User login
Our missing microbes: Short-term antibiotic courses have long-term consequences
Recent years have seen dramatic increases in the prevalences of chronic diseases such as type 1 diabetes,1 gastroesophageal reflux disease,2 asthma,3 inflammatory bowel disease,4 and, notably, obesity.5 I propose the hypothesis that much of this increase may be due to loss of diversity in the bacteria that make our guts their home.6 While multiple causes contribute, much of the blame may be attributed to the use—and overuse—of antibiotics.
FAT AND GETTING FATTER
Today, nearly 40% of US adults are obese, and nearly three-fourths are either obese or overweight.7 More alarming, the prevalence of obesity is also high and getting higher in children and adolescents,8 having increased from 10.0% in 1988–1994 to 17.8% in 2013–2016.
And not just in the United States. Trends in weight have been going up around the world, with a lag of about 30 years between developing countries and industrialized countries.5
OUR BACTERIA, OURSELVES
I believe that the bacteria we carry are not random, but rather have coevolved along with us, passed down from generation to generation in a state of dynamic equilibrium between microbes and host. Evidence supporting this comes from a study by Ochman et al,9 who analyzed the DNA from fecal samples from different hominid species (including Homo sapiens) and found that the phylogenic relationships among the bacteria mirrored those among the apes.
Interacting with each other and with us in complex ways, our bacteria are a diverse community to which we can apply the term microbiome. They are acquired in a standard, choreographed process,10 and their composition comes to resemble that of adults by the age of 3.11
Before modern times, microbes were transferred from mother to child during vaginal birth, from the mother’s breast during nursing, through skin-to-skin contact, and from the mother’s mouth by kissing. Now, widespread cesarean delivery, bottle-feeding, extensive bathing (especially with antibacterial soaps), and especially the use of antibiotics have changed the human ecology and altered transmission and maintenance of ancestral microbes, which affects the composition of the microbiota. The microbes, both good and bad, that are usually acquired early in life are especially important, since they affect a developmentally critical stage.12
Loss of microbial diversity in the mother appears to be cumulative over succeeding generations.13 For example, in a study in Japanese families, Urita et al14 found a decline in the prevalence of Helicobacter pylori colonization from 68.7% in the first generation to 43.4% in the second generation and 12.5% in the third. Clemente et al15 studied the intestinal microbiota in a previously uncontacted group of Yanomami people in the Amazon jungle and found they had the highest diversity of bacteria ever reported in a human group. By comparison, the research team calculated that we in the United States have already lost 50% of our microbial diversity, and 2 other groups, the Guahibo (another Amerindian group) and rural Malawians, were in between. More recent studies are confirming these observations.16,17
USE AND OVERUSE OF ANTIBIOTICS
More than 73 billion antibiotic doses are prescribed worldwide yearly,18 or about 10 doses for every man, woman, and child on Earth, and the numbers are rising. In the United States 262 million courses were prescribed in 2011, or 842 per 1,000 population.19 Children receive a mean of 2.7 courses by age 2, and 10.9 by age 10. More than 50% of women receive antibiotics during pregnancy or perinatally. This is in addition to an unknown level of exposure from agricultural use of antibiotics.
Repeated antibiotic exposure is common in early life, varies widely by country, and is often not medically justified.20 In the United States, antibiotic use varies by region, with the heaviest use in the South.19,21 It also varies widely among prescribers.22 Jones et al23 examined antibiotic prescribing for acute respiratory infections in US veterans and found that the top 10% of physicians gave an antibiotic more than 90% of the time. Physicians in Sweden prescribe about 60% fewer antibiotics than we do in the United States.21,24
Observational data indicate that people who receive antibiotics have a higher risk of chronic diseases later in life, eg:
- Type 2 diabetes (odds ratio 1.21, 95% confidence interval 1.19–1.23 with 2 to 4 courses, and odds ratio 1.53 (1.50–1.55) with 5 or more courses, up to 15 years after25
- Obesity: US states with the highest prevalence of antibiotic use also have the highest prevalence of obesity26
- Kidney stones: prior antibiotic exposure in a large UK study was associated with increased kidney stone risk, for exposures up to 5 years earlier.27
The meat industry has exploited the weight effect for decades, adding subtherapeutic doses of antibiotics to animals’ feed to make them gain weight.28
FINDINGS FROM STUDIES IN MICE
Laboratory studies of the relationship between antibiotic exposure and disease phenotypes in mice have yielded interesting findings.
Mice exposed to antibiotics had more body fat at 10 weeks (32.0%) than control mice (22.9%).29
Low-dose penicillin, started at birth, induces long-lasting effects on the expression of genes involved in immunity and enhances the effect of a high-fat diet in terms of weight gain.30 If the antibiotic exposure is limited to early life, the effect on the microbiota is transient, but the mice still gain weight. If the microbiota from the mice who received penicillin is transferred to germ-free mice, the recipients also become fat, indicating that the bacteria, not the antibiotics per se, cause the weight gain.
In other experiments,31 a series of short, therapeutic doses of antibiotics early in life modeled after those given to children to treat their acute infections caused long-term changes in the composition of the microbiome and in metabolism.
A single course of a macrolide antibiotic also had long-term effects on the microbial population and on the host’s ileal gene expression, T-cell populations, and secretory immunoglobulin A expression.32 These effects were seen only in mice that had a microbiome to begin with, not in germ-free mice, indicating that the antibiotics had their effect through the changes in the microbiome, not directly. But when germ-free mice received a fecal transplant of an impaired microbiome, it was sufficient to affect immunity.
In nonobese diabetic mice, treatment with antibiotics early in life altered the gut microbiome and its metabolic capacities, intestinal gene expression, and T-cell populations, accelerating the onset of type 1 diabetes.33
In a study in Danish children,34 the likelihood of inflammatory bowel disease increased with early-life antibiotic exposure: the more courses the child received, the greater the likelihood of disease. This observation led researchers to wonder if an antibiotic-altered microbiome affects the outcome of inflammatory bowel disease in the next generation.35 Germ-free female mice who received microbiota from mice who had received antibiotics passed the altered microbiome to their pups. Mice lacking the gene for interleukin 10 are genetically susceptible to colitis, and when this experiment was done in mice lacking this gene, the offspring developed markedly more colitis. This indicated the mothers could pass down their altered microbiome to the next generation and that it would affect their risk of disease.
WHAT CAN WE DO?
All physicians must adhere to the principles of antibiotic stewardship,36 not only to prevent the development of resistant strains of pathogens and the overgrowth of potentially dangerous species such as Clostridium difficile, but also, possibly, to prevent the loss of diversity in the human microbiome and thus discourage the development of chronic diseases.
In the future, as we discover more about the microbiome and the optimal mix of bacteria to carry, this information may find practical application in medicine. A pediatrician, for example, may want to analyze a child’s microbiome and, if it is abnormal, administer specific organisms to reshape it.
- TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study. Ann NY Acad Sci 2008; 1150:1–13. doi:10.1196/annals.1447.062
- El-Serag HB, Sonnenberg A. Associations between different forms of gastro-oesophageal reflux disease. Gut 1997; 41(5):594–599. pmid:9414963
- Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med 2006; 355(21):2226–2235. doi:10.1056/NEJMra054308
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017; 152(2):313–321. doi:10.1053/j.gastro.2016.10.020
- de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 2010; 92(5):1257–1264. doi:10.3945/ajcn.2010.29786
- Blaser MJ. The theory of disappearing microbiota and the epidemics of chronic disease. Nat Rev Immunol 2017; 17(8):461–463. doi:10.1038/nri.2017.77
- Centers for Disease Control and Prevention. National Center for Health Statistics. Obesity and overweight. www.cdc.gov/nchs/fastats/obesity-overweight.htm. Accessed November 6, 2018.
- Centers for Disease Control and Prevention. National Center for Health Statistics. Table 59. Obesity among children and adolescents aged 2-19 years, by selected characteristics: United States, selected years 1988–1994 through 2013–2016. www.cdc.gov/nchs/data/hus/2017/059.pdf. Accessed November 6, 2018.
- Ochman H, Worobey M, Kuo CH, et al. Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biology 2010; 8(11):e1000546. doi:10.1371/journal.pbio.1000546
- Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Trans Med 2016; 8(343):343ra82. doi:10.1126/scitranslmed.aad7121
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486(7402):222–227. doi:10.1038/nature11053
- Blaser MJ. The past and future biology of the human microbiome in an age of extinctions. Cell 2018; 172(6):1173–1177. doi:10.1016/j.cell.2018.02.040
- Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol 2009; 7(12):887–894. doi:10.1038/nrmicro2245
- Urita Y, Watanabe T, Kawagoe N, et al. Role of infected grandmothers in transmission of Helicobacter pylori to children in a Japanese rural town. J Ped Child Health 2013; 49(5):394–398. doi:10.1111/jpc.12191
- Clemente JC, Pehrsson EC, Blaser MJ, et al. The microbiome of uncontacted Amerindians. Sci Adv 2015; 1(3). Pii:e1500183. doi:10.1126/sciadv.1500183
- Smits SA, Leach J, Sonnenburg ED, et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017; 357(6353):802-806. doi:10.1126/science.aan4834
- Vangay P, Johnson AJ, Ward TL, et al. US immigration westernizes the human gut microbiome. Cell 2018; 175(4):962–972. doi:10.1016/j.cell.2018.10.029
- Van Broeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14(8):742–750. doi:10.1016/S1473-3099(14)70780-7
- Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015; 60(9):1308–1316. doi:10.1093/cid/civ076
- Rogawski ET, Platts-Mills JA, Seidman JC, et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull World Health Organ 2017; 95(1):49–61. doi:10.2471/BLT.16.176123
- Hicks LA, Taylor TH Jr, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010; N Engl J Med 2013; 368(15):1461–1462. doi:10.1056/NEJMc1212055
- Gerber JS, Prasad PA, Russell LA, et al. Variation in antibiotic prescribing across a pediatric primary care network. J Pediatric Infect Dis Soc 2015; 4(4):297–304. doi:10.1093/jpids/piu086
- Jones BE, Sauer B, Jones MM, et al. Variation in outpatient antibiotic prescribing for acute respiratory infections in the veteran population: a cross-sectional study. Ann Intern Med 2015; 163(2):73–80. doi:10.7326/M14-1933
- Ternhag A, Hellman J. More on U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013; 369(12):1175. doi:10.1056/NEJMc1306863
- Mikkelsen KH, Knop FK, Frost M, Hallas J, Pottegard A. Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J Clin Endocrinol Metab 2015; 100(10):3633–3640. doi:10.1210/jc.2015-2696
- Petschow B, Dore J, Hibbert P, et al. Probiotics, prebiotics, and the host microbiome: the science of translation. Ann NY Acad Sci 2013; 1306:1–17. doi:10.1111/nyas.12303
- Tasian GE, Jemielita T, Goldfarb DS, et al. Oral antibiotic exposure and kidney stone disease. J Am Soc Nephrol 2018; 29(6):1731–1740. doi:10.1681/ASN.2017111213
- Zimmerman DR. Role of subtherapeutic levels of antimicrobials in pig production. J Anim Sci 1986; 62(suppl 3):6–16.
- Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012; 488(7413):621–626. doi:10.1038/nature11400
- Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014; 158(4):705–721. doi:10.1016/j.cell.2014.05.052
- Nobel YR, Cox LM, Kirigin FF, et al. Metabolic and metagenomics outcomes from early-life pulsed antibiotic treatment. Nat Commun 2015; 6:7486. doi:10.1038/ncomms8486
- Ruiz VE, Battaglia T, Kurtz ZD, et al. A single early-in-life macrolide course has lasting effects on murine microbial network topology and immunity. Nat Commun 2017; 8(1):518. doi:10.1038/s41467-017-00531-6
- Livanos AE, Greiner TU, Vangay P, et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol 2016; 1(11):16149. doi:10.1038/nmicrobiol.2016.140
- Hvilid A, Svanström H, Frish M. Antibiotic use and inflammatory bowel disease in childhood. Gut 2011; 60(1):49–54. doi:10.1136/gut.2010.219683
- Schulfer AF, Battaglia T, Alvarez Y, et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat Microbiol 2018; 3(2):234–242. doi:10.1038/s41564-017-0075-5
- Srinivasan A. Antibiotic stewardship: why we must, how we can. Cleve Clin J Med 2017; 84(9):673–679. doi:10.3949/ccjm.84gr.17003
Recent years have seen dramatic increases in the prevalences of chronic diseases such as type 1 diabetes,1 gastroesophageal reflux disease,2 asthma,3 inflammatory bowel disease,4 and, notably, obesity.5 I propose the hypothesis that much of this increase may be due to loss of diversity in the bacteria that make our guts their home.6 While multiple causes contribute, much of the blame may be attributed to the use—and overuse—of antibiotics.
FAT AND GETTING FATTER
Today, nearly 40% of US adults are obese, and nearly three-fourths are either obese or overweight.7 More alarming, the prevalence of obesity is also high and getting higher in children and adolescents,8 having increased from 10.0% in 1988–1994 to 17.8% in 2013–2016.
And not just in the United States. Trends in weight have been going up around the world, with a lag of about 30 years between developing countries and industrialized countries.5
OUR BACTERIA, OURSELVES
I believe that the bacteria we carry are not random, but rather have coevolved along with us, passed down from generation to generation in a state of dynamic equilibrium between microbes and host. Evidence supporting this comes from a study by Ochman et al,9 who analyzed the DNA from fecal samples from different hominid species (including Homo sapiens) and found that the phylogenic relationships among the bacteria mirrored those among the apes.
Interacting with each other and with us in complex ways, our bacteria are a diverse community to which we can apply the term microbiome. They are acquired in a standard, choreographed process,10 and their composition comes to resemble that of adults by the age of 3.11
Before modern times, microbes were transferred from mother to child during vaginal birth, from the mother’s breast during nursing, through skin-to-skin contact, and from the mother’s mouth by kissing. Now, widespread cesarean delivery, bottle-feeding, extensive bathing (especially with antibacterial soaps), and especially the use of antibiotics have changed the human ecology and altered transmission and maintenance of ancestral microbes, which affects the composition of the microbiota. The microbes, both good and bad, that are usually acquired early in life are especially important, since they affect a developmentally critical stage.12
Loss of microbial diversity in the mother appears to be cumulative over succeeding generations.13 For example, in a study in Japanese families, Urita et al14 found a decline in the prevalence of Helicobacter pylori colonization from 68.7% in the first generation to 43.4% in the second generation and 12.5% in the third. Clemente et al15 studied the intestinal microbiota in a previously uncontacted group of Yanomami people in the Amazon jungle and found they had the highest diversity of bacteria ever reported in a human group. By comparison, the research team calculated that we in the United States have already lost 50% of our microbial diversity, and 2 other groups, the Guahibo (another Amerindian group) and rural Malawians, were in between. More recent studies are confirming these observations.16,17
USE AND OVERUSE OF ANTIBIOTICS
More than 73 billion antibiotic doses are prescribed worldwide yearly,18 or about 10 doses for every man, woman, and child on Earth, and the numbers are rising. In the United States 262 million courses were prescribed in 2011, or 842 per 1,000 population.19 Children receive a mean of 2.7 courses by age 2, and 10.9 by age 10. More than 50% of women receive antibiotics during pregnancy or perinatally. This is in addition to an unknown level of exposure from agricultural use of antibiotics.
Repeated antibiotic exposure is common in early life, varies widely by country, and is often not medically justified.20 In the United States, antibiotic use varies by region, with the heaviest use in the South.19,21 It also varies widely among prescribers.22 Jones et al23 examined antibiotic prescribing for acute respiratory infections in US veterans and found that the top 10% of physicians gave an antibiotic more than 90% of the time. Physicians in Sweden prescribe about 60% fewer antibiotics than we do in the United States.21,24
Observational data indicate that people who receive antibiotics have a higher risk of chronic diseases later in life, eg:
- Type 2 diabetes (odds ratio 1.21, 95% confidence interval 1.19–1.23 with 2 to 4 courses, and odds ratio 1.53 (1.50–1.55) with 5 or more courses, up to 15 years after25
- Obesity: US states with the highest prevalence of antibiotic use also have the highest prevalence of obesity26
- Kidney stones: prior antibiotic exposure in a large UK study was associated with increased kidney stone risk, for exposures up to 5 years earlier.27
The meat industry has exploited the weight effect for decades, adding subtherapeutic doses of antibiotics to animals’ feed to make them gain weight.28
FINDINGS FROM STUDIES IN MICE
Laboratory studies of the relationship between antibiotic exposure and disease phenotypes in mice have yielded interesting findings.
Mice exposed to antibiotics had more body fat at 10 weeks (32.0%) than control mice (22.9%).29
Low-dose penicillin, started at birth, induces long-lasting effects on the expression of genes involved in immunity and enhances the effect of a high-fat diet in terms of weight gain.30 If the antibiotic exposure is limited to early life, the effect on the microbiota is transient, but the mice still gain weight. If the microbiota from the mice who received penicillin is transferred to germ-free mice, the recipients also become fat, indicating that the bacteria, not the antibiotics per se, cause the weight gain.
In other experiments,31 a series of short, therapeutic doses of antibiotics early in life modeled after those given to children to treat their acute infections caused long-term changes in the composition of the microbiome and in metabolism.
A single course of a macrolide antibiotic also had long-term effects on the microbial population and on the host’s ileal gene expression, T-cell populations, and secretory immunoglobulin A expression.32 These effects were seen only in mice that had a microbiome to begin with, not in germ-free mice, indicating that the antibiotics had their effect through the changes in the microbiome, not directly. But when germ-free mice received a fecal transplant of an impaired microbiome, it was sufficient to affect immunity.
In nonobese diabetic mice, treatment with antibiotics early in life altered the gut microbiome and its metabolic capacities, intestinal gene expression, and T-cell populations, accelerating the onset of type 1 diabetes.33
In a study in Danish children,34 the likelihood of inflammatory bowel disease increased with early-life antibiotic exposure: the more courses the child received, the greater the likelihood of disease. This observation led researchers to wonder if an antibiotic-altered microbiome affects the outcome of inflammatory bowel disease in the next generation.35 Germ-free female mice who received microbiota from mice who had received antibiotics passed the altered microbiome to their pups. Mice lacking the gene for interleukin 10 are genetically susceptible to colitis, and when this experiment was done in mice lacking this gene, the offspring developed markedly more colitis. This indicated the mothers could pass down their altered microbiome to the next generation and that it would affect their risk of disease.
WHAT CAN WE DO?
All physicians must adhere to the principles of antibiotic stewardship,36 not only to prevent the development of resistant strains of pathogens and the overgrowth of potentially dangerous species such as Clostridium difficile, but also, possibly, to prevent the loss of diversity in the human microbiome and thus discourage the development of chronic diseases.
In the future, as we discover more about the microbiome and the optimal mix of bacteria to carry, this information may find practical application in medicine. A pediatrician, for example, may want to analyze a child’s microbiome and, if it is abnormal, administer specific organisms to reshape it.
Recent years have seen dramatic increases in the prevalences of chronic diseases such as type 1 diabetes,1 gastroesophageal reflux disease,2 asthma,3 inflammatory bowel disease,4 and, notably, obesity.5 I propose the hypothesis that much of this increase may be due to loss of diversity in the bacteria that make our guts their home.6 While multiple causes contribute, much of the blame may be attributed to the use—and overuse—of antibiotics.
FAT AND GETTING FATTER
Today, nearly 40% of US adults are obese, and nearly three-fourths are either obese or overweight.7 More alarming, the prevalence of obesity is also high and getting higher in children and adolescents,8 having increased from 10.0% in 1988–1994 to 17.8% in 2013–2016.
And not just in the United States. Trends in weight have been going up around the world, with a lag of about 30 years between developing countries and industrialized countries.5
OUR BACTERIA, OURSELVES
I believe that the bacteria we carry are not random, but rather have coevolved along with us, passed down from generation to generation in a state of dynamic equilibrium between microbes and host. Evidence supporting this comes from a study by Ochman et al,9 who analyzed the DNA from fecal samples from different hominid species (including Homo sapiens) and found that the phylogenic relationships among the bacteria mirrored those among the apes.
Interacting with each other and with us in complex ways, our bacteria are a diverse community to which we can apply the term microbiome. They are acquired in a standard, choreographed process,10 and their composition comes to resemble that of adults by the age of 3.11
Before modern times, microbes were transferred from mother to child during vaginal birth, from the mother’s breast during nursing, through skin-to-skin contact, and from the mother’s mouth by kissing. Now, widespread cesarean delivery, bottle-feeding, extensive bathing (especially with antibacterial soaps), and especially the use of antibiotics have changed the human ecology and altered transmission and maintenance of ancestral microbes, which affects the composition of the microbiota. The microbes, both good and bad, that are usually acquired early in life are especially important, since they affect a developmentally critical stage.12
Loss of microbial diversity in the mother appears to be cumulative over succeeding generations.13 For example, in a study in Japanese families, Urita et al14 found a decline in the prevalence of Helicobacter pylori colonization from 68.7% in the first generation to 43.4% in the second generation and 12.5% in the third. Clemente et al15 studied the intestinal microbiota in a previously uncontacted group of Yanomami people in the Amazon jungle and found they had the highest diversity of bacteria ever reported in a human group. By comparison, the research team calculated that we in the United States have already lost 50% of our microbial diversity, and 2 other groups, the Guahibo (another Amerindian group) and rural Malawians, were in between. More recent studies are confirming these observations.16,17
USE AND OVERUSE OF ANTIBIOTICS
More than 73 billion antibiotic doses are prescribed worldwide yearly,18 or about 10 doses for every man, woman, and child on Earth, and the numbers are rising. In the United States 262 million courses were prescribed in 2011, or 842 per 1,000 population.19 Children receive a mean of 2.7 courses by age 2, and 10.9 by age 10. More than 50% of women receive antibiotics during pregnancy or perinatally. This is in addition to an unknown level of exposure from agricultural use of antibiotics.
Repeated antibiotic exposure is common in early life, varies widely by country, and is often not medically justified.20 In the United States, antibiotic use varies by region, with the heaviest use in the South.19,21 It also varies widely among prescribers.22 Jones et al23 examined antibiotic prescribing for acute respiratory infections in US veterans and found that the top 10% of physicians gave an antibiotic more than 90% of the time. Physicians in Sweden prescribe about 60% fewer antibiotics than we do in the United States.21,24
Observational data indicate that people who receive antibiotics have a higher risk of chronic diseases later in life, eg:
- Type 2 diabetes (odds ratio 1.21, 95% confidence interval 1.19–1.23 with 2 to 4 courses, and odds ratio 1.53 (1.50–1.55) with 5 or more courses, up to 15 years after25
- Obesity: US states with the highest prevalence of antibiotic use also have the highest prevalence of obesity26
- Kidney stones: prior antibiotic exposure in a large UK study was associated with increased kidney stone risk, for exposures up to 5 years earlier.27
The meat industry has exploited the weight effect for decades, adding subtherapeutic doses of antibiotics to animals’ feed to make them gain weight.28
FINDINGS FROM STUDIES IN MICE
Laboratory studies of the relationship between antibiotic exposure and disease phenotypes in mice have yielded interesting findings.
Mice exposed to antibiotics had more body fat at 10 weeks (32.0%) than control mice (22.9%).29
Low-dose penicillin, started at birth, induces long-lasting effects on the expression of genes involved in immunity and enhances the effect of a high-fat diet in terms of weight gain.30 If the antibiotic exposure is limited to early life, the effect on the microbiota is transient, but the mice still gain weight. If the microbiota from the mice who received penicillin is transferred to germ-free mice, the recipients also become fat, indicating that the bacteria, not the antibiotics per se, cause the weight gain.
In other experiments,31 a series of short, therapeutic doses of antibiotics early in life modeled after those given to children to treat their acute infections caused long-term changes in the composition of the microbiome and in metabolism.
A single course of a macrolide antibiotic also had long-term effects on the microbial population and on the host’s ileal gene expression, T-cell populations, and secretory immunoglobulin A expression.32 These effects were seen only in mice that had a microbiome to begin with, not in germ-free mice, indicating that the antibiotics had their effect through the changes in the microbiome, not directly. But when germ-free mice received a fecal transplant of an impaired microbiome, it was sufficient to affect immunity.
In nonobese diabetic mice, treatment with antibiotics early in life altered the gut microbiome and its metabolic capacities, intestinal gene expression, and T-cell populations, accelerating the onset of type 1 diabetes.33
In a study in Danish children,34 the likelihood of inflammatory bowel disease increased with early-life antibiotic exposure: the more courses the child received, the greater the likelihood of disease. This observation led researchers to wonder if an antibiotic-altered microbiome affects the outcome of inflammatory bowel disease in the next generation.35 Germ-free female mice who received microbiota from mice who had received antibiotics passed the altered microbiome to their pups. Mice lacking the gene for interleukin 10 are genetically susceptible to colitis, and when this experiment was done in mice lacking this gene, the offspring developed markedly more colitis. This indicated the mothers could pass down their altered microbiome to the next generation and that it would affect their risk of disease.
WHAT CAN WE DO?
All physicians must adhere to the principles of antibiotic stewardship,36 not only to prevent the development of resistant strains of pathogens and the overgrowth of potentially dangerous species such as Clostridium difficile, but also, possibly, to prevent the loss of diversity in the human microbiome and thus discourage the development of chronic diseases.
In the future, as we discover more about the microbiome and the optimal mix of bacteria to carry, this information may find practical application in medicine. A pediatrician, for example, may want to analyze a child’s microbiome and, if it is abnormal, administer specific organisms to reshape it.
- TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study. Ann NY Acad Sci 2008; 1150:1–13. doi:10.1196/annals.1447.062
- El-Serag HB, Sonnenberg A. Associations between different forms of gastro-oesophageal reflux disease. Gut 1997; 41(5):594–599. pmid:9414963
- Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med 2006; 355(21):2226–2235. doi:10.1056/NEJMra054308
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017; 152(2):313–321. doi:10.1053/j.gastro.2016.10.020
- de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 2010; 92(5):1257–1264. doi:10.3945/ajcn.2010.29786
- Blaser MJ. The theory of disappearing microbiota and the epidemics of chronic disease. Nat Rev Immunol 2017; 17(8):461–463. doi:10.1038/nri.2017.77
- Centers for Disease Control and Prevention. National Center for Health Statistics. Obesity and overweight. www.cdc.gov/nchs/fastats/obesity-overweight.htm. Accessed November 6, 2018.
- Centers for Disease Control and Prevention. National Center for Health Statistics. Table 59. Obesity among children and adolescents aged 2-19 years, by selected characteristics: United States, selected years 1988–1994 through 2013–2016. www.cdc.gov/nchs/data/hus/2017/059.pdf. Accessed November 6, 2018.
