User login
Low-Dose Oral Minoxidil: Expert Consensus Provide Guidance for Treating Hair Loss
. With large randomized, controlled trials lacking, the guidelines authors and other dermatologists said the paper provides practical pointers that should increase clinicians’ confidence in prescribing LDOM for hair loss.
Comfort and Confidence
Benjamin N. Ungar, MD, director of the Alopecia Center of Excellence at Mount Sinai Icahn School of Medicine, New York City, said he hopes that the guidelines will “make dermatologists in practice more comfortable with the use of low-dose oral minoxidil to treat different kinds of hair loss, and therefore, more patients will benefit.” He was not an author of the paper, which was published online in JAMA Dermatology on November 20, but was asked to comment.
Members of the multidisciplinary Low-Dose Oral Minoxidil Initiation steering committee recruited dermatologists with hair loss expertise from 12 countries. Using a modified four-round Delphi process that required at least 70% agreement, the group of 43 dermatologists crafted 76 consensus statements. “Notably,” said Co-senior author Jennifer Fu, MD, director of the Hair Disorders Clinic at the University of California, San Francisco, “27 items achieved at least 90% consensus after the first two rounds, indicating broad agreement in expert practice.”
Indications for LDOM
At least 90% of experts concurred regarding the appropriateness of LDOM use for androgenetic alopecia (AGA) and age-related thinning and in cases where topical minoxidil proves ineffective or problematic. Additional situations in which LDOM might provide direct benefit involve follicular miniaturization, such as alopecia areata, or hair cycle disruption, such as chemotherapy. The authors also recommended considering LDOM over topical minoxidil when the latter is more expensive and when patients desire enhanced hypertrichosis.
Contraindications and Precautions
Before prescribing LDOM, the authors wrote, clinicians may consult with primary care or cardiology when contraindications (cardiovascular issues, pregnancy/nursing, and potential drug interactions) or precautions (history of tachycardia or arrhythmia, hypotension, or impaired kidney function) exist. Patients with precautions may require blood pressure monitoring, as well as monitoring for adverse effects of treatment. The panel also suggested the latter for all patients at the time of LDOM initiation and dose escalation. The authors advised against routine baseline laboratory and EKG testing in cases without relevant precautions.
Dosing Considerations
Along with systemic adverse event risk and baseline hair loss severity, key dosing considerations include patient age, sex, and whether patients desire hypertrichosis. Consensus on daily doses for adolescent females and males begins at 0.625 mg and 1.25 mg, respectively, and ranges up to 2.5 mg for adolescent females vs 5 mg for adult females and adolescent and adult males.
Presently, said Ungar, many dermatologists — including some who prescribe LDOM — remain uncomfortable even with very low doses, perhaps because of an invalid perception of cardiovascular safety issues including potential hypotension and pericardial effusions. However, recently published data include a review published November 7 in the Journal of the American Academy of Dermatology, which showed no significant effect of LDOM on blood pressure. And in a September Journal of Drugs in Dermatology article the authors found no impact on pericardial effusions in a 100-patient cohort.
Some dermatologists worry about the impact hypertrichosis may have on patients, Ungar added. Although incidence estimates range from 15% to 30%, he said, more than half of his patients experience hypertrichosis. “However, most continue treatment because the beneficial effects outweigh the effect of hypertrichosis.”
Practical Roadmap
Adam Friedman, MD, who was not involved with the publication, applauds its inclusion of pragmatic clinical guidance, which he said consensus papers often lack. “This paper sets a great roadmap for working low-dose oral minoxidil into your clinical practice, Friedman, professor and chair of dermatology at George Washington University, Washington, DC, said in an interview.
Rather than limiting LDOM use to AGA, he said, the paper is most helpful in showing the spectrum of disease states for which the expert panel prescribes LDOM. “We use it as adjunctive therapy for many other things, both scarring and nonscarring hair loss,” he added.
In appropriate clinical contexts, the authors wrote, clinicians may consider combining LDOM with spironolactone or beta-blockers. Friedman said that in his hands, combining LDOM with a 5-alpha reductase inhibitor (5ARI) is “absolutely outstanding.” Minoxidil increases blood flow to the scalp, he explained, while 5ARIs prevent production of dihydrotestosterone, which miniaturizes hair.
Fu said, “We hope these consensus outcomes will be helpful to dermatology colleagues as they consider using LDOM to treat hair loss in their adult and adolescent patient populations. We anticipate that these guidelines will be updated as additional evidence-based data emerges and are encouraged that we are already seeing new publications on this topic.”
Important areas for future research, she noted, include pediatric use of LDOM, the comparative efficacy of topical vs oral minoxidil, the safety of oral minoxidil for patients with a history of allergic contact dermatitis to topical minoxidil, and the use of other off-label forms of minoxidil, such as compounded oral minoxidil and sublingual minoxidil.
The study was funded by the University of California, San Francisco, Department of Dermatology Medical Student Summer Research Fellowship Program. Fu reported personal fees from Pfizer, Eli Lilly and Company, and Sun Pharma outside of the study. The full list of author disclosures can be found in the paper. Ungar and Friedman reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
. With large randomized, controlled trials lacking, the guidelines authors and other dermatologists said the paper provides practical pointers that should increase clinicians’ confidence in prescribing LDOM for hair loss.
Comfort and Confidence
Benjamin N. Ungar, MD, director of the Alopecia Center of Excellence at Mount Sinai Icahn School of Medicine, New York City, said he hopes that the guidelines will “make dermatologists in practice more comfortable with the use of low-dose oral minoxidil to treat different kinds of hair loss, and therefore, more patients will benefit.” He was not an author of the paper, which was published online in JAMA Dermatology on November 20, but was asked to comment.
Members of the multidisciplinary Low-Dose Oral Minoxidil Initiation steering committee recruited dermatologists with hair loss expertise from 12 countries. Using a modified four-round Delphi process that required at least 70% agreement, the group of 43 dermatologists crafted 76 consensus statements. “Notably,” said Co-senior author Jennifer Fu, MD, director of the Hair Disorders Clinic at the University of California, San Francisco, “27 items achieved at least 90% consensus after the first two rounds, indicating broad agreement in expert practice.”
Indications for LDOM
At least 90% of experts concurred regarding the appropriateness of LDOM use for androgenetic alopecia (AGA) and age-related thinning and in cases where topical minoxidil proves ineffective or problematic. Additional situations in which LDOM might provide direct benefit involve follicular miniaturization, such as alopecia areata, or hair cycle disruption, such as chemotherapy. The authors also recommended considering LDOM over topical minoxidil when the latter is more expensive and when patients desire enhanced hypertrichosis.
Contraindications and Precautions
Before prescribing LDOM, the authors wrote, clinicians may consult with primary care or cardiology when contraindications (cardiovascular issues, pregnancy/nursing, and potential drug interactions) or precautions (history of tachycardia or arrhythmia, hypotension, or impaired kidney function) exist. Patients with precautions may require blood pressure monitoring, as well as monitoring for adverse effects of treatment. The panel also suggested the latter for all patients at the time of LDOM initiation and dose escalation. The authors advised against routine baseline laboratory and EKG testing in cases without relevant precautions.
Dosing Considerations
Along with systemic adverse event risk and baseline hair loss severity, key dosing considerations include patient age, sex, and whether patients desire hypertrichosis. Consensus on daily doses for adolescent females and males begins at 0.625 mg and 1.25 mg, respectively, and ranges up to 2.5 mg for adolescent females vs 5 mg for adult females and adolescent and adult males.
Presently, said Ungar, many dermatologists — including some who prescribe LDOM — remain uncomfortable even with very low doses, perhaps because of an invalid perception of cardiovascular safety issues including potential hypotension and pericardial effusions. However, recently published data include a review published November 7 in the Journal of the American Academy of Dermatology, which showed no significant effect of LDOM on blood pressure. And in a September Journal of Drugs in Dermatology article the authors found no impact on pericardial effusions in a 100-patient cohort.
Some dermatologists worry about the impact hypertrichosis may have on patients, Ungar added. Although incidence estimates range from 15% to 30%, he said, more than half of his patients experience hypertrichosis. “However, most continue treatment because the beneficial effects outweigh the effect of hypertrichosis.”
Practical Roadmap
Adam Friedman, MD, who was not involved with the publication, applauds its inclusion of pragmatic clinical guidance, which he said consensus papers often lack. “This paper sets a great roadmap for working low-dose oral minoxidil into your clinical practice, Friedman, professor and chair of dermatology at George Washington University, Washington, DC, said in an interview.
Rather than limiting LDOM use to AGA, he said, the paper is most helpful in showing the spectrum of disease states for which the expert panel prescribes LDOM. “We use it as adjunctive therapy for many other things, both scarring and nonscarring hair loss,” he added.
In appropriate clinical contexts, the authors wrote, clinicians may consider combining LDOM with spironolactone or beta-blockers. Friedman said that in his hands, combining LDOM with a 5-alpha reductase inhibitor (5ARI) is “absolutely outstanding.” Minoxidil increases blood flow to the scalp, he explained, while 5ARIs prevent production of dihydrotestosterone, which miniaturizes hair.
Fu said, “We hope these consensus outcomes will be helpful to dermatology colleagues as they consider using LDOM to treat hair loss in their adult and adolescent patient populations. We anticipate that these guidelines will be updated as additional evidence-based data emerges and are encouraged that we are already seeing new publications on this topic.”
Important areas for future research, she noted, include pediatric use of LDOM, the comparative efficacy of topical vs oral minoxidil, the safety of oral minoxidil for patients with a history of allergic contact dermatitis to topical minoxidil, and the use of other off-label forms of minoxidil, such as compounded oral minoxidil and sublingual minoxidil.
The study was funded by the University of California, San Francisco, Department of Dermatology Medical Student Summer Research Fellowship Program. Fu reported personal fees from Pfizer, Eli Lilly and Company, and Sun Pharma outside of the study. The full list of author disclosures can be found in the paper. Ungar and Friedman reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
. With large randomized, controlled trials lacking, the guidelines authors and other dermatologists said the paper provides practical pointers that should increase clinicians’ confidence in prescribing LDOM for hair loss.
Comfort and Confidence
Benjamin N. Ungar, MD, director of the Alopecia Center of Excellence at Mount Sinai Icahn School of Medicine, New York City, said he hopes that the guidelines will “make dermatologists in practice more comfortable with the use of low-dose oral minoxidil to treat different kinds of hair loss, and therefore, more patients will benefit.” He was not an author of the paper, which was published online in JAMA Dermatology on November 20, but was asked to comment.
Members of the multidisciplinary Low-Dose Oral Minoxidil Initiation steering committee recruited dermatologists with hair loss expertise from 12 countries. Using a modified four-round Delphi process that required at least 70% agreement, the group of 43 dermatologists crafted 76 consensus statements. “Notably,” said Co-senior author Jennifer Fu, MD, director of the Hair Disorders Clinic at the University of California, San Francisco, “27 items achieved at least 90% consensus after the first two rounds, indicating broad agreement in expert practice.”
Indications for LDOM
At least 90% of experts concurred regarding the appropriateness of LDOM use for androgenetic alopecia (AGA) and age-related thinning and in cases where topical minoxidil proves ineffective or problematic. Additional situations in which LDOM might provide direct benefit involve follicular miniaturization, such as alopecia areata, or hair cycle disruption, such as chemotherapy. The authors also recommended considering LDOM over topical minoxidil when the latter is more expensive and when patients desire enhanced hypertrichosis.
Contraindications and Precautions
Before prescribing LDOM, the authors wrote, clinicians may consult with primary care or cardiology when contraindications (cardiovascular issues, pregnancy/nursing, and potential drug interactions) or precautions (history of tachycardia or arrhythmia, hypotension, or impaired kidney function) exist. Patients with precautions may require blood pressure monitoring, as well as monitoring for adverse effects of treatment. The panel also suggested the latter for all patients at the time of LDOM initiation and dose escalation. The authors advised against routine baseline laboratory and EKG testing in cases without relevant precautions.
