User login
Be alert for BAP1 mutations in hereditary melanomas
MIAMI – Although rare, patients who present with one or more skin cancers characteristic of those associated with loss of the BAP1 tumor suppressor protein may be at elevated risk for more aggressive uveal melanomas and other cancers such as kidney cancer and mesothelioma. For this reason, dermatologists who recognize the lesions and telltale pattern of this inherited mutation within families can do a great service, encouraging education, genetic counseling, and referral of patients to a nearby cancer center, according to Hensin Tsao, MD, PhD.
“ Dr. Tsao said at the 2018 Orlando Dermatology Aesthetic and Clinical Conference.
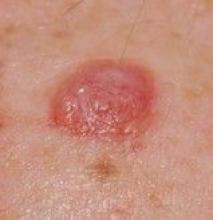
The BAP1-associated skin lesions can emerge when patients are relatively young, even as teenagers. The melanoma and renal cell cancers also can have an early onset, said Dr. Tsao, director of the melanoma genetics program at Massachusetts General Hospital, Boston. The skin lesion itself can be a tipoff for a BAP1 germline mutation. In general, they are small, dome shaped – not flat like a superficial basal cell – rarely pigmented and appear “orangey translucent.” Dr. Tsao added: “When you start seeing them, you’ll recognize them. However, to be sure, you’re going to have to biopsy to know what is going on.”
In one patient he described, the pattern of malignancies in the patient’s family was a hint that she had a BAP1 mutation, Dr. Tsao said. The proband had melanoma starting at age 31 years, a squamous cell carcinoma at 35 years, and basal cell carcinoma at age 40 years. “She had nine ‘nevoid melanomas’ over the years. Nevoid melanomas are rare, and with nine in a row, you know something odd is going on.” Dr. Tsao and his team performed a series of sentinel lymph node biopsies that ruled out metastasis. “What is also interesting is the father had ocular melanoma, which is what got us to thinking about BAP1 mutations in this family.” A sister who developed melanoma and a brother who also was diagnosed with melanoma plus kidney cancer at age 45 years were further clues to the germline mutation.
No longer ‘condemned proteins’
Under normal circumstances, BAP1 is a tumor suppressor protein involved in cellular process called “ubiquitination.” Often, ubiquitination serves to identify proteins “condemned” for destruction by the proteasome system. The BAP1 protein acts through a molecular relay and removes ubiquitin polypeptide groups on the protein. “In the absence of BAP1, proteins often linger longer because they accumulate ubiquitin groups, or alternatively, the protein’s function is somehow altered by mechanisms we don’t quite understand yet,” Dr. Tsao explained.
Once a dermatologist suspects a BAP1 mutation–associated cancer, they can order a BAP1 nuclear stain to confirm diagnosis. Formal documentation of a germline mutation, however, requires genetic testing of blood DNA.
A family history lesson
Ask patients not only about history of melanoma in their family, including if any close relative was diagnosed with eye melanoma, Dr. Tsao suggested. “We had an opportunity to look at cutaneous and ocular melanoma families. Overall, if your family has an ocular melanoma along with cutaneous melanoma, the risk of being a BAP1 mutation–bearing family is greater.” In addition, he and his colleagues did a case control study with Ivana K. Kim, MD, at the Massachusetts Eye and Ear Infirmary in Boston, and found people with metastatic ocular melanoma were more likely to have BAP1 mutations, compared with those with nonmetastatic ocular melanoma.
“The fear is, of course, patient who are BAP1 mutation carriers might be predisposed to more lethal variants of uveal melanoma.”
Although taking a family history is essential, some patients may be unfamiliar with mesothelioma. “So ask about any unusual lung cancers or eye cancers,” Dr. Tsao suggested. “And if it looks like there is an aggregation of rare tumors, get them to a nearby cancer center [for further work-up]. Mesothelioma is difficult to treat and a horrible disease,” he added. “So if there is any chance you can [catch] the mesothelioma early, that’s good.”
He also cautioned against over interpretation of patient reports about family malignancies, in part because lung and breast cancers are relatively common. “Sometimes, when you see a family with lung or breast cancers, it could just be a chance association since these are quite common in the general population.” In other words, determining if a lung cancer in a family with melanoma is an association beyond chance can take some “pretty large numbers to prove.”
In contrast, “the number of kidney cancers among BAP1 families I do believe are out of proportion with normal population expectations,” Dr. Tsao added.
Follow-up and genetic counseling
There is no standard protocol for follow-up once a patient is identified with a BAP1 mutation. “I refer them for uveal, kidney, and/or lung cancer evaluation and see them back two to four times a year for skin checks.”
A meeting attendee suggested that management of a patient with melanoma might not differ based on genetic-testing results. “I agree with you that I don’t need to know the genetic status within these families to help with their cutaneous melanomas,” Dr. Tsao replied. “But the question becomes, are there other internal malignancies you’re not screening for appropriately?”
Another attendee asked about genetic counseling. “I encourage genetic counseling since dermatologists often don’t have time to take at detailed family history of all cancers and ages of onset,” Dr. Tsao said. “Genetic counselors can help sort out the strength of the genetic pedigree in a family. My residents usually ask if someone has a history of melanoma in their family, and that’s it. But there is a big difference between having a cousin with melanoma and three brothers with melanoma.”
MIAMI – Although rare, patients who present with one or more skin cancers characteristic of those associated with loss of the BAP1 tumor suppressor protein may be at elevated risk for more aggressive uveal melanomas and other cancers such as kidney cancer and mesothelioma. For this reason, dermatologists who recognize the lesions and telltale pattern of this inherited mutation within families can do a great service, encouraging education, genetic counseling, and referral of patients to a nearby cancer center, according to Hensin Tsao, MD, PhD.
“ Dr. Tsao said at the 2018 Orlando Dermatology Aesthetic and Clinical Conference.
The BAP1-associated skin lesions can emerge when patients are relatively young, even as teenagers. The melanoma and renal cell cancers also can have an early onset, said Dr. Tsao, director of the melanoma genetics program at Massachusetts General Hospital, Boston. The skin lesion itself can be a tipoff for a BAP1 germline mutation. In general, they are small, dome shaped – not flat like a superficial basal cell – rarely pigmented and appear “orangey translucent.” Dr. Tsao added: “When you start seeing them, you’ll recognize them. However, to be sure, you’re going to have to biopsy to know what is going on.”
In one patient he described, the pattern of malignancies in the patient’s family was a hint that she had a BAP1 mutation, Dr. Tsao said. The proband had melanoma starting at age 31 years, a squamous cell carcinoma at 35 years, and basal cell carcinoma at age 40 years. “She had nine ‘nevoid melanomas’ over the years. Nevoid melanomas are rare, and with nine in a row, you know something odd is going on.” Dr. Tsao and his team performed a series of sentinel lymph node biopsies that ruled out metastasis. “What is also interesting is the father had ocular melanoma, which is what got us to thinking about BAP1 mutations in this family.” A sister who developed melanoma and a brother who also was diagnosed with melanoma plus kidney cancer at age 45 years were further clues to the germline mutation.
No longer ‘condemned proteins’
Under normal circumstances, BAP1 is a tumor suppressor protein involved in cellular process called “ubiquitination.” Often, ubiquitination serves to identify proteins “condemned” for destruction by the proteasome system. The BAP1 protein acts through a molecular relay and removes ubiquitin polypeptide groups on the protein. “In the absence of BAP1, proteins often linger longer because they accumulate ubiquitin groups, or alternatively, the protein’s function is somehow altered by mechanisms we don’t quite understand yet,” Dr. Tsao explained.
Once a dermatologist suspects a BAP1 mutation–associated cancer, they can order a BAP1 nuclear stain to confirm diagnosis. Formal documentation of a germline mutation, however, requires genetic testing of blood DNA.
A family history lesson
Ask patients not only about history of melanoma in their family, including if any close relative was diagnosed with eye melanoma, Dr. Tsao suggested. “We had an opportunity to look at cutaneous and ocular melanoma families. Overall, if your family has an ocular melanoma along with cutaneous melanoma, the risk of being a BAP1 mutation–bearing family is greater.” In addition, he and his colleagues did a case control study with Ivana K. Kim, MD, at the Massachusetts Eye and Ear Infirmary in Boston, and found people with metastatic ocular melanoma were more likely to have BAP1 mutations, compared with those with nonmetastatic ocular melanoma.
“The fear is, of course, patient who are BAP1 mutation carriers might be predisposed to more lethal variants of uveal melanoma.”
Although taking a family history is essential, some patients may be unfamiliar with mesothelioma. “So ask about any unusual lung cancers or eye cancers,” Dr. Tsao suggested. “And if it looks like there is an aggregation of rare tumors, get them to a nearby cancer center [for further work-up]. Mesothelioma is difficult to treat and a horrible disease,” he added. “So if there is any chance you can [catch] the mesothelioma early, that’s good.”
He also cautioned against over interpretation of patient reports about family malignancies, in part because lung and breast cancers are relatively common. “Sometimes, when you see a family with lung or breast cancers, it could just be a chance association since these are quite common in the general population.” In other words, determining if a lung cancer in a family with melanoma is an association beyond chance can take some “pretty large numbers to prove.”
In contrast, “the number of kidney cancers among BAP1 families I do believe are out of proportion with normal population expectations,” Dr. Tsao added.
Follow-up and genetic counseling
There is no standard protocol for follow-up once a patient is identified with a BAP1 mutation. “I refer them for uveal, kidney, and/or lung cancer evaluation and see them back two to four times a year for skin checks.”
A meeting attendee suggested that management of a patient with melanoma might not differ based on genetic-testing results. “I agree with you that I don’t need to know the genetic status within these families to help with their cutaneous melanomas,” Dr. Tsao replied. “But the question becomes, are there other internal malignancies you’re not screening for appropriately?”
Another attendee asked about genetic counseling. “I encourage genetic counseling since dermatologists often don’t have time to take at detailed family history of all cancers and ages of onset,” Dr. Tsao said. “Genetic counselors can help sort out the strength of the genetic pedigree in a family. My residents usually ask if someone has a history of melanoma in their family, and that’s it. But there is a big difference between having a cousin with melanoma and three brothers with melanoma.”
MIAMI – Although rare, patients who present with one or more skin cancers characteristic of those associated with loss of the BAP1 tumor suppressor protein may be at elevated risk for more aggressive uveal melanomas and other cancers such as kidney cancer and mesothelioma. For this reason, dermatologists who recognize the lesions and telltale pattern of this inherited mutation within families can do a great service, encouraging education, genetic counseling, and referral of patients to a nearby cancer center, according to Hensin Tsao, MD, PhD.
“ Dr. Tsao said at the 2018 Orlando Dermatology Aesthetic and Clinical Conference.
The BAP1-associated skin lesions can emerge when patients are relatively young, even as teenagers. The melanoma and renal cell cancers also can have an early onset, said Dr. Tsao, director of the melanoma genetics program at Massachusetts General Hospital, Boston. The skin lesion itself can be a tipoff for a BAP1 germline mutation. In general, they are small, dome shaped – not flat like a superficial basal cell – rarely pigmented and appear “orangey translucent.” Dr. Tsao added: “When you start seeing them, you’ll recognize them. However, to be sure, you’re going to have to biopsy to know what is going on.”
In one patient he described, the pattern of malignancies in the patient’s family was a hint that she had a BAP1 mutation, Dr. Tsao said. The proband had melanoma starting at age 31 years, a squamous cell carcinoma at 35 years, and basal cell carcinoma at age 40 years. “She had nine ‘nevoid melanomas’ over the years. Nevoid melanomas are rare, and with nine in a row, you know something odd is going on.” Dr. Tsao and his team performed a series of sentinel lymph node biopsies that ruled out metastasis. “What is also interesting is the father had ocular melanoma, which is what got us to thinking about BAP1 mutations in this family.” A sister who developed melanoma and a brother who also was diagnosed with melanoma plus kidney cancer at age 45 years were further clues to the germline mutation.
No longer ‘condemned proteins’
Under normal circumstances, BAP1 is a tumor suppressor protein involved in cellular process called “ubiquitination.” Often, ubiquitination serves to identify proteins “condemned” for destruction by the proteasome system. The BAP1 protein acts through a molecular relay and removes ubiquitin polypeptide groups on the protein. “In the absence of BAP1, proteins often linger longer because they accumulate ubiquitin groups, or alternatively, the protein’s function is somehow altered by mechanisms we don’t quite understand yet,” Dr. Tsao explained.
Once a dermatologist suspects a BAP1 mutation–associated cancer, they can order a BAP1 nuclear stain to confirm diagnosis. Formal documentation of a germline mutation, however, requires genetic testing of blood DNA.
A family history lesson
Ask patients not only about history of melanoma in their family, including if any close relative was diagnosed with eye melanoma, Dr. Tsao suggested. “We had an opportunity to look at cutaneous and ocular melanoma families. Overall, if your family has an ocular melanoma along with cutaneous melanoma, the risk of being a BAP1 mutation–bearing family is greater.” In addition, he and his colleagues did a case control study with Ivana K. Kim, MD, at the Massachusetts Eye and Ear Infirmary in Boston, and found people with metastatic ocular melanoma were more likely to have BAP1 mutations, compared with those with nonmetastatic ocular melanoma.
“The fear is, of course, patient who are BAP1 mutation carriers might be predisposed to more lethal variants of uveal melanoma.”
Although taking a family history is essential, some patients may be unfamiliar with mesothelioma. “So ask about any unusual lung cancers or eye cancers,” Dr. Tsao suggested. “And if it looks like there is an aggregation of rare tumors, get them to a nearby cancer center [for further work-up]. Mesothelioma is difficult to treat and a horrible disease,” he added. “So if there is any chance you can [catch] the mesothelioma early, that’s good.”
He also cautioned against over interpretation of patient reports about family malignancies, in part because lung and breast cancers are relatively common. “Sometimes, when you see a family with lung or breast cancers, it could just be a chance association since these are quite common in the general population.” In other words, determining if a lung cancer in a family with melanoma is an association beyond chance can take some “pretty large numbers to prove.”
In contrast, “the number of kidney cancers among BAP1 families I do believe are out of proportion with normal population expectations,” Dr. Tsao added.
Follow-up and genetic counseling
There is no standard protocol for follow-up once a patient is identified with a BAP1 mutation. “I refer them for uveal, kidney, and/or lung cancer evaluation and see them back two to four times a year for skin checks.”
A meeting attendee suggested that management of a patient with melanoma might not differ based on genetic-testing results. “I agree with you that I don’t need to know the genetic status within these families to help with their cutaneous melanomas,” Dr. Tsao replied. “But the question becomes, are there other internal malignancies you’re not screening for appropriately?”
Another attendee asked about genetic counseling. “I encourage genetic counseling since dermatologists often don’t have time to take at detailed family history of all cancers and ages of onset,” Dr. Tsao said. “Genetic counselors can help sort out the strength of the genetic pedigree in a family. My residents usually ask if someone has a history of melanoma in their family, and that’s it. But there is a big difference between having a cousin with melanoma and three brothers with melanoma.”
REPORTING FROM ODAC 2018
Mobile Medical Apps for Patient Education: A Graded Review of Available Dermatology Apps
According to industry estimates, roughly 64% of US adults were smartphone users in 2015.1 Smartphones enable users to utilize mobile applications (apps) that can perform a variety of functions in many categories, including business, music, photography, entertainment, education, social networking, travel, and lifestyle. The widespread adoption and use of mobile apps has implications for medical practice. Mobile apps have the capability to serve as information sources for patients, educational tools for students, and diagnostic aids for physicians.2 Consequently, a number of medical and health care–oriented apps have already been developed3 and are increasingly utilized by patients and providers.4
Given its visual nature, dermatology is particularly amenable to the integration of mobile medical apps. A study by Brewer et al5 identified more than 229 dermatology-related apps in categories ranging from general dermatology reference, self-surveillance and diagnosis, disease guides, educational aids, sunscreen and UV recommendations, and teledermatology. Patients served as the target audience and principal consumers of more than half of these dermatology apps.5
Mobile medical and health care apps demonstrate great potential for serving as valuable information sources for patients with dermatologic conditions; however, the content, functions, accuracy, and educational value of dermatology mobile apps are not well characterized, making it difficult for patients and health care providers to select and recommend appropriate apps.6 In this study, we created a rubric to objectively grade 44 publicly available mobile dermatology apps with the primary focus of patient education.
Methods
We conducted a search of dermatology-related educational mobile apps that were publicly available via the App Store (Apple Inc) from January 2016 to November 2016. (The pricing, availability, and other features of these apps may have changed since the study period.) The following search terms were used: dermatology, dermoscopy, melanoma, skin cancer, psoriasis, rosacea, acne, eczema, dermal fillers, and Mohs surgery. We excluded apps that were not in English; had a solely commercial focus; were mobile textbooks or scientific journals; were used to provide teledermatology services with no educational purpose; were solely focused on homeopathic, alternative, and/or complementary medicine; or were intended primarily as a reference for students or health care professionals. Our search yielded 44 apps with patient education as a primary objective. The apps were divided into 6 categories based on their focus: general dermatology, cosmetic dermatology, acne, eczema, psoriasis, and skin cancer.
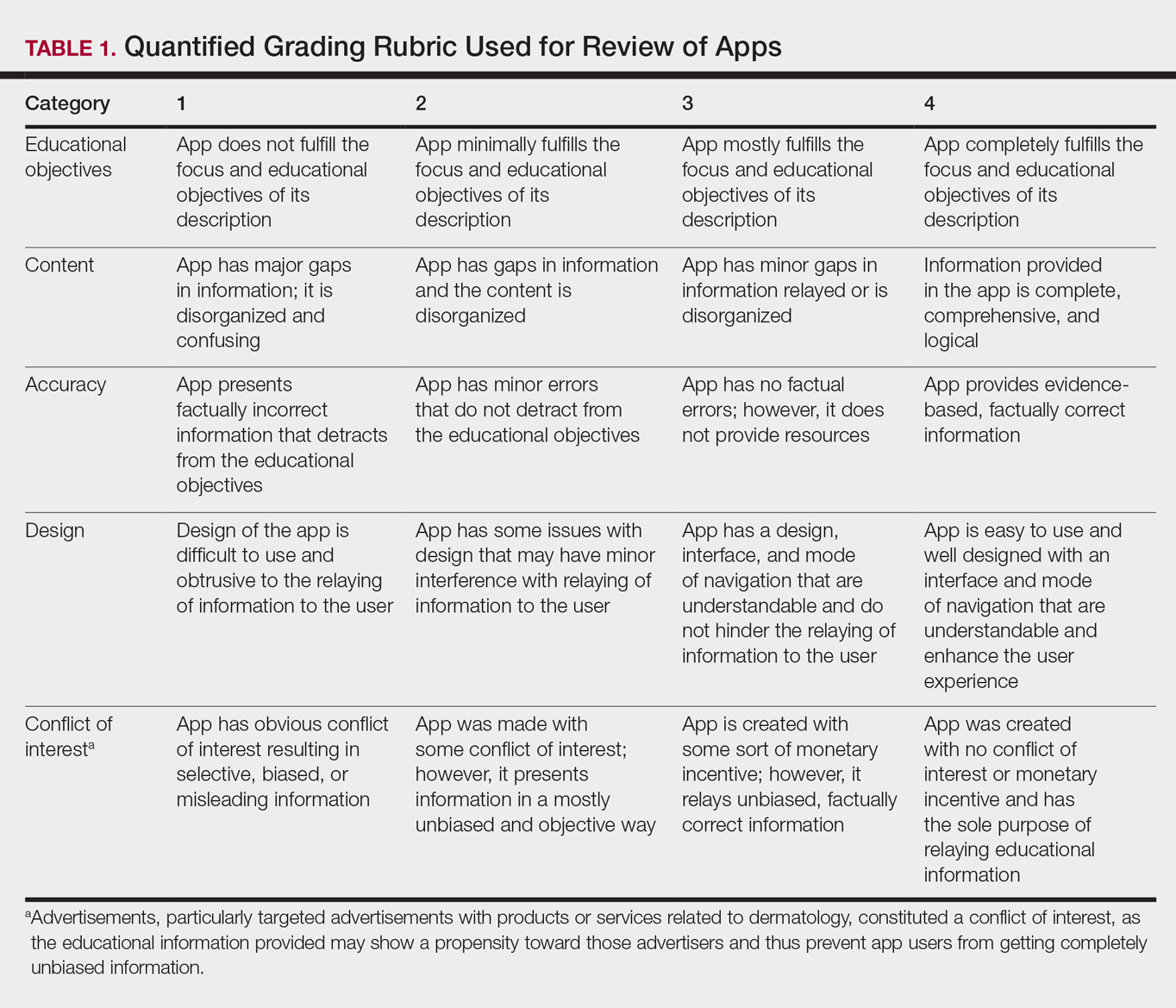
Each app was reviewed using a quantified grading rubric developed by the researchers. In a prior evaluation, Handel7 reviewed 35 health and wellness mobile apps utilizing the categories of ease of use, reliability, quality, scope of information, and aesthetics.4 These criteria were modified and adapted for the purposes of this study, and a 4-point scale was applied to each criterion. The final criteria were (1) educational objectives, (2) content, (3) accuracy, (4) design, and (5) conflict of interest. The quantified grading rubric is described in Table 1.
Results
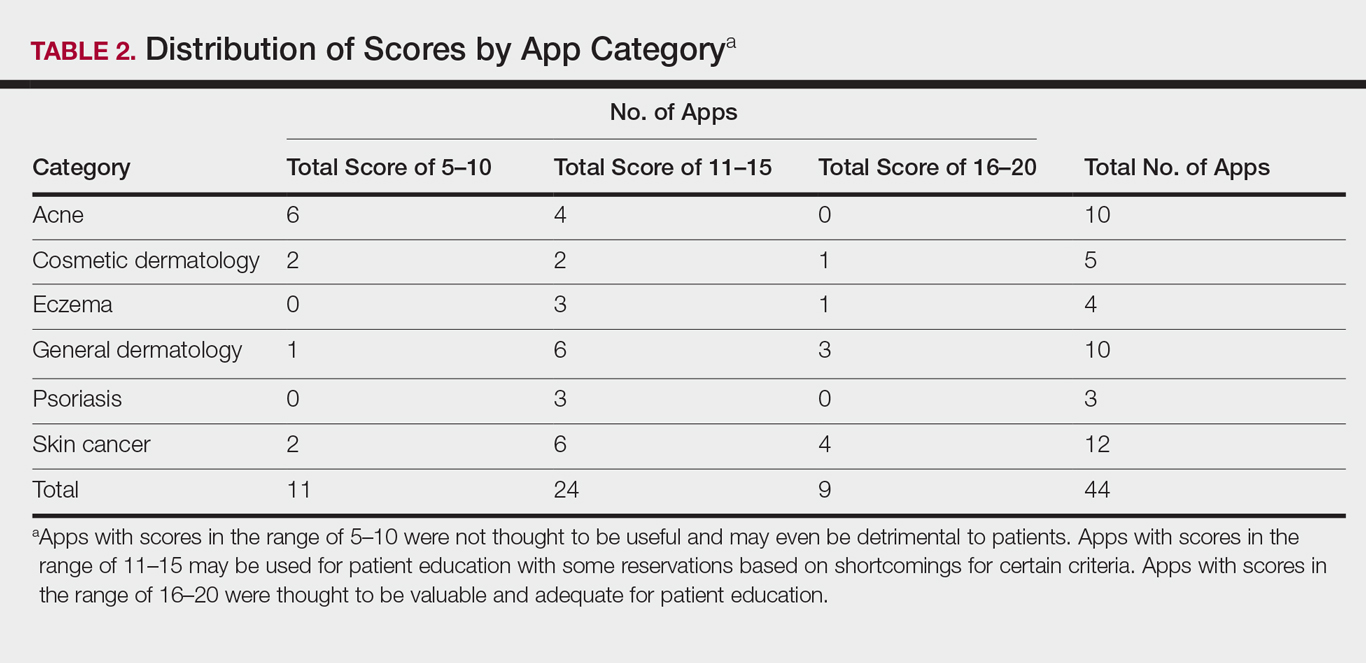
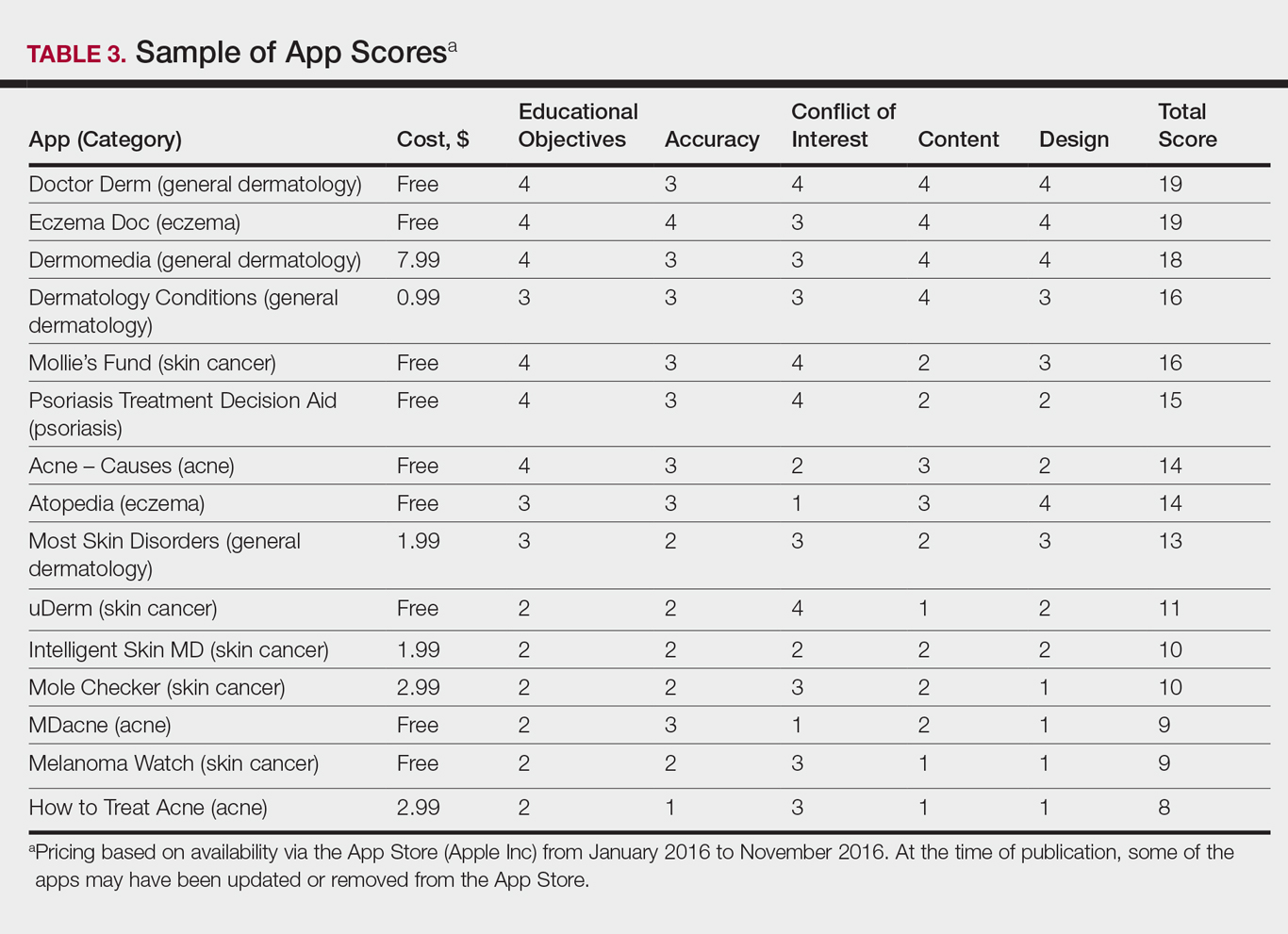
The possible range of scores based on the grading rubric was 5 to 20. The actual range of scores was 8 to 19 (Table 2). The 44 reviewed apps were categorized by topic as acne, cosmetic dermatology, eczema, general dermatology, psoriasis, or skin cancer. A sample of 15 apps selected to represent the distribution of scores and their grading on the rubric are presented in Table 3.
