User login
New AJCC guidance brings melanoma staging changes
WAILEA, HAWAII – The Eighth Edition of the American Joint Committee on Cancer Staging Manual includes significant changes in how melanoma is classified.
The manual has already been published and is available for purchase. However, its implementation will be delayed until Jan. 1, 2018, to give physicians, software vendors, and all other interested parties time to get up to speed. All cancers newly diagnosed through Dec. 31, 2017 should be staged in accord with the seventh edition, released in 2010.
The eighth edition breaks new ground, moving beyond TNM (Tumor, Node, Metastasis) anatomic staging to incorporate new evidence-based prognostic factors.
“There are some subtle differences here to be aware of. It can be a little bit tricky at first glance. You should become familiar with this,” advised Dr. Marchetti, a dermatologist at Memorial Sloan-Kettering Cancer Center in New York.
In addition to highlighting the changes in melanoma staging included in the new AJCC manual, he outlined key recommendations – some of them controversial – on the use of sentinel lymph node biopsy (SLNB) in melanoma patients incorporated in the 2017 National Comprehensive Cancer Network (NCCN) guidelines.
The biggest change for the dermatology community contained in the new edition of the AJCC staging manual is that the T1 classification of melanoma has changed. In the seventh edition, a melanoma was categorized as T1 if less than or equal to 1.0 mm thickness. The cancer was T1a if nonulcerated and had a mitosis rate of less than 1/mm2 and T1b if ulcerated or had at least 1 mitosis/mm2.
The eighth edition makes an evidence-based subcategorization of T1 based upon thickness in light of the prognostic implications of this distinction. A melanoma is defined as T1a if nonulcerated and less than 0.8 mm in thickness, and T1b if it is 0.8-1.0 mm thick or less than 0.8 mm with ulceration.
Of note, tumor mitotic rate has been dropped as a staging criterion for T1 tumors.
What this means is, for example, in 2017, a patient with a 0.9-mm nonulcerated melanoma with 1 mitosis/mm2 and a negative sentinel lymph node biopsy with wide local excision is T1bN0M0, pathologic Stage IB. Under the eighth edition of AJCC, the same patient is T1bN0M0, pathologic Stage IA, because that mitosis rate isn’t a factor.
Today, a patient with a 0.5-mm melanoma with 1 mitosis/mm2 with wide local excision is T1bN0M0, Pathologic Stage IB. Under the new system, the same tumor is downstaged to Pathologic Stage IA, Dr. Marchetti explained.
In the eighth edition, tumor thickness measurements are recorded with rounding to the nearest 0.1 mm, not to the nearest 0.01 mm as before. This change was prompted by the inherent lack of precision in measuring melanomas, especially thicker ones.
The T category definitions of primary tumors have been clarified in the eighth edition. A tumor should be classified as T0 only if there is no evidence of a primary tumor. T is utilized for melanoma in situ. TX is employed when the primary tumor thickness can’t be determined, as for example when the biopsy specimen was obtained through curettage.
The N categorization of regional lymph node status has become much more complicated in the eighth edition, the dermatologist cautioned. Plus, the terminology for nodal disease has changed. The term micrometastasis has been replaced by “clinically occult disease” as detected by SLNB. Macrometastasis has been supplanted by “clinically detected disease.” And while in-transit or satellite node metastasis or microsatellite metastasis with satellite nodes was formerly listed simply as N3, in the new system there are subcategories for N3 based upon the number of metastatic nodes involved. For example, in the eighth edition, a melanoma is pathologic Stage N3a if there are four or more clinically occult regional lymph nodes and no in-transit, satellite, or matted nodes. Pathologic Stage N3b is shorthand for four or more tumor-involved regional lymph nodes, at least one of which was clinically detected, or any number of matted lymph nodes, with no in-transit or satellite nodal involvement. Stage N3c is reserved for melanomas with two or more clinically occult or clinically detected regional lymph nodes and/or any number of matted nodes, plus the presence of in-transit or satellite nodal metastasis.
As a result of the changes in the N classification, there are now four pathologic Stage III groups rather than three. Stages IIIA-C have been joined by pathologic Stage IIID, reserved for patients who are T4b, N3a, b, or c, and M0.
The M categorization of distant metastatic disease status has also become more elaborate. In the AJCC seventh edition, if serum lactate dehydrogenase (LDH) is elevated and a patient has any distant metastatic disease, that’s automatically category M1c. Not any longer, though.
Under the eighth edition, if a patient has distant metastasis to skin, soft tissue including muscle, and/or nonregional lymph nodes and the LDH is unspecified, the categorization is M1a. If serum LDH is not elevated, it’s M1a(0). If elevated, then M1a(1).
Similarly, for distant metastasis to the lung, the range of possibilities based upon LDH is M1b, M1b(0), and M1b(1). For distant metastasis to non-CNS visceral sites, the possibilities are M1c, M1c(0), and M1c(1).
M1d is a new classification, a clear departure from the seventh edition. It applies to patients with distant metastasis to the CNS. The classification is M1d if LDH isn’t recorded, M1d(0) if LDH isn’t elevated, and M1d(1) if it is.
Turning to the updated 2017 NCCN guidelines Version 1.2017 on the role of SLNB in melanoma, Dr. Marchetti noted that the procedure is not recommended in patients with melanoma in situ or Stage IA or IB disease 0.75 mm or less in thickness, regardless of features. Neither are routine imaging or lab tests. That’s because the pretest probability of a positive SLNB is so low, at around 3%.
For Clinicopathologic Stage IA disease, 0.76-1.0 mm in thickness with no ulceration and a mitotic rate of less than 1 per mm2, the guidelines recommend that physicians “discuss and consider” SLNB, which the available evidence suggests has roughly a 7% pretest probability of a positive result.
For Stage IB disease, 0.76-1.0 mm in thickness with ulceration or a mitotic rate of at least 1 per mm2, as well as for Stage IB or Stage II disease greater than 1.0 mm in thickness, with any feature, the language of the recommendation shifts to “discuss and offer” rather than “discuss and consider” SLNB, since various studies have reported pretest probabilities of a positive result as high as 35%.
“The rationale here for performing sentinel lymph node biopsy is primarily to acquire more staging information. Is it a perfect test? Absolutely not. But it’s the current standard of care in terms of providing additional information for staging,” according to Dr. Marchetti.
If the SLNB generates a positive result, by definition the patient now has Stage III melanoma. The NCCN guidelines recommend consideration of imaging to establish a baseline, and state further that the primary treatment is to discuss and offer complete lymph node dissection in order to control the regional nodal basin and because of a possible favorable impact on overall survival. But the question of a survival benefit has been controversial for many years, and it’s unlikely to be resolved soon, Dr. Marchetti predicted.
The final report from the National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial–1 (MSLT-1) concluded that patients with primary cutaneous melanomas 1.2 mm or more in thickness who were randomized to undergo SLNB and, if positive, immediate complete lymphadenectomy, fared significantly better in terms of 10-year disease-free survival, compared with those assigned to observation and lymphadenectomy in the event of nodal relapse (N Engl J Med. 2014 Feb 13;370[7]:599-609).
This conclusion has generated numerous letters to the editor from melanoma experts who took issue with the analysis and conclusion. To try to put the MSLT-1 results in perspective, Dr. Marchetti applied the results to a hypothetical cohort of 100 patients with intermediate-thickness melanomas of 1.2-3.5 mm undergoing SLNB.
Eighty of these patients would be true SLNB-negative for regional nodal disease. Five others would have a false-negative SLNB and would later develop clinically detectable nodal disease. Fifteen patients with a positive SLNB would undergo prompt complete lymph node dissection, of whom 12 or 13 would derive no mortality benefit at 10 years, assuming the MSLT-1 investigators are correct in their analysis.
“Two or three patients with a positive SLNB will derive mortality benefit at 10 years, but we have no way to identify who those people are from the original 100,” he said.
Since the MSLT-1 report, a phase III German multicenter randomized trial of 241 melanoma patients with a positive screening SLNB has reported results. The participants assigned to complete lymph node dissection didn’t differ in terms of 3-year overall survival, distant metastasis-free survival, or recurrence-free survival, compared with those assigned to observation and lymphadenectomy if nodal disease occurred (Lancet Oncol. 2016 Jun;17[6]:757-67). However, as the investigators noted, the study, known as DeCOG-SLT, was underpowered, and Dr. Marchetti’s view is that it can’t be considered definitive.
“Ultimately I don’t think we’ll have a definitive answer to this question until the final results of the MSLT-II trial in the fall of 2022,” he said.
The MSLT-II trial has the same design as DeCOG-SLT.
Dr. Marchetti reported having no financial conflicts of interest regarding his presentation.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – The Eighth Edition of the American Joint Committee on Cancer Staging Manual includes significant changes in how melanoma is classified.
The manual has already been published and is available for purchase. However, its implementation will be delayed until Jan. 1, 2018, to give physicians, software vendors, and all other interested parties time to get up to speed. All cancers newly diagnosed through Dec. 31, 2017 should be staged in accord with the seventh edition, released in 2010.
The eighth edition breaks new ground, moving beyond TNM (Tumor, Node, Metastasis) anatomic staging to incorporate new evidence-based prognostic factors.
“There are some subtle differences here to be aware of. It can be a little bit tricky at first glance. You should become familiar with this,” advised Dr. Marchetti, a dermatologist at Memorial Sloan-Kettering Cancer Center in New York.
In addition to highlighting the changes in melanoma staging included in the new AJCC manual, he outlined key recommendations – some of them controversial – on the use of sentinel lymph node biopsy (SLNB) in melanoma patients incorporated in the 2017 National Comprehensive Cancer Network (NCCN) guidelines.
The biggest change for the dermatology community contained in the new edition of the AJCC staging manual is that the T1 classification of melanoma has changed. In the seventh edition, a melanoma was categorized as T1 if less than or equal to 1.0 mm thickness. The cancer was T1a if nonulcerated and had a mitosis rate of less than 1/mm2 and T1b if ulcerated or had at least 1 mitosis/mm2.
The eighth edition makes an evidence-based subcategorization of T1 based upon thickness in light of the prognostic implications of this distinction. A melanoma is defined as T1a if nonulcerated and less than 0.8 mm in thickness, and T1b if it is 0.8-1.0 mm thick or less than 0.8 mm with ulceration.
Of note, tumor mitotic rate has been dropped as a staging criterion for T1 tumors.
What this means is, for example, in 2017, a patient with a 0.9-mm nonulcerated melanoma with 1 mitosis/mm2 and a negative sentinel lymph node biopsy with wide local excision is T1bN0M0, pathologic Stage IB. Under the eighth edition of AJCC, the same patient is T1bN0M0, pathologic Stage IA, because that mitosis rate isn’t a factor.
Today, a patient with a 0.5-mm melanoma with 1 mitosis/mm2 with wide local excision is T1bN0M0, Pathologic Stage IB. Under the new system, the same tumor is downstaged to Pathologic Stage IA, Dr. Marchetti explained.
In the eighth edition, tumor thickness measurements are recorded with rounding to the nearest 0.1 mm, not to the nearest 0.01 mm as before. This change was prompted by the inherent lack of precision in measuring melanomas, especially thicker ones.
The T category definitions of primary tumors have been clarified in the eighth edition. A tumor should be classified as T0 only if there is no evidence of a primary tumor. T is utilized for melanoma in situ. TX is employed when the primary tumor thickness can’t be determined, as for example when the biopsy specimen was obtained through curettage.
The N categorization of regional lymph node status has become much more complicated in the eighth edition, the dermatologist cautioned. Plus, the terminology for nodal disease has changed. The term micrometastasis has been replaced by “clinically occult disease” as detected by SLNB. Macrometastasis has been supplanted by “clinically detected disease.” And while in-transit or satellite node metastasis or microsatellite metastasis with satellite nodes was formerly listed simply as N3, in the new system there are subcategories for N3 based upon the number of metastatic nodes involved. For example, in the eighth edition, a melanoma is pathologic Stage N3a if there are four or more clinically occult regional lymph nodes and no in-transit, satellite, or matted nodes. Pathologic Stage N3b is shorthand for four or more tumor-involved regional lymph nodes, at least one of which was clinically detected, or any number of matted lymph nodes, with no in-transit or satellite nodal involvement. Stage N3c is reserved for melanomas with two or more clinically occult or clinically detected regional lymph nodes and/or any number of matted nodes, plus the presence of in-transit or satellite nodal metastasis.
As a result of the changes in the N classification, there are now four pathologic Stage III groups rather than three. Stages IIIA-C have been joined by pathologic Stage IIID, reserved for patients who are T4b, N3a, b, or c, and M0.
The M categorization of distant metastatic disease status has also become more elaborate. In the AJCC seventh edition, if serum lactate dehydrogenase (LDH) is elevated and a patient has any distant metastatic disease, that’s automatically category M1c. Not any longer, though.
Under the eighth edition, if a patient has distant metastasis to skin, soft tissue including muscle, and/or nonregional lymph nodes and the LDH is unspecified, the categorization is M1a. If serum LDH is not elevated, it’s M1a(0). If elevated, then M1a(1).
Similarly, for distant metastasis to the lung, the range of possibilities based upon LDH is M1b, M1b(0), and M1b(1). For distant metastasis to non-CNS visceral sites, the possibilities are M1c, M1c(0), and M1c(1).
M1d is a new classification, a clear departure from the seventh edition. It applies to patients with distant metastasis to the CNS. The classification is M1d if LDH isn’t recorded, M1d(0) if LDH isn’t elevated, and M1d(1) if it is.
Turning to the updated 2017 NCCN guidelines Version 1.2017 on the role of SLNB in melanoma, Dr. Marchetti noted that the procedure is not recommended in patients with melanoma in situ or Stage IA or IB disease 0.75 mm or less in thickness, regardless of features. Neither are routine imaging or lab tests. That’s because the pretest probability of a positive SLNB is so low, at around 3%.
For Clinicopathologic Stage IA disease, 0.76-1.0 mm in thickness with no ulceration and a mitotic rate of less than 1 per mm2, the guidelines recommend that physicians “discuss and consider” SLNB, which the available evidence suggests has roughly a 7% pretest probability of a positive result.
For Stage IB disease, 0.76-1.0 mm in thickness with ulceration or a mitotic rate of at least 1 per mm2, as well as for Stage IB or Stage II disease greater than 1.0 mm in thickness, with any feature, the language of the recommendation shifts to “discuss and offer” rather than “discuss and consider” SLNB, since various studies have reported pretest probabilities of a positive result as high as 35%.
“The rationale here for performing sentinel lymph node biopsy is primarily to acquire more staging information. Is it a perfect test? Absolutely not. But it’s the current standard of care in terms of providing additional information for staging,” according to Dr. Marchetti.
If the SLNB generates a positive result, by definition the patient now has Stage III melanoma. The NCCN guidelines recommend consideration of imaging to establish a baseline, and state further that the primary treatment is to discuss and offer complete lymph node dissection in order to control the regional nodal basin and because of a possible favorable impact on overall survival. But the question of a survival benefit has been controversial for many years, and it’s unlikely to be resolved soon, Dr. Marchetti predicted.
The final report from the National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial–1 (MSLT-1) concluded that patients with primary cutaneous melanomas 1.2 mm or more in thickness who were randomized to undergo SLNB and, if positive, immediate complete lymphadenectomy, fared significantly better in terms of 10-year disease-free survival, compared with those assigned to observation and lymphadenectomy in the event of nodal relapse (N Engl J Med. 2014 Feb 13;370[7]:599-609).
This conclusion has generated numerous letters to the editor from melanoma experts who took issue with the analysis and conclusion. To try to put the MSLT-1 results in perspective, Dr. Marchetti applied the results to a hypothetical cohort of 100 patients with intermediate-thickness melanomas of 1.2-3.5 mm undergoing SLNB.
Eighty of these patients would be true SLNB-negative for regional nodal disease. Five others would have a false-negative SLNB and would later develop clinically detectable nodal disease. Fifteen patients with a positive SLNB would undergo prompt complete lymph node dissection, of whom 12 or 13 would derive no mortality benefit at 10 years, assuming the MSLT-1 investigators are correct in their analysis.
“Two or three patients with a positive SLNB will derive mortality benefit at 10 years, but we have no way to identify who those people are from the original 100,” he said.
Since the MSLT-1 report, a phase III German multicenter randomized trial of 241 melanoma patients with a positive screening SLNB has reported results. The participants assigned to complete lymph node dissection didn’t differ in terms of 3-year overall survival, distant metastasis-free survival, or recurrence-free survival, compared with those assigned to observation and lymphadenectomy if nodal disease occurred (Lancet Oncol. 2016 Jun;17[6]:757-67). However, as the investigators noted, the study, known as DeCOG-SLT, was underpowered, and Dr. Marchetti’s view is that it can’t be considered definitive.
“Ultimately I don’t think we’ll have a definitive answer to this question until the final results of the MSLT-II trial in the fall of 2022,” he said.
The MSLT-II trial has the same design as DeCOG-SLT.
Dr. Marchetti reported having no financial conflicts of interest regarding his presentation.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – The Eighth Edition of the American Joint Committee on Cancer Staging Manual includes significant changes in how melanoma is classified.
The manual has already been published and is available for purchase. However, its implementation will be delayed until Jan. 1, 2018, to give physicians, software vendors, and all other interested parties time to get up to speed. All cancers newly diagnosed through Dec. 31, 2017 should be staged in accord with the seventh edition, released in 2010.
The eighth edition breaks new ground, moving beyond TNM (Tumor, Node, Metastasis) anatomic staging to incorporate new evidence-based prognostic factors.
“There are some subtle differences here to be aware of. It can be a little bit tricky at first glance. You should become familiar with this,” advised Dr. Marchetti, a dermatologist at Memorial Sloan-Kettering Cancer Center in New York.
In addition to highlighting the changes in melanoma staging included in the new AJCC manual, he outlined key recommendations – some of them controversial – on the use of sentinel lymph node biopsy (SLNB) in melanoma patients incorporated in the 2017 National Comprehensive Cancer Network (NCCN) guidelines.
The biggest change for the dermatology community contained in the new edition of the AJCC staging manual is that the T1 classification of melanoma has changed. In the seventh edition, a melanoma was categorized as T1 if less than or equal to 1.0 mm thickness. The cancer was T1a if nonulcerated and had a mitosis rate of less than 1/mm2 and T1b if ulcerated or had at least 1 mitosis/mm2.
