User login
Is It All in the Eye of the Beholder? Comparing Pulmonologists’ and Radiologists’ Performance
Lung cancer remains a leading cause of cancer-related deaths, and screening with low-dose computed tomography (LDCT) has the potential to decrease the mortality rate of patients by 20%.1 Most major cancer societies have issued lung cancer screening recommendations. For example, the National Comprehensive Cancer Network recommends annual LDCT scans for high-risk patients (those at moderate or low risk need not be screened). High-risk patients are aged between 55 and 74 years (the U.S. Preventive Services Task Force upper age limit is 80 years) and have a smoking history of ≥ 30 pack-years, or if no longer smoking, a quit date within the past 15 years. Although length of screening needed is unclear, it is advised that patients have annual LDCT scans until they have been smoke free for 15 years, develop limited life expectancy, or are no longer eligible for definitive treatment for lung cancer. A strong antismoking commitment and a multidisciplinary approach are of paramount importance.2,3
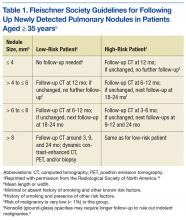
Fleischner Society criteria are the most established guidelines for risk-stratifying pulmonary nodules (Table 1). Nodules are stratified by size and change in size over a 2-year period. There is interest in evaluating change in volume as well, but techniques are still emerging and have not been universally adopted.4,5
Lung nodule screening likely will require significant involvement of radiologists and pulmonologists in the workup of patients with positive screens. Radiologists have demonstrated a fair amount of interobserver agreement with respect to diagnosis, but there are no data comparing pulmonologists with other pulmonologists or with radiologists.6-8 In addition, although health care professionals have access to validated models for predicting risk of malignancy, there is evidence they do not use them.9,10 This study was conducted to determine whether pulmonologists and radiologists experienced in thoracic abnormalities are consistent in accurately diagnosing malignant lung nodules and masses noted on CT scans.
Methods
After obtaining institutional review board approval for this study, the authors evaluated all the lung nodule or lung mass referrals that had been made to the University of Arkansas for Medical Sciences (UAMS) and Central Arkansas Veterans Healthcare System (CAVHS) interventional pulmonary clinics between March 2009 and March 2013. Of the 1,512 referrals made, 250 were randomly se
In each case, a pulmonologist and a radiologist reviewed the patient’s CT images from the first visit. Reviewers were asked to determine and document the single most likely diagnosis. Diagnoses were grouped into primary lung cancer, metastatic disease, lymphoma, infectious/inflammatory etiology, benign neoplasm, and other (eg, sarcoma). A lesion with a diagnostic biopsy and stability at 2 years was deemed benign. A lesion that was culture-positive or responded rapidly to antibacterial or antifungal therapy was deemed infectious/inflammatory. Lesions were grouped by size: group 1 (≤ 10 mm), group 2 (11-30 mm), group 3 (31-50 mm), group 4 (≥ 51 mm).
Statistical Analyses
Student t tests were used to compare means. Concordance of the pulmonary reviewers and FD was assessed with the κ coefficient. The concordance was also evaluated between the radiology reviewers and FD. These statistical analyses were performed with SAS Version 9.4 (SAS Institute). P values were interpreted using the sliding-scale approach of Mendenhall and colleagues: P < .01 (highly significant); .01 < P < .05 (statistically significant); .05 < P < .10 (trending toward significance); P > .10 (not significant).11
Results
Of the 250 patients selected for the study, 111 had the pertinent data available, along with a follow-up appointment > 2 years afterward at the center. The patients included 40 women and 71 men; 79 white patients, 29 black patients, and 3 patients of other races. Mean age was 58 years (range, 21-93 years).
Risk factors for malignancy were older age, larger lesion, and history of smoking. The malignancy rates for women and men were almost identical (53% and 54%, respectively), and the difference was not statistically significant (P = .40).
Diagnosis
Table 2 outlines the distribution of the reviewers’ diagnoses and the distribution of FD. Primary lung cancer was the dominant suspected diagnosis and accounted for 61%, 65%, and 54% of the cases reviewed by the pulmonologist, the radiologist, and FD, respectively. Metastatic disease was a distant second dominant diagnosis (17%, 15%, and 15%, respectively). There was no statistical difference between the reviews of the pulmonologist and radiologist, and the FD (P > .05).
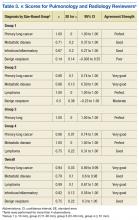
Table 3 lists the κ results for the strength of agreement between pulmonologist and radiologist. Agreement for primary lung cancer was very good: 0.94 (95% confidence interval [CI], 0.89-0.99). With respect to group 1, agreement was perfect: 1.0 (95% CI, 1.000-1.000). Benign neoplasm had the weakest agreement. There was no statistical difference between pulmonologist and radiologist determinations across size-based groups.Agreement between pulmonologist and FD was almost perfect. The major discrepancy between the sets of reviewers remained benign neoplasm and infectious/inflammatory etiology.
Of the 111 study patients, 68 (61%) and 72 (65%) were suspected of having primary lung cancer by pulmonologist and radiologist, respectively. However, only 60 (54%) actually had primary lung cancer; the differences were not statistically significant (P = .27 and .1, respectively). No cases were reclassified as primary lung cancer on final pathology.
Infectious/inflammatory etiologies did not always have positive cultures. Those with positive cultures included Streptococcus (S) viridans, Rhodococcus equi, Blastomyces dermatitidis, S constellatus, S anginosus, S intermedius, and Histoplasma capsulatum. Benign neoplasms included radiation injuries, benign fibrous tumor of the pleura, and hamartoma.
Pulmonologists and radiologists had identical high sensitivities for primary lung cancer: 1.0 (95% CI, 0.94-1.00). Specificities were 0.84 (95% CI, 0.77-0.84) for pulmonologists and 0.77(95% CI, 0.69-0.77) for radiologists, and the difference was not statistically significant (P = .28) (Table 4).
Discussion
Computed tomography scans are performed to evaluate a variety of diseases. An estimated 7 million CT scans are performed in the U.S. annually.6,12 As the National Lung Screening Trial recommendations are followed more routinely, almost 9 million people
Radiologists would understandably read most of these patients’ scans. However, patients referred to tertiary-care centers usually bring CT images with them; even scans performed at UAMS and CAVHS centers may not be read by a radiologist in time for an appointment. The result is that the clinic pulmonologist often must base decisions on a CT reading, but without the assistance of high-fidelity computer programs or a high-definition scan.5 These limitations indicate why it is important to know whether assessment by a pulmonologist compares favorably with assessment by a radiologist and with the eventual diagnosis.
The malignancy rate in the referred population is not insignificant. Halbert and colleagues found a 25% malignancy rate in their study,12 and the present study had an overall malignancy rate of 54%. The difference may be attributed to the possibility that the patients may have been prescreened prior to referral.
The reviewers overestimated the presence of malignant disease, though not to a level of statistical significance. About 88% of cases evaluated by a pulmonologist and 83% of cases evaluated by a radiologist were confirmed to be malignant. The reviewers’ sensitivity was perfect for all diagnoses except benign neoplasms, likely because these cases were classified malignant, thus increasing sensitivity but decreasing specificity.
This dynamic is important to understand, as it allows for a very high negative predictive value, which has real implications for resource management at VA hospitals, including CAVHS facility, where almost every CT scan with an abnormality is referred for pulmonologist consultation. In these cases, the radiologist not only lists the likely suspicion but includes a recommendation for follow-up or further workup based on Fleischner Society guidelines.4,14 The patient should be informed of findings as soon as the radiologist reads the CT scan, and a plan should be made on the basis of the recommendation. The patient should not have to unnecessarily wait—a potential source of anxiety—to see another specialist who would probably make the same recommendation.
Applying this study’s findings could improve workflow and the timing of CT scans. A patient should not be referred to a pulmonologist unless specifically recommended by a radiologist, thus decreasing the scheduling burden on the specialty clinic and allowing for appropriate patients to be scheduled at reasonable intervals. In addition, having only 1 person in charge of ordering CT scans could reduce the chance of duplicating orders and performing CT scans at inappropriate times.
Most important, these results should lead to more detailed physician–patient discussions about radiologic findings, hopefully alleviating any patient anxiety. A patient who still wants to see a specialist may, but with less stress that can accompany being told that there is “something abnormal” on the imaging and that the patient needs to see a lung doctor.
Limitations
This study had a few weaknesses. It was a small trial, and its data were collected retrospectively. In addition, generalizing its results may be difficult, as its reviewers had less than 5 years of training, and reviewers with more experience likely would be more accurate and have a higher rate of agreement.
Results could have been skewed by the study’s unusually large number of patients with malignant disease. Had the study been conducted with a larger population (patients at primary care offices), accuracy and agreement might have been lower.
Conclusion
This study answered its 2 questions. Although it is universally accepted that pulmonologists can review patients’ scans, to the authors’ knowledge this is the first study that asked, “Are pulmonologists as good as radiologists in reading CT scans?” The answer is yes. Also asked was, “Do pulmonologists’ and radiologists’ diagnoses predict the final path?” The reviewers’ were very accurate except in the case of benign neoplasms.
Experienced pulmonologists and radiologists are consistent in accurately diagnosing malignant lung nodules and lung masses noted on CT scans.
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
2. Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin. 2015;25(2):185-197.
3. Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services task force recommendation. Ann Intern Med. 2013;159(6):411-420.
4. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317.
5. Mehta HJ, Ravenel JG, Shaftman SR, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest. 2014;145(3):464-472.
6. Gierada DS, Pilgram TK, Ford M, et al. Lung cancer: interobserver agreement on interpretation of pulmonary findings at low-dose CT screening. Radiology. 2008;246(1):265-272.
7. McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology. 2006;239(2):514-520.
8. Bogot NR, Kazerooni EA, Kelly AM, Quint LE, Desjardins B, Nan B. Interobserver and intraobserver variability in the assessment of pulmonary nodule size on CT using film and computer display methods. Acad Radiol. 2005;12(8):948-956.
9. Schultz EM, Sanders GD, Trotter PR, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax. 2008;63(4):335-341.
10. Tanner NT, Aggarwal J, Gould MK, et al. Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest. 2015;148(6):1405-1414.
11. Mendenhall W, Beaver RJ, Beaver BM. Introduction to Probability and Statistics. 13th ed. Belmont, CA: Brooks/Cole, Cengage Learning; 2009.
12. Halbert CL, Madtes DK, Vaughan AE, et al. Expression of human alpha1-antitrypsin in mice and dogs following AAV6 vector-mediated gene transfer to the lungs. Mol Ther. 2010;18(6):1165-1172.
13. Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381-1385.
14. MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400.
Lung cancer remains a leading cause of cancer-related deaths, and screening with low-dose computed tomography (LDCT) has the potential to decrease the mortality rate of patients by 20%.1 Most major cancer societies have issued lung cancer screening recommendations. For example, the National Comprehensive Cancer Network recommends annual LDCT scans for high-risk patients (those at moderate or low risk need not be screened). High-risk patients are aged between 55 and 74 years (the U.S. Preventive Services Task Force upper age limit is 80 years) and have a smoking history of ≥ 30 pack-years, or if no longer smoking, a quit date within the past 15 years. Although length of screening needed is unclear, it is advised that patients have annual LDCT scans until they have been smoke free for 15 years, develop limited life expectancy, or are no longer eligible for definitive treatment for lung cancer. A strong antismoking commitment and a multidisciplinary approach are of paramount importance.2,3
Fleischner Society criteria are the most established guidelines for risk-stratifying pulmonary nodules (Table 1). Nodules are stratified by size and change in size over a 2-year period. There is interest in evaluating change in volume as well, but techniques are still emerging and have not been universally adopted.4,5
Lung nodule screening likely will require significant involvement of radiologists and pulmonologists in the workup of patients with positive screens. Radiologists have demonstrated a fair amount of interobserver agreement with respect to diagnosis, but there are no data comparing pulmonologists with other pulmonologists or with radiologists.6-8 In addition, although health care professionals have access to validated models for predicting risk of malignancy, there is evidence they do not use them.9,10 This study was conducted to determine whether pulmonologists and radiologists experienced in thoracic abnormalities are consistent in accurately diagnosing malignant lung nodules and masses noted on CT scans.
Methods
After obtaining institutional review board approval for this study, the authors evaluated all the lung nodule or lung mass referrals that had been made to the University of Arkansas for Medical Sciences (UAMS) and Central Arkansas Veterans Healthcare System (CAVHS) interventional pulmonary clinics between March 2009 and March 2013. Of the 1,512 referrals made, 250 were randomly se
In each case, a pulmonologist and a radiologist reviewed the patient’s CT images from the first visit. Reviewers were asked to determine and document the single most likely diagnosis. Diagnoses were grouped into primary lung cancer, metastatic disease, lymphoma, infectious/inflammatory etiology, benign neoplasm, and other (eg, sarcoma). A lesion with a diagnostic biopsy and stability at 2 years was deemed benign. A lesion that was culture-positive or responded rapidly to antibacterial or antifungal therapy was deemed infectious/inflammatory. Lesions were grouped by size: group 1 (≤ 10 mm), group 2 (11-30 mm), group 3 (31-50 mm), group 4 (≥ 51 mm).
Statistical Analyses
Student t tests were used to compare means. Concordance of the pulmonary reviewers and FD was assessed with the κ coefficient. The concordance was also evaluated between the radiology reviewers and FD. These statistical analyses were performed with SAS Version 9.4 (SAS Institute). P values were interpreted using the sliding-scale approach of Mendenhall and colleagues: P < .01 (highly significant); .01 < P < .05 (statistically significant); .05 < P < .10 (trending toward significance); P > .10 (not significant).11
Results
Of the 250 patients selected for the study, 111 had the pertinent data available, along with a follow-up appointment > 2 years afterward at the center. The patients included 40 women and 71 men; 79 white patients, 29 black patients, and 3 patients of other races. Mean age was 58 years (range, 21-93 years).
Risk factors for malignancy were older age, larger lesion, and history of smoking. The malignancy rates for women and men were almost identical (53% and 54%, respectively), and the difference was not statistically significant (P = .40).
Diagnosis
Table 2 outlines the distribution of the reviewers’ diagnoses and the distribution of FD. Primary lung cancer was the dominant suspected diagnosis and accounted for 61%, 65%, and 54% of the cases reviewed by the pulmonologist, the radiologist, and FD, respectively. Metastatic disease was a distant second dominant diagnosis (17%, 15%, and 15%, respectively). There was no statistical difference between the reviews of the pulmonologist and radiologist, and the FD (P > .05).
Table 3 lists the κ results for the strength of agreement between pulmonologist and radiologist. Agreement for primary lung cancer was very good: 0.94 (95% confidence interval [CI], 0.89-0.99). With respect to group 1, agreement was perfect: 1.0 (95% CI, 1.000-1.000). Benign neoplasm had the weakest agreement. There was no statistical difference between pulmonologist and radiologist determinations across size-based groups.Agreement between pulmonologist and FD was almost perfect. The major discrepancy between the sets of reviewers remained benign neoplasm and infectious/inflammatory etiology.
Of the 111 study patients, 68 (61%) and 72 (65%) were suspected of having primary lung cancer by pulmonologist and radiologist, respectively. However, only 60 (54%) actually had primary lung cancer; the differences were not statistically significant (P = .27 and .1, respectively). No cases were reclassified as primary lung cancer on final pathology.
Infectious/inflammatory etiologies did not always have positive cultures. Those with positive cultures included Streptococcus (S) viridans, Rhodococcus equi, Blastomyces dermatitidis, S constellatus, S anginosus, S intermedius, and Histoplasma capsulatum. Benign neoplasms included radiation injuries, benign fibrous tumor of the pleura, and hamartoma.
Pulmonologists and radiologists had identical high sensitivities for primary lung cancer: 1.0 (95% CI, 0.94-1.00). Specificities were 0.84 (95% CI, 0.77-0.84) for pulmonologists and 0.77(95% CI, 0.69-0.77) for radiologists, and the difference was not statistically significant (P = .28) (Table 4).
Discussion
Computed tomography scans are performed to evaluate a variety of diseases. An estimated 7 million CT scans are performed in the U.S. annually.6,12 As the National Lung Screening Trial recommendations are followed more routinely, almost 9 million people
Radiologists would understandably read most of these patients’ scans. However, patients referred to tertiary-care centers usually bring CT images with them; even scans performed at UAMS and CAVHS centers may not be read by a radiologist in time for an appointment. The result is that the clinic pulmonologist often must base decisions on a CT reading, but without the assistance of high-fidelity computer programs or a high-definition scan.5 These limitations indicate why it is important to know whether assessment by a pulmonologist compares favorably with assessment by a radiologist and with the eventual diagnosis.
The malignancy rate in the referred population is not insignificant. Halbert and colleagues found a 25% malignancy rate in their study,12 and the present study had an overall malignancy rate of 54%. The difference may be attributed to the possibility that the patients may have been prescreened prior to referral.
The reviewers overestimated the presence of malignant disease, though not to a level of statistical significance. About 88% of cases evaluated by a pulmonologist and 83% of cases evaluated by a radiologist were confirmed to be malignant. The reviewers’ sensitivity was perfect for all diagnoses except benign neoplasms, likely because these cases were classified malignant, thus increasing sensitivity but decreasing specificity.
This dynamic is important to understand, as it allows for a very high negative predictive value, which has real implications for resource management at VA hospitals, including CAVHS facility, where almost every CT scan with an abnormality is referred for pulmonologist consultation. In these cases, the radiologist not only lists the likely suspicion but includes a recommendation for follow-up or further workup based on Fleischner Society guidelines.4,14 The patient should be informed of findings as soon as the radiologist reads the CT scan, and a plan should be made on the basis of the recommendation. The patient should not have to unnecessarily wait—a potential source of anxiety—to see another specialist who would probably make the same recommendation.
Applying this study’s findings could improve workflow and the timing of CT scans. A patient should not be referred to a pulmonologist unless specifically recommended by a radiologist, thus decreasing the scheduling burden on the specialty clinic and allowing for appropriate patients to be scheduled at reasonable intervals. In addition, having only 1 person in charge of ordering CT scans could reduce the chance of duplicating orders and performing CT scans at inappropriate times.
Most important, these results should lead to more detailed physician–patient discussions about radiologic findings, hopefully alleviating any patient anxiety. A patient who still wants to see a specialist may, but with less stress that can accompany being told that there is “something abnormal” on the imaging and that the patient needs to see a lung doctor.
Limitations
This study had a few weaknesses. It was a small trial, and its data were collected retrospectively. In addition, generalizing its results may be difficult, as its reviewers had less than 5 years of training, and reviewers with more experience likely would be more accurate and have a higher rate of agreement.
Results could have been skewed by the study’s unusually large number of patients with malignant disease. Had the study been conducted with a larger population (patients at primary care offices), accuracy and agreement might have been lower.
Conclusion
This study answered its 2 questions. Although it is universally accepted that pulmonologists can review patients’ scans, to the authors’ knowledge this is the first study that asked, “Are pulmonologists as good as radiologists in reading CT scans?” The answer is yes. Also asked was, “Do pulmonologists’ and radiologists’ diagnoses predict the final path?” The reviewers’ were very accurate except in the case of benign neoplasms.
Experienced pulmonologists and radiologists are consistent in accurately diagnosing malignant lung nodules and lung masses noted on CT scans.
Lung cancer remains a leading cause of cancer-related deaths, and screening with low-dose computed tomography (LDCT) has the potential to decrease the mortality rate of patients by 20%.1 Most major cancer societies have issued lung cancer screening recommendations. For example, the National Comprehensive Cancer Network recommends annual LDCT scans for high-risk patients (those at moderate or low risk need not be screened). High-risk patients are aged between 55 and 74 years (the U.S. Preventive Services Task Force upper age limit is 80 years) and have a smoking history of ≥ 30 pack-years, or if no longer smoking, a quit date within the past 15 years. Although length of screening needed is unclear, it is advised that patients have annual LDCT scans until they have been smoke free for 15 years, develop limited life expectancy, or are no longer eligible for definitive treatment for lung cancer. A strong antismoking commitment and a multidisciplinary approach are of paramount importance.2,3
Fleischner Society criteria are the most established guidelines for risk-stratifying pulmonary nodules (Table 1). Nodules are stratified by size and change in size over a 2-year period. There is interest in evaluating change in volume as well, but techniques are still emerging and have not been universally adopted.4,5
Lung nodule screening likely will require significant involvement of radiologists and pulmonologists in the workup of patients with positive screens. Radiologists have demonstrated a fair amount of interobserver agreement with respect to diagnosis, but there are no data comparing pulmonologists with other pulmonologists or with radiologists.6-8 In addition, although health care professionals have access to validated models for predicting risk of malignancy, there is evidence they do not use them.9,10 This study was conducted to determine whether pulmonologists and radiologists experienced in thoracic abnormalities are consistent in accurately diagnosing malignant lung nodules and masses noted on CT scans.
