User login
Depression screens do not reduce suicidal acts in teens: Study
Screening adolescents for signs of depression does not reduce their emergency department visits, hospitalizations, or treatment for suicidal behaviors, according to research published in Preventive Medicine. Adolescents who underwent a depression screening were just as likely to need these services as those who did not.
In 2016, the U.S. Preventive Services Task Force recommended that adolescents aged 12-18 years be screened for major depressive disorder, provided that effective treatment options and follow-up strategies are in place.
“The main goal of depression screening is really to reduce adverse psychiatric outcomes. But I think a collateral hope is that, in reducing these adverse psychiatric outcomes, you would also reduce avoidable health services use,” such as ED visits or hospitalizations, said Kira Riehm, PhD, a postdoctoral fellow in epidemiology at Columbia University, New York, who led the research. Dr. Riehm designed the new study, which was part of her doctoral work at Johns Hopkins University, Baltimore, to test this premise.
Dr. Riehm and colleagues compared 14,433 adolescents aged 12-18 years who were screened for depression at least once during a wellness visit from 2014 to 2017 to 43,299 adolescents who were not screened for depression during such visits. Depression screenings were interspersed among a total of 281,463 adolescent wellness visits from 2014 to 2017, which represented 5% of all visits.
The researchers used diagnostic codes from a database of insurance claims to determine who had undergone depression screening. They then compared use of ED services, inpatient hospitalizations, and the number of treatments for suicidal behaviors between the two groups for the 2 years following the wellness visit.
The average age of the adolescents who underwent screening was 13-14 years, as was the average age of adolescents who were not screened. Both groups were evenly matched with respect to being male or female.
The researchers estimated that a high majority of adolescents in the sample were White (83%). Black persons represented 7% of the sample; Hispanic/Latino, 5%; and Asian, 3%. Insurance claims don’t always include racial and ethnicity data, Dr. Riehm said, so her group statistically imputed these proportions. The claims data also do not include details about which type of screening tool was used or the results of the screening, such as whether a teen exhibited mild or severe depression.
Adolescents in both groups were just as likely to go to the ED for any reason, be admitted to the hospital for any reason, or undergo treatment for suicidal behaviors. The researchers observed a slight association between being screened for depression and going to the ED specifically for a mental health reason (relative risk, 1.16; 95% confidence interval, 1.00-1.33). The sex of the adolescents had no bearing on whether they used these services.
“I think people think of [depression screening] as one event. But in reality, screening is a series of different events that all have to be happening in order for a screening program to work,” Dr. Riehm told this news organization.
These events could include ensuring that adolescents who exhibit signs of depression receive a proper assessment, receive medications if needed, and have access to psychotherapists who can help them. Without these supports in place, she said, a one-off depression screening may have limited benefit.
“There’s a lot of places where people could drop out of that care continuum,” Dr. Riehm said.
“One-time screening may not be enough,” said Trân Đoàn, PhD, MPH, a postdoctoral researcher in the University of Pittsburgh department of pediatrics.
Dr. Đoàn, who was not involved in the research, noted that the American Academy of Pediatrics recommends annual screening of all adolescents for depressive symptoms. Given that only 5% of the visits in this sample included any kind of depression screening, Dr. Đoàn said, some pediatric practices may not have felt they had the resources to adequately address positive screenings for depression.
Both Dr. Riehm and Dr. Đoàn are focusing on the link between depression screening and health outcomes. In her own doctoral work at the University of Michigan, Dr. Đoàn modeled the effects of universal annual depression screening in primary care settings on the health status of people aged 12-22 years. She is currently preparing this work for publication.
“I did find that, over the long term, there is improvement in health outcomes if we were to screen on an annual basis,” provided improved screening is coupled with comprehensive treatment plans, Dr. Đoàn said. The model’s health outcomes measures included an increase in life expectancy as well as a greater proportion of depression-free days among adolescents who receive appropriate treatment.
Dr. Riehm and Dr. Đoàn disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Screening adolescents for signs of depression does not reduce their emergency department visits, hospitalizations, or treatment for suicidal behaviors, according to research published in Preventive Medicine. Adolescents who underwent a depression screening were just as likely to need these services as those who did not.
In 2016, the U.S. Preventive Services Task Force recommended that adolescents aged 12-18 years be screened for major depressive disorder, provided that effective treatment options and follow-up strategies are in place.
“The main goal of depression screening is really to reduce adverse psychiatric outcomes. But I think a collateral hope is that, in reducing these adverse psychiatric outcomes, you would also reduce avoidable health services use,” such as ED visits or hospitalizations, said Kira Riehm, PhD, a postdoctoral fellow in epidemiology at Columbia University, New York, who led the research. Dr. Riehm designed the new study, which was part of her doctoral work at Johns Hopkins University, Baltimore, to test this premise.
Dr. Riehm and colleagues compared 14,433 adolescents aged 12-18 years who were screened for depression at least once during a wellness visit from 2014 to 2017 to 43,299 adolescents who were not screened for depression during such visits. Depression screenings were interspersed among a total of 281,463 adolescent wellness visits from 2014 to 2017, which represented 5% of all visits.
The researchers used diagnostic codes from a database of insurance claims to determine who had undergone depression screening. They then compared use of ED services, inpatient hospitalizations, and the number of treatments for suicidal behaviors between the two groups for the 2 years following the wellness visit.
The average age of the adolescents who underwent screening was 13-14 years, as was the average age of adolescents who were not screened. Both groups were evenly matched with respect to being male or female.
The researchers estimated that a high majority of adolescents in the sample were White (83%). Black persons represented 7% of the sample; Hispanic/Latino, 5%; and Asian, 3%. Insurance claims don’t always include racial and ethnicity data, Dr. Riehm said, so her group statistically imputed these proportions. The claims data also do not include details about which type of screening tool was used or the results of the screening, such as whether a teen exhibited mild or severe depression.
Adolescents in both groups were just as likely to go to the ED for any reason, be admitted to the hospital for any reason, or undergo treatment for suicidal behaviors. The researchers observed a slight association between being screened for depression and going to the ED specifically for a mental health reason (relative risk, 1.16; 95% confidence interval, 1.00-1.33). The sex of the adolescents had no bearing on whether they used these services.
“I think people think of [depression screening] as one event. But in reality, screening is a series of different events that all have to be happening in order for a screening program to work,” Dr. Riehm told this news organization.
These events could include ensuring that adolescents who exhibit signs of depression receive a proper assessment, receive medications if needed, and have access to psychotherapists who can help them. Without these supports in place, she said, a one-off depression screening may have limited benefit.
“There’s a lot of places where people could drop out of that care continuum,” Dr. Riehm said.
“One-time screening may not be enough,” said Trân Đoàn, PhD, MPH, a postdoctoral researcher in the University of Pittsburgh department of pediatrics.
Dr. Đoàn, who was not involved in the research, noted that the American Academy of Pediatrics recommends annual screening of all adolescents for depressive symptoms. Given that only 5% of the visits in this sample included any kind of depression screening, Dr. Đoàn said, some pediatric practices may not have felt they had the resources to adequately address positive screenings for depression.
Both Dr. Riehm and Dr. Đoàn are focusing on the link between depression screening and health outcomes. In her own doctoral work at the University of Michigan, Dr. Đoàn modeled the effects of universal annual depression screening in primary care settings on the health status of people aged 12-22 years. She is currently preparing this work for publication.
“I did find that, over the long term, there is improvement in health outcomes if we were to screen on an annual basis,” provided improved screening is coupled with comprehensive treatment plans, Dr. Đoàn said. The model’s health outcomes measures included an increase in life expectancy as well as a greater proportion of depression-free days among adolescents who receive appropriate treatment.
Dr. Riehm and Dr. Đoàn disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Screening adolescents for signs of depression does not reduce their emergency department visits, hospitalizations, or treatment for suicidal behaviors, according to research published in Preventive Medicine. Adolescents who underwent a depression screening were just as likely to need these services as those who did not.
In 2016, the U.S. Preventive Services Task Force recommended that adolescents aged 12-18 years be screened for major depressive disorder, provided that effective treatment options and follow-up strategies are in place.
“The main goal of depression screening is really to reduce adverse psychiatric outcomes. But I think a collateral hope is that, in reducing these adverse psychiatric outcomes, you would also reduce avoidable health services use,” such as ED visits or hospitalizations, said Kira Riehm, PhD, a postdoctoral fellow in epidemiology at Columbia University, New York, who led the research. Dr. Riehm designed the new study, which was part of her doctoral work at Johns Hopkins University, Baltimore, to test this premise.
Dr. Riehm and colleagues compared 14,433 adolescents aged 12-18 years who were screened for depression at least once during a wellness visit from 2014 to 2017 to 43,299 adolescents who were not screened for depression during such visits. Depression screenings were interspersed among a total of 281,463 adolescent wellness visits from 2014 to 2017, which represented 5% of all visits.
The researchers used diagnostic codes from a database of insurance claims to determine who had undergone depression screening. They then compared use of ED services, inpatient hospitalizations, and the number of treatments for suicidal behaviors between the two groups for the 2 years following the wellness visit.
The average age of the adolescents who underwent screening was 13-14 years, as was the average age of adolescents who were not screened. Both groups were evenly matched with respect to being male or female.
The researchers estimated that a high majority of adolescents in the sample were White (83%). Black persons represented 7% of the sample; Hispanic/Latino, 5%; and Asian, 3%. Insurance claims don’t always include racial and ethnicity data, Dr. Riehm said, so her group statistically imputed these proportions. The claims data also do not include details about which type of screening tool was used or the results of the screening, such as whether a teen exhibited mild or severe depression.
Adolescents in both groups were just as likely to go to the ED for any reason, be admitted to the hospital for any reason, or undergo treatment for suicidal behaviors. The researchers observed a slight association between being screened for depression and going to the ED specifically for a mental health reason (relative risk, 1.16; 95% confidence interval, 1.00-1.33). The sex of the adolescents had no bearing on whether they used these services.
“I think people think of [depression screening] as one event. But in reality, screening is a series of different events that all have to be happening in order for a screening program to work,” Dr. Riehm told this news organization.
These events could include ensuring that adolescents who exhibit signs of depression receive a proper assessment, receive medications if needed, and have access to psychotherapists who can help them. Without these supports in place, she said, a one-off depression screening may have limited benefit.
“There’s a lot of places where people could drop out of that care continuum,” Dr. Riehm said.
“One-time screening may not be enough,” said Trân Đoàn, PhD, MPH, a postdoctoral researcher in the University of Pittsburgh department of pediatrics.
Dr. Đoàn, who was not involved in the research, noted that the American Academy of Pediatrics recommends annual screening of all adolescents for depressive symptoms. Given that only 5% of the visits in this sample included any kind of depression screening, Dr. Đoàn said, some pediatric practices may not have felt they had the resources to adequately address positive screenings for depression.
Both Dr. Riehm and Dr. Đoàn are focusing on the link between depression screening and health outcomes. In her own doctoral work at the University of Michigan, Dr. Đoàn modeled the effects of universal annual depression screening in primary care settings on the health status of people aged 12-22 years. She is currently preparing this work for publication.
“I did find that, over the long term, there is improvement in health outcomes if we were to screen on an annual basis,” provided improved screening is coupled with comprehensive treatment plans, Dr. Đoàn said. The model’s health outcomes measures included an increase in life expectancy as well as a greater proportion of depression-free days among adolescents who receive appropriate treatment.
Dr. Riehm and Dr. Đoàn disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PREVENTIVE MEDICINE
Nocturnal sleep key to successful kindergarten adjustment
Children who regularly slept 10-plus hours per night, particularly just before starting kindergarten, transitioned more successfully to kindergarten than those with less regular sleeping patterns, an observational study found. The effect held across the kindergarten year regardless of socioeconomic and health covariates, according to a new study by Douglas M. Teti, PhD, a developmental scientist and a professor of pediatrics at Penn State University, University Park, and colleagues.
“These effects were ubiquitous, extending to socioemotional learning engagement and academic domains,” they wrote online in Pediatrics
Furthermore, it was the regularity of sufficient nocturnal sleep that appeared to be more important for school adjustment than overall amounts of sleep accumulated across the day or the proportion of 24-hour periods in which children got 10 or more hours of sleep.
The American Academy of Sleep Medicine has recommended that 3- to 5-year-olds get 10-13 hours of sleep per day, including naps.
The findings by Dr. Teti’s group suggest that family-based interventions to establish consistent patterns of sufficient nighttime sleep should begin 5 or 6 months before the start of kindergarten.
“The importance of sleep as a predictor of school functioning in children is well-established, but relatively less is known about how sleep impacts children as they make their first transition into formal schooling,” Dr. Teti told this news organization. “School readiness and adjustment can be impacted by many factors, including socioeconomic status, child health, and missed days of school, but few studies have isolated the role of sleep in the transition to kindergarten net of these other influences, and few studies have examined the role that sleep plays on children’s school functioning throughout the full kindergarten year.”
The study
During 2016-2019, the researcher recruited 230 families from three Pennsylvania school districts, of which 221 completed the study. At several time points, the study examined three different measures of child sleep duration in 7-day bursts: at pre-kindergarten (July to August), early kindergarten (late September), mid-kindergarten (late November), and late kindergarten (mid-to-late April), using wrist actigraphy. These measures included:
- mean amounts of child sleep per 24-hour period across the full week
- proportion of 24-hour periods per week that children slept 10 or more hours
- proportion of nighttime sleep periods per week that children slept 10 or more hours
Outcomes at the designated school year time points were provided by 64 teachers blinded to the pupils’ sleep histories and by assessments administered by project staff.
Among the sleep measures examined, regularity of nighttime sleep involving 10 or more hours of sleep over the nocturnal period, especially at the pre-kindergarten stage, consistently predicted more favorable outcomes in socioemotional, learning engagement, and academic domains. These findings were controlled for income-to-poverty threshold ratios, child health status, and number of missed school days.
The study results generally align with those of previous studies, showing the importance of sleep for children’s school functioning, Dr. Teti told this news organization. “But they differed significantly in terms of finding that it was the regularity of 10-plus hours concentrated during the nighttime sleep period that was most important for predicting school adjustment, in particular, regular or sufficient sleep that occurred prior to the start of kindergarten.”
Calling the study “thought provoking,” Michael B. Grosso, MD, chair of pediatrics at Huntington (N.Y.) Hospital, said it confirms a robust correlation between total sleep duration and outcomes important to successful adjustment to kindergarten. “And we find out that uninterrupted sleep time of 10 hours or more seems to matter as well.”
In his view, the biggest limitation to the analysis is the one inherent to any observational study, “which is that association cannot prove causality. The authors did attempt to control for other health factors, but that can be hard to do,” he said. “The point is that if a child faces any of several health challenges, from sleep apnea to uncontrolled asthma, to ADHD or an autistic spectrum disorder, those issues will cause disrupted, abnormal sleep and also interfere with the outcomes the study addresses. In other words, it’s hard to know if sleep is affecting kindergarten adjustment or whether some X factor is affecting sleep and also affecting kindergarten performance.”
Getting children into bed earlier in long bright evenings of spring and summer before onset of kindergarten may not be easy, Dr. Teti acknowledged. “Arranging children’s sleep schedule as they approach kindergarten so that most, if not all, of their sleep takes place during the night – and as a corollary, reducing the frequency of naps during the day – should help children shift into sleeping nighttime primarily if not exclusively,” he said.
If necessary, he added, parents can work with sleep professionals to gradually concentrate children’s sleep during the night. They should normalize earlier bedtimes by reducing access to electronic screens before bedtime and removing televisions from their bedrooms. “A consistent bedtime routine should be a central feature of parental attempts to shape better, more regular sleep in their children.”
Dr. Grosso added that pediatricians need to talk about the importance of consistent routines and especially adequate sleep when counseling parents during pre-school health supervision visits. “And as the authors mention, it’s hard to ensure good sleep hygiene for children if parents aren’t also getting a good night sleep. It all goes together.”
This study was supported by the National Institutes of Health. The authors had no competing interests to declare. Dr. Grosso disclosed no relevant conflicts of interest.
Children who regularly slept 10-plus hours per night, particularly just before starting kindergarten, transitioned more successfully to kindergarten than those with less regular sleeping patterns, an observational study found. The effect held across the kindergarten year regardless of socioeconomic and health covariates, according to a new study by Douglas M. Teti, PhD, a developmental scientist and a professor of pediatrics at Penn State University, University Park, and colleagues.
“These effects were ubiquitous, extending to socioemotional learning engagement and academic domains,” they wrote online in Pediatrics
Furthermore, it was the regularity of sufficient nocturnal sleep that appeared to be more important for school adjustment than overall amounts of sleep accumulated across the day or the proportion of 24-hour periods in which children got 10 or more hours of sleep.
The American Academy of Sleep Medicine has recommended that 3- to 5-year-olds get 10-13 hours of sleep per day, including naps.
The findings by Dr. Teti’s group suggest that family-based interventions to establish consistent patterns of sufficient nighttime sleep should begin 5 or 6 months before the start of kindergarten.
“The importance of sleep as a predictor of school functioning in children is well-established, but relatively less is known about how sleep impacts children as they make their first transition into formal schooling,” Dr. Teti told this news organization. “School readiness and adjustment can be impacted by many factors, including socioeconomic status, child health, and missed days of school, but few studies have isolated the role of sleep in the transition to kindergarten net of these other influences, and few studies have examined the role that sleep plays on children’s school functioning throughout the full kindergarten year.”
The study
During 2016-2019, the researcher recruited 230 families from three Pennsylvania school districts, of which 221 completed the study. At several time points, the study examined three different measures of child sleep duration in 7-day bursts: at pre-kindergarten (July to August), early kindergarten (late September), mid-kindergarten (late November), and late kindergarten (mid-to-late April), using wrist actigraphy. These measures included:
- mean amounts of child sleep per 24-hour period across the full week
- proportion of 24-hour periods per week that children slept 10 or more hours
- proportion of nighttime sleep periods per week that children slept 10 or more hours
Outcomes at the designated school year time points were provided by 64 teachers blinded to the pupils’ sleep histories and by assessments administered by project staff.
Among the sleep measures examined, regularity of nighttime sleep involving 10 or more hours of sleep over the nocturnal period, especially at the pre-kindergarten stage, consistently predicted more favorable outcomes in socioemotional, learning engagement, and academic domains. These findings were controlled for income-to-poverty threshold ratios, child health status, and number of missed school days.
