User login
Slowing, not stopping, Alzheimer’s a better goal for clinical trials?
and may be a more realistic goal for clinical AD drug trials, a new report suggests.
The report is a yearlong undertaking by an expert work group convened by the Alzheimer’s Association and was prompted, in part, by the fallout from the U.S. Food and Drug Administration’s controversial decision to grant aducanumab (Aduhelm) accelerated approval, which came over the objection of an advisory panel that found the drug was ineffective.
The report’s authors call for a “reframing” of how researchers define “clinically meaningful” in randomized controlled trials (RCTs), noting that it’s time to adjust expectations of outcomes from relatively short clinical trials.
“Without lowering the bar, are we expecting too much from a clinical trial by expecting that unless the disease is halted in its tracks and there’s no progression, we failed at treatment?” the report’s lead author and group leader Ronald C. Petersen, MD, PhD, lead author, chair of the work group, and professor of neurology at the Mayo Clinic, Rochester, Minn., told this news organization.
Interpretations of clinical meaningfulness are used in the drug approval process and in decisions about whether an insurer will cover the cost of treatment, the authors note.
While the report doesn’t provide a consensus definition of clinically meaningful benefit, it does offer a starting point for a conversation about how the phrase should be defined in the context of RCTs for disease-modifying therapies (DMTs) in AD, Dr. Petersen said.
“What we tried to do was to put it into some kind of perspective and at least have people reflect on this: If you’re going to design the perfect drug trial in Alzheimer’s disease, what would it be? We wanted to get people to think about it without digging in their heels for or against,” he added.
The report was published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
A proactive measure
The expert group began its work in January 2022, less than a year after the FDA approved aducanumab. Since the panel began its work, the FDA has approved a second AD drug, lecanemab (Leqembi), and denied accelerated approval of a third medication, donanemab.
“At the time we started this group, we had one approved treatment, and we just knew that there were others on the way, and we needed to be prepared to have this conversation and be more proactive than reactive,” Christopher Weber, PhD, director of global science initiatives for the Alzheimer’s Association and co-author of the report, said in an interview.
The work group suggests that simply slowing disease progression might be a desired goal for drug trials, especially early on, before cognition and memory are affected.
They also note that a benefit identified during an 18-month clinical trial may ultimately lead to even more meaningful changes over coming years, well beyond the trial’s end.
In addition, the report authors call for the development of better research tools to more accurately assess meaningful change. The Clinical Dementia Rating (CDR) scale is currently the key instrument used as a primary outcome measure in RCTs. However, the report’s authors note that it may not be adequate to measure meaningful change in early-stage disease.
“Developing better tools certainly should be on the radar screen for all of us, because I think we can do better,” Dr. Petersen said. “The CDR, as good as it is and as long as it’s been used in the field, is a pretty blunt instrument, and it’s the result of subjective ratings.”
‘Quality of mind’
Jason Karlawish, MD, professor of medicine, medical ethics, health policy, and neurology at the University of Pennsylvania, Philadelphia, said measuring the actual impact of a drug on a patient’s disease and quality of life has been a hot topic in the AD field for some time, but settling on a definition of “clinically meaningful” that everyone agrees upon will be a challenge.
“I think the idea of ‘clinically meaningful’ is truly a socially constructed idea,” said Dr. Karlawish, co-director of Penn’s Memory Center, who did not work on the report.
“You can come up with objective measures of cognition, but a measure to call something ‘clinically meaningful’ ultimately requires some sort of negotiated social order among clinicians and patients and others who have immediate interest in the health and well-being of the patient.”
Dr. Karlawish added that he’s interested in the conversations the report might prompt and the challenges it could highlight, especially when it comes to how meaningful clinical benefit can be measured, regardless of how it’s defined.
“Hidden in this conversation about clinically meaningful treatments in Alzheimer’s disease is, frankly, not quality of life, but quality of mind,” said Dr. Karlawish. “No measure captures acceptably the very thing that everyone actually cares a lot about and why we view this disease as so dreadful, which is damage to our mind.”
More evidence needed
The development of such tools will take time. What does that mean for drugs already in the pipeline? Members of the work group argue that those trials must move forward at the same time new tools are being created.
“We need to continue to refine, develop better instruments, [and] develop tools that are going to assess the disease in its more subtle features early on, even in the so-called ‘pre-symptomatic’ stage of the disease,” said lead author Dr. Petersen. “We shouldn’t wait for the development of that before intervening if we have a drug that seems to work.”
However, not everyone who agrees with the premise of the report agrees with this position, including Joel S. Perlmutter, MD, professor of neurology, Washington University School of Medicine, St. Louis, who also commented on the report.
As reported by this news organization, Dr. Perlmutter was one of three physicians who resigned from the FDA advisory panel that voted against approving aducanumab after the agency moved forward anyway.
“We have to be careful not to recommend DMTs that we hope will help without strong evidence, especially when potential side effects are not trivial,” Dr. Perlmutter said. “We have to have evidence before making these recommendations so we don’t end up harming people more than helping them.”
The report received no specific funding. Dr. Petersen received consulting fees from Roche, Nestle, Merck, Biogen, Eisai, and Genentech. Full disclosures are included in the original article. Dr. Perlmutter and Dr. Karlawish report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
and may be a more realistic goal for clinical AD drug trials, a new report suggests.
The report is a yearlong undertaking by an expert work group convened by the Alzheimer’s Association and was prompted, in part, by the fallout from the U.S. Food and Drug Administration’s controversial decision to grant aducanumab (Aduhelm) accelerated approval, which came over the objection of an advisory panel that found the drug was ineffective.
The report’s authors call for a “reframing” of how researchers define “clinically meaningful” in randomized controlled trials (RCTs), noting that it’s time to adjust expectations of outcomes from relatively short clinical trials.
“Without lowering the bar, are we expecting too much from a clinical trial by expecting that unless the disease is halted in its tracks and there’s no progression, we failed at treatment?” the report’s lead author and group leader Ronald C. Petersen, MD, PhD, lead author, chair of the work group, and professor of neurology at the Mayo Clinic, Rochester, Minn., told this news organization.
Interpretations of clinical meaningfulness are used in the drug approval process and in decisions about whether an insurer will cover the cost of treatment, the authors note.
While the report doesn’t provide a consensus definition of clinically meaningful benefit, it does offer a starting point for a conversation about how the phrase should be defined in the context of RCTs for disease-modifying therapies (DMTs) in AD, Dr. Petersen said.
“What we tried to do was to put it into some kind of perspective and at least have people reflect on this: If you’re going to design the perfect drug trial in Alzheimer’s disease, what would it be? We wanted to get people to think about it without digging in their heels for or against,” he added.
The report was published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
A proactive measure
The expert group began its work in January 2022, less than a year after the FDA approved aducanumab. Since the panel began its work, the FDA has approved a second AD drug, lecanemab (Leqembi), and denied accelerated approval of a third medication, donanemab.
“At the time we started this group, we had one approved treatment, and we just knew that there were others on the way, and we needed to be prepared to have this conversation and be more proactive than reactive,” Christopher Weber, PhD, director of global science initiatives for the Alzheimer’s Association and co-author of the report, said in an interview.
The work group suggests that simply slowing disease progression might be a desired goal for drug trials, especially early on, before cognition and memory are affected.
They also note that a benefit identified during an 18-month clinical trial may ultimately lead to even more meaningful changes over coming years, well beyond the trial’s end.
In addition, the report authors call for the development of better research tools to more accurately assess meaningful change. The Clinical Dementia Rating (CDR) scale is currently the key instrument used as a primary outcome measure in RCTs. However, the report’s authors note that it may not be adequate to measure meaningful change in early-stage disease.
“Developing better tools certainly should be on the radar screen for all of us, because I think we can do better,” Dr. Petersen said. “The CDR, as good as it is and as long as it’s been used in the field, is a pretty blunt instrument, and it’s the result of subjective ratings.”
‘Quality of mind’
Jason Karlawish, MD, professor of medicine, medical ethics, health policy, and neurology at the University of Pennsylvania, Philadelphia, said measuring the actual impact of a drug on a patient’s disease and quality of life has been a hot topic in the AD field for some time, but settling on a definition of “clinically meaningful” that everyone agrees upon will be a challenge.
“I think the idea of ‘clinically meaningful’ is truly a socially constructed idea,” said Dr. Karlawish, co-director of Penn’s Memory Center, who did not work on the report.
“You can come up with objective measures of cognition, but a measure to call something ‘clinically meaningful’ ultimately requires some sort of negotiated social order among clinicians and patients and others who have immediate interest in the health and well-being of the patient.”
Dr. Karlawish added that he’s interested in the conversations the report might prompt and the challenges it could highlight, especially when it comes to how meaningful clinical benefit can be measured, regardless of how it’s defined.
“Hidden in this conversation about clinically meaningful treatments in Alzheimer’s disease is, frankly, not quality of life, but quality of mind,” said Dr. Karlawish. “No measure captures acceptably the very thing that everyone actually cares a lot about and why we view this disease as so dreadful, which is damage to our mind.”
More evidence needed
The development of such tools will take time. What does that mean for drugs already in the pipeline? Members of the work group argue that those trials must move forward at the same time new tools are being created.
“We need to continue to refine, develop better instruments, [and] develop tools that are going to assess the disease in its more subtle features early on, even in the so-called ‘pre-symptomatic’ stage of the disease,” said lead author Dr. Petersen. “We shouldn’t wait for the development of that before intervening if we have a drug that seems to work.”
However, not everyone who agrees with the premise of the report agrees with this position, including Joel S. Perlmutter, MD, professor of neurology, Washington University School of Medicine, St. Louis, who also commented on the report.
As reported by this news organization, Dr. Perlmutter was one of three physicians who resigned from the FDA advisory panel that voted against approving aducanumab after the agency moved forward anyway.
“We have to be careful not to recommend DMTs that we hope will help without strong evidence, especially when potential side effects are not trivial,” Dr. Perlmutter said. “We have to have evidence before making these recommendations so we don’t end up harming people more than helping them.”
The report received no specific funding. Dr. Petersen received consulting fees from Roche, Nestle, Merck, Biogen, Eisai, and Genentech. Full disclosures are included in the original article. Dr. Perlmutter and Dr. Karlawish report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
and may be a more realistic goal for clinical AD drug trials, a new report suggests.
The report is a yearlong undertaking by an expert work group convened by the Alzheimer’s Association and was prompted, in part, by the fallout from the U.S. Food and Drug Administration’s controversial decision to grant aducanumab (Aduhelm) accelerated approval, which came over the objection of an advisory panel that found the drug was ineffective.
The report’s authors call for a “reframing” of how researchers define “clinically meaningful” in randomized controlled trials (RCTs), noting that it’s time to adjust expectations of outcomes from relatively short clinical trials.
“Without lowering the bar, are we expecting too much from a clinical trial by expecting that unless the disease is halted in its tracks and there’s no progression, we failed at treatment?” the report’s lead author and group leader Ronald C. Petersen, MD, PhD, lead author, chair of the work group, and professor of neurology at the Mayo Clinic, Rochester, Minn., told this news organization.
Interpretations of clinical meaningfulness are used in the drug approval process and in decisions about whether an insurer will cover the cost of treatment, the authors note.
While the report doesn’t provide a consensus definition of clinically meaningful benefit, it does offer a starting point for a conversation about how the phrase should be defined in the context of RCTs for disease-modifying therapies (DMTs) in AD, Dr. Petersen said.
“What we tried to do was to put it into some kind of perspective and at least have people reflect on this: If you’re going to design the perfect drug trial in Alzheimer’s disease, what would it be? We wanted to get people to think about it without digging in their heels for or against,” he added.
The report was published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
A proactive measure
The expert group began its work in January 2022, less than a year after the FDA approved aducanumab. Since the panel began its work, the FDA has approved a second AD drug, lecanemab (Leqembi), and denied accelerated approval of a third medication, donanemab.
“At the time we started this group, we had one approved treatment, and we just knew that there were others on the way, and we needed to be prepared to have this conversation and be more proactive than reactive,” Christopher Weber, PhD, director of global science initiatives for the Alzheimer’s Association and co-author of the report, said in an interview.
The work group suggests that simply slowing disease progression might be a desired goal for drug trials, especially early on, before cognition and memory are affected.
They also note that a benefit identified during an 18-month clinical trial may ultimately lead to even more meaningful changes over coming years, well beyond the trial’s end.
In addition, the report authors call for the development of better research tools to more accurately assess meaningful change. The Clinical Dementia Rating (CDR) scale is currently the key instrument used as a primary outcome measure in RCTs. However, the report’s authors note that it may not be adequate to measure meaningful change in early-stage disease.
“Developing better tools certainly should be on the radar screen for all of us, because I think we can do better,” Dr. Petersen said. “The CDR, as good as it is and as long as it’s been used in the field, is a pretty blunt instrument, and it’s the result of subjective ratings.”
‘Quality of mind’
Jason Karlawish, MD, professor of medicine, medical ethics, health policy, and neurology at the University of Pennsylvania, Philadelphia, said measuring the actual impact of a drug on a patient’s disease and quality of life has been a hot topic in the AD field for some time, but settling on a definition of “clinically meaningful” that everyone agrees upon will be a challenge.
“I think the idea of ‘clinically meaningful’ is truly a socially constructed idea,” said Dr. Karlawish, co-director of Penn’s Memory Center, who did not work on the report.
“You can come up with objective measures of cognition, but a measure to call something ‘clinically meaningful’ ultimately requires some sort of negotiated social order among clinicians and patients and others who have immediate interest in the health and well-being of the patient.”
Dr. Karlawish added that he’s interested in the conversations the report might prompt and the challenges it could highlight, especially when it comes to how meaningful clinical benefit can be measured, regardless of how it’s defined.
“Hidden in this conversation about clinically meaningful treatments in Alzheimer’s disease is, frankly, not quality of life, but quality of mind,” said Dr. Karlawish. “No measure captures acceptably the very thing that everyone actually cares a lot about and why we view this disease as so dreadful, which is damage to our mind.”
More evidence needed
The development of such tools will take time. What does that mean for drugs already in the pipeline? Members of the work group argue that those trials must move forward at the same time new tools are being created.
“We need to continue to refine, develop better instruments, [and] develop tools that are going to assess the disease in its more subtle features early on, even in the so-called ‘pre-symptomatic’ stage of the disease,” said lead author Dr. Petersen. “We shouldn’t wait for the development of that before intervening if we have a drug that seems to work.”
However, not everyone who agrees with the premise of the report agrees with this position, including Joel S. Perlmutter, MD, professor of neurology, Washington University School of Medicine, St. Louis, who also commented on the report.
As reported by this news organization, Dr. Perlmutter was one of three physicians who resigned from the FDA advisory panel that voted against approving aducanumab after the agency moved forward anyway.
“We have to be careful not to recommend DMTs that we hope will help without strong evidence, especially when potential side effects are not trivial,” Dr. Perlmutter said. “We have to have evidence before making these recommendations so we don’t end up harming people more than helping them.”
The report received no specific funding. Dr. Petersen received consulting fees from Roche, Nestle, Merck, Biogen, Eisai, and Genentech. Full disclosures are included in the original article. Dr. Perlmutter and Dr. Karlawish report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ALZHEIMER’S AND DEMENTIA
What’s new in brain health?
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
No benefit of long-acting antipsychotics in schizophrenia?
In a multicountry, randomized, open-label study of more than 500 adults with schizophrenia, participants received either LAI paliperidone, LAI aripiprazole, or the respective oral formulation of these antipsychotics.
Results showed no significant difference between the combined oral and combined LAI treatment groups in time to all-cause discontinuation.
“We found no substantial advantage for LAI antipsychotic treatment over oral treatment, regarding time to discontinuation in patients with early-phase schizophrenia,” write investigators, led by Inge Winter-van Rossum, PhD, assistant visiting professor at Mount Sinai, New York, and affiliated with King’s College London and UMC Utrecht (the Netherlands).
This indicates that “there is no reason to prescribe LAIs instead of oral antipsychotics if the goal is to prevent discontinuation of antipsychotic medication in daily clinical practice,” they add.
The findings were published online in The Lancet Psychiatry.
Previous conflicting results
Maintenance treatment with antipsychotic medication reduces risk for relapse considerably, with treatment discontinuation being “by far the most important reason for relapse,” the investigators write.
LAIs “seem theoretically to be a way to enhance medication continuation and thereby reduce the risk for relapse,” they add. This is because LAIs enable a rapid response to nonadherence and remove the need for patients to remember to take their medications on a daily basis.
However, previous research has “provided conflicting results,” regarding the effectiveness of LAIs in accomplishing this. Moreover, the subject has not been thoroughly investigated in early-stage schizophrenia, the researchers note.
Therefore, they decided to conduct the EULAST study to compare LAI and oral formulations in terms of all-cause discontinuation.
The trial was conducted at 50 general hospitals and psychiatric specialty clinics located in 15 European countries and Israel and included 511 participants in the intention-to-treat sample (67% men; mean age, 30.5 years).
All were randomly assigned 1:1:1 to receive either LAI paliperidone, LAI aripiprazole, or their respective oral formulations.
The combined OA treatment group consisted of 247 patients; the combined LAI group consisted of 264 patients.
Randomization was stratified by country and illness duration (5 months to 3 years vs. 4-7 years). Participants were followed up to 19 months, with all-cause discontinuation during that time serving as the primary endpoint.
All-cause discontinuation was defined as the allocated treatment was stopped or used at doses outside the allowed range, medication was switched or augmented with another antipsychotic after visit four, the patient missed a monthly visit and did not show up after being reminded, the patient withdrew consent for the study, or the clinician withdrew the patient from the study.
After the baseline visit, patients already taking antipsychotics were also randomly assigned. The next 4 weeks were then used to cross-taper between the prestudy antipsychotic and the agent they would be treated with during the study.
LAIs not superior
Results showed the LAI group did not have lower rates of hospitalization.
