User login
New Pill Successfully Lowers Lp(a) Levels
Concentrations of Lp(a) cholesterol are genetically determined and remain steady throughout life. Levels of 125 nmol/L or higher promote clotting and inflammation, significantly increasing the risk for heart attack, stroke, aortic stenosis, and peripheral artery disease. This affects about 20% of the population, particularly people of Black African and South Asian descent.
There are currently no approved therapies that lower Lp(a), said study author Stephen Nicholls, MBBS, PhD, director of the Victorian Heart Institute at Monash University in Melbourne, Australia. Several injectable therapies are currently in clinical trials, but muvalaplin is the only oral option. The new drug lowers Lp(a) levels by disrupting the bond between the two parts of the Lp(a) particle.
The KRAKEN Trial
In the KRAKEN trial, 233 adults from around the world with very high Lp(a) levels (> 175 nmol/L) were randomized either to one of three daily doses of muvalaplin — 10, 60, or 240 mg — or to placebo for 12 weeks.
The researchers measured Lp(a) levels with a standard blood test and with a novel test designed to specifically measure levels of intact Lp(a) particles in the blood. In addition to Lp(a), the standard test detects one of its components, apolipoprotein A particles, that are bound to the drug, which can lead to an underestimation of Lp(a) reductions.
Lp(a) levels were up to 70.0% lower in the muvalaplin group than in the placebo group when measured with the traditional blood test and by up to 85.5% lower when measured with the new test. Approximately 82% of participants achieved an Lp(a) level lower than 125 nmol/L when measured with the traditional blood test, and 97% achieved that level when the new test was used. Patients who received either 60 or 240 mg of muvalaplin had similar reductions in Lp(a) levels, which were greater than the reductions seen in the 10 mg group. The drug was safe and generally well tolerated.
“This is a very reassuring phase 2 result,” Nicholls said when he presented the KRAKEN findings at the American Heart Association (AHA) Scientific Sessions 2024 in Chicago, which were simultaneously published online in JAMA. “It encourages the ongoing development of this agent.”
Lp(a) levels are not affected by changes in lifestyle or diet or by traditional lipid-lowering treatments like statins, said Erin Michos, MD, a cardiologist at the Johns Hopkins University School of Medicine in Baltimore, Maryland, who was not involved in the study.
And high Lp(a) levels confer significant cardiovascular risk even when other risks are reduced. So muvalaplin is “a highly promising approach to treat a previously untreatable disorder,” she explained.
Larger and longer studies, with more diverse patient populations, are needed to confirm the results and to determine whether reducing Lp(a) also improves cardiovascular outcomes, Michos pointed out.
“While muvalaplin appears to be an effective approach to lowering Lp(a) levels, we still need to study whether Lp(a) lowering will result in fewer heart attacks and strokes,” Nicholls added.
A version of this article appeared on Medscape.com.
Concentrations of Lp(a) cholesterol are genetically determined and remain steady throughout life. Levels of 125 nmol/L or higher promote clotting and inflammation, significantly increasing the risk for heart attack, stroke, aortic stenosis, and peripheral artery disease. This affects about 20% of the population, particularly people of Black African and South Asian descent.
There are currently no approved therapies that lower Lp(a), said study author Stephen Nicholls, MBBS, PhD, director of the Victorian Heart Institute at Monash University in Melbourne, Australia. Several injectable therapies are currently in clinical trials, but muvalaplin is the only oral option. The new drug lowers Lp(a) levels by disrupting the bond between the two parts of the Lp(a) particle.
The KRAKEN Trial
In the KRAKEN trial, 233 adults from around the world with very high Lp(a) levels (> 175 nmol/L) were randomized either to one of three daily doses of muvalaplin — 10, 60, or 240 mg — or to placebo for 12 weeks.
The researchers measured Lp(a) levels with a standard blood test and with a novel test designed to specifically measure levels of intact Lp(a) particles in the blood. In addition to Lp(a), the standard test detects one of its components, apolipoprotein A particles, that are bound to the drug, which can lead to an underestimation of Lp(a) reductions.
Lp(a) levels were up to 70.0% lower in the muvalaplin group than in the placebo group when measured with the traditional blood test and by up to 85.5% lower when measured with the new test. Approximately 82% of participants achieved an Lp(a) level lower than 125 nmol/L when measured with the traditional blood test, and 97% achieved that level when the new test was used. Patients who received either 60 or 240 mg of muvalaplin had similar reductions in Lp(a) levels, which were greater than the reductions seen in the 10 mg group. The drug was safe and generally well tolerated.
“This is a very reassuring phase 2 result,” Nicholls said when he presented the KRAKEN findings at the American Heart Association (AHA) Scientific Sessions 2024 in Chicago, which were simultaneously published online in JAMA. “It encourages the ongoing development of this agent.”
Lp(a) levels are not affected by changes in lifestyle or diet or by traditional lipid-lowering treatments like statins, said Erin Michos, MD, a cardiologist at the Johns Hopkins University School of Medicine in Baltimore, Maryland, who was not involved in the study.
And high Lp(a) levels confer significant cardiovascular risk even when other risks are reduced. So muvalaplin is “a highly promising approach to treat a previously untreatable disorder,” she explained.
Larger and longer studies, with more diverse patient populations, are needed to confirm the results and to determine whether reducing Lp(a) also improves cardiovascular outcomes, Michos pointed out.
“While muvalaplin appears to be an effective approach to lowering Lp(a) levels, we still need to study whether Lp(a) lowering will result in fewer heart attacks and strokes,” Nicholls added.
A version of this article appeared on Medscape.com.
Concentrations of Lp(a) cholesterol are genetically determined and remain steady throughout life. Levels of 125 nmol/L or higher promote clotting and inflammation, significantly increasing the risk for heart attack, stroke, aortic stenosis, and peripheral artery disease. This affects about 20% of the population, particularly people of Black African and South Asian descent.
There are currently no approved therapies that lower Lp(a), said study author Stephen Nicholls, MBBS, PhD, director of the Victorian Heart Institute at Monash University in Melbourne, Australia. Several injectable therapies are currently in clinical trials, but muvalaplin is the only oral option. The new drug lowers Lp(a) levels by disrupting the bond between the two parts of the Lp(a) particle.
The KRAKEN Trial
In the KRAKEN trial, 233 adults from around the world with very high Lp(a) levels (> 175 nmol/L) were randomized either to one of three daily doses of muvalaplin — 10, 60, or 240 mg — or to placebo for 12 weeks.
The researchers measured Lp(a) levels with a standard blood test and with a novel test designed to specifically measure levels of intact Lp(a) particles in the blood. In addition to Lp(a), the standard test detects one of its components, apolipoprotein A particles, that are bound to the drug, which can lead to an underestimation of Lp(a) reductions.
Lp(a) levels were up to 70.0% lower in the muvalaplin group than in the placebo group when measured with the traditional blood test and by up to 85.5% lower when measured with the new test. Approximately 82% of participants achieved an Lp(a) level lower than 125 nmol/L when measured with the traditional blood test, and 97% achieved that level when the new test was used. Patients who received either 60 or 240 mg of muvalaplin had similar reductions in Lp(a) levels, which were greater than the reductions seen in the 10 mg group. The drug was safe and generally well tolerated.
“This is a very reassuring phase 2 result,” Nicholls said when he presented the KRAKEN findings at the American Heart Association (AHA) Scientific Sessions 2024 in Chicago, which were simultaneously published online in JAMA. “It encourages the ongoing development of this agent.”
Lp(a) levels are not affected by changes in lifestyle or diet or by traditional lipid-lowering treatments like statins, said Erin Michos, MD, a cardiologist at the Johns Hopkins University School of Medicine in Baltimore, Maryland, who was not involved in the study.
And high Lp(a) levels confer significant cardiovascular risk even when other risks are reduced. So muvalaplin is “a highly promising approach to treat a previously untreatable disorder,” she explained.
Larger and longer studies, with more diverse patient populations, are needed to confirm the results and to determine whether reducing Lp(a) also improves cardiovascular outcomes, Michos pointed out.
“While muvalaplin appears to be an effective approach to lowering Lp(a) levels, we still need to study whether Lp(a) lowering will result in fewer heart attacks and strokes,” Nicholls added.
A version of this article appeared on Medscape.com.
An Epidemiologist’s Guide to Debunking Nutritional Research
You’re invited to a dinner party but you struggle to make small talk. Do not worry; that will invariably crop up over cocktails. Because all journalism has been reduced to listicles, here are four ways to seem clever at dinner parties.
1. The Predinner Cocktails: A Lesson in Reverse Causation
Wine connoisseurs sniff, swirl, and gently swish the wine in their mouths before spitting out and cleansing their palates to better appreciate the subtlety of each vintage. If you’re not an oenophile, no matter. Whenever somebody claims that moderate amounts of alcohol are good for your heart, this is your moment to pounce. Interject yourself in the conversation and tell everybody about reverse causation.
Reverse causation, also known as protopathic bias, involves misinterpreting the directionality of an association. You assume that X leads to Y, when in fact Y leads to X. Temporal paradoxes are useful plot devices in science fiction movies, but they have no place in medical research. In our bland world, cause must precede effect. As such, smoking leads to lung cancer; lung cancer doesn’t make you smoke more.
But with alcohol, directionality is less obvious. Many studies of alcohol and cardiovascular disease have demonstrated a U-shaped association, with risk being lowest among those who drink moderate amounts of alcohol (usually one to two drinks per day) and higher in those who drink more and also those who drink very little.
But one must ask why some people drink little or no alcohol. There is an important difference between former drinkers and never drinkers. Former drinkers cut back for a reason. More likely than not, the reason for this newfound sobriety was medical. A new cancer diagnosis, the emergence of atrial fibrillation, the development of diabetes, or rising blood pressure are all good reasons to reduce or eliminate alcohol. A cross-sectional study will fail to capture that alcohol consumption changes over time — people who now don’t drink may have imbibed alcohol in years past. It was not abstinence that led to an increased risk for heart disease; it was the increased risk for heart disease that led to abstinence.
You see the same phenomenon with the so-called obesity paradox. The idea that being a little overweight is good for you may appeal when you no longer fit into last year’s pants. But people who are underweight are so for a reason. Malnutrition, cachexia from cancer, or some other cause is almost certainly driving up the risk at the left-hand side of the U-shaped curve that makes the middle part seem better than it actually is.
Food consumption changes over time. A cross-sectional survey at one point in time cannot accurately capture past habits and distant exposures, especially for diseases such as heart disease and cancer that develop slowly over time. Studies on alcohol that try to overcome these shortcomings by eliminating former drinkers, or by using Mendelian randomization to better account for past exposure, do not show a cardiovascular benefit for moderate red wine drinking.
2. The Hors D’oeuvres — The Importance of RCTs
Now that you have made yourself the center of attention, it is time to cement your newfound reputation as a font of scientific knowledge. Most self-respecting hosts will serve smoked salmon as an amuse-bouche before the main meal. When someone mentions the health benefits of fish oils, you should take the opportunity to teach them about confounding.
Fish, especially cold-water fish from northern climates, have relatively high amounts of omega-3 fatty acids. Despite the plethora of observational studies suggesting a cardiovascular benefit, it’s now relatively clear that fish oil or omega-3 supplements have no medical benefit.
This will probably come as a shock to the worried well, but many studies, including VITAL and ASCEND, have demonstrated no cardiovascular or cancer benefit to supplementation with omega-3s. The reason is straightforward and explains why hormone replacement therapy, vitamin D, and myriad purported game-changers never panned out. Confounding is hard to overcome in observational research.
Prior to the publication of the Women’s Health Initiative (WHI) Study, hormone replacement therapy was routinely prescribed to postmenopausal women because numerous observational studies suggested a cardiovascular benefit. But with the publication of the WHI study, it became clear that much of that “benefit” was due to confounding. The women choosing to take hormones were more health conscious at baseline and healthier overall.
A similar phenomenon occurred during COVID. Patients with low serum vitamin D levels had worse outcomes, prompting many to suggest vitamin D supplementation as a possible treatment. Trials did not support the intervention because we’d overlooked the obvious. People with vitamin D deficiency have underlying health problems that contribute to the vitamin D deficiency. They are probably older, frailer, possibly with a poorer diet. No amount of statistical adjustment can account for all those differences, and some degree of residual confounding will always persist.
The only way to overcome confounding is with randomization. When patients are randomly assigned to one group or another, their baseline differences largely balance out if the randomization was performed properly and the groups were large enough. There is a role for observational research, such as in situations where ethics, cost, and practicality do not allow for a randomized controlled trial. But randomized controlled trials have largely put to rest the purported health benefits of over-the-counter fish oils, omega-3s, and vitamin D.
3. The Main Course — Absolute vs Relative Risk
When you get to the main course, all eyes will now be on you. You will almost certainly be called upon to pronounce on the harms or benefits of red meat consumption. Begin by regaling your guests with a little trivia. Ask them if they know the definition of red meat and white meat. When someone says pork is white meat, you can reveal that “pork, the other white meat,” was a marketing slogan with no scientific underpinning. Now that everyone is lulled into a stupefied silence, tell them that red meat comes from mammals and white meat comes from birds. As they process this revelation, you can now launch into the deeply mathematical concept of absolute vs relative risk.
Many etiquette books will caution against bringing up math at a dinner party. These books are wrong. Everyone finds math interesting if they are primed properly. For example, you can point to a study claiming that berries reduce cardiovascular risk in women. Even if true — and there is reason to be cautious, given the observational nature of the research — we need to understand what the authors meant by a 32% risk reduction. (Side note: It was a reduction in hazard, with a hazard ratio of 0.68 (95% CI, 0.49-0.96), but we won’t dwell on the difference between hazard ratios and risk ratios right now.)
This relative risk reduction has to be interpreted carefully. The authors divided the population into quintiles based on their consumption of anthocyanins (the antioxidant in blueberries and strawberries) and compared the bottom fifth (average consumption, 2.5 mg/d) with the top fifth (average consumption, 25 mg/d). The bottom quintile had 126 myocardial infarctions (MIs) over 324,793 patient-years compared with 59 MIs over 332,143 patient-years. Some quick math shows an approximate reduction from 39 to 18 MIs per 100,000 patient-years. Or to put it another way, you must get 4762 women to increase their berry consumption 10-fold for 1 year to prevent one heart attack. Feel free to show people how you calculated this number. They will be impressed by your head for numbers. It is nothing more than 39 minus 18, divided by 100,000, to get the absolute risk reduction. Take the reciprocal of this (ie, 1 divided by this number) to get the number needed to treat.
Describing risks in absolute terms or using number needed to treat (or harm) can help conceptualize statistics that are sometimes hard to wrap your head around.
4. Dessert — Funding
By the time the coffee is served, everyone will be hanging on to your every word. This is as it should be, and you should not be afraid of your newfound power and influence.
Dessert will probably involve some form of chocolate, possibly in cake format. (Anyone who serves fruit as dessert is not someone you should associate with.) Take the opportunity to tell your follow diners that chocolate is not actually good for you and will not boost brain performance.
The health benefits of chocolate are often repeated but rarely scrutinized. In fact, much of the scientific research purporting to show that chocolate is good for you did not actually study chocolate. It usually involved a cocoa bean extract because the chocolate manufacturing process destroys the supposedly health-promoting antioxidants in the cocoa bean. It is true that dark chocolate has more antioxidants than milk chocolate, and that the addition of milk to chocolate further inactivates the potentially healthy antioxidants. But the amount of sugar and fat that has to be added to chocolate to make it palatable precludes any serious consideration about health benefits. Dark chocolate may have less fat and sugar than milk chocolate, but it still has a lot.
But even the cocoa bean extract doesn’t seem to do much for your heart or your brain. The long-awaited COSMOS study was published with surprisingly little fanfare. The largest randomized controlled trial of chocolate (or rather cocoa bean extract) was supposed to settle the issue definitively.
COSMOS showed no cardiovascular or neurocognitive benefit to the cocoa bean extract. But the health halo of chocolate continues to be bolstered by many studies funded by chocolate manufacturers.
We are appropriately critical of the pharmaceutical industry’s involvement in drug research. However, we should not forget that any private entity is prone to the same self-interest regardless of its product’s tastiness. How many of you knew that there was an avocado lobby funding research? No matter how many industry-funded observational studies using surrogate endpoints are out there telling you that chocolate is healthy, a randomized trial with hard clinical endpoints such as COSMOS should generally win the day.
The Final Goodbyes — Summarizing Your Case
As the party slowly winds down and everyone is saddened that you will soon take your leave, synthesize everything you have taught them over the evening. Like movies, not all studies are good. Some are just bad. They can be prone to reverse causation or confounding, and they may report relative risks when absolute risks would be more telling. Reading research studies critically is essential for separating the wheat from the chaff. With the knowledge you have now imparted to your friends, they will be much better consumers of medical news, especially when it comes to food.
And they will no doubt thank you for it by never inviting you to another dinner party!
Labos, a cardiologist at Hôpital, Notre-Dame, Montreal, Quebec, Canada, has disclosed no relevant financial relationships. He has a degree in epidemiology.
A version of this article appeared on Medscape.com.
You’re invited to a dinner party but you struggle to make small talk. Do not worry; that will invariably crop up over cocktails. Because all journalism has been reduced to listicles, here are four ways to seem clever at dinner parties.
1. The Predinner Cocktails: A Lesson in Reverse Causation
Wine connoisseurs sniff, swirl, and gently swish the wine in their mouths before spitting out and cleansing their palates to better appreciate the subtlety of each vintage. If you’re not an oenophile, no matter. Whenever somebody claims that moderate amounts of alcohol are good for your heart, this is your moment to pounce. Interject yourself in the conversation and tell everybody about reverse causation.
Reverse causation, also known as protopathic bias, involves misinterpreting the directionality of an association. You assume that X leads to Y, when in fact Y leads to X. Temporal paradoxes are useful plot devices in science fiction movies, but they have no place in medical research. In our bland world, cause must precede effect. As such, smoking leads to lung cancer; lung cancer doesn’t make you smoke more.
But with alcohol, directionality is less obvious. Many studies of alcohol and cardiovascular disease have demonstrated a U-shaped association, with risk being lowest among those who drink moderate amounts of alcohol (usually one to two drinks per day) and higher in those who drink more and also those who drink very little.
But one must ask why some people drink little or no alcohol. There is an important difference between former drinkers and never drinkers. Former drinkers cut back for a reason. More likely than not, the reason for this newfound sobriety was medical. A new cancer diagnosis, the emergence of atrial fibrillation, the development of diabetes, or rising blood pressure are all good reasons to reduce or eliminate alcohol. A cross-sectional study will fail to capture that alcohol consumption changes over time — people who now don’t drink may have imbibed alcohol in years past. It was not abstinence that led to an increased risk for heart disease; it was the increased risk for heart disease that led to abstinence.
You see the same phenomenon with the so-called obesity paradox. The idea that being a little overweight is good for you may appeal when you no longer fit into last year’s pants. But people who are underweight are so for a reason. Malnutrition, cachexia from cancer, or some other cause is almost certainly driving up the risk at the left-hand side of the U-shaped curve that makes the middle part seem better than it actually is.
Food consumption changes over time. A cross-sectional survey at one point in time cannot accurately capture past habits and distant exposures, especially for diseases such as heart disease and cancer that develop slowly over time. Studies on alcohol that try to overcome these shortcomings by eliminating former drinkers, or by using Mendelian randomization to better account for past exposure, do not show a cardiovascular benefit for moderate red wine drinking.
2. The Hors D’oeuvres — The Importance of RCTs
Now that you have made yourself the center of attention, it is time to cement your newfound reputation as a font of scientific knowledge. Most self-respecting hosts will serve smoked salmon as an amuse-bouche before the main meal. When someone mentions the health benefits of fish oils, you should take the opportunity to teach them about confounding.
Fish, especially cold-water fish from northern climates, have relatively high amounts of omega-3 fatty acids. Despite the plethora of observational studies suggesting a cardiovascular benefit, it’s now relatively clear that fish oil or omega-3 supplements have no medical benefit.
This will probably come as a shock to the worried well, but many studies, including VITAL and ASCEND, have demonstrated no cardiovascular or cancer benefit to supplementation with omega-3s. The reason is straightforward and explains why hormone replacement therapy, vitamin D, and myriad purported game-changers never panned out. Confounding is hard to overcome in observational research.
Prior to the publication of the Women’s Health Initiative (WHI) Study, hormone replacement therapy was routinely prescribed to postmenopausal women because numerous observational studies suggested a cardiovascular benefit. But with the publication of the WHI study, it became clear that much of that “benefit” was due to confounding. The women choosing to take hormones were more health conscious at baseline and healthier overall.