The study results generally align with those of previous studies, showing the importance of sleep for children’s school functioning, Dr. Teti told this news organization. “But they differed significantly in terms of finding that it was the regularity of 10-plus hours concentrated during the nighttime sleep period that was most important for predicting school adjustment, in particular, regular or sufficient sleep that occurred prior to the start of kindergarten.”
Calling the study “thought provoking,” Michael B. Grosso, MD, chair of pediatrics at Huntington (N.Y.) Hospital, said it confirms a robust correlation between total sleep duration and outcomes important to successful adjustment to kindergarten. “And we find out that uninterrupted sleep time of 10 hours or more seems to matter as well.”
In his view, the biggest limitation to the analysis is the one inherent to any observational study, “which is that association cannot prove causality. The authors did attempt to control for other health factors, but that can be hard to do,” he said. “The point is that if a child faces any of several health challenges, from sleep apnea to uncontrolled asthma, to ADHD or an autistic spectrum disorder, those issues will cause disrupted, abnormal sleep and also interfere with the outcomes the study addresses. In other words, it’s hard to know if sleep is affecting kindergarten adjustment or whether some X factor is affecting sleep and also affecting kindergarten performance.”
Getting children into bed earlier in long bright evenings of spring and summer before onset of kindergarten may not be easy, Dr. Teti acknowledged. “Arranging children’s sleep schedule as they approach kindergarten so that most, if not all, of their sleep takes place during the night – and as a corollary, reducing the frequency of naps during the day – should help children shift into sleeping nighttime primarily if not exclusively,” he said.
If necessary, he added, parents can work with sleep professionals to gradually concentrate children’s sleep during the night. They should normalize earlier bedtimes by reducing access to electronic screens before bedtime and removing televisions from their bedrooms. “A consistent bedtime routine should be a central feature of parental attempts to shape better, more regular sleep in their children.”
Dr. Grosso added that pediatricians need to talk about the importance of consistent routines and especially adequate sleep when counseling parents during pre-school health supervision visits. “And as the authors mention, it’s hard to ensure good sleep hygiene for children if parents aren’t also getting a good night sleep. It all goes together.”
This study was supported by the National Institutes of Health. The authors had no competing interests to declare. Dr. Grosso disclosed no relevant conflicts of interest.
Children who regularly slept 10-plus hours per night, particularly just before starting kindergarten, transitioned more successfully to kindergarten than those with less regular sleeping patterns, an observational study found. The effect held across the kindergarten year regardless of socioeconomic and health covariates, according to a new study by Douglas M. Teti, PhD, a developmental scientist and a professor of pediatrics at Penn State University, University Park, and colleagues.
“These effects were ubiquitous, extending to socioemotional learning engagement and academic domains,” they wrote online in Pediatrics
Furthermore, it was the regularity of sufficient nocturnal sleep that appeared to be more important for school adjustment than overall amounts of sleep accumulated across the day or the proportion of 24-hour periods in which children got 10 or more hours of sleep.
The American Academy of Sleep Medicine has recommended that 3- to 5-year-olds get 10-13 hours of sleep per day, including naps.
The findings by Dr. Teti’s group suggest that family-based interventions to establish consistent patterns of sufficient nighttime sleep should begin 5 or 6 months before the start of kindergarten.
“The importance of sleep as a predictor of school functioning in children is well-established, but relatively less is known about how sleep impacts children as they make their first transition into formal schooling,” Dr. Teti told this news organization. “School readiness and adjustment can be impacted by many factors, including socioeconomic status, child health, and missed days of school, but few studies have isolated the role of sleep in the transition to kindergarten net of these other influences, and few studies have examined the role that sleep plays on children’s school functioning throughout the full kindergarten year.”
The study
During 2016-2019, the researcher recruited 230 families from three Pennsylvania school districts, of which 221 completed the study. At several time points, the study examined three different measures of child sleep duration in 7-day bursts: at pre-kindergarten (July to August), early kindergarten (late September), mid-kindergarten (late November), and late kindergarten (mid-to-late April), using wrist actigraphy. These measures included:
- mean amounts of child sleep per 24-hour period across the full week
- proportion of 24-hour periods per week that children slept 10 or more hours
- proportion of nighttime sleep periods per week that children slept 10 or more hours
Outcomes at the designated school year time points were provided by 64 teachers blinded to the pupils’ sleep histories and by assessments administered by project staff.
Among the sleep measures examined, regularity of nighttime sleep involving 10 or more hours of sleep over the nocturnal period, especially at the pre-kindergarten stage, consistently predicted more favorable outcomes in socioemotional, learning engagement, and academic domains. These findings were controlled for income-to-poverty threshold ratios, child health status, and number of missed school days.
The study results generally align with those of previous studies, showing the importance of sleep for children’s school functioning, Dr. Teti told this news organization. “But they differed significantly in terms of finding that it was the regularity of 10-plus hours concentrated during the nighttime sleep period that was most important for predicting school adjustment, in particular, regular or sufficient sleep that occurred prior to the start of kindergarten.”
Calling the study “thought provoking,” Michael B. Grosso, MD, chair of pediatrics at Huntington (N.Y.) Hospital, said it confirms a robust correlation between total sleep duration and outcomes important to successful adjustment to kindergarten. “And we find out that uninterrupted sleep time of 10 hours or more seems to matter as well.”
In his view, the biggest limitation to the analysis is the one inherent to any observational study, “which is that association cannot prove causality. The authors did attempt to control for other health factors, but that can be hard to do,” he said. “The point is that if a child faces any of several health challenges, from sleep apnea to uncontrolled asthma, to ADHD or an autistic spectrum disorder, those issues will cause disrupted, abnormal sleep and also interfere with the outcomes the study addresses. In other words, it’s hard to know if sleep is affecting kindergarten adjustment or whether some X factor is affecting sleep and also affecting kindergarten performance.”
Getting children into bed earlier in long bright evenings of spring and summer before onset of kindergarten may not be easy, Dr. Teti acknowledged. “Arranging children’s sleep schedule as they approach kindergarten so that most, if not all, of their sleep takes place during the night – and as a corollary, reducing the frequency of naps during the day – should help children shift into sleeping nighttime primarily if not exclusively,” he said.
If necessary, he added, parents can work with sleep professionals to gradually concentrate children’s sleep during the night. They should normalize earlier bedtimes by reducing access to electronic screens before bedtime and removing televisions from their bedrooms. “A consistent bedtime routine should be a central feature of parental attempts to shape better, more regular sleep in their children.”
Dr. Grosso added that pediatricians need to talk about the importance of consistent routines and especially adequate sleep when counseling parents during pre-school health supervision visits. “And as the authors mention, it’s hard to ensure good sleep hygiene for children if parents aren’t also getting a good night sleep. It all goes together.”
This study was supported by the National Institutes of Health. The authors had no competing interests to declare. Dr. Grosso disclosed no relevant conflicts of interest.
FROM PEDIATRICS
Attacking childhood anxiety in primary care
Multiple media outlets and numerous children’s professional organizations are discussing the child and adolescent mental health crisis. Finally, society at large seems to be taking notice that our kids are not okay, and that they haven’t been okay for a long time.
Over the past 5-7 years, both in my practice in tertiary children’s hospital emergency departments and in primary care pediatrics, I have seen a disturbing decline in kids’ mental well-being. What can a primary care physician do to make a difference? How do we capitalize on these discussions about mental health and illness now that it is rising to a priority status?
The U.S. Preventive Services Task Force recently drafted a statement of recommendations specifically discussing anxiety in children and adolescents. It shows supporting evidence that there is a moderate benefit to screening children 8-18 years old for anxiety. We know from the 2018-2019 National Survey of Children’s Health that almost 8% of children/adolescents ages 3-17 years old have an anxiety disorder. And among those 13-18 years old, the lifetime prevalence rises to nearly 33%, according to National Institutes of Health statistics.
Childhood anxiety unquestionably increases the chances of persistent anxiety or depression in adulthood. I have followed children who had excessive social anxiety from age 3 or 4 who progressed to generalized anxiety disorder as adolescents, usually when no intervention was done or when the family waited for the child to “outgrow” it. The DSM-5 has six separate categories for anxiety disorders in children and adolescents: generalized anxiety disorder, separation anxiety disorder, specific phobias, social phobia, agoraphobia, and panic disorder. Unfortunately, these illnesses cannot be wished away.
Screening, diagnosis, and follow-up
A few simple screening tools can be used to check for anxiety in children and adolescents. These include SCARED (Screen for Child Anxiety Related Emotional Disorders), GAD-7 (Generalized Anxiety Disorder-7), and/or the PHQ-A (Patient Health Questionnaire for Adolescents). Keep in mind that a screening tool is just that – a screen. Diagnostic confirmation and follow-up are appropriate after a positive screen. I like all of these particular screens as they are easy to administer and can be incorporated into a busy practice without extra training to administer. They are also easy for parents and patients to complete prior to a visit or during a visit.
Ideally, after a positive screen, the next step is to consult a child and adolescent psychiatrist (CAP); however, according to statistics from the American Academy of Child and Adolescent Psychiatry (AACAP), there are only 8,300 CAPs in the United States. The reality is that not a single state in the entire country has a “mostly sufficient supply” of CAP’s (defined as ≥ 47 per 100,000 children). In fact, most have a “severe shortage,” defined as 1-17 per 100,000 children
Adding a child/adolescent therapist is also necessary for patients 8 years old and up, but the harsh truth is that it may take up to several months before the child is seen. If a patient is in a rural or other underserved area, it may be even longer.
So, what does this mean for primary care physicians? When you are faced with a positive screening for childhood anxiety, the next step is “tag, you’re it!” Understandably, this is frightening for many physicians who feel unqualified.
Don’t be afraid! Like the old adage says, a journey of a thousand miles begins with a single step. Starting the conversation with patients and families is foremost. Physicians must be first in line to end the stigma surrounding mental illness, and the easiest way to do that is to start the conversation. Remember that anxiety in kids can present as classic fear or worry, but it also can present as irritability, anger outbursts, and attention issues. There have been so many patients referred to me for “being out of control” or “always angry” or “probable ADHD” who turned out to have significant anxiety.
Part of a routine medical evaluation includes obtaining personal, family, and social history; there should be no difference when considering an anxiety disorder. Obtaining information about family history, personality traits, environmental components, early attachment issues, developmental history, parental style, parental conflict, occupants in the home, any adverse childhood events, and history of child maltreatment is crucial. Assessing other risk factors, including socioeconomic status, race, ethnicity, and gender, is key as well. I have seen families literally breathe a sigh of relief when these questions are asked. Parents feel heard and seen. And, equally significant, so does the child/adolescent.
The ‘Big 4’
An in-depth assessment of patient and family lifestyle factors such as nutrition, sleep, physical activity/exercise, and screen time habits is also basic and essential. This kind of evaluation usually cannot be done in the typical 15-minute visit and often will need to be done over several patient visits. I have had numerous conversations with my patients regarding what I call the “Big 4” – simple but not easy concepts and actions. They include nutrition, sleep, exercise, and screen time. Parents will look at me and say, “I can’t believe I never thought of this!” Some of my favorite moments with patients over the years have involved partnering with the patient and family and encouraging them to do the “simple” but not “easy” things.
Nutrition
Does the child have proper nutrition? That is not meant to be an exercise in labeling foods as “good” or “bad” but meant to confirm whether there is a balance of different foods. It’s also a way of exploring whether there are family meals in the home. Family meals have been shown to have a protective factor for children’s social development and emotional regulation.
Sleep
Review the child’s sleep habits, such as difficulty falling/staying asleep, bedtime routine (soothing, relaxing activities vs. the opposite), nightmares, snoring, nighttime cough, etc. The physical sleeping environment is important as well. Is it quiet? Is it a crowded room?
Exercise
Discuss physical activity with the family. Is there time for the child to play outside without a defined goal? So much of a child’s day is structured, in school or with after-school activities, but can the kid simply be a kid? Does the family take walks together? Is it safe to play outside?
Screen time
Reviewing screen time is important for multiple reasons, especially because the more time spent in front of a TV, computer, or video game, the less time there is to be physically active. Numerous experts, including the American Academy of Pediatrics, recommend limits on screen time for children. For adolescents, there appears to be some evidence that excessive screen time contributes to depression/anxiety.
I am not embarrassed to say that with my own kids I felt so strongly about screen time that we did not own any kind of video games or iPad (that was theirs alone), and they spent the summers until they turned 14 building a two-story bamboo fort in our backyard instead of vegging out in front of the TV or computer. It didn’t hurt them a bit; one is an engineer and the other is in nursing school.
It is easy to see that lifestyle factors can come into play with childhood anxiety and are often ignored in the clinical setting. They do not involve technologically advanced techniques or procedures, which are more likely to be reimbursed. They are straightforward – but not easy – concepts, and require active participation from the patient and family. Some of my most exciting moments with families is when they return for follow up and say, “It worked!”
We need to be as comfortable taking care of a child’s mind and spirit as we are taking care of a child’s physical body. Is this easy in a busy office? No. Is this easy in a 15-minute visit? No. Is this easy with poor reimbursement from insurance companies? No. Is it necessary? Unequivocally YES. Start the conversation.
Tag, you’re it!
Dr. Contrucci is an assistant professor of pediatrics, clinical education department, Philadelphia College of Osteopathic Medicine, Georgia Campus, Suwanee. She disclosed no relevant conflict of interest.
A version of this article first appeared on Medscape.com.
Multiple media outlets and numerous children’s professional organizations are discussing the child and adolescent mental health crisis. Finally, society at large seems to be taking notice that our kids are not okay, and that they haven’t been okay for a long time.
Over the past 5-7 years, both in my practice in tertiary children’s hospital emergency departments and in primary care pediatrics, I have seen a disturbing decline in kids’ mental well-being. What can a primary care physician do to make a difference? How do we capitalize on these discussions about mental health and illness now that it is rising to a priority status?
The U.S. Preventive Services Task Force recently drafted a statement of recommendations specifically discussing anxiety in children and adolescents. It shows supporting evidence that there is a moderate benefit to screening children 8-18 years old for anxiety. We know from the 2018-2019 National Survey of Children’s Health that almost 8% of children/adolescents ages 3-17 years old have an anxiety disorder. And among those 13-18 years old, the lifetime prevalence rises to nearly 33%, according to National Institutes of Health statistics.
Childhood anxiety unquestionably increases the chances of persistent anxiety or depression in adulthood. I have followed children who had excessive social anxiety from age 3 or 4 who progressed to generalized anxiety disorder as adolescents, usually when no intervention was done or when the family waited for the child to “outgrow” it. The DSM-5 has six separate categories for anxiety disorders in children and adolescents: generalized anxiety disorder, separation anxiety disorder, specific phobias, social phobia, agoraphobia, and panic disorder. Unfortunately, these illnesses cannot be wished away.
Screening, diagnosis, and follow-up
A few simple screening tools can be used to check for anxiety in children and adolescents. These include SCARED (Screen for Child Anxiety Related Emotional Disorders), GAD-7 (Generalized Anxiety Disorder-7), and/or the PHQ-A (Patient Health Questionnaire for Adolescents). Keep in mind that a screening tool is just that – a screen. Diagnostic confirmation and follow-up are appropriate after a positive screen. I like all of these particular screens as they are easy to administer and can be incorporated into a busy practice without extra training to administer. They are also easy for parents and patients to complete prior to a visit or during a visit.
Ideally, after a positive screen, the next step is to consult a child and adolescent psychiatrist (CAP); however, according to statistics from the American Academy of Child and Adolescent Psychiatry (AACAP), there are only 8,300 CAPs in the United States. The reality is that not a single state in the entire country has a “mostly sufficient supply” of CAP’s (defined as ≥ 47 per 100,000 children). In fact, most have a “severe shortage,” defined as 1-17 per 100,000 children
Adding a child/adolescent therapist is also necessary for patients 8 years old and up, but the harsh truth is that it may take up to several months before the child is seen. If a patient is in a rural or other underserved area, it may be even longer.
So, what does this mean for primary care physicians? When you are faced with a positive screening for childhood anxiety, the next step is “tag, you’re it!” Understandably, this is frightening for many physicians who feel unqualified.
Don’t be afraid! Like the old adage says, a journey of a thousand miles begins with a single step. Starting the conversation with patients and families is foremost. Physicians must be first in line to end the stigma surrounding mental illness, and the easiest way to do that is to start the conversation. Remember that anxiety in kids can present as classic fear or worry, but it also can present as irritability, anger outbursts, and attention issues. There have been so many patients referred to me for “being out of control” or “always angry” or “probable ADHD” who turned out to have significant anxiety.
Part of a routine medical evaluation includes obtaining personal, family, and social history; there should be no difference when considering an anxiety disorder. Obtaining information about family history, personality traits, environmental components, early attachment issues, developmental history, parental style, parental conflict, occupants in the home, any adverse childhood events, and history of child maltreatment is crucial. Assessing other risk factors, including socioeconomic status, race, ethnicity, and gender, is key as well. I have seen families literally breathe a sigh of relief when these questions are asked. Parents feel heard and seen. And, equally significant, so does the child/adolescent.
The ‘Big 4’
An in-depth assessment of patient and family lifestyle factors such as nutrition, sleep, physical activity/exercise, and screen time habits is also basic and essential. This kind of evaluation usually cannot be done in the typical 15-minute visit and often will need to be done over several patient visits. I have had numerous conversations with my patients regarding what I call the “Big 4” – simple but not easy concepts and actions. They include nutrition, sleep, exercise, and screen time. Parents will look at me and say, “I can’t believe I never thought of this!” Some of my favorite moments with patients over the years have involved partnering with the patient and family and encouraging them to do the “simple” but not “easy” things.
Nutrition
Does the child have proper nutrition? That is not meant to be an exercise in labeling foods as “good” or “bad” but meant to confirm whether there is a balance of different foods. It’s also a way of exploring whether there are family meals in the home. Family meals have been shown to have a protective factor for children’s social development and emotional regulation.
Sleep
Review the child’s sleep habits, such as difficulty falling/staying asleep, bedtime routine (soothing, relaxing activities vs. the opposite), nightmares, snoring, nighttime cough, etc. The physical sleeping environment is important as well. Is it quiet? Is it a crowded room?