Comment
The number of dermatology-related apps available to mobile users continues to grow at an increasing rate.8 The apps vary in many aspects, including their purpose, scope, intended audience, and goals of the app publisher. In turn, more individuals are turning to mobile apps for medical information,4 especially in dermatology, thus it is necessary to create a systematic way to evaluate the quality and utility of each app to assist users in making informed decisions about which apps will best meet their needs in the midst of a wide array of choices.
For the purpose of this study, an objective rubric was created that can be used to evaluate the quality of medical apps for patient education in dermatology. An app’s adequacy and usefulness for patient education was thought to depend on 3 possible score ranges into which the app could fall based on the grading rubric. An app with a total score in the range of 5 to 10 was not thought to be useful and may even be detrimental to patients. An app with a total score in the range of 11 to 15 may be used for patient education with some reservations based on shortcomings for certain criteria. An app with a score in the range of 16 to 20 was thought to be valuable and adequate for patient education. For example, the How to Treat Acne app received a total score of 8 and therefore would not be recommended to patients based on the grading rubric used in this study. This particular app provided sparse and sometimes inaccurate information, had a confusing user interface, and contained many obstructive advertisements. In contrast, the Eczema Doc app received a total score of 19, which indicates a quality app deemed to be useful for patient information based on the established rubric. This app met all the objectives that it advertised, contained accurate information with verified citation of sources, and was very easy for users to navigate.
Of the 44 graded apps, only 9 (20.5%) received scores in the highest range of 16 to 20, which indicates a need for improvements in mobile dermatology apps intended for patient education. Adopting the grading rubric developed in this study as a standard in the creation of medical apps could have beneficial implications in disseminating accurate, safe, unbiased, and easy-to-understand information to patients.
- Smith A. U.S. smartphone use in 2015. Pew Research Center website. http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015. Published April 1, 2015. Accessed August 29, 2017.
- Nilsen W, Kumar S, Shar A, et al. Advancing the science of mHealth. J Health Commun. 2012;17(suppl 1):5-10.
- West DM. How mobile devices are transforming healthcare issues in technology innovation. Issues Technol Innov. 2012;18:1-14.
- Boudreaux ED, Waring ME, Hayes RB, et al. Evaluating and selecting mobile health apps: strategies for healthcare providers and healthcare organizations. Transl Behav Med. 2014;4:363-371.
- Brewer AC, Endly DC, Henley J, et al. Mobile applications in dermatology. JAMA Dermatol. 2013;149:1300-1304.
- Cummings E, Borycki E, Roehrer E. Issues and considerations for healthcare consumers using mobile applications. Stud Health Technol Inform. 2013;183:227-231.
- Handel MJ. mHealth (mobile health)-using apps for health and wellness. Explore. 2011;7:256-261.
- Boulos MN, Brewer AC, Karimkhani C, et al. Mobile medical and health apps: state of the art, concerns, regulatory control and certification. Online J Public Health Inform. 2014;5:229.
According to industry estimates, roughly 64% of US adults were smartphone users in 2015.1 Smartphones enable users to utilize mobile applications (apps) that can perform a variety of functions in many categories, including business, music, photography, entertainment, education, social networking, travel, and lifestyle. The widespread adoption and use of mobile apps has implications for medical practice. Mobile apps have the capability to serve as information sources for patients, educational tools for students, and diagnostic aids for physicians.2 Consequently, a number of medical and health care–oriented apps have already been developed3 and are increasingly utilized by patients and providers.4
Given its visual nature, dermatology is particularly amenable to the integration of mobile medical apps. A study by Brewer et al5 identified more than 229 dermatology-related apps in categories ranging from general dermatology reference, self-surveillance and diagnosis, disease guides, educational aids, sunscreen and UV recommendations, and teledermatology. Patients served as the target audience and principal consumers of more than half of these dermatology apps.5
Mobile medical and health care apps demonstrate great potential for serving as valuable information sources for patients with dermatologic conditions; however, the content, functions, accuracy, and educational value of dermatology mobile apps are not well characterized, making it difficult for patients and health care providers to select and recommend appropriate apps.6 In this study, we created a rubric to objectively grade 44 publicly available mobile dermatology apps with the primary focus of patient education.
Methods
We conducted a search of dermatology-related educational mobile apps that were publicly available via the App Store (Apple Inc) from January 2016 to November 2016. (The pricing, availability, and other features of these apps may have changed since the study period.) The following search terms were used: dermatology, dermoscopy, melanoma, skin cancer, psoriasis, rosacea, acne, eczema, dermal fillers, and Mohs surgery. We excluded apps that were not in English; had a solely commercial focus; were mobile textbooks or scientific journals; were used to provide teledermatology services with no educational purpose; were solely focused on homeopathic, alternative, and/or complementary medicine; or were intended primarily as a reference for students or health care professionals. Our search yielded 44 apps with patient education as a primary objective. The apps were divided into 6 categories based on their focus: general dermatology, cosmetic dermatology, acne, eczema, psoriasis, and skin cancer.
Each app was reviewed using a quantified grading rubric developed by the researchers. In a prior evaluation, Handel7 reviewed 35 health and wellness mobile apps utilizing the categories of ease of use, reliability, quality, scope of information, and aesthetics.4 These criteria were modified and adapted for the purposes of this study, and a 4-point scale was applied to each criterion. The final criteria were (1) educational objectives, (2) content, (3) accuracy, (4) design, and (5) conflict of interest. The quantified grading rubric is described in Table 1.
Results
The possible range of scores based on the grading rubric was 5 to 20. The actual range of scores was 8 to 19 (Table 2). The 44 reviewed apps were categorized by topic as acne, cosmetic dermatology, eczema, general dermatology, psoriasis, or skin cancer. A sample of 15 apps selected to represent the distribution of scores and their grading on the rubric are presented in Table 3.
Comment
The number of dermatology-related apps available to mobile users continues to grow at an increasing rate.8 The apps vary in many aspects, including their purpose, scope, intended audience, and goals of the app publisher. In turn, more individuals are turning to mobile apps for medical information,4 especially in dermatology, thus it is necessary to create a systematic way to evaluate the quality and utility of each app to assist users in making informed decisions about which apps will best meet their needs in the midst of a wide array of choices.
For the purpose of this study, an objective rubric was created that can be used to evaluate the quality of medical apps for patient education in dermatology. An app’s adequacy and usefulness for patient education was thought to depend on 3 possible score ranges into which the app could fall based on the grading rubric. An app with a total score in the range of 5 to 10 was not thought to be useful and may even be detrimental to patients. An app with a total score in the range of 11 to 15 may be used for patient education with some reservations based on shortcomings for certain criteria. An app with a score in the range of 16 to 20 was thought to be valuable and adequate for patient education. For example, the How to Treat Acne app received a total score of 8 and therefore would not be recommended to patients based on the grading rubric used in this study. This particular app provided sparse and sometimes inaccurate information, had a confusing user interface, and contained many obstructive advertisements. In contrast, the Eczema Doc app received a total score of 19, which indicates a quality app deemed to be useful for patient information based on the established rubric. This app met all the objectives that it advertised, contained accurate information with verified citation of sources, and was very easy for users to navigate.
Of the 44 graded apps, only 9 (20.5%) received scores in the highest range of 16 to 20, which indicates a need for improvements in mobile dermatology apps intended for patient education. Adopting the grading rubric developed in this study as a standard in the creation of medical apps could have beneficial implications in disseminating accurate, safe, unbiased, and easy-to-understand information to patients.
According to industry estimates, roughly 64% of US adults were smartphone users in 2015.1 Smartphones enable users to utilize mobile applications (apps) that can perform a variety of functions in many categories, including business, music, photography, entertainment, education, social networking, travel, and lifestyle. The widespread adoption and use of mobile apps has implications for medical practice. Mobile apps have the capability to serve as information sources for patients, educational tools for students, and diagnostic aids for physicians.2 Consequently, a number of medical and health care–oriented apps have already been developed3 and are increasingly utilized by patients and providers.4
Given its visual nature, dermatology is particularly amenable to the integration of mobile medical apps. A study by Brewer et al5 identified more than 229 dermatology-related apps in categories ranging from general dermatology reference, self-surveillance and diagnosis, disease guides, educational aids, sunscreen and UV recommendations, and teledermatology. Patients served as the target audience and principal consumers of more than half of these dermatology apps.5
Mobile medical and health care apps demonstrate great potential for serving as valuable information sources for patients with dermatologic conditions; however, the content, functions, accuracy, and educational value of dermatology mobile apps are not well characterized, making it difficult for patients and health care providers to select and recommend appropriate apps.6 In this study, we created a rubric to objectively grade 44 publicly available mobile dermatology apps with the primary focus of patient education.
Methods
We conducted a search of dermatology-related educational mobile apps that were publicly available via the App Store (Apple Inc) from January 2016 to November 2016. (The pricing, availability, and other features of these apps may have changed since the study period.) The following search terms were used: dermatology, dermoscopy, melanoma, skin cancer, psoriasis, rosacea, acne, eczema, dermal fillers, and Mohs surgery. We excluded apps that were not in English; had a solely commercial focus; were mobile textbooks or scientific journals; were used to provide teledermatology services with no educational purpose; were solely focused on homeopathic, alternative, and/or complementary medicine; or were intended primarily as a reference for students or health care professionals. Our search yielded 44 apps with patient education as a primary objective. The apps were divided into 6 categories based on their focus: general dermatology, cosmetic dermatology, acne, eczema, psoriasis, and skin cancer.
Each app was reviewed using a quantified grading rubric developed by the researchers. In a prior evaluation, Handel7 reviewed 35 health and wellness mobile apps utilizing the categories of ease of use, reliability, quality, scope of information, and aesthetics.4 These criteria were modified and adapted for the purposes of this study, and a 4-point scale was applied to each criterion. The final criteria were (1) educational objectives, (2) content, (3) accuracy, (4) design, and (5) conflict of interest. The quantified grading rubric is described in Table 1.
Results
The possible range of scores based on the grading rubric was 5 to 20. The actual range of scores was 8 to 19 (Table 2). The 44 reviewed apps were categorized by topic as acne, cosmetic dermatology, eczema, general dermatology, psoriasis, or skin cancer. A sample of 15 apps selected to represent the distribution of scores and their grading on the rubric are presented in Table 3.
Comment
The number of dermatology-related apps available to mobile users continues to grow at an increasing rate.8 The apps vary in many aspects, including their purpose, scope, intended audience, and goals of the app publisher. In turn, more individuals are turning to mobile apps for medical information,4 especially in dermatology, thus it is necessary to create a systematic way to evaluate the quality and utility of each app to assist users in making informed decisions about which apps will best meet their needs in the midst of a wide array of choices.
For the purpose of this study, an objective rubric was created that can be used to evaluate the quality of medical apps for patient education in dermatology. An app’s adequacy and usefulness for patient education was thought to depend on 3 possible score ranges into which the app could fall based on the grading rubric. An app with a total score in the range of 5 to 10 was not thought to be useful and may even be detrimental to patients. An app with a total score in the range of 11 to 15 may be used for patient education with some reservations based on shortcomings for certain criteria. An app with a score in the range of 16 to 20 was thought to be valuable and adequate for patient education. For example, the How to Treat Acne app received a total score of 8 and therefore would not be recommended to patients based on the grading rubric used in this study. This particular app provided sparse and sometimes inaccurate information, had a confusing user interface, and contained many obstructive advertisements. In contrast, the Eczema Doc app received a total score of 19, which indicates a quality app deemed to be useful for patient information based on the established rubric. This app met all the objectives that it advertised, contained accurate information with verified citation of sources, and was very easy for users to navigate.
Of the 44 graded apps, only 9 (20.5%) received scores in the highest range of 16 to 20, which indicates a need for improvements in mobile dermatology apps intended for patient education. Adopting the grading rubric developed in this study as a standard in the creation of medical apps could have beneficial implications in disseminating accurate, safe, unbiased, and easy-to-understand information to patients.
- Smith A. U.S. smartphone use in 2015. Pew Research Center website. http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015. Published April 1, 2015. Accessed August 29, 2017.
- Nilsen W, Kumar S, Shar A, et al. Advancing the science of mHealth. J Health Commun. 2012;17(suppl 1):5-10.
- West DM. How mobile devices are transforming healthcare issues in technology innovation. Issues Technol Innov. 2012;18:1-14.
- Boudreaux ED, Waring ME, Hayes RB, et al. Evaluating and selecting mobile health apps: strategies for healthcare providers and healthcare organizations. Transl Behav Med. 2014;4:363-371.
- Brewer AC, Endly DC, Henley J, et al. Mobile applications in dermatology. JAMA Dermatol. 2013;149:1300-1304.
- Cummings E, Borycki E, Roehrer E. Issues and considerations for healthcare consumers using mobile applications. Stud Health Technol Inform. 2013;183:227-231.
- Handel MJ. mHealth (mobile health)-using apps for health and wellness. Explore. 2011;7:256-261.
- Boulos MN, Brewer AC, Karimkhani C, et al. Mobile medical and health apps: state of the art, concerns, regulatory control and certification. Online J Public Health Inform. 2014;5:229.
- Smith A. U.S. smartphone use in 2015. Pew Research Center website. http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015. Published April 1, 2015. Accessed August 29, 2017.
- Nilsen W, Kumar S, Shar A, et al. Advancing the science of mHealth. J Health Commun. 2012;17(suppl 1):5-10.
- West DM. How mobile devices are transforming healthcare issues in technology innovation. Issues Technol Innov. 2012;18:1-14.
- Boudreaux ED, Waring ME, Hayes RB, et al. Evaluating and selecting mobile health apps: strategies for healthcare providers and healthcare organizations. Transl Behav Med. 2014;4:363-371.
- Brewer AC, Endly DC, Henley J, et al. Mobile applications in dermatology. JAMA Dermatol. 2013;149:1300-1304.
- Cummings E, Borycki E, Roehrer E. Issues and considerations for healthcare consumers using mobile applications. Stud Health Technol Inform. 2013;183:227-231.
- Handel MJ. mHealth (mobile health)-using apps for health and wellness. Explore. 2011;7:256-261.
- Boulos MN, Brewer AC, Karimkhani C, et al. Mobile medical and health apps: state of the art, concerns, regulatory control and certification. Online J Public Health Inform. 2014;5:229.
Practice Points
- Mobile dermatology apps for educational purposes should be objectively reviewed before being used by patients.
- In our study, only 9 (20.5%) of the 44 dermatology apps evaluated were considered adequate for patient information based on our grading criteria.
Neoadjuvant dabrafenib and trametinib improves event-free survival in resectable melanoma
For patients with surgically resectable, BRAF-mutated melanoma, neoadjuvant and adjuvant treatment with the combination of dabrafenib and trametinib resulted in significantly longer event-free survival compared with standard care, according to results of a randomized study.
The trial was closed early because of the “large difference” in event-free survival favoring the neoadjuvant approach, the authors wrote (Lancet Oncol. 2018 Jan 17. doi: 10.1016/S1470-2045(18)30015-9.
While early closure limits interpretation of results, they do provide important proof-of-concept and data for future studies, wrote the authors, led by Rodabe N Amaria, MD, of the department of medical oncology, University of Texas MD Anderson Cancer Center, Houston.
“The clinical and translational results strongly support the rationale for further assessment of neoadjuvant therapy in patients with high-risk, surgically resectable melanoma,” Dr. Amaria and colleagues said in the report on the randomized trial, believed to be the first to evaluate the role of neoadjuvant therapy versus standard care in BRAF-mutated melanoma.
Dabrafenib and trametinib combination therapy is approved as a treatment for patients with unresectable or metastatic stage IV melanoma and a BRAFV600 mutation, which is found in about half of cutaneous melanomas, authors said.
To evaluate the combination in earlier stage disease, Dr. Amaria and coinvestigators conducted a single-center, open-label, randomized, phase 2 trial of 21 patients with surgically resectable clinical stage III or oligometastatic stage IV melanoma with BRAFV600E or BRAFV600K mutations.
Patients were randomized 2:1 to receive the neoadjuvant/adjuvant treatment or to standard of care, which consisted of standard surgery plus consideration for adjuvant therapy, the authors said. Those patients assigned to the targeted therapy arm received 8 weeks of neoadjuvant dabrafenib and trametinib followed by surgery, then adjuvant dabrafenib and trametinib for up to 44 weeks.
Event-free survival, the primary endpoint of the trial, was a median of 19.7 months for neoadjuvant plus adjuvant dabrafenib and trametinib, versus 2.9 months for standard care (P less than .0001), the investigators reported.
Dabrafenib and trametinib combination therapy was well tolerated as neoadjuvant and adjuvant therapy, with no grade 4 adverse events or treatment related deaths, according to the investigators. The most common grade 3 adverse event seen with the combination was diarrhea, occurring in 2 patients (15%).
The trial is continuing as a single-arm study of neoadjuvant plus adjuvant dabrafenib and trametinib.
Dr. Amaria and colleagues reported individual disclosures related to Merck, Bristol-Myers Squibb, Array Biopharma, and others, including Novartis Pharmaceuticals Corp., which supplied drugs and funded clinical aspects of the study.
SOURCE: Amaria et al. 2018 Jan 17. doi: 10.1016/S1470-2045(18)30015-9
Although results of the study by Amaria et al. are “promising,” the role of neoadjuvant therapy in treatment of stage III–IV oligometastatic melanoma in clinical practice “is unclear for now,” melanoma specialists Paolo A. Ascierto, MD, and Alexander M. M. Eggermont, MD, PhD, wrote in an editorial.
Amaria et al. have presented results of the first randomized trial to evaluate neoadjuvant therapy versus standard care in patients with high-risk resectable BRAF-mutated melanoma.
Patients who received both neoadjuvant and adjuvant treatment with the dabrafenib/trametinib combination had superior event-free survival versus standard surgery and consideration for adjuvant therapy, published results show.
However, previous studies have already shown good results on this, that adjuvant dabrafenib plus trametinib (as well as nivolumab monotherapy) in this setting, “raising the question of whether a neoadjuvant approach is really needed, especially given a possible reduction of the role of surgery in the future,” Dr. Ascierto and Dr. Eggermont wrote.
Alternatively, adjuvant therapy with newer, more effective agents may be a “better way forward,” they said, noting that three patients in the trial by Amaria et al. who progressed after neoadjuvant/adjuvant dabrafenib and trametinib relapsed at first with brain metastases, raising the question of whether the treatment “might induce a resistant phenotype predisposed to the development of CNS metastases.”
That said, effectively combining neoadjuvant with adjuvant therapy could reduce the extent of surgery, make radiotherapy redundant, or increase distant metastasis-free survival and overall survival, among other benefits.
“The next generation of adjuvant trials should aim to address these outstanding questions,” they concluded.
Dr. Ascierto is with Istituto Nazionale Tumori Fondazione “G Pascale,” Napoli, Italy, and Dr. Eggermont is with Cancer Institute Gustave Roussy, University Paris-Sud, France. This commentary is based on their editorial appearing in The Lancet Oncology (2018 Jan 17. doi: 10.1016/S1470-2045[18]30016-0). The authors reported disclosures related to Novartis, Merck Serono, Bristol-Myers Squibb, Amgen, and others.
Although results of the study by Amaria et al. are “promising,” the role of neoadjuvant therapy in treatment of stage III–IV oligometastatic melanoma in clinical practice “is unclear for now,” melanoma specialists Paolo A. Ascierto, MD, and Alexander M. M. Eggermont, MD, PhD, wrote in an editorial.
Amaria et al. have presented results of the first randomized trial to evaluate neoadjuvant therapy versus standard care in patients with high-risk resectable BRAF-mutated melanoma.
Patients who received both neoadjuvant and adjuvant treatment with the dabrafenib/trametinib combination had superior event-free survival versus standard surgery and consideration for adjuvant therapy, published results show.
However, previous studies have already shown good results on this, that adjuvant dabrafenib plus trametinib (as well as nivolumab monotherapy) in this setting, “raising the question of whether a neoadjuvant approach is really needed, especially given a possible reduction of the role of surgery in the future,” Dr. Ascierto and Dr. Eggermont wrote.
Alternatively, adjuvant therapy with newer, more effective agents may be a “better way forward,” they said, noting that three patients in the trial by Amaria et al. who progressed after neoadjuvant/adjuvant dabrafenib and trametinib relapsed at first with brain metastases, raising the question of whether the treatment “might induce a resistant phenotype predisposed to the development of CNS metastases.”
That said, effectively combining neoadjuvant with adjuvant therapy could reduce the extent of surgery, make radiotherapy redundant, or increase distant metastasis-free survival and overall survival, among other benefits.
“The next generation of adjuvant trials should aim to address these outstanding questions,” they concluded.
Dr. Ascierto is with Istituto Nazionale Tumori Fondazione “G Pascale,” Napoli, Italy, and Dr. Eggermont is with Cancer Institute Gustave Roussy, University Paris-Sud, France. This commentary is based on their editorial appearing in The Lancet Oncology (2018 Jan 17. doi: 10.1016/S1470-2045[18]30016-0). The authors reported disclosures related to Novartis, Merck Serono, Bristol-Myers Squibb, Amgen, and others.
Although results of the study by Amaria et al. are “promising,” the role of neoadjuvant therapy in treatment of stage III–IV oligometastatic melanoma in clinical practice “is unclear for now,” melanoma specialists Paolo A. Ascierto, MD, and Alexander M. M. Eggermont, MD, PhD, wrote in an editorial.
Amaria et al. have presented results of the first randomized trial to evaluate neoadjuvant therapy versus standard care in patients with high-risk resectable BRAF-mutated melanoma.
Patients who received both neoadjuvant and adjuvant treatment with the dabrafenib/trametinib combination had superior event-free survival versus standard surgery and consideration for adjuvant therapy, published results show.
However, previous studies have already shown good results on this, that adjuvant dabrafenib plus trametinib (as well as nivolumab monotherapy) in this setting, “raising the question of whether a neoadjuvant approach is really needed, especially given a possible reduction of the role of surgery in the future,” Dr. Ascierto and Dr. Eggermont wrote.
Alternatively, adjuvant therapy with newer, more effective agents may be a “better way forward,” they said, noting that three patients in the trial by Amaria et al. who progressed after neoadjuvant/adjuvant dabrafenib and trametinib relapsed at first with brain metastases, raising the question of whether the treatment “might induce a resistant phenotype predisposed to the development of CNS metastases.”
That said, effectively combining neoadjuvant with adjuvant therapy could reduce the extent of surgery, make radiotherapy redundant, or increase distant metastasis-free survival and overall survival, among other benefits.
“The next generation of adjuvant trials should aim to address these outstanding questions,” they concluded.
Dr. Ascierto is with Istituto Nazionale Tumori Fondazione “G Pascale,” Napoli, Italy, and Dr. Eggermont is with Cancer Institute Gustave Roussy, University Paris-Sud, France. This commentary is based on their editorial appearing in The Lancet Oncology (2018 Jan 17. doi: 10.1016/S1470-2045[18]30016-0). The authors reported disclosures related to Novartis, Merck Serono, Bristol-Myers Squibb, Amgen, and others.
For patients with surgically resectable, BRAF-mutated melanoma, neoadjuvant and adjuvant treatment with the combination of dabrafenib and trametinib resulted in significantly longer event-free survival compared with standard care, according to results of a randomized study.
The trial was closed early because of the “large difference” in event-free survival favoring the neoadjuvant approach, the authors wrote (Lancet Oncol. 2018 Jan 17. doi: 10.1016/S1470-2045(18)30015-9.
While early closure limits interpretation of results, they do provide important proof-of-concept and data for future studies, wrote the authors, led by Rodabe N Amaria, MD, of the department of medical oncology, University of Texas MD Anderson Cancer Center, Houston.
“The clinical and translational results strongly support the rationale for further assessment of neoadjuvant therapy in patients with high-risk, surgically resectable melanoma,” Dr. Amaria and colleagues said in the report on the randomized trial, believed to be the first to evaluate the role of neoadjuvant therapy versus standard care in BRAF-mutated melanoma.
Dabrafenib and trametinib combination therapy is approved as a treatment for patients with unresectable or metastatic stage IV melanoma and a BRAFV600 mutation, which is found in about half of cutaneous melanomas, authors said.
To evaluate the combination in earlier stage disease, Dr. Amaria and coinvestigators conducted a single-center, open-label, randomized, phase 2 trial of 21 patients with surgically resectable clinical stage III or oligometastatic stage IV melanoma with BRAFV600E or BRAFV600K mutations.
Patients were randomized 2:1 to receive the neoadjuvant/adjuvant treatment or to standard of care, which consisted of standard surgery plus consideration for adjuvant therapy, the authors said. Those patients assigned to the targeted therapy arm received 8 weeks of neoadjuvant dabrafenib and trametinib followed by surgery, then adjuvant dabrafenib and trametinib for up to 44 weeks.
Event-free survival, the primary endpoint of the trial, was a median of 19.7 months for neoadjuvant plus adjuvant dabrafenib and trametinib, versus 2.9 months for standard care (P less than .0001), the investigators reported.
Dabrafenib and trametinib combination therapy was well tolerated as neoadjuvant and adjuvant therapy, with no grade 4 adverse events or treatment related deaths, according to the investigators. The most common grade 3 adverse event seen with the combination was diarrhea, occurring in 2 patients (15%).
The trial is continuing as a single-arm study of neoadjuvant plus adjuvant dabrafenib and trametinib.
Dr. Amaria and colleagues reported individual disclosures related to Merck, Bristol-Myers Squibb, Array Biopharma, and others, including Novartis Pharmaceuticals Corp., which supplied drugs and funded clinical aspects of the study.
SOURCE: Amaria et al. 2018 Jan 17. doi: 10.1016/S1470-2045(18)30015-9
For patients with surgically resectable, BRAF-mutated melanoma, neoadjuvant and adjuvant treatment with the combination of dabrafenib and trametinib resulted in significantly longer event-free survival compared with standard care, according to results of a randomized study.