A similar phenomenon occurred during COVID. Patients with low serum vitamin D levels had worse outcomes, prompting many to suggest vitamin D supplementation as a possible treatment. Trials did not support the intervention because we’d overlooked the obvious. People with vitamin D deficiency have underlying health problems that contribute to the vitamin D deficiency. They are probably older, frailer, possibly with a poorer diet. No amount of statistical adjustment can account for all those differences, and some degree of residual confounding will always persist.
The only way to overcome confounding is with randomization. When patients are randomly assigned to one group or another, their baseline differences largely balance out if the randomization was performed properly and the groups were large enough. There is a role for observational research, such as in situations where ethics, cost, and practicality do not allow for a randomized controlled trial. But randomized controlled trials have largely put to rest the purported health benefits of over-the-counter fish oils, omega-3s, and vitamin D.
3. The Main Course — Absolute vs Relative Risk
When you get to the main course, all eyes will now be on you. You will almost certainly be called upon to pronounce on the harms or benefits of red meat consumption. Begin by regaling your guests with a little trivia. Ask them if they know the definition of red meat and white meat. When someone says pork is white meat, you can reveal that “pork, the other white meat,” was a marketing slogan with no scientific underpinning. Now that everyone is lulled into a stupefied silence, tell them that red meat comes from mammals and white meat comes from birds. As they process this revelation, you can now launch into the deeply mathematical concept of absolute vs relative risk.
Many etiquette books will caution against bringing up math at a dinner party. These books are wrong. Everyone finds math interesting if they are primed properly. For example, you can point to a study claiming that berries reduce cardiovascular risk in women. Even if true — and there is reason to be cautious, given the observational nature of the research — we need to understand what the authors meant by a 32% risk reduction. (Side note: It was a reduction in hazard, with a hazard ratio of 0.68 (95% CI, 0.49-0.96), but we won’t dwell on the difference between hazard ratios and risk ratios right now.)
This relative risk reduction has to be interpreted carefully. The authors divided the population into quintiles based on their consumption of anthocyanins (the antioxidant in blueberries and strawberries) and compared the bottom fifth (average consumption, 2.5 mg/d) with the top fifth (average consumption, 25 mg/d). The bottom quintile had 126 myocardial infarctions (MIs) over 324,793 patient-years compared with 59 MIs over 332,143 patient-years. Some quick math shows an approximate reduction from 39 to 18 MIs per 100,000 patient-years. Or to put it another way, you must get 4762 women to increase their berry consumption 10-fold for 1 year to prevent one heart attack. Feel free to show people how you calculated this number. They will be impressed by your head for numbers. It is nothing more than 39 minus 18, divided by 100,000, to get the absolute risk reduction. Take the reciprocal of this (ie, 1 divided by this number) to get the number needed to treat.
Describing risks in absolute terms or using number needed to treat (or harm) can help conceptualize statistics that are sometimes hard to wrap your head around.
4. Dessert — Funding
By the time the coffee is served, everyone will be hanging on to your every word. This is as it should be, and you should not be afraid of your newfound power and influence.
Dessert will probably involve some form of chocolate, possibly in cake format. (Anyone who serves fruit as dessert is not someone you should associate with.) Take the opportunity to tell your follow diners that chocolate is not actually good for you and will not boost brain performance.
The health benefits of chocolate are often repeated but rarely scrutinized. In fact, much of the scientific research purporting to show that chocolate is good for you did not actually study chocolate. It usually involved a cocoa bean extract because the chocolate manufacturing process destroys the supposedly health-promoting antioxidants in the cocoa bean. It is true that dark chocolate has more antioxidants than milk chocolate, and that the addition of milk to chocolate further inactivates the potentially healthy antioxidants. But the amount of sugar and fat that has to be added to chocolate to make it palatable precludes any serious consideration about health benefits. Dark chocolate may have less fat and sugar than milk chocolate, but it still has a lot.
But even the cocoa bean extract doesn’t seem to do much for your heart or your brain. The long-awaited COSMOS study was published with surprisingly little fanfare. The largest randomized controlled trial of chocolate (or rather cocoa bean extract) was supposed to settle the issue definitively.
COSMOS showed no cardiovascular or neurocognitive benefit to the cocoa bean extract. But the health halo of chocolate continues to be bolstered by many studies funded by chocolate manufacturers.
We are appropriately critical of the pharmaceutical industry’s involvement in drug research. However, we should not forget that any private entity is prone to the same self-interest regardless of its product’s tastiness. How many of you knew that there was an avocado lobby funding research? No matter how many industry-funded observational studies using surrogate endpoints are out there telling you that chocolate is healthy, a randomized trial with hard clinical endpoints such as COSMOS should generally win the day.
The Final Goodbyes — Summarizing Your Case
As the party slowly winds down and everyone is saddened that you will soon take your leave, synthesize everything you have taught them over the evening. Like movies, not all studies are good. Some are just bad. They can be prone to reverse causation or confounding, and they may report relative risks when absolute risks would be more telling. Reading research studies critically is essential for separating the wheat from the chaff. With the knowledge you have now imparted to your friends, they will be much better consumers of medical news, especially when it comes to food.
And they will no doubt thank you for it by never inviting you to another dinner party!
Labos, a cardiologist at Hôpital, Notre-Dame, Montreal, Quebec, Canada, has disclosed no relevant financial relationships. He has a degree in epidemiology.
A version of this article appeared on Medscape.com.
You’re invited to a dinner party but you struggle to make small talk. Do not worry; that will invariably crop up over cocktails. Because all journalism has been reduced to listicles, here are four ways to seem clever at dinner parties.
1. The Predinner Cocktails: A Lesson in Reverse Causation
Wine connoisseurs sniff, swirl, and gently swish the wine in their mouths before spitting out and cleansing their palates to better appreciate the subtlety of each vintage. If you’re not an oenophile, no matter. Whenever somebody claims that moderate amounts of alcohol are good for your heart, this is your moment to pounce. Interject yourself in the conversation and tell everybody about reverse causation.
Reverse causation, also known as protopathic bias, involves misinterpreting the directionality of an association. You assume that X leads to Y, when in fact Y leads to X. Temporal paradoxes are useful plot devices in science fiction movies, but they have no place in medical research. In our bland world, cause must precede effect. As such, smoking leads to lung cancer; lung cancer doesn’t make you smoke more.
But with alcohol, directionality is less obvious. Many studies of alcohol and cardiovascular disease have demonstrated a U-shaped association, with risk being lowest among those who drink moderate amounts of alcohol (usually one to two drinks per day) and higher in those who drink more and also those who drink very little.
But one must ask why some people drink little or no alcohol. There is an important difference between former drinkers and never drinkers. Former drinkers cut back for a reason. More likely than not, the reason for this newfound sobriety was medical. A new cancer diagnosis, the emergence of atrial fibrillation, the development of diabetes, or rising blood pressure are all good reasons to reduce or eliminate alcohol. A cross-sectional study will fail to capture that alcohol consumption changes over time — people who now don’t drink may have imbibed alcohol in years past. It was not abstinence that led to an increased risk for heart disease; it was the increased risk for heart disease that led to abstinence.
You see the same phenomenon with the so-called obesity paradox. The idea that being a little overweight is good for you may appeal when you no longer fit into last year’s pants. But people who are underweight are so for a reason. Malnutrition, cachexia from cancer, or some other cause is almost certainly driving up the risk at the left-hand side of the U-shaped curve that makes the middle part seem better than it actually is.
Food consumption changes over time. A cross-sectional survey at one point in time cannot accurately capture past habits and distant exposures, especially for diseases such as heart disease and cancer that develop slowly over time. Studies on alcohol that try to overcome these shortcomings by eliminating former drinkers, or by using Mendelian randomization to better account for past exposure, do not show a cardiovascular benefit for moderate red wine drinking.
2. The Hors D’oeuvres — The Importance of RCTs
Now that you have made yourself the center of attention, it is time to cement your newfound reputation as a font of scientific knowledge. Most self-respecting hosts will serve smoked salmon as an amuse-bouche before the main meal. When someone mentions the health benefits of fish oils, you should take the opportunity to teach them about confounding.
Fish, especially cold-water fish from northern climates, have relatively high amounts of omega-3 fatty acids. Despite the plethora of observational studies suggesting a cardiovascular benefit, it’s now relatively clear that fish oil or omega-3 supplements have no medical benefit.
This will probably come as a shock to the worried well, but many studies, including VITAL and ASCEND, have demonstrated no cardiovascular or cancer benefit to supplementation with omega-3s. The reason is straightforward and explains why hormone replacement therapy, vitamin D, and myriad purported game-changers never panned out. Confounding is hard to overcome in observational research.
Prior to the publication of the Women’s Health Initiative (WHI) Study, hormone replacement therapy was routinely prescribed to postmenopausal women because numerous observational studies suggested a cardiovascular benefit. But with the publication of the WHI study, it became clear that much of that “benefit” was due to confounding. The women choosing to take hormones were more health conscious at baseline and healthier overall.
A similar phenomenon occurred during COVID. Patients with low serum vitamin D levels had worse outcomes, prompting many to suggest vitamin D supplementation as a possible treatment. Trials did not support the intervention because we’d overlooked the obvious. People with vitamin D deficiency have underlying health problems that contribute to the vitamin D deficiency. They are probably older, frailer, possibly with a poorer diet. No amount of statistical adjustment can account for all those differences, and some degree of residual confounding will always persist.
The only way to overcome confounding is with randomization. When patients are randomly assigned to one group or another, their baseline differences largely balance out if the randomization was performed properly and the groups were large enough. There is a role for observational research, such as in situations where ethics, cost, and practicality do not allow for a randomized controlled trial. But randomized controlled trials have largely put to rest the purported health benefits of over-the-counter fish oils, omega-3s, and vitamin D.
3. The Main Course — Absolute vs Relative Risk
When you get to the main course, all eyes will now be on you. You will almost certainly be called upon to pronounce on the harms or benefits of red meat consumption. Begin by regaling your guests with a little trivia. Ask them if they know the definition of red meat and white meat. When someone says pork is white meat, you can reveal that “pork, the other white meat,” was a marketing slogan with no scientific underpinning. Now that everyone is lulled into a stupefied silence, tell them that red meat comes from mammals and white meat comes from birds. As they process this revelation, you can now launch into the deeply mathematical concept of absolute vs relative risk.
Many etiquette books will caution against bringing up math at a dinner party. These books are wrong. Everyone finds math interesting if they are primed properly. For example, you can point to a study claiming that berries reduce cardiovascular risk in women. Even if true — and there is reason to be cautious, given the observational nature of the research — we need to understand what the authors meant by a 32% risk reduction. (Side note: It was a reduction in hazard, with a hazard ratio of 0.68 (95% CI, 0.49-0.96), but we won’t dwell on the difference between hazard ratios and risk ratios right now.)
This relative risk reduction has to be interpreted carefully. The authors divided the population into quintiles based on their consumption of anthocyanins (the antioxidant in blueberries and strawberries) and compared the bottom fifth (average consumption, 2.5 mg/d) with the top fifth (average consumption, 25 mg/d). The bottom quintile had 126 myocardial infarctions (MIs) over 324,793 patient-years compared with 59 MIs over 332,143 patient-years. Some quick math shows an approximate reduction from 39 to 18 MIs per 100,000 patient-years. Or to put it another way, you must get 4762 women to increase their berry consumption 10-fold for 1 year to prevent one heart attack. Feel free to show people how you calculated this number. They will be impressed by your head for numbers. It is nothing more than 39 minus 18, divided by 100,000, to get the absolute risk reduction. Take the reciprocal of this (ie, 1 divided by this number) to get the number needed to treat.
Describing risks in absolute terms or using number needed to treat (or harm) can help conceptualize statistics that are sometimes hard to wrap your head around.
4. Dessert — Funding
By the time the coffee is served, everyone will be hanging on to your every word. This is as it should be, and you should not be afraid of your newfound power and influence.
Dessert will probably involve some form of chocolate, possibly in cake format. (Anyone who serves fruit as dessert is not someone you should associate with.) Take the opportunity to tell your follow diners that chocolate is not actually good for you and will not boost brain performance.
The health benefits of chocolate are often repeated but rarely scrutinized. In fact, much of the scientific research purporting to show that chocolate is good for you did not actually study chocolate. It usually involved a cocoa bean extract because the chocolate manufacturing process destroys the supposedly health-promoting antioxidants in the cocoa bean. It is true that dark chocolate has more antioxidants than milk chocolate, and that the addition of milk to chocolate further inactivates the potentially healthy antioxidants. But the amount of sugar and fat that has to be added to chocolate to make it palatable precludes any serious consideration about health benefits. Dark chocolate may have less fat and sugar than milk chocolate, but it still has a lot.
But even the cocoa bean extract doesn’t seem to do much for your heart or your brain. The long-awaited COSMOS study was published with surprisingly little fanfare. The largest randomized controlled trial of chocolate (or rather cocoa bean extract) was supposed to settle the issue definitively.
COSMOS showed no cardiovascular or neurocognitive benefit to the cocoa bean extract. But the health halo of chocolate continues to be bolstered by many studies funded by chocolate manufacturers.
We are appropriately critical of the pharmaceutical industry’s involvement in drug research. However, we should not forget that any private entity is prone to the same self-interest regardless of its product’s tastiness. How many of you knew that there was an avocado lobby funding research? No matter how many industry-funded observational studies using surrogate endpoints are out there telling you that chocolate is healthy, a randomized trial with hard clinical endpoints such as COSMOS should generally win the day.
The Final Goodbyes — Summarizing Your Case
As the party slowly winds down and everyone is saddened that you will soon take your leave, synthesize everything you have taught them over the evening. Like movies, not all studies are good. Some are just bad. They can be prone to reverse causation or confounding, and they may report relative risks when absolute risks would be more telling. Reading research studies critically is essential for separating the wheat from the chaff. With the knowledge you have now imparted to your friends, they will be much better consumers of medical news, especially when it comes to food.
And they will no doubt thank you for it by never inviting you to another dinner party!
Labos, a cardiologist at Hôpital, Notre-Dame, Montreal, Quebec, Canada, has disclosed no relevant financial relationships. He has a degree in epidemiology.
A version of this article appeared on Medscape.com.
Can We Repurpose Obesity Drugs to Reverse Liver Disease?
Metabolic dysfunction–associated steatotic liver disease (MASLD) has become the most common liver disease worldwide, with a global prevalence of 32.4%. Its growth over the past three decades has occurred in tandem with increasing rates of obesity and type 2 diabetes — two cornerstones of MASLD.
Higher rates of MASLD and metabolic dysfunction–associated steatohepatitis (MASH) with fibrosis are present in adults with obesity and diabetes, noted Arun Sanyal, MD, professor and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia.
The success surrounding the medications for obesity and type 2 diabetes, including glucagon-like peptide 1 receptor agonists (GLP-1 RAs), has sparked studies investigating whether they could also be an effective treatment for liver disease.
In particular, GLP-1 RAs help patients lose weight and/or control diabetes by mimicking the function of the gut hormone GLP-1, released in response to nutrient intake, and are able to increase insulin secretion and reduce glucagon secretion, delay gastric emptying, and reduce appetite and caloric intake.
The studies for MASLD are testing whether these functions will also work against liver disease, either directly or indirectly, through obesity and diabetes control. The early results are promising.
More Than One Risk Factor in Play
MASLD is defined by the presence of hepatic steatosis and at least one of five cardiometabolic risk factors: Overweight/obesity, hypertension, hyperglycemia, dyslipidemia with either low-plasma high-density lipoprotein cholesterol or high triglycerides, or treatment for these conditions.
It is a grim trajectory if the disease progresses to MASH, as the patient may accumulate hepatic fibrosis and go on to develop cirrhosis and/or hepatocellular carcinoma.
Typically, more than one risk factor is at play in MASLD, noted Adnan Said, MD, chief of the Division of Gastroenterology and Hepatology at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
“It most commonly occurs in the setting of weight gain and obesity, which are epidemics in the United States and worldwide, as well as the associated condition — metabolic syndrome — which goes along with obesity and includes type 2 diabetes, hyperlipidemia, hypertension, and sleep apnea,” Said, a hepatology and gastroenterology professor at the University of Wisconsin–Madison, told this news organization.
The research surrounding MASLD is investigating GLP-1 RAs as single agents and in combination with other drugs.
Finding treatment is critical, as there is only one drug — resmetirom — approved for the treatment of MASH with moderate to advanced fibrosis. But because it’s not approved for earlier stages, a treatment gap exists. The drug also doesn’t produce weight loss, which is key to treating MASLD. And while GLP-1 RAs help patients with the weight loss that is critical to MASLD, they are only approved by the US Food and Drug Administration (FDA) for obesity and type 2 diabetes.
Single Agents
The GLP-1 RAs liraglutide and semaglutide, both approved for diabetes and weight loss, are being studied as single agents against liver disease, Said said.
“Their action in the setting of MASLD and MASH is primarily indirect, through systemic pathways, improving these conditions via weight loss, as well as by improving insulin sensitivity and reducing lipotoxicity,” he added.
One of the first trials of these agents for liver disease was in 2016. In that double-blind, randomized, 48-week clinical trial of liraglutide in patients with MASH and overweight, 39% of patients who received liraglutide had a resolution of MASH compared with only 9% of those who received placebo. Moreover, only 9% vs 36% of patients in the treatment vs placebo group had progression of fibrosis.
Since then, a 72-week phase 2 trial in patients with MASH, liver fibrosis (stages F1-F3), and overweight or obesity found that once-daily subcutaneous semaglutide (0.1, 0.2, or 0.4 mg) outperformed placebo on MASH resolution without worsening of fibrosis (36%-59% vs 17%) and on weight loss (5%-13% vs 1%), with the greatest benefits at the largest dose. However, neoplasms were reported in 15% of patients receiving semaglutide vs 8% of those receiving placebo.
A phase 1 trial involving patients with liver stiffness, steatosis, and overweight or obesity found significantly greater reductions in liver fat at 48 weeks with semaglutide vs placebo, as well as decreases in liver enzymes, body weight, and A1c. There was no significant difference in liver stiffness.
Furthermore, a meta-analysis of eight studies found that treatment with 24 weeks of semaglutide significantly improved liver enzymes, reduced liver stiffness, and improved metabolic parameters in patients with MASLD/MASH. The authors cautioned that gastrointestinal adverse effects “could be a major concern.”
Several studies have found other GLP-1 RAs, including exenatide and dulaglutide, have a beneficial impact on liver injury indices and liver steatosis.
A new retrospective observational study offers evidence that GLP-1 RAs may have a direct impact on MASLD, independent of weight loss. Among the 28% of patients with type 2 diabetes and MASLD who received a GLP-1 RA, there was a significant reduction not only in body mass index but also in A1c, liver enzymes, and controlled attenuation parameter scores. A beneficial impact on liver parameters was observed even in patients who didn’t lose weight. While there was no difference in liver stiffness measurement, the median 60-month follow-up time may not have been long enough to capture such changes.
Another study indicated that the apparent benefits of GLP-1 RAs, in this case semaglutide, may not extend to patients whose disease has progressed to cirrhosis.
Dual and Triple Mechanisms of Action
Newer agents with double or triple mechanisms of action appear to have a more direct effect on the liver.
“Dual agents may have an added effect by improving MASLD directly through adipose regulation and thermogenesis, thereby improving fibrosis,” Said said.
An example is tirzepatide, a GLP-1 RA and an agonist of glucose-dependent insulinotropic polypeptide (GIP). Like GLP-1, GIP is an incretin. When used together as co-agonists, GLP-1 and GIP have been shown to increase insulin and glucagonostatic response and may work synergistically.
A new phase 2 trial that randomly assigned patients with biopsy-confirmed MASH and moderate or severe fibrosis to receive either once-weekly subcutaneous tirzepatide at one of three doses (5, 10, or 15 mg) or placebo found that tirzepatide at each dosage outperformed placebo in resolution of MASH without worsening of fibrosis.
“These findings were encouraging,” Said said. “We’ll see if the results continue into phase 3 trials.”
The combination of GLP-1 RAs with glucagon (GCG) receptor agonists also has garnered interest.
In a phase 2 trial, adults with biopsy-confirmed MASH and fibrosis stages F1-F3 were randomly assigned to receive either one of three doses of the GLP-1/GCG RA survodutide (2.4, 4.8, or 6 mg) or placebo. Survodutide at each dose was found to be superior to placebo in improving MASH without the worsening of fibrosis, reducing liver fat content by at least 30%, and decreasing liver fibrosis by at least one stage, with the 4.8-mg dose showing the best performance for each measure. However, adverse events, including nausea, diarrhea, and vomiting, were more frequent with survodutide than with placebo.