Exercise
Discuss physical activity with the family. Is there time for the child to play outside without a defined goal? So much of a child’s day is structured, in school or with after-school activities, but can the kid simply be a kid? Does the family take walks together? Is it safe to play outside?
Screen time
Reviewing screen time is important for multiple reasons, especially because the more time spent in front of a TV, computer, or video game, the less time there is to be physically active. Numerous experts, including the American Academy of Pediatrics, recommend limits on screen time for children. For adolescents, there appears to be some evidence that excessive screen time contributes to depression/anxiety.
I am not embarrassed to say that with my own kids I felt so strongly about screen time that we did not own any kind of video games or iPad (that was theirs alone), and they spent the summers until they turned 14 building a two-story bamboo fort in our backyard instead of vegging out in front of the TV or computer. It didn’t hurt them a bit; one is an engineer and the other is in nursing school.
It is easy to see that lifestyle factors can come into play with childhood anxiety and are often ignored in the clinical setting. They do not involve technologically advanced techniques or procedures, which are more likely to be reimbursed. They are straightforward – but not easy – concepts, and require active participation from the patient and family. Some of my most exciting moments with families is when they return for follow up and say, “It worked!”
We need to be as comfortable taking care of a child’s mind and spirit as we are taking care of a child’s physical body. Is this easy in a busy office? No. Is this easy in a 15-minute visit? No. Is this easy with poor reimbursement from insurance companies? No. Is it necessary? Unequivocally YES. Start the conversation.
Tag, you’re it!
Dr. Contrucci is an assistant professor of pediatrics, clinical education department, Philadelphia College of Osteopathic Medicine, Georgia Campus, Suwanee. She disclosed no relevant conflict of interest.
A version of this article first appeared on Medscape.com.
Multiple media outlets and numerous children’s professional organizations are discussing the child and adolescent mental health crisis. Finally, society at large seems to be taking notice that our kids are not okay, and that they haven’t been okay for a long time.
Over the past 5-7 years, both in my practice in tertiary children’s hospital emergency departments and in primary care pediatrics, I have seen a disturbing decline in kids’ mental well-being. What can a primary care physician do to make a difference? How do we capitalize on these discussions about mental health and illness now that it is rising to a priority status?
The U.S. Preventive Services Task Force recently drafted a statement of recommendations specifically discussing anxiety in children and adolescents. It shows supporting evidence that there is a moderate benefit to screening children 8-18 years old for anxiety. We know from the 2018-2019 National Survey of Children’s Health that almost 8% of children/adolescents ages 3-17 years old have an anxiety disorder. And among those 13-18 years old, the lifetime prevalence rises to nearly 33%, according to National Institutes of Health statistics.
Childhood anxiety unquestionably increases the chances of persistent anxiety or depression in adulthood. I have followed children who had excessive social anxiety from age 3 or 4 who progressed to generalized anxiety disorder as adolescents, usually when no intervention was done or when the family waited for the child to “outgrow” it. The DSM-5 has six separate categories for anxiety disorders in children and adolescents: generalized anxiety disorder, separation anxiety disorder, specific phobias, social phobia, agoraphobia, and panic disorder. Unfortunately, these illnesses cannot be wished away.
Screening, diagnosis, and follow-up
A few simple screening tools can be used to check for anxiety in children and adolescents. These include SCARED (Screen for Child Anxiety Related Emotional Disorders), GAD-7 (Generalized Anxiety Disorder-7), and/or the PHQ-A (Patient Health Questionnaire for Adolescents). Keep in mind that a screening tool is just that – a screen. Diagnostic confirmation and follow-up are appropriate after a positive screen. I like all of these particular screens as they are easy to administer and can be incorporated into a busy practice without extra training to administer. They are also easy for parents and patients to complete prior to a visit or during a visit.
Ideally, after a positive screen, the next step is to consult a child and adolescent psychiatrist (CAP); however, according to statistics from the American Academy of Child and Adolescent Psychiatry (AACAP), there are only 8,300 CAPs in the United States. The reality is that not a single state in the entire country has a “mostly sufficient supply” of CAP’s (defined as ≥ 47 per 100,000 children). In fact, most have a “severe shortage,” defined as 1-17 per 100,000 children
Adding a child/adolescent therapist is also necessary for patients 8 years old and up, but the harsh truth is that it may take up to several months before the child is seen. If a patient is in a rural or other underserved area, it may be even longer.
So, what does this mean for primary care physicians? When you are faced with a positive screening for childhood anxiety, the next step is “tag, you’re it!” Understandably, this is frightening for many physicians who feel unqualified.
Don’t be afraid! Like the old adage says, a journey of a thousand miles begins with a single step. Starting the conversation with patients and families is foremost. Physicians must be first in line to end the stigma surrounding mental illness, and the easiest way to do that is to start the conversation. Remember that anxiety in kids can present as classic fear or worry, but it also can present as irritability, anger outbursts, and attention issues. There have been so many patients referred to me for “being out of control” or “always angry” or “probable ADHD” who turned out to have significant anxiety.
Part of a routine medical evaluation includes obtaining personal, family, and social history; there should be no difference when considering an anxiety disorder. Obtaining information about family history, personality traits, environmental components, early attachment issues, developmental history, parental style, parental conflict, occupants in the home, any adverse childhood events, and history of child maltreatment is crucial. Assessing other risk factors, including socioeconomic status, race, ethnicity, and gender, is key as well. I have seen families literally breathe a sigh of relief when these questions are asked. Parents feel heard and seen. And, equally significant, so does the child/adolescent.
The ‘Big 4’
An in-depth assessment of patient and family lifestyle factors such as nutrition, sleep, physical activity/exercise, and screen time habits is also basic and essential. This kind of evaluation usually cannot be done in the typical 15-minute visit and often will need to be done over several patient visits. I have had numerous conversations with my patients regarding what I call the “Big 4” – simple but not easy concepts and actions. They include nutrition, sleep, exercise, and screen time. Parents will look at me and say, “I can’t believe I never thought of this!” Some of my favorite moments with patients over the years have involved partnering with the patient and family and encouraging them to do the “simple” but not “easy” things.
Nutrition
Does the child have proper nutrition? That is not meant to be an exercise in labeling foods as “good” or “bad” but meant to confirm whether there is a balance of different foods. It’s also a way of exploring whether there are family meals in the home. Family meals have been shown to have a protective factor for children’s social development and emotional regulation.
Sleep
Review the child’s sleep habits, such as difficulty falling/staying asleep, bedtime routine (soothing, relaxing activities vs. the opposite), nightmares, snoring, nighttime cough, etc. The physical sleeping environment is important as well. Is it quiet? Is it a crowded room?
Exercise
Discuss physical activity with the family. Is there time for the child to play outside without a defined goal? So much of a child’s day is structured, in school or with after-school activities, but can the kid simply be a kid? Does the family take walks together? Is it safe to play outside?
Screen time
Reviewing screen time is important for multiple reasons, especially because the more time spent in front of a TV, computer, or video game, the less time there is to be physically active. Numerous experts, including the American Academy of Pediatrics, recommend limits on screen time for children. For adolescents, there appears to be some evidence that excessive screen time contributes to depression/anxiety.
I am not embarrassed to say that with my own kids I felt so strongly about screen time that we did not own any kind of video games or iPad (that was theirs alone), and they spent the summers until they turned 14 building a two-story bamboo fort in our backyard instead of vegging out in front of the TV or computer. It didn’t hurt them a bit; one is an engineer and the other is in nursing school.
It is easy to see that lifestyle factors can come into play with childhood anxiety and are often ignored in the clinical setting. They do not involve technologically advanced techniques or procedures, which are more likely to be reimbursed. They are straightforward – but not easy – concepts, and require active participation from the patient and family. Some of my most exciting moments with families is when they return for follow up and say, “It worked!”
We need to be as comfortable taking care of a child’s mind and spirit as we are taking care of a child’s physical body. Is this easy in a busy office? No. Is this easy in a 15-minute visit? No. Is this easy with poor reimbursement from insurance companies? No. Is it necessary? Unequivocally YES. Start the conversation.
Tag, you’re it!
Dr. Contrucci is an assistant professor of pediatrics, clinical education department, Philadelphia College of Osteopathic Medicine, Georgia Campus, Suwanee. She disclosed no relevant conflict of interest.
A version of this article first appeared on Medscape.com.
Transgender youth on hormone therapy risk substantial bone loss
, and this is true regardless of gender assignment at birth.
The problem worsens as the time during which these patients receive sex steroid hormones increases. So far, the “bone mineral density effects of these therapies are understudied,” warned Natalie Nokoff, MD, who presented a cross-sectional study at the annual meeting of the Endocrine Society.
The study of bone density is part of a larger body of research being conducted by Dr. Nokoff and her co-investigators on the long-term health effects of gender-affirming therapy in children and adolescents. In one of several recent studies, transgender youths taking gonadotropin-releasing hormone (GnRH) agonists, which effectively block puberty, were shown to be at greater risk of adverse changes in body composition and markers of cardiometabolic health than youths who were not taking them.
“We need more information on the optimal length of treatment with puberty-delaying medications before either discontinuation or introduction of gender-affirming hormones,” said Dr. Nokoff, an assistant professor of pediatrics and endocrinology at the University of Colorado School of Medicine, Aurora.
In this study, 56 transgender youth underwent total body dual-energy x-ray absorptiometry (DEXA). The patients ranged in age from 10 years to almost 20 years. Just over half (53%) were assigned female sex at birth.
The mean Z scores, signifying deviation from age-matched norms, were lower regardless of current use or past use of GnRH agonists in both transgender males or transgender females, relative to age-matched norms.
Asked to comment, Michele A. O’Connell, MBBCh, department of endocrinology and diabetes, Royal Children’s Hospital, Victoria, Australia, said the risk of bone loss is real.
“Monitoring of bone health is recommended for all transgender-diverse adolescents treated with gonadotropin-releasing hormone agonists,” said Dr. O’Connell. He referred to multiple guidelines, including those issued by the World Professional Association of Transgender Health in 2012 and those from the Endocrine Society that were issued in 2017.
Inverse correlation between duration of GnRH agonist therapy and Z scores
In Dr. Nokoff’s study, for transgender males, the BMD Z score was reduced 0.2 relative to male norms and by 0.4 relative to female norms. For transgender females, the scores were reduced by 0.4 relative to male norms and by 0.2 relative to female norms.
Among transgender males who were taking testosterone and who had previously been exposed to GnRH agonists, the Z score was significantly lower than those taking testosterone alone (P = .004). There were no differences in Z score for transgender females taking estradiol alone relative to estradiol with current or past use of GnRH agonists.
There was a significant inverse correlation for duration of GnRH agonist therapy and Z scores for transgender females relative to male norms (P = .005) or female norms (P = .029). However, Z scores were unrelated to length of time receiving testosterone or estradiol therapy or to sex steroid concentrations.
The number of children and adolescents taking puberty-delaying or gender-affirming therapies is increasing. Although reliable data are limited, the exploration of gender identify appears to have become more common with the growing social acceptance of gender dysphoria. That term refers to a sense of unease among individuals who feel that their biological sex does not match their gender identity, according to Dr. Nokoff.
“It is now estimated that 2% of youths identify as transgender,” she said.
Findings from studies investigating the relationship between gender-affirming therapy and bone loss among adults have not been consistent. In a single-center study that followed 543 transgender men and 711 transgender women who had undergone DEXA scanning at baseline prior to starting hormone therapy, there did not appear to be any substantial negative effects on lumbar bone density over time (J Bone Min Res. 2018 Dec;34:447-54).
For adolescents, there is growing evidence of the risk of bone loss in relation to gender-affirming therapy, but there is limited agreement on clinical risks and how they can be avoided. Relevant variables include genetics and diet, as well as the types, doses, and length of time receiving gender-affirming therapy.
Monitor bone in transgender youth; Use vitamin D and weight-bearing exercise
Dr. O’Connell is the first author of a recent summary of the pharmacologic management of trans and gender-diverse adolescents. That summary covered multiple topics in addition to risk of bone loss, including the impact on growth, cognition, and mental health (J Clin Endocrinol Metab. 2022 Jan;107:241-257).
Overall, she believes that bone health should be monitored for children receiving puberty-delaying or gender-affirming therapies but agrees with Dr. Nokoff that the clinical impact remains poorly defined.
“Long-term follow-up studies will be required to assess the impact, if any, on functional outcomes such as fracture risk,” she reported. Still, she encouraged use of standard ways of improving bone health, including adequate vitamin D intake and weight-bearing exercise.
Dr. Nokoff and Dr. O’Connell have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, and this is true regardless of gender assignment at birth.
The problem worsens as the time during which these patients receive sex steroid hormones increases. So far, the “bone mineral density effects of these therapies are understudied,” warned Natalie Nokoff, MD, who presented a cross-sectional study at the annual meeting of the Endocrine Society.
The study of bone density is part of a larger body of research being conducted by Dr. Nokoff and her co-investigators on the long-term health effects of gender-affirming therapy in children and adolescents. In one of several recent studies, transgender youths taking gonadotropin-releasing hormone (GnRH) agonists, which effectively block puberty, were shown to be at greater risk of adverse changes in body composition and markers of cardiometabolic health than youths who were not taking them.
“We need more information on the optimal length of treatment with puberty-delaying medications before either discontinuation or introduction of gender-affirming hormones,” said Dr. Nokoff, an assistant professor of pediatrics and endocrinology at the University of Colorado School of Medicine, Aurora.
In this study, 56 transgender youth underwent total body dual-energy x-ray absorptiometry (DEXA). The patients ranged in age from 10 years to almost 20 years. Just over half (53%) were assigned female sex at birth.
The mean Z scores, signifying deviation from age-matched norms, were lower regardless of current use or past use of GnRH agonists in both transgender males or transgender females, relative to age-matched norms.
Asked to comment, Michele A. O’Connell, MBBCh, department of endocrinology and diabetes, Royal Children’s Hospital, Victoria, Australia, said the risk of bone loss is real.
“Monitoring of bone health is recommended for all transgender-diverse adolescents treated with gonadotropin-releasing hormone agonists,” said Dr. O’Connell. He referred to multiple guidelines, including those issued by the World Professional Association of Transgender Health in 2012 and those from the Endocrine Society that were issued in 2017.
Inverse correlation between duration of GnRH agonist therapy and Z scores
In Dr. Nokoff’s study, for transgender males, the BMD Z score was reduced 0.2 relative to male norms and by 0.4 relative to female norms. For transgender females, the scores were reduced by 0.4 relative to male norms and by 0.2 relative to female norms.
Among transgender males who were taking testosterone and who had previously been exposed to GnRH agonists, the Z score was significantly lower than those taking testosterone alone (P = .004). There were no differences in Z score for transgender females taking estradiol alone relative to estradiol with current or past use of GnRH agonists.
There was a significant inverse correlation for duration of GnRH agonist therapy and Z scores for transgender females relative to male norms (P = .005) or female norms (P = .029). However, Z scores were unrelated to length of time receiving testosterone or estradiol therapy or to sex steroid concentrations.
The number of children and adolescents taking puberty-delaying or gender-affirming therapies is increasing. Although reliable data are limited, the exploration of gender identify appears to have become more common with the growing social acceptance of gender dysphoria. That term refers to a sense of unease among individuals who feel that their biological sex does not match their gender identity, according to Dr. Nokoff.
“It is now estimated that 2% of youths identify as transgender,” she said.
Findings from studies investigating the relationship between gender-affirming therapy and bone loss among adults have not been consistent. In a single-center study that followed 543 transgender men and 711 transgender women who had undergone DEXA scanning at baseline prior to starting hormone therapy, there did not appear to be any substantial negative effects on lumbar bone density over time (J Bone Min Res. 2018 Dec;34:447-54).
For adolescents, there is growing evidence of the risk of bone loss in relation to gender-affirming therapy, but there is limited agreement on clinical risks and how they can be avoided. Relevant variables include genetics and diet, as well as the types, doses, and length of time receiving gender-affirming therapy.
Monitor bone in transgender youth; Use vitamin D and weight-bearing exercise
Dr. O’Connell is the first author of a recent summary of the pharmacologic management of trans and gender-diverse adolescents. That summary covered multiple topics in addition to risk of bone loss, including the impact on growth, cognition, and mental health (J Clin Endocrinol Metab. 2022 Jan;107:241-257).
Overall, she believes that bone health should be monitored for children receiving puberty-delaying or gender-affirming therapies but agrees with Dr. Nokoff that the clinical impact remains poorly defined.
“Long-term follow-up studies will be required to assess the impact, if any, on functional outcomes such as fracture risk,” she reported. Still, she encouraged use of standard ways of improving bone health, including adequate vitamin D intake and weight-bearing exercise.
Dr. Nokoff and Dr. O’Connell have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, and this is true regardless of gender assignment at birth.
The problem worsens as the time during which these patients receive sex steroid hormones increases. So far, the “bone mineral density effects of these therapies are understudied,” warned Natalie Nokoff, MD, who presented a cross-sectional study at the annual meeting of the Endocrine Society.
The study of bone density is part of a larger body of research being conducted by Dr. Nokoff and her co-investigators on the long-term health effects of gender-affirming therapy in children and adolescents. In one of several recent studies, transgender youths taking gonadotropin-releasing hormone (GnRH) agonists, which effectively block puberty, were shown to be at greater risk of adverse changes in body composition and markers of cardiometabolic health than youths who were not taking them.
“We need more information on the optimal length of treatment with puberty-delaying medications before either discontinuation or introduction of gender-affirming hormones,” said Dr. Nokoff, an assistant professor of pediatrics and endocrinology at the University of Colorado School of Medicine, Aurora.
In this study, 56 transgender youth underwent total body dual-energy x-ray absorptiometry (DEXA). The patients ranged in age from 10 years to almost 20 years. Just over half (53%) were assigned female sex at birth.
The mean Z scores, signifying deviation from age-matched norms, were lower regardless of current use or past use of GnRH agonists in both transgender males or transgender females, relative to age-matched norms.
Asked to comment, Michele A. O’Connell, MBBCh, department of endocrinology and diabetes, Royal Children’s Hospital, Victoria, Australia, said the risk of bone loss is real.
