User login
Bariatric surgery leads to less improvement in black patients
“Per this analysis, there are significant racial disparities in perioperative outcomes, weight loss, and quality of life after bariatric surgery,” wrote lead author Michael H. Wood, MD, of Wayne State University, Detroit, and his coauthors, adding that, “while biological differences may explain some of the disparity in outcomes, environmental, social, and behavioral factors likely play a role.” The study was published online in JAMA Surgery.
This study reviewed data from 14,210 participants in the Michigan Bariatric Surgery Collaborative (MBSC), a state-wide consortium and clinical registry of bariatric surgery patients. Matching cohorts were established for black (n = 7,105) and white (n = 7,105) patients who underwent a primary bariatric operation (Roux-en-Y gastric bypass, sleeve gastrectomy, or adjustable gastric banding) between June 2006 and January 2017. The only significant differences between cohorts – clarified as “never more than 1 or 2 percentage points” – were in regard to income brackets and procedure type.
At 30-day follow-up, the rate of overall complications was higher in black patients (628, 8.8%) than in white patients (481, 6.8%; adjusted odds ratio, 1.33; 95% confidence interval, 1.17-1.51; P = .02), as was the length of stay (mean, 2.2 days vs. 1.9 days; aOR, 0.30; 95% CI, 0.20-0.40; P less than .001). Black patients also had a higher rate of both ED visits (541 [11.6%] vs. 826 [7.6%]; aOR, 1.60; 95% CI, 1.43-1.79; P less than .001) and readmissions (414 [5.8%] vs. 245 [3.5%]; aOR, 1.73; 95% CI, 1.47-2.03; P less than .001).
In addition, at 1-year follow-up, black patients had a lower mean weight loss (32.0 kg vs. 38.3 kg; P less than .001) and percentage of total weight loss (26% vs. 29%; P less than .001) compared with white patients. And though black patients were more likely than white patients to report a high quality of life before surgery (2,672 [49.5%] vs. 2,354 [41.4%]; P less than .001), they were less likely to do so 1 year afterward (1,379 [87.2%] vs. 2,133 [90.4%]; P = .002).
The coauthors acknowledged the limitations of their study, including potential unmeasured factors between cohorts such as disease duration or severity. They also noted that a wider time horizon than 30 days post surgery could have altered the results, although “serious adverse events and resource use tend to be highest within the first month after surgery, and we anticipate that this effect would have been negligible.”
The study was funded by Blue Cross Blue Shield Michigan/Blue Care Network. Dr. Wood reported no conflicts of interest. Three of his coauthors reported receiving salary support from Blue Cross Blue Shield Michigan/Blue Care Network for their work with the MBSC, and one other coauthor reported receiving an honorarium for being the MBSC’s executive committee chair.
The AGA Practice guide on Obesity and Weight management, Education and Resources (POWER) white paper provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at https://www.gastro.org/practice-guidance/practice-updates/obesity-practice-guide.
SOURCE: Wood MH et al. JAMA Surg. 2019 Mar 6. doi: 10.1001/jamasurg.2019.0029.
The well-documented disparities between black and white patients after bariatric surgery are brought back to the forefront via to this study from Wood et al., according to Brian Hodgens, MD, and Kenric M. Murayama, MD, of the University of Hawaii, Honolulu.
Some of the findings hint at the cultural differences that permeate the time before and after a surgery like this: In particular, they highlighted how black patients were more likely to report good or very good quality of life before surgery but less likely after. This could be related to a “difference in perceptions of obesity by black patients,” where they are more hesitant to pursue the surgery than their white counterparts, Dr. Hodgens and Dr. Murayama wrote.
More work is needed, they added, but “this study and others like it can better equip practicing bariatric surgeons to educate themselves and patients on expectations before and after bariatric surgery.”
These comments are adapted from an accompanying editorial ( JAMA Surg. 2019 Mar 6. doi: 1 0.1001/jamasurg.2019.0067 ). Dr. Murayama reported receiving personal fees from Medtronic outside the submitted work.
The well-documented disparities between black and white patients after bariatric surgery are brought back to the forefront via to this study from Wood et al., according to Brian Hodgens, MD, and Kenric M. Murayama, MD, of the University of Hawaii, Honolulu.
Some of the findings hint at the cultural differences that permeate the time before and after a surgery like this: In particular, they highlighted how black patients were more likely to report good or very good quality of life before surgery but less likely after. This could be related to a “difference in perceptions of obesity by black patients,” where they are more hesitant to pursue the surgery than their white counterparts, Dr. Hodgens and Dr. Murayama wrote.
More work is needed, they added, but “this study and others like it can better equip practicing bariatric surgeons to educate themselves and patients on expectations before and after bariatric surgery.”
These comments are adapted from an accompanying editorial ( JAMA Surg. 2019 Mar 6. doi: 1 0.1001/jamasurg.2019.0067 ). Dr. Murayama reported receiving personal fees from Medtronic outside the submitted work.
The well-documented disparities between black and white patients after bariatric surgery are brought back to the forefront via to this study from Wood et al., according to Brian Hodgens, MD, and Kenric M. Murayama, MD, of the University of Hawaii, Honolulu.
Some of the findings hint at the cultural differences that permeate the time before and after a surgery like this: In particular, they highlighted how black patients were more likely to report good or very good quality of life before surgery but less likely after. This could be related to a “difference in perceptions of obesity by black patients,” where they are more hesitant to pursue the surgery than their white counterparts, Dr. Hodgens and Dr. Murayama wrote.
More work is needed, they added, but “this study and others like it can better equip practicing bariatric surgeons to educate themselves and patients on expectations before and after bariatric surgery.”
These comments are adapted from an accompanying editorial ( JAMA Surg. 2019 Mar 6. doi: 1 0.1001/jamasurg.2019.0067 ). Dr. Murayama reported receiving personal fees from Medtronic outside the submitted work.
“Per this analysis, there are significant racial disparities in perioperative outcomes, weight loss, and quality of life after bariatric surgery,” wrote lead author Michael H. Wood, MD, of Wayne State University, Detroit, and his coauthors, adding that, “while biological differences may explain some of the disparity in outcomes, environmental, social, and behavioral factors likely play a role.” The study was published online in JAMA Surgery.
This study reviewed data from 14,210 participants in the Michigan Bariatric Surgery Collaborative (MBSC), a state-wide consortium and clinical registry of bariatric surgery patients. Matching cohorts were established for black (n = 7,105) and white (n = 7,105) patients who underwent a primary bariatric operation (Roux-en-Y gastric bypass, sleeve gastrectomy, or adjustable gastric banding) between June 2006 and January 2017. The only significant differences between cohorts – clarified as “never more than 1 or 2 percentage points” – were in regard to income brackets and procedure type.
At 30-day follow-up, the rate of overall complications was higher in black patients (628, 8.8%) than in white patients (481, 6.8%; adjusted odds ratio, 1.33; 95% confidence interval, 1.17-1.51; P = .02), as was the length of stay (mean, 2.2 days vs. 1.9 days; aOR, 0.30; 95% CI, 0.20-0.40; P less than .001). Black patients also had a higher rate of both ED visits (541 [11.6%] vs. 826 [7.6%]; aOR, 1.60; 95% CI, 1.43-1.79; P less than .001) and readmissions (414 [5.8%] vs. 245 [3.5%]; aOR, 1.73; 95% CI, 1.47-2.03; P less than .001).
In addition, at 1-year follow-up, black patients had a lower mean weight loss (32.0 kg vs. 38.3 kg; P less than .001) and percentage of total weight loss (26% vs. 29%; P less than .001) compared with white patients. And though black patients were more likely than white patients to report a high quality of life before surgery (2,672 [49.5%] vs. 2,354 [41.4%]; P less than .001), they were less likely to do so 1 year afterward (1,379 [87.2%] vs. 2,133 [90.4%]; P = .002).
The coauthors acknowledged the limitations of their study, including potential unmeasured factors between cohorts such as disease duration or severity. They also noted that a wider time horizon than 30 days post surgery could have altered the results, although “serious adverse events and resource use tend to be highest within the first month after surgery, and we anticipate that this effect would have been negligible.”
The study was funded by Blue Cross Blue Shield Michigan/Blue Care Network. Dr. Wood reported no conflicts of interest. Three of his coauthors reported receiving salary support from Blue Cross Blue Shield Michigan/Blue Care Network for their work with the MBSC, and one other coauthor reported receiving an honorarium for being the MBSC’s executive committee chair.
The AGA Practice guide on Obesity and Weight management, Education and Resources (POWER) white paper provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at https://www.gastro.org/practice-guidance/practice-updates/obesity-practice-guide.
SOURCE: Wood MH et al. JAMA Surg. 2019 Mar 6. doi: 10.1001/jamasurg.2019.0029.
“Per this analysis, there are significant racial disparities in perioperative outcomes, weight loss, and quality of life after bariatric surgery,” wrote lead author Michael H. Wood, MD, of Wayne State University, Detroit, and his coauthors, adding that, “while biological differences may explain some of the disparity in outcomes, environmental, social, and behavioral factors likely play a role.” The study was published online in JAMA Surgery.
This study reviewed data from 14,210 participants in the Michigan Bariatric Surgery Collaborative (MBSC), a state-wide consortium and clinical registry of bariatric surgery patients. Matching cohorts were established for black (n = 7,105) and white (n = 7,105) patients who underwent a primary bariatric operation (Roux-en-Y gastric bypass, sleeve gastrectomy, or adjustable gastric banding) between June 2006 and January 2017. The only significant differences between cohorts – clarified as “never more than 1 or 2 percentage points” – were in regard to income brackets and procedure type.
At 30-day follow-up, the rate of overall complications was higher in black patients (628, 8.8%) than in white patients (481, 6.8%; adjusted odds ratio, 1.33; 95% confidence interval, 1.17-1.51; P = .02), as was the length of stay (mean, 2.2 days vs. 1.9 days; aOR, 0.30; 95% CI, 0.20-0.40; P less than .001). Black patients also had a higher rate of both ED visits (541 [11.6%] vs. 826 [7.6%]; aOR, 1.60; 95% CI, 1.43-1.79; P less than .001) and readmissions (414 [5.8%] vs. 245 [3.5%]; aOR, 1.73; 95% CI, 1.47-2.03; P less than .001).
In addition, at 1-year follow-up, black patients had a lower mean weight loss (32.0 kg vs. 38.3 kg; P less than .001) and percentage of total weight loss (26% vs. 29%; P less than .001) compared with white patients. And though black patients were more likely than white patients to report a high quality of life before surgery (2,672 [49.5%] vs. 2,354 [41.4%]; P less than .001), they were less likely to do so 1 year afterward (1,379 [87.2%] vs. 2,133 [90.4%]; P = .002).
The coauthors acknowledged the limitations of their study, including potential unmeasured factors between cohorts such as disease duration or severity. They also noted that a wider time horizon than 30 days post surgery could have altered the results, although “serious adverse events and resource use tend to be highest within the first month after surgery, and we anticipate that this effect would have been negligible.”
The study was funded by Blue Cross Blue Shield Michigan/Blue Care Network. Dr. Wood reported no conflicts of interest. Three of his coauthors reported receiving salary support from Blue Cross Blue Shield Michigan/Blue Care Network for their work with the MBSC, and one other coauthor reported receiving an honorarium for being the MBSC’s executive committee chair.
The AGA Practice guide on Obesity and Weight management, Education and Resources (POWER) white paper provides physicians with a comprehensive, multi-disciplinary process to guide and personalize innovative obesity care for safe and effective weight management. Learn more at https://www.gastro.org/practice-guidance/practice-updates/obesity-practice-guide.
SOURCE: Wood MH et al. JAMA Surg. 2019 Mar 6. doi: 10.1001/jamasurg.2019.0029.
FROM JAMA SURGERY
Key clinical point: Black patients who underwent bariatric surgery suffered more overall complications and reported a lower quality of life than white patients.
Major finding: The rate of overall complications was higher in black patients (628, 8.8%) than in white patients (481, 6.8%; adjusted odds ratio, 1.33; 95% confidence interval, 1.17-1.51; P = .02).
Study details: A matched cohort study of 14,210 patients, half black and half white, who underwent a primary bariatric operation in Michigan between June 2006 and January 2017.
Disclosures: The study was funded by Blue Cross Blue Shield Michigan/Blue Care Network. Dr. Wood reported no conflicts of interest. Three of his coauthors reported receiving salary support from Blue Cross Blue Shield Michigan/Blue Care Network for their work with the Michigan Bariatric Surgery Collaborative, and one other coauthor reported receiving an honorarium for being the collaborative’s executive committee chair.
Source: Wood MH et al. JAMA Surg. 2019 Mar 6. doi: 10.1001/jamasurg.2019.0029.
Bariatric surgery leads to less improvement in black patients
“Per this analysis, there are significant racial disparities in perioperative outcomes, weight loss, and quality of life after bariatric surgery,” wrote lead author Michael H. Wood, MD, of Wayne State University, Detroit, and his coauthors, adding that, “while biological differences may explain some of the disparity in outcomes, environmental, social, and behavioral factors likely play a role.” The study was published online in JAMA Surgery.
This study reviewed data from 14,210 participants in the Michigan Bariatric Surgery Collaborative (MBSC), a state-wide consortium and clinical registry of bariatric surgery patients. Matching cohorts were established for black (n = 7,105) and white (n = 7,105) patients who underwent a primary bariatric operation (Roux-en-Y gastric bypass, sleeve gastrectomy, or adjustable gastric banding) between June 2006 and January 2017. The only significant differences between cohorts – clarified as “never more than 1 or 2 percentage points” – were in regard to income brackets and procedure type.
At 30-day follow-up, the rate of overall complications was higher in black patients (628, 8.8%) than in white patients (481, 6.8%; adjusted odds ratio, 1.33; 95% confidence interval, 1.17-1.51; P = .02), as was the length of stay (mean, 2.2 days vs. 1.9 days; aOR, 0.30; 95% CI, 0.20-0.40; P less than .001). Black patients also had a higher rate of both ED visits (541 [11.6%] vs. 826 [7.6%]; aOR, 1.60; 95% CI, 1.43-1.79; P less than .001) and readmissions (414 [5.8%] vs. 245 [3.5%]; aOR, 1.73; 95% CI, 1.47-2.03; P less than .001).
In addition, at 1-year follow-up, black patients had a lower mean weight loss (32.0 kg vs. 38.3 kg; P less than .001) and percentage of total weight loss (26% vs. 29%; P less than .001) compared with white patients. And though black patients were more likely than white patients to report a high quality of life before surgery (2,672 [49.5%] vs. 2,354 [41.4%]; P less than .001), they were less likely to do so 1 year afterward (1,379 [87.2%] vs. 2,133 [90.4%]; P = .002).
The coauthors acknowledged the limitations of their study, including potential unmeasured factors between cohorts such as disease duration or severity. They also noted that a wider time horizon than 30 days post surgery could have altered the results, although “serious adverse events and resource use tend to be highest within the first month after surgery, and we anticipate that this effect would have been negligible.”
The study was funded by Blue Cross Blue Shield Michigan/Blue Care Network. Dr. Wood reported no conflicts of interest. Three of his coauthors reported receiving salary support from Blue Cross Blue Shield Michigan/Blue Care Network for their work with the MBSC, and one other coauthor reported receiving an honorarium for being the MBSC’s executive committee chair.
SOURCE: Wood MH et al. JAMA Surg. 2019 Mar 6. doi: 10.1001/jamasurg.2019.0029.
The well-documented disparities between black and white patients after bariatric surgery are brought back to the forefront via to this study from Wood et al., according to Brian Hodgens, MD, and Kenric M. Murayama, MD, of the University of Hawaii, Honolulu.
Some of the findings hint at the cultural differences that permeate the time before and after a surgery like this: In particular, they highlighted how black patients were more likely to report good or very good quality of life before surgery but less likely after. This could be related to a “difference in perceptions of obesity by black patients,” where they are more hesitant to pursue the surgery than their white counterparts, Dr. Hodgens and Dr. Murayama wrote.
More work is needed, they added, but “this study and others like it can better equip practicing bariatric surgeons to educate themselves and patients on expectations before and after bariatric surgery.”
These comments are adapted from an accompanying editorial ( JAMA Surg. 2019 Mar 6. doi: 1 0.1001/jamasurg.2019.0067 ). Dr. Murayama reported receiving personal fees from Medtronic outside the submitted work.
The well-documented disparities between black and white patients after bariatric surgery are brought back to the forefront via to this study from Wood et al., according to Brian Hodgens, MD, and Kenric M. Murayama, MD, of the University of Hawaii, Honolulu.
Some of the findings hint at the cultural differences that permeate the time before and after a surgery like this: In particular, they highlighted how black patients were more likely to report good or very good quality of life before surgery but less likely after. This could be related to a “difference in perceptions of obesity by black patients,” where they are more hesitant to pursue the surgery than their white counterparts, Dr. Hodgens and Dr. Murayama wrote.
More work is needed, they added, but “this study and others like it can better equip practicing bariatric surgeons to educate themselves and patients on expectations before and after bariatric surgery.”
These comments are adapted from an accompanying editorial ( JAMA Surg. 2019 Mar 6. doi: 1 0.1001/jamasurg.2019.0067 ). Dr. Murayama reported receiving personal fees from Medtronic outside the submitted work.
The well-documented disparities between black and white patients after bariatric surgery are brought back to the forefront via to this study from Wood et al., according to Brian Hodgens, MD, and Kenric M. Murayama, MD, of the University of Hawaii, Honolulu.
Some of the findings hint at the cultural differences that permeate the time before and after a surgery like this: In particular, they highlighted how black patients were more likely to report good or very good quality of life before surgery but less likely after. This could be related to a “difference in perceptions of obesity by black patients,” where they are more hesitant to pursue the surgery than their white counterparts, Dr. Hodgens and Dr. Murayama wrote.
More work is needed, they added, but “this study and others like it can better equip practicing bariatric surgeons to educate themselves and patients on expectations before and after bariatric surgery.”
These comments are adapted from an accompanying editorial ( JAMA Surg. 2019 Mar 6. doi: 1 0.1001/jamasurg.2019.0067 ). Dr. Murayama reported receiving personal fees from Medtronic outside the submitted work.
“Per this analysis, there are significant racial disparities in perioperative outcomes, weight loss, and quality of life after bariatric surgery,” wrote lead author Michael H. Wood, MD, of Wayne State University, Detroit, and his coauthors, adding that, “while biological differences may explain some of the disparity in outcomes, environmental, social, and behavioral factors likely play a role.” The study was published online in JAMA Surgery.
This study reviewed data from 14,210 participants in the Michigan Bariatric Surgery Collaborative (MBSC), a state-wide consortium and clinical registry of bariatric surgery patients. Matching cohorts were established for black (n = 7,105) and white (n = 7,105) patients who underwent a primary bariatric operation (Roux-en-Y gastric bypass, sleeve gastrectomy, or adjustable gastric banding) between June 2006 and January 2017. The only significant differences between cohorts – clarified as “never more than 1 or 2 percentage points” – were in regard to income brackets and procedure type.
At 30-day follow-up, the rate of overall complications was higher in black patients (628, 8.8%) than in white patients (481, 6.8%; adjusted odds ratio, 1.33; 95% confidence interval, 1.17-1.51; P = .02), as was the length of stay (mean, 2.2 days vs. 1.9 days; aOR, 0.30; 95% CI, 0.20-0.40; P less than .001). Black patients also had a higher rate of both ED visits (541 [11.6%] vs. 826 [7.6%]; aOR, 1.60; 95% CI, 1.43-1.79; P less than .001) and readmissions (414 [5.8%] vs. 245 [3.5%]; aOR, 1.73; 95% CI, 1.47-2.03; P less than .001).
In addition, at 1-year follow-up, black patients had a lower mean weight loss (32.0 kg vs. 38.3 kg; P less than .001) and percentage of total weight loss (26% vs. 29%; P less than .001) compared with white patients. And though black patients were more likely than white patients to report a high quality of life before surgery (2,672 [49.5%] vs. 2,354 [41.4%]; P less than .001), they were less likely to do so 1 year afterward (1,379 [87.2%] vs. 2,133 [90.4%]; P = .002).
The coauthors acknowledged the limitations of their study, including potential unmeasured factors between cohorts such as disease duration or severity. They also noted that a wider time horizon than 30 days post surgery could have altered the results, although “serious adverse events and resource use tend to be highest within the first month after surgery, and we anticipate that this effect would have been negligible.”
The study was funded by Blue Cross Blue Shield Michigan/Blue Care Network. Dr. Wood reported no conflicts of interest. Three of his coauthors reported receiving salary support from Blue Cross Blue Shield Michigan/Blue Care Network for their work with the MBSC, and one other coauthor reported receiving an honorarium for being the MBSC’s executive committee chair.
SOURCE: Wood MH et al. JAMA Surg. 2019 Mar 6. doi: 10.1001/jamasurg.2019.0029.
“Per this analysis, there are significant racial disparities in perioperative outcomes, weight loss, and quality of life after bariatric surgery,” wrote lead author Michael H. Wood, MD, of Wayne State University, Detroit, and his coauthors, adding that, “while biological differences may explain some of the disparity in outcomes, environmental, social, and behavioral factors likely play a role.” The study was published online in JAMA Surgery.