- Ochman H, Worobey M, Kuo CH, et al. Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biology 2010; 8(11):e1000546. doi:10.1371/journal.pbio.1000546
- Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Trans Med 2016; 8(343):343ra82. doi:10.1126/scitranslmed.aad7121
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486(7402):222–227. doi:10.1038/nature11053
- Blaser MJ. The past and future biology of the human microbiome in an age of extinctions. Cell 2018; 172(6):1173–1177. doi:10.1016/j.cell.2018.02.040
- Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol 2009; 7(12):887–894. doi:10.1038/nrmicro2245
- Urita Y, Watanabe T, Kawagoe N, et al. Role of infected grandmothers in transmission of Helicobacter pylori to children in a Japanese rural town. J Ped Child Health 2013; 49(5):394–398. doi:10.1111/jpc.12191
- Clemente JC, Pehrsson EC, Blaser MJ, et al. The microbiome of uncontacted Amerindians. Sci Adv 2015; 1(3). Pii:e1500183. doi:10.1126/sciadv.1500183
- Smits SA, Leach J, Sonnenburg ED, et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017; 357(6353):802-806. doi:10.1126/science.aan4834
- Vangay P, Johnson AJ, Ward TL, et al. US immigration westernizes the human gut microbiome. Cell 2018; 175(4):962–972. doi:10.1016/j.cell.2018.10.029
- Van Broeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14(8):742–750. doi:10.1016/S1473-3099(14)70780-7
- Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015; 60(9):1308–1316. doi:10.1093/cid/civ076
- Rogawski ET, Platts-Mills JA, Seidman JC, et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull World Health Organ 2017; 95(1):49–61. doi:10.2471/BLT.16.176123
- Hicks LA, Taylor TH Jr, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010; N Engl J Med 2013; 368(15):1461–1462. doi:10.1056/NEJMc1212055
- Gerber JS, Prasad PA, Russell LA, et al. Variation in antibiotic prescribing across a pediatric primary care network. J Pediatric Infect Dis Soc 2015; 4(4):297–304. doi:10.1093/jpids/piu086
- Jones BE, Sauer B, Jones MM, et al. Variation in outpatient antibiotic prescribing for acute respiratory infections in the veteran population: a cross-sectional study. Ann Intern Med 2015; 163(2):73–80. doi:10.7326/M14-1933
- Ternhag A, Hellman J. More on U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013; 369(12):1175. doi:10.1056/NEJMc1306863
- Mikkelsen KH, Knop FK, Frost M, Hallas J, Pottegard A. Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J Clin Endocrinol Metab 2015; 100(10):3633–3640. doi:10.1210/jc.2015-2696
- Petschow B, Dore J, Hibbert P, et al. Probiotics, prebiotics, and the host microbiome: the science of translation. Ann NY Acad Sci 2013; 1306:1–17. doi:10.1111/nyas.12303
- Tasian GE, Jemielita T, Goldfarb DS, et al. Oral antibiotic exposure and kidney stone disease. J Am Soc Nephrol 2018; 29(6):1731–1740. doi:10.1681/ASN.2017111213
- Zimmerman DR. Role of subtherapeutic levels of antimicrobials in pig production. J Anim Sci 1986; 62(suppl 3):6–16.
- Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012; 488(7413):621–626. doi:10.1038/nature11400
- Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014; 158(4):705–721. doi:10.1016/j.cell.2014.05.052
- Nobel YR, Cox LM, Kirigin FF, et al. Metabolic and metagenomics outcomes from early-life pulsed antibiotic treatment. Nat Commun 2015; 6:7486. doi:10.1038/ncomms8486
- Ruiz VE, Battaglia T, Kurtz ZD, et al. A single early-in-life macrolide course has lasting effects on murine microbial network topology and immunity. Nat Commun 2017; 8(1):518. doi:10.1038/s41467-017-00531-6
- Livanos AE, Greiner TU, Vangay P, et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol 2016; 1(11):16149. doi:10.1038/nmicrobiol.2016.140
- Hvilid A, Svanström H, Frish M. Antibiotic use and inflammatory bowel disease in childhood. Gut 2011; 60(1):49–54. doi:10.1136/gut.2010.219683
- Schulfer AF, Battaglia T, Alvarez Y, et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat Microbiol 2018; 3(2):234–242. doi:10.1038/s41564-017-0075-5
- Srinivasan A. Antibiotic stewardship: why we must, how we can. Cleve Clin J Med 2017; 84(9):673–679. doi:10.3949/ccjm.84gr.17003
- TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study. Ann NY Acad Sci 2008; 1150:1–13. doi:10.1196/annals.1447.062
- El-Serag HB, Sonnenberg A. Associations between different forms of gastro-oesophageal reflux disease. Gut 1997; 41(5):594–599. pmid:9414963
- Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med 2006; 355(21):2226–2235. doi:10.1056/NEJMra054308
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017; 152(2):313–321. doi:10.1053/j.gastro.2016.10.020
- de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 2010; 92(5):1257–1264. doi:10.3945/ajcn.2010.29786
- Blaser MJ. The theory of disappearing microbiota and the epidemics of chronic disease. Nat Rev Immunol 2017; 17(8):461–463. doi:10.1038/nri.2017.77
- Centers for Disease Control and Prevention. National Center for Health Statistics. Obesity and overweight. www.cdc.gov/nchs/fastats/obesity-overweight.htm. Accessed November 6, 2018.
- Centers for Disease Control and Prevention. National Center for Health Statistics. Table 59. Obesity among children and adolescents aged 2-19 years, by selected characteristics: United States, selected years 1988–1994 through 2013–2016. www.cdc.gov/nchs/data/hus/2017/059.pdf. Accessed November 6, 2018.
- Ochman H, Worobey M, Kuo CH, et al. Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biology 2010; 8(11):e1000546. doi:10.1371/journal.pbio.1000546
- Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Trans Med 2016; 8(343):343ra82. doi:10.1126/scitranslmed.aad7121
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012; 486(7402):222–227. doi:10.1038/nature11053
- Blaser MJ. The past and future biology of the human microbiome in an age of extinctions. Cell 2018; 172(6):1173–1177. doi:10.1016/j.cell.2018.02.040
- Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol 2009; 7(12):887–894. doi:10.1038/nrmicro2245
- Urita Y, Watanabe T, Kawagoe N, et al. Role of infected grandmothers in transmission of Helicobacter pylori to children in a Japanese rural town. J Ped Child Health 2013; 49(5):394–398. doi:10.1111/jpc.12191
- Clemente JC, Pehrsson EC, Blaser MJ, et al. The microbiome of uncontacted Amerindians. Sci Adv 2015; 1(3). Pii:e1500183. doi:10.1126/sciadv.1500183
- Smits SA, Leach J, Sonnenburg ED, et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017; 357(6353):802-806. doi:10.1126/science.aan4834
- Vangay P, Johnson AJ, Ward TL, et al. US immigration westernizes the human gut microbiome. Cell 2018; 175(4):962–972. doi:10.1016/j.cell.2018.10.029
- Van Broeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14(8):742–750. doi:10.1016/S1473-3099(14)70780-7
- Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015; 60(9):1308–1316. doi:10.1093/cid/civ076
- Rogawski ET, Platts-Mills JA, Seidman JC, et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull World Health Organ 2017; 95(1):49–61. doi:10.2471/BLT.16.176123
- Hicks LA, Taylor TH Jr, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010; N Engl J Med 2013; 368(15):1461–1462. doi:10.1056/NEJMc1212055
- Gerber JS, Prasad PA, Russell LA, et al. Variation in antibiotic prescribing across a pediatric primary care network. J Pediatric Infect Dis Soc 2015; 4(4):297–304. doi:10.1093/jpids/piu086
- Jones BE, Sauer B, Jones MM, et al. Variation in outpatient antibiotic prescribing for acute respiratory infections in the veteran population: a cross-sectional study. Ann Intern Med 2015; 163(2):73–80. doi:10.7326/M14-1933
- Ternhag A, Hellman J. More on U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 2013; 369(12):1175. doi:10.1056/NEJMc1306863
- Mikkelsen KH, Knop FK, Frost M, Hallas J, Pottegard A. Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J Clin Endocrinol Metab 2015; 100(10):3633–3640. doi:10.1210/jc.2015-2696
- Petschow B, Dore J, Hibbert P, et al. Probiotics, prebiotics, and the host microbiome: the science of translation. Ann NY Acad Sci 2013; 1306:1–17. doi:10.1111/nyas.12303
- Tasian GE, Jemielita T, Goldfarb DS, et al. Oral antibiotic exposure and kidney stone disease. J Am Soc Nephrol 2018; 29(6):1731–1740. doi:10.1681/ASN.2017111213
- Zimmerman DR. Role of subtherapeutic levels of antimicrobials in pig production. J Anim Sci 1986; 62(suppl 3):6–16.
- Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012; 488(7413):621–626. doi:10.1038/nature11400
- Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014; 158(4):705–721. doi:10.1016/j.cell.2014.05.052
- Nobel YR, Cox LM, Kirigin FF, et al. Metabolic and metagenomics outcomes from early-life pulsed antibiotic treatment. Nat Commun 2015; 6:7486. doi:10.1038/ncomms8486
- Ruiz VE, Battaglia T, Kurtz ZD, et al. A single early-in-life macrolide course has lasting effects on murine microbial network topology and immunity. Nat Commun 2017; 8(1):518. doi:10.1038/s41467-017-00531-6
- Livanos AE, Greiner TU, Vangay P, et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol 2016; 1(11):16149. doi:10.1038/nmicrobiol.2016.140
- Hvilid A, Svanström H, Frish M. Antibiotic use and inflammatory bowel disease in childhood. Gut 2011; 60(1):49–54. doi:10.1136/gut.2010.219683
- Schulfer AF, Battaglia T, Alvarez Y, et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat Microbiol 2018; 3(2):234–242. doi:10.1038/s41564-017-0075-5
- Srinivasan A. Antibiotic stewardship: why we must, how we can. Cleve Clin J Med 2017; 84(9):673–679. doi:10.3949/ccjm.84gr.17003
Narcolepsy: Diagnosis and management
Narcolepsy was originally described in the late 1800s by the French physician Jean-Baptiste-Edouard Gélineau, who reported the case of a wine merchant suffering from somnolence. In this first description, he coined the term narcolepsie by joining the Greek words narke (numbness or stupor) and lepsis (attack).1
Since then, the disorder has been further characterized, and some insight into its biological underpinnings has been established. Importantly, treatments have improved and expanded, facilitating its management and thereby improving quality of life for those with the disorder.
This review focuses on clinically relevant features of the disorder and proposes management strategies.
CLINICAL FEATURES
Narcolepsy is characterized by instability of sleep-wake transitions.
Daytime sleepiness
Clinically, narcolepsy manifests with excessive daytime sleepiness that can be personally and socially disabling. Cataplexy, sleep paralysis, and hypnagogic or hypnopompic hallucinations can also be present,2,3 but they are not necessary for diagnosis. In fact, a minority of patients with narcolepsy have all these symptoms.4 Narcolepsy is divided into type 1 (with cataplexy) and type 2 (without cataplexy).2
Sleepiness tends to be worse with inactivity, and sleep can often be irresistible. Sleep attacks can come on suddenly and may be brief enough to manifest as a lapse in consciousness.
Short naps tend to be refreshing. Rapid eye movement (REM) latency—the interval between falling asleep and the onset of the REM sleep—is short in narcolepsy, and since the REM stage is when dreaming occurs, naps often include dreaming. Therefore, when taking a history, it is worthwhile to ask patients whether they dream during naps; a yes answer supports the diagnosis of narcolepsy.5
In children, sleepiness can manifest in reduced concentration and behavioral issues.6 Napping after age 5 or 6 is considered abnormal and may reflect pathologic sleepiness.7
Cataplexy
Cataplexy—transient muscle weakness triggered by emotion—is a specific feature of narcolepsy type 1. It often begins in the facial muscles and can manifest with slackening of the jaw or brief dropping of the head. However, episodes can be more dramatic and, if the trunk and limb muscles are affected, can result in collapsing to the ground.
Cataplexy usually has its onset at about the same time as the sleepiness associated with narcolepsy, but it can arise even years later.8 Episodes can last from a few seconds to 2 minutes. Consciousness is always preserved. A range of emotions can trigger cataplexy, but typically the emotion is a positive one such as laughter or excitement.9 Deep tendon reflexes disappear in cataplexy, so checking reflexes during a witnessed episode can be clinically valuable.2
Cataplexy can worsen with stress and insufficient sleep, occasionally with “status cataplecticus,” in which repeated, persistent episodes of cataplexy occur over several hours.8 Status cataplecticus can be spontaneous or an effect of withdrawal from anticataplectic medications.2
Cataplexy is thought to represent intrusion of REM sleep and its associated muscle atonia during wakefulness.
Sleep paralysis, hallucinations
Sleep paralysis and hallucinations are other features of narcolepsy that reflect this REM dissociation from sleep.
Sleep paralysis occurs most commonly upon awakening, but sometimes just before sleep onset. In most cases, it is manifested by inability to move the limbs or speak, lasting several seconds or, in rare cases, minutes at a time. Sleep paralysis can be associated with a sensation of fear or suffocation, especially when initially experienced.8
Hypnopompic hallucinations, occurring upon awakening, are more common than hypnagogic hallucinations, which are experienced before falling asleep. The hallucinations are often vivid and usually visual, although other types of hallucinations are possible. Unlike those that occur in psychotic disorders, the hallucinations tend to be associated with preserved insight that they are not real.10
Notably, both sleep paralysis and hallucinations are nonspecific symptoms that are common in the general population.8,11,12
Fragmented sleep
Although they are very sleepy, people with narcolepsy generally cannot stay asleep for very long. Their sleep tends to be extremely fragmented, and they often wake up several times a night.2
This sleep pattern reflects the inherent instability of sleep-wake transitions in narcolepsy. In fact, over a 24-hour period, adults with narcolepsy have a normal amount of sleep.13 In children, however, when narcolepsy first arises, the 24-hour sleep time can increase abruptly and can sometimes be associated with persistent cataplexy that can manifest as a clumsy gait.14
Weight gain, obstructive sleep apnea
Weight gain is common, particularly after symptom onset, and especially in children. As a result, obesity is a frequent comorbidity.15 Because obstructive sleep apnea can consequently develop, all patients with narcolepsy require screening for sleep-disordered breathing.
Other sleep disorders often accompany narcolepsy and are more common than in the general population.16 In a study incorporating both clinical and polysomnographic data of 100 patients with narcolepsy, insomnia was the most common comorbid sleep disorder, with a prevalence of 28%; others were REM sleep behavior disorder (24%), restless legs syndrome (24%), obstructive sleep apnea (21%), and non-REM parasomnias.17
PSYCHOSOCIAL CONSEQUENCES
Narcolepsy has significant psychosocial consequences. As a result of their symptoms, people with narcolepsy may not be able to meet academic or work-related demands.
Additionally, their risk of a motor vehicle accident is 3 to 4 times higher than in the general population, and more than one-third of patients have been in an accident due to sleepiness.18 There is some evidence to show that treatment eliminates this risk.19
Few systematic studies have examined mood disorders in narcolepsy. However, studies tend to show a higher prevalence of psychiatric disorders than in the general population, with depression and anxiety the most com-mon.20,21
DIAGNOSIS IS OFTEN DELAYED
The prevalence of narcolepsy type 1 is between 25 and 100 per 100,000 people.22 In a Mayo Clinic study,23 the incidence of narcolepsy type 1 was estimated to be 0.74 per 100,000 person-years. Epidemiologic data on narcolepsy type 2 are sparse, but patients with narcolepsy without cataplexy are thought to represent only 36% of all narcolepsy patients.23
Diagnosis is often delayed, with the average time between the onset of symptoms and the diagnosis ranging from 8 to 22 years. With increasing awareness, the efficiency of the diagnostic process is improving, and this delay is expected to lessen accordingly.24
Symptoms most commonly arise in the second decade; but the age at onset ranges significantly, between the first and fifth decades. Narcolepsy has a bimodal distribution in incidence, with the biggest peak at approximately age 15 and second smaller peak in the mid-30s. Some studies have suggested a slight male predominance.23,25
DIAGNOSIS
History is key
The history should include specific questions about the hallmark features of narcolepsy, including cataplexy, sleep paralysis, and sleep-related hallucinations. For individual assessment of subjective sleepiness, the Epworth Sleepiness Scale or Pediatric Daytime Sleepiness Scale can be administered quickly in the office setting.26,27
The Epworth score is calculated from the self-rated likelihood of falling asleep in 8 different situations, with possible scores of 0 (would never doze) to 3 (high chance of dozing) on each question, for a total possible score of 0 to 24. Normal total scores are between 0 and 10, while scores greater than 10 reflect pathologic sleepiness. Scores on the Epworth Sleepiness Scale in those with narcolepsy tend to reflect moderate to severe sleepiness, or at least 13, as opposed to patients with obstructive sleep apnea, whose scores commonly reflect milder sleepiness.28
Testing with actigraphy and polysomnography
It is imperative to rule out insufficient sleep and other sleep disorders as a cause of daytime sleepiness. This can be done with a careful clinical history, actigraphy with sleep logs, and polysomnography.
In the 2 to 4 weeks before actigraphy and subsequent testing, all medications with alerting or sedating properties (including antidepressants) should be tapered off to prevent influence on the results of the study.
Delayed sleep-phase disorder presents at a similar age as narcolepsy and can be associated with similar degrees of sleepiness. However, individuals with delayed sleep phase disorder have an inappropriately timed sleep-wake cycle so that there is a shift in their desired sleep onset and awakening times. It is common—prevalence estimates vary but average about 1% in the general population.29
Insufficient sleep syndrome is even more common, especially in teenagers and young adults, with increasing family, social, and academic demands. Sleep needs vary across the life span. A teenager needs 8 to 10 hours of sleep per night, and a young adult needs 7 to 9 hours. A study of 1,285 high school students found that 10.4% were not getting enough sleep.30
If actigraphy data suggest a circadian rhythm disorder or insufficient sleep that could explain the symptoms of sleepiness, then further testing should be halted and these specific issues should be addressed. In these cases, working with the patient toward maintaining a regular sleep-wake schedule with 7 to 8 hours of nightly sleep will often resolve symptoms.
If actigraphy demonstrates the patient is maintaining a regular sleep schedule and allowing adequate time for nightly sleep, the next step is polysomnography.
Polysomnography is performed to detect other disorders that can disrupt sleep, such as sleep-disordered breathing or periodic limb movement disorder.2,5 In addition, polysomnography can provide assurance that adequate sleep was obtained prior to the next step in testing.
Multiple sleep latency test
If sufficient sleep is obtained on polysomnograpy (at least 6 hours for an adult) and no other sleep disorder is identified, a multiple sleep latency test is performed. A urine toxicology screen is typically performed on the day of the test to ensure that drugs are not affecting the results.
The multiple sleep latency test consists of 4 to 5 nap opportunities at 2-hour intervals in a quiet dark room conducive to sleep, during which both sleep and REM latency are recorded. The sleep latency of those with narcolepsy is significantly shortened, and the diagnosis of narcolepsy requires an average sleep latency of less than 8 minutes.
Given the propensity for REM sleep in narcolepsy, another essential feature for diagnosis is the sleep-onset REM period (SOREMP). A SOREMP is defined as a REM latency of less than 15 minutes. A diagnosis of narcolepsy re-quires a SOREMP in at least 2 of the naps in a multiple sleep latency test (or 1 nap if the shortened REM latency is seen during polysomnography).31
The multiple sleep latency test has an imperfect sensitivity, though, and should be repeated when there is a high suspicion of narcolepsy.32–34 It is not completely specific either, and false-positive results occur. In fact, SOREMPs can be seen in the general population, particularly in those with a circadian rhythm disorder, insufficient sleep, or sleep-disordered breathing. Two or more SOREMPs in an multiple sleep latency test can be seen in a small proportion of the general population.35 The results of a multiple sleep latency test should be interpreted in the clinical context.
Differential diagnosis
Narcolepsy type 1 is distinguished from type 2 by the presence of cataplexy. A cerebrospinal fluid hypocretin 1 level of 110 pg/mL or less, or less than one-third of the mean value obtained in normal individuals, can substitute for the multiple sleep latency test in diagnosing narcolepsy type 1.31 Currently, hypocretin testing is generally not performed in clinical practice, although it may become a routine part of the narcolepsy evaluation in the future.
Thus, according to the International Classification of Sleep Disorders, 3rd edition,31 the diagnosis of narcolepsy type 1 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder, and at least 1 of the following:
- Cataplexy and mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing (1 of which can be on the preceding night’s polysomography)
- Cerebrospinal fluid hypocretin 1 levels less than 110 pg/mL or one-third the baseline normal levels and mean sleep latency ≤ 8 minutes with ≥ 2 SOREMPs on multiple sleep latency testing.
Similarly, the diagnosis of narcolepsy type 2 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder, plus:
- Mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing.
Idiopathic hypersomnia, another disorder of central hypersomnolence, is also characterized by disabling sleepiness. It is diagnostically differentiated from narcolepsy, as there are fewer than 2 SOREMPs. As opposed to narcolepsy, in which naps tend to be refreshing, even prolonged naps in idiopathic hypersomnia are often not helpful in restoring wakefulness. In idiopathic hypersomnia, sleep is usually not fragmented, and there are few nocturnal arousals. Sleep times can often be prolonged as well, whereas in narcolepsy total sleep time through the day may not be increased but is not consolidated.
Kleine-Levin syndrome is a rarer disorder of hypersomnia. It is episodic compared with the relatively persistent sleepiness in narcolepsy and idiopathic hypersomnia. Periods of hypersomnia occur intermittently for days to weeks and are accompanied by cognitive and behavioral changes including hyperphagia and hypersexuality.4
LINKED TO HYPOCRETIN DEFICIENCY
Over the past 2 decades, the underlying pathophysiology of narcolepsy type 1 has been better characterized.
Narcolepsy type 1 has been linked to a deficiency in hypocretin in the central nervous system.36 Hypocretin (also known as orexin) is a hormone produced in the hypothalamus that acts on multiple brain regions and maintains alertness. For unclear reasons, hypothalamic neurons producing hypocretin are selectively reduced in narcolepsy type 1. Hypocretin also stabilizes wakefulness and inhibits REM sleep; therefore, hypocretin deficiency can lead to inappropriate intrusions of REM sleep onto wakefulness, leading to the hallmark features of narcolepsy—cataplexy, sleep-related hallucinations, and sleep paralysis.37 According to one theory, cataplexy is triggered by emotional stimuli because of a pathway between the medial prefrontal cortex and the amygdala to the pons.38
Cerebrospinal fluid levels of hypocretin in patients with narcolepsy type 2 tend to be normal, and the biologic underpinnings of narcolepsy type 2 remain mysterious. However, in the subgroup of those with narcolepsy type 2 in which hypocretin is low, many individuals go on to develop cataplexy, thereby evolving to narcolepsy type 1.36
POSSIBLE AUTOIMMUNE BASIS
Narcolepsy is typically a sporadic disorder, although familial cases have been described. The risk of a parent with narcolepsy having a child who is affected is approximately 1%.5
Narcolepsy type 1 is strongly associated with HLA-DQB1*0602, with up to 95% of those affected having at least one allele.39 Having 2 copies of the allele further increases the risk of developing narcolepsy.40 However, this allele is far from specific for narcolepsy with cataplexy, as it occurs in 12% to 38% of the general population.41 Therefore, HLA typing currently has limited clinical utility. The exact cause is as yet unknown, but substantial literature proposes an autoimmune basis of the disorder, given the strong association with the HLA subtype.42–44
After the 2009 H1N1 influenza pandemic, there was a significant increase in the incidence of narcolepsy with cataplexy, which again sparked interest in an autoimmune etiology underlying the disorder. Pandemrix, an H1N1 vaccine produced as a result of the 2009 pandemic, appeared to also be associated with an increase in the incidence of narcolepsy. An association with other upper respiratory infections has also been noted, further supporting a possible autoimmune basis.
A few studies have looked for serum autoantibodies involved in the pathogenesis of narcolepsy. Thus far, only one has been identified, an antibody to Tribbles homolog 2, found in 20% to 40% of those with new onset of nar-colepsy.42–44
TREATMENTS FOR DAYTIME SLEEPINESS
As with many chronic disorders, the treatment of narcolepsy consists of symptomatic rather than curative management, which can be done through both pharmacologic and nonpharmacologic means.
Nondrug measures
Scheduled naps lasting 15 to 20 minutes can help improve alertness.45 A consistent sleep schedule with good sleep hygiene, ensuring sufficient nightly sleep, is also important. In one study, the combination of scheduled naps and regular nocturnal sleep times reduced the level of daytime sleepiness and unintentional daytime sleep. Daytime naps were most helpful for those with the highest degree of daytime sleepiness.45
Strategic use of caffeine can be helpful and can reduce dependence on pharmacologic treatment.
Screening should be performed routinely for other sleep disorders, such as sleep-disordered breathing, which should be treated if identified.5,18 When being treated for other medical conditions, individuals with narcolepsy should avoid medications that can cause sedation, such as opiates or barbiturates; alcohol should be minimized or avoided.
Networking with other individuals with narcolepsy through support groups such as Narcolepsy Network can be valuable for learning coping skills and connecting with community resources. Psychological counseling for the patient, and sometimes the family, can also be useful. School-age children may need special accommodations such as schedule adjustments to allow for scheduled naps or frequent breaks to maintain alertness.
People with narcolepsy tend to function better in careers that do not require long periods of sitting, as sleepiness tends to be worse, but instead offer flexibility and require higher levels of activity that tend to combat sleepiness. They should not work as commercial drivers.18
Medications
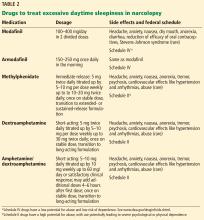
While behavioral interventions in narcolepsy are vital, they are rarely sufficient, and drugs that promote daytime wakefulness are used as an adjunct (Table 2).46
Realistic expectations should be established when starting, as some degree of residual sleepiness usually remains even with optimal medical therapy. Medications should be strategically scheduled to maximize alertness during necessary times such as at work or school or during driving. Patients should specifically be counseled to avoid driving if sleepy.18,47
Modafinil is often used as a first-line therapy, given its favorable side-effect profile and low potential for abuse. Its pharmacologic action has been debated but it probably acts as a selective dopamine reuptake inhibitor. It is typically taken twice daily (upon waking and early afternoon) and is usually well tolerated.
Potential side effects include headache, nausea, dry mouth, anorexia, diarrhea, and, rarely, Stevens-Johnson syndrome. Cardiovascular side effects are minimal, making it a favorable choice in older patients.18,48
A trial in 283 patients showed significantly lower levels of sleepiness in patients taking modafinil 200 mg or 400 mg than in a control group. Other trials have supported these findings and showed improved driving performance on modafinil.18
Notably, modafinil can increase the metabolism of oral contraceptives, thereby reducing their efficacy. Women of childbearing age should be warned about this interaction and should be transitioned to nonhormonal forms of contraception.2,47
Armodafinil, a purified R-isomer of modafinil, has a longer half-life and requires only once-daily dosing.5
If modafinil or armodafinil fails to optimally manage daytime sleepiness, a traditional stimulant such as methylphenidate or an amphetamine is often used.
Methylphenidate and amphetamines primarily inhibit the reuptake and increase the release of the monoamines, mainly dopamine, and to a lesser degree serotonin and norepinephrine.
These drugs have more significant adverse effects that can involve the cardiovascular system, causing hypertension and arrhythmias. Anorexia, weight loss, and, particularly with high doses, psychosis can occur.49
These drugs should be avoided in patients with a history of significant cardiovascular disease. Before starting stimulant therapy, a thorough cardiovascular examination should be done, often including electrocardiography to ensure there is no baseline arrhythmia.
Patients on these medications should be followed closely to ensure that blood pressure, pulse, and weight are not negatively affected.18,50 Addiction and tolerance can develop with these drugs, and follow-up should include assessment for dependence. Some states may require prescription drug monitoring to ensure the drugs are not being abused or diverted.
Short- and long-acting formulations of both methylphenidate and amphetamines are available, and a long-acting form is often used in conjunction with a short-acting form as needed.18
Addiction and drug-seeking behavior can develop but are unusual in those taking stimulants to treat narcolepsy.49
Follow-up
Residual daytime sleepiness can be measured subjectively through the Epworth Sleepiness Scale on follow-up. If necessary, a maintenance-of-wakefulness test can provide an objective assessment of treatment efficacy.18
As narcolepsy is a chronic disorder, treatment should evolve with time. Most medications that treat narcolepsy are categorized by the US Food and Drug Administration as pregnancy category C, as we do not have adequate studies in human pregnancies to evaluate their effects. When a patient with narcolepsy becomes pregnant, she should be counseled about the risks and benefits of remaining on therapy. Treatment should balance the risks of sleepiness with the potential risks of remaining on medications.50 In the elderly, as cardiovascular comorbidities tend to increase, the risks and benefits of therapy should be routinely reevaluated.
