User login
For MD-IQ use only
Prolonged Antibiotic Treatment in Newborns May Promote Multidrug Resistance
Antibiotics given to preterm infants can set them up for health problems later in life; research has shown, including allergies, psoriasis, diabetes, and inflammatory bowel disease. Researchers who conducted a National Institutes of Health (NIH)-funded study have added to that body of knowledge with their finding that treating preterm infants with long-term antibiotics could have lasting effects by promoting multidrug-resistant gut bacteria.
They used high-speed DNA sequencing and advanced computational analysis to study stool samples from 32 infants born very preterm who received antibiotic treatment for 21 months in the hospital and after discharge, then compared those with results from 9 very preterm infants treated with antibiotics for > 1 week and 17 healthy term and late-term infants who had not received antibiotics.
The infants on long-term antibiotics had less diverse bacterial populations in their gut, and those bacteria contained more antibiotic-resistant genes.
Strikingly, the genomes of the high-antibiotic-use samples contained genes for resistance to antibiotics typically not given to newborns, such as ciprofloxacin and chloramphenicol. The researchers say this may mean that the genes originate in multidrug-resistant bacteria. Using a particular antibiotic may trigger resistance to other antibiotics even if they were not used.
“The collateral damage of early-life antibiotic treatment and hospitalization in preterm infants is long lasting,” the researchers say. They urge development of strategies to protect these highly vulnerable patients.
Antibiotics given to preterm infants can set them up for health problems later in life; research has shown, including allergies, psoriasis, diabetes, and inflammatory bowel disease. Researchers who conducted a National Institutes of Health (NIH)-funded study have added to that body of knowledge with their finding that treating preterm infants with long-term antibiotics could have lasting effects by promoting multidrug-resistant gut bacteria.
They used high-speed DNA sequencing and advanced computational analysis to study stool samples from 32 infants born very preterm who received antibiotic treatment for 21 months in the hospital and after discharge, then compared those with results from 9 very preterm infants treated with antibiotics for > 1 week and 17 healthy term and late-term infants who had not received antibiotics.
The infants on long-term antibiotics had less diverse bacterial populations in their gut, and those bacteria contained more antibiotic-resistant genes.
Strikingly, the genomes of the high-antibiotic-use samples contained genes for resistance to antibiotics typically not given to newborns, such as ciprofloxacin and chloramphenicol. The researchers say this may mean that the genes originate in multidrug-resistant bacteria. Using a particular antibiotic may trigger resistance to other antibiotics even if they were not used.
“The collateral damage of early-life antibiotic treatment and hospitalization in preterm infants is long lasting,” the researchers say. They urge development of strategies to protect these highly vulnerable patients.
Antibiotics given to preterm infants can set them up for health problems later in life; research has shown, including allergies, psoriasis, diabetes, and inflammatory bowel disease. Researchers who conducted a National Institutes of Health (NIH)-funded study have added to that body of knowledge with their finding that treating preterm infants with long-term antibiotics could have lasting effects by promoting multidrug-resistant gut bacteria.
They used high-speed DNA sequencing and advanced computational analysis to study stool samples from 32 infants born very preterm who received antibiotic treatment for 21 months in the hospital and after discharge, then compared those with results from 9 very preterm infants treated with antibiotics for > 1 week and 17 healthy term and late-term infants who had not received antibiotics.
The infants on long-term antibiotics had less diverse bacterial populations in their gut, and those bacteria contained more antibiotic-resistant genes.
Strikingly, the genomes of the high-antibiotic-use samples contained genes for resistance to antibiotics typically not given to newborns, such as ciprofloxacin and chloramphenicol. The researchers say this may mean that the genes originate in multidrug-resistant bacteria. Using a particular antibiotic may trigger resistance to other antibiotics even if they were not used.
“The collateral damage of early-life antibiotic treatment and hospitalization in preterm infants is long lasting,” the researchers say. They urge development of strategies to protect these highly vulnerable patients.
Milrinone beats dobutamine in extending survival in HF
PHILADELPHIA – Milrinone was associated with a 50% reduction in mortality, compared with dobutamine, in patients with heart failure who were receiving home inotropes as a bridge to advanced therapies, according to results of a large U.S. registry study.
The survival profile for milrinone was more favorable than that for dobutamine across various indications for use, including bridge to transplant, bridge to mechanical support, and palliation.
The findings illustrate an “urgent need” for randomized trials that compare inotropic therapy strategies in patients with end-stage heart failure, said investigator Behram P. Mody, MD, of the division of cardiology at the University of California, San Diego, La Jolla.
“Although [there is] the possibility of selection bias and there are confounding variables, this consistent finding may reflect a true benefit of milrinone,” Dr. Mody said in a presentation at the annual scientific meeting of the Heart Failure Society of America
The evidence to support use of continuous outpatient intravenous inotropic therapy in stage D heart failure is limited and comes mainly from case series, and small, randomized trials demonstrating poor outcomes, Dr. Mody explained.
“Long-term survival associated with home inotropes in the modern era of guideline-directed medical and device therapy is poorly defined,” he said.
The retrospective study Dr. Mody presented at the meeting included 1,149 patients (mean age, 60 years; 29.9% women) who received continuous intravenous outpatient inotropic therapy (milrinone or dobutamine) from 2015 to 2017. This is possibly the largest body of data reported to date of patients receiving home inotropes, he said.
For the overall population, the estimated 1-year survival rate was 71% with milrinone, compared with 46% for dobutamine (P less than .0001), Dr. Mody reported. After adjusting for age, gender, weight, and indication, milrinone use was still significantly associated with lower mortality, compared with dobutamine (hazard ratio, 0.50; 95% confidence interval, 0.39-0.64; P less than .0001).
The survival benefit of milrinone over dobutamine was also significant in subsets of patients receiving home inotropes as bridge to transplant, bridge to mechanical support, or palliation. A similar trend, though not statistically significant, was seen for inotropes used as a bridge to decision, he added.
Mortality was generally higher in patients receiving inotropes as palliation, as opposed to bridge therapy, with 1-year survival of 55.0% and 65.6%, respectively, and median survival of 461 days and 584 days.
These findings suggest home inotropes can be “beneficial” in patients with end-stage heart failure in an era of guideline-directed medical therapy and greater defibrillator use, Dr. Mody said.
“I’m not saying that it should replace left ventricular assist devices as the bridge to transplant,” he added, “but it could be an alternative strategy for our patients.”
No funding source was given. Dr. Mody had no disclosures related to the study.
SOURCE: Mody BP et al. J Card Fail. 2019 Sep 14. doi: 10.1016/j.cardfail.2019.07.021.
PHILADELPHIA – Milrinone was associated with a 50% reduction in mortality, compared with dobutamine, in patients with heart failure who were receiving home inotropes as a bridge to advanced therapies, according to results of a large U.S. registry study.
The survival profile for milrinone was more favorable than that for dobutamine across various indications for use, including bridge to transplant, bridge to mechanical support, and palliation.
The findings illustrate an “urgent need” for randomized trials that compare inotropic therapy strategies in patients with end-stage heart failure, said investigator Behram P. Mody, MD, of the division of cardiology at the University of California, San Diego, La Jolla.
“Although [there is] the possibility of selection bias and there are confounding variables, this consistent finding may reflect a true benefit of milrinone,” Dr. Mody said in a presentation at the annual scientific meeting of the Heart Failure Society of America
The evidence to support use of continuous outpatient intravenous inotropic therapy in stage D heart failure is limited and comes mainly from case series, and small, randomized trials demonstrating poor outcomes, Dr. Mody explained.
“Long-term survival associated with home inotropes in the modern era of guideline-directed medical and device therapy is poorly defined,” he said.
The retrospective study Dr. Mody presented at the meeting included 1,149 patients (mean age, 60 years; 29.9% women) who received continuous intravenous outpatient inotropic therapy (milrinone or dobutamine) from 2015 to 2017. This is possibly the largest body of data reported to date of patients receiving home inotropes, he said.
For the overall population, the estimated 1-year survival rate was 71% with milrinone, compared with 46% for dobutamine (P less than .0001), Dr. Mody reported. After adjusting for age, gender, weight, and indication, milrinone use was still significantly associated with lower mortality, compared with dobutamine (hazard ratio, 0.50; 95% confidence interval, 0.39-0.64; P less than .0001).
The survival benefit of milrinone over dobutamine was also significant in subsets of patients receiving home inotropes as bridge to transplant, bridge to mechanical support, or palliation. A similar trend, though not statistically significant, was seen for inotropes used as a bridge to decision, he added.
Mortality was generally higher in patients receiving inotropes as palliation, as opposed to bridge therapy, with 1-year survival of 55.0% and 65.6%, respectively, and median survival of 461 days and 584 days.
These findings suggest home inotropes can be “beneficial” in patients with end-stage heart failure in an era of guideline-directed medical therapy and greater defibrillator use, Dr. Mody said.
“I’m not saying that it should replace left ventricular assist devices as the bridge to transplant,” he added, “but it could be an alternative strategy for our patients.”
No funding source was given. Dr. Mody had no disclosures related to the study.
SOURCE: Mody BP et al. J Card Fail. 2019 Sep 14. doi: 10.1016/j.cardfail.2019.07.021.
PHILADELPHIA – Milrinone was associated with a 50% reduction in mortality, compared with dobutamine, in patients with heart failure who were receiving home inotropes as a bridge to advanced therapies, according to results of a large U.S. registry study.
The survival profile for milrinone was more favorable than that for dobutamine across various indications for use, including bridge to transplant, bridge to mechanical support, and palliation.
