User login
For MD-IQ use only
Identifying VA Cancer Patients for Clinical Trials: Use of an Umbrella Pre-Screening Protocol to Improve Enrollment in Oncology/Hematology Clinical Trials Within the VA Healthcare System
Background: The VA Connecticut Healthcare System Cancer Center developed and implemented a standardized pre-screening process and infrastructure to help identify patients for clinical trials and increase enrollment. Participation rate of cancer patients in clinical trials in the US is low, and the rate of enrollment of veterans with cancer into clinical trials is even lower than in the non-VA population. We hypothesized that a standardized process to prospectively identify potential patients for available clinical trials would increase enrollment of veteran cancer patients.
Methods: Our process uses the Research Electronic Data Capture (REDCap) system to pre-screen patients for clinical trials and to create a registry of potential research subjects. Patients are initially identified through multiple resources (clinic lists, tumor boards, cancer registry), and a standardized medical chart review is used to manually populate customized data fields in REDCap. Limited data fields are entered on all cancer patients, and more detailed information including stage, previous treatments and comorbidities is captured in patients for which we have active clinical trials. Providers are alerted prior to clinic when a potential research subject is scheduled, and have the opportunity to confirm eligibility, review the protocol, and set aside adequate time in clinic to discuss the study with the patient. The pre-screening protocol is approved by our IRB.
Reasults: From March 2017 through March 2019, research coordinators pre-screened and entered 4,959 unique patients into REDCap and identified 1,282 potential participants for trials. Of these, 756 patients met study specific criteria. Of those who were approached, 439 patients consented and proceeded with study specific screening procedures. We also routinely use our REDCap™ database to help estimate the likelihood of meeting enrollment targets when considering new clinical trials. Our clinical trials enrollment numbers have increased from 66 in 2016 to 238 in 2018, and the number of open trials has increased from 16 to 26 during this period.
Conclusion: An IRB-approved prescreening protocol which utilizes a data capturing system such as REDCap can help increase cancer clinical trials enrollment. Next steps will be to expand this concept across VA sites and to automate portions of data capture.
Background: The VA Connecticut Healthcare System Cancer Center developed and implemented a standardized pre-screening process and infrastructure to help identify patients for clinical trials and increase enrollment. Participation rate of cancer patients in clinical trials in the US is low, and the rate of enrollment of veterans with cancer into clinical trials is even lower than in the non-VA population. We hypothesized that a standardized process to prospectively identify potential patients for available clinical trials would increase enrollment of veteran cancer patients.
Methods: Our process uses the Research Electronic Data Capture (REDCap) system to pre-screen patients for clinical trials and to create a registry of potential research subjects. Patients are initially identified through multiple resources (clinic lists, tumor boards, cancer registry), and a standardized medical chart review is used to manually populate customized data fields in REDCap. Limited data fields are entered on all cancer patients, and more detailed information including stage, previous treatments and comorbidities is captured in patients for which we have active clinical trials. Providers are alerted prior to clinic when a potential research subject is scheduled, and have the opportunity to confirm eligibility, review the protocol, and set aside adequate time in clinic to discuss the study with the patient. The pre-screening protocol is approved by our IRB.
Reasults: From March 2017 through March 2019, research coordinators pre-screened and entered 4,959 unique patients into REDCap and identified 1,282 potential participants for trials. Of these, 756 patients met study specific criteria. Of those who were approached, 439 patients consented and proceeded with study specific screening procedures. We also routinely use our REDCap™ database to help estimate the likelihood of meeting enrollment targets when considering new clinical trials. Our clinical trials enrollment numbers have increased from 66 in 2016 to 238 in 2018, and the number of open trials has increased from 16 to 26 during this period.
Conclusion: An IRB-approved prescreening protocol which utilizes a data capturing system such as REDCap can help increase cancer clinical trials enrollment. Next steps will be to expand this concept across VA sites and to automate portions of data capture.
Background: The VA Connecticut Healthcare System Cancer Center developed and implemented a standardized pre-screening process and infrastructure to help identify patients for clinical trials and increase enrollment. Participation rate of cancer patients in clinical trials in the US is low, and the rate of enrollment of veterans with cancer into clinical trials is even lower than in the non-VA population. We hypothesized that a standardized process to prospectively identify potential patients for available clinical trials would increase enrollment of veteran cancer patients.
Methods: Our process uses the Research Electronic Data Capture (REDCap) system to pre-screen patients for clinical trials and to create a registry of potential research subjects. Patients are initially identified through multiple resources (clinic lists, tumor boards, cancer registry), and a standardized medical chart review is used to manually populate customized data fields in REDCap. Limited data fields are entered on all cancer patients, and more detailed information including stage, previous treatments and comorbidities is captured in patients for which we have active clinical trials. Providers are alerted prior to clinic when a potential research subject is scheduled, and have the opportunity to confirm eligibility, review the protocol, and set aside adequate time in clinic to discuss the study with the patient. The pre-screening protocol is approved by our IRB.
Reasults: From March 2017 through March 2019, research coordinators pre-screened and entered 4,959 unique patients into REDCap and identified 1,282 potential participants for trials. Of these, 756 patients met study specific criteria. Of those who were approached, 439 patients consented and proceeded with study specific screening procedures. We also routinely use our REDCap™ database to help estimate the likelihood of meeting enrollment targets when considering new clinical trials. Our clinical trials enrollment numbers have increased from 66 in 2016 to 238 in 2018, and the number of open trials has increased from 16 to 26 during this period.
Conclusion: An IRB-approved prescreening protocol which utilizes a data capturing system such as REDCap can help increase cancer clinical trials enrollment. Next steps will be to expand this concept across VA sites and to automate portions of data capture.
A Focus on Implementation and Impact of a Pharmacy Driven Oral Chemotherapy Clinic
Background: The utilization of oral chemotherapy agents is becoming increasingly widespread due to expanding indications in the oncology world. This change represents a shift in managing patients with cancer from intermittent intravenous therapy to self-administered chronic oral therapy which presents unique issues regarding patient safety. A previous study conducted in Toronto, Canada showed that the formation of a multidisciplinary oral chemotherapy clinic helped improve patient outcomes (Disperati et al, 2017).
To address these concerns at our facility, an oral chemotherapy clinic was implemented to provide closer monitoring of patients on oral chemotherapeutic agents. The pharmacy driven oral chemotherapy clinic includes a multidisciplinary team of an oncology pharmacist, oncology physicians, and support staff. The oncology pharmacist provides counseling on proper medication administration, ensures medication adherence, and manages adverse drug events.
Physicians collaborate with the oncology pharmacist to enroll patients into the clinic by placing an intrafacility consult. Referred patients may be newly starting oral chemotherapy or continuing an oral chemotherapy regimen. Patients are not eligible if partial care is provided by a community oncologist. Pharmacist appointments may be face to face or telephone and are in addition to routine physician provider visits.
Results: After the first 4 months of initiating the oral chemotherapy clinic, there were 10 patients enrolled. There were 22 documented interventions, 16 pharmacist interventions and 6 physician interventions. The most common pharmacist interventions included medication adjustments and initiation of supplemental medications to treat adverse events. Patients engaged in 49 encounters, including 17 traditional visits, 21 oral chemotherapy clinic visits, 8 scheduled telehealth visits, and 3 unscheduled telehealth visits with only 1 emergency department visit. Notably, no emergency visits were due to a patient’s oral chemotherapy regimen.
Additional outcomes were analyzed showing 100% patient compliance, 100% proper renal/hepatic dosing and the oral chemo clinic achieved 84% appropriate lab monitoring (improved from 36% in the control group).
Implications: A multidisciplinary approach and integrating the pharmacist run oral chemotherapy clinic improved patient monitoring, drug compliance and patient access to care. With these positive results, we hope to expand the program and incorporate a fulltime pharmacist.
Background: The utilization of oral chemotherapy agents is becoming increasingly widespread due to expanding indications in the oncology world. This change represents a shift in managing patients with cancer from intermittent intravenous therapy to self-administered chronic oral therapy which presents unique issues regarding patient safety. A previous study conducted in Toronto, Canada showed that the formation of a multidisciplinary oral chemotherapy clinic helped improve patient outcomes (Disperati et al, 2017).
To address these concerns at our facility, an oral chemotherapy clinic was implemented to provide closer monitoring of patients on oral chemotherapeutic agents. The pharmacy driven oral chemotherapy clinic includes a multidisciplinary team of an oncology pharmacist, oncology physicians, and support staff. The oncology pharmacist provides counseling on proper medication administration, ensures medication adherence, and manages adverse drug events.
Physicians collaborate with the oncology pharmacist to enroll patients into the clinic by placing an intrafacility consult. Referred patients may be newly starting oral chemotherapy or continuing an oral chemotherapy regimen. Patients are not eligible if partial care is provided by a community oncologist. Pharmacist appointments may be face to face or telephone and are in addition to routine physician provider visits.
Results: After the first 4 months of initiating the oral chemotherapy clinic, there were 10 patients enrolled. There were 22 documented interventions, 16 pharmacist interventions and 6 physician interventions. The most common pharmacist interventions included medication adjustments and initiation of supplemental medications to treat adverse events. Patients engaged in 49 encounters, including 17 traditional visits, 21 oral chemotherapy clinic visits, 8 scheduled telehealth visits, and 3 unscheduled telehealth visits with only 1 emergency department visit. Notably, no emergency visits were due to a patient’s oral chemotherapy regimen.
