User login
First-in-class glutaminase inhibitor combats anti-PD-1/PD-L1 resistance
NATIONAL HARBOR, MD. – Combination treatment with the first-in-class glutaminase inhibitor CB-839 and nivolumab is well-tolerated and shows clinical activity in patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer, including anti-PD-1/PD-L1 refractory patients, according to initial results from a phase 1/2 study.
Responses in melanoma patients who were progressing on nivolumab at study entry and who were refractory to multiple prior immunotherapy regimens are particularly notable, as they highlight the potential for CB-839, when added to nivolumab (Opdivo), to help overcome resistance to anti-PD-L1 therapy, Funda Meric‐Bernstam, MD, reported at the annual meeting of the Society for Immunotherapy of Cancer.
CB‐839 is highly selective and targets tumor glutamine metabolism, said Dr. Meric-Bernstam of the University of Texas MD Anderson Cancer Center, Houston.
Competition between tumor cells and immune cells for nutrients such as glutamine in the tumor microenvironment can create a metabolic checkpoint that induces local immune suppression. CB‐839 inhibits tumor glutamine consumption, thereby increasing glutamine availability to support T‐cell activity, she explained, noting that in preclinical models, CB‐839 increased intra‐tumoral glutamine and enhanced antitumor activity of PD‐1/PD‐L1 inhibitors.
In the phase 1 dose escalation study, she and her colleagues evaluated the safety and efficacy of CB-839 in combination with the PD‐1 inhibitor nivolumab in patients with melanoma, non-small cell lung cancer (NSCLC), or renal cell carcinoma (RCC). Phase 2 expansion cohorts include a melanoma rescue cohort of patients progressing on anti-PD-L1 therapy at study entry (22 patients), an NSCLC and RCC rescue cohort of patients who were progressing on anti-PD-L1 therapy at study entry or who had stable disease for 6 months or longer without a response (11 NSCLC and 11 RCC), an RCC cohort of patients with prior immunotherapy exposure and no response (10 patients), and an RCC cohort of patents who had no prior immunotherapy exposure (28 patients).
During dose escalation, patients received oral CB‐839 at 600 mg or 800 mg twice daily in combination with standard‐dose nivolumab. In the ongoing phase 2 expansion study, which continues to enroll, patients are receiving 800 mg of CB-839 twice daily with standard‐dose nivolumab, Dr. Meric-Bernstam said.
Patients in each of the cohorts were high risk and/or had intermediate or poor prognostic status at study entry. For example, 50% of patients in the melanoma rescue cohort had liver metastases, 77% had other visceral metastases, and 18% had brain metastases, and the majority of patients in the lung cancer/RCC cohort had visceral metastases. Most had progressive disease as their best response on their last line of immunotherapy.
Of 16 response-evaluable melanoma patients, 1 experienced a complete response, 2 had partial responses, and 4 had stable disease.
“So overall in this patient population that was progressing on a PD-1/PD-L1 inhibitor at enrollment, 19% had an objective response. The disease control rate in this group was 44%,” she said.
In evaluable patients in the lung cancer rescue cohort (6 patients), RCC rescue cohort (8 patients), and RCC prior exposure cohort (7 patients), disease control rates ranged from 57% to 75%, and in the immunotherapy-naive RCC cohort (19 patients), the partial response rate was 21%, and 53% had stable disease, so the overall disease control rate was 74%. Half of the patients in that group remain on study, she noted.
A closer look at the melanoma rescue cohort showed dramatic and rapid responses in two patients who each achieved a partial response in about 8 weeks with response durations of 3.7 months and 5.4 months, respectively. Additionally, pre-treatment biopsies in this cohort showed an elevated T-cell inflamed signature associated with clinical benefit from the addition of CB-839, and in one patient who had both a pretreatment and on-treatment biopsy that was evaluable, the latter showed an increase in T-cell inflamed signature and T-cell effector genes.
In all cohorts, the combination therapy was generally well tolerated. A maximum tolerated dose was not reached. Dose-limiting toxicity – a grade 3 alanine aminotransferase (ALT) increase – occurred in one patient on the 800-mg dose. The most common grade 3 or greater adverse events were fatigue, nausea, photophobia, rash, and elevated ALT, she said, noting that two patients discontinued for treatment-related adverse events (one for a grade 3 rash and one for grade 2 pneumonitis).
“Overall there appeared to be no apparent increase in immune-related adverse events, either in rate or severity, compared with [nivolumab] monotherapy,” she said.
The combination of CB-839 and nivolumab was well tolerated, and in some patients – as seen in the melanoma cohort – adding CB-839 to checkpoint blockade can overcome checkpoint blockade resistance, Dr. Meric-Bernstam concluded, noting that the disease control rates seen in the majority of lung cancer and RCC patients who were progressing on checkpoint blockade is encouraging, as is the objective response rate seen thus far in the RCC therapy-naive patients, and the stable and deep responses seen in the melanoma rescue cohort.
“Based on our encouraging signal in the melanoma rescue cohort, this [cohort] has been expanded,” she said.
Calithera Biosciences sponsored the study. Bristol-Myers Squibb provided nivolumab for the study. Dr. Meric-Bernstam has received grant or research support from Calithera Biosciences and many other companies. She also reported being a paid consultant for several companies and serving on an advisory committee or review panel, or as a board member for multiple companies.
NATIONAL HARBOR, MD. – Combination treatment with the first-in-class glutaminase inhibitor CB-839 and nivolumab is well-tolerated and shows clinical activity in patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer, including anti-PD-1/PD-L1 refractory patients, according to initial results from a phase 1/2 study.
Responses in melanoma patients who were progressing on nivolumab at study entry and who were refractory to multiple prior immunotherapy regimens are particularly notable, as they highlight the potential for CB-839, when added to nivolumab (Opdivo), to help overcome resistance to anti-PD-L1 therapy, Funda Meric‐Bernstam, MD, reported at the annual meeting of the Society for Immunotherapy of Cancer.
CB‐839 is highly selective and targets tumor glutamine metabolism, said Dr. Meric-Bernstam of the University of Texas MD Anderson Cancer Center, Houston.
Competition between tumor cells and immune cells for nutrients such as glutamine in the tumor microenvironment can create a metabolic checkpoint that induces local immune suppression. CB‐839 inhibits tumor glutamine consumption, thereby increasing glutamine availability to support T‐cell activity, she explained, noting that in preclinical models, CB‐839 increased intra‐tumoral glutamine and enhanced antitumor activity of PD‐1/PD‐L1 inhibitors.
In the phase 1 dose escalation study, she and her colleagues evaluated the safety and efficacy of CB-839 in combination with the PD‐1 inhibitor nivolumab in patients with melanoma, non-small cell lung cancer (NSCLC), or renal cell carcinoma (RCC). Phase 2 expansion cohorts include a melanoma rescue cohort of patients progressing on anti-PD-L1 therapy at study entry (22 patients), an NSCLC and RCC rescue cohort of patients who were progressing on anti-PD-L1 therapy at study entry or who had stable disease for 6 months or longer without a response (11 NSCLC and 11 RCC), an RCC cohort of patients with prior immunotherapy exposure and no response (10 patients), and an RCC cohort of patents who had no prior immunotherapy exposure (28 patients).
During dose escalation, patients received oral CB‐839 at 600 mg or 800 mg twice daily in combination with standard‐dose nivolumab. In the ongoing phase 2 expansion study, which continues to enroll, patients are receiving 800 mg of CB-839 twice daily with standard‐dose nivolumab, Dr. Meric-Bernstam said.
Patients in each of the cohorts were high risk and/or had intermediate or poor prognostic status at study entry. For example, 50% of patients in the melanoma rescue cohort had liver metastases, 77% had other visceral metastases, and 18% had brain metastases, and the majority of patients in the lung cancer/RCC cohort had visceral metastases. Most had progressive disease as their best response on their last line of immunotherapy.
Of 16 response-evaluable melanoma patients, 1 experienced a complete response, 2 had partial responses, and 4 had stable disease.
“So overall in this patient population that was progressing on a PD-1/PD-L1 inhibitor at enrollment, 19% had an objective response. The disease control rate in this group was 44%,” she said.
In evaluable patients in the lung cancer rescue cohort (6 patients), RCC rescue cohort (8 patients), and RCC prior exposure cohort (7 patients), disease control rates ranged from 57% to 75%, and in the immunotherapy-naive RCC cohort (19 patients), the partial response rate was 21%, and 53% had stable disease, so the overall disease control rate was 74%. Half of the patients in that group remain on study, she noted.
A closer look at the melanoma rescue cohort showed dramatic and rapid responses in two patients who each achieved a partial response in about 8 weeks with response durations of 3.7 months and 5.4 months, respectively. Additionally, pre-treatment biopsies in this cohort showed an elevated T-cell inflamed signature associated with clinical benefit from the addition of CB-839, and in one patient who had both a pretreatment and on-treatment biopsy that was evaluable, the latter showed an increase in T-cell inflamed signature and T-cell effector genes.
In all cohorts, the combination therapy was generally well tolerated. A maximum tolerated dose was not reached. Dose-limiting toxicity – a grade 3 alanine aminotransferase (ALT) increase – occurred in one patient on the 800-mg dose. The most common grade 3 or greater adverse events were fatigue, nausea, photophobia, rash, and elevated ALT, she said, noting that two patients discontinued for treatment-related adverse events (one for a grade 3 rash and one for grade 2 pneumonitis).
“Overall there appeared to be no apparent increase in immune-related adverse events, either in rate or severity, compared with [nivolumab] monotherapy,” she said.
The combination of CB-839 and nivolumab was well tolerated, and in some patients – as seen in the melanoma cohort – adding CB-839 to checkpoint blockade can overcome checkpoint blockade resistance, Dr. Meric-Bernstam concluded, noting that the disease control rates seen in the majority of lung cancer and RCC patients who were progressing on checkpoint blockade is encouraging, as is the objective response rate seen thus far in the RCC therapy-naive patients, and the stable and deep responses seen in the melanoma rescue cohort.
“Based on our encouraging signal in the melanoma rescue cohort, this [cohort] has been expanded,” she said.
Calithera Biosciences sponsored the study. Bristol-Myers Squibb provided nivolumab for the study. Dr. Meric-Bernstam has received grant or research support from Calithera Biosciences and many other companies. She also reported being a paid consultant for several companies and serving on an advisory committee or review panel, or as a board member for multiple companies.
NATIONAL HARBOR, MD. – Combination treatment with the first-in-class glutaminase inhibitor CB-839 and nivolumab is well-tolerated and shows clinical activity in patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer, including anti-PD-1/PD-L1 refractory patients, according to initial results from a phase 1/2 study.
Responses in melanoma patients who were progressing on nivolumab at study entry and who were refractory to multiple prior immunotherapy regimens are particularly notable, as they highlight the potential for CB-839, when added to nivolumab (Opdivo), to help overcome resistance to anti-PD-L1 therapy, Funda Meric‐Bernstam, MD, reported at the annual meeting of the Society for Immunotherapy of Cancer.
CB‐839 is highly selective and targets tumor glutamine metabolism, said Dr. Meric-Bernstam of the University of Texas MD Anderson Cancer Center, Houston.
Competition between tumor cells and immune cells for nutrients such as glutamine in the tumor microenvironment can create a metabolic checkpoint that induces local immune suppression. CB‐839 inhibits tumor glutamine consumption, thereby increasing glutamine availability to support T‐cell activity, she explained, noting that in preclinical models, CB‐839 increased intra‐tumoral glutamine and enhanced antitumor activity of PD‐1/PD‐L1 inhibitors.
In the phase 1 dose escalation study, she and her colleagues evaluated the safety and efficacy of CB-839 in combination with the PD‐1 inhibitor nivolumab in patients with melanoma, non-small cell lung cancer (NSCLC), or renal cell carcinoma (RCC). Phase 2 expansion cohorts include a melanoma rescue cohort of patients progressing on anti-PD-L1 therapy at study entry (22 patients), an NSCLC and RCC rescue cohort of patients who were progressing on anti-PD-L1 therapy at study entry or who had stable disease for 6 months or longer without a response (11 NSCLC and 11 RCC), an RCC cohort of patients with prior immunotherapy exposure and no response (10 patients), and an RCC cohort of patents who had no prior immunotherapy exposure (28 patients).
During dose escalation, patients received oral CB‐839 at 600 mg or 800 mg twice daily in combination with standard‐dose nivolumab. In the ongoing phase 2 expansion study, which continues to enroll, patients are receiving 800 mg of CB-839 twice daily with standard‐dose nivolumab, Dr. Meric-Bernstam said.
Patients in each of the cohorts were high risk and/or had intermediate or poor prognostic status at study entry. For example, 50% of patients in the melanoma rescue cohort had liver metastases, 77% had other visceral metastases, and 18% had brain metastases, and the majority of patients in the lung cancer/RCC cohort had visceral metastases. Most had progressive disease as their best response on their last line of immunotherapy.
Of 16 response-evaluable melanoma patients, 1 experienced a complete response, 2 had partial responses, and 4 had stable disease.
“So overall in this patient population that was progressing on a PD-1/PD-L1 inhibitor at enrollment, 19% had an objective response. The disease control rate in this group was 44%,” she said.
In evaluable patients in the lung cancer rescue cohort (6 patients), RCC rescue cohort (8 patients), and RCC prior exposure cohort (7 patients), disease control rates ranged from 57% to 75%, and in the immunotherapy-naive RCC cohort (19 patients), the partial response rate was 21%, and 53% had stable disease, so the overall disease control rate was 74%. Half of the patients in that group remain on study, she noted.
A closer look at the melanoma rescue cohort showed dramatic and rapid responses in two patients who each achieved a partial response in about 8 weeks with response durations of 3.7 months and 5.4 months, respectively. Additionally, pre-treatment biopsies in this cohort showed an elevated T-cell inflamed signature associated with clinical benefit from the addition of CB-839, and in one patient who had both a pretreatment and on-treatment biopsy that was evaluable, the latter showed an increase in T-cell inflamed signature and T-cell effector genes.
In all cohorts, the combination therapy was generally well tolerated. A maximum tolerated dose was not reached. Dose-limiting toxicity – a grade 3 alanine aminotransferase (ALT) increase – occurred in one patient on the 800-mg dose. The most common grade 3 or greater adverse events were fatigue, nausea, photophobia, rash, and elevated ALT, she said, noting that two patients discontinued for treatment-related adverse events (one for a grade 3 rash and one for grade 2 pneumonitis).
“Overall there appeared to be no apparent increase in immune-related adverse events, either in rate or severity, compared with [nivolumab] monotherapy,” she said.
The combination of CB-839 and nivolumab was well tolerated, and in some patients – as seen in the melanoma cohort – adding CB-839 to checkpoint blockade can overcome checkpoint blockade resistance, Dr. Meric-Bernstam concluded, noting that the disease control rates seen in the majority of lung cancer and RCC patients who were progressing on checkpoint blockade is encouraging, as is the objective response rate seen thus far in the RCC therapy-naive patients, and the stable and deep responses seen in the melanoma rescue cohort.
“Based on our encouraging signal in the melanoma rescue cohort, this [cohort] has been expanded,” she said.
Calithera Biosciences sponsored the study. Bristol-Myers Squibb provided nivolumab for the study. Dr. Meric-Bernstam has received grant or research support from Calithera Biosciences and many other companies. She also reported being a paid consultant for several companies and serving on an advisory committee or review panel, or as a board member for multiple companies.
AT SITC 2017
Key clinical point:
Major finding: The objective response rate in advanced melanoma patients refractory to anti-PD-1/PD-L1 therapy was 19%.
Data source: A phase 1/2 study of 82 patients.
Disclosures: Calithera Biosciences sponsored the study. Bristol-Myers Squibb provided nivolumab for the study. Dr. Meric-Bernstam has received grant or research support from Calithera Biosciences and many other companies. She also reported being a paid consultant for several companies and serving on an advisory committee or review panel or as a board member for multiple companies.
The Effects of Sunscreen on Marine Environments
Coastal travel accounts for 80% of all tourism worldwide, a number that continues to grow. The number of travelers to the Mediterranean Sea alone is expected to rise to 350 million individuals per year within the next 20 years.1 As the number of tourists visiting the world’s oceans increases, the rate of sunscreen unintentionally washed into these marine environments also rises. One study estimated that approximately one-quarter of the sunscreen applied to the skin is washed off over a 20-minute period spent in the water.2 Four of the most common sunscreen agents—benzophenone-3 (BP-3),
Benzophenone-3
4-Methylbenzylidene Camphor
Environmental concerns have also been raised about another common chemical UV filter: 4-MBC, or enzacamene. In laboratory studies, 4-MBC has been shown to cause oxidative stress to Tetrahymena thermophila, an aquatic protozoan, which results in inhibited growth. At higher concentrations, damage to the cellular membrane was seen as soon as 4 hours after exposure.6 In embryonic zebrafish, elevated 4-MBC levels were correlated to improper nerve and muscular development, resulting in developmental defects.7 Another study demonstrated that 4-MBC was toxic to Mytilus galloprovincialis, known as the Mediterranean mussel, and Paracentrotus lividus, a species of sea urchin.8 Although these studies utilized highly controlled laboratory settings, further studies are needed to examine the effects of 4-MBC on these species at environmentally relevant concentrations.
Physical Sunscreens
Physical sunscreens, as compared to the chemical filters referenced above, use either zinc or titanium to protect the skin from the sun’s rays. Nanoparticles, in particular, are preferred because they do not leave a white film on the skin.9 Both titanium dioxide and zinc oxide nanoparticles have been found to inhibit the growth and photosynthesis of marine phytoplankton, the most abundant primary producers on Earth.10,11 These metal contaminants can be transferred to organisms of higher trophic levels, including zooplankton,12 and filter-feeding organisms, including marine abalone13 and the Mediterranean mussel.14 These nanoparticles have been shown to cause oxidative stress to these organisms, making them less fit to withstand environmental stressors. It is difficult to show their true impact, however, as it is challenging to accurately detect and quantify nanoparticle concentrations in vivo.15
Final Thoughts
- Marine problems: tourism & coastal development. World Wide Fund for Nature website. http://wwf.panda.org/about_our_earth/blue_planet/problems/tourism/. Published 2017. Accessed November 14, 2017.
