User login
Plasma genotyping yields actionable mutation in advanced NSCLC
Taking a deep dive into plasma cell-free DNA in patients with advanced non–small cell lung cancer may reveal targetable mutations and cancer resistance mechanisms in tumors, even when tissue biopsy samples are not adequate for genotyping, investigators say,
Noninvasive tumor genotyping of plasma cell-free DNA (cfDNA) with ultra-deep next generation sequencing (NGS) in plasma samples from 127 patients identified known oncogenic drivers with a sensitivity of 75% and ruled out the presence of driver mutations with a specificity of 100% in patients with tissue samples indicating no mutations, reported Bob T. Li, MD, MPH, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York, and his colleagues.
“These results reveal the potential utility of NGS assays that use cfDNA as input for detecting actionable driver alterations and both de novo and emergent resistance mechanisms in the clinical setting,” they wrote. The report is in Annals of Oncology.
Although the researchers did not directly assess clinical utility, the results suggest that NGS-based analysis of cfDNA may help guide treatment selection, they added.
Ultra-deep NGS is a kind of obsessive-compulsive form of sequencing in which the same genomic region is read repeatedly – in this study, 50,000 times over – with filtering of somatic mutations attributable to clonal hematopoiesis. The technique allows for detection of rare genetic alterations that can be missed by other methods.
“More recent studies employing plasma cfDNA NGS have shown promise in detecting a broader variety of genetic alterations with similar sensitivity to that of digital PCR, with potential to change clinical practice,” Dr. Li and his colleagues wrote.
They conducted a systematic study of a novel cfDNA assay in patients whose cancers had oncogenic driver mutations, those who were driver negative on tissue-based NGS, and those whose tumors had unknown mutational status.
A total of 127 patients from three centers (MSKCC, the Dana-Farber Cancer Center in Boston, and the University of Texas MD Anderson Cancer Center in Houston) were available for assessment.
Ultra-deep NGS was performed on cfDNA and matched white blood cells using a hybrid capture panel covering 37 lung cancer-related genes sequenced to 50,000 times raw-target coverage filtering somatic mutations attributable to clonal hematopoiesis.
Plasma NGS was able to detect driver mutations with variant allele frequencies ranging from as low as 0.14% to as high as 52%.
In 21 of 22 patients, plasma digital drop polymerase chain reaction (ddPCR) results for EGFR or KRAS mutations were nearly identical to those of NGS, with high concordance for variant allele frequencies (r = .98).
In analyses blinded to tissue genotyping results in 91 patients, plasma NGS detected de novo known oncogenic driver alterations in 68 samples, for a sensitivity of 75%, and in 19 of 19 patients who were driver negative by tissue sequencing, plasma NGS also showed an absence of mutations, for a specificity of 100%.
Furthermore, plasma NGS identified four KRAS mutations in plasma from 17 patients for whom tissues samples were not adequate for genotyping, and the plasma-based technique was able to identify potential resistance mutations in samples from 23 patients with EGFR mutations whose tumors had required resistance to targeted therapy.
“The sensitivity of detection by NGS was comparable to that of established ddPCR methods. Its high concordance with tissue genotyping and the detection of drivers in settings where tissue biopsy had failed or was not feasible lend credence to the potential clinical use of plasma cfDNA NGS and the development of cfDNA-guided intervention studies,” the investigators wrote.
The study was supported by Illumina. Authors from MSKCC and MD Anderson were supported by National Institutes of Health grants. Dr. Li received consulting/advisory board fees from Genentech, Thermo-Fisher Scientific, and Guardant Health outside of the submitted work. Multiple coauthors reported similar relationships, and eight coauthors were current or former employees of Illumina.
SOURCE: Source: Li BT et al. Ann Oncol. doi: 10.1093/annonc/mdz046.
Taking a deep dive into plasma cell-free DNA in patients with advanced non–small cell lung cancer may reveal targetable mutations and cancer resistance mechanisms in tumors, even when tissue biopsy samples are not adequate for genotyping, investigators say,
Noninvasive tumor genotyping of plasma cell-free DNA (cfDNA) with ultra-deep next generation sequencing (NGS) in plasma samples from 127 patients identified known oncogenic drivers with a sensitivity of 75% and ruled out the presence of driver mutations with a specificity of 100% in patients with tissue samples indicating no mutations, reported Bob T. Li, MD, MPH, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York, and his colleagues.
“These results reveal the potential utility of NGS assays that use cfDNA as input for detecting actionable driver alterations and both de novo and emergent resistance mechanisms in the clinical setting,” they wrote. The report is in Annals of Oncology.
Although the researchers did not directly assess clinical utility, the results suggest that NGS-based analysis of cfDNA may help guide treatment selection, they added.
Ultra-deep NGS is a kind of obsessive-compulsive form of sequencing in which the same genomic region is read repeatedly – in this study, 50,000 times over – with filtering of somatic mutations attributable to clonal hematopoiesis. The technique allows for detection of rare genetic alterations that can be missed by other methods.
“More recent studies employing plasma cfDNA NGS have shown promise in detecting a broader variety of genetic alterations with similar sensitivity to that of digital PCR, with potential to change clinical practice,” Dr. Li and his colleagues wrote.
They conducted a systematic study of a novel cfDNA assay in patients whose cancers had oncogenic driver mutations, those who were driver negative on tissue-based NGS, and those whose tumors had unknown mutational status.
A total of 127 patients from three centers (MSKCC, the Dana-Farber Cancer Center in Boston, and the University of Texas MD Anderson Cancer Center in Houston) were available for assessment.
Ultra-deep NGS was performed on cfDNA and matched white blood cells using a hybrid capture panel covering 37 lung cancer-related genes sequenced to 50,000 times raw-target coverage filtering somatic mutations attributable to clonal hematopoiesis.
Plasma NGS was able to detect driver mutations with variant allele frequencies ranging from as low as 0.14% to as high as 52%.
In 21 of 22 patients, plasma digital drop polymerase chain reaction (ddPCR) results for EGFR or KRAS mutations were nearly identical to those of NGS, with high concordance for variant allele frequencies (r = .98).
In analyses blinded to tissue genotyping results in 91 patients, plasma NGS detected de novo known oncogenic driver alterations in 68 samples, for a sensitivity of 75%, and in 19 of 19 patients who were driver negative by tissue sequencing, plasma NGS also showed an absence of mutations, for a specificity of 100%.
Furthermore, plasma NGS identified four KRAS mutations in plasma from 17 patients for whom tissues samples were not adequate for genotyping, and the plasma-based technique was able to identify potential resistance mutations in samples from 23 patients with EGFR mutations whose tumors had required resistance to targeted therapy.
“The sensitivity of detection by NGS was comparable to that of established ddPCR methods. Its high concordance with tissue genotyping and the detection of drivers in settings where tissue biopsy had failed or was not feasible lend credence to the potential clinical use of plasma cfDNA NGS and the development of cfDNA-guided intervention studies,” the investigators wrote.
The study was supported by Illumina. Authors from MSKCC and MD Anderson were supported by National Institutes of Health grants. Dr. Li received consulting/advisory board fees from Genentech, Thermo-Fisher Scientific, and Guardant Health outside of the submitted work. Multiple coauthors reported similar relationships, and eight coauthors were current or former employees of Illumina.
SOURCE: Source: Li BT et al. Ann Oncol. doi: 10.1093/annonc/mdz046.
Taking a deep dive into plasma cell-free DNA in patients with advanced non–small cell lung cancer may reveal targetable mutations and cancer resistance mechanisms in tumors, even when tissue biopsy samples are not adequate for genotyping, investigators say,
Noninvasive tumor genotyping of plasma cell-free DNA (cfDNA) with ultra-deep next generation sequencing (NGS) in plasma samples from 127 patients identified known oncogenic drivers with a sensitivity of 75% and ruled out the presence of driver mutations with a specificity of 100% in patients with tissue samples indicating no mutations, reported Bob T. Li, MD, MPH, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York, and his colleagues.
“These results reveal the potential utility of NGS assays that use cfDNA as input for detecting actionable driver alterations and both de novo and emergent resistance mechanisms in the clinical setting,” they wrote. The report is in Annals of Oncology.
Although the researchers did not directly assess clinical utility, the results suggest that NGS-based analysis of cfDNA may help guide treatment selection, they added.
Ultra-deep NGS is a kind of obsessive-compulsive form of sequencing in which the same genomic region is read repeatedly – in this study, 50,000 times over – with filtering of somatic mutations attributable to clonal hematopoiesis. The technique allows for detection of rare genetic alterations that can be missed by other methods.
“More recent studies employing plasma cfDNA NGS have shown promise in detecting a broader variety of genetic alterations with similar sensitivity to that of digital PCR, with potential to change clinical practice,” Dr. Li and his colleagues wrote.
They conducted a systematic study of a novel cfDNA assay in patients whose cancers had oncogenic driver mutations, those who were driver negative on tissue-based NGS, and those whose tumors had unknown mutational status.
A total of 127 patients from three centers (MSKCC, the Dana-Farber Cancer Center in Boston, and the University of Texas MD Anderson Cancer Center in Houston) were available for assessment.
Ultra-deep NGS was performed on cfDNA and matched white blood cells using a hybrid capture panel covering 37 lung cancer-related genes sequenced to 50,000 times raw-target coverage filtering somatic mutations attributable to clonal hematopoiesis.
Plasma NGS was able to detect driver mutations with variant allele frequencies ranging from as low as 0.14% to as high as 52%.
In 21 of 22 patients, plasma digital drop polymerase chain reaction (ddPCR) results for EGFR or KRAS mutations were nearly identical to those of NGS, with high concordance for variant allele frequencies (r = .98).
In analyses blinded to tissue genotyping results in 91 patients, plasma NGS detected de novo known oncogenic driver alterations in 68 samples, for a sensitivity of 75%, and in 19 of 19 patients who were driver negative by tissue sequencing, plasma NGS also showed an absence of mutations, for a specificity of 100%.
Furthermore, plasma NGS identified four KRAS mutations in plasma from 17 patients for whom tissues samples were not adequate for genotyping, and the plasma-based technique was able to identify potential resistance mutations in samples from 23 patients with EGFR mutations whose tumors had required resistance to targeted therapy.
“The sensitivity of detection by NGS was comparable to that of established ddPCR methods. Its high concordance with tissue genotyping and the detection of drivers in settings where tissue biopsy had failed or was not feasible lend credence to the potential clinical use of plasma cfDNA NGS and the development of cfDNA-guided intervention studies,” the investigators wrote.
The study was supported by Illumina. Authors from MSKCC and MD Anderson were supported by National Institutes of Health grants. Dr. Li received consulting/advisory board fees from Genentech, Thermo-Fisher Scientific, and Guardant Health outside of the submitted work. Multiple coauthors reported similar relationships, and eight coauthors were current or former employees of Illumina.
SOURCE: Source: Li BT et al. Ann Oncol. doi: 10.1093/annonc/mdz046.
FROM ANNALS OF ONCOLOGY
FDA approves atezolizumab for first-line ES-SCLC treatment
The Food and Drug Administration has approved atezolizumab (Tecentriq), in combination with carboplatin and etoposide, for the first-line treatment of adults with extensive-stage small cell lung cancer (ES-SCLC).