Additional outcomes were analyzed showing 100% patient compliance, 100% proper renal/hepatic dosing and the oral chemo clinic achieved 84% appropriate lab monitoring (improved from 36% in the control group).
Implications: A multidisciplinary approach and integrating the pharmacist run oral chemotherapy clinic improved patient monitoring, drug compliance and patient access to care. With these positive results, we hope to expand the program and incorporate a fulltime pharmacist.
Background: The utilization of oral chemotherapy agents is becoming increasingly widespread due to expanding indications in the oncology world. This change represents a shift in managing patients with cancer from intermittent intravenous therapy to self-administered chronic oral therapy which presents unique issues regarding patient safety. A previous study conducted in Toronto, Canada showed that the formation of a multidisciplinary oral chemotherapy clinic helped improve patient outcomes (Disperati et al, 2017).
To address these concerns at our facility, an oral chemotherapy clinic was implemented to provide closer monitoring of patients on oral chemotherapeutic agents. The pharmacy driven oral chemotherapy clinic includes a multidisciplinary team of an oncology pharmacist, oncology physicians, and support staff. The oncology pharmacist provides counseling on proper medication administration, ensures medication adherence, and manages adverse drug events.
Physicians collaborate with the oncology pharmacist to enroll patients into the clinic by placing an intrafacility consult. Referred patients may be newly starting oral chemotherapy or continuing an oral chemotherapy regimen. Patients are not eligible if partial care is provided by a community oncologist. Pharmacist appointments may be face to face or telephone and are in addition to routine physician provider visits.
Results: After the first 4 months of initiating the oral chemotherapy clinic, there were 10 patients enrolled. There were 22 documented interventions, 16 pharmacist interventions and 6 physician interventions. The most common pharmacist interventions included medication adjustments and initiation of supplemental medications to treat adverse events. Patients engaged in 49 encounters, including 17 traditional visits, 21 oral chemotherapy clinic visits, 8 scheduled telehealth visits, and 3 unscheduled telehealth visits with only 1 emergency department visit. Notably, no emergency visits were due to a patient’s oral chemotherapy regimen.
Additional outcomes were analyzed showing 100% patient compliance, 100% proper renal/hepatic dosing and the oral chemo clinic achieved 84% appropriate lab monitoring (improved from 36% in the control group).
Implications: A multidisciplinary approach and integrating the pharmacist run oral chemotherapy clinic improved patient monitoring, drug compliance and patient access to care. With these positive results, we hope to expand the program and incorporate a fulltime pharmacist.
Demographic Factors of Patients with Oligodendroglioma: A NCDB Analysis
Background: Oligodendrogliomas represent about 12% of all brain tumors. Our goal was to compare the demographic factors of patients diagnosed with oligodendroglioma from 2004-2014 identified in the National Cancer Database (NCDB). We also examined the survival of patients based off of the number of their comorbidities.
Methods: We identified 7525 patients diagnosed with oligodendroglioma in the NCDB diagnosed between 2004-2014. Many demographic factors were examined such as age, gender, race, facility treated at, comorbidities, and surgery type. Between-insurance survival differences were estimated by the Kaplan-Meier method and associated log-rank tests; Tukey-Kramer adjusted P < .05 indicated statistical significance.
Results: More men were diagnosed with the tumor than females (55% vs 45%). Average age of patients at diagnosis was 43.5 years old. 66% of patients had private insurance, while 7% of patients were uninsured. 88.9% of patients were white, while 5.5% of patients were black. Patients that were treated at an academic/ research program were 32% of the sample size. 17% of the sample size were treated at a comprehensive community cancer program. Those with no comorbidities had the highest mean survival time of 111 months, those with one comorbidity had a mean survival time of 97 months, and those with two comorbidities had the lowest mean survival time of 75 months. 12.8% of patients had radical, total gross resection of tumor, lesion, or mass in their brain and 10% of patients had less than half of the lobe involved with the tumor resection. 20.1% of patients had systemic therapy after surgery. 59% of patients had no systemic therapy or surgery.
Conclusion: Our study shows men were affected more than women and that the mean age at diagnosis was 44 years old. The greater number of comorbidities a patient had, the lower the mean survival time was. Majority of patients were treated at an academic/research program. This is one of the largest studies to examine the demographics of patients with oligodendroglioma. Understanding who and how patients are affected can allow us to provide better resources and treatment.
Background: Oligodendrogliomas represent about 12% of all brain tumors. Our goal was to compare the demographic factors of patients diagnosed with oligodendroglioma from 2004-2014 identified in the National Cancer Database (NCDB). We also examined the survival of patients based off of the number of their comorbidities.
Methods: We identified 7525 patients diagnosed with oligodendroglioma in the NCDB diagnosed between 2004-2014. Many demographic factors were examined such as age, gender, race, facility treated at, comorbidities, and surgery type. Between-insurance survival differences were estimated by the Kaplan-Meier method and associated log-rank tests; Tukey-Kramer adjusted P < .05 indicated statistical significance.
Results: More men were diagnosed with the tumor than females (55% vs 45%). Average age of patients at diagnosis was 43.5 years old. 66% of patients had private insurance, while 7% of patients were uninsured. 88.9% of patients were white, while 5.5% of patients were black. Patients that were treated at an academic/ research program were 32% of the sample size. 17% of the sample size were treated at a comprehensive community cancer program. Those with no comorbidities had the highest mean survival time of 111 months, those with one comorbidity had a mean survival time of 97 months, and those with two comorbidities had the lowest mean survival time of 75 months. 12.8% of patients had radical, total gross resection of tumor, lesion, or mass in their brain and 10% of patients had less than half of the lobe involved with the tumor resection. 20.1% of patients had systemic therapy after surgery. 59% of patients had no systemic therapy or surgery.
Conclusion: Our study shows men were affected more than women and that the mean age at diagnosis was 44 years old. The greater number of comorbidities a patient had, the lower the mean survival time was. Majority of patients were treated at an academic/research program. This is one of the largest studies to examine the demographics of patients with oligodendroglioma. Understanding who and how patients are affected can allow us to provide better resources and treatment.
Background: Oligodendrogliomas represent about 12% of all brain tumors. Our goal was to compare the demographic factors of patients diagnosed with oligodendroglioma from 2004-2014 identified in the National Cancer Database (NCDB). We also examined the survival of patients based off of the number of their comorbidities.
Methods: We identified 7525 patients diagnosed with oligodendroglioma in the NCDB diagnosed between 2004-2014. Many demographic factors were examined such as age, gender, race, facility treated at, comorbidities, and surgery type. Between-insurance survival differences were estimated by the Kaplan-Meier method and associated log-rank tests; Tukey-Kramer adjusted P < .05 indicated statistical significance.
Results: More men were diagnosed with the tumor than females (55% vs 45%). Average age of patients at diagnosis was 43.5 years old. 66% of patients had private insurance, while 7% of patients were uninsured. 88.9% of patients were white, while 5.5% of patients were black. Patients that were treated at an academic/ research program were 32% of the sample size. 17% of the sample size were treated at a comprehensive community cancer program. Those with no comorbidities had the highest mean survival time of 111 months, those with one comorbidity had a mean survival time of 97 months, and those with two comorbidities had the lowest mean survival time of 75 months. 12.8% of patients had radical, total gross resection of tumor, lesion, or mass in their brain and 10% of patients had less than half of the lobe involved with the tumor resection. 20.1% of patients had systemic therapy after surgery. 59% of patients had no systemic therapy or surgery.
Conclusion: Our study shows men were affected more than women and that the mean age at diagnosis was 44 years old. The greater number of comorbidities a patient had, the lower the mean survival time was. Majority of patients were treated at an academic/research program. This is one of the largest studies to examine the demographics of patients with oligodendroglioma. Understanding who and how patients are affected can allow us to provide better resources and treatment.
Characterization of Adverse Reactions to ‘4-week’ Nivolumab Dosing
Background: Nivolumab was recently approved for a new flat-dose schedule 480 mg IV every 4 weeks (“480 Q4w”) using data from pharmacokinetics simulations without being first tested directly in humans. We noted several unusual adverse drug reactions (ADRs) using the new dosing and hypothesized that this new dose schedule might generate more ADRs than prior dosing schedules.
Methods: This study attempts to summarize and characterize the types of ADRs seen on the new 480 Q4w dosing. We conducted a retrospective, descriptive chart review and case series including patients at the San Antonio VA Hematology/Oncology clinic treated with at least one dose of Nivolumab 480 mg between 2/1/18 and 10/1/18. We tracked whether these patients developed ADRs, and if so, the highest CTCAE 4.03 grade of reaction, the number of treatments before the reaction developed, and whether the reaction influenced treatment (hold treatment, stop treatment, dose change).
Results: 18 patients matched this criterion (all male, average age 67.6 years). 6 patients experienced an ADR during treatment with the 480 Q4w dose. Grade 1 toxicities included pruritis, abdominal pain, skin rash, fatigue, fever, cramping, myalgia, and diarrhea. There was a Grade 3 case of encephalopathy and a Grade 2 case of diplopia. Of the 6 patients who experienced an adverse drug reaction, 2 (with only Grade 1 toxicities) continued treatment at their same dose frequency; the others changed to 240 mg Q2w. All 4 patients who experienced an ADR and had their dose changed to 240 mg Q2w experienced resolution or improvement in their symptoms except for 1 patient’s complaint of abdominal pain.