This study reviewed data from 14,210 participants in the Michigan Bariatric Surgery Collaborative (MBSC), a state-wide consortium and clinical registry of bariatric surgery patients. Matching cohorts were established for black (n = 7,105) and white (n = 7,105) patients who underwent a primary bariatric operation (Roux-en-Y gastric bypass, sleeve gastrectomy, or adjustable gastric banding) between June 2006 and January 2017. The only significant differences between cohorts – clarified as “never more than 1 or 2 percentage points” – were in regard to income brackets and procedure type.
At 30-day follow-up, the rate of overall complications was higher in black patients (628, 8.8%) than in white patients (481, 6.8%; adjusted odds ratio, 1.33; 95% confidence interval, 1.17-1.51; P = .02), as was the length of stay (mean, 2.2 days vs. 1.9 days; aOR, 0.30; 95% CI, 0.20-0.40; P less than .001). Black patients also had a higher rate of both ED visits (541 [11.6%] vs. 826 [7.6%]; aOR, 1.60; 95% CI, 1.43-1.79; P less than .001) and readmissions (414 [5.8%] vs. 245 [3.5%]; aOR, 1.73; 95% CI, 1.47-2.03; P less than .001).
In addition, at 1-year follow-up, black patients had a lower mean weight loss (32.0 kg vs. 38.3 kg; P less than .001) and percentage of total weight loss (26% vs. 29%; P less than .001) compared with white patients. And though black patients were more likely than white patients to report a high quality of life before surgery (2,672 [49.5%] vs. 2,354 [41.4%]; P less than .001), they were less likely to do so 1 year afterward (1,379 [87.2%] vs. 2,133 [90.4%]; P = .002).
The coauthors acknowledged the limitations of their study, including potential unmeasured factors between cohorts such as disease duration or severity. They also noted that a wider time horizon than 30 days post surgery could have altered the results, although “serious adverse events and resource use tend to be highest within the first month after surgery, and we anticipate that this effect would have been negligible.”
The study was funded by Blue Cross Blue Shield Michigan/Blue Care Network. Dr. Wood reported no conflicts of interest. Three of his coauthors reported receiving salary support from Blue Cross Blue Shield Michigan/Blue Care Network for their work with the MBSC, and one other coauthor reported receiving an honorarium for being the MBSC’s executive committee chair.
SOURCE: Wood MH et al. JAMA Surg. 2019 Mar 6. doi: 10.1001/jamasurg.2019.0029.
FROM JAMA SURGERY
Sudden-onset rash on the trunk and limbs • morbid obesity • family history of diabetes mellitus • Dx?
THE CASE
A 37-year-old man presented with a sudden-onset, nonpruritic, nonpainful, papular rash of 1 month’s duration on his trunk and both arms and legs. Two weeks prior to the current presentation, he consulted a general practitioner, who treated the rash with a course of unknown oral antibiotics; the patient showed no improvement. He recalled that on a few occasions, he used his fingers to express a creamy discharge from some of the lesions. This temporarily reduced the size of those papules.
His medical history was unremarkable except for morbid obesity. He did not drink alcohol regularly and was not taking any medications prior to the onset of the rash. He had no family history of hyperlipidemia, but his mother had a history of diabetes mellitus.
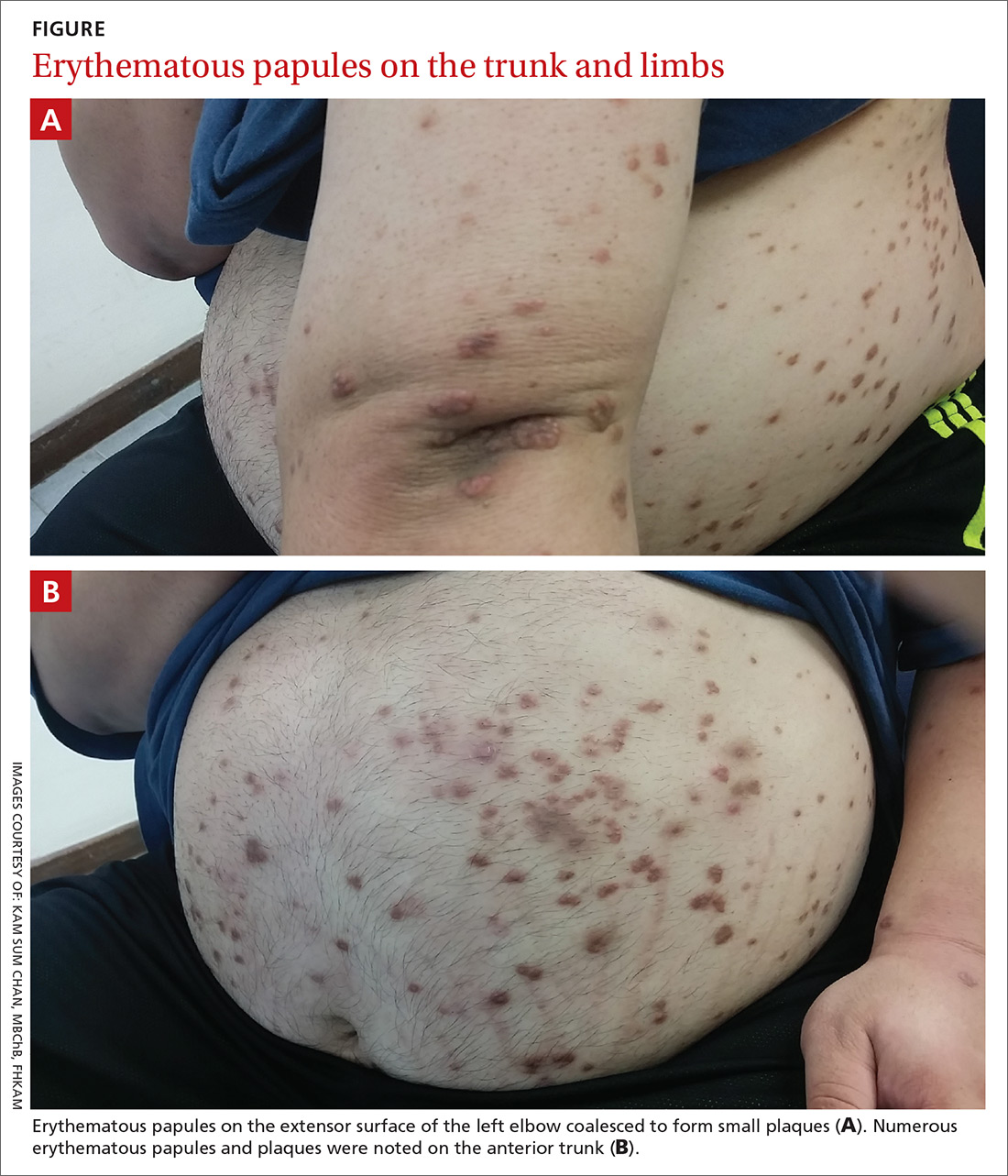
Physical examination showed numerous discrete erythematous papules with a creamy center on his trunk and his arms and legs. The lesions were more numerous on the extensor surfaces of the arms and legs. Some of the papules coalesced to form small plaques (FIGURE). There was no scaling, and the lesions were firm in texture. The patient’s face was spared, and there was no mucosal involvement. The patient was otherwise systemically well.
THE DIAGNOSIS
Based on the morphology, distribution, and abrupt onset of the diffuse nonpruritic papules in this morbidly obese (but otherwise systemically well) middle-aged man, a clinical diagnosis of eruptive xanthoma was suspected. Subsequent blood testing revealed an elevated serum triglyceride level of 47.8 mmol/L (reference range, <1.7 mmol/L), elevated serum total cholesterol of 7.1 mmol/L (reference range, <6.2 mmol/L), and low serum high-density lipoprotein cholesterol of 0.7 mmol/L (reference range, >1 mmol/L in men). He also had an elevated fasting serum glucose level of 12.9 mmol/L (reference range, 3.9–5.6 mmol/L) and an elevated hemoglobin A1c (glycated hemoglobin) level of 10.9%.
Subsequent thyroid, liver, and renal function tests were normal, but the patient had heavy proteinuria, with an elevated urine albumin-to-creatinine ratio of 355.6 mg/mmol (reference range, ≤2.5 mg/mmol). The patient was referred to a dermatologist, who confirmed the clinical diagnosis without the need for a skin biopsy.
DISCUSSION
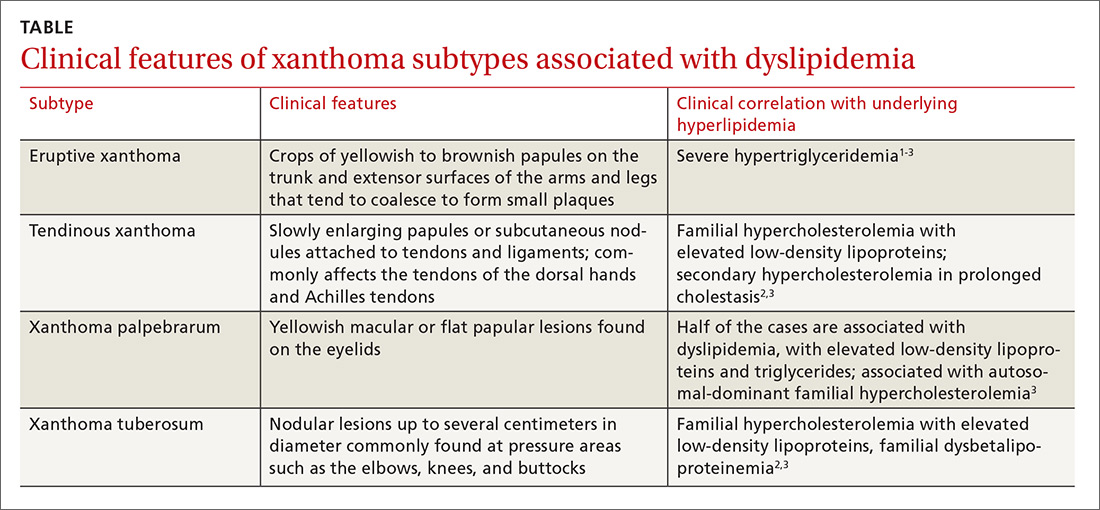
Eruptive xanthoma is characterized by an abrupt onset of crops of multiple yellowish to brownish papules that can coalesce into small plaques. The lesions can be generalized, but tend to be more densely distributed on the extensor surfaces of the arms and legs, buttocks, and thighs.5 Eruptive xanthoma often is associated with hypertriglyceridemia, which can be primary—as a result of a genetic defect caused by familial hypertriglyceridemia—or secondary, associated with poorly controlled diabetes mellitus, morbid obesity, excessive alcohol consumption, nephrotic syndrome, hypothyroidism, primary biliary cholangitis, and drugs like estrogen replacement therapies, corticosteroids, and isotretinoin.6 Pruritus and tenderness may or may not be present, and the Köbner phenomenon may occur.7
Continue to: The differential diagnosis
The differential diagnosis for eruptive xanthoma includes xanthoma disseminatum, non–Langerhans cell histiocytoses (eg, generalized eruptive histiocytosis), and cutaneous mastocytosis.1
Xanthoma disseminatum is an extremely rare, but benign, disorder of non–Langerhans cell origin. The average age of onset is older than 40 years. The rash consists of multiple red-yellow papules and nodules that most commonly present in flexural areas. Forty percent to 60% of patients have mucosal involvement, and rarely the central nervous system is involved.8
Generalized eruptive histiocytosis is another rare non–Langerhans cell histiocytosis that occurs mainly in adults and is characterized by widespread, symmetric, red-brown papules on the trunk, arms, and legs, and rarely the mucous membranes.9
Cutaneous mastocytosis, especially xanthelasmoid mastocytosis, consists of multiple pruritic, yellowish, papular or nodular lesions that may mimic eruptive xanthoma. It occurs mainly in children and rarely in adults.10
Confirming the diagnosis, initiating treatment
The diagnosis of eruptive xanthoma can be confirmed by skin biopsy if other differential diagnoses cannot be ruled out or the lesions do not resolve with treatment. Skin biopsy will reveal lipid-laden macrophages (known as foam cells) deposited in the dermis.7
Continue to: Treatment of eruptive xanthoma
Treatment of eruptive xanthoma involves management of the underlying causes of the condition. In most cases, dietary control, intensive triglyceride-lowering therapies, and treatment of other secondary causes of hypertriglyceridemia result in complete resolution of the lesions within several weeks.5
Our patient’s outcome
Our patient’s sudden-onset rash alerted us to the presence of type 2 diabetes mellitus, hypertriglyceridemia, and heavy proteinuria, which he was not aware of previously. We counselled him about stringent low-sugar, low-lipid diet control and exercise, and we started him on metformin and gemfibrozil. He was referred to an internal medicine specialist for further assessment and management of his severe hypertriglyceridemia and heavy proteinuria.
The rash started to wane 1 month after the patient started the metformin and gemfibrozil, and his drug regimen was changed to combination therapy with metformin/glimepiride and fenofibrate/simvastatin 6 weeks later when he was seen in the medical specialty clinic. Fundus photography performed 1 month after starting oral antidiabetic therapy showed no diabetic retinopathy or lipemia retinalis.
After 3 months of treatment, his serum triglycerides and hemoglobin A1c levels dropped to 3.8 mmol/L and 8.7%, respectively. The rash also resolved considerably, with only residual papules on the abdomen. This rapid clinical response to treatment of the underlying hypertriglyceridemia and diabetes further supported the clinical diagnosis of eruptive xanthoma.
THE TAKEAWAY
Eruptive xanthoma is relatively rare, but it is important for family physicians to recognize this clinical presentation as a potential indicator of severe hypertriglyceridemia. Recognizing hypertriglyceridemia early is important, as it can be associated with an increased risk for acute pancreatitis. Moreover, eruptive xanthoma might be the sole presenting symptom of underlying diabetes mellitus or familial hyperlipidemia, both of which can lead to a significant increase in cardiovascular risk if uncontrolled.
CORRESPONDENCE
Chan Kam Sum, MBChB, FRACGP, Tseung Kwan O Jockey Club General Out-patient Clinic, 99 Po Lam Road North, G/F, Tseung Kwan O, Kowloon, Hong Kong; cks048@ha.org.hk
1. Tang WK. Eruptive xanthoma. [case reports]. Hong Kong Dermatol Venereol Bull. 2001;9:172-175.
2. Frew J, Murrell D, Haber R. Fifty shades of yellow: a review of the xanthodermatoses. Int J Dermatol. 2015;54:1109-1123.
3. Zak A, Zeman M, Slaby A, et al. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:181-188.
4. Sandhu S, Al-Sarraf A, Taraboanta C, et al. Incidence of pancreatitis, secondary causes, and treatment of patients referred to specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids Health Dis. 2011;10:157.
5. Holsinger JM, Campbell SM, Witman P. Multiple erythematous-yellow, dome-shaped papules. Am Fam Physician. 2010;82:517.
6. Loeckermann S, Braun-Falco M. Eruptive xanthomas in association with metabolic syndrome. Clin Exp Dermatol. 2010;35:565-566.
7. Merola JF, Mengden SJ, Soldano A, et al. Eruptive xanthomas. Dermatol Online J. 2008;14:10.
8. Park M, Boone B, Devas S. Xanthoma disseminatum: case report and mini-review of the literature. Acta Dermatovenerol Croat. 2014;22:150-154.
9. Attia A, Seleit I, El Badawy N, et al. Photoletter to the editor: generalized eruptive histiocytoma. J Dermatol Case Rep. 2011;5:53-55.
10. Nabavi NS, Nejad MH, Feli S, et al. Adult onset of xanthelasmoid mastocytosis: report of a rare entity. Indian J Dermatol. 2016;61:468.
THE CASE
A 37-year-old man presented with a sudden-onset, nonpruritic, nonpainful, papular rash of 1 month’s duration on his trunk and both arms and legs. Two weeks prior to the current presentation, he consulted a general practitioner, who treated the rash with a course of unknown oral antibiotics; the patient showed no improvement. He recalled that on a few occasions, he used his fingers to express a creamy discharge from some of the lesions. This temporarily reduced the size of those papules.
His medical history was unremarkable except for morbid obesity. He did not drink alcohol regularly and was not taking any medications prior to the onset of the rash. He had no family history of hyperlipidemia, but his mother had a history of diabetes mellitus.
Physical examination showed numerous discrete erythematous papules with a creamy center on his trunk and his arms and legs. The lesions were more numerous on the extensor surfaces of the arms and legs. Some of the papules coalesced to form small plaques (FIGURE). There was no scaling, and the lesions were firm in texture. The patient’s face was spared, and there was no mucosal involvement. The patient was otherwise systemically well.
THE DIAGNOSIS
Based on the morphology, distribution, and abrupt onset of the diffuse nonpruritic papules in this morbidly obese (but otherwise systemically well) middle-aged man, a clinical diagnosis of eruptive xanthoma was suspected. Subsequent blood testing revealed an elevated serum triglyceride level of 47.8 mmol/L (reference range, <1.7 mmol/L), elevated serum total cholesterol of 7.1 mmol/L (reference range, <6.2 mmol/L), and low serum high-density lipoprotein cholesterol of 0.7 mmol/L (reference range, >1 mmol/L in men). He also had an elevated fasting serum glucose level of 12.9 mmol/L (reference range, 3.9–5.6 mmol/L) and an elevated hemoglobin A1c (glycated hemoglobin) level of 10.9%.
Subsequent thyroid, liver, and renal function tests were normal, but the patient had heavy proteinuria, with an elevated urine albumin-to-creatinine ratio of 355.6 mg/mmol (reference range, ≤2.5 mg/mmol). The patient was referred to a dermatologist, who confirmed the clinical diagnosis without the need for a skin biopsy.
DISCUSSION
Eruptive xanthoma is characterized by an abrupt onset of crops of multiple yellowish to brownish papules that can coalesce into small plaques. The lesions can be generalized, but tend to be more densely distributed on the extensor surfaces of the arms and legs, buttocks, and thighs.5 Eruptive xanthoma often is associated with hypertriglyceridemia, which can be primary—as a result of a genetic defect caused by familial hypertriglyceridemia—or secondary, associated with poorly controlled diabetes mellitus, morbid obesity, excessive alcohol consumption, nephrotic syndrome, hypothyroidism, primary biliary cholangitis, and drugs like estrogen replacement therapies, corticosteroids, and isotretinoin.6 Pruritus and tenderness may or may not be present, and the Köbner phenomenon may occur.7
Continue to: The differential diagnosis
The differential diagnosis for eruptive xanthoma includes xanthoma disseminatum, non–Langerhans cell histiocytoses (eg, generalized eruptive histiocytosis), and cutaneous mastocytosis.1
Xanthoma disseminatum is an extremely rare, but benign, disorder of non–Langerhans cell origin. The average age of onset is older than 40 years. The rash consists of multiple red-yellow papules and nodules that most commonly present in flexural areas. Forty percent to 60% of patients have mucosal involvement, and rarely the central nervous system is involved.8
Generalized eruptive histiocytosis is another rare non–Langerhans cell histiocytosis that occurs mainly in adults and is characterized by widespread, symmetric, red-brown papules on the trunk, arms, and legs, and rarely the mucous membranes.9
Cutaneous mastocytosis, especially xanthelasmoid mastocytosis, consists of multiple pruritic, yellowish, papular or nodular lesions that may mimic eruptive xanthoma. It occurs mainly in children and rarely in adults.10
Confirming the diagnosis, initiating treatment
The diagnosis of eruptive xanthoma can be confirmed by skin biopsy if other differential diagnoses cannot be ruled out or the lesions do not resolve with treatment. Skin biopsy will reveal lipid-laden macrophages (known as foam cells) deposited in the dermis.7
Continue to: Treatment of eruptive xanthoma
Treatment of eruptive xanthoma involves management of the underlying causes of the condition. In most cases, dietary control, intensive triglyceride-lowering therapies, and treatment of other secondary causes of hypertriglyceridemia result in complete resolution of the lesions within several weeks.5
Our patient’s outcome
Our patient’s sudden-onset rash alerted us to the presence of type 2 diabetes mellitus, hypertriglyceridemia, and heavy proteinuria, which he was not aware of previously. We counselled him about stringent low-sugar, low-lipid diet control and exercise, and we started him on metformin and gemfibrozil. He was referred to an internal medicine specialist for further assessment and management of his severe hypertriglyceridemia and heavy proteinuria.
The rash started to wane 1 month after the patient started the metformin and gemfibrozil, and his drug regimen was changed to combination therapy with metformin/glimepiride and fenofibrate/simvastatin 6 weeks later when he was seen in the medical specialty clinic. Fundus photography performed 1 month after starting oral antidiabetic therapy showed no diabetic retinopathy or lipemia retinalis.
After 3 months of treatment, his serum triglycerides and hemoglobin A1c levels dropped to 3.8 mmol/L and 8.7%, respectively. The rash also resolved considerably, with only residual papules on the abdomen. This rapid clinical response to treatment of the underlying hypertriglyceridemia and diabetes further supported the clinical diagnosis of eruptive xanthoma.
THE TAKEAWAY
Eruptive xanthoma is relatively rare, but it is important for family physicians to recognize this clinical presentation as a potential indicator of severe hypertriglyceridemia. Recognizing hypertriglyceridemia early is important, as it can be associated with an increased risk for acute pancreatitis. Moreover, eruptive xanthoma might be the sole presenting symptom of underlying diabetes mellitus or familial hyperlipidemia, both of which can lead to a significant increase in cardiovascular risk if uncontrolled.
