User login
Risk of cancer in NAFLD is 91% higher than in control subjects
SAN FRANCISCO –
Those are key findings from a community cohort study with up to 21 years of follow-up, which one of the authors, Alina M. Allen, MD, discussed during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases.
“NAFLD is the most common chronic liver disease in the Western world,” said Dr. Allen, a gastroenterologist at the Mayo Clinic, Rochester, Minn. “It affects one in four adults in the U.S. It is related to fat accumulation in the liver in people who are overweight or obese and can lead to cirrhosis and liver-related mortality. However, the most common cause of death in this population is not liver disease but malignancy and cardiovascular disease. There is a paucity of epidemiologic studies of extrahepatic cancer in NAFLD. It is not clear what types of cancers and how much higher their cancer risk is in reference to the general population.”
In an effort to determine the incidence of cancer diagnoses in NALFD, compared with controls, in a U.S. community, Dr. Allen and her colleagues drew from the Rochester Epidemiology Project to evaluate 4,791 adults diagnosed with NAFLD and 14,432 age- and sex-matched control subjects in Olmstead County, Minn., during 1997-2017. The researchers obtained corresponding Surveillance, Epidemiology, and End Results Program (SEER) rates as a quality check and used a regression model to assess the malignancy risk in NAFLD overall and by cancer type, age, sex, and body mass index. They recorded all new diagnoses of cancers that developed in both groups until 2018, for a total possible follow-up of 21 years, and they reported results in incidence rate ratios, a risk estimate similar to hazard ratios.
The mean age of the study population was 53 years, 53% were female, and the mean follow-up was 8 years with a range from 1 to 21 years. New cancers were identified in 16% of subjects with NALFD and in 12% of control subjects. The overall risk of malignancy was 91% higher in NAFLD subjects, compared with control subjects; there were higher rates in the NAFLD subjects for most types of cancers, but the largest increases were in GI cancers. The greatest malignancy risk was for cancer of the liver (RR, 3.24), followed by cancer of the uterus (RR, 2.39), stomach (RR, 2.34), pancreas (RR, 2.09), and colon (RR, 1.75). “Interestingly, the risk of colon cancer increased only in men but not in women,” Dr. Allen said. “These data provide an important hierarchical overview of the top most important malignancy risks associated with NAFLD that the medical community should be aware of.”
When the researchers looked for differences in age at cancer diagnosis between NAFLD and controls, they found that pancreas cancer occurred at a younger age among subjects with NAFLD. They also observed that colon cancer occurred at a younger age in men with NAFLD, but not in women with the disease. “What was most interesting to us was the assessment of cancer risk in NAFLD versus obesity alone,” Dr. Allen said. “Previous studies from the general population have linked obesity to a higher risk of cancer. Whether the presence of fatty liver disease would impact that risk has not been assessed. We showed that obesity is associated with a higher risk of cancer only in those with NAFLD, not in those without. If validated in independent cohorts, these findings could change our understanding of the relationship between obesity and cancer and the importance of screening for NAFLD – not only to risk-stratify liver disease but also for the risk of extrahepatic malignancy.”
Dr. Allen concluded her presentation by noting that findings from large population-based studies such as the Rochester Epidemiology Project “can offer important epidemiologic data regarding the biggest threats to the health of a community,” she said. “Such data increase awareness, enable appropriate counseling, and could inform screening policies. There is a signal in the fact that the GI cancers are increased [in NAFLD]. It’s an interesting signal that needs to be studied further.”
Dr. Allen reported having no financial conflicts.
Source: Allen AM. Hepatol. 2018;68[S1]: Abstract 31.
SAN FRANCISCO –
Those are key findings from a community cohort study with up to 21 years of follow-up, which one of the authors, Alina M. Allen, MD, discussed during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases.
“NAFLD is the most common chronic liver disease in the Western world,” said Dr. Allen, a gastroenterologist at the Mayo Clinic, Rochester, Minn. “It affects one in four adults in the U.S. It is related to fat accumulation in the liver in people who are overweight or obese and can lead to cirrhosis and liver-related mortality. However, the most common cause of death in this population is not liver disease but malignancy and cardiovascular disease. There is a paucity of epidemiologic studies of extrahepatic cancer in NAFLD. It is not clear what types of cancers and how much higher their cancer risk is in reference to the general population.”
In an effort to determine the incidence of cancer diagnoses in NALFD, compared with controls, in a U.S. community, Dr. Allen and her colleagues drew from the Rochester Epidemiology Project to evaluate 4,791 adults diagnosed with NAFLD and 14,432 age- and sex-matched control subjects in Olmstead County, Minn., during 1997-2017. The researchers obtained corresponding Surveillance, Epidemiology, and End Results Program (SEER) rates as a quality check and used a regression model to assess the malignancy risk in NAFLD overall and by cancer type, age, sex, and body mass index. They recorded all new diagnoses of cancers that developed in both groups until 2018, for a total possible follow-up of 21 years, and they reported results in incidence rate ratios, a risk estimate similar to hazard ratios.
The mean age of the study population was 53 years, 53% were female, and the mean follow-up was 8 years with a range from 1 to 21 years. New cancers were identified in 16% of subjects with NALFD and in 12% of control subjects. The overall risk of malignancy was 91% higher in NAFLD subjects, compared with control subjects; there were higher rates in the NAFLD subjects for most types of cancers, but the largest increases were in GI cancers. The greatest malignancy risk was for cancer of the liver (RR, 3.24), followed by cancer of the uterus (RR, 2.39), stomach (RR, 2.34), pancreas (RR, 2.09), and colon (RR, 1.75). “Interestingly, the risk of colon cancer increased only in men but not in women,” Dr. Allen said. “These data provide an important hierarchical overview of the top most important malignancy risks associated with NAFLD that the medical community should be aware of.”
When the researchers looked for differences in age at cancer diagnosis between NAFLD and controls, they found that pancreas cancer occurred at a younger age among subjects with NAFLD. They also observed that colon cancer occurred at a younger age in men with NAFLD, but not in women with the disease. “What was most interesting to us was the assessment of cancer risk in NAFLD versus obesity alone,” Dr. Allen said. “Previous studies from the general population have linked obesity to a higher risk of cancer. Whether the presence of fatty liver disease would impact that risk has not been assessed. We showed that obesity is associated with a higher risk of cancer only in those with NAFLD, not in those without. If validated in independent cohorts, these findings could change our understanding of the relationship between obesity and cancer and the importance of screening for NAFLD – not only to risk-stratify liver disease but also for the risk of extrahepatic malignancy.”
Dr. Allen concluded her presentation by noting that findings from large population-based studies such as the Rochester Epidemiology Project “can offer important epidemiologic data regarding the biggest threats to the health of a community,” she said. “Such data increase awareness, enable appropriate counseling, and could inform screening policies. There is a signal in the fact that the GI cancers are increased [in NAFLD]. It’s an interesting signal that needs to be studied further.”
Dr. Allen reported having no financial conflicts.
Source: Allen AM. Hepatol. 2018;68[S1]: Abstract 31.
SAN FRANCISCO –
Those are key findings from a community cohort study with up to 21 years of follow-up, which one of the authors, Alina M. Allen, MD, discussed during a press briefing at the annual meeting of the American Association for the Study of Liver Diseases.
“NAFLD is the most common chronic liver disease in the Western world,” said Dr. Allen, a gastroenterologist at the Mayo Clinic, Rochester, Minn. “It affects one in four adults in the U.S. It is related to fat accumulation in the liver in people who are overweight or obese and can lead to cirrhosis and liver-related mortality. However, the most common cause of death in this population is not liver disease but malignancy and cardiovascular disease. There is a paucity of epidemiologic studies of extrahepatic cancer in NAFLD. It is not clear what types of cancers and how much higher their cancer risk is in reference to the general population.”
In an effort to determine the incidence of cancer diagnoses in NALFD, compared with controls, in a U.S. community, Dr. Allen and her colleagues drew from the Rochester Epidemiology Project to evaluate 4,791 adults diagnosed with NAFLD and 14,432 age- and sex-matched control subjects in Olmstead County, Minn., during 1997-2017. The researchers obtained corresponding Surveillance, Epidemiology, and End Results Program (SEER) rates as a quality check and used a regression model to assess the malignancy risk in NAFLD overall and by cancer type, age, sex, and body mass index. They recorded all new diagnoses of cancers that developed in both groups until 2018, for a total possible follow-up of 21 years, and they reported results in incidence rate ratios, a risk estimate similar to hazard ratios.
The mean age of the study population was 53 years, 53% were female, and the mean follow-up was 8 years with a range from 1 to 21 years. New cancers were identified in 16% of subjects with NALFD and in 12% of control subjects. The overall risk of malignancy was 91% higher in NAFLD subjects, compared with control subjects; there were higher rates in the NAFLD subjects for most types of cancers, but the largest increases were in GI cancers. The greatest malignancy risk was for cancer of the liver (RR, 3.24), followed by cancer of the uterus (RR, 2.39), stomach (RR, 2.34), pancreas (RR, 2.09), and colon (RR, 1.75). “Interestingly, the risk of colon cancer increased only in men but not in women,” Dr. Allen said. “These data provide an important hierarchical overview of the top most important malignancy risks associated with NAFLD that the medical community should be aware of.”
When the researchers looked for differences in age at cancer diagnosis between NAFLD and controls, they found that pancreas cancer occurred at a younger age among subjects with NAFLD. They also observed that colon cancer occurred at a younger age in men with NAFLD, but not in women with the disease. “What was most interesting to us was the assessment of cancer risk in NAFLD versus obesity alone,” Dr. Allen said. “Previous studies from the general population have linked obesity to a higher risk of cancer. Whether the presence of fatty liver disease would impact that risk has not been assessed. We showed that obesity is associated with a higher risk of cancer only in those with NAFLD, not in those without. If validated in independent cohorts, these findings could change our understanding of the relationship between obesity and cancer and the importance of screening for NAFLD – not only to risk-stratify liver disease but also for the risk of extrahepatic malignancy.”
Dr. Allen concluded her presentation by noting that findings from large population-based studies such as the Rochester Epidemiology Project “can offer important epidemiologic data regarding the biggest threats to the health of a community,” she said. “Such data increase awareness, enable appropriate counseling, and could inform screening policies. There is a signal in the fact that the GI cancers are increased [in NAFLD]. It’s an interesting signal that needs to be studied further.”
Dr. Allen reported having no financial conflicts.
Source: Allen AM. Hepatol. 2018;68[S1]: Abstract 31.
REPORTING FROM THE LIVER MEETING 2018
Key clinical point: The risk of cancer in patients with nonalcoholic fatty liver disease is 91% higher than in control subjects.
Major finding: Among subjects with NAFLD, the greatest malignancy risk was for cancer of the liver (risk ratio, 3.24).
Study details: A cohort study of 4,791 adults diagnosed with NAFLD and 14,432 age- and sex-matched control subjects from the Rochester Epidemiology Project.
Disclosures: Dr. Allen reported having no financial conflicts.
Source: Allen AM. Hepatol. 2018;68[S1]:Abstract 31
Cardiologist: Obesity has basically stopped progress on cardiovascular disease
NASHVILLE – It’s all hands on deck to fight the obesity epidemic, according to a cardiologist who made a plea for collaboration at the opening session at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery. said Steven Nissen, MD, because when significant weight loss is achieved, “we can have an amazing effect on cardiovascular death, stroke, myocardial infarction, and these feared complications of obesity.”
From Dr. Nissen’s perspective, though, rates of death from cardiovascular disease have plateaued and are creeping up after decades of marked improvement.
“I am sorry to tell you that these rates are beginning to go up again – because of the obesity epidemic. That’s why we need to work together on this problem,” said Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic. “It has basically stopped progress on cardiovascular disease.”
Dr. Nissen drew from the broad literature intertwining obesity and cardiometabolic health to tell a story that went beyond weight loss to focus on outcomes.
For bariatric surgery, the evidence of reduction in cardiovascular risk is looking very good, said Dr. Nissen. “There are just huge changes in the metabolic risk factors. … Clearly we have evidence that if we get people to lose substantial weight, you can get people to normalize major metabolic risk factors.”
Recent data from a multisite, retrospective, matched cohort study of patients with diabetes and severe obesity show the promise of substantial weight loss in reducing risk. The study tracked 5,301 bariatric surgery patients and compared them with 14,934 “well-matched” control participants who did not have bariatric surgery but received usual care for diabetes, said Dr. Nissen.
During 7 years of follow-up, the bariatric surgery group had a hazard ratio for coronary events of 0.64 (95% confidence interval, 0.42-0.99; P less than .001). A post hoc analysis showed an HR of 0.34 for all-cause mortality among the bariatric surgery patients (95% CI, 0.15-0.74; P less than .001; JAMA. 2018;320[15]:1570-82).
“I’ve been practicing in this field for about 40 years,” said Dr. Nissen. “With statins, we get about 25% risk reduction … a 34% risk reduction is just a whopping big reduction.” Background risk was high among this population with diabetes, and this was a cohort study, not a randomized, controlled trial (RCT).
“We need RCTs,” he said. “I hope we can come together – all of us – and do a large, multicenter, global RCT on the effects of bariatric surgery on cardiovascular outcomes. But barring that, these are the best data we are going to have.”
But can these changes be achieved and sustained without surgery?
“Can diet or drug therapy favorably affect atherosclerotic cardiovascular outcomes? To me, this is the holy grail,” Dr. Nissen said.
A major cautionary note was sounded by a European Medicines Agency–mandated cardiovascular outcomes study of sibutramine, said Dr. Nissen. In clinical trials, patients taking sibutramine had seen modest weight loss, with increased HDL cholesterol and decreased triglycerides. However, blood pressure rose by 1-3 mm Hg, and heart rates also climbed by 4-5 beats per minute, changes consistent with sibutramine’s sympathomimetic effects, said Dr. Nissen. The EMA-mandated trial included over 10,000 patients and looked at a composite endpoint of major cardiovascular events, including death, MI, stroke, and resuscitated arrest. Patients were included if they were aged older than 55 years, had a body mass index of greater than 27 kg/m2, and had a history of cardiovascular disease or diabetes with an additional risk factor. Patients who had significant heart rate or blood pressure increases during the study run-in period were excluded.
In the end, patients taking sibutramine had an increased risk for the composite endpoint (11.4% vs. 10.0%; P = .02). The risk for nonfatal stroke and nonfatal MI was also significantly elevated for the sibutramine group (N Engl J Med. 2010; 363:905-17).
Phentermine is a pharmacologic relative of sibutramine, with similar effects on blood pressure and heart rate. Since it was approved prior to the current increased focus on real-world clinical outcomes in drug approvals, phentermine’s cardiovascular outcomes have never been studied by means of a RCT. “Nobody’s going to do this study unless we push for it, but it has to be done,” he said. “Although this drug reduces weight, there is considerable uncertainty whether it increases cardiovascular outcomes.”
Even looking at weight loss alone, pharmacologic treatments show marginal benefit over time, said Dr. Nissen, citing, as an example, recently published outcome data on lorcaserin. Over 40 months of treatment, there was a “complete absence of any benefit for lorcaserin,” compared with placebo, and participants saw an average weight loss of just 1.9 kg by the end of the study period, with no change in cardiovascular outcomes (N Engl J Med. 2018;379:1107-17).
To drive home the point, Dr. Nissen shared a slide entitled “Established Benefits of Weight Loss Drugs on Clinically Important Outcomes.” The slide’s text read, “This slide intentionally left blank.”
“It’s very hard for me to argue in favor of giving any of these drugs,” said Dr. Nissen. “In the absence of established outcome benefits, there are only risks and costs. I know this is not going to be popular with everyone in this audience, but I have to tell you what I really believe here: We have to do better.”
More broadly, “I think we have to demand outcome trials for obesity drugs,” said Dr. Nissen. He noted that such trials are underway for some glucagonlike peptide–1 agonists, “and I applaud them. ... I hope you will participate in those studies, because they are going to give us some answers.”
Calling for renewed efforts to improve the efficacy of lifestyle interventions, Dr. Nissen said, “What we have to do is try. .... You know as well as I that there are some outliers” who will achieve profound weight loss without surgery, and those patients are likely to reap big benefits in risk reduction.
“We’ve got a problem that affects tens of millions of people, and we’ve got to find a societal approach to this. But we share these patients; let’s work together on trying to make them better.”
Dr. Nissen did not report any relevant financial disclosures.
koakes@mdedge.com
NASHVILLE – It’s all hands on deck to fight the obesity epidemic, according to a cardiologist who made a plea for collaboration at the opening session at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery. said Steven Nissen, MD, because when significant weight loss is achieved, “we can have an amazing effect on cardiovascular death, stroke, myocardial infarction, and these feared complications of obesity.”
From Dr. Nissen’s perspective, though, rates of death from cardiovascular disease have plateaued and are creeping up after decades of marked improvement.
“I am sorry to tell you that these rates are beginning to go up again – because of the obesity epidemic. That’s why we need to work together on this problem,” said Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic. “It has basically stopped progress on cardiovascular disease.”
Dr. Nissen drew from the broad literature intertwining obesity and cardiometabolic health to tell a story that went beyond weight loss to focus on outcomes.
For bariatric surgery, the evidence of reduction in cardiovascular risk is looking very good, said Dr. Nissen. “There are just huge changes in the metabolic risk factors. … Clearly we have evidence that if we get people to lose substantial weight, you can get people to normalize major metabolic risk factors.”
Recent data from a multisite, retrospective, matched cohort study of patients with diabetes and severe obesity show the promise of substantial weight loss in reducing risk. The study tracked 5,301 bariatric surgery patients and compared them with 14,934 “well-matched” control participants who did not have bariatric surgery but received usual care for diabetes, said Dr. Nissen.
During 7 years of follow-up, the bariatric surgery group had a hazard ratio for coronary events of 0.64 (95% confidence interval, 0.42-0.99; P less than .001). A post hoc analysis showed an HR of 0.34 for all-cause mortality among the bariatric surgery patients (95% CI, 0.15-0.74; P less than .001; JAMA. 2018;320[15]:1570-82).
“I’ve been practicing in this field for about 40 years,” said Dr. Nissen. “With statins, we get about 25% risk reduction … a 34% risk reduction is just a whopping big reduction.” Background risk was high among this population with diabetes, and this was a cohort study, not a randomized, controlled trial (RCT).
“We need RCTs,” he said. “I hope we can come together – all of us – and do a large, multicenter, global RCT on the effects of bariatric surgery on cardiovascular outcomes. But barring that, these are the best data we are going to have.”
But can these changes be achieved and sustained without surgery?
“Can diet or drug therapy favorably affect atherosclerotic cardiovascular outcomes? To me, this is the holy grail,” Dr. Nissen said.
A major cautionary note was sounded by a European Medicines Agency–mandated cardiovascular outcomes study of sibutramine, said Dr. Nissen. In clinical trials, patients taking sibutramine had seen modest weight loss, with increased HDL cholesterol and decreased triglycerides. However, blood pressure rose by 1-3 mm Hg, and heart rates also climbed by 4-5 beats per minute, changes consistent with sibutramine’s sympathomimetic effects, said Dr. Nissen. The EMA-mandated trial included over 10,000 patients and looked at a composite endpoint of major cardiovascular events, including death, MI, stroke, and resuscitated arrest. Patients were included if they were aged older than 55 years, had a body mass index of greater than 27 kg/m2, and had a history of cardiovascular disease or diabetes with an additional risk factor. Patients who had significant heart rate or blood pressure increases during the study run-in period were excluded.
In the end, patients taking sibutramine had an increased risk for the composite endpoint (11.4% vs. 10.0%; P = .02). The risk for nonfatal stroke and nonfatal MI was also significantly elevated for the sibutramine group (N Engl J Med. 2010; 363:905-17).
Phentermine is a pharmacologic relative of sibutramine, with similar effects on blood pressure and heart rate. Since it was approved prior to the current increased focus on real-world clinical outcomes in drug approvals, phentermine’s cardiovascular outcomes have never been studied by means of a RCT. “Nobody’s going to do this study unless we push for it, but it has to be done,” he said. “Although this drug reduces weight, there is considerable uncertainty whether it increases cardiovascular outcomes.”
Even looking at weight loss alone, pharmacologic treatments show marginal benefit over time, said Dr. Nissen, citing, as an example, recently published outcome data on lorcaserin. Over 40 months of treatment, there was a “complete absence of any benefit for lorcaserin,” compared with placebo, and participants saw an average weight loss of just 1.9 kg by the end of the study period, with no change in cardiovascular outcomes (N Engl J Med. 2018;379:1107-17).
To drive home the point, Dr. Nissen shared a slide entitled “Established Benefits of Weight Loss Drugs on Clinically Important Outcomes.” The slide’s text read, “This slide intentionally left blank.”
“It’s very hard for me to argue in favor of giving any of these drugs,” said Dr. Nissen. “In the absence of established outcome benefits, there are only risks and costs. I know this is not going to be popular with everyone in this audience, but I have to tell you what I really believe here: We have to do better.”
More broadly, “I think we have to demand outcome trials for obesity drugs,” said Dr. Nissen. He noted that such trials are underway for some glucagonlike peptide–1 agonists, “and I applaud them. ... I hope you will participate in those studies, because they are going to give us some answers.”
Calling for renewed efforts to improve the efficacy of lifestyle interventions, Dr. Nissen said, “What we have to do is try. .... You know as well as I that there are some outliers” who will achieve profound weight loss without surgery, and those patients are likely to reap big benefits in risk reduction.
“We’ve got a problem that affects tens of millions of people, and we’ve got to find a societal approach to this. But we share these patients; let’s work together on trying to make them better.”
Dr. Nissen did not report any relevant financial disclosures.
koakes@mdedge.com
NASHVILLE – It’s all hands on deck to fight the obesity epidemic, according to a cardiologist who made a plea for collaboration at the opening session at a meeting presented by the Obesity Society and the American Society for Metabolic and Bariatric Surgery. said Steven Nissen, MD, because when significant weight loss is achieved, “we can have an amazing effect on cardiovascular death, stroke, myocardial infarction, and these feared complications of obesity.”
From Dr. Nissen’s perspective, though, rates of death from cardiovascular disease have plateaued and are creeping up after decades of marked improvement.
“I am sorry to tell you that these rates are beginning to go up again – because of the obesity epidemic. That’s why we need to work together on this problem,” said Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic. “It has basically stopped progress on cardiovascular disease.”
Dr. Nissen drew from the broad literature intertwining obesity and cardiometabolic health to tell a story that went beyond weight loss to focus on outcomes.
For bariatric surgery, the evidence of reduction in cardiovascular risk is looking very good, said Dr. Nissen. “There are just huge changes in the metabolic risk factors. … Clearly we have evidence that if we get people to lose substantial weight, you can get people to normalize major metabolic risk factors.”
Recent data from a multisite, retrospective, matched cohort study of patients with diabetes and severe obesity show the promise of substantial weight loss in reducing risk. The study tracked 5,301 bariatric surgery patients and compared them with 14,934 “well-matched” control participants who did not have bariatric surgery but received usual care for diabetes, said Dr. Nissen.
During 7 years of follow-up, the bariatric surgery group had a hazard ratio for coronary events of 0.64 (95% confidence interval, 0.42-0.99; P less than .001). A post hoc analysis showed an HR of 0.34 for all-cause mortality among the bariatric surgery patients (95% CI, 0.15-0.74; P less than .001; JAMA. 2018;320[15]:1570-82).
“I’ve been practicing in this field for about 40 years,” said Dr. Nissen. “With statins, we get about 25% risk reduction … a 34% risk reduction is just a whopping big reduction.” Background risk was high among this population with diabetes, and this was a cohort study, not a randomized, controlled trial (RCT).
“We need RCTs,” he said. “I hope we can come together – all of us – and do a large, multicenter, global RCT on the effects of bariatric surgery on cardiovascular outcomes. But barring that, these are the best data we are going to have.”
But can these changes be achieved and sustained without surgery?
“Can diet or drug therapy favorably affect atherosclerotic cardiovascular outcomes? To me, this is the holy grail,” Dr. Nissen said.
A major cautionary note was sounded by a European Medicines Agency–mandated cardiovascular outcomes study of sibutramine, said Dr. Nissen. In clinical trials, patients taking sibutramine had seen modest weight loss, with increased HDL cholesterol and decreased triglycerides. However, blood pressure rose by 1-3 mm Hg, and heart rates also climbed by 4-5 beats per minute, changes consistent with sibutramine’s sympathomimetic effects, said Dr. Nissen. The EMA-mandated trial included over 10,000 patients and looked at a composite endpoint of major cardiovascular events, including death, MI, stroke, and resuscitated arrest. Patients were included if they were aged older than 55 years, had a body mass index of greater than 27 kg/m2, and had a history of cardiovascular disease or diabetes with an additional risk factor. Patients who had significant heart rate or blood pressure increases during the study run-in period were excluded.
In the end, patients taking sibutramine had an increased risk for the composite endpoint (11.4% vs. 10.0%; P = .02). The risk for nonfatal stroke and nonfatal MI was also significantly elevated for the sibutramine group (N Engl J Med. 2010; 363:905-17).
