User login
Bariatric surgery tied to lower aortic dissection risk
The finding is the latest in a series of benefits researchers have linked to the surgery, not all of which appear to directly result from weight loss.
“It has an incredible impact on hyperlipidemia and hypertension,” said Luis Felipe Okida, MD, from Cleveland Clinic Florida, Weston. “Those are the main risk factors for aortic dissection.”
He presented the finding at the virtual American Congress of Surgeons Clinical Congress 2020. The study was also published online in the Journal of the American College of Surgeons.
Although uncommon, acute aortic dissection proves fatal to half the people it strikes if patients do not receive treatment within 72 hours, Dr. Okida said in an interview.
To learn whether there is an association between bariatric surgery and risk for aortic dissection, Dr. Okida and colleagues analyzed data from the National Inpatient Sample (NIS) database from 2010 to 2015. The NIS comprises about 20% of hospital inpatient admissions in the United States.
Among the patients in the sample, 296,041 adults had undergone bariatric surgery, and 2,004,804 adults had obesity (body mass index ≥35 kg/m2) but had never undergone bariatric surgery. This latter group represented the control group.
Among the control group, 1,411 patients (.070%) experienced aortic dissection; among the bariatric surgery group, 94 patients (0.032%) experienced aortic dissection. This was a statistically significant difference (P < .0001).
The groups differed significantly in many ways. The mean age of the patients in the control group was 54.4 years, which was a mean of 2.5 years older than the bariatric surgery group. Additionally, the control group included a higher percentage of women and a lower percentage of White persons.
Those in the control group were also more likely to have a history of tobacco use, hypertension (64.2% vs. 48.9% in the surgery group), hyperlipidemia (32.7% vs. 18.3%), diabetes, aortic aneurysm (20.6% vs. 12.0%), and bicuspid aortic valves but were less likely to have Marfan/Ehlers-Danlos syndrome.
A multivariate analysis showed that gender, age, history of tobacco use, hypertension, hyperlipidemia, and Marfan/Ehlers-Danlos syndrome were associated with an increased risk for aortic dissection. Diabetes was associated with a lower risk. All of these findings had previously been reported in the literature, Dr. Okida said, but the reasons for the negative association with diabetes is not well understood.
The association between the surgery and aortic dissection applied to younger patients as well as older ones.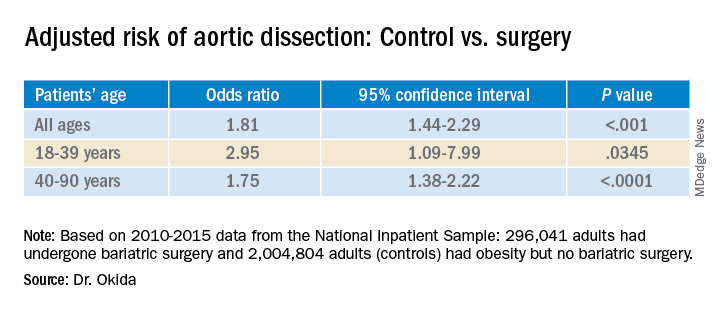
“In elderly patients, the main risk factor for aortic dissection is hypertension, and in younger patients, below 40 years old, the main risk factors are diseases of the collagen and diseases of the aorta,” said Dr. Okida during his presentation. “But these younger patients still have a high prevalence of hypertension, and that’s why bariatric surgery is beneficial.”
Although the finding regarding risk for aortic dissection supports the value of bariatric surgery, it does not in itself provide justification for undergoing the procedure. “It’s not even one of the comorbidities that insurance companies would recognize as key in approving this procedure,” said senior author Emanuele Lo Menzo, MD, PhD, also from the Cleveland Clinic Florida.
“I don’t think a physician would ever recommend this procedure specifically to avoid aortic dissection,” he said in an interview. “It’s sort of an extended benefit.”
The study raises interesting questions about the effects of the surgery, said Shanu Kothari, MD, president-elect of the American Society for Metabolic and Bariatric Surgery.
“We’ve known for a long time that patients with chronic obesity who undergo weight-loss surgery live longer than those who don’t,” he said in an interview. “They have less cardiovascular disease and cancer. Is this one more reason that they live longer?”
Bariatric surgery produces benefits for people with diabetes the day after the surgery, long before patients lose weight as a result of the procedure, Dr. Kothari said.
The effects on metabolism are complex, he added. Besides caloric restriction, they include changes in bile salt absorption and the gut microbiome, which in turn can affect hormones and inflammation.
A key question is how long after the surgery the risk for aortic dissection starts to decline, said Dr. Kothari.
The study could not answer such questions, and Dr. Okida could not find any previous studies that explored the association. He also couldn’t find any study that examined whether weight loss by other means might also reduce the risk for aortic dissection.
Dr. Okida, Dr. Lo Menzo, and Dr. Kothari disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The finding is the latest in a series of benefits researchers have linked to the surgery, not all of which appear to directly result from weight loss.
“It has an incredible impact on hyperlipidemia and hypertension,” said Luis Felipe Okida, MD, from Cleveland Clinic Florida, Weston. “Those are the main risk factors for aortic dissection.”
He presented the finding at the virtual American Congress of Surgeons Clinical Congress 2020. The study was also published online in the Journal of the American College of Surgeons.
Although uncommon, acute aortic dissection proves fatal to half the people it strikes if patients do not receive treatment within 72 hours, Dr. Okida said in an interview.
To learn whether there is an association between bariatric surgery and risk for aortic dissection, Dr. Okida and colleagues analyzed data from the National Inpatient Sample (NIS) database from 2010 to 2015. The NIS comprises about 20% of hospital inpatient admissions in the United States.
Among the patients in the sample, 296,041 adults had undergone bariatric surgery, and 2,004,804 adults had obesity (body mass index ≥35 kg/m2) but had never undergone bariatric surgery. This latter group represented the control group.
Among the control group, 1,411 patients (.070%) experienced aortic dissection; among the bariatric surgery group, 94 patients (0.032%) experienced aortic dissection. This was a statistically significant difference (P < .0001).
The groups differed significantly in many ways. The mean age of the patients in the control group was 54.4 years, which was a mean of 2.5 years older than the bariatric surgery group. Additionally, the control group included a higher percentage of women and a lower percentage of White persons.
Those in the control group were also more likely to have a history of tobacco use, hypertension (64.2% vs. 48.9% in the surgery group), hyperlipidemia (32.7% vs. 18.3%), diabetes, aortic aneurysm (20.6% vs. 12.0%), and bicuspid aortic valves but were less likely to have Marfan/Ehlers-Danlos syndrome.
A multivariate analysis showed that gender, age, history of tobacco use, hypertension, hyperlipidemia, and Marfan/Ehlers-Danlos syndrome were associated with an increased risk for aortic dissection. Diabetes was associated with a lower risk. All of these findings had previously been reported in the literature, Dr. Okida said, but the reasons for the negative association with diabetes is not well understood.
The association between the surgery and aortic dissection applied to younger patients as well as older ones.
“In elderly patients, the main risk factor for aortic dissection is hypertension, and in younger patients, below 40 years old, the main risk factors are diseases of the collagen and diseases of the aorta,” said Dr. Okida during his presentation. “But these younger patients still have a high prevalence of hypertension, and that’s why bariatric surgery is beneficial.”
Although the finding regarding risk for aortic dissection supports the value of bariatric surgery, it does not in itself provide justification for undergoing the procedure. “It’s not even one of the comorbidities that insurance companies would recognize as key in approving this procedure,” said senior author Emanuele Lo Menzo, MD, PhD, also from the Cleveland Clinic Florida.
“I don’t think a physician would ever recommend this procedure specifically to avoid aortic dissection,” he said in an interview. “It’s sort of an extended benefit.”
The study raises interesting questions about the effects of the surgery, said Shanu Kothari, MD, president-elect of the American Society for Metabolic and Bariatric Surgery.
“We’ve known for a long time that patients with chronic obesity who undergo weight-loss surgery live longer than those who don’t,” he said in an interview. “They have less cardiovascular disease and cancer. Is this one more reason that they live longer?”
Bariatric surgery produces benefits for people with diabetes the day after the surgery, long before patients lose weight as a result of the procedure, Dr. Kothari said.
The effects on metabolism are complex, he added. Besides caloric restriction, they include changes in bile salt absorption and the gut microbiome, which in turn can affect hormones and inflammation.
A key question is how long after the surgery the risk for aortic dissection starts to decline, said Dr. Kothari.
The study could not answer such questions, and Dr. Okida could not find any previous studies that explored the association. He also couldn’t find any study that examined whether weight loss by other means might also reduce the risk for aortic dissection.
Dr. Okida, Dr. Lo Menzo, and Dr. Kothari disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The finding is the latest in a series of benefits researchers have linked to the surgery, not all of which appear to directly result from weight loss.
“It has an incredible impact on hyperlipidemia and hypertension,” said Luis Felipe Okida, MD, from Cleveland Clinic Florida, Weston. “Those are the main risk factors for aortic dissection.”
He presented the finding at the virtual American Congress of Surgeons Clinical Congress 2020. The study was also published online in the Journal of the American College of Surgeons.
Although uncommon, acute aortic dissection proves fatal to half the people it strikes if patients do not receive treatment within 72 hours, Dr. Okida said in an interview.
To learn whether there is an association between bariatric surgery and risk for aortic dissection, Dr. Okida and colleagues analyzed data from the National Inpatient Sample (NIS) database from 2010 to 2015. The NIS comprises about 20% of hospital inpatient admissions in the United States.
Among the patients in the sample, 296,041 adults had undergone bariatric surgery, and 2,004,804 adults had obesity (body mass index ≥35 kg/m2) but had never undergone bariatric surgery. This latter group represented the control group.
Among the control group, 1,411 patients (.070%) experienced aortic dissection; among the bariatric surgery group, 94 patients (0.032%) experienced aortic dissection. This was a statistically significant difference (P < .0001).
The groups differed significantly in many ways. The mean age of the patients in the control group was 54.4 years, which was a mean of 2.5 years older than the bariatric surgery group. Additionally, the control group included a higher percentage of women and a lower percentage of White persons.
Those in the control group were also more likely to have a history of tobacco use, hypertension (64.2% vs. 48.9% in the surgery group), hyperlipidemia (32.7% vs. 18.3%), diabetes, aortic aneurysm (20.6% vs. 12.0%), and bicuspid aortic valves but were less likely to have Marfan/Ehlers-Danlos syndrome.
A multivariate analysis showed that gender, age, history of tobacco use, hypertension, hyperlipidemia, and Marfan/Ehlers-Danlos syndrome were associated with an increased risk for aortic dissection. Diabetes was associated with a lower risk. All of these findings had previously been reported in the literature, Dr. Okida said, but the reasons for the negative association with diabetes is not well understood.
The association between the surgery and aortic dissection applied to younger patients as well as older ones.
“In elderly patients, the main risk factor for aortic dissection is hypertension, and in younger patients, below 40 years old, the main risk factors are diseases of the collagen and diseases of the aorta,” said Dr. Okida during his presentation. “But these younger patients still have a high prevalence of hypertension, and that’s why bariatric surgery is beneficial.”
Although the finding regarding risk for aortic dissection supports the value of bariatric surgery, it does not in itself provide justification for undergoing the procedure. “It’s not even one of the comorbidities that insurance companies would recognize as key in approving this procedure,” said senior author Emanuele Lo Menzo, MD, PhD, also from the Cleveland Clinic Florida.
“I don’t think a physician would ever recommend this procedure specifically to avoid aortic dissection,” he said in an interview. “It’s sort of an extended benefit.”
The study raises interesting questions about the effects of the surgery, said Shanu Kothari, MD, president-elect of the American Society for Metabolic and Bariatric Surgery.
“We’ve known for a long time that patients with chronic obesity who undergo weight-loss surgery live longer than those who don’t,” he said in an interview. “They have less cardiovascular disease and cancer. Is this one more reason that they live longer?”
Bariatric surgery produces benefits for people with diabetes the day after the surgery, long before patients lose weight as a result of the procedure, Dr. Kothari said.
The effects on metabolism are complex, he added. Besides caloric restriction, they include changes in bile salt absorption and the gut microbiome, which in turn can affect hormones and inflammation.
A key question is how long after the surgery the risk for aortic dissection starts to decline, said Dr. Kothari.
The study could not answer such questions, and Dr. Okida could not find any previous studies that explored the association. He also couldn’t find any study that examined whether weight loss by other means might also reduce the risk for aortic dissection.
Dr. Okida, Dr. Lo Menzo, and Dr. Kothari disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Creeping fat in Crohn’s linked with microbial translocation
Creeping fat in Crohn’s disease is likely caused by microbial translocation from the gut to neighboring mesenteric adipose tissue (MAT), based on a recent study.
This finding may lead to early risk stratification for creeping fat, and nonsurgical interventions, according to principal author Suzanne Devkota, PhD, assistant professor at Cedars-Sinai Medical Center in Los Angeles.
Creeping fat, which is unique to Crohn’s disease, is characterized by hyperplastic MAT that grips areas of intestinal inflammation with invasive “fingerlike projections,” the investigators wrote in Cell. This phenomenon was first described by the eponymous Dr. Crohn in 1932; since then, despite associations with fibrotic strictures that may require surgical resection, underlying mechanisms have remained mysterious and largely unexplored.
That changed during a session of grand rounds at Cedars-Sinai in September 2016; Dr. Devkota was discussing adipose tissue when her surgeon colleague, Phillip Fleshner, MD, asked: “What about creeping fat?”
“Yeah, that’s cool,” Dr. Devkota replied, “but I don’t have access to creeping fat.”
“I see it all the time,” Dr. Fleshner said. “I can get you some.”
And so a partnership was born, allowing Dr. Devkota and colleagues to pursue the translocation hypothesis.
The present report involved tissue samples from 11 patients with Crohn’s disease and 13 patients with ulcerative colitis. Healthy tissue controls were taken from four subjects without inflammatory bowel disease who underwent ileostomy.
Microbial cultivation of Crohn’s disease and healthy patient samples revealed bacteria in the mesenteric tissue of both groups, suggesting that microbial translocation from the gut to MAT “may not be unusual;” however, Crohn’s disease samples were associated with an exclusive consortium of five species: Clostridium innocuum, Erysipeloclostridium ramosum, Parabacteroides distasonis, Clostridium symbiosum, and Bifidobacterium pseudolongum.
C. innocuum was isolated most frequently; and its unique characteristics increased suspicions that it was the creeping fat culprit.
“Core genomic features of C. innocuum include type IV pili and twitching motility, a preference for lipid-derived metabolic substrates, and multiple genes for lipid catabolism, as well as a functional substrate preference for b-hydroxybutyrate, a byproduct of fatty acid oxidation,” the investigators wrote. “This suggests that C. innocuum is well suited for, and perhaps prefers, a lipid-rich environment and seeks these out when the opportunity arises.”
To observe this opportunism firsthand, the investigators gavaged gnotobiotic mice with C. innocuum. Indeed, these mice demonstrated “dramatic mesenteric adiposity,” compared with controls.
Cotreatment with dextran sulfate sodium (DSS) was unnecessary to induce translocation of C. innocuum, which “suggests that overt inflammation is not a prerequisite for its translocation,” the investigators noted.
The profibrotic potential of C. innocuum was supported by in vitro experiments, in which adipose-derived stem cells and primary fibroblasts from Crohn’s disease MAT were exposed to either C. innocuum lysate or macrophage-conditioned media from C. innocuum–exposed macrophages. While the lysate alone did not alter genes involved in fibrosis and remodeling, the macrophage-conditioned media did, indicating that C. innocuum alters MAT indirectly via macrophage activity.
Although multiple signs suggest that C. innocuum causes creeping fat, Dr. Devkota noted that systematic testing is needed to confirm this likelihood.
“But I do think we’ve honed in on the consortium that is at play,” she said, referring to the five identified species.
According to Dr. Devkota, awareness of these microbes could lead to diagnostic and interventional benefits for patients with Crohn’s disease. For example, gut microbiota profiling could be used to measure levels of C. innocuum in newly diagnosed patients, thereby stratifying risk of creeping fat. And phage therapy, with its high specificity for bacterial species, could be an ideal intervention.
“I’m very eager to hear from the surgeons, and hear what their opinion is, and whether this will affect their treatment or how they approach [creeping fat],” Dr. Devkota said.
Beyond Crohn’s disease, the study findings could inform obesity research, as bacterial DNA has been found in obese adipose tissue, which is characteristically fibrotic.
“There are a lot of gene-expression patterns [in the present study], that are also seen in obesity literature,” Dr. Devkota said.
“Obviously there’s a lifestyle caloric aspect to [obesity],” she added. “I definitely don’t claim that microbes are the end-all and be-all of obesity – I want to make that clear. But it could be possible, and particularly related to abdominal fat. Expanded abdominal fat could be a sign that there’s underlying intestinal inflammation ... that there’s something deeper going on that may be unrelated to a metabolic defect.”
The study was funded by Leona M. and Harry B. Helmsley Charitable Trust and the National Institutes of Health. Dr. Devkota and Dr. Ha are inventors on U.S. patent application #62/679,624.
SOURCE: Ha CWY et al. Cell. 2020 Oct 29. doi: 10.1016/j.cell.2020.09.009.
Creeping fat in Crohn’s disease is likely caused by microbial translocation from the gut to neighboring mesenteric adipose tissue (MAT), based on a recent study.
This finding may lead to early risk stratification for creeping fat, and nonsurgical interventions, according to principal author Suzanne Devkota, PhD, assistant professor at Cedars-Sinai Medical Center in Los Angeles.
Creeping fat, which is unique to Crohn’s disease, is characterized by hyperplastic MAT that grips areas of intestinal inflammation with invasive “fingerlike projections,” the investigators wrote in Cell. This phenomenon was first described by the eponymous Dr. Crohn in 1932; since then, despite associations with fibrotic strictures that may require surgical resection, underlying mechanisms have remained mysterious and largely unexplored.
