User login
Physicians urged to write indications on drug scripts as methotrexate users face new barriers with SCOTUS decision
.
The Court’s 5-4 decision in Dobbs v. Jackson Women’s Health Organization, which halted abortion procedures across the country, also appears to be affecting certain drug regimens. Reports have emerged that pharmacies are denying access to methotrexate (MTX), a drug often used in patients with arthritis or cancer, as well as psoriasis and other skin diseases. In very high doses, MTX it is used to terminate an ectopic pregnancy after miscarriage. The drug can also lead to birth defects.
“It’s happening all over,” Donald Miller, PharmD, professor of pharmacy practice at North Dakota State University, Fargo, said in an interview. “Pharmacists are reluctant to dispense it, and rheumatologists are reluctant to prescribe it because they’re afraid of going to jail.”
Becky Schwartz, a patient who takes MTX for lupus, recently tweeted that her physician’s office stopped prescribing the drug because it is considered an abortifacient. “I had care that made my disabled life easier, and [the Supreme Court] took that from me,” Ms. Schwartz wrote.
Prior to the Supreme Court’s ruling, physicians were concerned about the impact an overturning of the 1973 law would have on patient access to MTX and other prescription medications with abortifacient properties. Doctors in general are becoming afraid of prescribing anything that’s a teratogen, said Dr. Miller.
MTX is used far more often for autoimmune disease than as an abortifacient, said rheumatologist Kristen Young, MD, clinical assistant professor at the University of Arizona College of Medicine, Phoenix. It’s a slippery slope if states reacting to the Supreme Court ruling start regulating oral abortifacients, she added. Specifically, this will have a significant impact on patients with rheumatic disease.
Texas pharmacies target two drugs
MTX denials have caught the attention of health care organizations. “Uncertainty in financial and criminal liability for health care professionals in certain state laws and regulations are possibly compromising continuity of care and access [to] medications proven to be safe and effective by the Food and Drug Administration for these indications,” warned the American Pharmacists Association (APhA) in a statement to this news organization.
The APhA said that it was monitoring this situation to assess the effect on patients and pharmacists.
The Arthritis Foundation was made aware of challenges from patients in accessing their MTX prescription for managing their arthritis and shared a statement on the Foundation’s website.
In Texas, pharmacists can refuse to fill scripts for misoprostol and MTX, a combination used for medical abortions. According to the foundation, “Already there are reports that people in Texas who miscarry or take methotrexate for arthritis [are] having trouble getting their prescriptions filled.”
MTX, approved by the FDA in 1985, “is the absolute cornerstone of rheumatoid arthritis. We cannot deny our patients this incredibly valuable drug,” said John Reveille, MD, vice-chair for the department of medicine at the University of Texas McGovern School of Medicine and a member of the Arthritis Foundation expert panel, in an interview.
“While it’s true that methotrexate can be lethal to the fetus, misoprostol is much more likely to cause a spontaneous abortion, and the combination is especially effective,” he said.
“If you look at Cochrane clinical studies, the dose of misoprostol contained in certain combinations with NSAIDs [nonsteroidal anti-inflammatory drugs] can induce spontaneous abortions. It’s surprising that pharmacists are targeting methotrexate, an essential drug in arthritis treatment, when there are medications available that do not have this benefit that can by themselves cause loss of the fetus, such as mifepristone,” added Dr. Reveille.
The Dobbs ruling could also affect the ability of oncologists to provide lifesaving cancer care, according to Jason Westin, MD, an oncologist at the University of Texas MD Anderson Cancer Center in the department of lymphoma and myeloma.
“We have heard of medications with multiple indications, such as methotrexate, not being dispensed by pharmacies due to confusion regarding the intended use and potential consequences for the health care team,” he said in an interview.
Conflicting laws pose challenges for physicians
In North Dakota, inconsistencies in several laws are making it difficult for physicians and pharmacists to make decisions. “Lots of confusion can result when people pass laws against abortion. There’s sometimes no insight into the ramifications of those laws,” said Dr. Miller.
North Dakota approved a trigger law several years ago that makes abortion illegal 30 days after an overturning of Roe. However, another law that regulates abortion conflicts with the trigger law. “Some of the language will need clarification in the next legislative session,” he said.
APhA and other pharmacy associations strongly favor not interfering with the doctor- or pharmacist-patient relationship. The law needs to defer to appropriate care between doctor and patient, said Dr. Miller. State pharmacy associations in North Dakota are working with legislatures to clarify any exceptions in the law, he added.
Arizona lawmakers are trying to reconcile two abortion laws on the books. One, based on an 1864 territorial law, deems abortion illegal. In addition, a newly approved law bans abortions after 15 weeks. The latter will go into effect in September 2022. In both laws, a risk to the mother’s life is the only exception for abortion, said Dr. Young.
Denials aren’t widespread
Not all doctors are seeing MTX denials, but they’re worried about the future. “To date, we have not encountered difficulty in obtaining methotrexate based upon state abortion restrictions but are concerned that this could occur and result in dangerous delays in care,” said Dr. Westin.
Dr. Reveille, who practices rheumatology in Houston, has not yet received any complaints from patients. Things may be different in more rural parts of Texas, where pharmacists could be denying prescriptions based on religious issues, he offered.
It’s a little soon to see what repercussions may result from the Supreme Court ruling and state actions, said Dr. Reveille. “In Texas, we’re a bit ahead of the tidal wave.”
Access problems also haven’t shown up at the university clinic where Dr. Young practices. “In Arizona, it’s unclear if there would be a legal basis to refuse a person methotrexate on the basis that it can be used as an abortifacient,” she said.
Specificity is key in writing Rx scripts
Physicians can make things easier for patients by writing the indication and dose for the drug on the prescription slip. For example, a 10-mg script for MTX is not going to be used for an abortion, said Dr. Miller.
Rheumatologists in Texas have been doing this for some time, even before the Supreme Court ruling, said Fehmida Zahabi, MD, FACR, president of the Society of Texas Association of Rheumatology. For MTX prescriptions in premenopausal women, “patients are told their doctor needs to call the pharmacist. In the small print, we are asked to give a diagnosis to make sure we aren’t using it to terminate pregnancies,” said Dr. Zahabi.
She further noted that if the diagnosis is already indicated on the script, pharmacies generally won’t give patients a hard time.
Patients can also ask their physicians for a letter of medical necessity that confirms a drug’s use for a specific medical condition.
Mail order is another option if a local pharmacy won’t fill a prescription, said Dr. Miller. “This is legal unless a state makes it illegal to send an abortifacient across state lines,” he added.
Many medications used in rheumatic diseases are harmful in pregnancy, and it’s important to routinely discuss pregnancy risk and planning in the rheumatology clinic, said Dr. Young. This should include a thorough discussion and referral for long-acting reversible contraception in most cases, she suggested.
Actions at the federal, state level
President Joe Biden recently signed an executive order prompting federal regulators to protect access to medication abortions, among other steps to safeguard access to reproductive services.
In a statement on Twitter, the American College of Rheumatology (ACR) said that it was “ ... following this issue closely to determine if rheumatology providers and patients are experiencing any widespread difficulty accessing methotrexate or if any initial disruptions are potentially temporary and due to the independent actions of pharmacists trying to figure out what is and isn’t allowed where they practice.”
ACR has assembled a task force of medical and policy experts to determine the best course of action for patients.
The Arthritis Foundation also continues to monitor the situation, encouraging patients to call its hotline, said Steven Schultz, director of state legislative affairs, in an interview.
“We are analyzing how medication abortion could cause confusion on the part of providers or pharmacists dispensing the medication and what this means for specific patients,” said Mr. Schultz. Through a survey, the foundation hopes to get a better idea of what’s going on in the states at a macro level.
This may take some time, as states go through a process of lawsuits, injunctions, or coming into session to do something that may affect access to MTX, said Mr. Schultz.
Being involved in local advocacy is more important than ever, stressed Dr. Young. “Additionally, being plugged into what the ACR and other advocacy groups are doing on the national level is helpful as well to know the status of these medication access issues.”
Rheumatologists have a unique voice in this discussion, she added. “We guide our patients to stability for a safe pregnancy, and even with careful planning, we see patients who become critically ill during pregnancy and require lifesaving treatment, which at times can mean an abortion is necessary.”
Oncologists also advocate for their patients on a regular basis to make sure they have access to the care they need, said Dr. Westin. This situation with Roe is no different, he added. “We will continue to use our unique expertise to advocate for policies that assure access to high-quality, evidence-based care – and to help our patients overcome barriers that may interfere.”
Dr. Reveille participated on an advisory board with Eli Lilly in October 2021.
A version of this article first appeared on Medscape.com.
.
The Court’s 5-4 decision in Dobbs v. Jackson Women’s Health Organization, which halted abortion procedures across the country, also appears to be affecting certain drug regimens. Reports have emerged that pharmacies are denying access to methotrexate (MTX), a drug often used in patients with arthritis or cancer, as well as psoriasis and other skin diseases. In very high doses, MTX it is used to terminate an ectopic pregnancy after miscarriage. The drug can also lead to birth defects.
“It’s happening all over,” Donald Miller, PharmD, professor of pharmacy practice at North Dakota State University, Fargo, said in an interview. “Pharmacists are reluctant to dispense it, and rheumatologists are reluctant to prescribe it because they’re afraid of going to jail.”
Becky Schwartz, a patient who takes MTX for lupus, recently tweeted that her physician’s office stopped prescribing the drug because it is considered an abortifacient. “I had care that made my disabled life easier, and [the Supreme Court] took that from me,” Ms. Schwartz wrote.
Prior to the Supreme Court’s ruling, physicians were concerned about the impact an overturning of the 1973 law would have on patient access to MTX and other prescription medications with abortifacient properties. Doctors in general are becoming afraid of prescribing anything that’s a teratogen, said Dr. Miller.
MTX is used far more often for autoimmune disease than as an abortifacient, said rheumatologist Kristen Young, MD, clinical assistant professor at the University of Arizona College of Medicine, Phoenix. It’s a slippery slope if states reacting to the Supreme Court ruling start regulating oral abortifacients, she added. Specifically, this will have a significant impact on patients with rheumatic disease.
Texas pharmacies target two drugs
MTX denials have caught the attention of health care organizations. “Uncertainty in financial and criminal liability for health care professionals in certain state laws and regulations are possibly compromising continuity of care and access [to] medications proven to be safe and effective by the Food and Drug Administration for these indications,” warned the American Pharmacists Association (APhA) in a statement to this news organization.
The APhA said that it was monitoring this situation to assess the effect on patients and pharmacists.
The Arthritis Foundation was made aware of challenges from patients in accessing their MTX prescription for managing their arthritis and shared a statement on the Foundation’s website.
In Texas, pharmacists can refuse to fill scripts for misoprostol and MTX, a combination used for medical abortions. According to the foundation, “Already there are reports that people in Texas who miscarry or take methotrexate for arthritis [are] having trouble getting their prescriptions filled.”
MTX, approved by the FDA in 1985, “is the absolute cornerstone of rheumatoid arthritis. We cannot deny our patients this incredibly valuable drug,” said John Reveille, MD, vice-chair for the department of medicine at the University of Texas McGovern School of Medicine and a member of the Arthritis Foundation expert panel, in an interview.
“While it’s true that methotrexate can be lethal to the fetus, misoprostol is much more likely to cause a spontaneous abortion, and the combination is especially effective,” he said.
“If you look at Cochrane clinical studies, the dose of misoprostol contained in certain combinations with NSAIDs [nonsteroidal anti-inflammatory drugs] can induce spontaneous abortions. It’s surprising that pharmacists are targeting methotrexate, an essential drug in arthritis treatment, when there are medications available that do not have this benefit that can by themselves cause loss of the fetus, such as mifepristone,” added Dr. Reveille.
The Dobbs ruling could also affect the ability of oncologists to provide lifesaving cancer care, according to Jason Westin, MD, an oncologist at the University of Texas MD Anderson Cancer Center in the department of lymphoma and myeloma.
“We have heard of medications with multiple indications, such as methotrexate, not being dispensed by pharmacies due to confusion regarding the intended use and potential consequences for the health care team,” he said in an interview.
