User login
Update on New Drugs in Dermatology
CenterWatch (http://www.centerwatch.com/) is an online resource that provides directories, analysis, and market research of medications that are either under clinical evaluation or available for use in patients. A list of currently approved drugs by the US Food and Drug Administration (FDA) also is available by specialty. It is important for dermatologists in-training to know about recently approved drugs and those that are in the pipeline, as these treatments may benefit patients who are unresponsive to other previously used medications. New drugs also may be useful for physicians who have a difficult time getting insurance to cover prescriptions for their patients, as most new medications have built-in patient assistance.
New Drugs in Dermatology
Actinic Keratosis
Ameluz (aminolevulinic acid hydrochloride)(Biofrontera AG) is a new drug that was approved in May 2016 for treatment of mild to moderate actinic keratosis on the face and scalp.1 It is only intended for in-office use on patients who may not be candidates for other treatment options for actinic keratosis. The product is a gel formulation that should be applied to cover the lesions and approximately 5 mm of the surrounding area with a film of approximately 1-mm thickness. The entire treatment area is then illuminated with a red light source, either with a narrow spectrum around 630 nm with a light dose of approximately 37 J/cm2 or a broader and continuous spectrum in the range of 570 to 670 nm with a light dose between 75 and 200 J/cm2.1 Similar to the previously used aminolevulinic acid treatment method for actinic keratosis, the patient may experience a burning stinging sensation throughout the treatment and the skin will then proceed to peel.
Psoriasis and Psoriatic Arthritis
Taltz (ixekizumab)(Eli Lilly and Company) was approved by the FDA in March 2016 for the treatment of moderate to severe plaque psoriasis.2 It is a humanized IL-17A antagonist that works when IgG4 monoclonal antibodies selectively bind with IL-17A cytokines and inhibit their interaction with the IL-17 receptor. Although this injectable medication is approved for the treatment of psoriasis, it also can potentially be used off label for the treatment of psoriatic arthritis and rheumatoid arthritis. The approved dosage is 160 mg (two 80-mg injections) at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.2 Injectable immunomodulatory medications such as ixekizumab are ideal for patients in whom topical treatments and light therapy failed and they continue to have serious psoriatic discomfort as well as for those who have substantial body surface area coverage.
In January 2015, Cosentyx (secukinumab)(Novartis Corporation) was approved by the FDA.3 Similar to ixekizumab, this injectable is an IgG1 monoclonal antibody that selectively binds to the IL-17A cytokine and inhibits its interaction with the IL-17 receptor. It is approved for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis. The approved dosage for plaque psoriasis is 300 mg (two 150-mg subcutaneous injections) at weeks 0 through 4 followed by 300 mg every 4 weeks as needed until clearance.3 Similar to ixekizumab, secukinumab may be used for the treatment of recalcitrant psoriasis or psoriasis with substantial body surface area involvement.
Melanoma
Cotellic (cobimetinib)(Genentech USA, Inc) was FDA approved in November 2015.4 Cobimetinib is a reversible inhibitor of mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase 1. Mitogen-activated protein kinase MEK1 and MEK2 are regulators of the extracellular signal-related kinase pathway, which promotes cellular proliferation. This pathway is key, as melanomas that have a BRAF V600E and kinase mutation continue to proliferate due to the constitutive activation of MEK1 and MEK2, further promoting cellular proliferation. Cobimetinib is approved for the treatment of melanoma in patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, in conjunction with vemurafenib. Zelboraf (vemurafenib)(Genentech USA, Inc), another inhibitor of BRAF V600E, also is used for the treatment of unresectable melanomas and was initially approved in 2011.5
BRAF is a serine/threonine protein kinase. When unregulated, it results in the deregulation of cell proliferation. According to Ascierto et al,6 50% of melanomas have a BRAF mutation, with nearly 90% of them with a V600E mutation. Hence, since the advent of direct chemotherapeutic agents such as BRAF inhibitors, clinical trials have shown notable reduction in mortality and morbidity of melanoma patients with BRAF mutations.6
Imlygic (talimogene laherparepvec)(Amgen, Inc) is a modified oncolytic viral therapy.7 This treatment was approved by the FDA in 2015 and replicates within tumors to produce granulocyte-macrophage colony-stimulating factor protein, which promotes an antitumor immune response within unresectable cutaneous, subcutaneous, and nodal melanoma lesions. Although it is not a gene-directed therapy, the melanoma does not require a specific mutation for treatment. Again, this medication is better served in conjunction with other melanoma chemotherapeutic and surgical interventions.
Submental Fat
Kybella (deoxycholic acid)(Allergan) is a nonhuman, nonanimal, synthetically created compound that is naturally found within the human body for the breakdown and absorption of dietary fat.8 This drug was FDA approved in 2015 for the improvement of the appearance of moderate subcutaneous fat under the chin. Patients are evaluated in clinic to determine if the submental fat would be responsive to an injectable or require more radical surgical intervention based on desired outcomes. The treatment is administered as 0.2-mL injections (up to a total of 10 mL) spaced 1-cm apart and ideally is repeated at regular intervals to evaluate for efficacy.
Basal Cell Carcinoma
Odomzo (sonidegib)(Novartis Corporation) was FDA approved in 2015 for locally advanced basal cell carcinoma.9 Odomzo is a smoothened antagonist that inhibits the hedgehog signaling pathway. Smoothened is a transmembrane protein that allows for signal transduction of hedgehog proteins.10 Protein patched homolog 1 binds to smoothened protein and prevents the signal transduction through the cell for Gli family zinc factor 1 to continue protein translation; however, when PTCH is mutated and can no longer bind to smoothened, tumor formation results, specifically basal cell carcinoma. Hence, sonidegib is for the treatment of basal cell carcinomas that have persisted despite radiation treatment and/or surgery as well as for patients who have multiple basal cell carcinomas that can no longer be treated with surgery or radiation.
Final Thoughts
Overall, although there are several medications that can be used in conjunction for treatment of dermatological conditions, it always is recommended to know what is in the pipeline as FDA-approved medications for dermatology.
- Ameluz [package insert]. Leverkusen, Germany: Biofrontera Bioscience GmbH; 2016.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Corporation; 2015.
- Cotellic [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Zelboraf [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85.
- Imlygic (talimogene laherparepvec). Thousand Oaks, CA: Amgen Inc; 2015.
- Kybella [package insert]. West Lake Village, CA: Kythera Biopharmaceuticals, Inc; 2015.
- Odomzo [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015.
- Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet. 2000;67:1047-1054.
CenterWatch (http://www.centerwatch.com/) is an online resource that provides directories, analysis, and market research of medications that are either under clinical evaluation or available for use in patients. A list of currently approved drugs by the US Food and Drug Administration (FDA) also is available by specialty. It is important for dermatologists in-training to know about recently approved drugs and those that are in the pipeline, as these treatments may benefit patients who are unresponsive to other previously used medications. New drugs also may be useful for physicians who have a difficult time getting insurance to cover prescriptions for their patients, as most new medications have built-in patient assistance.
New Drugs in Dermatology
Actinic Keratosis
Ameluz (aminolevulinic acid hydrochloride)(Biofrontera AG) is a new drug that was approved in May 2016 for treatment of mild to moderate actinic keratosis on the face and scalp.1 It is only intended for in-office use on patients who may not be candidates for other treatment options for actinic keratosis. The product is a gel formulation that should be applied to cover the lesions and approximately 5 mm of the surrounding area with a film of approximately 1-mm thickness. The entire treatment area is then illuminated with a red light source, either with a narrow spectrum around 630 nm with a light dose of approximately 37 J/cm2 or a broader and continuous spectrum in the range of 570 to 670 nm with a light dose between 75 and 200 J/cm2.1 Similar to the previously used aminolevulinic acid treatment method for actinic keratosis, the patient may experience a burning stinging sensation throughout the treatment and the skin will then proceed to peel.
Psoriasis and Psoriatic Arthritis
Taltz (ixekizumab)(Eli Lilly and Company) was approved by the FDA in March 2016 for the treatment of moderate to severe plaque psoriasis.2 It is a humanized IL-17A antagonist that works when IgG4 monoclonal antibodies selectively bind with IL-17A cytokines and inhibit their interaction with the IL-17 receptor. Although this injectable medication is approved for the treatment of psoriasis, it also can potentially be used off label for the treatment of psoriatic arthritis and rheumatoid arthritis. The approved dosage is 160 mg (two 80-mg injections) at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.2 Injectable immunomodulatory medications such as ixekizumab are ideal for patients in whom topical treatments and light therapy failed and they continue to have serious psoriatic discomfort as well as for those who have substantial body surface area coverage.
In January 2015, Cosentyx (secukinumab)(Novartis Corporation) was approved by the FDA.3 Similar to ixekizumab, this injectable is an IgG1 monoclonal antibody that selectively binds to the IL-17A cytokine and inhibits its interaction with the IL-17 receptor. It is approved for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis. The approved dosage for plaque psoriasis is 300 mg (two 150-mg subcutaneous injections) at weeks 0 through 4 followed by 300 mg every 4 weeks as needed until clearance.3 Similar to ixekizumab, secukinumab may be used for the treatment of recalcitrant psoriasis or psoriasis with substantial body surface area involvement.
Melanoma
Cotellic (cobimetinib)(Genentech USA, Inc) was FDA approved in November 2015.4 Cobimetinib is a reversible inhibitor of mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase 1. Mitogen-activated protein kinase MEK1 and MEK2 are regulators of the extracellular signal-related kinase pathway, which promotes cellular proliferation. This pathway is key, as melanomas that have a BRAF V600E and kinase mutation continue to proliferate due to the constitutive activation of MEK1 and MEK2, further promoting cellular proliferation. Cobimetinib is approved for the treatment of melanoma in patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, in conjunction with vemurafenib. Zelboraf (vemurafenib)(Genentech USA, Inc), another inhibitor of BRAF V600E, also is used for the treatment of unresectable melanomas and was initially approved in 2011.5
BRAF is a serine/threonine protein kinase. When unregulated, it results in the deregulation of cell proliferation. According to Ascierto et al,6 50% of melanomas have a BRAF mutation, with nearly 90% of them with a V600E mutation. Hence, since the advent of direct chemotherapeutic agents such as BRAF inhibitors, clinical trials have shown notable reduction in mortality and morbidity of melanoma patients with BRAF mutations.6
Imlygic (talimogene laherparepvec)(Amgen, Inc) is a modified oncolytic viral therapy.7 This treatment was approved by the FDA in 2015 and replicates within tumors to produce granulocyte-macrophage colony-stimulating factor protein, which promotes an antitumor immune response within unresectable cutaneous, subcutaneous, and nodal melanoma lesions. Although it is not a gene-directed therapy, the melanoma does not require a specific mutation for treatment. Again, this medication is better served in conjunction with other melanoma chemotherapeutic and surgical interventions.
Submental Fat
Kybella (deoxycholic acid)(Allergan) is a nonhuman, nonanimal, synthetically created compound that is naturally found within the human body for the breakdown and absorption of dietary fat.8 This drug was FDA approved in 2015 for the improvement of the appearance of moderate subcutaneous fat under the chin. Patients are evaluated in clinic to determine if the submental fat would be responsive to an injectable or require more radical surgical intervention based on desired outcomes. The treatment is administered as 0.2-mL injections (up to a total of 10 mL) spaced 1-cm apart and ideally is repeated at regular intervals to evaluate for efficacy.