Approval was based on results from the phase 3 IMpower133 study, in which 403 treatment-naive patients with ES-SCLC received atezolizumab at 1,200 mg with carboplatin at 5 mg/mL per minute on day 1 and etoposide 100 mg/m2 on days 1, 2, and 3 of a 21-day cycle for four cycles, followed by atezolizumab at 1,200 mg once every 3 weeks until disease progression or unacceptable toxicity; or received placebo with the same dosage of carboplatin and etoposide for a similar duration.
Overall survival was significantly better in patients who received atezolizumab, compared with placebo (12.3 vs. 10.3 months; hazard ratio, 0.70; 95% confidence interval, 0.54-0.91; P = .0069), as was progression-free survival (5.2 vs. 4.3 months; HR, 0.77; 95% CI, 0.62-0.96; P = .017).
The most common adverse events associated with atezolizumab in the study were fatigue/asthenia, nausea, alopecia, constipation, and decreased appetite.
According to the FDA, the recommended dose is 1,200 mg IV over 60 minutes every 3 weeks. When administered on the same day as chemotherapy, atezolizumab should be given first. If the first infusion is tolerated, all subsequent infusions can be delivered over 30 minutes.
Find the full press release on the FDA website.
The Food and Drug Administration has approved atezolizumab (Tecentriq), in combination with carboplatin and etoposide, for the first-line treatment of adults with extensive-stage small cell lung cancer (ES-SCLC).
Approval was based on results from the phase 3 IMpower133 study, in which 403 treatment-naive patients with ES-SCLC received atezolizumab at 1,200 mg with carboplatin at 5 mg/mL per minute on day 1 and etoposide 100 mg/m2 on days 1, 2, and 3 of a 21-day cycle for four cycles, followed by atezolizumab at 1,200 mg once every 3 weeks until disease progression or unacceptable toxicity; or received placebo with the same dosage of carboplatin and etoposide for a similar duration.
Overall survival was significantly better in patients who received atezolizumab, compared with placebo (12.3 vs. 10.3 months; hazard ratio, 0.70; 95% confidence interval, 0.54-0.91; P = .0069), as was progression-free survival (5.2 vs. 4.3 months; HR, 0.77; 95% CI, 0.62-0.96; P = .017).
The most common adverse events associated with atezolizumab in the study were fatigue/asthenia, nausea, alopecia, constipation, and decreased appetite.
According to the FDA, the recommended dose is 1,200 mg IV over 60 minutes every 3 weeks. When administered on the same day as chemotherapy, atezolizumab should be given first. If the first infusion is tolerated, all subsequent infusions can be delivered over 30 minutes.
Find the full press release on the FDA website.
The Food and Drug Administration has approved atezolizumab (Tecentriq), in combination with carboplatin and etoposide, for the first-line treatment of adults with extensive-stage small cell lung cancer (ES-SCLC).
Approval was based on results from the phase 3 IMpower133 study, in which 403 treatment-naive patients with ES-SCLC received atezolizumab at 1,200 mg with carboplatin at 5 mg/mL per minute on day 1 and etoposide 100 mg/m2 on days 1, 2, and 3 of a 21-day cycle for four cycles, followed by atezolizumab at 1,200 mg once every 3 weeks until disease progression or unacceptable toxicity; or received placebo with the same dosage of carboplatin and etoposide for a similar duration.
Overall survival was significantly better in patients who received atezolizumab, compared with placebo (12.3 vs. 10.3 months; hazard ratio, 0.70; 95% confidence interval, 0.54-0.91; P = .0069), as was progression-free survival (5.2 vs. 4.3 months; HR, 0.77; 95% CI, 0.62-0.96; P = .017).
The most common adverse events associated with atezolizumab in the study were fatigue/asthenia, nausea, alopecia, constipation, and decreased appetite.
According to the FDA, the recommended dose is 1,200 mg IV over 60 minutes every 3 weeks. When administered on the same day as chemotherapy, atezolizumab should be given first. If the first infusion is tolerated, all subsequent infusions can be delivered over 30 minutes.
Find the full press release on the FDA website.
Antibiotics gut checkpoint inhibitor efficacy
SAN FRANCISCO – Antibiotic exposure in the month before cancer immunotherapy starts may hamper the efficacy of immune checkpoint inhibitors, investigators caution.
A prospective study of 196 patients treated with immune checkpoint inhibitors for various cancers showed that the 29 patients who received antibiotics within 30 days of starting immunotherapy had significantly worse overall survival than patients without antibiotic exposure; this effect was seen across cancer types, reported David James Pinato, MD, PhD, from Imperial College London.
In contrast, concurrent antibiotic and checkpoint inhibitor use was not significantly associated with overall survival differences, he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
“I think these data are quite interesting in showing an independent detrimental effect, both on response and survival, in unselected patients treated with immune checkpoint inhibitors in routine clinical practice,” Dr. Pinato said.
The data also suggest “the timing of antibiotic exposure is crucial,” he added. Antibiotic treatment concurrent with immunotherapy did not appear to affect prognosis. Alternatively, prior antibiotic therapy appeared to have “a sort of a priming effect towards the immune system.”
Broad-spectrum antibiotics can affect the diversity of the gut microbiome, which influences mucosal immunity, dendritic cell function, and antigen presentation. Alternatively, enrichment of the microbiome with several bacterial species can enhance the potency of checkpoint inhibitors by facilitating the process of tumor rejection, Dr. Pinato explained.
To see whether antibiotic disruption, or “dysbiosis” of the gut microbiome, could hinder responsiveness to checkpoint inhibitors regardless of the tumor site and whether there were time-dependent effects of antibiotic exposure on response to checkpoint inhibitors, the investigators conducted a prospective, observational study in 196 patients treated with checkpoint inhibitors for non–small cell lung cancer (NSCLC), melanoma, renal cell carcinoma, head and neck cancer, transitional cell carcinoma of the bladder, and other cancers.
The researchers defined prior antibiotic exposure as more than 30 days before the start of checkpoint inhibitor therapy and concurrent exposure as antibiotics begun on the first day of the first cycle of checkpoint inhibitor dosing.
Of the 196 patients, 29 had previously received antibiotics, and 68 received them concurrently. The most frequently prescribed antibiotics were beta-lactam agents given in a single, short course. Other classes of drugs, used in eight or fewer patients each, included quinolones, macrolides, sulfonamides, tetracyclines, aminoglycosides, and nitroimidazole.
Median overall survival for the entire cohort, one of two primary outcomes, was 2 months for patients who had received prior antibiotics and 26 months for patients with no prior exposure. This difference was similar for patients with NSCLC (2.5 vs. 26 months), melanoma (3.9 vs. 14 months), and other cancers combined (1.1 vs. 11.0 months; log-rank P less than .01 for all comparisons).
In multivariate analysis, only response to checkpoints inhibitors (complete vs. partial response, stable disease, or progression) and prior antibiotic exposure were significantly associated with survival. The hazard ratio for survival for patients who had not previously received antibiotics was 3.5 (P less than .001).
In contrast, concurrent antibiotic and checkpoint inhibitor use did not have a significant effect on survival.
An analysis of radiologic responses also showed that patients with prior antibiotic exposure had a significantly higher probability of primary disease progression than those without (81% vs. 44%; P less than .001). There were no associations, however, between specific classes of antibiotics or corticosteroid use.
The findings indicate that “certainly, mechanistic studies are required here, not just to investigate the prognostic role of antibiotic-mediated dysbiosis, but perhaps transform this into an actual driver of antitumor immunity,” Dr. Pinato concluded.
The study was internally supported. Dr. Pinato reported receiving grant funding from Merck and Bristol-Myers Squibb unrelated to the study, as well as honoraria from ViiV Healthcare.
SOURCE: Pinato DJ et al. ASCO-SITC, Abstract 147.
SAN FRANCISCO – Antibiotic exposure in the month before cancer immunotherapy starts may hamper the efficacy of immune checkpoint inhibitors, investigators caution.
A prospective study of 196 patients treated with immune checkpoint inhibitors for various cancers showed that the 29 patients who received antibiotics within 30 days of starting immunotherapy had significantly worse overall survival than patients without antibiotic exposure; this effect was seen across cancer types, reported David James Pinato, MD, PhD, from Imperial College London.
In contrast, concurrent antibiotic and checkpoint inhibitor use was not significantly associated with overall survival differences, he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
“I think these data are quite interesting in showing an independent detrimental effect, both on response and survival, in unselected patients treated with immune checkpoint inhibitors in routine clinical practice,” Dr. Pinato said.
The data also suggest “the timing of antibiotic exposure is crucial,” he added. Antibiotic treatment concurrent with immunotherapy did not appear to affect prognosis. Alternatively, prior antibiotic therapy appeared to have “a sort of a priming effect towards the immune system.”
Broad-spectrum antibiotics can affect the diversity of the gut microbiome, which influences mucosal immunity, dendritic cell function, and antigen presentation. Alternatively, enrichment of the microbiome with several bacterial species can enhance the potency of checkpoint inhibitors by facilitating the process of tumor rejection, Dr. Pinato explained.
To see whether antibiotic disruption, or “dysbiosis” of the gut microbiome, could hinder responsiveness to checkpoint inhibitors regardless of the tumor site and whether there were time-dependent effects of antibiotic exposure on response to checkpoint inhibitors, the investigators conducted a prospective, observational study in 196 patients treated with checkpoint inhibitors for non–small cell lung cancer (NSCLC), melanoma, renal cell carcinoma, head and neck cancer, transitional cell carcinoma of the bladder, and other cancers.
The researchers defined prior antibiotic exposure as more than 30 days before the start of checkpoint inhibitor therapy and concurrent exposure as antibiotics begun on the first day of the first cycle of checkpoint inhibitor dosing.
Of the 196 patients, 29 had previously received antibiotics, and 68 received them concurrently. The most frequently prescribed antibiotics were beta-lactam agents given in a single, short course. Other classes of drugs, used in eight or fewer patients each, included quinolones, macrolides, sulfonamides, tetracyclines, aminoglycosides, and nitroimidazole.
Median overall survival for the entire cohort, one of two primary outcomes, was 2 months for patients who had received prior antibiotics and 26 months for patients with no prior exposure. This difference was similar for patients with NSCLC (2.5 vs. 26 months), melanoma (3.9 vs. 14 months), and other cancers combined (1.1 vs. 11.0 months; log-rank P less than .01 for all comparisons).
In multivariate analysis, only response to checkpoints inhibitors (complete vs. partial response, stable disease, or progression) and prior antibiotic exposure were significantly associated with survival. The hazard ratio for survival for patients who had not previously received antibiotics was 3.5 (P less than .001).
In contrast, concurrent antibiotic and checkpoint inhibitor use did not have a significant effect on survival.
An analysis of radiologic responses also showed that patients with prior antibiotic exposure had a significantly higher probability of primary disease progression than those without (81% vs. 44%; P less than .001). There were no associations, however, between specific classes of antibiotics or corticosteroid use.
The findings indicate that “certainly, mechanistic studies are required here, not just to investigate the prognostic role of antibiotic-mediated dysbiosis, but perhaps transform this into an actual driver of antitumor immunity,” Dr. Pinato concluded.
The study was internally supported. Dr. Pinato reported receiving grant funding from Merck and Bristol-Myers Squibb unrelated to the study, as well as honoraria from ViiV Healthcare.