Methods
After obtaining institutional review board approval for this study, the authors evaluated all the lung nodule or lung mass referrals that had been made to the University of Arkansas for Medical Sciences (UAMS) and Central Arkansas Veterans Healthcare System (CAVHS) interventional pulmonary clinics between March 2009 and March 2013. Of the 1,512 referrals made, 250 were randomly se
In each case, a pulmonologist and a radiologist reviewed the patient’s CT images from the first visit. Reviewers were asked to determine and document the single most likely diagnosis. Diagnoses were grouped into primary lung cancer, metastatic disease, lymphoma, infectious/inflammatory etiology, benign neoplasm, and other (eg, sarcoma). A lesion with a diagnostic biopsy and stability at 2 years was deemed benign. A lesion that was culture-positive or responded rapidly to antibacterial or antifungal therapy was deemed infectious/inflammatory. Lesions were grouped by size: group 1 (≤ 10 mm), group 2 (11-30 mm), group 3 (31-50 mm), group 4 (≥ 51 mm).
Statistical Analyses
Student t tests were used to compare means. Concordance of the pulmonary reviewers and FD was assessed with the κ coefficient. The concordance was also evaluated between the radiology reviewers and FD. These statistical analyses were performed with SAS Version 9.4 (SAS Institute). P values were interpreted using the sliding-scale approach of Mendenhall and colleagues: P < .01 (highly significant); .01 < P < .05 (statistically significant); .05 < P < .10 (trending toward significance); P > .10 (not significant).11
Results
Of the 250 patients selected for the study, 111 had the pertinent data available, along with a follow-up appointment > 2 years afterward at the center. The patients included 40 women and 71 men; 79 white patients, 29 black patients, and 3 patients of other races. Mean age was 58 years (range, 21-93 years).
Risk factors for malignancy were older age, larger lesion, and history of smoking. The malignancy rates for women and men were almost identical (53% and 54%, respectively), and the difference was not statistically significant (P = .40).
Diagnosis
Table 2 outlines the distribution of the reviewers’ diagnoses and the distribution of FD. Primary lung cancer was the dominant suspected diagnosis and accounted for 61%, 65%, and 54% of the cases reviewed by the pulmonologist, the radiologist, and FD, respectively. Metastatic disease was a distant second dominant diagnosis (17%, 15%, and 15%, respectively). There was no statistical difference between the reviews of the pulmonologist and radiologist, and the FD (P > .05).
Table 3 lists the κ results for the strength of agreement between pulmonologist and radiologist. Agreement for primary lung cancer was very good: 0.94 (95% confidence interval [CI], 0.89-0.99). With respect to group 1, agreement was perfect: 1.0 (95% CI, 1.000-1.000). Benign neoplasm had the weakest agreement. There was no statistical difference between pulmonologist and radiologist determinations across size-based groups.Agreement between pulmonologist and FD was almost perfect. The major discrepancy between the sets of reviewers remained benign neoplasm and infectious/inflammatory etiology.
Of the 111 study patients, 68 (61%) and 72 (65%) were suspected of having primary lung cancer by pulmonologist and radiologist, respectively. However, only 60 (54%) actually had primary lung cancer; the differences were not statistically significant (P = .27 and .1, respectively). No cases were reclassified as primary lung cancer on final pathology.
Infectious/inflammatory etiologies did not always have positive cultures. Those with positive cultures included Streptococcus (S) viridans, Rhodococcus equi, Blastomyces dermatitidis, S constellatus, S anginosus, S intermedius, and Histoplasma capsulatum. Benign neoplasms included radiation injuries, benign fibrous tumor of the pleura, and hamartoma.
Pulmonologists and radiologists had identical high sensitivities for primary lung cancer: 1.0 (95% CI, 0.94-1.00). Specificities were 0.84 (95% CI, 0.77-0.84) for pulmonologists and 0.77(95% CI, 0.69-0.77) for radiologists, and the difference was not statistically significant (P = .28) (Table 4).
Discussion
Computed tomography scans are performed to evaluate a variety of diseases. An estimated 7 million CT scans are performed in the U.S. annually.6,12 As the National Lung Screening Trial recommendations are followed more routinely, almost 9 million people
Radiologists would understandably read most of these patients’ scans. However, patients referred to tertiary-care centers usually bring CT images with them; even scans performed at UAMS and CAVHS centers may not be read by a radiologist in time for an appointment. The result is that the clinic pulmonologist often must base decisions on a CT reading, but without the assistance of high-fidelity computer programs or a high-definition scan.5 These limitations indicate why it is important to know whether assessment by a pulmonologist compares favorably with assessment by a radiologist and with the eventual diagnosis.
The malignancy rate in the referred population is not insignificant. Halbert and colleagues found a 25% malignancy rate in their study,12 and the present study had an overall malignancy rate of 54%. The difference may be attributed to the possibility that the patients may have been prescreened prior to referral.
The reviewers overestimated the presence of malignant disease, though not to a level of statistical significance. About 88% of cases evaluated by a pulmonologist and 83% of cases evaluated by a radiologist were confirmed to be malignant. The reviewers’ sensitivity was perfect for all diagnoses except benign neoplasms, likely because these cases were classified malignant, thus increasing sensitivity but decreasing specificity.
This dynamic is important to understand, as it allows for a very high negative predictive value, which has real implications for resource management at VA hospitals, including CAVHS facility, where almost every CT scan with an abnormality is referred for pulmonologist consultation. In these cases, the radiologist not only lists the likely suspicion but includes a recommendation for follow-up or further workup based on Fleischner Society guidelines.4,14 The patient should be informed of findings as soon as the radiologist reads the CT scan, and a plan should be made on the basis of the recommendation. The patient should not have to unnecessarily wait—a potential source of anxiety—to see another specialist who would probably make the same recommendation.
Applying this study’s findings could improve workflow and the timing of CT scans. A patient should not be referred to a pulmonologist unless specifically recommended by a radiologist, thus decreasing the scheduling burden on the specialty clinic and allowing for appropriate patients to be scheduled at reasonable intervals. In addition, having only 1 person in charge of ordering CT scans could reduce the chance of duplicating orders and performing CT scans at inappropriate times.
Most important, these results should lead to more detailed physician–patient discussions about radiologic findings, hopefully alleviating any patient anxiety. A patient who still wants to see a specialist may, but with less stress that can accompany being told that there is “something abnormal” on the imaging and that the patient needs to see a lung doctor.
Limitations
This study had a few weaknesses. It was a small trial, and its data were collected retrospectively. In addition, generalizing its results may be difficult, as its reviewers had less than 5 years of training, and reviewers with more experience likely would be more accurate and have a higher rate of agreement.
Results could have been skewed by the study’s unusually large number of patients with malignant disease. Had the study been conducted with a larger population (patients at primary care offices), accuracy and agreement might have been lower.
Conclusion
This study answered its 2 questions. Although it is universally accepted that pulmonologists can review patients’ scans, to the authors’ knowledge this is the first study that asked, “Are pulmonologists as good as radiologists in reading CT scans?” The answer is yes. Also asked was, “Do pulmonologists’ and radiologists’ diagnoses predict the final path?” The reviewers’ were very accurate except in the case of benign neoplasms.
Experienced pulmonologists and radiologists are consistent in accurately diagnosing malignant lung nodules and lung masses noted on CT scans.
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
2. Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin. 2015;25(2):185-197.
3. Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services task force recommendation. Ann Intern Med. 2013;159(6):411-420.
4. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317.
5. Mehta HJ, Ravenel JG, Shaftman SR, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest. 2014;145(3):464-472.
6. Gierada DS, Pilgram TK, Ford M, et al. Lung cancer: interobserver agreement on interpretation of pulmonary findings at low-dose CT screening. Radiology. 2008;246(1):265-272.
7. McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology. 2006;239(2):514-520.
8. Bogot NR, Kazerooni EA, Kelly AM, Quint LE, Desjardins B, Nan B. Interobserver and intraobserver variability in the assessment of pulmonary nodule size on CT using film and computer display methods. Acad Radiol. 2005;12(8):948-956.
9. Schultz EM, Sanders GD, Trotter PR, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax. 2008;63(4):335-341.
10. Tanner NT, Aggarwal J, Gould MK, et al. Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest. 2015;148(6):1405-1414.
11. Mendenhall W, Beaver RJ, Beaver BM. Introduction to Probability and Statistics. 13th ed. Belmont, CA: Brooks/Cole, Cengage Learning; 2009.
12. Halbert CL, Madtes DK, Vaughan AE, et al. Expression of human alpha1-antitrypsin in mice and dogs following AAV6 vector-mediated gene transfer to the lungs. Mol Ther. 2010;18(6):1165-1172.
13. Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381-1385.
14. MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400.
1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
2. Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin. 2015;25(2):185-197.
3. Humphrey LL, Deffebach M, Pappas M, et al. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services task force recommendation. Ann Intern Med. 2013;159(6):411-420.
4. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317.
5. Mehta HJ, Ravenel JG, Shaftman SR, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest. 2014;145(3):464-472.
6. Gierada DS, Pilgram TK, Ford M, et al. Lung cancer: interobserver agreement on interpretation of pulmonary findings at low-dose CT screening. Radiology. 2008;246(1):265-272.
7. McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology. 2006;239(2):514-520.
8. Bogot NR, Kazerooni EA, Kelly AM, Quint LE, Desjardins B, Nan B. Interobserver and intraobserver variability in the assessment of pulmonary nodule size on CT using film and computer display methods. Acad Radiol. 2005;12(8):948-956.
9. Schultz EM, Sanders GD, Trotter PR, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax. 2008;63(4):335-341.
10. Tanner NT, Aggarwal J, Gould MK, et al. Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest. 2015;148(6):1405-1414.
11. Mendenhall W, Beaver RJ, Beaver BM. Introduction to Probability and Statistics. 13th ed. Belmont, CA: Brooks/Cole, Cengage Learning; 2009.
12. Halbert CL, Madtes DK, Vaughan AE, et al. Expression of human alpha1-antitrypsin in mice and dogs following AAV6 vector-mediated gene transfer to the lungs. Mol Ther. 2010;18(6):1165-1172.
13. Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381-1385.
14. MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400.
Low caregiver self-care linked with depression, anxiety
There is increased anxiety and depression among family caregivers who do not take care of themselves, according to a study to be presented at the 2016 ASCO Palliative Care in Oncology Symposium.
Nearly a quarter of 294 caregivers of Medicare patients with advanced cancer reported high depression scores (23%) and 34% reported borderline or high anxiety scores. Worse caregiver anxiety, depression, and mental health–related quality of life scores were significantly associated with lower scores in every self-care measure (P less than .05 for all). Lower self-care behavior scores were associated with longer durations, higher hours, and more days/week of caregiving and with fair or poor patient health.
The cross-sectional survey was conducted in community settings of eight cancer centers in Alabama, Florida, and Tennessee. The family caregivers of Medicare beneficiaries diagnosed with pancreatic, lung, brain, ovarian, head & neck, hematologic, or stage IV cancer completed measures of self-care behaviors, including health responsibility, physical activity, nutrition, spiritual growth, interpersonal relations, stress management, and sleep. Caregivers averaged 66 years and were mostly female (72.8%), white (91.2%), Protestant (76.2%), retired (54.4%), and patients’ spouse/partner (60.2%). Approximately half were rural dwellers (46.9%) and had incomes less than $50,000 (53.8%). The majority provided support 6-7 days per week (71%) for greater than 1 year (68%).
“This research serves as an important call to action for the oncology community to implement support networks and services that care for the caregiver,” ASCO representative Andrew Epstein, MD, of Memorial Sloan Kettering Cancer Center, New York, said in a written statement ahead of the symposium.
“We hope our research rallies the oncology palliative care communities to develop assessment tools and services that support caregivers,” lead author James Nicholas Dionne-Odom, PhD, RN, of the University of Alabama at Birmingham, said in the statement.
On Twitter @jessnicolecraig
There is increased anxiety and depression among family caregivers who do not take care of themselves, according to a study to be presented at the 2016 ASCO Palliative Care in Oncology Symposium.
Nearly a quarter of 294 caregivers of Medicare patients with advanced cancer reported high depression scores (23%) and 34% reported borderline or high anxiety scores. Worse caregiver anxiety, depression, and mental health–related quality of life scores were significantly associated with lower scores in every self-care measure (P less than .05 for all). Lower self-care behavior scores were associated with longer durations, higher hours, and more days/week of caregiving and with fair or poor patient health.
The cross-sectional survey was conducted in community settings of eight cancer centers in Alabama, Florida, and Tennessee. The family caregivers of Medicare beneficiaries diagnosed with pancreatic, lung, brain, ovarian, head & neck, hematologic, or stage IV cancer completed measures of self-care behaviors, including health responsibility, physical activity, nutrition, spiritual growth, interpersonal relations, stress management, and sleep. Caregivers averaged 66 years and were mostly female (72.8%), white (91.2%), Protestant (76.2%), retired (54.4%), and patients’ spouse/partner (60.2%). Approximately half were rural dwellers (46.9%) and had incomes less than $50,000 (53.8%). The majority provided support 6-7 days per week (71%) for greater than 1 year (68%).
“This research serves as an important call to action for the oncology community to implement support networks and services that care for the caregiver,” ASCO representative Andrew Epstein, MD, of Memorial Sloan Kettering Cancer Center, New York, said in a written statement ahead of the symposium.
“We hope our research rallies the oncology palliative care communities to develop assessment tools and services that support caregivers,” lead author James Nicholas Dionne-Odom, PhD, RN, of the University of Alabama at Birmingham, said in the statement.
On Twitter @jessnicolecraig
There is increased anxiety and depression among family caregivers who do not take care of themselves, according to a study to be presented at the 2016 ASCO Palliative Care in Oncology Symposium.
Nearly a quarter of 294 caregivers of Medicare patients with advanced cancer reported high depression scores (23%) and 34% reported borderline or high anxiety scores. Worse caregiver anxiety, depression, and mental health–related quality of life scores were significantly associated with lower scores in every self-care measure (P less than .05 for all). Lower self-care behavior scores were associated with longer durations, higher hours, and more days/week of caregiving and with fair or poor patient health.
The cross-sectional survey was conducted in community settings of eight cancer centers in Alabama, Florida, and Tennessee. The family caregivers of Medicare beneficiaries diagnosed with pancreatic, lung, brain, ovarian, head & neck, hematologic, or stage IV cancer completed measures of self-care behaviors, including health responsibility, physical activity, nutrition, spiritual growth, interpersonal relations, stress management, and sleep. Caregivers averaged 66 years and were mostly female (72.8%), white (91.2%), Protestant (76.2%), retired (54.4%), and patients’ spouse/partner (60.2%). Approximately half were rural dwellers (46.9%) and had incomes less than $50,000 (53.8%). The majority provided support 6-7 days per week (71%) for greater than 1 year (68%).
“This research serves as an important call to action for the oncology community to implement support networks and services that care for the caregiver,” ASCO representative Andrew Epstein, MD, of Memorial Sloan Kettering Cancer Center, New York, said in a written statement ahead of the symposium.
“We hope our research rallies the oncology palliative care communities to develop assessment tools and services that support caregivers,” lead author James Nicholas Dionne-Odom, PhD, RN, of the University of Alabama at Birmingham, said in the statement.
On Twitter @jessnicolecraig
FROM THE 2016 ASCO PALLIATIVE CARE IN ONCOLOGY SYMPOSIUM
Key clinical point: There is increased anxiety and depression among family caregivers who do not take care of themselves.
Major finding: Worse caregiver anxiety, depression, and mental health–related quality of life scores were significantly associated with lower scores in every self-care measure (P less than .05 for all).
Data source: A multistate and cross-sectional survey of 294 family caregivers.
Disclosures: The University of Alabama at Birmingham funded the study. One investigator reported receiving financial compensation and honoraria from Medscape, Carevive Systems, and PackHealth.
New anticancer drugs linked to increased costs, life expectancy
New anticancer drugs are often expensive and have been accompanied by large increases in the cost of medical treatment, but they also are associated with gains in life expectancy, according to an analysis of Medicare data published online.
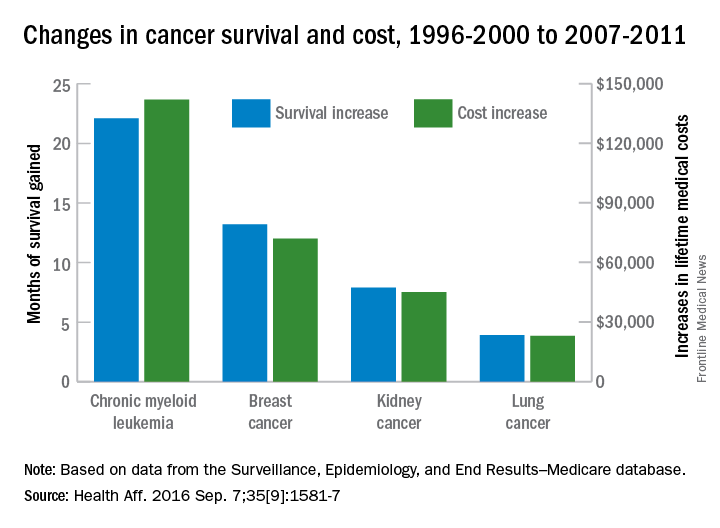
Investigators looked at four different types of cancer – breast, kidney, lung, and chronic myeloid leukemia (CML) – over two time periods: 1996-2000 and 2007-2011. Patients treated for CML during 2007-2011 had the largest increases in both average lifetime medical cost ($142,000) and months of life gained (22.1) over those treated during 1996-2000, reported David H. Howard, PhD, of Emory University, Atlanta, and his associates.
Breast cancer patients had the next-largest increases: 13.2 months of life expectancy and $72,000 in lifetime medical cost for those who received physician-administered intravenous drugs. For breast cancer patients who received only oral drugs, the increases were 2 months of life and $9,000 in lifetime cost, they noted.
Patients with kidney cancer had an average life-expectancy increase of 7.9 months and a cost increase of $45,000, but those estimates don’t fully reflect the effect of several oral drugs that were introduced after 2007 but did not come into widespread use during the entire study period, Dr. Howard and his associates noted (Health Aff. 2016 Sep 7;35[9]:1581-7).
Lung cancer patients experienced the smallest changes between the two time periods, with an increase in life expectancy of 3.9 months for those who received physician-administered anticancer drugs and a lifetime medical cost increase of $23,000. Patients with lung cancer who did not receive such drugs had increases of 0.7 months of life expectancy and $4,000 in lifetime medical costs.
The researchers used data from the Surveillance, Epidemiology, and End Results–Medicare database, and all costs are adjusted to 2012 dollars. Data collection was supported by the California Department of Health and funding for the study was provided by Pfizer. Three of Dr. Howard’s five coinvestigators are Pfizer employees.
New anticancer drugs are often expensive and have been accompanied by large increases in the cost of medical treatment, but they also are associated with gains in life expectancy, according to an analysis of Medicare data published online.
Investigators looked at four different types of cancer – breast, kidney, lung, and chronic myeloid leukemia (CML) – over two time periods: 1996-2000 and 2007-2011. Patients treated for CML during 2007-2011 had the largest increases in both average lifetime medical cost ($142,000) and months of life gained (22.1) over those treated during 1996-2000, reported David H. Howard, PhD, of Emory University, Atlanta, and his associates.
Breast cancer patients had the next-largest increases: 13.2 months of life expectancy and $72,000 in lifetime medical cost for those who received physician-administered intravenous drugs. For breast cancer patients who received only oral drugs, the increases were 2 months of life and $9,000 in lifetime cost, they noted.
Patients with kidney cancer had an average life-expectancy increase of 7.9 months and a cost increase of $45,000, but those estimates don’t fully reflect the effect of several oral drugs that were introduced after 2007 but did not come into widespread use during the entire study period, Dr. Howard and his associates noted (Health Aff. 2016 Sep 7;35[9]:1581-7).
Lung cancer patients experienced the smallest changes between the two time periods, with an increase in life expectancy of 3.9 months for those who received physician-administered anticancer drugs and a lifetime medical cost increase of $23,000. Patients with lung cancer who did not receive such drugs had increases of 0.7 months of life expectancy and $4,000 in lifetime medical costs.
The researchers used data from the Surveillance, Epidemiology, and End Results–Medicare database, and all costs are adjusted to 2012 dollars. Data collection was supported by the California Department of Health and funding for the study was provided by Pfizer. Three of Dr. Howard’s five coinvestigators are Pfizer employees.
New anticancer drugs are often expensive and have been accompanied by large increases in the cost of medical treatment, but they also are associated with gains in life expectancy, according to an analysis of Medicare data published online.
Investigators looked at four different types of cancer – breast, kidney, lung, and chronic myeloid leukemia (CML) – over two time periods: 1996-2000 and 2007-2011. Patients treated for CML during 2007-2011 had the largest increases in both average lifetime medical cost ($142,000) and months of life gained (22.1) over those treated during 1996-2000, reported David H. Howard, PhD, of Emory University, Atlanta, and his associates.
Breast cancer patients had the next-largest increases: 13.2 months of life expectancy and $72,000 in lifetime medical cost for those who received physician-administered intravenous drugs. For breast cancer patients who received only oral drugs, the increases were 2 months of life and $9,000 in lifetime cost, they noted.
Patients with kidney cancer had an average life-expectancy increase of 7.9 months and a cost increase of $45,000, but those estimates don’t fully reflect the effect of several oral drugs that were introduced after 2007 but did not come into widespread use during the entire study period, Dr. Howard and his associates noted (Health Aff. 2016 Sep 7;35[9]:1581-7).