Basal Cell Carcinoma
Odomzo (sonidegib)(Novartis Corporation) was FDA approved in 2015 for locally advanced basal cell carcinoma.9 Odomzo is a smoothened antagonist that inhibits the hedgehog signaling pathway. Smoothened is a transmembrane protein that allows for signal transduction of hedgehog proteins.10 Protein patched homolog 1 binds to smoothened protein and prevents the signal transduction through the cell for Gli family zinc factor 1 to continue protein translation; however, when PTCH is mutated and can no longer bind to smoothened, tumor formation results, specifically basal cell carcinoma. Hence, sonidegib is for the treatment of basal cell carcinomas that have persisted despite radiation treatment and/or surgery as well as for patients who have multiple basal cell carcinomas that can no longer be treated with surgery or radiation.
Final Thoughts
Overall, although there are several medications that can be used in conjunction for treatment of dermatological conditions, it always is recommended to know what is in the pipeline as FDA-approved medications for dermatology.
CenterWatch (http://www.centerwatch.com/) is an online resource that provides directories, analysis, and market research of medications that are either under clinical evaluation or available for use in patients. A list of currently approved drugs by the US Food and Drug Administration (FDA) also is available by specialty. It is important for dermatologists in-training to know about recently approved drugs and those that are in the pipeline, as these treatments may benefit patients who are unresponsive to other previously used medications. New drugs also may be useful for physicians who have a difficult time getting insurance to cover prescriptions for their patients, as most new medications have built-in patient assistance.
New Drugs in Dermatology
Actinic Keratosis
Ameluz (aminolevulinic acid hydrochloride)(Biofrontera AG) is a new drug that was approved in May 2016 for treatment of mild to moderate actinic keratosis on the face and scalp.1 It is only intended for in-office use on patients who may not be candidates for other treatment options for actinic keratosis. The product is a gel formulation that should be applied to cover the lesions and approximately 5 mm of the surrounding area with a film of approximately 1-mm thickness. The entire treatment area is then illuminated with a red light source, either with a narrow spectrum around 630 nm with a light dose of approximately 37 J/cm2 or a broader and continuous spectrum in the range of 570 to 670 nm with a light dose between 75 and 200 J/cm2.1 Similar to the previously used aminolevulinic acid treatment method for actinic keratosis, the patient may experience a burning stinging sensation throughout the treatment and the skin will then proceed to peel.
Psoriasis and Psoriatic Arthritis
Taltz (ixekizumab)(Eli Lilly and Company) was approved by the FDA in March 2016 for the treatment of moderate to severe plaque psoriasis.2 It is a humanized IL-17A antagonist that works when IgG4 monoclonal antibodies selectively bind with IL-17A cytokines and inhibit their interaction with the IL-17 receptor. Although this injectable medication is approved for the treatment of psoriasis, it also can potentially be used off label for the treatment of psoriatic arthritis and rheumatoid arthritis. The approved dosage is 160 mg (two 80-mg injections) at week 0, followed by 80 mg at weeks 2, 4, 6, 8, 10, and 12, then 80 mg every 4 weeks.2 Injectable immunomodulatory medications such as ixekizumab are ideal for patients in whom topical treatments and light therapy failed and they continue to have serious psoriatic discomfort as well as for those who have substantial body surface area coverage.
In January 2015, Cosentyx (secukinumab)(Novartis Corporation) was approved by the FDA.3 Similar to ixekizumab, this injectable is an IgG1 monoclonal antibody that selectively binds to the IL-17A cytokine and inhibits its interaction with the IL-17 receptor. It is approved for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis. The approved dosage for plaque psoriasis is 300 mg (two 150-mg subcutaneous injections) at weeks 0 through 4 followed by 300 mg every 4 weeks as needed until clearance.3 Similar to ixekizumab, secukinumab may be used for the treatment of recalcitrant psoriasis or psoriasis with substantial body surface area involvement.
Melanoma
Cotellic (cobimetinib)(Genentech USA, Inc) was FDA approved in November 2015.4 Cobimetinib is a reversible inhibitor of mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase 1. Mitogen-activated protein kinase MEK1 and MEK2 are regulators of the extracellular signal-related kinase pathway, which promotes cellular proliferation. This pathway is key, as melanomas that have a BRAF V600E and kinase mutation continue to proliferate due to the constitutive activation of MEK1 and MEK2, further promoting cellular proliferation. Cobimetinib is approved for the treatment of melanoma in patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, in conjunction with vemurafenib. Zelboraf (vemurafenib)(Genentech USA, Inc), another inhibitor of BRAF V600E, also is used for the treatment of unresectable melanomas and was initially approved in 2011.5
BRAF is a serine/threonine protein kinase. When unregulated, it results in the deregulation of cell proliferation. According to Ascierto et al,6 50% of melanomas have a BRAF mutation, with nearly 90% of them with a V600E mutation. Hence, since the advent of direct chemotherapeutic agents such as BRAF inhibitors, clinical trials have shown notable reduction in mortality and morbidity of melanoma patients with BRAF mutations.6
Imlygic (talimogene laherparepvec)(Amgen, Inc) is a modified oncolytic viral therapy.7 This treatment was approved by the FDA in 2015 and replicates within tumors to produce granulocyte-macrophage colony-stimulating factor protein, which promotes an antitumor immune response within unresectable cutaneous, subcutaneous, and nodal melanoma lesions. Although it is not a gene-directed therapy, the melanoma does not require a specific mutation for treatment. Again, this medication is better served in conjunction with other melanoma chemotherapeutic and surgical interventions.
Submental Fat
Kybella (deoxycholic acid)(Allergan) is a nonhuman, nonanimal, synthetically created compound that is naturally found within the human body for the breakdown and absorption of dietary fat.8 This drug was FDA approved in 2015 for the improvement of the appearance of moderate subcutaneous fat under the chin. Patients are evaluated in clinic to determine if the submental fat would be responsive to an injectable or require more radical surgical intervention based on desired outcomes. The treatment is administered as 0.2-mL injections (up to a total of 10 mL) spaced 1-cm apart and ideally is repeated at regular intervals to evaluate for efficacy.
Basal Cell Carcinoma
Odomzo (sonidegib)(Novartis Corporation) was FDA approved in 2015 for locally advanced basal cell carcinoma.9 Odomzo is a smoothened antagonist that inhibits the hedgehog signaling pathway. Smoothened is a transmembrane protein that allows for signal transduction of hedgehog proteins.10 Protein patched homolog 1 binds to smoothened protein and prevents the signal transduction through the cell for Gli family zinc factor 1 to continue protein translation; however, when PTCH is mutated and can no longer bind to smoothened, tumor formation results, specifically basal cell carcinoma. Hence, sonidegib is for the treatment of basal cell carcinomas that have persisted despite radiation treatment and/or surgery as well as for patients who have multiple basal cell carcinomas that can no longer be treated with surgery or radiation.
Final Thoughts
Overall, although there are several medications that can be used in conjunction for treatment of dermatological conditions, it always is recommended to know what is in the pipeline as FDA-approved medications for dermatology.
- Ameluz [package insert]. Leverkusen, Germany: Biofrontera Bioscience GmbH; 2016.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Corporation; 2015.
- Cotellic [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Zelboraf [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85.
- Imlygic (talimogene laherparepvec). Thousand Oaks, CA: Amgen Inc; 2015.
- Kybella [package insert]. West Lake Village, CA: Kythera Biopharmaceuticals, Inc; 2015.
- Odomzo [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015.
- Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet. 2000;67:1047-1054.
- Ameluz [package insert]. Leverkusen, Germany: Biofrontera Bioscience GmbH; 2016.
- Taltz [package insert]. Indianapolis, IN: Eli Lilly and Company; 2016.
- Cosentyx [package insert]. East Hanover, NJ: Novartis Corporation; 2015.
- Cotellic [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Zelboraf [package insert]. San Francisco, CA: Genentech, Inc; 2016.
- Ascierto PA, Kirkwood JM, Grob JJ, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85.
- Imlygic (talimogene laherparepvec). Thousand Oaks, CA: Amgen Inc; 2015.
- Kybella [package insert]. West Lake Village, CA: Kythera Biopharmaceuticals, Inc; 2015.
- Odomzo [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015.
- Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet. 2000;67:1047-1054.
Sex differences in T-cell profiles may drive anti–PD-L1 responses
NATIONAL HARBOR, MD. – Sex difference in immune regulatory responses may drive the poorer responses to treatment with immune checkpoint inhibitors targeted against programmed death–1 (PD-1 inhibitors) seen in women with advanced melanoma, investigators report.
Among patients with advanced melanoma treated with either pembrolizumab (Keytruda) or nivolumab (Opdivo) monotherapy in four clinical trials, the median objective response rate (ORR) among women was 33.1%, compared with 54.6% among men. Median progression-free survival (PFS), respectively, was 5.5 months vs. 18 months, reported Katy K. Tsai, MD, a clinical instructor in cutaneous oncology at the University of California, San Francisco.
“There has been a lot of interesting data coming out recently about the influence of sex hormones on the immune regulatory response in general, so I do think that is something that needs to be explored further,” Dr. Tsai said at the annual meeting of the Society for Immunotherapy of Cancer.
“There are some interesting data to suggest that perhaps women, and in particular pregnant women or perhaps even women who have higher parity than those who are nulliparous, may have higher circulating levels of T-regs that may contribute to dampening this immune response,” she said.
Response prediction model
Dr. Tsai and her colleagues had previously reported on a validated clinical scoring model for predicting response to anti–PD-1 therapy. In that study, they found that female sex was associated with a lower response rate with an odds ratio of 0.36 (95% confidence interval, 0.19-0.67).
In a separate study, they reported that relative abundance in tumors of a partially exhausted T-cell phenotype (PD-1high/CTLA-4–positive CD8 cells) was predictive of response to anti–PD-1 therapy.
In the current study, they looked at data on 118 women and 218 men who had advanced cutaneous melanoma and were treated in one of four clinical trials of pembrolizumab or nivolumab as monotherapy or in combination with an anti-CTLA4 agent such as ipilimumab (Yervoy) (NCT01295827, NCT01704287, NCT01721746, and NCT02156804).
On flow-cytometry analysis of pre-treatment tumor samples, women had a significantly lower proportion of PD-1high/CTLA-4–positive CD8 cells as compared with men (mean, 16.9% vs. 26%; P = .008).
“The mechanisms of this [discrepancy] may have an immunologic basis given the difference in pre-treatment T-cell profiles between women and men. Sex-related differences in tumor immunity and immunotherapy responses warrant further investigation,” the investigators wrote in a poster presentation.
The study was internally funded. Dr. Tsai and her colleagues reported no relevant disclosures.
NATIONAL HARBOR, MD. – Sex difference in immune regulatory responses may drive the poorer responses to treatment with immune checkpoint inhibitors targeted against programmed death–1 (PD-1 inhibitors) seen in women with advanced melanoma, investigators report.
Among patients with advanced melanoma treated with either pembrolizumab (Keytruda) or nivolumab (Opdivo) monotherapy in four clinical trials, the median objective response rate (ORR) among women was 33.1%, compared with 54.6% among men. Median progression-free survival (PFS), respectively, was 5.5 months vs. 18 months, reported Katy K. Tsai, MD, a clinical instructor in cutaneous oncology at the University of California, San Francisco.
“There has been a lot of interesting data coming out recently about the influence of sex hormones on the immune regulatory response in general, so I do think that is something that needs to be explored further,” Dr. Tsai said at the annual meeting of the Society for Immunotherapy of Cancer.
“There are some interesting data to suggest that perhaps women, and in particular pregnant women or perhaps even women who have higher parity than those who are nulliparous, may have higher circulating levels of T-regs that may contribute to dampening this immune response,” she said.