- Danovaro R, Bongiorni L, Corinaldesi C, et al. Sunscreens cause coral bleaching by promoting viral infections. Environ Health Perspect. 2008;116:441-447.
- Downs C, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- Sánchez Rodríguez A, Rodrigo Sanz M, Betancort Rodríguez JR. Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands)[published online March 17, 2015]. Chemosphere. 2015;131:85-90.
- Bratkovics S, Sapozhnikova Y. Determination of seven commonly used organic UV filters in fresh and saline waters by liquid chromatography-tandem mass spectrometry. Analytical Methods. 2011;3:2943-2950.
- Gao L, Yuan T, Zhou C, et al. Effects of four commonly used UV filters on the growth, cell viability and oxidative stress responses of the Tetrahymena thermophila. Chemosphere. 2013;93:2507-2513.
- Li VW, Tsui MP, Chen X, et al. Effects of 4-methylbenzylidene camphor (4-MBC) on neuronal and muscular development in zebrafish (Danio rerio) embryos [published online February 18, 2016]. Environ Sci Pollut Res Int. 2016;23:8275-8285.
- Paredes E, Perez S, Rodil R, et al. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere. 2014;104:44-50.
- Osterwalder U, Sohn M, Herzog B. Global state of sunscreens. Photodermatol Photoimmunol Photomed. 2014;30:62-80.
- Miller RJ, Bennett S, Keller AA, et al. TiO2 nanoparticles are phototoxic to marine phytoplankton. PloS One. 2012;7:E30321.
- Spisni E. Toxicity Assessment of Industrial- and Sunscreen-derived ZnO Nanoparticles [master’s thesis]. Coral Gables, FL: University of Miami Libraries Scholarly Repository; 2016. http://scholarlyrepository.miami.edu/cgi/viewcontent.cgi?article=1625&context=oa_theses. Accessed November 10, 2017.
- Jarvis TA, Miller RJ, Lenihan HS, et al. Toxicity of ZnO nanoparticles to the copepod Acartia tonsa, exposed through a phytoplankton diet [published online April 15, 2013]. Environ Toxicol Chem. 2013;32:1264-1269.
- Zhu X, Zhou J, Cai Z. The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta). Mar Pollut Bull. 2011;63:334-338.
- Barmo C, Ciacci C, Canonico B, et al. In vivo effects of n-TiO2 on digestive gland and immune function of the marine bivalve Mytilus galloprovincialis. Aquatic Toxicol. 2013;132:9-18.
- Sánchez-Quiles D, Tovar-Sánchez A. Are sunscreens a new environmental risk associated with coastal tourism? Environ Int. 2015;83:158-170.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Vesper I. Hawaii seeks to ban ‘reef-unfriendly’ sunscreen. Nature. February 3, 2017. https://www.nature.com/news/hawaii-seeks-to-ban-reef-unfriendly-sunscreen-1.21332. Accessed November 16, 2017.
Coastal travel accounts for 80% of all tourism worldwide, a number that continues to grow. The number of travelers to the Mediterranean Sea alone is expected to rise to 350 million individuals per year within the next 20 years.1 As the number of tourists visiting the world’s oceans increases, the rate of sunscreen unintentionally washed into these marine environments also rises. One study estimated that approximately one-quarter of the sunscreen applied to the skin is washed off over a 20-minute period spent in the water.2 Four of the most common sunscreen agents—benzophenone-3 (BP-3),
Benzophenone-3
4-Methylbenzylidene Camphor
Environmental concerns have also been raised about another common chemical UV filter: 4-MBC, or enzacamene. In laboratory studies, 4-MBC has been shown to cause oxidative stress to Tetrahymena thermophila, an aquatic protozoan, which results in inhibited growth. At higher concentrations, damage to the cellular membrane was seen as soon as 4 hours after exposure.6 In embryonic zebrafish, elevated 4-MBC levels were correlated to improper nerve and muscular development, resulting in developmental defects.7 Another study demonstrated that 4-MBC was toxic to Mytilus galloprovincialis, known as the Mediterranean mussel, and Paracentrotus lividus, a species of sea urchin.8 Although these studies utilized highly controlled laboratory settings, further studies are needed to examine the effects of 4-MBC on these species at environmentally relevant concentrations.
Physical Sunscreens
Physical sunscreens, as compared to the chemical filters referenced above, use either zinc or titanium to protect the skin from the sun’s rays. Nanoparticles, in particular, are preferred because they do not leave a white film on the skin.9 Both titanium dioxide and zinc oxide nanoparticles have been found to inhibit the growth and photosynthesis of marine phytoplankton, the most abundant primary producers on Earth.10,11 These metal contaminants can be transferred to organisms of higher trophic levels, including zooplankton,12 and filter-feeding organisms, including marine abalone13 and the Mediterranean mussel.14 These nanoparticles have been shown to cause oxidative stress to these organisms, making them less fit to withstand environmental stressors. It is difficult to show their true impact, however, as it is challenging to accurately detect and quantify nanoparticle concentrations in vivo.15
Final Thoughts
Coastal travel accounts for 80% of all tourism worldwide, a number that continues to grow. The number of travelers to the Mediterranean Sea alone is expected to rise to 350 million individuals per year within the next 20 years.1 As the number of tourists visiting the world’s oceans increases, the rate of sunscreen unintentionally washed into these marine environments also rises. One study estimated that approximately one-quarter of the sunscreen applied to the skin is washed off over a 20-minute period spent in the water.2 Four of the most common sunscreen agents—benzophenone-3 (BP-3),
Benzophenone-3
4-Methylbenzylidene Camphor
Environmental concerns have also been raised about another common chemical UV filter: 4-MBC, or enzacamene. In laboratory studies, 4-MBC has been shown to cause oxidative stress to Tetrahymena thermophila, an aquatic protozoan, which results in inhibited growth. At higher concentrations, damage to the cellular membrane was seen as soon as 4 hours after exposure.6 In embryonic zebrafish, elevated 4-MBC levels were correlated to improper nerve and muscular development, resulting in developmental defects.7 Another study demonstrated that 4-MBC was toxic to Mytilus galloprovincialis, known as the Mediterranean mussel, and Paracentrotus lividus, a species of sea urchin.8 Although these studies utilized highly controlled laboratory settings, further studies are needed to examine the effects of 4-MBC on these species at environmentally relevant concentrations.
Physical Sunscreens
Physical sunscreens, as compared to the chemical filters referenced above, use either zinc or titanium to protect the skin from the sun’s rays. Nanoparticles, in particular, are preferred because they do not leave a white film on the skin.9 Both titanium dioxide and zinc oxide nanoparticles have been found to inhibit the growth and photosynthesis of marine phytoplankton, the most abundant primary producers on Earth.10,11 These metal contaminants can be transferred to organisms of higher trophic levels, including zooplankton,12 and filter-feeding organisms, including marine abalone13 and the Mediterranean mussel.14 These nanoparticles have been shown to cause oxidative stress to these organisms, making them less fit to withstand environmental stressors. It is difficult to show their true impact, however, as it is challenging to accurately detect and quantify nanoparticle concentrations in vivo.15
Final Thoughts
- Marine problems: tourism & coastal development. World Wide Fund for Nature website. http://wwf.panda.org/about_our_earth/blue_planet/problems/tourism/. Published 2017. Accessed November 14, 2017.
- Danovaro R, Bongiorni L, Corinaldesi C, et al. Sunscreens cause coral bleaching by promoting viral infections. Environ Health Perspect. 2008;116:441-447.
- Downs C, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- Sánchez Rodríguez A, Rodrigo Sanz M, Betancort Rodríguez JR. Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands)[published online March 17, 2015]. Chemosphere. 2015;131:85-90.
- Bratkovics S, Sapozhnikova Y. Determination of seven commonly used organic UV filters in fresh and saline waters by liquid chromatography-tandem mass spectrometry. Analytical Methods. 2011;3:2943-2950.
- Gao L, Yuan T, Zhou C, et al. Effects of four commonly used UV filters on the growth, cell viability and oxidative stress responses of the Tetrahymena thermophila. Chemosphere. 2013;93:2507-2513.
- Li VW, Tsui MP, Chen X, et al. Effects of 4-methylbenzylidene camphor (4-MBC) on neuronal and muscular development in zebrafish (Danio rerio) embryos [published online February 18, 2016]. Environ Sci Pollut Res Int. 2016;23:8275-8285.
- Paredes E, Perez S, Rodil R, et al. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere. 2014;104:44-50.
- Osterwalder U, Sohn M, Herzog B. Global state of sunscreens. Photodermatol Photoimmunol Photomed. 2014;30:62-80.
- Miller RJ, Bennett S, Keller AA, et al. TiO2 nanoparticles are phototoxic to marine phytoplankton. PloS One. 2012;7:E30321.
- Spisni E. Toxicity Assessment of Industrial- and Sunscreen-derived ZnO Nanoparticles [master’s thesis]. Coral Gables, FL: University of Miami Libraries Scholarly Repository; 2016. http://scholarlyrepository.miami.edu/cgi/viewcontent.cgi?article=1625&context=oa_theses. Accessed November 10, 2017.
- Jarvis TA, Miller RJ, Lenihan HS, et al. Toxicity of ZnO nanoparticles to the copepod Acartia tonsa, exposed through a phytoplankton diet [published online April 15, 2013]. Environ Toxicol Chem. 2013;32:1264-1269.
- Zhu X, Zhou J, Cai Z. The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta). Mar Pollut Bull. 2011;63:334-338.
- Barmo C, Ciacci C, Canonico B, et al. In vivo effects of n-TiO2 on digestive gland and immune function of the marine bivalve Mytilus galloprovincialis. Aquatic Toxicol. 2013;132:9-18.
- Sánchez-Quiles D, Tovar-Sánchez A. Are sunscreens a new environmental risk associated with coastal tourism? Environ Int. 2015;83:158-170.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Vesper I. Hawaii seeks to ban ‘reef-unfriendly’ sunscreen. Nature. February 3, 2017. https://www.nature.com/news/hawaii-seeks-to-ban-reef-unfriendly-sunscreen-1.21332. Accessed November 16, 2017.
- Marine problems: tourism & coastal development. World Wide Fund for Nature website. http://wwf.panda.org/about_our_earth/blue_planet/problems/tourism/. Published 2017. Accessed November 14, 2017.
- Danovaro R, Bongiorni L, Corinaldesi C, et al. Sunscreens cause coral bleaching by promoting viral infections. Environ Health Perspect. 2008;116:441-447.
- Downs C, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- Sánchez Rodríguez A, Rodrigo Sanz M, Betancort Rodríguez JR. Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands)[published online March 17, 2015]. Chemosphere. 2015;131:85-90.
- Bratkovics S, Sapozhnikova Y. Determination of seven commonly used organic UV filters in fresh and saline waters by liquid chromatography-tandem mass spectrometry. Analytical Methods. 2011;3:2943-2950.
- Gao L, Yuan T, Zhou C, et al. Effects of four commonly used UV filters on the growth, cell viability and oxidative stress responses of the Tetrahymena thermophila. Chemosphere. 2013;93:2507-2513.
- Li VW, Tsui MP, Chen X, et al. Effects of 4-methylbenzylidene camphor (4-MBC) on neuronal and muscular development in zebrafish (Danio rerio) embryos [published online February 18, 2016]. Environ Sci Pollut Res Int. 2016;23:8275-8285.
- Paredes E, Perez S, Rodil R, et al. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere. 2014;104:44-50.
- Osterwalder U, Sohn M, Herzog B. Global state of sunscreens. Photodermatol Photoimmunol Photomed. 2014;30:62-80.
- Miller RJ, Bennett S, Keller AA, et al. TiO2 nanoparticles are phototoxic to marine phytoplankton. PloS One. 2012;7:E30321.
- Spisni E. Toxicity Assessment of Industrial- and Sunscreen-derived ZnO Nanoparticles [master’s thesis]. Coral Gables, FL: University of Miami Libraries Scholarly Repository; 2016. http://scholarlyrepository.miami.edu/cgi/viewcontent.cgi?article=1625&context=oa_theses. Accessed November 10, 2017.
- Jarvis TA, Miller RJ, Lenihan HS, et al. Toxicity of ZnO nanoparticles to the copepod Acartia tonsa, exposed through a phytoplankton diet [published online April 15, 2013]. Environ Toxicol Chem. 2013;32:1264-1269.
- Zhu X, Zhou J, Cai Z. The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta). Mar Pollut Bull. 2011;63:334-338.
- Barmo C, Ciacci C, Canonico B, et al. In vivo effects of n-TiO2 on digestive gland and immune function of the marine bivalve Mytilus galloprovincialis. Aquatic Toxicol. 2013;132:9-18.
- Sánchez-Quiles D, Tovar-Sánchez A. Are sunscreens a new environmental risk associated with coastal tourism? Environ Int. 2015;83:158-170.
- Xu S, Kwa M, Agarwal A, et al. Sunscreen product performance and other determinants of consumer preferences. JAMA Dermatol. 2016;152:920-927.
- Vesper I. Hawaii seeks to ban ‘reef-unfriendly’ sunscreen. Nature. February 3, 2017. https://www.nature.com/news/hawaii-seeks-to-ban-reef-unfriendly-sunscreen-1.21332. Accessed November 16, 2017.
Breakthrough cancer gene assay approved, CMS proposes coverage
The Food and Drug Administration approved a new genetic sequencing test that detects mutations across 324 genes in tumor biopsy specimens with an accuracy of 94.6%.
The FoundationOne CDx (F1CDx) test from Foundation Medicine “can identify which patients with any of five tumor types” – non–small-cell lung cancer, melanoma, breast cancer, colorectal cancer, or ovarian cancer – “may benefit from 15 different FDA-approved targeted treatment options,” as well as clinical trial eligibility, “with one test report, avoiding duplicative biopsies,” the agency said in a statement.
On the same day as the approval, the Centers for Medicare & Medicaid Services proposed nationwide coverage for Medicare beneficiaries with recurrent or metastatic disease. CMS is accepting public comments on the proposal for 30 days. The cost of the test is $5,800.
F1CDx went through the FDA and CMS Parallel Review Program, in which the agencies review medical devices concurrently to help reduce the time between approval and Medicare coverage.
F1CDx reads the order of nucleotides on DNA isolated from biopsy specimens to detect a range of genetic anomalies, including base substitutions, insertion and deletion alterations, copy number alterations, and select gene rearrangements, as well as genomic signatures including microsatellite instability and tumor mutational burden. Clinical performance was established by comparing the F1CDx to previously approved tests.
The Food and Drug Administration approved a new genetic sequencing test that detects mutations across 324 genes in tumor biopsy specimens with an accuracy of 94.6%.
The FoundationOne CDx (F1CDx) test from Foundation Medicine “can identify which patients with any of five tumor types” – non–small-cell lung cancer, melanoma, breast cancer, colorectal cancer, or ovarian cancer – “may benefit from 15 different FDA-approved targeted treatment options,” as well as clinical trial eligibility, “with one test report, avoiding duplicative biopsies,” the agency said in a statement.
On the same day as the approval, the Centers for Medicare & Medicaid Services proposed nationwide coverage for Medicare beneficiaries with recurrent or metastatic disease. CMS is accepting public comments on the proposal for 30 days. The cost of the test is $5,800.
F1CDx went through the FDA and CMS Parallel Review Program, in which the agencies review medical devices concurrently to help reduce the time between approval and Medicare coverage.
F1CDx reads the order of nucleotides on DNA isolated from biopsy specimens to detect a range of genetic anomalies, including base substitutions, insertion and deletion alterations, copy number alterations, and select gene rearrangements, as well as genomic signatures including microsatellite instability and tumor mutational burden. Clinical performance was established by comparing the F1CDx to previously approved tests.
The Food and Drug Administration approved a new genetic sequencing test that detects mutations across 324 genes in tumor biopsy specimens with an accuracy of 94.6%.
The FoundationOne CDx (F1CDx) test from Foundation Medicine “can identify which patients with any of five tumor types” – non–small-cell lung cancer, melanoma, breast cancer, colorectal cancer, or ovarian cancer – “may benefit from 15 different FDA-approved targeted treatment options,” as well as clinical trial eligibility, “with one test report, avoiding duplicative biopsies,” the agency said in a statement.
On the same day as the approval, the Centers for Medicare & Medicaid Services proposed nationwide coverage for Medicare beneficiaries with recurrent or metastatic disease. CMS is accepting public comments on the proposal for 30 days. The cost of the test is $5,800.
F1CDx went through the FDA and CMS Parallel Review Program, in which the agencies review medical devices concurrently to help reduce the time between approval and Medicare coverage.
F1CDx reads the order of nucleotides on DNA isolated from biopsy specimens to detect a range of genetic anomalies, including base substitutions, insertion and deletion alterations, copy number alterations, and select gene rearrangements, as well as genomic signatures including microsatellite instability and tumor mutational burden. Clinical performance was established by comparing the F1CDx to previously approved tests.
Gut bacteria influenced response to checkpoint inhibitors
The gut microbome may influence responses to immune checkpoint inhibitors, based on results from two studies, and one of the investigators is now gearing up for the next step - evaluating in a clinical trial whether altering the microflora will actually improve responses.
In the first study, investigators carried out a series of experiments using fecal microbiome samples from patients with metastatic melanoma embarking on therapy with a PD-1 (programmed cell death protein 1) inhibitor.