Dosing Considerations
Along with systemic adverse event risk and baseline hair loss severity, key dosing considerations include patient age, sex, and whether patients desire hypertrichosis. Consensus on daily doses for adolescent females and males begins at 0.625 mg and 1.25 mg, respectively, and ranges up to 2.5 mg for adolescent females vs 5 mg for adult females and adolescent and adult males.
Presently, said Ungar, many dermatologists — including some who prescribe LDOM — remain uncomfortable even with very low doses, perhaps because of an invalid perception of cardiovascular safety issues including potential hypotension and pericardial effusions. However, recently published data include a review published November 7 in the Journal of the American Academy of Dermatology, which showed no significant effect of LDOM on blood pressure. And in a September Journal of Drugs in Dermatology article the authors found no impact on pericardial effusions in a 100-patient cohort.
Some dermatologists worry about the impact hypertrichosis may have on patients, Ungar added. Although incidence estimates range from 15% to 30%, he said, more than half of his patients experience hypertrichosis. “However, most continue treatment because the beneficial effects outweigh the effect of hypertrichosis.”
Practical Roadmap
Adam Friedman, MD, who was not involved with the publication, applauds its inclusion of pragmatic clinical guidance, which he said consensus papers often lack. “This paper sets a great roadmap for working low-dose oral minoxidil into your clinical practice, Friedman, professor and chair of dermatology at George Washington University, Washington, DC, said in an interview.
Rather than limiting LDOM use to AGA, he said, the paper is most helpful in showing the spectrum of disease states for which the expert panel prescribes LDOM. “We use it as adjunctive therapy for many other things, both scarring and nonscarring hair loss,” he added.
In appropriate clinical contexts, the authors wrote, clinicians may consider combining LDOM with spironolactone or beta-blockers. Friedman said that in his hands, combining LDOM with a 5-alpha reductase inhibitor (5ARI) is “absolutely outstanding.” Minoxidil increases blood flow to the scalp, he explained, while 5ARIs prevent production of dihydrotestosterone, which miniaturizes hair.
Fu said, “We hope these consensus outcomes will be helpful to dermatology colleagues as they consider using LDOM to treat hair loss in their adult and adolescent patient populations. We anticipate that these guidelines will be updated as additional evidence-based data emerges and are encouraged that we are already seeing new publications on this topic.”
Important areas for future research, she noted, include pediatric use of LDOM, the comparative efficacy of topical vs oral minoxidil, the safety of oral minoxidil for patients with a history of allergic contact dermatitis to topical minoxidil, and the use of other off-label forms of minoxidil, such as compounded oral minoxidil and sublingual minoxidil.
The study was funded by the University of California, San Francisco, Department of Dermatology Medical Student Summer Research Fellowship Program. Fu reported personal fees from Pfizer, Eli Lilly and Company, and Sun Pharma outside of the study. The full list of author disclosures can be found in the paper. Ungar and Friedman reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Post COVID-19, Long-term Risk for Autoimmune, Autoinflammatory Skin Disorders Increased, Study Finds
In addition, the authors reported that COVID-19 vaccination appears to reduce these risks.
The study was published in JAMA Dermatology.
‘Compelling Evidence’
“This well-executed study by Heo et al provides compelling evidence to support an association between COVID-19 infection and the development of subsequent autoimmune and autoinflammatory skin diseases,” wrote authors led by Lisa M. Arkin, MD, of the Department of Dermatology, University of Wisconsin School of Medicine and Public Health in Madison, in an accompanying editorial.
Using databases from Korea’s National Health Insurance Service and the Korea Disease Control and Prevention Agency, investigators led by Yeon-Woo Heo, MD, a dermatology resident at Yonsei University Wonju College of Medicine, Wonju, Republic of Korea, compared 3.1 million people who had COVID-19 with 3.8 million controls, all with at least 180 days’ follow-up through December 31, 2022.
At a mean follow-up of 287 days in both cohorts, authors found significantly elevated risks for AA and vitiligo (adjusted hazard ratio [aHR], 1.11 for both), AT (aHR, 1.24), Behçet disease (aHR, 1.45), and BP (aHR, 1.62) in the post–COVID-19 cohort. The infection also raised the risk for other conditions such as systemic lupus erythematosus (aHR, 1.14) and Crohn’s disease (aHR, 1.35).
In subgroup analyses, demographic factors were associated with diverse effects: COVID-19 infection was associated with significantly higher odds of developing AA (for both men and women), vitiligo (men), Behçet disease (men and women), Crohn’s disease (men), ulcerative colitis (men), rheumatoid arthritis (men and women), systemic lupus erythematosus (men), ankylosing spondylitis (men), AT (women), and BP (women) than controls.
Those aged under 40 years were more likely to develop AA, primary cicatricial alopecia, Behçet disease, and ulcerative colitis, while those aged 40 years or older were more likely to develop AA, AT, vitiligo, Behçet disease, Crohn’s disease, rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and BP.
Additionally, severe COVID-19 requiring intensive care unit admission was associated with a significantly increased risk for autoimmune diseases, including AA, psoriasis, BP, and sarcoidosis. By timeframe, risks for AA, AT, and psoriasis were significantly higher during the initial Delta-dominant period.
Vaccination Effect
Moreover, vaccinated individuals were less likely to develop AA, AT, psoriasis, Behçet disease, and various nondermatologic conditions than were those who were unvaccinated. This finding, wrote Heo and colleagues, “may provide evidence to support the hypothesis that COVID-19 vaccines can help prevent autoimmune diseases.”
“That’s the part we all need to take into our offices tomorrow,” said Brett King, MD, PhD, a Fairfield, Connecticut–based dermatologist in private practice. He was not involved with the study but was asked to comment.
Overall, King said, the study carries two main messages. “The first is that COVID-19 infection increases the likelihood of developing an autoimmune or autoinflammatory disease in a large population.” The second and very important message is that being vaccinated against COVID-19 provides protection against developing an autoimmune or autoinflammatory disease.
“My concern is that the popular media highlights the first part,” said King, “and everybody who develops alopecia areata, vitiligo, or sarcoidosis blames COVID-19. That’s not what this work says.”
The foregoing distinction is especially important during the fall and winter, he added, when people getting influenza vaccines are routinely offered COVID-19 vaccines. “Many patients have said, ‘I got the COVID vaccine and developed alopecia areata 6 months later.’ Nearly everybody who has developed a new or worsening health condition in the last almost 5 years has had the perfect fall guy — the COVID vaccine or infection.”
With virtually all patients asking if they should get an updated COVID-19 vaccine or booster, he added, many report having heard that such vaccines cause AA, vitiligo, or other diseases. “To anchor these conversations in real data and not just anecdotes from a blog or Facebook is very useful,” said King, “and now we have very good data saying that the COVID vaccine is protective against these disorders.”
George Han, MD, PhD, associate professor of dermatology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, applauds investigators’ use of a large, robust database but suggests interpreting results cautiously. He was not involved with the study but was asked to comment.
“You could do a large, well-done study,” Han said, “but it could still not necessarily be generalizable. These autoimmune conditions they’re looking at have clear ethnic and racial biases.” Heo and colleagues acknowledged shortcomings including their study population’s monomorphic nature.
Additional issues that limit the study’s impact, said Han, include the difficulty of conceptualizing a 10%-20% increase in conditions that at baseline are rare. And many of the findings reflected natural patterns, he said. For instance, BP more commonly affects older people, COVID-19 notwithstanding.
Han said that for him, the study’s main value going forward is helping to explain a rash of worsening inflammatory skin disease that many dermatologists saw early in the pandemic. “We would regularly see patients who were well controlled with, for example, psoriasis or eczema. But after COVID-19 infection or a vaccine (usually mRNA-type), in some cases they would come in flaring badly.” This happened at least a dozen times during the first year of post-shutdown appointments, he said.
“We’ve seen patients who have flared multiple times — they get the booster, then flare again,” Han added. Similar patterns occurred with pyoderma gangrenosum and other inflammatory skin diseases, he said.
Given the modest effect sizes of the associations reported in the Korean study, Arkin and colleagues wrote in their JAMA Dermatology editorial that surveillance for autoimmune disease is probably not warranted without new examination findings or symptoms. “For certain,” King said, “we should not go hunting for things that aren’t obviously there.”
Rather, Arkin and colleagues wrote, the higher autoimmunity rates seen among the unvaccinated, as well as during the Delta phase (when patients were sicker and hospitalizations were more likely) and in patients requiring intensive care, suggest that “interventions that reduce disease severity could also potentially reduce long-term risk of subsequent autoimmune sequelae.”
Future research addressing whether people with preexisting autoimmune conditions are at greater risk for flares or developing new autoimmune diseases following COVID-19 infection “would help to frame an evidence-based approach for patients with autoimmune disorders who develop COVID-19 infection, including the role for antiviral treatments,” they added.
The study was supported by grants from the Research Program of the Korea Medical Institute, the Korea Health Industry Development Institute, and the National Research Foundation of Korea. Han and King reported no relevant financial relationships. Arkin disclosed receiving research grants to her institution from Amgen and Eli Lilly, personal fees from Sanofi/Regeneron for consulting, and personal consulting fees from Merck outside the submitted work. Another author reported personal consulting fees from Dexcel Pharma and Honeydew outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
In addition, the authors reported that COVID-19 vaccination appears to reduce these risks.
The study was published in JAMA Dermatology.
‘Compelling Evidence’
“This well-executed study by Heo et al provides compelling evidence to support an association between COVID-19 infection and the development of subsequent autoimmune and autoinflammatory skin diseases,” wrote authors led by Lisa M. Arkin, MD, of the Department of Dermatology, University of Wisconsin School of Medicine and Public Health in Madison, in an accompanying editorial.
Using databases from Korea’s National Health Insurance Service and the Korea Disease Control and Prevention Agency, investigators led by Yeon-Woo Heo, MD, a dermatology resident at Yonsei University Wonju College of Medicine, Wonju, Republic of Korea, compared 3.1 million people who had COVID-19 with 3.8 million controls, all with at least 180 days’ follow-up through December 31, 2022.
At a mean follow-up of 287 days in both cohorts, authors found significantly elevated risks for AA and vitiligo (adjusted hazard ratio [aHR], 1.11 for both), AT (aHR, 1.24), Behçet disease (aHR, 1.45), and BP (aHR, 1.62) in the post–COVID-19 cohort. The infection also raised the risk for other conditions such as systemic lupus erythematosus (aHR, 1.14) and Crohn’s disease (aHR, 1.35).
In subgroup analyses, demographic factors were associated with diverse effects: COVID-19 infection was associated with significantly higher odds of developing AA (for both men and women), vitiligo (men), Behçet disease (men and women), Crohn’s disease (men), ulcerative colitis (men), rheumatoid arthritis (men and women), systemic lupus erythematosus (men), ankylosing spondylitis (men), AT (women), and BP (women) than controls.
Those aged under 40 years were more likely to develop AA, primary cicatricial alopecia, Behçet disease, and ulcerative colitis, while those aged 40 years or older were more likely to develop AA, AT, vitiligo, Behçet disease, Crohn’s disease, rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and BP.
Additionally, severe COVID-19 requiring intensive care unit admission was associated with a significantly increased risk for autoimmune diseases, including AA, psoriasis, BP, and sarcoidosis. By timeframe, risks for AA, AT, and psoriasis were significantly higher during the initial Delta-dominant period.
Vaccination Effect
Moreover, vaccinated individuals were less likely to develop AA, AT, psoriasis, Behçet disease, and various nondermatologic conditions than were those who were unvaccinated. This finding, wrote Heo and colleagues, “may provide evidence to support the hypothesis that COVID-19 vaccines can help prevent autoimmune diseases.”
“That’s the part we all need to take into our offices tomorrow,” said Brett King, MD, PhD, a Fairfield, Connecticut–based dermatologist in private practice. He was not involved with the study but was asked to comment.
Overall, King said, the study carries two main messages. “The first is that COVID-19 infection increases the likelihood of developing an autoimmune or autoinflammatory disease in a large population.” The second and very important message is that being vaccinated against COVID-19 provides protection against developing an autoimmune or autoinflammatory disease.
