User login
Don’t Miss These Signs of Rosacea in Darker Skin Types
Don’t Miss These Signs of Rosacea in Darker Skin Types
THE COMPARISON:
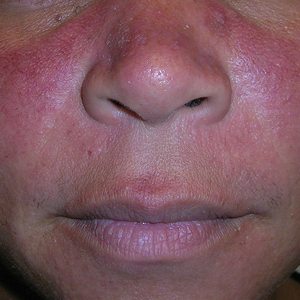
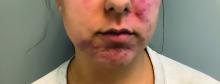
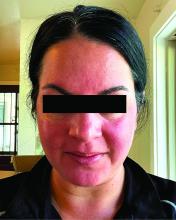
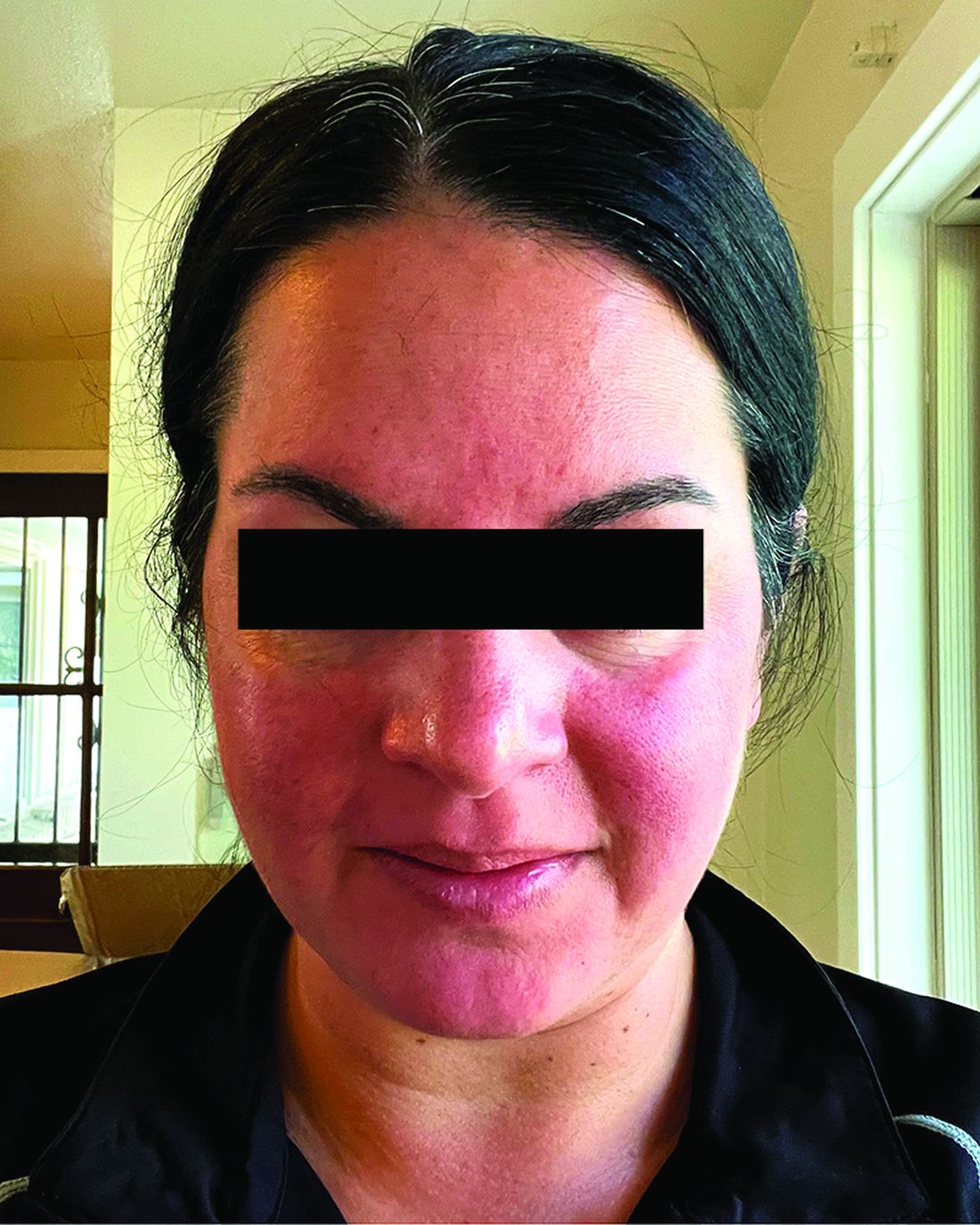
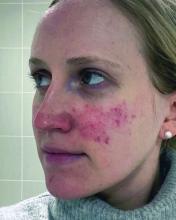
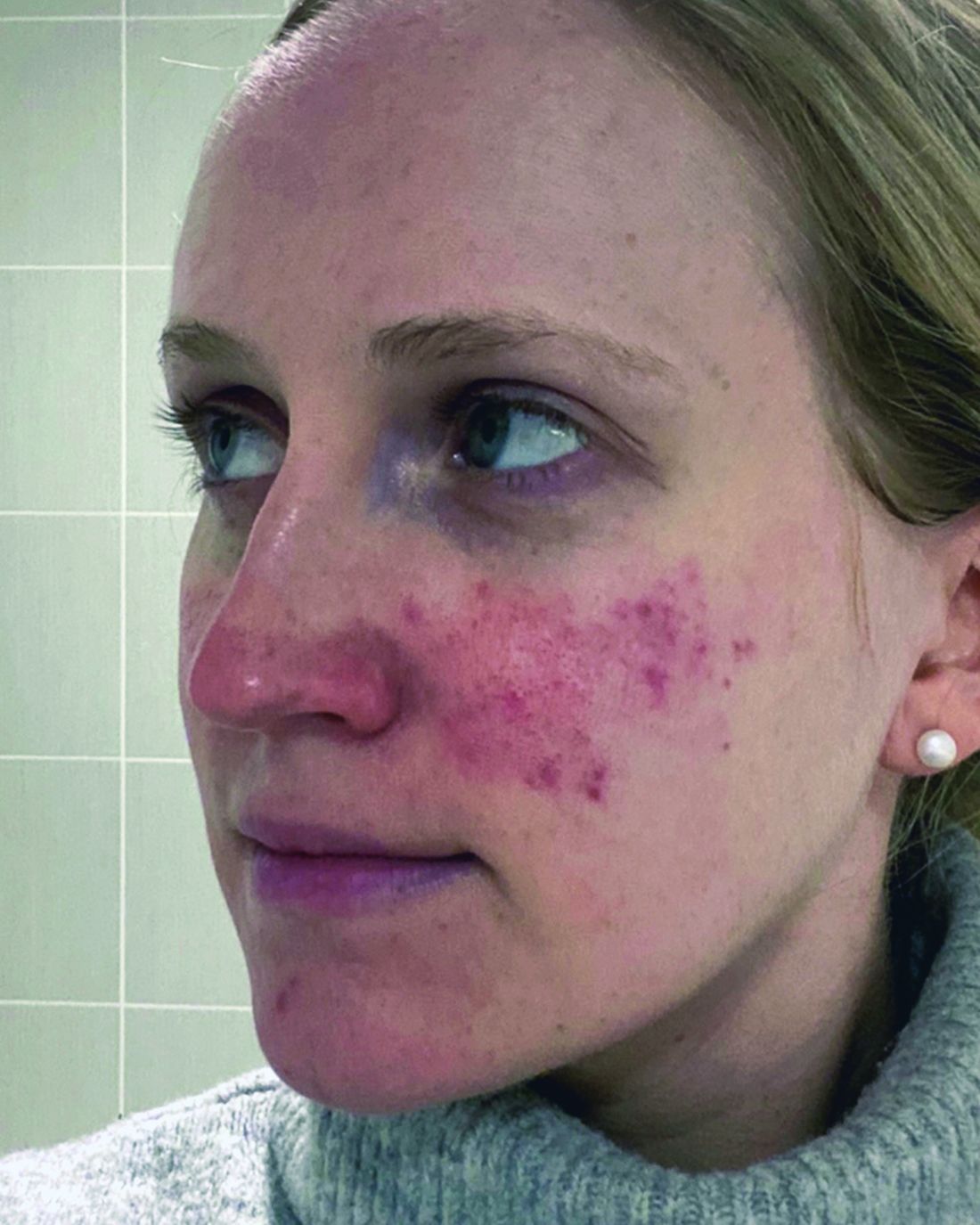
- A. Erythematotelangiectatic rosacea in a polygonal vascular pattern on the cheeks in a Black woman who also has eyelid hypopigmentation due to vitiligo.
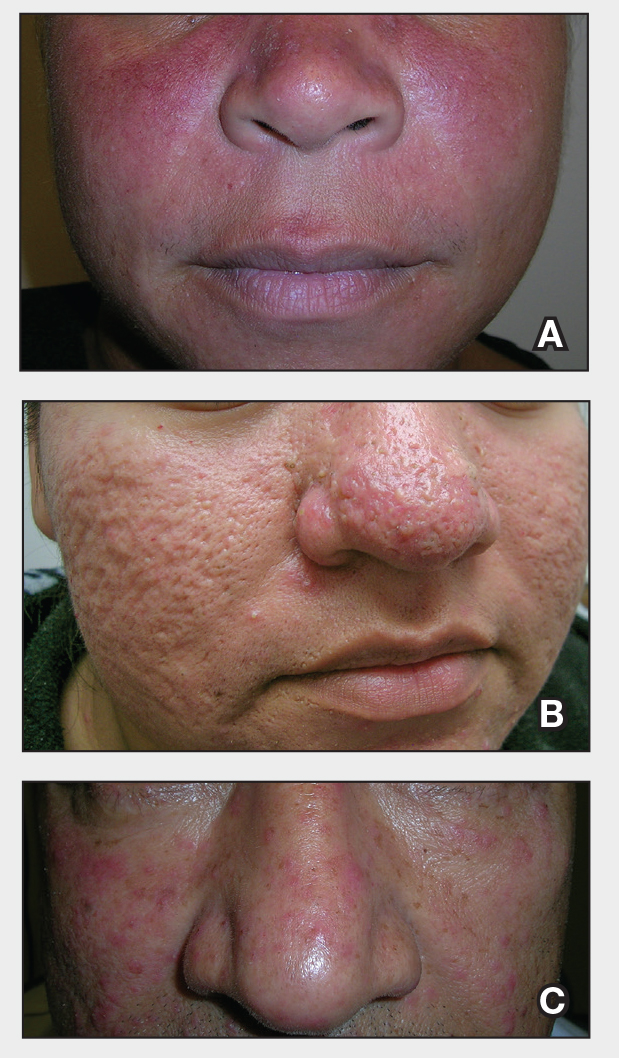
- B. Rhinophymatous rosacea in a Hispanic woman who also has papules and pustules on the chin and upper lip region as well as facial scarring from severe inflammatory acne during her teen years.
- C. Papulopustular rosacea in a Hispanic man.
Rosacea is a chronic inflammatory condition characterized by facial flushing and persistent erythema of the central face, typically affecting the cheeks and nose. It also may manifest with papules, pustules, and telangiectasias. The 4 main subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous (involving thickening of the skin, often of the nose), and ocular (dry, itchy, or irritated eyes).1 Patients also may report stinging, burning, dryness, and edema.2 The etiology of rosacea is unclear but is believed to involve immune dysfunction, neurovascular dysregulation, certain microorganisms, and genetic predisposition.1,2
Epidemiology
Rosacea often is associated with fair skin and more frequently is reported in individuals of Northern European descent.1,2 While it may be less common in darker skin types, rosacea is not rare in patients with skin of color (SOC). A review of US outpatient data from 1993 to 2010 found that 2% of patients with rosacea were Black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.3 Global estimates suggest that up to 40 million individuals with SOC may be affected by rosacea,4 with the reported prevalence as high as 10%.2 Although early research linked rosacea primarily to adults older than 30 years, newer data show peak prevalence between ages 25 to 39 years, suggesting that younger adults may be affected more than previously recognized.5
Key Clinical Features
In addition to the traditional subtypes, updated guidelines recommend a phenotype- based approach to diagnosing rosacea focusing on observable features such as persistent redness in the central face and thickened skin rather than classifying patients into broad categories. A diagnosis can be made when at least one diagnostic feature is present (eg, fixed facial erythema or phymatous changes) or when 2 or more major features are observed (eg, papules, pustules, flushing, visible blood vessels, or ocular findings).6
In individuals with darker skin types, erythema may not be bright red; rather, the skin may appear pink, reddish-brown, violaceous, or dusky brown.7 Postinflammatory hyperpigmentation, which is common in darker skin tones, can further mask erythema.2 Pressing a microscope slide or magnifying glass against the skin can help assess for blanching, which is indicative of erythema. Telangiectasias also may be more challenging to appreciate in patients with SOC and typically require bright, shadow-free lighting or dermoscopy for detection.2
Skin thickening across the cheeks and nose with overlying acneform papules can be diagnostic clues of rosacea in darker skin types and help distinguish it from acne.2 It also is important to distinguish rosacea from systemic lupus erythematosus, which typically manifests as a malar rash that spares the nasolabial folds and is nonpustular. If uncertain, consider serologic testing for antinuclear antibodies, patch testing, or biopsy.8
Worth Noting
Treatment of rosacea is focused on managing symptoms and reducing flares. First-line strategies include behavioral modifications and trigger avoidance, such as minimizing sun exposure and avoiding consumption of alcohol and spicy foods.9 Gentle skin care practices are essential, including the use of light, fragrance-free, nonirritating cleansers and moisturizers at least once daily. Application of sunscreen with an SPF of at least 30 also is routinely recommended.9,10 Additionally, patients should be counseled to avoid harsh cleansers, such as exfoliants, astringents, and chemicals that may further diminish the skin barrier.10
Treatment options approved by the US Food and Drug Administration for rosacea include oral doxycycline, oral minocycline, topical brimonidine, oxymetazoline, ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, encapsulated benzoyl peroxide cream, and minocycline.11-13
Topical treatment options commonly used off-label for rosacea include topical clindamycin, topical retinoids, and azithromycin. Oral tetracyclines should be avoided in children and pregnant women; instead, oral erythromycin and topical metronidazole commonly are used.14
Laser or intense pulsed light therapy may be considered, although results have been mixed, and the long-term benefits are uncertain. Given the higher risk for postinflammatory hyperpigmentation in patients with SOC, these modalities should be used cautiously.15 Among the available options, the Nd:YAG laser is preferred in darker skin types due to its safety profile.16 A small case series reported successful CO2 laser treatment for rhinophyma in patients with melanated skin; however, some patients developed localized scarring, suggesting that conservative depth settings should be used to reduce risk for this adverse event.17
Health Disparity Highlight
Rosacea may be underdiagnosed in individuals with darker skin types,2,15,18 likely due in part to reduced contrast between erythema and background skin tone, which can make features such as flushing and telangiectasias harder to appreciate.1,10,15
Although tools to assess erythema exist, they rarely are used in everyday clinical practice.10 In patients with deeply pigmented skin, ensuring adequate examination room lighting and using dermoscopy can help identify any subtle vascular or textural changes localized across the central face. While various imaging techniques are used in clinical trials to monitor treatment response, few have been studied and optimized across a wide range of skin tones.10 There is a need for dermatologic assessment tools that better capture the degree of erythema, inflammation, and vascular features of rosacea in pigmented skin. Emerging research is focused on developing more equitable imaging technologies.19
- Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9:E1361574.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Al-Dabagh A, Davis SA, McMichael AJ, el al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20:13030/qt1mv9r0ss.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
- Saurat JH, Halioua B, Baissac C, et al. Epidemiology of acne and rosacea: a worldwide global study. J Am Acad Dermatol. 2024;90:1016-1018.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Finlay AY, Griffiths TW, Belmo S, et al. Why we should abandon the misused descriptor ‘erythema’. Br J Dermatol. 2021;185:1240-1241.
- Callender VD, Barbosa V, Burgess CM, et al. Approach to treatment of medical and cosmetic facial concerns in skin of color patients. Cutis. 2017;100:375-380.
- Baldwin H, Alexis A, Andriessen A, et al. Supplement article: skin barrier deficiency in rosacea: an algorithm integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2022;21:SF3595563-SF35955610.
- Ohanenye C, Taliaferro S, Callender VD. Diagnosing disorders of facial erythema. Dermatol Clin. 2023;41:377-392.
- Thiboutot D, Anderson R, Cook-Bolden F, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82:1501-1510.
- Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
- van der Linden MMD, van Ratingen AR, van Rappard DC, et al. DOMINO, doxycycline 40 mg vs. minocycline 100 mg in the treatment of rosacea: a randomized, single-blinded, noninferiority trial, comparing efficacy and safety. Br J Dermatol. 2017;176:1465-1474.
- Geng R, Bourkas A, Sibbald RG, et al. Efficacy of treatments for rosacea in the pediatric population: a systematic review. JEADV Clinical Practice. 2024;3:17-48.
- Sarkar R, Podder I, Jagadeesan S. Rosacea in skin of color: a comprehensive review. Indian J Dermatol Venereol Leprol. 2020;86:611-621.
- Chen A, Choi J, Balazic E, et al. Review of laser and energy-based devices to treat rosacea in skin of color. J Cosmet Laser Ther. 2024;26:43-53.
- Nganzeu CG, Lopez A, Brennan TE. Ablative CO2 laser treatment of rhinophyma in people of color: a case series. Plast Reconstr Surg Glob Open. 2025;13:E6616.
- Kulthanan K, Andriessen A, Jiang X, et al. A review of the challenges and nuances in treating rosacea in Asian skin types using cleansers and moisturizers as adjuncts. J Drugs Dermatol. 2023;22:45-53.
- Jarang A, McGrath Q, Harunani M, et al. Multispectral SWIR imaging for equitable pigmentation-insensitive assessment of inflammatory acne in darkly pigmented skin. Presented at Photonics in Dermatology and Plastic Surgery 2025; January 25-27, 2025; San Francisco, California.
THE COMPARISON:
- A. Erythematotelangiectatic rosacea in a polygonal vascular pattern on the cheeks in a Black woman who also has eyelid hypopigmentation due to vitiligo.
- B. Rhinophymatous rosacea in a Hispanic woman who also has papules and pustules on the chin and upper lip region as well as facial scarring from severe inflammatory acne during her teen years.
- C. Papulopustular rosacea in a Hispanic man.