The findings illustrate an “urgent need” for randomized trials that compare inotropic therapy strategies in patients with end-stage heart failure, said investigator Behram P. Mody, MD, of the division of cardiology at the University of California, San Diego, La Jolla.
“Although [there is] the possibility of selection bias and there are confounding variables, this consistent finding may reflect a true benefit of milrinone,” Dr. Mody said in a presentation at the annual scientific meeting of the Heart Failure Society of America
The evidence to support use of continuous outpatient intravenous inotropic therapy in stage D heart failure is limited and comes mainly from case series, and small, randomized trials demonstrating poor outcomes, Dr. Mody explained.
“Long-term survival associated with home inotropes in the modern era of guideline-directed medical and device therapy is poorly defined,” he said.
The retrospective study Dr. Mody presented at the meeting included 1,149 patients (mean age, 60 years; 29.9% women) who received continuous intravenous outpatient inotropic therapy (milrinone or dobutamine) from 2015 to 2017. This is possibly the largest body of data reported to date of patients receiving home inotropes, he said.
For the overall population, the estimated 1-year survival rate was 71% with milrinone, compared with 46% for dobutamine (P less than .0001), Dr. Mody reported. After adjusting for age, gender, weight, and indication, milrinone use was still significantly associated with lower mortality, compared with dobutamine (hazard ratio, 0.50; 95% confidence interval, 0.39-0.64; P less than .0001).
The survival benefit of milrinone over dobutamine was also significant in subsets of patients receiving home inotropes as bridge to transplant, bridge to mechanical support, or palliation. A similar trend, though not statistically significant, was seen for inotropes used as a bridge to decision, he added.
Mortality was generally higher in patients receiving inotropes as palliation, as opposed to bridge therapy, with 1-year survival of 55.0% and 65.6%, respectively, and median survival of 461 days and 584 days.
These findings suggest home inotropes can be “beneficial” in patients with end-stage heart failure in an era of guideline-directed medical therapy and greater defibrillator use, Dr. Mody said.
“I’m not saying that it should replace left ventricular assist devices as the bridge to transplant,” he added, “but it could be an alternative strategy for our patients.”
No funding source was given. Dr. Mody had no disclosures related to the study.
SOURCE: Mody BP et al. J Card Fail. 2019 Sep 14. doi: 10.1016/j.cardfail.2019.07.021.
REPORTING FROM HFSA 2019
Rates of off-label prescribing for children continue to increase
Physicians continue to prescribe off-label drugs for children, with rates increasing over a 10-year period from 2006 to 2015, according to findings from a new study.
The increase occurred despite recent legislation aimed at encouraging pediatric clinical trials, with the intention of improving the “quality of evidence and the number of drugs approved for children,” Divya Hoon of Rutgers University in New Brunswick, N.J., and colleagues wrote in Pediatrics.
“[Our] results can help inform ongoing education, research, and policies around efficacious, effective, and safe use of medications in children,” the researchers said.
To determine trends in, and categories of, drugs prescribed off label, the researchers used data from the National Ambulatory Medical Care Surveys for all pediatric visits and subsequent drug orders from 2006 to 2015. They focused on 141 drugs that are predominantly or exclusively used in systemic formulations and that had been ordered at least 30 times.
At least one off-label systemic drug order occurred at 18.5% of the 1.74 billion estimated ambulatory pediatric visits (95% confidence interval, 17.7%-19.3%), totaling 41.2 million off-label orders per year. The primary reason for a drug being considered off label was that it was for an unapproved condition (74.6%), followed by patient age (17.6%) and weight (0.6%). Absolute and relative rates of off-label ordering increased throughout the study, especially in regard to antihistamines and psychotropic drugs, the investigators said.
In an accompanying editorial, Katelyn Yackey, MD, of the University of Kentucky Children’s Hospital, Lexington, and Rachel Stanley, MD, of Nationwide Children’s Hospital, Columbus, Ohio, stated that “off label is not synonymous with off evidence” and emphasized the need for more clinical trials of medications for children (Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1571).
“Although drugs are often used off label, there may be sufficient preliminary research about a medical condition and particular drugs to support their use,” they wrote. While recognizing that evaluating medications in pediatric patients has been challenging, they added that “children continue to receive medications off label and for unapproved conditions,” so studies that evaluate “safety, efficacy, pharmacokinetics, and optimal dosing in pediatric patients” remain a necessity.
Though the research featured a long study period and large study population, the authors recognized its possible limitations, including the exclusion of less commonly ordered drugs, the inability to determine drug formulation or dosage, and the fact that the survey data captured only ordered medicines and not whether they were actually dispensed or consumed.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship. The authors reported no conflicts of interest.
SOURCE: Hoon D et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-0896.
Physicians continue to prescribe off-label drugs for children, with rates increasing over a 10-year period from 2006 to 2015, according to findings from a new study.
The increase occurred despite recent legislation aimed at encouraging pediatric clinical trials, with the intention of improving the “quality of evidence and the number of drugs approved for children,” Divya Hoon of Rutgers University in New Brunswick, N.J., and colleagues wrote in Pediatrics.
“[Our] results can help inform ongoing education, research, and policies around efficacious, effective, and safe use of medications in children,” the researchers said.
To determine trends in, and categories of, drugs prescribed off label, the researchers used data from the National Ambulatory Medical Care Surveys for all pediatric visits and subsequent drug orders from 2006 to 2015. They focused on 141 drugs that are predominantly or exclusively used in systemic formulations and that had been ordered at least 30 times.
At least one off-label systemic drug order occurred at 18.5% of the 1.74 billion estimated ambulatory pediatric visits (95% confidence interval, 17.7%-19.3%), totaling 41.2 million off-label orders per year. The primary reason for a drug being considered off label was that it was for an unapproved condition (74.6%), followed by patient age (17.6%) and weight (0.6%). Absolute and relative rates of off-label ordering increased throughout the study, especially in regard to antihistamines and psychotropic drugs, the investigators said.
In an accompanying editorial, Katelyn Yackey, MD, of the University of Kentucky Children’s Hospital, Lexington, and Rachel Stanley, MD, of Nationwide Children’s Hospital, Columbus, Ohio, stated that “off label is not synonymous with off evidence” and emphasized the need for more clinical trials of medications for children (Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1571).
“Although drugs are often used off label, there may be sufficient preliminary research about a medical condition and particular drugs to support their use,” they wrote. While recognizing that evaluating medications in pediatric patients has been challenging, they added that “children continue to receive medications off label and for unapproved conditions,” so studies that evaluate “safety, efficacy, pharmacokinetics, and optimal dosing in pediatric patients” remain a necessity.
Though the research featured a long study period and large study population, the authors recognized its possible limitations, including the exclusion of less commonly ordered drugs, the inability to determine drug formulation or dosage, and the fact that the survey data captured only ordered medicines and not whether they were actually dispensed or consumed.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship. The authors reported no conflicts of interest.
SOURCE: Hoon D et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-0896.
Physicians continue to prescribe off-label drugs for children, with rates increasing over a 10-year period from 2006 to 2015, according to findings from a new study.
The increase occurred despite recent legislation aimed at encouraging pediatric clinical trials, with the intention of improving the “quality of evidence and the number of drugs approved for children,” Divya Hoon of Rutgers University in New Brunswick, N.J., and colleagues wrote in Pediatrics.
“[Our] results can help inform ongoing education, research, and policies around efficacious, effective, and safe use of medications in children,” the researchers said.
To determine trends in, and categories of, drugs prescribed off label, the researchers used data from the National Ambulatory Medical Care Surveys for all pediatric visits and subsequent drug orders from 2006 to 2015. They focused on 141 drugs that are predominantly or exclusively used in systemic formulations and that had been ordered at least 30 times.
At least one off-label systemic drug order occurred at 18.5% of the 1.74 billion estimated ambulatory pediatric visits (95% confidence interval, 17.7%-19.3%), totaling 41.2 million off-label orders per year. The primary reason for a drug being considered off label was that it was for an unapproved condition (74.6%), followed by patient age (17.6%) and weight (0.6%). Absolute and relative rates of off-label ordering increased throughout the study, especially in regard to antihistamines and psychotropic drugs, the investigators said.
In an accompanying editorial, Katelyn Yackey, MD, of the University of Kentucky Children’s Hospital, Lexington, and Rachel Stanley, MD, of Nationwide Children’s Hospital, Columbus, Ohio, stated that “off label is not synonymous with off evidence” and emphasized the need for more clinical trials of medications for children (Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-1571).
“Although drugs are often used off label, there may be sufficient preliminary research about a medical condition and particular drugs to support their use,” they wrote. While recognizing that evaluating medications in pediatric patients has been challenging, they added that “children continue to receive medications off label and for unapproved conditions,” so studies that evaluate “safety, efficacy, pharmacokinetics, and optimal dosing in pediatric patients” remain a necessity.
Though the research featured a long study period and large study population, the authors recognized its possible limitations, including the exclusion of less commonly ordered drugs, the inability to determine drug formulation or dosage, and the fact that the survey data captured only ordered medicines and not whether they were actually dispensed or consumed.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship. The authors reported no conflicts of interest.
SOURCE: Hoon D et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-0896.
FROM PEDIATRICS
Key clinical point:
Major finding: At least one off-label systemic drug order occurred at 18.5% of the 1.74 billion estimated ambulatory pediatric visits (95% CI, 17.7%-19.3%).
Study details: A retrospective study of serial, cross-sectional data from the National Ambulatory Medical Care Surveys (2006-2015).