Lung cancer patients experienced the smallest changes between the two time periods, with an increase in life expectancy of 3.9 months for those who received physician-administered anticancer drugs and a lifetime medical cost increase of $23,000. Patients with lung cancer who did not receive such drugs had increases of 0.7 months of life expectancy and $4,000 in lifetime medical costs.
The researchers used data from the Surveillance, Epidemiology, and End Results–Medicare database, and all costs are adjusted to 2012 dollars. Data collection was supported by the California Department of Health and funding for the study was provided by Pfizer. Three of Dr. Howard’s five coinvestigators are Pfizer employees.
FROM HEALTH AFFAIRS
Whole brain radiotherapy not beneficial for NSCLC metastasis
Whole brain radiotherapy, a standard treatment for patients with metastatic non–small-cell lung cancer, provided no clinical benefit in a noninferiority trial specifically designed to assess both patient survival and quality of life.
The findings were published online Sept. 4 in the Lancet.
Whole brain radiotherapy, with or without concomitant steroid treatment, has been widely used for decades in that patient population, even though no sufficiently powered, definitive studies support the approach. It is likely that patients and clinicians alike continue to embrace it because of the absence of alternative treatment options.
The Quality of Life After Treatment for Brain Metastases (QUARTZ) trial was intended to assess whether any improvement in survival offered by whole brain radiotherapy is balanced by deterioration in quality of life, said Paula Mulvenna, MBBS, of the Northern Center for Cancer Care, Newcastle (England) Hospitals, and her associates (Lancet 2016 Sep 4. doi: 10.1016/S0140-6736(16)30825-X).
QUARTZ involved 538 adults seen during a 7-year period who had NSCLC with brain metastases and who were not suited for either brain surgery or stereotactic radiotherapy. The median age was 66 years (range, 35-85 years), and 38% had a Karnofsky Performance Status score of less than 70.
The participants were randomly assigned to receive either optimal supportive care plus whole brain radiotherapy (269 patients) or optimal supportive care alone (269 patients) at 69 U.K. and 3 Australian medical centers. They reported on 20 symptoms and adverse effects, as well as health-related quality of life, approximately once per week.
The primary outcome measure – quality-adjusted life-years (QALY), which combines overall survival and quality of life – was 46.4 days with radiotherapy and 41.7 days without it.
Symptoms, adverse effects, and quality of life (QOL) were similar between the two study groups at 4 weeks, except that the radiotherapy group reported more moderate or severe episodes of drowsiness, hair loss, nausea, and dry or itchy scalp. The number and severity of serious adverse events were similar through 12 weeks of follow-up.
The percentage of patients whose QOL was either maintained or improved over time was similar between the two groups at 4 weeks (54% vs. 57%), 8 weeks (44% vs. 51%), and 12 weeks (44% vs. 49%). Changes in Karnofsky scores also were similar.
The study refuted the widely held belief that whole brain radiotherapy allows patients to reduce or discontinue steroid treatment, averting the associated adverse effects. Steroid doses were not significantly different between the two study groups through the first 8 weeks of treatment, which “challenges the dogma that whole brain radiotherapy can be seen as a steroid-sparing modality,” the investigators said.
Taken together, the findings “suggest that whole brain radiotherapy can be omitted and patients treated with optimal supportive care alone, without an important reduction in either overall survival or quality of life,” Dr. Mulvenna and her associates said.
The approximately 5-day difference between the two study groups in median overall survival highlights both the limited benefit offered by radiotherapy and the poor prognosis of this patient population, the researchers added.
Whole brain radiotherapy did offer a small survival benefit to the youngest patients who had good performance status and a “controlled” primary NSCLC. “For all other groups, [it] does not significantly affect QALY or overall survival,” they said.
Cancer Research U.K., the Medical Research Council in the U.K., the Trans Tasman Radiation Oncology Group, and the National Health and Medical Research Council Australia supported the study. Dr. Mulvenna and her associates reported having no relevant financial disclosures.
Managing brain metastases from NSCLC is a challenge, because the lesions may well produce life-threatening symptoms and serious impairment, which could be ameliorated with whole brain radiotherapy.
This is a large and well designed trial, but it was limited in that the maximal benefit of radiotherapy is believed to occur 6 weeks after the end of treatment. Given that median overall survival was only 8 weeks and considering the time it took to deliver the treatment, approximately half of the patients in this study died before an optimal assessment of symptoms could be done.
This might also explain why radiotherapy didn’t have an effect on steroid use in this study. Many patients didn’t live long enough for radiotherapy’s steroid-sparing effect to be observed.
Cécile Le Pechoux, MD, is in the department of radiation oncology at Gustave Roussy Cancer Campus in Villejuif, France. She and her associates reported having no relevant financial disclosures. They made these remarks in a comment accompanying the report on the QUARTZ trial (Lancet 2016 Sep 4. doi: 10.1016/S0140-6736[16]31391-5).
Managing brain metastases from NSCLC is a challenge, because the lesions may well produce life-threatening symptoms and serious impairment, which could be ameliorated with whole brain radiotherapy.
This is a large and well designed trial, but it was limited in that the maximal benefit of radiotherapy is believed to occur 6 weeks after the end of treatment. Given that median overall survival was only 8 weeks and considering the time it took to deliver the treatment, approximately half of the patients in this study died before an optimal assessment of symptoms could be done.
This might also explain why radiotherapy didn’t have an effect on steroid use in this study. Many patients didn’t live long enough for radiotherapy’s steroid-sparing effect to be observed.
Cécile Le Pechoux, MD, is in the department of radiation oncology at Gustave Roussy Cancer Campus in Villejuif, France. She and her associates reported having no relevant financial disclosures. They made these remarks in a comment accompanying the report on the QUARTZ trial (Lancet 2016 Sep 4. doi: 10.1016/S0140-6736[16]31391-5).
Managing brain metastases from NSCLC is a challenge, because the lesions may well produce life-threatening symptoms and serious impairment, which could be ameliorated with whole brain radiotherapy.
This is a large and well designed trial, but it was limited in that the maximal benefit of radiotherapy is believed to occur 6 weeks after the end of treatment. Given that median overall survival was only 8 weeks and considering the time it took to deliver the treatment, approximately half of the patients in this study died before an optimal assessment of symptoms could be done.
This might also explain why radiotherapy didn’t have an effect on steroid use in this study. Many patients didn’t live long enough for radiotherapy’s steroid-sparing effect to be observed.
Cécile Le Pechoux, MD, is in the department of radiation oncology at Gustave Roussy Cancer Campus in Villejuif, France. She and her associates reported having no relevant financial disclosures. They made these remarks in a comment accompanying the report on the QUARTZ trial (Lancet 2016 Sep 4. doi: 10.1016/S0140-6736[16]31391-5).
Whole brain radiotherapy, a standard treatment for patients with metastatic non–small-cell lung cancer, provided no clinical benefit in a noninferiority trial specifically designed to assess both patient survival and quality of life.
The findings were published online Sept. 4 in the Lancet.
Whole brain radiotherapy, with or without concomitant steroid treatment, has been widely used for decades in that patient population, even though no sufficiently powered, definitive studies support the approach. It is likely that patients and clinicians alike continue to embrace it because of the absence of alternative treatment options.
The Quality of Life After Treatment for Brain Metastases (QUARTZ) trial was intended to assess whether any improvement in survival offered by whole brain radiotherapy is balanced by deterioration in quality of life, said Paula Mulvenna, MBBS, of the Northern Center for Cancer Care, Newcastle (England) Hospitals, and her associates (Lancet 2016 Sep 4. doi: 10.1016/S0140-6736(16)30825-X).
QUARTZ involved 538 adults seen during a 7-year period who had NSCLC with brain metastases and who were not suited for either brain surgery or stereotactic radiotherapy. The median age was 66 years (range, 35-85 years), and 38% had a Karnofsky Performance Status score of less than 70.
The participants were randomly assigned to receive either optimal supportive care plus whole brain radiotherapy (269 patients) or optimal supportive care alone (269 patients) at 69 U.K. and 3 Australian medical centers. They reported on 20 symptoms and adverse effects, as well as health-related quality of life, approximately once per week.
The primary outcome measure – quality-adjusted life-years (QALY), which combines overall survival and quality of life – was 46.4 days with radiotherapy and 41.7 days without it.
Symptoms, adverse effects, and quality of life (QOL) were similar between the two study groups at 4 weeks, except that the radiotherapy group reported more moderate or severe episodes of drowsiness, hair loss, nausea, and dry or itchy scalp. The number and severity of serious adverse events were similar through 12 weeks of follow-up.
The percentage of patients whose QOL was either maintained or improved over time was similar between the two groups at 4 weeks (54% vs. 57%), 8 weeks (44% vs. 51%), and 12 weeks (44% vs. 49%). Changes in Karnofsky scores also were similar.
The study refuted the widely held belief that whole brain radiotherapy allows patients to reduce or discontinue steroid treatment, averting the associated adverse effects. Steroid doses were not significantly different between the two study groups through the first 8 weeks of treatment, which “challenges the dogma that whole brain radiotherapy can be seen as a steroid-sparing modality,” the investigators said.
Taken together, the findings “suggest that whole brain radiotherapy can be omitted and patients treated with optimal supportive care alone, without an important reduction in either overall survival or quality of life,” Dr. Mulvenna and her associates said.
The approximately 5-day difference between the two study groups in median overall survival highlights both the limited benefit offered by radiotherapy and the poor prognosis of this patient population, the researchers added.
Whole brain radiotherapy did offer a small survival benefit to the youngest patients who had good performance status and a “controlled” primary NSCLC. “For all other groups, [it] does not significantly affect QALY or overall survival,” they said.
Cancer Research U.K., the Medical Research Council in the U.K., the Trans Tasman Radiation Oncology Group, and the National Health and Medical Research Council Australia supported the study. Dr. Mulvenna and her associates reported having no relevant financial disclosures.
Whole brain radiotherapy, a standard treatment for patients with metastatic non–small-cell lung cancer, provided no clinical benefit in a noninferiority trial specifically designed to assess both patient survival and quality of life.
The findings were published online Sept. 4 in the Lancet.
Whole brain radiotherapy, with or without concomitant steroid treatment, has been widely used for decades in that patient population, even though no sufficiently powered, definitive studies support the approach. It is likely that patients and clinicians alike continue to embrace it because of the absence of alternative treatment options.
The Quality of Life After Treatment for Brain Metastases (QUARTZ) trial was intended to assess whether any improvement in survival offered by whole brain radiotherapy is balanced by deterioration in quality of life, said Paula Mulvenna, MBBS, of the Northern Center for Cancer Care, Newcastle (England) Hospitals, and her associates (Lancet 2016 Sep 4. doi: 10.1016/S0140-6736(16)30825-X).
QUARTZ involved 538 adults seen during a 7-year period who had NSCLC with brain metastases and who were not suited for either brain surgery or stereotactic radiotherapy. The median age was 66 years (range, 35-85 years), and 38% had a Karnofsky Performance Status score of less than 70.
The participants were randomly assigned to receive either optimal supportive care plus whole brain radiotherapy (269 patients) or optimal supportive care alone (269 patients) at 69 U.K. and 3 Australian medical centers. They reported on 20 symptoms and adverse effects, as well as health-related quality of life, approximately once per week.
The primary outcome measure – quality-adjusted life-years (QALY), which combines overall survival and quality of life – was 46.4 days with radiotherapy and 41.7 days without it.
Symptoms, adverse effects, and quality of life (QOL) were similar between the two study groups at 4 weeks, except that the radiotherapy group reported more moderate or severe episodes of drowsiness, hair loss, nausea, and dry or itchy scalp. The number and severity of serious adverse events were similar through 12 weeks of follow-up.
The percentage of patients whose QOL was either maintained or improved over time was similar between the two groups at 4 weeks (54% vs. 57%), 8 weeks (44% vs. 51%), and 12 weeks (44% vs. 49%). Changes in Karnofsky scores also were similar.
The study refuted the widely held belief that whole brain radiotherapy allows patients to reduce or discontinue steroid treatment, averting the associated adverse effects. Steroid doses were not significantly different between the two study groups through the first 8 weeks of treatment, which “challenges the dogma that whole brain radiotherapy can be seen as a steroid-sparing modality,” the investigators said.
Taken together, the findings “suggest that whole brain radiotherapy can be omitted and patients treated with optimal supportive care alone, without an important reduction in either overall survival or quality of life,” Dr. Mulvenna and her associates said.
The approximately 5-day difference between the two study groups in median overall survival highlights both the limited benefit offered by radiotherapy and the poor prognosis of this patient population, the researchers added.
Whole brain radiotherapy did offer a small survival benefit to the youngest patients who had good performance status and a “controlled” primary NSCLC. “For all other groups, [it] does not significantly affect QALY or overall survival,” they said.
Cancer Research U.K., the Medical Research Council in the U.K., the Trans Tasman Radiation Oncology Group, and the National Health and Medical Research Council Australia supported the study. Dr. Mulvenna and her associates reported having no relevant financial disclosures.
FROM THE LANCET
Key clinical point: Whole brain radiotherapy provided no clinically significant benefit for most patients with metastatic NSCLC.
Major finding: The primary outcome measure, quality-adjusted life-years, was 46.4 days with radiotherapy and 41.7 days without it.
Data source: An international, randomized, phase III noninferiority trial involving 538 patients treated during a 7-year period.
Disclosures: Cancer Research U.K., the Medical Research Council in the U.K., the Trans Tasman Radiation Oncology Group, and the Medical Research Council Australia supported the study. Dr. Mulvenna and her associates reported having no relevant financial disclosures.
Pneumonitis with nivolumab treatment shows common radiographic patterns
A study of cancer patients enrolled in trials of the programmed cell death-1 inhibiting medicine nivolumab found that among a minority who developed pneumonitis during treatment, distinct radiographic patterns were significantly associated with the level of pneumonitis severity.
Investigators found that cryptic organizing pneumonia pattern (COP) was the most common, though not the most severe. Led by Mizuki Nishino, MD, of Brigham and Women’s Hospital, Boston, the researchers looked at the 20 patients out of a cohort of 170 (11.8%) who had developed pneumonitis, and found that radiologic patterns indicating acute interstitial pneumonia/acute respiratory distress syndrome (n = 2) had the highest severity grade on a scale of 1-5 (median 3), followed by those with COP pattern (n = 13, median grade 2), hypersensitivity pneumonitis (n = 2, median grade 1), and nonspecific interstitial pneumonia (n = 3, median grade 1). The pattern was significantly associated with severity (P = .0006).
The study cohort included patients being treated with nivolumab for lung cancer, melanoma, and lymphoma; the COP patten was the most common across tumor types and observed in patients receiving monotherapy and combination therapy alike. Therapy with nivolumab was suspended for all 20 pneumonitis patients, and most (n = 17) received treatment for pneumonitis with corticosteroids with or without infliximab, for a median treatment time of 6 weeks. Seven patients were able to restart nivolumab, though pneumonitis recurred in two, the investigators reported (Clin Cancer Res. 2016 Aug 17. doi: 10.1158/1078-0432.CCR-16-1320).
“Time from initiation of therapy to the development of pneumonitis had a wide range (0.5-11.5 months), indicating an importance of careful observation and follow-up for signs and symptoms of pneumonitis throughout treatment,” Dr. Nishino and colleagues wrote in their analysis, adding that shorter times were observed for lung cancer patients, possibly because of their higher pulmonary burden, a lower threshold for performing chest scans in these patients, or both. “In most patients, clinical and radiographic improvements were noted after treatment, indicating that [PD-1 inhibitor-related pneumonitis], although potentially serious, is treatable if diagnosed and managed appropriately. The observation emphasizes the importance of timely recognition, accurate diagnosis, and early intervention.”
The lead author and several coauthors disclosed funding from Bristol-Myers Squibb, which sponsored the trial, as well as from other manufacturers.
A study of cancer patients enrolled in trials of the programmed cell death-1 inhibiting medicine nivolumab found that among a minority who developed pneumonitis during treatment, distinct radiographic patterns were significantly associated with the level of pneumonitis severity.
Investigators found that cryptic organizing pneumonia pattern (COP) was the most common, though not the most severe. Led by Mizuki Nishino, MD, of Brigham and Women’s Hospital, Boston, the researchers looked at the 20 patients out of a cohort of 170 (11.8%) who had developed pneumonitis, and found that radiologic patterns indicating acute interstitial pneumonia/acute respiratory distress syndrome (n = 2) had the highest severity grade on a scale of 1-5 (median 3), followed by those with COP pattern (n = 13, median grade 2), hypersensitivity pneumonitis (n = 2, median grade 1), and nonspecific interstitial pneumonia (n = 3, median grade 1). The pattern was significantly associated with severity (P = .0006).
The study cohort included patients being treated with nivolumab for lung cancer, melanoma, and lymphoma; the COP patten was the most common across tumor types and observed in patients receiving monotherapy and combination therapy alike. Therapy with nivolumab was suspended for all 20 pneumonitis patients, and most (n = 17) received treatment for pneumonitis with corticosteroids with or without infliximab, for a median treatment time of 6 weeks. Seven patients were able to restart nivolumab, though pneumonitis recurred in two, the investigators reported (Clin Cancer Res. 2016 Aug 17. doi: 10.1158/1078-0432.CCR-16-1320).
“Time from initiation of therapy to the development of pneumonitis had a wide range (0.5-11.5 months), indicating an importance of careful observation and follow-up for signs and symptoms of pneumonitis throughout treatment,” Dr. Nishino and colleagues wrote in their analysis, adding that shorter times were observed for lung cancer patients, possibly because of their higher pulmonary burden, a lower threshold for performing chest scans in these patients, or both. “In most patients, clinical and radiographic improvements were noted after treatment, indicating that [PD-1 inhibitor-related pneumonitis], although potentially serious, is treatable if diagnosed and managed appropriately. The observation emphasizes the importance of timely recognition, accurate diagnosis, and early intervention.”
The lead author and several coauthors disclosed funding from Bristol-Myers Squibb, which sponsored the trial, as well as from other manufacturers.
A study of cancer patients enrolled in trials of the programmed cell death-1 inhibiting medicine nivolumab found that among a minority who developed pneumonitis during treatment, distinct radiographic patterns were significantly associated with the level of pneumonitis severity.
Investigators found that cryptic organizing pneumonia pattern (COP) was the most common, though not the most severe. Led by Mizuki Nishino, MD, of Brigham and Women’s Hospital, Boston, the researchers looked at the 20 patients out of a cohort of 170 (11.8%) who had developed pneumonitis, and found that radiologic patterns indicating acute interstitial pneumonia/acute respiratory distress syndrome (n = 2) had the highest severity grade on a scale of 1-5 (median 3), followed by those with COP pattern (n = 13, median grade 2), hypersensitivity pneumonitis (n = 2, median grade 1), and nonspecific interstitial pneumonia (n = 3, median grade 1). The pattern was significantly associated with severity (P = .0006).
The study cohort included patients being treated with nivolumab for lung cancer, melanoma, and lymphoma; the COP patten was the most common across tumor types and observed in patients receiving monotherapy and combination therapy alike. Therapy with nivolumab was suspended for all 20 pneumonitis patients, and most (n = 17) received treatment for pneumonitis with corticosteroids with or without infliximab, for a median treatment time of 6 weeks. Seven patients were able to restart nivolumab, though pneumonitis recurred in two, the investigators reported (Clin Cancer Res. 2016 Aug 17. doi: 10.1158/1078-0432.CCR-16-1320).
“Time from initiation of therapy to the development of pneumonitis had a wide range (0.5-11.5 months), indicating an importance of careful observation and follow-up for signs and symptoms of pneumonitis throughout treatment,” Dr. Nishino and colleagues wrote in their analysis, adding that shorter times were observed for lung cancer patients, possibly because of their higher pulmonary burden, a lower threshold for performing chest scans in these patients, or both. “In most patients, clinical and radiographic improvements were noted after treatment, indicating that [PD-1 inhibitor-related pneumonitis], although potentially serious, is treatable if diagnosed and managed appropriately. The observation emphasizes the importance of timely recognition, accurate diagnosis, and early intervention.”
The lead author and several coauthors disclosed funding from Bristol-Myers Squibb, which sponsored the trial, as well as from other manufacturers.
FROM CLINICAL CANCER RESEARCH
Key clinical point: Pneumonitis related to treatment with PD-1 inhibitors showed distinct radiographic patterns associated with severity; most cases resolved with corticosteroid treatment.
Major finding: Of 20 patients in nivolumab trials who developed pneumonitis, a COP pattern was seen in 13, and other patterns in 7; different patterns were significantly associated with pneumonitis severity (P = .006).
Data source: 170 patients with melanoma, lung cancer or lymphoma enrolled in single-site open-label clinical trial of nivolumab.
Disclosures: The lead author and several coauthors disclosed funding from Bristol-Myers Squibb, which sponsored the trial, as well as from other manufacturers.
Metastatic Small Cell Carcinoma of the Lung: An Unusual Cause of Acute Fulminant Hepatic Failure
For patients with acute fulminant liver failure, imaging and histopathologic studies are indicated to reveal the underlying etiology, and metastatic small cell carcinoma should be included in the clinical differential diagnosis when appropriate.