The eighth edition makes an evidence-based subcategorization of T1 based upon thickness in light of the prognostic implications of this distinction. A melanoma is defined as T1a if nonulcerated and less than 0.8 mm in thickness, and T1b if it is 0.8-1.0 mm thick or less than 0.8 mm with ulceration.
Of note, tumor mitotic rate has been dropped as a staging criterion for T1 tumors.
What this means is, for example, in 2017, a patient with a 0.9-mm nonulcerated melanoma with 1 mitosis/mm2 and a negative sentinel lymph node biopsy with wide local excision is T1bN0M0, pathologic Stage IB. Under the eighth edition of AJCC, the same patient is T1bN0M0, pathologic Stage IA, because that mitosis rate isn’t a factor.
Today, a patient with a 0.5-mm melanoma with 1 mitosis/mm2 with wide local excision is T1bN0M0, Pathologic Stage IB. Under the new system, the same tumor is downstaged to Pathologic Stage IA, Dr. Marchetti explained.
In the eighth edition, tumor thickness measurements are recorded with rounding to the nearest 0.1 mm, not to the nearest 0.01 mm as before. This change was prompted by the inherent lack of precision in measuring melanomas, especially thicker ones.
The T category definitions of primary tumors have been clarified in the eighth edition. A tumor should be classified as T0 only if there is no evidence of a primary tumor. T is utilized for melanoma in situ. TX is employed when the primary tumor thickness can’t be determined, as for example when the biopsy specimen was obtained through curettage.
The N categorization of regional lymph node status has become much more complicated in the eighth edition, the dermatologist cautioned. Plus, the terminology for nodal disease has changed. The term micrometastasis has been replaced by “clinically occult disease” as detected by SLNB. Macrometastasis has been supplanted by “clinically detected disease.” And while in-transit or satellite node metastasis or microsatellite metastasis with satellite nodes was formerly listed simply as N3, in the new system there are subcategories for N3 based upon the number of metastatic nodes involved. For example, in the eighth edition, a melanoma is pathologic Stage N3a if there are four or more clinically occult regional lymph nodes and no in-transit, satellite, or matted nodes. Pathologic Stage N3b is shorthand for four or more tumor-involved regional lymph nodes, at least one of which was clinically detected, or any number of matted lymph nodes, with no in-transit or satellite nodal involvement. Stage N3c is reserved for melanomas with two or more clinically occult or clinically detected regional lymph nodes and/or any number of matted nodes, plus the presence of in-transit or satellite nodal metastasis.
As a result of the changes in the N classification, there are now four pathologic Stage III groups rather than three. Stages IIIA-C have been joined by pathologic Stage IIID, reserved for patients who are T4b, N3a, b, or c, and M0.
The M categorization of distant metastatic disease status has also become more elaborate. In the AJCC seventh edition, if serum lactate dehydrogenase (LDH) is elevated and a patient has any distant metastatic disease, that’s automatically category M1c. Not any longer, though.
Under the eighth edition, if a patient has distant metastasis to skin, soft tissue including muscle, and/or nonregional lymph nodes and the LDH is unspecified, the categorization is M1a. If serum LDH is not elevated, it’s M1a(0). If elevated, then M1a(1).
Similarly, for distant metastasis to the lung, the range of possibilities based upon LDH is M1b, M1b(0), and M1b(1). For distant metastasis to non-CNS visceral sites, the possibilities are M1c, M1c(0), and M1c(1).
M1d is a new classification, a clear departure from the seventh edition. It applies to patients with distant metastasis to the CNS. The classification is M1d if LDH isn’t recorded, M1d(0) if LDH isn’t elevated, and M1d(1) if it is.
Turning to the updated 2017 NCCN guidelines Version 1.2017 on the role of SLNB in melanoma, Dr. Marchetti noted that the procedure is not recommended in patients with melanoma in situ or Stage IA or IB disease 0.75 mm or less in thickness, regardless of features. Neither are routine imaging or lab tests. That’s because the pretest probability of a positive SLNB is so low, at around 3%.
For Clinicopathologic Stage IA disease, 0.76-1.0 mm in thickness with no ulceration and a mitotic rate of less than 1 per mm2, the guidelines recommend that physicians “discuss and consider” SLNB, which the available evidence suggests has roughly a 7% pretest probability of a positive result.
For Stage IB disease, 0.76-1.0 mm in thickness with ulceration or a mitotic rate of at least 1 per mm2, as well as for Stage IB or Stage II disease greater than 1.0 mm in thickness, with any feature, the language of the recommendation shifts to “discuss and offer” rather than “discuss and consider” SLNB, since various studies have reported pretest probabilities of a positive result as high as 35%.
“The rationale here for performing sentinel lymph node biopsy is primarily to acquire more staging information. Is it a perfect test? Absolutely not. But it’s the current standard of care in terms of providing additional information for staging,” according to Dr. Marchetti.
If the SLNB generates a positive result, by definition the patient now has Stage III melanoma. The NCCN guidelines recommend consideration of imaging to establish a baseline, and state further that the primary treatment is to discuss and offer complete lymph node dissection in order to control the regional nodal basin and because of a possible favorable impact on overall survival. But the question of a survival benefit has been controversial for many years, and it’s unlikely to be resolved soon, Dr. Marchetti predicted.
The final report from the National Cancer Institute–sponsored Multicenter Selective Lymphadenectomy Trial–1 (MSLT-1) concluded that patients with primary cutaneous melanomas 1.2 mm or more in thickness who were randomized to undergo SLNB and, if positive, immediate complete lymphadenectomy, fared significantly better in terms of 10-year disease-free survival, compared with those assigned to observation and lymphadenectomy in the event of nodal relapse (N Engl J Med. 2014 Feb 13;370[7]:599-609).
This conclusion has generated numerous letters to the editor from melanoma experts who took issue with the analysis and conclusion. To try to put the MSLT-1 results in perspective, Dr. Marchetti applied the results to a hypothetical cohort of 100 patients with intermediate-thickness melanomas of 1.2-3.5 mm undergoing SLNB.
Eighty of these patients would be true SLNB-negative for regional nodal disease. Five others would have a false-negative SLNB and would later develop clinically detectable nodal disease. Fifteen patients with a positive SLNB would undergo prompt complete lymph node dissection, of whom 12 or 13 would derive no mortality benefit at 10 years, assuming the MSLT-1 investigators are correct in their analysis.
“Two or three patients with a positive SLNB will derive mortality benefit at 10 years, but we have no way to identify who those people are from the original 100,” he said.
Since the MSLT-1 report, a phase III German multicenter randomized trial of 241 melanoma patients with a positive screening SLNB has reported results. The participants assigned to complete lymph node dissection didn’t differ in terms of 3-year overall survival, distant metastasis-free survival, or recurrence-free survival, compared with those assigned to observation and lymphadenectomy if nodal disease occurred (Lancet Oncol. 2016 Jun;17[6]:757-67). However, as the investigators noted, the study, known as DeCOG-SLT, was underpowered, and Dr. Marchetti’s view is that it can’t be considered definitive.
“Ultimately I don’t think we’ll have a definitive answer to this question until the final results of the MSLT-II trial in the fall of 2022,” he said.
The MSLT-II trial has the same design as DeCOG-SLT.
Dr. Marchetti reported having no financial conflicts of interest regarding his presentation.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
Photoprotection Prevents Skin Cancer: Let’s Make It Fashionable to Wear Sun-Protective Clothing
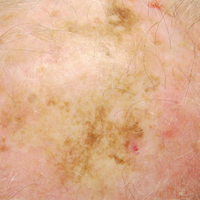
Photoprotection is the foundation of all skin cancer prevention, as UV radiation (UVR) exposure is the only known modifiable risk factor for skin cancer. With the majority of UVR exposure–induced skin cancers found on the scalp, ears, face, and neck, public health initiatives call for wise choices in personal fashion that emphasize the importance of covering these areas.1-3 From a science of fashion perspective, research has shown that wide-brimmed hats provide better means of ensuring the largest area of coverage compared to standard baseball-style hats.4 Thus, for maximum protection, wide-brimmed hats should be favored. However, in academic and military settings, individual style is not optional and is instead influenced or directed by policy, which may not be aligned with the goal of providing photoprotection and raises additional concern for individuals working in environments with longer periods of peak daylight UVR exposure.
In all military branches, service members don uniforms that include head coverage when operating outdoors; however, the choice of headgear is not always aimed at reducing UVR exposure. Similarly, in our counterpart civilian populations, wearing hats that provide the best photoprotection may be influenced by school policies, which frequently mandate clothing choices for children, or by the press or fashion industry in the general public, which might portray sun-protective garments as unfashionable or in some cases threatening if perceived as demonstrating gang affiliation.5 This article serves to encourage health care providers to not only discuss the use of sunscreen when educating patients on sun protection but also to emphasize the benefits of wearing photoprotective garments, particularly wide-brimmed hats given their simplicity, reusability, and affordability. Hat use is particularly important for men with comorbid androgenetic alopecia.6
Skin Cancer Risk
Unfortunately, the incidence of most common types of skin cancer, specifically nonmelanoma skin cancers such basal cell carcinomas and squamous cell carcinomas (ie, keratinocyte carcinomas [KCs]), is difficult to estimate properly, as these cases are not required to be reported to worldwide cancer registries. However, more than 5.4 million cases of skin cancers were diagnosed among 3.3 million Americans in 2016, with an estimated 13,650 deaths associated with skin cancers (not including KCs).3 Tracking and data analyses of cases diagnosed in the active and reserve component populations of the US Armed Forces reflect parallel findings.7 Keratinocyte carcinomas could be considered largely preventable, as most are the result of UVR exposure.1 Additionally, it has been suggested that the vast majority of mutations in melanoma skin cancers (up to 86%) are caused by UVR exposure.8
Prevention
United States–based national public health services such as the American Cancer Society, the Centers for Disease Control and Prevention, and the American Academy of Dermatology embrace photoprotection as the central practice in reducing risk factors for skin cancers. Guidelines put forth by these and other national preventive medical institutions specifically recommend the use of wide-brimmed hats as the best option for protection of the face, head, ears, and neck, in addition to more common recommendations such as seeking shade, avoiding sunlight during peak hours of the day, and using sunscreen.1-3 At state and local levels, policies should be adapted from these recommendations to support protective practices and skin cancer education that begins early for school-aged children. Unfortunately, in some school districts, wearing hats of any kind may be perceived as disruptive or in some cases baseball hats may be a sign of gang affiliation and are therefore banned in the schoolyard.5 The opposite is true in certain parts of the world where sun protection is embraced by the population as a whole, such as Australia where the widely accepted “slip, slop and slap, seek and slide” campaign has extended to some school policymakers who have considered adopting a “no hat, no play” policy.9,10
Sunscreen use as a primary component of photoprotection has its disadvantages in comparison to wearing protective clothing, as sunscreen cannot be reused and proper usage requires reapplication after swimming, when sweating, and following 2 hours of application.1-3 The need for reapplication of sunscreen can lead to considerable expense as well as time spent in application and reapplication. Additionally, for individuals who are physically active (eg, operationally engaged service members, outdoor athletes), sunscreen applied to the face may become a hindrance to function, as it may drip or enter the eyes with excessive sweating, possibly impairing vision. Some individuals may be averse to applying lotions or creams to the skin in general, as they do not prefer the textural changes or appearance of the skin after application. The application of sunscreen also could impair use of lifesaving military gear (eg, gas masks, helmets) from fitting or securing appropriately.
Patient Education
From a military perspective, a review of a recent targeted pilot study in which skin cancer patients at a US Veterans Administration hospital were surveyed on personal knowledge of UVR protection showed that respondents who had a history of skin cancer diagnosis did not feel that they had ever been at an increased risk for skin cancers and did not receive skin cancer prevention education during their tours of service. The overwhelming majority of all participants in this study agreed that the military should issue sun-protective clothing and sunscreen to active-duty personnel.11 Another 2015 survey of 356 current US Air Force flight line personnel noted that active-duty service members tend not to use sunscreen when at work or while at home, and 43% of participants reported using no sun-protective methods while working outdoors.12 Although these studies focused on military personal, the data mirror findings within the general public, as it was shown in a survey by the Centers for Disease Control and Prevention that Americans do not fully take advantage of the benefits of UVR protection, specifically with regard to sunscreen use. Little to no usage was correlated with low socioeconomic status, suggesting that a reusable form of protection could be preferred.13
Public health initiatives typically promote education on the use of sunscreen in populations that spend a considerable amount of time working outdoors (eg, construction workers, farmers, military personnel); however, we feel emphasis should be placed on the benefits of wearing hats, as the UVR exposure protection they provide does not wear off, is cost effective, does not require reapplication, and has the advantage of being a recyclable and affordable form of photoprotection.
History of the Military-Grade Wide-Brimmed Hat
One military-specific example of a sun-protective hat is the boonie hat, known at the time of its inception as the tropical or hot-weather hat, which first became popular during the Vietnam War. This hat option was initially proposed on April 7, 1966, when it was realized that a full-brimmed field hat was needed to protect soldiers’ faces and necks from rain and sun in harsh tropical climates.14 Unfortunately, despite the protective advantages of this style of head covering and favorable support from service members themselves, the boonie hat was not widely accepted, as commanders disliked its “unmilitary appearance.” Fervent protests by units throughout Vietnam eventually led to a compromise in policy that allowed unit-level commanders to authorize the use of boonie hats for units in combat or combat support field operations.14 Today, the boonie hat continues to garnish mixed emotions from unit commanders, as wearing this garment often is interpreted as not being in line with an appropriate military appearance, which is similar to the public fashion zeitgeist that also does not openly endorse the use of sun-protective garments. A change in fashion culture and policy (both military and civilian) that promotes sun-protective measures is needed.
Wide-Brimmed Hats Are Superior to Baseball Hats
The distribution of skin cancers across anatomic sites is consistent and proportional with the level and frequency of chronic UVR exposure, with the occurrence of most skin cancers being greatest on the nose, forehead/temples, cheeks/perioral areas, and ears.15 Additionally, higher incidences of skin cancers have been noted in chronically sun-exposed areas of the head and neck in men versus women. It is thought that hair distribution in these areas may be the causal factor.6
Baseball-style hats are worn by all branches of the US military as part of standard training and work duty uniform requirements, primarily for the sake of tradition by maintaining a standard appearance and uniform dress code but also to provide photoprotection to these vulnerable areas of the body. Standard, nonmilitary, baseball-style hats have been shown to provide UV protection factor (UPF) equivalents ranging from 2 to 10 on sites known for the highest levels of exposure.16 Military “patrol caps,” fashioned similar to the baseball-style hat but constructed from military-grade textiles, provide greater levels of photoprotection with UPF ratings from 35 to 50 and higher depending on the fabric color.17 Although patrol caps have a favorable UPF rating and are advantageous compared to former military headgear styles (eg, berets), wide-brimmed hats would provide greater overall coverage.4,6 Studies in school environments also revealed that wide-brimmed hats come out ahead in side-by-side testing against baseball hats and are shown to provide greater photoprotection for the cheeks, chin, ears, and neck.16
Final Thoughts
The battle to educate the public about adequate photoprotection to prevent skin cancers caused by UVR exposure applies to all providers, both military and civilian. Our ongoing initiatives should not only sustain current practices but should further stress the importance of wearing wide-brimmed hats as a vital part of coverage of the skin and protection from UVR. We must combat the public perception that wearing wide-brimmed hats is a detractor of personal fashion and that instead it is desirable to reduce the risk for skin cancer. The wide-brimmed hat is a simple, reusable, and easily executed recommendation that should be made to all patients, both military and civilian, young and old. In conclusion, by improving patients’ perceptions and acknowledgment of the importance of photoprotection as well as making a concerted effort to integrate our knowledge in the fashion industry, in policies at schools, in the military, and in popular culture, we will undoubtedly come to agree that it is not unfashionable to wear a wide-brimmed hat, but it is unfashionable to risk developing skin cancer.
- Prevent skin cancer. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent. Accessed January 4, 2017.
- What can I do to reduce my risk of skin cancer? Centers for Disease Control and Prevention website. http://www.cdc.gov/cancer/skin/basic_info/prevention.htm. Accessed January 4, 2017.
- Cancer facts & figures 2016. American Cancer Society website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed January 4, 2017.
- Diffey BL, Cheeseman J. Sun protection with hats. Br J Dermatol. 1992;127:10-12.
- Bray FN. Florida school boards restrict access to outdoor sun protection: an observational study. J Am Acad Dermatol. 2016;75:642-644.
- Yeung H, Luk KM, Chen SC. Focal photodamage on the occipital scalp. JAMA Dermatol. 2016;152:1060-1062.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Parkin DM, Mesher D, Sasieni P. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105(suppl 2):S66-S69.
- Casper K. Elementary schools consider “no hat no play policy.” Coolibar website. http://blog.coolibar.com/elementary-schools-consider-no-hat-no-play-policy/. Published March 27, 2012. Accessed January 4, 2017.
- Slip, slop, slap, seek & slide: Sid Seagull. SunSmart Victoria website. http://www.sunsmart.com.au/tools/videos/current-tv-campaigns/slip-slop-slap-seek-slide-sid-seagull.html. Accessed January 4, 2017.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns - a pilot study. Am J Prev Med. 2016;50:E62-E63.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Mil Med. 2015;180:26-31.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults [published online May 19, 2015]. J Am Acad Dermatol. 2015;73:83-92.e1.
- Stanton SL. Headgear. In: Stanton SL. U.S. Army Uniforms of the Vietnam War. Harrisburg, PA: Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population [published online July 31, 2008]. J Invest Dermatol. 2009;129:323-328.
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Winterhalter C, DiLuna K, Bide M. Characterization of the Ultraviolet Protection of Combat Uniform Fabrics. Natick, MA: US Army Solider and Biological Chemical Command; 2002. Technical report 02/006.
Photoprotection is the foundation of all skin cancer prevention, as UV radiation (UVR) exposure is the only known modifiable risk factor for skin cancer. With the majority of UVR exposure–induced skin cancers found on the scalp, ears, face, and neck, public health initiatives call for wise choices in personal fashion that emphasize the importance of covering these areas.1-3 From a science of fashion perspective, research has shown that wide-brimmed hats provide better means of ensuring the largest area of coverage compared to standard baseball-style hats.4 Thus, for maximum protection, wide-brimmed hats should be favored. However, in academic and military settings, individual style is not optional and is instead influenced or directed by policy, which may not be aligned with the goal of providing photoprotection and raises additional concern for individuals working in environments with longer periods of peak daylight UVR exposure.