“Monitoring of bone health is recommended for all transgender-diverse adolescents treated with gonadotropin-releasing hormone agonists,” said Dr. O’Connell. He referred to multiple guidelines, including those issued by the World Professional Association of Transgender Health in 2012 and those from the Endocrine Society that were issued in 2017.
Inverse correlation between duration of GnRH agonist therapy and Z scores
In Dr. Nokoff’s study, for transgender males, the BMD Z score was reduced 0.2 relative to male norms and by 0.4 relative to female norms. For transgender females, the scores were reduced by 0.4 relative to male norms and by 0.2 relative to female norms.
Among transgender males who were taking testosterone and who had previously been exposed to GnRH agonists, the Z score was significantly lower than those taking testosterone alone (P = .004). There were no differences in Z score for transgender females taking estradiol alone relative to estradiol with current or past use of GnRH agonists.
There was a significant inverse correlation for duration of GnRH agonist therapy and Z scores for transgender females relative to male norms (P = .005) or female norms (P = .029). However, Z scores were unrelated to length of time receiving testosterone or estradiol therapy or to sex steroid concentrations.
The number of children and adolescents taking puberty-delaying or gender-affirming therapies is increasing. Although reliable data are limited, the exploration of gender identify appears to have become more common with the growing social acceptance of gender dysphoria. That term refers to a sense of unease among individuals who feel that their biological sex does not match their gender identity, according to Dr. Nokoff.
“It is now estimated that 2% of youths identify as transgender,” she said.
Findings from studies investigating the relationship between gender-affirming therapy and bone loss among adults have not been consistent. In a single-center study that followed 543 transgender men and 711 transgender women who had undergone DEXA scanning at baseline prior to starting hormone therapy, there did not appear to be any substantial negative effects on lumbar bone density over time (J Bone Min Res. 2018 Dec;34:447-54).
For adolescents, there is growing evidence of the risk of bone loss in relation to gender-affirming therapy, but there is limited agreement on clinical risks and how they can be avoided. Relevant variables include genetics and diet, as well as the types, doses, and length of time receiving gender-affirming therapy.
Monitor bone in transgender youth; Use vitamin D and weight-bearing exercise
Dr. O’Connell is the first author of a recent summary of the pharmacologic management of trans and gender-diverse adolescents. That summary covered multiple topics in addition to risk of bone loss, including the impact on growth, cognition, and mental health (J Clin Endocrinol Metab. 2022 Jan;107:241-257).
Overall, she believes that bone health should be monitored for children receiving puberty-delaying or gender-affirming therapies but agrees with Dr. Nokoff that the clinical impact remains poorly defined.
“Long-term follow-up studies will be required to assess the impact, if any, on functional outcomes such as fracture risk,” she reported. Still, she encouraged use of standard ways of improving bone health, including adequate vitamin D intake and weight-bearing exercise.
Dr. Nokoff and Dr. O’Connell have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ENDO 2022
Early childhood allergies linked with ADHD and ASD
, according to a large retrospective study.
“Our study provides strong evidence for the association between allergic disorders in early childhood and the development of ADHD,” Shay Nemet, MD, of the Kaplan Medical Center, Rehovot, Israel, and colleagues write in Pediatric Allergy and Immunology. “The risk of those children to develop ASD was less significant.”
The researchers analyzed data from 117,022 consecutive children diagnosed with at least one allergic disorder – asthma, conjunctivitis, rhinitis, and drug, food, or skin allergy – and 116,968 children without allergies in the Clalit Health Services pediatric database. The children had been treated from 2000 to 2018; the mean follow-up period was 11 years.
The children who were diagnosed with one or more allergies (mean age, 4.5 years) were significantly more likely to develop ADHD (odds ratio, 2.45; 95% confidence interval, 2.39-2.51), ASD (OR, 1.17; 95% CI, 1.08-1.27), or both ADHD and ASD (OR, 1.56; 95% CI, 1.35-1.79) than were the control children who did not have allergies.
Children diagnosed with rhinitis (OR, 3.96; 95% CI, 3.80-4.12) and conjunctivitis (OR, 3.63; 95% CI, 3.53-3.74) were the most likely to develop ADHD.
Allergy correlation with ADHD and ASD
Cy B. Nadler, PhD, a clinical psychologist and the director of Autism Services at Children’s Mercy Kansas City, Missouri, told this news organization that children and adults with neurodevelopmental differences are also more likely to have other health problems.
“Clinicians practicing in subspecialties such as allergy and immunology may have opportunities to help psychologists identify developmental and behavioral concerns early in childhood,” he added.
“Studies like this can’t be accomplished without large health care databases, but this approach has drawbacks, too,” Dr. Nadler said in an email. “Without more information about these patients’ co-occurring medical and behavioral conditions, we are almost certainly missing important contributors to the observed associations.”
Dr. Nadler, who was not involved in the study, noted that in the multivariable analysis that controlled for age at study entry, gender, and number of annual visits, the link between allergy and ASD diagnosis was not significant.
“It is important to remember not to interpret these study results as causal,” he added.
Desha M. Jordan, MD, FAAP, an assistant professor of pediatrics at UPMC Children’s Hospital of Pittsburgh, called the study “an interesting new area that has been speculated about for some time” and “one of the first I have seen with statistically significant correlations found between ADHD, ASD, and allergic conditions.”
More questions for future studies
Health care providers need to understand the potential sequelae of allergic conditions so that they can manage their patients appropriately, she advised.
Although symptoms and diagnoses were confirmed for all patients, the study’s retrospective design and the possibility of recall bias were limitations, said Dr. Jordan in an email. She also was not involved in the study.
“For example, the family of a child diagnosed with ADHD or ASD may have been more mindful of anything out of the norm in that child’s past, while the family of a child without these conditions may not have recalled allergic symptoms as important,” she explained.
Another question that arises is whether some patients were treated and managed well while others were not and whether this disparity in care affected the development or severity of ADHD or ASD, she added.
“Is a patient with a well-controlled allergic condition less likely to develop ADHD or ASD than a patient with an uncontrolled allergic condition? Does a well-controlled patient ever return to the same probability of getting ADHD or ASD as a nonallergic patient?”
“While this study expands our understanding of these conditions and their interrelationships, it also brings up many additional questions and opens a new segment of research,” Dr. Jordan said. “More studies in this area are necessary to confirm the findings of this paper.”
The study was partially funded by the Israel Ambulatory Pediatric Association. The authors, Dr. Nadler, and Dr. Jordan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a large retrospective study.
“Our study provides strong evidence for the association between allergic disorders in early childhood and the development of ADHD,” Shay Nemet, MD, of the Kaplan Medical Center, Rehovot, Israel, and colleagues write in Pediatric Allergy and Immunology. “The risk of those children to develop ASD was less significant.”
The researchers analyzed data from 117,022 consecutive children diagnosed with at least one allergic disorder – asthma, conjunctivitis, rhinitis, and drug, food, or skin allergy – and 116,968 children without allergies in the Clalit Health Services pediatric database. The children had been treated from 2000 to 2018; the mean follow-up period was 11 years.
The children who were diagnosed with one or more allergies (mean age, 4.5 years) were significantly more likely to develop ADHD (odds ratio, 2.45; 95% confidence interval, 2.39-2.51), ASD (OR, 1.17; 95% CI, 1.08-1.27), or both ADHD and ASD (OR, 1.56; 95% CI, 1.35-1.79) than were the control children who did not have allergies.
Children diagnosed with rhinitis (OR, 3.96; 95% CI, 3.80-4.12) and conjunctivitis (OR, 3.63; 95% CI, 3.53-3.74) were the most likely to develop ADHD.
Allergy correlation with ADHD and ASD
Cy B. Nadler, PhD, a clinical psychologist and the director of Autism Services at Children’s Mercy Kansas City, Missouri, told this news organization that children and adults with neurodevelopmental differences are also more likely to have other health problems.
“Clinicians practicing in subspecialties such as allergy and immunology may have opportunities to help psychologists identify developmental and behavioral concerns early in childhood,” he added.
“Studies like this can’t be accomplished without large health care databases, but this approach has drawbacks, too,” Dr. Nadler said in an email. “Without more information about these patients’ co-occurring medical and behavioral conditions, we are almost certainly missing important contributors to the observed associations.”
Dr. Nadler, who was not involved in the study, noted that in the multivariable analysis that controlled for age at study entry, gender, and number of annual visits, the link between allergy and ASD diagnosis was not significant.
“It is important to remember not to interpret these study results as causal,” he added.
Desha M. Jordan, MD, FAAP, an assistant professor of pediatrics at UPMC Children’s Hospital of Pittsburgh, called the study “an interesting new area that has been speculated about for some time” and “one of the first I have seen with statistically significant correlations found between ADHD, ASD, and allergic conditions.”
More questions for future studies
Health care providers need to understand the potential sequelae of allergic conditions so that they can manage their patients appropriately, she advised.
Although symptoms and diagnoses were confirmed for all patients, the study’s retrospective design and the possibility of recall bias were limitations, said Dr. Jordan in an email. She also was not involved in the study.
“For example, the family of a child diagnosed with ADHD or ASD may have been more mindful of anything out of the norm in that child’s past, while the family of a child without these conditions may not have recalled allergic symptoms as important,” she explained.
Another question that arises is whether some patients were treated and managed well while others were not and whether this disparity in care affected the development or severity of ADHD or ASD, she added.
“Is a patient with a well-controlled allergic condition less likely to develop ADHD or ASD than a patient with an uncontrolled allergic condition? Does a well-controlled patient ever return to the same probability of getting ADHD or ASD as a nonallergic patient?”
“While this study expands our understanding of these conditions and their interrelationships, it also brings up many additional questions and opens a new segment of research,” Dr. Jordan said. “More studies in this area are necessary to confirm the findings of this paper.”
The study was partially funded by the Israel Ambulatory Pediatric Association. The authors, Dr. Nadler, and Dr. Jordan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a large retrospective study.
“Our study provides strong evidence for the association between allergic disorders in early childhood and the development of ADHD,” Shay Nemet, MD, of the Kaplan Medical Center, Rehovot, Israel, and colleagues write in Pediatric Allergy and Immunology. “The risk of those children to develop ASD was less significant.”
The researchers analyzed data from 117,022 consecutive children diagnosed with at least one allergic disorder – asthma, conjunctivitis, rhinitis, and drug, food, or skin allergy – and 116,968 children without allergies in the Clalit Health Services pediatric database. The children had been treated from 2000 to 2018; the mean follow-up period was 11 years.
The children who were diagnosed with one or more allergies (mean age, 4.5 years) were significantly more likely to develop ADHD (odds ratio, 2.45; 95% confidence interval, 2.39-2.51), ASD (OR, 1.17; 95% CI, 1.08-1.27), or both ADHD and ASD (OR, 1.56; 95% CI, 1.35-1.79) than were the control children who did not have allergies.
Children diagnosed with rhinitis (OR, 3.96; 95% CI, 3.80-4.12) and conjunctivitis (OR, 3.63; 95% CI, 3.53-3.74) were the most likely to develop ADHD.
Allergy correlation with ADHD and ASD
Cy B. Nadler, PhD, a clinical psychologist and the director of Autism Services at Children’s Mercy Kansas City, Missouri, told this news organization that children and adults with neurodevelopmental differences are also more likely to have other health problems.
“Clinicians practicing in subspecialties such as allergy and immunology may have opportunities to help psychologists identify developmental and behavioral concerns early in childhood,” he added.
“Studies like this can’t be accomplished without large health care databases, but this approach has drawbacks, too,” Dr. Nadler said in an email. “Without more information about these patients’ co-occurring medical and behavioral conditions, we are almost certainly missing important contributors to the observed associations.”
Dr. Nadler, who was not involved in the study, noted that in the multivariable analysis that controlled for age at study entry, gender, and number of annual visits, the link between allergy and ASD diagnosis was not significant.
“It is important to remember not to interpret these study results as causal,” he added.
Desha M. Jordan, MD, FAAP, an assistant professor of pediatrics at UPMC Children’s Hospital of Pittsburgh, called the study “an interesting new area that has been speculated about for some time” and “one of the first I have seen with statistically significant correlations found between ADHD, ASD, and allergic conditions.”
More questions for future studies
Health care providers need to understand the potential sequelae of allergic conditions so that they can manage their patients appropriately, she advised.
Although symptoms and diagnoses were confirmed for all patients, the study’s retrospective design and the possibility of recall bias were limitations, said Dr. Jordan in an email. She also was not involved in the study.
“For example, the family of a child diagnosed with ADHD or ASD may have been more mindful of anything out of the norm in that child’s past, while the family of a child without these conditions may not have recalled allergic symptoms as important,” she explained.
Another question that arises is whether some patients were treated and managed well while others were not and whether this disparity in care affected the development or severity of ADHD or ASD, she added.
“Is a patient with a well-controlled allergic condition less likely to develop ADHD or ASD than a patient with an uncontrolled allergic condition? Does a well-controlled patient ever return to the same probability of getting ADHD or ASD as a nonallergic patient?”
“While this study expands our understanding of these conditions and their interrelationships, it also brings up many additional questions and opens a new segment of research,” Dr. Jordan said. “More studies in this area are necessary to confirm the findings of this paper.”
The study was partially funded by the Israel Ambulatory Pediatric Association. The authors, Dr. Nadler, and Dr. Jordan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PEDIATRIC ALLERGY AND IMMUNOLOGY
Study explores gender differences in pediatric melanoma
INDIANAPOLIS – .
In addition, male gender was independently associated with increased mortality, but age was not.
Those are key findings from a retrospective cohort analysis of nearly 5,000 records from the National Cancer Database.
“There are multiple studies from primarily adult populations showing females with melanoma have a different presentation and better outcomes than males,” co-first author Rebecca M. Thiede, MD, a dermatologist at the University of Arizona, Tucson, said in an interview with this news organization in advance of the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session. “However, because melanoma is so rare in younger patients, little is known about gender differences in presentation and survival in pediatric and adolescent patients. To our knowledge, this is one of the largest studies to date in this population, and the first to explore gender differences in detail in pediatric and adolescent patients with melanoma.”
Working with co-first author Sabrina Dahak, a fourth-year medical student at the University of Arizona, Phoenix, Dr. Thiede and colleagues retrospectively analyzed the National Cancer Database to identify biopsy-confirmed invasive primary cutaneous melanoma cases diagnosed in patients 0-21 years of age between 2004 and 2018. The search yielded 4,645 cases, and the researchers used American Academy of Pediatrics definitions to categorize the patients by age, from infancy (birth to 2 years), to childhood (3-10 years), early adolescence (11-14 years), middle adolescence (15-17 years), and late adolescence (18-21 years). They used the Kaplan Meier analysis to determine overall survival and multivariate Cox regression to determine independent survival predictors.
Of the 4,645 pediatric melanoma cases, 63.4% were in females and 36.6% were in males, a difference that was significant (P < .001). Dr. Thiede and colleagues also observed a significant relationship between primary site and gender (P < .001). Primary sites included the trunk (34.3% of females vs. 32.9% of males, respectively), head and neck (16.4% vs. 30.9%), upper extremities (19.5% vs. 16%), lower extremities (27.9% vs. 16.5%), and “unspecified” (1.9% vs. 3.7%).
Females had higher rates of superficial spreading melanoma while males were affected by nodular melanoma more often. For example, the median Breslow depth was higher for males (1.05 mm; interquartile range [IQR] 0.50-2.31) than for females (0.80 mm; IQR, 0.40-1.67; P < .001).
Although females accounted for a higher percentage of cases than males overall, from birth to 17 years, a higher percentage of males than females were found to have later stage of melanoma at time of diagnosis: Females were more likely to be diagnosed with stage I disease (67.8%) than were males (53.6%), and males were more likely than were females to be diagnosed with stages II (15.9% vs. 12.3%), III (27.1% vs. 18.3%), and IV disease (3.3% vs. 1.6%; P < .001 for all).
In other findings, the 5- and 10-year overall survival rates were higher for females (95.9% and 93.9%, respectively) than for males (92.0% vs. 86.7%, respectively; P < .001). However, by age group, overall survival rates were similar between females and males among infants, children, and those in early adolescence – but not for those in middle adolescence (96.7% vs. 91.9%; P < .001) or late adolescence (95.7% vs. 90.4%; P < .001).
When the researchers adjusted for confounding variables, male gender was independently associated with an increased risk of death (adjusted hazard ratio 1.37; P < .001), but age was not.
“It was particularly surprising to see that even at such a young age, there is a significant difference in overall survival between males and females, where females have better outcomes than males,” Dr. Thiede said. “When examining pediatric and adolescent patients, it is essential to maintain cutaneous melanoma on the differential,” she advised. “It is important for clinicians to perform a thorough exam at annual visits particularly for those at high risk for melanoma to catch this rare but potentially devastating diagnosis.”
She acknowledged certain limitations of the study, including its reliance on one database, “as comparing multiple databases would strengthen the conclusions,” she said. “There was some missing data present in our dataset, and a large percentage of the histologic subtypes were unspecified, both of which are common issues with cancer registries. An additional limitation is related to the low death rates in adolescent and pediatric patients, which may impact the analysis related to survival and independent predictors of survival.”
Asked to comment on the study results, Carrie C. Coughlin, MD, who directs the section of pediatric dermatology Washington University/St. Louis Children’s Hospital, said that the finding that males were more likely to present with stage II or higher disease compared with females “could be related to their finding that females had more superficial spreading melanomas, whereas males had more nodular melanoma.” Those differences “could influence how providers evaluate melanocytic lesions in children,” she added.
Dr. Coughlin, who directs the pediatric dermatology fellowship at Washington University/St. Louis Children’s Hospital, said it was “interesting” that the authors found no association between older age and an increased risk of death. “It would be helpful to have more data about melanoma subtype, including information about Spitz or Spitzoid melanomas,” she said. “Also, knowing the distribution of melanoma across the age categories could provide more insight into their data.”
Ms. Dahak received an award from the National Cancer Institute to fund travel for presentation of this study at the SPD meeting. No other financial conflicts were reported by the researchers. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – .
In addition, male gender was independently associated with increased mortality, but age was not.
Those are key findings from a retrospective cohort analysis of nearly 5,000 records from the National Cancer Database.