Trials of triple-action agents (GLP-1/GIP/GCG RAs) are underway too.
The hope is the triple agonists could deliver greater reduction in hepatic fat in patients with MASLD, Sanyal said.
Sanyal further noted that a reduction in liver fat is important, citing a meta-analysis that showed ≥ 30% relative decline in liver fat is associated with higher odds of histologic response and MASH resolution.
Sanyal pointed to efocipegtrutide (HM15211), a GLP-1/GIP/GCG RA, which demonstrated significant liver fat reduction after 12 weeks in patients with MASLD in a phase 1b/2a randomized, placebo-controlled trial and is now in phase 2 development.
Another example is retatrutide (LY3437943), a once-weekly injectable, that was associated with up to a 24.2% reduction in body weight at 48 weeks, compared with 2.1% with placebo, in a phase 2 trial involving patients with obesity.
A sub-study assessed the mean relative change from baseline in liver fat at 24 weeks. These participants, who also had MASLD and ≥ 10% of liver fat content, were randomly assigned to receive either retatrutide in one of four doses (1, 4, 8, or 12 mg) or placebo for 48 weeks. All doses of retatrutide showed significantly greater reduction in liver fat content compared with placebo in weeks 24-48, with a mean relative liver fat reduction > 80% at the two higher doses. Moreover, ≥ 80% of participants on the higher retatrutide doses experienced ≥ 70% reduction in liver fat at 48 weeks, compared with 0% reduction in those on placebo, and hepatic steatosis resolved in > 85% of these participants.
This space “continues to evolve at a rapid rate,” Sanyal said. For example, oral dual-action agents are under development.
Obstacles and Warnings
Sanyal warned that GLP-1 RAs can cause nausea, so they have to be introduced at a low dose and slowly titrated upward. They should be used with caution in people with a history of multiple endocrine neoplasia. There is also a small but increased risk for gallstone formation and gallstone-induced pancreatitis with rapid weight loss.
GLP-1 RAs may increase the risk for suicidal ideation, with the authors of a recent study calling for “urgent clarification” regarding this possibility.
Following reports of suicidality submitted through its Adverse Events Reporting System, the FDA concluded that it could find no causal relationship between these agents and increased risk for suicidal ideation but also that it could not “definitively rule out that a small risk may exist” and would continue to investigate.
Access to GLP-1 RAs is an obstacle as well. Semaglutide continues to be on the FDA’s shortage list.
“This is improving, but there are still issues around getting approval from insurance companies,” Sanyal said.
Many patients discontinue use because of tolerability or access issues, which is problematic because most regain the weight they had lost while on the medication.
“Right now, we see GLP-1 RAs as a long-term therapeutic commitment, but there is a lot of research interest in figuring out if there’s a more modest benefit — almost an induction-remission maintenance approach to weight loss,” Sanyal said. These are “evolving trends,” and it’s unclear how they will unfold.
“As of now, you have to decide that if you’re putting your patient on these medications, they will have to take them on a long-term basis and include that consideration in your risk-benefit analysis, together with any concerns about adverse effects,” he said.
Sanyal reported consulting for Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Said received research support from Exact Sciences, Boehringer Ingelheim, and Mallinckrodt.
A version of this article first appeared on Medscape.com.
Metabolic dysfunction–associated steatotic liver disease (MASLD) has become the most common liver disease worldwide, with a global prevalence of 32.4%. Its growth over the past three decades has occurred in tandem with increasing rates of obesity and type 2 diabetes — two cornerstones of MASLD.
Higher rates of MASLD and metabolic dysfunction–associated steatohepatitis (MASH) with fibrosis are present in adults with obesity and diabetes, noted Arun Sanyal, MD, professor and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia.
The success surrounding the medications for obesity and type 2 diabetes, including glucagon-like peptide 1 receptor agonists (GLP-1 RAs), has sparked studies investigating whether they could also be an effective treatment for liver disease.
In particular, GLP-1 RAs help patients lose weight and/or control diabetes by mimicking the function of the gut hormone GLP-1, released in response to nutrient intake, and are able to increase insulin secretion and reduce glucagon secretion, delay gastric emptying, and reduce appetite and caloric intake.
The studies for MASLD are testing whether these functions will also work against liver disease, either directly or indirectly, through obesity and diabetes control. The early results are promising.
More Than One Risk Factor in Play
MASLD is defined by the presence of hepatic steatosis and at least one of five cardiometabolic risk factors: Overweight/obesity, hypertension, hyperglycemia, dyslipidemia with either low-plasma high-density lipoprotein cholesterol or high triglycerides, or treatment for these conditions.
It is a grim trajectory if the disease progresses to MASH, as the patient may accumulate hepatic fibrosis and go on to develop cirrhosis and/or hepatocellular carcinoma.
Typically, more than one risk factor is at play in MASLD, noted Adnan Said, MD, chief of the Division of Gastroenterology and Hepatology at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
“It most commonly occurs in the setting of weight gain and obesity, which are epidemics in the United States and worldwide, as well as the associated condition — metabolic syndrome — which goes along with obesity and includes type 2 diabetes, hyperlipidemia, hypertension, and sleep apnea,” Said, a hepatology and gastroenterology professor at the University of Wisconsin–Madison, told this news organization.
The research surrounding MASLD is investigating GLP-1 RAs as single agents and in combination with other drugs.
Finding treatment is critical, as there is only one drug — resmetirom — approved for the treatment of MASH with moderate to advanced fibrosis. But because it’s not approved for earlier stages, a treatment gap exists. The drug also doesn’t produce weight loss, which is key to treating MASLD. And while GLP-1 RAs help patients with the weight loss that is critical to MASLD, they are only approved by the US Food and Drug Administration (FDA) for obesity and type 2 diabetes.
Single Agents
The GLP-1 RAs liraglutide and semaglutide, both approved for diabetes and weight loss, are being studied as single agents against liver disease, Said said.
“Their action in the setting of MASLD and MASH is primarily indirect, through systemic pathways, improving these conditions via weight loss, as well as by improving insulin sensitivity and reducing lipotoxicity,” he added.
One of the first trials of these agents for liver disease was in 2016. In that double-blind, randomized, 48-week clinical trial of liraglutide in patients with MASH and overweight, 39% of patients who received liraglutide had a resolution of MASH compared with only 9% of those who received placebo. Moreover, only 9% vs 36% of patients in the treatment vs placebo group had progression of fibrosis.
Since then, a 72-week phase 2 trial in patients with MASH, liver fibrosis (stages F1-F3), and overweight or obesity found that once-daily subcutaneous semaglutide (0.1, 0.2, or 0.4 mg) outperformed placebo on MASH resolution without worsening of fibrosis (36%-59% vs 17%) and on weight loss (5%-13% vs 1%), with the greatest benefits at the largest dose. However, neoplasms were reported in 15% of patients receiving semaglutide vs 8% of those receiving placebo.
A phase 1 trial involving patients with liver stiffness, steatosis, and overweight or obesity found significantly greater reductions in liver fat at 48 weeks with semaglutide vs placebo, as well as decreases in liver enzymes, body weight, and A1c. There was no significant difference in liver stiffness.
Furthermore, a meta-analysis of eight studies found that treatment with 24 weeks of semaglutide significantly improved liver enzymes, reduced liver stiffness, and improved metabolic parameters in patients with MASLD/MASH. The authors cautioned that gastrointestinal adverse effects “could be a major concern.”
Several studies have found other GLP-1 RAs, including exenatide and dulaglutide, have a beneficial impact on liver injury indices and liver steatosis.
A new retrospective observational study offers evidence that GLP-1 RAs may have a direct impact on MASLD, independent of weight loss. Among the 28% of patients with type 2 diabetes and MASLD who received a GLP-1 RA, there was a significant reduction not only in body mass index but also in A1c, liver enzymes, and controlled attenuation parameter scores. A beneficial impact on liver parameters was observed even in patients who didn’t lose weight. While there was no difference in liver stiffness measurement, the median 60-month follow-up time may not have been long enough to capture such changes.
Another study indicated that the apparent benefits of GLP-1 RAs, in this case semaglutide, may not extend to patients whose disease has progressed to cirrhosis.
Dual and Triple Mechanisms of Action
Newer agents with double or triple mechanisms of action appear to have a more direct effect on the liver.
“Dual agents may have an added effect by improving MASLD directly through adipose regulation and thermogenesis, thereby improving fibrosis,” Said said.
An example is tirzepatide, a GLP-1 RA and an agonist of glucose-dependent insulinotropic polypeptide (GIP). Like GLP-1, GIP is an incretin. When used together as co-agonists, GLP-1 and GIP have been shown to increase insulin and glucagonostatic response and may work synergistically.
A new phase 2 trial that randomly assigned patients with biopsy-confirmed MASH and moderate or severe fibrosis to receive either once-weekly subcutaneous tirzepatide at one of three doses (5, 10, or 15 mg) or placebo found that tirzepatide at each dosage outperformed placebo in resolution of MASH without worsening of fibrosis.
“These findings were encouraging,” Said said. “We’ll see if the results continue into phase 3 trials.”
The combination of GLP-1 RAs with glucagon (GCG) receptor agonists also has garnered interest.
In a phase 2 trial, adults with biopsy-confirmed MASH and fibrosis stages F1-F3 were randomly assigned to receive either one of three doses of the GLP-1/GCG RA survodutide (2.4, 4.8, or 6 mg) or placebo. Survodutide at each dose was found to be superior to placebo in improving MASH without the worsening of fibrosis, reducing liver fat content by at least 30%, and decreasing liver fibrosis by at least one stage, with the 4.8-mg dose showing the best performance for each measure. However, adverse events, including nausea, diarrhea, and vomiting, were more frequent with survodutide than with placebo.
Trials of triple-action agents (GLP-1/GIP/GCG RAs) are underway too.
The hope is the triple agonists could deliver greater reduction in hepatic fat in patients with MASLD, Sanyal said.
Sanyal further noted that a reduction in liver fat is important, citing a meta-analysis that showed ≥ 30% relative decline in liver fat is associated with higher odds of histologic response and MASH resolution.
Sanyal pointed to efocipegtrutide (HM15211), a GLP-1/GIP/GCG RA, which demonstrated significant liver fat reduction after 12 weeks in patients with MASLD in a phase 1b/2a randomized, placebo-controlled trial and is now in phase 2 development.
Another example is retatrutide (LY3437943), a once-weekly injectable, that was associated with up to a 24.2% reduction in body weight at 48 weeks, compared with 2.1% with placebo, in a phase 2 trial involving patients with obesity.
A sub-study assessed the mean relative change from baseline in liver fat at 24 weeks. These participants, who also had MASLD and ≥ 10% of liver fat content, were randomly assigned to receive either retatrutide in one of four doses (1, 4, 8, or 12 mg) or placebo for 48 weeks. All doses of retatrutide showed significantly greater reduction in liver fat content compared with placebo in weeks 24-48, with a mean relative liver fat reduction > 80% at the two higher doses. Moreover, ≥ 80% of participants on the higher retatrutide doses experienced ≥ 70% reduction in liver fat at 48 weeks, compared with 0% reduction in those on placebo, and hepatic steatosis resolved in > 85% of these participants.
This space “continues to evolve at a rapid rate,” Sanyal said. For example, oral dual-action agents are under development.
Obstacles and Warnings
Sanyal warned that GLP-1 RAs can cause nausea, so they have to be introduced at a low dose and slowly titrated upward. They should be used with caution in people with a history of multiple endocrine neoplasia. There is also a small but increased risk for gallstone formation and gallstone-induced pancreatitis with rapid weight loss.
GLP-1 RAs may increase the risk for suicidal ideation, with the authors of a recent study calling for “urgent clarification” regarding this possibility.
Following reports of suicidality submitted through its Adverse Events Reporting System, the FDA concluded that it could find no causal relationship between these agents and increased risk for suicidal ideation but also that it could not “definitively rule out that a small risk may exist” and would continue to investigate.
Access to GLP-1 RAs is an obstacle as well. Semaglutide continues to be on the FDA’s shortage list.
“This is improving, but there are still issues around getting approval from insurance companies,” Sanyal said.
Many patients discontinue use because of tolerability or access issues, which is problematic because most regain the weight they had lost while on the medication.
“Right now, we see GLP-1 RAs as a long-term therapeutic commitment, but there is a lot of research interest in figuring out if there’s a more modest benefit — almost an induction-remission maintenance approach to weight loss,” Sanyal said. These are “evolving trends,” and it’s unclear how they will unfold.
“As of now, you have to decide that if you’re putting your patient on these medications, they will have to take them on a long-term basis and include that consideration in your risk-benefit analysis, together with any concerns about adverse effects,” he said.
Sanyal reported consulting for Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Said received research support from Exact Sciences, Boehringer Ingelheim, and Mallinckrodt.
A version of this article first appeared on Medscape.com.
Metabolic dysfunction–associated steatotic liver disease (MASLD) has become the most common liver disease worldwide, with a global prevalence of 32.4%. Its growth over the past three decades has occurred in tandem with increasing rates of obesity and type 2 diabetes — two cornerstones of MASLD.
Higher rates of MASLD and metabolic dysfunction–associated steatohepatitis (MASH) with fibrosis are present in adults with obesity and diabetes, noted Arun Sanyal, MD, professor and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health, Virginia Commonwealth University, Richmond, Virginia.
The success surrounding the medications for obesity and type 2 diabetes, including glucagon-like peptide 1 receptor agonists (GLP-1 RAs), has sparked studies investigating whether they could also be an effective treatment for liver disease.
In particular, GLP-1 RAs help patients lose weight and/or control diabetes by mimicking the function of the gut hormone GLP-1, released in response to nutrient intake, and are able to increase insulin secretion and reduce glucagon secretion, delay gastric emptying, and reduce appetite and caloric intake.
The studies for MASLD are testing whether these functions will also work against liver disease, either directly or indirectly, through obesity and diabetes control. The early results are promising.
More Than One Risk Factor in Play
MASLD is defined by the presence of hepatic steatosis and at least one of five cardiometabolic risk factors: Overweight/obesity, hypertension, hyperglycemia, dyslipidemia with either low-plasma high-density lipoprotein cholesterol or high triglycerides, or treatment for these conditions.
It is a grim trajectory if the disease progresses to MASH, as the patient may accumulate hepatic fibrosis and go on to develop cirrhosis and/or hepatocellular carcinoma.
Typically, more than one risk factor is at play in MASLD, noted Adnan Said, MD, chief of the Division of Gastroenterology and Hepatology at the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
“It most commonly occurs in the setting of weight gain and obesity, which are epidemics in the United States and worldwide, as well as the associated condition — metabolic syndrome — which goes along with obesity and includes type 2 diabetes, hyperlipidemia, hypertension, and sleep apnea,” Said, a hepatology and gastroenterology professor at the University of Wisconsin–Madison, told this news organization.
The research surrounding MASLD is investigating GLP-1 RAs as single agents and in combination with other drugs.
Finding treatment is critical, as there is only one drug — resmetirom — approved for the treatment of MASH with moderate to advanced fibrosis. But because it’s not approved for earlier stages, a treatment gap exists. The drug also doesn’t produce weight loss, which is key to treating MASLD. And while GLP-1 RAs help patients with the weight loss that is critical to MASLD, they are only approved by the US Food and Drug Administration (FDA) for obesity and type 2 diabetes.
Single Agents
The GLP-1 RAs liraglutide and semaglutide, both approved for diabetes and weight loss, are being studied as single agents against liver disease, Said said.
“Their action in the setting of MASLD and MASH is primarily indirect, through systemic pathways, improving these conditions via weight loss, as well as by improving insulin sensitivity and reducing lipotoxicity,” he added.
One of the first trials of these agents for liver disease was in 2016. In that double-blind, randomized, 48-week clinical trial of liraglutide in patients with MASH and overweight, 39% of patients who received liraglutide had a resolution of MASH compared with only 9% of those who received placebo. Moreover, only 9% vs 36% of patients in the treatment vs placebo group had progression of fibrosis.
Since then, a 72-week phase 2 trial in patients with MASH, liver fibrosis (stages F1-F3), and overweight or obesity found that once-daily subcutaneous semaglutide (0.1, 0.2, or 0.4 mg) outperformed placebo on MASH resolution without worsening of fibrosis (36%-59% vs 17%) and on weight loss (5%-13% vs 1%), with the greatest benefits at the largest dose. However, neoplasms were reported in 15% of patients receiving semaglutide vs 8% of those receiving placebo.
A phase 1 trial involving patients with liver stiffness, steatosis, and overweight or obesity found significantly greater reductions in liver fat at 48 weeks with semaglutide vs placebo, as well as decreases in liver enzymes, body weight, and A1c. There was no significant difference in liver stiffness.
Furthermore, a meta-analysis of eight studies found that treatment with 24 weeks of semaglutide significantly improved liver enzymes, reduced liver stiffness, and improved metabolic parameters in patients with MASLD/MASH. The authors cautioned that gastrointestinal adverse effects “could be a major concern.”
Several studies have found other GLP-1 RAs, including exenatide and dulaglutide, have a beneficial impact on liver injury indices and liver steatosis.
A new retrospective observational study offers evidence that GLP-1 RAs may have a direct impact on MASLD, independent of weight loss. Among the 28% of patients with type 2 diabetes and MASLD who received a GLP-1 RA, there was a significant reduction not only in body mass index but also in A1c, liver enzymes, and controlled attenuation parameter scores. A beneficial impact on liver parameters was observed even in patients who didn’t lose weight. While there was no difference in liver stiffness measurement, the median 60-month follow-up time may not have been long enough to capture such changes.
Another study indicated that the apparent benefits of GLP-1 RAs, in this case semaglutide, may not extend to patients whose disease has progressed to cirrhosis.
Dual and Triple Mechanisms of Action
Newer agents with double or triple mechanisms of action appear to have a more direct effect on the liver.
“Dual agents may have an added effect by improving MASLD directly through adipose regulation and thermogenesis, thereby improving fibrosis,” Said said.
An example is tirzepatide, a GLP-1 RA and an agonist of glucose-dependent insulinotropic polypeptide (GIP). Like GLP-1, GIP is an incretin. When used together as co-agonists, GLP-1 and GIP have been shown to increase insulin and glucagonostatic response and may work synergistically.
A new phase 2 trial that randomly assigned patients with biopsy-confirmed MASH and moderate or severe fibrosis to receive either once-weekly subcutaneous tirzepatide at one of three doses (5, 10, or 15 mg) or placebo found that tirzepatide at each dosage outperformed placebo in resolution of MASH without worsening of fibrosis.
“These findings were encouraging,” Said said. “We’ll see if the results continue into phase 3 trials.”
The combination of GLP-1 RAs with glucagon (GCG) receptor agonists also has garnered interest.
In a phase 2 trial, adults with biopsy-confirmed MASH and fibrosis stages F1-F3 were randomly assigned to receive either one of three doses of the GLP-1/GCG RA survodutide (2.4, 4.8, or 6 mg) or placebo. Survodutide at each dose was found to be superior to placebo in improving MASH without the worsening of fibrosis, reducing liver fat content by at least 30%, and decreasing liver fibrosis by at least one stage, with the 4.8-mg dose showing the best performance for each measure. However, adverse events, including nausea, diarrhea, and vomiting, were more frequent with survodutide than with placebo.
Trials of triple-action agents (GLP-1/GIP/GCG RAs) are underway too.
The hope is the triple agonists could deliver greater reduction in hepatic fat in patients with MASLD, Sanyal said.
Sanyal further noted that a reduction in liver fat is important, citing a meta-analysis that showed ≥ 30% relative decline in liver fat is associated with higher odds of histologic response and MASH resolution.
Sanyal pointed to efocipegtrutide (HM15211), a GLP-1/GIP/GCG RA, which demonstrated significant liver fat reduction after 12 weeks in patients with MASLD in a phase 1b/2a randomized, placebo-controlled trial and is now in phase 2 development.
Another example is retatrutide (LY3437943), a once-weekly injectable, that was associated with up to a 24.2% reduction in body weight at 48 weeks, compared with 2.1% with placebo, in a phase 2 trial involving patients with obesity.
A sub-study assessed the mean relative change from baseline in liver fat at 24 weeks. These participants, who also had MASLD and ≥ 10% of liver fat content, were randomly assigned to receive either retatrutide in one of four doses (1, 4, 8, or 12 mg) or placebo for 48 weeks. All doses of retatrutide showed significantly greater reduction in liver fat content compared with placebo in weeks 24-48, with a mean relative liver fat reduction > 80% at the two higher doses. Moreover, ≥ 80% of participants on the higher retatrutide doses experienced ≥ 70% reduction in liver fat at 48 weeks, compared with 0% reduction in those on placebo, and hepatic steatosis resolved in > 85% of these participants.