CORRESPONDENCE
Chan Kam Sum, MBChB, FRACGP, Tseung Kwan O Jockey Club General Out-patient Clinic, 99 Po Lam Road North, G/F, Tseung Kwan O, Kowloon, Hong Kong; cks048@ha.org.hk
THE CASE
A 37-year-old man presented with a sudden-onset, nonpruritic, nonpainful, papular rash of 1 month’s duration on his trunk and both arms and legs. Two weeks prior to the current presentation, he consulted a general practitioner, who treated the rash with a course of unknown oral antibiotics; the patient showed no improvement. He recalled that on a few occasions, he used his fingers to express a creamy discharge from some of the lesions. This temporarily reduced the size of those papules.
His medical history was unremarkable except for morbid obesity. He did not drink alcohol regularly and was not taking any medications prior to the onset of the rash. He had no family history of hyperlipidemia, but his mother had a history of diabetes mellitus.
Physical examination showed numerous discrete erythematous papules with a creamy center on his trunk and his arms and legs. The lesions were more numerous on the extensor surfaces of the arms and legs. Some of the papules coalesced to form small plaques (FIGURE). There was no scaling, and the lesions were firm in texture. The patient’s face was spared, and there was no mucosal involvement. The patient was otherwise systemically well.
THE DIAGNOSIS
Based on the morphology, distribution, and abrupt onset of the diffuse nonpruritic papules in this morbidly obese (but otherwise systemically well) middle-aged man, a clinical diagnosis of eruptive xanthoma was suspected. Subsequent blood testing revealed an elevated serum triglyceride level of 47.8 mmol/L (reference range, <1.7 mmol/L), elevated serum total cholesterol of 7.1 mmol/L (reference range, <6.2 mmol/L), and low serum high-density lipoprotein cholesterol of 0.7 mmol/L (reference range, >1 mmol/L in men). He also had an elevated fasting serum glucose level of 12.9 mmol/L (reference range, 3.9–5.6 mmol/L) and an elevated hemoglobin A1c (glycated hemoglobin) level of 10.9%.
Subsequent thyroid, liver, and renal function tests were normal, but the patient had heavy proteinuria, with an elevated urine albumin-to-creatinine ratio of 355.6 mg/mmol (reference range, ≤2.5 mg/mmol). The patient was referred to a dermatologist, who confirmed the clinical diagnosis without the need for a skin biopsy.
DISCUSSION
Eruptive xanthoma is characterized by an abrupt onset of crops of multiple yellowish to brownish papules that can coalesce into small plaques. The lesions can be generalized, but tend to be more densely distributed on the extensor surfaces of the arms and legs, buttocks, and thighs.5 Eruptive xanthoma often is associated with hypertriglyceridemia, which can be primary—as a result of a genetic defect caused by familial hypertriglyceridemia—or secondary, associated with poorly controlled diabetes mellitus, morbid obesity, excessive alcohol consumption, nephrotic syndrome, hypothyroidism, primary biliary cholangitis, and drugs like estrogen replacement therapies, corticosteroids, and isotretinoin.6 Pruritus and tenderness may or may not be present, and the Köbner phenomenon may occur.7
Continue to: The differential diagnosis
The differential diagnosis for eruptive xanthoma includes xanthoma disseminatum, non–Langerhans cell histiocytoses (eg, generalized eruptive histiocytosis), and cutaneous mastocytosis.1
Xanthoma disseminatum is an extremely rare, but benign, disorder of non–Langerhans cell origin. The average age of onset is older than 40 years. The rash consists of multiple red-yellow papules and nodules that most commonly present in flexural areas. Forty percent to 60% of patients have mucosal involvement, and rarely the central nervous system is involved.8
Generalized eruptive histiocytosis is another rare non–Langerhans cell histiocytosis that occurs mainly in adults and is characterized by widespread, symmetric, red-brown papules on the trunk, arms, and legs, and rarely the mucous membranes.9
Cutaneous mastocytosis, especially xanthelasmoid mastocytosis, consists of multiple pruritic, yellowish, papular or nodular lesions that may mimic eruptive xanthoma. It occurs mainly in children and rarely in adults.10
Confirming the diagnosis, initiating treatment
The diagnosis of eruptive xanthoma can be confirmed by skin biopsy if other differential diagnoses cannot be ruled out or the lesions do not resolve with treatment. Skin biopsy will reveal lipid-laden macrophages (known as foam cells) deposited in the dermis.7
Continue to: Treatment of eruptive xanthoma
Treatment of eruptive xanthoma involves management of the underlying causes of the condition. In most cases, dietary control, intensive triglyceride-lowering therapies, and treatment of other secondary causes of hypertriglyceridemia result in complete resolution of the lesions within several weeks.5
Our patient’s outcome
Our patient’s sudden-onset rash alerted us to the presence of type 2 diabetes mellitus, hypertriglyceridemia, and heavy proteinuria, which he was not aware of previously. We counselled him about stringent low-sugar, low-lipid diet control and exercise, and we started him on metformin and gemfibrozil. He was referred to an internal medicine specialist for further assessment and management of his severe hypertriglyceridemia and heavy proteinuria.
The rash started to wane 1 month after the patient started the metformin and gemfibrozil, and his drug regimen was changed to combination therapy with metformin/glimepiride and fenofibrate/simvastatin 6 weeks later when he was seen in the medical specialty clinic. Fundus photography performed 1 month after starting oral antidiabetic therapy showed no diabetic retinopathy or lipemia retinalis.
After 3 months of treatment, his serum triglycerides and hemoglobin A1c levels dropped to 3.8 mmol/L and 8.7%, respectively. The rash also resolved considerably, with only residual papules on the abdomen. This rapid clinical response to treatment of the underlying hypertriglyceridemia and diabetes further supported the clinical diagnosis of eruptive xanthoma.
THE TAKEAWAY
Eruptive xanthoma is relatively rare, but it is important for family physicians to recognize this clinical presentation as a potential indicator of severe hypertriglyceridemia. Recognizing hypertriglyceridemia early is important, as it can be associated with an increased risk for acute pancreatitis. Moreover, eruptive xanthoma might be the sole presenting symptom of underlying diabetes mellitus or familial hyperlipidemia, both of which can lead to a significant increase in cardiovascular risk if uncontrolled.
CORRESPONDENCE
Chan Kam Sum, MBChB, FRACGP, Tseung Kwan O Jockey Club General Out-patient Clinic, 99 Po Lam Road North, G/F, Tseung Kwan O, Kowloon, Hong Kong; cks048@ha.org.hk
1. Tang WK. Eruptive xanthoma. [case reports]. Hong Kong Dermatol Venereol Bull. 2001;9:172-175.
2. Frew J, Murrell D, Haber R. Fifty shades of yellow: a review of the xanthodermatoses. Int J Dermatol. 2015;54:1109-1123.
3. Zak A, Zeman M, Slaby A, et al. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:181-188.
4. Sandhu S, Al-Sarraf A, Taraboanta C, et al. Incidence of pancreatitis, secondary causes, and treatment of patients referred to specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids Health Dis. 2011;10:157.
5. Holsinger JM, Campbell SM, Witman P. Multiple erythematous-yellow, dome-shaped papules. Am Fam Physician. 2010;82:517.
6. Loeckermann S, Braun-Falco M. Eruptive xanthomas in association with metabolic syndrome. Clin Exp Dermatol. 2010;35:565-566.
7. Merola JF, Mengden SJ, Soldano A, et al. Eruptive xanthomas. Dermatol Online J. 2008;14:10.
8. Park M, Boone B, Devas S. Xanthoma disseminatum: case report and mini-review of the literature. Acta Dermatovenerol Croat. 2014;22:150-154.
9. Attia A, Seleit I, El Badawy N, et al. Photoletter to the editor: generalized eruptive histiocytoma. J Dermatol Case Rep. 2011;5:53-55.
10. Nabavi NS, Nejad MH, Feli S, et al. Adult onset of xanthelasmoid mastocytosis: report of a rare entity. Indian J Dermatol. 2016;61:468.
1. Tang WK. Eruptive xanthoma. [case reports]. Hong Kong Dermatol Venereol Bull. 2001;9:172-175.
2. Frew J, Murrell D, Haber R. Fifty shades of yellow: a review of the xanthodermatoses. Int J Dermatol. 2015;54:1109-1123.
3. Zak A, Zeman M, Slaby A, et al. Xanthomas: clinical and pathophysiological relations. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:181-188.
4. Sandhu S, Al-Sarraf A, Taraboanta C, et al. Incidence of pancreatitis, secondary causes, and treatment of patients referred to specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids Health Dis. 2011;10:157.
5. Holsinger JM, Campbell SM, Witman P. Multiple erythematous-yellow, dome-shaped papules. Am Fam Physician. 2010;82:517.
6. Loeckermann S, Braun-Falco M. Eruptive xanthomas in association with metabolic syndrome. Clin Exp Dermatol. 2010;35:565-566.
7. Merola JF, Mengden SJ, Soldano A, et al. Eruptive xanthomas. Dermatol Online J. 2008;14:10.
8. Park M, Boone B, Devas S. Xanthoma disseminatum: case report and mini-review of the literature. Acta Dermatovenerol Croat. 2014;22:150-154.
9. Attia A, Seleit I, El Badawy N, et al. Photoletter to the editor: generalized eruptive histiocytoma. J Dermatol Case Rep. 2011;5:53-55.
10. Nabavi NS, Nejad MH, Feli S, et al. Adult onset of xanthelasmoid mastocytosis: report of a rare entity. Indian J Dermatol. 2016;61:468.
Bariatric surgery + medical therapy: Effective Tx for T2DM?
ILLUSTRATIVE CASE
A 46-year-old woman presents with a body mass index (BMI) of 28 kg/m2, a 4-year history of type 2 diabetes mellitus (T2DM), and a glycated hemoglobin (HgbA1c) of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 units/d, with minimal change in HgbA1c. Should you recommend bariatric surgery as an option for the treatment of diabetes?
One in 11 Americans has diabetes and at least 95% of those have type 2.2,3 The treatment of T2DM is generally multimodal in order to target the various mechanisms that cause hyperglycemia. Treatment strategies may include lifestyle modifications, decreasing insulin resistance, increasing secretion of insulin, insulin replacement, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) currently recommends diet, exercise, and behavioral modifications as first-line therapy for the management of diabetes,2 but these by themselves are often inadequate. In addition to various pharmacotherapeutic strategies for other populations with T2DM (see the PURL, “How do these 3 diabetes agents compare in reducing mortality?”), the ADA recommends bariatric surgery for the treatment of patients with T2DM, a BMI ≥35 kg/m2, and uncontrolled hyperglycemia.2,4 However, this recommendation from the ADA supporting bariatric surgery is based only on short-term studies.
For example, one single-center nonblinded randomized controlled trial (RCT) involving 60 patients with a BMI ≥35 kg/m2 found reductions in HgbA1C levels from the average baseline of 8.65±1.45% to 7.7±0.6% in the IMT group and to 6.4±1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35 kg/m2), gastric bypass had better outcomes than sleeve gastrectomy, with 93% of patients in the gastric bypass group achieving remission of T2DM vs 47% of patients in the sleeve gastrectomy group (P=.02) over a 12-month period.6
The current study sought to examine the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up shows surgery + intensive medical therapy works
This study by Schauer et al was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were 20 to 60 years of age, had a BMI of 27 to 43 kg/m2, and had an HgbA1C >7%. Patients with previous bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Each patient was randomly placed in a 1:1:1 fashion into 3 groups: IMT only, IMT and gastric bypass, or IMT and sleeve gastrectomy. All patients underwent IMT as defined by the ADA. The primary outcome was the number of patients with an HgbA1c ≤6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period leaving 149; 134 completed the 5-year follow-up; 8 patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment; an additional 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
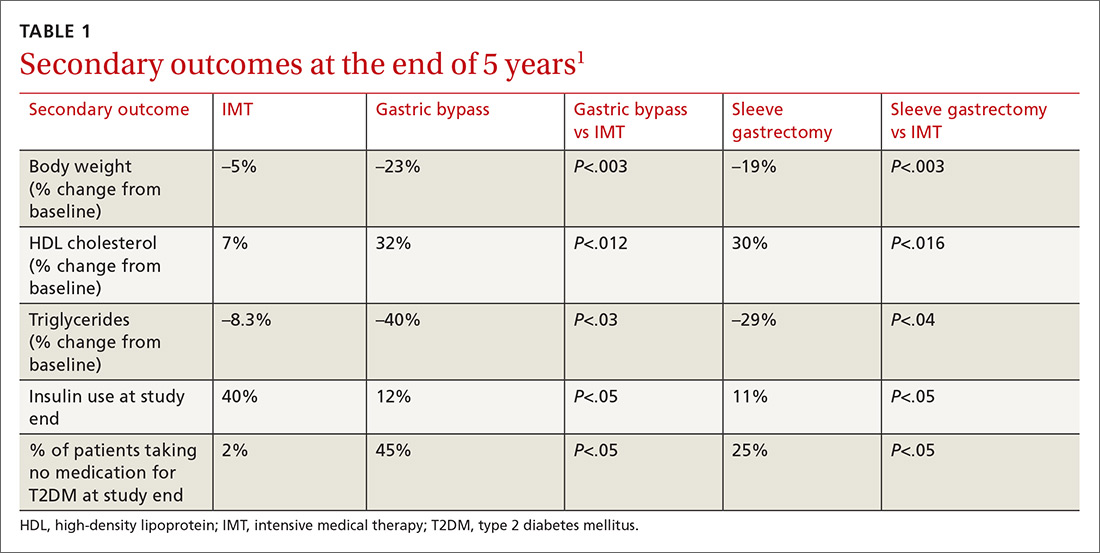
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an HgbA1c of ≤6% compared with the IMT group (14 of 49 gastric bypass patients vs 2 of 38 IMT patients; P=.01; 11 of 47 sleeve gastrectomy patients vs 2 of 38 IMT patients; P=.03). Compared with those in the IMT group, the patients in the bariatric surgery and sleeve gastrectomy groups showed greater reductions from baseline in body weight and triglyceride levels, and greater increases from baseline in high-density lipoprotein (HDL) cholesterol levels; they also required less diabetic medication for glycemic control (see TABLE 11). However, when data were imputed for the intention-to-treat analysis, P-values were P=0.08 for gastric bypass and P=0.17 for sleeve gastrectomy compared with the IMT group for lowering HgbA1c.
WHAT’S NEW?
Adding surgery has big benefits with minimal adverse effects
Prior studies that evaluated the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates bariatric surgery plus IMT has long-term benefits with minimal adverse events compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM for patients with a BMI ≥27 kg/m2, which is below the starting BMI (35 kg/m2) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications, such as gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis, in this patient population is significant.1 Complications can include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling regarding the possible complications is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1c, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size of the study...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who received gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up compared with the patients who received sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to that with IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and the cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Asssociation. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (suppl 1):S81-S89.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 1, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
ILLUSTRATIVE CASE
A 46-year-old woman presents with a body mass index (BMI) of 28 kg/m2, a 4-year history of type 2 diabetes mellitus (T2DM), and a glycated hemoglobin (HgbA1c) of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 units/d, with minimal change in HgbA1c. Should you recommend bariatric surgery as an option for the treatment of diabetes?
One in 11 Americans has diabetes and at least 95% of those have type 2.2,3 The treatment of T2DM is generally multimodal in order to target the various mechanisms that cause hyperglycemia. Treatment strategies may include lifestyle modifications, decreasing insulin resistance, increasing secretion of insulin, insulin replacement, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) currently recommends diet, exercise, and behavioral modifications as first-line therapy for the management of diabetes,2 but these by themselves are often inadequate. In addition to various pharmacotherapeutic strategies for other populations with T2DM (see the PURL, “How do these 3 diabetes agents compare in reducing mortality?”), the ADA recommends bariatric surgery for the treatment of patients with T2DM, a BMI ≥35 kg/m2, and uncontrolled hyperglycemia.2,4 However, this recommendation from the ADA supporting bariatric surgery is based only on short-term studies.
For example, one single-center nonblinded randomized controlled trial (RCT) involving 60 patients with a BMI ≥35 kg/m2 found reductions in HgbA1C levels from the average baseline of 8.65±1.45% to 7.7±0.6% in the IMT group and to 6.4±1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35 kg/m2), gastric bypass had better outcomes than sleeve gastrectomy, with 93% of patients in the gastric bypass group achieving remission of T2DM vs 47% of patients in the sleeve gastrectomy group (P=.02) over a 12-month period.6
The current study sought to examine the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up shows surgery + intensive medical therapy works
This study by Schauer et al was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were 20 to 60 years of age, had a BMI of 27 to 43 kg/m2, and had an HgbA1C >7%. Patients with previous bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Each patient was randomly placed in a 1:1:1 fashion into 3 groups: IMT only, IMT and gastric bypass, or IMT and sleeve gastrectomy. All patients underwent IMT as defined by the ADA. The primary outcome was the number of patients with an HgbA1c ≤6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period leaving 149; 134 completed the 5-year follow-up; 8 patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment; an additional 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an HgbA1c of ≤6% compared with the IMT group (14 of 49 gastric bypass patients vs 2 of 38 IMT patients; P=.01; 11 of 47 sleeve gastrectomy patients vs 2 of 38 IMT patients; P=.03). Compared with those in the IMT group, the patients in the bariatric surgery and sleeve gastrectomy groups showed greater reductions from baseline in body weight and triglyceride levels, and greater increases from baseline in high-density lipoprotein (HDL) cholesterol levels; they also required less diabetic medication for glycemic control (see TABLE 11). However, when data were imputed for the intention-to-treat analysis, P-values were P=0.08 for gastric bypass and P=0.17 for sleeve gastrectomy compared with the IMT group for lowering HgbA1c.
WHAT’S NEW?
Adding surgery has big benefits with minimal adverse effects
Prior studies that evaluated the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates bariatric surgery plus IMT has long-term benefits with minimal adverse events compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM for patients with a BMI ≥27 kg/m2, which is below the starting BMI (35 kg/m2) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications, such as gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis, in this patient population is significant.1 Complications can include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling regarding the possible complications is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1c, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size of the study...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who received gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up compared with the patients who received sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to that with IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and the cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 46-year-old woman presents with a body mass index (BMI) of 28 kg/m2, a 4-year history of type 2 diabetes mellitus (T2DM), and a glycated hemoglobin (HgbA1c) of 9.8%. The patient is currently being treated with intensive medical therapy (IMT), including metformin 2000 mg/d, sitagliptin 100 mg/d, and insulin glargine 12 units/d, with minimal change in HgbA1c. Should you recommend bariatric surgery as an option for the treatment of diabetes?
One in 11 Americans has diabetes and at least 95% of those have type 2.2,3 The treatment of T2DM is generally multimodal in order to target the various mechanisms that cause hyperglycemia. Treatment strategies may include lifestyle modifications, decreasing insulin resistance, increasing secretion of insulin, insulin replacement, and targeting incretin-hormonal pathways.
The American Diabetes Association (ADA) currently recommends diet, exercise, and behavioral modifications as first-line therapy for the management of diabetes,2 but these by themselves are often inadequate. In addition to various pharmacotherapeutic strategies for other populations with T2DM (see the PURL, “How do these 3 diabetes agents compare in reducing mortality?”), the ADA recommends bariatric surgery for the treatment of patients with T2DM, a BMI ≥35 kg/m2, and uncontrolled hyperglycemia.2,4 However, this recommendation from the ADA supporting bariatric surgery is based only on short-term studies.
For example, one single-center nonblinded randomized controlled trial (RCT) involving 60 patients with a BMI ≥35 kg/m2 found reductions in HgbA1C levels from the average baseline of 8.65±1.45% to 7.7±0.6% in the IMT group and to 6.4±1.4% in the gastric-bypass group at 2 years.5 In another study, a randomized double-blind trial involving 60 moderately obese patients (BMI, 25-35 kg/m2), gastric bypass had better outcomes than sleeve gastrectomy, with 93% of patients in the gastric bypass group achieving remission of T2DM vs 47% of patients in the sleeve gastrectomy group (P=.02) over a 12-month period.6
The current study sought to examine the long-term outcomes of IMT alone vs bariatric surgery with IMT for the treatment of T2DM in patients who are overweight or obese.1
STUDY SUMMARY
5-year follow-up shows surgery + intensive medical therapy works
This study by Schauer et al was a 5-year follow-up of a nonblinded, single-center RCT comparing IMT alone to IMT with Roux-en-Y gastric bypass or sleeve gastrectomy in 150 patients with T2DM.1 Patients were included if they were 20 to 60 years of age, had a BMI of 27 to 43 kg/m2, and had an HgbA1C >7%. Patients with previous bariatric surgery, complex abdominal surgery, or uncontrolled medical or psychiatric disorders were excluded.
Each patient was randomly placed in a 1:1:1 fashion into 3 groups: IMT only, IMT and gastric bypass, or IMT and sleeve gastrectomy. All patients underwent IMT as defined by the ADA. The primary outcome was the number of patients with an HgbA1c ≤6%. Secondary outcomes included weight loss, glucose control, lipid levels, blood pressure, medication use, renal function, adverse effects, ophthalmologic outcomes, and quality of life.