In addition, the discontinuation rates between the two combined groups were very similar at 71% for the oral antipsychotics group versus 64% in the LAIs group (hazard ratio, 1.6; 95% confidence interval, 0.94-1.43; P = .18).
Moreover, “no significant difference was found in the time to all-cause discontinuation between the combined oral and combined LAI treatment groups (P = .17),” the researchers report.
Reasons for discontinuation also did not differ significantly between the groups: 12% of patients in the OA group discontinued treatment because of efficacy vs. 17% of patients in the combined LAI group. The difference was not significant and the time to discontinuation also did not differ.
The main reason for discontinuation in both groups was safety concerns, affecting 10% and 13% of the combined OA and LAI groups, respectively, which was not a significant between-group difference.
Illness duration had a significant effect on time to all-cause discontinuation, with patients who had longer illness duration showing a poorer response, compared with those who had shorter duration (HR, 1.26; 95% CI, 1.01-1.56; P = .038).
However, stratifying participants by illness duration showed no significant difference between the subgroups (P = .25 and .34, respectively).
There was a significant between-group difference in discontinuation due to “other reasons,” with 49% vs. 34% of patients in the OA and LAI groups, respectively, discontinuing (HR, 1.51; 95% CI, 1.15-1.98; P = .0034). Moreover, the LAI group showed significantly longer continued use of medication vs the OA group (P = .0029).
“After separating the reasons for discontinuation into no efficacy, safety reasons, and other reasons, we only found a significant difference in favor of LAI for the ‘other reasons’ category; although the number of patients discontinuing medication for this reason over the follow-up period did not differ, patients on LAI continued treatment for a longer time,” the investigators write.
They acknowledge that this finding is “difficult to interpret, given the wide variety of reasons for discontinuation captured in this category,” which prevented an “informative subgroup analysis.”
Nevertheless, since there is “no consistent evidence supporting the use of LAI over oral antipsychotics” in patients with early-phase schizophrenia, their use should be “carefully considered on an individual risk-benefit basis,” they conclude.
No ‘real-world’ implications?
John M. Kane, MD, codirector and professor, Institute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, N.Y., said that overall, this was a “large, potentially valuable study.” However, he raised several concerns.
“I think the investigators made a much too emphatic statement about the lack of value of LAIs in early-phase patients when discontinuation is the primary outcome,” he said, noting that other studies have come to the opposite conclusion.
Dr. Kane, who is also a professor of psychiatry at Hofstra/Northwell, New York, was not involved with the current research.
“RCTs [randomized controlled trials] in general are not necessarily the best way to evaluate the impact of LAIs [which] usually represent a small percentage of potentially eligible patients and are likely to include patients who are more adherent than those who would not agree to participate in an RCT,” he said. He added that the investigators “did not report on how many patients were screened and refused to be considered.”
Also, Dr. Kane noted that half of the participants were recruited from inpatient services, and so may have been “more unstable” at baseline. “Patients with residual positive symptoms are more likely to relapse on LAIs than patients who are in remission. This could potentially reduce the advantage of the LAI,” he said.
In addition, he took issue with the definition of all-cause discontinuation, which included the need for augmentation with another antipsychotic or use outside the normal range.
“This happens often in clinical practice. If someone’s symptoms aren’t sufficiently controlled by an LAI alone, for example, they often receive more of that antipsychotic or another drug. This perhaps makes the EULAST study somewhat less ‘real-world’,” Dr. Kane said.
More information needed
In an accompanying editorial, Martina Hahn, PharmD, PhD, department of psychiatry, psychosomatics, and psychotherapy, University Hospital-Goethe University, Frankfurt, Germany, and Sibylle Christine Roll, MD, PHD, department of mental health, Varisano Hospital in Frankfurt, note that comedications were neither documented nor analyzed by the researchers.
“Drug-drug interactions could be responsible for relapse or poor tolerability,” they write.
Moreover, pharmacogenetic information was not available nor were serum concentrations that could have been used for dose optimization after switching antipsychotic formulations, they note.
This information would have provided “a deeper understanding of why some patients do not respond or show side effects,” the editorialists write. “The use of therapeutic drug monitoring, drug interaction checks, and pharmacogenetic testing could improve treatment outcomes in both study settings and clinical practice.”
Financial support and study medication was provided by Lundbeck and Otsuka. Dr. Winter-van Rossum reports no relevant financial relationships. Disclosures for the other investigators are fully listed in the original paper. Dr. Kane is or has been a consultant to or received honoraria for lectures from Alkermes , Biogen, Boehringer Ingelheim, Cerevel, Dainippon Sumitomo, H. Lundbeck, HLS, Intracellular Therapies, Janssen, Karuna, Merck, Newron, Otsuka, Roche, Saladax, Sunovion, and TEVA. He is also a shareholder in The Vanguard Research Group, LB Pharma, Health Rhythms, North Shore Therapeutics, and Medincell. Dr. Hahn reports having received honoraria for lecture from Otsuka and advisory board participation for Rovi. Dr. Roll reports advisory board participation for Recordati, Otsuka, and Janssen.
A version of this article first appeared on Medscape.com.
In a multicountry, randomized, open-label study of more than 500 adults with schizophrenia, participants received either LAI paliperidone, LAI aripiprazole, or the respective oral formulation of these antipsychotics.
Results showed no significant difference between the combined oral and combined LAI treatment groups in time to all-cause discontinuation.
“We found no substantial advantage for LAI antipsychotic treatment over oral treatment, regarding time to discontinuation in patients with early-phase schizophrenia,” write investigators, led by Inge Winter-van Rossum, PhD, assistant visiting professor at Mount Sinai, New York, and affiliated with King’s College London and UMC Utrecht (the Netherlands).
This indicates that “there is no reason to prescribe LAIs instead of oral antipsychotics if the goal is to prevent discontinuation of antipsychotic medication in daily clinical practice,” they add.
The findings were published online in The Lancet Psychiatry.
Previous conflicting results
Maintenance treatment with antipsychotic medication reduces risk for relapse considerably, with treatment discontinuation being “by far the most important reason for relapse,” the investigators write.
LAIs “seem theoretically to be a way to enhance medication continuation and thereby reduce the risk for relapse,” they add. This is because LAIs enable a rapid response to nonadherence and remove the need for patients to remember to take their medications on a daily basis.
However, previous research has “provided conflicting results,” regarding the effectiveness of LAIs in accomplishing this. Moreover, the subject has not been thoroughly investigated in early-stage schizophrenia, the researchers note.
Therefore, they decided to conduct the EULAST study to compare LAI and oral formulations in terms of all-cause discontinuation.
The trial was conducted at 50 general hospitals and psychiatric specialty clinics located in 15 European countries and Israel and included 511 participants in the intention-to-treat sample (67% men; mean age, 30.5 years).
All were randomly assigned 1:1:1 to receive either LAI paliperidone, LAI aripiprazole, or their respective oral formulations.
The combined OA treatment group consisted of 247 patients; the combined LAI group consisted of 264 patients.
Randomization was stratified by country and illness duration (5 months to 3 years vs. 4-7 years). Participants were followed up to 19 months, with all-cause discontinuation during that time serving as the primary endpoint.
All-cause discontinuation was defined as the allocated treatment was stopped or used at doses outside the allowed range, medication was switched or augmented with another antipsychotic after visit four, the patient missed a monthly visit and did not show up after being reminded, the patient withdrew consent for the study, or the clinician withdrew the patient from the study.
After the baseline visit, patients already taking antipsychotics were also randomly assigned. The next 4 weeks were then used to cross-taper between the prestudy antipsychotic and the agent they would be treated with during the study.
LAIs not superior
Results showed the LAI group did not have lower rates of hospitalization.
In addition, the discontinuation rates between the two combined groups were very similar at 71% for the oral antipsychotics group versus 64% in the LAIs group (hazard ratio, 1.6; 95% confidence interval, 0.94-1.43; P = .18).
Moreover, “no significant difference was found in the time to all-cause discontinuation between the combined oral and combined LAI treatment groups (P = .17),” the researchers report.
Reasons for discontinuation also did not differ significantly between the groups: 12% of patients in the OA group discontinued treatment because of efficacy vs. 17% of patients in the combined LAI group. The difference was not significant and the time to discontinuation also did not differ.
The main reason for discontinuation in both groups was safety concerns, affecting 10% and 13% of the combined OA and LAI groups, respectively, which was not a significant between-group difference.
Illness duration had a significant effect on time to all-cause discontinuation, with patients who had longer illness duration showing a poorer response, compared with those who had shorter duration (HR, 1.26; 95% CI, 1.01-1.56; P = .038).
However, stratifying participants by illness duration showed no significant difference between the subgroups (P = .25 and .34, respectively).
There was a significant between-group difference in discontinuation due to “other reasons,” with 49% vs. 34% of patients in the OA and LAI groups, respectively, discontinuing (HR, 1.51; 95% CI, 1.15-1.98; P = .0034). Moreover, the LAI group showed significantly longer continued use of medication vs the OA group (P = .0029).
“After separating the reasons for discontinuation into no efficacy, safety reasons, and other reasons, we only found a significant difference in favor of LAI for the ‘other reasons’ category; although the number of patients discontinuing medication for this reason over the follow-up period did not differ, patients on LAI continued treatment for a longer time,” the investigators write.
They acknowledge that this finding is “difficult to interpret, given the wide variety of reasons for discontinuation captured in this category,” which prevented an “informative subgroup analysis.”
Nevertheless, since there is “no consistent evidence supporting the use of LAI over oral antipsychotics” in patients with early-phase schizophrenia, their use should be “carefully considered on an individual risk-benefit basis,” they conclude.
No ‘real-world’ implications?
John M. Kane, MD, codirector and professor, Institute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, N.Y., said that overall, this was a “large, potentially valuable study.” However, he raised several concerns.
“I think the investigators made a much too emphatic statement about the lack of value of LAIs in early-phase patients when discontinuation is the primary outcome,” he said, noting that other studies have come to the opposite conclusion.
Dr. Kane, who is also a professor of psychiatry at Hofstra/Northwell, New York, was not involved with the current research.
“RCTs [randomized controlled trials] in general are not necessarily the best way to evaluate the impact of LAIs [which] usually represent a small percentage of potentially eligible patients and are likely to include patients who are more adherent than those who would not agree to participate in an RCT,” he said. He added that the investigators “did not report on how many patients were screened and refused to be considered.”
Also, Dr. Kane noted that half of the participants were recruited from inpatient services, and so may have been “more unstable” at baseline. “Patients with residual positive symptoms are more likely to relapse on LAIs than patients who are in remission. This could potentially reduce the advantage of the LAI,” he said.
In addition, he took issue with the definition of all-cause discontinuation, which included the need for augmentation with another antipsychotic or use outside the normal range.
“This happens often in clinical practice. If someone’s symptoms aren’t sufficiently controlled by an LAI alone, for example, they often receive more of that antipsychotic or another drug. This perhaps makes the EULAST study somewhat less ‘real-world’,” Dr. Kane said.
More information needed
In an accompanying editorial, Martina Hahn, PharmD, PhD, department of psychiatry, psychosomatics, and psychotherapy, University Hospital-Goethe University, Frankfurt, Germany, and Sibylle Christine Roll, MD, PHD, department of mental health, Varisano Hospital in Frankfurt, note that comedications were neither documented nor analyzed by the researchers.
“Drug-drug interactions could be responsible for relapse or poor tolerability,” they write.
Moreover, pharmacogenetic information was not available nor were serum concentrations that could have been used for dose optimization after switching antipsychotic formulations, they note.
This information would have provided “a deeper understanding of why some patients do not respond or show side effects,” the editorialists write. “The use of therapeutic drug monitoring, drug interaction checks, and pharmacogenetic testing could improve treatment outcomes in both study settings and clinical practice.”
Financial support and study medication was provided by Lundbeck and Otsuka. Dr. Winter-van Rossum reports no relevant financial relationships. Disclosures for the other investigators are fully listed in the original paper. Dr. Kane is or has been a consultant to or received honoraria for lectures from Alkermes , Biogen, Boehringer Ingelheim, Cerevel, Dainippon Sumitomo, H. Lundbeck, HLS, Intracellular Therapies, Janssen, Karuna, Merck, Newron, Otsuka, Roche, Saladax, Sunovion, and TEVA. He is also a shareholder in The Vanguard Research Group, LB Pharma, Health Rhythms, North Shore Therapeutics, and Medincell. Dr. Hahn reports having received honoraria for lecture from Otsuka and advisory board participation for Rovi. Dr. Roll reports advisory board participation for Recordati, Otsuka, and Janssen.
A version of this article first appeared on Medscape.com.
In a multicountry, randomized, open-label study of more than 500 adults with schizophrenia, participants received either LAI paliperidone, LAI aripiprazole, or the respective oral formulation of these antipsychotics.
Results showed no significant difference between the combined oral and combined LAI treatment groups in time to all-cause discontinuation.
“We found no substantial advantage for LAI antipsychotic treatment over oral treatment, regarding time to discontinuation in patients with early-phase schizophrenia,” write investigators, led by Inge Winter-van Rossum, PhD, assistant visiting professor at Mount Sinai, New York, and affiliated with King’s College London and UMC Utrecht (the Netherlands).
This indicates that “there is no reason to prescribe LAIs instead of oral antipsychotics if the goal is to prevent discontinuation of antipsychotic medication in daily clinical practice,” they add.
The findings were published online in The Lancet Psychiatry.
Previous conflicting results
Maintenance treatment with antipsychotic medication reduces risk for relapse considerably, with treatment discontinuation being “by far the most important reason for relapse,” the investigators write.
LAIs “seem theoretically to be a way to enhance medication continuation and thereby reduce the risk for relapse,” they add. This is because LAIs enable a rapid response to nonadherence and remove the need for patients to remember to take their medications on a daily basis.
However, previous research has “provided conflicting results,” regarding the effectiveness of LAIs in accomplishing this. Moreover, the subject has not been thoroughly investigated in early-stage schizophrenia, the researchers note.
Therefore, they decided to conduct the EULAST study to compare LAI and oral formulations in terms of all-cause discontinuation.
The trial was conducted at 50 general hospitals and psychiatric specialty clinics located in 15 European countries and Israel and included 511 participants in the intention-to-treat sample (67% men; mean age, 30.5 years).
All were randomly assigned 1:1:1 to receive either LAI paliperidone, LAI aripiprazole, or their respective oral formulations.
The combined OA treatment group consisted of 247 patients; the combined LAI group consisted of 264 patients.
Randomization was stratified by country and illness duration (5 months to 3 years vs. 4-7 years). Participants were followed up to 19 months, with all-cause discontinuation during that time serving as the primary endpoint.
All-cause discontinuation was defined as the allocated treatment was stopped or used at doses outside the allowed range, medication was switched or augmented with another antipsychotic after visit four, the patient missed a monthly visit and did not show up after being reminded, the patient withdrew consent for the study, or the clinician withdrew the patient from the study.
After the baseline visit, patients already taking antipsychotics were also randomly assigned. The next 4 weeks were then used to cross-taper between the prestudy antipsychotic and the agent they would be treated with during the study.
LAIs not superior
Results showed the LAI group did not have lower rates of hospitalization.
In addition, the discontinuation rates between the two combined groups were very similar at 71% for the oral antipsychotics group versus 64% in the LAIs group (hazard ratio, 1.6; 95% confidence interval, 0.94-1.43; P = .18).
Moreover, “no significant difference was found in the time to all-cause discontinuation between the combined oral and combined LAI treatment groups (P = .17),” the researchers report.
Reasons for discontinuation also did not differ significantly between the groups: 12% of patients in the OA group discontinued treatment because of efficacy vs. 17% of patients in the combined LAI group. The difference was not significant and the time to discontinuation also did not differ.
The main reason for discontinuation in both groups was safety concerns, affecting 10% and 13% of the combined OA and LAI groups, respectively, which was not a significant between-group difference.
Illness duration had a significant effect on time to all-cause discontinuation, with patients who had longer illness duration showing a poorer response, compared with those who had shorter duration (HR, 1.26; 95% CI, 1.01-1.56; P = .038).
However, stratifying participants by illness duration showed no significant difference between the subgroups (P = .25 and .34, respectively).
There was a significant between-group difference in discontinuation due to “other reasons,” with 49% vs. 34% of patients in the OA and LAI groups, respectively, discontinuing (HR, 1.51; 95% CI, 1.15-1.98; P = .0034). Moreover, the LAI group showed significantly longer continued use of medication vs the OA group (P = .0029).
“After separating the reasons for discontinuation into no efficacy, safety reasons, and other reasons, we only found a significant difference in favor of LAI for the ‘other reasons’ category; although the number of patients discontinuing medication for this reason over the follow-up period did not differ, patients on LAI continued treatment for a longer time,” the investigators write.
They acknowledge that this finding is “difficult to interpret, given the wide variety of reasons for discontinuation captured in this category,” which prevented an “informative subgroup analysis.”
Nevertheless, since there is “no consistent evidence supporting the use of LAI over oral antipsychotics” in patients with early-phase schizophrenia, their use should be “carefully considered on an individual risk-benefit basis,” they conclude.
No ‘real-world’ implications?
John M. Kane, MD, codirector and professor, Institute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, N.Y., said that overall, this was a “large, potentially valuable study.” However, he raised several concerns.
“I think the investigators made a much too emphatic statement about the lack of value of LAIs in early-phase patients when discontinuation is the primary outcome,” he said, noting that other studies have come to the opposite conclusion.
Dr. Kane, who is also a professor of psychiatry at Hofstra/Northwell, New York, was not involved with the current research.
“RCTs [randomized controlled trials] in general are not necessarily the best way to evaluate the impact of LAIs [which] usually represent a small percentage of potentially eligible patients and are likely to include patients who are more adherent than those who would not agree to participate in an RCT,” he said. He added that the investigators “did not report on how many patients were screened and refused to be considered.”
