User login
Stage III NSCLC: Preoperative FDG PET/CT tied to longer survival
Key clinical point: Use of preoperative fluorine 18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is linked to a longer overall survival in patients with stage IIIA or IIIB resectable non—small-cell lung cancer (NSCLC).
Major finding: Among patients who underwent vs did not undergo preoperative 18F-FDG PET/CT, those with stage IIIB and stage IIIA disease had 20% (adjusted hazard ratio [aHR] 0.80; 95% CI 0.71-0.90) and 10% (aHR 0.90; 95% CI 0.79-0.94) lower risks for mortality, respectively. Patients with stage I-II disease did not have a lower risk for mortality (aHR 1.19; 95% CI 0.89-1.30).
Study details: The data come from a Taiwanese retrospective cohort study of patients with resectable stage I-IIIB NSCLC who underwent (n = 6,754) and did not undergo (n = 6,754) preoperative 18F-FDG PET/CT.
Disclosures: This study was funded by the Lo-Hsu Medical Foundation and Lotung Poh-Ai Hospital, Taiwan. SY Wu is a member of the Taiwan Radiological Society and Taiwan Society for Therapeutic Radiology and Oncology. The other authors reported no relevant relationships.
Source: Chen W-M et al. Use of preoperative FDG PET/CT and survival of patients with resectable non–small cell lung cancer. Radiology. 2022 (Jun 21). Doi: 10.1148/radiol.212798
Key clinical point: Use of preoperative fluorine 18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is linked to a longer overall survival in patients with stage IIIA or IIIB resectable non—small-cell lung cancer (NSCLC).
Major finding: Among patients who underwent vs did not undergo preoperative 18F-FDG PET/CT, those with stage IIIB and stage IIIA disease had 20% (adjusted hazard ratio [aHR] 0.80; 95% CI 0.71-0.90) and 10% (aHR 0.90; 95% CI 0.79-0.94) lower risks for mortality, respectively. Patients with stage I-II disease did not have a lower risk for mortality (aHR 1.19; 95% CI 0.89-1.30).
Study details: The data come from a Taiwanese retrospective cohort study of patients with resectable stage I-IIIB NSCLC who underwent (n = 6,754) and did not undergo (n = 6,754) preoperative 18F-FDG PET/CT.
Disclosures: This study was funded by the Lo-Hsu Medical Foundation and Lotung Poh-Ai Hospital, Taiwan. SY Wu is a member of the Taiwan Radiological Society and Taiwan Society for Therapeutic Radiology and Oncology. The other authors reported no relevant relationships.
Source: Chen W-M et al. Use of preoperative FDG PET/CT and survival of patients with resectable non–small cell lung cancer. Radiology. 2022 (Jun 21). Doi: 10.1148/radiol.212798
Key clinical point: Use of preoperative fluorine 18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) is linked to a longer overall survival in patients with stage IIIA or IIIB resectable non—small-cell lung cancer (NSCLC).
Major finding: Among patients who underwent vs did not undergo preoperative 18F-FDG PET/CT, those with stage IIIB and stage IIIA disease had 20% (adjusted hazard ratio [aHR] 0.80; 95% CI 0.71-0.90) and 10% (aHR 0.90; 95% CI 0.79-0.94) lower risks for mortality, respectively. Patients with stage I-II disease did not have a lower risk for mortality (aHR 1.19; 95% CI 0.89-1.30).
Study details: The data come from a Taiwanese retrospective cohort study of patients with resectable stage I-IIIB NSCLC who underwent (n = 6,754) and did not undergo (n = 6,754) preoperative 18F-FDG PET/CT.
Disclosures: This study was funded by the Lo-Hsu Medical Foundation and Lotung Poh-Ai Hospital, Taiwan. SY Wu is a member of the Taiwan Radiological Society and Taiwan Society for Therapeutic Radiology and Oncology. The other authors reported no relevant relationships.
Source: Chen W-M et al. Use of preoperative FDG PET/CT and survival of patients with resectable non–small cell lung cancer. Radiology. 2022 (Jun 21). Doi: 10.1148/radiol.212798
NSCLC: Cell-free RNA beneficial for early detection and prognosi
Key clinical point: Circulating cell-free RNA (cfRNA) of the candidate transcript MORF4L2 demonstrates very good sensitivity and specificity for distinguishing patients with non—small-cell lung cancer (NSCLC) from healthy individuals.
Major finding: A greater concentration of MORF4L2 cfRNA was seen in patients with NSCLC compared with healthy donors (P < .0001). A cutoff value of 537.5 copies/mL of plasma was useful in distinguishing patients with NSCLC from healthy donors with very good sensitivity (0.73; 95% CI 0.61-0.82) and specificity (0.87; 95% CI 0.73-0.96). Low vs high levels of MORF4L2 cfRNA at baseline were associated with a better overall survival (hazard ratio 0.25; P = .009).
Study details: The data come from a cohort study of 41 patients with stage IV NSCLC, 38 patients with early-stage (stage I-III) NSCLC, and 39 healthy blood donors.
Disclosures: This study was supported by the European Transcan-2 project CEVIR (Cancer EVolution and Identification of Relapse-initiating cells) and the German Cancer Consortium. M Metzenmacher, JT Siveke, and M Schuler reported ties with ≥1 pharmaceutical companies. The other authors reported no conflicts of interest.
Source: Metzenmacher M et al. The clinical utility of cfRNA for disease detection and surveillance: A proof of concept study in non-small cell lung cancer. Thorac Cancer. 2022 (Jun 16). Doi: 10.1111/1759-7714.14540
Key clinical point: Circulating cell-free RNA (cfRNA) of the candidate transcript MORF4L2 demonstrates very good sensitivity and specificity for distinguishing patients with non—small-cell lung cancer (NSCLC) from healthy individuals.
Major finding: A greater concentration of MORF4L2 cfRNA was seen in patients with NSCLC compared with healthy donors (P < .0001). A cutoff value of 537.5 copies/mL of plasma was useful in distinguishing patients with NSCLC from healthy donors with very good sensitivity (0.73; 95% CI 0.61-0.82) and specificity (0.87; 95% CI 0.73-0.96). Low vs high levels of MORF4L2 cfRNA at baseline were associated with a better overall survival (hazard ratio 0.25; P = .009).
Study details: The data come from a cohort study of 41 patients with stage IV NSCLC, 38 patients with early-stage (stage I-III) NSCLC, and 39 healthy blood donors.
Disclosures: This study was supported by the European Transcan-2 project CEVIR (Cancer EVolution and Identification of Relapse-initiating cells) and the German Cancer Consortium. M Metzenmacher, JT Siveke, and M Schuler reported ties with ≥1 pharmaceutical companies. The other authors reported no conflicts of interest.
Source: Metzenmacher M et al. The clinical utility of cfRNA for disease detection and surveillance: A proof of concept study in non-small cell lung cancer. Thorac Cancer. 2022 (Jun 16). Doi: 10.1111/1759-7714.14540
Key clinical point: Circulating cell-free RNA (cfRNA) of the candidate transcript MORF4L2 demonstrates very good sensitivity and specificity for distinguishing patients with non—small-cell lung cancer (NSCLC) from healthy individuals.
Major finding: A greater concentration of MORF4L2 cfRNA was seen in patients with NSCLC compared with healthy donors (P < .0001). A cutoff value of 537.5 copies/mL of plasma was useful in distinguishing patients with NSCLC from healthy donors with very good sensitivity (0.73; 95% CI 0.61-0.82) and specificity (0.87; 95% CI 0.73-0.96). Low vs high levels of MORF4L2 cfRNA at baseline were associated with a better overall survival (hazard ratio 0.25; P = .009).
Study details: The data come from a cohort study of 41 patients with stage IV NSCLC, 38 patients with early-stage (stage I-III) NSCLC, and 39 healthy blood donors.
Disclosures: This study was supported by the European Transcan-2 project CEVIR (Cancer EVolution and Identification of Relapse-initiating cells) and the German Cancer Consortium. M Metzenmacher, JT Siveke, and M Schuler reported ties with ≥1 pharmaceutical companies. The other authors reported no conflicts of interest.
Source: Metzenmacher M et al. The clinical utility of cfRNA for disease detection and surveillance: A proof of concept study in non-small cell lung cancer. Thorac Cancer. 2022 (Jun 16). Doi: 10.1111/1759-7714.14540
Early-stage NSCLC: EGFR mutation predicts longer survival
Key clinical point: The presence of epidermal growth factor receptor (EGFR) mutation is associated with a longer median overall survival (OS) compared with absence of the EGFR mutation in patients with early-stage (stage I-IIIA) non—small-cell lung cancer (NSCLC).
Major finding: The presence vs absence of EGFR mutation was associated with a longer median OS (5.7 vs 4.4 years). The lower risk for all-cause mortality was consistent across all subgroups (stage at diagnosis, age, sex, comorbidity, and surgery receipt), with hazard ratios ranging from 0.48 to 0.83.
Study details: The data come from a Danish population-based cohort study involving 21,282 patients with NSCLC.
Disclosures: This study was funded by AstraZeneca. A Taylor and L Servidio reported being current or former employees of AstraZeneca. V Ehrenstein and K Eriksen are employees of Aarhus University or Aarhus University Hospital. E Jakobsen is an employee of Odense University Hospital.
Source: Ehrenstein V et al. Characteristics and overall survival of patients with early-stage non-small cell lung cancer: A cohort study in Denmark. Cancer Med. 2022 (Jun 20). Doi: 10.1002/cam4.4946
Key clinical point: The presence of epidermal growth factor receptor (EGFR) mutation is associated with a longer median overall survival (OS) compared with absence of the EGFR mutation in patients with early-stage (stage I-IIIA) non—small-cell lung cancer (NSCLC).
Major finding: The presence vs absence of EGFR mutation was associated with a longer median OS (5.7 vs 4.4 years). The lower risk for all-cause mortality was consistent across all subgroups (stage at diagnosis, age, sex, comorbidity, and surgery receipt), with hazard ratios ranging from 0.48 to 0.83.
Study details: The data come from a Danish population-based cohort study involving 21,282 patients with NSCLC.
Disclosures: This study was funded by AstraZeneca. A Taylor and L Servidio reported being current or former employees of AstraZeneca. V Ehrenstein and K Eriksen are employees of Aarhus University or Aarhus University Hospital. E Jakobsen is an employee of Odense University Hospital.
Source: Ehrenstein V et al. Characteristics and overall survival of patients with early-stage non-small cell lung cancer: A cohort study in Denmark. Cancer Med. 2022 (Jun 20). Doi: 10.1002/cam4.4946
Key clinical point: The presence of epidermal growth factor receptor (EGFR) mutation is associated with a longer median overall survival (OS) compared with absence of the EGFR mutation in patients with early-stage (stage I-IIIA) non—small-cell lung cancer (NSCLC).
Major finding: The presence vs absence of EGFR mutation was associated with a longer median OS (5.7 vs 4.4 years). The lower risk for all-cause mortality was consistent across all subgroups (stage at diagnosis, age, sex, comorbidity, and surgery receipt), with hazard ratios ranging from 0.48 to 0.83.
Study details: The data come from a Danish population-based cohort study involving 21,282 patients with NSCLC.
Disclosures: This study was funded by AstraZeneca. A Taylor and L Servidio reported being current or former employees of AstraZeneca. V Ehrenstein and K Eriksen are employees of Aarhus University or Aarhus University Hospital. E Jakobsen is an employee of Odense University Hospital.
Source: Ehrenstein V et al. Characteristics and overall survival of patients with early-stage non-small cell lung cancer: A cohort study in Denmark. Cancer Med. 2022 (Jun 20). Doi: 10.1002/cam4.4946
Lung cancer treatment combo may be effective after ICI failure
In a phase 2 clinical trial, the combination of an immune checkpoint inhibitor (ICI) and a vascular endothelial growth factor (VEGF) inhibitor led to improved overall survival versus standard of care in patients with non–small cell lung cancer (NSCLC) who had failed previous ICI therapy.
NSCLC patients usually receive immune checkpoint inhibitor therapy at some point, whether in the adjuvant or neoadjuvant setting, or among stage 3 patients after radiation. “The majority of patients who get diagnosed with lung cancer will get some sort of immunotherapy, and we know that at least from the advanced setting, about 15% of those will have long-term responses, which means the majority of patients will develop tumor resistance to immune checkpoint inhibitor therapy,” said Karen L. Reckamp, MD, who is the lead author of the study published online in Journal of Clinical Oncology.
That clinical need has led to the combination of ICIs with VEGF inhibitors. This approach is approved for first-line therapy of renal cell cancer, endometrial, and hepatocellular cancer. Along with its effect on tumor vasculature, VEGF inhibition assists in the activation and maturation of dendritic cells, as well as to attract cytotoxic T cells to the tumor. “By both changing the vasculature and changing the tumor milieu, there’s a potential to overcome that immune suppression and potentially overcome that (ICI) resistance,” said Dr. Reckamp, who is associate director of clinical research at Cedars Sinai Medical Center, Los Angeles. “The results of the study were encouraging. . We would like to confirm this finding in a phase 3 trial and potentially provide to patients an option that does not include chemotherapy and can potentially overcome resistance to their prior immune checkpoint inhibitor therapy,” Dr. Reckamp said.