Rosacea is a chronic inflammatory condition characterized by facial flushing and persistent erythema of the central face, typically affecting the cheeks and nose. It also may manifest with papules, pustules, and telangiectasias. The 4 main subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous (involving thickening of the skin, often of the nose), and ocular (dry, itchy, or irritated eyes).1 Patients also may report stinging, burning, dryness, and edema.2 The etiology of rosacea is unclear but is believed to involve immune dysfunction, neurovascular dysregulation, certain microorganisms, and genetic predisposition.1,2

Epidemiology
Rosacea often is associated with fair skin and more frequently is reported in individuals of Northern European descent.1,2 While it may be less common in darker skin types, rosacea is not rare in patients with skin of color (SOC). A review of US outpatient data from 1993 to 2010 found that 2% of patients with rosacea were Black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.3 Global estimates suggest that up to 40 million individuals with SOC may be affected by rosacea,4 with the reported prevalence as high as 10%.2 Although early research linked rosacea primarily to adults older than 30 years, newer data show peak prevalence between ages 25 to 39 years, suggesting that younger adults may be affected more than previously recognized.5
Key Clinical Features
In addition to the traditional subtypes, updated guidelines recommend a phenotype- based approach to diagnosing rosacea focusing on observable features such as persistent redness in the central face and thickened skin rather than classifying patients into broad categories. A diagnosis can be made when at least one diagnostic feature is present (eg, fixed facial erythema or phymatous changes) or when 2 or more major features are observed (eg, papules, pustules, flushing, visible blood vessels, or ocular findings).6
In individuals with darker skin types, erythema may not be bright red; rather, the skin may appear pink, reddish-brown, violaceous, or dusky brown.7 Postinflammatory hyperpigmentation, which is common in darker skin tones, can further mask erythema.2 Pressing a microscope slide or magnifying glass against the skin can help assess for blanching, which is indicative of erythema. Telangiectasias also may be more challenging to appreciate in patients with SOC and typically require bright, shadow-free lighting or dermoscopy for detection.2
Skin thickening across the cheeks and nose with overlying acneform papules can be diagnostic clues of rosacea in darker skin types and help distinguish it from acne.2 It also is important to distinguish rosacea from systemic lupus erythematosus, which typically manifests as a malar rash that spares the nasolabial folds and is nonpustular. If uncertain, consider serologic testing for antinuclear antibodies, patch testing, or biopsy.8
Worth Noting
Treatment of rosacea is focused on managing symptoms and reducing flares. First-line strategies include behavioral modifications and trigger avoidance, such as minimizing sun exposure and avoiding consumption of alcohol and spicy foods.9 Gentle skin care practices are essential, including the use of light, fragrance-free, nonirritating cleansers and moisturizers at least once daily. Application of sunscreen with an SPF of at least 30 also is routinely recommended.9,10 Additionally, patients should be counseled to avoid harsh cleansers, such as exfoliants, astringents, and chemicals that may further diminish the skin barrier.10
Treatment options approved by the US Food and Drug Administration for rosacea include oral doxycycline, oral minocycline, topical brimonidine, oxymetazoline, ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, encapsulated benzoyl peroxide cream, and minocycline.11-13
Topical treatment options commonly used off-label for rosacea include topical clindamycin, topical retinoids, and azithromycin. Oral tetracyclines should be avoided in children and pregnant women; instead, oral erythromycin and topical metronidazole commonly are used.14
Laser or intense pulsed light therapy may be considered, although results have been mixed, and the long-term benefits are uncertain. Given the higher risk for postinflammatory hyperpigmentation in patients with SOC, these modalities should be used cautiously.15 Among the available options, the Nd:YAG laser is preferred in darker skin types due to its safety profile.16 A small case series reported successful CO2 laser treatment for rhinophyma in patients with melanated skin; however, some patients developed localized scarring, suggesting that conservative depth settings should be used to reduce risk for this adverse event.17
Health Disparity Highlight
Rosacea may be underdiagnosed in individuals with darker skin types,2,15,18 likely due in part to reduced contrast between erythema and background skin tone, which can make features such as flushing and telangiectasias harder to appreciate.1,10,15
Although tools to assess erythema exist, they rarely are used in everyday clinical practice.10 In patients with deeply pigmented skin, ensuring adequate examination room lighting and using dermoscopy can help identify any subtle vascular or textural changes localized across the central face. While various imaging techniques are used in clinical trials to monitor treatment response, few have been studied and optimized across a wide range of skin tones.10 There is a need for dermatologic assessment tools that better capture the degree of erythema, inflammation, and vascular features of rosacea in pigmented skin. Emerging research is focused on developing more equitable imaging technologies.19
THE COMPARISON:
- A. Erythematotelangiectatic rosacea in a polygonal vascular pattern on the cheeks in a Black woman who also has eyelid hypopigmentation due to vitiligo.
- B. Rhinophymatous rosacea in a Hispanic woman who also has papules and pustules on the chin and upper lip region as well as facial scarring from severe inflammatory acne during her teen years.
- C. Papulopustular rosacea in a Hispanic man.
Rosacea is a chronic inflammatory condition characterized by facial flushing and persistent erythema of the central face, typically affecting the cheeks and nose. It also may manifest with papules, pustules, and telangiectasias. The 4 main subtypes of rosacea are erythematotelangiectatic, papulopustular, phymatous (involving thickening of the skin, often of the nose), and ocular (dry, itchy, or irritated eyes).1 Patients also may report stinging, burning, dryness, and edema.2 The etiology of rosacea is unclear but is believed to involve immune dysfunction, neurovascular dysregulation, certain microorganisms, and genetic predisposition.1,2

Epidemiology
Rosacea often is associated with fair skin and more frequently is reported in individuals of Northern European descent.1,2 While it may be less common in darker skin types, rosacea is not rare in patients with skin of color (SOC). A review of US outpatient data from 1993 to 2010 found that 2% of patients with rosacea were Black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.3 Global estimates suggest that up to 40 million individuals with SOC may be affected by rosacea,4 with the reported prevalence as high as 10%.2 Although early research linked rosacea primarily to adults older than 30 years, newer data show peak prevalence between ages 25 to 39 years, suggesting that younger adults may be affected more than previously recognized.5
Key Clinical Features
In addition to the traditional subtypes, updated guidelines recommend a phenotype- based approach to diagnosing rosacea focusing on observable features such as persistent redness in the central face and thickened skin rather than classifying patients into broad categories. A diagnosis can be made when at least one diagnostic feature is present (eg, fixed facial erythema or phymatous changes) or when 2 or more major features are observed (eg, papules, pustules, flushing, visible blood vessels, or ocular findings).6
In individuals with darker skin types, erythema may not be bright red; rather, the skin may appear pink, reddish-brown, violaceous, or dusky brown.7 Postinflammatory hyperpigmentation, which is common in darker skin tones, can further mask erythema.2 Pressing a microscope slide or magnifying glass against the skin can help assess for blanching, which is indicative of erythema. Telangiectasias also may be more challenging to appreciate in patients with SOC and typically require bright, shadow-free lighting or dermoscopy for detection.2
Skin thickening across the cheeks and nose with overlying acneform papules can be diagnostic clues of rosacea in darker skin types and help distinguish it from acne.2 It also is important to distinguish rosacea from systemic lupus erythematosus, which typically manifests as a malar rash that spares the nasolabial folds and is nonpustular. If uncertain, consider serologic testing for antinuclear antibodies, patch testing, or biopsy.8
Worth Noting
Treatment of rosacea is focused on managing symptoms and reducing flares. First-line strategies include behavioral modifications and trigger avoidance, such as minimizing sun exposure and avoiding consumption of alcohol and spicy foods.9 Gentle skin care practices are essential, including the use of light, fragrance-free, nonirritating cleansers and moisturizers at least once daily. Application of sunscreen with an SPF of at least 30 also is routinely recommended.9,10 Additionally, patients should be counseled to avoid harsh cleansers, such as exfoliants, astringents, and chemicals that may further diminish the skin barrier.10
Treatment options approved by the US Food and Drug Administration for rosacea include oral doxycycline, oral minocycline, topical brimonidine, oxymetazoline, ivermectin, metronidazole, azelaic acid, sodium sulfacetamide/sulfur, encapsulated benzoyl peroxide cream, and minocycline.11-13
Topical treatment options commonly used off-label for rosacea include topical clindamycin, topical retinoids, and azithromycin. Oral tetracyclines should be avoided in children and pregnant women; instead, oral erythromycin and topical metronidazole commonly are used.14
Laser or intense pulsed light therapy may be considered, although results have been mixed, and the long-term benefits are uncertain. Given the higher risk for postinflammatory hyperpigmentation in patients with SOC, these modalities should be used cautiously.15 Among the available options, the Nd:YAG laser is preferred in darker skin types due to its safety profile.16 A small case series reported successful CO2 laser treatment for rhinophyma in patients with melanated skin; however, some patients developed localized scarring, suggesting that conservative depth settings should be used to reduce risk for this adverse event.17
Health Disparity Highlight
Rosacea may be underdiagnosed in individuals with darker skin types,2,15,18 likely due in part to reduced contrast between erythema and background skin tone, which can make features such as flushing and telangiectasias harder to appreciate.1,10,15
Although tools to assess erythema exist, they rarely are used in everyday clinical practice.10 In patients with deeply pigmented skin, ensuring adequate examination room lighting and using dermoscopy can help identify any subtle vascular or textural changes localized across the central face. While various imaging techniques are used in clinical trials to monitor treatment response, few have been studied and optimized across a wide range of skin tones.10 There is a need for dermatologic assessment tools that better capture the degree of erythema, inflammation, and vascular features of rosacea in pigmented skin. Emerging research is focused on developing more equitable imaging technologies.19
- Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9:E1361574.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Al-Dabagh A, Davis SA, McMichael AJ, el al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20:13030/qt1mv9r0ss.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
- Saurat JH, Halioua B, Baissac C, et al. Epidemiology of acne and rosacea: a worldwide global study. J Am Acad Dermatol. 2024;90:1016-1018.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Finlay AY, Griffiths TW, Belmo S, et al. Why we should abandon the misused descriptor ‘erythema’. Br J Dermatol. 2021;185:1240-1241.
- Callender VD, Barbosa V, Burgess CM, et al. Approach to treatment of medical and cosmetic facial concerns in skin of color patients. Cutis. 2017;100:375-380.
- Baldwin H, Alexis A, Andriessen A, et al. Supplement article: skin barrier deficiency in rosacea: an algorithm integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2022;21:SF3595563-SF35955610.
- Ohanenye C, Taliaferro S, Callender VD. Diagnosing disorders of facial erythema. Dermatol Clin. 2023;41:377-392.
- Thiboutot D, Anderson R, Cook-Bolden F, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82:1501-1510.
- Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
- van der Linden MMD, van Ratingen AR, van Rappard DC, et al. DOMINO, doxycycline 40 mg vs. minocycline 100 mg in the treatment of rosacea: a randomized, single-blinded, noninferiority trial, comparing efficacy and safety. Br J Dermatol. 2017;176:1465-1474.
- Geng R, Bourkas A, Sibbald RG, et al. Efficacy of treatments for rosacea in the pediatric population: a systematic review. JEADV Clinical Practice. 2024;3:17-48.
- Sarkar R, Podder I, Jagadeesan S. Rosacea in skin of color: a comprehensive review. Indian J Dermatol Venereol Leprol. 2020;86:611-621.
- Chen A, Choi J, Balazic E, et al. Review of laser and energy-based devices to treat rosacea in skin of color. J Cosmet Laser Ther. 2024;26:43-53.
- Nganzeu CG, Lopez A, Brennan TE. Ablative CO2 laser treatment of rhinophyma in people of color: a case series. Plast Reconstr Surg Glob Open. 2025;13:E6616.
- Kulthanan K, Andriessen A, Jiang X, et al. A review of the challenges and nuances in treating rosacea in Asian skin types using cleansers and moisturizers as adjuncts. J Drugs Dermatol. 2023;22:45-53.
- Jarang A, McGrath Q, Harunani M, et al. Multispectral SWIR imaging for equitable pigmentation-insensitive assessment of inflammatory acne in darkly pigmented skin. Presented at Photonics in Dermatology and Plastic Surgery 2025; January 25-27, 2025; San Francisco, California.
- Rainer BM, Kang S, Chien AL. Rosacea: epidemiology, pathogenesis, and treatment. Dermatoendocrinol. 2017;9:E1361574.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Al-Dabagh A, Davis SA, McMichael AJ, el al. Rosacea in skin of color: not a rare diagnosis. Dermatol Online J. 2014;20:13030/qt1mv9r0ss.
- Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 suppl 1):S27-S35.
- Saurat JH, Halioua B, Baissac C, et al. Epidemiology of acne and rosacea: a worldwide global study. J Am Acad Dermatol. 2024;90:1016-1018.
- Gallo RL, Granstein RD, Kang S, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78:148-155.
- Finlay AY, Griffiths TW, Belmo S, et al. Why we should abandon the misused descriptor ‘erythema’. Br J Dermatol. 2021;185:1240-1241.
- Callender VD, Barbosa V, Burgess CM, et al. Approach to treatment of medical and cosmetic facial concerns in skin of color patients. Cutis. 2017;100:375-380.
- Baldwin H, Alexis A, Andriessen A, et al. Supplement article: skin barrier deficiency in rosacea: an algorithm integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2022;21:SF3595563-SF35955610.
- Ohanenye C, Taliaferro S, Callender VD. Diagnosing disorders of facial erythema. Dermatol Clin. 2023;41:377-392.
- Thiboutot D, Anderson R, Cook-Bolden F, et al. Standard management options for rosacea: the 2019 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2020;82:1501-1510.
- Del Rosso JQ, Schlessinger J, Werschler P. Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol. 2008;7:573-576.
- van der Linden MMD, van Ratingen AR, van Rappard DC, et al. DOMINO, doxycycline 40 mg vs. minocycline 100 mg in the treatment of rosacea: a randomized, single-blinded, noninferiority trial, comparing efficacy and safety. Br J Dermatol. 2017;176:1465-1474.
- Geng R, Bourkas A, Sibbald RG, et al. Efficacy of treatments for rosacea in the pediatric population: a systematic review. JEADV Clinical Practice. 2024;3:17-48.
- Sarkar R, Podder I, Jagadeesan S. Rosacea in skin of color: a comprehensive review. Indian J Dermatol Venereol Leprol. 2020;86:611-621.
- Chen A, Choi J, Balazic E, et al. Review of laser and energy-based devices to treat rosacea in skin of color. J Cosmet Laser Ther. 2024;26:43-53.
- Nganzeu CG, Lopez A, Brennan TE. Ablative CO2 laser treatment of rhinophyma in people of color: a case series. Plast Reconstr Surg Glob Open. 2025;13:E6616.
- Kulthanan K, Andriessen A, Jiang X, et al. A review of the challenges and nuances in treating rosacea in Asian skin types using cleansers and moisturizers as adjuncts. J Drugs Dermatol. 2023;22:45-53.
- Jarang A, McGrath Q, Harunani M, et al. Multispectral SWIR imaging for equitable pigmentation-insensitive assessment of inflammatory acne in darkly pigmented skin. Presented at Photonics in Dermatology and Plastic Surgery 2025; January 25-27, 2025; San Francisco, California.
Don’t Miss These Signs of Rosacea in Darker Skin Types
Don’t Miss These Signs of Rosacea in Darker Skin Types
The Skin Microbiome in Rosacea: Mechanisms, Gut-Skin Interactions, and Therapeutic Implications
The Skin Microbiome in Rosacea: Mechanisms, Gut-Skin Interactions, and Therapeutic Implications
Rosacea is a chronic inflammatory skin condition affecting the central face—including the cheeks, nose, chin, and forehead—that causes considerable discomfort.1 Its pathogenesis involves immune dysregulation, genetic predisposition, and microbial dysbiosis.2 While immune and environmental factors are known triggers of rosacea, recent research highlights the roles of the gut and skin microbiomes in disease progression. While the skin microbiome interacts directly with the immune system to regulate inflammation and skin homeostasis, the gut microbiome also influences cutaneous inflammation, emphasizing the need to address both topical and internal microbiome imbalances.3 In this article, we review gut and skin microbial alterations in rosacea, focusing on the skin microbiome and including the gut-skin axis implications as well as therapeutic strategies aimed at microbiome balance to enhance patient outcomes.
Skin Microbiome Alterations in Rosacea
The human skin microbiome interacts with the immune system, and microbial imbalances have been shown to contribute to immune dysregulation. Several key microbial species have been identified as playing a large role in rosacea, including Demodex folliculorum, Staphylococcus epidermidis, Bacillus oleronius, and Cutibacterium acnes (Figure).
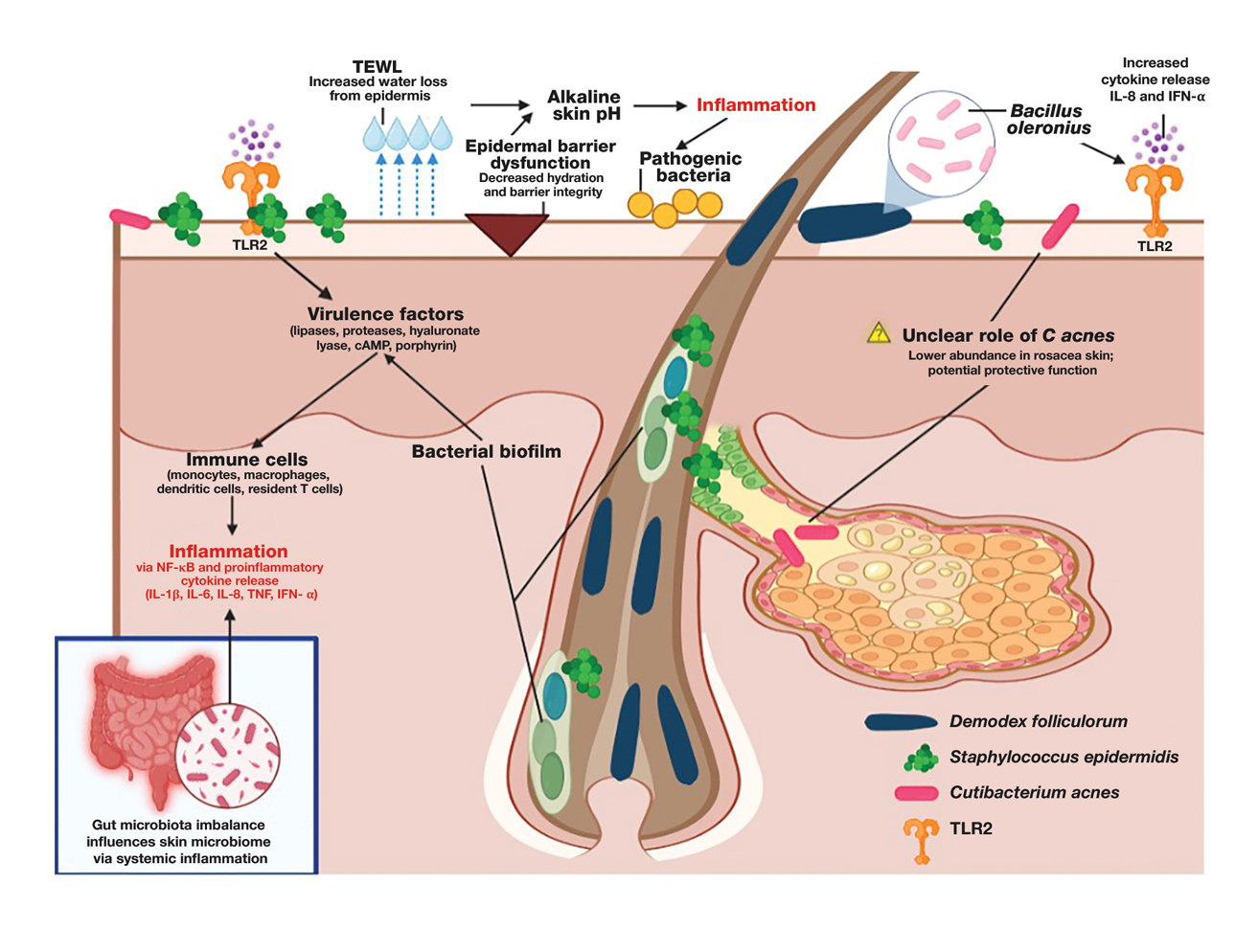
Demodex folliculorum is a microscopic mite is found in hair follicles and sebaceous glands. Patients with rosacea have higher densities of D folliculorum, which trigger follicular occlusion and immune activation.1 Bacillus oleronius be isolated from D folliculorum and can further activate toll-like receptor 2, leading to cytokine production and immune cell infiltration.3,4 Increased propagation of this mite correlates with shifts in skin microbiome composition, demonstrating increased inflammatory microbial populations.3
Staphylococcus epidermidis normally is commensal but can become pathogenic (pathobiont) in rosacea due to disruptions in the skin microenvironment, where it can form biofilms and produce virulence factors, particularly in papulopustular rosacea.5
Bacillus oleronius has been isolated from D folliculorum mites and provokes inflammatory responses in patients with rosacea by triggering toll-like receptor 2 activation and cytokine secretion.6
Cutibacterium acnes commonly is associated with acne vulgaris. Its role in rosacea is unclear, but recent research suggests it may have a protective effect. A single-arm trial investigated the effects of minocycline on rosacea and found that treatment significantly reduced C acnes but increased microbial species diversity, improving inflammation.7 One longitudinal cohort study of 12 patients with rosacea found that C acnes levels were lower in those older than 60 years. Rosacea severity increased with age and correlated with a decline in C acnes, suggesting that it may confer some protective effect in rosacea.8 This finding is supported by studies that have shown a reduction in C acnes levels in patients with rosacea compared to controls.4,8
Important mechanisms in rosacea include epidermal barrier dysfunction, transepidermal water loss, and decreased stratum corneum hydration, particularly in erythematotelangiectatic and papulopustular subtypes. The resulting alkaline skin pH contributes to barrier instability and heightened inflammation, permitting pathogenic bacteria to proliferate and disrupt skin microbial homeostasis.9 A recent study identified metabolic changes in the skin microbiome of patients with rosacea, showing that increased heme and hydrogen sulfide in rosacea skin microbiomes likely drive inflammation, while healthy skin microbiomes produce more anti-inflammatory adenosylcobalamin, thiazole, and L-isoleucine.1 These findings highlight the link between microbial imbalances and inflammation in rosacea.
