User login
FDA approves oteseconazole for chronic yeast infections
The Food and Drug Administration has approved oteseconazole capsules (Vivjoa), an azole antifungal agent, for the prevention of recurrent yeast infections in women who are not of reproductive potential.
Oteseconazole inhibits CYP51, an enzyme fungi require to preserve the integrity of their cell walls and to grow properly, according to Mycovia, the drug’s manufacturer. It is the first FDA-approved product for the treatment of recurrent vulvovaginal candidiasis (RVVC).
Recurrent vulvovaginal candidiasis, or chronic yeast infection, affects an estimated 138 million women worldwide annually. The condition is defined as three or more symptomatic acute episodes of yeast infection within a 12-month period. The primary symptoms of RVVC include vaginal itching, burning, irritation, and inflammation. Some patients may also experience abnormal vaginal discharge and pain during sex or urination.
“A medicine with Vivjoa’s sustained efficacy combined with the clinical safety profile has been long needed, as until now, physicians and their patients have had no FDA-approved medications for RVVC,” Stephen Brand, PhD, chief development officer of Mycovia, said in a statement. “We are excited to be the first to offer a medication designed specifically for RVVC, a challenging and chronic condition that is expected to increase in prevalence over the next decade.”
Approval for oteseconazole was based on results of three phase 3 trials involving 875 patients at 232 sites across 11 countries. In the U.S.-only ultraVIOLET trial, 89.7% of women with RVVC who received oteseconazole cleared their initial yeast infection and did not experience a recurrence during the 50-week maintenance period, compared with 57.1% of those who received fluconazole (Diflucan) followed by placebo (P < .001), according to Mycovia.
The most common side effects reported in phase 3 clinical studies were headache (7.4%) and nausea (3.6%), the company said. Patients with a hypersensitivity to oteseconazole should not take the drug, nor should those who are of reproductive potential, pregnant, or lactating.
Mycovia said it plans to launch the drug in the second quarter of 2022.
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved oteseconazole capsules (Vivjoa), an azole antifungal agent, for the prevention of recurrent yeast infections in women who are not of reproductive potential.
Oteseconazole inhibits CYP51, an enzyme fungi require to preserve the integrity of their cell walls and to grow properly, according to Mycovia, the drug’s manufacturer. It is the first FDA-approved product for the treatment of recurrent vulvovaginal candidiasis (RVVC).
Recurrent vulvovaginal candidiasis, or chronic yeast infection, affects an estimated 138 million women worldwide annually. The condition is defined as three or more symptomatic acute episodes of yeast infection within a 12-month period. The primary symptoms of RVVC include vaginal itching, burning, irritation, and inflammation. Some patients may also experience abnormal vaginal discharge and pain during sex or urination.
“A medicine with Vivjoa’s sustained efficacy combined with the clinical safety profile has been long needed, as until now, physicians and their patients have had no FDA-approved medications for RVVC,” Stephen Brand, PhD, chief development officer of Mycovia, said in a statement. “We are excited to be the first to offer a medication designed specifically for RVVC, a challenging and chronic condition that is expected to increase in prevalence over the next decade.”
Approval for oteseconazole was based on results of three phase 3 trials involving 875 patients at 232 sites across 11 countries. In the U.S.-only ultraVIOLET trial, 89.7% of women with RVVC who received oteseconazole cleared their initial yeast infection and did not experience a recurrence during the 50-week maintenance period, compared with 57.1% of those who received fluconazole (Diflucan) followed by placebo (P < .001), according to Mycovia.
The most common side effects reported in phase 3 clinical studies were headache (7.4%) and nausea (3.6%), the company said. Patients with a hypersensitivity to oteseconazole should not take the drug, nor should those who are of reproductive potential, pregnant, or lactating.
Mycovia said it plans to launch the drug in the second quarter of 2022.
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved oteseconazole capsules (Vivjoa), an azole antifungal agent, for the prevention of recurrent yeast infections in women who are not of reproductive potential.
Oteseconazole inhibits CYP51, an enzyme fungi require to preserve the integrity of their cell walls and to grow properly, according to Mycovia, the drug’s manufacturer. It is the first FDA-approved product for the treatment of recurrent vulvovaginal candidiasis (RVVC).
Recurrent vulvovaginal candidiasis, or chronic yeast infection, affects an estimated 138 million women worldwide annually. The condition is defined as three or more symptomatic acute episodes of yeast infection within a 12-month period. The primary symptoms of RVVC include vaginal itching, burning, irritation, and inflammation. Some patients may also experience abnormal vaginal discharge and pain during sex or urination.
“A medicine with Vivjoa’s sustained efficacy combined with the clinical safety profile has been long needed, as until now, physicians and their patients have had no FDA-approved medications for RVVC,” Stephen Brand, PhD, chief development officer of Mycovia, said in a statement. “We are excited to be the first to offer a medication designed specifically for RVVC, a challenging and chronic condition that is expected to increase in prevalence over the next decade.”
Approval for oteseconazole was based on results of three phase 3 trials involving 875 patients at 232 sites across 11 countries. In the U.S.-only ultraVIOLET trial, 89.7% of women with RVVC who received oteseconazole cleared their initial yeast infection and did not experience a recurrence during the 50-week maintenance period, compared with 57.1% of those who received fluconazole (Diflucan) followed by placebo (P < .001), according to Mycovia.
The most common side effects reported in phase 3 clinical studies were headache (7.4%) and nausea (3.6%), the company said. Patients with a hypersensitivity to oteseconazole should not take the drug, nor should those who are of reproductive potential, pregnant, or lactating.
Mycovia said it plans to launch the drug in the second quarter of 2022.
Full prescribing information is available online.
A version of this article first appeared on Medscape.com.
Erythematous Plaque on the Groin and Buttocks
The Diagnosis: Pseudomonas Pyoderma
A skin swab confirmed the presence of a ciprofloxacinsusceptible Pseudomonas aeruginosa strain. Our patient received oral ciprofloxacin 500 mg twice daily for 10 days with remarkable clinical improvement. The remaining skin lesion was successfully treated with more frequent diaper changes and the use of topical corticosteroids and emollients.
The topographical location, cutaneous morphology, clinical context, and sometimes the type of exudate are fundamental for the diagnosis of eruptions in intertriginous areas. Cutaneous Candida infections are common in these locations. They classically present as markedly erythematous plaques that occasionally are erosive, accompanied by satellite papules and pustules.1 Tinea cruris is a dermatophyte infection of the groin, proximal medial thighs, perineum, and buttocks. It usually presents as an erythematous patch that spreads centrifugally with partial central clearing and a slightly elevated, scaly border. Although candidiasis was higher on the differential, it was less likely, as our patient had a concomitant exudate inconsistent with Candida infections. Also, the lack of response to antifungal agents made hypotheses of fungal infections improbable.1
Inverse psoriasis is a variant of psoriasis identified by the development of well-demarcated, nonscaly, shiny plaques on body folds.2 Psoriasis is a chronic disease with several other cutaneous manifestations, such as nail and scalp involvement, as well as erythematous scaly plaques on the extensor surfaces of the limbs. The absence of a history of psoriasis, lack of other cutaneous manifestations, and no response to topical corticosteroids made the diagnosis of inverse psoriasis unlikely in our patient.
Erythrasma is a common superficial cutaneous infection caused by Corynebacterium minutissimum, a grampositive bacillus. It typically presents as an intertriginous eruption characterized by small erythematous to brown patches or thin plaques with fine scaling and sharp borders.3 Erythrasma displays a coral red fluorescence on Wood lamp examination that can be useful in the distinction from other causes of intertrigo.1 Although this examination had not been performed in our patient, the striking exudate made erythrasma less likely, and the culture performed on skin swab material would help to rule out this diagnosis.
Pseudomonas aeruginosa is a gram-negative strict aerobic bacillus of ubiquitous distribution with a preference for humid environments.4,5Pseudomonas aeruginosa infections were first reported in the 19th century by physicians who noticed a peculiar odorous condition that caused a blue-green discoloration on bandages. This coloration explains the species name aeruginosa which is derived from the Latin word for copper rust.4 It comes from several water-soluble pigments produced by this microorganism, the most prevalent of which are pyocyanin and pyoverdine. Pyocyanin has a greenish-blue color and is nonfluorescent, while pyoverdine is green-yellowish and fluoresces under Wood light.5 Other pigments, such as pyorubin and pyomelanin, can be produced by some Pseudomonas strains.4
Pseudomonas aeruginosa has become one of the main pathogens involved in hospital-acquired infections,6 especially in immunocompromised patients.6,7 It is a frequent cause of respiratory infections in patients with cystic fibrosis, as it is present in the airways of up to 70% of these patients in adulthood.7 Also, due to a variety of adaptive mechanisms with the development of resistance to a range of antibiotics, P aeruginosa has become a worldwide public health problem and is involved in several life-threatening nosocomial infections.7,8
Cutaneous P aeruginosa infections range from superficial to deep tissue involvement and can affect both immunocompromised and immunocompetent individuals.9 They are classified as primary when they originate directly from the skin or secondary when they occur in the context of bacteremia. Primary infections mostly are mild and often are seen in healthy individuals; they usually occur by inoculation and predominate in moist areas where skin breakdown is frequent. Secondary infections typically affect immunocompromised individuals and portend a poor prognosis.5,9
Denominated as Pseudomonas pyoderma, the superficial skin infection by P aeruginosa is described as a condition where the epidermis has a moth-eaten appearance with macerated or eroded borders.10 A blue-greenish exudate and a grape juice odor often are present. This infection usually occurs as a complication of several skin conditions such as tinea pedis, eczema, burns, wounds, and ulcers.5,10
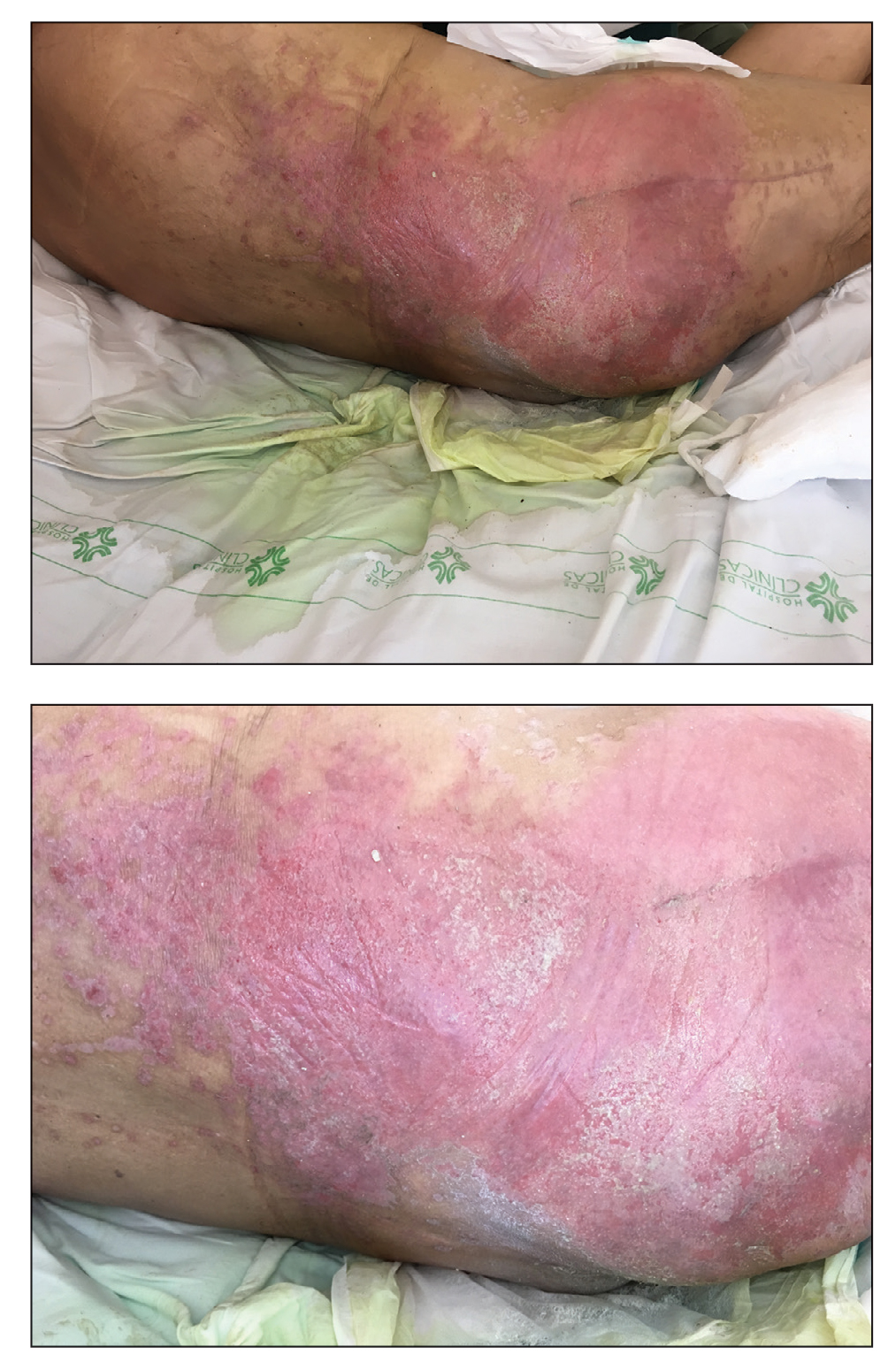
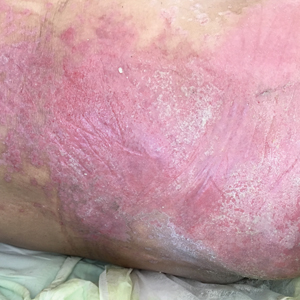
We believe that our patient developed Pseudomonas pyoderma as a complication of diaper dermatitis. His extended hospital stay with the use of different antibiotic regimens for the treatment of several infectious complications may have contributed to the development of infection by P aeruginosa.11 Despite its great clinical relevance, there are few studies in the literature on primary skin infections caused by P aeruginosa, and clinical descriptions with images are rare. Our patient had a nonspecific noneczematous dermatitis, and the projections on the periphery of the lesion resembled the moth-eaten appearance of the classic description of Pseudomonas pyoderma.5,10 The presence of a greenish exudate should promptly raise suspicion for this entity. We believe that the presentation of this case can illustrate this finding and help physicians to recognize this infection.
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Micali G, Verzi AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019; 12:953-959.
- Somerville DA. Erythrasma in normal young adults. J Med Microbiol. 1970;3:57-64.
- D’Agata E. Pseudomonas aeruginosa and other Pseudomonas species. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Vol 2. 8th ed. Elsevier; 2015:2518-2531.
- Silvestre JF, Betlloch MI. Cutaneous manifestations due to Pseudomonas infection. Int J Dermatol. 1999;38:419-431.
- Young LS, Armstrong D. Pseudomonas aeruginosa infections. CRC Crit Rev Clin Lab Sci. 1972;3:291-347.
- Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39.
- Rosenthal VD, Bat-Erdene I, Gupta D, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012-2017: device-associated module. Am J Infect Control. 2020;48:423-432.
- Wu DC, Chan WW, Metelitsa AI, et al. Pseudomonas skin infection: clinical features, epidemiology, and management. Am J Clin Dermatol. 2011;12:157-169.
- Hall JH, Callaway JL, Tindall JP, et al. Pseudomonas aeruginosa in dermatology. Arch Dermatol. 1968;97:312-324.
- Merchant S, Proudfoot EM, Quadri HN, et al. Risk factors for Pseudomonas aeruginosa infections in Asia-Pacific and consequences of inappropriate initial antimicrobial therapy: a systematic literature review and meta-analysis. J Glob Antimicrob Resist. 2018;14:33-44.
The Diagnosis: Pseudomonas Pyoderma
A skin swab confirmed the presence of a ciprofloxacinsusceptible Pseudomonas aeruginosa strain. Our patient received oral ciprofloxacin 500 mg twice daily for 10 days with remarkable clinical improvement. The remaining skin lesion was successfully treated with more frequent diaper changes and the use of topical corticosteroids and emollients.
The topographical location, cutaneous morphology, clinical context, and sometimes the type of exudate are fundamental for the diagnosis of eruptions in intertriginous areas. Cutaneous Candida infections are common in these locations. They classically present as markedly erythematous plaques that occasionally are erosive, accompanied by satellite papules and pustules.1 Tinea cruris is a dermatophyte infection of the groin, proximal medial thighs, perineum, and buttocks. It usually presents as an erythematous patch that spreads centrifugally with partial central clearing and a slightly elevated, scaly border. Although candidiasis was higher on the differential, it was less likely, as our patient had a concomitant exudate inconsistent with Candida infections. Also, the lack of response to antifungal agents made hypotheses of fungal infections improbable.1
Inverse psoriasis is a variant of psoriasis identified by the development of well-demarcated, nonscaly, shiny plaques on body folds.2 Psoriasis is a chronic disease with several other cutaneous manifestations, such as nail and scalp involvement, as well as erythematous scaly plaques on the extensor surfaces of the limbs. The absence of a history of psoriasis, lack of other cutaneous manifestations, and no response to topical corticosteroids made the diagnosis of inverse psoriasis unlikely in our patient.
Erythrasma is a common superficial cutaneous infection caused by Corynebacterium minutissimum, a grampositive bacillus. It typically presents as an intertriginous eruption characterized by small erythematous to brown patches or thin plaques with fine scaling and sharp borders.3 Erythrasma displays a coral red fluorescence on Wood lamp examination that can be useful in the distinction from other causes of intertrigo.1 Although this examination had not been performed in our patient, the striking exudate made erythrasma less likely, and the culture performed on skin swab material would help to rule out this diagnosis.
Pseudomonas aeruginosa is a gram-negative strict aerobic bacillus of ubiquitous distribution with a preference for humid environments.4,5Pseudomonas aeruginosa infections were first reported in the 19th century by physicians who noticed a peculiar odorous condition that caused a blue-green discoloration on bandages. This coloration explains the species name aeruginosa which is derived from the Latin word for copper rust.4 It comes from several water-soluble pigments produced by this microorganism, the most prevalent of which are pyocyanin and pyoverdine. Pyocyanin has a greenish-blue color and is nonfluorescent, while pyoverdine is green-yellowish and fluoresces under Wood light.5 Other pigments, such as pyorubin and pyomelanin, can be produced by some Pseudomonas strains.4
Pseudomonas aeruginosa has become one of the main pathogens involved in hospital-acquired infections,6 especially in immunocompromised patients.6,7 It is a frequent cause of respiratory infections in patients with cystic fibrosis, as it is present in the airways of up to 70% of these patients in adulthood.7 Also, due to a variety of adaptive mechanisms with the development of resistance to a range of antibiotics, P aeruginosa has become a worldwide public health problem and is involved in several life-threatening nosocomial infections.7,8
Cutaneous P aeruginosa infections range from superficial to deep tissue involvement and can affect both immunocompromised and immunocompetent individuals.9 They are classified as primary when they originate directly from the skin or secondary when they occur in the context of bacteremia. Primary infections mostly are mild and often are seen in healthy individuals; they usually occur by inoculation and predominate in moist areas where skin breakdown is frequent. Secondary infections typically affect immunocompromised individuals and portend a poor prognosis.5,9
Denominated as Pseudomonas pyoderma, the superficial skin infection by P aeruginosa is described as a condition where the epidermis has a moth-eaten appearance with macerated or eroded borders.10 A blue-greenish exudate and a grape juice odor often are present. This infection usually occurs as a complication of several skin conditions such as tinea pedis, eczema, burns, wounds, and ulcers.5,10
We believe that our patient developed Pseudomonas pyoderma as a complication of diaper dermatitis. His extended hospital stay with the use of different antibiotic regimens for the treatment of several infectious complications may have contributed to the development of infection by P aeruginosa.11 Despite its great clinical relevance, there are few studies in the literature on primary skin infections caused by P aeruginosa, and clinical descriptions with images are rare. Our patient had a nonspecific noneczematous dermatitis, and the projections on the periphery of the lesion resembled the moth-eaten appearance of the classic description of Pseudomonas pyoderma.5,10 The presence of a greenish exudate should promptly raise suspicion for this entity. We believe that the presentation of this case can illustrate this finding and help physicians to recognize this infection.