Conflicting laws pose challenges for physicians
In North Dakota, inconsistencies in several laws are making it difficult for physicians and pharmacists to make decisions. “Lots of confusion can result when people pass laws against abortion. There’s sometimes no insight into the ramifications of those laws,” said Dr. Miller.
North Dakota approved a trigger law several years ago that makes abortion illegal 30 days after an overturning of Roe. However, another law that regulates abortion conflicts with the trigger law. “Some of the language will need clarification in the next legislative session,” he said.
APhA and other pharmacy associations strongly favor not interfering with the doctor- or pharmacist-patient relationship. The law needs to defer to appropriate care between doctor and patient, said Dr. Miller. State pharmacy associations in North Dakota are working with legislatures to clarify any exceptions in the law, he added.
Arizona lawmakers are trying to reconcile two abortion laws on the books. One, based on an 1864 territorial law, deems abortion illegal. In addition, a newly approved law bans abortions after 15 weeks. The latter will go into effect in September 2022. In both laws, a risk to the mother’s life is the only exception for abortion, said Dr. Young.
Denials aren’t widespread
Not all doctors are seeing MTX denials, but they’re worried about the future. “To date, we have not encountered difficulty in obtaining methotrexate based upon state abortion restrictions but are concerned that this could occur and result in dangerous delays in care,” said Dr. Westin.
Dr. Reveille, who practices rheumatology in Houston, has not yet received any complaints from patients. Things may be different in more rural parts of Texas, where pharmacists could be denying prescriptions based on religious issues, he offered.
It’s a little soon to see what repercussions may result from the Supreme Court ruling and state actions, said Dr. Reveille. “In Texas, we’re a bit ahead of the tidal wave.”
Access problems also haven’t shown up at the university clinic where Dr. Young practices. “In Arizona, it’s unclear if there would be a legal basis to refuse a person methotrexate on the basis that it can be used as an abortifacient,” she said.
Specificity is key in writing Rx scripts
Physicians can make things easier for patients by writing the indication and dose for the drug on the prescription slip. For example, a 10-mg script for MTX is not going to be used for an abortion, said Dr. Miller.
Rheumatologists in Texas have been doing this for some time, even before the Supreme Court ruling, said Fehmida Zahabi, MD, FACR, president of the Society of Texas Association of Rheumatology. For MTX prescriptions in premenopausal women, “patients are told their doctor needs to call the pharmacist. In the small print, we are asked to give a diagnosis to make sure we aren’t using it to terminate pregnancies,” said Dr. Zahabi.
She further noted that if the diagnosis is already indicated on the script, pharmacies generally won’t give patients a hard time.
Patients can also ask their physicians for a letter of medical necessity that confirms a drug’s use for a specific medical condition.
Mail order is another option if a local pharmacy won’t fill a prescription, said Dr. Miller. “This is legal unless a state makes it illegal to send an abortifacient across state lines,” he added.
Many medications used in rheumatic diseases are harmful in pregnancy, and it’s important to routinely discuss pregnancy risk and planning in the rheumatology clinic, said Dr. Young. This should include a thorough discussion and referral for long-acting reversible contraception in most cases, she suggested.
Actions at the federal, state level
President Joe Biden recently signed an executive order prompting federal regulators to protect access to medication abortions, among other steps to safeguard access to reproductive services.
In a statement on Twitter, the American College of Rheumatology (ACR) said that it was “ ... following this issue closely to determine if rheumatology providers and patients are experiencing any widespread difficulty accessing methotrexate or if any initial disruptions are potentially temporary and due to the independent actions of pharmacists trying to figure out what is and isn’t allowed where they practice.”
ACR has assembled a task force of medical and policy experts to determine the best course of action for patients.
The Arthritis Foundation also continues to monitor the situation, encouraging patients to call its hotline, said Steven Schultz, director of state legislative affairs, in an interview.
“We are analyzing how medication abortion could cause confusion on the part of providers or pharmacists dispensing the medication and what this means for specific patients,” said Mr. Schultz. Through a survey, the foundation hopes to get a better idea of what’s going on in the states at a macro level.
This may take some time, as states go through a process of lawsuits, injunctions, or coming into session to do something that may affect access to MTX, said Mr. Schultz.
Being involved in local advocacy is more important than ever, stressed Dr. Young. “Additionally, being plugged into what the ACR and other advocacy groups are doing on the national level is helpful as well to know the status of these medication access issues.”
Rheumatologists have a unique voice in this discussion, she added. “We guide our patients to stability for a safe pregnancy, and even with careful planning, we see patients who become critically ill during pregnancy and require lifesaving treatment, which at times can mean an abortion is necessary.”
Oncologists also advocate for their patients on a regular basis to make sure they have access to the care they need, said Dr. Westin. This situation with Roe is no different, he added. “We will continue to use our unique expertise to advocate for policies that assure access to high-quality, evidence-based care – and to help our patients overcome barriers that may interfere.”
Dr. Reveille participated on an advisory board with Eli Lilly in October 2021.
A version of this article first appeared on Medscape.com.
.
The Court’s 5-4 decision in Dobbs v. Jackson Women’s Health Organization, which halted abortion procedures across the country, also appears to be affecting certain drug regimens. Reports have emerged that pharmacies are denying access to methotrexate (MTX), a drug often used in patients with arthritis or cancer, as well as psoriasis and other skin diseases. In very high doses, MTX it is used to terminate an ectopic pregnancy after miscarriage. The drug can also lead to birth defects.
“It’s happening all over,” Donald Miller, PharmD, professor of pharmacy practice at North Dakota State University, Fargo, said in an interview. “Pharmacists are reluctant to dispense it, and rheumatologists are reluctant to prescribe it because they’re afraid of going to jail.”
Becky Schwartz, a patient who takes MTX for lupus, recently tweeted that her physician’s office stopped prescribing the drug because it is considered an abortifacient. “I had care that made my disabled life easier, and [the Supreme Court] took that from me,” Ms. Schwartz wrote.
Prior to the Supreme Court’s ruling, physicians were concerned about the impact an overturning of the 1973 law would have on patient access to MTX and other prescription medications with abortifacient properties. Doctors in general are becoming afraid of prescribing anything that’s a teratogen, said Dr. Miller.
MTX is used far more often for autoimmune disease than as an abortifacient, said rheumatologist Kristen Young, MD, clinical assistant professor at the University of Arizona College of Medicine, Phoenix. It’s a slippery slope if states reacting to the Supreme Court ruling start regulating oral abortifacients, she added. Specifically, this will have a significant impact on patients with rheumatic disease.
Texas pharmacies target two drugs
MTX denials have caught the attention of health care organizations. “Uncertainty in financial and criminal liability for health care professionals in certain state laws and regulations are possibly compromising continuity of care and access [to] medications proven to be safe and effective by the Food and Drug Administration for these indications,” warned the American Pharmacists Association (APhA) in a statement to this news organization.
The APhA said that it was monitoring this situation to assess the effect on patients and pharmacists.
The Arthritis Foundation was made aware of challenges from patients in accessing their MTX prescription for managing their arthritis and shared a statement on the Foundation’s website.
In Texas, pharmacists can refuse to fill scripts for misoprostol and MTX, a combination used for medical abortions. According to the foundation, “Already there are reports that people in Texas who miscarry or take methotrexate for arthritis [are] having trouble getting their prescriptions filled.”
MTX, approved by the FDA in 1985, “is the absolute cornerstone of rheumatoid arthritis. We cannot deny our patients this incredibly valuable drug,” said John Reveille, MD, vice-chair for the department of medicine at the University of Texas McGovern School of Medicine and a member of the Arthritis Foundation expert panel, in an interview.
“While it’s true that methotrexate can be lethal to the fetus, misoprostol is much more likely to cause a spontaneous abortion, and the combination is especially effective,” he said.
“If you look at Cochrane clinical studies, the dose of misoprostol contained in certain combinations with NSAIDs [nonsteroidal anti-inflammatory drugs] can induce spontaneous abortions. It’s surprising that pharmacists are targeting methotrexate, an essential drug in arthritis treatment, when there are medications available that do not have this benefit that can by themselves cause loss of the fetus, such as mifepristone,” added Dr. Reveille.
The Dobbs ruling could also affect the ability of oncologists to provide lifesaving cancer care, according to Jason Westin, MD, an oncologist at the University of Texas MD Anderson Cancer Center in the department of lymphoma and myeloma.
“We have heard of medications with multiple indications, such as methotrexate, not being dispensed by pharmacies due to confusion regarding the intended use and potential consequences for the health care team,” he said in an interview.
Conflicting laws pose challenges for physicians
In North Dakota, inconsistencies in several laws are making it difficult for physicians and pharmacists to make decisions. “Lots of confusion can result when people pass laws against abortion. There’s sometimes no insight into the ramifications of those laws,” said Dr. Miller.
North Dakota approved a trigger law several years ago that makes abortion illegal 30 days after an overturning of Roe. However, another law that regulates abortion conflicts with the trigger law. “Some of the language will need clarification in the next legislative session,” he said.
APhA and other pharmacy associations strongly favor not interfering with the doctor- or pharmacist-patient relationship. The law needs to defer to appropriate care between doctor and patient, said Dr. Miller. State pharmacy associations in North Dakota are working with legislatures to clarify any exceptions in the law, he added.
Arizona lawmakers are trying to reconcile two abortion laws on the books. One, based on an 1864 territorial law, deems abortion illegal. In addition, a newly approved law bans abortions after 15 weeks. The latter will go into effect in September 2022. In both laws, a risk to the mother’s life is the only exception for abortion, said Dr. Young.
Denials aren’t widespread
Not all doctors are seeing MTX denials, but they’re worried about the future. “To date, we have not encountered difficulty in obtaining methotrexate based upon state abortion restrictions but are concerned that this could occur and result in dangerous delays in care,” said Dr. Westin.
Dr. Reveille, who practices rheumatology in Houston, has not yet received any complaints from patients. Things may be different in more rural parts of Texas, where pharmacists could be denying prescriptions based on religious issues, he offered.
It’s a little soon to see what repercussions may result from the Supreme Court ruling and state actions, said Dr. Reveille. “In Texas, we’re a bit ahead of the tidal wave.”
Access problems also haven’t shown up at the university clinic where Dr. Young practices. “In Arizona, it’s unclear if there would be a legal basis to refuse a person methotrexate on the basis that it can be used as an abortifacient,” she said.
Specificity is key in writing Rx scripts
Physicians can make things easier for patients by writing the indication and dose for the drug on the prescription slip. For example, a 10-mg script for MTX is not going to be used for an abortion, said Dr. Miller.
Rheumatologists in Texas have been doing this for some time, even before the Supreme Court ruling, said Fehmida Zahabi, MD, FACR, president of the Society of Texas Association of Rheumatology. For MTX prescriptions in premenopausal women, “patients are told their doctor needs to call the pharmacist. In the small print, we are asked to give a diagnosis to make sure we aren’t using it to terminate pregnancies,” said Dr. Zahabi.
She further noted that if the diagnosis is already indicated on the script, pharmacies generally won’t give patients a hard time.
Patients can also ask their physicians for a letter of medical necessity that confirms a drug’s use for a specific medical condition.
Mail order is another option if a local pharmacy won’t fill a prescription, said Dr. Miller. “This is legal unless a state makes it illegal to send an abortifacient across state lines,” he added.
Many medications used in rheumatic diseases are harmful in pregnancy, and it’s important to routinely discuss pregnancy risk and planning in the rheumatology clinic, said Dr. Young. This should include a thorough discussion and referral for long-acting reversible contraception in most cases, she suggested.
Actions at the federal, state level
President Joe Biden recently signed an executive order prompting federal regulators to protect access to medication abortions, among other steps to safeguard access to reproductive services.
In a statement on Twitter, the American College of Rheumatology (ACR) said that it was “ ... following this issue closely to determine if rheumatology providers and patients are experiencing any widespread difficulty accessing methotrexate or if any initial disruptions are potentially temporary and due to the independent actions of pharmacists trying to figure out what is and isn’t allowed where they practice.”
ACR has assembled a task force of medical and policy experts to determine the best course of action for patients.
The Arthritis Foundation also continues to monitor the situation, encouraging patients to call its hotline, said Steven Schultz, director of state legislative affairs, in an interview.