Conclusion: 480 Q4w dosing of Nivolumab may have a different ADR profile from prior dose regimens; further quantitative analysis will be required to answer this question. Dose frequency change may present an opportunity to relieve toxicities while allowing patients to continue treatment.
Background: Nivolumab was recently approved for a new flat-dose schedule 480 mg IV every 4 weeks (“480 Q4w”) using data from pharmacokinetics simulations without being first tested directly in humans. We noted several unusual adverse drug reactions (ADRs) using the new dosing and hypothesized that this new dose schedule might generate more ADRs than prior dosing schedules.
Methods: This study attempts to summarize and characterize the types of ADRs seen on the new 480 Q4w dosing. We conducted a retrospective, descriptive chart review and case series including patients at the San Antonio VA Hematology/Oncology clinic treated with at least one dose of Nivolumab 480 mg between 2/1/18 and 10/1/18. We tracked whether these patients developed ADRs, and if so, the highest CTCAE 4.03 grade of reaction, the number of treatments before the reaction developed, and whether the reaction influenced treatment (hold treatment, stop treatment, dose change).
Results: 18 patients matched this criterion (all male, average age 67.6 years). 6 patients experienced an ADR during treatment with the 480 Q4w dose. Grade 1 toxicities included pruritis, abdominal pain, skin rash, fatigue, fever, cramping, myalgia, and diarrhea. There was a Grade 3 case of encephalopathy and a Grade 2 case of diplopia. Of the 6 patients who experienced an adverse drug reaction, 2 (with only Grade 1 toxicities) continued treatment at their same dose frequency; the others changed to 240 mg Q2w. All 4 patients who experienced an ADR and had their dose changed to 240 mg Q2w experienced resolution or improvement in their symptoms except for 1 patient’s complaint of abdominal pain.
Conclusion: 480 Q4w dosing of Nivolumab may have a different ADR profile from prior dose regimens; further quantitative analysis will be required to answer this question. Dose frequency change may present an opportunity to relieve toxicities while allowing patients to continue treatment.
Background: Nivolumab was recently approved for a new flat-dose schedule 480 mg IV every 4 weeks (“480 Q4w”) using data from pharmacokinetics simulations without being first tested directly in humans. We noted several unusual adverse drug reactions (ADRs) using the new dosing and hypothesized that this new dose schedule might generate more ADRs than prior dosing schedules.
Methods: This study attempts to summarize and characterize the types of ADRs seen on the new 480 Q4w dosing. We conducted a retrospective, descriptive chart review and case series including patients at the San Antonio VA Hematology/Oncology clinic treated with at least one dose of Nivolumab 480 mg between 2/1/18 and 10/1/18. We tracked whether these patients developed ADRs, and if so, the highest CTCAE 4.03 grade of reaction, the number of treatments before the reaction developed, and whether the reaction influenced treatment (hold treatment, stop treatment, dose change).
Results: 18 patients matched this criterion (all male, average age 67.6 years). 6 patients experienced an ADR during treatment with the 480 Q4w dose. Grade 1 toxicities included pruritis, abdominal pain, skin rash, fatigue, fever, cramping, myalgia, and diarrhea. There was a Grade 3 case of encephalopathy and a Grade 2 case of diplopia. Of the 6 patients who experienced an adverse drug reaction, 2 (with only Grade 1 toxicities) continued treatment at their same dose frequency; the others changed to 240 mg Q2w. All 4 patients who experienced an ADR and had their dose changed to 240 mg Q2w experienced resolution or improvement in their symptoms except for 1 patient’s complaint of abdominal pain.
Conclusion: 480 Q4w dosing of Nivolumab may have a different ADR profile from prior dose regimens; further quantitative analysis will be required to answer this question. Dose frequency change may present an opportunity to relieve toxicities while allowing patients to continue treatment.
Atypical Presentation and Management of Refractory Multisystem Checkpoint Inhibitor Toxicities
Background: Immunotherapy with checkpoint inhibition represents a significant development in the management of advanced malignancies. Toxicity associated with this therapy, or immune-related adverse events (irAEs), is most frequently seen in the form of colitis, dermatitis, pneumonitis, or hepatitis. Prompt initiation of high-dose corticosteroids in severe cases is essential. Refractory toxicity can be seen and requires a multimodality approach.
Case Report: A 68 year old male with a history of stage IVB squamous cell carcinoma (SCC) and an illdefined left renal mass (4.8 × 4.1 cm2, presumed urothelial carcinoma) was successfully maintained on nivolumab monotherapy for nearly two years. However, after 45 cycles he presented to the emergency department with acute hypoxic respiratory failure. He was admitted and found to have multifocal pulmonary consolidations refractory to empiric antibiotics. Ultimately his symptoms were attributed to nivolumab-induced pneumonitis. He improved on corticosteroids and was discharged with a prednisone taper. One month later he returned with severe lower extremity weakness, an elevated creatinine kinase, grade IV hepatitis, and hematuria. Notwithstanding immediate escalation to intravenous methylprednisolone (2 mg/kg) and a trial of IVIG, his myositis and liver dysfunction worsened. Mycophenolate mofetil was added (1000 mg BID) and successfully reversed his creatinine kinase (from 3633 U/L); however, his hepatitis continued to progress (total bilirubin to 16.4 mg/dL). After further discussion with gastroenterology, tacrolimus was also started (2 mg BID) and caused gradual improvement in his transaminases. Once stabilized, he underwent a left nephrectomy for persistent hematuria; pathology results revealed an unusual focus of metastatic SCC. Unfortunately, he developed post-operative bleeding complications and CMV viremia. He elected to pursue comfort measures and passed 2 weeks later.
Conclusion: Checkpoint inhibitor toxicities can demonstrate delayed presentations despite numerous cycles of successful therapy. In cases where aggressive corticosteroid therapy is unsuccessful, additional immunomodulatory agents are required to curb progression of organ-specific damage. Close surveillance for opportunistic infections must be maintained. Additional studies are needed to assess whether earlier discontinuation of checkpoint inhibition is feasible in patients with sustained responses to minimize late irAEs.
Background: Immunotherapy with checkpoint inhibition represents a significant development in the management of advanced malignancies. Toxicity associated with this therapy, or immune-related adverse events (irAEs), is most frequently seen in the form of colitis, dermatitis, pneumonitis, or hepatitis. Prompt initiation of high-dose corticosteroids in severe cases is essential. Refractory toxicity can be seen and requires a multimodality approach.
Case Report: A 68 year old male with a history of stage IVB squamous cell carcinoma (SCC) and an illdefined left renal mass (4.8 × 4.1 cm2, presumed urothelial carcinoma) was successfully maintained on nivolumab monotherapy for nearly two years. However, after 45 cycles he presented to the emergency department with acute hypoxic respiratory failure. He was admitted and found to have multifocal pulmonary consolidations refractory to empiric antibiotics. Ultimately his symptoms were attributed to nivolumab-induced pneumonitis. He improved on corticosteroids and was discharged with a prednisone taper. One month later he returned with severe lower extremity weakness, an elevated creatinine kinase, grade IV hepatitis, and hematuria. Notwithstanding immediate escalation to intravenous methylprednisolone (2 mg/kg) and a trial of IVIG, his myositis and liver dysfunction worsened. Mycophenolate mofetil was added (1000 mg BID) and successfully reversed his creatinine kinase (from 3633 U/L); however, his hepatitis continued to progress (total bilirubin to 16.4 mg/dL). After further discussion with gastroenterology, tacrolimus was also started (2 mg BID) and caused gradual improvement in his transaminases. Once stabilized, he underwent a left nephrectomy for persistent hematuria; pathology results revealed an unusual focus of metastatic SCC. Unfortunately, he developed post-operative bleeding complications and CMV viremia. He elected to pursue comfort measures and passed 2 weeks later.
Conclusion: Checkpoint inhibitor toxicities can demonstrate delayed presentations despite numerous cycles of successful therapy. In cases where aggressive corticosteroid therapy is unsuccessful, additional immunomodulatory agents are required to curb progression of organ-specific damage. Close surveillance for opportunistic infections must be maintained. Additional studies are needed to assess whether earlier discontinuation of checkpoint inhibition is feasible in patients with sustained responses to minimize late irAEs.
Background: Immunotherapy with checkpoint inhibition represents a significant development in the management of advanced malignancies. Toxicity associated with this therapy, or immune-related adverse events (irAEs), is most frequently seen in the form of colitis, dermatitis, pneumonitis, or hepatitis. Prompt initiation of high-dose corticosteroids in severe cases is essential. Refractory toxicity can be seen and requires a multimodality approach.