Phentermine is a pharmacologic relative of sibutramine, with similar effects on blood pressure and heart rate. Since it was approved prior to the current increased focus on real-world clinical outcomes in drug approvals, phentermine’s cardiovascular outcomes have never been studied by means of a RCT. “Nobody’s going to do this study unless we push for it, but it has to be done,” he said. “Although this drug reduces weight, there is considerable uncertainty whether it increases cardiovascular outcomes.”
Even looking at weight loss alone, pharmacologic treatments show marginal benefit over time, said Dr. Nissen, citing, as an example, recently published outcome data on lorcaserin. Over 40 months of treatment, there was a “complete absence of any benefit for lorcaserin,” compared with placebo, and participants saw an average weight loss of just 1.9 kg by the end of the study period, with no change in cardiovascular outcomes (N Engl J Med. 2018;379:1107-17).
To drive home the point, Dr. Nissen shared a slide entitled “Established Benefits of Weight Loss Drugs on Clinically Important Outcomes.” The slide’s text read, “This slide intentionally left blank.”
“It’s very hard for me to argue in favor of giving any of these drugs,” said Dr. Nissen. “In the absence of established outcome benefits, there are only risks and costs. I know this is not going to be popular with everyone in this audience, but I have to tell you what I really believe here: We have to do better.”
More broadly, “I think we have to demand outcome trials for obesity drugs,” said Dr. Nissen. He noted that such trials are underway for some glucagonlike peptide–1 agonists, “and I applaud them. ... I hope you will participate in those studies, because they are going to give us some answers.”
Calling for renewed efforts to improve the efficacy of lifestyle interventions, Dr. Nissen said, “What we have to do is try. .... You know as well as I that there are some outliers” who will achieve profound weight loss without surgery, and those patients are likely to reap big benefits in risk reduction.
“We’ve got a problem that affects tens of millions of people, and we’ve got to find a societal approach to this. But we share these patients; let’s work together on trying to make them better.”
Dr. Nissen did not report any relevant financial disclosures.
koakes@mdedge.com
EXPERT ANALYSIS FROM OBESITY WEEK 2018
U.S. death rates from chronic liver disease continue to rise
SAN FRANCISCO –
“I believe it’s all related to a big increase in obesity and type 2 diabetes in this country,” lead study author Zobair M. Younossi, MD, MPH, said in an interview in advance of the annual meeting of the American Association for the Study of Liver Diseases. “Those two risk factors drive NAFLD and its progressive type, nonalcoholic steatohepatitis (NASH). That accounts for at least part of the increase in mortality related to liver disease.”
In an effort to evaluate recent mortality trends in chronic liver disease in the United States, Dr. Younossi and his colleagues drew from National Vital Statistics Data during 2007-2016. They used ICD-10 codes to select mortality data for alcoholic liver disease, chronic hepatitis B and C, iron overload, NAFLD, cirrhosis, and hepatocellular carcinoma. NAFLD cases were defined as those having an ICD-10 code for NAFLD/NASH or an ICD-10 code for “cirrhosis of unknown etiology.” Next, the researchers adjusted age-standardized death rates to the 2000 U.S. Census population and used logistic regression and propensity scores to estimate predictors of chronic liver disease-related deaths.
Dr. Younossi, who chairs the department of medicine at Inova Fairfax Medical Campus, in Falls Church, Va., and his colleagues reported findings from 838,809 chronic liver disease–related deaths during the study period. They found that the age-standardized death rate for chronic liver disease increased from 21.9/100,000 population in 2007 to 24.9/100,000 population in 2016, which translated into an annual percentage change of 1.3% for males and 2.5% for females. Chronic liver disease–related deaths increased with age and were highest among those aged 55-64 years, followed by those aged 65-74 years – an average annual percentage change of 3.4% and 3.1% in each group.
Among chronic liver disease–related deaths, the most common diagnostic etiology was NAFLD (34.7%), followed by alcoholic liver disease (28.8%) and chronic hepatitis C (21.1%). Between 2007 and 2016, death rates increased from 7.6 to 9.0 per 100,000 population for NAFLD (an average annual percentage change of 2.1%) and from 6.1 to 7.9 per 100,000 population for alcoholic liver disease (an average annual percentage change of 3.1%). “What surprised me was that, despite highly effective treatment for HCV, we still have a burden of hepatitis C in this country,” Dr. Younossi said. “It’s still the most common cause of liver disease in the U.S. But it seems like hepatitis C–related liver disease is being replaced quickly by liver disease from nonalcoholic steatohepatitis. This transition between hepatitis C as the most important cause of liver disease and liver mortality to NASH or obesity-related NASH is becoming more rapid than I expected.”
On multivariate analysis, three factors were independently associated with an increased risk of death in NAFLD: the presence of type 2 diabetes (odds ratio, 1.78), cardiovascular disease (OR, 1.07), and renal failure (OR, 1.08).
“One important message from this study is that NASH is very common in the U.S. population,” said Dr. Younossi, who is also a professor of medicine at Virginia Commonwealth University, Richmond. “These patients are underrecognized and underdiagnosed because they are asymptomatic. The second message is that there is a subtype of patients with fatty liver disease – even a subtype of NASH – that can progress to cirrhosis and its complications. We have to pay attention to this silent disease to identify patients who are at risk for progressive liver disease and try to address some of the risk issues, such as tight control of diabetes, obesity, and control of hypertension and hyperlipidemia. Short of that, right now we have very few medical treatments such as vitamin E and pioglitazone recommended for a very selected group. In contrast, there are plenty of new medications that are being developed. The first step in tackling this disease is to identify who the patients are with fatty liver disease who are at risk for bad outcomes and make sure they’re linked to care by a knowledgeable caregiver [who] understands the importance of NASH.”
Dr. Younossi acknowledged certain limitations of the study, including the fact that liver disease diagnoses were based on ICD-10 coding. He disclosed that he is a consultant for Gilead, Intercept, Novo Nordisk, BMS, AbbVie, Viking, Term Quest Diagnostics, Echosens,and Shionogi. He has also received grant/research support from Gilead, Intercept, and BMS.
Source: Hepatol. 2018;68[S1], Abstract 763.
SAN FRANCISCO –
“I believe it’s all related to a big increase in obesity and type 2 diabetes in this country,” lead study author Zobair M. Younossi, MD, MPH, said in an interview in advance of the annual meeting of the American Association for the Study of Liver Diseases. “Those two risk factors drive NAFLD and its progressive type, nonalcoholic steatohepatitis (NASH). That accounts for at least part of the increase in mortality related to liver disease.”
In an effort to evaluate recent mortality trends in chronic liver disease in the United States, Dr. Younossi and his colleagues drew from National Vital Statistics Data during 2007-2016. They used ICD-10 codes to select mortality data for alcoholic liver disease, chronic hepatitis B and C, iron overload, NAFLD, cirrhosis, and hepatocellular carcinoma. NAFLD cases were defined as those having an ICD-10 code for NAFLD/NASH or an ICD-10 code for “cirrhosis of unknown etiology.” Next, the researchers adjusted age-standardized death rates to the 2000 U.S. Census population and used logistic regression and propensity scores to estimate predictors of chronic liver disease-related deaths.
Dr. Younossi, who chairs the department of medicine at Inova Fairfax Medical Campus, in Falls Church, Va., and his colleagues reported findings from 838,809 chronic liver disease–related deaths during the study period. They found that the age-standardized death rate for chronic liver disease increased from 21.9/100,000 population in 2007 to 24.9/100,000 population in 2016, which translated into an annual percentage change of 1.3% for males and 2.5% for females. Chronic liver disease–related deaths increased with age and were highest among those aged 55-64 years, followed by those aged 65-74 years – an average annual percentage change of 3.4% and 3.1% in each group.
Among chronic liver disease–related deaths, the most common diagnostic etiology was NAFLD (34.7%), followed by alcoholic liver disease (28.8%) and chronic hepatitis C (21.1%). Between 2007 and 2016, death rates increased from 7.6 to 9.0 per 100,000 population for NAFLD (an average annual percentage change of 2.1%) and from 6.1 to 7.9 per 100,000 population for alcoholic liver disease (an average annual percentage change of 3.1%). “What surprised me was that, despite highly effective treatment for HCV, we still have a burden of hepatitis C in this country,” Dr. Younossi said. “It’s still the most common cause of liver disease in the U.S. But it seems like hepatitis C–related liver disease is being replaced quickly by liver disease from nonalcoholic steatohepatitis. This transition between hepatitis C as the most important cause of liver disease and liver mortality to NASH or obesity-related NASH is becoming more rapid than I expected.”
On multivariate analysis, three factors were independently associated with an increased risk of death in NAFLD: the presence of type 2 diabetes (odds ratio, 1.78), cardiovascular disease (OR, 1.07), and renal failure (OR, 1.08).
“One important message from this study is that NASH is very common in the U.S. population,” said Dr. Younossi, who is also a professor of medicine at Virginia Commonwealth University, Richmond. “These patients are underrecognized and underdiagnosed because they are asymptomatic. The second message is that there is a subtype of patients with fatty liver disease – even a subtype of NASH – that can progress to cirrhosis and its complications. We have to pay attention to this silent disease to identify patients who are at risk for progressive liver disease and try to address some of the risk issues, such as tight control of diabetes, obesity, and control of hypertension and hyperlipidemia. Short of that, right now we have very few medical treatments such as vitamin E and pioglitazone recommended for a very selected group. In contrast, there are plenty of new medications that are being developed. The first step in tackling this disease is to identify who the patients are with fatty liver disease who are at risk for bad outcomes and make sure they’re linked to care by a knowledgeable caregiver [who] understands the importance of NASH.”
Dr. Younossi acknowledged certain limitations of the study, including the fact that liver disease diagnoses were based on ICD-10 coding. He disclosed that he is a consultant for Gilead, Intercept, Novo Nordisk, BMS, AbbVie, Viking, Term Quest Diagnostics, Echosens,and Shionogi. He has also received grant/research support from Gilead, Intercept, and BMS.
Source: Hepatol. 2018;68[S1], Abstract 763.
SAN FRANCISCO –
“I believe it’s all related to a big increase in obesity and type 2 diabetes in this country,” lead study author Zobair M. Younossi, MD, MPH, said in an interview in advance of the annual meeting of the American Association for the Study of Liver Diseases. “Those two risk factors drive NAFLD and its progressive type, nonalcoholic steatohepatitis (NASH). That accounts for at least part of the increase in mortality related to liver disease.”
In an effort to evaluate recent mortality trends in chronic liver disease in the United States, Dr. Younossi and his colleagues drew from National Vital Statistics Data during 2007-2016. They used ICD-10 codes to select mortality data for alcoholic liver disease, chronic hepatitis B and C, iron overload, NAFLD, cirrhosis, and hepatocellular carcinoma. NAFLD cases were defined as those having an ICD-10 code for NAFLD/NASH or an ICD-10 code for “cirrhosis of unknown etiology.” Next, the researchers adjusted age-standardized death rates to the 2000 U.S. Census population and used logistic regression and propensity scores to estimate predictors of chronic liver disease-related deaths.
Dr. Younossi, who chairs the department of medicine at Inova Fairfax Medical Campus, in Falls Church, Va., and his colleagues reported findings from 838,809 chronic liver disease–related deaths during the study period. They found that the age-standardized death rate for chronic liver disease increased from 21.9/100,000 population in 2007 to 24.9/100,000 population in 2016, which translated into an annual percentage change of 1.3% for males and 2.5% for females. Chronic liver disease–related deaths increased with age and were highest among those aged 55-64 years, followed by those aged 65-74 years – an average annual percentage change of 3.4% and 3.1% in each group.
Among chronic liver disease–related deaths, the most common diagnostic etiology was NAFLD (34.7%), followed by alcoholic liver disease (28.8%) and chronic hepatitis C (21.1%). Between 2007 and 2016, death rates increased from 7.6 to 9.0 per 100,000 population for NAFLD (an average annual percentage change of 2.1%) and from 6.1 to 7.9 per 100,000 population for alcoholic liver disease (an average annual percentage change of 3.1%). “What surprised me was that, despite highly effective treatment for HCV, we still have a burden of hepatitis C in this country,” Dr. Younossi said. “It’s still the most common cause of liver disease in the U.S. But it seems like hepatitis C–related liver disease is being replaced quickly by liver disease from nonalcoholic steatohepatitis. This transition between hepatitis C as the most important cause of liver disease and liver mortality to NASH or obesity-related NASH is becoming more rapid than I expected.”
On multivariate analysis, three factors were independently associated with an increased risk of death in NAFLD: the presence of type 2 diabetes (odds ratio, 1.78), cardiovascular disease (OR, 1.07), and renal failure (OR, 1.08).
“One important message from this study is that NASH is very common in the U.S. population,” said Dr. Younossi, who is also a professor of medicine at Virginia Commonwealth University, Richmond. “These patients are underrecognized and underdiagnosed because they are asymptomatic. The second message is that there is a subtype of patients with fatty liver disease – even a subtype of NASH – that can progress to cirrhosis and its complications. We have to pay attention to this silent disease to identify patients who are at risk for progressive liver disease and try to address some of the risk issues, such as tight control of diabetes, obesity, and control of hypertension and hyperlipidemia. Short of that, right now we have very few medical treatments such as vitamin E and pioglitazone recommended for a very selected group. In contrast, there are plenty of new medications that are being developed. The first step in tackling this disease is to identify who the patients are with fatty liver disease who are at risk for bad outcomes and make sure they’re linked to care by a knowledgeable caregiver [who] understands the importance of NASH.”
Dr. Younossi acknowledged certain limitations of the study, including the fact that liver disease diagnoses were based on ICD-10 coding. He disclosed that he is a consultant for Gilead, Intercept, Novo Nordisk, BMS, AbbVie, Viking, Term Quest Diagnostics, Echosens,and Shionogi. He has also received grant/research support from Gilead, Intercept, and BMS.
Source: Hepatol. 2018;68[S1], Abstract 763.
AT THE LIVER MEETING 2018
Key clinical point: Nonalcoholic steatohepatitis is very common in the U.S. population.
Major finding: Between 2007 and 2016, the age-standardized death rate for chronic liver disease increased from 21.9/100,000 population to 24.9/100,000 population.
Study details: An analysis of 838,809 chronic liver disease–related deaths from 2007 to 2016.
Disclosures: Dr. Younossi disclosed that he is a consultant for Gilead, Intercept, Novo Nordisk, Bristol-Myers Squibb, AbbVie, Viking, Term Quest Diagnostics, Echosens, and Shionogi. He has also received grant/research support from Gilead, Intercept, and Bristol-Myers Squibb.
Source: Hepatol. 2018;68[S1], Abstract 763.
Providing Rural Veterans With Access to Exercise Through Gerofit
Clinical video telehealth can be used to deliver functional circuit exercise training to older veterans in remote locations.
Exercise increases endurance, muscle strength, and functional performance with corresponding gains in mobility, survival, and quality of life.1 However, even with these benefits and improvements in clinical outcomes, only 15% of adults aged ≥ 65 years follow current guidelines for exercise.2 Despite their prior military training, the majority of veterans do not meet physical activity recommendations.3 Time, travel, and support are common barriers to exercise participation and adherence—barriers that are further amplified among older adults.
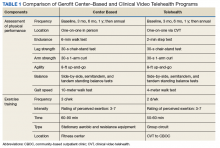
The Veterans Health Administration (VHA) is recognized as a world leader in telehealth service development. Currently, 677,000 veterans have received telehealth services, which represents 12% of the 5.6 million veterans under VHA care.4 Clinical video telehealth (CVT) is widely used within the VHA system to deliver health care that otherwise would not be available to veterans. Veterans who have difficulty traveling to the nearest US Department of Veteran Affairs (VA) medical center (VAMC) can access CVT programs at a participating VHA community-based outpatient clinic (CBOC). The VA has more than 45 CVT programs, including programs for mental health, weight management, cardiology, and dermatology. Outside the VA, cardiac exercise rehabilitation provided by CVT has been shown to be as effective as center-based programs in improving cardiovascular risk factors and functional capacity.5 A VHA exercise program that leveraged CVT resources and was dedicated to older adults with a wide range of comorbid conditions would have a high impact on the health and well-being of older veterans.
Gerofit is a VHA clinical demonstration program of supervised center-based exercise for veterans aged ≥ 65 years. Developed at the Durham VAMC Geriatric Research, Education, and Clinical Center (GRECC) in North Carolina, it has demonstrated improved clinical outcomes, including physical function, mobility, quality of life, and survival.6-10 The program offers veterans individualized exercise in a group setting that focuses on improving endurance, strength, and balance. The exercise prescription is based on the patient’s physical limitations as identified in a physical performance assessment.
With support from VHA Geriatric Extended Care (GEC) and the Office of Rural Health (ORH), Gerofit was implemented in 10 VAMCs across 8 VISNs. However, barriers such as travel time, distance, and transportation limit participation. Previously, we found that rural veterans lack access to exercise programs.11,12 Although some do aerobic exercise (AEX), most do not do resistance training (RT), though they are willing to learn. Access to Gerofit for rural veterans is expanding with recent support from the ORH Enterprise Wide Initiative. Rural program expansion includes several different Gerofit initiatives, many involving CBOCs.
The Salem VAMC Gerofit program sought to adapt the facility-based assessment and exercise procedures into a self-reliant CVT class for its CBOCs. This article describes the development of the Salem VAMC Gerofit CVT program, hereafter referred to as Tele-Gerofit.
Related: Expanding the Scope of Telemedicine in Gastroenterology
Program Design
Gerofit was established in 1986 at the Durham GRECC as an exercise and health promotion program for veterans aged ≥ 65 years.13 Its goal is to prevent or improve functional decline from physical inactivity and age-related conditions. Gerofit targets the geriatric patient population and thus extends beyond cardiac and pulmonary rehabilitation or weight loss programs. The primary exclusion criteria are based on safety issues in the context of a group exercise setting of older adults and include oxygen dependency, unstable cardiac disease, and moderate-to-severe cognitive impairment.
To participate in Gerofit, veterans must be able to perform activities of daily living and self-manage an exercise prescription developed by the exercise instructor based on physical performance testing. These physical performance tests include measures that are independent predictors of disability, loss of independent living, and death, as well as surrogate measures of exercise capacity (eg, strength, endurance, balance).14,15 A novel aspect of Gerofit is that the physical performance assessment is used not only to determine physical limitations, but also to individualize the exercise prescription based on the observed deficits in strength, endurance, or balance. These assessments are performed at initial enrollment; 3 months, 6 months, and 1 year later; and annually after that. Currently, the center-based Gerofit programs administer 5 items of the Senior Fitness Test: 6-minute walk, 10-meter walk (10-MWT), 30-second 1-arm curl, 30-second chair-stand test, and 8-foot up-and-go.15 The side-by-side, semitandem, and tandem standing balance tests from the short physical performance battery also are performed.16 In addition, participants complete a questionnaire that includes items from the physical functioning scale of the 12-Item Short Form Health Survey (SF-12).
After each assessment, the Gerofit exercise instructor reviews the results with the veteran and formulates an individualized exercise prescription along with goals for improvement. Veterans are encouraged to attend supervised center-based exercise sessions 3 times weekly. Classes are offered in a gym or fitness center at the VAMC or in leased space. Each patient uses a cue card that lists an exercise plan personalized for intensity and duration for aerobic exercise (AEX; eg, treadmill walking, stationary bicycling, arm ergometry), RT using dumbbells and weight equipment, and functional exercises for flexibility and balance. Some medical centers also offer yoga, tai chi, or dancing Gerofit classes.
For participants in the Durham Gerofit program, mortality decreased 25% over a decade (hazard ratio, 0.75; 95% CI, 0.61-0.91).9 A substudy that included the Psychological General Well-Being Index found that 81% of participants significantly increased their score after 1 year.7 Observed initial improvement in physical performance has been sustained over 5 years.10,17 One-year results from the recent Gerofit expansion to 6 other VAMCs showed clinically and statistically significantly improved physical performance from baseline to 3-, 6-, and 12-month follow-up.18
Adaptation of Gerofit to CVT Delivery
Initial work. The Greater Los Angeles VAMC Gerofit program conducted a pilot CVT exercise class of 6 veterans at the rural Bakersfield CBOC in California.19 Each week, an exercise instructor broadcast a 60-minute exercise class that included warm-up, RT with bands, progressive balance training, and flexibility. Trained student volunteers from California State University in Bakersfield kinesiology program were on site at the Bakersfield CBOC to perform the assessments and aid in exercises during the CVT sessions. Despite the lack of AEX per se, veterans showed significant improvement in endurance as measured by an increase in the number of steps completed in 2 minutes at the 3-month assessment (P = .049). Although exercises were not delivered in a circuit format, the improved endurance supported the potential for cardiovascular benefit from RT in older adults.
This pilot project also demonstrated that key components of the Gerofit program could be delivered safely by telehealth with onsite supervision. The Miami VA Healthcare System also offers CVT Gerofit exercise classes broadcast to the rural Florida CBOCs of Key Largo and Homestead.11 The exercise activities offered for the Miami CVT participants incorporate components of AEX (calisthenics) and RT (resistance bands). Veterans enjoyed the classes, and adherence was good. However, availability of staff and space are an ongoing challenge.
In Key Largo, 5 veterans participated before the CVT classes were placed on hold owing to the demands of other CVT programs and limited availability of the telehealth clinical technician (TCT). The Homestead CBOC continues to offer CVT Gerofit exercise classes and has 6 regular participants. Notably, the physical space at the Homestead CBOC is smaller than that at the Key Largo CBOC; the Homestead CBOC has adjusted by shifting to exercises performed while standing or sitting, ensuring participants’ safety and satisfaction.
The Baltimore, Maryland VAMC Gerofit program offers other innovative CVT exercise classes, including a tai chi class, and a class with exercise performed while sitting in a chair. Although the Baltimore VAMC CVT exercise classes do not have the scope of the center-based exercise prescriptions, they are unique in that they are broadcast not only to their affiliated CBOCs, but also other Gerofit programs in different VISNs.
Related: Telehealth for Rural Veterans With Neurologic Disorders
Salem VAMC Gerofit Program. The center-based Salem VAMC Gerofit program was established in July 2015. In fiscal year 2017, its dedicated exercise facility had more than 5,000 patient visits. Despite the program’s success, we prioritized establishing Tele-Gerofit because of the medical center’s rural location in southwest Virginia and the large number of veterans who receive care at CBOCs. Therefore, much as with the pilot CVT Gerofit classes in Los Angeles and Miami, the target setting was rural CBOCs. The goal for Salem VAMC Tele-Gerofit was to modify Gerofit delivery to the CVT format and a CBOC setting with minimal modification of the content and personnel requirements of both physical performance testing and exercise training procedures.
Adjustments for CBOC Setting. The enrollment process for Tele-Gerofit is the same as that for the center-based program. To start, a veteran’s primary care provider reviews the list of eligibility criteria and, if the veteran qualifies, places a consult. A Gerofit team member then contacts the veteran by phone to describe the program and schedule an assessment. At the baseline physical performance assessment, American College of Sports Medicine guidelines on exercise participation, health screening, and exercise intensity are used to evaluate veterans and rank them by their cardiovascular risk.20 All new program participants start with low-intensity exercise and gradually progress to recommended levels of exercise. Before starting an exercise class, participants are instructed on use of the 10-point rating of perceived exertion (RPE).
Each CBOC site is supplied with an RPE poster that is displayed for participants’ use. During a Tele-Gerofit class, the exercise instructor asks participants to periodically report their RPE. This class differs slightly from the center-based exercise sessions in which RPE is primarily assessed when a different exercise is introduced or the duration or intensity of an exercise is increased. The Gerofit instructor monitors exercise and treatment fidelity, but the onsite TCT observes for safety during class. The TCT also takes initial vital signs and sets up the room for the class. Emergency contacts and procedures are posted in each CBOC CVT room and are available to the center-based exercise instructor. Because the CBOCs are not inside medical facilities, some CBOC directors have asked that heart rate monitors be used as an extra safety precaution to ensure that high-risk participants do not exceed a heart rate limit that may be set by their cardiologists.
Modifications to Physical Performance Assessment. Physical performance testing had to be adapted to the small rooms available at the CBOCs. For measuring normal gait speed, the 10-MWT was replaced with the 4-meter walk test (4-MWT). The 4-MWT has excellent test–retest reliability with an intraclass correlation coefficient (ICC) of 0.93, but the discrepancy in gait speed between the 4-MWT and the 10-MWT is such that the tests cannot be used interchangeably.21 For measuring endurance, the 6-minute walk test was replaced with the 2-minute step test (2-MST). In older adults, the 2-MST has a moderate correlation with 6-minute walk distance (r = 0.36; P = .04) and high reliability (ICC, 0.90).15,22 The 30-second 1-arm curl, the 30-second chair-stand test, and the 8-foot up-and-go test are performed without modification and require only dumbbells, a chair without wheels, and a stopwatch.