Continue to: Of the 150 patients...
Of the 150 patients, 1 died during the follow-up period leaving 149; 134 completed the 5-year follow-up; 8 patients in the IMT group and 1 patient in the sleeve gastrectomy group never initiated assigned treatment; an additional 6 patients were lost to follow-up. One patient from the IMT group and 1 patient from the sleeve gastrectomy group crossed over to the gastric bypass group.
Results. More patients in the bariatric surgery and sleeve gastrectomy groups achieved an HgbA1c of ≤6% compared with the IMT group (14 of 49 gastric bypass patients vs 2 of 38 IMT patients; P=.01; 11 of 47 sleeve gastrectomy patients vs 2 of 38 IMT patients; P=.03). Compared with those in the IMT group, the patients in the bariatric surgery and sleeve gastrectomy groups showed greater reductions from baseline in body weight and triglyceride levels, and greater increases from baseline in high-density lipoprotein (HDL) cholesterol levels; they also required less diabetic medication for glycemic control (see TABLE 11). However, when data were imputed for the intention-to-treat analysis, P-values were P=0.08 for gastric bypass and P=0.17 for sleeve gastrectomy compared with the IMT group for lowering HgbA1c.
WHAT’S NEW?
Adding surgery has big benefits with minimal adverse effects
Prior studies that evaluated the effect of gastric bypass surgery on diabetes were observational or had a shorter follow-up duration. This study demonstrates bariatric surgery plus IMT has long-term benefits with minimal adverse events compared with IMT alone.1,5 Additionally, this study supports recommendations for bariatric surgery as treatment for T2DM for patients with a BMI ≥27 kg/m2, which is below the starting BMI (35 kg/m2) recommended by the ADA.1,4
CAVEATS
Surgery is not without risks
The risk for surgical complications, such as gastrointestinal bleeding, severe hypoglycemia requiring intervention, and ketoacidosis, in this patient population is significant.1 Complications can include gastrointestinal leak, stroke, and infection.1 Additionally, long-term complications from bariatric surgery are emerging and include choledocholithiasis, intestinal obstruction, and esophageal pathology.7 Extensive patient counseling regarding the possible complications is necessary to ensure that patients make an informed decision regarding surgery.
This study utilized surrogate markers (A1c, lipid levels, and body weight) as disease-oriented outcome measures. Patient-oriented outcomes, such as morbidity and mortality, were not explored in this study.
Continue to: Due to the small sample size of the study...
Due to the small sample size of the study, it is unclear if the outcomes of the 2 surgery groups were significantly different. Patients who received gastric bypass surgery had more weight loss and used less diabetes medication at the end of follow-up compared with the patients who received sleeve gastrectomy. More information is needed to determine which gastric surgery is preferable for the treatment of T2DM while minimizing adverse effects. However, both of the procedures had outcomes superior to that with IMT, and selection of a particular type of surgery should be a joint decision between the patient and provider.
CHALLENGES TO IMPLEMENTATION
Access and cost may be barriers
The major barriers to implementation are access to, and the cost of, bariatric surgery.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Asssociation. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (suppl 1):S81-S89.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 1, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
1. Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
2. American Diabetes Asssociation. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42 (suppl 1):S81-S89.
3. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 1, 2019.
4. Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861-877.
5. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585.
6. Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148.
7. Schulman AR, Thompson CC. Complications of bariatric surgery: what you can expect to see in your GI practice. Am J Gastroenterol. 2017;112:1640-1655.
PRACTICE CHANGER
Consider bariatric surgery with medical therapy as a treatment option for adults with uncontrolled type 2 diabetes and a body mass index ≥27 kg/m2.1
STRENGTH OF RECOMMENDATION
B: Based on a nonblinded, single-center, randomized controlled trial.
Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641-651.
Are You Sitting Down for This?
Not all sedentary behavior is equal, say researchers from Universidad Autónoma de Madrid in Spain, who evaluated the sedentary habits of 5,459 women and 4,740 men.
The researchers note that several studies have found that, unlike, for example, computer use and reading, TV watching is consistently associated with adverse health outcomes, such as metabolic syndrome, obesity, and diabetes mellitus (DM). But different sedentary behaviors (SBs) have different health effects, they add. They cite research that suggests TV and other “passive” SBs (eg, listening or talking while sitting) could be more harmful than “mentally active” SBs, such as computer use and reading. In this study, “passive” sedentary time, such as TV watching, was associated with less recreational activity and higher body weight. Time at the computer and reading were linked to more recreational physical activity but less light-intensity activity at home.
Moreover, each type of SB has a distinct demographic and lifestyle profile, the researchers say. Older age, lower education, unhealthy lifestyle (smoking, worse diet, less physical activity, higher BMI) and chronic morbidity, such as DM or osteomuscular disease, were linked to more TV time. Longer time at the computer or in commuting was linked to younger age, male gender, higher education, and a sedentary job.
Watching TV had no association with total time spent on the rest of leisure-time SBs. The researchers also found that “mentally active” SBs, such as using the computer and reading, tend to cluster.
Many studies have looked at the effects of and connections between SB, lifestyle choices, and health. The researchers of this study say theirs extends knowledge in the field by considering more types of SB (using the computer, commuting, lying in the sun, listening to music, and reading). To their knowledge, they say, no previous study on a representative sample of an entire country has examined the association between TV watching time and the rest of SB, or has reported the full profile of sociodemographic, lifestyle, and health variables associated with each type of SB.
Watching TV was the predominant SB (45% of total sitting time), followed by sitting at the computer (23%), reading (15%), and commuting (12%). The participants spent a mean of 1.96 hours a day watching TV, vs > 1 hour for the other behaviors.
Not all sedentary behavior is equal, say researchers from Universidad Autónoma de Madrid in Spain, who evaluated the sedentary habits of 5,459 women and 4,740 men.
The researchers note that several studies have found that, unlike, for example, computer use and reading, TV watching is consistently associated with adverse health outcomes, such as metabolic syndrome, obesity, and diabetes mellitus (DM). But different sedentary behaviors (SBs) have different health effects, they add. They cite research that suggests TV and other “passive” SBs (eg, listening or talking while sitting) could be more harmful than “mentally active” SBs, such as computer use and reading. In this study, “passive” sedentary time, such as TV watching, was associated with less recreational activity and higher body weight. Time at the computer and reading were linked to more recreational physical activity but less light-intensity activity at home.
Moreover, each type of SB has a distinct demographic and lifestyle profile, the researchers say. Older age, lower education, unhealthy lifestyle (smoking, worse diet, less physical activity, higher BMI) and chronic morbidity, such as DM or osteomuscular disease, were linked to more TV time. Longer time at the computer or in commuting was linked to younger age, male gender, higher education, and a sedentary job.
Watching TV had no association with total time spent on the rest of leisure-time SBs. The researchers also found that “mentally active” SBs, such as using the computer and reading, tend to cluster.
Many studies have looked at the effects of and connections between SB, lifestyle choices, and health. The researchers of this study say theirs extends knowledge in the field by considering more types of SB (using the computer, commuting, lying in the sun, listening to music, and reading). To their knowledge, they say, no previous study on a representative sample of an entire country has examined the association between TV watching time and the rest of SB, or has reported the full profile of sociodemographic, lifestyle, and health variables associated with each type of SB.
Watching TV was the predominant SB (45% of total sitting time), followed by sitting at the computer (23%), reading (15%), and commuting (12%). The participants spent a mean of 1.96 hours a day watching TV, vs > 1 hour for the other behaviors.
Not all sedentary behavior is equal, say researchers from Universidad Autónoma de Madrid in Spain, who evaluated the sedentary habits of 5,459 women and 4,740 men.
The researchers note that several studies have found that, unlike, for example, computer use and reading, TV watching is consistently associated with adverse health outcomes, such as metabolic syndrome, obesity, and diabetes mellitus (DM). But different sedentary behaviors (SBs) have different health effects, they add. They cite research that suggests TV and other “passive” SBs (eg, listening or talking while sitting) could be more harmful than “mentally active” SBs, such as computer use and reading. In this study, “passive” sedentary time, such as TV watching, was associated with less recreational activity and higher body weight. Time at the computer and reading were linked to more recreational physical activity but less light-intensity activity at home.
Moreover, each type of SB has a distinct demographic and lifestyle profile, the researchers say. Older age, lower education, unhealthy lifestyle (smoking, worse diet, less physical activity, higher BMI) and chronic morbidity, such as DM or osteomuscular disease, were linked to more TV time. Longer time at the computer or in commuting was linked to younger age, male gender, higher education, and a sedentary job.
Watching TV had no association with total time spent on the rest of leisure-time SBs. The researchers also found that “mentally active” SBs, such as using the computer and reading, tend to cluster.
Many studies have looked at the effects of and connections between SB, lifestyle choices, and health. The researchers of this study say theirs extends knowledge in the field by considering more types of SB (using the computer, commuting, lying in the sun, listening to music, and reading). To their knowledge, they say, no previous study on a representative sample of an entire country has examined the association between TV watching time and the rest of SB, or has reported the full profile of sociodemographic, lifestyle, and health variables associated with each type of SB.
Watching TV was the predominant SB (45% of total sitting time), followed by sitting at the computer (23%), reading (15%), and commuting (12%). The participants spent a mean of 1.96 hours a day watching TV, vs > 1 hour for the other behaviors.
Supplements and food-related therapy do not prevent depression in overweight adults
Multinutrient supplements and food-related therapy, together or separately, do not reduce major depressive disorder (MDD) episodes, according to a clinical trial of overweight adults with subsyndromal depressive symptoms.
“These findings do not support the use of these interventions for prevention of major depressive disorder in this population,” wrote lead author Mariska Bot, PhD, of Amsterdam University Medical Center, and her coauthors. The study was published in JAMA.
For this randomized clinical trial, Dr. Bot and her colleagues recruited 1,025 overweight adults from four European countries. All had at least mild depressive symptoms – determined through Patient Health Questionnaire–9 scores of 5 or higher – but no MDD episode in the last 6 months. The patients were allocated into four groups: placebo without therapy (n = 257), placebo with therapy (n = 256), supplements without therapy (n = 256), and supplements with therapy (n = 256). The supplements included 1,412 mg of omega-3 fatty acids, 30 mcg of selenium, 400 mcg of folic acid, and 20 mcg of vitamin D3 plus 100 mg of calcium. The therapy sessions were focused on food-related behavioral activation and emphasized a Mediterranean-style diet.
Only 779 (76%) of the patients completed the trial. Of the 105 participants who developed an MDD episode during 12-month follow-up, 25 (9.7%) were receiving placebo alone, 26 (10.2%) were receiving placebo with therapy, 32 (12.5%) were receiving supplements alone, and 22 (8.6%) were receiving supplements with therapy. Three of the four groups had 24 patients hospitalized, and the supplements-only group saw 26 patients hospitalized.
“This study showed that multinutrient supplements containing omega-3 [polyunsaturated fatty acids], vitamin D, folic acid, and selenium neither reduced depressive symptoms, anxiety symptoms nor improved health utility measures,” Dr. Bot and her coauthors wrote. “In fact, they appeared to result in slightly poorer depressive and anxiety symptoms scores compared with placebo.”
, roughly a quarter of patients lost to follow-up, and the likelihood that patients in the placebo group might have realized that they were not taking a multivitamin. In addition, participants were not selected based on deficiencies in the nutrients provided, making it possible that “deficient individuals will be more likely to benefit from supplementation.”
The study was funded by the European Union FP7 MooDFOOD Project Multi-country Collaborative Project on the Role of Diet, Food-related Behavior, and Obesity in the Prevention of Depression. Dr. Bot reported no disclosures. Her coauthors reported receiving funding from numerous pharmaceutical companies, the European Union, and Guilford Press.
SOURCE: Bot M et al. JAMA. 2019 Mar 5. doi: 10.1001/jama.2019.0556.
Though links between mental and physical health disorders have been established, this study from Bot et al. reinforces the murkiness of a nascent field like nutritional psychiatry, according to Michael Berk, MD, PhD, and Felice N. Jacka, PhD, of Deakin University in Victoria, Australia.
“Prevention of major depressive disorder is difficult to study,” they wrote, which makes a trial like this so important. However, its findings also emphasize the ambiguous nature of oft-touted remedies, specifically the “liberal and mostly non–evidence-based use of nutrient supplement combinations for psychiatric disorders.”
This study raises as many questions as it answers. Only 71% of those in the food-related behavioral activation therapy group attended more than 8 of the 21 offered sessions, and there is no way to prove how many followed the dietary restrictions. Those who did attend at least of 8 the sessions “showed a significant reduction in risk of depression,” however, which supports other trials that have associated dietary adherence and symptom improvement.
Diet is not a sole treatment for depression. However, the authors acknowledged that it is likely a piece of the complex puzzle that nutritional psychiatry wishes to solve. “These recent findings,” they wrote, “highlight that an integrated care package incorporating first-line psychological and pharmacological treatments, along with evidence-based lifestyle interventions addressing ... diet quality, may have a more robust effect on this burdensome disorder.”
These comments are adapted from an accompanying editorial (JAMA. 2019 Mar 5. doi: 10.1001/jama.2019.0273 ). Both coauthors reported conflicts of interest, including receiving grants, consulting fees, and research support from numerous boards, pharmaceutical companies, and foundations.
Though links between mental and physical health disorders have been established, this study from Bot et al. reinforces the murkiness of a nascent field like nutritional psychiatry, according to Michael Berk, MD, PhD, and Felice N. Jacka, PhD, of Deakin University in Victoria, Australia.
“Prevention of major depressive disorder is difficult to study,” they wrote, which makes a trial like this so important. However, its findings also emphasize the ambiguous nature of oft-touted remedies, specifically the “liberal and mostly non–evidence-based use of nutrient supplement combinations for psychiatric disorders.”
This study raises as many questions as it answers. Only 71% of those in the food-related behavioral activation therapy group attended more than 8 of the 21 offered sessions, and there is no way to prove how many followed the dietary restrictions. Those who did attend at least of 8 the sessions “showed a significant reduction in risk of depression,” however, which supports other trials that have associated dietary adherence and symptom improvement.
Diet is not a sole treatment for depression. However, the authors acknowledged that it is likely a piece of the complex puzzle that nutritional psychiatry wishes to solve. “These recent findings,” they wrote, “highlight that an integrated care package incorporating first-line psychological and pharmacological treatments, along with evidence-based lifestyle interventions addressing ... diet quality, may have a more robust effect on this burdensome disorder.”
These comments are adapted from an accompanying editorial (JAMA. 2019 Mar 5. doi: 10.1001/jama.2019.0273 ). Both coauthors reported conflicts of interest, including receiving grants, consulting fees, and research support from numerous boards, pharmaceutical companies, and foundations.
Though links between mental and physical health disorders have been established, this study from Bot et al. reinforces the murkiness of a nascent field like nutritional psychiatry, according to Michael Berk, MD, PhD, and Felice N. Jacka, PhD, of Deakin University in Victoria, Australia.
“Prevention of major depressive disorder is difficult to study,” they wrote, which makes a trial like this so important. However, its findings also emphasize the ambiguous nature of oft-touted remedies, specifically the “liberal and mostly non–evidence-based use of nutrient supplement combinations for psychiatric disorders.”
This study raises as many questions as it answers. Only 71% of those in the food-related behavioral activation therapy group attended more than 8 of the 21 offered sessions, and there is no way to prove how many followed the dietary restrictions. Those who did attend at least of 8 the sessions “showed a significant reduction in risk of depression,” however, which supports other trials that have associated dietary adherence and symptom improvement.
Diet is not a sole treatment for depression. However, the authors acknowledged that it is likely a piece of the complex puzzle that nutritional psychiatry wishes to solve. “These recent findings,” they wrote, “highlight that an integrated care package incorporating first-line psychological and pharmacological treatments, along with evidence-based lifestyle interventions addressing ... diet quality, may have a more robust effect on this burdensome disorder.”
These comments are adapted from an accompanying editorial (JAMA. 2019 Mar 5. doi: 10.1001/jama.2019.0273 ). Both coauthors reported conflicts of interest, including receiving grants, consulting fees, and research support from numerous boards, pharmaceutical companies, and foundations.
Multinutrient supplements and food-related therapy, together or separately, do not reduce major depressive disorder (MDD) episodes, according to a clinical trial of overweight adults with subsyndromal depressive symptoms.
“These findings do not support the use of these interventions for prevention of major depressive disorder in this population,” wrote lead author Mariska Bot, PhD, of Amsterdam University Medical Center, and her coauthors. The study was published in JAMA.
For this randomized clinical trial, Dr. Bot and her colleagues recruited 1,025 overweight adults from four European countries. All had at least mild depressive symptoms – determined through Patient Health Questionnaire–9 scores of 5 or higher – but no MDD episode in the last 6 months. The patients were allocated into four groups: placebo without therapy (n = 257), placebo with therapy (n = 256), supplements without therapy (n = 256), and supplements with therapy (n = 256). The supplements included 1,412 mg of omega-3 fatty acids, 30 mcg of selenium, 400 mcg of folic acid, and 20 mcg of vitamin D3 plus 100 mg of calcium. The therapy sessions were focused on food-related behavioral activation and emphasized a Mediterranean-style diet.
Only 779 (76%) of the patients completed the trial. Of the 105 participants who developed an MDD episode during 12-month follow-up, 25 (9.7%) were receiving placebo alone, 26 (10.2%) were receiving placebo with therapy, 32 (12.5%) were receiving supplements alone, and 22 (8.6%) were receiving supplements with therapy. Three of the four groups had 24 patients hospitalized, and the supplements-only group saw 26 patients hospitalized.
“This study showed that multinutrient supplements containing omega-3 [polyunsaturated fatty acids], vitamin D, folic acid, and selenium neither reduced depressive symptoms, anxiety symptoms nor improved health utility measures,” Dr. Bot and her coauthors wrote. “In fact, they appeared to result in slightly poorer depressive and anxiety symptoms scores compared with placebo.”
, roughly a quarter of patients lost to follow-up, and the likelihood that patients in the placebo group might have realized that they were not taking a multivitamin. In addition, participants were not selected based on deficiencies in the nutrients provided, making it possible that “deficient individuals will be more likely to benefit from supplementation.”
The study was funded by the European Union FP7 MooDFOOD Project Multi-country Collaborative Project on the Role of Diet, Food-related Behavior, and Obesity in the Prevention of Depression. Dr. Bot reported no disclosures. Her coauthors reported receiving funding from numerous pharmaceutical companies, the European Union, and Guilford Press.
SOURCE: Bot M et al. JAMA. 2019 Mar 5. doi: 10.1001/jama.2019.0556.
Multinutrient supplements and food-related therapy, together or separately, do not reduce major depressive disorder (MDD) episodes, according to a clinical trial of overweight adults with subsyndromal depressive symptoms.
“These findings do not support the use of these interventions for prevention of major depressive disorder in this population,” wrote lead author Mariska Bot, PhD, of Amsterdam University Medical Center, and her coauthors. The study was published in JAMA.
For this randomized clinical trial, Dr. Bot and her colleagues recruited 1,025 overweight adults from four European countries. All had at least mild depressive symptoms – determined through Patient Health Questionnaire–9 scores of 5 or higher – but no MDD episode in the last 6 months. The patients were allocated into four groups: placebo without therapy (n = 257), placebo with therapy (n = 256), supplements without therapy (n = 256), and supplements with therapy (n = 256). The supplements included 1,412 mg of omega-3 fatty acids, 30 mcg of selenium, 400 mcg of folic acid, and 20 mcg of vitamin D3 plus 100 mg of calcium. The therapy sessions were focused on food-related behavioral activation and emphasized a Mediterranean-style diet.
Only 779 (76%) of the patients completed the trial. Of the 105 participants who developed an MDD episode during 12-month follow-up, 25 (9.7%) were receiving placebo alone, 26 (10.2%) were receiving placebo with therapy, 32 (12.5%) were receiving supplements alone, and 22 (8.6%) were receiving supplements with therapy. Three of the four groups had 24 patients hospitalized, and the supplements-only group saw 26 patients hospitalized.
“This study showed that multinutrient supplements containing omega-3 [polyunsaturated fatty acids], vitamin D, folic acid, and selenium neither reduced depressive symptoms, anxiety symptoms nor improved health utility measures,” Dr. Bot and her coauthors wrote. “In fact, they appeared to result in slightly poorer depressive and anxiety symptoms scores compared with placebo.”
, roughly a quarter of patients lost to follow-up, and the likelihood that patients in the placebo group might have realized that they were not taking a multivitamin. In addition, participants were not selected based on deficiencies in the nutrients provided, making it possible that “deficient individuals will be more likely to benefit from supplementation.”
The study was funded by the European Union FP7 MooDFOOD Project Multi-country Collaborative Project on the Role of Diet, Food-related Behavior, and Obesity in the Prevention of Depression. Dr. Bot reported no disclosures. Her coauthors reported receiving funding from numerous pharmaceutical companies, the European Union, and Guilford Press.
SOURCE: Bot M et al. JAMA. 2019 Mar 5. doi: 10.1001/jama.2019.0556.
FROM JAMA
Key clinical point: A combination of multinutrient supplements and food-related behavioral activation therapy did not reduce episodes of major depressive disorder in overweight adults.