The Diagnosis: Pseudomonas Pyoderma
A skin swab confirmed the presence of a ciprofloxacinsusceptible Pseudomonas aeruginosa strain. Our patient received oral ciprofloxacin 500 mg twice daily for 10 days with remarkable clinical improvement. The remaining skin lesion was successfully treated with more frequent diaper changes and the use of topical corticosteroids and emollients.
The topographical location, cutaneous morphology, clinical context, and sometimes the type of exudate are fundamental for the diagnosis of eruptions in intertriginous areas. Cutaneous Candida infections are common in these locations. They classically present as markedly erythematous plaques that occasionally are erosive, accompanied by satellite papules and pustules.1 Tinea cruris is a dermatophyte infection of the groin, proximal medial thighs, perineum, and buttocks. It usually presents as an erythematous patch that spreads centrifugally with partial central clearing and a slightly elevated, scaly border. Although candidiasis was higher on the differential, it was less likely, as our patient had a concomitant exudate inconsistent with Candida infections. Also, the lack of response to antifungal agents made hypotheses of fungal infections improbable.1
Inverse psoriasis is a variant of psoriasis identified by the development of well-demarcated, nonscaly, shiny plaques on body folds.2 Psoriasis is a chronic disease with several other cutaneous manifestations, such as nail and scalp involvement, as well as erythematous scaly plaques on the extensor surfaces of the limbs. The absence of a history of psoriasis, lack of other cutaneous manifestations, and no response to topical corticosteroids made the diagnosis of inverse psoriasis unlikely in our patient.
Erythrasma is a common superficial cutaneous infection caused by Corynebacterium minutissimum, a grampositive bacillus. It typically presents as an intertriginous eruption characterized by small erythematous to brown patches or thin plaques with fine scaling and sharp borders.3 Erythrasma displays a coral red fluorescence on Wood lamp examination that can be useful in the distinction from other causes of intertrigo.1 Although this examination had not been performed in our patient, the striking exudate made erythrasma less likely, and the culture performed on skin swab material would help to rule out this diagnosis.
Pseudomonas aeruginosa is a gram-negative strict aerobic bacillus of ubiquitous distribution with a preference for humid environments.4,5Pseudomonas aeruginosa infections were first reported in the 19th century by physicians who noticed a peculiar odorous condition that caused a blue-green discoloration on bandages. This coloration explains the species name aeruginosa which is derived from the Latin word for copper rust.4 It comes from several water-soluble pigments produced by this microorganism, the most prevalent of which are pyocyanin and pyoverdine. Pyocyanin has a greenish-blue color and is nonfluorescent, while pyoverdine is green-yellowish and fluoresces under Wood light.5 Other pigments, such as pyorubin and pyomelanin, can be produced by some Pseudomonas strains.4
Pseudomonas aeruginosa has become one of the main pathogens involved in hospital-acquired infections,6 especially in immunocompromised patients.6,7 It is a frequent cause of respiratory infections in patients with cystic fibrosis, as it is present in the airways of up to 70% of these patients in adulthood.7 Also, due to a variety of adaptive mechanisms with the development of resistance to a range of antibiotics, P aeruginosa has become a worldwide public health problem and is involved in several life-threatening nosocomial infections.7,8
Cutaneous P aeruginosa infections range from superficial to deep tissue involvement and can affect both immunocompromised and immunocompetent individuals.9 They are classified as primary when they originate directly from the skin or secondary when they occur in the context of bacteremia. Primary infections mostly are mild and often are seen in healthy individuals; they usually occur by inoculation and predominate in moist areas where skin breakdown is frequent. Secondary infections typically affect immunocompromised individuals and portend a poor prognosis.5,9
Denominated as Pseudomonas pyoderma, the superficial skin infection by P aeruginosa is described as a condition where the epidermis has a moth-eaten appearance with macerated or eroded borders.10 A blue-greenish exudate and a grape juice odor often are present. This infection usually occurs as a complication of several skin conditions such as tinea pedis, eczema, burns, wounds, and ulcers.5,10
We believe that our patient developed Pseudomonas pyoderma as a complication of diaper dermatitis. His extended hospital stay with the use of different antibiotic regimens for the treatment of several infectious complications may have contributed to the development of infection by P aeruginosa.11 Despite its great clinical relevance, there are few studies in the literature on primary skin infections caused by P aeruginosa, and clinical descriptions with images are rare. Our patient had a nonspecific noneczematous dermatitis, and the projections on the periphery of the lesion resembled the moth-eaten appearance of the classic description of Pseudomonas pyoderma.5,10 The presence of a greenish exudate should promptly raise suspicion for this entity. We believe that the presentation of this case can illustrate this finding and help physicians to recognize this infection.
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Micali G, Verzi AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019; 12:953-959.
- Somerville DA. Erythrasma in normal young adults. J Med Microbiol. 1970;3:57-64.
- D’Agata E. Pseudomonas aeruginosa and other Pseudomonas species. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Vol 2. 8th ed. Elsevier; 2015:2518-2531.
- Silvestre JF, Betlloch MI. Cutaneous manifestations due to Pseudomonas infection. Int J Dermatol. 1999;38:419-431.
- Young LS, Armstrong D. Pseudomonas aeruginosa infections. CRC Crit Rev Clin Lab Sci. 1972;3:291-347.
- Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39.
- Rosenthal VD, Bat-Erdene I, Gupta D, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012-2017: device-associated module. Am J Infect Control. 2020;48:423-432.
- Wu DC, Chan WW, Metelitsa AI, et al. Pseudomonas skin infection: clinical features, epidemiology, and management. Am J Clin Dermatol. 2011;12:157-169.
- Hall JH, Callaway JL, Tindall JP, et al. Pseudomonas aeruginosa in dermatology. Arch Dermatol. 1968;97:312-324.
- Merchant S, Proudfoot EM, Quadri HN, et al. Risk factors for Pseudomonas aeruginosa infections in Asia-Pacific and consequences of inappropriate initial antimicrobial therapy: a systematic literature review and meta-analysis. J Glob Antimicrob Resist. 2018;14:33-44.
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Micali G, Verzi AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019; 12:953-959.
- Somerville DA. Erythrasma in normal young adults. J Med Microbiol. 1970;3:57-64.
- D’Agata E. Pseudomonas aeruginosa and other Pseudomonas species. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Vol 2. 8th ed. Elsevier; 2015:2518-2531.
- Silvestre JF, Betlloch MI. Cutaneous manifestations due to Pseudomonas infection. Int J Dermatol. 1999;38:419-431.
- Young LS, Armstrong D. Pseudomonas aeruginosa infections. CRC Crit Rev Clin Lab Sci. 1972;3:291-347.
- Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39.
- Rosenthal VD, Bat-Erdene I, Gupta D, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012-2017: device-associated module. Am J Infect Control. 2020;48:423-432.
- Wu DC, Chan WW, Metelitsa AI, et al. Pseudomonas skin infection: clinical features, epidemiology, and management. Am J Clin Dermatol. 2011;12:157-169.
- Hall JH, Callaway JL, Tindall JP, et al. Pseudomonas aeruginosa in dermatology. Arch Dermatol. 1968;97:312-324.
- Merchant S, Proudfoot EM, Quadri HN, et al. Risk factors for Pseudomonas aeruginosa infections in Asia-Pacific and consequences of inappropriate initial antimicrobial therapy: a systematic literature review and meta-analysis. J Glob Antimicrob Resist. 2018;14:33-44.
A 68-year-old man presented with an extensive erythematous plaque of 3 weeks’ duration that started in the groin and spread to the buttocks. It was associated with pruritus and a burning sensation. He was admitted to the palliative care unit 1 year prior for the management of terminal lung cancer. Despite the use of topical corticosteroids and antifungals, the lesions gradually worsened with dissemination to the back. Physical examination revealed an erythematous macerated plaque that extended from the buttocks and groin region to the scapular area (top). Its borders had an eroded appearance with projections compatible with radial spread (bottom). A greenish exudate soaked the diaper and sheets. No other cutaneous lesions were noted.
How do we distinguish between viral and bacterial meningitis?
Bacteria and viruses are the leading causes of community-acquired meningitis. Bacterial meningitis is associated with high morbidity and mortality, and prompt treatment with appropriate antibiotics is essential to optimize outcomes. Early diagnosis is therefore crucial for selecting patients who need antibiotics. On the other hand, the course of viral meningitis is generally benign, and there is usually no specific antimicrobial treatment required. Distinguishing between viral and bacterial causes of meningitis can be challenging; therefore, many patients receive empiric antibiotic treatment.
Etiology
Among the etiologic agents of viral meningitis, the nonpolio enteroviruses (Echovirus 30, 11, 9, 6, 7, 18, 16, 71, 25; Coxsackie B2, A9, B1, B3, B4) are the most common, responsible for more than 85% of cases. Other viruses potentially responsible for meningitis include the herpes simplex virus (HSV), primarily type 2, and flavivirus (such as the Dengue virus).
Clinical presentation
The clinical presentation of bacterial meningitis is more severe than that of viral meningitis. The classic clinical triad of bacterial meningitis consists of fever, neck stiffness, and altered mental status. Only 41% of cases present with these three symptoms, however. Other clinical characteristics include severe headaches, decreased level of consciousness, nausea, vomiting, seizures, focal neurologic signs, and skin rash.
Viral meningitis is usually not associated with a decreased level of consciousness or significant decline in overall health status. The most frequently reported symptoms are unusual headaches, fever, nausea, vomiting, sensitivity to light, and neck stiffness. Patients may also present with skin changes and lymphadenopathy, and, depending on etiology, genital ulcers.
Diagnosis
The diagnosis of bacterial meningitis is based on clinical symptoms, blood panels (blood count, inflammation markers, cultures), and cerebrospinal fluid (CSF) cultures. Gram staining and latex agglutination may lead to false-negative results, and cultures may take a few days to provide a definitive result. Therefore, empiric antibiotic treatment is often started until the etiology can be determined.
A spinal tap must always be performed, preferably after a scan is taken, to rule out the risk of herniation. After CSF samples have been collected, they must undergo complete analysis, including cytological, biochemical, and microbiological evaluation, using conventional and molecular testing methods, when available.
Cytological and biochemical analyses of CSF may be helpful, as findings may indicate a higher probability of either bacterial or viral etiology.
CSF samples collected from patients with acute bacterial meningitis present characteristic neutrophilic pleocytosis (cell count usually ranging from hundreds to a few thousand, with >80% polymorphonuclear cells). In some cases of L. monocytogenes meningitis (from 25% to 30%), a lymphocytic predominance may occur. Normally, glucose is low (CSF glucose-to-blood-glucose ratio of ≤0.4 or <40 mg/dL), protein is very high (>200 mg/dL), and the CSF lactate level is high (≥31.53 mg/dL).
In viral meningitis, the white blood cell count is generally 10-300 cells/mm3. Although glucose levels are normal in most cases, they may be below normal limits in lymphocytic choriomeningitis virus (LCMV), HSV, mumps virus, and poliovirus meningitis. Protein levels tend to be slightly elevated, but they may still be within the reference range.
A recent study investigated which of the cytological or biochemical markers best correlate with the definite etiologic diagnosis. This study, in which CSF samples were collected and analyzed from 2013 to 2017, considered cases of bacterial or viral meningitis confirmed via microbiological evaluation or polymerase chain reaction (PCR). CSF lactate was the best single CSF parameter, and CSF lactate above 30 mg/dL virtually excludes the possibility of a viral etiology.
Etiologic determination
Despite the major contribution of globally analyzing CSF and secondary parameters, particularly CSF lactate, the precise etiologic definition is of great importance in cases of acute meningitis. Such precise definition is not simple, as identification of the causative microorganism is often difficult. Moreover, there are limits to conventional microbiological methods. Bacterioscopy is poorly sensitive, and although bacterial cultures are more sensitive, they can delay diagnosis because of the time it takes for the bacteria to grow in culture media.
Targeted molecular detection methods are usually more sensitive than conventional microbiological methods. Panel-based molecular tests identify multiple pathogens in a single test. In 2015, the U.S. Food and Drug Administration authorized the first commercial multiplex detection system for infectious causes of community-acquired meningitis and encephalitis. This test, the BioFire FilmArray system, detects 14 bacterial, viral, and fungal pathogens in a turnaround time of about 1 hour, including S. pneumoniae, N. meningitidis, H. influenzae, S. agalactiae (i.e., group B Streptococcus), E. coli (serotype K1), L. monocytogenes, HSV-1, HSV-2, varicella-zoster virus (VZV), cytomegalovirus (CMV), human herpesvirus 6 (HHV-6), human parechovirus (HPeV), and Cryptococcus neoformans/gattii.
A meta-analysis of eight precise diagnostic studies evaluating the BioFire FilmArray system showed a high sensitivity of 90% (95% confidence interval, 86%-93%) and specificity of 97% (95% CI, 94%-99%). The FilmArray ME panel can halve the time to microbiological result, allowing for earlier discontinuation of antimicrobial agents and hospital discharge in cases of viral meningitis.
Conclusion
Acute community-acquired meningitis is usually the result of viral or bacterial infections. Given the low specificity of clinical symptoms and, very often, of the general laboratory panel findings, many patients are empirically treated with antibiotics. High-sensitivity and -specificity molecular techniques allow for rapid identification of the bacterial etiology (which requires antibiotic therapy) or the viral etiology of meningitis. The latter can be managed only with symptom-specific medications and does not usually require extended hospitalization. Therefore, these new techniques can improve the quality of care for these patients with viral meningitis.
A version of this article first appeared on Medscape.com.
Bacteria and viruses are the leading causes of community-acquired meningitis. Bacterial meningitis is associated with high morbidity and mortality, and prompt treatment with appropriate antibiotics is essential to optimize outcomes. Early diagnosis is therefore crucial for selecting patients who need antibiotics. On the other hand, the course of viral meningitis is generally benign, and there is usually no specific antimicrobial treatment required. Distinguishing between viral and bacterial causes of meningitis can be challenging; therefore, many patients receive empiric antibiotic treatment.
Etiology
Among the etiologic agents of viral meningitis, the nonpolio enteroviruses (Echovirus 30, 11, 9, 6, 7, 18, 16, 71, 25; Coxsackie B2, A9, B1, B3, B4) are the most common, responsible for more than 85% of cases. Other viruses potentially responsible for meningitis include the herpes simplex virus (HSV), primarily type 2, and flavivirus (such as the Dengue virus).
Clinical presentation
The clinical presentation of bacterial meningitis is more severe than that of viral meningitis. The classic clinical triad of bacterial meningitis consists of fever, neck stiffness, and altered mental status. Only 41% of cases present with these three symptoms, however. Other clinical characteristics include severe headaches, decreased level of consciousness, nausea, vomiting, seizures, focal neurologic signs, and skin rash.
Viral meningitis is usually not associated with a decreased level of consciousness or significant decline in overall health status. The most frequently reported symptoms are unusual headaches, fever, nausea, vomiting, sensitivity to light, and neck stiffness. Patients may also present with skin changes and lymphadenopathy, and, depending on etiology, genital ulcers.
Diagnosis
The diagnosis of bacterial meningitis is based on clinical symptoms, blood panels (blood count, inflammation markers, cultures), and cerebrospinal fluid (CSF) cultures. Gram staining and latex agglutination may lead to false-negative results, and cultures may take a few days to provide a definitive result. Therefore, empiric antibiotic treatment is often started until the etiology can be determined.
A spinal tap must always be performed, preferably after a scan is taken, to rule out the risk of herniation. After CSF samples have been collected, they must undergo complete analysis, including cytological, biochemical, and microbiological evaluation, using conventional and molecular testing methods, when available.
Cytological and biochemical analyses of CSF may be helpful, as findings may indicate a higher probability of either bacterial or viral etiology.
CSF samples collected from patients with acute bacterial meningitis present characteristic neutrophilic pleocytosis (cell count usually ranging from hundreds to a few thousand, with >80% polymorphonuclear cells). In some cases of L. monocytogenes meningitis (from 25% to 30%), a lymphocytic predominance may occur. Normally, glucose is low (CSF glucose-to-blood-glucose ratio of ≤0.4 or <40 mg/dL), protein is very high (>200 mg/dL), and the CSF lactate level is high (≥31.53 mg/dL).
In viral meningitis, the white blood cell count is generally 10-300 cells/mm3. Although glucose levels are normal in most cases, they may be below normal limits in lymphocytic choriomeningitis virus (LCMV), HSV, mumps virus, and poliovirus meningitis. Protein levels tend to be slightly elevated, but they may still be within the reference range.
A recent study investigated which of the cytological or biochemical markers best correlate with the definite etiologic diagnosis. This study, in which CSF samples were collected and analyzed from 2013 to 2017, considered cases of bacterial or viral meningitis confirmed via microbiological evaluation or polymerase chain reaction (PCR). CSF lactate was the best single CSF parameter, and CSF lactate above 30 mg/dL virtually excludes the possibility of a viral etiology.
Etiologic determination
Despite the major contribution of globally analyzing CSF and secondary parameters, particularly CSF lactate, the precise etiologic definition is of great importance in cases of acute meningitis. Such precise definition is not simple, as identification of the causative microorganism is often difficult. Moreover, there are limits to conventional microbiological methods. Bacterioscopy is poorly sensitive, and although bacterial cultures are more sensitive, they can delay diagnosis because of the time it takes for the bacteria to grow in culture media.
Targeted molecular detection methods are usually more sensitive than conventional microbiological methods. Panel-based molecular tests identify multiple pathogens in a single test. In 2015, the U.S. Food and Drug Administration authorized the first commercial multiplex detection system for infectious causes of community-acquired meningitis and encephalitis. This test, the BioFire FilmArray system, detects 14 bacterial, viral, and fungal pathogens in a turnaround time of about 1 hour, including S. pneumoniae, N. meningitidis, H. influenzae, S. agalactiae (i.e., group B Streptococcus), E. coli (serotype K1), L. monocytogenes, HSV-1, HSV-2, varicella-zoster virus (VZV), cytomegalovirus (CMV), human herpesvirus 6 (HHV-6), human parechovirus (HPeV), and Cryptococcus neoformans/gattii.