The Gut-Skin Axis in Rosacea
Gut microbiota play a critical role in managing systemic inflammation, and microbial dysbiosis in the intestine can influence the skin microbiome in rosacea. Patients with rosacea who have gastrointestinal conditions such as small intestinal bacterial overgrowth and Helicobacter pylori infection experience more severe rosacea symptoms.3,10
Patients with rosacea have distinctive gut microbiota compositions, with an increased prevalence of proinflammatory bacterial species, potentially affecting the skin microbiome.8,11 Systemic antibiotics have been shown to modulate the gut microbiome, indirectly influencing the skin microbiome.11 A recent study demonstrated that doxycycline treatment in patients with rosacea altered skin microbial diversity, reducing C acnes while increasing Weissella confusa—highlighting the complicated relationship between systemic antibiotics and the gut-skin axis.8
Specific probiotics, such as Escherichia coli Nissle, when given orally shifted gut microbial balance to protective microbiota with increased Lactobacillus and Bifidobacteria species and decreased pathogenic bacteria. This improved rosacea symptoms, normalized immunoglobulin A levels, and suppressed cytokine interleukin 8 levels.10 Recent studies also suggest oral sarecycline, a narrow-spectrum antibiotic, may improve papulopustular rosacea symptoms through its anti-inflammatory effects while having minimal impact on gut microbiota diversity.11,12
Gut-derived short-chain fatty acids, which are known to regulate immune function, also have been shown to influence the composition of skin microbiota, suggesting a direct link between gut dysbiosis and skin microbial imbalances. Notably, antibiotic and probiotic treatments targeting the gut microbiome (eg, rifaximin for small intestinal bacterial overgrowth) have been associated with improvements in rosacea symptoms, further underscoring the interconnectedness of the gut-skin axis.13 Understanding how gut-derived inflammation alters the skin microbiome may provide new therapeutic avenues for restoring microbial balance and reducing rosacea severity.
Immune Dysregulation and Inflammatory Pathways
Mechanisms of microbiome-driven inflammation via the innate immune system contribute to rosacea pathogenesis. Toll-like receptor 2 is upregulated in rosacea, producing increased peptides including cathelicidins.13 When abnormally processed, cathelicidins produce proinflammatory peptides and worsen rosacea symptoms such as erythema, telangiectasias, and neutrophilic infiltration by dysregulating the immune system and the skin barrier.6
Heightened levels of cytokines interleukin 8 and interferon α have been identified in patients with rosacea. These cytokines are involved in rosacea pathogenesis, including leukocyte recruitment, angiogenesis, and tissue remodeling and further activate the inflammatory cascade.8,14
Mendelian randomization studies have provided confirmation of a causal link between skin microbiota alterations and inflammatory skin diseases including rosacea.2 Specific alterations in bacteria such as Cutibacterium and Staphylococcus microbial species have been associated with shifts in host immune gene expression, potentially predisposing individuals to abnormal immune activation and inflammation.2,8 These studies show the potential of leveraging precision medicine to design therapies that target pathways that improve microbial imbalances seen in rosacea.
Environmental and Lifestyle Factors Affecting the Skin Microbiome
Individuals with rosacea often have increased sensitivity to environmental and lifestyle stressors such as high temperatures, UV exposure, and sugar and alcohol consumption. These factors influence the composition of the skin microbiome and potentially contribute to rosacea development and disease exacerbation; therefore, trigger avoidance is an important way to manage rosacea.
High temperatures and UV exposure—Demodex activity increases in response to heat exposure and subsequently worsens rosacea symptoms, while exposure to UV radiation can change the composition of the skin microbiome by encouraging inflammatory responses such as oxidative stress reactions.4 This effect on the skin microbiome is driven partly by the increased presence of certain skin microbial species, such as S epidermidis, which secrete virulence factors at higher temperatures and further contribute to inflammation.1,4
High-glycemic diet and alcohol consumption—High-glycemic diets and alcohol intake have been associated with gut dysbiosis and increased disease severity in rosacea. Processed foods and high sugar consumption can promote proinflammatory reactions that cause skin dysbiosis and exacerbate symptoms.15 Increased consumption of anti-inflammatory foods or consumption of probiotics and prebiotics can improve microbial balance.
Therapeutic Implications
The influence of the skin and gut microbiome on rosacea have been well described in the medical literature; therefore, many therapeutic strategies aim to address microbiome dysbiosis, including the use of antibiotics, anthelmintics, and a range of topical agents as well as probiotics, microbiome-friendly skin care products, and dietary modifications.
Antibiotics and Anthelmintics—Topical and oral antibiotics such as metronidazole and doxycycline reduce microbial load and inflammation.5,7,8 Ivermectin, an anthelmintic, has demonstrated efficacy in decreasing Demodex colonization and associated inflammation by interfering with mite survival and reducing bacterial interactions on the skin.5 Recent literature also has explored next-generation antibiotics that disrupt biofilm production by bacteria, which could positively affect outcomes while safeguarding antibiotic stewardship.15 Given its targeted antimicrobial activity and low propensity for microbial resistance, sarecycline represents a promising therapeutic option for managing rosacea symptoms with reduced risk for microbiome-related adverse events.12,16
Probiotics and Skin Care Interventions—Probiotics, prebiotics, and postbiotics have emerged as promising approaches to improve rosacea outcomes. Topical probiotics have been shown to maintain skin microbiome homeostasis, reduce inflammation, and enhance epidermal barrier function, making them a promising adjunctive therapy for rosacea.17,18 Physiological pH cleansers and moisturizers formulated with microbiome-friendly ingredients may reduce transepidermal water loss and improve skin hydration, which are critical in microbial equilibrium.9 Oral administration of E coli Nissle, Lactobacillus, and Bifidobacterium have shown potential in improving microbial balance and reducing disease severity.10
Other Topical Therapies—Azelaic acid and benzoyl peroxide can improve rosacea symptoms by decreasing inflammation and also may shift the skin microbiome.19,20 Formulations of topical therapies, including microencapsulated benzoyl peroxide, show improved efficacy in targeting pathogenic bacteria while maintaining tolerability.19
Dietary Modifications—Avoiding triggers such as alcohol and high-glycemic foods can help reduce gut and skin dysbiosis.13 Polyphenol-rich foods and prebiotic fiber may promote beneficial gut and skin microbial composition and currently are being studied.13
Emerging Therapies—Long-pulsed alexandrite laser therapy has been shown to reduce facial erythema and modulate skin microbiota.21 Patients with treatment-resistant rosacea may benefit from advanced precision targeted antimicrobials.
The future of rosacea treatment may involve integrating established and emerging microbiome-targeted treatment strategies to improve short- and long-term patient outcomes in rosacea.
Conclusion
As our understanding of rosacea, its pathogenesis, and the role of the skin microbiome continues to grow, so does our ability to develop increasingly effective and well-tolerated treatments. Future research should focus on how changes to the skin microbiome can influence disease progression and treatment responses as well as potential therapies targeting the skin microbiome. Integrating precision treatments that restore microbial balance alongside more traditional therapies may improve outcomes by addressing both inflammation and epidermal barrier dysfunction. Additionally, strategies that support a healthy skin microbiome, such as microbiome-friendly skin care and topical probiotics, should be further explored to enhance long-term disease management. There remains a dearth of literature addressing how the skin microbiome of patients with rosacea can be optimized to maximize treatment, highlighting the need for more research into these interventions.
- Joura MI, Jobbágy A, Dunai ZA, et al. Characteristics of the stool, blood and skin microbiome in rosacea patients. Microorganisms. 2024;12:2667. doi:10.3390/microorganisms12122667
- Li X, Chen S, Chen S, et al. Skin microbiome and causal relationships in three dermatological diseases: evidence from Mendelian randomization and Bayesian weighting. Skin Res Technol. 2024;30:E70035. doi:10.1111/srt.70035
- GulbasC aran F, Sar.mustafa S, Ozbag. c.van O, et al. Investigation of factors associated with gut microbiota in Demodex-associated skin conditions. Turkiye Parazitol Derg. 2024;48:171-177. doi:10.4274 /tpd.galenos.2024.93064
- Xiong J, Chen S, Wang P, et al. Characterisation of the bacterial microbiome in patients with rosacea and healthy controls. Eur J Dermatol. 2023;33:612-617. doi:10.1684/ejd.2023.4619
- Nakatsuji T, Cheng JY, Butcher A, et al. Topical ivermectin treatment of rosacea changes the bacterial microbiome of the skin. J Invest Dermatol. Published online October 29, 2024. doi:10.1016 /j.jid.2024.10.592
- Mylonas A, Hawerkamp HC, Wang Y, et al. Type I IFNs link skin-associated dysbiotic commensal bacteria to pathogenic inflammation and angiogenesis in rosacea. JCI Insight. 2023;8:e151846. doi:10.1172/jci.insight.151846
- Zhang Y, Zhou Y, Humbert P, et al. Effect on the skin microbiota of oral minocycline for rosacea. Acta Derm Venereol. 2023;103:adv10331. doi:10.2340/actadv.v103.10331
- Woo YR, Lee SH, Cho SH, et al. Characterization and analysis of the skin microbiota in rosacea: impact of systemic antibiotics. J Clin Med. 2020;9:185. doi:10.3390/jcm9010185
- Marson J, Bhatia N, Graber E, et al. Supplement article: the role of epidermal barrier dysfunction and cutaneous microbiome dysbiosis in the pathogenesis and management of acne vulgaris and rosacea. J Drugs Dermatol. 2022;21:SF3502915-SF35029114. doi:10.36849 /JDD.m0922
- Manzhalii E, Hornuss D, Stremmel W. Intestinal-borne dermatoses significantly improved by oral application of Escherichia coli Nissle 1917. World J Gastroenterol. 2016;22:5415-5421. doi:10.3748 /wjg.v22.i23.5415
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424. doi:10.1007/s12325-021-01624-x
- del Rosso JQ, Draelos ZD, Effron C, et al. Oral sarecycline for treatment of papulopustular rosacea: results of a pilot study of effectiveness and safety. J Drugs Dermatol. 2021;20:426-431. doi:10.36849 /JDD.2021.5923
- Qi X, Xiao Y, Zhang X, et al. Probiotics suppress LL37-generated rosacea-like skin inflammation by modulating the TLR2/MyD88 /NF-êB signaling pathway. Food Funct. 2024;15:8916-8934. doi:10.1039 /d4fo03083d
- Pan L, Li C, Liang Z, et al. Exploring the association between skin microbiota and inflammatory skin diseases: a two-sample Mendelian randomization analysis. Arch Dermatol Res. 2024;316:677. doi:10.1007/s00403-024-03433-y
- Sánchez-Pellicer P, Eguren-Michelena C, García-Gavín J, et al. Rosacea, microbiome and probiotics: the gut-skin axis. Front Microbiol. 2024;14:1323644. doi:10.3389/fmicb.2023.1323644
- Moura IB, Grada A, Spittal W, et al. Profiling the effects of systemic antibiotics for acne, including the narrow-spectrum antibiotic sarecycline, on the human gut microbiota. Front Microbiol. 2022;13:901911. doi:10.3389/fmicb.2022.901911
- Habeebuddin M, Karnati RK, Shiroorkar PN, et al. Topical probiotics: more than a skin deep. Pharmaceutics. 2022;14:557. doi:10.3390/pharmaceutics14030557
- Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020; 29:15-21. doi:10.1111/exd.14032
- Nong Y, Sugarman J, York JP, et al. Effect of topical microencapsulated benzoyl peroxide on the skin microbiome in rosacea: a randomized, double-blind, crossover, vehicle-controlled clinical trial. J Clin Aesthet Dermatol. 2024;17:19-26.
- Bojar RA, Cunliffe WJ, Holland KT. Disruption of the transmembrane pH gradient—a possible mechanism for the antibacterial action of azelaic acid in Propionibacterium acnes and Staphylococcus epidermidis. J Antimicrob Chemother. 1994;34:321-330. doi:10.1093/jac/34.3.321
- Park S, Jang H, Seong SH, et al. The effects of long-pulsed alexandrite laser therapy on facial redness and skin microbiota compositions in rosacea: a prospective, multicentre, single-arm clinical trial. Photodermatol Photoimmunol Photomed. 2024;40:10.1111/phpp.12921. doi:10.1111/phpp.12921
Rosacea is a chronic inflammatory skin condition affecting the central face—including the cheeks, nose, chin, and forehead—that causes considerable discomfort.1 Its pathogenesis involves immune dysregulation, genetic predisposition, and microbial dysbiosis.2 While immune and environmental factors are known triggers of rosacea, recent research highlights the roles of the gut and skin microbiomes in disease progression. While the skin microbiome interacts directly with the immune system to regulate inflammation and skin homeostasis, the gut microbiome also influences cutaneous inflammation, emphasizing the need to address both topical and internal microbiome imbalances.3 In this article, we review gut and skin microbial alterations in rosacea, focusing on the skin microbiome and including the gut-skin axis implications as well as therapeutic strategies aimed at microbiome balance to enhance patient outcomes.
Skin Microbiome Alterations in Rosacea
The human skin microbiome interacts with the immune system, and microbial imbalances have been shown to contribute to immune dysregulation. Several key microbial species have been identified as playing a large role in rosacea, including Demodex folliculorum, Staphylococcus epidermidis, Bacillus oleronius, and Cutibacterium acnes (Figure).

Demodex folliculorum is a microscopic mite is found in hair follicles and sebaceous glands. Patients with rosacea have higher densities of D folliculorum, which trigger follicular occlusion and immune activation.1 Bacillus oleronius be isolated from D folliculorum and can further activate toll-like receptor 2, leading to cytokine production and immune cell infiltration.3,4 Increased propagation of this mite correlates with shifts in skin microbiome composition, demonstrating increased inflammatory microbial populations.3
Staphylococcus epidermidis normally is commensal but can become pathogenic (pathobiont) in rosacea due to disruptions in the skin microenvironment, where it can form biofilms and produce virulence factors, particularly in papulopustular rosacea.5
Bacillus oleronius has been isolated from D folliculorum mites and provokes inflammatory responses in patients with rosacea by triggering toll-like receptor 2 activation and cytokine secretion.6
Cutibacterium acnes commonly is associated with acne vulgaris. Its role in rosacea is unclear, but recent research suggests it may have a protective effect. A single-arm trial investigated the effects of minocycline on rosacea and found that treatment significantly reduced C acnes but increased microbial species diversity, improving inflammation.7 One longitudinal cohort study of 12 patients with rosacea found that C acnes levels were lower in those older than 60 years. Rosacea severity increased with age and correlated with a decline in C acnes, suggesting that it may confer some protective effect in rosacea.8 This finding is supported by studies that have shown a reduction in C acnes levels in patients with rosacea compared to controls.4,8
Important mechanisms in rosacea include epidermal barrier dysfunction, transepidermal water loss, and decreased stratum corneum hydration, particularly in erythematotelangiectatic and papulopustular subtypes. The resulting alkaline skin pH contributes to barrier instability and heightened inflammation, permitting pathogenic bacteria to proliferate and disrupt skin microbial homeostasis.9 A recent study identified metabolic changes in the skin microbiome of patients with rosacea, showing that increased heme and hydrogen sulfide in rosacea skin microbiomes likely drive inflammation, while healthy skin microbiomes produce more anti-inflammatory adenosylcobalamin, thiazole, and L-isoleucine.1 These findings highlight the link between microbial imbalances and inflammation in rosacea.
The Gut-Skin Axis in Rosacea
Gut microbiota play a critical role in managing systemic inflammation, and microbial dysbiosis in the intestine can influence the skin microbiome in rosacea. Patients with rosacea who have gastrointestinal conditions such as small intestinal bacterial overgrowth and Helicobacter pylori infection experience more severe rosacea symptoms.3,10
Patients with rosacea have distinctive gut microbiota compositions, with an increased prevalence of proinflammatory bacterial species, potentially affecting the skin microbiome.8,11 Systemic antibiotics have been shown to modulate the gut microbiome, indirectly influencing the skin microbiome.11 A recent study demonstrated that doxycycline treatment in patients with rosacea altered skin microbial diversity, reducing C acnes while increasing Weissella confusa—highlighting the complicated relationship between systemic antibiotics and the gut-skin axis.8
Specific probiotics, such as Escherichia coli Nissle, when given orally shifted gut microbial balance to protective microbiota with increased Lactobacillus and Bifidobacteria species and decreased pathogenic bacteria. This improved rosacea symptoms, normalized immunoglobulin A levels, and suppressed cytokine interleukin 8 levels.10 Recent studies also suggest oral sarecycline, a narrow-spectrum antibiotic, may improve papulopustular rosacea symptoms through its anti-inflammatory effects while having minimal impact on gut microbiota diversity.11,12
Gut-derived short-chain fatty acids, which are known to regulate immune function, also have been shown to influence the composition of skin microbiota, suggesting a direct link between gut dysbiosis and skin microbial imbalances. Notably, antibiotic and probiotic treatments targeting the gut microbiome (eg, rifaximin for small intestinal bacterial overgrowth) have been associated with improvements in rosacea symptoms, further underscoring the interconnectedness of the gut-skin axis.13 Understanding how gut-derived inflammation alters the skin microbiome may provide new therapeutic avenues for restoring microbial balance and reducing rosacea severity.