This space “continues to evolve at a rapid rate,” Sanyal said. For example, oral dual-action agents are under development.
Obstacles and Warnings
Sanyal warned that GLP-1 RAs can cause nausea, so they have to be introduced at a low dose and slowly titrated upward. They should be used with caution in people with a history of multiple endocrine neoplasia. There is also a small but increased risk for gallstone formation and gallstone-induced pancreatitis with rapid weight loss.
GLP-1 RAs may increase the risk for suicidal ideation, with the authors of a recent study calling for “urgent clarification” regarding this possibility.
Following reports of suicidality submitted through its Adverse Events Reporting System, the FDA concluded that it could find no causal relationship between these agents and increased risk for suicidal ideation but also that it could not “definitively rule out that a small risk may exist” and would continue to investigate.
Access to GLP-1 RAs is an obstacle as well. Semaglutide continues to be on the FDA’s shortage list.
“This is improving, but there are still issues around getting approval from insurance companies,” Sanyal said.
Many patients discontinue use because of tolerability or access issues, which is problematic because most regain the weight they had lost while on the medication.
“Right now, we see GLP-1 RAs as a long-term therapeutic commitment, but there is a lot of research interest in figuring out if there’s a more modest benefit — almost an induction-remission maintenance approach to weight loss,” Sanyal said. These are “evolving trends,” and it’s unclear how they will unfold.
“As of now, you have to decide that if you’re putting your patient on these medications, they will have to take them on a long-term basis and include that consideration in your risk-benefit analysis, together with any concerns about adverse effects,” he said.
Sanyal reported consulting for Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. Said received research support from Exact Sciences, Boehringer Ingelheim, and Mallinckrodt.
A version of this article first appeared on Medscape.com.
State of Confusion: Should All Children Get Lipid Labs for High Cholesterol?
Clinicians receive conflicting advice on whether to order blood tests to screen for lipids in children. A new study could add to the confusion. Researchers found that a combination of physical proxy measures such as hypertension and body mass index (BMI) predicted the risk for future cardiovascular events as well as the physical model plus lipid labs, questioning the value of those blood tests.
Some medical organizations advise screening only for high-risk children because more research is needed to define the harms and benefits of universal screening. Diet and behavioral changes are sufficient for most children, and universal screening could lead to false positives and unnecessary further testing, they said.
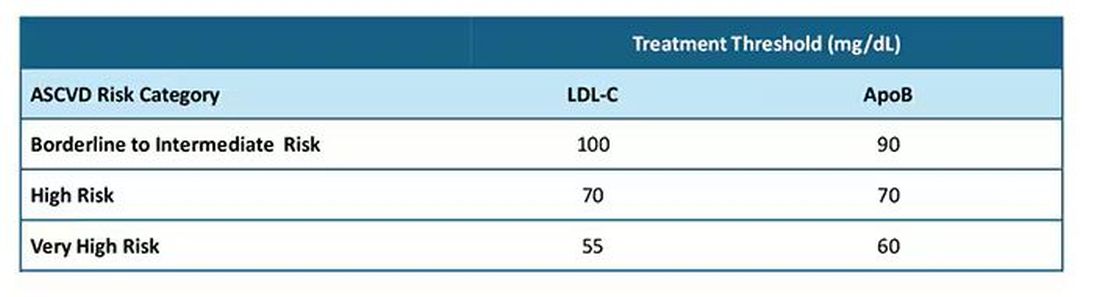
Groups that favor lipid tests for all children say these measurements detect familial hypercholesterolemia (FH) that would not otherwise be diagnosed, leading to treatment with drugs like statins and a greater chance of preventing cardiovascular disease (CVD) in adulthood.
Researchers from the new study said their findings do not address screenings for FH, which affects 1 in 250 US children and puts them at a risk for atherosclerotic CVD.
Recommending Blood Tests in Age Groups
One of the seminal guidelines on screening lipids in children came from the National Heart, Lung, and Blood Institute (NHLBI), which in 2011 recommended children undergo dyslipidemia screening between the ages of 9 and 11 years and again between 17 and 21 years. Children should receive a screening starting at age 2 years if they have a family history of CVD or dyslipidemia or have diabetes, an elevated BMI, or hypertension. The American Academy of Pediatrics shortly followed suit, issuing similar recommendations.
Screening for the two subsets of ages was an expansion from the original 1992 guidelines from the National Cholesterol Education Program, which recommended screening only for children with either a family history of early CVD or elevated total cholesterol levels.
A 2011 panel for the NHLBI said the older approach identified significantly fewer children with abnormal levels of low-density lipoprotein cholesterol (LDL-C) than the addition of two age groups for screening, adding that many children do not have a complete family history. The American College of Cardiology and American Heart Association later supported NHLBI’s stance in their joint guidelines on the management of cholesterol.
Mark Corkins, MD, chair of the AAP’s Committee on Nutrition, told Medscape Medical News that if children are screened only because they have obesity or a family history of FH, some with elevated lipid levels will be missed. For instance, studies indicate caregiver recall of FH often is inaccurate, and the genetic disorder that causes the condition is not related to obesity.
“The screening is to find familial hypercholesterolemia, to try to find the ones that need therapy,” that would not be caught by the risk-based screening earlier on in childhood, Corkins said.
Only Screen Children With Risk Factors
But other groups do not agree. The US Preventive Services Task Force (USPSTF) found insufficient evidence to recommend for or against screening for lipid disorders in asymptomatic children and teens.
The group also said it found inadequate evidence that lipid-lowering interventions in the general pediatric population lead to reductions in cardiovascular events or all-cause mortality once they reached adulthood. USPSTF also raised questions about the safety of lipid-lowering drugs in children.
“The current evidence is insufficient to assess the balance of benefits and harms of screening for lipid disorders in children and adolescents 20 years or younger,” the panel wrote.
The American Academy of Family Physicians supports USPSTF’s recommendations.
Low Rate of Screening
While the uncertainty over screening in children continues, the practice has been adopted by a minority of clinicians.
A study published in JAMA Network Open in July found 9% of 700,000 9- to 11-year-olds had a documented result from a lipid screening. Among more than 1.3 million 17- to 21-year-olds, 13% had received a screening.
As BMI went up, so did screening rates. A little over 9% children and teens with a healthy weight were screened compared with 14.7% of those with moderate obesity and 21.9% of those with severe obesity.
Among those screened, 32.3% of 9- to 11-year-olds and 30.2% of 17- to 21-year-olds had abnormal lipid levels, defined as having one elevated measure out of five, including total cholesterol of 200 mg/dL or higher or LDL-C levels of 130 mg/dL or higher.
Justin Zachariah, MD, MPH, an associate professor of pediatrics-cardiology at Baylor College of Medicine in Houston, spoke about physicians screening children based only on factors like obesity during a presentation at the recent annual meeting of the American Academy of Pediatrics. He cited research showing roughly one in four children with abnormal lipids had a normal weight.
If a clinician is reserving a lipid screening for a child who is overweight or has obesity, “you’re missing nearly half the problem,” Zachariah said during his presentation.
One reason for the low rate of universal screening may be inattention to FH by clinicians, according to Samuel S. Gidding, MD, a professor in the Department of Genomic Health at Geisinger College of Health Sciences in Bridgewater Corners, Vermont.
For instance, a clinician has only a set amount of time during a well-child visit and other issues may take precedence, “so it doesn’t make sense to broach preventive screening for something that could happen 30 or 40 years from now, vs this [other] very immediate problem,” he said.
Clinicians “are triggered to act on the LDL level, but don’t think about FH as a possible diagnosis,” Gidding told Medscape Medical News.
Another barrier is that in some settings, caregivers must take children and teens to another facility on a different day to fulfill an order for a lipid test.
“It’s reluctance of doctors to order it, knowing patients won’t go through with it,” Gidding said.
Gidding is a consultant for Esperion Therapeutics. Other sources in this story reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Clinicians receive conflicting advice on whether to order blood tests to screen for lipids in children. A new study could add to the confusion. Researchers found that a combination of physical proxy measures such as hypertension and body mass index (BMI) predicted the risk for future cardiovascular events as well as the physical model plus lipid labs, questioning the value of those blood tests.
Some medical organizations advise screening only for high-risk children because more research is needed to define the harms and benefits of universal screening. Diet and behavioral changes are sufficient for most children, and universal screening could lead to false positives and unnecessary further testing, they said.
Groups that favor lipid tests for all children say these measurements detect familial hypercholesterolemia (FH) that would not otherwise be diagnosed, leading to treatment with drugs like statins and a greater chance of preventing cardiovascular disease (CVD) in adulthood.
Researchers from the new study said their findings do not address screenings for FH, which affects 1 in 250 US children and puts them at a risk for atherosclerotic CVD.
Recommending Blood Tests in Age Groups
One of the seminal guidelines on screening lipids in children came from the National Heart, Lung, and Blood Institute (NHLBI), which in 2011 recommended children undergo dyslipidemia screening between the ages of 9 and 11 years and again between 17 and 21 years. Children should receive a screening starting at age 2 years if they have a family history of CVD or dyslipidemia or have diabetes, an elevated BMI, or hypertension. The American Academy of Pediatrics shortly followed suit, issuing similar recommendations.
Screening for the two subsets of ages was an expansion from the original 1992 guidelines from the National Cholesterol Education Program, which recommended screening only for children with either a family history of early CVD or elevated total cholesterol levels.
A 2011 panel for the NHLBI said the older approach identified significantly fewer children with abnormal levels of low-density lipoprotein cholesterol (LDL-C) than the addition of two age groups for screening, adding that many children do not have a complete family history. The American College of Cardiology and American Heart Association later supported NHLBI’s stance in their joint guidelines on the management of cholesterol.
Mark Corkins, MD, chair of the AAP’s Committee on Nutrition, told Medscape Medical News that if children are screened only because they have obesity or a family history of FH, some with elevated lipid levels will be missed. For instance, studies indicate caregiver recall of FH often is inaccurate, and the genetic disorder that causes the condition is not related to obesity.
“The screening is to find familial hypercholesterolemia, to try to find the ones that need therapy,” that would not be caught by the risk-based screening earlier on in childhood, Corkins said.
Only Screen Children With Risk Factors
But other groups do not agree. The US Preventive Services Task Force (USPSTF) found insufficient evidence to recommend for or against screening for lipid disorders in asymptomatic children and teens.
The group also said it found inadequate evidence that lipid-lowering interventions in the general pediatric population lead to reductions in cardiovascular events or all-cause mortality once they reached adulthood. USPSTF also raised questions about the safety of lipid-lowering drugs in children.
“The current evidence is insufficient to assess the balance of benefits and harms of screening for lipid disorders in children and adolescents 20 years or younger,” the panel wrote.
The American Academy of Family Physicians supports USPSTF’s recommendations.
Low Rate of Screening
While the uncertainty over screening in children continues, the practice has been adopted by a minority of clinicians.
A study published in JAMA Network Open in July found 9% of 700,000 9- to 11-year-olds had a documented result from a lipid screening. Among more than 1.3 million 17- to 21-year-olds, 13% had received a screening.
As BMI went up, so did screening rates. A little over 9% children and teens with a healthy weight were screened compared with 14.7% of those with moderate obesity and 21.9% of those with severe obesity.
Among those screened, 32.3% of 9- to 11-year-olds and 30.2% of 17- to 21-year-olds had abnormal lipid levels, defined as having one elevated measure out of five, including total cholesterol of 200 mg/dL or higher or LDL-C levels of 130 mg/dL or higher.
Justin Zachariah, MD, MPH, an associate professor of pediatrics-cardiology at Baylor College of Medicine in Houston, spoke about physicians screening children based only on factors like obesity during a presentation at the recent annual meeting of the American Academy of Pediatrics. He cited research showing roughly one in four children with abnormal lipids had a normal weight.
If a clinician is reserving a lipid screening for a child who is overweight or has obesity, “you’re missing nearly half the problem,” Zachariah said during his presentation.
One reason for the low rate of universal screening may be inattention to FH by clinicians, according to Samuel S. Gidding, MD, a professor in the Department of Genomic Health at Geisinger College of Health Sciences in Bridgewater Corners, Vermont.
For instance, a clinician has only a set amount of time during a well-child visit and other issues may take precedence, “so it doesn’t make sense to broach preventive screening for something that could happen 30 or 40 years from now, vs this [other] very immediate problem,” he said.
Clinicians “are triggered to act on the LDL level, but don’t think about FH as a possible diagnosis,” Gidding told Medscape Medical News.
Another barrier is that in some settings, caregivers must take children and teens to another facility on a different day to fulfill an order for a lipid test.
“It’s reluctance of doctors to order it, knowing patients won’t go through with it,” Gidding said.
Gidding is a consultant for Esperion Therapeutics. Other sources in this story reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Clinicians receive conflicting advice on whether to order blood tests to screen for lipids in children. A new study could add to the confusion. Researchers found that a combination of physical proxy measures such as hypertension and body mass index (BMI) predicted the risk for future cardiovascular events as well as the physical model plus lipid labs, questioning the value of those blood tests.
Some medical organizations advise screening only for high-risk children because more research is needed to define the harms and benefits of universal screening. Diet and behavioral changes are sufficient for most children, and universal screening could lead to false positives and unnecessary further testing, they said.
Groups that favor lipid tests for all children say these measurements detect familial hypercholesterolemia (FH) that would not otherwise be diagnosed, leading to treatment with drugs like statins and a greater chance of preventing cardiovascular disease (CVD) in adulthood.
Researchers from the new study said their findings do not address screenings for FH, which affects 1 in 250 US children and puts them at a risk for atherosclerotic CVD.
Recommending Blood Tests in Age Groups
One of the seminal guidelines on screening lipids in children came from the National Heart, Lung, and Blood Institute (NHLBI), which in 2011 recommended children undergo dyslipidemia screening between the ages of 9 and 11 years and again between 17 and 21 years. Children should receive a screening starting at age 2 years if they have a family history of CVD or dyslipidemia or have diabetes, an elevated BMI, or hypertension. The American Academy of Pediatrics shortly followed suit, issuing similar recommendations.
Screening for the two subsets of ages was an expansion from the original 1992 guidelines from the National Cholesterol Education Program, which recommended screening only for children with either a family history of early CVD or elevated total cholesterol levels.
A 2011 panel for the NHLBI said the older approach identified significantly fewer children with abnormal levels of low-density lipoprotein cholesterol (LDL-C) than the addition of two age groups for screening, adding that many children do not have a complete family history. The American College of Cardiology and American Heart Association later supported NHLBI’s stance in their joint guidelines on the management of cholesterol.
Mark Corkins, MD, chair of the AAP’s Committee on Nutrition, told Medscape Medical News that if children are screened only because they have obesity or a family history of FH, some with elevated lipid levels will be missed. For instance, studies indicate caregiver recall of FH often is inaccurate, and the genetic disorder that causes the condition is not related to obesity.
“The screening is to find familial hypercholesterolemia, to try to find the ones that need therapy,” that would not be caught by the risk-based screening earlier on in childhood, Corkins said.
Only Screen Children With Risk Factors
But other groups do not agree. The US Preventive Services Task Force (USPSTF) found insufficient evidence to recommend for or against screening for lipid disorders in asymptomatic children and teens.
The group also said it found inadequate evidence that lipid-lowering interventions in the general pediatric population lead to reductions in cardiovascular events or all-cause mortality once they reached adulthood. USPSTF also raised questions about the safety of lipid-lowering drugs in children.
“The current evidence is insufficient to assess the balance of benefits and harms of screening for lipid disorders in children and adolescents 20 years or younger,” the panel wrote.
The American Academy of Family Physicians supports USPSTF’s recommendations.
Low Rate of Screening
While the uncertainty over screening in children continues, the practice has been adopted by a minority of clinicians.
A study published in JAMA Network Open in July found 9% of 700,000 9- to 11-year-olds had a documented result from a lipid screening. Among more than 1.3 million 17- to 21-year-olds, 13% had received a screening.
As BMI went up, so did screening rates. A little over 9% children and teens with a healthy weight were screened compared with 14.7% of those with moderate obesity and 21.9% of those with severe obesity.
Among those screened, 32.3% of 9- to 11-year-olds and 30.2% of 17- to 21-year-olds had abnormal lipid levels, defined as having one elevated measure out of five, including total cholesterol of 200 mg/dL or higher or LDL-C levels of 130 mg/dL or higher.
Justin Zachariah, MD, MPH, an associate professor of pediatrics-cardiology at Baylor College of Medicine in Houston, spoke about physicians screening children based only on factors like obesity during a presentation at the recent annual meeting of the American Academy of Pediatrics. He cited research showing roughly one in four children with abnormal lipids had a normal weight.
If a clinician is reserving a lipid screening for a child who is overweight or has obesity, “you’re missing nearly half the problem,” Zachariah said during his presentation.
One reason for the low rate of universal screening may be inattention to FH by clinicians, according to Samuel S. Gidding, MD, a professor in the Department of Genomic Health at Geisinger College of Health Sciences in Bridgewater Corners, Vermont.
For instance, a clinician has only a set amount of time during a well-child visit and other issues may take precedence, “so it doesn’t make sense to broach preventive screening for something that could happen 30 or 40 years from now, vs this [other] very immediate problem,” he said.
Clinicians “are triggered to act on the LDL level, but don’t think about FH as a possible diagnosis,” Gidding told Medscape Medical News.
Another barrier is that in some settings, caregivers must take children and teens to another facility on a different day to fulfill an order for a lipid test.
“It’s reluctance of doctors to order it, knowing patients won’t go through with it,” Gidding said.
Gidding is a consultant for Esperion Therapeutics. Other sources in this story reported no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Heard of ApoB Testing? New Guidelines
This transcript has been edited for clarity.
I've been hearing a lot about apolipoprotein B (apoB) lately. It keeps popping up, but I've not been sure where it fits in or what I should do about it. The new Expert Clinical Consensus from the National Lipid Association now finally gives us clear guidance.
ApoB is the main protein that is found on all atherogenic lipoproteins. It is found on low-density lipoprotein (LDL) but also on other atherogenic lipoprotein particles. Because it is a part of all atherogenic particles, it predicts cardiovascular (CV) risk more accurately than does LDL cholesterol (LDL-C).
ApoB and LDL-C tend to run together, but not always. While they are correlated fairly well on a population level, for a given individual they can diverge; and when they do, apoB is the better predictor of future CV outcomes. This divergence occurs frequently, and it can occur even more frequently after treatment with statins. When LDL decreases to reach the LDL threshold for treatment, but apoB remains elevated, there is the potential for misclassification of CV risk and essentially the risk for undertreatment of someone whose CV risk is actually higher than it appears to be if we only look at their LDL-C. The consensus statement says, "Where there is discordance between apoB and LDL-C, risk follows apoB."
This understanding leads to the places where measurement of apoB may be helpful:
In patients with borderline atherosclerotic cardiovascular disease risk in whom a shared decision about statin therapy is being determined and the patient prefers not to start a statin, apoB can be useful for further risk stratification. If apoB suggests low risk, then statin therapy could be withheld, and if apoB is high, that would favor starting statin therapy. Certain common conditions, such as obesity and insulin resistance, can lead to smaller cholesterol-depleted LDL particles that result in lower LDL-C, but elevated apoB levels in this circumstance may drive the decision to treat with a statin.
In patients already treated with statins, but a decision must be made about whether treatment intensification is warranted. If the LDL-C is to goal and apoB is above threshold, treatment intensification may be considered. In patients who are not yet to goal, based on an elevated apoB, the first step is intensification of statin therapy. After that, intensification would be the same as has already been addressed in my review of the 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering.
After clarifying the importance of apoB in providing additional discrimination of CV risk, the consensus statement clarifies the treatment thresholds, or goals for treatment, for apoB that correlate with established LDL-C thresholds, as shown in this table:
Let me be really clear: The consensus statement does not say that we need to measure apoB in all patients or that such measurement is the standard of care. It is not. It says, and I'll quote, "At present, the use of apoB to assess the effectiveness of lipid-lowering therapies remains a matter of clinical judgment." This guideline is helpful in pointing out the patients most likely to benefit from this additional measurement, including those with hypertriglyceridemia, diabetes, visceral adiposity, insulin resistance/metabolic syndrome, low HDL-C, or very low LDL-C levels.
In summary, measurement of apoB can be helpful for further risk stratification in patients with borderline or intermediate LDL-C levels, and for deciding whether further intensification of lipid-lowering therapy may be warranted when the LDL threshold has been reached.
Lipid management is something that we do every day in the office. This is new information, or at least clarifying information, for most of us. Hopefully it is helpful. I'm interested in your thoughts on this topic, including whether and how you plan to use apoB measurements.