A meta-analysis of eight precise diagnostic studies evaluating the BioFire FilmArray system showed a high sensitivity of 90% (95% confidence interval, 86%-93%) and specificity of 97% (95% CI, 94%-99%). The FilmArray ME panel can halve the time to microbiological result, allowing for earlier discontinuation of antimicrobial agents and hospital discharge in cases of viral meningitis.
Conclusion
Acute community-acquired meningitis is usually the result of viral or bacterial infections. Given the low specificity of clinical symptoms and, very often, of the general laboratory panel findings, many patients are empirically treated with antibiotics. High-sensitivity and -specificity molecular techniques allow for rapid identification of the bacterial etiology (which requires antibiotic therapy) or the viral etiology of meningitis. The latter can be managed only with symptom-specific medications and does not usually require extended hospitalization. Therefore, these new techniques can improve the quality of care for these patients with viral meningitis.
A version of this article first appeared on Medscape.com.
Bacteria and viruses are the leading causes of community-acquired meningitis. Bacterial meningitis is associated with high morbidity and mortality, and prompt treatment with appropriate antibiotics is essential to optimize outcomes. Early diagnosis is therefore crucial for selecting patients who need antibiotics. On the other hand, the course of viral meningitis is generally benign, and there is usually no specific antimicrobial treatment required. Distinguishing between viral and bacterial causes of meningitis can be challenging; therefore, many patients receive empiric antibiotic treatment.
Etiology
Among the etiologic agents of viral meningitis, the nonpolio enteroviruses (Echovirus 30, 11, 9, 6, 7, 18, 16, 71, 25; Coxsackie B2, A9, B1, B3, B4) are the most common, responsible for more than 85% of cases. Other viruses potentially responsible for meningitis include the herpes simplex virus (HSV), primarily type 2, and flavivirus (such as the Dengue virus).
Clinical presentation
The clinical presentation of bacterial meningitis is more severe than that of viral meningitis. The classic clinical triad of bacterial meningitis consists of fever, neck stiffness, and altered mental status. Only 41% of cases present with these three symptoms, however. Other clinical characteristics include severe headaches, decreased level of consciousness, nausea, vomiting, seizures, focal neurologic signs, and skin rash.
Viral meningitis is usually not associated with a decreased level of consciousness or significant decline in overall health status. The most frequently reported symptoms are unusual headaches, fever, nausea, vomiting, sensitivity to light, and neck stiffness. Patients may also present with skin changes and lymphadenopathy, and, depending on etiology, genital ulcers.
Diagnosis
The diagnosis of bacterial meningitis is based on clinical symptoms, blood panels (blood count, inflammation markers, cultures), and cerebrospinal fluid (CSF) cultures. Gram staining and latex agglutination may lead to false-negative results, and cultures may take a few days to provide a definitive result. Therefore, empiric antibiotic treatment is often started until the etiology can be determined.
A spinal tap must always be performed, preferably after a scan is taken, to rule out the risk of herniation. After CSF samples have been collected, they must undergo complete analysis, including cytological, biochemical, and microbiological evaluation, using conventional and molecular testing methods, when available.
Cytological and biochemical analyses of CSF may be helpful, as findings may indicate a higher probability of either bacterial or viral etiology.
CSF samples collected from patients with acute bacterial meningitis present characteristic neutrophilic pleocytosis (cell count usually ranging from hundreds to a few thousand, with >80% polymorphonuclear cells). In some cases of L. monocytogenes meningitis (from 25% to 30%), a lymphocytic predominance may occur. Normally, glucose is low (CSF glucose-to-blood-glucose ratio of ≤0.4 or <40 mg/dL), protein is very high (>200 mg/dL), and the CSF lactate level is high (≥31.53 mg/dL).
In viral meningitis, the white blood cell count is generally 10-300 cells/mm3. Although glucose levels are normal in most cases, they may be below normal limits in lymphocytic choriomeningitis virus (LCMV), HSV, mumps virus, and poliovirus meningitis. Protein levels tend to be slightly elevated, but they may still be within the reference range.
A recent study investigated which of the cytological or biochemical markers best correlate with the definite etiologic diagnosis. This study, in which CSF samples were collected and analyzed from 2013 to 2017, considered cases of bacterial or viral meningitis confirmed via microbiological evaluation or polymerase chain reaction (PCR). CSF lactate was the best single CSF parameter, and CSF lactate above 30 mg/dL virtually excludes the possibility of a viral etiology.
Etiologic determination
Despite the major contribution of globally analyzing CSF and secondary parameters, particularly CSF lactate, the precise etiologic definition is of great importance in cases of acute meningitis. Such precise definition is not simple, as identification of the causative microorganism is often difficult. Moreover, there are limits to conventional microbiological methods. Bacterioscopy is poorly sensitive, and although bacterial cultures are more sensitive, they can delay diagnosis because of the time it takes for the bacteria to grow in culture media.
Targeted molecular detection methods are usually more sensitive than conventional microbiological methods. Panel-based molecular tests identify multiple pathogens in a single test. In 2015, the U.S. Food and Drug Administration authorized the first commercial multiplex detection system for infectious causes of community-acquired meningitis and encephalitis. This test, the BioFire FilmArray system, detects 14 bacterial, viral, and fungal pathogens in a turnaround time of about 1 hour, including S. pneumoniae, N. meningitidis, H. influenzae, S. agalactiae (i.e., group B Streptococcus), E. coli (serotype K1), L. monocytogenes, HSV-1, HSV-2, varicella-zoster virus (VZV), cytomegalovirus (CMV), human herpesvirus 6 (HHV-6), human parechovirus (HPeV), and Cryptococcus neoformans/gattii.
A meta-analysis of eight precise diagnostic studies evaluating the BioFire FilmArray system showed a high sensitivity of 90% (95% confidence interval, 86%-93%) and specificity of 97% (95% CI, 94%-99%). The FilmArray ME panel can halve the time to microbiological result, allowing for earlier discontinuation of antimicrobial agents and hospital discharge in cases of viral meningitis.
Conclusion
Acute community-acquired meningitis is usually the result of viral or bacterial infections. Given the low specificity of clinical symptoms and, very often, of the general laboratory panel findings, many patients are empirically treated with antibiotics. High-sensitivity and -specificity molecular techniques allow for rapid identification of the bacterial etiology (which requires antibiotic therapy) or the viral etiology of meningitis. The latter can be managed only with symptom-specific medications and does not usually require extended hospitalization. Therefore, these new techniques can improve the quality of care for these patients with viral meningitis.
A version of this article first appeared on Medscape.com.
Children and COVID: Weekly cases rise again, but more slowly
New cases of COVID-19 in U.S. children went up for a second consecutive week, but the pace of increase slowed considerably, based on a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week’s count – about 33,000 new COVID cases for April 8-14 – was almost 30% higher than the week before and marked the first rise in incidence after 11 straight weeks of declines, the AAP and CHA said in their weekly COVID-19 report, which is based on data from state and territorial health departments.
The cumulative number of child COVID-19 cases since the start of the pandemic is now over 12.9 million, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention, which uses a different age range for children (0-17 years) than many states, reports corresponding figures of 12.4 million and 17.6%, along with 1,501 deaths.
ED visits show a similar rising trend over recent weeks, as the 7-day average of ED visits with confirmed COVID has crept up from 0.5% in late March/early April to 0.8% on April 22 for children aged 0-11 years, from 0.3% for 0.5% for those aged 12-15, and from 0.3% to 0.6% for 16- and 17-year-olds, based on CDC data.
The daily rate for new admissions for children with confirmed COVID has also moved up slightly, rising from 0.13 per 100,000 population as late as April 13 to 0.15 per 100,000 on April 23. For the number of actual admissions, the latest 7-day (April 17-23) average was 107 in children aged 0-17, compared with 102 for the week of April 10-16, the CDC reported.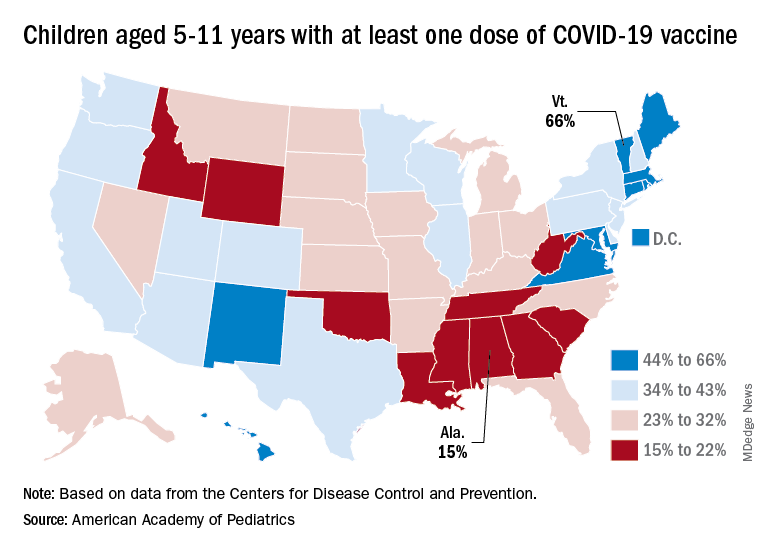
Uptake of the COVID vaccine, however, continued to slide since spiking in January. Initial vaccinations for the latest available week (April 14-20) were down to 48,000 from 59,000 the week before in children aged 5-11 years and 35,000 (vs. 47,000) for those aged 12-17. The weekly highs hit 500,000 and 331,000, respectively, during the Omicron surge, the AAP reported based on CDC data.
Among children aged 5-11, the CDC said that 35.0% had received at least one dose of COVID vaccine as of April 25 and that 28.3% are fully vaccinated, with corresponding figures of 68.8% and 58.8% for 12- to 17-year-olds on April 25.
Among the states, the highest vaccination rates generally are found in New England and the lowest in the Southeast. In Alabama, just 15% of children aged 5-11 have received an initial dose of the vaccine, compared with 66% in Vermont, while Wyoming is the lowest (41%) for children aged 12-17 and Massachusetts is the highest (96%), the AAP said in a separate report.
New cases of COVID-19 in U.S. children went up for a second consecutive week, but the pace of increase slowed considerably, based on a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week’s count – about 33,000 new COVID cases for April 8-14 – was almost 30% higher than the week before and marked the first rise in incidence after 11 straight weeks of declines, the AAP and CHA said in their weekly COVID-19 report, which is based on data from state and territorial health departments.
The cumulative number of child COVID-19 cases since the start of the pandemic is now over 12.9 million, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention, which uses a different age range for children (0-17 years) than many states, reports corresponding figures of 12.4 million and 17.6%, along with 1,501 deaths.
ED visits show a similar rising trend over recent weeks, as the 7-day average of ED visits with confirmed COVID has crept up from 0.5% in late March/early April to 0.8% on April 22 for children aged 0-11 years, from 0.3% for 0.5% for those aged 12-15, and from 0.3% to 0.6% for 16- and 17-year-olds, based on CDC data.
The daily rate for new admissions for children with confirmed COVID has also moved up slightly, rising from 0.13 per 100,000 population as late as April 13 to 0.15 per 100,000 on April 23. For the number of actual admissions, the latest 7-day (April 17-23) average was 107 in children aged 0-17, compared with 102 for the week of April 10-16, the CDC reported.
Uptake of the COVID vaccine, however, continued to slide since spiking in January. Initial vaccinations for the latest available week (April 14-20) were down to 48,000 from 59,000 the week before in children aged 5-11 years and 35,000 (vs. 47,000) for those aged 12-17. The weekly highs hit 500,000 and 331,000, respectively, during the Omicron surge, the AAP reported based on CDC data.
Among children aged 5-11, the CDC said that 35.0% had received at least one dose of COVID vaccine as of April 25 and that 28.3% are fully vaccinated, with corresponding figures of 68.8% and 58.8% for 12- to 17-year-olds on April 25.
Among the states, the highest vaccination rates generally are found in New England and the lowest in the Southeast. In Alabama, just 15% of children aged 5-11 have received an initial dose of the vaccine, compared with 66% in Vermont, while Wyoming is the lowest (41%) for children aged 12-17 and Massachusetts is the highest (96%), the AAP said in a separate report.
New cases of COVID-19 in U.S. children went up for a second consecutive week, but the pace of increase slowed considerably, based on a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week’s count – about 33,000 new COVID cases for April 8-14 – was almost 30% higher than the week before and marked the first rise in incidence after 11 straight weeks of declines, the AAP and CHA said in their weekly COVID-19 report, which is based on data from state and territorial health departments.
The cumulative number of child COVID-19 cases since the start of the pandemic is now over 12.9 million, with children representing 19.0% of cases among all ages. The Centers for Disease Control and Prevention, which uses a different age range for children (0-17 years) than many states, reports corresponding figures of 12.4 million and 17.6%, along with 1,501 deaths.
ED visits show a similar rising trend over recent weeks, as the 7-day average of ED visits with confirmed COVID has crept up from 0.5% in late March/early April to 0.8% on April 22 for children aged 0-11 years, from 0.3% for 0.5% for those aged 12-15, and from 0.3% to 0.6% for 16- and 17-year-olds, based on CDC data.
The daily rate for new admissions for children with confirmed COVID has also moved up slightly, rising from 0.13 per 100,000 population as late as April 13 to 0.15 per 100,000 on April 23. For the number of actual admissions, the latest 7-day (April 17-23) average was 107 in children aged 0-17, compared with 102 for the week of April 10-16, the CDC reported.
Uptake of the COVID vaccine, however, continued to slide since spiking in January. Initial vaccinations for the latest available week (April 14-20) were down to 48,000 from 59,000 the week before in children aged 5-11 years and 35,000 (vs. 47,000) for those aged 12-17. The weekly highs hit 500,000 and 331,000, respectively, during the Omicron surge, the AAP reported based on CDC data.
Among children aged 5-11, the CDC said that 35.0% had received at least one dose of COVID vaccine as of April 25 and that 28.3% are fully vaccinated, with corresponding figures of 68.8% and 58.8% for 12- to 17-year-olds on April 25.
Among the states, the highest vaccination rates generally are found in New England and the lowest in the Southeast. In Alabama, just 15% of children aged 5-11 have received an initial dose of the vaccine, compared with 66% in Vermont, while Wyoming is the lowest (41%) for children aged 12-17 and Massachusetts is the highest (96%), the AAP said in a separate report.
Polypharmacy common among patients aged 65 or older with HIV
People aged 65 or older with human immunodeficiency virus (HIV) receive significantly more nonantiretroviral therapy (non-ART) medications, compared with patients with HIV who are between ages 50 and 64, according to a new study.
Moreover, in a sample of more than 900 patients with HIV, about 60% were taking at least one potentially inappropriate medication (PIM).
“Clinicians looking after persons living with HIV need to provide medication reconciliation with prioritization of medications based on the patients’ wishes and patients’ goals and life expectancy,” lead author Jacqueline McMillan, MD, clinical assistant professor of geriatric medicine at the University of Calgary (Alt.) told this news organization.
The findings were published online in the Canadian Journal of General Internal Medicine.
Examining the pill burden
A geriatrician by training and a clinical researcher with an interest in aging in patients with HIV, Dr. McMillan said she began to observe that many older adults with HIV were on polypharmacy. “There are many other things that aging people with HIV experience, such as frailty, falls, cognitive impairment, medication nonadherence, and mortality, but in this study, we focused just on the polypharmacy,” said Dr. McMillan.
Her aim was to see if there was a way to improve the pill burden in these older adults.
“Do they need to be on all of these medications? Is there anything that we were overprescribing that they no longer needed, or possibly not prescribing and undertreating people because they were older? I wanted to have a better sense that the medications we were prescribing were appropriate and that we minimized the pill burden for older adults,” Dr. McMillan said.
Persons with HIV are at a particularly increased risk of polypharmacy and potential drug-drug interactions because they need antiretroviral therapy medications and medications to treat comorbidities.
“Certainly, when the ARTs were first discovered, sometimes that regimen required several pills a day, but as time has gone on and our retrovirals have gotten better, some of those requirements have narrowed down to one-pill-a-day regimens. We are now replacing that pill burden with non-HIV drugs,” said Dr. McMillan.
The researchers obtained medication reconciliation data for 951 persons with HIV aged 50 or older as of Feb. 1, 2020. The study population was receiving HIV care through the Southern Alberta HIV Clinic in Calgary. The researchers defined polypharmacy as taking five or more non-ART drugs. They defined PIMs according to the 2019 Beers criteria.
In their analysis, the researchers compared patients aged 65 or older with patients aged 50-64, as well as patients with shorter (< 10 years) and longer (> 10 years) duration of HIV infection.
PIM use common
The population’s mean age was 59 years, and 82% were men. The mean time since HIV diagnosis was 17.8 years, and the median time was 17 years. Most (80%) of the patients were aged 50-64 years, and 20% were 65 and older.
The researchers collected sociodemographic, clinical, medication, and laboratory data for all patients at each clinical visit.
The mean number of non-ART medications was 6.7 for the population. Patients aged 65 years or older were taking significantly more non-ART medications than patients aged 50-64 (8.4 vs. 6.3; P < .001).
Similarly, those living with HIV for more than 10 years were taking significantly more non-ART medications (mean, 6.9) than those living with HIV for 10 or fewer years (mean 6.1; P = .0168).
In all, almost 60% of patients were taking at least one PIM. The mean number of PIMs per patient was 1.6.
Patients living with diagnosed HIV infection for more than 10 years were at greater risk of PIMs (1.6 PIMs) than those with shorter duration of HIV diagnosis (1.4 PIMs; P = .06).
Dr. McMillan says she hopes her study reminds clinicians to review patients’ medications at each visit and ensure they are neither over- nor underprescribing.
“From my perspective as a geriatrician, I hope that we do more dedicated medication reconciliation to actually make sure we know what people are taking,” she said. She asks patients to bring all their medications to the office so that they can review which ones match their diagnoses.
“I want to do more patient-centered personalized care for older adults, with a focus on people who are frail and who may have a limited life expectancy, so that we don’t have someone with a short life expectancy still taking 15 medications a day,” said Dr. McMillan.
‘Carefully document medications’
“This study identifies potentially inappropriate medication use in a group of older people living with HIV who are particularly vulnerable to it at an earlier age because of their medical complexity or frailty than perhaps healthy older adults,” Adrian Wagg, MD, professor of healthy aging in the department of medicine at the University of Alberta, Edmonton, told this news organization.
The study emphasizes the importance of careful documentation of medications that the patient is taking at every clinical visit, he said.
“Make sure you carefully document medications which are taken whenever you see the individual. Also try to limit the number of prescribers, because we know multiple prescribers are associated with greater likelihood of inappropriate prescribing,” Dr. Wagg said.
The move to wean patients from inappropriate medications is gaining momentum, he added.
“There is a huge movement now around actively deprescribing medications which are either no longer indicated or potentially of little benefit, given remaining life expectancy,” said Dr. Wagg. Drugs such as proton pump inhibitors, hypnotics, unrequired antidepressants, and benzodiazepines are the first targets for elimination, he concluded.