“There are multiple studies from primarily adult populations showing females with melanoma have a different presentation and better outcomes than males,” co-first author Rebecca M. Thiede, MD, a dermatologist at the University of Arizona, Tucson, said in an interview with this news organization in advance of the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session. “However, because melanoma is so rare in younger patients, little is known about gender differences in presentation and survival in pediatric and adolescent patients. To our knowledge, this is one of the largest studies to date in this population, and the first to explore gender differences in detail in pediatric and adolescent patients with melanoma.”
Working with co-first author Sabrina Dahak, a fourth-year medical student at the University of Arizona, Phoenix, Dr. Thiede and colleagues retrospectively analyzed the National Cancer Database to identify biopsy-confirmed invasive primary cutaneous melanoma cases diagnosed in patients 0-21 years of age between 2004 and 2018. The search yielded 4,645 cases, and the researchers used American Academy of Pediatrics definitions to categorize the patients by age, from infancy (birth to 2 years), to childhood (3-10 years), early adolescence (11-14 years), middle adolescence (15-17 years), and late adolescence (18-21 years). They used the Kaplan Meier analysis to determine overall survival and multivariate Cox regression to determine independent survival predictors.
Of the 4,645 pediatric melanoma cases, 63.4% were in females and 36.6% were in males, a difference that was significant (P < .001). Dr. Thiede and colleagues also observed a significant relationship between primary site and gender (P < .001). Primary sites included the trunk (34.3% of females vs. 32.9% of males, respectively), head and neck (16.4% vs. 30.9%), upper extremities (19.5% vs. 16%), lower extremities (27.9% vs. 16.5%), and “unspecified” (1.9% vs. 3.7%).
Females had higher rates of superficial spreading melanoma while males were affected by nodular melanoma more often. For example, the median Breslow depth was higher for males (1.05 mm; interquartile range [IQR] 0.50-2.31) than for females (0.80 mm; IQR, 0.40-1.67; P < .001).
Although females accounted for a higher percentage of cases than males overall, from birth to 17 years, a higher percentage of males than females were found to have later stage of melanoma at time of diagnosis: Females were more likely to be diagnosed with stage I disease (67.8%) than were males (53.6%), and males were more likely than were females to be diagnosed with stages II (15.9% vs. 12.3%), III (27.1% vs. 18.3%), and IV disease (3.3% vs. 1.6%; P < .001 for all).
In other findings, the 5- and 10-year overall survival rates were higher for females (95.9% and 93.9%, respectively) than for males (92.0% vs. 86.7%, respectively; P < .001). However, by age group, overall survival rates were similar between females and males among infants, children, and those in early adolescence – but not for those in middle adolescence (96.7% vs. 91.9%; P < .001) or late adolescence (95.7% vs. 90.4%; P < .001).
When the researchers adjusted for confounding variables, male gender was independently associated with an increased risk of death (adjusted hazard ratio 1.37; P < .001), but age was not.
“It was particularly surprising to see that even at such a young age, there is a significant difference in overall survival between males and females, where females have better outcomes than males,” Dr. Thiede said. “When examining pediatric and adolescent patients, it is essential to maintain cutaneous melanoma on the differential,” she advised. “It is important for clinicians to perform a thorough exam at annual visits particularly for those at high risk for melanoma to catch this rare but potentially devastating diagnosis.”
She acknowledged certain limitations of the study, including its reliance on one database, “as comparing multiple databases would strengthen the conclusions,” she said. “There was some missing data present in our dataset, and a large percentage of the histologic subtypes were unspecified, both of which are common issues with cancer registries. An additional limitation is related to the low death rates in adolescent and pediatric patients, which may impact the analysis related to survival and independent predictors of survival.”
Asked to comment on the study results, Carrie C. Coughlin, MD, who directs the section of pediatric dermatology Washington University/St. Louis Children’s Hospital, said that the finding that males were more likely to present with stage II or higher disease compared with females “could be related to their finding that females had more superficial spreading melanomas, whereas males had more nodular melanoma.” Those differences “could influence how providers evaluate melanocytic lesions in children,” she added.
Dr. Coughlin, who directs the pediatric dermatology fellowship at Washington University/St. Louis Children’s Hospital, said it was “interesting” that the authors found no association between older age and an increased risk of death. “It would be helpful to have more data about melanoma subtype, including information about Spitz or Spitzoid melanomas,” she said. “Also, knowing the distribution of melanoma across the age categories could provide more insight into their data.”
Ms. Dahak received an award from the National Cancer Institute to fund travel for presentation of this study at the SPD meeting. No other financial conflicts were reported by the researchers. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – .
In addition, male gender was independently associated with increased mortality, but age was not.
Those are key findings from a retrospective cohort analysis of nearly 5,000 records from the National Cancer Database.
“There are multiple studies from primarily adult populations showing females with melanoma have a different presentation and better outcomes than males,” co-first author Rebecca M. Thiede, MD, a dermatologist at the University of Arizona, Tucson, said in an interview with this news organization in advance of the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session. “However, because melanoma is so rare in younger patients, little is known about gender differences in presentation and survival in pediatric and adolescent patients. To our knowledge, this is one of the largest studies to date in this population, and the first to explore gender differences in detail in pediatric and adolescent patients with melanoma.”
Working with co-first author Sabrina Dahak, a fourth-year medical student at the University of Arizona, Phoenix, Dr. Thiede and colleagues retrospectively analyzed the National Cancer Database to identify biopsy-confirmed invasive primary cutaneous melanoma cases diagnosed in patients 0-21 years of age between 2004 and 2018. The search yielded 4,645 cases, and the researchers used American Academy of Pediatrics definitions to categorize the patients by age, from infancy (birth to 2 years), to childhood (3-10 years), early adolescence (11-14 years), middle adolescence (15-17 years), and late adolescence (18-21 years). They used the Kaplan Meier analysis to determine overall survival and multivariate Cox regression to determine independent survival predictors.
Of the 4,645 pediatric melanoma cases, 63.4% were in females and 36.6% were in males, a difference that was significant (P < .001). Dr. Thiede and colleagues also observed a significant relationship between primary site and gender (P < .001). Primary sites included the trunk (34.3% of females vs. 32.9% of males, respectively), head and neck (16.4% vs. 30.9%), upper extremities (19.5% vs. 16%), lower extremities (27.9% vs. 16.5%), and “unspecified” (1.9% vs. 3.7%).
Females had higher rates of superficial spreading melanoma while males were affected by nodular melanoma more often. For example, the median Breslow depth was higher for males (1.05 mm; interquartile range [IQR] 0.50-2.31) than for females (0.80 mm; IQR, 0.40-1.67; P < .001).
Although females accounted for a higher percentage of cases than males overall, from birth to 17 years, a higher percentage of males than females were found to have later stage of melanoma at time of diagnosis: Females were more likely to be diagnosed with stage I disease (67.8%) than were males (53.6%), and males were more likely than were females to be diagnosed with stages II (15.9% vs. 12.3%), III (27.1% vs. 18.3%), and IV disease (3.3% vs. 1.6%; P < .001 for all).
In other findings, the 5- and 10-year overall survival rates were higher for females (95.9% and 93.9%, respectively) than for males (92.0% vs. 86.7%, respectively; P < .001). However, by age group, overall survival rates were similar between females and males among infants, children, and those in early adolescence – but not for those in middle adolescence (96.7% vs. 91.9%; P < .001) or late adolescence (95.7% vs. 90.4%; P < .001).
When the researchers adjusted for confounding variables, male gender was independently associated with an increased risk of death (adjusted hazard ratio 1.37; P < .001), but age was not.
“It was particularly surprising to see that even at such a young age, there is a significant difference in overall survival between males and females, where females have better outcomes than males,” Dr. Thiede said. “When examining pediatric and adolescent patients, it is essential to maintain cutaneous melanoma on the differential,” she advised. “It is important for clinicians to perform a thorough exam at annual visits particularly for those at high risk for melanoma to catch this rare but potentially devastating diagnosis.”
She acknowledged certain limitations of the study, including its reliance on one database, “as comparing multiple databases would strengthen the conclusions,” she said. “There was some missing data present in our dataset, and a large percentage of the histologic subtypes were unspecified, both of which are common issues with cancer registries. An additional limitation is related to the low death rates in adolescent and pediatric patients, which may impact the analysis related to survival and independent predictors of survival.”
Asked to comment on the study results, Carrie C. Coughlin, MD, who directs the section of pediatric dermatology Washington University/St. Louis Children’s Hospital, said that the finding that males were more likely to present with stage II or higher disease compared with females “could be related to their finding that females had more superficial spreading melanomas, whereas males had more nodular melanoma.” Those differences “could influence how providers evaluate melanocytic lesions in children,” she added.
Dr. Coughlin, who directs the pediatric dermatology fellowship at Washington University/St. Louis Children’s Hospital, said it was “interesting” that the authors found no association between older age and an increased risk of death. “It would be helpful to have more data about melanoma subtype, including information about Spitz or Spitzoid melanomas,” she said. “Also, knowing the distribution of melanoma across the age categories could provide more insight into their data.”
Ms. Dahak received an award from the National Cancer Institute to fund travel for presentation of this study at the SPD meeting. No other financial conflicts were reported by the researchers. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance (PeDRA) and the International Immunosuppression and Transplant Skin Cancer Collaborative.
AT SPD 2022
Ruxolitinib found to benefit adolescents with vitiligo up to one year
INDIANAPOLIS – and a higher proportion responded at week 52, results from a pooled analysis of phase 3 data showed.
Currently, there is no treatment approved by the Food and Drug Administration to repigment patients with vitiligo, but the cream formulation of the Janus kinase inhibitor ruxolitinib was shown to be effective and have a favorable safety profile in patients aged 12 years and up in the phase 3 clinical trials, TRuE-V1 and TruE-V2. “We know that about half of patients will develop vitiligo by the age of 20, so there is a significant need to have treatments available for the pediatric population,” lead study author David Rosmarin, MD, told this news organization in advance of the annual meeting of the Society for Pediatric Dermatology.
In September 2021, topical ruxolitinib (Opzelura) was approved by the FDA for treating atopic dermatitis in nonimmunocompromised patients aged 12 years and older. The manufacturer, Incyte, has submitted an application for approval to the agency for treating vitiligo in patients ages 12 years and older based on 24-week results; the FDA is expected to make a decision by July 18.
For the current study, presented during a poster session at the meeting, Dr. Rosmarin, of the department of dermatology at Tufts Medical Center, Boston, and colleagues pooled efficacy and safety data for adolescent patients aged 12-17 years from the TRuE-V studies, which enrolled patients 12 years of age and older diagnosed with nonsegmental vitiligo with depigmentation covering up to 10% of total body surface area (BSA), including facial and total Vitiligo Area Scoring Index (F-VASI/T-VASI) scores of ≥ 0.5/≥ 3. Investigators randomized patients 2:1 to twice-daily 1.5% ruxolitinib cream or vehicle for 24 weeks, after which all patients could apply 1.5% ruxolitinib cream through week 52. Efficacy endpoints included the proportions of patients who achieved at least 75%, 50%, and 90% improvement from baseline in F-VASI scores (F-VASI75, F-VASI50, F-VASI90); the proportion of patients who achieved at least a 50% improvement from baseline in T-VASI (T-VASI50); the proportion of patients who achieved a Vitiligo Noticeability Scale (VNS) rating of 4 or 5; and percentage change from baseline in facial BSA (F-BSA). Safety and tolerability were also assessed.
For the pooled analysis, Dr. Rosmarin and colleagues reported results on 72 adolescents: 55 who received ruxolitinib cream and 17 who received vehicle. At week 24, 32.1% of adolescents treated with ruxolitinib cream achieved F-VASI75, compared with none of those in the vehicle group. Further, response rates at week 52 for patients who applied ruxolitinib cream from day 1 were as follows: F-VASI75, 48.0%; F-VASI50, 70.0%; F-VASI90, 24.0%; T-VASI50, 60.0%; VNS score of 4/5, 56.0%; and F-BSA mean percentage change from baseline, –41.9%.
Efficacy at week 52 among crossover patients (after 28 weeks of ruxolitinib cream) was consistent with week 24 data in patients who applied ruxolitinib cream from day 1.
“As we know that repigmentation takes time, about half of the patients achieved the F-VASI75 at the 52-week endpoint,” said Dr. Rosmarin, who is also vice-chair for research and education at Tufts Medical Center, Boston. “Particularly remarkable is that 60% of adolescents achieved a T-VASI50 [50% or more repigmentation of the whole body at the year mark] and over half the patients described their vitiligo as a lot less noticeable or no longer noticeable at the year mark.”
In terms of safety, treatment-related adverse events occurred in 12.9% of patients treated with ruxolitinib (no information was available on the specific events). Serious adverse events occurred in 1.4% of patients; none were considered related to treatment.
“Overall, these results are quite impressive,” Dr. Rosmarin said. “While it can be very challenging to repigment patients with vitiligo, ruxolitinib cream provides an effective option which can help many of my patients.” He acknowledged certain limitations of the analysis, including the fact that the TRuE-V studies were conducted during the COVID-19 pandemic, “which may have contributed to patients being lost to follow-up. Also, the majority of the patients had skin phototypes 1-3.”
Carrie C. Coughlin, MD, who was asked to comment on the study, said that patients with vitiligo need treatment options that are well-studied and covered by insurance. “This study is a great step forward in developing medications for this underserved patient population,” said Dr. Coughlin, who directs the section of pediatric dermatology at Washington University/St. Louis Children’s Hospital.
However, she continued, “the authors mention approximately 13% of patients had a treatment-related adverse reaction, but the abstract does not delineate these reactions.” In addition, the study was limited to children who had less than or equal to 10% body surface area involvement of vitiligo, she noted, adding that “more work is needed to learn about safety of application to larger surface areas.”
Going forward, “it will be important to learn the durability of response,” said Dr. Coughlin, who is also assistant professor of dermatology at Washington University in St. Louis. “Does the vitiligo return if patients stop applying the ruxolitinib cream?”
Dr. Rosmarin disclosed that he has received honoraria as a consultant for Incyte, AbbVie, Abcuro, AltruBio, Arena, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and VielaBio. He has also received research support from Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Dermira, Galderma, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron; and has served as a paid speaker for Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Incyte, Janssen, Lilly, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – and a higher proportion responded at week 52, results from a pooled analysis of phase 3 data showed.
Currently, there is no treatment approved by the Food and Drug Administration to repigment patients with vitiligo, but the cream formulation of the Janus kinase inhibitor ruxolitinib was shown to be effective and have a favorable safety profile in patients aged 12 years and up in the phase 3 clinical trials, TRuE-V1 and TruE-V2. “We know that about half of patients will develop vitiligo by the age of 20, so there is a significant need to have treatments available for the pediatric population,” lead study author David Rosmarin, MD, told this news organization in advance of the annual meeting of the Society for Pediatric Dermatology.
In September 2021, topical ruxolitinib (Opzelura) was approved by the FDA for treating atopic dermatitis in nonimmunocompromised patients aged 12 years and older. The manufacturer, Incyte, has submitted an application for approval to the agency for treating vitiligo in patients ages 12 years and older based on 24-week results; the FDA is expected to make a decision by July 18.
For the current study, presented during a poster session at the meeting, Dr. Rosmarin, of the department of dermatology at Tufts Medical Center, Boston, and colleagues pooled efficacy and safety data for adolescent patients aged 12-17 years from the TRuE-V studies, which enrolled patients 12 years of age and older diagnosed with nonsegmental vitiligo with depigmentation covering up to 10% of total body surface area (BSA), including facial and total Vitiligo Area Scoring Index (F-VASI/T-VASI) scores of ≥ 0.5/≥ 3. Investigators randomized patients 2:1 to twice-daily 1.5% ruxolitinib cream or vehicle for 24 weeks, after which all patients could apply 1.5% ruxolitinib cream through week 52. Efficacy endpoints included the proportions of patients who achieved at least 75%, 50%, and 90% improvement from baseline in F-VASI scores (F-VASI75, F-VASI50, F-VASI90); the proportion of patients who achieved at least a 50% improvement from baseline in T-VASI (T-VASI50); the proportion of patients who achieved a Vitiligo Noticeability Scale (VNS) rating of 4 or 5; and percentage change from baseline in facial BSA (F-BSA). Safety and tolerability were also assessed.
For the pooled analysis, Dr. Rosmarin and colleagues reported results on 72 adolescents: 55 who received ruxolitinib cream and 17 who received vehicle. At week 24, 32.1% of adolescents treated with ruxolitinib cream achieved F-VASI75, compared with none of those in the vehicle group. Further, response rates at week 52 for patients who applied ruxolitinib cream from day 1 were as follows: F-VASI75, 48.0%; F-VASI50, 70.0%; F-VASI90, 24.0%; T-VASI50, 60.0%; VNS score of 4/5, 56.0%; and F-BSA mean percentage change from baseline, –41.9%.
Efficacy at week 52 among crossover patients (after 28 weeks of ruxolitinib cream) was consistent with week 24 data in patients who applied ruxolitinib cream from day 1.
“As we know that repigmentation takes time, about half of the patients achieved the F-VASI75 at the 52-week endpoint,” said Dr. Rosmarin, who is also vice-chair for research and education at Tufts Medical Center, Boston. “Particularly remarkable is that 60% of adolescents achieved a T-VASI50 [50% or more repigmentation of the whole body at the year mark] and over half the patients described their vitiligo as a lot less noticeable or no longer noticeable at the year mark.”
In terms of safety, treatment-related adverse events occurred in 12.9% of patients treated with ruxolitinib (no information was available on the specific events). Serious adverse events occurred in 1.4% of patients; none were considered related to treatment.
“Overall, these results are quite impressive,” Dr. Rosmarin said. “While it can be very challenging to repigment patients with vitiligo, ruxolitinib cream provides an effective option which can help many of my patients.” He acknowledged certain limitations of the analysis, including the fact that the TRuE-V studies were conducted during the COVID-19 pandemic, “which may have contributed to patients being lost to follow-up. Also, the majority of the patients had skin phototypes 1-3.”
Carrie C. Coughlin, MD, who was asked to comment on the study, said that patients with vitiligo need treatment options that are well-studied and covered by insurance. “This study is a great step forward in developing medications for this underserved patient population,” said Dr. Coughlin, who directs the section of pediatric dermatology at Washington University/St. Louis Children’s Hospital.