“In melanoma patients, there were differential signals in the gut microbiome of responders versus nonresponders, and I think the clincher was when we transplanted fecal samples from responders to nonresponders in germ-free mice, essentially reconstituting the microbiome and showing that it equally affected the systemic immunity and antitumor immunity when we implanted tumors, as well as response to checkpoint blockade,” lead author Jennifer A. Wargo, MD, MMSc, of the University of Texas MD Anderson Cancer Center in Houston, said in an interview.
Dr. Wargo and her colleagues first collected buccal and fecal microbiome samples from 112 patients with metastatic melanoma before they began therapy with a PD-1 inhibitor. After performing taxonomic profiling on all samples, they found that there was a clustering effect by response status in the gut microbiome, but not the oral microbiome, and because changes in the oral microbiome did not appear to be related to treatment response, they focused on the gut.
When Dr. Wargo and her colleagues studied the posttherapy microbiomes of 43 patients (30 responders and 13 nonresponders) according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1), they found that the responders had a significantly higher degree of alpha diversity, a measure of species diversity within a specific environment, compared with nonresponders (P less than .01). In addition, responders had a relative abundance of Ruminococcaceae, commonly occurring gut microbes that break down complex carbohydrates, the investigators reported (Science. 2017 Nov. 2. doi: 10.1126/science.aan4236).
They found that patients whose microbiomes were diverse in general, and in particular were enriched with Faecalibacterium and Clostridiales species, were more likely to respond to immunotherapy with a PD-1 inhibitor and have a longer duration of progression-free survival. In contrast, patients whose microbiomes were more enriched with Bacteroidales species were more likely to be nonresponders.
To get a better understanding of the mechanisms whereby gut bacteria may influence response to PD-1 inhibitors, they performed metagenomic analysis on samples from 14 responders and 11 nonresponders, and found that responders had micro-organisms predominantly associated with anabolic functions that may support host immunity, whereas nonresponders had microbiomes where catabolic functions were more common.
The investigators next performed immune profiling, and found that both systemic immunity and local immunity in the tumor microenvironment in responders were associated with the aforementioned favorable gut microbiome.
The researchers then transplanted feces from the human donors into germ-free mice and then injected tumor cells into the mice, and found that tumor growth was significantly reduced, and response to PD-1 inhibition was significantly enhanced, in mice who received feces from responders.
“An obvious next step is to run a clinical trial to test the hypothesis that by modulating the microbiome, you can actually enhance responses to therapy,” Dr. Wargo said. Details of the clinical trial are still being worked out, but will likely involve fecal transfers and other mechanisms for modulating the microbiome in hopes of improving responses to PD-1 inhibitors.
“It’s going to be a very biomarker-heavy trial,” she said. “We’re going to look, certainly, for changes in the microbiome, and will also do a lot of profiling in the blood, the tumor, and in the microbiome to see if there are changes that occur by modulating that microbiome. Then of course we’ll look for differences in response rates in patients as well.”
Bacteria also affect epithelial cancers
In a separate study, also published in Science, investigators led by Bertrand Routy, MD, of the Gustave Roussy Cancer Institute in Villejuif, France, reported that patients with non–small cell lung cancer and urothelial carcinoma who had previously used systemic antibiotics had reduced survival when treated with a PD-1 inhibitor, compared with patients who had never taken antibiotics (Science. 2017 Nov. 2 doi: 10.1126/science.aan3706).
Analysis of the gut microbiome in these patients showed that higher levels of Akkermansia muciniphila were associated with the best clinical outcomes, with the species detectable in the microbiome of 69% of patients who had partial responses to anti–PD-1 therapy, and in 58% of those with stable disease. In contrast, the bacterium was detectable in only 34% of patients who experienced disease progression.
As in the experiments by Dr. Wargo and her associates, when the French investigators first treated mice with antibiotics and then gave them oral supplements containing the bacteria, the supplements restored response to PD-1 blockade,
“We conclude from the study that the gut microbiome markedly influences the outcome of PD-1 blockade in mice and patients,” Dr. Routy and his associates wrote.
They acknowledged that the mechanism whereby a common organism such as Akkermansia muciniphila might have an immunomodulatory effect is still unknown,
“Irrespective of these remaining questions, our findings suggest that the microbiome governs the cancer-immune set point of cancer-bearing individuals and offer[s] novel avenues for manipulating the gut ecosystem to circumvent primary resistance to [immune checkpoint inhibitors],” they wrote.
The study by Dr. Wargo and her colleagues was supported by contributions to the University of Texas MD Anderson Melanoma Moon Shots program. Dr. Wargo is supported by the Binational Science Foundation, Melanoma Research Alliance, Stand Up to Cancer, and the MDACC Melanoma Moon Shots Program. The work by Dr. Routy and his associates was supported by the Goustave Roussy Cancer Institute and McGill University. Coauthors were supported by the National Cancer Institute of France and other agencies and philanthropies.
The gut microbome may influence responses to immune checkpoint inhibitors, based on results from two studies, and one of the investigators is now gearing up for the next step - evaluating in a clinical trial whether altering the microflora will actually improve responses.
In the first study, investigators carried out a series of experiments using fecal microbiome samples from patients with metastatic melanoma embarking on therapy with a PD-1 (programmed cell death protein 1) inhibitor.
“In melanoma patients, there were differential signals in the gut microbiome of responders versus nonresponders, and I think the clincher was when we transplanted fecal samples from responders to nonresponders in germ-free mice, essentially reconstituting the microbiome and showing that it equally affected the systemic immunity and antitumor immunity when we implanted tumors, as well as response to checkpoint blockade,” lead author Jennifer A. Wargo, MD, MMSc, of the University of Texas MD Anderson Cancer Center in Houston, said in an interview.
Dr. Wargo and her colleagues first collected buccal and fecal microbiome samples from 112 patients with metastatic melanoma before they began therapy with a PD-1 inhibitor. After performing taxonomic profiling on all samples, they found that there was a clustering effect by response status in the gut microbiome, but not the oral microbiome, and because changes in the oral microbiome did not appear to be related to treatment response, they focused on the gut.
When Dr. Wargo and her colleagues studied the posttherapy microbiomes of 43 patients (30 responders and 13 nonresponders) according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1), they found that the responders had a significantly higher degree of alpha diversity, a measure of species diversity within a specific environment, compared with nonresponders (P less than .01). In addition, responders had a relative abundance of Ruminococcaceae, commonly occurring gut microbes that break down complex carbohydrates, the investigators reported (Science. 2017 Nov. 2. doi: 10.1126/science.aan4236).
They found that patients whose microbiomes were diverse in general, and in particular were enriched with Faecalibacterium and Clostridiales species, were more likely to respond to immunotherapy with a PD-1 inhibitor and have a longer duration of progression-free survival. In contrast, patients whose microbiomes were more enriched with Bacteroidales species were more likely to be nonresponders.
To get a better understanding of the mechanisms whereby gut bacteria may influence response to PD-1 inhibitors, they performed metagenomic analysis on samples from 14 responders and 11 nonresponders, and found that responders had micro-organisms predominantly associated with anabolic functions that may support host immunity, whereas nonresponders had microbiomes where catabolic functions were more common.
The investigators next performed immune profiling, and found that both systemic immunity and local immunity in the tumor microenvironment in responders were associated with the aforementioned favorable gut microbiome.
The researchers then transplanted feces from the human donors into germ-free mice and then injected tumor cells into the mice, and found that tumor growth was significantly reduced, and response to PD-1 inhibition was significantly enhanced, in mice who received feces from responders.
“An obvious next step is to run a clinical trial to test the hypothesis that by modulating the microbiome, you can actually enhance responses to therapy,” Dr. Wargo said. Details of the clinical trial are still being worked out, but will likely involve fecal transfers and other mechanisms for modulating the microbiome in hopes of improving responses to PD-1 inhibitors.
“It’s going to be a very biomarker-heavy trial,” she said. “We’re going to look, certainly, for changes in the microbiome, and will also do a lot of profiling in the blood, the tumor, and in the microbiome to see if there are changes that occur by modulating that microbiome. Then of course we’ll look for differences in response rates in patients as well.”
Bacteria also affect epithelial cancers
In a separate study, also published in Science, investigators led by Bertrand Routy, MD, of the Gustave Roussy Cancer Institute in Villejuif, France, reported that patients with non–small cell lung cancer and urothelial carcinoma who had previously used systemic antibiotics had reduced survival when treated with a PD-1 inhibitor, compared with patients who had never taken antibiotics (Science. 2017 Nov. 2 doi: 10.1126/science.aan3706).
Analysis of the gut microbiome in these patients showed that higher levels of Akkermansia muciniphila were associated with the best clinical outcomes, with the species detectable in the microbiome of 69% of patients who had partial responses to anti–PD-1 therapy, and in 58% of those with stable disease. In contrast, the bacterium was detectable in only 34% of patients who experienced disease progression.
As in the experiments by Dr. Wargo and her associates, when the French investigators first treated mice with antibiotics and then gave them oral supplements containing the bacteria, the supplements restored response to PD-1 blockade,
“We conclude from the study that the gut microbiome markedly influences the outcome of PD-1 blockade in mice and patients,” Dr. Routy and his associates wrote.
They acknowledged that the mechanism whereby a common organism such as Akkermansia muciniphila might have an immunomodulatory effect is still unknown,
“Irrespective of these remaining questions, our findings suggest that the microbiome governs the cancer-immune set point of cancer-bearing individuals and offer[s] novel avenues for manipulating the gut ecosystem to circumvent primary resistance to [immune checkpoint inhibitors],” they wrote.
The study by Dr. Wargo and her colleagues was supported by contributions to the University of Texas MD Anderson Melanoma Moon Shots program. Dr. Wargo is supported by the Binational Science Foundation, Melanoma Research Alliance, Stand Up to Cancer, and the MDACC Melanoma Moon Shots Program. The work by Dr. Routy and his associates was supported by the Goustave Roussy Cancer Institute and McGill University. Coauthors were supported by the National Cancer Institute of France and other agencies and philanthropies.
The gut microbome may influence responses to immune checkpoint inhibitors, based on results from two studies, and one of the investigators is now gearing up for the next step - evaluating in a clinical trial whether altering the microflora will actually improve responses.
In the first study, investigators carried out a series of experiments using fecal microbiome samples from patients with metastatic melanoma embarking on therapy with a PD-1 (programmed cell death protein 1) inhibitor.
“In melanoma patients, there were differential signals in the gut microbiome of responders versus nonresponders, and I think the clincher was when we transplanted fecal samples from responders to nonresponders in germ-free mice, essentially reconstituting the microbiome and showing that it equally affected the systemic immunity and antitumor immunity when we implanted tumors, as well as response to checkpoint blockade,” lead author Jennifer A. Wargo, MD, MMSc, of the University of Texas MD Anderson Cancer Center in Houston, said in an interview.
Dr. Wargo and her colleagues first collected buccal and fecal microbiome samples from 112 patients with metastatic melanoma before they began therapy with a PD-1 inhibitor. After performing taxonomic profiling on all samples, they found that there was a clustering effect by response status in the gut microbiome, but not the oral microbiome, and because changes in the oral microbiome did not appear to be related to treatment response, they focused on the gut.
When Dr. Wargo and her colleagues studied the posttherapy microbiomes of 43 patients (30 responders and 13 nonresponders) according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1), they found that the responders had a significantly higher degree of alpha diversity, a measure of species diversity within a specific environment, compared with nonresponders (P less than .01). In addition, responders had a relative abundance of Ruminococcaceae, commonly occurring gut microbes that break down complex carbohydrates, the investigators reported (Science. 2017 Nov. 2. doi: 10.1126/science.aan4236).
They found that patients whose microbiomes were diverse in general, and in particular were enriched with Faecalibacterium and Clostridiales species, were more likely to respond to immunotherapy with a PD-1 inhibitor and have a longer duration of progression-free survival. In contrast, patients whose microbiomes were more enriched with Bacteroidales species were more likely to be nonresponders.
To get a better understanding of the mechanisms whereby gut bacteria may influence response to PD-1 inhibitors, they performed metagenomic analysis on samples from 14 responders and 11 nonresponders, and found that responders had micro-organisms predominantly associated with anabolic functions that may support host immunity, whereas nonresponders had microbiomes where catabolic functions were more common.
The investigators next performed immune profiling, and found that both systemic immunity and local immunity in the tumor microenvironment in responders were associated with the aforementioned favorable gut microbiome.
The researchers then transplanted feces from the human donors into germ-free mice and then injected tumor cells into the mice, and found that tumor growth was significantly reduced, and response to PD-1 inhibition was significantly enhanced, in mice who received feces from responders.
“An obvious next step is to run a clinical trial to test the hypothesis that by modulating the microbiome, you can actually enhance responses to therapy,” Dr. Wargo said. Details of the clinical trial are still being worked out, but will likely involve fecal transfers and other mechanisms for modulating the microbiome in hopes of improving responses to PD-1 inhibitors.
“It’s going to be a very biomarker-heavy trial,” she said. “We’re going to look, certainly, for changes in the microbiome, and will also do a lot of profiling in the blood, the tumor, and in the microbiome to see if there are changes that occur by modulating that microbiome. Then of course we’ll look for differences in response rates in patients as well.”
Bacteria also affect epithelial cancers
In a separate study, also published in Science, investigators led by Bertrand Routy, MD, of the Gustave Roussy Cancer Institute in Villejuif, France, reported that patients with non–small cell lung cancer and urothelial carcinoma who had previously used systemic antibiotics had reduced survival when treated with a PD-1 inhibitor, compared with patients who had never taken antibiotics (Science. 2017 Nov. 2 doi: 10.1126/science.aan3706).
Analysis of the gut microbiome in these patients showed that higher levels of Akkermansia muciniphila were associated with the best clinical outcomes, with the species detectable in the microbiome of 69% of patients who had partial responses to anti–PD-1 therapy, and in 58% of those with stable disease. In contrast, the bacterium was detectable in only 34% of patients who experienced disease progression.
As in the experiments by Dr. Wargo and her associates, when the French investigators first treated mice with antibiotics and then gave them oral supplements containing the bacteria, the supplements restored response to PD-1 blockade,
“We conclude from the study that the gut microbiome markedly influences the outcome of PD-1 blockade in mice and patients,” Dr. Routy and his associates wrote.
They acknowledged that the mechanism whereby a common organism such as Akkermansia muciniphila might have an immunomodulatory effect is still unknown,
“Irrespective of these remaining questions, our findings suggest that the microbiome governs the cancer-immune set point of cancer-bearing individuals and offer[s] novel avenues for manipulating the gut ecosystem to circumvent primary resistance to [immune checkpoint inhibitors],” they wrote.
The study by Dr. Wargo and her colleagues was supported by contributions to the University of Texas MD Anderson Melanoma Moon Shots program. Dr. Wargo is supported by the Binational Science Foundation, Melanoma Research Alliance, Stand Up to Cancer, and the MDACC Melanoma Moon Shots Program. The work by Dr. Routy and his associates was supported by the Goustave Roussy Cancer Institute and McGill University. Coauthors were supported by the National Cancer Institute of France and other agencies and philanthropies.
FROM SCIENCE
Key clinical point: Modulating the gut microbome may improve responses to immune checkpoint inhibitors in patients with advanced melanoma, non–small cell lung cancer, and urothelial carcinoma.
Major finding: Responders to a checkpoint inhibitor had a significantly higher degree of alpha diversity, a measure of species diversity within a specific environment, compared with nonresponders (P less than .01).
Data source: A series of studies using microbiome samples from cancer patients receiving immune checkpoint inhibitors.
Disclosures: The study by Dr. Wargo and her colleagues was supported by contributions to the University of Texas MD Anderson Melanoma Moon Shots Program. Dr. Wargo is supported by the Binational Science Foundation, Melanoma Research Alliance, Stand Up to Cancer, and the MDACC Melanoma Moon Shots Program. The work by Dr. Routy and his colleagues was supported by the Goustave Roussy Cancer Institute and McGill University. Coauthors were supported by the National Cancer Institute of France and other agencies and philanthropies.
Neoantigen profiling predicts response to immunotherapy
In antitumor immunity and immunotherapy, quality and fitness count.
Specifically, the quality and fitness of neoantigens – tumor-specific mutated peptides on the surface of cancer cells – can influence a patient’s response to immune checkpoint inhibitors, and mathematical models of neoantigen fitness can serve as biomarkers for response to immunotherapy, according to investigators of two separate but related studies published in Nature.
In one study, Marta Łuksza, PhD, from the Simons Center for Systems Biology at the Institute for Advanced Study in Princeton, N.J., and colleagues propose a neoantigen fitness model that can predict tumor response to checkpoint blockade immunotherapy.
Importantly, low-fitness neoantigens identified by our method may be leveraged for developing novel immunotherapies,” they wrote (Nature. 2017 Nov 8. doi: 10.1038/nature24473).
In a related study, Vinod P. Balachandran, MD, from the David M. Rubinstein Center for Pancreatic Cancer Research at Memorial Sloan Kettering Cancer Center in New York and colleagues, including Dr. Łuksza and others, looked at T-cell antigens in long-term survivors of pancreatic cancer and identified specific neoantigens as T-cell targets.
“More broadly, we identify neoantigen quality as a biomarker for immunogenic tumors that may guide the application of immunotherapies,” Dr. Balachandran and colleagues wrote (Nature. 2017 Nov 8. doi: 10.1038/nature24462).
Proof of concept
The studies provide a proof of concept that mathematical modeling of tumor evolution and the interactions of tumors with the immune system may soon provide clinicians with valuable and actionable information about responses to immunotherapy, Benjamin Greenbaum, PhD, senior author on the study by Łuksza et al., and a coauthor on the pancreatic cancer study said in an interview.
“We’re trying to come up with measures that take into account what we think the underlying processes are and what lies behind therapy response, and that should lead to better predictive models associated with response in the future,” said Dr. Greenbaum, of the Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai Medical Center, New York.
One of the key findings of the studies is that neoantigen quality – the ability of neoantigens to spark T-cell recognition – seems to be as or more important than neoantigen quantity for influencing immune responses during tumor evolution.
