User login
Counterfeit HIV drugs: Justice Department opens investigation
Since the start of the pandemic, supply-chain problems have permeated just about every industry sector. While most of the media attention has focused on toilet paper and retail shipment delays, a darker, more sinister supply chain disruption has been unfolding, one that entails a sophisticated criminal enterprise that has been operating at scale to distribute and profit from counterfeit HIV drugs.
Recently, news has emerged – most notably in the Wall Street Journal – with reports of a Justice Department investigation into what appears to be a national drug trafficking network comprising more than 70 distributors and marketers.
The details read like a best-selling crime novel.
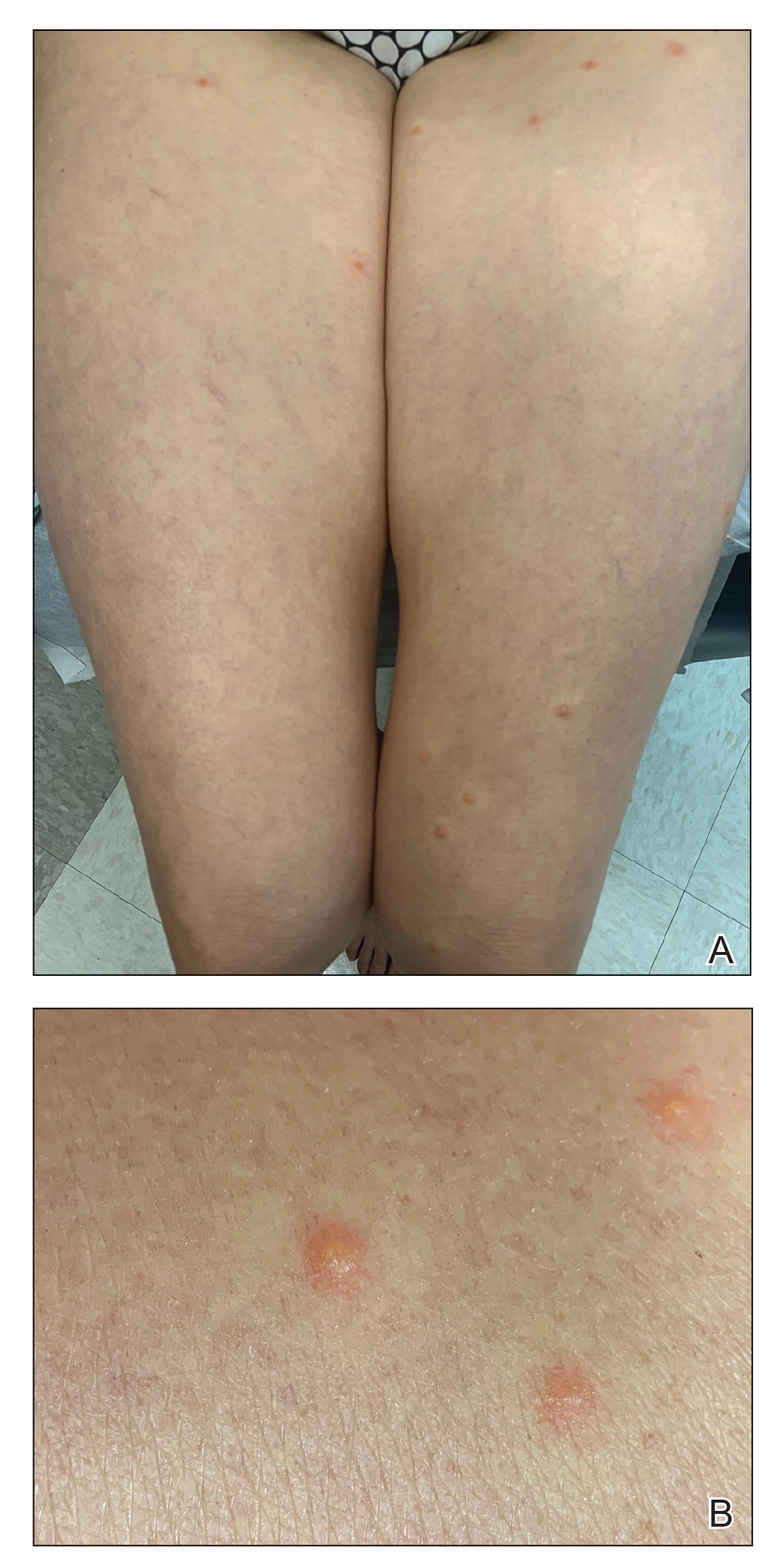
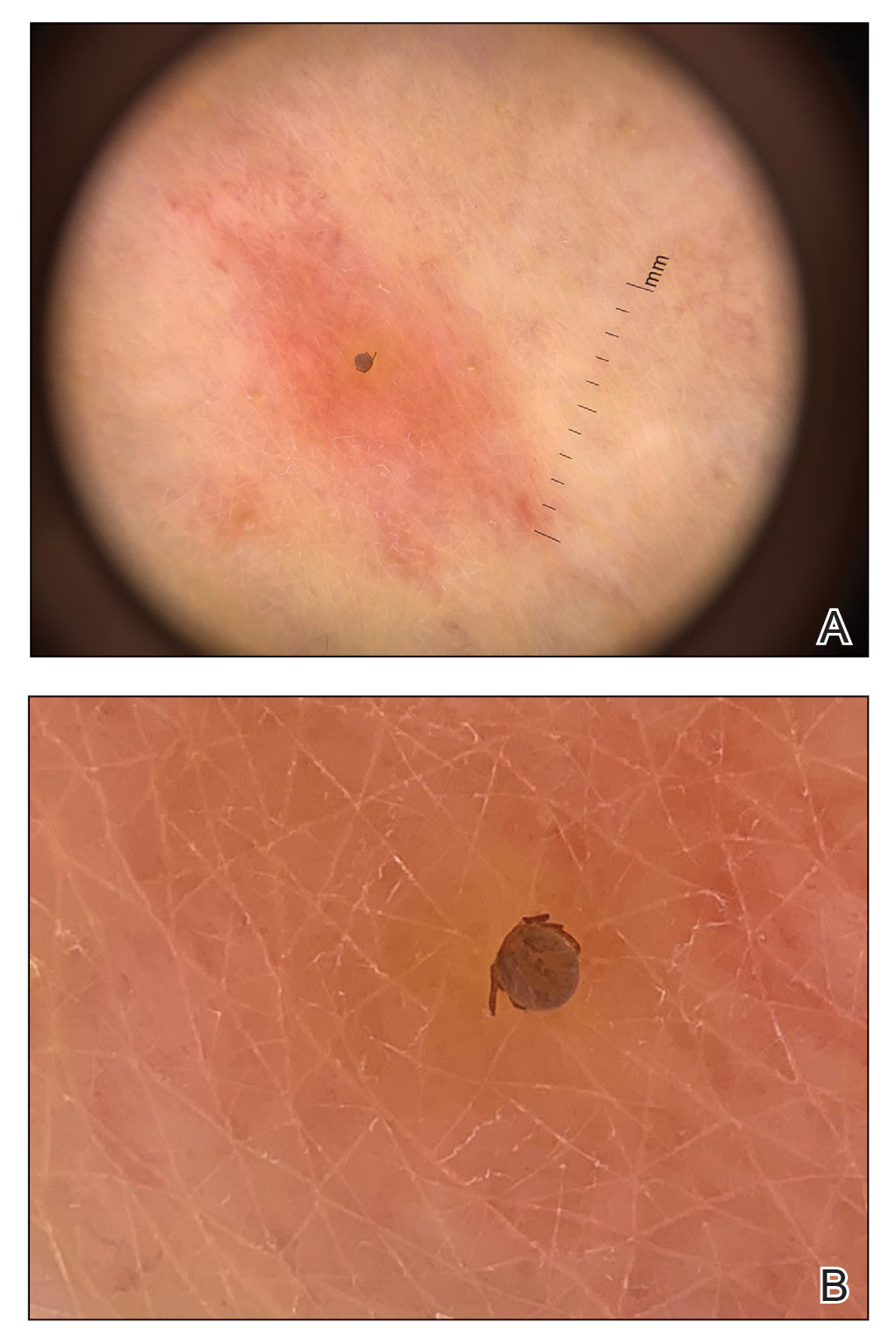
Since last year, authorities have seized 85,247 bottles of counterfeit HIV drugs, both Biktarvy (bictegravir 50 mg, emtricitabine 200 mg, and tenofovir alafenamide 25 mg tablets) and Descovy (emtricitabine 200 mg and tenofovir alafenamide 25 mg tablets). Law enforcement has conducted raids at 17 locations in eight states. Doctored supply chain papers have provided cover for the fake medicines and the individuals behind them.
But unlike the inconvenience of sparse toilet paper, this crime poses life-threatening risks to millions of patients with HIV who rely on Biktarvy to suppress the virus or Descovy to prevent infection from it. Even worse, some patients have been exposed to over-the-counter painkillers or the antipsychotic drug quetiapine fumarate masquerading as HIV drugs in legitimate but repurposed bottles.
Gilead Sciences (Foster City, Calif.), which manufactures both Biktarvy and Descovy, declined to comment when contacted, instead referring this news organization to previous press statements.
Falsified HIV medications, illicit purchases over 2 Years
On Aug. 5, 2021, Gilead first warned the public that it had become aware of tampered and counterfeit Biktarvy and Descovy tablets. In coordination with the Food and Drug Administration, it alerted pharmacies to “investigate the potential for counterfeit or tampered Gilead medication sold by [unauthorized] distributors that may be within their recent supply.”
On Jan. 19, 2022, Gilead issued a second statement outlining ongoing actions in coordination with U.S. marshals and local law enforcement to remove these illegal medications from circulation and prevent further distribution.
The timing of the most recent announcement was not accidental. The day before, a federal judge serving the U.S. District Court for the Eastern District of New York unsealed documents detailing the company’s lawsuit against dozens of individuals and entities who they alleged had engaged in a highly coordinated effort to defraud pharmacies and consumers. The suit followed two prior Gilead filings that ultimately resulted in court-issued ex parte seizure orders (orders that allow a court to seize property without the property owner’s consent) and the recovery of more than 1,000 bottles containing questionable Gilead medications.
The lawsuit centered on Cambridge, Mass.–based wholesale pharmaceutical distributor Safe Chain Solutions and its two cofounders. The document is peppered with terms such as “shifting series of fly-by-night corporate entities,” “gray market” distributors, a “dedicated sales force,” and “shell entities,” along with accusations that the defendants were believed to have made purchases of gold bullion, jewelry, and other luxury items for conversion into cash.
In a curious twist of fate, this sinister effort appeared to have been first revealed not by a pharmacist but by a patient who had returned a bottle of Biktarvy with “foreign medication inside” to the California pharmacy that dispensed it.
“Specifically with HIV medications, there’s no point in which the pharmacy is actually opening the bottle, breaking the seal, and counting out pills to put into a smaller prescription bottle,” Emily Heil, PharmD, BCIDP, AAHIVP, associate professor of infectious diseases in the department of pharmacy practice and science at the University of Maryland School of Pharmacy, Baltimore, told this news organization.
“But that’s also why pharmacies work with these centralized groups of distributors that maintain a chain of command and fidelity with drug manufacturers so that we don’t run into these situations,” she said.
This is the link in the chain where that tightly coordinated and highly regulated process was broken.
Although Gilead and Safe Chain Solutions were informed of the incident as early as August 2020, the distributor repeatedly refused to identify the supplier and the pedigree (the record demonstrating the chain of all sales or transfers of a specific drug, going back to the manufacturer, as required by the FDA’s Drug Supply Chain Security Act in 2013).
Later that year, Janssen Pharmaceutical Companies of Johnson & Johnson issued a media statement saying that they had been alerted to the distribution of counterfeit Symtuza (darunavir/cobicistat/emtricitabine/tenofovir alafenamide) to three pharmacies in the United States.
A spokesperson for the FDA declined to comment on the ongoing investigation when contacted by this news organization and instead wrote in an email that the agency “will continue to use all available tools to ensure consumers and patients have access to a safe and effective medical product supply.”
Old dog, new tricks
This is not the first time that HIV drugs have been targeted for criminal benefit. An analysis published in September 2014 in JAMA highlighted a federal investigation that year into a $32 million dollar scheme to defraud Medicare’s Part D program for HIV drugs and divert them for resale on the black market.
What’s more, prior research and news reports highlight the attractiveness of HIV drug diversion both for the buyer and the seller – not only because of the cost of the drugs themselves but also because of institutional or systemic deficiencies that exclude certain individuals from obtaining treatment through federal initiatives such as the Ryan White/AIDS Drug Assistance program.
In its most recent statement, Gilead reinforced that this practice remains alive and well.
On the buyer side, the company stated, many of the counterfeits originated from suppliers who purchased Gilead HIV medication from individuals after it was first dispensed to them. Unfortunately, the exploitation of individuals with low incomes who experience homelessness or substance use/abuse echoes a pattern whereby HIV patients sell medications to cover personal needs or are forced to buy them on the black market to keep up with their treatment regimens.
On the supply side, All of these counterfeits were sold as though they were legitimate Gilead products.”
But counterfeit pedigrees make it impossible to verify where the products came from, how they have been handled and stored, and what pills are in the bottles – all of which can have dire consequences for patients who ingest them.
The ramifications can be devastating.
“With HIV meds specifically, the worst case scenario would be if the medication is not actually the medication they’re supposed to be on,” said Dr. Heil, reinforcing that the increased safety net provided with viral suppression and against transmission is lost.
Dr. Heil pointed to another significant risk: resistance.
“In a situation like this, where maybe it’s not the full strength of the medication, maybe it’s expired and lost potency or was not stored correctly or is not even the accurate medication, changing those drug level exposures potentially puts the patient at risk for developing resistance to their regimen without them knowing.”
Yet another risk was posed by the replacement of HIV drugs with other medications, such as quetiapine, which increased the risk for life-threatening and irreversible side effects. The lawsuit included a story of a patient who unknowingly took quetiapine after receiving a counterfeit bottle of Biktarvy and could not speak or walk afterward.
Where this tale will ultimately end is unclear. There’s no telling what other activities or bad actors the Justice Department investigation will uncover as it works to unravel the counterfeit network’s activities and deal with its aftermath.
Regardless, clinicians are encouraged to inform HIV patients about the risks associated with counterfeit medications, how to determine whether the drugs they’ve been dispensed are authentic, and to report any product they believe to be counterfeit or to have been tampered with to their doctors, pharmacies, and to Gilead or other drug manufacturers.
“It’s okay to ask questions of your pharmacy about where they get their medications from,” noted Dr. Heil. “If patients have access to an independent pharmacy, it’s a great way for them to have a relationship with their pharmacist.
“We went into this profession to be able to have those conversations with patients,” Dr. Heil said.
The FDA recommends that patients receiving these medications who believe that their drugs may be counterfeit or who experience any adverse effects report the event to FDA’s MedWatch Safety Information and Adverse Event Reporting Program (1-800-FDA-1088 or www.fda.gov/medwatch).
Dr. Heil reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Since the start of the pandemic, supply-chain problems have permeated just about every industry sector. While most of the media attention has focused on toilet paper and retail shipment delays, a darker, more sinister supply chain disruption has been unfolding, one that entails a sophisticated criminal enterprise that has been operating at scale to distribute and profit from counterfeit HIV drugs.
Recently, news has emerged – most notably in the Wall Street Journal – with reports of a Justice Department investigation into what appears to be a national drug trafficking network comprising more than 70 distributors and marketers.
The details read like a best-selling crime novel.
Since last year, authorities have seized 85,247 bottles of counterfeit HIV drugs, both Biktarvy (bictegravir 50 mg, emtricitabine 200 mg, and tenofovir alafenamide 25 mg tablets) and Descovy (emtricitabine 200 mg and tenofovir alafenamide 25 mg tablets). Law enforcement has conducted raids at 17 locations in eight states. Doctored supply chain papers have provided cover for the fake medicines and the individuals behind them.
But unlike the inconvenience of sparse toilet paper, this crime poses life-threatening risks to millions of patients with HIV who rely on Biktarvy to suppress the virus or Descovy to prevent infection from it. Even worse, some patients have been exposed to over-the-counter painkillers or the antipsychotic drug quetiapine fumarate masquerading as HIV drugs in legitimate but repurposed bottles.
Gilead Sciences (Foster City, Calif.), which manufactures both Biktarvy and Descovy, declined to comment when contacted, instead referring this news organization to previous press statements.
Falsified HIV medications, illicit purchases over 2 Years
On Aug. 5, 2021, Gilead first warned the public that it had become aware of tampered and counterfeit Biktarvy and Descovy tablets. In coordination with the Food and Drug Administration, it alerted pharmacies to “investigate the potential for counterfeit or tampered Gilead medication sold by [unauthorized] distributors that may be within their recent supply.”
On Jan. 19, 2022, Gilead issued a second statement outlining ongoing actions in coordination with U.S. marshals and local law enforcement to remove these illegal medications from circulation and prevent further distribution.
The timing of the most recent announcement was not accidental. The day before, a federal judge serving the U.S. District Court for the Eastern District of New York unsealed documents detailing the company’s lawsuit against dozens of individuals and entities who they alleged had engaged in a highly coordinated effort to defraud pharmacies and consumers. The suit followed two prior Gilead filings that ultimately resulted in court-issued ex parte seizure orders (orders that allow a court to seize property without the property owner’s consent) and the recovery of more than 1,000 bottles containing questionable Gilead medications.
The lawsuit centered on Cambridge, Mass.–based wholesale pharmaceutical distributor Safe Chain Solutions and its two cofounders. The document is peppered with terms such as “shifting series of fly-by-night corporate entities,” “gray market” distributors, a “dedicated sales force,” and “shell entities,” along with accusations that the defendants were believed to have made purchases of gold bullion, jewelry, and other luxury items for conversion into cash.
In a curious twist of fate, this sinister effort appeared to have been first revealed not by a pharmacist but by a patient who had returned a bottle of Biktarvy with “foreign medication inside” to the California pharmacy that dispensed it.
“Specifically with HIV medications, there’s no point in which the pharmacy is actually opening the bottle, breaking the seal, and counting out pills to put into a smaller prescription bottle,” Emily Heil, PharmD, BCIDP, AAHIVP, associate professor of infectious diseases in the department of pharmacy practice and science at the University of Maryland School of Pharmacy, Baltimore, told this news organization.
“But that’s also why pharmacies work with these centralized groups of distributors that maintain a chain of command and fidelity with drug manufacturers so that we don’t run into these situations,” she said.
This is the link in the chain where that tightly coordinated and highly regulated process was broken.
Although Gilead and Safe Chain Solutions were informed of the incident as early as August 2020, the distributor repeatedly refused to identify the supplier and the pedigree (the record demonstrating the chain of all sales or transfers of a specific drug, going back to the manufacturer, as required by the FDA’s Drug Supply Chain Security Act in 2013).
Later that year, Janssen Pharmaceutical Companies of Johnson & Johnson issued a media statement saying that they had been alerted to the distribution of counterfeit Symtuza (darunavir/cobicistat/emtricitabine/tenofovir alafenamide) to three pharmacies in the United States.
A spokesperson for the FDA declined to comment on the ongoing investigation when contacted by this news organization and instead wrote in an email that the agency “will continue to use all available tools to ensure consumers and patients have access to a safe and effective medical product supply.”