However, she continued, “the authors mention approximately 13% of patients had a treatment-related adverse reaction, but the abstract does not delineate these reactions.” In addition, the study was limited to children who had less than or equal to 10% body surface area involvement of vitiligo, she noted, adding that “more work is needed to learn about safety of application to larger surface areas.”
Going forward, “it will be important to learn the durability of response,” said Dr. Coughlin, who is also assistant professor of dermatology at Washington University in St. Louis. “Does the vitiligo return if patients stop applying the ruxolitinib cream?”
Dr. Rosmarin disclosed that he has received honoraria as a consultant for Incyte, AbbVie, Abcuro, AltruBio, Arena, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and VielaBio. He has also received research support from Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Dermira, Galderma, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron; and has served as a paid speaker for Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Incyte, Janssen, Lilly, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance and the International Immunosuppression and Transplant Skin Cancer Collaborative.
INDIANAPOLIS – and a higher proportion responded at week 52, results from a pooled analysis of phase 3 data showed.
Currently, there is no treatment approved by the Food and Drug Administration to repigment patients with vitiligo, but the cream formulation of the Janus kinase inhibitor ruxolitinib was shown to be effective and have a favorable safety profile in patients aged 12 years and up in the phase 3 clinical trials, TRuE-V1 and TruE-V2. “We know that about half of patients will develop vitiligo by the age of 20, so there is a significant need to have treatments available for the pediatric population,” lead study author David Rosmarin, MD, told this news organization in advance of the annual meeting of the Society for Pediatric Dermatology.
In September 2021, topical ruxolitinib (Opzelura) was approved by the FDA for treating atopic dermatitis in nonimmunocompromised patients aged 12 years and older. The manufacturer, Incyte, has submitted an application for approval to the agency for treating vitiligo in patients ages 12 years and older based on 24-week results; the FDA is expected to make a decision by July 18.
For the current study, presented during a poster session at the meeting, Dr. Rosmarin, of the department of dermatology at Tufts Medical Center, Boston, and colleagues pooled efficacy and safety data for adolescent patients aged 12-17 years from the TRuE-V studies, which enrolled patients 12 years of age and older diagnosed with nonsegmental vitiligo with depigmentation covering up to 10% of total body surface area (BSA), including facial and total Vitiligo Area Scoring Index (F-VASI/T-VASI) scores of ≥ 0.5/≥ 3. Investigators randomized patients 2:1 to twice-daily 1.5% ruxolitinib cream or vehicle for 24 weeks, after which all patients could apply 1.5% ruxolitinib cream through week 52. Efficacy endpoints included the proportions of patients who achieved at least 75%, 50%, and 90% improvement from baseline in F-VASI scores (F-VASI75, F-VASI50, F-VASI90); the proportion of patients who achieved at least a 50% improvement from baseline in T-VASI (T-VASI50); the proportion of patients who achieved a Vitiligo Noticeability Scale (VNS) rating of 4 or 5; and percentage change from baseline in facial BSA (F-BSA). Safety and tolerability were also assessed.
For the pooled analysis, Dr. Rosmarin and colleagues reported results on 72 adolescents: 55 who received ruxolitinib cream and 17 who received vehicle. At week 24, 32.1% of adolescents treated with ruxolitinib cream achieved F-VASI75, compared with none of those in the vehicle group. Further, response rates at week 52 for patients who applied ruxolitinib cream from day 1 were as follows: F-VASI75, 48.0%; F-VASI50, 70.0%; F-VASI90, 24.0%; T-VASI50, 60.0%; VNS score of 4/5, 56.0%; and F-BSA mean percentage change from baseline, –41.9%.
Efficacy at week 52 among crossover patients (after 28 weeks of ruxolitinib cream) was consistent with week 24 data in patients who applied ruxolitinib cream from day 1.
“As we know that repigmentation takes time, about half of the patients achieved the F-VASI75 at the 52-week endpoint,” said Dr. Rosmarin, who is also vice-chair for research and education at Tufts Medical Center, Boston. “Particularly remarkable is that 60% of adolescents achieved a T-VASI50 [50% or more repigmentation of the whole body at the year mark] and over half the patients described their vitiligo as a lot less noticeable or no longer noticeable at the year mark.”
In terms of safety, treatment-related adverse events occurred in 12.9% of patients treated with ruxolitinib (no information was available on the specific events). Serious adverse events occurred in 1.4% of patients; none were considered related to treatment.
“Overall, these results are quite impressive,” Dr. Rosmarin said. “While it can be very challenging to repigment patients with vitiligo, ruxolitinib cream provides an effective option which can help many of my patients.” He acknowledged certain limitations of the analysis, including the fact that the TRuE-V studies were conducted during the COVID-19 pandemic, “which may have contributed to patients being lost to follow-up. Also, the majority of the patients had skin phototypes 1-3.”
Carrie C. Coughlin, MD, who was asked to comment on the study, said that patients with vitiligo need treatment options that are well-studied and covered by insurance. “This study is a great step forward in developing medications for this underserved patient population,” said Dr. Coughlin, who directs the section of pediatric dermatology at Washington University/St. Louis Children’s Hospital.
However, she continued, “the authors mention approximately 13% of patients had a treatment-related adverse reaction, but the abstract does not delineate these reactions.” In addition, the study was limited to children who had less than or equal to 10% body surface area involvement of vitiligo, she noted, adding that “more work is needed to learn about safety of application to larger surface areas.”
Going forward, “it will be important to learn the durability of response,” said Dr. Coughlin, who is also assistant professor of dermatology at Washington University in St. Louis. “Does the vitiligo return if patients stop applying the ruxolitinib cream?”
Dr. Rosmarin disclosed that he has received honoraria as a consultant for Incyte, AbbVie, Abcuro, AltruBio, Arena, Boehringer Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and VielaBio. He has also received research support from Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Dermira, Galderma, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron; and has served as a paid speaker for Incyte, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Incyte, Janssen, Lilly, Novartis, Pfizer, Regeneron, and Sanofi. Dr. Coughlin is on the board of the Pediatric Dermatology Research Alliance and the International Immunosuppression and Transplant Skin Cancer Collaborative.
AT SPD 2022
To vaccinate 6-month- to 5-year-olds against SARS-CoV-2 or not to vaccinate
A family’s decision to vaccinate their child is best made jointly with a trusted medical provider who knows the child and family. The American Academy of Pediatrics created a toolkit with resources for answering questions about the recently authorized SARS-CoV-2 mRNA vaccines (Pfizer and Moderna) for 6-month- to 5-year-olds with science-backed vaccine facts, including links to other useful AAP information websites, talking points, graphics, and videos.1
SARS-CoV-2 seasonality
SARS-CoV-2 is now endemic, not a once-a-year seasonal virus. Seasons (aka surges) will occur whenever a new variant arises (twice yearly since 2020, Omicron BA.4/BA.5 currently), or when enough vaccine holdouts, newborns, and/or those with waning of prior immunity (vaccine or infection induced) accrue.
Emergency use authorization submission data for mRNA vaccine responses in young children2,3
Moderna in 6-month- through 5-year-olds. Two 25-mcg doses given 4-8 weeks apart produced 37.8% (95% confidence interval, 20.9%-51.1%) protection against symptomatic Omicron SARS-CoV-2 infections through 3 months of follow-up. Immunobridging analysis of antibody responses compared to 18- to 25-year-olds (100-mcg doses) showed the children’s responses were noninferior. Thus, the committee inferred that vaccine effectiveness in children should be similar to that in 18- to 25-year-olds. Fever, irritability, or local reaction/pain occurred in two-thirds after the second dose. Grade 3 reactions were noted in less than 5%.
Pfizer in 6-month- through 4-year-olds. Three 3-mcg doses, two doses 3-8 weeks apart and the third dose at least 8 weeks later (median 16 weeks), produced 80.3% (95% CI, 13.9%-96.7%) protection against symptomatic COVID-19 during the 6 weeks after the third dose. Local and systemic reactions occurred in 63.8%; less than 5% had grade 3 reactions (fever in about 3%, irritability in 1.3%, fatigue in 0.8%) mostly after second dose.
Neither duration of follow-up is very long. The Moderna data tell me that a third primary dose would have been better but restarting the trial to evaluate third doses would have delayed Moderna’s EUA another 4-6 months. The three-dose Pfizer data look better but may not have been as good with another 6 weeks of follow-up.
Additional post-EUA data will be collected. Boosters will be needed when immunity from both vaccines wanes (one estimate is about 6 months after the primary series). The Advisory Committee on Immunization Practices noted in their deliberations that vaccine-induced antibody responses are higher and cross-neutralize variants (even Omicron) better than infection-induced immunity.4
Are there downsides to the vaccines? Naysayers question vaccinating children less than 5 years old with reasons containing enough “truth” that they catch people’s attention, for example, “young children don’t get very sick with COVID-19,” “most have been infected already,” “RNA for the spike protein stays in the body for months,” or “myocarditis.” Naysayers can quote references in reputable journals but seem to spin selected data out of context or quote unconfirmed data from the Vaccine Adverse Event Reporting System.
Reasons to vaccinate
- While children have milder disease than adults, mid-June 2022 surveillance indicated 50 hospitalizations and 1 pediatric death each day from SARS-CoV-2.5
- Vaccinating young children endows a foundation of vaccine-induced SARS-CoV-2 immunity that is superior to infection-induced immunity.4
- Long-term effects of large numbers of SARS-CoV-2 particles that enter every organ of a developing child have not been determined.
- Viral loads are lowered by prior vaccine; fewer viral replications lessen chances for newer variants to arise.
- Transmission is less in breakthrough infections than infections in the unvaccinated.
- Thirty percent of 5- to 11-year-olds hospitalized for SARS-CoV-2 had no underlying conditions;6 hospitalization rates in newborn to 4-year-olds have been the highest in the Omicron surge.7
- No myocarditis or pericarditis episodes have been detected in 6-month- to 11-year-old trials.
- The AAP and ACIP recommend the mRNA vaccines.
My thoughts are that SARS-CoV-2 vaccine is just another “routine” childhood vaccine that prepares children for healthier futures, pandemic or not, and the vaccines are as safe as other routine vaccines.
And like other pediatric vaccines, it should be no surprise that boosters will be needed, even if no newer variants than Omicron BA.4/BA.5 arise. But we know newer variants will arise and, similar to influenza vaccine, new formulations, perhaps with multiple SARS-CoV-2 strain antigens, will be needed every year or so. Everyone will get SARS-CoV-2 multiple times in their lives no matter how careful they are. So isn’t it good medical practice to establish early the best available foundation for maintaining lifelong SARS-CoV-2 immunity?
To me it is like pertussis. Most pertussis-infected children are sick enough to be hospitalized; very few die. They are miserable with illnesses that take weeks to months to subside. The worst disease usually occurs in unvaccinated young children or those with underlying conditions. Reactogenicity was reduced with acellular vaccine but resulted in less immunogenicity, so we give boosters at intervals that best match waning immunity. Circulating strains can be different than the vaccine strain, so protection against infection is 80%. Finally, even the safest vaccine may very rarely have sequelae. That is why The National Vaccine Injury Compensation Program was created. Yet the benefit-to-harm ratio for children and society favors universal pertussis vaccine use. And we vaccinate even those who have had pertussis because even infection-based immunity is incomplete and protection wanes. If arguments similar to those by SARS-CoV-2 vaccine naysayers were applied to acellular pertussis vaccine, it seems they would argue against pertussis vaccine for young children.
Another major issue has been “safety concerns” about the vaccines’ small amount of mRNA for the spike protein encased in microscopic lipid bubbles injected in the arm or leg. This mRNA is picked up by human cells, and in the cytoplasm (not the nucleus where our DNA resides) produces a limited supply of spike protein that is then picked up by antigen-presenting cells for short-lived distribution (days to 2 weeks at most) to regional lymph nodes where immune-memory processes are jump-started. Contrast that to even asymptomatic SARS-CoV-2 infection where multibillions of virus particles are produced for up to 14 days with access to every bodily organ that contains ACE-2 receptors (they all do). Each virus particle hijacks a human cell producing thousands of mRNA for spike protein (and multiple other SARS-CoV-2 proteins), eventually releasing multibillions of lipid fragments from the ruptured cell. Comparing the amount of these components in the mRNA vaccines to those from infection is like comparing a campfire to the many-thousand-acre wildfire. So, if one is worried about the effects of spike protein and lipid fragments, the limited localized amounts in mRNA vaccines should make one much less concerned than the enormous amounts circulating throughout the body as a result of a SARS-CoV-2 infection.
My take is that children 6-months to 5-years-old deserve SARS-CoV-2–induced vaccine protection and we can and should strongly recommend it as medical providers and child advocates.
*Dr. Harrison is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at pdnews@mdedge.com.
References
1. AAP. 2022 Jun 21. As COVID-19 vaccines become available for children ages 6 months to 4 years, AAP urges families to reach out to pediatricians to ask questions and access vaccine. www.aap.org.
2. CDC. Grading of recommendations, assessment, development, and evaluation (GRADE): Moderna COVID-19 vaccine for children aged 6 months–5 years. www.cdc.gov.
3. CDC. ACIP evidence to recommendations for use of Moderna COVID-19 vaccine in children ages 6 months–5 years and Pfizer-BioNTech COVID-19 vaccine in children ages 6 months–4 years under an emergency use authorization. www.cdc.gov.
4. Tang J et al. Nat Commun. 2022;13:2979.
5. Children and COVID-19: State Data Report. 2022 Jun 30. www.aap.org.
6. Shi DS et al. MMWR Morb Mortal Wkly Rep. 2022;71:574-81.
7. Marks KJ et al. MMWR Morb Mortal Wkly Rep. 2022;71:429-36.
Other good resources for families are https://getvaccineanswers.org/ or www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-in-babies-and-children/art-20484405.
*This story was updated on July 19, 2022.
A family’s decision to vaccinate their child is best made jointly with a trusted medical provider who knows the child and family. The American Academy of Pediatrics created a toolkit with resources for answering questions about the recently authorized SARS-CoV-2 mRNA vaccines (Pfizer and Moderna) for 6-month- to 5-year-olds with science-backed vaccine facts, including links to other useful AAP information websites, talking points, graphics, and videos.1
SARS-CoV-2 seasonality
SARS-CoV-2 is now endemic, not a once-a-year seasonal virus. Seasons (aka surges) will occur whenever a new variant arises (twice yearly since 2020, Omicron BA.4/BA.5 currently), or when enough vaccine holdouts, newborns, and/or those with waning of prior immunity (vaccine or infection induced) accrue.
Emergency use authorization submission data for mRNA vaccine responses in young children2,3
Moderna in 6-month- through 5-year-olds. Two 25-mcg doses given 4-8 weeks apart produced 37.8% (95% confidence interval, 20.9%-51.1%) protection against symptomatic Omicron SARS-CoV-2 infections through 3 months of follow-up. Immunobridging analysis of antibody responses compared to 18- to 25-year-olds (100-mcg doses) showed the children’s responses were noninferior. Thus, the committee inferred that vaccine effectiveness in children should be similar to that in 18- to 25-year-olds. Fever, irritability, or local reaction/pain occurred in two-thirds after the second dose. Grade 3 reactions were noted in less than 5%.
Pfizer in 6-month- through 4-year-olds. Three 3-mcg doses, two doses 3-8 weeks apart and the third dose at least 8 weeks later (median 16 weeks), produced 80.3% (95% CI, 13.9%-96.7%) protection against symptomatic COVID-19 during the 6 weeks after the third dose. Local and systemic reactions occurred in 63.8%; less than 5% had grade 3 reactions (fever in about 3%, irritability in 1.3%, fatigue in 0.8%) mostly after second dose.
Neither duration of follow-up is very long. The Moderna data tell me that a third primary dose would have been better but restarting the trial to evaluate third doses would have delayed Moderna’s EUA another 4-6 months. The three-dose Pfizer data look better but may not have been as good with another 6 weeks of follow-up.
Additional post-EUA data will be collected. Boosters will be needed when immunity from both vaccines wanes (one estimate is about 6 months after the primary series). The Advisory Committee on Immunization Practices noted in their deliberations that vaccine-induced antibody responses are higher and cross-neutralize variants (even Omicron) better than infection-induced immunity.4
Are there downsides to the vaccines? Naysayers question vaccinating children less than 5 years old with reasons containing enough “truth” that they catch people’s attention, for example, “young children don’t get very sick with COVID-19,” “most have been infected already,” “RNA for the spike protein stays in the body for months,” or “myocarditis.” Naysayers can quote references in reputable journals but seem to spin selected data out of context or quote unconfirmed data from the Vaccine Adverse Event Reporting System.
Reasons to vaccinate
- While children have milder disease than adults, mid-June 2022 surveillance indicated 50 hospitalizations and 1 pediatric death each day from SARS-CoV-2.5
- Vaccinating young children endows a foundation of vaccine-induced SARS-CoV-2 immunity that is superior to infection-induced immunity.4
- Long-term effects of large numbers of SARS-CoV-2 particles that enter every organ of a developing child have not been determined.
- Viral loads are lowered by prior vaccine; fewer viral replications lessen chances for newer variants to arise.
- Transmission is less in breakthrough infections than infections in the unvaccinated.
- Thirty percent of 5- to 11-year-olds hospitalized for SARS-CoV-2 had no underlying conditions;6 hospitalization rates in newborn to 4-year-olds have been the highest in the Omicron surge.7
- No myocarditis or pericarditis episodes have been detected in 6-month- to 11-year-old trials.
- The AAP and ACIP recommend the mRNA vaccines.
My thoughts are that SARS-CoV-2 vaccine is just another “routine” childhood vaccine that prepares children for healthier futures, pandemic or not, and the vaccines are as safe as other routine vaccines.
And like other pediatric vaccines, it should be no surprise that boosters will be needed, even if no newer variants than Omicron BA.4/BA.5 arise. But we know newer variants will arise and, similar to influenza vaccine, new formulations, perhaps with multiple SARS-CoV-2 strain antigens, will be needed every year or so. Everyone will get SARS-CoV-2 multiple times in their lives no matter how careful they are. So isn’t it good medical practice to establish early the best available foundation for maintaining lifelong SARS-CoV-2 immunity?