SOURCE: Pinato DJ et al. ASCO-SITC, Abstract 147.
SAN FRANCISCO – Antibiotic exposure in the month before cancer immunotherapy starts may hamper the efficacy of immune checkpoint inhibitors, investigators caution.
A prospective study of 196 patients treated with immune checkpoint inhibitors for various cancers showed that the 29 patients who received antibiotics within 30 days of starting immunotherapy had significantly worse overall survival than patients without antibiotic exposure; this effect was seen across cancer types, reported David James Pinato, MD, PhD, from Imperial College London.
In contrast, concurrent antibiotic and checkpoint inhibitor use was not significantly associated with overall survival differences, he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
“I think these data are quite interesting in showing an independent detrimental effect, both on response and survival, in unselected patients treated with immune checkpoint inhibitors in routine clinical practice,” Dr. Pinato said.
The data also suggest “the timing of antibiotic exposure is crucial,” he added. Antibiotic treatment concurrent with immunotherapy did not appear to affect prognosis. Alternatively, prior antibiotic therapy appeared to have “a sort of a priming effect towards the immune system.”
Broad-spectrum antibiotics can affect the diversity of the gut microbiome, which influences mucosal immunity, dendritic cell function, and antigen presentation. Alternatively, enrichment of the microbiome with several bacterial species can enhance the potency of checkpoint inhibitors by facilitating the process of tumor rejection, Dr. Pinato explained.
To see whether antibiotic disruption, or “dysbiosis” of the gut microbiome, could hinder responsiveness to checkpoint inhibitors regardless of the tumor site and whether there were time-dependent effects of antibiotic exposure on response to checkpoint inhibitors, the investigators conducted a prospective, observational study in 196 patients treated with checkpoint inhibitors for non–small cell lung cancer (NSCLC), melanoma, renal cell carcinoma, head and neck cancer, transitional cell carcinoma of the bladder, and other cancers.
The researchers defined prior antibiotic exposure as more than 30 days before the start of checkpoint inhibitor therapy and concurrent exposure as antibiotics begun on the first day of the first cycle of checkpoint inhibitor dosing.
Of the 196 patients, 29 had previously received antibiotics, and 68 received them concurrently. The most frequently prescribed antibiotics were beta-lactam agents given in a single, short course. Other classes of drugs, used in eight or fewer patients each, included quinolones, macrolides, sulfonamides, tetracyclines, aminoglycosides, and nitroimidazole.
Median overall survival for the entire cohort, one of two primary outcomes, was 2 months for patients who had received prior antibiotics and 26 months for patients with no prior exposure. This difference was similar for patients with NSCLC (2.5 vs. 26 months), melanoma (3.9 vs. 14 months), and other cancers combined (1.1 vs. 11.0 months; log-rank P less than .01 for all comparisons).
In multivariate analysis, only response to checkpoints inhibitors (complete vs. partial response, stable disease, or progression) and prior antibiotic exposure were significantly associated with survival. The hazard ratio for survival for patients who had not previously received antibiotics was 3.5 (P less than .001).
In contrast, concurrent antibiotic and checkpoint inhibitor use did not have a significant effect on survival.
An analysis of radiologic responses also showed that patients with prior antibiotic exposure had a significantly higher probability of primary disease progression than those without (81% vs. 44%; P less than .001). There were no associations, however, between specific classes of antibiotics or corticosteroid use.
The findings indicate that “certainly, mechanistic studies are required here, not just to investigate the prognostic role of antibiotic-mediated dysbiosis, but perhaps transform this into an actual driver of antitumor immunity,” Dr. Pinato concluded.
The study was internally supported. Dr. Pinato reported receiving grant funding from Merck and Bristol-Myers Squibb unrelated to the study, as well as honoraria from ViiV Healthcare.
SOURCE: Pinato DJ et al. ASCO-SITC, Abstract 147.
REPORTING FROM ASCO-SITC
Higher dose of checkpoint inhibitor every 4 weeks feasible in NSCLC
SAN FRANCISCO – For patients with advanced non–small cell lung cancer (NSCLC) who previously had disease control with the checkpoint inhibitor nivolumab (Opdivo), second-line nivolumab at a higher dose every 4 weeks appeared to be comparable in efficacy and safety with standard-dose nivolumab every 2 weeks.
The key word in that last sentence is “appeared,” because the Checkmate 384 trial that was designed to show noninferiority of the every-4-weeks regimen lacked the statistical muscle to get the job done, reported Edward B. Garon, MD, from the University of California, Los Angeles.
“In many respects, extending the dosing frequency of nivolumab fulfills some of the promise of immunotherapy: The idea that we would be able to decrease the medicalization of the lives of our patients. For some people this would lead to them being able to resume a more normal work schedule, and for other people it would allow them to do things for fun, like travel on trips that would take longer than a couple of weeks,” he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
However, because of difficulties in recruitment, the investigators had to stop enrollment early and settle for a sample size of 363 patients, instead of the 600 planned that would be necessary to meet a 10% noninferiority margin and one-sided 95% confidence interval. Thus, the trial analysis can only be reported as descriptive rather than definitive, Dr. Garon acknowledged.
Nivolumab is approved at a fixed dose of 240 mg every 2 weeks for the treatment of multiple tumor types in several different nations, and in the United States and Canada it is approved at a dose of 480 mg every 4 weeks for the treatment of NSCLC.
The CheckMate 384 study enrolled patients with advanced or metastatic NSCLC who had received 3 mg/kg or 240 mg of nivolumab every 2 weeks for up to 1 year. The patients had to have had relatively good performance status (Eastern Cooperative Oncology Group 0-2) and two consecutive assessments of either complete response, partial response, or stable disease.
The patients were stratified by tumor histology (squamous or nonsquamous) and response to prior nivolumab therapy at randomization, and were then randomized to receive nivolumab 240 mg every 2 weeks or 480 mg every 4 weeks until disease progression or unacceptable toxicity for up to 2 years.
Dr. Garon presented an interim analysis including data on 329 of the 363 patients; the final analysis will occur after all patients have had a minimum of 12 months of follow-up. Here, he reported on 6-month progression-free survival, a coprimary endpoint with 12-month PFS.
After a median follow-up of 9.5 months in the Q4-week group and 10.2 months in the Q2-week group, the 6-month PFS rates were identical between the two dosing strategies, at 72%. The median PFS was 12.1 months and 12.2 months, respectively.
“Although the study is no longer formally powered to show noninferiority, there’s certainly nothing in these curves that makes me concerned that this 480 mg every-4-week dose would be inferior,” Dr. Garon said.
There was a slightly higher rate of treatment-related adverse events of any grade in the lower, more frequent dose group: 48% in the Q4-week versus 61% in the Q2-week arm. The respective rates of grade 3 or 4 adverse events were 8% and 12%. Rates of serious adverse events and events leading to treatment discontinuation were similar between the group; there were no treatment-related deaths.
The investigators hypothesize that the higher rate of overall events in the lower-dose group may be attributable to more frequent visits and more opportunities to report adverse events, Dr. Garon said.
“Overall, the clinical data are in agreement with the pharmacokinetic modeling and give further evidence for this 480 mg every 4 week nivolumab dosing option,” he concluded.
The study was supported by Bristol-Myers Squibb. Dr. Garon reported receiving research support from Bristol-Myers Squibb and others and consulting fees from Dracen Pharmaceuticals.
SOURCE: Garon EB et al. ASCO-SITC, Abstract 100.
SAN FRANCISCO – For patients with advanced non–small cell lung cancer (NSCLC) who previously had disease control with the checkpoint inhibitor nivolumab (Opdivo), second-line nivolumab at a higher dose every 4 weeks appeared to be comparable in efficacy and safety with standard-dose nivolumab every 2 weeks.
The key word in that last sentence is “appeared,” because the Checkmate 384 trial that was designed to show noninferiority of the every-4-weeks regimen lacked the statistical muscle to get the job done, reported Edward B. Garon, MD, from the University of California, Los Angeles.
“In many respects, extending the dosing frequency of nivolumab fulfills some of the promise of immunotherapy: The idea that we would be able to decrease the medicalization of the lives of our patients. For some people this would lead to them being able to resume a more normal work schedule, and for other people it would allow them to do things for fun, like travel on trips that would take longer than a couple of weeks,” he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
However, because of difficulties in recruitment, the investigators had to stop enrollment early and settle for a sample size of 363 patients, instead of the 600 planned that would be necessary to meet a 10% noninferiority margin and one-sided 95% confidence interval. Thus, the trial analysis can only be reported as descriptive rather than definitive, Dr. Garon acknowledged.
Nivolumab is approved at a fixed dose of 240 mg every 2 weeks for the treatment of multiple tumor types in several different nations, and in the United States and Canada it is approved at a dose of 480 mg every 4 weeks for the treatment of NSCLC.
The CheckMate 384 study enrolled patients with advanced or metastatic NSCLC who had received 3 mg/kg or 240 mg of nivolumab every 2 weeks for up to 1 year. The patients had to have had relatively good performance status (Eastern Cooperative Oncology Group 0-2) and two consecutive assessments of either complete response, partial response, or stable disease.
The patients were stratified by tumor histology (squamous or nonsquamous) and response to prior nivolumab therapy at randomization, and were then randomized to receive nivolumab 240 mg every 2 weeks or 480 mg every 4 weeks until disease progression or unacceptable toxicity for up to 2 years.
Dr. Garon presented an interim analysis including data on 329 of the 363 patients; the final analysis will occur after all patients have had a minimum of 12 months of follow-up. Here, he reported on 6-month progression-free survival, a coprimary endpoint with 12-month PFS.
After a median follow-up of 9.5 months in the Q4-week group and 10.2 months in the Q2-week group, the 6-month PFS rates were identical between the two dosing strategies, at 72%. The median PFS was 12.1 months and 12.2 months, respectively.
“Although the study is no longer formally powered to show noninferiority, there’s certainly nothing in these curves that makes me concerned that this 480 mg every-4-week dose would be inferior,” Dr. Garon said.
There was a slightly higher rate of treatment-related adverse events of any grade in the lower, more frequent dose group: 48% in the Q4-week versus 61% in the Q2-week arm. The respective rates of grade 3 or 4 adverse events were 8% and 12%. Rates of serious adverse events and events leading to treatment discontinuation were similar between the group; there were no treatment-related deaths.
The investigators hypothesize that the higher rate of overall events in the lower-dose group may be attributable to more frequent visits and more opportunities to report adverse events, Dr. Garon said.
“Overall, the clinical data are in agreement with the pharmacokinetic modeling and give further evidence for this 480 mg every 4 week nivolumab dosing option,” he concluded.
The study was supported by Bristol-Myers Squibb. Dr. Garon reported receiving research support from Bristol-Myers Squibb and others and consulting fees from Dracen Pharmaceuticals.
SOURCE: Garon EB et al. ASCO-SITC, Abstract 100.
SAN FRANCISCO – For patients with advanced non–small cell lung cancer (NSCLC) who previously had disease control with the checkpoint inhibitor nivolumab (Opdivo), second-line nivolumab at a higher dose every 4 weeks appeared to be comparable in efficacy and safety with standard-dose nivolumab every 2 weeks.