“We are analyzing how medication abortion could cause confusion on the part of providers or pharmacists dispensing the medication and what this means for specific patients,” said Mr. Schultz. Through a survey, the foundation hopes to get a better idea of what’s going on in the states at a macro level.
This may take some time, as states go through a process of lawsuits, injunctions, or coming into session to do something that may affect access to MTX, said Mr. Schultz.
Being involved in local advocacy is more important than ever, stressed Dr. Young. “Additionally, being plugged into what the ACR and other advocacy groups are doing on the national level is helpful as well to know the status of these medication access issues.”
Rheumatologists have a unique voice in this discussion, she added. “We guide our patients to stability for a safe pregnancy, and even with careful planning, we see patients who become critically ill during pregnancy and require lifesaving treatment, which at times can mean an abortion is necessary.”
Oncologists also advocate for their patients on a regular basis to make sure they have access to the care they need, said Dr. Westin. This situation with Roe is no different, he added. “We will continue to use our unique expertise to advocate for policies that assure access to high-quality, evidence-based care – and to help our patients overcome barriers that may interfere.”
Dr. Reveille participated on an advisory board with Eli Lilly in October 2021.
A version of this article first appeared on Medscape.com.
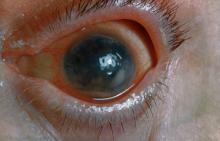
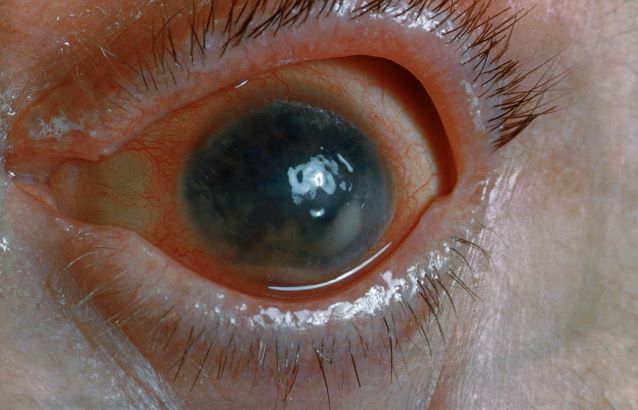
Pain and photophobia
On the basis of the patient's medical history and presentation, this is probably a case of uveitis, a common extra-articular manifestation of psoriatic disease. In fact, the presence of uveitis can help distinguish PsA from osteoarthritis. Uveitis is characterized by inflammation of the uvea tract, with the retina, optic nerve, vitreous body, and sclera potentially becoming inflamed as well. Among patients with PsA, the prevalence of uveitis rises with ongoing disease duration, though the condition may also precede the development of PsA in patients with psoriasis, and is common among patients with severe psoriatic disease in Western and Asian populations. Overall, the prevalence of uveitis has been estimated to be 6%-9%. HLA-B27 genotype is strongly associated with uveitis in patients with concomitant PsA.
Symptoms of uveitis, as seen in the present case, include blurred vision, photophobia, pain, and ciliary flush. The condition is classified as anterior, intermediate, posterior, or panuveitis, with the majority of cases diagnosed as anterior. In anterior uveitis, the inflamed pupil may become constricted or take on an irregular shape caused by iris adhesions to the anterior lens capsule. Uveitis in PsA is bilateral and has a chronic relapsing course. Onset is typically insidious.
Workup for uveitis should comprise visual acuity testing, slit lamp biomicroscopy, measurement of intraocular pressures, and a dilated eye exam. Conditions in the differential which threaten a patient's sight include retinal vasculitis, vitritis, cystoid macular edema, Behçet disease, and tubulo-interstitial nephritis. Other autoimmune diseases which can cause uveitis with systemic manifestations (multiple sclerosis, sarcoidosis, lupus) should be investigated. Infectious causes must also be eliminated. However, considering this patient's history of psoriatic disease, uveitis should be highly suspected.
Uveitis demands urgent treatment to control ocular inflammation. Tumor necrosis factor (TNF) inhibitors are the recommended first-line and second-line treatment for PsA, including in patients with complications such as uveitis. However, etanercept should not be used as it is less effective than adalimumab or other TNF inhibitors for uveitis. Because uveitis may sometimes respond to MTX therapy, patients with severe PsA may use a biologic agent in combination with MTX if they have had a partial response to current MTX therapy, as recommended by the American College of Rheumatology.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of the patient's medical history and presentation, this is probably a case of uveitis, a common extra-articular manifestation of psoriatic disease. In fact, the presence of uveitis can help distinguish PsA from osteoarthritis. Uveitis is characterized by inflammation of the uvea tract, with the retina, optic nerve, vitreous body, and sclera potentially becoming inflamed as well. Among patients with PsA, the prevalence of uveitis rises with ongoing disease duration, though the condition may also precede the development of PsA in patients with psoriasis, and is common among patients with severe psoriatic disease in Western and Asian populations. Overall, the prevalence of uveitis has been estimated to be 6%-9%. HLA-B27 genotype is strongly associated with uveitis in patients with concomitant PsA.
Symptoms of uveitis, as seen in the present case, include blurred vision, photophobia, pain, and ciliary flush. The condition is classified as anterior, intermediate, posterior, or panuveitis, with the majority of cases diagnosed as anterior. In anterior uveitis, the inflamed pupil may become constricted or take on an irregular shape caused by iris adhesions to the anterior lens capsule. Uveitis in PsA is bilateral and has a chronic relapsing course. Onset is typically insidious.
Workup for uveitis should comprise visual acuity testing, slit lamp biomicroscopy, measurement of intraocular pressures, and a dilated eye exam. Conditions in the differential which threaten a patient's sight include retinal vasculitis, vitritis, cystoid macular edema, Behçet disease, and tubulo-interstitial nephritis. Other autoimmune diseases which can cause uveitis with systemic manifestations (multiple sclerosis, sarcoidosis, lupus) should be investigated. Infectious causes must also be eliminated. However, considering this patient's history of psoriatic disease, uveitis should be highly suspected.
Uveitis demands urgent treatment to control ocular inflammation. Tumor necrosis factor (TNF) inhibitors are the recommended first-line and second-line treatment for PsA, including in patients with complications such as uveitis. However, etanercept should not be used as it is less effective than adalimumab or other TNF inhibitors for uveitis. Because uveitis may sometimes respond to MTX therapy, patients with severe PsA may use a biologic agent in combination with MTX if they have had a partial response to current MTX therapy, as recommended by the American College of Rheumatology.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of the patient's medical history and presentation, this is probably a case of uveitis, a common extra-articular manifestation of psoriatic disease. In fact, the presence of uveitis can help distinguish PsA from osteoarthritis. Uveitis is characterized by inflammation of the uvea tract, with the retina, optic nerve, vitreous body, and sclera potentially becoming inflamed as well. Among patients with PsA, the prevalence of uveitis rises with ongoing disease duration, though the condition may also precede the development of PsA in patients with psoriasis, and is common among patients with severe psoriatic disease in Western and Asian populations. Overall, the prevalence of uveitis has been estimated to be 6%-9%. HLA-B27 genotype is strongly associated with uveitis in patients with concomitant PsA.
Symptoms of uveitis, as seen in the present case, include blurred vision, photophobia, pain, and ciliary flush. The condition is classified as anterior, intermediate, posterior, or panuveitis, with the majority of cases diagnosed as anterior. In anterior uveitis, the inflamed pupil may become constricted or take on an irregular shape caused by iris adhesions to the anterior lens capsule. Uveitis in PsA is bilateral and has a chronic relapsing course. Onset is typically insidious.
Workup for uveitis should comprise visual acuity testing, slit lamp biomicroscopy, measurement of intraocular pressures, and a dilated eye exam. Conditions in the differential which threaten a patient's sight include retinal vasculitis, vitritis, cystoid macular edema, Behçet disease, and tubulo-interstitial nephritis. Other autoimmune diseases which can cause uveitis with systemic manifestations (multiple sclerosis, sarcoidosis, lupus) should be investigated. Infectious causes must also be eliminated. However, considering this patient's history of psoriatic disease, uveitis should be highly suspected.
Uveitis demands urgent treatment to control ocular inflammation. Tumor necrosis factor (TNF) inhibitors are the recommended first-line and second-line treatment for PsA, including in patients with complications such as uveitis. However, etanercept should not be used as it is less effective than adalimumab or other TNF inhibitors for uveitis. Because uveitis may sometimes respond to MTX therapy, patients with severe PsA may use a biologic agent in combination with MTX if they have had a partial response to current MTX therapy, as recommended by the American College of Rheumatology.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 48-year-old male patient presents with blurred vision, pain, and photophobia. He recently had a routine visit with an ophthalmologist, which was normal. The affected pupil appears irregular in shape. The anterior chamber appears foggy. Local ciliary flush is observed on slit lamp exam. The physical examination is also notable for axial arthropathy. The patient has an 11-year history of moderate to severe psoriatic arthritis (PsA) which he typically manages with methotrexate (MTX) therapy, to which he has had a partial response. He was initially diagnosed when he presented with worsening psoriasis and enthesitis on the insertion sites of the plantar fascia, as well as dactylitis.
Zoster vaccination does not appear to increase flare risk in patients with immune-mediated inflammatory disease
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
, according to research published in Arthritis & Rheumatology.
The authors of the study noted that individuals with IMIDs are at increased risk for herpes zoster and related complications, including postherpetic neuralgia, and that vaccination has been recommended for certain groups of patients with rheumatoid arthritis, inflammatory bowel disease, and psoriasis, by the American College of Rheumatology and other professional organizations for individuals aged 50 and older.
The study investigators used medical claims from IBM MarketScan, which provided data on patients aged 50-64 years, and data from the Centers for Medicare and Medicaid Services’ Medicare on patients aged 65 and older.
They defined presumed flares in three ways: hospitalization/emergency department visits for IMIDs, steroid treatment with a short-acting oral glucocorticoid, or treatment with a parenteral glucocorticoid injection. The investigators conducted a self-controlled case series (SCCS) analysis to examine any temporal link between the RZV and disease flares.
Among enrollees with IMIDs, 14.8% of the 55,654 patients in the MarketScan database and 43.2% of the 160,545 patients in the Medicare database received at least one dose of RZV during 2018-2019. The two-dose series completion within 6 months was 76.6% in the MarketScan group (age range, 50-64 years) and 85.4% among Medicare enrollees (age range, 65 years and older). In the SCCS analysis, 10% and 13% of patients developed flares in the control group as compared to 9%, and 11%-12% in the risk window following one or two doses of RZV among MarketScan and Medicare enrollees, respectively.
Based on these findings, the investigators concluded there was no statistically significant increase in flares subsequent to RZV administration for any IMID in either patients aged 50-64 years or patients aged 65 years and older following the first dose or second dose.
Nilanjana Bose, MD, a rheumatologist with Lonestar Rheumatology, Houston, Texas, who was not involved with the study, said that the research addresses a topic where there is uneasiness, namely vaccination in patients with IMIDs.
“Anytime you are vaccinating a patient with an autoimmune disease, especially one on a biologic, you always worry about the risk of flares,” said Dr. Bose. “Any time you tamper with the immune system, there is a risk of flares.”
The study serves as a clarification for the primary care setting, said Dr. Bose. “A lot of the time, the shingles vaccine is administered not by rheumatology but by primary care or through the pharmacy,” she said. “This study puts them [primary care physicians] at ease.”
Findings from the study reflect that most RZV vaccinations were administered in pharmacies.
One of the weaknesses of the study is that the investigators did not include patients younger than 50 years old, said Dr. Bose. “It would have been nice if they could have looked at younger patients,” she said. “We try to vaccinate all our [immunocompromised] adult patients, even the younger ones, because they are also at risk for shingles.”
Given that there are increasing options of medical therapies in rheumatology that are immunomodulatory, the subject of vaccination for patients is often one of discussion, added Dr. Bose.