That changed during a session of grand rounds at Cedars-Sinai in September 2016; Dr. Devkota was discussing adipose tissue when her surgeon colleague, Phillip Fleshner, MD, asked: “What about creeping fat?”
“Yeah, that’s cool,” Dr. Devkota replied, “but I don’t have access to creeping fat.”
“I see it all the time,” Dr. Fleshner said. “I can get you some.”
And so a partnership was born, allowing Dr. Devkota and colleagues to pursue the translocation hypothesis.
The present report involved tissue samples from 11 patients with Crohn’s disease and 13 patients with ulcerative colitis. Healthy tissue controls were taken from four subjects without inflammatory bowel disease who underwent ileostomy.
Microbial cultivation of Crohn’s disease and healthy patient samples revealed bacteria in the mesenteric tissue of both groups, suggesting that microbial translocation from the gut to MAT “may not be unusual;” however, Crohn’s disease samples were associated with an exclusive consortium of five species: Clostridium innocuum, Erysipeloclostridium ramosum, Parabacteroides distasonis, Clostridium symbiosum, and Bifidobacterium pseudolongum.
C. innocuum was isolated most frequently; and its unique characteristics increased suspicions that it was the creeping fat culprit.
“Core genomic features of C. innocuum include type IV pili and twitching motility, a preference for lipid-derived metabolic substrates, and multiple genes for lipid catabolism, as well as a functional substrate preference for b-hydroxybutyrate, a byproduct of fatty acid oxidation,” the investigators wrote. “This suggests that C. innocuum is well suited for, and perhaps prefers, a lipid-rich environment and seeks these out when the opportunity arises.”
To observe this opportunism firsthand, the investigators gavaged gnotobiotic mice with C. innocuum. Indeed, these mice demonstrated “dramatic mesenteric adiposity,” compared with controls.
Cotreatment with dextran sulfate sodium (DSS) was unnecessary to induce translocation of C. innocuum, which “suggests that overt inflammation is not a prerequisite for its translocation,” the investigators noted.
The profibrotic potential of C. innocuum was supported by in vitro experiments, in which adipose-derived stem cells and primary fibroblasts from Crohn’s disease MAT were exposed to either C. innocuum lysate or macrophage-conditioned media from C. innocuum–exposed macrophages. While the lysate alone did not alter genes involved in fibrosis and remodeling, the macrophage-conditioned media did, indicating that C. innocuum alters MAT indirectly via macrophage activity.
Although multiple signs suggest that C. innocuum causes creeping fat, Dr. Devkota noted that systematic testing is needed to confirm this likelihood.
“But I do think we’ve honed in on the consortium that is at play,” she said, referring to the five identified species.
According to Dr. Devkota, awareness of these microbes could lead to diagnostic and interventional benefits for patients with Crohn’s disease. For example, gut microbiota profiling could be used to measure levels of C. innocuum in newly diagnosed patients, thereby stratifying risk of creeping fat. And phage therapy, with its high specificity for bacterial species, could be an ideal intervention.
“I’m very eager to hear from the surgeons, and hear what their opinion is, and whether this will affect their treatment or how they approach [creeping fat],” Dr. Devkota said.
Beyond Crohn’s disease, the study findings could inform obesity research, as bacterial DNA has been found in obese adipose tissue, which is characteristically fibrotic.
“There are a lot of gene-expression patterns [in the present study], that are also seen in obesity literature,” Dr. Devkota said.
“Obviously there’s a lifestyle caloric aspect to [obesity],” she added. “I definitely don’t claim that microbes are the end-all and be-all of obesity – I want to make that clear. But it could be possible, and particularly related to abdominal fat. Expanded abdominal fat could be a sign that there’s underlying intestinal inflammation ... that there’s something deeper going on that may be unrelated to a metabolic defect.”
The study was funded by Leona M. and Harry B. Helmsley Charitable Trust and the National Institutes of Health. Dr. Devkota and Dr. Ha are inventors on U.S. patent application #62/679,624.
SOURCE: Ha CWY et al. Cell. 2020 Oct 29. doi: 10.1016/j.cell.2020.09.009.
Creeping fat in Crohn’s disease is likely caused by microbial translocation from the gut to neighboring mesenteric adipose tissue (MAT), based on a recent study.
This finding may lead to early risk stratification for creeping fat, and nonsurgical interventions, according to principal author Suzanne Devkota, PhD, assistant professor at Cedars-Sinai Medical Center in Los Angeles.
Creeping fat, which is unique to Crohn’s disease, is characterized by hyperplastic MAT that grips areas of intestinal inflammation with invasive “fingerlike projections,” the investigators wrote in Cell. This phenomenon was first described by the eponymous Dr. Crohn in 1932; since then, despite associations with fibrotic strictures that may require surgical resection, underlying mechanisms have remained mysterious and largely unexplored.
That changed during a session of grand rounds at Cedars-Sinai in September 2016; Dr. Devkota was discussing adipose tissue when her surgeon colleague, Phillip Fleshner, MD, asked: “What about creeping fat?”
“Yeah, that’s cool,” Dr. Devkota replied, “but I don’t have access to creeping fat.”
“I see it all the time,” Dr. Fleshner said. “I can get you some.”
And so a partnership was born, allowing Dr. Devkota and colleagues to pursue the translocation hypothesis.
The present report involved tissue samples from 11 patients with Crohn’s disease and 13 patients with ulcerative colitis. Healthy tissue controls were taken from four subjects without inflammatory bowel disease who underwent ileostomy.
Microbial cultivation of Crohn’s disease and healthy patient samples revealed bacteria in the mesenteric tissue of both groups, suggesting that microbial translocation from the gut to MAT “may not be unusual;” however, Crohn’s disease samples were associated with an exclusive consortium of five species: Clostridium innocuum, Erysipeloclostridium ramosum, Parabacteroides distasonis, Clostridium symbiosum, and Bifidobacterium pseudolongum.
C. innocuum was isolated most frequently; and its unique characteristics increased suspicions that it was the creeping fat culprit.
“Core genomic features of C. innocuum include type IV pili and twitching motility, a preference for lipid-derived metabolic substrates, and multiple genes for lipid catabolism, as well as a functional substrate preference for b-hydroxybutyrate, a byproduct of fatty acid oxidation,” the investigators wrote. “This suggests that C. innocuum is well suited for, and perhaps prefers, a lipid-rich environment and seeks these out when the opportunity arises.”
To observe this opportunism firsthand, the investigators gavaged gnotobiotic mice with C. innocuum. Indeed, these mice demonstrated “dramatic mesenteric adiposity,” compared with controls.
Cotreatment with dextran sulfate sodium (DSS) was unnecessary to induce translocation of C. innocuum, which “suggests that overt inflammation is not a prerequisite for its translocation,” the investigators noted.
The profibrotic potential of C. innocuum was supported by in vitro experiments, in which adipose-derived stem cells and primary fibroblasts from Crohn’s disease MAT were exposed to either C. innocuum lysate or macrophage-conditioned media from C. innocuum–exposed macrophages. While the lysate alone did not alter genes involved in fibrosis and remodeling, the macrophage-conditioned media did, indicating that C. innocuum alters MAT indirectly via macrophage activity.
Although multiple signs suggest that C. innocuum causes creeping fat, Dr. Devkota noted that systematic testing is needed to confirm this likelihood.
“But I do think we’ve honed in on the consortium that is at play,” she said, referring to the five identified species.
According to Dr. Devkota, awareness of these microbes could lead to diagnostic and interventional benefits for patients with Crohn’s disease. For example, gut microbiota profiling could be used to measure levels of C. innocuum in newly diagnosed patients, thereby stratifying risk of creeping fat. And phage therapy, with its high specificity for bacterial species, could be an ideal intervention.
“I’m very eager to hear from the surgeons, and hear what their opinion is, and whether this will affect their treatment or how they approach [creeping fat],” Dr. Devkota said.
Beyond Crohn’s disease, the study findings could inform obesity research, as bacterial DNA has been found in obese adipose tissue, which is characteristically fibrotic.
“There are a lot of gene-expression patterns [in the present study], that are also seen in obesity literature,” Dr. Devkota said.
“Obviously there’s a lifestyle caloric aspect to [obesity],” she added. “I definitely don’t claim that microbes are the end-all and be-all of obesity – I want to make that clear. But it could be possible, and particularly related to abdominal fat. Expanded abdominal fat could be a sign that there’s underlying intestinal inflammation ... that there’s something deeper going on that may be unrelated to a metabolic defect.”
The study was funded by Leona M. and Harry B. Helmsley Charitable Trust and the National Institutes of Health. Dr. Devkota and Dr. Ha are inventors on U.S. patent application #62/679,624.
SOURCE: Ha CWY et al. Cell. 2020 Oct 29. doi: 10.1016/j.cell.2020.09.009.
FROM CELL
Psychosocial resilience associated with better cardiovascular health in Blacks
Resilience might deserve targeting
Increased psychosocial resilience, which captures a sense of purpose, optimism, and life-coping strategies, correlates with improved cardiovascular (CV) health in Black Americans, according to a study that might hold a key for identifying new strategies for CV disease prevention.
“Our findings highlight the importance of individual psychosocial factors that promote cardiovascular health among Black adults, traditionally considered to be a high-risk population,” according to a team of authors collaborating on a study produced by the Morehouse-Emory Cardiovascular Center for Health Equity in Atlanta.
Studies associating psychosocial resilience with improved health outcomes have been published before. In a 12-study review of this concept, it was emphasized that resilience is a dynamic process, not a personality trait, and has shown promise as a target of efforts to relieve the burden of disease (Johnston MC et al. Psychosomatics 2015;56:168-80).
In this study, which received partial support from the American Heart Association, psychosocial resilience was evaluated at both the individual level and at the community level among 389 Black adults living in Atlanta. The senior author was Tené T. Lewis, PhD, of the department of epidemiology at Emory’s Rollins School of Public Health (Circ Cardiovasc Qual Outcomes 2020 Oct 7;13:3006638).
Psychosocial resilience was calculated across the domains of environmental mastery, purpose of life, optimism, coping, and lack of depression with standardized tests, such as the Life Orientation Test-Revised questionnaire for optimism and the Ryff Scales of Psychological Well-Being for the domains of environmental mastery and purpose of life. A composite score for psychosocial resilience was reached by calculating the median score across the measured domains.
Patients with high psychosocial resilience, defined as a composite score above the median, or low resilience, defined as a lower score, were then compared for CV health based on the AHA’s Life’s Simple 7 (LS7) score.
LS7 scores incorporate measures for exercise, diet, smoking history, blood pressure, glucose, cholesterol, and body mass index. Composite LS7 scores range from 0 to 14. Prior work cited by the authors have associated each 1-unit increase in LS7 score with a 13% lower risk of CVD.
As a continuous variable for CV risk at the individual level, each higher standard-deviation increment in the composite psychosocial resilience score was associated with a highly significant 0.42-point increase in LS7 score (P < .001) for study participants. In other words, increasing resilience predicted lower CV risk scores.
Resilience was also calculated at the community level by looking at census tract-level rates of CV mortality and morbidity relative to socioeconomic status. Again, high CV resilience, defined as scores above the median, were compared with lower scores across neighborhoods with similar median household income. As a continuous variable in this analysis, each higher standard-deviation increment in the resilience score was associated with a 0.27-point increase in LS7 score (P = .01).
After adjustment for sociodemographic factors, the association between psychosocial resilience and CV health remained significant for both the individual and community calculations, according to the authors. When examined jointly, high individual psychosocial resilience remained independently associated with improved CV health, but living in a high-resilience neighborhood was not an independent predictor.
When evaluated individually, each of the domains in the psychosocial resistance score were positively correlated with higher LS7 scores, meaning lower CV risk. The strongest associations on a statistical level were low depressive symptoms (P = .001), environmental mastery (P = .006), and purpose in life (P = .009).
The impact of high psychosocial resistance scores was greatest in Black adults living in low-resilience neighborhoods. Among these subjects, high resilience was associated with a nearly 1-point increase in LS7 score relative to low resilience (8.38 vs. 7.42). This was unexpected, but it “is consistent with some broader conceptual literature that posits that individual psychosocial resilience matters more under conditions of adversity,” the authors reported.
Understanding disparities is key
Black race has repeatedly been associated with an increased risk of CV events, but this study is valuable for providing a fresh perspective on the potential reasons, according to the authors of an accompanying editorial, Amber E. Johnson, MD, and Jared Magnani, MD, who are both affiliated with the division of cardiology at the University of Pittsburgh (Circ Cardiovasc Qual Outcomes 2020 Oct 7. doi: 10.1161/CIRCOUTCOMES.120.007357.
“Clinicians increasingly recognize that race-based disparities do not stem inherently from race; instead, the disparities stem from the underlying social determinations of health,” they wrote, citing such variables as unequal access to pay and acceptable living conditions “and the structural racism that perpetuates them.”
They agreed with the authors that promotion of psychosocial resilience among Black people living in communities with poor CV health has the potential to improve CV outcomes, but they warned that this is complex. Although they contend that resilience techniques can be taught, they cautioned there might be limitations if the underlying factors associated with poor psychosocial resilience remain unchanged.
“Thus, the superficial application of positive psychology strategies is likely insufficient to bring parity to CV health outcomes,” they wrote, concluding that strategies to promote health equity would negate the need for interventions to bolster resilience.
Studies that focus on Black adults and cardiovascular health, including this investigation into the role of psychosocial factors “are much needed and very welcome,” said Harlan M. Krumholz, MD, a cardiologist and professor in the Institute for Social and Policy Studies at Yale University, New Haven, Conn.
He sees a broad array of potential directions of research.
“The study opens many questions about whether the resilience can be strengthened by interventions; whether addressing structural racism could reduce the need for such resilience, and whether this association is specific to Black adults in an urban center or is generally present in other settings and in other populations,” Dr. Krumholz said.
An effort is now needed to determine “whether this is a marker or a mediator of cardiovascular health,” he added.
In either case, resilience is a potentially important factor for understanding racial disparities in CV-disease prevalence and outcomes, according to the authors of the accompanying editorial and Dr. Krumholz.
SOURCE: Kim JH et al. Circ Cardiovasc Qual Outcomes. 2020 Oct 7;13:e006638.
Resilience might deserve targeting
Resilience might deserve targeting
Increased psychosocial resilience, which captures a sense of purpose, optimism, and life-coping strategies, correlates with improved cardiovascular (CV) health in Black Americans, according to a study that might hold a key for identifying new strategies for CV disease prevention.
“Our findings highlight the importance of individual psychosocial factors that promote cardiovascular health among Black adults, traditionally considered to be a high-risk population,” according to a team of authors collaborating on a study produced by the Morehouse-Emory Cardiovascular Center for Health Equity in Atlanta.
Studies associating psychosocial resilience with improved health outcomes have been published before. In a 12-study review of this concept, it was emphasized that resilience is a dynamic process, not a personality trait, and has shown promise as a target of efforts to relieve the burden of disease (Johnston MC et al. Psychosomatics 2015;56:168-80).
In this study, which received partial support from the American Heart Association, psychosocial resilience was evaluated at both the individual level and at the community level among 389 Black adults living in Atlanta. The senior author was Tené T. Lewis, PhD, of the department of epidemiology at Emory’s Rollins School of Public Health (Circ Cardiovasc Qual Outcomes 2020 Oct 7;13:3006638).
Psychosocial resilience was calculated across the domains of environmental mastery, purpose of life, optimism, coping, and lack of depression with standardized tests, such as the Life Orientation Test-Revised questionnaire for optimism and the Ryff Scales of Psychological Well-Being for the domains of environmental mastery and purpose of life. A composite score for psychosocial resilience was reached by calculating the median score across the measured domains.
Patients with high psychosocial resilience, defined as a composite score above the median, or low resilience, defined as a lower score, were then compared for CV health based on the AHA’s Life’s Simple 7 (LS7) score.
LS7 scores incorporate measures for exercise, diet, smoking history, blood pressure, glucose, cholesterol, and body mass index. Composite LS7 scores range from 0 to 14. Prior work cited by the authors have associated each 1-unit increase in LS7 score with a 13% lower risk of CVD.
As a continuous variable for CV risk at the individual level, each higher standard-deviation increment in the composite psychosocial resilience score was associated with a highly significant 0.42-point increase in LS7 score (P < .001) for study participants. In other words, increasing resilience predicted lower CV risk scores.
Resilience was also calculated at the community level by looking at census tract-level rates of CV mortality and morbidity relative to socioeconomic status. Again, high CV resilience, defined as scores above the median, were compared with lower scores across neighborhoods with similar median household income. As a continuous variable in this analysis, each higher standard-deviation increment in the resilience score was associated with a 0.27-point increase in LS7 score (P = .01).
After adjustment for sociodemographic factors, the association between psychosocial resilience and CV health remained significant for both the individual and community calculations, according to the authors. When examined jointly, high individual psychosocial resilience remained independently associated with improved CV health, but living in a high-resilience neighborhood was not an independent predictor.
When evaluated individually, each of the domains in the psychosocial resistance score were positively correlated with higher LS7 scores, meaning lower CV risk. The strongest associations on a statistical level were low depressive symptoms (P = .001), environmental mastery (P = .006), and purpose in life (P = .009).
The impact of high psychosocial resistance scores was greatest in Black adults living in low-resilience neighborhoods. Among these subjects, high resilience was associated with a nearly 1-point increase in LS7 score relative to low resilience (8.38 vs. 7.42). This was unexpected, but it “is consistent with some broader conceptual literature that posits that individual psychosocial resilience matters more under conditions of adversity,” the authors reported.
Understanding disparities is key
Black race has repeatedly been associated with an increased risk of CV events, but this study is valuable for providing a fresh perspective on the potential reasons, according to the authors of an accompanying editorial, Amber E. Johnson, MD, and Jared Magnani, MD, who are both affiliated with the division of cardiology at the University of Pittsburgh (Circ Cardiovasc Qual Outcomes 2020 Oct 7. doi: 10.1161/CIRCOUTCOMES.120.007357.