The key word in that last sentence is “appeared,” because the Checkmate 384 trial that was designed to show noninferiority of the every-4-weeks regimen lacked the statistical muscle to get the job done, reported Edward B. Garon, MD, from the University of California, Los Angeles.
“In many respects, extending the dosing frequency of nivolumab fulfills some of the promise of immunotherapy: The idea that we would be able to decrease the medicalization of the lives of our patients. For some people this would lead to them being able to resume a more normal work schedule, and for other people it would allow them to do things for fun, like travel on trips that would take longer than a couple of weeks,” he said at the American Society of Clinical Oncology (ASCO) – Society for Immunotherapy of Cancer (SITC): Clinical Immuno-Oncology Symposium.
However, because of difficulties in recruitment, the investigators had to stop enrollment early and settle for a sample size of 363 patients, instead of the 600 planned that would be necessary to meet a 10% noninferiority margin and one-sided 95% confidence interval. Thus, the trial analysis can only be reported as descriptive rather than definitive, Dr. Garon acknowledged.
Nivolumab is approved at a fixed dose of 240 mg every 2 weeks for the treatment of multiple tumor types in several different nations, and in the United States and Canada it is approved at a dose of 480 mg every 4 weeks for the treatment of NSCLC.
The CheckMate 384 study enrolled patients with advanced or metastatic NSCLC who had received 3 mg/kg or 240 mg of nivolumab every 2 weeks for up to 1 year. The patients had to have had relatively good performance status (Eastern Cooperative Oncology Group 0-2) and two consecutive assessments of either complete response, partial response, or stable disease.
The patients were stratified by tumor histology (squamous or nonsquamous) and response to prior nivolumab therapy at randomization, and were then randomized to receive nivolumab 240 mg every 2 weeks or 480 mg every 4 weeks until disease progression or unacceptable toxicity for up to 2 years.
Dr. Garon presented an interim analysis including data on 329 of the 363 patients; the final analysis will occur after all patients have had a minimum of 12 months of follow-up. Here, he reported on 6-month progression-free survival, a coprimary endpoint with 12-month PFS.
After a median follow-up of 9.5 months in the Q4-week group and 10.2 months in the Q2-week group, the 6-month PFS rates were identical between the two dosing strategies, at 72%. The median PFS was 12.1 months and 12.2 months, respectively.
“Although the study is no longer formally powered to show noninferiority, there’s certainly nothing in these curves that makes me concerned that this 480 mg every-4-week dose would be inferior,” Dr. Garon said.
There was a slightly higher rate of treatment-related adverse events of any grade in the lower, more frequent dose group: 48% in the Q4-week versus 61% in the Q2-week arm. The respective rates of grade 3 or 4 adverse events were 8% and 12%. Rates of serious adverse events and events leading to treatment discontinuation were similar between the group; there were no treatment-related deaths.
The investigators hypothesize that the higher rate of overall events in the lower-dose group may be attributable to more frequent visits and more opportunities to report adverse events, Dr. Garon said.
“Overall, the clinical data are in agreement with the pharmacokinetic modeling and give further evidence for this 480 mg every 4 week nivolumab dosing option,” he concluded.
The study was supported by Bristol-Myers Squibb. Dr. Garon reported receiving research support from Bristol-Myers Squibb and others and consulting fees from Dracen Pharmaceuticals.
SOURCE: Garon EB et al. ASCO-SITC, Abstract 100.
REPORTING FROM ASCO-SITC
FDA Expanded Access benefits heavily pretreated patients, especially children
Single-patient use (SPU) of investigational therapies via the Food and Drug Administration’s Expanded Access program is an option worth considering for heavily pretreated cancer patients, according to a retrospective analysis of SPUs at Memorial Sloan Kettering Cancer Center.
Although approximately 2% of cancer cases at Kettering are pediatric, 34.1% of SPUs were for children, reported lead author Noah Z. Feit of Cornell University, New York, and his colleagues.
Therefore, “SPUs may provide an important means of pediatric drug access,” the investigators wrote in a JAMA Oncology letter.
The analysis involved 179 patients with 43 cancer types; these were more often solid tumors than hematologic malignancies (57.9% vs. 42.1%). The most common solid tumor type was neuroblastoma (15.3%), followed by lung (7.9%), primary brain (7.9%), and breast (5.9%). Sixty-six investigational products were given; the top three types were kinase inhibitors (28.8%), naked antibodies (12.5%), and allogeneic cell therapy (12.0%). Therapies were in various stages of development, including phase 3 (39.4%), phase 2 (36.1%), and phase 1 (18.8%). SPU approval was most often based on previous clinical experience (61.5%), although genomic data (38.0%) and preclinical evidence (30.8%) were also cited. The median number of prior treatments was four, suggesting a heavily pretreated patient population.
Analysis showed that the overall response rate to SPU agents was 20.1%, and patients with hematologic cancers responded more often than did those with solid tumors (30.4% vs. 12.2%). Median progression-free survival and overall survival were 3.9 months and 11.4 months, respectively. About one-third of patients (29.7%) had at least one serious treatment-related adverse event, with adults more often affected than children (35.3% vs. 19.1%). No treatment-related deaths occurred.
“In summary, our data provide an initial evidence basis to evaluate the FDA Expanded Access mechanism. We find its use is broad, involving a wide variety of patients and products, and clinical benefit was observed,” the investigators concluded. “Routine prospective collection of key safety and efficacy metrics should be considered moving forward.”
The study was funded by National Institutes of Health, the St. Baldrick’s Foundation, and the Nonna’s Garden Foundation Initiative in Precision Oncology. The investigators reported financial relationships with Mylan, Atara Biotherapeutics, Chugai Pharma, Boehringer Ingelheim, and others.
SOURCE: Feit et al. JAMA Onc. 2019 Feb 28. doi: 10.1001/jamaoncol.2018.7002.
Single-patient use (SPU) of investigational therapies via the Food and Drug Administration’s Expanded Access program is an option worth considering for heavily pretreated cancer patients, according to a retrospective analysis of SPUs at Memorial Sloan Kettering Cancer Center.
Although approximately 2% of cancer cases at Kettering are pediatric, 34.1% of SPUs were for children, reported lead author Noah Z. Feit of Cornell University, New York, and his colleagues.
Therefore, “SPUs may provide an important means of pediatric drug access,” the investigators wrote in a JAMA Oncology letter.
The analysis involved 179 patients with 43 cancer types; these were more often solid tumors than hematologic malignancies (57.9% vs. 42.1%). The most common solid tumor type was neuroblastoma (15.3%), followed by lung (7.9%), primary brain (7.9%), and breast (5.9%). Sixty-six investigational products were given; the top three types were kinase inhibitors (28.8%), naked antibodies (12.5%), and allogeneic cell therapy (12.0%). Therapies were in various stages of development, including phase 3 (39.4%), phase 2 (36.1%), and phase 1 (18.8%). SPU approval was most often based on previous clinical experience (61.5%), although genomic data (38.0%) and preclinical evidence (30.8%) were also cited. The median number of prior treatments was four, suggesting a heavily pretreated patient population.
Analysis showed that the overall response rate to SPU agents was 20.1%, and patients with hematologic cancers responded more often than did those with solid tumors (30.4% vs. 12.2%). Median progression-free survival and overall survival were 3.9 months and 11.4 months, respectively. About one-third of patients (29.7%) had at least one serious treatment-related adverse event, with adults more often affected than children (35.3% vs. 19.1%). No treatment-related deaths occurred.
“In summary, our data provide an initial evidence basis to evaluate the FDA Expanded Access mechanism. We find its use is broad, involving a wide variety of patients and products, and clinical benefit was observed,” the investigators concluded. “Routine prospective collection of key safety and efficacy metrics should be considered moving forward.”
The study was funded by National Institutes of Health, the St. Baldrick’s Foundation, and the Nonna’s Garden Foundation Initiative in Precision Oncology. The investigators reported financial relationships with Mylan, Atara Biotherapeutics, Chugai Pharma, Boehringer Ingelheim, and others.
SOURCE: Feit et al. JAMA Onc. 2019 Feb 28. doi: 10.1001/jamaoncol.2018.7002.
Single-patient use (SPU) of investigational therapies via the Food and Drug Administration’s Expanded Access program is an option worth considering for heavily pretreated cancer patients, according to a retrospective analysis of SPUs at Memorial Sloan Kettering Cancer Center.
Although approximately 2% of cancer cases at Kettering are pediatric, 34.1% of SPUs were for children, reported lead author Noah Z. Feit of Cornell University, New York, and his colleagues.
Therefore, “SPUs may provide an important means of pediatric drug access,” the investigators wrote in a JAMA Oncology letter.
The analysis involved 179 patients with 43 cancer types; these were more often solid tumors than hematologic malignancies (57.9% vs. 42.1%). The most common solid tumor type was neuroblastoma (15.3%), followed by lung (7.9%), primary brain (7.9%), and breast (5.9%). Sixty-six investigational products were given; the top three types were kinase inhibitors (28.8%), naked antibodies (12.5%), and allogeneic cell therapy (12.0%). Therapies were in various stages of development, including phase 3 (39.4%), phase 2 (36.1%), and phase 1 (18.8%). SPU approval was most often based on previous clinical experience (61.5%), although genomic data (38.0%) and preclinical evidence (30.8%) were also cited. The median number of prior treatments was four, suggesting a heavily pretreated patient population.
Analysis showed that the overall response rate to SPU agents was 20.1%, and patients with hematologic cancers responded more often than did those with solid tumors (30.4% vs. 12.2%). Median progression-free survival and overall survival were 3.9 months and 11.4 months, respectively. About one-third of patients (29.7%) had at least one serious treatment-related adverse event, with adults more often affected than children (35.3% vs. 19.1%). No treatment-related deaths occurred.
“In summary, our data provide an initial evidence basis to evaluate the FDA Expanded Access mechanism. We find its use is broad, involving a wide variety of patients and products, and clinical benefit was observed,” the investigators concluded. “Routine prospective collection of key safety and efficacy metrics should be considered moving forward.”
The study was funded by National Institutes of Health, the St. Baldrick’s Foundation, and the Nonna’s Garden Foundation Initiative in Precision Oncology. The investigators reported financial relationships with Mylan, Atara Biotherapeutics, Chugai Pharma, Boehringer Ingelheim, and others.
SOURCE: Feit et al. JAMA Onc. 2019 Feb 28. doi: 10.1001/jamaoncol.2018.7002.
FROM JAMA ONCOLOGY
Rare, aggressive NSCLC type yields to pembrolizumab
SAN FRANCISCO – The immune checkpoint inhibitor pembrolizumab (Keytruda) was associated in a small case series with remarkable overall and progression-free survival of patients with pulmonary sarcomatoid carcinoma (PSC), a rare variant of non–small cell lung cancer (NSCLC) with a grim prognosis.
Among five patients with PSC, three of whom were treatment naive, none experienced disease progression on pembrolizumab after a median follow-up of 13 months – although one died from a fungal infection unrelated to therapy – with the longest overall survival to date out to 33 months.