Response prediction model
Dr. Tsai and her colleagues had previously reported on a validated clinical scoring model for predicting response to anti–PD-1 therapy. In that study, they found that female sex was associated with a lower response rate with an odds ratio of 0.36 (95% confidence interval, 0.19-0.67).
In a separate study, they reported that relative abundance in tumors of a partially exhausted T-cell phenotype (PD-1high/CTLA-4–positive CD8 cells) was predictive of response to anti–PD-1 therapy.
In the current study, they looked at data on 118 women and 218 men who had advanced cutaneous melanoma and were treated in one of four clinical trials of pembrolizumab or nivolumab as monotherapy or in combination with an anti-CTLA4 agent such as ipilimumab (Yervoy) (NCT01295827, NCT01704287, NCT01721746, and NCT02156804).
On flow-cytometry analysis of pre-treatment tumor samples, women had a significantly lower proportion of PD-1high/CTLA-4–positive CD8 cells as compared with men (mean, 16.9% vs. 26%; P = .008).
“The mechanisms of this [discrepancy] may have an immunologic basis given the difference in pre-treatment T-cell profiles between women and men. Sex-related differences in tumor immunity and immunotherapy responses warrant further investigation,” the investigators wrote in a poster presentation.
The study was internally funded. Dr. Tsai and her colleagues reported no relevant disclosures.
NATIONAL HARBOR, MD. – Sex difference in immune regulatory responses may drive the poorer responses to treatment with immune checkpoint inhibitors targeted against programmed death–1 (PD-1 inhibitors) seen in women with advanced melanoma, investigators report.
Among patients with advanced melanoma treated with either pembrolizumab (Keytruda) or nivolumab (Opdivo) monotherapy in four clinical trials, the median objective response rate (ORR) among women was 33.1%, compared with 54.6% among men. Median progression-free survival (PFS), respectively, was 5.5 months vs. 18 months, reported Katy K. Tsai, MD, a clinical instructor in cutaneous oncology at the University of California, San Francisco.
“There has been a lot of interesting data coming out recently about the influence of sex hormones on the immune regulatory response in general, so I do think that is something that needs to be explored further,” Dr. Tsai said at the annual meeting of the Society for Immunotherapy of Cancer.
“There are some interesting data to suggest that perhaps women, and in particular pregnant women or perhaps even women who have higher parity than those who are nulliparous, may have higher circulating levels of T-regs that may contribute to dampening this immune response,” she said.
Response prediction model
Dr. Tsai and her colleagues had previously reported on a validated clinical scoring model for predicting response to anti–PD-1 therapy. In that study, they found that female sex was associated with a lower response rate with an odds ratio of 0.36 (95% confidence interval, 0.19-0.67).
In a separate study, they reported that relative abundance in tumors of a partially exhausted T-cell phenotype (PD-1high/CTLA-4–positive CD8 cells) was predictive of response to anti–PD-1 therapy.
In the current study, they looked at data on 118 women and 218 men who had advanced cutaneous melanoma and were treated in one of four clinical trials of pembrolizumab or nivolumab as monotherapy or in combination with an anti-CTLA4 agent such as ipilimumab (Yervoy) (NCT01295827, NCT01704287, NCT01721746, and NCT02156804).
On flow-cytometry analysis of pre-treatment tumor samples, women had a significantly lower proportion of PD-1high/CTLA-4–positive CD8 cells as compared with men (mean, 16.9% vs. 26%; P = .008).
“The mechanisms of this [discrepancy] may have an immunologic basis given the difference in pre-treatment T-cell profiles between women and men. Sex-related differences in tumor immunity and immunotherapy responses warrant further investigation,” the investigators wrote in a poster presentation.
The study was internally funded. Dr. Tsai and her colleagues reported no relevant disclosures.
AT SITC 2016
Key clinical point: Sex differences in response to PD-1 inhibitors may be caused by differences in immune regulation.
Major finding: On flow-cytometry analysis of pre-treatment tumor samples, women had a significantly lower proportion of PD-1high/CTLA-4–positive CD8 cells as compared with men (mean, 16.9% vs. 26%; P = .008).
Data source: Analysis of data on 336 patients enrolled in four clinical trials of the PD-1 inhibitors pembrolizumab and nivolumab.
Disclosures: The study was internally funded. Dr. Tsai and her colleagues reported no relevant disclosures.
Nivolumab’s safety profile further clarified
In patients taking nivolumab for advanced melanoma, most immunologic adverse effects are mild to moderate in intensity and resolve when existing management guidelines are followed, investigators report.
Nivolumab, a programmed death-1 (PD-1) checkpoint inhibitor antibody, is effective in several tumor types, including melanoma, but its safety profile has not been well characterized to date. Researchers pooled data from two phase I exploratory and two phase III comparative clinical trials of advanced melanoma to better examine safety outcomes, in what they described as “the largest and most comprehensive analysis to date of ... anti-PD-1 monotherapy.”
The study included 576 patients (median age, 61 years) taking nivolumab. Slightly more than half had received ipilimumab, another immune checkpoint inhibitor, previously. Twelve percent had brain metastases. The median duration of nivolumab therapy was 3.7 months, reflecting a median of nine doses of the agent per patient. Median follow-up was 7.2 months (range, 0.3-62.5 months), said Jeffrey S. Weber, MD, PhD, of Dana-Farber Cancer Institute, Boston, and his associates.
The overall rate of adverse events likely to have an immunologic etiology was 49%, and most of these were mild to moderate; severe immunologic adverse effects occurred in less than 4% of patients. Prior ipilimumab therapy did not influence the number or severity of adverse reactions to nivolumab. Most immunologic adverse effects involved the skin and GI tract, and most of them, including the few severe effects, resolved when treated according to safety management guidelines.
The types of immunologic adverse events in this study were similar to those previously reported for this class of drug. However, pneumonitis developed less frequently (less than 2% of patients) in this study population than has been reported previously and also was less severe. Rare and unusual immunologic adverse events remain a possibility with PD-1 checkpoint inhibitor antibodies. In particular, five of these study participants developed grade 3 neurologic toxicities. Further information on such effects and on optimal management is needed, the investigators wrote (J Clin Oncol. 2016 Nov 14. doi: 10.1200/JCO.66.1389).
Immune modulators – chiefly systemic, topical, or inhaled corticosteroids – were frequently used to treat immunologic adverse effects. “Although there is a theoretic concern that [this] might interfere with an anticancer immune response, the results of our analysis suggest that immune modulators do not negatively affect the rate or quality of antitumor responses [to] nivolumab therapy,” Dr. Weber and his associates said.
In patients taking nivolumab for advanced melanoma, most immunologic adverse effects are mild to moderate in intensity and resolve when existing management guidelines are followed, investigators report.
Nivolumab, a programmed death-1 (PD-1) checkpoint inhibitor antibody, is effective in several tumor types, including melanoma, but its safety profile has not been well characterized to date. Researchers pooled data from two phase I exploratory and two phase III comparative clinical trials of advanced melanoma to better examine safety outcomes, in what they described as “the largest and most comprehensive analysis to date of ... anti-PD-1 monotherapy.”
The study included 576 patients (median age, 61 years) taking nivolumab. Slightly more than half had received ipilimumab, another immune checkpoint inhibitor, previously. Twelve percent had brain metastases. The median duration of nivolumab therapy was 3.7 months, reflecting a median of nine doses of the agent per patient. Median follow-up was 7.2 months (range, 0.3-62.5 months), said Jeffrey S. Weber, MD, PhD, of Dana-Farber Cancer Institute, Boston, and his associates.
The overall rate of adverse events likely to have an immunologic etiology was 49%, and most of these were mild to moderate; severe immunologic adverse effects occurred in less than 4% of patients. Prior ipilimumab therapy did not influence the number or severity of adverse reactions to nivolumab. Most immunologic adverse effects involved the skin and GI tract, and most of them, including the few severe effects, resolved when treated according to safety management guidelines.
The types of immunologic adverse events in this study were similar to those previously reported for this class of drug. However, pneumonitis developed less frequently (less than 2% of patients) in this study population than has been reported previously and also was less severe. Rare and unusual immunologic adverse events remain a possibility with PD-1 checkpoint inhibitor antibodies. In particular, five of these study participants developed grade 3 neurologic toxicities. Further information on such effects and on optimal management is needed, the investigators wrote (J Clin Oncol. 2016 Nov 14. doi: 10.1200/JCO.66.1389).
Immune modulators – chiefly systemic, topical, or inhaled corticosteroids – were frequently used to treat immunologic adverse effects. “Although there is a theoretic concern that [this] might interfere with an anticancer immune response, the results of our analysis suggest that immune modulators do not negatively affect the rate or quality of antitumor responses [to] nivolumab therapy,” Dr. Weber and his associates said.
In patients taking nivolumab for advanced melanoma, most immunologic adverse effects are mild to moderate in intensity and resolve when existing management guidelines are followed, investigators report.
Nivolumab, a programmed death-1 (PD-1) checkpoint inhibitor antibody, is effective in several tumor types, including melanoma, but its safety profile has not been well characterized to date. Researchers pooled data from two phase I exploratory and two phase III comparative clinical trials of advanced melanoma to better examine safety outcomes, in what they described as “the largest and most comprehensive analysis to date of ... anti-PD-1 monotherapy.”
The study included 576 patients (median age, 61 years) taking nivolumab. Slightly more than half had received ipilimumab, another immune checkpoint inhibitor, previously. Twelve percent had brain metastases. The median duration of nivolumab therapy was 3.7 months, reflecting a median of nine doses of the agent per patient. Median follow-up was 7.2 months (range, 0.3-62.5 months), said Jeffrey S. Weber, MD, PhD, of Dana-Farber Cancer Institute, Boston, and his associates.
The overall rate of adverse events likely to have an immunologic etiology was 49%, and most of these were mild to moderate; severe immunologic adverse effects occurred in less than 4% of patients. Prior ipilimumab therapy did not influence the number or severity of adverse reactions to nivolumab. Most immunologic adverse effects involved the skin and GI tract, and most of them, including the few severe effects, resolved when treated according to safety management guidelines.
The types of immunologic adverse events in this study were similar to those previously reported for this class of drug. However, pneumonitis developed less frequently (less than 2% of patients) in this study population than has been reported previously and also was less severe. Rare and unusual immunologic adverse events remain a possibility with PD-1 checkpoint inhibitor antibodies. In particular, five of these study participants developed grade 3 neurologic toxicities. Further information on such effects and on optimal management is needed, the investigators wrote (J Clin Oncol. 2016 Nov 14. doi: 10.1200/JCO.66.1389).
Immune modulators – chiefly systemic, topical, or inhaled corticosteroids – were frequently used to treat immunologic adverse effects. “Although there is a theoretic concern that [this] might interfere with an anticancer immune response, the results of our analysis suggest that immune modulators do not negatively affect the rate or quality of antitumor responses [to] nivolumab therapy,” Dr. Weber and his associates said.
Key clinical point: In patients taking nivolumab for advanced melanoma, most immunologic adverse effects are mild to moderate in intensity and resolve when existing management guidelines are followed.
Major finding: The overall rate of adverse events likely to have an immunologic etiology was 49%, while the rate of severe immunologic adverse effects was less than 4%.
Data source: A pooled analysis of data from four clinical trials involving 576 patients who took nivolumab for a median of 4 months and were followed for a median of 7 months.
Disclosures: This study was supported in part by Bristol-Myers Squibb and the Royal Marsden/Institute of Cancer Research Biomedical Research Centre. Dr. Weber and his associates reported ties to numerous industry sources.
Sunscreen and Sperm: Can Chemical UV Filters Alter Sperm Function?