“Clinicians increasingly recognize that race-based disparities do not stem inherently from race; instead, the disparities stem from the underlying social determinations of health,” they wrote, citing such variables as unequal access to pay and acceptable living conditions “and the structural racism that perpetuates them.”
They agreed with the authors that promotion of psychosocial resilience among Black people living in communities with poor CV health has the potential to improve CV outcomes, but they warned that this is complex. Although they contend that resilience techniques can be taught, they cautioned there might be limitations if the underlying factors associated with poor psychosocial resilience remain unchanged.
“Thus, the superficial application of positive psychology strategies is likely insufficient to bring parity to CV health outcomes,” they wrote, concluding that strategies to promote health equity would negate the need for interventions to bolster resilience.
Studies that focus on Black adults and cardiovascular health, including this investigation into the role of psychosocial factors “are much needed and very welcome,” said Harlan M. Krumholz, MD, a cardiologist and professor in the Institute for Social and Policy Studies at Yale University, New Haven, Conn.
He sees a broad array of potential directions of research.
“The study opens many questions about whether the resilience can be strengthened by interventions; whether addressing structural racism could reduce the need for such resilience, and whether this association is specific to Black adults in an urban center or is generally present in other settings and in other populations,” Dr. Krumholz said.
An effort is now needed to determine “whether this is a marker or a mediator of cardiovascular health,” he added.
In either case, resilience is a potentially important factor for understanding racial disparities in CV-disease prevalence and outcomes, according to the authors of the accompanying editorial and Dr. Krumholz.
SOURCE: Kim JH et al. Circ Cardiovasc Qual Outcomes. 2020 Oct 7;13:e006638.
Increased psychosocial resilience, which captures a sense of purpose, optimism, and life-coping strategies, correlates with improved cardiovascular (CV) health in Black Americans, according to a study that might hold a key for identifying new strategies for CV disease prevention.
“Our findings highlight the importance of individual psychosocial factors that promote cardiovascular health among Black adults, traditionally considered to be a high-risk population,” according to a team of authors collaborating on a study produced by the Morehouse-Emory Cardiovascular Center for Health Equity in Atlanta.
Studies associating psychosocial resilience with improved health outcomes have been published before. In a 12-study review of this concept, it was emphasized that resilience is a dynamic process, not a personality trait, and has shown promise as a target of efforts to relieve the burden of disease (Johnston MC et al. Psychosomatics 2015;56:168-80).
In this study, which received partial support from the American Heart Association, psychosocial resilience was evaluated at both the individual level and at the community level among 389 Black adults living in Atlanta. The senior author was Tené T. Lewis, PhD, of the department of epidemiology at Emory’s Rollins School of Public Health (Circ Cardiovasc Qual Outcomes 2020 Oct 7;13:3006638).
Psychosocial resilience was calculated across the domains of environmental mastery, purpose of life, optimism, coping, and lack of depression with standardized tests, such as the Life Orientation Test-Revised questionnaire for optimism and the Ryff Scales of Psychological Well-Being for the domains of environmental mastery and purpose of life. A composite score for psychosocial resilience was reached by calculating the median score across the measured domains.
Patients with high psychosocial resilience, defined as a composite score above the median, or low resilience, defined as a lower score, were then compared for CV health based on the AHA’s Life’s Simple 7 (LS7) score.
LS7 scores incorporate measures for exercise, diet, smoking history, blood pressure, glucose, cholesterol, and body mass index. Composite LS7 scores range from 0 to 14. Prior work cited by the authors have associated each 1-unit increase in LS7 score with a 13% lower risk of CVD.
As a continuous variable for CV risk at the individual level, each higher standard-deviation increment in the composite psychosocial resilience score was associated with a highly significant 0.42-point increase in LS7 score (P < .001) for study participants. In other words, increasing resilience predicted lower CV risk scores.
Resilience was also calculated at the community level by looking at census tract-level rates of CV mortality and morbidity relative to socioeconomic status. Again, high CV resilience, defined as scores above the median, were compared with lower scores across neighborhoods with similar median household income. As a continuous variable in this analysis, each higher standard-deviation increment in the resilience score was associated with a 0.27-point increase in LS7 score (P = .01).
After adjustment for sociodemographic factors, the association between psychosocial resilience and CV health remained significant for both the individual and community calculations, according to the authors. When examined jointly, high individual psychosocial resilience remained independently associated with improved CV health, but living in a high-resilience neighborhood was not an independent predictor.
When evaluated individually, each of the domains in the psychosocial resistance score were positively correlated with higher LS7 scores, meaning lower CV risk. The strongest associations on a statistical level were low depressive symptoms (P = .001), environmental mastery (P = .006), and purpose in life (P = .009).
The impact of high psychosocial resistance scores was greatest in Black adults living in low-resilience neighborhoods. Among these subjects, high resilience was associated with a nearly 1-point increase in LS7 score relative to low resilience (8.38 vs. 7.42). This was unexpected, but it “is consistent with some broader conceptual literature that posits that individual psychosocial resilience matters more under conditions of adversity,” the authors reported.
Understanding disparities is key
Black race has repeatedly been associated with an increased risk of CV events, but this study is valuable for providing a fresh perspective on the potential reasons, according to the authors of an accompanying editorial, Amber E. Johnson, MD, and Jared Magnani, MD, who are both affiliated with the division of cardiology at the University of Pittsburgh (Circ Cardiovasc Qual Outcomes 2020 Oct 7. doi: 10.1161/CIRCOUTCOMES.120.007357.
“Clinicians increasingly recognize that race-based disparities do not stem inherently from race; instead, the disparities stem from the underlying social determinations of health,” they wrote, citing such variables as unequal access to pay and acceptable living conditions “and the structural racism that perpetuates them.”
They agreed with the authors that promotion of psychosocial resilience among Black people living in communities with poor CV health has the potential to improve CV outcomes, but they warned that this is complex. Although they contend that resilience techniques can be taught, they cautioned there might be limitations if the underlying factors associated with poor psychosocial resilience remain unchanged.
“Thus, the superficial application of positive psychology strategies is likely insufficient to bring parity to CV health outcomes,” they wrote, concluding that strategies to promote health equity would negate the need for interventions to bolster resilience.
Studies that focus on Black adults and cardiovascular health, including this investigation into the role of psychosocial factors “are much needed and very welcome,” said Harlan M. Krumholz, MD, a cardiologist and professor in the Institute for Social and Policy Studies at Yale University, New Haven, Conn.
He sees a broad array of potential directions of research.
“The study opens many questions about whether the resilience can be strengthened by interventions; whether addressing structural racism could reduce the need for such resilience, and whether this association is specific to Black adults in an urban center or is generally present in other settings and in other populations,” Dr. Krumholz said.
An effort is now needed to determine “whether this is a marker or a mediator of cardiovascular health,” he added.
In either case, resilience is a potentially important factor for understanding racial disparities in CV-disease prevalence and outcomes, according to the authors of the accompanying editorial and Dr. Krumholz.
SOURCE: Kim JH et al. Circ Cardiovasc Qual Outcomes. 2020 Oct 7;13:e006638.
FROM CIRCULATION: CARDIOVASCULAR QUALITY AND OUTCOMES
Screening algorithm safely selects patients for OSA treatment before bariatric surgery
A novel algorithm for selecting patients who require treatment for obstructive sleep apnea (OSA) before undergoing bariatric surgery proved safe in a prospective cohort study of 1,103 patients.
Screening for OSA is recommended before bariatric surgery. OSA has been associated in several meta-analyses with increased risk for postoperative complications – not limited to bariatric surgery – and some studies have suggested that this increased risk may be limited to severe OSA, said Frédéric Series, MD, of Université Laval, Quebec City, at the virtual annual meeting of the Associated Sleep Societies.
The preoperative screening algorithm, which utilizes the results of nocturnal home oximetry and morning capillary gas measurements, effectively stratified patients for the risk of postoperative adverse events and “safely selected patients who don’t need [continuous positive airway pressure] before bariatric surgery,” he said. “The risk of postoperative adverse events following bariatric surgery was not increased in untreated OSA patients with low or moderate risk of severe OSA and hypoventilation.”
The study also demonstrated, he said, that patients with severe OSA with or without hypoventilation, even when correctly treated, remain at higher risk for complications.
The algorithm utilizes an oxygen desaturation index (ODI) corresponding to 3% drops in SaO2 and the percent of the total recording time with an SaO2 below 90%, as well as capillary gas measurements (PCO2). Treatment was initiated for those with severe OSA (ODI ≥ 25/hr, < 10% of recording time with a SaO2 below 90%) or OSA with hypoventilation (PCO2 ≥ 45).
“When the ODI was less than 25 per hour, and when the total recording time spent below 90% SaO2 was less than 10%, with PCO2 < 45 mmHg, we expected no need for CPAP treatment,” Dr. Series said. For analysis, the investigators considered part of the untreated group – those with an ODI < 10/hr (no or mild OSA) – as a control group.
Treated patients underwent CPAP/BiPAP for a mean duration of 1.5 months. Good treatment compliance was mandatory for surgery, and treatment was continued immediately after extubation, in the recovery room, in nearly all patients, Dr. Series reported.
The analysis covered 1,103 patients: 447 controls (40.8%), 358 untreated (32.7%), 289 treated for OSA (26.4%) and 9 (0.8%) treated for OSA + hypoventilation. Patients with OSA, particularly those with severe OSA and those with hypoventilation, were older and heavier and significantly more likely to have hypertension and diabetes than controls.
There were no differences between the four groups in 10-day reoperation or 30-day readmission occurrence, and postoperative complications were “particularly infrequent in the control and OSA-untreated groups, with no differences between these two groups,” Dr. Series said.
Cardiac arrhythmia (mainly atrial fibrillation) occurred more frequently in the OSA-treated group (2.4%) and the OSA/hypoventilation patients (11%) than in the other groups (0.5%-0.6%).
Respiratory failure occurred in about one-third of patients with hypoventilation, and admission to the ICU was “dramatically higher” in patients with hypoventilation (67%), because of respiratory failure, arrhythmia, or other unstable medical conditions, Dr. Series said.
There were no differences between the groups in the duration of surgery or the amount of anesthetic used, but the length of stay in the recovery room was significantly longer in the OSA-treated and hypoventilation groups. The length of hospital stay was also longer in these groups. Sleeve gastrectomy was the most frequent bariatric surgical procedure across all groups, including 100% of patients with hypoventilation, he noted.
Asked to comment on the study, Octavian C. Ioachimescu, MD, PhD, of Emory University in Atlanta and the Atlanta Veterans Affairs Medical Center in Decatur, said the algorithm “clearly deserves further validation in other clinical-based cohorts and longer-term outcome assessment.”
Dr. Series reported that he has no relevant disclosures. Dr. Ioachimescu also said he has no relevant disclosures.
A novel algorithm for selecting patients who require treatment for obstructive sleep apnea (OSA) before undergoing bariatric surgery proved safe in a prospective cohort study of 1,103 patients.
Screening for OSA is recommended before bariatric surgery. OSA has been associated in several meta-analyses with increased risk for postoperative complications – not limited to bariatric surgery – and some studies have suggested that this increased risk may be limited to severe OSA, said Frédéric Series, MD, of Université Laval, Quebec City, at the virtual annual meeting of the Associated Sleep Societies.
The preoperative screening algorithm, which utilizes the results of nocturnal home oximetry and morning capillary gas measurements, effectively stratified patients for the risk of postoperative adverse events and “safely selected patients who don’t need [continuous positive airway pressure] before bariatric surgery,” he said. “The risk of postoperative adverse events following bariatric surgery was not increased in untreated OSA patients with low or moderate risk of severe OSA and hypoventilation.”
The study also demonstrated, he said, that patients with severe OSA with or without hypoventilation, even when correctly treated, remain at higher risk for complications.
The algorithm utilizes an oxygen desaturation index (ODI) corresponding to 3% drops in SaO2 and the percent of the total recording time with an SaO2 below 90%, as well as capillary gas measurements (PCO2). Treatment was initiated for those with severe OSA (ODI ≥ 25/hr, < 10% of recording time with a SaO2 below 90%) or OSA with hypoventilation (PCO2 ≥ 45).
“When the ODI was less than 25 per hour, and when the total recording time spent below 90% SaO2 was less than 10%, with PCO2 < 45 mmHg, we expected no need for CPAP treatment,” Dr. Series said. For analysis, the investigators considered part of the untreated group – those with an ODI < 10/hr (no or mild OSA) – as a control group.
Treated patients underwent CPAP/BiPAP for a mean duration of 1.5 months. Good treatment compliance was mandatory for surgery, and treatment was continued immediately after extubation, in the recovery room, in nearly all patients, Dr. Series reported.
The analysis covered 1,103 patients: 447 controls (40.8%), 358 untreated (32.7%), 289 treated for OSA (26.4%) and 9 (0.8%) treated for OSA + hypoventilation. Patients with OSA, particularly those with severe OSA and those with hypoventilation, were older and heavier and significantly more likely to have hypertension and diabetes than controls.
There were no differences between the four groups in 10-day reoperation or 30-day readmission occurrence, and postoperative complications were “particularly infrequent in the control and OSA-untreated groups, with no differences between these two groups,” Dr. Series said.
Cardiac arrhythmia (mainly atrial fibrillation) occurred more frequently in the OSA-treated group (2.4%) and the OSA/hypoventilation patients (11%) than in the other groups (0.5%-0.6%).
Respiratory failure occurred in about one-third of patients with hypoventilation, and admission to the ICU was “dramatically higher” in patients with hypoventilation (67%), because of respiratory failure, arrhythmia, or other unstable medical conditions, Dr. Series said.
There were no differences between the groups in the duration of surgery or the amount of anesthetic used, but the length of stay in the recovery room was significantly longer in the OSA-treated and hypoventilation groups. The length of hospital stay was also longer in these groups. Sleeve gastrectomy was the most frequent bariatric surgical procedure across all groups, including 100% of patients with hypoventilation, he noted.
Asked to comment on the study, Octavian C. Ioachimescu, MD, PhD, of Emory University in Atlanta and the Atlanta Veterans Affairs Medical Center in Decatur, said the algorithm “clearly deserves further validation in other clinical-based cohorts and longer-term outcome assessment.”
Dr. Series reported that he has no relevant disclosures. Dr. Ioachimescu also said he has no relevant disclosures.
A novel algorithm for selecting patients who require treatment for obstructive sleep apnea (OSA) before undergoing bariatric surgery proved safe in a prospective cohort study of 1,103 patients.
Screening for OSA is recommended before bariatric surgery. OSA has been associated in several meta-analyses with increased risk for postoperative complications – not limited to bariatric surgery – and some studies have suggested that this increased risk may be limited to severe OSA, said Frédéric Series, MD, of Université Laval, Quebec City, at the virtual annual meeting of the Associated Sleep Societies.
The preoperative screening algorithm, which utilizes the results of nocturnal home oximetry and morning capillary gas measurements, effectively stratified patients for the risk of postoperative adverse events and “safely selected patients who don’t need [continuous positive airway pressure] before bariatric surgery,” he said. “The risk of postoperative adverse events following bariatric surgery was not increased in untreated OSA patients with low or moderate risk of severe OSA and hypoventilation.”
The study also demonstrated, he said, that patients with severe OSA with or without hypoventilation, even when correctly treated, remain at higher risk for complications.
The algorithm utilizes an oxygen desaturation index (ODI) corresponding to 3% drops in SaO2 and the percent of the total recording time with an SaO2 below 90%, as well as capillary gas measurements (PCO2). Treatment was initiated for those with severe OSA (ODI ≥ 25/hr, < 10% of recording time with a SaO2 below 90%) or OSA with hypoventilation (PCO2 ≥ 45).
“When the ODI was less than 25 per hour, and when the total recording time spent below 90% SaO2 was less than 10%, with PCO2 < 45 mmHg, we expected no need for CPAP treatment,” Dr. Series said. For analysis, the investigators considered part of the untreated group – those with an ODI < 10/hr (no or mild OSA) – as a control group.
Treated patients underwent CPAP/BiPAP for a mean duration of 1.5 months. Good treatment compliance was mandatory for surgery, and treatment was continued immediately after extubation, in the recovery room, in nearly all patients, Dr. Series reported.
The analysis covered 1,103 patients: 447 controls (40.8%), 358 untreated (32.7%), 289 treated for OSA (26.4%) and 9 (0.8%) treated for OSA + hypoventilation. Patients with OSA, particularly those with severe OSA and those with hypoventilation, were older and heavier and significantly more likely to have hypertension and diabetes than controls.
There were no differences between the four groups in 10-day reoperation or 30-day readmission occurrence, and postoperative complications were “particularly infrequent in the control and OSA-untreated groups, with no differences between these two groups,” Dr. Series said.
Cardiac arrhythmia (mainly atrial fibrillation) occurred more frequently in the OSA-treated group (2.4%) and the OSA/hypoventilation patients (11%) than in the other groups (0.5%-0.6%).
Respiratory failure occurred in about one-third of patients with hypoventilation, and admission to the ICU was “dramatically higher” in patients with hypoventilation (67%), because of respiratory failure, arrhythmia, or other unstable medical conditions, Dr. Series said.
There were no differences between the groups in the duration of surgery or the amount of anesthetic used, but the length of stay in the recovery room was significantly longer in the OSA-treated and hypoventilation groups. The length of hospital stay was also longer in these groups. Sleeve gastrectomy was the most frequent bariatric surgical procedure across all groups, including 100% of patients with hypoventilation, he noted.
Asked to comment on the study, Octavian C. Ioachimescu, MD, PhD, of Emory University in Atlanta and the Atlanta Veterans Affairs Medical Center in Decatur, said the algorithm “clearly deserves further validation in other clinical-based cohorts and longer-term outcome assessment.”