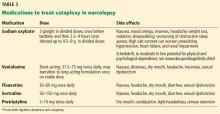
For cataplexy
Sodium oxybate,51–53 the most potent anticataplectic drug, is the sodium salt of gamma hydroxybutyrate, a metabolite of gamma-aminobutyric acid. Sodium oxybate can be prescribed in the United States, Canada, and Europe. The American Academy of Sleep Medicine recommends sodium oxybate for cataplexy, daytime sleepiness, and disrupted sleep based on 3 level-1 studies and 2 level-4 studies.46
Sodium oxybate increases slow-wave sleep, improves sleep continuity, and often helps to mitigate daytime sleepiness. Due to its short half-life, its administration is unusual: the first dose is taken before bedtime and the second dose 2.5 to 4 hours later. Some patients set an alarm clock to take the second dose, while others awaken spontaneously to take the second dose. Most patients find that with adherence to dosing and safety instructions, sodium oxybate can serve as a highly effective form of treatment of both excessive sleepiness and cataplexy and may reduce the need for stimulant-based therapies.
The most common adverse effects are nausea, mood swings, and enuresis. Occasionally, psychosis can result and limit use of the drug. Obstructive sleep apnea can also develop or worsen.52 Because of its high salt content, sodium oxybate should be used with caution in those with heart failure, hypertension, or renal impairment. Its relative, gamma hydroxybutyrate, causes rapid sedation and has been notorious for illegal use as a date rape drug.
In the United States, sodium oxybate is distributed only through a central pharmacy to mitigate potential abuse. Due to this system, the rates of diversion are extremely low, estimated in a postmarketing analysis to be 1 instance per 5,200 patients treated. In the same study, abuse and dependence were both rare as well, about 1 case for every 2,600 and 6,500 patients treated.6,18,52,53
Antidepressants promote the action of norepinephrine and, to a lesser degree, serotonin, thereby suppressing REM sleep.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is often used as a first-line treatment for cataplexy. Selective serotonin reuptake inhibitors such as fluoxetine are also used with success. Tricyclic antidepressants such as protriptyline or clomipramine are extremely effective for cataplexy, but are rarely used due to their adverse effects.2,47
FUTURE WORK
While our understanding of narcolepsy has advanced, there are still gaps in our knowledge of the disorder—namely, the specific trigger for the loss of hypocretin neurons in type 1 narcolepsy and the underlying pathophysiology of type 2.
A number of emerging therapies target the hypocretin system, including peptide replacement, neuronal transplant, and immunotherapy preventing hypocretin neuronal cell death.50,54,55 Additional drugs designed to improve alertness that do not involve the hypocretin system are also being developed, including a histamine inverse agonist.50,56 Sodium oxybate and modafinil, although currently approved for use in adults, are still off-label in pediatric practice. Studies of the safety and efficacy of these medications in children are needed.7,57
- Gélineau J. De la narcolepsie. Gazette des Hôpitaux Civils et Militaires 1880; part a, 53:626–628, part b, 54:635–637.
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet 2007; 369(9560):499–511. doi:10.1016/S0140-6736(07)60237-2
- Scammell TE. Clinical features and diagnosis of narcolepsy in adults. In: Eichler AF, ed. UpToDate. Waltham, MA: UpToDate; 2018. www.uptodate.com. Accessed October 31, 2018.
- Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med 2004; 5(1):37–41. pmid:14725825
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373(27):2654–2662. doi:10.1056/NEJMra1500587
- Babiker MO, Prasad M. Narcolepsy in children: a diagnostic and management approach. Pediatr Neurol 2015; 52(6):557–565. doi:10.1016/j.pediatrneurol.2015.02.020
- Kotagal S. Narcolepsy in children. In: UpToDate, Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol 2003; 53(2):154–166. doi:10.1002/ana.10444
- Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med 2011; 12(1):12–18. doi:10.1016/j.sleep.2010.05.010
- Plazzi G, Fabbri C, Pizza F, Serretti A. Schizophrenia-like symptoms in narcolepsy type 1: shared and distinctive clinical characteristics. Neuropsychobiology 2015; 71(4):218–224. doi:10.1159/000432400
- Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res 2000; 97(2-3):153–164. pmid:11166087
- Sharpless BA, Barber JP. Lifetime prevalence rates of sleep paralysis: a systematic review. Sleep Med Rev 2011;5(5):311–315. doi:10.1016/j.smrv.2011.01.007
- Broughton R, Dunham W, Newman J, Lutley K, Duschesne P, Rivers M. Ambulatory 24 hour sleep-wake monitoring in narcolepsy-cataplexy compared to matched controls. Electroencephalogr Clin Neurophysiol 1988; 70(6):473–481. pmid:2461281
- Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain 2013; 136(pt 12):3787–3795. doi:10.1093/brain/awt277
- Kotagal S, Krahn LE, Slocumb N. A putative link between childhood narcolepsy and obesity. Sleep Med 2004; 5(2):147–150. doi:10.1016/j.sleep.2003.10.006
- Pizza F, Tartarotti S, Poryazova R, Baumann CR, Bassetti CL. Sleep-disordered breathing and periodic limb movements in narcolepsy with cataplexy: a systematic analysis of 35 consecutive patients. Eur Neurol 2013; 70(1-2):22–26. doi:10.1159/000348719
- Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck narcolepsy cohort. J Clin Sleep Med 2013; 9(8):805–812. doi:10.5664/jcsm.2926
- Scammell TE. Treatment of narcolepsy in adults. In: Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Pizza F, Jaussent I, Lopez R, et al. Car crashes and central disorders of hypersomnolence: a French study. PLoS One 2015; 10(6):e0129386. doi:10.1371/journal.pone.0129386
- Fortuyn HD, Lappenschaar MA, Furer JW, et al. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry 2010; 32(1):49–56. doi:10.1016/j.genhosppsych.2009.08.007
- Ruoff CM, Reaven NL, Funk SE, et al. High rates of psychiatric comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study of 9,312 patients in the United States. J Clin Psychiatry 2017; 78(2):171–176. doi:10.4088/JCP.15m10262
- Longstreth WT Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007; 30(1):13–26. pmid:17310860
- Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep 2002; 25(2):197–202. pmid:11902429
- Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med 2014; 15(5):502–507. doi:10.1016/j.sleep.2014.01.015
- Dauvilliers Y, Montplaisir J, Molinari N, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001; 57(11):2029–2033. pmid:11739821
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14(6):540–545. pmid:1798888
- Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Badia P. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep 2003; 26(4):455–458. pmid:12841372
- van der Heide A, van Schie MK, Lammers GJ, et al. Comparing treatment effect measurements in narcolepsy: the sustained attention to response task, Epworth sleepiness scale and maintenance of wakefulness test. Sleep 2015; 38(7):1051–1058. doi:10.5665/sleep.4810
- Nesbitt AD. Delayed sleep-wake phase disorder. J Thorac Dis 2018; 10(suppl 1):S103–S111. doi:10.21037/jtd.2018.01.11
- Pallesen S, Saxvig IW, Molde H, Sørensen E, Wilhelmsen-Langeland A, Bjorvatn B. Brief report: behaviorally induced insufficient sleep syndrome in older adolescents: prevalence and correlates. J Adolesc 2011; 34(2):391–395. doi:10.1016/j.adolescence.2010.02.005
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Disorders; 2014.
- Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med 2013; 9(8):789–795. doi:10.5664/jcsm.2922
- Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol 2013; 70(7):891–902. doi:10.1001/jamaneurol.2013.1589
- Cairns A, Bogan R. Prevalence and clinical correlates of a short onset REM period (SOREMP) during routine PSG. Sleep 2015; 38(10):1575–1581. doi:10.5665/sleep.5050
- Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the multiple sleep latency test in community adults. Brain 2006; 129(6):1609–1623. doi:10.1093/brain/awl079
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000; 355(9197):39–40. doi:10.1016/S0140-6736(99)05582-8
- Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000; 6(9):991–997. doi:10.1038/79690
- Oishi Y, Williams RH, Agostinelli L, et al. Role of the medial prefrontal cortex in cataplexy. J Neurosci 2013; 33(23):9743–9751. doi:10.1523/JNEUROSCI.0499-13.2013
- Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients.. Sleep 1997; 20(11):1012–1020. pmid:9456467
- Pelin Z, Guilleminault C, Risch N, Grumet FC, Mignot E. HLA-DQB1*0602 homozygosity increases relative risk for narcolepsy but not disease severity in two ethnic groups. US Modafinil in Narcolepsy Multicenter Study Group. Tissue Antigens 1998; 51(1):96–100. pmid:9459509
- Akintomide GS, Rickards H. Narcolepsy: a review. Neuropsychiatr Dis Treat 2011; 7(1):507–518. doi:10.2147/NDT.S23624
- Mahlios J, De la Herrán-Arita AK, Mignot E. The autoimmune basis of narcolepsy. Curr Opin Neurobiol 2013; 23(5):767–773. doi:10.1016/j.conb.2013.04.013
- Degn M, Kornum BR. Type 1 narcolepsy: a CD8(+) T cell-mediated disease? Ann N Y Acad Sci 2015;1 351:80–88. doi:10.1111/nyas.12793
- Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol 2015; 14(3):318–328. doi:10.1016/S1474-4422(14)70218-2
- Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep 2001; 24(4):385–391. pmid:11403522
- Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007; 30(12):1705–1711. pmid:18246980
- Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics 2012; 9(4):739–752. doi:10.1007/s13311-012-0150-9
- Roth T, Schwartz JR, Hirshkowitz M, Erman MK, Dayno JM, Arora S. Evaluation of the safety of modafinil for treatment of excessive sleepiness. J Clin Sleep Med 2007; 3(6):595–602. pmid:17993041
- Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL. Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study. Sleep 2005; 28(6):667–672. pmid:16477952
- Abad VC, Guilleminault C. New developments in the management of narcolepsy. Nat Sci Sleep 2017; 9:39–57. doi:10.2147/NSS.S103467
- Drakatos P, Lykouras D, D’Ancona G, et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med 2017; 35:80–84. doi:10.1016/j.sleep.2017.03.028
- Mansukhani MP, Kotagal S. Sodium oxybate in the treatment of childhood narcolepsy–cataplexy: a retrospective study. Sleep Med 2012; 13(6):606–610. doi:10.1016/j.sleep.2011.10.032
- Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J Clin Sleep Med 2009; 5(4):365–371. pmid:19968016
- Weinhold SL, Seeck-Hirschner M, Nowak A, Hallschmid M, Göder R, Baier PC. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav Brain Res 2014; 262:8–13. doi:10.1016/j.bbr.2013.12.045
- Arias-Carrión O, Murillo-Rodriguez E. Effects of hypocretin/orexin cell transplantation on narcoleptic-like sleep behavior in rats. PLoS One 2014; 9(4):e95342. doi:10.1371/journal.pone.0095342
- Leu-Semenescu S, Nittur N, Golmard JL, Arnulf I. Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review. Sleep Med 2014; 15(6):681–687. doi:10.1016/j.sleep.2014.01.021
- Lecendreux M, Bruni O, Franco P, et al. Clinical experience suggests that modafinil is an effective and safe treatment for paediatric narcolepsy. J Sleep Res 2012; 21(4):481–483. doi:10.1111/j.1365-2869.2011.00991.x
Narcolepsy was originally described in the late 1800s by the French physician Jean-Baptiste-Edouard Gélineau, who reported the case of a wine merchant suffering from somnolence. In this first description, he coined the term narcolepsie by joining the Greek words narke (numbness or stupor) and lepsis (attack).1
Since then, the disorder has been further characterized, and some insight into its biological underpinnings has been established. Importantly, treatments have improved and expanded, facilitating its management and thereby improving quality of life for those with the disorder.
This review focuses on clinically relevant features of the disorder and proposes management strategies.
CLINICAL FEATURES
Narcolepsy is characterized by instability of sleep-wake transitions.
Daytime sleepiness
Clinically, narcolepsy manifests with excessive daytime sleepiness that can be personally and socially disabling. Cataplexy, sleep paralysis, and hypnagogic or hypnopompic hallucinations can also be present,2,3 but they are not necessary for diagnosis. In fact, a minority of patients with narcolepsy have all these symptoms.4 Narcolepsy is divided into type 1 (with cataplexy) and type 2 (without cataplexy).2
Sleepiness tends to be worse with inactivity, and sleep can often be irresistible. Sleep attacks can come on suddenly and may be brief enough to manifest as a lapse in consciousness.
Short naps tend to be refreshing. Rapid eye movement (REM) latency—the interval between falling asleep and the onset of the REM sleep—is short in narcolepsy, and since the REM stage is when dreaming occurs, naps often include dreaming. Therefore, when taking a history, it is worthwhile to ask patients whether they dream during naps; a yes answer supports the diagnosis of narcolepsy.5
In children, sleepiness can manifest in reduced concentration and behavioral issues.6 Napping after age 5 or 6 is considered abnormal and may reflect pathologic sleepiness.7
Cataplexy
Cataplexy—transient muscle weakness triggered by emotion—is a specific feature of narcolepsy type 1. It often begins in the facial muscles and can manifest with slackening of the jaw or brief dropping of the head. However, episodes can be more dramatic and, if the trunk and limb muscles are affected, can result in collapsing to the ground.
Cataplexy usually has its onset at about the same time as the sleepiness associated with narcolepsy, but it can arise even years later.8 Episodes can last from a few seconds to 2 minutes. Consciousness is always preserved. A range of emotions can trigger cataplexy, but typically the emotion is a positive one such as laughter or excitement.9 Deep tendon reflexes disappear in cataplexy, so checking reflexes during a witnessed episode can be clinically valuable.2
Cataplexy can worsen with stress and insufficient sleep, occasionally with “status cataplecticus,” in which repeated, persistent episodes of cataplexy occur over several hours.8 Status cataplecticus can be spontaneous or an effect of withdrawal from anticataplectic medications.2
Cataplexy is thought to represent intrusion of REM sleep and its associated muscle atonia during wakefulness.
Sleep paralysis, hallucinations
Sleep paralysis and hallucinations are other features of narcolepsy that reflect this REM dissociation from sleep.
Sleep paralysis occurs most commonly upon awakening, but sometimes just before sleep onset. In most cases, it is manifested by inability to move the limbs or speak, lasting several seconds or, in rare cases, minutes at a time. Sleep paralysis can be associated with a sensation of fear or suffocation, especially when initially experienced.8
Hypnopompic hallucinations, occurring upon awakening, are more common than hypnagogic hallucinations, which are experienced before falling asleep. The hallucinations are often vivid and usually visual, although other types of hallucinations are possible. Unlike those that occur in psychotic disorders, the hallucinations tend to be associated with preserved insight that they are not real.10
Notably, both sleep paralysis and hallucinations are nonspecific symptoms that are common in the general population.8,11,12
Fragmented sleep
Although they are very sleepy, people with narcolepsy generally cannot stay asleep for very long. Their sleep tends to be extremely fragmented, and they often wake up several times a night.2
This sleep pattern reflects the inherent instability of sleep-wake transitions in narcolepsy. In fact, over a 24-hour period, adults with narcolepsy have a normal amount of sleep.13 In children, however, when narcolepsy first arises, the 24-hour sleep time can increase abruptly and can sometimes be associated with persistent cataplexy that can manifest as a clumsy gait.14
Weight gain, obstructive sleep apnea
Weight gain is common, particularly after symptom onset, and especially in children. As a result, obesity is a frequent comorbidity.15 Because obstructive sleep apnea can consequently develop, all patients with narcolepsy require screening for sleep-disordered breathing.
Other sleep disorders often accompany narcolepsy and are more common than in the general population.16 In a study incorporating both clinical and polysomnographic data of 100 patients with narcolepsy, insomnia was the most common comorbid sleep disorder, with a prevalence of 28%; others were REM sleep behavior disorder (24%), restless legs syndrome (24%), obstructive sleep apnea (21%), and non-REM parasomnias.17
PSYCHOSOCIAL CONSEQUENCES
Narcolepsy has significant psychosocial consequences. As a result of their symptoms, people with narcolepsy may not be able to meet academic or work-related demands.
Additionally, their risk of a motor vehicle accident is 3 to 4 times higher than in the general population, and more than one-third of patients have been in an accident due to sleepiness.18 There is some evidence to show that treatment eliminates this risk.19
Few systematic studies have examined mood disorders in narcolepsy. However, studies tend to show a higher prevalence of psychiatric disorders than in the general population, with depression and anxiety the most com-mon.20,21
DIAGNOSIS IS OFTEN DELAYED
The prevalence of narcolepsy type 1 is between 25 and 100 per 100,000 people.22 In a Mayo Clinic study,23 the incidence of narcolepsy type 1 was estimated to be 0.74 per 100,000 person-years. Epidemiologic data on narcolepsy type 2 are sparse, but patients with narcolepsy without cataplexy are thought to represent only 36% of all narcolepsy patients.23
Diagnosis is often delayed, with the average time between the onset of symptoms and the diagnosis ranging from 8 to 22 years. With increasing awareness, the efficiency of the diagnostic process is improving, and this delay is expected to lessen accordingly.24
Symptoms most commonly arise in the second decade; but the age at onset ranges significantly, between the first and fifth decades. Narcolepsy has a bimodal distribution in incidence, with the biggest peak at approximately age 15 and second smaller peak in the mid-30s. Some studies have suggested a slight male predominance.23,25
DIAGNOSIS
History is key
The history should include specific questions about the hallmark features of narcolepsy, including cataplexy, sleep paralysis, and sleep-related hallucinations. For individual assessment of subjective sleepiness, the Epworth Sleepiness Scale or Pediatric Daytime Sleepiness Scale can be administered quickly in the office setting.26,27
The Epworth score is calculated from the self-rated likelihood of falling asleep in 8 different situations, with possible scores of 0 (would never doze) to 3 (high chance of dozing) on each question, for a total possible score of 0 to 24. Normal total scores are between 0 and 10, while scores greater than 10 reflect pathologic sleepiness. Scores on the Epworth Sleepiness Scale in those with narcolepsy tend to reflect moderate to severe sleepiness, or at least 13, as opposed to patients with obstructive sleep apnea, whose scores commonly reflect milder sleepiness.28
Testing with actigraphy and polysomnography
It is imperative to rule out insufficient sleep and other sleep disorders as a cause of daytime sleepiness. This can be done with a careful clinical history, actigraphy with sleep logs, and polysomnography.
In the 2 to 4 weeks before actigraphy and subsequent testing, all medications with alerting or sedating properties (including antidepressants) should be tapered off to prevent influence on the results of the study.
Delayed sleep-phase disorder presents at a similar age as narcolepsy and can be associated with similar degrees of sleepiness. However, individuals with delayed sleep phase disorder have an inappropriately timed sleep-wake cycle so that there is a shift in their desired sleep onset and awakening times. It is common—prevalence estimates vary but average about 1% in the general population.29
Insufficient sleep syndrome is even more common, especially in teenagers and young adults, with increasing family, social, and academic demands. Sleep needs vary across the life span. A teenager needs 8 to 10 hours of sleep per night, and a young adult needs 7 to 9 hours. A study of 1,285 high school students found that 10.4% were not getting enough sleep.30
If actigraphy data suggest a circadian rhythm disorder or insufficient sleep that could explain the symptoms of sleepiness, then further testing should be halted and these specific issues should be addressed. In these cases, working with the patient toward maintaining a regular sleep-wake schedule with 7 to 8 hours of nightly sleep will often resolve symptoms.
If actigraphy demonstrates the patient is maintaining a regular sleep schedule and allowing adequate time for nightly sleep, the next step is polysomnography.
Polysomnography is performed to detect other disorders that can disrupt sleep, such as sleep-disordered breathing or periodic limb movement disorder.2,5 In addition, polysomnography can provide assurance that adequate sleep was obtained prior to the next step in testing.
Multiple sleep latency test
If sufficient sleep is obtained on polysomnograpy (at least 6 hours for an adult) and no other sleep disorder is identified, a multiple sleep latency test is performed. A urine toxicology screen is typically performed on the day of the test to ensure that drugs are not affecting the results.
The multiple sleep latency test consists of 4 to 5 nap opportunities at 2-hour intervals in a quiet dark room conducive to sleep, during which both sleep and REM latency are recorded. The sleep latency of those with narcolepsy is significantly shortened, and the diagnosis of narcolepsy requires an average sleep latency of less than 8 minutes.
Given the propensity for REM sleep in narcolepsy, another essential feature for diagnosis is the sleep-onset REM period (SOREMP). A SOREMP is defined as a REM latency of less than 15 minutes. A diagnosis of narcolepsy re-quires a SOREMP in at least 2 of the naps in a multiple sleep latency test (or 1 nap if the shortened REM latency is seen during polysomnography).31
The multiple sleep latency test has an imperfect sensitivity, though, and should be repeated when there is a high suspicion of narcolepsy.32–34 It is not completely specific either, and false-positive results occur. In fact, SOREMPs can be seen in the general population, particularly in those with a circadian rhythm disorder, insufficient sleep, or sleep-disordered breathing. Two or more SOREMPs in an multiple sleep latency test can be seen in a small proportion of the general population.35 The results of a multiple sleep latency test should be interpreted in the clinical context.
Differential diagnosis
Narcolepsy type 1 is distinguished from type 2 by the presence of cataplexy. A cerebrospinal fluid hypocretin 1 level of 110 pg/mL or less, or less than one-third of the mean value obtained in normal individuals, can substitute for the multiple sleep latency test in diagnosing narcolepsy type 1.31 Currently, hypocretin testing is generally not performed in clinical practice, although it may become a routine part of the narcolepsy evaluation in the future.
Thus, according to the International Classification of Sleep Disorders, 3rd edition,31 the diagnosis of narcolepsy type 1 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder, and at least 1 of the following:
- Cataplexy and mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing (1 of which can be on the preceding night’s polysomography)
- Cerebrospinal fluid hypocretin 1 levels less than 110 pg/mL or one-third the baseline normal levels and mean sleep latency ≤ 8 minutes with ≥ 2 SOREMPs on multiple sleep latency testing.
Similarly, the diagnosis of narcolepsy type 2 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder, plus:
- Mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing.
Idiopathic hypersomnia, another disorder of central hypersomnolence, is also characterized by disabling sleepiness. It is diagnostically differentiated from narcolepsy, as there are fewer than 2 SOREMPs. As opposed to narcolepsy, in which naps tend to be refreshing, even prolonged naps in idiopathic hypersomnia are often not helpful in restoring wakefulness. In idiopathic hypersomnia, sleep is usually not fragmented, and there are few nocturnal arousals. Sleep times can often be prolonged as well, whereas in narcolepsy total sleep time through the day may not be increased but is not consolidated.
Kleine-Levin syndrome is a rarer disorder of hypersomnia. It is episodic compared with the relatively persistent sleepiness in narcolepsy and idiopathic hypersomnia. Periods of hypersomnia occur intermittently for days to weeks and are accompanied by cognitive and behavioral changes including hyperphagia and hypersexuality.4
LINKED TO HYPOCRETIN DEFICIENCY
Over the past 2 decades, the underlying pathophysiology of narcolepsy type 1 has been better characterized.
Narcolepsy type 1 has been linked to a deficiency in hypocretin in the central nervous system.36 Hypocretin (also known as orexin) is a hormone produced in the hypothalamus that acts on multiple brain regions and maintains alertness. For unclear reasons, hypothalamic neurons producing hypocretin are selectively reduced in narcolepsy type 1. Hypocretin also stabilizes wakefulness and inhibits REM sleep; therefore, hypocretin deficiency can lead to inappropriate intrusions of REM sleep onto wakefulness, leading to the hallmark features of narcolepsy—cataplexy, sleep-related hallucinations, and sleep paralysis.37 According to one theory, cataplexy is triggered by emotional stimuli because of a pathway between the medial prefrontal cortex and the amygdala to the pons.38
Cerebrospinal fluid levels of hypocretin in patients with narcolepsy type 2 tend to be normal, and the biologic underpinnings of narcolepsy type 2 remain mysterious. However, in the subgroup of those with narcolepsy type 2 in which hypocretin is low, many individuals go on to develop cataplexy, thereby evolving to narcolepsy type 1.36
POSSIBLE AUTOIMMUNE BASIS
Narcolepsy is typically a sporadic disorder, although familial cases have been described. The risk of a parent with narcolepsy having a child who is affected is approximately 1%.5
Narcolepsy type 1 is strongly associated with HLA-DQB1*0602, with up to 95% of those affected having at least one allele.39 Having 2 copies of the allele further increases the risk of developing narcolepsy.40 However, this allele is far from specific for narcolepsy with cataplexy, as it occurs in 12% to 38% of the general population.41 Therefore, HLA typing currently has limited clinical utility. The exact cause is as yet unknown, but substantial literature proposes an autoimmune basis of the disorder, given the strong association with the HLA subtype.42–44
After the 2009 H1N1 influenza pandemic, there was a significant increase in the incidence of narcolepsy with cataplexy, which again sparked interest in an autoimmune etiology underlying the disorder. Pandemrix, an H1N1 vaccine produced as a result of the 2009 pandemic, appeared to also be associated with an increase in the incidence of narcolepsy. An association with other upper respiratory infections has also been noted, further supporting a possible autoimmune basis.
A few studies have looked for serum autoantibodies involved in the pathogenesis of narcolepsy. Thus far, only one has been identified, an antibody to Tribbles homolog 2, found in 20% to 40% of those with new onset of nar-colepsy.42–44
TREATMENTS FOR DAYTIME SLEEPINESS
As with many chronic disorders, the treatment of narcolepsy consists of symptomatic rather than curative management, which can be done through both pharmacologic and nonpharmacologic means.
Nondrug measures
Scheduled naps lasting 15 to 20 minutes can help improve alertness.45 A consistent sleep schedule with good sleep hygiene, ensuring sufficient nightly sleep, is also important. In one study, the combination of scheduled naps and regular nocturnal sleep times reduced the level of daytime sleepiness and unintentional daytime sleep. Daytime naps were most helpful for those with the highest degree of daytime sleepiness.45
Strategic use of caffeine can be helpful and can reduce dependence on pharmacologic treatment.
Screening should be performed routinely for other sleep disorders, such as sleep-disordered breathing, which should be treated if identified.5,18 When being treated for other medical conditions, individuals with narcolepsy should avoid medications that can cause sedation, such as opiates or barbiturates; alcohol should be minimized or avoided.
Networking with other individuals with narcolepsy through support groups such as Narcolepsy Network can be valuable for learning coping skills and connecting with community resources. Psychological counseling for the patient, and sometimes the family, can also be useful. School-age children may need special accommodations such as schedule adjustments to allow for scheduled naps or frequent breaks to maintain alertness.
People with narcolepsy tend to function better in careers that do not require long periods of sitting, as sleepiness tends to be worse, but instead offer flexibility and require higher levels of activity that tend to combat sleepiness. They should not work as commercial drivers.18
Medications
While behavioral interventions in narcolepsy are vital, they are rarely sufficient, and drugs that promote daytime wakefulness are used as an adjunct (Table 2).46
Realistic expectations should be established when starting, as some degree of residual sleepiness usually remains even with optimal medical therapy. Medications should be strategically scheduled to maximize alertness during necessary times such as at work or school or during driving. Patients should specifically be counseled to avoid driving if sleepy.18,47
Modafinil is often used as a first-line therapy, given its favorable side-effect profile and low potential for abuse. Its pharmacologic action has been debated but it probably acts as a selective dopamine reuptake inhibitor. It is typically taken twice daily (upon waking and early afternoon) and is usually well tolerated.
Potential side effects include headache, nausea, dry mouth, anorexia, diarrhea, and, rarely, Stevens-Johnson syndrome. Cardiovascular side effects are minimal, making it a favorable choice in older patients.18,48
A trial in 283 patients showed significantly lower levels of sleepiness in patients taking modafinil 200 mg or 400 mg than in a control group. Other trials have supported these findings and showed improved driving performance on modafinil.18
Notably, modafinil can increase the metabolism of oral contraceptives, thereby reducing their efficacy. Women of childbearing age should be warned about this interaction and should be transitioned to nonhormonal forms of contraception.2,47
Armodafinil, a purified R-isomer of modafinil, has a longer half-life and requires only once-daily dosing.5
If modafinil or armodafinil fails to optimally manage daytime sleepiness, a traditional stimulant such as methylphenidate or an amphetamine is often used.