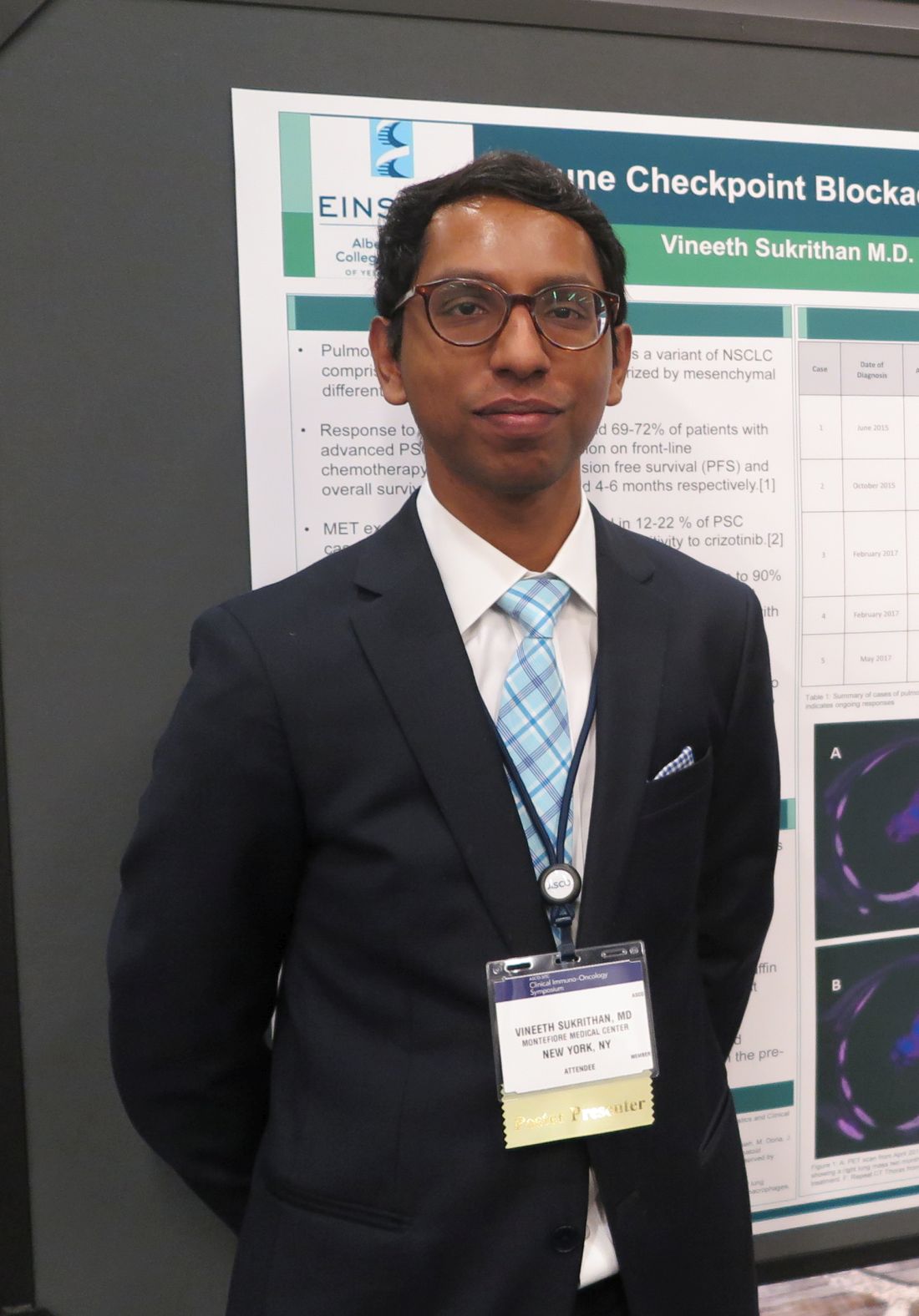
In contrast, patients with PSC treated before the advent of immunotherapy had a median progression-free survival of just 2 months and median overall survival a brief 4-6 months, reported Vineeth Sukrithan, MD, and his colleagues at the Albert Einstein College of Medicine, New York.
“It’s a uniquely enriched population of patients with lung cancer that have the best prognosis in terms of benefits from checkpoint inhibitors,” he said in an interview at the ASCO-SITC Clinical Immuno-Oncology Symposium.
PSC, a poorly differentiated subtype of NSCLC, accounts for about 0.3%-1.3% of all cases of lung cancer. It is closely associated with a history of heavy cigarette smoking and is rapidly fatal, with a poor response to conventional chemotherapy, although approximately 20% of patients with PSC have MET exon 14–skipping mutations that are “exquisitely” sensitive to crizotinib (Xalkori), Dr. Sukrithan explained.
PSC tumors are also unique in that they have extraordinarily high levels of programmed death-ligand 1 (PD-L1), the target of immune checkpoint inhibitors, with tumor proportion scores exceeding 90% in some cases.
Additionally, up to 43% of PSC tumors have been found to have a high mutational burden, with more than 10 mutations per megabase, suggesting that these tumors may be especially attractive targets for checkpoint inhibitor therapy, he said.
The investigators retrospectively studied surgical pathology and treatment records for all patients with advanced PSC diagnosed at their center from June 2015 to June 2018 who received pembrolizumab. They performed immunohistochemistry testing on tissue samples from the patients to quantify PD-L1 expression.
They compared the results with a cohort of patients with advanced PSC diagnosed from June 2012 to June 2015, prior to the clinical availability of anti-PD-1/PD-L1 checkpoint inhibitors.
The PD-L1-treated cohort included two men and three women, ranging from 48 to 67 years, with smoking pack-years ranging from 24 to 50. Two of the patients had received prior chemotherapy followed by pembrolizumab, and the remaining three received pembrolizumab monotherapy.
Tumor proportion scores ranged from more than 75% of tumor cells examined in one patient to 100% of cells in another.
The objective response rate to pembrolizumab was 80% consisting of one complete response and three partial responses. The fifth patient continued to have stable disease out to more than 17 months.
“This highly treatment-refractory disease now should be carefully assessed for immuno-oncologic and molecularly targeted options, which are associated with significant improvement in outcomes,” the investigators wrote in a poster presentation.
The study was internally funded. Dr. Sukrithan reported having no disclosures.
SOURCE: Sukrithan V et al. ASCO-SITC, Abstract 115.
SAN FRANCISCO – The immune checkpoint inhibitor pembrolizumab (Keytruda) was associated in a small case series with remarkable overall and progression-free survival of patients with pulmonary sarcomatoid carcinoma (PSC), a rare variant of non–small cell lung cancer (NSCLC) with a grim prognosis.
Among five patients with PSC, three of whom were treatment naive, none experienced disease progression on pembrolizumab after a median follow-up of 13 months – although one died from a fungal infection unrelated to therapy – with the longest overall survival to date out to 33 months.
In contrast, patients with PSC treated before the advent of immunotherapy had a median progression-free survival of just 2 months and median overall survival a brief 4-6 months, reported Vineeth Sukrithan, MD, and his colleagues at the Albert Einstein College of Medicine, New York.
“It’s a uniquely enriched population of patients with lung cancer that have the best prognosis in terms of benefits from checkpoint inhibitors,” he said in an interview at the ASCO-SITC Clinical Immuno-Oncology Symposium.
PSC, a poorly differentiated subtype of NSCLC, accounts for about 0.3%-1.3% of all cases of lung cancer. It is closely associated with a history of heavy cigarette smoking and is rapidly fatal, with a poor response to conventional chemotherapy, although approximately 20% of patients with PSC have MET exon 14–skipping mutations that are “exquisitely” sensitive to crizotinib (Xalkori), Dr. Sukrithan explained.
PSC tumors are also unique in that they have extraordinarily high levels of programmed death-ligand 1 (PD-L1), the target of immune checkpoint inhibitors, with tumor proportion scores exceeding 90% in some cases.
Additionally, up to 43% of PSC tumors have been found to have a high mutational burden, with more than 10 mutations per megabase, suggesting that these tumors may be especially attractive targets for checkpoint inhibitor therapy, he said.
The investigators retrospectively studied surgical pathology and treatment records for all patients with advanced PSC diagnosed at their center from June 2015 to June 2018 who received pembrolizumab. They performed immunohistochemistry testing on tissue samples from the patients to quantify PD-L1 expression.
They compared the results with a cohort of patients with advanced PSC diagnosed from June 2012 to June 2015, prior to the clinical availability of anti-PD-1/PD-L1 checkpoint inhibitors.
The PD-L1-treated cohort included two men and three women, ranging from 48 to 67 years, with smoking pack-years ranging from 24 to 50. Two of the patients had received prior chemotherapy followed by pembrolizumab, and the remaining three received pembrolizumab monotherapy.
Tumor proportion scores ranged from more than 75% of tumor cells examined in one patient to 100% of cells in another.
The objective response rate to pembrolizumab was 80% consisting of one complete response and three partial responses. The fifth patient continued to have stable disease out to more than 17 months.
“This highly treatment-refractory disease now should be carefully assessed for immuno-oncologic and molecularly targeted options, which are associated with significant improvement in outcomes,” the investigators wrote in a poster presentation.
The study was internally funded. Dr. Sukrithan reported having no disclosures.
SOURCE: Sukrithan V et al. ASCO-SITC, Abstract 115.
SAN FRANCISCO – The immune checkpoint inhibitor pembrolizumab (Keytruda) was associated in a small case series with remarkable overall and progression-free survival of patients with pulmonary sarcomatoid carcinoma (PSC), a rare variant of non–small cell lung cancer (NSCLC) with a grim prognosis.
Among five patients with PSC, three of whom were treatment naive, none experienced disease progression on pembrolizumab after a median follow-up of 13 months – although one died from a fungal infection unrelated to therapy – with the longest overall survival to date out to 33 months.
In contrast, patients with PSC treated before the advent of immunotherapy had a median progression-free survival of just 2 months and median overall survival a brief 4-6 months, reported Vineeth Sukrithan, MD, and his colleagues at the Albert Einstein College of Medicine, New York.
“It’s a uniquely enriched population of patients with lung cancer that have the best prognosis in terms of benefits from checkpoint inhibitors,” he said in an interview at the ASCO-SITC Clinical Immuno-Oncology Symposium.
PSC, a poorly differentiated subtype of NSCLC, accounts for about 0.3%-1.3% of all cases of lung cancer. It is closely associated with a history of heavy cigarette smoking and is rapidly fatal, with a poor response to conventional chemotherapy, although approximately 20% of patients with PSC have MET exon 14–skipping mutations that are “exquisitely” sensitive to crizotinib (Xalkori), Dr. Sukrithan explained.
PSC tumors are also unique in that they have extraordinarily high levels of programmed death-ligand 1 (PD-L1), the target of immune checkpoint inhibitors, with tumor proportion scores exceeding 90% in some cases.
Additionally, up to 43% of PSC tumors have been found to have a high mutational burden, with more than 10 mutations per megabase, suggesting that these tumors may be especially attractive targets for checkpoint inhibitor therapy, he said.
The investigators retrospectively studied surgical pathology and treatment records for all patients with advanced PSC diagnosed at their center from June 2015 to June 2018 who received pembrolizumab. They performed immunohistochemistry testing on tissue samples from the patients to quantify PD-L1 expression.
They compared the results with a cohort of patients with advanced PSC diagnosed from June 2012 to June 2015, prior to the clinical availability of anti-PD-1/PD-L1 checkpoint inhibitors.