To me it is like pertussis. Most pertussis-infected children are sick enough to be hospitalized; very few die. They are miserable with illnesses that take weeks to months to subside. The worst disease usually occurs in unvaccinated young children or those with underlying conditions. Reactogenicity was reduced with acellular vaccine but resulted in less immunogenicity, so we give boosters at intervals that best match waning immunity. Circulating strains can be different than the vaccine strain, so protection against infection is 80%. Finally, even the safest vaccine may very rarely have sequelae. That is why The National Vaccine Injury Compensation Program was created. Yet the benefit-to-harm ratio for children and society favors universal pertussis vaccine use. And we vaccinate even those who have had pertussis because even infection-based immunity is incomplete and protection wanes. If arguments similar to those by SARS-CoV-2 vaccine naysayers were applied to acellular pertussis vaccine, it seems they would argue against pertussis vaccine for young children.
Another major issue has been “safety concerns” about the vaccines’ small amount of mRNA for the spike protein encased in microscopic lipid bubbles injected in the arm or leg. This mRNA is picked up by human cells, and in the cytoplasm (not the nucleus where our DNA resides) produces a limited supply of spike protein that is then picked up by antigen-presenting cells for short-lived distribution (days to 2 weeks at most) to regional lymph nodes where immune-memory processes are jump-started. Contrast that to even asymptomatic SARS-CoV-2 infection where multibillions of virus particles are produced for up to 14 days with access to every bodily organ that contains ACE-2 receptors (they all do). Each virus particle hijacks a human cell producing thousands of mRNA for spike protein (and multiple other SARS-CoV-2 proteins), eventually releasing multibillions of lipid fragments from the ruptured cell. Comparing the amount of these components in the mRNA vaccines to those from infection is like comparing a campfire to the many-thousand-acre wildfire. So, if one is worried about the effects of spike protein and lipid fragments, the limited localized amounts in mRNA vaccines should make one much less concerned than the enormous amounts circulating throughout the body as a result of a SARS-CoV-2 infection.
My take is that children 6-months to 5-years-old deserve SARS-CoV-2–induced vaccine protection and we can and should strongly recommend it as medical providers and child advocates.
*Dr. Harrison is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at pdnews@mdedge.com.
References
1. AAP. 2022 Jun 21. As COVID-19 vaccines become available for children ages 6 months to 4 years, AAP urges families to reach out to pediatricians to ask questions and access vaccine. www.aap.org.
2. CDC. Grading of recommendations, assessment, development, and evaluation (GRADE): Moderna COVID-19 vaccine for children aged 6 months–5 years. www.cdc.gov.
3. CDC. ACIP evidence to recommendations for use of Moderna COVID-19 vaccine in children ages 6 months–5 years and Pfizer-BioNTech COVID-19 vaccine in children ages 6 months–4 years under an emergency use authorization. www.cdc.gov.
4. Tang J et al. Nat Commun. 2022;13:2979.
5. Children and COVID-19: State Data Report. 2022 Jun 30. www.aap.org.
6. Shi DS et al. MMWR Morb Mortal Wkly Rep. 2022;71:574-81.
7. Marks KJ et al. MMWR Morb Mortal Wkly Rep. 2022;71:429-36.
Other good resources for families are https://getvaccineanswers.org/ or www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-in-babies-and-children/art-20484405.
*This story was updated on July 19, 2022.
A family’s decision to vaccinate their child is best made jointly with a trusted medical provider who knows the child and family. The American Academy of Pediatrics created a toolkit with resources for answering questions about the recently authorized SARS-CoV-2 mRNA vaccines (Pfizer and Moderna) for 6-month- to 5-year-olds with science-backed vaccine facts, including links to other useful AAP information websites, talking points, graphics, and videos.1
SARS-CoV-2 seasonality
SARS-CoV-2 is now endemic, not a once-a-year seasonal virus. Seasons (aka surges) will occur whenever a new variant arises (twice yearly since 2020, Omicron BA.4/BA.5 currently), or when enough vaccine holdouts, newborns, and/or those with waning of prior immunity (vaccine or infection induced) accrue.
Emergency use authorization submission data for mRNA vaccine responses in young children2,3
Moderna in 6-month- through 5-year-olds. Two 25-mcg doses given 4-8 weeks apart produced 37.8% (95% confidence interval, 20.9%-51.1%) protection against symptomatic Omicron SARS-CoV-2 infections through 3 months of follow-up. Immunobridging analysis of antibody responses compared to 18- to 25-year-olds (100-mcg doses) showed the children’s responses were noninferior. Thus, the committee inferred that vaccine effectiveness in children should be similar to that in 18- to 25-year-olds. Fever, irritability, or local reaction/pain occurred in two-thirds after the second dose. Grade 3 reactions were noted in less than 5%.
Pfizer in 6-month- through 4-year-olds. Three 3-mcg doses, two doses 3-8 weeks apart and the third dose at least 8 weeks later (median 16 weeks), produced 80.3% (95% CI, 13.9%-96.7%) protection against symptomatic COVID-19 during the 6 weeks after the third dose. Local and systemic reactions occurred in 63.8%; less than 5% had grade 3 reactions (fever in about 3%, irritability in 1.3%, fatigue in 0.8%) mostly after second dose.
Neither duration of follow-up is very long. The Moderna data tell me that a third primary dose would have been better but restarting the trial to evaluate third doses would have delayed Moderna’s EUA another 4-6 months. The three-dose Pfizer data look better but may not have been as good with another 6 weeks of follow-up.
Additional post-EUA data will be collected. Boosters will be needed when immunity from both vaccines wanes (one estimate is about 6 months after the primary series). The Advisory Committee on Immunization Practices noted in their deliberations that vaccine-induced antibody responses are higher and cross-neutralize variants (even Omicron) better than infection-induced immunity.4
Are there downsides to the vaccines? Naysayers question vaccinating children less than 5 years old with reasons containing enough “truth” that they catch people’s attention, for example, “young children don’t get very sick with COVID-19,” “most have been infected already,” “RNA for the spike protein stays in the body for months,” or “myocarditis.” Naysayers can quote references in reputable journals but seem to spin selected data out of context or quote unconfirmed data from the Vaccine Adverse Event Reporting System.
Reasons to vaccinate
- While children have milder disease than adults, mid-June 2022 surveillance indicated 50 hospitalizations and 1 pediatric death each day from SARS-CoV-2.5
- Vaccinating young children endows a foundation of vaccine-induced SARS-CoV-2 immunity that is superior to infection-induced immunity.4
- Long-term effects of large numbers of SARS-CoV-2 particles that enter every organ of a developing child have not been determined.
- Viral loads are lowered by prior vaccine; fewer viral replications lessen chances for newer variants to arise.
- Transmission is less in breakthrough infections than infections in the unvaccinated.
- Thirty percent of 5- to 11-year-olds hospitalized for SARS-CoV-2 had no underlying conditions;6 hospitalization rates in newborn to 4-year-olds have been the highest in the Omicron surge.7
- No myocarditis or pericarditis episodes have been detected in 6-month- to 11-year-old trials.
- The AAP and ACIP recommend the mRNA vaccines.
My thoughts are that SARS-CoV-2 vaccine is just another “routine” childhood vaccine that prepares children for healthier futures, pandemic or not, and the vaccines are as safe as other routine vaccines.
And like other pediatric vaccines, it should be no surprise that boosters will be needed, even if no newer variants than Omicron BA.4/BA.5 arise. But we know newer variants will arise and, similar to influenza vaccine, new formulations, perhaps with multiple SARS-CoV-2 strain antigens, will be needed every year or so. Everyone will get SARS-CoV-2 multiple times in their lives no matter how careful they are. So isn’t it good medical practice to establish early the best available foundation for maintaining lifelong SARS-CoV-2 immunity?
To me it is like pertussis. Most pertussis-infected children are sick enough to be hospitalized; very few die. They are miserable with illnesses that take weeks to months to subside. The worst disease usually occurs in unvaccinated young children or those with underlying conditions. Reactogenicity was reduced with acellular vaccine but resulted in less immunogenicity, so we give boosters at intervals that best match waning immunity. Circulating strains can be different than the vaccine strain, so protection against infection is 80%. Finally, even the safest vaccine may very rarely have sequelae. That is why The National Vaccine Injury Compensation Program was created. Yet the benefit-to-harm ratio for children and society favors universal pertussis vaccine use. And we vaccinate even those who have had pertussis because even infection-based immunity is incomplete and protection wanes. If arguments similar to those by SARS-CoV-2 vaccine naysayers were applied to acellular pertussis vaccine, it seems they would argue against pertussis vaccine for young children.
Another major issue has been “safety concerns” about the vaccines’ small amount of mRNA for the spike protein encased in microscopic lipid bubbles injected in the arm or leg. This mRNA is picked up by human cells, and in the cytoplasm (not the nucleus where our DNA resides) produces a limited supply of spike protein that is then picked up by antigen-presenting cells for short-lived distribution (days to 2 weeks at most) to regional lymph nodes where immune-memory processes are jump-started. Contrast that to even asymptomatic SARS-CoV-2 infection where multibillions of virus particles are produced for up to 14 days with access to every bodily organ that contains ACE-2 receptors (they all do). Each virus particle hijacks a human cell producing thousands of mRNA for spike protein (and multiple other SARS-CoV-2 proteins), eventually releasing multibillions of lipid fragments from the ruptured cell. Comparing the amount of these components in the mRNA vaccines to those from infection is like comparing a campfire to the many-thousand-acre wildfire. So, if one is worried about the effects of spike protein and lipid fragments, the limited localized amounts in mRNA vaccines should make one much less concerned than the enormous amounts circulating throughout the body as a result of a SARS-CoV-2 infection.
My take is that children 6-months to 5-years-old deserve SARS-CoV-2–induced vaccine protection and we can and should strongly recommend it as medical providers and child advocates.
*Dr. Harrison is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. Email him at pdnews@mdedge.com.
References
1. AAP. 2022 Jun 21. As COVID-19 vaccines become available for children ages 6 months to 4 years, AAP urges families to reach out to pediatricians to ask questions and access vaccine. www.aap.org.
2. CDC. Grading of recommendations, assessment, development, and evaluation (GRADE): Moderna COVID-19 vaccine for children aged 6 months–5 years. www.cdc.gov.
3. CDC. ACIP evidence to recommendations for use of Moderna COVID-19 vaccine in children ages 6 months–5 years and Pfizer-BioNTech COVID-19 vaccine in children ages 6 months–4 years under an emergency use authorization. www.cdc.gov.
4. Tang J et al. Nat Commun. 2022;13:2979.
5. Children and COVID-19: State Data Report. 2022 Jun 30. www.aap.org.
6. Shi DS et al. MMWR Morb Mortal Wkly Rep. 2022;71:574-81.
7. Marks KJ et al. MMWR Morb Mortal Wkly Rep. 2022;71:429-36.
Other good resources for families are https://getvaccineanswers.org/ or www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-in-babies-and-children/art-20484405.
*This story was updated on July 19, 2022.
Precocious puberty – how early is too soon?
A 6-year-old girl presents with breast development. Her medical history is unremarkable. The parents are of average height, and the mother reports her thelarche was age 11 years. The girl is at the 97th percentile for her height and 90th percentile for her weight. She has Tanner stage 3 breast development and Tanner stage 2 pubic hair development. She has grown slightly more than 3 inches over the past year. How should she be evaluated and managed (N Engl J Med. 2008;358:2366-77)?
The premature onset of puberty, i.e., precocious puberty (PP), can be an emotionally traumatic event for the child and parents. Over the past century, improvements in public health and nutrition, and, more recently, increased obesity, have been associated with earlier puberty and the dominant factor has been attributed to genetics (Curr Opin Endocrinol Diabetes Obes. 2018;25[1]:49-54). This month’s article will focus on understanding what is considered “early” puberty, evaluating for causes, and managing precocious puberty.
More commonly seen in girls than boys, PP is defined as the onset of secondary sexual characteristics before age 7.5 years in Black and Hispanic girls, and prior to 8 years in White girls, which is 2-2.5 standard deviations below the average age of pubertal onset in healthy children (J Pediatr Adolesc Gynecol. 2019;32:455-9). As a comparison, PP is diagnosed with onset before age 9 years in boys. For White compared with Black girls, the average timing of thelarche is age 10 vs. 9.5 years, peak growth velocity is age 11.5, menarche is age 12.5 vs. 12, while completion of puberty is near age 14.5 vs. 13.5, respectively (J Pediatr. 1985;107:317). Fortunately, most girls with PP have common variants rather than serious pathology.
Classification: Central (CPP) vs. peripheral (PPP)
CPP is gonadotropin dependent, meaning the hypothalamic-pituitary-ovarian axis (HPO) is prematurely activated resulting in the normal progression of puberty.
PPP is gonadotropin independent, caused by sex steroid secretion from any source – ovaries, adrenal gland, exogenous or ectopic production, e.g., germ-cell tumor. This results in a disordered progression of pubertal milestones.
Whereas CPP is typically isosexual development, i.e., consistent with the child’s gender, PPP can be isosexual or contrasexual, e.g., virilization of girls. A third classification is “benign or nonprogressive pubertal variants” manifesting as isolated premature thelarche or adrenarche.
Causes (see table)
CPP. Idiopathic causes account for 80%-90% of presentations in girls and 25%-80% in boys. Remarkably, international and domestic adoption, as well as a family history of PP increases the likelihood of CPP in girls. Other etiologies include CNS lesions, e.g., hamartomas, which are the most common cause of PP in young children. MRI with contrast has been the traditional mode of diagnosis for CNS tumors, yet the yield is dubious in girls above age 6. Genetic causes are found in only a small percentage of PP cases. Rarely, CPP can result from gonadotropin-secreting tumors because of elevated luteinizing hormone levels.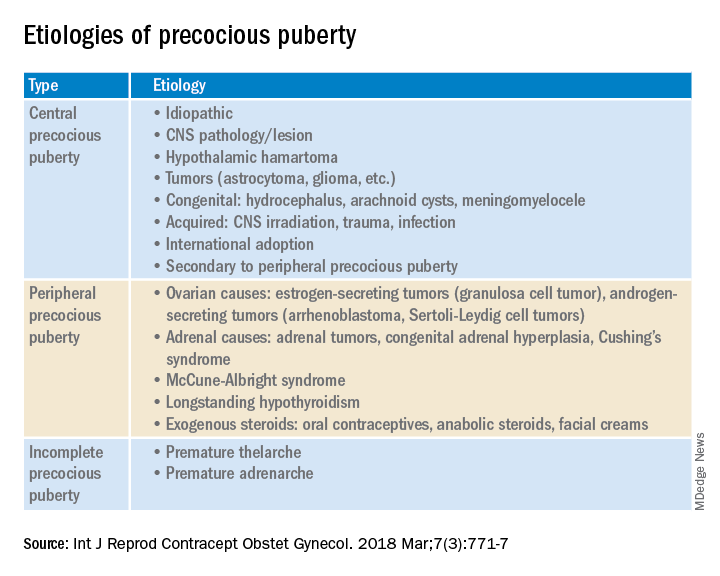
PPP. As a result of sex steroid secretion, peripheral causes of PPP include ovarian cysts and ovarian tumors that increase circulating estradiol, such as granulosa cell tumors, which would cause isosexual PPP and Sertoli-Leydig cell tumors that secrete testosterone, which can result in contrasexual PPP. Mild congenital adrenal hyperplasia can result in PPP with virilization (contrasexual) and markedly advanced bone age.
McCune-Albright syndrome is rare and presents with the classic triad of PPP, skin pigmentation called café-au-lait, and fibrous dysplasia of bone. The pathophysiology of McCune-Albright syndrome is autoactivation of the G-protein leading to activation of ovarian tissue that results in formation of large ovarian cysts and extreme elevations in serum estradiol as well as the potential production of other hormones, e.g., thyrotoxicosis, excess growth hormone (acromegaly), and Cushing syndrome.
Premature thelarche. Premature thelarche typically occurs in girls between the ages of 1 and 3 years and is limited to breast enlargement. While no cause has been determined, the plausible explanations include partial activation of the HPO axis, endocrine-disrupting chemicals (EDCs), or a genetic origin. A small percentage of these girls progress to CPP.
EDCs have been considered as potential influencers of early puberty, but no consensus has been established. (Examples of EDCs in the environment include air, soil, or water supply along with food sources, personal care products, and manufactured products that can affect the endocrine system.)
Premature adenarche. Premature adrenarche presents with adult body odor and/or body hair (pubic and/or axillary) in girls who have an elevated body mass index, most commonly at the ages of 6-7 years. The presumed mechanism is normal maturation of the adrenal gland with resultant elevation of circulating androgens. Bone age may be mildly accelerated and DHEAS is prematurely elevated for age. These girls appear to be at increased risk for polycystic ovary syndrome.
Evaluation
The initial step in the evaluation of PP is to determine whether the cause is CPP or PPP; the latter includes distinguishing isosexual from contrasexual development. A thorough history (growth, headaches, behavior or visual change, seizures, abdominal pain), physical exam, including Tanner staging, and bone age is required. However, with isolated premature thelarche or adrenarche, a bone age may not be necessary, as initial close clinical observation for pubertal progression is likely sufficient.
For CPP, the diagnosis is based on serum LH, whether random levels or elevations follow GnRH stimulation. Puberty milestones progress normally although adrenarche is not consistently apparent. For girls younger than age 6, a brain MRI is recommended but not in asymptomatic older girls with CPP. LH and FSH along with estradiol or testosterone, the latter especially in boys, are the first line of serum testing. Serum TSH is recommended for suspicion of primary hypothyroidism. In girls with premature adrenarche, a bone age, testosterone, DHEAS, and 17-OHP to rule out adrenal hyperplasia should be obtained. Pelvic ultrasound may be a useful adjunct to assess uterine volume and/or ovarian cysts/tumors.
Rapidity of onset can also lead the evaluation since a normal growth chart and skeletal maturation suggests a benign pubertal variant whereas a more rapid rate can signal CPP or PPP. Of note, health care providers should ensure prescription, over-the-counter oral or topical sources of hormones, and EDCs are ruled out.
Consequences
An association between childhood sexual abuse and earlier pubertal onset has been cited. These girls may be at increased risk for psychosocial difficulties, menstrual and fertility problems, and even reproductive cancers because of prolonged exposure to sex hormones (J Adolesc Health. 2016;60[1]:65-71).