The trial was closed early because of the “large difference” in event-free survival favoring the neoadjuvant approach, the authors wrote (Lancet Oncol. 2018 Jan 17. doi: 10.1016/S1470-2045(18)30015-9.
While early closure limits interpretation of results, they do provide important proof-of-concept and data for future studies, wrote the authors, led by Rodabe N Amaria, MD, of the department of medical oncology, University of Texas MD Anderson Cancer Center, Houston.
“The clinical and translational results strongly support the rationale for further assessment of neoadjuvant therapy in patients with high-risk, surgically resectable melanoma,” Dr. Amaria and colleagues said in the report on the randomized trial, believed to be the first to evaluate the role of neoadjuvant therapy versus standard care in BRAF-mutated melanoma.
Dabrafenib and trametinib combination therapy is approved as a treatment for patients with unresectable or metastatic stage IV melanoma and a BRAFV600 mutation, which is found in about half of cutaneous melanomas, authors said.
To evaluate the combination in earlier stage disease, Dr. Amaria and coinvestigators conducted a single-center, open-label, randomized, phase 2 trial of 21 patients with surgically resectable clinical stage III or oligometastatic stage IV melanoma with BRAFV600E or BRAFV600K mutations.
Patients were randomized 2:1 to receive the neoadjuvant/adjuvant treatment or to standard of care, which consisted of standard surgery plus consideration for adjuvant therapy, the authors said. Those patients assigned to the targeted therapy arm received 8 weeks of neoadjuvant dabrafenib and trametinib followed by surgery, then adjuvant dabrafenib and trametinib for up to 44 weeks.
Event-free survival, the primary endpoint of the trial, was a median of 19.7 months for neoadjuvant plus adjuvant dabrafenib and trametinib, versus 2.9 months for standard care (P less than .0001), the investigators reported.
Dabrafenib and trametinib combination therapy was well tolerated as neoadjuvant and adjuvant therapy, with no grade 4 adverse events or treatment related deaths, according to the investigators. The most common grade 3 adverse event seen with the combination was diarrhea, occurring in 2 patients (15%).
The trial is continuing as a single-arm study of neoadjuvant plus adjuvant dabrafenib and trametinib.
Dr. Amaria and colleagues reported individual disclosures related to Merck, Bristol-Myers Squibb, Array Biopharma, and others, including Novartis Pharmaceuticals Corp., which supplied drugs and funded clinical aspects of the study.
SOURCE: Amaria et al. 2018 Jan 17. doi: 10.1016/S1470-2045(18)30015-9
FROM THE LANCET ONCOLOGY
Key clinical point: In patients with high-risk, surgically resectable, clinical stage III-IV melanoma, dabrafenib and trametinib given in both the neoadjuvant and adjuvant setting improved event-free survival compared with standard care.
Major finding: Median event-free survival was 19.7 months for neoadjuvant/adjuvant dabrafenib and trametinib versus 2.9 months for standard upfront surgery including consideration for standard adjuvant therapy (P less than .0001).
Data source: A single-center, open-label, randomized, phase 2 trial including 21 patients with surgically resectable clinical stage III or oligometastatic stage IV BRAF-mutated melanoma.
Disclosures: Investigators reported ties to Novartis Pharmaceuticals Corp., which supplied drugs and funded clinical aspects of the study, and disclosures related to Merck, Bristol-Myers Squibb, Array Biopharma, and others.
Source: Amaria et al. 2018 Jan 17. doi: 10.1016/S1470-2045(18)30015-9.
Supportive oncodermatology: Cancer advances spawn new subspecialty
Not too long ago at the Dana-Farber/Brigham and Women’s Cancer Center in Boston, a woman with widely metastatic melanoma, who had been planning her own funeral, was surprised when she had a phenomenal response to immunotherapy.
She was shocked to learn that her cancer was almost completely gone after 12 weeks, but she was stunned when she developed a rash that made her oncologist think she needed to stop treatment.
With traditional cytotoxic chemotherapies, there were a few well-defined skin side effects that oncologists were comfortable managing on their own with steroids or by reducing or stopping treatment for a bit.
But over the last decade, new cancer options have become available, most notably immunotherapies and targeted biologics, which are keeping some people alive longer but also causing cutaneous side effects that have never been seen before in oncology and are being reported frequently.
An urgent need
Currently in the United States, there’s only a handful of dedicated supportive oncodermatology services, which can be found at major academic cancer centers such as Dana-Farber/Brigham and Women’s, but the residents and fellows being trained at these centers are starting to fan out across the country and set up new services.
One day, it’s likely that every major cancer institution will have “a toxicities team with expert dermatologists,” said Dr. LeBoeuf, who launched the supportive oncodermatology program at Dana-Farber in 2014 and who now runs it with a team of dermatologists and clinics every week. Dr. LeBoeuf is a leader in the field, like the other dermatologists interviewed for this story.
With all the new treatments and with even more on the way, “there’s an urgent need for dermatologists to be involved in care of cancer patients,” Dr. LeBoeuf said.
The problem
Immunotherapies like the PD-1 blocking agents pembrolizumab (Keytruda) and nivolumab (Opdivo) – both used for an ever-expanding list of tumors – amp up the immune system to fight cancer, but they also tend to cause adverse events that mimic autoimmune diseases such as lupus, psoriasis, lichen planus, and vitiligo. Dermatologists are familiar with those problems and how to manage them, but oncologists generally are not.
Meanwhile, the many targeted therapies approved over the past decade interfere with specific molecules needed for tumor growth, but they also are associated with a wide range of skin, hair, and nail side effects that include skin growths, itching, paronychia, and more.
Agents that target vascular endothelial growth factors, such as sorafenib (Nexavar) and bevacizumab (Avastin), can trigger a painful hand-foot skin reaction that’s different from the hand-foot syndrome reported with older cytotoxic agents.
Epidermal growth factor receptor (EGFR) inhibitors, such as erlotinib (Tarceva) or gefitinib (Iressa), often cause miserable acne-like eruptions, but that can mean the drug is working.
It’s hard for oncologists to know what’s life-threatening and what isn’t; that’s where dermatologists come in.
A solution
When problems come up, oncologists and patients need answers right away, she said. There’s no time to wait a month or two for a dermatology appointment to find out whether, for instance, a new mouth ulcer is a minor inconvenience or the first sign of Stevens-Johnson syndrome, and the last thing an exhausted cancer patient needs is to be told to go to yet another clinic for a dermatology consult.
For supportive oncodermatology, that means being where the patients are: in the cancer centers. “Our clinic is situated on the same cancer floor as all the other oncology clinics,” which means easy access for both patients and oncologists, Dr. Choi said. “They just come down the hall.”
Build it, and they will come
The Stanford (Calif.) Cancer Center is a good example of what happens once a supportive oncodermatology service is up and running.
The program there was the brainchild of dermatologist Bernice Kwong, MD, who helped launch it in 2012 with 2 half-day outpatient clinics per week.
“Once people knew we were there seeing patients, we needed to expand it to 3 half days, and within 6 months, we knew we had to be” in the cancer center daily, she said. “The oncologists felt we were helping them keep their patients on treatment longer; they didn’t have to stop therapy to sort out a rash.”
Currently, the clinic sees about 15 to 20 patients a day, but “we have more need than that,” said Dr. Kwong, who is trying to recruit more dermatologists to help.
“The need is huge. There’s so much room for growth,” she noted, but first, “you need the oncologists to be on board.”
Dermatologist Adam Friedman, MD, director of supportive oncodermatology at the George Washington University Cancer Center, Washington, says his program is on the other end of the growth curve since it was only launched in the spring of 2017. Only about 80 patients have been treated so far, and there’s one dedicated clinic day a month, although he is on call for urgent cases, as is the case for many of the other dermatologists interviewed for this story.
Dr. Friedman expects business will pick up soon once word gets out, just like at Dana-Farber/Brigham and Women’s, Stanford, and elsewhere. “The places with the greatest need are going to have these services first, and then you’ll see them pop up elsewhere. I think we are going to see more,” he said.
The birth of supportive oncodermatology
Dermatologist Mario Lacouture, MD, director of the oncodermatology program at Memorial Sloan Kettering Cancer Center, New York, is considered by many oncodermatologists to be the father of the field.
He started the very first program in 2005 at Northwestern University, Chicago, followed by the program at Sloan Kettering a few years later. He has helped train many of the leaders in the field and coined the phrase “supportive oncodermatology” as the senior author in the field’s seminal paper, published in 2011 (J Am Acad Dermatol. 2011 Sep;65[3]:624-35). That article, in turn, inspired at least a few young dermatologists to make supportive oncodermatology their career choice. Dr. Lacouture speaks regularly at oncology and dermatology meetings to raise awareness about how dermatologists can improve cancer care.
Cancer survivors were also a concern. “Cancer treatment has improved so much that people are living longer, but the majority of survivors have either temporary or permanent cutaneous problems that would benefit from dermatologic care. However, the oncology community and patients are usually not aware that there are things we can do to help,” Dr. Lacouture said.
The message seems to have gotten out, however, among the hundreds of oncologists affiliated with Sloan Kettering. Dr. Lacouture needs a team of supportive oncodermatologists to meet the demand, with walk-in clinics every day and round-the-clock call.
He anticipates a day when visiting a supportive oncodermatologist will be routine, even before the start of cancer treatment, just as people visit a dentist before bone marrow transplants or radiation treatment to the head and neck. The idea would be to prevent cutaneous toxicity, something Dr. Lacouture and his team are already doing at Sloan Kettering. In time, supportive oncodermatology “is something that is going to be instituted early on” in treatment, he said.
“It’s important for dermatologists to reach out to their local oncologists; they will see there are many, many cancer patients and survivors who would benefit immensely from their care,” he said.
Dr. Lacouture is a consultant for Galderma, Janssen, and Johnson & Johnson. The other dermatologists interviewed for this story had no relevant industry disclosures. La Roche-Posay, a subsidiary of L’Oreal, is helping fund the supportive oncodermatology program at George Washington University. The company is interested in using cosmetics to camouflage cancer treatment skin lesions, Dr. Friedman said. Dr. Friedman is a member of the Dermatology News advisory board.
aotto@frontlinemedcom.com
Not too long ago at the Dana-Farber/Brigham and Women’s Cancer Center in Boston, a woman with widely metastatic melanoma, who had been planning her own funeral, was surprised when she had a phenomenal response to immunotherapy.
She was shocked to learn that her cancer was almost completely gone after 12 weeks, but she was stunned when she developed a rash that made her oncologist think she needed to stop treatment.
With traditional cytotoxic chemotherapies, there were a few well-defined skin side effects that oncologists were comfortable managing on their own with steroids or by reducing or stopping treatment for a bit.
But over the last decade, new cancer options have become available, most notably immunotherapies and targeted biologics, which are keeping some people alive longer but also causing cutaneous side effects that have never been seen before in oncology and are being reported frequently.
An urgent need
Currently in the United States, there’s only a handful of dedicated supportive oncodermatology services, which can be found at major academic cancer centers such as Dana-Farber/Brigham and Women’s, but the residents and fellows being trained at these centers are starting to fan out across the country and set up new services.
One day, it’s likely that every major cancer institution will have “a toxicities team with expert dermatologists,” said Dr. LeBoeuf, who launched the supportive oncodermatology program at Dana-Farber in 2014 and who now runs it with a team of dermatologists and clinics every week. Dr. LeBoeuf is a leader in the field, like the other dermatologists interviewed for this story.
With all the new treatments and with even more on the way, “there’s an urgent need for dermatologists to be involved in care of cancer patients,” Dr. LeBoeuf said.
The problem
Immunotherapies like the PD-1 blocking agents pembrolizumab (Keytruda) and nivolumab (Opdivo) – both used for an ever-expanding list of tumors – amp up the immune system to fight cancer, but they also tend to cause adverse events that mimic autoimmune diseases such as lupus, psoriasis, lichen planus, and vitiligo. Dermatologists are familiar with those problems and how to manage them, but oncologists generally are not.
Meanwhile, the many targeted therapies approved over the past decade interfere with specific molecules needed for tumor growth, but they also are associated with a wide range of skin, hair, and nail side effects that include skin growths, itching, paronychia, and more.
Agents that target vascular endothelial growth factors, such as sorafenib (Nexavar) and bevacizumab (Avastin), can trigger a painful hand-foot skin reaction that’s different from the hand-foot syndrome reported with older cytotoxic agents.
Epidermal growth factor receptor (EGFR) inhibitors, such as erlotinib (Tarceva) or gefitinib (Iressa), often cause miserable acne-like eruptions, but that can mean the drug is working.
It’s hard for oncologists to know what’s life-threatening and what isn’t; that’s where dermatologists come in.
A solution
When problems come up, oncologists and patients need answers right away, she said. There’s no time to wait a month or two for a dermatology appointment to find out whether, for instance, a new mouth ulcer is a minor inconvenience or the first sign of Stevens-Johnson syndrome, and the last thing an exhausted cancer patient needs is to be told to go to yet another clinic for a dermatology consult.
For supportive oncodermatology, that means being where the patients are: in the cancer centers. “Our clinic is situated on the same cancer floor as all the other oncology clinics,” which means easy access for both patients and oncologists, Dr. Choi said. “They just come down the hall.”
Build it, and they will come
The Stanford (Calif.) Cancer Center is a good example of what happens once a supportive oncodermatology service is up and running.
The program there was the brainchild of dermatologist Bernice Kwong, MD, who helped launch it in 2012 with 2 half-day outpatient clinics per week.
“Once people knew we were there seeing patients, we needed to expand it to 3 half days, and within 6 months, we knew we had to be” in the cancer center daily, she said. “The oncologists felt we were helping them keep their patients on treatment longer; they didn’t have to stop therapy to sort out a rash.”
Currently, the clinic sees about 15 to 20 patients a day, but “we have more need than that,” said Dr. Kwong, who is trying to recruit more dermatologists to help.
“The need is huge. There’s so much room for growth,” she noted, but first, “you need the oncologists to be on board.”
Dermatologist Adam Friedman, MD, director of supportive oncodermatology at the George Washington University Cancer Center, Washington, says his program is on the other end of the growth curve since it was only launched in the spring of 2017. Only about 80 patients have been treated so far, and there’s one dedicated clinic day a month, although he is on call for urgent cases, as is the case for many of the other dermatologists interviewed for this story.
Dr. Friedman expects business will pick up soon once word gets out, just like at Dana-Farber/Brigham and Women’s, Stanford, and elsewhere. “The places with the greatest need are going to have these services first, and then you’ll see them pop up elsewhere. I think we are going to see more,” he said.
The birth of supportive oncodermatology
Dermatologist Mario Lacouture, MD, director of the oncodermatology program at Memorial Sloan Kettering Cancer Center, New York, is considered by many oncodermatologists to be the father of the field.
He started the very first program in 2005 at Northwestern University, Chicago, followed by the program at Sloan Kettering a few years later. He has helped train many of the leaders in the field and coined the phrase “supportive oncodermatology” as the senior author in the field’s seminal paper, published in 2011 (J Am Acad Dermatol. 2011 Sep;65[3]:624-35). That article, in turn, inspired at least a few young dermatologists to make supportive oncodermatology their career choice. Dr. Lacouture speaks regularly at oncology and dermatology meetings to raise awareness about how dermatologists can improve cancer care.
Cancer survivors were also a concern. “Cancer treatment has improved so much that people are living longer, but the majority of survivors have either temporary or permanent cutaneous problems that would benefit from dermatologic care. However, the oncology community and patients are usually not aware that there are things we can do to help,” Dr. Lacouture said.
The message seems to have gotten out, however, among the hundreds of oncologists affiliated with Sloan Kettering. Dr. Lacouture needs a team of supportive oncodermatologists to meet the demand, with walk-in clinics every day and round-the-clock call.
He anticipates a day when visiting a supportive oncodermatologist will be routine, even before the start of cancer treatment, just as people visit a dentist before bone marrow transplants or radiation treatment to the head and neck. The idea would be to prevent cutaneous toxicity, something Dr. Lacouture and his team are already doing at Sloan Kettering. In time, supportive oncodermatology “is something that is going to be instituted early on” in treatment, he said.
“It’s important for dermatologists to reach out to their local oncologists; they will see there are many, many cancer patients and survivors who would benefit immensely from their care,” he said.
Dr. Lacouture is a consultant for Galderma, Janssen, and Johnson & Johnson. The other dermatologists interviewed for this story had no relevant industry disclosures. La Roche-Posay, a subsidiary of L’Oreal, is helping fund the supportive oncodermatology program at George Washington University. The company is interested in using cosmetics to camouflage cancer treatment skin lesions, Dr. Friedman said. Dr. Friedman is a member of the Dermatology News advisory board.
aotto@frontlinemedcom.com
Not too long ago at the Dana-Farber/Brigham and Women’s Cancer Center in Boston, a woman with widely metastatic melanoma, who had been planning her own funeral, was surprised when she had a phenomenal response to immunotherapy.
She was shocked to learn that her cancer was almost completely gone after 12 weeks, but she was stunned when she developed a rash that made her oncologist think she needed to stop treatment.
With traditional cytotoxic chemotherapies, there were a few well-defined skin side effects that oncologists were comfortable managing on their own with steroids or by reducing or stopping treatment for a bit.
But over the last decade, new cancer options have become available, most notably immunotherapies and targeted biologics, which are keeping some people alive longer but also causing cutaneous side effects that have never been seen before in oncology and are being reported frequently.
An urgent need
Currently in the United States, there’s only a handful of dedicated supportive oncodermatology services, which can be found at major academic cancer centers such as Dana-Farber/Brigham and Women’s, but the residents and fellows being trained at these centers are starting to fan out across the country and set up new services.
One day, it’s likely that every major cancer institution will have “a toxicities team with expert dermatologists,” said Dr. LeBoeuf, who launched the supportive oncodermatology program at Dana-Farber in 2014 and who now runs it with a team of dermatologists and clinics every week. Dr. LeBoeuf is a leader in the field, like the other dermatologists interviewed for this story.
With all the new treatments and with even more on the way, “there’s an urgent need for dermatologists to be involved in care of cancer patients,” Dr. LeBoeuf said.
The problem
Immunotherapies like the PD-1 blocking agents pembrolizumab (Keytruda) and nivolumab (Opdivo) – both used for an ever-expanding list of tumors – amp up the immune system to fight cancer, but they also tend to cause adverse events that mimic autoimmune diseases such as lupus, psoriasis, lichen planus, and vitiligo. Dermatologists are familiar with those problems and how to manage them, but oncologists generally are not.
Meanwhile, the many targeted therapies approved over the past decade interfere with specific molecules needed for tumor growth, but they also are associated with a wide range of skin, hair, and nail side effects that include skin growths, itching, paronychia, and more.
Agents that target vascular endothelial growth factors, such as sorafenib (Nexavar) and bevacizumab (Avastin), can trigger a painful hand-foot skin reaction that’s different from the hand-foot syndrome reported with older cytotoxic agents.
Epidermal growth factor receptor (EGFR) inhibitors, such as erlotinib (Tarceva) or gefitinib (Iressa), often cause miserable acne-like eruptions, but that can mean the drug is working.
It’s hard for oncologists to know what’s life-threatening and what isn’t; that’s where dermatologists come in.
A solution
When problems come up, oncologists and patients need answers right away, she said. There’s no time to wait a month or two for a dermatology appointment to find out whether, for instance, a new mouth ulcer is a minor inconvenience or the first sign of Stevens-Johnson syndrome, and the last thing an exhausted cancer patient needs is to be told to go to yet another clinic for a dermatology consult.
For supportive oncodermatology, that means being where the patients are: in the cancer centers. “Our clinic is situated on the same cancer floor as all the other oncology clinics,” which means easy access for both patients and oncologists, Dr. Choi said. “They just come down the hall.”
Build it, and they will come
The Stanford (Calif.) Cancer Center is a good example of what happens once a supportive oncodermatology service is up and running.
The program there was the brainchild of dermatologist Bernice Kwong, MD, who helped launch it in 2012 with 2 half-day outpatient clinics per week.
“Once people knew we were there seeing patients, we needed to expand it to 3 half days, and within 6 months, we knew we had to be” in the cancer center daily, she said. “The oncologists felt we were helping them keep their patients on treatment longer; they didn’t have to stop therapy to sort out a rash.”
Currently, the clinic sees about 15 to 20 patients a day, but “we have more need than that,” said Dr. Kwong, who is trying to recruit more dermatologists to help.
“The need is huge. There’s so much room for growth,” she noted, but first, “you need the oncologists to be on board.”
Dermatologist Adam Friedman, MD, director of supportive oncodermatology at the George Washington University Cancer Center, Washington, says his program is on the other end of the growth curve since it was only launched in the spring of 2017. Only about 80 patients have been treated so far, and there’s one dedicated clinic day a month, although he is on call for urgent cases, as is the case for many of the other dermatologists interviewed for this story.
Dr. Friedman expects business will pick up soon once word gets out, just like at Dana-Farber/Brigham and Women’s, Stanford, and elsewhere. “The places with the greatest need are going to have these services first, and then you’ll see them pop up elsewhere. I think we are going to see more,” he said.
The birth of supportive oncodermatology
Dermatologist Mario Lacouture, MD, director of the oncodermatology program at Memorial Sloan Kettering Cancer Center, New York, is considered by many oncodermatologists to be the father of the field.
He started the very first program in 2005 at Northwestern University, Chicago, followed by the program at Sloan Kettering a few years later. He has helped train many of the leaders in the field and coined the phrase “supportive oncodermatology” as the senior author in the field’s seminal paper, published in 2011 (J Am Acad Dermatol. 2011 Sep;65[3]:624-35). That article, in turn, inspired at least a few young dermatologists to make supportive oncodermatology their career choice. Dr. Lacouture speaks regularly at oncology and dermatology meetings to raise awareness about how dermatologists can improve cancer care.
Cancer survivors were also a concern. “Cancer treatment has improved so much that people are living longer, but the majority of survivors have either temporary or permanent cutaneous problems that would benefit from dermatologic care. However, the oncology community and patients are usually not aware that there are things we can do to help,” Dr. Lacouture said.
The message seems to have gotten out, however, among the hundreds of oncologists affiliated with Sloan Kettering. Dr. Lacouture needs a team of supportive oncodermatologists to meet the demand, with walk-in clinics every day and round-the-clock call.
He anticipates a day when visiting a supportive oncodermatologist will be routine, even before the start of cancer treatment, just as people visit a dentist before bone marrow transplants or radiation treatment to the head and neck. The idea would be to prevent cutaneous toxicity, something Dr. Lacouture and his team are already doing at Sloan Kettering. In time, supportive oncodermatology “is something that is going to be instituted early on” in treatment, he said.
“It’s important for dermatologists to reach out to their local oncologists; they will see there are many, many cancer patients and survivors who would benefit immensely from their care,” he said.
Dr. Lacouture is a consultant for Galderma, Janssen, and Johnson & Johnson. The other dermatologists interviewed for this story had no relevant industry disclosures. La Roche-Posay, a subsidiary of L’Oreal, is helping fund the supportive oncodermatology program at George Washington University. The company is interested in using cosmetics to camouflage cancer treatment skin lesions, Dr. Friedman said. Dr. Friedman is a member of the Dermatology News advisory board.
aotto@frontlinemedcom.com
Sentinel node biopsy: Who needs it?
More than 17 years ago, I published an article that was largely ignored, predicating that patient benefit from the sentinel node biopsy procedure was unlikely.
I asserted that the lymph nodes are not a reliable filter for melanoma cells, lymphatic drainage is capricious, and many individuals (especially younger ones) have benign neval rests in their lymph nodes that cannot be distinguished from melanoma deposits, since they are both positive for the S100 protein (Int J Dermatol. 2000 Nov;39[11]:807-11). In addition, multiple uncontrolled studies had shown that locating sentinel nodes, followed by a complete lymph node dissection, had no survival benefit. At the time, I argued that sentinel node biopsy should be performed only if the patient was going to be enrolled in a clinical study.
Many surgical oncologists have built their careers around the flawed premise that removing the draining nodes would cure melanoma. It doesn’t. It is past time to admit it and move on.
So, if completion node dissection does not save lives, why do a sentinel node biopsy? I recently asked a dermatologist friend, who is a committed acolyte of the sentinel node biopsy school, why he continues to recommend sentinel node biopsy if there is no benefit from complete node dissection. His quick response was that patients want to know if they are at higher risk of metastatic disease so that they can be followed closely with high-resolution ultrasound at a major cancer center and can be eligible for clinical trials. His reply gave me pause, so I asked why completion node dissection was still being recommended. I was told that some patients with positive sentinel nodes lived far away, and if they would not make regular follow-up visits for high-resolution ultrasound, the surgical oncologists do completion node dissection to ensure “local nodal control.” Yipes! You’re going to rip my groin out because I like quiet county living?
I doubt that patients would be enthusiastic if told beforehand that sentinel node biopsies costs $14,000-$18,000, and has a 9% complication rate, and one-third of those patients who have complications end up with permanent lymphedema. I wondered if the patients were told they could have a genetic test done on their already excised melanoma tissue that would tell them if they were in a high-risk group without having an additional invasive surgical procedure. I wondered if they were told that 10%-30% of people with negative sentinel nodes go on to develop metastatic disease. I also wondered if they had been told they would have to walk around with their melanoma, which could spread at any time, for several additional weeks, while waiting for the results of their sentinel node biopsy, instead of having the melanoma immediately removed by their dermatologist. I also wondered if they had been told that high-resolution ultrasound has not definitively been shown to be superior to clinical palpation of the lymph nodes.
I looked into the possibility of clinical trials for patients with positive sentinel nodes, as well. Based on my search of clinical trials.gov in January, there are 33 trials in the United States studying patients with stage 3 (positive sentinel node) or greater disease. If I had a positive sentinel node, I would look for a study in which I had a chance of getting nivolumab, which recently has been shown to be superior to ipilimumab in the phase 3 Checkmate 238 trial published in 2017 (N Engl J Med 2017 Nov 9; 377:1824-35).
But I am getting ahead of myself.
As a thinking man, if I had a thick melanoma (that was less than 2 mm), I would opt for a genetic test of my already excised melanoma tissue. If the results of that genetic test (which has near identical sensitivity and specificity for developing metastatic disease as a sentinel node) put me in the low-risk group, I would pass on the sentinel node biopsy. This would eliminate a lot of unnecessary surgery. If I fell into the high-risk group, I would consider a sentinel node biopsy so I could get into a study, or determine if I needed to find a way to get my insurance to pay, or if I could personally afford nivolumab. Even if I opted not to take the drug, because of the potential risk of high-grade side effects, the high-risk genetic profile tells me I would still need more frequent follow-up.
These are exciting times. I am looking forward to clinical trials that allow a patient with a high-risk genetic profile to go directly into a trial. We are moving into the realm of individualized genomic medicine in which metastatic melanoma truly becomes a curable disease.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Dr. Coldiron has no financial or other conflicts of interest with Castle Biosciences, the manufacturer of the DecisionDx-Melanoma genetic expression profile test. Email him at dermnews@frontlinemedcom.com.