Old dog, new tricks
This is not the first time that HIV drugs have been targeted for criminal benefit. An analysis published in September 2014 in JAMA highlighted a federal investigation that year into a $32 million dollar scheme to defraud Medicare’s Part D program for HIV drugs and divert them for resale on the black market.
What’s more, prior research and news reports highlight the attractiveness of HIV drug diversion both for the buyer and the seller – not only because of the cost of the drugs themselves but also because of institutional or systemic deficiencies that exclude certain individuals from obtaining treatment through federal initiatives such as the Ryan White/AIDS Drug Assistance program.
In its most recent statement, Gilead reinforced that this practice remains alive and well.
On the buyer side, the company stated, many of the counterfeits originated from suppliers who purchased Gilead HIV medication from individuals after it was first dispensed to them. Unfortunately, the exploitation of individuals with low incomes who experience homelessness or substance use/abuse echoes a pattern whereby HIV patients sell medications to cover personal needs or are forced to buy them on the black market to keep up with their treatment regimens.
On the supply side, All of these counterfeits were sold as though they were legitimate Gilead products.”
But counterfeit pedigrees make it impossible to verify where the products came from, how they have been handled and stored, and what pills are in the bottles – all of which can have dire consequences for patients who ingest them.
The ramifications can be devastating.
“With HIV meds specifically, the worst case scenario would be if the medication is not actually the medication they’re supposed to be on,” said Dr. Heil, reinforcing that the increased safety net provided with viral suppression and against transmission is lost.
Dr. Heil pointed to another significant risk: resistance.
“In a situation like this, where maybe it’s not the full strength of the medication, maybe it’s expired and lost potency or was not stored correctly or is not even the accurate medication, changing those drug level exposures potentially puts the patient at risk for developing resistance to their regimen without them knowing.”
Yet another risk was posed by the replacement of HIV drugs with other medications, such as quetiapine, which increased the risk for life-threatening and irreversible side effects. The lawsuit included a story of a patient who unknowingly took quetiapine after receiving a counterfeit bottle of Biktarvy and could not speak or walk afterward.
Where this tale will ultimately end is unclear. There’s no telling what other activities or bad actors the Justice Department investigation will uncover as it works to unravel the counterfeit network’s activities and deal with its aftermath.
Regardless, clinicians are encouraged to inform HIV patients about the risks associated with counterfeit medications, how to determine whether the drugs they’ve been dispensed are authentic, and to report any product they believe to be counterfeit or to have been tampered with to their doctors, pharmacies, and to Gilead or other drug manufacturers.
“It’s okay to ask questions of your pharmacy about where they get their medications from,” noted Dr. Heil. “If patients have access to an independent pharmacy, it’s a great way for them to have a relationship with their pharmacist.
“We went into this profession to be able to have those conversations with patients,” Dr. Heil said.
The FDA recommends that patients receiving these medications who believe that their drugs may be counterfeit or who experience any adverse effects report the event to FDA’s MedWatch Safety Information and Adverse Event Reporting Program (1-800-FDA-1088 or www.fda.gov/medwatch).
Dr. Heil reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Since the start of the pandemic, supply-chain problems have permeated just about every industry sector. While most of the media attention has focused on toilet paper and retail shipment delays, a darker, more sinister supply chain disruption has been unfolding, one that entails a sophisticated criminal enterprise that has been operating at scale to distribute and profit from counterfeit HIV drugs.
Recently, news has emerged – most notably in the Wall Street Journal – with reports of a Justice Department investigation into what appears to be a national drug trafficking network comprising more than 70 distributors and marketers.
The details read like a best-selling crime novel.
Since last year, authorities have seized 85,247 bottles of counterfeit HIV drugs, both Biktarvy (bictegravir 50 mg, emtricitabine 200 mg, and tenofovir alafenamide 25 mg tablets) and Descovy (emtricitabine 200 mg and tenofovir alafenamide 25 mg tablets). Law enforcement has conducted raids at 17 locations in eight states. Doctored supply chain papers have provided cover for the fake medicines and the individuals behind them.
But unlike the inconvenience of sparse toilet paper, this crime poses life-threatening risks to millions of patients with HIV who rely on Biktarvy to suppress the virus or Descovy to prevent infection from it. Even worse, some patients have been exposed to over-the-counter painkillers or the antipsychotic drug quetiapine fumarate masquerading as HIV drugs in legitimate but repurposed bottles.
Gilead Sciences (Foster City, Calif.), which manufactures both Biktarvy and Descovy, declined to comment when contacted, instead referring this news organization to previous press statements.
Falsified HIV medications, illicit purchases over 2 Years
On Aug. 5, 2021, Gilead first warned the public that it had become aware of tampered and counterfeit Biktarvy and Descovy tablets. In coordination with the Food and Drug Administration, it alerted pharmacies to “investigate the potential for counterfeit or tampered Gilead medication sold by [unauthorized] distributors that may be within their recent supply.”
On Jan. 19, 2022, Gilead issued a second statement outlining ongoing actions in coordination with U.S. marshals and local law enforcement to remove these illegal medications from circulation and prevent further distribution.
The timing of the most recent announcement was not accidental. The day before, a federal judge serving the U.S. District Court for the Eastern District of New York unsealed documents detailing the company’s lawsuit against dozens of individuals and entities who they alleged had engaged in a highly coordinated effort to defraud pharmacies and consumers. The suit followed two prior Gilead filings that ultimately resulted in court-issued ex parte seizure orders (orders that allow a court to seize property without the property owner’s consent) and the recovery of more than 1,000 bottles containing questionable Gilead medications.
The lawsuit centered on Cambridge, Mass.–based wholesale pharmaceutical distributor Safe Chain Solutions and its two cofounders. The document is peppered with terms such as “shifting series of fly-by-night corporate entities,” “gray market” distributors, a “dedicated sales force,” and “shell entities,” along with accusations that the defendants were believed to have made purchases of gold bullion, jewelry, and other luxury items for conversion into cash.
In a curious twist of fate, this sinister effort appeared to have been first revealed not by a pharmacist but by a patient who had returned a bottle of Biktarvy with “foreign medication inside” to the California pharmacy that dispensed it.
“Specifically with HIV medications, there’s no point in which the pharmacy is actually opening the bottle, breaking the seal, and counting out pills to put into a smaller prescription bottle,” Emily Heil, PharmD, BCIDP, AAHIVP, associate professor of infectious diseases in the department of pharmacy practice and science at the University of Maryland School of Pharmacy, Baltimore, told this news organization.
“But that’s also why pharmacies work with these centralized groups of distributors that maintain a chain of command and fidelity with drug manufacturers so that we don’t run into these situations,” she said.
This is the link in the chain where that tightly coordinated and highly regulated process was broken.
Although Gilead and Safe Chain Solutions were informed of the incident as early as August 2020, the distributor repeatedly refused to identify the supplier and the pedigree (the record demonstrating the chain of all sales or transfers of a specific drug, going back to the manufacturer, as required by the FDA’s Drug Supply Chain Security Act in 2013).
Later that year, Janssen Pharmaceutical Companies of Johnson & Johnson issued a media statement saying that they had been alerted to the distribution of counterfeit Symtuza (darunavir/cobicistat/emtricitabine/tenofovir alafenamide) to three pharmacies in the United States.
A spokesperson for the FDA declined to comment on the ongoing investigation when contacted by this news organization and instead wrote in an email that the agency “will continue to use all available tools to ensure consumers and patients have access to a safe and effective medical product supply.”
Old dog, new tricks
This is not the first time that HIV drugs have been targeted for criminal benefit. An analysis published in September 2014 in JAMA highlighted a federal investigation that year into a $32 million dollar scheme to defraud Medicare’s Part D program for HIV drugs and divert them for resale on the black market.
What’s more, prior research and news reports highlight the attractiveness of HIV drug diversion both for the buyer and the seller – not only because of the cost of the drugs themselves but also because of institutional or systemic deficiencies that exclude certain individuals from obtaining treatment through federal initiatives such as the Ryan White/AIDS Drug Assistance program.
In its most recent statement, Gilead reinforced that this practice remains alive and well.
On the buyer side, the company stated, many of the counterfeits originated from suppliers who purchased Gilead HIV medication from individuals after it was first dispensed to them. Unfortunately, the exploitation of individuals with low incomes who experience homelessness or substance use/abuse echoes a pattern whereby HIV patients sell medications to cover personal needs or are forced to buy them on the black market to keep up with their treatment regimens.
On the supply side, All of these counterfeits were sold as though they were legitimate Gilead products.”
But counterfeit pedigrees make it impossible to verify where the products came from, how they have been handled and stored, and what pills are in the bottles – all of which can have dire consequences for patients who ingest them.
The ramifications can be devastating.
“With HIV meds specifically, the worst case scenario would be if the medication is not actually the medication they’re supposed to be on,” said Dr. Heil, reinforcing that the increased safety net provided with viral suppression and against transmission is lost.
Dr. Heil pointed to another significant risk: resistance.
“In a situation like this, where maybe it’s not the full strength of the medication, maybe it’s expired and lost potency or was not stored correctly or is not even the accurate medication, changing those drug level exposures potentially puts the patient at risk for developing resistance to their regimen without them knowing.”
Yet another risk was posed by the replacement of HIV drugs with other medications, such as quetiapine, which increased the risk for life-threatening and irreversible side effects. The lawsuit included a story of a patient who unknowingly took quetiapine after receiving a counterfeit bottle of Biktarvy and could not speak or walk afterward.
Where this tale will ultimately end is unclear. There’s no telling what other activities or bad actors the Justice Department investigation will uncover as it works to unravel the counterfeit network’s activities and deal with its aftermath.
Regardless, clinicians are encouraged to inform HIV patients about the risks associated with counterfeit medications, how to determine whether the drugs they’ve been dispensed are authentic, and to report any product they believe to be counterfeit or to have been tampered with to their doctors, pharmacies, and to Gilead or other drug manufacturers.
“It’s okay to ask questions of your pharmacy about where they get their medications from,” noted Dr. Heil. “If patients have access to an independent pharmacy, it’s a great way for them to have a relationship with their pharmacist.
“We went into this profession to be able to have those conversations with patients,” Dr. Heil said.
The FDA recommends that patients receiving these medications who believe that their drugs may be counterfeit or who experience any adverse effects report the event to FDA’s MedWatch Safety Information and Adverse Event Reporting Program (1-800-FDA-1088 or www.fda.gov/medwatch).
Dr. Heil reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Can gram stains guide antibiotics for pneumonia in critical care?
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Similar outcomes in patients with ventilator-associated pneumonia (VAP) suggest that antibiotics selected by Gram staining were noninferior to those based on guidelines and also significantly decreased the use of broad-spectrum antibiotics in this patient population.
The findings were published in JAMA Network Open. The multicenter, open-label, noninferiority, randomized trial, Gram Stain-Guided Antibiotics Choice for VAP (GRACE-VAP), was conducted for 2 years in intensive care units (ICUs) of a dozen tertiary referral hospitals in Japan, from April 1, 2018, through May 31, 2020.
The authors noted in their paper that the 2016 clinical practice guidelines for VAP published by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society recommend antibiotic agents active against both methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa as an empirical treatment. Adherence to these guidelines may lead to overuse of broad-spectrum antibiotic agents and could be associated with the accelerated emergence of antimicrobial-resistant organisms, the authors postulated.
The study sought to answer the question: Can Gram staining be used as an alternative to established guidelines to direct antibiotic use – thereby curbing the use of broad-spectrum antibiotics – without compromising patient safety and clinical outcomes?
A total of 206 patients, with a mean age of 69, took part in the study. The same number of patients were assigned to each arm. Patients aged 15 years or older with a VAP diagnosis and a modified Clinical Pulmonary Infection Score of 5 or higher were included.
Investigators reported that 79 patients (76.7%) responded to antibiotics in the Gram stain-guided group and 74 (71.8%) responded in the guideline-based group (risk difference, 0.05; 95% confidence interval, –0.07 to 0.17; P < .001, for noninferiority).
There was a decrease in antipseudomonal agent use comparing the Gram stain-guided group with the guideline-based group (30.1%; 95% CI, 21.5% to 39.9%; P < .001). There also was a decrease in anti-MRSA agents in the Gram stain-guided group, compared with the guideline-based group (38.8%; 95% CI, 29.4% to 48.9%; P < .001).
The 28-day cumulative incidence of mortality was 13.6% (n = 14) in the Gram stain-guided group versus 17.5% (n = 18) in the guideline-based group. Escalation of antibiotics according to culture results was performed in seven patients (6.8%) in the Gram stain-guided group and in one patient (1.0%) in the guideline-based group. No significant differences in study arms were observed on other measures, such as ICU-free days, ventilator-free days, and adverse events.
The authors concluded that their findings support the use of Gram staining as a strategy to manage infectious diseases and contain the development of multidrug resistant organisms (MDROs) in the setting of critical care.
“In the GRACE-VAP trial, we used the time-honored Gram stain technique as part of the daily management of infectious diseases. We believe that the trial results are acceptable and have the potential to change the strategy of antibiotic choice worldwide,” the authors wrote.
Benjamin D. Galvan MLS(ASCP), CIC, an infection preventionist with a professional background in clinical microbiology, noted that Gram staining is more accessible and significantly less costly than the rapid polymerase chain reaction testing certain institutions use to rapidly identify MDROs to help tailor therapy.
But one of the pitfalls with relying on Gram stain collection to guide antibiotic use is that it is operator dependent and subject to extrinsic factors, like prior antibiotic use, he pointed out.
“If it is not collected, set up, and read properly, the Gram stain is not going to necessarily be reliable” said Mr. Galvan, also a member of the national communications committee for the Association for Professionals in Infection Control and Epidemiology. He added that the sample in the study was not representative of institutions dealing with elevated rates of multidrug resistance.
“Even from their own results, they were looking at hospitals that have a low rate of multidrug resistance,” he said. “It was not clear if MRSA or just Staphylococcus aureus was identified in significant quantities upon review, and they recognized a lower-than-expected number of isolates of Pseudomonas aeruginosa.”
Establishing antibiotic treatment from the results of Gram-stain collection may not be sufficiently comprehensive, he said.
“Generally speaking, basing it (antibiotic therapy) solely off of a Gram stain is not looking at the whole picture,” said Mr. Galvan, noting that the 2016 IDSA guidelines call for an evaluation of the clinical status, including risk, of the individual patient, as well as locally available antibiotic resistance data.
Moreover, the evidence-based IDSA guidelines are in place to help address the issue of antimicrobial resistance trends, already recommending tailoring empiric antibiotic therapy based upon the levels of resistance in the local population, according to Galvan.
While the study suggests that this Gram-stain-driven tailoring of empiric antibiotic therapy may be noninferior to current guidelines in health care settings with low MDRO rates, its utility may not be suitable in hospitals that are already dealing with high rates of MDROs, such as Pseudomonas aeruginosa and Acinetobacter baumannii, or severe clinical cases of VAP, Mr. Galvan explained.
The researchers and Mr. Galvan disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Adolescents are undertested for STIs
Approximately 20% of sexually active high schoolers reported testing for a sexually transmitted infection in the previous year, based on data from 2,501 respondents to the 2019 national Youth Risk Behavior Survey.
Data suggest that half of all new STIs in the United States occur in youth aged 15-24 years, and that 25% of sexually active young women in the United States have an STI, wrote Nicole Liddon, PhD, of the Centers for Disease Control and Prevention, Atlanta, and colleagues.
Although organizations including the American Academy of Pediatrics and the U.S. Preventive Services Task Force recommend varying degrees of routine STI screening for adolescents, data on the prevalence of testing in this population are limited, the researchers said.
However, the addition in 2019 of a question on STI testing to the national Youth Risk Behavior Survey (YRBS) provided an opportunity to assess prevalence of STI testing, identify potential barriers, and increase screening rates, they wrote.
In a study published in Pediatrics, the researchers reviewed data from the 2019 national YRBS, an anonymous survey administered biannually to public and private high school students across the United States.
The study population included 2,501 individuals who reported sexual activity with at least one person during the 3 months prior to the survey.
Overall, 20.4% of the respondents reported being tested for an STI in the previous year, including significantly more females than males (26.1% vs. 13.7%).
The prevalence of testing among females was not significantly different according to race/ethnicity, sexual identity, or the sex of sexual contacts, but the prevalence increased with age; 12.6%, 22.8%, 28.5%, and 36.9% for females aged 15 years and younger, 16 years, 17 years, and 18 years, respectively.
Among males, no significant differences in STI testing were noted according to race/ethnicity, age, sexual identity, or sex of sexual contacts.
The researchers also analyzed prevalence of STI tested based on sexual behaviors. Among female students, the prevalence of STI testing was higher among those who reported the following behaviors, compared with those who did not: nonuse of condoms at last sexual intercourse (34.1% vs. 18.2%), substance use at last sexual intercourse (32.0% vs. 24.7%), and having four or more lifetime sex partners (31.9% vs. 24.7%).
Among male students, the prevalence of STI testing was higher among those who reported the following behaviors, compared with those who did not: sex before age 13 years (27.1% vs. 12.1%), having two or more recent sex partners (22.4% vs. 10.4%), having four or more lifetime sex partners (22.3% vs. 9.5%), and substance use at last sexual intercourse (19.2% vs. 12.1%).
The low prevalence of STI testing in teens has become more urgent in the wake of the coronavirus pandemic, the researchers wrote. “These prevalence estimates were derived before the possible profound impacts of the pandemic on adolescent sexual behavior and access to and use of health care services.”
Current guidelines allow health care providers the options for opt-out STI screening as a strategy to improve screening rates and testing; however, this option does not eliminate the need for conversations with adolescent patients about sexual activity, they emphasized.
The study findings were limited by several other factors including the inability to directly assess adherence to screening recommendations specifically, the inability to determine whether low testing rates resulted from limited access to health care or missed screening opportunities at visits, and the inclusion only of high school students but not out-of-school youth who may have more limited access to testing.
However, the results highlight the need to improve STI testing services for adolescents, and to address barriers at the individual and clinic level, they said. The addition of a question about past-year STI testing to the 2019 and future YRBS survey will promote ongoing monitoring of efforts to increase testing rates.
Teen sexual health goes beyond testing
The current study shows that routine testing for STIs according to published guidelines is low, Cynthia Holland-Hall, MD, and Andrea E. Bonny, MD, of Nationwide Children’s Hospital and Ohio State University, both in Columbus, wrote in an accompanying editorial.
Notably, sexually active females and males who had sex with male partners, two groups for whom annual testing is specifically recommended by multiple organizations, had testing rates of less than 30%, they said. The authors highlighted the study’s lack of information on which specific barriers, such as lack of access to the health care system, lack of knowledge, and fear of disclosure, contributed to overall low rates of testing.
However, STI testing is only one element of sexual and reproductive health care. Although opt-out testing may improve detection rates, the editorialists emphasized the need for patient-provider conversations about sex, citing recent studies showing that adolescents who spent some time alone with providers were more likely to receive sexual and reproductive health (SRH) services in the past year.
“Resources such as confidentiality policies, checklists, and written screening tools may facilitate SRH discussions,” Dr. Holland-Hall and Dr. Bonny said. “With a little practice, respect, and intention, a caring provider can take the awkward out of discussing sexual health but must not opt out of the conversation.”
Privacy and time issues exacerbate low testing rates
The current study is especially important at this time because many adolescents have likely missed well visits, and therefore important STI screens, because of disruptions caused by the COVID-19 pandemic, Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
“I was surprised that the rate of screening was only one in five,” said Dr. Kinsella. “I knew it would be suboptimal, but not that low.”