Acute fulminant hepatic failure (FHF) is an uncommon but highly fatal condition that results from the massive destruction of liver tissue. Viral hepatitis and drug-induced liver damage predominate in North America and Europe, but the underlying precipitating factors differ around the world.1 In children, indeterminate causes account for more than 50% of cases.2 Other conditions associated with FHF are Budd-Chiari syndrome, vascular hypoperfusion, mushroom poisoning, Wilson disease, autoimmune hepatitis, and fatty liver of pregnancy.3
Neoplastic lesions of the liver, mostly metastatic carcinomas, present with ductular obstruction with occasional mild elevations in aminotransferases. Rarely do space-occupying lesions lead to acute liver failure (ALF) with massive hepatocyte necrosis.
The authors report a case of rapidly progressing ALF due to metastatic small cell carcinoma to the liver. Small cell lung carcinoma (SCLC) is an aggressive tumor that often presents at an advanced stage. Although liver metastasis is common in this disease, development of FHF is extremely uncommon.
Case Presentation
A 90-year-old African American man presented to the emergency department (ED) of the Brooklyn Campus of the VA New York Harbor Health Care System (VANYHHS), with a persistent cough, worsening of shortness of breath, increasing right upper quadrant abdominal pain, and chronic constipation. He noted that he had smoked 1 pack per day for 40 years but quit 30 years ago. He had a medical history of chronic obstructive pulmonary disease (COPD), hypertension, prostate cancer treated 20 years earlier with external beam radiation therapy and with intramuscular leuprolide every 6 months for the previous 6.5 years, and gout. He was taking no hepatotoxic prescription medications and never used over-the-counter analgesics or abused alcohol. Five days before admission, he was treated for COPD exacerbation in the ED.
Blood chemistry at the time revealed significantly elevated liver function enzymes, including aspartate aminotransferase, alanine aminotransferase (ALT), alkaline phosphatase (AST), and total bilirubin compared with baseline levels taken 3 months earlier (Table). Primary care follow-up was recommended. Physical examination on the day of admission was remarkable for normal blood pressure (137/74), emaciated appearance, and a large liver with right upper quadrant tenderness.
Repeat blood chemistries showed a further rise in liver function tests. Acetaminophen level was < 1.0 μg/mL (therapeutic range 10-20 μg/mL). Hepatitis A, B, and C serologic testing was negative. Serum creatinine was elevated at 1.7 mg/dL and steadily increased to 3.2 mg/dL at the end of the hospital course. A chest X-ray and a noncontrast computed tomography (CT) scan of the chest showed left upper lobe ill-defined infiltrates/opacities. Noncontrast abdominal and pelvic CT revealed hepatomegaly and ascites. Hepatic ultrasound showed that the liver was enlarged, diffusely heterogeneous, and nodular in appearance. The patient was admitted for evaluation.
On day 2 of admission, the patient reported “numbness of digits.” Serum glucose was measured and found to be low (36 mg/dL) (reference range: 70-110 mg/dL). He was subsequently managed for refractory hypoglycemia, which was presumed to be a result of liver disease. On day 3, he was transferred to the intensive care unit for close monitoring and management. On day 4, the patient was still experiencing episodes of hypoglycemia despite glucagon and dextrose administration. He developed altered mental status and metabolic acidosis and was intubated. Repeat laboratory tests showed a significant increase in AST and ALT with an AST:ALT ratio of about 4. Serum ammonia levels also were increased at 198.6 μg/dL (reference range: 17-80 μg/dL). The platelet count decreased to as low as 86 x 103/μL (reference range:150-450 x 103/μL). The prothrombin time (PT) increased continuously to as high as 21.4 sec (reference range: 9.6-12.4 sec) as did the activated partial thromboplastin time (aPTT) to 65.1 sec (reference range: 28-36.3 sec). Afterward, the patient developed multiple organ failure, including hemodynamic instability requiring fluid resuscitation. On day 5, the patient died.
At autopsy, the left upper lobe of the patient’s lung was found to have a tan-white, firm, irregularly shaped 4.8-cm mass. The liver weighed 2,980 g (reference range: 1,400-1,600 g) and was diffusely infiltrated by tan-white masses comprising about 70% of the liver (Figure 1).
Histologic examination of the lung (Figure 2) and liver (Figure 3) masses revealed small, round, blue cells with high nucleocytoplasmic ratios, nuclear molding, and crushing artifact. The tumor cells were found to be positive for chromogranin and synaptophysin. The liver showed diffuse hepatocyte necrosis with few viable hepatocytes present. The autopsy case was signed out as SCLC with diffuse liver metastasis.
Discussion
Acute FHF is a rare condition that often presents with sudden onset in which patients become encephalopathic due to hyperammonemia and exhibit marked elevations in the 2 aminotransferases, AST and ALT. A prior study of this condition reported on 6 patients, 5 of whom succumbed to the condition and 3 of whom were autopsied.4 The study found that both AST and ALT became rapidly elevated markedly such that the AST to ALT ratio was significantly greater than 1 and often exceeding 2, a pattern suggesting mitochondrial damage in hepatocytes resulting in release of intramitochondrial AST in addition to extramitochondrial AST.4
In addition, total protein and albumin were significantly decreased, and serum ammonia levels were markedly increased. All patients were encepaholopathic and were found to have disseminated intravascular coagulopathy. Five of the 6 patients had renal failure, including 2 with acute tubular necrosis, and electrolyte abnormalities, including hypernatremia, in one case due to circulating elevated levels of aldosterone. Two of the 6 patients were found to be consistently hypoglycemic, possibly caused by impaired glycogenolysis. Three of these patients were found to have had lactic acidosis. In this study, liver biopsy was unrevealing and showed only minimal changes even during the earlier noted changes in laboratory values. Total hepatocyte necrosis was found only at postmortem examination.
Causes of FHF
Previous studies have identified possible causes of FHF that include alcohol abuse and IV drug abuse giving rise to pan-hepatic hepatitis—both conditions giving rise to cirrhosis; multiple abdominal surgeries; drug (acetaminophen) overdose; fatty liver of pregnancy resulting in microvesicular steatosis of hepatocytes; hypotension (shock liver); and Reye syndrome, mainly in children but also reported in adults, in which there is a viral prodrome with fever followed by treatment with aspirin that progresses to acute FHF.
Metastatic cancer is not generally listed as a potential cause of FHF. Although cancer is a less common cause of this condition, metastasis-induced FHF that has been documented in the literature includes tumors of the breast, gastrointestinal tract, lung, nasopharynx, melanoma, and hematolymphoid malignancies, including leukemia, Hodgkin disease, non-Hodgkin lymphomas, and malignant histiocytosis.5-12
Small Cell Carcinoma as a Cause of FHF
Small cell carcinoma of the lung is a highly malignant neoplasm that often presents at an advanced stage. Most often, metastatic disease to the liver may result in some mild increase in ALT and obstructive symptoms. However, diffuse sinusoidal infiltration of the tumor is most likely to present with hyperacute liver failure.13 A literature review of all small cell carcinomas in the liver presenting with acute FHF shows a consistent morphologic pattern of diffuse parenchymal infiltration,some that initially present with acute hepatic failure with no known history of liver disease.13-25 Imaging studies sometimes are difficult to interpret and may fail to detect infiltration of the tumor because of diffuse involvement of the liver parenchyma. Malignant infiltration of the liver should be one of the considerations in cases of unexplained hepatomegaly.
As found in the authors’ prior study, coagulopathy, renal failure (final creatinine was 3.2 mg/dL) as well as hypoglycemia are oftentimes seen, all of which were found in the patient in this study.4 (Coagulopathy was indicated by the low platelet count and elevated PT and aPTT.) Laboratory findings for FHF include rapid increases in serum ALTs such that the AST:ALT ratio is significantly greater than 1 and in which total protein and albumin are significantly decreased. Often there is hyperammonemia as was present in the current case.
A study has been performed to develop serodiagnostic markers to distinguish malignant from nonmalignant causes of FHF on 4 patients with tumor-induced FHF and 12 patients with FHF due to other causes. It was found that that there was an increase in the lactate dehydrogenase (LDH) to ALT ratio as well as elevated uric acid levels in the 4 patients with FHF not found in any of the 12 patients with nonmalignant causes of this condition.19 Although LDH was not measured in this case, in view of the patient’s history of gout, the LDH/uric acid ratio may not have been discriminating.
Conclusion
Although rare, metastatic small cell carcinoma should be included in the clinical differential diagnosis of patients presenting with acute FHF with no other obvious medical etiology. Accurate and timely diagnosis is important to better guide management of these patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Hoofnagle JH, Carithers RL Jr, Shapiro C, Ascher N. Fulminant hepatic failure: summary of workshop. Hepatology. 1995;21(1):240-252.
2. D’Agata ID, Balister WF. Pediatric aspects of acute liver failure. In: Lee WM, Williams R, eds. Acute Liver Failure. Cambridge, UK: Cambridge University Press, 1997:53-66.
3. Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases position paper on acute liver failure 2011. Hepatology. 2012;55(3):965-967.
4. Sunheimer R, Capaldo G, Kashanian F, et al. Serum analyte pattern characteristic of fulminant hepatic failure. Ann Clin Lab Sci. 1994;24(2):101-109.
5. Athanasakis E, Mouloudi E, Prinianakis G, Kostaki M, Tzardi M, Georgopoulos D. Metastatic liver disease and fulminant hepatic failure: presentation of a case and review of the literature. Eur J Gastroenterol Hepatol. 2003;15(11):1235-1240.
6. Preissler G, Graeb C, Steib C, et al. Acute liver failure, rupture and hemorrhagic shock as primary manifestation of advanced metastatic disease. Anticancer Res. 2012;32(8):3449-3454.
7. Alexopoulou A, Koskinas J, Deutsch M, Delladetsima J, Kountouras D, Dourakis SP. Acute liver failure as the initial manifestation of hepatic infiltration by a solid tumor: report of 5 cases and review of the literature. Tumori. 2006;92(4):354-357.
8. Shah KG, Modi PR, Rizvi J. Breast carcinoma metastasizing to the urinary bladder and retroperitoneum presenting as acute renal failure. Indian J Urol. 2011;27(1):135-136.
9. Nazario HE, Lepe R, Trotter JF. Metastatic breast cancer presenting as acute liver failure. Gastroenterol Hepatol (NY). 2011;7(1):65-66.
10. Rajvanshi P, Kowdley KV, Hirota WK, Meyers JB, Keeffe EB. Fulminant hepatic failure secondary to neoplastic infiltration of the liver. J Clin Gastroenterol. 2005;39(4):339-343.
11. Fairbank WH. Three atypical cases of Hodgkin’s Disease, presenting with liver failure. Can Med Assoc J. 1953;69(3):315-317.
12. Braude S, Portmann B, Gimson AE, Williams R. Fulminant hepatic failure in non-Hodgkin’s lymphoma. Postgrad Med J. 1982;58(679):301-304.
13. Lo AA, Lo EC, Li H, et al. Unique morphologic and clinical features of liver predominant/primary small cell carcinoma—autopsy and biopsy case series. Ann Diagn Pathol. 2014;18(3):151-156.
14. Hwang YT, Shin JW, Lee JH, et al. A case of fulminant hepatic failure secondary to hepatic metastasis of small cell lung carcinoma [in Korean]. Korean J Hepatol. 2007;13(4):565-570.
15. Miyaaki H, Ichikawa T, Taura N, et al. Diffuse liver metastasis of small cell lung cancer causing marked hepatomegaly and fulminant hepatic failure. Intern Med. 2010;49(14):1383-1386.
16. Sato K, Takeyama Y, Tanaka T, Fukui Y, Gonda H, Suzuki R. Fulminant hepatic failure and hepatomegaly caused by diffuse liver metastases from small cell lung carcinoma: 2 autopsy cases. Respir Investig. 2013;51(2):98-102.
17. Galus M. Liver failure due to metastatic small-cell carcinoma of the lung. Mayo Clin Proc. 1997;72(8):791.
18. Kovalev Y, Lurie M, Naschitz JE, Yeshurun D, Zuckerman E. Metastatic small cell carcinoma presenting as acute hepatic failure. Am J Gastroenterol. 2001;96(12):3471-3473.
19. McGuire BM, Cherwitz DL, Rabe KM, Ho SB. Small-cell carcinoma of the lung manifesting as acute hepatic failure. Mayo Clin Proc. 1997;72(2):133-139.
20. Richecoeur M, Massoure MP, Le Coadou G, Lipovac AS, Bronstein JA, Delluc C. Acute hepatic failure as the presenting manifestation of a metastatic lung carcinoma to liver [in French]. Rev Med Interne. 2009;30(10):911-913.
21. Valladares Ayerbes MJ, Canadas Garcia de Leon M, Reina Zoilo JJ, Valenzuela Claros JC, Ruiz Borrego M, Barea Bejarano JL. Acute liver failure as presentation form of small cell carcinoma of the lung [in Spanish]. An Med Interna. 1997;14(3):128-130.
22. Gilbert J, Rutledge H, Koch A. Diffuse malignant infiltration of the liver manifesting as a case of acute liver failure. Nat Clin Pract Gastroenterol Hepatol. 2008;5(7):405-408.
23. Vaideeswar P, Munot S, Rojekar A, Deodhar K. Hepatic diffuse intra-sinusoidal metastases of pulmonary small-cell carcinoma. J Postgrad Med. 2012;58(3):230-231.
24. Krauss EA, Ludwig PW, Sumner HW. Metastatic carcinoma presenting as fulminant hepatic failure. Am J Gastroenterol. 1979;72(6):651-654.
25. Ke E, Gomez JD, Tang K, Sriram KB. Metastatic small-cell lung cancer presenting
as fulminant hepatic failure. BMJ Case Rep. 2013;2013.
For patients with acute fulminant liver failure, imaging and histopathologic studies are indicated to reveal the underlying etiology, and metastatic small cell carcinoma should be included in the clinical differential diagnosis when appropriate.
Acute fulminant hepatic failure (FHF) is an uncommon but highly fatal condition that results from the massive destruction of liver tissue. Viral hepatitis and drug-induced liver damage predominate in North America and Europe, but the underlying precipitating factors differ around the world.1 In children, indeterminate causes account for more than 50% of cases.2 Other conditions associated with FHF are Budd-Chiari syndrome, vascular hypoperfusion, mushroom poisoning, Wilson disease, autoimmune hepatitis, and fatty liver of pregnancy.3
Neoplastic lesions of the liver, mostly metastatic carcinomas, present with ductular obstruction with occasional mild elevations in aminotransferases. Rarely do space-occupying lesions lead to acute liver failure (ALF) with massive hepatocyte necrosis.
The authors report a case of rapidly progressing ALF due to metastatic small cell carcinoma to the liver. Small cell lung carcinoma (SCLC) is an aggressive tumor that often presents at an advanced stage. Although liver metastasis is common in this disease, development of FHF is extremely uncommon.
Case Presentation
A 90-year-old African American man presented to the emergency department (ED) of the Brooklyn Campus of the VA New York Harbor Health Care System (VANYHHS), with a persistent cough, worsening of shortness of breath, increasing right upper quadrant abdominal pain, and chronic constipation. He noted that he had smoked 1 pack per day for 40 years but quit 30 years ago. He had a medical history of chronic obstructive pulmonary disease (COPD), hypertension, prostate cancer treated 20 years earlier with external beam radiation therapy and with intramuscular leuprolide every 6 months for the previous 6.5 years, and gout. He was taking no hepatotoxic prescription medications and never used over-the-counter analgesics or abused alcohol. Five days before admission, he was treated for COPD exacerbation in the ED.
Blood chemistry at the time revealed significantly elevated liver function enzymes, including aspartate aminotransferase, alanine aminotransferase (ALT), alkaline phosphatase (AST), and total bilirubin compared with baseline levels taken 3 months earlier (Table). Primary care follow-up was recommended. Physical examination on the day of admission was remarkable for normal blood pressure (137/74), emaciated appearance, and a large liver with right upper quadrant tenderness.
Repeat blood chemistries showed a further rise in liver function tests. Acetaminophen level was < 1.0 μg/mL (therapeutic range 10-20 μg/mL). Hepatitis A, B, and C serologic testing was negative. Serum creatinine was elevated at 1.7 mg/dL and steadily increased to 3.2 mg/dL at the end of the hospital course. A chest X-ray and a noncontrast computed tomography (CT) scan of the chest showed left upper lobe ill-defined infiltrates/opacities. Noncontrast abdominal and pelvic CT revealed hepatomegaly and ascites. Hepatic ultrasound showed that the liver was enlarged, diffusely heterogeneous, and nodular in appearance. The patient was admitted for evaluation.
On day 2 of admission, the patient reported “numbness of digits.” Serum glucose was measured and found to be low (36 mg/dL) (reference range: 70-110 mg/dL). He was subsequently managed for refractory hypoglycemia, which was presumed to be a result of liver disease. On day 3, he was transferred to the intensive care unit for close monitoring and management. On day 4, the patient was still experiencing episodes of hypoglycemia despite glucagon and dextrose administration. He developed altered mental status and metabolic acidosis and was intubated. Repeat laboratory tests showed a significant increase in AST and ALT with an AST:ALT ratio of about 4. Serum ammonia levels also were increased at 198.6 μg/dL (reference range: 17-80 μg/dL). The platelet count decreased to as low as 86 x 103/μL (reference range:150-450 x 103/μL). The prothrombin time (PT) increased continuously to as high as 21.4 sec (reference range: 9.6-12.4 sec) as did the activated partial thromboplastin time (aPTT) to 65.1 sec (reference range: 28-36.3 sec). Afterward, the patient developed multiple organ failure, including hemodynamic instability requiring fluid resuscitation. On day 5, the patient died.
At autopsy, the left upper lobe of the patient’s lung was found to have a tan-white, firm, irregularly shaped 4.8-cm mass. The liver weighed 2,980 g (reference range: 1,400-1,600 g) and was diffusely infiltrated by tan-white masses comprising about 70% of the liver (Figure 1).
Histologic examination of the lung (Figure 2) and liver (Figure 3) masses revealed small, round, blue cells with high nucleocytoplasmic ratios, nuclear molding, and crushing artifact. The tumor cells were found to be positive for chromogranin and synaptophysin. The liver showed diffuse hepatocyte necrosis with few viable hepatocytes present. The autopsy case was signed out as SCLC with diffuse liver metastasis.
Discussion
Acute FHF is a rare condition that often presents with sudden onset in which patients become encephalopathic due to hyperammonemia and exhibit marked elevations in the 2 aminotransferases, AST and ALT. A prior study of this condition reported on 6 patients, 5 of whom succumbed to the condition and 3 of whom were autopsied.4 The study found that both AST and ALT became rapidly elevated markedly such that the AST to ALT ratio was significantly greater than 1 and often exceeding 2, a pattern suggesting mitochondrial damage in hepatocytes resulting in release of intramitochondrial AST in addition to extramitochondrial AST.4
In addition, total protein and albumin were significantly decreased, and serum ammonia levels were markedly increased. All patients were encepaholopathic and were found to have disseminated intravascular coagulopathy. Five of the 6 patients had renal failure, including 2 with acute tubular necrosis, and electrolyte abnormalities, including hypernatremia, in one case due to circulating elevated levels of aldosterone. Two of the 6 patients were found to be consistently hypoglycemic, possibly caused by impaired glycogenolysis. Three of these patients were found to have had lactic acidosis. In this study, liver biopsy was unrevealing and showed only minimal changes even during the earlier noted changes in laboratory values. Total hepatocyte necrosis was found only at postmortem examination.
Causes of FHF
Previous studies have identified possible causes of FHF that include alcohol abuse and IV drug abuse giving rise to pan-hepatic hepatitis—both conditions giving rise to cirrhosis; multiple abdominal surgeries; drug (acetaminophen) overdose; fatty liver of pregnancy resulting in microvesicular steatosis of hepatocytes; hypotension (shock liver); and Reye syndrome, mainly in children but also reported in adults, in which there is a viral prodrome with fever followed by treatment with aspirin that progresses to acute FHF.
Metastatic cancer is not generally listed as a potential cause of FHF. Although cancer is a less common cause of this condition, metastasis-induced FHF that has been documented in the literature includes tumors of the breast, gastrointestinal tract, lung, nasopharynx, melanoma, and hematolymphoid malignancies, including leukemia, Hodgkin disease, non-Hodgkin lymphomas, and malignant histiocytosis.5-12
Small Cell Carcinoma as a Cause of FHF
Small cell carcinoma of the lung is a highly malignant neoplasm that often presents at an advanced stage. Most often, metastatic disease to the liver may result in some mild increase in ALT and obstructive symptoms. However, diffuse sinusoidal infiltration of the tumor is most likely to present with hyperacute liver failure.13 A literature review of all small cell carcinomas in the liver presenting with acute FHF shows a consistent morphologic pattern of diffuse parenchymal infiltration,some that initially present with acute hepatic failure with no known history of liver disease.13-25 Imaging studies sometimes are difficult to interpret and may fail to detect infiltration of the tumor because of diffuse involvement of the liver parenchyma. Malignant infiltration of the liver should be one of the considerations in cases of unexplained hepatomegaly.
As found in the authors’ prior study, coagulopathy, renal failure (final creatinine was 3.2 mg/dL) as well as hypoglycemia are oftentimes seen, all of which were found in the patient in this study.4 (Coagulopathy was indicated by the low platelet count and elevated PT and aPTT.) Laboratory findings for FHF include rapid increases in serum ALTs such that the AST:ALT ratio is significantly greater than 1 and in which total protein and albumin are significantly decreased. Often there is hyperammonemia as was present in the current case.