Case Report: A 68 year old male with a history of stage IVB squamous cell carcinoma (SCC) and an illdefined left renal mass (4.8 × 4.1 cm2, presumed urothelial carcinoma) was successfully maintained on nivolumab monotherapy for nearly two years. However, after 45 cycles he presented to the emergency department with acute hypoxic respiratory failure. He was admitted and found to have multifocal pulmonary consolidations refractory to empiric antibiotics. Ultimately his symptoms were attributed to nivolumab-induced pneumonitis. He improved on corticosteroids and was discharged with a prednisone taper. One month later he returned with severe lower extremity weakness, an elevated creatinine kinase, grade IV hepatitis, and hematuria. Notwithstanding immediate escalation to intravenous methylprednisolone (2 mg/kg) and a trial of IVIG, his myositis and liver dysfunction worsened. Mycophenolate mofetil was added (1000 mg BID) and successfully reversed his creatinine kinase (from 3633 U/L); however, his hepatitis continued to progress (total bilirubin to 16.4 mg/dL). After further discussion with gastroenterology, tacrolimus was also started (2 mg BID) and caused gradual improvement in his transaminases. Once stabilized, he underwent a left nephrectomy for persistent hematuria; pathology results revealed an unusual focus of metastatic SCC. Unfortunately, he developed post-operative bleeding complications and CMV viremia. He elected to pursue comfort measures and passed 2 weeks later.
Conclusion: Checkpoint inhibitor toxicities can demonstrate delayed presentations despite numerous cycles of successful therapy. In cases where aggressive corticosteroid therapy is unsuccessful, additional immunomodulatory agents are required to curb progression of organ-specific damage. Close surveillance for opportunistic infections must be maintained. Additional studies are needed to assess whether earlier discontinuation of checkpoint inhibition is feasible in patients with sustained responses to minimize late irAEs.
A Rural VA Utilizing Telehealth Platform to Address Dietary Issues of Veterans With Cancer
Background: The Salisbury VA Medical Center (SVA) is a rural VA and some of our veterans with cancer are treated at VA Health Care Center (HCCs) in Kernersville or Charlotte. The VA telehealth platform provides a bridge to address dietary issues for veterans that cannot travel to Salisbury. The SVA offers virtual nutrition counseling sessions conveniently scheduled in conjunction with veterans HCC oncology visit and eliminates the need for additional appointments or having to arrange transportation to SVA.
Dietary counseling for veterans with cancer is an integral part of the SVA cancer care program. This commitment is shown by SVA Medical Centers commitment to a board certified oncology dietician FTE. The oncology dietician staffs the SVA outpatient medical oncology clinic and manages dietary issues that are present at diagnosis or arise during treatment. Annually, the oncology dietician averages a case load of 334 unique veterans and averages 1395 visits with these veterans. Most of these dietary encounters occur at the SVA infusion center while veterans are getting treatment or in the SVA oncology exam room after the veteran visits with their oncologic provider.
Methods: To provide this same dietary service to Kernersville and Charlotte veterans, the dietary oncology telehealth program was established. The program has performed 99 telehealth visits. The telehealth visits accomplish the same objectives as the live clinic appointments.
Common dietary issues that are managed in the clinic involve weight loss in lung cancer veterans, weight gain in prostate cancer veterans, and malabsorption in colorectal cancer veterans. The oncology dietician has competency and resources in managing these nutrition impact symptoms.
Implizations: Ideas for expansion of the Salisbury oncology dietary telehealth program would be to utilize the new Anywhere to Anywhere initiative, to improve access to veterans in the SVA system and to possibly aid other VAs oncology programs that do not have a dedicated oncology dietician.
Background: The Salisbury VA Medical Center (SVA) is a rural VA and some of our veterans with cancer are treated at VA Health Care Center (HCCs) in Kernersville or Charlotte. The VA telehealth platform provides a bridge to address dietary issues for veterans that cannot travel to Salisbury. The SVA offers virtual nutrition counseling sessions conveniently scheduled in conjunction with veterans HCC oncology visit and eliminates the need for additional appointments or having to arrange transportation to SVA.
Dietary counseling for veterans with cancer is an integral part of the SVA cancer care program. This commitment is shown by SVA Medical Centers commitment to a board certified oncology dietician FTE. The oncology dietician staffs the SVA outpatient medical oncology clinic and manages dietary issues that are present at diagnosis or arise during treatment. Annually, the oncology dietician averages a case load of 334 unique veterans and averages 1395 visits with these veterans. Most of these dietary encounters occur at the SVA infusion center while veterans are getting treatment or in the SVA oncology exam room after the veteran visits with their oncologic provider.
Methods: To provide this same dietary service to Kernersville and Charlotte veterans, the dietary oncology telehealth program was established. The program has performed 99 telehealth visits. The telehealth visits accomplish the same objectives as the live clinic appointments.
Common dietary issues that are managed in the clinic involve weight loss in lung cancer veterans, weight gain in prostate cancer veterans, and malabsorption in colorectal cancer veterans. The oncology dietician has competency and resources in managing these nutrition impact symptoms.
Implizations: Ideas for expansion of the Salisbury oncology dietary telehealth program would be to utilize the new Anywhere to Anywhere initiative, to improve access to veterans in the SVA system and to possibly aid other VAs oncology programs that do not have a dedicated oncology dietician.
Background: The Salisbury VA Medical Center (SVA) is a rural VA and some of our veterans with cancer are treated at VA Health Care Center (HCCs) in Kernersville or Charlotte. The VA telehealth platform provides a bridge to address dietary issues for veterans that cannot travel to Salisbury. The SVA offers virtual nutrition counseling sessions conveniently scheduled in conjunction with veterans HCC oncology visit and eliminates the need for additional appointments or having to arrange transportation to SVA.
Dietary counseling for veterans with cancer is an integral part of the SVA cancer care program. This commitment is shown by SVA Medical Centers commitment to a board certified oncology dietician FTE. The oncology dietician staffs the SVA outpatient medical oncology clinic and manages dietary issues that are present at diagnosis or arise during treatment. Annually, the oncology dietician averages a case load of 334 unique veterans and averages 1395 visits with these veterans. Most of these dietary encounters occur at the SVA infusion center while veterans are getting treatment or in the SVA oncology exam room after the veteran visits with their oncologic provider.
Methods: To provide this same dietary service to Kernersville and Charlotte veterans, the dietary oncology telehealth program was established. The program has performed 99 telehealth visits. The telehealth visits accomplish the same objectives as the live clinic appointments.
Common dietary issues that are managed in the clinic involve weight loss in lung cancer veterans, weight gain in prostate cancer veterans, and malabsorption in colorectal cancer veterans. The oncology dietician has competency and resources in managing these nutrition impact symptoms.
Implizations: Ideas for expansion of the Salisbury oncology dietary telehealth program would be to utilize the new Anywhere to Anywhere initiative, to improve access to veterans in the SVA system and to possibly aid other VAs oncology programs that do not have a dedicated oncology dietician.
A Rare Case of Immunotactoid Glomerulopathy and Monoclonal Gammopathy of Renal Significance due to an IgM Kappa Clone at the VA Pittsburgh Healthcare System
Introduction: Monoclonal gammopathy of renal significance (MGRS) is a recently recognized disorder from pathologic M protein causing renal disease and minimal hematologic disease burden. Failure to treat leads to poor outcomes from progression to advanced monoclonal gammopathies and end stage renal disease (ESRD). We present a case of MGRS with immunotactoid glomerulopathy.
Case Report: A 66-year-old female presented in December 2015 with mild granulocytopenia and anemia. Workup revealed serum 0.28 mg/dL IgM kappa monoclonal M-protein and kappa/lambda ratio of 2.23. She underwent surveillance for MGUS. Due to acute kidney injury, peripheral edema and hypertension, nephrology workup was obtained in December 2017. She had nephrotic range proteinuria and hematuria. Urine studies suggested paraproteinemia. Renal biopsy demonstrated immunotactoid glomerulopathy with membranoproliferative glomerulonephritis pattern. Immunofluorescence showed kappa light chain in mesangial and capillary loop, and heavy IgM and moderate C3 staining. Electron microscopy revealed numerous immunotactoid deposits beneath the glomerular basement membrane and mesangium. M-protein burden remained stable. Her bone marrow biopsy was nondiagnostic, however peripheral flow cytometry identified a small CD20+, CD5-, CD10-, CD23-, B-cell population with kappa light chain restriction. Diagnosis was reclassified as MGRS and she was treated with rituximab weekly for four doses. Follow-up demonstrated stability of M-protein and light chains, improvement of AKI and hypertension, but persistent nephrotic range proteinuria. We are planning an additional eight-week course of weekly rituximab. Treatment outcome and further studies are pending.
Discussion: MGRS is a rare monoclonal gammopathy that was formerly subclassified under MGUS. Patients were undertreated due to under-recognition of the disorder and its renal sequalae. Treatment with regimens targeting a plasma cell or B-cell clone can reduce the clone and improve renal outcomes. Our patient experienced a partial response to clone directed therapy with rituximab. Further treatment is pending.
Conclusion: Clinicians should be aware of MGRS. Collaboration with nephrology is key for proper diagnosis and prognosis. Consider treating more aggressively than MGUS to improve renal and hematologic outcomes. Prospective interventional studies are needed.
Introduction: Monoclonal gammopathy of renal significance (MGRS) is a recently recognized disorder from pathologic M protein causing renal disease and minimal hematologic disease burden. Failure to treat leads to poor outcomes from progression to advanced monoclonal gammopathies and end stage renal disease (ESRD). We present a case of MGRS with immunotactoid glomerulopathy.