According to Dr. Kinsella, there are two major barriers to increasing STI testing in adolescents in primary care. One barrier is that insurance companies will often state on the bill what the testing was for, which will lead to an uncomfortable conversation at a later date for the adolescent and parent when the bill arrives in the mail. A second barrier is when to test during a visit,. “If we obtain urine samples on all adolescents and many of them are not sexually active, we are wasting a lot of time in the short visit to obtain urine,” she explained. “If testing is scheduled for the end of the visit, they often leave without providing a urine sample.”
Overall, the study is an important reminder to general pediatricians about STI testing for sexually active teens, she emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Approximately 20% of sexually active high schoolers reported testing for a sexually transmitted infection in the previous year, based on data from 2,501 respondents to the 2019 national Youth Risk Behavior Survey.
Data suggest that half of all new STIs in the United States occur in youth aged 15-24 years, and that 25% of sexually active young women in the United States have an STI, wrote Nicole Liddon, PhD, of the Centers for Disease Control and Prevention, Atlanta, and colleagues.
Although organizations including the American Academy of Pediatrics and the U.S. Preventive Services Task Force recommend varying degrees of routine STI screening for adolescents, data on the prevalence of testing in this population are limited, the researchers said.
However, the addition in 2019 of a question on STI testing to the national Youth Risk Behavior Survey (YRBS) provided an opportunity to assess prevalence of STI testing, identify potential barriers, and increase screening rates, they wrote.
In a study published in Pediatrics, the researchers reviewed data from the 2019 national YRBS, an anonymous survey administered biannually to public and private high school students across the United States.
The study population included 2,501 individuals who reported sexual activity with at least one person during the 3 months prior to the survey.
Overall, 20.4% of the respondents reported being tested for an STI in the previous year, including significantly more females than males (26.1% vs. 13.7%).
The prevalence of testing among females was not significantly different according to race/ethnicity, sexual identity, or the sex of sexual contacts, but the prevalence increased with age; 12.6%, 22.8%, 28.5%, and 36.9% for females aged 15 years and younger, 16 years, 17 years, and 18 years, respectively.
Among males, no significant differences in STI testing were noted according to race/ethnicity, age, sexual identity, or sex of sexual contacts.
The researchers also analyzed prevalence of STI tested based on sexual behaviors. Among female students, the prevalence of STI testing was higher among those who reported the following behaviors, compared with those who did not: nonuse of condoms at last sexual intercourse (34.1% vs. 18.2%), substance use at last sexual intercourse (32.0% vs. 24.7%), and having four or more lifetime sex partners (31.9% vs. 24.7%).
Among male students, the prevalence of STI testing was higher among those who reported the following behaviors, compared with those who did not: sex before age 13 years (27.1% vs. 12.1%), having two or more recent sex partners (22.4% vs. 10.4%), having four or more lifetime sex partners (22.3% vs. 9.5%), and substance use at last sexual intercourse (19.2% vs. 12.1%).
The low prevalence of STI testing in teens has become more urgent in the wake of the coronavirus pandemic, the researchers wrote. “These prevalence estimates were derived before the possible profound impacts of the pandemic on adolescent sexual behavior and access to and use of health care services.”
Current guidelines allow health care providers the options for opt-out STI screening as a strategy to improve screening rates and testing; however, this option does not eliminate the need for conversations with adolescent patients about sexual activity, they emphasized.
The study findings were limited by several other factors including the inability to directly assess adherence to screening recommendations specifically, the inability to determine whether low testing rates resulted from limited access to health care or missed screening opportunities at visits, and the inclusion only of high school students but not out-of-school youth who may have more limited access to testing.
However, the results highlight the need to improve STI testing services for adolescents, and to address barriers at the individual and clinic level, they said. The addition of a question about past-year STI testing to the 2019 and future YRBS survey will promote ongoing monitoring of efforts to increase testing rates.
Teen sexual health goes beyond testing
The current study shows that routine testing for STIs according to published guidelines is low, Cynthia Holland-Hall, MD, and Andrea E. Bonny, MD, of Nationwide Children’s Hospital and Ohio State University, both in Columbus, wrote in an accompanying editorial.
Notably, sexually active females and males who had sex with male partners, two groups for whom annual testing is specifically recommended by multiple organizations, had testing rates of less than 30%, they said. The authors highlighted the study’s lack of information on which specific barriers, such as lack of access to the health care system, lack of knowledge, and fear of disclosure, contributed to overall low rates of testing.
However, STI testing is only one element of sexual and reproductive health care. Although opt-out testing may improve detection rates, the editorialists emphasized the need for patient-provider conversations about sex, citing recent studies showing that adolescents who spent some time alone with providers were more likely to receive sexual and reproductive health (SRH) services in the past year.
“Resources such as confidentiality policies, checklists, and written screening tools may facilitate SRH discussions,” Dr. Holland-Hall and Dr. Bonny said. “With a little practice, respect, and intention, a caring provider can take the awkward out of discussing sexual health but must not opt out of the conversation.”
Privacy and time issues exacerbate low testing rates
The current study is especially important at this time because many adolescents have likely missed well visits, and therefore important STI screens, because of disruptions caused by the COVID-19 pandemic, Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
“I was surprised that the rate of screening was only one in five,” said Dr. Kinsella. “I knew it would be suboptimal, but not that low.”
According to Dr. Kinsella, there are two major barriers to increasing STI testing in adolescents in primary care. One barrier is that insurance companies will often state on the bill what the testing was for, which will lead to an uncomfortable conversation at a later date for the adolescent and parent when the bill arrives in the mail. A second barrier is when to test during a visit,. “If we obtain urine samples on all adolescents and many of them are not sexually active, we are wasting a lot of time in the short visit to obtain urine,” she explained. “If testing is scheduled for the end of the visit, they often leave without providing a urine sample.”
Overall, the study is an important reminder to general pediatricians about STI testing for sexually active teens, she emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
Approximately 20% of sexually active high schoolers reported testing for a sexually transmitted infection in the previous year, based on data from 2,501 respondents to the 2019 national Youth Risk Behavior Survey.
Data suggest that half of all new STIs in the United States occur in youth aged 15-24 years, and that 25% of sexually active young women in the United States have an STI, wrote Nicole Liddon, PhD, of the Centers for Disease Control and Prevention, Atlanta, and colleagues.
Although organizations including the American Academy of Pediatrics and the U.S. Preventive Services Task Force recommend varying degrees of routine STI screening for adolescents, data on the prevalence of testing in this population are limited, the researchers said.
However, the addition in 2019 of a question on STI testing to the national Youth Risk Behavior Survey (YRBS) provided an opportunity to assess prevalence of STI testing, identify potential barriers, and increase screening rates, they wrote.
In a study published in Pediatrics, the researchers reviewed data from the 2019 national YRBS, an anonymous survey administered biannually to public and private high school students across the United States.
The study population included 2,501 individuals who reported sexual activity with at least one person during the 3 months prior to the survey.
Overall, 20.4% of the respondents reported being tested for an STI in the previous year, including significantly more females than males (26.1% vs. 13.7%).
The prevalence of testing among females was not significantly different according to race/ethnicity, sexual identity, or the sex of sexual contacts, but the prevalence increased with age; 12.6%, 22.8%, 28.5%, and 36.9% for females aged 15 years and younger, 16 years, 17 years, and 18 years, respectively.
Among males, no significant differences in STI testing were noted according to race/ethnicity, age, sexual identity, or sex of sexual contacts.
The researchers also analyzed prevalence of STI tested based on sexual behaviors. Among female students, the prevalence of STI testing was higher among those who reported the following behaviors, compared with those who did not: nonuse of condoms at last sexual intercourse (34.1% vs. 18.2%), substance use at last sexual intercourse (32.0% vs. 24.7%), and having four or more lifetime sex partners (31.9% vs. 24.7%).
Among male students, the prevalence of STI testing was higher among those who reported the following behaviors, compared with those who did not: sex before age 13 years (27.1% vs. 12.1%), having two or more recent sex partners (22.4% vs. 10.4%), having four or more lifetime sex partners (22.3% vs. 9.5%), and substance use at last sexual intercourse (19.2% vs. 12.1%).
The low prevalence of STI testing in teens has become more urgent in the wake of the coronavirus pandemic, the researchers wrote. “These prevalence estimates were derived before the possible profound impacts of the pandemic on adolescent sexual behavior and access to and use of health care services.”
Current guidelines allow health care providers the options for opt-out STI screening as a strategy to improve screening rates and testing; however, this option does not eliminate the need for conversations with adolescent patients about sexual activity, they emphasized.
The study findings were limited by several other factors including the inability to directly assess adherence to screening recommendations specifically, the inability to determine whether low testing rates resulted from limited access to health care or missed screening opportunities at visits, and the inclusion only of high school students but not out-of-school youth who may have more limited access to testing.
However, the results highlight the need to improve STI testing services for adolescents, and to address barriers at the individual and clinic level, they said. The addition of a question about past-year STI testing to the 2019 and future YRBS survey will promote ongoing monitoring of efforts to increase testing rates.
Teen sexual health goes beyond testing
The current study shows that routine testing for STIs according to published guidelines is low, Cynthia Holland-Hall, MD, and Andrea E. Bonny, MD, of Nationwide Children’s Hospital and Ohio State University, both in Columbus, wrote in an accompanying editorial.
Notably, sexually active females and males who had sex with male partners, two groups for whom annual testing is specifically recommended by multiple organizations, had testing rates of less than 30%, they said. The authors highlighted the study’s lack of information on which specific barriers, such as lack of access to the health care system, lack of knowledge, and fear of disclosure, contributed to overall low rates of testing.
However, STI testing is only one element of sexual and reproductive health care. Although opt-out testing may improve detection rates, the editorialists emphasized the need for patient-provider conversations about sex, citing recent studies showing that adolescents who spent some time alone with providers were more likely to receive sexual and reproductive health (SRH) services in the past year.
“Resources such as confidentiality policies, checklists, and written screening tools may facilitate SRH discussions,” Dr. Holland-Hall and Dr. Bonny said. “With a little practice, respect, and intention, a caring provider can take the awkward out of discussing sexual health but must not opt out of the conversation.”
Privacy and time issues exacerbate low testing rates
The current study is especially important at this time because many adolescents have likely missed well visits, and therefore important STI screens, because of disruptions caused by the COVID-19 pandemic, Karalyn Kinsella, MD, a pediatrician in private practice in Cheshire, Conn., said in an interview.
“I was surprised that the rate of screening was only one in five,” said Dr. Kinsella. “I knew it would be suboptimal, but not that low.”
According to Dr. Kinsella, there are two major barriers to increasing STI testing in adolescents in primary care. One barrier is that insurance companies will often state on the bill what the testing was for, which will lead to an uncomfortable conversation at a later date for the adolescent and parent when the bill arrives in the mail. A second barrier is when to test during a visit,. “If we obtain urine samples on all adolescents and many of them are not sexually active, we are wasting a lot of time in the short visit to obtain urine,” she explained. “If testing is scheduled for the end of the visit, they often leave without providing a urine sample.”
Overall, the study is an important reminder to general pediatricians about STI testing for sexually active teens, she emphasized.
The study received no outside funding. The researchers had no financial conflicts to disclose. The editorialists had no financial conflicts to disclose. Dr. Kinsella had no financial conflicts to disclose and serves on the editorial advisory board of Pediatric News.
FROM PEDIATRICS
Protease inhibitors increase small-for-gestational-age but not other pregnancy risks
Pregnant women with HIV can be reassured that protease inhibitors are safer than previously thought in terms of risk to the fetus, according to research from the National Perinatal Epidemiology Unit (NPEU) at Oxford Population Health, a research institute based at the University of Oxford (England).
Antiretroviral therapy (ART) is recommended for all pregnant women living with HIV and plays a crucial role both in improving maternal health and in reducing transmission of HIV from mother to child. However, there has been a critical lack of evidence about the effects of ART on the risk of adverse pregnancy outcomes, with particular concern about protease inhibitors.
Current guidelines recommend that protease inhibitor-based therapies should be used in pregnancy only if first-line treatments (such as integrase and reverse-transcriptase based treatments) are either unsuitable or unavailable. These guidelines also often advise against the use of a specific protease inhibitor, lopinavir/ritonavir, citing an increased risk of preterm birth. However, such advice may restrict treatment options for pregnant women with HIV on the basis of limited evidence.
Largest review to date
The NPEU researchers, therefore, conducted the largest systematic review to date of adverse perinatal outcomes after a range of antiretroviral therapies. It included 34 cohort studies published between 1980 and 2020 and involving over 57,000 pregnant women with HIV in 22 different countries. The review, published in eClinicalMedicine, looked for evidence of 11 perinatal outcomes:
- Preterm birth, very preterm birth, and spontaneous preterm birth
- Low birth weight, very low birth weight, term low birth weight, and preterm low birth weight
- Small for gestational age and very small for gestational age
- Stillbirth, and neonatal death
Using pairwise random-effects meta-analyses, researchers compared protease inhibitor versus non-protease inhibitor-based ART, as well as specifically looking at the comparative risks associated with different protease inhibitor regimens.
They found that protease inhibitor-based ART significantly increased the risk of small or very small for gestational age babies, with relative risks of 1.24 (95% confidence interval, 1.08-1.43; I2 = 66.7%) and 1.40 (95% CI, 1.09-1.81; I2 = 0.0%), respectively. However there were no significant differences in other adverse pregnancy outcomes for protease inhibitors, compared with other therapies.
In addition, researchers found no significant differences in perinatal outcomes between ART regimens containing lopinavir/ritonavir, atazanavir/ritonavir, or darunavir/ritonavir, which are the most frequently used protease inhibitors.
No increased risk of preterm birth
Senior author Dr. Joris Hemelaar, senior clinical research fellow at the NPEU and honorary consultant in obstetrics at the John Radcliffe Hospital, Oxford (England), said: “Antiretroviral therapy in pregnancy has clear benefits for maternal health and prevention of HIV transmission to the child, but our study has shown for the first time that protease inhibitors are associated with babies being small or very small for their gestational age.”
“However, there was no increased risk of preterm birth, or any other adverse pregnancy outcomes. This means protease inhibitors remain an important option for pregnant women living with HIV if other treatments are unsuitable, for example due to drug resistance, or unavailable. The evidence presented here indicates that the commonly used protease inhibitors atazanavir, lopinavir, and darunavir are comparable with regard to perinatal outcomes, which should inform international treatment guidelines.”
Over 70% of the studies assessed were conducted in high-income countries, and Dr. Hemelaar added that there is an urgent need for more research on pregnancy outcomes after different ART in low- to middle-income countries, where the burden of HIV is highest.
Professor Yvonne Gilleece, a spokesperson for the British HIV Association (BHIVA) and immediate past chair of the BHIVA guidelines on the management of HIV in pregnancy and the postpartum period commented: “Pregnancy is a unique life situation in which we must consider the safety of both the birthing parent and the baby. Due to ongoing under-representation of all women in clinical trials, but particularly pregnant women, we do not have enough evidence on which to base all our management decisions. This systematic review includes large numbers of pregnant women living with HIV and can, therefore, improve an informed discussion regarding the safety of the use of protease inhibitors during pregnancy.”
Dr. Hemelaar told Medscape UK: “Many international treatment guidelines cite adverse pregnancy outcomes, in particular preterm birth, associated with protease inhibitor (PI)-drugs as a reason for caution for their use in pregnancy. However, PI drugs are not associated with preterm birth in our analysis. This suggests that PI drugs may not be as detrimental as previously thought (and we found no differences between different PI drugs used), and, hence, these drugs may have a more favourable profile for use in pregnancy.
“However, many other aspects of treatment, including the extent to which the virus can be suppressed, adverse drug effects, adherence to drug prescriptions, antiretroviral drug resistance, drug interactions, drug cost, and availability, should also be taken into account by clinicians and guideline development committees.”
A version of this article first appeared on Medscape UK.
Pregnant women with HIV can be reassured that protease inhibitors are safer than previously thought in terms of risk to the fetus, according to research from the National Perinatal Epidemiology Unit (NPEU) at Oxford Population Health, a research institute based at the University of Oxford (England).
Antiretroviral therapy (ART) is recommended for all pregnant women living with HIV and plays a crucial role both in improving maternal health and in reducing transmission of HIV from mother to child. However, there has been a critical lack of evidence about the effects of ART on the risk of adverse pregnancy outcomes, with particular concern about protease inhibitors.
Current guidelines recommend that protease inhibitor-based therapies should be used in pregnancy only if first-line treatments (such as integrase and reverse-transcriptase based treatments) are either unsuitable or unavailable. These guidelines also often advise against the use of a specific protease inhibitor, lopinavir/ritonavir, citing an increased risk of preterm birth. However, such advice may restrict treatment options for pregnant women with HIV on the basis of limited evidence.
Largest review to date
The NPEU researchers, therefore, conducted the largest systematic review to date of adverse perinatal outcomes after a range of antiretroviral therapies. It included 34 cohort studies published between 1980 and 2020 and involving over 57,000 pregnant women with HIV in 22 different countries. The review, published in eClinicalMedicine, looked for evidence of 11 perinatal outcomes:
- Preterm birth, very preterm birth, and spontaneous preterm birth
- Low birth weight, very low birth weight, term low birth weight, and preterm low birth weight
- Small for gestational age and very small for gestational age
- Stillbirth, and neonatal death
Using pairwise random-effects meta-analyses, researchers compared protease inhibitor versus non-protease inhibitor-based ART, as well as specifically looking at the comparative risks associated with different protease inhibitor regimens.
They found that protease inhibitor-based ART significantly increased the risk of small or very small for gestational age babies, with relative risks of 1.24 (95% confidence interval, 1.08-1.43; I2 = 66.7%) and 1.40 (95% CI, 1.09-1.81; I2 = 0.0%), respectively. However there were no significant differences in other adverse pregnancy outcomes for protease inhibitors, compared with other therapies.
In addition, researchers found no significant differences in perinatal outcomes between ART regimens containing lopinavir/ritonavir, atazanavir/ritonavir, or darunavir/ritonavir, which are the most frequently used protease inhibitors.
No increased risk of preterm birth
Senior author Dr. Joris Hemelaar, senior clinical research fellow at the NPEU and honorary consultant in obstetrics at the John Radcliffe Hospital, Oxford (England), said: “Antiretroviral therapy in pregnancy has clear benefits for maternal health and prevention of HIV transmission to the child, but our study has shown for the first time that protease inhibitors are associated with babies being small or very small for their gestational age.”
“However, there was no increased risk of preterm birth, or any other adverse pregnancy outcomes. This means protease inhibitors remain an important option for pregnant women living with HIV if other treatments are unsuitable, for example due to drug resistance, or unavailable. The evidence presented here indicates that the commonly used protease inhibitors atazanavir, lopinavir, and darunavir are comparable with regard to perinatal outcomes, which should inform international treatment guidelines.”
Over 70% of the studies assessed were conducted in high-income countries, and Dr. Hemelaar added that there is an urgent need for more research on pregnancy outcomes after different ART in low- to middle-income countries, where the burden of HIV is highest.
Professor Yvonne Gilleece, a spokesperson for the British HIV Association (BHIVA) and immediate past chair of the BHIVA guidelines on the management of HIV in pregnancy and the postpartum period commented: “Pregnancy is a unique life situation in which we must consider the safety of both the birthing parent and the baby. Due to ongoing under-representation of all women in clinical trials, but particularly pregnant women, we do not have enough evidence on which to base all our management decisions. This systematic review includes large numbers of pregnant women living with HIV and can, therefore, improve an informed discussion regarding the safety of the use of protease inhibitors during pregnancy.”