A study has been performed to develop serodiagnostic markers to distinguish malignant from nonmalignant causes of FHF on 4 patients with tumor-induced FHF and 12 patients with FHF due to other causes. It was found that that there was an increase in the lactate dehydrogenase (LDH) to ALT ratio as well as elevated uric acid levels in the 4 patients with FHF not found in any of the 12 patients with nonmalignant causes of this condition.19 Although LDH was not measured in this case, in view of the patient’s history of gout, the LDH/uric acid ratio may not have been discriminating.
Conclusion
Although rare, metastatic small cell carcinoma should be included in the clinical differential diagnosis of patients presenting with acute FHF with no other obvious medical etiology. Accurate and timely diagnosis is important to better guide management of these patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
For patients with acute fulminant liver failure, imaging and histopathologic studies are indicated to reveal the underlying etiology, and metastatic small cell carcinoma should be included in the clinical differential diagnosis when appropriate.
Acute fulminant hepatic failure (FHF) is an uncommon but highly fatal condition that results from the massive destruction of liver tissue. Viral hepatitis and drug-induced liver damage predominate in North America and Europe, but the underlying precipitating factors differ around the world.1 In children, indeterminate causes account for more than 50% of cases.2 Other conditions associated with FHF are Budd-Chiari syndrome, vascular hypoperfusion, mushroom poisoning, Wilson disease, autoimmune hepatitis, and fatty liver of pregnancy.3
Neoplastic lesions of the liver, mostly metastatic carcinomas, present with ductular obstruction with occasional mild elevations in aminotransferases. Rarely do space-occupying lesions lead to acute liver failure (ALF) with massive hepatocyte necrosis.
The authors report a case of rapidly progressing ALF due to metastatic small cell carcinoma to the liver. Small cell lung carcinoma (SCLC) is an aggressive tumor that often presents at an advanced stage. Although liver metastasis is common in this disease, development of FHF is extremely uncommon.
Case Presentation
A 90-year-old African American man presented to the emergency department (ED) of the Brooklyn Campus of the VA New York Harbor Health Care System (VANYHHS), with a persistent cough, worsening of shortness of breath, increasing right upper quadrant abdominal pain, and chronic constipation. He noted that he had smoked 1 pack per day for 40 years but quit 30 years ago. He had a medical history of chronic obstructive pulmonary disease (COPD), hypertension, prostate cancer treated 20 years earlier with external beam radiation therapy and with intramuscular leuprolide every 6 months for the previous 6.5 years, and gout. He was taking no hepatotoxic prescription medications and never used over-the-counter analgesics or abused alcohol. Five days before admission, he was treated for COPD exacerbation in the ED.
Blood chemistry at the time revealed significantly elevated liver function enzymes, including aspartate aminotransferase, alanine aminotransferase (ALT), alkaline phosphatase (AST), and total bilirubin compared with baseline levels taken 3 months earlier (Table). Primary care follow-up was recommended. Physical examination on the day of admission was remarkable for normal blood pressure (137/74), emaciated appearance, and a large liver with right upper quadrant tenderness.
Repeat blood chemistries showed a further rise in liver function tests. Acetaminophen level was < 1.0 μg/mL (therapeutic range 10-20 μg/mL). Hepatitis A, B, and C serologic testing was negative. Serum creatinine was elevated at 1.7 mg/dL and steadily increased to 3.2 mg/dL at the end of the hospital course. A chest X-ray and a noncontrast computed tomography (CT) scan of the chest showed left upper lobe ill-defined infiltrates/opacities. Noncontrast abdominal and pelvic CT revealed hepatomegaly and ascites. Hepatic ultrasound showed that the liver was enlarged, diffusely heterogeneous, and nodular in appearance. The patient was admitted for evaluation.
On day 2 of admission, the patient reported “numbness of digits.” Serum glucose was measured and found to be low (36 mg/dL) (reference range: 70-110 mg/dL). He was subsequently managed for refractory hypoglycemia, which was presumed to be a result of liver disease. On day 3, he was transferred to the intensive care unit for close monitoring and management. On day 4, the patient was still experiencing episodes of hypoglycemia despite glucagon and dextrose administration. He developed altered mental status and metabolic acidosis and was intubated. Repeat laboratory tests showed a significant increase in AST and ALT with an AST:ALT ratio of about 4. Serum ammonia levels also were increased at 198.6 μg/dL (reference range: 17-80 μg/dL). The platelet count decreased to as low as 86 x 103/μL (reference range:150-450 x 103/μL). The prothrombin time (PT) increased continuously to as high as 21.4 sec (reference range: 9.6-12.4 sec) as did the activated partial thromboplastin time (aPTT) to 65.1 sec (reference range: 28-36.3 sec). Afterward, the patient developed multiple organ failure, including hemodynamic instability requiring fluid resuscitation. On day 5, the patient died.
At autopsy, the left upper lobe of the patient’s lung was found to have a tan-white, firm, irregularly shaped 4.8-cm mass. The liver weighed 2,980 g (reference range: 1,400-1,600 g) and was diffusely infiltrated by tan-white masses comprising about 70% of the liver (Figure 1).
Histologic examination of the lung (Figure 2) and liver (Figure 3) masses revealed small, round, blue cells with high nucleocytoplasmic ratios, nuclear molding, and crushing artifact. The tumor cells were found to be positive for chromogranin and synaptophysin. The liver showed diffuse hepatocyte necrosis with few viable hepatocytes present. The autopsy case was signed out as SCLC with diffuse liver metastasis.
Discussion
Acute FHF is a rare condition that often presents with sudden onset in which patients become encephalopathic due to hyperammonemia and exhibit marked elevations in the 2 aminotransferases, AST and ALT. A prior study of this condition reported on 6 patients, 5 of whom succumbed to the condition and 3 of whom were autopsied.4 The study found that both AST and ALT became rapidly elevated markedly such that the AST to ALT ratio was significantly greater than 1 and often exceeding 2, a pattern suggesting mitochondrial damage in hepatocytes resulting in release of intramitochondrial AST in addition to extramitochondrial AST.4
In addition, total protein and albumin were significantly decreased, and serum ammonia levels were markedly increased. All patients were encepaholopathic and were found to have disseminated intravascular coagulopathy. Five of the 6 patients had renal failure, including 2 with acute tubular necrosis, and electrolyte abnormalities, including hypernatremia, in one case due to circulating elevated levels of aldosterone. Two of the 6 patients were found to be consistently hypoglycemic, possibly caused by impaired glycogenolysis. Three of these patients were found to have had lactic acidosis. In this study, liver biopsy was unrevealing and showed only minimal changes even during the earlier noted changes in laboratory values. Total hepatocyte necrosis was found only at postmortem examination.
Causes of FHF
Previous studies have identified possible causes of FHF that include alcohol abuse and IV drug abuse giving rise to pan-hepatic hepatitis—both conditions giving rise to cirrhosis; multiple abdominal surgeries; drug (acetaminophen) overdose; fatty liver of pregnancy resulting in microvesicular steatosis of hepatocytes; hypotension (shock liver); and Reye syndrome, mainly in children but also reported in adults, in which there is a viral prodrome with fever followed by treatment with aspirin that progresses to acute FHF.
Metastatic cancer is not generally listed as a potential cause of FHF. Although cancer is a less common cause of this condition, metastasis-induced FHF that has been documented in the literature includes tumors of the breast, gastrointestinal tract, lung, nasopharynx, melanoma, and hematolymphoid malignancies, including leukemia, Hodgkin disease, non-Hodgkin lymphomas, and malignant histiocytosis.5-12
Small Cell Carcinoma as a Cause of FHF
Small cell carcinoma of the lung is a highly malignant neoplasm that often presents at an advanced stage. Most often, metastatic disease to the liver may result in some mild increase in ALT and obstructive symptoms. However, diffuse sinusoidal infiltration of the tumor is most likely to present with hyperacute liver failure.13 A literature review of all small cell carcinomas in the liver presenting with acute FHF shows a consistent morphologic pattern of diffuse parenchymal infiltration,some that initially present with acute hepatic failure with no known history of liver disease.13-25 Imaging studies sometimes are difficult to interpret and may fail to detect infiltration of the tumor because of diffuse involvement of the liver parenchyma. Malignant infiltration of the liver should be one of the considerations in cases of unexplained hepatomegaly.
As found in the authors’ prior study, coagulopathy, renal failure (final creatinine was 3.2 mg/dL) as well as hypoglycemia are oftentimes seen, all of which were found in the patient in this study.4 (Coagulopathy was indicated by the low platelet count and elevated PT and aPTT.) Laboratory findings for FHF include rapid increases in serum ALTs such that the AST:ALT ratio is significantly greater than 1 and in which total protein and albumin are significantly decreased. Often there is hyperammonemia as was present in the current case.
A study has been performed to develop serodiagnostic markers to distinguish malignant from nonmalignant causes of FHF on 4 patients with tumor-induced FHF and 12 patients with FHF due to other causes. It was found that that there was an increase in the lactate dehydrogenase (LDH) to ALT ratio as well as elevated uric acid levels in the 4 patients with FHF not found in any of the 12 patients with nonmalignant causes of this condition.19 Although LDH was not measured in this case, in view of the patient’s history of gout, the LDH/uric acid ratio may not have been discriminating.
Conclusion
Although rare, metastatic small cell carcinoma should be included in the clinical differential diagnosis of patients presenting with acute FHF with no other obvious medical etiology. Accurate and timely diagnosis is important to better guide management of these patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Hoofnagle JH, Carithers RL Jr, Shapiro C, Ascher N. Fulminant hepatic failure: summary of workshop. Hepatology. 1995;21(1):240-252.
2. D’Agata ID, Balister WF. Pediatric aspects of acute liver failure. In: Lee WM, Williams R, eds. Acute Liver Failure. Cambridge, UK: Cambridge University Press, 1997:53-66.
3. Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases position paper on acute liver failure 2011. Hepatology. 2012;55(3):965-967.
4. Sunheimer R, Capaldo G, Kashanian F, et al. Serum analyte pattern characteristic of fulminant hepatic failure. Ann Clin Lab Sci. 1994;24(2):101-109.
5. Athanasakis E, Mouloudi E, Prinianakis G, Kostaki M, Tzardi M, Georgopoulos D. Metastatic liver disease and fulminant hepatic failure: presentation of a case and review of the literature. Eur J Gastroenterol Hepatol. 2003;15(11):1235-1240.
6. Preissler G, Graeb C, Steib C, et al. Acute liver failure, rupture and hemorrhagic shock as primary manifestation of advanced metastatic disease. Anticancer Res. 2012;32(8):3449-3454.
7. Alexopoulou A, Koskinas J, Deutsch M, Delladetsima J, Kountouras D, Dourakis SP. Acute liver failure as the initial manifestation of hepatic infiltration by a solid tumor: report of 5 cases and review of the literature. Tumori. 2006;92(4):354-357.
8. Shah KG, Modi PR, Rizvi J. Breast carcinoma metastasizing to the urinary bladder and retroperitoneum presenting as acute renal failure. Indian J Urol. 2011;27(1):135-136.
9. Nazario HE, Lepe R, Trotter JF. Metastatic breast cancer presenting as acute liver failure. Gastroenterol Hepatol (NY). 2011;7(1):65-66.
10. Rajvanshi P, Kowdley KV, Hirota WK, Meyers JB, Keeffe EB. Fulminant hepatic failure secondary to neoplastic infiltration of the liver. J Clin Gastroenterol. 2005;39(4):339-343.
11. Fairbank WH. Three atypical cases of Hodgkin’s Disease, presenting with liver failure. Can Med Assoc J. 1953;69(3):315-317.
12. Braude S, Portmann B, Gimson AE, Williams R. Fulminant hepatic failure in non-Hodgkin’s lymphoma. Postgrad Med J. 1982;58(679):301-304.
13. Lo AA, Lo EC, Li H, et al. Unique morphologic and clinical features of liver predominant/primary small cell carcinoma—autopsy and biopsy case series. Ann Diagn Pathol. 2014;18(3):151-156.
14. Hwang YT, Shin JW, Lee JH, et al. A case of fulminant hepatic failure secondary to hepatic metastasis of small cell lung carcinoma [in Korean]. Korean J Hepatol. 2007;13(4):565-570.
15. Miyaaki H, Ichikawa T, Taura N, et al. Diffuse liver metastasis of small cell lung cancer causing marked hepatomegaly and fulminant hepatic failure. Intern Med. 2010;49(14):1383-1386.
16. Sato K, Takeyama Y, Tanaka T, Fukui Y, Gonda H, Suzuki R. Fulminant hepatic failure and hepatomegaly caused by diffuse liver metastases from small cell lung carcinoma: 2 autopsy cases. Respir Investig. 2013;51(2):98-102.
17. Galus M. Liver failure due to metastatic small-cell carcinoma of the lung. Mayo Clin Proc. 1997;72(8):791.
18. Kovalev Y, Lurie M, Naschitz JE, Yeshurun D, Zuckerman E. Metastatic small cell carcinoma presenting as acute hepatic failure. Am J Gastroenterol. 2001;96(12):3471-3473.
19. McGuire BM, Cherwitz DL, Rabe KM, Ho SB. Small-cell carcinoma of the lung manifesting as acute hepatic failure. Mayo Clin Proc. 1997;72(2):133-139.
20. Richecoeur M, Massoure MP, Le Coadou G, Lipovac AS, Bronstein JA, Delluc C. Acute hepatic failure as the presenting manifestation of a metastatic lung carcinoma to liver [in French]. Rev Med Interne. 2009;30(10):911-913.
21. Valladares Ayerbes MJ, Canadas Garcia de Leon M, Reina Zoilo JJ, Valenzuela Claros JC, Ruiz Borrego M, Barea Bejarano JL. Acute liver failure as presentation form of small cell carcinoma of the lung [in Spanish]. An Med Interna. 1997;14(3):128-130.
22. Gilbert J, Rutledge H, Koch A. Diffuse malignant infiltration of the liver manifesting as a case of acute liver failure. Nat Clin Pract Gastroenterol Hepatol. 2008;5(7):405-408.
23. Vaideeswar P, Munot S, Rojekar A, Deodhar K. Hepatic diffuse intra-sinusoidal metastases of pulmonary small-cell carcinoma. J Postgrad Med. 2012;58(3):230-231.
24. Krauss EA, Ludwig PW, Sumner HW. Metastatic carcinoma presenting as fulminant hepatic failure. Am J Gastroenterol. 1979;72(6):651-654.
25. Ke E, Gomez JD, Tang K, Sriram KB. Metastatic small-cell lung cancer presenting
as fulminant hepatic failure. BMJ Case Rep. 2013;2013.
1. Hoofnagle JH, Carithers RL Jr, Shapiro C, Ascher N. Fulminant hepatic failure: summary of workshop. Hepatology. 1995;21(1):240-252.
2. D’Agata ID, Balister WF. Pediatric aspects of acute liver failure. In: Lee WM, Williams R, eds. Acute Liver Failure. Cambridge, UK: Cambridge University Press, 1997:53-66.
3. Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases position paper on acute liver failure 2011. Hepatology. 2012;55(3):965-967.
4. Sunheimer R, Capaldo G, Kashanian F, et al. Serum analyte pattern characteristic of fulminant hepatic failure. Ann Clin Lab Sci. 1994;24(2):101-109.
5. Athanasakis E, Mouloudi E, Prinianakis G, Kostaki M, Tzardi M, Georgopoulos D. Metastatic liver disease and fulminant hepatic failure: presentation of a case and review of the literature. Eur J Gastroenterol Hepatol. 2003;15(11):1235-1240.
6. Preissler G, Graeb C, Steib C, et al. Acute liver failure, rupture and hemorrhagic shock as primary manifestation of advanced metastatic disease. Anticancer Res. 2012;32(8):3449-3454.
7. Alexopoulou A, Koskinas J, Deutsch M, Delladetsima J, Kountouras D, Dourakis SP. Acute liver failure as the initial manifestation of hepatic infiltration by a solid tumor: report of 5 cases and review of the literature. Tumori. 2006;92(4):354-357.
8. Shah KG, Modi PR, Rizvi J. Breast carcinoma metastasizing to the urinary bladder and retroperitoneum presenting as acute renal failure. Indian J Urol. 2011;27(1):135-136.
9. Nazario HE, Lepe R, Trotter JF. Metastatic breast cancer presenting as acute liver failure. Gastroenterol Hepatol (NY). 2011;7(1):65-66.
10. Rajvanshi P, Kowdley KV, Hirota WK, Meyers JB, Keeffe EB. Fulminant hepatic failure secondary to neoplastic infiltration of the liver. J Clin Gastroenterol. 2005;39(4):339-343.
11. Fairbank WH. Three atypical cases of Hodgkin’s Disease, presenting with liver failure. Can Med Assoc J. 1953;69(3):315-317.
12. Braude S, Portmann B, Gimson AE, Williams R. Fulminant hepatic failure in non-Hodgkin’s lymphoma. Postgrad Med J. 1982;58(679):301-304.
13. Lo AA, Lo EC, Li H, et al. Unique morphologic and clinical features of liver predominant/primary small cell carcinoma—autopsy and biopsy case series. Ann Diagn Pathol. 2014;18(3):151-156.
14. Hwang YT, Shin JW, Lee JH, et al. A case of fulminant hepatic failure secondary to hepatic metastasis of small cell lung carcinoma [in Korean]. Korean J Hepatol. 2007;13(4):565-570.
15. Miyaaki H, Ichikawa T, Taura N, et al. Diffuse liver metastasis of small cell lung cancer causing marked hepatomegaly and fulminant hepatic failure. Intern Med. 2010;49(14):1383-1386.
16. Sato K, Takeyama Y, Tanaka T, Fukui Y, Gonda H, Suzuki R. Fulminant hepatic failure and hepatomegaly caused by diffuse liver metastases from small cell lung carcinoma: 2 autopsy cases. Respir Investig. 2013;51(2):98-102.
17. Galus M. Liver failure due to metastatic small-cell carcinoma of the lung. Mayo Clin Proc. 1997;72(8):791.
18. Kovalev Y, Lurie M, Naschitz JE, Yeshurun D, Zuckerman E. Metastatic small cell carcinoma presenting as acute hepatic failure. Am J Gastroenterol. 2001;96(12):3471-3473.
19. McGuire BM, Cherwitz DL, Rabe KM, Ho SB. Small-cell carcinoma of the lung manifesting as acute hepatic failure. Mayo Clin Proc. 1997;72(2):133-139.
20. Richecoeur M, Massoure MP, Le Coadou G, Lipovac AS, Bronstein JA, Delluc C. Acute hepatic failure as the presenting manifestation of a metastatic lung carcinoma to liver [in French]. Rev Med Interne. 2009;30(10):911-913.
21. Valladares Ayerbes MJ, Canadas Garcia de Leon M, Reina Zoilo JJ, Valenzuela Claros JC, Ruiz Borrego M, Barea Bejarano JL. Acute liver failure as presentation form of small cell carcinoma of the lung [in Spanish]. An Med Interna. 1997;14(3):128-130.
22. Gilbert J, Rutledge H, Koch A. Diffuse malignant infiltration of the liver manifesting as a case of acute liver failure. Nat Clin Pract Gastroenterol Hepatol. 2008;5(7):405-408.
23. Vaideeswar P, Munot S, Rojekar A, Deodhar K. Hepatic diffuse intra-sinusoidal metastases of pulmonary small-cell carcinoma. J Postgrad Med. 2012;58(3):230-231.
24. Krauss EA, Ludwig PW, Sumner HW. Metastatic carcinoma presenting as fulminant hepatic failure. Am J Gastroenterol. 1979;72(6):651-654.
25. Ke E, Gomez JD, Tang K, Sriram KB. Metastatic small-cell lung cancer presenting
as fulminant hepatic failure. BMJ Case Rep. 2013;2013.
An Unusual Cause of Shortness of Breath: Primary Tracheal Basal Cell Adenocarcinoma
Salivary gland lung tumors are extremely rare intrathoracic malignancies, accounting for only 0.2% of all lung tumors.1 It has been postulated that these lung tumors arise from pluripotential cells in the epithelium of the submucosal bronchial glands and usually present as endoluminal lesions. The cause of salivary gland tumors is unclear. They seem to be unrelated to exposure to smoking, air pollutants, or other chemicals.2 Associated symptoms are generally related to endoluminal obstruction by the tumors, which are centrally located. Thus, presenting symptoms commonly include chronic cough, progressive dyspnea, hoarseness, wheezing, and occasional hemoptysis.3 Chest radiographs seem normal in most cases except those in which obstruction is present. Computed tomography usually shows well-defined endotracheal or endobronchial lesions that are lobulated, polypoid, or smooth, without infiltration into surrounding tissues.