Dr. Series reported that he has no relevant disclosures. Dr. Ioachimescu also said he has no relevant disclosures.
REPORTING FROM SLEEP 2020
Address root causes to manage NASH
Not only the prevalence, but the impact of nonalcoholic fatty liver disease (NAFLD) is increasing in much of the world, Arun J. Sanyal, MD, said in a virtual presentation at the meeting jointly provided by Rutgers and Global Academy for Medical Education. “It is currently estimated that the number of people living with cirrhosis or with decompensated cirrhosis will increase two- to threefold from 2015 to 2030,” which underlines the public health impact and the need for improved treatment paradigms, he emphasized.
“The thing to remember about NAFLD is that it does not exist in a vacuum,” Dr. Sanyal said. NAFLD is a multisystem disorder. Most patients have concomitant cardiovascular disease, but others may have type 2 diabetes, hypertension, and dyslipidemia, all of which are now accepted as risk factors for nonalcoholic steatohepatitis (NASH), he said.
“What ties these conditions together is metabolic stress leading to systemic inflammation and fibrosis. This is primarily due to diet-induced obesity. If you think about treating all of these competing risks to the patient’s life, the optimal way is to treat the root cause,” he said.
Various options exist to manage the conditions that can lead to NASH, but several of these also appear promising as a treatment of NASH, Dr. Sanyal said. Glucagonlike peptide–1 agonists and sodium-glucose transporter 2 inhibitors have been shown to improve multiple outcomes of interest in type 2 diabetes. However, insulin can cause weight gain at the expense of controlling HbA1C levels, he said.
Bariatric surgery can improve histology, but many patients with advanced fibrosis do not demonstrate improvement in fibrosis. Also, bariatric surgery has its own associated morbidity, including an increased suicide rate across multiple studies, Dr. Sanyal noted.
A new and interesting option is duodenal mucosal resurfacing (DMR) “a novel, minimally invasive outpatient upper-endoscopic procedure,” said Dr. Sanyal. DMR involves use of a catheter to perform a submucosal lift and hydrothermal mucosal ablation, prompting healthy epithelial regrowth, he explained. “The mucosa sloughs off, fresh epithelium grows in, and the hormonal signal from the gut to the rest of the body is restored to a more normal pattern,” he noted.
In the REVITA-2 study of patients with diabetes and NAFLD, the average fat loss was 5.4% in those randomized to DMR vs. 2.4% in sham-procedure patients and represented “quite significant defatting of the liver,” Dr. Sanyal said.
Dr. Sanyal then focused on fatty liver disease. “The first step when you see a patient with fatty liver disease is to see how scarred is the liver, and whether the patient has silent cirrhosis. The more scarred the liver, the greater risk of liver-related outcomes,” he said. The goal of therapy for these patients is to reduce the risk of progression to cirrhosis, he added. Dr. Sanyal recommended evaluating fibrosis using the Fibrosis 4 score (Fib4). “If the Fib4 is less than 1.3, the likelihood of significant scarring in the liver is less than 10%,” he said. On the other hand, a Fib4 greater than 2.67 suggests advanced fibrosis, he noted.
Overall, the goals of treatment for NASH patients are to prevent cirrhosis, reduce decompensation, and prevent hepatocellular carcinoma, said Dr. Sanyal.
“The ideal drug for NASH should also help other end organs, or at least be neutral,” said Dr. Sanyal.
Current frontline therapies for precirrhotic NASH include thiazolidinediones (TZD), farnesoid X receptor (FXR)/fibroblast growth factor 19 (FGF-19), FGF21, thyroxine B-R, and glucagonlike peptide-1. Clinical evidence varies based on different populations, endpoints, assessment methods, and treatment duration, he said.
Looking ahead to the next decade, a NASH management paradigm will likely play out that can be applied in the clinic today, Dr. Sanyal said. First, make an initial assessment of the status of the end organs. Start with a weight-loss regimen; use statins and GLP-1 and SGLT2 inhibitors as needed. Follow and reassess, and if the patient still has disease, progress to targeted therapy for active NASH while continuing to encourage weight loss and healthy living, he said.
“The ultimate proof that what we are doing is working is that we are improving mortality, reducing health care costs, and improving patients’ function and quality of life,” he concluded.
Dr. Sanyal is president of Sanyal Biotechnologies. He also disclosed stock options for Durect, Exhalenz, Galmed, Genfit, Immuton, Indalo, and Tiziana, as well as various relationships with Allergan, AMRA, Astra Zeneca-Medimmune, Birdrock, Boehringer Ingelheim, Bristol Myers, Echosense, GE, Genentech, Gilead, Hemoshear, IFMO, Innovate, Intercept, Lilly, Lipocine, Merck, Novartis, Novo Nordisk, OWL, Pfizer, RedX, Sundise, Tern, and Zydus.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Not only the prevalence, but the impact of nonalcoholic fatty liver disease (NAFLD) is increasing in much of the world, Arun J. Sanyal, MD, said in a virtual presentation at the meeting jointly provided by Rutgers and Global Academy for Medical Education. “It is currently estimated that the number of people living with cirrhosis or with decompensated cirrhosis will increase two- to threefold from 2015 to 2030,” which underlines the public health impact and the need for improved treatment paradigms, he emphasized.
“The thing to remember about NAFLD is that it does not exist in a vacuum,” Dr. Sanyal said. NAFLD is a multisystem disorder. Most patients have concomitant cardiovascular disease, but others may have type 2 diabetes, hypertension, and dyslipidemia, all of which are now accepted as risk factors for nonalcoholic steatohepatitis (NASH), he said.
“What ties these conditions together is metabolic stress leading to systemic inflammation and fibrosis. This is primarily due to diet-induced obesity. If you think about treating all of these competing risks to the patient’s life, the optimal way is to treat the root cause,” he said.
Various options exist to manage the conditions that can lead to NASH, but several of these also appear promising as a treatment of NASH, Dr. Sanyal said. Glucagonlike peptide–1 agonists and sodium-glucose transporter 2 inhibitors have been shown to improve multiple outcomes of interest in type 2 diabetes. However, insulin can cause weight gain at the expense of controlling HbA1C levels, he said.
Bariatric surgery can improve histology, but many patients with advanced fibrosis do not demonstrate improvement in fibrosis. Also, bariatric surgery has its own associated morbidity, including an increased suicide rate across multiple studies, Dr. Sanyal noted.
A new and interesting option is duodenal mucosal resurfacing (DMR) “a novel, minimally invasive outpatient upper-endoscopic procedure,” said Dr. Sanyal. DMR involves use of a catheter to perform a submucosal lift and hydrothermal mucosal ablation, prompting healthy epithelial regrowth, he explained. “The mucosa sloughs off, fresh epithelium grows in, and the hormonal signal from the gut to the rest of the body is restored to a more normal pattern,” he noted.
In the REVITA-2 study of patients with diabetes and NAFLD, the average fat loss was 5.4% in those randomized to DMR vs. 2.4% in sham-procedure patients and represented “quite significant defatting of the liver,” Dr. Sanyal said.
Dr. Sanyal then focused on fatty liver disease. “The first step when you see a patient with fatty liver disease is to see how scarred is the liver, and whether the patient has silent cirrhosis. The more scarred the liver, the greater risk of liver-related outcomes,” he said. The goal of therapy for these patients is to reduce the risk of progression to cirrhosis, he added. Dr. Sanyal recommended evaluating fibrosis using the Fibrosis 4 score (Fib4). “If the Fib4 is less than 1.3, the likelihood of significant scarring in the liver is less than 10%,” he said. On the other hand, a Fib4 greater than 2.67 suggests advanced fibrosis, he noted.
Overall, the goals of treatment for NASH patients are to prevent cirrhosis, reduce decompensation, and prevent hepatocellular carcinoma, said Dr. Sanyal.
“The ideal drug for NASH should also help other end organs, or at least be neutral,” said Dr. Sanyal.
Current frontline therapies for precirrhotic NASH include thiazolidinediones (TZD), farnesoid X receptor (FXR)/fibroblast growth factor 19 (FGF-19), FGF21, thyroxine B-R, and glucagonlike peptide-1. Clinical evidence varies based on different populations, endpoints, assessment methods, and treatment duration, he said.
Looking ahead to the next decade, a NASH management paradigm will likely play out that can be applied in the clinic today, Dr. Sanyal said. First, make an initial assessment of the status of the end organs. Start with a weight-loss regimen; use statins and GLP-1 and SGLT2 inhibitors as needed. Follow and reassess, and if the patient still has disease, progress to targeted therapy for active NASH while continuing to encourage weight loss and healthy living, he said.
“The ultimate proof that what we are doing is working is that we are improving mortality, reducing health care costs, and improving patients’ function and quality of life,” he concluded.
Dr. Sanyal is president of Sanyal Biotechnologies. He also disclosed stock options for Durect, Exhalenz, Galmed, Genfit, Immuton, Indalo, and Tiziana, as well as various relationships with Allergan, AMRA, Astra Zeneca-Medimmune, Birdrock, Boehringer Ingelheim, Bristol Myers, Echosense, GE, Genentech, Gilead, Hemoshear, IFMO, Innovate, Intercept, Lilly, Lipocine, Merck, Novartis, Novo Nordisk, OWL, Pfizer, RedX, Sundise, Tern, and Zydus.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Not only the prevalence, but the impact of nonalcoholic fatty liver disease (NAFLD) is increasing in much of the world, Arun J. Sanyal, MD, said in a virtual presentation at the meeting jointly provided by Rutgers and Global Academy for Medical Education. “It is currently estimated that the number of people living with cirrhosis or with decompensated cirrhosis will increase two- to threefold from 2015 to 2030,” which underlines the public health impact and the need for improved treatment paradigms, he emphasized.
“The thing to remember about NAFLD is that it does not exist in a vacuum,” Dr. Sanyal said. NAFLD is a multisystem disorder. Most patients have concomitant cardiovascular disease, but others may have type 2 diabetes, hypertension, and dyslipidemia, all of which are now accepted as risk factors for nonalcoholic steatohepatitis (NASH), he said.
“What ties these conditions together is metabolic stress leading to systemic inflammation and fibrosis. This is primarily due to diet-induced obesity. If you think about treating all of these competing risks to the patient’s life, the optimal way is to treat the root cause,” he said.
Various options exist to manage the conditions that can lead to NASH, but several of these also appear promising as a treatment of NASH, Dr. Sanyal said. Glucagonlike peptide–1 agonists and sodium-glucose transporter 2 inhibitors have been shown to improve multiple outcomes of interest in type 2 diabetes. However, insulin can cause weight gain at the expense of controlling HbA1C levels, he said.
Bariatric surgery can improve histology, but many patients with advanced fibrosis do not demonstrate improvement in fibrosis. Also, bariatric surgery has its own associated morbidity, including an increased suicide rate across multiple studies, Dr. Sanyal noted.
A new and interesting option is duodenal mucosal resurfacing (DMR) “a novel, minimally invasive outpatient upper-endoscopic procedure,” said Dr. Sanyal. DMR involves use of a catheter to perform a submucosal lift and hydrothermal mucosal ablation, prompting healthy epithelial regrowth, he explained. “The mucosa sloughs off, fresh epithelium grows in, and the hormonal signal from the gut to the rest of the body is restored to a more normal pattern,” he noted.
In the REVITA-2 study of patients with diabetes and NAFLD, the average fat loss was 5.4% in those randomized to DMR vs. 2.4% in sham-procedure patients and represented “quite significant defatting of the liver,” Dr. Sanyal said.
Dr. Sanyal then focused on fatty liver disease. “The first step when you see a patient with fatty liver disease is to see how scarred is the liver, and whether the patient has silent cirrhosis. The more scarred the liver, the greater risk of liver-related outcomes,” he said. The goal of therapy for these patients is to reduce the risk of progression to cirrhosis, he added. Dr. Sanyal recommended evaluating fibrosis using the Fibrosis 4 score (Fib4). “If the Fib4 is less than 1.3, the likelihood of significant scarring in the liver is less than 10%,” he said. On the other hand, a Fib4 greater than 2.67 suggests advanced fibrosis, he noted.
Overall, the goals of treatment for NASH patients are to prevent cirrhosis, reduce decompensation, and prevent hepatocellular carcinoma, said Dr. Sanyal.
“The ideal drug for NASH should also help other end organs, or at least be neutral,” said Dr. Sanyal.
Current frontline therapies for precirrhotic NASH include thiazolidinediones (TZD), farnesoid X receptor (FXR)/fibroblast growth factor 19 (FGF-19), FGF21, thyroxine B-R, and glucagonlike peptide-1. Clinical evidence varies based on different populations, endpoints, assessment methods, and treatment duration, he said.
Looking ahead to the next decade, a NASH management paradigm will likely play out that can be applied in the clinic today, Dr. Sanyal said. First, make an initial assessment of the status of the end organs. Start with a weight-loss regimen; use statins and GLP-1 and SGLT2 inhibitors as needed. Follow and reassess, and if the patient still has disease, progress to targeted therapy for active NASH while continuing to encourage weight loss and healthy living, he said.
“The ultimate proof that what we are doing is working is that we are improving mortality, reducing health care costs, and improving patients’ function and quality of life,” he concluded.
Dr. Sanyal is president of Sanyal Biotechnologies. He also disclosed stock options for Durect, Exhalenz, Galmed, Genfit, Immuton, Indalo, and Tiziana, as well as various relationships with Allergan, AMRA, Astra Zeneca-Medimmune, Birdrock, Boehringer Ingelheim, Bristol Myers, Echosense, GE, Genentech, Gilead, Hemoshear, IFMO, Innovate, Intercept, Lilly, Lipocine, Merck, Novartis, Novo Nordisk, OWL, Pfizer, RedX, Sundise, Tern, and Zydus.
Global Academy for Medical Education and this news organization are owned by the same parent company.
FROM DIGESTIVE DISEASES: NEW ADVANCES
Smart health devices – promises and pitfalls
What needs to be done before the data deluge hits the office
Hurricane Sally recently crossed the Gulf of Mexico and landed with torrential rainfalls along the Alabama coast. A little rainfall is important for crops; too much leads to devastation. As physicians, we need data in order to help manage patients’ illnesses and to help to keep them healthy. Our fear though is that too much data provided too quickly may have the opposite effect.
Personal monitoring devices
When I bought my first Fitbit 7 years ago, I was enamored with the technology. The Fitbit was little more than a step tracker, yet I proudly wore its black rubber strap on my wrist. It was my first foray into wearable technology, and it felt quite empowering to have an objective way to track my fitness beyond just using my bathroom scale. Now less than a decade later, that Fitbit looks archaic in comparison with the wrist-top technology currently available.
As I write this, the world’s largest technology company is in the process of releasing its sixth-generation Apple Watch. In addition to acting as a smartphone, this new device, which is barely larger than a postage stamp, offers GPS-based movement tracking, the ability to detect falls, continuous heart rate monitoring, a built-in EKG capable of diagnosing atrial fibrillation, and an oxygen saturation sensor. These features weren’t added thoughtlessly. Apple is marketing this as a health-focused device, with their primary advertising campaign claiming that “the future of health is on your wrist,” and they aren’t the only company making this play.
Along with Apple, Samsung, Withings, Fitbit, and other companies continue to bring products to market that monitor our activity and provide new insights into our health. Typically linked to smartphone-based apps, these devices record all of their measurements for later review, while software helps interpret the findings to make them actionable. From heart rate tracking to sleep analysis, these options now provide access to volumes of data that promise to improve our wellness and change our lives. Of course, those promises will only be fulfilled if our behavior is altered as a consequence of having more detailed information. Whether that will happen remains to be seen.
Health system–linked devices
Major advancements in medical monitoring technology are now enabling physicians to get much deeper insight into their patients’ health status. Internet-connected scales, blood pressure cuffs, and exercise equipment offer the ability to upload information into patient portals and integrate that information into EHRs. New devices provide access to information that previously was impossible to obtain. For example, wearable continuous blood glucose monitors, such as the FreeStyle Libre or DexCom’s G6, allow patients and physicians to follow blood sugar readings 24 hours a day. This provides unprecedented awareness of diabetes control and relieves the pain and inconvenience of finger sticks and blood draws. It also aids with compliance because patients don’t need to remember to check their sugar levels on a schedule.
Other compliance-boosting breakthroughs, such as Bluetooth-enabled asthma inhalers and cellular-connected continuous positive airway pressure machines, assist patients with managing chronic respiratory conditions. Many companies are developing technologies to manage acute conditions as well. One such company, an on-demand telemedicine provider called TytoCare, has developed a $299 suite of instruments that includes a digital stethoscope, thermometer, and camera-based otoscope. In concert with a virtual visit, their providers can remotely use these tools to examine and assess sick individuals. This virtual “laying on of hands” may have sounded like science fiction and likely would have been rejected by patients just a few years ago. Now it is becoming commonplace and will soon be an expectation of many seeking care.
But if we are to be successful, everyone must acknowledge that this revolution in health care brings many challenges along with it. One of those is the deluge of data that connected devices provide.
Information overload
There is such a thing as “too much of a good thing.” Described by journalist David Shenk as “data smog” in his 1997 book of the same name, the idea is clear: There is only so much information we can assimilate.
Even after years of using EHRs and with government-implemented incentives that promote “meaningful use,” physicians are still struggling with EHRs. Additionally, many have expressed frustration with the connectedness that EHRs provide and lament their inability to ever really “leave the office.” As more and more data become available to physicians, the challenge of how to assimilate and act on those data will continue to grow. The addition of patient-provided health statistics will only make information overload worse, with clinicians will feeling an ever-growing burden to know, understand, and act on this information.