In an article published online on September 1 in Endocrinology, Rehfeld et al discussed their results after testing 29 UV filters. They found that 13 of 29 filters tested had in vitro effects on Ca2+: 4-methylbenzylidene camphor, 3-benzylidene camphor, menthyl anthranilate, isoamyl p-methoxycinnamate, ethylhexyl salicylate, benzylidene camphor sulfonic acid, homosalate, ethylhexyl methoxycinnamate, octcrylene, butyl methoxydibenzoylmethane, and diethylamino hydroxybenzoyl hexyl benzoate.
This study was prompted by a prior study by Schiffer et al (EMBO Rep. 2014;15:758-765) on multiple endocrine disrupting chemicals of which 33 of 96 tested chemicals induced Ca2+ signals in human sperm cells in vitro. Of these previously tested chemicals, some of the chemical sunscreen filters were the most potent, leading to the current study.
Rehfeld et al sought to determine how the UV filters affected calcium signaling, which is a pathway that is essential for sperm cells to be able to swim healthily. These calcium-signaling pathways usually are triggered by progesterone, but the authors showed that 13 of 29 UV filters (45%) also commenced calcium signaling. This effect began at low doses of the chemicals, below the levels of some UV filters found in people after whole-body application of sunscreens.
What’s the issue?
Are these chemical UV filters mimicking progesterone in vivo and could it be interfering with sperm motility? A suboptimal progesterone-induced Ca2+ influx has been associated with reduced male fertility and CatSper (cation channel of sperm) is essential for male fertility (Hum Reprod. 1995;10:120-124).
The UV filters tested are widely available in Europe and the United States. Although this study was in vitro, the in vivo effects will need to be explored. It has been reported by Chivsvert et al (Anal Chim Acta. 2012;752:11-29) that some UV filters can be transcutaneously absorbed into bodily tissues, which could be potentially important for men trying to conceive or for reproductively challenged couples.
What do you discuss with your patients regarding sunscreen safety?
In an article published online on September 1 in Endocrinology, Rehfeld et al discussed their results after testing 29 UV filters. They found that 13 of 29 filters tested had in vitro effects on Ca2+: 4-methylbenzylidene camphor, 3-benzylidene camphor, menthyl anthranilate, isoamyl p-methoxycinnamate, ethylhexyl salicylate, benzylidene camphor sulfonic acid, homosalate, ethylhexyl methoxycinnamate, octcrylene, butyl methoxydibenzoylmethane, and diethylamino hydroxybenzoyl hexyl benzoate.
This study was prompted by a prior study by Schiffer et al (EMBO Rep. 2014;15:758-765) on multiple endocrine disrupting chemicals of which 33 of 96 tested chemicals induced Ca2+ signals in human sperm cells in vitro. Of these previously tested chemicals, some of the chemical sunscreen filters were the most potent, leading to the current study.
Rehfeld et al sought to determine how the UV filters affected calcium signaling, which is a pathway that is essential for sperm cells to be able to swim healthily. These calcium-signaling pathways usually are triggered by progesterone, but the authors showed that 13 of 29 UV filters (45%) also commenced calcium signaling. This effect began at low doses of the chemicals, below the levels of some UV filters found in people after whole-body application of sunscreens.
What’s the issue?
Are these chemical UV filters mimicking progesterone in vivo and could it be interfering with sperm motility? A suboptimal progesterone-induced Ca2+ influx has been associated with reduced male fertility and CatSper (cation channel of sperm) is essential for male fertility (Hum Reprod. 1995;10:120-124).
The UV filters tested are widely available in Europe and the United States. Although this study was in vitro, the in vivo effects will need to be explored. It has been reported by Chivsvert et al (Anal Chim Acta. 2012;752:11-29) that some UV filters can be transcutaneously absorbed into bodily tissues, which could be potentially important for men trying to conceive or for reproductively challenged couples.
What do you discuss with your patients regarding sunscreen safety?
In an article published online on September 1 in Endocrinology, Rehfeld et al discussed their results after testing 29 UV filters. They found that 13 of 29 filters tested had in vitro effects on Ca2+: 4-methylbenzylidene camphor, 3-benzylidene camphor, menthyl anthranilate, isoamyl p-methoxycinnamate, ethylhexyl salicylate, benzylidene camphor sulfonic acid, homosalate, ethylhexyl methoxycinnamate, octcrylene, butyl methoxydibenzoylmethane, and diethylamino hydroxybenzoyl hexyl benzoate.
This study was prompted by a prior study by Schiffer et al (EMBO Rep. 2014;15:758-765) on multiple endocrine disrupting chemicals of which 33 of 96 tested chemicals induced Ca2+ signals in human sperm cells in vitro. Of these previously tested chemicals, some of the chemical sunscreen filters were the most potent, leading to the current study.
Rehfeld et al sought to determine how the UV filters affected calcium signaling, which is a pathway that is essential for sperm cells to be able to swim healthily. These calcium-signaling pathways usually are triggered by progesterone, but the authors showed that 13 of 29 UV filters (45%) also commenced calcium signaling. This effect began at low doses of the chemicals, below the levels of some UV filters found in people after whole-body application of sunscreens.
What’s the issue?
Are these chemical UV filters mimicking progesterone in vivo and could it be interfering with sperm motility? A suboptimal progesterone-induced Ca2+ influx has been associated with reduced male fertility and CatSper (cation channel of sperm) is essential for male fertility (Hum Reprod. 1995;10:120-124).
The UV filters tested are widely available in Europe and the United States. Although this study was in vitro, the in vivo effects will need to be explored. It has been reported by Chivsvert et al (Anal Chim Acta. 2012;752:11-29) that some UV filters can be transcutaneously absorbed into bodily tissues, which could be potentially important for men trying to conceive or for reproductively challenged couples.
What do you discuss with your patients regarding sunscreen safety?
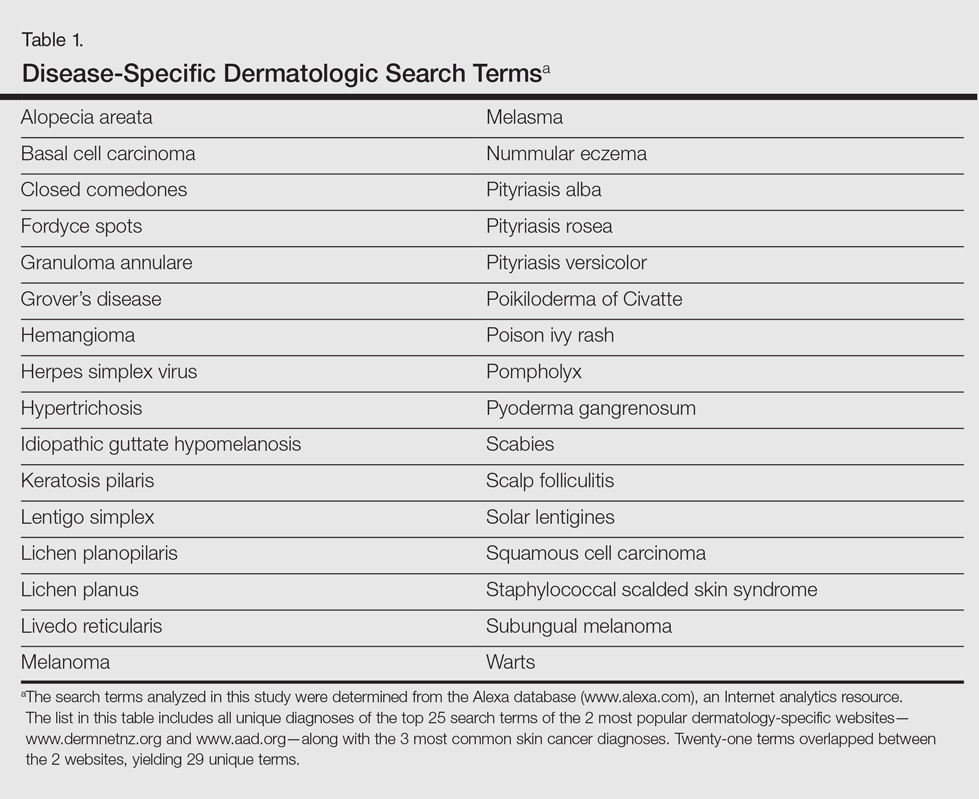
Accuracy and Sources of Images From Direct Google Image Searches for Common Dermatology Terms
To the Editor:
Prior studies have assessed the quality of text-based dermatology information on the Internet using traditional search engine queries.1 However, little is understood about the sources, accuracy, and quality of online dermatology images derived from direct image searches. Previous work has shown that direct search engine image queries were largely accurate for 3 pediatric dermatology diagnosis searches: atopic dermatitis, lichen striatus, and subcutaneous fat necrosis.2 We assessed images obtained for common dermatologic conditions from a Google image search (GIS) compared to a traditional text-based Google web search (GWS).
Image results for 32 unique dermatologic search terms were analyzed (Table 1). These search terms were selected using the results of a prior study that identified the most common dermatologic diagnoses that led users to the 2 most popular dermatology-specific websites worldwide: the American Academy of Dermatology (www.aad.org) and DermNet New Zealand (www.dermnetnz.org).3 The Alexa directory (www.alexa.com), a large publicly available Internet analytics resource, was used to determine the most common dermatology search terms that led a user to either www.dermnetnz.org or www.aad.org. In addition, searches for the 3 most common types of skin cancer—melanoma, squamous cell carcinoma, and basal cell carcinoma—were included. Each term was entered into a GIS and a GWS. The first 10 results, which represent 92% of the websites ultimately visited by users,4 were analyzed. The source, diagnostic accuracy, and Fitzpatrick skin type of the images was determined. Website sources were organized into 11 categories. All data collection occurred within a 1-week period in August 2015.
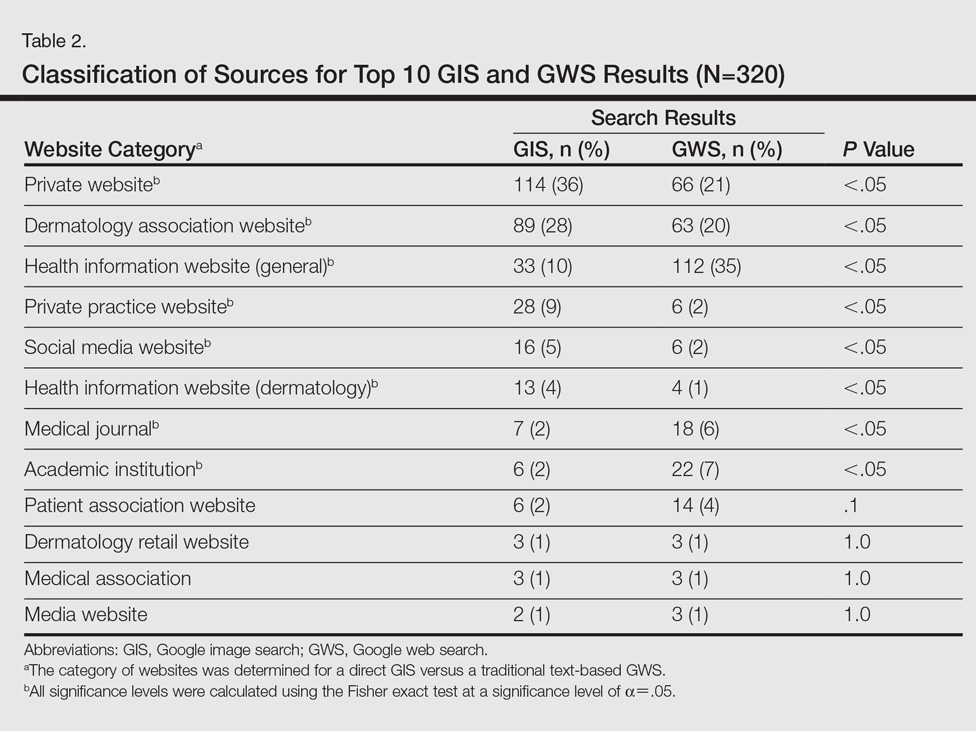
A total of 320 images were analyzed. In the GIS, private websites (36%), dermatology association websites (28%), and general health information websites (10%) were the 3 most common sources. In the GWS, health information websites (35%), private websites (21%), and dermatology association websites (20%) accounted for the most common sources (Table 2). The majority of images were of Fitzpatrick skin types I and II (89%) and nearly all images were diagnostically accurate (98%). There was no statistically significant difference in accuracy of diagnosis between physician-associated websites (100% accuracy) versus nonphysician-associated sites (98% accuracy, P=.25).