Major finding: Of the 105 participants who developed an MDD episode, 25 (9.7%) were receiving placebo alone, 26 (10.2%) were receiving placebo with therapy, 32 (12.5%) were receiving supplements alone, and 22 (8.6%) were receiving supplements with therapy.
Study details: A 2 x 2 factorial randomized clinical trial of 1,025 overweight adults from four European countries with elevated depressive symptoms and no major depressive disorder episode in the past 6 months.
Disclosures: The study was funded by the European Union FP7 MooDFOOD Project Multi-country Collaborative Project on the Role of Diet, Food-related Behavior, and Obesity in the Prevention of Depression. Dr. Bot reported no disclosures. Her coauthors reported receiving funding from numerous pharmaceutical companies, the European Union, and Guilford Press.
Source: Bot M et al. JAMA. 2019 Mar 5. doi: 10.1001/jama.2019.0556.
Assessing liver fibrosis without biopsy in patients with HCV or NAFLD
Staging of liver fibrosis, important for determining prognosis in patients with chronic liver disease and for the need to start screening for complications of cirrhosis, was traditionally done only by liver biopsy. While biopsy is still the gold standard method to stage fibrosis, noninvasive methods have been developed that can also assess disease severity.
This article briefly reviews the epidemiology and physiology of chronic liver disease and the traditional role of liver biopsy. Pros and cons of alternative fibrosis assessment methods are discussed, with a focus on their utility for patients with nonalcoholic fatty liver disease (NAFLD) and hepatitis C virus (HCV) infection.
CHRONIC LIVER DISEASE: A HUGE HEALTH BURDEN
Chronic liver disease is associated with enormous health and financial costs in the United States. Its prevalence is about 15%,1 and it is the 12th leading cause of death.2 Hospital costs are estimated at about $4 billion annually.3
The most common causes of chronic liver disease are NAFLD (which may be present in up to one-third of the US population and is increasing with the epidemic of obesity), its aggressive variant, nonalcoholic steatohepatitis (NASH) (present in about 3% of the population), and HCV infection (1%).4,5
Since direct-acting antiviral agents were introduced, HCV infection dropped from being the leading cause of liver transplant to third place.6 But at the same time, the number of patients on the transplant waiting list who have NASH has risen faster than for any other cause of chronic liver disease.7
FIBROSIS: A KEY INDICATOR OF DISEASE SEVERITY
In HCV infection, advanced fibrosis is defined as either stage 4 to 6 using the Ishak system10 or stage 3 to 4 using the Meta-analysis of Histological Data in Viral Hepatitis (METAVIR) system.11
In NAFLD, advanced fibrosis is defined as stage 3 to 4 using the NASH Clinical Research Network system.12
Staging fibrosis is also important so that patients with cirrhosis can be identified early to begin screening for hepatocellular carcinoma and esophageal varices to reduce the risks of illness and death. In addition, insurance companies often require documentation of fibrosis stage before treating HCV with the new direct-acting antiviral agents.
LIVER BIOPSY IS STILL THE GOLD STANDARD
Although invasive, liver biopsy remains the gold standard for determining fibrosis stage. Liver biopsies were performed “blindly” (without imaging) until the 1990s, but imaging-guided biopsy using ultrasonography was then developed, which entailed less pain and lower complication and hospitalization rates. Slightly more hepatic tissue is obtained with guided liver biopsy, but the difference was deemed clinically insignificant.13 Concern initially arose about the added cost involved with imaging, but imaging-guided biopsy was actually found to be more cost-effective.14
In the 2000s, transjugular liver biopsy via the right internal jugular vein became available. This method was originally used primarily in patients with ascites or significant coagulopathy. At first, there were concerns about the adequacy of specimens obtained to make an accurate diagnosis or establish fibrosis stage, but this limitation was overcome with improved techniques.15,16 Transjugular liver biopsy has the additional advantage of enabling one to measure the hepatic venous pressure gradient, which also has prognostic significance; a gradient greater than 10 mm Hg is associated with worse prognosis.17
Disadvantages of biopsy: Complications, sampling errors
Liver biopsy has disadvantages. Reported rates of complications necessitating hospitalization using the blind method were as high as 6% in the 1970s,18 dropping to 3.2% in a 1993 study.19 Bleeding remains the most worrisome complication. With the transjugular method, major and minor complication rates are less than 1% and 7%, respectively.15,16 Complication rates with imaging-guided biopsy are also low.
Liver biopsy is also prone to sampling error. The number of portal tracts obtained in the biopsy correlates with the accuracy of fibrosis staging, and smaller samples may lead to underestimating fibrosis stage. In patients with HCV, samples more than 15 mm long led to accurate staging diagnosis in 65% of patients, and those longer than 25 mm conferred 75% accuracy.20 Also, different stages can be diagnosed from samples obtained from separate locations in the liver, although rarely is the difference more than a single stage.21
Histologic evaluation of liver biopsies is operator-dependent. Although significant interobserver variation has been reported for degree of inflammation, there tends to be good concordance for fibrosis staging.22,23
STAGING BASED ON DEMOGRAPHIC AND LABORATORY VARIABLES
Several scores based on patient characteristics and laboratory values have been developed for assessing liver fibrosis and have been specifically validated for HCV infection, NAFLD, or both. They can serve as inexpensive initial screening tests for the presence or absence of advanced fibrosis.
FIB-4 index for HCV, NAFLD
The FIB-4 index predicts the presence of advanced fibrosis using, as its name indicates, a combination of 4 factors in fibrosis: age, platelet count, and the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), according to the formula:
FIB-4 index = (age × AST [U/L]) /
(platelet count [× 109/L] × √ALT [U/L]).
The index was derived from data from 832 patients co-infected with HCV and human immunodeficiency virus.24 The Ishak staging system10 for fibrosis on liver biopsy was used for confirmation, with stage 4 to 6 defined as advanced fibrosis. A cutoff value of more than 3.25 had a positive predictive value of 65% for advanced fibrosis, and to exclude advanced fibrosis, a cutoff value of less than 1.45 had a negative predictive value of 90%.
The FIB-4 index has since been validated in patients with HCV infection25 and NAFLD.26 In a subsequent study in 142 patients with NAFLD, the FIB-4 index was more accurate in diagnosing advanced fibrosis than the other noninvasive prediction models discussed below.27
NAFLD fibrosis score
The NAFLD fibrosis score, constructed and validated only in patients with biopsy-confirmed NAFLD, incorporates age, body mass index, presence of diabetes or prediabetes, albumin level, platelet count, and AST and ALT levels.
A group of 480 patients was used to construct the score, and 253 patients were used to validate it. Using the high cutoff value of 0.676, the presence of advanced fibrosis was diagnosed with a positive predictive value of 90% in the group used to construct the model (82% in the validation group). Using the low cutoff score of –1.455, advanced fibrosis could be excluded with a negative predictive value of 93% in the construction group and 88% in the validation group.28 A score between the cutoff values merits liver biopsy to determine fibrosis stage. The score is more accurate in patients with diabetes.29 When used by primary care physicians, the NAFLD fibrosis score is more cost-effective than transient elastography and liver biopsy for accurately predicting advanced fibrosis.30
AST-to-platelet ratio index score for HCV, NAFLD
The AST-to-platelet ratio index (APRI) score was developed in 2003 using a cohort of 270 patients with HCV and liver biopsy as the standard. A cutoff value of less than or equal to 0.5 had a negative predictive value of 86% for the absence of significant fibrosis, while a score of more than 1.5 detected the presence of significant fibrosis with a positive predictive value of 88%.31 The APRI score was subsequently validated for NAFLD.27,32
FibroSure uses a patented formula
FibroSure (LabCorp; labcorp.com) uses a patented mathematical formula that takes into account age, sex, and levels of gamma-glutamyl transferase, total bilirubin, haptoglobin, apolipoprotein-A, and alpha-2 macroglobulin to assess fibrosis. Developed in 2001 for use in patients with HCV infection, it was reported to have a positive predictive value of greater than 90% and a negative predictive value of 100% for clinically significant fibrosis, defined as stage 2 to 4 based on the METAVIR staging system in the prediction model.33 The use of FibroSure in patients with HCV was subsequently validated in various meta-analyses and systematic reviews.34,35 It is less accurate in patients with normal ALT levels.36
FibroSure also has good accuracy for predicting fibrosis stage in chronic liver disease due to other causes, including NAFLD.37
The prediction models discussed above use routine laboratory tests for chronic liver disease and thus are inexpensive. The high cost of additional testing needed for FibroSure, coupled with the risk of misdiagnosis, makes its cost-effectiveness questionable.38
IMAGING TO PREDICT FIBROSIS STAGE
Conventional ultrasonography (with or without vascular imaging) and computed tomography can detect cirrhosis on the basis of certain imaging characteristics,39,40 including the nodular contour of the liver, caudate lobe hypertrophy, ascites, reversal of blood flow in the portal vein, and splenomegaly. However, they cannot detect fibrosis in its early stages.
The 3 methods discussed below provide more accurate fibrosis staging by measuring the velocity of shear waves sent across hepatic tissue. Because shear-wave velocity increases with liver stiffness, the fibrosis stage can be estimated from this information.41
Transient elastography
Transient elastography uses a special ultrasound transducer. It is highly accurate for predicting advanced fibrosis for almost all causes of chronic liver disease, including HCV infection42,43 and NAFLD.44 The cutoff values of wave velocity to estimate fibrosis stage differ by liver disease etiology.
Transient elastography should not be used to evaluate fibrosis in patients with acute hepatitis, which transiently increases liver stiffness, resulting in a falsely high fibrosis stage diagnosis.45 It is also not a good method for evaluating fibrosis in patients with biliary obstruction or extrahepatic venous congestion. Because liver stiffness can increase after eating,46 the test should be done under fasting conditions.
A significant limitation of transient elastography has been its poor accuracy in patients with obesity.47 This has been largely overcome with the use of a more powerful (XL) probe but is still a limitation for those with morbid obesity.48 Because many patients with NAFLD are obese, this limitation can be significant.
Transient elastography has gained popularity for evaluating fibrosis in patients with chronic liver disease for multiple reasons: it is cost-effective and results are highly reproducible, with low variation in results among different observers and in individual observers.49 Combined with a platelet count, it can also be used to detect the development of clinically significant portal hypertension in patients with cirrhosis, thus determining the need to screen for esophageal varices using endoscopy.50 Screening endoscopy can be avoided in patients whose liver stiffness remains below 20 kPa or whose platelet count is above 150 × 109/L.
Acoustic radiation force imaging
Unlike transient elastography, which requires a separate transducer probe to assess shear- wave velocity, acoustic radiation force imaging uses the same transducer for both this function and imaging. Different image modes are available when testing for liver stiffness, so a region of interest that is optimal for avoiding vascular structures or masses can be selected, increasing accuracy.51
Acoustic radiation force imaging has been tested in different causes of chronic liver disease, including HCV and NAFLD,52 with accuracy similar to that of transient elastography.53 For overweight and obese patients, acoustic radiation force imaging is more accurate than transient elastography using the XL probe.54 However, this method is still new, and we need more data to support using one method over the other.
Magnetic resonance elastography
Magnetic resonance elastography uses a special transducer placed under the rib cage to transmit shear waves concurrently with magnetic resonance imaging. It has been tested in patients with HCV and NAFLD and has been found to have better diagnostic accuracy than transient elastography and acoustic radiation force imaging.55,56 Patients must be fasting for better diagnostic accuracy57 and must hold their breath while elastography is performed. The need for breath-holding and the high cost limit the use of this method for assessing fibrosis.
BOTTOM LINE FOR ASSESSING FIBROSIS
- Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011; 9(6):524–530.e1. doi:10.1016/j.cgh.2011.03.020
- Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. Deaths: final data for 2009. Natl Vital Stat Rep 2011; 60(3):1–116. pmid:24974587
- Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 2012; 107(2):247–252. doi:10.1038/ajg.2011.314
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34(3):274–285. doi:10.1111/j.1365-2036.2011.04724.x
- Udompap P, Kim D, Kim WR. Current and future burden of chronic nonmalignant liver disease. Clin Gastroenterol Hepatol 2015; 13(12):2031–2041. doi:10.1016/j.cgh.2015.08.015
- Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 annual data report: liver. Am J Transplant 2018; 18(suppl 1):172–253. doi:10.1111/ajt.14559
- Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148(3):547–555. doi:10.1053/j.gastro.2014.11.039
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22(6):696–699. pmid:7560864
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996; 24(2):289–293. doi:10.1002/hep.510240201
- Kleiner DE, Brunt EM, Van Natta M, et al; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41(6):1313–1321. doi:10.1002/hep.20701
- Everhart JE, Wright EC, Goodman ZD, et al; HALT-C Trial Group. Prognostic value of Ishak fibrosis stage: findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology 2010; 51(2):585–594. doi:10.1002/hep.23315
- Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015; 149(2):389–397.e10. doi:10.1053/j.gastro.2015.04.043
- Lindor KD, Bru C, Jorgensen RA, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology 1996; 23(5):1079–1083. doi:10.1002/hep.510230522
- Pasha T, Gabriel S, Therneau T, Dickson ER, Lindor KD. Cost-effectiveness of ultrasound-guided liver biopsy. Hepatology 1998; 27(5):1220–1226. doi:10.1002/hep.510270506
- Alessandria C, Debernardi-Venon W, Rizzetto M, Marzano A. Transjugular liver biopsy: a relatively simple procedure with an indefinite past and an expected brilliant future. J Hepatol 2008; 48(1):171–173. doi:10.1016/j.jhep.2007.10.001
- Kalambokis G, Manousou P, Vibhakorn S, et al. Transjugular liver biopsy—indications, adequacy, quality of specimens, and complications—a systematic review. J Hepatol 2007; 47(2):284–294. doi:10.1016/j.jhep.2007.05.001
- Ripoll C, Groszmann R, Garcia-Tsao G, et al; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007; 133(2):481–488. doi:10.1053/j.gastro.2007.05.024
- Perrault J, McGill DB, Ott BJ, Taylor WF. Liver biopsy: complications in 1000 inpatients and outpatients. Gastroenterology 1978; 74(1):103–106. pmid:618417
- Janes CH, Lindor KD. Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med 1993; 118(2):96–98. pmid:8416324
- Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003; 38(6):1449–1457. doi:10.1016/j.hep.2003.09.022
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97(10):2614–2618. doi:10.1111/j.1572-0241.2002.06038.x
- Goldin RD, Goldin JG, Burt AD, et al. Intra-observer and inter-observer variation in the histopathological assessment of chronic viral hepatitis. J Hepatol 1996; 25(5):649–654. pmid:8938541
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994; 20(1 Pt 1):15–20. pmid:8020885
- Sterling RK, Lissen E, Clumeck N, et al; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43(6):1317–1325. doi:10.1002/hep.21178
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007; 46(1):32–36. doi:10.1002/hep.21669
- Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7(10):1104–1112. doi:10.1016/j.cgh.2009.05.033
- McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010; 59(9):1265–1269. doi:10.1136/gut.2010.216077
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846–854. doi:10.1002/hep.21496
- Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin 2015; 3:141–145. doi:10.1016/j.bbacli.2014.09.001
- Tapper EB, Hunink MG, Afdhal NH, Lai M, Sengupta N. Cost-effectiveness analysis: risk stratification of nonalcoholic fatty liver disease (NAFLD) by the primary care physician using the NAFLD fibrosis score. PLoS One 2016; 11(2):e0147237. doi:10.1371/journal.pone.0147237
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38(2):518–526. doi:10.1053/jhep.2003.50346
- Calès P, Lainé F, Boursier J, et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol 2009; 50(1):165–173. doi:10.1016/j.jhep.2008.07.035
- Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T; MULTIVIRC Group. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001; 357(9262):1069–1075. doi:10.1016/S0140-6736(00)04258-6
- Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol 2007; 102(11):2589–2600. doi:10.1111/j.1572-0241.2007.01466.x
- Smith JO, Sterling RK. Systematic review: non-invasive methods of fibrosis analysis in chronic hepatitis C. Aliment Pharmacol Ther 2009; 30(6):557–576. doi:10.1111/j.1365-2036.2009.04062.x
- Sebastiani G, Vario A, Guido M, Alberti A. Performance of noninvasive markers for liver fibrosis is reduced in chronic hepatitis C with normal transaminases. J Viral Hepat 2007; 15(3):212–218. doi:10.1111/j.1365-2893.2007.00932.x
- Poynard T, Morra R, Halfon P, et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol 2007; 7:40. doi:10.1186/1471-230X-7-40
- Carlson JJ, Kowdley KV, Sullivan SD, Ramsey SD, Veenstra DL. An evaluation of the potential cost-effectiveness of non-invasive testing strategies in the diagnosis of significant liver fibrosis. J Gastroenterol Hepatol 2009; 24(5):786–791. doi:10.1111/j.1440-1746.2009.05778.x
- Aubé C, Oberti F, Korali N, et al. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol 1999; 30(3):472–478. pmid:10190731
- Di Lelio A, Cestari C, Lomazzi A, Beretta L. Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology 1989; 172(2):389–392. doi:10.1148/radiology.172.2.2526349
- Wong VW, Chan HL. Transient elastography. J Gastroenterol Hepatol 2010; 25(11):1726–1731. doi:10.1111/j.1440-1746.2010.06437.x
- Arena U, Vizzutti F, Abraldes JG, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut 2008; 57(9):1288–1293. doi:10.1136/gut.2008.149708
- Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41(1):48–54. doi:10.1002/hep.20506
- Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010; 51(2):454–462. doi:10.1002/hep.23312
- Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2007; 48(2):592–595. doi:10.1002/hep.22056
- Mederacke I, Wursthorn K, Kirschner J, et al. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int 2009; 29(10):1500–1506. doi:10.1111/j.1478-3231.2009.02100.x
- Castéra L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2010; 51(3):828–835. doi:10.1002/hep.23425
- Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 2012; 107(12):1862–1871. doi:10.1038/ajg.2012.331
- Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007; 56(7):968–973. doi:10.1136/gut.2006.111302
- de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63(3):743–752. doi:10.1016/j.jhep.2015.05.022
- Friedrich-Rust M, Wunder K, Kriener S, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 2009; 252(2):595–604. doi:10.1148/radiol.2523081928
- Yoneda M, Suzuki K, Kato S, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology 2010; 256(2):640–647. doi:10.1148/radiol.10091662
- Bota S, Herkner H, Sporea I, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int 2013; 33(8):1138–1147. doi:10.1111/liv.12240
- Attia D, Bantel H, Lenzen H, Manns MP, Gebel MJ, Potthoff A. Liver stiffness measurement using acoustic radiation force impulse elastography in overweight and obese patients. Aliment Pharmacol Ther 2016; 44(4):366–379. doi:10.1111/apt.13710
- Cui J, Heba E, Hernandez C, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. Hepatology 2016; 63(2):453–461. doi:10.1002/hep.28337
- Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008; 135(1):32–40. doi:10.1053/j.gastro.2008.03.076
- Jajamovich GH, Dyvorne H, Donnerhack C, Taouli B. Quantitative liver MRI combining phase contrast imaging, elastography, and DWI: assessment of reproducibility and postprandial effect at 3.0 T. PLoS One 2014; 9(5):e97355. doi:10.1371/journal.pone.0097355
- Lim JK, Flamm SL, Singh S, Falck-Ytter YT; Clinical Guidelines Committee of the American Gastroenterological Association. American Gastroenterological Association Institute guideline on the role of elastography in the evaluation of liver fibrosis. Gastroenterology 2017; 152(6):1536–1543. doi:10.1053/j.gastro.2017.03.017
- N, Feldstein AE. Noninvasive diagnosis of nonalcoholic fatty liver disease: are we there yet? Metabolism 2016; 65(8):1087–1095. doi:10.1016/j.metabol.2016.01.013
Staging of liver fibrosis, important for determining prognosis in patients with chronic liver disease and for the need to start screening for complications of cirrhosis, was traditionally done only by liver biopsy. While biopsy is still the gold standard method to stage fibrosis, noninvasive methods have been developed that can also assess disease severity.
This article briefly reviews the epidemiology and physiology of chronic liver disease and the traditional role of liver biopsy. Pros and cons of alternative fibrosis assessment methods are discussed, with a focus on their utility for patients with nonalcoholic fatty liver disease (NAFLD) and hepatitis C virus (HCV) infection.
CHRONIC LIVER DISEASE: A HUGE HEALTH BURDEN
Chronic liver disease is associated with enormous health and financial costs in the United States. Its prevalence is about 15%,1 and it is the 12th leading cause of death.2 Hospital costs are estimated at about $4 billion annually.3
The most common causes of chronic liver disease are NAFLD (which may be present in up to one-third of the US population and is increasing with the epidemic of obesity), its aggressive variant, nonalcoholic steatohepatitis (NASH) (present in about 3% of the population), and HCV infection (1%).4,5
Since direct-acting antiviral agents were introduced, HCV infection dropped from being the leading cause of liver transplant to third place.6 But at the same time, the number of patients on the transplant waiting list who have NASH has risen faster than for any other cause of chronic liver disease.7
FIBROSIS: A KEY INDICATOR OF DISEASE SEVERITY
In HCV infection, advanced fibrosis is defined as either stage 4 to 6 using the Ishak system10 or stage 3 to 4 using the Meta-analysis of Histological Data in Viral Hepatitis (METAVIR) system.11
In NAFLD, advanced fibrosis is defined as stage 3 to 4 using the NASH Clinical Research Network system.12
Staging fibrosis is also important so that patients with cirrhosis can be identified early to begin screening for hepatocellular carcinoma and esophageal varices to reduce the risks of illness and death. In addition, insurance companies often require documentation of fibrosis stage before treating HCV with the new direct-acting antiviral agents.