The exercise instructor at the Salem VAMC conducts physical performance testing by 2-way videoconferencing with the veteran in a room at the CBOC. The TCT at the CBOC assists by measuring and demarcating 4 meters on the floor and a designated height on the wall for knee elevation for 4-MWT and 2-MST, respectively. The TCT remains in the room during the assessment visit. Except for taking vital signs before and after the physical performance assessment, the TCT does not participate in the testing. To date, more than 20 physical performance assessments have been conducted without difficulty at Salem-affiliated CBOCs. The primary challenge has been scheduling the room with CVT equipment (ie, camera and screen) for the 30-minute individual assessment session, which occurs on a rolling basis as individuals are enrolled and followed.
After the assessment is completed, the exercise instructor reviews the results with the participant and provides feedback on areas in need of improvement. However, these education sessions can be lengthy and are best supported by giving the patient a personalized handout.
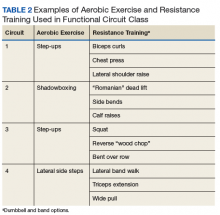
Functional Circuit Exercise. In Tele-Gerofit, exercise training is delivered by CVT broadcast from the Salem VAMC to veterans in a room (equipped with steps, dumbbells, chairs, and bands) at the CBOC. This type of exercise training, which uses only mobile equipment and plyometric (weight-bearing) exercises, is referred to as functional exercise. The AEX includes marching in place, moving on and off a raised step, and body-weight exercises, while RT uses dumbbells, resistance bands, and plyometric exercises (Table 2).
Progression of intensity is achieved by increasing the rate of stepping and the size of the steps (AEX) or the number of repetitions and the weight of the dumbbells or bands (RT). Each veteran exercises at an intensity level that is appropriate for his or her baseline limitations and medical conditions. The exercise instructor uses different forms of the same equipment (eg, heavier dumbbells, higher steps) to vary intensity among individuals while having them perform the same exercises as a group. The challenge is to adjust the pace of the AEX or the timing of the RT repetitions for individuals new to the class.
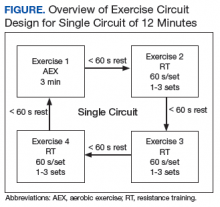
Delivery of exercise training in the form of circuits allows for a diverse exercise program in a setting with limited space. Circuit training is an exercise modality that consists of a series of different exercises, each usually completed in 30 to 60 seconds, with minimal rest between each type of exercise. Each Tele-Gerofit circuit has a mix of AEX and RT exercises performed for 3 minutes consecutively (Figure).
The design of the circuit training can be adjusted based on the number of individuals in the class. Larger classes can be split into 2 groups that alternate between exercise sets, while smaller classes have 1 group performing the same exercise set and then rotating to either the AEX or RT set. Total exercise time to complete the circuit depends on the number of different exercises, number of repetitions, and the rest between repetitions and the different exercises. In this way, total exercise time can be made shorter or longer depending on the veteran’s capacity.
Frequency. Tele-Gerofit exercise classes are currently offered twice weekly and last about 1 hour, which includes warm-up (8-10 minutes), functional circuit training (40 minutes), and cooldown/stretching (8-10 minutes). A challenge for the exercise instructor is the need to provide ongoing clear instructions both to the class and to individuals as needed. As the exercise prescription for each patient is based on physical performance testing, the exercise instructor for the training must be familiar with the test results. Derivation of the exercise prescription in Tele-Gerofit follows the same process as center-based Gerofit.
Each patient is given an exercise prescription written to address any impairments noted in the different domains of the physical performance assessment, scored using age and sex percentiles. For instance, individuals scoring poorly on lower body strength are given specific lower body strengthening exercises. Participants are given an exercise program that guides them toward achieving recommended physical activity guidelines using their RPE to modulate each exercise. Duration and intensity of each type of planned exercise are formally discussed after initial and follow-up assessments. In addition, exercise training is informally progressed throughout the program. For Tele-Gerofit, instructors must design each class with the group in mind while being prepared for modifications and specific changes for individuals.
Discussion
Tele-Gerofit adapts the well-established center-based Gerofit program to be executed without an exercise facility while maintaining the content of the evidence-based procedures. Physical performance testing and exercise training were modified, adding elements necessary for CVT assessments and classes to be broadcast from the Salem VAMC to its affiliated CBOCs. Tele-Gerofit exercises are performed in a circuit style that allows a veteran or small structured groups of veterans to move among exercises and requires less space than traditional group exercise does. Safety and monitoring concerns are addressed with a safety procedure that includes emergency plans for each site, prescreening of enrolled participants, and monitoring of exercise intensity in accordance with national guidelines.1 Similar to the center-based Gerofit program, the exercise prescription is tailored to each veteran’s physical limitations based on initial and ongoing assessment of physical performance. Tele-Gerofit physical performance testing fulfills the same need with only a few modifications using validated measures. Tele-Gerofit assessments are administered by CVT without the need for additional staff on site.
Adaptation of center-based Gerofit exercise classes to Tele-Gerofit is a major innovation. Use of a circuit exercise design was supported by findings in older adults that RT alone, when performed quickly with minimal rest between each set and exercise station, increases both aerobic capacity and strength.23,24 Older adult RT trials that compared circuit RT with traditional RT found that strength gains are comparable between circuit and traditional RT.24-26 Working with adults aged > 60 years, Takeshima and colleagues conducted a trial of circuit exercise with added callisthenic exercises performed in place between RT on exercise machines.27 This dual-modality (AEX+RT) circuit approach was well tolerated and effective, increasing aerobic capacity and strength. Unfortunately, the resistance exercise machines used in those circuit exercise studies and in the center-based Gerofit program are not an option for Tele-Gerofit.
The requirement for an exercise facility was removed by designing Tele-Gerofit exercise to include only functional exercises that rely on body weight or small mobile exercise equipment. Although popular among young adults, functional circuit exercise is understudied in older adults. Recently, a 12-week functional circuit exercise intervention in frail elderly adults demonstrated significant improvements in gait speed and the timed chair-stand test.28 A pilot observational study of Gerofit participants at the Canandaigua VAMC offered 27 veterans functional circuit exercise instead of their traditional exercise facility class and found larger increases in the timed chair-stand test and 6-minute walk distance compared with 11 Gerofit participants in the traditional program.29
This Tele-Gerofit exercise training combines functional and circuit exercise strategies into telehealth delivery. However, its effect on physical performance remains to be demonstrated. To address this question, we are conducting a single-arm pilot study of Tele-Gerofit with CVT broadcast to 3 Salem CBOC affiliates (Wytheville, Staunton, and Danville, Virginia). The goal is to determine the effect on physical performance and collect feasibility data, including attendance rate and patient satisfaction with the video broadcast. In addition, we are planning an effectiveness trial to compare the impact of functional circuit exercise delivered in person (center based, not CVT) with the parent Gerofit exercise program on direct measures of endurance and strength, in addition to physical performance.
Related: Setting and Method of Measurement Affect Blood Pressure Readings in Older Veterans
Implementation research is needed to determine how Tele-Gerofit can be disseminated to other VAMCs and community-based centers beyond CBOCs. Although the cost of the equipment used to implement Tele-Gerofit is minimal, the program requires dedicated and experienced exercise instructors, and the sharing of telehealth resources with other clinical programs. The authors expect that a diverse group of stakeholders is needed across service lines of primary care, geriatrics and extended care, physical medicine and rehabilitation, and telehealth. Of note, this multidisciplinary collaboration is a hallmark of the Gerofit program. The recent success of the implementation of center-based Gerofit in VAMCs across the US demonstrates the program’s flexibility and robust results.18
Plans also include refining strategies for physical performance testing and exercise monitoring. For instance, we would like to adapt telehealth technology for heart rate monitors that can be worn by high-risk veterans at the CBOC and viewed in real time by the exercise instructor.
Conclusion
Gerofit, which is designed to help older veterans maintain independent living and prevent disability, has been demonstrated to improve quality of life and survival. Our goal has been to adapt Gerofit to CVT and provide a supervised, individualized exercise program in a group setting—a program that can be widely disseminated. Salem VAMC Tele-Gerofit is an innovative and prescriptive program that delivers CVT functional circuit exercise training to remote locations without the need for stationary exercise equipment. This approach has the potential to become an effective and feasible exercise strategy for preventing and minimizing disability in the increasing population of older veterans. Work is needed to determine whether Tele-Gerofit provides a rapid translation of Gerofit to clinical practice and improved outcomes with substantial cost savings from reduced hospitalization and institutionalization.
Acknowledgments
Gerofit has been funded by the Veterans Health Affairs Office of Geriatrics and Extended Care Non-Institutional Long-Term Care Funding and Mentored Partnership Program, and the Veterans Health Affairs Office of Rural Health Rural Enterprise-Wide Initiative.
The authors thank Kim Birkett, MPH, for assistance in editing, references, and graphics and the staff at the Wytheville, Staunton, and Danville community-based outpatient clinics for their support.
1. American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510-1530.
2. Centers for Disease Control and Prevention. Adult participation in aerobic and muscle-strengthening physical activities—United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62(17):326-330.
3. Littman AJ, Forsberg CW, Koepsell TD. Physical activity in a national sample of veterans. Med Sci Sports Exerc. 2009;41(5):1006-1013.
4. US Department of Veterans Affairs, Office of Rural Health. Annual Report: Thrive 2015. https://www.ruralhealth.va.gov/docs/ORH_Annual_Report_2015_FINAL.pdf. Published 2015. Accessed July 16, 2018.
5. Rawstorn JC, Gant N, Direito A, Beckmann C, Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart. 2016;102(15):1183-1192.
6. Morey MC. Celebrating 20 years of excellence in exercise for the older veteran. Fed Pract. 2007;24(10):49-65.
7. Cowper PA, Morey MC, Bearon LB, et al. The impact of supervised exercise on the psychological well-being and health status of older veterans. J Appl Gerontol. 1991;10(4):469-485.
8. Morey MC, Cowper PA, Feussner JR, et al. Evaluation of a supervised exercise program in a geriatric population. J Am Geriatr Soc. 1989;37(4):348-354.
9. Morey MC, Pieper CF, Crowley GM, Sullivan RJ, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50(12):1929-1933.
10. Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44(10):1226-1231.
11. Valencia WM, Botros D, Pendlebury D, et al. Proactive reach and telehealth monitoring (Gerofit) enhance resistance exercise at rural setting. Innovat Aging. 2017;1(suppl 1):225.12. Pendlebury D, Botros D VW. Proactive Reach: an innovative access approach to identify & deliver GEROFIT exercise telehealth counseling to rural veterans & enhance CBOC services. J Am Geriatr Soc. 2017(suppl 1):S208. Poster presented at: Annual Scientific Meeting of the American Geriatrics Society; May 18, 2017; San Antonio, TX.
13. Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit program: a VA innovation. South Med J. 1994;87(5):S83-S87.
14. Cooper R, Kuh D, Hardy R; Mortality Review Group; FALCon and HALCyon Study Teams. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467.
15. Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7(2):129-161.
16. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-M94.
17. Morey MC, Cowper PA, Feussner JR, et al. Two-year trends in physical performance following supervised exercise among community-dwelling older veterans. J Am Geriatr Soc. 1991;39(10):986-992.
18. Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit program. J Am Geriatr Soc. 2018;66(5):1009-1016.
19. Blanchard E, Castle S, Ines E, et al. Delivering a clinical exercise program to rural veterans via video telehealth. Poster C167 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
20. Riebe D, Ehrman JK, Liguori G, Magal M, eds; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Philadelphia, PA: Wolters Kluwer Health; 2018.
21. Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-meter walk test for measurements of gait speed in healthy, older adults. J Geriatr Phys Ther. 2013;36(1):24-30.
22. Pedrosa R, Holanda G. Correlation between the walk, 2-minute step and TUG tests among hypertensive older women. Rev Bras Fisioter. 2009;13(3):252-256.
23. Romero-Arenas S, Blazevich AJ, Martinez-Pascual M, et al. Effects of high-resistance circuit training in an elderly population. Exp Gerontol. 2013;48(3):334-340.
24. Brentano MA, Cadore EL, Da Silva EM, et al. Physiological adaptations to strength and circuit training in postmenopausal women with bone loss. J Strength Cond Res. 2008;22(6):1816-1825.
25. Romero-Arenas S, Martinez-Pascual M, Alcaraz PE. Impact of resistance circuit training on neuromuscular, cardiorespiratory and body composition adaptations in the elderly. Aging Dis. 2013;4(5):256-263.
26. Paoli A, Pacelli F, Bargossi AM, et al. Effects of three distinct protocols of fitness training on body composition, strength and blood lactate. J Sports Med Phys Fitness. 2010;50(1):43-51.
27. Takeshima N, Rogers ME, Islam MM, Yamauchi T, Watanabe E, Okada A. Effect of concurrent aerobic and resistance circuit exercise training on fitness in older adults. Eur J Appl Physiol. 2004;93(1-2):173-182.
28. Giné-Garriga M, Guerra M, Pagés E, Manini TM, Jiménez R, Unnithan VB. The effect of functional circuit training on physical frailty in frail older adults: a randomized controlled trial. J Aging Phys Act. 2010;18(4):401-424.
29. Biddle ED, Reynolds P, Kopp T, Cammarata H, Conway P. Implementation of functional training tools elicits improvements in aerobic fitness and lower body strength in older veterans. Poster C169 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
Clinical video telehealth can be used to deliver functional circuit exercise training to older veterans in remote locations.
Clinical video telehealth can be used to deliver functional circuit exercise training to older veterans in remote locations.
Exercise increases endurance, muscle strength, and functional performance with corresponding gains in mobility, survival, and quality of life.1 However, even with these benefits and improvements in clinical outcomes, only 15% of adults aged ≥ 65 years follow current guidelines for exercise.2 Despite their prior military training, the majority of veterans do not meet physical activity recommendations.3 Time, travel, and support are common barriers to exercise participation and adherence—barriers that are further amplified among older adults.
The Veterans Health Administration (VHA) is recognized as a world leader in telehealth service development. Currently, 677,000 veterans have received telehealth services, which represents 12% of the 5.6 million veterans under VHA care.4 Clinical video telehealth (CVT) is widely used within the VHA system to deliver health care that otherwise would not be available to veterans. Veterans who have difficulty traveling to the nearest US Department of Veteran Affairs (VA) medical center (VAMC) can access CVT programs at a participating VHA community-based outpatient clinic (CBOC). The VA has more than 45 CVT programs, including programs for mental health, weight management, cardiology, and dermatology. Outside the VA, cardiac exercise rehabilitation provided by CVT has been shown to be as effective as center-based programs in improving cardiovascular risk factors and functional capacity.5 A VHA exercise program that leveraged CVT resources and was dedicated to older adults with a wide range of comorbid conditions would have a high impact on the health and well-being of older veterans.
Gerofit is a VHA clinical demonstration program of supervised center-based exercise for veterans aged ≥ 65 years. Developed at the Durham VAMC Geriatric Research, Education, and Clinical Center (GRECC) in North Carolina, it has demonstrated improved clinical outcomes, including physical function, mobility, quality of life, and survival.6-10 The program offers veterans individualized exercise in a group setting that focuses on improving endurance, strength, and balance. The exercise prescription is based on the patient’s physical limitations as identified in a physical performance assessment.
With support from VHA Geriatric Extended Care (GEC) and the Office of Rural Health (ORH), Gerofit was implemented in 10 VAMCs across 8 VISNs. However, barriers such as travel time, distance, and transportation limit participation. Previously, we found that rural veterans lack access to exercise programs.11,12 Although some do aerobic exercise (AEX), most do not do resistance training (RT), though they are willing to learn. Access to Gerofit for rural veterans is expanding with recent support from the ORH Enterprise Wide Initiative. Rural program expansion includes several different Gerofit initiatives, many involving CBOCs.
The Salem VAMC Gerofit program sought to adapt the facility-based assessment and exercise procedures into a self-reliant CVT class for its CBOCs. This article describes the development of the Salem VAMC Gerofit CVT program, hereafter referred to as Tele-Gerofit.
Related: Expanding the Scope of Telemedicine in Gastroenterology
Program Design
Gerofit was established in 1986 at the Durham GRECC as an exercise and health promotion program for veterans aged ≥ 65 years.13 Its goal is to prevent or improve functional decline from physical inactivity and age-related conditions. Gerofit targets the geriatric patient population and thus extends beyond cardiac and pulmonary rehabilitation or weight loss programs. The primary exclusion criteria are based on safety issues in the context of a group exercise setting of older adults and include oxygen dependency, unstable cardiac disease, and moderate-to-severe cognitive impairment.
To participate in Gerofit, veterans must be able to perform activities of daily living and self-manage an exercise prescription developed by the exercise instructor based on physical performance testing. These physical performance tests include measures that are independent predictors of disability, loss of independent living, and death, as well as surrogate measures of exercise capacity (eg, strength, endurance, balance).14,15 A novel aspect of Gerofit is that the physical performance assessment is used not only to determine physical limitations, but also to individualize the exercise prescription based on the observed deficits in strength, endurance, or balance. These assessments are performed at initial enrollment; 3 months, 6 months, and 1 year later; and annually after that. Currently, the center-based Gerofit programs administer 5 items of the Senior Fitness Test: 6-minute walk, 10-meter walk (10-MWT), 30-second 1-arm curl, 30-second chair-stand test, and 8-foot up-and-go.15 The side-by-side, semitandem, and tandem standing balance tests from the short physical performance battery also are performed.16 In addition, participants complete a questionnaire that includes items from the physical functioning scale of the 12-Item Short Form Health Survey (SF-12).
After each assessment, the Gerofit exercise instructor reviews the results with the veteran and formulates an individualized exercise prescription along with goals for improvement. Veterans are encouraged to attend supervised center-based exercise sessions 3 times weekly. Classes are offered in a gym or fitness center at the VAMC or in leased space. Each patient uses a cue card that lists an exercise plan personalized for intensity and duration for aerobic exercise (AEX; eg, treadmill walking, stationary bicycling, arm ergometry), RT using dumbbells and weight equipment, and functional exercises for flexibility and balance. Some medical centers also offer yoga, tai chi, or dancing Gerofit classes.
For participants in the Durham Gerofit program, mortality decreased 25% over a decade (hazard ratio, 0.75; 95% CI, 0.61-0.91).9 A substudy that included the Psychological General Well-Being Index found that 81% of participants significantly increased their score after 1 year.7 Observed initial improvement in physical performance has been sustained over 5 years.10,17 One-year results from the recent Gerofit expansion to 6 other VAMCs showed clinically and statistically significantly improved physical performance from baseline to 3-, 6-, and 12-month follow-up.18
Adaptation of Gerofit to CVT Delivery
Initial work. The Greater Los Angeles VAMC Gerofit program conducted a pilot CVT exercise class of 6 veterans at the rural Bakersfield CBOC in California.19 Each week, an exercise instructor broadcast a 60-minute exercise class that included warm-up, RT with bands, progressive balance training, and flexibility. Trained student volunteers from California State University in Bakersfield kinesiology program were on site at the Bakersfield CBOC to perform the assessments and aid in exercises during the CVT sessions. Despite the lack of AEX per se, veterans showed significant improvement in endurance as measured by an increase in the number of steps completed in 2 minutes at the 3-month assessment (P = .049). Although exercises were not delivered in a circuit format, the improved endurance supported the potential for cardiovascular benefit from RT in older adults.
This pilot project also demonstrated that key components of the Gerofit program could be delivered safely by telehealth with onsite supervision. The Miami VA Healthcare System also offers CVT Gerofit exercise classes broadcast to the rural Florida CBOCs of Key Largo and Homestead.11 The exercise activities offered for the Miami CVT participants incorporate components of AEX (calisthenics) and RT (resistance bands). Veterans enjoyed the classes, and adherence was good. However, availability of staff and space are an ongoing challenge.
In Key Largo, 5 veterans participated before the CVT classes were placed on hold owing to the demands of other CVT programs and limited availability of the telehealth clinical technician (TCT). The Homestead CBOC continues to offer CVT Gerofit exercise classes and has 6 regular participants. Notably, the physical space at the Homestead CBOC is smaller than that at the Key Largo CBOC; the Homestead CBOC has adjusted by shifting to exercises performed while standing or sitting, ensuring participants’ safety and satisfaction.
The Baltimore, Maryland VAMC Gerofit program offers other innovative CVT exercise classes, including a tai chi class, and a class with exercise performed while sitting in a chair. Although the Baltimore VAMC CVT exercise classes do not have the scope of the center-based exercise prescriptions, they are unique in that they are broadcast not only to their affiliated CBOCs, but also other Gerofit programs in different VISNs.
Related: Telehealth for Rural Veterans With Neurologic Disorders
Salem VAMC Gerofit Program. The center-based Salem VAMC Gerofit program was established in July 2015. In fiscal year 2017, its dedicated exercise facility had more than 5,000 patient visits. Despite the program’s success, we prioritized establishing Tele-Gerofit because of the medical center’s rural location in southwest Virginia and the large number of veterans who receive care at CBOCs. Therefore, much as with the pilot CVT Gerofit classes in Los Angeles and Miami, the target setting was rural CBOCs. The goal for Salem VAMC Tele-Gerofit was to modify Gerofit delivery to the CVT format and a CBOC setting with minimal modification of the content and personnel requirements of both physical performance testing and exercise training procedures.
Adjustments for CBOC Setting. The enrollment process for Tele-Gerofit is the same as that for the center-based program. To start, a veteran’s primary care provider reviews the list of eligibility criteria and, if the veteran qualifies, places a consult. A Gerofit team member then contacts the veteran by phone to describe the program and schedule an assessment. At the baseline physical performance assessment, American College of Sports Medicine guidelines on exercise participation, health screening, and exercise intensity are used to evaluate veterans and rank them by their cardiovascular risk.20 All new program participants start with low-intensity exercise and gradually progress to recommended levels of exercise. Before starting an exercise class, participants are instructed on use of the 10-point rating of perceived exertion (RPE).
Each CBOC site is supplied with an RPE poster that is displayed for participants’ use. During a Tele-Gerofit class, the exercise instructor asks participants to periodically report their RPE. This class differs slightly from the center-based exercise sessions in which RPE is primarily assessed when a different exercise is introduced or the duration or intensity of an exercise is increased. The Gerofit instructor monitors exercise and treatment fidelity, but the onsite TCT observes for safety during class. The TCT also takes initial vital signs and sets up the room for the class. Emergency contacts and procedures are posted in each CBOC CVT room and are available to the center-based exercise instructor. Because the CBOCs are not inside medical facilities, some CBOC directors have asked that heart rate monitors be used as an extra safety precaution to ensure that high-risk participants do not exceed a heart rate limit that may be set by their cardiologists.
Modifications to Physical Performance Assessment. Physical performance testing had to be adapted to the small rooms available at the CBOCs. For measuring normal gait speed, the 10-MWT was replaced with the 4-meter walk test (4-MWT). The 4-MWT has excellent test–retest reliability with an intraclass correlation coefficient (ICC) of 0.93, but the discrepancy in gait speed between the 4-MWT and the 10-MWT is such that the tests cannot be used interchangeably.21 For measuring endurance, the 6-minute walk test was replaced with the 2-minute step test (2-MST). In older adults, the 2-MST has a moderate correlation with 6-minute walk distance (r = 0.36; P = .04) and high reliability (ICC, 0.90).15,22 The 30-second 1-arm curl, the 30-second chair-stand test, and the 8-foot up-and-go test are performed without modification and require only dumbbells, a chair without wheels, and a stopwatch.
The exercise instructor at the Salem VAMC conducts physical performance testing by 2-way videoconferencing with the veteran in a room at the CBOC. The TCT at the CBOC assists by measuring and demarcating 4 meters on the floor and a designated height on the wall for knee elevation for 4-MWT and 2-MST, respectively. The TCT remains in the room during the assessment visit. Except for taking vital signs before and after the physical performance assessment, the TCT does not participate in the testing. To date, more than 20 physical performance assessments have been conducted without difficulty at Salem-affiliated CBOCs. The primary challenge has been scheduling the room with CVT equipment (ie, camera and screen) for the 30-minute individual assessment session, which occurs on a rolling basis as individuals are enrolled and followed.
After the assessment is completed, the exercise instructor reviews the results with the participant and provides feedback on areas in need of improvement. However, these education sessions can be lengthy and are best supported by giving the patient a personalized handout.
Functional Circuit Exercise. In Tele-Gerofit, exercise training is delivered by CVT broadcast from the Salem VAMC to veterans in a room (equipped with steps, dumbbells, chairs, and bands) at the CBOC. This type of exercise training, which uses only mobile equipment and plyometric (weight-bearing) exercises, is referred to as functional exercise. The AEX includes marching in place, moving on and off a raised step, and body-weight exercises, while RT uses dumbbells, resistance bands, and plyometric exercises (Table 2).
Progression of intensity is achieved by increasing the rate of stepping and the size of the steps (AEX) or the number of repetitions and the weight of the dumbbells or bands (RT). Each veteran exercises at an intensity level that is appropriate for his or her baseline limitations and medical conditions. The exercise instructor uses different forms of the same equipment (eg, heavier dumbbells, higher steps) to vary intensity among individuals while having them perform the same exercises as a group. The challenge is to adjust the pace of the AEX or the timing of the RT repetitions for individuals new to the class.