Also, Dr. Kane noted that half of the participants were recruited from inpatient services, and so may have been “more unstable” at baseline. “Patients with residual positive symptoms are more likely to relapse on LAIs than patients who are in remission. This could potentially reduce the advantage of the LAI,” he said.
In addition, he took issue with the definition of all-cause discontinuation, which included the need for augmentation with another antipsychotic or use outside the normal range.
“This happens often in clinical practice. If someone’s symptoms aren’t sufficiently controlled by an LAI alone, for example, they often receive more of that antipsychotic or another drug. This perhaps makes the EULAST study somewhat less ‘real-world’,” Dr. Kane said.
More information needed
In an accompanying editorial, Martina Hahn, PharmD, PhD, department of psychiatry, psychosomatics, and psychotherapy, University Hospital-Goethe University, Frankfurt, Germany, and Sibylle Christine Roll, MD, PHD, department of mental health, Varisano Hospital in Frankfurt, note that comedications were neither documented nor analyzed by the researchers.
“Drug-drug interactions could be responsible for relapse or poor tolerability,” they write.
Moreover, pharmacogenetic information was not available nor were serum concentrations that could have been used for dose optimization after switching antipsychotic formulations, they note.
This information would have provided “a deeper understanding of why some patients do not respond or show side effects,” the editorialists write. “The use of therapeutic drug monitoring, drug interaction checks, and pharmacogenetic testing could improve treatment outcomes in both study settings and clinical practice.”
Financial support and study medication was provided by Lundbeck and Otsuka. Dr. Winter-van Rossum reports no relevant financial relationships. Disclosures for the other investigators are fully listed in the original paper. Dr. Kane is or has been a consultant to or received honoraria for lectures from Alkermes , Biogen, Boehringer Ingelheim, Cerevel, Dainippon Sumitomo, H. Lundbeck, HLS, Intracellular Therapies, Janssen, Karuna, Merck, Newron, Otsuka, Roche, Saladax, Sunovion, and TEVA. He is also a shareholder in The Vanguard Research Group, LB Pharma, Health Rhythms, North Shore Therapeutics, and Medincell. Dr. Hahn reports having received honoraria for lecture from Otsuka and advisory board participation for Rovi. Dr. Roll reports advisory board participation for Recordati, Otsuka, and Janssen.
A version of this article first appeared on Medscape.com.
FROM THE LANCET PSYCHIATRY
‘Sighing’ tops mindfulness for reduced stress, improved mood
In a randomized controlled study, daily breathwork – especially cyclic breathing, which emphasizes shorter inhalations and prolonged exhalations – was associated with greater improvement in mood and a slower respiratory rate than mindfulness meditation.
“We were pleased that just 5 minutes a day of the breathing exercises positively affected mood and resulted in slower respiratory rate, indicating reduced arousal,” coinvestigator David Spiegel, MD, who directs the Center for Stress and Health at Stanford (Calif.) University, told this news organization.
The findings were published online in Cell Reports Medicine.
Intentional breath control
Controlled breathwork has emerged as a potential tool to manage stress and boost well-being.
In the new study, researchers compared three different daily 5-minute breathwork exercises to an equal amount of mindfulness meditation over 1 month in 108 healthy adults recruited mostly from an undergraduate psychology class at Stanford: 33 participants practiced cyclic hyperventilation, which emphasizes robust inhalation, short retention and rapid exhalation, 30 did exhale-focused cyclic sighing, 21 performed box breathing, which emphasizes equal duration of inhalation, breath retention, and exhalation, and 24 practiced mindfulness meditation (the control group).
The primary endpoints were improvement in mood and anxiety, as well as reduced physiologic arousal (respiratory rate, heart rate, and heart rate variability). Physiological data was collected using a wearable WHOOP strap.
All four groups showed significant daily improvement in mood, as well as reduction in anxiety and negative mood, but there were significant differences between mindfulness meditation and breathwork.
Using a mixed-effects model, the researchers showed that breathwork, especially the exhale-focused cyclic sighing, produced greater improvement in mood (P < .05) and reduction in respiratory rate (P < .05), compared with mindfulness meditation.
Specific patterns vs. passive attention
The finding supports the team’s hypothesis that intentional control over breath with specific breathing patterns produces more benefit to mood than passive attention to one’s breath, as in mindfulness meditation practice.
“It turned out that the cyclic sighing was indeed most soothing,” Dr. Spiegel noted.
“We expected that because of respiratory sinus arrhythmia. Exhaling is accomplished by increasing pressure in the chest, which increases venous return to the heart, triggering parasympathetic slowing of heart rate via the sinoatrial node,” he said.
Dr. Spiegel added that, conversely, inspiration reduces venous return, triggering sympathetic activity and increased heart rate.
“The magnitude of this heart rate variability is associated with better health, including recovery from myocardial infarction and even cancer survival. So self-soothing is a good thing, and we expected an advantage for cyclic sighing,” he said.
“If you’re looking to improve sleep and reduce daytime stress, recover from intense work, life, and/or training, then interventions that facilitate autonomic control (and indeed you can control it), brief (5 minutes) structured breathwork is among the more powerful (and zero cost) tools,” tweeted senior investigator Andrew Huberman, PhD, professor of neurobiology at Stanford.
Immediate application?
Sara Lazar, PhD, Massachusetts General Hospital and Harvard Medical School, Boston, said the findings are “interesting” but cautioned that this is “just one study with a pretty small sample size,” and it only enrolled healthy college students.
Dr. Lazar, who also runs the Lazar Lab for Meditation Research at Mass General, noted that she would want to see a future study “done with working-age adults and with clinical populations.”
“It should also be noted that mindfulness had a bigger effect on negative affect, which could have implications for conditions such as depression or trauma,” said Dr. Lazar, who was not involved with the current research.
Also weighing in, Steven R. Thorp, PhD, professor at California School of Professional Psychology, Alliant International University, San Diego, said in an interview the study is “really interesting and well done.”
“Although breathing exercises and breathing retraining are commonly found in psychosocial interventions, especially for anxiety disorders, there have been few empirical studies comparing different breathing protocols,” Dr. Thorp said.
In this study, the passive observation of breaths (mindfulness) and specific breathwork interventions “all worked to decrease state anxiety; but the breathwork, particularly the cyclic sighing protocol, produced a greater overall reduction in respiratory rate and increase in positive mood,” he noted.
“These techniques can be recommended by all clinicians because all clients have access to their breath at all times – and only 5 minutes of daily practice can yield the benefits. Moreover, as the authors note, the immediate benefits may encourage clients to engage with the breathwork and potentially other aspects of treatment,” Dr. Thorp said.
The study was funded by Victor and Winnie Koo and Tianren Culture and a Stanford School of Medicine Discovery Innovation Award. WHOOP donated the wrist straps used in the study, but was not involved in the study’s design or analysis. Dr. Huberman is an advisor to WHOOP. Dr. Lazar and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized controlled study, daily breathwork – especially cyclic breathing, which emphasizes shorter inhalations and prolonged exhalations – was associated with greater improvement in mood and a slower respiratory rate than mindfulness meditation.
“We were pleased that just 5 minutes a day of the breathing exercises positively affected mood and resulted in slower respiratory rate, indicating reduced arousal,” coinvestigator David Spiegel, MD, who directs the Center for Stress and Health at Stanford (Calif.) University, told this news organization.
The findings were published online in Cell Reports Medicine.
Intentional breath control
Controlled breathwork has emerged as a potential tool to manage stress and boost well-being.
In the new study, researchers compared three different daily 5-minute breathwork exercises to an equal amount of mindfulness meditation over 1 month in 108 healthy adults recruited mostly from an undergraduate psychology class at Stanford: 33 participants practiced cyclic hyperventilation, which emphasizes robust inhalation, short retention and rapid exhalation, 30 did exhale-focused cyclic sighing, 21 performed box breathing, which emphasizes equal duration of inhalation, breath retention, and exhalation, and 24 practiced mindfulness meditation (the control group).
The primary endpoints were improvement in mood and anxiety, as well as reduced physiologic arousal (respiratory rate, heart rate, and heart rate variability). Physiological data was collected using a wearable WHOOP strap.
All four groups showed significant daily improvement in mood, as well as reduction in anxiety and negative mood, but there were significant differences between mindfulness meditation and breathwork.
Using a mixed-effects model, the researchers showed that breathwork, especially the exhale-focused cyclic sighing, produced greater improvement in mood (P < .05) and reduction in respiratory rate (P < .05), compared with mindfulness meditation.
Specific patterns vs. passive attention
The finding supports the team’s hypothesis that intentional control over breath with specific breathing patterns produces more benefit to mood than passive attention to one’s breath, as in mindfulness meditation practice.
“It turned out that the cyclic sighing was indeed most soothing,” Dr. Spiegel noted.
“We expected that because of respiratory sinus arrhythmia. Exhaling is accomplished by increasing pressure in the chest, which increases venous return to the heart, triggering parasympathetic slowing of heart rate via the sinoatrial node,” he said.
Dr. Spiegel added that, conversely, inspiration reduces venous return, triggering sympathetic activity and increased heart rate.
“The magnitude of this heart rate variability is associated with better health, including recovery from myocardial infarction and even cancer survival. So self-soothing is a good thing, and we expected an advantage for cyclic sighing,” he said.
“If you’re looking to improve sleep and reduce daytime stress, recover from intense work, life, and/or training, then interventions that facilitate autonomic control (and indeed you can control it), brief (5 minutes) structured breathwork is among the more powerful (and zero cost) tools,” tweeted senior investigator Andrew Huberman, PhD, professor of neurobiology at Stanford.
Immediate application?
Sara Lazar, PhD, Massachusetts General Hospital and Harvard Medical School, Boston, said the findings are “interesting” but cautioned that this is “just one study with a pretty small sample size,” and it only enrolled healthy college students.
Dr. Lazar, who also runs the Lazar Lab for Meditation Research at Mass General, noted that she would want to see a future study “done with working-age adults and with clinical populations.”
“It should also be noted that mindfulness had a bigger effect on negative affect, which could have implications for conditions such as depression or trauma,” said Dr. Lazar, who was not involved with the current research.
Also weighing in, Steven R. Thorp, PhD, professor at California School of Professional Psychology, Alliant International University, San Diego, said in an interview the study is “really interesting and well done.”
“Although breathing exercises and breathing retraining are commonly found in psychosocial interventions, especially for anxiety disorders, there have been few empirical studies comparing different breathing protocols,” Dr. Thorp said.
In this study, the passive observation of breaths (mindfulness) and specific breathwork interventions “all worked to decrease state anxiety; but the breathwork, particularly the cyclic sighing protocol, produced a greater overall reduction in respiratory rate and increase in positive mood,” he noted.
“These techniques can be recommended by all clinicians because all clients have access to their breath at all times – and only 5 minutes of daily practice can yield the benefits. Moreover, as the authors note, the immediate benefits may encourage clients to engage with the breathwork and potentially other aspects of treatment,” Dr. Thorp said.
The study was funded by Victor and Winnie Koo and Tianren Culture and a Stanford School of Medicine Discovery Innovation Award. WHOOP donated the wrist straps used in the study, but was not involved in the study’s design or analysis. Dr. Huberman is an advisor to WHOOP. Dr. Lazar and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized controlled study, daily breathwork – especially cyclic breathing, which emphasizes shorter inhalations and prolonged exhalations – was associated with greater improvement in mood and a slower respiratory rate than mindfulness meditation.
“We were pleased that just 5 minutes a day of the breathing exercises positively affected mood and resulted in slower respiratory rate, indicating reduced arousal,” coinvestigator David Spiegel, MD, who directs the Center for Stress and Health at Stanford (Calif.) University, told this news organization.
The findings were published online in Cell Reports Medicine.
Intentional breath control
Controlled breathwork has emerged as a potential tool to manage stress and boost well-being.
In the new study, researchers compared three different daily 5-minute breathwork exercises to an equal amount of mindfulness meditation over 1 month in 108 healthy adults recruited mostly from an undergraduate psychology class at Stanford: 33 participants practiced cyclic hyperventilation, which emphasizes robust inhalation, short retention and rapid exhalation, 30 did exhale-focused cyclic sighing, 21 performed box breathing, which emphasizes equal duration of inhalation, breath retention, and exhalation, and 24 practiced mindfulness meditation (the control group).
The primary endpoints were improvement in mood and anxiety, as well as reduced physiologic arousal (respiratory rate, heart rate, and heart rate variability). Physiological data was collected using a wearable WHOOP strap.
All four groups showed significant daily improvement in mood, as well as reduction in anxiety and negative mood, but there were significant differences between mindfulness meditation and breathwork.
Using a mixed-effects model, the researchers showed that breathwork, especially the exhale-focused cyclic sighing, produced greater improvement in mood (P < .05) and reduction in respiratory rate (P < .05), compared with mindfulness meditation.
Specific patterns vs. passive attention
The finding supports the team’s hypothesis that intentional control over breath with specific breathing patterns produces more benefit to mood than passive attention to one’s breath, as in mindfulness meditation practice.
“It turned out that the cyclic sighing was indeed most soothing,” Dr. Spiegel noted.
“We expected that because of respiratory sinus arrhythmia. Exhaling is accomplished by increasing pressure in the chest, which increases venous return to the heart, triggering parasympathetic slowing of heart rate via the sinoatrial node,” he said.
Dr. Spiegel added that, conversely, inspiration reduces venous return, triggering sympathetic activity and increased heart rate.
“The magnitude of this heart rate variability is associated with better health, including recovery from myocardial infarction and even cancer survival. So self-soothing is a good thing, and we expected an advantage for cyclic sighing,” he said.
“If you’re looking to improve sleep and reduce daytime stress, recover from intense work, life, and/or training, then interventions that facilitate autonomic control (and indeed you can control it), brief (5 minutes) structured breathwork is among the more powerful (and zero cost) tools,” tweeted senior investigator Andrew Huberman, PhD, professor of neurobiology at Stanford.
Immediate application?
Sara Lazar, PhD, Massachusetts General Hospital and Harvard Medical School, Boston, said the findings are “interesting” but cautioned that this is “just one study with a pretty small sample size,” and it only enrolled healthy college students.
Dr. Lazar, who also runs the Lazar Lab for Meditation Research at Mass General, noted that she would want to see a future study “done with working-age adults and with clinical populations.”
“It should also be noted that mindfulness had a bigger effect on negative affect, which could have implications for conditions such as depression or trauma,” said Dr. Lazar, who was not involved with the current research.
Also weighing in, Steven R. Thorp, PhD, professor at California School of Professional Psychology, Alliant International University, San Diego, said in an interview the study is “really interesting and well done.”
“Although breathing exercises and breathing retraining are commonly found in psychosocial interventions, especially for anxiety disorders, there have been few empirical studies comparing different breathing protocols,” Dr. Thorp said.
In this study, the passive observation of breaths (mindfulness) and specific breathwork interventions “all worked to decrease state anxiety; but the breathwork, particularly the cyclic sighing protocol, produced a greater overall reduction in respiratory rate and increase in positive mood,” he noted.
“These techniques can be recommended by all clinicians because all clients have access to their breath at all times – and only 5 minutes of daily practice can yield the benefits. Moreover, as the authors note, the immediate benefits may encourage clients to engage with the breathwork and potentially other aspects of treatment,” Dr. Thorp said.
The study was funded by Victor and Winnie Koo and Tianren Culture and a Stanford School of Medicine Discovery Innovation Award. WHOOP donated the wrist straps used in the study, but was not involved in the study’s design or analysis. Dr. Huberman is an advisor to WHOOP. Dr. Lazar and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CELL REPORTS MEDICINE
Time for a national ketamine registry, experts say
The number of ketamine clinics has risen dramatically, with little to no oversight. Prescriptions are being written by providers who lack training in safe ketamine use and online startups are selling the drug for at-home use, taking advantage of a temporary federal regulation that makes it easier to prescribe controlled substances without an in-person patient assessment.
All of this comes at a time when recreational use of ketamine, known on the street as “Special K,” is rising, and reports to poison control centers and drug seizures by the U.S. Drug Enforcement Agency (DEA) are climbing.
In a scenario where enthusiasm for the drug is larger than the body of evidence supporting its clinical use, support is growing for the creation of a ketamine registry to collect data on dosage, treatment frequency, adverse events, and long-term outcomes in patients receiving the therapy for depression and other mental health conditions.
“In the past, there was this question of whether a registry was even needed,” said Gerard Sanacora, MD, PhD, a professor of psychiatry at Yale University, New Haven, Conn., who has pushed for a registry for more than 5 years.
“Now, not only are people being treated with this in large numbers, but it’s also started to push the envelope with at-home dosing,” Dr. Sanacora said in an interview. “It’s come to the point that everybody agrees we do need some way to track it.”
An idea whose time has come
Interest in ketamine’s antidepressant effects has grown since 2000, when a small study suggested the drug rapidly improved depressive symptoms. Research now suggests ketamine reduces symptoms in patients with treatment-resistant depression (TRD).
Studies linking ketamine to relief of depressive symptoms are small and mostly retrospective, and none has offered longitudinal information on long-term outcomes, including side effects and the risk of addiction.
Still, clinicians desperate to help the one-third of patients with major depression who fail to respond to first-line treatments often prescribe the drug anyway.
In 2017, Dr. Sanacora, who also is director of the Yale Depression Research Program at the Yale School of Medicine, was the lead author of a consensus statement that sought to help physicians administer ketamine safely and appropriately in patients with severe depression and other mood disorders.
In that paper, Dr. Sanacora and his coauthors advocated for the creation of a ketamine registry. Such a database, they argued, would provide much-needed data for large, long-term studies, which could be used to develop treatment guidelines, certification programs, and possibly even accreditation standards for providers. Meanwhile, researchers and clinicians in the United Kingdom were also calling for a ketamine registry.
While there seemed to be wide consensus that such a registry was needed, there was no clear path to creating one and no clear line to an agency that would take responsibility for maintaining it.