The PD-L1-treated cohort included two men and three women, ranging from 48 to 67 years, with smoking pack-years ranging from 24 to 50. Two of the patients had received prior chemotherapy followed by pembrolizumab, and the remaining three received pembrolizumab monotherapy.
Tumor proportion scores ranged from more than 75% of tumor cells examined in one patient to 100% of cells in another.
The objective response rate to pembrolizumab was 80% consisting of one complete response and three partial responses. The fifth patient continued to have stable disease out to more than 17 months.
“This highly treatment-refractory disease now should be carefully assessed for immuno-oncologic and molecularly targeted options, which are associated with significant improvement in outcomes,” the investigators wrote in a poster presentation.
The study was internally funded. Dr. Sukrithan reported having no disclosures.
SOURCE: Sukrithan V et al. ASCO-SITC, Abstract 115.
REPORTING FROM ASCO-SITC
NILE: Liquid biopsy bests tissue testing for targetable mutations in NSCLC
A cell-free DNA (cfDNA) test, or “liquid biopsy,” identifies more biomarkers and does so more quickly than tissue-based genotyping for guiding treatment in newly diagnosed advanced non–small cell lung cancer (NSCLC), according to a finding from a prospective study.
In 282 patients with newly diagnosed advanced NSCLC who were enrolled in the multicenter Noninvasive versus Invasive Lung Evaluation (NILE) study between July 2016 and April 2018, the “well-validated, comprehensive, and highly sensitive test” – Guardant360 – detected at least one guideline-recommended biomarker mutation in significantly more cases than did tissue-based tests alone (77 vs. 60 patients), Vassiliki A. Papadimitrakopoulou, MD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research annual meeting in Atlanta.
“Additionally, the cfDNA results were delivered significantly faster than the standard-of-care tissue results [median, 9 vs. 15 days],” said Dr. Papadimitrakopoulou, chief of the section of thoracic medical oncology and the Jay and Lori Eisenberg Distinguished Professor in the department of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Guardant360 assesses for all guideline-recommended genomic biomarkers, Dr. Papadimitrakopoulou said, noting that nine such biomarkers have been identified. All biomarkers identified using the liquid biopsy were also detected in tissue every time.
“Plasma cfDNA testing therefore had 100% positive predictive value,” she said.
This is important, because “we know that about 30% of patients with newly diagnosed advanced non–small lung cancer have therapeutically targetable genomic alterations that make them eligible for targeted therapies,” she said.
“Identifying these patients is important, as the response rate to the properly identified targeted therapy is higher than response rates to first-line chemotherapy or immune checkpoint inhibitor therapy,” she added, explaining that tissue-based assessment has long been the standard of care option for identifying genomic biomarkers, but is limited by the risks associated with the biopsy procedure, the inability to test for all relevant mutations, and the time it takes – up to 30 days – to obtain results.
“[The NILE] results have very exciting implications for clinical practice, especially in light of the expanding list of genomic biomarkers to be assessed,” she said, concluding that the findings from NILE – the largest study of newly diagnosed advanced NSCLC – demonstrate that the clinical utility of this well-validated, comprehensive, sensitive cfDNA test “is cardinal in identification of patients with guideline-recommended biomarker-positive tumors, and it is an alternative to SOC [standard of care] tissue testing in the first-line testing.”
Clinical follow-up of patients is ongoing, she noted.
This study was funded by Guardant Health. Dr. Papadimitrakopoulou serves on the advisory boards of several pharmaceutical companies. She reported receiving CME speaker fees from F. Hoffmann–La Roche, and has received research support from Eli Lilly, Novartis, Merck, AstraZeneca, F. Hoffmann–La Roche, Nektar Therapeutics, Janssen, Bristol-Myers Squibb, Checkmate, Incyte and Guardant Health.
SOURCE: Papadimitrakopoulou VA et al. AACR 2019, Abstract 4460..
A cell-free DNA (cfDNA) test, or “liquid biopsy,” identifies more biomarkers and does so more quickly than tissue-based genotyping for guiding treatment in newly diagnosed advanced non–small cell lung cancer (NSCLC), according to a finding from a prospective study.
In 282 patients with newly diagnosed advanced NSCLC who were enrolled in the multicenter Noninvasive versus Invasive Lung Evaluation (NILE) study between July 2016 and April 2018, the “well-validated, comprehensive, and highly sensitive test” – Guardant360 – detected at least one guideline-recommended biomarker mutation in significantly more cases than did tissue-based tests alone (77 vs. 60 patients), Vassiliki A. Papadimitrakopoulou, MD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research annual meeting in Atlanta.
“Additionally, the cfDNA results were delivered significantly faster than the standard-of-care tissue results [median, 9 vs. 15 days],” said Dr. Papadimitrakopoulou, chief of the section of thoracic medical oncology and the Jay and Lori Eisenberg Distinguished Professor in the department of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Guardant360 assesses for all guideline-recommended genomic biomarkers, Dr. Papadimitrakopoulou said, noting that nine such biomarkers have been identified. All biomarkers identified using the liquid biopsy were also detected in tissue every time.
“Plasma cfDNA testing therefore had 100% positive predictive value,” she said.
This is important, because “we know that about 30% of patients with newly diagnosed advanced non–small lung cancer have therapeutically targetable genomic alterations that make them eligible for targeted therapies,” she said.
“Identifying these patients is important, as the response rate to the properly identified targeted therapy is higher than response rates to first-line chemotherapy or immune checkpoint inhibitor therapy,” she added, explaining that tissue-based assessment has long been the standard of care option for identifying genomic biomarkers, but is limited by the risks associated with the biopsy procedure, the inability to test for all relevant mutations, and the time it takes – up to 30 days – to obtain results.
“[The NILE] results have very exciting implications for clinical practice, especially in light of the expanding list of genomic biomarkers to be assessed,” she said, concluding that the findings from NILE – the largest study of newly diagnosed advanced NSCLC – demonstrate that the clinical utility of this well-validated, comprehensive, sensitive cfDNA test “is cardinal in identification of patients with guideline-recommended biomarker-positive tumors, and it is an alternative to SOC [standard of care] tissue testing in the first-line testing.”
Clinical follow-up of patients is ongoing, she noted.
This study was funded by Guardant Health. Dr. Papadimitrakopoulou serves on the advisory boards of several pharmaceutical companies. She reported receiving CME speaker fees from F. Hoffmann–La Roche, and has received research support from Eli Lilly, Novartis, Merck, AstraZeneca, F. Hoffmann–La Roche, Nektar Therapeutics, Janssen, Bristol-Myers Squibb, Checkmate, Incyte and Guardant Health.
SOURCE: Papadimitrakopoulou VA et al. AACR 2019, Abstract 4460..
A cell-free DNA (cfDNA) test, or “liquid biopsy,” identifies more biomarkers and does so more quickly than tissue-based genotyping for guiding treatment in newly diagnosed advanced non–small cell lung cancer (NSCLC), according to a finding from a prospective study.
In 282 patients with newly diagnosed advanced NSCLC who were enrolled in the multicenter Noninvasive versus Invasive Lung Evaluation (NILE) study between July 2016 and April 2018, the “well-validated, comprehensive, and highly sensitive test” – Guardant360 – detected at least one guideline-recommended biomarker mutation in significantly more cases than did tissue-based tests alone (77 vs. 60 patients), Vassiliki A. Papadimitrakopoulou, MD, reported during a press conference highlighting data to be presented at the upcoming American Association for Cancer Research annual meeting in Atlanta.
“Additionally, the cfDNA results were delivered significantly faster than the standard-of-care tissue results [median, 9 vs. 15 days],” said Dr. Papadimitrakopoulou, chief of the section of thoracic medical oncology and the Jay and Lori Eisenberg Distinguished Professor in the department of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Guardant360 assesses for all guideline-recommended genomic biomarkers, Dr. Papadimitrakopoulou said, noting that nine such biomarkers have been identified. All biomarkers identified using the liquid biopsy were also detected in tissue every time.
“Plasma cfDNA testing therefore had 100% positive predictive value,” she said.
This is important, because “we know that about 30% of patients with newly diagnosed advanced non–small lung cancer have therapeutically targetable genomic alterations that make them eligible for targeted therapies,” she said.
“Identifying these patients is important, as the response rate to the properly identified targeted therapy is higher than response rates to first-line chemotherapy or immune checkpoint inhibitor therapy,” she added, explaining that tissue-based assessment has long been the standard of care option for identifying genomic biomarkers, but is limited by the risks associated with the biopsy procedure, the inability to test for all relevant mutations, and the time it takes – up to 30 days – to obtain results.
“[The NILE] results have very exciting implications for clinical practice, especially in light of the expanding list of genomic biomarkers to be assessed,” she said, concluding that the findings from NILE – the largest study of newly diagnosed advanced NSCLC – demonstrate that the clinical utility of this well-validated, comprehensive, sensitive cfDNA test “is cardinal in identification of patients with guideline-recommended biomarker-positive tumors, and it is an alternative to SOC [standard of care] tissue testing in the first-line testing.”
Clinical follow-up of patients is ongoing, she noted.
This study was funded by Guardant Health. Dr. Papadimitrakopoulou serves on the advisory boards of several pharmaceutical companies. She reported receiving CME speaker fees from F. Hoffmann–La Roche, and has received research support from Eli Lilly, Novartis, Merck, AstraZeneca, F. Hoffmann–La Roche, Nektar Therapeutics, Janssen, Bristol-Myers Squibb, Checkmate, Incyte and Guardant Health.
SOURCE: Papadimitrakopoulou VA et al. AACR 2019, Abstract 4460..
Caring for aging HIV-infected patients requires close attention to unrelated diseases
A substantial proportion of non–AIDS-defining cancers, and other noninfectious comorbid diseases, could be prevented with interventions on traditional risk factors in HIV-infected patients, according to the results of large database analysis published online in The Lancet HIV.
The researchers analyzed traditional and HIV-related risk factors for four validated noncommunicable disease outcomes (non–AIDS-defining cancers, myocardial infarction, end-stage liver disease, and end-stage renal disease) among participants of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), according to Keri N. Althoff, PhD, of Johns Hopkins University, Baltimore, and her colleagues on behalf of the NA-ACCORD.
The study comprised individuals with the assessed disease conditions from among more than 180,000 adults (aged 18 years or older) with HIV from more than 200 sites who had at least two care visits within 12 months. The researchers used a population attributable fraction (PAF) approach to quantify the proportion of noncommunicable diseases that could be eliminated if particular risk factors were not present. According to the researchers, PAF can be used to inform prioritization of interventions.
Dr. Althoff and her colleagues found that, for non–AIDS-defining cancer, the significant preventable or modifiable risk factors were smoking, low CD4 cell count, detectable HIV RNA, a history of clinical AIDS diagnosis, and hepatitis B infection.
For myocardial infarction, the significant factors were smoking, elevated total cholesterol, hypertension, stage 4 chronic kidney disease, a low CD4 cell count, detectable HIV RNA, and hepatitis C infection.
For end-stage liver disease, the significant factors were low CD4 cell count, detectable HIV RNA, a history of a clinical AIDS diagnosis, and hepatitis B or C infection.
For end-stage renal disease, the significantly associated risk factors were elevated total cholesterol, hypertension, diabetes, low CD4 cell count, detectable HIV RNA, and a history of clinical AIDS diagnosis.