Methylphenidate and amphetamines primarily inhibit the reuptake and increase the release of the monoamines, mainly dopamine, and to a lesser degree serotonin and norepinephrine.
These drugs have more significant adverse effects that can involve the cardiovascular system, causing hypertension and arrhythmias. Anorexia, weight loss, and, particularly with high doses, psychosis can occur.49
These drugs should be avoided in patients with a history of significant cardiovascular disease. Before starting stimulant therapy, a thorough cardiovascular examination should be done, often including electrocardiography to ensure there is no baseline arrhythmia.
Patients on these medications should be followed closely to ensure that blood pressure, pulse, and weight are not negatively affected.18,50 Addiction and tolerance can develop with these drugs, and follow-up should include assessment for dependence. Some states may require prescription drug monitoring to ensure the drugs are not being abused or diverted.
Short- and long-acting formulations of both methylphenidate and amphetamines are available, and a long-acting form is often used in conjunction with a short-acting form as needed.18
Addiction and drug-seeking behavior can develop but are unusual in those taking stimulants to treat narcolepsy.49
Follow-up
Residual daytime sleepiness can be measured subjectively through the Epworth Sleepiness Scale on follow-up. If necessary, a maintenance-of-wakefulness test can provide an objective assessment of treatment efficacy.18
As narcolepsy is a chronic disorder, treatment should evolve with time. Most medications that treat narcolepsy are categorized by the US Food and Drug Administration as pregnancy category C, as we do not have adequate studies in human pregnancies to evaluate their effects. When a patient with narcolepsy becomes pregnant, she should be counseled about the risks and benefits of remaining on therapy. Treatment should balance the risks of sleepiness with the potential risks of remaining on medications.50 In the elderly, as cardiovascular comorbidities tend to increase, the risks and benefits of therapy should be routinely reevaluated.
For cataplexy
Sodium oxybate,51–53 the most potent anticataplectic drug, is the sodium salt of gamma hydroxybutyrate, a metabolite of gamma-aminobutyric acid. Sodium oxybate can be prescribed in the United States, Canada, and Europe. The American Academy of Sleep Medicine recommends sodium oxybate for cataplexy, daytime sleepiness, and disrupted sleep based on 3 level-1 studies and 2 level-4 studies.46
Sodium oxybate increases slow-wave sleep, improves sleep continuity, and often helps to mitigate daytime sleepiness. Due to its short half-life, its administration is unusual: the first dose is taken before bedtime and the second dose 2.5 to 4 hours later. Some patients set an alarm clock to take the second dose, while others awaken spontaneously to take the second dose. Most patients find that with adherence to dosing and safety instructions, sodium oxybate can serve as a highly effective form of treatment of both excessive sleepiness and cataplexy and may reduce the need for stimulant-based therapies.
The most common adverse effects are nausea, mood swings, and enuresis. Occasionally, psychosis can result and limit use of the drug. Obstructive sleep apnea can also develop or worsen.52 Because of its high salt content, sodium oxybate should be used with caution in those with heart failure, hypertension, or renal impairment. Its relative, gamma hydroxybutyrate, causes rapid sedation and has been notorious for illegal use as a date rape drug.
In the United States, sodium oxybate is distributed only through a central pharmacy to mitigate potential abuse. Due to this system, the rates of diversion are extremely low, estimated in a postmarketing analysis to be 1 instance per 5,200 patients treated. In the same study, abuse and dependence were both rare as well, about 1 case for every 2,600 and 6,500 patients treated.6,18,52,53
Antidepressants promote the action of norepinephrine and, to a lesser degree, serotonin, thereby suppressing REM sleep.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is often used as a first-line treatment for cataplexy. Selective serotonin reuptake inhibitors such as fluoxetine are also used with success. Tricyclic antidepressants such as protriptyline or clomipramine are extremely effective for cataplexy, but are rarely used due to their adverse effects.2,47
FUTURE WORK
While our understanding of narcolepsy has advanced, there are still gaps in our knowledge of the disorder—namely, the specific trigger for the loss of hypocretin neurons in type 1 narcolepsy and the underlying pathophysiology of type 2.
A number of emerging therapies target the hypocretin system, including peptide replacement, neuronal transplant, and immunotherapy preventing hypocretin neuronal cell death.50,54,55 Additional drugs designed to improve alertness that do not involve the hypocretin system are also being developed, including a histamine inverse agonist.50,56 Sodium oxybate and modafinil, although currently approved for use in adults, are still off-label in pediatric practice. Studies of the safety and efficacy of these medications in children are needed.7,57
Narcolepsy was originally described in the late 1800s by the French physician Jean-Baptiste-Edouard Gélineau, who reported the case of a wine merchant suffering from somnolence. In this first description, he coined the term narcolepsie by joining the Greek words narke (numbness or stupor) and lepsis (attack).1
Since then, the disorder has been further characterized, and some insight into its biological underpinnings has been established. Importantly, treatments have improved and expanded, facilitating its management and thereby improving quality of life for those with the disorder.
This review focuses on clinically relevant features of the disorder and proposes management strategies.
CLINICAL FEATURES
Narcolepsy is characterized by instability of sleep-wake transitions.
Daytime sleepiness
Clinically, narcolepsy manifests with excessive daytime sleepiness that can be personally and socially disabling. Cataplexy, sleep paralysis, and hypnagogic or hypnopompic hallucinations can also be present,2,3 but they are not necessary for diagnosis. In fact, a minority of patients with narcolepsy have all these symptoms.4 Narcolepsy is divided into type 1 (with cataplexy) and type 2 (without cataplexy).2
Sleepiness tends to be worse with inactivity, and sleep can often be irresistible. Sleep attacks can come on suddenly and may be brief enough to manifest as a lapse in consciousness.
Short naps tend to be refreshing. Rapid eye movement (REM) latency—the interval between falling asleep and the onset of the REM sleep—is short in narcolepsy, and since the REM stage is when dreaming occurs, naps often include dreaming. Therefore, when taking a history, it is worthwhile to ask patients whether they dream during naps; a yes answer supports the diagnosis of narcolepsy.5
In children, sleepiness can manifest in reduced concentration and behavioral issues.6 Napping after age 5 or 6 is considered abnormal and may reflect pathologic sleepiness.7
Cataplexy
Cataplexy—transient muscle weakness triggered by emotion—is a specific feature of narcolepsy type 1. It often begins in the facial muscles and can manifest with slackening of the jaw or brief dropping of the head. However, episodes can be more dramatic and, if the trunk and limb muscles are affected, can result in collapsing to the ground.
Cataplexy usually has its onset at about the same time as the sleepiness associated with narcolepsy, but it can arise even years later.8 Episodes can last from a few seconds to 2 minutes. Consciousness is always preserved. A range of emotions can trigger cataplexy, but typically the emotion is a positive one such as laughter or excitement.9 Deep tendon reflexes disappear in cataplexy, so checking reflexes during a witnessed episode can be clinically valuable.2
Cataplexy can worsen with stress and insufficient sleep, occasionally with “status cataplecticus,” in which repeated, persistent episodes of cataplexy occur over several hours.8 Status cataplecticus can be spontaneous or an effect of withdrawal from anticataplectic medications.2
Cataplexy is thought to represent intrusion of REM sleep and its associated muscle atonia during wakefulness.
Sleep paralysis, hallucinations
Sleep paralysis and hallucinations are other features of narcolepsy that reflect this REM dissociation from sleep.
Sleep paralysis occurs most commonly upon awakening, but sometimes just before sleep onset. In most cases, it is manifested by inability to move the limbs or speak, lasting several seconds or, in rare cases, minutes at a time. Sleep paralysis can be associated with a sensation of fear or suffocation, especially when initially experienced.8
Hypnopompic hallucinations, occurring upon awakening, are more common than hypnagogic hallucinations, which are experienced before falling asleep. The hallucinations are often vivid and usually visual, although other types of hallucinations are possible. Unlike those that occur in psychotic disorders, the hallucinations tend to be associated with preserved insight that they are not real.10
Notably, both sleep paralysis and hallucinations are nonspecific symptoms that are common in the general population.8,11,12
Fragmented sleep
Although they are very sleepy, people with narcolepsy generally cannot stay asleep for very long. Their sleep tends to be extremely fragmented, and they often wake up several times a night.2
This sleep pattern reflects the inherent instability of sleep-wake transitions in narcolepsy. In fact, over a 24-hour period, adults with narcolepsy have a normal amount of sleep.13 In children, however, when narcolepsy first arises, the 24-hour sleep time can increase abruptly and can sometimes be associated with persistent cataplexy that can manifest as a clumsy gait.14
Weight gain, obstructive sleep apnea
Weight gain is common, particularly after symptom onset, and especially in children. As a result, obesity is a frequent comorbidity.15 Because obstructive sleep apnea can consequently develop, all patients with narcolepsy require screening for sleep-disordered breathing.
Other sleep disorders often accompany narcolepsy and are more common than in the general population.16 In a study incorporating both clinical and polysomnographic data of 100 patients with narcolepsy, insomnia was the most common comorbid sleep disorder, with a prevalence of 28%; others were REM sleep behavior disorder (24%), restless legs syndrome (24%), obstructive sleep apnea (21%), and non-REM parasomnias.17
PSYCHOSOCIAL CONSEQUENCES
Narcolepsy has significant psychosocial consequences. As a result of their symptoms, people with narcolepsy may not be able to meet academic or work-related demands.
Additionally, their risk of a motor vehicle accident is 3 to 4 times higher than in the general population, and more than one-third of patients have been in an accident due to sleepiness.18 There is some evidence to show that treatment eliminates this risk.19
Few systematic studies have examined mood disorders in narcolepsy. However, studies tend to show a higher prevalence of psychiatric disorders than in the general population, with depression and anxiety the most com-mon.20,21
DIAGNOSIS IS OFTEN DELAYED
The prevalence of narcolepsy type 1 is between 25 and 100 per 100,000 people.22 In a Mayo Clinic study,23 the incidence of narcolepsy type 1 was estimated to be 0.74 per 100,000 person-years. Epidemiologic data on narcolepsy type 2 are sparse, but patients with narcolepsy without cataplexy are thought to represent only 36% of all narcolepsy patients.23
Diagnosis is often delayed, with the average time between the onset of symptoms and the diagnosis ranging from 8 to 22 years. With increasing awareness, the efficiency of the diagnostic process is improving, and this delay is expected to lessen accordingly.24
Symptoms most commonly arise in the second decade; but the age at onset ranges significantly, between the first and fifth decades. Narcolepsy has a bimodal distribution in incidence, with the biggest peak at approximately age 15 and second smaller peak in the mid-30s. Some studies have suggested a slight male predominance.23,25
DIAGNOSIS
History is key
The history should include specific questions about the hallmark features of narcolepsy, including cataplexy, sleep paralysis, and sleep-related hallucinations. For individual assessment of subjective sleepiness, the Epworth Sleepiness Scale or Pediatric Daytime Sleepiness Scale can be administered quickly in the office setting.26,27
The Epworth score is calculated from the self-rated likelihood of falling asleep in 8 different situations, with possible scores of 0 (would never doze) to 3 (high chance of dozing) on each question, for a total possible score of 0 to 24. Normal total scores are between 0 and 10, while scores greater than 10 reflect pathologic sleepiness. Scores on the Epworth Sleepiness Scale in those with narcolepsy tend to reflect moderate to severe sleepiness, or at least 13, as opposed to patients with obstructive sleep apnea, whose scores commonly reflect milder sleepiness.28
Testing with actigraphy and polysomnography
It is imperative to rule out insufficient sleep and other sleep disorders as a cause of daytime sleepiness. This can be done with a careful clinical history, actigraphy with sleep logs, and polysomnography.
In the 2 to 4 weeks before actigraphy and subsequent testing, all medications with alerting or sedating properties (including antidepressants) should be tapered off to prevent influence on the results of the study.
Delayed sleep-phase disorder presents at a similar age as narcolepsy and can be associated with similar degrees of sleepiness. However, individuals with delayed sleep phase disorder have an inappropriately timed sleep-wake cycle so that there is a shift in their desired sleep onset and awakening times. It is common—prevalence estimates vary but average about 1% in the general population.29
Insufficient sleep syndrome is even more common, especially in teenagers and young adults, with increasing family, social, and academic demands. Sleep needs vary across the life span. A teenager needs 8 to 10 hours of sleep per night, and a young adult needs 7 to 9 hours. A study of 1,285 high school students found that 10.4% were not getting enough sleep.30
If actigraphy data suggest a circadian rhythm disorder or insufficient sleep that could explain the symptoms of sleepiness, then further testing should be halted and these specific issues should be addressed. In these cases, working with the patient toward maintaining a regular sleep-wake schedule with 7 to 8 hours of nightly sleep will often resolve symptoms.
If actigraphy demonstrates the patient is maintaining a regular sleep schedule and allowing adequate time for nightly sleep, the next step is polysomnography.
Polysomnography is performed to detect other disorders that can disrupt sleep, such as sleep-disordered breathing or periodic limb movement disorder.2,5 In addition, polysomnography can provide assurance that adequate sleep was obtained prior to the next step in testing.
Multiple sleep latency test
If sufficient sleep is obtained on polysomnograpy (at least 6 hours for an adult) and no other sleep disorder is identified, a multiple sleep latency test is performed. A urine toxicology screen is typically performed on the day of the test to ensure that drugs are not affecting the results.
The multiple sleep latency test consists of 4 to 5 nap opportunities at 2-hour intervals in a quiet dark room conducive to sleep, during which both sleep and REM latency are recorded. The sleep latency of those with narcolepsy is significantly shortened, and the diagnosis of narcolepsy requires an average sleep latency of less than 8 minutes.
Given the propensity for REM sleep in narcolepsy, another essential feature for diagnosis is the sleep-onset REM period (SOREMP). A SOREMP is defined as a REM latency of less than 15 minutes. A diagnosis of narcolepsy re-quires a SOREMP in at least 2 of the naps in a multiple sleep latency test (or 1 nap if the shortened REM latency is seen during polysomnography).31
The multiple sleep latency test has an imperfect sensitivity, though, and should be repeated when there is a high suspicion of narcolepsy.32–34 It is not completely specific either, and false-positive results occur. In fact, SOREMPs can be seen in the general population, particularly in those with a circadian rhythm disorder, insufficient sleep, or sleep-disordered breathing. Two or more SOREMPs in an multiple sleep latency test can be seen in a small proportion of the general population.35 The results of a multiple sleep latency test should be interpreted in the clinical context.
Differential diagnosis
Narcolepsy type 1 is distinguished from type 2 by the presence of cataplexy. A cerebrospinal fluid hypocretin 1 level of 110 pg/mL or less, or less than one-third of the mean value obtained in normal individuals, can substitute for the multiple sleep latency test in diagnosing narcolepsy type 1.31 Currently, hypocretin testing is generally not performed in clinical practice, although it may become a routine part of the narcolepsy evaluation in the future.
Thus, according to the International Classification of Sleep Disorders, 3rd edition,31 the diagnosis of narcolepsy type 1 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder, and at least 1 of the following:
- Cataplexy and mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing (1 of which can be on the preceding night’s polysomography)
- Cerebrospinal fluid hypocretin 1 levels less than 110 pg/mL or one-third the baseline normal levels and mean sleep latency ≤ 8 minutes with ≥ 2 SOREMPs on multiple sleep latency testing.
Similarly, the diagnosis of narcolepsy type 2 requires excessive daytime sleepiness for at least 3 months that cannot be explained by another sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder, plus:
- Mean sleep latency of 8 minutes or less with at least 2 SOREMPs on multiple sleep latency testing.
Idiopathic hypersomnia, another disorder of central hypersomnolence, is also characterized by disabling sleepiness. It is diagnostically differentiated from narcolepsy, as there are fewer than 2 SOREMPs. As opposed to narcolepsy, in which naps tend to be refreshing, even prolonged naps in idiopathic hypersomnia are often not helpful in restoring wakefulness. In idiopathic hypersomnia, sleep is usually not fragmented, and there are few nocturnal arousals. Sleep times can often be prolonged as well, whereas in narcolepsy total sleep time through the day may not be increased but is not consolidated.
Kleine-Levin syndrome is a rarer disorder of hypersomnia. It is episodic compared with the relatively persistent sleepiness in narcolepsy and idiopathic hypersomnia. Periods of hypersomnia occur intermittently for days to weeks and are accompanied by cognitive and behavioral changes including hyperphagia and hypersexuality.4
LINKED TO HYPOCRETIN DEFICIENCY
Over the past 2 decades, the underlying pathophysiology of narcolepsy type 1 has been better characterized.
Narcolepsy type 1 has been linked to a deficiency in hypocretin in the central nervous system.36 Hypocretin (also known as orexin) is a hormone produced in the hypothalamus that acts on multiple brain regions and maintains alertness. For unclear reasons, hypothalamic neurons producing hypocretin are selectively reduced in narcolepsy type 1. Hypocretin also stabilizes wakefulness and inhibits REM sleep; therefore, hypocretin deficiency can lead to inappropriate intrusions of REM sleep onto wakefulness, leading to the hallmark features of narcolepsy—cataplexy, sleep-related hallucinations, and sleep paralysis.37 According to one theory, cataplexy is triggered by emotional stimuli because of a pathway between the medial prefrontal cortex and the amygdala to the pons.38
Cerebrospinal fluid levels of hypocretin in patients with narcolepsy type 2 tend to be normal, and the biologic underpinnings of narcolepsy type 2 remain mysterious. However, in the subgroup of those with narcolepsy type 2 in which hypocretin is low, many individuals go on to develop cataplexy, thereby evolving to narcolepsy type 1.36
POSSIBLE AUTOIMMUNE BASIS
Narcolepsy is typically a sporadic disorder, although familial cases have been described. The risk of a parent with narcolepsy having a child who is affected is approximately 1%.5
Narcolepsy type 1 is strongly associated with HLA-DQB1*0602, with up to 95% of those affected having at least one allele.39 Having 2 copies of the allele further increases the risk of developing narcolepsy.40 However, this allele is far from specific for narcolepsy with cataplexy, as it occurs in 12% to 38% of the general population.41 Therefore, HLA typing currently has limited clinical utility. The exact cause is as yet unknown, but substantial literature proposes an autoimmune basis of the disorder, given the strong association with the HLA subtype.42–44
After the 2009 H1N1 influenza pandemic, there was a significant increase in the incidence of narcolepsy with cataplexy, which again sparked interest in an autoimmune etiology underlying the disorder. Pandemrix, an H1N1 vaccine produced as a result of the 2009 pandemic, appeared to also be associated with an increase in the incidence of narcolepsy. An association with other upper respiratory infections has also been noted, further supporting a possible autoimmune basis.
A few studies have looked for serum autoantibodies involved in the pathogenesis of narcolepsy. Thus far, only one has been identified, an antibody to Tribbles homolog 2, found in 20% to 40% of those with new onset of nar-colepsy.42–44
TREATMENTS FOR DAYTIME SLEEPINESS
As with many chronic disorders, the treatment of narcolepsy consists of symptomatic rather than curative management, which can be done through both pharmacologic and nonpharmacologic means.
Nondrug measures
Scheduled naps lasting 15 to 20 minutes can help improve alertness.45 A consistent sleep schedule with good sleep hygiene, ensuring sufficient nightly sleep, is also important. In one study, the combination of scheduled naps and regular nocturnal sleep times reduced the level of daytime sleepiness and unintentional daytime sleep. Daytime naps were most helpful for those with the highest degree of daytime sleepiness.45
Strategic use of caffeine can be helpful and can reduce dependence on pharmacologic treatment.
Screening should be performed routinely for other sleep disorders, such as sleep-disordered breathing, which should be treated if identified.5,18 When being treated for other medical conditions, individuals with narcolepsy should avoid medications that can cause sedation, such as opiates or barbiturates; alcohol should be minimized or avoided.
Networking with other individuals with narcolepsy through support groups such as Narcolepsy Network can be valuable for learning coping skills and connecting with community resources. Psychological counseling for the patient, and sometimes the family, can also be useful. School-age children may need special accommodations such as schedule adjustments to allow for scheduled naps or frequent breaks to maintain alertness.
People with narcolepsy tend to function better in careers that do not require long periods of sitting, as sleepiness tends to be worse, but instead offer flexibility and require higher levels of activity that tend to combat sleepiness. They should not work as commercial drivers.18
Medications
While behavioral interventions in narcolepsy are vital, they are rarely sufficient, and drugs that promote daytime wakefulness are used as an adjunct (Table 2).46
Realistic expectations should be established when starting, as some degree of residual sleepiness usually remains even with optimal medical therapy. Medications should be strategically scheduled to maximize alertness during necessary times such as at work or school or during driving. Patients should specifically be counseled to avoid driving if sleepy.18,47
Modafinil is often used as a first-line therapy, given its favorable side-effect profile and low potential for abuse. Its pharmacologic action has been debated but it probably acts as a selective dopamine reuptake inhibitor. It is typically taken twice daily (upon waking and early afternoon) and is usually well tolerated.
Potential side effects include headache, nausea, dry mouth, anorexia, diarrhea, and, rarely, Stevens-Johnson syndrome. Cardiovascular side effects are minimal, making it a favorable choice in older patients.18,48
A trial in 283 patients showed significantly lower levels of sleepiness in patients taking modafinil 200 mg or 400 mg than in a control group. Other trials have supported these findings and showed improved driving performance on modafinil.18
Notably, modafinil can increase the metabolism of oral contraceptives, thereby reducing their efficacy. Women of childbearing age should be warned about this interaction and should be transitioned to nonhormonal forms of contraception.2,47
Armodafinil, a purified R-isomer of modafinil, has a longer half-life and requires only once-daily dosing.5
If modafinil or armodafinil fails to optimally manage daytime sleepiness, a traditional stimulant such as methylphenidate or an amphetamine is often used.
Methylphenidate and amphetamines primarily inhibit the reuptake and increase the release of the monoamines, mainly dopamine, and to a lesser degree serotonin and norepinephrine.
These drugs have more significant adverse effects that can involve the cardiovascular system, causing hypertension and arrhythmias. Anorexia, weight loss, and, particularly with high doses, psychosis can occur.49
These drugs should be avoided in patients with a history of significant cardiovascular disease. Before starting stimulant therapy, a thorough cardiovascular examination should be done, often including electrocardiography to ensure there is no baseline arrhythmia.
Patients on these medications should be followed closely to ensure that blood pressure, pulse, and weight are not negatively affected.18,50 Addiction and tolerance can develop with these drugs, and follow-up should include assessment for dependence. Some states may require prescription drug monitoring to ensure the drugs are not being abused or diverted.
Short- and long-acting formulations of both methylphenidate and amphetamines are available, and a long-acting form is often used in conjunction with a short-acting form as needed.18
Addiction and drug-seeking behavior can develop but are unusual in those taking stimulants to treat narcolepsy.49
Follow-up
Residual daytime sleepiness can be measured subjectively through the Epworth Sleepiness Scale on follow-up. If necessary, a maintenance-of-wakefulness test can provide an objective assessment of treatment efficacy.18
As narcolepsy is a chronic disorder, treatment should evolve with time. Most medications that treat narcolepsy are categorized by the US Food and Drug Administration as pregnancy category C, as we do not have adequate studies in human pregnancies to evaluate their effects. When a patient with narcolepsy becomes pregnant, she should be counseled about the risks and benefits of remaining on therapy. Treatment should balance the risks of sleepiness with the potential risks of remaining on medications.50 In the elderly, as cardiovascular comorbidities tend to increase, the risks and benefits of therapy should be routinely reevaluated.
For cataplexy
Sodium oxybate,51–53 the most potent anticataplectic drug, is the sodium salt of gamma hydroxybutyrate, a metabolite of gamma-aminobutyric acid. Sodium oxybate can be prescribed in the United States, Canada, and Europe. The American Academy of Sleep Medicine recommends sodium oxybate for cataplexy, daytime sleepiness, and disrupted sleep based on 3 level-1 studies and 2 level-4 studies.46
Sodium oxybate increases slow-wave sleep, improves sleep continuity, and often helps to mitigate daytime sleepiness. Due to its short half-life, its administration is unusual: the first dose is taken before bedtime and the second dose 2.5 to 4 hours later. Some patients set an alarm clock to take the second dose, while others awaken spontaneously to take the second dose. Most patients find that with adherence to dosing and safety instructions, sodium oxybate can serve as a highly effective form of treatment of both excessive sleepiness and cataplexy and may reduce the need for stimulant-based therapies.
The most common adverse effects are nausea, mood swings, and enuresis. Occasionally, psychosis can result and limit use of the drug. Obstructive sleep apnea can also develop or worsen.52 Because of its high salt content, sodium oxybate should be used with caution in those with heart failure, hypertension, or renal impairment. Its relative, gamma hydroxybutyrate, causes rapid sedation and has been notorious for illegal use as a date rape drug.
In the United States, sodium oxybate is distributed only through a central pharmacy to mitigate potential abuse. Due to this system, the rates of diversion are extremely low, estimated in a postmarketing analysis to be 1 instance per 5,200 patients treated. In the same study, abuse and dependence were both rare as well, about 1 case for every 2,600 and 6,500 patients treated.6,18,52,53
Antidepressants promote the action of norepinephrine and, to a lesser degree, serotonin, thereby suppressing REM sleep.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is often used as a first-line treatment for cataplexy. Selective serotonin reuptake inhibitors such as fluoxetine are also used with success. Tricyclic antidepressants such as protriptyline or clomipramine are extremely effective for cataplexy, but are rarely used due to their adverse effects.2,47
FUTURE WORK
While our understanding of narcolepsy has advanced, there are still gaps in our knowledge of the disorder—namely, the specific trigger for the loss of hypocretin neurons in type 1 narcolepsy and the underlying pathophysiology of type 2.
A number of emerging therapies target the hypocretin system, including peptide replacement, neuronal transplant, and immunotherapy preventing hypocretin neuronal cell death.50,54,55 Additional drugs designed to improve alertness that do not involve the hypocretin system are also being developed, including a histamine inverse agonist.50,56 Sodium oxybate and modafinil, although currently approved for use in adults, are still off-label in pediatric practice. Studies of the safety and efficacy of these medications in children are needed.7,57
- Gélineau J. De la narcolepsie. Gazette des Hôpitaux Civils et Militaires 1880; part a, 53:626–628, part b, 54:635–637.
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet 2007; 369(9560):499–511. doi:10.1016/S0140-6736(07)60237-2
- Scammell TE. Clinical features and diagnosis of narcolepsy in adults. In: Eichler AF, ed. UpToDate. Waltham, MA: UpToDate; 2018. www.uptodate.com. Accessed October 31, 2018.
- Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med 2004; 5(1):37–41. pmid:14725825
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373(27):2654–2662. doi:10.1056/NEJMra1500587
- Babiker MO, Prasad M. Narcolepsy in children: a diagnostic and management approach. Pediatr Neurol 2015; 52(6):557–565. doi:10.1016/j.pediatrneurol.2015.02.020
- Kotagal S. Narcolepsy in children. In: UpToDate, Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol 2003; 53(2):154–166. doi:10.1002/ana.10444
- Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med 2011; 12(1):12–18. doi:10.1016/j.sleep.2010.05.010
- Plazzi G, Fabbri C, Pizza F, Serretti A. Schizophrenia-like symptoms in narcolepsy type 1: shared and distinctive clinical characteristics. Neuropsychobiology 2015; 71(4):218–224. doi:10.1159/000432400
- Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res 2000; 97(2-3):153–164. pmid:11166087
- Sharpless BA, Barber JP. Lifetime prevalence rates of sleep paralysis: a systematic review. Sleep Med Rev 2011;5(5):311–315. doi:10.1016/j.smrv.2011.01.007
- Broughton R, Dunham W, Newman J, Lutley K, Duschesne P, Rivers M. Ambulatory 24 hour sleep-wake monitoring in narcolepsy-cataplexy compared to matched controls. Electroencephalogr Clin Neurophysiol 1988; 70(6):473–481. pmid:2461281
- Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain 2013; 136(pt 12):3787–3795. doi:10.1093/brain/awt277
- Kotagal S, Krahn LE, Slocumb N. A putative link between childhood narcolepsy and obesity. Sleep Med 2004; 5(2):147–150. doi:10.1016/j.sleep.2003.10.006
- Pizza F, Tartarotti S, Poryazova R, Baumann CR, Bassetti CL. Sleep-disordered breathing and periodic limb movements in narcolepsy with cataplexy: a systematic analysis of 35 consecutive patients. Eur Neurol 2013; 70(1-2):22–26. doi:10.1159/000348719
- Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck narcolepsy cohort. J Clin Sleep Med 2013; 9(8):805–812. doi:10.5664/jcsm.2926
- Scammell TE. Treatment of narcolepsy in adults. In: Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Pizza F, Jaussent I, Lopez R, et al. Car crashes and central disorders of hypersomnolence: a French study. PLoS One 2015; 10(6):e0129386. doi:10.1371/journal.pone.0129386
- Fortuyn HD, Lappenschaar MA, Furer JW, et al. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry 2010; 32(1):49–56. doi:10.1016/j.genhosppsych.2009.08.007
- Ruoff CM, Reaven NL, Funk SE, et al. High rates of psychiatric comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study of 9,312 patients in the United States. J Clin Psychiatry 2017; 78(2):171–176. doi:10.4088/JCP.15m10262
- Longstreth WT Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007; 30(1):13–26. pmid:17310860
- Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep 2002; 25(2):197–202. pmid:11902429
- Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med 2014; 15(5):502–507. doi:10.1016/j.sleep.2014.01.015
- Dauvilliers Y, Montplaisir J, Molinari N, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001; 57(11):2029–2033. pmid:11739821
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14(6):540–545. pmid:1798888
- Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Badia P. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep 2003; 26(4):455–458. pmid:12841372
- van der Heide A, van Schie MK, Lammers GJ, et al. Comparing treatment effect measurements in narcolepsy: the sustained attention to response task, Epworth sleepiness scale and maintenance of wakefulness test. Sleep 2015; 38(7):1051–1058. doi:10.5665/sleep.4810
- Nesbitt AD. Delayed sleep-wake phase disorder. J Thorac Dis 2018; 10(suppl 1):S103–S111. doi:10.21037/jtd.2018.01.11
- Pallesen S, Saxvig IW, Molde H, Sørensen E, Wilhelmsen-Langeland A, Bjorvatn B. Brief report: behaviorally induced insufficient sleep syndrome in older adolescents: prevalence and correlates. J Adolesc 2011; 34(2):391–395. doi:10.1016/j.adolescence.2010.02.005
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Disorders; 2014.
- Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med 2013; 9(8):789–795. doi:10.5664/jcsm.2922
- Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol 2013; 70(7):891–902. doi:10.1001/jamaneurol.2013.1589
- Cairns A, Bogan R. Prevalence and clinical correlates of a short onset REM period (SOREMP) during routine PSG. Sleep 2015; 38(10):1575–1581. doi:10.5665/sleep.5050
- Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the multiple sleep latency test in community adults. Brain 2006; 129(6):1609–1623. doi:10.1093/brain/awl079
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000; 355(9197):39–40. doi:10.1016/S0140-6736(99)05582-8
- Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000; 6(9):991–997. doi:10.1038/79690
- Oishi Y, Williams RH, Agostinelli L, et al. Role of the medial prefrontal cortex in cataplexy. J Neurosci 2013; 33(23):9743–9751. doi:10.1523/JNEUROSCI.0499-13.2013
- Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients.. Sleep 1997; 20(11):1012–1020. pmid:9456467
- Pelin Z, Guilleminault C, Risch N, Grumet FC, Mignot E. HLA-DQB1*0602 homozygosity increases relative risk for narcolepsy but not disease severity in two ethnic groups. US Modafinil in Narcolepsy Multicenter Study Group. Tissue Antigens 1998; 51(1):96–100. pmid:9459509
- Akintomide GS, Rickards H. Narcolepsy: a review. Neuropsychiatr Dis Treat 2011; 7(1):507–518. doi:10.2147/NDT.S23624
- Mahlios J, De la Herrán-Arita AK, Mignot E. The autoimmune basis of narcolepsy. Curr Opin Neurobiol 2013; 23(5):767–773. doi:10.1016/j.conb.2013.04.013
- Degn M, Kornum BR. Type 1 narcolepsy: a CD8(+) T cell-mediated disease? Ann N Y Acad Sci 2015;1 351:80–88. doi:10.1111/nyas.12793
- Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol 2015; 14(3):318–328. doi:10.1016/S1474-4422(14)70218-2
- Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep 2001; 24(4):385–391. pmid:11403522
- Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007; 30(12):1705–1711. pmid:18246980
- Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics 2012; 9(4):739–752. doi:10.1007/s13311-012-0150-9
- Roth T, Schwartz JR, Hirshkowitz M, Erman MK, Dayno JM, Arora S. Evaluation of the safety of modafinil for treatment of excessive sleepiness. J Clin Sleep Med 2007; 3(6):595–602. pmid:17993041
- Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL. Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study. Sleep 2005; 28(6):667–672. pmid:16477952
- Abad VC, Guilleminault C. New developments in the management of narcolepsy. Nat Sci Sleep 2017; 9:39–57. doi:10.2147/NSS.S103467
- Drakatos P, Lykouras D, D’Ancona G, et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med 2017; 35:80–84. doi:10.1016/j.sleep.2017.03.028
- Mansukhani MP, Kotagal S. Sodium oxybate in the treatment of childhood narcolepsy–cataplexy: a retrospective study. Sleep Med 2012; 13(6):606–610. doi:10.1016/j.sleep.2011.10.032
- Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J Clin Sleep Med 2009; 5(4):365–371. pmid:19968016
- Weinhold SL, Seeck-Hirschner M, Nowak A, Hallschmid M, Göder R, Baier PC. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav Brain Res 2014; 262:8–13. doi:10.1016/j.bbr.2013.12.045
- Arias-Carrión O, Murillo-Rodriguez E. Effects of hypocretin/orexin cell transplantation on narcoleptic-like sleep behavior in rats. PLoS One 2014; 9(4):e95342. doi:10.1371/journal.pone.0095342
- Leu-Semenescu S, Nittur N, Golmard JL, Arnulf I. Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review. Sleep Med 2014; 15(6):681–687. doi:10.1016/j.sleep.2014.01.021
- Lecendreux M, Bruni O, Franco P, et al. Clinical experience suggests that modafinil is an effective and safe treatment for paediatric narcolepsy. J Sleep Res 2012; 21(4):481–483. doi:10.1111/j.1365-2869.2011.00991.x
- Gélineau J. De la narcolepsie. Gazette des Hôpitaux Civils et Militaires 1880; part a, 53:626–628, part b, 54:635–637.
- Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet 2007; 369(9560):499–511. doi:10.1016/S0140-6736(07)60237-2
- Scammell TE. Clinical features and diagnosis of narcolepsy in adults. In: Eichler AF, ed. UpToDate. Waltham, MA: UpToDate; 2018. www.uptodate.com. Accessed October 31, 2018.
- Morrish E, King MA, Smith IE, Shneerson JM. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med 2004; 5(1):37–41. pmid:14725825
- Scammell TE. Narcolepsy. N Engl J Med 2015; 373(27):2654–2662. doi:10.1056/NEJMra1500587
- Babiker MO, Prasad M. Narcolepsy in children: a diagnostic and management approach. Pediatr Neurol 2015; 52(6):557–565. doi:10.1016/j.pediatrneurol.2015.02.020
- Kotagal S. Narcolepsy in children. In: UpToDate, Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol 2003; 53(2):154–166. doi:10.1002/ana.10444
- Overeem S, van Nues SJ, van der Zande WL, Donjacour CE, van Mierlo P, Lammers GJ. The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med 2011; 12(1):12–18. doi:10.1016/j.sleep.2010.05.010
- Plazzi G, Fabbri C, Pizza F, Serretti A. Schizophrenia-like symptoms in narcolepsy type 1: shared and distinctive clinical characteristics. Neuropsychobiology 2015; 71(4):218–224. doi:10.1159/000432400
- Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res 2000; 97(2-3):153–164. pmid:11166087
- Sharpless BA, Barber JP. Lifetime prevalence rates of sleep paralysis: a systematic review. Sleep Med Rev 2011;5(5):311–315. doi:10.1016/j.smrv.2011.01.007
- Broughton R, Dunham W, Newman J, Lutley K, Duschesne P, Rivers M. Ambulatory 24 hour sleep-wake monitoring in narcolepsy-cataplexy compared to matched controls. Electroencephalogr Clin Neurophysiol 1988; 70(6):473–481. pmid:2461281
- Pizza F, Franceschini C, Peltola H, et al. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain 2013; 136(pt 12):3787–3795. doi:10.1093/brain/awt277
- Kotagal S, Krahn LE, Slocumb N. A putative link between childhood narcolepsy and obesity. Sleep Med 2004; 5(2):147–150. doi:10.1016/j.sleep.2003.10.006
- Pizza F, Tartarotti S, Poryazova R, Baumann CR, Bassetti CL. Sleep-disordered breathing and periodic limb movements in narcolepsy with cataplexy: a systematic analysis of 35 consecutive patients. Eur Neurol 2013; 70(1-2):22–26. doi:10.1159/000348719
- Frauscher B, Ehrmann L, Mitterling T, et al. Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck narcolepsy cohort. J Clin Sleep Med 2013; 9(8):805–812. doi:10.5664/jcsm.2926
- Scammell TE. Treatment of narcolepsy in adults. In: Eichler AF, ed. UpToDate, Waltham, MA. www.uptodate.com. Accessed October 31, 2018.
- Pizza F, Jaussent I, Lopez R, et al. Car crashes and central disorders of hypersomnolence: a French study. PLoS One 2015; 10(6):e0129386. doi:10.1371/journal.pone.0129386
- Fortuyn HD, Lappenschaar MA, Furer JW, et al. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry 2010; 32(1):49–56. doi:10.1016/j.genhosppsych.2009.08.007
- Ruoff CM, Reaven NL, Funk SE, et al. High rates of psychiatric comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study of 9,312 patients in the United States. J Clin Psychiatry 2017; 78(2):171–176. doi:10.4088/JCP.15m10262
- Longstreth WT Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007; 30(1):13–26. pmid:17310860
- Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep 2002; 25(2):197–202. pmid:11902429
- Thorpy MJ, Krieger AC. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med 2014; 15(5):502–507. doi:10.1016/j.sleep.2014.01.015
- Dauvilliers Y, Montplaisir J, Molinari N, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology 2001; 57(11):2029–2033. pmid:11739821
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14(6):540–545. pmid:1798888
- Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Badia P. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep 2003; 26(4):455–458. pmid:12841372
- van der Heide A, van Schie MK, Lammers GJ, et al. Comparing treatment effect measurements in narcolepsy: the sustained attention to response task, Epworth sleepiness scale and maintenance of wakefulness test. Sleep 2015; 38(7):1051–1058. doi:10.5665/sleep.4810
- Nesbitt AD. Delayed sleep-wake phase disorder. J Thorac Dis 2018; 10(suppl 1):S103–S111. doi:10.21037/jtd.2018.01.11
- Pallesen S, Saxvig IW, Molde H, Sørensen E, Wilhelmsen-Langeland A, Bjorvatn B. Brief report: behaviorally induced insufficient sleep syndrome in older adolescents: prevalence and correlates. J Adolesc 2011; 34(2):391–395. doi:10.1016/j.adolescence.2010.02.005
- American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Disorders; 2014.
- Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med 2013; 9(8):789–795. doi:10.5664/jcsm.2922
- Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol 2013; 70(7):891–902. doi:10.1001/jamaneurol.2013.1589
- Cairns A, Bogan R. Prevalence and clinical correlates of a short onset REM period (SOREMP) during routine PSG. Sleep 2015; 38(10):1575–1581. doi:10.5665/sleep.5050
- Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the multiple sleep latency test in community adults. Brain 2006; 129(6):1609–1623. doi:10.1093/brain/awl079
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000; 355(9197):39–40. doi:10.1016/S0140-6736(99)05582-8
- Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000; 6(9):991–997. doi:10.1038/79690
- Oishi Y, Williams RH, Agostinelli L, et al. Role of the medial prefrontal cortex in cataplexy. J Neurosci 2013; 33(23):9743–9751. doi:10.1523/JNEUROSCI.0499-13.2013
- Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C. HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients.. Sleep 1997; 20(11):1012–1020. pmid:9456467
- Pelin Z, Guilleminault C, Risch N, Grumet FC, Mignot E. HLA-DQB1*0602 homozygosity increases relative risk for narcolepsy but not disease severity in two ethnic groups. US Modafinil in Narcolepsy Multicenter Study Group. Tissue Antigens 1998; 51(1):96–100. pmid:9459509
- Akintomide GS, Rickards H. Narcolepsy: a review. Neuropsychiatr Dis Treat 2011; 7(1):507–518. doi:10.2147/NDT.S23624
- Mahlios J, De la Herrán-Arita AK, Mignot E. The autoimmune basis of narcolepsy. Curr Opin Neurobiol 2013; 23(5):767–773. doi:10.1016/j.conb.2013.04.013
- Degn M, Kornum BR. Type 1 narcolepsy: a CD8(+) T cell-mediated disease? Ann N Y Acad Sci 2015;1 351:80–88. doi:10.1111/nyas.12793
- Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol 2015; 14(3):318–328. doi:10.1016/S1474-4422(14)70218-2
- Rogers AE, Aldrich MS, Lin X. A comparison of three different sleep schedules for reducing daytime sleepiness in narcolepsy. Sleep 2001; 24(4):385–391. pmid:11403522
- Morgenthaler TI, Kapur VK, Brown TM, et al; Standards of Practice Committee of the American Academy of Sleep Medicine. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep 2007; 30(12):1705–1711. pmid:18246980
- Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics 2012; 9(4):739–752. doi:10.1007/s13311-012-0150-9
- Roth T, Schwartz JR, Hirshkowitz M, Erman MK, Dayno JM, Arora S. Evaluation of the safety of modafinil for treatment of excessive sleepiness. J Clin Sleep Med 2007; 3(6):595–602. pmid:17993041
- Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL. Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study. Sleep 2005; 28(6):667–672. pmid:16477952
- Abad VC, Guilleminault C. New developments in the management of narcolepsy. Nat Sci Sleep 2017; 9:39–57. doi:10.2147/NSS.S103467
- Drakatos P, Lykouras D, D’Ancona G, et al. Safety and efficacy of long-term use of sodium oxybate for narcolepsy with cataplexy in routine clinical practice. Sleep Med 2017; 35:80–84. doi:10.1016/j.sleep.2017.03.028
- Mansukhani MP, Kotagal S. Sodium oxybate in the treatment of childhood narcolepsy–cataplexy: a retrospective study. Sleep Med 2012; 13(6):606–610. doi:10.1016/j.sleep.2011.10.032
- Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J Clin Sleep Med 2009; 5(4):365–371. pmid:19968016
- Weinhold SL, Seeck-Hirschner M, Nowak A, Hallschmid M, Göder R, Baier PC. The effect of intranasal orexin-A (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav Brain Res 2014; 262:8–13. doi:10.1016/j.bbr.2013.12.045
- Arias-Carrión O, Murillo-Rodriguez E. Effects of hypocretin/orexin cell transplantation on narcoleptic-like sleep behavior in rats. PLoS One 2014; 9(4):e95342. doi:10.1371/journal.pone.0095342
- Leu-Semenescu S, Nittur N, Golmard JL, Arnulf I. Effects of pitolisant, a histamine H3 inverse agonist, in drug-resistant idiopathic and symptomatic hypersomnia: a chart review. Sleep Med 2014; 15(6):681–687. doi:10.1016/j.sleep.2014.01.021
- Lecendreux M, Bruni O, Franco P, et al. Clinical experience suggests that modafinil is an effective and safe treatment for paediatric narcolepsy. J Sleep Res 2012; 21(4):481–483. doi:10.1111/j.1365-2869.2011.00991.x
KEY POINTS
- Features of narcolepsy include daytime sleepiness, sleep attacks, cataplexy (in narcolepsy type 1), sleep paralysis, and sleep-related hallucinations.
- People with narcolepsy feel sleepy and can fall asleep quickly, but they do not stay asleep for long. They go into rapid eye movement sleep soon after falling asleep. Total sleep time is normal, but sleep is fragmented.
- Scheduled naps lasting 15 to 20 minutes can improve alertness. A consistent sleep schedule with good sleep hygiene is also important.
- Modafinil, methylphenidate, and amphetamines are used to manage daytime sleepiness, and sodium oxybate and antidepressants are used for cataplexy.
A new reason to reconsider that antibiotic prescription: The microbiome
But, after the results of many recent studies, it turns out I should not have been so comfortable after all. This should not be a surprise. We should never be overly confident with our understanding of anything in clinical practice.
In this issue, Dr. Martin Blaser discusses his work, which supports the hypothesis that the currently increased prevalence of obesity and diabetes is at least in part due to reduced diversity in the gut microbiome. The increased exposure to antibiotics through prescriptions for women before and during pregnancy, as well as perhaps their exposure to antibiotics in the environment, results in changes to the gut and vaginal flora that influence the developing gut and likely other anatomic microbiomes in the neonate and infant. Fascinating research done in mice, utilizing fecal transfer experiments, is building an evidence trail to support the concept that the microbiome plays a major role in the development of childhood and adult obesity, and the gut microbiome is influenced by its exposure to antibiotics, perhaps given years earlier.
Knowledge of the gastrointestinal and other human microbiomes is exploding. I now wonder how many seemingly random clinical events associated with antibiotic use that were not understood and were easily dismissed as stochastic warrant formal study. Some of my patients with rheumatoid arthritis have described flares after eating certain foods and transient remissions or exacerbations after treatment with antibiotics. An epidemiologic study has linked the likelihood of developing childhood inflammatory bowel disease with exposure to antibiotics. Even more fascinating are observations that the microbiota composition (influenced by antibiotics) can influence the outcome of cardiac allografts in a murine model and the response of certain tumors to immune checkpoint inhibitors in murine and human studies. The mechanism may relate to the effects of the microbiome on immune cell activation and migration. Several disorders have been linked to specific bacteria in the gut microbiome, and others as diverse as cardiovascular events and the acute inflammatory response to monosodium urate crystals (gout) are affected by metabolites generated by bacteria in the gut.
The use of germ-free and antibiotic-treated mice in the laboratory, with selective repopulation of their gut microbiome with flora harvested from other strains of mice or selected humans, will continue to teach us much about the role that these microbes and other inhabitants play in controlling normal and disease-disrupted homeostasis. C difficile overgrowth after antibiotic exposure, and the successful treatment of refractory C difficile with fecal transplantation,1 was just the beginning.
The simple writing of a prescription for an antibiotic is a far more complicated and long-lasting affair than most of us have thought.
- Agito MD, Atreja A, Rizk MK. Fecal microbiota transplantation for recurrent C difficile infection: ready for prime time? Cleve Clin J Med 2013; 80(2):101–108. doi:10.3949/ccjm.80a.12110
But, after the results of many recent studies, it turns out I should not have been so comfortable after all. This should not be a surprise. We should never be overly confident with our understanding of anything in clinical practice.
In this issue, Dr. Martin Blaser discusses his work, which supports the hypothesis that the currently increased prevalence of obesity and diabetes is at least in part due to reduced diversity in the gut microbiome. The increased exposure to antibiotics through prescriptions for women before and during pregnancy, as well as perhaps their exposure to antibiotics in the environment, results in changes to the gut and vaginal flora that influence the developing gut and likely other anatomic microbiomes in the neonate and infant. Fascinating research done in mice, utilizing fecal transfer experiments, is building an evidence trail to support the concept that the microbiome plays a major role in the development of childhood and adult obesity, and the gut microbiome is influenced by its exposure to antibiotics, perhaps given years earlier.
Knowledge of the gastrointestinal and other human microbiomes is exploding. I now wonder how many seemingly random clinical events associated with antibiotic use that were not understood and were easily dismissed as stochastic warrant formal study. Some of my patients with rheumatoid arthritis have described flares after eating certain foods and transient remissions or exacerbations after treatment with antibiotics. An epidemiologic study has linked the likelihood of developing childhood inflammatory bowel disease with exposure to antibiotics. Even more fascinating are observations that the microbiota composition (influenced by antibiotics) can influence the outcome of cardiac allografts in a murine model and the response of certain tumors to immune checkpoint inhibitors in murine and human studies. The mechanism may relate to the effects of the microbiome on immune cell activation and migration. Several disorders have been linked to specific bacteria in the gut microbiome, and others as diverse as cardiovascular events and the acute inflammatory response to monosodium urate crystals (gout) are affected by metabolites generated by bacteria in the gut.
The use of germ-free and antibiotic-treated mice in the laboratory, with selective repopulation of their gut microbiome with flora harvested from other strains of mice or selected humans, will continue to teach us much about the role that these microbes and other inhabitants play in controlling normal and disease-disrupted homeostasis. C difficile overgrowth after antibiotic exposure, and the successful treatment of refractory C difficile with fecal transplantation,1 was just the beginning.
The simple writing of a prescription for an antibiotic is a far more complicated and long-lasting affair than most of us have thought.
But, after the results of many recent studies, it turns out I should not have been so comfortable after all. This should not be a surprise. We should never be overly confident with our understanding of anything in clinical practice.
In this issue, Dr. Martin Blaser discusses his work, which supports the hypothesis that the currently increased prevalence of obesity and diabetes is at least in part due to reduced diversity in the gut microbiome. The increased exposure to antibiotics through prescriptions for women before and during pregnancy, as well as perhaps their exposure to antibiotics in the environment, results in changes to the gut and vaginal flora that influence the developing gut and likely other anatomic microbiomes in the neonate and infant. Fascinating research done in mice, utilizing fecal transfer experiments, is building an evidence trail to support the concept that the microbiome plays a major role in the development of childhood and adult obesity, and the gut microbiome is influenced by its exposure to antibiotics, perhaps given years earlier.
Knowledge of the gastrointestinal and other human microbiomes is exploding. I now wonder how many seemingly random clinical events associated with antibiotic use that were not understood and were easily dismissed as stochastic warrant formal study. Some of my patients with rheumatoid arthritis have described flares after eating certain foods and transient remissions or exacerbations after treatment with antibiotics. An epidemiologic study has linked the likelihood of developing childhood inflammatory bowel disease with exposure to antibiotics. Even more fascinating are observations that the microbiota composition (influenced by antibiotics) can influence the outcome of cardiac allografts in a murine model and the response of certain tumors to immune checkpoint inhibitors in murine and human studies. The mechanism may relate to the effects of the microbiome on immune cell activation and migration. Several disorders have been linked to specific bacteria in the gut microbiome, and others as diverse as cardiovascular events and the acute inflammatory response to monosodium urate crystals (gout) are affected by metabolites generated by bacteria in the gut.
The use of germ-free and antibiotic-treated mice in the laboratory, with selective repopulation of their gut microbiome with flora harvested from other strains of mice or selected humans, will continue to teach us much about the role that these microbes and other inhabitants play in controlling normal and disease-disrupted homeostasis. C difficile overgrowth after antibiotic exposure, and the successful treatment of refractory C difficile with fecal transplantation,1 was just the beginning.
The simple writing of a prescription for an antibiotic is a far more complicated and long-lasting affair than most of us have thought.
- Agito MD, Atreja A, Rizk MK. Fecal microbiota transplantation for recurrent C difficile infection: ready for prime time? Cleve Clin J Med 2013; 80(2):101–108. doi:10.3949/ccjm.80a.12110
- Agito MD, Atreja A, Rizk MK. Fecal microbiota transplantation for recurrent C difficile infection: ready for prime time? Cleve Clin J Med 2013; 80(2):101–108. doi:10.3949/ccjm.80a.12110
Physical activity may count more for women who keep the pounds off
NASHVILLE – , according to new analysis of a small study.
Physical activity is a key component in successful maintenance of weight loss, but differential effects of physical activity between men and women had not been well explored, Ann Caldwell, PhD, said in an interview at Obesity Week 2018, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
Dr. Caldwell and her colleagues at the University of Colorado Anschutz Medical Campus, Aurora, conducted a secondary analysis of case-control data of individuals with healthy weight, overweight, or obesity, and those who had successfully maintained weight loss. They compared total daily energy expenditure (TDEE) and physical activity energy expenditure (PAEE), looking at men and women in all three groups separately.
The study included 20 women and 5 men who had successfully maintained a weight loss of at least 13.6 kg for at least 1 year. These were matched with 20 women and 7 men with a body mass index within the healthy range, as controls for the weight loss maintainers at their post–weight loss BMI.
Another group of 22 women and 6 men with BMIs in the overweight or obese category served as controls for the weight loss maintainers at their pre–weight loss BMI.
For all participants, TDEE was measured using the doubly labeled water method for 7 days. This method tracks elimination of a set quantity of ingested water made up of two uncommon isotopes (hydrogen-2 and oxygen-18) to measure energy expenditure. Since the oxygen is lost both as water and carbon dioxide as a result of metabolism, the presence of less oxygen-18 over time indicates a higher total energy expenditure.
Indirect calorimetry was used to measure resting energy expenditure (REE), and energy expenditure related to physical activity was calculated by subtracting REE and a 10% fraction of TDEE (to account for the thermic effect of feeding) from total TDEE.
“There were significant sex-group interactions for TDEE, PAEE, and PAEE/TEE,” said Dr. Caldwell. She explained that the cutoff for statistical significance for the investigators’ analysis was set at P = .1, since sample sizes were so small for men.
For women who were weight-loss maintainers, both PAEE and PAEE/TDEE ratios were higher than for the female healthy-BMI and high-BMI control participants: PAEE was 822 kcal/day for the maintainers, 536 kcal/day for the healthy-BMI, and 669 kcal/day for the high-BMI controls (P less than .01 for both comparisons).
Dr. Caldwell and her colleagues saw no difference when comparing the PAEE/TDEE ratio for women in each of the control groups.
For men, by contrast, PAEE was highest for those with healthy BMIs, at 815 kcal/day, and lowest for those in the high-BMI control group, at 506 kcal/day. Men who were weight loss maintainers fell in the middle, at 772 kcal/day of PAEE. The PAEE/TDEE ratio was significantly higher for both weight loss maintainers and normal-BMI participants than for the high-BMI participants (P less than .07).
“These cross-sectional data suggest potential sex differences in the importance of [physical activity] for successful weight loss maintenance that should be explored further with objective measures,” wrote Dr. Caldwell and her coauthors.
The investigators are planning further work that incorporates objective physical activity data via actigraphy, and that will include a larger sample of men. Through a prospective study that overcomes the limitation of the present study, they hope to develop a clearer picture of sex differences in weight loss maintenance.
The National Institutes of Health supported the study. Dr. Caldwell reported no relevant conflicts of interest.
SOURCE: Caldwell A et al. Obesity Week 2018, Abstract TP-3233.
NASHVILLE – , according to new analysis of a small study.
Physical activity is a key component in successful maintenance of weight loss, but differential effects of physical activity between men and women had not been well explored, Ann Caldwell, PhD, said in an interview at Obesity Week 2018, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
Dr. Caldwell and her colleagues at the University of Colorado Anschutz Medical Campus, Aurora, conducted a secondary analysis of case-control data of individuals with healthy weight, overweight, or obesity, and those who had successfully maintained weight loss. They compared total daily energy expenditure (TDEE) and physical activity energy expenditure (PAEE), looking at men and women in all three groups separately.
The study included 20 women and 5 men who had successfully maintained a weight loss of at least 13.6 kg for at least 1 year. These were matched with 20 women and 7 men with a body mass index within the healthy range, as controls for the weight loss maintainers at their post–weight loss BMI.
Another group of 22 women and 6 men with BMIs in the overweight or obese category served as controls for the weight loss maintainers at their pre–weight loss BMI.