Our results showed high diagnostic accuracy among the top GIS results for common dermatology search terms. Diagnostic accuracy did not vary between websites that were physician associated versus those that were not. Our results are comparable to the reported accuracy of online dermatologic health information.1 In GIS results, the majority of images were provided by private websites, whereas the top websites in GWS results were health information websites.
Only 1% of images were of Fitzpatrick skin types VI and VII. Presentation of skin diseases is remarkably different based on the patient’s skin type.5 The shortage of readily accessible images of skin of color is in line with the lack of familiarity physicians and trainees have with dermatologic conditions in ethnic skin.6
Based on the results from this analysis, providers and patients searching for dermatologic conditions via a direct GIS should be cognizant of several considerations. Although our results showed that GIS was accurate, the searcher should note that image-based searches are not accompanied by relevant text that can help confirm relevancy and accuracy. Image searches depend on textual tags added by the source website. Websites that represent dermatological associations and academic centers can add an additional layer of confidence for users. Patients and clinicians also should be aware that the consideration of a patient’s Fitzpatrick skin type is critical when assessing the relevancy of a GIS result. In conclusion, search results via GIS queries are accurate for the dermatological diagnoses tested but may be lacking in skin of color variations, suggesting a potential unmet need based on our growing ethnic skin population.
- Jensen JD, Dunnick CA, Arbuckle HA, et al. Dermatology information on the Internet: an appraisal by dermatologists and dermatology residents. J Am Acad Dermatol. 2010;63:1101-1103.
- Cutrone M, Grimalt R. Dermatological image search engines on the Internet: do they work? J Eur Acad Dermatol Venereol. 2007;21:175-177.
- Xu S, Nault A, Bhatia A. Search and engagement analysis of association websites representing dermatologists—implications and opportunities for web visibility and patient education: website rankings of dermatology associations. Pract Dermatol. In press.
- comScore releases July 2015 U.S. desktop search engine rankings [press release]. Reston, VA: comScore, Inc; August 14, 2015. http://www.comscore.com/Insights/Market-Rankings/comScore-Releases-July-2015-U.S.-Desktop-Search-Engine-Rankings. Accessed October 18, 2016.
- Kundu RV, Patterson S. Dermatologic conditions in skin of color: part I. special considerations for common skin disorders. Am Fam Physician. 2013;87:850-856.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
To the Editor:
Prior studies have assessed the quality of text-based dermatology information on the Internet using traditional search engine queries.1 However, little is understood about the sources, accuracy, and quality of online dermatology images derived from direct image searches. Previous work has shown that direct search engine image queries were largely accurate for 3 pediatric dermatology diagnosis searches: atopic dermatitis, lichen striatus, and subcutaneous fat necrosis.2 We assessed images obtained for common dermatologic conditions from a Google image search (GIS) compared to a traditional text-based Google web search (GWS).
Image results for 32 unique dermatologic search terms were analyzed (Table 1). These search terms were selected using the results of a prior study that identified the most common dermatologic diagnoses that led users to the 2 most popular dermatology-specific websites worldwide: the American Academy of Dermatology (www.aad.org) and DermNet New Zealand (www.dermnetnz.org).3 The Alexa directory (www.alexa.com), a large publicly available Internet analytics resource, was used to determine the most common dermatology search terms that led a user to either www.dermnetnz.org or www.aad.org. In addition, searches for the 3 most common types of skin cancer—melanoma, squamous cell carcinoma, and basal cell carcinoma—were included. Each term was entered into a GIS and a GWS. The first 10 results, which represent 92% of the websites ultimately visited by users,4 were analyzed. The source, diagnostic accuracy, and Fitzpatrick skin type of the images was determined. Website sources were organized into 11 categories. All data collection occurred within a 1-week period in August 2015.
A total of 320 images were analyzed. In the GIS, private websites (36%), dermatology association websites (28%), and general health information websites (10%) were the 3 most common sources. In the GWS, health information websites (35%), private websites (21%), and dermatology association websites (20%) accounted for the most common sources (Table 2). The majority of images were of Fitzpatrick skin types I and II (89%) and nearly all images were diagnostically accurate (98%). There was no statistically significant difference in accuracy of diagnosis between physician-associated websites (100% accuracy) versus nonphysician-associated sites (98% accuracy, P=.25).
Our results showed high diagnostic accuracy among the top GIS results for common dermatology search terms. Diagnostic accuracy did not vary between websites that were physician associated versus those that were not. Our results are comparable to the reported accuracy of online dermatologic health information.1 In GIS results, the majority of images were provided by private websites, whereas the top websites in GWS results were health information websites.
Only 1% of images were of Fitzpatrick skin types VI and VII. Presentation of skin diseases is remarkably different based on the patient’s skin type.5 The shortage of readily accessible images of skin of color is in line with the lack of familiarity physicians and trainees have with dermatologic conditions in ethnic skin.6
Based on the results from this analysis, providers and patients searching for dermatologic conditions via a direct GIS should be cognizant of several considerations. Although our results showed that GIS was accurate, the searcher should note that image-based searches are not accompanied by relevant text that can help confirm relevancy and accuracy. Image searches depend on textual tags added by the source website. Websites that represent dermatological associations and academic centers can add an additional layer of confidence for users. Patients and clinicians also should be aware that the consideration of a patient’s Fitzpatrick skin type is critical when assessing the relevancy of a GIS result. In conclusion, search results via GIS queries are accurate for the dermatological diagnoses tested but may be lacking in skin of color variations, suggesting a potential unmet need based on our growing ethnic skin population.
To the Editor:
Prior studies have assessed the quality of text-based dermatology information on the Internet using traditional search engine queries.1 However, little is understood about the sources, accuracy, and quality of online dermatology images derived from direct image searches. Previous work has shown that direct search engine image queries were largely accurate for 3 pediatric dermatology diagnosis searches: atopic dermatitis, lichen striatus, and subcutaneous fat necrosis.2 We assessed images obtained for common dermatologic conditions from a Google image search (GIS) compared to a traditional text-based Google web search (GWS).
Image results for 32 unique dermatologic search terms were analyzed (Table 1). These search terms were selected using the results of a prior study that identified the most common dermatologic diagnoses that led users to the 2 most popular dermatology-specific websites worldwide: the American Academy of Dermatology (www.aad.org) and DermNet New Zealand (www.dermnetnz.org).3 The Alexa directory (www.alexa.com), a large publicly available Internet analytics resource, was used to determine the most common dermatology search terms that led a user to either www.dermnetnz.org or www.aad.org. In addition, searches for the 3 most common types of skin cancer—melanoma, squamous cell carcinoma, and basal cell carcinoma—were included. Each term was entered into a GIS and a GWS. The first 10 results, which represent 92% of the websites ultimately visited by users,4 were analyzed. The source, diagnostic accuracy, and Fitzpatrick skin type of the images was determined. Website sources were organized into 11 categories. All data collection occurred within a 1-week period in August 2015.
A total of 320 images were analyzed. In the GIS, private websites (36%), dermatology association websites (28%), and general health information websites (10%) were the 3 most common sources. In the GWS, health information websites (35%), private websites (21%), and dermatology association websites (20%) accounted for the most common sources (Table 2). The majority of images were of Fitzpatrick skin types I and II (89%) and nearly all images were diagnostically accurate (98%). There was no statistically significant difference in accuracy of diagnosis between physician-associated websites (100% accuracy) versus nonphysician-associated sites (98% accuracy, P=.25).
Our results showed high diagnostic accuracy among the top GIS results for common dermatology search terms. Diagnostic accuracy did not vary between websites that were physician associated versus those that were not. Our results are comparable to the reported accuracy of online dermatologic health information.1 In GIS results, the majority of images were provided by private websites, whereas the top websites in GWS results were health information websites.
Only 1% of images were of Fitzpatrick skin types VI and VII. Presentation of skin diseases is remarkably different based on the patient’s skin type.5 The shortage of readily accessible images of skin of color is in line with the lack of familiarity physicians and trainees have with dermatologic conditions in ethnic skin.6
Based on the results from this analysis, providers and patients searching for dermatologic conditions via a direct GIS should be cognizant of several considerations. Although our results showed that GIS was accurate, the searcher should note that image-based searches are not accompanied by relevant text that can help confirm relevancy and accuracy. Image searches depend on textual tags added by the source website. Websites that represent dermatological associations and academic centers can add an additional layer of confidence for users. Patients and clinicians also should be aware that the consideration of a patient’s Fitzpatrick skin type is critical when assessing the relevancy of a GIS result. In conclusion, search results via GIS queries are accurate for the dermatological diagnoses tested but may be lacking in skin of color variations, suggesting a potential unmet need based on our growing ethnic skin population.
- Jensen JD, Dunnick CA, Arbuckle HA, et al. Dermatology information on the Internet: an appraisal by dermatologists and dermatology residents. J Am Acad Dermatol. 2010;63:1101-1103.
- Cutrone M, Grimalt R. Dermatological image search engines on the Internet: do they work? J Eur Acad Dermatol Venereol. 2007;21:175-177.
- Xu S, Nault A, Bhatia A. Search and engagement analysis of association websites representing dermatologists—implications and opportunities for web visibility and patient education: website rankings of dermatology associations. Pract Dermatol. In press.
- comScore releases July 2015 U.S. desktop search engine rankings [press release]. Reston, VA: comScore, Inc; August 14, 2015. http://www.comscore.com/Insights/Market-Rankings/comScore-Releases-July-2015-U.S.-Desktop-Search-Engine-Rankings. Accessed October 18, 2016.
- Kundu RV, Patterson S. Dermatologic conditions in skin of color: part I. special considerations for common skin disorders. Am Fam Physician. 2013;87:850-856.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
- Jensen JD, Dunnick CA, Arbuckle HA, et al. Dermatology information on the Internet: an appraisal by dermatologists and dermatology residents. J Am Acad Dermatol. 2010;63:1101-1103.
- Cutrone M, Grimalt R. Dermatological image search engines on the Internet: do they work? J Eur Acad Dermatol Venereol. 2007;21:175-177.
- Xu S, Nault A, Bhatia A. Search and engagement analysis of association websites representing dermatologists—implications and opportunities for web visibility and patient education: website rankings of dermatology associations. Pract Dermatol. In press.
- comScore releases July 2015 U.S. desktop search engine rankings [press release]. Reston, VA: comScore, Inc; August 14, 2015. http://www.comscore.com/Insights/Market-Rankings/comScore-Releases-July-2015-U.S.-Desktop-Search-Engine-Rankings. Accessed October 18, 2016.
- Kundu RV, Patterson S. Dermatologic conditions in skin of color: part I. special considerations for common skin disorders. Am Fam Physician. 2013;87:850-856.
- Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol. 2008;59:615-618.