Dr. Skolnik, Professor, Department of Family Medicine, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia; Associate Director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I've been hearing a lot about apolipoprotein B (apoB) lately. It keeps popping up, but I've not been sure where it fits in or what I should do about it. The new Expert Clinical Consensus from the National Lipid Association now finally gives us clear guidance.
ApoB is the main protein that is found on all atherogenic lipoproteins. It is found on low-density lipoprotein (LDL) but also on other atherogenic lipoprotein particles. Because it is a part of all atherogenic particles, it predicts cardiovascular (CV) risk more accurately than does LDL cholesterol (LDL-C).
ApoB and LDL-C tend to run together, but not always. While they are correlated fairly well on a population level, for a given individual they can diverge; and when they do, apoB is the better predictor of future CV outcomes. This divergence occurs frequently, and it can occur even more frequently after treatment with statins. When LDL decreases to reach the LDL threshold for treatment, but apoB remains elevated, there is the potential for misclassification of CV risk and essentially the risk for undertreatment of someone whose CV risk is actually higher than it appears to be if we only look at their LDL-C. The consensus statement says, "Where there is discordance between apoB and LDL-C, risk follows apoB."
This understanding leads to the places where measurement of apoB may be helpful:
In patients with borderline atherosclerotic cardiovascular disease risk in whom a shared decision about statin therapy is being determined and the patient prefers not to start a statin, apoB can be useful for further risk stratification. If apoB suggests low risk, then statin therapy could be withheld, and if apoB is high, that would favor starting statin therapy. Certain common conditions, such as obesity and insulin resistance, can lead to smaller cholesterol-depleted LDL particles that result in lower LDL-C, but elevated apoB levels in this circumstance may drive the decision to treat with a statin.
In patients already treated with statins, but a decision must be made about whether treatment intensification is warranted. If the LDL-C is to goal and apoB is above threshold, treatment intensification may be considered. In patients who are not yet to goal, based on an elevated apoB, the first step is intensification of statin therapy. After that, intensification would be the same as has already been addressed in my review of the 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering.
After clarifying the importance of apoB in providing additional discrimination of CV risk, the consensus statement clarifies the treatment thresholds, or goals for treatment, for apoB that correlate with established LDL-C thresholds, as shown in this table:
Let me be really clear: The consensus statement does not say that we need to measure apoB in all patients or that such measurement is the standard of care. It is not. It says, and I'll quote, "At present, the use of apoB to assess the effectiveness of lipid-lowering therapies remains a matter of clinical judgment." This guideline is helpful in pointing out the patients most likely to benefit from this additional measurement, including those with hypertriglyceridemia, diabetes, visceral adiposity, insulin resistance/metabolic syndrome, low HDL-C, or very low LDL-C levels.
In summary, measurement of apoB can be helpful for further risk stratification in patients with borderline or intermediate LDL-C levels, and for deciding whether further intensification of lipid-lowering therapy may be warranted when the LDL threshold has been reached.
Lipid management is something that we do every day in the office. This is new information, or at least clarifying information, for most of us. Hopefully it is helpful. I'm interested in your thoughts on this topic, including whether and how you plan to use apoB measurements.
Dr. Skolnik, Professor, Department of Family Medicine, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia; Associate Director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I've been hearing a lot about apolipoprotein B (apoB) lately. It keeps popping up, but I've not been sure where it fits in or what I should do about it. The new Expert Clinical Consensus from the National Lipid Association now finally gives us clear guidance.
ApoB is the main protein that is found on all atherogenic lipoproteins. It is found on low-density lipoprotein (LDL) but also on other atherogenic lipoprotein particles. Because it is a part of all atherogenic particles, it predicts cardiovascular (CV) risk more accurately than does LDL cholesterol (LDL-C).
ApoB and LDL-C tend to run together, but not always. While they are correlated fairly well on a population level, for a given individual they can diverge; and when they do, apoB is the better predictor of future CV outcomes. This divergence occurs frequently, and it can occur even more frequently after treatment with statins. When LDL decreases to reach the LDL threshold for treatment, but apoB remains elevated, there is the potential for misclassification of CV risk and essentially the risk for undertreatment of someone whose CV risk is actually higher than it appears to be if we only look at their LDL-C. The consensus statement says, "Where there is discordance between apoB and LDL-C, risk follows apoB."
This understanding leads to the places where measurement of apoB may be helpful:
In patients with borderline atherosclerotic cardiovascular disease risk in whom a shared decision about statin therapy is being determined and the patient prefers not to start a statin, apoB can be useful for further risk stratification. If apoB suggests low risk, then statin therapy could be withheld, and if apoB is high, that would favor starting statin therapy. Certain common conditions, such as obesity and insulin resistance, can lead to smaller cholesterol-depleted LDL particles that result in lower LDL-C, but elevated apoB levels in this circumstance may drive the decision to treat with a statin.
In patients already treated with statins, but a decision must be made about whether treatment intensification is warranted. If the LDL-C is to goal and apoB is above threshold, treatment intensification may be considered. In patients who are not yet to goal, based on an elevated apoB, the first step is intensification of statin therapy. After that, intensification would be the same as has already been addressed in my review of the 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering.
After clarifying the importance of apoB in providing additional discrimination of CV risk, the consensus statement clarifies the treatment thresholds, or goals for treatment, for apoB that correlate with established LDL-C thresholds, as shown in this table:
Let me be really clear: The consensus statement does not say that we need to measure apoB in all patients or that such measurement is the standard of care. It is not. It says, and I'll quote, "At present, the use of apoB to assess the effectiveness of lipid-lowering therapies remains a matter of clinical judgment." This guideline is helpful in pointing out the patients most likely to benefit from this additional measurement, including those with hypertriglyceridemia, diabetes, visceral adiposity, insulin resistance/metabolic syndrome, low HDL-C, or very low LDL-C levels.
In summary, measurement of apoB can be helpful for further risk stratification in patients with borderline or intermediate LDL-C levels, and for deciding whether further intensification of lipid-lowering therapy may be warranted when the LDL threshold has been reached.
Lipid management is something that we do every day in the office. This is new information, or at least clarifying information, for most of us. Hopefully it is helpful. I'm interested in your thoughts on this topic, including whether and how you plan to use apoB measurements.
Dr. Skolnik, Professor, Department of Family Medicine, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia; Associate Director, Department of Family Medicine, Abington Jefferson Health, Abington, Pennsylvania, disclosed ties with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
‘Call to Action’: Greater CVD Focus Urged for Type 1 Diabetes
MADRID — Emerging data points to the urgent need for cardiovascular risk reduction in all adults with type 1 diabetes (T1D), including those who are young and those diagnosed in adulthood.
At the European Association for the Study of Diabetes (EASD) 2024 Annual Meeting, two entire oral abstract sessions were devoted to research examining cardiovascular risk specifically in people with T1D. There is increasing evidence that as with type 2 diabetes (T2D), clinical visits need to focus on other cardiovascular risk factors and glucose.
Findings included the evidence of severe coronary artery disease (CAD) in asymptomatic adults with T1D, increased risks for mortality and cardiac events in people diagnosed with T1D in adulthood, and a greater cardiovascular risk for those with overweight/obesity and among those with more cumulative exposure to both hyperglycemia and dyslipidemia.
One speaker, Dr. Rebecka Johanna Bergdal, of the Folkhälsan Research Center and the University of Helsinki, Finland, issued a “call to action,” saying, “We call on healthcare professionals to continue supporting and encouraging individuals with T1D towards better management of diabetes, including both glucose and lipid management.”
Session Moderator Krzysztof Strojek, MD, PhD, head of the Department of Internal Medicine, Diabetology and Cardiometabolic Diseases at the Medical University of Silesia, Katowice, Poland, told this news organization that all the data point in the same direction for T1D management, to “look not only at A1c and blood glucose control but also lipids, hypertension, smoking status, all these risk factors recognized in type 2 ... are also important in T1D.”
The ‘Alarming’ Finding of CAD in Asymptomatic Patients
Michal Dubsky, MD, PhD, of the Diabetes Centre, Institute for Clinical and Experimental Medicine, Prague, Czech Republic, presented findings from 62 asymptomatic patients with T1D for > 10 years (mean, 36 years), with a mean A1c of 7.5% (58 mmol/mol), and no prior history of cardiovascular disease (CVD). They had slightly elevated CVD biomarkers, including a mean low-density lipoprotein (LDL) cholesterol level of 2.33 mmol/L, lipoprotein (a) level of 15 nmol/L, and N-terminal pro-B-type natriuretic peptide level of 125.3 ng/L.
All underwent a noninvasive carotid ultrasound and coronary artery calcium (CAC) scoring. Of those, 12 patients had a CAC score > 400 and/or presence of two or more carotid plaques identified as high-risk.
Those 12 patients underwent coronary angiography and had a total of 29 vessels examined by optical coherence tomography (OCT), “an invasive intravascular method for assessing coronary atherosclerosis that is far more sensitive than standard coronary angiography, especially for the detection of high-risk vulnerable plaques without a hemodynamically significant stenosis,” Dr. Dubsky explained.
Coronary angiography showed obstructive CAD in 5 of the 12 patients. Their mean calcium score was 950 and mean number of carotid plaques was 2.8. Features associated with plaque vulnerability included microphage accumulation in 24 vessels, lipid-rich plaques in 23, spotty calcium in 19, and neovascularizations in 13.
Thin-cap fibroatheroma, a strong predictor of plaque rupture, was present in 7 of the 12 patients (58.3%), and four had features of very high-risk plaques, defined as thin-cap fibroatheroma with a minimal lumen area < 3.5 mm2, a lipid arch > 180 degrees, and macrophages.
“Our study showed that asymptomatic T1D patients with high CAC score and carotid plaques had very severe OCT findings. We observed a significant proportion of high-risk lesions potentially associated with plaque rupture and risk of CV death. Therefore, we believe these patients should be treated as very high-risk with target LDL below 1.4 mmol/L (55 mg/dL), even though they are completely asymptomatic,” Dr. Dubsky concluded.
He added that because OCT is invasive and costly, the CAC score can be used to guide the decision for statin use, with any score above 100 considered elevated risk.
Study coauthor Martin Haluzik, MD, professor of internal medicine in the Charles University, Prague, Czech Republic, told this news organization, “I think it’s very alarming because some of these are basically very healthy-looking young people, so you don’t really expect them to have significant cardiovascular complications already or significant plaques. I think it shows that we should be more proactive in looking into the risk of cardiovascular complications and in looking into the early cardiovascular changes.”
Later Diagnosis Doesn’t Always Protect: Risk Seen in Adult-Onset T1D
Yuxia Wei, a PhD student at the Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden, presented an analysis of data from Sweden’s national health databases comparing cardiovascular outcomes between 10,184 people diagnosed with T1D at ages 18-29 years, 30-39 years, and ≥ 40 years; another 375,523 people diagnosed with T2D at those ages; and 509,172 population controls matched for age, sex, and county.
Those diagnosed after age 40 years had higher A1c levels and were less likely to be using insulin pumps than those diagnosed at younger adult ages.
Compared with population controls, at a median of about 7 years of follow-up, people with T1D had significantly higher all-cause mortality at all diagnosis age groups, with a hazard ratio of 1.71. This rose to 2.78 for those diagnosed at age 30-39 years.
Compared with those with T2D, the mortality risks weren’t significantly different at any age, but the risks for non-cardiovascular death, including from cancer and infection, were significantly higher among those diagnosed after age 40 years (1.31 overall). Those diagnosed with T1D at any adult age had lower risks for major cardiovascular events than those diagnosed with T2D. Hazard ratios ranged from 0.27 for those diagnosed at age 18-29 years to 0.78 for those diagnosed after the age of 40 years.
Smoking and A1c above target were the greatest contributors to mortality. Those two factors, along with body mass index (BMI), were the strongest contributors to major adverse cardiovascular events (MACE).
“Adult-onset T1D carries excess risk of death and cardiovascular disease, without obvious attenuation over age at diagnosis…Smoking, A1c, and BMI are the key factors to be managed to improve prognosis in adult-onset T1D,” Ms. Wei concluded.
BMI: Often Overlooked in T1D, but a Major CVD Risk Factor
Two studies examined the link between overweight/obesity and cardiovascular risk in T1D. One, by Laurence Salle, MCU PH, of the Endocrinology, Diabetes and Metabolic Diseases Department at CHU Limoges, France, was a prospective, longitudinal cohort study of 2367 people with T1D at 68 centers in France who didn’t have a cardiovascular history at baseline.
Of those, 51% had normal BMI (18.5-24.9), 31% had overweight (25-29.9), and 18% had obesity (≥ 30). Cardiovascular risk factors, including LDL cholesterol, triglycerides, and hypertension increased with an increasing BMI. The 10-year CVD risk was significantly higher for those with overweight (9.61%) and obesity (9.93%) than for those with normal weight (7.24%), in both men and women.
However, BMI was found to be an independent predictor of 10-year high cardiovascular risk in men but not women, while waist:height ratio independently predicted risk in both men and women, Dr. Salle reported.
The second BMI study, from Enrique Soto-Pedre, MBBS, of the Division of Population Health and Genomics at the University of Dundee, Scotland, presented data on a retrospective follow-up from 1995 to 2019 of 1973 people with T1D aged > 18 years at diagnosis (42% women; mean age, 34.2 years; 18.9% had obesity.
After 10 years of follow-up, those with overweight and obesity had significantly higher odds of developing arterial hypertension, even among those taking angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, with statistically significant adjusted hazard ratios of 1.73 and 3.37 for the obese and overweight groups, respectively.
MACE were significantly more common among those with obesity, with an adjusted hazard ratio of 2.95, as was acute myocardial infarction, 3.33.
“These results emphasize the importance of incorporating weight management into the overall management of individuals with T1D. No one has doubts about weight management in T2D, but in type 1, it’s not so clear. One of the main [concerns] would be safety [in terms of hypoglycemia],” Dr. Soto-Pedre concluded.
Call for Action: Cumulative Glucose and Lipid Exposures Increase Risk
Dr. Bergdal presented data on the effects of cumulative glycemia and lipids on the risk for CAD in 3495 adults with T1D who had been diagnosed prior to the age of 40 years. The history of CAD or stroke was exclusion criteria. There were a total of 534 CAD events within a median follow-up of 19.4 years.
Cumulative glycemia, LDL cholesterol, triglycerides, and non–high-density lipoprotein cholesterol exposures were all significantly associated with CAD risk (P < .001 for all). With an adjustment for confounders, the highest tertile of glycemia was associated with a twofold increased risk for CAD. Both hyperglycemia and dyslipidemia were independently associated with CAD risk, Dr. Bergdal reported.
“It’s important to minimize the time spent above A1c 7%, and lipid management in T1D must not be forgotten,” she said, prior to issuing her call for action.
Dr. Haluzik reported receiving honoraria for talks and/or consultancy and/or research funding from Eli Lilly, Novo Nordisk, Sanofi, AstraZeneca, Mundipharma, Bristol Myers Squibb, Amgen, Boehringer Ingelheim, Janssen, Ypsomed, and Johnson & Johnson. The presenters had no disclosures.
A version of this article first appeared on Medscape.com.
MADRID — Emerging data points to the urgent need for cardiovascular risk reduction in all adults with type 1 diabetes (T1D), including those who are young and those diagnosed in adulthood.
At the European Association for the Study of Diabetes (EASD) 2024 Annual Meeting, two entire oral abstract sessions were devoted to research examining cardiovascular risk specifically in people with T1D. There is increasing evidence that as with type 2 diabetes (T2D), clinical visits need to focus on other cardiovascular risk factors and glucose.
Findings included the evidence of severe coronary artery disease (CAD) in asymptomatic adults with T1D, increased risks for mortality and cardiac events in people diagnosed with T1D in adulthood, and a greater cardiovascular risk for those with overweight/obesity and among those with more cumulative exposure to both hyperglycemia and dyslipidemia.
One speaker, Dr. Rebecka Johanna Bergdal, of the Folkhälsan Research Center and the University of Helsinki, Finland, issued a “call to action,” saying, “We call on healthcare professionals to continue supporting and encouraging individuals with T1D towards better management of diabetes, including both glucose and lipid management.”
Session Moderator Krzysztof Strojek, MD, PhD, head of the Department of Internal Medicine, Diabetology and Cardiometabolic Diseases at the Medical University of Silesia, Katowice, Poland, told this news organization that all the data point in the same direction for T1D management, to “look not only at A1c and blood glucose control but also lipids, hypertension, smoking status, all these risk factors recognized in type 2 ... are also important in T1D.”
The ‘Alarming’ Finding of CAD in Asymptomatic Patients
Michal Dubsky, MD, PhD, of the Diabetes Centre, Institute for Clinical and Experimental Medicine, Prague, Czech Republic, presented findings from 62 asymptomatic patients with T1D for > 10 years (mean, 36 years), with a mean A1c of 7.5% (58 mmol/mol), and no prior history of cardiovascular disease (CVD). They had slightly elevated CVD biomarkers, including a mean low-density lipoprotein (LDL) cholesterol level of 2.33 mmol/L, lipoprotein (a) level of 15 nmol/L, and N-terminal pro-B-type natriuretic peptide level of 125.3 ng/L.
All underwent a noninvasive carotid ultrasound and coronary artery calcium (CAC) scoring. Of those, 12 patients had a CAC score > 400 and/or presence of two or more carotid plaques identified as high-risk.
Those 12 patients underwent coronary angiography and had a total of 29 vessels examined by optical coherence tomography (OCT), “an invasive intravascular method for assessing coronary atherosclerosis that is far more sensitive than standard coronary angiography, especially for the detection of high-risk vulnerable plaques without a hemodynamically significant stenosis,” Dr. Dubsky explained.
Coronary angiography showed obstructive CAD in 5 of the 12 patients. Their mean calcium score was 950 and mean number of carotid plaques was 2.8. Features associated with plaque vulnerability included microphage accumulation in 24 vessels, lipid-rich plaques in 23, spotty calcium in 19, and neovascularizations in 13.
Thin-cap fibroatheroma, a strong predictor of plaque rupture, was present in 7 of the 12 patients (58.3%), and four had features of very high-risk plaques, defined as thin-cap fibroatheroma with a minimal lumen area < 3.5 mm2, a lipid arch > 180 degrees, and macrophages.
“Our study showed that asymptomatic T1D patients with high CAC score and carotid plaques had very severe OCT findings. We observed a significant proportion of high-risk lesions potentially associated with plaque rupture and risk of CV death. Therefore, we believe these patients should be treated as very high-risk with target LDL below 1.4 mmol/L (55 mg/dL), even though they are completely asymptomatic,” Dr. Dubsky concluded.
He added that because OCT is invasive and costly, the CAC score can be used to guide the decision for statin use, with any score above 100 considered elevated risk.
Study coauthor Martin Haluzik, MD, professor of internal medicine in the Charles University, Prague, Czech Republic, told this news organization, “I think it’s very alarming because some of these are basically very healthy-looking young people, so you don’t really expect them to have significant cardiovascular complications already or significant plaques. I think it shows that we should be more proactive in looking into the risk of cardiovascular complications and in looking into the early cardiovascular changes.”
Later Diagnosis Doesn’t Always Protect: Risk Seen in Adult-Onset T1D
Yuxia Wei, a PhD student at the Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden, presented an analysis of data from Sweden’s national health databases comparing cardiovascular outcomes between 10,184 people diagnosed with T1D at ages 18-29 years, 30-39 years, and ≥ 40 years; another 375,523 people diagnosed with T2D at those ages; and 509,172 population controls matched for age, sex, and county.
Those diagnosed after age 40 years had higher A1c levels and were less likely to be using insulin pumps than those diagnosed at younger adult ages.
Compared with population controls, at a median of about 7 years of follow-up, people with T1D had significantly higher all-cause mortality at all diagnosis age groups, with a hazard ratio of 1.71. This rose to 2.78 for those diagnosed at age 30-39 years.
Compared with those with T2D, the mortality risks weren’t significantly different at any age, but the risks for non-cardiovascular death, including from cancer and infection, were significantly higher among those diagnosed after age 40 years (1.31 overall). Those diagnosed with T1D at any adult age had lower risks for major cardiovascular events than those diagnosed with T2D. Hazard ratios ranged from 0.27 for those diagnosed at age 18-29 years to 0.78 for those diagnosed after the age of 40 years.
Smoking and A1c above target were the greatest contributors to mortality. Those two factors, along with body mass index (BMI), were the strongest contributors to major adverse cardiovascular events (MACE).
“Adult-onset T1D carries excess risk of death and cardiovascular disease, without obvious attenuation over age at diagnosis…Smoking, A1c, and BMI are the key factors to be managed to improve prognosis in adult-onset T1D,” Ms. Wei concluded.