Disclosures: The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rutgers Robert Wood Johnson Medical School Summer Research Fellowship. The authors reported no conflicts of interest.
Source: Hoon D et al. Pediatrics. 2019 Sep 16. doi: 10.1542/peds.2019-0896.
Severe Pretibial Myxedema Refractory to Systemic Immunosuppressants
To the Editor:
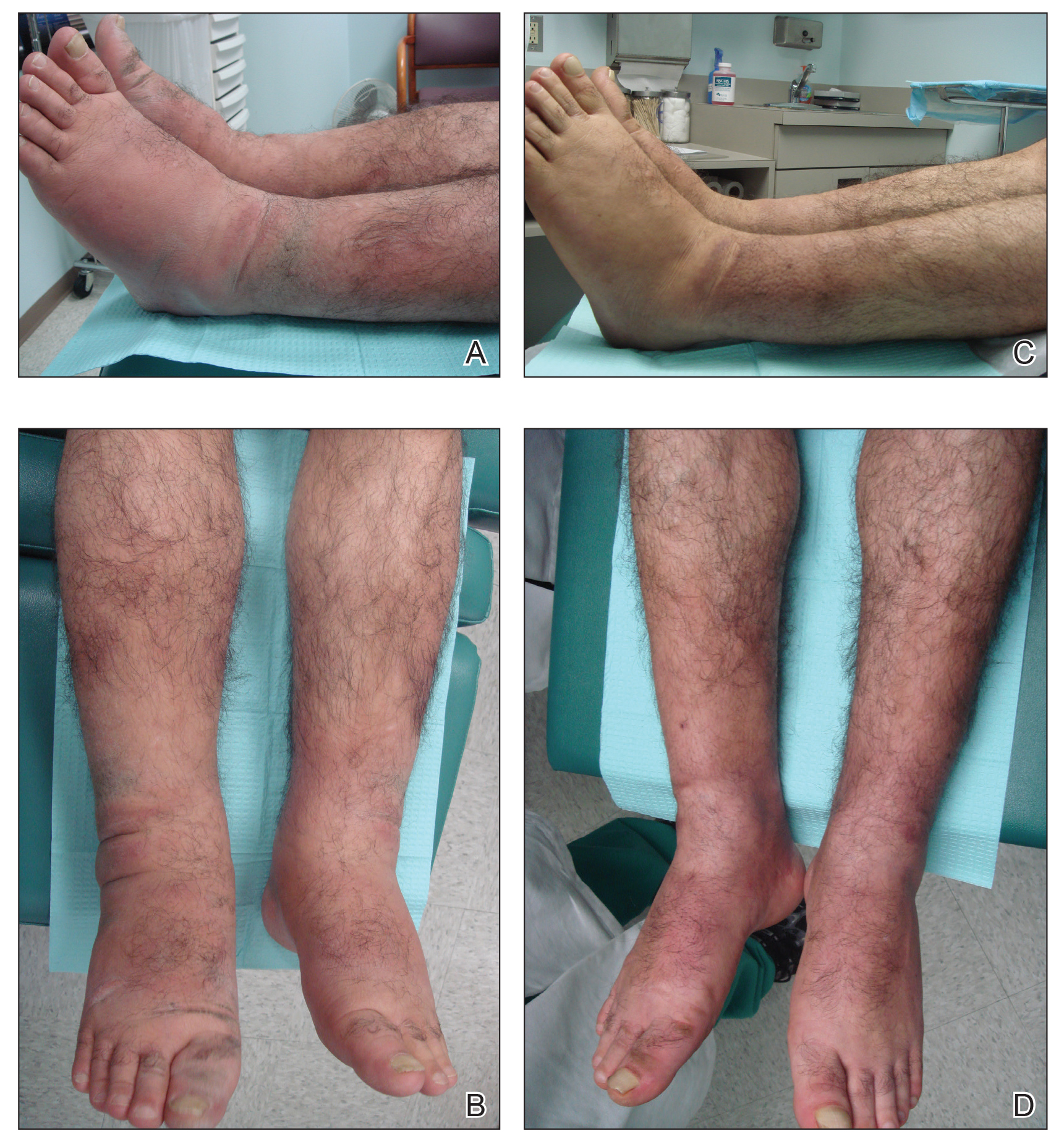
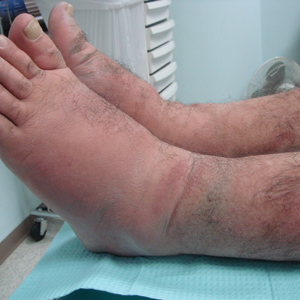
A 55-year-old man with a history of Graves disease treated with radioactive iodine and Graves ophthalmopathy was referred to our dermatology clinic by his endocrinologist with a 2-year history of severe pretibial myxedema (PM) that had failed treatment with systemic immunosuppressants after diagnosis by an outside dermatologist in the United Kingdom approximately 2 years prior. In addition to burning pain and difficulty walking associated with progressive “enlarging” of the lower legs and feet (Figure, A and B), the patient reported that he consistently had to find larger shoes (size 13 at the current presentation). His medications included gabapentin for foot pain and levothyroxine for hypothyroidism.
Physical examination revealed diffuse, waxy, indurated, flesh-colored and erythematous plaques and nodules with a peau d’orange appearance on the dorsal feet, ankles, and lower legs. Laboratory evaluation revealed a thyroid stimulating immunoglobulin level of 617% (reference range, <140%) and mild anemia. His thyroid stimulating hormone and free T4 levels, a comprehensive metabolic panel, and lipid panel were all within reference range.
Treatment with oral, intravenous, and intralesional steroids; cyclosporine; and azathioprine were tried prior to presentation to our clinic with no improvement. The patient was started on pentoxifylline (400 mg 3 times daily), intralesional triamcinolone acetonide (5 mg/mL every 3–4 weeks), clobetasol propionate ointment 0.05% under occlusion twice daily, short-stretch bandages, and compression stockings (20–30 mm Hg). The baseline circumference of the extremities also were measured (right ankle, 12 in; left ankle, 11.5 in; right and left mid-plantar feet, 12 in).
At 3-week follow-up, the lesions had flattened with softening of the skin. The patient reported his legs were smaller and he had bought a new pair of shoes at size 8.5 (Figure, C). He noted less pain and difficulty with walking. The circumference of the extremities was measured again (right ankle, 10.2 in; left ankle, 10 in; right and left mid-plantar feet, 10.5 in). The patient continued treatment and was followed for 3 months. At each visit, clinical improvement was noted as well as report of decreased pain while walking (Figure, D).
Pretibial myxedema is a known manifestation of Graves disease that almost always occurs in the presence of Graves ophthalmopathy. Pretibial myxedema occurs in 0.5% to 4.3% of patients with Graves disease and variably manifests as diffuse nonpitting edema or localized, waxy, indurated plaques or nodules.1,2
The proposed pathogenesis of PM is that autoantibodies directed against the thyroid receptors cross-react with the fibroblasts of the skin,2,3 which stimulates the fibroblasts to produce high amounts of glycosaminoglycans, especially hyaluronic acid, in the dermis and subcutis of the pretibial area. It is not known why there is a predilection for the anterior shins, but mechanical factors and dependent position (ie, leg position is lower than the level of the heart) may be involved.4
The mainstay of treatment for PM is topical and intralesional corticosteroids, which may have a benefit in mild to moderate disease; however, in cases of severe disease that is refractory to intralesional and topical corticosteroids under occlusion, more aggressive treatment is required. Systemic immunosuppressants such as cyclosporine, azathioprine, and corticosteroids have proven useful in some but not all cases.5,6
Our patient did not respond to treatment with systemic and intralesional corticosteroids, cyclosporine, or azathioprine before he presented to our clinic; however, the lesions were dramatically improved after 3 weeks of treatment with pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression stockings.
Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves ophthalmology and PM.7 It has been shown to reduce thickness of skin lesions when used in combination with topical or intralesional steroids.3,8 Corticosteroids are thought to block fibroblast-mediated glycosaminoglycan production.3,9 The deposition of mucin, which is comprised of glycosaminoglycans, expands the dermal tissue and causes fluid to accumulate; it also causes compression of dermal lymphatics, worsening the dermal edema. Because fluid accumulates, the use of short-stretch bandages and compression stockings may provide additional benefit, as was seen in our patient, whose shoe size decreased from a 13 to an 8.5 within 3 weeks of treatment.
In conclusion, the combination of pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression garments can cause substantial improvement in severe PM refractory to systemic immunosuppressants.
- Susser WS, Heermans AG, Chapman MS, et al. Elephantiasic pretibial myxedema: a novel treatment for an uncommon disorder. J Am Acad Dermatol. 2002;46:723-726.
- Kriss J. Pathogenesis and treatment of pretibial myxedema. Endocrinol Metab Clin North Am. 1987;16:409-415.
- Pineda AM, Tianco EA, Tan JB, et al. Oral pentoxifylline and topical clobetasol propionate ointment in the treatment of pretibial myxoedema, with concomitant improvement of Graves’ ophthalmopathy. J Eur Acad Dermatol Venereol. 2007; 21:1441-1443.
- Fatourechi V. Pretibial myxedema. Am J Clin Dermatol. 2005;6:295-309.
- Benoit FL, Greenspan FS. Corticoid therapy for pretibial myxedema: observations on the long-acting thyroid stimulator. Ann Intern Med. 1967;66:711-720.