Arthur Kavanaugh, MD, professor of medicine, University of California San Diego (UCSD), La Jolla, Calif., and director of the Center for Innovative Therapy in the UCSD Division of Rheumatology, Allergy, and Immunology, told this news organization that a strength of the study is its large numbers of patients but noted the shortcoming of using claims data. “Claims data has inherent limitations, such as the lack of detailed granular data on the patients,” wrote Dr. Kavanaugh, who was not involved with the study. He described this investigation as “really about the first evidence that I am aware of addressing this issue.”
No funding source was listed. One author disclosed having received research grants and consulting fees received from Pfizer and GSK for unrelated work; the other authors had no disclosures. Dr. Bose and Dr. Kavanaugh had no relevant disclosures.
Large study reaffirms rare risk of TNF inhibitor–induced psoriasis in patients with RA, IBD
according to a new study published in JAMA Dermatology.
Despite this finding, the authors of the large Danish nationwide cohort study noted that TNFi-induced psoriasis is still a rare adverse event. “Practitioners and patients should be aware and observant of the potential for TNFi-associated psoriasis during TNFi treatment but keep in mind that the absolute risk appears to be low,” David Thein, MB, of the department of dermatology at Bispebjerg Hospital, University of Copenhagen, and colleagues wrote in the study.
They analyzed 109,085 patients with RA and IBD enrolled in Danish national registries between 1995 and 2018 without a previous diagnosis of psoriasis, who received either TNFi (20,910 patients) or conventional treatments (108,024 patients) and were followed for 5 years. They were a mean of 50 years old when they started treatment, 62% were women, with 87.8% of patients in the TNFi group receiving prior conventional therapy and 1% of patients in the conventional therapy group receiving prior TNFi treatment.
The investigators assessed the risk of developing any psoriasis, nonpustular psoriasis, and pustular psoriasis in the two groups using ICD-10 codes as well as a record of two consecutive prescriptions for topical vitamin D analogs.
Overall, 1,471 patients (1.4%) developed psoriasis of any type; 1,332 had non-pustular psoriasis, 127 had palmoplantar pustulosis, and 12 had generalized pustulosis.
The incidence rate of developing any psoriasis was 3.0 per 1,000 patient-years (95% confidence interval, 2.9-3.2) for patients receiving conventional therapy and 7.8 per 1,000 patient-years (95% CI, 7.5-8.9) for patients receiving TNFi treatment. Compared with conventional treatment, the risk of developing nonpustular psoriasis was twofold higher among patients receiving TNFi treatment (hazard ratio, 2.12; 95% CI, 1.87-2.40; P < .001). The risk of developing pustular psoriasis was more than sixfold higher among those on a TNFi (HR, 6.50; 95% CI, 4.60-9.23; P < .001).
Dr. Thein and colleagues estimated that the exposure needed to harm 1 additional patient was 241 patient-years for any psoriasis type, 342 patient-years for nonpustular psoriasis, and 909 patient-years for pustular psoriasis, with an estimated absolute risk difference of 5 per 1,000 patient-years.
Best evidence to date on risk
Asked to comment on the study findings, Anthony Fernandez, MD, PhD, director of medical dermatology at the Cleveland Clinic, said that he applauded the researchers for performing this well-designed study to determine the risk of TNF inhibitor–induced psoriasis in patients with RA and IBD.
The strengths of the study include excluding patients with a history of psoriasis to rule out disease recurrence and having a large comparator group of patients with IBD and RA who were taking medications other than TNF inhibitors, while one limitation was the potential accuracy of the ICD-10 codes used as the basis for diagnosing psoriasis. “It’s probably closer to the truth of what the true risk is compared to studies done in the past,” he said in an interview.
Dr. Fernandez noted that the results aren’t likely to change how dermatologists, rheumatologists, or gastroenterologists practice, but the message to stay the course in initially treating TNFi-induced psoriasis also holds value. “We don’t need to change anything in our clinical practice when it comes to TNF-alpha inhibitors.”
For patients with RA or IBD who develop TNFi-induced psoriasis with disease that is well controlled with TNFi treatment, keeping them on that treatment is a priority, Dr. Fernandez explained. “The first and foremost goal is, if the TNF inhibitor is working very well to control the disease that it was prescribed for, then you exhaust your efforts to try to control the psoriasis and allow those patients to stay on the TNF inhibitor.”
In his experience, most patients with RA and IBD who develop TNFi-induced psoriasis are controlled with topical medications. Switching to another TNFi is not recommended, he noted, as patients are “likely to have that reaction with any TNF inhibitor.”
However, Dr. Fernandez said that won’t be an option for all patients with RA and IBD. “In some patients you do simply have to stop the TNF inhibitor” and try an alternative treatment with a different mechanism of action.
The cause of TNFi-induced psoriasis is still not well understood. “There certainly is evidence to support that interferon alpha production by plasmacytoid dendritic cells is playing some role in this phenomenon,” but there is “more to the story” and unanswered questions remain, Dr. Fernandez said.
What’s most interesting about this phenomenon, he added, is that “patients can develop it at any time when exposed to a TNF inhibitor.” For instance, most patients develop drug reactions within 2-3 weeks of starting a treatment, but TNFi-induced psoriasis can appear after a single dose or several years after initiating treatment.
“Why so few patients, and why is there such variability in terms of how long they’re on the TNF inhibitor before the reaction occurs?” he asked. “That really points to ... some other trigger besides exposure to the TNF inhibitor needed for the initiation of this reaction.”
He noted that it would be valuable to identify triggers – or the most likely triggers – which would be challenging, but could “potentially impact clinical practice.”
The authors reported personal and institutional relationships in the form of personal and institutional research grants, honoraria, personal fees, investigator fees paid to university, consultancies, and speaker’s bureau positions for a variety of pharmaceutical companies, data companies, hospitals, and foundations. Dr. Fernandez reported he has nonbranded speaking, consulting, and research relationships with AbbVie and Novartis; and is a consultant for UCB, Bristol-Myers Squibb, and Boehringer Ingelheim on related products.
according to a new study published in JAMA Dermatology.
Despite this finding, the authors of the large Danish nationwide cohort study noted that TNFi-induced psoriasis is still a rare adverse event. “Practitioners and patients should be aware and observant of the potential for TNFi-associated psoriasis during TNFi treatment but keep in mind that the absolute risk appears to be low,” David Thein, MB, of the department of dermatology at Bispebjerg Hospital, University of Copenhagen, and colleagues wrote in the study.
They analyzed 109,085 patients with RA and IBD enrolled in Danish national registries between 1995 and 2018 without a previous diagnosis of psoriasis, who received either TNFi (20,910 patients) or conventional treatments (108,024 patients) and were followed for 5 years. They were a mean of 50 years old when they started treatment, 62% were women, with 87.8% of patients in the TNFi group receiving prior conventional therapy and 1% of patients in the conventional therapy group receiving prior TNFi treatment.
The investigators assessed the risk of developing any psoriasis, nonpustular psoriasis, and pustular psoriasis in the two groups using ICD-10 codes as well as a record of two consecutive prescriptions for topical vitamin D analogs.
Overall, 1,471 patients (1.4%) developed psoriasis of any type; 1,332 had non-pustular psoriasis, 127 had palmoplantar pustulosis, and 12 had generalized pustulosis.
The incidence rate of developing any psoriasis was 3.0 per 1,000 patient-years (95% confidence interval, 2.9-3.2) for patients receiving conventional therapy and 7.8 per 1,000 patient-years (95% CI, 7.5-8.9) for patients receiving TNFi treatment. Compared with conventional treatment, the risk of developing nonpustular psoriasis was twofold higher among patients receiving TNFi treatment (hazard ratio, 2.12; 95% CI, 1.87-2.40; P < .001). The risk of developing pustular psoriasis was more than sixfold higher among those on a TNFi (HR, 6.50; 95% CI, 4.60-9.23; P < .001).
Dr. Thein and colleagues estimated that the exposure needed to harm 1 additional patient was 241 patient-years for any psoriasis type, 342 patient-years for nonpustular psoriasis, and 909 patient-years for pustular psoriasis, with an estimated absolute risk difference of 5 per 1,000 patient-years.
Best evidence to date on risk
Asked to comment on the study findings, Anthony Fernandez, MD, PhD, director of medical dermatology at the Cleveland Clinic, said that he applauded the researchers for performing this well-designed study to determine the risk of TNF inhibitor–induced psoriasis in patients with RA and IBD.
The strengths of the study include excluding patients with a history of psoriasis to rule out disease recurrence and having a large comparator group of patients with IBD and RA who were taking medications other than TNF inhibitors, while one limitation was the potential accuracy of the ICD-10 codes used as the basis for diagnosing psoriasis. “It’s probably closer to the truth of what the true risk is compared to studies done in the past,” he said in an interview.
Dr. Fernandez noted that the results aren’t likely to change how dermatologists, rheumatologists, or gastroenterologists practice, but the message to stay the course in initially treating TNFi-induced psoriasis also holds value. “We don’t need to change anything in our clinical practice when it comes to TNF-alpha inhibitors.”
For patients with RA or IBD who develop TNFi-induced psoriasis with disease that is well controlled with TNFi treatment, keeping them on that treatment is a priority, Dr. Fernandez explained. “The first and foremost goal is, if the TNF inhibitor is working very well to control the disease that it was prescribed for, then you exhaust your efforts to try to control the psoriasis and allow those patients to stay on the TNF inhibitor.”
In his experience, most patients with RA and IBD who develop TNFi-induced psoriasis are controlled with topical medications. Switching to another TNFi is not recommended, he noted, as patients are “likely to have that reaction with any TNF inhibitor.”
However, Dr. Fernandez said that won’t be an option for all patients with RA and IBD. “In some patients you do simply have to stop the TNF inhibitor” and try an alternative treatment with a different mechanism of action.
The cause of TNFi-induced psoriasis is still not well understood. “There certainly is evidence to support that interferon alpha production by plasmacytoid dendritic cells is playing some role in this phenomenon,” but there is “more to the story” and unanswered questions remain, Dr. Fernandez said.
What’s most interesting about this phenomenon, he added, is that “patients can develop it at any time when exposed to a TNF inhibitor.” For instance, most patients develop drug reactions within 2-3 weeks of starting a treatment, but TNFi-induced psoriasis can appear after a single dose or several years after initiating treatment.
“Why so few patients, and why is there such variability in terms of how long they’re on the TNF inhibitor before the reaction occurs?” he asked. “That really points to ... some other trigger besides exposure to the TNF inhibitor needed for the initiation of this reaction.”
He noted that it would be valuable to identify triggers – or the most likely triggers – which would be challenging, but could “potentially impact clinical practice.”
The authors reported personal and institutional relationships in the form of personal and institutional research grants, honoraria, personal fees, investigator fees paid to university, consultancies, and speaker’s bureau positions for a variety of pharmaceutical companies, data companies, hospitals, and foundations. Dr. Fernandez reported he has nonbranded speaking, consulting, and research relationships with AbbVie and Novartis; and is a consultant for UCB, Bristol-Myers Squibb, and Boehringer Ingelheim on related products.
according to a new study published in JAMA Dermatology.
Despite this finding, the authors of the large Danish nationwide cohort study noted that TNFi-induced psoriasis is still a rare adverse event. “Practitioners and patients should be aware and observant of the potential for TNFi-associated psoriasis during TNFi treatment but keep in mind that the absolute risk appears to be low,” David Thein, MB, of the department of dermatology at Bispebjerg Hospital, University of Copenhagen, and colleagues wrote in the study.
They analyzed 109,085 patients with RA and IBD enrolled in Danish national registries between 1995 and 2018 without a previous diagnosis of psoriasis, who received either TNFi (20,910 patients) or conventional treatments (108,024 patients) and were followed for 5 years. They were a mean of 50 years old when they started treatment, 62% were women, with 87.8% of patients in the TNFi group receiving prior conventional therapy and 1% of patients in the conventional therapy group receiving prior TNFi treatment.
The investigators assessed the risk of developing any psoriasis, nonpustular psoriasis, and pustular psoriasis in the two groups using ICD-10 codes as well as a record of two consecutive prescriptions for topical vitamin D analogs.
Overall, 1,471 patients (1.4%) developed psoriasis of any type; 1,332 had non-pustular psoriasis, 127 had palmoplantar pustulosis, and 12 had generalized pustulosis.