BMI: Often Overlooked in T1D, but a Major CVD Risk Factor
Two studies examined the link between overweight/obesity and cardiovascular risk in T1D. One, by Laurence Salle, MCU PH, of the Endocrinology, Diabetes and Metabolic Diseases Department at CHU Limoges, France, was a prospective, longitudinal cohort study of 2367 people with T1D at 68 centers in France who didn’t have a cardiovascular history at baseline.
Of those, 51% had normal BMI (18.5-24.9), 31% had overweight (25-29.9), and 18% had obesity (≥ 30). Cardiovascular risk factors, including LDL cholesterol, triglycerides, and hypertension increased with an increasing BMI. The 10-year CVD risk was significantly higher for those with overweight (9.61%) and obesity (9.93%) than for those with normal weight (7.24%), in both men and women.
However, BMI was found to be an independent predictor of 10-year high cardiovascular risk in men but not women, while waist:height ratio independently predicted risk in both men and women, Dr. Salle reported.
The second BMI study, from Enrique Soto-Pedre, MBBS, of the Division of Population Health and Genomics at the University of Dundee, Scotland, presented data on a retrospective follow-up from 1995 to 2019 of 1973 people with T1D aged > 18 years at diagnosis (42% women; mean age, 34.2 years; 18.9% had obesity.
After 10 years of follow-up, those with overweight and obesity had significantly higher odds of developing arterial hypertension, even among those taking angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, with statistically significant adjusted hazard ratios of 1.73 and 3.37 for the obese and overweight groups, respectively.
MACE were significantly more common among those with obesity, with an adjusted hazard ratio of 2.95, as was acute myocardial infarction, 3.33.
“These results emphasize the importance of incorporating weight management into the overall management of individuals with T1D. No one has doubts about weight management in T2D, but in type 1, it’s not so clear. One of the main [concerns] would be safety [in terms of hypoglycemia],” Dr. Soto-Pedre concluded.
Call for Action: Cumulative Glucose and Lipid Exposures Increase Risk
Dr. Bergdal presented data on the effects of cumulative glycemia and lipids on the risk for CAD in 3495 adults with T1D who had been diagnosed prior to the age of 40 years. The history of CAD or stroke was exclusion criteria. There were a total of 534 CAD events within a median follow-up of 19.4 years.
Cumulative glycemia, LDL cholesterol, triglycerides, and non–high-density lipoprotein cholesterol exposures were all significantly associated with CAD risk (P < .001 for all). With an adjustment for confounders, the highest tertile of glycemia was associated with a twofold increased risk for CAD. Both hyperglycemia and dyslipidemia were independently associated with CAD risk, Dr. Bergdal reported.
“It’s important to minimize the time spent above A1c 7%, and lipid management in T1D must not be forgotten,” she said, prior to issuing her call for action.
Dr. Haluzik reported receiving honoraria for talks and/or consultancy and/or research funding from Eli Lilly, Novo Nordisk, Sanofi, AstraZeneca, Mundipharma, Bristol Myers Squibb, Amgen, Boehringer Ingelheim, Janssen, Ypsomed, and Johnson & Johnson. The presenters had no disclosures.
A version of this article first appeared on Medscape.com.
MADRID — Emerging data points to the urgent need for cardiovascular risk reduction in all adults with type 1 diabetes (T1D), including those who are young and those diagnosed in adulthood.
At the European Association for the Study of Diabetes (EASD) 2024 Annual Meeting, two entire oral abstract sessions were devoted to research examining cardiovascular risk specifically in people with T1D. There is increasing evidence that as with type 2 diabetes (T2D), clinical visits need to focus on other cardiovascular risk factors and glucose.
Findings included the evidence of severe coronary artery disease (CAD) in asymptomatic adults with T1D, increased risks for mortality and cardiac events in people diagnosed with T1D in adulthood, and a greater cardiovascular risk for those with overweight/obesity and among those with more cumulative exposure to both hyperglycemia and dyslipidemia.
One speaker, Dr. Rebecka Johanna Bergdal, of the Folkhälsan Research Center and the University of Helsinki, Finland, issued a “call to action,” saying, “We call on healthcare professionals to continue supporting and encouraging individuals with T1D towards better management of diabetes, including both glucose and lipid management.”
Session Moderator Krzysztof Strojek, MD, PhD, head of the Department of Internal Medicine, Diabetology and Cardiometabolic Diseases at the Medical University of Silesia, Katowice, Poland, told this news organization that all the data point in the same direction for T1D management, to “look not only at A1c and blood glucose control but also lipids, hypertension, smoking status, all these risk factors recognized in type 2 ... are also important in T1D.”
The ‘Alarming’ Finding of CAD in Asymptomatic Patients
Michal Dubsky, MD, PhD, of the Diabetes Centre, Institute for Clinical and Experimental Medicine, Prague, Czech Republic, presented findings from 62 asymptomatic patients with T1D for > 10 years (mean, 36 years), with a mean A1c of 7.5% (58 mmol/mol), and no prior history of cardiovascular disease (CVD). They had slightly elevated CVD biomarkers, including a mean low-density lipoprotein (LDL) cholesterol level of 2.33 mmol/L, lipoprotein (a) level of 15 nmol/L, and N-terminal pro-B-type natriuretic peptide level of 125.3 ng/L.
All underwent a noninvasive carotid ultrasound and coronary artery calcium (CAC) scoring. Of those, 12 patients had a CAC score > 400 and/or presence of two or more carotid plaques identified as high-risk.
Those 12 patients underwent coronary angiography and had a total of 29 vessels examined by optical coherence tomography (OCT), “an invasive intravascular method for assessing coronary atherosclerosis that is far more sensitive than standard coronary angiography, especially for the detection of high-risk vulnerable plaques without a hemodynamically significant stenosis,” Dr. Dubsky explained.
Coronary angiography showed obstructive CAD in 5 of the 12 patients. Their mean calcium score was 950 and mean number of carotid plaques was 2.8. Features associated with plaque vulnerability included microphage accumulation in 24 vessels, lipid-rich plaques in 23, spotty calcium in 19, and neovascularizations in 13.
Thin-cap fibroatheroma, a strong predictor of plaque rupture, was present in 7 of the 12 patients (58.3%), and four had features of very high-risk plaques, defined as thin-cap fibroatheroma with a minimal lumen area < 3.5 mm2, a lipid arch > 180 degrees, and macrophages.
“Our study showed that asymptomatic T1D patients with high CAC score and carotid plaques had very severe OCT findings. We observed a significant proportion of high-risk lesions potentially associated with plaque rupture and risk of CV death. Therefore, we believe these patients should be treated as very high-risk with target LDL below 1.4 mmol/L (55 mg/dL), even though they are completely asymptomatic,” Dr. Dubsky concluded.
He added that because OCT is invasive and costly, the CAC score can be used to guide the decision for statin use, with any score above 100 considered elevated risk.
Study coauthor Martin Haluzik, MD, professor of internal medicine in the Charles University, Prague, Czech Republic, told this news organization, “I think it’s very alarming because some of these are basically very healthy-looking young people, so you don’t really expect them to have significant cardiovascular complications already or significant plaques. I think it shows that we should be more proactive in looking into the risk of cardiovascular complications and in looking into the early cardiovascular changes.”
Later Diagnosis Doesn’t Always Protect: Risk Seen in Adult-Onset T1D
Yuxia Wei, a PhD student at the Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden, presented an analysis of data from Sweden’s national health databases comparing cardiovascular outcomes between 10,184 people diagnosed with T1D at ages 18-29 years, 30-39 years, and ≥ 40 years; another 375,523 people diagnosed with T2D at those ages; and 509,172 population controls matched for age, sex, and county.
Those diagnosed after age 40 years had higher A1c levels and were less likely to be using insulin pumps than those diagnosed at younger adult ages.
Compared with population controls, at a median of about 7 years of follow-up, people with T1D had significantly higher all-cause mortality at all diagnosis age groups, with a hazard ratio of 1.71. This rose to 2.78 for those diagnosed at age 30-39 years.
Compared with those with T2D, the mortality risks weren’t significantly different at any age, but the risks for non-cardiovascular death, including from cancer and infection, were significantly higher among those diagnosed after age 40 years (1.31 overall). Those diagnosed with T1D at any adult age had lower risks for major cardiovascular events than those diagnosed with T2D. Hazard ratios ranged from 0.27 for those diagnosed at age 18-29 years to 0.78 for those diagnosed after the age of 40 years.
Smoking and A1c above target were the greatest contributors to mortality. Those two factors, along with body mass index (BMI), were the strongest contributors to major adverse cardiovascular events (MACE).
“Adult-onset T1D carries excess risk of death and cardiovascular disease, without obvious attenuation over age at diagnosis…Smoking, A1c, and BMI are the key factors to be managed to improve prognosis in adult-onset T1D,” Ms. Wei concluded.
BMI: Often Overlooked in T1D, but a Major CVD Risk Factor
Two studies examined the link between overweight/obesity and cardiovascular risk in T1D. One, by Laurence Salle, MCU PH, of the Endocrinology, Diabetes and Metabolic Diseases Department at CHU Limoges, France, was a prospective, longitudinal cohort study of 2367 people with T1D at 68 centers in France who didn’t have a cardiovascular history at baseline.
Of those, 51% had normal BMI (18.5-24.9), 31% had overweight (25-29.9), and 18% had obesity (≥ 30). Cardiovascular risk factors, including LDL cholesterol, triglycerides, and hypertension increased with an increasing BMI. The 10-year CVD risk was significantly higher for those with overweight (9.61%) and obesity (9.93%) than for those with normal weight (7.24%), in both men and women.
However, BMI was found to be an independent predictor of 10-year high cardiovascular risk in men but not women, while waist:height ratio independently predicted risk in both men and women, Dr. Salle reported.
The second BMI study, from Enrique Soto-Pedre, MBBS, of the Division of Population Health and Genomics at the University of Dundee, Scotland, presented data on a retrospective follow-up from 1995 to 2019 of 1973 people with T1D aged > 18 years at diagnosis (42% women; mean age, 34.2 years; 18.9% had obesity.
After 10 years of follow-up, those with overweight and obesity had significantly higher odds of developing arterial hypertension, even among those taking angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, with statistically significant adjusted hazard ratios of 1.73 and 3.37 for the obese and overweight groups, respectively.
MACE were significantly more common among those with obesity, with an adjusted hazard ratio of 2.95, as was acute myocardial infarction, 3.33.
“These results emphasize the importance of incorporating weight management into the overall management of individuals with T1D. No one has doubts about weight management in T2D, but in type 1, it’s not so clear. One of the main [concerns] would be safety [in terms of hypoglycemia],” Dr. Soto-Pedre concluded.
Call for Action: Cumulative Glucose and Lipid Exposures Increase Risk
Dr. Bergdal presented data on the effects of cumulative glycemia and lipids on the risk for CAD in 3495 adults with T1D who had been diagnosed prior to the age of 40 years. The history of CAD or stroke was exclusion criteria. There were a total of 534 CAD events within a median follow-up of 19.4 years.
Cumulative glycemia, LDL cholesterol, triglycerides, and non–high-density lipoprotein cholesterol exposures were all significantly associated with CAD risk (P < .001 for all). With an adjustment for confounders, the highest tertile of glycemia was associated with a twofold increased risk for CAD. Both hyperglycemia and dyslipidemia were independently associated with CAD risk, Dr. Bergdal reported.
“It’s important to minimize the time spent above A1c 7%, and lipid management in T1D must not be forgotten,” she said, prior to issuing her call for action.
Dr. Haluzik reported receiving honoraria for talks and/or consultancy and/or research funding from Eli Lilly, Novo Nordisk, Sanofi, AstraZeneca, Mundipharma, Bristol Myers Squibb, Amgen, Boehringer Ingelheim, Janssen, Ypsomed, and Johnson & Johnson. The presenters had no disclosures.
A version of this article first appeared on Medscape.com.
FROM EASD 2024
Coffee’s ‘Sweet Spot’: Daily Consumption and Cardiometabolic Risk
Each and every day, 1 billion people on this planet ingest a particular psychoactive substance. This chemical has fairly profound physiologic effects. It increases levels of nitric oxide in the blood, leads to vasodilation, and, of course, makes you feel more awake. The substance comes in many forms but almost always in a liquid medium. Do you have it yet? That’s right. The substance is caffeine, quite possibly the healthiest recreational drug that has ever been discovered.
This might be my New England upbringing speaking, but when it comes to lifestyle and health, one of the rules I’ve internalized is that things that are pleasurable are generally bad for you. I know, I know — some of you love to exercise. Some of you love doing crosswords. But you know what I mean. I’m talking French fries, smoked meats, drugs, smoking, alcohol, binge-watching Firefly. You’d be suspicious if a study came out suggesting that eating ice cream in bed reduces your risk for heart attack, and so would I. So I’m always on the lookout for those unicorns of lifestyle factors, those rare things that you want to do and are also good for you.
So far, the data are strong for three things: sleeping, (safe) sexual activity, and coffee. You’ll have to stay tuned for articles about the first two. Today, we’re brewing up some deeper insights about the power of java.
I was inspired to write this article because of a paper, “Habitual Coffee, Tea, and Caffeine Consumption, Circulating Metabolites, and the Risk of Cardiometabolic Multimorbidity,” appearing September 17 in The Journal of Clinical Endocrinology and Metabolism (JCEM).
This is not the first study to suggest that coffee intake may be beneficial. A 2013 meta-analysis summarized the results of 36 studies with more than a million participants and found a U-shaped relationship between coffee intake and cardiovascular risk. The sweet spot was at three to five cups a day; people drinking that much coffee had about a 15% reduced risk for cardiovascular disease compared with nondrinkers.
But here’s the thing. Coffee contains caffeine, but it is much more than that. It is a heady brew of various chemicals and compounds, phenols, and chlorogenic acids. And, of course, you can get caffeine from stuff that isn’t coffee — natural things like tea — and decidedly unnatural things like energy drinks. How do you figure out where the benefit really lies?
The JCEM study leveraged the impressive UK Biobank dataset to figure this out. The Biobank recruited more than half a million people from the UK between 2006 and 2010 and collected a wealth of data from each of them: surveys, blood samples, biometrics, medical imaging — the works. And then they followed what would happen to those people medically over time. It’s a pretty amazing resource.
But for the purposes of this study, what you need to know is that just under 200,000 of those participants met the key criteria for this study: being free from cardiovascular disease at baseline; having completed a detailed survey about their coffee, tea, and other caffeinated beverage intake; and having adequate follow-up. A subset of that number, just under 100,000, had metabolomic data — which is where this study really gets interesting.
We’ll dive into the metabolome in a moment, but first let’s just talk about the main finding, the relationship between coffee, tea, or caffeine and cardiovascular disease. But to do that, we need to acknowledge that people who drink a lot of coffee are different from people who don’t, and it might be those differences, not the coffee itself, that are beneficial.
What were those differences? People who drank more coffee tended to be a bit older, were less likely to be female, and were slightly more likely to engage in physical activity. They ate less processed meat but also fewer vegetables. Some of those factors, like being female, are generally protective against cardiovascular disease; but some, like age, are definitely not. The authors adjusted for these and multiple other factors, including alcohol intake, BMI, kidney function, and many others to try to disentangle the effect of being the type of person who drinks a lot of coffee from the drinking a lot of coffee itself.
These are the results of the fully adjusted model. Compared with nonconsumers, you can see that people in the higher range of coffee, tea, or just caffeine intake have almost a 40% reduction in cardiovascular disease in follow-up.
Looking at the benefit across the spectrum of intake, you again see that U-shaped curve, suggesting that a sweet spot for daily consumption can be found around 3 cups of coffee or tea (or 250 mg of caffeine). A standard energy drink contains about 120 mg of caffeine.
But if this is true, it would be good to know why. To figure that out, the authors turned to the metabolome. The idea here is that your body is constantly breaking stuff down, taking all these proteins and chemicals and compounds that we ingest and turning them into metabolites. Using advanced measurement techniques, researchers can measure hundreds or even thousands of metabolites from a single blood sample. They provide information, obviously, about the food you eat and the drinks you drink, but what is really intriguing is that some metabolites are associated with better health and some with worse
In this study, researchers measured 168 individual metabolites. Eighty of them, nearly half, were significantly altered in people who drank more coffee.
This figure summarizes the findings, and yes, this is way too complicated.
But here’s how to interpret it. The inner ring shows you how certain metabolites are associated with cardiovascular disease. The outer rings show you how those metabolites are associated with coffee, tea, or caffeine. The interesting part is that the sections of the ring (outer rings and inner rings) are very different colors.
Like here.
What you see here is a fairly profound effect that coffee, tea, or caffeine intake has on metabolites of VLDL — bad cholesterol. The beverages lower it, and, of course, higher levels lead to cardiovascular disease. This means that this is a potential causal pathway from coffee intake to heart protection.
And that’s not the only one.
You see a similar relationship for saturated fatty acids. Higher levels lead to cardiovascular disease, and coffee intake lowers levels. The reverse works too: Lower levels of histidine (an amino acid) increase cardiovascular risk, and coffee seems to raise those levels.
Is this all too good to be true? It’s hard to say. The data on coffee’s benefits have been remarkably consistent. Still, I wouldn’t be a good doctor if I didn’t mention that clearly there is a difference between a cup of black coffee and a venti caramel Frappuccino.
Nevertheless, coffee remains firmly in my holy trinity of enjoyable things that are, for whatever reason, still good for you. So, when you’re having that second, or third, or maybe fourth cup of the day, you can take that to heart.
Dr. Wilson, associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Each and every day, 1 billion people on this planet ingest a particular psychoactive substance. This chemical has fairly profound physiologic effects. It increases levels of nitric oxide in the blood, leads to vasodilation, and, of course, makes you feel more awake. The substance comes in many forms but almost always in a liquid medium. Do you have it yet? That’s right. The substance is caffeine, quite possibly the healthiest recreational drug that has ever been discovered.
This might be my New England upbringing speaking, but when it comes to lifestyle and health, one of the rules I’ve internalized is that things that are pleasurable are generally bad for you. I know, I know — some of you love to exercise. Some of you love doing crosswords. But you know what I mean. I’m talking French fries, smoked meats, drugs, smoking, alcohol, binge-watching Firefly. You’d be suspicious if a study came out suggesting that eating ice cream in bed reduces your risk for heart attack, and so would I. So I’m always on the lookout for those unicorns of lifestyle factors, those rare things that you want to do and are also good for you.
So far, the data are strong for three things: sleeping, (safe) sexual activity, and coffee. You’ll have to stay tuned for articles about the first two. Today, we’re brewing up some deeper insights about the power of java.
I was inspired to write this article because of a paper, “Habitual Coffee, Tea, and Caffeine Consumption, Circulating Metabolites, and the Risk of Cardiometabolic Multimorbidity,” appearing September 17 in The Journal of Clinical Endocrinology and Metabolism (JCEM).
This is not the first study to suggest that coffee intake may be beneficial. A 2013 meta-analysis summarized the results of 36 studies with more than a million participants and found a U-shaped relationship between coffee intake and cardiovascular risk. The sweet spot was at three to five cups a day; people drinking that much coffee had about a 15% reduced risk for cardiovascular disease compared with nondrinkers.
But here’s the thing. Coffee contains caffeine, but it is much more than that. It is a heady brew of various chemicals and compounds, phenols, and chlorogenic acids. And, of course, you can get caffeine from stuff that isn’t coffee — natural things like tea — and decidedly unnatural things like energy drinks. How do you figure out where the benefit really lies?
The JCEM study leveraged the impressive UK Biobank dataset to figure this out. The Biobank recruited more than half a million people from the UK between 2006 and 2010 and collected a wealth of data from each of them: surveys, blood samples, biometrics, medical imaging — the works. And then they followed what would happen to those people medically over time. It’s a pretty amazing resource.
But for the purposes of this study, what you need to know is that just under 200,000 of those participants met the key criteria for this study: being free from cardiovascular disease at baseline; having completed a detailed survey about their coffee, tea, and other caffeinated beverage intake; and having adequate follow-up. A subset of that number, just under 100,000, had metabolomic data — which is where this study really gets interesting.
We’ll dive into the metabolome in a moment, but first let’s just talk about the main finding, the relationship between coffee, tea, or caffeine and cardiovascular disease. But to do that, we need to acknowledge that people who drink a lot of coffee are different from people who don’t, and it might be those differences, not the coffee itself, that are beneficial.
What were those differences? People who drank more coffee tended to be a bit older, were less likely to be female, and were slightly more likely to engage in physical activity. They ate less processed meat but also fewer vegetables. Some of those factors, like being female, are generally protective against cardiovascular disease; but some, like age, are definitely not. The authors adjusted for these and multiple other factors, including alcohol intake, BMI, kidney function, and many others to try to disentangle the effect of being the type of person who drinks a lot of coffee from the drinking a lot of coffee itself.