More than 17 years ago, I published an article that was largely ignored, predicating that patient benefit from the sentinel node biopsy procedure was unlikely.
I asserted that the lymph nodes are not a reliable filter for melanoma cells, lymphatic drainage is capricious, and many individuals (especially younger ones) have benign neval rests in their lymph nodes that cannot be distinguished from melanoma deposits, since they are both positive for the S100 protein (Int J Dermatol. 2000 Nov;39[11]:807-11). In addition, multiple uncontrolled studies had shown that locating sentinel nodes, followed by a complete lymph node dissection, had no survival benefit. At the time, I argued that sentinel node biopsy should be performed only if the patient was going to be enrolled in a clinical study.
Many surgical oncologists have built their careers around the flawed premise that removing the draining nodes would cure melanoma. It doesn’t. It is past time to admit it and move on.
So, if completion node dissection does not save lives, why do a sentinel node biopsy? I recently asked a dermatologist friend, who is a committed acolyte of the sentinel node biopsy school, why he continues to recommend sentinel node biopsy if there is no benefit from complete node dissection. His quick response was that patients want to know if they are at higher risk of metastatic disease so that they can be followed closely with high-resolution ultrasound at a major cancer center and can be eligible for clinical trials. His reply gave me pause, so I asked why completion node dissection was still being recommended. I was told that some patients with positive sentinel nodes lived far away, and if they would not make regular follow-up visits for high-resolution ultrasound, the surgical oncologists do completion node dissection to ensure “local nodal control.” Yipes! You’re going to rip my groin out because I like quiet county living?
I doubt that patients would be enthusiastic if told beforehand that sentinel node biopsies costs $14,000-$18,000, and has a 9% complication rate, and one-third of those patients who have complications end up with permanent lymphedema. I wondered if the patients were told they could have a genetic test done on their already excised melanoma tissue that would tell them if they were in a high-risk group without having an additional invasive surgical procedure. I wondered if they were told that 10%-30% of people with negative sentinel nodes go on to develop metastatic disease. I also wondered if they had been told they would have to walk around with their melanoma, which could spread at any time, for several additional weeks, while waiting for the results of their sentinel node biopsy, instead of having the melanoma immediately removed by their dermatologist. I also wondered if they had been told that high-resolution ultrasound has not definitively been shown to be superior to clinical palpation of the lymph nodes.
I looked into the possibility of clinical trials for patients with positive sentinel nodes, as well. Based on my search of clinical trials.gov in January, there are 33 trials in the United States studying patients with stage 3 (positive sentinel node) or greater disease. If I had a positive sentinel node, I would look for a study in which I had a chance of getting nivolumab, which recently has been shown to be superior to ipilimumab in the phase 3 Checkmate 238 trial published in 2017 (N Engl J Med 2017 Nov 9; 377:1824-35).
But I am getting ahead of myself.
As a thinking man, if I had a thick melanoma (that was less than 2 mm), I would opt for a genetic test of my already excised melanoma tissue. If the results of that genetic test (which has near identical sensitivity and specificity for developing metastatic disease as a sentinel node) put me in the low-risk group, I would pass on the sentinel node biopsy. This would eliminate a lot of unnecessary surgery. If I fell into the high-risk group, I would consider a sentinel node biopsy so I could get into a study, or determine if I needed to find a way to get my insurance to pay, or if I could personally afford nivolumab. Even if I opted not to take the drug, because of the potential risk of high-grade side effects, the high-risk genetic profile tells me I would still need more frequent follow-up.
These are exciting times. I am looking forward to clinical trials that allow a patient with a high-risk genetic profile to go directly into a trial. We are moving into the realm of individualized genomic medicine in which metastatic melanoma truly becomes a curable disease.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Dr. Coldiron has no financial or other conflicts of interest with Castle Biosciences, the manufacturer of the DecisionDx-Melanoma genetic expression profile test. Email him at dermnews@frontlinemedcom.com.
More than 17 years ago, I published an article that was largely ignored, predicating that patient benefit from the sentinel node biopsy procedure was unlikely.
I asserted that the lymph nodes are not a reliable filter for melanoma cells, lymphatic drainage is capricious, and many individuals (especially younger ones) have benign neval rests in their lymph nodes that cannot be distinguished from melanoma deposits, since they are both positive for the S100 protein (Int J Dermatol. 2000 Nov;39[11]:807-11). In addition, multiple uncontrolled studies had shown that locating sentinel nodes, followed by a complete lymph node dissection, had no survival benefit. At the time, I argued that sentinel node biopsy should be performed only if the patient was going to be enrolled in a clinical study.
Many surgical oncologists have built their careers around the flawed premise that removing the draining nodes would cure melanoma. It doesn’t. It is past time to admit it and move on.
So, if completion node dissection does not save lives, why do a sentinel node biopsy? I recently asked a dermatologist friend, who is a committed acolyte of the sentinel node biopsy school, why he continues to recommend sentinel node biopsy if there is no benefit from complete node dissection. His quick response was that patients want to know if they are at higher risk of metastatic disease so that they can be followed closely with high-resolution ultrasound at a major cancer center and can be eligible for clinical trials. His reply gave me pause, so I asked why completion node dissection was still being recommended. I was told that some patients with positive sentinel nodes lived far away, and if they would not make regular follow-up visits for high-resolution ultrasound, the surgical oncologists do completion node dissection to ensure “local nodal control.” Yipes! You’re going to rip my groin out because I like quiet county living?
I doubt that patients would be enthusiastic if told beforehand that sentinel node biopsies costs $14,000-$18,000, and has a 9% complication rate, and one-third of those patients who have complications end up with permanent lymphedema. I wondered if the patients were told they could have a genetic test done on their already excised melanoma tissue that would tell them if they were in a high-risk group without having an additional invasive surgical procedure. I wondered if they were told that 10%-30% of people with negative sentinel nodes go on to develop metastatic disease. I also wondered if they had been told they would have to walk around with their melanoma, which could spread at any time, for several additional weeks, while waiting for the results of their sentinel node biopsy, instead of having the melanoma immediately removed by their dermatologist. I also wondered if they had been told that high-resolution ultrasound has not definitively been shown to be superior to clinical palpation of the lymph nodes.
I looked into the possibility of clinical trials for patients with positive sentinel nodes, as well. Based on my search of clinical trials.gov in January, there are 33 trials in the United States studying patients with stage 3 (positive sentinel node) or greater disease. If I had a positive sentinel node, I would look for a study in which I had a chance of getting nivolumab, which recently has been shown to be superior to ipilimumab in the phase 3 Checkmate 238 trial published in 2017 (N Engl J Med 2017 Nov 9; 377:1824-35).
But I am getting ahead of myself.
As a thinking man, if I had a thick melanoma (that was less than 2 mm), I would opt for a genetic test of my already excised melanoma tissue. If the results of that genetic test (which has near identical sensitivity and specificity for developing metastatic disease as a sentinel node) put me in the low-risk group, I would pass on the sentinel node biopsy. This would eliminate a lot of unnecessary surgery. If I fell into the high-risk group, I would consider a sentinel node biopsy so I could get into a study, or determine if I needed to find a way to get my insurance to pay, or if I could personally afford nivolumab. Even if I opted not to take the drug, because of the potential risk of high-grade side effects, the high-risk genetic profile tells me I would still need more frequent follow-up.
These are exciting times. I am looking forward to clinical trials that allow a patient with a high-risk genetic profile to go directly into a trial. We are moving into the realm of individualized genomic medicine in which metastatic melanoma truly becomes a curable disease.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Dr. Coldiron has no financial or other conflicts of interest with Castle Biosciences, the manufacturer of the DecisionDx-Melanoma genetic expression profile test. Email him at dermnews@frontlinemedcom.com.
Desmoplastic melanoma yields to checkpoint inhibitors
Desmoplastic melanoma, a rare chemotherapy-resistant cutaneous malignancy, appears to be particularly responsive to immunotherapy with inhibitors of programmed death 1 (PD-1) or PD ligand 1 (PD-L1), investigators found.
Of 60 patients with desmoplastic melanoma (DM) treated with pembrolizumab (Keytruda), nivolumab (Opdivo), or an experimental PD-L1 inhibitor (BMS 936559) and followed for a median of 22 months, 42 (70%) had an objective response to immunotherapy, including 19 patients (32%) with a complete response (CR), and 38% with a partial response, reported Antoni Ribas, MD, PhD, of the University of California, Los Angeles, and colleagues.
Desmoplastic melanoma is frequently a consequence of DNA damage to cells exposed to ultraviolet light. The malignancy is characterized by spindle-shaped melanoma cells in dense, fibrous stroma. It is known to be resistant to conventional chemotherapy, and although DM tumors typically have high mutational loads, they generally lack driver mutations that could be treated with targeted agents, the investigators noted.
Nonetheless, the mutational burden of DM tumors may make them good candidates for immune checkpoint inhibitor therapy.
“As recognition of neoantigens that result from somatic nonsynonymous mutations is associated with improved clinical responses to anti–PD-1 and anti–PD-L1 therapy, we hypothesized that patients with DM might respond well to anti–PD-1 or anti–PD-L1 therapies, owing to their high mutational load,” Dr. Ribas and colleagues wrote.
To support their hypothesis, they identified 60 patients with DM from a retrospective review of pathology records on 1,058 patients with advanced melanoma treated with a PD-1 or PD-L1 inhibitor at 10 international sites from 2011 through 2016. Four of the patients had received the CTLA-4 inhibitor ipilimumab (Yervoy) in addition to anti–PD-1 agents.
Of the 60 patients, 35 (58%) had markers for a poor prognosis, either extrapulmonary visceral metastases or elevated lactate dehydrogenase levels.
The objective response rates were as noted before. Of the 23 patients with partial responses, 9 had tumor progression, whereas no patients with a CR had progression.
When the investigators looked at whole-exome sequencing results on 17 of the patients, they saw a high frequency of nonsynonymous mutations – in this instance, a change in the amino acid sequence of proteins from cytosine to thymine – “as part of a strong signature of ultraviolet light–induced DNA damage that is common to cutaneous melanoma.”
The most common driver mutations were in NF1, seen in 14 of the 17 cases. In contrast, targetable mutations in BRAF or RAS were absent.
Immunohistochemistry comparisons of samples from 19 cases of DM with 13 non-DM melanomas showed that the DM tumors had a significantly higher proportion of PD-L1–positive cells in the tumor parenchyma (P = .004). DM cells from invasive tumor margins showed increased CD8 cell density PD-L1 expression.
“Therefore, patients with advanced desmoplastic melanoma derive substantial clinical benefit from PD-1 or PD-L1 immune checkpoint blockade therapy, even though desmoplastic melanoma is defined by its dense desmoplastic fibrous stroma. The benefit is likely to result from the high mutational burden and a frequent preexisting adaptive immune response limited by PD-L1 expression,” Dr. Ribas and colleagues wrote.
The study was funded in part by the Grimaldi Family Fund, the Parker Institute for Cancer Immunotherapy, National Institutes of Health grants, the Ressler Family Fund, the Samuels Family Fund, and the Garcia-Corsini Family Fund. The authors reported having no competing financial interests.
SOURCE: Ribas A et al. Nature. 2018 Jan 10. doi: 10.1038/nature25187.
Desmoplastic melanoma, a rare chemotherapy-resistant cutaneous malignancy, appears to be particularly responsive to immunotherapy with inhibitors of programmed death 1 (PD-1) or PD ligand 1 (PD-L1), investigators found.
Of 60 patients with desmoplastic melanoma (DM) treated with pembrolizumab (Keytruda), nivolumab (Opdivo), or an experimental PD-L1 inhibitor (BMS 936559) and followed for a median of 22 months, 42 (70%) had an objective response to immunotherapy, including 19 patients (32%) with a complete response (CR), and 38% with a partial response, reported Antoni Ribas, MD, PhD, of the University of California, Los Angeles, and colleagues.
Desmoplastic melanoma is frequently a consequence of DNA damage to cells exposed to ultraviolet light. The malignancy is characterized by spindle-shaped melanoma cells in dense, fibrous stroma. It is known to be resistant to conventional chemotherapy, and although DM tumors typically have high mutational loads, they generally lack driver mutations that could be treated with targeted agents, the investigators noted.
Nonetheless, the mutational burden of DM tumors may make them good candidates for immune checkpoint inhibitor therapy.
“As recognition of neoantigens that result from somatic nonsynonymous mutations is associated with improved clinical responses to anti–PD-1 and anti–PD-L1 therapy, we hypothesized that patients with DM might respond well to anti–PD-1 or anti–PD-L1 therapies, owing to their high mutational load,” Dr. Ribas and colleagues wrote.
To support their hypothesis, they identified 60 patients with DM from a retrospective review of pathology records on 1,058 patients with advanced melanoma treated with a PD-1 or PD-L1 inhibitor at 10 international sites from 2011 through 2016. Four of the patients had received the CTLA-4 inhibitor ipilimumab (Yervoy) in addition to anti–PD-1 agents.
Of the 60 patients, 35 (58%) had markers for a poor prognosis, either extrapulmonary visceral metastases or elevated lactate dehydrogenase levels.
The objective response rates were as noted before. Of the 23 patients with partial responses, 9 had tumor progression, whereas no patients with a CR had progression.
When the investigators looked at whole-exome sequencing results on 17 of the patients, they saw a high frequency of nonsynonymous mutations – in this instance, a change in the amino acid sequence of proteins from cytosine to thymine – “as part of a strong signature of ultraviolet light–induced DNA damage that is common to cutaneous melanoma.”
The most common driver mutations were in NF1, seen in 14 of the 17 cases. In contrast, targetable mutations in BRAF or RAS were absent.
Immunohistochemistry comparisons of samples from 19 cases of DM with 13 non-DM melanomas showed that the DM tumors had a significantly higher proportion of PD-L1–positive cells in the tumor parenchyma (P = .004). DM cells from invasive tumor margins showed increased CD8 cell density PD-L1 expression.
“Therefore, patients with advanced desmoplastic melanoma derive substantial clinical benefit from PD-1 or PD-L1 immune checkpoint blockade therapy, even though desmoplastic melanoma is defined by its dense desmoplastic fibrous stroma. The benefit is likely to result from the high mutational burden and a frequent preexisting adaptive immune response limited by PD-L1 expression,” Dr. Ribas and colleagues wrote.
The study was funded in part by the Grimaldi Family Fund, the Parker Institute for Cancer Immunotherapy, National Institutes of Health grants, the Ressler Family Fund, the Samuels Family Fund, and the Garcia-Corsini Family Fund. The authors reported having no competing financial interests.
SOURCE: Ribas A et al. Nature. 2018 Jan 10. doi: 10.1038/nature25187.
Desmoplastic melanoma, a rare chemotherapy-resistant cutaneous malignancy, appears to be particularly responsive to immunotherapy with inhibitors of programmed death 1 (PD-1) or PD ligand 1 (PD-L1), investigators found.
Of 60 patients with desmoplastic melanoma (DM) treated with pembrolizumab (Keytruda), nivolumab (Opdivo), or an experimental PD-L1 inhibitor (BMS 936559) and followed for a median of 22 months, 42 (70%) had an objective response to immunotherapy, including 19 patients (32%) with a complete response (CR), and 38% with a partial response, reported Antoni Ribas, MD, PhD, of the University of California, Los Angeles, and colleagues.
Desmoplastic melanoma is frequently a consequence of DNA damage to cells exposed to ultraviolet light. The malignancy is characterized by spindle-shaped melanoma cells in dense, fibrous stroma. It is known to be resistant to conventional chemotherapy, and although DM tumors typically have high mutational loads, they generally lack driver mutations that could be treated with targeted agents, the investigators noted.
Nonetheless, the mutational burden of DM tumors may make them good candidates for immune checkpoint inhibitor therapy.
“As recognition of neoantigens that result from somatic nonsynonymous mutations is associated with improved clinical responses to anti–PD-1 and anti–PD-L1 therapy, we hypothesized that patients with DM might respond well to anti–PD-1 or anti–PD-L1 therapies, owing to their high mutational load,” Dr. Ribas and colleagues wrote.
To support their hypothesis, they identified 60 patients with DM from a retrospective review of pathology records on 1,058 patients with advanced melanoma treated with a PD-1 or PD-L1 inhibitor at 10 international sites from 2011 through 2016. Four of the patients had received the CTLA-4 inhibitor ipilimumab (Yervoy) in addition to anti–PD-1 agents.
Of the 60 patients, 35 (58%) had markers for a poor prognosis, either extrapulmonary visceral metastases or elevated lactate dehydrogenase levels.
The objective response rates were as noted before. Of the 23 patients with partial responses, 9 had tumor progression, whereas no patients with a CR had progression.
When the investigators looked at whole-exome sequencing results on 17 of the patients, they saw a high frequency of nonsynonymous mutations – in this instance, a change in the amino acid sequence of proteins from cytosine to thymine – “as part of a strong signature of ultraviolet light–induced DNA damage that is common to cutaneous melanoma.”
The most common driver mutations were in NF1, seen in 14 of the 17 cases. In contrast, targetable mutations in BRAF or RAS were absent.
Immunohistochemistry comparisons of samples from 19 cases of DM with 13 non-DM melanomas showed that the DM tumors had a significantly higher proportion of PD-L1–positive cells in the tumor parenchyma (P = .004). DM cells from invasive tumor margins showed increased CD8 cell density PD-L1 expression.
“Therefore, patients with advanced desmoplastic melanoma derive substantial clinical benefit from PD-1 or PD-L1 immune checkpoint blockade therapy, even though desmoplastic melanoma is defined by its dense desmoplastic fibrous stroma. The benefit is likely to result from the high mutational burden and a frequent preexisting adaptive immune response limited by PD-L1 expression,” Dr. Ribas and colleagues wrote.
The study was funded in part by the Grimaldi Family Fund, the Parker Institute for Cancer Immunotherapy, National Institutes of Health grants, the Ressler Family Fund, the Samuels Family Fund, and the Garcia-Corsini Family Fund. The authors reported having no competing financial interests.
SOURCE: Ribas A et al. Nature. 2018 Jan 10. doi: 10.1038/nature25187.
FROM NATURE
Key clinical point: Desmoplastic melanoma (DM) has a high mutational load that may make it susceptible to anti–PD-1 and PD-L1 therapy.
Major finding: The objective response rate was 70%, including 32% complete and 38% partial responses.
Data source: A retrospective review of data on 60 patients with desmoplastic melanoma treated with immune checkpoint inhibitors.
Disclosures: The study was funded in part by the Grimaldi Family Fund, the Parker Institute for Cancer Immunotherapy, National Institutes of Health grants, the Ressler Family Fund, the Samuels Family Fund, and the Garcia-Corsini Family Fund. The authors reported having no competing financial interests.
Source: Ribas A et al. Nature. 2018 Jan 10. doi: 10.1038/nature25187.
Nonmalignant Cutaneous Findings Associated With Vemurafenib
To the Editor:
A 53-year-old woman was referred by her oncologist to our dermatology office with lesions on the face and body that presented 8 days after starting vemurafenib 960 mg twice daily for metastatic melanoma. The patient denied any symptoms from the lesions but was concerned they would spread to cover her entire face and body.
The patient's medical history included a diagnosis of metastatic melanoma 6 years prior to presentation. She stated that the primary cutaneous melanoma site was unknown. The patient had endured numerous surgeries to excise lymph node tumors, with some lesions up to 3 cm. The patient recently started vemurafenib, a treatment for BRAF V600E mutation-positive metastatic melanoma. The patient's personal history was notable for hepatitis A, B, and C, and her family history revealed her mother had metastatic lung cancer.
Physical examination revealed numerous 2- to 3-mm, round-oval, flesh-colored to light-brown papules on the cheeks, chest, abdomen (Figure 1), back, and both arms and legs. Some papules were inflamed and some had a stuck-on appearance. Lesions on the chest between the breasts and inframammary region were slightly inflamed. Two skin biopsies were performed. Biopsy of the lesion on the right lateral back revealed solar lentigo, early macular seborrheic keratosis, and a focus of inflamed mild solar keratosis. The dermis showed a mild superficial perivascular and interstitial inflammatory infiltrate composed mostly of lymphocytes, histiocytes, and eosinophils. There were occasional melanophages present (Figure 2). Biopsy of the lesion between the breasts revealed inflamed verrucous seborrheic keratosis (Figure 3).


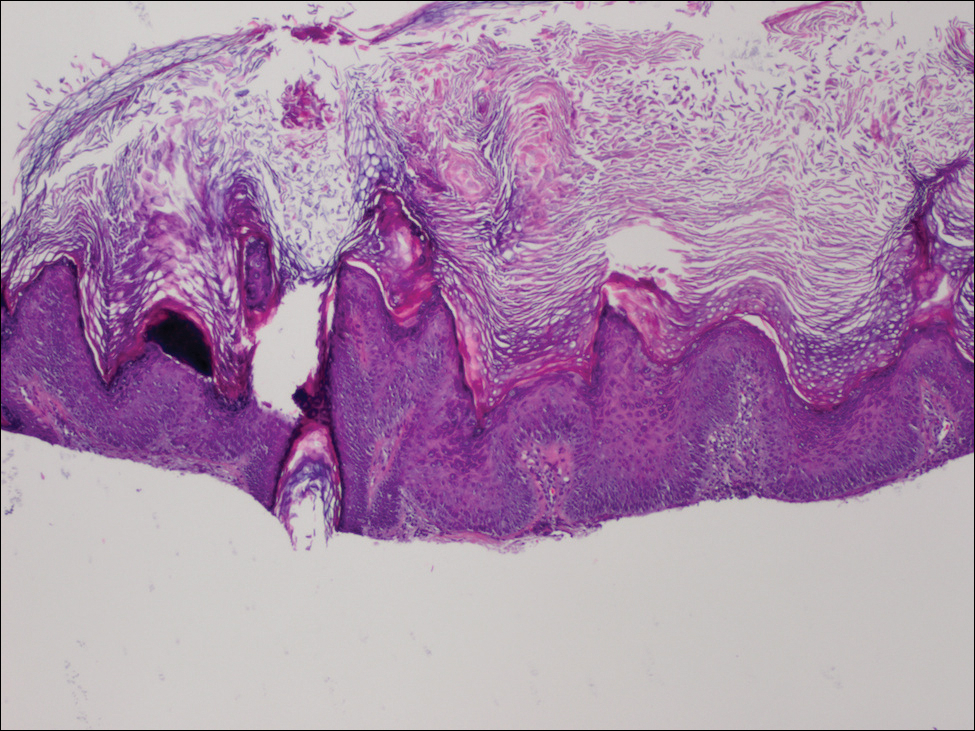
We treated the lesion on the right lateral back with cycles of cryotherapy and explained to the patient that the lesion between the breasts was benign. We also reiterated to the patient the importance of wearing sun-protective clothing and UVA/UVB sunblock with a sun protection factor of 30 or higher.
Our patient was diagnosed with pneumonia and subsequently had to discontinue vemurafenib. During the period of nontreatment, the keratotic lesions cleared with postinflammatory hyperpigmentation and no epidermal changes, which showed a possible inference of a direct relationship between the vemurafenib and the appearance of the nonmalignant cutaneous lesions. Although this report only represents 1 patient, other patients possibly can benefit from a modified dose of vemurafenib, which either would resolve or lessen the quantity of these lesions.
Vemurafenib is the first US Food and Drug Administration-approved treatment for nonresectable metastatic melanoma with the BRAF V600E mutation as detected by a US Food and Drug Administration-approved test.1,2 Mutated BRAF is present in approximately 60% of cutaneous melanomas.3 Vemurafenib targets the oncogenic BRAF V600E making the protein inactive, thus inhibiting cell proliferation and leading to apoptosis and shrinkage of the metastatic tumors.3-5 Vemurafenib has a response rate of more than 50% and is associated with rapid improvement in quality of life.3
Cutaneous side effects include increased incidence of squamous cell carcinoma and keratoacanthomas, appearing approximately 7 to 8 weeks after starting vemurafenib.4 The incidence of these lesions increases in patients 65 years and older and in patients with prior skin cancer and chronic sun exposure. The paradoxical activation of the mitogen-activated protein kinase pathway by mutant BRAF-selective inhibitors provides an explanation of the induction of squamous cell carcinomas.4 Prior to the initiation of vemurafenib, all patients should receive a total-body skin examination and every 2 months thereafter while on treatment. After discontinuation of the medicine, the patient should continue to receive total-body skin evaluations every 6 months indefinitely.
Patients should be aware of the potential for mild to severe photosensitivity reactions. They should be advised to limit their sun exposure time and to wear sun-protective clothing when outdoors. The use of broad-spectrum UVA/UVB sunscreen and lip protectant with a sun protection factor of 30 or higher also should be stressed.6,7 Patients should be aware that UVA rays penetrate glass; therefore, UV-protective clothing should be worn throughout the day and during all seasons.7
In clinical trials of vemurafenib, Stevens-Johnson syndrome and toxic epidermal necrolysis was reported in 2 patients.8,9 Clinical trials also reported patients developing new primary malignant melanoma lesions.10 These findings further emphasize the need for patients to undergo total-body skin examinations during and after treatment.
Other possible dermatologic reactions include a generalized rash, erythema, alopecia, and pruritus.2,3 The development of benign growths associated with patients on vemurafenib include follicular plugging seen in keratosis pilaris, palmar and plantar hyperkeratosis, seborrheic dermatitis-like rashes, verrucous keratosis, and acantholytic dyskeratosis.8,11,12
We report a case of nonmalignant growths occurring 8 days after starting vemurafenib. This case illustrates potential cutaneous adverse reactions that were benign yet still of great concern to our patient. Many of these nonmalignant cutaneous findings are associated with abnormal follicular keratinization thought to be secondary to abnormal signaling of the mitogen-activated protein kinase pathway that occurs with the use of BRAF inhibitors.8 Although in this case malignant lesions were not discovered, the need for total-body skin examinations exists during all stages of treatment. Supportive care and reassurance should be given to patients along with local treatments including topical therapies (steroids, retinoids), cryotherapy, and biopsies or excisions when necessary.13,14
- Holstein S, Hohl R. Therapeutic additions and possible deletions in oncology in 2011. Clin Pharmacol Ther. 2011;91:15-17.
- Zambon A, Niculescu-Dovaz I, Niculescu-Dovaz D, et al. Small molecule inhibitors of BRAF in clinical trials. Bioorg Med Chem Lett. 2012;22:789-792.
- Luke JJ, Hodi FS. Vemurafenib and BRAF inhibition: a new class of treatment for metastatic melanoma [published online November 14, 2011]. Clin Cancer Res. 2012;18:9-14.
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010; 363:809-819.
- Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA. 2008;105:3041-3046.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Dummer R, Rinderknecht J, Goldinger SM. Ultraviolet A and photosensitivity during vemuranefib therapy. N Engl J Med. 2012;366:480-481.
- Bovd KP, Vincent B, Andrea A, et al. Nonmalignant cutaneous findings associated with vemurafenib use in patients with metastatic melanoma. J Am Acad Dermatol. 2012;67:1375-1379.