Because the registry wouldn’t be tied to a drug indication, Dr. Sanacora was told the U.S. Food and Drug Administration wouldn’t take it on. The project also fell outside the purview of the U.S. Department of Health & Human Services, the National Institute of Mental Health (NIMH), and the DEA.
“I haven’t met anybody who has said this is a terrible idea, but nobody seems to have a clear mechanism of doing it, and it doesn’t seem to fall directly under anybody’s jurisdiction,” Dr. Sanacora said.
Dr. Sanacora and other ketamine registry advocates were met with an endless stream of questions. Who would pay for it? How would they get providers to participate? Who would run it and how would the data be shared? The barriers to implementation seemed insurmountable.
A changing landscape
Five years later, these barriers remain. However, advocates note support for a registry is growing, due in large part to a series of developments over the past 6 years that they believe have altered the ketamine landscape.
Chief among these was the 2019 FDA approval of esketamine, a nasal formulation of ketamine, for the treatment of resistant depression. The drug’s indication was expanded in 2020 to include major depressive disorder and acute suicidal ideation or behavior. The drug is only available through a restricted distribution system – the Spravato Risk Evaluation and Mitigation Strategy (REMS) – because of the risk for serious adverse events, including sedation and dissociation, as well as the potential for abuse or misuse.
A sharp increase in the number of ketamine prescribers and clinics has also heightened interest in a ketamine registry. In the last year alone, membership in the American Society of Ketamine Physicians, Psychotherapists, and Practitioners (ASKP) – a nonprofit trade organization for clinicians who prescribe ketamine for mental health disorders and pain conditions – swelled from 300 individual providers to more than 500.
The number of ketamine clinics in the United States has also grown exponentially and is estimated to be anywhere from 500 to 750. A spokesperson with HHS said such clinics are not regulated by the department or any other federal agency but instead are subject to oversight by individual states.
Although recreational use of ketamine remains low overall, there are signs that illicit use is rising, including an increase in DEA seizures of illicit ketamine and reports of ketamine-related poisonings to the nation’s poison control centers. Data on recreational use is spotty, at best. The Centers for Disease Control and Prevention National Vital Statistics System – the primary source of information on drug-related mortality in the United States – does not report on ketamine.
At-home ketamine use soars
Perhaps the most significant development came in March 2020 in the early days of the pandemic. To ease access to therapeutic schedule II-V controlled substances, the DEA issued a waiver that relaxed restrictions in the Ryan Haight Act, legislation that requires that patients be seen at least once in person before receiving a prescription for this class of drugs.
Under the waiver, DEA-registered practitioners are allowed to prescribe these substances – including ketamine, a schedule III substance – via telemedicine, without an in-person exam.
Startup companies cropped up almost overnight to prescribe oral ketamine online for at-home use, with almost no oversight. A spokesperson with the DEA told this news organization that the agency is working to make these “temporary” regulations permanent.
Under the relaxed DEA guidelines, a prescriber only needs to have a DEA license to dispense a ketamine prescription. An alarming number of clinics and online startups are staffed by individuals with no training in ketamine use and, in some cases, no formal mental health training at all, said Lisa Harding, MD, vice president of ASKP and a clinical instructor of psychiatry at Yale School of Medicine.
“The biggest problem is not the ketamine itself, it’s that the majority of practitioners are not psychiatrists, so they don’t have mental health training,” Dr. Harding said. “The fact that an untrained person, any practitioner with no mental health training, can administer this treatment once they have a state license to give ketamine ... then how are you protecting the patients?”
That question prompted ASKP to create the first known program to train psychiatrists, and other qualified mental health practitioners who prescribe ketamine, how to use the drug safely and effectively. The program, scheduled for June, will also include discussion by leaders in the field about how a ketamine registry might address these and other patient safety concerns.
“Nobody is really investigating the standard to which these clinics and online companies should be held, and I think a registry would help with that,” she said in an interview.
The path forward
While ASKP leadership supports the idea of a ketamine registry, Dr. Harding said the organization would need assurances the effort would not create a barrier to treatment.
“It will take somebody bringing all of us to the table and figuring that out,” Dr. Harding said.
Conversations like that with stakeholders would be one of the first steps toward creating a registry, Dr. Sanacora said.
“The more complicated we make this registry, the less compliance we’re going to get,” Dr. Sanacora said. “Our first step is to understand the major impediments and figure out how we can make this easier for people.”
Ideally, the registry would take advantage of existing data-collection tools, such as electronic health records (EHR), and include some sort of patient data entry mechanism, Dr. Sanacora said. The effort will also require skilled biostatisticians and a database system that is easy to manage.
And, of course, the registry will need a large number of patients to gather sufficient data to conduct high-quality research to develop treatment guidelines, training, and accreditation standards. A good target would be about 10,000 patients, Dr. Sanacora said.
All of this requires funding, which is the first hurdle registry advocates must clear. Dr. Sanacora is working on identifying funding sources and said that after working on this for years, he is hopeful that progress can be made.
“I had reached a point where it felt like there was no path forward,” Dr. Sanacora said. “But now I have renewed optimism that something can be done. And something does need to be done, largely for public health reasons but also to optimize the treatment.”
A version of this article first appeared on Medscape.com.
The number of ketamine clinics has risen dramatically, with little to no oversight. Prescriptions are being written by providers who lack training in safe ketamine use and online startups are selling the drug for at-home use, taking advantage of a temporary federal regulation that makes it easier to prescribe controlled substances without an in-person patient assessment.
All of this comes at a time when recreational use of ketamine, known on the street as “Special K,” is rising, and reports to poison control centers and drug seizures by the U.S. Drug Enforcement Agency (DEA) are climbing.
In a scenario where enthusiasm for the drug is larger than the body of evidence supporting its clinical use, support is growing for the creation of a ketamine registry to collect data on dosage, treatment frequency, adverse events, and long-term outcomes in patients receiving the therapy for depression and other mental health conditions.
“In the past, there was this question of whether a registry was even needed,” said Gerard Sanacora, MD, PhD, a professor of psychiatry at Yale University, New Haven, Conn., who has pushed for a registry for more than 5 years.
“Now, not only are people being treated with this in large numbers, but it’s also started to push the envelope with at-home dosing,” Dr. Sanacora said in an interview. “It’s come to the point that everybody agrees we do need some way to track it.”
An idea whose time has come
Interest in ketamine’s antidepressant effects has grown since 2000, when a small study suggested the drug rapidly improved depressive symptoms. Research now suggests ketamine reduces symptoms in patients with treatment-resistant depression (TRD).
Studies linking ketamine to relief of depressive symptoms are small and mostly retrospective, and none has offered longitudinal information on long-term outcomes, including side effects and the risk of addiction.
Still, clinicians desperate to help the one-third of patients with major depression who fail to respond to first-line treatments often prescribe the drug anyway.
In 2017, Dr. Sanacora, who also is director of the Yale Depression Research Program at the Yale School of Medicine, was the lead author of a consensus statement that sought to help physicians administer ketamine safely and appropriately in patients with severe depression and other mood disorders.
In that paper, Dr. Sanacora and his coauthors advocated for the creation of a ketamine registry. Such a database, they argued, would provide much-needed data for large, long-term studies, which could be used to develop treatment guidelines, certification programs, and possibly even accreditation standards for providers. Meanwhile, researchers and clinicians in the United Kingdom were also calling for a ketamine registry.
While there seemed to be wide consensus that such a registry was needed, there was no clear path to creating one and no clear line to an agency that would take responsibility for maintaining it.
Because the registry wouldn’t be tied to a drug indication, Dr. Sanacora was told the U.S. Food and Drug Administration wouldn’t take it on. The project also fell outside the purview of the U.S. Department of Health & Human Services, the National Institute of Mental Health (NIMH), and the DEA.
“I haven’t met anybody who has said this is a terrible idea, but nobody seems to have a clear mechanism of doing it, and it doesn’t seem to fall directly under anybody’s jurisdiction,” Dr. Sanacora said.
Dr. Sanacora and other ketamine registry advocates were met with an endless stream of questions. Who would pay for it? How would they get providers to participate? Who would run it and how would the data be shared? The barriers to implementation seemed insurmountable.
A changing landscape
Five years later, these barriers remain. However, advocates note support for a registry is growing, due in large part to a series of developments over the past 6 years that they believe have altered the ketamine landscape.
Chief among these was the 2019 FDA approval of esketamine, a nasal formulation of ketamine, for the treatment of resistant depression. The drug’s indication was expanded in 2020 to include major depressive disorder and acute suicidal ideation or behavior. The drug is only available through a restricted distribution system – the Spravato Risk Evaluation and Mitigation Strategy (REMS) – because of the risk for serious adverse events, including sedation and dissociation, as well as the potential for abuse or misuse.
A sharp increase in the number of ketamine prescribers and clinics has also heightened interest in a ketamine registry. In the last year alone, membership in the American Society of Ketamine Physicians, Psychotherapists, and Practitioners (ASKP) – a nonprofit trade organization for clinicians who prescribe ketamine for mental health disorders and pain conditions – swelled from 300 individual providers to more than 500.
The number of ketamine clinics in the United States has also grown exponentially and is estimated to be anywhere from 500 to 750. A spokesperson with HHS said such clinics are not regulated by the department or any other federal agency but instead are subject to oversight by individual states.
Although recreational use of ketamine remains low overall, there are signs that illicit use is rising, including an increase in DEA seizures of illicit ketamine and reports of ketamine-related poisonings to the nation’s poison control centers. Data on recreational use is spotty, at best. The Centers for Disease Control and Prevention National Vital Statistics System – the primary source of information on drug-related mortality in the United States – does not report on ketamine.
At-home ketamine use soars
Perhaps the most significant development came in March 2020 in the early days of the pandemic. To ease access to therapeutic schedule II-V controlled substances, the DEA issued a waiver that relaxed restrictions in the Ryan Haight Act, legislation that requires that patients be seen at least once in person before receiving a prescription for this class of drugs.
Under the waiver, DEA-registered practitioners are allowed to prescribe these substances – including ketamine, a schedule III substance – via telemedicine, without an in-person exam.
Startup companies cropped up almost overnight to prescribe oral ketamine online for at-home use, with almost no oversight. A spokesperson with the DEA told this news organization that the agency is working to make these “temporary” regulations permanent.
Under the relaxed DEA guidelines, a prescriber only needs to have a DEA license to dispense a ketamine prescription. An alarming number of clinics and online startups are staffed by individuals with no training in ketamine use and, in some cases, no formal mental health training at all, said Lisa Harding, MD, vice president of ASKP and a clinical instructor of psychiatry at Yale School of Medicine.
“The biggest problem is not the ketamine itself, it’s that the majority of practitioners are not psychiatrists, so they don’t have mental health training,” Dr. Harding said. “The fact that an untrained person, any practitioner with no mental health training, can administer this treatment once they have a state license to give ketamine ... then how are you protecting the patients?”
That question prompted ASKP to create the first known program to train psychiatrists, and other qualified mental health practitioners who prescribe ketamine, how to use the drug safely and effectively. The program, scheduled for June, will also include discussion by leaders in the field about how a ketamine registry might address these and other patient safety concerns.
“Nobody is really investigating the standard to which these clinics and online companies should be held, and I think a registry would help with that,” she said in an interview.
The path forward
While ASKP leadership supports the idea of a ketamine registry, Dr. Harding said the organization would need assurances the effort would not create a barrier to treatment.
“It will take somebody bringing all of us to the table and figuring that out,” Dr. Harding said.
Conversations like that with stakeholders would be one of the first steps toward creating a registry, Dr. Sanacora said.
“The more complicated we make this registry, the less compliance we’re going to get,” Dr. Sanacora said. “Our first step is to understand the major impediments and figure out how we can make this easier for people.”
Ideally, the registry would take advantage of existing data-collection tools, such as electronic health records (EHR), and include some sort of patient data entry mechanism, Dr. Sanacora said. The effort will also require skilled biostatisticians and a database system that is easy to manage.
And, of course, the registry will need a large number of patients to gather sufficient data to conduct high-quality research to develop treatment guidelines, training, and accreditation standards. A good target would be about 10,000 patients, Dr. Sanacora said.
All of this requires funding, which is the first hurdle registry advocates must clear. Dr. Sanacora is working on identifying funding sources and said that after working on this for years, he is hopeful that progress can be made.
“I had reached a point where it felt like there was no path forward,” Dr. Sanacora said. “But now I have renewed optimism that something can be done. And something does need to be done, largely for public health reasons but also to optimize the treatment.”
A version of this article first appeared on Medscape.com.
The number of ketamine clinics has risen dramatically, with little to no oversight. Prescriptions are being written by providers who lack training in safe ketamine use and online startups are selling the drug for at-home use, taking advantage of a temporary federal regulation that makes it easier to prescribe controlled substances without an in-person patient assessment.
All of this comes at a time when recreational use of ketamine, known on the street as “Special K,” is rising, and reports to poison control centers and drug seizures by the U.S. Drug Enforcement Agency (DEA) are climbing.
In a scenario where enthusiasm for the drug is larger than the body of evidence supporting its clinical use, support is growing for the creation of a ketamine registry to collect data on dosage, treatment frequency, adverse events, and long-term outcomes in patients receiving the therapy for depression and other mental health conditions.
“In the past, there was this question of whether a registry was even needed,” said Gerard Sanacora, MD, PhD, a professor of psychiatry at Yale University, New Haven, Conn., who has pushed for a registry for more than 5 years.
“Now, not only are people being treated with this in large numbers, but it’s also started to push the envelope with at-home dosing,” Dr. Sanacora said in an interview. “It’s come to the point that everybody agrees we do need some way to track it.”
An idea whose time has come
Interest in ketamine’s antidepressant effects has grown since 2000, when a small study suggested the drug rapidly improved depressive symptoms. Research now suggests ketamine reduces symptoms in patients with treatment-resistant depression (TRD).
Studies linking ketamine to relief of depressive symptoms are small and mostly retrospective, and none has offered longitudinal information on long-term outcomes, including side effects and the risk of addiction.
Still, clinicians desperate to help the one-third of patients with major depression who fail to respond to first-line treatments often prescribe the drug anyway.
In 2017, Dr. Sanacora, who also is director of the Yale Depression Research Program at the Yale School of Medicine, was the lead author of a consensus statement that sought to help physicians administer ketamine safely and appropriately in patients with severe depression and other mood disorders.
In that paper, Dr. Sanacora and his coauthors advocated for the creation of a ketamine registry. Such a database, they argued, would provide much-needed data for large, long-term studies, which could be used to develop treatment guidelines, certification programs, and possibly even accreditation standards for providers. Meanwhile, researchers and clinicians in the United Kingdom were also calling for a ketamine registry.
While there seemed to be wide consensus that such a registry was needed, there was no clear path to creating one and no clear line to an agency that would take responsibility for maintaining it.
Because the registry wouldn’t be tied to a drug indication, Dr. Sanacora was told the U.S. Food and Drug Administration wouldn’t take it on. The project also fell outside the purview of the U.S. Department of Health & Human Services, the National Institute of Mental Health (NIMH), and the DEA.
“I haven’t met anybody who has said this is a terrible idea, but nobody seems to have a clear mechanism of doing it, and it doesn’t seem to fall directly under anybody’s jurisdiction,” Dr. Sanacora said.
Dr. Sanacora and other ketamine registry advocates were met with an endless stream of questions. Who would pay for it? How would they get providers to participate? Who would run it and how would the data be shared? The barriers to implementation seemed insurmountable.
A changing landscape
Five years later, these barriers remain. However, advocates note support for a registry is growing, due in large part to a series of developments over the past 6 years that they believe have altered the ketamine landscape.
Chief among these was the 2019 FDA approval of esketamine, a nasal formulation of ketamine, for the treatment of resistant depression. The drug’s indication was expanded in 2020 to include major depressive disorder and acute suicidal ideation or behavior. The drug is only available through a restricted distribution system – the Spravato Risk Evaluation and Mitigation Strategy (REMS) – because of the risk for serious adverse events, including sedation and dissociation, as well as the potential for abuse or misuse.
A sharp increase in the number of ketamine prescribers and clinics has also heightened interest in a ketamine registry. In the last year alone, membership in the American Society of Ketamine Physicians, Psychotherapists, and Practitioners (ASKP) – a nonprofit trade organization for clinicians who prescribe ketamine for mental health disorders and pain conditions – swelled from 300 individual providers to more than 500.
The number of ketamine clinics in the United States has also grown exponentially and is estimated to be anywhere from 500 to 750. A spokesperson with HHS said such clinics are not regulated by the department or any other federal agency but instead are subject to oversight by individual states.
Although recreational use of ketamine remains low overall, there are signs that illicit use is rising, including an increase in DEA seizures of illicit ketamine and reports of ketamine-related poisonings to the nation’s poison control centers. Data on recreational use is spotty, at best. The Centers for Disease Control and Prevention National Vital Statistics System – the primary source of information on drug-related mortality in the United States – does not report on ketamine.
At-home ketamine use soars
Perhaps the most significant development came in March 2020 in the early days of the pandemic. To ease access to therapeutic schedule II-V controlled substances, the DEA issued a waiver that relaxed restrictions in the Ryan Haight Act, legislation that requires that patients be seen at least once in person before receiving a prescription for this class of drugs.
Under the waiver, DEA-registered practitioners are allowed to prescribe these substances – including ketamine, a schedule III substance – via telemedicine, without an in-person exam.
Startup companies cropped up almost overnight to prescribe oral ketamine online for at-home use, with almost no oversight. A spokesperson with the DEA told this news organization that the agency is working to make these “temporary” regulations permanent.
Under the relaxed DEA guidelines, a prescriber only needs to have a DEA license to dispense a ketamine prescription. An alarming number of clinics and online startups are staffed by individuals with no training in ketamine use and, in some cases, no formal mental health training at all, said Lisa Harding, MD, vice president of ASKP and a clinical instructor of psychiatry at Yale School of Medicine.