- Hanke CW, Bergfeld WF, Guirguis MN, et al. Pretibial myxedema (elephantiasis form): treatment with cytotoxic therapy. Cleve Clin Q. 1983;50:183-188.
- Chang CC, Chang TC, Kao SC, et al. Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves’ ophthalmopathy and pretibial myxoedema. Acta Endocrinol (Copenh). 1993;129:322-327.
- Engin B, Gümüs¸el M, Ozdemir M, et al. Successful combined pentoxifylline and intralesional triamcinolone acetonide treatment of severe pretibial myxedema. Dermatol Online J. 2007;13:16.
- Lang PG, Sisson JC, Lynch PJ. Intralesional triamcinolone therapy for pretibial myxedema. Arch Dermatol. 1975;111:197-202.
To the Editor:
A 55-year-old man with a history of Graves disease treated with radioactive iodine and Graves ophthalmopathy was referred to our dermatology clinic by his endocrinologist with a 2-year history of severe pretibial myxedema (PM) that had failed treatment with systemic immunosuppressants after diagnosis by an outside dermatologist in the United Kingdom approximately 2 years prior. In addition to burning pain and difficulty walking associated with progressive “enlarging” of the lower legs and feet (Figure, A and B), the patient reported that he consistently had to find larger shoes (size 13 at the current presentation). His medications included gabapentin for foot pain and levothyroxine for hypothyroidism.
Physical examination revealed diffuse, waxy, indurated, flesh-colored and erythematous plaques and nodules with a peau d’orange appearance on the dorsal feet, ankles, and lower legs. Laboratory evaluation revealed a thyroid stimulating immunoglobulin level of 617% (reference range, <140%) and mild anemia. His thyroid stimulating hormone and free T4 levels, a comprehensive metabolic panel, and lipid panel were all within reference range.
Treatment with oral, intravenous, and intralesional steroids; cyclosporine; and azathioprine were tried prior to presentation to our clinic with no improvement. The patient was started on pentoxifylline (400 mg 3 times daily), intralesional triamcinolone acetonide (5 mg/mL every 3–4 weeks), clobetasol propionate ointment 0.05% under occlusion twice daily, short-stretch bandages, and compression stockings (20–30 mm Hg). The baseline circumference of the extremities also were measured (right ankle, 12 in; left ankle, 11.5 in; right and left mid-plantar feet, 12 in).
At 3-week follow-up, the lesions had flattened with softening of the skin. The patient reported his legs were smaller and he had bought a new pair of shoes at size 8.5 (Figure, C). He noted less pain and difficulty with walking. The circumference of the extremities was measured again (right ankle, 10.2 in; left ankle, 10 in; right and left mid-plantar feet, 10.5 in). The patient continued treatment and was followed for 3 months. At each visit, clinical improvement was noted as well as report of decreased pain while walking (Figure, D).
Pretibial myxedema is a known manifestation of Graves disease that almost always occurs in the presence of Graves ophthalmopathy. Pretibial myxedema occurs in 0.5% to 4.3% of patients with Graves disease and variably manifests as diffuse nonpitting edema or localized, waxy, indurated plaques or nodules.1,2
The proposed pathogenesis of PM is that autoantibodies directed against the thyroid receptors cross-react with the fibroblasts of the skin,2,3 which stimulates the fibroblasts to produce high amounts of glycosaminoglycans, especially hyaluronic acid, in the dermis and subcutis of the pretibial area. It is not known why there is a predilection for the anterior shins, but mechanical factors and dependent position (ie, leg position is lower than the level of the heart) may be involved.4
The mainstay of treatment for PM is topical and intralesional corticosteroids, which may have a benefit in mild to moderate disease; however, in cases of severe disease that is refractory to intralesional and topical corticosteroids under occlusion, more aggressive treatment is required. Systemic immunosuppressants such as cyclosporine, azathioprine, and corticosteroids have proven useful in some but not all cases.5,6
Our patient did not respond to treatment with systemic and intralesional corticosteroids, cyclosporine, or azathioprine before he presented to our clinic; however, the lesions were dramatically improved after 3 weeks of treatment with pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression stockings.
Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves ophthalmology and PM.7 It has been shown to reduce thickness of skin lesions when used in combination with topical or intralesional steroids.3,8 Corticosteroids are thought to block fibroblast-mediated glycosaminoglycan production.3,9 The deposition of mucin, which is comprised of glycosaminoglycans, expands the dermal tissue and causes fluid to accumulate; it also causes compression of dermal lymphatics, worsening the dermal edema. Because fluid accumulates, the use of short-stretch bandages and compression stockings may provide additional benefit, as was seen in our patient, whose shoe size decreased from a 13 to an 8.5 within 3 weeks of treatment.
In conclusion, the combination of pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression garments can cause substantial improvement in severe PM refractory to systemic immunosuppressants.
To the Editor:
A 55-year-old man with a history of Graves disease treated with radioactive iodine and Graves ophthalmopathy was referred to our dermatology clinic by his endocrinologist with a 2-year history of severe pretibial myxedema (PM) that had failed treatment with systemic immunosuppressants after diagnosis by an outside dermatologist in the United Kingdom approximately 2 years prior. In addition to burning pain and difficulty walking associated with progressive “enlarging” of the lower legs and feet (Figure, A and B), the patient reported that he consistently had to find larger shoes (size 13 at the current presentation). His medications included gabapentin for foot pain and levothyroxine for hypothyroidism.
Physical examination revealed diffuse, waxy, indurated, flesh-colored and erythematous plaques and nodules with a peau d’orange appearance on the dorsal feet, ankles, and lower legs. Laboratory evaluation revealed a thyroid stimulating immunoglobulin level of 617% (reference range, <140%) and mild anemia. His thyroid stimulating hormone and free T4 levels, a comprehensive metabolic panel, and lipid panel were all within reference range.
Treatment with oral, intravenous, and intralesional steroids; cyclosporine; and azathioprine were tried prior to presentation to our clinic with no improvement. The patient was started on pentoxifylline (400 mg 3 times daily), intralesional triamcinolone acetonide (5 mg/mL every 3–4 weeks), clobetasol propionate ointment 0.05% under occlusion twice daily, short-stretch bandages, and compression stockings (20–30 mm Hg). The baseline circumference of the extremities also were measured (right ankle, 12 in; left ankle, 11.5 in; right and left mid-plantar feet, 12 in).
At 3-week follow-up, the lesions had flattened with softening of the skin. The patient reported his legs were smaller and he had bought a new pair of shoes at size 8.5 (Figure, C). He noted less pain and difficulty with walking. The circumference of the extremities was measured again (right ankle, 10.2 in; left ankle, 10 in; right and left mid-plantar feet, 10.5 in). The patient continued treatment and was followed for 3 months. At each visit, clinical improvement was noted as well as report of decreased pain while walking (Figure, D).
Pretibial myxedema is a known manifestation of Graves disease that almost always occurs in the presence of Graves ophthalmopathy. Pretibial myxedema occurs in 0.5% to 4.3% of patients with Graves disease and variably manifests as diffuse nonpitting edema or localized, waxy, indurated plaques or nodules.1,2
The proposed pathogenesis of PM is that autoantibodies directed against the thyroid receptors cross-react with the fibroblasts of the skin,2,3 which stimulates the fibroblasts to produce high amounts of glycosaminoglycans, especially hyaluronic acid, in the dermis and subcutis of the pretibial area. It is not known why there is a predilection for the anterior shins, but mechanical factors and dependent position (ie, leg position is lower than the level of the heart) may be involved.4
The mainstay of treatment for PM is topical and intralesional corticosteroids, which may have a benefit in mild to moderate disease; however, in cases of severe disease that is refractory to intralesional and topical corticosteroids under occlusion, more aggressive treatment is required. Systemic immunosuppressants such as cyclosporine, azathioprine, and corticosteroids have proven useful in some but not all cases.5,6
Our patient did not respond to treatment with systemic and intralesional corticosteroids, cyclosporine, or azathioprine before he presented to our clinic; however, the lesions were dramatically improved after 3 weeks of treatment with pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression stockings.
Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves ophthalmology and PM.7 It has been shown to reduce thickness of skin lesions when used in combination with topical or intralesional steroids.3,8 Corticosteroids are thought to block fibroblast-mediated glycosaminoglycan production.3,9 The deposition of mucin, which is comprised of glycosaminoglycans, expands the dermal tissue and causes fluid to accumulate; it also causes compression of dermal lymphatics, worsening the dermal edema. Because fluid accumulates, the use of short-stretch bandages and compression stockings may provide additional benefit, as was seen in our patient, whose shoe size decreased from a 13 to an 8.5 within 3 weeks of treatment.
In conclusion, the combination of pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression garments can cause substantial improvement in severe PM refractory to systemic immunosuppressants.
- Susser WS, Heermans AG, Chapman MS, et al. Elephantiasic pretibial myxedema: a novel treatment for an uncommon disorder. J Am Acad Dermatol. 2002;46:723-726.
- Kriss J. Pathogenesis and treatment of pretibial myxedema. Endocrinol Metab Clin North Am. 1987;16:409-415.
- Pineda AM, Tianco EA, Tan JB, et al. Oral pentoxifylline and topical clobetasol propionate ointment in the treatment of pretibial myxoedema, with concomitant improvement of Graves’ ophthalmopathy. J Eur Acad Dermatol Venereol. 2007; 21:1441-1443.
- Fatourechi V. Pretibial myxedema. Am J Clin Dermatol. 2005;6:295-309.