The study was funded by the University of Calgary. Dr. McMillan and Dr. Wagg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People aged 65 or older with human immunodeficiency virus (HIV) receive significantly more nonantiretroviral therapy (non-ART) medications, compared with patients with HIV who are between ages 50 and 64, according to a new study.
Moreover, in a sample of more than 900 patients with HIV, about 60% were taking at least one potentially inappropriate medication (PIM).
“Clinicians looking after persons living with HIV need to provide medication reconciliation with prioritization of medications based on the patients’ wishes and patients’ goals and life expectancy,” lead author Jacqueline McMillan, MD, clinical assistant professor of geriatric medicine at the University of Calgary (Alt.) told this news organization.
The findings were published online in the Canadian Journal of General Internal Medicine.
Examining the pill burden
A geriatrician by training and a clinical researcher with an interest in aging in patients with HIV, Dr. McMillan said she began to observe that many older adults with HIV were on polypharmacy. “There are many other things that aging people with HIV experience, such as frailty, falls, cognitive impairment, medication nonadherence, and mortality, but in this study, we focused just on the polypharmacy,” said Dr. McMillan.
Her aim was to see if there was a way to improve the pill burden in these older adults.
“Do they need to be on all of these medications? Is there anything that we were overprescribing that they no longer needed, or possibly not prescribing and undertreating people because they were older? I wanted to have a better sense that the medications we were prescribing were appropriate and that we minimized the pill burden for older adults,” Dr. McMillan said.
Persons with HIV are at a particularly increased risk of polypharmacy and potential drug-drug interactions because they need antiretroviral therapy medications and medications to treat comorbidities.
“Certainly, when the ARTs were first discovered, sometimes that regimen required several pills a day, but as time has gone on and our retrovirals have gotten better, some of those requirements have narrowed down to one-pill-a-day regimens. We are now replacing that pill burden with non-HIV drugs,” said Dr. McMillan.
The researchers obtained medication reconciliation data for 951 persons with HIV aged 50 or older as of Feb. 1, 2020. The study population was receiving HIV care through the Southern Alberta HIV Clinic in Calgary. The researchers defined polypharmacy as taking five or more non-ART drugs. They defined PIMs according to the 2019 Beers criteria.
In their analysis, the researchers compared patients aged 65 or older with patients aged 50-64, as well as patients with shorter (< 10 years) and longer (> 10 years) duration of HIV infection.
PIM use common
The population’s mean age was 59 years, and 82% were men. The mean time since HIV diagnosis was 17.8 years, and the median time was 17 years. Most (80%) of the patients were aged 50-64 years, and 20% were 65 and older.
The researchers collected sociodemographic, clinical, medication, and laboratory data for all patients at each clinical visit.
The mean number of non-ART medications was 6.7 for the population. Patients aged 65 years or older were taking significantly more non-ART medications than patients aged 50-64 (8.4 vs. 6.3; P < .001).
Similarly, those living with HIV for more than 10 years were taking significantly more non-ART medications (mean, 6.9) than those living with HIV for 10 or fewer years (mean 6.1; P = .0168).
In all, almost 60% of patients were taking at least one PIM. The mean number of PIMs per patient was 1.6.
Patients living with diagnosed HIV infection for more than 10 years were at greater risk of PIMs (1.6 PIMs) than those with shorter duration of HIV diagnosis (1.4 PIMs; P = .06).
Dr. McMillan says she hopes her study reminds clinicians to review patients’ medications at each visit and ensure they are neither over- nor underprescribing.
“From my perspective as a geriatrician, I hope that we do more dedicated medication reconciliation to actually make sure we know what people are taking,” she said. She asks patients to bring all their medications to the office so that they can review which ones match their diagnoses.
“I want to do more patient-centered personalized care for older adults, with a focus on people who are frail and who may have a limited life expectancy, so that we don’t have someone with a short life expectancy still taking 15 medications a day,” said Dr. McMillan.
‘Carefully document medications’
“This study identifies potentially inappropriate medication use in a group of older people living with HIV who are particularly vulnerable to it at an earlier age because of their medical complexity or frailty than perhaps healthy older adults,” Adrian Wagg, MD, professor of healthy aging in the department of medicine at the University of Alberta, Edmonton, told this news organization.
The study emphasizes the importance of careful documentation of medications that the patient is taking at every clinical visit, he said.
“Make sure you carefully document medications which are taken whenever you see the individual. Also try to limit the number of prescribers, because we know multiple prescribers are associated with greater likelihood of inappropriate prescribing,” Dr. Wagg said.
The move to wean patients from inappropriate medications is gaining momentum, he added.
“There is a huge movement now around actively deprescribing medications which are either no longer indicated or potentially of little benefit, given remaining life expectancy,” said Dr. Wagg. Drugs such as proton pump inhibitors, hypnotics, unrequired antidepressants, and benzodiazepines are the first targets for elimination, he concluded.
The study was funded by the University of Calgary. Dr. McMillan and Dr. Wagg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People aged 65 or older with human immunodeficiency virus (HIV) receive significantly more nonantiretroviral therapy (non-ART) medications, compared with patients with HIV who are between ages 50 and 64, according to a new study.
Moreover, in a sample of more than 900 patients with HIV, about 60% were taking at least one potentially inappropriate medication (PIM).
“Clinicians looking after persons living with HIV need to provide medication reconciliation with prioritization of medications based on the patients’ wishes and patients’ goals and life expectancy,” lead author Jacqueline McMillan, MD, clinical assistant professor of geriatric medicine at the University of Calgary (Alt.) told this news organization.
The findings were published online in the Canadian Journal of General Internal Medicine.
Examining the pill burden
A geriatrician by training and a clinical researcher with an interest in aging in patients with HIV, Dr. McMillan said she began to observe that many older adults with HIV were on polypharmacy. “There are many other things that aging people with HIV experience, such as frailty, falls, cognitive impairment, medication nonadherence, and mortality, but in this study, we focused just on the polypharmacy,” said Dr. McMillan.
Her aim was to see if there was a way to improve the pill burden in these older adults.
“Do they need to be on all of these medications? Is there anything that we were overprescribing that they no longer needed, or possibly not prescribing and undertreating people because they were older? I wanted to have a better sense that the medications we were prescribing were appropriate and that we minimized the pill burden for older adults,” Dr. McMillan said.
Persons with HIV are at a particularly increased risk of polypharmacy and potential drug-drug interactions because they need antiretroviral therapy medications and medications to treat comorbidities.
“Certainly, when the ARTs were first discovered, sometimes that regimen required several pills a day, but as time has gone on and our retrovirals have gotten better, some of those requirements have narrowed down to one-pill-a-day regimens. We are now replacing that pill burden with non-HIV drugs,” said Dr. McMillan.
The researchers obtained medication reconciliation data for 951 persons with HIV aged 50 or older as of Feb. 1, 2020. The study population was receiving HIV care through the Southern Alberta HIV Clinic in Calgary. The researchers defined polypharmacy as taking five or more non-ART drugs. They defined PIMs according to the 2019 Beers criteria.
In their analysis, the researchers compared patients aged 65 or older with patients aged 50-64, as well as patients with shorter (< 10 years) and longer (> 10 years) duration of HIV infection.
PIM use common
The population’s mean age was 59 years, and 82% were men. The mean time since HIV diagnosis was 17.8 years, and the median time was 17 years. Most (80%) of the patients were aged 50-64 years, and 20% were 65 and older.
The researchers collected sociodemographic, clinical, medication, and laboratory data for all patients at each clinical visit.
The mean number of non-ART medications was 6.7 for the population. Patients aged 65 years or older were taking significantly more non-ART medications than patients aged 50-64 (8.4 vs. 6.3; P < .001).
Similarly, those living with HIV for more than 10 years were taking significantly more non-ART medications (mean, 6.9) than those living with HIV for 10 or fewer years (mean 6.1; P = .0168).
In all, almost 60% of patients were taking at least one PIM. The mean number of PIMs per patient was 1.6.
Patients living with diagnosed HIV infection for more than 10 years were at greater risk of PIMs (1.6 PIMs) than those with shorter duration of HIV diagnosis (1.4 PIMs; P = .06).
Dr. McMillan says she hopes her study reminds clinicians to review patients’ medications at each visit and ensure they are neither over- nor underprescribing.
“From my perspective as a geriatrician, I hope that we do more dedicated medication reconciliation to actually make sure we know what people are taking,” she said. She asks patients to bring all their medications to the office so that they can review which ones match their diagnoses.
“I want to do more patient-centered personalized care for older adults, with a focus on people who are frail and who may have a limited life expectancy, so that we don’t have someone with a short life expectancy still taking 15 medications a day,” said Dr. McMillan.
‘Carefully document medications’
“This study identifies potentially inappropriate medication use in a group of older people living with HIV who are particularly vulnerable to it at an earlier age because of their medical complexity or frailty than perhaps healthy older adults,” Adrian Wagg, MD, professor of healthy aging in the department of medicine at the University of Alberta, Edmonton, told this news organization.
The study emphasizes the importance of careful documentation of medications that the patient is taking at every clinical visit, he said.
“Make sure you carefully document medications which are taken whenever you see the individual. Also try to limit the number of prescribers, because we know multiple prescribers are associated with greater likelihood of inappropriate prescribing,” Dr. Wagg said.
The move to wean patients from inappropriate medications is gaining momentum, he added.
“There is a huge movement now around actively deprescribing medications which are either no longer indicated or potentially of little benefit, given remaining life expectancy,” said Dr. Wagg. Drugs such as proton pump inhibitors, hypnotics, unrequired antidepressants, and benzodiazepines are the first targets for elimination, he concluded.
The study was funded by the University of Calgary. Dr. McMillan and Dr. Wagg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF GENERAL INTERNAL MEDICINE
Malaria: Testing parasite DNA in travelers’ blood may help predict drug resistance
Testing the DNA of antimicrobial-resistant Plasmodium falciparum in the blood of travelers from malaria-endemic regions may help researchers monitor how drug resistance changes over time, a study from Canada reports.
“Malaria remains the deadliest vector-borne infectious disease worldwide. Plasmodium spp., most commonly P. falciparum, are responsible for [approximately] 229 million cases and 500,000 deaths from malaria annually,” the authors write in Emerging Infectious Diseases.
“Our findings demonstrate an absence of genetic markers of resistance to the most powerful antimalarials on the planet – the artemisinins – in potentially deadly malaria imported primarily from sub-Saharan Africa over time. This is good news,” senior study author Andrea K. Boggild, MD, MSc, DTMH, told this news organization.
“We also showed that over 90% of falciparum malaria imports were resistant to the proguanil component of the fixed drug combination atovaquone-proguanil, a popular oral antimalarial that is first-line treatment for uncomplicated malaria in Canada,” Dr. Boggild, an associate professor in the department of medicine at the University of Toronto, Canada, added in an email. “We documented no genetic markers of atovaquone resistance.”
Search for global patterns of emerging drug resistance
Dr. Boggild, the medical director of the tropical disease unit at Toronto General Hospital, and colleagues analyzed 243 whole-blood specimens that contained P. falciparum and no other Plasmodium species from the malaria biobank at the Public Health Ontario Laboratory in Toronto. They analyzed specimens from the years 2008-2009, 2013-2014, and 2017-2018 from patients ranging in age from 3 to 88 years. Of the 186 patients with a documented travel history, 81 had traveled in West Africa, the most common region, and 40 in Nigeria, the most common country. Five specimens came from travelers to Southeast Asia, and one came from a traveler to the Caribbean.
The researchers extracted DNA from whole blood and detected the parasite’s DNA by real-time quantitative polymerase chain reaction (qPCR). They analyzed 23 different single-nucleotide polymorphisms (SNPs) in six genes, and quantified the prevalence of resistance markers, including genes that provoke resistance to the most common antimalarial drugs: chloroquine, mefloquine, atovaquone/proguanil, and the artemisinins.
They analyzed SNPs at atpase6 (pfATPase6), pfcrt (chloroquine resistance transporter, cytb (cytochrome b), dhfr (dihydrofolate reductase), dhps (dihydropteroate synthetase), mdr1 (multidrug resistance protein) and mdr1 copy number, and kelch13 (kelch protein gene on chromosome 13).
Over time, they detected increasing mutant genotypes for dhfr S108N (P = .001) and dhps A613T (P = .029) but decreasing mutant genotypes for mdr1 N86Y (P < .001), D1246Y (P = .003), pfcrt K76T (P = .011), and pfcrt 74-75 (P = .014). They found no kelch13 mutations. They detected fewer mutations indicating chloroquine resistance over time, suggesting less chloroquine pressure in specimens from travelers to Africa, but mutations that provided proguanil resistance increased.
“Antimalarial resistance – particularly resistance to the powerful artemisinins – continues to expand globally, and it is important to conduct routine surveillance for resistant parasites in order to inform appropriate prevention and treatment guidelines,” Dr. Boggild explained. “It cannot be presumed that a drug’s efficacy will be durable over time given the global landscape of antimalarial resistance.”
Dr. Boggild acknowledged limitations to the study, including incomplete travel history in about half of the patients, relatively few patients from Southeast Asia, and the small sample set.
“Clinicians caring for travelers before or after travel should familiarize themselves with the options for malaria prevention and treatment and understand the risk–benefit profile of each drug,” Dr. Boggild advised.
“Resistance to proguanil means that we are reliant on the partner drug atovaquone for the antimalarial action of this formulation, which is effective only when taken with food,” she added.
Anne N. Cowell, MD, MPH, of the division of infectious diseases at the University of California, San Diego, was not surprised by the findings.
“The study demonstrates how quickly malaria parasites adapt and evolve to survive changes in malaria treatment,” Dr. Cowell, who was not involved in the study, told this news organization.
“These changes reflect changing malaria treatment and thus drug pressure during the time period,” she said in an email. “Because the majority of the clinical samples with a known travel history came from West Africa, and there was no clear evidence of artemisinin resistance in the area during the final time period studied, it is not surprising that they did not find kelch13 resistance mutations.
“The increase in mutations associated with proguanil resistance is concerning because atovaquone-proguanil is frequently used for prophylaxis during travel,” Dr. Cowell added. “There is no widespread evidence of resistance in travelers at this time, but it warrants monitoring.”
Sean C. Murphy, MD, PhD, an associate professor of laboratory medicine and the director of the malaria molecular diagnostic laboratory at the University of Washington in Seattle, also was not surprised by the study’s results.
“It may be just a matter of time before evidence of artemisinin resistance crops up among returning travelers,” he said in an email. “When that happens, we may lose the opportunity to easily use common go-to drugs like atovaquone/proguanil to treat these patients.
“The biggest takeaway of this study is the reminder that drug-resistant malaria (including the future potential for artemisinin-resistant malaria) is just an airplane flight or two away from nonendemic places like Canada and the United States,” Dr. Murphy noted. He was not involved with this Canadian study.
“Continued investment is needed to support malaria control, drug resistance monitoring, and vaccine efforts in order to fight this relentless, terrible parasite,” he urged.
The Project Initiation Fund of Public Health Ontario funded the study. The study authors, Dr. Cowell, and Dr. Murphy have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Testing the DNA of antimicrobial-resistant Plasmodium falciparum in the blood of travelers from malaria-endemic regions may help researchers monitor how drug resistance changes over time, a study from Canada reports.
“Malaria remains the deadliest vector-borne infectious disease worldwide. Plasmodium spp., most commonly P. falciparum, are responsible for [approximately] 229 million cases and 500,000 deaths from malaria annually,” the authors write in Emerging Infectious Diseases.
“Our findings demonstrate an absence of genetic markers of resistance to the most powerful antimalarials on the planet – the artemisinins – in potentially deadly malaria imported primarily from sub-Saharan Africa over time. This is good news,” senior study author Andrea K. Boggild, MD, MSc, DTMH, told this news organization.
“We also showed that over 90% of falciparum malaria imports were resistant to the proguanil component of the fixed drug combination atovaquone-proguanil, a popular oral antimalarial that is first-line treatment for uncomplicated malaria in Canada,” Dr. Boggild, an associate professor in the department of medicine at the University of Toronto, Canada, added in an email. “We documented no genetic markers of atovaquone resistance.”
Search for global patterns of emerging drug resistance
Dr. Boggild, the medical director of the tropical disease unit at Toronto General Hospital, and colleagues analyzed 243 whole-blood specimens that contained P. falciparum and no other Plasmodium species from the malaria biobank at the Public Health Ontario Laboratory in Toronto. They analyzed specimens from the years 2008-2009, 2013-2014, and 2017-2018 from patients ranging in age from 3 to 88 years. Of the 186 patients with a documented travel history, 81 had traveled in West Africa, the most common region, and 40 in Nigeria, the most common country. Five specimens came from travelers to Southeast Asia, and one came from a traveler to the Caribbean.
The researchers extracted DNA from whole blood and detected the parasite’s DNA by real-time quantitative polymerase chain reaction (qPCR). They analyzed 23 different single-nucleotide polymorphisms (SNPs) in six genes, and quantified the prevalence of resistance markers, including genes that provoke resistance to the most common antimalarial drugs: chloroquine, mefloquine, atovaquone/proguanil, and the artemisinins.
They analyzed SNPs at atpase6 (pfATPase6), pfcrt (chloroquine resistance transporter, cytb (cytochrome b), dhfr (dihydrofolate reductase), dhps (dihydropteroate synthetase), mdr1 (multidrug resistance protein) and mdr1 copy number, and kelch13 (kelch protein gene on chromosome 13).
Over time, they detected increasing mutant genotypes for dhfr S108N (P = .001) and dhps A613T (P = .029) but decreasing mutant genotypes for mdr1 N86Y (P < .001), D1246Y (P = .003), pfcrt K76T (P = .011), and pfcrt 74-75 (P = .014). They found no kelch13 mutations. They detected fewer mutations indicating chloroquine resistance over time, suggesting less chloroquine pressure in specimens from travelers to Africa, but mutations that provided proguanil resistance increased.
“Antimalarial resistance – particularly resistance to the powerful artemisinins – continues to expand globally, and it is important to conduct routine surveillance for resistant parasites in order to inform appropriate prevention and treatment guidelines,” Dr. Boggild explained. “It cannot be presumed that a drug’s efficacy will be durable over time given the global landscape of antimalarial resistance.”
Dr. Boggild acknowledged limitations to the study, including incomplete travel history in about half of the patients, relatively few patients from Southeast Asia, and the small sample set.
“Clinicians caring for travelers before or after travel should familiarize themselves with the options for malaria prevention and treatment and understand the risk–benefit profile of each drug,” Dr. Boggild advised.
“Resistance to proguanil means that we are reliant on the partner drug atovaquone for the antimalarial action of this formulation, which is effective only when taken with food,” she added.
Anne N. Cowell, MD, MPH, of the division of infectious diseases at the University of California, San Diego, was not surprised by the findings.
“The study demonstrates how quickly malaria parasites adapt and evolve to survive changes in malaria treatment,” Dr. Cowell, who was not involved in the study, told this news organization.
“These changes reflect changing malaria treatment and thus drug pressure during the time period,” she said in an email. “Because the majority of the clinical samples with a known travel history came from West Africa, and there was no clear evidence of artemisinin resistance in the area during the final time period studied, it is not surprising that they did not find kelch13 resistance mutations.
