to be associated with more severe COVID-19; however, the study found that children and young people with obesity (body mass index ≥ 30) were more likely to be hospitalized, although only 6% of patients in this study had a BMI in this category. Three patients died – two from areas of lower resources who were diagnosed with systemic lupus erythematosus (SLE) at approximately the same time they were diagnosed with COVID-19, and one with a preexisting autoinflammatory syndrome who was being treated with low-dose glucocorticoids and methotrexate.
Published in Annals of the Rheumatic Diseases, the study was led by Kimme L. Hyrich, MD, PhD, and Lianne Kearsley-Fleet, PhD, both from the University of Manchester (England). Dr. Hyrich is also a consultant rheumatologist at Manchester University Hospitals NHS Foundation Trust.
In an interview, Dr. Hyrich explained that overall these data are reassuring and show that the majority of children and young people with RMDs are not at high risk of severe COVID-19.
“Many parents and families with children who have RMDs have lived with great fear over the pandemic about whether or not their children are at an increased risk of severe COVID-19,” said Dr. Hyrich. “Many are immunosuppressed or take other immunomodulatory medications. This has also had a great impact on schooling and children’s well-being.”
In the study, children with SLE, mixed connective tissue disease (MCTD), or vasculitis were more likely to have severe COVID-19. “[This] is not surprising given the typically greater systemic involvement and need for more aggressive immunosuppressive therapy than the majority of individuals with JIA,” the researchers wrote.
Dr. Hyrich added: “There may be times when children are on particularly high doses of immunosuppression or their disease is particularly active, when they may need more protection, and rheumatology teams can advise parents and young people about this.”
Studies such as those by Zimmerman and Curtis and Viner and colleagues have found that generally, children with no underlying disease are less susceptible to symptomatic COVID-19 and that reports of death are rare. Findings show that the younger the child, the less likely they will be symptomatic.
Adult data suggest a higher risk of COVID-related death among patients with arthritis, lupus, or psoriasis. A recent systematic review of the literature suggested that increased risk of COVID-related death only applies to subgroups of people with RMDs.
However, whether children and young people with RMDs are likely to have more severe COVID-19 and whether there is additional risk attributable to either their underlying disease or its therapy remain unknown. The goal of the study by Dr. Hyrich and colleagues was to address these questions.
The global analysis aimed to describe characteristics of those children and young people (younger than 19 years) with preexisting RMDs who also had COVID-19; to describe outcomes following COVID-19; and to identify characteristics associated with more severe COVID-19 outcomes.
Data were drawn from the European Alliance of Associations for Rheumatology COVID-19 Registry, the Childhood Arthritis and Rheumatology Research Alliance Registry, and the CARRA-sponsored COVID-19 Global Paediatric Rheumatology Database.
Demographic information included primary RMD diagnosis; RMD disease activity (remission, low, moderate, high, or unknown); RMD treatments, including glucocorticoid use and which disease-modifying antirheumatic drug (DMARD) the patient was taking at the time of COVID-19; and comorbidities (none, ocular inflammation, interstitial lung disease, asthma, diabetes, obesity, hypertension, cerebrovascular accident, renal disease, inflammatory bowel disease, and heart disease).
With respect to COVID-19, information collected included diagnosis date, whether the case was presumptive or confirmed, clinical symptoms, hospitalization and/or death because of COVID-19, and whether the patient stopped receiving rheumatic therapies.
Rheumatology diagnoses were categorized into four groups: JIA; SLE, MCTD, vasculitis, or other RMD; autoinflammatory syndromes; and “other,” including chronic recurrent multifocal osteomyelitis, sarcoidosis, or ocular inflammation.
Of the 607 children and young people with reported SARS-CoV-2 infection from 25 different countries (464 from the EULAR COVID-19 Registry), 499 (82%) cases were polymerase chain reaction confirmed, and 399 (66%) patients were female (median age, 14 years). Most (62%) had JIA: 37%, polyarticular JIA; 30%, oligoarticular JIA; 12%, enthesitis-related JIA; 9%, systemic JIA; 4%, psoriatic JIA; and 9%, JIA of unknown subcategory. Furthermore, 13% of patients had autoinflammatory syndromes, 8% with SLE or MCTD, 3% with vasculitis, and 2% with inflammatory myopathy.
No associations were seen between DMARD treatment (conventional-synthetic, biologic/targeted-synthetic, or combination therapy), compared with no DMARD treatment, glucocorticoid use, and hospitalization.
Owing to substantial differences in reporting of race and ethnicity between data sources, the researchers were unable to analyze whether Black, Asian, and minority ethnic groups with pediatric RMDs are at higher risk of COVID-19–related death, compared with those of White ethnicity, as has been reported for the general population.
The study also did not account for variants of SARS-CoV-2 other than to note that data were collected prior to the spread of the Omicron variant. Also, the registries did not capture vaccination status (though very few children had received vaccines at the time of data collection) or information on long COVID or multisystem inflammatory syndrome in children.
Dr. Hyrich and Dr. Kearsley-Fleet have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF THE RHEUMATIC DISEASES
52-year-old man • hematemesis • history of cirrhosis • persistent fevers • Dx?
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).
Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.
THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; talbert@uw.edu
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).
Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.
THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; talbert@uw.edu
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).
Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.
THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; talbert@uw.edu
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
Children and COVID: Cases drop again, admission rate up slightly
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.