Dr. Hemelaar told Medscape UK: “Many international treatment guidelines cite adverse pregnancy outcomes, in particular preterm birth, associated with protease inhibitor (PI)-drugs as a reason for caution for their use in pregnancy. However, PI drugs are not associated with preterm birth in our analysis. This suggests that PI drugs may not be as detrimental as previously thought (and we found no differences between different PI drugs used), and, hence, these drugs may have a more favourable profile for use in pregnancy.
“However, many other aspects of treatment, including the extent to which the virus can be suppressed, adverse drug effects, adherence to drug prescriptions, antiretroviral drug resistance, drug interactions, drug cost, and availability, should also be taken into account by clinicians and guideline development committees.”
A version of this article first appeared on Medscape UK.
Pregnant women with HIV can be reassured that protease inhibitors are safer than previously thought in terms of risk to the fetus, according to research from the National Perinatal Epidemiology Unit (NPEU) at Oxford Population Health, a research institute based at the University of Oxford (England).
Antiretroviral therapy (ART) is recommended for all pregnant women living with HIV and plays a crucial role both in improving maternal health and in reducing transmission of HIV from mother to child. However, there has been a critical lack of evidence about the effects of ART on the risk of adverse pregnancy outcomes, with particular concern about protease inhibitors.
Current guidelines recommend that protease inhibitor-based therapies should be used in pregnancy only if first-line treatments (such as integrase and reverse-transcriptase based treatments) are either unsuitable or unavailable. These guidelines also often advise against the use of a specific protease inhibitor, lopinavir/ritonavir, citing an increased risk of preterm birth. However, such advice may restrict treatment options for pregnant women with HIV on the basis of limited evidence.
Largest review to date
The NPEU researchers, therefore, conducted the largest systematic review to date of adverse perinatal outcomes after a range of antiretroviral therapies. It included 34 cohort studies published between 1980 and 2020 and involving over 57,000 pregnant women with HIV in 22 different countries. The review, published in eClinicalMedicine, looked for evidence of 11 perinatal outcomes:
- Preterm birth, very preterm birth, and spontaneous preterm birth
- Low birth weight, very low birth weight, term low birth weight, and preterm low birth weight
- Small for gestational age and very small for gestational age
- Stillbirth, and neonatal death
Using pairwise random-effects meta-analyses, researchers compared protease inhibitor versus non-protease inhibitor-based ART, as well as specifically looking at the comparative risks associated with different protease inhibitor regimens.
They found that protease inhibitor-based ART significantly increased the risk of small or very small for gestational age babies, with relative risks of 1.24 (95% confidence interval, 1.08-1.43; I2 = 66.7%) and 1.40 (95% CI, 1.09-1.81; I2 = 0.0%), respectively. However there were no significant differences in other adverse pregnancy outcomes for protease inhibitors, compared with other therapies.
In addition, researchers found no significant differences in perinatal outcomes between ART regimens containing lopinavir/ritonavir, atazanavir/ritonavir, or darunavir/ritonavir, which are the most frequently used protease inhibitors.
No increased risk of preterm birth
Senior author Dr. Joris Hemelaar, senior clinical research fellow at the NPEU and honorary consultant in obstetrics at the John Radcliffe Hospital, Oxford (England), said: “Antiretroviral therapy in pregnancy has clear benefits for maternal health and prevention of HIV transmission to the child, but our study has shown for the first time that protease inhibitors are associated with babies being small or very small for their gestational age.”
“However, there was no increased risk of preterm birth, or any other adverse pregnancy outcomes. This means protease inhibitors remain an important option for pregnant women living with HIV if other treatments are unsuitable, for example due to drug resistance, or unavailable. The evidence presented here indicates that the commonly used protease inhibitors atazanavir, lopinavir, and darunavir are comparable with regard to perinatal outcomes, which should inform international treatment guidelines.”
Over 70% of the studies assessed were conducted in high-income countries, and Dr. Hemelaar added that there is an urgent need for more research on pregnancy outcomes after different ART in low- to middle-income countries, where the burden of HIV is highest.
Professor Yvonne Gilleece, a spokesperson for the British HIV Association (BHIVA) and immediate past chair of the BHIVA guidelines on the management of HIV in pregnancy and the postpartum period commented: “Pregnancy is a unique life situation in which we must consider the safety of both the birthing parent and the baby. Due to ongoing under-representation of all women in clinical trials, but particularly pregnant women, we do not have enough evidence on which to base all our management decisions. This systematic review includes large numbers of pregnant women living with HIV and can, therefore, improve an informed discussion regarding the safety of the use of protease inhibitors during pregnancy.”
Dr. Hemelaar told Medscape UK: “Many international treatment guidelines cite adverse pregnancy outcomes, in particular preterm birth, associated with protease inhibitor (PI)-drugs as a reason for caution for their use in pregnancy. However, PI drugs are not associated with preterm birth in our analysis. This suggests that PI drugs may not be as detrimental as previously thought (and we found no differences between different PI drugs used), and, hence, these drugs may have a more favourable profile for use in pregnancy.
“However, many other aspects of treatment, including the extent to which the virus can be suppressed, adverse drug effects, adherence to drug prescriptions, antiretroviral drug resistance, drug interactions, drug cost, and availability, should also be taken into account by clinicians and guideline development committees.”
A version of this article first appeared on Medscape UK.
FROM ECLINICALMEDICINE
Pneumonia shows strong connection to chronic otitis media
Individuals with a prior diagnosis of pneumonia were significantly more likely to develop chronic otitis media (COM) than were those without a history of pneumonia, based on data from a nationwide cohort study of more than 100,000 patients.
“Recently, middle ear diseases, including COM, have been recognized as respiratory tract diseases beyond the pathophysiological concepts of ventilation dysfunction, with recurrent infection that occurs from anatomically adjacent structures such as the middle ear, mastoid cavity, and eustachian tube,” but the potential link between pneumonia and chronic otitis media and adults in particular has not been examined, wrote Sung Kyun Kim, MD, of Hallym University, Dongtan, South Korea, and colleagues.
In a study recently published in the International Journal of Infectious Diseases, the researchers identified 23,436 adults with COM and 93,744 controls aged 40 years and older from a Korean health insurance database between 2002 and 2015.
The overall incidence of pneumonia in the study population was significantly higher in the COM group compared with controls (9.3% vs. 7.2%, P <.001). The odds ratios of pneumonia were significantly higher in the COM group compared with controls, and a history of pneumonia increased the odds of COM regardless of sex and across all ages.
Pneumonia was defined as when a patient had a diagnosis of pneumonia based on ICD-10 codes and underwent a chest x-ray or chest CT scan. Chronic otitis media was defined as when a patient had a diagnosis based on ICD-10 codes at least two times with one of the following conditions: chronic serous otitis media, chronic mucoid otitis media, other chronic nonsuppurative otitis media, unspecified nonsuppurative otitis media, chronic tubotympanic suppurative otitis media, chronic atticoantral suppurative otitis media, other chronic suppurative otitis media, or unspecified suppurative otitis media.
Age groups were divided into 5-year intervals, and patients were classified into income groups and rural vs. urban residence.
In a further sensitivity analysis, individuals who were diagnosed with pneumonia five or more times before the index date had a significantly higher odds ratio for COM compared with those with less than five diagnoses of pneumonia (adjusted odds ratio, 1.34; P < .001).
Microbiome dysbiosis may explain part of the connection between pneumonia and COM, the researchers wrote in their discussion. Pathogens in the lungs can prompt changes in the microbiome dynamics, as might the use of antibiotics, they said. In addition, “Mucus plugging in the airway caused by pneumonia induces hypoxic conditions and leads to the expression of inflammatory markers in the eustachian tube and middle ear mucosa,” they noted.
The study findings were limited by several factors, including the retrospective design and lack of data on microbiological cultures for antibiotic susceptibility, radiologic findings on the severity of pneumonia, results of pulmonary function tests, and hearing thresholds, the researchers noted. Other limitations were the exclusion of the frequency of upper respiratory infections and antibiotic use due to lack of data, they said.
However, the results show an association between pneumonia diagnoses and increased incidence of COM, which suggests a novel perspective that “infection of the lower respiratory tract may affect the function of the eustachian tube and the middle ear to later cause COM,” they concluded.
The study received no outside funding. The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals with a prior diagnosis of pneumonia were significantly more likely to develop chronic otitis media (COM) than were those without a history of pneumonia, based on data from a nationwide cohort study of more than 100,000 patients.
“Recently, middle ear diseases, including COM, have been recognized as respiratory tract diseases beyond the pathophysiological concepts of ventilation dysfunction, with recurrent infection that occurs from anatomically adjacent structures such as the middle ear, mastoid cavity, and eustachian tube,” but the potential link between pneumonia and chronic otitis media and adults in particular has not been examined, wrote Sung Kyun Kim, MD, of Hallym University, Dongtan, South Korea, and colleagues.
In a study recently published in the International Journal of Infectious Diseases, the researchers identified 23,436 adults with COM and 93,744 controls aged 40 years and older from a Korean health insurance database between 2002 and 2015.
The overall incidence of pneumonia in the study population was significantly higher in the COM group compared with controls (9.3% vs. 7.2%, P <.001). The odds ratios of pneumonia were significantly higher in the COM group compared with controls, and a history of pneumonia increased the odds of COM regardless of sex and across all ages.
Pneumonia was defined as when a patient had a diagnosis of pneumonia based on ICD-10 codes and underwent a chest x-ray or chest CT scan. Chronic otitis media was defined as when a patient had a diagnosis based on ICD-10 codes at least two times with one of the following conditions: chronic serous otitis media, chronic mucoid otitis media, other chronic nonsuppurative otitis media, unspecified nonsuppurative otitis media, chronic tubotympanic suppurative otitis media, chronic atticoantral suppurative otitis media, other chronic suppurative otitis media, or unspecified suppurative otitis media.
Age groups were divided into 5-year intervals, and patients were classified into income groups and rural vs. urban residence.
In a further sensitivity analysis, individuals who were diagnosed with pneumonia five or more times before the index date had a significantly higher odds ratio for COM compared with those with less than five diagnoses of pneumonia (adjusted odds ratio, 1.34; P < .001).
Microbiome dysbiosis may explain part of the connection between pneumonia and COM, the researchers wrote in their discussion. Pathogens in the lungs can prompt changes in the microbiome dynamics, as might the use of antibiotics, they said. In addition, “Mucus plugging in the airway caused by pneumonia induces hypoxic conditions and leads to the expression of inflammatory markers in the eustachian tube and middle ear mucosa,” they noted.
The study findings were limited by several factors, including the retrospective design and lack of data on microbiological cultures for antibiotic susceptibility, radiologic findings on the severity of pneumonia, results of pulmonary function tests, and hearing thresholds, the researchers noted. Other limitations were the exclusion of the frequency of upper respiratory infections and antibiotic use due to lack of data, they said.
However, the results show an association between pneumonia diagnoses and increased incidence of COM, which suggests a novel perspective that “infection of the lower respiratory tract may affect the function of the eustachian tube and the middle ear to later cause COM,” they concluded.
The study received no outside funding. The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals with a prior diagnosis of pneumonia were significantly more likely to develop chronic otitis media (COM) than were those without a history of pneumonia, based on data from a nationwide cohort study of more than 100,000 patients.
“Recently, middle ear diseases, including COM, have been recognized as respiratory tract diseases beyond the pathophysiological concepts of ventilation dysfunction, with recurrent infection that occurs from anatomically adjacent structures such as the middle ear, mastoid cavity, and eustachian tube,” but the potential link between pneumonia and chronic otitis media and adults in particular has not been examined, wrote Sung Kyun Kim, MD, of Hallym University, Dongtan, South Korea, and colleagues.
In a study recently published in the International Journal of Infectious Diseases, the researchers identified 23,436 adults with COM and 93,744 controls aged 40 years and older from a Korean health insurance database between 2002 and 2015.
The overall incidence of pneumonia in the study population was significantly higher in the COM group compared with controls (9.3% vs. 7.2%, P <.001). The odds ratios of pneumonia were significantly higher in the COM group compared with controls, and a history of pneumonia increased the odds of COM regardless of sex and across all ages.
Pneumonia was defined as when a patient had a diagnosis of pneumonia based on ICD-10 codes and underwent a chest x-ray or chest CT scan. Chronic otitis media was defined as when a patient had a diagnosis based on ICD-10 codes at least two times with one of the following conditions: chronic serous otitis media, chronic mucoid otitis media, other chronic nonsuppurative otitis media, unspecified nonsuppurative otitis media, chronic tubotympanic suppurative otitis media, chronic atticoantral suppurative otitis media, other chronic suppurative otitis media, or unspecified suppurative otitis media.
Age groups were divided into 5-year intervals, and patients were classified into income groups and rural vs. urban residence.
In a further sensitivity analysis, individuals who were diagnosed with pneumonia five or more times before the index date had a significantly higher odds ratio for COM compared with those with less than five diagnoses of pneumonia (adjusted odds ratio, 1.34; P < .001).
Microbiome dysbiosis may explain part of the connection between pneumonia and COM, the researchers wrote in their discussion. Pathogens in the lungs can prompt changes in the microbiome dynamics, as might the use of antibiotics, they said. In addition, “Mucus plugging in the airway caused by pneumonia induces hypoxic conditions and leads to the expression of inflammatory markers in the eustachian tube and middle ear mucosa,” they noted.
The study findings were limited by several factors, including the retrospective design and lack of data on microbiological cultures for antibiotic susceptibility, radiologic findings on the severity of pneumonia, results of pulmonary function tests, and hearing thresholds, the researchers noted. Other limitations were the exclusion of the frequency of upper respiratory infections and antibiotic use due to lack of data, they said.
However, the results show an association between pneumonia diagnoses and increased incidence of COM, which suggests a novel perspective that “infection of the lower respiratory tract may affect the function of the eustachian tube and the middle ear to later cause COM,” they concluded.
The study received no outside funding. The researchers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
Acral Papulovesicular Eruption in a Soldier Following Smallpox Vaccination
Following the attacks of September 11, 2001, heightened concerns over bioterrorism and the potential use of smallpox as a biological weapon made smallpox vaccination a critical component of military readiness. Therefore, the US Military resumed its smallpox vaccination program in 2002 using the first-generation smallpox vaccine (Dryvax, Wyeth Pharmaceuticals), a live vaccinia virus vaccine created in the late 19th century. This vaccine was developed by pooling vaccinia strains from the skin of infected cows1 and had previously been used during the worldwide vaccination campaign in the 1970s. Dryvax was associated with various cardiac and cutaneous complications, from benign hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
Due to concerns that the remaining supply of Dryvax was insufficient to vaccinate the US population in the case of a bioterrorism attack, investigators developed the second-generation smallpox vaccine (ACAM2000, Sanofi Pasteur Biologics Co) using advances in vaccine technology.2 ACAM2000 is a plaque-purified isolate of vaccinia virus propagated in cell culture, thereby reducing contaminants and lot-to-lot variation.1 Clinical trials demonstrated comparable immunogenicity and frequency of adverse events compared with Dryvax,2 and ACAM2000 replaced Dryvax in 2008. However, these trials focused on serious adverse events, such as cardiac complications and postvaccinal encephalitis, with less specific characterization and description of cutaneous eruptions.3
Since 2008, there have been few reports of cutaneous adverse reactions following vaccination with ACAM2000. Beachkofsky et al4 described 7 cases of papulovesicular eruptions and 1 case of generalized vaccinia. Freeman and Lenz5 described 4 cases of papulovesicular eruptions, and there has been 1 case of progressive vaccinia reported in a soldier with newly diagnosed acute myelogenous leukemia.6 Kramer7 described a patient with multiple vesiculopustular lesions secondary to autoinoculation. The distinct pruritic acral papulovesicular eruptions following ACAM2000 vaccination have occurred in healthy military service members at different locations since the introduction of ACAM2000. We describe an additional case of this unique cutaneous eruption, followed by a review of previously described cutaneous adverse events associated with smallpox vaccination.
Case Report
A 21-year-old female soldier who was otherwise healthy presented to the dermatology clinic with a pruritic papular eruption involving the upper and lower extremities of 1 week’s duration. The lesions first appeared 8 days after she received the ACAM2000 vaccine. She received no other concurrent vaccines, had no history of atopic dermatitis, and had no systemic symptoms. Physical examination revealed numerous erythematous indurated papules involving the dorsolateral hands and fingers, as well as the extensor surfaces of the elbows, knees, and thighs (Figures 1 and 2). Based on the clinical presentation, the differential diagnosis included lichen planus, verruca plana, dyshidrotic eczema, and smallpox vaccine reaction. Erythema multiforme was considered; however, the absence of palmoplantar involvement and typical targetoid lesions made this diagnosis less likely.
Biopsies of lesions on the arm and thigh were performed. Histologic findings revealed interface and spongiotic dermatitis with scattered necrotic keratinocytes and extravasated erythrocytes (Figure 3). There was no evidence of viral cytopathic effects. Similar clinical and histologic findings have been reported in the literature as acral papulovesicular eruptions following smallpox vaccination or papular spongiotic dermatitis of smallpox vaccination.8 The presence of eosinophils was not conspicuous in the current case and was only a notable finding in 1 of 2 cases previously described by Gaertner et al.8 This may simply be due to an idiosyncratic drug reaction. Furthermore, in the cases described by Beachkofsky et al,4 there were essentially 2 histologic groups. The first group demonstrated a dermal hypersensitivity-type reaction, and the second group demonstrated a lymphocytic capillaritis.
Based on these findings, the patient was diagnosed with an acral papulovesicular eruption following smallpox vaccination. Of note, the patient’s presentation was not consistent with other described smallpox vaccine reactions, which included eczema vaccinatum, autoinoculation, generalized vaccinia, and progressive vaccinia. The patient was treated supportively with triamcinolone acetonide cream 0.1%, cool compresses, and oral diphenhydramine as needed for pruritus. The lesions notably improved within the first week of treatment.
Comment
Reported cases of acral papulovesicular eruption4-6 demonstrated an onset of cutaneous symptoms an average of 14 days following vaccination (range, 8–18 days postvaccination). Lesions were benign and self-limited in all cases, with resolution within an average of 25 days (range, 7–71 days). All patients were active-duty military adults with a mean age of 24 years. Supportive treatment varied from topical steroids and oral antihistamines to tapering oral prednisone doses. Of note, all previously reported cases of this reaction occurred in patients who also had received other concurrent or near-concurrent vaccines, including anthrax, hepatitis B, influenza, and typhoid. Our patient represents a unique case of a papulovesicular eruption following smallpox vaccination with no history of concurrent vaccines.