The most substantial PAF for each of the respective diseases was as follows: smoking for non–AIDS-related cancers (24%; 95% confidence interval, 13%-35%), elevated total cholesterol for myocardial infarction (44%; 95% CI, 30%-58%), and hepatitis C infection for end-stage liver disease (30%; 95% CI, 21%-39%). In addition, hypertension had the highest PAF for end-stage renal disease (39%; 95% CI, 26%-51%).
“Modifications to individual-level interventions and models of HIV care, and the implementation of structural and policy-level interventions that focus on prevention and modification of traditional risk factors are necessary to avoid noncommunicable diseases and preserve health among successfully antiretroviral-treated adults aging with HIV,” the researchers concluded.
The study was funded by the National Institutes of Health and the NA-ACCORD. Dr. Althoff reported having no relevant disclosures.
SOURCE: Althoff KN et al. The Lancet HIV. 2019 Jan 22. doi: 10.1016/S2352-3018(18)30295-9.
A substantial proportion of non–AIDS-defining cancers, and other noninfectious comorbid diseases, could be prevented with interventions on traditional risk factors in HIV-infected patients, according to the results of large database analysis published online in The Lancet HIV.
The researchers analyzed traditional and HIV-related risk factors for four validated noncommunicable disease outcomes (non–AIDS-defining cancers, myocardial infarction, end-stage liver disease, and end-stage renal disease) among participants of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), according to Keri N. Althoff, PhD, of Johns Hopkins University, Baltimore, and her colleagues on behalf of the NA-ACCORD.
The study comprised individuals with the assessed disease conditions from among more than 180,000 adults (aged 18 years or older) with HIV from more than 200 sites who had at least two care visits within 12 months. The researchers used a population attributable fraction (PAF) approach to quantify the proportion of noncommunicable diseases that could be eliminated if particular risk factors were not present. According to the researchers, PAF can be used to inform prioritization of interventions.
Dr. Althoff and her colleagues found that, for non–AIDS-defining cancer, the significant preventable or modifiable risk factors were smoking, low CD4 cell count, detectable HIV RNA, a history of clinical AIDS diagnosis, and hepatitis B infection.
For myocardial infarction, the significant factors were smoking, elevated total cholesterol, hypertension, stage 4 chronic kidney disease, a low CD4 cell count, detectable HIV RNA, and hepatitis C infection.
For end-stage liver disease, the significant factors were low CD4 cell count, detectable HIV RNA, a history of a clinical AIDS diagnosis, and hepatitis B or C infection.
For end-stage renal disease, the significantly associated risk factors were elevated total cholesterol, hypertension, diabetes, low CD4 cell count, detectable HIV RNA, and a history of clinical AIDS diagnosis.
The most substantial PAF for each of the respective diseases was as follows: smoking for non–AIDS-related cancers (24%; 95% confidence interval, 13%-35%), elevated total cholesterol for myocardial infarction (44%; 95% CI, 30%-58%), and hepatitis C infection for end-stage liver disease (30%; 95% CI, 21%-39%). In addition, hypertension had the highest PAF for end-stage renal disease (39%; 95% CI, 26%-51%).
“Modifications to individual-level interventions and models of HIV care, and the implementation of structural and policy-level interventions that focus on prevention and modification of traditional risk factors are necessary to avoid noncommunicable diseases and preserve health among successfully antiretroviral-treated adults aging with HIV,” the researchers concluded.
The study was funded by the National Institutes of Health and the NA-ACCORD. Dr. Althoff reported having no relevant disclosures.
SOURCE: Althoff KN et al. The Lancet HIV. 2019 Jan 22. doi: 10.1016/S2352-3018(18)30295-9.
A substantial proportion of non–AIDS-defining cancers, and other noninfectious comorbid diseases, could be prevented with interventions on traditional risk factors in HIV-infected patients, according to the results of large database analysis published online in The Lancet HIV.
The researchers analyzed traditional and HIV-related risk factors for four validated noncommunicable disease outcomes (non–AIDS-defining cancers, myocardial infarction, end-stage liver disease, and end-stage renal disease) among participants of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), according to Keri N. Althoff, PhD, of Johns Hopkins University, Baltimore, and her colleagues on behalf of the NA-ACCORD.
The study comprised individuals with the assessed disease conditions from among more than 180,000 adults (aged 18 years or older) with HIV from more than 200 sites who had at least two care visits within 12 months. The researchers used a population attributable fraction (PAF) approach to quantify the proportion of noncommunicable diseases that could be eliminated if particular risk factors were not present. According to the researchers, PAF can be used to inform prioritization of interventions.
Dr. Althoff and her colleagues found that, for non–AIDS-defining cancer, the significant preventable or modifiable risk factors were smoking, low CD4 cell count, detectable HIV RNA, a history of clinical AIDS diagnosis, and hepatitis B infection.
For myocardial infarction, the significant factors were smoking, elevated total cholesterol, hypertension, stage 4 chronic kidney disease, a low CD4 cell count, detectable HIV RNA, and hepatitis C infection.
For end-stage liver disease, the significant factors were low CD4 cell count, detectable HIV RNA, a history of a clinical AIDS diagnosis, and hepatitis B or C infection.
For end-stage renal disease, the significantly associated risk factors were elevated total cholesterol, hypertension, diabetes, low CD4 cell count, detectable HIV RNA, and a history of clinical AIDS diagnosis.
The most substantial PAF for each of the respective diseases was as follows: smoking for non–AIDS-related cancers (24%; 95% confidence interval, 13%-35%), elevated total cholesterol for myocardial infarction (44%; 95% CI, 30%-58%), and hepatitis C infection for end-stage liver disease (30%; 95% CI, 21%-39%). In addition, hypertension had the highest PAF for end-stage renal disease (39%; 95% CI, 26%-51%).
“Modifications to individual-level interventions and models of HIV care, and the implementation of structural and policy-level interventions that focus on prevention and modification of traditional risk factors are necessary to avoid noncommunicable diseases and preserve health among successfully antiretroviral-treated adults aging with HIV,” the researchers concluded.
The study was funded by the National Institutes of Health and the NA-ACCORD. Dr. Althoff reported having no relevant disclosures.
SOURCE: Althoff KN et al. The Lancet HIV. 2019 Jan 22. doi: 10.1016/S2352-3018(18)30295-9.
FROM THE LANCET HIV
SABR response rate falls short in early NSCLC
For patients with resectable stage I non–small cell lung cancer (NSCLC), stereotactic ablative radiotherapy (SABR) may lack the efficacy needed to replace surgical intervention, according to a recent phase II trial.
SABR provided a pathologic complete response rate (pCR) of 60%, which was “lower than hypothesized,” reported lead author David A. Palma, MD, PhD, of London (Ont.) Health Sciences Centre, and his colleagues.
“In patients with cancer who are fit for resection ... the role of SABR is controversial,” the investigators wrote in JAMA Oncology. “Although some recent studies suggest that SABR may achieve outcomes similar to surgery, others do not, and randomized clinical trials are currently under way to compare these 2 modalities.”
The present trial involved 40 adult patients with stage I NSCLC, good pulmonary function, and good performance status. Of these, 35 were evaluable for the primary endpoint, which was tumor pCR rate after SABR. Secondary endpoints were toxic effects, quality of life, distant control, regional control, and local control. Patients with tumors no larger than 3 cm in diameter were given 54 Gy in 3 fractions, while bigger tumors received 55 Gy in 5 fractions. Patients with tumors 2 cm or closer to the brachial plexus or mediastinum were given 60 Gy in 8 fractions. Ten weeks after SABR, sublobar resection or lobectomy was performed, via an open approach or with video-assisted thoracoscopic surgery (VATS).
Analysis revealed a pCR rate of 60%, which was lower than anticipated. One-month and 3-month survival rates were both 100%. After a median follow-up of 19 months, local control rate was 100%, but only three out of four patients (76%) had distant control, and half (53%) had regional control. After 2 years, three out of four patients were still alive (77%). Eighteen percent of patients experienced grade 3 or higher toxic effects.
“[T]he pCR rate of 60% at 10 weeks suggests that practitioners should be cautious in the use of SABR in patients with cancers who are fit for resection,” the investigators concluded.
The Ontario Institute for Cancer Research funded the study. Dr. Palma and Dr. Ward are patent holders for a computed tomography technology that assesses responses after radiotherapy. Dr. Louie reported honoraria from AstraZeneca and Varian Medical Systems Inc.
SOURCE: Palma et al. 2019 Feb 21. doi: 10.1001/jamaoncol.2018.6993.
For patients with resectable stage I non–small cell lung cancer (NSCLC), stereotactic ablative radiotherapy (SABR) may lack the efficacy needed to replace surgical intervention, according to a recent phase II trial.
SABR provided a pathologic complete response rate (pCR) of 60%, which was “lower than hypothesized,” reported lead author David A. Palma, MD, PhD, of London (Ont.) Health Sciences Centre, and his colleagues.
“In patients with cancer who are fit for resection ... the role of SABR is controversial,” the investigators wrote in JAMA Oncology. “Although some recent studies suggest that SABR may achieve outcomes similar to surgery, others do not, and randomized clinical trials are currently under way to compare these 2 modalities.”
The present trial involved 40 adult patients with stage I NSCLC, good pulmonary function, and good performance status. Of these, 35 were evaluable for the primary endpoint, which was tumor pCR rate after SABR. Secondary endpoints were toxic effects, quality of life, distant control, regional control, and local control. Patients with tumors no larger than 3 cm in diameter were given 54 Gy in 3 fractions, while bigger tumors received 55 Gy in 5 fractions. Patients with tumors 2 cm or closer to the brachial plexus or mediastinum were given 60 Gy in 8 fractions. Ten weeks after SABR, sublobar resection or lobectomy was performed, via an open approach or with video-assisted thoracoscopic surgery (VATS).
Analysis revealed a pCR rate of 60%, which was lower than anticipated. One-month and 3-month survival rates were both 100%. After a median follow-up of 19 months, local control rate was 100%, but only three out of four patients (76%) had distant control, and half (53%) had regional control. After 2 years, three out of four patients were still alive (77%). Eighteen percent of patients experienced grade 3 or higher toxic effects.
“[T]he pCR rate of 60% at 10 weeks suggests that practitioners should be cautious in the use of SABR in patients with cancers who are fit for resection,” the investigators concluded.
The Ontario Institute for Cancer Research funded the study. Dr. Palma and Dr. Ward are patent holders for a computed tomography technology that assesses responses after radiotherapy. Dr. Louie reported honoraria from AstraZeneca and Varian Medical Systems Inc.
SOURCE: Palma et al. 2019 Feb 21. doi: 10.1001/jamaoncol.2018.6993.
For patients with resectable stage I non–small cell lung cancer (NSCLC), stereotactic ablative radiotherapy (SABR) may lack the efficacy needed to replace surgical intervention, according to a recent phase II trial.
SABR provided a pathologic complete response rate (pCR) of 60%, which was “lower than hypothesized,” reported lead author David A. Palma, MD, PhD, of London (Ont.) Health Sciences Centre, and his colleagues.
“In patients with cancer who are fit for resection ... the role of SABR is controversial,” the investigators wrote in JAMA Oncology. “Although some recent studies suggest that SABR may achieve outcomes similar to surgery, others do not, and randomized clinical trials are currently under way to compare these 2 modalities.”