“My concern is that the popular media highlights the first part,” said King, “and everybody who develops alopecia areata, vitiligo, or sarcoidosis blames COVID-19. That’s not what this work says.”
The foregoing distinction is especially important during the fall and winter, he added, when people getting influenza vaccines are routinely offered COVID-19 vaccines. “Many patients have said, ‘I got the COVID vaccine and developed alopecia areata 6 months later.’ Nearly everybody who has developed a new or worsening health condition in the last almost 5 years has had the perfect fall guy — the COVID vaccine or infection.”
With virtually all patients asking if they should get an updated COVID-19 vaccine or booster, he added, many report having heard that such vaccines cause AA, vitiligo, or other diseases. “To anchor these conversations in real data and not just anecdotes from a blog or Facebook is very useful,” said King, “and now we have very good data saying that the COVID vaccine is protective against these disorders.”
George Han, MD, PhD, associate professor of dermatology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, applauds investigators’ use of a large, robust database but suggests interpreting results cautiously. He was not involved with the study but was asked to comment.
“You could do a large, well-done study,” Han said, “but it could still not necessarily be generalizable. These autoimmune conditions they’re looking at have clear ethnic and racial biases.” Heo and colleagues acknowledged shortcomings including their study population’s monomorphic nature.
Additional issues that limit the study’s impact, said Han, include the difficulty of conceptualizing a 10%-20% increase in conditions that at baseline are rare. And many of the findings reflected natural patterns, he said. For instance, BP more commonly affects older people, COVID-19 notwithstanding.
Han said that for him, the study’s main value going forward is helping to explain a rash of worsening inflammatory skin disease that many dermatologists saw early in the pandemic. “We would regularly see patients who were well controlled with, for example, psoriasis or eczema. But after COVID-19 infection or a vaccine (usually mRNA-type), in some cases they would come in flaring badly.” This happened at least a dozen times during the first year of post-shutdown appointments, he said.
“We’ve seen patients who have flared multiple times — they get the booster, then flare again,” Han added. Similar patterns occurred with pyoderma gangrenosum and other inflammatory skin diseases, he said.
Given the modest effect sizes of the associations reported in the Korean study, Arkin and colleagues wrote in their JAMA Dermatology editorial that surveillance for autoimmune disease is probably not warranted without new examination findings or symptoms. “For certain,” King said, “we should not go hunting for things that aren’t obviously there.”
Rather, Arkin and colleagues wrote, the higher autoimmunity rates seen among the unvaccinated, as well as during the Delta phase (when patients were sicker and hospitalizations were more likely) and in patients requiring intensive care, suggest that “interventions that reduce disease severity could also potentially reduce long-term risk of subsequent autoimmune sequelae.”
Future research addressing whether people with preexisting autoimmune conditions are at greater risk for flares or developing new autoimmune diseases following COVID-19 infection “would help to frame an evidence-based approach for patients with autoimmune disorders who develop COVID-19 infection, including the role for antiviral treatments,” they added.
The study was supported by grants from the Research Program of the Korea Medical Institute, the Korea Health Industry Development Institute, and the National Research Foundation of Korea. Han and King reported no relevant financial relationships. Arkin disclosed receiving research grants to her institution from Amgen and Eli Lilly, personal fees from Sanofi/Regeneron for consulting, and personal consulting fees from Merck outside the submitted work. Another author reported personal consulting fees from Dexcel Pharma and Honeydew outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
In addition, the authors reported that COVID-19 vaccination appears to reduce these risks.
The study was published in JAMA Dermatology.
‘Compelling Evidence’
“This well-executed study by Heo et al provides compelling evidence to support an association between COVID-19 infection and the development of subsequent autoimmune and autoinflammatory skin diseases,” wrote authors led by Lisa M. Arkin, MD, of the Department of Dermatology, University of Wisconsin School of Medicine and Public Health in Madison, in an accompanying editorial.
Using databases from Korea’s National Health Insurance Service and the Korea Disease Control and Prevention Agency, investigators led by Yeon-Woo Heo, MD, a dermatology resident at Yonsei University Wonju College of Medicine, Wonju, Republic of Korea, compared 3.1 million people who had COVID-19 with 3.8 million controls, all with at least 180 days’ follow-up through December 31, 2022.
At a mean follow-up of 287 days in both cohorts, authors found significantly elevated risks for AA and vitiligo (adjusted hazard ratio [aHR], 1.11 for both), AT (aHR, 1.24), Behçet disease (aHR, 1.45), and BP (aHR, 1.62) in the post–COVID-19 cohort. The infection also raised the risk for other conditions such as systemic lupus erythematosus (aHR, 1.14) and Crohn’s disease (aHR, 1.35).
In subgroup analyses, demographic factors were associated with diverse effects: COVID-19 infection was associated with significantly higher odds of developing AA (for both men and women), vitiligo (men), Behçet disease (men and women), Crohn’s disease (men), ulcerative colitis (men), rheumatoid arthritis (men and women), systemic lupus erythematosus (men), ankylosing spondylitis (men), AT (women), and BP (women) than controls.
Those aged under 40 years were more likely to develop AA, primary cicatricial alopecia, Behçet disease, and ulcerative colitis, while those aged 40 years or older were more likely to develop AA, AT, vitiligo, Behçet disease, Crohn’s disease, rheumatoid arthritis, systemic lupus erythematosus, Sjögren’s syndrome, ankylosing spondylitis, and BP.
Additionally, severe COVID-19 requiring intensive care unit admission was associated with a significantly increased risk for autoimmune diseases, including AA, psoriasis, BP, and sarcoidosis. By timeframe, risks for AA, AT, and psoriasis were significantly higher during the initial Delta-dominant period.
Vaccination Effect
Moreover, vaccinated individuals were less likely to develop AA, AT, psoriasis, Behçet disease, and various nondermatologic conditions than were those who were unvaccinated. This finding, wrote Heo and colleagues, “may provide evidence to support the hypothesis that COVID-19 vaccines can help prevent autoimmune diseases.”
“That’s the part we all need to take into our offices tomorrow,” said Brett King, MD, PhD, a Fairfield, Connecticut–based dermatologist in private practice. He was not involved with the study but was asked to comment.
Overall, King said, the study carries two main messages. “The first is that COVID-19 infection increases the likelihood of developing an autoimmune or autoinflammatory disease in a large population.” The second and very important message is that being vaccinated against COVID-19 provides protection against developing an autoimmune or autoinflammatory disease.
“My concern is that the popular media highlights the first part,” said King, “and everybody who develops alopecia areata, vitiligo, or sarcoidosis blames COVID-19. That’s not what this work says.”
The foregoing distinction is especially important during the fall and winter, he added, when people getting influenza vaccines are routinely offered COVID-19 vaccines. “Many patients have said, ‘I got the COVID vaccine and developed alopecia areata 6 months later.’ Nearly everybody who has developed a new or worsening health condition in the last almost 5 years has had the perfect fall guy — the COVID vaccine or infection.”
With virtually all patients asking if they should get an updated COVID-19 vaccine or booster, he added, many report having heard that such vaccines cause AA, vitiligo, or other diseases. “To anchor these conversations in real data and not just anecdotes from a blog or Facebook is very useful,” said King, “and now we have very good data saying that the COVID vaccine is protective against these disorders.”
George Han, MD, PhD, associate professor of dermatology at the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, applauds investigators’ use of a large, robust database but suggests interpreting results cautiously. He was not involved with the study but was asked to comment.
“You could do a large, well-done study,” Han said, “but it could still not necessarily be generalizable. These autoimmune conditions they’re looking at have clear ethnic and racial biases.” Heo and colleagues acknowledged shortcomings including their study population’s monomorphic nature.
Additional issues that limit the study’s impact, said Han, include the difficulty of conceptualizing a 10%-20% increase in conditions that at baseline are rare. And many of the findings reflected natural patterns, he said. For instance, BP more commonly affects older people, COVID-19 notwithstanding.
Han said that for him, the study’s main value going forward is helping to explain a rash of worsening inflammatory skin disease that many dermatologists saw early in the pandemic. “We would regularly see patients who were well controlled with, for example, psoriasis or eczema. But after COVID-19 infection or a vaccine (usually mRNA-type), in some cases they would come in flaring badly.” This happened at least a dozen times during the first year of post-shutdown appointments, he said.
“We’ve seen patients who have flared multiple times — they get the booster, then flare again,” Han added. Similar patterns occurred with pyoderma gangrenosum and other inflammatory skin diseases, he said.
Given the modest effect sizes of the associations reported in the Korean study, Arkin and colleagues wrote in their JAMA Dermatology editorial that surveillance for autoimmune disease is probably not warranted without new examination findings or symptoms. “For certain,” King said, “we should not go hunting for things that aren’t obviously there.”
Rather, Arkin and colleagues wrote, the higher autoimmunity rates seen among the unvaccinated, as well as during the Delta phase (when patients were sicker and hospitalizations were more likely) and in patients requiring intensive care, suggest that “interventions that reduce disease severity could also potentially reduce long-term risk of subsequent autoimmune sequelae.”
Future research addressing whether people with preexisting autoimmune conditions are at greater risk for flares or developing new autoimmune diseases following COVID-19 infection “would help to frame an evidence-based approach for patients with autoimmune disorders who develop COVID-19 infection, including the role for antiviral treatments,” they added.
The study was supported by grants from the Research Program of the Korea Medical Institute, the Korea Health Industry Development Institute, and the National Research Foundation of Korea. Han and King reported no relevant financial relationships. Arkin disclosed receiving research grants to her institution from Amgen and Eli Lilly, personal fees from Sanofi/Regeneron for consulting, and personal consulting fees from Merck outside the submitted work. Another author reported personal consulting fees from Dexcel Pharma and Honeydew outside the submitted work. No other disclosures were reported.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Smoldering MS May Warrant Unique Diagnosis, Treatment, and Research Strategies
Smoldering-associated worsening (SAW) of multiple sclerosis (MS) deserves a broader, more comprehensive approach to diagnosis, treatment, and research that goes beyond neurologists’ understanding of progression independent of relapse activity (PIRA), according to a recently published international consensus. However, an outside expert said that promulgating the “smoldering” concept may stoke patient and provider confusion.
Although current disease-modifying therapies (DMTs) for MS exclusively target focal white matter (WM) inflammation, wrote authors lead by Antonio Scalfari, MD, PhD, of Charing Cross Hospital, Imperial College London in England, many people with MS experience worsening disability in a more indolent fashion — despite stable inflammatory markers.
“The gradual accumulation of physical and cognitive disability is driven by smoldering pathological processes via biological substrates, which are different from those of acute focal damage, remain an important unmet therapeutic target,” they wrote.
The same research team first described smoldering MS in a 2022 publication. In the present paper, Scalfari and colleagues reviewed emerging clinical, radiological, and pathological evidence and presented 29 consensus statements in areas ranging from the definition, pathology, and clinical manifestations of smoldering MS to appropriate biomarkers and best clinical practices.
Definition
By definition, the authors wrote, SAW encompasses PIRA but also includes a range of gradually worsening, relapse-independent symptoms that remain undetectable on standard assessments, including the Expanded Disability Status Scale (EDSS) or EDSS-Plus, especially in early disease. To capture symptoms such as subtle motor impairment, cognitive slowing, and fatigue, Scalfari and colleagues recommend tools such as neurological stress tests, fatigue/mood scales, wearable devices, and patient reported outcomes.
Disease Mechanisms
Pathologically, the authors wrote, smoldering MS may stem from intrinsic central nervous system processes that likely incorporate various glial, immune, and neural cells. Smoldering MS also could contribute to aging, and vice versa, the latter possibly through dynamics such as age-related exhaustion of compensatory mechanisms, reduction in remyelination efficiency, and telomere shortening, they added.
Clinical Implementation
Current MS management rests on crude estimates of physical disability and overemphasizes identifying relapses and new MRI lesions as the principal markers of disease activity, wrote Scalfari and colleagues. Instead, they suggested combining motor-associated assessments such as EDSS-Plus with cognitive gauges such as the Brief International Cognitive Assessment for Multiple Sclerosis.