LIVER BIOPSY IS STILL THE GOLD STANDARD
Although invasive, liver biopsy remains the gold standard for determining fibrosis stage. Liver biopsies were performed “blindly” (without imaging) until the 1990s, but imaging-guided biopsy using ultrasonography was then developed, which entailed less pain and lower complication and hospitalization rates. Slightly more hepatic tissue is obtained with guided liver biopsy, but the difference was deemed clinically insignificant.13 Concern initially arose about the added cost involved with imaging, but imaging-guided biopsy was actually found to be more cost-effective.14
In the 2000s, transjugular liver biopsy via the right internal jugular vein became available. This method was originally used primarily in patients with ascites or significant coagulopathy. At first, there were concerns about the adequacy of specimens obtained to make an accurate diagnosis or establish fibrosis stage, but this limitation was overcome with improved techniques.15,16 Transjugular liver biopsy has the additional advantage of enabling one to measure the hepatic venous pressure gradient, which also has prognostic significance; a gradient greater than 10 mm Hg is associated with worse prognosis.17
Disadvantages of biopsy: Complications, sampling errors
Liver biopsy has disadvantages. Reported rates of complications necessitating hospitalization using the blind method were as high as 6% in the 1970s,18 dropping to 3.2% in a 1993 study.19 Bleeding remains the most worrisome complication. With the transjugular method, major and minor complication rates are less than 1% and 7%, respectively.15,16 Complication rates with imaging-guided biopsy are also low.
Liver biopsy is also prone to sampling error. The number of portal tracts obtained in the biopsy correlates with the accuracy of fibrosis staging, and smaller samples may lead to underestimating fibrosis stage. In patients with HCV, samples more than 15 mm long led to accurate staging diagnosis in 65% of patients, and those longer than 25 mm conferred 75% accuracy.20 Also, different stages can be diagnosed from samples obtained from separate locations in the liver, although rarely is the difference more than a single stage.21
Histologic evaluation of liver biopsies is operator-dependent. Although significant interobserver variation has been reported for degree of inflammation, there tends to be good concordance for fibrosis staging.22,23
STAGING BASED ON DEMOGRAPHIC AND LABORATORY VARIABLES
Several scores based on patient characteristics and laboratory values have been developed for assessing liver fibrosis and have been specifically validated for HCV infection, NAFLD, or both. They can serve as inexpensive initial screening tests for the presence or absence of advanced fibrosis.
FIB-4 index for HCV, NAFLD
The FIB-4 index predicts the presence of advanced fibrosis using, as its name indicates, a combination of 4 factors in fibrosis: age, platelet count, and the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), according to the formula:
FIB-4 index = (age × AST [U/L]) /
(platelet count [× 109/L] × √ALT [U/L]).
The index was derived from data from 832 patients co-infected with HCV and human immunodeficiency virus.24 The Ishak staging system10 for fibrosis on liver biopsy was used for confirmation, with stage 4 to 6 defined as advanced fibrosis. A cutoff value of more than 3.25 had a positive predictive value of 65% for advanced fibrosis, and to exclude advanced fibrosis, a cutoff value of less than 1.45 had a negative predictive value of 90%.
The FIB-4 index has since been validated in patients with HCV infection25 and NAFLD.26 In a subsequent study in 142 patients with NAFLD, the FIB-4 index was more accurate in diagnosing advanced fibrosis than the other noninvasive prediction models discussed below.27
NAFLD fibrosis score
The NAFLD fibrosis score, constructed and validated only in patients with biopsy-confirmed NAFLD, incorporates age, body mass index, presence of diabetes or prediabetes, albumin level, platelet count, and AST and ALT levels.
A group of 480 patients was used to construct the score, and 253 patients were used to validate it. Using the high cutoff value of 0.676, the presence of advanced fibrosis was diagnosed with a positive predictive value of 90% in the group used to construct the model (82% in the validation group). Using the low cutoff score of –1.455, advanced fibrosis could be excluded with a negative predictive value of 93% in the construction group and 88% in the validation group.28 A score between the cutoff values merits liver biopsy to determine fibrosis stage. The score is more accurate in patients with diabetes.29 When used by primary care physicians, the NAFLD fibrosis score is more cost-effective than transient elastography and liver biopsy for accurately predicting advanced fibrosis.30
AST-to-platelet ratio index score for HCV, NAFLD
The AST-to-platelet ratio index (APRI) score was developed in 2003 using a cohort of 270 patients with HCV and liver biopsy as the standard. A cutoff value of less than or equal to 0.5 had a negative predictive value of 86% for the absence of significant fibrosis, while a score of more than 1.5 detected the presence of significant fibrosis with a positive predictive value of 88%.31 The APRI score was subsequently validated for NAFLD.27,32
FibroSure uses a patented formula
FibroSure (LabCorp; labcorp.com) uses a patented mathematical formula that takes into account age, sex, and levels of gamma-glutamyl transferase, total bilirubin, haptoglobin, apolipoprotein-A, and alpha-2 macroglobulin to assess fibrosis. Developed in 2001 for use in patients with HCV infection, it was reported to have a positive predictive value of greater than 90% and a negative predictive value of 100% for clinically significant fibrosis, defined as stage 2 to 4 based on the METAVIR staging system in the prediction model.33 The use of FibroSure in patients with HCV was subsequently validated in various meta-analyses and systematic reviews.34,35 It is less accurate in patients with normal ALT levels.36
FibroSure also has good accuracy for predicting fibrosis stage in chronic liver disease due to other causes, including NAFLD.37
The prediction models discussed above use routine laboratory tests for chronic liver disease and thus are inexpensive. The high cost of additional testing needed for FibroSure, coupled with the risk of misdiagnosis, makes its cost-effectiveness questionable.38
IMAGING TO PREDICT FIBROSIS STAGE
Conventional ultrasonography (with or without vascular imaging) and computed tomography can detect cirrhosis on the basis of certain imaging characteristics,39,40 including the nodular contour of the liver, caudate lobe hypertrophy, ascites, reversal of blood flow in the portal vein, and splenomegaly. However, they cannot detect fibrosis in its early stages.
The 3 methods discussed below provide more accurate fibrosis staging by measuring the velocity of shear waves sent across hepatic tissue. Because shear-wave velocity increases with liver stiffness, the fibrosis stage can be estimated from this information.41
Transient elastography
Transient elastography uses a special ultrasound transducer. It is highly accurate for predicting advanced fibrosis for almost all causes of chronic liver disease, including HCV infection42,43 and NAFLD.44 The cutoff values of wave velocity to estimate fibrosis stage differ by liver disease etiology.
Transient elastography should not be used to evaluate fibrosis in patients with acute hepatitis, which transiently increases liver stiffness, resulting in a falsely high fibrosis stage diagnosis.45 It is also not a good method for evaluating fibrosis in patients with biliary obstruction or extrahepatic venous congestion. Because liver stiffness can increase after eating,46 the test should be done under fasting conditions.
A significant limitation of transient elastography has been its poor accuracy in patients with obesity.47 This has been largely overcome with the use of a more powerful (XL) probe but is still a limitation for those with morbid obesity.48 Because many patients with NAFLD are obese, this limitation can be significant.
Transient elastography has gained popularity for evaluating fibrosis in patients with chronic liver disease for multiple reasons: it is cost-effective and results are highly reproducible, with low variation in results among different observers and in individual observers.49 Combined with a platelet count, it can also be used to detect the development of clinically significant portal hypertension in patients with cirrhosis, thus determining the need to screen for esophageal varices using endoscopy.50 Screening endoscopy can be avoided in patients whose liver stiffness remains below 20 kPa or whose platelet count is above 150 × 109/L.
Acoustic radiation force imaging
Unlike transient elastography, which requires a separate transducer probe to assess shear- wave velocity, acoustic radiation force imaging uses the same transducer for both this function and imaging. Different image modes are available when testing for liver stiffness, so a region of interest that is optimal for avoiding vascular structures or masses can be selected, increasing accuracy.51
Acoustic radiation force imaging has been tested in different causes of chronic liver disease, including HCV and NAFLD,52 with accuracy similar to that of transient elastography.53 For overweight and obese patients, acoustic radiation force imaging is more accurate than transient elastography using the XL probe.54 However, this method is still new, and we need more data to support using one method over the other.
Magnetic resonance elastography
Magnetic resonance elastography uses a special transducer placed under the rib cage to transmit shear waves concurrently with magnetic resonance imaging. It has been tested in patients with HCV and NAFLD and has been found to have better diagnostic accuracy than transient elastography and acoustic radiation force imaging.55,56 Patients must be fasting for better diagnostic accuracy57 and must hold their breath while elastography is performed. The need for breath-holding and the high cost limit the use of this method for assessing fibrosis.
BOTTOM LINE FOR ASSESSING FIBROSIS
Staging of liver fibrosis, important for determining prognosis in patients with chronic liver disease and for the need to start screening for complications of cirrhosis, was traditionally done only by liver biopsy. While biopsy is still the gold standard method to stage fibrosis, noninvasive methods have been developed that can also assess disease severity.
This article briefly reviews the epidemiology and physiology of chronic liver disease and the traditional role of liver biopsy. Pros and cons of alternative fibrosis assessment methods are discussed, with a focus on their utility for patients with nonalcoholic fatty liver disease (NAFLD) and hepatitis C virus (HCV) infection.
CHRONIC LIVER DISEASE: A HUGE HEALTH BURDEN
Chronic liver disease is associated with enormous health and financial costs in the United States. Its prevalence is about 15%,1 and it is the 12th leading cause of death.2 Hospital costs are estimated at about $4 billion annually.3
The most common causes of chronic liver disease are NAFLD (which may be present in up to one-third of the US population and is increasing with the epidemic of obesity), its aggressive variant, nonalcoholic steatohepatitis (NASH) (present in about 3% of the population), and HCV infection (1%).4,5
Since direct-acting antiviral agents were introduced, HCV infection dropped from being the leading cause of liver transplant to third place.6 But at the same time, the number of patients on the transplant waiting list who have NASH has risen faster than for any other cause of chronic liver disease.7
FIBROSIS: A KEY INDICATOR OF DISEASE SEVERITY
In HCV infection, advanced fibrosis is defined as either stage 4 to 6 using the Ishak system10 or stage 3 to 4 using the Meta-analysis of Histological Data in Viral Hepatitis (METAVIR) system.11
In NAFLD, advanced fibrosis is defined as stage 3 to 4 using the NASH Clinical Research Network system.12
Staging fibrosis is also important so that patients with cirrhosis can be identified early to begin screening for hepatocellular carcinoma and esophageal varices to reduce the risks of illness and death. In addition, insurance companies often require documentation of fibrosis stage before treating HCV with the new direct-acting antiviral agents.
LIVER BIOPSY IS STILL THE GOLD STANDARD
Although invasive, liver biopsy remains the gold standard for determining fibrosis stage. Liver biopsies were performed “blindly” (without imaging) until the 1990s, but imaging-guided biopsy using ultrasonography was then developed, which entailed less pain and lower complication and hospitalization rates. Slightly more hepatic tissue is obtained with guided liver biopsy, but the difference was deemed clinically insignificant.13 Concern initially arose about the added cost involved with imaging, but imaging-guided biopsy was actually found to be more cost-effective.14
In the 2000s, transjugular liver biopsy via the right internal jugular vein became available. This method was originally used primarily in patients with ascites or significant coagulopathy. At first, there were concerns about the adequacy of specimens obtained to make an accurate diagnosis or establish fibrosis stage, but this limitation was overcome with improved techniques.15,16 Transjugular liver biopsy has the additional advantage of enabling one to measure the hepatic venous pressure gradient, which also has prognostic significance; a gradient greater than 10 mm Hg is associated with worse prognosis.17
Disadvantages of biopsy: Complications, sampling errors
Liver biopsy has disadvantages. Reported rates of complications necessitating hospitalization using the blind method were as high as 6% in the 1970s,18 dropping to 3.2% in a 1993 study.19 Bleeding remains the most worrisome complication. With the transjugular method, major and minor complication rates are less than 1% and 7%, respectively.15,16 Complication rates with imaging-guided biopsy are also low.
Liver biopsy is also prone to sampling error. The number of portal tracts obtained in the biopsy correlates with the accuracy of fibrosis staging, and smaller samples may lead to underestimating fibrosis stage. In patients with HCV, samples more than 15 mm long led to accurate staging diagnosis in 65% of patients, and those longer than 25 mm conferred 75% accuracy.20 Also, different stages can be diagnosed from samples obtained from separate locations in the liver, although rarely is the difference more than a single stage.21
Histologic evaluation of liver biopsies is operator-dependent. Although significant interobserver variation has been reported for degree of inflammation, there tends to be good concordance for fibrosis staging.22,23
STAGING BASED ON DEMOGRAPHIC AND LABORATORY VARIABLES
Several scores based on patient characteristics and laboratory values have been developed for assessing liver fibrosis and have been specifically validated for HCV infection, NAFLD, or both. They can serve as inexpensive initial screening tests for the presence or absence of advanced fibrosis.
FIB-4 index for HCV, NAFLD
The FIB-4 index predicts the presence of advanced fibrosis using, as its name indicates, a combination of 4 factors in fibrosis: age, platelet count, and the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), according to the formula:
FIB-4 index = (age × AST [U/L]) /
(platelet count [× 109/L] × √ALT [U/L]).
The index was derived from data from 832 patients co-infected with HCV and human immunodeficiency virus.24 The Ishak staging system10 for fibrosis on liver biopsy was used for confirmation, with stage 4 to 6 defined as advanced fibrosis. A cutoff value of more than 3.25 had a positive predictive value of 65% for advanced fibrosis, and to exclude advanced fibrosis, a cutoff value of less than 1.45 had a negative predictive value of 90%.
The FIB-4 index has since been validated in patients with HCV infection25 and NAFLD.26 In a subsequent study in 142 patients with NAFLD, the FIB-4 index was more accurate in diagnosing advanced fibrosis than the other noninvasive prediction models discussed below.27
NAFLD fibrosis score
The NAFLD fibrosis score, constructed and validated only in patients with biopsy-confirmed NAFLD, incorporates age, body mass index, presence of diabetes or prediabetes, albumin level, platelet count, and AST and ALT levels.
A group of 480 patients was used to construct the score, and 253 patients were used to validate it. Using the high cutoff value of 0.676, the presence of advanced fibrosis was diagnosed with a positive predictive value of 90% in the group used to construct the model (82% in the validation group). Using the low cutoff score of –1.455, advanced fibrosis could be excluded with a negative predictive value of 93% in the construction group and 88% in the validation group.28 A score between the cutoff values merits liver biopsy to determine fibrosis stage. The score is more accurate in patients with diabetes.29 When used by primary care physicians, the NAFLD fibrosis score is more cost-effective than transient elastography and liver biopsy for accurately predicting advanced fibrosis.30
AST-to-platelet ratio index score for HCV, NAFLD
The AST-to-platelet ratio index (APRI) score was developed in 2003 using a cohort of 270 patients with HCV and liver biopsy as the standard. A cutoff value of less than or equal to 0.5 had a negative predictive value of 86% for the absence of significant fibrosis, while a score of more than 1.5 detected the presence of significant fibrosis with a positive predictive value of 88%.31 The APRI score was subsequently validated for NAFLD.27,32
FibroSure uses a patented formula
FibroSure (LabCorp; labcorp.com) uses a patented mathematical formula that takes into account age, sex, and levels of gamma-glutamyl transferase, total bilirubin, haptoglobin, apolipoprotein-A, and alpha-2 macroglobulin to assess fibrosis. Developed in 2001 for use in patients with HCV infection, it was reported to have a positive predictive value of greater than 90% and a negative predictive value of 100% for clinically significant fibrosis, defined as stage 2 to 4 based on the METAVIR staging system in the prediction model.33 The use of FibroSure in patients with HCV was subsequently validated in various meta-analyses and systematic reviews.34,35 It is less accurate in patients with normal ALT levels.36
FibroSure also has good accuracy for predicting fibrosis stage in chronic liver disease due to other causes, including NAFLD.37
The prediction models discussed above use routine laboratory tests for chronic liver disease and thus are inexpensive. The high cost of additional testing needed for FibroSure, coupled with the risk of misdiagnosis, makes its cost-effectiveness questionable.38
IMAGING TO PREDICT FIBROSIS STAGE
Conventional ultrasonography (with or without vascular imaging) and computed tomography can detect cirrhosis on the basis of certain imaging characteristics,39,40 including the nodular contour of the liver, caudate lobe hypertrophy, ascites, reversal of blood flow in the portal vein, and splenomegaly. However, they cannot detect fibrosis in its early stages.
The 3 methods discussed below provide more accurate fibrosis staging by measuring the velocity of shear waves sent across hepatic tissue. Because shear-wave velocity increases with liver stiffness, the fibrosis stage can be estimated from this information.41
Transient elastography
Transient elastography uses a special ultrasound transducer. It is highly accurate for predicting advanced fibrosis for almost all causes of chronic liver disease, including HCV infection42,43 and NAFLD.44 The cutoff values of wave velocity to estimate fibrosis stage differ by liver disease etiology.
Transient elastography should not be used to evaluate fibrosis in patients with acute hepatitis, which transiently increases liver stiffness, resulting in a falsely high fibrosis stage diagnosis.45 It is also not a good method for evaluating fibrosis in patients with biliary obstruction or extrahepatic venous congestion. Because liver stiffness can increase after eating,46 the test should be done under fasting conditions.
A significant limitation of transient elastography has been its poor accuracy in patients with obesity.47 This has been largely overcome with the use of a more powerful (XL) probe but is still a limitation for those with morbid obesity.48 Because many patients with NAFLD are obese, this limitation can be significant.
Transient elastography has gained popularity for evaluating fibrosis in patients with chronic liver disease for multiple reasons: it is cost-effective and results are highly reproducible, with low variation in results among different observers and in individual observers.49 Combined with a platelet count, it can also be used to detect the development of clinically significant portal hypertension in patients with cirrhosis, thus determining the need to screen for esophageal varices using endoscopy.50 Screening endoscopy can be avoided in patients whose liver stiffness remains below 20 kPa or whose platelet count is above 150 × 109/L.
Acoustic radiation force imaging
Unlike transient elastography, which requires a separate transducer probe to assess shear- wave velocity, acoustic radiation force imaging uses the same transducer for both this function and imaging. Different image modes are available when testing for liver stiffness, so a region of interest that is optimal for avoiding vascular structures or masses can be selected, increasing accuracy.51
Acoustic radiation force imaging has been tested in different causes of chronic liver disease, including HCV and NAFLD,52 with accuracy similar to that of transient elastography.53 For overweight and obese patients, acoustic radiation force imaging is more accurate than transient elastography using the XL probe.54 However, this method is still new, and we need more data to support using one method over the other.
Magnetic resonance elastography
Magnetic resonance elastography uses a special transducer placed under the rib cage to transmit shear waves concurrently with magnetic resonance imaging. It has been tested in patients with HCV and NAFLD and has been found to have better diagnostic accuracy than transient elastography and acoustic radiation force imaging.55,56 Patients must be fasting for better diagnostic accuracy57 and must hold their breath while elastography is performed. The need for breath-holding and the high cost limit the use of this method for assessing fibrosis.