The study included 136 patients. The median patient age was 66 years and 61% were male. The ICI/VEGF arm had better overall survival (hazard ratio, 0.69; SLR one-sided P = .05). The median overall survival was 14.5 months in the ICI/VEGF arm, versus 11.6 months in the standard care arm. Both arms had similar response rates, and grade 3 or higher treatment-related adverse events were more common in the chemotherapy arm (60% versus 42%).
The next step is a phase 3 trial and Dr. Reckamp hopes to improve patient selection for VEGF inhibitor and VEGF receptor inhibitor therapy. “The precision medicine that’s associated with other tumor alterations has kind of been elusive for VEGF therapies, but I would hope with potentially a larger trial and understanding of some of the biomarkers that we might find a more select patient population who will benefit the most,” Dr. Reckamp said.
She also noted that the comparative arm in the phase 2 study was a combination of docetaxel and ramucirumab. “That combination has shown to be more effective than single agent docetaxel alone so [the new study] was really improved overall survival over the best standard of care therapy we have,” Dr. Reckamp said.
The study was funded, in part, by Eli Lilly and Company and Merck Sharp & Dohme Corp. Dr. Reckamp disclosed ties to Amgen, Tesaro, Takeda, AstraZeneca, Seattle Genetics, Genentech, Blueprint Medicines, Daiichi Sankyo/Lilly, EMD Serono, Janssen Oncology, Merck KGaA, GlaxoSmithKline, and Mirati Therapeutics.
In a phase 2 clinical trial, the combination of an immune checkpoint inhibitor (ICI) and a vascular endothelial growth factor (VEGF) inhibitor led to improved overall survival versus standard of care in patients with non–small cell lung cancer (NSCLC) who had failed previous ICI therapy.
NSCLC patients usually receive immune checkpoint inhibitor therapy at some point, whether in the adjuvant or neoadjuvant setting, or among stage 3 patients after radiation. “The majority of patients who get diagnosed with lung cancer will get some sort of immunotherapy, and we know that at least from the advanced setting, about 15% of those will have long-term responses, which means the majority of patients will develop tumor resistance to immune checkpoint inhibitor therapy,” said Karen L. Reckamp, MD, who is the lead author of the study published online in Journal of Clinical Oncology.
That clinical need has led to the combination of ICIs with VEGF inhibitors. This approach is approved for first-line therapy of renal cell cancer, endometrial, and hepatocellular cancer. Along with its effect on tumor vasculature, VEGF inhibition assists in the activation and maturation of dendritic cells, as well as to attract cytotoxic T cells to the tumor. “By both changing the vasculature and changing the tumor milieu, there’s a potential to overcome that immune suppression and potentially overcome that (ICI) resistance,” said Dr. Reckamp, who is associate director of clinical research at Cedars Sinai Medical Center, Los Angeles. “The results of the study were encouraging. . We would like to confirm this finding in a phase 3 trial and potentially provide to patients an option that does not include chemotherapy and can potentially overcome resistance to their prior immune checkpoint inhibitor therapy,” Dr. Reckamp said.
The study included 136 patients. The median patient age was 66 years and 61% were male. The ICI/VEGF arm had better overall survival (hazard ratio, 0.69; SLR one-sided P = .05). The median overall survival was 14.5 months in the ICI/VEGF arm, versus 11.6 months in the standard care arm. Both arms had similar response rates, and grade 3 or higher treatment-related adverse events were more common in the chemotherapy arm (60% versus 42%).
The next step is a phase 3 trial and Dr. Reckamp hopes to improve patient selection for VEGF inhibitor and VEGF receptor inhibitor therapy. “The precision medicine that’s associated with other tumor alterations has kind of been elusive for VEGF therapies, but I would hope with potentially a larger trial and understanding of some of the biomarkers that we might find a more select patient population who will benefit the most,” Dr. Reckamp said.
She also noted that the comparative arm in the phase 2 study was a combination of docetaxel and ramucirumab. “That combination has shown to be more effective than single agent docetaxel alone so [the new study] was really improved overall survival over the best standard of care therapy we have,” Dr. Reckamp said.
The study was funded, in part, by Eli Lilly and Company and Merck Sharp & Dohme Corp. Dr. Reckamp disclosed ties to Amgen, Tesaro, Takeda, AstraZeneca, Seattle Genetics, Genentech, Blueprint Medicines, Daiichi Sankyo/Lilly, EMD Serono, Janssen Oncology, Merck KGaA, GlaxoSmithKline, and Mirati Therapeutics.
In a phase 2 clinical trial, the combination of an immune checkpoint inhibitor (ICI) and a vascular endothelial growth factor (VEGF) inhibitor led to improved overall survival versus standard of care in patients with non–small cell lung cancer (NSCLC) who had failed previous ICI therapy.
NSCLC patients usually receive immune checkpoint inhibitor therapy at some point, whether in the adjuvant or neoadjuvant setting, or among stage 3 patients after radiation. “The majority of patients who get diagnosed with lung cancer will get some sort of immunotherapy, and we know that at least from the advanced setting, about 15% of those will have long-term responses, which means the majority of patients will develop tumor resistance to immune checkpoint inhibitor therapy,” said Karen L. Reckamp, MD, who is the lead author of the study published online in Journal of Clinical Oncology.
That clinical need has led to the combination of ICIs with VEGF inhibitors. This approach is approved for first-line therapy of renal cell cancer, endometrial, and hepatocellular cancer. Along with its effect on tumor vasculature, VEGF inhibition assists in the activation and maturation of dendritic cells, as well as to attract cytotoxic T cells to the tumor. “By both changing the vasculature and changing the tumor milieu, there’s a potential to overcome that immune suppression and potentially overcome that (ICI) resistance,” said Dr. Reckamp, who is associate director of clinical research at Cedars Sinai Medical Center, Los Angeles. “The results of the study were encouraging. . We would like to confirm this finding in a phase 3 trial and potentially provide to patients an option that does not include chemotherapy and can potentially overcome resistance to their prior immune checkpoint inhibitor therapy,” Dr. Reckamp said.
The study included 136 patients. The median patient age was 66 years and 61% were male. The ICI/VEGF arm had better overall survival (hazard ratio, 0.69; SLR one-sided P = .05). The median overall survival was 14.5 months in the ICI/VEGF arm, versus 11.6 months in the standard care arm. Both arms had similar response rates, and grade 3 or higher treatment-related adverse events were more common in the chemotherapy arm (60% versus 42%).
The next step is a phase 3 trial and Dr. Reckamp hopes to improve patient selection for VEGF inhibitor and VEGF receptor inhibitor therapy. “The precision medicine that’s associated with other tumor alterations has kind of been elusive for VEGF therapies, but I would hope with potentially a larger trial and understanding of some of the biomarkers that we might find a more select patient population who will benefit the most,” Dr. Reckamp said.
She also noted that the comparative arm in the phase 2 study was a combination of docetaxel and ramucirumab. “That combination has shown to be more effective than single agent docetaxel alone so [the new study] was really improved overall survival over the best standard of care therapy we have,” Dr. Reckamp said.
The study was funded, in part, by Eli Lilly and Company and Merck Sharp & Dohme Corp. Dr. Reckamp disclosed ties to Amgen, Tesaro, Takeda, AstraZeneca, Seattle Genetics, Genentech, Blueprint Medicines, Daiichi Sankyo/Lilly, EMD Serono, Janssen Oncology, Merck KGaA, GlaxoSmithKline, and Mirati Therapeutics.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
The shifting sands of lung cancer screening
An analysis of trends in lung cancer screening since March 2021 when the U.S. Preventive Services Task Force (USPSTF) expanded the eligibility criteria for lung cancer screening, shows that significantly more Black men have been screened for lung cancer, but not women or undereducated people.
The eligibility for lung cancer screening was expanded in 2021 to include men and women under 50 years old and people who smoke at least one pack of cigarettes a day for the last 20 years. “
“Expansion of screening criteria is a critical first step to achieving equity in lung cancer screening for all high-risk populations, but myriad challenges remain before individuals enter the door for screening,” wrote the authors, led by Julie A. Barta, MD, Thomas Jefferson University, Philadelphia. “Health policy changes must occur simultaneously with efforts to expand community outreach, overcome logistical barriers, and facilitate screening adherence. Only after comprehensive strategies to dismantle screening barriers are identified, validated, and implemented can there be a truly equitable landscape for lung cancer screening.”
For the study, published in JAMA Open Network, researchers examined rates of centralized lung cancer screening in the Baltimore area. In addition to expanding lung cancer screening generally, there was hope that the expanded criteria might increase uptake of screening in populations that are traditionally underserved, such as African American, Hispanic, and female patients. Of 815 people screened during the study period (March-December 2021), 161 were newly eligible for screening under the 2021 criteria.
“There’s been quite a bit of work in the field demonstrating that Black men and women develop lung cancer at more advanced stages of disease, and they often are diagnosed at younger ages and have fewer pack-years of smoking. So the hypothesis was that this would reduce some of the disparities seen in lung cancer screening by making more people eligible,” Dr. Barta said in an interview.
The researchers categorized participants as those who would have been eligible for screening under the USPSTF 2013 guideline (age 55 or older, 30 or more pack-years, quit within the past 15 years), and those who would be eligible under the 2021 guideline (age 50 or older, 20 or more pack-years, quit within the past 15 years). Of the 2021 cohort, 54.5% were African American, versus 39.5% of the 2013 cohort (P = .002). There were no differences between the cohorts with respect to education level or gender.
“Although we’ve seen some encouraging improvement in terms of getting more eligible patients into our screening program, there’s still a lot of work to be done in the field,” Dr. Barta said. “Diagnosing lung cancer at earlier stages of disease is more cost effective in general for the health care system than fighting lung cancer at advanced stages, which requires more complex and multimodal and prolonged therapies.”
New evidence: Chest CTs for lung cancer screening reduces incidence of advanced lung cancer
In an analysis of the SEER database presented in June at the annual meeting of the American Society of Clinical Oncology, the adoption of low-dose chest computed tomography (LDCT) led to fewer diagnoses of advanced lung cancer, although these declines varied significantly by race and ethnicity. Non-Hispanic Blacks seemed to benefit the most with a 55% decline (P < .01), while Hispanics had the lowest rate of decline at 41% (P < .01). The change was recommended by USPSTF in 2013 after the National Lung Screening Trial revealed a 20% relative reduction in mortality when CT scans were used instead of chest radiography. The Centers for Medicare and Medicaid Services approved coverage of the screen in 2015.
The SEER study looked at data from 400,343 individuals from 2004-2014 (preintervention) and 2015-2018 (postintervention). The age-adjusted incidence of advanced lung cancer declined during both periods, but the decline was sharper between 2015 and 2018, with three fewer cases per 100,000 people than 2004-2014 (P < .01). Similar patterns were seen in subanalyses of males and females, non-Hispanic Whites, non-Hispanic Blacks, and Hispanics. The relative declines were largest in women, non-Hispanic Blacks, and people who lived outside of Metropolitan areas.
During a Q&A session that followed the presentation, Robert Smith, PhD, pointed out that the bar for eligibility of lung cancer risk has been set quite high, following the eligibility criteria for clinical trials. He noted that . “We are missing opportunities to prevent avertable lung cancer deaths,” said Dr. Smith, senior vice president of cancer screening at the American Cancer Society.
On the other hand, screening-prompted biopsies have the potential to cause harm, particularly in patients who already have lung disease, said Douglas Allen Arenberg, MD, professor at the University of Michigan, Ann Arbor. “I think that’s what scares most people is the potential downside, which is very hard to measure outside of a clinical trial,” said Dr. Arenberg, who served as a discussant for the presentation.
One way to reduce that risk is to identify biomarkers, either for screens or for incidentally-detected nodules, that have good negative predictive value. “If I had a blood test that is as good as a negative PET scan, I’m going to be much more likely to say, ‘Yeah, you’re 40 and your grandfather had lung cancer. Maybe you should get a CT. If we had that, we could screen a lot more people. Right now, I would discourage anybody who is at low risk from getting screened because when they come to me, the biggest opportunity I have to do harm is when I do a biopsy, and you always remember the ones that go wrong,” he said.
Dr. Arenberg also called for improvements in electronic medical records to better flag at-risk patients. “I think we as physicians have to demand more of the software developers that create these EMRs for us,” he said.
Another study in the same session used data from 1,391,088 patients drawn from the National Cancer Database between 2010 and 2017 to examine trends in diagnosis of stage I cancer. In 2010, 23.5% of patients were diagnosed as stage I, versus 29.1% in 2017. Stage I incidence increased from 25.8% to 31.7% in non–small cell lung cancer, but there was no statistically significant change in small cell lung cancer. As with the SEER database study, the researchers noted that the shift toward stage I diagnoses predated the recommendation of LDCT.
Dr. Arenberg suggested that the trend may come down to increased frequency of CT scans, which often collect incidental images of the lungs. He added that better access to care may also be helping to drive the change. “How much of that might have had something to do with the introduction 5 or 10 years earlier of the Affordable Care Act and people just simply having access to care and taking advantage of that?” Dr. Arenberg said.
But Dr. Arenberg said that not even screening can explain all the data. He referenced a stage shift in patients of all age groups in the National Cancer Database study, even those too young to be eligible for screening. “There’s something else going on here. It would be nice for us to understand what caused these trends, so perhaps we could accentuate that trend even more, but stage shifts are clearly occurring in lung cancer,” Dr. Arenberg said.
Dr. Barta has received grants from Genentech Health Equity Innovations Fund. Dr. Arenberg has no relevant financial disclosures. Dr. Smith’s potential disclosures could not be ascertained.