Unless we develop systems to sort, filter, and prioritize the flow of information, there is potential for liability from not acting on the amount of virtual information doctors receive. This new risk for already fatigued and overburdened physicians combined with an increase in the amount of virtual information at doctors’ fingertips may lead to the value of patient data being lost.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
What needs to be done before the data deluge hits the office
What needs to be done before the data deluge hits the office
Hurricane Sally recently crossed the Gulf of Mexico and landed with torrential rainfalls along the Alabama coast. A little rainfall is important for crops; too much leads to devastation. As physicians, we need data in order to help manage patients’ illnesses and to help to keep them healthy. Our fear though is that too much data provided too quickly may have the opposite effect.
Personal monitoring devices
When I bought my first Fitbit 7 years ago, I was enamored with the technology. The Fitbit was little more than a step tracker, yet I proudly wore its black rubber strap on my wrist. It was my first foray into wearable technology, and it felt quite empowering to have an objective way to track my fitness beyond just using my bathroom scale. Now less than a decade later, that Fitbit looks archaic in comparison with the wrist-top technology currently available.
As I write this, the world’s largest technology company is in the process of releasing its sixth-generation Apple Watch. In addition to acting as a smartphone, this new device, which is barely larger than a postage stamp, offers GPS-based movement tracking, the ability to detect falls, continuous heart rate monitoring, a built-in EKG capable of diagnosing atrial fibrillation, and an oxygen saturation sensor. These features weren’t added thoughtlessly. Apple is marketing this as a health-focused device, with their primary advertising campaign claiming that “the future of health is on your wrist,” and they aren’t the only company making this play.
Along with Apple, Samsung, Withings, Fitbit, and other companies continue to bring products to market that monitor our activity and provide new insights into our health. Typically linked to smartphone-based apps, these devices record all of their measurements for later review, while software helps interpret the findings to make them actionable. From heart rate tracking to sleep analysis, these options now provide access to volumes of data that promise to improve our wellness and change our lives. Of course, those promises will only be fulfilled if our behavior is altered as a consequence of having more detailed information. Whether that will happen remains to be seen.
Health system–linked devices
Major advancements in medical monitoring technology are now enabling physicians to get much deeper insight into their patients’ health status. Internet-connected scales, blood pressure cuffs, and exercise equipment offer the ability to upload information into patient portals and integrate that information into EHRs. New devices provide access to information that previously was impossible to obtain. For example, wearable continuous blood glucose monitors, such as the FreeStyle Libre or DexCom’s G6, allow patients and physicians to follow blood sugar readings 24 hours a day. This provides unprecedented awareness of diabetes control and relieves the pain and inconvenience of finger sticks and blood draws. It also aids with compliance because patients don’t need to remember to check their sugar levels on a schedule.
Other compliance-boosting breakthroughs, such as Bluetooth-enabled asthma inhalers and cellular-connected continuous positive airway pressure machines, assist patients with managing chronic respiratory conditions. Many companies are developing technologies to manage acute conditions as well. One such company, an on-demand telemedicine provider called TytoCare, has developed a $299 suite of instruments that includes a digital stethoscope, thermometer, and camera-based otoscope. In concert with a virtual visit, their providers can remotely use these tools to examine and assess sick individuals. This virtual “laying on of hands” may have sounded like science fiction and likely would have been rejected by patients just a few years ago. Now it is becoming commonplace and will soon be an expectation of many seeking care.
But if we are to be successful, everyone must acknowledge that this revolution in health care brings many challenges along with it. One of those is the deluge of data that connected devices provide.
Information overload
There is such a thing as “too much of a good thing.” Described by journalist David Shenk as “data smog” in his 1997 book of the same name, the idea is clear: There is only so much information we can assimilate.
Even after years of using EHRs and with government-implemented incentives that promote “meaningful use,” physicians are still struggling with EHRs. Additionally, many have expressed frustration with the connectedness that EHRs provide and lament their inability to ever really “leave the office.” As more and more data become available to physicians, the challenge of how to assimilate and act on those data will continue to grow. The addition of patient-provided health statistics will only make information overload worse, with clinicians will feeling an ever-growing burden to know, understand, and act on this information.
Unless we develop systems to sort, filter, and prioritize the flow of information, there is potential for liability from not acting on the amount of virtual information doctors receive. This new risk for already fatigued and overburdened physicians combined with an increase in the amount of virtual information at doctors’ fingertips may lead to the value of patient data being lost.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Hurricane Sally recently crossed the Gulf of Mexico and landed with torrential rainfalls along the Alabama coast. A little rainfall is important for crops; too much leads to devastation. As physicians, we need data in order to help manage patients’ illnesses and to help to keep them healthy. Our fear though is that too much data provided too quickly may have the opposite effect.
Personal monitoring devices
When I bought my first Fitbit 7 years ago, I was enamored with the technology. The Fitbit was little more than a step tracker, yet I proudly wore its black rubber strap on my wrist. It was my first foray into wearable technology, and it felt quite empowering to have an objective way to track my fitness beyond just using my bathroom scale. Now less than a decade later, that Fitbit looks archaic in comparison with the wrist-top technology currently available.
As I write this, the world’s largest technology company is in the process of releasing its sixth-generation Apple Watch. In addition to acting as a smartphone, this new device, which is barely larger than a postage stamp, offers GPS-based movement tracking, the ability to detect falls, continuous heart rate monitoring, a built-in EKG capable of diagnosing atrial fibrillation, and an oxygen saturation sensor. These features weren’t added thoughtlessly. Apple is marketing this as a health-focused device, with their primary advertising campaign claiming that “the future of health is on your wrist,” and they aren’t the only company making this play.
Along with Apple, Samsung, Withings, Fitbit, and other companies continue to bring products to market that monitor our activity and provide new insights into our health. Typically linked to smartphone-based apps, these devices record all of their measurements for later review, while software helps interpret the findings to make them actionable. From heart rate tracking to sleep analysis, these options now provide access to volumes of data that promise to improve our wellness and change our lives. Of course, those promises will only be fulfilled if our behavior is altered as a consequence of having more detailed information. Whether that will happen remains to be seen.
Health system–linked devices
Major advancements in medical monitoring technology are now enabling physicians to get much deeper insight into their patients’ health status. Internet-connected scales, blood pressure cuffs, and exercise equipment offer the ability to upload information into patient portals and integrate that information into EHRs. New devices provide access to information that previously was impossible to obtain. For example, wearable continuous blood glucose monitors, such as the FreeStyle Libre or DexCom’s G6, allow patients and physicians to follow blood sugar readings 24 hours a day. This provides unprecedented awareness of diabetes control and relieves the pain and inconvenience of finger sticks and blood draws. It also aids with compliance because patients don’t need to remember to check their sugar levels on a schedule.
Other compliance-boosting breakthroughs, such as Bluetooth-enabled asthma inhalers and cellular-connected continuous positive airway pressure machines, assist patients with managing chronic respiratory conditions. Many companies are developing technologies to manage acute conditions as well. One such company, an on-demand telemedicine provider called TytoCare, has developed a $299 suite of instruments that includes a digital stethoscope, thermometer, and camera-based otoscope. In concert with a virtual visit, their providers can remotely use these tools to examine and assess sick individuals. This virtual “laying on of hands” may have sounded like science fiction and likely would have been rejected by patients just a few years ago. Now it is becoming commonplace and will soon be an expectation of many seeking care.
But if we are to be successful, everyone must acknowledge that this revolution in health care brings many challenges along with it. One of those is the deluge of data that connected devices provide.
Information overload
There is such a thing as “too much of a good thing.” Described by journalist David Shenk as “data smog” in his 1997 book of the same name, the idea is clear: There is only so much information we can assimilate.
Even after years of using EHRs and with government-implemented incentives that promote “meaningful use,” physicians are still struggling with EHRs. Additionally, many have expressed frustration with the connectedness that EHRs provide and lament their inability to ever really “leave the office.” As more and more data become available to physicians, the challenge of how to assimilate and act on those data will continue to grow. The addition of patient-provided health statistics will only make information overload worse, with clinicians will feeling an ever-growing burden to know, understand, and act on this information.
Unless we develop systems to sort, filter, and prioritize the flow of information, there is potential for liability from not acting on the amount of virtual information doctors receive. This new risk for already fatigued and overburdened physicians combined with an increase in the amount of virtual information at doctors’ fingertips may lead to the value of patient data being lost.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Bariatric surgery achieved long-term resolution of NASH without worsening fibrosis
Bariatric surgery resolved nonalcoholic steatohepatitis (NASH) without worsening fibrosis in 84% of patients with evaluable biopsies, according to the findings of a prospective study.
The study included 180 severely or morbidly obese adults (body mass index >35 kg/m2) with NASH who underwent bariatric surgery at a center in France. Among 94 patients evaluated 5 years later, 68% had follow-up liver biopsies, of whom 84% (95% confidence interval, 73.1%-92.2%) met the primary endpoint of resolution of NASH without worsening of fibrosis. All histologic aspects of NASH had improved, median nonalcoholic fatty liver disease scores (NAS) fell from 5 (interquartile range, 4 to 5) to 1 (IQR, 0-2; P < .001), and 90% of patients achieved at least a 2-point NAS improvement. Hepatocellular ballooning also improved in 87.5% of patients. Baseline severity of NASH did not affect the chances of it resolving at 5 years. “The reduction of fibrosis [was] progressive, beginning during the first year and continuing through 5 years,” Guillaume Lassailly, MD, and associates wrote in Gastroenterology.
NASH is a priority for clinical research because of the substantial risk for subsequent cirrhosis, added Dr. Lassailly of CHU Lille (France). For NASH to resolve, most patients need to lose at least 7%-10% of their body weight, but “only 10% of patients reach this objective with lifestyle therapy at 1 year, and less than half maintain the weight loss 5 years later.” Despite ongoing drug development efforts, no medications have been approved for treating NASH. Although weight loss after bariatric surgery has been reported to resolve NASH in approximately 80% of patients at 1 year, longer-term data have been unavailable, and it has remained unclear whether bariatric surgery can slow or halt fibrosis progression.
All patients in this study had biopsy-confirmed NASH and at least a 5-year history of severe or morbid obesity as well as at least one comorbidity, such as diabetes mellitus or arterial hypertension. Patients were not heavy drinkers, and none had detectable markers of chronic liver disease.
Bariatric surgery produced a median 12-kg/m2 drop in body mass index. At 5-year follow-up, 93% of patients meeting or exceeding this threshold who had biopsies performed showed resolution of NASH without worsening of fibrosis. Furthermore, 56% of patients (95% CI, 42.4%-69.3%) had no histologic evidence of fibrosis, including 45.5% of patients who had bridging fibrosis at baseline.
Participants in this study received intensive preoperative support, including evaluations by numerous specialists, a nutrition plan, and a 6- to 12-month therapeutic education program. Bariatric surgery techniques included Roux-en-Y gastric bypass, gastric banding, and sleeve gastrectomy. A subgroup analysis linked gastric bypass to a significantly higher probability of meeting the primary endpoint, compared with gastric banding. Refusal was the most common reason for not having a follow-up biopsy, the researchers said. “Patients without liver biopsy after bariatric surgery were not significantly different from those with a histological follow-up except for a lower BMI at 1 year. Baseline fibrosis did not influence the probability of undergoing histological reevaluation at 5 years.”
Two study participants died from surgical complications within 1 month after surgery, and one patient died from cardiac dysfunction 4 years later. No fatality was deemed liver related.
The study was funded by the French Ministry of Health, Conseil Régional Nord-Pas de Calais, National de la Recherche, and the European commission (FEDER). The researchers reported having no conflicts of interest.
SOURCE: Lassailly G et al. Gastroenterology. 2020 Jun 15. doi: 10.1053/j.gastro.2020.06.006.
As obesity prevalence increases at an alarming pace, nonalcoholic steatohepatitis (NASH) has become the most common indication for liver transplantation in women and the second most common in men in the United States. Impeding the inflammation and reversing the resultant fibrosis prior to the development of end-stage liver disease and needing liver transplantation are essential goals in NASH management. The lack of Food and Drug Administration–approved pharmacotherapy triggered interest in the effect of weight loss on NASH and short-term benefits were noted.
In this article, Lassailly et al. demonstrated long-term benefits of bariatric surgery in patients with NASH. They prospectively enrolled 180 patients and histologically followed 64 patients at 1 year and 5 years postoperatively. NASH resolved in 84% of patients and fibrosis regressed in >70%. Importantly, advanced fibrosis (F3) regressed in 15/19 patients. Cirrhosis regressed to F3 in two-thirds of patients. No liver-related mortality or decompensation was observed.
These favorable outcomes embolden the practice of referring NASH patients with morbid obesity to bariatric surgery before liver disease severity becomes prohibitive of this approach. NASH pharmacotherapy may become available in the future. However, we must not forget that cardiovascular disease remains a common cause of morbidity and mortality in NASH patients.
With these study findings and previously established benefits of bariatric surgery on mitigating cardiovascular risk and treating relevant metabolic derangements (e.g., diabetes mellitus), pursuing bariatric surgery in NASH patients may be the seed that, if planted early on, can later flourish with resolution of NASH, prevention of cardiovascular disease, metabolic optimization, and potentially longer and healthier life.
Manhal J. Izzy, MD, is assistant professor of medicine, Vanderbilt Digestive Disease Center, Vanderbilt University, Nashville, Tenn.
As obesity prevalence increases at an alarming pace, nonalcoholic steatohepatitis (NASH) has become the most common indication for liver transplantation in women and the second most common in men in the United States. Impeding the inflammation and reversing the resultant fibrosis prior to the development of end-stage liver disease and needing liver transplantation are essential goals in NASH management. The lack of Food and Drug Administration–approved pharmacotherapy triggered interest in the effect of weight loss on NASH and short-term benefits were noted.
In this article, Lassailly et al. demonstrated long-term benefits of bariatric surgery in patients with NASH. They prospectively enrolled 180 patients and histologically followed 64 patients at 1 year and 5 years postoperatively. NASH resolved in 84% of patients and fibrosis regressed in >70%. Importantly, advanced fibrosis (F3) regressed in 15/19 patients. Cirrhosis regressed to F3 in two-thirds of patients. No liver-related mortality or decompensation was observed.
These favorable outcomes embolden the practice of referring NASH patients with morbid obesity to bariatric surgery before liver disease severity becomes prohibitive of this approach. NASH pharmacotherapy may become available in the future. However, we must not forget that cardiovascular disease remains a common cause of morbidity and mortality in NASH patients.
With these study findings and previously established benefits of bariatric surgery on mitigating cardiovascular risk and treating relevant metabolic derangements (e.g., diabetes mellitus), pursuing bariatric surgery in NASH patients may be the seed that, if planted early on, can later flourish with resolution of NASH, prevention of cardiovascular disease, metabolic optimization, and potentially longer and healthier life.
Manhal J. Izzy, MD, is assistant professor of medicine, Vanderbilt Digestive Disease Center, Vanderbilt University, Nashville, Tenn.
As obesity prevalence increases at an alarming pace, nonalcoholic steatohepatitis (NASH) has become the most common indication for liver transplantation in women and the second most common in men in the United States. Impeding the inflammation and reversing the resultant fibrosis prior to the development of end-stage liver disease and needing liver transplantation are essential goals in NASH management. The lack of Food and Drug Administration–approved pharmacotherapy triggered interest in the effect of weight loss on NASH and short-term benefits were noted.
In this article, Lassailly et al. demonstrated long-term benefits of bariatric surgery in patients with NASH. They prospectively enrolled 180 patients and histologically followed 64 patients at 1 year and 5 years postoperatively. NASH resolved in 84% of patients and fibrosis regressed in >70%. Importantly, advanced fibrosis (F3) regressed in 15/19 patients. Cirrhosis regressed to F3 in two-thirds of patients. No liver-related mortality or decompensation was observed.
These favorable outcomes embolden the practice of referring NASH patients with morbid obesity to bariatric surgery before liver disease severity becomes prohibitive of this approach. NASH pharmacotherapy may become available in the future. However, we must not forget that cardiovascular disease remains a common cause of morbidity and mortality in NASH patients.
With these study findings and previously established benefits of bariatric surgery on mitigating cardiovascular risk and treating relevant metabolic derangements (e.g., diabetes mellitus), pursuing bariatric surgery in NASH patients may be the seed that, if planted early on, can later flourish with resolution of NASH, prevention of cardiovascular disease, metabolic optimization, and potentially longer and healthier life.
Manhal J. Izzy, MD, is assistant professor of medicine, Vanderbilt Digestive Disease Center, Vanderbilt University, Nashville, Tenn.
Bariatric surgery resolved nonalcoholic steatohepatitis (NASH) without worsening fibrosis in 84% of patients with evaluable biopsies, according to the findings of a prospective study.
The study included 180 severely or morbidly obese adults (body mass index >35 kg/m2) with NASH who underwent bariatric surgery at a center in France. Among 94 patients evaluated 5 years later, 68% had follow-up liver biopsies, of whom 84% (95% confidence interval, 73.1%-92.2%) met the primary endpoint of resolution of NASH without worsening of fibrosis. All histologic aspects of NASH had improved, median nonalcoholic fatty liver disease scores (NAS) fell from 5 (interquartile range, 4 to 5) to 1 (IQR, 0-2; P < .001), and 90% of patients achieved at least a 2-point NAS improvement. Hepatocellular ballooning also improved in 87.5% of patients. Baseline severity of NASH did not affect the chances of it resolving at 5 years. “The reduction of fibrosis [was] progressive, beginning during the first year and continuing through 5 years,” Guillaume Lassailly, MD, and associates wrote in Gastroenterology.
NASH is a priority for clinical research because of the substantial risk for subsequent cirrhosis, added Dr. Lassailly of CHU Lille (France). For NASH to resolve, most patients need to lose at least 7%-10% of their body weight, but “only 10% of patients reach this objective with lifestyle therapy at 1 year, and less than half maintain the weight loss 5 years later.” Despite ongoing drug development efforts, no medications have been approved for treating NASH. Although weight loss after bariatric surgery has been reported to resolve NASH in approximately 80% of patients at 1 year, longer-term data have been unavailable, and it has remained unclear whether bariatric surgery can slow or halt fibrosis progression.