In all military branches, service members don uniforms that include head coverage when operating outdoors; however, the choice of headgear is not always aimed at reducing UVR exposure. Similarly, in our counterpart civilian populations, wearing hats that provide the best photoprotection may be influenced by school policies, which frequently mandate clothing choices for children, or by the press or fashion industry in the general public, which might portray sun-protective garments as unfashionable or in some cases threatening if perceived as demonstrating gang affiliation.5 This article serves to encourage health care providers to not only discuss the use of sunscreen when educating patients on sun protection but also to emphasize the benefits of wearing photoprotective garments, particularly wide-brimmed hats given their simplicity, reusability, and affordability. Hat use is particularly important for men with comorbid androgenetic alopecia.6
Skin Cancer Risk
Unfortunately, the incidence of most common types of skin cancer, specifically nonmelanoma skin cancers such basal cell carcinomas and squamous cell carcinomas (ie, keratinocyte carcinomas [KCs]), is difficult to estimate properly, as these cases are not required to be reported to worldwide cancer registries. However, more than 5.4 million cases of skin cancers were diagnosed among 3.3 million Americans in 2016, with an estimated 13,650 deaths associated with skin cancers (not including KCs).3 Tracking and data analyses of cases diagnosed in the active and reserve component populations of the US Armed Forces reflect parallel findings.7 Keratinocyte carcinomas could be considered largely preventable, as most are the result of UVR exposure.1 Additionally, it has been suggested that the vast majority of mutations in melanoma skin cancers (up to 86%) are caused by UVR exposure.8
Prevention
United States–based national public health services such as the American Cancer Society, the Centers for Disease Control and Prevention, and the American Academy of Dermatology embrace photoprotection as the central practice in reducing risk factors for skin cancers. Guidelines put forth by these and other national preventive medical institutions specifically recommend the use of wide-brimmed hats as the best option for protection of the face, head, ears, and neck, in addition to more common recommendations such as seeking shade, avoiding sunlight during peak hours of the day, and using sunscreen.1-3 At state and local levels, policies should be adapted from these recommendations to support protective practices and skin cancer education that begins early for school-aged children. Unfortunately, in some school districts, wearing hats of any kind may be perceived as disruptive or in some cases baseball hats may be a sign of gang affiliation and are therefore banned in the schoolyard.5 The opposite is true in certain parts of the world where sun protection is embraced by the population as a whole, such as Australia where the widely accepted “slip, slop and slap, seek and slide” campaign has extended to some school policymakers who have considered adopting a “no hat, no play” policy.9,10
Sunscreen use as a primary component of photoprotection has its disadvantages in comparison to wearing protective clothing, as sunscreen cannot be reused and proper usage requires reapplication after swimming, when sweating, and following 2 hours of application.1-3 The need for reapplication of sunscreen can lead to considerable expense as well as time spent in application and reapplication. Additionally, for individuals who are physically active (eg, operationally engaged service members, outdoor athletes), sunscreen applied to the face may become a hindrance to function, as it may drip or enter the eyes with excessive sweating, possibly impairing vision. Some individuals may be averse to applying lotions or creams to the skin in general, as they do not prefer the textural changes or appearance of the skin after application. The application of sunscreen also could impair use of lifesaving military gear (eg, gas masks, helmets) from fitting or securing appropriately.
Patient Education
From a military perspective, a review of a recent targeted pilot study in which skin cancer patients at a US Veterans Administration hospital were surveyed on personal knowledge of UVR protection showed that respondents who had a history of skin cancer diagnosis did not feel that they had ever been at an increased risk for skin cancers and did not receive skin cancer prevention education during their tours of service. The overwhelming majority of all participants in this study agreed that the military should issue sun-protective clothing and sunscreen to active-duty personnel.11 Another 2015 survey of 356 current US Air Force flight line personnel noted that active-duty service members tend not to use sunscreen when at work or while at home, and 43% of participants reported using no sun-protective methods while working outdoors.12 Although these studies focused on military personal, the data mirror findings within the general public, as it was shown in a survey by the Centers for Disease Control and Prevention that Americans do not fully take advantage of the benefits of UVR protection, specifically with regard to sunscreen use. Little to no usage was correlated with low socioeconomic status, suggesting that a reusable form of protection could be preferred.13
Public health initiatives typically promote education on the use of sunscreen in populations that spend a considerable amount of time working outdoors (eg, construction workers, farmers, military personnel); however, we feel emphasis should be placed on the benefits of wearing hats, as the UVR exposure protection they provide does not wear off, is cost effective, does not require reapplication, and has the advantage of being a recyclable and affordable form of photoprotection.
History of the Military-Grade Wide-Brimmed Hat
One military-specific example of a sun-protective hat is the boonie hat, known at the time of its inception as the tropical or hot-weather hat, which first became popular during the Vietnam War. This hat option was initially proposed on April 7, 1966, when it was realized that a full-brimmed field hat was needed to protect soldiers’ faces and necks from rain and sun in harsh tropical climates.14 Unfortunately, despite the protective advantages of this style of head covering and favorable support from service members themselves, the boonie hat was not widely accepted, as commanders disliked its “unmilitary appearance.” Fervent protests by units throughout Vietnam eventually led to a compromise in policy that allowed unit-level commanders to authorize the use of boonie hats for units in combat or combat support field operations.14 Today, the boonie hat continues to garnish mixed emotions from unit commanders, as wearing this garment often is interpreted as not being in line with an appropriate military appearance, which is similar to the public fashion zeitgeist that also does not openly endorse the use of sun-protective garments. A change in fashion culture and policy (both military and civilian) that promotes sun-protective measures is needed.
Wide-Brimmed Hats Are Superior to Baseball Hats
The distribution of skin cancers across anatomic sites is consistent and proportional with the level and frequency of chronic UVR exposure, with the occurrence of most skin cancers being greatest on the nose, forehead/temples, cheeks/perioral areas, and ears.15 Additionally, higher incidences of skin cancers have been noted in chronically sun-exposed areas of the head and neck in men versus women. It is thought that hair distribution in these areas may be the causal factor.6
Baseball-style hats are worn by all branches of the US military as part of standard training and work duty uniform requirements, primarily for the sake of tradition by maintaining a standard appearance and uniform dress code but also to provide photoprotection to these vulnerable areas of the body. Standard, nonmilitary, baseball-style hats have been shown to provide UV protection factor (UPF) equivalents ranging from 2 to 10 on sites known for the highest levels of exposure.16 Military “patrol caps,” fashioned similar to the baseball-style hat but constructed from military-grade textiles, provide greater levels of photoprotection with UPF ratings from 35 to 50 and higher depending on the fabric color.17 Although patrol caps have a favorable UPF rating and are advantageous compared to former military headgear styles (eg, berets), wide-brimmed hats would provide greater overall coverage.4,6 Studies in school environments also revealed that wide-brimmed hats come out ahead in side-by-side testing against baseball hats and are shown to provide greater photoprotection for the cheeks, chin, ears, and neck.16
Final Thoughts
The battle to educate the public about adequate photoprotection to prevent skin cancers caused by UVR exposure applies to all providers, both military and civilian. Our ongoing initiatives should not only sustain current practices but should further stress the importance of wearing wide-brimmed hats as a vital part of coverage of the skin and protection from UVR. We must combat the public perception that wearing wide-brimmed hats is a detractor of personal fashion and that instead it is desirable to reduce the risk for skin cancer. The wide-brimmed hat is a simple, reusable, and easily executed recommendation that should be made to all patients, both military and civilian, young and old. In conclusion, by improving patients’ perceptions and acknowledgment of the importance of photoprotection as well as making a concerted effort to integrate our knowledge in the fashion industry, in policies at schools, in the military, and in popular culture, we will undoubtedly come to agree that it is not unfashionable to wear a wide-brimmed hat, but it is unfashionable to risk developing skin cancer.
Photoprotection is the foundation of all skin cancer prevention, as UV radiation (UVR) exposure is the only known modifiable risk factor for skin cancer. With the majority of UVR exposure–induced skin cancers found on the scalp, ears, face, and neck, public health initiatives call for wise choices in personal fashion that emphasize the importance of covering these areas.1-3 From a science of fashion perspective, research has shown that wide-brimmed hats provide better means of ensuring the largest area of coverage compared to standard baseball-style hats.4 Thus, for maximum protection, wide-brimmed hats should be favored. However, in academic and military settings, individual style is not optional and is instead influenced or directed by policy, which may not be aligned with the goal of providing photoprotection and raises additional concern for individuals working in environments with longer periods of peak daylight UVR exposure.
In all military branches, service members don uniforms that include head coverage when operating outdoors; however, the choice of headgear is not always aimed at reducing UVR exposure. Similarly, in our counterpart civilian populations, wearing hats that provide the best photoprotection may be influenced by school policies, which frequently mandate clothing choices for children, or by the press or fashion industry in the general public, which might portray sun-protective garments as unfashionable or in some cases threatening if perceived as demonstrating gang affiliation.5 This article serves to encourage health care providers to not only discuss the use of sunscreen when educating patients on sun protection but also to emphasize the benefits of wearing photoprotective garments, particularly wide-brimmed hats given their simplicity, reusability, and affordability. Hat use is particularly important for men with comorbid androgenetic alopecia.6
Skin Cancer Risk
Unfortunately, the incidence of most common types of skin cancer, specifically nonmelanoma skin cancers such basal cell carcinomas and squamous cell carcinomas (ie, keratinocyte carcinomas [KCs]), is difficult to estimate properly, as these cases are not required to be reported to worldwide cancer registries. However, more than 5.4 million cases of skin cancers were diagnosed among 3.3 million Americans in 2016, with an estimated 13,650 deaths associated with skin cancers (not including KCs).3 Tracking and data analyses of cases diagnosed in the active and reserve component populations of the US Armed Forces reflect parallel findings.7 Keratinocyte carcinomas could be considered largely preventable, as most are the result of UVR exposure.1 Additionally, it has been suggested that the vast majority of mutations in melanoma skin cancers (up to 86%) are caused by UVR exposure.8
Prevention
United States–based national public health services such as the American Cancer Society, the Centers for Disease Control and Prevention, and the American Academy of Dermatology embrace photoprotection as the central practice in reducing risk factors for skin cancers. Guidelines put forth by these and other national preventive medical institutions specifically recommend the use of wide-brimmed hats as the best option for protection of the face, head, ears, and neck, in addition to more common recommendations such as seeking shade, avoiding sunlight during peak hours of the day, and using sunscreen.1-3 At state and local levels, policies should be adapted from these recommendations to support protective practices and skin cancer education that begins early for school-aged children. Unfortunately, in some school districts, wearing hats of any kind may be perceived as disruptive or in some cases baseball hats may be a sign of gang affiliation and are therefore banned in the schoolyard.5 The opposite is true in certain parts of the world where sun protection is embraced by the population as a whole, such as Australia where the widely accepted “slip, slop and slap, seek and slide” campaign has extended to some school policymakers who have considered adopting a “no hat, no play” policy.9,10
Sunscreen use as a primary component of photoprotection has its disadvantages in comparison to wearing protective clothing, as sunscreen cannot be reused and proper usage requires reapplication after swimming, when sweating, and following 2 hours of application.1-3 The need for reapplication of sunscreen can lead to considerable expense as well as time spent in application and reapplication. Additionally, for individuals who are physically active (eg, operationally engaged service members, outdoor athletes), sunscreen applied to the face may become a hindrance to function, as it may drip or enter the eyes with excessive sweating, possibly impairing vision. Some individuals may be averse to applying lotions or creams to the skin in general, as they do not prefer the textural changes or appearance of the skin after application. The application of sunscreen also could impair use of lifesaving military gear (eg, gas masks, helmets) from fitting or securing appropriately.
Patient Education
From a military perspective, a review of a recent targeted pilot study in which skin cancer patients at a US Veterans Administration hospital were surveyed on personal knowledge of UVR protection showed that respondents who had a history of skin cancer diagnosis did not feel that they had ever been at an increased risk for skin cancers and did not receive skin cancer prevention education during their tours of service. The overwhelming majority of all participants in this study agreed that the military should issue sun-protective clothing and sunscreen to active-duty personnel.11 Another 2015 survey of 356 current US Air Force flight line personnel noted that active-duty service members tend not to use sunscreen when at work or while at home, and 43% of participants reported using no sun-protective methods while working outdoors.12 Although these studies focused on military personal, the data mirror findings within the general public, as it was shown in a survey by the Centers for Disease Control and Prevention that Americans do not fully take advantage of the benefits of UVR protection, specifically with regard to sunscreen use. Little to no usage was correlated with low socioeconomic status, suggesting that a reusable form of protection could be preferred.13
Public health initiatives typically promote education on the use of sunscreen in populations that spend a considerable amount of time working outdoors (eg, construction workers, farmers, military personnel); however, we feel emphasis should be placed on the benefits of wearing hats, as the UVR exposure protection they provide does not wear off, is cost effective, does not require reapplication, and has the advantage of being a recyclable and affordable form of photoprotection.
History of the Military-Grade Wide-Brimmed Hat
One military-specific example of a sun-protective hat is the boonie hat, known at the time of its inception as the tropical or hot-weather hat, which first became popular during the Vietnam War. This hat option was initially proposed on April 7, 1966, when it was realized that a full-brimmed field hat was needed to protect soldiers’ faces and necks from rain and sun in harsh tropical climates.14 Unfortunately, despite the protective advantages of this style of head covering and favorable support from service members themselves, the boonie hat was not widely accepted, as commanders disliked its “unmilitary appearance.” Fervent protests by units throughout Vietnam eventually led to a compromise in policy that allowed unit-level commanders to authorize the use of boonie hats for units in combat or combat support field operations.14 Today, the boonie hat continues to garnish mixed emotions from unit commanders, as wearing this garment often is interpreted as not being in line with an appropriate military appearance, which is similar to the public fashion zeitgeist that also does not openly endorse the use of sun-protective garments. A change in fashion culture and policy (both military and civilian) that promotes sun-protective measures is needed.
Wide-Brimmed Hats Are Superior to Baseball Hats
The distribution of skin cancers across anatomic sites is consistent and proportional with the level and frequency of chronic UVR exposure, with the occurrence of most skin cancers being greatest on the nose, forehead/temples, cheeks/perioral areas, and ears.15 Additionally, higher incidences of skin cancers have been noted in chronically sun-exposed areas of the head and neck in men versus women. It is thought that hair distribution in these areas may be the causal factor.6
Baseball-style hats are worn by all branches of the US military as part of standard training and work duty uniform requirements, primarily for the sake of tradition by maintaining a standard appearance and uniform dress code but also to provide photoprotection to these vulnerable areas of the body. Standard, nonmilitary, baseball-style hats have been shown to provide UV protection factor (UPF) equivalents ranging from 2 to 10 on sites known for the highest levels of exposure.16 Military “patrol caps,” fashioned similar to the baseball-style hat but constructed from military-grade textiles, provide greater levels of photoprotection with UPF ratings from 35 to 50 and higher depending on the fabric color.17 Although patrol caps have a favorable UPF rating and are advantageous compared to former military headgear styles (eg, berets), wide-brimmed hats would provide greater overall coverage.4,6 Studies in school environments also revealed that wide-brimmed hats come out ahead in side-by-side testing against baseball hats and are shown to provide greater photoprotection for the cheeks, chin, ears, and neck.16
Final Thoughts
The battle to educate the public about adequate photoprotection to prevent skin cancers caused by UVR exposure applies to all providers, both military and civilian. Our ongoing initiatives should not only sustain current practices but should further stress the importance of wearing wide-brimmed hats as a vital part of coverage of the skin and protection from UVR. We must combat the public perception that wearing wide-brimmed hats is a detractor of personal fashion and that instead it is desirable to reduce the risk for skin cancer. The wide-brimmed hat is a simple, reusable, and easily executed recommendation that should be made to all patients, both military and civilian, young and old. In conclusion, by improving patients’ perceptions and acknowledgment of the importance of photoprotection as well as making a concerted effort to integrate our knowledge in the fashion industry, in policies at schools, in the military, and in popular culture, we will undoubtedly come to agree that it is not unfashionable to wear a wide-brimmed hat, but it is unfashionable to risk developing skin cancer.
- Prevent skin cancer. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent. Accessed January 4, 2017.
- What can I do to reduce my risk of skin cancer? Centers for Disease Control and Prevention website. http://www.cdc.gov/cancer/skin/basic_info/prevention.htm. Accessed January 4, 2017.
- Cancer facts & figures 2016. American Cancer Society website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed January 4, 2017.
- Diffey BL, Cheeseman J. Sun protection with hats. Br J Dermatol. 1992;127:10-12.
- Bray FN. Florida school boards restrict access to outdoor sun protection: an observational study. J Am Acad Dermatol. 2016;75:642-644.
- Yeung H, Luk KM, Chen SC. Focal photodamage on the occipital scalp. JAMA Dermatol. 2016;152:1060-1062.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Parkin DM, Mesher D, Sasieni P. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105(suppl 2):S66-S69.
- Casper K. Elementary schools consider “no hat no play policy.” Coolibar website. http://blog.coolibar.com/elementary-schools-consider-no-hat-no-play-policy/. Published March 27, 2012. Accessed January 4, 2017.
- Slip, slop, slap, seek & slide: Sid Seagull. SunSmart Victoria website. http://www.sunsmart.com.au/tools/videos/current-tv-campaigns/slip-slop-slap-seek-slide-sid-seagull.html. Accessed January 4, 2017.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns - a pilot study. Am J Prev Med. 2016;50:E62-E63.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Mil Med. 2015;180:26-31.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults [published online May 19, 2015]. J Am Acad Dermatol. 2015;73:83-92.e1.
- Stanton SL. Headgear. In: Stanton SL. U.S. Army Uniforms of the Vietnam War. Harrisburg, PA: Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population [published online July 31, 2008]. J Invest Dermatol. 2009;129:323-328.
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Winterhalter C, DiLuna K, Bide M. Characterization of the Ultraviolet Protection of Combat Uniform Fabrics. Natick, MA: US Army Solider and Biological Chemical Command; 2002. Technical report 02/006.