- Wang CM, Fleming KF Hsu S. A case of vemurafenib-induced keratosis pilaris-like eruption. Dermatol Online J. 2012;18:7.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Gupta M, Huang V, Linette G, et al. Unusual complication of vemurafenib treatment of metastatic melanoma: exacerbation of acantholytic dyskeratosis complicated by Kaposi varicelliform eruption. Arch Dermatol. 2012;148:966-968;
- Sinha R, Edmonds K, Newton-Bishop JA, et al. Cutaneous adverse events associated with vemurafenib in patients with metastatic melanoma: practical advice on diagnosis, preventions and management of the main treatment related skin toxicities. Br J Dermatol. 2012;167:987-994.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
To the Editor:
A 53-year-old woman was referred by her oncologist to our dermatology office with lesions on the face and body that presented 8 days after starting vemurafenib 960 mg twice daily for metastatic melanoma. The patient denied any symptoms from the lesions but was concerned they would spread to cover her entire face and body.
The patient's medical history included a diagnosis of metastatic melanoma 6 years prior to presentation. She stated that the primary cutaneous melanoma site was unknown. The patient had endured numerous surgeries to excise lymph node tumors, with some lesions up to 3 cm. The patient recently started vemurafenib, a treatment for BRAF V600E mutation-positive metastatic melanoma. The patient's personal history was notable for hepatitis A, B, and C, and her family history revealed her mother had metastatic lung cancer.
Physical examination revealed numerous 2- to 3-mm, round-oval, flesh-colored to light-brown papules on the cheeks, chest, abdomen (Figure 1), back, and both arms and legs. Some papules were inflamed and some had a stuck-on appearance. Lesions on the chest between the breasts and inframammary region were slightly inflamed. Two skin biopsies were performed. Biopsy of the lesion on the right lateral back revealed solar lentigo, early macular seborrheic keratosis, and a focus of inflamed mild solar keratosis. The dermis showed a mild superficial perivascular and interstitial inflammatory infiltrate composed mostly of lymphocytes, histiocytes, and eosinophils. There were occasional melanophages present (Figure 2). Biopsy of the lesion between the breasts revealed inflamed verrucous seborrheic keratosis (Figure 3).



We treated the lesion on the right lateral back with cycles of cryotherapy and explained to the patient that the lesion between the breasts was benign. We also reiterated to the patient the importance of wearing sun-protective clothing and UVA/UVB sunblock with a sun protection factor of 30 or higher.
Our patient was diagnosed with pneumonia and subsequently had to discontinue vemurafenib. During the period of nontreatment, the keratotic lesions cleared with postinflammatory hyperpigmentation and no epidermal changes, which showed a possible inference of a direct relationship between the vemurafenib and the appearance of the nonmalignant cutaneous lesions. Although this report only represents 1 patient, other patients possibly can benefit from a modified dose of vemurafenib, which either would resolve or lessen the quantity of these lesions.
Vemurafenib is the first US Food and Drug Administration-approved treatment for nonresectable metastatic melanoma with the BRAF V600E mutation as detected by a US Food and Drug Administration-approved test.1,2 Mutated BRAF is present in approximately 60% of cutaneous melanomas.3 Vemurafenib targets the oncogenic BRAF V600E making the protein inactive, thus inhibiting cell proliferation and leading to apoptosis and shrinkage of the metastatic tumors.3-5 Vemurafenib has a response rate of more than 50% and is associated with rapid improvement in quality of life.3
Cutaneous side effects include increased incidence of squamous cell carcinoma and keratoacanthomas, appearing approximately 7 to 8 weeks after starting vemurafenib.4 The incidence of these lesions increases in patients 65 years and older and in patients with prior skin cancer and chronic sun exposure. The paradoxical activation of the mitogen-activated protein kinase pathway by mutant BRAF-selective inhibitors provides an explanation of the induction of squamous cell carcinomas.4 Prior to the initiation of vemurafenib, all patients should receive a total-body skin examination and every 2 months thereafter while on treatment. After discontinuation of the medicine, the patient should continue to receive total-body skin evaluations every 6 months indefinitely.
Patients should be aware of the potential for mild to severe photosensitivity reactions. They should be advised to limit their sun exposure time and to wear sun-protective clothing when outdoors. The use of broad-spectrum UVA/UVB sunscreen and lip protectant with a sun protection factor of 30 or higher also should be stressed.6,7 Patients should be aware that UVA rays penetrate glass; therefore, UV-protective clothing should be worn throughout the day and during all seasons.7
In clinical trials of vemurafenib, Stevens-Johnson syndrome and toxic epidermal necrolysis was reported in 2 patients.8,9 Clinical trials also reported patients developing new primary malignant melanoma lesions.10 These findings further emphasize the need for patients to undergo total-body skin examinations during and after treatment.
Other possible dermatologic reactions include a generalized rash, erythema, alopecia, and pruritus.2,3 The development of benign growths associated with patients on vemurafenib include follicular plugging seen in keratosis pilaris, palmar and plantar hyperkeratosis, seborrheic dermatitis-like rashes, verrucous keratosis, and acantholytic dyskeratosis.8,11,12
We report a case of nonmalignant growths occurring 8 days after starting vemurafenib. This case illustrates potential cutaneous adverse reactions that were benign yet still of great concern to our patient. Many of these nonmalignant cutaneous findings are associated with abnormal follicular keratinization thought to be secondary to abnormal signaling of the mitogen-activated protein kinase pathway that occurs with the use of BRAF inhibitors.8 Although in this case malignant lesions were not discovered, the need for total-body skin examinations exists during all stages of treatment. Supportive care and reassurance should be given to patients along with local treatments including topical therapies (steroids, retinoids), cryotherapy, and biopsies or excisions when necessary.13,14
To the Editor:
A 53-year-old woman was referred by her oncologist to our dermatology office with lesions on the face and body that presented 8 days after starting vemurafenib 960 mg twice daily for metastatic melanoma. The patient denied any symptoms from the lesions but was concerned they would spread to cover her entire face and body.
The patient's medical history included a diagnosis of metastatic melanoma 6 years prior to presentation. She stated that the primary cutaneous melanoma site was unknown. The patient had endured numerous surgeries to excise lymph node tumors, with some lesions up to 3 cm. The patient recently started vemurafenib, a treatment for BRAF V600E mutation-positive metastatic melanoma. The patient's personal history was notable for hepatitis A, B, and C, and her family history revealed her mother had metastatic lung cancer.
Physical examination revealed numerous 2- to 3-mm, round-oval, flesh-colored to light-brown papules on the cheeks, chest, abdomen (Figure 1), back, and both arms and legs. Some papules were inflamed and some had a stuck-on appearance. Lesions on the chest between the breasts and inframammary region were slightly inflamed. Two skin biopsies were performed. Biopsy of the lesion on the right lateral back revealed solar lentigo, early macular seborrheic keratosis, and a focus of inflamed mild solar keratosis. The dermis showed a mild superficial perivascular and interstitial inflammatory infiltrate composed mostly of lymphocytes, histiocytes, and eosinophils. There were occasional melanophages present (Figure 2). Biopsy of the lesion between the breasts revealed inflamed verrucous seborrheic keratosis (Figure 3).



We treated the lesion on the right lateral back with cycles of cryotherapy and explained to the patient that the lesion between the breasts was benign. We also reiterated to the patient the importance of wearing sun-protective clothing and UVA/UVB sunblock with a sun protection factor of 30 or higher.
Our patient was diagnosed with pneumonia and subsequently had to discontinue vemurafenib. During the period of nontreatment, the keratotic lesions cleared with postinflammatory hyperpigmentation and no epidermal changes, which showed a possible inference of a direct relationship between the vemurafenib and the appearance of the nonmalignant cutaneous lesions. Although this report only represents 1 patient, other patients possibly can benefit from a modified dose of vemurafenib, which either would resolve or lessen the quantity of these lesions.
Vemurafenib is the first US Food and Drug Administration-approved treatment for nonresectable metastatic melanoma with the BRAF V600E mutation as detected by a US Food and Drug Administration-approved test.1,2 Mutated BRAF is present in approximately 60% of cutaneous melanomas.3 Vemurafenib targets the oncogenic BRAF V600E making the protein inactive, thus inhibiting cell proliferation and leading to apoptosis and shrinkage of the metastatic tumors.3-5 Vemurafenib has a response rate of more than 50% and is associated with rapid improvement in quality of life.3
Cutaneous side effects include increased incidence of squamous cell carcinoma and keratoacanthomas, appearing approximately 7 to 8 weeks after starting vemurafenib.4 The incidence of these lesions increases in patients 65 years and older and in patients with prior skin cancer and chronic sun exposure. The paradoxical activation of the mitogen-activated protein kinase pathway by mutant BRAF-selective inhibitors provides an explanation of the induction of squamous cell carcinomas.4 Prior to the initiation of vemurafenib, all patients should receive a total-body skin examination and every 2 months thereafter while on treatment. After discontinuation of the medicine, the patient should continue to receive total-body skin evaluations every 6 months indefinitely.
Patients should be aware of the potential for mild to severe photosensitivity reactions. They should be advised to limit their sun exposure time and to wear sun-protective clothing when outdoors. The use of broad-spectrum UVA/UVB sunscreen and lip protectant with a sun protection factor of 30 or higher also should be stressed.6,7 Patients should be aware that UVA rays penetrate glass; therefore, UV-protective clothing should be worn throughout the day and during all seasons.7
In clinical trials of vemurafenib, Stevens-Johnson syndrome and toxic epidermal necrolysis was reported in 2 patients.8,9 Clinical trials also reported patients developing new primary malignant melanoma lesions.10 These findings further emphasize the need for patients to undergo total-body skin examinations during and after treatment.
Other possible dermatologic reactions include a generalized rash, erythema, alopecia, and pruritus.2,3 The development of benign growths associated with patients on vemurafenib include follicular plugging seen in keratosis pilaris, palmar and plantar hyperkeratosis, seborrheic dermatitis-like rashes, verrucous keratosis, and acantholytic dyskeratosis.8,11,12
We report a case of nonmalignant growths occurring 8 days after starting vemurafenib. This case illustrates potential cutaneous adverse reactions that were benign yet still of great concern to our patient. Many of these nonmalignant cutaneous findings are associated with abnormal follicular keratinization thought to be secondary to abnormal signaling of the mitogen-activated protein kinase pathway that occurs with the use of BRAF inhibitors.8 Although in this case malignant lesions were not discovered, the need for total-body skin examinations exists during all stages of treatment. Supportive care and reassurance should be given to patients along with local treatments including topical therapies (steroids, retinoids), cryotherapy, and biopsies or excisions when necessary.13,14
- Holstein S, Hohl R. Therapeutic additions and possible deletions in oncology in 2011. Clin Pharmacol Ther. 2011;91:15-17.
- Zambon A, Niculescu-Dovaz I, Niculescu-Dovaz D, et al. Small molecule inhibitors of BRAF in clinical trials. Bioorg Med Chem Lett. 2012;22:789-792.
- Luke JJ, Hodi FS. Vemurafenib and BRAF inhibition: a new class of treatment for metastatic melanoma [published online November 14, 2011]. Clin Cancer Res. 2012;18:9-14.
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010; 363:809-819.
- Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA. 2008;105:3041-3046.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Dummer R, Rinderknecht J, Goldinger SM. Ultraviolet A and photosensitivity during vemuranefib therapy. N Engl J Med. 2012;366:480-481.
- Bovd KP, Vincent B, Andrea A, et al. Nonmalignant cutaneous findings associated with vemurafenib use in patients with metastatic melanoma. J Am Acad Dermatol. 2012;67:1375-1379.
- Wang CM, Fleming KF Hsu S. A case of vemurafenib-induced keratosis pilaris-like eruption. Dermatol Online J. 2012;18:7.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Gupta M, Huang V, Linette G, et al. Unusual complication of vemurafenib treatment of metastatic melanoma: exacerbation of acantholytic dyskeratosis complicated by Kaposi varicelliform eruption. Arch Dermatol. 2012;148:966-968;
- Sinha R, Edmonds K, Newton-Bishop JA, et al. Cutaneous adverse events associated with vemurafenib in patients with metastatic melanoma: practical advice on diagnosis, preventions and management of the main treatment related skin toxicities. Br J Dermatol. 2012;167:987-994.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Holstein S, Hohl R. Therapeutic additions and possible deletions in oncology in 2011. Clin Pharmacol Ther. 2011;91:15-17.
- Zambon A, Niculescu-Dovaz I, Niculescu-Dovaz D, et al. Small molecule inhibitors of BRAF in clinical trials. Bioorg Med Chem Lett. 2012;22:789-792.
- Luke JJ, Hodi FS. Vemurafenib and BRAF inhibition: a new class of treatment for metastatic melanoma [published online November 14, 2011]. Clin Cancer Res. 2012;18:9-14.
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010; 363:809-819.
- Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA. 2008;105:3041-3046.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Dummer R, Rinderknecht J, Goldinger SM. Ultraviolet A and photosensitivity during vemuranefib therapy. N Engl J Med. 2012;366:480-481.
- Bovd KP, Vincent B, Andrea A, et al. Nonmalignant cutaneous findings associated with vemurafenib use in patients with metastatic melanoma. J Am Acad Dermatol. 2012;67:1375-1379.
- Wang CM, Fleming KF Hsu S. A case of vemurafenib-induced keratosis pilaris-like eruption. Dermatol Online J. 2012;18:7.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Huang V, Hepper D, Anadkat M, et al. Cutaneous toxic effects associated with vemurafenib and inhibition of the BRAF pathway. Arch Dermatol. 2012;148:628-633.
- Gupta M, Huang V, Linette G, et al. Unusual complication of vemurafenib treatment of metastatic melanoma: exacerbation of acantholytic dyskeratosis complicated by Kaposi varicelliform eruption. Arch Dermatol. 2012;148:966-968;
- Sinha R, Edmonds K, Newton-Bishop JA, et al. Cutaneous adverse events associated with vemurafenib in patients with metastatic melanoma: practical advice on diagnosis, preventions and management of the main treatment related skin toxicities. Br J Dermatol. 2012;167:987-994.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
Practice Points
- Prior to starting a BRAF inhibitor, clinicians should perform a baseline total-body skin examination and follow-up every 2 months.
- Take photographs of the patient's entire body on initial total-body skin examination.
- Encourage sun protection for exposed areas on the body in all seasons.
Perceptions of Tanning Risk Among Melanoma Patients With a History of Indoor Tanning
The incidence of melanoma is increasing at a rate greater than any other cancer,1 possibly due to the increasing use of indoor tanning devices. These devices emit unnaturally high levels of UVA and low levels of UVA and UVB rays.2 The risks of using these devices include increased incidence of melanoma (3438 cases attributed to indoor tanning in 2008) and keratinocytes cancer (increased risk of squamous cell carcinoma by 67% and basal cell carcinoma by 29%), severe sunburns (61.1% of female users and 44.6% of male users have reported sunburns), and aggravation of underlying disorders such as systemic lupus erythematosus.3-5
The literature varies in its explanation of how indoor tanning increases the risk of developing melanoma. Some authors suggest it is due to increased frequency of use, duration of sessions, and years of using tanning devices.1,6 Others suggest the increased cancer risk is the result of starting to tan at an earlier age.2,3,6-10 There is conflicting literature on the level of increased risk of melanoma in those who tan indoors at a young age (<35 years). Although the estimated rate of increased skin cancer risk varies, with rates up to 75% compared to nonusers, nearly all sources support an increased rate.6 Despite the growing body of knowledge that indoor tanning is dangerous, as well as the academic publication of these risks (eg, carcinogenesis, short-term and long-term eye injury, burns, UV sensitivity when combined with certain medications), teenagers in the United States and affluent countries appear to disregard the risks of tanning.11
Tanning companies have promoted the misconception that only UVB rays cause cell damage and UVA rays, which the devices emit, result in “damage-free” or “safe” tans.2,3 Until 2013, indoor tanning devices were classified by the US Food and Drug Administration (FDA) as class I, indicating that they are safe in terms of electrical shock. Many indoor tanning facilities have promoted the FDA “safe” label without clarifying that the safety indications only referred to electrical-shock potential. Nonetheless, it is known now that these devices, which emit high UVA and low UVB rays, promote melanoma, nonmelanoma skin cancers, and severe sunburns, as well as aggravate existing conditions (eg, systemic lupus erythematosus).4 As a result of an unacceptably high incidence of these disease complications, a 2014 FDA regulation categorized tanning beds as class II, requiring that tanning bed users be informed of the risk of skin cancer in an effort to reverse the growing trend of indoor tanning.12 Despite these regulatory interventions, it is not clear if this knowledge of cancer risk deters patients from indoor tanning.
The purpose of this study was to investigate the patients’ perspective on indoor tanning behaviors as associated with the severity of their melanoma and the time frame in which they were diagnosed as well as their perceived views on the safety of indoor tanning and the frequency in which they continue to tan indoors. This information is highly relevant in helping to determine if requiring a warning of the risk of skin cancer will deter patients from this unhealthy habit, especially given recent reclassification of sunbeds as class II by the FDA. Additional insights from these data may clarify if indoor tanning decreases the time frame in which melanoma is diagnosed or increases the severity of the resulting melanoma. Moreover, it will help elucidate whether or not the age at which indoor tanning is initiated affects the time frame to melanoma onset and corresponding severity.
Methods
An original unvalidated online survey was conducted worldwide via a link distributed to the following supporting institutions: Advanced Dermatology & Cosmetic Surgery, Ameriderm Research, Melanoma Research Foundation (a melanoma patient advocacy group), Florida State University Department of Dermatology, Moffitt Cancer Center Cutaneous Oncology Program, Cleveland Clinic, Ohio State University Division of Medical Oncology, Harvard Medical School Department of Dermatology, The University of Texas MD Anderson Cancer Center Department of Dermatology, University of Colorado Department of Dermatology, and Northwestern University Department of Dermatology. However, there was not confirmation that all of these institutions promoted the survey. Additionally, respondents were recruited through patient advocacy groups and social media sites including Facebook, Twitter, LinkedIn, Tumblr, and Instagram. The patient advocacy groups and social media sites invited participation through recruitment announcements, including DermNetNZ (a global dermatology patient information site), with additional help from the International Federation of Dermatology Clinical Trial Network.
The survey was restricted to those who were self-identified as 18 years or older and who self-reported a diagnosis of melanoma following the use of indoor tanning devices. The survey was hosted by SurveyMonkey, which allowed consent to be obtained and responses to remain anonymous. Access to the survey was sponsored by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. The University of Central Florida (Orlando, Florida) institutional review board reviewed and approved this study as exempt human research.
Survey responses collected from January 2014 to June 2015 were analyzed herein. The survey contained 58 questions and was divided into different topics including indoor tanning background (eg, states/countries in which participants tanned indoors, age when they first tanned, frequency of tanning), consenting process (eg, length, did someone review the consent with participants, what was contained in the consent), indoor tanning and melanoma (eg, how long after tanning did melanoma develop, age at development, location of melanoma), indoor tanning postmelanoma (eg, did participants tan after diagnosis and why), and other risk factors (eg, did participants smoke or drink pre- or postmelanoma).
Statistical Analysis
The data consist of both categorical and continuous variables. The categorical variables included age (<35 years or ≥35 years), frequency of indoor tanning (≤1 time weekly or >1 time weekly), and onset of melanoma diagnosis (within or after 5 years
Difference in proportions among groups, age, frequency of tanning, onset of melanoma diagnosis within or after 5 years of starting indoor tanning, and knowledge of cancer risks was tested for significance using the χ² test. Reported P values were 2-tailed, corresponding with a significance level of P<.05. All data were analyzed using SPSS (version 21.0). All statistical analyses were conducted independent of the participants’ sex.
Results
Of the 454 participants who accessed the survey, 448 were analyzed in this study; 6 participants did not complete the questionnaire. Both males and females were analyzed: 289 females, 12 males, and 153 who did not report gender. The age range of participants was 18 to 69 years. The age at start of indoor tanning ranged from 8 to 54 years, with a mean of 22 years. Additional participant characteristics are described in Table 1. The mean frequency of indoor tanning was reported as 2 times weekly. When participants were asked if they were warned of the risk of skin cancer, 21.5% reported yes while 78.4% reported not being told of the risk. This knowledge was compared to their frequency of indoor tanning. Having the knowledge of the risk of skin cancer had no influence on their frequency of indoor tanning (Table 2).
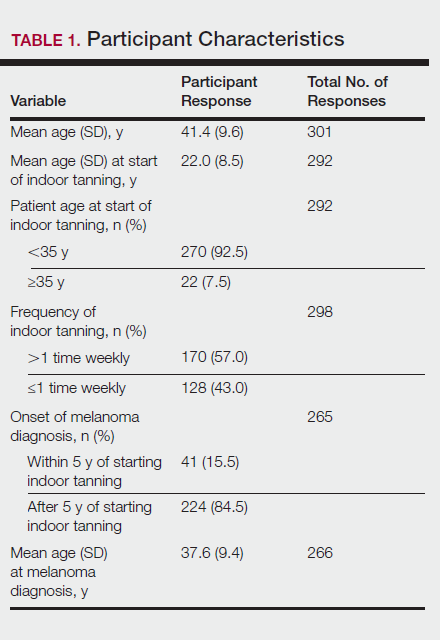
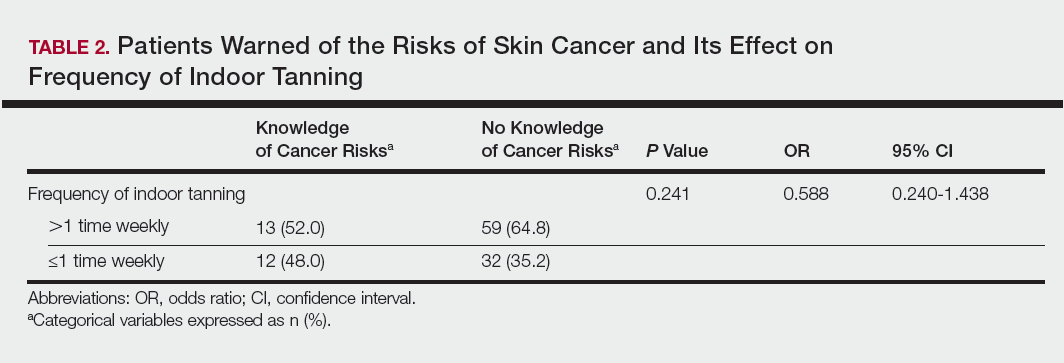
Among responders, those who perceived indoor tanning as safer than outdoor tanning tanned indoors more frequently than those who do not (Spearman r=−0.224; P<.05)(Table 3). The frequency of indoor tanning was divided into those who tanned indoors more than once weekly and those who tanned indoors once a week or less. This study showed that the frequency of indoor tanning had no effect on the latency time between the commencement of indoor tanning and diagnosis of melanoma (Table 4). The time frame from the onset of melanoma diagnosis also was compared to the age at which the participants started to tan indoors. Age was divided into those younger than 35 years and those 35 years and older. There was no correlation between the age when indoor tanning began and the time frame in which the melanoma was diagnosed (eTable).
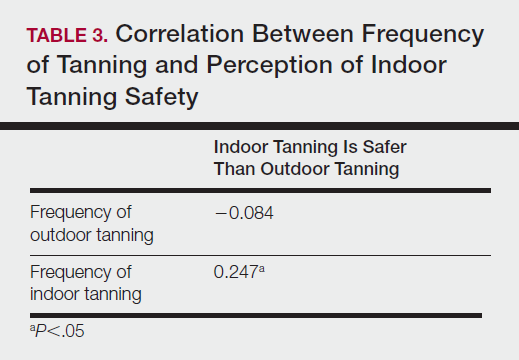
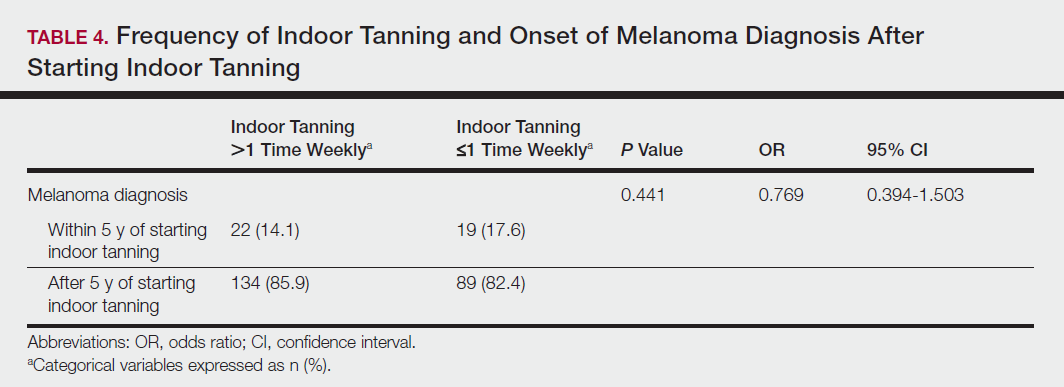
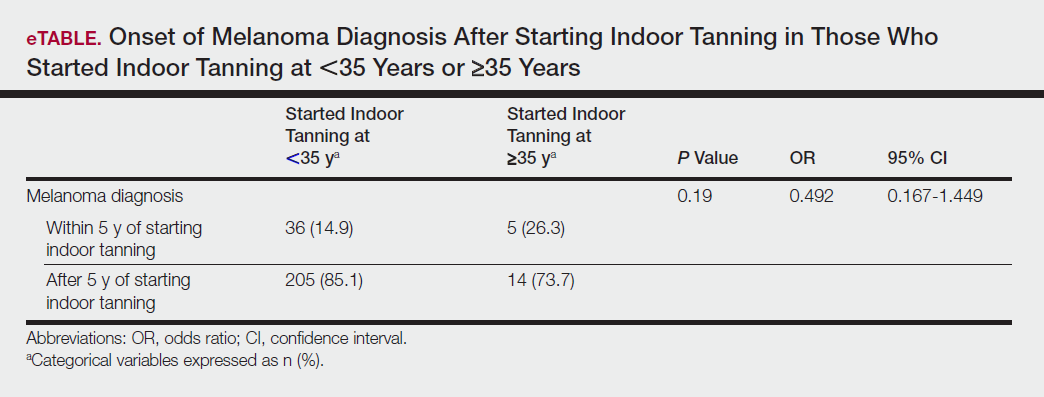
Table 5 shows the correlations between indoor tanning behaviors and melanoma characteristics. Those who started indoor tanning at an earlier age were diagnosed with melanoma at an earlier age compared to those who started indoor tanning later in life (r=0.549; P<.01). Moreover, those who started indoor tanning at a later age reported being diagnosed with a melanoma of greater Breslow depth (r=0.173; P<.01). Those who reported being diagnosed with a greater Breslow depth also reported a higher Clark level (r=0.608; P<.01). Among responders, those who more frequently tanned indoors also reported greater frequency of outdoor tanning (r=0.197; P<.01). This study showed no correlation between the age at melanoma diagnosis and the frequency of indoor (r=0.004; P>.05 not significant) or outdoor (r=0.093; P>.05 not significant) tanning. Having the knowledge of the risk of skin cancer had no relationship on the frequency of indoor tanning (r=−0.04; P>.05 not significant).