All patients in this study had biopsy-confirmed NASH and at least a 5-year history of severe or morbid obesity as well as at least one comorbidity, such as diabetes mellitus or arterial hypertension. Patients were not heavy drinkers, and none had detectable markers of chronic liver disease.
Bariatric surgery produced a median 12-kg/m2 drop in body mass index. At 5-year follow-up, 93% of patients meeting or exceeding this threshold who had biopsies performed showed resolution of NASH without worsening of fibrosis. Furthermore, 56% of patients (95% CI, 42.4%-69.3%) had no histologic evidence of fibrosis, including 45.5% of patients who had bridging fibrosis at baseline.
Participants in this study received intensive preoperative support, including evaluations by numerous specialists, a nutrition plan, and a 6- to 12-month therapeutic education program. Bariatric surgery techniques included Roux-en-Y gastric bypass, gastric banding, and sleeve gastrectomy. A subgroup analysis linked gastric bypass to a significantly higher probability of meeting the primary endpoint, compared with gastric banding. Refusal was the most common reason for not having a follow-up biopsy, the researchers said. “Patients without liver biopsy after bariatric surgery were not significantly different from those with a histological follow-up except for a lower BMI at 1 year. Baseline fibrosis did not influence the probability of undergoing histological reevaluation at 5 years.”
Two study participants died from surgical complications within 1 month after surgery, and one patient died from cardiac dysfunction 4 years later. No fatality was deemed liver related.
The study was funded by the French Ministry of Health, Conseil Régional Nord-Pas de Calais, National de la Recherche, and the European commission (FEDER). The researchers reported having no conflicts of interest.
SOURCE: Lassailly G et al. Gastroenterology. 2020 Jun 15. doi: 10.1053/j.gastro.2020.06.006.
Bariatric surgery resolved nonalcoholic steatohepatitis (NASH) without worsening fibrosis in 84% of patients with evaluable biopsies, according to the findings of a prospective study.
The study included 180 severely or morbidly obese adults (body mass index >35 kg/m2) with NASH who underwent bariatric surgery at a center in France. Among 94 patients evaluated 5 years later, 68% had follow-up liver biopsies, of whom 84% (95% confidence interval, 73.1%-92.2%) met the primary endpoint of resolution of NASH without worsening of fibrosis. All histologic aspects of NASH had improved, median nonalcoholic fatty liver disease scores (NAS) fell from 5 (interquartile range, 4 to 5) to 1 (IQR, 0-2; P < .001), and 90% of patients achieved at least a 2-point NAS improvement. Hepatocellular ballooning also improved in 87.5% of patients. Baseline severity of NASH did not affect the chances of it resolving at 5 years. “The reduction of fibrosis [was] progressive, beginning during the first year and continuing through 5 years,” Guillaume Lassailly, MD, and associates wrote in Gastroenterology.
NASH is a priority for clinical research because of the substantial risk for subsequent cirrhosis, added Dr. Lassailly of CHU Lille (France). For NASH to resolve, most patients need to lose at least 7%-10% of their body weight, but “only 10% of patients reach this objective with lifestyle therapy at 1 year, and less than half maintain the weight loss 5 years later.” Despite ongoing drug development efforts, no medications have been approved for treating NASH. Although weight loss after bariatric surgery has been reported to resolve NASH in approximately 80% of patients at 1 year, longer-term data have been unavailable, and it has remained unclear whether bariatric surgery can slow or halt fibrosis progression.
All patients in this study had biopsy-confirmed NASH and at least a 5-year history of severe or morbid obesity as well as at least one comorbidity, such as diabetes mellitus or arterial hypertension. Patients were not heavy drinkers, and none had detectable markers of chronic liver disease.
Bariatric surgery produced a median 12-kg/m2 drop in body mass index. At 5-year follow-up, 93% of patients meeting or exceeding this threshold who had biopsies performed showed resolution of NASH without worsening of fibrosis. Furthermore, 56% of patients (95% CI, 42.4%-69.3%) had no histologic evidence of fibrosis, including 45.5% of patients who had bridging fibrosis at baseline.
Participants in this study received intensive preoperative support, including evaluations by numerous specialists, a nutrition plan, and a 6- to 12-month therapeutic education program. Bariatric surgery techniques included Roux-en-Y gastric bypass, gastric banding, and sleeve gastrectomy. A subgroup analysis linked gastric bypass to a significantly higher probability of meeting the primary endpoint, compared with gastric banding. Refusal was the most common reason for not having a follow-up biopsy, the researchers said. “Patients without liver biopsy after bariatric surgery were not significantly different from those with a histological follow-up except for a lower BMI at 1 year. Baseline fibrosis did not influence the probability of undergoing histological reevaluation at 5 years.”
Two study participants died from surgical complications within 1 month after surgery, and one patient died from cardiac dysfunction 4 years later. No fatality was deemed liver related.
The study was funded by the French Ministry of Health, Conseil Régional Nord-Pas de Calais, National de la Recherche, and the European commission (FEDER). The researchers reported having no conflicts of interest.
SOURCE: Lassailly G et al. Gastroenterology. 2020 Jun 15. doi: 10.1053/j.gastro.2020.06.006.
FROM GASTROENTEROLOGY
Small weight loss produces impressive drop in type 2 diabetes risk
Intentional loss of a median of just 13% of body weight reduces the relative risk of developing type 2 diabetes by around 40% in people with obesity, among many other health benefits, shows a large real-world study in half a million adults.
Other findings associated with the same modest weight loss included a reduction in the risk of sleep apnea by 22%-27%, hypertension by 18%-25%, and dyslipidemia by 20%-22%.
Christiane Haase, PhD, of Novo Nordisk, led the work together with Nick Finer, MD, senior principal clinical scientist, Novo Nordisk.
“This is powerful evidence to say it is worthwhile to help people lose weight and that it is hugely beneficial. These are not small effects, and they show that weight loss has a huge impact on health. It’s extraordinary,” Dr. Finer asserted.
“These data show that if we treat obesity first, rather than the complications, we actually get big results in terms of health. This really should be a game-changer for those health care systems that are still prevaricating about treating obesity seriously,” he added.
The size of the study, of over 550,000 U.K. adults in primary care, makes it unique. In the real-world cohort, people who had lost 10%-25% of their body weight were followed for a mean 8 years to see how this affected their subsequent risk of obesity-related conditions. The results were presented during the virtual European and International Congress on Obesity.
“Weight loss was real-world without any artificial intervention and they experienced a real-life reduction in risk of various obesity-related conditions,” Dr. Haase said in an interview.
Carel Le Roux, MD, PhD, from the Diabetes Complications Research Centre, University College Dublin, welcomed the study because it showed those with obesity who maintained more than 10% weight loss experienced a significant reduction in the complications of obesity.
“In the study, intentional weight loss was achieved using mainly diets and exercise, but also some medications and surgical treatments. However, it did not matter how patients were able to maintain the 10% or more weight loss as regards the positive impact on complications of obesity,” he highlighted.
From a clinician standpoint, “it helps to consider all the weight-loss options available, but also for those who are not able to achieve weight-loss maintenance, to escalate treatment. This is now possible as we gain access to more effective treatments,” he added.
Also commenting on the findings, Matt Petersen, vice president of medical information and professional engagement at the American Diabetes Association, said: “It’s helpful to have further evidence that weight loss reduces risk for type 2 diabetes.”
However, “finding effective strategies to achieve and maintain long-term weight loss and maintenance remains a significant challenge,” he observed.
Large database of half a million people with obesity
For the research, anonymized data from over half a million patients documented in the Clinical Practice Research Datalink database, which holds information from 674 general practices in the United Kingdom, were linked to Hospital Episode Statistics and prescribing data to determine comorbidity outcomes.
At baseline, characteristics for the full study population included a median age of 54 years, around 50% of participants had hypertension, around 40% had dyslipidemia, and around 20% had type 2 diabetes. Less than 10% had sleep apnea, hip/knee osteoarthritis, or history of cardiovascular disease. All participants had a body mass index (BMI) of 25.0-50.0 kg/m2 at the start of the follow-up, between January 2001 and December 2010.
Patients may have been advised to lose weight, or take more exercise, or have been referred to a dietitian. Some had been prescribed antiobesity medications available between 2001 and 2010. (Novo Nordisk medications for obesity were unavailable during this period.) Less than 1% had been referred for bariatric surgery.
“This is typical of real-world management of obesity,” Dr. Haase pointed out.
Participants were divided into two categories based on their weight pattern during the 4-year period: one whose weight remained stable (492,380 individuals with BMI change within –5% to 5%) and one who lost weight (60,573 with BMI change –10% to –25%).
The median change in BMI in the weight-loss group was –13%. The researchers also extracted information on weight loss interventions and dietary advice to confirm intention to lose weight.
The benefits of losing 13% of body weight were then determined for three risk profiles: BMI reduction from 34.5 to 30 (obesity class I level); from 40.3 to 35 (obesity class II level), and from46 to 40 (obesity class III level).
Individuals with a baseline history of any particular outcome were excluded from the risk analysis for that same outcome. All analyses were adjusted for BMI, age, gender, smoking status, and baseline comorbidities.
Study strengths include the large number of participants and the relatively long follow-up period. But the observational nature of the study limits the ability to know the ways in which the participants who lost weight may have differed from those who maintained or gained weight, the authors said.
Type 2 diabetes, sleep apnea showed greatest risk reductions
The researchers looked at the risk reduction for various comorbidities after weight loss, compared with before weight loss. They also examined the risk reductions after weight loss, compared with someone who had always had a median 13% lower weight.
Effectively, the analysis provided a measure of the effect of risk reduction because of weight loss, compared with having that lower weight as a stable weight.
“The analysis asks if the person’s risk was reversed by the weight loss to the risk associated with that of the lower weight level,” explained Dr. Haase.
“We found that the risks of type 2 diabetes, dyslipidemia, and hypertension were reversed while the risk of sleep apnea and hip/knee osteoarthritis showed some residual risk,” she added.
With sleep apnea there was a risk reduction of up to 27%, compared with before weight loss.
“This is a condition that can’t be easily reversed except with mechanical sleeping devices and it is underrecognized and causes a lot of distress. There’s actually a link between sleep apnea, diabetes, and hypertension in a two-way connection,” noted Dr. Finer, who is also honorary professor of cardiovascular medicine at University College London.
“A reduction of this proportion is impressive,” he stressed.
Dyslipidemia, hypertension, and type 2 diabetes are well-known cardiovascular risk factors. “We did not see any impact on myocardial infarction,” which “might be due to length of follow-up,” noted Dr. Haase.
Response of type 2 diabetes to weight loss
Most patients in the study did not have type 2 diabetes at baseline, and Dr. Finer commented on how weight loss might affect type 2 diabetes risk.
“The complications of obesity resolve with weight loss at different speeds,” he said.
“Type 2 diabetes is very sensitive to weight loss and improvements are obvious in weeks to months.”
In contrast, reductions in risk of obstructive sleep apnea “take longer and might depend on the amount of weight lost.” And with osteoarthritis, “It’s hard to show improvement with weight loss because irreparable damage has [already] been done,” he explained.
The degree of improvement in diabetes because of weight loss is partly dependent on how long the person has had diabetes, Dr. Finer further explained. “If someone has less excess weight then the diabetes might have had a shorter duration and therefore response might be greater.”
Lucy Chambers, PhD, head of research communications at Diabetes UK, said: “We’ve known for a long time that carrying extra weight can increase your risk of developing type 2 diabetes, and this new study adds to the extensive body of evidence showing that losing some of this weight is associated with reduced risk.”
She acknowledged, however, that losing weight is difficult and that support is important: “We need government to urgently review provision of weight management services and take action to address the barriers to accessing them.”
Dr. Finer and Dr. Haase are both employees of Novo Nordisk. Dr. Le Roux reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Intentional loss of a median of just 13% of body weight reduces the relative risk of developing type 2 diabetes by around 40% in people with obesity, among many other health benefits, shows a large real-world study in half a million adults.
Other findings associated with the same modest weight loss included a reduction in the risk of sleep apnea by 22%-27%, hypertension by 18%-25%, and dyslipidemia by 20%-22%.
Christiane Haase, PhD, of Novo Nordisk, led the work together with Nick Finer, MD, senior principal clinical scientist, Novo Nordisk.
“This is powerful evidence to say it is worthwhile to help people lose weight and that it is hugely beneficial. These are not small effects, and they show that weight loss has a huge impact on health. It’s extraordinary,” Dr. Finer asserted.
“These data show that if we treat obesity first, rather than the complications, we actually get big results in terms of health. This really should be a game-changer for those health care systems that are still prevaricating about treating obesity seriously,” he added.
The size of the study, of over 550,000 U.K. adults in primary care, makes it unique. In the real-world cohort, people who had lost 10%-25% of their body weight were followed for a mean 8 years to see how this affected their subsequent risk of obesity-related conditions. The results were presented during the virtual European and International Congress on Obesity.
“Weight loss was real-world without any artificial intervention and they experienced a real-life reduction in risk of various obesity-related conditions,” Dr. Haase said in an interview.
Carel Le Roux, MD, PhD, from the Diabetes Complications Research Centre, University College Dublin, welcomed the study because it showed those with obesity who maintained more than 10% weight loss experienced a significant reduction in the complications of obesity.
“In the study, intentional weight loss was achieved using mainly diets and exercise, but also some medications and surgical treatments. However, it did not matter how patients were able to maintain the 10% or more weight loss as regards the positive impact on complications of obesity,” he highlighted.
From a clinician standpoint, “it helps to consider all the weight-loss options available, but also for those who are not able to achieve weight-loss maintenance, to escalate treatment. This is now possible as we gain access to more effective treatments,” he added.
Also commenting on the findings, Matt Petersen, vice president of medical information and professional engagement at the American Diabetes Association, said: “It’s helpful to have further evidence that weight loss reduces risk for type 2 diabetes.”
However, “finding effective strategies to achieve and maintain long-term weight loss and maintenance remains a significant challenge,” he observed.
Large database of half a million people with obesity
For the research, anonymized data from over half a million patients documented in the Clinical Practice Research Datalink database, which holds information from 674 general practices in the United Kingdom, were linked to Hospital Episode Statistics and prescribing data to determine comorbidity outcomes.
At baseline, characteristics for the full study population included a median age of 54 years, around 50% of participants had hypertension, around 40% had dyslipidemia, and around 20% had type 2 diabetes. Less than 10% had sleep apnea, hip/knee osteoarthritis, or history of cardiovascular disease. All participants had a body mass index (BMI) of 25.0-50.0 kg/m2 at the start of the follow-up, between January 2001 and December 2010.
Patients may have been advised to lose weight, or take more exercise, or have been referred to a dietitian. Some had been prescribed antiobesity medications available between 2001 and 2010. (Novo Nordisk medications for obesity were unavailable during this period.) Less than 1% had been referred for bariatric surgery.
“This is typical of real-world management of obesity,” Dr. Haase pointed out.
Participants were divided into two categories based on their weight pattern during the 4-year period: one whose weight remained stable (492,380 individuals with BMI change within –5% to 5%) and one who lost weight (60,573 with BMI change –10% to –25%).
The median change in BMI in the weight-loss group was –13%. The researchers also extracted information on weight loss interventions and dietary advice to confirm intention to lose weight.
The benefits of losing 13% of body weight were then determined for three risk profiles: BMI reduction from 34.5 to 30 (obesity class I level); from 40.3 to 35 (obesity class II level), and from46 to 40 (obesity class III level).
Individuals with a baseline history of any particular outcome were excluded from the risk analysis for that same outcome. All analyses were adjusted for BMI, age, gender, smoking status, and baseline comorbidities.
Study strengths include the large number of participants and the relatively long follow-up period. But the observational nature of the study limits the ability to know the ways in which the participants who lost weight may have differed from those who maintained or gained weight, the authors said.
Type 2 diabetes, sleep apnea showed greatest risk reductions
The researchers looked at the risk reduction for various comorbidities after weight loss, compared with before weight loss. They also examined the risk reductions after weight loss, compared with someone who had always had a median 13% lower weight.
Effectively, the analysis provided a measure of the effect of risk reduction because of weight loss, compared with having that lower weight as a stable weight.
“The analysis asks if the person’s risk was reversed by the weight loss to the risk associated with that of the lower weight level,” explained Dr. Haase.
“We found that the risks of type 2 diabetes, dyslipidemia, and hypertension were reversed while the risk of sleep apnea and hip/knee osteoarthritis showed some residual risk,” she added.
With sleep apnea there was a risk reduction of up to 27%, compared with before weight loss.
“This is a condition that can’t be easily reversed except with mechanical sleeping devices and it is underrecognized and causes a lot of distress. There’s actually a link between sleep apnea, diabetes, and hypertension in a two-way connection,” noted Dr. Finer, who is also honorary professor of cardiovascular medicine at University College London.
“A reduction of this proportion is impressive,” he stressed.
Dyslipidemia, hypertension, and type 2 diabetes are well-known cardiovascular risk factors. “We did not see any impact on myocardial infarction,” which “might be due to length of follow-up,” noted Dr. Haase.
Response of type 2 diabetes to weight loss
Most patients in the study did not have type 2 diabetes at baseline, and Dr. Finer commented on how weight loss might affect type 2 diabetes risk.
“The complications of obesity resolve with weight loss at different speeds,” he said.
“Type 2 diabetes is very sensitive to weight loss and improvements are obvious in weeks to months.”
In contrast, reductions in risk of obstructive sleep apnea “take longer and might depend on the amount of weight lost.” And with osteoarthritis, “It’s hard to show improvement with weight loss because irreparable damage has [already] been done,” he explained.
The degree of improvement in diabetes because of weight loss is partly dependent on how long the person has had diabetes, Dr. Finer further explained. “If someone has less excess weight then the diabetes might have had a shorter duration and therefore response might be greater.”