An analysis of trends in lung cancer screening since March 2021 when the U.S. Preventive Services Task Force (USPSTF) expanded the eligibility criteria for lung cancer screening, shows that significantly more Black men have been screened for lung cancer, but not women or undereducated people.
The eligibility for lung cancer screening was expanded in 2021 to include men and women under 50 years old and people who smoke at least one pack of cigarettes a day for the last 20 years. “
“Expansion of screening criteria is a critical first step to achieving equity in lung cancer screening for all high-risk populations, but myriad challenges remain before individuals enter the door for screening,” wrote the authors, led by Julie A. Barta, MD, Thomas Jefferson University, Philadelphia. “Health policy changes must occur simultaneously with efforts to expand community outreach, overcome logistical barriers, and facilitate screening adherence. Only after comprehensive strategies to dismantle screening barriers are identified, validated, and implemented can there be a truly equitable landscape for lung cancer screening.”
For the study, published in JAMA Open Network, researchers examined rates of centralized lung cancer screening in the Baltimore area. In addition to expanding lung cancer screening generally, there was hope that the expanded criteria might increase uptake of screening in populations that are traditionally underserved, such as African American, Hispanic, and female patients. Of 815 people screened during the study period (March-December 2021), 161 were newly eligible for screening under the 2021 criteria.
“There’s been quite a bit of work in the field demonstrating that Black men and women develop lung cancer at more advanced stages of disease, and they often are diagnosed at younger ages and have fewer pack-years of smoking. So the hypothesis was that this would reduce some of the disparities seen in lung cancer screening by making more people eligible,” Dr. Barta said in an interview.
The researchers categorized participants as those who would have been eligible for screening under the USPSTF 2013 guideline (age 55 or older, 30 or more pack-years, quit within the past 15 years), and those who would be eligible under the 2021 guideline (age 50 or older, 20 or more pack-years, quit within the past 15 years). Of the 2021 cohort, 54.5% were African American, versus 39.5% of the 2013 cohort (P = .002). There were no differences between the cohorts with respect to education level or gender.
“Although we’ve seen some encouraging improvement in terms of getting more eligible patients into our screening program, there’s still a lot of work to be done in the field,” Dr. Barta said. “Diagnosing lung cancer at earlier stages of disease is more cost effective in general for the health care system than fighting lung cancer at advanced stages, which requires more complex and multimodal and prolonged therapies.”
New evidence: Chest CTs for lung cancer screening reduces incidence of advanced lung cancer
In an analysis of the SEER database presented in June at the annual meeting of the American Society of Clinical Oncology, the adoption of low-dose chest computed tomography (LDCT) led to fewer diagnoses of advanced lung cancer, although these declines varied significantly by race and ethnicity. Non-Hispanic Blacks seemed to benefit the most with a 55% decline (P < .01), while Hispanics had the lowest rate of decline at 41% (P < .01). The change was recommended by USPSTF in 2013 after the National Lung Screening Trial revealed a 20% relative reduction in mortality when CT scans were used instead of chest radiography. The Centers for Medicare and Medicaid Services approved coverage of the screen in 2015.
The SEER study looked at data from 400,343 individuals from 2004-2014 (preintervention) and 2015-2018 (postintervention). The age-adjusted incidence of advanced lung cancer declined during both periods, but the decline was sharper between 2015 and 2018, with three fewer cases per 100,000 people than 2004-2014 (P < .01). Similar patterns were seen in subanalyses of males and females, non-Hispanic Whites, non-Hispanic Blacks, and Hispanics. The relative declines were largest in women, non-Hispanic Blacks, and people who lived outside of Metropolitan areas.
During a Q&A session that followed the presentation, Robert Smith, PhD, pointed out that the bar for eligibility of lung cancer risk has been set quite high, following the eligibility criteria for clinical trials. He noted that . “We are missing opportunities to prevent avertable lung cancer deaths,” said Dr. Smith, senior vice president of cancer screening at the American Cancer Society.
On the other hand, screening-prompted biopsies have the potential to cause harm, particularly in patients who already have lung disease, said Douglas Allen Arenberg, MD, professor at the University of Michigan, Ann Arbor. “I think that’s what scares most people is the potential downside, which is very hard to measure outside of a clinical trial,” said Dr. Arenberg, who served as a discussant for the presentation.
One way to reduce that risk is to identify biomarkers, either for screens or for incidentally-detected nodules, that have good negative predictive value. “If I had a blood test that is as good as a negative PET scan, I’m going to be much more likely to say, ‘Yeah, you’re 40 and your grandfather had lung cancer. Maybe you should get a CT. If we had that, we could screen a lot more people. Right now, I would discourage anybody who is at low risk from getting screened because when they come to me, the biggest opportunity I have to do harm is when I do a biopsy, and you always remember the ones that go wrong,” he said.
Dr. Arenberg also called for improvements in electronic medical records to better flag at-risk patients. “I think we as physicians have to demand more of the software developers that create these EMRs for us,” he said.
Another study in the same session used data from 1,391,088 patients drawn from the National Cancer Database between 2010 and 2017 to examine trends in diagnosis of stage I cancer. In 2010, 23.5% of patients were diagnosed as stage I, versus 29.1% in 2017. Stage I incidence increased from 25.8% to 31.7% in non–small cell lung cancer, but there was no statistically significant change in small cell lung cancer. As with the SEER database study, the researchers noted that the shift toward stage I diagnoses predated the recommendation of LDCT.
Dr. Arenberg suggested that the trend may come down to increased frequency of CT scans, which often collect incidental images of the lungs. He added that better access to care may also be helping to drive the change. “How much of that might have had something to do with the introduction 5 or 10 years earlier of the Affordable Care Act and people just simply having access to care and taking advantage of that?” Dr. Arenberg said.
But Dr. Arenberg said that not even screening can explain all the data. He referenced a stage shift in patients of all age groups in the National Cancer Database study, even those too young to be eligible for screening. “There’s something else going on here. It would be nice for us to understand what caused these trends, so perhaps we could accentuate that trend even more, but stage shifts are clearly occurring in lung cancer,” Dr. Arenberg said.
Dr. Barta has received grants from Genentech Health Equity Innovations Fund. Dr. Arenberg has no relevant financial disclosures. Dr. Smith’s potential disclosures could not be ascertained.
An analysis of trends in lung cancer screening since March 2021 when the U.S. Preventive Services Task Force (USPSTF) expanded the eligibility criteria for lung cancer screening, shows that significantly more Black men have been screened for lung cancer, but not women or undereducated people.
The eligibility for lung cancer screening was expanded in 2021 to include men and women under 50 years old and people who smoke at least one pack of cigarettes a day for the last 20 years. “
“Expansion of screening criteria is a critical first step to achieving equity in lung cancer screening for all high-risk populations, but myriad challenges remain before individuals enter the door for screening,” wrote the authors, led by Julie A. Barta, MD, Thomas Jefferson University, Philadelphia. “Health policy changes must occur simultaneously with efforts to expand community outreach, overcome logistical barriers, and facilitate screening adherence. Only after comprehensive strategies to dismantle screening barriers are identified, validated, and implemented can there be a truly equitable landscape for lung cancer screening.”
For the study, published in JAMA Open Network, researchers examined rates of centralized lung cancer screening in the Baltimore area. In addition to expanding lung cancer screening generally, there was hope that the expanded criteria might increase uptake of screening in populations that are traditionally underserved, such as African American, Hispanic, and female patients. Of 815 people screened during the study period (March-December 2021), 161 were newly eligible for screening under the 2021 criteria.
“There’s been quite a bit of work in the field demonstrating that Black men and women develop lung cancer at more advanced stages of disease, and they often are diagnosed at younger ages and have fewer pack-years of smoking. So the hypothesis was that this would reduce some of the disparities seen in lung cancer screening by making more people eligible,” Dr. Barta said in an interview.
The researchers categorized participants as those who would have been eligible for screening under the USPSTF 2013 guideline (age 55 or older, 30 or more pack-years, quit within the past 15 years), and those who would be eligible under the 2021 guideline (age 50 or older, 20 or more pack-years, quit within the past 15 years). Of the 2021 cohort, 54.5% were African American, versus 39.5% of the 2013 cohort (P = .002). There were no differences between the cohorts with respect to education level or gender.
“Although we’ve seen some encouraging improvement in terms of getting more eligible patients into our screening program, there’s still a lot of work to be done in the field,” Dr. Barta said. “Diagnosing lung cancer at earlier stages of disease is more cost effective in general for the health care system than fighting lung cancer at advanced stages, which requires more complex and multimodal and prolonged therapies.”
New evidence: Chest CTs for lung cancer screening reduces incidence of advanced lung cancer
In an analysis of the SEER database presented in June at the annual meeting of the American Society of Clinical Oncology, the adoption of low-dose chest computed tomography (LDCT) led to fewer diagnoses of advanced lung cancer, although these declines varied significantly by race and ethnicity. Non-Hispanic Blacks seemed to benefit the most with a 55% decline (P < .01), while Hispanics had the lowest rate of decline at 41% (P < .01). The change was recommended by USPSTF in 2013 after the National Lung Screening Trial revealed a 20% relative reduction in mortality when CT scans were used instead of chest radiography. The Centers for Medicare and Medicaid Services approved coverage of the screen in 2015.
The SEER study looked at data from 400,343 individuals from 2004-2014 (preintervention) and 2015-2018 (postintervention). The age-adjusted incidence of advanced lung cancer declined during both periods, but the decline was sharper between 2015 and 2018, with three fewer cases per 100,000 people than 2004-2014 (P < .01). Similar patterns were seen in subanalyses of males and females, non-Hispanic Whites, non-Hispanic Blacks, and Hispanics. The relative declines were largest in women, non-Hispanic Blacks, and people who lived outside of Metropolitan areas.
During a Q&A session that followed the presentation, Robert Smith, PhD, pointed out that the bar for eligibility of lung cancer risk has been set quite high, following the eligibility criteria for clinical trials. He noted that . “We are missing opportunities to prevent avertable lung cancer deaths,” said Dr. Smith, senior vice president of cancer screening at the American Cancer Society.
On the other hand, screening-prompted biopsies have the potential to cause harm, particularly in patients who already have lung disease, said Douglas Allen Arenberg, MD, professor at the University of Michigan, Ann Arbor. “I think that’s what scares most people is the potential downside, which is very hard to measure outside of a clinical trial,” said Dr. Arenberg, who served as a discussant for the presentation.
One way to reduce that risk is to identify biomarkers, either for screens or for incidentally-detected nodules, that have good negative predictive value. “If I had a blood test that is as good as a negative PET scan, I’m going to be much more likely to say, ‘Yeah, you’re 40 and your grandfather had lung cancer. Maybe you should get a CT. If we had that, we could screen a lot more people. Right now, I would discourage anybody who is at low risk from getting screened because when they come to me, the biggest opportunity I have to do harm is when I do a biopsy, and you always remember the ones that go wrong,” he said.
Dr. Arenberg also called for improvements in electronic medical records to better flag at-risk patients. “I think we as physicians have to demand more of the software developers that create these EMRs for us,” he said.
Another study in the same session used data from 1,391,088 patients drawn from the National Cancer Database between 2010 and 2017 to examine trends in diagnosis of stage I cancer. In 2010, 23.5% of patients were diagnosed as stage I, versus 29.1% in 2017. Stage I incidence increased from 25.8% to 31.7% in non–small cell lung cancer, but there was no statistically significant change in small cell lung cancer. As with the SEER database study, the researchers noted that the shift toward stage I diagnoses predated the recommendation of LDCT.
Dr. Arenberg suggested that the trend may come down to increased frequency of CT scans, which often collect incidental images of the lungs. He added that better access to care may also be helping to drive the change. “How much of that might have had something to do with the introduction 5 or 10 years earlier of the Affordable Care Act and people just simply having access to care and taking advantage of that?” Dr. Arenberg said.
But Dr. Arenberg said that not even screening can explain all the data. He referenced a stage shift in patients of all age groups in the National Cancer Database study, even those too young to be eligible for screening. “There’s something else going on here. It would be nice for us to understand what caused these trends, so perhaps we could accentuate that trend even more, but stage shifts are clearly occurring in lung cancer,” Dr. Arenberg said.
Dr. Barta has received grants from Genentech Health Equity Innovations Fund. Dr. Arenberg has no relevant financial disclosures. Dr. Smith’s potential disclosures could not be ascertained.
FROM JAMA NETWORK OPEN
New KRAS inhibitor shows promise in NSCLC
In a phase 2 cohort study, who had previously been treated with platinum-based chemotherapy and immune therapy.
Adagrasib targets KRAS (G12C), which had long been thought undruggable until research published in 2013 revealed a new binding pocket that did not compete directly against the protein’s natural binding partner. The new trial further validates the approach. “It supports that clinically effective targeted therapies can be developed for patients with KRAS (G12C)–mutant NSCLC,” said Pasi Jänne, MD, PhD, who is the lead author of the study describing the new results published online in the New England Journal of Medicine.
KRAS is the most frequently mutated oncogene in human cancers. A mutated form is found in about 25% of NSCLCs. KRAS plays a key role in cell signaling governing growth, maturation, and cell death. The mutated form is linked to cancer growth and spread. Patients with mutated KRAS have few effective treatment options.