Delivery of exercise training in the form of circuits allows for a diverse exercise program in a setting with limited space. Circuit training is an exercise modality that consists of a series of different exercises, each usually completed in 30 to 60 seconds, with minimal rest between each type of exercise. Each Tele-Gerofit circuit has a mix of AEX and RT exercises performed for 3 minutes consecutively (Figure).
The design of the circuit training can be adjusted based on the number of individuals in the class. Larger classes can be split into 2 groups that alternate between exercise sets, while smaller classes have 1 group performing the same exercise set and then rotating to either the AEX or RT set. Total exercise time to complete the circuit depends on the number of different exercises, number of repetitions, and the rest between repetitions and the different exercises. In this way, total exercise time can be made shorter or longer depending on the veteran’s capacity.
Frequency. Tele-Gerofit exercise classes are currently offered twice weekly and last about 1 hour, which includes warm-up (8-10 minutes), functional circuit training (40 minutes), and cooldown/stretching (8-10 minutes). A challenge for the exercise instructor is the need to provide ongoing clear instructions both to the class and to individuals as needed. As the exercise prescription for each patient is based on physical performance testing, the exercise instructor for the training must be familiar with the test results. Derivation of the exercise prescription in Tele-Gerofit follows the same process as center-based Gerofit.
Each patient is given an exercise prescription written to address any impairments noted in the different domains of the physical performance assessment, scored using age and sex percentiles. For instance, individuals scoring poorly on lower body strength are given specific lower body strengthening exercises. Participants are given an exercise program that guides them toward achieving recommended physical activity guidelines using their RPE to modulate each exercise. Duration and intensity of each type of planned exercise are formally discussed after initial and follow-up assessments. In addition, exercise training is informally progressed throughout the program. For Tele-Gerofit, instructors must design each class with the group in mind while being prepared for modifications and specific changes for individuals.
Discussion
Tele-Gerofit adapts the well-established center-based Gerofit program to be executed without an exercise facility while maintaining the content of the evidence-based procedures. Physical performance testing and exercise training were modified, adding elements necessary for CVT assessments and classes to be broadcast from the Salem VAMC to its affiliated CBOCs. Tele-Gerofit exercises are performed in a circuit style that allows a veteran or small structured groups of veterans to move among exercises and requires less space than traditional group exercise does. Safety and monitoring concerns are addressed with a safety procedure that includes emergency plans for each site, prescreening of enrolled participants, and monitoring of exercise intensity in accordance with national guidelines.1 Similar to the center-based Gerofit program, the exercise prescription is tailored to each veteran’s physical limitations based on initial and ongoing assessment of physical performance. Tele-Gerofit physical performance testing fulfills the same need with only a few modifications using validated measures. Tele-Gerofit assessments are administered by CVT without the need for additional staff on site.
Adaptation of center-based Gerofit exercise classes to Tele-Gerofit is a major innovation. Use of a circuit exercise design was supported by findings in older adults that RT alone, when performed quickly with minimal rest between each set and exercise station, increases both aerobic capacity and strength.23,24 Older adult RT trials that compared circuit RT with traditional RT found that strength gains are comparable between circuit and traditional RT.24-26 Working with adults aged > 60 years, Takeshima and colleagues conducted a trial of circuit exercise with added callisthenic exercises performed in place between RT on exercise machines.27 This dual-modality (AEX+RT) circuit approach was well tolerated and effective, increasing aerobic capacity and strength. Unfortunately, the resistance exercise machines used in those circuit exercise studies and in the center-based Gerofit program are not an option for Tele-Gerofit.
The requirement for an exercise facility was removed by designing Tele-Gerofit exercise to include only functional exercises that rely on body weight or small mobile exercise equipment. Although popular among young adults, functional circuit exercise is understudied in older adults. Recently, a 12-week functional circuit exercise intervention in frail elderly adults demonstrated significant improvements in gait speed and the timed chair-stand test.28 A pilot observational study of Gerofit participants at the Canandaigua VAMC offered 27 veterans functional circuit exercise instead of their traditional exercise facility class and found larger increases in the timed chair-stand test and 6-minute walk distance compared with 11 Gerofit participants in the traditional program.29
This Tele-Gerofit exercise training combines functional and circuit exercise strategies into telehealth delivery. However, its effect on physical performance remains to be demonstrated. To address this question, we are conducting a single-arm pilot study of Tele-Gerofit with CVT broadcast to 3 Salem CBOC affiliates (Wytheville, Staunton, and Danville, Virginia). The goal is to determine the effect on physical performance and collect feasibility data, including attendance rate and patient satisfaction with the video broadcast. In addition, we are planning an effectiveness trial to compare the impact of functional circuit exercise delivered in person (center based, not CVT) with the parent Gerofit exercise program on direct measures of endurance and strength, in addition to physical performance.
Related: Setting and Method of Measurement Affect Blood Pressure Readings in Older Veterans
Implementation research is needed to determine how Tele-Gerofit can be disseminated to other VAMCs and community-based centers beyond CBOCs. Although the cost of the equipment used to implement Tele-Gerofit is minimal, the program requires dedicated and experienced exercise instructors, and the sharing of telehealth resources with other clinical programs. The authors expect that a diverse group of stakeholders is needed across service lines of primary care, geriatrics and extended care, physical medicine and rehabilitation, and telehealth. Of note, this multidisciplinary collaboration is a hallmark of the Gerofit program. The recent success of the implementation of center-based Gerofit in VAMCs across the US demonstrates the program’s flexibility and robust results.18
Plans also include refining strategies for physical performance testing and exercise monitoring. For instance, we would like to adapt telehealth technology for heart rate monitors that can be worn by high-risk veterans at the CBOC and viewed in real time by the exercise instructor.
Conclusion
Gerofit, which is designed to help older veterans maintain independent living and prevent disability, has been demonstrated to improve quality of life and survival. Our goal has been to adapt Gerofit to CVT and provide a supervised, individualized exercise program in a group setting—a program that can be widely disseminated. Salem VAMC Tele-Gerofit is an innovative and prescriptive program that delivers CVT functional circuit exercise training to remote locations without the need for stationary exercise equipment. This approach has the potential to become an effective and feasible exercise strategy for preventing and minimizing disability in the increasing population of older veterans. Work is needed to determine whether Tele-Gerofit provides a rapid translation of Gerofit to clinical practice and improved outcomes with substantial cost savings from reduced hospitalization and institutionalization.
Acknowledgments
Gerofit has been funded by the Veterans Health Affairs Office of Geriatrics and Extended Care Non-Institutional Long-Term Care Funding and Mentored Partnership Program, and the Veterans Health Affairs Office of Rural Health Rural Enterprise-Wide Initiative.
The authors thank Kim Birkett, MPH, for assistance in editing, references, and graphics and the staff at the Wytheville, Staunton, and Danville community-based outpatient clinics for their support.
Exercise increases endurance, muscle strength, and functional performance with corresponding gains in mobility, survival, and quality of life.1 However, even with these benefits and improvements in clinical outcomes, only 15% of adults aged ≥ 65 years follow current guidelines for exercise.2 Despite their prior military training, the majority of veterans do not meet physical activity recommendations.3 Time, travel, and support are common barriers to exercise participation and adherence—barriers that are further amplified among older adults.
The Veterans Health Administration (VHA) is recognized as a world leader in telehealth service development. Currently, 677,000 veterans have received telehealth services, which represents 12% of the 5.6 million veterans under VHA care.4 Clinical video telehealth (CVT) is widely used within the VHA system to deliver health care that otherwise would not be available to veterans. Veterans who have difficulty traveling to the nearest US Department of Veteran Affairs (VA) medical center (VAMC) can access CVT programs at a participating VHA community-based outpatient clinic (CBOC). The VA has more than 45 CVT programs, including programs for mental health, weight management, cardiology, and dermatology. Outside the VA, cardiac exercise rehabilitation provided by CVT has been shown to be as effective as center-based programs in improving cardiovascular risk factors and functional capacity.5 A VHA exercise program that leveraged CVT resources and was dedicated to older adults with a wide range of comorbid conditions would have a high impact on the health and well-being of older veterans.
Gerofit is a VHA clinical demonstration program of supervised center-based exercise for veterans aged ≥ 65 years. Developed at the Durham VAMC Geriatric Research, Education, and Clinical Center (GRECC) in North Carolina, it has demonstrated improved clinical outcomes, including physical function, mobility, quality of life, and survival.6-10 The program offers veterans individualized exercise in a group setting that focuses on improving endurance, strength, and balance. The exercise prescription is based on the patient’s physical limitations as identified in a physical performance assessment.
With support from VHA Geriatric Extended Care (GEC) and the Office of Rural Health (ORH), Gerofit was implemented in 10 VAMCs across 8 VISNs. However, barriers such as travel time, distance, and transportation limit participation. Previously, we found that rural veterans lack access to exercise programs.11,12 Although some do aerobic exercise (AEX), most do not do resistance training (RT), though they are willing to learn. Access to Gerofit for rural veterans is expanding with recent support from the ORH Enterprise Wide Initiative. Rural program expansion includes several different Gerofit initiatives, many involving CBOCs.
The Salem VAMC Gerofit program sought to adapt the facility-based assessment and exercise procedures into a self-reliant CVT class for its CBOCs. This article describes the development of the Salem VAMC Gerofit CVT program, hereafter referred to as Tele-Gerofit.
Related: Expanding the Scope of Telemedicine in Gastroenterology
Program Design
Gerofit was established in 1986 at the Durham GRECC as an exercise and health promotion program for veterans aged ≥ 65 years.13 Its goal is to prevent or improve functional decline from physical inactivity and age-related conditions. Gerofit targets the geriatric patient population and thus extends beyond cardiac and pulmonary rehabilitation or weight loss programs. The primary exclusion criteria are based on safety issues in the context of a group exercise setting of older adults and include oxygen dependency, unstable cardiac disease, and moderate-to-severe cognitive impairment.
To participate in Gerofit, veterans must be able to perform activities of daily living and self-manage an exercise prescription developed by the exercise instructor based on physical performance testing. These physical performance tests include measures that are independent predictors of disability, loss of independent living, and death, as well as surrogate measures of exercise capacity (eg, strength, endurance, balance).14,15 A novel aspect of Gerofit is that the physical performance assessment is used not only to determine physical limitations, but also to individualize the exercise prescription based on the observed deficits in strength, endurance, or balance. These assessments are performed at initial enrollment; 3 months, 6 months, and 1 year later; and annually after that. Currently, the center-based Gerofit programs administer 5 items of the Senior Fitness Test: 6-minute walk, 10-meter walk (10-MWT), 30-second 1-arm curl, 30-second chair-stand test, and 8-foot up-and-go.15 The side-by-side, semitandem, and tandem standing balance tests from the short physical performance battery also are performed.16 In addition, participants complete a questionnaire that includes items from the physical functioning scale of the 12-Item Short Form Health Survey (SF-12).
After each assessment, the Gerofit exercise instructor reviews the results with the veteran and formulates an individualized exercise prescription along with goals for improvement. Veterans are encouraged to attend supervised center-based exercise sessions 3 times weekly. Classes are offered in a gym or fitness center at the VAMC or in leased space. Each patient uses a cue card that lists an exercise plan personalized for intensity and duration for aerobic exercise (AEX; eg, treadmill walking, stationary bicycling, arm ergometry), RT using dumbbells and weight equipment, and functional exercises for flexibility and balance. Some medical centers also offer yoga, tai chi, or dancing Gerofit classes.
For participants in the Durham Gerofit program, mortality decreased 25% over a decade (hazard ratio, 0.75; 95% CI, 0.61-0.91).9 A substudy that included the Psychological General Well-Being Index found that 81% of participants significantly increased their score after 1 year.7 Observed initial improvement in physical performance has been sustained over 5 years.10,17 One-year results from the recent Gerofit expansion to 6 other VAMCs showed clinically and statistically significantly improved physical performance from baseline to 3-, 6-, and 12-month follow-up.18
Adaptation of Gerofit to CVT Delivery
Initial work. The Greater Los Angeles VAMC Gerofit program conducted a pilot CVT exercise class of 6 veterans at the rural Bakersfield CBOC in California.19 Each week, an exercise instructor broadcast a 60-minute exercise class that included warm-up, RT with bands, progressive balance training, and flexibility. Trained student volunteers from California State University in Bakersfield kinesiology program were on site at the Bakersfield CBOC to perform the assessments and aid in exercises during the CVT sessions. Despite the lack of AEX per se, veterans showed significant improvement in endurance as measured by an increase in the number of steps completed in 2 minutes at the 3-month assessment (P = .049). Although exercises were not delivered in a circuit format, the improved endurance supported the potential for cardiovascular benefit from RT in older adults.
This pilot project also demonstrated that key components of the Gerofit program could be delivered safely by telehealth with onsite supervision. The Miami VA Healthcare System also offers CVT Gerofit exercise classes broadcast to the rural Florida CBOCs of Key Largo and Homestead.11 The exercise activities offered for the Miami CVT participants incorporate components of AEX (calisthenics) and RT (resistance bands). Veterans enjoyed the classes, and adherence was good. However, availability of staff and space are an ongoing challenge.
In Key Largo, 5 veterans participated before the CVT classes were placed on hold owing to the demands of other CVT programs and limited availability of the telehealth clinical technician (TCT). The Homestead CBOC continues to offer CVT Gerofit exercise classes and has 6 regular participants. Notably, the physical space at the Homestead CBOC is smaller than that at the Key Largo CBOC; the Homestead CBOC has adjusted by shifting to exercises performed while standing or sitting, ensuring participants’ safety and satisfaction.
The Baltimore, Maryland VAMC Gerofit program offers other innovative CVT exercise classes, including a tai chi class, and a class with exercise performed while sitting in a chair. Although the Baltimore VAMC CVT exercise classes do not have the scope of the center-based exercise prescriptions, they are unique in that they are broadcast not only to their affiliated CBOCs, but also other Gerofit programs in different VISNs.
Related: Telehealth for Rural Veterans With Neurologic Disorders
Salem VAMC Gerofit Program. The center-based Salem VAMC Gerofit program was established in July 2015. In fiscal year 2017, its dedicated exercise facility had more than 5,000 patient visits. Despite the program’s success, we prioritized establishing Tele-Gerofit because of the medical center’s rural location in southwest Virginia and the large number of veterans who receive care at CBOCs. Therefore, much as with the pilot CVT Gerofit classes in Los Angeles and Miami, the target setting was rural CBOCs. The goal for Salem VAMC Tele-Gerofit was to modify Gerofit delivery to the CVT format and a CBOC setting with minimal modification of the content and personnel requirements of both physical performance testing and exercise training procedures.
Adjustments for CBOC Setting. The enrollment process for Tele-Gerofit is the same as that for the center-based program. To start, a veteran’s primary care provider reviews the list of eligibility criteria and, if the veteran qualifies, places a consult. A Gerofit team member then contacts the veteran by phone to describe the program and schedule an assessment. At the baseline physical performance assessment, American College of Sports Medicine guidelines on exercise participation, health screening, and exercise intensity are used to evaluate veterans and rank them by their cardiovascular risk.20 All new program participants start with low-intensity exercise and gradually progress to recommended levels of exercise. Before starting an exercise class, participants are instructed on use of the 10-point rating of perceived exertion (RPE).
Each CBOC site is supplied with an RPE poster that is displayed for participants’ use. During a Tele-Gerofit class, the exercise instructor asks participants to periodically report their RPE. This class differs slightly from the center-based exercise sessions in which RPE is primarily assessed when a different exercise is introduced or the duration or intensity of an exercise is increased. The Gerofit instructor monitors exercise and treatment fidelity, but the onsite TCT observes for safety during class. The TCT also takes initial vital signs and sets up the room for the class. Emergency contacts and procedures are posted in each CBOC CVT room and are available to the center-based exercise instructor. Because the CBOCs are not inside medical facilities, some CBOC directors have asked that heart rate monitors be used as an extra safety precaution to ensure that high-risk participants do not exceed a heart rate limit that may be set by their cardiologists.
Modifications to Physical Performance Assessment. Physical performance testing had to be adapted to the small rooms available at the CBOCs. For measuring normal gait speed, the 10-MWT was replaced with the 4-meter walk test (4-MWT). The 4-MWT has excellent test–retest reliability with an intraclass correlation coefficient (ICC) of 0.93, but the discrepancy in gait speed between the 4-MWT and the 10-MWT is such that the tests cannot be used interchangeably.21 For measuring endurance, the 6-minute walk test was replaced with the 2-minute step test (2-MST). In older adults, the 2-MST has a moderate correlation with 6-minute walk distance (r = 0.36; P = .04) and high reliability (ICC, 0.90).15,22 The 30-second 1-arm curl, the 30-second chair-stand test, and the 8-foot up-and-go test are performed without modification and require only dumbbells, a chair without wheels, and a stopwatch.
The exercise instructor at the Salem VAMC conducts physical performance testing by 2-way videoconferencing with the veteran in a room at the CBOC. The TCT at the CBOC assists by measuring and demarcating 4 meters on the floor and a designated height on the wall for knee elevation for 4-MWT and 2-MST, respectively. The TCT remains in the room during the assessment visit. Except for taking vital signs before and after the physical performance assessment, the TCT does not participate in the testing. To date, more than 20 physical performance assessments have been conducted without difficulty at Salem-affiliated CBOCs. The primary challenge has been scheduling the room with CVT equipment (ie, camera and screen) for the 30-minute individual assessment session, which occurs on a rolling basis as individuals are enrolled and followed.
After the assessment is completed, the exercise instructor reviews the results with the participant and provides feedback on areas in need of improvement. However, these education sessions can be lengthy and are best supported by giving the patient a personalized handout.
Functional Circuit Exercise. In Tele-Gerofit, exercise training is delivered by CVT broadcast from the Salem VAMC to veterans in a room (equipped with steps, dumbbells, chairs, and bands) at the CBOC. This type of exercise training, which uses only mobile equipment and plyometric (weight-bearing) exercises, is referred to as functional exercise. The AEX includes marching in place, moving on and off a raised step, and body-weight exercises, while RT uses dumbbells, resistance bands, and plyometric exercises (Table 2).
Progression of intensity is achieved by increasing the rate of stepping and the size of the steps (AEX) or the number of repetitions and the weight of the dumbbells or bands (RT). Each veteran exercises at an intensity level that is appropriate for his or her baseline limitations and medical conditions. The exercise instructor uses different forms of the same equipment (eg, heavier dumbbells, higher steps) to vary intensity among individuals while having them perform the same exercises as a group. The challenge is to adjust the pace of the AEX or the timing of the RT repetitions for individuals new to the class.
Delivery of exercise training in the form of circuits allows for a diverse exercise program in a setting with limited space. Circuit training is an exercise modality that consists of a series of different exercises, each usually completed in 30 to 60 seconds, with minimal rest between each type of exercise. Each Tele-Gerofit circuit has a mix of AEX and RT exercises performed for 3 minutes consecutively (Figure).
The design of the circuit training can be adjusted based on the number of individuals in the class. Larger classes can be split into 2 groups that alternate between exercise sets, while smaller classes have 1 group performing the same exercise set and then rotating to either the AEX or RT set. Total exercise time to complete the circuit depends on the number of different exercises, number of repetitions, and the rest between repetitions and the different exercises. In this way, total exercise time can be made shorter or longer depending on the veteran’s capacity.
Frequency. Tele-Gerofit exercise classes are currently offered twice weekly and last about 1 hour, which includes warm-up (8-10 minutes), functional circuit training (40 minutes), and cooldown/stretching (8-10 minutes). A challenge for the exercise instructor is the need to provide ongoing clear instructions both to the class and to individuals as needed. As the exercise prescription for each patient is based on physical performance testing, the exercise instructor for the training must be familiar with the test results. Derivation of the exercise prescription in Tele-Gerofit follows the same process as center-based Gerofit.
Each patient is given an exercise prescription written to address any impairments noted in the different domains of the physical performance assessment, scored using age and sex percentiles. For instance, individuals scoring poorly on lower body strength are given specific lower body strengthening exercises. Participants are given an exercise program that guides them toward achieving recommended physical activity guidelines using their RPE to modulate each exercise. Duration and intensity of each type of planned exercise are formally discussed after initial and follow-up assessments. In addition, exercise training is informally progressed throughout the program. For Tele-Gerofit, instructors must design each class with the group in mind while being prepared for modifications and specific changes for individuals.
Discussion
Tele-Gerofit adapts the well-established center-based Gerofit program to be executed without an exercise facility while maintaining the content of the evidence-based procedures. Physical performance testing and exercise training were modified, adding elements necessary for CVT assessments and classes to be broadcast from the Salem VAMC to its affiliated CBOCs. Tele-Gerofit exercises are performed in a circuit style that allows a veteran or small structured groups of veterans to move among exercises and requires less space than traditional group exercise does. Safety and monitoring concerns are addressed with a safety procedure that includes emergency plans for each site, prescreening of enrolled participants, and monitoring of exercise intensity in accordance with national guidelines.1 Similar to the center-based Gerofit program, the exercise prescription is tailored to each veteran’s physical limitations based on initial and ongoing assessment of physical performance. Tele-Gerofit physical performance testing fulfills the same need with only a few modifications using validated measures. Tele-Gerofit assessments are administered by CVT without the need for additional staff on site.
Adaptation of center-based Gerofit exercise classes to Tele-Gerofit is a major innovation. Use of a circuit exercise design was supported by findings in older adults that RT alone, when performed quickly with minimal rest between each set and exercise station, increases both aerobic capacity and strength.23,24 Older adult RT trials that compared circuit RT with traditional RT found that strength gains are comparable between circuit and traditional RT.24-26 Working with adults aged > 60 years, Takeshima and colleagues conducted a trial of circuit exercise with added callisthenic exercises performed in place between RT on exercise machines.27 This dual-modality (AEX+RT) circuit approach was well tolerated and effective, increasing aerobic capacity and strength. Unfortunately, the resistance exercise machines used in those circuit exercise studies and in the center-based Gerofit program are not an option for Tele-Gerofit.
The requirement for an exercise facility was removed by designing Tele-Gerofit exercise to include only functional exercises that rely on body weight or small mobile exercise equipment. Although popular among young adults, functional circuit exercise is understudied in older adults. Recently, a 12-week functional circuit exercise intervention in frail elderly adults demonstrated significant improvements in gait speed and the timed chair-stand test.28 A pilot observational study of Gerofit participants at the Canandaigua VAMC offered 27 veterans functional circuit exercise instead of their traditional exercise facility class and found larger increases in the timed chair-stand test and 6-minute walk distance compared with 11 Gerofit participants in the traditional program.29
This Tele-Gerofit exercise training combines functional and circuit exercise strategies into telehealth delivery. However, its effect on physical performance remains to be demonstrated. To address this question, we are conducting a single-arm pilot study of Tele-Gerofit with CVT broadcast to 3 Salem CBOC affiliates (Wytheville, Staunton, and Danville, Virginia). The goal is to determine the effect on physical performance and collect feasibility data, including attendance rate and patient satisfaction with the video broadcast. In addition, we are planning an effectiveness trial to compare the impact of functional circuit exercise delivered in person (center based, not CVT) with the parent Gerofit exercise program on direct measures of endurance and strength, in addition to physical performance.
Related: Setting and Method of Measurement Affect Blood Pressure Readings in Older Veterans
Implementation research is needed to determine how Tele-Gerofit can be disseminated to other VAMCs and community-based centers beyond CBOCs. Although the cost of the equipment used to implement Tele-Gerofit is minimal, the program requires dedicated and experienced exercise instructors, and the sharing of telehealth resources with other clinical programs. The authors expect that a diverse group of stakeholders is needed across service lines of primary care, geriatrics and extended care, physical medicine and rehabilitation, and telehealth. Of note, this multidisciplinary collaboration is a hallmark of the Gerofit program. The recent success of the implementation of center-based Gerofit in VAMCs across the US demonstrates the program’s flexibility and robust results.18
Plans also include refining strategies for physical performance testing and exercise monitoring. For instance, we would like to adapt telehealth technology for heart rate monitors that can be worn by high-risk veterans at the CBOC and viewed in real time by the exercise instructor.
Conclusion
Gerofit, which is designed to help older veterans maintain independent living and prevent disability, has been demonstrated to improve quality of life and survival. Our goal has been to adapt Gerofit to CVT and provide a supervised, individualized exercise program in a group setting—a program that can be widely disseminated. Salem VAMC Tele-Gerofit is an innovative and prescriptive program that delivers CVT functional circuit exercise training to remote locations without the need for stationary exercise equipment. This approach has the potential to become an effective and feasible exercise strategy for preventing and minimizing disability in the increasing population of older veterans. Work is needed to determine whether Tele-Gerofit provides a rapid translation of Gerofit to clinical practice and improved outcomes with substantial cost savings from reduced hospitalization and institutionalization.
Acknowledgments
Gerofit has been funded by the Veterans Health Affairs Office of Geriatrics and Extended Care Non-Institutional Long-Term Care Funding and Mentored Partnership Program, and the Veterans Health Affairs Office of Rural Health Rural Enterprise-Wide Initiative.
The authors thank Kim Birkett, MPH, for assistance in editing, references, and graphics and the staff at the Wytheville, Staunton, and Danville community-based outpatient clinics for their support.