“The general logic behind the idea that mutational burden can be a good predictor of response is that the more mutations you have, the more likely that you have a neoantigen, a peptide generated by a tumor mutation, that elicits productive T-cell recognition. We tried to model that process that might lead to productive T-cell recognition, to assign a kind of number to every neoantigen to provide some estimate of how likely it was to undergo a productive process,” Dr. Greenbaum explained.
Melanoma and lung cancer survivors
In the study by Łuksza et al., the investigators created a mathematical fitness model that can predict how tumors respond to immunotherapy based on how neoantigens interact with the immune system and applied the model to data on three previously reported patient cohorts, including two groups of patients with malignant melanoma treated with a cytotoxic T-lymphocyte associated protein 4 (CTLA4) immune checkpoint such as ipilimumab (Yervoy), and one group of patients with non–small cell lung cancer treated with a programmed death-1 (PD-1) inhibitor (for example, nivolumab [Opdivo]).
They found that their proposed model is more accurate than genomic biomarkers for predicting how a specific tumor may respond to immunotherapy.
“Importantly, low-fitness neoantigens identified by our method may be leveraged for developing novel immunotherapies. By using an immune fitness model to study immunotherapy, we reveal broad similarities between the evolution of tumors and rapidly evolving pathogens,” they wrote.
Pancreatic cancer survivors
Fewer than 7% of patients diagnosed with pancreatic ductal adenocarcinoma (PDAC) survive more than 5 years, despite the best surgical and medical therapy. But a few lucky patients are long-term survivors, and Dr. Balachandran and associates sought to examine what aspects of T-cell immunity contributed to their longevity.
Rather than relying on genomic analysis of tumor samples, however, they used a combination of genetic, immunohistochemical, and transcriptional immunoprofiling, as well as computational biophysics and function to identify T-cell antigens in the long-term survivors.
When they compared surgically resected patients matched by tumor stage, they found that tumors from those with a median overall survival (OS) of 6 years had a 3-fold greater density of CD8-positive T cells and a 12-fold greater density of cytolytic CD8-positive cells, as well as more mature dendritic cells, regulatory T cells, and macrophages, but decreased numbers of CD4-positive T cells, compared with patients with a more typical course of survival (median OS, 0.8 years). There were no differences between long- and short-term survivors in either B cells or major histocompatibility complex (MHC) class I–positive cells.
They then performed whole-exome sequencing on tumor samples to determine the frequency of neoantigens and found a median of 38 predicted neoantigens per tumor.
“Notably, patients with both the highest predicted neoantigen number and either the greatest CD3+, CD8+, or polyclonal T-cell repertoire, but neither alone, exhibited the longest survival,” they wrote.
When they looked for qualities of neoantigens responsible for promoting T-cell activation in the long-term survivors, they found that the tumors from the survivors, compared with others, were enriched in neoantigen qualities that could be described by a mathematical fitness model.
“Our results provide insight into the heterogeneous immunobiology of PDAC, a presumed poorly immunogenic and checkpoint blockade–refractory tumor, demonstrating that neoantigens may be T-cell targets in [long-term survivors]”, they wrote.
The investigators propose that immunity to neoantigens that are generated during the outgrowth of a primary tumor could at least partially explain the lower incidence of relapse and prolonged survival of a small minority of patients with pancreatic cancer.
“Our findings support the development of strategies to harness neoantigen-specific immunity to treat checkpoint blockade–refractory cancers, and the identification of immunogenic hot spots for directed neoantigen targeting,” they concluded.
The studies were supported by grants from Stand Up to Cancer, American Cancer Society, National Science Foundation, Lustgarten Foundation, Janssen Research & Development, the STARR Cancer Consortium, the Pershing Square Sohn Cancer Research Alliance, the National Institutes of Health, the V Foundation, Swim Across America, Ludwig Institute for Cancer Research, the Parker Institute for Cancer Immunotherapy, a National Cancer Institute Career Development Award, and a Memorial Sloan Kettering Cancer Center core grant. Dr. Łuksza and Dr. Greenbaum disclosed consulting for Merck. Dr. Balachandran disclosed research funding from Bristol-Myers Squibb.
In antitumor immunity and immunotherapy, quality and fitness count.
Specifically, the quality and fitness of neoantigens – tumor-specific mutated peptides on the surface of cancer cells – can influence a patient’s response to immune checkpoint inhibitors, and mathematical models of neoantigen fitness can serve as biomarkers for response to immunotherapy, according to investigators of two separate but related studies published in Nature.
In one study, Marta Łuksza, PhD, from the Simons Center for Systems Biology at the Institute for Advanced Study in Princeton, N.J., and colleagues propose a neoantigen fitness model that can predict tumor response to checkpoint blockade immunotherapy.
Importantly, low-fitness neoantigens identified by our method may be leveraged for developing novel immunotherapies,” they wrote (Nature. 2017 Nov 8. doi: 10.1038/nature24473).
In a related study, Vinod P. Balachandran, MD, from the David M. Rubinstein Center for Pancreatic Cancer Research at Memorial Sloan Kettering Cancer Center in New York and colleagues, including Dr. Łuksza and others, looked at T-cell antigens in long-term survivors of pancreatic cancer and identified specific neoantigens as T-cell targets.
“More broadly, we identify neoantigen quality as a biomarker for immunogenic tumors that may guide the application of immunotherapies,” Dr. Balachandran and colleagues wrote (Nature. 2017 Nov 8. doi: 10.1038/nature24462).
Proof of concept
The studies provide a proof of concept that mathematical modeling of tumor evolution and the interactions of tumors with the immune system may soon provide clinicians with valuable and actionable information about responses to immunotherapy, Benjamin Greenbaum, PhD, senior author on the study by Łuksza et al., and a coauthor on the pancreatic cancer study said in an interview.
“We’re trying to come up with measures that take into account what we think the underlying processes are and what lies behind therapy response, and that should lead to better predictive models associated with response in the future,” said Dr. Greenbaum, of the Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai Medical Center, New York.
One of the key findings of the studies is that neoantigen quality – the ability of neoantigens to spark T-cell recognition – seems to be as or more important than neoantigen quantity for influencing immune responses during tumor evolution.
“The general logic behind the idea that mutational burden can be a good predictor of response is that the more mutations you have, the more likely that you have a neoantigen, a peptide generated by a tumor mutation, that elicits productive T-cell recognition. We tried to model that process that might lead to productive T-cell recognition, to assign a kind of number to every neoantigen to provide some estimate of how likely it was to undergo a productive process,” Dr. Greenbaum explained.
Melanoma and lung cancer survivors
In the study by Łuksza et al., the investigators created a mathematical fitness model that can predict how tumors respond to immunotherapy based on how neoantigens interact with the immune system and applied the model to data on three previously reported patient cohorts, including two groups of patients with malignant melanoma treated with a cytotoxic T-lymphocyte associated protein 4 (CTLA4) immune checkpoint such as ipilimumab (Yervoy), and one group of patients with non–small cell lung cancer treated with a programmed death-1 (PD-1) inhibitor (for example, nivolumab [Opdivo]).
They found that their proposed model is more accurate than genomic biomarkers for predicting how a specific tumor may respond to immunotherapy.
“Importantly, low-fitness neoantigens identified by our method may be leveraged for developing novel immunotherapies. By using an immune fitness model to study immunotherapy, we reveal broad similarities between the evolution of tumors and rapidly evolving pathogens,” they wrote.
Pancreatic cancer survivors
Fewer than 7% of patients diagnosed with pancreatic ductal adenocarcinoma (PDAC) survive more than 5 years, despite the best surgical and medical therapy. But a few lucky patients are long-term survivors, and Dr. Balachandran and associates sought to examine what aspects of T-cell immunity contributed to their longevity.
Rather than relying on genomic analysis of tumor samples, however, they used a combination of genetic, immunohistochemical, and transcriptional immunoprofiling, as well as computational biophysics and function to identify T-cell antigens in the long-term survivors.
When they compared surgically resected patients matched by tumor stage, they found that tumors from those with a median overall survival (OS) of 6 years had a 3-fold greater density of CD8-positive T cells and a 12-fold greater density of cytolytic CD8-positive cells, as well as more mature dendritic cells, regulatory T cells, and macrophages, but decreased numbers of CD4-positive T cells, compared with patients with a more typical course of survival (median OS, 0.8 years). There were no differences between long- and short-term survivors in either B cells or major histocompatibility complex (MHC) class I–positive cells.
They then performed whole-exome sequencing on tumor samples to determine the frequency of neoantigens and found a median of 38 predicted neoantigens per tumor.
“Notably, patients with both the highest predicted neoantigen number and either the greatest CD3+, CD8+, or polyclonal T-cell repertoire, but neither alone, exhibited the longest survival,” they wrote.
When they looked for qualities of neoantigens responsible for promoting T-cell activation in the long-term survivors, they found that the tumors from the survivors, compared with others, were enriched in neoantigen qualities that could be described by a mathematical fitness model.
“Our results provide insight into the heterogeneous immunobiology of PDAC, a presumed poorly immunogenic and checkpoint blockade–refractory tumor, demonstrating that neoantigens may be T-cell targets in [long-term survivors]”, they wrote.
The investigators propose that immunity to neoantigens that are generated during the outgrowth of a primary tumor could at least partially explain the lower incidence of relapse and prolonged survival of a small minority of patients with pancreatic cancer.
“Our findings support the development of strategies to harness neoantigen-specific immunity to treat checkpoint blockade–refractory cancers, and the identification of immunogenic hot spots for directed neoantigen targeting,” they concluded.
The studies were supported by grants from Stand Up to Cancer, American Cancer Society, National Science Foundation, Lustgarten Foundation, Janssen Research & Development, the STARR Cancer Consortium, the Pershing Square Sohn Cancer Research Alliance, the National Institutes of Health, the V Foundation, Swim Across America, Ludwig Institute for Cancer Research, the Parker Institute for Cancer Immunotherapy, a National Cancer Institute Career Development Award, and a Memorial Sloan Kettering Cancer Center core grant. Dr. Łuksza and Dr. Greenbaum disclosed consulting for Merck. Dr. Balachandran disclosed research funding from Bristol-Myers Squibb.
In antitumor immunity and immunotherapy, quality and fitness count.
Specifically, the quality and fitness of neoantigens – tumor-specific mutated peptides on the surface of cancer cells – can influence a patient’s response to immune checkpoint inhibitors, and mathematical models of neoantigen fitness can serve as biomarkers for response to immunotherapy, according to investigators of two separate but related studies published in Nature.
In one study, Marta Łuksza, PhD, from the Simons Center for Systems Biology at the Institute for Advanced Study in Princeton, N.J., and colleagues propose a neoantigen fitness model that can predict tumor response to checkpoint blockade immunotherapy.
Importantly, low-fitness neoantigens identified by our method may be leveraged for developing novel immunotherapies,” they wrote (Nature. 2017 Nov 8. doi: 10.1038/nature24473).
In a related study, Vinod P. Balachandran, MD, from the David M. Rubinstein Center for Pancreatic Cancer Research at Memorial Sloan Kettering Cancer Center in New York and colleagues, including Dr. Łuksza and others, looked at T-cell antigens in long-term survivors of pancreatic cancer and identified specific neoantigens as T-cell targets.
“More broadly, we identify neoantigen quality as a biomarker for immunogenic tumors that may guide the application of immunotherapies,” Dr. Balachandran and colleagues wrote (Nature. 2017 Nov 8. doi: 10.1038/nature24462).
Proof of concept
The studies provide a proof of concept that mathematical modeling of tumor evolution and the interactions of tumors with the immune system may soon provide clinicians with valuable and actionable information about responses to immunotherapy, Benjamin Greenbaum, PhD, senior author on the study by Łuksza et al., and a coauthor on the pancreatic cancer study said in an interview.
“We’re trying to come up with measures that take into account what we think the underlying processes are and what lies behind therapy response, and that should lead to better predictive models associated with response in the future,” said Dr. Greenbaum, of the Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai Medical Center, New York.
One of the key findings of the studies is that neoantigen quality – the ability of neoantigens to spark T-cell recognition – seems to be as or more important than neoantigen quantity for influencing immune responses during tumor evolution.
“The general logic behind the idea that mutational burden can be a good predictor of response is that the more mutations you have, the more likely that you have a neoantigen, a peptide generated by a tumor mutation, that elicits productive T-cell recognition. We tried to model that process that might lead to productive T-cell recognition, to assign a kind of number to every neoantigen to provide some estimate of how likely it was to undergo a productive process,” Dr. Greenbaum explained.
Melanoma and lung cancer survivors
In the study by Łuksza et al., the investigators created a mathematical fitness model that can predict how tumors respond to immunotherapy based on how neoantigens interact with the immune system and applied the model to data on three previously reported patient cohorts, including two groups of patients with malignant melanoma treated with a cytotoxic T-lymphocyte associated protein 4 (CTLA4) immune checkpoint such as ipilimumab (Yervoy), and one group of patients with non–small cell lung cancer treated with a programmed death-1 (PD-1) inhibitor (for example, nivolumab [Opdivo]).
They found that their proposed model is more accurate than genomic biomarkers for predicting how a specific tumor may respond to immunotherapy.
“Importantly, low-fitness neoantigens identified by our method may be leveraged for developing novel immunotherapies. By using an immune fitness model to study immunotherapy, we reveal broad similarities between the evolution of tumors and rapidly evolving pathogens,” they wrote.
Pancreatic cancer survivors
Fewer than 7% of patients diagnosed with pancreatic ductal adenocarcinoma (PDAC) survive more than 5 years, despite the best surgical and medical therapy. But a few lucky patients are long-term survivors, and Dr. Balachandran and associates sought to examine what aspects of T-cell immunity contributed to their longevity.
Rather than relying on genomic analysis of tumor samples, however, they used a combination of genetic, immunohistochemical, and transcriptional immunoprofiling, as well as computational biophysics and function to identify T-cell antigens in the long-term survivors.
When they compared surgically resected patients matched by tumor stage, they found that tumors from those with a median overall survival (OS) of 6 years had a 3-fold greater density of CD8-positive T cells and a 12-fold greater density of cytolytic CD8-positive cells, as well as more mature dendritic cells, regulatory T cells, and macrophages, but decreased numbers of CD4-positive T cells, compared with patients with a more typical course of survival (median OS, 0.8 years). There were no differences between long- and short-term survivors in either B cells or major histocompatibility complex (MHC) class I–positive cells.
They then performed whole-exome sequencing on tumor samples to determine the frequency of neoantigens and found a median of 38 predicted neoantigens per tumor.
“Notably, patients with both the highest predicted neoantigen number and either the greatest CD3+, CD8+, or polyclonal T-cell repertoire, but neither alone, exhibited the longest survival,” they wrote.
When they looked for qualities of neoantigens responsible for promoting T-cell activation in the long-term survivors, they found that the tumors from the survivors, compared with others, were enriched in neoantigen qualities that could be described by a mathematical fitness model.
“Our results provide insight into the heterogeneous immunobiology of PDAC, a presumed poorly immunogenic and checkpoint blockade–refractory tumor, demonstrating that neoantigens may be T-cell targets in [long-term survivors]”, they wrote.
The investigators propose that immunity to neoantigens that are generated during the outgrowth of a primary tumor could at least partially explain the lower incidence of relapse and prolonged survival of a small minority of patients with pancreatic cancer.
“Our findings support the development of strategies to harness neoantigen-specific immunity to treat checkpoint blockade–refractory cancers, and the identification of immunogenic hot spots for directed neoantigen targeting,” they concluded.
The studies were supported by grants from Stand Up to Cancer, American Cancer Society, National Science Foundation, Lustgarten Foundation, Janssen Research & Development, the STARR Cancer Consortium, the Pershing Square Sohn Cancer Research Alliance, the National Institutes of Health, the V Foundation, Swim Across America, Ludwig Institute for Cancer Research, the Parker Institute for Cancer Immunotherapy, a National Cancer Institute Career Development Award, and a Memorial Sloan Kettering Cancer Center core grant. Dr. Łuksza and Dr. Greenbaum disclosed consulting for Merck. Dr. Balachandran disclosed research funding from Bristol-Myers Squibb.
FROM NATURE
Key clinical point: Proof-of-concept studies show that mathematical modeling of neoantigens can be used to predict tumor responses to immune checkpoint inhibitors.
Major finding: Neoantigen quality may be a better biomarker for guiding immunotherapy than tumor genomic profiling.
Data source: Basic science reports focusing on neoantigens and their potential influence on tumor interactions with the immune system.
Disclosures: The studies were supported by grants from Stand Up to Cancer, American Cancer Society, National Science Foundation, Lustgarten Foundation, Janssen Research & Development, the STARR Cancer Consortium, the Pershing Square Sohn Cancer Research Alliance, the National Institutes of Health, the V Foundation, Swim Across America, Ludwig Institute for Cancer Research, the Parker Institute for Cancer Immunotherapy, a National Cancer Institute Career Development Award, and a Memorial Sloan Kettering Cancer Center core grant. Dr. Łuksza and Dr. Greenbaum disclosed consulting for Merck. Dr. Balachandran disclosed research funding from Bristol-Myers Squibb.
T-VEC improves melanoma response without toxicity increase
In patients with advanced, unresectable melanoma, the combination of talimogene laherparepvec (T-VEC) and ipilimumab yielded a higher objective response rate vs. ipilimumab alone, with a similar rate of severe or life-threatening ipilimumab-related toxicities, according to results of a 198-patient randomized phase II study.
Moreover, the incidence of grade 3/4 toxicities attributed to ipilimumab was similar between the two arms of the study, with no unexpected increases in treatment-related adverse events (AEs), reported Jason A. Chesney, MD, PhD, of the James Graham Brown Cancer Center, University of Louisville (Ky.), and his coinvestigators.