Adagrasib is currently under study and not yet approved by the Food and Drug Administration. However, sotorasib (Lumakras, Amgen), which also inhibits KRAS (G12C), was approved in May 2021 by the FDA for KRAS (G12C)–mutated NSCLC. There are some key differences between the drugs. Adagrasib has a half-life of 23 hours versus 5 hours for sotorasib, and the newer drug has the potential to penetrate the central nervous system. That could be an important consideration in NSCLC since it often metastasizes to the brain. “Having pharmacological approaches to treat brain metastases is a wonderful new therapeutic option for lung cancer patients,” said Dr. Jänne, who is director of the Lowe Center for Thoracic Oncology at Dana Farber Cancer Institute, Boston.
Adagrasib is being investigated as part of the KRYSTAL-1 study, alone and as part of combinations in various solid tumors. Previously treated NSCLC KRAS (G12C) patients are also being enrolled in a phase 3 study of adagrasib combined with docetaxel, as well as another phase 2 study of adagrasib combined with pembrolizumab as first-line therapy for NSCLC KRAS (G12C).
Adagrasib is likely to remain a second-line therapy following chemotherapy and immunotherapy. “The activity by itself at the moment is not sufficient to be a first-line treatment. That may change in the future in combination with a standard of care agent or in a subset of patients with KRAS (G12C)–mutant NSCLC, although no subset with higher efficacy has been identified to date. Identification of predictive biomarkers for patients likely to benefit from single agent or an adagrasib combination treatment remains a high priority,” Dr. Jänne said.
The study included 116 patients who had previously been treated with platinum-based chemotherapy and anti–programmed death 1 or programmed death–ligand 1 therapy. They received 600 mg oral adagrasib twice per day over a median follow-up period of 12.9 months. About 42.9% (95% confidence interval, 33.5%-52.6%) experienced a confirmed objective response with a median duration of 8.5 months (95% CI, 6.2-13.8 months). The median progression-free survival was 6.5 months (95% CI, 4.7-8.4 months). After a median follow-up of 15.6 months, the median overall survival was 12.6 months (95% CI, 9.2-19.2 months). The estimated overall survival at 1 year was 50.8% (95% CI, 40.9%-60.0%).
33 patients had stable central nervous system metastases that had been previously treated. About 33.3% had an intracranial confirmed objective response (95% CI, 18.0-51.8%) with a median duration of response of 11.2 months (95% CI, 2.99 months to not available).
Adverse events are similar to what is seen with other targeted therapies, according to Dr. Jänne. 97.4% of patient reported a treatment-related adverse event; 52.6% had grade 1-2 adverse events, and 44.8% had grade 3 adverse events. 6.9% discontinued the drug as a result.
Dr. Jänne has consulted for Mirati Therapeutics and is a member of its scientific advisory board. The study was funded by Mirati Therapeutics.
In a phase 2 cohort study, who had previously been treated with platinum-based chemotherapy and immune therapy.
Adagrasib targets KRAS (G12C), which had long been thought undruggable until research published in 2013 revealed a new binding pocket that did not compete directly against the protein’s natural binding partner. The new trial further validates the approach. “It supports that clinically effective targeted therapies can be developed for patients with KRAS (G12C)–mutant NSCLC,” said Pasi Jänne, MD, PhD, who is the lead author of the study describing the new results published online in the New England Journal of Medicine.
KRAS is the most frequently mutated oncogene in human cancers. A mutated form is found in about 25% of NSCLCs. KRAS plays a key role in cell signaling governing growth, maturation, and cell death. The mutated form is linked to cancer growth and spread. Patients with mutated KRAS have few effective treatment options.
Adagrasib is currently under study and not yet approved by the Food and Drug Administration. However, sotorasib (Lumakras, Amgen), which also inhibits KRAS (G12C), was approved in May 2021 by the FDA for KRAS (G12C)–mutated NSCLC. There are some key differences between the drugs. Adagrasib has a half-life of 23 hours versus 5 hours for sotorasib, and the newer drug has the potential to penetrate the central nervous system. That could be an important consideration in NSCLC since it often metastasizes to the brain. “Having pharmacological approaches to treat brain metastases is a wonderful new therapeutic option for lung cancer patients,” said Dr. Jänne, who is director of the Lowe Center for Thoracic Oncology at Dana Farber Cancer Institute, Boston.
Adagrasib is being investigated as part of the KRYSTAL-1 study, alone and as part of combinations in various solid tumors. Previously treated NSCLC KRAS (G12C) patients are also being enrolled in a phase 3 study of adagrasib combined with docetaxel, as well as another phase 2 study of adagrasib combined with pembrolizumab as first-line therapy for NSCLC KRAS (G12C).
Adagrasib is likely to remain a second-line therapy following chemotherapy and immunotherapy. “The activity by itself at the moment is not sufficient to be a first-line treatment. That may change in the future in combination with a standard of care agent or in a subset of patients with KRAS (G12C)–mutant NSCLC, although no subset with higher efficacy has been identified to date. Identification of predictive biomarkers for patients likely to benefit from single agent or an adagrasib combination treatment remains a high priority,” Dr. Jänne said.
The study included 116 patients who had previously been treated with platinum-based chemotherapy and anti–programmed death 1 or programmed death–ligand 1 therapy. They received 600 mg oral adagrasib twice per day over a median follow-up period of 12.9 months. About 42.9% (95% confidence interval, 33.5%-52.6%) experienced a confirmed objective response with a median duration of 8.5 months (95% CI, 6.2-13.8 months). The median progression-free survival was 6.5 months (95% CI, 4.7-8.4 months). After a median follow-up of 15.6 months, the median overall survival was 12.6 months (95% CI, 9.2-19.2 months). The estimated overall survival at 1 year was 50.8% (95% CI, 40.9%-60.0%).
33 patients had stable central nervous system metastases that had been previously treated. About 33.3% had an intracranial confirmed objective response (95% CI, 18.0-51.8%) with a median duration of response of 11.2 months (95% CI, 2.99 months to not available).
Adverse events are similar to what is seen with other targeted therapies, according to Dr. Jänne. 97.4% of patient reported a treatment-related adverse event; 52.6% had grade 1-2 adverse events, and 44.8% had grade 3 adverse events. 6.9% discontinued the drug as a result.
Dr. Jänne has consulted for Mirati Therapeutics and is a member of its scientific advisory board. The study was funded by Mirati Therapeutics.
In a phase 2 cohort study, who had previously been treated with platinum-based chemotherapy and immune therapy.
Adagrasib targets KRAS (G12C), which had long been thought undruggable until research published in 2013 revealed a new binding pocket that did not compete directly against the protein’s natural binding partner. The new trial further validates the approach. “It supports that clinically effective targeted therapies can be developed for patients with KRAS (G12C)–mutant NSCLC,” said Pasi Jänne, MD, PhD, who is the lead author of the study describing the new results published online in the New England Journal of Medicine.
KRAS is the most frequently mutated oncogene in human cancers. A mutated form is found in about 25% of NSCLCs. KRAS plays a key role in cell signaling governing growth, maturation, and cell death. The mutated form is linked to cancer growth and spread. Patients with mutated KRAS have few effective treatment options.
Adagrasib is currently under study and not yet approved by the Food and Drug Administration. However, sotorasib (Lumakras, Amgen), which also inhibits KRAS (G12C), was approved in May 2021 by the FDA for KRAS (G12C)–mutated NSCLC. There are some key differences between the drugs. Adagrasib has a half-life of 23 hours versus 5 hours for sotorasib, and the newer drug has the potential to penetrate the central nervous system. That could be an important consideration in NSCLC since it often metastasizes to the brain. “Having pharmacological approaches to treat brain metastases is a wonderful new therapeutic option for lung cancer patients,” said Dr. Jänne, who is director of the Lowe Center for Thoracic Oncology at Dana Farber Cancer Institute, Boston.
Adagrasib is being investigated as part of the KRYSTAL-1 study, alone and as part of combinations in various solid tumors. Previously treated NSCLC KRAS (G12C) patients are also being enrolled in a phase 3 study of adagrasib combined with docetaxel, as well as another phase 2 study of adagrasib combined with pembrolizumab as first-line therapy for NSCLC KRAS (G12C).
Adagrasib is likely to remain a second-line therapy following chemotherapy and immunotherapy. “The activity by itself at the moment is not sufficient to be a first-line treatment. That may change in the future in combination with a standard of care agent or in a subset of patients with KRAS (G12C)–mutant NSCLC, although no subset with higher efficacy has been identified to date. Identification of predictive biomarkers for patients likely to benefit from single agent or an adagrasib combination treatment remains a high priority,” Dr. Jänne said.
The study included 116 patients who had previously been treated with platinum-based chemotherapy and anti–programmed death 1 or programmed death–ligand 1 therapy. They received 600 mg oral adagrasib twice per day over a median follow-up period of 12.9 months. About 42.9% (95% confidence interval, 33.5%-52.6%) experienced a confirmed objective response with a median duration of 8.5 months (95% CI, 6.2-13.8 months). The median progression-free survival was 6.5 months (95% CI, 4.7-8.4 months). After a median follow-up of 15.6 months, the median overall survival was 12.6 months (95% CI, 9.2-19.2 months). The estimated overall survival at 1 year was 50.8% (95% CI, 40.9%-60.0%).
33 patients had stable central nervous system metastases that had been previously treated. About 33.3% had an intracranial confirmed objective response (95% CI, 18.0-51.8%) with a median duration of response of 11.2 months (95% CI, 2.99 months to not available).
Adverse events are similar to what is seen with other targeted therapies, according to Dr. Jänne. 97.4% of patient reported a treatment-related adverse event; 52.6% had grade 1-2 adverse events, and 44.8% had grade 3 adverse events. 6.9% discontinued the drug as a result.
Dr. Jänne has consulted for Mirati Therapeutics and is a member of its scientific advisory board. The study was funded by Mirati Therapeutics.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Dry cough and dyspnea
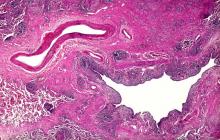
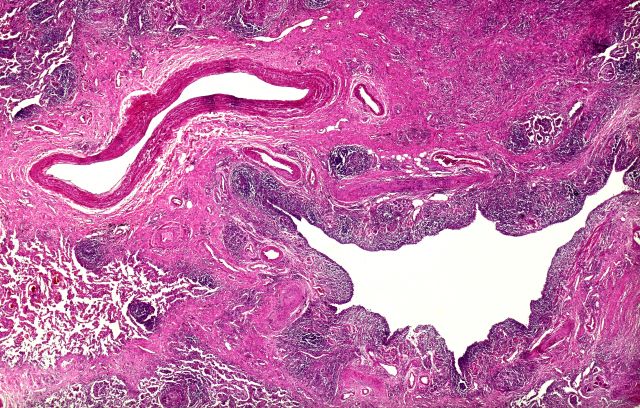
Based on the patient's presentation and workup, the likely diagnosis is adenosquamous carcinoma of the lung, a relatively rare subtype of non–small cell lung cancer (NSCLC). Adenosquamous carcinoma displays qualities of both squamous cell carcinoma and adenocarcinoma; for definitive diagnosis, the cancer must contain 10% of each of these major NSCLC subtypes. Maeda and colleagues concluded that adenosquamous carcinoma occurs more frequently among men and that the age at the time of diagnosis is higher among such cancers compared with adenocarcinoma. Several studies have confirmed that adenosquamous carcinoma of the lung is also more prevalent among smokers.
Though a diagnosis of adenosquamous carcinoma may be suspected after small biopsies, cytology, or excisional biopsies, definitive diagnosis necessitates a resection specimen. If any adenocarcinoma component is observed in a biopsy specimen that is otherwise squamous, as in the present case, this finding is an indication for molecular testing. Epidermal growth factor receptor (EGFR) mutations may be present in adenosquamous carcinoma cancers, despite a majority of cancers with EGFR mutations being among nonsmokers or former light smokers with adenocarcinoma histology. In addition, even for patients diagnosed with squamous cell carcinoma, adenosquamous carcinoma should be considered if genetic testing suggests EGFR mutations.
Relative to adenocarcinoma and squamous cell carcinoma, adenosquamous carcinoma has higher grade malignancy, more advanced postoperative stage, and stronger lymph nodal invasiveness. In terms of treatment, surgical resection is the curative option for adenosquamous carcinoma of the lung, with lobectomy with lymphadenectomy considered for first-line treatment. Though the most beneficial chemotherapy regimen for patients with adenosquamous carcinoma of the lung remains the subject of investigation, platinum-based doublet chemotherapy is the current standard treatment option. EGFR tyrosine kinase inhibitors may be an effective option for EGFR-positive patients.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Based on the patient's presentation and workup, the likely diagnosis is adenosquamous carcinoma of the lung, a relatively rare subtype of non–small cell lung cancer (NSCLC). Adenosquamous carcinoma displays qualities of both squamous cell carcinoma and adenocarcinoma; for definitive diagnosis, the cancer must contain 10% of each of these major NSCLC subtypes. Maeda and colleagues concluded that adenosquamous carcinoma occurs more frequently among men and that the age at the time of diagnosis is higher among such cancers compared with adenocarcinoma. Several studies have confirmed that adenosquamous carcinoma of the lung is also more prevalent among smokers.
Though a diagnosis of adenosquamous carcinoma may be suspected after small biopsies, cytology, or excisional biopsies, definitive diagnosis necessitates a resection specimen. If any adenocarcinoma component is observed in a biopsy specimen that is otherwise squamous, as in the present case, this finding is an indication for molecular testing. Epidermal growth factor receptor (EGFR) mutations may be present in adenosquamous carcinoma cancers, despite a majority of cancers with EGFR mutations being among nonsmokers or former light smokers with adenocarcinoma histology. In addition, even for patients diagnosed with squamous cell carcinoma, adenosquamous carcinoma should be considered if genetic testing suggests EGFR mutations.