1. American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510-1530.
2. Centers for Disease Control and Prevention. Adult participation in aerobic and muscle-strengthening physical activities—United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62(17):326-330.
3. Littman AJ, Forsberg CW, Koepsell TD. Physical activity in a national sample of veterans. Med Sci Sports Exerc. 2009;41(5):1006-1013.
4. US Department of Veterans Affairs, Office of Rural Health. Annual Report: Thrive 2015. https://www.ruralhealth.va.gov/docs/ORH_Annual_Report_2015_FINAL.pdf. Published 2015. Accessed July 16, 2018.
5. Rawstorn JC, Gant N, Direito A, Beckmann C, Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart. 2016;102(15):1183-1192.
6. Morey MC. Celebrating 20 years of excellence in exercise for the older veteran. Fed Pract. 2007;24(10):49-65.
7. Cowper PA, Morey MC, Bearon LB, et al. The impact of supervised exercise on the psychological well-being and health status of older veterans. J Appl Gerontol. 1991;10(4):469-485.
8. Morey MC, Cowper PA, Feussner JR, et al. Evaluation of a supervised exercise program in a geriatric population. J Am Geriatr Soc. 1989;37(4):348-354.
9. Morey MC, Pieper CF, Crowley GM, Sullivan RJ, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50(12):1929-1933.
10. Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44(10):1226-1231.
11. Valencia WM, Botros D, Pendlebury D, et al. Proactive reach and telehealth monitoring (Gerofit) enhance resistance exercise at rural setting. Innovat Aging. 2017;1(suppl 1):225.12. Pendlebury D, Botros D VW. Proactive Reach: an innovative access approach to identify & deliver GEROFIT exercise telehealth counseling to rural veterans & enhance CBOC services. J Am Geriatr Soc. 2017(suppl 1):S208. Poster presented at: Annual Scientific Meeting of the American Geriatrics Society; May 18, 2017; San Antonio, TX.
13. Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit program: a VA innovation. South Med J. 1994;87(5):S83-S87.
14. Cooper R, Kuh D, Hardy R; Mortality Review Group; FALCon and HALCyon Study Teams. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467.
15. Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7(2):129-161.
16. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-M94.
17. Morey MC, Cowper PA, Feussner JR, et al. Two-year trends in physical performance following supervised exercise among community-dwelling older veterans. J Am Geriatr Soc. 1991;39(10):986-992.
18. Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit program. J Am Geriatr Soc. 2018;66(5):1009-1016.
19. Blanchard E, Castle S, Ines E, et al. Delivering a clinical exercise program to rural veterans via video telehealth. Poster C167 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
20. Riebe D, Ehrman JK, Liguori G, Magal M, eds; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Philadelphia, PA: Wolters Kluwer Health; 2018.
21. Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-meter walk test for measurements of gait speed in healthy, older adults. J Geriatr Phys Ther. 2013;36(1):24-30.
22. Pedrosa R, Holanda G. Correlation between the walk, 2-minute step and TUG tests among hypertensive older women. Rev Bras Fisioter. 2009;13(3):252-256.
23. Romero-Arenas S, Blazevich AJ, Martinez-Pascual M, et al. Effects of high-resistance circuit training in an elderly population. Exp Gerontol. 2013;48(3):334-340.
24. Brentano MA, Cadore EL, Da Silva EM, et al. Physiological adaptations to strength and circuit training in postmenopausal women with bone loss. J Strength Cond Res. 2008;22(6):1816-1825.
25. Romero-Arenas S, Martinez-Pascual M, Alcaraz PE. Impact of resistance circuit training on neuromuscular, cardiorespiratory and body composition adaptations in the elderly. Aging Dis. 2013;4(5):256-263.
26. Paoli A, Pacelli F, Bargossi AM, et al. Effects of three distinct protocols of fitness training on body composition, strength and blood lactate. J Sports Med Phys Fitness. 2010;50(1):43-51.
27. Takeshima N, Rogers ME, Islam MM, Yamauchi T, Watanabe E, Okada A. Effect of concurrent aerobic and resistance circuit exercise training on fitness in older adults. Eur J Appl Physiol. 2004;93(1-2):173-182.
28. Giné-Garriga M, Guerra M, Pagés E, Manini TM, Jiménez R, Unnithan VB. The effect of functional circuit training on physical frailty in frail older adults: a randomized controlled trial. J Aging Phys Act. 2010;18(4):401-424.
29. Biddle ED, Reynolds P, Kopp T, Cammarata H, Conway P. Implementation of functional training tools elicits improvements in aerobic fitness and lower body strength in older veterans. Poster C169 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
1. American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510-1530.
2. Centers for Disease Control and Prevention. Adult participation in aerobic and muscle-strengthening physical activities—United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62(17):326-330.
3. Littman AJ, Forsberg CW, Koepsell TD. Physical activity in a national sample of veterans. Med Sci Sports Exerc. 2009;41(5):1006-1013.
4. US Department of Veterans Affairs, Office of Rural Health. Annual Report: Thrive 2015. https://www.ruralhealth.va.gov/docs/ORH_Annual_Report_2015_FINAL.pdf. Published 2015. Accessed July 16, 2018.
5. Rawstorn JC, Gant N, Direito A, Beckmann C, Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart. 2016;102(15):1183-1192.
6. Morey MC. Celebrating 20 years of excellence in exercise for the older veteran. Fed Pract. 2007;24(10):49-65.
7. Cowper PA, Morey MC, Bearon LB, et al. The impact of supervised exercise on the psychological well-being and health status of older veterans. J Appl Gerontol. 1991;10(4):469-485.
8. Morey MC, Cowper PA, Feussner JR, et al. Evaluation of a supervised exercise program in a geriatric population. J Am Geriatr Soc. 1989;37(4):348-354.
9. Morey MC, Pieper CF, Crowley GM, Sullivan RJ, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50(12):1929-1933.
10. Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44(10):1226-1231.
11. Valencia WM, Botros D, Pendlebury D, et al. Proactive reach and telehealth monitoring (Gerofit) enhance resistance exercise at rural setting. Innovat Aging. 2017;1(suppl 1):225.12. Pendlebury D, Botros D VW. Proactive Reach: an innovative access approach to identify & deliver GEROFIT exercise telehealth counseling to rural veterans & enhance CBOC services. J Am Geriatr Soc. 2017(suppl 1):S208. Poster presented at: Annual Scientific Meeting of the American Geriatrics Society; May 18, 2017; San Antonio, TX.
13. Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit program: a VA innovation. South Med J. 1994;87(5):S83-S87.
14. Cooper R, Kuh D, Hardy R; Mortality Review Group; FALCon and HALCyon Study Teams. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467.
15. Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7(2):129-161.
16. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-M94.
17. Morey MC, Cowper PA, Feussner JR, et al. Two-year trends in physical performance following supervised exercise among community-dwelling older veterans. J Am Geriatr Soc. 1991;39(10):986-992.
18. Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit program. J Am Geriatr Soc. 2018;66(5):1009-1016.
19. Blanchard E, Castle S, Ines E, et al. Delivering a clinical exercise program to rural veterans via video telehealth. Poster C167 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
20. Riebe D, Ehrman JK, Liguori G, Magal M, eds; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Philadelphia, PA: Wolters Kluwer Health; 2018.
21. Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-meter walk test for measurements of gait speed in healthy, older adults. J Geriatr Phys Ther. 2013;36(1):24-30.
22. Pedrosa R, Holanda G. Correlation between the walk, 2-minute step and TUG tests among hypertensive older women. Rev Bras Fisioter. 2009;13(3):252-256.
23. Romero-Arenas S, Blazevich AJ, Martinez-Pascual M, et al. Effects of high-resistance circuit training in an elderly population. Exp Gerontol. 2013;48(3):334-340.
24. Brentano MA, Cadore EL, Da Silva EM, et al. Physiological adaptations to strength and circuit training in postmenopausal women with bone loss. J Strength Cond Res. 2008;22(6):1816-1825.
25. Romero-Arenas S, Martinez-Pascual M, Alcaraz PE. Impact of resistance circuit training on neuromuscular, cardiorespiratory and body composition adaptations in the elderly. Aging Dis. 2013;4(5):256-263.
26. Paoli A, Pacelli F, Bargossi AM, et al. Effects of three distinct protocols of fitness training on body composition, strength and blood lactate. J Sports Med Phys Fitness. 2010;50(1):43-51.
27. Takeshima N, Rogers ME, Islam MM, Yamauchi T, Watanabe E, Okada A. Effect of concurrent aerobic and resistance circuit exercise training on fitness in older adults. Eur J Appl Physiol. 2004;93(1-2):173-182.
28. Giné-Garriga M, Guerra M, Pagés E, Manini TM, Jiménez R, Unnithan VB. The effect of functional circuit training on physical frailty in frail older adults: a randomized controlled trial. J Aging Phys Act. 2010;18(4):401-424.
29. Biddle ED, Reynolds P, Kopp T, Cammarata H, Conway P. Implementation of functional training tools elicits improvements in aerobic fitness and lower body strength in older veterans. Poster C169 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
Antibiotics, antacids before age 2 linked to obesity
Acid-suppressing medications were also associated with childhood obesity, although to a lesser extent, according to results of the study, which included more than a quarter of a million children receiving care in the U.S. military health system.
Antibiotics and antacids are both microbiota-altering medications, researchers said, noting that obesity has been linked to variations in the native gut microbiota.
While the evidence from previous studies is conflicting on whether microbiota-altering medications may play a role in development of childhood obesity, this study does suggest such medications may lead to weight gain early in life, they said in the journal Gut.
“Providers should practice appropriate stewardship as the first-line response to these findings,” said Christopher M. Stark, MD, of the William Beaumont Army Medical Center, El Paso, Tex., and his co-authors.
This retrospective analysis included the largest cohort of pediatric patients ever studied for the link between antibiotics and obesity, according to Dr. Stark and colleagues, and was the first to look at the link between acid-suppressing medications and obesity in that age group.
The analysis included a total of 333,353 U.S. Department of Defense TRICARE beneficiaries born between October 2006 and September 2013 who were exposed to antibiotics, histamine-2-receptor antagonists (H2RAs), or proton pump inhibitors (PPIs) within the first 2 years of life.
Patients were followed past their initial exposure period, up to 8 years of age in some cases, investigators said.
Antibiotics were prescribed in 72.4% of those children, while H2RAs and PPIs were prescribed in 11.8% and 3.3%, respectively, with a substantial number of children receiving more than one of the medications of interest for this study.
A total of 46,993 (14.1%) of the children developed obesity. Of those obese children only 9,268, or 11%, had no antibiotic or acid suppressant prescriptions on record in the first 2 years of life, the reported data show.
Antibiotic prescriptions were associated with a 26% increase in obesity risk (unadjusted hazard ratio, 1.26; 95% CI, 1.23-1.28), investigators reported. That association strengthened steadily with the number of antibiotic prescriptions, with adjusted hazard ratios of 1.12 (95% CI, 1.09-1.15) for a single prescription, up to 1.42 (95% CI, 1.37-1.46) for 4 or more prescriptions
Likewise, H2RAs and PPIs were associated, albeit weakly, with an increased hazard of obesity, investigators said. The adjusted hazard ratios and 95% confidence intervals were 1.02 (1.01-1.03) for PPIs and 1.01 (1.004-1.02) for H2RA prescriptions.
The risk of obesity steadily climbed for those receiving multiple medications, researchers added, from a hazard ratio of 1.21 for one medication, 1.31 for two, and 1.42 for three, data show.
Dr. Stark and co-authors declared no competing interests related to the study.
SOURCE: Stark CM, et al. Gut. 2018 Oct 30. pii: gutjnl-2017-314971.
Acid-suppressing medications were also associated with childhood obesity, although to a lesser extent, according to results of the study, which included more than a quarter of a million children receiving care in the U.S. military health system.
Antibiotics and antacids are both microbiota-altering medications, researchers said, noting that obesity has been linked to variations in the native gut microbiota.
While the evidence from previous studies is conflicting on whether microbiota-altering medications may play a role in development of childhood obesity, this study does suggest such medications may lead to weight gain early in life, they said in the journal Gut.
“Providers should practice appropriate stewardship as the first-line response to these findings,” said Christopher M. Stark, MD, of the William Beaumont Army Medical Center, El Paso, Tex., and his co-authors.
This retrospective analysis included the largest cohort of pediatric patients ever studied for the link between antibiotics and obesity, according to Dr. Stark and colleagues, and was the first to look at the link between acid-suppressing medications and obesity in that age group.
The analysis included a total of 333,353 U.S. Department of Defense TRICARE beneficiaries born between October 2006 and September 2013 who were exposed to antibiotics, histamine-2-receptor antagonists (H2RAs), or proton pump inhibitors (PPIs) within the first 2 years of life.
Patients were followed past their initial exposure period, up to 8 years of age in some cases, investigators said.
Antibiotics were prescribed in 72.4% of those children, while H2RAs and PPIs were prescribed in 11.8% and 3.3%, respectively, with a substantial number of children receiving more than one of the medications of interest for this study.
A total of 46,993 (14.1%) of the children developed obesity. Of those obese children only 9,268, or 11%, had no antibiotic or acid suppressant prescriptions on record in the first 2 years of life, the reported data show.
Antibiotic prescriptions were associated with a 26% increase in obesity risk (unadjusted hazard ratio, 1.26; 95% CI, 1.23-1.28), investigators reported. That association strengthened steadily with the number of antibiotic prescriptions, with adjusted hazard ratios of 1.12 (95% CI, 1.09-1.15) for a single prescription, up to 1.42 (95% CI, 1.37-1.46) for 4 or more prescriptions
Likewise, H2RAs and PPIs were associated, albeit weakly, with an increased hazard of obesity, investigators said. The adjusted hazard ratios and 95% confidence intervals were 1.02 (1.01-1.03) for PPIs and 1.01 (1.004-1.02) for H2RA prescriptions.
The risk of obesity steadily climbed for those receiving multiple medications, researchers added, from a hazard ratio of 1.21 for one medication, 1.31 for two, and 1.42 for three, data show.
Dr. Stark and co-authors declared no competing interests related to the study.
SOURCE: Stark CM, et al. Gut. 2018 Oct 30. pii: gutjnl-2017-314971.
Acid-suppressing medications were also associated with childhood obesity, although to a lesser extent, according to results of the study, which included more than a quarter of a million children receiving care in the U.S. military health system.
Antibiotics and antacids are both microbiota-altering medications, researchers said, noting that obesity has been linked to variations in the native gut microbiota.
While the evidence from previous studies is conflicting on whether microbiota-altering medications may play a role in development of childhood obesity, this study does suggest such medications may lead to weight gain early in life, they said in the journal Gut.
“Providers should practice appropriate stewardship as the first-line response to these findings,” said Christopher M. Stark, MD, of the William Beaumont Army Medical Center, El Paso, Tex., and his co-authors.
This retrospective analysis included the largest cohort of pediatric patients ever studied for the link between antibiotics and obesity, according to Dr. Stark and colleagues, and was the first to look at the link between acid-suppressing medications and obesity in that age group.
The analysis included a total of 333,353 U.S. Department of Defense TRICARE beneficiaries born between October 2006 and September 2013 who were exposed to antibiotics, histamine-2-receptor antagonists (H2RAs), or proton pump inhibitors (PPIs) within the first 2 years of life.
Patients were followed past their initial exposure period, up to 8 years of age in some cases, investigators said.
Antibiotics were prescribed in 72.4% of those children, while H2RAs and PPIs were prescribed in 11.8% and 3.3%, respectively, with a substantial number of children receiving more than one of the medications of interest for this study.
A total of 46,993 (14.1%) of the children developed obesity. Of those obese children only 9,268, or 11%, had no antibiotic or acid suppressant prescriptions on record in the first 2 years of life, the reported data show.
Antibiotic prescriptions were associated with a 26% increase in obesity risk (unadjusted hazard ratio, 1.26; 95% CI, 1.23-1.28), investigators reported. That association strengthened steadily with the number of antibiotic prescriptions, with adjusted hazard ratios of 1.12 (95% CI, 1.09-1.15) for a single prescription, up to 1.42 (95% CI, 1.37-1.46) for 4 or more prescriptions
Likewise, H2RAs and PPIs were associated, albeit weakly, with an increased hazard of obesity, investigators said. The adjusted hazard ratios and 95% confidence intervals were 1.02 (1.01-1.03) for PPIs and 1.01 (1.004-1.02) for H2RA prescriptions.
The risk of obesity steadily climbed for those receiving multiple medications, researchers added, from a hazard ratio of 1.21 for one medication, 1.31 for two, and 1.42 for three, data show.
Dr. Stark and co-authors declared no competing interests related to the study.
SOURCE: Stark CM, et al. Gut. 2018 Oct 30. pii: gutjnl-2017-314971.
FROM GUT
Key clinical point: When prescribed early in life, antibiotics, and to a lesser extent antacids, were associated with childhood obesity.
Major finding: Antibiotic prescriptions were associated with a 26% increase in obesity risk (unadjusted hazard ratio, 1.26; 95% CI, 1.23-1.28).
Study details: Retrospective study of 333,353 children in the U.S. military health system exposed in the first two years of life to antibiotics, histamine-2-receptor antagonists, or proton pump inhibitors.
Disclosures: Study authors declared no competing interests related to the work.
Source: Stark CM, et al. Gut. 2018 Oct 30. pii: gutjnl-2017-314971.
Endoscopic management of obesity
Editor's Note
Gastroenterologists are becoming increasingly involved in the management of obesity. While prior therapy for obesity was mainly based on lifestyle changes, medication, or surgery, the new and exciting field of endoscopic bariatric and metabolic therapies has recently garnered incredible attention and momentum.
In this quarter’s In Focus article, brought to you by The New Gastroenterologist, Pichamol Jirapinyo and Christopher Thompson (Brigham and Women’s Hospital) provide an outstanding overview of the gastric and small bowel endoscopic interventions that are either already approved for use in obesity or currently being studied. This field is moving incredibly fast, and knowledge and understanding of these endoscopic therapies for obesity will undoubtedly be important for our field.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Introduction
Obesity is a rising pandemic. As of 2016, 93.3 million U.S. adults had obesity, representing 39.8% of our adult population.1 It is estimated that approximately $147 billion is spent annually on caring for patients with obesity. Traditionally, the management of obesity includes lifestyle therapy (diet and exercise), pharmacotherapy (six Food and Drug Administration–approved medications for obesity), and bariatric surgery (sleeve gastrectomy [SG] and Roux-en-Y gastric bypass [RYGB]). Nevertheless, intensive lifestyle intervention and pharmacotherapy are associated with approximately 3.1%-6.6% total weight loss (TWL),2-7 and bariatric surgery is associated with 20%-33.3% TWL.8 However, less than 2% of patients who are eligible for bariatric surgery elect to undergo surgery, leaving a large proportion of patients with obesity untreated or undertreated.9
Endoscopic bariatric and metabolic therapies (EBMTs) encompass an emerging field for the treatment of obesity. In general, EBMTs are associated with greater weight loss than are lifestyle intervention and pharmacotherapy, but with a less- invasive risk profile than bariatric surgery. EBMTs may be divided into two general categories – gastric and small bowel interventions (Figure 1 and Table 1). Gastric EBMTs are effective at treating obesity, while small bowel EBMTs are effective at treating metabolic diseases with a variable weight loss profile depending on the device.10,11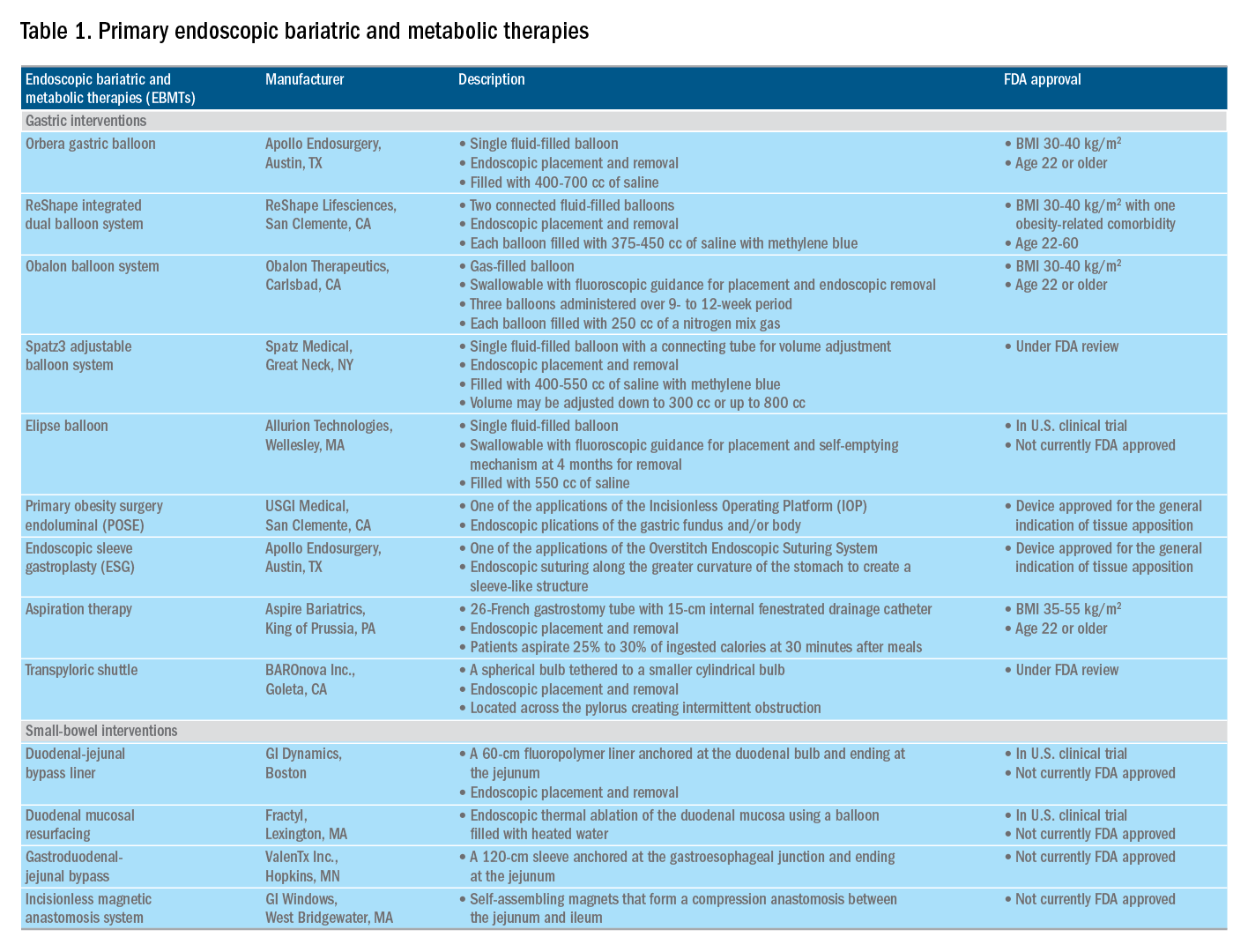
Of note, a variety of study designs (including retrospective series, prospective series, and randomized trials with and without shams) have been employed, which can affect outcomes. Therefore, weight loss comparisons among studies are challenging and should be considered in this context.
Gastric interventions
Currently, there are three types of EBMTs that are FDA approved and used for the treatment of obesity. These include intragastric balloons (IGBs), plications and suturing, and aspiration therapy (AT). Other technologies that are under investigation also will be briefly covered.
Intragastric balloons
An intragastric balloon is a space-occupying device that is placed in the stomach. The mechanism of action of IGBs involves delaying gastric emptying, which leads to increased satiety.12 There are several types of IGBs available worldwide differing in techniques of placement and removal (endoscopic versus fluoroscopic versus swallowable), materials used to fill the balloon (fluid-filled versus air-filled), and the number of balloons placed (single versus duo versus three-balloon). At the time of this writing, three IGBs are approved by the FDA (Orbera, ReShape, and Obalon), all for patients with body mass indexes of 30-40 kg/m2, and two others are in the process of obtaining FDA approval (Spatz and Elipse).