These are the results of the fully adjusted model. Compared with nonconsumers, you can see that people in the higher range of coffee, tea, or just caffeine intake have almost a 40% reduction in cardiovascular disease in follow-up.
Looking at the benefit across the spectrum of intake, you again see that U-shaped curve, suggesting that a sweet spot for daily consumption can be found around 3 cups of coffee or tea (or 250 mg of caffeine). A standard energy drink contains about 120 mg of caffeine.
But if this is true, it would be good to know why. To figure that out, the authors turned to the metabolome. The idea here is that your body is constantly breaking stuff down, taking all these proteins and chemicals and compounds that we ingest and turning them into metabolites. Using advanced measurement techniques, researchers can measure hundreds or even thousands of metabolites from a single blood sample. They provide information, obviously, about the food you eat and the drinks you drink, but what is really intriguing is that some metabolites are associated with better health and some with worse
In this study, researchers measured 168 individual metabolites. Eighty of them, nearly half, were significantly altered in people who drank more coffee.
This figure summarizes the findings, and yes, this is way too complicated.
But here’s how to interpret it. The inner ring shows you how certain metabolites are associated with cardiovascular disease. The outer rings show you how those metabolites are associated with coffee, tea, or caffeine. The interesting part is that the sections of the ring (outer rings and inner rings) are very different colors.
Like here.
What you see here is a fairly profound effect that coffee, tea, or caffeine intake has on metabolites of VLDL — bad cholesterol. The beverages lower it, and, of course, higher levels lead to cardiovascular disease. This means that this is a potential causal pathway from coffee intake to heart protection.
And that’s not the only one.
You see a similar relationship for saturated fatty acids. Higher levels lead to cardiovascular disease, and coffee intake lowers levels. The reverse works too: Lower levels of histidine (an amino acid) increase cardiovascular risk, and coffee seems to raise those levels.
Is this all too good to be true? It’s hard to say. The data on coffee’s benefits have been remarkably consistent. Still, I wouldn’t be a good doctor if I didn’t mention that clearly there is a difference between a cup of black coffee and a venti caramel Frappuccino.
Nevertheless, coffee remains firmly in my holy trinity of enjoyable things that are, for whatever reason, still good for you. So, when you’re having that second, or third, or maybe fourth cup of the day, you can take that to heart.
Dr. Wilson, associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Each and every day, 1 billion people on this planet ingest a particular psychoactive substance. This chemical has fairly profound physiologic effects. It increases levels of nitric oxide in the blood, leads to vasodilation, and, of course, makes you feel more awake. The substance comes in many forms but almost always in a liquid medium. Do you have it yet? That’s right. The substance is caffeine, quite possibly the healthiest recreational drug that has ever been discovered.
This might be my New England upbringing speaking, but when it comes to lifestyle and health, one of the rules I’ve internalized is that things that are pleasurable are generally bad for you. I know, I know — some of you love to exercise. Some of you love doing crosswords. But you know what I mean. I’m talking French fries, smoked meats, drugs, smoking, alcohol, binge-watching Firefly. You’d be suspicious if a study came out suggesting that eating ice cream in bed reduces your risk for heart attack, and so would I. So I’m always on the lookout for those unicorns of lifestyle factors, those rare things that you want to do and are also good for you.
So far, the data are strong for three things: sleeping, (safe) sexual activity, and coffee. You’ll have to stay tuned for articles about the first two. Today, we’re brewing up some deeper insights about the power of java.
I was inspired to write this article because of a paper, “Habitual Coffee, Tea, and Caffeine Consumption, Circulating Metabolites, and the Risk of Cardiometabolic Multimorbidity,” appearing September 17 in The Journal of Clinical Endocrinology and Metabolism (JCEM).
This is not the first study to suggest that coffee intake may be beneficial. A 2013 meta-analysis summarized the results of 36 studies with more than a million participants and found a U-shaped relationship between coffee intake and cardiovascular risk. The sweet spot was at three to five cups a day; people drinking that much coffee had about a 15% reduced risk for cardiovascular disease compared with nondrinkers.
But here’s the thing. Coffee contains caffeine, but it is much more than that. It is a heady brew of various chemicals and compounds, phenols, and chlorogenic acids. And, of course, you can get caffeine from stuff that isn’t coffee — natural things like tea — and decidedly unnatural things like energy drinks. How do you figure out where the benefit really lies?
The JCEM study leveraged the impressive UK Biobank dataset to figure this out. The Biobank recruited more than half a million people from the UK between 2006 and 2010 and collected a wealth of data from each of them: surveys, blood samples, biometrics, medical imaging — the works. And then they followed what would happen to those people medically over time. It’s a pretty amazing resource.
But for the purposes of this study, what you need to know is that just under 200,000 of those participants met the key criteria for this study: being free from cardiovascular disease at baseline; having completed a detailed survey about their coffee, tea, and other caffeinated beverage intake; and having adequate follow-up. A subset of that number, just under 100,000, had metabolomic data — which is where this study really gets interesting.
We’ll dive into the metabolome in a moment, but first let’s just talk about the main finding, the relationship between coffee, tea, or caffeine and cardiovascular disease. But to do that, we need to acknowledge that people who drink a lot of coffee are different from people who don’t, and it might be those differences, not the coffee itself, that are beneficial.
What were those differences? People who drank more coffee tended to be a bit older, were less likely to be female, and were slightly more likely to engage in physical activity. They ate less processed meat but also fewer vegetables. Some of those factors, like being female, are generally protective against cardiovascular disease; but some, like age, are definitely not. The authors adjusted for these and multiple other factors, including alcohol intake, BMI, kidney function, and many others to try to disentangle the effect of being the type of person who drinks a lot of coffee from the drinking a lot of coffee itself.
These are the results of the fully adjusted model. Compared with nonconsumers, you can see that people in the higher range of coffee, tea, or just caffeine intake have almost a 40% reduction in cardiovascular disease in follow-up.
Looking at the benefit across the spectrum of intake, you again see that U-shaped curve, suggesting that a sweet spot for daily consumption can be found around 3 cups of coffee or tea (or 250 mg of caffeine). A standard energy drink contains about 120 mg of caffeine.
But if this is true, it would be good to know why. To figure that out, the authors turned to the metabolome. The idea here is that your body is constantly breaking stuff down, taking all these proteins and chemicals and compounds that we ingest and turning them into metabolites. Using advanced measurement techniques, researchers can measure hundreds or even thousands of metabolites from a single blood sample. They provide information, obviously, about the food you eat and the drinks you drink, but what is really intriguing is that some metabolites are associated with better health and some with worse
In this study, researchers measured 168 individual metabolites. Eighty of them, nearly half, were significantly altered in people who drank more coffee.
This figure summarizes the findings, and yes, this is way too complicated.
But here’s how to interpret it. The inner ring shows you how certain metabolites are associated with cardiovascular disease. The outer rings show you how those metabolites are associated with coffee, tea, or caffeine. The interesting part is that the sections of the ring (outer rings and inner rings) are very different colors.
Like here.
What you see here is a fairly profound effect that coffee, tea, or caffeine intake has on metabolites of VLDL — bad cholesterol. The beverages lower it, and, of course, higher levels lead to cardiovascular disease. This means that this is a potential causal pathway from coffee intake to heart protection.
And that’s not the only one.
You see a similar relationship for saturated fatty acids. Higher levels lead to cardiovascular disease, and coffee intake lowers levels. The reverse works too: Lower levels of histidine (an amino acid) increase cardiovascular risk, and coffee seems to raise those levels.
Is this all too good to be true? It’s hard to say. The data on coffee’s benefits have been remarkably consistent. Still, I wouldn’t be a good doctor if I didn’t mention that clearly there is a difference between a cup of black coffee and a venti caramel Frappuccino.
Nevertheless, coffee remains firmly in my holy trinity of enjoyable things that are, for whatever reason, still good for you. So, when you’re having that second, or third, or maybe fourth cup of the day, you can take that to heart.
Dr. Wilson, associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Genitourinary Symptoms in Men: Canaries in the Coal Mine for Underlying Chronic Disease
At age 57, a senior scientific researcher in Santa Barbara, California, complained of chronic erectile dysfunction (ED) in what had been a sexually active marriage. “I just couldn’t get an erection, let alone sustain one. Apart from that, I maybe felt a bit tired but generally okay,” he said. Though seemingly well otherwise, 18 months later he was dead of a hereditary right-sided colon cancer.
While not all cases of ED are associated with a dire outcome, the genitourinary signals of ED and lower urinary tract symptoms (LUTS), especially nocturia, serve as sentinel indicators of the presence of, or risk factors for, serious chronic conditions. These commonly include cardiovascular disease (CVD), diabetes, and metabolic syndrome and are associated with obesity, depression, and obstructive sleep apnea.
Sometimes these serious conditions may stay under the radar until men seek help for ED or LUTS.
“We know that among men who had a heart attack, 50% had some degree of ED within 3 years of their cardiac event,” Sam Tafari, MBBS, of the Endocrine and Metabolic Unit at Royal Adelaide Hospital in Adelaide, South Australia, said in an interview.
That’s the bad news. The good news is that these two problems may specifically incentivize men to seek timely care for serious conditions they might otherwise not get, according to Dr. Tafari. And primary care doctors are ideally positioned to get men early multifaceted care. He recently coauthored a call to action on this issue in a review appearing in the Journal of Men’s Health.
In Dr. Tafari’s experience, most patients seeking urological care are unaware of the multiple conditions linked to ED and LUTS. “Many consider these to be due to issues like low testosterone, which actually make up a very small proportion of cases of ED,” he said. Aging, obesity, inactivity, smoking, alcohol abuse, and prescription and street drugs can also contribute to the development of ED.
In most affected men, ED is of vascular etiology, with endothelial dysfunction of the inner lining of blood vessels and smooth muscle the common denominator.
This dysfunction causes inadequate blood supply to both the coronary and the penile arteries, so ED and CVD are considered different manifestations of the same systemic disorder. Because the tumescence-controlling cavernosal vessels of the penis are considerably smaller, the same level of arteriopathy causes a more severe reduction in blood in the erectile tissue. As a result, ED often precedes CVD and presents an early opportunity to screen men for CVD.
As to the mechanisms behind LUTS, Peter N. Tsambarlis, MD, a urologist at Northwestern Medicine in Chicago, subscribes to the inflammation theory. “Suboptimal health issues such as high [blood] pressure, blood lipids, and blood glucose lead to chronic widespread inflammation, which makes the bladder less flexible as a storage vessel,” he explained. “It’s not able to stretch adequately overnight to hold the urine until morning.”
Ask Early, Ask Often
Jeffrey P. Weiss, MD, PhD, chair of the Department of Urology at SUNY Downstate Health Sciences University in Brooklyn, New York, has done research that uncovered a relationship between structural cardiac disease and nocturia. “So if you had to ask a patient a single question that would point to a global health issue, it would be ‘Do you have frequent nighttime urination,’ ” he said.
It’s never too soon to ask men about these symptoms, said Dr. Tsambarlis. The best time to raise issues of ED and LUTS is when a man enters primary care — regardless of age or absence of symptoms. “That way you have a baseline and can watch for changes and do early intervention as needed. Men don’t usually want to bring up sexual dysfunction or urinary health, but asking doesn’t need to dominate the visit,” he said.
Dr. Tafari recommends that primary care physicians adopt a targeted approach using ED and nocturia as entry points for engaging men in their healthcare. While acknowledging that primary care physicians have an ever-growing checklist of questions to ask patients and hardly need one more thing to screen for, he suggests asking two quick, and easy “before you go” genitourinary queries:
- Are you having trouble with erections or having sex?
- Are you getting up at night to pass urine more than once?
“The men really appreciate being asked,” he said. “But what worries me is all the men we don’t see who have these symptoms but don’t know they’re important, and no one is asking about them.”
Gideon Richards, MD, a urologist at the Northwell Health Physician Partners Smith Institute for Urology at Garden City, and director of Men’s Health, Central Region, for Northwell Health in New Hyde Park, both in New York, said erectile problems should not wait for specialty care. By the time men with ED are referred to urology, they may already have failed treatment with first-line phosphodiesterase 5 inhibitor therapy, he said. “A significant proportion will have arteriogenic erectile dysfunction, a measurable decrease in the amount of blood flow into the erectile bodies.”
Addressing the Issue
Addressing genitourinary-signaled issues has the double benefit of easing ED and LUTS and improving men’s health and longevity and may help narrow the worldwide gender gap in life expectancy. As a recent global analysis found, there’s a 5-year longevity disparity favoring women over men. Biology aside, men do not access healthcare as often as women, who consult their general practitioners regularly throughout their lifespan for multiple reasons, including reproductive care, and more screening programs are aimed at women.
Added Dr. Tsambarlis, “Men should know that losing weight and switching to a healthy lifestyle can improve sexual function about half as much as phosphodiesterase 5 inhibitors such as sildenafil [Viagra] or tadalafil [Cialis].”
“Many, however, would prefer just to take drugs rather than change their lifestyle and lose weight. There are certainly effective options available, but these are not uniformly effective,” said Dr. Weiss.
Dr. Tafari’s group is designing a short, simple, culturally acceptable screening tool for use in primary care practice and will monitor its impact on physician prescribing habits and overall men’s health outcomes.
Dr. Tafari received funding from the Hospital Research Foundation and Freemasons Centre for Male Health and Wellbeing in Adelaide, South Australia. Dr. Tafari, Dr. Tsambarlis, Dr. Weiss, and Dr. Richards had no relevant conflicts of interest to declare.
A version of this article appeared on Medscape.com.
At age 57, a senior scientific researcher in Santa Barbara, California, complained of chronic erectile dysfunction (ED) in what had been a sexually active marriage. “I just couldn’t get an erection, let alone sustain one. Apart from that, I maybe felt a bit tired but generally okay,” he said. Though seemingly well otherwise, 18 months later he was dead of a hereditary right-sided colon cancer.
While not all cases of ED are associated with a dire outcome, the genitourinary signals of ED and lower urinary tract symptoms (LUTS), especially nocturia, serve as sentinel indicators of the presence of, or risk factors for, serious chronic conditions. These commonly include cardiovascular disease (CVD), diabetes, and metabolic syndrome and are associated with obesity, depression, and obstructive sleep apnea.
Sometimes these serious conditions may stay under the radar until men seek help for ED or LUTS.
“We know that among men who had a heart attack, 50% had some degree of ED within 3 years of their cardiac event,” Sam Tafari, MBBS, of the Endocrine and Metabolic Unit at Royal Adelaide Hospital in Adelaide, South Australia, said in an interview.
That’s the bad news. The good news is that these two problems may specifically incentivize men to seek timely care for serious conditions they might otherwise not get, according to Dr. Tafari. And primary care doctors are ideally positioned to get men early multifaceted care. He recently coauthored a call to action on this issue in a review appearing in the Journal of Men’s Health.
In Dr. Tafari’s experience, most patients seeking urological care are unaware of the multiple conditions linked to ED and LUTS. “Many consider these to be due to issues like low testosterone, which actually make up a very small proportion of cases of ED,” he said. Aging, obesity, inactivity, smoking, alcohol abuse, and prescription and street drugs can also contribute to the development of ED.
In most affected men, ED is of vascular etiology, with endothelial dysfunction of the inner lining of blood vessels and smooth muscle the common denominator.
This dysfunction causes inadequate blood supply to both the coronary and the penile arteries, so ED and CVD are considered different manifestations of the same systemic disorder. Because the tumescence-controlling cavernosal vessels of the penis are considerably smaller, the same level of arteriopathy causes a more severe reduction in blood in the erectile tissue. As a result, ED often precedes CVD and presents an early opportunity to screen men for CVD.
As to the mechanisms behind LUTS, Peter N. Tsambarlis, MD, a urologist at Northwestern Medicine in Chicago, subscribes to the inflammation theory. “Suboptimal health issues such as high [blood] pressure, blood lipids, and blood glucose lead to chronic widespread inflammation, which makes the bladder less flexible as a storage vessel,” he explained. “It’s not able to stretch adequately overnight to hold the urine until morning.”
Ask Early, Ask Often
Jeffrey P. Weiss, MD, PhD, chair of the Department of Urology at SUNY Downstate Health Sciences University in Brooklyn, New York, has done research that uncovered a relationship between structural cardiac disease and nocturia. “So if you had to ask a patient a single question that would point to a global health issue, it would be ‘Do you have frequent nighttime urination,’ ” he said.
It’s never too soon to ask men about these symptoms, said Dr. Tsambarlis. The best time to raise issues of ED and LUTS is when a man enters primary care — regardless of age or absence of symptoms. “That way you have a baseline and can watch for changes and do early intervention as needed. Men don’t usually want to bring up sexual dysfunction or urinary health, but asking doesn’t need to dominate the visit,” he said.
Dr. Tafari recommends that primary care physicians adopt a targeted approach using ED and nocturia as entry points for engaging men in their healthcare. While acknowledging that primary care physicians have an ever-growing checklist of questions to ask patients and hardly need one more thing to screen for, he suggests asking two quick, and easy “before you go” genitourinary queries:
- Are you having trouble with erections or having sex?
- Are you getting up at night to pass urine more than once?
“The men really appreciate being asked,” he said. “But what worries me is all the men we don’t see who have these symptoms but don’t know they’re important, and no one is asking about them.”
Gideon Richards, MD, a urologist at the Northwell Health Physician Partners Smith Institute for Urology at Garden City, and director of Men’s Health, Central Region, for Northwell Health in New Hyde Park, both in New York, said erectile problems should not wait for specialty care. By the time men with ED are referred to urology, they may already have failed treatment with first-line phosphodiesterase 5 inhibitor therapy, he said. “A significant proportion will have arteriogenic erectile dysfunction, a measurable decrease in the amount of blood flow into the erectile bodies.”
Addressing the Issue
Addressing genitourinary-signaled issues has the double benefit of easing ED and LUTS and improving men’s health and longevity and may help narrow the worldwide gender gap in life expectancy. As a recent global analysis found, there’s a 5-year longevity disparity favoring women over men. Biology aside, men do not access healthcare as often as women, who consult their general practitioners regularly throughout their lifespan for multiple reasons, including reproductive care, and more screening programs are aimed at women.
Added Dr. Tsambarlis, “Men should know that losing weight and switching to a healthy lifestyle can improve sexual function about half as much as phosphodiesterase 5 inhibitors such as sildenafil [Viagra] or tadalafil [Cialis].”
“Many, however, would prefer just to take drugs rather than change their lifestyle and lose weight. There are certainly effective options available, but these are not uniformly effective,” said Dr. Weiss.
Dr. Tafari’s group is designing a short, simple, culturally acceptable screening tool for use in primary care practice and will monitor its impact on physician prescribing habits and overall men’s health outcomes.
Dr. Tafari received funding from the Hospital Research Foundation and Freemasons Centre for Male Health and Wellbeing in Adelaide, South Australia. Dr. Tafari, Dr. Tsambarlis, Dr. Weiss, and Dr. Richards had no relevant conflicts of interest to declare.
A version of this article appeared on Medscape.com.
At age 57, a senior scientific researcher in Santa Barbara, California, complained of chronic erectile dysfunction (ED) in what had been a sexually active marriage. “I just couldn’t get an erection, let alone sustain one. Apart from that, I maybe felt a bit tired but generally okay,” he said. Though seemingly well otherwise, 18 months later he was dead of a hereditary right-sided colon cancer.
While not all cases of ED are associated with a dire outcome, the genitourinary signals of ED and lower urinary tract symptoms (LUTS), especially nocturia, serve as sentinel indicators of the presence of, or risk factors for, serious chronic conditions. These commonly include cardiovascular disease (CVD), diabetes, and metabolic syndrome and are associated with obesity, depression, and obstructive sleep apnea.
Sometimes these serious conditions may stay under the radar until men seek help for ED or LUTS.
“We know that among men who had a heart attack, 50% had some degree of ED within 3 years of their cardiac event,” Sam Tafari, MBBS, of the Endocrine and Metabolic Unit at Royal Adelaide Hospital in Adelaide, South Australia, said in an interview.
That’s the bad news. The good news is that these two problems may specifically incentivize men to seek timely care for serious conditions they might otherwise not get, according to Dr. Tafari. And primary care doctors are ideally positioned to get men early multifaceted care. He recently coauthored a call to action on this issue in a review appearing in the Journal of Men’s Health.