- Prevent skin cancer. American Academy of Dermatology website. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent. Accessed January 4, 2017.
- What can I do to reduce my risk of skin cancer? Centers for Disease Control and Prevention website. http://www.cdc.gov/cancer/skin/basic_info/prevention.htm. Accessed January 4, 2017.
- Cancer facts & figures 2016. American Cancer Society website. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed January 4, 2017.
- Diffey BL, Cheeseman J. Sun protection with hats. Br J Dermatol. 1992;127:10-12.
- Bray FN. Florida school boards restrict access to outdoor sun protection: an observational study. J Am Acad Dermatol. 2016;75:642-644.
- Yeung H, Luk KM, Chen SC. Focal photodamage on the occipital scalp. JAMA Dermatol. 2016;152:1060-1062.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Parkin DM, Mesher D, Sasieni P. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105(suppl 2):S66-S69.
- Casper K. Elementary schools consider “no hat no play policy.” Coolibar website. http://blog.coolibar.com/elementary-schools-consider-no-hat-no-play-policy/. Published March 27, 2012. Accessed January 4, 2017.
- Slip, slop, slap, seek & slide: Sid Seagull. SunSmart Victoria website. http://www.sunsmart.com.au/tools/videos/current-tv-campaigns/slip-slop-slap-seek-slide-sid-seagull.html. Accessed January 4, 2017.
- McGrath JM, Fisher V, Krejci-Manwaring J. Skin cancer warnings and the need for new preventive campaigns - a pilot study. Am J Prev Med. 2016;50:E62-E63.
- Parker G, Williams B, Driggers P. Sun exposure knowledge and practices survey of maintenance squadrons at Travis AFB. Mil Med. 2015;180:26-31.
- Holman DM, Berkowitz Z, Guy GP Jr, et al. Patterns of sunscreen use on the face and other exposed skin among US adults [published online May 19, 2015]. J Am Acad Dermatol. 2015;73:83-92.e1.
- Stanton SL. Headgear. In: Stanton SL. U.S. Army Uniforms of the Vietnam War. Harrisburg, PA: Stackpole Books; 1992:26-61.
- Richmond-Sinclair NM, Pandeya N, Ware RS, et al. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population [published online July 31, 2008]. J Invest Dermatol. 2009;129:323-328.
- Gies P, Javorniczky J, Roy C, et al. Measurements of the UVR protection provided by hats used at school. Photochem Photobiol. 2006;82:750-754.
- Winterhalter C, DiLuna K, Bide M. Characterization of the Ultraviolet Protection of Combat Uniform Fabrics. Natick, MA: US Army Solider and Biological Chemical Command; 2002. Technical report 02/006.
Practice Points
- Routine wear of wide-brimmed hats is the simplest, most inexpensive, and only reusable form of photoprotection for the head and neck and should be an everyday practice for reducing the risk for preventable skin cancers.
- The regular wear of clothing and head cover with adequate UV protection factor is equally as important to utilize in the prevention of UV-induced skin cancers as the application of topical sunscreens and sunblocks.
- The medical community should make a concerted effort to dispel any public policy or fashion trend that does not promote personal protection from sun-induced skin cancers. Policies that restrict wearing photoprotective garments, such as in schools and in the military, need to be changed.
Improving sunscreen use entails patient counseling
WAILEA, HAWAII – When sunscreens are tested for their SPF, testers apply 2 mg/cm2, but most people use only 20%-50% of that amount, which significantly reduces their protection, according to Dr. Julie C. Harper, director of the Dermatology & Skin Care Center of Birmingham, Ala.
The correct amount is 1 teaspoon of sunscreen on the face/head/neck, 1 teaspoon on each arm, 2 teaspoons on the torso, and 2 teaspoons on each leg, Dr. Harper said in a presentation at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. Reapplication every 90 minutes to 2 hours is key to effective protection, Dr. Harper said.
However, “most people use less than one bottle of sunscreen per year,” she noted.
Prompting patients to improve their sunscreen use involves disproving some myths, Dr. Harper pointed out. When patients cite concerns about low vitamin D as a reason to avoid sunscreens, she recommended that they be counseled that there are three sources of vitamin D: foods such as fatty fish, vitamin D fortified foods, cheese, and egg yolks; vitamin D supplements; and skin synthesis through UVB exposure; and that only one of these – UVB exposure – is a known carcinogen.
Also, some patients express concern that sunscreen itself may be a carcinogen. Oxybenzone, a common sunscreen ingredient, has demonstrated some estrogenic effects in vitro and in vivo studies. However, the rat studies often cited in support of that finding involved the use of very high doses – approximately the equivalent of 277 years of daily sunscreen application with 6% oxybenzone, a much higher concentration than is found in commercial sunscreens, she said.
For patients interested in nontopical sun protection, polypodium leucotomos extract (PLE) is an option, Dr. Harper said. PLE, an antioxidant extract from a tropical fern, can be part of a skin cancer prevention strategy that also includes good sunscreen and protective clothing. PLE works by counteracting UV-induced immunosuppression, activating the tumor suppressor p53 gene, and inhibiting cyclooxygenase-2, all of which can help protect the skin from burning.
In addition, oral nicotinamide has been shown to help repair DNA damage in human keratinocytes, and in a clinical trial, has been associated with fewer actinic keratoses and squamous cell carcinoma, compared with placebo, she said.
However, more research in these options is needed, and patients should be encouraged to follow consistent sun protection practices, Dr. Harper emphasized.
Dr. Harper disclosed relationships with companies including Allergan, Bayer, Galderma, LaRoche-Posay, Promius, Valeant, and BioPharmX.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – When sunscreens are tested for their SPF, testers apply 2 mg/cm2, but most people use only 20%-50% of that amount, which significantly reduces their protection, according to Dr. Julie C. Harper, director of the Dermatology & Skin Care Center of Birmingham, Ala.
The correct amount is 1 teaspoon of sunscreen on the face/head/neck, 1 teaspoon on each arm, 2 teaspoons on the torso, and 2 teaspoons on each leg, Dr. Harper said in a presentation at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. Reapplication every 90 minutes to 2 hours is key to effective protection, Dr. Harper said.
However, “most people use less than one bottle of sunscreen per year,” she noted.
Prompting patients to improve their sunscreen use involves disproving some myths, Dr. Harper pointed out. When patients cite concerns about low vitamin D as a reason to avoid sunscreens, she recommended that they be counseled that there are three sources of vitamin D: foods such as fatty fish, vitamin D fortified foods, cheese, and egg yolks; vitamin D supplements; and skin synthesis through UVB exposure; and that only one of these – UVB exposure – is a known carcinogen.
Also, some patients express concern that sunscreen itself may be a carcinogen. Oxybenzone, a common sunscreen ingredient, has demonstrated some estrogenic effects in vitro and in vivo studies. However, the rat studies often cited in support of that finding involved the use of very high doses – approximately the equivalent of 277 years of daily sunscreen application with 6% oxybenzone, a much higher concentration than is found in commercial sunscreens, she said.
For patients interested in nontopical sun protection, polypodium leucotomos extract (PLE) is an option, Dr. Harper said. PLE, an antioxidant extract from a tropical fern, can be part of a skin cancer prevention strategy that also includes good sunscreen and protective clothing. PLE works by counteracting UV-induced immunosuppression, activating the tumor suppressor p53 gene, and inhibiting cyclooxygenase-2, all of which can help protect the skin from burning.
In addition, oral nicotinamide has been shown to help repair DNA damage in human keratinocytes, and in a clinical trial, has been associated with fewer actinic keratoses and squamous cell carcinoma, compared with placebo, she said.
However, more research in these options is needed, and patients should be encouraged to follow consistent sun protection practices, Dr. Harper emphasized.
Dr. Harper disclosed relationships with companies including Allergan, Bayer, Galderma, LaRoche-Posay, Promius, Valeant, and BioPharmX.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – When sunscreens are tested for their SPF, testers apply 2 mg/cm2, but most people use only 20%-50% of that amount, which significantly reduces their protection, according to Dr. Julie C. Harper, director of the Dermatology & Skin Care Center of Birmingham, Ala.
The correct amount is 1 teaspoon of sunscreen on the face/head/neck, 1 teaspoon on each arm, 2 teaspoons on the torso, and 2 teaspoons on each leg, Dr. Harper said in a presentation at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. Reapplication every 90 minutes to 2 hours is key to effective protection, Dr. Harper said.
However, “most people use less than one bottle of sunscreen per year,” she noted.
Prompting patients to improve their sunscreen use involves disproving some myths, Dr. Harper pointed out. When patients cite concerns about low vitamin D as a reason to avoid sunscreens, she recommended that they be counseled that there are three sources of vitamin D: foods such as fatty fish, vitamin D fortified foods, cheese, and egg yolks; vitamin D supplements; and skin synthesis through UVB exposure; and that only one of these – UVB exposure – is a known carcinogen.
Also, some patients express concern that sunscreen itself may be a carcinogen. Oxybenzone, a common sunscreen ingredient, has demonstrated some estrogenic effects in vitro and in vivo studies. However, the rat studies often cited in support of that finding involved the use of very high doses – approximately the equivalent of 277 years of daily sunscreen application with 6% oxybenzone, a much higher concentration than is found in commercial sunscreens, she said.
For patients interested in nontopical sun protection, polypodium leucotomos extract (PLE) is an option, Dr. Harper said. PLE, an antioxidant extract from a tropical fern, can be part of a skin cancer prevention strategy that also includes good sunscreen and protective clothing. PLE works by counteracting UV-induced immunosuppression, activating the tumor suppressor p53 gene, and inhibiting cyclooxygenase-2, all of which can help protect the skin from burning.
In addition, oral nicotinamide has been shown to help repair DNA damage in human keratinocytes, and in a clinical trial, has been associated with fewer actinic keratoses and squamous cell carcinoma, compared with placebo, she said.
However, more research in these options is needed, and patients should be encouraged to follow consistent sun protection practices, Dr. Harper emphasized.
Dr. Harper disclosed relationships with companies including Allergan, Bayer, Galderma, LaRoche-Posay, Promius, Valeant, and BioPharmX.
SDEF and this news organization are owned by the same parent company.
Wheat in skin care
Gluten-free food products have inundated the marketplace in recent years as the food industry has responded to greater awareness of celiac sprue disease and wheat sensitivity. Gluten is the primary form of wheat protein.
Wheat is a versatile and globally popular member of the Poaceae or Gramineae family known as grasses; it is of the Triticum species with Triticum aestivum and Triticum vulgare being particularly pervasive.1 In traditional Iranian medicine, the topical application of wheat germ oil has been used to treat psoriasis.2
Moisturization
In 2008, N. Akhtar and Y. Yazan investigated the effects of a stable emulsion containing two ingredients included to exert anti-aging effects: vitamin C and wheat protein. The antioxidant vitamin C was entrapped in the inner aqueous phase of the water-in-oil-in-water emulsion while wheat protein was incorporated in the oily phase. The investigators prepared and applied stable emulsions to the cheeks of 11 volunteers over 4 weeks, finding that the formulation increased skin moisture.7
Melanoma and wheat supplementation
Demidov et al. reported in 2008 on a randomized, pilot, phase II clinical trial to assess the impact of the adjuvant use of a fermented wheat germ extract nutraceutical (Avemar) in high-risk cutaneous melanoma patients. Investigators compared the efficacy of dacarbazine-based adjuvant chemotherapy on survival parameters of melanoma patients to that of the identical treatment supplemented with a 1-year administration of fermented wheat germ extract nutraceutical (FWGE), which is generally recognized as safe. They reported that after a 7-year follow-up, significant differences favoring the nutraceutical group were observed in progression-free and overall survival. Including nutraceutical as an adjuvant treatment for such patients was recommended by the authors.8
Telekes et al. noted that nutraceutical is registered as a special nutriment for cancer patients in Hungary that has exhibited potent anticancer activity on cell lines and immunomodulatory activity in vivo.9 In 2005, Boros et al. reported that orally administered FWGE suppressed metastatic tumor dissemination and proliferation during and after chemotherapy, surgery, or radiation, with benefits seen in some human cancers and cultured cells as well as some autoimmune disorders and in chemical carcinogenesis prevention.10
Hypersensitivity and allergic reactions
The risks of sensitization to topical wheat proteins are thought to be higher in patients with atopic dermatitis, who have an impaired skin barrier.1 Indeed, Codreanu et al. have suggested that topical products containing food proteins of known allergenicity (including wheat) are contraindicated for neonates and infants with atopic dermatitis.11
In 2015, Bonciolini et al. studied 17 patients (13 females and 4 males, median age 36 years) with nonceliac gluten sensitivity presenting with nonspecific skin lesions. The eczema-, psoriasis-, or dermatitis herpetiformis-like lesions on the extensor surfaces of the upper and lower limbs, especially, were confirmed histologically, but immunopathological evaluations revealed pervasive C3 deposits along the dermoepidermal junction in a microgranular/granular pattern (82%). Notably, all of the patients improved markedly after initiating a gluten-free diet.12
In 2014, Fukutomi et al. conducted a case-control study of Japanese women aged 20-54 years (157 cases) who self-reported wheat allergy to ascertain the epidemiologic relationship between food allergy to wheat after exposure to facial soaps containing hydrolyzed wheat protein. There were 449 age-matched controls without wheat allergy. Participants answered a Web-based questionnaire about their use of skin and hair care products. The investigators found that current use of the facial soap Cha no Shizuku (Drop of Tea), which contains hydrolyzed wheat protein, was significantly linked to a greater risk of wheat allergy, with use of the soap more frequent in consumers whose wheat allergy had newly emerged (11% vs. 6% in controls).13
Cha no Shizuku had earlier been implicated in provoking hundreds of cases of allergic reactions between 2009 and June 2013. R. Teshima noted that the soap contains acid-hydrolyzed wheat protein produced from gluten after partial hydrolysis with hydrogen chloride at 95 ° C for 40 minutes.14
It is worth noting that case reports of allergic reactions to facial soap containing hydrolyzed wheat protein continue to appear. Iseki et al. described in 2014 a 38-year-old woman who experienced irregular headaches, sleepiness, and an episode of facial rash eruption after daily use for about 1 year of a facial soap with hydrolyzed wheat proteins (Glupearl 19s, which is also used in Cha no Shizuku). The investigators added that the patient’s serum contained wheat-specific IgE antibodies. Symptoms disappeared after the patient abstained from wheat.15
In 2012, Tammaro studied cutaneous hypersensitivity to gluten in 14 female patients (aged 12-60 years) with celiac disease who presented with eczema on the face, neck, and arms, after topical application of gluten-containing emollient cream, bath or face powder, or contact with foods containing wheat and durum. Five of the patients tested positive for wheat and durum wheat, while none of the 14 control patients tested positive. Improvement in cutaneous lesions, with no relapses during a 6-month follow-up, resulted when these patients used gluten-free cream and bath powder, and wore gloves before handling wheat-containing food.16
In 2011, Celakovská et al. studied the impact of wheat allergy in 179 adults with atopic eczema (128 females, 51 males; average age 26 years), using open exposure and double-blind, placebo-controlled food challenge tests, as well as specific serum IgE, skin prick, and atopy patch tests. The double-blind, placebo-controlled food challenge test showed that the course of atopic eczema was exacerbated by wheat allergy in eight patients (4.5%). A positive trend revealing that the course of atopic eczema was impacted by wheat allergy emerged during follow-up (at 3, 6, 9, and 12 months).17
Contact urticaria also has been reported to have been induced by hydrolyzed wheat proteins in cosmetics and is notable for the potential to precede food allergies.2,3 A wide variety of protein hydrolysates found in hair products have been associated with inducing contact urticaria, particularly in patients with atopic dermatitis.4
In 2006, Laurière et al. studied nine female patients without common wheat allergy who presented with contact urticaria to cosmetics containing hydrolyzed wheat proteins; six also had experienced generalized urticaria or anaphylaxis in response to foods containing such wheat proteins. Analyses revealed the importance of hydrolysis in augmenting the allergenicity of wheat proteins through contact or consumption.18 Immediate contact urticaria in reaction to hydrolyzed wheat protein in topical products also has been reported in a child.19
Conclusion
Can the presence of wheat hydrolysates in personal care products adversely affect a patient with celiac sprue or wheat sensitivity? The short answer appears to be “yes.” Given the use of hydrolyzed wheat protein in various skin care products, it is important that consumers who have celiac disease or sensitivity to wheat be advised to avoid skin care formulations with such active ingredients. On the positive side of the wheat ledger, there are some indications (albeit in very limited research) that the plant protein may impart beneficial health effects. Much more research is necessary to delineate the full impact of wheat on skin health.
I thank my dermatologist colleague Sharon E. Jacob, MD, at the University of Miami, for suggesting this topic.
References
1. Dermatitis. 2013 Nov-Dec;24(6):291-5.
2. Iran J Med Sci. 2016 May;41(3 Suppl):S54.
3. Contact Dermatitis. 2007 Feb;56(2):119-20.
4. Ann Dermatol Venereol. 2010 Apr;137(4):281-4.
5. Allergy. 1998 Nov;53(11):1078-82.
6. J Drugs Dermatol. 2013 Sep;12(9 Suppl):s133-6.
7. Pak J Pharm Sci. 2008 Jan;21(1):45-50.
8. Cancer Biother Radiopharm. 2008 Aug;23(4):477-82.
9. Nutr Cancer. 2009;61(6):891-9.
10. Ann N Y Acad Sci. 2005 Jun;1051:529-42.
11. Eur Ann Allergy Clin Immunol. 2006 Apr;38(4):126-30.
12. Nutrients. 2015 Sep 15;7(9):7798-805.
13. Allergy. 2014 Oct;69(10):1405-11.
14. Yakugaku Zasshi. 2014;134(1):33-8.
15. Intern Med. 2014;53(2):151-4.
16. Dermatitis. 2012 Sep-Oct;23(5):220-1.
17. Acta Medica (Hradec Kralove). 2011;54(4):157-62.
18. Contact Dermatitis. 2006 May;54(5):283-9.
19. Contact Dermatitis. 2013 Jun;68(6):379-80.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
Gluten-free food products have inundated the marketplace in recent years as the food industry has responded to greater awareness of celiac sprue disease and wheat sensitivity. Gluten is the primary form of wheat protein.