“The biggest problem is not the ketamine itself, it’s that the majority of practitioners are not psychiatrists, so they don’t have mental health training,” Dr. Harding said. “The fact that an untrained person, any practitioner with no mental health training, can administer this treatment once they have a state license to give ketamine ... then how are you protecting the patients?”
That question prompted ASKP to create the first known program to train psychiatrists, and other qualified mental health practitioners who prescribe ketamine, how to use the drug safely and effectively. The program, scheduled for June, will also include discussion by leaders in the field about how a ketamine registry might address these and other patient safety concerns.
“Nobody is really investigating the standard to which these clinics and online companies should be held, and I think a registry would help with that,” she said in an interview.
The path forward
While ASKP leadership supports the idea of a ketamine registry, Dr. Harding said the organization would need assurances the effort would not create a barrier to treatment.
“It will take somebody bringing all of us to the table and figuring that out,” Dr. Harding said.
Conversations like that with stakeholders would be one of the first steps toward creating a registry, Dr. Sanacora said.
“The more complicated we make this registry, the less compliance we’re going to get,” Dr. Sanacora said. “Our first step is to understand the major impediments and figure out how we can make this easier for people.”
Ideally, the registry would take advantage of existing data-collection tools, such as electronic health records (EHR), and include some sort of patient data entry mechanism, Dr. Sanacora said. The effort will also require skilled biostatisticians and a database system that is easy to manage.
And, of course, the registry will need a large number of patients to gather sufficient data to conduct high-quality research to develop treatment guidelines, training, and accreditation standards. A good target would be about 10,000 patients, Dr. Sanacora said.
All of this requires funding, which is the first hurdle registry advocates must clear. Dr. Sanacora is working on identifying funding sources and said that after working on this for years, he is hopeful that progress can be made.
“I had reached a point where it felt like there was no path forward,” Dr. Sanacora said. “But now I have renewed optimism that something can be done. And something does need to be done, largely for public health reasons but also to optimize the treatment.”
A version of this article first appeared on Medscape.com.
Can medication management smooth the journey for families of autistic children?
Caring for a child with autism is a long haul for families and not often a smooth ride. Medications can help improve child functioning and family quality of life but the evidence may require our careful consideration.
Although I am discussing medication treatment here, the best evidence-based treatments for autism symptoms such as poor social communication and repetitive restricted behavior (RRB) are behavioral (for example, applied behavior analysis), cognitive-behavioral therapy (CBT), and parent training. These modalities also augment the effectiveness of medications in many cases. Educational adjustments and specific therapies, when indicated, such as speech-language, occupational, and physical therapy are also beneficial.
Autism symptoms change with age from early regression, later to RRB, then depression, and they are complicated by coexisting conditions. Not surprisingly then, studying the effectiveness and side effects of medications is complex and guidance is in flux as reliable data emerge.
Because of the shortage of specialists and increasing prevalence of autism, we need to be prepared to manage, monitor, and sometimes start medications for our autistic patients. With great individual differences and the hopes and fears of distressed parents making them desperate for help, we need to be as evidence-based as possible to avoid serious side effects or delay effective behavioral treatments.
Our assistance is mainly to address the many co-occurring symptoms in autism: 37%-85% ADHD, 50% anxiety, 7.3% bipolar disorder, and 54.1% depression (by age 30). Many autistic children have problematic irritability, explosive episodes, repetitive or rigid routines, difficulty with social engagement, or trouble sleeping.
We need to be clear with families about the evidence and, whenever possible, use our own time-limited trials with placebos and objective measures that target symptoms and goals for improvement. This is complicated by that fact that the child may have trouble communicating about how they feel, have hypo- or hypersensitivity to feelings, as well as confounding coexisting conditions.
The Food and Drug Administration has approved the atypical antipsychotics risperidone (ages 5-16) and aripiprazole (ages 6-17) for reducing symptoms of irritability by 25%-50% – such as agitation, stereotypy, anger outbursts, self-injurious behavior, and hyperactivity within 8 weeks. The Aberrant Behavior Checklist can be used for monitoring. These benefits are largest with behavior therapy and at doses of 1.25-1.75 mg/day (risperidone) or 2-15 mg/day (aripiprazole). Unfortunately, side effects of these medications include somnolence, increased appetite and weight gain (average of 5.1 kg), abnormal blood lipids and glucose, dyskinesia, and elevated prolactin (sometimes galactorrhea). Aripiprazole is equivalent to risperidone for irritability, has less prolactin and fewer metabolic effects, but sometimes has extrapyramidal symptoms. Other second-generation atypical antipsychotics have less evidence but may have fewer side effects. With careful monitoring, these medications can make a major difference in child behavior.
ADHD symptoms often respond to methylphenidate within 4 weeks but at a lower dose and with more side effects of irritability, social withdrawal, and emotional outbursts than for children with ADHD without autism. Formulations such as liquid (short or long acting) or dermal patch may facilitate the important small-dose adjustments and slow ramp-up we should use with checklist monitoring (for example, Vanderbilt Assessment). Atomoxetine also reduces hyperactivity, especially when used with parent training, but has associated nausea, anorexia, early awakening, and rare unpredictable liver failure. Mixed amphetamine salts have not been studied. Clonidine (oral or patch) and guanfacine extended release have also shown some effectiveness for hyperarousal, social interaction, and sleep although they can cause drowsiness/hypotension.
Sleep issues such as sleep onset, duration, and disruptions can improve with melatonin, especially combined with CBT, and it can even sometimes help with anxiety, rigidity, and communication. Use a certified brand and prevent accidental ingestion of gummy forms. Note that obstructive sleep apnea is significantly more common in children with autism spectrum disorder (ASD) and should be evaluated if there are signs. The Childhood Sleep Questionnaire can be used to monitor.
There aren’t data available for selective serotonin reuptake inhibitors in treating depression and anxiety in autistic children but CBT may help. Buspirone improved RRB (at 2.5 mg b.i.d.) but did not help mood. Mood-stabilizing antiepileptics have had mixed results (valproate reduced irritability but with serious side effects), no benefits (lamotrigine and levetiracetam), or no trials (lithium, oxcarbazepine, and topiramate). In spite of this, a Cochrane report recommends antidepressants “on a case by case basis” for children with ASD, keeping in mind the higher risks of behavioral activation (consider comorbid bipolar disorder), irritability, akathisia, and sleep disturbance. We can monitor with Short Moods and Feelings Questionnaire and Problem Behavior Checklist.
NMDA and GABA receptors are implicated in the genesis of ASD. Bumetanide, a GABA modulator, at 1 mg b.i.d., improved social communication and restricted interests, but had dose-related hypokalemia, increased urination, dehydration, loss of appetite, and asthenia. Donepezil (cholinesterase inhibitor) in small studies improved autism scores and expressive/receptive language. N-acetylcysteine, D-cycloserine, and arbaclofen did not show efficacy.
Currently, 64% of children with ASD are prescribed one psychotropic medication, 35% more than two classes, and 15% more than three. While we may look askance at polypharmacy, several medications not effective as monotherapy for children with ASD have significant effects in combination with risperidone; notably memantine, riluzole, N-acetylcysteine, amantadine, topiramate, and buspirone, compared with placebo. Memantine alone has shown benefits in 60% of autistic patients on social, language, and self-stimulatory behaviors at 2.5-30 mg; effective enough that 80% chose continuation.
Families often use complementary or alternative medicines (CAM) so we need to ask about them because CAM may interact with prescribed drugs or complicate determining the source of side effects or benefits. Oxytocin has promising but inconclusive data for improving social cognition but only for 3-8 year olds. Omega-3 fatty acid had benefits for young child stereotypy and lethargy but only by parent report. Vitamin B12, folinic acid, vitamin D3, and digestive enzymes may help but lack data. It is important that families not replace evidence-based treatments with CAM when there are significant symptoms needing treatment.
Being a person with autism, beyond the stress of rigid routines and social difficulties, may include being a target of physical or sexual abuse or bullying, which are risks for suicide. Suicide is eightfold greater in people with autism, especially those who are high functioning; thus, we need to include children with ASD in our routine suicide screening.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
References
Goel R et al. Int Rev Psychiatry. 2018;30(1):78-95.
Stepanova E et al. Dialogues Clin Neurosci. 2017;19(4):395-402.
Caring for a child with autism is a long haul for families and not often a smooth ride. Medications can help improve child functioning and family quality of life but the evidence may require our careful consideration.
Although I am discussing medication treatment here, the best evidence-based treatments for autism symptoms such as poor social communication and repetitive restricted behavior (RRB) are behavioral (for example, applied behavior analysis), cognitive-behavioral therapy (CBT), and parent training. These modalities also augment the effectiveness of medications in many cases. Educational adjustments and specific therapies, when indicated, such as speech-language, occupational, and physical therapy are also beneficial.
Autism symptoms change with age from early regression, later to RRB, then depression, and they are complicated by coexisting conditions. Not surprisingly then, studying the effectiveness and side effects of medications is complex and guidance is in flux as reliable data emerge.
Because of the shortage of specialists and increasing prevalence of autism, we need to be prepared to manage, monitor, and sometimes start medications for our autistic patients. With great individual differences and the hopes and fears of distressed parents making them desperate for help, we need to be as evidence-based as possible to avoid serious side effects or delay effective behavioral treatments.
Our assistance is mainly to address the many co-occurring symptoms in autism: 37%-85% ADHD, 50% anxiety, 7.3% bipolar disorder, and 54.1% depression (by age 30). Many autistic children have problematic irritability, explosive episodes, repetitive or rigid routines, difficulty with social engagement, or trouble sleeping.
We need to be clear with families about the evidence and, whenever possible, use our own time-limited trials with placebos and objective measures that target symptoms and goals for improvement. This is complicated by that fact that the child may have trouble communicating about how they feel, have hypo- or hypersensitivity to feelings, as well as confounding coexisting conditions.
The Food and Drug Administration has approved the atypical antipsychotics risperidone (ages 5-16) and aripiprazole (ages 6-17) for reducing symptoms of irritability by 25%-50% – such as agitation, stereotypy, anger outbursts, self-injurious behavior, and hyperactivity within 8 weeks. The Aberrant Behavior Checklist can be used for monitoring. These benefits are largest with behavior therapy and at doses of 1.25-1.75 mg/day (risperidone) or 2-15 mg/day (aripiprazole). Unfortunately, side effects of these medications include somnolence, increased appetite and weight gain (average of 5.1 kg), abnormal blood lipids and glucose, dyskinesia, and elevated prolactin (sometimes galactorrhea). Aripiprazole is equivalent to risperidone for irritability, has less prolactin and fewer metabolic effects, but sometimes has extrapyramidal symptoms. Other second-generation atypical antipsychotics have less evidence but may have fewer side effects. With careful monitoring, these medications can make a major difference in child behavior.
ADHD symptoms often respond to methylphenidate within 4 weeks but at a lower dose and with more side effects of irritability, social withdrawal, and emotional outbursts than for children with ADHD without autism. Formulations such as liquid (short or long acting) or dermal patch may facilitate the important small-dose adjustments and slow ramp-up we should use with checklist monitoring (for example, Vanderbilt Assessment). Atomoxetine also reduces hyperactivity, especially when used with parent training, but has associated nausea, anorexia, early awakening, and rare unpredictable liver failure. Mixed amphetamine salts have not been studied. Clonidine (oral or patch) and guanfacine extended release have also shown some effectiveness for hyperarousal, social interaction, and sleep although they can cause drowsiness/hypotension.
Sleep issues such as sleep onset, duration, and disruptions can improve with melatonin, especially combined with CBT, and it can even sometimes help with anxiety, rigidity, and communication. Use a certified brand and prevent accidental ingestion of gummy forms. Note that obstructive sleep apnea is significantly more common in children with autism spectrum disorder (ASD) and should be evaluated if there are signs. The Childhood Sleep Questionnaire can be used to monitor.
There aren’t data available for selective serotonin reuptake inhibitors in treating depression and anxiety in autistic children but CBT may help. Buspirone improved RRB (at 2.5 mg b.i.d.) but did not help mood. Mood-stabilizing antiepileptics have had mixed results (valproate reduced irritability but with serious side effects), no benefits (lamotrigine and levetiracetam), or no trials (lithium, oxcarbazepine, and topiramate). In spite of this, a Cochrane report recommends antidepressants “on a case by case basis” for children with ASD, keeping in mind the higher risks of behavioral activation (consider comorbid bipolar disorder), irritability, akathisia, and sleep disturbance. We can monitor with Short Moods and Feelings Questionnaire and Problem Behavior Checklist.
NMDA and GABA receptors are implicated in the genesis of ASD. Bumetanide, a GABA modulator, at 1 mg b.i.d., improved social communication and restricted interests, but had dose-related hypokalemia, increased urination, dehydration, loss of appetite, and asthenia. Donepezil (cholinesterase inhibitor) in small studies improved autism scores and expressive/receptive language. N-acetylcysteine, D-cycloserine, and arbaclofen did not show efficacy.
Currently, 64% of children with ASD are prescribed one psychotropic medication, 35% more than two classes, and 15% more than three. While we may look askance at polypharmacy, several medications not effective as monotherapy for children with ASD have significant effects in combination with risperidone; notably memantine, riluzole, N-acetylcysteine, amantadine, topiramate, and buspirone, compared with placebo. Memantine alone has shown benefits in 60% of autistic patients on social, language, and self-stimulatory behaviors at 2.5-30 mg; effective enough that 80% chose continuation.
Families often use complementary or alternative medicines (CAM) so we need to ask about them because CAM may interact with prescribed drugs or complicate determining the source of side effects or benefits. Oxytocin has promising but inconclusive data for improving social cognition but only for 3-8 year olds. Omega-3 fatty acid had benefits for young child stereotypy and lethargy but only by parent report. Vitamin B12, folinic acid, vitamin D3, and digestive enzymes may help but lack data. It is important that families not replace evidence-based treatments with CAM when there are significant symptoms needing treatment.
Being a person with autism, beyond the stress of rigid routines and social difficulties, may include being a target of physical or sexual abuse or bullying, which are risks for suicide. Suicide is eightfold greater in people with autism, especially those who are high functioning; thus, we need to include children with ASD in our routine suicide screening.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
References
Goel R et al. Int Rev Psychiatry. 2018;30(1):78-95.
Stepanova E et al. Dialogues Clin Neurosci. 2017;19(4):395-402.
Caring for a child with autism is a long haul for families and not often a smooth ride. Medications can help improve child functioning and family quality of life but the evidence may require our careful consideration.
Although I am discussing medication treatment here, the best evidence-based treatments for autism symptoms such as poor social communication and repetitive restricted behavior (RRB) are behavioral (for example, applied behavior analysis), cognitive-behavioral therapy (CBT), and parent training. These modalities also augment the effectiveness of medications in many cases. Educational adjustments and specific therapies, when indicated, such as speech-language, occupational, and physical therapy are also beneficial.
Autism symptoms change with age from early regression, later to RRB, then depression, and they are complicated by coexisting conditions. Not surprisingly then, studying the effectiveness and side effects of medications is complex and guidance is in flux as reliable data emerge.
Because of the shortage of specialists and increasing prevalence of autism, we need to be prepared to manage, monitor, and sometimes start medications for our autistic patients. With great individual differences and the hopes and fears of distressed parents making them desperate for help, we need to be as evidence-based as possible to avoid serious side effects or delay effective behavioral treatments.
Our assistance is mainly to address the many co-occurring symptoms in autism: 37%-85% ADHD, 50% anxiety, 7.3% bipolar disorder, and 54.1% depression (by age 30). Many autistic children have problematic irritability, explosive episodes, repetitive or rigid routines, difficulty with social engagement, or trouble sleeping.
We need to be clear with families about the evidence and, whenever possible, use our own time-limited trials with placebos and objective measures that target symptoms and goals for improvement. This is complicated by that fact that the child may have trouble communicating about how they feel, have hypo- or hypersensitivity to feelings, as well as confounding coexisting conditions.
The Food and Drug Administration has approved the atypical antipsychotics risperidone (ages 5-16) and aripiprazole (ages 6-17) for reducing symptoms of irritability by 25%-50% – such as agitation, stereotypy, anger outbursts, self-injurious behavior, and hyperactivity within 8 weeks. The Aberrant Behavior Checklist can be used for monitoring. These benefits are largest with behavior therapy and at doses of 1.25-1.75 mg/day (risperidone) or 2-15 mg/day (aripiprazole). Unfortunately, side effects of these medications include somnolence, increased appetite and weight gain (average of 5.1 kg), abnormal blood lipids and glucose, dyskinesia, and elevated prolactin (sometimes galactorrhea). Aripiprazole is equivalent to risperidone for irritability, has less prolactin and fewer metabolic effects, but sometimes has extrapyramidal symptoms. Other second-generation atypical antipsychotics have less evidence but may have fewer side effects. With careful monitoring, these medications can make a major difference in child behavior.
ADHD symptoms often respond to methylphenidate within 4 weeks but at a lower dose and with more side effects of irritability, social withdrawal, and emotional outbursts than for children with ADHD without autism. Formulations such as liquid (short or long acting) or dermal patch may facilitate the important small-dose adjustments and slow ramp-up we should use with checklist monitoring (for example, Vanderbilt Assessment). Atomoxetine also reduces hyperactivity, especially when used with parent training, but has associated nausea, anorexia, early awakening, and rare unpredictable liver failure. Mixed amphetamine salts have not been studied. Clonidine (oral or patch) and guanfacine extended release have also shown some effectiveness for hyperarousal, social interaction, and sleep although they can cause drowsiness/hypotension.
Sleep issues such as sleep onset, duration, and disruptions can improve with melatonin, especially combined with CBT, and it can even sometimes help with anxiety, rigidity, and communication. Use a certified brand and prevent accidental ingestion of gummy forms. Note that obstructive sleep apnea is significantly more common in children with autism spectrum disorder (ASD) and should be evaluated if there are signs. The Childhood Sleep Questionnaire can be used to monitor.