“The increase in mutations associated with proguanil resistance is concerning because atovaquone-proguanil is frequently used for prophylaxis during travel,” Dr. Cowell added. “There is no widespread evidence of resistance in travelers at this time, but it warrants monitoring.”
Sean C. Murphy, MD, PhD, an associate professor of laboratory medicine and the director of the malaria molecular diagnostic laboratory at the University of Washington in Seattle, also was not surprised by the study’s results.
“It may be just a matter of time before evidence of artemisinin resistance crops up among returning travelers,” he said in an email. “When that happens, we may lose the opportunity to easily use common go-to drugs like atovaquone/proguanil to treat these patients.
“The biggest takeaway of this study is the reminder that drug-resistant malaria (including the future potential for artemisinin-resistant malaria) is just an airplane flight or two away from nonendemic places like Canada and the United States,” Dr. Murphy noted. He was not involved with this Canadian study.
“Continued investment is needed to support malaria control, drug resistance monitoring, and vaccine efforts in order to fight this relentless, terrible parasite,” he urged.
The Project Initiation Fund of Public Health Ontario funded the study. The study authors, Dr. Cowell, and Dr. Murphy have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Testing the DNA of antimicrobial-resistant Plasmodium falciparum in the blood of travelers from malaria-endemic regions may help researchers monitor how drug resistance changes over time, a study from Canada reports.
“Malaria remains the deadliest vector-borne infectious disease worldwide. Plasmodium spp., most commonly P. falciparum, are responsible for [approximately] 229 million cases and 500,000 deaths from malaria annually,” the authors write in Emerging Infectious Diseases.
“Our findings demonstrate an absence of genetic markers of resistance to the most powerful antimalarials on the planet – the artemisinins – in potentially deadly malaria imported primarily from sub-Saharan Africa over time. This is good news,” senior study author Andrea K. Boggild, MD, MSc, DTMH, told this news organization.
“We also showed that over 90% of falciparum malaria imports were resistant to the proguanil component of the fixed drug combination atovaquone-proguanil, a popular oral antimalarial that is first-line treatment for uncomplicated malaria in Canada,” Dr. Boggild, an associate professor in the department of medicine at the University of Toronto, Canada, added in an email. “We documented no genetic markers of atovaquone resistance.”
Search for global patterns of emerging drug resistance
Dr. Boggild, the medical director of the tropical disease unit at Toronto General Hospital, and colleagues analyzed 243 whole-blood specimens that contained P. falciparum and no other Plasmodium species from the malaria biobank at the Public Health Ontario Laboratory in Toronto. They analyzed specimens from the years 2008-2009, 2013-2014, and 2017-2018 from patients ranging in age from 3 to 88 years. Of the 186 patients with a documented travel history, 81 had traveled in West Africa, the most common region, and 40 in Nigeria, the most common country. Five specimens came from travelers to Southeast Asia, and one came from a traveler to the Caribbean.
The researchers extracted DNA from whole blood and detected the parasite’s DNA by real-time quantitative polymerase chain reaction (qPCR). They analyzed 23 different single-nucleotide polymorphisms (SNPs) in six genes, and quantified the prevalence of resistance markers, including genes that provoke resistance to the most common antimalarial drugs: chloroquine, mefloquine, atovaquone/proguanil, and the artemisinins.
They analyzed SNPs at atpase6 (pfATPase6), pfcrt (chloroquine resistance transporter, cytb (cytochrome b), dhfr (dihydrofolate reductase), dhps (dihydropteroate synthetase), mdr1 (multidrug resistance protein) and mdr1 copy number, and kelch13 (kelch protein gene on chromosome 13).
Over time, they detected increasing mutant genotypes for dhfr S108N (P = .001) and dhps A613T (P = .029) but decreasing mutant genotypes for mdr1 N86Y (P < .001), D1246Y (P = .003), pfcrt K76T (P = .011), and pfcrt 74-75 (P = .014). They found no kelch13 mutations. They detected fewer mutations indicating chloroquine resistance over time, suggesting less chloroquine pressure in specimens from travelers to Africa, but mutations that provided proguanil resistance increased.
“Antimalarial resistance – particularly resistance to the powerful artemisinins – continues to expand globally, and it is important to conduct routine surveillance for resistant parasites in order to inform appropriate prevention and treatment guidelines,” Dr. Boggild explained. “It cannot be presumed that a drug’s efficacy will be durable over time given the global landscape of antimalarial resistance.”
Dr. Boggild acknowledged limitations to the study, including incomplete travel history in about half of the patients, relatively few patients from Southeast Asia, and the small sample set.
“Clinicians caring for travelers before or after travel should familiarize themselves with the options for malaria prevention and treatment and understand the risk–benefit profile of each drug,” Dr. Boggild advised.
“Resistance to proguanil means that we are reliant on the partner drug atovaquone for the antimalarial action of this formulation, which is effective only when taken with food,” she added.
Anne N. Cowell, MD, MPH, of the division of infectious diseases at the University of California, San Diego, was not surprised by the findings.
“The study demonstrates how quickly malaria parasites adapt and evolve to survive changes in malaria treatment,” Dr. Cowell, who was not involved in the study, told this news organization.
“These changes reflect changing malaria treatment and thus drug pressure during the time period,” she said in an email. “Because the majority of the clinical samples with a known travel history came from West Africa, and there was no clear evidence of artemisinin resistance in the area during the final time period studied, it is not surprising that they did not find kelch13 resistance mutations.
“The increase in mutations associated with proguanil resistance is concerning because atovaquone-proguanil is frequently used for prophylaxis during travel,” Dr. Cowell added. “There is no widespread evidence of resistance in travelers at this time, but it warrants monitoring.”
Sean C. Murphy, MD, PhD, an associate professor of laboratory medicine and the director of the malaria molecular diagnostic laboratory at the University of Washington in Seattle, also was not surprised by the study’s results.
“It may be just a matter of time before evidence of artemisinin resistance crops up among returning travelers,” he said in an email. “When that happens, we may lose the opportunity to easily use common go-to drugs like atovaquone/proguanil to treat these patients.
“The biggest takeaway of this study is the reminder that drug-resistant malaria (including the future potential for artemisinin-resistant malaria) is just an airplane flight or two away from nonendemic places like Canada and the United States,” Dr. Murphy noted. He was not involved with this Canadian study.
“Continued investment is needed to support malaria control, drug resistance monitoring, and vaccine efforts in order to fight this relentless, terrible parasite,” he urged.
The Project Initiation Fund of Public Health Ontario funded the study. The study authors, Dr. Cowell, and Dr. Murphy have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EMERGING INFECTIOUS DISEASES
Experts decry CDC’s long pause on neglected tropical disease testing
The Centers for Disease Control and Prevention has long been the premier reference lab for the United States and, for some diseases, internationally.
In September 2021, the CDC stated on its website that it would stop testing for parasites, herpesvirus encephalitis, human herpesvirus 6 and 7, Epstein-Barr virus, and other viruses, saying, “We are working diligently to implement laboratory system improvements.”
At the time, the CDC said testing would be halted only for a few months.
In response to a query from this news organization, a CDC spokesperson replied, “While at present we are unable to share a detailed timeline, our highest priority is to resume high-quality testing operations in a phased, prioritized approach as soon as possible and to offer the same tests that were available before the pause.”
Several global health clinicians told this news organization that they were not aware of the halt and that they are now uncertain about the specific diagnosis and best treatment for some patients. Other patients have been lost to follow-up.
In response, a group of tropical disease specialists who focus on neglected tropical diseases (NTDs) wrote an editorial, “Neglected Testing for Neglected Tropical Diseases at the CDC,” which recently appeared in the American Journal of Tropical Medicine and Hygiene (AJTMH).
NTDs are caused by viruses, bacteria, and parasites. They include leprosy and worms; many such diseases are disfiguring, such as filariasis (which causes the hugely swollen extremities of elephantiasis) and onchocerciasis (river blindness). They also include some viral and bacterial diseases. Their common denominator is that they are diseases of poverty, primarily in Africa, Asia, and Latin America, so they garner little attention from “first world” countries.
The loss of testing for two devastating parasites – Chagas and Leishmania – was particularly significant. Few other labs in the United States test for these, and the tests can be expensive and of variable quality, experts said.
Norman Beatty, MD, a global health physician at the University of Florida, told this news organization, “Chagas confirmatory testing is only available at the CDC and is the most reliable testing we have access to in the United States. Leishmania species identification is also only available at the CDC and is important in determining which antiparasitic medications we will use.”
Chagas disease is caused by the parasite Trypanosoma cruzi and is transmitted by triatomine bugs, also known as kissing bugs. Chagas is a major cause of an enlarged heart and congestive heart failure, as well as a dramatically enlarged esophagus or colon.
Prior to the cuts and before COVID-19, the CDC reported that they ran 10,000 to 15,000 tests for parasitic diseases annually. Testing requests declined during COVID. In 2021, they ran 1,003 tests for Chagas.
Dr. Beatty said that he first became aware of the CDC’s testing cuts last fall when he sought care for a patient. He was first told the delay would be 2-3 weeks, then another 2-3 weeks. It’s now been 7 months, and only three tests have been resumed.
Dr. Beatty added that for Chagas disease in particular, there is urgency in testing because cardiac complications can be life-threatening. He said that “a lot of these diseases can be considered rare, but they also have a tremendous ability to cause morbidity and mortality.”
Leishmania infections are also serious. Following the bite of an infected sandfly, they can cause disfiguring skin infections, but, more importantly, they can affect the liver, spleen, and bone marrow. Dr. Beatty said that since testing was dropped at the CDC, some colleagues had to send specimens outside of the country.
Dr. Beatty emphasized that the cuts in testing at the CDC highlight disparities in our society. “There are other commercial reference laboratories who may have some of these tests available, but the vast majority of people who suffer from diseases are underserved and vulnerable. [My patients] most definitely will not have access to advanced testing commercial laboratories,” Dr. Beatty said. Those laboratories include Associated Regional University Pathologists laboratories, Quest Diagnostics, and LabCorp Diagnostics. But for some parasitic infections, there will simply be no testing, and patients will not receive appropriate therapy.
The CDC’s website says, “USAID and CDC work together on a shared agenda to advance global progress towards the control and elimination of NTDs that can be addressed with preventive chemotherapy. ... CDC has strong working relationships with WHO, regional reference laboratories/bodies, [and] national NTD programs ... working with these partners through the provision of unique laboratory, diagnostic, and epidemiological technical assistance.”
The WHO Roadmap for 2030 aims to prevent and control many NTDs, in part by “providing new interventions and effective, standardized, and affordable diagnostics.” Last year, the CDC said that they “will continue working with WHO and other global partners to meet the established goals.”
But testing for a number of NTDs is not currently available at the CDC. In response to questions from this news organization, a CDC spokesperson said the agency “supports the development of country capacity for NTD testing required ... but does not perform testing related to the WHO Roadmap.”
A group of CDC officials wrote an editorial response that was published in AJTMH, saying the agency has “three main priorities: reducing parasitic disease-related death, illness, and disability in the United States; reducing the global burden of malaria; and eliminating targeted neglected tropical diseases.”
In response to this news organization’s interview request, a CDC spokesperson wrote, “CDC is unwavering in our commitment to provide the highest quality laboratory diagnostic services for parasitic diseases. We understand the concerns expressed in the editorial and the challenges the pause in testing for parasitic diseases presents for health care providers, particularly those treating people at elevated risk for parasitic diseases.”
Michael Reich, PhD, Dr. Beatty’s co-author, is an international health policy expert at Harvard. He and the physicians had approached CDC about the elimination of services. He said in an interview, “We’re still unable to get clear responses except for something along the lines of, ‘We are working on it. It is complicated. It takes time. We’re doing our best.’”
Dr. Reich added, “For me, this raises troubling issues both of transparency and accountability – transparency about what is going on and what the problems are, and accountability in terms of who’s being held responsible for the closures and the impacts on both public health and patient treatment.”
Dr. Beatty concluded, “I think the goal of our group was to bring more awareness to the importance of having a national laboratory that can service all people, even the most underserved and vulnerable populations.” He added, “Chagas disease is a disease of inequity in Latin Americans. Without having access to an appropriate laboratory such as the CDC, we would be taking a backwards approach to tackle neglected tropical diseases in our country and worldwide.”
Dr. Beatty and Dr. Reich report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has long been the premier reference lab for the United States and, for some diseases, internationally.
In September 2021, the CDC stated on its website that it would stop testing for parasites, herpesvirus encephalitis, human herpesvirus 6 and 7, Epstein-Barr virus, and other viruses, saying, “We are working diligently to implement laboratory system improvements.”
At the time, the CDC said testing would be halted only for a few months.
In response to a query from this news organization, a CDC spokesperson replied, “While at present we are unable to share a detailed timeline, our highest priority is to resume high-quality testing operations in a phased, prioritized approach as soon as possible and to offer the same tests that were available before the pause.”
Several global health clinicians told this news organization that they were not aware of the halt and that they are now uncertain about the specific diagnosis and best treatment for some patients. Other patients have been lost to follow-up.
In response, a group of tropical disease specialists who focus on neglected tropical diseases (NTDs) wrote an editorial, “Neglected Testing for Neglected Tropical Diseases at the CDC,” which recently appeared in the American Journal of Tropical Medicine and Hygiene (AJTMH).
NTDs are caused by viruses, bacteria, and parasites. They include leprosy and worms; many such diseases are disfiguring, such as filariasis (which causes the hugely swollen extremities of elephantiasis) and onchocerciasis (river blindness). They also include some viral and bacterial diseases. Their common denominator is that they are diseases of poverty, primarily in Africa, Asia, and Latin America, so they garner little attention from “first world” countries.
The loss of testing for two devastating parasites – Chagas and Leishmania – was particularly significant. Few other labs in the United States test for these, and the tests can be expensive and of variable quality, experts said.
Norman Beatty, MD, a global health physician at the University of Florida, told this news organization, “Chagas confirmatory testing is only available at the CDC and is the most reliable testing we have access to in the United States. Leishmania species identification is also only available at the CDC and is important in determining which antiparasitic medications we will use.”
Chagas disease is caused by the parasite Trypanosoma cruzi and is transmitted by triatomine bugs, also known as kissing bugs. Chagas is a major cause of an enlarged heart and congestive heart failure, as well as a dramatically enlarged esophagus or colon.
Prior to the cuts and before COVID-19, the CDC reported that they ran 10,000 to 15,000 tests for parasitic diseases annually. Testing requests declined during COVID. In 2021, they ran 1,003 tests for Chagas.
Dr. Beatty said that he first became aware of the CDC’s testing cuts last fall when he sought care for a patient. He was first told the delay would be 2-3 weeks, then another 2-3 weeks. It’s now been 7 months, and only three tests have been resumed.
Dr. Beatty added that for Chagas disease in particular, there is urgency in testing because cardiac complications can be life-threatening. He said that “a lot of these diseases can be considered rare, but they also have a tremendous ability to cause morbidity and mortality.”
Leishmania infections are also serious. Following the bite of an infected sandfly, they can cause disfiguring skin infections, but, more importantly, they can affect the liver, spleen, and bone marrow. Dr. Beatty said that since testing was dropped at the CDC, some colleagues had to send specimens outside of the country.
Dr. Beatty emphasized that the cuts in testing at the CDC highlight disparities in our society. “There are other commercial reference laboratories who may have some of these tests available, but the vast majority of people who suffer from diseases are underserved and vulnerable. [My patients] most definitely will not have access to advanced testing commercial laboratories,” Dr. Beatty said. Those laboratories include Associated Regional University Pathologists laboratories, Quest Diagnostics, and LabCorp Diagnostics. But for some parasitic infections, there will simply be no testing, and patients will not receive appropriate therapy.
The CDC’s website says, “USAID and CDC work together on a shared agenda to advance global progress towards the control and elimination of NTDs that can be addressed with preventive chemotherapy. ... CDC has strong working relationships with WHO, regional reference laboratories/bodies, [and] national NTD programs ... working with these partners through the provision of unique laboratory, diagnostic, and epidemiological technical assistance.”
The WHO Roadmap for 2030 aims to prevent and control many NTDs, in part by “providing new interventions and effective, standardized, and affordable diagnostics.” Last year, the CDC said that they “will continue working with WHO and other global partners to meet the established goals.”
But testing for a number of NTDs is not currently available at the CDC. In response to questions from this news organization, a CDC spokesperson said the agency “supports the development of country capacity for NTD testing required ... but does not perform testing related to the WHO Roadmap.”
A group of CDC officials wrote an editorial response that was published in AJTMH, saying the agency has “three main priorities: reducing parasitic disease-related death, illness, and disability in the United States; reducing the global burden of malaria; and eliminating targeted neglected tropical diseases.”
In response to this news organization’s interview request, a CDC spokesperson wrote, “CDC is unwavering in our commitment to provide the highest quality laboratory diagnostic services for parasitic diseases. We understand the concerns expressed in the editorial and the challenges the pause in testing for parasitic diseases presents for health care providers, particularly those treating people at elevated risk for parasitic diseases.”
Michael Reich, PhD, Dr. Beatty’s co-author, is an international health policy expert at Harvard. He and the physicians had approached CDC about the elimination of services. He said in an interview, “We’re still unable to get clear responses except for something along the lines of, ‘We are working on it. It is complicated. It takes time. We’re doing our best.’”
Dr. Reich added, “For me, this raises troubling issues both of transparency and accountability – transparency about what is going on and what the problems are, and accountability in terms of who’s being held responsible for the closures and the impacts on both public health and patient treatment.”
Dr. Beatty concluded, “I think the goal of our group was to bring more awareness to the importance of having a national laboratory that can service all people, even the most underserved and vulnerable populations.” He added, “Chagas disease is a disease of inequity in Latin Americans. Without having access to an appropriate laboratory such as the CDC, we would be taking a backwards approach to tackle neglected tropical diseases in our country and worldwide.”
Dr. Beatty and Dr. Reich report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention has long been the premier reference lab for the United States and, for some diseases, internationally.
In September 2021, the CDC stated on its website that it would stop testing for parasites, herpesvirus encephalitis, human herpesvirus 6 and 7, Epstein-Barr virus, and other viruses, saying, “We are working diligently to implement laboratory system improvements.”
At the time, the CDC said testing would be halted only for a few months.
In response to a query from this news organization, a CDC spokesperson replied, “While at present we are unable to share a detailed timeline, our highest priority is to resume high-quality testing operations in a phased, prioritized approach as soon as possible and to offer the same tests that were available before the pause.”
Several global health clinicians told this news organization that they were not aware of the halt and that they are now uncertain about the specific diagnosis and best treatment for some patients. Other patients have been lost to follow-up.
In response, a group of tropical disease specialists who focus on neglected tropical diseases (NTDs) wrote an editorial, “Neglected Testing for Neglected Tropical Diseases at the CDC,” which recently appeared in the American Journal of Tropical Medicine and Hygiene (AJTMH).