- Benoit FL, Greenspan FS. Corticoid therapy for pretibial myxedema: observations on the long-acting thyroid stimulator. Ann Intern Med. 1967;66:711-720.
- Hanke CW, Bergfeld WF, Guirguis MN, et al. Pretibial myxedema (elephantiasis form): treatment with cytotoxic therapy. Cleve Clin Q. 1983;50:183-188.
- Chang CC, Chang TC, Kao SC, et al. Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves’ ophthalmopathy and pretibial myxoedema. Acta Endocrinol (Copenh). 1993;129:322-327.
- Engin B, Gümüs¸el M, Ozdemir M, et al. Successful combined pentoxifylline and intralesional triamcinolone acetonide treatment of severe pretibial myxedema. Dermatol Online J. 2007;13:16.
- Lang PG, Sisson JC, Lynch PJ. Intralesional triamcinolone therapy for pretibial myxedema. Arch Dermatol. 1975;111:197-202.
- Susser WS, Heermans AG, Chapman MS, et al. Elephantiasic pretibial myxedema: a novel treatment for an uncommon disorder. J Am Acad Dermatol. 2002;46:723-726.
- Kriss J. Pathogenesis and treatment of pretibial myxedema. Endocrinol Metab Clin North Am. 1987;16:409-415.
- Pineda AM, Tianco EA, Tan JB, et al. Oral pentoxifylline and topical clobetasol propionate ointment in the treatment of pretibial myxoedema, with concomitant improvement of Graves’ ophthalmopathy. J Eur Acad Dermatol Venereol. 2007; 21:1441-1443.
- Fatourechi V. Pretibial myxedema. Am J Clin Dermatol. 2005;6:295-309.
- Benoit FL, Greenspan FS. Corticoid therapy for pretibial myxedema: observations on the long-acting thyroid stimulator. Ann Intern Med. 1967;66:711-720.
- Hanke CW, Bergfeld WF, Guirguis MN, et al. Pretibial myxedema (elephantiasis form): treatment with cytotoxic therapy. Cleve Clin Q. 1983;50:183-188.
- Chang CC, Chang TC, Kao SC, et al. Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves’ ophthalmopathy and pretibial myxoedema. Acta Endocrinol (Copenh). 1993;129:322-327.
- Engin B, Gümüs¸el M, Ozdemir M, et al. Successful combined pentoxifylline and intralesional triamcinolone acetonide treatment of severe pretibial myxedema. Dermatol Online J. 2007;13:16.
- Lang PG, Sisson JC, Lynch PJ. Intralesional triamcinolone therapy for pretibial myxedema. Arch Dermatol. 1975;111:197-202.
Practice Points
- Pretibial myxedema (PM) is a known manifestation of Graves disease that almost always occurs in the presence of Graves ophthalmopathy.
- The proposed pathogenesis of PM is cross-reaction of autoantibodies directed against the thyroid receptors with the fibroblasts of the skin. It is not known why there is a predilection for the anterior shins, but mechanical factors and dependent position may be involved.
- The mainstay of treatment for PM is topical and intralesional corticosteroids, which may have a benefit in mild to moderate disease; however, in cases of severe disease that is refractory to intralesional and topical corticosteroids under occlusion, more aggressive treatment is required.
Cancer Care Coordination Perspectives of Veteran Patients with Cancer
Background: Coordinating cancer care can prove especially challenging because of the complexity of both the disease and its treatment. Typically, the care of cancer patients involves multiple types of therapies and providers (surgery, radiation therapy, chemotherapy) and ongoing treatment of cancer and other health conditions. Cancer care coordination depends upon effective, regular communication among physicians, support staff, and the patient. The objective of this study is to explore veteran patients’ experiences of cancer care coordination in order to develop a deeper understanding of the issues veterans consider important and actionable.
Methods: Semi-structured interviews were conducted among patients diagnosed with cancer types and stages that commonly receive inter-disciplinary care (eg, colorectal [stage II and up], prostate [high risk], head and neck [all stages], and lung [all stages] cancer) were eligible to participate in the study. The constant comparison technique was used to identify emerging themes.
Results: Twenty-five veteran cancer patients participated in this study. Our analysis identified four general categories representing different parts of the trajectory of the patient cancer care experience in need of coordination. Each phase had a distinct set of needs and challenges. Diagnosis: After receiving a diagnosis of cancer, Veterans reported initial feelings of shock and fear. Pre-Treatment: This phase was characterized as busy and chaotic, consisting of many appointments in different locations, and ultimately left many veterans feeling a lack of control. Onset of Treatment: Uncertainty about what to expect when receiving advanced treatments, like radiation and chemotherapy, was often exacerbated by poor provider-provider communication issues. Ongoing Treatment: As treatment progressed, many veterans experienced a growing familiarity with their routine, and simultaneously, began to regain a sense of control. Care Team: Nurse navigators were considered vital to cancer care coordination. Veterans valued honesty and transparency from their providers which built a sense of trust.
Conclusion: Veterans expressed a need to have care coordination needs and steps more explicitly addressed early, as well as active mechanisms to meet those needs. The patient experience of coordination gradually improved over the course of the care trajectory, often facilitated by relationships fostered with members of the healthcare team.
Background: Coordinating cancer care can prove especially challenging because of the complexity of both the disease and its treatment. Typically, the care of cancer patients involves multiple types of therapies and providers (surgery, radiation therapy, chemotherapy) and ongoing treatment of cancer and other health conditions. Cancer care coordination depends upon effective, regular communication among physicians, support staff, and the patient. The objective of this study is to explore veteran patients’ experiences of cancer care coordination in order to develop a deeper understanding of the issues veterans consider important and actionable.
Methods: Semi-structured interviews were conducted among patients diagnosed with cancer types and stages that commonly receive inter-disciplinary care (eg, colorectal [stage II and up], prostate [high risk], head and neck [all stages], and lung [all stages] cancer) were eligible to participate in the study. The constant comparison technique was used to identify emerging themes.
Results: Twenty-five veteran cancer patients participated in this study. Our analysis identified four general categories representing different parts of the trajectory of the patient cancer care experience in need of coordination. Each phase had a distinct set of needs and challenges. Diagnosis: After receiving a diagnosis of cancer, Veterans reported initial feelings of shock and fear. Pre-Treatment: This phase was characterized as busy and chaotic, consisting of many appointments in different locations, and ultimately left many veterans feeling a lack of control. Onset of Treatment: Uncertainty about what to expect when receiving advanced treatments, like radiation and chemotherapy, was often exacerbated by poor provider-provider communication issues. Ongoing Treatment: As treatment progressed, many veterans experienced a growing familiarity with their routine, and simultaneously, began to regain a sense of control. Care Team: Nurse navigators were considered vital to cancer care coordination. Veterans valued honesty and transparency from their providers which built a sense of trust.
Conclusion: Veterans expressed a need to have care coordination needs and steps more explicitly addressed early, as well as active mechanisms to meet those needs. The patient experience of coordination gradually improved over the course of the care trajectory, often facilitated by relationships fostered with members of the healthcare team.
Background: Coordinating cancer care can prove especially challenging because of the complexity of both the disease and its treatment. Typically, the care of cancer patients involves multiple types of therapies and providers (surgery, radiation therapy, chemotherapy) and ongoing treatment of cancer and other health conditions. Cancer care coordination depends upon effective, regular communication among physicians, support staff, and the patient. The objective of this study is to explore veteran patients’ experiences of cancer care coordination in order to develop a deeper understanding of the issues veterans consider important and actionable.
Methods: Semi-structured interviews were conducted among patients diagnosed with cancer types and stages that commonly receive inter-disciplinary care (eg, colorectal [stage II and up], prostate [high risk], head and neck [all stages], and lung [all stages] cancer) were eligible to participate in the study. The constant comparison technique was used to identify emerging themes.
Results: Twenty-five veteran cancer patients participated in this study. Our analysis identified four general categories representing different parts of the trajectory of the patient cancer care experience in need of coordination. Each phase had a distinct set of needs and challenges. Diagnosis: After receiving a diagnosis of cancer, Veterans reported initial feelings of shock and fear. Pre-Treatment: This phase was characterized as busy and chaotic, consisting of many appointments in different locations, and ultimately left many veterans feeling a lack of control. Onset of Treatment: Uncertainty about what to expect when receiving advanced treatments, like radiation and chemotherapy, was often exacerbated by poor provider-provider communication issues. Ongoing Treatment: As treatment progressed, many veterans experienced a growing familiarity with their routine, and simultaneously, began to regain a sense of control. Care Team: Nurse navigators were considered vital to cancer care coordination. Veterans valued honesty and transparency from their providers which built a sense of trust.
Conclusion: Veterans expressed a need to have care coordination needs and steps more explicitly addressed early, as well as active mechanisms to meet those needs. The patient experience of coordination gradually improved over the course of the care trajectory, often facilitated by relationships fostered with members of the healthcare team.
Decreasing Chemotherapy Administration Wait Time for Veterans with Cancer: A Minneapolis VA Medical Center Quality Improvement Project
Background: Cancer diagnosis is a devastating and painful process for patients and their families, resulting in significant stress and uncertainty. Chemotherapy treatments may provide a cure, control or palliate symptoms caused by cancer. However, delivery of chemotherapy in outpatient clinics is challenging and timeconsuming. Long wait is a common patient complaint, affects work-flow and chair time usage, increases costs, and compromises safety. We sought to review our system and implement changes that may result in decrease wait time.