Providers are uncomfortable identifying and discussing smoldering MS, authors allowed, because no licensed treatments target SAW. However, the authors wrote, a principal reason for discussing smoldering MS with patients is to help manage their expectations of current DMTs, which may have little effect on SAW.
‘More Than Lesions’
Bruce Cree, MD, PhD, MAS, professor of neurology at the University of California, San Francisco, said that it is extremely important to raise awareness of physicians’ emerging understanding that “there is more going on in MS than lesions and relapses,” a concept that has been a work in progress for several years. He was not involved with the study but was asked to comment.
A 2019 report on the EPIC cohort coauthored by Cree labeled the disconnect between disability accumulation and relapse occurrence “silent progression.” The observation that disability accumulates in early relapsing MS independent of relapsing activity has been replicated in virtually every dataset worldwide, he added.
“What I don’t like about this article is the reliance on the term ‘smoldering’ and the acceptance that this is an actual phenomenon supported by data.” And authors’ leveraging “smoldering” into additional acronyms such as SAW likely will confuse rather than clarify physicians’ and patients’ understanding of the situation, Cree added. “Clinicians don’t need yet another snappy acronym.” Many are still trying to grasp the PIRA concept in relapsing MS, he said.
“One of the reasons this topic has become so important is that we recognize that even when we have very good control of relapsing disease activity — clinical relapses as well as radiographic large lesion formation on MRI — some patients still develop insidious worsening of disability. And the reasons for that are not well understood,” said Cree.
Accumulating disability absent relapse activity could stem from any number of microscopic inflammatory processes, possibly involving abnormal microglial activation, fibrinogen deposition, microscopic inflammatory infiltrates of CD8-positive T cells, or mitochondrial damage from iron deposition, he said. Or the processes driving PIRA may not even involve inflammation, he added. “We still don’t have a unifying way of understanding how these processes work.”
Cree suspects that, despite investigators’ good intentions, the study’s sponsor, Sanofi, may have influenced the resultant messaging. The company’s tolebrutinib recently completed phase 3 trials in secondary progressive MS and relapsing MS, and a phase 3 trial in primary progressive MS is scheduled for completion in 2025. “A hallmark of Sanofi’s messaging has been this idea that there is smoldering inflammation occurring in MS that tolebrutinib is going to address,” he said.
If clinicians really knew what drove progressive MS, said Cree, “we would be keen on developing therapies targeting that fundamental process. But because we don’t know what’s driving it, we don’t know what to go after.”
The study was supported by Sanofi. Cree is a coauthor of the GEMINI 1 and GEMINI 2 tolebrutinib studies.
A version of this article first appeared on Medscape.com.
Smoldering-associated worsening (SAW) of multiple sclerosis (MS) deserves a broader, more comprehensive approach to diagnosis, treatment, and research that goes beyond neurologists’ understanding of progression independent of relapse activity (PIRA), according to a recently published international consensus. However, an outside expert said that promulgating the “smoldering” concept may stoke patient and provider confusion.
Although current disease-modifying therapies (DMTs) for MS exclusively target focal white matter (WM) inflammation, wrote authors lead by Antonio Scalfari, MD, PhD, of Charing Cross Hospital, Imperial College London in England, many people with MS experience worsening disability in a more indolent fashion — despite stable inflammatory markers.
“The gradual accumulation of physical and cognitive disability is driven by smoldering pathological processes via biological substrates, which are different from those of acute focal damage, remain an important unmet therapeutic target,” they wrote.
The same research team first described smoldering MS in a 2022 publication. In the present paper, Scalfari and colleagues reviewed emerging clinical, radiological, and pathological evidence and presented 29 consensus statements in areas ranging from the definition, pathology, and clinical manifestations of smoldering MS to appropriate biomarkers and best clinical practices.
Definition
By definition, the authors wrote, SAW encompasses PIRA but also includes a range of gradually worsening, relapse-independent symptoms that remain undetectable on standard assessments, including the Expanded Disability Status Scale (EDSS) or EDSS-Plus, especially in early disease. To capture symptoms such as subtle motor impairment, cognitive slowing, and fatigue, Scalfari and colleagues recommend tools such as neurological stress tests, fatigue/mood scales, wearable devices, and patient reported outcomes.
Disease Mechanisms
Pathologically, the authors wrote, smoldering MS may stem from intrinsic central nervous system processes that likely incorporate various glial, immune, and neural cells. Smoldering MS also could contribute to aging, and vice versa, the latter possibly through dynamics such as age-related exhaustion of compensatory mechanisms, reduction in remyelination efficiency, and telomere shortening, they added.
Clinical Implementation
Current MS management rests on crude estimates of physical disability and overemphasizes identifying relapses and new MRI lesions as the principal markers of disease activity, wrote Scalfari and colleagues. Instead, they suggested combining motor-associated assessments such as EDSS-Plus with cognitive gauges such as the Brief International Cognitive Assessment for Multiple Sclerosis.
Providers are uncomfortable identifying and discussing smoldering MS, authors allowed, because no licensed treatments target SAW. However, the authors wrote, a principal reason for discussing smoldering MS with patients is to help manage their expectations of current DMTs, which may have little effect on SAW.
‘More Than Lesions’
Bruce Cree, MD, PhD, MAS, professor of neurology at the University of California, San Francisco, said that it is extremely important to raise awareness of physicians’ emerging understanding that “there is more going on in MS than lesions and relapses,” a concept that has been a work in progress for several years. He was not involved with the study but was asked to comment.
A 2019 report on the EPIC cohort coauthored by Cree labeled the disconnect between disability accumulation and relapse occurrence “silent progression.” The observation that disability accumulates in early relapsing MS independent of relapsing activity has been replicated in virtually every dataset worldwide, he added.
“What I don’t like about this article is the reliance on the term ‘smoldering’ and the acceptance that this is an actual phenomenon supported by data.” And authors’ leveraging “smoldering” into additional acronyms such as SAW likely will confuse rather than clarify physicians’ and patients’ understanding of the situation, Cree added. “Clinicians don’t need yet another snappy acronym.” Many are still trying to grasp the PIRA concept in relapsing MS, he said.
“One of the reasons this topic has become so important is that we recognize that even when we have very good control of relapsing disease activity — clinical relapses as well as radiographic large lesion formation on MRI — some patients still develop insidious worsening of disability. And the reasons for that are not well understood,” said Cree.
Accumulating disability absent relapse activity could stem from any number of microscopic inflammatory processes, possibly involving abnormal microglial activation, fibrinogen deposition, microscopic inflammatory infiltrates of CD8-positive T cells, or mitochondrial damage from iron deposition, he said. Or the processes driving PIRA may not even involve inflammation, he added. “We still don’t have a unifying way of understanding how these processes work.”
Cree suspects that, despite investigators’ good intentions, the study’s sponsor, Sanofi, may have influenced the resultant messaging. The company’s tolebrutinib recently completed phase 3 trials in secondary progressive MS and relapsing MS, and a phase 3 trial in primary progressive MS is scheduled for completion in 2025. “A hallmark of Sanofi’s messaging has been this idea that there is smoldering inflammation occurring in MS that tolebrutinib is going to address,” he said.
If clinicians really knew what drove progressive MS, said Cree, “we would be keen on developing therapies targeting that fundamental process. But because we don’t know what’s driving it, we don’t know what to go after.”
The study was supported by Sanofi. Cree is a coauthor of the GEMINI 1 and GEMINI 2 tolebrutinib studies.
A version of this article first appeared on Medscape.com.
Smoldering-associated worsening (SAW) of multiple sclerosis (MS) deserves a broader, more comprehensive approach to diagnosis, treatment, and research that goes beyond neurologists’ understanding of progression independent of relapse activity (PIRA), according to a recently published international consensus. However, an outside expert said that promulgating the “smoldering” concept may stoke patient and provider confusion.
Although current disease-modifying therapies (DMTs) for MS exclusively target focal white matter (WM) inflammation, wrote authors lead by Antonio Scalfari, MD, PhD, of Charing Cross Hospital, Imperial College London in England, many people with MS experience worsening disability in a more indolent fashion — despite stable inflammatory markers.
“The gradual accumulation of physical and cognitive disability is driven by smoldering pathological processes via biological substrates, which are different from those of acute focal damage, remain an important unmet therapeutic target,” they wrote.
The same research team first described smoldering MS in a 2022 publication. In the present paper, Scalfari and colleagues reviewed emerging clinical, radiological, and pathological evidence and presented 29 consensus statements in areas ranging from the definition, pathology, and clinical manifestations of smoldering MS to appropriate biomarkers and best clinical practices.
Definition
By definition, the authors wrote, SAW encompasses PIRA but also includes a range of gradually worsening, relapse-independent symptoms that remain undetectable on standard assessments, including the Expanded Disability Status Scale (EDSS) or EDSS-Plus, especially in early disease. To capture symptoms such as subtle motor impairment, cognitive slowing, and fatigue, Scalfari and colleagues recommend tools such as neurological stress tests, fatigue/mood scales, wearable devices, and patient reported outcomes.
Disease Mechanisms
Pathologically, the authors wrote, smoldering MS may stem from intrinsic central nervous system processes that likely incorporate various glial, immune, and neural cells. Smoldering MS also could contribute to aging, and vice versa, the latter possibly through dynamics such as age-related exhaustion of compensatory mechanisms, reduction in remyelination efficiency, and telomere shortening, they added.
Clinical Implementation
Current MS management rests on crude estimates of physical disability and overemphasizes identifying relapses and new MRI lesions as the principal markers of disease activity, wrote Scalfari and colleagues. Instead, they suggested combining motor-associated assessments such as EDSS-Plus with cognitive gauges such as the Brief International Cognitive Assessment for Multiple Sclerosis.
Providers are uncomfortable identifying and discussing smoldering MS, authors allowed, because no licensed treatments target SAW. However, the authors wrote, a principal reason for discussing smoldering MS with patients is to help manage their expectations of current DMTs, which may have little effect on SAW.
‘More Than Lesions’
Bruce Cree, MD, PhD, MAS, professor of neurology at the University of California, San Francisco, said that it is extremely important to raise awareness of physicians’ emerging understanding that “there is more going on in MS than lesions and relapses,” a concept that has been a work in progress for several years. He was not involved with the study but was asked to comment.
A 2019 report on the EPIC cohort coauthored by Cree labeled the disconnect between disability accumulation and relapse occurrence “silent progression.” The observation that disability accumulates in early relapsing MS independent of relapsing activity has been replicated in virtually every dataset worldwide, he added.
“What I don’t like about this article is the reliance on the term ‘smoldering’ and the acceptance that this is an actual phenomenon supported by data.” And authors’ leveraging “smoldering” into additional acronyms such as SAW likely will confuse rather than clarify physicians’ and patients’ understanding of the situation, Cree added. “Clinicians don’t need yet another snappy acronym.” Many are still trying to grasp the PIRA concept in relapsing MS, he said.
“One of the reasons this topic has become so important is that we recognize that even when we have very good control of relapsing disease activity — clinical relapses as well as radiographic large lesion formation on MRI — some patients still develop insidious worsening of disability. And the reasons for that are not well understood,” said Cree.
Accumulating disability absent relapse activity could stem from any number of microscopic inflammatory processes, possibly involving abnormal microglial activation, fibrinogen deposition, microscopic inflammatory infiltrates of CD8-positive T cells, or mitochondrial damage from iron deposition, he said. Or the processes driving PIRA may not even involve inflammation, he added. “We still don’t have a unifying way of understanding how these processes work.”
Cree suspects that, despite investigators’ good intentions, the study’s sponsor, Sanofi, may have influenced the resultant messaging. The company’s tolebrutinib recently completed phase 3 trials in secondary progressive MS and relapsing MS, and a phase 3 trial in primary progressive MS is scheduled for completion in 2025. “A hallmark of Sanofi’s messaging has been this idea that there is smoldering inflammation occurring in MS that tolebrutinib is going to address,” he said.