Since the 1970s, smallpox vaccination has been associated with numerous cutaneous reactions, most of which have been reported with the first-generation Dryvax. Minor local reactions occurred in approximately 2% to 6% of vaccinees in clinical trials.9 These reactions included local edema involving the upper arm, satellite lesions within 2.5 cm of the vaccination site, local lymphadenopathy, intense inflammation or viral cellulitis surrounding the inoculation site, and viral lymphangitis tracking to axillary lymph nodes. In clinical trials, these reactions were self-limited and required only symptomatic treatment.9
Autoinoculation is another cutaneous reaction that can occur because Dryvax and ACAM2000 both contain live-attenuated replicating vaccinia virus. Accidental implantation may occur when the high titers of virus present at the vaccine site are subsequently transferred to other sites, especially abnormal mucosa or skin, resulting in an additional primary inoculation site.10
Eczema vaccinatum is a potentially life-threatening reaction that may occur in patients with disruptive skin disorders, such as atopic dermatitis. These patients are at risk for massive confluent vaccinia infection of the skin.10 In patients with atopic dermatitis, the virus rapidly disseminates due to both skin barrier dysfunction and impaired immunomodulation, resulting in large confluent skin lesions and the potential for viremia, septic shock, and death.10,11 Mortality from eczema vaccinatum may be reduced by administration of vaccinia immune globulin.10
The vaccinia virus also may spread hematogenously in healthy individuals,10 resulting in a benign reaction called generalized vaccinia. These patients develop pustules on areas of the skin other than the vaccination site. Although typically benign and self-limited, Beachkofsky et al4 described a case of generalized vaccinia in a healthy 34-year-old man resulting in a rapidly progressive vesiculopustular eruption with associated fever and pancytopenia. The patient made a complete recovery over the course of the following month.4
Alternatively, progressive vaccinia is a severe complication of smallpox vaccination seen in patients with impaired cell-mediated immunity. It also is known as vaccinia gangrenosum or vaccinia necrosum. These patients develop expanding ulcers due to exaggerated viral replication and cell-to-cell spread of the vaccinia virus.10,11 Hematogenous spread may result in viral implantation at distant sites of the body. This disease slowly progresses over weeks to months, and it often is resistant to treatment and fatal in patients with severe T-cell deficiency.10
Acral papulovesicular eruption is a distinct cutaneous adverse event following smallpox vaccination. Although further research is needed to discern the pathogenesis of this reaction, it is benign and self-limited, and patients have fully recovered with supportive care. In addition, a modified vaccinia Ankara vaccine (Bavarian Nordic) was approved by the US Food and Drug Administration in 2019.12,13 It is a nonreplicating attenuated viral vaccine that had fewer adverse events compared to ACAM2000 in clinical trials.13 To date, papulovesicular eruptions have not been reported following vaccination with the modified vaccinia Ankara vaccine; however, continued monitoring will help to further characterize any cutaneous reactions to this newer vaccine.
- Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79.
- Monath TP, Caldwell JR, Mundt W, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8:S31-S44.
- Thomas TN, Reef S, Neff L, et al. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis. 2008;46:S212-S220.
- Beachkofsky TM, Carrizales SC, Bidinger JJ, et al. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch Dermatol. 2010;146:656-661.
- Freeman R, Lenz B. Cutaneous reactions associated with ACAM2000 smallpox vaccination in a deploying U.S. Army unit. Mil Med. 2015;180:E152-E156.
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Kramer TR. Post–smallpox vaccination skin eruption in a marine. Mil Med. 2018;183:E649-E653.
- Gaertner EM, Groo S, Kim J. Papular spongiotic dermatitis of smallpox vaccination: report of 2 cases with review of the literature. Arch Pathol Lab Med. 2004;128:1173-1175.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part I. background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241-250.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Bray M. Understanding smallpox vaccination. J Infect Dis. 2011;203:1037-1039.
- Greenberg RN, Hay CM, Stapleton JT, et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11:E0157335.
- Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897-1908.
Following the attacks of September 11, 2001, heightened concerns over bioterrorism and the potential use of smallpox as a biological weapon made smallpox vaccination a critical component of military readiness. Therefore, the US Military resumed its smallpox vaccination program in 2002 using the first-generation smallpox vaccine (Dryvax, Wyeth Pharmaceuticals), a live vaccinia virus vaccine created in the late 19th century. This vaccine was developed by pooling vaccinia strains from the skin of infected cows1 and had previously been used during the worldwide vaccination campaign in the 1970s. Dryvax was associated with various cardiac and cutaneous complications, from benign hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
Due to concerns that the remaining supply of Dryvax was insufficient to vaccinate the US population in the case of a bioterrorism attack, investigators developed the second-generation smallpox vaccine (ACAM2000, Sanofi Pasteur Biologics Co) using advances in vaccine technology.2 ACAM2000 is a plaque-purified isolate of vaccinia virus propagated in cell culture, thereby reducing contaminants and lot-to-lot variation.1 Clinical trials demonstrated comparable immunogenicity and frequency of adverse events compared with Dryvax,2 and ACAM2000 replaced Dryvax in 2008. However, these trials focused on serious adverse events, such as cardiac complications and postvaccinal encephalitis, with less specific characterization and description of cutaneous eruptions.3
Since 2008, there have been few reports of cutaneous adverse reactions following vaccination with ACAM2000. Beachkofsky et al4 described 7 cases of papulovesicular eruptions and 1 case of generalized vaccinia. Freeman and Lenz5 described 4 cases of papulovesicular eruptions, and there has been 1 case of progressive vaccinia reported in a soldier with newly diagnosed acute myelogenous leukemia.6 Kramer7 described a patient with multiple vesiculopustular lesions secondary to autoinoculation. The distinct pruritic acral papulovesicular eruptions following ACAM2000 vaccination have occurred in healthy military service members at different locations since the introduction of ACAM2000. We describe an additional case of this unique cutaneous eruption, followed by a review of previously described cutaneous adverse events associated with smallpox vaccination.
Case Report
A 21-year-old female soldier who was otherwise healthy presented to the dermatology clinic with a pruritic papular eruption involving the upper and lower extremities of 1 week’s duration. The lesions first appeared 8 days after she received the ACAM2000 vaccine. She received no other concurrent vaccines, had no history of atopic dermatitis, and had no systemic symptoms. Physical examination revealed numerous erythematous indurated papules involving the dorsolateral hands and fingers, as well as the extensor surfaces of the elbows, knees, and thighs (Figures 1 and 2). Based on the clinical presentation, the differential diagnosis included lichen planus, verruca plana, dyshidrotic eczema, and smallpox vaccine reaction. Erythema multiforme was considered; however, the absence of palmoplantar involvement and typical targetoid lesions made this diagnosis less likely.
Biopsies of lesions on the arm and thigh were performed. Histologic findings revealed interface and spongiotic dermatitis with scattered necrotic keratinocytes and extravasated erythrocytes (Figure 3). There was no evidence of viral cytopathic effects. Similar clinical and histologic findings have been reported in the literature as acral papulovesicular eruptions following smallpox vaccination or papular spongiotic dermatitis of smallpox vaccination.8 The presence of eosinophils was not conspicuous in the current case and was only a notable finding in 1 of 2 cases previously described by Gaertner et al.8 This may simply be due to an idiosyncratic drug reaction. Furthermore, in the cases described by Beachkofsky et al,4 there were essentially 2 histologic groups. The first group demonstrated a dermal hypersensitivity-type reaction, and the second group demonstrated a lymphocytic capillaritis.
Based on these findings, the patient was diagnosed with an acral papulovesicular eruption following smallpox vaccination. Of note, the patient’s presentation was not consistent with other described smallpox vaccine reactions, which included eczema vaccinatum, autoinoculation, generalized vaccinia, and progressive vaccinia. The patient was treated supportively with triamcinolone acetonide cream 0.1%, cool compresses, and oral diphenhydramine as needed for pruritus. The lesions notably improved within the first week of treatment.
Comment
Reported cases of acral papulovesicular eruption4-6 demonstrated an onset of cutaneous symptoms an average of 14 days following vaccination (range, 8–18 days postvaccination). Lesions were benign and self-limited in all cases, with resolution within an average of 25 days (range, 7–71 days). All patients were active-duty military adults with a mean age of 24 years. Supportive treatment varied from topical steroids and oral antihistamines to tapering oral prednisone doses. Of note, all previously reported cases of this reaction occurred in patients who also had received other concurrent or near-concurrent vaccines, including anthrax, hepatitis B, influenza, and typhoid. Our patient represents a unique case of a papulovesicular eruption following smallpox vaccination with no history of concurrent vaccines.
Since the 1970s, smallpox vaccination has been associated with numerous cutaneous reactions, most of which have been reported with the first-generation Dryvax. Minor local reactions occurred in approximately 2% to 6% of vaccinees in clinical trials.9 These reactions included local edema involving the upper arm, satellite lesions within 2.5 cm of the vaccination site, local lymphadenopathy, intense inflammation or viral cellulitis surrounding the inoculation site, and viral lymphangitis tracking to axillary lymph nodes. In clinical trials, these reactions were self-limited and required only symptomatic treatment.9
Autoinoculation is another cutaneous reaction that can occur because Dryvax and ACAM2000 both contain live-attenuated replicating vaccinia virus. Accidental implantation may occur when the high titers of virus present at the vaccine site are subsequently transferred to other sites, especially abnormal mucosa or skin, resulting in an additional primary inoculation site.10
Eczema vaccinatum is a potentially life-threatening reaction that may occur in patients with disruptive skin disorders, such as atopic dermatitis. These patients are at risk for massive confluent vaccinia infection of the skin.10 In patients with atopic dermatitis, the virus rapidly disseminates due to both skin barrier dysfunction and impaired immunomodulation, resulting in large confluent skin lesions and the potential for viremia, septic shock, and death.10,11 Mortality from eczema vaccinatum may be reduced by administration of vaccinia immune globulin.10
The vaccinia virus also may spread hematogenously in healthy individuals,10 resulting in a benign reaction called generalized vaccinia. These patients develop pustules on areas of the skin other than the vaccination site. Although typically benign and self-limited, Beachkofsky et al4 described a case of generalized vaccinia in a healthy 34-year-old man resulting in a rapidly progressive vesiculopustular eruption with associated fever and pancytopenia. The patient made a complete recovery over the course of the following month.4
Alternatively, progressive vaccinia is a severe complication of smallpox vaccination seen in patients with impaired cell-mediated immunity. It also is known as vaccinia gangrenosum or vaccinia necrosum. These patients develop expanding ulcers due to exaggerated viral replication and cell-to-cell spread of the vaccinia virus.10,11 Hematogenous spread may result in viral implantation at distant sites of the body. This disease slowly progresses over weeks to months, and it often is resistant to treatment and fatal in patients with severe T-cell deficiency.10
Acral papulovesicular eruption is a distinct cutaneous adverse event following smallpox vaccination. Although further research is needed to discern the pathogenesis of this reaction, it is benign and self-limited, and patients have fully recovered with supportive care. In addition, a modified vaccinia Ankara vaccine (Bavarian Nordic) was approved by the US Food and Drug Administration in 2019.12,13 It is a nonreplicating attenuated viral vaccine that had fewer adverse events compared to ACAM2000 in clinical trials.13 To date, papulovesicular eruptions have not been reported following vaccination with the modified vaccinia Ankara vaccine; however, continued monitoring will help to further characterize any cutaneous reactions to this newer vaccine.
Following the attacks of September 11, 2001, heightened concerns over bioterrorism and the potential use of smallpox as a biological weapon made smallpox vaccination a critical component of military readiness. Therefore, the US Military resumed its smallpox vaccination program in 2002 using the first-generation smallpox vaccine (Dryvax, Wyeth Pharmaceuticals), a live vaccinia virus vaccine created in the late 19th century. This vaccine was developed by pooling vaccinia strains from the skin of infected cows1 and had previously been used during the worldwide vaccination campaign in the 1970s. Dryvax was associated with various cardiac and cutaneous complications, from benign hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
Due to concerns that the remaining supply of Dryvax was insufficient to vaccinate the US population in the case of a bioterrorism attack, investigators developed the second-generation smallpox vaccine (ACAM2000, Sanofi Pasteur Biologics Co) using advances in vaccine technology.2 ACAM2000 is a plaque-purified isolate of vaccinia virus propagated in cell culture, thereby reducing contaminants and lot-to-lot variation.1 Clinical trials demonstrated comparable immunogenicity and frequency of adverse events compared with Dryvax,2 and ACAM2000 replaced Dryvax in 2008. However, these trials focused on serious adverse events, such as cardiac complications and postvaccinal encephalitis, with less specific characterization and description of cutaneous eruptions.3
Since 2008, there have been few reports of cutaneous adverse reactions following vaccination with ACAM2000. Beachkofsky et al4 described 7 cases of papulovesicular eruptions and 1 case of generalized vaccinia. Freeman and Lenz5 described 4 cases of papulovesicular eruptions, and there has been 1 case of progressive vaccinia reported in a soldier with newly diagnosed acute myelogenous leukemia.6 Kramer7 described a patient with multiple vesiculopustular lesions secondary to autoinoculation. The distinct pruritic acral papulovesicular eruptions following ACAM2000 vaccination have occurred in healthy military service members at different locations since the introduction of ACAM2000. We describe an additional case of this unique cutaneous eruption, followed by a review of previously described cutaneous adverse events associated with smallpox vaccination.
Case Report
A 21-year-old female soldier who was otherwise healthy presented to the dermatology clinic with a pruritic papular eruption involving the upper and lower extremities of 1 week’s duration. The lesions first appeared 8 days after she received the ACAM2000 vaccine. She received no other concurrent vaccines, had no history of atopic dermatitis, and had no systemic symptoms. Physical examination revealed numerous erythematous indurated papules involving the dorsolateral hands and fingers, as well as the extensor surfaces of the elbows, knees, and thighs (Figures 1 and 2). Based on the clinical presentation, the differential diagnosis included lichen planus, verruca plana, dyshidrotic eczema, and smallpox vaccine reaction. Erythema multiforme was considered; however, the absence of palmoplantar involvement and typical targetoid lesions made this diagnosis less likely.
Biopsies of lesions on the arm and thigh were performed. Histologic findings revealed interface and spongiotic dermatitis with scattered necrotic keratinocytes and extravasated erythrocytes (Figure 3). There was no evidence of viral cytopathic effects. Similar clinical and histologic findings have been reported in the literature as acral papulovesicular eruptions following smallpox vaccination or papular spongiotic dermatitis of smallpox vaccination.8 The presence of eosinophils was not conspicuous in the current case and was only a notable finding in 1 of 2 cases previously described by Gaertner et al.8 This may simply be due to an idiosyncratic drug reaction. Furthermore, in the cases described by Beachkofsky et al,4 there were essentially 2 histologic groups. The first group demonstrated a dermal hypersensitivity-type reaction, and the second group demonstrated a lymphocytic capillaritis.
Based on these findings, the patient was diagnosed with an acral papulovesicular eruption following smallpox vaccination. Of note, the patient’s presentation was not consistent with other described smallpox vaccine reactions, which included eczema vaccinatum, autoinoculation, generalized vaccinia, and progressive vaccinia. The patient was treated supportively with triamcinolone acetonide cream 0.1%, cool compresses, and oral diphenhydramine as needed for pruritus. The lesions notably improved within the first week of treatment.
Comment
Reported cases of acral papulovesicular eruption4-6 demonstrated an onset of cutaneous symptoms an average of 14 days following vaccination (range, 8–18 days postvaccination). Lesions were benign and self-limited in all cases, with resolution within an average of 25 days (range, 7–71 days). All patients were active-duty military adults with a mean age of 24 years. Supportive treatment varied from topical steroids and oral antihistamines to tapering oral prednisone doses. Of note, all previously reported cases of this reaction occurred in patients who also had received other concurrent or near-concurrent vaccines, including anthrax, hepatitis B, influenza, and typhoid. Our patient represents a unique case of a papulovesicular eruption following smallpox vaccination with no history of concurrent vaccines.
Since the 1970s, smallpox vaccination has been associated with numerous cutaneous reactions, most of which have been reported with the first-generation Dryvax. Minor local reactions occurred in approximately 2% to 6% of vaccinees in clinical trials.9 These reactions included local edema involving the upper arm, satellite lesions within 2.5 cm of the vaccination site, local lymphadenopathy, intense inflammation or viral cellulitis surrounding the inoculation site, and viral lymphangitis tracking to axillary lymph nodes. In clinical trials, these reactions were self-limited and required only symptomatic treatment.9
Autoinoculation is another cutaneous reaction that can occur because Dryvax and ACAM2000 both contain live-attenuated replicating vaccinia virus. Accidental implantation may occur when the high titers of virus present at the vaccine site are subsequently transferred to other sites, especially abnormal mucosa or skin, resulting in an additional primary inoculation site.10
Eczema vaccinatum is a potentially life-threatening reaction that may occur in patients with disruptive skin disorders, such as atopic dermatitis. These patients are at risk for massive confluent vaccinia infection of the skin.10 In patients with atopic dermatitis, the virus rapidly disseminates due to both skin barrier dysfunction and impaired immunomodulation, resulting in large confluent skin lesions and the potential for viremia, septic shock, and death.10,11 Mortality from eczema vaccinatum may be reduced by administration of vaccinia immune globulin.10
The vaccinia virus also may spread hematogenously in healthy individuals,10 resulting in a benign reaction called generalized vaccinia. These patients develop pustules on areas of the skin other than the vaccination site. Although typically benign and self-limited, Beachkofsky et al4 described a case of generalized vaccinia in a healthy 34-year-old man resulting in a rapidly progressive vesiculopustular eruption with associated fever and pancytopenia. The patient made a complete recovery over the course of the following month.4
Alternatively, progressive vaccinia is a severe complication of smallpox vaccination seen in patients with impaired cell-mediated immunity. It also is known as vaccinia gangrenosum or vaccinia necrosum. These patients develop expanding ulcers due to exaggerated viral replication and cell-to-cell spread of the vaccinia virus.10,11 Hematogenous spread may result in viral implantation at distant sites of the body. This disease slowly progresses over weeks to months, and it often is resistant to treatment and fatal in patients with severe T-cell deficiency.10
Acral papulovesicular eruption is a distinct cutaneous adverse event following smallpox vaccination. Although further research is needed to discern the pathogenesis of this reaction, it is benign and self-limited, and patients have fully recovered with supportive care. In addition, a modified vaccinia Ankara vaccine (Bavarian Nordic) was approved by the US Food and Drug Administration in 2019.12,13 It is a nonreplicating attenuated viral vaccine that had fewer adverse events compared to ACAM2000 in clinical trials.13 To date, papulovesicular eruptions have not been reported following vaccination with the modified vaccinia Ankara vaccine; however, continued monitoring will help to further characterize any cutaneous reactions to this newer vaccine.
- Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79.
- Monath TP, Caldwell JR, Mundt W, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8:S31-S44.
- Thomas TN, Reef S, Neff L, et al. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis. 2008;46:S212-S220.
- Beachkofsky TM, Carrizales SC, Bidinger JJ, et al. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch Dermatol. 2010;146:656-661.
- Freeman R, Lenz B. Cutaneous reactions associated with ACAM2000 smallpox vaccination in a deploying U.S. Army unit. Mil Med. 2015;180:E152-E156.
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Kramer TR. Post–smallpox vaccination skin eruption in a marine. Mil Med. 2018;183:E649-E653.
- Gaertner EM, Groo S, Kim J. Papular spongiotic dermatitis of smallpox vaccination: report of 2 cases with review of the literature. Arch Pathol Lab Med. 2004;128:1173-1175.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part I. background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241-250.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Bray M. Understanding smallpox vaccination. J Infect Dis. 2011;203:1037-1039.
- Greenberg RN, Hay CM, Stapleton JT, et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11:E0157335.
- Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897-1908.
- Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79.
- Monath TP, Caldwell JR, Mundt W, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8:S31-S44.
- Thomas TN, Reef S, Neff L, et al. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis. 2008;46:S212-S220.
- Beachkofsky TM, Carrizales SC, Bidinger JJ, et al. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch Dermatol. 2010;146:656-661.