Taken together, the efficacy and safety findings suggest that the combination of T-VEC and ipilimumab “may have significant clinical utility in treatment of advanced melanoma,” Dr. Chesney and his colleagues wrote (J Clin Oncol. 2017 Oct. 5 doi: 10.1200/JCO.2017.73.7379).
The study included patients with unresectable stage IIIB/IV melanoma who had received no more than one previous treatment if BRAF wild type and no more than two treatments if BRAF mutant. Patients randomized to the combination arm received T-VEC starting in week 1 of the study and ipilimumab starting on week 6, while those in the single-agent arm received ipilimumab starting on week 1.
The primary endpoint of the phase II study was objective response rate by immune-related response criteria. Objective responses were seen in 38 of the 98 patients (39%) receiving T-VEC/ipilimumab, vs. 18 of the 100 patients (18%) who received ipilimumab alone (P = 0.002), the investigators said.
The incidence of grade 3 or greater AEs was 45% for the combination arm and 35% for the single-agent arm. There were three fatal AEs in the combination arm, but none was related to treatment, according to the investigators.
“Overall, combination treatment was not associated with unexpected AEs or increase in incidence or severity of AEs, suggesting that the combination therapy is tolerable for patients with advanced melanoma,” Dr. Chesney and his associates wrote.
Median progression-free survival (PFS) was 8.2 months for the combination arm and 6.4 months for ipilimumab alone (P = .35). Although the difference was not statistically significant, investigators remarked that ipilimumab was started later in the combination arm, per study design. Moreover, the 8.2-month median PFS exceeds the 2.8- to 2.9-month median PFS seen in previous ipilimumab studies, they said.
Combination immunotherapy is of great interest now in melanoma research. Ipilimumab is an anticytotoxic T-lymphocyte antigen-4 antibody, while T-VEC is an attenuated herpes simplex 1 virus that expresses the immunostimulatory cytokine granulocyte–macrophage colony-stimulating factor. Some other combinations have shown promise, but with higher rates of toxicity, including the combination of ipilimumab plus nivolumab, which resulted in an increase in clinically significant AEs of grade 3 or greater, Dr. Chesney and his colleagues said.
“Combination regimens with lower toxicity may allow for their use in a broader range of patients,” they added.
The study was funded by Amgen, which manufactures talimogene laherparepvec. Dr. Chesney has a relationship with Amgen that involves consulting or advising; research funding; and travel, accommodation, and expenses. His associates reported financial relationships with Amgen and other companies; three of the investigators are Amgen employees.
In patients with advanced, unresectable melanoma, the combination of talimogene laherparepvec (T-VEC) and ipilimumab yielded a higher objective response rate vs. ipilimumab alone, with a similar rate of severe or life-threatening ipilimumab-related toxicities, according to results of a 198-patient randomized phase II study.
Moreover, the incidence of grade 3/4 toxicities attributed to ipilimumab was similar between the two arms of the study, with no unexpected increases in treatment-related adverse events (AEs), reported Jason A. Chesney, MD, PhD, of the James Graham Brown Cancer Center, University of Louisville (Ky.), and his coinvestigators.
Taken together, the efficacy and safety findings suggest that the combination of T-VEC and ipilimumab “may have significant clinical utility in treatment of advanced melanoma,” Dr. Chesney and his colleagues wrote (J Clin Oncol. 2017 Oct. 5 doi: 10.1200/JCO.2017.73.7379).
The study included patients with unresectable stage IIIB/IV melanoma who had received no more than one previous treatment if BRAF wild type and no more than two treatments if BRAF mutant. Patients randomized to the combination arm received T-VEC starting in week 1 of the study and ipilimumab starting on week 6, while those in the single-agent arm received ipilimumab starting on week 1.
The primary endpoint of the phase II study was objective response rate by immune-related response criteria. Objective responses were seen in 38 of the 98 patients (39%) receiving T-VEC/ipilimumab, vs. 18 of the 100 patients (18%) who received ipilimumab alone (P = 0.002), the investigators said.
The incidence of grade 3 or greater AEs was 45% for the combination arm and 35% for the single-agent arm. There were three fatal AEs in the combination arm, but none was related to treatment, according to the investigators.
“Overall, combination treatment was not associated with unexpected AEs or increase in incidence or severity of AEs, suggesting that the combination therapy is tolerable for patients with advanced melanoma,” Dr. Chesney and his associates wrote.
Median progression-free survival (PFS) was 8.2 months for the combination arm and 6.4 months for ipilimumab alone (P = .35). Although the difference was not statistically significant, investigators remarked that ipilimumab was started later in the combination arm, per study design. Moreover, the 8.2-month median PFS exceeds the 2.8- to 2.9-month median PFS seen in previous ipilimumab studies, they said.
Combination immunotherapy is of great interest now in melanoma research. Ipilimumab is an anticytotoxic T-lymphocyte antigen-4 antibody, while T-VEC is an attenuated herpes simplex 1 virus that expresses the immunostimulatory cytokine granulocyte–macrophage colony-stimulating factor. Some other combinations have shown promise, but with higher rates of toxicity, including the combination of ipilimumab plus nivolumab, which resulted in an increase in clinically significant AEs of grade 3 or greater, Dr. Chesney and his colleagues said.
“Combination regimens with lower toxicity may allow for their use in a broader range of patients,” they added.
The study was funded by Amgen, which manufactures talimogene laherparepvec. Dr. Chesney has a relationship with Amgen that involves consulting or advising; research funding; and travel, accommodation, and expenses. His associates reported financial relationships with Amgen and other companies; three of the investigators are Amgen employees.
In patients with advanced, unresectable melanoma, the combination of talimogene laherparepvec (T-VEC) and ipilimumab yielded a higher objective response rate vs. ipilimumab alone, with a similar rate of severe or life-threatening ipilimumab-related toxicities, according to results of a 198-patient randomized phase II study.
Moreover, the incidence of grade 3/4 toxicities attributed to ipilimumab was similar between the two arms of the study, with no unexpected increases in treatment-related adverse events (AEs), reported Jason A. Chesney, MD, PhD, of the James Graham Brown Cancer Center, University of Louisville (Ky.), and his coinvestigators.
Taken together, the efficacy and safety findings suggest that the combination of T-VEC and ipilimumab “may have significant clinical utility in treatment of advanced melanoma,” Dr. Chesney and his colleagues wrote (J Clin Oncol. 2017 Oct. 5 doi: 10.1200/JCO.2017.73.7379).
The study included patients with unresectable stage IIIB/IV melanoma who had received no more than one previous treatment if BRAF wild type and no more than two treatments if BRAF mutant. Patients randomized to the combination arm received T-VEC starting in week 1 of the study and ipilimumab starting on week 6, while those in the single-agent arm received ipilimumab starting on week 1.
The primary endpoint of the phase II study was objective response rate by immune-related response criteria. Objective responses were seen in 38 of the 98 patients (39%) receiving T-VEC/ipilimumab, vs. 18 of the 100 patients (18%) who received ipilimumab alone (P = 0.002), the investigators said.
The incidence of grade 3 or greater AEs was 45% for the combination arm and 35% for the single-agent arm. There were three fatal AEs in the combination arm, but none was related to treatment, according to the investigators.
“Overall, combination treatment was not associated with unexpected AEs or increase in incidence or severity of AEs, suggesting that the combination therapy is tolerable for patients with advanced melanoma,” Dr. Chesney and his associates wrote.
Median progression-free survival (PFS) was 8.2 months for the combination arm and 6.4 months for ipilimumab alone (P = .35). Although the difference was not statistically significant, investigators remarked that ipilimumab was started later in the combination arm, per study design. Moreover, the 8.2-month median PFS exceeds the 2.8- to 2.9-month median PFS seen in previous ipilimumab studies, they said.
Combination immunotherapy is of great interest now in melanoma research. Ipilimumab is an anticytotoxic T-lymphocyte antigen-4 antibody, while T-VEC is an attenuated herpes simplex 1 virus that expresses the immunostimulatory cytokine granulocyte–macrophage colony-stimulating factor. Some other combinations have shown promise, but with higher rates of toxicity, including the combination of ipilimumab plus nivolumab, which resulted in an increase in clinically significant AEs of grade 3 or greater, Dr. Chesney and his colleagues said.
“Combination regimens with lower toxicity may allow for their use in a broader range of patients,” they added.
The study was funded by Amgen, which manufactures talimogene laherparepvec. Dr. Chesney has a relationship with Amgen that involves consulting or advising; research funding; and travel, accommodation, and expenses. His associates reported financial relationships with Amgen and other companies; three of the investigators are Amgen employees.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: Of the 98 patients receiving T-VEC/ipilimumab, 38 (39%) had objective responses, vs. 18 of the 100 patients receiving ipilimumab alone (P = .002).
Data source: Analysis of a 198-patient randomized, open-label phase II study of T-VEC/ipilimumab vs. ipilimumab alone.
Disclosures: The study was funded by Amgen, which manufactures talimogene laherparepvec. Dr. Chesney has a relationship with Amgen that involves consulting or advising; research funding; and travel, accommodation, and expenses. His associates reported financial relationships with Amgen and other companies; three of the investigators are Amgen employees.
Debunking Actinic Keratosis Myths: Are Patients With Darker Skin At Risk for Actinic Keratoses?
Myth: Actinic keratoses are only seen in patients with lighter skin
Actinic keratoses (AKs) are precancerous lesions that may turn into squamous cell carcinoma if left untreated. UV rays cause AKs, either from outdoor sun exposure or tanning beds. According to the American Academy of Dermatology, AKs are more likely to develop in patients 40 years or older with fair skin; hair color that is naturally blonde or red; eye color that is naturally blue, green, or hazel; skin that freckles or burns when in the sun; a weakened immune system; and occupations involving substances that contain polycyclic aromatic hydrocarbons such as coal or tar.
A 2007 study compared the most common diagnoses among patients of different racial and ethnic groups in New York City. Alexis et al found that AK was in the top 10 diagnoses in white patients but not for black patients. They postulated that photoprotective factors in darkly pigmented skin such as larger and more numerous melanosomes that contain more melanin and are more dispersed throughout the epidermis result in a lower incidence of skin cancers in the skin of color (SOC) population.
RELATED ARTICLE: Common Dermatologic Disorders in Skin of Color: A Comparative Practice Survey
However, a recent skin cancer awareness study in Cutis reported that even though SOC populations have lower incidences of skin cancer such as melanoma, basal cell carcinoma, and squamous cell carcinoma, they exhibit higher death rates. Furthermore, black individuals are more likely to present with advanced-stage melanoma and acral lentiginous melanomas compared to white individuals. Kailas et al stated, “Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.” They evaluated several knowledge-based interventions for increasing skin cancer awareness, knowledge, and protective behaviors in SOC populations, including the use of visuals such as photographs to allow SOC patients to visualize different skin tones, educational interventions in another language, and pamphlets.
Dermatologists should be aware that education of SOC patients is important to eradicate the common misconception that these patients do not have to worry about AKs and other skin cancers. Remind these patients that they need to protect their skin from the sun, just as patients with fair skin do. Further research in the dermatology community should focus on educational interventions that will help increase knowledge regarding skin cancer in SOC populations.
Expert Commentary
Although more common in patients with lighter skin, actinic keratosis and skin cancer can be seen in patients of all skin types. Many patients are unaware of this risk and do not use sunscreen and other sun-protective measures. We, as a specialty, have to educate our patients and the public of the risk for actinic keratosis and skin cancer in all skin types.
—Gary Goldenberg, MD (New York, New York)
Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
American Academy of Dermatology. Actinic keratosis. https://www.aad.org/public/diseases/scaly-skin/actinic-keratosis. Accessed October 17, 2017.
Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Myth: Actinic keratoses are only seen in patients with lighter skin
Actinic keratoses (AKs) are precancerous lesions that may turn into squamous cell carcinoma if left untreated. UV rays cause AKs, either from outdoor sun exposure or tanning beds. According to the American Academy of Dermatology, AKs are more likely to develop in patients 40 years or older with fair skin; hair color that is naturally blonde or red; eye color that is naturally blue, green, or hazel; skin that freckles or burns when in the sun; a weakened immune system; and occupations involving substances that contain polycyclic aromatic hydrocarbons such as coal or tar.
A 2007 study compared the most common diagnoses among patients of different racial and ethnic groups in New York City. Alexis et al found that AK was in the top 10 diagnoses in white patients but not for black patients. They postulated that photoprotective factors in darkly pigmented skin such as larger and more numerous melanosomes that contain more melanin and are more dispersed throughout the epidermis result in a lower incidence of skin cancers in the skin of color (SOC) population.
RELATED ARTICLE: Common Dermatologic Disorders in Skin of Color: A Comparative Practice Survey
However, a recent skin cancer awareness study in Cutis reported that even though SOC populations have lower incidences of skin cancer such as melanoma, basal cell carcinoma, and squamous cell carcinoma, they exhibit higher death rates. Furthermore, black individuals are more likely to present with advanced-stage melanoma and acral lentiginous melanomas compared to white individuals. Kailas et al stated, “Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.” They evaluated several knowledge-based interventions for increasing skin cancer awareness, knowledge, and protective behaviors in SOC populations, including the use of visuals such as photographs to allow SOC patients to visualize different skin tones, educational interventions in another language, and pamphlets.
Dermatologists should be aware that education of SOC patients is important to eradicate the common misconception that these patients do not have to worry about AKs and other skin cancers. Remind these patients that they need to protect their skin from the sun, just as patients with fair skin do. Further research in the dermatology community should focus on educational interventions that will help increase knowledge regarding skin cancer in SOC populations.
Expert Commentary
Although more common in patients with lighter skin, actinic keratosis and skin cancer can be seen in patients of all skin types. Many patients are unaware of this risk and do not use sunscreen and other sun-protective measures. We, as a specialty, have to educate our patients and the public of the risk for actinic keratosis and skin cancer in all skin types.
—Gary Goldenberg, MD (New York, New York)
Myth: Actinic keratoses are only seen in patients with lighter skin
Actinic keratoses (AKs) are precancerous lesions that may turn into squamous cell carcinoma if left untreated. UV rays cause AKs, either from outdoor sun exposure or tanning beds. According to the American Academy of Dermatology, AKs are more likely to develop in patients 40 years or older with fair skin; hair color that is naturally blonde or red; eye color that is naturally blue, green, or hazel; skin that freckles or burns when in the sun; a weakened immune system; and occupations involving substances that contain polycyclic aromatic hydrocarbons such as coal or tar.
A 2007 study compared the most common diagnoses among patients of different racial and ethnic groups in New York City. Alexis et al found that AK was in the top 10 diagnoses in white patients but not for black patients. They postulated that photoprotective factors in darkly pigmented skin such as larger and more numerous melanosomes that contain more melanin and are more dispersed throughout the epidermis result in a lower incidence of skin cancers in the skin of color (SOC) population.
RELATED ARTICLE: Common Dermatologic Disorders in Skin of Color: A Comparative Practice Survey
However, a recent skin cancer awareness study in Cutis reported that even though SOC populations have lower incidences of skin cancer such as melanoma, basal cell carcinoma, and squamous cell carcinoma, they exhibit higher death rates. Furthermore, black individuals are more likely to present with advanced-stage melanoma and acral lentiginous melanomas compared to white individuals. Kailas et al stated, “Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.” They evaluated several knowledge-based interventions for increasing skin cancer awareness, knowledge, and protective behaviors in SOC populations, including the use of visuals such as photographs to allow SOC patients to visualize different skin tones, educational interventions in another language, and pamphlets.
Dermatologists should be aware that education of SOC patients is important to eradicate the common misconception that these patients do not have to worry about AKs and other skin cancers. Remind these patients that they need to protect their skin from the sun, just as patients with fair skin do. Further research in the dermatology community should focus on educational interventions that will help increase knowledge regarding skin cancer in SOC populations.
Expert Commentary
Although more common in patients with lighter skin, actinic keratosis and skin cancer can be seen in patients of all skin types. Many patients are unaware of this risk and do not use sunscreen and other sun-protective measures. We, as a specialty, have to educate our patients and the public of the risk for actinic keratosis and skin cancer in all skin types.
—Gary Goldenberg, MD (New York, New York)
Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
American Academy of Dermatology. Actinic keratosis. https://www.aad.org/public/diseases/scaly-skin/actinic-keratosis. Accessed October 17, 2017.
Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
American Academy of Dermatology. Actinic keratosis. https://www.aad.org/public/diseases/scaly-skin/actinic-keratosis. Accessed October 17, 2017.
Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Assessing the Effectiveness of Knowledge-Based Interventions in Increasing Skin Cancer Awareness, Knowledge, and Protective Behaviors in Skin of Color Populations
Malignant melanoma, basal cell carcinoma, and squamous cell carcinoma account for approximately 40% of all neoplasms among the white population in the United States. Skin cancer is the most common malignancy in the United States.1 However, despite this occurrence, there are limited data regarding skin cancer in individuals with skin of color (SOC). The 5-year survival rates for melanoma are 58.2% for black individuals, 69.7% for Hispanics, and 70.9% for Asians compared to 79.8% for white individuals in the United States.2 Even though SOC populations have lower incidences of skin cancer—melanoma, basal cell carcinoma, and squamous cell carcinoma—they exhibit higher death rates.3-7 Nonetheless, no specific guidelines exist to address sun exposure and safety habits in SOC populations.6,8 Furthermore, current demographics suggest that by the year 2050, approximately half of the US population will be nonwhite.4 Paradoxically, despite having increased sun protection from greater amounts of melanin in their skin, black individuals are more likely to present with advanced-stage melanoma (eg, stage III/IV) compared to white individuals.8-12 Furthermore, those of nonwhite populations are more likely to present with more advanced stages of acral lentiginous melanomas than white individuals.13,14 Hispanics also face an increasing incidence of more invasive acral lentiginous melanomas.15 Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.1
Although skin cancer is largely a preventable condition, the literature suggests that lack of awareness of melanoma among ethnic minorities is one of the main reasons for their poor skin cancer prognosis.16 This lack of awareness decreases the likelihood that an SOC patient would be alert to early detection of cancerous changes.17 Because educating at-risk SOC populations is key to decreasing skin cancer risk, this study focused on determining the efficacy of major knowledge-based interventions conducted to date.1 Overall, we sought to answer the question, do knowledge-based interventions increase skin cancer awareness, knowledge, and protective behavior among people of color?