Methods: Utilizing the Institute for Healthcare Improvement (IHI) model, the plan-do-study-act (PDSA) method to test and implement changes, we established a preintervention Ishikawa diagram over a 4 weeks period to study the process and determine cause of delay. Changes were implemented in a stepwise fashion, assess for improvement and revised our process over another 4 week period. We collect and analyze data for a total of 2 months. The objective of the study was to decrease wait-time to initiate chemotherapy treatment from the end of clinic visit to start of chemotherapy infusion by 20-30 minutes for 50% of patients in the oncology clinic over a three-month period.
Results: Pre-intervention data was collected on 245 patients. 55% (n=136) of these patients waited an average of 90 minutes and 45% (n=110) waited an average of 42 minutes from check-in to infusion clinic to start of chemotherapy. Identified barriers causing delayed chemotherapy administration included no consent, no prior authorization, unwritten/unsigned orders, pharmacy release delay, incomplete required labs, delay drug delivery from pharmacy, and difficulty with IV access. After the first cycle of PDSA, post-intervention data reveal a small improvement. 52% (n=199) patients waited an average of 87 minutes and 48% (n=183) average wait was 41 minutes.
Conclusions: By utilizing the PDSA cycle to test the modification of the revised workflow system and eliminate barriers to release of chemotherapy we could potentially reduce wait times for patient receiving chemotherapy at the Minneapolis VA. Updated data will be presented at the AVAHO Annual Meeting.
Background: Cancer diagnosis is a devastating and painful process for patients and their families, resulting in significant stress and uncertainty. Chemotherapy treatments may provide a cure, control or palliate symptoms caused by cancer. However, delivery of chemotherapy in outpatient clinics is challenging and timeconsuming. Long wait is a common patient complaint, affects work-flow and chair time usage, increases costs, and compromises safety. We sought to review our system and implement changes that may result in decrease wait time.
Methods: Utilizing the Institute for Healthcare Improvement (IHI) model, the plan-do-study-act (PDSA) method to test and implement changes, we established a preintervention Ishikawa diagram over a 4 weeks period to study the process and determine cause of delay. Changes were implemented in a stepwise fashion, assess for improvement and revised our process over another 4 week period. We collect and analyze data for a total of 2 months. The objective of the study was to decrease wait-time to initiate chemotherapy treatment from the end of clinic visit to start of chemotherapy infusion by 20-30 minutes for 50% of patients in the oncology clinic over a three-month period.
Results: Pre-intervention data was collected on 245 patients. 55% (n=136) of these patients waited an average of 90 minutes and 45% (n=110) waited an average of 42 minutes from check-in to infusion clinic to start of chemotherapy. Identified barriers causing delayed chemotherapy administration included no consent, no prior authorization, unwritten/unsigned orders, pharmacy release delay, incomplete required labs, delay drug delivery from pharmacy, and difficulty with IV access. After the first cycle of PDSA, post-intervention data reveal a small improvement. 52% (n=199) patients waited an average of 87 minutes and 48% (n=183) average wait was 41 minutes.
Conclusions: By utilizing the PDSA cycle to test the modification of the revised workflow system and eliminate barriers to release of chemotherapy we could potentially reduce wait times for patient receiving chemotherapy at the Minneapolis VA. Updated data will be presented at the AVAHO Annual Meeting.
Background: Cancer diagnosis is a devastating and painful process for patients and their families, resulting in significant stress and uncertainty. Chemotherapy treatments may provide a cure, control or palliate symptoms caused by cancer. However, delivery of chemotherapy in outpatient clinics is challenging and timeconsuming. Long wait is a common patient complaint, affects work-flow and chair time usage, increases costs, and compromises safety. We sought to review our system and implement changes that may result in decrease wait time.
Methods: Utilizing the Institute for Healthcare Improvement (IHI) model, the plan-do-study-act (PDSA) method to test and implement changes, we established a preintervention Ishikawa diagram over a 4 weeks period to study the process and determine cause of delay. Changes were implemented in a stepwise fashion, assess for improvement and revised our process over another 4 week period. We collect and analyze data for a total of 2 months. The objective of the study was to decrease wait-time to initiate chemotherapy treatment from the end of clinic visit to start of chemotherapy infusion by 20-30 minutes for 50% of patients in the oncology clinic over a three-month period.
Results: Pre-intervention data was collected on 245 patients. 55% (n=136) of these patients waited an average of 90 minutes and 45% (n=110) waited an average of 42 minutes from check-in to infusion clinic to start of chemotherapy. Identified barriers causing delayed chemotherapy administration included no consent, no prior authorization, unwritten/unsigned orders, pharmacy release delay, incomplete required labs, delay drug delivery from pharmacy, and difficulty with IV access. After the first cycle of PDSA, post-intervention data reveal a small improvement. 52% (n=199) patients waited an average of 87 minutes and 48% (n=183) average wait was 41 minutes.
Conclusions: By utilizing the PDSA cycle to test the modification of the revised workflow system and eliminate barriers to release of chemotherapy we could potentially reduce wait times for patient receiving chemotherapy at the Minneapolis VA. Updated data will be presented at the AVAHO Annual Meeting.
The Impact of Using Ideal Body Weight for Dosing of Intravenous Immune Globulin on Potential Grams Averted
Purpose: The primary objective of this study was to evaluate the total potential grams of intravenous immune globulin (IVIG) averted using ideal body weight (IBW) versus actual body weight (ABW) for dosing calculations. The secondary objectives assessed the indication for use of IVIG, change in serum immunoglobulin G (IgG) levels, and potential cost savings of using IBW to dose IVIG as an alternative to ABW.
Background: Dosing of IVIG in clinical studies is based on ABW. Increasing evidence suggests it is more appropriate to dose IVIG using IBW in all patients, given it primarily distributes throughout intravascular and extravascular fluid compartments. Recent studies have demonstrated benefit for the use of IBW for IVIG dosing in regard to outcomes such as grams of drug averted, guideline compliance, and changes in serum IgG levels. This study aimed to add to the body of literature supporting use of IBW for dosing of IVIG.
Methods: A retrospective chart review was conducted for patients administered IVIG therapy between May 1, 2018 and November 30, 2018. IBW was calculated per patient using the Devine equation.
Data Anaysis: Descriptive statistics were used to assess all primary and secondary objectives. This included examining medians and interquartile ranges for the primary objective as well as potential cost savings per dose.
Results: In regard to the primary objective, the total potential grams of IVIG averted was 965 grams. In terms per dose, a median of 45 grams of IVIG per patient could have been averted, with a range from 0 to 75 grams. In regard to secondary objectives, the total potential cost savings was $41,038.57. In terms per dose, a median of $252.43 per patient could have been avoided, with a range from 0 to $634.51. IVIG was most commonly being prescribed for primary humoral immunodeficiency states and chronic lymphocytic leukemia.
Implications: This data may help guide the decision to transition to utilization of IBW for IVIG dosing. In addition, it may give further insight regarding the need to create a pharmacy-to-dose protocol for IVIG.
Purpose: The primary objective of this study was to evaluate the total potential grams of intravenous immune globulin (IVIG) averted using ideal body weight (IBW) versus actual body weight (ABW) for dosing calculations. The secondary objectives assessed the indication for use of IVIG, change in serum immunoglobulin G (IgG) levels, and potential cost savings of using IBW to dose IVIG as an alternative to ABW.
Background: Dosing of IVIG in clinical studies is based on ABW. Increasing evidence suggests it is more appropriate to dose IVIG using IBW in all patients, given it primarily distributes throughout intravascular and extravascular fluid compartments. Recent studies have demonstrated benefit for the use of IBW for IVIG dosing in regard to outcomes such as grams of drug averted, guideline compliance, and changes in serum IgG levels. This study aimed to add to the body of literature supporting use of IBW for dosing of IVIG.
Methods: A retrospective chart review was conducted for patients administered IVIG therapy between May 1, 2018 and November 30, 2018. IBW was calculated per patient using the Devine equation.
Data Anaysis: Descriptive statistics were used to assess all primary and secondary objectives. This included examining medians and interquartile ranges for the primary objective as well as potential cost savings per dose.
Results: In regard to the primary objective, the total potential grams of IVIG averted was 965 grams. In terms per dose, a median of 45 grams of IVIG per patient could have been averted, with a range from 0 to 75 grams. In regard to secondary objectives, the total potential cost savings was $41,038.57. In terms per dose, a median of $252.43 per patient could have been avoided, with a range from 0 to $634.51. IVIG was most commonly being prescribed for primary humoral immunodeficiency states and chronic lymphocytic leukemia.
Implications: This data may help guide the decision to transition to utilization of IBW for IVIG dosing. In addition, it may give further insight regarding the need to create a pharmacy-to-dose protocol for IVIG.
Purpose: The primary objective of this study was to evaluate the total potential grams of intravenous immune globulin (IVIG) averted using ideal body weight (IBW) versus actual body weight (ABW) for dosing calculations. The secondary objectives assessed the indication for use of IVIG, change in serum immunoglobulin G (IgG) levels, and potential cost savings of using IBW to dose IVIG as an alternative to ABW.
Background: Dosing of IVIG in clinical studies is based on ABW. Increasing evidence suggests it is more appropriate to dose IVIG using IBW in all patients, given it primarily distributes throughout intravascular and extravascular fluid compartments. Recent studies have demonstrated benefit for the use of IBW for IVIG dosing in regard to outcomes such as grams of drug averted, guideline compliance, and changes in serum IgG levels. This study aimed to add to the body of literature supporting use of IBW for dosing of IVIG.