Case Report: A 66-year-old female presented in December 2015 with mild granulocytopenia and anemia. Workup revealed serum 0.28 mg/dL IgM kappa monoclonal M-protein and kappa/lambda ratio of 2.23. She underwent surveillance for MGUS. Due to acute kidney injury, peripheral edema and hypertension, nephrology workup was obtained in December 2017. She had nephrotic range proteinuria and hematuria. Urine studies suggested paraproteinemia. Renal biopsy demonstrated immunotactoid glomerulopathy with membranoproliferative glomerulonephritis pattern. Immunofluorescence showed kappa light chain in mesangial and capillary loop, and heavy IgM and moderate C3 staining. Electron microscopy revealed numerous immunotactoid deposits beneath the glomerular basement membrane and mesangium. M-protein burden remained stable. Her bone marrow biopsy was nondiagnostic, however peripheral flow cytometry identified a small CD20+, CD5-, CD10-, CD23-, B-cell population with kappa light chain restriction. Diagnosis was reclassified as MGRS and she was treated with rituximab weekly for four doses. Follow-up demonstrated stability of M-protein and light chains, improvement of AKI and hypertension, but persistent nephrotic range proteinuria. We are planning an additional eight-week course of weekly rituximab. Treatment outcome and further studies are pending.
Discussion: MGRS is a rare monoclonal gammopathy that was formerly subclassified under MGUS. Patients were undertreated due to under-recognition of the disorder and its renal sequalae. Treatment with regimens targeting a plasma cell or B-cell clone can reduce the clone and improve renal outcomes. Our patient experienced a partial response to clone directed therapy with rituximab. Further treatment is pending.
Conclusion: Clinicians should be aware of MGRS. Collaboration with nephrology is key for proper diagnosis and prognosis. Consider treating more aggressively than MGUS to improve renal and hematologic outcomes. Prospective interventional studies are needed.
Introduction: Monoclonal gammopathy of renal significance (MGRS) is a recently recognized disorder from pathologic M protein causing renal disease and minimal hematologic disease burden. Failure to treat leads to poor outcomes from progression to advanced monoclonal gammopathies and end stage renal disease (ESRD). We present a case of MGRS with immunotactoid glomerulopathy.
Case Report: A 66-year-old female presented in December 2015 with mild granulocytopenia and anemia. Workup revealed serum 0.28 mg/dL IgM kappa monoclonal M-protein and kappa/lambda ratio of 2.23. She underwent surveillance for MGUS. Due to acute kidney injury, peripheral edema and hypertension, nephrology workup was obtained in December 2017. She had nephrotic range proteinuria and hematuria. Urine studies suggested paraproteinemia. Renal biopsy demonstrated immunotactoid glomerulopathy with membranoproliferative glomerulonephritis pattern. Immunofluorescence showed kappa light chain in mesangial and capillary loop, and heavy IgM and moderate C3 staining. Electron microscopy revealed numerous immunotactoid deposits beneath the glomerular basement membrane and mesangium. M-protein burden remained stable. Her bone marrow biopsy was nondiagnostic, however peripheral flow cytometry identified a small CD20+, CD5-, CD10-, CD23-, B-cell population with kappa light chain restriction. Diagnosis was reclassified as MGRS and she was treated with rituximab weekly for four doses. Follow-up demonstrated stability of M-protein and light chains, improvement of AKI and hypertension, but persistent nephrotic range proteinuria. We are planning an additional eight-week course of weekly rituximab. Treatment outcome and further studies are pending.
Discussion: MGRS is a rare monoclonal gammopathy that was formerly subclassified under MGUS. Patients were undertreated due to under-recognition of the disorder and its renal sequalae. Treatment with regimens targeting a plasma cell or B-cell clone can reduce the clone and improve renal outcomes. Our patient experienced a partial response to clone directed therapy with rituximab. Further treatment is pending.
Conclusion: Clinicians should be aware of MGRS. Collaboration with nephrology is key for proper diagnosis and prognosis. Consider treating more aggressively than MGUS to improve renal and hematologic outcomes. Prospective interventional studies are needed.
Cutaneous Sarcoidosis Presenting as a Cutaneous Horn
To the Editor:
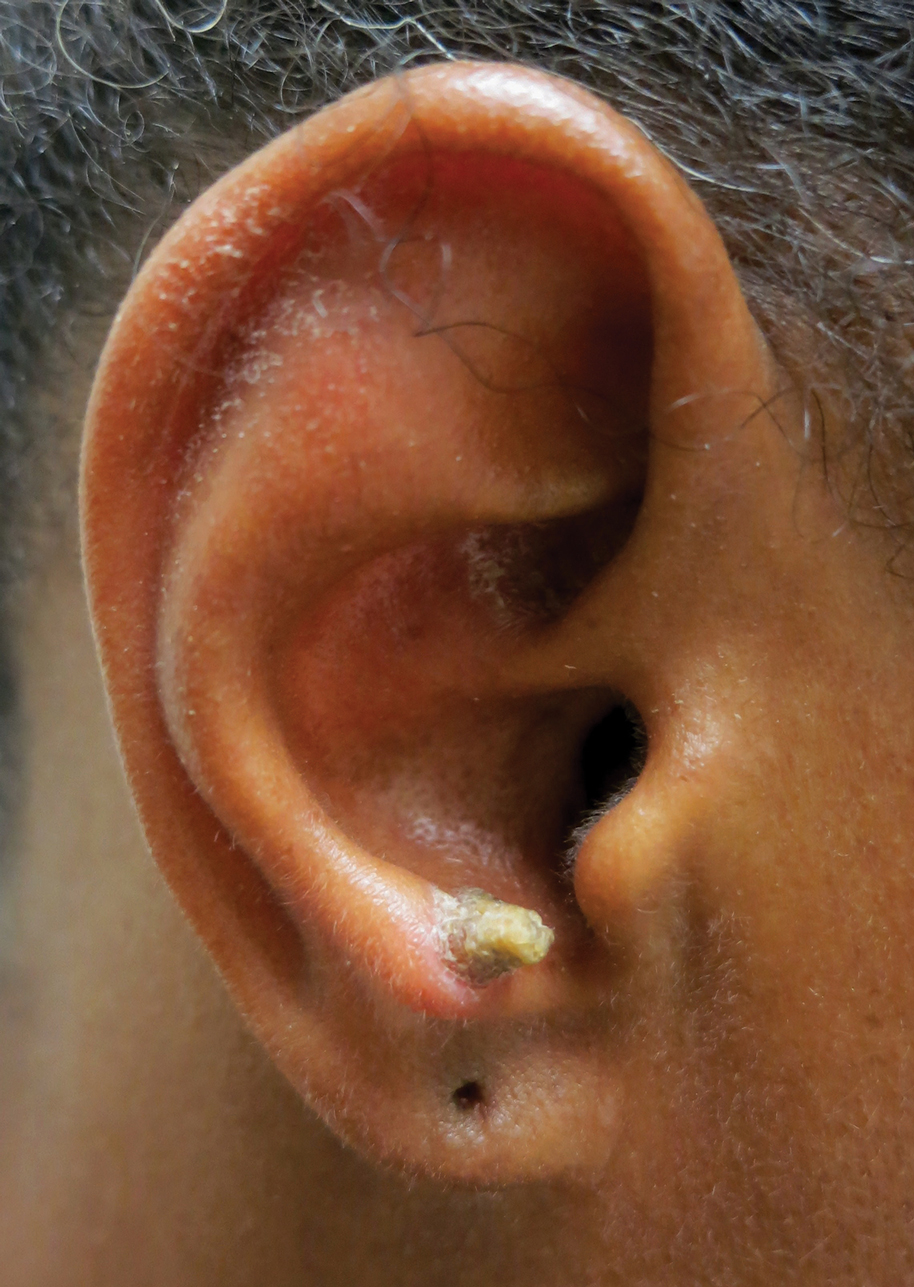
A 53-year-old woman presented to our dermatology clinic with a painful growth on the right ear of 2 months’ duration. A complete review of systems was negative except for an isolated episode of shortness of breath prior to presentation that resolved without intervention. During this episode, her primary care physician made a diagnosis of chronic obstructive pulmonary disease based on a chest radiograph. The patient reported minimal tobacco use, specifically that she had smoked a few cigarettes daily for several years but had quit 6 months prior to the current presentation.