NTDs are caused by viruses, bacteria, and parasites. They include leprosy and worms; many such diseases are disfiguring, such as filariasis (which causes the hugely swollen extremities of elephantiasis) and onchocerciasis (river blindness). They also include some viral and bacterial diseases. Their common denominator is that they are diseases of poverty, primarily in Africa, Asia, and Latin America, so they garner little attention from “first world” countries.
The loss of testing for two devastating parasites – Chagas and Leishmania – was particularly significant. Few other labs in the United States test for these, and the tests can be expensive and of variable quality, experts said.
Norman Beatty, MD, a global health physician at the University of Florida, told this news organization, “Chagas confirmatory testing is only available at the CDC and is the most reliable testing we have access to in the United States. Leishmania species identification is also only available at the CDC and is important in determining which antiparasitic medications we will use.”
Chagas disease is caused by the parasite Trypanosoma cruzi and is transmitted by triatomine bugs, also known as kissing bugs. Chagas is a major cause of an enlarged heart and congestive heart failure, as well as a dramatically enlarged esophagus or colon.
Prior to the cuts and before COVID-19, the CDC reported that they ran 10,000 to 15,000 tests for parasitic diseases annually. Testing requests declined during COVID. In 2021, they ran 1,003 tests for Chagas.
Dr. Beatty said that he first became aware of the CDC’s testing cuts last fall when he sought care for a patient. He was first told the delay would be 2-3 weeks, then another 2-3 weeks. It’s now been 7 months, and only three tests have been resumed.
Dr. Beatty added that for Chagas disease in particular, there is urgency in testing because cardiac complications can be life-threatening. He said that “a lot of these diseases can be considered rare, but they also have a tremendous ability to cause morbidity and mortality.”
Leishmania infections are also serious. Following the bite of an infected sandfly, they can cause disfiguring skin infections, but, more importantly, they can affect the liver, spleen, and bone marrow. Dr. Beatty said that since testing was dropped at the CDC, some colleagues had to send specimens outside of the country.
Dr. Beatty emphasized that the cuts in testing at the CDC highlight disparities in our society. “There are other commercial reference laboratories who may have some of these tests available, but the vast majority of people who suffer from diseases are underserved and vulnerable. [My patients] most definitely will not have access to advanced testing commercial laboratories,” Dr. Beatty said. Those laboratories include Associated Regional University Pathologists laboratories, Quest Diagnostics, and LabCorp Diagnostics. But for some parasitic infections, there will simply be no testing, and patients will not receive appropriate therapy.
The CDC’s website says, “USAID and CDC work together on a shared agenda to advance global progress towards the control and elimination of NTDs that can be addressed with preventive chemotherapy. ... CDC has strong working relationships with WHO, regional reference laboratories/bodies, [and] national NTD programs ... working with these partners through the provision of unique laboratory, diagnostic, and epidemiological technical assistance.”
The WHO Roadmap for 2030 aims to prevent and control many NTDs, in part by “providing new interventions and effective, standardized, and affordable diagnostics.” Last year, the CDC said that they “will continue working with WHO and other global partners to meet the established goals.”
But testing for a number of NTDs is not currently available at the CDC. In response to questions from this news organization, a CDC spokesperson said the agency “supports the development of country capacity for NTD testing required ... but does not perform testing related to the WHO Roadmap.”
A group of CDC officials wrote an editorial response that was published in AJTMH, saying the agency has “three main priorities: reducing parasitic disease-related death, illness, and disability in the United States; reducing the global burden of malaria; and eliminating targeted neglected tropical diseases.”
In response to this news organization’s interview request, a CDC spokesperson wrote, “CDC is unwavering in our commitment to provide the highest quality laboratory diagnostic services for parasitic diseases. We understand the concerns expressed in the editorial and the challenges the pause in testing for parasitic diseases presents for health care providers, particularly those treating people at elevated risk for parasitic diseases.”
Michael Reich, PhD, Dr. Beatty’s co-author, is an international health policy expert at Harvard. He and the physicians had approached CDC about the elimination of services. He said in an interview, “We’re still unable to get clear responses except for something along the lines of, ‘We are working on it. It is complicated. It takes time. We’re doing our best.’”
Dr. Reich added, “For me, this raises troubling issues both of transparency and accountability – transparency about what is going on and what the problems are, and accountability in terms of who’s being held responsible for the closures and the impacts on both public health and patient treatment.”
Dr. Beatty concluded, “I think the goal of our group was to bring more awareness to the importance of having a national laboratory that can service all people, even the most underserved and vulnerable populations.” He added, “Chagas disease is a disease of inequity in Latin Americans. Without having access to an appropriate laboratory such as the CDC, we would be taking a backwards approach to tackle neglected tropical diseases in our country and worldwide.”
Dr. Beatty and Dr. Reich report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Emerging tick-borne pathogen has spread to state of Georgia
Heartland virus (HRTV), an emerging infection first detected in lone star ticks in Missouri in 2009, has spread to lone star ticks in Georgia, a study published in Emerging Infectious Diseases reports.
HRTV disease is transmitted by the bite of an infected Amblyomma americanum tick, named “lone star” because of the silver-white spot on the female scutum (back).
“By … sampling … in an area with reported exposure to HRTV in wildlife and humans and testing for infection in thousands of ticks from multiple sites and physiologic stages, we confirmed the presence of HRTV in Georgia,” the authors write.
“This information about the expanding geographic range of lone star ticks, combined with increased human presence in tick-infested habitats, can be used to improve strategies for preventing tick bites and to alert physicians about this emerging tickborne virus infection,” a press release by the Centers for Disease Control and Prevention notes.
Persistent field and lab work led to HRTV discovery in Georgia
The search for infected lone star ticks began after a retroactive analysis confirmed that a person who died in Georgia in 2005 from an unidentified illness was infected with HRTV. A subsequent analysis of serum samples collected earlier from local white-tailed deer showed that the deer had been exposed to HRTV since at least 2001, according to a press release by Emory University.
These discoveries prompted local researchers to investigate whether lone star ticks in rural, woodsy central Georgia were carrying HRTV.
Lead study author Yamila Romer, MD, an infectious disease clinician and microbiologist in the department of environmental sciences at Emory University in Atlanta, and her colleagues collected samples of ticks in 2018 at 26 sites near the location of the patient who died and the seropositive deer. In 2019, they focused their collections on the two sites that had provided the most ticks in 2018.
From April to October in both years, the research team visited sites weekly to swish white flannel flags through underbrush. They picked off adult and nymph Amblyomma americanum ticks, placed them into vials, and transported them to their lab. They sorted 9,294 ticks by sex, life stage, and collection site. Then they crushed the ticks and extracted their RNA.
To confirm viral infection, the team tested RNA extracted from cell culture supernatants using a real-time polymerase chain reaction test specific for HRTV.
In the three pools of ticks that tested positive for HRTV, the researchers found a minimum infection rate of 0.46/1,000 ticks, suggesting that about 1 of every 2,000 ticks carried HRTV. They sequenced the genome of the three isolates and found that the genomes were similar to one another but were very different from the genomes from HRTV samples taken outside Georgia.
Catherine A. Hill, PhD, a professor of entomology and vector biology and the interim head of the department of entomology at Purdue University in West Lafayette, Ind., was impressed with the researchers’ discovery.
“Heartland virus is difficult to detect,” she said in an email. “The prevalence of human cases is low, and the virus appears to be present at very low levels in populations of lone star tick. The investigators went to some lengths to survey for the virus, collect, and process thousands of ticks – and they found the needle in the haystack.” Dr. Hill was not involved in the study.
Georgia data help researchers monitor HRTV spread
HRTV was first identified in 2009 in Missouri in two people hospitalized with fever, muscle pain, diarrhea, and low white blood cell and platelet counts. Researchers traced the infections to lone star ticks, and they found antibodies to the virus in blood samples from deer and other wild mammals.
According to the CDC, U.S. cases of tick-borne diseases more than doubled between 2004 and 2016. As of January 2021, more than 50 human cases of HRTV disease had been reported in 11 Midwestern and Southeastern states: Arkansas, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Missouri, North Carolina, Oklahoma, and Tennessee.
Precautions, signs, symptoms, testing, and treatment
“The lone star tick is aggressive and will actively seek out a human host to bite,” Dr. Hill noted.
She recommends that health care providers advise patients to avoid tick habitat, wear protective clothing, apply repellants, know the signs and symptoms of tick-borne disease, and seek immediate medical care if they become ill.
Common symptoms of HRTV disease include fatigue, fever, nausea, diarrhea, and anorexia. Treatment is supportive. Many patients have been hospitalized, and some with comorbidities have died.
HRTV infection is rarely tested for, and the disease burden is unknown. With no commercial tests available in the United States, the CDC performs molecular and serologic testing for HRTV infection. The agency advises doctors to contact their state health department if they suspect a patient may have HRTV disease.
Further research is needed
Samantha M. Wisely, PhD, a professor of wildlife ecology and the director of the Cervidae Health Research Initiative at the University of Florida in Gainesville, was not surprised by the study finding.
“The more we look for heartland virus, the more places we find it,” Dr. Wisely told this news organization in an email.
“Little is known about which wildlife play a role in maintaining the virus on the landscape,” said Dr. Wisely, who was not involved in the study. “White-tailed deer have been shown to produce antibodies, meaning they have been exposed to the virus, but no one has actually found the virus in a wildlife species.”
The whole-genome sequencing of the virus was particularly important, Dr. Wisely explained. “Whole-genome data allow researchers to better understand viral evolution, pathogenicity, and viral dynamics across space and time – how it is evolving.”
The study was supported by a grant from the Emory University Research Council. The authors, Dr. Wisely, and Dr. Hill have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Heartland virus (HRTV), an emerging infection first detected in lone star ticks in Missouri in 2009, has spread to lone star ticks in Georgia, a study published in Emerging Infectious Diseases reports.
HRTV disease is transmitted by the bite of an infected Amblyomma americanum tick, named “lone star” because of the silver-white spot on the female scutum (back).
“By … sampling … in an area with reported exposure to HRTV in wildlife and humans and testing for infection in thousands of ticks from multiple sites and physiologic stages, we confirmed the presence of HRTV in Georgia,” the authors write.
“This information about the expanding geographic range of lone star ticks, combined with increased human presence in tick-infested habitats, can be used to improve strategies for preventing tick bites and to alert physicians about this emerging tickborne virus infection,” a press release by the Centers for Disease Control and Prevention notes.
Persistent field and lab work led to HRTV discovery in Georgia
The search for infected lone star ticks began after a retroactive analysis confirmed that a person who died in Georgia in 2005 from an unidentified illness was infected with HRTV. A subsequent analysis of serum samples collected earlier from local white-tailed deer showed that the deer had been exposed to HRTV since at least 2001, according to a press release by Emory University.
These discoveries prompted local researchers to investigate whether lone star ticks in rural, woodsy central Georgia were carrying HRTV.
Lead study author Yamila Romer, MD, an infectious disease clinician and microbiologist in the department of environmental sciences at Emory University in Atlanta, and her colleagues collected samples of ticks in 2018 at 26 sites near the location of the patient who died and the seropositive deer. In 2019, they focused their collections on the two sites that had provided the most ticks in 2018.
From April to October in both years, the research team visited sites weekly to swish white flannel flags through underbrush. They picked off adult and nymph Amblyomma americanum ticks, placed them into vials, and transported them to their lab. They sorted 9,294 ticks by sex, life stage, and collection site. Then they crushed the ticks and extracted their RNA.
To confirm viral infection, the team tested RNA extracted from cell culture supernatants using a real-time polymerase chain reaction test specific for HRTV.
In the three pools of ticks that tested positive for HRTV, the researchers found a minimum infection rate of 0.46/1,000 ticks, suggesting that about 1 of every 2,000 ticks carried HRTV. They sequenced the genome of the three isolates and found that the genomes were similar to one another but were very different from the genomes from HRTV samples taken outside Georgia.
Catherine A. Hill, PhD, a professor of entomology and vector biology and the interim head of the department of entomology at Purdue University in West Lafayette, Ind., was impressed with the researchers’ discovery.
“Heartland virus is difficult to detect,” she said in an email. “The prevalence of human cases is low, and the virus appears to be present at very low levels in populations of lone star tick. The investigators went to some lengths to survey for the virus, collect, and process thousands of ticks – and they found the needle in the haystack.” Dr. Hill was not involved in the study.
Georgia data help researchers monitor HRTV spread
HRTV was first identified in 2009 in Missouri in two people hospitalized with fever, muscle pain, diarrhea, and low white blood cell and platelet counts. Researchers traced the infections to lone star ticks, and they found antibodies to the virus in blood samples from deer and other wild mammals.
According to the CDC, U.S. cases of tick-borne diseases more than doubled between 2004 and 2016. As of January 2021, more than 50 human cases of HRTV disease had been reported in 11 Midwestern and Southeastern states: Arkansas, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Missouri, North Carolina, Oklahoma, and Tennessee.
Precautions, signs, symptoms, testing, and treatment
“The lone star tick is aggressive and will actively seek out a human host to bite,” Dr. Hill noted.
She recommends that health care providers advise patients to avoid tick habitat, wear protective clothing, apply repellants, know the signs and symptoms of tick-borne disease, and seek immediate medical care if they become ill.
Common symptoms of HRTV disease include fatigue, fever, nausea, diarrhea, and anorexia. Treatment is supportive. Many patients have been hospitalized, and some with comorbidities have died.
HRTV infection is rarely tested for, and the disease burden is unknown. With no commercial tests available in the United States, the CDC performs molecular and serologic testing for HRTV infection. The agency advises doctors to contact their state health department if they suspect a patient may have HRTV disease.
Further research is needed
Samantha M. Wisely, PhD, a professor of wildlife ecology and the director of the Cervidae Health Research Initiative at the University of Florida in Gainesville, was not surprised by the study finding.
“The more we look for heartland virus, the more places we find it,” Dr. Wisely told this news organization in an email.
“Little is known about which wildlife play a role in maintaining the virus on the landscape,” said Dr. Wisely, who was not involved in the study. “White-tailed deer have been shown to produce antibodies, meaning they have been exposed to the virus, but no one has actually found the virus in a wildlife species.”
The whole-genome sequencing of the virus was particularly important, Dr. Wisely explained. “Whole-genome data allow researchers to better understand viral evolution, pathogenicity, and viral dynamics across space and time – how it is evolving.”
The study was supported by a grant from the Emory University Research Council. The authors, Dr. Wisely, and Dr. Hill have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Heartland virus (HRTV), an emerging infection first detected in lone star ticks in Missouri in 2009, has spread to lone star ticks in Georgia, a study published in Emerging Infectious Diseases reports.
HRTV disease is transmitted by the bite of an infected Amblyomma americanum tick, named “lone star” because of the silver-white spot on the female scutum (back).
“By … sampling … in an area with reported exposure to HRTV in wildlife and humans and testing for infection in thousands of ticks from multiple sites and physiologic stages, we confirmed the presence of HRTV in Georgia,” the authors write.
“This information about the expanding geographic range of lone star ticks, combined with increased human presence in tick-infested habitats, can be used to improve strategies for preventing tick bites and to alert physicians about this emerging tickborne virus infection,” a press release by the Centers for Disease Control and Prevention notes.
Persistent field and lab work led to HRTV discovery in Georgia
The search for infected lone star ticks began after a retroactive analysis confirmed that a person who died in Georgia in 2005 from an unidentified illness was infected with HRTV. A subsequent analysis of serum samples collected earlier from local white-tailed deer showed that the deer had been exposed to HRTV since at least 2001, according to a press release by Emory University.
These discoveries prompted local researchers to investigate whether lone star ticks in rural, woodsy central Georgia were carrying HRTV.
Lead study author Yamila Romer, MD, an infectious disease clinician and microbiologist in the department of environmental sciences at Emory University in Atlanta, and her colleagues collected samples of ticks in 2018 at 26 sites near the location of the patient who died and the seropositive deer. In 2019, they focused their collections on the two sites that had provided the most ticks in 2018.
From April to October in both years, the research team visited sites weekly to swish white flannel flags through underbrush. They picked off adult and nymph Amblyomma americanum ticks, placed them into vials, and transported them to their lab. They sorted 9,294 ticks by sex, life stage, and collection site. Then they crushed the ticks and extracted their RNA.
To confirm viral infection, the team tested RNA extracted from cell culture supernatants using a real-time polymerase chain reaction test specific for HRTV.
In the three pools of ticks that tested positive for HRTV, the researchers found a minimum infection rate of 0.46/1,000 ticks, suggesting that about 1 of every 2,000 ticks carried HRTV. They sequenced the genome of the three isolates and found that the genomes were similar to one another but were very different from the genomes from HRTV samples taken outside Georgia.
Catherine A. Hill, PhD, a professor of entomology and vector biology and the interim head of the department of entomology at Purdue University in West Lafayette, Ind., was impressed with the researchers’ discovery.
“Heartland virus is difficult to detect,” she said in an email. “The prevalence of human cases is low, and the virus appears to be present at very low levels in populations of lone star tick. The investigators went to some lengths to survey for the virus, collect, and process thousands of ticks – and they found the needle in the haystack.” Dr. Hill was not involved in the study.
Georgia data help researchers monitor HRTV spread
HRTV was first identified in 2009 in Missouri in two people hospitalized with fever, muscle pain, diarrhea, and low white blood cell and platelet counts. Researchers traced the infections to lone star ticks, and they found antibodies to the virus in blood samples from deer and other wild mammals.
According to the CDC, U.S. cases of tick-borne diseases more than doubled between 2004 and 2016. As of January 2021, more than 50 human cases of HRTV disease had been reported in 11 Midwestern and Southeastern states: Arkansas, Georgia, Illinois, Indiana, Iowa, Kansas, Kentucky, Missouri, North Carolina, Oklahoma, and Tennessee.
Precautions, signs, symptoms, testing, and treatment
“The lone star tick is aggressive and will actively seek out a human host to bite,” Dr. Hill noted.
She recommends that health care providers advise patients to avoid tick habitat, wear protective clothing, apply repellants, know the signs and symptoms of tick-borne disease, and seek immediate medical care if they become ill.
Common symptoms of HRTV disease include fatigue, fever, nausea, diarrhea, and anorexia. Treatment is supportive. Many patients have been hospitalized, and some with comorbidities have died.
HRTV infection is rarely tested for, and the disease burden is unknown. With no commercial tests available in the United States, the CDC performs molecular and serologic testing for HRTV infection. The agency advises doctors to contact their state health department if they suspect a patient may have HRTV disease.
Further research is needed
Samantha M. Wisely, PhD, a professor of wildlife ecology and the director of the Cervidae Health Research Initiative at the University of Florida in Gainesville, was not surprised by the study finding.
“The more we look for heartland virus, the more places we find it,” Dr. Wisely told this news organization in an email.
“Little is known about which wildlife play a role in maintaining the virus on the landscape,” said Dr. Wisely, who was not involved in the study. “White-tailed deer have been shown to produce antibodies, meaning they have been exposed to the virus, but no one has actually found the virus in a wildlife species.”
The whole-genome sequencing of the virus was particularly important, Dr. Wisely explained. “Whole-genome data allow researchers to better understand viral evolution, pathogenicity, and viral dynamics across space and time – how it is evolving.”