If clinicians really knew what drove progressive MS, said Cree, “we would be keen on developing therapies targeting that fundamental process. But because we don’t know what’s driving it, we don’t know what to go after.”
The study was supported by Sanofi. Cree is a coauthor of the GEMINI 1 and GEMINI 2 tolebrutinib studies.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF NEUROLOGY
Increase in Troublesome Fungal Infections Requires All-Out Approach
As dermatologists, public health officials, and infectious disease specialists scramble to raise awareness about prevention and treatment, challenges ranging from a dearth of testing facilities and data to payer pushback over longer therapeutic courses remain.
Dermatophyte Discourse Changing
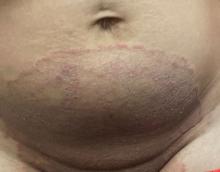
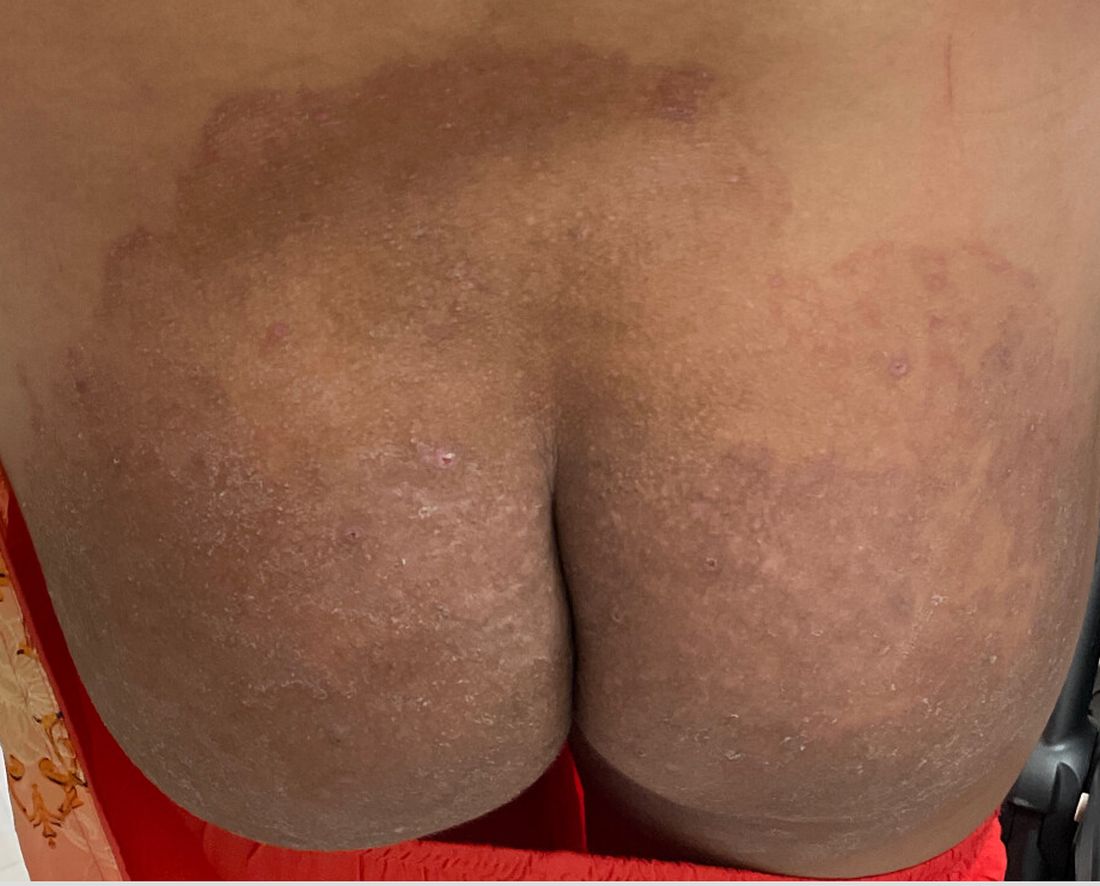
“Trichophyton indotineae is changing the way we talk about dermatophyte infections,” Avrom S. Caplan, MD, assistant professor in the Department of Dermatology at New York University, New York City, said in an interview. Called T mentagrophytes VIII (TMVIII) before a 2020 report in the journal Mycopathologia proposed the name T indotineae, this species requires clinicians to expand their conception of how tinea looks, acts, and responds to treatment.
Boni E. Elewski, MD, professor and chair of dermatology, at The University of Alabama at Birmingham, saw her first case of probable T indotineae in a patient in early 2020. “He was covered with fine scale, and he itched all over. I thought he had atopic dermatitis. This didn’t look like any tinea. His face, arms, back, and legs were scaly.”
Nevertheless, KOH and biopsy confirmed dermatophytosis. Culture (performed at the Center for Medical Mycology [CMM] in Cleveland) identified T mentagrophytes. Back then, Elewski told this news organization, labs did not routinely go beyond genus and species. But based on the patient’s symptoms, history of unresponsiveness to terbinafine, borderline sensitivity to fluconazole, and travel to India and Spain, Elewski strongly suspected T indotineae.
The patient refused itraconazole, to which the fungus was sensitive, and did not respond to fluconazole 400 mg daily. Ultimately, he was lost to follow-up. “Last I saw him,” said Elewski, “he was not cured.”
Tracking Cases
Because T indotineae does not require reporting to public health agencies, said Jeremy Gold, MD, MS, a medical officer with the US Centers for Disease Control and Prevention (CDC) Mycotic Diseases Branch in Atlanta, “there is no official public health surveillance keeping track of exactly how many cases have occurred.”
The same is true for TMVII and terbinafine-resistant T rubrum, which are also on the rise. Regarding T indotineae, authors from the University of Texas Health Science Center at San Antonio retrospectively reported 21 terbinafine-resistant isolates from North America in the July 2023 Journal of Clinical Microbiology .
Caplan has seen approximately 12 T indotineae cases to date, including the first two confirmed US cases, which he and co-authors, including Gold, reported in the CDC’s Morbidity and Mortality Weekly Report in May 2023. T indotineae is likely underreported, he said, because it eludes standard culture-based techniques, and identifying it requires molecular testing, which is available at only a handful of labs nationally.
To help educate providers, in July, the American Academy of Dermatology (AAD) and the International League of Dermatological Societies unveiled an Emerging Diseases Resource Center, which includes resources for providers and a registry for reporting confirmed and suspected resistant dermatophytes.
“Our goal is to provide easy-to-access and easy-to-understand resources to healthcare providers,” Esther Freeman, MD, PhD, told this news organization. She is director of Global Health Dermatology at Massachusetts General Hospital, associate professor of dermatology at Harvard Medical School, both in Boston, and chair of the AAD’s Emerging Diseases Task Force.
“Our resources include an algorithm for when to suspect a drug-resistant case and how to think through treatment options. We cover issues related to diagnosis and treatment, as well as linking to our case registry reporting system,” said Freeman.
The new registry resides within the AAD’s existing COVID-19, Mpox, and Emerging Infections Registry. “Our registry efforts have already captured 2500 COVID-19 and mpox cases from 72 different countries,” Freeman said. For all these infections, she added, “we hope that real-time data analysis of cases worldwide will provide information that helps physicians recognize and treat cases.”
Consistent with the registry’s approach, said Caplan and Gold, there is no silver bullet for battling dermatophyte resistance. What is needed, said Gold, is a coordinated approach involving public health officials, dermatologists, primary care providers, infectious disease specialists, pharmacists, and patients. “It’s going to be a team effort to address the challenge of emerging complex dermatophytosis,” he said.
Resistant T rubrum
“The biggest difference with T rubrum resistance is you may not see that widespread infection that we see with T indotineae,” said Caplan. T rubrum is probably the most common dermatophyte that dermatologists see, added Elewski, who encounters a resistant case at least monthly. One such patient, featured in a January 2021 British Journal of Dermatology research letter, cleared on itraconazole and ciclopirox cream but subsequently returned with itraconazole-resistant T rubrum because he had been doctor-shopping for the drug intermittently for years, she said. He cleared on posaconazole 300 mg daily, then was lost to follow-up.
TMVII
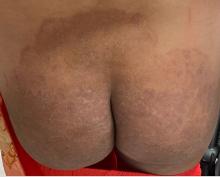
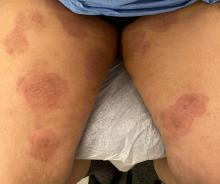
A 2023 Emerging Infectious Diseases report highlighted the potential for this dermatophyte to spread among men who have sex with men (MSM), presenting as an itchy, scaly rash affecting the pubic, genital, and buttocks skin. “People don’t generally think of a fungal infection as something that could behave like a sexually transmitted infection (STI),” said Gold.
Caplan and coauthors recently reported the first confirmed US TMVII case in JAMA Dermatology. Many experts suspect that unreported US cases existed previously, he said. “When it circulates in Europe and there’s so much travel, it’s probably here too.”
The fact that T indotineae was formerly called TMVIII has created confusion, added Caplan. “I’ve had patients say, ‘I’m worried I have that resistant ringworm that’s spreading among MSM.’ Whenever we talk about STIs and introduce the word ‘resistant,’ that comes with the potential for stigma, anxiety, and concern.” Fortunately, he said, TMVII has shown no resistance to first-line antifungals.
Why the Rise
Gold said, “We don’t know for sure why we’re seeing these different drug-resistant species popping up.” One possibility, he said, is the common misuse and overuse of topical antifungals — especially those available overseas in combination with high-potency steroids, such as clobetasol. Consumers use these products for a few weeks until symptoms resolve, then reapply them off and on over years, fueling resistance, said Gold.
“We are worried that with warming temperatures, there’s potential to see expansion of the geographic range of epidemic fungi,” he added. “That could be part of what has fueled recent increases in resistant dermatophytes. But it’s hard to prove.”
Climate change may be behind the emergence of Candida auris, according to a 2022 article in The Lancet Regional Health – Americas. This potentially fatal multidrug-resistant infection spreads easily among sick patients in healthcare facilities, according to a CDC information page on C auris.
Confirming Dermatophyte Infection
“A biopsy will only confirm the presence of fungus,” said Elewski. “Here you will need a lab that knows how to do a fungal culture.” Most state laboratories can do this, she said, as can some hospitals and special labs such as CMM in Cleveland.
It takes a Clinical Laboratory Improvement Amendments–certified lab to perform KOH prep in-house, added Caplan, plus up-to-date gear and knowledge of where and how to scrape and what to look for microscopically. Moreover, identifying T indotineae requires molecular testing available at only a handful of laboratories — listed on the AAD Emerging Dermatophytes webpage — nationwide.
Nevertheless, said Caplan, nailing down a diagnosis can guide treatment, often supplanting empirically prescribed antifungal steroid creams. “Those are probably not going to help. And people may be using those on areas of the body they shouldn’t. Both the clinical clues and the steps to make the diagnosis need to come together. But that’s often easier said than done, especially in a busy practice.”
Identifying resistance requires antifungal sensitivity testing, he added, which few labs perform. “Practically speaking,” said Elewski, “if the patient failed terbinafine, I would try itraconazole. You don’t necessarily need proof” of resistance. But if a patient does not respond to itraconazole and terbinafine clinically, she said that she might consider fungal susceptibility testing.
Treatment Tips
To address any resistant dermatophyte, Elewski recommended getting comfortable with itraconazole. For decades, she said, dermatologists have avoided itraconazole because terbinafine typically costs patients $10 for 3 months. “Itraconazole could be $200 per month,” said Elewski. Because of potential drug-drug interactions and absorption issues — and a boxed warning regarding congestive heart failure — physicians historically reserved itraconazole for severe fungal infections.
Itraconazole labeled dosing for onychomycosis is 200 mg daily for 12 weeks. Elewski favors a two-pronged attack, often combining an -azole antifungal with topical ciclopirox.
Another element that emerging tinea pathogens share is slower response to treatment. For T indotineae, reports appearing in the Journal of the American Academy of Dermatology in 2022 and 2024 suggest duration from 6-8 weeks up to 20 weeks.