For all participants, TDEE was measured using the doubly labeled water method for 7 days. This method tracks elimination of a set quantity of ingested water made up of two uncommon isotopes (hydrogen-2 and oxygen-18) to measure energy expenditure. Since the oxygen is lost both as water and carbon dioxide as a result of metabolism, the presence of less oxygen-18 over time indicates a higher total energy expenditure.
Indirect calorimetry was used to measure resting energy expenditure (REE), and energy expenditure related to physical activity was calculated by subtracting REE and a 10% fraction of TDEE (to account for the thermic effect of feeding) from total TDEE.
“There were significant sex-group interactions for TDEE, PAEE, and PAEE/TEE,” said Dr. Caldwell. She explained that the cutoff for statistical significance for the investigators’ analysis was set at P = .1, since sample sizes were so small for men.
For women who were weight-loss maintainers, both PAEE and PAEE/TDEE ratios were higher than for the female healthy-BMI and high-BMI control participants: PAEE was 822 kcal/day for the maintainers, 536 kcal/day for the healthy-BMI, and 669 kcal/day for the high-BMI controls (P less than .01 for both comparisons).
Dr. Caldwell and her colleagues saw no difference when comparing the PAEE/TDEE ratio for women in each of the control groups.
For men, by contrast, PAEE was highest for those with healthy BMIs, at 815 kcal/day, and lowest for those in the high-BMI control group, at 506 kcal/day. Men who were weight loss maintainers fell in the middle, at 772 kcal/day of PAEE. The PAEE/TDEE ratio was significantly higher for both weight loss maintainers and normal-BMI participants than for the high-BMI participants (P less than .07).
“These cross-sectional data suggest potential sex differences in the importance of [physical activity] for successful weight loss maintenance that should be explored further with objective measures,” wrote Dr. Caldwell and her coauthors.
The investigators are planning further work that incorporates objective physical activity data via actigraphy, and that will include a larger sample of men. Through a prospective study that overcomes the limitation of the present study, they hope to develop a clearer picture of sex differences in weight loss maintenance.
The National Institutes of Health supported the study. Dr. Caldwell reported no relevant conflicts of interest.
SOURCE: Caldwell A et al. Obesity Week 2018, Abstract TP-3233.
NASHVILLE – , according to new analysis of a small study.
Physical activity is a key component in successful maintenance of weight loss, but differential effects of physical activity between men and women had not been well explored, Ann Caldwell, PhD, said in an interview at Obesity Week 2018, presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
Dr. Caldwell and her colleagues at the University of Colorado Anschutz Medical Campus, Aurora, conducted a secondary analysis of case-control data of individuals with healthy weight, overweight, or obesity, and those who had successfully maintained weight loss. They compared total daily energy expenditure (TDEE) and physical activity energy expenditure (PAEE), looking at men and women in all three groups separately.
The study included 20 women and 5 men who had successfully maintained a weight loss of at least 13.6 kg for at least 1 year. These were matched with 20 women and 7 men with a body mass index within the healthy range, as controls for the weight loss maintainers at their post–weight loss BMI.
Another group of 22 women and 6 men with BMIs in the overweight or obese category served as controls for the weight loss maintainers at their pre–weight loss BMI.
For all participants, TDEE was measured using the doubly labeled water method for 7 days. This method tracks elimination of a set quantity of ingested water made up of two uncommon isotopes (hydrogen-2 and oxygen-18) to measure energy expenditure. Since the oxygen is lost both as water and carbon dioxide as a result of metabolism, the presence of less oxygen-18 over time indicates a higher total energy expenditure.
Indirect calorimetry was used to measure resting energy expenditure (REE), and energy expenditure related to physical activity was calculated by subtracting REE and a 10% fraction of TDEE (to account for the thermic effect of feeding) from total TDEE.
“There were significant sex-group interactions for TDEE, PAEE, and PAEE/TEE,” said Dr. Caldwell. She explained that the cutoff for statistical significance for the investigators’ analysis was set at P = .1, since sample sizes were so small for men.
For women who were weight-loss maintainers, both PAEE and PAEE/TDEE ratios were higher than for the female healthy-BMI and high-BMI control participants: PAEE was 822 kcal/day for the maintainers, 536 kcal/day for the healthy-BMI, and 669 kcal/day for the high-BMI controls (P less than .01 for both comparisons).
Dr. Caldwell and her colleagues saw no difference when comparing the PAEE/TDEE ratio for women in each of the control groups.
For men, by contrast, PAEE was highest for those with healthy BMIs, at 815 kcal/day, and lowest for those in the high-BMI control group, at 506 kcal/day. Men who were weight loss maintainers fell in the middle, at 772 kcal/day of PAEE. The PAEE/TDEE ratio was significantly higher for both weight loss maintainers and normal-BMI participants than for the high-BMI participants (P less than .07).
“These cross-sectional data suggest potential sex differences in the importance of [physical activity] for successful weight loss maintenance that should be explored further with objective measures,” wrote Dr. Caldwell and her coauthors.
The investigators are planning further work that incorporates objective physical activity data via actigraphy, and that will include a larger sample of men. Through a prospective study that overcomes the limitation of the present study, they hope to develop a clearer picture of sex differences in weight loss maintenance.
The National Institutes of Health supported the study. Dr. Caldwell reported no relevant conflicts of interest.
SOURCE: Caldwell A et al. Obesity Week 2018, Abstract TP-3233.
REPORTING FROM OBESITY WEEK 2018
Key clinical point: Women who kept weight off burned more calories in physical activity than did normal or high-BMI controls.
Major finding: In women, the ratio of physical activity energy expenditure to total daily energy expenditure was higher for successful weight-loss maintainers (P less than .01).
Study details: Secondary analysis of case-control study enrolling 80 individuals.
Disclosures: The National Institutes of Health funded the study. Dr. Caldwell reported no conflicts of interest.
Source: Caldwell A et al. Obesity Week 2018, abstract TP-3233.
Routine markers predicted histologic response to obeticholic acid in NASH
Routine clinical and laboratory markers predicted histologic response to obeticholic acid therapy among patients with nonalcoholic steatohepatitis (NASH), investigators reported in Gastroenterology.
In a secondary analysis of data from the FLINT trial, histologic response at treatment week 24 correlated significantly with baseline nonalcoholic fatty liver disease activity score (NAS) greater than 5, baseline triglycerides 154 mg/dL or less, baseline international normalized ratio no greater than 1, baseline aspartate aminotransferase (AST) level no greater than 49 U/L, and at least a 17-U/L decrease from baseline in alanine aminotransferase (ALT) level.
A stepwise logistic regression model including these variables and receipt of obeticholic acid distinguished histologic responders from nonresponders with an area under the receiver operating characteristic curve (AUROC) of 0.83 (95% confidence interval, 0.77-0.89; P less than .0001). These parameters “are readily available clinical and biochemical characteristics that are routinely available to clinicians and may be applied to daily practice,” wrote Rohit Loomba, MD, of the University of California, San Diego, with his associates. They may show “that the patients most likely to achieve histologic response are those with higher disease activity, but still with largely conserved liver function, allowing for potential healing or improvement.”
NASH is expected to become the leading reason for liver transplantation in the next few decades. Several treatments can induce histologic hepatic improvement, but none are approved for NASH. Obeticholic acid (Ocaliva) is a selective agonist of the farsenoid X receptor ligand and is indicated for treating primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA).
In the 72-week, multicenter, randomized, double-blind FLINT trial, noncirrhotic adults with biopsy-confirmed NASH received once-daily obeticholic acid (25 mg) or placebo. Blinded pathologists interpreted biopsies. The primary endpoint (improvement in liver histology) was met in the interim analysis, so the researchers stopped collecting final liver biopsies.
The secondary analysis included all patients with baseline and final biopsies, including 73 histologic responders and 127 nonresponders. “[The] trends for each of the selected predictors was the same when comparing histologic responders to nonresponders, regardless of treatment group (obeticholic acid versus placebo),” the researchers wrote. The predictors are biologically feasible, the researchers contended – for example, high baseline NAS would be more susceptible to significant improvement, while lower baseline triglyceride levels might reflect a liver that “is less burdened by triglyceride secretion” and, therefore, might have greater capacity to heal. Both AST and ALT “are metrics of liver injury,” and lower baseline AST, in combination with greater reduction in ALT at week 24, probably reflected “AST and ALT levels that are closer to normal,” they added.
Nonetheless, the researchers acknowledged several possible sources of bias. Trial participants were recruited from tertiary care settings and had complete biopsy data, which might not reflect the overall NASH population. Overfitting also could have biased the model because the number of variables assessed approached the number of events being predicted. Furthermore, the model assessed no treatment other than obeticholic acid. “A more robust model could potentially be developed if multiple pharmacological interventions could be considered simultaneously,” the researchers noted. The ongoing phase 3 REGENERATE trial aims to confirm the benefit of obeticholic acid in patients with NASH, they added. Topline results are expected in October 2022.
The FLINT trial was funded by Intercept Pharmaceuticals and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Loomba cochaired the FLINT trial protocol writing committee, is on the steering committee of the ongoing REGENERATE trial, and has received research funding from Intercept Pharmaceuticals, which developed and markets obeticholic acid. Several other coinvestigators reported ties to Intercept and to other pharmaceutical companies.
SOURCE: Loomba R et al. Gastroenterology. 2018 Sep 14. doi: 10.1053/j.gastro.2018.09.021.
Routine clinical and laboratory markers predicted histologic response to obeticholic acid therapy among patients with nonalcoholic steatohepatitis (NASH), investigators reported in Gastroenterology.
In a secondary analysis of data from the FLINT trial, histologic response at treatment week 24 correlated significantly with baseline nonalcoholic fatty liver disease activity score (NAS) greater than 5, baseline triglycerides 154 mg/dL or less, baseline international normalized ratio no greater than 1, baseline aspartate aminotransferase (AST) level no greater than 49 U/L, and at least a 17-U/L decrease from baseline in alanine aminotransferase (ALT) level.
A stepwise logistic regression model including these variables and receipt of obeticholic acid distinguished histologic responders from nonresponders with an area under the receiver operating characteristic curve (AUROC) of 0.83 (95% confidence interval, 0.77-0.89; P less than .0001). These parameters “are readily available clinical and biochemical characteristics that are routinely available to clinicians and may be applied to daily practice,” wrote Rohit Loomba, MD, of the University of California, San Diego, with his associates. They may show “that the patients most likely to achieve histologic response are those with higher disease activity, but still with largely conserved liver function, allowing for potential healing or improvement.”
NASH is expected to become the leading reason for liver transplantation in the next few decades. Several treatments can induce histologic hepatic improvement, but none are approved for NASH. Obeticholic acid (Ocaliva) is a selective agonist of the farsenoid X receptor ligand and is indicated for treating primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA).
In the 72-week, multicenter, randomized, double-blind FLINT trial, noncirrhotic adults with biopsy-confirmed NASH received once-daily obeticholic acid (25 mg) or placebo. Blinded pathologists interpreted biopsies. The primary endpoint (improvement in liver histology) was met in the interim analysis, so the researchers stopped collecting final liver biopsies.
The secondary analysis included all patients with baseline and final biopsies, including 73 histologic responders and 127 nonresponders. “[The] trends for each of the selected predictors was the same when comparing histologic responders to nonresponders, regardless of treatment group (obeticholic acid versus placebo),” the researchers wrote. The predictors are biologically feasible, the researchers contended – for example, high baseline NAS would be more susceptible to significant improvement, while lower baseline triglyceride levels might reflect a liver that “is less burdened by triglyceride secretion” and, therefore, might have greater capacity to heal. Both AST and ALT “are metrics of liver injury,” and lower baseline AST, in combination with greater reduction in ALT at week 24, probably reflected “AST and ALT levels that are closer to normal,” they added.
Nonetheless, the researchers acknowledged several possible sources of bias. Trial participants were recruited from tertiary care settings and had complete biopsy data, which might not reflect the overall NASH population. Overfitting also could have biased the model because the number of variables assessed approached the number of events being predicted. Furthermore, the model assessed no treatment other than obeticholic acid. “A more robust model could potentially be developed if multiple pharmacological interventions could be considered simultaneously,” the researchers noted. The ongoing phase 3 REGENERATE trial aims to confirm the benefit of obeticholic acid in patients with NASH, they added. Topline results are expected in October 2022.
The FLINT trial was funded by Intercept Pharmaceuticals and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Loomba cochaired the FLINT trial protocol writing committee, is on the steering committee of the ongoing REGENERATE trial, and has received research funding from Intercept Pharmaceuticals, which developed and markets obeticholic acid. Several other coinvestigators reported ties to Intercept and to other pharmaceutical companies.
SOURCE: Loomba R et al. Gastroenterology. 2018 Sep 14. doi: 10.1053/j.gastro.2018.09.021.
Routine clinical and laboratory markers predicted histologic response to obeticholic acid therapy among patients with nonalcoholic steatohepatitis (NASH), investigators reported in Gastroenterology.
In a secondary analysis of data from the FLINT trial, histologic response at treatment week 24 correlated significantly with baseline nonalcoholic fatty liver disease activity score (NAS) greater than 5, baseline triglycerides 154 mg/dL or less, baseline international normalized ratio no greater than 1, baseline aspartate aminotransferase (AST) level no greater than 49 U/L, and at least a 17-U/L decrease from baseline in alanine aminotransferase (ALT) level.
A stepwise logistic regression model including these variables and receipt of obeticholic acid distinguished histologic responders from nonresponders with an area under the receiver operating characteristic curve (AUROC) of 0.83 (95% confidence interval, 0.77-0.89; P less than .0001). These parameters “are readily available clinical and biochemical characteristics that are routinely available to clinicians and may be applied to daily practice,” wrote Rohit Loomba, MD, of the University of California, San Diego, with his associates. They may show “that the patients most likely to achieve histologic response are those with higher disease activity, but still with largely conserved liver function, allowing for potential healing or improvement.”
NASH is expected to become the leading reason for liver transplantation in the next few decades. Several treatments can induce histologic hepatic improvement, but none are approved for NASH. Obeticholic acid (Ocaliva) is a selective agonist of the farsenoid X receptor ligand and is indicated for treating primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA).
In the 72-week, multicenter, randomized, double-blind FLINT trial, noncirrhotic adults with biopsy-confirmed NASH received once-daily obeticholic acid (25 mg) or placebo. Blinded pathologists interpreted biopsies. The primary endpoint (improvement in liver histology) was met in the interim analysis, so the researchers stopped collecting final liver biopsies.
The secondary analysis included all patients with baseline and final biopsies, including 73 histologic responders and 127 nonresponders. “[The] trends for each of the selected predictors was the same when comparing histologic responders to nonresponders, regardless of treatment group (obeticholic acid versus placebo),” the researchers wrote. The predictors are biologically feasible, the researchers contended – for example, high baseline NAS would be more susceptible to significant improvement, while lower baseline triglyceride levels might reflect a liver that “is less burdened by triglyceride secretion” and, therefore, might have greater capacity to heal. Both AST and ALT “are metrics of liver injury,” and lower baseline AST, in combination with greater reduction in ALT at week 24, probably reflected “AST and ALT levels that are closer to normal,” they added.
Nonetheless, the researchers acknowledged several possible sources of bias. Trial participants were recruited from tertiary care settings and had complete biopsy data, which might not reflect the overall NASH population. Overfitting also could have biased the model because the number of variables assessed approached the number of events being predicted. Furthermore, the model assessed no treatment other than obeticholic acid. “A more robust model could potentially be developed if multiple pharmacological interventions could be considered simultaneously,” the researchers noted. The ongoing phase 3 REGENERATE trial aims to confirm the benefit of obeticholic acid in patients with NASH, they added. Topline results are expected in October 2022.
The FLINT trial was funded by Intercept Pharmaceuticals and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Loomba cochaired the FLINT trial protocol writing committee, is on the steering committee of the ongoing REGENERATE trial, and has received research funding from Intercept Pharmaceuticals, which developed and markets obeticholic acid. Several other coinvestigators reported ties to Intercept and to other pharmaceutical companies.
SOURCE: Loomba R et al. Gastroenterology. 2018 Sep 14. doi: 10.1053/j.gastro.2018.09.021.
FROM GASTROENTEROLOGY
Key clinical point: Routine clinical and laboratory parameters were significant correlates of histologic response to obeticholic acid therapy in patients with nonalcoholic steatohepatitis.
Major finding: Significant predictors included baseline nonalcoholic fatty liver disease activity score (NAS) greater than 5, baseline triglycerides up to 154 mg/dL, baseline international normalized ratio up to 1, baseline aspartate aminotransferase up to 49 U/L, and at least a 17-U/L decrease in alanine aminotransferase at week 24.
Study details: Secondary analysis of data from 200 patients with nonalcoholic steatohepatitis in the randomized, double-blind FLINT trial.
Disclosures: The FLINT trial was funded by Intercept Pharmaceuticals and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Loomba cochaired the FLINT trial protocol writing committee, is on the steering committee of the ongoing REGENERATE trial, and has received research funding from Intercept Pharmaceuticals, which developed and markets obeticholic acid. Several other coinvestigators reported ties to Intercept and to other pharmaceutical companies.
Source: Loomba R et al. Gastroenterology. 2018 Sep 14. doi: 10.1053/j.gastro.2018.09.021.
Bariatric surgery ups risk of suicide, self-harm
NASHVILLE – , according to findings from a meta-analysis presented at the meeting.
Overall, the odds ratio was 1.69 for self-harm or suicide after bariatric surgery (95% confidence interval, 1.62-1.76; P less than .001), “indicating a nearly 70% increase in risk for self-harm or suicide following bariatric surgery,” wrote Dawn Roberts, PhD, of Bradley University, Peoria, Ill., and her coauthor, Nicole Pearl of Washington University, St. Louis, in the poster accompanying the presentation.
Further, as elapsed time from surgery grew, the suicide rate dropped (effect size covariance, r = –0.25). “Thus, the greatest risk for self-harm or suicide appears to emerge in the years immediately following surgery,” Dr. Roberts said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
The investigators had a primary objective of characterizing the association between bariatric surgery and suicide or self-harm. The secondary purpose of the meta-analysis was to find moderators of the association that could explain some of the variability that had previously been seen in studies looking at mental health outcomes after bariatric surgery.
Some of the potential moderators, explained the investigators, included the surgery type. With Roux-en-Y gastric bypass (RYGB), more tissue is removed, potentially causing “more extensive disruption of neural pathways,” the investigators wrote. With greater loss of small-intestine surface area might come more disruption of the gut-brain axis, along with unknown effects on metabolism and pharmacokinetics of psychiatric medication. Additionally, alcohol use disorder might have a more profound effect after gastric bypass surgery.
It had previously been shown that more than two-thirds of suicides happen within the first 3 years after bariatric surgery. With the initial weight loss comes renegotiation of personal relationships, and the potential for more mobility and perhaps expanded career choices; stress accompanies even positive changes in these major life domains. Some patients will also experience weight regain within the first 3 years, after an initial nadir. This also can cause deterioration in quality of life, the investigators explained.
Dr. Roberts and her coinvestigator acknowledge that some of the potential moderators may have been missed in the initial data reporting. For example, the “presurgical sample may be at higher risk for suicide or self-injury but withhold psychiatric history during evaluation for fear of being rejected for surgery.”
From an initial 2,676 studies identified for consideration, investigators in the end extracted data from 28 studies from the United States, Canada, Sweden, and Brazil. The studies had considerable heterogeneity in methods; some included presurgery/postsurgery analyses of the same patients, some had a comparator nonsurgical group, and some used a single postsurgical assessment.
In studies where no nonbariatric comparison sample was available, the investigators assigned interpolated comparison. To arrive at this measure, they drew on the World Health Organization–reported base rate of suicides in the study country at the approximate year of assessment. Suicide was the only interpolated outcome.
Various measures of suicide and self-harm were captured, including completed and probable suicides, suicide attempts, and self-harm events. In some studies, information was drawn from a suicide-specific questionnaire, or from a suicide item on another type of questionnaire.
There was significant variability in the odds ratios for suicide or self-harm across studies, Dr. Roberts said.
The researchers plan to continue analyzing additional measures captured in the meta-analysis, such as gender, age, initial body mass index, surgery type, and the percent of excess weight lost at the time of assessment for suicide or self-harm risk. They reported no outside sources of funding, and no conflicts of interest.
SOURCE: Roberts, D et al. Obesity Week 2018, Poster A433.
NASHVILLE – , according to findings from a meta-analysis presented at the meeting.
Overall, the odds ratio was 1.69 for self-harm or suicide after bariatric surgery (95% confidence interval, 1.62-1.76; P less than .001), “indicating a nearly 70% increase in risk for self-harm or suicide following bariatric surgery,” wrote Dawn Roberts, PhD, of Bradley University, Peoria, Ill., and her coauthor, Nicole Pearl of Washington University, St. Louis, in the poster accompanying the presentation.
Further, as elapsed time from surgery grew, the suicide rate dropped (effect size covariance, r = –0.25). “Thus, the greatest risk for self-harm or suicide appears to emerge in the years immediately following surgery,” Dr. Roberts said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
The investigators had a primary objective of characterizing the association between bariatric surgery and suicide or self-harm. The secondary purpose of the meta-analysis was to find moderators of the association that could explain some of the variability that had previously been seen in studies looking at mental health outcomes after bariatric surgery.
Some of the potential moderators, explained the investigators, included the surgery type. With Roux-en-Y gastric bypass (RYGB), more tissue is removed, potentially causing “more extensive disruption of neural pathways,” the investigators wrote. With greater loss of small-intestine surface area might come more disruption of the gut-brain axis, along with unknown effects on metabolism and pharmacokinetics of psychiatric medication. Additionally, alcohol use disorder might have a more profound effect after gastric bypass surgery.
It had previously been shown that more than two-thirds of suicides happen within the first 3 years after bariatric surgery. With the initial weight loss comes renegotiation of personal relationships, and the potential for more mobility and perhaps expanded career choices; stress accompanies even positive changes in these major life domains. Some patients will also experience weight regain within the first 3 years, after an initial nadir. This also can cause deterioration in quality of life, the investigators explained.
Dr. Roberts and her coinvestigator acknowledge that some of the potential moderators may have been missed in the initial data reporting. For example, the “presurgical sample may be at higher risk for suicide or self-injury but withhold psychiatric history during evaluation for fear of being rejected for surgery.”
From an initial 2,676 studies identified for consideration, investigators in the end extracted data from 28 studies from the United States, Canada, Sweden, and Brazil. The studies had considerable heterogeneity in methods; some included presurgery/postsurgery analyses of the same patients, some had a comparator nonsurgical group, and some used a single postsurgical assessment.
In studies where no nonbariatric comparison sample was available, the investigators assigned interpolated comparison. To arrive at this measure, they drew on the World Health Organization–reported base rate of suicides in the study country at the approximate year of assessment. Suicide was the only interpolated outcome.
Various measures of suicide and self-harm were captured, including completed and probable suicides, suicide attempts, and self-harm events. In some studies, information was drawn from a suicide-specific questionnaire, or from a suicide item on another type of questionnaire.
There was significant variability in the odds ratios for suicide or self-harm across studies, Dr. Roberts said.
The researchers plan to continue analyzing additional measures captured in the meta-analysis, such as gender, age, initial body mass index, surgery type, and the percent of excess weight lost at the time of assessment for suicide or self-harm risk. They reported no outside sources of funding, and no conflicts of interest.
SOURCE: Roberts, D et al. Obesity Week 2018, Poster A433.
NASHVILLE – , according to findings from a meta-analysis presented at the meeting.
Overall, the odds ratio was 1.69 for self-harm or suicide after bariatric surgery (95% confidence interval, 1.62-1.76; P less than .001), “indicating a nearly 70% increase in risk for self-harm or suicide following bariatric surgery,” wrote Dawn Roberts, PhD, of Bradley University, Peoria, Ill., and her coauthor, Nicole Pearl of Washington University, St. Louis, in the poster accompanying the presentation.
Further, as elapsed time from surgery grew, the suicide rate dropped (effect size covariance, r = –0.25). “Thus, the greatest risk for self-harm or suicide appears to emerge in the years immediately following surgery,” Dr. Roberts said at the meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery.
The investigators had a primary objective of characterizing the association between bariatric surgery and suicide or self-harm. The secondary purpose of the meta-analysis was to find moderators of the association that could explain some of the variability that had previously been seen in studies looking at mental health outcomes after bariatric surgery.
Some of the potential moderators, explained the investigators, included the surgery type. With Roux-en-Y gastric bypass (RYGB), more tissue is removed, potentially causing “more extensive disruption of neural pathways,” the investigators wrote. With greater loss of small-intestine surface area might come more disruption of the gut-brain axis, along with unknown effects on metabolism and pharmacokinetics of psychiatric medication. Additionally, alcohol use disorder might have a more profound effect after gastric bypass surgery.
It had previously been shown that more than two-thirds of suicides happen within the first 3 years after bariatric surgery. With the initial weight loss comes renegotiation of personal relationships, and the potential for more mobility and perhaps expanded career choices; stress accompanies even positive changes in these major life domains. Some patients will also experience weight regain within the first 3 years, after an initial nadir. This also can cause deterioration in quality of life, the investigators explained.
Dr. Roberts and her coinvestigator acknowledge that some of the potential moderators may have been missed in the initial data reporting. For example, the “presurgical sample may be at higher risk for suicide or self-injury but withhold psychiatric history during evaluation for fear of being rejected for surgery.”
From an initial 2,676 studies identified for consideration, investigators in the end extracted data from 28 studies from the United States, Canada, Sweden, and Brazil. The studies had considerable heterogeneity in methods; some included presurgery/postsurgery analyses of the same patients, some had a comparator nonsurgical group, and some used a single postsurgical assessment.
In studies where no nonbariatric comparison sample was available, the investigators assigned interpolated comparison. To arrive at this measure, they drew on the World Health Organization–reported base rate of suicides in the study country at the approximate year of assessment. Suicide was the only interpolated outcome.
Various measures of suicide and self-harm were captured, including completed and probable suicides, suicide attempts, and self-harm events. In some studies, information was drawn from a suicide-specific questionnaire, or from a suicide item on another type of questionnaire.
There was significant variability in the odds ratios for suicide or self-harm across studies, Dr. Roberts said.
The researchers plan to continue analyzing additional measures captured in the meta-analysis, such as gender, age, initial body mass index, surgery type, and the percent of excess weight lost at the time of assessment for suicide or self-harm risk. They reported no outside sources of funding, and no conflicts of interest.
SOURCE: Roberts, D et al. Obesity Week 2018, Poster A433.
REPORTING FROM OBESITY WEEK 2018
Key clinical point: Bariatric surgery patients have an elevated risk for suicide or self-harm.
Major finding: The odds ratio for suicide or self-harm was 1.69 after bariatric surgery.
Study details: Meta-analysis of 28 studies of bariatric surgery patients.
Disclosures: The authors reported no conflicts of interest and no outside sources of funding.
Source: Roberts D et al. Obesity Week 2018, Poster A433.
ADA releases guidelines for type 2 diabetes in children, youth
The American Diabetes Association’s guidelines for the evaluation and management of pediatric patients with type 2 diabetes differ from those for adults.
“Puberty-related physiologic insulin resistance, particularly in obese youth, may play a role” in the fact that youth are more insulin resistant than adults. Also, type 2 diabetes apparently is “more aggressive in youth than adults, with a faster rate of deterioration of beta-cell function and poorer response to glucose-lowering medications,” wrote Silva Arslanian, MD, from the division of pediatric endocrinology, metabolism, and diabetes mellitus at the University of Pittsburgh, and her colleagues. “Even though our knowledge of youth-onset type 2 diabetes has increased tremendously over the last 2 decades, robust and evidence-based data are still limited regarding diagnostic and therapeutic approaches and prevention of complications.”
The ADA position statement by Dr. Arslanian and her colleagues outlines management of type 2 diabetes in children and youth.