The study was supported by a grant from the Emory University Research Council. The authors, Dr. Wisely, and Dr. Hill have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EMERGING INFECTIOUS DISEASES
Children and COVID: Decline in new cases comes to an end
It was a good run while it lasted.
according to the American Academy of Pediatrics and the Children’s Hospital Association.
The number of reported pediatric cases for the week was 33,146, and the actual increase from the previous week was just 7,231 cases, the AAP and CHA said, but some reports suggest that the new COVID variants and subvariants are starting to have an effect on incidence in some areas while mask mandates continue to fall.
Data from the Centers for Disease Control and Prevention show that, over the last week or two, the 7-day average for percentage of emergency department visits with diagnosed COVID has risen from 0.5% to 0.6% in children aged 0-11 years, from 0.3% to 0.5% among 12- to 15-year-olds, and from 0.3% to 0.4% in 16- and 17-year-olds. Small increases, to be sure, but increases nonetheless.
A somewhat similar scenario is playing out for new admissions of children aged 0-17, which have leveled out after dropping from a high of 1.25 per 100,000 population in mid-January to 0.13 per 100,000 in early April. Over the last 2 weeks, the rate has been alternating between 0.13 and 0.14 per 100,000, the CDC said on its COVID Data Tracker.
The latest news on the vaccination front came from Pfizer and BIoNTech, which announced that a third dose of its COVID-19 vaccine boosted immune protection in children aged 5-11 years in a phase 2/3 trial. Protection against the Omicron strain was 36 times higher than the two previous doses, the companies said, adding that they plan to submit a request for emergency use authorization of a booster dose in the near future.
The ongoing vaccination effort, however, produced mixed results in the last week. Initial vaccinations among children aged 5-11 years fell 14.5% to another new low while initial doses were up 9.3% for those aged 12-17, the AAP said. Overall, just 28.2% of the country’s 5- to 11-year-olds are fully vaccinated, compared with 58.7% of those aged 12-17, the CDC reported.
It was a good run while it lasted.
according to the American Academy of Pediatrics and the Children’s Hospital Association.
The number of reported pediatric cases for the week was 33,146, and the actual increase from the previous week was just 7,231 cases, the AAP and CHA said, but some reports suggest that the new COVID variants and subvariants are starting to have an effect on incidence in some areas while mask mandates continue to fall.
Data from the Centers for Disease Control and Prevention show that, over the last week or two, the 7-day average for percentage of emergency department visits with diagnosed COVID has risen from 0.5% to 0.6% in children aged 0-11 years, from 0.3% to 0.5% among 12- to 15-year-olds, and from 0.3% to 0.4% in 16- and 17-year-olds. Small increases, to be sure, but increases nonetheless.
A somewhat similar scenario is playing out for new admissions of children aged 0-17, which have leveled out after dropping from a high of 1.25 per 100,000 population in mid-January to 0.13 per 100,000 in early April. Over the last 2 weeks, the rate has been alternating between 0.13 and 0.14 per 100,000, the CDC said on its COVID Data Tracker.
The latest news on the vaccination front came from Pfizer and BIoNTech, which announced that a third dose of its COVID-19 vaccine boosted immune protection in children aged 5-11 years in a phase 2/3 trial. Protection against the Omicron strain was 36 times higher than the two previous doses, the companies said, adding that they plan to submit a request for emergency use authorization of a booster dose in the near future.
The ongoing vaccination effort, however, produced mixed results in the last week. Initial vaccinations among children aged 5-11 years fell 14.5% to another new low while initial doses were up 9.3% for those aged 12-17, the AAP said. Overall, just 28.2% of the country’s 5- to 11-year-olds are fully vaccinated, compared with 58.7% of those aged 12-17, the CDC reported.
It was a good run while it lasted.
according to the American Academy of Pediatrics and the Children’s Hospital Association.
The number of reported pediatric cases for the week was 33,146, and the actual increase from the previous week was just 7,231 cases, the AAP and CHA said, but some reports suggest that the new COVID variants and subvariants are starting to have an effect on incidence in some areas while mask mandates continue to fall.
Data from the Centers for Disease Control and Prevention show that, over the last week or two, the 7-day average for percentage of emergency department visits with diagnosed COVID has risen from 0.5% to 0.6% in children aged 0-11 years, from 0.3% to 0.5% among 12- to 15-year-olds, and from 0.3% to 0.4% in 16- and 17-year-olds. Small increases, to be sure, but increases nonetheless.
A somewhat similar scenario is playing out for new admissions of children aged 0-17, which have leveled out after dropping from a high of 1.25 per 100,000 population in mid-January to 0.13 per 100,000 in early April. Over the last 2 weeks, the rate has been alternating between 0.13 and 0.14 per 100,000, the CDC said on its COVID Data Tracker.
The latest news on the vaccination front came from Pfizer and BIoNTech, which announced that a third dose of its COVID-19 vaccine boosted immune protection in children aged 5-11 years in a phase 2/3 trial. Protection against the Omicron strain was 36 times higher than the two previous doses, the companies said, adding that they plan to submit a request for emergency use authorization of a booster dose in the near future.
The ongoing vaccination effort, however, produced mixed results in the last week. Initial vaccinations among children aged 5-11 years fell 14.5% to another new low while initial doses were up 9.3% for those aged 12-17, the AAP said. Overall, just 28.2% of the country’s 5- to 11-year-olds are fully vaccinated, compared with 58.7% of those aged 12-17, the CDC reported.
Cupping in dermatology
My inspiration to write about cupping this month stems from the perception that everyone seems to be talking about it, from a facialist who suggested it for me to a coworker who swears by cupping to treat her allergies. Cupping is by no means a novel procedure. Its use as a health therapy dates back thousands of years to ancient Egypt (1500 BCE), ancient Greece (described by Hippocrates), ancient Rome (described by the Greek physician Galen), China (during the Han dynasty, 206 BCE to 220 CE) and traditional Islamic culture.1 Over the past decade, the popularity of this ancient procedure has been increasing in the United States.1 Cupping has been applied as a remedy for various dermatologic and medical conditions, including herpes zoster, headaches, diminished appetite, maldigestion, abscess evacuation, narcolepsy, pain, fever, dysmenorrhea, and gout.1,2
Theories on the mechanism(s) of action
The practice of cupping is differentiated into dry and wet cupping.1,2 Traditionally, with dry cupping, a flame is applied to heat the air inside a thick glass cup (rather than the cup itself).1 The cup is placed on the skin surface, and negative pressure suctions the skin into the cup. Wet cupping differs mainly from dry cupping in that it involves blood-letting. Cups made of either silicone or glass of varying size and shapes are used. Modern adaptations to cupping include needle, herbal, and pulsatile cupping, as well as a “moving cupping” technique (vs. traditionally stationary cups).1
There are several theories, many of which are derived from the nondermatologic literature (that is, pain management), as to how cupping may deliver a clinical benefit. Some theories are based in scientific and medical principles, whereas other theories are more whimsical – specifically, that cupping draws out evil spirits.2 Studies of dry cupping have suggested that the procedure results in increased oxygenation of muscles via a local increase in oxygenated hemoglobin, which may help improve muscular activity and reduce pain.1 As theorized by Lowe in 2017, negative pressure exerted by dry cupping leads to stretching and dilation of capillaries, which increases blood flow.3 Wet cupping has been shown to increase heat shock protein 70 (HSP70) and beta-endorphin expression in rat models, which is thought to facilitate pain management.1 Removal of oxidants and reduction of reactive oxygen species in the blood is believed to be among the benefits of wet cupping.1
Cupping in general dermatology
While , as well as various inflammatory conditions.
Herpes zoster
In 2010, Cao et al. reported on their systematic review of wet cupping after completing searches of multiple databases (that is, PubMed, the Cochrane Library [Issue 3, 2008], China Network Knowledge Infrastructure, Chinese Scientific Journal Database, and Wan Fang Database). They identified eight randomized controlled trials involving 651 patients, with meta-analyses revealing that wet cupping performed better than medications in terms of the number of “cured” patients, number of patients with improved symptoms, and a lower incidence of postherpetic neuralgia. Wet cupping, in addition to medication, was also found to be superior to medication alone in multiple patients. The researchers concluded that wet cupping appears to effectively treat herpes zoster.4 However, the study failed to identify which medications were used to treat herpes zoster. In the United States, common medications for herpes zoster include acyclovir, valacyclovir, steroids, gabapentin, and other neuromodulators. Without knowing which medications were used, it is difficult to compare cupping to medication in terms of efficacy in treating herpes zoster.
Urticaria
Urticaria (hives) is an inflammatory skin condition that can be very uncomfortable for patients but often resolves without intervention within several months after onset. In 2001, Li and Ding reported on the treatment with cupping of 40 patients with urticaria. The cure rate among the treatment group was cited as 55%, compared with 30% in the control group, who were treated with a traditional Chinese remedy and an unidentified first-generation antihistamine.1,5 In 2020, Xiao et al. conducted a systematic review and meta-analysis of cupping therapy for patients with chronic urticaria. They identified 13 comparisons from 12 randomized controlled trials involving 842 subjects. The investigators found no significant differences between wet cupping and medication usage. They also found that cupping combined with antihistamine treatment was superior to antihistamines alone, and cupping therapy with acupuncture was more effective than acupuncture alone. The investigators did call for caution, citing the poor quality of the studies reviewed.6
It is important to note that it is difficult to attribute resolution of urticaria to the use of cupping given the self-resolution often associated with this condition. Antihistamines are the mainstay of therapy for urticaria, but in my personal experience, patients are not entirely satisfied with the level of symptom control with antihistamines alone and often search for alternative therapies to control the pesky hives and associated itch. In 2014, omalizumab (Xolair) was approved for treating chronic idiopathic urticaria, which has helped patients control symptoms of chronic idiopathic urticaria without needing to take antihistamines. There was no indication that the studies reviewed by Xiao et al. compared cupping against this new effective treatment. Therefore, these studies comparing cupping to medical management are outdated.
Acne, eczema, and psoriasis
Soliman’s 2018 review of cupping in dermatology included a few studies on these common cutaneous conditions. For instance, a 2013 single-blind prospective study by Xu et al. reported on the results of patients with moderate acne who received wet cupping (in the form of prickling bloodletting) twice weekly for 6 weeks.7 They reported that patients demonstrated improvement in the global acne grading system (GAGS) score by the end of the trial.1,7 Unfortunately, cupping was not compared with standard acne treatments (that is, benzoyl peroxide, topical and oral antibiotics, isotretinoin, topical retinoids, spironolactone).
In evaluating cupping for acute eczema, wet cupping was compared with oral loratadine and topical ointments in a 2007 study by Yao and Li. They divided 88 cases into treatment and control groups, with the former group (n = 46) receiving bloodletting puncturing and cupping and the control group (n = 42) receiving oral loratadine and topical Pairuisong (an herbal ointment used in Chinese medicine). The investigators observed no significant difference in total effective rates but a superior difference in the rates of responses that were considered “cured” and “markedly effective” in favor of the cupping treatment.1,8 However, a case report by Hon et al. has indicated that cupping therapy may be associated with more harm than benefit when used as an eczema treatment.1,9
In addition, it is important to note that the past 5 years have been gamechanging in the management of chronic eczema in terms of the array of novel and effective therapies (e.g., dupilumab and JAK inhibitors) and chronic moderate-to-severe eczema has become very treatable. Similarly, acute eczema is often successfully managed with topical steroids, calcineurin inhibitors, and emollients. As such, there is no compelling reason to consider an unproven treatment such as cupping.
In 2020, Xing et al. reviewed 16 randomized controlled trials assessing the use of “moving cupping” for plaque psoriasis, with 1,164 patients meeting inclusion criteria. Moving cupping was found to be significantly more effective than “no-moving” cupping therapy, and moving cupping, combined with medications, performed better than medications alone.10 None of the trials evaluated in this study included randomized controlled trials that compared patients using any of the more modern psoriasis medications, specifically biologics. And, again, the studies evaluated were not of the highest quality.
The data that support cupping, as summarized above, are based mostly on case reports, and strong double-blind prospective studies are lacking. Additionally, most of the studies cited gauged the efficacy of cupping using qualitative endpoints, rather than standardized quantitative endpoints and scales. Moreover, spontaneous remission of various dermatoses can occur, or they can improve over time, including acute eczema, psoriasis, and, especially, urticaria.
Adverse effects of cupping
Often alternative therapies are seen as “benign” and without adverse effects. However, complications can result from cupping. Trauma can be induced from the cupping itself by damaging superficial blood vessels and causing bruising.1,11 Blistering can also occur secondary to the suction effect, and the epidermal and dermal layers of the skin can be separated.1,11 Further, burns and discoloration have also been noted secondary to heat, trauma, and post inflammatory pigmentary changes.1,11 Another risk of cupping is the Koebner phenomenon, which occurs with psoriasis, with new lesions appearing in traumatized skin.12 Other adverse outcomes that have been reported with cupping include reactivation of herpes simplex virus secondary to skin trauma, iron deficiency anemia (secondary to blood loss), panniculitis, infections, and residual marks mistaken for signs of child abuse.1,11
Cupping in aesthetic dermatology
Facial cupping, a distinct practice from body cupping used to treat general dermatology conditions described previously, is also increasing in popularity. This practice is usually conducted in association with a facial or facial acupuncture by an aesthetician or other licensed professional. It can also be performed using at-home kits. The marketing claims for facial cupping cite improved tightening and contouring of facial skin, increased facial microcirculation and collagen synthesis, and enhanced lymphatic flow to aid with facial puffiness or swelling. One supposed mechanism for these benefits is that cupping increases blood flow. Interestingly, there was a 2020 animal study in which photoacoustic imaging of a mouse ear revealed increased temporary blood flow in the cupping microenvironment.13 Currently, however, there is no evidence in the English scientific literature that supports facial cupping. The benefits attributed to facial cupping for aesthetic purposes have emerged only in personal anecdotes. The temporary increase in blood flow may induce inflammation and swelling that adds volume to the face and temporarily diminishes wrinkles. However, this temporary plumpness may be associated with adverse effects, such as local trauma, irritation, bruising, postinflammatory pigmentary alteration, or even herpes reactivation. In my opinion, the possible adverse effects of cupping outweigh any potential benefit, especially given the insufficient evidence supporting the utility of cupping for cosmetic enhancement.
Summary
There is increasing interest among patients to incorporate complementary and alternative medicine – including the ancient tradition of cupping – in managing medical dermatologic conditions. However, current evidence supporting cupping as an effective therapeutic strategy is not strong, with most studies to date appearing to be of poor quality or not sufficiently convincing to displace standard therapies. Our medical strategies for managing chronic dermatologic conditions, particularly inflammatory disorders, continue to improve from both a safety and a proven efficacy standpoint. Therefore, I would not forgo medical management in favor of cupping. While cupping can be used as an adjunct therapy, I would caution patients about possible adverse side effects. In the aesthetic world, cupping is also gaining popularity, but this trend is also not supported by current evidence or studies, at least in the Western literature.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Write to her at dermnews@mdedge.com or message her on Instragram @DrChloeGoldman. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other disclosures.
References
1. Soliman Y et al. Acta Dermatovenerol Alp Pannonica Adriat. 2018 Jun;27(2):103-7.
2. França K and Lotti T. Advances in Integrative Dermatology. John Wiley & Sons, 2019.
3. Lowe DT. Complement Ther Clin Pract. 2017 Nov;29:162-8.
4.Cao H et al. Altern Ther Health Med. 2010 Nov-Dec;16(6):48-54.
5. Li L and Ding J. J Tradit Chin Med. 2001 Mar;21(1):37-8.
6. Xiao XJ et al. J Integr Med. 2020 Jul;18(4):303-12.
7. Xu J et al. J Tradit Chin Med. 2013 Dec;33(6):752-6.
8. Yao J et al. Zhongguo Zhen Jiu. 2007; Jun;27(6):424-6.
9. Hon KL et al. Case Rep Pediatr. 2013;2013:605829.
10. Xing M et al. Medicine (Baltimore). 2020 Oct 9;99(41):e22539.
11. Kim TH et al. Eur J Integr Med. 2014 Aug 1;6(4):434-40.
12. Vender R and Vender R. J Cutan Med Surg. 2015 May-Jun;19(3):320-2.
13. Zhou Y et al. Biomed Opt Express. 2020 Apr 6;11(5):2394-401.
This article was updated 4/25/22.
My inspiration to write about cupping this month stems from the perception that everyone seems to be talking about it, from a facialist who suggested it for me to a coworker who swears by cupping to treat her allergies. Cupping is by no means a novel procedure. Its use as a health therapy dates back thousands of years to ancient Egypt (1500 BCE), ancient Greece (described by Hippocrates), ancient Rome (described by the Greek physician Galen), China (during the Han dynasty, 206 BCE to 220 CE) and traditional Islamic culture.1 Over the past decade, the popularity of this ancient procedure has been increasing in the United States.1 Cupping has been applied as a remedy for various dermatologic and medical conditions, including herpes zoster, headaches, diminished appetite, maldigestion, abscess evacuation, narcolepsy, pain, fever, dysmenorrhea, and gout.1,2
Theories on the mechanism(s) of action
The practice of cupping is differentiated into dry and wet cupping.1,2 Traditionally, with dry cupping, a flame is applied to heat the air inside a thick glass cup (rather than the cup itself).1 The cup is placed on the skin surface, and negative pressure suctions the skin into the cup. Wet cupping differs mainly from dry cupping in that it involves blood-letting. Cups made of either silicone or glass of varying size and shapes are used. Modern adaptations to cupping include needle, herbal, and pulsatile cupping, as well as a “moving cupping” technique (vs. traditionally stationary cups).1
There are several theories, many of which are derived from the nondermatologic literature (that is, pain management), as to how cupping may deliver a clinical benefit. Some theories are based in scientific and medical principles, whereas other theories are more whimsical – specifically, that cupping draws out evil spirits.2 Studies of dry cupping have suggested that the procedure results in increased oxygenation of muscles via a local increase in oxygenated hemoglobin, which may help improve muscular activity and reduce pain.1 As theorized by Lowe in 2017, negative pressure exerted by dry cupping leads to stretching and dilation of capillaries, which increases blood flow.3 Wet cupping has been shown to increase heat shock protein 70 (HSP70) and beta-endorphin expression in rat models, which is thought to facilitate pain management.1 Removal of oxidants and reduction of reactive oxygen species in the blood is believed to be among the benefits of wet cupping.1
Cupping in general dermatology
While , as well as various inflammatory conditions.
Herpes zoster
In 2010, Cao et al. reported on their systematic review of wet cupping after completing searches of multiple databases (that is, PubMed, the Cochrane Library [Issue 3, 2008], China Network Knowledge Infrastructure, Chinese Scientific Journal Database, and Wan Fang Database). They identified eight randomized controlled trials involving 651 patients, with meta-analyses revealing that wet cupping performed better than medications in terms of the number of “cured” patients, number of patients with improved symptoms, and a lower incidence of postherpetic neuralgia. Wet cupping, in addition to medication, was also found to be superior to medication alone in multiple patients. The researchers concluded that wet cupping appears to effectively treat herpes zoster.4 However, the study failed to identify which medications were used to treat herpes zoster. In the United States, common medications for herpes zoster include acyclovir, valacyclovir, steroids, gabapentin, and other neuromodulators. Without knowing which medications were used, it is difficult to compare cupping to medication in terms of efficacy in treating herpes zoster.