Treatment
The mainstay of CPP treatment is maximizing adult height, typically through the use of a GnRH agonist for HPO suppression from pituitary downregulation. For girls above age 8 years, attempts at improving adult height have not shown a benefit.
In girls with PPP, treatment is directed at the prevailing pathology. Interestingly, early PPP can activate the HPO axis thereby converting to “secondary” CPP. In PPP, McCune-Albright syndrome treatment targets reducing circulating estrogens through letrozole or tamoxifen as well as addressing other autoactivated hormone production. Ovarian and adrenal tumors, albeit rare, can cause PP; therefore, surgical excision is the goal of treatment.
PP should be approached with equal concerns about the physical and emotional effects while including the family to help them understand the pathophysiology and psychosocial risks.
Dr. Mark P. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
A 6-year-old girl presents with breast development. Her medical history is unremarkable. The parents are of average height, and the mother reports her thelarche was age 11 years. The girl is at the 97th percentile for her height and 90th percentile for her weight. She has Tanner stage 3 breast development and Tanner stage 2 pubic hair development. She has grown slightly more than 3 inches over the past year. How should she be evaluated and managed (N Engl J Med. 2008;358:2366-77)?
The premature onset of puberty, i.e., precocious puberty (PP), can be an emotionally traumatic event for the child and parents. Over the past century, improvements in public health and nutrition, and, more recently, increased obesity, have been associated with earlier puberty and the dominant factor has been attributed to genetics (Curr Opin Endocrinol Diabetes Obes. 2018;25[1]:49-54). This month’s article will focus on understanding what is considered “early” puberty, evaluating for causes, and managing precocious puberty.
More commonly seen in girls than boys, PP is defined as the onset of secondary sexual characteristics before age 7.5 years in Black and Hispanic girls, and prior to 8 years in White girls, which is 2-2.5 standard deviations below the average age of pubertal onset in healthy children (J Pediatr Adolesc Gynecol. 2019;32:455-9). As a comparison, PP is diagnosed with onset before age 9 years in boys. For White compared with Black girls, the average timing of thelarche is age 10 vs. 9.5 years, peak growth velocity is age 11.5, menarche is age 12.5 vs. 12, while completion of puberty is near age 14.5 vs. 13.5, respectively (J Pediatr. 1985;107:317). Fortunately, most girls with PP have common variants rather than serious pathology.
Classification: Central (CPP) vs. peripheral (PPP)
CPP is gonadotropin dependent, meaning the hypothalamic-pituitary-ovarian axis (HPO) is prematurely activated resulting in the normal progression of puberty.
PPP is gonadotropin independent, caused by sex steroid secretion from any source – ovaries, adrenal gland, exogenous or ectopic production, e.g., germ-cell tumor. This results in a disordered progression of pubertal milestones.
Whereas CPP is typically isosexual development, i.e., consistent with the child’s gender, PPP can be isosexual or contrasexual, e.g., virilization of girls. A third classification is “benign or nonprogressive pubertal variants” manifesting as isolated premature thelarche or adrenarche.
Causes (see table)
CPP. Idiopathic causes account for 80%-90% of presentations in girls and 25%-80% in boys. Remarkably, international and domestic adoption, as well as a family history of PP increases the likelihood of CPP in girls. Other etiologies include CNS lesions, e.g., hamartomas, which are the most common cause of PP in young children. MRI with contrast has been the traditional mode of diagnosis for CNS tumors, yet the yield is dubious in girls above age 6. Genetic causes are found in only a small percentage of PP cases. Rarely, CPP can result from gonadotropin-secreting tumors because of elevated luteinizing hormone levels.
PPP. As a result of sex steroid secretion, peripheral causes of PPP include ovarian cysts and ovarian tumors that increase circulating estradiol, such as granulosa cell tumors, which would cause isosexual PPP and Sertoli-Leydig cell tumors that secrete testosterone, which can result in contrasexual PPP. Mild congenital adrenal hyperplasia can result in PPP with virilization (contrasexual) and markedly advanced bone age.
McCune-Albright syndrome is rare and presents with the classic triad of PPP, skin pigmentation called café-au-lait, and fibrous dysplasia of bone. The pathophysiology of McCune-Albright syndrome is autoactivation of the G-protein leading to activation of ovarian tissue that results in formation of large ovarian cysts and extreme elevations in serum estradiol as well as the potential production of other hormones, e.g., thyrotoxicosis, excess growth hormone (acromegaly), and Cushing syndrome.
Premature thelarche. Premature thelarche typically occurs in girls between the ages of 1 and 3 years and is limited to breast enlargement. While no cause has been determined, the plausible explanations include partial activation of the HPO axis, endocrine-disrupting chemicals (EDCs), or a genetic origin. A small percentage of these girls progress to CPP.
EDCs have been considered as potential influencers of early puberty, but no consensus has been established. (Examples of EDCs in the environment include air, soil, or water supply along with food sources, personal care products, and manufactured products that can affect the endocrine system.)
Premature adenarche. Premature adrenarche presents with adult body odor and/or body hair (pubic and/or axillary) in girls who have an elevated body mass index, most commonly at the ages of 6-7 years. The presumed mechanism is normal maturation of the adrenal gland with resultant elevation of circulating androgens. Bone age may be mildly accelerated and DHEAS is prematurely elevated for age. These girls appear to be at increased risk for polycystic ovary syndrome.
Evaluation
The initial step in the evaluation of PP is to determine whether the cause is CPP or PPP; the latter includes distinguishing isosexual from contrasexual development. A thorough history (growth, headaches, behavior or visual change, seizures, abdominal pain), physical exam, including Tanner staging, and bone age is required. However, with isolated premature thelarche or adrenarche, a bone age may not be necessary, as initial close clinical observation for pubertal progression is likely sufficient.
For CPP, the diagnosis is based on serum LH, whether random levels or elevations follow GnRH stimulation. Puberty milestones progress normally although adrenarche is not consistently apparent. For girls younger than age 6, a brain MRI is recommended but not in asymptomatic older girls with CPP. LH and FSH along with estradiol or testosterone, the latter especially in boys, are the first line of serum testing. Serum TSH is recommended for suspicion of primary hypothyroidism. In girls with premature adrenarche, a bone age, testosterone, DHEAS, and 17-OHP to rule out adrenal hyperplasia should be obtained. Pelvic ultrasound may be a useful adjunct to assess uterine volume and/or ovarian cysts/tumors.
Rapidity of onset can also lead the evaluation since a normal growth chart and skeletal maturation suggests a benign pubertal variant whereas a more rapid rate can signal CPP or PPP. Of note, health care providers should ensure prescription, over-the-counter oral or topical sources of hormones, and EDCs are ruled out.
Consequences
An association between childhood sexual abuse and earlier pubertal onset has been cited. These girls may be at increased risk for psychosocial difficulties, menstrual and fertility problems, and even reproductive cancers because of prolonged exposure to sex hormones (J Adolesc Health. 2016;60[1]:65-71).
Treatment
The mainstay of CPP treatment is maximizing adult height, typically through the use of a GnRH agonist for HPO suppression from pituitary downregulation. For girls above age 8 years, attempts at improving adult height have not shown a benefit.
In girls with PPP, treatment is directed at the prevailing pathology. Interestingly, early PPP can activate the HPO axis thereby converting to “secondary” CPP. In PPP, McCune-Albright syndrome treatment targets reducing circulating estrogens through letrozole or tamoxifen as well as addressing other autoactivated hormone production. Ovarian and adrenal tumors, albeit rare, can cause PP; therefore, surgical excision is the goal of treatment.
PP should be approached with equal concerns about the physical and emotional effects while including the family to help them understand the pathophysiology and psychosocial risks.
Dr. Mark P. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
A 6-year-old girl presents with breast development. Her medical history is unremarkable. The parents are of average height, and the mother reports her thelarche was age 11 years. The girl is at the 97th percentile for her height and 90th percentile for her weight. She has Tanner stage 3 breast development and Tanner stage 2 pubic hair development. She has grown slightly more than 3 inches over the past year. How should she be evaluated and managed (N Engl J Med. 2008;358:2366-77)?
The premature onset of puberty, i.e., precocious puberty (PP), can be an emotionally traumatic event for the child and parents. Over the past century, improvements in public health and nutrition, and, more recently, increased obesity, have been associated with earlier puberty and the dominant factor has been attributed to genetics (Curr Opin Endocrinol Diabetes Obes. 2018;25[1]:49-54). This month’s article will focus on understanding what is considered “early” puberty, evaluating for causes, and managing precocious puberty.
More commonly seen in girls than boys, PP is defined as the onset of secondary sexual characteristics before age 7.5 years in Black and Hispanic girls, and prior to 8 years in White girls, which is 2-2.5 standard deviations below the average age of pubertal onset in healthy children (J Pediatr Adolesc Gynecol. 2019;32:455-9). As a comparison, PP is diagnosed with onset before age 9 years in boys. For White compared with Black girls, the average timing of thelarche is age 10 vs. 9.5 years, peak growth velocity is age 11.5, menarche is age 12.5 vs. 12, while completion of puberty is near age 14.5 vs. 13.5, respectively (J Pediatr. 1985;107:317). Fortunately, most girls with PP have common variants rather than serious pathology.
Classification: Central (CPP) vs. peripheral (PPP)
CPP is gonadotropin dependent, meaning the hypothalamic-pituitary-ovarian axis (HPO) is prematurely activated resulting in the normal progression of puberty.
PPP is gonadotropin independent, caused by sex steroid secretion from any source – ovaries, adrenal gland, exogenous or ectopic production, e.g., germ-cell tumor. This results in a disordered progression of pubertal milestones.
Whereas CPP is typically isosexual development, i.e., consistent with the child’s gender, PPP can be isosexual or contrasexual, e.g., virilization of girls. A third classification is “benign or nonprogressive pubertal variants” manifesting as isolated premature thelarche or adrenarche.
Causes (see table)
CPP. Idiopathic causes account for 80%-90% of presentations in girls and 25%-80% in boys. Remarkably, international and domestic adoption, as well as a family history of PP increases the likelihood of CPP in girls. Other etiologies include CNS lesions, e.g., hamartomas, which are the most common cause of PP in young children. MRI with contrast has been the traditional mode of diagnosis for CNS tumors, yet the yield is dubious in girls above age 6. Genetic causes are found in only a small percentage of PP cases. Rarely, CPP can result from gonadotropin-secreting tumors because of elevated luteinizing hormone levels.
PPP. As a result of sex steroid secretion, peripheral causes of PPP include ovarian cysts and ovarian tumors that increase circulating estradiol, such as granulosa cell tumors, which would cause isosexual PPP and Sertoli-Leydig cell tumors that secrete testosterone, which can result in contrasexual PPP. Mild congenital adrenal hyperplasia can result in PPP with virilization (contrasexual) and markedly advanced bone age.
McCune-Albright syndrome is rare and presents with the classic triad of PPP, skin pigmentation called café-au-lait, and fibrous dysplasia of bone. The pathophysiology of McCune-Albright syndrome is autoactivation of the G-protein leading to activation of ovarian tissue that results in formation of large ovarian cysts and extreme elevations in serum estradiol as well as the potential production of other hormones, e.g., thyrotoxicosis, excess growth hormone (acromegaly), and Cushing syndrome.
Premature thelarche. Premature thelarche typically occurs in girls between the ages of 1 and 3 years and is limited to breast enlargement. While no cause has been determined, the plausible explanations include partial activation of the HPO axis, endocrine-disrupting chemicals (EDCs), or a genetic origin. A small percentage of these girls progress to CPP.
EDCs have been considered as potential influencers of early puberty, but no consensus has been established. (Examples of EDCs in the environment include air, soil, or water supply along with food sources, personal care products, and manufactured products that can affect the endocrine system.)
Premature adenarche. Premature adrenarche presents with adult body odor and/or body hair (pubic and/or axillary) in girls who have an elevated body mass index, most commonly at the ages of 6-7 years. The presumed mechanism is normal maturation of the adrenal gland with resultant elevation of circulating androgens. Bone age may be mildly accelerated and DHEAS is prematurely elevated for age. These girls appear to be at increased risk for polycystic ovary syndrome.
Evaluation
The initial step in the evaluation of PP is to determine whether the cause is CPP or PPP; the latter includes distinguishing isosexual from contrasexual development. A thorough history (growth, headaches, behavior or visual change, seizures, abdominal pain), physical exam, including Tanner staging, and bone age is required. However, with isolated premature thelarche or adrenarche, a bone age may not be necessary, as initial close clinical observation for pubertal progression is likely sufficient.
For CPP, the diagnosis is based on serum LH, whether random levels or elevations follow GnRH stimulation. Puberty milestones progress normally although adrenarche is not consistently apparent. For girls younger than age 6, a brain MRI is recommended but not in asymptomatic older girls with CPP. LH and FSH along with estradiol or testosterone, the latter especially in boys, are the first line of serum testing. Serum TSH is recommended for suspicion of primary hypothyroidism. In girls with premature adrenarche, a bone age, testosterone, DHEAS, and 17-OHP to rule out adrenal hyperplasia should be obtained. Pelvic ultrasound may be a useful adjunct to assess uterine volume and/or ovarian cysts/tumors.
Rapidity of onset can also lead the evaluation since a normal growth chart and skeletal maturation suggests a benign pubertal variant whereas a more rapid rate can signal CPP or PPP. Of note, health care providers should ensure prescription, over-the-counter oral or topical sources of hormones, and EDCs are ruled out.
Consequences
An association between childhood sexual abuse and earlier pubertal onset has been cited. These girls may be at increased risk for psychosocial difficulties, menstrual and fertility problems, and even reproductive cancers because of prolonged exposure to sex hormones (J Adolesc Health. 2016;60[1]:65-71).
Treatment
The mainstay of CPP treatment is maximizing adult height, typically through the use of a GnRH agonist for HPO suppression from pituitary downregulation. For girls above age 8 years, attempts at improving adult height have not shown a benefit.
In girls with PPP, treatment is directed at the prevailing pathology. Interestingly, early PPP can activate the HPO axis thereby converting to “secondary” CPP. In PPP, McCune-Albright syndrome treatment targets reducing circulating estrogens through letrozole or tamoxifen as well as addressing other autoactivated hormone production. Ovarian and adrenal tumors, albeit rare, can cause PP; therefore, surgical excision is the goal of treatment.
PP should be approached with equal concerns about the physical and emotional effects while including the family to help them understand the pathophysiology and psychosocial risks.
Dr. Mark P. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Neck floats may not be right for certain babies, FDA warns
The FDA is warning that parents should avoid using neck floats for infants with special needs or developmental delays.
According to the agency, companies have been advertising the products as having health benefits for children with physical and developmental problems, despite a lack of evidence for such claims. The companies, which the FDA did not name, claimed that water therapy with floats could help babies with special needs – like those with spina bifida – to increase muscle tone, boost flexibility and range of motion, and build lung capacity, among other benefits.
But used improperly, neck floats can lead to serious injury and death. At least one baby has died, and one was hospitalized, after using the floats, FDA officials said.
The inflatable plastic rings are worn around a baby’s neck, allowing them to float freely in water. Some of these products are being marketed for infants as young as 2 weeks old, as well as for premature babies. But the FDA said the safety and effectiveness of the products for these children have not been proven.
The floats “have not been evaluated by the FDA, and we are not aware of any demonstrated benefit with the use of neck floats for water therapy interventions,” the agency said in the June 28 statement.
While injuries and deaths from neck floats are rare, the FDA said families and caregivers should be aware that these incidents can and do occur.
People who have problems with the neck floats are encouraged to report them through MedWatch, the FDA Safety Information and Adverse Event Reporting Program. Health care personnel employed by the FDA are required to file new reports with the FDA.
A version of this article first appeared on WebMD.com.
The FDA is warning that parents should avoid using neck floats for infants with special needs or developmental delays.
According to the agency, companies have been advertising the products as having health benefits for children with physical and developmental problems, despite a lack of evidence for such claims. The companies, which the FDA did not name, claimed that water therapy with floats could help babies with special needs – like those with spina bifida – to increase muscle tone, boost flexibility and range of motion, and build lung capacity, among other benefits.
But used improperly, neck floats can lead to serious injury and death. At least one baby has died, and one was hospitalized, after using the floats, FDA officials said.
The inflatable plastic rings are worn around a baby’s neck, allowing them to float freely in water. Some of these products are being marketed for infants as young as 2 weeks old, as well as for premature babies. But the FDA said the safety and effectiveness of the products for these children have not been proven.
The floats “have not been evaluated by the FDA, and we are not aware of any demonstrated benefit with the use of neck floats for water therapy interventions,” the agency said in the June 28 statement.
While injuries and deaths from neck floats are rare, the FDA said families and caregivers should be aware that these incidents can and do occur.
People who have problems with the neck floats are encouraged to report them through MedWatch, the FDA Safety Information and Adverse Event Reporting Program. Health care personnel employed by the FDA are required to file new reports with the FDA.
A version of this article first appeared on WebMD.com.
The FDA is warning that parents should avoid using neck floats for infants with special needs or developmental delays.
According to the agency, companies have been advertising the products as having health benefits for children with physical and developmental problems, despite a lack of evidence for such claims. The companies, which the FDA did not name, claimed that water therapy with floats could help babies with special needs – like those with spina bifida – to increase muscle tone, boost flexibility and range of motion, and build lung capacity, among other benefits.
But used improperly, neck floats can lead to serious injury and death. At least one baby has died, and one was hospitalized, after using the floats, FDA officials said.
The inflatable plastic rings are worn around a baby’s neck, allowing them to float freely in water. Some of these products are being marketed for infants as young as 2 weeks old, as well as for premature babies. But the FDA said the safety and effectiveness of the products for these children have not been proven.
The floats “have not been evaluated by the FDA, and we are not aware of any demonstrated benefit with the use of neck floats for water therapy interventions,” the agency said in the June 28 statement.
While injuries and deaths from neck floats are rare, the FDA said families and caregivers should be aware that these incidents can and do occur.
People who have problems with the neck floats are encouraged to report them through MedWatch, the FDA Safety Information and Adverse Event Reporting Program. Health care personnel employed by the FDA are required to file new reports with the FDA.
A version of this article first appeared on WebMD.com.