Practice Points
- Direct Google image searches largely deliver accurate results for common dermatological diagnoses.
- Greater effort should be made to include more publicly available images for dermatological diseases in darker skin types.
Nivolumab + ipilimumab induced fulminant, fatal myocarditis
Two patients taking the immune checkpoint inhibitors nivolumab and ipilimumab for metastatic melanoma developed fulminant, fatal myocarditis, investigators reported in the New England Journal of Medicine.
Even though this adverse effect is rare, “clinicians should be vigilant for immune-mediated myocarditis, particularly because of its early onset, nonspecific symptomatology, and fulminant progression,” said Douglas B. Johnson, MD, of Vanderbilt University Medical Center, Nashville, and his associates.
The first case involved a 65-year-old woman with no cardiac risk factors who was admitted to the hospital with chest pain, dyspnea, and fatigue 12 days after she received her first dose of the combination therapy. She was found to have myocarditis and myositis with rhabdomylysis. Despite treatment with high-dose glucocorticoids, she developed intraventricular conduction delay within 24 hours, followed by complete heart block. She died from multisystem organ failure and refractory ventricular tachycardia.
The second case involved a 63-year-old man with no cardiac risk factors who was admitted with fatigue and myalgias 15 days after he received his first dose of the combination therapy. He showed profound ST-segment depression, an intraventricular conduction delay, myocarditis, and myositis. He also was treated with high-dose glucocorticoids but developed complete heart block and died from cardiac arrest.
Both patients had “strikingly elevated troponin levels and refractory conduction-system abnormalities with preserved cardiac function,” the investigators noted. Postmortem assessments showed intense lymphocytic infiltrates only in striated cardiac and skeletal muscle and in metastases; adjacent smooth muscle and other tissues were unaffected. Pathology results “were reminiscent of those observed in patients with acute allograft rejection after cardiac transplantation,” Dr. Johnson and his associates said (N Engl J Med. 2016 Nov 3. doi: 10.1056/NEJMoa1609214).
To assess the frequency of myocarditis and myositis in patients receiving immune checkpoint inhibitors for many different cancers, the investigators searched Bristol-Myers Squibb safety databases. They found 18 drug-related cases of severe myocarditis among 20,594 patients, for a frequency of 0.09%. Patients who received combined nivolumab and ipilimumab had more frequent and more severe myocarditis than those who took either agent alone.
“There are no known data regarding what monitoring strategy may be of value; in our practice, we are performing baseline ECG and weekly testing of troponin levels during weeks 1-3 for patients receiving combination immunotherapy,” the researchers noted.
This work was supported by the Bready Family Foundation, the National Cancer Institute, Vanderbilt-Ingram Cancer Center Ambassadors, the Breast Cancer Specialized Program of Research Excellence, the National Comprehensive Cancer Network, the National Institutes of Health, the Howard Hughes Medical Institute, and Gilead Life Sciences. Dr. Johnson reported receiving personal fees from Genoptix and Bristol-Myers Squibb, and his associates reported ties to numerous industry sources.
Two patients taking the immune checkpoint inhibitors nivolumab and ipilimumab for metastatic melanoma developed fulminant, fatal myocarditis, investigators reported in the New England Journal of Medicine.
Even though this adverse effect is rare, “clinicians should be vigilant for immune-mediated myocarditis, particularly because of its early onset, nonspecific symptomatology, and fulminant progression,” said Douglas B. Johnson, MD, of Vanderbilt University Medical Center, Nashville, and his associates.
The first case involved a 65-year-old woman with no cardiac risk factors who was admitted to the hospital with chest pain, dyspnea, and fatigue 12 days after she received her first dose of the combination therapy. She was found to have myocarditis and myositis with rhabdomylysis. Despite treatment with high-dose glucocorticoids, she developed intraventricular conduction delay within 24 hours, followed by complete heart block. She died from multisystem organ failure and refractory ventricular tachycardia.
The second case involved a 63-year-old man with no cardiac risk factors who was admitted with fatigue and myalgias 15 days after he received his first dose of the combination therapy. He showed profound ST-segment depression, an intraventricular conduction delay, myocarditis, and myositis. He also was treated with high-dose glucocorticoids but developed complete heart block and died from cardiac arrest.
Both patients had “strikingly elevated troponin levels and refractory conduction-system abnormalities with preserved cardiac function,” the investigators noted. Postmortem assessments showed intense lymphocytic infiltrates only in striated cardiac and skeletal muscle and in metastases; adjacent smooth muscle and other tissues were unaffected. Pathology results “were reminiscent of those observed in patients with acute allograft rejection after cardiac transplantation,” Dr. Johnson and his associates said (N Engl J Med. 2016 Nov 3. doi: 10.1056/NEJMoa1609214).
To assess the frequency of myocarditis and myositis in patients receiving immune checkpoint inhibitors for many different cancers, the investigators searched Bristol-Myers Squibb safety databases. They found 18 drug-related cases of severe myocarditis among 20,594 patients, for a frequency of 0.09%. Patients who received combined nivolumab and ipilimumab had more frequent and more severe myocarditis than those who took either agent alone.
“There are no known data regarding what monitoring strategy may be of value; in our practice, we are performing baseline ECG and weekly testing of troponin levels during weeks 1-3 for patients receiving combination immunotherapy,” the researchers noted.
This work was supported by the Bready Family Foundation, the National Cancer Institute, Vanderbilt-Ingram Cancer Center Ambassadors, the Breast Cancer Specialized Program of Research Excellence, the National Comprehensive Cancer Network, the National Institutes of Health, the Howard Hughes Medical Institute, and Gilead Life Sciences. Dr. Johnson reported receiving personal fees from Genoptix and Bristol-Myers Squibb, and his associates reported ties to numerous industry sources.
Two patients taking the immune checkpoint inhibitors nivolumab and ipilimumab for metastatic melanoma developed fulminant, fatal myocarditis, investigators reported in the New England Journal of Medicine.
Even though this adverse effect is rare, “clinicians should be vigilant for immune-mediated myocarditis, particularly because of its early onset, nonspecific symptomatology, and fulminant progression,” said Douglas B. Johnson, MD, of Vanderbilt University Medical Center, Nashville, and his associates.
The first case involved a 65-year-old woman with no cardiac risk factors who was admitted to the hospital with chest pain, dyspnea, and fatigue 12 days after she received her first dose of the combination therapy. She was found to have myocarditis and myositis with rhabdomylysis. Despite treatment with high-dose glucocorticoids, she developed intraventricular conduction delay within 24 hours, followed by complete heart block. She died from multisystem organ failure and refractory ventricular tachycardia.
The second case involved a 63-year-old man with no cardiac risk factors who was admitted with fatigue and myalgias 15 days after he received his first dose of the combination therapy. He showed profound ST-segment depression, an intraventricular conduction delay, myocarditis, and myositis. He also was treated with high-dose glucocorticoids but developed complete heart block and died from cardiac arrest.
Both patients had “strikingly elevated troponin levels and refractory conduction-system abnormalities with preserved cardiac function,” the investigators noted. Postmortem assessments showed intense lymphocytic infiltrates only in striated cardiac and skeletal muscle and in metastases; adjacent smooth muscle and other tissues were unaffected. Pathology results “were reminiscent of those observed in patients with acute allograft rejection after cardiac transplantation,” Dr. Johnson and his associates said (N Engl J Med. 2016 Nov 3. doi: 10.1056/NEJMoa1609214).
To assess the frequency of myocarditis and myositis in patients receiving immune checkpoint inhibitors for many different cancers, the investigators searched Bristol-Myers Squibb safety databases. They found 18 drug-related cases of severe myocarditis among 20,594 patients, for a frequency of 0.09%. Patients who received combined nivolumab and ipilimumab had more frequent and more severe myocarditis than those who took either agent alone.
“There are no known data regarding what monitoring strategy may be of value; in our practice, we are performing baseline ECG and weekly testing of troponin levels during weeks 1-3 for patients receiving combination immunotherapy,” the researchers noted.
This work was supported by the Bready Family Foundation, the National Cancer Institute, Vanderbilt-Ingram Cancer Center Ambassadors, the Breast Cancer Specialized Program of Research Excellence, the National Comprehensive Cancer Network, the National Institutes of Health, the Howard Hughes Medical Institute, and Gilead Life Sciences. Dr. Johnson reported receiving personal fees from Genoptix and Bristol-Myers Squibb, and his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Two patients taking the immune checkpoint inhibitors nivolumab and ipilimumab for metastatic melanoma developed fulminant, fatal myocarditis.
Major finding: A search of Bristol-Myers Squibb safety databases found 18 drug-related cases of severe myocarditis among 20,594 patients, for a frequency of 0.09%.
Data source: Two case reports of a rare adverse effect of treatment with immune checkpoint inhibitors.
Disclosures: This work was supported by the Bready Family Foundation, the National Cancer Institute, Vanderbilt-Ingram Cancer Center Ambassadors, the Breast Cancer Specialized Program of Research Excellence, the National Comprehensive Cancer Network, the National Institutes of Health, the Howard Hughes Medical Institute, and Gilead Life Sciences. Dr. Johnson reported receiving personal fees from Genoptix and Bristol-Myers Squibb, and his associates reported ties to numerous industry sources.
Cancer survivors report two times greater medication use for anxiety and depression
Approximately 20% of adult cancer survivors in the United States – roughly 2.5 million – take medication for anxiety or depression, a rate that is approximately twice that of the general population, according to a report published online in Journal of Clinical Oncology.
Considering that previous research reported that more than 30% of cancer survivors discuss psychosocial concerns with their medical providers, this finding suggests that “even more survivors might benefit from pharmacologic treatment than were receiving treatment at the time of this study,” said Nikki A. Hawkins, PhD, of the Centers for Disease Control and Prevention, and her associates.
“If left unaddressed and untreated, anxiety and depression have been found to negatively affect health behaviors, the body’s inflammatory response, and even survival.” Yet rates of medication use have not been examined until now, the investigators noted.
Dr. Hawkins and her associates analyzed data from the National Health Interview Surveys for 2010 through 2013 to determine population-based prevalence rates. Their study population comprised a nationally representative sample of 48,181 adults, of whom 3,184 were cancer survivors.
Compared with the general population, cancer survivors were approximately twice as likely to self-report taking medication for anxiety (16.8% vs 8.6%), depression (14.1% vs 7.8%), both conditions (11.8% vs 6.1%), and one or both conditions combined (19.1% vs 10.3%). When these results were extrapolated to the entire country, an estimated 2.5 million cancer survivors were found to currently use these medications, the investigators reported (J Clin Oncol. 2016 Oct 26. doi: 10.1200/JCO.2016.67.7690).
“Interestingly, medication use did not vary significantly by time since cancer diagnosis, which is consistent with recent research that has shown elevated rates of depression and mental disorders for cancer survivors as much as 10 years after diagnosis,” they wrote.
The highest rates (greater than 20%) of antianxiety and antidepressant use occurred among patients who were middle aged (those aged 40-64 years), had never married, had three or more chronic health conditions, expected to have a short survival time, or had ovarian or uterine cancer.
Nine types of cancer were included in this study: breast, prostate, melanoma, cervical, colorectal, hematologic, ovarian/uterine, “short survival,” and other. Of these, patients with prostate cancer were the least likely to use antianxiety or antidepressant medications, and patients with ovarian/uterine and short survival cancers were the most likely to.
“Efforts to improve the psychosocial care of cancer survivors will be aided by continued tracking of the treatment received for mental health. Good medical care requires systematic evaluation, screening for new problems, and making adjustments to the prescribed therapies as needed, and survivors’ mental health deserves the same detailed, evidence-based, and ongoing attention,” Dr. Hawkins and her associates said.