Lucy Chambers, PhD, head of research communications at Diabetes UK, said: “We’ve known for a long time that carrying extra weight can increase your risk of developing type 2 diabetes, and this new study adds to the extensive body of evidence showing that losing some of this weight is associated with reduced risk.”
She acknowledged, however, that losing weight is difficult and that support is important: “We need government to urgently review provision of weight management services and take action to address the barriers to accessing them.”
Dr. Finer and Dr. Haase are both employees of Novo Nordisk. Dr. Le Roux reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Intentional loss of a median of just 13% of body weight reduces the relative risk of developing type 2 diabetes by around 40% in people with obesity, among many other health benefits, shows a large real-world study in half a million adults.
Other findings associated with the same modest weight loss included a reduction in the risk of sleep apnea by 22%-27%, hypertension by 18%-25%, and dyslipidemia by 20%-22%.
Christiane Haase, PhD, of Novo Nordisk, led the work together with Nick Finer, MD, senior principal clinical scientist, Novo Nordisk.
“This is powerful evidence to say it is worthwhile to help people lose weight and that it is hugely beneficial. These are not small effects, and they show that weight loss has a huge impact on health. It’s extraordinary,” Dr. Finer asserted.
“These data show that if we treat obesity first, rather than the complications, we actually get big results in terms of health. This really should be a game-changer for those health care systems that are still prevaricating about treating obesity seriously,” he added.
The size of the study, of over 550,000 U.K. adults in primary care, makes it unique. In the real-world cohort, people who had lost 10%-25% of their body weight were followed for a mean 8 years to see how this affected their subsequent risk of obesity-related conditions. The results were presented during the virtual European and International Congress on Obesity.
“Weight loss was real-world without any artificial intervention and they experienced a real-life reduction in risk of various obesity-related conditions,” Dr. Haase said in an interview.
Carel Le Roux, MD, PhD, from the Diabetes Complications Research Centre, University College Dublin, welcomed the study because it showed those with obesity who maintained more than 10% weight loss experienced a significant reduction in the complications of obesity.
“In the study, intentional weight loss was achieved using mainly diets and exercise, but also some medications and surgical treatments. However, it did not matter how patients were able to maintain the 10% or more weight loss as regards the positive impact on complications of obesity,” he highlighted.
From a clinician standpoint, “it helps to consider all the weight-loss options available, but also for those who are not able to achieve weight-loss maintenance, to escalate treatment. This is now possible as we gain access to more effective treatments,” he added.
Also commenting on the findings, Matt Petersen, vice president of medical information and professional engagement at the American Diabetes Association, said: “It’s helpful to have further evidence that weight loss reduces risk for type 2 diabetes.”
However, “finding effective strategies to achieve and maintain long-term weight loss and maintenance remains a significant challenge,” he observed.
Large database of half a million people with obesity
For the research, anonymized data from over half a million patients documented in the Clinical Practice Research Datalink database, which holds information from 674 general practices in the United Kingdom, were linked to Hospital Episode Statistics and prescribing data to determine comorbidity outcomes.
At baseline, characteristics for the full study population included a median age of 54 years, around 50% of participants had hypertension, around 40% had dyslipidemia, and around 20% had type 2 diabetes. Less than 10% had sleep apnea, hip/knee osteoarthritis, or history of cardiovascular disease. All participants had a body mass index (BMI) of 25.0-50.0 kg/m2 at the start of the follow-up, between January 2001 and December 2010.
Patients may have been advised to lose weight, or take more exercise, or have been referred to a dietitian. Some had been prescribed antiobesity medications available between 2001 and 2010. (Novo Nordisk medications for obesity were unavailable during this period.) Less than 1% had been referred for bariatric surgery.
“This is typical of real-world management of obesity,” Dr. Haase pointed out.
Participants were divided into two categories based on their weight pattern during the 4-year period: one whose weight remained stable (492,380 individuals with BMI change within –5% to 5%) and one who lost weight (60,573 with BMI change –10% to –25%).
The median change in BMI in the weight-loss group was –13%. The researchers also extracted information on weight loss interventions and dietary advice to confirm intention to lose weight.
The benefits of losing 13% of body weight were then determined for three risk profiles: BMI reduction from 34.5 to 30 (obesity class I level); from 40.3 to 35 (obesity class II level), and from46 to 40 (obesity class III level).
Individuals with a baseline history of any particular outcome were excluded from the risk analysis for that same outcome. All analyses were adjusted for BMI, age, gender, smoking status, and baseline comorbidities.
Study strengths include the large number of participants and the relatively long follow-up period. But the observational nature of the study limits the ability to know the ways in which the participants who lost weight may have differed from those who maintained or gained weight, the authors said.
Type 2 diabetes, sleep apnea showed greatest risk reductions
The researchers looked at the risk reduction for various comorbidities after weight loss, compared with before weight loss. They also examined the risk reductions after weight loss, compared with someone who had always had a median 13% lower weight.
Effectively, the analysis provided a measure of the effect of risk reduction because of weight loss, compared with having that lower weight as a stable weight.
“The analysis asks if the person’s risk was reversed by the weight loss to the risk associated with that of the lower weight level,” explained Dr. Haase.
“We found that the risks of type 2 diabetes, dyslipidemia, and hypertension were reversed while the risk of sleep apnea and hip/knee osteoarthritis showed some residual risk,” she added.
With sleep apnea there was a risk reduction of up to 27%, compared with before weight loss.
“This is a condition that can’t be easily reversed except with mechanical sleeping devices and it is underrecognized and causes a lot of distress. There’s actually a link between sleep apnea, diabetes, and hypertension in a two-way connection,” noted Dr. Finer, who is also honorary professor of cardiovascular medicine at University College London.
“A reduction of this proportion is impressive,” he stressed.
Dyslipidemia, hypertension, and type 2 diabetes are well-known cardiovascular risk factors. “We did not see any impact on myocardial infarction,” which “might be due to length of follow-up,” noted Dr. Haase.
Response of type 2 diabetes to weight loss
Most patients in the study did not have type 2 diabetes at baseline, and Dr. Finer commented on how weight loss might affect type 2 diabetes risk.
“The complications of obesity resolve with weight loss at different speeds,” he said.
“Type 2 diabetes is very sensitive to weight loss and improvements are obvious in weeks to months.”
In contrast, reductions in risk of obstructive sleep apnea “take longer and might depend on the amount of weight lost.” And with osteoarthritis, “It’s hard to show improvement with weight loss because irreparable damage has [already] been done,” he explained.
The degree of improvement in diabetes because of weight loss is partly dependent on how long the person has had diabetes, Dr. Finer further explained. “If someone has less excess weight then the diabetes might have had a shorter duration and therefore response might be greater.”
Lucy Chambers, PhD, head of research communications at Diabetes UK, said: “We’ve known for a long time that carrying extra weight can increase your risk of developing type 2 diabetes, and this new study adds to the extensive body of evidence showing that losing some of this weight is associated with reduced risk.”
She acknowledged, however, that losing weight is difficult and that support is important: “We need government to urgently review provision of weight management services and take action to address the barriers to accessing them.”
Dr. Finer and Dr. Haase are both employees of Novo Nordisk. Dr. Le Roux reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Obesity-related hypoventilation increased morbidity risk after bariatric surgery
Patients with obesity-associated sleep hypoventilation had a heightened risk of postoperative morbidities after bariatric surgery, according to a retrospective study.
Reena Mehra, MD, director of sleep disorders research for the Sleep Disorders Center at the Cleveland Clinic, led the team and the findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies. Her research team examined the outcomes of 1,665 patients who underwent polysomnography prior to bariatric surgery performed at the Cleveland Clinic from 2011 to 2018.
More than two-thirds – 68.5% – had obesity-associated sleep hypoventilation as defined by body mass index (BMI) of ≥30 kg/m2 and either polysomnography-based end-tidal CO2 ≥45 mm Hg or serum bicarbonate ≥27 mEq/L.
These patients represent “a subset, if you will, of obesity hypoventilation syndrome – a subset that we were able to capture from our sleep studies … [because] we do CO2 monitoring during sleep studies uniformly,” Dr. Mehra said in an interview after the meeting.
Pornprapa Chindamporn, MD, a former fellow at the center and first author on the abstract, presented the findings. Patients in the study had a mean age of 45.2 ± 12.0 years and a BMI of 48.7 ± 9.0. Approximately 20% were male and 63.6% were White.
Those with obesity-associated sleep hypoventilation were more likely to be male and have a higher BMI and higher hemoglobin A1c than those without the condition. They also had a significantly higher apnea-hypopnea index (17.0 vs. 13.8) in those without the condition, she reported.
A number of outcomes (ICU stay, intubation, tracheostomy, discharge disposition, and 30-day readmission) were compared individually and as a composite outcome between those with and without obesity-associated sleep hypoventilation. While some of these postoperative morbidities were more common in patients with the condition, the differences between those with and without OHS were not statistically significant for intubation (1.5% vs. 1.3%, P = .81) and 30-day readmission (13.8% vs. 11.3%, P = .16). However, the composite outcome was significantly higher: 18.9% vs. 14.3% (P = .021), including in multivariable analysis that considered age, gender, BMI, Apnea Hypopnea Index, and diabetes.
All-cause mortality was not significantly different between the groups, likely because of its low overall rate (hazard ratio, 1.39; 95% confidence interval, 0.56-3.42).
“In this largest sample to date of systematically phenotyped obesity-associated sleep hypoventilation in patients undergoing bariatric surgery, we identified increased postoperative morbidity,” said Dr. Chindamporn, now a pulmonologist and sleep specialist practicing in Bangkok.
Dr. Mehra said in the interview that patients considering bariatric surgery are typically assessed for obstructive sleep apnea, but “not so much obesity hypoventilation syndrome or obesity-associated sleep-related hypoventilation syndrome.” The findings, “support the notion that we should be closely examining sleep-related hypoventilation in these patients.”
At the Cleveland Clinic, “clinically, we make sure we’re identifying these individuals and communicating the findings to bariatric surgery colleagues and to anesthesia,” said Dr. Mehra, also professor of medicine at Case Western Reserve University, Cleveland.
OHS is defined, according to the 2019 American Thoracic Society clinical practice guideline on evaluation and management of OHS, by the combination of obesity, sleep-disordered breathing, and awake daytime hypercapnia, after excluding other causes for hypoventilation (Am J Respir Crit Care Med. 2019;200[3]:e6-24).
A European Respiratory Society task force has proposed severity grading for OHS, with early stages defined by sleep-related hypoventilation and the highest grade of severity defined by morbidity-associated daytime hypercapnia (Eur Respir Rev. 2019;28:180097). However, Dr. Mehra said she is “not sure that we know enough [from long-term studies of OHS] to say definitively that there’s such an evolution.”
Certainly, she said, future research on OHS should consider its heterogeneity. It is possible that a subset of patients with OHS, “maybe these individuals with sleep-related hypoventilation,” are most likely to have adverse postsurgical outcomes.
Atul Malhotra, MD, professor of medicine at the University of California, San Diego, who was asked to comment on the study, said that OHS is understudied in general and particularly in the perioperative setting. “With the obesity pandemic, issues around OHS are likely to be [increasingly] important. And with increasing [use of] bariatric surgery, strategies to minimize risks are clearly needed,” he said, adding that the potential risks of nonbariatric surgery in patients with OHS require further study.
He noted that mortality rates in good hospitals “have become quite low for many elective surgeries, making it hard to show mortality benefit to most interventions.”
The ATS guideline on OHS states that it is the most severe form of obesity-induced respiratory compromise and leads to serious sequelae, including increased rates of mortality, chronic heart failure, pulmonary hypertension, and hospitalization caused by acute-on-chronic hypercapnic respiratory failure.
Dr. Chindamporn said in her presentation that she had no disclosures. Dr. Mehra’s research program is funded by the National Institute of Health, but she has also procured funding from the American College of Chest Physicians, American Heart Association, Clinical Translational Science Collaborative, and Central Society of Clinical Research. Dr. Malhotra disclosed that he is funded by the NIH and has received income from Merck and LIvanova related to medical education.
CORRECTION 9/15/2020: The original story misstated the presenter of the study. Dr. Chindamporn presented the findings.
Patients with obesity-associated sleep hypoventilation had a heightened risk of postoperative morbidities after bariatric surgery, according to a retrospective study.
Reena Mehra, MD, director of sleep disorders research for the Sleep Disorders Center at the Cleveland Clinic, led the team and the findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies. Her research team examined the outcomes of 1,665 patients who underwent polysomnography prior to bariatric surgery performed at the Cleveland Clinic from 2011 to 2018.
More than two-thirds – 68.5% – had obesity-associated sleep hypoventilation as defined by body mass index (BMI) of ≥30 kg/m2 and either polysomnography-based end-tidal CO2 ≥45 mm Hg or serum bicarbonate ≥27 mEq/L.
These patients represent “a subset, if you will, of obesity hypoventilation syndrome – a subset that we were able to capture from our sleep studies … [because] we do CO2 monitoring during sleep studies uniformly,” Dr. Mehra said in an interview after the meeting.
Pornprapa Chindamporn, MD, a former fellow at the center and first author on the abstract, presented the findings. Patients in the study had a mean age of 45.2 ± 12.0 years and a BMI of 48.7 ± 9.0. Approximately 20% were male and 63.6% were White.
Those with obesity-associated sleep hypoventilation were more likely to be male and have a higher BMI and higher hemoglobin A1c than those without the condition. They also had a significantly higher apnea-hypopnea index (17.0 vs. 13.8) in those without the condition, she reported.
A number of outcomes (ICU stay, intubation, tracheostomy, discharge disposition, and 30-day readmission) were compared individually and as a composite outcome between those with and without obesity-associated sleep hypoventilation. While some of these postoperative morbidities were more common in patients with the condition, the differences between those with and without OHS were not statistically significant for intubation (1.5% vs. 1.3%, P = .81) and 30-day readmission (13.8% vs. 11.3%, P = .16). However, the composite outcome was significantly higher: 18.9% vs. 14.3% (P = .021), including in multivariable analysis that considered age, gender, BMI, Apnea Hypopnea Index, and diabetes.
All-cause mortality was not significantly different between the groups, likely because of its low overall rate (hazard ratio, 1.39; 95% confidence interval, 0.56-3.42).
“In this largest sample to date of systematically phenotyped obesity-associated sleep hypoventilation in patients undergoing bariatric surgery, we identified increased postoperative morbidity,” said Dr. Chindamporn, now a pulmonologist and sleep specialist practicing in Bangkok.
Dr. Mehra said in the interview that patients considering bariatric surgery are typically assessed for obstructive sleep apnea, but “not so much obesity hypoventilation syndrome or obesity-associated sleep-related hypoventilation syndrome.” The findings, “support the notion that we should be closely examining sleep-related hypoventilation in these patients.”
At the Cleveland Clinic, “clinically, we make sure we’re identifying these individuals and communicating the findings to bariatric surgery colleagues and to anesthesia,” said Dr. Mehra, also professor of medicine at Case Western Reserve University, Cleveland.
OHS is defined, according to the 2019 American Thoracic Society clinical practice guideline on evaluation and management of OHS, by the combination of obesity, sleep-disordered breathing, and awake daytime hypercapnia, after excluding other causes for hypoventilation (Am J Respir Crit Care Med. 2019;200[3]:e6-24).
A European Respiratory Society task force has proposed severity grading for OHS, with early stages defined by sleep-related hypoventilation and the highest grade of severity defined by morbidity-associated daytime hypercapnia (Eur Respir Rev. 2019;28:180097). However, Dr. Mehra said she is “not sure that we know enough [from long-term studies of OHS] to say definitively that there’s such an evolution.”
Certainly, she said, future research on OHS should consider its heterogeneity. It is possible that a subset of patients with OHS, “maybe these individuals with sleep-related hypoventilation,” are most likely to have adverse postsurgical outcomes.
Atul Malhotra, MD, professor of medicine at the University of California, San Diego, who was asked to comment on the study, said that OHS is understudied in general and particularly in the perioperative setting. “With the obesity pandemic, issues around OHS are likely to be [increasingly] important. And with increasing [use of] bariatric surgery, strategies to minimize risks are clearly needed,” he said, adding that the potential risks of nonbariatric surgery in patients with OHS require further study.
He noted that mortality rates in good hospitals “have become quite low for many elective surgeries, making it hard to show mortality benefit to most interventions.”
The ATS guideline on OHS states that it is the most severe form of obesity-induced respiratory compromise and leads to serious sequelae, including increased rates of mortality, chronic heart failure, pulmonary hypertension, and hospitalization caused by acute-on-chronic hypercapnic respiratory failure.
Dr. Chindamporn said in her presentation that she had no disclosures. Dr. Mehra’s research program is funded by the National Institute of Health, but she has also procured funding from the American College of Chest Physicians, American Heart Association, Clinical Translational Science Collaborative, and Central Society of Clinical Research. Dr. Malhotra disclosed that he is funded by the NIH and has received income from Merck and LIvanova related to medical education.
CORRECTION 9/15/2020: The original story misstated the presenter of the study. Dr. Chindamporn presented the findings.
Patients with obesity-associated sleep hypoventilation had a heightened risk of postoperative morbidities after bariatric surgery, according to a retrospective study.
Reena Mehra, MD, director of sleep disorders research for the Sleep Disorders Center at the Cleveland Clinic, led the team and the findings were presented at the virtual annual meeting of the Associated Professional Sleep Societies. Her research team examined the outcomes of 1,665 patients who underwent polysomnography prior to bariatric surgery performed at the Cleveland Clinic from 2011 to 2018.
More than two-thirds – 68.5% – had obesity-associated sleep hypoventilation as defined by body mass index (BMI) of ≥30 kg/m2 and either polysomnography-based end-tidal CO2 ≥45 mm Hg or serum bicarbonate ≥27 mEq/L.