Diagnosis
and repeat testing should occur at least every 3 years for these patients. Pancreatic autoantibody tests should also be considered in this patient population to rule out autoimmune type 1 diabetes, and genetic evaluation should be performed to test for monogenic diabetes, “based on clinical characteristics and presentation,” they wrote.
Use fasting plasma glucose, 2-hour fasting plasma glucose after a 75-g oral glucose tolerance test, or glycosylated hemoglobin (HbA1C) to test for diabetes or prediabetes. Also consider factors like medication adherence and treatment effects when prescribing glucose-lowering or other medications for overweight or obese children and adolescents with type 2 diabetes.
Lifestyle management
With regard to lifestyle management programs, the intervention should be introduced as a part of diabetes care – aimed at reducing between 7% and 10% of body weight – and be based on a chronic care model. The intervention should include 30-60 minutes of moderate to intense physical activity for 5 days each week, strength training 3 days per week, and incorporate healthy eating plans. Dr. Arslanian and her associates noted there was limited evidence for pharmacotherapy for weight reduction in children and adolescents with type 2 diabetes.
Pharmacologic therapy
Pharmacologic therapy should be started together with lifestyle therapy once a diagnosis is made, according to the recommendations.
Metformin is the preferred initial pharmacologic treatment for patients with normal renal function who are asymptomatic and with HbA1C levels of less than 8.5%.
Patients with blood glucose greater than or equal to 250 mg/dL and HbA1C greater than or equal to 8.5% with symptoms such as weight loss, polydipsia, polyuria, or nocturia should receive basal insulin during initiation and titration of metformin.
Patients with ketosis or ketoacidosis should receive intravenous insulin to address hyperglycemia. Once the acidosis is corrected, initiate metformin with subcutaneous insulin therapy. For patients who are reaching home-based glucose monitoring targets, consider tapering the dose over 2-6 weeks with a 10%-30% reduction in insulin every few days.
In patients where metformin alone is not meeting the glycemic target, consider basal insulin therapy and, if that fails to help achieve glycemic targets, more intensive approaches should be considered, such as metabolic surgery.
Jay Cohen, MD, FACE, medical director at the Endocrine Clinic in Memphis, said in an interview that he agreed with the ADA position statement except for the pharmacologic therapy recommendations.
“The pharmacology therapy is 4 years outdated,” he said. “We routinely use all of the medications that are not Food and Drug Administration [approved] for kids, but are FDA approved for adults.”
He also questioned the ADA’s recommendation to give basal insulin to patients who are insulin resistant.
“Why give insulin if these people are insulin resistant?” said Dr. Cohen, who also is a Clinical Endocrinology News editorial board member. “The oral and injectable noninsulins work fabulously with less weight gain – already a problem for these patients – and less hypoglycemia, less side effects, and better compliance.”
Treatment goals
The HbA1C goal for children and adolescents with type 2 diabetes is less than 7% when treated with oral agents alone. HbA1C should be tested every 3 months and should be individualized, according to the ADA recommendations. In some patients, such as those with a shorter diabetes duration, lesser degrees of beta-cell dysfunction, and those who achieve significant weight improvement through lifestyle changes or taking metformin, consider lowering the HbA1C goal to less than 6.5%.
Give individualized care with regard to home self-monitoring of blood glucose. Also provide patients and their families with “culturally competent” diabetes self-management tools and lifestyle programs. Consider social factors such as housing stability, food insecurity, and financial barriers when making treatment decisions.
Screening for complications
To screen for nephropathy, take BP measurements at every visit and promote lifestyle management to reduce risk of diabetic kidney disease and improve weight loss. After 6 months, if a patient’s BP remains greater than the 95th percentile for their age, gender, and height, ACE inhibitors, or angiotensin receptor blockers are initial therapeutic options, according to the position statement. Other BP-lowering treatments may be added as necessary.
Also monitor protein intake (0.8 g/kg per day) as well as urine albumin/creatinine ratio (UACR) and estimated glomerular filtration rate (eGFR) annually. Patients with diabetes and hypertension who are not pregnant should receive an ACE inhibitor or angiotensin receptor blocker if their UACR is modestly elevated (30-299 mg/g creatinine). Such a regimen is strongly recommended if their UACR is above 300 mg/g creatinine and/or if their eGFR is less than 60 mL/min per 1.73 m2.
Screening issues
When considering diabetes distress and mental or behavioral health in children and adolescents with type 2 diabetes, use standardized and validated tools to assess symptoms such as depression and disordered eating behaviors. Regularly screen for smoking and alcohol use and provide preconception counseling for female patients of child-bearing age.
Screen for neuropathy, retinopathy, and nonalcoholic fatty liver disease annually and for obstructive sleep apnea at each visit. Lipid testing should be performed annually once patients have achieved glycemic control. Polycystic ovary syndrome should be considered in female patients with type 2 diabetes and treated with metformin together with lifestyle changes to address menstrual cyclicity and hyperandrogenism, the authors recommended.
Dr. Arslanian is on a data monitoring committee for AstraZeneca; data safety monitoring board for Boehringer Ingelheim; and advisory boards for Eli Lilly, Novo Nordisk, and Sanofi-Aventis; and has received research grants from Eli Lilly and Novo Nordisk. Other authors reported various relationships with a number of pharmaceutical companies.
SOURCE: Arslanian S et al. Diabetes Care. 2018 Nov 13. doi: 10.2337/dci18-0052.
The American Diabetes Association’s guidelines for the evaluation and management of pediatric patients with type 2 diabetes differ from those for adults.
“Puberty-related physiologic insulin resistance, particularly in obese youth, may play a role” in the fact that youth are more insulin resistant than adults. Also, type 2 diabetes apparently is “more aggressive in youth than adults, with a faster rate of deterioration of beta-cell function and poorer response to glucose-lowering medications,” wrote Silva Arslanian, MD, from the division of pediatric endocrinology, metabolism, and diabetes mellitus at the University of Pittsburgh, and her colleagues. “Even though our knowledge of youth-onset type 2 diabetes has increased tremendously over the last 2 decades, robust and evidence-based data are still limited regarding diagnostic and therapeutic approaches and prevention of complications.”
The ADA position statement by Dr. Arslanian and her colleagues outlines management of type 2 diabetes in children and youth.
Diagnosis
and repeat testing should occur at least every 3 years for these patients. Pancreatic autoantibody tests should also be considered in this patient population to rule out autoimmune type 1 diabetes, and genetic evaluation should be performed to test for monogenic diabetes, “based on clinical characteristics and presentation,” they wrote.
Use fasting plasma glucose, 2-hour fasting plasma glucose after a 75-g oral glucose tolerance test, or glycosylated hemoglobin (HbA1C) to test for diabetes or prediabetes. Also consider factors like medication adherence and treatment effects when prescribing glucose-lowering or other medications for overweight or obese children and adolescents with type 2 diabetes.
Lifestyle management
With regard to lifestyle management programs, the intervention should be introduced as a part of diabetes care – aimed at reducing between 7% and 10% of body weight – and be based on a chronic care model. The intervention should include 30-60 minutes of moderate to intense physical activity for 5 days each week, strength training 3 days per week, and incorporate healthy eating plans. Dr. Arslanian and her associates noted there was limited evidence for pharmacotherapy for weight reduction in children and adolescents with type 2 diabetes.
Pharmacologic therapy
Pharmacologic therapy should be started together with lifestyle therapy once a diagnosis is made, according to the recommendations.
Metformin is the preferred initial pharmacologic treatment for patients with normal renal function who are asymptomatic and with HbA1C levels of less than 8.5%.
Patients with blood glucose greater than or equal to 250 mg/dL and HbA1C greater than or equal to 8.5% with symptoms such as weight loss, polydipsia, polyuria, or nocturia should receive basal insulin during initiation and titration of metformin.
Patients with ketosis or ketoacidosis should receive intravenous insulin to address hyperglycemia. Once the acidosis is corrected, initiate metformin with subcutaneous insulin therapy. For patients who are reaching home-based glucose monitoring targets, consider tapering the dose over 2-6 weeks with a 10%-30% reduction in insulin every few days.
In patients where metformin alone is not meeting the glycemic target, consider basal insulin therapy and, if that fails to help achieve glycemic targets, more intensive approaches should be considered, such as metabolic surgery.
Jay Cohen, MD, FACE, medical director at the Endocrine Clinic in Memphis, said in an interview that he agreed with the ADA position statement except for the pharmacologic therapy recommendations.
“The pharmacology therapy is 4 years outdated,” he said. “We routinely use all of the medications that are not Food and Drug Administration [approved] for kids, but are FDA approved for adults.”
He also questioned the ADA’s recommendation to give basal insulin to patients who are insulin resistant.
“Why give insulin if these people are insulin resistant?” said Dr. Cohen, who also is a Clinical Endocrinology News editorial board member. “The oral and injectable noninsulins work fabulously with less weight gain – already a problem for these patients – and less hypoglycemia, less side effects, and better compliance.”
Treatment goals
The HbA1C goal for children and adolescents with type 2 diabetes is less than 7% when treated with oral agents alone. HbA1C should be tested every 3 months and should be individualized, according to the ADA recommendations. In some patients, such as those with a shorter diabetes duration, lesser degrees of beta-cell dysfunction, and those who achieve significant weight improvement through lifestyle changes or taking metformin, consider lowering the HbA1C goal to less than 6.5%.
Give individualized care with regard to home self-monitoring of blood glucose. Also provide patients and their families with “culturally competent” diabetes self-management tools and lifestyle programs. Consider social factors such as housing stability, food insecurity, and financial barriers when making treatment decisions.
Screening for complications
To screen for nephropathy, take BP measurements at every visit and promote lifestyle management to reduce risk of diabetic kidney disease and improve weight loss. After 6 months, if a patient’s BP remains greater than the 95th percentile for their age, gender, and height, ACE inhibitors, or angiotensin receptor blockers are initial therapeutic options, according to the position statement. Other BP-lowering treatments may be added as necessary.
Also monitor protein intake (0.8 g/kg per day) as well as urine albumin/creatinine ratio (UACR) and estimated glomerular filtration rate (eGFR) annually. Patients with diabetes and hypertension who are not pregnant should receive an ACE inhibitor or angiotensin receptor blocker if their UACR is modestly elevated (30-299 mg/g creatinine). Such a regimen is strongly recommended if their UACR is above 300 mg/g creatinine and/or if their eGFR is less than 60 mL/min per 1.73 m2.
Screening issues
When considering diabetes distress and mental or behavioral health in children and adolescents with type 2 diabetes, use standardized and validated tools to assess symptoms such as depression and disordered eating behaviors. Regularly screen for smoking and alcohol use and provide preconception counseling for female patients of child-bearing age.
Screen for neuropathy, retinopathy, and nonalcoholic fatty liver disease annually and for obstructive sleep apnea at each visit. Lipid testing should be performed annually once patients have achieved glycemic control. Polycystic ovary syndrome should be considered in female patients with type 2 diabetes and treated with metformin together with lifestyle changes to address menstrual cyclicity and hyperandrogenism, the authors recommended.
Dr. Arslanian is on a data monitoring committee for AstraZeneca; data safety monitoring board for Boehringer Ingelheim; and advisory boards for Eli Lilly, Novo Nordisk, and Sanofi-Aventis; and has received research grants from Eli Lilly and Novo Nordisk. Other authors reported various relationships with a number of pharmaceutical companies.
SOURCE: Arslanian S et al. Diabetes Care. 2018 Nov 13. doi: 10.2337/dci18-0052.
The American Diabetes Association’s guidelines for the evaluation and management of pediatric patients with type 2 diabetes differ from those for adults.
“Puberty-related physiologic insulin resistance, particularly in obese youth, may play a role” in the fact that youth are more insulin resistant than adults. Also, type 2 diabetes apparently is “more aggressive in youth than adults, with a faster rate of deterioration of beta-cell function and poorer response to glucose-lowering medications,” wrote Silva Arslanian, MD, from the division of pediatric endocrinology, metabolism, and diabetes mellitus at the University of Pittsburgh, and her colleagues. “Even though our knowledge of youth-onset type 2 diabetes has increased tremendously over the last 2 decades, robust and evidence-based data are still limited regarding diagnostic and therapeutic approaches and prevention of complications.”
The ADA position statement by Dr. Arslanian and her colleagues outlines management of type 2 diabetes in children and youth.
Diagnosis
and repeat testing should occur at least every 3 years for these patients. Pancreatic autoantibody tests should also be considered in this patient population to rule out autoimmune type 1 diabetes, and genetic evaluation should be performed to test for monogenic diabetes, “based on clinical characteristics and presentation,” they wrote.
Use fasting plasma glucose, 2-hour fasting plasma glucose after a 75-g oral glucose tolerance test, or glycosylated hemoglobin (HbA1C) to test for diabetes or prediabetes. Also consider factors like medication adherence and treatment effects when prescribing glucose-lowering or other medications for overweight or obese children and adolescents with type 2 diabetes.
Lifestyle management
With regard to lifestyle management programs, the intervention should be introduced as a part of diabetes care – aimed at reducing between 7% and 10% of body weight – and be based on a chronic care model. The intervention should include 30-60 minutes of moderate to intense physical activity for 5 days each week, strength training 3 days per week, and incorporate healthy eating plans. Dr. Arslanian and her associates noted there was limited evidence for pharmacotherapy for weight reduction in children and adolescents with type 2 diabetes.
Pharmacologic therapy
Pharmacologic therapy should be started together with lifestyle therapy once a diagnosis is made, according to the recommendations.
Metformin is the preferred initial pharmacologic treatment for patients with normal renal function who are asymptomatic and with HbA1C levels of less than 8.5%.
Patients with blood glucose greater than or equal to 250 mg/dL and HbA1C greater than or equal to 8.5% with symptoms such as weight loss, polydipsia, polyuria, or nocturia should receive basal insulin during initiation and titration of metformin.
Patients with ketosis or ketoacidosis should receive intravenous insulin to address hyperglycemia. Once the acidosis is corrected, initiate metformin with subcutaneous insulin therapy. For patients who are reaching home-based glucose monitoring targets, consider tapering the dose over 2-6 weeks with a 10%-30% reduction in insulin every few days.
In patients where metformin alone is not meeting the glycemic target, consider basal insulin therapy and, if that fails to help achieve glycemic targets, more intensive approaches should be considered, such as metabolic surgery.
Jay Cohen, MD, FACE, medical director at the Endocrine Clinic in Memphis, said in an interview that he agreed with the ADA position statement except for the pharmacologic therapy recommendations.
“The pharmacology therapy is 4 years outdated,” he said. “We routinely use all of the medications that are not Food and Drug Administration [approved] for kids, but are FDA approved for adults.”
He also questioned the ADA’s recommendation to give basal insulin to patients who are insulin resistant.
“Why give insulin if these people are insulin resistant?” said Dr. Cohen, who also is a Clinical Endocrinology News editorial board member. “The oral and injectable noninsulins work fabulously with less weight gain – already a problem for these patients – and less hypoglycemia, less side effects, and better compliance.”
Treatment goals
The HbA1C goal for children and adolescents with type 2 diabetes is less than 7% when treated with oral agents alone. HbA1C should be tested every 3 months and should be individualized, according to the ADA recommendations. In some patients, such as those with a shorter diabetes duration, lesser degrees of beta-cell dysfunction, and those who achieve significant weight improvement through lifestyle changes or taking metformin, consider lowering the HbA1C goal to less than 6.5%.
Give individualized care with regard to home self-monitoring of blood glucose. Also provide patients and their families with “culturally competent” diabetes self-management tools and lifestyle programs. Consider social factors such as housing stability, food insecurity, and financial barriers when making treatment decisions.
Screening for complications
To screen for nephropathy, take BP measurements at every visit and promote lifestyle management to reduce risk of diabetic kidney disease and improve weight loss. After 6 months, if a patient’s BP remains greater than the 95th percentile for their age, gender, and height, ACE inhibitors, or angiotensin receptor blockers are initial therapeutic options, according to the position statement. Other BP-lowering treatments may be added as necessary.
Also monitor protein intake (0.8 g/kg per day) as well as urine albumin/creatinine ratio (UACR) and estimated glomerular filtration rate (eGFR) annually. Patients with diabetes and hypertension who are not pregnant should receive an ACE inhibitor or angiotensin receptor blocker if their UACR is modestly elevated (30-299 mg/g creatinine). Such a regimen is strongly recommended if their UACR is above 300 mg/g creatinine and/or if their eGFR is less than 60 mL/min per 1.73 m2.
Screening issues
When considering diabetes distress and mental or behavioral health in children and adolescents with type 2 diabetes, use standardized and validated tools to assess symptoms such as depression and disordered eating behaviors. Regularly screen for smoking and alcohol use and provide preconception counseling for female patients of child-bearing age.
Screen for neuropathy, retinopathy, and nonalcoholic fatty liver disease annually and for obstructive sleep apnea at each visit. Lipid testing should be performed annually once patients have achieved glycemic control. Polycystic ovary syndrome should be considered in female patients with type 2 diabetes and treated with metformin together with lifestyle changes to address menstrual cyclicity and hyperandrogenism, the authors recommended.
Dr. Arslanian is on a data monitoring committee for AstraZeneca; data safety monitoring board for Boehringer Ingelheim; and advisory boards for Eli Lilly, Novo Nordisk, and Sanofi-Aventis; and has received research grants from Eli Lilly and Novo Nordisk. Other authors reported various relationships with a number of pharmaceutical companies.
SOURCE: Arslanian S et al. Diabetes Care. 2018 Nov 13. doi: 10.2337/dci18-0052.
FROM DIABETES CARE
New HHS physical activity guidelines break fresh ground
CHICAGO – The newly released comprehensive second edition of the federal physical activity guidelines have a lofty goal.
Adm. Brett P. Giroir, MD, declared in introducing the recommendations at the American Heart Association scientific sessions.
“Physical activity is one of the most effective preventive health interventions available, and we need more emphasis on prevention as we transition to a value-based reimbursement structure that rewards better health maintenance and avoids chronic conditions,” added Adm. Giroir, assistant secretary for health at the U.S. Department of Health & Human Services.
Although the agency opted to unveil the new guidelines before an audience of cardiologists at the AHA scientific sessions, the report includes sections relevant for a wide range of medical specialists, including primary care physicians, pediatricians, psychiatrists, neurologists, endocrinologists, and geriatricians.
Before launching into a description of what’s new in the second edition, Adm. Giroir set the stage with blunt talk about the nation’s poor state of physical fitness.
“Inactivity causes 10% of premature mortality in the United States. That means if we can just get 25% of inactive people to be active and meet the recommendations, almost 75,000 deaths per year would be prevented in the United States. And on an even larger scale worldwide, if 25% of those same people who are inactive started moving and met the guidelines, more than 1.3 million deaths would be prevented,” according to Adm. Giroir.
At present, only 26% of men, 19% of women, and 20% of teenagers meet the physical activity recommendations.
Failure to meet the federal aerobic physical activity recommendations accounts for an estimated nearly $117 billion in annual health care costs. And it poses a national security threat, too: Nearly one-third of all 17- to 24-year-olds are disqualified from military service because of obesity. Even more eye-opening, he continued, is that fully 71% of all 17- to 24-year-olds are ineligible for military service because of obesity, lack of physical fitness, lack of education, or substance use.
The actual recommendations contained in the second edition of the Physical Activity Guidelines for Americans remain unchanged from those in the first, issued a decade earlier. That is, in order to gain substantial health benefits, adults and adolescents should engage in at least 150-300 min/week of moderate intensity aerobic physical activity or 75-150 min/week of vigorous intensity aerobic activity. Plus they should do muscle-strengthening exercises such as weight lifting or push-ups at moderate or greater intensity at least 2 days/week.
Asked why the guidelines are sticking with time-based physical activity recommendations in an era when popular smartwatches and other mobile devices can readily spit out number of steps walked, calories burned, and heart-rate data, cardiologist William E. Kraus, MD, one of the 17 members of the scientific advisory committee who reviewed and graded the scientific evidence on physical activity, sedentary behavior, and health, answered. He said the group’s careful review concluded that “there’s just not enough evidence at this time to make a recommendation” with regard to mobile device–based measurements of physical activity and their relationship with health benefits.
“We’re hoping to spur more research in this area, so that the next time we make recommendations, that can be incorporated,” added Dr. Kraus, a professor of medicine and cardiologist at Duke University, Durham, N.C., as well as president-elect of the American College of Sports Medicine.
What’s new in the guidelines
“This edition tells us that it’s easier to meet the recommendations in the physical activity guidelines,” according to Adm. Giroir. “The new guidelines demonstrate, based on the best science, everyone can dramatically improve their health just by moving: anytime, anywhere, and by any means that gets you active.” He broke the guidelines down as follows:
- We have new evidence about the risks of sedentary behavior, and new evidence that any amount – any amount – of moderate to vigorous physical activity, like walking, dancing, line dancing if you’re from Texas, and household chores is beneficial,” Adm. Giroir observed.
- While the first edition of the federal guidelines cited strong evidence that physical activity reduces the risk of two types of cancer, breast and colon, the intervening decade has brought forth strong evidence of a protective effect against an additional six types of cancer: bladder, endometrial, kidney, stomach, esophageal, and lung cancer.
- The guidelines formulate for the first time physical activity standards for children aged 3-5 years. The recommended target is at least 3 hr/day of varied physical activity, consistent with existing guidelines in Australia Canada, and the United Kingdom.
- Updated recommendations for children aged 6-17 years call for an hour or more/day of moderate- or vigorous-intensity physical activity on a daily basis, with that activity level falling within the vigorous category on at least 3 days/week. Plus, it recommends bone- and muscle-strengthening activity on at least 3 days.
- The pediatric guidelines are linked to a planned HHS national strategy to expand children’s participation in youth sports as part of an effort to curb childhood obesity, rates of which have more than tripled since the 1970s.
“We’ll soon announce funding opportunities for communities to increase participation in sports among children and teens through participation in affordable programs with trained coaches,” said Dr. Giroir, a pediatrician.
The new guidelines endorse what is called “the comprehensive school physical activity model.”
“I strongly believe our schools should take action to implement this approach. There is a lot of interest right now to affect change in the schools across our country. Part of the answer, I think, is to provide kids with high-quality physical education, but I think we recognize that alone will not be enough.” The comprehensive school activity model calls for not only enriching school PE programs but also incorporating active transport to school, classroom activity, active learning, and after school programs – activity in all settings during the school day. “I’m very hopeful that this model, which is endorsed in the guidelines document, will be widely adopted by schools in this country over the next decade,” Dr. Giroir said.
The first edition declared that only bouts of physical activity of at least 10 minutes duration should count toward meeting the guidelines. That requirement is gone in the second edition. It was an impediment to being active, and upon close examination it wasn’t based on scientific evidence. That means taking the stairs instead of the escalator or parking farther away from the store count toward the weekly physical activity goal, Dr. Kraus said.
“It makes it easier to achieve the guidelines and to encourage Americans to move more and sit less just by making a better choice at many times during the day,” observed Dr. Giroir, a four-star admiral in the U.S. Public Health Service Commissioned Corps.
The latest guidelines contain up-to-date information on the benefits of regular physical activity in terms of brain health, including reduced risk of developing Alzheimer’s disease, and improved cognition, including performance on academic achievement tests and measures of executive function, memory, and processing speed, in preadolescent children as well as older adults. Solid evidence also is cited for improved cognition in patients with MS, dementia, ADHD, and Parkinson’s disease.
The guidelines provide new recommendations for physical activity for women during pregnancy and post partum.
A section of the guidelines is devoted to proven evidence-based strategies to promote physical activity at the individual, small group, and community level.
Physicians now have a resource to aid them in prescribing an individualized physical activity prescription for their patients with existing health conditions, including osteoarthritis, type 2 diabetes, cancer survivors, and physical disabilities.
The new physical activity guidelines and related resources for health care professionals are available at the Health.gov website.
SOURCE: Giroir BP. AHA Scientific Sessions, Session ME.05.
CHICAGO – The newly released comprehensive second edition of the federal physical activity guidelines have a lofty goal.
Adm. Brett P. Giroir, MD, declared in introducing the recommendations at the American Heart Association scientific sessions.
“Physical activity is one of the most effective preventive health interventions available, and we need more emphasis on prevention as we transition to a value-based reimbursement structure that rewards better health maintenance and avoids chronic conditions,” added Adm. Giroir, assistant secretary for health at the U.S. Department of Health & Human Services.
Although the agency opted to unveil the new guidelines before an audience of cardiologists at the AHA scientific sessions, the report includes sections relevant for a wide range of medical specialists, including primary care physicians, pediatricians, psychiatrists, neurologists, endocrinologists, and geriatricians.
Before launching into a description of what’s new in the second edition, Adm. Giroir set the stage with blunt talk about the nation’s poor state of physical fitness.
“Inactivity causes 10% of premature mortality in the United States. That means if we can just get 25% of inactive people to be active and meet the recommendations, almost 75,000 deaths per year would be prevented in the United States. And on an even larger scale worldwide, if 25% of those same people who are inactive started moving and met the guidelines, more than 1.3 million deaths would be prevented,” according to Adm. Giroir.
At present, only 26% of men, 19% of women, and 20% of teenagers meet the physical activity recommendations.
Failure to meet the federal aerobic physical activity recommendations accounts for an estimated nearly $117 billion in annual health care costs. And it poses a national security threat, too: Nearly one-third of all 17- to 24-year-olds are disqualified from military service because of obesity. Even more eye-opening, he continued, is that fully 71% of all 17- to 24-year-olds are ineligible for military service because of obesity, lack of physical fitness, lack of education, or substance use.
The actual recommendations contained in the second edition of the Physical Activity Guidelines for Americans remain unchanged from those in the first, issued a decade earlier. That is, in order to gain substantial health benefits, adults and adolescents should engage in at least 150-300 min/week of moderate intensity aerobic physical activity or 75-150 min/week of vigorous intensity aerobic activity. Plus they should do muscle-strengthening exercises such as weight lifting or push-ups at moderate or greater intensity at least 2 days/week.
Asked why the guidelines are sticking with time-based physical activity recommendations in an era when popular smartwatches and other mobile devices can readily spit out number of steps walked, calories burned, and heart-rate data, cardiologist William E. Kraus, MD, one of the 17 members of the scientific advisory committee who reviewed and graded the scientific evidence on physical activity, sedentary behavior, and health, answered. He said the group’s careful review concluded that “there’s just not enough evidence at this time to make a recommendation” with regard to mobile device–based measurements of physical activity and their relationship with health benefits.
“We’re hoping to spur more research in this area, so that the next time we make recommendations, that can be incorporated,” added Dr. Kraus, a professor of medicine and cardiologist at Duke University, Durham, N.C., as well as president-elect of the American College of Sports Medicine.
What’s new in the guidelines
“This edition tells us that it’s easier to meet the recommendations in the physical activity guidelines,” according to Adm. Giroir. “The new guidelines demonstrate, based on the best science, everyone can dramatically improve their health just by moving: anytime, anywhere, and by any means that gets you active.” He broke the guidelines down as follows:
- We have new evidence about the risks of sedentary behavior, and new evidence that any amount – any amount – of moderate to vigorous physical activity, like walking, dancing, line dancing if you’re from Texas, and household chores is beneficial,” Adm. Giroir observed.
- While the first edition of the federal guidelines cited strong evidence that physical activity reduces the risk of two types of cancer, breast and colon, the intervening decade has brought forth strong evidence of a protective effect against an additional six types of cancer: bladder, endometrial, kidney, stomach, esophageal, and lung cancer.
- The guidelines formulate for the first time physical activity standards for children aged 3-5 years. The recommended target is at least 3 hr/day of varied physical activity, consistent with existing guidelines in Australia Canada, and the United Kingdom.
- Updated recommendations for children aged 6-17 years call for an hour or more/day of moderate- or vigorous-intensity physical activity on a daily basis, with that activity level falling within the vigorous category on at least 3 days/week. Plus, it recommends bone- and muscle-strengthening activity on at least 3 days.
- The pediatric guidelines are linked to a planned HHS national strategy to expand children’s participation in youth sports as part of an effort to curb childhood obesity, rates of which have more than tripled since the 1970s.