Methods
For this review, the Cochrane method of analysis was used to conduct a thorough search of PubMed articles indexed for MEDLINE (1994-2016), as well as a search of CINAHL (1997-2016), PsycINFO (1999-2016), and Web of Science (1965-2016), using a combination of more than 100 search terms including but not limited to skin cancer, skin of color, intervention, and ethnic skin. The search yielded a total of 52 articles (Figure). Following review, only 8 articles met inclusion criteria, which were as follows: (1) study was related to skin cancer in SOC patients, which included an intervention to increase skin cancer awareness and knowledge; (2) study included adult participants or adolescents aged 12 to 18 years; (3) study was written in English; and (4) study was published in a peer-reviewed journal. Of the remaining 8 articles, 4 were excluded due to the following criteria: (1) study failed to provide both preintervention and postintervention data, (2) study failed to provide quantitative data, and (3) study included participants who worked as health care professionals or ancillary staff. As a result, a total of 4 articles were analyzed and discussed in this review (Table).
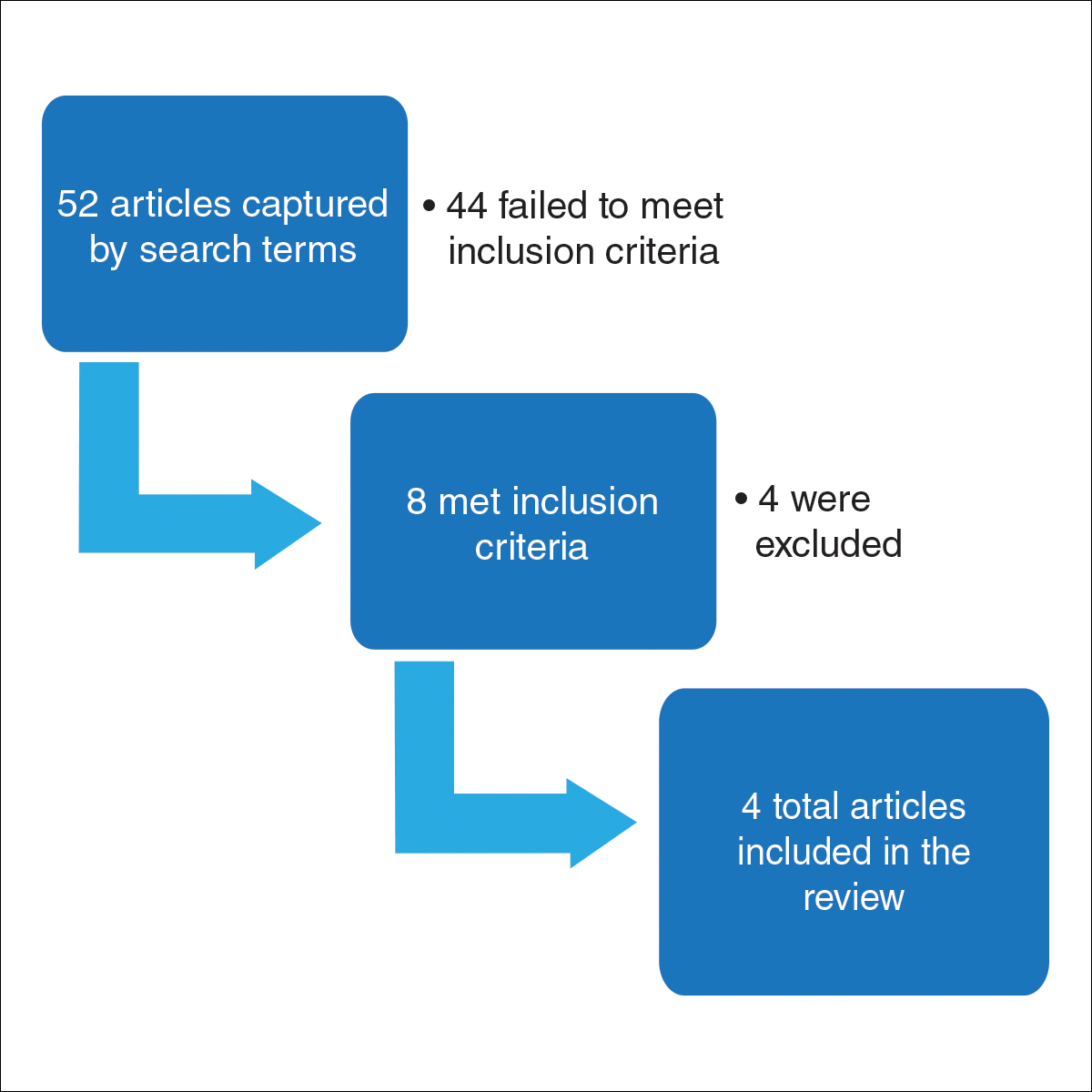
Results
Robinson et al18 conducted 12 focus groups with 120 total participants (40 black, 40 Asian, and 40 Hispanic patients). Participants engaged in a 2-hour tape-recorded focus group with a moderator guide on melanoma and skin cancer. Furthermore, they also were asked to assess skin cancer risk in 5 celebrities with different skin tones. The statistically significant preintervention results of the study (χ2=4.6, P<.001) were as follows: only 2%, 4%, and 14% correctly reported that celebrities with a very fair skin type, a fair skin type, and very dark skin type, respectively, could get sunburn, compared to 75%, 76%, and 62% post-intervention. Additionally, prior to intervention, 14% of the study population believed that dark brown skin type could get sunburn compared to 62% of the same group postintervention. This study demonstrated that the intervention helped SOC patients better identify their ability to get sunburn and identify their skin cancer risk.18
Hernandez et al19 used a video-based intervention in a Hispanic community, which was in contrast to the multiracial focus group intervention conducted by Robinson et al.18 Eighty Hispanic individuals were recruited from beauty salons to participate in the study. Participants watched two 3-minute videos in Spanish and completed a preintervention and postintervention survey. The first video emphasized the photoaging benefits of sun protection, while the second focused on skin cancer prevention. Preintervention surveys indicated that only 54 (68%) participants believed that fair-skinned Hispanics were at risk for skin cancer, which improved to 72 (90%) participants postintervention. Furthermore, initially only 44 (55%) participants thought those with darker skin types could develop skin cancer, but this number increased to 69 (86%) postintervention. For both questions regarding fair and dark skin, the agreement proportion was significantly different between the preeducation and posteducation videos (P<.0002 for the fair skin question and P<.0001 for the dark skin question). This study greatly increased awareness of skin cancer risk among Hispanics,19 similar to the Robinson et al18 study.
In contrast to 2-hour focus groups or 3-minute video–based interventions, a study by Kundu et al17 employed a 20-minute educational class-based intervention with both verbal and visual instruction. This study assessed the efficacy of an educational tutorial on improving awareness and early detection of melanoma in SOC individuals. Photographs were used to help participants recognize the ABCDEs of melanoma and to show examples of acral lentiginous melanomas in white individuals. A total of 71 participants completed a preintervention questionnaire, participated in a 20-minute class, and completed a postintervention questionnaire immediately after and 3 months following the class. The study population included 44 black, 15 Asian, 10 Hispanic, and 2 multiethnic participants. Knowledge that melanoma is a skin cancer increased from 83.9% to 100% immediately postintervention (P=.0001) and 97.2% at 3 months postintervention (P=.0075). Additionally, knowledge that people of color are at risk for melanoma increased from 48.4% preintervention to 82.8% immediately postintervention (P<.0001). However, only 40.8% of participants retained this knowledge at 3 months postintervention. Because only 1 participant reported a family history of skin cancer, the authors hypothesized that the reason for this loss of knowledge was that most participants were not personally affected by friends or family members with melanoma. A future study with an appropriate control group would be needed to support this claim. This study shed light on the potential of class-based interventions to increase both awareness and knowledge of skin cancer in SOC populations.17
A study by Chapman et al20 examined the effects of a sun protection educational program on increasing awareness of skin cancer in Hispanic and black middle school students in southern Los Angeles, California. It was the only study we reviewed that focused primarily on adolescents. Furthermore, it included the largest sample size (N=148) analyzed here. Students were given a preintervention questionnaire to evaluate their awareness of skin cancer and current sun-protection practices. Based on these results, the investigators devised a set of learning goals and incorporated them into an educational pamphlet. The intervention, called “Skin Teaching Day,” was a 1-day program discussing skin cancer and the importance of sun protection. Prior to the intervention, 68% of participants reported that they used sunscreen. Three months after completing the program, 80% of participants reported sunscreen use, an increase of 12% prior to the intervention. The results of this study demonstrated the unique effectiveness and potential of pamphlets in increasing sunscreen use.20
Comment
Overall, various methods of interventions such as focus groups, videos, pamphlets, and lectures improved knowledge of skin cancer risk and sun-protection behaviors in SOC populations. Furthermore, the unique differences of each study provided important insights into the successful design of an intervention.
An important characteristic of the Robinson et al18 study was the addition of photographs, which allowed participants not only to visualize different skin tones but also provided them with the opportunity to relate themselves to the photographs; by doing so, participants could effectively pick out the skin tone that best suited them. Written SOC scales are limited to mere descriptions and thus make it more difficult for participants to accurately identify the tone that best fits them. Kundu et al17 used photographs to teach skin self-examination and ABCDEs for detection of melanoma. Additionally, both studies used photographs to demonstrate examples of skin cancer.17,18 Recent evidence suggests the use of visuals can be efficacious for improving skin cancer knowledge and awareness; a study in 16 SOC kidney transplant recipients found that the addition of photographs of squamous cell carcinoma in various skin tones to a sun-protection educational pamphlet was more effective than the original pamphlet without photographs.21
In contrast to the Robinson et al18 study and Hernandez et al19 study, the Kundu et al17 study showed photographs of acral lentiginous melanomas in white patients rather than SOC patients. However, SOC populations may be less likely to relate to or identify skin changes in skin types that are different from their own. This technique was still beneficial, as acral lentiginous melanoma is the most common type of melanoma in SOC populations. Another benefit of the study was that it was the only study reviewed that included a follow-up postintervention questionnaire. Such data is useful, as it demonstrates how muchinformation is retained by participants and may be more likely to predict compliance with skin cancer protective behaviors.17
The Hernandez et al19 study is unique in that it was the only one to include an educational intervention entirely in Spanish, which is important to consider, as language may be a hindrance to participants’ understanding in the other studies, particularly Hispanics, possibly leading to a lack of information retention regarding sun-protective behaviors. Furthermore, it also was the only study to utilize videos as a method for interventions. The 3-minute videos demonstrated that interventions could be efficient as compared to the 2-hour in-class intervention used by Robinson et al18 and the 20-minute intervention used by Kundu et al.17 Additionally, videos also could be more cost-effective, as incentives for large focus groups would no longer be needed. Furthermore, in the Hernandez et al19 study, there was minimal to no disruption in the participants’ daily routine, as the participants were getting cosmetic services while watching the videos, perhaps allowing them to be more attentive. In contrast, both the Robinson et al18 and Kundu et al17 studies required time out from the participants’ daily schedules. In addition, these studies were notably longer than the Hernandez et al19 study. The 8-hour intervention in the Chapman et al20 study also may not be feasible for the general population because of its excessive length. However, the intervention was successful among the adolescent participants, which suggested that shorter durations are effective in the adult population and longer interventions may be more appropriate for adolescents because they benefit from peer activity.
Despite the success of the educational interventions as outlined in the 4 studies described here, a major epidemiologic flaw is that these interventions included only a small percentage of the target population. The largest total number of adults surveyed and undergoing an intervention in any of the populations was only 120.17 By failing to reach a substantial proportion of the population at risk, the number of preventable deaths likely will not decrease. The authors believe a larger-scale intervention would provide meaningful change. Australia’s SunSmart campaign to increase skin cancer awareness in the Australian population is an example of one such large-scale national intervention. The campaign focused on massive television advertisements in the summer to educate participants about the dangers of skin cancer and the importance of protective behaviors. Telephone surveys conducted from 1987 to 2011 demonstrated that more exposure to the advertisements in the SunSmart campaign meant that individuals were more likely to use sunscreen and avoid sun exposure.22 In the United States, a similar intervention would be of great benefit in educating SOC populations regarding skin cancer risk. Additionally, dermatology residents need to be adequately trained to educate patients of color about the risk for skin cancer, as survey data indicated more than 80% of Australian dermatologists desired more SOC teaching during their training and 50% indicated that they would have time to learn it during their training if offered.23 Furthermore, one study suggested that future interventions must include primary-, secondary-, and tertiary-prevention methods to effectively reduce skin cancer risk among patients of color.24 Primary prevention involves sun avoidance, secondary prevention involves detecting cancerous lesions, and tertiary prevention involves undergoing treatment of skin malignancies. However, increased knowledge does not necessarily mean increased preventative action will be employed (eg, sunscreen use, wearing sun-protective clothing and sunglasses, avoiding tanning beds and excessive sun exposure). Additional studies that demonstrate a notable increase in sun-protective behaviors related to increased knowledge are needed.
Because retention of skin cancer knowledge decreased in several postintervention surveys, there also is a dire need for continuing skin cancer education in patients of color, which may be accomplished through a combination effort of television advertisement campaigns, pamphlets, social media, community health departments, or even community members. For example, a pilot program found that Hispanic lay health workers who are educated about skin cancer may serve as a bridge between medical providers and the Hispanic community by encouraging individuals in this population to get regular skin examinations from a physician.25 Overall, there are currently gaps in the understanding and treatment of skin cancer in people of color.26 Identifying the advantages and disadvantages of all relevant skin cancer interventions conducted in the SOC population will hopefully guide future studies to help close these gaps by allowing others to design the best possible intervention. By doing so, researchers can generate an intervention that is precise, well-informed, and effective in decreasing mortality rates from skin cancer among SOC populations.
Conclusion
All of the studies reviewed demonstrated that instructional and educational interventions are promising methods for improving either knowledge, awareness, or safe skin practices and sun-protective behaviors in SOC populations to differing degrees (Table). Although each of the 4 interventions employed their own methods, they all increased 1 or more of the 3 aforementioned concepts—knowledge, awareness, or safe skin practices and sun-protective behaviors—when comparing postsurvey to presurvey data. However, the critically important message derived from this research is that there is a tremendous need for a substantial large-scale educational intervention to increase knowledge regarding skin cancer in SOC populations.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Byrd KM, Wilson DC, Hoyler SS, et al. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21-24.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5, suppl 1):S26-S37.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708.
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;5:1031-1032.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- Shin S, Palis BE, Phillips JL, et al. Cutaneous melanoma in Asian-Americans. J Surg Oncol. 2009;99:114-118.
- Stubblefield J, Kelly B. Melanoma in non-caucasian populations. Surg Clin North Am. 2014;94:1115-1126.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Pichon LC, Corral I, Landrine H, et al. Perceived skin cancer risk and sunscreen use among African American adults. J Health Psychol. 2010;15:1181-1189.
- Kundu RV, Kamaria M, Ortiz S, et al. Effectiveness of a knowledge-based intervention for melanoma among those with ethnic skin. J Am Acad Dermatol. 2010;62:777-784.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2010;20:313-320.
- Hernandez C, Wang S, Abraham I, et al. Evaluation of educational videos to increase skin cancer risk awareness and sun safe behaviors among adult Hispanics. J Cancer Educ. 2014;29:563-569.
- Chapman LW, Ochoa A, Tenconi F, et al. Dermatologic health literacy in underserved communities: a case report of south Los Angeles middle schools. Dermatol Online J. 2015;21. pii:13030/qt8671p40n.
- Yanina G, Gaber R, Clayman ML, et al. Sun protection education for diverse audiences: need for skin cancer pictures. J Cancer Educ. 2015;30:187-189.
- Dobbinson SJ, Volkov A, Wakefield MA. Continued impact of sunsmart advertising on youth and adults’ behaviors. Am J Prev Med. 2015;49:20-28.
- Rodrigues MA, Ross AL, Gilmore S, et al. Australian dermatologists’ perspective on skin of colour: results of a national survey [published online December 9, 2016]. Australas J Dermatol. doi:10.1111/ajd.12556.
- Jacobsen A, Galvan A, Lachapelle CC, et al. Defining the need for skin cancer prevention education in uninsured, minority, and immigrant communities. JAMA Dermatol. 2016;152:1342-1347.
- Hernandez C, Kim H, Mauleon G, et al. A pilot program in collaboration with community centers to increase awareness and participation in skin cancer screening among Latinos in Chicago. J Cancer Educ. 2013;28:342-345.
- Kailas A, Solomon JA, Mostow EN, et al. Gaps in the understanding and treatment of skin cancer in people of color. J Am Acad Dermatol. 2016;74:144-149.