This study was supported by the Centers for Disease Control and Prevention. Dr. Hawkins and her associates reported having no relevant financial disclosures.
Approximately 20% of adult cancer survivors in the United States – roughly 2.5 million – take medication for anxiety or depression, a rate that is approximately twice that of the general population, according to a report published online in Journal of Clinical Oncology.
Considering that previous research reported that more than 30% of cancer survivors discuss psychosocial concerns with their medical providers, this finding suggests that “even more survivors might benefit from pharmacologic treatment than were receiving treatment at the time of this study,” said Nikki A. Hawkins, PhD, of the Centers for Disease Control and Prevention, and her associates.
“If left unaddressed and untreated, anxiety and depression have been found to negatively affect health behaviors, the body’s inflammatory response, and even survival.” Yet rates of medication use have not been examined until now, the investigators noted.
Dr. Hawkins and her associates analyzed data from the National Health Interview Surveys for 2010 through 2013 to determine population-based prevalence rates. Their study population comprised a nationally representative sample of 48,181 adults, of whom 3,184 were cancer survivors.
Compared with the general population, cancer survivors were approximately twice as likely to self-report taking medication for anxiety (16.8% vs 8.6%), depression (14.1% vs 7.8%), both conditions (11.8% vs 6.1%), and one or both conditions combined (19.1% vs 10.3%). When these results were extrapolated to the entire country, an estimated 2.5 million cancer survivors were found to currently use these medications, the investigators reported (J Clin Oncol. 2016 Oct 26. doi: 10.1200/JCO.2016.67.7690).
“Interestingly, medication use did not vary significantly by time since cancer diagnosis, which is consistent with recent research that has shown elevated rates of depression and mental disorders for cancer survivors as much as 10 years after diagnosis,” they wrote.
The highest rates (greater than 20%) of antianxiety and antidepressant use occurred among patients who were middle aged (those aged 40-64 years), had never married, had three or more chronic health conditions, expected to have a short survival time, or had ovarian or uterine cancer.
Nine types of cancer were included in this study: breast, prostate, melanoma, cervical, colorectal, hematologic, ovarian/uterine, “short survival,” and other. Of these, patients with prostate cancer were the least likely to use antianxiety or antidepressant medications, and patients with ovarian/uterine and short survival cancers were the most likely to.
“Efforts to improve the psychosocial care of cancer survivors will be aided by continued tracking of the treatment received for mental health. Good medical care requires systematic evaluation, screening for new problems, and making adjustments to the prescribed therapies as needed, and survivors’ mental health deserves the same detailed, evidence-based, and ongoing attention,” Dr. Hawkins and her associates said.
This study was supported by the Centers for Disease Control and Prevention. Dr. Hawkins and her associates reported having no relevant financial disclosures.
Approximately 20% of adult cancer survivors in the United States – roughly 2.5 million – take medication for anxiety or depression, a rate that is approximately twice that of the general population, according to a report published online in Journal of Clinical Oncology.
Considering that previous research reported that more than 30% of cancer survivors discuss psychosocial concerns with their medical providers, this finding suggests that “even more survivors might benefit from pharmacologic treatment than were receiving treatment at the time of this study,” said Nikki A. Hawkins, PhD, of the Centers for Disease Control and Prevention, and her associates.
“If left unaddressed and untreated, anxiety and depression have been found to negatively affect health behaviors, the body’s inflammatory response, and even survival.” Yet rates of medication use have not been examined until now, the investigators noted.
Dr. Hawkins and her associates analyzed data from the National Health Interview Surveys for 2010 through 2013 to determine population-based prevalence rates. Their study population comprised a nationally representative sample of 48,181 adults, of whom 3,184 were cancer survivors.
Compared with the general population, cancer survivors were approximately twice as likely to self-report taking medication for anxiety (16.8% vs 8.6%), depression (14.1% vs 7.8%), both conditions (11.8% vs 6.1%), and one or both conditions combined (19.1% vs 10.3%). When these results were extrapolated to the entire country, an estimated 2.5 million cancer survivors were found to currently use these medications, the investigators reported (J Clin Oncol. 2016 Oct 26. doi: 10.1200/JCO.2016.67.7690).
“Interestingly, medication use did not vary significantly by time since cancer diagnosis, which is consistent with recent research that has shown elevated rates of depression and mental disorders for cancer survivors as much as 10 years after diagnosis,” they wrote.
The highest rates (greater than 20%) of antianxiety and antidepressant use occurred among patients who were middle aged (those aged 40-64 years), had never married, had three or more chronic health conditions, expected to have a short survival time, or had ovarian or uterine cancer.
Nine types of cancer were included in this study: breast, prostate, melanoma, cervical, colorectal, hematologic, ovarian/uterine, “short survival,” and other. Of these, patients with prostate cancer were the least likely to use antianxiety or antidepressant medications, and patients with ovarian/uterine and short survival cancers were the most likely to.
“Efforts to improve the psychosocial care of cancer survivors will be aided by continued tracking of the treatment received for mental health. Good medical care requires systematic evaluation, screening for new problems, and making adjustments to the prescribed therapies as needed, and survivors’ mental health deserves the same detailed, evidence-based, and ongoing attention,” Dr. Hawkins and her associates said.
This study was supported by the Centers for Disease Control and Prevention. Dr. Hawkins and her associates reported having no relevant financial disclosures.
Key clinical point: Cancer survivors take medications for anxiety and depression at approximately double the rate in the general population.
Major finding: Compared with the general population, cancer survivors were approximately twice as likely to self-report taking medication for anxiety (16.8% vs 8.6%), depression (14.1% vs 7.8%), both conditions (11.8% vs 6.1%), and one or both conditions combined (19.1% vs 10.3%).
Data source: A cross-sectional analysis of data from nationwide surveys of 48,181 adults, including 3,184 cancer survivors, during a 4-year period.
Disclosures: This study was supported by the Centers for Disease Control and Prevention. Dr. Hawkins and her associates reported having no relevant financial disclosures.
Evaluating the Malignant Potential of Nevus Spilus
PD-L1 positivity correlated with pembrolizumab response in advanced melanoma
Programmed death ligand–1 expression correlated positively and significantly with pembrolizumab response in advanced melanoma, based on an analysis of 405 patients from the international, multicohort, open-label phase I KEYNOTE-001 trial.
Among patients for whom 33%-65% of tumor cells expressed PD-L1, the objective rate of response was 57%, but the rate was only 8% among patients whose specimens did not express PD-L1, Adil Daud, MD, of the University of California, San Francisco, and associates reported. Taken together, the findings suggest that melanoma is most likely to respond to pembrolizumab when specimens show at least 10% positivity, the investigators reported (J Clin Oncol. 2016 Oct 10. doi: 10.1200/JCO.2016.67.2477).
Among the 405 patients who also were evaluable for tumor response, the overall objective response rate was 33% (95% confidence interval, 28%-37%). Grouping patients based on the melanoma scoring system for PD-L1 expression showed that PD-L1 positivity correlated significantly with the objective response rate (P less than .001). Furthermore, a higher PD-L1 melanoma score correlated significantly with both progression-free survival (hazard ratio, 0.76; 95% CI, 0.7-0.82) and overall survival (HR, 0.76; 95% CI, 0.69-0.83), with P values less than .001 for each association.
Median progression-free survival was 5.6 months in PD-L1–positive patients and 2.8 months in PD-L1–negative patients, while median overall survival was 30 months in PD-L1–positive patients and 12.6 months in PD-L1–negative patients, the researchers reported. “The high prevalence of PD-L1 positivity observed in this study, along with the durable responses observed in PD-L1–negative tumors, suggest that pembrolizumab treatment should not be limited to patients with PD-L1–positive tumors,” they concluded. “Ongoing clinical trials with correlative studies will further delineate the role of PD-L1 expression in melanoma.”
Merck sponsored the trial. Dr. Daud disclosed ties to Merck, OncoSec, Novartis, Genentech, Bristol-Myers Squibb, and Array BioPharma.
Programmed death ligand–1 expression correlated positively and significantly with pembrolizumab response in advanced melanoma, based on an analysis of 405 patients from the international, multicohort, open-label phase I KEYNOTE-001 trial.
Among patients for whom 33%-65% of tumor cells expressed PD-L1, the objective rate of response was 57%, but the rate was only 8% among patients whose specimens did not express PD-L1, Adil Daud, MD, of the University of California, San Francisco, and associates reported. Taken together, the findings suggest that melanoma is most likely to respond to pembrolizumab when specimens show at least 10% positivity, the investigators reported (J Clin Oncol. 2016 Oct 10. doi: 10.1200/JCO.2016.67.2477).
Among the 405 patients who also were evaluable for tumor response, the overall objective response rate was 33% (95% confidence interval, 28%-37%). Grouping patients based on the melanoma scoring system for PD-L1 expression showed that PD-L1 positivity correlated significantly with the objective response rate (P less than .001). Furthermore, a higher PD-L1 melanoma score correlated significantly with both progression-free survival (hazard ratio, 0.76; 95% CI, 0.7-0.82) and overall survival (HR, 0.76; 95% CI, 0.69-0.83), with P values less than .001 for each association.
Median progression-free survival was 5.6 months in PD-L1–positive patients and 2.8 months in PD-L1–negative patients, while median overall survival was 30 months in PD-L1–positive patients and 12.6 months in PD-L1–negative patients, the researchers reported. “The high prevalence of PD-L1 positivity observed in this study, along with the durable responses observed in PD-L1–negative tumors, suggest that pembrolizumab treatment should not be limited to patients with PD-L1–positive tumors,” they concluded. “Ongoing clinical trials with correlative studies will further delineate the role of PD-L1 expression in melanoma.”
Merck sponsored the trial. Dr. Daud disclosed ties to Merck, OncoSec, Novartis, Genentech, Bristol-Myers Squibb, and Array BioPharma.
Programmed death ligand–1 expression correlated positively and significantly with pembrolizumab response in advanced melanoma, based on an analysis of 405 patients from the international, multicohort, open-label phase I KEYNOTE-001 trial.
Among patients for whom 33%-65% of tumor cells expressed PD-L1, the objective rate of response was 57%, but the rate was only 8% among patients whose specimens did not express PD-L1, Adil Daud, MD, of the University of California, San Francisco, and associates reported. Taken together, the findings suggest that melanoma is most likely to respond to pembrolizumab when specimens show at least 10% positivity, the investigators reported (J Clin Oncol. 2016 Oct 10. doi: 10.1200/JCO.2016.67.2477).
Among the 405 patients who also were evaluable for tumor response, the overall objective response rate was 33% (95% confidence interval, 28%-37%). Grouping patients based on the melanoma scoring system for PD-L1 expression showed that PD-L1 positivity correlated significantly with the objective response rate (P less than .001). Furthermore, a higher PD-L1 melanoma score correlated significantly with both progression-free survival (hazard ratio, 0.76; 95% CI, 0.7-0.82) and overall survival (HR, 0.76; 95% CI, 0.69-0.83), with P values less than .001 for each association.
Median progression-free survival was 5.6 months in PD-L1–positive patients and 2.8 months in PD-L1–negative patients, while median overall survival was 30 months in PD-L1–positive patients and 12.6 months in PD-L1–negative patients, the researchers reported. “The high prevalence of PD-L1 positivity observed in this study, along with the durable responses observed in PD-L1–negative tumors, suggest that pembrolizumab treatment should not be limited to patients with PD-L1–positive tumors,” they concluded. “Ongoing clinical trials with correlative studies will further delineate the role of PD-L1 expression in melanoma.”