- Freeman R, Lenz B. Cutaneous reactions associated with ACAM2000 smallpox vaccination in a deploying U.S. Army unit. Mil Med. 2015;180:E152-E156.
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Kramer TR. Post–smallpox vaccination skin eruption in a marine. Mil Med. 2018;183:E649-E653.
- Gaertner EM, Groo S, Kim J. Papular spongiotic dermatitis of smallpox vaccination: report of 2 cases with review of the literature. Arch Pathol Lab Med. 2004;128:1173-1175.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part I. background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241-250.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Bray M. Understanding smallpox vaccination. J Infect Dis. 2011;203:1037-1039.
- Greenberg RN, Hay CM, Stapleton JT, et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11:E0157335.
- Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897-1908.
Practice Points
- There are several potential cutaneous adverse reactions associated with smallpox vaccination, ranging from benign self-limited hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
- Acral papulovesicular eruption is a distinct presentation that has been described in the US Military following vaccination with the second-generation live smallpox vaccine (ACAM2000).
Vesicular Eruption Secondary to Bites by Larval Amblyomma americanum
Case Report
A 58-year-old woman presented to the dermatology office with a widespread pruritic eruption of 3 days’ duration that started in the groin and spread to the rest of the body. No treatments had been attempted. She had no notable medical history, and she denied any recent illness, change in personal care products, or new medications or supplements. She reported a camping trip 2 weeks prior to presentation on the east end of Long Island, New York. She later learned that others on the same trip developed a similar, albeit less widespread, eruption.
Physical examination revealed clear vesicles on the arms, legs, trunk, and pubic area (Figure 1). Dermoscopy revealed a small lone star tick larva in the center of one of the vesicles (Figure 2). The type of tick larva was identified using resources from the Centers for Disease Control and Prevention (Figure 3).1 Careful inspection revealed dark marks on various vesicles, mostly in the perineum, yielding nearly 20 larvae, which were removed with forceps. The patient was counseled to cover herself in petrolatum for 2 to 3 hours with the hope of smothering any remaining tick larvae. She was given triamcinolone cream and was encouraged to take a nonsedating antihistamine for itch. The patient was seen back in clinic 2 weeks later and the eruption had resolved.
Comment
Spread of Tick-Borne Disease—Ticks and tick-borne disease are increasing major health concerns for humans, domesticated animals, and livestock. Reported cases of bacterial and protozoan tick-borne disease doubled in the United States between 2004 and 2016. Ninety percent of the nearly 60,000 cases of nationally notifiable vector-borne diseases reported in 2017 were linked to ticks.2 Geographic ranges of multiple tick species continue to expand, which is thought to be secondary to rising global temperatures, ecologic changes, reforestation, and increases in commerce and travel (Figure 4).3 Not only have warming temperatures contributed to geographic range expansion, they also may extend ticks’ active season. The lone star tick (Amblyomma americanum) is widely distributed throughout much of the eastern United States.4 The range of A americanum has expanded north in recent years from its prior core range in the southeastern United States.2 One study found that from 2006 to 2016, the vector tick species most commonly collected from humans and submitted to a tick surveillance system in New Jersey shifted from Ixodes scapularis to A americanum.5
Bites by Amblyomma Ticks—As with most hard ticks, the life cycle of A americanum lasts 2 years and includes the egg, the 6-legged larva or “seed tick,” the 8-legged immature nymph, and the 8-legged reproductively mature adult (Figure 3). Amblyomma americanum can lay several thousand eggs.2 Because our patient had numerous bites, it is plausible that she came into contact with a nest of newly hatched tick larvae. Morphogenesis from larva to nymph, then nymph to adult, requires a blood meal.6,7 The larvae emerge from eggs deposited on the ground and then crawl up low vegetation where they can easily attach to passing hosts. The tick clings to hair or clothing and waits until the host is at rest before moving to a favorable location and then bites.8 When attaching, ticks inject an anesthetic akin to lidocaine, making the bite painless. A tick may spend up to 24 hours on the host prior to biting and then feed for 2 hours to 7 days before releasing.9 For the majority of tick-borne illnesses, the tick must remain attached for 24 to 48 hours before disease is transmitted.10
All stages of
Even when the ticks do not transmit disease, tick bites can cause impressive local reactions. Uncomplicated bites can be painful and leave a puncture wound that can take 1 to 2 weeks to heal.13 Rarely, bites can cause a delayed hypersensitivity reaction including fever, pruritus, and urticaria. Granulomas can develop if a tick is improperly removed.9 Other reports describe prurigo lesions, skin hemorrhage, papular urticaria, diffuse papules, vesicles and bullae, necrotic ulcers, and patchy alopecia.14,15 A 2015 systematic controlled study of human bite reactions from A americanum demonstrated the development of itchy erythematous papules and vesicles within 48 hours of larval tick attachment to research participants. The study found tissue damage from A americanum mouthparts, and degranulating mast cells may be evident in as little as 15 minutes.16 The severity of individual skin reaction is hypothesized to depend on several variables, such as the duration of feeding, size of mouthparts, type of tick secretions, changes in secretions during feeding, and prior exposures of the host.14
Tick Removal—If patients present to clinic with ticks attached, removal can be challenging. Removal recommendations call for use of blunt forceps or tweezers. Ticks should be grasped near the skin with consistent pressure, and the tick should be pulled straight out, perpendicular to the skin. Twisting motions can cause the head to separate from the body and remain in the bite wound. Immediately following removal, the area should be cleansed with a disinfectant.10,17 After the tick is removed, some studies recommend storing the tick at −20 °C; should the patient develop disease, the tick could be sent for evaluation.6,17 If there is no clinical or serologic evidence of infection, testing for the presence of antibodies against tick-borne bacteria at presentation and at 3 and 6 weeks is not recommended due to low sensitivity, low positive predictive value, and cost. Clinicians must only observe and treat if disease occurs.17
Prevention of Tick Bites—Tick bites are best prevented by avoiding tick-infested areas; when these areas are unavoidable, tick bites may be prevented by wearing long pants with the pant legs tucked into boots. In addition, applying topical DEET (N,N-diethyl-m-toluamide) repellent to exposed skin and treating clothing with permethrin can be helpful.17 When used alone, DEET provides greater than 90% protection for up to 2.7 hours against A americanum.18 Permethrin-treated clothing alone is 79% to 100% effective at killing A americanum ticks or disabling them for several hours.19
Conclusion
Tick-borne illness is an increasingly important cause of human infectious disease. In addition to their role as a disease vector, ticks can produce primary skin disorders. This case posed a diagnostic challenge because of the unusually large number and wide distribution of bites as well as the subsequent vesicular reaction that ensued. It is important to keep tick larvae or adult tick bites in the differential when evaluating a patient to expedite tick removal and begin clinical monitoring. Recognition of A americanum larvae as a potential cause of pruritic papules may be helpful in similar cases. In addition, it is important for dermatologists to be aware of the tick species in their area.
- Centers for Disease Control and Prevention. Tick ID. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tickID.html
- Molaei G, Little EAH, Williams SC, et al. Bracing for the worst—range expansion of the lone star tick in the northeastern United States. N Engl J Med. 2019;381:2189-2192.
- Centers for Disease Control and Prevention, Division of Vector-Borne Diseases. Lone star tick (Amblyomma americanum). Accessed March 23, 2022. https://www.cdc.gov/ticks/maps/lone_star_tick.pdf
- Reynolds HH, Elston DM. What’s eating you? lone star tick (Amblyomma americanum). Cutis. 2017;99:111-114.
- Jordan RA, Egizi A. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One. 2019;14:E0211778.
- Singh-Behl D, La Rosa SP, Tomecki KJ. Tick-borne infections. Dermatol Clin. 2003;21:237-244, v.
- Spach DH, Liles WC, Campbell GL, et al. Tick-borne diseases in the United States. N Engl J Med. 1993;329:936-947.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Middleton DB. Tick-borne infections. what starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Moody EK, Barker RW, White JL, et al. Ticks and tick-borne diseases in Oklahoma. J Okla State Med Assoc. 1998;91:438-445.
- Jones BE. Human ‘seed tick’ infestation. Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Centers for Disease Control and Prevention. Tick bite prophylaxis. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tick-bite-prophylaxis.html
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Krinsky WL. Dermatoses associated with the bites of mites and ticks (Arthropoda: Acari). Int J Dermatol. 1983;22:75-91.
- Yesudian P, Thambiah AS. Persistent papules after tick-bites. Dermatologica. 1973;147:214-218.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Solberg VB, Klein TA, McPherson KR, et al. Field evaluation of DEET and a piperidine repellent (AI3-37220) against Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1995;32:870-875.
- Evans SR, Korch GW Jr, Lawson MA. Comparative field evaluation of permethrin and DEET-treated military uniforms for personal protection against ticks (Acari). J Med Entomol. 1990;27:829-834.
Case Report
A 58-year-old woman presented to the dermatology office with a widespread pruritic eruption of 3 days’ duration that started in the groin and spread to the rest of the body. No treatments had been attempted. She had no notable medical history, and she denied any recent illness, change in personal care products, or new medications or supplements. She reported a camping trip 2 weeks prior to presentation on the east end of Long Island, New York. She later learned that others on the same trip developed a similar, albeit less widespread, eruption.
Physical examination revealed clear vesicles on the arms, legs, trunk, and pubic area (Figure 1). Dermoscopy revealed a small lone star tick larva in the center of one of the vesicles (Figure 2). The type of tick larva was identified using resources from the Centers for Disease Control and Prevention (Figure 3).1 Careful inspection revealed dark marks on various vesicles, mostly in the perineum, yielding nearly 20 larvae, which were removed with forceps. The patient was counseled to cover herself in petrolatum for 2 to 3 hours with the hope of smothering any remaining tick larvae. She was given triamcinolone cream and was encouraged to take a nonsedating antihistamine for itch. The patient was seen back in clinic 2 weeks later and the eruption had resolved.
Comment
Spread of Tick-Borne Disease—Ticks and tick-borne disease are increasing major health concerns for humans, domesticated animals, and livestock. Reported cases of bacterial and protozoan tick-borne disease doubled in the United States between 2004 and 2016. Ninety percent of the nearly 60,000 cases of nationally notifiable vector-borne diseases reported in 2017 were linked to ticks.2 Geographic ranges of multiple tick species continue to expand, which is thought to be secondary to rising global temperatures, ecologic changes, reforestation, and increases in commerce and travel (Figure 4).3 Not only have warming temperatures contributed to geographic range expansion, they also may extend ticks’ active season. The lone star tick (Amblyomma americanum) is widely distributed throughout much of the eastern United States.4 The range of A americanum has expanded north in recent years from its prior core range in the southeastern United States.2 One study found that from 2006 to 2016, the vector tick species most commonly collected from humans and submitted to a tick surveillance system in New Jersey shifted from Ixodes scapularis to A americanum.5
Bites by Amblyomma Ticks—As with most hard ticks, the life cycle of A americanum lasts 2 years and includes the egg, the 6-legged larva or “seed tick,” the 8-legged immature nymph, and the 8-legged reproductively mature adult (Figure 3). Amblyomma americanum can lay several thousand eggs.2 Because our patient had numerous bites, it is plausible that she came into contact with a nest of newly hatched tick larvae. Morphogenesis from larva to nymph, then nymph to adult, requires a blood meal.6,7 The larvae emerge from eggs deposited on the ground and then crawl up low vegetation where they can easily attach to passing hosts. The tick clings to hair or clothing and waits until the host is at rest before moving to a favorable location and then bites.8 When attaching, ticks inject an anesthetic akin to lidocaine, making the bite painless. A tick may spend up to 24 hours on the host prior to biting and then feed for 2 hours to 7 days before releasing.9 For the majority of tick-borne illnesses, the tick must remain attached for 24 to 48 hours before disease is transmitted.10
All stages of
Even when the ticks do not transmit disease, tick bites can cause impressive local reactions. Uncomplicated bites can be painful and leave a puncture wound that can take 1 to 2 weeks to heal.13 Rarely, bites can cause a delayed hypersensitivity reaction including fever, pruritus, and urticaria. Granulomas can develop if a tick is improperly removed.9 Other reports describe prurigo lesions, skin hemorrhage, papular urticaria, diffuse papules, vesicles and bullae, necrotic ulcers, and patchy alopecia.14,15 A 2015 systematic controlled study of human bite reactions from A americanum demonstrated the development of itchy erythematous papules and vesicles within 48 hours of larval tick attachment to research participants. The study found tissue damage from A americanum mouthparts, and degranulating mast cells may be evident in as little as 15 minutes.16 The severity of individual skin reaction is hypothesized to depend on several variables, such as the duration of feeding, size of mouthparts, type of tick secretions, changes in secretions during feeding, and prior exposures of the host.14
Tick Removal—If patients present to clinic with ticks attached, removal can be challenging. Removal recommendations call for use of blunt forceps or tweezers. Ticks should be grasped near the skin with consistent pressure, and the tick should be pulled straight out, perpendicular to the skin. Twisting motions can cause the head to separate from the body and remain in the bite wound. Immediately following removal, the area should be cleansed with a disinfectant.10,17 After the tick is removed, some studies recommend storing the tick at −20 °C; should the patient develop disease, the tick could be sent for evaluation.6,17 If there is no clinical or serologic evidence of infection, testing for the presence of antibodies against tick-borne bacteria at presentation and at 3 and 6 weeks is not recommended due to low sensitivity, low positive predictive value, and cost. Clinicians must only observe and treat if disease occurs.17
Prevention of Tick Bites—Tick bites are best prevented by avoiding tick-infested areas; when these areas are unavoidable, tick bites may be prevented by wearing long pants with the pant legs tucked into boots. In addition, applying topical DEET (N,N-diethyl-m-toluamide) repellent to exposed skin and treating clothing with permethrin can be helpful.17 When used alone, DEET provides greater than 90% protection for up to 2.7 hours against A americanum.18 Permethrin-treated clothing alone is 79% to 100% effective at killing A americanum ticks or disabling them for several hours.19
Conclusion
Tick-borne illness is an increasingly important cause of human infectious disease. In addition to their role as a disease vector, ticks can produce primary skin disorders. This case posed a diagnostic challenge because of the unusually large number and wide distribution of bites as well as the subsequent vesicular reaction that ensued. It is important to keep tick larvae or adult tick bites in the differential when evaluating a patient to expedite tick removal and begin clinical monitoring. Recognition of A americanum larvae as a potential cause of pruritic papules may be helpful in similar cases. In addition, it is important for dermatologists to be aware of the tick species in their area.
Case Report
A 58-year-old woman presented to the dermatology office with a widespread pruritic eruption of 3 days’ duration that started in the groin and spread to the rest of the body. No treatments had been attempted. She had no notable medical history, and she denied any recent illness, change in personal care products, or new medications or supplements. She reported a camping trip 2 weeks prior to presentation on the east end of Long Island, New York. She later learned that others on the same trip developed a similar, albeit less widespread, eruption.
Physical examination revealed clear vesicles on the arms, legs, trunk, and pubic area (Figure 1). Dermoscopy revealed a small lone star tick larva in the center of one of the vesicles (Figure 2). The type of tick larva was identified using resources from the Centers for Disease Control and Prevention (Figure 3).1 Careful inspection revealed dark marks on various vesicles, mostly in the perineum, yielding nearly 20 larvae, which were removed with forceps. The patient was counseled to cover herself in petrolatum for 2 to 3 hours with the hope of smothering any remaining tick larvae. She was given triamcinolone cream and was encouraged to take a nonsedating antihistamine for itch. The patient was seen back in clinic 2 weeks later and the eruption had resolved.
Comment
Spread of Tick-Borne Disease—Ticks and tick-borne disease are increasing major health concerns for humans, domesticated animals, and livestock. Reported cases of bacterial and protozoan tick-borne disease doubled in the United States between 2004 and 2016. Ninety percent of the nearly 60,000 cases of nationally notifiable vector-borne diseases reported in 2017 were linked to ticks.2 Geographic ranges of multiple tick species continue to expand, which is thought to be secondary to rising global temperatures, ecologic changes, reforestation, and increases in commerce and travel (Figure 4).3 Not only have warming temperatures contributed to geographic range expansion, they also may extend ticks’ active season. The lone star tick (Amblyomma americanum) is widely distributed throughout much of the eastern United States.4 The range of A americanum has expanded north in recent years from its prior core range in the southeastern United States.2 One study found that from 2006 to 2016, the vector tick species most commonly collected from humans and submitted to a tick surveillance system in New Jersey shifted from Ixodes scapularis to A americanum.5
Bites by Amblyomma Ticks—As with most hard ticks, the life cycle of A americanum lasts 2 years and includes the egg, the 6-legged larva or “seed tick,” the 8-legged immature nymph, and the 8-legged reproductively mature adult (Figure 3). Amblyomma americanum can lay several thousand eggs.2 Because our patient had numerous bites, it is plausible that she came into contact with a nest of newly hatched tick larvae. Morphogenesis from larva to nymph, then nymph to adult, requires a blood meal.6,7 The larvae emerge from eggs deposited on the ground and then crawl up low vegetation where they can easily attach to passing hosts. The tick clings to hair or clothing and waits until the host is at rest before moving to a favorable location and then bites.8 When attaching, ticks inject an anesthetic akin to lidocaine, making the bite painless. A tick may spend up to 24 hours on the host prior to biting and then feed for 2 hours to 7 days before releasing.9 For the majority of tick-borne illnesses, the tick must remain attached for 24 to 48 hours before disease is transmitted.10
All stages of
Even when the ticks do not transmit disease, tick bites can cause impressive local reactions. Uncomplicated bites can be painful and leave a puncture wound that can take 1 to 2 weeks to heal.13 Rarely, bites can cause a delayed hypersensitivity reaction including fever, pruritus, and urticaria. Granulomas can develop if a tick is improperly removed.9 Other reports describe prurigo lesions, skin hemorrhage, papular urticaria, diffuse papules, vesicles and bullae, necrotic ulcers, and patchy alopecia.14,15 A 2015 systematic controlled study of human bite reactions from A americanum demonstrated the development of itchy erythematous papules and vesicles within 48 hours of larval tick attachment to research participants. The study found tissue damage from A americanum mouthparts, and degranulating mast cells may be evident in as little as 15 minutes.16 The severity of individual skin reaction is hypothesized to depend on several variables, such as the duration of feeding, size of mouthparts, type of tick secretions, changes in secretions during feeding, and prior exposures of the host.14
Tick Removal—If patients present to clinic with ticks attached, removal can be challenging. Removal recommendations call for use of blunt forceps or tweezers. Ticks should be grasped near the skin with consistent pressure, and the tick should be pulled straight out, perpendicular to the skin. Twisting motions can cause the head to separate from the body and remain in the bite wound. Immediately following removal, the area should be cleansed with a disinfectant.10,17 After the tick is removed, some studies recommend storing the tick at −20 °C; should the patient develop disease, the tick could be sent for evaluation.6,17 If there is no clinical or serologic evidence of infection, testing for the presence of antibodies against tick-borne bacteria at presentation and at 3 and 6 weeks is not recommended due to low sensitivity, low positive predictive value, and cost. Clinicians must only observe and treat if disease occurs.17
Prevention of Tick Bites—Tick bites are best prevented by avoiding tick-infested areas; when these areas are unavoidable, tick bites may be prevented by wearing long pants with the pant legs tucked into boots. In addition, applying topical DEET (N,N-diethyl-m-toluamide) repellent to exposed skin and treating clothing with permethrin can be helpful.17 When used alone, DEET provides greater than 90% protection for up to 2.7 hours against A americanum.18 Permethrin-treated clothing alone is 79% to 100% effective at killing A americanum ticks or disabling them for several hours.19
Conclusion
Tick-borne illness is an increasingly important cause of human infectious disease. In addition to their role as a disease vector, ticks can produce primary skin disorders. This case posed a diagnostic challenge because of the unusually large number and wide distribution of bites as well as the subsequent vesicular reaction that ensued. It is important to keep tick larvae or adult tick bites in the differential when evaluating a patient to expedite tick removal and begin clinical monitoring. Recognition of A americanum larvae as a potential cause of pruritic papules may be helpful in similar cases. In addition, it is important for dermatologists to be aware of the tick species in their area.