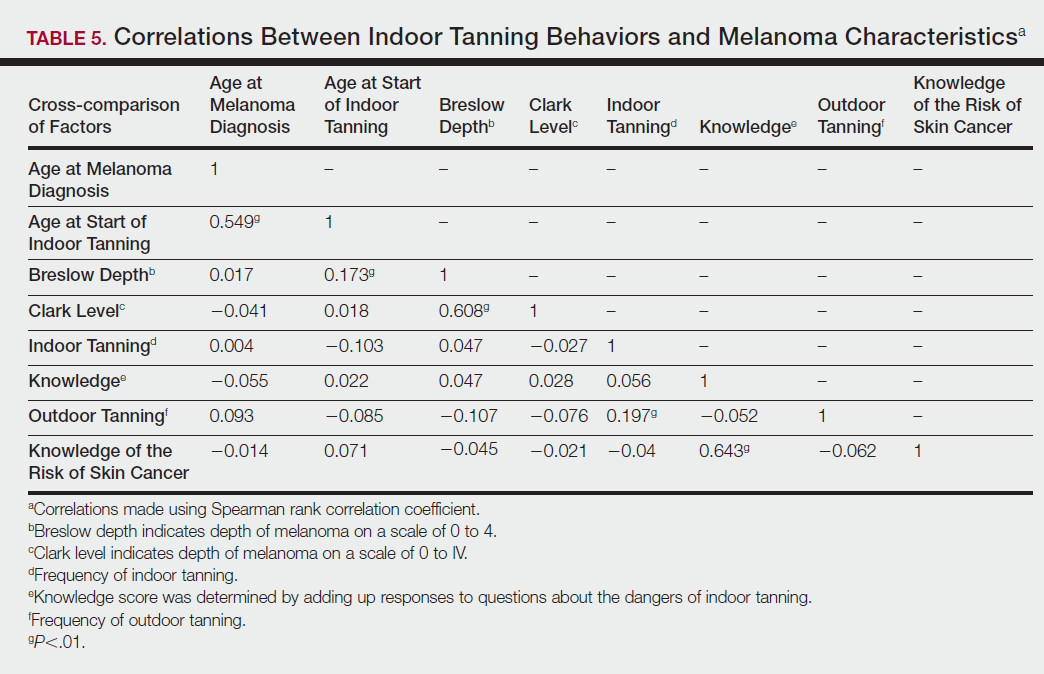
Comment
Thirty million Americans utilize indoor tanning devices at least once a year.13 UVA light comprises the majority of the spectrum used by indoor tanning devices, with a fraction (<5%) being UVB light. Until recently, UVB light was the only solar spectrum considered carcinogenic. In 2009, the International Agency for Research on Cancer classified the whole spectrum as carcinogenic to humans.5,11 Despite this evidence, indoor tanning facilities have promoted indoor tanning as damage free.3 The goal of this study was to collect the patient perspective on the safety of indoor tanning, indoor tanning behaviors, time frame of onset of melanoma, and the severity (ie, Breslow depth) of those melanomas.
Melanoma is the most prevalent cancer in females aged 25 to 29 years.3 The median age of diagnosis of melanoma (with and without the use of indoor tanning devices) is approximately 60 years14 versus our study, which found the average age at diagnosis was 37.6 years. Our findings are consistent with other literature in that those who start indoor tanning earlier (<35 years of age) develop melanoma at an earlier age.14,15 Cust et al14 also promoted the idea that patients develop melanoma earlier because younger individuals are more biologically susceptible to the carcinogenic effects of artificial UV light. However, our study found that those who started indoor tanning at an older age reported being diagnosed with a melanoma of greater Breslow depth, seemingly incongruent with the aforementioned hypothesis. One limitation is the age range for this research sample (18–69 years). The young age range may be attributable to the recruitment through social media, which is geared toward a younger population. Additionally, indoor tanning is a relatively new phenomenon practiced since the 1980s,2 which may contribute to the younger sample size. However, 2.7 billion individuals use social media worldwide with 40% of those older than 65 years on social media.16
Prior research has shown that those who start indoor tanning before the age of 35 years have a 75% increased risk of developing melanoma.14 Another study also has suggested that UVA-rich sunlamps may shorten the latency period for induction of melanoma and nonmelanoma skin cancers.3 Our study used similar age cutoffs in concluding that there was no earlier onset of melanoma diagnosis between those who started indoor tanning before the age of 35 years and those who started at the age of 35 years or older. Limitations include that our study is cross-sectional, and therefore time course cannot be established. Also, survey responses were self-reported, allowing the possibility of recall bias.
A plethora of research has been conducted to determine if there is a connection between the use of indoor tanning devices and developing melanoma. Cust et al14 suggested the risk of melanoma was 41% higher for those who had ever used a sunbed in comparison to those who had not. Other studies describe the difficulty in making the connection between indoor tanning and melanoma, as those who more frequently tan indoors also more frequently tan outdoors,11 as suggested by this study. However, there is a paucity of literature on the patients’ perspectives on the safety of indoor tanning. This study determined that those who more frequently tan indoors believed that indoor tanning is safer than outdoor tanning. With this altered perception promoted by the indoor tanning industry, the FDA has added a warning label to all indoor tanning devices about the risk of skin cancer. Our study revealed that having the knowledge of the risk of skin cancer had no influence on the frequency of indoor tanning. This concerning finding highlights a pressing need for an alternative approach to increase awareness of the harmful consequences that accompany indoor tanning. Further studies may elaborate on potential effective methods and messages to relate to an indoor tanning population comprised mostly of young females.
Acknowledgments
Supported and funded by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. This research project was completed as part of the FIRE Module at the University of Central Florida, College of Medicine. We thank the FIRE Module faculty and staff for their assistance with this project.
- Fisher DE, James WD. Indoor tanning—science, behavior, and policy. N Engl J Med. 2010;363:901-903.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Coelho SG, Hearing VJ. UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Melanoma Res. 2010;23:57-63.
- Klein RS, Sayre RM, Dowdy JC, et al. The risk of ultraviolet radiation exposure from indoor lamps in lupus erythematosus. Autoimmun Rev. 2009;8:320-324.
- O’Sullivan NA, Tait CP. Tanning bed and nail lamp use and the risk of cutaneous malignancy: a review of the literature. Australas J Dermatol. 2014;55:99-106.
- Schmidt CW. UV radiation and skin cancer: the science behind age restrictions for tanning beds. Environ Health Perspect. 2012;120:a308-a313.
- Lazovich D, Vogel RI, Berwick M, et al. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557-1568.
- Centers for Disease Control and Prevention (CDC). Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- Nielsen K, Masback A, Olsson H, et al. A prospective, population-based study of 40,000 women regarding host factors, UV exposure and sunbed use in relation to risk and anatomic site of cutaneous melanoma. Int J Cancer. 2012;131:706-715.
- Gandini S, Autier P, Boniol M. Reviews on sun exposure and artificial light and melanoma. Prog Biophys Mol Biol. 2011;107:362-366.
- Indoor tanning: the risks of ultraviolet rays. US Food and Drug Administration website. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm186687.htm. Updated September 11, 2017. Accessed November 2, 2017.
- Food and Drug Administration, HHS. General and plastic surgery devices: reclassification of ultraviolet lamps for tanning, henceforth to be known as sunlamp products and ultraviolet lamps intended for use in sunlamp products. Fed Regist. 2014;79:31205-31214.
- Brady MS. Public health and the tanning bed controversy. J Clin Oncol. 2012;30:1571-1573.
- Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128:2425-2435.
- International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer. 2007;120:1116-1122.
- Greenwood S, Perrin A, Duggan M. Social media update 2016. Pew Research Center website. http://www.pewinternet.org/2016/11/11/social-media-update-2016/. Published November 11, 2016. Accessed December 12, 2017.
The incidence of melanoma is increasing at a rate greater than any other cancer,1 possibly due to the increasing use of indoor tanning devices. These devices emit unnaturally high levels of UVA and low levels of UVA and UVB rays.2 The risks of using these devices include increased incidence of melanoma (3438 cases attributed to indoor tanning in 2008) and keratinocytes cancer (increased risk of squamous cell carcinoma by 67% and basal cell carcinoma by 29%), severe sunburns (61.1% of female users and 44.6% of male users have reported sunburns), and aggravation of underlying disorders such as systemic lupus erythematosus.3-5
The literature varies in its explanation of how indoor tanning increases the risk of developing melanoma. Some authors suggest it is due to increased frequency of use, duration of sessions, and years of using tanning devices.1,6 Others suggest the increased cancer risk is the result of starting to tan at an earlier age.2,3,6-10 There is conflicting literature on the level of increased risk of melanoma in those who tan indoors at a young age (<35 years). Although the estimated rate of increased skin cancer risk varies, with rates up to 75% compared to nonusers, nearly all sources support an increased rate.6 Despite the growing body of knowledge that indoor tanning is dangerous, as well as the academic publication of these risks (eg, carcinogenesis, short-term and long-term eye injury, burns, UV sensitivity when combined with certain medications), teenagers in the United States and affluent countries appear to disregard the risks of tanning.11
Tanning companies have promoted the misconception that only UVB rays cause cell damage and UVA rays, which the devices emit, result in “damage-free” or “safe” tans.2,3 Until 2013, indoor tanning devices were classified by the US Food and Drug Administration (FDA) as class I, indicating that they are safe in terms of electrical shock. Many indoor tanning facilities have promoted the FDA “safe” label without clarifying that the safety indications only referred to electrical-shock potential. Nonetheless, it is known now that these devices, which emit high UVA and low UVB rays, promote melanoma, nonmelanoma skin cancers, and severe sunburns, as well as aggravate existing conditions (eg, systemic lupus erythematosus).4 As a result of an unacceptably high incidence of these disease complications, a 2014 FDA regulation categorized tanning beds as class II, requiring that tanning bed users be informed of the risk of skin cancer in an effort to reverse the growing trend of indoor tanning.12 Despite these regulatory interventions, it is not clear if this knowledge of cancer risk deters patients from indoor tanning.
The purpose of this study was to investigate the patients’ perspective on indoor tanning behaviors as associated with the severity of their melanoma and the time frame in which they were diagnosed as well as their perceived views on the safety of indoor tanning and the frequency in which they continue to tan indoors. This information is highly relevant in helping to determine if requiring a warning of the risk of skin cancer will deter patients from this unhealthy habit, especially given recent reclassification of sunbeds as class II by the FDA. Additional insights from these data may clarify if indoor tanning decreases the time frame in which melanoma is diagnosed or increases the severity of the resulting melanoma. Moreover, it will help elucidate whether or not the age at which indoor tanning is initiated affects the time frame to melanoma onset and corresponding severity.
Methods
An original unvalidated online survey was conducted worldwide via a link distributed to the following supporting institutions: Advanced Dermatology & Cosmetic Surgery, Ameriderm Research, Melanoma Research Foundation (a melanoma patient advocacy group), Florida State University Department of Dermatology, Moffitt Cancer Center Cutaneous Oncology Program, Cleveland Clinic, Ohio State University Division of Medical Oncology, Harvard Medical School Department of Dermatology, The University of Texas MD Anderson Cancer Center Department of Dermatology, University of Colorado Department of Dermatology, and Northwestern University Department of Dermatology. However, there was not confirmation that all of these institutions promoted the survey. Additionally, respondents were recruited through patient advocacy groups and social media sites including Facebook, Twitter, LinkedIn, Tumblr, and Instagram. The patient advocacy groups and social media sites invited participation through recruitment announcements, including DermNetNZ (a global dermatology patient information site), with additional help from the International Federation of Dermatology Clinical Trial Network.
The survey was restricted to those who were self-identified as 18 years or older and who self-reported a diagnosis of melanoma following the use of indoor tanning devices. The survey was hosted by SurveyMonkey, which allowed consent to be obtained and responses to remain anonymous. Access to the survey was sponsored by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. The University of Central Florida (Orlando, Florida) institutional review board reviewed and approved this study as exempt human research.
Survey responses collected from January 2014 to June 2015 were analyzed herein. The survey contained 58 questions and was divided into different topics including indoor tanning background (eg, states/countries in which participants tanned indoors, age when they first tanned, frequency of tanning), consenting process (eg, length, did someone review the consent with participants, what was contained in the consent), indoor tanning and melanoma (eg, how long after tanning did melanoma develop, age at development, location of melanoma), indoor tanning postmelanoma (eg, did participants tan after diagnosis and why), and other risk factors (eg, did participants smoke or drink pre- or postmelanoma).
Statistical Analysis
The data consist of both categorical and continuous variables. The categorical variables included age (<35 years or ≥35 years), frequency of indoor tanning (≤1 time weekly or >1 time weekly), and onset of melanoma diagnosis (within or after 5 years
Difference in proportions among groups, age, frequency of tanning, onset of melanoma diagnosis within or after 5 years of starting indoor tanning, and knowledge of cancer risks was tested for significance using the χ² test. Reported P values were 2-tailed, corresponding with a significance level of P<.05. All data were analyzed using SPSS (version 21.0). All statistical analyses were conducted independent of the participants’ sex.
Results
Of the 454 participants who accessed the survey, 448 were analyzed in this study; 6 participants did not complete the questionnaire. Both males and females were analyzed: 289 females, 12 males, and 153 who did not report gender. The age range of participants was 18 to 69 years. The age at start of indoor tanning ranged from 8 to 54 years, with a mean of 22 years. Additional participant characteristics are described in Table 1. The mean frequency of indoor tanning was reported as 2 times weekly. When participants were asked if they were warned of the risk of skin cancer, 21.5% reported yes while 78.4% reported not being told of the risk. This knowledge was compared to their frequency of indoor tanning. Having the knowledge of the risk of skin cancer had no influence on their frequency of indoor tanning (Table 2).


Among responders, those who perceived indoor tanning as safer than outdoor tanning tanned indoors more frequently than those who do not (Spearman r=−0.224; P<.05)(Table 3). The frequency of indoor tanning was divided into those who tanned indoors more than once weekly and those who tanned indoors once a week or less. This study showed that the frequency of indoor tanning had no effect on the latency time between the commencement of indoor tanning and diagnosis of melanoma (Table 4). The time frame from the onset of melanoma diagnosis also was compared to the age at which the participants started to tan indoors. Age was divided into those younger than 35 years and those 35 years and older. There was no correlation between the age when indoor tanning began and the time frame in which the melanoma was diagnosed (eTable).



Table 5 shows the correlations between indoor tanning behaviors and melanoma characteristics. Those who started indoor tanning at an earlier age were diagnosed with melanoma at an earlier age compared to those who started indoor tanning later in life (r=0.549; P<.01). Moreover, those who started indoor tanning at a later age reported being diagnosed with a melanoma of greater Breslow depth (r=0.173; P<.01). Those who reported being diagnosed with a greater Breslow depth also reported a higher Clark level (r=0.608; P<.01). Among responders, those who more frequently tanned indoors also reported greater frequency of outdoor tanning (r=0.197; P<.01). This study showed no correlation between the age at melanoma diagnosis and the frequency of indoor (r=0.004; P>.05 not significant) or outdoor (r=0.093; P>.05 not significant) tanning. Having the knowledge of the risk of skin cancer had no relationship on the frequency of indoor tanning (r=−0.04; P>.05 not significant).

Comment
Thirty million Americans utilize indoor tanning devices at least once a year.13 UVA light comprises the majority of the spectrum used by indoor tanning devices, with a fraction (<5%) being UVB light. Until recently, UVB light was the only solar spectrum considered carcinogenic. In 2009, the International Agency for Research on Cancer classified the whole spectrum as carcinogenic to humans.5,11 Despite this evidence, indoor tanning facilities have promoted indoor tanning as damage free.3 The goal of this study was to collect the patient perspective on the safety of indoor tanning, indoor tanning behaviors, time frame of onset of melanoma, and the severity (ie, Breslow depth) of those melanomas.
Melanoma is the most prevalent cancer in females aged 25 to 29 years.3 The median age of diagnosis of melanoma (with and without the use of indoor tanning devices) is approximately 60 years14 versus our study, which found the average age at diagnosis was 37.6 years. Our findings are consistent with other literature in that those who start indoor tanning earlier (<35 years of age) develop melanoma at an earlier age.14,15 Cust et al14 also promoted the idea that patients develop melanoma earlier because younger individuals are more biologically susceptible to the carcinogenic effects of artificial UV light. However, our study found that those who started indoor tanning at an older age reported being diagnosed with a melanoma of greater Breslow depth, seemingly incongruent with the aforementioned hypothesis. One limitation is the age range for this research sample (18–69 years). The young age range may be attributable to the recruitment through social media, which is geared toward a younger population. Additionally, indoor tanning is a relatively new phenomenon practiced since the 1980s,2 which may contribute to the younger sample size. However, 2.7 billion individuals use social media worldwide with 40% of those older than 65 years on social media.16
Prior research has shown that those who start indoor tanning before the age of 35 years have a 75% increased risk of developing melanoma.14 Another study also has suggested that UVA-rich sunlamps may shorten the latency period for induction of melanoma and nonmelanoma skin cancers.3 Our study used similar age cutoffs in concluding that there was no earlier onset of melanoma diagnosis between those who started indoor tanning before the age of 35 years and those who started at the age of 35 years or older. Limitations include that our study is cross-sectional, and therefore time course cannot be established. Also, survey responses were self-reported, allowing the possibility of recall bias.
A plethora of research has been conducted to determine if there is a connection between the use of indoor tanning devices and developing melanoma. Cust et al14 suggested the risk of melanoma was 41% higher for those who had ever used a sunbed in comparison to those who had not. Other studies describe the difficulty in making the connection between indoor tanning and melanoma, as those who more frequently tan indoors also more frequently tan outdoors,11 as suggested by this study. However, there is a paucity of literature on the patients’ perspectives on the safety of indoor tanning. This study determined that those who more frequently tan indoors believed that indoor tanning is safer than outdoor tanning. With this altered perception promoted by the indoor tanning industry, the FDA has added a warning label to all indoor tanning devices about the risk of skin cancer. Our study revealed that having the knowledge of the risk of skin cancer had no influence on the frequency of indoor tanning. This concerning finding highlights a pressing need for an alternative approach to increase awareness of the harmful consequences that accompany indoor tanning. Further studies may elaborate on potential effective methods and messages to relate to an indoor tanning population comprised mostly of young females.
Acknowledgments
Supported and funded by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. This research project was completed as part of the FIRE Module at the University of Central Florida, College of Medicine. We thank the FIRE Module faculty and staff for their assistance with this project.
The incidence of melanoma is increasing at a rate greater than any other cancer,1 possibly due to the increasing use of indoor tanning devices. These devices emit unnaturally high levels of UVA and low levels of UVA and UVB rays.2 The risks of using these devices include increased incidence of melanoma (3438 cases attributed to indoor tanning in 2008) and keratinocytes cancer (increased risk of squamous cell carcinoma by 67% and basal cell carcinoma by 29%), severe sunburns (61.1% of female users and 44.6% of male users have reported sunburns), and aggravation of underlying disorders such as systemic lupus erythematosus.3-5
The literature varies in its explanation of how indoor tanning increases the risk of developing melanoma. Some authors suggest it is due to increased frequency of use, duration of sessions, and years of using tanning devices.1,6 Others suggest the increased cancer risk is the result of starting to tan at an earlier age.2,3,6-10 There is conflicting literature on the level of increased risk of melanoma in those who tan indoors at a young age (<35 years). Although the estimated rate of increased skin cancer risk varies, with rates up to 75% compared to nonusers, nearly all sources support an increased rate.6 Despite the growing body of knowledge that indoor tanning is dangerous, as well as the academic publication of these risks (eg, carcinogenesis, short-term and long-term eye injury, burns, UV sensitivity when combined with certain medications), teenagers in the United States and affluent countries appear to disregard the risks of tanning.11
Tanning companies have promoted the misconception that only UVB rays cause cell damage and UVA rays, which the devices emit, result in “damage-free” or “safe” tans.2,3 Until 2013, indoor tanning devices were classified by the US Food and Drug Administration (FDA) as class I, indicating that they are safe in terms of electrical shock. Many indoor tanning facilities have promoted the FDA “safe” label without clarifying that the safety indications only referred to electrical-shock potential. Nonetheless, it is known now that these devices, which emit high UVA and low UVB rays, promote melanoma, nonmelanoma skin cancers, and severe sunburns, as well as aggravate existing conditions (eg, systemic lupus erythematosus).4 As a result of an unacceptably high incidence of these disease complications, a 2014 FDA regulation categorized tanning beds as class II, requiring that tanning bed users be informed of the risk of skin cancer in an effort to reverse the growing trend of indoor tanning.12 Despite these regulatory interventions, it is not clear if this knowledge of cancer risk deters patients from indoor tanning.
The purpose of this study was to investigate the patients’ perspective on indoor tanning behaviors as associated with the severity of their melanoma and the time frame in which they were diagnosed as well as their perceived views on the safety of indoor tanning and the frequency in which they continue to tan indoors. This information is highly relevant in helping to determine if requiring a warning of the risk of skin cancer will deter patients from this unhealthy habit, especially given recent reclassification of sunbeds as class II by the FDA. Additional insights from these data may clarify if indoor tanning decreases the time frame in which melanoma is diagnosed or increases the severity of the resulting melanoma. Moreover, it will help elucidate whether or not the age at which indoor tanning is initiated affects the time frame to melanoma onset and corresponding severity.
Methods
An original unvalidated online survey was conducted worldwide via a link distributed to the following supporting institutions: Advanced Dermatology & Cosmetic Surgery, Ameriderm Research, Melanoma Research Foundation (a melanoma patient advocacy group), Florida State University Department of Dermatology, Moffitt Cancer Center Cutaneous Oncology Program, Cleveland Clinic, Ohio State University Division of Medical Oncology, Harvard Medical School Department of Dermatology, The University of Texas MD Anderson Cancer Center Department of Dermatology, University of Colorado Department of Dermatology, and Northwestern University Department of Dermatology. However, there was not confirmation that all of these institutions promoted the survey. Additionally, respondents were recruited through patient advocacy groups and social media sites including Facebook, Twitter, LinkedIn, Tumblr, and Instagram. The patient advocacy groups and social media sites invited participation through recruitment announcements, including DermNetNZ (a global dermatology patient information site), with additional help from the International Federation of Dermatology Clinical Trial Network.
The survey was restricted to those who were self-identified as 18 years or older and who self-reported a diagnosis of melanoma following the use of indoor tanning devices. The survey was hosted by SurveyMonkey, which allowed consent to be obtained and responses to remain anonymous. Access to the survey was sponsored by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. The University of Central Florida (Orlando, Florida) institutional review board reviewed and approved this study as exempt human research.
Survey responses collected from January 2014 to June 2015 were analyzed herein. The survey contained 58 questions and was divided into different topics including indoor tanning background (eg, states/countries in which participants tanned indoors, age when they first tanned, frequency of tanning), consenting process (eg, length, did someone review the consent with participants, what was contained in the consent), indoor tanning and melanoma (eg, how long after tanning did melanoma develop, age at development, location of melanoma), indoor tanning postmelanoma (eg, did participants tan after diagnosis and why), and other risk factors (eg, did participants smoke or drink pre- or postmelanoma).
Statistical Analysis
The data consist of both categorical and continuous variables. The categorical variables included age (<35 years or ≥35 years), frequency of indoor tanning (≤1 time weekly or >1 time weekly), and onset of melanoma diagnosis (within or after 5 years
Difference in proportions among groups, age, frequency of tanning, onset of melanoma diagnosis within or after 5 years of starting indoor tanning, and knowledge of cancer risks was tested for significance using the χ² test. Reported P values were 2-tailed, corresponding with a significance level of P<.05. All data were analyzed using SPSS (version 21.0). All statistical analyses were conducted independent of the participants’ sex.
Results
Of the 454 participants who accessed the survey, 448 were analyzed in this study; 6 participants did not complete the questionnaire. Both males and females were analyzed: 289 females, 12 males, and 153 who did not report gender. The age range of participants was 18 to 69 years. The age at start of indoor tanning ranged from 8 to 54 years, with a mean of 22 years. Additional participant characteristics are described in Table 1. The mean frequency of indoor tanning was reported as 2 times weekly. When participants were asked if they were warned of the risk of skin cancer, 21.5% reported yes while 78.4% reported not being told of the risk. This knowledge was compared to their frequency of indoor tanning. Having the knowledge of the risk of skin cancer had no influence on their frequency of indoor tanning (Table 2).


Among responders, those who perceived indoor tanning as safer than outdoor tanning tanned indoors more frequently than those who do not (Spearman r=−0.224; P<.05)(Table 3). The frequency of indoor tanning was divided into those who tanned indoors more than once weekly and those who tanned indoors once a week or less. This study showed that the frequency of indoor tanning had no effect on the latency time between the commencement of indoor tanning and diagnosis of melanoma (Table 4). The time frame from the onset of melanoma diagnosis also was compared to the age at which the participants started to tan indoors. Age was divided into those younger than 35 years and those 35 years and older. There was no correlation between the age when indoor tanning began and the time frame in which the melanoma was diagnosed (eTable).



Table 5 shows the correlations between indoor tanning behaviors and melanoma characteristics. Those who started indoor tanning at an earlier age were diagnosed with melanoma at an earlier age compared to those who started indoor tanning later in life (r=0.549; P<.01). Moreover, those who started indoor tanning at a later age reported being diagnosed with a melanoma of greater Breslow depth (r=0.173; P<.01). Those who reported being diagnosed with a greater Breslow depth also reported a higher Clark level (r=0.608; P<.01). Among responders, those who more frequently tanned indoors also reported greater frequency of outdoor tanning (r=0.197; P<.01). This study showed no correlation between the age at melanoma diagnosis and the frequency of indoor (r=0.004; P>.05 not significant) or outdoor (r=0.093; P>.05 not significant) tanning. Having the knowledge of the risk of skin cancer had no relationship on the frequency of indoor tanning (r=−0.04; P>.05 not significant).

Comment
Thirty million Americans utilize indoor tanning devices at least once a year.13 UVA light comprises the majority of the spectrum used by indoor tanning devices, with a fraction (<5%) being UVB light. Until recently, UVB light was the only solar spectrum considered carcinogenic. In 2009, the International Agency for Research on Cancer classified the whole spectrum as carcinogenic to humans.5,11 Despite this evidence, indoor tanning facilities have promoted indoor tanning as damage free.3 The goal of this study was to collect the patient perspective on the safety of indoor tanning, indoor tanning behaviors, time frame of onset of melanoma, and the severity (ie, Breslow depth) of those melanomas.