Malignant melanoma, basal cell carcinoma, and squamous cell carcinoma account for approximately 40% of all neoplasms among the white population in the United States. Skin cancer is the most common malignancy in the United States.1 However, despite this occurrence, there are limited data regarding skin cancer in individuals with skin of color (SOC). The 5-year survival rates for melanoma are 58.2% for black individuals, 69.7% for Hispanics, and 70.9% for Asians compared to 79.8% for white individuals in the United States.2 Even though SOC populations have lower incidences of skin cancer—melanoma, basal cell carcinoma, and squamous cell carcinoma—they exhibit higher death rates.3-7 Nonetheless, no specific guidelines exist to address sun exposure and safety habits in SOC populations.6,8 Furthermore, current demographics suggest that by the year 2050, approximately half of the US population will be nonwhite.4 Paradoxically, despite having increased sun protection from greater amounts of melanin in their skin, black individuals are more likely to present with advanced-stage melanoma (eg, stage III/IV) compared to white individuals.8-12 Furthermore, those of nonwhite populations are more likely to present with more advanced stages of acral lentiginous melanomas than white individuals.13,14 Hispanics also face an increasing incidence of more invasive acral lentiginous melanomas.15 Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.1
Although skin cancer is largely a preventable condition, the literature suggests that lack of awareness of melanoma among ethnic minorities is one of the main reasons for their poor skin cancer prognosis.16 This lack of awareness decreases the likelihood that an SOC patient would be alert to early detection of cancerous changes.17 Because educating at-risk SOC populations is key to decreasing skin cancer risk, this study focused on determining the efficacy of major knowledge-based interventions conducted to date.1 Overall, we sought to answer the question, do knowledge-based interventions increase skin cancer awareness, knowledge, and protective behavior among people of color?
Methods
For this review, the Cochrane method of analysis was used to conduct a thorough search of PubMed articles indexed for MEDLINE (1994-2016), as well as a search of CINAHL (1997-2016), PsycINFO (1999-2016), and Web of Science (1965-2016), using a combination of more than 100 search terms including but not limited to skin cancer, skin of color, intervention, and ethnic skin. The search yielded a total of 52 articles (Figure). Following review, only 8 articles met inclusion criteria, which were as follows: (1) study was related to skin cancer in SOC patients, which included an intervention to increase skin cancer awareness and knowledge; (2) study included adult participants or adolescents aged 12 to 18 years; (3) study was written in English; and (4) study was published in a peer-reviewed journal. Of the remaining 8 articles, 4 were excluded due to the following criteria: (1) study failed to provide both preintervention and postintervention data, (2) study failed to provide quantitative data, and (3) study included participants who worked as health care professionals or ancillary staff. As a result, a total of 4 articles were analyzed and discussed in this review (Table).

Results
Robinson et al18 conducted 12 focus groups with 120 total participants (40 black, 40 Asian, and 40 Hispanic patients). Participants engaged in a 2-hour tape-recorded focus group with a moderator guide on melanoma and skin cancer. Furthermore, they also were asked to assess skin cancer risk in 5 celebrities with different skin tones. The statistically significant preintervention results of the study (χ2=4.6, P<.001) were as follows: only 2%, 4%, and 14% correctly reported that celebrities with a very fair skin type, a fair skin type, and very dark skin type, respectively, could get sunburn, compared to 75%, 76%, and 62% post-intervention. Additionally, prior to intervention, 14% of the study population believed that dark brown skin type could get sunburn compared to 62% of the same group postintervention. This study demonstrated that the intervention helped SOC patients better identify their ability to get sunburn and identify their skin cancer risk.18
Hernandez et al19 used a video-based intervention in a Hispanic community, which was in contrast to the multiracial focus group intervention conducted by Robinson et al.18 Eighty Hispanic individuals were recruited from beauty salons to participate in the study. Participants watched two 3-minute videos in Spanish and completed a preintervention and postintervention survey. The first video emphasized the photoaging benefits of sun protection, while the second focused on skin cancer prevention. Preintervention surveys indicated that only 54 (68%) participants believed that fair-skinned Hispanics were at risk for skin cancer, which improved to 72 (90%) participants postintervention. Furthermore, initially only 44 (55%) participants thought those with darker skin types could develop skin cancer, but this number increased to 69 (86%) postintervention. For both questions regarding fair and dark skin, the agreement proportion was significantly different between the preeducation and posteducation videos (P<.0002 for the fair skin question and P<.0001 for the dark skin question). This study greatly increased awareness of skin cancer risk among Hispanics,19 similar to the Robinson et al18 study.
In contrast to 2-hour focus groups or 3-minute video–based interventions, a study by Kundu et al17 employed a 20-minute educational class-based intervention with both verbal and visual instruction. This study assessed the efficacy of an educational tutorial on improving awareness and early detection of melanoma in SOC individuals. Photographs were used to help participants recognize the ABCDEs of melanoma and to show examples of acral lentiginous melanomas in white individuals. A total of 71 participants completed a preintervention questionnaire, participated in a 20-minute class, and completed a postintervention questionnaire immediately after and 3 months following the class. The study population included 44 black, 15 Asian, 10 Hispanic, and 2 multiethnic participants. Knowledge that melanoma is a skin cancer increased from 83.9% to 100% immediately postintervention (P=.0001) and 97.2% at 3 months postintervention (P=.0075). Additionally, knowledge that people of color are at risk for melanoma increased from 48.4% preintervention to 82.8% immediately postintervention (P<.0001). However, only 40.8% of participants retained this knowledge at 3 months postintervention. Because only 1 participant reported a family history of skin cancer, the authors hypothesized that the reason for this loss of knowledge was that most participants were not personally affected by friends or family members with melanoma. A future study with an appropriate control group would be needed to support this claim. This study shed light on the potential of class-based interventions to increase both awareness and knowledge of skin cancer in SOC populations.17
A study by Chapman et al20 examined the effects of a sun protection educational program on increasing awareness of skin cancer in Hispanic and black middle school students in southern Los Angeles, California. It was the only study we reviewed that focused primarily on adolescents. Furthermore, it included the largest sample size (N=148) analyzed here. Students were given a preintervention questionnaire to evaluate their awareness of skin cancer and current sun-protection practices. Based on these results, the investigators devised a set of learning goals and incorporated them into an educational pamphlet. The intervention, called “Skin Teaching Day,” was a 1-day program discussing skin cancer and the importance of sun protection. Prior to the intervention, 68% of participants reported that they used sunscreen. Three months after completing the program, 80% of participants reported sunscreen use, an increase of 12% prior to the intervention. The results of this study demonstrated the unique effectiveness and potential of pamphlets in increasing sunscreen use.20
Comment
Overall, various methods of interventions such as focus groups, videos, pamphlets, and lectures improved knowledge of skin cancer risk and sun-protection behaviors in SOC populations. Furthermore, the unique differences of each study provided important insights into the successful design of an intervention.
An important characteristic of the Robinson et al18 study was the addition of photographs, which allowed participants not only to visualize different skin tones but also provided them with the opportunity to relate themselves to the photographs; by doing so, participants could effectively pick out the skin tone that best suited them. Written SOC scales are limited to mere descriptions and thus make it more difficult for participants to accurately identify the tone that best fits them. Kundu et al17 used photographs to teach skin self-examination and ABCDEs for detection of melanoma. Additionally, both studies used photographs to demonstrate examples of skin cancer.17,18 Recent evidence suggests the use of visuals can be efficacious for improving skin cancer knowledge and awareness; a study in 16 SOC kidney transplant recipients found that the addition of photographs of squamous cell carcinoma in various skin tones to a sun-protection educational pamphlet was more effective than the original pamphlet without photographs.21
In contrast to the Robinson et al18 study and Hernandez et al19 study, the Kundu et al17 study showed photographs of acral lentiginous melanomas in white patients rather than SOC patients. However, SOC populations may be less likely to relate to or identify skin changes in skin types that are different from their own. This technique was still beneficial, as acral lentiginous melanoma is the most common type of melanoma in SOC populations. Another benefit of the study was that it was the only study reviewed that included a follow-up postintervention questionnaire. Such data is useful, as it demonstrates how muchinformation is retained by participants and may be more likely to predict compliance with skin cancer protective behaviors.17
The Hernandez et al19 study is unique in that it was the only one to include an educational intervention entirely in Spanish, which is important to consider, as language may be a hindrance to participants’ understanding in the other studies, particularly Hispanics, possibly leading to a lack of information retention regarding sun-protective behaviors. Furthermore, it also was the only study to utilize videos as a method for interventions. The 3-minute videos demonstrated that interventions could be efficient as compared to the 2-hour in-class intervention used by Robinson et al18 and the 20-minute intervention used by Kundu et al.17 Additionally, videos also could be more cost-effective, as incentives for large focus groups would no longer be needed. Furthermore, in the Hernandez et al19 study, there was minimal to no disruption in the participants’ daily routine, as the participants were getting cosmetic services while watching the videos, perhaps allowing them to be more attentive. In contrast, both the Robinson et al18 and Kundu et al17 studies required time out from the participants’ daily schedules. In addition, these studies were notably longer than the Hernandez et al19 study. The 8-hour intervention in the Chapman et al20 study also may not be feasible for the general population because of its excessive length. However, the intervention was successful among the adolescent participants, which suggested that shorter durations are effective in the adult population and longer interventions may be more appropriate for adolescents because they benefit from peer activity.
Despite the success of the educational interventions as outlined in the 4 studies described here, a major epidemiologic flaw is that these interventions included only a small percentage of the target population. The largest total number of adults surveyed and undergoing an intervention in any of the populations was only 120.17 By failing to reach a substantial proportion of the population at risk, the number of preventable deaths likely will not decrease. The authors believe a larger-scale intervention would provide meaningful change. Australia’s SunSmart campaign to increase skin cancer awareness in the Australian population is an example of one such large-scale national intervention. The campaign focused on massive television advertisements in the summer to educate participants about the dangers of skin cancer and the importance of protective behaviors. Telephone surveys conducted from 1987 to 2011 demonstrated that more exposure to the advertisements in the SunSmart campaign meant that individuals were more likely to use sunscreen and avoid sun exposure.22 In the United States, a similar intervention would be of great benefit in educating SOC populations regarding skin cancer risk. Additionally, dermatology residents need to be adequately trained to educate patients of color about the risk for skin cancer, as survey data indicated more than 80% of Australian dermatologists desired more SOC teaching during their training and 50% indicated that they would have time to learn it during their training if offered.23 Furthermore, one study suggested that future interventions must include primary-, secondary-, and tertiary-prevention methods to effectively reduce skin cancer risk among patients of color.24 Primary prevention involves sun avoidance, secondary prevention involves detecting cancerous lesions, and tertiary prevention involves undergoing treatment of skin malignancies. However, increased knowledge does not necessarily mean increased preventative action will be employed (eg, sunscreen use, wearing sun-protective clothing and sunglasses, avoiding tanning beds and excessive sun exposure). Additional studies that demonstrate a notable increase in sun-protective behaviors related to increased knowledge are needed.
Because retention of skin cancer knowledge decreased in several postintervention surveys, there also is a dire need for continuing skin cancer education in patients of color, which may be accomplished through a combination effort of television advertisement campaigns, pamphlets, social media, community health departments, or even community members. For example, a pilot program found that Hispanic lay health workers who are educated about skin cancer may serve as a bridge between medical providers and the Hispanic community by encouraging individuals in this population to get regular skin examinations from a physician.25 Overall, there are currently gaps in the understanding and treatment of skin cancer in people of color.26 Identifying the advantages and disadvantages of all relevant skin cancer interventions conducted in the SOC population will hopefully guide future studies to help close these gaps by allowing others to design the best possible intervention. By doing so, researchers can generate an intervention that is precise, well-informed, and effective in decreasing mortality rates from skin cancer among SOC populations.
Conclusion
All of the studies reviewed demonstrated that instructional and educational interventions are promising methods for improving either knowledge, awareness, or safe skin practices and sun-protective behaviors in SOC populations to differing degrees (Table). Although each of the 4 interventions employed their own methods, they all increased 1 or more of the 3 aforementioned concepts—knowledge, awareness, or safe skin practices and sun-protective behaviors—when comparing postsurvey to presurvey data. However, the critically important message derived from this research is that there is a tremendous need for a substantial large-scale educational intervention to increase knowledge regarding skin cancer in SOC populations.
Malignant melanoma, basal cell carcinoma, and squamous cell carcinoma account for approximately 40% of all neoplasms among the white population in the United States. Skin cancer is the most common malignancy in the United States.1 However, despite this occurrence, there are limited data regarding skin cancer in individuals with skin of color (SOC). The 5-year survival rates for melanoma are 58.2% for black individuals, 69.7% for Hispanics, and 70.9% for Asians compared to 79.8% for white individuals in the United States.2 Even though SOC populations have lower incidences of skin cancer—melanoma, basal cell carcinoma, and squamous cell carcinoma—they exhibit higher death rates.3-7 Nonetheless, no specific guidelines exist to address sun exposure and safety habits in SOC populations.6,8 Furthermore, current demographics suggest that by the year 2050, approximately half of the US population will be nonwhite.4 Paradoxically, despite having increased sun protection from greater amounts of melanin in their skin, black individuals are more likely to present with advanced-stage melanoma (eg, stage III/IV) compared to white individuals.8-12 Furthermore, those of nonwhite populations are more likely to present with more advanced stages of acral lentiginous melanomas than white individuals.13,14 Hispanics also face an increasing incidence of more invasive acral lentiginous melanomas.15 Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.1
Although skin cancer is largely a preventable condition, the literature suggests that lack of awareness of melanoma among ethnic minorities is one of the main reasons for their poor skin cancer prognosis.16 This lack of awareness decreases the likelihood that an SOC patient would be alert to early detection of cancerous changes.17 Because educating at-risk SOC populations is key to decreasing skin cancer risk, this study focused on determining the efficacy of major knowledge-based interventions conducted to date.1 Overall, we sought to answer the question, do knowledge-based interventions increase skin cancer awareness, knowledge, and protective behavior among people of color?
Methods
For this review, the Cochrane method of analysis was used to conduct a thorough search of PubMed articles indexed for MEDLINE (1994-2016), as well as a search of CINAHL (1997-2016), PsycINFO (1999-2016), and Web of Science (1965-2016), using a combination of more than 100 search terms including but not limited to skin cancer, skin of color, intervention, and ethnic skin. The search yielded a total of 52 articles (Figure). Following review, only 8 articles met inclusion criteria, which were as follows: (1) study was related to skin cancer in SOC patients, which included an intervention to increase skin cancer awareness and knowledge; (2) study included adult participants or adolescents aged 12 to 18 years; (3) study was written in English; and (4) study was published in a peer-reviewed journal. Of the remaining 8 articles, 4 were excluded due to the following criteria: (1) study failed to provide both preintervention and postintervention data, (2) study failed to provide quantitative data, and (3) study included participants who worked as health care professionals or ancillary staff. As a result, a total of 4 articles were analyzed and discussed in this review (Table).

Results
Robinson et al18 conducted 12 focus groups with 120 total participants (40 black, 40 Asian, and 40 Hispanic patients). Participants engaged in a 2-hour tape-recorded focus group with a moderator guide on melanoma and skin cancer. Furthermore, they also were asked to assess skin cancer risk in 5 celebrities with different skin tones. The statistically significant preintervention results of the study (χ2=4.6, P<.001) were as follows: only 2%, 4%, and 14% correctly reported that celebrities with a very fair skin type, a fair skin type, and very dark skin type, respectively, could get sunburn, compared to 75%, 76%, and 62% post-intervention. Additionally, prior to intervention, 14% of the study population believed that dark brown skin type could get sunburn compared to 62% of the same group postintervention. This study demonstrated that the intervention helped SOC patients better identify their ability to get sunburn and identify their skin cancer risk.18
Hernandez et al19 used a video-based intervention in a Hispanic community, which was in contrast to the multiracial focus group intervention conducted by Robinson et al.18 Eighty Hispanic individuals were recruited from beauty salons to participate in the study. Participants watched two 3-minute videos in Spanish and completed a preintervention and postintervention survey. The first video emphasized the photoaging benefits of sun protection, while the second focused on skin cancer prevention. Preintervention surveys indicated that only 54 (68%) participants believed that fair-skinned Hispanics were at risk for skin cancer, which improved to 72 (90%) participants postintervention. Furthermore, initially only 44 (55%) participants thought those with darker skin types could develop skin cancer, but this number increased to 69 (86%) postintervention. For both questions regarding fair and dark skin, the agreement proportion was significantly different between the preeducation and posteducation videos (P<.0002 for the fair skin question and P<.0001 for the dark skin question). This study greatly increased awareness of skin cancer risk among Hispanics,19 similar to the Robinson et al18 study.
In contrast to 2-hour focus groups or 3-minute video–based interventions, a study by Kundu et al17 employed a 20-minute educational class-based intervention with both verbal and visual instruction. This study assessed the efficacy of an educational tutorial on improving awareness and early detection of melanoma in SOC individuals. Photographs were used to help participants recognize the ABCDEs of melanoma and to show examples of acral lentiginous melanomas in white individuals. A total of 71 participants completed a preintervention questionnaire, participated in a 20-minute class, and completed a postintervention questionnaire immediately after and 3 months following the class. The study population included 44 black, 15 Asian, 10 Hispanic, and 2 multiethnic participants. Knowledge that melanoma is a skin cancer increased from 83.9% to 100% immediately postintervention (P=.0001) and 97.2% at 3 months postintervention (P=.0075). Additionally, knowledge that people of color are at risk for melanoma increased from 48.4% preintervention to 82.8% immediately postintervention (P<.0001). However, only 40.8% of participants retained this knowledge at 3 months postintervention. Because only 1 participant reported a family history of skin cancer, the authors hypothesized that the reason for this loss of knowledge was that most participants were not personally affected by friends or family members with melanoma. A future study with an appropriate control group would be needed to support this claim. This study shed light on the potential of class-based interventions to increase both awareness and knowledge of skin cancer in SOC populations.17
A study by Chapman et al20 examined the effects of a sun protection educational program on increasing awareness of skin cancer in Hispanic and black middle school students in southern Los Angeles, California. It was the only study we reviewed that focused primarily on adolescents. Furthermore, it included the largest sample size (N=148) analyzed here. Students were given a preintervention questionnaire to evaluate their awareness of skin cancer and current sun-protection practices. Based on these results, the investigators devised a set of learning goals and incorporated them into an educational pamphlet. The intervention, called “Skin Teaching Day,” was a 1-day program discussing skin cancer and the importance of sun protection. Prior to the intervention, 68% of participants reported that they used sunscreen. Three months after completing the program, 80% of participants reported sunscreen use, an increase of 12% prior to the intervention. The results of this study demonstrated the unique effectiveness and potential of pamphlets in increasing sunscreen use.20
Comment
Overall, various methods of interventions such as focus groups, videos, pamphlets, and lectures improved knowledge of skin cancer risk and sun-protection behaviors in SOC populations. Furthermore, the unique differences of each study provided important insights into the successful design of an intervention.