Relative to adenocarcinoma and squamous cell carcinoma, adenosquamous carcinoma has higher grade malignancy, more advanced postoperative stage, and stronger lymph nodal invasiveness. In terms of treatment, surgical resection is the curative option for adenosquamous carcinoma of the lung, with lobectomy with lymphadenectomy considered for first-line treatment. Though the most beneficial chemotherapy regimen for patients with adenosquamous carcinoma of the lung remains the subject of investigation, platinum-based doublet chemotherapy is the current standard treatment option. EGFR tyrosine kinase inhibitors may be an effective option for EGFR-positive patients.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Based on the patient's presentation and workup, the likely diagnosis is adenosquamous carcinoma of the lung, a relatively rare subtype of non–small cell lung cancer (NSCLC). Adenosquamous carcinoma displays qualities of both squamous cell carcinoma and adenocarcinoma; for definitive diagnosis, the cancer must contain 10% of each of these major NSCLC subtypes. Maeda and colleagues concluded that adenosquamous carcinoma occurs more frequently among men and that the age at the time of diagnosis is higher among such cancers compared with adenocarcinoma. Several studies have confirmed that adenosquamous carcinoma of the lung is also more prevalent among smokers.
Though a diagnosis of adenosquamous carcinoma may be suspected after small biopsies, cytology, or excisional biopsies, definitive diagnosis necessitates a resection specimen. If any adenocarcinoma component is observed in a biopsy specimen that is otherwise squamous, as in the present case, this finding is an indication for molecular testing. Epidermal growth factor receptor (EGFR) mutations may be present in adenosquamous carcinoma cancers, despite a majority of cancers with EGFR mutations being among nonsmokers or former light smokers with adenocarcinoma histology. In addition, even for patients diagnosed with squamous cell carcinoma, adenosquamous carcinoma should be considered if genetic testing suggests EGFR mutations.
Relative to adenocarcinoma and squamous cell carcinoma, adenosquamous carcinoma has higher grade malignancy, more advanced postoperative stage, and stronger lymph nodal invasiveness. In terms of treatment, surgical resection is the curative option for adenosquamous carcinoma of the lung, with lobectomy with lymphadenectomy considered for first-line treatment. Though the most beneficial chemotherapy regimen for patients with adenosquamous carcinoma of the lung remains the subject of investigation, platinum-based doublet chemotherapy is the current standard treatment option. EGFR tyrosine kinase inhibitors may be an effective option for EGFR-positive patients.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 58-year-old man with a 20-year–pack history of smoking initially presented with a persistent dry cough and dyspnea. Clubbing was noted on physical examination and breath sounds in the right upper lung were weak. Other than hypertension, which the patient manages with angiotensin-converting enzyme (ACE) inhibitors, medical history is unremarkable. The patient notes that this medication has always made him cough, but dyspnea has only developed over the past 6 weeks. Respiratory symptoms prompted a chest radiograph which revealed a mass in the upper lobe of the right lung. Transbronchial lung biopsy of the right lung reveals components of adenocarcinoma; the specimen is otherwise squamous.
Drugging the undruggable
including 68% of pancreatic tumors and 20% of all non–small cell lung cancers (NSCLC).
We now have a treatment – sotorasib – for patients with locally advanced or metastatic NSCLC that is driven by a KRAS mutation (G12C). And, now, there is a second treatment – adagrasib – under study, which, according to a presentation recently made at the annual meeting of the American Society of Clinical Oncology, looks promising.
Ras is a membrane-bound regulatory protein (G protein) belonging to the family of guanosine triphosphatases (GTPases). Ras functions as a guanosine diphosphate/triphosphate binary switch by cycling between the active GTP-bound and the inactive GDP-bound states in response to extracellular stimuli. The KRAS (G12C) mutation affects the active form of KRAS and results in abnormally high concentrations of GTP-bound KRAS leading to hyperactivation of downstream oncogenic pathways and uncontrolled cell growth, specifically of ERK and MEK signaling pathways.
At the ASCO annual meeting in June, Spira and colleagues reported the results of cohort A of the KRYSTAL-1 study evaluating adagrasib as second-line therapy patients with advanced solid tumors harboring a KRAS (G12C) mutation. Like sotorasib, adagrasib is a KRAS (G12C) inhibitor that irreversibly and selectively binds KRAS (G12C), locking it in its inactive state. In this study, patients had to have failed first-line chemotherapy and immunotherapy with 43% of lung cancer patients responding. The 12-month overall survival (OS) was 51%, median overall survival was 12.6 and median progression-free survival (PFS) was 6.5 months. Twenty-five patients with KRAS (G12C)–mutant NSCLC and active, untreated central nervous system metastases received adagrasib in a phase 1b cohort. The intracranial overall response rate was 31.6% and median intracranial PFS was 4.2 months. Systemic ORR was 35.0% (7/20), the disease control rate was 80.0% (16/20) and median duration of response was 9.6 months. Based on these data, a phase 3 trial evaluating adagrasib monotherapy versus docetaxel in previously treated patients with KRAS (G12C) mutant NSCLC is ongoing.
The Food and Drug Administration approval of sotorasib in 2021 was, in part, based on the results of a single-arm, phase 2, second-line study of patients who had previously received platinum-based chemotherapy and/or immunotherapy. An ORR rate of 37.1% was reported with a median PFS of 6.8 months and median OS of 12.5 months leading to the FDA approval. Responses were observed across the range of baseline PD-L1 expression levels: 48% of PD-L1 negative, 39% with PD-L1 between 1%-49%, and 22% of patients with a PD-L1 of greater than 50% having a response.
The major toxicities observed in these studies were gastrointestinal (diarrhea, nausea, vomiting) and hepatic (elevated liver enzymes). About 97% of patients on adagrasib experienced any treatment-related adverse events, and 43% experienced a grade 3 or 4 treatment-related adverse event leading to dose reduction in 52% of patients, a dose interruption in 61% of patients, and a 7% discontinuation rate. About 70% of patients treated with sotorasib had a treatment-related adverse event of any grade, and 21% reported grade 3 or 4 treatment-related adverse events.
A subgroup in the KRYSTAL-1 trial reported an intracranial ORR of 32% in patients with active, untreated CNS metastases. Median overall survival has not yet reached concordance between systemic and intracranial disease control was 88%. In addition, preliminary data from two patients with untreated CNS metastases from a phase 1b cohort found cerebrospinal fluid concentrations of adagrasib with a mean ratio of unbound brain-to-plasma concentration of 0.47, which is comparable or exceeds values for known CNS-penetrant tyrosine kinase inhibitors.
Unfortunately, KRAS (G12C) is not the only KRAS mutation out there. There are a myriad of others, such as G12V and G12D. Hopefully, we will be seeing more drugs aimed at this set of important mutations. Another question, of course, is when and if these drugs will move to the first-line setting.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
including 68% of pancreatic tumors and 20% of all non–small cell lung cancers (NSCLC).
We now have a treatment – sotorasib – for patients with locally advanced or metastatic NSCLC that is driven by a KRAS mutation (G12C). And, now, there is a second treatment – adagrasib – under study, which, according to a presentation recently made at the annual meeting of the American Society of Clinical Oncology, looks promising.
Ras is a membrane-bound regulatory protein (G protein) belonging to the family of guanosine triphosphatases (GTPases). Ras functions as a guanosine diphosphate/triphosphate binary switch by cycling between the active GTP-bound and the inactive GDP-bound states in response to extracellular stimuli. The KRAS (G12C) mutation affects the active form of KRAS and results in abnormally high concentrations of GTP-bound KRAS leading to hyperactivation of downstream oncogenic pathways and uncontrolled cell growth, specifically of ERK and MEK signaling pathways.
At the ASCO annual meeting in June, Spira and colleagues reported the results of cohort A of the KRYSTAL-1 study evaluating adagrasib as second-line therapy patients with advanced solid tumors harboring a KRAS (G12C) mutation. Like sotorasib, adagrasib is a KRAS (G12C) inhibitor that irreversibly and selectively binds KRAS (G12C), locking it in its inactive state. In this study, patients had to have failed first-line chemotherapy and immunotherapy with 43% of lung cancer patients responding. The 12-month overall survival (OS) was 51%, median overall survival was 12.6 and median progression-free survival (PFS) was 6.5 months. Twenty-five patients with KRAS (G12C)–mutant NSCLC and active, untreated central nervous system metastases received adagrasib in a phase 1b cohort. The intracranial overall response rate was 31.6% and median intracranial PFS was 4.2 months. Systemic ORR was 35.0% (7/20), the disease control rate was 80.0% (16/20) and median duration of response was 9.6 months. Based on these data, a phase 3 trial evaluating adagrasib monotherapy versus docetaxel in previously treated patients with KRAS (G12C) mutant NSCLC is ongoing.
The Food and Drug Administration approval of sotorasib in 2021 was, in part, based on the results of a single-arm, phase 2, second-line study of patients who had previously received platinum-based chemotherapy and/or immunotherapy. An ORR rate of 37.1% was reported with a median PFS of 6.8 months and median OS of 12.5 months leading to the FDA approval. Responses were observed across the range of baseline PD-L1 expression levels: 48% of PD-L1 negative, 39% with PD-L1 between 1%-49%, and 22% of patients with a PD-L1 of greater than 50% having a response.
The major toxicities observed in these studies were gastrointestinal (diarrhea, nausea, vomiting) and hepatic (elevated liver enzymes). About 97% of patients on adagrasib experienced any treatment-related adverse events, and 43% experienced a grade 3 or 4 treatment-related adverse event leading to dose reduction in 52% of patients, a dose interruption in 61% of patients, and a 7% discontinuation rate. About 70% of patients treated with sotorasib had a treatment-related adverse event of any grade, and 21% reported grade 3 or 4 treatment-related adverse events.
A subgroup in the KRYSTAL-1 trial reported an intracranial ORR of 32% in patients with active, untreated CNS metastases. Median overall survival has not yet reached concordance between systemic and intracranial disease control was 88%. In addition, preliminary data from two patients with untreated CNS metastases from a phase 1b cohort found cerebrospinal fluid concentrations of adagrasib with a mean ratio of unbound brain-to-plasma concentration of 0.47, which is comparable or exceeds values for known CNS-penetrant tyrosine kinase inhibitors.
Unfortunately, KRAS (G12C) is not the only KRAS mutation out there. There are a myriad of others, such as G12V and G12D. Hopefully, we will be seeing more drugs aimed at this set of important mutations. Another question, of course, is when and if these drugs will move to the first-line setting.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
including 68% of pancreatic tumors and 20% of all non–small cell lung cancers (NSCLC).
We now have a treatment – sotorasib – for patients with locally advanced or metastatic NSCLC that is driven by a KRAS mutation (G12C). And, now, there is a second treatment – adagrasib – under study, which, according to a presentation recently made at the annual meeting of the American Society of Clinical Oncology, looks promising.
Ras is a membrane-bound regulatory protein (G protein) belonging to the family of guanosine triphosphatases (GTPases). Ras functions as a guanosine diphosphate/triphosphate binary switch by cycling between the active GTP-bound and the inactive GDP-bound states in response to extracellular stimuli. The KRAS (G12C) mutation affects the active form of KRAS and results in abnormally high concentrations of GTP-bound KRAS leading to hyperactivation of downstream oncogenic pathways and uncontrolled cell growth, specifically of ERK and MEK signaling pathways.
At the ASCO annual meeting in June, Spira and colleagues reported the results of cohort A of the KRYSTAL-1 study evaluating adagrasib as second-line therapy patients with advanced solid tumors harboring a KRAS (G12C) mutation. Like sotorasib, adagrasib is a KRAS (G12C) inhibitor that irreversibly and selectively binds KRAS (G12C), locking it in its inactive state. In this study, patients had to have failed first-line chemotherapy and immunotherapy with 43% of lung cancer patients responding. The 12-month overall survival (OS) was 51%, median overall survival was 12.6 and median progression-free survival (PFS) was 6.5 months. Twenty-five patients with KRAS (G12C)–mutant NSCLC and active, untreated central nervous system metastases received adagrasib in a phase 1b cohort. The intracranial overall response rate was 31.6% and median intracranial PFS was 4.2 months. Systemic ORR was 35.0% (7/20), the disease control rate was 80.0% (16/20) and median duration of response was 9.6 months. Based on these data, a phase 3 trial evaluating adagrasib monotherapy versus docetaxel in previously treated patients with KRAS (G12C) mutant NSCLC is ongoing.
The Food and Drug Administration approval of sotorasib in 2021 was, in part, based on the results of a single-arm, phase 2, second-line study of patients who had previously received platinum-based chemotherapy and/or immunotherapy. An ORR rate of 37.1% was reported with a median PFS of 6.8 months and median OS of 12.5 months leading to the FDA approval. Responses were observed across the range of baseline PD-L1 expression levels: 48% of PD-L1 negative, 39% with PD-L1 between 1%-49%, and 22% of patients with a PD-L1 of greater than 50% having a response.