In Dr. Tafari’s experience, most patients seeking urological care are unaware of the multiple conditions linked to ED and LUTS. “Many consider these to be due to issues like low testosterone, which actually make up a very small proportion of cases of ED,” he said. Aging, obesity, inactivity, smoking, alcohol abuse, and prescription and street drugs can also contribute to the development of ED.
In most affected men, ED is of vascular etiology, with endothelial dysfunction of the inner lining of blood vessels and smooth muscle the common denominator.
This dysfunction causes inadequate blood supply to both the coronary and the penile arteries, so ED and CVD are considered different manifestations of the same systemic disorder. Because the tumescence-controlling cavernosal vessels of the penis are considerably smaller, the same level of arteriopathy causes a more severe reduction in blood in the erectile tissue. As a result, ED often precedes CVD and presents an early opportunity to screen men for CVD.
As to the mechanisms behind LUTS, Peter N. Tsambarlis, MD, a urologist at Northwestern Medicine in Chicago, subscribes to the inflammation theory. “Suboptimal health issues such as high [blood] pressure, blood lipids, and blood glucose lead to chronic widespread inflammation, which makes the bladder less flexible as a storage vessel,” he explained. “It’s not able to stretch adequately overnight to hold the urine until morning.”
Ask Early, Ask Often
Jeffrey P. Weiss, MD, PhD, chair of the Department of Urology at SUNY Downstate Health Sciences University in Brooklyn, New York, has done research that uncovered a relationship between structural cardiac disease and nocturia. “So if you had to ask a patient a single question that would point to a global health issue, it would be ‘Do you have frequent nighttime urination,’ ” he said.
It’s never too soon to ask men about these symptoms, said Dr. Tsambarlis. The best time to raise issues of ED and LUTS is when a man enters primary care — regardless of age or absence of symptoms. “That way you have a baseline and can watch for changes and do early intervention as needed. Men don’t usually want to bring up sexual dysfunction or urinary health, but asking doesn’t need to dominate the visit,” he said.
Dr. Tafari recommends that primary care physicians adopt a targeted approach using ED and nocturia as entry points for engaging men in their healthcare. While acknowledging that primary care physicians have an ever-growing checklist of questions to ask patients and hardly need one more thing to screen for, he suggests asking two quick, and easy “before you go” genitourinary queries:
- Are you having trouble with erections or having sex?
- Are you getting up at night to pass urine more than once?
“The men really appreciate being asked,” he said. “But what worries me is all the men we don’t see who have these symptoms but don’t know they’re important, and no one is asking about them.”
Gideon Richards, MD, a urologist at the Northwell Health Physician Partners Smith Institute for Urology at Garden City, and director of Men’s Health, Central Region, for Northwell Health in New Hyde Park, both in New York, said erectile problems should not wait for specialty care. By the time men with ED are referred to urology, they may already have failed treatment with first-line phosphodiesterase 5 inhibitor therapy, he said. “A significant proportion will have arteriogenic erectile dysfunction, a measurable decrease in the amount of blood flow into the erectile bodies.”
Addressing the Issue
Addressing genitourinary-signaled issues has the double benefit of easing ED and LUTS and improving men’s health and longevity and may help narrow the worldwide gender gap in life expectancy. As a recent global analysis found, there’s a 5-year longevity disparity favoring women over men. Biology aside, men do not access healthcare as often as women, who consult their general practitioners regularly throughout their lifespan for multiple reasons, including reproductive care, and more screening programs are aimed at women.
Added Dr. Tsambarlis, “Men should know that losing weight and switching to a healthy lifestyle can improve sexual function about half as much as phosphodiesterase 5 inhibitors such as sildenafil [Viagra] or tadalafil [Cialis].”
“Many, however, would prefer just to take drugs rather than change their lifestyle and lose weight. There are certainly effective options available, but these are not uniformly effective,” said Dr. Weiss.
Dr. Tafari’s group is designing a short, simple, culturally acceptable screening tool for use in primary care practice and will monitor its impact on physician prescribing habits and overall men’s health outcomes.
Dr. Tafari received funding from the Hospital Research Foundation and Freemasons Centre for Male Health and Wellbeing in Adelaide, South Australia. Dr. Tafari, Dr. Tsambarlis, Dr. Weiss, and Dr. Richards had no relevant conflicts of interest to declare.
A version of this article appeared on Medscape.com.
A New Focus for Cushing Syndrome Screening in Obesity
TOPLINE:
METHODOLOGY:
- Obesity is a key clinical feature of Cushing syndrome and shares many overlapping characteristics. An ongoing debate continues about the need to screen patients with obesity for the rare endocrine disease, but phenotypes known as metabolically healthy or unhealthy obesity may help better define an at-risk population.
- To assess the prevalence of Cushing syndrome by metabolic health status, researchers conducted a retrospective study of 1008 patients with obesity (mean age, 40 years; 83% women; body mass index ≥ 30) seen at an endocrinology outpatient clinic in Turkey between December 2020 and June 2022.
- They screened patients for Cushing syndrome with an overnight dexamethasone suppression test (1 mg DST), an oral dexamethasone dose given at 11 PM followed by a fasting blood sample for cortisol measurement the next morning. A serum cortisol level < 1.8 mcg/dL indicated normal suppression.
- Patients were categorized into those with metabolically healthy obesity (n = 229) or metabolically unhealthy obesity (n = 779) based on the absence or presence of comorbidities such as diabetes, prediabetes, coronary artery disease, hypertension, or dyslipidemia.
TAKEAWAY:
- The overall prevalence of Cushing syndrome in the study cohort was 0.2%, with only two patients definitively diagnosed after more tests and the remaining 10 classified as having subclinical hypercortisolism.
- Cortisol levels following the 1 mg DST were higher in the metabolically unhealthy obesity group than in the metabolically healthy obesity group (P = .001).
- Among the 12 patients with unsuppressed levels of cortisol, 11 belonged to the metabolically unhealthy obesity group, indicating a strong association between metabolic health and the levels of cortisol.
- The test demonstrated a specificity of 99% and sensitivity of 100% for screening Cushing syndrome in patients with obesity.
IN PRACTICE:
“Screening all patients with obesity for CS [Cushing syndrome] without considering any associated metabolic conditions appears impractical and unnecessary in everyday clinical practice,” the authors wrote. “However, it may be more reasonable and applicable to selectively screen the patients with obesity having comorbidities such as DM [diabetes mellitus], hypertension, dyslipidemia, or coronary artery disease, which lead to a metabolically unhealthy phenotype, rather than all individuals with obesity,” they added.
SOURCE:
The study, led by Sema Hepsen, Ankara Etlik City Hospital, Department of Endocrinology and Metabolism, Ankara, Turkey, was published online in the International Journal of Obesity.
LIMITATIONS:
The single-center design of the study and inclusion of patients from a single racial group may limit the generalizability of the findings. The retrospective design prevented the retrieval of all relevant data on clinical features and fat distribution.
DISCLOSURES:
The study was supported by an open access funding provided by the Scientific and Technological Research Council of Türkiye. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Obesity is a key clinical feature of Cushing syndrome and shares many overlapping characteristics. An ongoing debate continues about the need to screen patients with obesity for the rare endocrine disease, but phenotypes known as metabolically healthy or unhealthy obesity may help better define an at-risk population.
- To assess the prevalence of Cushing syndrome by metabolic health status, researchers conducted a retrospective study of 1008 patients with obesity (mean age, 40 years; 83% women; body mass index ≥ 30) seen at an endocrinology outpatient clinic in Turkey between December 2020 and June 2022.
- They screened patients for Cushing syndrome with an overnight dexamethasone suppression test (1 mg DST), an oral dexamethasone dose given at 11 PM followed by a fasting blood sample for cortisol measurement the next morning. A serum cortisol level < 1.8 mcg/dL indicated normal suppression.
- Patients were categorized into those with metabolically healthy obesity (n = 229) or metabolically unhealthy obesity (n = 779) based on the absence or presence of comorbidities such as diabetes, prediabetes, coronary artery disease, hypertension, or dyslipidemia.
TAKEAWAY:
- The overall prevalence of Cushing syndrome in the study cohort was 0.2%, with only two patients definitively diagnosed after more tests and the remaining 10 classified as having subclinical hypercortisolism.
- Cortisol levels following the 1 mg DST were higher in the metabolically unhealthy obesity group than in the metabolically healthy obesity group (P = .001).
- Among the 12 patients with unsuppressed levels of cortisol, 11 belonged to the metabolically unhealthy obesity group, indicating a strong association between metabolic health and the levels of cortisol.
- The test demonstrated a specificity of 99% and sensitivity of 100% for screening Cushing syndrome in patients with obesity.
IN PRACTICE:
“Screening all patients with obesity for CS [Cushing syndrome] without considering any associated metabolic conditions appears impractical and unnecessary in everyday clinical practice,” the authors wrote. “However, it may be more reasonable and applicable to selectively screen the patients with obesity having comorbidities such as DM [diabetes mellitus], hypertension, dyslipidemia, or coronary artery disease, which lead to a metabolically unhealthy phenotype, rather than all individuals with obesity,” they added.
SOURCE:
The study, led by Sema Hepsen, Ankara Etlik City Hospital, Department of Endocrinology and Metabolism, Ankara, Turkey, was published online in the International Journal of Obesity.
LIMITATIONS:
The single-center design of the study and inclusion of patients from a single racial group may limit the generalizability of the findings. The retrospective design prevented the retrieval of all relevant data on clinical features and fat distribution.
DISCLOSURES:
The study was supported by an open access funding provided by the Scientific and Technological Research Council of Türkiye. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Obesity is a key clinical feature of Cushing syndrome and shares many overlapping characteristics. An ongoing debate continues about the need to screen patients with obesity for the rare endocrine disease, but phenotypes known as metabolically healthy or unhealthy obesity may help better define an at-risk population.
- To assess the prevalence of Cushing syndrome by metabolic health status, researchers conducted a retrospective study of 1008 patients with obesity (mean age, 40 years; 83% women; body mass index ≥ 30) seen at an endocrinology outpatient clinic in Turkey between December 2020 and June 2022.
- They screened patients for Cushing syndrome with an overnight dexamethasone suppression test (1 mg DST), an oral dexamethasone dose given at 11 PM followed by a fasting blood sample for cortisol measurement the next morning. A serum cortisol level < 1.8 mcg/dL indicated normal suppression.
- Patients were categorized into those with metabolically healthy obesity (n = 229) or metabolically unhealthy obesity (n = 779) based on the absence or presence of comorbidities such as diabetes, prediabetes, coronary artery disease, hypertension, or dyslipidemia.
TAKEAWAY:
- The overall prevalence of Cushing syndrome in the study cohort was 0.2%, with only two patients definitively diagnosed after more tests and the remaining 10 classified as having subclinical hypercortisolism.
- Cortisol levels following the 1 mg DST were higher in the metabolically unhealthy obesity group than in the metabolically healthy obesity group (P = .001).
- Among the 12 patients with unsuppressed levels of cortisol, 11 belonged to the metabolically unhealthy obesity group, indicating a strong association between metabolic health and the levels of cortisol.
- The test demonstrated a specificity of 99% and sensitivity of 100% for screening Cushing syndrome in patients with obesity.
IN PRACTICE:
“Screening all patients with obesity for CS [Cushing syndrome] without considering any associated metabolic conditions appears impractical and unnecessary in everyday clinical practice,” the authors wrote. “However, it may be more reasonable and applicable to selectively screen the patients with obesity having comorbidities such as DM [diabetes mellitus], hypertension, dyslipidemia, or coronary artery disease, which lead to a metabolically unhealthy phenotype, rather than all individuals with obesity,” they added.
SOURCE:
The study, led by Sema Hepsen, Ankara Etlik City Hospital, Department of Endocrinology and Metabolism, Ankara, Turkey, was published online in the International Journal of Obesity.
LIMITATIONS:
The single-center design of the study and inclusion of patients from a single racial group may limit the generalizability of the findings. The retrospective design prevented the retrieval of all relevant data on clinical features and fat distribution.
DISCLOSURES:
The study was supported by an open access funding provided by the Scientific and Technological Research Council of Türkiye. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Semaglutide Coverage Could Raise Medicare Costs by Billions
TOPLINE:
after Medicare allowed Part D plans to cover the drug for patients with a BMI ≥ 27 and a history of cardiovascular disease (CVD), regardless of their diabetes status.
METHODOLOGY:
- In March 2024, Medicare approved the coverage of semaglutide by Part D plans for patients with a high BMI and existing CVD, irrespective of their diabetes status. This decision follows the SELECT trial results, showing that semaglutide lowered the risk for cardiovascular events in some patients without diabetes.
- This study aimed to describe the Medicare beneficiaries most likely to be newly eligible for semaglutide treatment and estimated maximum costs to Medicare Part D.
- The researchers included 5111 individuals aged ≥ 65 years with self-reported Medicare enrollment in the National Health and Nutrition Examination Survey between 2011 and 2020, all of whom had a BMI ≥ 27 and were likely to benefit from semaglutide treatment.
- They evaluated the following potential definitions of established CVD that could be considered by the Part D plan: physician-provided diagnosis of myocardial infarction, stroke, coronary artery disease, or angina; a 10-year risk for atherosclerotic CVD between 7.5% and < 20.0%; a 10-year risk for atherosclerotic CVD of ≥ 20%; or fulfillment of any of the previous three criteria.
- Data on interview responses, medication use, clinical examinations, laboratory results, and diabetes diagnoses were obtained from the participants.
TAKEAWAY:
- This study found that 3.6 million individuals (14.2%) were deemed highly likely to qualify for semaglutide treatment for the first time, and broadening the criteria for established CVD could increase this number to 15.2 million individuals (60.9%).
- If all newly eligible beneficiaries were to receive semaglutide treatment, Medicare spending could increase by $34-$145 billion annually.
- Even with more conservative definitions of CVD and a significant portion of individuals not maintaining long-term adherence to semaglutide treatment, costs could still increase by $10 billion annually.
- Younger, generally healthier, female Medicare beneficiaries were still likely to remain ineligible for semaglutide treatment according to the coverage provided by Part D Medicare plans.
IN PRACTICE:
“Although approximately one in seven Medicare beneficiaries with elevated BMI is likely to be newly eligible for semaglutide, the majority will remain ineligible if a narrow definition of established CVD is used by Part D plans. Weight control has benefits for patients with elevated BMI, so the definition of established CVD used by Part D plans for coverage of semaglutide could have outsized public health implications,” the authors wrote.
SOURCE:
The study was led by Alexander Chaitoff, MD, MPH, Center for Healthcare Delivery Sciences, Department of Medicine, Brigham and Women’s Hospital, Boston. It was published online in Annals of Internal Medicine.
LIMITATIONS:
This analysis relied on self-reported cases of CVD. The study was also limited to only community-dwelling adults. It estimated maximum budgetary impacts but did not account for payment reforms introduced by the Inflation Reduction Act or for absolute contraindications to semaglutide.
DISCLOSURES:
This study did not disclose any sources of funding. Some authors declared receiving grants, serving as consultants, and having other ties with some institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
after Medicare allowed Part D plans to cover the drug for patients with a BMI ≥ 27 and a history of cardiovascular disease (CVD), regardless of their diabetes status.
METHODOLOGY:
- In March 2024, Medicare approved the coverage of semaglutide by Part D plans for patients with a high BMI and existing CVD, irrespective of their diabetes status. This decision follows the SELECT trial results, showing that semaglutide lowered the risk for cardiovascular events in some patients without diabetes.
- This study aimed to describe the Medicare beneficiaries most likely to be newly eligible for semaglutide treatment and estimated maximum costs to Medicare Part D.
- The researchers included 5111 individuals aged ≥ 65 years with self-reported Medicare enrollment in the National Health and Nutrition Examination Survey between 2011 and 2020, all of whom had a BMI ≥ 27 and were likely to benefit from semaglutide treatment.
- They evaluated the following potential definitions of established CVD that could be considered by the Part D plan: physician-provided diagnosis of myocardial infarction, stroke, coronary artery disease, or angina; a 10-year risk for atherosclerotic CVD between 7.5% and < 20.0%; a 10-year risk for atherosclerotic CVD of ≥ 20%; or fulfillment of any of the previous three criteria.
- Data on interview responses, medication use, clinical examinations, laboratory results, and diabetes diagnoses were obtained from the participants.
TAKEAWAY:
- This study found that 3.6 million individuals (14.2%) were deemed highly likely to qualify for semaglutide treatment for the first time, and broadening the criteria for established CVD could increase this number to 15.2 million individuals (60.9%).
- If all newly eligible beneficiaries were to receive semaglutide treatment, Medicare spending could increase by $34-$145 billion annually.
- Even with more conservative definitions of CVD and a significant portion of individuals not maintaining long-term adherence to semaglutide treatment, costs could still increase by $10 billion annually.
- Younger, generally healthier, female Medicare beneficiaries were still likely to remain ineligible for semaglutide treatment according to the coverage provided by Part D Medicare plans.
IN PRACTICE:
“Although approximately one in seven Medicare beneficiaries with elevated BMI is likely to be newly eligible for semaglutide, the majority will remain ineligible if a narrow definition of established CVD is used by Part D plans. Weight control has benefits for patients with elevated BMI, so the definition of established CVD used by Part D plans for coverage of semaglutide could have outsized public health implications,” the authors wrote.
SOURCE:
The study was led by Alexander Chaitoff, MD, MPH, Center for Healthcare Delivery Sciences, Department of Medicine, Brigham and Women’s Hospital, Boston. It was published online in Annals of Internal Medicine.
LIMITATIONS:
This analysis relied on self-reported cases of CVD. The study was also limited to only community-dwelling adults. It estimated maximum budgetary impacts but did not account for payment reforms introduced by the Inflation Reduction Act or for absolute contraindications to semaglutide.
DISCLOSURES:
This study did not disclose any sources of funding. Some authors declared receiving grants, serving as consultants, and having other ties with some institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
after Medicare allowed Part D plans to cover the drug for patients with a BMI ≥ 27 and a history of cardiovascular disease (CVD), regardless of their diabetes status.
METHODOLOGY:
- In March 2024, Medicare approved the coverage of semaglutide by Part D plans for patients with a high BMI and existing CVD, irrespective of their diabetes status. This decision follows the SELECT trial results, showing that semaglutide lowered the risk for cardiovascular events in some patients without diabetes.
- This study aimed to describe the Medicare beneficiaries most likely to be newly eligible for semaglutide treatment and estimated maximum costs to Medicare Part D.
- The researchers included 5111 individuals aged ≥ 65 years with self-reported Medicare enrollment in the National Health and Nutrition Examination Survey between 2011 and 2020, all of whom had a BMI ≥ 27 and were likely to benefit from semaglutide treatment.
- They evaluated the following potential definitions of established CVD that could be considered by the Part D plan: physician-provided diagnosis of myocardial infarction, stroke, coronary artery disease, or angina; a 10-year risk for atherosclerotic CVD between 7.5% and < 20.0%; a 10-year risk for atherosclerotic CVD of ≥ 20%; or fulfillment of any of the previous three criteria.
- Data on interview responses, medication use, clinical examinations, laboratory results, and diabetes diagnoses were obtained from the participants.
TAKEAWAY:
- This study found that 3.6 million individuals (14.2%) were deemed highly likely to qualify for semaglutide treatment for the first time, and broadening the criteria for established CVD could increase this number to 15.2 million individuals (60.9%).
- If all newly eligible beneficiaries were to receive semaglutide treatment, Medicare spending could increase by $34-$145 billion annually.
- Even with more conservative definitions of CVD and a significant portion of individuals not maintaining long-term adherence to semaglutide treatment, costs could still increase by $10 billion annually.
- Younger, generally healthier, female Medicare beneficiaries were still likely to remain ineligible for semaglutide treatment according to the coverage provided by Part D Medicare plans.
IN PRACTICE:
“Although approximately one in seven Medicare beneficiaries with elevated BMI is likely to be newly eligible for semaglutide, the majority will remain ineligible if a narrow definition of established CVD is used by Part D plans. Weight control has benefits for patients with elevated BMI, so the definition of established CVD used by Part D plans for coverage of semaglutide could have outsized public health implications,” the authors wrote.
SOURCE:
The study was led by Alexander Chaitoff, MD, MPH, Center for Healthcare Delivery Sciences, Department of Medicine, Brigham and Women’s Hospital, Boston. It was published online in Annals of Internal Medicine.
LIMITATIONS:
This analysis relied on self-reported cases of CVD. The study was also limited to only community-dwelling adults. It estimated maximum budgetary impacts but did not account for payment reforms introduced by the Inflation Reduction Act or for absolute contraindications to semaglutide.
DISCLOSURES:
This study did not disclose any sources of funding. Some authors declared receiving grants, serving as consultants, and having other ties with some institutions.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.