The present trial involved 40 adult patients with stage I NSCLC, good pulmonary function, and good performance status. Of these, 35 were evaluable for the primary endpoint, which was tumor pCR rate after SABR. Secondary endpoints were toxic effects, quality of life, distant control, regional control, and local control. Patients with tumors no larger than 3 cm in diameter were given 54 Gy in 3 fractions, while bigger tumors received 55 Gy in 5 fractions. Patients with tumors 2 cm or closer to the brachial plexus or mediastinum were given 60 Gy in 8 fractions. Ten weeks after SABR, sublobar resection or lobectomy was performed, via an open approach or with video-assisted thoracoscopic surgery (VATS).
Analysis revealed a pCR rate of 60%, which was lower than anticipated. One-month and 3-month survival rates were both 100%. After a median follow-up of 19 months, local control rate was 100%, but only three out of four patients (76%) had distant control, and half (53%) had regional control. After 2 years, three out of four patients were still alive (77%). Eighteen percent of patients experienced grade 3 or higher toxic effects.
“[T]he pCR rate of 60% at 10 weeks suggests that practitioners should be cautious in the use of SABR in patients with cancers who are fit for resection,” the investigators concluded.
The Ontario Institute for Cancer Research funded the study. Dr. Palma and Dr. Ward are patent holders for a computed tomography technology that assesses responses after radiotherapy. Dr. Louie reported honoraria from AstraZeneca and Varian Medical Systems Inc.
SOURCE: Palma et al. 2019 Feb 21. doi: 10.1001/jamaoncol.2018.6993.
FROM JAMA ONCOLOGY
Checkpoint inhibitors ‘viable treatment option’ in HIV-infected individuals
Immune checkpoint inhibitors are safe and effective in HIV-infected patients with advanced cancers, according to authors of a recently published systematic review.
The treatment was well tolerated and associated with a 9% rate of grade 3 or higher immune-related adverse events, according to results of the review of 73 patient cases.
There were no adverse impacts on HIV load or CD4 cell count detected in the patients, according to researchers Michael R. Cook, MD, and Chul Kim, MD, MPH, of Georgetown University, Washington.
Antitumor activity of the checkpoint inhibitors in lung cancer patients was comparable to what has been seen in previous randomized clinical trials that excluded HIV-infected individuals, Dr. Cook and Dr. Kim reported in JAMA Oncology.
“Based on the results of the present systematic review, and in the absence of definitive prospective data suggesting an unfavorable risk-to-benefit ratio, immune checkpoint inhibitor therapy may be considered as a viable treatment option for HIV-infected patients with advanced cancer,” they said.
There are preclinical data suggesting that immune checkpoint modulation could improve function of HIV-specific T cells, the investigators added.
“Prospective trials of immune checkpoint inhibitors are necessary to elucidate the antiviral efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and cancer,” they said.
Several such trials are underway to evaluate the role of the pembrolizumab, nivolumab, nivolumab plus ipilimumab, and durvalumab in HIV-infected patients with advanced-stage cancers, according to the review authors.
In the present systematic review, Dr. Cook and Dr. Kim conducted a literature search and reviewed presentations from major annual medical conferences.
Of the 73 HIV-infected patients they identified, most had non–small cell lung cancer (34.2%), melanoma (21.9%), or Kaposi sarcoma (12.3%), while the rest had anal cancer, head and neck cancer, or other malignancies. Most patients had received either nivolumab (39.7%) or pembrolizumab (35.6%).
There were “no concerning findings” among these patients with regard to immune-mediated toxicities or changes in HIV-related parameters.
Six of 70 patients had immune-related adverse events of grade 3 or greater.
Thirty-four patients had documented HIV loads before and after receiving an immune checkpoint inhibitor. Of those, 28 had undetectable HIV loads at baseline, and all but 2 (7%) maintained undetectable loads in the posttreatment evaluation.
Of the remaining six with detectable HIV loads before treatment, five had a decrease in viral load, to the point that four had undetectable HIV viral load in the posttreatment evaluation, the investigators reported.
The overall response rate was 30% for the lung cancer patients, 27% for melanoma, and 63% for Kaposi sarcoma.
In the non–small cell lung cancer subset, response rates were 26% for those who had received previous systemic treatment, and 50% for those who had not, which was similar to findings from major checkpoint inhibitor trials that excluded HIV-infected individuals, the investigators said.
The American Society of Clinical Oncology Conquer Cancer Foundation and Georgetown University supported the study. Dr. Kim reported disclosures related to CARIS Life Science and AstraZeneca.
SOURCE: Cook MR and Kim C. JAMA Oncol. 2019 Feb 7. doi: 10.1001/jamaoncol.2018.6737.
Immune checkpoint inhibitors are safe and effective in HIV-infected patients with advanced cancers, according to authors of a recently published systematic review.
The treatment was well tolerated and associated with a 9% rate of grade 3 or higher immune-related adverse events, according to results of the review of 73 patient cases.
There were no adverse impacts on HIV load or CD4 cell count detected in the patients, according to researchers Michael R. Cook, MD, and Chul Kim, MD, MPH, of Georgetown University, Washington.
Antitumor activity of the checkpoint inhibitors in lung cancer patients was comparable to what has been seen in previous randomized clinical trials that excluded HIV-infected individuals, Dr. Cook and Dr. Kim reported in JAMA Oncology.
“Based on the results of the present systematic review, and in the absence of definitive prospective data suggesting an unfavorable risk-to-benefit ratio, immune checkpoint inhibitor therapy may be considered as a viable treatment option for HIV-infected patients with advanced cancer,” they said.
There are preclinical data suggesting that immune checkpoint modulation could improve function of HIV-specific T cells, the investigators added.
“Prospective trials of immune checkpoint inhibitors are necessary to elucidate the antiviral efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and cancer,” they said.
Several such trials are underway to evaluate the role of the pembrolizumab, nivolumab, nivolumab plus ipilimumab, and durvalumab in HIV-infected patients with advanced-stage cancers, according to the review authors.
In the present systematic review, Dr. Cook and Dr. Kim conducted a literature search and reviewed presentations from major annual medical conferences.
Of the 73 HIV-infected patients they identified, most had non–small cell lung cancer (34.2%), melanoma (21.9%), or Kaposi sarcoma (12.3%), while the rest had anal cancer, head and neck cancer, or other malignancies. Most patients had received either nivolumab (39.7%) or pembrolizumab (35.6%).
There were “no concerning findings” among these patients with regard to immune-mediated toxicities or changes in HIV-related parameters.
Six of 70 patients had immune-related adverse events of grade 3 or greater.
Thirty-four patients had documented HIV loads before and after receiving an immune checkpoint inhibitor. Of those, 28 had undetectable HIV loads at baseline, and all but 2 (7%) maintained undetectable loads in the posttreatment evaluation.
Of the remaining six with detectable HIV loads before treatment, five had a decrease in viral load, to the point that four had undetectable HIV viral load in the posttreatment evaluation, the investigators reported.
The overall response rate was 30% for the lung cancer patients, 27% for melanoma, and 63% for Kaposi sarcoma.
In the non–small cell lung cancer subset, response rates were 26% for those who had received previous systemic treatment, and 50% for those who had not, which was similar to findings from major checkpoint inhibitor trials that excluded HIV-infected individuals, the investigators said.
The American Society of Clinical Oncology Conquer Cancer Foundation and Georgetown University supported the study. Dr. Kim reported disclosures related to CARIS Life Science and AstraZeneca.
SOURCE: Cook MR and Kim C. JAMA Oncol. 2019 Feb 7. doi: 10.1001/jamaoncol.2018.6737.
Immune checkpoint inhibitors are safe and effective in HIV-infected patients with advanced cancers, according to authors of a recently published systematic review.
The treatment was well tolerated and associated with a 9% rate of grade 3 or higher immune-related adverse events, according to results of the review of 73 patient cases.
There were no adverse impacts on HIV load or CD4 cell count detected in the patients, according to researchers Michael R. Cook, MD, and Chul Kim, MD, MPH, of Georgetown University, Washington.
Antitumor activity of the checkpoint inhibitors in lung cancer patients was comparable to what has been seen in previous randomized clinical trials that excluded HIV-infected individuals, Dr. Cook and Dr. Kim reported in JAMA Oncology.
“Based on the results of the present systematic review, and in the absence of definitive prospective data suggesting an unfavorable risk-to-benefit ratio, immune checkpoint inhibitor therapy may be considered as a viable treatment option for HIV-infected patients with advanced cancer,” they said.
There are preclinical data suggesting that immune checkpoint modulation could improve function of HIV-specific T cells, the investigators added.
“Prospective trials of immune checkpoint inhibitors are necessary to elucidate the antiviral efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and cancer,” they said.
Several such trials are underway to evaluate the role of the pembrolizumab, nivolumab, nivolumab plus ipilimumab, and durvalumab in HIV-infected patients with advanced-stage cancers, according to the review authors.
In the present systematic review, Dr. Cook and Dr. Kim conducted a literature search and reviewed presentations from major annual medical conferences.
Of the 73 HIV-infected patients they identified, most had non–small cell lung cancer (34.2%), melanoma (21.9%), or Kaposi sarcoma (12.3%), while the rest had anal cancer, head and neck cancer, or other malignancies. Most patients had received either nivolumab (39.7%) or pembrolizumab (35.6%).
There were “no concerning findings” among these patients with regard to immune-mediated toxicities or changes in HIV-related parameters.
Six of 70 patients had immune-related adverse events of grade 3 or greater.
Thirty-four patients had documented HIV loads before and after receiving an immune checkpoint inhibitor. Of those, 28 had undetectable HIV loads at baseline, and all but 2 (7%) maintained undetectable loads in the posttreatment evaluation.
Of the remaining six with detectable HIV loads before treatment, five had a decrease in viral load, to the point that four had undetectable HIV viral load in the posttreatment evaluation, the investigators reported.
The overall response rate was 30% for the lung cancer patients, 27% for melanoma, and 63% for Kaposi sarcoma.
In the non–small cell lung cancer subset, response rates were 26% for those who had received previous systemic treatment, and 50% for those who had not, which was similar to findings from major checkpoint inhibitor trials that excluded HIV-infected individuals, the investigators said.
The American Society of Clinical Oncology Conquer Cancer Foundation and Georgetown University supported the study. Dr. Kim reported disclosures related to CARIS Life Science and AstraZeneca.
SOURCE: Cook MR and Kim C. JAMA Oncol. 2019 Feb 7. doi: 10.1001/jamaoncol.2018.6737.
FROM JAMA ONCOLOGY
Key clinical point: Immune checkpoint inhibitors are a viable treatment option for HIV-infected patients, according to data supporting their safety and efficacy in this patient population.
Major finding: The treatment was well tolerated, with an 8.6% rate of grade 3 or greater immune-related adverse events, and no impact on HIV-related parameters.
Study details: A systematic review of 73 patients with HIV infection who had received treatment with a checkpoint inhibitor.
Disclosures: The American Society of Clinical Oncology Conquer Cancer Foundation and Georgetown University supported the study. One study author reported disclosures related to CARIS Life Science and AstraZeneca.
Source: Cook MR and Kim C. JAMA Oncol. 2019 Feb 7. doi: 10.1001/jamaoncol.2018.6737.