Urticaria
Urticaria (hives) is an inflammatory skin condition that can be very uncomfortable for patients but often resolves without intervention within several months after onset. In 2001, Li and Ding reported on the treatment with cupping of 40 patients with urticaria. The cure rate among the treatment group was cited as 55%, compared with 30% in the control group, who were treated with a traditional Chinese remedy and an unidentified first-generation antihistamine.1,5 In 2020, Xiao et al. conducted a systematic review and meta-analysis of cupping therapy for patients with chronic urticaria. They identified 13 comparisons from 12 randomized controlled trials involving 842 subjects. The investigators found no significant differences between wet cupping and medication usage. They also found that cupping combined with antihistamine treatment was superior to antihistamines alone, and cupping therapy with acupuncture was more effective than acupuncture alone. The investigators did call for caution, citing the poor quality of the studies reviewed.6
It is important to note that it is difficult to attribute resolution of urticaria to the use of cupping given the self-resolution often associated with this condition. Antihistamines are the mainstay of therapy for urticaria, but in my personal experience, patients are not entirely satisfied with the level of symptom control with antihistamines alone and often search for alternative therapies to control the pesky hives and associated itch. In 2014, omalizumab (Xolair) was approved for treating chronic idiopathic urticaria, which has helped patients control symptoms of chronic idiopathic urticaria without needing to take antihistamines. There was no indication that the studies reviewed by Xiao et al. compared cupping against this new effective treatment. Therefore, these studies comparing cupping to medical management are outdated.
Acne, eczema, and psoriasis
Soliman’s 2018 review of cupping in dermatology included a few studies on these common cutaneous conditions. For instance, a 2013 single-blind prospective study by Xu et al. reported on the results of patients with moderate acne who received wet cupping (in the form of prickling bloodletting) twice weekly for 6 weeks.7 They reported that patients demonstrated improvement in the global acne grading system (GAGS) score by the end of the trial.1,7 Unfortunately, cupping was not compared with standard acne treatments (that is, benzoyl peroxide, topical and oral antibiotics, isotretinoin, topical retinoids, spironolactone).
In evaluating cupping for acute eczema, wet cupping was compared with oral loratadine and topical ointments in a 2007 study by Yao and Li. They divided 88 cases into treatment and control groups, with the former group (n = 46) receiving bloodletting puncturing and cupping and the control group (n = 42) receiving oral loratadine and topical Pairuisong (an herbal ointment used in Chinese medicine). The investigators observed no significant difference in total effective rates but a superior difference in the rates of responses that were considered “cured” and “markedly effective” in favor of the cupping treatment.1,8 However, a case report by Hon et al. has indicated that cupping therapy may be associated with more harm than benefit when used as an eczema treatment.1,9
In addition, it is important to note that the past 5 years have been gamechanging in the management of chronic eczema in terms of the array of novel and effective therapies (e.g., dupilumab and JAK inhibitors) and chronic moderate-to-severe eczema has become very treatable. Similarly, acute eczema is often successfully managed with topical steroids, calcineurin inhibitors, and emollients. As such, there is no compelling reason to consider an unproven treatment such as cupping.
In 2020, Xing et al. reviewed 16 randomized controlled trials assessing the use of “moving cupping” for plaque psoriasis, with 1,164 patients meeting inclusion criteria. Moving cupping was found to be significantly more effective than “no-moving” cupping therapy, and moving cupping, combined with medications, performed better than medications alone.10 None of the trials evaluated in this study included randomized controlled trials that compared patients using any of the more modern psoriasis medications, specifically biologics. And, again, the studies evaluated were not of the highest quality.
The data that support cupping, as summarized above, are based mostly on case reports, and strong double-blind prospective studies are lacking. Additionally, most of the studies cited gauged the efficacy of cupping using qualitative endpoints, rather than standardized quantitative endpoints and scales. Moreover, spontaneous remission of various dermatoses can occur, or they can improve over time, including acute eczema, psoriasis, and, especially, urticaria.
Adverse effects of cupping
Often alternative therapies are seen as “benign” and without adverse effects. However, complications can result from cupping. Trauma can be induced from the cupping itself by damaging superficial blood vessels and causing bruising.1,11 Blistering can also occur secondary to the suction effect, and the epidermal and dermal layers of the skin can be separated.1,11 Further, burns and discoloration have also been noted secondary to heat, trauma, and post inflammatory pigmentary changes.1,11 Another risk of cupping is the Koebner phenomenon, which occurs with psoriasis, with new lesions appearing in traumatized skin.12 Other adverse outcomes that have been reported with cupping include reactivation of herpes simplex virus secondary to skin trauma, iron deficiency anemia (secondary to blood loss), panniculitis, infections, and residual marks mistaken for signs of child abuse.1,11
Cupping in aesthetic dermatology
Facial cupping, a distinct practice from body cupping used to treat general dermatology conditions described previously, is also increasing in popularity. This practice is usually conducted in association with a facial or facial acupuncture by an aesthetician or other licensed professional. It can also be performed using at-home kits. The marketing claims for facial cupping cite improved tightening and contouring of facial skin, increased facial microcirculation and collagen synthesis, and enhanced lymphatic flow to aid with facial puffiness or swelling. One supposed mechanism for these benefits is that cupping increases blood flow. Interestingly, there was a 2020 animal study in which photoacoustic imaging of a mouse ear revealed increased temporary blood flow in the cupping microenvironment.13 Currently, however, there is no evidence in the English scientific literature that supports facial cupping. The benefits attributed to facial cupping for aesthetic purposes have emerged only in personal anecdotes. The temporary increase in blood flow may induce inflammation and swelling that adds volume to the face and temporarily diminishes wrinkles. However, this temporary plumpness may be associated with adverse effects, such as local trauma, irritation, bruising, postinflammatory pigmentary alteration, or even herpes reactivation. In my opinion, the possible adverse effects of cupping outweigh any potential benefit, especially given the insufficient evidence supporting the utility of cupping for cosmetic enhancement.
Summary
There is increasing interest among patients to incorporate complementary and alternative medicine – including the ancient tradition of cupping – in managing medical dermatologic conditions. However, current evidence supporting cupping as an effective therapeutic strategy is not strong, with most studies to date appearing to be of poor quality or not sufficiently convincing to displace standard therapies. Our medical strategies for managing chronic dermatologic conditions, particularly inflammatory disorders, continue to improve from both a safety and a proven efficacy standpoint. Therefore, I would not forgo medical management in favor of cupping. While cupping can be used as an adjunct therapy, I would caution patients about possible adverse side effects. In the aesthetic world, cupping is also gaining popularity, but this trend is also not supported by current evidence or studies, at least in the Western literature.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Write to her at dermnews@mdedge.com or message her on Instragram @DrChloeGoldman. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other disclosures.
References
1. Soliman Y et al. Acta Dermatovenerol Alp Pannonica Adriat. 2018 Jun;27(2):103-7.
2. França K and Lotti T. Advances in Integrative Dermatology. John Wiley & Sons, 2019.
3. Lowe DT. Complement Ther Clin Pract. 2017 Nov;29:162-8.
4.Cao H et al. Altern Ther Health Med. 2010 Nov-Dec;16(6):48-54.
5. Li L and Ding J. J Tradit Chin Med. 2001 Mar;21(1):37-8.
6. Xiao XJ et al. J Integr Med. 2020 Jul;18(4):303-12.
7. Xu J et al. J Tradit Chin Med. 2013 Dec;33(6):752-6.
8. Yao J et al. Zhongguo Zhen Jiu. 2007; Jun;27(6):424-6.
9. Hon KL et al. Case Rep Pediatr. 2013;2013:605829.
10. Xing M et al. Medicine (Baltimore). 2020 Oct 9;99(41):e22539.
11. Kim TH et al. Eur J Integr Med. 2014 Aug 1;6(4):434-40.
12. Vender R and Vender R. J Cutan Med Surg. 2015 May-Jun;19(3):320-2.
13. Zhou Y et al. Biomed Opt Express. 2020 Apr 6;11(5):2394-401.
This article was updated 4/25/22.
My inspiration to write about cupping this month stems from the perception that everyone seems to be talking about it, from a facialist who suggested it for me to a coworker who swears by cupping to treat her allergies. Cupping is by no means a novel procedure. Its use as a health therapy dates back thousands of years to ancient Egypt (1500 BCE), ancient Greece (described by Hippocrates), ancient Rome (described by the Greek physician Galen), China (during the Han dynasty, 206 BCE to 220 CE) and traditional Islamic culture.1 Over the past decade, the popularity of this ancient procedure has been increasing in the United States.1 Cupping has been applied as a remedy for various dermatologic and medical conditions, including herpes zoster, headaches, diminished appetite, maldigestion, abscess evacuation, narcolepsy, pain, fever, dysmenorrhea, and gout.1,2
Theories on the mechanism(s) of action
The practice of cupping is differentiated into dry and wet cupping.1,2 Traditionally, with dry cupping, a flame is applied to heat the air inside a thick glass cup (rather than the cup itself).1 The cup is placed on the skin surface, and negative pressure suctions the skin into the cup. Wet cupping differs mainly from dry cupping in that it involves blood-letting. Cups made of either silicone or glass of varying size and shapes are used. Modern adaptations to cupping include needle, herbal, and pulsatile cupping, as well as a “moving cupping” technique (vs. traditionally stationary cups).1
There are several theories, many of which are derived from the nondermatologic literature (that is, pain management), as to how cupping may deliver a clinical benefit. Some theories are based in scientific and medical principles, whereas other theories are more whimsical – specifically, that cupping draws out evil spirits.2 Studies of dry cupping have suggested that the procedure results in increased oxygenation of muscles via a local increase in oxygenated hemoglobin, which may help improve muscular activity and reduce pain.1 As theorized by Lowe in 2017, negative pressure exerted by dry cupping leads to stretching and dilation of capillaries, which increases blood flow.3 Wet cupping has been shown to increase heat shock protein 70 (HSP70) and beta-endorphin expression in rat models, which is thought to facilitate pain management.1 Removal of oxidants and reduction of reactive oxygen species in the blood is believed to be among the benefits of wet cupping.1
Cupping in general dermatology
While , as well as various inflammatory conditions.
Herpes zoster
In 2010, Cao et al. reported on their systematic review of wet cupping after completing searches of multiple databases (that is, PubMed, the Cochrane Library [Issue 3, 2008], China Network Knowledge Infrastructure, Chinese Scientific Journal Database, and Wan Fang Database). They identified eight randomized controlled trials involving 651 patients, with meta-analyses revealing that wet cupping performed better than medications in terms of the number of “cured” patients, number of patients with improved symptoms, and a lower incidence of postherpetic neuralgia. Wet cupping, in addition to medication, was also found to be superior to medication alone in multiple patients. The researchers concluded that wet cupping appears to effectively treat herpes zoster.4 However, the study failed to identify which medications were used to treat herpes zoster. In the United States, common medications for herpes zoster include acyclovir, valacyclovir, steroids, gabapentin, and other neuromodulators. Without knowing which medications were used, it is difficult to compare cupping to medication in terms of efficacy in treating herpes zoster.
Urticaria
Urticaria (hives) is an inflammatory skin condition that can be very uncomfortable for patients but often resolves without intervention within several months after onset. In 2001, Li and Ding reported on the treatment with cupping of 40 patients with urticaria. The cure rate among the treatment group was cited as 55%, compared with 30% in the control group, who were treated with a traditional Chinese remedy and an unidentified first-generation antihistamine.1,5 In 2020, Xiao et al. conducted a systematic review and meta-analysis of cupping therapy for patients with chronic urticaria. They identified 13 comparisons from 12 randomized controlled trials involving 842 subjects. The investigators found no significant differences between wet cupping and medication usage. They also found that cupping combined with antihistamine treatment was superior to antihistamines alone, and cupping therapy with acupuncture was more effective than acupuncture alone. The investigators did call for caution, citing the poor quality of the studies reviewed.6
It is important to note that it is difficult to attribute resolution of urticaria to the use of cupping given the self-resolution often associated with this condition. Antihistamines are the mainstay of therapy for urticaria, but in my personal experience, patients are not entirely satisfied with the level of symptom control with antihistamines alone and often search for alternative therapies to control the pesky hives and associated itch. In 2014, omalizumab (Xolair) was approved for treating chronic idiopathic urticaria, which has helped patients control symptoms of chronic idiopathic urticaria without needing to take antihistamines. There was no indication that the studies reviewed by Xiao et al. compared cupping against this new effective treatment. Therefore, these studies comparing cupping to medical management are outdated.
Acne, eczema, and psoriasis
Soliman’s 2018 review of cupping in dermatology included a few studies on these common cutaneous conditions. For instance, a 2013 single-blind prospective study by Xu et al. reported on the results of patients with moderate acne who received wet cupping (in the form of prickling bloodletting) twice weekly for 6 weeks.7 They reported that patients demonstrated improvement in the global acne grading system (GAGS) score by the end of the trial.1,7 Unfortunately, cupping was not compared with standard acne treatments (that is, benzoyl peroxide, topical and oral antibiotics, isotretinoin, topical retinoids, spironolactone).
In evaluating cupping for acute eczema, wet cupping was compared with oral loratadine and topical ointments in a 2007 study by Yao and Li. They divided 88 cases into treatment and control groups, with the former group (n = 46) receiving bloodletting puncturing and cupping and the control group (n = 42) receiving oral loratadine and topical Pairuisong (an herbal ointment used in Chinese medicine). The investigators observed no significant difference in total effective rates but a superior difference in the rates of responses that were considered “cured” and “markedly effective” in favor of the cupping treatment.1,8 However, a case report by Hon et al. has indicated that cupping therapy may be associated with more harm than benefit when used as an eczema treatment.1,9
In addition, it is important to note that the past 5 years have been gamechanging in the management of chronic eczema in terms of the array of novel and effective therapies (e.g., dupilumab and JAK inhibitors) and chronic moderate-to-severe eczema has become very treatable. Similarly, acute eczema is often successfully managed with topical steroids, calcineurin inhibitors, and emollients. As such, there is no compelling reason to consider an unproven treatment such as cupping.
In 2020, Xing et al. reviewed 16 randomized controlled trials assessing the use of “moving cupping” for plaque psoriasis, with 1,164 patients meeting inclusion criteria. Moving cupping was found to be significantly more effective than “no-moving” cupping therapy, and moving cupping, combined with medications, performed better than medications alone.10 None of the trials evaluated in this study included randomized controlled trials that compared patients using any of the more modern psoriasis medications, specifically biologics. And, again, the studies evaluated were not of the highest quality.
The data that support cupping, as summarized above, are based mostly on case reports, and strong double-blind prospective studies are lacking. Additionally, most of the studies cited gauged the efficacy of cupping using qualitative endpoints, rather than standardized quantitative endpoints and scales. Moreover, spontaneous remission of various dermatoses can occur, or they can improve over time, including acute eczema, psoriasis, and, especially, urticaria.
Adverse effects of cupping
Often alternative therapies are seen as “benign” and without adverse effects. However, complications can result from cupping. Trauma can be induced from the cupping itself by damaging superficial blood vessels and causing bruising.1,11 Blistering can also occur secondary to the suction effect, and the epidermal and dermal layers of the skin can be separated.1,11 Further, burns and discoloration have also been noted secondary to heat, trauma, and post inflammatory pigmentary changes.1,11 Another risk of cupping is the Koebner phenomenon, which occurs with psoriasis, with new lesions appearing in traumatized skin.12 Other adverse outcomes that have been reported with cupping include reactivation of herpes simplex virus secondary to skin trauma, iron deficiency anemia (secondary to blood loss), panniculitis, infections, and residual marks mistaken for signs of child abuse.1,11
Cupping in aesthetic dermatology
Facial cupping, a distinct practice from body cupping used to treat general dermatology conditions described previously, is also increasing in popularity. This practice is usually conducted in association with a facial or facial acupuncture by an aesthetician or other licensed professional. It can also be performed using at-home kits. The marketing claims for facial cupping cite improved tightening and contouring of facial skin, increased facial microcirculation and collagen synthesis, and enhanced lymphatic flow to aid with facial puffiness or swelling. One supposed mechanism for these benefits is that cupping increases blood flow. Interestingly, there was a 2020 animal study in which photoacoustic imaging of a mouse ear revealed increased temporary blood flow in the cupping microenvironment.13 Currently, however, there is no evidence in the English scientific literature that supports facial cupping. The benefits attributed to facial cupping for aesthetic purposes have emerged only in personal anecdotes. The temporary increase in blood flow may induce inflammation and swelling that adds volume to the face and temporarily diminishes wrinkles. However, this temporary plumpness may be associated with adverse effects, such as local trauma, irritation, bruising, postinflammatory pigmentary alteration, or even herpes reactivation. In my opinion, the possible adverse effects of cupping outweigh any potential benefit, especially given the insufficient evidence supporting the utility of cupping for cosmetic enhancement.
Summary
There is increasing interest among patients to incorporate complementary and alternative medicine – including the ancient tradition of cupping – in managing medical dermatologic conditions. However, current evidence supporting cupping as an effective therapeutic strategy is not strong, with most studies to date appearing to be of poor quality or not sufficiently convincing to displace standard therapies. Our medical strategies for managing chronic dermatologic conditions, particularly inflammatory disorders, continue to improve from both a safety and a proven efficacy standpoint. Therefore, I would not forgo medical management in favor of cupping. While cupping can be used as an adjunct therapy, I would caution patients about possible adverse side effects. In the aesthetic world, cupping is also gaining popularity, but this trend is also not supported by current evidence or studies, at least in the Western literature.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Write to her at dermnews@mdedge.com or message her on Instragram @DrChloeGoldman. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other disclosures.
References
1. Soliman Y et al. Acta Dermatovenerol Alp Pannonica Adriat. 2018 Jun;27(2):103-7.
2. França K and Lotti T. Advances in Integrative Dermatology. John Wiley & Sons, 2019.
3. Lowe DT. Complement Ther Clin Pract. 2017 Nov;29:162-8.
4.Cao H et al. Altern Ther Health Med. 2010 Nov-Dec;16(6):48-54.
5. Li L and Ding J. J Tradit Chin Med. 2001 Mar;21(1):37-8.
6. Xiao XJ et al. J Integr Med. 2020 Jul;18(4):303-12.
7. Xu J et al. J Tradit Chin Med. 2013 Dec;33(6):752-6.
8. Yao J et al. Zhongguo Zhen Jiu. 2007; Jun;27(6):424-6.
9. Hon KL et al. Case Rep Pediatr. 2013;2013:605829.
10. Xing M et al. Medicine (Baltimore). 2020 Oct 9;99(41):e22539.
11. Kim TH et al. Eur J Integr Med. 2014 Aug 1;6(4):434-40.
12. Vender R and Vender R. J Cutan Med Surg. 2015 May-Jun;19(3):320-2.
13. Zhou Y et al. Biomed Opt Express. 2020 Apr 6;11(5):2394-401.
This article was updated 4/25/22.