Wheat is a versatile and globally popular member of the Poaceae or Gramineae family known as grasses; it is of the Triticum species with Triticum aestivum and Triticum vulgare being particularly pervasive.1 In traditional Iranian medicine, the topical application of wheat germ oil has been used to treat psoriasis.2
Moisturization
In 2008, N. Akhtar and Y. Yazan investigated the effects of a stable emulsion containing two ingredients included to exert anti-aging effects: vitamin C and wheat protein. The antioxidant vitamin C was entrapped in the inner aqueous phase of the water-in-oil-in-water emulsion while wheat protein was incorporated in the oily phase. The investigators prepared and applied stable emulsions to the cheeks of 11 volunteers over 4 weeks, finding that the formulation increased skin moisture.7
Melanoma and wheat supplementation
Demidov et al. reported in 2008 on a randomized, pilot, phase II clinical trial to assess the impact of the adjuvant use of a fermented wheat germ extract nutraceutical (Avemar) in high-risk cutaneous melanoma patients. Investigators compared the efficacy of dacarbazine-based adjuvant chemotherapy on survival parameters of melanoma patients to that of the identical treatment supplemented with a 1-year administration of fermented wheat germ extract nutraceutical (FWGE), which is generally recognized as safe. They reported that after a 7-year follow-up, significant differences favoring the nutraceutical group were observed in progression-free and overall survival. Including nutraceutical as an adjuvant treatment for such patients was recommended by the authors.8
Telekes et al. noted that nutraceutical is registered as a special nutriment for cancer patients in Hungary that has exhibited potent anticancer activity on cell lines and immunomodulatory activity in vivo.9 In 2005, Boros et al. reported that orally administered FWGE suppressed metastatic tumor dissemination and proliferation during and after chemotherapy, surgery, or radiation, with benefits seen in some human cancers and cultured cells as well as some autoimmune disorders and in chemical carcinogenesis prevention.10
Hypersensitivity and allergic reactions
The risks of sensitization to topical wheat proteins are thought to be higher in patients with atopic dermatitis, who have an impaired skin barrier.1 Indeed, Codreanu et al. have suggested that topical products containing food proteins of known allergenicity (including wheat) are contraindicated for neonates and infants with atopic dermatitis.11
In 2015, Bonciolini et al. studied 17 patients (13 females and 4 males, median age 36 years) with nonceliac gluten sensitivity presenting with nonspecific skin lesions. The eczema-, psoriasis-, or dermatitis herpetiformis-like lesions on the extensor surfaces of the upper and lower limbs, especially, were confirmed histologically, but immunopathological evaluations revealed pervasive C3 deposits along the dermoepidermal junction in a microgranular/granular pattern (82%). Notably, all of the patients improved markedly after initiating a gluten-free diet.12
In 2014, Fukutomi et al. conducted a case-control study of Japanese women aged 20-54 years (157 cases) who self-reported wheat allergy to ascertain the epidemiologic relationship between food allergy to wheat after exposure to facial soaps containing hydrolyzed wheat protein. There were 449 age-matched controls without wheat allergy. Participants answered a Web-based questionnaire about their use of skin and hair care products. The investigators found that current use of the facial soap Cha no Shizuku (Drop of Tea), which contains hydrolyzed wheat protein, was significantly linked to a greater risk of wheat allergy, with use of the soap more frequent in consumers whose wheat allergy had newly emerged (11% vs. 6% in controls).13
Cha no Shizuku had earlier been implicated in provoking hundreds of cases of allergic reactions between 2009 and June 2013. R. Teshima noted that the soap contains acid-hydrolyzed wheat protein produced from gluten after partial hydrolysis with hydrogen chloride at 95 ° C for 40 minutes.14
It is worth noting that case reports of allergic reactions to facial soap containing hydrolyzed wheat protein continue to appear. Iseki et al. described in 2014 a 38-year-old woman who experienced irregular headaches, sleepiness, and an episode of facial rash eruption after daily use for about 1 year of a facial soap with hydrolyzed wheat proteins (Glupearl 19s, which is also used in Cha no Shizuku). The investigators added that the patient’s serum contained wheat-specific IgE antibodies. Symptoms disappeared after the patient abstained from wheat.15
In 2012, Tammaro studied cutaneous hypersensitivity to gluten in 14 female patients (aged 12-60 years) with celiac disease who presented with eczema on the face, neck, and arms, after topical application of gluten-containing emollient cream, bath or face powder, or contact with foods containing wheat and durum. Five of the patients tested positive for wheat and durum wheat, while none of the 14 control patients tested positive. Improvement in cutaneous lesions, with no relapses during a 6-month follow-up, resulted when these patients used gluten-free cream and bath powder, and wore gloves before handling wheat-containing food.16
In 2011, Celakovská et al. studied the impact of wheat allergy in 179 adults with atopic eczema (128 females, 51 males; average age 26 years), using open exposure and double-blind, placebo-controlled food challenge tests, as well as specific serum IgE, skin prick, and atopy patch tests. The double-blind, placebo-controlled food challenge test showed that the course of atopic eczema was exacerbated by wheat allergy in eight patients (4.5%). A positive trend revealing that the course of atopic eczema was impacted by wheat allergy emerged during follow-up (at 3, 6, 9, and 12 months).17
Contact urticaria also has been reported to have been induced by hydrolyzed wheat proteins in cosmetics and is notable for the potential to precede food allergies.2,3 A wide variety of protein hydrolysates found in hair products have been associated with inducing contact urticaria, particularly in patients with atopic dermatitis.4
In 2006, Laurière et al. studied nine female patients without common wheat allergy who presented with contact urticaria to cosmetics containing hydrolyzed wheat proteins; six also had experienced generalized urticaria or anaphylaxis in response to foods containing such wheat proteins. Analyses revealed the importance of hydrolysis in augmenting the allergenicity of wheat proteins through contact or consumption.18 Immediate contact urticaria in reaction to hydrolyzed wheat protein in topical products also has been reported in a child.19
Conclusion
Can the presence of wheat hydrolysates in personal care products adversely affect a patient with celiac sprue or wheat sensitivity? The short answer appears to be “yes.” Given the use of hydrolyzed wheat protein in various skin care products, it is important that consumers who have celiac disease or sensitivity to wheat be advised to avoid skin care formulations with such active ingredients. On the positive side of the wheat ledger, there are some indications (albeit in very limited research) that the plant protein may impart beneficial health effects. Much more research is necessary to delineate the full impact of wheat on skin health.
I thank my dermatologist colleague Sharon E. Jacob, MD, at the University of Miami, for suggesting this topic.
References
1. Dermatitis. 2013 Nov-Dec;24(6):291-5.
2. Iran J Med Sci. 2016 May;41(3 Suppl):S54.
3. Contact Dermatitis. 2007 Feb;56(2):119-20.
4. Ann Dermatol Venereol. 2010 Apr;137(4):281-4.
5. Allergy. 1998 Nov;53(11):1078-82.
6. J Drugs Dermatol. 2013 Sep;12(9 Suppl):s133-6.
7. Pak J Pharm Sci. 2008 Jan;21(1):45-50.
8. Cancer Biother Radiopharm. 2008 Aug;23(4):477-82.
9. Nutr Cancer. 2009;61(6):891-9.
10. Ann N Y Acad Sci. 2005 Jun;1051:529-42.
11. Eur Ann Allergy Clin Immunol. 2006 Apr;38(4):126-30.
12. Nutrients. 2015 Sep 15;7(9):7798-805.
13. Allergy. 2014 Oct;69(10):1405-11.
14. Yakugaku Zasshi. 2014;134(1):33-8.
15. Intern Med. 2014;53(2):151-4.
16. Dermatitis. 2012 Sep-Oct;23(5):220-1.
17. Acta Medica (Hradec Kralove). 2011;54(4):157-62.
18. Contact Dermatitis. 2006 May;54(5):283-9.
19. Contact Dermatitis. 2013 Jun;68(6):379-80.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
Gluten-free food products have inundated the marketplace in recent years as the food industry has responded to greater awareness of celiac sprue disease and wheat sensitivity. Gluten is the primary form of wheat protein.
Wheat is a versatile and globally popular member of the Poaceae or Gramineae family known as grasses; it is of the Triticum species with Triticum aestivum and Triticum vulgare being particularly pervasive.1 In traditional Iranian medicine, the topical application of wheat germ oil has been used to treat psoriasis.2
Moisturization
In 2008, N. Akhtar and Y. Yazan investigated the effects of a stable emulsion containing two ingredients included to exert anti-aging effects: vitamin C and wheat protein. The antioxidant vitamin C was entrapped in the inner aqueous phase of the water-in-oil-in-water emulsion while wheat protein was incorporated in the oily phase. The investigators prepared and applied stable emulsions to the cheeks of 11 volunteers over 4 weeks, finding that the formulation increased skin moisture.7
Melanoma and wheat supplementation
Demidov et al. reported in 2008 on a randomized, pilot, phase II clinical trial to assess the impact of the adjuvant use of a fermented wheat germ extract nutraceutical (Avemar) in high-risk cutaneous melanoma patients. Investigators compared the efficacy of dacarbazine-based adjuvant chemotherapy on survival parameters of melanoma patients to that of the identical treatment supplemented with a 1-year administration of fermented wheat germ extract nutraceutical (FWGE), which is generally recognized as safe. They reported that after a 7-year follow-up, significant differences favoring the nutraceutical group were observed in progression-free and overall survival. Including nutraceutical as an adjuvant treatment for such patients was recommended by the authors.8
Telekes et al. noted that nutraceutical is registered as a special nutriment for cancer patients in Hungary that has exhibited potent anticancer activity on cell lines and immunomodulatory activity in vivo.9 In 2005, Boros et al. reported that orally administered FWGE suppressed metastatic tumor dissemination and proliferation during and after chemotherapy, surgery, or radiation, with benefits seen in some human cancers and cultured cells as well as some autoimmune disorders and in chemical carcinogenesis prevention.10
Hypersensitivity and allergic reactions
The risks of sensitization to topical wheat proteins are thought to be higher in patients with atopic dermatitis, who have an impaired skin barrier.1 Indeed, Codreanu et al. have suggested that topical products containing food proteins of known allergenicity (including wheat) are contraindicated for neonates and infants with atopic dermatitis.11
In 2015, Bonciolini et al. studied 17 patients (13 females and 4 males, median age 36 years) with nonceliac gluten sensitivity presenting with nonspecific skin lesions. The eczema-, psoriasis-, or dermatitis herpetiformis-like lesions on the extensor surfaces of the upper and lower limbs, especially, were confirmed histologically, but immunopathological evaluations revealed pervasive C3 deposits along the dermoepidermal junction in a microgranular/granular pattern (82%). Notably, all of the patients improved markedly after initiating a gluten-free diet.12
In 2014, Fukutomi et al. conducted a case-control study of Japanese women aged 20-54 years (157 cases) who self-reported wheat allergy to ascertain the epidemiologic relationship between food allergy to wheat after exposure to facial soaps containing hydrolyzed wheat protein. There were 449 age-matched controls without wheat allergy. Participants answered a Web-based questionnaire about their use of skin and hair care products. The investigators found that current use of the facial soap Cha no Shizuku (Drop of Tea), which contains hydrolyzed wheat protein, was significantly linked to a greater risk of wheat allergy, with use of the soap more frequent in consumers whose wheat allergy had newly emerged (11% vs. 6% in controls).13
Cha no Shizuku had earlier been implicated in provoking hundreds of cases of allergic reactions between 2009 and June 2013. R. Teshima noted that the soap contains acid-hydrolyzed wheat protein produced from gluten after partial hydrolysis with hydrogen chloride at 95 ° C for 40 minutes.14
It is worth noting that case reports of allergic reactions to facial soap containing hydrolyzed wheat protein continue to appear. Iseki et al. described in 2014 a 38-year-old woman who experienced irregular headaches, sleepiness, and an episode of facial rash eruption after daily use for about 1 year of a facial soap with hydrolyzed wheat proteins (Glupearl 19s, which is also used in Cha no Shizuku). The investigators added that the patient’s serum contained wheat-specific IgE antibodies. Symptoms disappeared after the patient abstained from wheat.15
In 2012, Tammaro studied cutaneous hypersensitivity to gluten in 14 female patients (aged 12-60 years) with celiac disease who presented with eczema on the face, neck, and arms, after topical application of gluten-containing emollient cream, bath or face powder, or contact with foods containing wheat and durum. Five of the patients tested positive for wheat and durum wheat, while none of the 14 control patients tested positive. Improvement in cutaneous lesions, with no relapses during a 6-month follow-up, resulted when these patients used gluten-free cream and bath powder, and wore gloves before handling wheat-containing food.16
In 2011, Celakovská et al. studied the impact of wheat allergy in 179 adults with atopic eczema (128 females, 51 males; average age 26 years), using open exposure and double-blind, placebo-controlled food challenge tests, as well as specific serum IgE, skin prick, and atopy patch tests. The double-blind, placebo-controlled food challenge test showed that the course of atopic eczema was exacerbated by wheat allergy in eight patients (4.5%). A positive trend revealing that the course of atopic eczema was impacted by wheat allergy emerged during follow-up (at 3, 6, 9, and 12 months).17
Contact urticaria also has been reported to have been induced by hydrolyzed wheat proteins in cosmetics and is notable for the potential to precede food allergies.2,3 A wide variety of protein hydrolysates found in hair products have been associated with inducing contact urticaria, particularly in patients with atopic dermatitis.4
In 2006, Laurière et al. studied nine female patients without common wheat allergy who presented with contact urticaria to cosmetics containing hydrolyzed wheat proteins; six also had experienced generalized urticaria or anaphylaxis in response to foods containing such wheat proteins. Analyses revealed the importance of hydrolysis in augmenting the allergenicity of wheat proteins through contact or consumption.18 Immediate contact urticaria in reaction to hydrolyzed wheat protein in topical products also has been reported in a child.19
Conclusion
Can the presence of wheat hydrolysates in personal care products adversely affect a patient with celiac sprue or wheat sensitivity? The short answer appears to be “yes.” Given the use of hydrolyzed wheat protein in various skin care products, it is important that consumers who have celiac disease or sensitivity to wheat be advised to avoid skin care formulations with such active ingredients. On the positive side of the wheat ledger, there are some indications (albeit in very limited research) that the plant protein may impart beneficial health effects. Much more research is necessary to delineate the full impact of wheat on skin health.
I thank my dermatologist colleague Sharon E. Jacob, MD, at the University of Miami, for suggesting this topic.
References
1. Dermatitis. 2013 Nov-Dec;24(6):291-5.
2. Iran J Med Sci. 2016 May;41(3 Suppl):S54.
3. Contact Dermatitis. 2007 Feb;56(2):119-20.
4. Ann Dermatol Venereol. 2010 Apr;137(4):281-4.
5. Allergy. 1998 Nov;53(11):1078-82.
6. J Drugs Dermatol. 2013 Sep;12(9 Suppl):s133-6.
7. Pak J Pharm Sci. 2008 Jan;21(1):45-50.
8. Cancer Biother Radiopharm. 2008 Aug;23(4):477-82.
9. Nutr Cancer. 2009;61(6):891-9.
10. Ann N Y Acad Sci. 2005 Jun;1051:529-42.
11. Eur Ann Allergy Clin Immunol. 2006 Apr;38(4):126-30.
12. Nutrients. 2015 Sep 15;7(9):7798-805.
13. Allergy. 2014 Oct;69(10):1405-11.
14. Yakugaku Zasshi. 2014;134(1):33-8.
15. Intern Med. 2014;53(2):151-4.
16. Dermatitis. 2012 Sep-Oct;23(5):220-1.
17. Acta Medica (Hradec Kralove). 2011;54(4):157-62.
18. Contact Dermatitis. 2006 May;54(5):283-9.
19. Contact Dermatitis. 2013 Jun;68(6):379-80.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
Topical Imiquimod Clears Invasive Melanoma
Malignant melanoma has continually shown a pattern of increased incidence and mortality over the last 50 years, especially in fair-skinned individuals. In fact, malignant melanoma has the highest mortality rate of all skin cancers in white individuals. Currently, wide local surgical excision is the mainstay of treatment of primary cutaneous melanomas.1 The margins vary in size according to the Breslow thickness (or depth) of the involved tumor. As such, advancements in melanoma treatment continue to be studied. We present the case of a patient with invasive melanoma that was cleared with topical imiquimod.
Case Report
A 71-year-old man presented with biopsy-proven malignant melanoma on the right posterior scalp that was diagnosed a few weeks prior. The melanoma was invasive with a depth of 0.73 mm. The patient also had an approximately 8-cm, irregular, patchy area of hyperpigmentation involving almost the entire crown of the head (Figure 1A). The biopsy site used for melanoma diagnosis was on the right posterior aspect of the hyperpigmented area where a symptomatic pigmented papule was located. To determine if the rest of this macule represented an extension of the proven malignancy, surveillance biopsies were taken at the 12 o'clock (anterior aspect), 3 o'clock, 6 o'clock, and 9 o'clock positions on the head. All of the biopsies came back as lentigo simplex, which presented a clinical problem in that the boundaries of the invasive melanoma merged with the lentigo simplex and were not clinically apparent. Because an exact boundary could not be visualized, the entire area was treated with imiquimod cream 5% once nightly at bedtime for 4 weeks prior to excision of the original biopsy site. There was a notable decrease in hyperpigmentation in the treated area after 4 weeks of therapy (Figure 1B). The original biopsy site was then excised with a 0.6-cm margin and a complex linear repair was performed. Histologic examination of the excised specimen showed no residual melanoma.