The major toxicities observed in these studies were gastrointestinal (diarrhea, nausea, vomiting) and hepatic (elevated liver enzymes). About 97% of patients on adagrasib experienced any treatment-related adverse events, and 43% experienced a grade 3 or 4 treatment-related adverse event leading to dose reduction in 52% of patients, a dose interruption in 61% of patients, and a 7% discontinuation rate. About 70% of patients treated with sotorasib had a treatment-related adverse event of any grade, and 21% reported grade 3 or 4 treatment-related adverse events.
A subgroup in the KRYSTAL-1 trial reported an intracranial ORR of 32% in patients with active, untreated CNS metastases. Median overall survival has not yet reached concordance between systemic and intracranial disease control was 88%. In addition, preliminary data from two patients with untreated CNS metastases from a phase 1b cohort found cerebrospinal fluid concentrations of adagrasib with a mean ratio of unbound brain-to-plasma concentration of 0.47, which is comparable or exceeds values for known CNS-penetrant tyrosine kinase inhibitors.
Unfortunately, KRAS (G12C) is not the only KRAS mutation out there. There are a myriad of others, such as G12V and G12D. Hopefully, we will be seeing more drugs aimed at this set of important mutations. Another question, of course, is when and if these drugs will move to the first-line setting.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
Quality of life benefit exaggerated in some cancer studies
, according to a study published in JAMA Oncology.
The study found trials that failed to show improved quality of life often reported their quality of life outcomes more favorably. Non–immunotherapy-targeted drugs were found to lead to worse quality of life outcomes more often than did cytotoxic agents. And, while there is an association between quality of life benefit and overall survival, no such association was found with progression-free survival.
“In this study, we evaluated the outcomes of cancer drug trials with regard to patients’ quality of life and found that only a quarter of phase 3 cancer drug trials in the advanced-disease setting demonstrated improved quality of life,” wrote authors who were led by Bishal Gyawali, MD, PhD, of the Cancer Research Institute, Queen’s University, Kingston, Ont.
“Improved quality of life outcomes were associated with improved overall survival but not with improved progression-free survival. Importantly, almost half of the cancer drugs drug trials that showed improved progression-free survival showed no improved overall survival or quality of life (i.e., PFS-only benefit). Some reports included conclusions regarding quality of life (QOL) findings that were not directly supported by the trial data, particularly for inferior or non–statistically significant QOL outcomes, thereby framing the findings in a favorable light or downplaying detrimental effects of the study intervention on QOL. Furthermore, contrary to common perception, inferior QOL outcomes were more common with targeted drugs than cytotoxic drugs. Taken together, these findings have important policy implications,” the authors wrote.
These findings are based on the results of a cohort study of 45 phase 3 research clinical trials of 24,806 patients. Only a small percentage of patients showed QOL benefits. The study found that industry-funded clinical trial reports often framed QOL findings more favorably than was warranted by the data.
The study found improved QOL with experimental agents in 11 of 45 randomized controlled trials (24.4%). Studies that reported improved QOL were more likely to also show improved overall survival as compared with trials in which quality of life was not improved (7 of 11 [64%] versus 10 of 34 [29%] trials). For improved progression-free survival, however, there was no positive association (6 of 11 [55%] trials versus 17 of 34 [50%] trials without improved QOL). Among six trials reporting worsening QOL, three (50%) were trials of targeted drugs. Among 11 trials reporting improved QOL, 6 (55%) were trials of immunotherapy drugs. Among the 34 trials in which QOL was not improved compared with controls, the findings were framed favorably (versus neutrally or negatively) in the abstract or conclusions in 16 (47%), an observation that was statistically significantly associated with industry funding (chi-squared = 6.35; P = .01).
“It is important to clearly understand and communicate the effects of cancer drugs”
To fulfill the obligation to inform patients about proposed treatments, the authors wrote that it is important to clearly understand and communicate the effects of cancer drugs on patient quality of life alongside their effects on overall survival and intermediate end points such as progression-free survival. “Patients with advanced cancer expect treatment to help them live longer or have better lives,” the authors wrote. In that respect, in clinical trials of cancer medicines, overall survival and quality of life are the most important measures. Toxicity profiles and disease progression delays do not reliably predict quality of life, and studies have shown poor correlations between quality of life, overall survival, and progression-free survival. This raises the question of validity of progression-free survival as a surrogate endpoint. “Progression-free survival is meaningless without overall survival or quality of life gains,” Dr. Gyawali said in an interview.
Writing in The Lancet Oncology in March, Dr. Gyawali stated that, because progression free survival “does not directly measure how a patient feels or functions, or how long a patient lives, progression-free survival was not intended to inform clinical practice or establish whether a new therapy provides clinically meaningful benefits for patients. However, over the past 2 decades, it has become the most common primary endpoint in oncology clinical trials. We are deeply worried about how the term survival in this phrase can influence clinical practice and patient choices. We propose replacing the phrase progression-free survival with a less ambiguous term: progression-free interval.”
In JAMA Oncology, Dr. Gyawali aimed to elucidate relationships between QOL, overall survival, and progression-free survival, and to assess, as well, how QOL results are framed, especially in industry-sponsored research. When drug trials they analyzed showed no change in QOL but reported that QOL did not worsen or QOL was maintained rather than stating that QOL did not improve, or if there was downplaying of worse QOL outcomes, the study had favorable interpretation, Dr. Gyawali and associates wrote. The expectation of patients receiving cancer drugs would be improved QOL rather than “not worse” QOL, Dr. Gyawali said.
Regarding the finding that QOL outcomes were described as favorable in 47% of trials with unimproved QOL outcomes, Dr. Gyawali said, “the bias in reporting should be corrected by the reviewers and editors of journals. Also, quality of life reporting should be made mandatory. Without unbiased quality of life information, informed decision making on whether or not to use a certain drug is impossible. Patients and physicians need to know that information. Regulators can demand that this should be mandatory in all trials in noncurative settings.”
He remarked further on the worsening QOL in some targeted drug trials, “People tout chemo-free regimens as automatically having better quality of life, but that doesn’t seem to be the case. Targeted drugs can have a severe impact on quality of life, probably due to prolonged duration of side effects. Quality of life should be measured and reported for all drugs.”
Dr. Gyawali and associates noted the limitation in that several studies with negative QOL results are not published at all or are published after a considerable delay, so the present observations may understate the issues that have been raised.
Dr. Gyawali declared that he received no funding and disclosed no conflicts of interest for this study.
, according to a study published in JAMA Oncology.
The study found trials that failed to show improved quality of life often reported their quality of life outcomes more favorably. Non–immunotherapy-targeted drugs were found to lead to worse quality of life outcomes more often than did cytotoxic agents. And, while there is an association between quality of life benefit and overall survival, no such association was found with progression-free survival.
“In this study, we evaluated the outcomes of cancer drug trials with regard to patients’ quality of life and found that only a quarter of phase 3 cancer drug trials in the advanced-disease setting demonstrated improved quality of life,” wrote authors who were led by Bishal Gyawali, MD, PhD, of the Cancer Research Institute, Queen’s University, Kingston, Ont.
“Improved quality of life outcomes were associated with improved overall survival but not with improved progression-free survival. Importantly, almost half of the cancer drugs drug trials that showed improved progression-free survival showed no improved overall survival or quality of life (i.e., PFS-only benefit). Some reports included conclusions regarding quality of life (QOL) findings that were not directly supported by the trial data, particularly for inferior or non–statistically significant QOL outcomes, thereby framing the findings in a favorable light or downplaying detrimental effects of the study intervention on QOL. Furthermore, contrary to common perception, inferior QOL outcomes were more common with targeted drugs than cytotoxic drugs. Taken together, these findings have important policy implications,” the authors wrote.
These findings are based on the results of a cohort study of 45 phase 3 research clinical trials of 24,806 patients. Only a small percentage of patients showed QOL benefits. The study found that industry-funded clinical trial reports often framed QOL findings more favorably than was warranted by the data.
The study found improved QOL with experimental agents in 11 of 45 randomized controlled trials (24.4%). Studies that reported improved QOL were more likely to also show improved overall survival as compared with trials in which quality of life was not improved (7 of 11 [64%] versus 10 of 34 [29%] trials). For improved progression-free survival, however, there was no positive association (6 of 11 [55%] trials versus 17 of 34 [50%] trials without improved QOL). Among six trials reporting worsening QOL, three (50%) were trials of targeted drugs. Among 11 trials reporting improved QOL, 6 (55%) were trials of immunotherapy drugs. Among the 34 trials in which QOL was not improved compared with controls, the findings were framed favorably (versus neutrally or negatively) in the abstract or conclusions in 16 (47%), an observation that was statistically significantly associated with industry funding (chi-squared = 6.35; P = .01).
“It is important to clearly understand and communicate the effects of cancer drugs”
To fulfill the obligation to inform patients about proposed treatments, the authors wrote that it is important to clearly understand and communicate the effects of cancer drugs on patient quality of life alongside their effects on overall survival and intermediate end points such as progression-free survival. “Patients with advanced cancer expect treatment to help them live longer or have better lives,” the authors wrote. In that respect, in clinical trials of cancer medicines, overall survival and quality of life are the most important measures. Toxicity profiles and disease progression delays do not reliably predict quality of life, and studies have shown poor correlations between quality of life, overall survival, and progression-free survival. This raises the question of validity of progression-free survival as a surrogate endpoint. “Progression-free survival is meaningless without overall survival or quality of life gains,” Dr. Gyawali said in an interview.
Writing in The Lancet Oncology in March, Dr. Gyawali stated that, because progression free survival “does not directly measure how a patient feels or functions, or how long a patient lives, progression-free survival was not intended to inform clinical practice or establish whether a new therapy provides clinically meaningful benefits for patients. However, over the past 2 decades, it has become the most common primary endpoint in oncology clinical trials. We are deeply worried about how the term survival in this phrase can influence clinical practice and patient choices. We propose replacing the phrase progression-free survival with a less ambiguous term: progression-free interval.”
In JAMA Oncology, Dr. Gyawali aimed to elucidate relationships between QOL, overall survival, and progression-free survival, and to assess, as well, how QOL results are framed, especially in industry-sponsored research. When drug trials they analyzed showed no change in QOL but reported that QOL did not worsen or QOL was maintained rather than stating that QOL did not improve, or if there was downplaying of worse QOL outcomes, the study had favorable interpretation, Dr. Gyawali and associates wrote. The expectation of patients receiving cancer drugs would be improved QOL rather than “not worse” QOL, Dr. Gyawali said.
Regarding the finding that QOL outcomes were described as favorable in 47% of trials with unimproved QOL outcomes, Dr. Gyawali said, “the bias in reporting should be corrected by the reviewers and editors of journals. Also, quality of life reporting should be made mandatory. Without unbiased quality of life information, informed decision making on whether or not to use a certain drug is impossible. Patients and physicians need to know that information. Regulators can demand that this should be mandatory in all trials in noncurative settings.”
He remarked further on the worsening QOL in some targeted drug trials, “People tout chemo-free regimens as automatically having better quality of life, but that doesn’t seem to be the case. Targeted drugs can have a severe impact on quality of life, probably due to prolonged duration of side effects. Quality of life should be measured and reported for all drugs.”
Dr. Gyawali and associates noted the limitation in that several studies with negative QOL results are not published at all or are published after a considerable delay, so the present observations may understate the issues that have been raised.
Dr. Gyawali declared that he received no funding and disclosed no conflicts of interest for this study.
, according to a study published in JAMA Oncology.
The study found trials that failed to show improved quality of life often reported their quality of life outcomes more favorably. Non–immunotherapy-targeted drugs were found to lead to worse quality of life outcomes more often than did cytotoxic agents. And, while there is an association between quality of life benefit and overall survival, no such association was found with progression-free survival.
“In this study, we evaluated the outcomes of cancer drug trials with regard to patients’ quality of life and found that only a quarter of phase 3 cancer drug trials in the advanced-disease setting demonstrated improved quality of life,” wrote authors who were led by Bishal Gyawali, MD, PhD, of the Cancer Research Institute, Queen’s University, Kingston, Ont.
“Improved quality of life outcomes were associated with improved overall survival but not with improved progression-free survival. Importantly, almost half of the cancer drugs drug trials that showed improved progression-free survival showed no improved overall survival or quality of life (i.e., PFS-only benefit). Some reports included conclusions regarding quality of life (QOL) findings that were not directly supported by the trial data, particularly for inferior or non–statistically significant QOL outcomes, thereby framing the findings in a favorable light or downplaying detrimental effects of the study intervention on QOL. Furthermore, contrary to common perception, inferior QOL outcomes were more common with targeted drugs than cytotoxic drugs. Taken together, these findings have important policy implications,” the authors wrote.
These findings are based on the results of a cohort study of 45 phase 3 research clinical trials of 24,806 patients. Only a small percentage of patients showed QOL benefits. The study found that industry-funded clinical trial reports often framed QOL findings more favorably than was warranted by the data.
The study found improved QOL with experimental agents in 11 of 45 randomized controlled trials (24.4%). Studies that reported improved QOL were more likely to also show improved overall survival as compared with trials in which quality of life was not improved (7 of 11 [64%] versus 10 of 34 [29%] trials). For improved progression-free survival, however, there was no positive association (6 of 11 [55%] trials versus 17 of 34 [50%] trials without improved QOL). Among six trials reporting worsening QOL, three (50%) were trials of targeted drugs. Among 11 trials reporting improved QOL, 6 (55%) were trials of immunotherapy drugs. Among the 34 trials in which QOL was not improved compared with controls, the findings were framed favorably (versus neutrally or negatively) in the abstract or conclusions in 16 (47%), an observation that was statistically significantly associated with industry funding (chi-squared = 6.35; P = .01).