Melanoma is the most prevalent cancer in females aged 25 to 29 years.3 The median age of diagnosis of melanoma (with and without the use of indoor tanning devices) is approximately 60 years14 versus our study, which found the average age at diagnosis was 37.6 years. Our findings are consistent with other literature in that those who start indoor tanning earlier (<35 years of age) develop melanoma at an earlier age.14,15 Cust et al14 also promoted the idea that patients develop melanoma earlier because younger individuals are more biologically susceptible to the carcinogenic effects of artificial UV light. However, our study found that those who started indoor tanning at an older age reported being diagnosed with a melanoma of greater Breslow depth, seemingly incongruent with the aforementioned hypothesis. One limitation is the age range for this research sample (18–69 years). The young age range may be attributable to the recruitment through social media, which is geared toward a younger population. Additionally, indoor tanning is a relatively new phenomenon practiced since the 1980s,2 which may contribute to the younger sample size. However, 2.7 billion individuals use social media worldwide with 40% of those older than 65 years on social media.16
Prior research has shown that those who start indoor tanning before the age of 35 years have a 75% increased risk of developing melanoma.14 Another study also has suggested that UVA-rich sunlamps may shorten the latency period for induction of melanoma and nonmelanoma skin cancers.3 Our study used similar age cutoffs in concluding that there was no earlier onset of melanoma diagnosis between those who started indoor tanning before the age of 35 years and those who started at the age of 35 years or older. Limitations include that our study is cross-sectional, and therefore time course cannot be established. Also, survey responses were self-reported, allowing the possibility of recall bias.
A plethora of research has been conducted to determine if there is a connection between the use of indoor tanning devices and developing melanoma. Cust et al14 suggested the risk of melanoma was 41% higher for those who had ever used a sunbed in comparison to those who had not. Other studies describe the difficulty in making the connection between indoor tanning and melanoma, as those who more frequently tan indoors also more frequently tan outdoors,11 as suggested by this study. However, there is a paucity of literature on the patients’ perspectives on the safety of indoor tanning. This study determined that those who more frequently tan indoors believed that indoor tanning is safer than outdoor tanning. With this altered perception promoted by the indoor tanning industry, the FDA has added a warning label to all indoor tanning devices about the risk of skin cancer. Our study revealed that having the knowledge of the risk of skin cancer had no influence on the frequency of indoor tanning. This concerning finding highlights a pressing need for an alternative approach to increase awareness of the harmful consequences that accompany indoor tanning. Further studies may elaborate on potential effective methods and messages to relate to an indoor tanning population comprised mostly of young females.
Acknowledgments
Supported and funded by the Basal Cell Carcinoma Nevus Syndrome Life Support Network. This research project was completed as part of the FIRE Module at the University of Central Florida, College of Medicine. We thank the FIRE Module faculty and staff for their assistance with this project.
- Fisher DE, James WD. Indoor tanning—science, behavior, and policy. N Engl J Med. 2010;363:901-903.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Coelho SG, Hearing VJ. UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Melanoma Res. 2010;23:57-63.
- Klein RS, Sayre RM, Dowdy JC, et al. The risk of ultraviolet radiation exposure from indoor lamps in lupus erythematosus. Autoimmun Rev. 2009;8:320-324.
- O’Sullivan NA, Tait CP. Tanning bed and nail lamp use and the risk of cutaneous malignancy: a review of the literature. Australas J Dermatol. 2014;55:99-106.
- Schmidt CW. UV radiation and skin cancer: the science behind age restrictions for tanning beds. Environ Health Perspect. 2012;120:a308-a313.
- Lazovich D, Vogel RI, Berwick M, et al. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557-1568.
- Centers for Disease Control and Prevention (CDC). Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- Nielsen K, Masback A, Olsson H, et al. A prospective, population-based study of 40,000 women regarding host factors, UV exposure and sunbed use in relation to risk and anatomic site of cutaneous melanoma. Int J Cancer. 2012;131:706-715.
- Gandini S, Autier P, Boniol M. Reviews on sun exposure and artificial light and melanoma. Prog Biophys Mol Biol. 2011;107:362-366.
- Indoor tanning: the risks of ultraviolet rays. US Food and Drug Administration website. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm186687.htm. Updated September 11, 2017. Accessed November 2, 2017.
- Food and Drug Administration, HHS. General and plastic surgery devices: reclassification of ultraviolet lamps for tanning, henceforth to be known as sunlamp products and ultraviolet lamps intended for use in sunlamp products. Fed Regist. 2014;79:31205-31214.
- Brady MS. Public health and the tanning bed controversy. J Clin Oncol. 2012;30:1571-1573.
- Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128:2425-2435.
- International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer. 2007;120:1116-1122.
- Greenwood S, Perrin A, Duggan M. Social media update 2016. Pew Research Center website. http://www.pewinternet.org/2016/11/11/social-media-update-2016/. Published November 11, 2016. Accessed December 12, 2017.
- Fisher DE, James WD. Indoor tanning—science, behavior, and policy. N Engl J Med. 2010;363:901-903.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanoma attributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:e4757.
- Coelho SG, Hearing VJ. UVA tanning is involved in the increased incidence of skin cancers in fair-skinned young women. Pigment Cell Melanoma Res. 2010;23:57-63.
- Klein RS, Sayre RM, Dowdy JC, et al. The risk of ultraviolet radiation exposure from indoor lamps in lupus erythematosus. Autoimmun Rev. 2009;8:320-324.
- O’Sullivan NA, Tait CP. Tanning bed and nail lamp use and the risk of cutaneous malignancy: a review of the literature. Australas J Dermatol. 2014;55:99-106.
- Schmidt CW. UV radiation and skin cancer: the science behind age restrictions for tanning beds. Environ Health Perspect. 2012;120:a308-a313.
- Lazovich D, Vogel RI, Berwick M, et al. Indoor tanning and risk of melanoma: a case-control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19:1557-1568.
- Centers for Disease Control and Prevention (CDC). Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- Nielsen K, Masback A, Olsson H, et al. A prospective, population-based study of 40,000 women regarding host factors, UV exposure and sunbed use in relation to risk and anatomic site of cutaneous melanoma. Int J Cancer. 2012;131:706-715.
- Gandini S, Autier P, Boniol M. Reviews on sun exposure and artificial light and melanoma. Prog Biophys Mol Biol. 2011;107:362-366.
- Indoor tanning: the risks of ultraviolet rays. US Food and Drug Administration website. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm186687.htm. Updated September 11, 2017. Accessed November 2, 2017.
- Food and Drug Administration, HHS. General and plastic surgery devices: reclassification of ultraviolet lamps for tanning, henceforth to be known as sunlamp products and ultraviolet lamps intended for use in sunlamp products. Fed Regist. 2014;79:31205-31214.
- Brady MS. Public health and the tanning bed controversy. J Clin Oncol. 2012;30:1571-1573.
- Cust AE, Armstrong BK, Goumas C, et al. Sunbed use during adolescence and early adulthood is associated with increased risk of early-onset melanoma. Int J Cancer. 2011;128:2425-2435.
- International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer. 2007;120:1116-1122.
- Greenwood S, Perrin A, Duggan M. Social media update 2016. Pew Research Center website. http://www.pewinternet.org/2016/11/11/social-media-update-2016/. Published November 11, 2016. Accessed December 12, 2017.
Practice Points
- Despite US Food and Drug Administration reclassification and publicity of the risks of skin cancer, many patients continue to use sunbeds.
- It is important to assess how patients are obtaining information regarding sunbed safety, as indoor tanning companies are promoting sunbeds as “safe” tans.
- The increased combination of sunbed use and outdoor tanning is putting people at greater risk for the development of melanoma and nonmelanoma skin cancer.
FDA expands approval of nivolumab for melanoma treatment
The Food and Drug Administration has approved nivolumab for the adjuvant treatment of patients with melanoma with involvement of lymph nodes or in patients with metastatic disease who have undergone complete resection.
Nivolumab was previously approved for the treatment of patients with unresectable or metastatic melanoma, the FDA said in a press statement.
Approval was based on results from the CHECKMATE-238 trial, where 906 patients with completely resected stage IIIB/C or stage IV melanoma received either nivolumab or ipilimumab for up to 1 year. Recurrence-free survival was superior in patients who received nivolumab, with 34% of patients in the nivolumab group experiencing recurrence/death, compared to 45.5% in the ipilimumab group.
The recommended dose and schedule of nivolumab in adjuvant melanoma is 240 mg administered as an IV infusion over 60 minutes every 2 weeks until disease recurrence or unacceptable toxicity, for a maximum of 1 year, according to the FDA.
Nivolumab is marketed as Opdivo by Bristol-Myers Squibb Company.
Find the full press release on the FDA website.
The Food and Drug Administration has approved nivolumab for the adjuvant treatment of patients with melanoma with involvement of lymph nodes or in patients with metastatic disease who have undergone complete resection.
Nivolumab was previously approved for the treatment of patients with unresectable or metastatic melanoma, the FDA said in a press statement.
Approval was based on results from the CHECKMATE-238 trial, where 906 patients with completely resected stage IIIB/C or stage IV melanoma received either nivolumab or ipilimumab for up to 1 year. Recurrence-free survival was superior in patients who received nivolumab, with 34% of patients in the nivolumab group experiencing recurrence/death, compared to 45.5% in the ipilimumab group.
The recommended dose and schedule of nivolumab in adjuvant melanoma is 240 mg administered as an IV infusion over 60 minutes every 2 weeks until disease recurrence or unacceptable toxicity, for a maximum of 1 year, according to the FDA.
Nivolumab is marketed as Opdivo by Bristol-Myers Squibb Company.
Find the full press release on the FDA website.
The Food and Drug Administration has approved nivolumab for the adjuvant treatment of patients with melanoma with involvement of lymph nodes or in patients with metastatic disease who have undergone complete resection.
Nivolumab was previously approved for the treatment of patients with unresectable or metastatic melanoma, the FDA said in a press statement.
Approval was based on results from the CHECKMATE-238 trial, where 906 patients with completely resected stage IIIB/C or stage IV melanoma received either nivolumab or ipilimumab for up to 1 year. Recurrence-free survival was superior in patients who received nivolumab, with 34% of patients in the nivolumab group experiencing recurrence/death, compared to 45.5% in the ipilimumab group.
The recommended dose and schedule of nivolumab in adjuvant melanoma is 240 mg administered as an IV infusion over 60 minutes every 2 weeks until disease recurrence or unacceptable toxicity, for a maximum of 1 year, according to the FDA.
Nivolumab is marketed as Opdivo by Bristol-Myers Squibb Company.
Find the full press release on the FDA website.
Metastatic eccrine carcinoma with stomach and pericardial involvement
Skin adnexal tumors (SAT) are rare tumors that make up about 1%-2% of all cutaneous malignancies. They represent a various group of benign and malignant tumors that arise from skin adnexal epithelial structures: hair follicle, pilosebaceous unit, and apocrine or eccrine sweat glands. Although this derivation provides a practical basis for classification, some tumors may exhibit a mixed or more than one line of differentiation, rendering precise classification of those neoplasms difficult, and such cases should be categorized according to prevailing phenotype. In this report, we present a patient with metastatic eccrine carcinoma. Clinical experience for metastatic disease treatment is derived from a few reports, and there are no universal treatment guidelines. Given the few reported cases and the absence of randomized clinical trials for these patients, it is important to collect clinical experiences.
Case presentation and summary
A 56-year-old African man presented with a 5-week history of multiple nontender subcutaneous skin nodules all over his body except for his palms and soles, and associated with generalized itching. He had a mass in the sole of his right foot 35 years previously in another country. The mass had recurred 15 years later and was excised again. The exact etiology of the mass was unknown to the patient. He had no other medical problems. He was on no medications and did not smoke, drink, or use recreational drugs.
His vital signs on admission were normal. Examination was significant for innumerable superficial skin nodules in the scalp, back, torso, and abdomen. The largest was in the neck and measured 4 x 2 cm. A firm right inguinal mass of 7 x 4 cm was palpable. An abdominal exam revealed large ascites but no organomegaly.
The results of laboratory tests were significant for hyponatremia 126 mEq/L (normal, 135-145), hypercalcemia of 12.2 mg/dL (8.5-10.5), with normal phosphorous of 2.5 mg/dL (2.5-4.5), parathyroid of 11.5 pg/ml (6-65), and low vitamin D level of <7 ng/ml (30-100). Other test results were: carcinoembryonic antigen (CEA), 4.36 ng/ml (0.00-2.99); alpha fetoprotein, 2.39 IU/ml (0.00-9.0); calcium 11.6 mg/dL (8.5-10.2); lactate dehydrogenase, 325 U/L (85-210); aspartate aminotransferase, 59 U/L (0-40); alanine aminotransferase 43 U/L (5-35); alkaline phosphatase, 65 u/L (50-120); albumin, 2.7 g/dL (3.8-5.2); white blood cell count, 14.1 k/uL (4.4-10.6); h
A chest and abdomen computed-tomography scan on presentation showed presence of innumerable subcutaneous and intramuscular nodules throughout the chest, abdomen, and pelvis (Figure 1).
Extensive peritoneal carcinomatosis in addition to moderate ascites and perivascular lymphadenopathy were evident in the abdomen cuts. Remarkably, multiple lytic, osseous metastases were seen with subacute pathologic fracture of right fourth rib in addition to mediastinal lymphadenopathy with small pericardial effusion in the chest cuts. The right thigh mass was described as a large lobulated solid and cystic mass. Ascitic fluid analysis was negative for malignant cells. Biopsy of one the skin nodules in the upper back showed carcinoma involving the skin with focal tubular differentiation (Figure 2).
Immunohistochemical stains were positive for p63, epithelial membrane antigen, high molecular weight keratin, and p40. The lesional cells were negative for CEA, bcl-2, Ber-Ep4, CK7, and CK20. The profile was compatible with a skin adnexal carcinoma of sweat gland origin. The groin lymph node showed eccrine acrospiroma.
The patient underwent an upper endoscopy to assess for recurrent vomiting and it revealed diffuse areas of large erythematous ulcerated nodules noted in the cardia, fundus, and body of the stomach (Figure 3). A biopsy of the gastric nodules revealed gastric mucosa with metastatic carcinoma.
After a thorough review of the literature, he was started on palliative chemotherapy 13 days after initial presentation with docetaxel 75 mg/m2, carboplatin AUC 5 (470 mg), and 5-FU (5-fluorouracil, 750 mg/m2) over 24 hours on days 1 through 5. However, on day 2 of the chemotherapy, he became hypotensive and was found to have cardiac tamponade. He underwent an emergent pericardial window procedure. Analysis of the pericardial fluid was consistent with metastatic carcinoma (Figure 4). Chemotherapy was discontinued while he remained hypotensive requiring multiple vasopressors. His clinical condition did not improve and he passed away 27 days from initial presentation.
Discussion
Sweat gland carcinomas are very rare malignant tumors of the adnexal epithelial structures of the skin, sebaceous, hair follicle, apocrine or eccrine glands that were first described by Cornil in 1865.1 They occur primarily in adult patients, with a peak incidence in fifth and sixth decades of life.2,3 The etiology is unknown, but some cases have been reported to be a consequence of radiation therapy.4 They are almost always an incidental histologic diagnosis.2,5 The tumors usually appear as single nodule, and multinodularity usually associated with both local and metastatic disease.6 There are no characteristic findings to suggest that a particular nodule may represent sweat gland carcinoma, and even if sweat gland tumor is suspected, benign counterparts are more common.
Eccrine carcinoma is the most aggressive among skin adnexal tumors. They can arise on the lower limbs, trunk, head and neck, scalp and ears, upper extremities, abdomen, and genital sites.7
The cells of eccrine sweat glands express low molecular weight keratin, epithelial membrane antigen, carcinoembryonic antigen, as well as S100 protein, smooth muscle actin, p63, calponin, cytokeratin 14, and bcl-2.8 Skin tumors with eccrine differentiation may stain for estrogen and progesterone, which has important clinical implications because those patients can be treated with hormonal therapy.9 Positivity for estrogen receptors does not differentiate cutaneous eccrine tumors from cutaneous metastases of breast cancers.8,9 Androgen receptor evaluation in these cases can help distinguish between the two.10 Human epidermal growth factor receptor 2 (HER-2) is expressed in 3.5% of skin adnexal tumors.11
The molecular pathogenesis of malignant adnexal tumors is not clear, but overexpression of tumor suppressor protein p16 has been described as a common feature in eccrine carcinomas.12
Prognostic factors for sweat gland carcinoma are difficult to identify, because of the small number of reported cases. The likely prognostic factors include size, histological type, lymph node involvement, and presence of distant metastasis. Absent of lymph node involvement correlates with 10-year disease-free survival rate of 56%, which falls to 9% if nodes are involved.13
There are no uniform guidelines for the treatment sweat gland carcinomas, and the clinical experience described in the literature is the only source of available information.
The treatment of choice of all subtypes of localized sweat gland carcinomas is wide surgical excision with broad tumor margins, given the propensity for local recurrences along with regional lymph node dissection in the presence of clinically positive nodes. Prophylactic lymph node resection does not seem to improve survival or decrease recurrence rates.7 The use of adjuvant radiotherapy to prevent local recurrence also is not well established. One report suggested radiosensitivity of these tumors, and adjuvant radiation was therefore recommended in high-risk cases (ie, large tumors of 5 cm and positive surgical margins of 1 cm) and moderate to poorly differentiated tumors with lymphovascular invasion.14 Adjuvant radiation to the involved lymph node basin is suggested in the setting of extranodal extension or extensive involvement, that is, 4 lymph nodes.15 The role of lymphadenectomy has not been adequately addressed in the literature.
The role of chemotherapy in metastatic disease is not clear, but sweat gland carcinomas are considered chemoresistant (Table). Several combinations have been used with short-term responses. In one case treated with doxorubicin, mitomycin, vincristine, and 5-FU followed by maintenance therapy, the patient achieved a complete response that lasted for 16 months.16 In another report, the treatment response was 2 years with treatment consisted of anthracyclin, cyclophosphamide, vincristine, and bloemycin.17 Other combinations used in the literature include carboplatin and paclitaxel, which led to prolonged remission.14 Cisplatin and 5-FU, or cisplatin plus cetuximab have been reported but with discouraging results.18 Results to taxanes showed conflicting results.19,20
Hormonal therapy can be effective in cases in which estrogen and progesterone receptors are expressed, which can range from 19%-30% of eccrine sweat gland carcinomas.21,22 Two cases have reported complete regression of lymph nodes in patients with metastatic disease, and in 1 patient relief from pain caused by bone metastases with durable response of around 3 years.23,24 a
Experience with targeted therapy is very limited. Sunitinib has been reported to have some activity in metastatic adnexal tumors as a second-line therapy in 2 patients, with disease control for 8 and 10 months respectively.25 Trastuzumab has been reported as having activity in 1 patient with strong HER2 expression (IHC score of 3+, denoting HER2 positivity), with complete regression of metastatic tumor. Upon progression in the same patient, a combination of lapatinib and capecitabine also showed positive response.26
In conclusion, metastatic sweat gland tumors treatment has not been standardized because of a dearth of reports in the literatures. Its early identification and complete excision gives the best chance of a cure. Neither chemotherapy nor radiation therapy has been proven to be of clinical benefit in treating metastatic disease.
1. Gates O, Warren S, Warvi WN. Tumors of sweat glands. Am J Pathol. 1943;19(4):591-631.
2. Mitts DL, Smith MT, Russell L, Bannayan GA, Cruz AB. Sweat gland carcinoma: a clinico-pathological reappraisal. J Surg Oncol. 1976;8(1):23-29.
3. Panoussopoulos D, Darom A, Lazaris AC, Misthos P, Papadimitriou K, Androulakis G. Sweat gland carcinoma with multiple local recurrences: a case report. Adv Clin Path. 1999;3(3):63-68.
4. Marone U, Caracò C, Anniciello AM, et al. Metastatic eccrine porocarcinoma : report of a case and review of the literature. World J Surg Oncol. 2011;9:32.
5. Yildirim S, Aköz T, Akan M, Ege GA. De novo malignant eccrine spiradenoma with an interesting and unusual location. Dermatol Surg. 2001;27(4):417-420.
6. Shaw M, McKee PH, Lowe D, Black MM. Malignant eccrine poroma: a study of twenty-seven cases. Br J Dermatol. 1982;107(6):675-680.
7. De Iuliis F, Amoroso L, Taglieri L, et al. Chemotherapy of rare skin adnexal tumors: a review of literature. Anticancer Res. 2014;34(10):5263-5268.
8. Alsaad KO, Obaidat NA, Ghazarian D. Skin adnexal neoplasms – part 1: an approach to tumours of the pilosebaceous unit. J Clin Pathol. 2007;60(2):129-144.
9. Serhrouchni KI, Harmouch T, Chbani L, et al. Eccrine carcinoma : a rare cutaneous neoplasm. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3570399/. Published online February 4, 2013. Accessed October 11, 2017.
10. Shidham VB, Komorowski RA, Machhi JK. Androgen receptor expression in metastatic adenocarcinoma in females favors a breast primary. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1601970/. Published online October 4, 2006. Accessed October 11, 2017.
11. Hiatt KM, Pillow JL, Smoller BR. Her-2 expression in cutaneous eccrine and apocrine neoplasms. Mod Pathol. 2004;17(1):28-32.
12. Gu L-H, Ichiki Y, Kitajima Y. Aberrant expression of p16 and RB protein in eccrine porocarcinoma. J Cutan Pathol. 2002;29(8):473-479.
13. el-Domeiri AA, Brasfield RD, Huvos AG, Strong EW. Sweat gland carcinoma: a clinico-pathologic study of 83 patients. Ann Surg. 1971;173(2):270-274.
14. Tlemcani K, Levine D, Smith R V, et al. Metastatic apocrine carcinoma of the scalp: prolonged response to systemic chemotherapy. J Clin Oncol. 2010;28(24):e412-e414.
15. Chamberlain RS, Huber K, White JC, Travaglino-Parda R. Apocrine gland carcinoma of the axilla: review of the literature and recommendations for treatment. Am J Clin Oncol. 1999;22(2):131-135.
16. Gutermuth J, Audring H, Voit C, Trefzer U, Haas N. Antitumour activity of paclitaxel and interferon-alpha in a case of metastatic eccrine porocarcinoma. J Eur Acad Dermatol Venereol. 2004;18(4):477-479.
17. Mezger J, Remberger K, Schalhorn A, Wohlrab A, Wilmanns W. Treatment of metastatic sweat gland carcinoma by a four drug combination chemotherapy: response in two cases. Med Oncol Tumor Pharmacother. 1986;3(1):29-34.
18. Aaribi I, Mohtaram A, Ben Ameur El Youbi M, et al. Successful management of metastatic eccrine porocarcinoma. https://www.hindawi.com/journals/crionm/2013/282536/. Published 2013. Accessed October 10, 2017.
19. Shiohara J, Koga H, Uhara H, Takata M, Saida T. Eccrine porocarcinoma: clinical and pathological studies of 12 cases. J Dermatol. 2007;34(8):516-522.
20. Swanson PE, Mazoujian G, Mills SE, Campbell RJ, Wick MR. Immunoreactivity for estrogen receptor protein in sweat gland tumors. Am J Surg Pathol. 1991;15(9):835-841.
21. Busam KJ, Tan LK, Granter SR, et al. Epidermal growth factor, estrogen, and progesterone receptor expression in primary sweat gland carcinomas and primary and metastatic mammary carcinomas. Mod Pathol. 1999;12(8):786-793.
22. Sridhar KS, Benedetto P, Otrakji CL, Charyulu KK. Response of eccrine adenocarcinoma to tamoxifen. Cancer. 1989;64(2):366-370.
23. Daniel SJ, Nader R, Kost K, Hüttner I. Facial sweat gland carcinoma metastasizing to neck nodes: a diagnostic and therapeutic challenge. Arch Otolaryngol Head Neck Surg. 2001;127(12):1495-1498.
24. Battistella M, Mateus C, Lassau N, et al. Sunitinib efficacy in the treatment of metastatic skin adnexal carcinomas: report of two patients with hidradenocarcinoma and trichoblastic carcinoma. J Eur Acad Dermatol Venereol. 2010;24(2):199-203.
25. Hidaka T, Fujimura T, Watabe A, et al. Successful treatment of HER-2-positive metastatic apocrine carcinoma of the skin with lapatinib and capecitabine. Acta Derm Venereol. 2012;92(6):654-655.
26. Mandaliya H, Nordman I. Metastatic eccrine porocarcinoma: a rare case of successful treatment. Case Rep Oncol. 2016;9(2):454-456.
27. de Bree E, Volalakis E, Tsetis D, et al. Treatment of advanced malignant eccrine poroma with locoregional chemotherapy. Br J Dermatol. 2005;152(5):1051-1055.
28. Bahl A, Sharma DN, Julka PK, Das A, Rath GK. Sweat gland carcinoma with lung metastases. J Cancer Res Ther. 2(4):209-211.
29. Wang X-X, Wang H-Y, Zheng J-N, Sui J-C. Primary cutaneous sweat gland carcinoma. J Cancer Res Ther. 10(2):390-392.
Skin adnexal tumors (SAT) are rare tumors that make up about 1%-2% of all cutaneous malignancies. They represent a various group of benign and malignant tumors that arise from skin adnexal epithelial structures: hair follicle, pilosebaceous unit, and apocrine or eccrine sweat glands. Although this derivation provides a practical basis for classification, some tumors may exhibit a mixed or more than one line of differentiation, rendering precise classification of those neoplasms difficult, and such cases should be categorized according to prevailing phenotype. In this report, we present a patient with metastatic eccrine carcinoma. Clinical experience for metastatic disease treatment is derived from a few reports, and there are no universal treatment guidelines. Given the few reported cases and the absence of randomized clinical trials for these patients, it is important to collect clinical experiences.
Case presentation and summary
A 56-year-old African man presented with a 5-week history of multiple nontender subcutaneous skin nodules all over his body except for his palms and soles, and associated with generalized itching. He had a mass in the sole of his right foot 35 years previously in another country. The mass had recurred 15 years later and was excised again. The exact etiology of the mass was unknown to the patient. He had no other medical problems. He was on no medications and did not smoke, drink, or use recreational drugs.
His vital signs on admission were normal. Examination was significant for innumerable superficial skin nodules in the scalp, back, torso, and abdomen. The largest was in the neck and measured 4 x 2 cm. A firm right inguinal mass of 7 x 4 cm was palpable. An abdominal exam revealed large ascites but no organomegaly.
The results of laboratory tests were significant for hyponatremia 126 mEq/L (normal, 135-145), hypercalcemia of 12.2 mg/dL (8.5-10.5), with normal phosphorous of 2.5 mg/dL (2.5-4.5), parathyroid of 11.5 pg/ml (6-65), and low vitamin D level of <7 ng/ml (30-100). Other test results were: carcinoembryonic antigen (CEA), 4.36 ng/ml (0.00-2.99); alpha fetoprotein, 2.39 IU/ml (0.00-9.0); calcium 11.6 mg/dL (8.5-10.2); lactate dehydrogenase, 325 U/L (85-210); aspartate aminotransferase, 59 U/L (0-40); alanine aminotransferase 43 U/L (5-35); alkaline phosphatase, 65 u/L (50-120); albumin, 2.7 g/dL (3.8-5.2); white blood cell count, 14.1 k/uL (4.4-10.6); h
A chest and abdomen computed-tomography scan on presentation showed presence of innumerable subcutaneous and intramuscular nodules throughout the chest, abdomen, and pelvis (Figure 1).