Methods: A retrospective chart review was conducted for patients administered IVIG therapy between May 1, 2018 and November 30, 2018. IBW was calculated per patient using the Devine equation.
Data Anaysis: Descriptive statistics were used to assess all primary and secondary objectives. This included examining medians and interquartile ranges for the primary objective as well as potential cost savings per dose.
Results: In regard to the primary objective, the total potential grams of IVIG averted was 965 grams. In terms per dose, a median of 45 grams of IVIG per patient could have been averted, with a range from 0 to 75 grams. In regard to secondary objectives, the total potential cost savings was $41,038.57. In terms per dose, a median of $252.43 per patient could have been avoided, with a range from 0 to $634.51. IVIG was most commonly being prescribed for primary humoral immunodeficiency states and chronic lymphocytic leukemia.
Implications: This data may help guide the decision to transition to utilization of IBW for IVIG dosing. In addition, it may give further insight regarding the need to create a pharmacy-to-dose protocol for IVIG.
Primary Site and Other Prognostic Factors Affecting Myxoid Liposarcoma Survivorship
Background: Myxoid liposarcomas (MLS) are intermediate to high grade liposarcomas, which are the most common type of soft tissue sarcoma. Although MLS most frequently develops in the legs, it can arise in any of the body’s soft tissue adipose. Previous studies of MLS survival have been limited in terms of geography, cohort size, variety of treatment settings, and assessment of primary site role. We retrospectively studied survival data for a nationwide cohort of MLS patients to identify prognostic factors and assess their effects on survival.
Methods: Using the National Cancer Database, we obtained data on 4636 patients diagnosed with MLS (ICD-O-3 8852) between 2004 and 2016. 5- and 10-year survival curves were estimated with Kaplan-Meier analysis and compared with log-rank analysis. Cox hazard regression was also used to compare multiple variables’ effects on survival.
Results: Approximately 59.2% of the cohort was male with a median age of presentation of 49 years. The most common site of metastasis was the bone followed by lung, liver, and brain. The majority (46.8%) of patients were stage I at time of diagnosis, followed by stage II with 18.1%, stage III with 13.1%, and stage IV at 5.3%. Overall 5- and 10-year survival probabilities for the cohort were 76.1% and 62.0%. Female patients (5-year: 79.6% and 10-year: 65.5%) had better survivals than male patients (5-year: 73.8% and 10-years: 59.6%). As stage increased, overall survival decreased with stage IV patients having 5- and 10-year survival probabilities of 21.2% and 9.4%, respectively. Patients with tumors localized to the extremities (5-year: 81.2%) had the best overall survival followed by tumors in the head or neck (5-year: 79.9%), pelvis (5-year: 77.3%), pelvis (5-year: 78.0%), thorax or trunk (5-year: 66.5%), and the retroperitoneum or abdomen (5-year: 53.1%). Finally, adjuvant radiation treatment correlated with decreased mortality to surgical resection of primary tumor alone (HR: 0.847; 95% CI: 0.726-0.989), whereas adjuvant chemotherapy correlated with increased mortality (HR: 2.769; 95% CI: 1.995-3.841).
Conclusion: Primary anatomical site was determined to be a major prognostic factor along with treatment facility type, sex, stage, and surgical margins for patients with myxoid liposarcoma.
Background: Myxoid liposarcomas (MLS) are intermediate to high grade liposarcomas, which are the most common type of soft tissue sarcoma. Although MLS most frequently develops in the legs, it can arise in any of the body’s soft tissue adipose. Previous studies of MLS survival have been limited in terms of geography, cohort size, variety of treatment settings, and assessment of primary site role. We retrospectively studied survival data for a nationwide cohort of MLS patients to identify prognostic factors and assess their effects on survival.
Methods: Using the National Cancer Database, we obtained data on 4636 patients diagnosed with MLS (ICD-O-3 8852) between 2004 and 2016. 5- and 10-year survival curves were estimated with Kaplan-Meier analysis and compared with log-rank analysis. Cox hazard regression was also used to compare multiple variables’ effects on survival.
Results: Approximately 59.2% of the cohort was male with a median age of presentation of 49 years. The most common site of metastasis was the bone followed by lung, liver, and brain. The majority (46.8%) of patients were stage I at time of diagnosis, followed by stage II with 18.1%, stage III with 13.1%, and stage IV at 5.3%. Overall 5- and 10-year survival probabilities for the cohort were 76.1% and 62.0%. Female patients (5-year: 79.6% and 10-year: 65.5%) had better survivals than male patients (5-year: 73.8% and 10-years: 59.6%). As stage increased, overall survival decreased with stage IV patients having 5- and 10-year survival probabilities of 21.2% and 9.4%, respectively. Patients with tumors localized to the extremities (5-year: 81.2%) had the best overall survival followed by tumors in the head or neck (5-year: 79.9%), pelvis (5-year: 77.3%), pelvis (5-year: 78.0%), thorax or trunk (5-year: 66.5%), and the retroperitoneum or abdomen (5-year: 53.1%). Finally, adjuvant radiation treatment correlated with decreased mortality to surgical resection of primary tumor alone (HR: 0.847; 95% CI: 0.726-0.989), whereas adjuvant chemotherapy correlated with increased mortality (HR: 2.769; 95% CI: 1.995-3.841).
Conclusion: Primary anatomical site was determined to be a major prognostic factor along with treatment facility type, sex, stage, and surgical margins for patients with myxoid liposarcoma.
Background: Myxoid liposarcomas (MLS) are intermediate to high grade liposarcomas, which are the most common type of soft tissue sarcoma. Although MLS most frequently develops in the legs, it can arise in any of the body’s soft tissue adipose. Previous studies of MLS survival have been limited in terms of geography, cohort size, variety of treatment settings, and assessment of primary site role. We retrospectively studied survival data for a nationwide cohort of MLS patients to identify prognostic factors and assess their effects on survival.
Methods: Using the National Cancer Database, we obtained data on 4636 patients diagnosed with MLS (ICD-O-3 8852) between 2004 and 2016. 5- and 10-year survival curves were estimated with Kaplan-Meier analysis and compared with log-rank analysis. Cox hazard regression was also used to compare multiple variables’ effects on survival.
Results: Approximately 59.2% of the cohort was male with a median age of presentation of 49 years. The most common site of metastasis was the bone followed by lung, liver, and brain. The majority (46.8%) of patients were stage I at time of diagnosis, followed by stage II with 18.1%, stage III with 13.1%, and stage IV at 5.3%. Overall 5- and 10-year survival probabilities for the cohort were 76.1% and 62.0%. Female patients (5-year: 79.6% and 10-year: 65.5%) had better survivals than male patients (5-year: 73.8% and 10-years: 59.6%). As stage increased, overall survival decreased with stage IV patients having 5- and 10-year survival probabilities of 21.2% and 9.4%, respectively. Patients with tumors localized to the extremities (5-year: 81.2%) had the best overall survival followed by tumors in the head or neck (5-year: 79.9%), pelvis (5-year: 77.3%), pelvis (5-year: 78.0%), thorax or trunk (5-year: 66.5%), and the retroperitoneum or abdomen (5-year: 53.1%). Finally, adjuvant radiation treatment correlated with decreased mortality to surgical resection of primary tumor alone (HR: 0.847; 95% CI: 0.726-0.989), whereas adjuvant chemotherapy correlated with increased mortality (HR: 2.769; 95% CI: 1.995-3.841).
Conclusion: Primary anatomical site was determined to be a major prognostic factor along with treatment facility type, sex, stage, and surgical margins for patients with myxoid liposarcoma.
Primary Anatomical Site as a Prognostic Factor for Pleomorphic Liposarcoma
Background: Pleomorphic liposarcomas is an aggressive, high grade subtype of soft tissue sarcoma representing < 15% of liposarcomas. It most commonly arises in the retroperitoneum and proximal upper extremities. Current prognostic factors are centered around staging, which accounts for the grade, size, and location of the tumor in relation to the superficial fascia.
Methods: A total of 1778 patients diagnosed with pleomorphic liposarcoma were identified in the National Cancer Database and stratified by primary anatomical site: head/neck, upper limb, lower limb/hip, thorax/lung, pelvis, and retroperitoneum/abdomen. Kaplan-Meier survival tables were produced to estimate 1-, 5-, and 10-year overall survival. Log-rank tests with a Bonferroni correction for multiple comparisons and a multivariable Cox proportional hazards analysis were utilized to compare the primary site groups; P < 0.05 was considered significant.
Results: The most common primary anatomical site was the lower limb/hip. The head/neck primary anatomical site demonstrated the highest 10-year overall survival probability, while retroperitoneum/abdomen had the lowest (50% and 18.4%, respectively). Median survival was 9.19 years for the head/neck group, and 3.08 years for the retroperitoneum/abdomen group. Significant overall survival differences were found between the retroperitoneum/abdomen group and each of the following groups: head/neck (P=0.001), upper limb (P<0.001), lower limb/hip (P<0.001), and pelvis (P=0.001). After adjusting for age, biological sex, race and ethnicity, Charlson-Deyo comorbidity index, and AJCC pathologic stage, a 48.4% increased risk of death was found for retroperitoneum/abdomen vs. lower limb/hip primary site (95% CI: 14.6% to 92.1%; P=0.003). Thorax/lung vs. lower limb/hip was also associated with a 55.1% increased risk of death (95% CI: 8.4% to 121.9%; P=0.016).
Conclusion: The current prognostic modality for pleomorphic liposarcoma is limited. There are statistically significant differences in survival based on primary anatomical sites, which may serve as a useful prognostic indicator. Tumors manifesting in the retroperitoneum/abdomen demonstrated the lowest survival.