Immune Dysregulation and Inflammatory Pathways
Mechanisms of microbiome-driven inflammation via the innate immune system contribute to rosacea pathogenesis. Toll-like receptor 2 is upregulated in rosacea, producing increased peptides including cathelicidins.13 When abnormally processed, cathelicidins produce proinflammatory peptides and worsen rosacea symptoms such as erythema, telangiectasias, and neutrophilic infiltration by dysregulating the immune system and the skin barrier.6
Heightened levels of cytokines interleukin 8 and interferon α have been identified in patients with rosacea. These cytokines are involved in rosacea pathogenesis, including leukocyte recruitment, angiogenesis, and tissue remodeling and further activate the inflammatory cascade.8,14
Mendelian randomization studies have provided confirmation of a causal link between skin microbiota alterations and inflammatory skin diseases including rosacea.2 Specific alterations in bacteria such as Cutibacterium and Staphylococcus microbial species have been associated with shifts in host immune gene expression, potentially predisposing individuals to abnormal immune activation and inflammation.2,8 These studies show the potential of leveraging precision medicine to design therapies that target pathways that improve microbial imbalances seen in rosacea.
Environmental and Lifestyle Factors Affecting the Skin Microbiome
Individuals with rosacea often have increased sensitivity to environmental and lifestyle stressors such as high temperatures, UV exposure, and sugar and alcohol consumption. These factors influence the composition of the skin microbiome and potentially contribute to rosacea development and disease exacerbation; therefore, trigger avoidance is an important way to manage rosacea.
High temperatures and UV exposure—Demodex activity increases in response to heat exposure and subsequently worsens rosacea symptoms, while exposure to UV radiation can change the composition of the skin microbiome by encouraging inflammatory responses such as oxidative stress reactions.4 This effect on the skin microbiome is driven partly by the increased presence of certain skin microbial species, such as S epidermidis, which secrete virulence factors at higher temperatures and further contribute to inflammation.1,4
High-glycemic diet and alcohol consumption—High-glycemic diets and alcohol intake have been associated with gut dysbiosis and increased disease severity in rosacea. Processed foods and high sugar consumption can promote proinflammatory reactions that cause skin dysbiosis and exacerbate symptoms.15 Increased consumption of anti-inflammatory foods or consumption of probiotics and prebiotics can improve microbial balance.
Therapeutic Implications
The influence of the skin and gut microbiome on rosacea have been well described in the medical literature; therefore, many therapeutic strategies aim to address microbiome dysbiosis, including the use of antibiotics, anthelmintics, and a range of topical agents as well as probiotics, microbiome-friendly skin care products, and dietary modifications.
Antibiotics and Anthelmintics—Topical and oral antibiotics such as metronidazole and doxycycline reduce microbial load and inflammation.5,7,8 Ivermectin, an anthelmintic, has demonstrated efficacy in decreasing Demodex colonization and associated inflammation by interfering with mite survival and reducing bacterial interactions on the skin.5 Recent literature also has explored next-generation antibiotics that disrupt biofilm production by bacteria, which could positively affect outcomes while safeguarding antibiotic stewardship.15 Given its targeted antimicrobial activity and low propensity for microbial resistance, sarecycline represents a promising therapeutic option for managing rosacea symptoms with reduced risk for microbiome-related adverse events.12,16
Probiotics and Skin Care Interventions—Probiotics, prebiotics, and postbiotics have emerged as promising approaches to improve rosacea outcomes. Topical probiotics have been shown to maintain skin microbiome homeostasis, reduce inflammation, and enhance epidermal barrier function, making them a promising adjunctive therapy for rosacea.17,18 Physiological pH cleansers and moisturizers formulated with microbiome-friendly ingredients may reduce transepidermal water loss and improve skin hydration, which are critical in microbial equilibrium.9 Oral administration of E coli Nissle, Lactobacillus, and Bifidobacterium have shown potential in improving microbial balance and reducing disease severity.10
Other Topical Therapies—Azelaic acid and benzoyl peroxide can improve rosacea symptoms by decreasing inflammation and also may shift the skin microbiome.19,20 Formulations of topical therapies, including microencapsulated benzoyl peroxide, show improved efficacy in targeting pathogenic bacteria while maintaining tolerability.19
Dietary Modifications—Avoiding triggers such as alcohol and high-glycemic foods can help reduce gut and skin dysbiosis.13 Polyphenol-rich foods and prebiotic fiber may promote beneficial gut and skin microbial composition and currently are being studied.13
Emerging Therapies—Long-pulsed alexandrite laser therapy has been shown to reduce facial erythema and modulate skin microbiota.21 Patients with treatment-resistant rosacea may benefit from advanced precision targeted antimicrobials.
The future of rosacea treatment may involve integrating established and emerging microbiome-targeted treatment strategies to improve short- and long-term patient outcomes in rosacea.
Conclusion
As our understanding of rosacea, its pathogenesis, and the role of the skin microbiome continues to grow, so does our ability to develop increasingly effective and well-tolerated treatments. Future research should focus on how changes to the skin microbiome can influence disease progression and treatment responses as well as potential therapies targeting the skin microbiome. Integrating precision treatments that restore microbial balance alongside more traditional therapies may improve outcomes by addressing both inflammation and epidermal barrier dysfunction. Additionally, strategies that support a healthy skin microbiome, such as microbiome-friendly skin care and topical probiotics, should be further explored to enhance long-term disease management. There remains a dearth of literature addressing how the skin microbiome of patients with rosacea can be optimized to maximize treatment, highlighting the need for more research into these interventions.
Rosacea is a chronic inflammatory skin condition affecting the central face—including the cheeks, nose, chin, and forehead—that causes considerable discomfort.1 Its pathogenesis involves immune dysregulation, genetic predisposition, and microbial dysbiosis.2 While immune and environmental factors are known triggers of rosacea, recent research highlights the roles of the gut and skin microbiomes in disease progression. While the skin microbiome interacts directly with the immune system to regulate inflammation and skin homeostasis, the gut microbiome also influences cutaneous inflammation, emphasizing the need to address both topical and internal microbiome imbalances.3 In this article, we review gut and skin microbial alterations in rosacea, focusing on the skin microbiome and including the gut-skin axis implications as well as therapeutic strategies aimed at microbiome balance to enhance patient outcomes.
Skin Microbiome Alterations in Rosacea
The human skin microbiome interacts with the immune system, and microbial imbalances have been shown to contribute to immune dysregulation. Several key microbial species have been identified as playing a large role in rosacea, including Demodex folliculorum, Staphylococcus epidermidis, Bacillus oleronius, and Cutibacterium acnes (Figure).

Demodex folliculorum is a microscopic mite is found in hair follicles and sebaceous glands. Patients with rosacea have higher densities of D folliculorum, which trigger follicular occlusion and immune activation.1 Bacillus oleronius be isolated from D folliculorum and can further activate toll-like receptor 2, leading to cytokine production and immune cell infiltration.3,4 Increased propagation of this mite correlates with shifts in skin microbiome composition, demonstrating increased inflammatory microbial populations.3
Staphylococcus epidermidis normally is commensal but can become pathogenic (pathobiont) in rosacea due to disruptions in the skin microenvironment, where it can form biofilms and produce virulence factors, particularly in papulopustular rosacea.5
Bacillus oleronius has been isolated from D folliculorum mites and provokes inflammatory responses in patients with rosacea by triggering toll-like receptor 2 activation and cytokine secretion.6
Cutibacterium acnes commonly is associated with acne vulgaris. Its role in rosacea is unclear, but recent research suggests it may have a protective effect. A single-arm trial investigated the effects of minocycline on rosacea and found that treatment significantly reduced C acnes but increased microbial species diversity, improving inflammation.7 One longitudinal cohort study of 12 patients with rosacea found that C acnes levels were lower in those older than 60 years. Rosacea severity increased with age and correlated with a decline in C acnes, suggesting that it may confer some protective effect in rosacea.8 This finding is supported by studies that have shown a reduction in C acnes levels in patients with rosacea compared to controls.4,8
Important mechanisms in rosacea include epidermal barrier dysfunction, transepidermal water loss, and decreased stratum corneum hydration, particularly in erythematotelangiectatic and papulopustular subtypes. The resulting alkaline skin pH contributes to barrier instability and heightened inflammation, permitting pathogenic bacteria to proliferate and disrupt skin microbial homeostasis.9 A recent study identified metabolic changes in the skin microbiome of patients with rosacea, showing that increased heme and hydrogen sulfide in rosacea skin microbiomes likely drive inflammation, while healthy skin microbiomes produce more anti-inflammatory adenosylcobalamin, thiazole, and L-isoleucine.1 These findings highlight the link between microbial imbalances and inflammation in rosacea.
The Gut-Skin Axis in Rosacea
Gut microbiota play a critical role in managing systemic inflammation, and microbial dysbiosis in the intestine can influence the skin microbiome in rosacea. Patients with rosacea who have gastrointestinal conditions such as small intestinal bacterial overgrowth and Helicobacter pylori infection experience more severe rosacea symptoms.3,10
Patients with rosacea have distinctive gut microbiota compositions, with an increased prevalence of proinflammatory bacterial species, potentially affecting the skin microbiome.8,11 Systemic antibiotics have been shown to modulate the gut microbiome, indirectly influencing the skin microbiome.11 A recent study demonstrated that doxycycline treatment in patients with rosacea altered skin microbial diversity, reducing C acnes while increasing Weissella confusa—highlighting the complicated relationship between systemic antibiotics and the gut-skin axis.8
Specific probiotics, such as Escherichia coli Nissle, when given orally shifted gut microbial balance to protective microbiota with increased Lactobacillus and Bifidobacteria species and decreased pathogenic bacteria. This improved rosacea symptoms, normalized immunoglobulin A levels, and suppressed cytokine interleukin 8 levels.10 Recent studies also suggest oral sarecycline, a narrow-spectrum antibiotic, may improve papulopustular rosacea symptoms through its anti-inflammatory effects while having minimal impact on gut microbiota diversity.11,12
Gut-derived short-chain fatty acids, which are known to regulate immune function, also have been shown to influence the composition of skin microbiota, suggesting a direct link between gut dysbiosis and skin microbial imbalances. Notably, antibiotic and probiotic treatments targeting the gut microbiome (eg, rifaximin for small intestinal bacterial overgrowth) have been associated with improvements in rosacea symptoms, further underscoring the interconnectedness of the gut-skin axis.13 Understanding how gut-derived inflammation alters the skin microbiome may provide new therapeutic avenues for restoring microbial balance and reducing rosacea severity.
Immune Dysregulation and Inflammatory Pathways
Mechanisms of microbiome-driven inflammation via the innate immune system contribute to rosacea pathogenesis. Toll-like receptor 2 is upregulated in rosacea, producing increased peptides including cathelicidins.13 When abnormally processed, cathelicidins produce proinflammatory peptides and worsen rosacea symptoms such as erythema, telangiectasias, and neutrophilic infiltration by dysregulating the immune system and the skin barrier.6
Heightened levels of cytokines interleukin 8 and interferon α have been identified in patients with rosacea. These cytokines are involved in rosacea pathogenesis, including leukocyte recruitment, angiogenesis, and tissue remodeling and further activate the inflammatory cascade.8,14
Mendelian randomization studies have provided confirmation of a causal link between skin microbiota alterations and inflammatory skin diseases including rosacea.2 Specific alterations in bacteria such as Cutibacterium and Staphylococcus microbial species have been associated with shifts in host immune gene expression, potentially predisposing individuals to abnormal immune activation and inflammation.2,8 These studies show the potential of leveraging precision medicine to design therapies that target pathways that improve microbial imbalances seen in rosacea.
Environmental and Lifestyle Factors Affecting the Skin Microbiome
Individuals with rosacea often have increased sensitivity to environmental and lifestyle stressors such as high temperatures, UV exposure, and sugar and alcohol consumption. These factors influence the composition of the skin microbiome and potentially contribute to rosacea development and disease exacerbation; therefore, trigger avoidance is an important way to manage rosacea.
High temperatures and UV exposure—Demodex activity increases in response to heat exposure and subsequently worsens rosacea symptoms, while exposure to UV radiation can change the composition of the skin microbiome by encouraging inflammatory responses such as oxidative stress reactions.4 This effect on the skin microbiome is driven partly by the increased presence of certain skin microbial species, such as S epidermidis, which secrete virulence factors at higher temperatures and further contribute to inflammation.1,4
High-glycemic diet and alcohol consumption—High-glycemic diets and alcohol intake have been associated with gut dysbiosis and increased disease severity in rosacea. Processed foods and high sugar consumption can promote proinflammatory reactions that cause skin dysbiosis and exacerbate symptoms.15 Increased consumption of anti-inflammatory foods or consumption of probiotics and prebiotics can improve microbial balance.
Therapeutic Implications
The influence of the skin and gut microbiome on rosacea have been well described in the medical literature; therefore, many therapeutic strategies aim to address microbiome dysbiosis, including the use of antibiotics, anthelmintics, and a range of topical agents as well as probiotics, microbiome-friendly skin care products, and dietary modifications.
Antibiotics and Anthelmintics—Topical and oral antibiotics such as metronidazole and doxycycline reduce microbial load and inflammation.5,7,8 Ivermectin, an anthelmintic, has demonstrated efficacy in decreasing Demodex colonization and associated inflammation by interfering with mite survival and reducing bacterial interactions on the skin.5 Recent literature also has explored next-generation antibiotics that disrupt biofilm production by bacteria, which could positively affect outcomes while safeguarding antibiotic stewardship.15 Given its targeted antimicrobial activity and low propensity for microbial resistance, sarecycline represents a promising therapeutic option for managing rosacea symptoms with reduced risk for microbiome-related adverse events.12,16
Probiotics and Skin Care Interventions—Probiotics, prebiotics, and postbiotics have emerged as promising approaches to improve rosacea outcomes. Topical probiotics have been shown to maintain skin microbiome homeostasis, reduce inflammation, and enhance epidermal barrier function, making them a promising adjunctive therapy for rosacea.17,18 Physiological pH cleansers and moisturizers formulated with microbiome-friendly ingredients may reduce transepidermal water loss and improve skin hydration, which are critical in microbial equilibrium.9 Oral administration of E coli Nissle, Lactobacillus, and Bifidobacterium have shown potential in improving microbial balance and reducing disease severity.10
Other Topical Therapies—Azelaic acid and benzoyl peroxide can improve rosacea symptoms by decreasing inflammation and also may shift the skin microbiome.19,20 Formulations of topical therapies, including microencapsulated benzoyl peroxide, show improved efficacy in targeting pathogenic bacteria while maintaining tolerability.19
Dietary Modifications—Avoiding triggers such as alcohol and high-glycemic foods can help reduce gut and skin dysbiosis.13 Polyphenol-rich foods and prebiotic fiber may promote beneficial gut and skin microbial composition and currently are being studied.13
Emerging Therapies—Long-pulsed alexandrite laser therapy has been shown to reduce facial erythema and modulate skin microbiota.21 Patients with treatment-resistant rosacea may benefit from advanced precision targeted antimicrobials.
The future of rosacea treatment may involve integrating established and emerging microbiome-targeted treatment strategies to improve short- and long-term patient outcomes in rosacea.
Conclusion
As our understanding of rosacea, its pathogenesis, and the role of the skin microbiome continues to grow, so does our ability to develop increasingly effective and well-tolerated treatments. Future research should focus on how changes to the skin microbiome can influence disease progression and treatment responses as well as potential therapies targeting the skin microbiome. Integrating precision treatments that restore microbial balance alongside more traditional therapies may improve outcomes by addressing both inflammation and epidermal barrier dysfunction. Additionally, strategies that support a healthy skin microbiome, such as microbiome-friendly skin care and topical probiotics, should be further explored to enhance long-term disease management. There remains a dearth of literature addressing how the skin microbiome of patients with rosacea can be optimized to maximize treatment, highlighting the need for more research into these interventions.
- Joura MI, Jobbágy A, Dunai ZA, et al. Characteristics of the stool, blood and skin microbiome in rosacea patients. Microorganisms. 2024;12:2667. doi:10.3390/microorganisms12122667
- Li X, Chen S, Chen S, et al. Skin microbiome and causal relationships in three dermatological diseases: evidence from Mendelian randomization and Bayesian weighting. Skin Res Technol. 2024;30:E70035. doi:10.1111/srt.70035
- GulbasC aran F, Sar.mustafa S, Ozbag. c.van O, et al. Investigation of factors associated with gut microbiota in Demodex-associated skin conditions. Turkiye Parazitol Derg. 2024;48:171-177. doi:10.4274 /tpd.galenos.2024.93064
- Xiong J, Chen S, Wang P, et al. Characterisation of the bacterial microbiome in patients with rosacea and healthy controls. Eur J Dermatol. 2023;33:612-617. doi:10.1684/ejd.2023.4619
- Nakatsuji T, Cheng JY, Butcher A, et al. Topical ivermectin treatment of rosacea changes the bacterial microbiome of the skin. J Invest Dermatol. Published online October 29, 2024. doi:10.1016 /j.jid.2024.10.592
- Mylonas A, Hawerkamp HC, Wang Y, et al. Type I IFNs link skin-associated dysbiotic commensal bacteria to pathogenic inflammation and angiogenesis in rosacea. JCI Insight. 2023;8:e151846. doi:10.1172/jci.insight.151846
- Zhang Y, Zhou Y, Humbert P, et al. Effect on the skin microbiota of oral minocycline for rosacea. Acta Derm Venereol. 2023;103:adv10331. doi:10.2340/actadv.v103.10331
- Woo YR, Lee SH, Cho SH, et al. Characterization and analysis of the skin microbiota in rosacea: impact of systemic antibiotics. J Clin Med. 2020;9:185. doi:10.3390/jcm9010185
- Marson J, Bhatia N, Graber E, et al. Supplement article: the role of epidermal barrier dysfunction and cutaneous microbiome dysbiosis in the pathogenesis and management of acne vulgaris and rosacea. J Drugs Dermatol. 2022;21:SF3502915-SF35029114. doi:10.36849 /JDD.m0922
- Manzhalii E, Hornuss D, Stremmel W. Intestinal-borne dermatoses significantly improved by oral application of Escherichia coli Nissle 1917. World J Gastroenterol. 2016;22:5415-5421. doi:10.3748 /wjg.v22.i23.5415
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424. doi:10.1007/s12325-021-01624-x
- del Rosso JQ, Draelos ZD, Effron C, et al. Oral sarecycline for treatment of papulopustular rosacea: results of a pilot study of effectiveness and safety. J Drugs Dermatol. 2021;20:426-431. doi:10.36849 /JDD.2021.5923
- Qi X, Xiao Y, Zhang X, et al. Probiotics suppress LL37-generated rosacea-like skin inflammation by modulating the TLR2/MyD88 /NF-êB signaling pathway. Food Funct. 2024;15:8916-8934. doi:10.1039 /d4fo03083d
- Pan L, Li C, Liang Z, et al. Exploring the association between skin microbiota and inflammatory skin diseases: a two-sample Mendelian randomization analysis. Arch Dermatol Res. 2024;316:677. doi:10.1007/s00403-024-03433-y
- Sánchez-Pellicer P, Eguren-Michelena C, García-Gavín J, et al. Rosacea, microbiome and probiotics: the gut-skin axis. Front Microbiol. 2024;14:1323644. doi:10.3389/fmicb.2023.1323644
- Moura IB, Grada A, Spittal W, et al. Profiling the effects of systemic antibiotics for acne, including the narrow-spectrum antibiotic sarecycline, on the human gut microbiota. Front Microbiol. 2022;13:901911. doi:10.3389/fmicb.2022.901911
- Habeebuddin M, Karnati RK, Shiroorkar PN, et al. Topical probiotics: more than a skin deep. Pharmaceutics. 2022;14:557. doi:10.3390/pharmaceutics14030557
- Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020; 29:15-21. doi:10.1111/exd.14032
- Nong Y, Sugarman J, York JP, et al. Effect of topical microencapsulated benzoyl peroxide on the skin microbiome in rosacea: a randomized, double-blind, crossover, vehicle-controlled clinical trial. J Clin Aesthet Dermatol. 2024;17:19-26.