“We’ll soon announce funding opportunities for communities to increase participation in sports among children and teens through participation in affordable programs with trained coaches,” said Dr. Giroir, a pediatrician.
The new guidelines endorse what is called “the comprehensive school physical activity model.”
“I strongly believe our schools should take action to implement this approach. There is a lot of interest right now to affect change in the schools across our country. Part of the answer, I think, is to provide kids with high-quality physical education, but I think we recognize that alone will not be enough.” The comprehensive school activity model calls for not only enriching school PE programs but also incorporating active transport to school, classroom activity, active learning, and after school programs – activity in all settings during the school day. “I’m very hopeful that this model, which is endorsed in the guidelines document, will be widely adopted by schools in this country over the next decade,” Dr. Giroir said.
The first edition declared that only bouts of physical activity of at least 10 minutes duration should count toward meeting the guidelines. That requirement is gone in the second edition. It was an impediment to being active, and upon close examination it wasn’t based on scientific evidence. That means taking the stairs instead of the escalator or parking farther away from the store count toward the weekly physical activity goal, Dr. Kraus said.
“It makes it easier to achieve the guidelines and to encourage Americans to move more and sit less just by making a better choice at many times during the day,” observed Dr. Giroir, a four-star admiral in the U.S. Public Health Service Commissioned Corps.
The latest guidelines contain up-to-date information on the benefits of regular physical activity in terms of brain health, including reduced risk of developing Alzheimer’s disease, and improved cognition, including performance on academic achievement tests and measures of executive function, memory, and processing speed, in preadolescent children as well as older adults. Solid evidence also is cited for improved cognition in patients with MS, dementia, ADHD, and Parkinson’s disease.
The guidelines provide new recommendations for physical activity for women during pregnancy and post partum.
A section of the guidelines is devoted to proven evidence-based strategies to promote physical activity at the individual, small group, and community level.
Physicians now have a resource to aid them in prescribing an individualized physical activity prescription for their patients with existing health conditions, including osteoarthritis, type 2 diabetes, cancer survivors, and physical disabilities.
The new physical activity guidelines and related resources for health care professionals are available at the Health.gov website.
SOURCE: Giroir BP. AHA Scientific Sessions, Session ME.05.
CHICAGO – The newly released comprehensive second edition of the federal physical activity guidelines have a lofty goal.
Adm. Brett P. Giroir, MD, declared in introducing the recommendations at the American Heart Association scientific sessions.
“Physical activity is one of the most effective preventive health interventions available, and we need more emphasis on prevention as we transition to a value-based reimbursement structure that rewards better health maintenance and avoids chronic conditions,” added Adm. Giroir, assistant secretary for health at the U.S. Department of Health & Human Services.
Although the agency opted to unveil the new guidelines before an audience of cardiologists at the AHA scientific sessions, the report includes sections relevant for a wide range of medical specialists, including primary care physicians, pediatricians, psychiatrists, neurologists, endocrinologists, and geriatricians.
Before launching into a description of what’s new in the second edition, Adm. Giroir set the stage with blunt talk about the nation’s poor state of physical fitness.
“Inactivity causes 10% of premature mortality in the United States. That means if we can just get 25% of inactive people to be active and meet the recommendations, almost 75,000 deaths per year would be prevented in the United States. And on an even larger scale worldwide, if 25% of those same people who are inactive started moving and met the guidelines, more than 1.3 million deaths would be prevented,” according to Adm. Giroir.
At present, only 26% of men, 19% of women, and 20% of teenagers meet the physical activity recommendations.
Failure to meet the federal aerobic physical activity recommendations accounts for an estimated nearly $117 billion in annual health care costs. And it poses a national security threat, too: Nearly one-third of all 17- to 24-year-olds are disqualified from military service because of obesity. Even more eye-opening, he continued, is that fully 71% of all 17- to 24-year-olds are ineligible for military service because of obesity, lack of physical fitness, lack of education, or substance use.
The actual recommendations contained in the second edition of the Physical Activity Guidelines for Americans remain unchanged from those in the first, issued a decade earlier. That is, in order to gain substantial health benefits, adults and adolescents should engage in at least 150-300 min/week of moderate intensity aerobic physical activity or 75-150 min/week of vigorous intensity aerobic activity. Plus they should do muscle-strengthening exercises such as weight lifting or push-ups at moderate or greater intensity at least 2 days/week.
Asked why the guidelines are sticking with time-based physical activity recommendations in an era when popular smartwatches and other mobile devices can readily spit out number of steps walked, calories burned, and heart-rate data, cardiologist William E. Kraus, MD, one of the 17 members of the scientific advisory committee who reviewed and graded the scientific evidence on physical activity, sedentary behavior, and health, answered. He said the group’s careful review concluded that “there’s just not enough evidence at this time to make a recommendation” with regard to mobile device–based measurements of physical activity and their relationship with health benefits.
“We’re hoping to spur more research in this area, so that the next time we make recommendations, that can be incorporated,” added Dr. Kraus, a professor of medicine and cardiologist at Duke University, Durham, N.C., as well as president-elect of the American College of Sports Medicine.
What’s new in the guidelines
“This edition tells us that it’s easier to meet the recommendations in the physical activity guidelines,” according to Adm. Giroir. “The new guidelines demonstrate, based on the best science, everyone can dramatically improve their health just by moving: anytime, anywhere, and by any means that gets you active.” He broke the guidelines down as follows:
- We have new evidence about the risks of sedentary behavior, and new evidence that any amount – any amount – of moderate to vigorous physical activity, like walking, dancing, line dancing if you’re from Texas, and household chores is beneficial,” Adm. Giroir observed.
- While the first edition of the federal guidelines cited strong evidence that physical activity reduces the risk of two types of cancer, breast and colon, the intervening decade has brought forth strong evidence of a protective effect against an additional six types of cancer: bladder, endometrial, kidney, stomach, esophageal, and lung cancer.
- The guidelines formulate for the first time physical activity standards for children aged 3-5 years. The recommended target is at least 3 hr/day of varied physical activity, consistent with existing guidelines in Australia Canada, and the United Kingdom.
- Updated recommendations for children aged 6-17 years call for an hour or more/day of moderate- or vigorous-intensity physical activity on a daily basis, with that activity level falling within the vigorous category on at least 3 days/week. Plus, it recommends bone- and muscle-strengthening activity on at least 3 days.
- The pediatric guidelines are linked to a planned HHS national strategy to expand children’s participation in youth sports as part of an effort to curb childhood obesity, rates of which have more than tripled since the 1970s.
“We’ll soon announce funding opportunities for communities to increase participation in sports among children and teens through participation in affordable programs with trained coaches,” said Dr. Giroir, a pediatrician.
The new guidelines endorse what is called “the comprehensive school physical activity model.”
“I strongly believe our schools should take action to implement this approach. There is a lot of interest right now to affect change in the schools across our country. Part of the answer, I think, is to provide kids with high-quality physical education, but I think we recognize that alone will not be enough.” The comprehensive school activity model calls for not only enriching school PE programs but also incorporating active transport to school, classroom activity, active learning, and after school programs – activity in all settings during the school day. “I’m very hopeful that this model, which is endorsed in the guidelines document, will be widely adopted by schools in this country over the next decade,” Dr. Giroir said.
The first edition declared that only bouts of physical activity of at least 10 minutes duration should count toward meeting the guidelines. That requirement is gone in the second edition. It was an impediment to being active, and upon close examination it wasn’t based on scientific evidence. That means taking the stairs instead of the escalator or parking farther away from the store count toward the weekly physical activity goal, Dr. Kraus said.
“It makes it easier to achieve the guidelines and to encourage Americans to move more and sit less just by making a better choice at many times during the day,” observed Dr. Giroir, a four-star admiral in the U.S. Public Health Service Commissioned Corps.
The latest guidelines contain up-to-date information on the benefits of regular physical activity in terms of brain health, including reduced risk of developing Alzheimer’s disease, and improved cognition, including performance on academic achievement tests and measures of executive function, memory, and processing speed, in preadolescent children as well as older adults. Solid evidence also is cited for improved cognition in patients with MS, dementia, ADHD, and Parkinson’s disease.
The guidelines provide new recommendations for physical activity for women during pregnancy and post partum.
A section of the guidelines is devoted to proven evidence-based strategies to promote physical activity at the individual, small group, and community level.
Physicians now have a resource to aid them in prescribing an individualized physical activity prescription for their patients with existing health conditions, including osteoarthritis, type 2 diabetes, cancer survivors, and physical disabilities.
The new physical activity guidelines and related resources for health care professionals are available at the Health.gov website.
SOURCE: Giroir BP. AHA Scientific Sessions, Session ME.05.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Children with poor cardiorespiratory fitness have a higher risk of type 2 diabetes and cardiovascular disease
according to the analysis of an ongoing Finnish study of physical activity and dietary intervention in school children.
“Our results are in agreement with previous findings that cardiorespiratory fitness measured in exercise test laboratories or using field tests and scaled by body mass (BM) using the ratio standard method had a strong inverse association with cardiometabolic risk in children,” lead author Andrew O. Agbaje, MD, MPH, and his coauthors wrote in the Scandinavian Journal of Medicine & Science in Sports.
The coauthors assessed the cardiorespiratory fitness of 352 primary school children – 186 boys and 166 girls – from Kuopio, Finland, all of whom were already participating in the ongoing PANIC (Physical Activity and Nutrition in Children) Study. The children were asked to perform a maximal exercise test, upon which fitness was assessed by measuring peak oxygen uptake (VO2 peak), noted Dr. Agbaje, a PhD student at the University of Eastern Finland’s Institute of Biomedicine in Kuopio, and his colleagues.
Body mass and lean mass were also measured by bioelectrical impedance and used to scale VO2 peak, while variables such as waist circumference, insulin, glucose, HDL cholesterol, and triglycerides were used to calculate a continuous cardiometabolic risk score. Upon analysis, VO2 peak less than 45.8 mL/kg BM-1 min-1 in boys and less than 44.1 mL/kg BM-1 min-1 in girls was associated with increased cardiometabolic risk.
The coauthors noted that cardiorespiratory fitness can be influenced by genetics and that adjustments for puberty had “no effect on the relationships between VO2 peak and cardiometabolic risk.” As such, they recommended that “longitudinal studies are needed to clarify the role of CRF in cardiometabolic health during growth and maturation.”
That said, despite advocating caution in regard to determining proper CRF thresholds, the coauthors suggested that CRF scaled by BM could be used to screen children and improve prevention efforts. “Cardiometabolic risk tracks from childhood into adulthood and the early identification of individuals at increased risk is essential in developing public health actions targeted at preventing cardiometabolic diseases,” they wrote.
The study was funded by grants from the Ministry of Education and Culture of Finland, Ministry of Social Affairs and Health of Finland, Research Committee of the Kuopio University Hospital Catchment Area (State Research Funding), Finnish Innovation Fund Sitra, Social Insurance Institution of Finland, Finnish Cultural Foundation, Foundation for Paediatric Research, Diabetes Research Foundation in Finland, Finnish Foundation for Cardiovascular Research, Juho Vainio Foundation, Paavo Nurmi Foundation, and the Yrjö Jahnsson Foundation. Dr. Agbaje reported grant support from the Olvi Foundation and the Urho Känkanen Foundation.
SOURCE: Agbaje AO et al. Scand J Med Sci Sports. 2018 Sep 19. doi: 10.1111/sms.13307.
according to the analysis of an ongoing Finnish study of physical activity and dietary intervention in school children.
“Our results are in agreement with previous findings that cardiorespiratory fitness measured in exercise test laboratories or using field tests and scaled by body mass (BM) using the ratio standard method had a strong inverse association with cardiometabolic risk in children,” lead author Andrew O. Agbaje, MD, MPH, and his coauthors wrote in the Scandinavian Journal of Medicine & Science in Sports.
The coauthors assessed the cardiorespiratory fitness of 352 primary school children – 186 boys and 166 girls – from Kuopio, Finland, all of whom were already participating in the ongoing PANIC (Physical Activity and Nutrition in Children) Study. The children were asked to perform a maximal exercise test, upon which fitness was assessed by measuring peak oxygen uptake (VO2 peak), noted Dr. Agbaje, a PhD student at the University of Eastern Finland’s Institute of Biomedicine in Kuopio, and his colleagues.
Body mass and lean mass were also measured by bioelectrical impedance and used to scale VO2 peak, while variables such as waist circumference, insulin, glucose, HDL cholesterol, and triglycerides were used to calculate a continuous cardiometabolic risk score. Upon analysis, VO2 peak less than 45.8 mL/kg BM-1 min-1 in boys and less than 44.1 mL/kg BM-1 min-1 in girls was associated with increased cardiometabolic risk.
The coauthors noted that cardiorespiratory fitness can be influenced by genetics and that adjustments for puberty had “no effect on the relationships between VO2 peak and cardiometabolic risk.” As such, they recommended that “longitudinal studies are needed to clarify the role of CRF in cardiometabolic health during growth and maturation.”
That said, despite advocating caution in regard to determining proper CRF thresholds, the coauthors suggested that CRF scaled by BM could be used to screen children and improve prevention efforts. “Cardiometabolic risk tracks from childhood into adulthood and the early identification of individuals at increased risk is essential in developing public health actions targeted at preventing cardiometabolic diseases,” they wrote.
The study was funded by grants from the Ministry of Education and Culture of Finland, Ministry of Social Affairs and Health of Finland, Research Committee of the Kuopio University Hospital Catchment Area (State Research Funding), Finnish Innovation Fund Sitra, Social Insurance Institution of Finland, Finnish Cultural Foundation, Foundation for Paediatric Research, Diabetes Research Foundation in Finland, Finnish Foundation for Cardiovascular Research, Juho Vainio Foundation, Paavo Nurmi Foundation, and the Yrjö Jahnsson Foundation. Dr. Agbaje reported grant support from the Olvi Foundation and the Urho Känkanen Foundation.
SOURCE: Agbaje AO et al. Scand J Med Sci Sports. 2018 Sep 19. doi: 10.1111/sms.13307.
according to the analysis of an ongoing Finnish study of physical activity and dietary intervention in school children.
“Our results are in agreement with previous findings that cardiorespiratory fitness measured in exercise test laboratories or using field tests and scaled by body mass (BM) using the ratio standard method had a strong inverse association with cardiometabolic risk in children,” lead author Andrew O. Agbaje, MD, MPH, and his coauthors wrote in the Scandinavian Journal of Medicine & Science in Sports.
The coauthors assessed the cardiorespiratory fitness of 352 primary school children – 186 boys and 166 girls – from Kuopio, Finland, all of whom were already participating in the ongoing PANIC (Physical Activity and Nutrition in Children) Study. The children were asked to perform a maximal exercise test, upon which fitness was assessed by measuring peak oxygen uptake (VO2 peak), noted Dr. Agbaje, a PhD student at the University of Eastern Finland’s Institute of Biomedicine in Kuopio, and his colleagues.
Body mass and lean mass were also measured by bioelectrical impedance and used to scale VO2 peak, while variables such as waist circumference, insulin, glucose, HDL cholesterol, and triglycerides were used to calculate a continuous cardiometabolic risk score. Upon analysis, VO2 peak less than 45.8 mL/kg BM-1 min-1 in boys and less than 44.1 mL/kg BM-1 min-1 in girls was associated with increased cardiometabolic risk.
The coauthors noted that cardiorespiratory fitness can be influenced by genetics and that adjustments for puberty had “no effect on the relationships between VO2 peak and cardiometabolic risk.” As such, they recommended that “longitudinal studies are needed to clarify the role of CRF in cardiometabolic health during growth and maturation.”
That said, despite advocating caution in regard to determining proper CRF thresholds, the coauthors suggested that CRF scaled by BM could be used to screen children and improve prevention efforts. “Cardiometabolic risk tracks from childhood into adulthood and the early identification of individuals at increased risk is essential in developing public health actions targeted at preventing cardiometabolic diseases,” they wrote.
The study was funded by grants from the Ministry of Education and Culture of Finland, Ministry of Social Affairs and Health of Finland, Research Committee of the Kuopio University Hospital Catchment Area (State Research Funding), Finnish Innovation Fund Sitra, Social Insurance Institution of Finland, Finnish Cultural Foundation, Foundation for Paediatric Research, Diabetes Research Foundation in Finland, Finnish Foundation for Cardiovascular Research, Juho Vainio Foundation, Paavo Nurmi Foundation, and the Yrjö Jahnsson Foundation. Dr. Agbaje reported grant support from the Olvi Foundation and the Urho Känkanen Foundation.
SOURCE: Agbaje AO et al. Scand J Med Sci Sports. 2018 Sep 19. doi: 10.1111/sms.13307.
FROM THE SCANDINAVIAN JOURNAL OF MEDICINE & SCIENCE IN SPORTS
Key clinical point: Peak oxygen uptake less than 45.8 mL/kg BM-1 min-1 in boys and less than 44.1 mL/kg BM-1 min-1 in girls was associated with increased cardiometabolic risk.
Major finding: Cardiorespiratory fitness scaled by body mass could be used to screen for cardiometabolic risk in children.
Study details: An analysis of 352 Finnish children, all aged 9-11 years, who took a maximal exercise test as part of an ongoing physical activity and dietary intervention study.
Disclosures: The study was funded by grants from the Ministry of Education and Culture of Finland, Ministry of Social Affairs and Health of Finland, Research Committee of the Kuopio University Hospital Catchment Area, Finnish Innovation Fund Sitra, Social Insurance Institution of Finland, Finnish Cultural Foundation, Foundation for Paediatric Research, Diabetes Research Foundation in Finland, Finnish Foundation for Cardiovascular Research, Juho Vainio Foundation, Paavo Nurmi Foundation, and the Yrjö Jahnsson Foundation. Dr. Agbaje reported grant support from the Olvi Foundation and the Urho Känkanen Foundation.
Source: Agbaje AO et al. Scand J Med Sci Sports. 2018 Sep 19. doi: 10.1111/sms.13307.
Antibiotic-obesity link ‘clinically insignificant’ at age 10 years
NASHVILLE, tenn. – Antibiotic use in the first 2 years of life was associated with a small amount of weight gain by 10 years of age, but the amount is “likely clinically insignificant,” according to new data presented at Obesity Week.
At 10 years of age, children without chronic health conditions who received any antibiotics had an odds ratio of 1.02 for being overweight or obese; for children with complex chronic conditions, the OR was 1.07 (95% confidence intervals, 0.97-1.07 and 0.96-1.19, respectively). The findings were based on data from almost 60,000 children studied in a large, multi-institutional national cohort.
“This is good news,” said first author Sheryl Rifas-Shiman, MPH, discussing the findings during a poster session. She noted that the 10-year data from the longitudinal study are consistent with findings at the 5-year mark that had previously been reported.
The study comes against the background of a recent meta-analysis finding that children given any antibiotics before age 24 months had a higher body mass index z-score (BMI-z) in childhood than children who didn’t take antibiotics. Disruptions that antibiotics can cause in the gut microbiome have been hypothesized to promote overweight and obesity in children.
The present study was conducted using electronic medical record data from 2009-2016 drawn from institutions participating in PCORnet, a national research collaboration and clearinghouse.
The analysis dichotomized the cohort into those who, by 24 months of age, had received any antibiotics and those who received none. Ms. Rifas-Shiman and her colleagues also looked at a categorical count of the number of antibiotic prescriptions, from 0 to 4 or more.
Finally, they broke the type of antibiotics into narrow- or broad-spectrum, she said in an interview during the poster session. In order for exposure to be considered narrow-spectrum only, the analysis was limited to participants who had no broad-spectrum antibiotic exposure during the same time frame.
The study’s multivariable analysis also took into account complex chronic conditions the children may have had.
Fifty-seven percent of children received antibiotics before the age of 24 months. Patients were overall just under half (48%) female, and about half (49%) were white. Black children made up 37% of the cohort, and Hispanic children constituted 12%.
By 10 years of age, 36% of participants were overweight or obese, with BMIs at or above the 85th percentile, according to 2000 Centers for Disease Control growth charts.
There was a suggestion of a dose-response relationship for both narrow- and broad-spectrum antibiotics because only at the higher antibiotic exposures did increased BMI-z reach statistical significance. However, broad-spectrum antibiotics were not more likely than narrow-spectrum antibiotics to be associated with increased BMI-z.
Other multivariable adjustment took into account site clustering and adjusted for sex, race, ethnicity, preterm birth, asthma or infectious disease diagnoses, and corticosteroid exposure, as well as health care visits in the first 2 years of life.
Limitations of the study included the fact that it was observational, raising the potential for undetected confounders. Also, since the study looked at prescription, rather than dispensing data, there could be some exposure misclassification by which children who were classified as having taken antibiotics did not actually take them or did not take the full amount prescribed.
“Antibiotics should always be used judiciously; however, the long-term risk of childhood obesity from antibiotics in infancy appears to be small and clinically insignificant,” wrote Ms. Rifas-Shiman, of the department of population medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, both in Boston.
The study was funded by an award from the Patient-Centered Outcomes Research Institute.
SOURCE: Rifas-Shiman et al. Obesity Week 2018, Poster TP-3155.
NASHVILLE, tenn. – Antibiotic use in the first 2 years of life was associated with a small amount of weight gain by 10 years of age, but the amount is “likely clinically insignificant,” according to new data presented at Obesity Week.
At 10 years of age, children without chronic health conditions who received any antibiotics had an odds ratio of 1.02 for being overweight or obese; for children with complex chronic conditions, the OR was 1.07 (95% confidence intervals, 0.97-1.07 and 0.96-1.19, respectively). The findings were based on data from almost 60,000 children studied in a large, multi-institutional national cohort.
“This is good news,” said first author Sheryl Rifas-Shiman, MPH, discussing the findings during a poster session. She noted that the 10-year data from the longitudinal study are consistent with findings at the 5-year mark that had previously been reported.
The study comes against the background of a recent meta-analysis finding that children given any antibiotics before age 24 months had a higher body mass index z-score (BMI-z) in childhood than children who didn’t take antibiotics. Disruptions that antibiotics can cause in the gut microbiome have been hypothesized to promote overweight and obesity in children.
The present study was conducted using electronic medical record data from 2009-2016 drawn from institutions participating in PCORnet, a national research collaboration and clearinghouse.
The analysis dichotomized the cohort into those who, by 24 months of age, had received any antibiotics and those who received none. Ms. Rifas-Shiman and her colleagues also looked at a categorical count of the number of antibiotic prescriptions, from 0 to 4 or more.
Finally, they broke the type of antibiotics into narrow- or broad-spectrum, she said in an interview during the poster session. In order for exposure to be considered narrow-spectrum only, the analysis was limited to participants who had no broad-spectrum antibiotic exposure during the same time frame.
The study’s multivariable analysis also took into account complex chronic conditions the children may have had.
Fifty-seven percent of children received antibiotics before the age of 24 months. Patients were overall just under half (48%) female, and about half (49%) were white. Black children made up 37% of the cohort, and Hispanic children constituted 12%.
By 10 years of age, 36% of participants were overweight or obese, with BMIs at or above the 85th percentile, according to 2000 Centers for Disease Control growth charts.
There was a suggestion of a dose-response relationship for both narrow- and broad-spectrum antibiotics because only at the higher antibiotic exposures did increased BMI-z reach statistical significance. However, broad-spectrum antibiotics were not more likely than narrow-spectrum antibiotics to be associated with increased BMI-z.
Other multivariable adjustment took into account site clustering and adjusted for sex, race, ethnicity, preterm birth, asthma or infectious disease diagnoses, and corticosteroid exposure, as well as health care visits in the first 2 years of life.
Limitations of the study included the fact that it was observational, raising the potential for undetected confounders. Also, since the study looked at prescription, rather than dispensing data, there could be some exposure misclassification by which children who were classified as having taken antibiotics did not actually take them or did not take the full amount prescribed.
“Antibiotics should always be used judiciously; however, the long-term risk of childhood obesity from antibiotics in infancy appears to be small and clinically insignificant,” wrote Ms. Rifas-Shiman, of the department of population medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, both in Boston.
The study was funded by an award from the Patient-Centered Outcomes Research Institute.
SOURCE: Rifas-Shiman et al. Obesity Week 2018, Poster TP-3155.
NASHVILLE, tenn. – Antibiotic use in the first 2 years of life was associated with a small amount of weight gain by 10 years of age, but the amount is “likely clinically insignificant,” according to new data presented at Obesity Week.
At 10 years of age, children without chronic health conditions who received any antibiotics had an odds ratio of 1.02 for being overweight or obese; for children with complex chronic conditions, the OR was 1.07 (95% confidence intervals, 0.97-1.07 and 0.96-1.19, respectively). The findings were based on data from almost 60,000 children studied in a large, multi-institutional national cohort.
“This is good news,” said first author Sheryl Rifas-Shiman, MPH, discussing the findings during a poster session. She noted that the 10-year data from the longitudinal study are consistent with findings at the 5-year mark that had previously been reported.
The study comes against the background of a recent meta-analysis finding that children given any antibiotics before age 24 months had a higher body mass index z-score (BMI-z) in childhood than children who didn’t take antibiotics. Disruptions that antibiotics can cause in the gut microbiome have been hypothesized to promote overweight and obesity in children.
The present study was conducted using electronic medical record data from 2009-2016 drawn from institutions participating in PCORnet, a national research collaboration and clearinghouse.
The analysis dichotomized the cohort into those who, by 24 months of age, had received any antibiotics and those who received none. Ms. Rifas-Shiman and her colleagues also looked at a categorical count of the number of antibiotic prescriptions, from 0 to 4 or more.
Finally, they broke the type of antibiotics into narrow- or broad-spectrum, she said in an interview during the poster session. In order for exposure to be considered narrow-spectrum only, the analysis was limited to participants who had no broad-spectrum antibiotic exposure during the same time frame.
The study’s multivariable analysis also took into account complex chronic conditions the children may have had.
Fifty-seven percent of children received antibiotics before the age of 24 months. Patients were overall just under half (48%) female, and about half (49%) were white. Black children made up 37% of the cohort, and Hispanic children constituted 12%.
By 10 years of age, 36% of participants were overweight or obese, with BMIs at or above the 85th percentile, according to 2000 Centers for Disease Control growth charts.
There was a suggestion of a dose-response relationship for both narrow- and broad-spectrum antibiotics because only at the higher antibiotic exposures did increased BMI-z reach statistical significance. However, broad-spectrum antibiotics were not more likely than narrow-spectrum antibiotics to be associated with increased BMI-z.
Other multivariable adjustment took into account site clustering and adjusted for sex, race, ethnicity, preterm birth, asthma or infectious disease diagnoses, and corticosteroid exposure, as well as health care visits in the first 2 years of life.
Limitations of the study included the fact that it was observational, raising the potential for undetected confounders. Also, since the study looked at prescription, rather than dispensing data, there could be some exposure misclassification by which children who were classified as having taken antibiotics did not actually take them or did not take the full amount prescribed.
“Antibiotics should always be used judiciously; however, the long-term risk of childhood obesity from antibiotics in infancy appears to be small and clinically insignificant,” wrote Ms. Rifas-Shiman, of the department of population medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, both in Boston.
The study was funded by an award from the Patient-Centered Outcomes Research Institute.
SOURCE: Rifas-Shiman et al. Obesity Week 2018, Poster TP-3155.
REPORTING FROM OBESITY WEEK 2018
Key clinical point: Antibiotic use before the age of 2 years was not associated with clinically significant weight gain at age 10 years.
Major finding: By age 10 years, children given antibiotics without chronic health conditions had an odds ratio of 1.02 for being overweight or obese, and those with chronic health conditions had an OR of 1.07, with 95% confidence intervals for both groups crossing unity.
Study details: Prospective, multisite, national cohort study of 56,727 children.
Disclosures: The study was funded by the Patient-Centered Outcomes Research Institute. Ms. Rifas-Shiman reported that she had no conflicts of interest.
Source: Rifas-Shiman S et al. Obesity Week 2018, poster TP-3155,