There aren’t data available for selective serotonin reuptake inhibitors in treating depression and anxiety in autistic children but CBT may help. Buspirone improved RRB (at 2.5 mg b.i.d.) but did not help mood. Mood-stabilizing antiepileptics have had mixed results (valproate reduced irritability but with serious side effects), no benefits (lamotrigine and levetiracetam), or no trials (lithium, oxcarbazepine, and topiramate). In spite of this, a Cochrane report recommends antidepressants “on a case by case basis” for children with ASD, keeping in mind the higher risks of behavioral activation (consider comorbid bipolar disorder), irritability, akathisia, and sleep disturbance. We can monitor with Short Moods and Feelings Questionnaire and Problem Behavior Checklist.
NMDA and GABA receptors are implicated in the genesis of ASD. Bumetanide, a GABA modulator, at 1 mg b.i.d., improved social communication and restricted interests, but had dose-related hypokalemia, increased urination, dehydration, loss of appetite, and asthenia. Donepezil (cholinesterase inhibitor) in small studies improved autism scores and expressive/receptive language. N-acetylcysteine, D-cycloserine, and arbaclofen did not show efficacy.
Currently, 64% of children with ASD are prescribed one psychotropic medication, 35% more than two classes, and 15% more than three. While we may look askance at polypharmacy, several medications not effective as monotherapy for children with ASD have significant effects in combination with risperidone; notably memantine, riluzole, N-acetylcysteine, amantadine, topiramate, and buspirone, compared with placebo. Memantine alone has shown benefits in 60% of autistic patients on social, language, and self-stimulatory behaviors at 2.5-30 mg; effective enough that 80% chose continuation.
Families often use complementary or alternative medicines (CAM) so we need to ask about them because CAM may interact with prescribed drugs or complicate determining the source of side effects or benefits. Oxytocin has promising but inconclusive data for improving social cognition but only for 3-8 year olds. Omega-3 fatty acid had benefits for young child stereotypy and lethargy but only by parent report. Vitamin B12, folinic acid, vitamin D3, and digestive enzymes may help but lack data. It is important that families not replace evidence-based treatments with CAM when there are significant symptoms needing treatment.
Being a person with autism, beyond the stress of rigid routines and social difficulties, may include being a target of physical or sexual abuse or bullying, which are risks for suicide. Suicide is eightfold greater in people with autism, especially those who are high functioning; thus, we need to include children with ASD in our routine suicide screening.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at pdnews@mdedge.com.
References
Goel R et al. Int Rev Psychiatry. 2018;30(1):78-95.
Stepanova E et al. Dialogues Clin Neurosci. 2017;19(4):395-402.
Using devices to calm children can backfire long term
according to developmental behavioral pediatricians at University of Michigan Health C. S. Mott Children’s Hospital, Ann Arbor.
What to know
- Using a mobile device to distract children from how they are feeling may displace opportunities for them to develop independent, alternative methods to self-regulate, especially in early childhood.
- Signs of increased dysregulation could include rapid shifts between sadness and excitement, a sudden change in mood or feelings, and heightened impulsivity.
- The association between device-calming and emotional consequences may be particularly high among young boys and children who are already experiencing hyperactivity, impulsiveness, and a strong temperament that makes them more likely to react intensely to feelings such as anger, frustration, and sadness.
- While occasional use of media to occupy children is expected and understandable, it is important that it not become a primary or regular soothing tool, and children should be given clear expectations of when and where devices can be used.
- The preschool-to-kindergarten period is a developmental stage in which children may be more likely to exhibit difficult behaviors, such as tantrums, defiance, and intense emotions, but parents should resist using devices as a parenting strategy.
This is a summary of the article, “Longitudinal Association Between Use of Mobile Devices for Calming and Emotional Reactivity and Executive Functioning in Children Aged 3 to 5 Years,” published in JAMA Pediatrics on Dec. 20, 2022. The full article can be found on jamanetwork.com. A version of this article originally appeared on Medscape.com.
according to developmental behavioral pediatricians at University of Michigan Health C. S. Mott Children’s Hospital, Ann Arbor.
What to know
- Using a mobile device to distract children from how they are feeling may displace opportunities for them to develop independent, alternative methods to self-regulate, especially in early childhood.
- Signs of increased dysregulation could include rapid shifts between sadness and excitement, a sudden change in mood or feelings, and heightened impulsivity.
- The association between device-calming and emotional consequences may be particularly high among young boys and children who are already experiencing hyperactivity, impulsiveness, and a strong temperament that makes them more likely to react intensely to feelings such as anger, frustration, and sadness.
- While occasional use of media to occupy children is expected and understandable, it is important that it not become a primary or regular soothing tool, and children should be given clear expectations of when and where devices can be used.
- The preschool-to-kindergarten period is a developmental stage in which children may be more likely to exhibit difficult behaviors, such as tantrums, defiance, and intense emotions, but parents should resist using devices as a parenting strategy.
This is a summary of the article, “Longitudinal Association Between Use of Mobile Devices for Calming and Emotional Reactivity and Executive Functioning in Children Aged 3 to 5 Years,” published in JAMA Pediatrics on Dec. 20, 2022. The full article can be found on jamanetwork.com. A version of this article originally appeared on Medscape.com.
according to developmental behavioral pediatricians at University of Michigan Health C. S. Mott Children’s Hospital, Ann Arbor.
What to know
- Using a mobile device to distract children from how they are feeling may displace opportunities for them to develop independent, alternative methods to self-regulate, especially in early childhood.
- Signs of increased dysregulation could include rapid shifts between sadness and excitement, a sudden change in mood or feelings, and heightened impulsivity.
- The association between device-calming and emotional consequences may be particularly high among young boys and children who are already experiencing hyperactivity, impulsiveness, and a strong temperament that makes them more likely to react intensely to feelings such as anger, frustration, and sadness.
- While occasional use of media to occupy children is expected and understandable, it is important that it not become a primary or regular soothing tool, and children should be given clear expectations of when and where devices can be used.
- The preschool-to-kindergarten period is a developmental stage in which children may be more likely to exhibit difficult behaviors, such as tantrums, defiance, and intense emotions, but parents should resist using devices as a parenting strategy.
This is a summary of the article, “Longitudinal Association Between Use of Mobile Devices for Calming and Emotional Reactivity and Executive Functioning in Children Aged 3 to 5 Years,” published in JAMA Pediatrics on Dec. 20, 2022. The full article can be found on jamanetwork.com. A version of this article originally appeared on Medscape.com.
Physicians don’t feel safe with some patients: Here’s how to reduce the danger
“I talked to him about whether he was okay seeing me and he said yes,” Dr. Cheng said. “But I remained vigilant and conscious of what the patient was doing the whole time so he couldn’t take advantage of the situation.”
Dr. Cheng never turned his back to the patient and even backed out of the exam room. That encounter passed without incident. However, a urologist Dr. Cheng knew from residency wasn’t so fortunate. Ronald Gilbert, MD, of Newport Beach, Calif., was shot and killed by a patient in his office. The patient blamed him for complications following prostate surgery 25 years earlier.
In 2022, a gunman in Tulsa, Okla., blamed his physician for pain from a recent back surgery and shot and killed him, another physician, and two others in a medical building before taking his own life.
Nearly 9 in 10 physicians reported in a recent Medscape poll that they had experienced one or more violent or potentially violent incidents in the past year. The most common patient behaviors were verbal abuse, getting angry and leaving, and behaving erratically.
About one in three respondents said that the patients threatened to harm them, and about one in five said that the patients became violent.
Experts say that many factors contribute to this potentially lethal situation: Health care services have become more impersonal, patients experience longer wait times, some abuse prescription drugs, mental health services are lacking, and security is poor or nonexistent at some health care facilities.
Violence against hospital workers has become so common that a bill was introduced in 2022 in Congress to better protect them. The Safety From Violence for Healthcare Employees Act includes stiffer penalties for acts involving the use of a dangerous weapon or committed during a public emergency and would also provide $25 million in grants to hospitals for programs aimed at reducing violent incidents in health care settings, including de-escalation training. The American Hospital Association and American College of Emergency Physicians support the bill, which is now before the House Judiciary Subcommittee on Crime, Terrorism, and Homeland Security.
The worst day of their lives
“You have people who already are having the worst day of their lives and feeling on edge. If they already have a short fuse or substance abuse issues, that can translate into agitation, violence, or aggression,” said Scott Zeller, MD, vice president of acute psychiatry at Vituity, a physician-owned multispecialty group that operates in several states.
Health care workers in psychiatric and substance abuse hospitals were 10 times more likely to experience nonfatal injuries by others in 2018 than were health care workers in ambulatory settings, according to an April 2020 Bureau of Labor Statistics report. In addition, health care workers were five times more likely to suffer a workplace violence injury than were workers overall in 2018.
Psychiatrists who responded to the poll were the specialists most likely to report that they encountered violent patients and potentially violent patients. “Historically, inpatient psychiatry, which requires more acute care and monitoring, is considered the most dangerous profession outside of the police,” said Dr. Zeller.
Emergency physicians have reported an uptick in violence from patients; 85% said in a survey by ACEP in 2022 that they believed the rate of violence in emergency departments has increased over the past 5 years, whereas 45% indicated that it has greatly increased.
Some doctors have been threatened with violence or actually killed by family members. Alex Skog, MD, president-elect of ACEP’s Oregon chapter, told HealthCare Dive that “a patient’s family member with a gun holster on his hip threatened to kill me and kill my entire family after I told his father that he needed to be admitted because he had coronavirus.”
“I’ve been scared for my safety as well as the safety of my family,” Dr. Skog said. “That was just not something that we were seeing 3, 4, or 5 years ago.”
Many patients are already upset by the time they see doctors, according to the poll.
“The most common reason patients are upset is that they’re already in a lot of pain, which can be expressed as anger, hostility, or aggression. They’re very anxious and afraid of what’s happening and may be thinking about the worst-case scenario – that a bump or lump is cancer,” Dr. Zeller said.
Patients may also get upset if they disagree with their doctors’ diagnosis or treatment plan or the doctor refuses to prescribe them the drugs or tests they want.
“One doctor commented recently: ‘After over 30 years in this business, I can say patients are worse now than at any point in my career. Entitled, demanding, obnoxious. Any denial is met with outrage and indignity, whether it’s an opioid request or a demand for MRI of something because they ‘want to know.’ ”
An orthopedic surgeon in Indiana lost his life after he refused to prescribe opioids to a patient. Her angry husband shot and killed the doctor in the parking lot only 2 hours after confronting him in his office.
Decreased physician-patient trust
“When doctors experience something frightening, they become more apprehensive in the future. There’s no doubt that after the first violent experience, they think of things differently,” said Dr. Zeller.
More than half of the doctors who reported experiencing at least one violent or potentially violent incident in the poll said they trusted patients less.
This diminished trust can negatively impact the physician-patient relationship, said the authors of a recent Health Affairs article.
“The more patients harm their health care providers, intentionally or unintentionally, the more difficult it will be for those providers to trust them, leading to yet another unfortunate pattern: physicians pulling back on some of the behaviors thought to be most trust-building, for example, talking about their personal lives, building rapport, displaying compassion, or giving out their personal cell phone numbers,” the article stated.
What doctors can do
Most doctors who experienced a violent or potentially violent incident said they had tried to defuse the situation and that they succeeded at least some of the time, the poll results show.
One of the best ways to defuse a situation is to be empathetic and show the person that you’re on their side and not the enemy, said Dr. Cheng,.
“Rather than making general statements like ‘I understand that you’re upset,’ it’s better to be specific about the reason the person is upset. For example: ‘I understand that you’re upset that the pharmacy didn’t fill your prescription’ or ‘I understand how you’re feeling about Doctor So-and-so, who didn’t treat you right,’ ” Dr. Cheng stated.
Dr. Zeller urged physicians to talk to patients about why they’re upset and how they can help them. That approach worked with a patient who was having a psychotic episode.
“I told the staff, who wanted to forcibly restrain him and inject him with medication, that I would talk to him. I asked the patient, who was screaming ‘ya ya ya ya,’ whether he would take his medication if I gave it to him and he said yes. When he was calm, he explained that he was screaming to stop the voices telling him to kill his parents. He then got the help he needed,” said Dr. Zeller.
Dr. Cheng was trained in de-escalation techniques as an Orange County reserve deputy sheriff. He and Dr. Zeller recommended that physicians and staff receive training in how to spot potentially violent behavior and defuse these situations before they escalate.
Dr. Cheng suggests looking at the person’s body language for signs of increasing agitation or tension, such as clenched fists, tense posture, tight jaw, or fidgeting that may be accompanied by shouting and/or verbal abuse.
Physicians also need to consider where they are physically in relation to patients they see. “You don’t want to be too close to the patient or stand in front of them, which can be seen as confrontational. Instead, stand or sit off to the side, and never block the door if the patient’s upset,” said Dr. Cheng.
He recommended that physician practices prepare for violent incidents by developing detailed plans, including how and when to escape, how to protect patients, and how to cooperate with law enforcement.
“If a violent incident is inescapable, physicians and staff must be ready to fight back with whatever tools they have available, which may include fire extinguishers, chairs, or scalpels,” said Dr. Cheng.
A version of this article originally appeared on Medscape.com.
“I talked to him about whether he was okay seeing me and he said yes,” Dr. Cheng said. “But I remained vigilant and conscious of what the patient was doing the whole time so he couldn’t take advantage of the situation.”
Dr. Cheng never turned his back to the patient and even backed out of the exam room. That encounter passed without incident. However, a urologist Dr. Cheng knew from residency wasn’t so fortunate. Ronald Gilbert, MD, of Newport Beach, Calif., was shot and killed by a patient in his office. The patient blamed him for complications following prostate surgery 25 years earlier.
In 2022, a gunman in Tulsa, Okla., blamed his physician for pain from a recent back surgery and shot and killed him, another physician, and two others in a medical building before taking his own life.
Nearly 9 in 10 physicians reported in a recent Medscape poll that they had experienced one or more violent or potentially violent incidents in the past year. The most common patient behaviors were verbal abuse, getting angry and leaving, and behaving erratically.
About one in three respondents said that the patients threatened to harm them, and about one in five said that the patients became violent.
Experts say that many factors contribute to this potentially lethal situation: Health care services have become more impersonal, patients experience longer wait times, some abuse prescription drugs, mental health services are lacking, and security is poor or nonexistent at some health care facilities.
Violence against hospital workers has become so common that a bill was introduced in 2022 in Congress to better protect them. The Safety From Violence for Healthcare Employees Act includes stiffer penalties for acts involving the use of a dangerous weapon or committed during a public emergency and would also provide $25 million in grants to hospitals for programs aimed at reducing violent incidents in health care settings, including de-escalation training. The American Hospital Association and American College of Emergency Physicians support the bill, which is now before the House Judiciary Subcommittee on Crime, Terrorism, and Homeland Security.
The worst day of their lives
“You have people who already are having the worst day of their lives and feeling on edge. If they already have a short fuse or substance abuse issues, that can translate into agitation, violence, or aggression,” said Scott Zeller, MD, vice president of acute psychiatry at Vituity, a physician-owned multispecialty group that operates in several states.
Health care workers in psychiatric and substance abuse hospitals were 10 times more likely to experience nonfatal injuries by others in 2018 than were health care workers in ambulatory settings, according to an April 2020 Bureau of Labor Statistics report. In addition, health care workers were five times more likely to suffer a workplace violence injury than were workers overall in 2018.
Psychiatrists who responded to the poll were the specialists most likely to report that they encountered violent patients and potentially violent patients. “Historically, inpatient psychiatry, which requires more acute care and monitoring, is considered the most dangerous profession outside of the police,” said Dr. Zeller.
Emergency physicians have reported an uptick in violence from patients; 85% said in a survey by ACEP in 2022 that they believed the rate of violence in emergency departments has increased over the past 5 years, whereas 45% indicated that it has greatly increased.
Some doctors have been threatened with violence or actually killed by family members. Alex Skog, MD, president-elect of ACEP’s Oregon chapter, told HealthCare Dive that “a patient’s family member with a gun holster on his hip threatened to kill me and kill my entire family after I told his father that he needed to be admitted because he had coronavirus.”
“I’ve been scared for my safety as well as the safety of my family,” Dr. Skog said. “That was just not something that we were seeing 3, 4, or 5 years ago.”
Many patients are already upset by the time they see doctors, according to the poll.
“The most common reason patients are upset is that they’re already in a lot of pain, which can be expressed as anger, hostility, or aggression. They’re very anxious and afraid of what’s happening and may be thinking about the worst-case scenario – that a bump or lump is cancer,” Dr. Zeller said.
Patients may also get upset if they disagree with their doctors’ diagnosis or treatment plan or the doctor refuses to prescribe them the drugs or tests they want.
“One doctor commented recently: ‘After over 30 years in this business, I can say patients are worse now than at any point in my career. Entitled, demanding, obnoxious. Any denial is met with outrage and indignity, whether it’s an opioid request or a demand for MRI of something because they ‘want to know.’ ”
An orthopedic surgeon in Indiana lost his life after he refused to prescribe opioids to a patient. Her angry husband shot and killed the doctor in the parking lot only 2 hours after confronting him in his office.
Decreased physician-patient trust
“When doctors experience something frightening, they become more apprehensive in the future. There’s no doubt that after the first violent experience, they think of things differently,” said Dr. Zeller.
More than half of the doctors who reported experiencing at least one violent or potentially violent incident in the poll said they trusted patients less.
This diminished trust can negatively impact the physician-patient relationship, said the authors of a recent Health Affairs article.
“The more patients harm their health care providers, intentionally or unintentionally, the more difficult it will be for those providers to trust them, leading to yet another unfortunate pattern: physicians pulling back on some of the behaviors thought to be most trust-building, for example, talking about their personal lives, building rapport, displaying compassion, or giving out their personal cell phone numbers,” the article stated.