- Bojar RA, Cunliffe WJ, Holland KT. Disruption of the transmembrane pH gradient—a possible mechanism for the antibacterial action of azelaic acid in Propionibacterium acnes and Staphylococcus epidermidis. J Antimicrob Chemother. 1994;34:321-330. doi:10.1093/jac/34.3.321
- Park S, Jang H, Seong SH, et al. The effects of long-pulsed alexandrite laser therapy on facial redness and skin microbiota compositions in rosacea: a prospective, multicentre, single-arm clinical trial. Photodermatol Photoimmunol Photomed. 2024;40:10.1111/phpp.12921. doi:10.1111/phpp.12921
- Joura MI, Jobbágy A, Dunai ZA, et al. Characteristics of the stool, blood and skin microbiome in rosacea patients. Microorganisms. 2024;12:2667. doi:10.3390/microorganisms12122667
- Li X, Chen S, Chen S, et al. Skin microbiome and causal relationships in three dermatological diseases: evidence from Mendelian randomization and Bayesian weighting. Skin Res Technol. 2024;30:E70035. doi:10.1111/srt.70035
- GulbasC aran F, Sar.mustafa S, Ozbag. c.van O, et al. Investigation of factors associated with gut microbiota in Demodex-associated skin conditions. Turkiye Parazitol Derg. 2024;48:171-177. doi:10.4274 /tpd.galenos.2024.93064
- Xiong J, Chen S, Wang P, et al. Characterisation of the bacterial microbiome in patients with rosacea and healthy controls. Eur J Dermatol. 2023;33:612-617. doi:10.1684/ejd.2023.4619
- Nakatsuji T, Cheng JY, Butcher A, et al. Topical ivermectin treatment of rosacea changes the bacterial microbiome of the skin. J Invest Dermatol. Published online October 29, 2024. doi:10.1016 /j.jid.2024.10.592
- Mylonas A, Hawerkamp HC, Wang Y, et al. Type I IFNs link skin-associated dysbiotic commensal bacteria to pathogenic inflammation and angiogenesis in rosacea. JCI Insight. 2023;8:e151846. doi:10.1172/jci.insight.151846
- Zhang Y, Zhou Y, Humbert P, et al. Effect on the skin microbiota of oral minocycline for rosacea. Acta Derm Venereol. 2023;103:adv10331. doi:10.2340/actadv.v103.10331
- Woo YR, Lee SH, Cho SH, et al. Characterization and analysis of the skin microbiota in rosacea: impact of systemic antibiotics. J Clin Med. 2020;9:185. doi:10.3390/jcm9010185
- Marson J, Bhatia N, Graber E, et al. Supplement article: the role of epidermal barrier dysfunction and cutaneous microbiome dysbiosis in the pathogenesis and management of acne vulgaris and rosacea. J Drugs Dermatol. 2022;21:SF3502915-SF35029114. doi:10.36849 /JDD.m0922
- Manzhalii E, Hornuss D, Stremmel W. Intestinal-borne dermatoses significantly improved by oral application of Escherichia coli Nissle 1917. World J Gastroenterol. 2016;22:5415-5421. doi:10.3748 /wjg.v22.i23.5415
- Wang FY, Chi CC. Rosacea, germs, and bowels: a review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv Ther. 2021;38:1415-1424. doi:10.1007/s12325-021-01624-x
- del Rosso JQ, Draelos ZD, Effron C, et al. Oral sarecycline for treatment of papulopustular rosacea: results of a pilot study of effectiveness and safety. J Drugs Dermatol. 2021;20:426-431. doi:10.36849 /JDD.2021.5923
- Qi X, Xiao Y, Zhang X, et al. Probiotics suppress LL37-generated rosacea-like skin inflammation by modulating the TLR2/MyD88 /NF-êB signaling pathway. Food Funct. 2024;15:8916-8934. doi:10.1039 /d4fo03083d
- Pan L, Li C, Liang Z, et al. Exploring the association between skin microbiota and inflammatory skin diseases: a two-sample Mendelian randomization analysis. Arch Dermatol Res. 2024;316:677. doi:10.1007/s00403-024-03433-y
- Sánchez-Pellicer P, Eguren-Michelena C, García-Gavín J, et al. Rosacea, microbiome and probiotics: the gut-skin axis. Front Microbiol. 2024;14:1323644. doi:10.3389/fmicb.2023.1323644
- Moura IB, Grada A, Spittal W, et al. Profiling the effects of systemic antibiotics for acne, including the narrow-spectrum antibiotic sarecycline, on the human gut microbiota. Front Microbiol. 2022;13:901911. doi:10.3389/fmicb.2022.901911
- Habeebuddin M, Karnati RK, Shiroorkar PN, et al. Topical probiotics: more than a skin deep. Pharmaceutics. 2022;14:557. doi:10.3390/pharmaceutics14030557
- Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020; 29:15-21. doi:10.1111/exd.14032
- Nong Y, Sugarman J, York JP, et al. Effect of topical microencapsulated benzoyl peroxide on the skin microbiome in rosacea: a randomized, double-blind, crossover, vehicle-controlled clinical trial. J Clin Aesthet Dermatol. 2024;17:19-26.
- Bojar RA, Cunliffe WJ, Holland KT. Disruption of the transmembrane pH gradient—a possible mechanism for the antibacterial action of azelaic acid in Propionibacterium acnes and Staphylococcus epidermidis. J Antimicrob Chemother. 1994;34:321-330. doi:10.1093/jac/34.3.321
- Park S, Jang H, Seong SH, et al. The effects of long-pulsed alexandrite laser therapy on facial redness and skin microbiota compositions in rosacea: a prospective, multicentre, single-arm clinical trial. Photodermatol Photoimmunol Photomed. 2024;40:10.1111/phpp.12921. doi:10.1111/phpp.12921
The Skin Microbiome in Rosacea: Mechanisms, Gut-Skin Interactions, and Therapeutic Implications
The Skin Microbiome in Rosacea: Mechanisms, Gut-Skin Interactions, and Therapeutic Implications
PRACTICE POINTS:
- It is important to assess both the gut and skin microbiomes in patients with rosacea (eg, incorporate evaluation of Demodex folliculorum density, take a gut-health history).
- Narrow-spectrum antibiotics such as sarecycline or anthelmintics such as topical ivermectin target pathogens while preserving beneficial flora.
- Patients with rosacea should be counseled on trigger avoidance as well as pH-balanced, microbiomefriendly skin care and lifestyle tips to strengthen the skin barrier.
Managing Rosacea: Tips for Reducing Facial Erythema, Flushing
LAS VEGAS —
These agents “work fast” and “improve redness quickly,” Harper, a dermatologist who practices in Birmingham, Alabama, said at the Society of Dermatology Physician Associates (SDPA) 22nd Annual Fall Dermatology Conference. In addition, “you’re going to know within 30 minutes or an hour whether it’s going to work or not.”
Brimonidine 0.33% gel, an alpha-2 adrenergic receptor agonist, was approved by the Food and Drug Administration (FDA) in 2014 for persistent facial erythema of rosacea. It does not treat telangiectasia and is not approved for flushing (transient erythema). Patients are advised to apply the gel daily in the morning. In phase 3 pivotal trials of patients with moderate to severe erythema of rosacea, which excluded individuals with more than two papules, a composite (investigator- and patient-reported) 2-grade improvement was seen as early as 30 minutes after application on day 1, and erythema was reduced for 9-12 hours.
Oxymetazoline 1% cream, an alpha-1a adrenergic receptor agonist, was approved by the FDA in 2017 for persistent facial erythema of rosacea. It neither treats telangiectasia nor is approved for flushing. Phase 3 trials of patients with moderate to severe persistent erythema of rosacea excluded individuals with more than three inflammatory papules or pustules. A composite (investigator- and subject-reported) 2-grade improvement was seen as early as 1 hour after application on day 1, and erythema was reduced for 9-12 hours.
Receptor Selectivity Differences
According to Harper, there are more reports of worsening erythema with brimonidine 0.33% gel than with oxymetazoline 1% cream, perhaps because of the different receptor selectivity between the two products. She explained that alpha-1 receptors are located only postsynaptically in vascular smooth muscle, while alpha-2 receptors are located presynaptically, which can inhibit norepinephrine and lead to vasodilation. Alpha-2 receptors are also located postsynaptically in vascular smooth muscle and in the endothelial wall, which can mediate nitric oxide release and cause vasodilation.
No head-to-head studies exist that compare brimonidine 0.33% gel with oxymetazoline 1% cream. But in a 52-week study of oxymetazoline 1% cream for persistent facial erythema associated with rosacea published in 2018, at week 52, 36.7% and 43.4% of patients achieved a 2-grade or greater composite improvement from baseline in both Clinician Erythema Assessment and Subject Self-Assessment 3 and 6 hours after a dose, respectively. Also, fewer than 1% of patients experienced a rebound effect following treatment cessation.
“What we learned from this study is that maybe patients do better if they use oxymetazoline 1% cream consistently,” Harper said. “Does that mean that everybody I give this to uses it daily? Probably not, but I think we can change the vascular tone by using it consistently every day.”
Oral Beta-Blockers Another Option
Alpha agonists can also help quell flushing associated with rosacea, Harper continued, but oral beta-blockers may be the better choice. In a 2020 review that drew from nine studies, researchers evaluated the use of carvedilol, propranolol, nadolol, and beta-blockers in general for rosacea-associated facial erythema and flushing. Articles studying carvedilol and propranolol showed a large reduction of erythema and flushing during treatment with a rapid onset of symptom control, while bradycardia and hypotension were the most commonly reported adverse events. “All of these agents are studied in rosacea, but none of them are FDA approved for rosacea,” Harper noted.
In a separate study, five patients with rosacea who had either severe frequent flushing episodes or persistent erythema and burning sensations were treated with carvedilol, a nonselective beta-blocker. Prior treatments included cetirizine and doxycycline, or isotretinoin combined with topical application of metronidazole gel or ivermectin without sufficient improvement in erythema. Carvedilol was added to the above treatments and titrated up to 12.5 mg twice a day and continued for at least 6 months.
The Clinician Erythema Assessment 5-point scale before therapy was 3.4 and dropped to 0.4 during therapy, while the patient self-assessment before therapy was 3.8 and dropped to 0.8 during therapy.
Another study evaluated the use of propranolol and/or doxycycline in 78 patients with rosacea. The propranolol and combination treatment groups showed more rapid improvement at weeks 4 and 8, but there was no statistically significant difference between them by week 12. Rosacea clinical scores also decreased in all groups, but there were no significant differences between them. Reduction of Assessment of Rosacea Clinical Score was 51%, 52.2%, and 57.3% in the propranolol, doxycycline, and combination groups, respectively.
Harper disclosed ties with Almirall, Cutera, Galderma, Journey, Ortho Dermatologics, and Sun Pharmaceutical Industries.
A version of this article appeared on Medscape.com.
LAS VEGAS —
These agents “work fast” and “improve redness quickly,” Harper, a dermatologist who practices in Birmingham, Alabama, said at the Society of Dermatology Physician Associates (SDPA) 22nd Annual Fall Dermatology Conference. In addition, “you’re going to know within 30 minutes or an hour whether it’s going to work or not.”
Brimonidine 0.33% gel, an alpha-2 adrenergic receptor agonist, was approved by the Food and Drug Administration (FDA) in 2014 for persistent facial erythema of rosacea. It does not treat telangiectasia and is not approved for flushing (transient erythema). Patients are advised to apply the gel daily in the morning. In phase 3 pivotal trials of patients with moderate to severe erythema of rosacea, which excluded individuals with more than two papules, a composite (investigator- and patient-reported) 2-grade improvement was seen as early as 30 minutes after application on day 1, and erythema was reduced for 9-12 hours.
Oxymetazoline 1% cream, an alpha-1a adrenergic receptor agonist, was approved by the FDA in 2017 for persistent facial erythema of rosacea. It neither treats telangiectasia nor is approved for flushing. Phase 3 trials of patients with moderate to severe persistent erythema of rosacea excluded individuals with more than three inflammatory papules or pustules. A composite (investigator- and subject-reported) 2-grade improvement was seen as early as 1 hour after application on day 1, and erythema was reduced for 9-12 hours.
Receptor Selectivity Differences
According to Harper, there are more reports of worsening erythema with brimonidine 0.33% gel than with oxymetazoline 1% cream, perhaps because of the different receptor selectivity between the two products. She explained that alpha-1 receptors are located only postsynaptically in vascular smooth muscle, while alpha-2 receptors are located presynaptically, which can inhibit norepinephrine and lead to vasodilation. Alpha-2 receptors are also located postsynaptically in vascular smooth muscle and in the endothelial wall, which can mediate nitric oxide release and cause vasodilation.
No head-to-head studies exist that compare brimonidine 0.33% gel with oxymetazoline 1% cream. But in a 52-week study of oxymetazoline 1% cream for persistent facial erythema associated with rosacea published in 2018, at week 52, 36.7% and 43.4% of patients achieved a 2-grade or greater composite improvement from baseline in both Clinician Erythema Assessment and Subject Self-Assessment 3 and 6 hours after a dose, respectively. Also, fewer than 1% of patients experienced a rebound effect following treatment cessation.
“What we learned from this study is that maybe patients do better if they use oxymetazoline 1% cream consistently,” Harper said. “Does that mean that everybody I give this to uses it daily? Probably not, but I think we can change the vascular tone by using it consistently every day.”
Oral Beta-Blockers Another Option
Alpha agonists can also help quell flushing associated with rosacea, Harper continued, but oral beta-blockers may be the better choice. In a 2020 review that drew from nine studies, researchers evaluated the use of carvedilol, propranolol, nadolol, and beta-blockers in general for rosacea-associated facial erythema and flushing. Articles studying carvedilol and propranolol showed a large reduction of erythema and flushing during treatment with a rapid onset of symptom control, while bradycardia and hypotension were the most commonly reported adverse events. “All of these agents are studied in rosacea, but none of them are FDA approved for rosacea,” Harper noted.
In a separate study, five patients with rosacea who had either severe frequent flushing episodes or persistent erythema and burning sensations were treated with carvedilol, a nonselective beta-blocker. Prior treatments included cetirizine and doxycycline, or isotretinoin combined with topical application of metronidazole gel or ivermectin without sufficient improvement in erythema. Carvedilol was added to the above treatments and titrated up to 12.5 mg twice a day and continued for at least 6 months.
The Clinician Erythema Assessment 5-point scale before therapy was 3.4 and dropped to 0.4 during therapy, while the patient self-assessment before therapy was 3.8 and dropped to 0.8 during therapy.
Another study evaluated the use of propranolol and/or doxycycline in 78 patients with rosacea. The propranolol and combination treatment groups showed more rapid improvement at weeks 4 and 8, but there was no statistically significant difference between them by week 12. Rosacea clinical scores also decreased in all groups, but there were no significant differences between them. Reduction of Assessment of Rosacea Clinical Score was 51%, 52.2%, and 57.3% in the propranolol, doxycycline, and combination groups, respectively.
Harper disclosed ties with Almirall, Cutera, Galderma, Journey, Ortho Dermatologics, and Sun Pharmaceutical Industries.
A version of this article appeared on Medscape.com.
LAS VEGAS —
These agents “work fast” and “improve redness quickly,” Harper, a dermatologist who practices in Birmingham, Alabama, said at the Society of Dermatology Physician Associates (SDPA) 22nd Annual Fall Dermatology Conference. In addition, “you’re going to know within 30 minutes or an hour whether it’s going to work or not.”
Brimonidine 0.33% gel, an alpha-2 adrenergic receptor agonist, was approved by the Food and Drug Administration (FDA) in 2014 for persistent facial erythema of rosacea. It does not treat telangiectasia and is not approved for flushing (transient erythema). Patients are advised to apply the gel daily in the morning. In phase 3 pivotal trials of patients with moderate to severe erythema of rosacea, which excluded individuals with more than two papules, a composite (investigator- and patient-reported) 2-grade improvement was seen as early as 30 minutes after application on day 1, and erythema was reduced for 9-12 hours.
Oxymetazoline 1% cream, an alpha-1a adrenergic receptor agonist, was approved by the FDA in 2017 for persistent facial erythema of rosacea. It neither treats telangiectasia nor is approved for flushing. Phase 3 trials of patients with moderate to severe persistent erythema of rosacea excluded individuals with more than three inflammatory papules or pustules. A composite (investigator- and subject-reported) 2-grade improvement was seen as early as 1 hour after application on day 1, and erythema was reduced for 9-12 hours.
Receptor Selectivity Differences
According to Harper, there are more reports of worsening erythema with brimonidine 0.33% gel than with oxymetazoline 1% cream, perhaps because of the different receptor selectivity between the two products. She explained that alpha-1 receptors are located only postsynaptically in vascular smooth muscle, while alpha-2 receptors are located presynaptically, which can inhibit norepinephrine and lead to vasodilation. Alpha-2 receptors are also located postsynaptically in vascular smooth muscle and in the endothelial wall, which can mediate nitric oxide release and cause vasodilation.
No head-to-head studies exist that compare brimonidine 0.33% gel with oxymetazoline 1% cream. But in a 52-week study of oxymetazoline 1% cream for persistent facial erythema associated with rosacea published in 2018, at week 52, 36.7% and 43.4% of patients achieved a 2-grade or greater composite improvement from baseline in both Clinician Erythema Assessment and Subject Self-Assessment 3 and 6 hours after a dose, respectively. Also, fewer than 1% of patients experienced a rebound effect following treatment cessation.