To avoid recurrences of resistant T rubrum, Elewski treats for a year. However, she has problems getting itraconazole approved, when often it is the only agent that works. “I’ve written more letters than I like to insurance companies” to document terbinafine failure, she said.
Rarely, said Gold, dermatophyte infections resist both terbinafine and itraconazole. Next-line agents such as voriconazole, which some dermatologists have used for resistant T indotineae, can be much harder to tolerate, with more drug interactions, he said.
And because itraconazole, voriconazole, and posaconazole are all triazoles, added Elewski, the latter two might not work better than the former. But because these drugs might outperform itraconazole in selected cases, she said, “that’s when you want to do fungal susceptibility testing.”
TMVII is so new, said Caplan, that optimal therapy duration remains unclear. “One of the challenges with TMVII is when it gets into the genital skin, it’s a hair-bearing area. And based on various grooming practices, there’s an opportunity for the tinea to get deeper into the hair follicle and dermis. That may also be true of T indotineae.”
Anemic Arsenal
Unfortunately, said Gold, the arsenal of antifungals available in the United States remains limited. “Depending on how you count, there are only three to four classes of antifungal drugs designed to treat severe or invasive infections. So whenever we hear about a new fungal pathogen that’s causing resistant infections, it causes public health concern.”
Promising drugs in development include olorofim (F2G) and fosmanogepix (Basilea), according to Gold. However, he said, the development of these drugs to date has targeted invasive fungal infections such as aspergillosis. In June 2023, the Food and Drug Administration rejected the new drug application for olorofim, requesting additional data and analyses. Regarding fosmanogepix, a double-blinded noninferiority phase 3 trial in invasive yeast infections was recently launched, according to a September 24 press release.
Gold, Caplan, and Elewski reported no relevant financial disclosures. Freeman is a COVID-19 co-author for UpToDate and chair of the AAD Emerging Diseases Task Force.
A version of this article appeared on Medscape.com.
As dermatologists, public health officials, and infectious disease specialists scramble to raise awareness about prevention and treatment, challenges ranging from a dearth of testing facilities and data to payer pushback over longer therapeutic courses remain.
Dermatophyte Discourse Changing
“Trichophyton indotineae is changing the way we talk about dermatophyte infections,” Avrom S. Caplan, MD, assistant professor in the Department of Dermatology at New York University, New York City, said in an interview. Called T mentagrophytes VIII (TMVIII) before a 2020 report in the journal Mycopathologia proposed the name T indotineae, this species requires clinicians to expand their conception of how tinea looks, acts, and responds to treatment.
Boni E. Elewski, MD, professor and chair of dermatology, at The University of Alabama at Birmingham, saw her first case of probable T indotineae in a patient in early 2020. “He was covered with fine scale, and he itched all over. I thought he had atopic dermatitis. This didn’t look like any tinea. His face, arms, back, and legs were scaly.”
Nevertheless, KOH and biopsy confirmed dermatophytosis. Culture (performed at the Center for Medical Mycology [CMM] in Cleveland) identified T mentagrophytes. Back then, Elewski told this news organization, labs did not routinely go beyond genus and species. But based on the patient’s symptoms, history of unresponsiveness to terbinafine, borderline sensitivity to fluconazole, and travel to India and Spain, Elewski strongly suspected T indotineae.
The patient refused itraconazole, to which the fungus was sensitive, and did not respond to fluconazole 400 mg daily. Ultimately, he was lost to follow-up. “Last I saw him,” said Elewski, “he was not cured.”
Tracking Cases
Because T indotineae does not require reporting to public health agencies, said Jeremy Gold, MD, MS, a medical officer with the US Centers for Disease Control and Prevention (CDC) Mycotic Diseases Branch in Atlanta, “there is no official public health surveillance keeping track of exactly how many cases have occurred.”
The same is true for TMVII and terbinafine-resistant T rubrum, which are also on the rise. Regarding T indotineae, authors from the University of Texas Health Science Center at San Antonio retrospectively reported 21 terbinafine-resistant isolates from North America in the July 2023 Journal of Clinical Microbiology .
Caplan has seen approximately 12 T indotineae cases to date, including the first two confirmed US cases, which he and co-authors, including Gold, reported in the CDC’s Morbidity and Mortality Weekly Report in May 2023. T indotineae is likely underreported, he said, because it eludes standard culture-based techniques, and identifying it requires molecular testing, which is available at only a handful of labs nationally.
To help educate providers, in July, the American Academy of Dermatology (AAD) and the International League of Dermatological Societies unveiled an Emerging Diseases Resource Center, which includes resources for providers and a registry for reporting confirmed and suspected resistant dermatophytes.
“Our goal is to provide easy-to-access and easy-to-understand resources to healthcare providers,” Esther Freeman, MD, PhD, told this news organization. She is director of Global Health Dermatology at Massachusetts General Hospital, associate professor of dermatology at Harvard Medical School, both in Boston, and chair of the AAD’s Emerging Diseases Task Force.
“Our resources include an algorithm for when to suspect a drug-resistant case and how to think through treatment options. We cover issues related to diagnosis and treatment, as well as linking to our case registry reporting system,” said Freeman.
The new registry resides within the AAD’s existing COVID-19, Mpox, and Emerging Infections Registry. “Our registry efforts have already captured 2500 COVID-19 and mpox cases from 72 different countries,” Freeman said. For all these infections, she added, “we hope that real-time data analysis of cases worldwide will provide information that helps physicians recognize and treat cases.”
Consistent with the registry’s approach, said Caplan and Gold, there is no silver bullet for battling dermatophyte resistance. What is needed, said Gold, is a coordinated approach involving public health officials, dermatologists, primary care providers, infectious disease specialists, pharmacists, and patients. “It’s going to be a team effort to address the challenge of emerging complex dermatophytosis,” he said.
Resistant T rubrum
“The biggest difference with T rubrum resistance is you may not see that widespread infection that we see with T indotineae,” said Caplan. T rubrum is probably the most common dermatophyte that dermatologists see, added Elewski, who encounters a resistant case at least monthly. One such patient, featured in a January 2021 British Journal of Dermatology research letter, cleared on itraconazole and ciclopirox cream but subsequently returned with itraconazole-resistant T rubrum because he had been doctor-shopping for the drug intermittently for years, she said. He cleared on posaconazole 300 mg daily, then was lost to follow-up.
TMVII
A 2023 Emerging Infectious Diseases report highlighted the potential for this dermatophyte to spread among men who have sex with men (MSM), presenting as an itchy, scaly rash affecting the pubic, genital, and buttocks skin. “People don’t generally think of a fungal infection as something that could behave like a sexually transmitted infection (STI),” said Gold.
Caplan and coauthors recently reported the first confirmed US TMVII case in JAMA Dermatology. Many experts suspect that unreported US cases existed previously, he said. “When it circulates in Europe and there’s so much travel, it’s probably here too.”
The fact that T indotineae was formerly called TMVIII has created confusion, added Caplan. “I’ve had patients say, ‘I’m worried I have that resistant ringworm that’s spreading among MSM.’ Whenever we talk about STIs and introduce the word ‘resistant,’ that comes with the potential for stigma, anxiety, and concern.” Fortunately, he said, TMVII has shown no resistance to first-line antifungals.
Why the Rise
Gold said, “We don’t know for sure why we’re seeing these different drug-resistant species popping up.” One possibility, he said, is the common misuse and overuse of topical antifungals — especially those available overseas in combination with high-potency steroids, such as clobetasol. Consumers use these products for a few weeks until symptoms resolve, then reapply them off and on over years, fueling resistance, said Gold.
“We are worried that with warming temperatures, there’s potential to see expansion of the geographic range of epidemic fungi,” he added. “That could be part of what has fueled recent increases in resistant dermatophytes. But it’s hard to prove.”
Climate change may be behind the emergence of Candida auris, according to a 2022 article in The Lancet Regional Health – Americas. This potentially fatal multidrug-resistant infection spreads easily among sick patients in healthcare facilities, according to a CDC information page on C auris.
Confirming Dermatophyte Infection
“A biopsy will only confirm the presence of fungus,” said Elewski. “Here you will need a lab that knows how to do a fungal culture.” Most state laboratories can do this, she said, as can some hospitals and special labs such as CMM in Cleveland.
It takes a Clinical Laboratory Improvement Amendments–certified lab to perform KOH prep in-house, added Caplan, plus up-to-date gear and knowledge of where and how to scrape and what to look for microscopically. Moreover, identifying T indotineae requires molecular testing available at only a handful of laboratories — listed on the AAD Emerging Dermatophytes webpage — nationwide.
Nevertheless, said Caplan, nailing down a diagnosis can guide treatment, often supplanting empirically prescribed antifungal steroid creams. “Those are probably not going to help. And people may be using those on areas of the body they shouldn’t. Both the clinical clues and the steps to make the diagnosis need to come together. But that’s often easier said than done, especially in a busy practice.”
Identifying resistance requires antifungal sensitivity testing, he added, which few labs perform. “Practically speaking,” said Elewski, “if the patient failed terbinafine, I would try itraconazole. You don’t necessarily need proof” of resistance. But if a patient does not respond to itraconazole and terbinafine clinically, she said that she might consider fungal susceptibility testing.
Treatment Tips
To address any resistant dermatophyte, Elewski recommended getting comfortable with itraconazole. For decades, she said, dermatologists have avoided itraconazole because terbinafine typically costs patients $10 for 3 months. “Itraconazole could be $200 per month,” said Elewski. Because of potential drug-drug interactions and absorption issues — and a boxed warning regarding congestive heart failure — physicians historically reserved itraconazole for severe fungal infections.
Itraconazole labeled dosing for onychomycosis is 200 mg daily for 12 weeks. Elewski favors a two-pronged attack, often combining an -azole antifungal with topical ciclopirox.
Another element that emerging tinea pathogens share is slower response to treatment. For T indotineae, reports appearing in the Journal of the American Academy of Dermatology in 2022 and 2024 suggest duration from 6-8 weeks up to 20 weeks.
To avoid recurrences of resistant T rubrum, Elewski treats for a year. However, she has problems getting itraconazole approved, when often it is the only agent that works. “I’ve written more letters than I like to insurance companies” to document terbinafine failure, she said.
Rarely, said Gold, dermatophyte infections resist both terbinafine and itraconazole. Next-line agents such as voriconazole, which some dermatologists have used for resistant T indotineae, can be much harder to tolerate, with more drug interactions, he said.
And because itraconazole, voriconazole, and posaconazole are all triazoles, added Elewski, the latter two might not work better than the former. But because these drugs might outperform itraconazole in selected cases, she said, “that’s when you want to do fungal susceptibility testing.”
TMVII is so new, said Caplan, that optimal therapy duration remains unclear. “One of the challenges with TMVII is when it gets into the genital skin, it’s a hair-bearing area. And based on various grooming practices, there’s an opportunity for the tinea to get deeper into the hair follicle and dermis. That may also be true of T indotineae.”
Anemic Arsenal
Unfortunately, said Gold, the arsenal of antifungals available in the United States remains limited. “Depending on how you count, there are only three to four classes of antifungal drugs designed to treat severe or invasive infections. So whenever we hear about a new fungal pathogen that’s causing resistant infections, it causes public health concern.”
Promising drugs in development include olorofim (F2G) and fosmanogepix (Basilea), according to Gold. However, he said, the development of these drugs to date has targeted invasive fungal infections such as aspergillosis. In June 2023, the Food and Drug Administration rejected the new drug application for olorofim, requesting additional data and analyses. Regarding fosmanogepix, a double-blinded noninferiority phase 3 trial in invasive yeast infections was recently launched, according to a September 24 press release.
Gold, Caplan, and Elewski reported no relevant financial disclosures. Freeman is a COVID-19 co-author for UpToDate and chair of the AAD Emerging Diseases Task Force.
A version of this article appeared on Medscape.com.
As dermatologists, public health officials, and infectious disease specialists scramble to raise awareness about prevention and treatment, challenges ranging from a dearth of testing facilities and data to payer pushback over longer therapeutic courses remain.