“It is important to clearly understand and communicate the effects of cancer drugs”
To fulfill the obligation to inform patients about proposed treatments, the authors wrote that it is important to clearly understand and communicate the effects of cancer drugs on patient quality of life alongside their effects on overall survival and intermediate end points such as progression-free survival. “Patients with advanced cancer expect treatment to help them live longer or have better lives,” the authors wrote. In that respect, in clinical trials of cancer medicines, overall survival and quality of life are the most important measures. Toxicity profiles and disease progression delays do not reliably predict quality of life, and studies have shown poor correlations between quality of life, overall survival, and progression-free survival. This raises the question of validity of progression-free survival as a surrogate endpoint. “Progression-free survival is meaningless without overall survival or quality of life gains,” Dr. Gyawali said in an interview.
Writing in The Lancet Oncology in March, Dr. Gyawali stated that, because progression free survival “does not directly measure how a patient feels or functions, or how long a patient lives, progression-free survival was not intended to inform clinical practice or establish whether a new therapy provides clinically meaningful benefits for patients. However, over the past 2 decades, it has become the most common primary endpoint in oncology clinical trials. We are deeply worried about how the term survival in this phrase can influence clinical practice and patient choices. We propose replacing the phrase progression-free survival with a less ambiguous term: progression-free interval.”
In JAMA Oncology, Dr. Gyawali aimed to elucidate relationships between QOL, overall survival, and progression-free survival, and to assess, as well, how QOL results are framed, especially in industry-sponsored research. When drug trials they analyzed showed no change in QOL but reported that QOL did not worsen or QOL was maintained rather than stating that QOL did not improve, or if there was downplaying of worse QOL outcomes, the study had favorable interpretation, Dr. Gyawali and associates wrote. The expectation of patients receiving cancer drugs would be improved QOL rather than “not worse” QOL, Dr. Gyawali said.
Regarding the finding that QOL outcomes were described as favorable in 47% of trials with unimproved QOL outcomes, Dr. Gyawali said, “the bias in reporting should be corrected by the reviewers and editors of journals. Also, quality of life reporting should be made mandatory. Without unbiased quality of life information, informed decision making on whether or not to use a certain drug is impossible. Patients and physicians need to know that information. Regulators can demand that this should be mandatory in all trials in noncurative settings.”
He remarked further on the worsening QOL in some targeted drug trials, “People tout chemo-free regimens as automatically having better quality of life, but that doesn’t seem to be the case. Targeted drugs can have a severe impact on quality of life, probably due to prolonged duration of side effects. Quality of life should be measured and reported for all drugs.”
Dr. Gyawali and associates noted the limitation in that several studies with negative QOL results are not published at all or are published after a considerable delay, so the present observations may understate the issues that have been raised.
Dr. Gyawali declared that he received no funding and disclosed no conflicts of interest for this study.
FROM JAMA ONCOLOGY
Commentary: New Horizons in NSCLC Treatment: Adagrasib, Aumolertinib, and Lorlatinib, July 2022
The Direct KRASG12C Inhibitor Adagrasib in Advanced KRASG12C-Mutant NSCLC: Results From a Registrational Phase 2 Study
KRAS mutations are detected in about one quarter of all lung adenocarcinomas and are the most common oncogene driver in non–small-cell lung cancer (NSCLC). KRASG12C amino acid substitutions are the most common KRAS mutations in NSCLC, comprising just about half of all KRAS mutations in this tumor type. Despite being the most common and first detected oncogene driver in lung cancer, until recently there were no targeted therapies in KRAS mutant NSCLC. The development of direct KRASG12C inhibitors represents an important step forward in targeting KRAS mutations. These inhibitors bind inactive guanosine diphosphate (GDP)–bound RAS and trap it in its inactive state.
Dr Jänne and colleagues recently published a phase 2 registrational trial of the direct KRASG12C inhibitor adagrasib. In this study of 112 patients with measurable disease at baseline treated with adagrasib, 48 (42.9%) had a confirmed objective response. The median duration of response was 8.5 months (95% CI 6.2-13.8), and the median progression-free survival (PFS) was 6.5 months (95% CI 4.7-8.4). The median overall survival (OS) was 12.6 months (95% CI 9.2-19.2). Among 33 patients with previously treated, stable central nervous system (CNS) metastases, the intracranial confirmed objective response rate was 33.3% (95% CI 18.0-51.8). Treatment-related adverse events occurred in 97.4% of the patients: grade 1 or 2 in 52.6% and grade 3 or higher in 44.8% (including two grade 5 events). The most frequent toxicities were fatigue and gastrointestinal-related issues (nausea, vomiting, diarrhea, aspartate transaminase/alanine transaminase elevation). Adagrasib was discontinued in 6.9% of patients.
These results further demonstrate that the KRASG12C mutation is an actionable target in NSCLC. Sotorasib, another direct KRASG12C inhibitor, is currently US Food and Drug Administration approved after initial systemic treatment. The clinical activity of sotorasib and adagrasib are comparable; for sotorasib the rates are an overall response rate (ORR) of 37.1% (95% CI 28.6-46.2), median PFS of 6.8 months (95% CI 5.1-8.2), and median OS of 12.5 months (95% CI 10.0 to nonestimable). Adagrasib also has published evidence of CNS activity that tracks with its systemic activity. Overall, these direct KRASG12C inhibitors represent a major advance in the treatment of KRASG12C-mutant NSCLC.
EGFR-Mutated NSCLC: Aumolertinib vs Gefitinib Extends PFS
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI) have improved clinical outcomes in EGFR-mutant NSCLC.The current standard of care for first-line treatment of advanced NSCLC with the most frequent EGFR activating mutations (EGFR E19del and L858R)is the third-generation EGFR TKI osimertinib. In the FLAURA trial, patients randomly assigned tofirst-lineosimertinib had a substantial PFS benefit (median PFS 18.9 vs 10.2 months) and OS benefit (median OS 38.6 vs 31.8 months)when receivingosimertinib compared with gefitinib or erlotinib.
In the AENEAS trial, published in the Journal of Clinical Oncologyby Dr Lu and colleagues, 420 patients from China with advanced NSCLC harboring EGFR E19del or L858R activating mutations and naive to systemic treatment were enrolled. Patients were randomly assigned to the next-generation EGFR TKI aumolertinib or the first-generation EGFR TKI gefitinib with the primary endpoint of PFS by investigator assessment.Of note, patients with asymptomatic, untreated brain metastases were allowed into the trial. Upon disease progression, patients in the gefitinib group who acquired an EGFR T790M mutation were eligible to crossover to aumolertinib.
The study met its primary endpoint: Patients treated with aumolertinib compared with those treated with gefitinib had a significantly longer median PFS (19.3 vs 9.9 months; hazard ratio [HR] 0.46; P< .0001). This PFS advantage of aumolertinib over gefitinib was also present in the subgroup of patients with CNS metastases (15.3 vs 8.2 months; HR 0.38; P< .0001). The objective response rate was similar inthe aumolertinib and gefitinib groups (objective response rate 73.8% and 72.1%, respectively). The median duration of response was 18.1 months (95% CI 15.2 to not reached) with aumolertinib vs 8.3 months (95% CI 6.9-11.1) with gefitinib. Treatment-emergent adverse events of grade 3 or more were similar in the aumolertinib and gefitinib groups (36.4% vs 35.8%, respectively). There was less rash and diarrhea as well as transaminitis in the aumolertinib arm compared with the gefitinib arm. However, 35.5% of patients developed an elevation in creatinine phosphokinase (CPK), including 7% with grade 3 CPK elevation. However, no rhabdomyolysis was observed.
Overall, the AENEAS study showed comparable median PFS for first-line aumolertinib comparedwith what was observed with osimertinib in the FLAURA study. We still await the OSdata on aumolertinib compared with gefitinib. In the FLAURA study, investigators could choose between erlotinib or gefitinib in the control arm, whereas in AENEAS only gefitinib was allowed, which may have less CNS activity than erlotinib. Moreover, the FLAURA trial was conducted worldwide, whereas the AENEAS trial only enrolled patients in China. This study provides further support for the use of third-generation EGFR TKI over first-generation EGFR TKI as first-line treatment in advanced/metastatic NSCLC harboring EGFR E19del or L858R mutations.
Advanced ALK+ NSCLC With Brain Metastases: Lorlatinib Boosts PFS, Reduces CNS Progression
The CROWN trial was a pivotal randomized phase 3 trial that demonstrated an impressive improvement in PFS in patients treated with the third-generationALK inhibitor lorlatinib compared with the first-generation ALK inhibitor crizotinib as initial treatment for advanced ALK-postive (ALK+) NSCLC (HR for disease progression or death0.28; 95% CI0.19-0.41; P≤ .001). A major driver of this PFS benefit in ALK+ NSCLC in the CROWN study is the superior CNS penetration of lorlatinib compared with crizotinib. Obtaining CNS control in ALK+ lung cancers is important because up to 40% of patients with ALK+ NSCLC have brain metastases at initial evaluation, and CNS progression is often observed in patients with ALK+ lung cancer whether it be intracranial metastases or leptomeningeal carcinomatosis. A potential challenge in treating patients with lorlatinib is a unique side effect profile including neurocognitive side effects from lorlatinib. In a recently published study in the Journal of Clinical Oncology, Dr Solomon and colleagues conducted a post hoc exploratory analysis of intracranial efficacy and safety of lorlatinib in ALK+ NSCLC from a phase 3 trial. PFS by blinded independent central review was improved with lorlatinib vs crizotinib in patients with and without brain metastases at baseline (12-month PFS rates: 78% vs 22% and 78% vs 45%, respectively). Lorlatinib was associated with lower 12-month cumulative incidence of CNS progression compared with crizotinib in patients with (7.4% vs 72%) and without (1% vs 18%) brain metastases at baseline. Complete CNS responses with lorlatinib were seen in 23/38(61%) patients with any brain metastases at baseline compared with 6/40 (15%) with crizotinib. In total, 35% of patients had CNS adverse events with lorlatinib: grade 1 (21%), grade 2 (10%), and grade 3 (3%)in severity. These included cognitive (21%), mood (16%), speech (5%), and psychotic effects (3%), some of which overlapped. Half of all CNS adverse events resolved without intervention or with lorlatinib dose modification. Dose reductions of lorlatinib did not appear to affect PFS on the basis of a landmark analysis. Overall, this study demonstrates the exceptional CNS activity of lorlatinib in ALK+ NSCLC and that the neurocognitive side effects can often be managed. There are several next-generation ALK inhibitors now approved in the first-line setting —alectinib, lorlatinib, and brigatinib — notably all with enhanced CNS penetration and improved PFS compared with crizotinib. This posthoc study further supports the impressive CNS activity of lorlatinib in ALK+ NSCLC and supports the use of lorlatinib as a first-line treatment option in these patients, particularly those with ALK+ NSCLC diagnosed with baseline CNS disease.
The Direct KRASG12C Inhibitor Adagrasib in Advanced KRASG12C-Mutant NSCLC: Results From a Registrational Phase 2 Study
KRAS mutations are detected in about one quarter of all lung adenocarcinomas and are the most common oncogene driver in non–small-cell lung cancer (NSCLC). KRASG12C amino acid substitutions are the most common KRAS mutations in NSCLC, comprising just about half of all KRAS mutations in this tumor type. Despite being the most common and first detected oncogene driver in lung cancer, until recently there were no targeted therapies in KRAS mutant NSCLC. The development of direct KRASG12C inhibitors represents an important step forward in targeting KRAS mutations. These inhibitors bind inactive guanosine diphosphate (GDP)–bound RAS and trap it in its inactive state.
Dr Jänne and colleagues recently published a phase 2 registrational trial of the direct KRASG12C inhibitor adagrasib. In this study of 112 patients with measurable disease at baseline treated with adagrasib, 48 (42.9%) had a confirmed objective response. The median duration of response was 8.5 months (95% CI 6.2-13.8), and the median progression-free survival (PFS) was 6.5 months (95% CI 4.7-8.4). The median overall survival (OS) was 12.6 months (95% CI 9.2-19.2). Among 33 patients with previously treated, stable central nervous system (CNS) metastases, the intracranial confirmed objective response rate was 33.3% (95% CI 18.0-51.8). Treatment-related adverse events occurred in 97.4% of the patients: grade 1 or 2 in 52.6% and grade 3 or higher in 44.8% (including two grade 5 events). The most frequent toxicities were fatigue and gastrointestinal-related issues (nausea, vomiting, diarrhea, aspartate transaminase/alanine transaminase elevation). Adagrasib was discontinued in 6.9% of patients.
These results further demonstrate that the KRASG12C mutation is an actionable target in NSCLC. Sotorasib, another direct KRASG12C inhibitor, is currently US Food and Drug Administration approved after initial systemic treatment. The clinical activity of sotorasib and adagrasib are comparable; for sotorasib the rates are an overall response rate (ORR) of 37.1% (95% CI 28.6-46.2), median PFS of 6.8 months (95% CI 5.1-8.2), and median OS of 12.5 months (95% CI 10.0 to nonestimable). Adagrasib also has published evidence of CNS activity that tracks with its systemic activity. Overall, these direct KRASG12C inhibitors represent a major advance in the treatment of KRASG12C-mutant NSCLC.