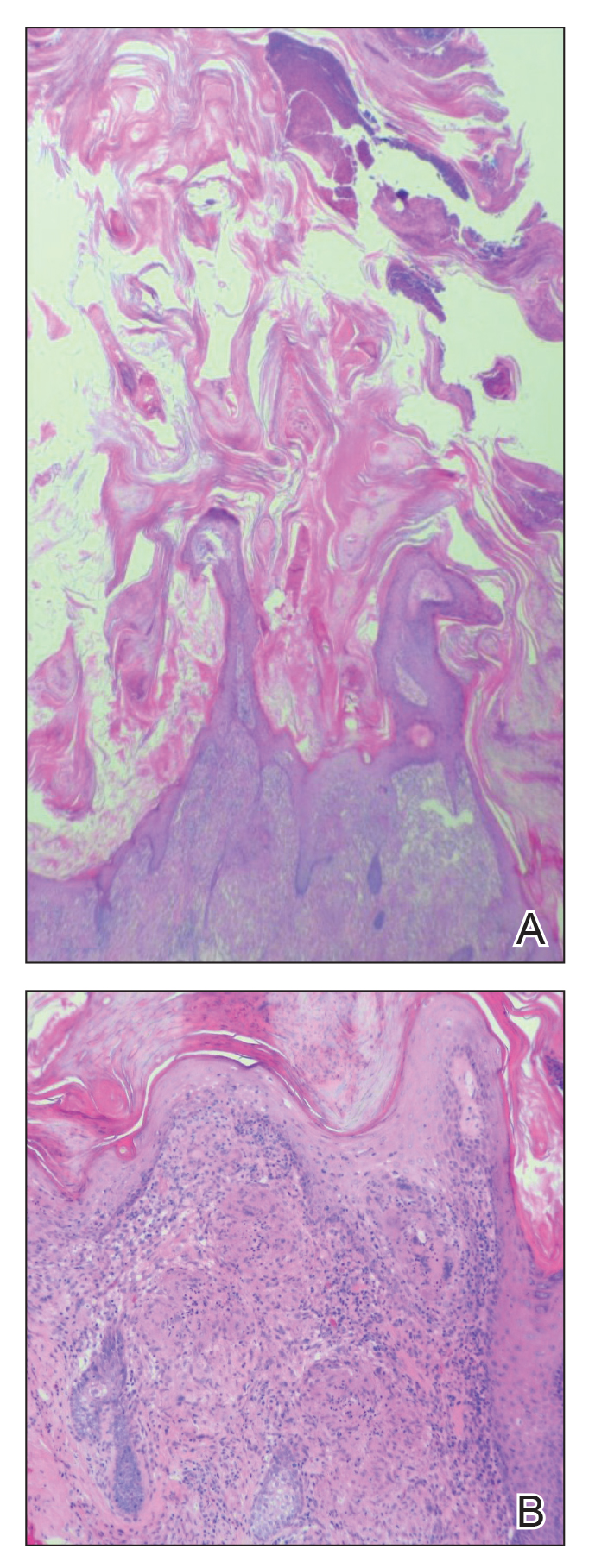
Cutaneous horn is a clinical term used to describe hyperkeratotic horn-shaped growths of highly variable shapes and sizes. Although the pathogenesis and incidence of cutaneous horns remain unknown, these lesions most often are the result of a neoplastic rather than an inflammatory process. The differential diagnosis typically includes entities characterized by marked hyperkeratosis, including hypertrophic actinic keratosis, squamous cell carcinoma (SCC), seborrheic keratosis, and verruca vulgaris. The base of the horn must be biopsied to determine the underlying etiology, paying careful attention to avoid a superficial biopsy, as it may be nondiagnostic.
Studies analyzing the underlying diagnoses and clinical features of cutaneous horns are limited. In a large retrospective study of 643 cutaneous horns, 61% were benign, 23% were premalignant, and 16% were malignant. In this study, 4 features were associated with premalignant or malignant pathology: (1) older age (mid- 60s to 70s); (2) male sex; (3) location on the nose, pinnae, dorsal hands, scalp, forearms, or face; and (4) a wide base (4.4 mm or larger) and a lower height-to-base ratio than benign lesions.1 Two additional studies of more than 200 horns each showed higher rates of premalignant horns (42% and 38%, respectively) with malignancy found in 7% and 20% of horns, respectively.2,3 One prospective study sought to identify clinical and dermatoscopic features of SCCs underlying cutaneous horns, concluding that SCC diagnosis was more likely if a horn had (1) a height less than the diameter of its base, (2) a lack of terrace morphology (a dermatoscopic feature defined as horizontal parallel layers of keratin), (3) erythema at the base, and (4) the presence of pain.4
Our patient had a cutaneous horn on the pinna that was painful, wider than it was tall, and erythematous at the base, suggesting a malignant process; however, a complete cutaneous physical examination revealed other skin lesions that were concerning for sarcoidosis and raised suspicion that the horn also was a manifestation of the same inflammatory process.
Although unusual, cutaneous sarcoidosis presenting as a cutaneous horn is not unexpected. In a histopathologic study of 62 cases of cutaneous sarcoidosis, 79% (49/62) showed epidermal changes and 13% (8/62) demonstrated hyperkeratosis. Other epidermal changes included parakeratosis (16% [10/62]), acanthosis (10% [6/62]), and epidermal atrophy (57% [35/62]).5 The spectrum of epidermal pathology in cutaneous sarcoidosis is evident in its well-documented verrucous, psoriasiform, and ichthyosiform presentations. For completeness, cutaneous horn is added to the list of clinical morphologies for this “great imitator” of cutaneous diseases.
- Yu RC, Pryce DW, Macfarlane AW, et al. A histopathological study of 643 cutaneous horns. Br J Dermatol. 1991;124:449-452.
- Schosser RH, Hodge SJ, Gaba CR, et al. Cutaneous horns: a histopathologic study. South Med J. 1979;72:1129-1131.
- Mantese SA, Diogo PM, Rocha A, et al. Cutaneous horn: a retrospective histopathological study of 222 cases. An Bras Dermatol. 2010;85:157-163.
- Pyne J, Sapkota D, Wong JC. Cutaneous horns: clues to invasive squamous cell carcinoma being present in the horn base. Dermatol Pract Concept. 2013;3:3-7.
- Hiroyuki O. Epidermal changes in cutaneous lesions of sarcoidosis. Am J Dermatopathol. 1999;21:229-233.
To the Editor:
A 53-year-old woman presented to our dermatology clinic with a painful growth on the right ear of 2 months’ duration. A complete review of systems was negative except for an isolated episode of shortness of breath prior to presentation that resolved without intervention. During this episode, her primary care physician made a diagnosis of chronic obstructive pulmonary disease based on a chest radiograph. The patient reported minimal tobacco use, specifically that she had smoked a few cigarettes daily for several years but had quit 6 months prior to the current presentation.
Cutaneous horn is a clinical term used to describe hyperkeratotic horn-shaped growths of highly variable shapes and sizes. Although the pathogenesis and incidence of cutaneous horns remain unknown, these lesions most often are the result of a neoplastic rather than an inflammatory process. The differential diagnosis typically includes entities characterized by marked hyperkeratosis, including hypertrophic actinic keratosis, squamous cell carcinoma (SCC), seborrheic keratosis, and verruca vulgaris. The base of the horn must be biopsied to determine the underlying etiology, paying careful attention to avoid a superficial biopsy, as it may be nondiagnostic.
Studies analyzing the underlying diagnoses and clinical features of cutaneous horns are limited. In a large retrospective study of 643 cutaneous horns, 61% were benign, 23% were premalignant, and 16% were malignant. In this study, 4 features were associated with premalignant or malignant pathology: (1) older age (mid- 60s to 70s); (2) male sex; (3) location on the nose, pinnae, dorsal hands, scalp, forearms, or face; and (4) a wide base (4.4 mm or larger) and a lower height-to-base ratio than benign lesions.1 Two additional studies of more than 200 horns each showed higher rates of premalignant horns (42% and 38%, respectively) with malignancy found in 7% and 20% of horns, respectively.2,3 One prospective study sought to identify clinical and dermatoscopic features of SCCs underlying cutaneous horns, concluding that SCC diagnosis was more likely if a horn had (1) a height less than the diameter of its base, (2) a lack of terrace morphology (a dermatoscopic feature defined as horizontal parallel layers of keratin), (3) erythema at the base, and (4) the presence of pain.4
Our patient had a cutaneous horn on the pinna that was painful, wider than it was tall, and erythematous at the base, suggesting a malignant process; however, a complete cutaneous physical examination revealed other skin lesions that were concerning for sarcoidosis and raised suspicion that the horn also was a manifestation of the same inflammatory process.
Although unusual, cutaneous sarcoidosis presenting as a cutaneous horn is not unexpected. In a histopathologic study of 62 cases of cutaneous sarcoidosis, 79% (49/62) showed epidermal changes and 13% (8/62) demonstrated hyperkeratosis. Other epidermal changes included parakeratosis (16% [10/62]), acanthosis (10% [6/62]), and epidermal atrophy (57% [35/62]).5 The spectrum of epidermal pathology in cutaneous sarcoidosis is evident in its well-documented verrucous, psoriasiform, and ichthyosiform presentations. For completeness, cutaneous horn is added to the list of clinical morphologies for this “great imitator” of cutaneous diseases.
To the Editor:
A 53-year-old woman presented to our dermatology clinic with a painful growth on the right ear of 2 months’ duration. A complete review of systems was negative except for an isolated episode of shortness of breath prior to presentation that resolved without intervention. During this episode, her primary care physician made a diagnosis of chronic obstructive pulmonary disease based on a chest radiograph. The patient reported minimal tobacco use, specifically that she had smoked a few cigarettes daily for several years but had quit 6 months prior to the current presentation.
Cutaneous horn is a clinical term used to describe hyperkeratotic horn-shaped growths of highly variable shapes and sizes. Although the pathogenesis and incidence of cutaneous horns remain unknown, these lesions most often are the result of a neoplastic rather than an inflammatory process. The differential diagnosis typically includes entities characterized by marked hyperkeratosis, including hypertrophic actinic keratosis, squamous cell carcinoma (SCC), seborrheic keratosis, and verruca vulgaris. The base of the horn must be biopsied to determine the underlying etiology, paying careful attention to avoid a superficial biopsy, as it may be nondiagnostic.