- Centers for Disease Control and Prevention. Tick ID. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tickID.html
- Molaei G, Little EAH, Williams SC, et al. Bracing for the worst—range expansion of the lone star tick in the northeastern United States. N Engl J Med. 2019;381:2189-2192.
- Centers for Disease Control and Prevention, Division of Vector-Borne Diseases. Lone star tick (Amblyomma americanum). Accessed March 23, 2022. https://www.cdc.gov/ticks/maps/lone_star_tick.pdf
- Reynolds HH, Elston DM. What’s eating you? lone star tick (Amblyomma americanum). Cutis. 2017;99:111-114.
- Jordan RA, Egizi A. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One. 2019;14:E0211778.
- Singh-Behl D, La Rosa SP, Tomecki KJ. Tick-borne infections. Dermatol Clin. 2003;21:237-244, v.
- Spach DH, Liles WC, Campbell GL, et al. Tick-borne diseases in the United States. N Engl J Med. 1993;329:936-947.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Middleton DB. Tick-borne infections. what starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Moody EK, Barker RW, White JL, et al. Ticks and tick-borne diseases in Oklahoma. J Okla State Med Assoc. 1998;91:438-445.
- Jones BE. Human ‘seed tick’ infestation. Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Centers for Disease Control and Prevention. Tick bite prophylaxis. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tick-bite-prophylaxis.html
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Krinsky WL. Dermatoses associated with the bites of mites and ticks (Arthropoda: Acari). Int J Dermatol. 1983;22:75-91.
- Yesudian P, Thambiah AS. Persistent papules after tick-bites. Dermatologica. 1973;147:214-218.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Solberg VB, Klein TA, McPherson KR, et al. Field evaluation of DEET and a piperidine repellent (AI3-37220) against Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1995;32:870-875.
- Evans SR, Korch GW Jr, Lawson MA. Comparative field evaluation of permethrin and DEET-treated military uniforms for personal protection against ticks (Acari). J Med Entomol. 1990;27:829-834.
- Centers for Disease Control and Prevention. Tick ID. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tickID.html
- Molaei G, Little EAH, Williams SC, et al. Bracing for the worst—range expansion of the lone star tick in the northeastern United States. N Engl J Med. 2019;381:2189-2192.
- Centers for Disease Control and Prevention, Division of Vector-Borne Diseases. Lone star tick (Amblyomma americanum). Accessed March 23, 2022. https://www.cdc.gov/ticks/maps/lone_star_tick.pdf
- Reynolds HH, Elston DM. What’s eating you? lone star tick (Amblyomma americanum). Cutis. 2017;99:111-114.
- Jordan RA, Egizi A. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One. 2019;14:E0211778.
- Singh-Behl D, La Rosa SP, Tomecki KJ. Tick-borne infections. Dermatol Clin. 2003;21:237-244, v.
- Spach DH, Liles WC, Campbell GL, et al. Tick-borne diseases in the United States. N Engl J Med. 1993;329:936-947.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Middleton DB. Tick-borne infections. what starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Moody EK, Barker RW, White JL, et al. Ticks and tick-borne diseases in Oklahoma. J Okla State Med Assoc. 1998;91:438-445.
- Jones BE. Human ‘seed tick’ infestation. Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Centers for Disease Control and Prevention. Tick bite prophylaxis. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tick-bite-prophylaxis.html
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Krinsky WL. Dermatoses associated with the bites of mites and ticks (Arthropoda: Acari). Int J Dermatol. 1983;22:75-91.
- Yesudian P, Thambiah AS. Persistent papules after tick-bites. Dermatologica. 1973;147:214-218.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Solberg VB, Klein TA, McPherson KR, et al. Field evaluation of DEET and a piperidine repellent (AI3-37220) against Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1995;32:870-875.
- Evans SR, Korch GW Jr, Lawson MA. Comparative field evaluation of permethrin and DEET-treated military uniforms for personal protection against ticks (Acari). J Med Entomol. 1990;27:829-834.
Practice Points
- The range of Amblyomma americanum has expanded north in recent years from its core range in the southeastern United States. Warming temperatures also have increased the duration of the ticks’ active season.
- Amblyomma americanum can lay several thousand eggs. A person happening upon a newly hatched nest of larval ticks could sustain a widespread vesicular eruption secondary to tick bites.
- It is important to keep larval tick infestation in the differential when evaluating a patient with a new widespread vesicular eruption to expedite prompt removal of the offending ticks and to begin clinical monitoring.
Tebipenem pivoxil hydrobromide offers oral option for complex UTIs
“No new oral antibiotic alternative has emerged to treat these conditions in more than 25 years,” corresponding author Angela K. Talley, MD, said in an interview. The new research was published in the New England Journal of Medicine.
Patients with complicated urinary tract infection (cUTI), including acute pyelonephritis (AP), are often hospitalized and treated with intravenous therapy because of the lack of oral options, especially in cases of antibiotic-resistant pathogens, explained Dr. Talley, of Spero Therapeutics.
In their new phase 3, double-blind randomized trial, the researchers evaluated the safety and effectiveness of oral TBP-PI-HBr, compared with intravenous ertapenem in hospitalized patients with cUTIs or AP. Oral tebipenem is an investigational carbapenem with demonstrated activity against uropathogenic Enterobacterales, and it has shown effectiveness in animal models, the researchers noted in their paper.
Methods and results
The researchers randomized 1,372 adult patients. The microbiologic intent-to-treat population included 449 patients who received TBP-PI-HBr (600 mg every 8 hours) and 419 who received ertapenem (1 g every 24 hours) for 7-10 days or up to 14 days for patients with bacteremia.
The primary endpoint was a composite of clinical cure and favorable microbiologic response, assessed at a test-of-cure visit on day 19. Clinical cure was defined as “complete resolution or clinically significant alleviation of baseline signs and symptoms of complicated urinary tract infection or acute pyelonephritis and no new symptoms, such that no further antimicrobial therapy was warranted,” the researchers wrote. Microbiologic response was defined as a reduction to less than 103 CFU per milliliter in uropathogen levels from baseline at day 19.
Overall, the clinical response occurred in 58.8% of patients who received TBP-PI-HBr and 61.6% of those who received ertapenem at the test-of-cure visit.
Clinical cure rates were similar in the TBP-PI-HBr and ertapenem groups (93.1% vs. 93.6%) at the test-of-cure visit.
Both treatment groups showed similar responses to Enterobacterales pathogens at the test-of-cure visit (62.7% for TBP-PI-HBr and 65.2% for ertapenem).
Among patients with bacteremia at baseline, overall response rates were 72.3% and 66.0% for TBP-PI-HBr and ertapenem, respectively, at the test-of-cure visit, and 93.6% and 96.2%, respectively, at the end-of-treatment visit on or around day 25.
The overall incidence of adverse events was approximately 26% in both treatment groups. Most adverse events were mild or moderate in severity and did not limit treatment, the researchers wrote.
The mean age of the patients was 58.1 years; 46.1% were aged 65 and older, and 11.5% had bacteremia at baseline.
The study findings were limited by several factors, including the mandated 7- to 10-day course of antibiotics, which may not reflect the standard of care in other settings in the United States. The study’s trial sites were located in the United States, South Africa, and Europe. The study population was primarily White and from Central and Eastern Europe. Other limitations included the randomization of patients before confirming the baseline pathogen, although this was done to limit potential confounding from previous antibiotics, the researchers noted.
Safety and efficacy support application for approval
“To our knowledge, this is the first head-to-head evaluation of an IV vs. an oral drug for the treatment of cUTI and acute pyelonephritis,” Dr. Talley said in an interview.
“The findings demonstrate that almost all patients in the study achieved complete resolution of the signs and symptoms of their infection,” she said.
TBP-PI-HBr has not been approved by the Food and Drug Administration, but a new drug application that included data from the current study was submitted to the FDA and is currently under review, Dr. Talley noted.
As for additional research, the current study was conducted in hospitalized patients, and the use of TBP-PI-HBr in the outpatient setting has not yet been evaluated, she said.
Approval and use of oral carbapenem will change practice
The current study is very important because it provides a viable and effective alternative form of antibiotic delivery for the patients with complicated UTI, Noel N. Deep, MD, emphasized in an interview.
“Currently these patients have to be treated with IV carbapenem antibiotics either in a hospital or through a home health nurse,” Dr. Deep, a general internist in group practice in Antigo, Wisc., explained.
Current IV strategies also carry the inherent risk associated with the insertion of an IV catheter that is left in place for several days or replaced periodically. “The oral antibiotic eliminates these risks and higher health care costs and provides a safer and equally efficacious option,” Dr. Deep said.
In the current study, “I was definitely surprised at the effectiveness of the oral carbapenem,” Dr. Deep said. “I am absolutely delighted with this new treatment option that physicians can now add to their armamentarium [assuming FDA approval] as we provide care to our patients,” he said.
If approved, TBP-PI-HBr will definitely change the treatment spectrum for the multidrug-resistant bacterial UTIs, said Dr. Deep. “Carbapenems have continued to be effective and low antibiotic resistance to carbapenems has been recorded.”
As for additional research, “I would like to see studies done in other ethnicities and different countries to ascertain the effectiveness of this antibiotic in those populations and against other bacterial strains with potentially different resistance mechanisms,” Dr. Deep said.
The study was supported by Spero Therapeutics and the Department of Health and Human Services. Lead author Paul B. Eckburg, MD, of Stanford (Calif.) University, and Dr. Talley are employees of Spero Therapeutics. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
“No new oral antibiotic alternative has emerged to treat these conditions in more than 25 years,” corresponding author Angela K. Talley, MD, said in an interview. The new research was published in the New England Journal of Medicine.
Patients with complicated urinary tract infection (cUTI), including acute pyelonephritis (AP), are often hospitalized and treated with intravenous therapy because of the lack of oral options, especially in cases of antibiotic-resistant pathogens, explained Dr. Talley, of Spero Therapeutics.
In their new phase 3, double-blind randomized trial, the researchers evaluated the safety and effectiveness of oral TBP-PI-HBr, compared with intravenous ertapenem in hospitalized patients with cUTIs or AP. Oral tebipenem is an investigational carbapenem with demonstrated activity against uropathogenic Enterobacterales, and it has shown effectiveness in animal models, the researchers noted in their paper.
Methods and results
The researchers randomized 1,372 adult patients. The microbiologic intent-to-treat population included 449 patients who received TBP-PI-HBr (600 mg every 8 hours) and 419 who received ertapenem (1 g every 24 hours) for 7-10 days or up to 14 days for patients with bacteremia.
The primary endpoint was a composite of clinical cure and favorable microbiologic response, assessed at a test-of-cure visit on day 19. Clinical cure was defined as “complete resolution or clinically significant alleviation of baseline signs and symptoms of complicated urinary tract infection or acute pyelonephritis and no new symptoms, such that no further antimicrobial therapy was warranted,” the researchers wrote. Microbiologic response was defined as a reduction to less than 103 CFU per milliliter in uropathogen levels from baseline at day 19.
Overall, the clinical response occurred in 58.8% of patients who received TBP-PI-HBr and 61.6% of those who received ertapenem at the test-of-cure visit.
Clinical cure rates were similar in the TBP-PI-HBr and ertapenem groups (93.1% vs. 93.6%) at the test-of-cure visit.
Both treatment groups showed similar responses to Enterobacterales pathogens at the test-of-cure visit (62.7% for TBP-PI-HBr and 65.2% for ertapenem).
Among patients with bacteremia at baseline, overall response rates were 72.3% and 66.0% for TBP-PI-HBr and ertapenem, respectively, at the test-of-cure visit, and 93.6% and 96.2%, respectively, at the end-of-treatment visit on or around day 25.
The overall incidence of adverse events was approximately 26% in both treatment groups. Most adverse events were mild or moderate in severity and did not limit treatment, the researchers wrote.
The mean age of the patients was 58.1 years; 46.1% were aged 65 and older, and 11.5% had bacteremia at baseline.
The study findings were limited by several factors, including the mandated 7- to 10-day course of antibiotics, which may not reflect the standard of care in other settings in the United States. The study’s trial sites were located in the United States, South Africa, and Europe. The study population was primarily White and from Central and Eastern Europe. Other limitations included the randomization of patients before confirming the baseline pathogen, although this was done to limit potential confounding from previous antibiotics, the researchers noted.
Safety and efficacy support application for approval
“To our knowledge, this is the first head-to-head evaluation of an IV vs. an oral drug for the treatment of cUTI and acute pyelonephritis,” Dr. Talley said in an interview.
“The findings demonstrate that almost all patients in the study achieved complete resolution of the signs and symptoms of their infection,” she said.
TBP-PI-HBr has not been approved by the Food and Drug Administration, but a new drug application that included data from the current study was submitted to the FDA and is currently under review, Dr. Talley noted.
As for additional research, the current study was conducted in hospitalized patients, and the use of TBP-PI-HBr in the outpatient setting has not yet been evaluated, she said.
Approval and use of oral carbapenem will change practice
The current study is very important because it provides a viable and effective alternative form of antibiotic delivery for the patients with complicated UTI, Noel N. Deep, MD, emphasized in an interview.
“Currently these patients have to be treated with IV carbapenem antibiotics either in a hospital or through a home health nurse,” Dr. Deep, a general internist in group practice in Antigo, Wisc., explained.
Current IV strategies also carry the inherent risk associated with the insertion of an IV catheter that is left in place for several days or replaced periodically. “The oral antibiotic eliminates these risks and higher health care costs and provides a safer and equally efficacious option,” Dr. Deep said.
In the current study, “I was definitely surprised at the effectiveness of the oral carbapenem,” Dr. Deep said. “I am absolutely delighted with this new treatment option that physicians can now add to their armamentarium [assuming FDA approval] as we provide care to our patients,” he said.
If approved, TBP-PI-HBr will definitely change the treatment spectrum for the multidrug-resistant bacterial UTIs, said Dr. Deep. “Carbapenems have continued to be effective and low antibiotic resistance to carbapenems has been recorded.”
As for additional research, “I would like to see studies done in other ethnicities and different countries to ascertain the effectiveness of this antibiotic in those populations and against other bacterial strains with potentially different resistance mechanisms,” Dr. Deep said.
The study was supported by Spero Therapeutics and the Department of Health and Human Services. Lead author Paul B. Eckburg, MD, of Stanford (Calif.) University, and Dr. Talley are employees of Spero Therapeutics. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
“No new oral antibiotic alternative has emerged to treat these conditions in more than 25 years,” corresponding author Angela K. Talley, MD, said in an interview. The new research was published in the New England Journal of Medicine.
Patients with complicated urinary tract infection (cUTI), including acute pyelonephritis (AP), are often hospitalized and treated with intravenous therapy because of the lack of oral options, especially in cases of antibiotic-resistant pathogens, explained Dr. Talley, of Spero Therapeutics.
In their new phase 3, double-blind randomized trial, the researchers evaluated the safety and effectiveness of oral TBP-PI-HBr, compared with intravenous ertapenem in hospitalized patients with cUTIs or AP. Oral tebipenem is an investigational carbapenem with demonstrated activity against uropathogenic Enterobacterales, and it has shown effectiveness in animal models, the researchers noted in their paper.
Methods and results
The researchers randomized 1,372 adult patients. The microbiologic intent-to-treat population included 449 patients who received TBP-PI-HBr (600 mg every 8 hours) and 419 who received ertapenem (1 g every 24 hours) for 7-10 days or up to 14 days for patients with bacteremia.
The primary endpoint was a composite of clinical cure and favorable microbiologic response, assessed at a test-of-cure visit on day 19. Clinical cure was defined as “complete resolution or clinically significant alleviation of baseline signs and symptoms of complicated urinary tract infection or acute pyelonephritis and no new symptoms, such that no further antimicrobial therapy was warranted,” the researchers wrote. Microbiologic response was defined as a reduction to less than 103 CFU per milliliter in uropathogen levels from baseline at day 19.
Overall, the clinical response occurred in 58.8% of patients who received TBP-PI-HBr and 61.6% of those who received ertapenem at the test-of-cure visit.
Clinical cure rates were similar in the TBP-PI-HBr and ertapenem groups (93.1% vs. 93.6%) at the test-of-cure visit.
Both treatment groups showed similar responses to Enterobacterales pathogens at the test-of-cure visit (62.7% for TBP-PI-HBr and 65.2% for ertapenem).
Among patients with bacteremia at baseline, overall response rates were 72.3% and 66.0% for TBP-PI-HBr and ertapenem, respectively, at the test-of-cure visit, and 93.6% and 96.2%, respectively, at the end-of-treatment visit on or around day 25.
The overall incidence of adverse events was approximately 26% in both treatment groups. Most adverse events were mild or moderate in severity and did not limit treatment, the researchers wrote.
The mean age of the patients was 58.1 years; 46.1% were aged 65 and older, and 11.5% had bacteremia at baseline.
The study findings were limited by several factors, including the mandated 7- to 10-day course of antibiotics, which may not reflect the standard of care in other settings in the United States. The study’s trial sites were located in the United States, South Africa, and Europe. The study population was primarily White and from Central and Eastern Europe. Other limitations included the randomization of patients before confirming the baseline pathogen, although this was done to limit potential confounding from previous antibiotics, the researchers noted.
Safety and efficacy support application for approval
“To our knowledge, this is the first head-to-head evaluation of an IV vs. an oral drug for the treatment of cUTI and acute pyelonephritis,” Dr. Talley said in an interview.
“The findings demonstrate that almost all patients in the study achieved complete resolution of the signs and symptoms of their infection,” she said.
TBP-PI-HBr has not been approved by the Food and Drug Administration, but a new drug application that included data from the current study was submitted to the FDA and is currently under review, Dr. Talley noted.
As for additional research, the current study was conducted in hospitalized patients, and the use of TBP-PI-HBr in the outpatient setting has not yet been evaluated, she said.
Approval and use of oral carbapenem will change practice
The current study is very important because it provides a viable and effective alternative form of antibiotic delivery for the patients with complicated UTI, Noel N. Deep, MD, emphasized in an interview.
“Currently these patients have to be treated with IV carbapenem antibiotics either in a hospital or through a home health nurse,” Dr. Deep, a general internist in group practice in Antigo, Wisc., explained.
Current IV strategies also carry the inherent risk associated with the insertion of an IV catheter that is left in place for several days or replaced periodically. “The oral antibiotic eliminates these risks and higher health care costs and provides a safer and equally efficacious option,” Dr. Deep said.
In the current study, “I was definitely surprised at the effectiveness of the oral carbapenem,” Dr. Deep said. “I am absolutely delighted with this new treatment option that physicians can now add to their armamentarium [assuming FDA approval] as we provide care to our patients,” he said.
If approved, TBP-PI-HBr will definitely change the treatment spectrum for the multidrug-resistant bacterial UTIs, said Dr. Deep. “Carbapenems have continued to be effective and low antibiotic resistance to carbapenems has been recorded.”