Dermatophyte Discourse Changing
“Trichophyton indotineae is changing the way we talk about dermatophyte infections,” Avrom S. Caplan, MD, assistant professor in the Department of Dermatology at New York University, New York City, said in an interview. Called T mentagrophytes VIII (TMVIII) before a 2020 report in the journal Mycopathologia proposed the name T indotineae, this species requires clinicians to expand their conception of how tinea looks, acts, and responds to treatment.
Boni E. Elewski, MD, professor and chair of dermatology, at The University of Alabama at Birmingham, saw her first case of probable T indotineae in a patient in early 2020. “He was covered with fine scale, and he itched all over. I thought he had atopic dermatitis. This didn’t look like any tinea. His face, arms, back, and legs were scaly.”
Nevertheless, KOH and biopsy confirmed dermatophytosis. Culture (performed at the Center for Medical Mycology [CMM] in Cleveland) identified T mentagrophytes. Back then, Elewski told this news organization, labs did not routinely go beyond genus and species. But based on the patient’s symptoms, history of unresponsiveness to terbinafine, borderline sensitivity to fluconazole, and travel to India and Spain, Elewski strongly suspected T indotineae.
The patient refused itraconazole, to which the fungus was sensitive, and did not respond to fluconazole 400 mg daily. Ultimately, he was lost to follow-up. “Last I saw him,” said Elewski, “he was not cured.”
Tracking Cases
Because T indotineae does not require reporting to public health agencies, said Jeremy Gold, MD, MS, a medical officer with the US Centers for Disease Control and Prevention (CDC) Mycotic Diseases Branch in Atlanta, “there is no official public health surveillance keeping track of exactly how many cases have occurred.”
The same is true for TMVII and terbinafine-resistant T rubrum, which are also on the rise. Regarding T indotineae, authors from the University of Texas Health Science Center at San Antonio retrospectively reported 21 terbinafine-resistant isolates from North America in the July 2023 Journal of Clinical Microbiology .
Caplan has seen approximately 12 T indotineae cases to date, including the first two confirmed US cases, which he and co-authors, including Gold, reported in the CDC’s Morbidity and Mortality Weekly Report in May 2023. T indotineae is likely underreported, he said, because it eludes standard culture-based techniques, and identifying it requires molecular testing, which is available at only a handful of labs nationally.
To help educate providers, in July, the American Academy of Dermatology (AAD) and the International League of Dermatological Societies unveiled an Emerging Diseases Resource Center, which includes resources for providers and a registry for reporting confirmed and suspected resistant dermatophytes.
“Our goal is to provide easy-to-access and easy-to-understand resources to healthcare providers,” Esther Freeman, MD, PhD, told this news organization. She is director of Global Health Dermatology at Massachusetts General Hospital, associate professor of dermatology at Harvard Medical School, both in Boston, and chair of the AAD’s Emerging Diseases Task Force.
“Our resources include an algorithm for when to suspect a drug-resistant case and how to think through treatment options. We cover issues related to diagnosis and treatment, as well as linking to our case registry reporting system,” said Freeman.
The new registry resides within the AAD’s existing COVID-19, Mpox, and Emerging Infections Registry. “Our registry efforts have already captured 2500 COVID-19 and mpox cases from 72 different countries,” Freeman said. For all these infections, she added, “we hope that real-time data analysis of cases worldwide will provide information that helps physicians recognize and treat cases.”
Consistent with the registry’s approach, said Caplan and Gold, there is no silver bullet for battling dermatophyte resistance. What is needed, said Gold, is a coordinated approach involving public health officials, dermatologists, primary care providers, infectious disease specialists, pharmacists, and patients. “It’s going to be a team effort to address the challenge of emerging complex dermatophytosis,” he said.
Resistant T rubrum
“The biggest difference with T rubrum resistance is you may not see that widespread infection that we see with T indotineae,” said Caplan. T rubrum is probably the most common dermatophyte that dermatologists see, added Elewski, who encounters a resistant case at least monthly. One such patient, featured in a January 2021 British Journal of Dermatology research letter, cleared on itraconazole and ciclopirox cream but subsequently returned with itraconazole-resistant T rubrum because he had been doctor-shopping for the drug intermittently for years, she said. He cleared on posaconazole 300 mg daily, then was lost to follow-up.
TMVII
A 2023 Emerging Infectious Diseases report highlighted the potential for this dermatophyte to spread among men who have sex with men (MSM), presenting as an itchy, scaly rash affecting the pubic, genital, and buttocks skin. “People don’t generally think of a fungal infection as something that could behave like a sexually transmitted infection (STI),” said Gold.
Caplan and coauthors recently reported the first confirmed US TMVII case in JAMA Dermatology. Many experts suspect that unreported US cases existed previously, he said. “When it circulates in Europe and there’s so much travel, it’s probably here too.”
The fact that T indotineae was formerly called TMVIII has created confusion, added Caplan. “I’ve had patients say, ‘I’m worried I have that resistant ringworm that’s spreading among MSM.’ Whenever we talk about STIs and introduce the word ‘resistant,’ that comes with the potential for stigma, anxiety, and concern.” Fortunately, he said, TMVII has shown no resistance to first-line antifungals.
Why the Rise
Gold said, “We don’t know for sure why we’re seeing these different drug-resistant species popping up.” One possibility, he said, is the common misuse and overuse of topical antifungals — especially those available overseas in combination with high-potency steroids, such as clobetasol. Consumers use these products for a few weeks until symptoms resolve, then reapply them off and on over years, fueling resistance, said Gold.
“We are worried that with warming temperatures, there’s potential to see expansion of the geographic range of epidemic fungi,” he added. “That could be part of what has fueled recent increases in resistant dermatophytes. But it’s hard to prove.”
Climate change may be behind the emergence of Candida auris, according to a 2022 article in The Lancet Regional Health – Americas. This potentially fatal multidrug-resistant infection spreads easily among sick patients in healthcare facilities, according to a CDC information page on C auris.
Confirming Dermatophyte Infection
“A biopsy will only confirm the presence of fungus,” said Elewski. “Here you will need a lab that knows how to do a fungal culture.” Most state laboratories can do this, she said, as can some hospitals and special labs such as CMM in Cleveland.
It takes a Clinical Laboratory Improvement Amendments–certified lab to perform KOH prep in-house, added Caplan, plus up-to-date gear and knowledge of where and how to scrape and what to look for microscopically. Moreover, identifying T indotineae requires molecular testing available at only a handful of laboratories — listed on the AAD Emerging Dermatophytes webpage — nationwide.
Nevertheless, said Caplan, nailing down a diagnosis can guide treatment, often supplanting empirically prescribed antifungal steroid creams. “Those are probably not going to help. And people may be using those on areas of the body they shouldn’t. Both the clinical clues and the steps to make the diagnosis need to come together. But that’s often easier said than done, especially in a busy practice.”
Identifying resistance requires antifungal sensitivity testing, he added, which few labs perform. “Practically speaking,” said Elewski, “if the patient failed terbinafine, I would try itraconazole. You don’t necessarily need proof” of resistance. But if a patient does not respond to itraconazole and terbinafine clinically, she said that she might consider fungal susceptibility testing.
Treatment Tips
To address any resistant dermatophyte, Elewski recommended getting comfortable with itraconazole. For decades, she said, dermatologists have avoided itraconazole because terbinafine typically costs patients $10 for 3 months. “Itraconazole could be $200 per month,” said Elewski. Because of potential drug-drug interactions and absorption issues — and a boxed warning regarding congestive heart failure — physicians historically reserved itraconazole for severe fungal infections.
Itraconazole labeled dosing for onychomycosis is 200 mg daily for 12 weeks. Elewski favors a two-pronged attack, often combining an -azole antifungal with topical ciclopirox.
Another element that emerging tinea pathogens share is slower response to treatment. For T indotineae, reports appearing in the Journal of the American Academy of Dermatology in 2022 and 2024 suggest duration from 6-8 weeks up to 20 weeks.
To avoid recurrences of resistant T rubrum, Elewski treats for a year. However, she has problems getting itraconazole approved, when often it is the only agent that works. “I’ve written more letters than I like to insurance companies” to document terbinafine failure, she said.
Rarely, said Gold, dermatophyte infections resist both terbinafine and itraconazole. Next-line agents such as voriconazole, which some dermatologists have used for resistant T indotineae, can be much harder to tolerate, with more drug interactions, he said.
And because itraconazole, voriconazole, and posaconazole are all triazoles, added Elewski, the latter two might not work better than the former. But because these drugs might outperform itraconazole in selected cases, she said, “that’s when you want to do fungal susceptibility testing.”
TMVII is so new, said Caplan, that optimal therapy duration remains unclear. “One of the challenges with TMVII is when it gets into the genital skin, it’s a hair-bearing area. And based on various grooming practices, there’s an opportunity for the tinea to get deeper into the hair follicle and dermis. That may also be true of T indotineae.”
Anemic Arsenal
Unfortunately, said Gold, the arsenal of antifungals available in the United States remains limited. “Depending on how you count, there are only three to four classes of antifungal drugs designed to treat severe or invasive infections. So whenever we hear about a new fungal pathogen that’s causing resistant infections, it causes public health concern.”
Promising drugs in development include olorofim (F2G) and fosmanogepix (Basilea), according to Gold. However, he said, the development of these drugs to date has targeted invasive fungal infections such as aspergillosis. In June 2023, the Food and Drug Administration rejected the new drug application for olorofim, requesting additional data and analyses. Regarding fosmanogepix, a double-blinded noninferiority phase 3 trial in invasive yeast infections was recently launched, according to a September 24 press release.
Gold, Caplan, and Elewski reported no relevant financial disclosures. Freeman is a COVID-19 co-author for UpToDate and chair of the AAD Emerging Diseases Task Force.
A version of this article appeared on Medscape.com.
Erenumab Reduces Nonopioid Medication Overuse Headache in Chronic Migraine
In a recent study of 6 monthly injections of 140 mg erenumab (Aimovig, Amgen), most patients with chronic migraine and nonopioid medication overuse headache (MOH) achieved remission. Published online in JAMA Neurology, the study is the first prospective, double-blind, randomized, placebo-controlled attempt to investigate patients with chronic migraine and MOH related to nonopioid medications, according to lead author Stewart J. Tepper, MD, and his coauthors.
Prior Studies Did Not Focus on MOH
Several prior phase 2 and 3 trials of calcitonin gene-related peptide (CGRP) ligand or receptor inhibitors that have been FDA-approved for migraine prevention have been performed. These drugs include erenumab, fremanezumab (Ajovy, Teva), galcanezumab (Emgality, Lilly), and eptinezumab (Vyepti, Lundbeck), for patients with and without medication overuse, said Alan M. Rapoport, MD, who was not involved with the new study. Dr. Rapoport is a clinical professor of neurology at the David Geffen School of Medicine of the University of California, in Los Angeles; past president of the International Headache Society; and founder and director emeritus of The New England Center for Headache in Stamford, Connecticut.
“But we could not call them patients with MOH because they weren’t studied prospectively, so that they had medication overuse according to International Classification of Headache Disorders (ICHD-3) criteria,” said Dr. Rapoport.
Phase 4, Randomized, Placebo-Controlled Trial
In the present clinical trial, investigators enrolled 584 patients with nonopioid MOH and history of failing at least one preventive treatment. After a 4-week baseline phase, researchers randomized patients 1:1:1 to 6 months’ treatment with erenumab 70 mg, erenumab 140 mg, or placebo.
Investigators defined remission as either of the following through months 4-6:
- < 10 mean monthly acute headache medication days per month (AHMD)
- < 14 mean monthly headache days (MHD)
In the primary analysis, 69.1% of patients in the 140 mg cohort achieved remission (P < .001) versus placebo. Remission rates in the 70 mg and the placebo cohorts were 60.3% (P < .13) and 52.6%, respectively. AHMD for the 140-mg, 70-mg, and placebo groups fell by 9.4, 7.8, and 6.6 days per month, respectively. Migraine Physical Function Impact Diary (non-EU sites) and Headache Impact Test-6 (EU sites) scores also showed greater improvement for patients treated with erenumab.