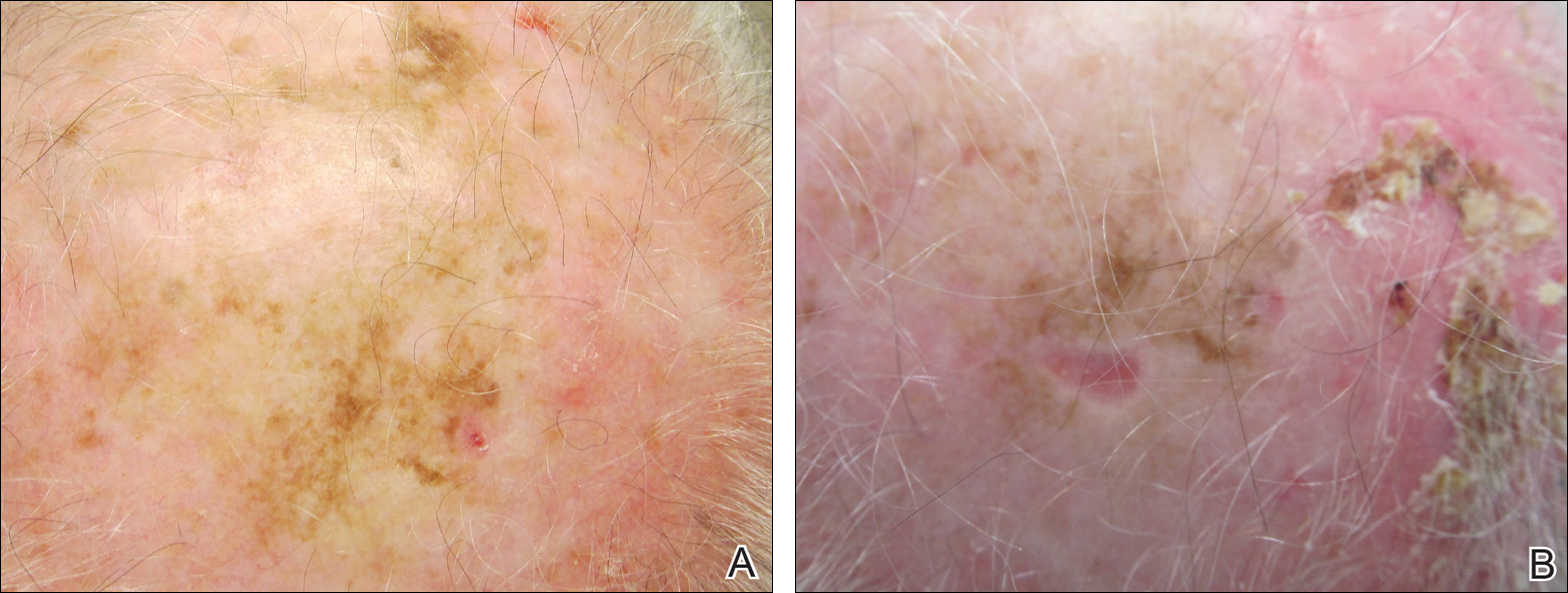
Comment
Although surgical excision is the recommended treatment of cutaneous melanoma,1 in some cases the defect following an excision can be quite large or even disfiguring. To minimize the size of the excision site, other treatment modalities should be studied. Imiquimod is an immunomodulating agent that exerts antitumor and antiviral effects. The US Food and Drug Administration has approved imiquimod for treatment of genital warts, actinic keratoses, and superficial basal cell carcinoma.2 The most common side effects of topical imiquimod involve application-site reactions such as erythema, swelling, and crusting of the treated area. Ulceration of the skin also is possible. A small percentage of individuals have experienced systemic flulike symptoms after using topical imiquimod. Topical imiquimod has been used off label to treat noninvasive forms of melanoma. The topical therapy has been reported to clear melanoma in situ and lentigo maligna.2,3 In addition, imiquimod has been used as a palliative therapy for cutaneous metastatic melanoma.4,5 In another case of a primary melanoma that responded to topical imiquimod, clinical and histological clearance of a recurrent oral mucosa melanoma was obtained.6
Moon and Spencer7 reported a case of an invasive melanoma that was cleared with topical imiquimod. A 93-year-old woman presented with a central 2.75-mm thick invasive melanoma surrounded by a large area of melanoma in situ involving the left cheek and eyelid. The excised tissue was stained for CD31 and D2-40 to rule out intravascular and intralymphatic spread (Figure 2A). The standard-of-care treatment for this case would involve surgical excision with 2-cm margins and a sentinel lymph node biopsy, but given the morbidity involved with the surgery, an alternative treatment plan was made with the patient. The patient completed 5 weeks of topical imiquimod therapy and then underwent wide local excision with a 1-cm margin. Extensive histological examination of the excised specimen showed no residual melanoma; in fact, there was a near absence of junctional melanocytes that would normally have been seen. The specimen underwent immunoperoxidase staining for Melan-A (Figure 2B). The patient was followed for 14 months with no evidence of recurrence.7
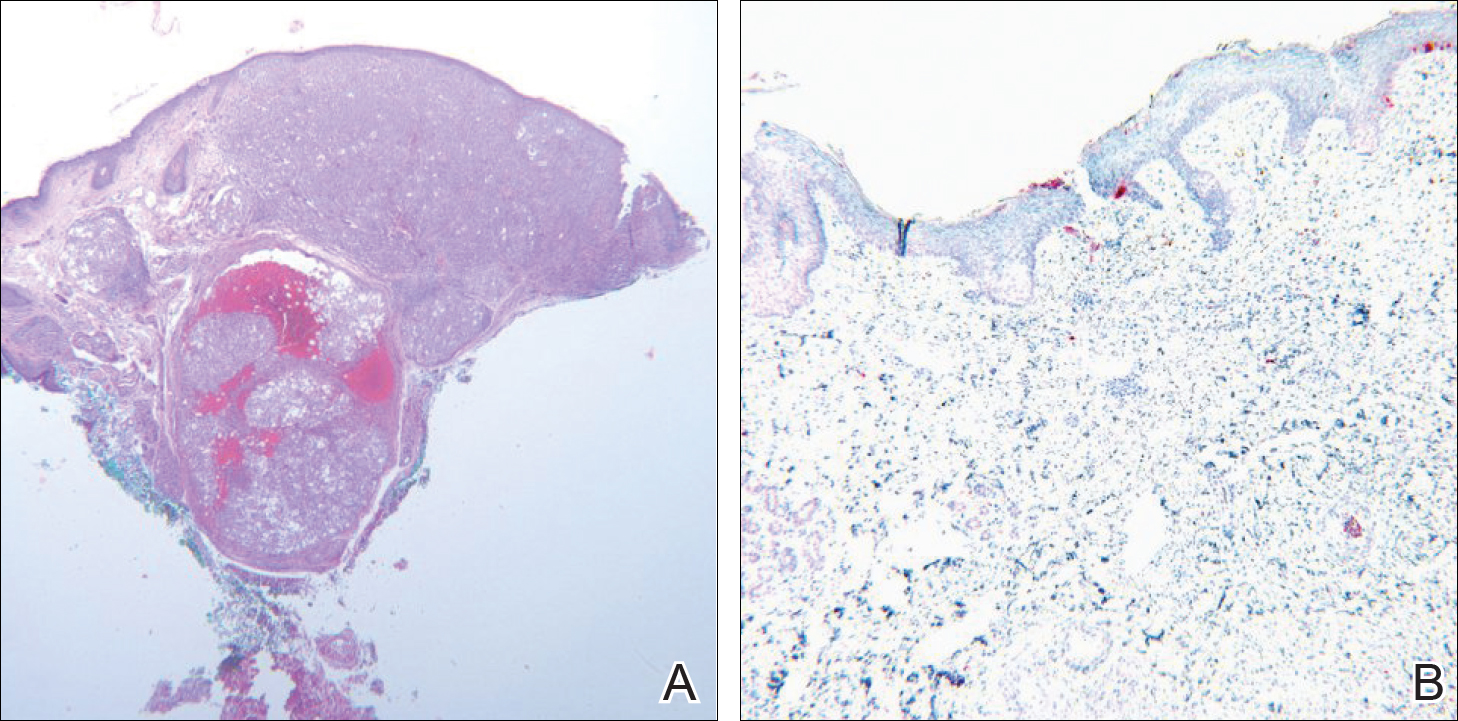
Conclusion
We describe a patient who achieved complete histologic clearance of invasive melanoma following treatment with topical imiquimod. Four weeks of topical therapy completely cleared an invasive melanoma that was 0.73-mm thick. Follow-up was recommended for the patient because long-term outcomes of this therapy are unknown. More studies demonstrating reliability and reproducibility are needed to evaluate the role of topical imiquimod in melanoma treatment; however, our case shows the potential of this topical modality.
- Rastrelli M, Alaibac M, Stramare R, et al. Melanoma m (zero): diagnosis and therapy. ISRN Dermatol. 2013;2013:616170.
- Ellis LZ, Cohen JL, High W, et al. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: review of the literature. Dermatol Surg. 2012;38:937-946.
- Cotter MA, McKenna JK, Bowen GM. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008;34:147-151.
- Li X, Naylor MF, Le H, et al. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol Ther. 2010;10:1081-1087.
- Steinmann A, Funk JO, Schuler G, et al. Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol. 2000;43:555-556.
- Spieth K, Kovács A, Wolter M, et al. Topical imiquimod: effectiveness in intraepithelial melanoma of oral mucosa. Lancet Oncol. 2006;7:1036-1037.
- Moon SD, Spencer JM. Clearance of invasive melanoma with topical imiquimod. J Drugs Dermatol. 2013;12:107-108.
Malignant melanoma has continually shown a pattern of increased incidence and mortality over the last 50 years, especially in fair-skinned individuals. In fact, malignant melanoma has the highest mortality rate of all skin cancers in white individuals. Currently, wide local surgical excision is the mainstay of treatment of primary cutaneous melanomas.1 The margins vary in size according to the Breslow thickness (or depth) of the involved tumor. As such, advancements in melanoma treatment continue to be studied. We present the case of a patient with invasive melanoma that was cleared with topical imiquimod.
Case Report
A 71-year-old man presented with biopsy-proven malignant melanoma on the right posterior scalp that was diagnosed a few weeks prior. The melanoma was invasive with a depth of 0.73 mm. The patient also had an approximately 8-cm, irregular, patchy area of hyperpigmentation involving almost the entire crown of the head (Figure 1A). The biopsy site used for melanoma diagnosis was on the right posterior aspect of the hyperpigmented area where a symptomatic pigmented papule was located. To determine if the rest of this macule represented an extension of the proven malignancy, surveillance biopsies were taken at the 12 o'clock (anterior aspect), 3 o'clock, 6 o'clock, and 9 o'clock positions on the head. All of the biopsies came back as lentigo simplex, which presented a clinical problem in that the boundaries of the invasive melanoma merged with the lentigo simplex and were not clinically apparent. Because an exact boundary could not be visualized, the entire area was treated with imiquimod cream 5% once nightly at bedtime for 4 weeks prior to excision of the original biopsy site. There was a notable decrease in hyperpigmentation in the treated area after 4 weeks of therapy (Figure 1B). The original biopsy site was then excised with a 0.6-cm margin and a complex linear repair was performed. Histologic examination of the excised specimen showed no residual melanoma.

Comment
Although surgical excision is the recommended treatment of cutaneous melanoma,1 in some cases the defect following an excision can be quite large or even disfiguring. To minimize the size of the excision site, other treatment modalities should be studied. Imiquimod is an immunomodulating agent that exerts antitumor and antiviral effects. The US Food and Drug Administration has approved imiquimod for treatment of genital warts, actinic keratoses, and superficial basal cell carcinoma.2 The most common side effects of topical imiquimod involve application-site reactions such as erythema, swelling, and crusting of the treated area. Ulceration of the skin also is possible. A small percentage of individuals have experienced systemic flulike symptoms after using topical imiquimod. Topical imiquimod has been used off label to treat noninvasive forms of melanoma. The topical therapy has been reported to clear melanoma in situ and lentigo maligna.2,3 In addition, imiquimod has been used as a palliative therapy for cutaneous metastatic melanoma.4,5 In another case of a primary melanoma that responded to topical imiquimod, clinical and histological clearance of a recurrent oral mucosa melanoma was obtained.6
Moon and Spencer7 reported a case of an invasive melanoma that was cleared with topical imiquimod. A 93-year-old woman presented with a central 2.75-mm thick invasive melanoma surrounded by a large area of melanoma in situ involving the left cheek and eyelid. The excised tissue was stained for CD31 and D2-40 to rule out intravascular and intralymphatic spread (Figure 2A). The standard-of-care treatment for this case would involve surgical excision with 2-cm margins and a sentinel lymph node biopsy, but given the morbidity involved with the surgery, an alternative treatment plan was made with the patient. The patient completed 5 weeks of topical imiquimod therapy and then underwent wide local excision with a 1-cm margin. Extensive histological examination of the excised specimen showed no residual melanoma; in fact, there was a near absence of junctional melanocytes that would normally have been seen. The specimen underwent immunoperoxidase staining for Melan-A (Figure 2B). The patient was followed for 14 months with no evidence of recurrence.7

Conclusion
We describe a patient who achieved complete histologic clearance of invasive melanoma following treatment with topical imiquimod. Four weeks of topical therapy completely cleared an invasive melanoma that was 0.73-mm thick. Follow-up was recommended for the patient because long-term outcomes of this therapy are unknown. More studies demonstrating reliability and reproducibility are needed to evaluate the role of topical imiquimod in melanoma treatment; however, our case shows the potential of this topical modality.
Malignant melanoma has continually shown a pattern of increased incidence and mortality over the last 50 years, especially in fair-skinned individuals. In fact, malignant melanoma has the highest mortality rate of all skin cancers in white individuals. Currently, wide local surgical excision is the mainstay of treatment of primary cutaneous melanomas.1 The margins vary in size according to the Breslow thickness (or depth) of the involved tumor. As such, advancements in melanoma treatment continue to be studied. We present the case of a patient with invasive melanoma that was cleared with topical imiquimod.
Case Report
A 71-year-old man presented with biopsy-proven malignant melanoma on the right posterior scalp that was diagnosed a few weeks prior. The melanoma was invasive with a depth of 0.73 mm. The patient also had an approximately 8-cm, irregular, patchy area of hyperpigmentation involving almost the entire crown of the head (Figure 1A). The biopsy site used for melanoma diagnosis was on the right posterior aspect of the hyperpigmented area where a symptomatic pigmented papule was located. To determine if the rest of this macule represented an extension of the proven malignancy, surveillance biopsies were taken at the 12 o'clock (anterior aspect), 3 o'clock, 6 o'clock, and 9 o'clock positions on the head. All of the biopsies came back as lentigo simplex, which presented a clinical problem in that the boundaries of the invasive melanoma merged with the lentigo simplex and were not clinically apparent. Because an exact boundary could not be visualized, the entire area was treated with imiquimod cream 5% once nightly at bedtime for 4 weeks prior to excision of the original biopsy site. There was a notable decrease in hyperpigmentation in the treated area after 4 weeks of therapy (Figure 1B). The original biopsy site was then excised with a 0.6-cm margin and a complex linear repair was performed. Histologic examination of the excised specimen showed no residual melanoma.

Comment
Although surgical excision is the recommended treatment of cutaneous melanoma,1 in some cases the defect following an excision can be quite large or even disfiguring. To minimize the size of the excision site, other treatment modalities should be studied. Imiquimod is an immunomodulating agent that exerts antitumor and antiviral effects. The US Food and Drug Administration has approved imiquimod for treatment of genital warts, actinic keratoses, and superficial basal cell carcinoma.2 The most common side effects of topical imiquimod involve application-site reactions such as erythema, swelling, and crusting of the treated area. Ulceration of the skin also is possible. A small percentage of individuals have experienced systemic flulike symptoms after using topical imiquimod. Topical imiquimod has been used off label to treat noninvasive forms of melanoma. The topical therapy has been reported to clear melanoma in situ and lentigo maligna.2,3 In addition, imiquimod has been used as a palliative therapy for cutaneous metastatic melanoma.4,5 In another case of a primary melanoma that responded to topical imiquimod, clinical and histological clearance of a recurrent oral mucosa melanoma was obtained.6
Moon and Spencer7 reported a case of an invasive melanoma that was cleared with topical imiquimod. A 93-year-old woman presented with a central 2.75-mm thick invasive melanoma surrounded by a large area of melanoma in situ involving the left cheek and eyelid. The excised tissue was stained for CD31 and D2-40 to rule out intravascular and intralymphatic spread (Figure 2A). The standard-of-care treatment for this case would involve surgical excision with 2-cm margins and a sentinel lymph node biopsy, but given the morbidity involved with the surgery, an alternative treatment plan was made with the patient. The patient completed 5 weeks of topical imiquimod therapy and then underwent wide local excision with a 1-cm margin. Extensive histological examination of the excised specimen showed no residual melanoma; in fact, there was a near absence of junctional melanocytes that would normally have been seen. The specimen underwent immunoperoxidase staining for Melan-A (Figure 2B). The patient was followed for 14 months with no evidence of recurrence.7

Conclusion
We describe a patient who achieved complete histologic clearance of invasive melanoma following treatment with topical imiquimod. Four weeks of topical therapy completely cleared an invasive melanoma that was 0.73-mm thick. Follow-up was recommended for the patient because long-term outcomes of this therapy are unknown. More studies demonstrating reliability and reproducibility are needed to evaluate the role of topical imiquimod in melanoma treatment; however, our case shows the potential of this topical modality.
- Rastrelli M, Alaibac M, Stramare R, et al. Melanoma m (zero): diagnosis and therapy. ISRN Dermatol. 2013;2013:616170.
- Ellis LZ, Cohen JL, High W, et al. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: review of the literature. Dermatol Surg. 2012;38:937-946.
- Cotter MA, McKenna JK, Bowen GM. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008;34:147-151.
- Li X, Naylor MF, Le H, et al. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol Ther. 2010;10:1081-1087.
- Steinmann A, Funk JO, Schuler G, et al. Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol. 2000;43:555-556.
- Spieth K, Kovács A, Wolter M, et al. Topical imiquimod: effectiveness in intraepithelial melanoma of oral mucosa. Lancet Oncol. 2006;7:1036-1037.
- Moon SD, Spencer JM. Clearance of invasive melanoma with topical imiquimod. J Drugs Dermatol. 2013;12:107-108.
- Rastrelli M, Alaibac M, Stramare R, et al. Melanoma m (zero): diagnosis and therapy. ISRN Dermatol. 2013;2013:616170.
- Ellis LZ, Cohen JL, High W, et al. Melanoma in situ treated successfully using imiquimod after nonclearance with surgery: review of the literature. Dermatol Surg. 2012;38:937-946.
- Cotter MA, McKenna JK, Bowen GM. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008;34:147-151.
- Li X, Naylor MF, Le H, et al. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol Ther. 2010;10:1081-1087.
- Steinmann A, Funk JO, Schuler G, et al. Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol. 2000;43:555-556.
- Spieth K, Kovács A, Wolter M, et al. Topical imiquimod: effectiveness in intraepithelial melanoma of oral mucosa. Lancet Oncol. 2006;7:1036-1037.