The incidence rate of developing any psoriasis was 3.0 per 1,000 patient-years (95% confidence interval, 2.9-3.2) for patients receiving conventional therapy and 7.8 per 1,000 patient-years (95% CI, 7.5-8.9) for patients receiving TNFi treatment. Compared with conventional treatment, the risk of developing nonpustular psoriasis was twofold higher among patients receiving TNFi treatment (hazard ratio, 2.12; 95% CI, 1.87-2.40; P < .001). The risk of developing pustular psoriasis was more than sixfold higher among those on a TNFi (HR, 6.50; 95% CI, 4.60-9.23; P < .001).
Dr. Thein and colleagues estimated that the exposure needed to harm 1 additional patient was 241 patient-years for any psoriasis type, 342 patient-years for nonpustular psoriasis, and 909 patient-years for pustular psoriasis, with an estimated absolute risk difference of 5 per 1,000 patient-years.
Best evidence to date on risk
Asked to comment on the study findings, Anthony Fernandez, MD, PhD, director of medical dermatology at the Cleveland Clinic, said that he applauded the researchers for performing this well-designed study to determine the risk of TNF inhibitor–induced psoriasis in patients with RA and IBD.
The strengths of the study include excluding patients with a history of psoriasis to rule out disease recurrence and having a large comparator group of patients with IBD and RA who were taking medications other than TNF inhibitors, while one limitation was the potential accuracy of the ICD-10 codes used as the basis for diagnosing psoriasis. “It’s probably closer to the truth of what the true risk is compared to studies done in the past,” he said in an interview.
Dr. Fernandez noted that the results aren’t likely to change how dermatologists, rheumatologists, or gastroenterologists practice, but the message to stay the course in initially treating TNFi-induced psoriasis also holds value. “We don’t need to change anything in our clinical practice when it comes to TNF-alpha inhibitors.”
For patients with RA or IBD who develop TNFi-induced psoriasis with disease that is well controlled with TNFi treatment, keeping them on that treatment is a priority, Dr. Fernandez explained. “The first and foremost goal is, if the TNF inhibitor is working very well to control the disease that it was prescribed for, then you exhaust your efforts to try to control the psoriasis and allow those patients to stay on the TNF inhibitor.”
In his experience, most patients with RA and IBD who develop TNFi-induced psoriasis are controlled with topical medications. Switching to another TNFi is not recommended, he noted, as patients are “likely to have that reaction with any TNF inhibitor.”
However, Dr. Fernandez said that won’t be an option for all patients with RA and IBD. “In some patients you do simply have to stop the TNF inhibitor” and try an alternative treatment with a different mechanism of action.
The cause of TNFi-induced psoriasis is still not well understood. “There certainly is evidence to support that interferon alpha production by plasmacytoid dendritic cells is playing some role in this phenomenon,” but there is “more to the story” and unanswered questions remain, Dr. Fernandez said.
What’s most interesting about this phenomenon, he added, is that “patients can develop it at any time when exposed to a TNF inhibitor.” For instance, most patients develop drug reactions within 2-3 weeks of starting a treatment, but TNFi-induced psoriasis can appear after a single dose or several years after initiating treatment.
“Why so few patients, and why is there such variability in terms of how long they’re on the TNF inhibitor before the reaction occurs?” he asked. “That really points to ... some other trigger besides exposure to the TNF inhibitor needed for the initiation of this reaction.”
He noted that it would be valuable to identify triggers – or the most likely triggers – which would be challenging, but could “potentially impact clinical practice.”
The authors reported personal and institutional relationships in the form of personal and institutional research grants, honoraria, personal fees, investigator fees paid to university, consultancies, and speaker’s bureau positions for a variety of pharmaceutical companies, data companies, hospitals, and foundations. Dr. Fernandez reported he has nonbranded speaking, consulting, and research relationships with AbbVie and Novartis; and is a consultant for UCB, Bristol-Myers Squibb, and Boehringer Ingelheim on related products.
FROM JAMA DERMATOLOGY
Commentary: Evaluating New Treatments and Cardiovascular Risk in PsA, July 2022
Inhibition of the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway by JAK inhibitors is efficacious in psoriatic arthritis (PsA). On the basis of the results of the pivotal SELECT-PsA 1 and SELECT-PsA 2 trials, upadacitinib, a selective JAK1 inhibitor, was recently approved for the treatment of PsA. However, data on longer-term disease control is still of interest. In a post hoc analysis of SELECT-PsA 1 and SELECT-PsA 2, Mease and colleagues assessed the proportion of patients achieving low disease activity or remission, as defined by validated measures such as the Disease Activity Index in Psoriatic Arthritis, Psoriatic Arthritis Disease Activity Scores, and minimal disease activity at 24 and 56 weeks. They showed that at week 24,a higher proportion of patients receiving 15 mg upadacitinib vs placebo achieved low disease activityon the Disease Activity Index in Psoriatic Arthritis (range, 35%-48% vs 4%-16%; P< .05) and remission (range, 7%-11% vs 0%-3%; P< .05), with the responses sustained until week 56. Thus, upadacitinib provides sustained disease control in PsA and is an effective oral therapy.
Advanced targeted therapies have proven safety and efficacy over conventional therapies, often dramatically improving signs and symptoms. However, it is also desirable that such expensive therapies also show benefit in other outcomes, such as work productivity and quality of life. To evaluate work productivity and daily activity impairment and health-related quality of life in patients with inflammatory arthritis (rheumatoid arthritis, n=95;PsA,n=69, and axial spondyloarthritis, n=95) treated with golimumab, Dejaco and colleagues conducted a prospective, multicenter study in Austria. A total of 110 of these patients were followed for 24 months. At 24 months after golimumab initiation, there was significant improvement in total work productivity, presenteeism, activity impairment, and quality-of-life scores. Thus, golimumab, in addition to reducing disease activity, improved work productivity, activity, and health-related quality of life in patients with inflammatory arthritis, including PsA.
Cardiovascular disease (CVD) remains a major comorbidity in patients with PsA. This observation was once again confirmed in an observational, cross-sectional, case-control study including 207 patients with PsA and 414 matched controls from France. Degboe and colleagues demonstrated that patients with PsA had a higher prevalence of cardiovascular events and cardiovascular risk factors, such as high body mass index, triglyceride level, and hypertension, compared with controls. The proportion of patients with PsA who were estimated to have very high cardiovascular risk factors (≥10%) increased when SCORE (European Society of Cardiology Systematic Coronary Risk Evaluation) and QRISK2 (British Heart Foundation) equations considered the additional risk attributable to PsA. However, risk predictions scores such as SCORE and QRISK2 perform poorly in patients with PsA. To identify novel inflammatory and metabolic parameters associated with cardiovascular disease, Schwartz and colleagues looked at18F-fluorodeoxyglucose(FDG) PET-CT uptakeina cross-sectional analysis of a prospective study including 39 patients with biologic-treatment-naive PsA and 56 age-sex matched controls without PsA. They found that coronary artery disease (CAD) was significantly associated with visceral adiposity and FDG uptake in the bone marrow, liver, spleen, and subcutaneous adipose tissue. Thus, inflammatory and metabolic parameters, including visceral adiposity, potentially contribute to subclinical CAD in patients with PsA and may in the future be used to refine CVD risk and be targets for CAD preventive treatments.
Inhibition of the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway by JAK inhibitors is efficacious in psoriatic arthritis (PsA). On the basis of the results of the pivotal SELECT-PsA 1 and SELECT-PsA 2 trials, upadacitinib, a selective JAK1 inhibitor, was recently approved for the treatment of PsA. However, data on longer-term disease control is still of interest. In a post hoc analysis of SELECT-PsA 1 and SELECT-PsA 2, Mease and colleagues assessed the proportion of patients achieving low disease activity or remission, as defined by validated measures such as the Disease Activity Index in Psoriatic Arthritis, Psoriatic Arthritis Disease Activity Scores, and minimal disease activity at 24 and 56 weeks. They showed that at week 24,a higher proportion of patients receiving 15 mg upadacitinib vs placebo achieved low disease activityon the Disease Activity Index in Psoriatic Arthritis (range, 35%-48% vs 4%-16%; P< .05) and remission (range, 7%-11% vs 0%-3%; P< .05), with the responses sustained until week 56. Thus, upadacitinib provides sustained disease control in PsA and is an effective oral therapy.
Advanced targeted therapies have proven safety and efficacy over conventional therapies, often dramatically improving signs and symptoms. However, it is also desirable that such expensive therapies also show benefit in other outcomes, such as work productivity and quality of life. To evaluate work productivity and daily activity impairment and health-related quality of life in patients with inflammatory arthritis (rheumatoid arthritis, n=95;PsA,n=69, and axial spondyloarthritis, n=95) treated with golimumab, Dejaco and colleagues conducted a prospective, multicenter study in Austria. A total of 110 of these patients were followed for 24 months. At 24 months after golimumab initiation, there was significant improvement in total work productivity, presenteeism, activity impairment, and quality-of-life scores. Thus, golimumab, in addition to reducing disease activity, improved work productivity, activity, and health-related quality of life in patients with inflammatory arthritis, including PsA.
Cardiovascular disease (CVD) remains a major comorbidity in patients with PsA. This observation was once again confirmed in an observational, cross-sectional, case-control study including 207 patients with PsA and 414 matched controls from France. Degboe and colleagues demonstrated that patients with PsA had a higher prevalence of cardiovascular events and cardiovascular risk factors, such as high body mass index, triglyceride level, and hypertension, compared with controls. The proportion of patients with PsA who were estimated to have very high cardiovascular risk factors (≥10%) increased when SCORE (European Society of Cardiology Systematic Coronary Risk Evaluation) and QRISK2 (British Heart Foundation) equations considered the additional risk attributable to PsA. However, risk predictions scores such as SCORE and QRISK2 perform poorly in patients with PsA. To identify novel inflammatory and metabolic parameters associated with cardiovascular disease, Schwartz and colleagues looked at18F-fluorodeoxyglucose(FDG) PET-CT uptakeina cross-sectional analysis of a prospective study including 39 patients with biologic-treatment-naive PsA and 56 age-sex matched controls without PsA. They found that coronary artery disease (CAD) was significantly associated with visceral adiposity and FDG uptake in the bone marrow, liver, spleen, and subcutaneous adipose tissue. Thus, inflammatory and metabolic parameters, including visceral adiposity, potentially contribute to subclinical CAD in patients with PsA and may in the future be used to refine CVD risk and be targets for CAD preventive treatments.
Inhibition of the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway by JAK inhibitors is efficacious in psoriatic arthritis (PsA). On the basis of the results of the pivotal SELECT-PsA 1 and SELECT-PsA 2 trials, upadacitinib, a selective JAK1 inhibitor, was recently approved for the treatment of PsA. However, data on longer-term disease control is still of interest. In a post hoc analysis of SELECT-PsA 1 and SELECT-PsA 2, Mease and colleagues assessed the proportion of patients achieving low disease activity or remission, as defined by validated measures such as the Disease Activity Index in Psoriatic Arthritis, Psoriatic Arthritis Disease Activity Scores, and minimal disease activity at 24 and 56 weeks. They showed that at week 24,a higher proportion of patients receiving 15 mg upadacitinib vs placebo achieved low disease activityon the Disease Activity Index in Psoriatic Arthritis (range, 35%-48% vs 4%-16%; P< .05) and remission (range, 7%-11% vs 0%-3%; P< .05), with the responses sustained until week 56. Thus, upadacitinib provides sustained disease control in PsA and is an effective oral therapy.
Advanced targeted therapies have proven safety and efficacy over conventional therapies, often dramatically improving signs and symptoms. However, it is also desirable that such expensive therapies also show benefit in other outcomes, such as work productivity and quality of life. To evaluate work productivity and daily activity impairment and health-related quality of life in patients with inflammatory arthritis (rheumatoid arthritis, n=95;PsA,n=69, and axial spondyloarthritis, n=95) treated with golimumab, Dejaco and colleagues conducted a prospective, multicenter study in Austria. A total of 110 of these patients were followed for 24 months. At 24 months after golimumab initiation, there was significant improvement in total work productivity, presenteeism, activity impairment, and quality-of-life scores. Thus, golimumab, in addition to reducing disease activity, improved work productivity, activity, and health-related quality of life in patients with inflammatory arthritis, including PsA.