What doctors can do
Most doctors who experienced a violent or potentially violent incident said they had tried to defuse the situation and that they succeeded at least some of the time, the poll results show.
One of the best ways to defuse a situation is to be empathetic and show the person that you’re on their side and not the enemy, said Dr. Cheng,.
“Rather than making general statements like ‘I understand that you’re upset,’ it’s better to be specific about the reason the person is upset. For example: ‘I understand that you’re upset that the pharmacy didn’t fill your prescription’ or ‘I understand how you’re feeling about Doctor So-and-so, who didn’t treat you right,’ ” Dr. Cheng stated.
Dr. Zeller urged physicians to talk to patients about why they’re upset and how they can help them. That approach worked with a patient who was having a psychotic episode.
“I told the staff, who wanted to forcibly restrain him and inject him with medication, that I would talk to him. I asked the patient, who was screaming ‘ya ya ya ya,’ whether he would take his medication if I gave it to him and he said yes. When he was calm, he explained that he was screaming to stop the voices telling him to kill his parents. He then got the help he needed,” said Dr. Zeller.
Dr. Cheng was trained in de-escalation techniques as an Orange County reserve deputy sheriff. He and Dr. Zeller recommended that physicians and staff receive training in how to spot potentially violent behavior and defuse these situations before they escalate.
Dr. Cheng suggests looking at the person’s body language for signs of increasing agitation or tension, such as clenched fists, tense posture, tight jaw, or fidgeting that may be accompanied by shouting and/or verbal abuse.
Physicians also need to consider where they are physically in relation to patients they see. “You don’t want to be too close to the patient or stand in front of them, which can be seen as confrontational. Instead, stand or sit off to the side, and never block the door if the patient’s upset,” said Dr. Cheng.
He recommended that physician practices prepare for violent incidents by developing detailed plans, including how and when to escape, how to protect patients, and how to cooperate with law enforcement.
“If a violent incident is inescapable, physicians and staff must be ready to fight back with whatever tools they have available, which may include fire extinguishers, chairs, or scalpels,” said Dr. Cheng.
A version of this article originally appeared on Medscape.com.
“I talked to him about whether he was okay seeing me and he said yes,” Dr. Cheng said. “But I remained vigilant and conscious of what the patient was doing the whole time so he couldn’t take advantage of the situation.”
Dr. Cheng never turned his back to the patient and even backed out of the exam room. That encounter passed without incident. However, a urologist Dr. Cheng knew from residency wasn’t so fortunate. Ronald Gilbert, MD, of Newport Beach, Calif., was shot and killed by a patient in his office. The patient blamed him for complications following prostate surgery 25 years earlier.
In 2022, a gunman in Tulsa, Okla., blamed his physician for pain from a recent back surgery and shot and killed him, another physician, and two others in a medical building before taking his own life.
Nearly 9 in 10 physicians reported in a recent Medscape poll that they had experienced one or more violent or potentially violent incidents in the past year. The most common patient behaviors were verbal abuse, getting angry and leaving, and behaving erratically.
About one in three respondents said that the patients threatened to harm them, and about one in five said that the patients became violent.
Experts say that many factors contribute to this potentially lethal situation: Health care services have become more impersonal, patients experience longer wait times, some abuse prescription drugs, mental health services are lacking, and security is poor or nonexistent at some health care facilities.
Violence against hospital workers has become so common that a bill was introduced in 2022 in Congress to better protect them. The Safety From Violence for Healthcare Employees Act includes stiffer penalties for acts involving the use of a dangerous weapon or committed during a public emergency and would also provide $25 million in grants to hospitals for programs aimed at reducing violent incidents in health care settings, including de-escalation training. The American Hospital Association and American College of Emergency Physicians support the bill, which is now before the House Judiciary Subcommittee on Crime, Terrorism, and Homeland Security.
The worst day of their lives
“You have people who already are having the worst day of their lives and feeling on edge. If they already have a short fuse or substance abuse issues, that can translate into agitation, violence, or aggression,” said Scott Zeller, MD, vice president of acute psychiatry at Vituity, a physician-owned multispecialty group that operates in several states.
Health care workers in psychiatric and substance abuse hospitals were 10 times more likely to experience nonfatal injuries by others in 2018 than were health care workers in ambulatory settings, according to an April 2020 Bureau of Labor Statistics report. In addition, health care workers were five times more likely to suffer a workplace violence injury than were workers overall in 2018.
Psychiatrists who responded to the poll were the specialists most likely to report that they encountered violent patients and potentially violent patients. “Historically, inpatient psychiatry, which requires more acute care and monitoring, is considered the most dangerous profession outside of the police,” said Dr. Zeller.
Emergency physicians have reported an uptick in violence from patients; 85% said in a survey by ACEP in 2022 that they believed the rate of violence in emergency departments has increased over the past 5 years, whereas 45% indicated that it has greatly increased.
Some doctors have been threatened with violence or actually killed by family members. Alex Skog, MD, president-elect of ACEP’s Oregon chapter, told HealthCare Dive that “a patient’s family member with a gun holster on his hip threatened to kill me and kill my entire family after I told his father that he needed to be admitted because he had coronavirus.”
“I’ve been scared for my safety as well as the safety of my family,” Dr. Skog said. “That was just not something that we were seeing 3, 4, or 5 years ago.”
Many patients are already upset by the time they see doctors, according to the poll.
“The most common reason patients are upset is that they’re already in a lot of pain, which can be expressed as anger, hostility, or aggression. They’re very anxious and afraid of what’s happening and may be thinking about the worst-case scenario – that a bump or lump is cancer,” Dr. Zeller said.
Patients may also get upset if they disagree with their doctors’ diagnosis or treatment plan or the doctor refuses to prescribe them the drugs or tests they want.
“One doctor commented recently: ‘After over 30 years in this business, I can say patients are worse now than at any point in my career. Entitled, demanding, obnoxious. Any denial is met with outrage and indignity, whether it’s an opioid request or a demand for MRI of something because they ‘want to know.’ ”
An orthopedic surgeon in Indiana lost his life after he refused to prescribe opioids to a patient. Her angry husband shot and killed the doctor in the parking lot only 2 hours after confronting him in his office.
Decreased physician-patient trust
“When doctors experience something frightening, they become more apprehensive in the future. There’s no doubt that after the first violent experience, they think of things differently,” said Dr. Zeller.
More than half of the doctors who reported experiencing at least one violent or potentially violent incident in the poll said they trusted patients less.
This diminished trust can negatively impact the physician-patient relationship, said the authors of a recent Health Affairs article.
“The more patients harm their health care providers, intentionally or unintentionally, the more difficult it will be for those providers to trust them, leading to yet another unfortunate pattern: physicians pulling back on some of the behaviors thought to be most trust-building, for example, talking about their personal lives, building rapport, displaying compassion, or giving out their personal cell phone numbers,” the article stated.
What doctors can do
Most doctors who experienced a violent or potentially violent incident said they had tried to defuse the situation and that they succeeded at least some of the time, the poll results show.
One of the best ways to defuse a situation is to be empathetic and show the person that you’re on their side and not the enemy, said Dr. Cheng,.
“Rather than making general statements like ‘I understand that you’re upset,’ it’s better to be specific about the reason the person is upset. For example: ‘I understand that you’re upset that the pharmacy didn’t fill your prescription’ or ‘I understand how you’re feeling about Doctor So-and-so, who didn’t treat you right,’ ” Dr. Cheng stated.
Dr. Zeller urged physicians to talk to patients about why they’re upset and how they can help them. That approach worked with a patient who was having a psychotic episode.
“I told the staff, who wanted to forcibly restrain him and inject him with medication, that I would talk to him. I asked the patient, who was screaming ‘ya ya ya ya,’ whether he would take his medication if I gave it to him and he said yes. When he was calm, he explained that he was screaming to stop the voices telling him to kill his parents. He then got the help he needed,” said Dr. Zeller.
Dr. Cheng was trained in de-escalation techniques as an Orange County reserve deputy sheriff. He and Dr. Zeller recommended that physicians and staff receive training in how to spot potentially violent behavior and defuse these situations before they escalate.
Dr. Cheng suggests looking at the person’s body language for signs of increasing agitation or tension, such as clenched fists, tense posture, tight jaw, or fidgeting that may be accompanied by shouting and/or verbal abuse.
Physicians also need to consider where they are physically in relation to patients they see. “You don’t want to be too close to the patient or stand in front of them, which can be seen as confrontational. Instead, stand or sit off to the side, and never block the door if the patient’s upset,” said Dr. Cheng.
He recommended that physician practices prepare for violent incidents by developing detailed plans, including how and when to escape, how to protect patients, and how to cooperate with law enforcement.
“If a violent incident is inescapable, physicians and staff must be ready to fight back with whatever tools they have available, which may include fire extinguishers, chairs, or scalpels,” said Dr. Cheng.
A version of this article originally appeared on Medscape.com.
Teen girls report record levels of sadness, sexual violence: CDC
Teenage girls are experiencing record high levels of sexual violence, and nearly three in five girls report feeling persistently sad or hopeless, according to a new report by the Centers for Disease Control and Prevention.
Nearly 70% of teens who identified as lesbian, bisexual, gay, or questioning (LGBQ+) report experiencing feelings of persistent sadness and hopeless, and nearly one in four (22%) LGBQ+ had attempted suicide in 2021, according to the report.
“High school should be a time for trailblazing, not trauma. These data show our kids need far more support to cope, hope, and thrive,” said Debra Houry, MD, MPH, the CDC’s acting principal deputy director, in a press release about the findings.
The new analysis looked at data from 2011 to 2021 from the CDC’s Youth Risk and Behavior Survey (YRBS), a semiannual analysis of the health behaviors of students in grades 9-12. The 2021 survey is the first YRBS conducted since the COVID-19 pandemic began and included 17,232 respondents.
Although the researchers saw signs of improvement in risky sexual behaviors and substance abuse, as well as fewer experiences of bullying, the analysis found youth mental health worsened over the past 10 years. This trend was particularly troubling for teenage girls: 57% said they felt persistently sad or hopeless in 2021, a 60% increase from a decade ago. By comparison, 29% of teenage boys reported feeling persistently sad or hopeless, compared with 21% in 2011.
Nearly one-third of girls (30%) reported seriously considering suicide, up from 19% in 2011. In teenage boys, serious thoughts of suicide increased from 13% to 14% from 2011 to 2021. The percentage of teenage girls who had attempted suicide in 2021 was 13%, nearly twice that of teenage boys (7%).
More than half of students with a same-sex partner (58%) reported seriously considering suicide, and 45% of LGBQ+ teens reported the same thoughts. One third of students with a same-sex partner reported attempting suicide in the past year.
The report did not have trend data on LGBQ+ students because of changes in survey methods. The 2021 survey did not have a question accessing gender identity, but this will be incorporated into future surveys, according to the researchers.
Hispanic and multiracial students were more likely to experience persistent feelings of sadness or hopelessness, compared with their peers, with 46% and 49%, respectively, reporting these feelings. From 2011-2021, the percentage of students reporting feelings of hopelessness increased in each racial and ethnic group. The percentage of Black, Hispanic, and White teens who seriously considered suicide also increased over the decade. (A different report released by the CDC on Feb. 10 found that the rate of suicide among Blacks in the United States aged 10-24 jumped 36.6% between 2018 and 2021, the largest increase for any racial or ethnic group.)
The survey also found an alarming spike in sexual violence toward teenage girls. Nearly one in five females (18%) experienced sexual violence in the past year, a 20% increase from 2017. More than 1 in 10 teen girls (14%) said they had been forced to have sex, according to the researchers.
Rates of sexual violence was even higher in LGBQ+ teens. Nearly two in five teens with a partner of the same sex (39%) experienced sexual violence, and 37% reported being sexually assaulted. More than one in five LGBQ+ teens (22%) had experienced sexual violence, and 20% said they had been forced to have sex, the report found.
Among racial and ethnic groups, American Indian and Alaskan Native and multiracial students were more likely to experience sexual violence. The percentage of White students reporting sexual violence increased from 2017 to 2021, but that trend was not observed in other racial and ethnic groups.
Delaney Ruston, MD, an internal medicine specialist in Seattle and creator of “Screenagers,” a 2016 documentary about how technology affects youth, said excessive exposure to social media can compound feelings of depression in teens – particularly, but not only, girls. “They can scroll and consume media for hours, and rather than do activities and have interactions that would help heal from depression symptoms, they stay stuck,” Ruston said in an interview. “As a primary care physician working with teens, this is an extremely common problem I see in my clinic.”
One approach that can help, Dr. Ruston added, is behavioral activation. “This is a strategy where you get them, usually with the support of other people, to do small activities that help to reset brain reward pathways so they start to experience doses of well-being and hope that eventually reverses the depression. Being stuck on screens prevents these healing actions from happening.”
The report also emphasized the importance of school-based services to support students and combat these troubling trends in worsening mental health. “Schools are the gateway to needed services for many young people,” the report stated. “Schools can provide health, behavioral, and mental health services directly or establish referral systems to connect to community sources of care.”
“Young people are experiencing a level of distress that calls on us to act with urgency and compassion,” Kathleen Ethier, PhD, director of the CDC’s division of adolescent and school health, added in a statement. “With the right programs and services in place, schools have the unique ability to help our youth flourish.”
A version of this article first appeared on Medscape.com.
Teenage girls are experiencing record high levels of sexual violence, and nearly three in five girls report feeling persistently sad or hopeless, according to a new report by the Centers for Disease Control and Prevention.
Nearly 70% of teens who identified as lesbian, bisexual, gay, or questioning (LGBQ+) report experiencing feelings of persistent sadness and hopeless, and nearly one in four (22%) LGBQ+ had attempted suicide in 2021, according to the report.
“High school should be a time for trailblazing, not trauma. These data show our kids need far more support to cope, hope, and thrive,” said Debra Houry, MD, MPH, the CDC’s acting principal deputy director, in a press release about the findings.
The new analysis looked at data from 2011 to 2021 from the CDC’s Youth Risk and Behavior Survey (YRBS), a semiannual analysis of the health behaviors of students in grades 9-12. The 2021 survey is the first YRBS conducted since the COVID-19 pandemic began and included 17,232 respondents.
Although the researchers saw signs of improvement in risky sexual behaviors and substance abuse, as well as fewer experiences of bullying, the analysis found youth mental health worsened over the past 10 years. This trend was particularly troubling for teenage girls: 57% said they felt persistently sad or hopeless in 2021, a 60% increase from a decade ago. By comparison, 29% of teenage boys reported feeling persistently sad or hopeless, compared with 21% in 2011.
Nearly one-third of girls (30%) reported seriously considering suicide, up from 19% in 2011. In teenage boys, serious thoughts of suicide increased from 13% to 14% from 2011 to 2021. The percentage of teenage girls who had attempted suicide in 2021 was 13%, nearly twice that of teenage boys (7%).
More than half of students with a same-sex partner (58%) reported seriously considering suicide, and 45% of LGBQ+ teens reported the same thoughts. One third of students with a same-sex partner reported attempting suicide in the past year.
The report did not have trend data on LGBQ+ students because of changes in survey methods. The 2021 survey did not have a question accessing gender identity, but this will be incorporated into future surveys, according to the researchers.
Hispanic and multiracial students were more likely to experience persistent feelings of sadness or hopelessness, compared with their peers, with 46% and 49%, respectively, reporting these feelings. From 2011-2021, the percentage of students reporting feelings of hopelessness increased in each racial and ethnic group. The percentage of Black, Hispanic, and White teens who seriously considered suicide also increased over the decade. (A different report released by the CDC on Feb. 10 found that the rate of suicide among Blacks in the United States aged 10-24 jumped 36.6% between 2018 and 2021, the largest increase for any racial or ethnic group.)
The survey also found an alarming spike in sexual violence toward teenage girls. Nearly one in five females (18%) experienced sexual violence in the past year, a 20% increase from 2017. More than 1 in 10 teen girls (14%) said they had been forced to have sex, according to the researchers.
Rates of sexual violence was even higher in LGBQ+ teens. Nearly two in five teens with a partner of the same sex (39%) experienced sexual violence, and 37% reported being sexually assaulted. More than one in five LGBQ+ teens (22%) had experienced sexual violence, and 20% said they had been forced to have sex, the report found.
Among racial and ethnic groups, American Indian and Alaskan Native and multiracial students were more likely to experience sexual violence. The percentage of White students reporting sexual violence increased from 2017 to 2021, but that trend was not observed in other racial and ethnic groups.
Delaney Ruston, MD, an internal medicine specialist in Seattle and creator of “Screenagers,” a 2016 documentary about how technology affects youth, said excessive exposure to social media can compound feelings of depression in teens – particularly, but not only, girls. “They can scroll and consume media for hours, and rather than do activities and have interactions that would help heal from depression symptoms, they stay stuck,” Ruston said in an interview. “As a primary care physician working with teens, this is an extremely common problem I see in my clinic.”
One approach that can help, Dr. Ruston added, is behavioral activation. “This is a strategy where you get them, usually with the support of other people, to do small activities that help to reset brain reward pathways so they start to experience doses of well-being and hope that eventually reverses the depression. Being stuck on screens prevents these healing actions from happening.”
The report also emphasized the importance of school-based services to support students and combat these troubling trends in worsening mental health. “Schools are the gateway to needed services for many young people,” the report stated. “Schools can provide health, behavioral, and mental health services directly or establish referral systems to connect to community sources of care.”
“Young people are experiencing a level of distress that calls on us to act with urgency and compassion,” Kathleen Ethier, PhD, director of the CDC’s division of adolescent and school health, added in a statement. “With the right programs and services in place, schools have the unique ability to help our youth flourish.”
A version of this article first appeared on Medscape.com.
Teenage girls are experiencing record high levels of sexual violence, and nearly three in five girls report feeling persistently sad or hopeless, according to a new report by the Centers for Disease Control and Prevention.