- Moon SD, Spencer JM. Clearance of invasive melanoma with topical imiquimod. J Drugs Dermatol. 2013;12:107-108.
Practice Points
- Topical imiquimod may clear invasive melanoma as well as melanoma in situ.
- Further study is required to confirm the role of topical imiquimod in melanoma treatment.
Skin cancer a concern in pediatric solid organ transplant recipients
As survival rates among pediatric organ transplant recipients increase, so do the rates of cutaneous malignancies later in life for this population, who are at a greater risk for skin cancers that include nonmelanoma skin cancers (NMSCs), melanoma, Kaposi sarcoma, and anogenital carcinoma, according to the authors of a literature review.
In studies, skin cancers account for 13%-55% of all cancers in pediatric organ transplant recipients (POTRs), according to Alexander L. Fogel of Stanford (Calif.) University and his coauthors. The review article provides an update on this topic, as well as information on the prevention and management of skin cancers in this population, and the differences between this group and adult organ transplant recipients (AOTRs).
NMSC is the most common type of skin cancer in the pediatric group – and the second most common type of malignancy (NMSCs are the most common type of cancer affecting adult organ transplant recipients). NMSCs typically appear an average of 12-18 years post transplantation in this population (at an average age of 26-34 years). Length of posttransplantation follow-up, sunlight exposure, fair skin, and Northern European ancestry are among the factors associated with increased risk. This type of cancer involves the lip nearly twice as often as in adult recipients: 23% vs. 12%. The pediatric cohort also experiences more nonmelanoma cancer spreading to the lymph nodes than do adults: 9% vs. 6%.
Among pediatric transplant recipients, squamous cell carcinomas appear 2.8 times more often than basal cell carcinomas, “a trend that is opposite that observed in the nontransplant population,” the authors wrote.
In one study, anogenital carcinomas accounted for 4% of posttransplant cancers in this cohort, at an average of 12 years after the transplant, at a mean age of 27 years.
Some data indicate that in adult transplant recipients, there is an association between the human papillomavirus, and anal and genital warts and posttransplant anogenital cancer, but there are little data looking at this association in the pediatric group, the authors noted.
Although melanoma and Kaposi sarcoma are also found in this cohort at rates greater than in the general population, and are associated with high mortality rates, the data are too few to draw conclusions, the authors wrote.
In 2014, 1,795 pediatric solid organ transplants were performed, accounting for 6% of all such transplants. The absolute number of pediatric transplants has remained fairly stable over 5 years, yet very little pediatric-specific literature exists for prevention and management of skin cancers post transplantation, the authors pointed out.
Changing immunosuppressive medications used in transplantation may be effective in reducing skin cancer risk, they said, noting that including rapamycin inhibitors in combination therapy has been shown to reduce the risk of developing skin cancers in some transplant patients by more than half.
The authors emphasized that regular sunscreen use and dermatologic checkups are also essential in this population, and that “the importance of regular dermatologic evaluation should be stressed to patients and their families.”
Mr. Fogel’s coauthors were Mari Miyar, MD, of the department of dermatology, Kaiser Permanente, San Jose, Calif., and Joyce Teng, MD, of the departments of dermatology and pediatrics, Stanford. The authors had no disclosures listed, and no funding source for the review was listed.
This article was updated 12/8/16.
As survival rates among pediatric organ transplant recipients increase, so do the rates of cutaneous malignancies later in life for this population, who are at a greater risk for skin cancers that include nonmelanoma skin cancers (NMSCs), melanoma, Kaposi sarcoma, and anogenital carcinoma, according to the authors of a literature review.
In studies, skin cancers account for 13%-55% of all cancers in pediatric organ transplant recipients (POTRs), according to Alexander L. Fogel of Stanford (Calif.) University and his coauthors. The review article provides an update on this topic, as well as information on the prevention and management of skin cancers in this population, and the differences between this group and adult organ transplant recipients (AOTRs).
NMSC is the most common type of skin cancer in the pediatric group – and the second most common type of malignancy (NMSCs are the most common type of cancer affecting adult organ transplant recipients). NMSCs typically appear an average of 12-18 years post transplantation in this population (at an average age of 26-34 years). Length of posttransplantation follow-up, sunlight exposure, fair skin, and Northern European ancestry are among the factors associated with increased risk. This type of cancer involves the lip nearly twice as often as in adult recipients: 23% vs. 12%. The pediatric cohort also experiences more nonmelanoma cancer spreading to the lymph nodes than do adults: 9% vs. 6%.
Among pediatric transplant recipients, squamous cell carcinomas appear 2.8 times more often than basal cell carcinomas, “a trend that is opposite that observed in the nontransplant population,” the authors wrote.
In one study, anogenital carcinomas accounted for 4% of posttransplant cancers in this cohort, at an average of 12 years after the transplant, at a mean age of 27 years.
Some data indicate that in adult transplant recipients, there is an association between the human papillomavirus, and anal and genital warts and posttransplant anogenital cancer, but there are little data looking at this association in the pediatric group, the authors noted.
Although melanoma and Kaposi sarcoma are also found in this cohort at rates greater than in the general population, and are associated with high mortality rates, the data are too few to draw conclusions, the authors wrote.
In 2014, 1,795 pediatric solid organ transplants were performed, accounting for 6% of all such transplants. The absolute number of pediatric transplants has remained fairly stable over 5 years, yet very little pediatric-specific literature exists for prevention and management of skin cancers post transplantation, the authors pointed out.
Changing immunosuppressive medications used in transplantation may be effective in reducing skin cancer risk, they said, noting that including rapamycin inhibitors in combination therapy has been shown to reduce the risk of developing skin cancers in some transplant patients by more than half.
The authors emphasized that regular sunscreen use and dermatologic checkups are also essential in this population, and that “the importance of regular dermatologic evaluation should be stressed to patients and their families.”
Mr. Fogel’s coauthors were Mari Miyar, MD, of the department of dermatology, Kaiser Permanente, San Jose, Calif., and Joyce Teng, MD, of the departments of dermatology and pediatrics, Stanford. The authors had no disclosures listed, and no funding source for the review was listed.
This article was updated 12/8/16.
As survival rates among pediatric organ transplant recipients increase, so do the rates of cutaneous malignancies later in life for this population, who are at a greater risk for skin cancers that include nonmelanoma skin cancers (NMSCs), melanoma, Kaposi sarcoma, and anogenital carcinoma, according to the authors of a literature review.
In studies, skin cancers account for 13%-55% of all cancers in pediatric organ transplant recipients (POTRs), according to Alexander L. Fogel of Stanford (Calif.) University and his coauthors. The review article provides an update on this topic, as well as information on the prevention and management of skin cancers in this population, and the differences between this group and adult organ transplant recipients (AOTRs).
NMSC is the most common type of skin cancer in the pediatric group – and the second most common type of malignancy (NMSCs are the most common type of cancer affecting adult organ transplant recipients). NMSCs typically appear an average of 12-18 years post transplantation in this population (at an average age of 26-34 years). Length of posttransplantation follow-up, sunlight exposure, fair skin, and Northern European ancestry are among the factors associated with increased risk. This type of cancer involves the lip nearly twice as often as in adult recipients: 23% vs. 12%. The pediatric cohort also experiences more nonmelanoma cancer spreading to the lymph nodes than do adults: 9% vs. 6%.
Among pediatric transplant recipients, squamous cell carcinomas appear 2.8 times more often than basal cell carcinomas, “a trend that is opposite that observed in the nontransplant population,” the authors wrote.
In one study, anogenital carcinomas accounted for 4% of posttransplant cancers in this cohort, at an average of 12 years after the transplant, at a mean age of 27 years.
Some data indicate that in adult transplant recipients, there is an association between the human papillomavirus, and anal and genital warts and posttransplant anogenital cancer, but there are little data looking at this association in the pediatric group, the authors noted.
Although melanoma and Kaposi sarcoma are also found in this cohort at rates greater than in the general population, and are associated with high mortality rates, the data are too few to draw conclusions, the authors wrote.
In 2014, 1,795 pediatric solid organ transplants were performed, accounting for 6% of all such transplants. The absolute number of pediatric transplants has remained fairly stable over 5 years, yet very little pediatric-specific literature exists for prevention and management of skin cancers post transplantation, the authors pointed out.
Changing immunosuppressive medications used in transplantation may be effective in reducing skin cancer risk, they said, noting that including rapamycin inhibitors in combination therapy has been shown to reduce the risk of developing skin cancers in some transplant patients by more than half.
The authors emphasized that regular sunscreen use and dermatologic checkups are also essential in this population, and that “the importance of regular dermatologic evaluation should be stressed to patients and their families.”
Mr. Fogel’s coauthors were Mari Miyar, MD, of the department of dermatology, Kaiser Permanente, San Jose, Calif., and Joyce Teng, MD, of the departments of dermatology and pediatrics, Stanford. The authors had no disclosures listed, and no funding source for the review was listed.
This article was updated 12/8/16.
FROM PEDIATRIC DERMATOLOGY
Key clinical point:
Major finding: Pediatric solid organ transplant recipients experience skin cancer rates between 13% and 55%.
Data source: A literature review of malignancies among pediatric organ transplant recipients.
Disclosures: The authors listed no disclosures, and no funding source for the review was listed.
Dermoscopy Update and Noninvasive Imaging Devices for Skin Cancer: Report From the Mount Sinai Winter Symposium
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz provided an update on dermoscopy as a first-line noninvasive imaging modality for skin cancer screening and diagnosis along with reflectance confocal microscopy and dynamic optical coherence tomography. She explained how noninvasive imaging offers a more complete picture of lesions along with what is seen clinically and on pathology and discussed how it can help catch aggressive melanomas and other skin cancers at earlier stages. For these reasons, she emphasized that increased use of dermoscopy can be used to justify the need for regular skin cancer screenings. Finally, she discussed how noninvasive imaging can be used to guide dermatologists in performing optimal biposies of suspicious lesions.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz provided an update on dermoscopy as a first-line noninvasive imaging modality for skin cancer screening and diagnosis along with reflectance confocal microscopy and dynamic optical coherence tomography. She explained how noninvasive imaging offers a more complete picture of lesions along with what is seen clinically and on pathology and discussed how it can help catch aggressive melanomas and other skin cancers at earlier stages. For these reasons, she emphasized that increased use of dermoscopy can be used to justify the need for regular skin cancer screenings. Finally, she discussed how noninvasive imaging can be used to guide dermatologists in performing optimal biposies of suspicious lesions.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz provided an update on dermoscopy as a first-line noninvasive imaging modality for skin cancer screening and diagnosis along with reflectance confocal microscopy and dynamic optical coherence tomography. She explained how noninvasive imaging offers a more complete picture of lesions along with what is seen clinically and on pathology and discussed how it can help catch aggressive melanomas and other skin cancers at earlier stages. For these reasons, she emphasized that increased use of dermoscopy can be used to justify the need for regular skin cancer screenings. Finally, she discussed how noninvasive imaging can be used to guide dermatologists in performing optimal biposies of suspicious lesions.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dermoscopy Pearls: Report From the Mount Sinai Winter Symposium
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz addressed some common questions physicians have about dermoscopy, including what kind of dermatoscope to buy, how to incorporate dermoscopy into a dermatology practice, and how to efficiently perform skin examinations using a dermatoscope. She also emphasized the importance of attending courses and workshops to learn how to utilize dermoscopy and other noninvasive imaging devices effectively.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz addressed some common questions physicians have about dermoscopy, including what kind of dermatoscope to buy, how to incorporate dermoscopy into a dermatology practice, and how to efficiently perform skin examinations using a dermatoscope. She also emphasized the importance of attending courses and workshops to learn how to utilize dermoscopy and other noninvasive imaging devices effectively.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Orit Markowitz addressed some common questions physicians have about dermoscopy, including what kind of dermatoscope to buy, how to incorporate dermoscopy into a dermatology practice, and how to efficiently perform skin examinations using a dermatoscope. She also emphasized the importance of attending courses and workshops to learn how to utilize dermoscopy and other noninvasive imaging devices effectively.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
A Potpourri of Things to Do Correctly
When you pick up the Current Procedural Terminology (CPT) manual and read it, you may wonder what certain terms mean and how they may be looked at by payers and auditors. As your eyes glaze over from reading mind-numbing descriptions, a few points should be obvious, but conversations with friends, colleagues, and US Office of Inspector General and Centers for Medicare &
Excisions
For excisions (11400–11646), size is easy to determine. You measure the longest diameter of the lesion and the smallest margin required based on your judgment. The sum of the diameter and twice the margin is your lesion size. For benign lesions, the margin can be as small as 0 to 1 mm. For malignancies, it might be 5 to 9 mm for a melanoma in situ, 1 cm or more for an invasive melanoma with similar margins for squamous cell carcinoma, and somewhat less than 1 cm for basal cell carcinomas and more than 1 cm for Merkel cell carcinomas or spindle cell neoplasms.
There are times when you may delay a repair for medical reasons, which you would document in the medical record, but if you systematically delay a repair overnight to avoid the multiple procedure payment reduction, you may become “a person of interest,” which is a bad thing.
The shave removal codes (11300–11313) do not require repair and hemostasis is included. The size of the lesion determines the size of the lesion reported, and margins are not included. Hemostasis is included in the value of the CPT code and is not separately reportable.
It is not uncommon for a patient, usually one well known to you, to present with another skin cancer that has classic clinical findings. You review options with your patient and proceed to take one of the following approaches.
Option 1: You can tangentially remove or curette the tumor bulk and send the specimen for pathology review. At the same time, you curette and cauterize the base. In this case, you should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant destruction code (17260–17286) only. If it is benign, you would report a biopsy based on site or a benign destruction (17110) if for some reason the destruction was medically necessary. If it is an actinic keratosis, you could report either a biopsy or a premalignant destruction (17000).
Option 2: You perform a full-thickness excision of the lesion with a margin to remove it and send the specimen for pathology review. You should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant excision (11600–11646) and repair as discussed above. If it is benign, you would report the appropriate benign excision (11400–11446) and repair as discussed above.
If a shave, excision, or destruction is performed, a biopsy of the tissue should never be reported separately simply because the tissue may be sent to the laboratory. In other words, a biopsy is not separately reportable when another procedure was done at the same site on the same day.
Biopsy
Biopsies come in 2 varieties: general and site specific. All dermatologists are familiar with the basic skin biopsy codes 11110 and 11101 (biopsy of skin, subcutaneous tissue and/or mucous membrane [including simple closure], unless otherwise listed). Many are not aware of site-specific biopsy codes that often are more appropriate and should be used when their localization is more precise than the general skin biopsy.
Biopsies of the nail unit (eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds) are reported using CPT code 11755. A simple nail clipping for culture or periodic acid–Schiff stain is not a nail biopsy and should not be separately reported from the evaluation and management component of the visit.
The lip biopsy code (40490) is used appropriately when the vermilion is sampled, not the skin around it. If the skin and vermilion are contiguously sampled, only report 40490. Specific codes exist for the vestibule of the mouth (40808), the anterior two-thirds (41100) and posterior one-third (41105) of the tongue, the floor (41108) and roof (42100) of the mouth, and the salivary glands by needle (42400) or by incision (42405).
The penis can be biopsied on the surface (54100) or deep structures can be sampled (54105), though the latter is uncommon in dermatology practices. The vulva can be sampled with codes comparable to general biopsy, with 54605 for the first biopsy and 54606 used for each additional one.
An incisional biopsy of the eyelid margin is reported with 67810, while conjunctival biopsy is reported with 68100; 68510 describes a lacrimal gland biopsy. The ear, not to be left out, has its own biopsy codes, with 69100 for the external ear and 69105 for the auditory canal.
Clipping of hair or tape stripping of skin (similar to nail clipping described above) are not biopsies and are not separately reportable, as the work involved is considered incident to the cognitive visit taking place.
Final Thoughts
These points should all be fairly straightforward—yes, the skin biopsy includes mucosa, but if a mucosal site such as the mouth has a more specific code, then that code is correct—and the simplest test for the clinician is to ask yourself, “If I were reviewing the claim, what would I expect to see?” As always, document what you do, do what you document, and report that which is medically necessary.
When you pick up the Current Procedural Terminology (CPT) manual and read it, you may wonder what certain terms mean and how they may be looked at by payers and auditors. As your eyes glaze over from reading mind-numbing descriptions, a few points should be obvious, but conversations with friends, colleagues, and US Office of Inspector General and Centers for Medicare &
Excisions
For excisions (11400–11646), size is easy to determine. You measure the longest diameter of the lesion and the smallest margin required based on your judgment. The sum of the diameter and twice the margin is your lesion size. For benign lesions, the margin can be as small as 0 to 1 mm. For malignancies, it might be 5 to 9 mm for a melanoma in situ, 1 cm or more for an invasive melanoma with similar margins for squamous cell carcinoma, and somewhat less than 1 cm for basal cell carcinomas and more than 1 cm for Merkel cell carcinomas or spindle cell neoplasms.
There are times when you may delay a repair for medical reasons, which you would document in the medical record, but if you systematically delay a repair overnight to avoid the multiple procedure payment reduction, you may become “a person of interest,” which is a bad thing.
The shave removal codes (11300–11313) do not require repair and hemostasis is included. The size of the lesion determines the size of the lesion reported, and margins are not included. Hemostasis is included in the value of the CPT code and is not separately reportable.
It is not uncommon for a patient, usually one well known to you, to present with another skin cancer that has classic clinical findings. You review options with your patient and proceed to take one of the following approaches.
Option 1: You can tangentially remove or curette the tumor bulk and send the specimen for pathology review. At the same time, you curette and cauterize the base. In this case, you should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant destruction code (17260–17286) only. If it is benign, you would report a biopsy based on site or a benign destruction (17110) if for some reason the destruction was medically necessary. If it is an actinic keratosis, you could report either a biopsy or a premalignant destruction (17000).
Option 2: You perform a full-thickness excision of the lesion with a margin to remove it and send the specimen for pathology review. You should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant excision (11600–11646) and repair as discussed above. If it is benign, you would report the appropriate benign excision (11400–11446) and repair as discussed above.
If a shave, excision, or destruction is performed, a biopsy of the tissue should never be reported separately simply because the tissue may be sent to the laboratory. In other words, a biopsy is not separately reportable when another procedure was done at the same site on the same day.