Orbera gastric balloon (Apollo Endosurgery, Austin, Tex.) is a single fluid-filled IGB that is endoscopically placed and removed at 6 months. The balloon is filled with 400-700 cc of saline with or without methylene blue (to identify leakage or rupture). Recently, Orbera365, which allows the balloon to stay for 12 months instead of 6 months, has become available in Europe; however, it is yet to be approved in the United States. The U.S. pivotal trial (Orbera trial) including 255 subjects (125 Orbera arm versus 130 non-sham control arm) demonstrated 10.2% TWL in the Orbera group compared with 3.3% TWL in the control group at 6 months based on intention-to-treat (ITT) analysis. This difference persisted at 12 months (6 months after explantation) with 7.6% TWL for the Orbera group versus 3.1% TWL for the control group.13,14
ReShape integrated dual balloon system (ReShape Lifesciences, San Clemente, Calif.) consists of two connected fluid-filled balloons that are endoscopically placed and removed at 6 months. Each balloon is filled with 375-450 cc of saline mixed with methylene blue. The U.S. pivotal trial (REDUCE trial) including 326 subjects (187 ReShape arm versus 139 sham arm) demonstrated 6.8% TWL in the ReShape group compared with 3.3% TWL in the sham group at 6 months based on ITT analysis.15,16
Obalon balloon system (Obalon Therapeutics, Carlsbad, Calif.) is a swallowable, gas-filled balloon system that requires endoscopy only for removal. During placement, a capsule is swallowed under fluoroscopic guidance. The balloon is then inflated with 250 cc of nitrogen mix gas prior to tube detachment. Up to three balloons may be swallowed sequentially at 1-month intervals. At 6 months from the first balloon placement, all balloons are removed endoscopically. The U.S. pivotal trial (SMART trial) including 366 subjects (185 Obalon arm versus 181 sham capsule arm) demonstrated 6.6% TWL in the Obalon group compared with 3.4% TWL in the sham group at 6 months based on ITT analysis.17,18
Two other balloons that are currently under investigation in the United States are the Spatz3 adjustable balloon system (Spatz Medical, Great Neck, N.Y.) and Elipse balloon (Allurion Technologies, Wellesley, Mass.). The Spatz3 is a fluid-filled balloon that is placed and removed endoscopically. It consists of a single balloon and a connecting tube that allows volume adjustment for control of symptoms and possible augmentation of weight loss. The U.S. pivotal trial was recently completed and the data are being reviewed by the FDA. The Elipse is a swallowable fluid-filled balloon that does not require endoscopy for placement or removal. At 4 months, the balloon releases fluid allowing it to empty and pass naturally. The U.S. pivotal trial (ENLIGHTEN trial) is currently underway.
A meta-analysis of randomized controlled trials revealed improvement in most metabolic parameters (diastolic blood pressure, fasting glucose, hemoglobin A1c, and waist circumference) following IGB compared with controls.19 Nausea and vomiting are seen in approximately 30% and should be addressed appropriately. Pooled serious adverse event (SAE) rate was 1.5%, which included migration, perforation, and death. Since 2016, 14 deaths have been reported according to the FDA MAUDE database. Corporate response was that over 295,000 balloons had been distributed worldwide with a mortality rate of less than 0.01%.20
Plication and suturing
Currently, there are two endoscopic devices that are approved for the general indication of tissue apposition. These include the Incisionless Operating Platform (IOP) (USGI Medical, San Clemente, Calif.) and the Overstitch endoscopic suturing system (Apollo Endosurgery, Austin, Tex.). These devices are used to remodel the stomach to create a sleeve-like structure to induce weight loss.
The IOP system consists of a transport, which is a 54-Fr flexible endoscope. It consists of four working channels that accommodate a G-Prox (for tissue approximation), a G-Lix (for tissue grasping), and an ultrathin endoscope (for visualization). In April 2008, Horgan performed the first-in-human primary obesity surgery endoluminal (POSE) procedure in Argentina. The procedure involves the use of the IOP system to place plications primarily in the fundus to modify gastric accommodation.21 The U.S. pivotal trial (ESSENTIAL trial) including 332 subjects (221 POSE arm versus 111 sham arm) demonstrated 5.0% TWL in the POSE group compared with 1.4% in the sham group at 12 months based on ITT analysis.22 A European multicenter randomized controlled trial (MILEPOST trial) including 44 subjects (34 POSE arm versus 10 non-sham control arm) demonstrated 13.0% TWL in the POSE group compared with 5.3% TWL in the control group at 12 months.23 A recent meta-analysis including five studies with 586 subjects showed pooled weight loss of 13.2% at 12-15 months following POSE with a pooled serious adverse event rate of 3.2%.24 These included extraluminal bleeding, minor bleeding at the suture site, hepatic abscess, chest pain, nausea, vomiting, and abdominal pain. A distal POSE procedure with a new plication pattern focusing on the gastric body to augment the effect on gastric emptying has also been described.25
The Overstitch is an endoscopic suturing device that is mounted on a double-channel endoscope. At the tip of the scope, there is a curved suture arm and an anchor exchange that allow the needle to pass back and forth to perform full-thickness bites. The tissue helix may also be placed through the second channel to grasp tissue. In April 2012, Thompson performed the first-in-human endoscopic sutured/sleeve gastroplasty (ESG) procedure in India, which was published together with cases performed in Panama and the Dominican Republic.26-28 This procedure involves the use of the Overstitch device to place several sets of running sutures along the greater curvature of the stomach to create a sleeve-like structure. It is thought to delay gastric emptying and therefore increase satiety.29 The largest multicenter retrospective study including 248 patients demonstrated 18.6% TWL at 2 years with 2% SAE rate including perigastric fluid collections, extraluminal hemorrhage, pulmonary embolism, pneumoperitoneum, and pneumothorax.30
Aspiration therapy
Aspiration therapy (AT; Aspire Bariatrics, King of Prussia, Pa.) allows patients to remove 25%-30% of ingested calories at approximately 30 minutes after meals. AT consists of an A-tube, which is a 26-Fr gastrostomy tube with a 15-cm fenestrated drainage catheter placed endoscopically via a standard pull technique. At 1-2 weeks after A-tube placement, the tube is cut down to the skin and connected to the port prior to aspiration. AT is approved for patients with a BMI of 35-55 kg/m2.31 The U.S. pivotal trial (PATHWAY trial) including 207 subjects (137 AT arm versus 70 non-sham control arm) demonstrated 12.1% TWL in the AT group compared to 3.5% in the control group at 12 months based on ITT analysis. The SAE rate was 3.6% including severe abdominal pain, peritonitis, prepyloric ulcer, and A-tube replacement due to skin-port malfunction.32
Transpyloric shuttle
The transpyloric shuttle (TPS; BAROnova, Goleta, Calif.) consists of a spherical bulb that is attached to a smaller cylindrical bulb by a flexible tether. It is placed and removed endoscopically at 6 months. TPS resides across the pylorus creating intermittent obstruction that may result in delayed gastric emptying. A pilot study including 20 patients demonstrated 14.5% TWL at 6 months.33 The U.S. pivotal trial (ENDObesity II trial) was recently completed and the data are being reviewed by the FDA.
Revision for weight regain following bariatric surgery
Weight regain is common following RYGB34,35 and can be associated with dilation of the gastrojejunal anastomosis (GJA).36 Several procedures have been developed to treat this condition by focusing on reduction of GJA size and are available in the United States (Figure 2). These procedures have level I evidence supporting their use and include transoral outlet reduction (TORe) and restorative obesity surgery endoluminal (ROSE).37 TORe involves the use of the Overstitch to place sutures at the GJA. At 1 year, patients had 8.4% TWL with improvement in comorbidities.38 Weight loss remained significant up to 3-5 years.39,40 The modern ROSE procedure utilizes the IOP system to place plications at the GJA and distal gastric pouch following argon plasma coagulation (APC). A small series showed 12.4% TWL at 6 months.41 APC is also currently being investigated as a standalone therapy for weight regain in this population.
Small bowel interventions
There are several small bowel interventions, with different mechanisms of action, available internationally. Many of these are under investigation in the United States; however, none are currently FDA approved.
Duodenal-jejunal bypass liner
Duodenal-jejunal bypass liner (DJBL; GI Dynamics, Boston, Mass.) is a 60-cm fluoropolymer liner that is endoscopically placed and removed at 12 months. It is anchored at the duodenal bulb and ends at the jejunum. By excluding direct contact between chyme and the proximal small bowel, DJBL is thought to work via foregut mechanism where there is less inhibition of the incretin effect (greater increase in insulin secretion following oral glucose administration compared to intravenous glucose administration due to gut-derived factors that enhance insulin secretion) leading to improved insulin resistance. In addition, the enteral transit of chyme and bile is altered suggesting the possible role of the hindgut mechanism. The previous U.S. pivotal trial (ENDO trial) met efficacy endpoints. However, the study was stopped early by the company because of a hepatic abscess rate of 3.5%, all of which were treated conservatively.42 A new U.S. pivotal study is currently planned. A meta-analysis of 17 published studies, all of which were from outside the United States, demonstrated a significant decrease in hemoglobin A1c of 1.3% and 18.9% TWL at 1 year following implantation in patients with obesity with concomitant diabetes.43
Duodenal mucosal resurfacing
Duodenal mucosal resurfacing (Fractyl, Lexington, Mass.) involves saline lifting of the duodenal mucosa circumferentially prior to thermal ablation using an inflated balloon filled with heated water. It is hypothesized that this may reset the diseased duodenal enteroendocrine cells leading to restoration of the incretin effect. A pilot study including 39 patients with poorly controlled diabetes demonstrated a decrease in hemoglobin A1c of 1.2%. The SAE rate was 7.7% including duodenal stenosis, all of which were treated with balloon dilation.44 The U.S. pivotal trial is currently planned.
Gastroduodenal-jejunal bypass
Gastroduodenal-jejunal bypass (ValenTx., Hopkins, Minn.) is a 120-cm sleeve that is anchored at the gastroesophageal junction to create the anatomic changes of RYGB. It is placed and removed endoscopically with laparoscopic assistance. A pilot study including 12 patients demonstrated 35.9% excess weight loss at 12 months. Two out of 12 patients had early device removal due to intolerance and they were not included in the weight loss analysis.45
Incisionless magnetic anastomosis system
The incisionless magnetic anastomosis system (GI Windows, West Bridgewater, Mass.) consists of self-assembling magnets that are deployed under fluoroscopic guidance through the working channel of colonoscopes to form magnetic octagons in the jejunum and ileum. After a week, a compression anastomosis is formed and the coupled magnets pass spontaneously. A pilot study including 10 patients showed 14.6% TWL and a decrease in hemoglobin A1c of 1.9% (for patients with diabetes) at 1 year.46 A randomized study outside the United States is currently underway.
Summary
Endoscopic bariatric and metabolic therapies are emerging as first-line treatments for obesity in many populations. They can serve as a gap therapy for patients who do not qualify for surgery, but also may have a specific role in the treatment of metabolic comorbidities. This field will continue to develop and improve with the introduction of personalized medicine leading to better patient selection, and newer combination therapies. It is time for gastroenterologists to become more involved in the management of this challenging condition.
Dr. Jirapinyo is an advanced and bariatric endoscopy fellow, Brigham and Women’s Hospital, Harvard Medical School, Boston; Dr. Thompson is director of therapeutic endoscopy, Brigham and Women’s Hospital, and associate professor of medicine, Harvard Medical School. Dr. Jirapinyo has served as a consultant for GI Dynamics and holds royalties for Endosim. Dr. Thompson has contracted research for Aspire Bariatrics, USGI Medical, Spatz, and Apollo Endosurgery; has served as a consultant for Boston Scientific, Covidien, USGI Medical, Olympus, and Fractyl; holds stocks and royalties for GI Windows and Endosim, and has served as an expert reviewer for GI Dynamics.
References
1. CDC. From https://www.cdc.gov/obesity/data/adult.html. Accessed on 11 September 2018.
2. Aronne LJ et al. Obesity. 2013;21:2163-71.
3. Torgerson JS et al. Diabetes Care. 2004;27:155-61.
4. Allison DB et al. Obesity. 2012;20:330-42.
5. Smith SR et al. N Engl J Med. 2010;363:245-56.
6. Apovian CM et al. Obesity. 2013;21:935-43.
7. Pi-Sunyer X et al. N Engl J Med. 2015;373:11-22.
8. Colguitt JL et al. Cochrane Database Syst Rev. 2014;8(8):CD003641.
9. Ponce J et al. Surg Obes Relat Dis. 2015;11(6):1199-200.
10. Jirapinyo P, Thompson CC et al. Clin Gastroenterol Hepatol. 2017;15(5):619-30.
11. Sullivan S et al.Gastroenterology. 2017;152(7):1791-801.
12. Gomez V et al. Obesity. 2016;24(9):1849-53.
13. Food and Drug Administration. Summary of safety and effectiveness data (SSED) ORBERA Intragastric Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140008b.pdf. 2015:1-32.
14. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;81:AB147.
15. Food and Drug Administration. Summary of safety and effectiveness data (SSED) ReShape Integrated Dual Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140012b.pdf. 2015:1-43.
16. Ponce J et al. Surg Obes Relat Dis. 2015;11:874-81.
17. Food and Drug Administration. Summary and effectiveness data (SSED): Obalon Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160001b.pdf. 2016:1-46.
18. Sullivan S et al. Gastroenterology. 2016;150:S1267.
19. Popov VB et al. Am J Gastroenterol. 2017;112:429-39.
20. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;82(3):425-38.
21. Espinos JC et al. Obes Surg. 2013;23(9):1375-83.
22. Sullivan S et al. Obesity. 2017;25:294-301.
23. Miller K et al. Obesity Surg. 2017;27(2):310-22.
24. Jirapinyo P et al. Gastrointest Endosc. 2018;87(6):AB604-AB605.
25. Jirapinyo P, Thompson CC. Video GIE. 2018;3(10):296-300.
26. Campos J et al. SAGES 2013 Presentation. Baltimore, MD. 19 April 2013.
27. Kumar N et al. Gastroenterology. 2014;146(5):S571-2.
28. Kumar N et al. Surg Endosc. 2018;32(4):2159-64.
29. Abu Dayyeh BK et al. Clin Gastroenterol Hepatol. 2017;15:37-43.
30. Lopez-Nava G et al. Obes Surg. 2017;27(10):2649-55.
31. Food and Drug Administration. Summary of safety and effectiveness (SSED): AspireAssist. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf15/p150024b.pdf. FDA,ed,2016:1-36.
32. Thompson CC et al. Am J Gastroenterol. 2017;112:447-57.
33. SAGES abstract archives. SAGES. Available from: http://www.sages.org/meetings/annual-meeting/abstracts-archive/first-clinical-experience-with-the-transpyloric-shuttle-tpsr-device-a-non-surgical-endoscopic treatment-for-obesity-results-from-a-3-month-and-6-month-study. Accessed Sept. 12, 2018.
34. Sjostrom L et al. N Engl J Med. 2007;357:741-52.
35. Adams TD et al. N Engl J Med. 2017;377:1143-55.
36. Abu Dayyeh BK et al. Clin Gastroenterol Hepatol. 2011;9:228-33.
37. Thompson CC et al. Gastroenterology. 2013;145(1):129-37.
38. Jirapinyo P et al. Endoscopy. 2018;50(4):371-7.
39. Kumar N, Thompson CC. Gastrointest Endosc. 2016;83(4):776-9.
40. Jirapinyo P et al. Gastrointest Endosc. 2017;85(5):AB93-94.
41. Jirapinyo P, Thompson CC et al. Comparison of a novel plication technique to suturing for endoscopic outlet reduction for the treatment of weight regain after Roux-en-Y gastric bypass. Obesity Week 2018. Poster presentation.
42. Kaplan LM et al. EndoBarrier therapy is associated with glycemic improvement, weight loss and safety issues in patients with obesity and type 2 diabetes on oral anti-hyperglycemic agents (The ENDO Trial). In: Oral Presentation at the 76th American Diabetes Association (ADA) Annual Meeting: 2016 June 10-14: New Orleans. Abstract number 362-LB.
43. Jirapinyo P et al. Diabetes Care. 2018;41(5):1106-15.
44. Rajagopalan H et al. Diabetes Care. 2016;39(12):2254-61.
45. Sandler BJ et al. Surgical Endosc. 2015;29:3298-303.
46. Machytka E et al. Gastrointest Endosc. 2017;86(5):904-12.
Editor's Note
Gastroenterologists are becoming increasingly involved in the management of obesity. While prior therapy for obesity was mainly based on lifestyle changes, medication, or surgery, the new and exciting field of endoscopic bariatric and metabolic therapies has recently garnered incredible attention and momentum.
In this quarter’s In Focus article, brought to you by The New Gastroenterologist, Pichamol Jirapinyo and Christopher Thompson (Brigham and Women’s Hospital) provide an outstanding overview of the gastric and small bowel endoscopic interventions that are either already approved for use in obesity or currently being studied. This field is moving incredibly fast, and knowledge and understanding of these endoscopic therapies for obesity will undoubtedly be important for our field.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Introduction
Obesity is a rising pandemic. As of 2016, 93.3 million U.S. adults had obesity, representing 39.8% of our adult population.1 It is estimated that approximately $147 billion is spent annually on caring for patients with obesity. Traditionally, the management of obesity includes lifestyle therapy (diet and exercise), pharmacotherapy (six Food and Drug Administration–approved medications for obesity), and bariatric surgery (sleeve gastrectomy [SG] and Roux-en-Y gastric bypass [RYGB]). Nevertheless, intensive lifestyle intervention and pharmacotherapy are associated with approximately 3.1%-6.6% total weight loss (TWL),2-7 and bariatric surgery is associated with 20%-33.3% TWL.8 However, less than 2% of patients who are eligible for bariatric surgery elect to undergo surgery, leaving a large proportion of patients with obesity untreated or undertreated.9

Endoscopic bariatric and metabolic therapies (EBMTs) encompass an emerging field for the treatment of obesity. In general, EBMTs are associated with greater weight loss than are lifestyle intervention and pharmacotherapy, but with a less- invasive risk profile than bariatric surgery. EBMTs may be divided into two general categories – gastric and small bowel interventions (Figure 1 and Table 1). Gastric EBMTs are effective at treating obesity, while small bowel EBMTs are effective at treating metabolic diseases with a variable weight loss profile depending on the device.10,11
Of note, a variety of study designs (including retrospective series, prospective series, and randomized trials with and without shams) have been employed, which can affect outcomes. Therefore, weight loss comparisons among studies are challenging and should be considered in this context.
Gastric interventions
Currently, there are three types of EBMTs that are FDA approved and used for the treatment of obesity. These include intragastric balloons (IGBs), plications and suturing, and aspiration therapy (AT). Other technologies that are under investigation also will be briefly covered.
Intragastric balloons
An intragastric balloon is a space-occupying device that is placed in the stomach. The mechanism of action of IGBs involves delaying gastric emptying, which leads to increased satiety.12 There are several types of IGBs available worldwide differing in techniques of placement and removal (endoscopic versus fluoroscopic versus swallowable), materials used to fill the balloon (fluid-filled versus air-filled), and the number of balloons placed (single versus duo versus three-balloon). At the time of this writing, three IGBs are approved by the FDA (Orbera, ReShape, and Obalon), all for patients with body mass indexes of 30-40 kg/m2, and two others are in the process of obtaining FDA approval (Spatz and Elipse).
Orbera gastric balloon (Apollo Endosurgery, Austin, Tex.) is a single fluid-filled IGB that is endoscopically placed and removed at 6 months. The balloon is filled with 400-700 cc of saline with or without methylene blue (to identify leakage or rupture). Recently, Orbera365, which allows the balloon to stay for 12 months instead of 6 months, has become available in Europe; however, it is yet to be approved in the United States. The U.S. pivotal trial (Orbera trial) including 255 subjects (125 Orbera arm versus 130 non-sham control arm) demonstrated 10.2% TWL in the Orbera group compared with 3.3% TWL in the control group at 6 months based on intention-to-treat (ITT) analysis. This difference persisted at 12 months (6 months after explantation) with 7.6% TWL for the Orbera group versus 3.1% TWL for the control group.13,14
ReShape integrated dual balloon system (ReShape Lifesciences, San Clemente, Calif.) consists of two connected fluid-filled balloons that are endoscopically placed and removed at 6 months. Each balloon is filled with 375-450 cc of saline mixed with methylene blue. The U.S. pivotal trial (REDUCE trial) including 326 subjects (187 ReShape arm versus 139 sham arm) demonstrated 6.8% TWL in the ReShape group compared with 3.3% TWL in the sham group at 6 months based on ITT analysis.15,16
Obalon balloon system (Obalon Therapeutics, Carlsbad, Calif.) is a swallowable, gas-filled balloon system that requires endoscopy only for removal. During placement, a capsule is swallowed under fluoroscopic guidance. The balloon is then inflated with 250 cc of nitrogen mix gas prior to tube detachment. Up to three balloons may be swallowed sequentially at 1-month intervals. At 6 months from the first balloon placement, all balloons are removed endoscopically. The U.S. pivotal trial (SMART trial) including 366 subjects (185 Obalon arm versus 181 sham capsule arm) demonstrated 6.6% TWL in the Obalon group compared with 3.4% TWL in the sham group at 6 months based on ITT analysis.17,18
Two other balloons that are currently under investigation in the United States are the Spatz3 adjustable balloon system (Spatz Medical, Great Neck, N.Y.) and Elipse balloon (Allurion Technologies, Wellesley, Mass.). The Spatz3 is a fluid-filled balloon that is placed and removed endoscopically. It consists of a single balloon and a connecting tube that allows volume adjustment for control of symptoms and possible augmentation of weight loss. The U.S. pivotal trial was recently completed and the data are being reviewed by the FDA. The Elipse is a swallowable fluid-filled balloon that does not require endoscopy for placement or removal. At 4 months, the balloon releases fluid allowing it to empty and pass naturally. The U.S. pivotal trial (ENLIGHTEN trial) is currently underway.
A meta-analysis of randomized controlled trials revealed improvement in most metabolic parameters (diastolic blood pressure, fasting glucose, hemoglobin A1c, and waist circumference) following IGB compared with controls.19 Nausea and vomiting are seen in approximately 30% and should be addressed appropriately. Pooled serious adverse event (SAE) rate was 1.5%, which included migration, perforation, and death. Since 2016, 14 deaths have been reported according to the FDA MAUDE database. Corporate response was that over 295,000 balloons had been distributed worldwide with a mortality rate of less than 0.01%.20
Plication and suturing
Currently, there are two endoscopic devices that are approved for the general indication of tissue apposition. These include the Incisionless Operating Platform (IOP) (USGI Medical, San Clemente, Calif.) and the Overstitch endoscopic suturing system (Apollo Endosurgery, Austin, Tex.). These devices are used to remodel the stomach to create a sleeve-like structure to induce weight loss.
The IOP system consists of a transport, which is a 54-Fr flexible endoscope. It consists of four working channels that accommodate a G-Prox (for tissue approximation), a G-Lix (for tissue grasping), and an ultrathin endoscope (for visualization). In April 2008, Horgan performed the first-in-human primary obesity surgery endoluminal (POSE) procedure in Argentina. The procedure involves the use of the IOP system to place plications primarily in the fundus to modify gastric accommodation.21 The U.S. pivotal trial (ESSENTIAL trial) including 332 subjects (221 POSE arm versus 111 sham arm) demonstrated 5.0% TWL in the POSE group compared with 1.4% in the sham group at 12 months based on ITT analysis.22 A European multicenter randomized controlled trial (MILEPOST trial) including 44 subjects (34 POSE arm versus 10 non-sham control arm) demonstrated 13.0% TWL in the POSE group compared with 5.3% TWL in the control group at 12 months.23 A recent meta-analysis including five studies with 586 subjects showed pooled weight loss of 13.2% at 12-15 months following POSE with a pooled serious adverse event rate of 3.2%.24 These included extraluminal bleeding, minor bleeding at the suture site, hepatic abscess, chest pain, nausea, vomiting, and abdominal pain. A distal POSE procedure with a new plication pattern focusing on the gastric body to augment the effect on gastric emptying has also been described.25
The Overstitch is an endoscopic suturing device that is mounted on a double-channel endoscope. At the tip of the scope, there is a curved suture arm and an anchor exchange that allow the needle to pass back and forth to perform full-thickness bites. The tissue helix may also be placed through the second channel to grasp tissue. In April 2012, Thompson performed the first-in-human endoscopic sutured/sleeve gastroplasty (ESG) procedure in India, which was published together with cases performed in Panama and the Dominican Republic.26-28 This procedure involves the use of the Overstitch device to place several sets of running sutures along the greater curvature of the stomach to create a sleeve-like structure. It is thought to delay gastric emptying and therefore increase satiety.29 The largest multicenter retrospective study including 248 patients demonstrated 18.6% TWL at 2 years with 2% SAE rate including perigastric fluid collections, extraluminal hemorrhage, pulmonary embolism, pneumoperitoneum, and pneumothorax.30
Aspiration therapy
Aspiration therapy (AT; Aspire Bariatrics, King of Prussia, Pa.) allows patients to remove 25%-30% of ingested calories at approximately 30 minutes after meals. AT consists of an A-tube, which is a 26-Fr gastrostomy tube with a 15-cm fenestrated drainage catheter placed endoscopically via a standard pull technique. At 1-2 weeks after A-tube placement, the tube is cut down to the skin and connected to the port prior to aspiration. AT is approved for patients with a BMI of 35-55 kg/m2.31 The U.S. pivotal trial (PATHWAY trial) including 207 subjects (137 AT arm versus 70 non-sham control arm) demonstrated 12.1% TWL in the AT group compared to 3.5% in the control group at 12 months based on ITT analysis. The SAE rate was 3.6% including severe abdominal pain, peritonitis, prepyloric ulcer, and A-tube replacement due to skin-port malfunction.32
Transpyloric shuttle
The transpyloric shuttle (TPS; BAROnova, Goleta, Calif.) consists of a spherical bulb that is attached to a smaller cylindrical bulb by a flexible tether. It is placed and removed endoscopically at 6 months. TPS resides across the pylorus creating intermittent obstruction that may result in delayed gastric emptying. A pilot study including 20 patients demonstrated 14.5% TWL at 6 months.33 The U.S. pivotal trial (ENDObesity II trial) was recently completed and the data are being reviewed by the FDA.