“What we learned from this study is that maybe patients do better if they use oxymetazoline 1% cream consistently,” Harper said. “Does that mean that everybody I give this to uses it daily? Probably not, but I think we can change the vascular tone by using it consistently every day.”
Oral Beta-Blockers Another Option
Alpha agonists can also help quell flushing associated with rosacea, Harper continued, but oral beta-blockers may be the better choice. In a 2020 review that drew from nine studies, researchers evaluated the use of carvedilol, propranolol, nadolol, and beta-blockers in general for rosacea-associated facial erythema and flushing. Articles studying carvedilol and propranolol showed a large reduction of erythema and flushing during treatment with a rapid onset of symptom control, while bradycardia and hypotension were the most commonly reported adverse events. “All of these agents are studied in rosacea, but none of them are FDA approved for rosacea,” Harper noted.
In a separate study, five patients with rosacea who had either severe frequent flushing episodes or persistent erythema and burning sensations were treated with carvedilol, a nonselective beta-blocker. Prior treatments included cetirizine and doxycycline, or isotretinoin combined with topical application of metronidazole gel or ivermectin without sufficient improvement in erythema. Carvedilol was added to the above treatments and titrated up to 12.5 mg twice a day and continued for at least 6 months.
The Clinician Erythema Assessment 5-point scale before therapy was 3.4 and dropped to 0.4 during therapy, while the patient self-assessment before therapy was 3.8 and dropped to 0.8 during therapy.
Another study evaluated the use of propranolol and/or doxycycline in 78 patients with rosacea. The propranolol and combination treatment groups showed more rapid improvement at weeks 4 and 8, but there was no statistically significant difference between them by week 12. Rosacea clinical scores also decreased in all groups, but there were no significant differences between them. Reduction of Assessment of Rosacea Clinical Score was 51%, 52.2%, and 57.3% in the propranolol, doxycycline, and combination groups, respectively.
Harper disclosed ties with Almirall, Cutera, Galderma, Journey, Ortho Dermatologics, and Sun Pharmaceutical Industries.
A version of this article appeared on Medscape.com.
FROM SDPA 2024
Rosacea: Ivermectin’s Benefits May Include Impact on Skin Microbiome
TOPLINE:
in a small study.
METHODOLOGY:
- In this single-center, open label study, 10 adults (mean age, 66.4 years) with papulopustular rosacea were treated with 1% ivermectin cream daily for 12 weeks.
- Skin swabs from lesional and nonlesional sites were collected at baseline and after 3 months of treatment to assess changes in the bacterial microbiome and the density of Demodex mites.
- The average baseline total papule count was 26.9, and the Clinician’s Erythema Assessment (CEA) score was 2 (average value on a scale of 0-4 from five locations on the face).
- For comparison, baseline swabs were taken from 10 healthy age-matched individuals.
TAKEAWAY:
- The density of Demodex mites was significantly reduced on lesional skin (P = .002) with topical ivermectin, which has anthelmintic effects against Demodex and is an effective treatment for rosacea.
- The absolute abundance of S epidermidis increased after ivermectin treatment on lesional skin (P = .039), while no changes were seen in Cutibacterium acnes.
- No changes were noted on nonlesional skin in the patients with rosacea.
- Topical ivermectin also reduced the number of papules and CEA scores (both P = .002) in individuals with rosacea.
IN PRACTICE:
“Treatment with topical ivermectin may improve the symptoms of rosacea through modulation of the skin microbiome beyond decreasing Demodex,” the authors concluded. “The results of this study,” they added, “provide valuable insights into the intricacies of the cutaneous microbiome in the pathophysiology of rosacea and highlight the potential therapeutic interventions targeting the skin microbiome.”
SOURCE:
The study was led by Teruaki Nakatsuji, PhD, of the department of dermatology, University of California, San Diego. It was published online on October 29 in the Journal of Investigative Dermatology.
LIMITATIONS:
The small sample size of 10 patients with rosacea limits the generalizability of the findings, and the study’s open-label design may introduce bias in the clinical assessments. Further research with larger sample sizes and randomized controlled trials is needed to confirm these findings.
DISCLOSURES:
This work was funded by a grant from the National Rosacea Society. One author disclosed being the cofounder and consultant, with equity interest in MatriSys Bioscience. The other authors reported no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
in a small study.
METHODOLOGY:
- In this single-center, open label study, 10 adults (mean age, 66.4 years) with papulopustular rosacea were treated with 1% ivermectin cream daily for 12 weeks.
- Skin swabs from lesional and nonlesional sites were collected at baseline and after 3 months of treatment to assess changes in the bacterial microbiome and the density of Demodex mites.
- The average baseline total papule count was 26.9, and the Clinician’s Erythema Assessment (CEA) score was 2 (average value on a scale of 0-4 from five locations on the face).
- For comparison, baseline swabs were taken from 10 healthy age-matched individuals.
TAKEAWAY:
- The density of Demodex mites was significantly reduced on lesional skin (P = .002) with topical ivermectin, which has anthelmintic effects against Demodex and is an effective treatment for rosacea.
- The absolute abundance of S epidermidis increased after ivermectin treatment on lesional skin (P = .039), while no changes were seen in Cutibacterium acnes.
- No changes were noted on nonlesional skin in the patients with rosacea.
- Topical ivermectin also reduced the number of papules and CEA scores (both P = .002) in individuals with rosacea.
IN PRACTICE:
“Treatment with topical ivermectin may improve the symptoms of rosacea through modulation of the skin microbiome beyond decreasing Demodex,” the authors concluded. “The results of this study,” they added, “provide valuable insights into the intricacies of the cutaneous microbiome in the pathophysiology of rosacea and highlight the potential therapeutic interventions targeting the skin microbiome.”
SOURCE:
The study was led by Teruaki Nakatsuji, PhD, of the department of dermatology, University of California, San Diego. It was published online on October 29 in the Journal of Investigative Dermatology.
LIMITATIONS:
The small sample size of 10 patients with rosacea limits the generalizability of the findings, and the study’s open-label design may introduce bias in the clinical assessments. Further research with larger sample sizes and randomized controlled trials is needed to confirm these findings.
DISCLOSURES:
This work was funded by a grant from the National Rosacea Society. One author disclosed being the cofounder and consultant, with equity interest in MatriSys Bioscience. The other authors reported no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
in a small study.
METHODOLOGY:
- In this single-center, open label study, 10 adults (mean age, 66.4 years) with papulopustular rosacea were treated with 1% ivermectin cream daily for 12 weeks.
- Skin swabs from lesional and nonlesional sites were collected at baseline and after 3 months of treatment to assess changes in the bacterial microbiome and the density of Demodex mites.
- The average baseline total papule count was 26.9, and the Clinician’s Erythema Assessment (CEA) score was 2 (average value on a scale of 0-4 from five locations on the face).
- For comparison, baseline swabs were taken from 10 healthy age-matched individuals.
TAKEAWAY:
- The density of Demodex mites was significantly reduced on lesional skin (P = .002) with topical ivermectin, which has anthelmintic effects against Demodex and is an effective treatment for rosacea.
- The absolute abundance of S epidermidis increased after ivermectin treatment on lesional skin (P = .039), while no changes were seen in Cutibacterium acnes.
- No changes were noted on nonlesional skin in the patients with rosacea.
- Topical ivermectin also reduced the number of papules and CEA scores (both P = .002) in individuals with rosacea.
IN PRACTICE:
“Treatment with topical ivermectin may improve the symptoms of rosacea through modulation of the skin microbiome beyond decreasing Demodex,” the authors concluded. “The results of this study,” they added, “provide valuable insights into the intricacies of the cutaneous microbiome in the pathophysiology of rosacea and highlight the potential therapeutic interventions targeting the skin microbiome.”
SOURCE:
The study was led by Teruaki Nakatsuji, PhD, of the department of dermatology, University of California, San Diego. It was published online on October 29 in the Journal of Investigative Dermatology.
LIMITATIONS:
The small sample size of 10 patients with rosacea limits the generalizability of the findings, and the study’s open-label design may introduce bias in the clinical assessments. Further research with larger sample sizes and randomized controlled trials is needed to confirm these findings.
DISCLOSURES:
This work was funded by a grant from the National Rosacea Society. One author disclosed being the cofounder and consultant, with equity interest in MatriSys Bioscience. The other authors reported no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
A young adult with a 1-year history of erythema, papules, and pustules on her cheeks and skin
. It typically presents with a sudden onset of papules, pustules, cysts, painful inflammatory nodules, and erythema on the centrofacial areas. The etiology is unknown but has been speculated to be hormone-related as it is more common in women and can be triggered by acute changes such as stress or medications.
Because of overlapping symptoms with other conditions, an accurate clinical assessment is crucial. Typically, there are no comedones and about half of the patients have a history of acne. Some cases have shown a possible link between pyoderma faciale with inflammatory bowel disease, thyroid disease and liver disease, highlighting the importance of considering these associations in treatment decisions.
Treatment options for pyoderma faciale include isotretinoin, corticosteroids, dapsone, and antibiotics such as doxycycline. Isotretinoin is usually the first-line treatment, with dapsone reserved for cases where other methods have failed. Despite concerns about isotretinoin exacerbating inflammatory bowel disease (IBD), there has been at least one reported case where a patient with ulcerative colitis who had pyoderma faciale that was successfully treated with isotretinoin with no adverse effects.
Isotretinoin has been shown to be effective in treating pyoderma faciale by significantly reducing inflammation and scarring. This is imperative because the scarring from pyoderma faciale can be disfiguring and psychologically harmful for patients. Therefore, an early diagnosis and effective treatment method are essential in preventing these scars and improving patients’ confidence and overall dermatological care.
This patient’s initial bacterial culture was negative. She was treated with a course of low dose isotretinoin. Prednisone was initiated two weeks before starting isotretinoin and then was tapered off during the first month of isotretinoin treatment. The patient was also started on spironolactone. The course of isotretinoin was 9 months. She has remained clear and still takes oral contraceptive pills and low dose spironolactone.
This case and the photos were submitted by Ms. Towe, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Donna Bilu Martin, MD, of Premier Dermatology, MD, Aventura, Florida. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Angileri L et al. J Dermatolog Treat. 2021 Feb;32(1):110-3. doi: 10.1080/09546634.2019.1628175.
Coutinho JC et al. An Bras Dermatol. 2016 Sep-Oct;91(5 suppl 1):151-3. doi: 10.1590/abd1806-4841.20164943.
Rosen T and Unkefer RP. Cutis. 1999 Aug;64(2):107-9.
. It typically presents with a sudden onset of papules, pustules, cysts, painful inflammatory nodules, and erythema on the centrofacial areas. The etiology is unknown but has been speculated to be hormone-related as it is more common in women and can be triggered by acute changes such as stress or medications.
Because of overlapping symptoms with other conditions, an accurate clinical assessment is crucial. Typically, there are no comedones and about half of the patients have a history of acne. Some cases have shown a possible link between pyoderma faciale with inflammatory bowel disease, thyroid disease and liver disease, highlighting the importance of considering these associations in treatment decisions.
Treatment options for pyoderma faciale include isotretinoin, corticosteroids, dapsone, and antibiotics such as doxycycline. Isotretinoin is usually the first-line treatment, with dapsone reserved for cases where other methods have failed. Despite concerns about isotretinoin exacerbating inflammatory bowel disease (IBD), there has been at least one reported case where a patient with ulcerative colitis who had pyoderma faciale that was successfully treated with isotretinoin with no adverse effects.
Isotretinoin has been shown to be effective in treating pyoderma faciale by significantly reducing inflammation and scarring. This is imperative because the scarring from pyoderma faciale can be disfiguring and psychologically harmful for patients. Therefore, an early diagnosis and effective treatment method are essential in preventing these scars and improving patients’ confidence and overall dermatological care.
This patient’s initial bacterial culture was negative. She was treated with a course of low dose isotretinoin. Prednisone was initiated two weeks before starting isotretinoin and then was tapered off during the first month of isotretinoin treatment. The patient was also started on spironolactone. The course of isotretinoin was 9 months. She has remained clear and still takes oral contraceptive pills and low dose spironolactone.
This case and the photos were submitted by Ms. Towe, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Donna Bilu Martin, MD, of Premier Dermatology, MD, Aventura, Florida. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Angileri L et al. J Dermatolog Treat. 2021 Feb;32(1):110-3. doi: 10.1080/09546634.2019.1628175.
Coutinho JC et al. An Bras Dermatol. 2016 Sep-Oct;91(5 suppl 1):151-3. doi: 10.1590/abd1806-4841.20164943.
Rosen T and Unkefer RP. Cutis. 1999 Aug;64(2):107-9.
. It typically presents with a sudden onset of papules, pustules, cysts, painful inflammatory nodules, and erythema on the centrofacial areas. The etiology is unknown but has been speculated to be hormone-related as it is more common in women and can be triggered by acute changes such as stress or medications.
Because of overlapping symptoms with other conditions, an accurate clinical assessment is crucial. Typically, there are no comedones and about half of the patients have a history of acne. Some cases have shown a possible link between pyoderma faciale with inflammatory bowel disease, thyroid disease and liver disease, highlighting the importance of considering these associations in treatment decisions.
Treatment options for pyoderma faciale include isotretinoin, corticosteroids, dapsone, and antibiotics such as doxycycline. Isotretinoin is usually the first-line treatment, with dapsone reserved for cases where other methods have failed. Despite concerns about isotretinoin exacerbating inflammatory bowel disease (IBD), there has been at least one reported case where a patient with ulcerative colitis who had pyoderma faciale that was successfully treated with isotretinoin with no adverse effects.
Isotretinoin has been shown to be effective in treating pyoderma faciale by significantly reducing inflammation and scarring. This is imperative because the scarring from pyoderma faciale can be disfiguring and psychologically harmful for patients. Therefore, an early diagnosis and effective treatment method are essential in preventing these scars and improving patients’ confidence and overall dermatological care.
This patient’s initial bacterial culture was negative. She was treated with a course of low dose isotretinoin. Prednisone was initiated two weeks before starting isotretinoin and then was tapered off during the first month of isotretinoin treatment. The patient was also started on spironolactone. The course of isotretinoin was 9 months. She has remained clear and still takes oral contraceptive pills and low dose spironolactone.
This case and the photos were submitted by Ms. Towe, Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Donna Bilu Martin, MD, of Premier Dermatology, MD, Aventura, Florida. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Angileri L et al. J Dermatolog Treat. 2021 Feb;32(1):110-3. doi: 10.1080/09546634.2019.1628175.
Coutinho JC et al. An Bras Dermatol. 2016 Sep-Oct;91(5 suppl 1):151-3. doi: 10.1590/abd1806-4841.20164943.
Rosen T and Unkefer RP. Cutis. 1999 Aug;64(2):107-9.
Migraine Drug Reduces Rosacea Flushing, Erythema in Small Study
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
In. Skin-related quality-of-life (QOL) measures also improved, albeit modestly.
The study was published in JAMA Dermatology.
“The transient erythema of rosacea is one of the most challenging rosacea symptoms to treat,” Emmy Graber, MD, MBA, who was not involved with the study, said in an interview. “As flushing can adversely impact quality of life in our rosacea patients, it is important to find therapeutic options for our patients. This study is exciting, not only because the treatment was successful for a notable number of patients, but also because it involved a drug with a novel mode of action in rosacea.” Dr. Graber practices in Boston and is an affiliate clinical instructor at Northeastern University, Boston.
Guy F. Webster, MD, PhD, clinical professor of dermatology, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, added, “The interesting thing about this study is that it gives us a new target to think about for therapy. But it’s a long way from saying we can use it tomorrow.” He was not involved with the study but was also asked to comment on the findings.
Spotlight on CGRP
Rosacea’s pathophysiology remains incompletely understood, wrote Nita K.F. Wienholtz, MD, PhD, Department of Dermatology, Bispebjerg and Frederiksberg Hospital, University of Copenhagen, Denmark, and coinvestigators. However, they added, mounting evidence suggests a possible role for CGRP. For example, a study published in JAMA Dermatology in 2015 revealed elevated CGRP levels in facial skin biopsies from patients with rosacea.
For the present study, the investigators enrolled 30 adults (including 23 women) with rosacea who experienced at least 15 days of moderate to severe erythema or extreme flushing during a 4-week, treatment-free run-in period. Most participants (87%) had previously failed one or more rosacea treatments because of a lack of efficacy or adverse reactions, and 43% had failed three or more treatments.
Participants received 3-monthly 140-mg doses of erenumab, which is approved by the Food and Drug Administration for migraine prevention. Patients recorded scores on the Patient Self-Assessment (PSA) and item 2 of the Flushing Assessment Tool online daily and made a final follow-up visit 12 weeks after the third dose.
Among the 27 patients who completed the study, the mean number of days with moderate to severe flushing from week 9 to week 12 fell by 6.9 from 23.6 days over 4 weeks at baseline (P < .001). Patients most severely affected by flushing at baseline experienced an 81% decline in days with severe to extreme flushing. Overall, 26% of patients experienced at least 50% reductions in moderate to extreme flushing days. The number of days with moderate to severe erythema as measured by PSA fell by 8.1 (mean) from baseline, and 56% of patients experienced at least 50% reductions in PSA scores. No unexpected safety signals emerged.
Questions Over QOL Data
“Although there were significant decreases in flushing and erythema,” wrote John S. Barbieri, MD, MBA, in an accompanying Editor’s Note, “the present study had relatively modest improvements in quality of life.” He is director of the Advanced Acne Therapeutics Clinic, Brigham and Women’s Hospital, Boston, and associate editor and evidence-based practice editor of JAMA Dermatology.
Compared with baseline (6.22), mean Dermatology Life Quality Index scores fell 2.08 points and 2.73 points at weeks 8 and 20, respectively (P = .004 and .003). At the same intervals, the mean baseline Rosacea Quality of Life score (48.22) decreased by 2.58 points and 4.14 points, respectively (P = .04 and .02).
No significant changes appeared in gauges of anxiety and depression. These findings, authors wrote, could stem from their decision to omit a follow-up visit at week 12 — where they may have seen mental-health effects which disappeared by week 20 — in response to patients’ logistical concerns.
However, Dr. Webster questioned the value of QOL measurements in rosacea. “Quality-of-life measures are blunt instruments,” he explained, and reducing severe itching or chronic pain improves the lives of affected patients. “But what question are you going to ask to tease out whether being less red-cheeked has made someone’s life easier? It’s not a problem that lends itself to quality-of-life assessments.” Moreover, he said, regulators who increasingly require such measures in clinical trials ignore this point, creating challenges for drug developers and researchers.
Because the study was neither blinded nor controlled, Dr. Webster suggested considering it a tantalizing proof of concept. “If I were putting money into a CGRP inhibitor, I’d want at least a small, placebo-controlled, double-blinded study.”
Study authors and Dr. Barbieri recommended larger randomized studies involving different populations and erenumab doses. For now, Dr. Barbieri wrote, CGRP inhibition represents a promising potential strategy for patients who have rosacea with comorbid migraine or recalcitrant flushing and erythema.