Biopsy
Biopsies come in 2 varieties: general and site specific. All dermatologists are familiar with the basic skin biopsy codes 11110 and 11101 (biopsy of skin, subcutaneous tissue and/or mucous membrane [including simple closure], unless otherwise listed). Many are not aware of site-specific biopsy codes that often are more appropriate and should be used when their localization is more precise than the general skin biopsy.
Biopsies of the nail unit (eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds) are reported using CPT code 11755. A simple nail clipping for culture or periodic acid–Schiff stain is not a nail biopsy and should not be separately reported from the evaluation and management component of the visit.
The lip biopsy code (40490) is used appropriately when the vermilion is sampled, not the skin around it. If the skin and vermilion are contiguously sampled, only report 40490. Specific codes exist for the vestibule of the mouth (40808), the anterior two-thirds (41100) and posterior one-third (41105) of the tongue, the floor (41108) and roof (42100) of the mouth, and the salivary glands by needle (42400) or by incision (42405).
The penis can be biopsied on the surface (54100) or deep structures can be sampled (54105), though the latter is uncommon in dermatology practices. The vulva can be sampled with codes comparable to general biopsy, with 54605 for the first biopsy and 54606 used for each additional one.
An incisional biopsy of the eyelid margin is reported with 67810, while conjunctival biopsy is reported with 68100; 68510 describes a lacrimal gland biopsy. The ear, not to be left out, has its own biopsy codes, with 69100 for the external ear and 69105 for the auditory canal.
Clipping of hair or tape stripping of skin (similar to nail clipping described above) are not biopsies and are not separately reportable, as the work involved is considered incident to the cognitive visit taking place.
Final Thoughts
These points should all be fairly straightforward—yes, the skin biopsy includes mucosa, but if a mucosal site such as the mouth has a more specific code, then that code is correct—and the simplest test for the clinician is to ask yourself, “If I were reviewing the claim, what would I expect to see?” As always, document what you do, do what you document, and report that which is medically necessary.
When you pick up the Current Procedural Terminology (CPT) manual and read it, you may wonder what certain terms mean and how they may be looked at by payers and auditors. As your eyes glaze over from reading mind-numbing descriptions, a few points should be obvious, but conversations with friends, colleagues, and US Office of Inspector General and Centers for Medicare &
Excisions
For excisions (11400–11646), size is easy to determine. You measure the longest diameter of the lesion and the smallest margin required based on your judgment. The sum of the diameter and twice the margin is your lesion size. For benign lesions, the margin can be as small as 0 to 1 mm. For malignancies, it might be 5 to 9 mm for a melanoma in situ, 1 cm or more for an invasive melanoma with similar margins for squamous cell carcinoma, and somewhat less than 1 cm for basal cell carcinomas and more than 1 cm for Merkel cell carcinomas or spindle cell neoplasms.
There are times when you may delay a repair for medical reasons, which you would document in the medical record, but if you systematically delay a repair overnight to avoid the multiple procedure payment reduction, you may become “a person of interest,” which is a bad thing.
The shave removal codes (11300–11313) do not require repair and hemostasis is included. The size of the lesion determines the size of the lesion reported, and margins are not included. Hemostasis is included in the value of the CPT code and is not separately reportable.
It is not uncommon for a patient, usually one well known to you, to present with another skin cancer that has classic clinical findings. You review options with your patient and proceed to take one of the following approaches.
Option 1: You can tangentially remove or curette the tumor bulk and send the specimen for pathology review. At the same time, you curette and cauterize the base. In this case, you should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant destruction code (17260–17286) only. If it is benign, you would report a biopsy based on site or a benign destruction (17110) if for some reason the destruction was medically necessary. If it is an actinic keratosis, you could report either a biopsy or a premalignant destruction (17000).
Option 2: You perform a full-thickness excision of the lesion with a margin to remove it and send the specimen for pathology review. You should hold your bill and await pathology. If the lesion is malignant, you would report the appropriate malignant excision (11600–11646) and repair as discussed above. If it is benign, you would report the appropriate benign excision (11400–11446) and repair as discussed above.
If a shave, excision, or destruction is performed, a biopsy of the tissue should never be reported separately simply because the tissue may be sent to the laboratory. In other words, a biopsy is not separately reportable when another procedure was done at the same site on the same day.
Biopsy
Biopsies come in 2 varieties: general and site specific. All dermatologists are familiar with the basic skin biopsy codes 11110 and 11101 (biopsy of skin, subcutaneous tissue and/or mucous membrane [including simple closure], unless otherwise listed). Many are not aware of site-specific biopsy codes that often are more appropriate and should be used when their localization is more precise than the general skin biopsy.
Biopsies of the nail unit (eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds) are reported using CPT code 11755. A simple nail clipping for culture or periodic acid–Schiff stain is not a nail biopsy and should not be separately reported from the evaluation and management component of the visit.
The lip biopsy code (40490) is used appropriately when the vermilion is sampled, not the skin around it. If the skin and vermilion are contiguously sampled, only report 40490. Specific codes exist for the vestibule of the mouth (40808), the anterior two-thirds (41100) and posterior one-third (41105) of the tongue, the floor (41108) and roof (42100) of the mouth, and the salivary glands by needle (42400) or by incision (42405).
The penis can be biopsied on the surface (54100) or deep structures can be sampled (54105), though the latter is uncommon in dermatology practices. The vulva can be sampled with codes comparable to general biopsy, with 54605 for the first biopsy and 54606 used for each additional one.
An incisional biopsy of the eyelid margin is reported with 67810, while conjunctival biopsy is reported with 68100; 68510 describes a lacrimal gland biopsy. The ear, not to be left out, has its own biopsy codes, with 69100 for the external ear and 69105 for the auditory canal.
Clipping of hair or tape stripping of skin (similar to nail clipping described above) are not biopsies and are not separately reportable, as the work involved is considered incident to the cognitive visit taking place.
Final Thoughts
These points should all be fairly straightforward—yes, the skin biopsy includes mucosa, but if a mucosal site such as the mouth has a more specific code, then that code is correct—and the simplest test for the clinician is to ask yourself, “If I were reviewing the claim, what would I expect to see?” As always, document what you do, do what you document, and report that which is medically necessary.
Practice Points
- A biopsy is not separately reportable when another procedure was done at the same site on the same day (eg, shave, excision, destruction).
- Use site-specific biopsy codes when their localization is more precise than the general skin biopsy.
- A simple nail clipping for culture or periodic acid-Schiff stain is not a nail biopsy and should not be separately reported from the evaluation and management component of the visit.
Debunking Melanoma Myths: Do Sunscreens Cause Cancer?
Myth: Sunscreens cause cancer
Regular sunscreen use is recommended by the American Academy of Dermatology as a primary method of sun protection to reduce the risk of melanoma and other nonmelanoma skin cancers. However, due to reports in the media, patients often inquire if sunscreen ingredients, specifically oxybenzone and retinyl palmitate as well as nanoparticles, are toxic and actually cause malignant melanoma and other skin cancers rather than prevent them.
Overall, the known benefits of sunscreen use to minimize short-term and long-term damage to the skin from UV radiation outweigh any unproven claims of toxicity or human health hazard. Active ingredients in sunscreens, such as oxybenzone and retinyl palmitate, are regulated as over-the-counter drugs by the US Food and Drug Administration and have a long-standing history of providing effective broad-spectrum protection from UV radiation. Despite concerns that oxybenzone can penetrate the skin and effect hormone levels, there is no evidence supporting this claim. Although oxybenzone is absorbed by the body, it is subsequently excreted and has no potential for harmful buildup. It also has been suggested that retinyl palmitate generates free radicals that can lead to cancer formation; however, the risk has only been linked to UV exposure in isolation, and antioxidants in the body can theoretically neutralize these free radicals before they lead to cancer development.
Sunscreens containing nanoparticles of inorganic filters such as zinc oxide and titanium dioxide also have been scrutinized. These formulations have largely proven effective in protecting against UVA and UVB radiation, and claims that nanoparticles are small enough to penetrate the epidermis and be absorbed in the human bloodstream have been refuted.
The positive association between sunscreen use and risk of developing malignant melanoma may be due to selection bias and uncontrolled confounding in studies rather than proven toxicity of sunscreen ingredients. Results from a meta-analysis of 11 case-control studies indicated that there is no association and the researchers discussed the role of selection bias in contributing to the positive association between sunscreen use and melanoma development. For instance, some studies failed to control for factors that commonly are linked with increased melanoma risk (eg, red or fair hair color, blue eye color, presence of nevi, freckling). Also, increased sun exposure among patients who use sunscreens may have impacted study results.
Dermatologists should emphasize to concerned patients that long-term sunscreen use has been proven to reduce the incidence of melanoma. A 2011 Australian study evaluated the effects of long-term application of sunscreen on the risk of cutaneous melanoma in 1621 randomly selected participants who applied sunscreen in combination with 30 mg of beta-carotene or placebo supplements for 4 years and were observed for 10 more years. They observed a reduction in primary melanomas and invasive melanomas in the sunscreen group, concluding that melanoma may be preventable with regular sunscreen use in adults.
For patients who are still concerned, dermatologists can recommend sunscreens containing organic UV filters only. Education about factors that contribute to the increased rate of melanoma also is necessary. Longer lifespans, the thinning ozone layer, increased popularity of outdoor activities, exposed skin due to clothing style, use of tanning beds, earlier detection of skin cancer, and other factors may be responsible. Greater exposure to UV radiation rather than commercial sunscreens is the likely cause of skin cancer.
Ask the expert: does sunscreen cause cancer? Skin Cancer Foundation website. http://www.skincancer.org/skin-cancer-information/ask-the-experts/does-sunscreen-cause-cancer. Published Fall 2008. Accessed November 17, 2016.
Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up [published online December 6, 2010]. J Clin Oncol. 2011;29:257-263.
Huncharek M, Kupelnick B. Use of topical sunscreens and the risk of malignant melanoma: a meta-analysis of 9067 patients from 11 case-control studies. Am J Public Health. 2002;92:1173-1177.
Morrison WL, Wang SQ. Sunscreens: safe and effective? Skin Cancer Foundation website. http://www.skincancer.org/prevention/sun-protection/sunscreen/sunscreens-safe-and-effective. Published November 17, 2011. Accessed November 17, 2016.
Sunscreen remains a safe, effective form of sun protection [press release]. Schaumburg, IL: American Academy of Dermatology; May 16, 2012. https://www.aad.org/media/news-releases/sunscreen-remains-a-safe-effective-form-of-sun-protection. Accessed November 17, 2016.
Myth: Sunscreens cause cancer
Regular sunscreen use is recommended by the American Academy of Dermatology as a primary method of sun protection to reduce the risk of melanoma and other nonmelanoma skin cancers. However, due to reports in the media, patients often inquire if sunscreen ingredients, specifically oxybenzone and retinyl palmitate as well as nanoparticles, are toxic and actually cause malignant melanoma and other skin cancers rather than prevent them.
Overall, the known benefits of sunscreen use to minimize short-term and long-term damage to the skin from UV radiation outweigh any unproven claims of toxicity or human health hazard. Active ingredients in sunscreens, such as oxybenzone and retinyl palmitate, are regulated as over-the-counter drugs by the US Food and Drug Administration and have a long-standing history of providing effective broad-spectrum protection from UV radiation. Despite concerns that oxybenzone can penetrate the skin and effect hormone levels, there is no evidence supporting this claim. Although oxybenzone is absorbed by the body, it is subsequently excreted and has no potential for harmful buildup. It also has been suggested that retinyl palmitate generates free radicals that can lead to cancer formation; however, the risk has only been linked to UV exposure in isolation, and antioxidants in the body can theoretically neutralize these free radicals before they lead to cancer development.
Sunscreens containing nanoparticles of inorganic filters such as zinc oxide and titanium dioxide also have been scrutinized. These formulations have largely proven effective in protecting against UVA and UVB radiation, and claims that nanoparticles are small enough to penetrate the epidermis and be absorbed in the human bloodstream have been refuted.
The positive association between sunscreen use and risk of developing malignant melanoma may be due to selection bias and uncontrolled confounding in studies rather than proven toxicity of sunscreen ingredients. Results from a meta-analysis of 11 case-control studies indicated that there is no association and the researchers discussed the role of selection bias in contributing to the positive association between sunscreen use and melanoma development. For instance, some studies failed to control for factors that commonly are linked with increased melanoma risk (eg, red or fair hair color, blue eye color, presence of nevi, freckling). Also, increased sun exposure among patients who use sunscreens may have impacted study results.
Dermatologists should emphasize to concerned patients that long-term sunscreen use has been proven to reduce the incidence of melanoma. A 2011 Australian study evaluated the effects of long-term application of sunscreen on the risk of cutaneous melanoma in 1621 randomly selected participants who applied sunscreen in combination with 30 mg of beta-carotene or placebo supplements for 4 years and were observed for 10 more years. They observed a reduction in primary melanomas and invasive melanomas in the sunscreen group, concluding that melanoma may be preventable with regular sunscreen use in adults.
For patients who are still concerned, dermatologists can recommend sunscreens containing organic UV filters only. Education about factors that contribute to the increased rate of melanoma also is necessary. Longer lifespans, the thinning ozone layer, increased popularity of outdoor activities, exposed skin due to clothing style, use of tanning beds, earlier detection of skin cancer, and other factors may be responsible. Greater exposure to UV radiation rather than commercial sunscreens is the likely cause of skin cancer.
Myth: Sunscreens cause cancer
Regular sunscreen use is recommended by the American Academy of Dermatology as a primary method of sun protection to reduce the risk of melanoma and other nonmelanoma skin cancers. However, due to reports in the media, patients often inquire if sunscreen ingredients, specifically oxybenzone and retinyl palmitate as well as nanoparticles, are toxic and actually cause malignant melanoma and other skin cancers rather than prevent them.
Overall, the known benefits of sunscreen use to minimize short-term and long-term damage to the skin from UV radiation outweigh any unproven claims of toxicity or human health hazard. Active ingredients in sunscreens, such as oxybenzone and retinyl palmitate, are regulated as over-the-counter drugs by the US Food and Drug Administration and have a long-standing history of providing effective broad-spectrum protection from UV radiation. Despite concerns that oxybenzone can penetrate the skin and effect hormone levels, there is no evidence supporting this claim. Although oxybenzone is absorbed by the body, it is subsequently excreted and has no potential for harmful buildup. It also has been suggested that retinyl palmitate generates free radicals that can lead to cancer formation; however, the risk has only been linked to UV exposure in isolation, and antioxidants in the body can theoretically neutralize these free radicals before they lead to cancer development.
Sunscreens containing nanoparticles of inorganic filters such as zinc oxide and titanium dioxide also have been scrutinized. These formulations have largely proven effective in protecting against UVA and UVB radiation, and claims that nanoparticles are small enough to penetrate the epidermis and be absorbed in the human bloodstream have been refuted.
The positive association between sunscreen use and risk of developing malignant melanoma may be due to selection bias and uncontrolled confounding in studies rather than proven toxicity of sunscreen ingredients. Results from a meta-analysis of 11 case-control studies indicated that there is no association and the researchers discussed the role of selection bias in contributing to the positive association between sunscreen use and melanoma development. For instance, some studies failed to control for factors that commonly are linked with increased melanoma risk (eg, red or fair hair color, blue eye color, presence of nevi, freckling). Also, increased sun exposure among patients who use sunscreens may have impacted study results.
Dermatologists should emphasize to concerned patients that long-term sunscreen use has been proven to reduce the incidence of melanoma. A 2011 Australian study evaluated the effects of long-term application of sunscreen on the risk of cutaneous melanoma in 1621 randomly selected participants who applied sunscreen in combination with 30 mg of beta-carotene or placebo supplements for 4 years and were observed for 10 more years. They observed a reduction in primary melanomas and invasive melanomas in the sunscreen group, concluding that melanoma may be preventable with regular sunscreen use in adults.
For patients who are still concerned, dermatologists can recommend sunscreens containing organic UV filters only. Education about factors that contribute to the increased rate of melanoma also is necessary. Longer lifespans, the thinning ozone layer, increased popularity of outdoor activities, exposed skin due to clothing style, use of tanning beds, earlier detection of skin cancer, and other factors may be responsible. Greater exposure to UV radiation rather than commercial sunscreens is the likely cause of skin cancer.
Ask the expert: does sunscreen cause cancer? Skin Cancer Foundation website. http://www.skincancer.org/skin-cancer-information/ask-the-experts/does-sunscreen-cause-cancer. Published Fall 2008. Accessed November 17, 2016.
Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up [published online December 6, 2010]. J Clin Oncol. 2011;29:257-263.
Huncharek M, Kupelnick B. Use of topical sunscreens and the risk of malignant melanoma: a meta-analysis of 9067 patients from 11 case-control studies. Am J Public Health. 2002;92:1173-1177.
Morrison WL, Wang SQ. Sunscreens: safe and effective? Skin Cancer Foundation website. http://www.skincancer.org/prevention/sun-protection/sunscreen/sunscreens-safe-and-effective. Published November 17, 2011. Accessed November 17, 2016.
Sunscreen remains a safe, effective form of sun protection [press release]. Schaumburg, IL: American Academy of Dermatology; May 16, 2012. https://www.aad.org/media/news-releases/sunscreen-remains-a-safe-effective-form-of-sun-protection. Accessed November 17, 2016.
Ask the expert: does sunscreen cause cancer? Skin Cancer Foundation website. http://www.skincancer.org/skin-cancer-information/ask-the-experts/does-sunscreen-cause-cancer. Published Fall 2008. Accessed November 17, 2016.
Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up [published online December 6, 2010]. J Clin Oncol. 2011;29:257-263.
Huncharek M, Kupelnick B. Use of topical sunscreens and the risk of malignant melanoma: a meta-analysis of 9067 patients from 11 case-control studies. Am J Public Health. 2002;92:1173-1177.
Morrison WL, Wang SQ. Sunscreens: safe and effective? Skin Cancer Foundation website. http://www.skincancer.org/prevention/sun-protection/sunscreen/sunscreens-safe-and-effective. Published November 17, 2011. Accessed November 17, 2016.
Sunscreen remains a safe, effective form of sun protection [press release]. Schaumburg, IL: American Academy of Dermatology; May 16, 2012. https://www.aad.org/media/news-releases/sunscreen-remains-a-safe-effective-form-of-sun-protection. Accessed November 17, 2016.