Nearly 70% of teens who identified as lesbian, bisexual, gay, or questioning (LGBQ+) report experiencing feelings of persistent sadness and hopeless, and nearly one in four (22%) LGBQ+ had attempted suicide in 2021, according to the report.
“High school should be a time for trailblazing, not trauma. These data show our kids need far more support to cope, hope, and thrive,” said Debra Houry, MD, MPH, the CDC’s acting principal deputy director, in a press release about the findings.
The new analysis looked at data from 2011 to 2021 from the CDC’s Youth Risk and Behavior Survey (YRBS), a semiannual analysis of the health behaviors of students in grades 9-12. The 2021 survey is the first YRBS conducted since the COVID-19 pandemic began and included 17,232 respondents.
Although the researchers saw signs of improvement in risky sexual behaviors and substance abuse, as well as fewer experiences of bullying, the analysis found youth mental health worsened over the past 10 years. This trend was particularly troubling for teenage girls: 57% said they felt persistently sad or hopeless in 2021, a 60% increase from a decade ago. By comparison, 29% of teenage boys reported feeling persistently sad or hopeless, compared with 21% in 2011.
Nearly one-third of girls (30%) reported seriously considering suicide, up from 19% in 2011. In teenage boys, serious thoughts of suicide increased from 13% to 14% from 2011 to 2021. The percentage of teenage girls who had attempted suicide in 2021 was 13%, nearly twice that of teenage boys (7%).
More than half of students with a same-sex partner (58%) reported seriously considering suicide, and 45% of LGBQ+ teens reported the same thoughts. One third of students with a same-sex partner reported attempting suicide in the past year.
The report did not have trend data on LGBQ+ students because of changes in survey methods. The 2021 survey did not have a question accessing gender identity, but this will be incorporated into future surveys, according to the researchers.
Hispanic and multiracial students were more likely to experience persistent feelings of sadness or hopelessness, compared with their peers, with 46% and 49%, respectively, reporting these feelings. From 2011-2021, the percentage of students reporting feelings of hopelessness increased in each racial and ethnic group. The percentage of Black, Hispanic, and White teens who seriously considered suicide also increased over the decade. (A different report released by the CDC on Feb. 10 found that the rate of suicide among Blacks in the United States aged 10-24 jumped 36.6% between 2018 and 2021, the largest increase for any racial or ethnic group.)
The survey also found an alarming spike in sexual violence toward teenage girls. Nearly one in five females (18%) experienced sexual violence in the past year, a 20% increase from 2017. More than 1 in 10 teen girls (14%) said they had been forced to have sex, according to the researchers.
Rates of sexual violence was even higher in LGBQ+ teens. Nearly two in five teens with a partner of the same sex (39%) experienced sexual violence, and 37% reported being sexually assaulted. More than one in five LGBQ+ teens (22%) had experienced sexual violence, and 20% said they had been forced to have sex, the report found.
Among racial and ethnic groups, American Indian and Alaskan Native and multiracial students were more likely to experience sexual violence. The percentage of White students reporting sexual violence increased from 2017 to 2021, but that trend was not observed in other racial and ethnic groups.
Delaney Ruston, MD, an internal medicine specialist in Seattle and creator of “Screenagers,” a 2016 documentary about how technology affects youth, said excessive exposure to social media can compound feelings of depression in teens – particularly, but not only, girls. “They can scroll and consume media for hours, and rather than do activities and have interactions that would help heal from depression symptoms, they stay stuck,” Ruston said in an interview. “As a primary care physician working with teens, this is an extremely common problem I see in my clinic.”
One approach that can help, Dr. Ruston added, is behavioral activation. “This is a strategy where you get them, usually with the support of other people, to do small activities that help to reset brain reward pathways so they start to experience doses of well-being and hope that eventually reverses the depression. Being stuck on screens prevents these healing actions from happening.”
The report also emphasized the importance of school-based services to support students and combat these troubling trends in worsening mental health. “Schools are the gateway to needed services for many young people,” the report stated. “Schools can provide health, behavioral, and mental health services directly or establish referral systems to connect to community sources of care.”
“Young people are experiencing a level of distress that calls on us to act with urgency and compassion,” Kathleen Ethier, PhD, director of the CDC’s division of adolescent and school health, added in a statement. “With the right programs and services in place, schools have the unique ability to help our youth flourish.”
A version of this article first appeared on Medscape.com.
Joint effort: CBD not just innocent bystander in weed
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
I visited a legal cannabis dispensary in Massachusetts a few years ago, mostly to see what the hype was about. There I was, knowing basically nothing about pot, as the gentle stoner behind the counter explained to me the differences between the various strains. Acapulco Gold is buoyant and energizing; Purple Kush is sleepy, relaxed, dissociative. Here’s a strain that makes you feel nostalgic; here’s one that helps you focus. It was as complicated and as oddly specific as a fancy wine tasting – and, I had a feeling, about as reliable.
It’s a plant, after all, and though delta-9-tetrahydrocannabinol (THC) is the chemical responsible for its euphoric effects, it is far from the only substance in there.
The second most important compound in cannabis is cannabidiol, and most people will tell you that CBD is the gentle yin to THC’s paranoiac yang. Hence your local ganja barista reminding you that, if you don›t want all those anxiety-inducing side effects of THC, grab a strain with a nice CBD balance.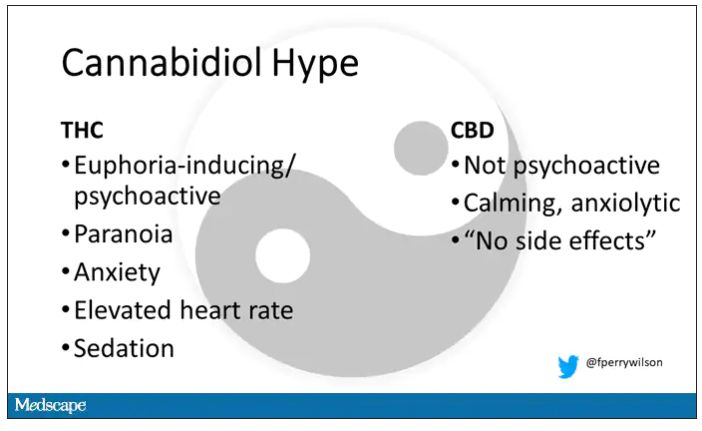
But is it true? A new study appearing in JAMA Network Open suggests, in fact, that it’s quite the opposite. This study is from Austin Zamarripa and colleagues, who clearly sit at the researcher cool kids table.
Eighteen adults who had abstained from marijuana use for at least a month participated in this trial (which is way more fun than anything we do in my lab at Yale). In random order, separated by at least a week, they ate some special brownies.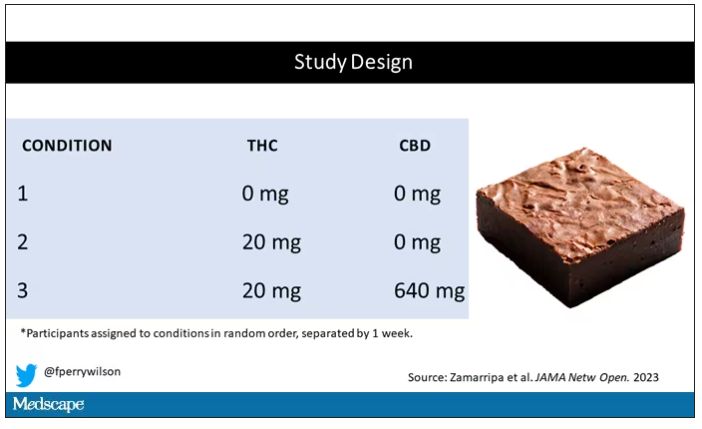
Condition one was a control brownie, condition two was a brownie containing 20 mg of THC, and condition three was a brownie containing 20 mg of THC and 640 mg of CBD. Participants were assigned each condition in random order, separated by at least a week.
A side note on doses for those of you who, like me, are not totally weed literate. A dose of 20 mg of THC is about a third of what you might find in a typical joint these days (though it’s about double the THC content of a joint in the ‘70s – I believe the technical term is “doobie”). And 640 mg of CBD is a decent dose, as 5 mg per kilogram is what some folks start with to achieve therapeutic effects.
Both THC and CBD interact with the cytochrome p450 system in the liver. This matters when you’re ingesting them instead of smoking them because you have first-pass metabolism to contend with. And, because of that p450 inhibition, it’s possible that CBD might actually increase the amount of THC that gets into your bloodstream from the brownie, or gummy, or pizza sauce, or whatever.
Let’s get to the results, starting with blood THC concentration. It’s not subtle. With CBD on board the THC concentration rises higher faster, with roughly double the area under the curve.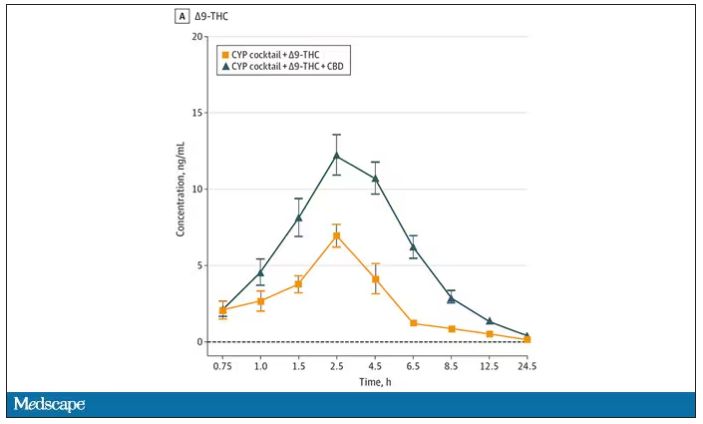
And, unsurprisingly, the subjective experience correlated with those higher levels. Individuals rated the “drug effect” higher with the combo. But, interestingly, the “pleasant” drug effect didn’t change much, while the unpleasant effects were substantially higher. No mitigation of THC anxiety here – quite the opposite. CBD made the anxiety worse.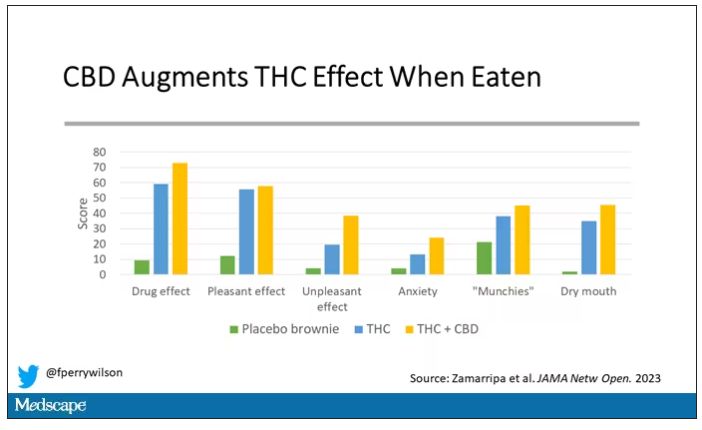
Cognitive effects were equally profound. Scores on a digit symbol substitution test and a paced serial addition task were all substantially worse when CBD was mixed with THC.
And for those of you who want some more objective measures, check out the heart rate. Despite the purported “calming” nature of CBD, heart rates were way higher when individuals were exposed to both chemicals.
The picture here is quite clear, though the mechanism is not. At least when talking edibles, CBD enhances the effects of THC, and not necessarily for the better. It may be that CBD is competing with some of the proteins that metabolize THC, thus prolonging its effects. CBD may also directly inhibit those enzymes. But whatever the case, I think we can safely say the myth that CBD makes the effects of THC more mild or more tolerable is busted.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
I visited a legal cannabis dispensary in Massachusetts a few years ago, mostly to see what the hype was about. There I was, knowing basically nothing about pot, as the gentle stoner behind the counter explained to me the differences between the various strains. Acapulco Gold is buoyant and energizing; Purple Kush is sleepy, relaxed, dissociative. Here’s a strain that makes you feel nostalgic; here’s one that helps you focus. It was as complicated and as oddly specific as a fancy wine tasting – and, I had a feeling, about as reliable.
It’s a plant, after all, and though delta-9-tetrahydrocannabinol (THC) is the chemical responsible for its euphoric effects, it is far from the only substance in there.
The second most important compound in cannabis is cannabidiol, and most people will tell you that CBD is the gentle yin to THC’s paranoiac yang. Hence your local ganja barista reminding you that, if you don›t want all those anxiety-inducing side effects of THC, grab a strain with a nice CBD balance.
But is it true? A new study appearing in JAMA Network Open suggests, in fact, that it’s quite the opposite. This study is from Austin Zamarripa and colleagues, who clearly sit at the researcher cool kids table.
Eighteen adults who had abstained from marijuana use for at least a month participated in this trial (which is way more fun than anything we do in my lab at Yale). In random order, separated by at least a week, they ate some special brownies.
Condition one was a control brownie, condition two was a brownie containing 20 mg of THC, and condition three was a brownie containing 20 mg of THC and 640 mg of CBD. Participants were assigned each condition in random order, separated by at least a week.
A side note on doses for those of you who, like me, are not totally weed literate. A dose of 20 mg of THC is about a third of what you might find in a typical joint these days (though it’s about double the THC content of a joint in the ‘70s – I believe the technical term is “doobie”). And 640 mg of CBD is a decent dose, as 5 mg per kilogram is what some folks start with to achieve therapeutic effects.
Both THC and CBD interact with the cytochrome p450 system in the liver. This matters when you’re ingesting them instead of smoking them because you have first-pass metabolism to contend with. And, because of that p450 inhibition, it’s possible that CBD might actually increase the amount of THC that gets into your bloodstream from the brownie, or gummy, or pizza sauce, or whatever.
Let’s get to the results, starting with blood THC concentration. It’s not subtle. With CBD on board the THC concentration rises higher faster, with roughly double the area under the curve.
And, unsurprisingly, the subjective experience correlated with those higher levels. Individuals rated the “drug effect” higher with the combo. But, interestingly, the “pleasant” drug effect didn’t change much, while the unpleasant effects were substantially higher. No mitigation of THC anxiety here – quite the opposite. CBD made the anxiety worse.
Cognitive effects were equally profound. Scores on a digit symbol substitution test and a paced serial addition task were all substantially worse when CBD was mixed with THC.
And for those of you who want some more objective measures, check out the heart rate. Despite the purported “calming” nature of CBD, heart rates were way higher when individuals were exposed to both chemicals.
The picture here is quite clear, though the mechanism is not. At least when talking edibles, CBD enhances the effects of THC, and not necessarily for the better. It may be that CBD is competing with some of the proteins that metabolize THC, thus prolonging its effects. CBD may also directly inhibit those enzymes. But whatever the case, I think we can safely say the myth that CBD makes the effects of THC more mild or more tolerable is busted.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
I visited a legal cannabis dispensary in Massachusetts a few years ago, mostly to see what the hype was about. There I was, knowing basically nothing about pot, as the gentle stoner behind the counter explained to me the differences between the various strains. Acapulco Gold is buoyant and energizing; Purple Kush is sleepy, relaxed, dissociative. Here’s a strain that makes you feel nostalgic; here’s one that helps you focus. It was as complicated and as oddly specific as a fancy wine tasting – and, I had a feeling, about as reliable.
It’s a plant, after all, and though delta-9-tetrahydrocannabinol (THC) is the chemical responsible for its euphoric effects, it is far from the only substance in there.
The second most important compound in cannabis is cannabidiol, and most people will tell you that CBD is the gentle yin to THC’s paranoiac yang. Hence your local ganja barista reminding you that, if you don›t want all those anxiety-inducing side effects of THC, grab a strain with a nice CBD balance.
But is it true? A new study appearing in JAMA Network Open suggests, in fact, that it’s quite the opposite. This study is from Austin Zamarripa and colleagues, who clearly sit at the researcher cool kids table.
Eighteen adults who had abstained from marijuana use for at least a month participated in this trial (which is way more fun than anything we do in my lab at Yale). In random order, separated by at least a week, they ate some special brownies.
Condition one was a control brownie, condition two was a brownie containing 20 mg of THC, and condition three was a brownie containing 20 mg of THC and 640 mg of CBD. Participants were assigned each condition in random order, separated by at least a week.
A side note on doses for those of you who, like me, are not totally weed literate. A dose of 20 mg of THC is about a third of what you might find in a typical joint these days (though it’s about double the THC content of a joint in the ‘70s – I believe the technical term is “doobie”). And 640 mg of CBD is a decent dose, as 5 mg per kilogram is what some folks start with to achieve therapeutic effects.
Both THC and CBD interact with the cytochrome p450 system in the liver. This matters when you’re ingesting them instead of smoking them because you have first-pass metabolism to contend with. And, because of that p450 inhibition, it’s possible that CBD might actually increase the amount of THC that gets into your bloodstream from the brownie, or gummy, or pizza sauce, or whatever.
Let’s get to the results, starting with blood THC concentration. It’s not subtle. With CBD on board the THC concentration rises higher faster, with roughly double the area under the curve.
And, unsurprisingly, the subjective experience correlated with those higher levels. Individuals rated the “drug effect” higher with the combo. But, interestingly, the “pleasant” drug effect didn’t change much, while the unpleasant effects were substantially higher. No mitigation of THC anxiety here – quite the opposite. CBD made the anxiety worse.
Cognitive effects were equally profound. Scores on a digit symbol substitution test and a paced serial addition task were all substantially worse when CBD was mixed with THC.
And for those of you who want some more objective measures, check out the heart rate. Despite the purported “calming” nature of CBD, heart rates were way higher when individuals were exposed to both chemicals.
The picture here is quite clear, though the mechanism is not. At least when talking edibles, CBD enhances the effects of THC, and not necessarily for the better. It may be that CBD is competing with some of the proteins that metabolize THC, thus prolonging its effects. CBD may also directly inhibit those enzymes. But whatever the case, I think we can safely say the myth that CBD makes the effects of THC more mild or more tolerable is busted.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn.
A version of this article first appeared on Medscape.com.