Background: Pleomorphic liposarcomas is an aggressive, high grade subtype of soft tissue sarcoma representing < 15% of liposarcomas. It most commonly arises in the retroperitoneum and proximal upper extremities. Current prognostic factors are centered around staging, which accounts for the grade, size, and location of the tumor in relation to the superficial fascia.
Methods: A total of 1778 patients diagnosed with pleomorphic liposarcoma were identified in the National Cancer Database and stratified by primary anatomical site: head/neck, upper limb, lower limb/hip, thorax/lung, pelvis, and retroperitoneum/abdomen. Kaplan-Meier survival tables were produced to estimate 1-, 5-, and 10-year overall survival. Log-rank tests with a Bonferroni correction for multiple comparisons and a multivariable Cox proportional hazards analysis were utilized to compare the primary site groups; P < 0.05 was considered significant.
Results: The most common primary anatomical site was the lower limb/hip. The head/neck primary anatomical site demonstrated the highest 10-year overall survival probability, while retroperitoneum/abdomen had the lowest (50% and 18.4%, respectively). Median survival was 9.19 years for the head/neck group, and 3.08 years for the retroperitoneum/abdomen group. Significant overall survival differences were found between the retroperitoneum/abdomen group and each of the following groups: head/neck (P=0.001), upper limb (P<0.001), lower limb/hip (P<0.001), and pelvis (P=0.001). After adjusting for age, biological sex, race and ethnicity, Charlson-Deyo comorbidity index, and AJCC pathologic stage, a 48.4% increased risk of death was found for retroperitoneum/abdomen vs. lower limb/hip primary site (95% CI: 14.6% to 92.1%; P=0.003). Thorax/lung vs. lower limb/hip was also associated with a 55.1% increased risk of death (95% CI: 8.4% to 121.9%; P=0.016).
Conclusion: The current prognostic modality for pleomorphic liposarcoma is limited. There are statistically significant differences in survival based on primary anatomical sites, which may serve as a useful prognostic indicator. Tumors manifesting in the retroperitoneum/abdomen demonstrated the lowest survival.
Background: Pleomorphic liposarcomas is an aggressive, high grade subtype of soft tissue sarcoma representing < 15% of liposarcomas. It most commonly arises in the retroperitoneum and proximal upper extremities. Current prognostic factors are centered around staging, which accounts for the grade, size, and location of the tumor in relation to the superficial fascia.
Methods: A total of 1778 patients diagnosed with pleomorphic liposarcoma were identified in the National Cancer Database and stratified by primary anatomical site: head/neck, upper limb, lower limb/hip, thorax/lung, pelvis, and retroperitoneum/abdomen. Kaplan-Meier survival tables were produced to estimate 1-, 5-, and 10-year overall survival. Log-rank tests with a Bonferroni correction for multiple comparisons and a multivariable Cox proportional hazards analysis were utilized to compare the primary site groups; P < 0.05 was considered significant.
Results: The most common primary anatomical site was the lower limb/hip. The head/neck primary anatomical site demonstrated the highest 10-year overall survival probability, while retroperitoneum/abdomen had the lowest (50% and 18.4%, respectively). Median survival was 9.19 years for the head/neck group, and 3.08 years for the retroperitoneum/abdomen group. Significant overall survival differences were found between the retroperitoneum/abdomen group and each of the following groups: head/neck (P=0.001), upper limb (P<0.001), lower limb/hip (P<0.001), and pelvis (P=0.001). After adjusting for age, biological sex, race and ethnicity, Charlson-Deyo comorbidity index, and AJCC pathologic stage, a 48.4% increased risk of death was found for retroperitoneum/abdomen vs. lower limb/hip primary site (95% CI: 14.6% to 92.1%; P=0.003). Thorax/lung vs. lower limb/hip was also associated with a 55.1% increased risk of death (95% CI: 8.4% to 121.9%; P=0.016).
Conclusion: The current prognostic modality for pleomorphic liposarcoma is limited. There are statistically significant differences in survival based on primary anatomical sites, which may serve as a useful prognostic indicator. Tumors manifesting in the retroperitoneum/abdomen demonstrated the lowest survival.
Prescribing Practices Based on Recommendations of the Veterans Health Administration’s National Precision Oncology Program
Background: Next-generation sequencing (NGS) of cancer gene panels is now standard-of-care for patients with advanced solid tumors. In July 2016, the Veterans Health Administration (VHA) launched the National Precision Oncology Program (NPOP) to increase access to NGS testing to VHA cancer patients across the country. A review of the prescription patterns among patients with highly actionable mutations is warranted to measure the impact of NPOP.
Purpose: The objective of this study is to assess the use of targeted therapies among patients with advanced solid tumors who received a Level 1, 2A, or R1 recommendation based on NGS results. For cases in which patients failed to receive targeted agents, underlying reasons will be identified. Study results will be used to improve outcomes of veterans undergoing NGS testing and the cost-benefit of NPOP.
Methods: This study will be conducted as a retrospective analysis of veterans who received oncologic care through the VHA and underwent NGS testing. From program inception in July 2016 until January 2019, the tumor samples of 5,897 patients have undergone NGS testing through NPOP. NGS results were categorized by Watson for Genomics (WfG), an artificial intelligence decision-support system. Among these, 608 (10.3%) samples noted to have at least one genetic variant with Level 1 or 2A actionability. The NPOP database will be queried to identify these patients who had a recommendation to receive a targeted agent. Prescribed and dispensed drugs will be identified from the Corporate Data Warehouse to indicate patients who have received targeted agents through VHA and compute the percentage of those who were not prescribed therapy through VHA. The medical records of patients who did not receive a corresponding targeted drug will be reviewed to identify non-VA drug use and code reasons if no record of drug administration is recorded. These codes will be examined for association with patients and tumor characteristics, sites of treating oncologists, and types of cancers. The most frequent coded reasons will be recorded, and assessment of this data will be performed to identify potential interventions to improve the utility of NGS testing for veterans.
Background: Next-generation sequencing (NGS) of cancer gene panels is now standard-of-care for patients with advanced solid tumors. In July 2016, the Veterans Health Administration (VHA) launched the National Precision Oncology Program (NPOP) to increase access to NGS testing to VHA cancer patients across the country. A review of the prescription patterns among patients with highly actionable mutations is warranted to measure the impact of NPOP.
Purpose: The objective of this study is to assess the use of targeted therapies among patients with advanced solid tumors who received a Level 1, 2A, or R1 recommendation based on NGS results. For cases in which patients failed to receive targeted agents, underlying reasons will be identified. Study results will be used to improve outcomes of veterans undergoing NGS testing and the cost-benefit of NPOP.
Methods: This study will be conducted as a retrospective analysis of veterans who received oncologic care through the VHA and underwent NGS testing. From program inception in July 2016 until January 2019, the tumor samples of 5,897 patients have undergone NGS testing through NPOP. NGS results were categorized by Watson for Genomics (WfG), an artificial intelligence decision-support system. Among these, 608 (10.3%) samples noted to have at least one genetic variant with Level 1 or 2A actionability. The NPOP database will be queried to identify these patients who had a recommendation to receive a targeted agent. Prescribed and dispensed drugs will be identified from the Corporate Data Warehouse to indicate patients who have received targeted agents through VHA and compute the percentage of those who were not prescribed therapy through VHA. The medical records of patients who did not receive a corresponding targeted drug will be reviewed to identify non-VA drug use and code reasons if no record of drug administration is recorded. These codes will be examined for association with patients and tumor characteristics, sites of treating oncologists, and types of cancers. The most frequent coded reasons will be recorded, and assessment of this data will be performed to identify potential interventions to improve the utility of NGS testing for veterans.
Background: Next-generation sequencing (NGS) of cancer gene panels is now standard-of-care for patients with advanced solid tumors. In July 2016, the Veterans Health Administration (VHA) launched the National Precision Oncology Program (NPOP) to increase access to NGS testing to VHA cancer patients across the country. A review of the prescription patterns among patients with highly actionable mutations is warranted to measure the impact of NPOP.
Purpose: The objective of this study is to assess the use of targeted therapies among patients with advanced solid tumors who received a Level 1, 2A, or R1 recommendation based on NGS results. For cases in which patients failed to receive targeted agents, underlying reasons will be identified. Study results will be used to improve outcomes of veterans undergoing NGS testing and the cost-benefit of NPOP.
Methods: This study will be conducted as a retrospective analysis of veterans who received oncologic care through the VHA and underwent NGS testing. From program inception in July 2016 until January 2019, the tumor samples of 5,897 patients have undergone NGS testing through NPOP. NGS results were categorized by Watson for Genomics (WfG), an artificial intelligence decision-support system. Among these, 608 (10.3%) samples noted to have at least one genetic variant with Level 1 or 2A actionability. The NPOP database will be queried to identify these patients who had a recommendation to receive a targeted agent. Prescribed and dispensed drugs will be identified from the Corporate Data Warehouse to indicate patients who have received targeted agents through VHA and compute the percentage of those who were not prescribed therapy through VHA. The medical records of patients who did not receive a corresponding targeted drug will be reviewed to identify non-VA drug use and code reasons if no record of drug administration is recorded. These codes will be examined for association with patients and tumor characteristics, sites of treating oncologists, and types of cancers. The most frequent coded reasons will be recorded, and assessment of this data will be performed to identify potential interventions to improve the utility of NGS testing for veterans.