Studies analyzing the underlying diagnoses and clinical features of cutaneous horns are limited. In a large retrospective study of 643 cutaneous horns, 61% were benign, 23% were premalignant, and 16% were malignant. In this study, 4 features were associated with premalignant or malignant pathology: (1) older age (mid- 60s to 70s); (2) male sex; (3) location on the nose, pinnae, dorsal hands, scalp, forearms, or face; and (4) a wide base (4.4 mm or larger) and a lower height-to-base ratio than benign lesions.1 Two additional studies of more than 200 horns each showed higher rates of premalignant horns (42% and 38%, respectively) with malignancy found in 7% and 20% of horns, respectively.2,3 One prospective study sought to identify clinical and dermatoscopic features of SCCs underlying cutaneous horns, concluding that SCC diagnosis was more likely if a horn had (1) a height less than the diameter of its base, (2) a lack of terrace morphology (a dermatoscopic feature defined as horizontal parallel layers of keratin), (3) erythema at the base, and (4) the presence of pain.4
Our patient had a cutaneous horn on the pinna that was painful, wider than it was tall, and erythematous at the base, suggesting a malignant process; however, a complete cutaneous physical examination revealed other skin lesions that were concerning for sarcoidosis and raised suspicion that the horn also was a manifestation of the same inflammatory process.
Although unusual, cutaneous sarcoidosis presenting as a cutaneous horn is not unexpected. In a histopathologic study of 62 cases of cutaneous sarcoidosis, 79% (49/62) showed epidermal changes and 13% (8/62) demonstrated hyperkeratosis. Other epidermal changes included parakeratosis (16% [10/62]), acanthosis (10% [6/62]), and epidermal atrophy (57% [35/62]).5 The spectrum of epidermal pathology in cutaneous sarcoidosis is evident in its well-documented verrucous, psoriasiform, and ichthyosiform presentations. For completeness, cutaneous horn is added to the list of clinical morphologies for this “great imitator” of cutaneous diseases.
- Yu RC, Pryce DW, Macfarlane AW, et al. A histopathological study of 643 cutaneous horns. Br J Dermatol. 1991;124:449-452.
- Schosser RH, Hodge SJ, Gaba CR, et al. Cutaneous horns: a histopathologic study. South Med J. 1979;72:1129-1131.
- Mantese SA, Diogo PM, Rocha A, et al. Cutaneous horn: a retrospective histopathological study of 222 cases. An Bras Dermatol. 2010;85:157-163.
- Pyne J, Sapkota D, Wong JC. Cutaneous horns: clues to invasive squamous cell carcinoma being present in the horn base. Dermatol Pract Concept. 2013;3:3-7.
- Hiroyuki O. Epidermal changes in cutaneous lesions of sarcoidosis. Am J Dermatopathol. 1999;21:229-233.
- Yu RC, Pryce DW, Macfarlane AW, et al. A histopathological study of 643 cutaneous horns. Br J Dermatol. 1991;124:449-452.
- Schosser RH, Hodge SJ, Gaba CR, et al. Cutaneous horns: a histopathologic study. South Med J. 1979;72:1129-1131.
- Mantese SA, Diogo PM, Rocha A, et al. Cutaneous horn: a retrospective histopathological study of 222 cases. An Bras Dermatol. 2010;85:157-163.
- Pyne J, Sapkota D, Wong JC. Cutaneous horns: clues to invasive squamous cell carcinoma being present in the horn base. Dermatol Pract Concept. 2013;3:3-7.
- Hiroyuki O. Epidermal changes in cutaneous lesions of sarcoidosis. Am J Dermatopathol. 1999;21:229-233.
Practice Points
- Biopsy of a cutaneous horn should be deep enough to capture the neoplastic or inflammatory process at the base of the lesion.
- Cutaneous sarcoidosis can present with variable morphologies including the epidermal changes of a cutaneous horn.
New study confirms rise in U.S. suicide rates, particularly in rural areas
County-by-county analysis cites links to higher density of gun shops, other factors
Suicide rates in the United States climbed from 1999 to 2016, a new cross-sectional study found, and the increases were highest in rural areas.
“These findings are consistent with previous studies demonstrating higher and more rapidly increasing suicide rates in rural areas and are of considerable interest in light of the work by [Anne] Case and [Angus] Deaton,” wrote Danielle L. Steelesmith, PhD, and associates. “While increasing rates of suicide are well documented, little is known about contextual factors associated with county-level suicide rates.” The findings appear in JAMA Network Open.
To examine those contextual factors, Dr. Steelesmith, of the department of psychiatry and behavioral health at the Ohio State University, Columbus, and associates analyzed county-by-county suicide statistics from 1999 to 2016 for adults aged 25-64 years, noting that they “focused on this age range because most studies on mortality trends have focused on this age range.”
The researchers developed 3-year suicide averages for counties for rate “stabilization” purposes. They placed the counties into four categories (large metropolitan, small metropolitan, micropolitan, and rural), and used various data sources to gather various types of statistics about the communities.
Most of those who died by suicide were men (77%), and most (51%) were aged 45-64 years. The median suicide rate per county rose from 15 per 100,000 (1999-2001) to 21 per 100,000 (2014-2016), reported Dr. Steelesmith and associates.
Rural counties only made up 2% of the suicides, compared with 81% in large and small metropolitan counties, but suicide rates were “increasing most rapidly in rural areas, although all county types saw increases during the period studied,” Dr. Steelesmith and associates wrote.
They added that “counties with the highest excess risk of suicide tended to be in Western states (e.g., Colorado, New Mexico, Utah, and Wyoming), Appalachia (e.g., Kentucky, Virginia, and West Virginia), and the Ozarks (e.g., Arkansas and Missouri).”
In addition to the connections between increasing suicide rates, living in a rural area, and a higher density of gun shops, the researchers cited other contextual factors. Among those factors were higher median age and higher percentages of non-Hispanic whites, numbers of residents without health insurance, and veterans. They also found links between higher suicide rates and worse numbers on indexes designed to measure social capital; social fragmentation; and deprivation, a measure encompassing lower education, employment levels, and income.
“Long-term and persistent poverty appears to be more entrenched and economic opportunities more constrained in rural areas,” Dr. Steelesmith and associates wrote. “Greater social isolation, challenges related to transportation and interpersonal communication, and associated difficulties accessing health and mental health services likely contribute to the disproportionate association of deprivation with suicide in rural counties.”
Dr. Steelesmith and associates cited several limitations. One key limitation is that, because the study looked only at adults aged 25-64 years, the results might not be generalizable to youth or elderly adults.
No study funding was reported. One study author reported serving on the scientific advisory board of Clarigent Health and receiving grant support from the National Institute of Mental Health outside of the submitted work. No other disclosures were reported.
SOURCE: Steelesmith DL et al. JAMA Netw Open. 2019 Sep 6. doi: 10.1001/jamanetworkopen.2019.10936.
County-by-county analysis cites links to higher density of gun shops, other factors
County-by-county analysis cites links to higher density of gun shops, other factors
Suicide rates in the United States climbed from 1999 to 2016, a new cross-sectional study found, and the increases were highest in rural areas.
“These findings are consistent with previous studies demonstrating higher and more rapidly increasing suicide rates in rural areas and are of considerable interest in light of the work by [Anne] Case and [Angus] Deaton,” wrote Danielle L. Steelesmith, PhD, and associates. “While increasing rates of suicide are well documented, little is known about contextual factors associated with county-level suicide rates.” The findings appear in JAMA Network Open.
To examine those contextual factors, Dr. Steelesmith, of the department of psychiatry and behavioral health at the Ohio State University, Columbus, and associates analyzed county-by-county suicide statistics from 1999 to 2016 for adults aged 25-64 years, noting that they “focused on this age range because most studies on mortality trends have focused on this age range.”
The researchers developed 3-year suicide averages for counties for rate “stabilization” purposes. They placed the counties into four categories (large metropolitan, small metropolitan, micropolitan, and rural), and used various data sources to gather various types of statistics about the communities.
Most of those who died by suicide were men (77%), and most (51%) were aged 45-64 years. The median suicide rate per county rose from 15 per 100,000 (1999-2001) to 21 per 100,000 (2014-2016), reported Dr. Steelesmith and associates.
Rural counties only made up 2% of the suicides, compared with 81% in large and small metropolitan counties, but suicide rates were “increasing most rapidly in rural areas, although all county types saw increases during the period studied,” Dr. Steelesmith and associates wrote.
They added that “counties with the highest excess risk of suicide tended to be in Western states (e.g., Colorado, New Mexico, Utah, and Wyoming), Appalachia (e.g., Kentucky, Virginia, and West Virginia), and the Ozarks (e.g., Arkansas and Missouri).”
In addition to the connections between increasing suicide rates, living in a rural area, and a higher density of gun shops, the researchers cited other contextual factors. Among those factors were higher median age and higher percentages of non-Hispanic whites, numbers of residents without health insurance, and veterans. They also found links between higher suicide rates and worse numbers on indexes designed to measure social capital; social fragmentation; and deprivation, a measure encompassing lower education, employment levels, and income.