Although basal cell adenocarcinoma (BCAC) was included in World Health Organization’s (WHO) Pathology and Genetics of Head and Neck Tumours in 1991, its definition—“an epithelial neoplasm that has cytological characteristics of basal cell adenoma (BCA), but a morphologic growth pattern indicative of malignancy”—did not appear until 2005.4 Basal cell adenocarcinoma generally is classified as a low-grade malignancy with a good long-term prognosis. It usually affects parotid and submandibular minor salivary glands.5
Histologic differentiation of BCAC and BCA is difficult and depends on whether local structures have been invaded or on which histologic features of perineural or vascular invasion are present.6 Basal cell adenocarcinoma also shows strong immunoreactivity to cytokeratin 7 (CK-7) and variable myoepithelial staining with S100.7 Because of the prognosis and potential treatment differences involved, BCAC must be differentiated from other basaloid cell tumors, such as BCA, adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, myoepithelial tumor, epithelial-myoepithelial carcinoma, and basaloid squamous cell carcinoma (SCC).8 Surgical excision is the primary treatment of choice.5 Rare in the salivary glands, BCA and BCAC are even rarer in salivary gland tissue outside the head and neck region. The authors report on a case of BCAC of the salivary gland tissue in the trachea.
Case Report
An 84-year-old man with diabetes mellitus and hypertension and a nonsmoker presented to the emergency department of the VA Caribbean Healthcare System in San Juan, Puerto Rico, with a dry cough and shortness of breath lasting 1 week, which worsened the day before
presentation. On physical examination, the patient was afebrile and in no respiratory distress, and his vital signs were within normal limits. There was no barrel chest, no prolonged expiratory phase, and occasional wheezing more prominent on the left side. A chest radiograph showed atelectasis and/or associated changes (Figure 1).
After the initial biopsy results led to a provisional diagnosis of basaloid neoplasm with squamous features, the patient underwent rigid bronchoscopic tracheal tumor debridement followed by cryotherapy at the base of the tumor.
Discussion
Primary tracheal tumors are rare, accounting for < 1% of all malignancies.9,10 According to the National Cancer Institute Surveillance, Epidemiology, and End Results database, the rate of new cases of primary carcinoma of the trachea was 2.6 per 1 million people per year.11 Of all primary tumors of the trachea, 80% are malignant9,10; the rest vary widely and include both malignant and benign histotypes.11
The abundant minor salivary gland tissue in the upper respiratory tract can potentially develop neoplasia the same as the wide spectrum seen in head and neck salivary gland tumors. The most recent (2004) WHO Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart includes a brief section on salivary gland tumors and mentions only mucoepidermoid carcinoma, adenoid cystic carcinoma, myoepithelial carcinoma, epithelial-myoepithelial carcinoma, pleomorphic adenoma, and carcinoma ex-pleomorphic adenoma.12 However, there are reports of other salivary gland tumors in the tracheopulmonary location.
García and colleagues recently described a case of hyalinizing clear cell carcinoma with its defining EWSR1-ATF1 (Ewing sarcoma breakpoint region 1–activating transcription factor 1) fusion transcript.13 Similarly, no tracheopulmonary BCAC cases have been reported in the English-language literature since 2004, when Damiani and colleagues described a case of basal adenocarcinoma of the lung.14 In 2012, Yamada and colleagues reported a case of “basaloid carcinoma with central cavitation,”15 which showed a peculiar immunohistochemical profile similar
to the tumor described in the present article—suggestive of a myoepithelial origin if the morphologic architectural features match.
Salivary gland tumors are prone to manifest the squamous phenotype. Depending on the biopsy modality, these squamous areas (squamous eddies) can be sampled and can lead to the erroneous diagnosis of SCC. However, the histologic features of squamous malignancy are
lacking in these squamous components, and the pathologist should be able to distinguish additional phenotypes that can generate a wider differential diagnosis.
On follow-up, this patient has maintained satisfactory respiratory function. Starting 2 years after the initial resection, he has had annual checkups. Recent imaging and bronchoscopy have revealed tumor recurrence in the same site. Because of the patient’s extensive comorbidities, surgical intervention has been deferred in favor of cryotherapy, a less aggressive therapy.
Author disclosure
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Moran CA. Primary salivary gland-type tumors of the lung. Semin Diagn Pathol.
1995;12(2):106-122.
2. Molina JR, Aubry MC, Lewis JE, et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer. 2007;110(10):2253-2259.
3. Brutinel WM, Cortese DA, McDougall JC, Gillio RG, Bergstralh EJ. A two-year experience with the neodymium-YAG laser in endobronchial obstruction. Chest. 1987;91(2):159-165.
4. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. Lyon, France: IARC Press; 2005. International Agency for Research on Cancer website. https://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb9/BB9.pdf. Accessed June 16, 2016.
5. Sarath PV, Kannan N, Patil R, Manne RK, Swapna B, Suneel Kumar KV. Basal cell adenocarcinoma of the minor salivary glands involving palate and maxillary sinus. J Clin Imaging Sci. 2013;3(suppl 1):4.
6. Farrell T, Chang YL. Basal cell adenocarcinoma of minor salivary glands. Arch Pathol Lab Med. 2007;131(10):1602-1604.
7. Jung MJ, Roh JL, Choi SH, et al. Basal cell adenocarcinoma of the salivary gland: a morphological and immunohistochemical comparison with basal cell adenoma with and without capsular invasion. Diagn Pathol. 2013;8:171.
8. Jäkel KT, Löning T. Differential diagnosis of basaloid salivary gland tumors [in German]. Pathologe. 2004;25(1):46-55.
9. Urdaneta AI, Yu JB, Wilson LD. Population based cancer registry analysis of primary tracheal carcinoma. Am J Clin Oncol. 2011;34(1):32-37.
10. Varadhachary GR, Rabe MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-765.
11. Pelosi G, Fraggetta F, Maffini F, Solli P, Cavallon A, Viale G. Pulmonary epithelialmyoepithelial tumor of unproven malignant potential: report of a case and review
of the literature. Mod Pathol. 2001;14(5):521-526.
12. Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004. International Agency for Research on Cancer website. https://www.iarc.fr/en/publications/pdfs-online /pat-gen/bb10/BB10.pdf. Accessed June 29, 2016.
13. García JJ, Jin L, Jackson SB, et al. Primary pulmonary hyalinizing clear cell carcinoma of bronchial submucosal gland origin. Hum Pathol. 2015;46(3):471-475.
14. Damiani S, Magrini E, Farnedi A, Pession A. Basal cell (myoepithelial) adenocarcinoma of the lung. First case with cytogenetic findings. Histopathology. 2004;45(4):422-424.
15. Yamada S, Noguchi H, Nabeshima A, et al. Basaloid carcinoma of the lung associated with central cavitation: a unique surgical case focusing on cytological and immunohistochemical findings. Diagn Pathol. 2012;7:175-180.
Note: Page numbers differ between the print issue and digital edition.
Salivary gland lung tumors are extremely rare intrathoracic malignancies, accounting for only 0.2% of all lung tumors.1 It has been postulated that these lung tumors arise from pluripotential cells in the epithelium of the submucosal bronchial glands and usually present as endoluminal lesions. The cause of salivary gland tumors is unclear. They seem to be unrelated to exposure to smoking, air pollutants, or other chemicals.2 Associated symptoms are generally related to endoluminal obstruction by the tumors, which are centrally located. Thus, presenting symptoms commonly include chronic cough, progressive dyspnea, hoarseness, wheezing, and occasional hemoptysis.3 Chest radiographs seem normal in most cases except those in which obstruction is present. Computed tomography usually shows well-defined endotracheal or endobronchial lesions that are lobulated, polypoid, or smooth, without infiltration into surrounding tissues.
Although basal cell adenocarcinoma (BCAC) was included in World Health Organization’s (WHO) Pathology and Genetics of Head and Neck Tumours in 1991, its definition—“an epithelial neoplasm that has cytological characteristics of basal cell adenoma (BCA), but a morphologic growth pattern indicative of malignancy”—did not appear until 2005.4 Basal cell adenocarcinoma generally is classified as a low-grade malignancy with a good long-term prognosis. It usually affects parotid and submandibular minor salivary glands.5
Histologic differentiation of BCAC and BCA is difficult and depends on whether local structures have been invaded or on which histologic features of perineural or vascular invasion are present.6 Basal cell adenocarcinoma also shows strong immunoreactivity to cytokeratin 7 (CK-7) and variable myoepithelial staining with S100.7 Because of the prognosis and potential treatment differences involved, BCAC must be differentiated from other basaloid cell tumors, such as BCA, adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, myoepithelial tumor, epithelial-myoepithelial carcinoma, and basaloid squamous cell carcinoma (SCC).8 Surgical excision is the primary treatment of choice.5 Rare in the salivary glands, BCA and BCAC are even rarer in salivary gland tissue outside the head and neck region. The authors report on a case of BCAC of the salivary gland tissue in the trachea.
Case Report
An 84-year-old man with diabetes mellitus and hypertension and a nonsmoker presented to the emergency department of the VA Caribbean Healthcare System in San Juan, Puerto Rico, with a dry cough and shortness of breath lasting 1 week, which worsened the day before
presentation. On physical examination, the patient was afebrile and in no respiratory distress, and his vital signs were within normal limits. There was no barrel chest, no prolonged expiratory phase, and occasional wheezing more prominent on the left side. A chest radiograph showed atelectasis and/or associated changes (Figure 1).
After the initial biopsy results led to a provisional diagnosis of basaloid neoplasm with squamous features, the patient underwent rigid bronchoscopic tracheal tumor debridement followed by cryotherapy at the base of the tumor.
Discussion
Primary tracheal tumors are rare, accounting for < 1% of all malignancies.9,10 According to the National Cancer Institute Surveillance, Epidemiology, and End Results database, the rate of new cases of primary carcinoma of the trachea was 2.6 per 1 million people per year.11 Of all primary tumors of the trachea, 80% are malignant9,10; the rest vary widely and include both malignant and benign histotypes.11
The abundant minor salivary gland tissue in the upper respiratory tract can potentially develop neoplasia the same as the wide spectrum seen in head and neck salivary gland tumors. The most recent (2004) WHO Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart includes a brief section on salivary gland tumors and mentions only mucoepidermoid carcinoma, adenoid cystic carcinoma, myoepithelial carcinoma, epithelial-myoepithelial carcinoma, pleomorphic adenoma, and carcinoma ex-pleomorphic adenoma.12 However, there are reports of other salivary gland tumors in the tracheopulmonary location.
García and colleagues recently described a case of hyalinizing clear cell carcinoma with its defining EWSR1-ATF1 (Ewing sarcoma breakpoint region 1–activating transcription factor 1) fusion transcript.13 Similarly, no tracheopulmonary BCAC cases have been reported in the English-language literature since 2004, when Damiani and colleagues described a case of basal adenocarcinoma of the lung.14 In 2012, Yamada and colleagues reported a case of “basaloid carcinoma with central cavitation,”15 which showed a peculiar immunohistochemical profile similar
to the tumor described in the present article—suggestive of a myoepithelial origin if the morphologic architectural features match.
Salivary gland tumors are prone to manifest the squamous phenotype. Depending on the biopsy modality, these squamous areas (squamous eddies) can be sampled and can lead to the erroneous diagnosis of SCC. However, the histologic features of squamous malignancy are
lacking in these squamous components, and the pathologist should be able to distinguish additional phenotypes that can generate a wider differential diagnosis.
On follow-up, this patient has maintained satisfactory respiratory function. Starting 2 years after the initial resection, he has had annual checkups. Recent imaging and bronchoscopy have revealed tumor recurrence in the same site. Because of the patient’s extensive comorbidities, surgical intervention has been deferred in favor of cryotherapy, a less aggressive therapy.
Author disclosure
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Salivary gland lung tumors are extremely rare intrathoracic malignancies, accounting for only 0.2% of all lung tumors.1 It has been postulated that these lung tumors arise from pluripotential cells in the epithelium of the submucosal bronchial glands and usually present as endoluminal lesions. The cause of salivary gland tumors is unclear. They seem to be unrelated to exposure to smoking, air pollutants, or other chemicals.2 Associated symptoms are generally related to endoluminal obstruction by the tumors, which are centrally located. Thus, presenting symptoms commonly include chronic cough, progressive dyspnea, hoarseness, wheezing, and occasional hemoptysis.3 Chest radiographs seem normal in most cases except those in which obstruction is present. Computed tomography usually shows well-defined endotracheal or endobronchial lesions that are lobulated, polypoid, or smooth, without infiltration into surrounding tissues.
Although basal cell adenocarcinoma (BCAC) was included in World Health Organization’s (WHO) Pathology and Genetics of Head and Neck Tumours in 1991, its definition—“an epithelial neoplasm that has cytological characteristics of basal cell adenoma (BCA), but a morphologic growth pattern indicative of malignancy”—did not appear until 2005.4 Basal cell adenocarcinoma generally is classified as a low-grade malignancy with a good long-term prognosis. It usually affects parotid and submandibular minor salivary glands.5
Histologic differentiation of BCAC and BCA is difficult and depends on whether local structures have been invaded or on which histologic features of perineural or vascular invasion are present.6 Basal cell adenocarcinoma also shows strong immunoreactivity to cytokeratin 7 (CK-7) and variable myoepithelial staining with S100.7 Because of the prognosis and potential treatment differences involved, BCAC must be differentiated from other basaloid cell tumors, such as BCA, adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, myoepithelial tumor, epithelial-myoepithelial carcinoma, and basaloid squamous cell carcinoma (SCC).8 Surgical excision is the primary treatment of choice.5 Rare in the salivary glands, BCA and BCAC are even rarer in salivary gland tissue outside the head and neck region. The authors report on a case of BCAC of the salivary gland tissue in the trachea.
Case Report
An 84-year-old man with diabetes mellitus and hypertension and a nonsmoker presented to the emergency department of the VA Caribbean Healthcare System in San Juan, Puerto Rico, with a dry cough and shortness of breath lasting 1 week, which worsened the day before
presentation. On physical examination, the patient was afebrile and in no respiratory distress, and his vital signs were within normal limits. There was no barrel chest, no prolonged expiratory phase, and occasional wheezing more prominent on the left side. A chest radiograph showed atelectasis and/or associated changes (Figure 1).
After the initial biopsy results led to a provisional diagnosis of basaloid neoplasm with squamous features, the patient underwent rigid bronchoscopic tracheal tumor debridement followed by cryotherapy at the base of the tumor.
Discussion
Primary tracheal tumors are rare, accounting for < 1% of all malignancies.9,10 According to the National Cancer Institute Surveillance, Epidemiology, and End Results database, the rate of new cases of primary carcinoma of the trachea was 2.6 per 1 million people per year.11 Of all primary tumors of the trachea, 80% are malignant9,10; the rest vary widely and include both malignant and benign histotypes.11
The abundant minor salivary gland tissue in the upper respiratory tract can potentially develop neoplasia the same as the wide spectrum seen in head and neck salivary gland tumors. The most recent (2004) WHO Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart includes a brief section on salivary gland tumors and mentions only mucoepidermoid carcinoma, adenoid cystic carcinoma, myoepithelial carcinoma, epithelial-myoepithelial carcinoma, pleomorphic adenoma, and carcinoma ex-pleomorphic adenoma.12 However, there are reports of other salivary gland tumors in the tracheopulmonary location.
García and colleagues recently described a case of hyalinizing clear cell carcinoma with its defining EWSR1-ATF1 (Ewing sarcoma breakpoint region 1–activating transcription factor 1) fusion transcript.13 Similarly, no tracheopulmonary BCAC cases have been reported in the English-language literature since 2004, when Damiani and colleagues described a case of basal adenocarcinoma of the lung.14 In 2012, Yamada and colleagues reported a case of “basaloid carcinoma with central cavitation,”15 which showed a peculiar immunohistochemical profile similar
to the tumor described in the present article—suggestive of a myoepithelial origin if the morphologic architectural features match.
Salivary gland tumors are prone to manifest the squamous phenotype. Depending on the biopsy modality, these squamous areas (squamous eddies) can be sampled and can lead to the erroneous diagnosis of SCC. However, the histologic features of squamous malignancy are
lacking in these squamous components, and the pathologist should be able to distinguish additional phenotypes that can generate a wider differential diagnosis.
On follow-up, this patient has maintained satisfactory respiratory function. Starting 2 years after the initial resection, he has had annual checkups. Recent imaging and bronchoscopy have revealed tumor recurrence in the same site. Because of the patient’s extensive comorbidities, surgical intervention has been deferred in favor of cryotherapy, a less aggressive therapy.
Author disclosure
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Moran CA. Primary salivary gland-type tumors of the lung. Semin Diagn Pathol.
1995;12(2):106-122.
2. Molina JR, Aubry MC, Lewis JE, et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer. 2007;110(10):2253-2259.
3. Brutinel WM, Cortese DA, McDougall JC, Gillio RG, Bergstralh EJ. A two-year experience with the neodymium-YAG laser in endobronchial obstruction. Chest. 1987;91(2):159-165.
4. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. Lyon, France: IARC Press; 2005. International Agency for Research on Cancer website. https://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb9/BB9.pdf. Accessed June 16, 2016.
5. Sarath PV, Kannan N, Patil R, Manne RK, Swapna B, Suneel Kumar KV. Basal cell adenocarcinoma of the minor salivary glands involving palate and maxillary sinus. J Clin Imaging Sci. 2013;3(suppl 1):4.
6. Farrell T, Chang YL. Basal cell adenocarcinoma of minor salivary glands. Arch Pathol Lab Med. 2007;131(10):1602-1604.
7. Jung MJ, Roh JL, Choi SH, et al. Basal cell adenocarcinoma of the salivary gland: a morphological and immunohistochemical comparison with basal cell adenoma with and without capsular invasion. Diagn Pathol. 2013;8:171.
8. Jäkel KT, Löning T. Differential diagnosis of basaloid salivary gland tumors [in German]. Pathologe. 2004;25(1):46-55.
9. Urdaneta AI, Yu JB, Wilson LD. Population based cancer registry analysis of primary tracheal carcinoma. Am J Clin Oncol. 2011;34(1):32-37.
10. Varadhachary GR, Rabe MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-765.
11. Pelosi G, Fraggetta F, Maffini F, Solli P, Cavallon A, Viale G. Pulmonary epithelialmyoepithelial tumor of unproven malignant potential: report of a case and review
of the literature. Mod Pathol. 2001;14(5):521-526.
12. Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004. International Agency for Research on Cancer website. https://www.iarc.fr/en/publications/pdfs-online /pat-gen/bb10/BB10.pdf. Accessed June 29, 2016.
13. García JJ, Jin L, Jackson SB, et al. Primary pulmonary hyalinizing clear cell carcinoma of bronchial submucosal gland origin. Hum Pathol. 2015;46(3):471-475.
14. Damiani S, Magrini E, Farnedi A, Pession A. Basal cell (myoepithelial) adenocarcinoma of the lung. First case with cytogenetic findings. Histopathology. 2004;45(4):422-424.
15. Yamada S, Noguchi H, Nabeshima A, et al. Basaloid carcinoma of the lung associated with central cavitation: a unique surgical case focusing on cytological and immunohistochemical findings. Diagn Pathol. 2012;7:175-180.
Note: Page numbers differ between the print issue and digital edition.
1. Moran CA. Primary salivary gland-type tumors of the lung. Semin Diagn Pathol.
1995;12(2):106-122.
2. Molina JR, Aubry MC, Lewis JE, et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer. 2007;110(10):2253-2259.
3. Brutinel WM, Cortese DA, McDougall JC, Gillio RG, Bergstralh EJ. A two-year experience with the neodymium-YAG laser in endobronchial obstruction. Chest. 1987;91(2):159-165.
4. Barnes L, Eveson JW, Reichart P, Sidransky D, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. Lyon, France: IARC Press; 2005. International Agency for Research on Cancer website. https://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb9/BB9.pdf. Accessed June 16, 2016.
5. Sarath PV, Kannan N, Patil R, Manne RK, Swapna B, Suneel Kumar KV. Basal cell adenocarcinoma of the minor salivary glands involving palate and maxillary sinus. J Clin Imaging Sci. 2013;3(suppl 1):4.
6. Farrell T, Chang YL. Basal cell adenocarcinoma of minor salivary glands. Arch Pathol Lab Med. 2007;131(10):1602-1604.
7. Jung MJ, Roh JL, Choi SH, et al. Basal cell adenocarcinoma of the salivary gland: a morphological and immunohistochemical comparison with basal cell adenoma with and without capsular invasion. Diagn Pathol. 2013;8:171.
8. Jäkel KT, Löning T. Differential diagnosis of basaloid salivary gland tumors [in German]. Pathologe. 2004;25(1):46-55.
9. Urdaneta AI, Yu JB, Wilson LD. Population based cancer registry analysis of primary tracheal carcinoma. Am J Clin Oncol. 2011;34(1):32-37.
10. Varadhachary GR, Rabe MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-765.
11. Pelosi G, Fraggetta F, Maffini F, Solli P, Cavallon A, Viale G. Pulmonary epithelialmyoepithelial tumor of unproven malignant potential: report of a case and review
of the literature. Mod Pathol. 2001;14(5):521-526.
12. Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004. International Agency for Research on Cancer website. https://www.iarc.fr/en/publications/pdfs-online /pat-gen/bb10/BB10.pdf. Accessed June 29, 2016.
13. García JJ, Jin L, Jackson SB, et al. Primary pulmonary hyalinizing clear cell carcinoma of bronchial submucosal gland origin. Hum Pathol. 2015;46(3):471-475.