Dr. Wienholtz reported no relevant financial interests. Dr. Barbieri had no related disclosures. Dr. Webster reported no relevant financial interests. Dr. Graber reported no conflicts related to erenumab but consults for other companies with rosacea-related products including Galderma. The study was supported by and conducted in collaboration with Novartis Pharma AG. Additional funding came from the Novo Nordisk Foundation and the Lundbeck Foundation.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
A Neurotoxin, an Antidepressant, and More Emerging Options for Treating Rosacea
ORLANDO, FLORIDA — At the same time, there is new recognition that systemic inflammation can occur with rosacea, and targeting treatment to the phenotype continues to gain steam as a way to help people with this difficult-to-manage condition.
“Anyone here think they’ve got rosacea under control? No, I wish — not yet,” Diane Dr. Thiboutot, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Botulinum Toxin Benefits
With that in mind, Dr. Thiboutot highlighted emerging therapies for treating rosacea. “Last year, there were a couple of reports … looking at the use of botulinum toxin injections for patients with rosacea,” said Dr. Thiboutot, professor of dermatology and vice chair for research in the Department of Dermatology at Penn State College of Medicine, Hershey, Pennsylvania.
One report describes the case of a woman with rosacea who had severe recurrent episodes of erythema and flushing. She also experienced occasional papules and pustules and had been recalcitrant to multiple treatments for rosacea, according to the report published in the Journal of Drugs in Dermatology in June 2023. The patient was treated with a total of 150-180 units of botulinum toxin administered as 3-6 units spaced 1 cm apart every 2-4 months. She was “eventually maintained every 6 months with excellent improvement,” Dr. Thiboutot said.
In another case, a man with refractory vascular and papulopustular rosacea was treated with half of a unit of botulinum toxin spaced every 0.5 cm. Images taken at baseline, 1 month, and 3 months after treatment demonstrated improvements, as reported in June 2023.
Regarding botulinum toxin for rosacea, Dr. Thiboutot said, “it’s a very interesting thing to think about.”
Susan Weinkle, MD, ODAC conference cochair, session moderator, and collaborative associate professor of dermatology at the University of South Florida, Tampa, Florida, agreed. “I do think it holds some interesting potential,” she said. “How good are your hands? Because administering 0.5-unit injections evenly is a little bit challenging.”
However, one approach that might help is “if we could be a little more innovative like they are in Europe.” Physicians in Europe can use a metered syringe, one where they dial in the exact amount per injection, which allows them to be consistent, she added.
With rosacea erythema, Dr. Thiboutot noted, a spotted effect can result if injections are not administered uniformly.
Potential Role for Paroxetine
The antidepressant paroxetine, a potent selective serotonin reuptake inhibitor, could be an effective treatment for refractory erythema of rosacea, Dr. Thiboutot said. It is approved for treating depression, obsessive-compulsive disorder, and social phobia. The agent has also shown effectiveness in alleviating hot flashes associated with vascular dysregulation in menopause.
Uptake in serotonin and changes in receptors are closely related to vascular dilation and constriction, Dr. Thiboutot added, so paroxetine “may be beneficial in treating vascular dysfunction” including in people with rosacea. Evidence to support this potential approach comes from the primary results of a randomized controlled trial published in June 2023. Based on the results, the researchers concluded that paroxetine “appears to be an efficacious and well-tolerated treatment for refractory erythema in rosacea.”
In the trial, almost 43% of people treated with paroxetine met the primary endpoint for improving recalcitrant erythema at week 12 compared with almost 21% who took a placebo, a statistically significant difference.
Heparan Sulfate Analog in a Cream
Evidence suggests that a low-molecular-weight heparan sulfate analog is another agent that holds potential for treating rosacea. For example, a 2023 randomized controlled trial evaluated the immune response in rosacea, focusing on a specific cathelicidin peptide called LL-37 that activates an inflammasome in rosacea. Low-molecular-weight heparan sulfate holds the potential to inhibit LL-37 activity, as LL-37 is inhibited by binding to heparan sulfate, a cell surface glycosaminoglycan.
The study of 16 people assessed the ability of the analog to modulate this response; they were also treated with the pulsed dye laser. Participants who applied a dermal repair cream that contained this ingredient experienced a one-grade reduction in erythema at weeks 4 and 8 compared with a control group applying a moisturizer.
A Growing Case for Systemic Inflammation
In the meantime, treating rosacea with more traditional therapies remains challenging.
But there’s hope. Success has been reported in the few years since an expert panel recommended treating based on phenotype — a treat-what-you-see approach, Dr. Thiboutot said.
“We don’t have a single treatment that is one-size-fits-all. We have to individualize our treatment [based] more on what we are seeing and what the patient is experiencing.”
Eventually, therapies to treat systemic inflammation could provide benefits as well. As with hidradenitis suppurativa and psoriasis, “there’s evidence of systemic inflammation in some of our rosacea patients,” Dr. Thiboutot said.
For example, researchers compared blood taken from people with and without rosacea and found increased levels of some inflammatory markers among participants with the condition.
The retrospective study published in June 2023 in Scientific Reports included 100 patients with rosacea and 58 controls. The investigators found significantly higher elevations in the SII index, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels in the patients with rosacea.
“There was no significant link between the severity of rosacea and the ESR, CRP, or SII index values, Dr. Thiboutot added. “This study suggests inflammation beyond the skin in rosacea patients.”
For more guidance on treating rosacea through standard management options, including how to tailor therapy to each individual, she recommended the 2019 Update by the National Rosacea Society Expert Committee. “It’s a nice quick way to see, based on expert opinion, the most effective treatments and what the evidence base is,” said Dr. Thiboutot, lead author of the paper, published in the Journal of the American Academy of Dermatology in February 2020.
Dr. Thiboutot reported no relevant financial relationships.
ORLANDO, FLORIDA — At the same time, there is new recognition that systemic inflammation can occur with rosacea, and targeting treatment to the phenotype continues to gain steam as a way to help people with this difficult-to-manage condition.
“Anyone here think they’ve got rosacea under control? No, I wish — not yet,” Diane Dr. Thiboutot, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Botulinum Toxin Benefits
With that in mind, Dr. Thiboutot highlighted emerging therapies for treating rosacea. “Last year, there were a couple of reports … looking at the use of botulinum toxin injections for patients with rosacea,” said Dr. Thiboutot, professor of dermatology and vice chair for research in the Department of Dermatology at Penn State College of Medicine, Hershey, Pennsylvania.
One report describes the case of a woman with rosacea who had severe recurrent episodes of erythema and flushing. She also experienced occasional papules and pustules and had been recalcitrant to multiple treatments for rosacea, according to the report published in the Journal of Drugs in Dermatology in June 2023. The patient was treated with a total of 150-180 units of botulinum toxin administered as 3-6 units spaced 1 cm apart every 2-4 months. She was “eventually maintained every 6 months with excellent improvement,” Dr. Thiboutot said.
In another case, a man with refractory vascular and papulopustular rosacea was treated with half of a unit of botulinum toxin spaced every 0.5 cm. Images taken at baseline, 1 month, and 3 months after treatment demonstrated improvements, as reported in June 2023.
Regarding botulinum toxin for rosacea, Dr. Thiboutot said, “it’s a very interesting thing to think about.”
Susan Weinkle, MD, ODAC conference cochair, session moderator, and collaborative associate professor of dermatology at the University of South Florida, Tampa, Florida, agreed. “I do think it holds some interesting potential,” she said. “How good are your hands? Because administering 0.5-unit injections evenly is a little bit challenging.”
However, one approach that might help is “if we could be a little more innovative like they are in Europe.” Physicians in Europe can use a metered syringe, one where they dial in the exact amount per injection, which allows them to be consistent, she added.
With rosacea erythema, Dr. Thiboutot noted, a spotted effect can result if injections are not administered uniformly.
Potential Role for Paroxetine
The antidepressant paroxetine, a potent selective serotonin reuptake inhibitor, could be an effective treatment for refractory erythema of rosacea, Dr. Thiboutot said. It is approved for treating depression, obsessive-compulsive disorder, and social phobia. The agent has also shown effectiveness in alleviating hot flashes associated with vascular dysregulation in menopause.
Uptake in serotonin and changes in receptors are closely related to vascular dilation and constriction, Dr. Thiboutot added, so paroxetine “may be beneficial in treating vascular dysfunction” including in people with rosacea. Evidence to support this potential approach comes from the primary results of a randomized controlled trial published in June 2023. Based on the results, the researchers concluded that paroxetine “appears to be an efficacious and well-tolerated treatment for refractory erythema in rosacea.”
In the trial, almost 43% of people treated with paroxetine met the primary endpoint for improving recalcitrant erythema at week 12 compared with almost 21% who took a placebo, a statistically significant difference.
Heparan Sulfate Analog in a Cream
Evidence suggests that a low-molecular-weight heparan sulfate analog is another agent that holds potential for treating rosacea. For example, a 2023 randomized controlled trial evaluated the immune response in rosacea, focusing on a specific cathelicidin peptide called LL-37 that activates an inflammasome in rosacea. Low-molecular-weight heparan sulfate holds the potential to inhibit LL-37 activity, as LL-37 is inhibited by binding to heparan sulfate, a cell surface glycosaminoglycan.
The study of 16 people assessed the ability of the analog to modulate this response; they were also treated with the pulsed dye laser. Participants who applied a dermal repair cream that contained this ingredient experienced a one-grade reduction in erythema at weeks 4 and 8 compared with a control group applying a moisturizer.
A Growing Case for Systemic Inflammation
In the meantime, treating rosacea with more traditional therapies remains challenging.
But there’s hope. Success has been reported in the few years since an expert panel recommended treating based on phenotype — a treat-what-you-see approach, Dr. Thiboutot said.
“We don’t have a single treatment that is one-size-fits-all. We have to individualize our treatment [based] more on what we are seeing and what the patient is experiencing.”
Eventually, therapies to treat systemic inflammation could provide benefits as well. As with hidradenitis suppurativa and psoriasis, “there’s evidence of systemic inflammation in some of our rosacea patients,” Dr. Thiboutot said.
For example, researchers compared blood taken from people with and without rosacea and found increased levels of some inflammatory markers among participants with the condition.
The retrospective study published in June 2023 in Scientific Reports included 100 patients with rosacea and 58 controls. The investigators found significantly higher elevations in the SII index, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels in the patients with rosacea.
“There was no significant link between the severity of rosacea and the ESR, CRP, or SII index values, Dr. Thiboutot added. “This study suggests inflammation beyond the skin in rosacea patients.”
For more guidance on treating rosacea through standard management options, including how to tailor therapy to each individual, she recommended the 2019 Update by the National Rosacea Society Expert Committee. “It’s a nice quick way to see, based on expert opinion, the most effective treatments and what the evidence base is,” said Dr. Thiboutot, lead author of the paper, published in the Journal of the American Academy of Dermatology in February 2020.
Dr. Thiboutot reported no relevant financial relationships.
ORLANDO, FLORIDA — At the same time, there is new recognition that systemic inflammation can occur with rosacea, and targeting treatment to the phenotype continues to gain steam as a way to help people with this difficult-to-manage condition.
“Anyone here think they’ve got rosacea under control? No, I wish — not yet,” Diane Dr. Thiboutot, MD, said at the annual ODAC Dermatology, Aesthetic & Surgical Conference.
Botulinum Toxin Benefits
With that in mind, Dr. Thiboutot highlighted emerging therapies for treating rosacea. “Last year, there were a couple of reports … looking at the use of botulinum toxin injections for patients with rosacea,” said Dr. Thiboutot, professor of dermatology and vice chair for research in the Department of Dermatology at Penn State College of Medicine, Hershey, Pennsylvania.
One report describes the case of a woman with rosacea who had severe recurrent episodes of erythema and flushing. She also experienced occasional papules and pustules and had been recalcitrant to multiple treatments for rosacea, according to the report published in the Journal of Drugs in Dermatology in June 2023. The patient was treated with a total of 150-180 units of botulinum toxin administered as 3-6 units spaced 1 cm apart every 2-4 months. She was “eventually maintained every 6 months with excellent improvement,” Dr. Thiboutot said.
In another case, a man with refractory vascular and papulopustular rosacea was treated with half of a unit of botulinum toxin spaced every 0.5 cm. Images taken at baseline, 1 month, and 3 months after treatment demonstrated improvements, as reported in June 2023.
Regarding botulinum toxin for rosacea, Dr. Thiboutot said, “it’s a very interesting thing to think about.”
Susan Weinkle, MD, ODAC conference cochair, session moderator, and collaborative associate professor of dermatology at the University of South Florida, Tampa, Florida, agreed. “I do think it holds some interesting potential,” she said. “How good are your hands? Because administering 0.5-unit injections evenly is a little bit challenging.”
However, one approach that might help is “if we could be a little more innovative like they are in Europe.” Physicians in Europe can use a metered syringe, one where they dial in the exact amount per injection, which allows them to be consistent, she added.
With rosacea erythema, Dr. Thiboutot noted, a spotted effect can result if injections are not administered uniformly.
Potential Role for Paroxetine
The antidepressant paroxetine, a potent selective serotonin reuptake inhibitor, could be an effective treatment for refractory erythema of rosacea, Dr. Thiboutot said. It is approved for treating depression, obsessive-compulsive disorder, and social phobia. The agent has also shown effectiveness in alleviating hot flashes associated with vascular dysregulation in menopause.
Uptake in serotonin and changes in receptors are closely related to vascular dilation and constriction, Dr. Thiboutot added, so paroxetine “may be beneficial in treating vascular dysfunction” including in people with rosacea. Evidence to support this potential approach comes from the primary results of a randomized controlled trial published in June 2023. Based on the results, the researchers concluded that paroxetine “appears to be an efficacious and well-tolerated treatment for refractory erythema in rosacea.”
In the trial, almost 43% of people treated with paroxetine met the primary endpoint for improving recalcitrant erythema at week 12 compared with almost 21% who took a placebo, a statistically significant difference.
Heparan Sulfate Analog in a Cream
Evidence suggests that a low-molecular-weight heparan sulfate analog is another agent that holds potential for treating rosacea. For example, a 2023 randomized controlled trial evaluated the immune response in rosacea, focusing on a specific cathelicidin peptide called LL-37 that activates an inflammasome in rosacea. Low-molecular-weight heparan sulfate holds the potential to inhibit LL-37 activity, as LL-37 is inhibited by binding to heparan sulfate, a cell surface glycosaminoglycan.
The study of 16 people assessed the ability of the analog to modulate this response; they were also treated with the pulsed dye laser. Participants who applied a dermal repair cream that contained this ingredient experienced a one-grade reduction in erythema at weeks 4 and 8 compared with a control group applying a moisturizer.
A Growing Case for Systemic Inflammation
In the meantime, treating rosacea with more traditional therapies remains challenging.
But there’s hope. Success has been reported in the few years since an expert panel recommended treating based on phenotype — a treat-what-you-see approach, Dr. Thiboutot said.
“We don’t have a single treatment that is one-size-fits-all. We have to individualize our treatment [based] more on what we are seeing and what the patient is experiencing.”
Eventually, therapies to treat systemic inflammation could provide benefits as well. As with hidradenitis suppurativa and psoriasis, “there’s evidence of systemic inflammation in some of our rosacea patients,” Dr. Thiboutot said.
For example, researchers compared blood taken from people with and without rosacea and found increased levels of some inflammatory markers among participants with the condition.
The retrospective study published in June 2023 in Scientific Reports included 100 patients with rosacea and 58 controls. The investigators found significantly higher elevations in the SII index, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels in the patients with rosacea.
“There was no significant link between the severity of rosacea and the ESR, CRP, or SII index values, Dr. Thiboutot added. “This study suggests inflammation beyond the skin in rosacea patients.”
For more guidance on treating rosacea through standard management options, including how to tailor therapy to each individual, she recommended the 2019 Update by the National Rosacea Society Expert Committee. “It’s a nice quick way to see, based on expert opinion, the most effective treatments and what the evidence base is,” said Dr. Thiboutot, lead author of the paper, published in the Journal of the American Academy of Dermatology in February 2020.
Dr. Thiboutot reported no relevant financial relationships.
FROM ODAC 2024
National Rosacea Society adds imprimatur to skin products
, according to a press release from the NRS.
The seal is meant to be a resource to easily identify skin care products and cosmetic products that have been evaluated as unlikely to cause rosacea flares or skin irritation, according to the press release.
Surveys conducted by the NRS indicate that 92% of rosacea patients report burning, stinging, or itching on their skin, 66% identified specific skin products as triggers for their symptoms, and 84% were “very interested” in skin care guidance.
Patients and clinicians can find a searchable list of currently approved products in the Seal of Acceptance section of the NRS website. New skin care and cosmetic products will be added to the list of those with the Seal of Acceptance on an ongoing basis.
Products under consideration to earn the Seal of Acceptance must be free of ingredients that can cause skin barrier disruption, flushing, burning, itching, or other unwanted neurosensory stimulation, according to the press release.
Each accepted product also must pass clinical testing to confirm safety and low risk for irritation and sensitization for individuals with rosacea. Applications for the Seal of Acceptance are reviewed anonymously by an independent panel of dermatologists. The NRS created the program under the guidance of Zoe D. Draelos, MD, a clinical and research dermatologist in High Point, North Carolina, who also serves on the NRS board of directors.
More information about products carrying the seal and how companies can apply to have their products considered to carry the seal is available at rosacea.org/seal-of-acceptance/.
, according to a press release from the NRS.
The seal is meant to be a resource to easily identify skin care products and cosmetic products that have been evaluated as unlikely to cause rosacea flares or skin irritation, according to the press release.
Surveys conducted by the NRS indicate that 92% of rosacea patients report burning, stinging, or itching on their skin, 66% identified specific skin products as triggers for their symptoms, and 84% were “very interested” in skin care guidance.
Patients and clinicians can find a searchable list of currently approved products in the Seal of Acceptance section of the NRS website. New skin care and cosmetic products will be added to the list of those with the Seal of Acceptance on an ongoing basis.
Products under consideration to earn the Seal of Acceptance must be free of ingredients that can cause skin barrier disruption, flushing, burning, itching, or other unwanted neurosensory stimulation, according to the press release.
Each accepted product also must pass clinical testing to confirm safety and low risk for irritation and sensitization for individuals with rosacea. Applications for the Seal of Acceptance are reviewed anonymously by an independent panel of dermatologists. The NRS created the program under the guidance of Zoe D. Draelos, MD, a clinical and research dermatologist in High Point, North Carolina, who also serves on the NRS board of directors.
More information about products carrying the seal and how companies can apply to have their products considered to carry the seal is available at rosacea.org/seal-of-acceptance/.
, according to a press release from the NRS.
The seal is meant to be a resource to easily identify skin care products and cosmetic products that have been evaluated as unlikely to cause rosacea flares or skin irritation, according to the press release.
Surveys conducted by the NRS indicate that 92% of rosacea patients report burning, stinging, or itching on their skin, 66% identified specific skin products as triggers for their symptoms, and 84% were “very interested” in skin care guidance.
Patients and clinicians can find a searchable list of currently approved products in the Seal of Acceptance section of the NRS website. New skin care and cosmetic products will be added to the list of those with the Seal of Acceptance on an ongoing basis.