Merck sponsored the trial. Dr. Daud disclosed ties to Merck, OncoSec, Novartis, Genentech, Bristol-Myers Squibb, and Array BioPharma.
Key clinical point: PD-L1 expression correlates with pembrolizumab response in advanced melanoma.
Major finding: Higher levels of PD-L1 staining correlated positively with tumor response (P less than .001).
Data source: Analyses of 405 patients from KEYNOTE-001, an international, multicohort, open-label phase I trial of pembrolizumab in advanced melanoma.
Disclosures: Merck sponsored the trial. Dr. Daud disclosed ties to Merck, OncoSec, Novartis, Genentech, Bristol-Myers Squibb, and Array BioPharma.
Survival benefit maintained long term with ipilimumab for high-risk melanoma
COPENHAGEN – Five years on, patients with high-risk stage III melanoma treated with the checkpoint inhibitor ipilimumab, following complete resection, continue to have significantly better overall, recurrence-free, and distant metastasis–free survival, compared with patients treated with placebo, reported investigators in a phase III trial.
Five-year overall survival among patients who received a 10-mg/kg dose of ipilimumab (Yervoy) in the EORTC 18071 trial was 65.4%, compared with 54.4% for patients who received placebo. This difference translated into a hazard ratio for death with ipilimumab of 0.72 (P = .001), reported Alexander M.M. Eggermont, MD, from the Gustave Roussy Cancer Center in Villejuif, France.
The survival benefit with ipilimumab “was consistent across all survival endpoints,” he said at a briefing prior to his presentation of the data in a symposium at the European Society for Medical Oncology Congress.
He noted, however, that the 10-mg/kg dose of ipilimumab selected in phase II trials is associated with significant toxicities.
“Ipilimumab is not an easy drug to handle. My recommendation is to keep it in [cancer] centers,” he said.
In the trial, 951 patients with high-risk, stage III, completely resected melanoma were randomly assigned to receive induction therapy with ipilimumab 10 mg/kg every 3 weeks for four cycles or placebo, followed by maintenance with the assigned therapy every 12 weeks for up to 3 years.
The investigators previously reported that, at a median follow-up of 2.7 years, ipilimumab was associated with significantly prolonged overall survival (the primary endpoint), with a hazard ratio vs. placebo of 0.75 (P = .001).
At ESMO 2016, Dr. Eggermont reported final survival results from the trial, at a median follow-up of 5.3 years.
The rate of 5-year overall survival for the 475 patients assigned to ipilimumab was 65.4%, compared with 54.4% among the 476 patients assigned to placebo (HR, 0.72, P = .001).
At 5 years, 41% of patients assigned to ipilimumab were free of recurrences, compared with 30% of patients on placebo, The median recurrence-free survival was 27.6 months vs. 17.1 months, respectively (HR, 0.76, P = .0008).
Similarly, the rate of distant metastasis-free survival at 5 years was 48.3% for patients assigned to ipilimumab, vs. 38.9% in the placebo group (HR for death or distant metastasis, 0.76; P = .002).
The safety analysis showed that twice as many patients assigned to ipilimumab had grade 3 or 4 adverse events (54.1% vs. 26.2%). Grade 3 or 4 adverse immune events occurred in 41.6% vs. 2.7%, respectively.
Five patients assigned to ipilimumab died from immune-related causes: three from colitis (two of whom had intestinal perforations), one from myocarditis, and one from multiorgan failure associated with Guillain-Barré syndrome.
“The final analysis shows, for the first time, that checkpoint blockade is effective in the adjuvant setting,” he said.
The data suggest, however, that the benefit appears to be concentrated in patients with higher-risk features, such as involvement of four or more lymph nodes, microscopic nodal disease, or ulceration, he said.
The discussant also agreed with Dr. Eggermont’s assertions that the decision to treat patients with ipilimumab should factor in toxicity, and that treatment should be administered only in centers experienced in using the drug.
The trial was sponsored by Bristol-Myers Squibb. Dr. Eggermont disclosed serving on an advisory board for Bristol-Myers Squibb and Merck. Dr. Michielin disclosed consulting and/or honoraria from Amgen, Bristol-Myers Squibb, Roche, Merck Sharp & Dohme, Novartis, and GlaxoSmithKline.
COPENHAGEN – Five years on, patients with high-risk stage III melanoma treated with the checkpoint inhibitor ipilimumab, following complete resection, continue to have significantly better overall, recurrence-free, and distant metastasis–free survival, compared with patients treated with placebo, reported investigators in a phase III trial.
Five-year overall survival among patients who received a 10-mg/kg dose of ipilimumab (Yervoy) in the EORTC 18071 trial was 65.4%, compared with 54.4% for patients who received placebo. This difference translated into a hazard ratio for death with ipilimumab of 0.72 (P = .001), reported Alexander M.M. Eggermont, MD, from the Gustave Roussy Cancer Center in Villejuif, France.
The survival benefit with ipilimumab “was consistent across all survival endpoints,” he said at a briefing prior to his presentation of the data in a symposium at the European Society for Medical Oncology Congress.
He noted, however, that the 10-mg/kg dose of ipilimumab selected in phase II trials is associated with significant toxicities.
“Ipilimumab is not an easy drug to handle. My recommendation is to keep it in [cancer] centers,” he said.
In the trial, 951 patients with high-risk, stage III, completely resected melanoma were randomly assigned to receive induction therapy with ipilimumab 10 mg/kg every 3 weeks for four cycles or placebo, followed by maintenance with the assigned therapy every 12 weeks for up to 3 years.
The investigators previously reported that, at a median follow-up of 2.7 years, ipilimumab was associated with significantly prolonged overall survival (the primary endpoint), with a hazard ratio vs. placebo of 0.75 (P = .001).
At ESMO 2016, Dr. Eggermont reported final survival results from the trial, at a median follow-up of 5.3 years.
The rate of 5-year overall survival for the 475 patients assigned to ipilimumab was 65.4%, compared with 54.4% among the 476 patients assigned to placebo (HR, 0.72, P = .001).
At 5 years, 41% of patients assigned to ipilimumab were free of recurrences, compared with 30% of patients on placebo, The median recurrence-free survival was 27.6 months vs. 17.1 months, respectively (HR, 0.76, P = .0008).
Similarly, the rate of distant metastasis-free survival at 5 years was 48.3% for patients assigned to ipilimumab, vs. 38.9% in the placebo group (HR for death or distant metastasis, 0.76; P = .002).
The safety analysis showed that twice as many patients assigned to ipilimumab had grade 3 or 4 adverse events (54.1% vs. 26.2%). Grade 3 or 4 adverse immune events occurred in 41.6% vs. 2.7%, respectively.
Five patients assigned to ipilimumab died from immune-related causes: three from colitis (two of whom had intestinal perforations), one from myocarditis, and one from multiorgan failure associated with Guillain-Barré syndrome.
“The final analysis shows, for the first time, that checkpoint blockade is effective in the adjuvant setting,” he said.
The data suggest, however, that the benefit appears to be concentrated in patients with higher-risk features, such as involvement of four or more lymph nodes, microscopic nodal disease, or ulceration, he said.
The discussant also agreed with Dr. Eggermont’s assertions that the decision to treat patients with ipilimumab should factor in toxicity, and that treatment should be administered only in centers experienced in using the drug.
The trial was sponsored by Bristol-Myers Squibb. Dr. Eggermont disclosed serving on an advisory board for Bristol-Myers Squibb and Merck. Dr. Michielin disclosed consulting and/or honoraria from Amgen, Bristol-Myers Squibb, Roche, Merck Sharp & Dohme, Novartis, and GlaxoSmithKline.
COPENHAGEN – Five years on, patients with high-risk stage III melanoma treated with the checkpoint inhibitor ipilimumab, following complete resection, continue to have significantly better overall, recurrence-free, and distant metastasis–free survival, compared with patients treated with placebo, reported investigators in a phase III trial.
Five-year overall survival among patients who received a 10-mg/kg dose of ipilimumab (Yervoy) in the EORTC 18071 trial was 65.4%, compared with 54.4% for patients who received placebo. This difference translated into a hazard ratio for death with ipilimumab of 0.72 (P = .001), reported Alexander M.M. Eggermont, MD, from the Gustave Roussy Cancer Center in Villejuif, France.
The survival benefit with ipilimumab “was consistent across all survival endpoints,” he said at a briefing prior to his presentation of the data in a symposium at the European Society for Medical Oncology Congress.
He noted, however, that the 10-mg/kg dose of ipilimumab selected in phase II trials is associated with significant toxicities.
“Ipilimumab is not an easy drug to handle. My recommendation is to keep it in [cancer] centers,” he said.
In the trial, 951 patients with high-risk, stage III, completely resected melanoma were randomly assigned to receive induction therapy with ipilimumab 10 mg/kg every 3 weeks for four cycles or placebo, followed by maintenance with the assigned therapy every 12 weeks for up to 3 years.
The investigators previously reported that, at a median follow-up of 2.7 years, ipilimumab was associated with significantly prolonged overall survival (the primary endpoint), with a hazard ratio vs. placebo of 0.75 (P = .001).
At ESMO 2016, Dr. Eggermont reported final survival results from the trial, at a median follow-up of 5.3 years.
The rate of 5-year overall survival for the 475 patients assigned to ipilimumab was 65.4%, compared with 54.4% among the 476 patients assigned to placebo (HR, 0.72, P = .001).
At 5 years, 41% of patients assigned to ipilimumab were free of recurrences, compared with 30% of patients on placebo, The median recurrence-free survival was 27.6 months vs. 17.1 months, respectively (HR, 0.76, P = .0008).
Similarly, the rate of distant metastasis-free survival at 5 years was 48.3% for patients assigned to ipilimumab, vs. 38.9% in the placebo group (HR for death or distant metastasis, 0.76; P = .002).
The safety analysis showed that twice as many patients assigned to ipilimumab had grade 3 or 4 adverse events (54.1% vs. 26.2%). Grade 3 or 4 adverse immune events occurred in 41.6% vs. 2.7%, respectively.
Five patients assigned to ipilimumab died from immune-related causes: three from colitis (two of whom had intestinal perforations), one from myocarditis, and one from multiorgan failure associated with Guillain-Barré syndrome.
“The final analysis shows, for the first time, that checkpoint blockade is effective in the adjuvant setting,” he said.
The data suggest, however, that the benefit appears to be concentrated in patients with higher-risk features, such as involvement of four or more lymph nodes, microscopic nodal disease, or ulceration, he said.
The discussant also agreed with Dr. Eggermont’s assertions that the decision to treat patients with ipilimumab should factor in toxicity, and that treatment should be administered only in centers experienced in using the drug.
The trial was sponsored by Bristol-Myers Squibb. Dr. Eggermont disclosed serving on an advisory board for Bristol-Myers Squibb and Merck. Dr. Michielin disclosed consulting and/or honoraria from Amgen, Bristol-Myers Squibb, Roche, Merck Sharp & Dohme, Novartis, and GlaxoSmithKline.
Key clinical point: The CTLA-4 checkpoint inhibitor ipilimumab offers survival benefit, compared with placebo in patients with malignant melanoma.
Major finding: The hazard ratio for death with ipilimumab vs. placebo was 0.72 (P = .001).
Data source: Randomized, controlled, phase III trial in 951 patients with high-risk stage III malignant melanoma following complete resection.
Disclosures: The trial was sponsored by Bristol-Myers Squibb. Dr. Eggermont disclosed serving on an advisory board for Bristol-Myers Squibb and Merck. Dr. Michielin disclosed consulting and/or honoraria from Amgen, Bristol-Myers Squibb, Roche, Merck Sharp & Dohme, Novartis, and GlaxoSmithKline.