Extensive peritoneal carcinomatosis in addition to moderate ascites and perivascular lymphadenopathy were evident in the abdomen cuts. Remarkably, multiple lytic, osseous metastases were seen with subacute pathologic fracture of right fourth rib in addition to mediastinal lymphadenopathy with small pericardial effusion in the chest cuts. The right thigh mass was described as a large lobulated solid and cystic mass. Ascitic fluid analysis was negative for malignant cells. Biopsy of one the skin nodules in the upper back showed carcinoma involving the skin with focal tubular differentiation (Figure 2).
Immunohistochemical stains were positive for p63, epithelial membrane antigen, high molecular weight keratin, and p40. The lesional cells were negative for CEA, bcl-2, Ber-Ep4, CK7, and CK20. The profile was compatible with a skin adnexal carcinoma of sweat gland origin. The groin lymph node showed eccrine acrospiroma.
The patient underwent an upper endoscopy to assess for recurrent vomiting and it revealed diffuse areas of large erythematous ulcerated nodules noted in the cardia, fundus, and body of the stomach (Figure 3). A biopsy of the gastric nodules revealed gastric mucosa with metastatic carcinoma.
After a thorough review of the literature, he was started on palliative chemotherapy 13 days after initial presentation with docetaxel 75 mg/m2, carboplatin AUC 5 (470 mg), and 5-FU (5-fluorouracil, 750 mg/m2) over 24 hours on days 1 through 5. However, on day 2 of the chemotherapy, he became hypotensive and was found to have cardiac tamponade. He underwent an emergent pericardial window procedure. Analysis of the pericardial fluid was consistent with metastatic carcinoma (Figure 4). Chemotherapy was discontinued while he remained hypotensive requiring multiple vasopressors. His clinical condition did not improve and he passed away 27 days from initial presentation.
Discussion
Sweat gland carcinomas are very rare malignant tumors of the adnexal epithelial structures of the skin, sebaceous, hair follicle, apocrine or eccrine glands that were first described by Cornil in 1865.1 They occur primarily in adult patients, with a peak incidence in fifth and sixth decades of life.2,3 The etiology is unknown, but some cases have been reported to be a consequence of radiation therapy.4 They are almost always an incidental histologic diagnosis.2,5 The tumors usually appear as single nodule, and multinodularity usually associated with both local and metastatic disease.6 There are no characteristic findings to suggest that a particular nodule may represent sweat gland carcinoma, and even if sweat gland tumor is suspected, benign counterparts are more common.
Eccrine carcinoma is the most aggressive among skin adnexal tumors. They can arise on the lower limbs, trunk, head and neck, scalp and ears, upper extremities, abdomen, and genital sites.7
The cells of eccrine sweat glands express low molecular weight keratin, epithelial membrane antigen, carcinoembryonic antigen, as well as S100 protein, smooth muscle actin, p63, calponin, cytokeratin 14, and bcl-2.8 Skin tumors with eccrine differentiation may stain for estrogen and progesterone, which has important clinical implications because those patients can be treated with hormonal therapy.9 Positivity for estrogen receptors does not differentiate cutaneous eccrine tumors from cutaneous metastases of breast cancers.8,9 Androgen receptor evaluation in these cases can help distinguish between the two.10 Human epidermal growth factor receptor 2 (HER-2) is expressed in 3.5% of skin adnexal tumors.11
The molecular pathogenesis of malignant adnexal tumors is not clear, but overexpression of tumor suppressor protein p16 has been described as a common feature in eccrine carcinomas.12
Prognostic factors for sweat gland carcinoma are difficult to identify, because of the small number of reported cases. The likely prognostic factors include size, histological type, lymph node involvement, and presence of distant metastasis. Absent of lymph node involvement correlates with 10-year disease-free survival rate of 56%, which falls to 9% if nodes are involved.13
There are no uniform guidelines for the treatment sweat gland carcinomas, and the clinical experience described in the literature is the only source of available information.
The treatment of choice of all subtypes of localized sweat gland carcinomas is wide surgical excision with broad tumor margins, given the propensity for local recurrences along with regional lymph node dissection in the presence of clinically positive nodes. Prophylactic lymph node resection does not seem to improve survival or decrease recurrence rates.7 The use of adjuvant radiotherapy to prevent local recurrence also is not well established. One report suggested radiosensitivity of these tumors, and adjuvant radiation was therefore recommended in high-risk cases (ie, large tumors of 5 cm and positive surgical margins of 1 cm) and moderate to poorly differentiated tumors with lymphovascular invasion.14 Adjuvant radiation to the involved lymph node basin is suggested in the setting of extranodal extension or extensive involvement, that is, 4 lymph nodes.15 The role of lymphadenectomy has not been adequately addressed in the literature.
The role of chemotherapy in metastatic disease is not clear, but sweat gland carcinomas are considered chemoresistant (Table). Several combinations have been used with short-term responses. In one case treated with doxorubicin, mitomycin, vincristine, and 5-FU followed by maintenance therapy, the patient achieved a complete response that lasted for 16 months.16 In another report, the treatment response was 2 years with treatment consisted of anthracyclin, cyclophosphamide, vincristine, and bloemycin.17 Other combinations used in the literature include carboplatin and paclitaxel, which led to prolonged remission.14 Cisplatin and 5-FU, or cisplatin plus cetuximab have been reported but with discouraging results.18 Results to taxanes showed conflicting results.19,20
Hormonal therapy can be effective in cases in which estrogen and progesterone receptors are expressed, which can range from 19%-30% of eccrine sweat gland carcinomas.21,22 Two cases have reported complete regression of lymph nodes in patients with metastatic disease, and in 1 patient relief from pain caused by bone metastases with durable response of around 3 years.23,24 a
Experience with targeted therapy is very limited. Sunitinib has been reported to have some activity in metastatic adnexal tumors as a second-line therapy in 2 patients, with disease control for 8 and 10 months respectively.25 Trastuzumab has been reported as having activity in 1 patient with strong HER2 expression (IHC score of 3+, denoting HER2 positivity), with complete regression of metastatic tumor. Upon progression in the same patient, a combination of lapatinib and capecitabine also showed positive response.26
In conclusion, metastatic sweat gland tumors treatment has not been standardized because of a dearth of reports in the literatures. Its early identification and complete excision gives the best chance of a cure. Neither chemotherapy nor radiation therapy has been proven to be of clinical benefit in treating metastatic disease.
Skin adnexal tumors (SAT) are rare tumors that make up about 1%-2% of all cutaneous malignancies. They represent a various group of benign and malignant tumors that arise from skin adnexal epithelial structures: hair follicle, pilosebaceous unit, and apocrine or eccrine sweat glands. Although this derivation provides a practical basis for classification, some tumors may exhibit a mixed or more than one line of differentiation, rendering precise classification of those neoplasms difficult, and such cases should be categorized according to prevailing phenotype. In this report, we present a patient with metastatic eccrine carcinoma. Clinical experience for metastatic disease treatment is derived from a few reports, and there are no universal treatment guidelines. Given the few reported cases and the absence of randomized clinical trials for these patients, it is important to collect clinical experiences.
Case presentation and summary
A 56-year-old African man presented with a 5-week history of multiple nontender subcutaneous skin nodules all over his body except for his palms and soles, and associated with generalized itching. He had a mass in the sole of his right foot 35 years previously in another country. The mass had recurred 15 years later and was excised again. The exact etiology of the mass was unknown to the patient. He had no other medical problems. He was on no medications and did not smoke, drink, or use recreational drugs.
His vital signs on admission were normal. Examination was significant for innumerable superficial skin nodules in the scalp, back, torso, and abdomen. The largest was in the neck and measured 4 x 2 cm. A firm right inguinal mass of 7 x 4 cm was palpable. An abdominal exam revealed large ascites but no organomegaly.
The results of laboratory tests were significant for hyponatremia 126 mEq/L (normal, 135-145), hypercalcemia of 12.2 mg/dL (8.5-10.5), with normal phosphorous of 2.5 mg/dL (2.5-4.5), parathyroid of 11.5 pg/ml (6-65), and low vitamin D level of <7 ng/ml (30-100). Other test results were: carcinoembryonic antigen (CEA), 4.36 ng/ml (0.00-2.99); alpha fetoprotein, 2.39 IU/ml (0.00-9.0); calcium 11.6 mg/dL (8.5-10.2); lactate dehydrogenase, 325 U/L (85-210); aspartate aminotransferase, 59 U/L (0-40); alanine aminotransferase 43 U/L (5-35); alkaline phosphatase, 65 u/L (50-120); albumin, 2.7 g/dL (3.8-5.2); white blood cell count, 14.1 k/uL (4.4-10.6); h
A chest and abdomen computed-tomography scan on presentation showed presence of innumerable subcutaneous and intramuscular nodules throughout the chest, abdomen, and pelvis (Figure 1).
Extensive peritoneal carcinomatosis in addition to moderate ascites and perivascular lymphadenopathy were evident in the abdomen cuts. Remarkably, multiple lytic, osseous metastases were seen with subacute pathologic fracture of right fourth rib in addition to mediastinal lymphadenopathy with small pericardial effusion in the chest cuts. The right thigh mass was described as a large lobulated solid and cystic mass. Ascitic fluid analysis was negative for malignant cells. Biopsy of one the skin nodules in the upper back showed carcinoma involving the skin with focal tubular differentiation (Figure 2).
Immunohistochemical stains were positive for p63, epithelial membrane antigen, high molecular weight keratin, and p40. The lesional cells were negative for CEA, bcl-2, Ber-Ep4, CK7, and CK20. The profile was compatible with a skin adnexal carcinoma of sweat gland origin. The groin lymph node showed eccrine acrospiroma.
The patient underwent an upper endoscopy to assess for recurrent vomiting and it revealed diffuse areas of large erythematous ulcerated nodules noted in the cardia, fundus, and body of the stomach (Figure 3). A biopsy of the gastric nodules revealed gastric mucosa with metastatic carcinoma.
After a thorough review of the literature, he was started on palliative chemotherapy 13 days after initial presentation with docetaxel 75 mg/m2, carboplatin AUC 5 (470 mg), and 5-FU (5-fluorouracil, 750 mg/m2) over 24 hours on days 1 through 5. However, on day 2 of the chemotherapy, he became hypotensive and was found to have cardiac tamponade. He underwent an emergent pericardial window procedure. Analysis of the pericardial fluid was consistent with metastatic carcinoma (Figure 4). Chemotherapy was discontinued while he remained hypotensive requiring multiple vasopressors. His clinical condition did not improve and he passed away 27 days from initial presentation.
Discussion
Sweat gland carcinomas are very rare malignant tumors of the adnexal epithelial structures of the skin, sebaceous, hair follicle, apocrine or eccrine glands that were first described by Cornil in 1865.1 They occur primarily in adult patients, with a peak incidence in fifth and sixth decades of life.2,3 The etiology is unknown, but some cases have been reported to be a consequence of radiation therapy.4 They are almost always an incidental histologic diagnosis.2,5 The tumors usually appear as single nodule, and multinodularity usually associated with both local and metastatic disease.6 There are no characteristic findings to suggest that a particular nodule may represent sweat gland carcinoma, and even if sweat gland tumor is suspected, benign counterparts are more common.
Eccrine carcinoma is the most aggressive among skin adnexal tumors. They can arise on the lower limbs, trunk, head and neck, scalp and ears, upper extremities, abdomen, and genital sites.7
The cells of eccrine sweat glands express low molecular weight keratin, epithelial membrane antigen, carcinoembryonic antigen, as well as S100 protein, smooth muscle actin, p63, calponin, cytokeratin 14, and bcl-2.8 Skin tumors with eccrine differentiation may stain for estrogen and progesterone, which has important clinical implications because those patients can be treated with hormonal therapy.9 Positivity for estrogen receptors does not differentiate cutaneous eccrine tumors from cutaneous metastases of breast cancers.8,9 Androgen receptor evaluation in these cases can help distinguish between the two.10 Human epidermal growth factor receptor 2 (HER-2) is expressed in 3.5% of skin adnexal tumors.11
The molecular pathogenesis of malignant adnexal tumors is not clear, but overexpression of tumor suppressor protein p16 has been described as a common feature in eccrine carcinomas.12
Prognostic factors for sweat gland carcinoma are difficult to identify, because of the small number of reported cases. The likely prognostic factors include size, histological type, lymph node involvement, and presence of distant metastasis. Absent of lymph node involvement correlates with 10-year disease-free survival rate of 56%, which falls to 9% if nodes are involved.13
There are no uniform guidelines for the treatment sweat gland carcinomas, and the clinical experience described in the literature is the only source of available information.
The treatment of choice of all subtypes of localized sweat gland carcinomas is wide surgical excision with broad tumor margins, given the propensity for local recurrences along with regional lymph node dissection in the presence of clinically positive nodes. Prophylactic lymph node resection does not seem to improve survival or decrease recurrence rates.7 The use of adjuvant radiotherapy to prevent local recurrence also is not well established. One report suggested radiosensitivity of these tumors, and adjuvant radiation was therefore recommended in high-risk cases (ie, large tumors of 5 cm and positive surgical margins of 1 cm) and moderate to poorly differentiated tumors with lymphovascular invasion.14 Adjuvant radiation to the involved lymph node basin is suggested in the setting of extranodal extension or extensive involvement, that is, 4 lymph nodes.15 The role of lymphadenectomy has not been adequately addressed in the literature.
The role of chemotherapy in metastatic disease is not clear, but sweat gland carcinomas are considered chemoresistant (Table). Several combinations have been used with short-term responses. In one case treated with doxorubicin, mitomycin, vincristine, and 5-FU followed by maintenance therapy, the patient achieved a complete response that lasted for 16 months.16 In another report, the treatment response was 2 years with treatment consisted of anthracyclin, cyclophosphamide, vincristine, and bloemycin.17 Other combinations used in the literature include carboplatin and paclitaxel, which led to prolonged remission.14 Cisplatin and 5-FU, or cisplatin plus cetuximab have been reported but with discouraging results.18 Results to taxanes showed conflicting results.19,20
Hormonal therapy can be effective in cases in which estrogen and progesterone receptors are expressed, which can range from 19%-30% of eccrine sweat gland carcinomas.21,22 Two cases have reported complete regression of lymph nodes in patients with metastatic disease, and in 1 patient relief from pain caused by bone metastases with durable response of around 3 years.23,24 a
Experience with targeted therapy is very limited. Sunitinib has been reported to have some activity in metastatic adnexal tumors as a second-line therapy in 2 patients, with disease control for 8 and 10 months respectively.25 Trastuzumab has been reported as having activity in 1 patient with strong HER2 expression (IHC score of 3+, denoting HER2 positivity), with complete regression of metastatic tumor. Upon progression in the same patient, a combination of lapatinib and capecitabine also showed positive response.26
In conclusion, metastatic sweat gland tumors treatment has not been standardized because of a dearth of reports in the literatures. Its early identification and complete excision gives the best chance of a cure. Neither chemotherapy nor radiation therapy has been proven to be of clinical benefit in treating metastatic disease.
1. Gates O, Warren S, Warvi WN. Tumors of sweat glands. Am J Pathol. 1943;19(4):591-631.
2. Mitts DL, Smith MT, Russell L, Bannayan GA, Cruz AB. Sweat gland carcinoma: a clinico-pathological reappraisal. J Surg Oncol. 1976;8(1):23-29.
3. Panoussopoulos D, Darom A, Lazaris AC, Misthos P, Papadimitriou K, Androulakis G. Sweat gland carcinoma with multiple local recurrences: a case report. Adv Clin Path. 1999;3(3):63-68.
4. Marone U, Caracò C, Anniciello AM, et al. Metastatic eccrine porocarcinoma : report of a case and review of the literature. World J Surg Oncol. 2011;9:32.
5. Yildirim S, Aköz T, Akan M, Ege GA. De novo malignant eccrine spiradenoma with an interesting and unusual location. Dermatol Surg. 2001;27(4):417-420.
6. Shaw M, McKee PH, Lowe D, Black MM. Malignant eccrine poroma: a study of twenty-seven cases. Br J Dermatol. 1982;107(6):675-680.
7. De Iuliis F, Amoroso L, Taglieri L, et al. Chemotherapy of rare skin adnexal tumors: a review of literature. Anticancer Res. 2014;34(10):5263-5268.
8. Alsaad KO, Obaidat NA, Ghazarian D. Skin adnexal neoplasms – part 1: an approach to tumours of the pilosebaceous unit. J Clin Pathol. 2007;60(2):129-144.
9. Serhrouchni KI, Harmouch T, Chbani L, et al. Eccrine carcinoma : a rare cutaneous neoplasm. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3570399/. Published online February 4, 2013. Accessed October 11, 2017.
10. Shidham VB, Komorowski RA, Machhi JK. Androgen receptor expression in metastatic adenocarcinoma in females favors a breast primary. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1601970/. Published online October 4, 2006. Accessed October 11, 2017.
11. Hiatt KM, Pillow JL, Smoller BR. Her-2 expression in cutaneous eccrine and apocrine neoplasms. Mod Pathol. 2004;17(1):28-32.
12. Gu L-H, Ichiki Y, Kitajima Y. Aberrant expression of p16 and RB protein in eccrine porocarcinoma. J Cutan Pathol. 2002;29(8):473-479.
13. el-Domeiri AA, Brasfield RD, Huvos AG, Strong EW. Sweat gland carcinoma: a clinico-pathologic study of 83 patients. Ann Surg. 1971;173(2):270-274.
14. Tlemcani K, Levine D, Smith R V, et al. Metastatic apocrine carcinoma of the scalp: prolonged response to systemic chemotherapy. J Clin Oncol. 2010;28(24):e412-e414.
15. Chamberlain RS, Huber K, White JC, Travaglino-Parda R. Apocrine gland carcinoma of the axilla: review of the literature and recommendations for treatment. Am J Clin Oncol. 1999;22(2):131-135.
16. Gutermuth J, Audring H, Voit C, Trefzer U, Haas N. Antitumour activity of paclitaxel and interferon-alpha in a case of metastatic eccrine porocarcinoma. J Eur Acad Dermatol Venereol. 2004;18(4):477-479.
17. Mezger J, Remberger K, Schalhorn A, Wohlrab A, Wilmanns W. Treatment of metastatic sweat gland carcinoma by a four drug combination chemotherapy: response in two cases. Med Oncol Tumor Pharmacother. 1986;3(1):29-34.
18. Aaribi I, Mohtaram A, Ben Ameur El Youbi M, et al. Successful management of metastatic eccrine porocarcinoma. https://www.hindawi.com/journals/crionm/2013/282536/. Published 2013. Accessed October 10, 2017.
19. Shiohara J, Koga H, Uhara H, Takata M, Saida T. Eccrine porocarcinoma: clinical and pathological studies of 12 cases. J Dermatol. 2007;34(8):516-522.
20. Swanson PE, Mazoujian G, Mills SE, Campbell RJ, Wick MR. Immunoreactivity for estrogen receptor protein in sweat gland tumors. Am J Surg Pathol. 1991;15(9):835-841.
21. Busam KJ, Tan LK, Granter SR, et al. Epidermal growth factor, estrogen, and progesterone receptor expression in primary sweat gland carcinomas and primary and metastatic mammary carcinomas. Mod Pathol. 1999;12(8):786-793.
22. Sridhar KS, Benedetto P, Otrakji CL, Charyulu KK. Response of eccrine adenocarcinoma to tamoxifen. Cancer. 1989;64(2):366-370.
23. Daniel SJ, Nader R, Kost K, Hüttner I. Facial sweat gland carcinoma metastasizing to neck nodes: a diagnostic and therapeutic challenge. Arch Otolaryngol Head Neck Surg. 2001;127(12):1495-1498.
24. Battistella M, Mateus C, Lassau N, et al. Sunitinib efficacy in the treatment of metastatic skin adnexal carcinomas: report of two patients with hidradenocarcinoma and trichoblastic carcinoma. J Eur Acad Dermatol Venereol. 2010;24(2):199-203.
25. Hidaka T, Fujimura T, Watabe A, et al. Successful treatment of HER-2-positive metastatic apocrine carcinoma of the skin with lapatinib and capecitabine. Acta Derm Venereol. 2012;92(6):654-655.
26. Mandaliya H, Nordman I. Metastatic eccrine porocarcinoma: a rare case of successful treatment. Case Rep Oncol. 2016;9(2):454-456.
27. de Bree E, Volalakis E, Tsetis D, et al. Treatment of advanced malignant eccrine poroma with locoregional chemotherapy. Br J Dermatol. 2005;152(5):1051-1055.
28. Bahl A, Sharma DN, Julka PK, Das A, Rath GK. Sweat gland carcinoma with lung metastases. J Cancer Res Ther. 2(4):209-211.
29. Wang X-X, Wang H-Y, Zheng J-N, Sui J-C. Primary cutaneous sweat gland carcinoma. J Cancer Res Ther. 10(2):390-392.
1. Gates O, Warren S, Warvi WN. Tumors of sweat glands. Am J Pathol. 1943;19(4):591-631.
2. Mitts DL, Smith MT, Russell L, Bannayan GA, Cruz AB. Sweat gland carcinoma: a clinico-pathological reappraisal. J Surg Oncol. 1976;8(1):23-29.
3. Panoussopoulos D, Darom A, Lazaris AC, Misthos P, Papadimitriou K, Androulakis G. Sweat gland carcinoma with multiple local recurrences: a case report. Adv Clin Path. 1999;3(3):63-68.
4. Marone U, Caracò C, Anniciello AM, et al. Metastatic eccrine porocarcinoma : report of a case and review of the literature. World J Surg Oncol. 2011;9:32.
5. Yildirim S, Aköz T, Akan M, Ege GA. De novo malignant eccrine spiradenoma with an interesting and unusual location. Dermatol Surg. 2001;27(4):417-420.
6. Shaw M, McKee PH, Lowe D, Black MM. Malignant eccrine poroma: a study of twenty-seven cases. Br J Dermatol. 1982;107(6):675-680.
7. De Iuliis F, Amoroso L, Taglieri L, et al. Chemotherapy of rare skin adnexal tumors: a review of literature. Anticancer Res. 2014;34(10):5263-5268.
8. Alsaad KO, Obaidat NA, Ghazarian D. Skin adnexal neoplasms – part 1: an approach to tumours of the pilosebaceous unit. J Clin Pathol. 2007;60(2):129-144.
9. Serhrouchni KI, Harmouch T, Chbani L, et al. Eccrine carcinoma : a rare cutaneous neoplasm. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3570399/. Published online February 4, 2013. Accessed October 11, 2017.
10. Shidham VB, Komorowski RA, Machhi JK. Androgen receptor expression in metastatic adenocarcinoma in females favors a breast primary. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1601970/. Published online October 4, 2006. Accessed October 11, 2017.
11. Hiatt KM, Pillow JL, Smoller BR. Her-2 expression in cutaneous eccrine and apocrine neoplasms. Mod Pathol. 2004;17(1):28-32.
12. Gu L-H, Ichiki Y, Kitajima Y. Aberrant expression of p16 and RB protein in eccrine porocarcinoma. J Cutan Pathol. 2002;29(8):473-479.
13. el-Domeiri AA, Brasfield RD, Huvos AG, Strong EW. Sweat gland carcinoma: a clinico-pathologic study of 83 patients. Ann Surg. 1971;173(2):270-274.
14. Tlemcani K, Levine D, Smith R V, et al. Metastatic apocrine carcinoma of the scalp: prolonged response to systemic chemotherapy. J Clin Oncol. 2010;28(24):e412-e414.
15. Chamberlain RS, Huber K, White JC, Travaglino-Parda R. Apocrine gland carcinoma of the axilla: review of the literature and recommendations for treatment. Am J Clin Oncol. 1999;22(2):131-135.
16. Gutermuth J, Audring H, Voit C, Trefzer U, Haas N. Antitumour activity of paclitaxel and interferon-alpha in a case of metastatic eccrine porocarcinoma. J Eur Acad Dermatol Venereol. 2004;18(4):477-479.
17. Mezger J, Remberger K, Schalhorn A, Wohlrab A, Wilmanns W. Treatment of metastatic sweat gland carcinoma by a four drug combination chemotherapy: response in two cases. Med Oncol Tumor Pharmacother. 1986;3(1):29-34.
18. Aaribi I, Mohtaram A, Ben Ameur El Youbi M, et al. Successful management of metastatic eccrine porocarcinoma. https://www.hindawi.com/journals/crionm/2013/282536/. Published 2013. Accessed October 10, 2017.
19. Shiohara J, Koga H, Uhara H, Takata M, Saida T. Eccrine porocarcinoma: clinical and pathological studies of 12 cases. J Dermatol. 2007;34(8):516-522.
20. Swanson PE, Mazoujian G, Mills SE, Campbell RJ, Wick MR. Immunoreactivity for estrogen receptor protein in sweat gland tumors. Am J Surg Pathol. 1991;15(9):835-841.
21. Busam KJ, Tan LK, Granter SR, et al. Epidermal growth factor, estrogen, and progesterone receptor expression in primary sweat gland carcinomas and primary and metastatic mammary carcinomas. Mod Pathol. 1999;12(8):786-793.
22. Sridhar KS, Benedetto P, Otrakji CL, Charyulu KK. Response of eccrine adenocarcinoma to tamoxifen. Cancer. 1989;64(2):366-370.
23. Daniel SJ, Nader R, Kost K, Hüttner I. Facial sweat gland carcinoma metastasizing to neck nodes: a diagnostic and therapeutic challenge. Arch Otolaryngol Head Neck Surg. 2001;127(12):1495-1498.
24. Battistella M, Mateus C, Lassau N, et al. Sunitinib efficacy in the treatment of metastatic skin adnexal carcinomas: report of two patients with hidradenocarcinoma and trichoblastic carcinoma. J Eur Acad Dermatol Venereol. 2010;24(2):199-203.
25. Hidaka T, Fujimura T, Watabe A, et al. Successful treatment of HER-2-positive metastatic apocrine carcinoma of the skin with lapatinib and capecitabine. Acta Derm Venereol. 2012;92(6):654-655.
26. Mandaliya H, Nordman I. Metastatic eccrine porocarcinoma: a rare case of successful treatment. Case Rep Oncol. 2016;9(2):454-456.
27. de Bree E, Volalakis E, Tsetis D, et al. Treatment of advanced malignant eccrine poroma with locoregional chemotherapy. Br J Dermatol. 2005;152(5):1051-1055.
28. Bahl A, Sharma DN, Julka PK, Das A, Rath GK. Sweat gland carcinoma with lung metastases. J Cancer Res Ther. 2(4):209-211.
29. Wang X-X, Wang H-Y, Zheng J-N, Sui J-C. Primary cutaneous sweat gland carcinoma. J Cancer Res Ther. 10(2):390-392.