Cardiovascular disease (CVD) remains a major comorbidity in patients with PsA. This observation was once again confirmed in an observational, cross-sectional, case-control study including 207 patients with PsA and 414 matched controls from France. Degboe and colleagues demonstrated that patients with PsA had a higher prevalence of cardiovascular events and cardiovascular risk factors, such as high body mass index, triglyceride level, and hypertension, compared with controls. The proportion of patients with PsA who were estimated to have very high cardiovascular risk factors (≥10%) increased when SCORE (European Society of Cardiology Systematic Coronary Risk Evaluation) and QRISK2 (British Heart Foundation) equations considered the additional risk attributable to PsA. However, risk predictions scores such as SCORE and QRISK2 perform poorly in patients with PsA. To identify novel inflammatory and metabolic parameters associated with cardiovascular disease, Schwartz and colleagues looked at18F-fluorodeoxyglucose(FDG) PET-CT uptakeina cross-sectional analysis of a prospective study including 39 patients with biologic-treatment-naive PsA and 56 age-sex matched controls without PsA. They found that coronary artery disease (CAD) was significantly associated with visceral adiposity and FDG uptake in the bone marrow, liver, spleen, and subcutaneous adipose tissue. Thus, inflammatory and metabolic parameters, including visceral adiposity, potentially contribute to subclinical CAD in patients with PsA and may in the future be used to refine CVD risk and be targets for CAD preventive treatments.
Upadacitinib offers effective disease control in PsA
Key clinical point: A higher proportion of patients with psoriatic arthritis (PsA) receiving 15 mg upadacitinib achieved low disease activity (LDA) or remission after the first 6 months of treatment, with the difference being visible even after 1 year of treatment, compared to those who received a placebo.
Major finding: At week 24, a higher proportion of patients receiving 15 mg upadacitinib vs. placebo achieved Disease Activity in PsA LDA (range 35%-48% vs. 4%-16%; P < .05) and remission (range 7%-11% vs. 0%-3%; P < .05), with the responses sustained until 56 weeks.
Study details: This was a post hoc analysis of the SELECT-PsA 1 and SELECT-PsA 2 trials including 1386 adults with PsA and prior inadequate response/intolerance to ≥1 non-biologic or biologic disease-modifying antirheumatic drugs who were randomly assigned to receive upadacitinib (15 or 30 mg), adalimumab, or placebo.
Disclosures: This study was funded by AbbVie, Inc. Four authors declared being current or former employees or stockholders of AbbVie, and other authors reported ties with various sources.
Source: Mease P et al. Disease control with upadacitinib in patients with psoriatic arthritis: A post hoc analysis of the randomized, placebo-controlled SELECT-PsA 1 and 2 phase 3 trials. Rheumatol Ther. 2022 (May 23). Doi: 10.1007/s40744-022-00449-6
Key clinical point: A higher proportion of patients with psoriatic arthritis (PsA) receiving 15 mg upadacitinib achieved low disease activity (LDA) or remission after the first 6 months of treatment, with the difference being visible even after 1 year of treatment, compared to those who received a placebo.
Major finding: At week 24, a higher proportion of patients receiving 15 mg upadacitinib vs. placebo achieved Disease Activity in PsA LDA (range 35%-48% vs. 4%-16%; P < .05) and remission (range 7%-11% vs. 0%-3%; P < .05), with the responses sustained until 56 weeks.
Study details: This was a post hoc analysis of the SELECT-PsA 1 and SELECT-PsA 2 trials including 1386 adults with PsA and prior inadequate response/intolerance to ≥1 non-biologic or biologic disease-modifying antirheumatic drugs who were randomly assigned to receive upadacitinib (15 or 30 mg), adalimumab, or placebo.
Disclosures: This study was funded by AbbVie, Inc. Four authors declared being current or former employees or stockholders of AbbVie, and other authors reported ties with various sources.
Source: Mease P et al. Disease control with upadacitinib in patients with psoriatic arthritis: A post hoc analysis of the randomized, placebo-controlled SELECT-PsA 1 and 2 phase 3 trials. Rheumatol Ther. 2022 (May 23). Doi: 10.1007/s40744-022-00449-6
Key clinical point: A higher proportion of patients with psoriatic arthritis (PsA) receiving 15 mg upadacitinib achieved low disease activity (LDA) or remission after the first 6 months of treatment, with the difference being visible even after 1 year of treatment, compared to those who received a placebo.
Major finding: At week 24, a higher proportion of patients receiving 15 mg upadacitinib vs. placebo achieved Disease Activity in PsA LDA (range 35%-48% vs. 4%-16%; P < .05) and remission (range 7%-11% vs. 0%-3%; P < .05), with the responses sustained until 56 weeks.
Study details: This was a post hoc analysis of the SELECT-PsA 1 and SELECT-PsA 2 trials including 1386 adults with PsA and prior inadequate response/intolerance to ≥1 non-biologic or biologic disease-modifying antirheumatic drugs who were randomly assigned to receive upadacitinib (15 or 30 mg), adalimumab, or placebo.
Disclosures: This study was funded by AbbVie, Inc. Four authors declared being current or former employees or stockholders of AbbVie, and other authors reported ties with various sources.
Source: Mease P et al. Disease control with upadacitinib in patients with psoriatic arthritis: A post hoc analysis of the randomized, placebo-controlled SELECT-PsA 1 and 2 phase 3 trials. Rheumatol Ther. 2022 (May 23). Doi: 10.1007/s40744-022-00449-6
Enthesitis resolves regardless of medication used in PsA
Key clinical point: A substantial proportion of patients with psoriatic arthritis (PsA) achieved resolution of enthesitis within a year of initiating nonsteroidal anti-inflammatory drugs (NSAID) or disease-modifying antirheumatic drugs (DMARD), although the odds were lower in patients with high joint disease activity at baseline.
Major finding: Complete resolution of enthesitis was achieved by 86.12% of patients within a mean period of 8.73 months from therapy initiation, with higher joint activity at baseline being associated with a lower chance of enthesitis resolution (odds ratio 0.97; P = .01).
Study details: Findings are from a retrospective analysis of prospectively collected data of 526 patients with PsA and enthesitis who received no treatment/only NSAID (n = 142), conventional DMARD ± NSAID but without targeted DMARD (n = 196), or targeted DMARD with or without other medications (n = 188).
Disclosures: Dr. Mathew and Dr. Chandran received funding from the National Psoriasis Foundation and University of Toronto, respectively. The authors declared no conflicts of interest.
Source: Mathew AJ et al. Effectiveness of disease modifying anti-rheumatic drugs for enthesitis in a prospective longitudinal psoriatic arthritis cohort. J Rheumatol. 2022 (Jun 1). Doi: 10.3899/jrheum.211231
Key clinical point: A substantial proportion of patients with psoriatic arthritis (PsA) achieved resolution of enthesitis within a year of initiating nonsteroidal anti-inflammatory drugs (NSAID) or disease-modifying antirheumatic drugs (DMARD), although the odds were lower in patients with high joint disease activity at baseline.
Major finding: Complete resolution of enthesitis was achieved by 86.12% of patients within a mean period of 8.73 months from therapy initiation, with higher joint activity at baseline being associated with a lower chance of enthesitis resolution (odds ratio 0.97; P = .01).
Study details: Findings are from a retrospective analysis of prospectively collected data of 526 patients with PsA and enthesitis who received no treatment/only NSAID (n = 142), conventional DMARD ± NSAID but without targeted DMARD (n = 196), or targeted DMARD with or without other medications (n = 188).
Disclosures: Dr. Mathew and Dr. Chandran received funding from the National Psoriasis Foundation and University of Toronto, respectively. The authors declared no conflicts of interest.
Source: Mathew AJ et al. Effectiveness of disease modifying anti-rheumatic drugs for enthesitis in a prospective longitudinal psoriatic arthritis cohort. J Rheumatol. 2022 (Jun 1). Doi: 10.3899/jrheum.211231
Key clinical point: A substantial proportion of patients with psoriatic arthritis (PsA) achieved resolution of enthesitis within a year of initiating nonsteroidal anti-inflammatory drugs (NSAID) or disease-modifying antirheumatic drugs (DMARD), although the odds were lower in patients with high joint disease activity at baseline.
Major finding: Complete resolution of enthesitis was achieved by 86.12% of patients within a mean period of 8.73 months from therapy initiation, with higher joint activity at baseline being associated with a lower chance of enthesitis resolution (odds ratio 0.97; P = .01).
Study details: Findings are from a retrospective analysis of prospectively collected data of 526 patients with PsA and enthesitis who received no treatment/only NSAID (n = 142), conventional DMARD ± NSAID but without targeted DMARD (n = 196), or targeted DMARD with or without other medications (n = 188).
Disclosures: Dr. Mathew and Dr. Chandran received funding from the National Psoriasis Foundation and University of Toronto, respectively. The authors declared no conflicts of interest.
Source: Mathew AJ et al. Effectiveness of disease modifying anti-rheumatic drugs for enthesitis in a prospective longitudinal psoriatic arthritis cohort. J Rheumatol. 2022 (Jun 1). Doi: 10.3899/jrheum.211231
Commentary: Evaluating New Treatments and Cardiovascular Risk in PsA, July 2022
Advanced targeted therapies have proven safety and efficacy over conventional therapies, often dramatically improving signs and symptoms. However, it is also desirable that such expensive therapies also show benefit in other outcomes, such as work productivity and quality of life. To evaluate work productivity and daily activity impairment and health-related quality of life in patients with inflammatory arthritis (rheumatoid arthritis, n = 95; PsA, n = 69, and axial spondyloarthritis, n = 95) treated with golimumab, Dejaco and colleagues conducted a prospective, multicenter study in Austria. A total of 110 of these patients were followed for 24 months. At 24 months after golimumab initiation, there was significant improvement in total work productivity, presenteeism, activity impairment, and quality-of-life scores. Thus, golimumab, in addition to reducing disease activity, improved work productivity, activity, and health-related quality of life in patients with inflammatory arthritis, including PsA.
Cardiovascular disease (CVD) remains a major comorbidity in patients with PsA. This observation was once again confirmed in an observational, cross-sectional, case-control study including 207 patients with PsA and 414 matched controls from France. Degboe and colleagues demonstrated that patients with PsA had a higher prevalence of cardiovascular events and cardiovascular risk factors, such as high body mass index, triglyceride level, and hypertension, compared with controls. The proportion of patients with PsA who were estimated to have very high cardiovascular risk factors (≥ 10%) increased when SCORE (European Society of Cardiology Systematic Coronary Risk Evaluation) and QRISK2 (British Heart Foundation) equations considered the additional risk attributable to PsA. However, risk predictions scores such as SCORE and QRISK2 perform poorly in patients with PsA. To identify novel inflammatory and metabolic parameters associated with cardiovascular disease, Schwartz and colleagues looked at 18F-fluorodeoxyglucose (FDG) PET-CT uptake in a cross-sectional analysis of a prospective study including 39 patients with biologic-treatment-naive PsA and 56 age-sex matched controls without PsA. They found that coronary artery disease (CAD) was significantly associated with visceral adiposity and FDG uptake in the bone marrow, liver, spleen, and subcutaneous adipose tissue. Thus, inflammatory and metabolic parameters, including visceral adiposity, potentially contribute to subclinical CAD in patients with PsA and may in the future be used to refine CVD risk and be targets for CAD preventive treatments.
Advanced targeted therapies have proven safety and efficacy over conventional therapies, often dramatically improving signs and symptoms. However, it is also desirable that such expensive therapies also show benefit in other outcomes, such as work productivity and quality of life. To evaluate work productivity and daily activity impairment and health-related quality of life in patients with inflammatory arthritis (rheumatoid arthritis, n = 95; PsA, n = 69, and axial spondyloarthritis, n = 95) treated with golimumab, Dejaco and colleagues conducted a prospective, multicenter study in Austria. A total of 110 of these patients were followed for 24 months. At 24 months after golimumab initiation, there was significant improvement in total work productivity, presenteeism, activity impairment, and quality-of-life scores. Thus, golimumab, in addition to reducing disease activity, improved work productivity, activity, and health-related quality of life in patients with inflammatory arthritis, including PsA.