14. Damiani S, Magrini E, Farnedi A, Pession A. Basal cell (myoepithelial) adenocarcinoma of the lung. First case with cytogenetic findings. Histopathology. 2004;45(4):422-424.
15. Yamada S, Noguchi H, Nabeshima A, et al. Basaloid carcinoma of the lung associated with central cavitation: a unique surgical case focusing on cytological and immunohistochemical findings. Diagn Pathol. 2012;7:175-180.
Note: Page numbers differ between the print issue and digital edition.
Long-Term Survival of a Patient With Late-Stage Non-Small Cell Lung Cancer
Lung cancer is the leading cause of cancer death in the world, with non-small cell lung cancer (NSCLC) a significant component of those deaths.1,2 Treatments for advanced-stage NSCLC, however, are limited. Erlotinib, a small-molecule tyrosine kinase inhibitor of epidermal growth factor receptor (EGFR), has aided in advancing NSCLC therapy. Erlotinib has been shown to increase survival by 2 months compared with placebo in a phase 3, randomized controlled trial when used as second- or third-line therapy.3 The authors present a case of a man surviving almost 8 years with late-stage NSCLC on treatment with erlotinib at the VA West Los Angeles Medical Center (WLAMC).
Note: Page numbers differ between the print issue and digital edition.
Lung cancer is the leading cause of cancer death in the world, with non-small cell lung cancer (NSCLC) a significant component of those deaths.1,2 Treatments for advanced-stage NSCLC, however, are limited. Erlotinib, a small-molecule tyrosine kinase inhibitor of epidermal growth factor receptor (EGFR), has aided in advancing NSCLC therapy. Erlotinib has been shown to increase survival by 2 months compared with placebo in a phase 3, randomized controlled trial when used as second- or third-line therapy.3 The authors present a case of a man surviving almost 8 years with late-stage NSCLC on treatment with erlotinib at the VA West Los Angeles Medical Center (WLAMC).
Lung cancer is the leading cause of cancer death in the world, with non-small cell lung cancer (NSCLC) a significant component of those deaths.1,2 Treatments for advanced-stage NSCLC, however, are limited. Erlotinib, a small-molecule tyrosine kinase inhibitor of epidermal growth factor receptor (EGFR), has aided in advancing NSCLC therapy. Erlotinib has been shown to increase survival by 2 months compared with placebo in a phase 3, randomized controlled trial when used as second- or third-line therapy.3 The authors present a case of a man surviving almost 8 years with late-stage NSCLC on treatment with erlotinib at the VA West Los Angeles Medical Center (WLAMC).
Note: Page numbers differ between the print issue and digital edition.
Note: Page numbers differ between the print issue and digital edition.
Long-Term Survival of a Patient With Late-Stage Non-Small Cell Lung Cancer
Lung cancer is the leading cause of cancer death in the world, with non-small cell lung cancer (NSCLC) a significant component of those deaths.1,2 Treatments for advanced-stage NSCLC, however, are limited. Erlotinib, a small-molecule tyrosine kinase inhibitor of epidermal growth factor receptor (EGFR), has aided in advancing NSCLC therapy. Erlotinib has been shown to increase survival by 2 months compared with placebo in a phase 3, randomized controlled trial when used as second- or third-line therapy.3 The authors present a case of a man surviving almost 8 years with late-stage NSCLC on treatment with erlotinib at the VA West Los Angeles Medical Center (WLAMC).
Case Report
Mr. J is a 59-year-old man with a medical history of hepatitis C. He smoked 2 packs of cigarettes a day for 25 years and quit in 2003. He also had a known history of IV drug use. He was unaware of his family history because he was adopted, but his twin sister, who has no known medical problems, is also a smoker. In 2005, when Mr. J was evaluated for treatment options of long-standing hepatitis C with liver ultrasound, a large, irregular right adrenal mass was found, measuring 6.4 x 3.5 cm. A subsequent positron emission tomography (PET) scan identified a lung nodule measuring 3.8 x 3.6 x 3.3 cm. A biopsy guided by computed tomography (CT) showed NSCLC. Subsequently, metastases to the liver and adrenal glands were noted, and Mr. J was started on chemotherapy. He received 4 cycles of carboplatin 725 mg and gemcitabine 2,000 mg as well as a thoracotomy and left upper lung wedge resection in December 2005. His pain was controlled with slow-release morphine 15 mg 2 times per day and oxycodone 5 mg and acetaminophen 325 mg 4 times per day as needed for breakthrough pain.
In 2006, after 4 cycles of chemotherapy, the size of the adrenal mass and the lung mass had decreased; however, he developed new abdominal pain. A CT scan showed new intrahepatic and extrahepatic biliary dilatation and worsening pancreatic function. He could not tolerate the recommended endoscopic ultrasound and left WLAMC, later presenting to an outside hospital for his abdominal pain.
At the outside hospital, 2 masses that were surgically removed from the head of the pancreas were confirmed to be EGFR-positive NSCLC, and he was given 4 cycles of cisplatin and irinotecan at unknown doses. The only adverse effects (AEs) Mr. J reported during this period were nausea and vomiting immediately after chemotherapy. He failed to respond to this treatment and was started on bevacizumab, also at an unknown dose. The patient again did not respond and was transitioned to erlotinib 150 mg daily. The patient showed remarkable response, with lesions decreasing in size.
The patient returned to the WLAMC with multiple ulcerated lesions on his face, chest, back, and extremities and hair loss, which he reported all began within weeks of starting erlotinib. Later, he also developed trichomegaly, also presumed to be a consequence of erlotinib. Despite these AEs, erlotinib was continued at the same dose, given his impressive response to this treatment, the absence of response to other therapy, and the patient’s insistence on continuing the medication.
Of note, after his transition to the outside hospital, Mr. J and his family paid all his medical expenses because he had no insurance. His family was very supportive, and the patient described their motivation and support as paramount in his receiving treatment.
In 2008, Mr. J presented to a dermatologist and was treated with cleocin solution. Although this helped to control his symptoms, the rash persisted. As a complication of these lesions, he also experienced several superinfections for which he was treated with cephalexin. At this same time, a PET scan showed no evidence of disease. He presented to the pain service for persistent chest wall pain around the surgical site, and his pain regimen was changed to slow-release morphine 200 mg 3 times per day and morphine sulfate solution 20 mg/mL 80 mg every 4 hours for breakthrough pain.
The PET scans, which were repeated every 3 months after Mr. J resumed treatment at WLAMC, showed continued absence of disease. In 2009, when he presented to the hospital with pneumonia, a PET scan showed 2 new areas of tracer uptake measuring 1 cm. His chest wall was irradiated, but radiation therapy was stopped after the biopsy returned benign. In 2015, an annual PET scan showed only evidence of postsurgical changes.
Discussion
The benefits of EGFR therapy have been established for treatment of late-stage NSCLC, but such therapy has limitations. For advanced-stage NSCLC, erlotinib has been shown to improve disease-free progression by 2.7 to 3.25 months and overall survival by 6.7 to 7.9 months.4-6 However, 1-year survival estimates remain as low as 35.0 to 37.7%,5,6 and its utility as first-line therapy has been questioned; randomized control trials have shown EGFR therapy to be of benefit only as secondor third-line therapy, when used with platinum-based chemotherapeutics.3,4 The few reports of complete response, however, have not included a definition of duration of survival.5,6
Occasionally, there have been reports of patients surviving for significantly longer periods, including 1 report of a patient who survived with complete remission for 2 years.7 In another case report, a patient experienced partial remission for more than 1 year with erlotinib as a third-line therapy.8 Although several reports indicated prolonged survival with erlotinib, or induction of complete remission of metastasis, survival has not been longer than 2 years.9-12
Important considerations for use of erlotinib are factors that predict a positive treatment response, including female sex, no previous exposure to tobacco, Asian origin, and adenocarcinoma on histologic examination.3,13 Mr. J did not meet any of these criteria. Interestingly, one study examining characteristics predictive of a positive response to erlotinib did not show that EGFR gene mutations were associated with response, although other studies have shown this to be a significant predictor of response.3,14,15
In this patient, his impressive response to erlotinib was most likely augmented by the presence of the EGFR mutation. Additionally, some reports indicate that pretreatment with platinum-based therapy can induce genetic changes resulting in EGFR mutations, thus enabling the benefit of erlotinib.10 Given that his biopsy results were not tested for the EGFR mutation prior to initiating carboplatin, this is a possibility.
Other factors specific to Mr. J that may have influenced his response to therapy include his personal wealth, which may have given him direct access to physicians outside the VA. His family support also may have motivated him to pursue and continue treatment, thus augmenting his survival. This support likely contributed in large part to his continuing erlotinib therapy despite the severe rash, hair loss, and trichomegaly. Other AEs associated with long-term erlotinib therapy include folliculitis, diarrhea, fatigue, and paronychia,although Mr. J did not experience these.16
Conclusion
Mr. J continues to follow up regularly at WLAMC. To the authors’ knowledge, this patient’s 8 year survival is the longest length of survival for any patient with NSCLC on erlotinib therapy. While the therapeutic benefits of erlotinib as a second-line therapy have been shown, EGFR therapy may be more effective than previously thought. Further research is needed to fully understand the benefits of erlotinib.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Ridge CA, McErlean AM, Ginsberg MS. Epidemiology of lung cancer. Semin Intervent Radiol. 2013;30(2):93-98.
2. Gridelli C, Bareschino MA, Schettino C, Rossi A, Maione P, Ciardiello F. Erlotinib in non-small cell lung cancer treatment: current status and future development. Oncologist. 2007;12(7):840-849.
3. Shepherd FA, Pereira JR, Ciuleanu T, et al; National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non–small-cell lung cancer. N Engl J Med. 2005;353(2):123-132.
4. Smith J. Erlotinib: small-molecule targeted therapy in the treatment of non-smallcell lung cancer. Clin Ther. 2005;27(10):1513-1534.
5. Boyer M, Horwood K, Pavlakis N, et al. Efficacy of erlotinib in patients with advanced non-small-cell lung cancer (NSCLC): analysis of the Australian subpopulation of the TRUST study. Asia Pac J Clin Oncol. 2012;8(3):248-254.
6. Reck M, van Zandwijk N, Gridelli C, et al. Erlotinib in advanced non-small cell lung cancer: efficacy and safety findings of the global phase IV Tarceva Lung Cancer Survival Treatment study. J Thorac Oncol. 2010;5(10):1616-1622.
7. Vitale MG, Riccardi F, Mocerino C, et al. Erlotinib-induced complete response in a patient with epidermal growth factor receptor wild-type lung adenocarcinoma after chemotherapy failure: a case report. J Med Case Rep. 2014;8:102.
8. Duchnowska R, Siemiatkowska A, Grala B, Smoter M. Long-term remission after erlotinib therapy in an elderly patient with advanced non-small-cell lung cancer. Case report and conclusions for clinical practice [in Polish]. Pneumonol Alergol Pol. 2008;76(6):451-455.
9. Lai CSL, Boshoff C, Falzon M, Lee SM. Complete response to erlotinib treatment in brain metastases from recurrent NSCLC. Thorax. 2006;61(1):91.
10. Karam I, Melosky B. Response to second-line erlotinib in an EGFR mutationnegative patient with non-small-cell lung cancer: make no assumptions. Curr Oncol. 2012;19(1):42-46.
11. Kobayashi T, Koizumi T, Agatsuma T, et al. A phase II trial of erlotinib in patients with EGFR wild-type advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2012;69(5):1241-1246.
12. Gridelli C, Maione P, Galetta D, et al. Three cases of long-lasting tumor control with erlotinib after progression with gefitinib in advanced non-small cell lung cancer. J Thorac Oncol. 2007;2(8):758-761.
13. Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21(12):2237-2246.
14. Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med. 2005;353(2):133-144.
15. Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25(5):587-595.
16. Becker A, van Wijk A, Smit EF, Postmus PE. Side-effects of long-term administration of erlotinib in patients with non-small cell lung cancer. J Thorac Oncol. 2010;5(9):1477-1480.
Note: Page numbers differ between the print issue and digital edition.
Lung cancer is the leading cause of cancer death in the world, with non-small cell lung cancer (NSCLC) a significant component of those deaths.1,2 Treatments for advanced-stage NSCLC, however, are limited. Erlotinib, a small-molecule tyrosine kinase inhibitor of epidermal growth factor receptor (EGFR), has aided in advancing NSCLC therapy. Erlotinib has been shown to increase survival by 2 months compared with placebo in a phase 3, randomized controlled trial when used as second- or third-line therapy.3 The authors present a case of a man surviving almost 8 years with late-stage NSCLC on treatment with erlotinib at the VA West Los Angeles Medical Center (WLAMC).
Case Report
Mr. J is a 59-year-old man with a medical history of hepatitis C. He smoked 2 packs of cigarettes a day for 25 years and quit in 2003. He also had a known history of IV drug use. He was unaware of his family history because he was adopted, but his twin sister, who has no known medical problems, is also a smoker. In 2005, when Mr. J was evaluated for treatment options of long-standing hepatitis C with liver ultrasound, a large, irregular right adrenal mass was found, measuring 6.4 x 3.5 cm. A subsequent positron emission tomography (PET) scan identified a lung nodule measuring 3.8 x 3.6 x 3.3 cm. A biopsy guided by computed tomography (CT) showed NSCLC. Subsequently, metastases to the liver and adrenal glands were noted, and Mr. J was started on chemotherapy. He received 4 cycles of carboplatin 725 mg and gemcitabine 2,000 mg as well as a thoracotomy and left upper lung wedge resection in December 2005. His pain was controlled with slow-release morphine 15 mg 2 times per day and oxycodone 5 mg and acetaminophen 325 mg 4 times per day as needed for breakthrough pain.
In 2006, after 4 cycles of chemotherapy, the size of the adrenal mass and the lung mass had decreased; however, he developed new abdominal pain. A CT scan showed new intrahepatic and extrahepatic biliary dilatation and worsening pancreatic function. He could not tolerate the recommended endoscopic ultrasound and left WLAMC, later presenting to an outside hospital for his abdominal pain.
At the outside hospital, 2 masses that were surgically removed from the head of the pancreas were confirmed to be EGFR-positive NSCLC, and he was given 4 cycles of cisplatin and irinotecan at unknown doses. The only adverse effects (AEs) Mr. J reported during this period were nausea and vomiting immediately after chemotherapy. He failed to respond to this treatment and was started on bevacizumab, also at an unknown dose. The patient again did not respond and was transitioned to erlotinib 150 mg daily. The patient showed remarkable response, with lesions decreasing in size.
The patient returned to the WLAMC with multiple ulcerated lesions on his face, chest, back, and extremities and hair loss, which he reported all began within weeks of starting erlotinib. Later, he also developed trichomegaly, also presumed to be a consequence of erlotinib. Despite these AEs, erlotinib was continued at the same dose, given his impressive response to this treatment, the absence of response to other therapy, and the patient’s insistence on continuing the medication.
Of note, after his transition to the outside hospital, Mr. J and his family paid all his medical expenses because he had no insurance. His family was very supportive, and the patient described their motivation and support as paramount in his receiving treatment.
In 2008, Mr. J presented to a dermatologist and was treated with cleocin solution. Although this helped to control his symptoms, the rash persisted. As a complication of these lesions, he also experienced several superinfections for which he was treated with cephalexin. At this same time, a PET scan showed no evidence of disease. He presented to the pain service for persistent chest wall pain around the surgical site, and his pain regimen was changed to slow-release morphine 200 mg 3 times per day and morphine sulfate solution 20 mg/mL 80 mg every 4 hours for breakthrough pain.
The PET scans, which were repeated every 3 months after Mr. J resumed treatment at WLAMC, showed continued absence of disease. In 2009, when he presented to the hospital with pneumonia, a PET scan showed 2 new areas of tracer uptake measuring 1 cm. His chest wall was irradiated, but radiation therapy was stopped after the biopsy returned benign. In 2015, an annual PET scan showed only evidence of postsurgical changes.
Discussion
The benefits of EGFR therapy have been established for treatment of late-stage NSCLC, but such therapy has limitations. For advanced-stage NSCLC, erlotinib has been shown to improve disease-free progression by 2.7 to 3.25 months and overall survival by 6.7 to 7.9 months.4-6 However, 1-year survival estimates remain as low as 35.0 to 37.7%,5,6 and its utility as first-line therapy has been questioned; randomized control trials have shown EGFR therapy to be of benefit only as secondor third-line therapy, when used with platinum-based chemotherapeutics.3,4 The few reports of complete response, however, have not included a definition of duration of survival.5,6
Occasionally, there have been reports of patients surviving for significantly longer periods, including 1 report of a patient who survived with complete remission for 2 years.7 In another case report, a patient experienced partial remission for more than 1 year with erlotinib as a third-line therapy.8 Although several reports indicated prolonged survival with erlotinib, or induction of complete remission of metastasis, survival has not been longer than 2 years.9-12
Important considerations for use of erlotinib are factors that predict a positive treatment response, including female sex, no previous exposure to tobacco, Asian origin, and adenocarcinoma on histologic examination.3,13 Mr. J did not meet any of these criteria. Interestingly, one study examining characteristics predictive of a positive response to erlotinib did not show that EGFR gene mutations were associated with response, although other studies have shown this to be a significant predictor of response.3,14,15
In this patient, his impressive response to erlotinib was most likely augmented by the presence of the EGFR mutation. Additionally, some reports indicate that pretreatment with platinum-based therapy can induce genetic changes resulting in EGFR mutations, thus enabling the benefit of erlotinib.10 Given that his biopsy results were not tested for the EGFR mutation prior to initiating carboplatin, this is a possibility.
Other factors specific to Mr. J that may have influenced his response to therapy include his personal wealth, which may have given him direct access to physicians outside the VA. His family support also may have motivated him to pursue and continue treatment, thus augmenting his survival. This support likely contributed in large part to his continuing erlotinib therapy despite the severe rash, hair loss, and trichomegaly. Other AEs associated with long-term erlotinib therapy include folliculitis, diarrhea, fatigue, and paronychia,although Mr. J did not experience these.16
Conclusion
Mr. J continues to follow up regularly at WLAMC. To the authors’ knowledge, this patient’s 8 year survival is the longest length of survival for any patient with NSCLC on erlotinib therapy. While the therapeutic benefits of erlotinib as a second-line therapy have been shown, EGFR therapy may be more effective than previously thought. Further research is needed to fully understand the benefits of erlotinib.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Lung cancer is the leading cause of cancer death in the world, with non-small cell lung cancer (NSCLC) a significant component of those deaths.1,2 Treatments for advanced-stage NSCLC, however, are limited. Erlotinib, a small-molecule tyrosine kinase inhibitor of epidermal growth factor receptor (EGFR), has aided in advancing NSCLC therapy. Erlotinib has been shown to increase survival by 2 months compared with placebo in a phase 3, randomized controlled trial when used as second- or third-line therapy.3 The authors present a case of a man surviving almost 8 years with late-stage NSCLC on treatment with erlotinib at the VA West Los Angeles Medical Center (WLAMC).
Case Report
Mr. J is a 59-year-old man with a medical history of hepatitis C. He smoked 2 packs of cigarettes a day for 25 years and quit in 2003. He also had a known history of IV drug use. He was unaware of his family history because he was adopted, but his twin sister, who has no known medical problems, is also a smoker. In 2005, when Mr. J was evaluated for treatment options of long-standing hepatitis C with liver ultrasound, a large, irregular right adrenal mass was found, measuring 6.4 x 3.5 cm. A subsequent positron emission tomography (PET) scan identified a lung nodule measuring 3.8 x 3.6 x 3.3 cm. A biopsy guided by computed tomography (CT) showed NSCLC. Subsequently, metastases to the liver and adrenal glands were noted, and Mr. J was started on chemotherapy. He received 4 cycles of carboplatin 725 mg and gemcitabine 2,000 mg as well as a thoracotomy and left upper lung wedge resection in December 2005. His pain was controlled with slow-release morphine 15 mg 2 times per day and oxycodone 5 mg and acetaminophen 325 mg 4 times per day as needed for breakthrough pain.
In 2006, after 4 cycles of chemotherapy, the size of the adrenal mass and the lung mass had decreased; however, he developed new abdominal pain. A CT scan showed new intrahepatic and extrahepatic biliary dilatation and worsening pancreatic function. He could not tolerate the recommended endoscopic ultrasound and left WLAMC, later presenting to an outside hospital for his abdominal pain.
At the outside hospital, 2 masses that were surgically removed from the head of the pancreas were confirmed to be EGFR-positive NSCLC, and he was given 4 cycles of cisplatin and irinotecan at unknown doses. The only adverse effects (AEs) Mr. J reported during this period were nausea and vomiting immediately after chemotherapy. He failed to respond to this treatment and was started on bevacizumab, also at an unknown dose. The patient again did not respond and was transitioned to erlotinib 150 mg daily. The patient showed remarkable response, with lesions decreasing in size.
The patient returned to the WLAMC with multiple ulcerated lesions on his face, chest, back, and extremities and hair loss, which he reported all began within weeks of starting erlotinib. Later, he also developed trichomegaly, also presumed to be a consequence of erlotinib. Despite these AEs, erlotinib was continued at the same dose, given his impressive response to this treatment, the absence of response to other therapy, and the patient’s insistence on continuing the medication.