As for additional research, “I would like to see studies done in other ethnicities and different countries to ascertain the effectiveness of this antibiotic in those populations and against other bacterial strains with potentially different resistance mechanisms,” Dr. Deep said.
The study was supported by Spero Therapeutics and the Department of Health and Human Services. Lead author Paul B. Eckburg, MD, of Stanford (Calif.) University, and Dr. Talley are employees of Spero Therapeutics. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Children and COVID-19: Decline in new cases may be leveling off
Even as a number of states see increases in new COVID-19 cases among all ages, the trend remains downward for children, albeit at a slower pace than in recent weeks, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
New pediatric cases in the United States totaled 27,521 for the most recent week, March 25-31, down by 5.2% from the previous week. Earlier weekly declines, going backward through March and into late February, were 9.3%, 23%, 39.5%, and 46%, according to data collected by the AAP and CHA from state and territorial health agencies. The lowest weekly total recorded since the initial wave in 2020 was just under 8,500 during the week of June 18-24, 2021.
Reported COVID-19 cases in children now total over 12.8 million since the beginning of the pandemic in March 2020, and those infections represent 19.0% of all cases. That share of new cases has not increased in the last 7 weeks, the AAP and CHA noted in their weekly COVID report, suggesting that children have not been bearing a disproportionate share of the declining Omicron burden.
As for Omicron, the BA.2 subvariant now makes up about 55% of COVID-19 infections, the Centers for Disease Control and Prevention said in its COVID Data Tracker Weekly Review, and New York, Massachusetts, and New Jersey are among the states reporting BA.2-driven increases in new cases of as much as 30%, the New York Times said.
Rates of new cases for the latest week available (March 27 to April 2) and at their Omicron peaks in January were 11.3 per 100,000 and 1,011 per 100,000 (ages 0-4 years), 12.5 and 1,505 per 100,000 (5-11 years), 12.7 and 1,779 per 100,000 (12-15 years), and 13.1 and 1,982 per 100,000 (16-17 years), the CDC said on its COVID Data Tracker.
Hospitalization rates, however, were a bit of a mixed bag. The last 2 weeks (March 13-19 and March 20-26) of data available from the CDC’s COVID-NET show that hospitalizations were up slightly in children aged 0-4 years (1.3 per 100,000 to 1.4 per 100,000), down for 5- to 11-year-olds (0.6 to 0.2), and steady for those aged 12-17 (0.4 to 0.4). COVID-NET collects data from nearly 100 counties in 10 states and from a separate four-state network.
Vaccinations got a small boost in the last week, the first one since early February. Initial doses and completions climbed slightly in the 12- to 17-year-olds, while just first doses were up a bit among the 5- to 11-year-olds during the week of March 24-30, compared with the previous week, although both groups are still well below the highest counts recorded so far in 2022, which are, in turn, far short of 2021’s peaks, according to CDC data analyzed by the AAP.
Even as a number of states see increases in new COVID-19 cases among all ages, the trend remains downward for children, albeit at a slower pace than in recent weeks, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
New pediatric cases in the United States totaled 27,521 for the most recent week, March 25-31, down by 5.2% from the previous week. Earlier weekly declines, going backward through March and into late February, were 9.3%, 23%, 39.5%, and 46%, according to data collected by the AAP and CHA from state and territorial health agencies. The lowest weekly total recorded since the initial wave in 2020 was just under 8,500 during the week of June 18-24, 2021.
Reported COVID-19 cases in children now total over 12.8 million since the beginning of the pandemic in March 2020, and those infections represent 19.0% of all cases. That share of new cases has not increased in the last 7 weeks, the AAP and CHA noted in their weekly COVID report, suggesting that children have not been bearing a disproportionate share of the declining Omicron burden.
As for Omicron, the BA.2 subvariant now makes up about 55% of COVID-19 infections, the Centers for Disease Control and Prevention said in its COVID Data Tracker Weekly Review, and New York, Massachusetts, and New Jersey are among the states reporting BA.2-driven increases in new cases of as much as 30%, the New York Times said.
Rates of new cases for the latest week available (March 27 to April 2) and at their Omicron peaks in January were 11.3 per 100,000 and 1,011 per 100,000 (ages 0-4 years), 12.5 and 1,505 per 100,000 (5-11 years), 12.7 and 1,779 per 100,000 (12-15 years), and 13.1 and 1,982 per 100,000 (16-17 years), the CDC said on its COVID Data Tracker.
Hospitalization rates, however, were a bit of a mixed bag. The last 2 weeks (March 13-19 and March 20-26) of data available from the CDC’s COVID-NET show that hospitalizations were up slightly in children aged 0-4 years (1.3 per 100,000 to 1.4 per 100,000), down for 5- to 11-year-olds (0.6 to 0.2), and steady for those aged 12-17 (0.4 to 0.4). COVID-NET collects data from nearly 100 counties in 10 states and from a separate four-state network.
Vaccinations got a small boost in the last week, the first one since early February. Initial doses and completions climbed slightly in the 12- to 17-year-olds, while just first doses were up a bit among the 5- to 11-year-olds during the week of March 24-30, compared with the previous week, although both groups are still well below the highest counts recorded so far in 2022, which are, in turn, far short of 2021’s peaks, according to CDC data analyzed by the AAP.
Even as a number of states see increases in new COVID-19 cases among all ages, the trend remains downward for children, albeit at a slower pace than in recent weeks, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
New pediatric cases in the United States totaled 27,521 for the most recent week, March 25-31, down by 5.2% from the previous week. Earlier weekly declines, going backward through March and into late February, were 9.3%, 23%, 39.5%, and 46%, according to data collected by the AAP and CHA from state and territorial health agencies. The lowest weekly total recorded since the initial wave in 2020 was just under 8,500 during the week of June 18-24, 2021.
Reported COVID-19 cases in children now total over 12.8 million since the beginning of the pandemic in March 2020, and those infections represent 19.0% of all cases. That share of new cases has not increased in the last 7 weeks, the AAP and CHA noted in their weekly COVID report, suggesting that children have not been bearing a disproportionate share of the declining Omicron burden.
As for Omicron, the BA.2 subvariant now makes up about 55% of COVID-19 infections, the Centers for Disease Control and Prevention said in its COVID Data Tracker Weekly Review, and New York, Massachusetts, and New Jersey are among the states reporting BA.2-driven increases in new cases of as much as 30%, the New York Times said.
Rates of new cases for the latest week available (March 27 to April 2) and at their Omicron peaks in January were 11.3 per 100,000 and 1,011 per 100,000 (ages 0-4 years), 12.5 and 1,505 per 100,000 (5-11 years), 12.7 and 1,779 per 100,000 (12-15 years), and 13.1 and 1,982 per 100,000 (16-17 years), the CDC said on its COVID Data Tracker.
Hospitalization rates, however, were a bit of a mixed bag. The last 2 weeks (March 13-19 and March 20-26) of data available from the CDC’s COVID-NET show that hospitalizations were up slightly in children aged 0-4 years (1.3 per 100,000 to 1.4 per 100,000), down for 5- to 11-year-olds (0.6 to 0.2), and steady for those aged 12-17 (0.4 to 0.4). COVID-NET collects data from nearly 100 counties in 10 states and from a separate four-state network.
Vaccinations got a small boost in the last week, the first one since early February. Initial doses and completions climbed slightly in the 12- to 17-year-olds, while just first doses were up a bit among the 5- to 11-year-olds during the week of March 24-30, compared with the previous week, although both groups are still well below the highest counts recorded so far in 2022, which are, in turn, far short of 2021’s peaks, according to CDC data analyzed by the AAP.
Flu vaccines cut seasonal death in heart failure patients
WASHINGTON – Patients with heart failure who received an annual influenza vaccine for 3 years running had significantly fewer all-cause hospitalizations and significantly fewer cases of pneumonia during that time, compared with placebo-treated patients with heart failure, in a prospective, randomized, global trial with 5,129 participants.
Although the results failed to show a significant reduction in all-cause deaths linked to influenza vaccination, compared with controls during the entire 3 years of the study, the results did show a significant 21% relative mortality-risk reduction by vaccination during periods of peak influenza circulation, and a significant 23% reduction in cardiovascular deaths, compared with controls during peak seasons.
“This is the first randomized, controlled trial of influenza vaccine in patients with heart failure, and we showed that vaccination reduces deaths” during peak influenza seasons, Mark Loeb, MD, said during a press briefing at the annual scientific sessions of the American College of Cardiology. The results send “an important global message that patients with heart failure should receive the influenza vaccine,” said Dr. Loeb, a professor at McMaster University, Hamilton, Ont., who specializes in clinical epidemiology and infectious diseases.
Dr. Loeb admitted that he and his associates erred when they picked the time window to assess the two primary endpoints for the trial: the combined rate of cardiovascular death, nonfatal MI, and nonfatal stroke, and this combined endpoint plus hospitalizations for heart failure.
The time window they selected was the entirety of all 3 years following three annual immunizations. That was a mistake.
No flu vaccine benefit outside flu season
“We know that the influenza vaccine will not have any effect outside of when influenza is circulating. In retrospect, we should have done that,” Dr. Loeb bemoaned during his talk. He chalked up the bad choice to concern over collecting enough endpoints to see a significant between-group difference when the researchers designed the study.
For the entire 3 years of follow-up, influenza vaccination was tied to a nonsignificant 7% relative risk reduction for the first primary endpoint, and a nonsignificant 9% relative risk reduction for the second primary endpoint, he reported.
But Dr. Loeb lobbied for the relevance of several significant secondary endpoints that collectively showed a compelling pattern of benefit during his talk. These included, for the full 3-years of follow-up, important, significant reductions relative to placebo of 16% for first all-cause hospitalizations (P = .01), and a 42% relative risk reduction in first cases of pneumonia (P = .0006).
Then there were the benefits that appeared during influenza season. In that analysis, first events for the first primary endpoint fell after vaccination by a significant 18% relative to placebo. The in-season analysis also showed the significant cuts in both all-cause and cardiovascular deaths.
Despite the neutral primary endpoints, “if you look at these data as a whole I think they speak to the importance of vaccinating patients with heart failure against influenza,” Dr. Loeb maintained.
‘Totality of evidence supports vaccination’
“I agree that the totality of evidence supports influenza vaccination,” commented Mark H. Drazner, MD, professor and clinical chief of cardiology at the University of Texas Southwestern Medical Center, Dallas, who was designated discussant for the report.
“The message should be to offer influenza vaccine to patients with heart failure,” Dr. Drazner said in an interview. “Previous data on influenza vaccine in patients with heart failure were largely observational. This was a randomized, prospective, placebo-controlled trial. That’s a step forward. Proving efficacy in a randomized trial is important.”
Dr Drazner added that his institution already promotes a “strong mandate” to vaccinate patients with heart failure against influenza.
“The influenza vaccine is a very effective and cost-efficient public health measure. Preventing hospitalizations of patients with heart failure has so many benefits,” commented Craig Beavers, PharmD, vice president of professional services at Baptist Health in Paducah, Ky., and a discussant during the press briefing.
The Influenza Vaccine To Prevent Adverse Vascular Events (IVVE) trial enrolled people with heart failure in New York Heart Association functional class II, III, or IV from any of 10 low- and middle-income countries including China, India, the Philippines, and multiple countries from Africa and the Middle East. They averaged 57 years of age, and slightly more than half were women.
IVVE was sponsored by McMaster University; the only commercial support that IVVE received was a free supply of influenza vaccine from Sanofi Pasteur. Dr. Loeb, Dr. Drazner, and Dr. Beavers had no disclosures.
WASHINGTON – Patients with heart failure who received an annual influenza vaccine for 3 years running had significantly fewer all-cause hospitalizations and significantly fewer cases of pneumonia during that time, compared with placebo-treated patients with heart failure, in a prospective, randomized, global trial with 5,129 participants.
Although the results failed to show a significant reduction in all-cause deaths linked to influenza vaccination, compared with controls during the entire 3 years of the study, the results did show a significant 21% relative mortality-risk reduction by vaccination during periods of peak influenza circulation, and a significant 23% reduction in cardiovascular deaths, compared with controls during peak seasons.
“This is the first randomized, controlled trial of influenza vaccine in patients with heart failure, and we showed that vaccination reduces deaths” during peak influenza seasons, Mark Loeb, MD, said during a press briefing at the annual scientific sessions of the American College of Cardiology. The results send “an important global message that patients with heart failure should receive the influenza vaccine,” said Dr. Loeb, a professor at McMaster University, Hamilton, Ont., who specializes in clinical epidemiology and infectious diseases.
Dr. Loeb admitted that he and his associates erred when they picked the time window to assess the two primary endpoints for the trial: the combined rate of cardiovascular death, nonfatal MI, and nonfatal stroke, and this combined endpoint plus hospitalizations for heart failure.
The time window they selected was the entirety of all 3 years following three annual immunizations. That was a mistake.
No flu vaccine benefit outside flu season
“We know that the influenza vaccine will not have any effect outside of when influenza is circulating. In retrospect, we should have done that,” Dr. Loeb bemoaned during his talk. He chalked up the bad choice to concern over collecting enough endpoints to see a significant between-group difference when the researchers designed the study.
For the entire 3 years of follow-up, influenza vaccination was tied to a nonsignificant 7% relative risk reduction for the first primary endpoint, and a nonsignificant 9% relative risk reduction for the second primary endpoint, he reported.
But Dr. Loeb lobbied for the relevance of several significant secondary endpoints that collectively showed a compelling pattern of benefit during his talk. These included, for the full 3-years of follow-up, important, significant reductions relative to placebo of 16% for first all-cause hospitalizations (P = .01), and a 42% relative risk reduction in first cases of pneumonia (P = .0006).
Then there were the benefits that appeared during influenza season. In that analysis, first events for the first primary endpoint fell after vaccination by a significant 18% relative to placebo. The in-season analysis also showed the significant cuts in both all-cause and cardiovascular deaths.
Despite the neutral primary endpoints, “if you look at these data as a whole I think they speak to the importance of vaccinating patients with heart failure against influenza,” Dr. Loeb maintained.
‘Totality of evidence supports vaccination’
“I agree that the totality of evidence supports influenza vaccination,” commented Mark H. Drazner, MD, professor and clinical chief of cardiology at the University of Texas Southwestern Medical Center, Dallas, who was designated discussant for the report.
“The message should be to offer influenza vaccine to patients with heart failure,” Dr. Drazner said in an interview. “Previous data on influenza vaccine in patients with heart failure were largely observational. This was a randomized, prospective, placebo-controlled trial. That’s a step forward. Proving efficacy in a randomized trial is important.”
Dr Drazner added that his institution already promotes a “strong mandate” to vaccinate patients with heart failure against influenza.
“The influenza vaccine is a very effective and cost-efficient public health measure. Preventing hospitalizations of patients with heart failure has so many benefits,” commented Craig Beavers, PharmD, vice president of professional services at Baptist Health in Paducah, Ky., and a discussant during the press briefing.
The Influenza Vaccine To Prevent Adverse Vascular Events (IVVE) trial enrolled people with heart failure in New York Heart Association functional class II, III, or IV from any of 10 low- and middle-income countries including China, India, the Philippines, and multiple countries from Africa and the Middle East. They averaged 57 years of age, and slightly more than half were women.
IVVE was sponsored by McMaster University; the only commercial support that IVVE received was a free supply of influenza vaccine from Sanofi Pasteur. Dr. Loeb, Dr. Drazner, and Dr. Beavers had no disclosures.
WASHINGTON – Patients with heart failure who received an annual influenza vaccine for 3 years running had significantly fewer all-cause hospitalizations and significantly fewer cases of pneumonia during that time, compared with placebo-treated patients with heart failure, in a prospective, randomized, global trial with 5,129 participants.
Although the results failed to show a significant reduction in all-cause deaths linked to influenza vaccination, compared with controls during the entire 3 years of the study, the results did show a significant 21% relative mortality-risk reduction by vaccination during periods of peak influenza circulation, and a significant 23% reduction in cardiovascular deaths, compared with controls during peak seasons.
“This is the first randomized, controlled trial of influenza vaccine in patients with heart failure, and we showed that vaccination reduces deaths” during peak influenza seasons, Mark Loeb, MD, said during a press briefing at the annual scientific sessions of the American College of Cardiology. The results send “an important global message that patients with heart failure should receive the influenza vaccine,” said Dr. Loeb, a professor at McMaster University, Hamilton, Ont., who specializes in clinical epidemiology and infectious diseases.
Dr. Loeb admitted that he and his associates erred when they picked the time window to assess the two primary endpoints for the trial: the combined rate of cardiovascular death, nonfatal MI, and nonfatal stroke, and this combined endpoint plus hospitalizations for heart failure.
The time window they selected was the entirety of all 3 years following three annual immunizations. That was a mistake.
No flu vaccine benefit outside flu season
“We know that the influenza vaccine will not have any effect outside of when influenza is circulating. In retrospect, we should have done that,” Dr. Loeb bemoaned during his talk. He chalked up the bad choice to concern over collecting enough endpoints to see a significant between-group difference when the researchers designed the study.
For the entire 3 years of follow-up, influenza vaccination was tied to a nonsignificant 7% relative risk reduction for the first primary endpoint, and a nonsignificant 9% relative risk reduction for the second primary endpoint, he reported.
But Dr. Loeb lobbied for the relevance of several significant secondary endpoints that collectively showed a compelling pattern of benefit during his talk. These included, for the full 3-years of follow-up, important, significant reductions relative to placebo of 16% for first all-cause hospitalizations (P = .01), and a 42% relative risk reduction in first cases of pneumonia (P = .0006).
Then there were the benefits that appeared during influenza season. In that analysis, first events for the first primary endpoint fell after vaccination by a significant 18% relative to placebo. The in-season analysis also showed the significant cuts in both all-cause and cardiovascular deaths.
Despite the neutral primary endpoints, “if you look at these data as a whole I think they speak to the importance of vaccinating patients with heart failure against influenza,” Dr. Loeb maintained.
‘Totality of evidence supports vaccination’
“I agree that the totality of evidence supports influenza vaccination,” commented Mark H. Drazner, MD, professor and clinical chief of cardiology at the University of Texas Southwestern Medical Center, Dallas, who was designated discussant for the report.
“The message should be to offer influenza vaccine to patients with heart failure,” Dr. Drazner said in an interview. “Previous data on influenza vaccine in patients with heart failure were largely observational. This was a randomized, prospective, placebo-controlled trial. That’s a step forward. Proving efficacy in a randomized trial is important.”
Dr Drazner added that his institution already promotes a “strong mandate” to vaccinate patients with heart failure against influenza.
“The influenza vaccine is a very effective and cost-efficient public health measure. Preventing hospitalizations of patients with heart failure has so many benefits,” commented Craig Beavers, PharmD, vice president of professional services at Baptist Health in Paducah, Ky., and a discussant during the press briefing.
The Influenza Vaccine To Prevent Adverse Vascular Events (IVVE) trial enrolled people with heart failure in New York Heart Association functional class II, III, or IV from any of 10 low- and middle-income countries including China, India, the Philippines, and multiple countries from Africa and the Middle East. They averaged 57 years of age, and slightly more than half were women.
IVVE was sponsored by McMaster University; the only commercial support that IVVE received was a free supply of influenza vaccine from Sanofi Pasteur. Dr. Loeb, Dr. Drazner, and Dr. Beavers had no disclosures.
AT ACC 2022