Products under consideration to earn the Seal of Acceptance must be free of ingredients that can cause skin barrier disruption, flushing, burning, itching, or other unwanted neurosensory stimulation, according to the press release.
Each accepted product also must pass clinical testing to confirm safety and low risk for irritation and sensitization for individuals with rosacea. Applications for the Seal of Acceptance are reviewed anonymously by an independent panel of dermatologists. The NRS created the program under the guidance of Zoe D. Draelos, MD, a clinical and research dermatologist in High Point, North Carolina, who also serves on the NRS board of directors.
More information about products carrying the seal and how companies can apply to have their products considered to carry the seal is available at rosacea.org/seal-of-acceptance/.
A Look at the Evidence Linking Diet to Skin Conditions
ORLANDO, FLORIDA — Amid all the hype, claims, and confusion, there is evidence linking some foods and drinks to an increased risk for acne, psoriasis, atopic dermatitis, rosacea, and other common skin conditions. So, what is the connection in each case? And how can people with any of these skin conditions potentially improve their health and quality of life with dietary changes?
What is clear is that there has been an explosion of interest in learning which foods can improve or worsen skin issues in recent years. It’s a good idea to familiarize yourself with the research and also to Google ‘diet’ and ‘skin’, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock. “As practitioners, we should be well prepared to talk about what patients want to talk about.”
Acne
One of the major areas of interest is diet and acne. “We’ve all heard sugar and dairy are bad, and the Western diet is high in sugar and dairy,” Dr. Shi said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Dairy, red meat, and carbohydrates can break down into leucine, an essential amino acid found in protein. Leucine and sugar together, in turn, can produce insulin and insulin-like growth factor 1 (IGF-1), which, through different pathways, can reach the androgen receptors throughout the body, including the skin. This results in sebogenesis, lipogenesis, and keratinization, which triggers follicular inflammation and results in more of the acne-causing bacteria Cutibacterium acnes.
Milk and other dairy products also can increase IGF-1 levels, which can alter hormonal mediators and increase acne.
Not all types of dairy milk are created equal, however, when it comes to acne. Dr. Shi wondered why 2% milk has overall color and nutritional content very similar to that of whole milk. “I looked into this.” She discovered that when milk manufacturers remove the fat, they often add whey proteins to restore some nutrients. Whey protein can increase acne, Dr. Shi added.
“So, if you’re going to choose any milk to drink, I think from an acne perspective, it’s better to use whole milk. If you can get it organic, even better.” Skim milk is the most acnegenic, she said.
Psoriasis
A systematic review of 55 studies evaluating diet and psoriasis found obesity can be an exacerbating factor. The strongest evidence for dietary weight reduction points to a hypocaloric diet in people with overweight or obesity, according to the review. Other evidence suggests alcohol can lower response to treatment and is linked with more severe psoriasis. Furthermore, a gluten-free diet or vitamin D supplements can help some subpopulations of people with psoriasis.
“An overwhelming majority of our psoriasis patients are vitamin D deficient,” Dr. Shi said.
The National Psoriasis Foundation (NPF) publishes dietary modification guidelines, updated as recently as November 2023. The NPF states that “there is no diet that will cure psoriatic disease, but there are many ways in which eating healthful food may lessen the severity of symptoms and play a role in lowering the likelihood of developing comorbidities.”
Healthier choices include fruits, vegetables, whole grains, and fat-free or low-fat dairy products. Include lean meats, poultry, fish, beans, eggs, and nuts. Adherence to a Mediterranean diet has been linked to a lower severity of psoriasis.
Atopic Dermatitis
Atopic dermatitis (AD) is “one of the prototypical diseases related to diet,” Dr. Shi said. A different meta-analysis looked at randomized controlled trials of synbiotics (a combination of prebiotics and probiotics) for treatment of AD.
These researchers found that synbiotics do not prevent AD, but they can help treat it in adults and children older than 1 year. In addition, synbiotics are more beneficial than probiotics in treating the condition, although there are no head-to-head comparison studies. In addition, the meta-analysis found that prebiotics alone can lower AD severity.
However, Dr. Shi said, there are no recommendations from the American Academy of Dermatology (AAD) on prebiotics or probiotics for AD, and the AAD does not recommend any supplement or essential oil for AD.
In a 2022 review, investigators ranked the efficacy of different supplements for AD based on available evidence. They found the greatest benefit associated with vitamin D supplementation, followed by vitamin E, probiotics, hemp seed oil, histidine, and oolong tea. They also noted the ‘Six Food Elimination Diet and Autoimmune Protocol’ featured the least amount of evidence to back it up.
Rosacea
Rosacea appears to be caused by “all the fun things in life” like sunlight, alcohol, chocolate, spicy foods, and caffeine, Dr. Shi said. In people with rosacea, they can cause facial flushing, edema, burning, and an inflammatory response.
Certain foods can activate skin receptors and sensory neurons, which can release neuropeptides that act on mast cells in blood that lead to flushing. The skin-gut axis may also be involved, evidence suggests. “And that is why food has a pretty profound impact on rosacea,” Dr. Shi said.
Dr. Shi reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
ORLANDO, FLORIDA — Amid all the hype, claims, and confusion, there is evidence linking some foods and drinks to an increased risk for acne, psoriasis, atopic dermatitis, rosacea, and other common skin conditions. So, what is the connection in each case? And how can people with any of these skin conditions potentially improve their health and quality of life with dietary changes?
What is clear is that there has been an explosion of interest in learning which foods can improve or worsen skin issues in recent years. It’s a good idea to familiarize yourself with the research and also to Google ‘diet’ and ‘skin’, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock. “As practitioners, we should be well prepared to talk about what patients want to talk about.”
Acne
One of the major areas of interest is diet and acne. “We’ve all heard sugar and dairy are bad, and the Western diet is high in sugar and dairy,” Dr. Shi said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Dairy, red meat, and carbohydrates can break down into leucine, an essential amino acid found in protein. Leucine and sugar together, in turn, can produce insulin and insulin-like growth factor 1 (IGF-1), which, through different pathways, can reach the androgen receptors throughout the body, including the skin. This results in sebogenesis, lipogenesis, and keratinization, which triggers follicular inflammation and results in more of the acne-causing bacteria Cutibacterium acnes.
Milk and other dairy products also can increase IGF-1 levels, which can alter hormonal mediators and increase acne.
Not all types of dairy milk are created equal, however, when it comes to acne. Dr. Shi wondered why 2% milk has overall color and nutritional content very similar to that of whole milk. “I looked into this.” She discovered that when milk manufacturers remove the fat, they often add whey proteins to restore some nutrients. Whey protein can increase acne, Dr. Shi added.
“So, if you’re going to choose any milk to drink, I think from an acne perspective, it’s better to use whole milk. If you can get it organic, even better.” Skim milk is the most acnegenic, she said.
Psoriasis
A systematic review of 55 studies evaluating diet and psoriasis found obesity can be an exacerbating factor. The strongest evidence for dietary weight reduction points to a hypocaloric diet in people with overweight or obesity, according to the review. Other evidence suggests alcohol can lower response to treatment and is linked with more severe psoriasis. Furthermore, a gluten-free diet or vitamin D supplements can help some subpopulations of people with psoriasis.
“An overwhelming majority of our psoriasis patients are vitamin D deficient,” Dr. Shi said.
The National Psoriasis Foundation (NPF) publishes dietary modification guidelines, updated as recently as November 2023. The NPF states that “there is no diet that will cure psoriatic disease, but there are many ways in which eating healthful food may lessen the severity of symptoms and play a role in lowering the likelihood of developing comorbidities.”
Healthier choices include fruits, vegetables, whole grains, and fat-free or low-fat dairy products. Include lean meats, poultry, fish, beans, eggs, and nuts. Adherence to a Mediterranean diet has been linked to a lower severity of psoriasis.
Atopic Dermatitis
Atopic dermatitis (AD) is “one of the prototypical diseases related to diet,” Dr. Shi said. A different meta-analysis looked at randomized controlled trials of synbiotics (a combination of prebiotics and probiotics) for treatment of AD.
These researchers found that synbiotics do not prevent AD, but they can help treat it in adults and children older than 1 year. In addition, synbiotics are more beneficial than probiotics in treating the condition, although there are no head-to-head comparison studies. In addition, the meta-analysis found that prebiotics alone can lower AD severity.
However, Dr. Shi said, there are no recommendations from the American Academy of Dermatology (AAD) on prebiotics or probiotics for AD, and the AAD does not recommend any supplement or essential oil for AD.
In a 2022 review, investigators ranked the efficacy of different supplements for AD based on available evidence. They found the greatest benefit associated with vitamin D supplementation, followed by vitamin E, probiotics, hemp seed oil, histidine, and oolong tea. They also noted the ‘Six Food Elimination Diet and Autoimmune Protocol’ featured the least amount of evidence to back it up.
Rosacea
Rosacea appears to be caused by “all the fun things in life” like sunlight, alcohol, chocolate, spicy foods, and caffeine, Dr. Shi said. In people with rosacea, they can cause facial flushing, edema, burning, and an inflammatory response.
Certain foods can activate skin receptors and sensory neurons, which can release neuropeptides that act on mast cells in blood that lead to flushing. The skin-gut axis may also be involved, evidence suggests. “And that is why food has a pretty profound impact on rosacea,” Dr. Shi said.
Dr. Shi reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
ORLANDO, FLORIDA — Amid all the hype, claims, and confusion, there is evidence linking some foods and drinks to an increased risk for acne, psoriasis, atopic dermatitis, rosacea, and other common skin conditions. So, what is the connection in each case? And how can people with any of these skin conditions potentially improve their health and quality of life with dietary changes?
What is clear is that there has been an explosion of interest in learning which foods can improve or worsen skin issues in recent years. It’s a good idea to familiarize yourself with the research and also to Google ‘diet’ and ‘skin’, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock. “As practitioners, we should be well prepared to talk about what patients want to talk about.”
Acne
One of the major areas of interest is diet and acne. “We’ve all heard sugar and dairy are bad, and the Western diet is high in sugar and dairy,” Dr. Shi said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Dairy, red meat, and carbohydrates can break down into leucine, an essential amino acid found in protein. Leucine and sugar together, in turn, can produce insulin and insulin-like growth factor 1 (IGF-1), which, through different pathways, can reach the androgen receptors throughout the body, including the skin. This results in sebogenesis, lipogenesis, and keratinization, which triggers follicular inflammation and results in more of the acne-causing bacteria Cutibacterium acnes.
Milk and other dairy products also can increase IGF-1 levels, which can alter hormonal mediators and increase acne.
Not all types of dairy milk are created equal, however, when it comes to acne. Dr. Shi wondered why 2% milk has overall color and nutritional content very similar to that of whole milk. “I looked into this.” She discovered that when milk manufacturers remove the fat, they often add whey proteins to restore some nutrients. Whey protein can increase acne, Dr. Shi added.
“So, if you’re going to choose any milk to drink, I think from an acne perspective, it’s better to use whole milk. If you can get it organic, even better.” Skim milk is the most acnegenic, she said.
Psoriasis
A systematic review of 55 studies evaluating diet and psoriasis found obesity can be an exacerbating factor. The strongest evidence for dietary weight reduction points to a hypocaloric diet in people with overweight or obesity, according to the review. Other evidence suggests alcohol can lower response to treatment and is linked with more severe psoriasis. Furthermore, a gluten-free diet or vitamin D supplements can help some subpopulations of people with psoriasis.
“An overwhelming majority of our psoriasis patients are vitamin D deficient,” Dr. Shi said.
The National Psoriasis Foundation (NPF) publishes dietary modification guidelines, updated as recently as November 2023. The NPF states that “there is no diet that will cure psoriatic disease, but there are many ways in which eating healthful food may lessen the severity of symptoms and play a role in lowering the likelihood of developing comorbidities.”
Healthier choices include fruits, vegetables, whole grains, and fat-free or low-fat dairy products. Include lean meats, poultry, fish, beans, eggs, and nuts. Adherence to a Mediterranean diet has been linked to a lower severity of psoriasis.
Atopic Dermatitis
Atopic dermatitis (AD) is “one of the prototypical diseases related to diet,” Dr. Shi said. A different meta-analysis looked at randomized controlled trials of synbiotics (a combination of prebiotics and probiotics) for treatment of AD.
These researchers found that synbiotics do not prevent AD, but they can help treat it in adults and children older than 1 year. In addition, synbiotics are more beneficial than probiotics in treating the condition, although there are no head-to-head comparison studies. In addition, the meta-analysis found that prebiotics alone can lower AD severity.
However, Dr. Shi said, there are no recommendations from the American Academy of Dermatology (AAD) on prebiotics or probiotics for AD, and the AAD does not recommend any supplement or essential oil for AD.
In a 2022 review, investigators ranked the efficacy of different supplements for AD based on available evidence. They found the greatest benefit associated with vitamin D supplementation, followed by vitamin E, probiotics, hemp seed oil, histidine, and oolong tea. They also noted the ‘Six Food Elimination Diet and Autoimmune Protocol’ featured the least amount of evidence to back it up.
Rosacea
Rosacea appears to be caused by “all the fun things in life” like sunlight, alcohol, chocolate, spicy foods, and caffeine, Dr. Shi said. In people with rosacea, they can cause facial flushing, edema, burning, and an inflammatory response.
Certain foods can activate skin receptors and sensory neurons, which can release neuropeptides that act on mast cells in blood that lead to flushing. The skin-gut axis may also be involved, evidence suggests. “And that is why food has a pretty profound impact on rosacea,” Dr. Shi said.
Dr. Shi reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Impact of Pregnancy on Rosacea Unpredictable, Study Suggests
TOPLINE:
Among women diagnosed with rosacea, the impact of pregnancy on the disease is unpredictable.
METHODOLOGY:
- Researchers conducted a telephone survey of 39 women with a diagnosis of rosacea in the electronic medical records prior to the onset of pregnancy who had been admitted to Oregon Health & Science University for labor and delivery from June 27, 2015, to June 27, 2020.
- Patient global assessment of clear (0), mild (1), moderate (2), or severe (3) rosacea was rated across five timepoints: 1-3 months preconception; first, second, and third trimesters; and 6 weeks postpartum.
TAKEAWAY:
- The mean age of the survey participants was 35.5 years, the mean gestational age at delivery was 39.4 weeks, and most had singleton pregnancies.
- All but one study participant (97.4%) reported symptoms of erythematotelangiectatic rosacea, while 26 (67%) reported symptoms of papulopustular rosacea.
- Nearly half of the participants (19, 48.7%) said their rosacea worsened during pregnancy, 13 (33.3%) reported no change in rosacea severity during pregnancy, and 7 (17.9%) reported that their rosacea improved during pregnancy.
- Before conceiving, the mean rosacea severity score among participants was mild (1.10; 95% CI, 0.92-1.29) and did not change significantly over time, a reflection of individual variations. In addition, 83.3% of participants did not use prescription rosacea treatments prior to pregnancy, and 89.6% did not use them during pregnancy.
IN PRACTICE:
“Rosacea, like acne, lacks a predictable group effect, and instead, each individual may have a different response to the physiologic changes of pregnancy,” the authors concluded.
SOURCE:
Genevieve Benedetti, MD, MPP, of the Department of Dermatology at Oregon Health & Science University, Portland, Oregon, led the research, published as a research letter in the International Journal of Women’s Dermatology.
LIMITATIONS:
The small sample size, single-center design, and overall prevalence of mild disease limit the ability to detect change.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women diagnosed with rosacea, the impact of pregnancy on the disease is unpredictable.
METHODOLOGY:
- Researchers conducted a telephone survey of 39 women with a diagnosis of rosacea in the electronic medical records prior to the onset of pregnancy who had been admitted to Oregon Health & Science University for labor and delivery from June 27, 2015, to June 27, 2020.
- Patient global assessment of clear (0), mild (1), moderate (2), or severe (3) rosacea was rated across five timepoints: 1-3 months preconception; first, second, and third trimesters; and 6 weeks postpartum.
TAKEAWAY:
- The mean age of the survey participants was 35.5 years, the mean gestational age at delivery was 39.4 weeks, and most had singleton pregnancies.
- All but one study participant (97.4%) reported symptoms of erythematotelangiectatic rosacea, while 26 (67%) reported symptoms of papulopustular rosacea.
- Nearly half of the participants (19, 48.7%) said their rosacea worsened during pregnancy, 13 (33.3%) reported no change in rosacea severity during pregnancy, and 7 (17.9%) reported that their rosacea improved during pregnancy.
- Before conceiving, the mean rosacea severity score among participants was mild (1.10; 95% CI, 0.92-1.29) and did not change significantly over time, a reflection of individual variations. In addition, 83.3% of participants did not use prescription rosacea treatments prior to pregnancy, and 89.6% did not use them during pregnancy.
IN PRACTICE:
“Rosacea, like acne, lacks a predictable group effect, and instead, each individual may have a different response to the physiologic changes of pregnancy,” the authors concluded.
SOURCE:
Genevieve Benedetti, MD, MPP, of the Department of Dermatology at Oregon Health & Science University, Portland, Oregon, led the research, published as a research letter in the International Journal of Women’s Dermatology.
LIMITATIONS:
The small sample size, single-center design, and overall prevalence of mild disease limit the ability to detect change.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women diagnosed with rosacea, the impact of pregnancy on the disease is unpredictable.
METHODOLOGY:
- Researchers conducted a telephone survey of 39 women with a diagnosis of rosacea in the electronic medical records prior to the onset of pregnancy who had been admitted to Oregon Health & Science University for labor and delivery from June 27, 2015, to June 27, 2020.
- Patient global assessment of clear (0), mild (1), moderate (2), or severe (3) rosacea was rated across five timepoints: 1-3 months preconception; first, second, and third trimesters; and 6 weeks postpartum.
TAKEAWAY:
- The mean age of the survey participants was 35.5 years, the mean gestational age at delivery was 39.4 weeks, and most had singleton pregnancies.
- All but one study participant (97.4%) reported symptoms of erythematotelangiectatic rosacea, while 26 (67%) reported symptoms of papulopustular rosacea.
- Nearly half of the participants (19, 48.7%) said their rosacea worsened during pregnancy, 13 (33.3%) reported no change in rosacea severity during pregnancy, and 7 (17.9%) reported that their rosacea improved during pregnancy.
- Before conceiving, the mean rosacea severity score among participants was mild (1.10; 95% CI, 0.92-1.29) and did not change significantly over time, a reflection of individual variations. In addition, 83.3% of participants did not use prescription rosacea treatments prior to pregnancy, and 89.6% did not use them during pregnancy.
IN PRACTICE:
“Rosacea, like acne, lacks a predictable group effect, and instead, each individual may have a different response to the physiologic changes of pregnancy,” the authors concluded.
SOURCE:
Genevieve Benedetti, MD, MPP, of the Department of Dermatology at Oregon Health & Science University, Portland, Oregon, led the research, published as a research letter in the International Journal of Women’s Dermatology.
LIMITATIONS:
The small sample size, single-center design, and overall prevalence of mild disease limit the ability to detect change.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.