Of note, after his transition to the outside hospital, Mr. J and his family paid all his medical expenses because he had no insurance. His family was very supportive, and the patient described their motivation and support as paramount in his receiving treatment.
In 2008, Mr. J presented to a dermatologist and was treated with cleocin solution. Although this helped to control his symptoms, the rash persisted. As a complication of these lesions, he also experienced several superinfections for which he was treated with cephalexin. At this same time, a PET scan showed no evidence of disease. He presented to the pain service for persistent chest wall pain around the surgical site, and his pain regimen was changed to slow-release morphine 200 mg 3 times per day and morphine sulfate solution 20 mg/mL 80 mg every 4 hours for breakthrough pain.
The PET scans, which were repeated every 3 months after Mr. J resumed treatment at WLAMC, showed continued absence of disease. In 2009, when he presented to the hospital with pneumonia, a PET scan showed 2 new areas of tracer uptake measuring 1 cm. His chest wall was irradiated, but radiation therapy was stopped after the biopsy returned benign. In 2015, an annual PET scan showed only evidence of postsurgical changes.
Discussion
The benefits of EGFR therapy have been established for treatment of late-stage NSCLC, but such therapy has limitations. For advanced-stage NSCLC, erlotinib has been shown to improve disease-free progression by 2.7 to 3.25 months and overall survival by 6.7 to 7.9 months.4-6 However, 1-year survival estimates remain as low as 35.0 to 37.7%,5,6 and its utility as first-line therapy has been questioned; randomized control trials have shown EGFR therapy to be of benefit only as secondor third-line therapy, when used with platinum-based chemotherapeutics.3,4 The few reports of complete response, however, have not included a definition of duration of survival.5,6
Occasionally, there have been reports of patients surviving for significantly longer periods, including 1 report of a patient who survived with complete remission for 2 years.7 In another case report, a patient experienced partial remission for more than 1 year with erlotinib as a third-line therapy.8 Although several reports indicated prolonged survival with erlotinib, or induction of complete remission of metastasis, survival has not been longer than 2 years.9-12
Important considerations for use of erlotinib are factors that predict a positive treatment response, including female sex, no previous exposure to tobacco, Asian origin, and adenocarcinoma on histologic examination.3,13 Mr. J did not meet any of these criteria. Interestingly, one study examining characteristics predictive of a positive response to erlotinib did not show that EGFR gene mutations were associated with response, although other studies have shown this to be a significant predictor of response.3,14,15
In this patient, his impressive response to erlotinib was most likely augmented by the presence of the EGFR mutation. Additionally, some reports indicate that pretreatment with platinum-based therapy can induce genetic changes resulting in EGFR mutations, thus enabling the benefit of erlotinib.10 Given that his biopsy results were not tested for the EGFR mutation prior to initiating carboplatin, this is a possibility.
Other factors specific to Mr. J that may have influenced his response to therapy include his personal wealth, which may have given him direct access to physicians outside the VA. His family support also may have motivated him to pursue and continue treatment, thus augmenting his survival. This support likely contributed in large part to his continuing erlotinib therapy despite the severe rash, hair loss, and trichomegaly. Other AEs associated with long-term erlotinib therapy include folliculitis, diarrhea, fatigue, and paronychia,although Mr. J did not experience these.16
Conclusion
Mr. J continues to follow up regularly at WLAMC. To the authors’ knowledge, this patient’s 8 year survival is the longest length of survival for any patient with NSCLC on erlotinib therapy. While the therapeutic benefits of erlotinib as a second-line therapy have been shown, EGFR therapy may be more effective than previously thought. Further research is needed to fully understand the benefits of erlotinib.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Ridge CA, McErlean AM, Ginsberg MS. Epidemiology of lung cancer. Semin Intervent Radiol. 2013;30(2):93-98.
2. Gridelli C, Bareschino MA, Schettino C, Rossi A, Maione P, Ciardiello F. Erlotinib in non-small cell lung cancer treatment: current status and future development. Oncologist. 2007;12(7):840-849.
3. Shepherd FA, Pereira JR, Ciuleanu T, et al; National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non–small-cell lung cancer. N Engl J Med. 2005;353(2):123-132.
4. Smith J. Erlotinib: small-molecule targeted therapy in the treatment of non-smallcell lung cancer. Clin Ther. 2005;27(10):1513-1534.
5. Boyer M, Horwood K, Pavlakis N, et al. Efficacy of erlotinib in patients with advanced non-small-cell lung cancer (NSCLC): analysis of the Australian subpopulation of the TRUST study. Asia Pac J Clin Oncol. 2012;8(3):248-254.
6. Reck M, van Zandwijk N, Gridelli C, et al. Erlotinib in advanced non-small cell lung cancer: efficacy and safety findings of the global phase IV Tarceva Lung Cancer Survival Treatment study. J Thorac Oncol. 2010;5(10):1616-1622.
7. Vitale MG, Riccardi F, Mocerino C, et al. Erlotinib-induced complete response in a patient with epidermal growth factor receptor wild-type lung adenocarcinoma after chemotherapy failure: a case report. J Med Case Rep. 2014;8:102.
8. Duchnowska R, Siemiatkowska A, Grala B, Smoter M. Long-term remission after erlotinib therapy in an elderly patient with advanced non-small-cell lung cancer. Case report and conclusions for clinical practice [in Polish]. Pneumonol Alergol Pol. 2008;76(6):451-455.
9. Lai CSL, Boshoff C, Falzon M, Lee SM. Complete response to erlotinib treatment in brain metastases from recurrent NSCLC. Thorax. 2006;61(1):91.
10. Karam I, Melosky B. Response to second-line erlotinib in an EGFR mutationnegative patient with non-small-cell lung cancer: make no assumptions. Curr Oncol. 2012;19(1):42-46.
11. Kobayashi T, Koizumi T, Agatsuma T, et al. A phase II trial of erlotinib in patients with EGFR wild-type advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2012;69(5):1241-1246.
12. Gridelli C, Maione P, Galetta D, et al. Three cases of long-lasting tumor control with erlotinib after progression with gefitinib in advanced non-small cell lung cancer. J Thorac Oncol. 2007;2(8):758-761.
13. Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21(12):2237-2246.
14. Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med. 2005;353(2):133-144.
15. Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25(5):587-595.
16. Becker A, van Wijk A, Smit EF, Postmus PE. Side-effects of long-term administration of erlotinib in patients with non-small cell lung cancer. J Thorac Oncol. 2010;5(9):1477-1480.
Note: Page numbers differ between the print issue and digital edition.
1. Ridge CA, McErlean AM, Ginsberg MS. Epidemiology of lung cancer. Semin Intervent Radiol. 2013;30(2):93-98.
2. Gridelli C, Bareschino MA, Schettino C, Rossi A, Maione P, Ciardiello F. Erlotinib in non-small cell lung cancer treatment: current status and future development. Oncologist. 2007;12(7):840-849.
3. Shepherd FA, Pereira JR, Ciuleanu T, et al; National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non–small-cell lung cancer. N Engl J Med. 2005;353(2):123-132.
4. Smith J. Erlotinib: small-molecule targeted therapy in the treatment of non-smallcell lung cancer. Clin Ther. 2005;27(10):1513-1534.
5. Boyer M, Horwood K, Pavlakis N, et al. Efficacy of erlotinib in patients with advanced non-small-cell lung cancer (NSCLC): analysis of the Australian subpopulation of the TRUST study. Asia Pac J Clin Oncol. 2012;8(3):248-254.
6. Reck M, van Zandwijk N, Gridelli C, et al. Erlotinib in advanced non-small cell lung cancer: efficacy and safety findings of the global phase IV Tarceva Lung Cancer Survival Treatment study. J Thorac Oncol. 2010;5(10):1616-1622.
7. Vitale MG, Riccardi F, Mocerino C, et al. Erlotinib-induced complete response in a patient with epidermal growth factor receptor wild-type lung adenocarcinoma after chemotherapy failure: a case report. J Med Case Rep. 2014;8:102.
8. Duchnowska R, Siemiatkowska A, Grala B, Smoter M. Long-term remission after erlotinib therapy in an elderly patient with advanced non-small-cell lung cancer. Case report and conclusions for clinical practice [in Polish]. Pneumonol Alergol Pol. 2008;76(6):451-455.
9. Lai CSL, Boshoff C, Falzon M, Lee SM. Complete response to erlotinib treatment in brain metastases from recurrent NSCLC. Thorax. 2006;61(1):91.
10. Karam I, Melosky B. Response to second-line erlotinib in an EGFR mutationnegative patient with non-small-cell lung cancer: make no assumptions. Curr Oncol. 2012;19(1):42-46.
11. Kobayashi T, Koizumi T, Agatsuma T, et al. A phase II trial of erlotinib in patients with EGFR wild-type advanced non-small-cell lung cancer. Cancer Chemother Pharmacol. 2012;69(5):1241-1246.
12. Gridelli C, Maione P, Galetta D, et al. Three cases of long-lasting tumor control with erlotinib after progression with gefitinib in advanced non-small cell lung cancer. J Thorac Oncol. 2007;2(8):758-761.
13. Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 2003;21(12):2237-2246.
14. Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med. 2005;353(2):133-144.
15. Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25(5):587-595.
16. Becker A, van Wijk A, Smit EF, Postmus PE. Side-effects of long-term administration of erlotinib in patients with non-small cell lung cancer. J Thorac Oncol. 2010;5(9):1477-1480.
Note: Page numbers differ between the print issue and digital edition.
Cancer trends shifting in HIV-positive patients
DURBAN, SOUTH AFRICA – The rates of the AIDS-defining cancers Kaposi’s sarcoma and non-Hodgkin’s lymphoma have plummeted in the antiretroviral era, yet they are still the two top cancers in terms of cumulative incidence in HIV-infected patients by age 75, Benigno Rodriguez, MD, said at the 21st International AIDS Conference.
Lung cancer also remains a major concern in the HIV-infected population. Each of these three cancers carries about a 1 in 25 lifetime risk to age 75, the estimated lifespan of HIV-positive patients on combination antiretroviral therapy (ART), according to Dr. Rodriguez of Case Western Reserve University in Cleveland.
ART has resulted in a marked change in cancer trends among HIV-infected patients. The incidence of AIDS-defining cancers has decreased “massively,” Dr. Rodriguez observed; but because ART has extended the lifespan, patients are now living long enough to get other cancers. And they do so at a higher rate than that of the general population because of their impaired immune function, higher rate of risk factors such as smoking, and greater prevalence of oncogenic viral coinfections such as hepatitis B and C and Epstein-Barr virus.
The increase in the incidence and risk of colorectal, liver, and anal cancers among HIV-positive individuals since the introduction of combination ART can largely be explained by the population’s longer exposure to risk due to increasing survival, he said.
Dr. Rodriguez presented highlights of an analysis of cancer trends in North America during 1996-2009. The study was based on 86,620 HIV-infected persons with 475,660 person-years of follow-up and 196,987 subjects without HIV infection and with more than 1.8 million person-years of follow-up. Trends over time were assessed for nine cancers: Kaposi’s sarcoma, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, and melanoma, as well as anal, lung, colorectal, liver, and oropharyngeal cancers (Ann Intern Med. 2015;163:507-18).
Examining trends in three periods – 1996-1999, 2000-2004, and 2005-2009 – the investigators looked at the impact of ART over time on rates of AIDS-related and non–AIDS-related cancers in HIV-infected patients and compared them to results in the general HIV-uninfected population.
By conducting a competing risk analysis, Dr. Rodriguez and his coworkers were able to estimate the cumulative lifetime risk of the various cancers by age 75, a metric that provides readily understandable information for counseling HIV-infected patients about their long-term cancer risk.
The measure “is more intuitive than using incidence rates,” Dr. Rodriguez said. In a study of 1,578 HIV-infected patients who received the hepatitis B vaccine, for example, those patients whose immune function did not to respond to the vaccine were more likely to be among the 6% of patients who subsequently developed cancer during up to 20 years of follow-up.
The findings on cancer trends in the ART era have clinical implications for cancer screening and prevention in HIV-infected patients. The high rates of smoking and lung cancer in this population make HIV-positive smokers a logical target for lung cancer screening. The rising risk of colorectal cancer – the cumulative lifetime risk to age 75 was 0.4% in 1996-1999, 0.7% in 2000-2004, and 1.3% in 2005-2009 – suggests a need for increased colorectal cancer screening in the older HIV-positive population.
Early and sustained HIV suppression with combination ART remains the only known method of preventing AIDS-defining cancers. Dr. Rodriguez and his coinvestigators in the Centers for AIDS Research Network of Integrated Clinical Systems demonstrated the crucial role of suppressing HIV in a study of 6,036 HIV-infected patients who started on ART and were followed for more than 21,000 person-years. Compared with HIV-infected patients with a 3-month lagged HIV viremia of no more than 50 copies/mL, patients’ risk of developing non-Hodgkin’s lymphoma was 2.1-fold greater if their 3-month lagged HIV viremia was 51-500 copies/mL and 3.56-fold greater if it exceeded 500 copies/mL (Clin Infect Dis. 2014 Jun;58[11]:1599-606).
The study on cancer trends over time was funded by the National Institutes of Health. Dr. Rodriguez reported receiving honoraria from Gilead Sciences.
DURBAN, SOUTH AFRICA – The rates of the AIDS-defining cancers Kaposi’s sarcoma and non-Hodgkin’s lymphoma have plummeted in the antiretroviral era, yet they are still the two top cancers in terms of cumulative incidence in HIV-infected patients by age 75, Benigno Rodriguez, MD, said at the 21st International AIDS Conference.
Lung cancer also remains a major concern in the HIV-infected population. Each of these three cancers carries about a 1 in 25 lifetime risk to age 75, the estimated lifespan of HIV-positive patients on combination antiretroviral therapy (ART), according to Dr. Rodriguez of Case Western Reserve University in Cleveland.
ART has resulted in a marked change in cancer trends among HIV-infected patients. The incidence of AIDS-defining cancers has decreased “massively,” Dr. Rodriguez observed; but because ART has extended the lifespan, patients are now living long enough to get other cancers. And they do so at a higher rate than that of the general population because of their impaired immune function, higher rate of risk factors such as smoking, and greater prevalence of oncogenic viral coinfections such as hepatitis B and C and Epstein-Barr virus.
The increase in the incidence and risk of colorectal, liver, and anal cancers among HIV-positive individuals since the introduction of combination ART can largely be explained by the population’s longer exposure to risk due to increasing survival, he said.
Dr. Rodriguez presented highlights of an analysis of cancer trends in North America during 1996-2009. The study was based on 86,620 HIV-infected persons with 475,660 person-years of follow-up and 196,987 subjects without HIV infection and with more than 1.8 million person-years of follow-up. Trends over time were assessed for nine cancers: Kaposi’s sarcoma, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, and melanoma, as well as anal, lung, colorectal, liver, and oropharyngeal cancers (Ann Intern Med. 2015;163:507-18).
Examining trends in three periods – 1996-1999, 2000-2004, and 2005-2009 – the investigators looked at the impact of ART over time on rates of AIDS-related and non–AIDS-related cancers in HIV-infected patients and compared them to results in the general HIV-uninfected population.

By conducting a competing risk analysis, Dr. Rodriguez and his coworkers were able to estimate the cumulative lifetime risk of the various cancers by age 75, a metric that provides readily understandable information for counseling HIV-infected patients about their long-term cancer risk.
The measure “is more intuitive than using incidence rates,” Dr. Rodriguez said. In a study of 1,578 HIV-infected patients who received the hepatitis B vaccine, for example, those patients whose immune function did not to respond to the vaccine were more likely to be among the 6% of patients who subsequently developed cancer during up to 20 years of follow-up.
The findings on cancer trends in the ART era have clinical implications for cancer screening and prevention in HIV-infected patients. The high rates of smoking and lung cancer in this population make HIV-positive smokers a logical target for lung cancer screening. The rising risk of colorectal cancer – the cumulative lifetime risk to age 75 was 0.4% in 1996-1999, 0.7% in 2000-2004, and 1.3% in 2005-2009 – suggests a need for increased colorectal cancer screening in the older HIV-positive population.
Early and sustained HIV suppression with combination ART remains the only known method of preventing AIDS-defining cancers. Dr. Rodriguez and his coinvestigators in the Centers for AIDS Research Network of Integrated Clinical Systems demonstrated the crucial role of suppressing HIV in a study of 6,036 HIV-infected patients who started on ART and were followed for more than 21,000 person-years. Compared with HIV-infected patients with a 3-month lagged HIV viremia of no more than 50 copies/mL, patients’ risk of developing non-Hodgkin’s lymphoma was 2.1-fold greater if their 3-month lagged HIV viremia was 51-500 copies/mL and 3.56-fold greater if it exceeded 500 copies/mL (Clin Infect Dis. 2014 Jun;58[11]:1599-606).
The study on cancer trends over time was funded by the National Institutes of Health. Dr. Rodriguez reported receiving honoraria from Gilead Sciences.
DURBAN, SOUTH AFRICA – The rates of the AIDS-defining cancers Kaposi’s sarcoma and non-Hodgkin’s lymphoma have plummeted in the antiretroviral era, yet they are still the two top cancers in terms of cumulative incidence in HIV-infected patients by age 75, Benigno Rodriguez, MD, said at the 21st International AIDS Conference.
Lung cancer also remains a major concern in the HIV-infected population. Each of these three cancers carries about a 1 in 25 lifetime risk to age 75, the estimated lifespan of HIV-positive patients on combination antiretroviral therapy (ART), according to Dr. Rodriguez of Case Western Reserve University in Cleveland.
ART has resulted in a marked change in cancer trends among HIV-infected patients. The incidence of AIDS-defining cancers has decreased “massively,” Dr. Rodriguez observed; but because ART has extended the lifespan, patients are now living long enough to get other cancers. And they do so at a higher rate than that of the general population because of their impaired immune function, higher rate of risk factors such as smoking, and greater prevalence of oncogenic viral coinfections such as hepatitis B and C and Epstein-Barr virus.
The increase in the incidence and risk of colorectal, liver, and anal cancers among HIV-positive individuals since the introduction of combination ART can largely be explained by the population’s longer exposure to risk due to increasing survival, he said.
Dr. Rodriguez presented highlights of an analysis of cancer trends in North America during 1996-2009. The study was based on 86,620 HIV-infected persons with 475,660 person-years of follow-up and 196,987 subjects without HIV infection and with more than 1.8 million person-years of follow-up. Trends over time were assessed for nine cancers: Kaposi’s sarcoma, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, and melanoma, as well as anal, lung, colorectal, liver, and oropharyngeal cancers (Ann Intern Med. 2015;163:507-18).
Examining trends in three periods – 1996-1999, 2000-2004, and 2005-2009 – the investigators looked at the impact of ART over time on rates of AIDS-related and non–AIDS-related cancers in HIV-infected patients and compared them to results in the general HIV-uninfected population.

By conducting a competing risk analysis, Dr. Rodriguez and his coworkers were able to estimate the cumulative lifetime risk of the various cancers by age 75, a metric that provides readily understandable information for counseling HIV-infected patients about their long-term cancer risk.
The measure “is more intuitive than using incidence rates,” Dr. Rodriguez said. In a study of 1,578 HIV-infected patients who received the hepatitis B vaccine, for example, those patients whose immune function did not to respond to the vaccine were more likely to be among the 6% of patients who subsequently developed cancer during up to 20 years of follow-up.
The findings on cancer trends in the ART era have clinical implications for cancer screening and prevention in HIV-infected patients. The high rates of smoking and lung cancer in this population make HIV-positive smokers a logical target for lung cancer screening. The rising risk of colorectal cancer – the cumulative lifetime risk to age 75 was 0.4% in 1996-1999, 0.7% in 2000-2004, and 1.3% in 2005-2009 – suggests a need for increased colorectal cancer screening in the older HIV-positive population.
Early and sustained HIV suppression with combination ART remains the only known method of preventing AIDS-defining cancers. Dr. Rodriguez and his coinvestigators in the Centers for AIDS Research Network of Integrated Clinical Systems demonstrated the crucial role of suppressing HIV in a study of 6,036 HIV-infected patients who started on ART and were followed for more than 21,000 person-years. Compared with HIV-infected patients with a 3-month lagged HIV viremia of no more than 50 copies/mL, patients’ risk of developing non-Hodgkin’s lymphoma was 2.1-fold greater if their 3-month lagged HIV viremia was 51-500 copies/mL and 3.56-fold greater if it exceeded 500 copies/mL (Clin Infect Dis. 2014 Jun;58[11]:1599-606).
The study on cancer trends over time was funded by the National Institutes of Health. Dr. Rodriguez reported receiving honoraria from Gilead Sciences.
AT AIDS 2016
Key clinical point: HIV-infected persons in North America have roughly a 1 in 25 cumulative lifetime risk of developing lung cancer, Kaposi’s sarcoma, or non-Hodgkin’s lymphoma.
Major finding: The cumulative lifetime risk of developing non-Hodgkin’s lymphoma in HIV-infected patients on combination antiretroviral therapy is sevenfold greater than the risk in the general population.
Data source: A competing risk analysis for nine cancers based upon 86,620 HIV-infected persons followed for 475,660 person-years and 196,987 subjects not infected with HIV and with more than 1.8 million person-years of follow-up.
Disclosures: The National Institutes of Health funded the study. The presenter reported receiving honoraria from Gilead Sciences.