EGFR-Mutated NSCLC: Aumolertinib vs Gefitinib Extends PFS
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI) have improved clinical outcomes in EGFR-mutant NSCLC.The current standard of care for first-line treatment of advanced NSCLC with the most frequent EGFR activating mutations (EGFR E19del and L858R)is the third-generation EGFR TKI osimertinib. In the FLAURA trial, patients randomly assigned tofirst-lineosimertinib had a substantial PFS benefit (median PFS 18.9 vs 10.2 months) and OS benefit (median OS 38.6 vs 31.8 months)when receivingosimertinib compared with gefitinib or erlotinib.
In the AENEAS trial, published in the Journal of Clinical Oncologyby Dr Lu and colleagues, 420 patients from China with advanced NSCLC harboring EGFR E19del or L858R activating mutations and naive to systemic treatment were enrolled. Patients were randomly assigned to the next-generation EGFR TKI aumolertinib or the first-generation EGFR TKI gefitinib with the primary endpoint of PFS by investigator assessment.Of note, patients with asymptomatic, untreated brain metastases were allowed into the trial. Upon disease progression, patients in the gefitinib group who acquired an EGFR T790M mutation were eligible to crossover to aumolertinib.
The study met its primary endpoint: Patients treated with aumolertinib compared with those treated with gefitinib had a significantly longer median PFS (19.3 vs 9.9 months; hazard ratio [HR] 0.46; P< .0001). This PFS advantage of aumolertinib over gefitinib was also present in the subgroup of patients with CNS metastases (15.3 vs 8.2 months; HR 0.38; P< .0001). The objective response rate was similar inthe aumolertinib and gefitinib groups (objective response rate 73.8% and 72.1%, respectively). The median duration of response was 18.1 months (95% CI 15.2 to not reached) with aumolertinib vs 8.3 months (95% CI 6.9-11.1) with gefitinib. Treatment-emergent adverse events of grade 3 or more were similar in the aumolertinib and gefitinib groups (36.4% vs 35.8%, respectively). There was less rash and diarrhea as well as transaminitis in the aumolertinib arm compared with the gefitinib arm. However, 35.5% of patients developed an elevation in creatinine phosphokinase (CPK), including 7% with grade 3 CPK elevation. However, no rhabdomyolysis was observed.
Overall, the AENEAS study showed comparable median PFS for first-line aumolertinib comparedwith what was observed with osimertinib in the FLAURA study. We still await the OSdata on aumolertinib compared with gefitinib. In the FLAURA study, investigators could choose between erlotinib or gefitinib in the control arm, whereas in AENEAS only gefitinib was allowed, which may have less CNS activity than erlotinib. Moreover, the FLAURA trial was conducted worldwide, whereas the AENEAS trial only enrolled patients in China. This study provides further support for the use of third-generation EGFR TKI over first-generation EGFR TKI as first-line treatment in advanced/metastatic NSCLC harboring EGFR E19del or L858R mutations.
Advanced ALK+ NSCLC With Brain Metastases: Lorlatinib Boosts PFS, Reduces CNS Progression
The CROWN trial was a pivotal randomized phase 3 trial that demonstrated an impressive improvement in PFS in patients treated with the third-generationALK inhibitor lorlatinib compared with the first-generation ALK inhibitor crizotinib as initial treatment for advanced ALK-postive (ALK+) NSCLC (HR for disease progression or death0.28; 95% CI0.19-0.41; P≤ .001). A major driver of this PFS benefit in ALK+ NSCLC in the CROWN study is the superior CNS penetration of lorlatinib compared with crizotinib. Obtaining CNS control in ALK+ lung cancers is important because up to 40% of patients with ALK+ NSCLC have brain metastases at initial evaluation, and CNS progression is often observed in patients with ALK+ lung cancer whether it be intracranial metastases or leptomeningeal carcinomatosis. A potential challenge in treating patients with lorlatinib is a unique side effect profile including neurocognitive side effects from lorlatinib. In a recently published study in the Journal of Clinical Oncology, Dr Solomon and colleagues conducted a post hoc exploratory analysis of intracranial efficacy and safety of lorlatinib in ALK+ NSCLC from a phase 3 trial. PFS by blinded independent central review was improved with lorlatinib vs crizotinib in patients with and without brain metastases at baseline (12-month PFS rates: 78% vs 22% and 78% vs 45%, respectively). Lorlatinib was associated with lower 12-month cumulative incidence of CNS progression compared with crizotinib in patients with (7.4% vs 72%) and without (1% vs 18%) brain metastases at baseline. Complete CNS responses with lorlatinib were seen in 23/38(61%) patients with any brain metastases at baseline compared with 6/40 (15%) with crizotinib. In total, 35% of patients had CNS adverse events with lorlatinib: grade 1 (21%), grade 2 (10%), and grade 3 (3%)in severity. These included cognitive (21%), mood (16%), speech (5%), and psychotic effects (3%), some of which overlapped. Half of all CNS adverse events resolved without intervention or with lorlatinib dose modification. Dose reductions of lorlatinib did not appear to affect PFS on the basis of a landmark analysis. Overall, this study demonstrates the exceptional CNS activity of lorlatinib in ALK+ NSCLC and that the neurocognitive side effects can often be managed. There are several next-generation ALK inhibitors now approved in the first-line setting —alectinib, lorlatinib, and brigatinib — notably all with enhanced CNS penetration and improved PFS compared with crizotinib. This posthoc study further supports the impressive CNS activity of lorlatinib in ALK+ NSCLC and supports the use of lorlatinib as a first-line treatment option in these patients, particularly those with ALK+ NSCLC diagnosed with baseline CNS disease.
The Direct KRASG12C Inhibitor Adagrasib in Advanced KRASG12C-Mutant NSCLC: Results From a Registrational Phase 2 Study
KRAS mutations are detected in about one quarter of all lung adenocarcinomas and are the most common oncogene driver in non–small-cell lung cancer (NSCLC). KRASG12C amino acid substitutions are the most common KRAS mutations in NSCLC, comprising just about half of all KRAS mutations in this tumor type. Despite being the most common and first detected oncogene driver in lung cancer, until recently there were no targeted therapies in KRAS mutant NSCLC. The development of direct KRASG12C inhibitors represents an important step forward in targeting KRAS mutations. These inhibitors bind inactive guanosine diphosphate (GDP)–bound RAS and trap it in its inactive state.
Dr Jänne and colleagues recently published a phase 2 registrational trial of the direct KRASG12C inhibitor adagrasib. In this study of 112 patients with measurable disease at baseline treated with adagrasib, 48 (42.9%) had a confirmed objective response. The median duration of response was 8.5 months (95% CI 6.2-13.8), and the median progression-free survival (PFS) was 6.5 months (95% CI 4.7-8.4). The median overall survival (OS) was 12.6 months (95% CI 9.2-19.2). Among 33 patients with previously treated, stable central nervous system (CNS) metastases, the intracranial confirmed objective response rate was 33.3% (95% CI 18.0-51.8). Treatment-related adverse events occurred in 97.4% of the patients: grade 1 or 2 in 52.6% and grade 3 or higher in 44.8% (including two grade 5 events). The most frequent toxicities were fatigue and gastrointestinal-related issues (nausea, vomiting, diarrhea, aspartate transaminase/alanine transaminase elevation). Adagrasib was discontinued in 6.9% of patients.
These results further demonstrate that the KRASG12C mutation is an actionable target in NSCLC. Sotorasib, another direct KRASG12C inhibitor, is currently US Food and Drug Administration approved after initial systemic treatment. The clinical activity of sotorasib and adagrasib are comparable; for sotorasib the rates are an overall response rate (ORR) of 37.1% (95% CI 28.6-46.2), median PFS of 6.8 months (95% CI 5.1-8.2), and median OS of 12.5 months (95% CI 10.0 to nonestimable). Adagrasib also has published evidence of CNS activity that tracks with its systemic activity. Overall, these direct KRASG12C inhibitors represent a major advance in the treatment of KRASG12C-mutant NSCLC.
EGFR-Mutated NSCLC: Aumolertinib vs Gefitinib Extends PFS
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI) have improved clinical outcomes in EGFR-mutant NSCLC.The current standard of care for first-line treatment of advanced NSCLC with the most frequent EGFR activating mutations (EGFR E19del and L858R)is the third-generation EGFR TKI osimertinib. In the FLAURA trial, patients randomly assigned tofirst-lineosimertinib had a substantial PFS benefit (median PFS 18.9 vs 10.2 months) and OS benefit (median OS 38.6 vs 31.8 months)when receivingosimertinib compared with gefitinib or erlotinib.
In the AENEAS trial, published in the Journal of Clinical Oncologyby Dr Lu and colleagues, 420 patients from China with advanced NSCLC harboring EGFR E19del or L858R activating mutations and naive to systemic treatment were enrolled. Patients were randomly assigned to the next-generation EGFR TKI aumolertinib or the first-generation EGFR TKI gefitinib with the primary endpoint of PFS by investigator assessment.Of note, patients with asymptomatic, untreated brain metastases were allowed into the trial. Upon disease progression, patients in the gefitinib group who acquired an EGFR T790M mutation were eligible to crossover to aumolertinib.
The study met its primary endpoint: Patients treated with aumolertinib compared with those treated with gefitinib had a significantly longer median PFS (19.3 vs 9.9 months; hazard ratio [HR] 0.46; P< .0001). This PFS advantage of aumolertinib over gefitinib was also present in the subgroup of patients with CNS metastases (15.3 vs 8.2 months; HR 0.38; P< .0001). The objective response rate was similar inthe aumolertinib and gefitinib groups (objective response rate 73.8% and 72.1%, respectively). The median duration of response was 18.1 months (95% CI 15.2 to not reached) with aumolertinib vs 8.3 months (95% CI 6.9-11.1) with gefitinib. Treatment-emergent adverse events of grade 3 or more were similar in the aumolertinib and gefitinib groups (36.4% vs 35.8%, respectively). There was less rash and diarrhea as well as transaminitis in the aumolertinib arm compared with the gefitinib arm. However, 35.5% of patients developed an elevation in creatinine phosphokinase (CPK), including 7% with grade 3 CPK elevation. However, no rhabdomyolysis was observed.
Overall, the AENEAS study showed comparable median PFS for first-line aumolertinib comparedwith what was observed with osimertinib in the FLAURA study. We still await the OSdata on aumolertinib compared with gefitinib. In the FLAURA study, investigators could choose between erlotinib or gefitinib in the control arm, whereas in AENEAS only gefitinib was allowed, which may have less CNS activity than erlotinib. Moreover, the FLAURA trial was conducted worldwide, whereas the AENEAS trial only enrolled patients in China. This study provides further support for the use of third-generation EGFR TKI over first-generation EGFR TKI as first-line treatment in advanced/metastatic NSCLC harboring EGFR E19del or L858R mutations.
Advanced ALK+ NSCLC With Brain Metastases: Lorlatinib Boosts PFS, Reduces CNS Progression
The CROWN trial was a pivotal randomized phase 3 trial that demonstrated an impressive improvement in PFS in patients treated with the third-generationALK inhibitor lorlatinib compared with the first-generation ALK inhibitor crizotinib as initial treatment for advanced ALK-postive (ALK+) NSCLC (HR for disease progression or death0.28; 95% CI0.19-0.41; P≤ .001). A major driver of this PFS benefit in ALK+ NSCLC in the CROWN study is the superior CNS penetration of lorlatinib compared with crizotinib. Obtaining CNS control in ALK+ lung cancers is important because up to 40% of patients with ALK+ NSCLC have brain metastases at initial evaluation, and CNS progression is often observed in patients with ALK+ lung cancer whether it be intracranial metastases or leptomeningeal carcinomatosis. A potential challenge in treating patients with lorlatinib is a unique side effect profile including neurocognitive side effects from lorlatinib. In a recently published study in the Journal of Clinical Oncology, Dr Solomon and colleagues conducted a post hoc exploratory analysis of intracranial efficacy and safety of lorlatinib in ALK+ NSCLC from a phase 3 trial. PFS by blinded independent central review was improved with lorlatinib vs crizotinib in patients with and without brain metastases at baseline (12-month PFS rates: 78% vs 22% and 78% vs 45%, respectively). Lorlatinib was associated with lower 12-month cumulative incidence of CNS progression compared with crizotinib in patients with (7.4% vs 72%) and without (1% vs 18%) brain metastases at baseline. Complete CNS responses with lorlatinib were seen in 23/38(61%) patients with any brain metastases at baseline compared with 6/40 (15%) with crizotinib. In total, 35% of patients had CNS adverse events with lorlatinib: grade 1 (21%), grade 2 (10%), and grade 3 (3%)in severity. These included cognitive (21%), mood (16%), speech (5%), and psychotic effects (3%), some of which overlapped. Half of all CNS adverse events resolved without intervention or with lorlatinib dose modification. Dose reductions of lorlatinib did not appear to affect PFS on the basis of a landmark analysis. Overall, this study demonstrates the exceptional CNS activity of lorlatinib in ALK+ NSCLC and that the neurocognitive side effects can often be managed. There are several next-generation ALK inhibitors now approved in the first-line setting —alectinib, lorlatinib, and brigatinib — notably all with enhanced CNS penetration and improved PFS compared with crizotinib. This posthoc study further supports the impressive CNS activity of lorlatinib in ALK+ NSCLC and supports the use of lorlatinib as a first-line treatment option in these patients, particularly those with ALK+ NSCLC diagnosed with baseline CNS disease.