Cardiovascular disease (CVD) remains a major comorbidity in patients with PsA. This observation was once again confirmed in an observational, cross-sectional, case-control study including 207 patients with PsA and 414 matched controls from France. Degboe and colleagues demonstrated that patients with PsA had a higher prevalence of cardiovascular events and cardiovascular risk factors, such as high body mass index, triglyceride level, and hypertension, compared with controls. The proportion of patients with PsA who were estimated to have very high cardiovascular risk factors (≥ 10%) increased when SCORE (European Society of Cardiology Systematic Coronary Risk Evaluation) and QRISK2 (British Heart Foundation) equations considered the additional risk attributable to PsA. However, risk predictions scores such as SCORE and QRISK2 perform poorly in patients with PsA. To identify novel inflammatory and metabolic parameters associated with cardiovascular disease, Schwartz and colleagues looked at 18F-fluorodeoxyglucose (FDG) PET-CT uptake in a cross-sectional analysis of a prospective study including 39 patients with biologic-treatment-naive PsA and 56 age-sex matched controls without PsA. They found that coronary artery disease (CAD) was significantly associated with visceral adiposity and FDG uptake in the bone marrow, liver, spleen, and subcutaneous adipose tissue. Thus, inflammatory and metabolic parameters, including visceral adiposity, potentially contribute to subclinical CAD in patients with PsA and may in the future be used to refine CVD risk and be targets for CAD preventive treatments.
Advanced targeted therapies have proven safety and efficacy over conventional therapies, often dramatically improving signs and symptoms. However, it is also desirable that such expensive therapies also show benefit in other outcomes, such as work productivity and quality of life. To evaluate work productivity and daily activity impairment and health-related quality of life in patients with inflammatory arthritis (rheumatoid arthritis, n = 95; PsA, n = 69, and axial spondyloarthritis, n = 95) treated with golimumab, Dejaco and colleagues conducted a prospective, multicenter study in Austria. A total of 110 of these patients were followed for 24 months. At 24 months after golimumab initiation, there was significant improvement in total work productivity, presenteeism, activity impairment, and quality-of-life scores. Thus, golimumab, in addition to reducing disease activity, improved work productivity, activity, and health-related quality of life in patients with inflammatory arthritis, including PsA.
Cardiovascular disease (CVD) remains a major comorbidity in patients with PsA. This observation was once again confirmed in an observational, cross-sectional, case-control study including 207 patients with PsA and 414 matched controls from France. Degboe and colleagues demonstrated that patients with PsA had a higher prevalence of cardiovascular events and cardiovascular risk factors, such as high body mass index, triglyceride level, and hypertension, compared with controls. The proportion of patients with PsA who were estimated to have very high cardiovascular risk factors (≥ 10%) increased when SCORE (European Society of Cardiology Systematic Coronary Risk Evaluation) and QRISK2 (British Heart Foundation) equations considered the additional risk attributable to PsA. However, risk predictions scores such as SCORE and QRISK2 perform poorly in patients with PsA. To identify novel inflammatory and metabolic parameters associated with cardiovascular disease, Schwartz and colleagues looked at 18F-fluorodeoxyglucose (FDG) PET-CT uptake in a cross-sectional analysis of a prospective study including 39 patients with biologic-treatment-naive PsA and 56 age-sex matched controls without PsA. They found that coronary artery disease (CAD) was significantly associated with visceral adiposity and FDG uptake in the bone marrow, liver, spleen, and subcutaneous adipose tissue. Thus, inflammatory and metabolic parameters, including visceral adiposity, potentially contribute to subclinical CAD in patients with PsA and may in the future be used to refine CVD risk and be targets for CAD preventive treatments.
No more ‘escape hatch’: Post Roe, new worries about meds linked to birth defects
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
As states ban or limit abortion in the wake of the demise of Roe v. Wade, physicians are turning their attention to widely-used drugs that can cause birth defects. At issue: Should these drugs still be prescribed to women of childbearing age if they don’t have the option of terminating their pregnancies?
“Doctors are going to understandably be terrified that a patient may become pregnant using a teratogen that they have prescribed,” said University of Pittsburgh rheumatologist Mehret Birru Talabi, MD, PhD, who works in a state where the future of abortion rights is uncertain. “While this was a feared outcome before Roe v. Wade was overturned, abortion provided an escape hatch by which women could avoid having to continue a pregnancy and potentially raise a child with congenital anomalies. I believe that prescribing is going to become much more defensive and conservative. Some clinicians may choose not to prescribe these medications to patients who have childbearing potential, even if they don’t have much risk for pregnancy.”
Other physicians expressed similar concerns in interviews. Duke University, Durham, N.C., rheumatologist Megan E. B. Clowse, MD, MPH, fears that physicians will be wary of prescribing a variety of medications – including new ones for which there are few pregnancy data – if abortion is unavailable. “Women who receive these new or teratogenic medications will likely lose their reproductive autonomy and be forced to choose between having sexual relationships with men, obtaining procedures that make them permanently sterile, or using contraception that may cause intolerable side effects,” she said. “I am very concerned that young women with rheumatic disease will now be left with active disease resulting in joint damage and renal failure.”
Abortion is now banned in at least six states, according to The New York Times. That number may rise to 16 as more restrictions become law. Another five states aren’t expected to ban abortion soon but have implemented gestational age limits on abortion or are expected to adopt them. In another nine states, courts or lawmakers will decide whether abortion remains legal.
Only 20 states and the District of Columbia have firm abortion protections in place.
Numerous drugs are considered teratogens, which means they may cause birth defects. Thalidomide is the most infamous, but there are many more, including several used in rheumatology, dermatology, and gastroenterology. Among the most widely used teratogenic medications are the acne drugs isotretinoin and methotrexate, which are used to treat a variety of conditions, such as cancer, rheumatoid arthritis, and psoriasis.
Dr. Clowse, who helps manage an industry-supported website devoted to reproductive care for women with lupus (www.LupusPregnancy.org), noted that several drugs linked to birth defects and pregnancy loss are commonly prescribed in rheumatology.
“Methotrexate is the most common medication and has been the cornerstone of rheumatoid arthritis [treatment] for at least two decades,” she said. “Mycophenolate is our best medication to treat lupus nephritis, which is inflammation in the kidneys caused by lupus. This is a common complication for young women with lupus, and all of our guideline-recommended treatment regimens include a medication that causes pregnancy loss and birth defects, either mycophenolate or cyclophosphamide.”
Rheumatologists also prescribe a large number of new drugs for which there are few data about pregnancy risks. “It typically takes about two decades to have sufficient data about the safety of our medications,” she said.
Reflecting the sensitivity of the topic, Dr. Clowse made clear that her opinions don’t represent the views of her institution. She works in North Carolina, where the fate of abortion rights is uncertain, according to The New York Times.
What about alternatives? “The short answer is that some of these medications work really well and sometimes much better than the nonteratogenic alternatives,” said Dr. Birru Talabi. “I’m worried about methotrexate. It has been used to induce abortions but is primarily used in the United States as a highly effective treatment for cancer as well as a myriad of rheumatic diseases. If legislators try to restrict access to methotrexate, we may see increasing disability and even death among people who need this medication but cannot access it.”
Rheumatologists aren’t the only physicians who are worrying about the fates of their patients in a new era of abortion restrictions. Gastroenterologist Sunanda Kane, MD, MSPH, of the Mayo Clinic, Rochester, Minn., said several teratogenic medications are used in her field to treat constipation, viral hepatitis, and inflammatory bowel disease.
“When treating women of childbearing age, there are usually alternatives. If we do prescribe a medication with a high teratogenic potential, we counsel and document that we have discussed two forms of birth control to avoid pregnancy. We usually do not prescribe a drug with teratogenic potential with the ‘out’ being an abortion if a pregnancy does occur,” she said. However, “if abortion is not even on the table as an option, we may be much less likely to prescribe these medications. This will be particularly true in patients who clearly do not have the means to travel to have an abortion in any situation.”
Abortion is expected to remain legal in Minnesota, where Dr. Kane practices, but it may be restricted or banned in nearby Wisconsin, depending on the state legislature. None of her patients have had abortions after becoming pregnant while taking the medications, she said, although she “did have a patient who because of her religious faith did not have an abortion after exposure and ended up with a stillbirth.”
The crackdown on abortion won’t just pose risks to patients who take potentially dangerous medications, physicians said. Dr. Kane said pregnancy itself is a significant risk for patients with “very active, uncontrolled gastrointestinal conditions where a pregnancy could be harmful to the mother’s health or result in offspring that are very unhealthy.” These include decompensated cirrhosis, uncontrolled Crohn’s disease or ulcerative colitis, refractory gastroparesis, uncontrolled celiac sprue, and chronic pancreatitis, she said.
“There have been times when after shared decisionmaking, a patient with very active inflammatory bowel disease has decided to terminate the pregnancy because of her own ongoing health issues,” she said. “Not having this option will potentially lead to disastrous results.”
Dr. Clowse, the Duke University rheumatologist, echoed Dr. Kane’s concerns about women who are too sick to bear children. “The removal of abortion rights puts the lives and quality of life for women with rheumatic disease at risk. For patients with lupus and other systemic rheumatic disease, pregnancy can be medically catastrophic, leading to permanent harm and even death to the woman and her offspring. I am worried that women in these conditions will die without lifesaving pregnancy terminations, due to worries about the legal consequences for their physicians.”
The U.S. Supreme Court’s ruling that overturned Roe v. Wade has also raised the prospect that the court could ultimately allow birth control to be restricted or outlawed.
While the ruling states that “nothing in this opinion should be understood to cast doubt on precedents that do not concern abortion,” Justice Clarence Thomas wrote a concurrence in which he said that the court should reconsider a 1960s ruling that forbids the banning of contraceptives. Republicans have dismissed concerns about bans being allowed, although Democrats, including the president and vice president, starkly warn that they could happen.
“If we as providers have to be concerned that there will be an unplanned pregnancy because of the lack of access to contraception,” Dr. Kane said, “this will have significant downstream consequences to the kind of care we can provide and might just drive some providers to not give care to female patients at all given this concern.”
The physicians quoted in this article report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treat-to-target strategy with tapering proves effective in PsA and axSpA
Aiming for a disease activity target while reducing biologic therapy could be a winning approach for patients with psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA), according to the results of a new study presented at the annual European Congress of Rheumatology.
The findings show that a treat-to-target (T2T) strategy with tapering using a tumor necrosis factor (TNF) inhibitor produces results that are noninferior to a T2T strategy that doesn’t include tapering in these patients.
“Our study has for the first time shown that a treat-to-target tapering strategy is just as good as full-dose continuation, while reducing medication use substantially,” first author Celia Michielsens, MD, a PhD student and researcher at Sint Maartenskliniek in Nijmegen, the Netherlands, said in an interview before her presentation of the study during an oral abstract session at the congress. “Stepwise tapering is also better than fixed-dose reduction or discontinuation, since it is much more individualized.”
The study is now published in Annals of the Rheumatic Diseases.
In the randomized, controlled, open-label, noninferiority study, researchers enrolled patients with PsA or axSpA who were using a TNF inhibitor such as etanercept, adalimumab, or infliximab, and had stable low disease activity for at least 6 months. Patients needed to have a Psoriatic Arthritis Disease Activity Score (PASDAS) of 3.2 or less, or an Ankylosing Spondylitis Disease Activity Score (ASDAS) of at 2.1 or less. In cases of flare, patients were treated with NSAIDs and/or glucorticoids, and if they still had not reached low disease activity after a month, their previous TNF inhibitor dose was reinstated to the last effective interval or dosage, which was maintained throughout the study period. When the patient was already using a full TNF-inhibitor dose or if dose adjustment did not suffice, patients were switched to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD).
Participants were randomized, from January 2019 to June 2021, to a tapering or a nontapering T2T strategy in a 2:1 fashion. Then researchers then followed them for 12 months and aimed to determine if the tapering strategy proved noninferior to not tapering within a predefined 20% margin for noninferiority, which Dr. Michielsens said was derived from other studies and what her group determined to be “an acceptable risk.”