BOTTOM LINE FOR ASSESSING FIBROSIS
- Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011; 9(6):524–530.e1. doi:10.1016/j.cgh.2011.03.020
- Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. Deaths: final data for 2009. Natl Vital Stat Rep 2011; 60(3):1–116. pmid:24974587
- Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 2012; 107(2):247–252. doi:10.1038/ajg.2011.314
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34(3):274–285. doi:10.1111/j.1365-2036.2011.04724.x
- Udompap P, Kim D, Kim WR. Current and future burden of chronic nonmalignant liver disease. Clin Gastroenterol Hepatol 2015; 13(12):2031–2041. doi:10.1016/j.cgh.2015.08.015
- Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 annual data report: liver. Am J Transplant 2018; 18(suppl 1):172–253. doi:10.1111/ajt.14559
- Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148(3):547–555. doi:10.1053/j.gastro.2014.11.039
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22(6):696–699. pmid:7560864
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996; 24(2):289–293. doi:10.1002/hep.510240201
- Kleiner DE, Brunt EM, Van Natta M, et al; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41(6):1313–1321. doi:10.1002/hep.20701
- Everhart JE, Wright EC, Goodman ZD, et al; HALT-C Trial Group. Prognostic value of Ishak fibrosis stage: findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology 2010; 51(2):585–594. doi:10.1002/hep.23315
- Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015; 149(2):389–397.e10. doi:10.1053/j.gastro.2015.04.043
- Lindor KD, Bru C, Jorgensen RA, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology 1996; 23(5):1079–1083. doi:10.1002/hep.510230522
- Pasha T, Gabriel S, Therneau T, Dickson ER, Lindor KD. Cost-effectiveness of ultrasound-guided liver biopsy. Hepatology 1998; 27(5):1220–1226. doi:10.1002/hep.510270506
- Alessandria C, Debernardi-Venon W, Rizzetto M, Marzano A. Transjugular liver biopsy: a relatively simple procedure with an indefinite past and an expected brilliant future. J Hepatol 2008; 48(1):171–173. doi:10.1016/j.jhep.2007.10.001
- Kalambokis G, Manousou P, Vibhakorn S, et al. Transjugular liver biopsy—indications, adequacy, quality of specimens, and complications—a systematic review. J Hepatol 2007; 47(2):284–294. doi:10.1016/j.jhep.2007.05.001
- Ripoll C, Groszmann R, Garcia-Tsao G, et al; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007; 133(2):481–488. doi:10.1053/j.gastro.2007.05.024
- Perrault J, McGill DB, Ott BJ, Taylor WF. Liver biopsy: complications in 1000 inpatients and outpatients. Gastroenterology 1978; 74(1):103–106. pmid:618417
- Janes CH, Lindor KD. Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med 1993; 118(2):96–98. pmid:8416324
- Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003; 38(6):1449–1457. doi:10.1016/j.hep.2003.09.022
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97(10):2614–2618. doi:10.1111/j.1572-0241.2002.06038.x
- Goldin RD, Goldin JG, Burt AD, et al. Intra-observer and inter-observer variation in the histopathological assessment of chronic viral hepatitis. J Hepatol 1996; 25(5):649–654. pmid:8938541
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994; 20(1 Pt 1):15–20. pmid:8020885
- Sterling RK, Lissen E, Clumeck N, et al; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43(6):1317–1325. doi:10.1002/hep.21178
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007; 46(1):32–36. doi:10.1002/hep.21669
- Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7(10):1104–1112. doi:10.1016/j.cgh.2009.05.033
- McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010; 59(9):1265–1269. doi:10.1136/gut.2010.216077
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846–854. doi:10.1002/hep.21496
- Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin 2015; 3:141–145. doi:10.1016/j.bbacli.2014.09.001
- Tapper EB, Hunink MG, Afdhal NH, Lai M, Sengupta N. Cost-effectiveness analysis: risk stratification of nonalcoholic fatty liver disease (NAFLD) by the primary care physician using the NAFLD fibrosis score. PLoS One 2016; 11(2):e0147237. doi:10.1371/journal.pone.0147237
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38(2):518–526. doi:10.1053/jhep.2003.50346
- Calès P, Lainé F, Boursier J, et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol 2009; 50(1):165–173. doi:10.1016/j.jhep.2008.07.035
- Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T; MULTIVIRC Group. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001; 357(9262):1069–1075. doi:10.1016/S0140-6736(00)04258-6
- Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol 2007; 102(11):2589–2600. doi:10.1111/j.1572-0241.2007.01466.x
- Smith JO, Sterling RK. Systematic review: non-invasive methods of fibrosis analysis in chronic hepatitis C. Aliment Pharmacol Ther 2009; 30(6):557–576. doi:10.1111/j.1365-2036.2009.04062.x
- Sebastiani G, Vario A, Guido M, Alberti A. Performance of noninvasive markers for liver fibrosis is reduced in chronic hepatitis C with normal transaminases. J Viral Hepat 2007; 15(3):212–218. doi:10.1111/j.1365-2893.2007.00932.x
- Poynard T, Morra R, Halfon P, et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol 2007; 7:40. doi:10.1186/1471-230X-7-40
- Carlson JJ, Kowdley KV, Sullivan SD, Ramsey SD, Veenstra DL. An evaluation of the potential cost-effectiveness of non-invasive testing strategies in the diagnosis of significant liver fibrosis. J Gastroenterol Hepatol 2009; 24(5):786–791. doi:10.1111/j.1440-1746.2009.05778.x
- Aubé C, Oberti F, Korali N, et al. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol 1999; 30(3):472–478. pmid:10190731
- Di Lelio A, Cestari C, Lomazzi A, Beretta L. Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology 1989; 172(2):389–392. doi:10.1148/radiology.172.2.2526349
- Wong VW, Chan HL. Transient elastography. J Gastroenterol Hepatol 2010; 25(11):1726–1731. doi:10.1111/j.1440-1746.2010.06437.x
- Arena U, Vizzutti F, Abraldes JG, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut 2008; 57(9):1288–1293. doi:10.1136/gut.2008.149708
- Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41(1):48–54. doi:10.1002/hep.20506
- Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010; 51(2):454–462. doi:10.1002/hep.23312
- Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2007; 48(2):592–595. doi:10.1002/hep.22056
- Mederacke I, Wursthorn K, Kirschner J, et al. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int 2009; 29(10):1500–1506. doi:10.1111/j.1478-3231.2009.02100.x
- Castéra L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2010; 51(3):828–835. doi:10.1002/hep.23425
- Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 2012; 107(12):1862–1871. doi:10.1038/ajg.2012.331
- Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007; 56(7):968–973. doi:10.1136/gut.2006.111302
- de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63(3):743–752. doi:10.1016/j.jhep.2015.05.022
- Friedrich-Rust M, Wunder K, Kriener S, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 2009; 252(2):595–604. doi:10.1148/radiol.2523081928
- Yoneda M, Suzuki K, Kato S, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology 2010; 256(2):640–647. doi:10.1148/radiol.10091662
- Bota S, Herkner H, Sporea I, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int 2013; 33(8):1138–1147. doi:10.1111/liv.12240
- Attia D, Bantel H, Lenzen H, Manns MP, Gebel MJ, Potthoff A. Liver stiffness measurement using acoustic radiation force impulse elastography in overweight and obese patients. Aliment Pharmacol Ther 2016; 44(4):366–379. doi:10.1111/apt.13710
- Cui J, Heba E, Hernandez C, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. Hepatology 2016; 63(2):453–461. doi:10.1002/hep.28337
- Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008; 135(1):32–40. doi:10.1053/j.gastro.2008.03.076
- Jajamovich GH, Dyvorne H, Donnerhack C, Taouli B. Quantitative liver MRI combining phase contrast imaging, elastography, and DWI: assessment of reproducibility and postprandial effect at 3.0 T. PLoS One 2014; 9(5):e97355. doi:10.1371/journal.pone.0097355
- Lim JK, Flamm SL, Singh S, Falck-Ytter YT; Clinical Guidelines Committee of the American Gastroenterological Association. American Gastroenterological Association Institute guideline on the role of elastography in the evaluation of liver fibrosis. Gastroenterology 2017; 152(6):1536–1543. doi:10.1053/j.gastro.2017.03.017
- N, Feldstein AE. Noninvasive diagnosis of nonalcoholic fatty liver disease: are we there yet? Metabolism 2016; 65(8):1087–1095. doi:10.1016/j.metabol.2016.01.013
- Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011; 9(6):524–530.e1. doi:10.1016/j.cgh.2011.03.020
- Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung H-C. Deaths: final data for 2009. Natl Vital Stat Rep 2011; 60(3):1–116. pmid:24974587
- Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 2012; 107(2):247–252. doi:10.1038/ajg.2011.314
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34(3):274–285. doi:10.1111/j.1365-2036.2011.04724.x
- Udompap P, Kim D, Kim WR. Current and future burden of chronic nonmalignant liver disease. Clin Gastroenterol Hepatol 2015; 13(12):2031–2041. doi:10.1016/j.cgh.2015.08.015
- Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2016 annual data report: liver. Am J Transplant 2018; 18(suppl 1):172–253. doi:10.1111/ajt.14559
- Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015; 148(3):547–555. doi:10.1053/j.gastro.2014.11.039
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22(6):696–699. pmid:7560864
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996; 24(2):289–293. doi:10.1002/hep.510240201
- Kleiner DE, Brunt EM, Van Natta M, et al; Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41(6):1313–1321. doi:10.1002/hep.20701
- Everhart JE, Wright EC, Goodman ZD, et al; HALT-C Trial Group. Prognostic value of Ishak fibrosis stage: findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology 2010; 51(2):585–594. doi:10.1002/hep.23315
- Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015; 149(2):389–397.e10. doi:10.1053/j.gastro.2015.04.043
- Lindor KD, Bru C, Jorgensen RA, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology 1996; 23(5):1079–1083. doi:10.1002/hep.510230522
- Pasha T, Gabriel S, Therneau T, Dickson ER, Lindor KD. Cost-effectiveness of ultrasound-guided liver biopsy. Hepatology 1998; 27(5):1220–1226. doi:10.1002/hep.510270506
- Alessandria C, Debernardi-Venon W, Rizzetto M, Marzano A. Transjugular liver biopsy: a relatively simple procedure with an indefinite past and an expected brilliant future. J Hepatol 2008; 48(1):171–173. doi:10.1016/j.jhep.2007.10.001
- Kalambokis G, Manousou P, Vibhakorn S, et al. Transjugular liver biopsy—indications, adequacy, quality of specimens, and complications—a systematic review. J Hepatol 2007; 47(2):284–294. doi:10.1016/j.jhep.2007.05.001
- Ripoll C, Groszmann R, Garcia-Tsao G, et al; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007; 133(2):481–488. doi:10.1053/j.gastro.2007.05.024
- Perrault J, McGill DB, Ott BJ, Taylor WF. Liver biopsy: complications in 1000 inpatients and outpatients. Gastroenterology 1978; 74(1):103–106. pmid:618417
- Janes CH, Lindor KD. Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med 1993; 118(2):96–98. pmid:8416324
- Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003; 38(6):1449–1457. doi:10.1016/j.hep.2003.09.022
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 2002; 97(10):2614–2618. doi:10.1111/j.1572-0241.2002.06038.x
- Goldin RD, Goldin JG, Burt AD, et al. Intra-observer and inter-observer variation in the histopathological assessment of chronic viral hepatitis. J Hepatol 1996; 25(5):649–654. pmid:8938541
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994; 20(1 Pt 1):15–20. pmid:8020885
- Sterling RK, Lissen E, Clumeck N, et al; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43(6):1317–1325. doi:10.1002/hep.21178
- Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007; 46(1):32–36. doi:10.1002/hep.21669
- Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009; 7(10):1104–1112. doi:10.1016/j.cgh.2009.05.033
- McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010; 59(9):1265–1269. doi:10.1136/gut.2010.216077
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846–854. doi:10.1002/hep.21496
- Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin 2015; 3:141–145. doi:10.1016/j.bbacli.2014.09.001
- Tapper EB, Hunink MG, Afdhal NH, Lai M, Sengupta N. Cost-effectiveness analysis: risk stratification of nonalcoholic fatty liver disease (NAFLD) by the primary care physician using the NAFLD fibrosis score. PLoS One 2016; 11(2):e0147237. doi:10.1371/journal.pone.0147237
- Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38(2):518–526. doi:10.1053/jhep.2003.50346
- Calès P, Lainé F, Boursier J, et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol 2009; 50(1):165–173. doi:10.1016/j.jhep.2008.07.035
- Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T; MULTIVIRC Group. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001; 357(9262):1069–1075. doi:10.1016/S0140-6736(00)04258-6
- Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol 2007; 102(11):2589–2600. doi:10.1111/j.1572-0241.2007.01466.x
- Smith JO, Sterling RK. Systematic review: non-invasive methods of fibrosis analysis in chronic hepatitis C. Aliment Pharmacol Ther 2009; 30(6):557–576. doi:10.1111/j.1365-2036.2009.04062.x
- Sebastiani G, Vario A, Guido M, Alberti A. Performance of noninvasive markers for liver fibrosis is reduced in chronic hepatitis C with normal transaminases. J Viral Hepat 2007; 15(3):212–218. doi:10.1111/j.1365-2893.2007.00932.x
- Poynard T, Morra R, Halfon P, et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol 2007; 7:40. doi:10.1186/1471-230X-7-40
- Carlson JJ, Kowdley KV, Sullivan SD, Ramsey SD, Veenstra DL. An evaluation of the potential cost-effectiveness of non-invasive testing strategies in the diagnosis of significant liver fibrosis. J Gastroenterol Hepatol 2009; 24(5):786–791. doi:10.1111/j.1440-1746.2009.05778.x
- Aubé C, Oberti F, Korali N, et al. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol 1999; 30(3):472–478. pmid:10190731
- Di Lelio A, Cestari C, Lomazzi A, Beretta L. Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology 1989; 172(2):389–392. doi:10.1148/radiology.172.2.2526349
- Wong VW, Chan HL. Transient elastography. J Gastroenterol Hepatol 2010; 25(11):1726–1731. doi:10.1111/j.1440-1746.2010.06437.x
- Arena U, Vizzutti F, Abraldes JG, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut 2008; 57(9):1288–1293. doi:10.1136/gut.2008.149708
- Ziol M, Handra-Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005; 41(1):48–54. doi:10.1002/hep.20506
- Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010; 51(2):454–462. doi:10.1002/hep.23312
- Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2007; 48(2):592–595. doi:10.1002/hep.22056
- Mederacke I, Wursthorn K, Kirschner J, et al. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int 2009; 29(10):1500–1506. doi:10.1111/j.1478-3231.2009.02100.x
- Castéra L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2010; 51(3):828–835. doi:10.1002/hep.23425
- Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 2012; 107(12):1862–1871. doi:10.1038/ajg.2012.331
- Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 2007; 56(7):968–973. doi:10.1136/gut.2006.111302
- de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63(3):743–752. doi:10.1016/j.jhep.2015.05.022
- Friedrich-Rust M, Wunder K, Kriener S, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology 2009; 252(2):595–604. doi:10.1148/radiol.2523081928
- Yoneda M, Suzuki K, Kato S, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology 2010; 256(2):640–647. doi:10.1148/radiol.10091662
- Bota S, Herkner H, Sporea I, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int 2013; 33(8):1138–1147. doi:10.1111/liv.12240
- Attia D, Bantel H, Lenzen H, Manns MP, Gebel MJ, Potthoff A. Liver stiffness measurement using acoustic radiation force impulse elastography in overweight and obese patients. Aliment Pharmacol Ther 2016; 44(4):366–379. doi:10.1111/apt.13710
- Cui J, Heba E, Hernandez C, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: a prospective study. Hepatology 2016; 63(2):453–461. doi:10.1002/hep.28337
- Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008; 135(1):32–40. doi:10.1053/j.gastro.2008.03.076
- Jajamovich GH, Dyvorne H, Donnerhack C, Taouli B. Quantitative liver MRI combining phase contrast imaging, elastography, and DWI: assessment of reproducibility and postprandial effect at 3.0 T. PLoS One 2014; 9(5):e97355. doi:10.1371/journal.pone.0097355
- Lim JK, Flamm SL, Singh S, Falck-Ytter YT; Clinical Guidelines Committee of the American Gastroenterological Association. American Gastroenterological Association Institute guideline on the role of elastography in the evaluation of liver fibrosis. Gastroenterology 2017; 152(6):1536–1543. doi:10.1053/j.gastro.2017.03.017
- N, Feldstein AE. Noninvasive diagnosis of nonalcoholic fatty liver disease: are we there yet? Metabolism 2016; 65(8):1087–1095. doi:10.1016/j.metabol.2016.01.013
KEY POINTS
- Liver biopsy remains the gold standard for determining fibrosis stage but is expensive and entails risk of complications.
- For patients infected with HCV, fibrosis stage should be determined with transient elastography, a transthoracic ultrasonographic technique that measures shear-wave velocity.
- For patients with cirrhosis, transient elastography combined with a platelet count can detect developing portal hypertension and determine whether to screen for esophageal varices.
- For NAFLD, combined elastography and NAFLD fibrosis score—which incorporates patient characteristics and laboratory test results—should be used to determine the need for liver biopsy.
FDA accepts supplemental New Drug Application to expand lorcaserin label
The Food and Drug Administration has accepted a supplemental New Drug Application from Eisai to update the label for the weight-loss drug lorcaserin (Belviq) with long-term efficacy and safety data from the CAMELLIA-TIMI 61 trial.
In CAMELLIA-TIMI 61, a double-blind, placebo-controlled, parallel-group, phase 3b/4 clinical trial, about 12,000 people who were overweight or obese and had cardiovascular disease or CVD risk factors, such as type 2 diabetes, received lorcaserin or placebo. In a study published in the New England Journal of Medicine last fall, lorcaserin, a serotonin 2C receptor agonist, met the primary safety endpoint of noninferiority to placebo in the rate of major adverse cardiovascular events (hazard ratio, 0.99; 95% confidence interval, 0.85-1.14; P less than .001).
After meeting the primary endpoint, the trial investigators continued the study, assessing for a broader composite endpoint consisting of cardiovascular death, nonfatal MI, nonfatal stroke, hospitalization caused by unstable angina, heart failure, or coronary revascularization. Although lorcaserin was not superior to placebo in this expanded study, it remained noninferior.
Lorcaserin is contraindicated in women who are pregnant and in patients hypersensitive to lorcaserin. The most common adverse events in patients without diabetes were headache, dizziness, fatigue, nausea, dry mouth, and constipation; in patients with diabetes, the most common adverse events were hypoglycemia, headache, back pain, cough, and fatigue.
“This sNDA file acceptance brings us one step closer to potentially incorporating data into our label based on the first completed large-scale cardiovascular outcomes trial for a weight loss agent,” Lynn Kramer, MD, chief clinical officer and chief medical officer of the Neurology Business Group at Eisai, said in the press release.
Find the full press release on the Eisai website.
The Food and Drug Administration has accepted a supplemental New Drug Application from Eisai to update the label for the weight-loss drug lorcaserin (Belviq) with long-term efficacy and safety data from the CAMELLIA-TIMI 61 trial.
In CAMELLIA-TIMI 61, a double-blind, placebo-controlled, parallel-group, phase 3b/4 clinical trial, about 12,000 people who were overweight or obese and had cardiovascular disease or CVD risk factors, such as type 2 diabetes, received lorcaserin or placebo. In a study published in the New England Journal of Medicine last fall, lorcaserin, a serotonin 2C receptor agonist, met the primary safety endpoint of noninferiority to placebo in the rate of major adverse cardiovascular events (hazard ratio, 0.99; 95% confidence interval, 0.85-1.14; P less than .001).
After meeting the primary endpoint, the trial investigators continued the study, assessing for a broader composite endpoint consisting of cardiovascular death, nonfatal MI, nonfatal stroke, hospitalization caused by unstable angina, heart failure, or coronary revascularization. Although lorcaserin was not superior to placebo in this expanded study, it remained noninferior.
Lorcaserin is contraindicated in women who are pregnant and in patients hypersensitive to lorcaserin. The most common adverse events in patients without diabetes were headache, dizziness, fatigue, nausea, dry mouth, and constipation; in patients with diabetes, the most common adverse events were hypoglycemia, headache, back pain, cough, and fatigue.
“This sNDA file acceptance brings us one step closer to potentially incorporating data into our label based on the first completed large-scale cardiovascular outcomes trial for a weight loss agent,” Lynn Kramer, MD, chief clinical officer and chief medical officer of the Neurology Business Group at Eisai, said in the press release.
Find the full press release on the Eisai website.
The Food and Drug Administration has accepted a supplemental New Drug Application from Eisai to update the label for the weight-loss drug lorcaserin (Belviq) with long-term efficacy and safety data from the CAMELLIA-TIMI 61 trial.
In CAMELLIA-TIMI 61, a double-blind, placebo-controlled, parallel-group, phase 3b/4 clinical trial, about 12,000 people who were overweight or obese and had cardiovascular disease or CVD risk factors, such as type 2 diabetes, received lorcaserin or placebo. In a study published in the New England Journal of Medicine last fall, lorcaserin, a serotonin 2C receptor agonist, met the primary safety endpoint of noninferiority to placebo in the rate of major adverse cardiovascular events (hazard ratio, 0.99; 95% confidence interval, 0.85-1.14; P less than .001).
After meeting the primary endpoint, the trial investigators continued the study, assessing for a broader composite endpoint consisting of cardiovascular death, nonfatal MI, nonfatal stroke, hospitalization caused by unstable angina, heart failure, or coronary revascularization. Although lorcaserin was not superior to placebo in this expanded study, it remained noninferior.
Lorcaserin is contraindicated in women who are pregnant and in patients hypersensitive to lorcaserin. The most common adverse events in patients without diabetes were headache, dizziness, fatigue, nausea, dry mouth, and constipation; in patients with diabetes, the most common adverse events were hypoglycemia, headache, back pain, cough, and fatigue.
“This sNDA file acceptance brings us one step closer to potentially incorporating data into our label based on the first completed large-scale cardiovascular outcomes trial for a weight loss agent,” Lynn Kramer, MD, chief clinical officer and chief medical officer of the Neurology Business Group at Eisai, said in the press release.
Find the full press release on the Eisai website.
Asthma, obesity, and the risk for severe sleep apnea in children
CORONADO, CALIF. –
“We have a good idea that obesity and asthma independently increase the risk of OSA, but a lot of the time in the pediatric population, these risk factors are found comorbid,” Ajay Narayanan at the Triological Society’s Combined Sections Meeting. “For this study we asked, how does the presence of asthma change the likelihood of having severe OSA in a cohort of obese patients? Knowing that both asthma and obesity independently increase the risk for OSA, we hypothesized that when they were comorbid, asthma would have a synergistic effect with obesity, causing severe OSA.”
Mr. Narayanan, a third-year student at the University of Texas Southwestern Medical Center, Dallas, and his colleagues performed a retrospective chart review of 367 children aged 9-17 years referred for a full-night polysomnography (PSG) for suspicion of having OSA. Demographic variables recorded included race, body mass index, rhinitis, gastroesophageal reflux disease, and tonsillar hypertrophy. Sleep variables recorded included apnea hypopnea index (AHI), sleep efficiency, rapid eye movement, and the peripheral capillary oxygen saturation (SpO2) nadir. The primary outcome was severe OSA defined as an AHI of 10 or greater on the PSG. They used logistic modeling to determine the association between asthma, obesity, and severe OSA.