Revision for weight regain following bariatric surgery

Weight regain is common following RYGB34,35 and can be associated with dilation of the gastrojejunal anastomosis (GJA).36 Several procedures have been developed to treat this condition by focusing on reduction of GJA size and are available in the United States (Figure 2). These procedures have level I evidence supporting their use and include transoral outlet reduction (TORe) and restorative obesity surgery endoluminal (ROSE).37 TORe involves the use of the Overstitch to place sutures at the GJA. At 1 year, patients had 8.4% TWL with improvement in comorbidities.38 Weight loss remained significant up to 3-5 years.39,40 The modern ROSE procedure utilizes the IOP system to place plications at the GJA and distal gastric pouch following argon plasma coagulation (APC). A small series showed 12.4% TWL at 6 months.41 APC is also currently being investigated as a standalone therapy for weight regain in this population.
Small bowel interventions
There are several small bowel interventions, with different mechanisms of action, available internationally. Many of these are under investigation in the United States; however, none are currently FDA approved.
Duodenal-jejunal bypass liner
Duodenal-jejunal bypass liner (DJBL; GI Dynamics, Boston, Mass.) is a 60-cm fluoropolymer liner that is endoscopically placed and removed at 12 months. It is anchored at the duodenal bulb and ends at the jejunum. By excluding direct contact between chyme and the proximal small bowel, DJBL is thought to work via foregut mechanism where there is less inhibition of the incretin effect (greater increase in insulin secretion following oral glucose administration compared to intravenous glucose administration due to gut-derived factors that enhance insulin secretion) leading to improved insulin resistance. In addition, the enteral transit of chyme and bile is altered suggesting the possible role of the hindgut mechanism. The previous U.S. pivotal trial (ENDO trial) met efficacy endpoints. However, the study was stopped early by the company because of a hepatic abscess rate of 3.5%, all of which were treated conservatively.42 A new U.S. pivotal study is currently planned. A meta-analysis of 17 published studies, all of which were from outside the United States, demonstrated a significant decrease in hemoglobin A1c of 1.3% and 18.9% TWL at 1 year following implantation in patients with obesity with concomitant diabetes.43
Duodenal mucosal resurfacing
Duodenal mucosal resurfacing (Fractyl, Lexington, Mass.) involves saline lifting of the duodenal mucosa circumferentially prior to thermal ablation using an inflated balloon filled with heated water. It is hypothesized that this may reset the diseased duodenal enteroendocrine cells leading to restoration of the incretin effect. A pilot study including 39 patients with poorly controlled diabetes demonstrated a decrease in hemoglobin A1c of 1.2%. The SAE rate was 7.7% including duodenal stenosis, all of which were treated with balloon dilation.44 The U.S. pivotal trial is currently planned.
Gastroduodenal-jejunal bypass
Gastroduodenal-jejunal bypass (ValenTx., Hopkins, Minn.) is a 120-cm sleeve that is anchored at the gastroesophageal junction to create the anatomic changes of RYGB. It is placed and removed endoscopically with laparoscopic assistance. A pilot study including 12 patients demonstrated 35.9% excess weight loss at 12 months. Two out of 12 patients had early device removal due to intolerance and they were not included in the weight loss analysis.45
Incisionless magnetic anastomosis system
The incisionless magnetic anastomosis system (GI Windows, West Bridgewater, Mass.) consists of self-assembling magnets that are deployed under fluoroscopic guidance through the working channel of colonoscopes to form magnetic octagons in the jejunum and ileum. After a week, a compression anastomosis is formed and the coupled magnets pass spontaneously. A pilot study including 10 patients showed 14.6% TWL and a decrease in hemoglobin A1c of 1.9% (for patients with diabetes) at 1 year.46 A randomized study outside the United States is currently underway.
Summary
Endoscopic bariatric and metabolic therapies are emerging as first-line treatments for obesity in many populations. They can serve as a gap therapy for patients who do not qualify for surgery, but also may have a specific role in the treatment of metabolic comorbidities. This field will continue to develop and improve with the introduction of personalized medicine leading to better patient selection, and newer combination therapies. It is time for gastroenterologists to become more involved in the management of this challenging condition.
Dr. Jirapinyo is an advanced and bariatric endoscopy fellow, Brigham and Women’s Hospital, Harvard Medical School, Boston; Dr. Thompson is director of therapeutic endoscopy, Brigham and Women’s Hospital, and associate professor of medicine, Harvard Medical School. Dr. Jirapinyo has served as a consultant for GI Dynamics and holds royalties for Endosim. Dr. Thompson has contracted research for Aspire Bariatrics, USGI Medical, Spatz, and Apollo Endosurgery; has served as a consultant for Boston Scientific, Covidien, USGI Medical, Olympus, and Fractyl; holds stocks and royalties for GI Windows and Endosim, and has served as an expert reviewer for GI Dynamics.
References
1. CDC. From https://www.cdc.gov/obesity/data/adult.html. Accessed on 11 September 2018.
2. Aronne LJ et al. Obesity. 2013;21:2163-71.
3. Torgerson JS et al. Diabetes Care. 2004;27:155-61.
4. Allison DB et al. Obesity. 2012;20:330-42.
5. Smith SR et al. N Engl J Med. 2010;363:245-56.
6. Apovian CM et al. Obesity. 2013;21:935-43.
7. Pi-Sunyer X et al. N Engl J Med. 2015;373:11-22.
8. Colguitt JL et al. Cochrane Database Syst Rev. 2014;8(8):CD003641.
9. Ponce J et al. Surg Obes Relat Dis. 2015;11(6):1199-200.
10. Jirapinyo P, Thompson CC et al. Clin Gastroenterol Hepatol. 2017;15(5):619-30.
11. Sullivan S et al.Gastroenterology. 2017;152(7):1791-801.
12. Gomez V et al. Obesity. 2016;24(9):1849-53.
13. Food and Drug Administration. Summary of safety and effectiveness data (SSED) ORBERA Intragastric Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140008b.pdf. 2015:1-32.
14. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;81:AB147.
15. Food and Drug Administration. Summary of safety and effectiveness data (SSED) ReShape Integrated Dual Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140012b.pdf. 2015:1-43.
16. Ponce J et al. Surg Obes Relat Dis. 2015;11:874-81.
17. Food and Drug Administration. Summary and effectiveness data (SSED): Obalon Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160001b.pdf. 2016:1-46.
18. Sullivan S et al. Gastroenterology. 2016;150:S1267.
19. Popov VB et al. Am J Gastroenterol. 2017;112:429-39.
20. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;82(3):425-38.
21. Espinos JC et al. Obes Surg. 2013;23(9):1375-83.
22. Sullivan S et al. Obesity. 2017;25:294-301.
23. Miller K et al. Obesity Surg. 2017;27(2):310-22.
24. Jirapinyo P et al. Gastrointest Endosc. 2018;87(6):AB604-AB605.
25. Jirapinyo P, Thompson CC. Video GIE. 2018;3(10):296-300.
26. Campos J et al. SAGES 2013 Presentation. Baltimore, MD. 19 April 2013.
27. Kumar N et al. Gastroenterology. 2014;146(5):S571-2.
28. Kumar N et al. Surg Endosc. 2018;32(4):2159-64.
29. Abu Dayyeh BK et al. Clin Gastroenterol Hepatol. 2017;15:37-43.
30. Lopez-Nava G et al. Obes Surg. 2017;27(10):2649-55.
31. Food and Drug Administration. Summary of safety and effectiveness (SSED): AspireAssist. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf15/p150024b.pdf. FDA,ed,2016:1-36.
32. Thompson CC et al. Am J Gastroenterol. 2017;112:447-57.
33. SAGES abstract archives. SAGES. Available from: http://www.sages.org/meetings/annual-meeting/abstracts-archive/first-clinical-experience-with-the-transpyloric-shuttle-tpsr-device-a-non-surgical-endoscopic treatment-for-obesity-results-from-a-3-month-and-6-month-study. Accessed Sept. 12, 2018.
34. Sjostrom L et al. N Engl J Med. 2007;357:741-52.
35. Adams TD et al. N Engl J Med. 2017;377:1143-55.
36. Abu Dayyeh BK et al. Clin Gastroenterol Hepatol. 2011;9:228-33.
37. Thompson CC et al. Gastroenterology. 2013;145(1):129-37.
38. Jirapinyo P et al. Endoscopy. 2018;50(4):371-7.
39. Kumar N, Thompson CC. Gastrointest Endosc. 2016;83(4):776-9.
40. Jirapinyo P et al. Gastrointest Endosc. 2017;85(5):AB93-94.
41. Jirapinyo P, Thompson CC et al. Comparison of a novel plication technique to suturing for endoscopic outlet reduction for the treatment of weight regain after Roux-en-Y gastric bypass. Obesity Week 2018. Poster presentation.
42. Kaplan LM et al. EndoBarrier therapy is associated with glycemic improvement, weight loss and safety issues in patients with obesity and type 2 diabetes on oral anti-hyperglycemic agents (The ENDO Trial). In: Oral Presentation at the 76th American Diabetes Association (ADA) Annual Meeting: 2016 June 10-14: New Orleans. Abstract number 362-LB.
43. Jirapinyo P et al. Diabetes Care. 2018;41(5):1106-15.
44. Rajagopalan H et al. Diabetes Care. 2016;39(12):2254-61.
45. Sandler BJ et al. Surgical Endosc. 2015;29:3298-303.
46. Machytka E et al. Gastrointest Endosc. 2017;86(5):904-12.
Editor's Note
Gastroenterologists are becoming increasingly involved in the management of obesity. While prior therapy for obesity was mainly based on lifestyle changes, medication, or surgery, the new and exciting field of endoscopic bariatric and metabolic therapies has recently garnered incredible attention and momentum.
In this quarter’s In Focus article, brought to you by The New Gastroenterologist, Pichamol Jirapinyo and Christopher Thompson (Brigham and Women’s Hospital) provide an outstanding overview of the gastric and small bowel endoscopic interventions that are either already approved for use in obesity or currently being studied. This field is moving incredibly fast, and knowledge and understanding of these endoscopic therapies for obesity will undoubtedly be important for our field.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Introduction
Obesity is a rising pandemic. As of 2016, 93.3 million U.S. adults had obesity, representing 39.8% of our adult population.1 It is estimated that approximately $147 billion is spent annually on caring for patients with obesity. Traditionally, the management of obesity includes lifestyle therapy (diet and exercise), pharmacotherapy (six Food and Drug Administration–approved medications for obesity), and bariatric surgery (sleeve gastrectomy [SG] and Roux-en-Y gastric bypass [RYGB]). Nevertheless, intensive lifestyle intervention and pharmacotherapy are associated with approximately 3.1%-6.6% total weight loss (TWL),2-7 and bariatric surgery is associated with 20%-33.3% TWL.8 However, less than 2% of patients who are eligible for bariatric surgery elect to undergo surgery, leaving a large proportion of patients with obesity untreated or undertreated.9

Endoscopic bariatric and metabolic therapies (EBMTs) encompass an emerging field for the treatment of obesity. In general, EBMTs are associated with greater weight loss than are lifestyle intervention and pharmacotherapy, but with a less- invasive risk profile than bariatric surgery. EBMTs may be divided into two general categories – gastric and small bowel interventions (Figure 1 and Table 1). Gastric EBMTs are effective at treating obesity, while small bowel EBMTs are effective at treating metabolic diseases with a variable weight loss profile depending on the device.10,11
Of note, a variety of study designs (including retrospective series, prospective series, and randomized trials with and without shams) have been employed, which can affect outcomes. Therefore, weight loss comparisons among studies are challenging and should be considered in this context.
Gastric interventions
Currently, there are three types of EBMTs that are FDA approved and used for the treatment of obesity. These include intragastric balloons (IGBs), plications and suturing, and aspiration therapy (AT). Other technologies that are under investigation also will be briefly covered.
Intragastric balloons
An intragastric balloon is a space-occupying device that is placed in the stomach. The mechanism of action of IGBs involves delaying gastric emptying, which leads to increased satiety.12 There are several types of IGBs available worldwide differing in techniques of placement and removal (endoscopic versus fluoroscopic versus swallowable), materials used to fill the balloon (fluid-filled versus air-filled), and the number of balloons placed (single versus duo versus three-balloon). At the time of this writing, three IGBs are approved by the FDA (Orbera, ReShape, and Obalon), all for patients with body mass indexes of 30-40 kg/m2, and two others are in the process of obtaining FDA approval (Spatz and Elipse).
Orbera gastric balloon (Apollo Endosurgery, Austin, Tex.) is a single fluid-filled IGB that is endoscopically placed and removed at 6 months. The balloon is filled with 400-700 cc of saline with or without methylene blue (to identify leakage or rupture). Recently, Orbera365, which allows the balloon to stay for 12 months instead of 6 months, has become available in Europe; however, it is yet to be approved in the United States. The U.S. pivotal trial (Orbera trial) including 255 subjects (125 Orbera arm versus 130 non-sham control arm) demonstrated 10.2% TWL in the Orbera group compared with 3.3% TWL in the control group at 6 months based on intention-to-treat (ITT) analysis. This difference persisted at 12 months (6 months after explantation) with 7.6% TWL for the Orbera group versus 3.1% TWL for the control group.13,14
ReShape integrated dual balloon system (ReShape Lifesciences, San Clemente, Calif.) consists of two connected fluid-filled balloons that are endoscopically placed and removed at 6 months. Each balloon is filled with 375-450 cc of saline mixed with methylene blue. The U.S. pivotal trial (REDUCE trial) including 326 subjects (187 ReShape arm versus 139 sham arm) demonstrated 6.8% TWL in the ReShape group compared with 3.3% TWL in the sham group at 6 months based on ITT analysis.15,16
Obalon balloon system (Obalon Therapeutics, Carlsbad, Calif.) is a swallowable, gas-filled balloon system that requires endoscopy only for removal. During placement, a capsule is swallowed under fluoroscopic guidance. The balloon is then inflated with 250 cc of nitrogen mix gas prior to tube detachment. Up to three balloons may be swallowed sequentially at 1-month intervals. At 6 months from the first balloon placement, all balloons are removed endoscopically. The U.S. pivotal trial (SMART trial) including 366 subjects (185 Obalon arm versus 181 sham capsule arm) demonstrated 6.6% TWL in the Obalon group compared with 3.4% TWL in the sham group at 6 months based on ITT analysis.17,18
Two other balloons that are currently under investigation in the United States are the Spatz3 adjustable balloon system (Spatz Medical, Great Neck, N.Y.) and Elipse balloon (Allurion Technologies, Wellesley, Mass.). The Spatz3 is a fluid-filled balloon that is placed and removed endoscopically. It consists of a single balloon and a connecting tube that allows volume adjustment for control of symptoms and possible augmentation of weight loss. The U.S. pivotal trial was recently completed and the data are being reviewed by the FDA. The Elipse is a swallowable fluid-filled balloon that does not require endoscopy for placement or removal. At 4 months, the balloon releases fluid allowing it to empty and pass naturally. The U.S. pivotal trial (ENLIGHTEN trial) is currently underway.
A meta-analysis of randomized controlled trials revealed improvement in most metabolic parameters (diastolic blood pressure, fasting glucose, hemoglobin A1c, and waist circumference) following IGB compared with controls.19 Nausea and vomiting are seen in approximately 30% and should be addressed appropriately. Pooled serious adverse event (SAE) rate was 1.5%, which included migration, perforation, and death. Since 2016, 14 deaths have been reported according to the FDA MAUDE database. Corporate response was that over 295,000 balloons had been distributed worldwide with a mortality rate of less than 0.01%.20
Plication and suturing
Currently, there are two endoscopic devices that are approved for the general indication of tissue apposition. These include the Incisionless Operating Platform (IOP) (USGI Medical, San Clemente, Calif.) and the Overstitch endoscopic suturing system (Apollo Endosurgery, Austin, Tex.). These devices are used to remodel the stomach to create a sleeve-like structure to induce weight loss.
The IOP system consists of a transport, which is a 54-Fr flexible endoscope. It consists of four working channels that accommodate a G-Prox (for tissue approximation), a G-Lix (for tissue grasping), and an ultrathin endoscope (for visualization). In April 2008, Horgan performed the first-in-human primary obesity surgery endoluminal (POSE) procedure in Argentina. The procedure involves the use of the IOP system to place plications primarily in the fundus to modify gastric accommodation.21 The U.S. pivotal trial (ESSENTIAL trial) including 332 subjects (221 POSE arm versus 111 sham arm) demonstrated 5.0% TWL in the POSE group compared with 1.4% in the sham group at 12 months based on ITT analysis.22 A European multicenter randomized controlled trial (MILEPOST trial) including 44 subjects (34 POSE arm versus 10 non-sham control arm) demonstrated 13.0% TWL in the POSE group compared with 5.3% TWL in the control group at 12 months.23 A recent meta-analysis including five studies with 586 subjects showed pooled weight loss of 13.2% at 12-15 months following POSE with a pooled serious adverse event rate of 3.2%.24 These included extraluminal bleeding, minor bleeding at the suture site, hepatic abscess, chest pain, nausea, vomiting, and abdominal pain. A distal POSE procedure with a new plication pattern focusing on the gastric body to augment the effect on gastric emptying has also been described.25
The Overstitch is an endoscopic suturing device that is mounted on a double-channel endoscope. At the tip of the scope, there is a curved suture arm and an anchor exchange that allow the needle to pass back and forth to perform full-thickness bites. The tissue helix may also be placed through the second channel to grasp tissue. In April 2012, Thompson performed the first-in-human endoscopic sutured/sleeve gastroplasty (ESG) procedure in India, which was published together with cases performed in Panama and the Dominican Republic.26-28 This procedure involves the use of the Overstitch device to place several sets of running sutures along the greater curvature of the stomach to create a sleeve-like structure. It is thought to delay gastric emptying and therefore increase satiety.29 The largest multicenter retrospective study including 248 patients demonstrated 18.6% TWL at 2 years with 2% SAE rate including perigastric fluid collections, extraluminal hemorrhage, pulmonary embolism, pneumoperitoneum, and pneumothorax.30
Aspiration therapy
Aspiration therapy (AT; Aspire Bariatrics, King of Prussia, Pa.) allows patients to remove 25%-30% of ingested calories at approximately 30 minutes after meals. AT consists of an A-tube, which is a 26-Fr gastrostomy tube with a 15-cm fenestrated drainage catheter placed endoscopically via a standard pull technique. At 1-2 weeks after A-tube placement, the tube is cut down to the skin and connected to the port prior to aspiration. AT is approved for patients with a BMI of 35-55 kg/m2.31 The U.S. pivotal trial (PATHWAY trial) including 207 subjects (137 AT arm versus 70 non-sham control arm) demonstrated 12.1% TWL in the AT group compared to 3.5% in the control group at 12 months based on ITT analysis. The SAE rate was 3.6% including severe abdominal pain, peritonitis, prepyloric ulcer, and A-tube replacement due to skin-port malfunction.32
Transpyloric shuttle
The transpyloric shuttle (TPS; BAROnova, Goleta, Calif.) consists of a spherical bulb that is attached to a smaller cylindrical bulb by a flexible tether. It is placed and removed endoscopically at 6 months. TPS resides across the pylorus creating intermittent obstruction that may result in delayed gastric emptying. A pilot study including 20 patients demonstrated 14.5% TWL at 6 months.33 The U.S. pivotal trial (ENDObesity II trial) was recently completed and the data are being reviewed by the FDA.
Revision for weight regain following bariatric surgery

Weight regain is common following RYGB34,35 and can be associated with dilation of the gastrojejunal anastomosis (GJA).36 Several procedures have been developed to treat this condition by focusing on reduction of GJA size and are available in the United States (Figure 2). These procedures have level I evidence supporting their use and include transoral outlet reduction (TORe) and restorative obesity surgery endoluminal (ROSE).37 TORe involves the use of the Overstitch to place sutures at the GJA. At 1 year, patients had 8.4% TWL with improvement in comorbidities.38 Weight loss remained significant up to 3-5 years.39,40 The modern ROSE procedure utilizes the IOP system to place plications at the GJA and distal gastric pouch following argon plasma coagulation (APC). A small series showed 12.4% TWL at 6 months.41 APC is also currently being investigated as a standalone therapy for weight regain in this population.
Small bowel interventions
There are several small bowel interventions, with different mechanisms of action, available internationally. Many of these are under investigation in the United States; however, none are currently FDA approved.
Duodenal-jejunal bypass liner
Duodenal-jejunal bypass liner (DJBL; GI Dynamics, Boston, Mass.) is a 60-cm fluoropolymer liner that is endoscopically placed and removed at 12 months. It is anchored at the duodenal bulb and ends at the jejunum. By excluding direct contact between chyme and the proximal small bowel, DJBL is thought to work via foregut mechanism where there is less inhibition of the incretin effect (greater increase in insulin secretion following oral glucose administration compared to intravenous glucose administration due to gut-derived factors that enhance insulin secretion) leading to improved insulin resistance. In addition, the enteral transit of chyme and bile is altered suggesting the possible role of the hindgut mechanism. The previous U.S. pivotal trial (ENDO trial) met efficacy endpoints. However, the study was stopped early by the company because of a hepatic abscess rate of 3.5%, all of which were treated conservatively.42 A new U.S. pivotal study is currently planned. A meta-analysis of 17 published studies, all of which were from outside the United States, demonstrated a significant decrease in hemoglobin A1c of 1.3% and 18.9% TWL at 1 year following implantation in patients with obesity with concomitant diabetes.43
Duodenal mucosal resurfacing
Duodenal mucosal resurfacing (Fractyl, Lexington, Mass.) involves saline lifting of the duodenal mucosa circumferentially prior to thermal ablation using an inflated balloon filled with heated water. It is hypothesized that this may reset the diseased duodenal enteroendocrine cells leading to restoration of the incretin effect. A pilot study including 39 patients with poorly controlled diabetes demonstrated a decrease in hemoglobin A1c of 1.2%. The SAE rate was 7.7% including duodenal stenosis, all of which were treated with balloon dilation.44 The U.S. pivotal trial is currently planned.
Gastroduodenal-jejunal bypass
Gastroduodenal-jejunal bypass (ValenTx., Hopkins, Minn.) is a 120-cm sleeve that is anchored at the gastroesophageal junction to create the anatomic changes of RYGB. It is placed and removed endoscopically with laparoscopic assistance. A pilot study including 12 patients demonstrated 35.9% excess weight loss at 12 months. Two out of 12 patients had early device removal due to intolerance and they were not included in the weight loss analysis.45
Incisionless magnetic anastomosis system
The incisionless magnetic anastomosis system (GI Windows, West Bridgewater, Mass.) consists of self-assembling magnets that are deployed under fluoroscopic guidance through the working channel of colonoscopes to form magnetic octagons in the jejunum and ileum. After a week, a compression anastomosis is formed and the coupled magnets pass spontaneously. A pilot study including 10 patients showed 14.6% TWL and a decrease in hemoglobin A1c of 1.9% (for patients with diabetes) at 1 year.46 A randomized study outside the United States is currently underway.
Summary
Endoscopic bariatric and metabolic therapies are emerging as first-line treatments for obesity in many populations. They can serve as a gap therapy for patients who do not qualify for surgery, but also may have a specific role in the treatment of metabolic comorbidities. This field will continue to develop and improve with the introduction of personalized medicine leading to better patient selection, and newer combination therapies. It is time for gastroenterologists to become more involved in the management of this challenging condition.
Dr. Jirapinyo is an advanced and bariatric endoscopy fellow, Brigham and Women’s Hospital, Harvard Medical School, Boston; Dr. Thompson is director of therapeutic endoscopy, Brigham and Women’s Hospital, and associate professor of medicine, Harvard Medical School. Dr. Jirapinyo has served as a consultant for GI Dynamics and holds royalties for Endosim. Dr. Thompson has contracted research for Aspire Bariatrics, USGI Medical, Spatz, and Apollo Endosurgery; has served as a consultant for Boston Scientific, Covidien, USGI Medical, Olympus, and Fractyl; holds stocks and royalties for GI Windows and Endosim, and has served as an expert reviewer for GI Dynamics.
References
1. CDC. From https://www.cdc.gov/obesity/data/adult.html. Accessed on 11 September 2018.
2. Aronne LJ et al. Obesity. 2013;21:2163-71.
3. Torgerson JS et al. Diabetes Care. 2004;27:155-61.
4. Allison DB et al. Obesity. 2012;20:330-42.
5. Smith SR et al. N Engl J Med. 2010;363:245-56.
6. Apovian CM et al. Obesity. 2013;21:935-43.
7. Pi-Sunyer X et al. N Engl J Med. 2015;373:11-22.
8. Colguitt JL et al. Cochrane Database Syst Rev. 2014;8(8):CD003641.
9. Ponce J et al. Surg Obes Relat Dis. 2015;11(6):1199-200.
10. Jirapinyo P, Thompson CC et al. Clin Gastroenterol Hepatol. 2017;15(5):619-30.
11. Sullivan S et al.Gastroenterology. 2017;152(7):1791-801.
12. Gomez V et al. Obesity. 2016;24(9):1849-53.
13. Food and Drug Administration. Summary of safety and effectiveness data (SSED) ORBERA Intragastric Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140008b.pdf. 2015:1-32.
14. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;81:AB147.
15. Food and Drug Administration. Summary of safety and effectiveness data (SSED) ReShape Integrated Dual Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf14/P140012b.pdf. 2015:1-43.
16. Ponce J et al. Surg Obes Relat Dis. 2015;11:874-81.
17. Food and Drug Administration. Summary and effectiveness data (SSED): Obalon Balloon System. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf16/P160001b.pdf. 2016:1-46.
18. Sullivan S et al. Gastroenterology. 2016;150:S1267.
19. Popov VB et al. Am J Gastroenterol. 2017;112:429-39.
20. Abu Dayyeh BK et al. Gastrointest Endosc. 2015;82(3):425-38.
21. Espinos JC et al. Obes Surg. 2013;23(9):1375-83.
22. Sullivan S et al. Obesity. 2017;25:294-301.
23. Miller K et al. Obesity Surg. 2017;27(2):310-22.
24. Jirapinyo P et al. Gastrointest Endosc. 2018;87(6):AB604-AB605.
25. Jirapinyo P, Thompson CC. Video GIE. 2018;3(10):296-300.
26. Campos J et al. SAGES 2013 Presentation. Baltimore, MD. 19 April 2013.
27. Kumar N et al. Gastroenterology. 2014;146(5):S571-2.
28. Kumar N et al. Surg Endosc. 2018;32(4):2159-64.
29. Abu Dayyeh BK et al. Clin Gastroenterol Hepatol. 2017;15:37-43.
30. Lopez-Nava G et al. Obes Surg. 2017;27(10):2649-55.
31. Food and Drug Administration. Summary of safety and effectiveness (SSED): AspireAssist. Available at https://www.accessdata.fda.gov/cdrh_docs/pdf15/p150024b.pdf. FDA,ed,2016:1-36.
32. Thompson CC et al. Am J Gastroenterol. 2017;112:447-57.
33. SAGES abstract archives. SAGES. Available from: http://www.sages.org/meetings/annual-meeting/abstracts-archive/first-clinical-experience-with-the-transpyloric-shuttle-tpsr-device-a-non-surgical-endoscopic treatment-for-obesity-results-from-a-3-month-and-6-month-study. Accessed Sept. 12, 2018.
34. Sjostrom L et al. N Engl J Med. 2007;357:741-52.
35. Adams TD et al. N Engl J Med. 2017;377:1143-55.
36. Abu Dayyeh BK et al. Clin Gastroenterol Hepatol. 2011;9:228-33.
37. Thompson CC et al. Gastroenterology. 2013;145(1):129-37.
38. Jirapinyo P et al. Endoscopy. 2018;50(4):371-7.
39. Kumar N, Thompson CC. Gastrointest Endosc. 2016;83(4):776-9.
40. Jirapinyo P et al. Gastrointest Endosc. 2017;85(5):AB93-94.
41. Jirapinyo P, Thompson CC et al. Comparison of a novel plication technique to suturing for endoscopic outlet reduction for the treatment of weight regain after Roux-en-Y gastric bypass. Obesity Week 2018. Poster presentation.
42. Kaplan LM et al. EndoBarrier therapy is associated with glycemic improvement, weight loss and safety issues in patients with obesity and type 2 diabetes on oral anti-hyperglycemic agents (The ENDO Trial). In: Oral Presentation at the 76th American Diabetes Association (ADA) Annual Meeting: 2016 June 10-14: New Orleans. Abstract number 362-LB.
43. Jirapinyo P et al. Diabetes Care. 2018;41(5):1106-15.
44. Rajagopalan H et al. Diabetes Care. 2016;39(12):2254-61.
45. Sandler BJ et al. Surgical Endosc. 2015;29:3298-303.
46. Machytka E et al. Gastrointest Endosc. 2017;86(5):904-12.
Diet Tips for Diabetes Management & Metabolic Health
Obesity, weight gain linked to CRC risk in younger women
Obesity and weight gain are linked to increased risk of colorectal cancer in younger women, according to an analysis of a large, prospective U.S. cohort study.
, authors of the study reported in JAMA Oncology.
The findings suggest body weight could be used to “personalize and complement” early cancer screening strategies among adults younger than 50 years, said investigator Po-Hong Liu, MD, of Washington University, St. Louis, and coauthors.
“Given that most of these younger cases are diagnosed symptomatically with more advanced tumors and with a significant influence on years of life lost, our findings reinforce the benefits of maintaining a healthy weight throughout life,” Dr. Liu and coinvestigators said in their report.
Their analysis was based on the ongoing Nurses Health Study II, which began in 1989 and enrolled a total of 116,430 women between the ages of 25 and 42 years in 14 U.S. states. Women completed questionnaires on demographics, medical and health information, and lifestyle factors every 2 years after enrollment.
Dr. Liu and colleagues were able to document 114 cases of colorectal cancer over a median of 13.9 years of follow-up in 85,256 women who had no cancer or inflammatory bowel disease when they were enrolled in the study. The median age at diagnosis for these cancers was 45 years.
Obesity was independently associated with increased risk of these early-onset colorectal cancers, the investigators found in multivariable analysis.
Women with a body mass index of 30 kg/m2 or higher had a relative risk of 1.93 (95% confidence interval, 1.15-3.25) versus women with normal BMIs in the 18.5 to 22.9-kg/m2 range, according to results of the analysis, they reported.
There was an apparent linear trend between increasing weight and increasing colorectal cancer risk, they added in their report.
They also found links between BMI in early adulthood and risk of early-onset colorectal cancer, including a relative risk of 1.63 for women who reported a BMI of 23 kg/m2 or higher at 18 years of age, versus women with a BMI of 18.5-20.9 kg/m2 at that age.
Similarly, increase in weight since early adulthood was associated with increased cancer risk, they reported.
While the link between excess weight and colorectal cancer incidence and mortality is well established in previous studies, this study is one of few reports looking at the association in younger individuals, according to Dr. Liu and colleagues.
This is thought to be the first prospective study looking at the link between obesity and risk of colo-rectal cancer diagnosed before the age of 50, they added.
The study was funded by grants from the National Institutes of Health. Dr. Liu had no conflict of interest disclosures related to the study. One coauthor reported consulting fees from Bayer Pharma AG, Janssen, and Pfizer.
SOURCE: Liu P-H et al. JAMA Oncol. 2018 Oct 11. doi: 10.1001/jamaoncol.2018.4280.
AGA Resource
Visit the AGA GI Patient Center for education to share with your patients about obesity, colorectal cancer and other GI disorders at patient.gastro.org.
Obesity and weight gain are linked to increased risk of colorectal cancer in younger women, according to an analysis of a large, prospective U.S. cohort study.
, authors of the study reported in JAMA Oncology.
The findings suggest body weight could be used to “personalize and complement” early cancer screening strategies among adults younger than 50 years, said investigator Po-Hong Liu, MD, of Washington University, St. Louis, and coauthors.
“Given that most of these younger cases are diagnosed symptomatically with more advanced tumors and with a significant influence on years of life lost, our findings reinforce the benefits of maintaining a healthy weight throughout life,” Dr. Liu and coinvestigators said in their report.
Their analysis was based on the ongoing Nurses Health Study II, which began in 1989 and enrolled a total of 116,430 women between the ages of 25 and 42 years in 14 U.S. states. Women completed questionnaires on demographics, medical and health information, and lifestyle factors every 2 years after enrollment.
Dr. Liu and colleagues were able to document 114 cases of colorectal cancer over a median of 13.9 years of follow-up in 85,256 women who had no cancer or inflammatory bowel disease when they were enrolled in the study. The median age at diagnosis for these cancers was 45 years.
Obesity was independently associated with increased risk of these early-onset colorectal cancers, the investigators found in multivariable analysis.
Women with a body mass index of 30 kg/m2 or higher had a relative risk of 1.93 (95% confidence interval, 1.15-3.25) versus women with normal BMIs in the 18.5 to 22.9-kg/m2 range, according to results of the analysis, they reported.
There was an apparent linear trend between increasing weight and increasing colorectal cancer risk, they added in their report.
They also found links between BMI in early adulthood and risk of early-onset colorectal cancer, including a relative risk of 1.63 for women who reported a BMI of 23 kg/m2 or higher at 18 years of age, versus women with a BMI of 18.5-20.9 kg/m2 at that age.
Similarly, increase in weight since early adulthood was associated with increased cancer risk, they reported.
While the link between excess weight and colorectal cancer incidence and mortality is well established in previous studies, this study is one of few reports looking at the association in younger individuals, according to Dr. Liu and colleagues.
This is thought to be the first prospective study looking at the link between obesity and risk of colo-rectal cancer diagnosed before the age of 50, they added.
The study was funded by grants from the National Institutes of Health. Dr. Liu had no conflict of interest disclosures related to the study. One coauthor reported consulting fees from Bayer Pharma AG, Janssen, and Pfizer.
SOURCE: Liu P-H et al. JAMA Oncol. 2018 Oct 11. doi: 10.1001/jamaoncol.2018.4280.
AGA Resource
Visit the AGA GI Patient Center for education to share with your patients about obesity, colorectal cancer and other GI disorders at patient.gastro.org.
Obesity and weight gain are linked to increased risk of colorectal cancer in younger women, according to an analysis of a large, prospective U.S. cohort study.
, authors of the study reported in JAMA Oncology.
The findings suggest body weight could be used to “personalize and complement” early cancer screening strategies among adults younger than 50 years, said investigator Po-Hong Liu, MD, of Washington University, St. Louis, and coauthors.
“Given that most of these younger cases are diagnosed symptomatically with more advanced tumors and with a significant influence on years of life lost, our findings reinforce the benefits of maintaining a healthy weight throughout life,” Dr. Liu and coinvestigators said in their report.
Their analysis was based on the ongoing Nurses Health Study II, which began in 1989 and enrolled a total of 116,430 women between the ages of 25 and 42 years in 14 U.S. states. Women completed questionnaires on demographics, medical and health information, and lifestyle factors every 2 years after enrollment.
Dr. Liu and colleagues were able to document 114 cases of colorectal cancer over a median of 13.9 years of follow-up in 85,256 women who had no cancer or inflammatory bowel disease when they were enrolled in the study. The median age at diagnosis for these cancers was 45 years.
Obesity was independently associated with increased risk of these early-onset colorectal cancers, the investigators found in multivariable analysis.
Women with a body mass index of 30 kg/m2 or higher had a relative risk of 1.93 (95% confidence interval, 1.15-3.25) versus women with normal BMIs in the 18.5 to 22.9-kg/m2 range, according to results of the analysis, they reported.
There was an apparent linear trend between increasing weight and increasing colorectal cancer risk, they added in their report.
They also found links between BMI in early adulthood and risk of early-onset colorectal cancer, including a relative risk of 1.63 for women who reported a BMI of 23 kg/m2 or higher at 18 years of age, versus women with a BMI of 18.5-20.9 kg/m2 at that age.
Similarly, increase in weight since early adulthood was associated with increased cancer risk, they reported.
While the link between excess weight and colorectal cancer incidence and mortality is well established in previous studies, this study is one of few reports looking at the association in younger individuals, according to Dr. Liu and colleagues.
This is thought to be the first prospective study looking at the link between obesity and risk of colo-rectal cancer diagnosed before the age of 50, they added.
The study was funded by grants from the National Institutes of Health. Dr. Liu had no conflict of interest disclosures related to the study. One coauthor reported consulting fees from Bayer Pharma AG, Janssen, and Pfizer.
SOURCE: Liu P-H et al. JAMA Oncol. 2018 Oct 11. doi: 10.1001/jamaoncol.2018.4280.
AGA Resource
Visit the AGA GI Patient Center for education to share with your patients about obesity, colorectal cancer and other GI disorders at patient.gastro.org.
Obesity Rates Are Up From 2016
In 2017, 7 states reported an adult obesity prevalence at or above 35%, up from 5 states in 2016. In 2012, obesity prevalence was lower than 35% in all states. The new data, from the Behavioral Risk Factor Surveillance System, are detailed in the CDC’s 2017 Adult Obesity Prevalence Maps, www.cdc.gov/obesity/data/prevalence-maps.html.
The highest levels were in Alabama, Arkansas, Iowa, Louisiana, Mississippi, Oklahoma, and West Virginia. The lowest prevalence was 22.6% in Colorado. Adults aged 45 to54 years were twice as likely as young adults to report obesity (36% vs 17%). Non-Hispanic blacks had the highest prevalence (39%), followed by Hispanics (32.4%), and non-Hispanic whites (29.3%). When education was factored in, adults without a high school degree had the highest prevalence at 35.6%; high school graduates, 32.9%; adults with some college, 31.9%; and college graduates, 22.7%.
Maps showing obesity levels overall, as well as by race and ethnicity, are also available for 2011 through 2016.
In 2017, 7 states reported an adult obesity prevalence at or above 35%, up from 5 states in 2016. In 2012, obesity prevalence was lower than 35% in all states. The new data, from the Behavioral Risk Factor Surveillance System, are detailed in the CDC’s 2017 Adult Obesity Prevalence Maps, www.cdc.gov/obesity/data/prevalence-maps.html.
The highest levels were in Alabama, Arkansas, Iowa, Louisiana, Mississippi, Oklahoma, and West Virginia. The lowest prevalence was 22.6% in Colorado. Adults aged 45 to54 years were twice as likely as young adults to report obesity (36% vs 17%). Non-Hispanic blacks had the highest prevalence (39%), followed by Hispanics (32.4%), and non-Hispanic whites (29.3%). When education was factored in, adults without a high school degree had the highest prevalence at 35.6%; high school graduates, 32.9%; adults with some college, 31.9%; and college graduates, 22.7%.
Maps showing obesity levels overall, as well as by race and ethnicity, are also available for 2011 through 2016.
In 2017, 7 states reported an adult obesity prevalence at or above 35%, up from 5 states in 2016. In 2012, obesity prevalence was lower than 35% in all states. The new data, from the Behavioral Risk Factor Surveillance System, are detailed in the CDC’s 2017 Adult Obesity Prevalence Maps, www.cdc.gov/obesity/data/prevalence-maps.html.
The highest levels were in Alabama, Arkansas, Iowa, Louisiana, Mississippi, Oklahoma, and West Virginia. The lowest prevalence was 22.6% in Colorado. Adults aged 45 to54 years were twice as likely as young adults to report obesity (36% vs 17%). Non-Hispanic blacks had the highest prevalence (39%), followed by Hispanics (32.4%), and non-Hispanic whites (29.3%). When education was factored in, adults without a high school degree had the highest prevalence at 35.6%; high school graduates, 32.9%; adults with some college, 31.9%; and college graduates, 22.7%.
Maps showing obesity levels overall, as well as by race and ethnicity, are also available for 2011 through 2016.
Obesity tied to improved inpatient survival of patients with PAD
The obesity paradox appears alive and well in the treatment of peripheral arterial disease (PAD), according to the results of a 10-year, 5.6-million patient database study.
The researchers found that coding for obesity is associated with lower in-hospital mortality in PAD patients relative to those who were normal weight or overweight. This obesity survival paradox was independent of age, sex, and comorbidities and was seen in all obesity classes, according to Karsten Keller, MD, of the University Medical Center Mainz (Germany), and his colleagues.
In total, 5,611,827 inpatients aged 18 years or older with PAD were treated between 2005 and 2015 in Germany, 5,611,484 of whom (64.8% men) were eligible for analysis. Among these, 500,027 (8.9%) were coded with obesity and 16,620 (0.3%) were coded as underweight; 5,094,837 (90.8%) were in neither classification (considered healthy/overweight) and served as the reference group for comparison, according to Dr. Keller and his colleagues.
Obese PAD patients were younger, more frequently women, and had less cancer but were diagnosed more often with cardiovascular disease risk factors such as diabetes and hypertension, compared with the reference group. In addition, there were higher levels of coronary artery disease, heart failure, renal insufficiency, and chronic obstructive pulmonary disease (COPD) in obese patients.
Obese patients had lower mortality (3.2% vs. 5.1%; P less than .001), compared with the reference group, and showed a reduced risk of in-hospital mortality (odds ratio, 0.617; P less than .001). Univariate logistic regression analyses showed the association of obesity and reduced in-hospital mortality was consistent and significant, even with adjustment for age, sex, and comorbidities.
In contrast, underweight patients were significantly more likely to die than those in the reference group (6% vs. 5.1%; P less than .001), according to the researchers. Underweight was associated with an increased risk for in-hospital mortality (OR, 1.18; P less than .001), and this was consistent throughout univariate analysis.
Underweight PAD patients also had significantly higher frequencies of cancer and COPD, but lower rates of diabetes mellitus, hypertension, coronary artery disease, and heart failure, compared with the reference group. Both obese and underweight PAD patients stayed longer in the hospital than the PAD patients who were not coded as underweight or obese.
Obese PAD patients had slight but significantly higher rates of MI (3.9% vs. 3.4%; P less than .001) and venous thromboembolic events, and more often had to undergo amputation surgery (8.3% vs. 8.1%; P less than .001), including a higher relative number of minor amputations (6.3% vs. 5.5%; P less than .001). However, major amputation rates were significantly lower in obese patients (2.6% vs. 3.2%; P less than .001), with univariate analysis showing a significant association between obesity and a lower risk of major amputation (OR, 0.82; P less than .001), which remained stable after multivariate adjustment.
Limitations of the study reported by the researchers included a lower than expected percent obesity in the 10-year database, compared with current rates, and the inability to follow tobacco use or to determine the socioeconomic status of the patients.
“Obesity is associated with lower in-hospital mortality in PAD patients relative to those with normal weight/overweight. ... Therefore, greater concern should be directed to the thinner patients with PAD who are particularly at increased risk of mortality,” the researchers concluded.
This study was supported by the German Federal Ministry of Education and Research; the authors reported that they had no disclosures.
SOURCE: Keller K et al. Clin Nutr. 2018 Oct 3. doi: 10.1016/j.clnu.2018.09.031.
The obesity paradox appears alive and well in the treatment of peripheral arterial disease (PAD), according to the results of a 10-year, 5.6-million patient database study.
The researchers found that coding for obesity is associated with lower in-hospital mortality in PAD patients relative to those who were normal weight or overweight. This obesity survival paradox was independent of age, sex, and comorbidities and was seen in all obesity classes, according to Karsten Keller, MD, of the University Medical Center Mainz (Germany), and his colleagues.
In total, 5,611,827 inpatients aged 18 years or older with PAD were treated between 2005 and 2015 in Germany, 5,611,484 of whom (64.8% men) were eligible for analysis. Among these, 500,027 (8.9%) were coded with obesity and 16,620 (0.3%) were coded as underweight; 5,094,837 (90.8%) were in neither classification (considered healthy/overweight) and served as the reference group for comparison, according to Dr. Keller and his colleagues.
Obese PAD patients were younger, more frequently women, and had less cancer but were diagnosed more often with cardiovascular disease risk factors such as diabetes and hypertension, compared with the reference group. In addition, there were higher levels of coronary artery disease, heart failure, renal insufficiency, and chronic obstructive pulmonary disease (COPD) in obese patients.
Obese patients had lower mortality (3.2% vs. 5.1%; P less than .001), compared with the reference group, and showed a reduced risk of in-hospital mortality (odds ratio, 0.617; P less than .001). Univariate logistic regression analyses showed the association of obesity and reduced in-hospital mortality was consistent and significant, even with adjustment for age, sex, and comorbidities.
In contrast, underweight patients were significantly more likely to die than those in the reference group (6% vs. 5.1%; P less than .001), according to the researchers. Underweight was associated with an increased risk for in-hospital mortality (OR, 1.18; P less than .001), and this was consistent throughout univariate analysis.
Underweight PAD patients also had significantly higher frequencies of cancer and COPD, but lower rates of diabetes mellitus, hypertension, coronary artery disease, and heart failure, compared with the reference group. Both obese and underweight PAD patients stayed longer in the hospital than the PAD patients who were not coded as underweight or obese.
Obese PAD patients had slight but significantly higher rates of MI (3.9% vs. 3.4%; P less than .001) and venous thromboembolic events, and more often had to undergo amputation surgery (8.3% vs. 8.1%; P less than .001), including a higher relative number of minor amputations (6.3% vs. 5.5%; P less than .001). However, major amputation rates were significantly lower in obese patients (2.6% vs. 3.2%; P less than .001), with univariate analysis showing a significant association between obesity and a lower risk of major amputation (OR, 0.82; P less than .001), which remained stable after multivariate adjustment.
Limitations of the study reported by the researchers included a lower than expected percent obesity in the 10-year database, compared with current rates, and the inability to follow tobacco use or to determine the socioeconomic status of the patients.
“Obesity is associated with lower in-hospital mortality in PAD patients relative to those with normal weight/overweight. ... Therefore, greater concern should be directed to the thinner patients with PAD who are particularly at increased risk of mortality,” the researchers concluded.
This study was supported by the German Federal Ministry of Education and Research; the authors reported that they had no disclosures.
SOURCE: Keller K et al. Clin Nutr. 2018 Oct 3. doi: 10.1016/j.clnu.2018.09.031.
The obesity paradox appears alive and well in the treatment of peripheral arterial disease (PAD), according to the results of a 10-year, 5.6-million patient database study.
The researchers found that coding for obesity is associated with lower in-hospital mortality in PAD patients relative to those who were normal weight or overweight. This obesity survival paradox was independent of age, sex, and comorbidities and was seen in all obesity classes, according to Karsten Keller, MD, of the University Medical Center Mainz (Germany), and his colleagues.
In total, 5,611,827 inpatients aged 18 years or older with PAD were treated between 2005 and 2015 in Germany, 5,611,484 of whom (64.8% men) were eligible for analysis. Among these, 500,027 (8.9%) were coded with obesity and 16,620 (0.3%) were coded as underweight; 5,094,837 (90.8%) were in neither classification (considered healthy/overweight) and served as the reference group for comparison, according to Dr. Keller and his colleagues.
Obese PAD patients were younger, more frequently women, and had less cancer but were diagnosed more often with cardiovascular disease risk factors such as diabetes and hypertension, compared with the reference group. In addition, there were higher levels of coronary artery disease, heart failure, renal insufficiency, and chronic obstructive pulmonary disease (COPD) in obese patients.
Obese patients had lower mortality (3.2% vs. 5.1%; P less than .001), compared with the reference group, and showed a reduced risk of in-hospital mortality (odds ratio, 0.617; P less than .001). Univariate logistic regression analyses showed the association of obesity and reduced in-hospital mortality was consistent and significant, even with adjustment for age, sex, and comorbidities.
In contrast, underweight patients were significantly more likely to die than those in the reference group (6% vs. 5.1%; P less than .001), according to the researchers. Underweight was associated with an increased risk for in-hospital mortality (OR, 1.18; P less than .001), and this was consistent throughout univariate analysis.
Underweight PAD patients also had significantly higher frequencies of cancer and COPD, but lower rates of diabetes mellitus, hypertension, coronary artery disease, and heart failure, compared with the reference group. Both obese and underweight PAD patients stayed longer in the hospital than the PAD patients who were not coded as underweight or obese.
Obese PAD patients had slight but significantly higher rates of MI (3.9% vs. 3.4%; P less than .001) and venous thromboembolic events, and more often had to undergo amputation surgery (8.3% vs. 8.1%; P less than .001), including a higher relative number of minor amputations (6.3% vs. 5.5%; P less than .001). However, major amputation rates were significantly lower in obese patients (2.6% vs. 3.2%; P less than .001), with univariate analysis showing a significant association between obesity and a lower risk of major amputation (OR, 0.82; P less than .001), which remained stable after multivariate adjustment.
Limitations of the study reported by the researchers included a lower than expected percent obesity in the 10-year database, compared with current rates, and the inability to follow tobacco use or to determine the socioeconomic status of the patients.
“Obesity is associated with lower in-hospital mortality in PAD patients relative to those with normal weight/overweight. ... Therefore, greater concern should be directed to the thinner patients with PAD who are particularly at increased risk of mortality,” the researchers concluded.
This study was supported by the German Federal Ministry of Education and Research; the authors reported that they had no disclosures.
SOURCE: Keller K et al. Clin Nutr. 2018 Oct 3. doi: 10.1016/j.clnu.2018.09.031.
FROM CLINICAL NUTRITION
Key clinical point: Obesity is associated with lower in-hospital mortality in patients with peripheral arterial disease relative to those who were healthy weight or overweight.
Major finding: Obese patients had a lower mortality (3.2% vs. 5.1%; P less than .001), compared with the reference group.
Study details: A database study of 5,611,484 inpatients diagnosed with peripheral arterial disease.
Disclosures: This study was supported by the German Federal Ministry of Education and Research; the authors reported that they had no disclosures.
Source: Keller K et al. Clin Nutr. 2018 Oct 3. doi: 10.1016/j.clnu.2018.09.031.