Results show strategy is ‘feasible in daily clinical care’
A total of 81 patients – 42 with PsA and 39 with axSpA – were in the group with tapering, and 41 were in the group without tapering: 22 with PsA and 19 with axSpA.
At 12 months, researchers found that 69% of the patients in the group with tapering had low disease activity, measured via the PASDAS and ASDAS, compared with 73% in patients who did not taper. And those in the tapering group saw their medication use dramatically reduced. At the 12-month mark, they were taking just 53% of the defined daily dose for maintenance, compared with 91% of the defined daily dose for the group that didn’t taper.
The researchers were able to successfully taper 72% of the patients in the tapering group, with 28% of them discontinuing their TNF-inhibitor medication entirely. The incidence of flares was 85% in the tapering group and 78% in the nontapering group, a nonsignificant difference (P = .32).
The start of a new medication or an increase in use of an existing medication was more frequent in the tapering group, and significantly so for NSAIDs. An increase in NSAID use was seen in 54% of the tapering group and in just 24% of the nontapering group (P = .002).
Conventional synthetic DMARD use went up in the tapering group, compared with the nontapering group, but this was only among the PsA patients and the change in use was not statistically significant. There were also more frequent increases in glucocorticoid use in the tapering group, compared with the nontapering group, but this was not significant.
Dr. Michielsens said the findings show the value of an individualized approach in treating patients with PsA or axSpA.
“Our study – and those [studies] in rheumatoid arthritis earlier – deliver the highest quality of evidence that disease activity–guided dose personalization can, and in fact should, be used in clinical practice,” she said. “Our pragmatic treat-to-target tapering strategy is feasible in daily clinical care, although treat-to-target using PASDAS and ASDAS needs some implementation. In shared decision-making with patients, a 50% reduction in TNFi use is obtainable, while maintaining low disease activity.”
The increase in the use of NSAIDs is something to be aware of, but it is “not concerning,” Dr. Michielsens added. She pointed out that the NSAID use was typically temporary, used when flares arose, and that the drugs are effective, safe, and inexpensive. She also noted that the use of TNF blockers decreased more than the use of NSAIDs increased.
“This seems a perfectly acceptable trade-off that can be discussed with your patient,” she said.
The 12-month duration of the study is likely long enough to show that the tapering strategy works, Dr. Michielsens said. In rheumatoid arthritis studies, for example, differences in strategies didn’t change after 1 year.
“That said, we are doing an observational extension study to provide more insights in the long-term effects of this treat-to-target strategy,” she said. “At the end of this summer, all patients will have completed their extended follow-up period – a 12-month observational period – so hopefully we can present the results next year at EULAR.”
This study received funding from ReumaNederland. Dr. Michielsens did not have any financial interests to disclose. Two coauthors reported financial relationships with numerous pharmaceutical companies.
Aiming for a disease activity target while reducing biologic therapy could be a winning approach for patients with psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA), according to the results of a new study presented at the annual European Congress of Rheumatology.
The findings show that a treat-to-target (T2T) strategy with tapering using a tumor necrosis factor (TNF) inhibitor produces results that are noninferior to a T2T strategy that doesn’t include tapering in these patients.
“Our study has for the first time shown that a treat-to-target tapering strategy is just as good as full-dose continuation, while reducing medication use substantially,” first author Celia Michielsens, MD, a PhD student and researcher at Sint Maartenskliniek in Nijmegen, the Netherlands, said in an interview before her presentation of the study during an oral abstract session at the congress. “Stepwise tapering is also better than fixed-dose reduction or discontinuation, since it is much more individualized.”
The study is now published in Annals of the Rheumatic Diseases.
In the randomized, controlled, open-label, noninferiority study, researchers enrolled patients with PsA or axSpA who were using a TNF inhibitor such as etanercept, adalimumab, or infliximab, and had stable low disease activity for at least 6 months. Patients needed to have a Psoriatic Arthritis Disease Activity Score (PASDAS) of 3.2 or less, or an Ankylosing Spondylitis Disease Activity Score (ASDAS) of at 2.1 or less. In cases of flare, patients were treated with NSAIDs and/or glucorticoids, and if they still had not reached low disease activity after a month, their previous TNF inhibitor dose was reinstated to the last effective interval or dosage, which was maintained throughout the study period. When the patient was already using a full TNF-inhibitor dose or if dose adjustment did not suffice, patients were switched to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD).
Participants were randomized, from January 2019 to June 2021, to a tapering or a nontapering T2T strategy in a 2:1 fashion. Then researchers then followed them for 12 months and aimed to determine if the tapering strategy proved noninferior to not tapering within a predefined 20% margin for noninferiority, which Dr. Michielsens said was derived from other studies and what her group determined to be “an acceptable risk.”
Results show strategy is ‘feasible in daily clinical care’
A total of 81 patients – 42 with PsA and 39 with axSpA – were in the group with tapering, and 41 were in the group without tapering: 22 with PsA and 19 with axSpA.
At 12 months, researchers found that 69% of the patients in the group with tapering had low disease activity, measured via the PASDAS and ASDAS, compared with 73% in patients who did not taper. And those in the tapering group saw their medication use dramatically reduced. At the 12-month mark, they were taking just 53% of the defined daily dose for maintenance, compared with 91% of the defined daily dose for the group that didn’t taper.
The researchers were able to successfully taper 72% of the patients in the tapering group, with 28% of them discontinuing their TNF-inhibitor medication entirely. The incidence of flares was 85% in the tapering group and 78% in the nontapering group, a nonsignificant difference (P = .32).
The start of a new medication or an increase in use of an existing medication was more frequent in the tapering group, and significantly so for NSAIDs. An increase in NSAID use was seen in 54% of the tapering group and in just 24% of the nontapering group (P = .002).
Conventional synthetic DMARD use went up in the tapering group, compared with the nontapering group, but this was only among the PsA patients and the change in use was not statistically significant. There were also more frequent increases in glucocorticoid use in the tapering group, compared with the nontapering group, but this was not significant.
Dr. Michielsens said the findings show the value of an individualized approach in treating patients with PsA or axSpA.
“Our study – and those [studies] in rheumatoid arthritis earlier – deliver the highest quality of evidence that disease activity–guided dose personalization can, and in fact should, be used in clinical practice,” she said. “Our pragmatic treat-to-target tapering strategy is feasible in daily clinical care, although treat-to-target using PASDAS and ASDAS needs some implementation. In shared decision-making with patients, a 50% reduction in TNFi use is obtainable, while maintaining low disease activity.”
The increase in the use of NSAIDs is something to be aware of, but it is “not concerning,” Dr. Michielsens added. She pointed out that the NSAID use was typically temporary, used when flares arose, and that the drugs are effective, safe, and inexpensive. She also noted that the use of TNF blockers decreased more than the use of NSAIDs increased.
“This seems a perfectly acceptable trade-off that can be discussed with your patient,” she said.
The 12-month duration of the study is likely long enough to show that the tapering strategy works, Dr. Michielsens said. In rheumatoid arthritis studies, for example, differences in strategies didn’t change after 1 year.
“That said, we are doing an observational extension study to provide more insights in the long-term effects of this treat-to-target strategy,” she said. “At the end of this summer, all patients will have completed their extended follow-up period – a 12-month observational period – so hopefully we can present the results next year at EULAR.”
This study received funding from ReumaNederland. Dr. Michielsens did not have any financial interests to disclose. Two coauthors reported financial relationships with numerous pharmaceutical companies.
Aiming for a disease activity target while reducing biologic therapy could be a winning approach for patients with psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA), according to the results of a new study presented at the annual European Congress of Rheumatology.
The findings show that a treat-to-target (T2T) strategy with tapering using a tumor necrosis factor (TNF) inhibitor produces results that are noninferior to a T2T strategy that doesn’t include tapering in these patients.
“Our study has for the first time shown that a treat-to-target tapering strategy is just as good as full-dose continuation, while reducing medication use substantially,” first author Celia Michielsens, MD, a PhD student and researcher at Sint Maartenskliniek in Nijmegen, the Netherlands, said in an interview before her presentation of the study during an oral abstract session at the congress. “Stepwise tapering is also better than fixed-dose reduction or discontinuation, since it is much more individualized.”
The study is now published in Annals of the Rheumatic Diseases.
In the randomized, controlled, open-label, noninferiority study, researchers enrolled patients with PsA or axSpA who were using a TNF inhibitor such as etanercept, adalimumab, or infliximab, and had stable low disease activity for at least 6 months. Patients needed to have a Psoriatic Arthritis Disease Activity Score (PASDAS) of 3.2 or less, or an Ankylosing Spondylitis Disease Activity Score (ASDAS) of at 2.1 or less. In cases of flare, patients were treated with NSAIDs and/or glucorticoids, and if they still had not reached low disease activity after a month, their previous TNF inhibitor dose was reinstated to the last effective interval or dosage, which was maintained throughout the study period. When the patient was already using a full TNF-inhibitor dose or if dose adjustment did not suffice, patients were switched to another biologic or targeted synthetic disease-modifying antirheumatic drug (DMARD).
Participants were randomized, from January 2019 to June 2021, to a tapering or a nontapering T2T strategy in a 2:1 fashion. Then researchers then followed them for 12 months and aimed to determine if the tapering strategy proved noninferior to not tapering within a predefined 20% margin for noninferiority, which Dr. Michielsens said was derived from other studies and what her group determined to be “an acceptable risk.”
Results show strategy is ‘feasible in daily clinical care’
A total of 81 patients – 42 with PsA and 39 with axSpA – were in the group with tapering, and 41 were in the group without tapering: 22 with PsA and 19 with axSpA.
At 12 months, researchers found that 69% of the patients in the group with tapering had low disease activity, measured via the PASDAS and ASDAS, compared with 73% in patients who did not taper. And those in the tapering group saw their medication use dramatically reduced. At the 12-month mark, they were taking just 53% of the defined daily dose for maintenance, compared with 91% of the defined daily dose for the group that didn’t taper.
The researchers were able to successfully taper 72% of the patients in the tapering group, with 28% of them discontinuing their TNF-inhibitor medication entirely. The incidence of flares was 85% in the tapering group and 78% in the nontapering group, a nonsignificant difference (P = .32).
The start of a new medication or an increase in use of an existing medication was more frequent in the tapering group, and significantly so for NSAIDs. An increase in NSAID use was seen in 54% of the tapering group and in just 24% of the nontapering group (P = .002).
Conventional synthetic DMARD use went up in the tapering group, compared with the nontapering group, but this was only among the PsA patients and the change in use was not statistically significant. There were also more frequent increases in glucocorticoid use in the tapering group, compared with the nontapering group, but this was not significant.
Dr. Michielsens said the findings show the value of an individualized approach in treating patients with PsA or axSpA.
“Our study – and those [studies] in rheumatoid arthritis earlier – deliver the highest quality of evidence that disease activity–guided dose personalization can, and in fact should, be used in clinical practice,” she said. “Our pragmatic treat-to-target tapering strategy is feasible in daily clinical care, although treat-to-target using PASDAS and ASDAS needs some implementation. In shared decision-making with patients, a 50% reduction in TNFi use is obtainable, while maintaining low disease activity.”
The increase in the use of NSAIDs is something to be aware of, but it is “not concerning,” Dr. Michielsens added. She pointed out that the NSAID use was typically temporary, used when flares arose, and that the drugs are effective, safe, and inexpensive. She also noted that the use of TNF blockers decreased more than the use of NSAIDs increased.
“This seems a perfectly acceptable trade-off that can be discussed with your patient,” she said.
The 12-month duration of the study is likely long enough to show that the tapering strategy works, Dr. Michielsens said. In rheumatoid arthritis studies, for example, differences in strategies didn’t change after 1 year.
“That said, we are doing an observational extension study to provide more insights in the long-term effects of this treat-to-target strategy,” she said. “At the end of this summer, all patients will have completed their extended follow-up period – a 12-month observational period – so hopefully we can present the results next year at EULAR.”
This study received funding from ReumaNederland. Dr. Michielsens did not have any financial interests to disclose. Two coauthors reported financial relationships with numerous pharmaceutical companies.
FROM THE EULAR 2022 CONGRESS