“Long-term and persistent poverty appears to be more entrenched and economic opportunities more constrained in rural areas,” Dr. Steelesmith and associates wrote. “Greater social isolation, challenges related to transportation and interpersonal communication, and associated difficulties accessing health and mental health services likely contribute to the disproportionate association of deprivation with suicide in rural counties.”
Dr. Steelesmith and associates cited several limitations. One key limitation is that, because the study looked only at adults aged 25-64 years, the results might not be generalizable to youth or elderly adults.
No study funding was reported. One study author reported serving on the scientific advisory board of Clarigent Health and receiving grant support from the National Institute of Mental Health outside of the submitted work. No other disclosures were reported.
SOURCE: Steelesmith DL et al. JAMA Netw Open. 2019 Sep 6. doi: 10.1001/jamanetworkopen.2019.10936.
Suicide rates in the United States climbed from 1999 to 2016, a new cross-sectional study found, and the increases were highest in rural areas.
“These findings are consistent with previous studies demonstrating higher and more rapidly increasing suicide rates in rural areas and are of considerable interest in light of the work by [Anne] Case and [Angus] Deaton,” wrote Danielle L. Steelesmith, PhD, and associates. “While increasing rates of suicide are well documented, little is known about contextual factors associated with county-level suicide rates.” The findings appear in JAMA Network Open.
To examine those contextual factors, Dr. Steelesmith, of the department of psychiatry and behavioral health at the Ohio State University, Columbus, and associates analyzed county-by-county suicide statistics from 1999 to 2016 for adults aged 25-64 years, noting that they “focused on this age range because most studies on mortality trends have focused on this age range.”
The researchers developed 3-year suicide averages for counties for rate “stabilization” purposes. They placed the counties into four categories (large metropolitan, small metropolitan, micropolitan, and rural), and used various data sources to gather various types of statistics about the communities.
Most of those who died by suicide were men (77%), and most (51%) were aged 45-64 years. The median suicide rate per county rose from 15 per 100,000 (1999-2001) to 21 per 100,000 (2014-2016), reported Dr. Steelesmith and associates.
Rural counties only made up 2% of the suicides, compared with 81% in large and small metropolitan counties, but suicide rates were “increasing most rapidly in rural areas, although all county types saw increases during the period studied,” Dr. Steelesmith and associates wrote.
They added that “counties with the highest excess risk of suicide tended to be in Western states (e.g., Colorado, New Mexico, Utah, and Wyoming), Appalachia (e.g., Kentucky, Virginia, and West Virginia), and the Ozarks (e.g., Arkansas and Missouri).”
In addition to the connections between increasing suicide rates, living in a rural area, and a higher density of gun shops, the researchers cited other contextual factors. Among those factors were higher median age and higher percentages of non-Hispanic whites, numbers of residents without health insurance, and veterans. They also found links between higher suicide rates and worse numbers on indexes designed to measure social capital; social fragmentation; and deprivation, a measure encompassing lower education, employment levels, and income.
“Long-term and persistent poverty appears to be more entrenched and economic opportunities more constrained in rural areas,” Dr. Steelesmith and associates wrote. “Greater social isolation, challenges related to transportation and interpersonal communication, and associated difficulties accessing health and mental health services likely contribute to the disproportionate association of deprivation with suicide in rural counties.”
Dr. Steelesmith and associates cited several limitations. One key limitation is that, because the study looked only at adults aged 25-64 years, the results might not be generalizable to youth or elderly adults.
No study funding was reported. One study author reported serving on the scientific advisory board of Clarigent Health and receiving grant support from the National Institute of Mental Health outside of the submitted work. No other disclosures were reported.
SOURCE: Steelesmith DL et al. JAMA Netw Open. 2019 Sep 6. doi: 10.1001/jamanetworkopen.2019.10936.
FROM JAMA NETWORK OPEN
Cancer Survivorship Clinic Utilizing an NP-led Model With Oncology Fellows
Background: Cancer survivors face unique posttreatment issues and require ongoing follow-up care. Per Commission on Cancer (CoC) and other cancer organizations, a survivorship care plan including a treatment summary and follow-up plan is standard of care. There are significant barriers to implementation of survivorship care plans due to the resources required. Our facility lacked a process to implement survivorship care plans. A need to expand clinical experiences for oncology fellows across the care continuum was also identified.
Methods: Researched existing private sector and VA models of providing cancer survivorship care. Analyzed literature regarding the unique care needs of veteran cancer survivors. NP led model was determined to support a holistic clinical care model including post treatment assessment, education, resources, and referrals.
Intervention: The Cancer Survivorship Clinic was implemented in August 2018, staffed by an oncology nurse practitioner and medical oncology fellows. Visits are face-to-face or by phone and one hour in length. Patients receive a survivorship care plan. The clinic provider addresses post-treatment health concerns and refers patients to other services when indicated. The clinic was created utilizing existing staffing and clinic space, no additional resources were needed.
Results: There were 30 Cancer Survivorship Clinic visits completed for veterans between 8/1/18 and 5/1/19. The clinic is part of an ongoing rotation for fellows and included in their annual orientation. Implementation of a Cancer Survivorship Clinic was effective in meeting the CoC Survivorship Care Plan standard. Oncology fellow rotation in the clinic has broadened their educational experience. Upon entering practice, fellows will be better equipped to address survivorship needs.
Discussion: The clinic was created utilizing existing medical oncology resources and staffing. Medical oncology is familiar with all cancer diagnoses and can therefore serve the entire cancer population. An NP-led model supports a holistic care approach, ensuring that physical and mental/emotional needs are addressed during the clinic visit. Oncology fellows receive an opportunity to care for patients following cancer treatment, expanding their understanding of cancer care. One drawback is the lack of fellow availability at certain times of the year.
Background: Cancer survivors face unique posttreatment issues and require ongoing follow-up care. Per Commission on Cancer (CoC) and other cancer organizations, a survivorship care plan including a treatment summary and follow-up plan is standard of care. There are significant barriers to implementation of survivorship care plans due to the resources required. Our facility lacked a process to implement survivorship care plans. A need to expand clinical experiences for oncology fellows across the care continuum was also identified.
Methods: Researched existing private sector and VA models of providing cancer survivorship care. Analyzed literature regarding the unique care needs of veteran cancer survivors. NP led model was determined to support a holistic clinical care model including post treatment assessment, education, resources, and referrals.
Intervention: The Cancer Survivorship Clinic was implemented in August 2018, staffed by an oncology nurse practitioner and medical oncology fellows. Visits are face-to-face or by phone and one hour in length. Patients receive a survivorship care plan. The clinic provider addresses post-treatment health concerns and refers patients to other services when indicated. The clinic was created utilizing existing staffing and clinic space, no additional resources were needed.
Results: There were 30 Cancer Survivorship Clinic visits completed for veterans between 8/1/18 and 5/1/19. The clinic is part of an ongoing rotation for fellows and included in their annual orientation. Implementation of a Cancer Survivorship Clinic was effective in meeting the CoC Survivorship Care Plan standard. Oncology fellow rotation in the clinic has broadened their educational experience. Upon entering practice, fellows will be better equipped to address survivorship needs.
Discussion: The clinic was created utilizing existing medical oncology resources and staffing. Medical oncology is familiar with all cancer diagnoses and can therefore serve the entire cancer population. An NP-led model supports a holistic care approach, ensuring that physical and mental/emotional needs are addressed during the clinic visit. Oncology fellows receive an opportunity to care for patients following cancer treatment, expanding their understanding of cancer care. One drawback is the lack of fellow availability at certain times of the year.
Background: Cancer survivors face unique posttreatment issues and require ongoing follow-up care. Per Commission on Cancer (CoC) and other cancer organizations, a survivorship care plan including a treatment summary and follow-up plan is standard of care. There are significant barriers to implementation of survivorship care plans due to the resources required. Our facility lacked a process to implement survivorship care plans. A need to expand clinical experiences for oncology fellows across the care continuum was also identified.
Methods: Researched existing private sector and VA models of providing cancer survivorship care. Analyzed literature regarding the unique care needs of veteran cancer survivors. NP led model was determined to support a holistic clinical care model including post treatment assessment, education, resources, and referrals.
Intervention: The Cancer Survivorship Clinic was implemented in August 2018, staffed by an oncology nurse practitioner and medical oncology fellows. Visits are face-to-face or by phone and one hour in length. Patients receive a survivorship care plan. The clinic provider addresses post-treatment health concerns and refers patients to other services when indicated. The clinic was created utilizing existing staffing and clinic space, no additional resources were needed.
Results: There were 30 Cancer Survivorship Clinic visits completed for veterans between 8/1/18 and 5/1/19. The clinic is part of an ongoing rotation for fellows and included in their annual orientation. Implementation of a Cancer Survivorship Clinic was effective in meeting the CoC Survivorship Care Plan standard. Oncology fellow rotation in the clinic has broadened their educational experience. Upon entering practice, fellows will be better equipped to address survivorship needs.
Discussion: The clinic was created utilizing existing medical oncology resources and staffing. Medical oncology is familiar with all cancer diagnoses and can therefore serve the entire cancer population. An NP-led model supports a holistic care approach, ensuring that physical and mental/emotional needs are addressed during the clinic visit. Oncology fellows receive an opportunity to care for patients following cancer treatment, expanding their understanding of cancer care. One drawback is the lack of fellow availability at certain times of the year.