An important characteristic of the Robinson et al18 study was the addition of photographs, which allowed participants not only to visualize different skin tones but also provided them with the opportunity to relate themselves to the photographs; by doing so, participants could effectively pick out the skin tone that best suited them. Written SOC scales are limited to mere descriptions and thus make it more difficult for participants to accurately identify the tone that best fits them. Kundu et al17 used photographs to teach skin self-examination and ABCDEs for detection of melanoma. Additionally, both studies used photographs to demonstrate examples of skin cancer.17,18 Recent evidence suggests the use of visuals can be efficacious for improving skin cancer knowledge and awareness; a study in 16 SOC kidney transplant recipients found that the addition of photographs of squamous cell carcinoma in various skin tones to a sun-protection educational pamphlet was more effective than the original pamphlet without photographs.21
In contrast to the Robinson et al18 study and Hernandez et al19 study, the Kundu et al17 study showed photographs of acral lentiginous melanomas in white patients rather than SOC patients. However, SOC populations may be less likely to relate to or identify skin changes in skin types that are different from their own. This technique was still beneficial, as acral lentiginous melanoma is the most common type of melanoma in SOC populations. Another benefit of the study was that it was the only study reviewed that included a follow-up postintervention questionnaire. Such data is useful, as it demonstrates how muchinformation is retained by participants and may be more likely to predict compliance with skin cancer protective behaviors.17
The Hernandez et al19 study is unique in that it was the only one to include an educational intervention entirely in Spanish, which is important to consider, as language may be a hindrance to participants’ understanding in the other studies, particularly Hispanics, possibly leading to a lack of information retention regarding sun-protective behaviors. Furthermore, it also was the only study to utilize videos as a method for interventions. The 3-minute videos demonstrated that interventions could be efficient as compared to the 2-hour in-class intervention used by Robinson et al18 and the 20-minute intervention used by Kundu et al.17 Additionally, videos also could be more cost-effective, as incentives for large focus groups would no longer be needed. Furthermore, in the Hernandez et al19 study, there was minimal to no disruption in the participants’ daily routine, as the participants were getting cosmetic services while watching the videos, perhaps allowing them to be more attentive. In contrast, both the Robinson et al18 and Kundu et al17 studies required time out from the participants’ daily schedules. In addition, these studies were notably longer than the Hernandez et al19 study. The 8-hour intervention in the Chapman et al20 study also may not be feasible for the general population because of its excessive length. However, the intervention was successful among the adolescent participants, which suggested that shorter durations are effective in the adult population and longer interventions may be more appropriate for adolescents because they benefit from peer activity.
Despite the success of the educational interventions as outlined in the 4 studies described here, a major epidemiologic flaw is that these interventions included only a small percentage of the target population. The largest total number of adults surveyed and undergoing an intervention in any of the populations was only 120.17 By failing to reach a substantial proportion of the population at risk, the number of preventable deaths likely will not decrease. The authors believe a larger-scale intervention would provide meaningful change. Australia’s SunSmart campaign to increase skin cancer awareness in the Australian population is an example of one such large-scale national intervention. The campaign focused on massive television advertisements in the summer to educate participants about the dangers of skin cancer and the importance of protective behaviors. Telephone surveys conducted from 1987 to 2011 demonstrated that more exposure to the advertisements in the SunSmart campaign meant that individuals were more likely to use sunscreen and avoid sun exposure.22 In the United States, a similar intervention would be of great benefit in educating SOC populations regarding skin cancer risk. Additionally, dermatology residents need to be adequately trained to educate patients of color about the risk for skin cancer, as survey data indicated more than 80% of Australian dermatologists desired more SOC teaching during their training and 50% indicated that they would have time to learn it during their training if offered.23 Furthermore, one study suggested that future interventions must include primary-, secondary-, and tertiary-prevention methods to effectively reduce skin cancer risk among patients of color.24 Primary prevention involves sun avoidance, secondary prevention involves detecting cancerous lesions, and tertiary prevention involves undergoing treatment of skin malignancies. However, increased knowledge does not necessarily mean increased preventative action will be employed (eg, sunscreen use, wearing sun-protective clothing and sunglasses, avoiding tanning beds and excessive sun exposure). Additional studies that demonstrate a notable increase in sun-protective behaviors related to increased knowledge are needed.
Because retention of skin cancer knowledge decreased in several postintervention surveys, there also is a dire need for continuing skin cancer education in patients of color, which may be accomplished through a combination effort of television advertisement campaigns, pamphlets, social media, community health departments, or even community members. For example, a pilot program found that Hispanic lay health workers who are educated about skin cancer may serve as a bridge between medical providers and the Hispanic community by encouraging individuals in this population to get regular skin examinations from a physician.25 Overall, there are currently gaps in the understanding and treatment of skin cancer in people of color.26 Identifying the advantages and disadvantages of all relevant skin cancer interventions conducted in the SOC population will hopefully guide future studies to help close these gaps by allowing others to design the best possible intervention. By doing so, researchers can generate an intervention that is precise, well-informed, and effective in decreasing mortality rates from skin cancer among SOC populations.
Conclusion
All of the studies reviewed demonstrated that instructional and educational interventions are promising methods for improving either knowledge, awareness, or safe skin practices and sun-protective behaviors in SOC populations to differing degrees (Table). Although each of the 4 interventions employed their own methods, they all increased 1 or more of the 3 aforementioned concepts—knowledge, awareness, or safe skin practices and sun-protective behaviors—when comparing postsurvey to presurvey data. However, the critically important message derived from this research is that there is a tremendous need for a substantial large-scale educational intervention to increase knowledge regarding skin cancer in SOC populations.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Byrd KM, Wilson DC, Hoyler SS, et al. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21-24.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5, suppl 1):S26-S37.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708.
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;5:1031-1032.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- Shin S, Palis BE, Phillips JL, et al. Cutaneous melanoma in Asian-Americans. J Surg Oncol. 2009;99:114-118.
- Stubblefield J, Kelly B. Melanoma in non-caucasian populations. Surg Clin North Am. 2014;94:1115-1126.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Pichon LC, Corral I, Landrine H, et al. Perceived skin cancer risk and sunscreen use among African American adults. J Health Psychol. 2010;15:1181-1189.
- Kundu RV, Kamaria M, Ortiz S, et al. Effectiveness of a knowledge-based intervention for melanoma among those with ethnic skin. J Am Acad Dermatol. 2010;62:777-784.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2010;20:313-320.
- Hernandez C, Wang S, Abraham I, et al. Evaluation of educational videos to increase skin cancer risk awareness and sun safe behaviors among adult Hispanics. J Cancer Educ. 2014;29:563-569.
- Chapman LW, Ochoa A, Tenconi F, et al. Dermatologic health literacy in underserved communities: a case report of south Los Angeles middle schools. Dermatol Online J. 2015;21. pii:13030/qt8671p40n.
- Yanina G, Gaber R, Clayman ML, et al. Sun protection education for diverse audiences: need for skin cancer pictures. J Cancer Educ. 2015;30:187-189.
- Dobbinson SJ, Volkov A, Wakefield MA. Continued impact of sunsmart advertising on youth and adults’ behaviors. Am J Prev Med. 2015;49:20-28.
- Rodrigues MA, Ross AL, Gilmore S, et al. Australian dermatologists’ perspective on skin of colour: results of a national survey [published online December 9, 2016]. Australas J Dermatol. doi:10.1111/ajd.12556.
- Jacobsen A, Galvan A, Lachapelle CC, et al. Defining the need for skin cancer prevention education in uninsured, minority, and immigrant communities. JAMA Dermatol. 2016;152:1342-1347.
- Hernandez C, Kim H, Mauleon G, et al. A pilot program in collaboration with community centers to increase awareness and participation in skin cancer screening among Latinos in Chicago. J Cancer Educ. 2013;28:342-345.
- Kailas A, Solomon JA, Mostow EN, et al. Gaps in the understanding and treatment of skin cancer in people of color. J Am Acad Dermatol. 2016;74:144-149.
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760.
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75:983-991.
- Byrd KM, Wilson DC, Hoyler SS, et al. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:21-24.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5, suppl 1):S26-S37.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708.
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;5:1031-1032.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Pritchett EN, Doyle A, Shaver CM, et al. Nonmelanoma skin cancer in nonwhite organ transplant recipients. JAMA Dermatol. 2016;152:1348-1353.
- Shin S, Palis BE, Phillips JL, et al. Cutaneous melanoma in Asian-Americans. J Surg Oncol. 2009;99:114-118.
- Stubblefield J, Kelly B. Melanoma in non-caucasian populations. Surg Clin North Am. 2014;94:1115-1126.
- Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145:427-434.
- Pichon LC, Corral I, Landrine H, et al. Perceived skin cancer risk and sunscreen use among African American adults. J Health Psychol. 2010;15:1181-1189.
- Kundu RV, Kamaria M, Ortiz S, et al. Effectiveness of a knowledge-based intervention for melanoma among those with ethnic skin. J Am Acad Dermatol. 2010;62:777-784.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2010;20:313-320.
- Hernandez C, Wang S, Abraham I, et al. Evaluation of educational videos to increase skin cancer risk awareness and sun safe behaviors among adult Hispanics. J Cancer Educ. 2014;29:563-569.
- Chapman LW, Ochoa A, Tenconi F, et al. Dermatologic health literacy in underserved communities: a case report of south Los Angeles middle schools. Dermatol Online J. 2015;21. pii:13030/qt8671p40n.
- Yanina G, Gaber R, Clayman ML, et al. Sun protection education for diverse audiences: need for skin cancer pictures. J Cancer Educ. 2015;30:187-189.
- Dobbinson SJ, Volkov A, Wakefield MA. Continued impact of sunsmart advertising on youth and adults’ behaviors. Am J Prev Med. 2015;49:20-28.
- Rodrigues MA, Ross AL, Gilmore S, et al. Australian dermatologists’ perspective on skin of colour: results of a national survey [published online December 9, 2016]. Australas J Dermatol. doi:10.1111/ajd.12556.
- Jacobsen A, Galvan A, Lachapelle CC, et al. Defining the need for skin cancer prevention education in uninsured, minority, and immigrant communities. JAMA Dermatol. 2016;152:1342-1347.
- Hernandez C, Kim H, Mauleon G, et al. A pilot program in collaboration with community centers to increase awareness and participation in skin cancer screening among Latinos in Chicago. J Cancer Educ. 2013;28:342-345.
- Kailas A, Solomon JA, Mostow EN, et al. Gaps in the understanding and treatment of skin cancer in people of color. J Am Acad Dermatol. 2016;74:144-149.
Practice Points
- Patients of color should be informed that they are at risk for skin cancer including melanoma.
- Patients of color should be taught to identify suspicious skin lesions including the ABCDEs of melanoma.
- Patients of color should be instructed to perform self-body skin examinations, especially of the palms and soles, for any evolving skin lesions. Patients should be instructed on the importance of visiting a physician for an evolving or suspicious mole or lesion.
Do Infants Fed Rice and Rice Products Have an Increased Risk for Skin Cancer?
To the Editor:
Rice and rice products, such as rice cereal and rice snacks, contain inorganic arsenic. Exposure to arsenicin utero and during early life may be associated with adverse fetal growth, adverse infant and child immune response, and adverse neurodevelopmental outcomes. Therefore, the World Health Organization, the Food and Agriculture Organization of the United Nations, the European Union, and the US Food and Drug Administration have suggested maximum arsenic ingestion recommendations for infants: 100 ng/g for inorganic arsenic in products geared toward infants. However, infants consuming only a few servings of rice products may exceed the weekly tolerable intake of arsenic.
Karagas et al1 obtained dietary data on 759 infants who were enrolled in the New Hampshire Birth Cohort Study from 2011 to 2014. They noted that 80% of the infants had been introduced to rice cereal during the first year. Additional data on diet and total urinary arsenic at 12 months was available for 129 infants: 32.6% of these infants were fed rice snacks. In addition, the total urinary arsenic concentration was higher among infants who ate rice cereal or rice snacks as compared to infants who did not eat rice or rice products.
Chronic arsenic exposure can result in patchy dark brown hyperpigmentation with scattered pale spots referred to as “raindrops on a dusty road.” The axilla, eyelids, groin, neck, nipples, and temples often are affected. However, the hyperpigmentation can extend across the chest, abdomen, and back in severe cases.
Horizontal white lines across the nails (Mees lines) may develop. Keratoses, often on the palms (arsenic keratoses), may appear; they persist and may progress to skin cancers. In addition, patients with arsenic exposure are more susceptible to developing nonmelanoma skin cancers.2
It is unknown if exposure to inorganic arsenic in infancy predisposes these individuals to skin cancer when they become adults. Long-term longitudinal follow-up of the participants in this study may provide additional insight. Perhaps infants should not receive rice cereals and rice snacks or their parents should more carefully monitor the amount of rice and rice products that they ingest.
- Karagas MR, Punshon T, Sayarath V, et al. Association of rice and rice-product consumption with arsenic exposure early in life. JAMA Pediatr. 2016;170:609-616.
- Mayer JE, Goldman RH. Arsenic and skin cancer in the USA: the current evidence regarding arsenic-contaminated drinking water. Int J Dermatol. 2016;55;e585-e591.
To the Editor:
Rice and rice products, such as rice cereal and rice snacks, contain inorganic arsenic. Exposure to arsenicin utero and during early life may be associated with adverse fetal growth, adverse infant and child immune response, and adverse neurodevelopmental outcomes. Therefore, the World Health Organization, the Food and Agriculture Organization of the United Nations, the European Union, and the US Food and Drug Administration have suggested maximum arsenic ingestion recommendations for infants: 100 ng/g for inorganic arsenic in products geared toward infants. However, infants consuming only a few servings of rice products may exceed the weekly tolerable intake of arsenic.
Karagas et al1 obtained dietary data on 759 infants who were enrolled in the New Hampshire Birth Cohort Study from 2011 to 2014. They noted that 80% of the infants had been introduced to rice cereal during the first year. Additional data on diet and total urinary arsenic at 12 months was available for 129 infants: 32.6% of these infants were fed rice snacks. In addition, the total urinary arsenic concentration was higher among infants who ate rice cereal or rice snacks as compared to infants who did not eat rice or rice products.
Chronic arsenic exposure can result in patchy dark brown hyperpigmentation with scattered pale spots referred to as “raindrops on a dusty road.” The axilla, eyelids, groin, neck, nipples, and temples often are affected. However, the hyperpigmentation can extend across the chest, abdomen, and back in severe cases.
Horizontal white lines across the nails (Mees lines) may develop. Keratoses, often on the palms (arsenic keratoses), may appear; they persist and may progress to skin cancers. In addition, patients with arsenic exposure are more susceptible to developing nonmelanoma skin cancers.2
It is unknown if exposure to inorganic arsenic in infancy predisposes these individuals to skin cancer when they become adults. Long-term longitudinal follow-up of the participants in this study may provide additional insight. Perhaps infants should not receive rice cereals and rice snacks or their parents should more carefully monitor the amount of rice and rice products that they ingest.
To the Editor:
Rice and rice products, such as rice cereal and rice snacks, contain inorganic arsenic. Exposure to arsenicin utero and during early life may be associated with adverse fetal growth, adverse infant and child immune response, and adverse neurodevelopmental outcomes. Therefore, the World Health Organization, the Food and Agriculture Organization of the United Nations, the European Union, and the US Food and Drug Administration have suggested maximum arsenic ingestion recommendations for infants: 100 ng/g for inorganic arsenic in products geared toward infants. However, infants consuming only a few servings of rice products may exceed the weekly tolerable intake of arsenic.
Karagas et al1 obtained dietary data on 759 infants who were enrolled in the New Hampshire Birth Cohort Study from 2011 to 2014. They noted that 80% of the infants had been introduced to rice cereal during the first year. Additional data on diet and total urinary arsenic at 12 months was available for 129 infants: 32.6% of these infants were fed rice snacks. In addition, the total urinary arsenic concentration was higher among infants who ate rice cereal or rice snacks as compared to infants who did not eat rice or rice products.
Chronic arsenic exposure can result in patchy dark brown hyperpigmentation with scattered pale spots referred to as “raindrops on a dusty road.” The axilla, eyelids, groin, neck, nipples, and temples often are affected. However, the hyperpigmentation can extend across the chest, abdomen, and back in severe cases.
Horizontal white lines across the nails (Mees lines) may develop. Keratoses, often on the palms (arsenic keratoses), may appear; they persist and may progress to skin cancers. In addition, patients with arsenic exposure are more susceptible to developing nonmelanoma skin cancers.2
It is unknown if exposure to inorganic arsenic in infancy predisposes these individuals to skin cancer when they become adults. Long-term longitudinal follow-up of the participants in this study may provide additional insight. Perhaps infants should not receive rice cereals and rice snacks or their parents should more carefully monitor the amount of rice and rice products that they ingest.
- Karagas MR, Punshon T, Sayarath V, et al. Association of rice and rice-product consumption with arsenic exposure early in life. JAMA Pediatr. 2016;170:609-616.
- Mayer JE, Goldman RH. Arsenic and skin cancer in the USA: the current evidence regarding arsenic-contaminated drinking water. Int J Dermatol. 2016;55;e585-e591.
- Karagas MR, Punshon T, Sayarath V, et al. Association of rice and rice-product consumption with arsenic exposure early in life. JAMA Pediatr. 2016;170:609-616.
- Mayer JE, Goldman RH. Arsenic and skin cancer in the USA: the current evidence regarding arsenic-contaminated drinking water. Int J Dermatol. 2016;55;e585-e591.