The mean age of the study population was 14 years, 56% were male, and 43% were Hispanic. Of the 367 patients, 77 were neither obese nor asthmatic, 93 were nonobese but were asthmatic, 102 were obese but were nonasthmatic, and 95 were both obese and asthmatic. PSG results confirmed that obesity was associated with more signs of sleep apnea. For example, the nonobese, nonasthmatic group had a mean AHI of 11 events per hour, while the obese, nonasthmatic group had a mean AHI of 19 events per hour. “We observed a similar trend amongst our asthmatic population,” Mr. Narayanan said. “We observed an increase in the mean AHI amongst our asthmatic kids when we added obesity to the picture. Surprisingly, we found that asthma was associated with having fewer signs of sleep apnea.” Specifically, while the nonobese, nonasthmatic group had a mean AHI of 11 events per hour, those in the nonobese, asthmatic group had a mean of 5.6 events per hour (P = .005). “The finding was similar amongst our obese kids,” he said. “We saw a decrease in the mean AHI of our obese kids when we added asthma to the picture.”
On logistic regression analysis using obesity and asthma as independent variables, the researchers found that obesity increased the risk of severe OSA by 2.4-fold, but asthma decreased the odds of having severe OSA by about half (0.55). On multiple logistic regression controlling for commonly associated factors such as tonsillar hypertrophy, black race, and Hispanic ethnicity, obesity increased the risk of severe OSA by 2.2-fold, while asthma decreased the odds of having severe OSA by about half (0.51).
“In trying to explain this finding, we can turn to how these diseases are treated,” Mr. Narayanan said. “I say this because of the proven association between preexisting asthma and new onset OSA. Some of the reasons for this association include the tendency for airway collapsibility and systemwide inflammation seen in asthma, which then might contribute to the development of OSA. If we treat asthma symptoms early on, it might prevent the progression to sleep apnea down the line.”
Considering how prevalent comorbid asthma and OSA is, he continued, “we need to confirm that it is in fact well-controlled asthma that is associated with lowering the risk of severe OSA. Once we do this, we can ask the question: Can we use asthma pharmacotherapy to treat OSA? Some studies have shown that inhaled corticosteroids and montelukast (Singulair) may be effective treatment options for kids with OSA, but there’s definitely room for more research in this field, [such as determining] which patients would most benefit from this pharmacotherapy.” The researchers reported having no financial disclosures.
SOURCE: Narayanan A et al. Triological CSM, Abstracts.
CORONADO, CALIF. –
“We have a good idea that obesity and asthma independently increase the risk of OSA, but a lot of the time in the pediatric population, these risk factors are found comorbid,” Ajay Narayanan at the Triological Society’s Combined Sections Meeting. “For this study we asked, how does the presence of asthma change the likelihood of having severe OSA in a cohort of obese patients? Knowing that both asthma and obesity independently increase the risk for OSA, we hypothesized that when they were comorbid, asthma would have a synergistic effect with obesity, causing severe OSA.”
Mr. Narayanan, a third-year student at the University of Texas Southwestern Medical Center, Dallas, and his colleagues performed a retrospective chart review of 367 children aged 9-17 years referred for a full-night polysomnography (PSG) for suspicion of having OSA. Demographic variables recorded included race, body mass index, rhinitis, gastroesophageal reflux disease, and tonsillar hypertrophy. Sleep variables recorded included apnea hypopnea index (AHI), sleep efficiency, rapid eye movement, and the peripheral capillary oxygen saturation (SpO2) nadir. The primary outcome was severe OSA defined as an AHI of 10 or greater on the PSG. They used logistic modeling to determine the association between asthma, obesity, and severe OSA.
The mean age of the study population was 14 years, 56% were male, and 43% were Hispanic. Of the 367 patients, 77 were neither obese nor asthmatic, 93 were nonobese but were asthmatic, 102 were obese but were nonasthmatic, and 95 were both obese and asthmatic. PSG results confirmed that obesity was associated with more signs of sleep apnea. For example, the nonobese, nonasthmatic group had a mean AHI of 11 events per hour, while the obese, nonasthmatic group had a mean AHI of 19 events per hour. “We observed a similar trend amongst our asthmatic population,” Mr. Narayanan said. “We observed an increase in the mean AHI amongst our asthmatic kids when we added obesity to the picture. Surprisingly, we found that asthma was associated with having fewer signs of sleep apnea.” Specifically, while the nonobese, nonasthmatic group had a mean AHI of 11 events per hour, those in the nonobese, asthmatic group had a mean of 5.6 events per hour (P = .005). “The finding was similar amongst our obese kids,” he said. “We saw a decrease in the mean AHI of our obese kids when we added asthma to the picture.”
On logistic regression analysis using obesity and asthma as independent variables, the researchers found that obesity increased the risk of severe OSA by 2.4-fold, but asthma decreased the odds of having severe OSA by about half (0.55). On multiple logistic regression controlling for commonly associated factors such as tonsillar hypertrophy, black race, and Hispanic ethnicity, obesity increased the risk of severe OSA by 2.2-fold, while asthma decreased the odds of having severe OSA by about half (0.51).
“In trying to explain this finding, we can turn to how these diseases are treated,” Mr. Narayanan said. “I say this because of the proven association between preexisting asthma and new onset OSA. Some of the reasons for this association include the tendency for airway collapsibility and systemwide inflammation seen in asthma, which then might contribute to the development of OSA. If we treat asthma symptoms early on, it might prevent the progression to sleep apnea down the line.”
Considering how prevalent comorbid asthma and OSA is, he continued, “we need to confirm that it is in fact well-controlled asthma that is associated with lowering the risk of severe OSA. Once we do this, we can ask the question: Can we use asthma pharmacotherapy to treat OSA? Some studies have shown that inhaled corticosteroids and montelukast (Singulair) may be effective treatment options for kids with OSA, but there’s definitely room for more research in this field, [such as determining] which patients would most benefit from this pharmacotherapy.” The researchers reported having no financial disclosures.
SOURCE: Narayanan A et al. Triological CSM, Abstracts.
CORONADO, CALIF. –
“We have a good idea that obesity and asthma independently increase the risk of OSA, but a lot of the time in the pediatric population, these risk factors are found comorbid,” Ajay Narayanan at the Triological Society’s Combined Sections Meeting. “For this study we asked, how does the presence of asthma change the likelihood of having severe OSA in a cohort of obese patients? Knowing that both asthma and obesity independently increase the risk for OSA, we hypothesized that when they were comorbid, asthma would have a synergistic effect with obesity, causing severe OSA.”
Mr. Narayanan, a third-year student at the University of Texas Southwestern Medical Center, Dallas, and his colleagues performed a retrospective chart review of 367 children aged 9-17 years referred for a full-night polysomnography (PSG) for suspicion of having OSA. Demographic variables recorded included race, body mass index, rhinitis, gastroesophageal reflux disease, and tonsillar hypertrophy. Sleep variables recorded included apnea hypopnea index (AHI), sleep efficiency, rapid eye movement, and the peripheral capillary oxygen saturation (SpO2) nadir. The primary outcome was severe OSA defined as an AHI of 10 or greater on the PSG. They used logistic modeling to determine the association between asthma, obesity, and severe OSA.
The mean age of the study population was 14 years, 56% were male, and 43% were Hispanic. Of the 367 patients, 77 were neither obese nor asthmatic, 93 were nonobese but were asthmatic, 102 were obese but were nonasthmatic, and 95 were both obese and asthmatic. PSG results confirmed that obesity was associated with more signs of sleep apnea. For example, the nonobese, nonasthmatic group had a mean AHI of 11 events per hour, while the obese, nonasthmatic group had a mean AHI of 19 events per hour. “We observed a similar trend amongst our asthmatic population,” Mr. Narayanan said. “We observed an increase in the mean AHI amongst our asthmatic kids when we added obesity to the picture. Surprisingly, we found that asthma was associated with having fewer signs of sleep apnea.” Specifically, while the nonobese, nonasthmatic group had a mean AHI of 11 events per hour, those in the nonobese, asthmatic group had a mean of 5.6 events per hour (P = .005). “The finding was similar amongst our obese kids,” he said. “We saw a decrease in the mean AHI of our obese kids when we added asthma to the picture.”
On logistic regression analysis using obesity and asthma as independent variables, the researchers found that obesity increased the risk of severe OSA by 2.4-fold, but asthma decreased the odds of having severe OSA by about half (0.55). On multiple logistic regression controlling for commonly associated factors such as tonsillar hypertrophy, black race, and Hispanic ethnicity, obesity increased the risk of severe OSA by 2.2-fold, while asthma decreased the odds of having severe OSA by about half (0.51).
“In trying to explain this finding, we can turn to how these diseases are treated,” Mr. Narayanan said. “I say this because of the proven association between preexisting asthma and new onset OSA. Some of the reasons for this association include the tendency for airway collapsibility and systemwide inflammation seen in asthma, which then might contribute to the development of OSA. If we treat asthma symptoms early on, it might prevent the progression to sleep apnea down the line.”
Considering how prevalent comorbid asthma and OSA is, he continued, “we need to confirm that it is in fact well-controlled asthma that is associated with lowering the risk of severe OSA. Once we do this, we can ask the question: Can we use asthma pharmacotherapy to treat OSA? Some studies have shown that inhaled corticosteroids and montelukast (Singulair) may be effective treatment options for kids with OSA, but there’s definitely room for more research in this field, [such as determining] which patients would most benefit from this pharmacotherapy.” The researchers reported having no financial disclosures.
SOURCE: Narayanan A et al. Triological CSM, Abstracts.
REPORTING FROM THE TRIOLOGICAL CSM
Key clinical point: In children, having asthma could decrease the risk of having severe obstructive sleep apnea, regardless of their obesity status.
Major finding: On multiple logistic regression, obesity increased the risk of severe OSA by 2.2-fold, while asthma decreased the odds of having severe OSA by about half.
Study details: A retrospective review of 367 children referred for a full-night polysomnography for suspicion of having OSA.
Disclosures: The researchers reported having no financial disclosures.
Source: Narayanan A et al. Triological CSM, Abstracts.
Roux-en-Y achieves diabetes remission in majority of patients
Around three-quarters of people with type 2 diabetes mellitus (T2DM) who undergo Roux-en-Y gastric bypass experience remission of their disease within a year of the surgery, according to published findings from a population-based observational study. However, one in four of those people will have relapsed by 5 years, the authors noted.
Researchers looked at the effect of Roux-en-Y gastric bypass (RYGB) in 1,111 individuals with T2DM, compared with 1,074 controls who also had T2DM but did not undergo gastric bypass.
By 6 months after surgery, 65% of those who had undergone RYGB met the criteria for remission – defined as no use of glucose-lowering drugs and an HbA1c below 48 mmol/mol (less than 6.5%) or metformin monotherapy with HbA1c below 42 mmol/mol (less than 6.0%).
By 1 year, 74% of those who had surgery had achieved remission, and 73% of those remained in remission 5 years after surgery. However, at 2 years, 6% of those who had achieved remission in the first year had already relapsed; by 3 years, 12% had relapsed; and by 4 years, 18% had relapsed. By 5 years after surgery, a total of 27% of those who originally achieved remission in the first year had relapsed.
The overall prevalence of remission remained at 70% for every 6-month period during the duration of the study, which suggests that, although some achieved remission early and then relapsed, others achieved remission later.
Individuals who were aged 50-60 years were 12% less likely to achieve remission, compared with those who were younger than 40 years, whereas those aged 60 years or more were 17% less likely to achieve remission.
A longer duration of diabetes was also associated with a lower likelihood of achieving remission after RYGB; individuals who had had diabetes for 8 years or more had a 27% lower likelihood of remission, compared with those who had had the disease for less than 2 years.
A higher HbA1c (greater than 53 mmol/mol) was associated with a 19% lower likelihood of remission, and individuals using insulin had a 43% lower likelihood of remission.
“Overall, our findings add evidence to the importance of regular check-ups following RYGB, despite initial diabetes remission, and also suggest that timing of RYGB is important (i.e., consider RYGB while there are still functional pancreatic beta cells),” wrote Lene R. Madsen, MD, from the department of endocrinology and internal medicine at Aarhus (Denmark) University Hospital and her colleagues.
The study also examined the effect of RYGB on microvascular and macrovascular diabetes complications. This revealed that the incidence of diabetic retinopathy was nearly halved among individuals who had undergone gastric bypass, the incidence of hospital-coded diabetic kidney disease was 46% lower, and the incidence of diabetic neuropathy was 16% lower.
In particular, individuals who achieved remission in the first year after surgery had a 57% lower incidence of microvascular events, compared with those who did not have surgery.
The authors noted that individuals who did not reach the threshold for diabetes remission after surgery still showed signs of better glycemic control, compared with individuals who had not undergone surgery.
“This aligns with the theory of ‘metabolic memory’ introduced by Coleman et al. [Diabetes Care. 2016;39(8):1400-07], suggesting that time spent in diabetes remission after RYGB is not spent in vain when it comes to reducing the risk of subsequent microvascular complications,” they wrote.
The surgery was also associated with a 46% reduction in the incidence of ischemic heart disease. In the first 30 days after surgery, 7.5% of patients were readmitted to hospital for any surgical complication, but the 90-day mortality rate after surgery was less than 0.5%.
The study was supported by the Health Research Fund of Central Denmark, the Novo Nordisk Foundation, and the A.P. Møller Foundation. The authors reported no conflicts of interest.
SOURCE: Madsen LR et al. Diabetologia. 2019, Feb 6. doi: 10.1007/s00125-019-4816-2.
Around three-quarters of people with type 2 diabetes mellitus (T2DM) who undergo Roux-en-Y gastric bypass experience remission of their disease within a year of the surgery, according to published findings from a population-based observational study. However, one in four of those people will have relapsed by 5 years, the authors noted.
Researchers looked at the effect of Roux-en-Y gastric bypass (RYGB) in 1,111 individuals with T2DM, compared with 1,074 controls who also had T2DM but did not undergo gastric bypass.
By 6 months after surgery, 65% of those who had undergone RYGB met the criteria for remission – defined as no use of glucose-lowering drugs and an HbA1c below 48 mmol/mol (less than 6.5%) or metformin monotherapy with HbA1c below 42 mmol/mol (less than 6.0%).
By 1 year, 74% of those who had surgery had achieved remission, and 73% of those remained in remission 5 years after surgery. However, at 2 years, 6% of those who had achieved remission in the first year had already relapsed; by 3 years, 12% had relapsed; and by 4 years, 18% had relapsed. By 5 years after surgery, a total of 27% of those who originally achieved remission in the first year had relapsed.
The overall prevalence of remission remained at 70% for every 6-month period during the duration of the study, which suggests that, although some achieved remission early and then relapsed, others achieved remission later.
Individuals who were aged 50-60 years were 12% less likely to achieve remission, compared with those who were younger than 40 years, whereas those aged 60 years or more were 17% less likely to achieve remission.
A longer duration of diabetes was also associated with a lower likelihood of achieving remission after RYGB; individuals who had had diabetes for 8 years or more had a 27% lower likelihood of remission, compared with those who had had the disease for less than 2 years.
A higher HbA1c (greater than 53 mmol/mol) was associated with a 19% lower likelihood of remission, and individuals using insulin had a 43% lower likelihood of remission.
“Overall, our findings add evidence to the importance of regular check-ups following RYGB, despite initial diabetes remission, and also suggest that timing of RYGB is important (i.e., consider RYGB while there are still functional pancreatic beta cells),” wrote Lene R. Madsen, MD, from the department of endocrinology and internal medicine at Aarhus (Denmark) University Hospital and her colleagues.
The study also examined the effect of RYGB on microvascular and macrovascular diabetes complications. This revealed that the incidence of diabetic retinopathy was nearly halved among individuals who had undergone gastric bypass, the incidence of hospital-coded diabetic kidney disease was 46% lower, and the incidence of diabetic neuropathy was 16% lower.
In particular, individuals who achieved remission in the first year after surgery had a 57% lower incidence of microvascular events, compared with those who did not have surgery.
The authors noted that individuals who did not reach the threshold for diabetes remission after surgery still showed signs of better glycemic control, compared with individuals who had not undergone surgery.
“This aligns with the theory of ‘metabolic memory’ introduced by Coleman et al. [Diabetes Care. 2016;39(8):1400-07], suggesting that time spent in diabetes remission after RYGB is not spent in vain when it comes to reducing the risk of subsequent microvascular complications,” they wrote.
The surgery was also associated with a 46% reduction in the incidence of ischemic heart disease. In the first 30 days after surgery, 7.5% of patients were readmitted to hospital for any surgical complication, but the 90-day mortality rate after surgery was less than 0.5%.
The study was supported by the Health Research Fund of Central Denmark, the Novo Nordisk Foundation, and the A.P. Møller Foundation. The authors reported no conflicts of interest.
SOURCE: Madsen LR et al. Diabetologia. 2019, Feb 6. doi: 10.1007/s00125-019-4816-2.
Around three-quarters of people with type 2 diabetes mellitus (T2DM) who undergo Roux-en-Y gastric bypass experience remission of their disease within a year of the surgery, according to published findings from a population-based observational study. However, one in four of those people will have relapsed by 5 years, the authors noted.
Researchers looked at the effect of Roux-en-Y gastric bypass (RYGB) in 1,111 individuals with T2DM, compared with 1,074 controls who also had T2DM but did not undergo gastric bypass.
By 6 months after surgery, 65% of those who had undergone RYGB met the criteria for remission – defined as no use of glucose-lowering drugs and an HbA1c below 48 mmol/mol (less than 6.5%) or metformin monotherapy with HbA1c below 42 mmol/mol (less than 6.0%).
By 1 year, 74% of those who had surgery had achieved remission, and 73% of those remained in remission 5 years after surgery. However, at 2 years, 6% of those who had achieved remission in the first year had already relapsed; by 3 years, 12% had relapsed; and by 4 years, 18% had relapsed. By 5 years after surgery, a total of 27% of those who originally achieved remission in the first year had relapsed.
The overall prevalence of remission remained at 70% for every 6-month period during the duration of the study, which suggests that, although some achieved remission early and then relapsed, others achieved remission later.
Individuals who were aged 50-60 years were 12% less likely to achieve remission, compared with those who were younger than 40 years, whereas those aged 60 years or more were 17% less likely to achieve remission.
A longer duration of diabetes was also associated with a lower likelihood of achieving remission after RYGB; individuals who had had diabetes for 8 years or more had a 27% lower likelihood of remission, compared with those who had had the disease for less than 2 years.
A higher HbA1c (greater than 53 mmol/mol) was associated with a 19% lower likelihood of remission, and individuals using insulin had a 43% lower likelihood of remission.
“Overall, our findings add evidence to the importance of regular check-ups following RYGB, despite initial diabetes remission, and also suggest that timing of RYGB is important (i.e., consider RYGB while there are still functional pancreatic beta cells),” wrote Lene R. Madsen, MD, from the department of endocrinology and internal medicine at Aarhus (Denmark) University Hospital and her colleagues.
The study also examined the effect of RYGB on microvascular and macrovascular diabetes complications. This revealed that the incidence of diabetic retinopathy was nearly halved among individuals who had undergone gastric bypass, the incidence of hospital-coded diabetic kidney disease was 46% lower, and the incidence of diabetic neuropathy was 16% lower.
In particular, individuals who achieved remission in the first year after surgery had a 57% lower incidence of microvascular events, compared with those who did not have surgery.
The authors noted that individuals who did not reach the threshold for diabetes remission after surgery still showed signs of better glycemic control, compared with individuals who had not undergone surgery.
“This aligns with the theory of ‘metabolic memory’ introduced by Coleman et al. [Diabetes Care. 2016;39(8):1400-07], suggesting that time spent in diabetes remission after RYGB is not spent in vain when it comes to reducing the risk of subsequent microvascular complications,” they wrote.
The surgery was also associated with a 46% reduction in the incidence of ischemic heart disease. In the first 30 days after surgery, 7.5% of patients were readmitted to hospital for any surgical complication, but the 90-day mortality rate after surgery was less than 0.5%.
The study was supported by the Health Research Fund of Central Denmark, the Novo Nordisk Foundation, and the A.P. Møller Foundation. The authors reported no conflicts of interest.
SOURCE: Madsen LR et al. Diabetologia. 2019, Feb 6. doi: 10.1007/s00125-019-4816-2.
FROM DIABETOLOGIA
Key clinical point: Diabetes remission was achieved in three-quarters of Roux-en-Y surgical patients.
Major finding: The incidence of diabetes remission 1 year after Roux-en-Y gastric bypass was 74%.
Study details: A population-based cohort study in 1,111 individuals with type 2 diabetes mellitus who underwent Roux-en-Y gastric bypass, compared with 1,074 nonsurgical controls with diabetes.
Disclosures: The study was supported by the Health Research Fund of Central Denmark, the Novo Nordisk Foundation, and the A.P. Møller Foundation. The authors reported no conflicts of interest.
Source: Madsen LR et al. Diabetologia. 2019, Feb 6. doi: 10.1007/s00125-019-4816-2.