These patients represent “a subset, if you will, of obesity hypoventilation syndrome – a subset that we were able to capture from our sleep studies … [because] we do CO2 monitoring during sleep studies uniformly,” Dr. Mehra said in an interview after the meeting.
Pornprapa Chindamporn, MD, a former fellow at the center and first author on the abstract, presented the findings. Patients in the study had a mean age of 45.2 ± 12.0 years and a BMI of 48.7 ± 9.0. Approximately 20% were male and 63.6% were White.
Those with obesity-associated sleep hypoventilation were more likely to be male and have a higher BMI and higher hemoglobin A1c than those without the condition. They also had a significantly higher apnea-hypopnea index (17.0 vs. 13.8) in those without the condition, she reported.
A number of outcomes (ICU stay, intubation, tracheostomy, discharge disposition, and 30-day readmission) were compared individually and as a composite outcome between those with and without obesity-associated sleep hypoventilation. While some of these postoperative morbidities were more common in patients with the condition, the differences between those with and without OHS were not statistically significant for intubation (1.5% vs. 1.3%, P = .81) and 30-day readmission (13.8% vs. 11.3%, P = .16). However, the composite outcome was significantly higher: 18.9% vs. 14.3% (P = .021), including in multivariable analysis that considered age, gender, BMI, Apnea Hypopnea Index, and diabetes.
All-cause mortality was not significantly different between the groups, likely because of its low overall rate (hazard ratio, 1.39; 95% confidence interval, 0.56-3.42).
“In this largest sample to date of systematically phenotyped obesity-associated sleep hypoventilation in patients undergoing bariatric surgery, we identified increased postoperative morbidity,” said Dr. Chindamporn, now a pulmonologist and sleep specialist practicing in Bangkok.
Dr. Mehra said in the interview that patients considering bariatric surgery are typically assessed for obstructive sleep apnea, but “not so much obesity hypoventilation syndrome or obesity-associated sleep-related hypoventilation syndrome.” The findings, “support the notion that we should be closely examining sleep-related hypoventilation in these patients.”
At the Cleveland Clinic, “clinically, we make sure we’re identifying these individuals and communicating the findings to bariatric surgery colleagues and to anesthesia,” said Dr. Mehra, also professor of medicine at Case Western Reserve University, Cleveland.
OHS is defined, according to the 2019 American Thoracic Society clinical practice guideline on evaluation and management of OHS, by the combination of obesity, sleep-disordered breathing, and awake daytime hypercapnia, after excluding other causes for hypoventilation (Am J Respir Crit Care Med. 2019;200[3]:e6-24).
A European Respiratory Society task force has proposed severity grading for OHS, with early stages defined by sleep-related hypoventilation and the highest grade of severity defined by morbidity-associated daytime hypercapnia (Eur Respir Rev. 2019;28:180097). However, Dr. Mehra said she is “not sure that we know enough [from long-term studies of OHS] to say definitively that there’s such an evolution.”
Certainly, she said, future research on OHS should consider its heterogeneity. It is possible that a subset of patients with OHS, “maybe these individuals with sleep-related hypoventilation,” are most likely to have adverse postsurgical outcomes.
Atul Malhotra, MD, professor of medicine at the University of California, San Diego, who was asked to comment on the study, said that OHS is understudied in general and particularly in the perioperative setting. “With the obesity pandemic, issues around OHS are likely to be [increasingly] important. And with increasing [use of] bariatric surgery, strategies to minimize risks are clearly needed,” he said, adding that the potential risks of nonbariatric surgery in patients with OHS require further study.
He noted that mortality rates in good hospitals “have become quite low for many elective surgeries, making it hard to show mortality benefit to most interventions.”
The ATS guideline on OHS states that it is the most severe form of obesity-induced respiratory compromise and leads to serious sequelae, including increased rates of mortality, chronic heart failure, pulmonary hypertension, and hospitalization caused by acute-on-chronic hypercapnic respiratory failure.
Dr. Chindamporn said in her presentation that she had no disclosures. Dr. Mehra’s research program is funded by the National Institute of Health, but she has also procured funding from the American College of Chest Physicians, American Heart Association, Clinical Translational Science Collaborative, and Central Society of Clinical Research. Dr. Malhotra disclosed that he is funded by the NIH and has received income from Merck and LIvanova related to medical education.
CORRECTION 9/15/2020: The original story misstated the presenter of the study. Dr. Chindamporn presented the findings.
FROM SLEEP 2020
Obesity boosts risks in COVID-19 from diagnosis to death
A new analysis of existing research confirms a stark link between excess weight and COVID-19:
Obese patients faced the greatest bump in risk on the hospitalization front, with their odds of being admitted listed as 113% higher. The odds of diagnosis, ICU admission, and death were 46% higher (odds ratio [OR], 1.46; 95% confidence interval [CI], 1.30-1.65; P < .0001); 74% higher (OR, 1.74, CI, 1.46-2.08, P < .0001); 48% (OR, 1.48, CI, 1.22–1.80, P < .001, all pooled analyses and 95% CI), respectively. All differences were highly significantly different, investigators reported in a systematic review and meta-analysis published online Aug. 26 in Obesity Reviews.
“Essentially, these are pretty scary statistics,” nutrition researcher and study lead author Barry M. Popkin, PhD, of the University of North Carolina at Chapel Hill School of Public Health, said in an interview. “Other studies have talked about an increase in mortality, and we were thinking there’d be a little increase like 10% – nothing like 48%.”
According to the Johns Hopkins University of Medicine tracker, nearly 6 million people in the United States had been diagnosed with COVID-19 as of Aug. 30. The number of deaths had surpassed 183,000.
The authors of the new review launched their project to better understand the link between obesity and COVID-19 “all the way from being diagnosed to death,” Dr. Popkin said, adding that the meta-analysis is the largest of its kind to examine the link.
Dr. Popkin and colleagues analyzed 75 studies during January to June 2020 that tracked 399,461 patients (55% of whom were male) diagnosed with COVID-19. They found that 18 of 20 studies linked obesity with a 46% higher risk of diagnosis, but Dr. Popkin cautioned that this may be misleading. “I suspect it’s because they’re sicker and getting tested more for COVID,” he said. “I don’t think obesity enhances your likelihood of getting COVID. We don’t have a biological rationale for that.”
The researchers examined 19 studies that explored a link between obesity and hospitalization; all 19 found a higher risk of hospitalization in patients with obesity (pooled OR, 2.13). Twenty-one of 22 studies that looked at ICU admissions discovered a higher risk for patients with obesity (pooled OR, 1.74). And 27 of 35 studies that examined COVID-19 mortality found a higher death rate in patients with obesity (pooled OR, 1.48).
The review also looked at 14 studies that examined links between obesity and administration of invasive mechanical ventilation. All the studies found a higher risk for patients with obesity (pooled OR, 1.66; 95% CI, 1.38-1.99; P < .0001).
Could socioeconomic factors explain the difference in risk for people with obesity? It’s not clear. According to Dr. Popkin, most of the studies don’t examine factors such as income. While he believes physical factors are the key to the higher risk, he said “there’s clearly a social side to this.”
On the biological front, it appears that “the immune system is much weaker if you’re obese,” he said, and excess weight may worsen the course of a respiratory disease such as COVID-19 because of lung disorders such as sleep apnea.
In addition to highlighting inflammation and a weakened immune system, the review offers multiple explanations for why patients with obesity face worse outcomes in COVID-19. It may be more difficult for medical professionals to care for them in the hospital because of their weight, the authors wrote, and “obesity may also impair therapeutic treatments during COVID-19 infections.” The authors noted that ACE inhibitors may worsen COVID-19 in patients with type 2 diabetes.
The researchers noted that “potentially the vaccines developed to address COVID-19 will be less effective for individuals with obesity due to a weakened immune response.” They pointed to research that suggests T-cell responses are weaker and antibody titers wane at a faster rate in people with obesity who are vaccinated against influenza.
Pulmonologist Joshua L. Denson, MD, MS, of Tulane University, New Orleans, praised the review in an interview, but noted that some of the included studies have wide confidence intervals. One study that links COVID-19 to a sixfold higher mortality rate (OR, 6.29) has a confidence interval of 1.76-22.45.
Dr. Denson said he’s seen about 100 patients with COVID-19, and many are obese and have metabolic syndrome.
Like the authors of the study, he believes higher levels of inflammation play a crucial role in making these patients more vulnerable. “For whatever reason, the virus tends to really like that state. That’s driving these people to get sick,” he said.
Moving forward, Dr. Popkin urged physicians to redouble their efforts to warn patients about the risks of obesity and the importance of healthy eating. He also said COVID-19 vaccine researchers must stratify obese vs. nonobese subjects in clinical trials.
The review was funded by Bloomberg Philanthropies, the Carolina Population Center, World Bank, and Saudi Health Council. The review authors report no relevant disclosures. Dr. Denson reports no relevant disclosures.
SOURCE: Popkin BM et al. Obes Rev. 2020 Aug 26. doi: 10.1111/obr.13128.
A new analysis of existing research confirms a stark link between excess weight and COVID-19:
Obese patients faced the greatest bump in risk on the hospitalization front, with their odds of being admitted listed as 113% higher. The odds of diagnosis, ICU admission, and death were 46% higher (odds ratio [OR], 1.46; 95% confidence interval [CI], 1.30-1.65; P < .0001); 74% higher (OR, 1.74, CI, 1.46-2.08, P < .0001); 48% (OR, 1.48, CI, 1.22–1.80, P < .001, all pooled analyses and 95% CI), respectively. All differences were highly significantly different, investigators reported in a systematic review and meta-analysis published online Aug. 26 in Obesity Reviews.
“Essentially, these are pretty scary statistics,” nutrition researcher and study lead author Barry M. Popkin, PhD, of the University of North Carolina at Chapel Hill School of Public Health, said in an interview. “Other studies have talked about an increase in mortality, and we were thinking there’d be a little increase like 10% – nothing like 48%.”
According to the Johns Hopkins University of Medicine tracker, nearly 6 million people in the United States had been diagnosed with COVID-19 as of Aug. 30. The number of deaths had surpassed 183,000.
The authors of the new review launched their project to better understand the link between obesity and COVID-19 “all the way from being diagnosed to death,” Dr. Popkin said, adding that the meta-analysis is the largest of its kind to examine the link.
Dr. Popkin and colleagues analyzed 75 studies during January to June 2020 that tracked 399,461 patients (55% of whom were male) diagnosed with COVID-19. They found that 18 of 20 studies linked obesity with a 46% higher risk of diagnosis, but Dr. Popkin cautioned that this may be misleading. “I suspect it’s because they’re sicker and getting tested more for COVID,” he said. “I don’t think obesity enhances your likelihood of getting COVID. We don’t have a biological rationale for that.”
The researchers examined 19 studies that explored a link between obesity and hospitalization; all 19 found a higher risk of hospitalization in patients with obesity (pooled OR, 2.13). Twenty-one of 22 studies that looked at ICU admissions discovered a higher risk for patients with obesity (pooled OR, 1.74). And 27 of 35 studies that examined COVID-19 mortality found a higher death rate in patients with obesity (pooled OR, 1.48).
The review also looked at 14 studies that examined links between obesity and administration of invasive mechanical ventilation. All the studies found a higher risk for patients with obesity (pooled OR, 1.66; 95% CI, 1.38-1.99; P < .0001).
Could socioeconomic factors explain the difference in risk for people with obesity? It’s not clear. According to Dr. Popkin, most of the studies don’t examine factors such as income. While he believes physical factors are the key to the higher risk, he said “there’s clearly a social side to this.”
On the biological front, it appears that “the immune system is much weaker if you’re obese,” he said, and excess weight may worsen the course of a respiratory disease such as COVID-19 because of lung disorders such as sleep apnea.
In addition to highlighting inflammation and a weakened immune system, the review offers multiple explanations for why patients with obesity face worse outcomes in COVID-19. It may be more difficult for medical professionals to care for them in the hospital because of their weight, the authors wrote, and “obesity may also impair therapeutic treatments during COVID-19 infections.” The authors noted that ACE inhibitors may worsen COVID-19 in patients with type 2 diabetes.
The researchers noted that “potentially the vaccines developed to address COVID-19 will be less effective for individuals with obesity due to a weakened immune response.” They pointed to research that suggests T-cell responses are weaker and antibody titers wane at a faster rate in people with obesity who are vaccinated against influenza.
Pulmonologist Joshua L. Denson, MD, MS, of Tulane University, New Orleans, praised the review in an interview, but noted that some of the included studies have wide confidence intervals. One study that links COVID-19 to a sixfold higher mortality rate (OR, 6.29) has a confidence interval of 1.76-22.45.
Dr. Denson said he’s seen about 100 patients with COVID-19, and many are obese and have metabolic syndrome.
Like the authors of the study, he believes higher levels of inflammation play a crucial role in making these patients more vulnerable. “For whatever reason, the virus tends to really like that state. That’s driving these people to get sick,” he said.
Moving forward, Dr. Popkin urged physicians to redouble their efforts to warn patients about the risks of obesity and the importance of healthy eating. He also said COVID-19 vaccine researchers must stratify obese vs. nonobese subjects in clinical trials.
The review was funded by Bloomberg Philanthropies, the Carolina Population Center, World Bank, and Saudi Health Council. The review authors report no relevant disclosures. Dr. Denson reports no relevant disclosures.
SOURCE: Popkin BM et al. Obes Rev. 2020 Aug 26. doi: 10.1111/obr.13128.
A new analysis of existing research confirms a stark link between excess weight and COVID-19:
Obese patients faced the greatest bump in risk on the hospitalization front, with their odds of being admitted listed as 113% higher. The odds of diagnosis, ICU admission, and death were 46% higher (odds ratio [OR], 1.46; 95% confidence interval [CI], 1.30-1.65; P < .0001); 74% higher (OR, 1.74, CI, 1.46-2.08, P < .0001); 48% (OR, 1.48, CI, 1.22–1.80, P < .001, all pooled analyses and 95% CI), respectively. All differences were highly significantly different, investigators reported in a systematic review and meta-analysis published online Aug. 26 in Obesity Reviews.
“Essentially, these are pretty scary statistics,” nutrition researcher and study lead author Barry M. Popkin, PhD, of the University of North Carolina at Chapel Hill School of Public Health, said in an interview. “Other studies have talked about an increase in mortality, and we were thinking there’d be a little increase like 10% – nothing like 48%.”
According to the Johns Hopkins University of Medicine tracker, nearly 6 million people in the United States had been diagnosed with COVID-19 as of Aug. 30. The number of deaths had surpassed 183,000.
The authors of the new review launched their project to better understand the link between obesity and COVID-19 “all the way from being diagnosed to death,” Dr. Popkin said, adding that the meta-analysis is the largest of its kind to examine the link.
Dr. Popkin and colleagues analyzed 75 studies during January to June 2020 that tracked 399,461 patients (55% of whom were male) diagnosed with COVID-19. They found that 18 of 20 studies linked obesity with a 46% higher risk of diagnosis, but Dr. Popkin cautioned that this may be misleading. “I suspect it’s because they’re sicker and getting tested more for COVID,” he said. “I don’t think obesity enhances your likelihood of getting COVID. We don’t have a biological rationale for that.”
The researchers examined 19 studies that explored a link between obesity and hospitalization; all 19 found a higher risk of hospitalization in patients with obesity (pooled OR, 2.13). Twenty-one of 22 studies that looked at ICU admissions discovered a higher risk for patients with obesity (pooled OR, 1.74). And 27 of 35 studies that examined COVID-19 mortality found a higher death rate in patients with obesity (pooled OR, 1.48).
The review also looked at 14 studies that examined links between obesity and administration of invasive mechanical ventilation. All the studies found a higher risk for patients with obesity (pooled OR, 1.66; 95% CI, 1.38-1.99; P < .0001).
Could socioeconomic factors explain the difference in risk for people with obesity? It’s not clear. According to Dr. Popkin, most of the studies don’t examine factors such as income. While he believes physical factors are the key to the higher risk, he said “there’s clearly a social side to this.”
On the biological front, it appears that “the immune system is much weaker if you’re obese,” he said, and excess weight may worsen the course of a respiratory disease such as COVID-19 because of lung disorders such as sleep apnea.
In addition to highlighting inflammation and a weakened immune system, the review offers multiple explanations for why patients with obesity face worse outcomes in COVID-19. It may be more difficult for medical professionals to care for them in the hospital because of their weight, the authors wrote, and “obesity may also impair therapeutic treatments during COVID-19 infections.” The authors noted that ACE inhibitors may worsen COVID-19 in patients with type 2 diabetes.
The researchers noted that “potentially the vaccines developed to address COVID-19 will be less effective for individuals with obesity due to a weakened immune response.” They pointed to research that suggests T-cell responses are weaker and antibody titers wane at a faster rate in people with obesity who are vaccinated against influenza.
Pulmonologist Joshua L. Denson, MD, MS, of Tulane University, New Orleans, praised the review in an interview, but noted that some of the included studies have wide confidence intervals. One study that links COVID-19 to a sixfold higher mortality rate (OR, 6.29) has a confidence interval of 1.76-22.45.
Dr. Denson said he’s seen about 100 patients with COVID-19, and many are obese and have metabolic syndrome.
Like the authors of the study, he believes higher levels of inflammation play a crucial role in making these patients more vulnerable. “For whatever reason, the virus tends to really like that state. That’s driving these people to get sick,” he said.
Moving forward, Dr. Popkin urged physicians to redouble their efforts to warn patients about the risks of obesity and the importance of healthy eating. He also said COVID-19 vaccine researchers must stratify obese vs. nonobese subjects in clinical trials.
The review was funded by Bloomberg Philanthropies, the Carolina Population Center, World Bank, and Saudi Health Council. The review authors report no relevant disclosures. Dr. Denson reports no relevant disclosures.
SOURCE: Popkin BM et al. Obes Rev. 2020 Aug 26. doi: 10.1111/obr.13128.
FROM OBESITY REVIEWS