No new safety signals emerged, although erenumab-treated participants experienced 2-2.5 times as much COVID-19 disease.
Regarding the primary endpoint, said Dr. Rapoport, the 70-mg dose might also have yielded statistically significant improvement over placebo with a larger sample size. “I have seen that the higher dose of erenumab can be superior for efficacy than the lower in some of the double-blind trials,” he said. The 52.6% placebo response rate was rather high, he added, but not necessarily higher than in other migraine prevention trials.
“Placebo is a type of treatment,” Dr. Rapoport said. “It’s not as strong as the actual medication, which is specific for prevention, but it does work on the brain to some extent.”
He was more concerned, however, that authors did not counsel study patients about reducing or discontinuing their overused medications in a unified manner. Rather, it was left to individual investigators’ discretion, in different countries, as to whether to educate patients about the harms of medication overuse. “The fascinating aspect of this paper was that no patient was asked to detoxify from the overused medication,” said Dr. Rapoport, “and yet so many patients no longer had MOH at 6 months.”
Detox Versus No Detox
In a pioneering study of migraine medication overuse headache (then called rebound headache) published by Lee Kudrow, MD, in Advances in Neurology in 1982, patients who discontinued the overused medication fared much better than those who did not. Adding amitriptyline for migraine prevention further improved results, mostly in those who discontinued their overused medication.
Anticipating possible concerns, the authors wrote that their approach “may also be seen as a strength, as it represents a scenario closer to real life and avoids undue interference with the physician-patient relationship.” Indeed, said Dr. Rapoport, study results are perhaps more impressive because they were achieved through treatment with erenumab alone, without detoxification.
Managing Chronic Migraine and MOH
Until erenumab’s 2018 approval, migraine prevention options were limited to tricyclic antidepressants, beta blockers, and antiseizure medicines – though these medicines never seemed to work very well without detoxification, said Dr. Rapoport. Neurologists still use these categories for migraine prevention, he added, “because insurance companies insist that before we give the more expensive, newer medications like those that block CGRP, patients must fail 2 of those 3 categories of older medications which are not approved for chronic migraine.” Only onabotulinumtoxinA (Botox) is FDA-approved for chronic migraine. “There has been no head-to-head comparison of it and any of the monoclonal antibodies against CGRP,” he said.
In a March 2024 publication in Headache, the American Headache Society stated that requiring patients to fail older drugs is inappropriate, and that CGRP inhibitors, though costly, should be first-line for headache prevention. The key advantage of any drug that blocks CGRP in treating MOH is that unlike older drugs, CGRP inhibitors appear to work well even without detoxification, said Dr. Rapoport.
Additional study limitations included the possibility that the 24-week treatment period might not have allowed complete evaluation of long-term efficacy, the authors wrote. “These are usually pretty sick patients,” said Dr. Rapoport, who acknowledged the difficulty of keeping placebo patients off preventive medication altogether for 6 months. The study was extended to 12 months, and the results of an opiate overusers cohort also will be published.
Authors noted that according to a study published in Headache in 2022, most Americans with chronic migraine commonly go without preventive medications. Moreover, such medications do not always work. Accordingly, Dr. Rapoport said, the study duration was reasonable provided patients understood that they had a 33% chance of receiving no effective preventive medication over 6 months.
Extending the study’s month-long baseline period to 3 months before starting erenumab might have been helpful, he added, as that is the timeframe required to confirm MOH diagnosis according to ICHD-3. “However,” said Dr. Rapoport, “3 months with only usual medications, and then 1/3 of patients going 6-12 months with only placebo, would be tough for some patients.”
Dr. Rapoport reports no relevant financial conflicts.
In a recent study of 6 monthly injections of 140 mg erenumab (Aimovig, Amgen), most patients with chronic migraine and nonopioid medication overuse headache (MOH) achieved remission. Published online in JAMA Neurology, the study is the first prospective, double-blind, randomized, placebo-controlled attempt to investigate patients with chronic migraine and MOH related to nonopioid medications, according to lead author Stewart J. Tepper, MD, and his coauthors.
Prior Studies Did Not Focus on MOH
Several prior phase 2 and 3 trials of calcitonin gene-related peptide (CGRP) ligand or receptor inhibitors that have been FDA-approved for migraine prevention have been performed. These drugs include erenumab, fremanezumab (Ajovy, Teva), galcanezumab (Emgality, Lilly), and eptinezumab (Vyepti, Lundbeck), for patients with and without medication overuse, said Alan M. Rapoport, MD, who was not involved with the new study. Dr. Rapoport is a clinical professor of neurology at the David Geffen School of Medicine of the University of California, in Los Angeles; past president of the International Headache Society; and founder and director emeritus of The New England Center for Headache in Stamford, Connecticut.
“But we could not call them patients with MOH because they weren’t studied prospectively, so that they had medication overuse according to International Classification of Headache Disorders (ICHD-3) criteria,” said Dr. Rapoport.
Phase 4, Randomized, Placebo-Controlled Trial
In the present clinical trial, investigators enrolled 584 patients with nonopioid MOH and history of failing at least one preventive treatment. After a 4-week baseline phase, researchers randomized patients 1:1:1 to 6 months’ treatment with erenumab 70 mg, erenumab 140 mg, or placebo.
Investigators defined remission as either of the following through months 4-6:
- < 10 mean monthly acute headache medication days per month (AHMD)
- < 14 mean monthly headache days (MHD)
In the primary analysis, 69.1% of patients in the 140 mg cohort achieved remission (P < .001) versus placebo. Remission rates in the 70 mg and the placebo cohorts were 60.3% (P < .13) and 52.6%, respectively. AHMD for the 140-mg, 70-mg, and placebo groups fell by 9.4, 7.8, and 6.6 days per month, respectively. Migraine Physical Function Impact Diary (non-EU sites) and Headache Impact Test-6 (EU sites) scores also showed greater improvement for patients treated with erenumab.
No new safety signals emerged, although erenumab-treated participants experienced 2-2.5 times as much COVID-19 disease.
Regarding the primary endpoint, said Dr. Rapoport, the 70-mg dose might also have yielded statistically significant improvement over placebo with a larger sample size. “I have seen that the higher dose of erenumab can be superior for efficacy than the lower in some of the double-blind trials,” he said. The 52.6% placebo response rate was rather high, he added, but not necessarily higher than in other migraine prevention trials.
“Placebo is a type of treatment,” Dr. Rapoport said. “It’s not as strong as the actual medication, which is specific for prevention, but it does work on the brain to some extent.”
He was more concerned, however, that authors did not counsel study patients about reducing or discontinuing their overused medications in a unified manner. Rather, it was left to individual investigators’ discretion, in different countries, as to whether to educate patients about the harms of medication overuse. “The fascinating aspect of this paper was that no patient was asked to detoxify from the overused medication,” said Dr. Rapoport, “and yet so many patients no longer had MOH at 6 months.”
Detox Versus No Detox
In a pioneering study of migraine medication overuse headache (then called rebound headache) published by Lee Kudrow, MD, in Advances in Neurology in 1982, patients who discontinued the overused medication fared much better than those who did not. Adding amitriptyline for migraine prevention further improved results, mostly in those who discontinued their overused medication.
Anticipating possible concerns, the authors wrote that their approach “may also be seen as a strength, as it represents a scenario closer to real life and avoids undue interference with the physician-patient relationship.” Indeed, said Dr. Rapoport, study results are perhaps more impressive because they were achieved through treatment with erenumab alone, without detoxification.
Managing Chronic Migraine and MOH
Until erenumab’s 2018 approval, migraine prevention options were limited to tricyclic antidepressants, beta blockers, and antiseizure medicines – though these medicines never seemed to work very well without detoxification, said Dr. Rapoport. Neurologists still use these categories for migraine prevention, he added, “because insurance companies insist that before we give the more expensive, newer medications like those that block CGRP, patients must fail 2 of those 3 categories of older medications which are not approved for chronic migraine.” Only onabotulinumtoxinA (Botox) is FDA-approved for chronic migraine. “There has been no head-to-head comparison of it and any of the monoclonal antibodies against CGRP,” he said.
In a March 2024 publication in Headache, the American Headache Society stated that requiring patients to fail older drugs is inappropriate, and that CGRP inhibitors, though costly, should be first-line for headache prevention. The key advantage of any drug that blocks CGRP in treating MOH is that unlike older drugs, CGRP inhibitors appear to work well even without detoxification, said Dr. Rapoport.
Additional study limitations included the possibility that the 24-week treatment period might not have allowed complete evaluation of long-term efficacy, the authors wrote. “These are usually pretty sick patients,” said Dr. Rapoport, who acknowledged the difficulty of keeping placebo patients off preventive medication altogether for 6 months. The study was extended to 12 months, and the results of an opiate overusers cohort also will be published.
Authors noted that according to a study published in Headache in 2022, most Americans with chronic migraine commonly go without preventive medications. Moreover, such medications do not always work. Accordingly, Dr. Rapoport said, the study duration was reasonable provided patients understood that they had a 33% chance of receiving no effective preventive medication over 6 months.
Extending the study’s month-long baseline period to 3 months before starting erenumab might have been helpful, he added, as that is the timeframe required to confirm MOH diagnosis according to ICHD-3. “However,” said Dr. Rapoport, “3 months with only usual medications, and then 1/3 of patients going 6-12 months with only placebo, would be tough for some patients.”
Dr. Rapoport reports no relevant financial conflicts.
In a recent study of 6 monthly injections of 140 mg erenumab (Aimovig, Amgen), most patients with chronic migraine and nonopioid medication overuse headache (MOH) achieved remission. Published online in JAMA Neurology, the study is the first prospective, double-blind, randomized, placebo-controlled attempt to investigate patients with chronic migraine and MOH related to nonopioid medications, according to lead author Stewart J. Tepper, MD, and his coauthors.
Prior Studies Did Not Focus on MOH
Several prior phase 2 and 3 trials of calcitonin gene-related peptide (CGRP) ligand or receptor inhibitors that have been FDA-approved for migraine prevention have been performed. These drugs include erenumab, fremanezumab (Ajovy, Teva), galcanezumab (Emgality, Lilly), and eptinezumab (Vyepti, Lundbeck), for patients with and without medication overuse, said Alan M. Rapoport, MD, who was not involved with the new study. Dr. Rapoport is a clinical professor of neurology at the David Geffen School of Medicine of the University of California, in Los Angeles; past president of the International Headache Society; and founder and director emeritus of The New England Center for Headache in Stamford, Connecticut.
“But we could not call them patients with MOH because they weren’t studied prospectively, so that they had medication overuse according to International Classification of Headache Disorders (ICHD-3) criteria,” said Dr. Rapoport.
Phase 4, Randomized, Placebo-Controlled Trial
In the present clinical trial, investigators enrolled 584 patients with nonopioid MOH and history of failing at least one preventive treatment. After a 4-week baseline phase, researchers randomized patients 1:1:1 to 6 months’ treatment with erenumab 70 mg, erenumab 140 mg, or placebo.
Investigators defined remission as either of the following through months 4-6:
- < 10 mean monthly acute headache medication days per month (AHMD)
- < 14 mean monthly headache days (MHD)
In the primary analysis, 69.1% of patients in the 140 mg cohort achieved remission (P < .001) versus placebo. Remission rates in the 70 mg and the placebo cohorts were 60.3% (P < .13) and 52.6%, respectively. AHMD for the 140-mg, 70-mg, and placebo groups fell by 9.4, 7.8, and 6.6 days per month, respectively. Migraine Physical Function Impact Diary (non-EU sites) and Headache Impact Test-6 (EU sites) scores also showed greater improvement for patients treated with erenumab.
No new safety signals emerged, although erenumab-treated participants experienced 2-2.5 times as much COVID-19 disease.
Regarding the primary endpoint, said Dr. Rapoport, the 70-mg dose might also have yielded statistically significant improvement over placebo with a larger sample size. “I have seen that the higher dose of erenumab can be superior for efficacy than the lower in some of the double-blind trials,” he said. The 52.6% placebo response rate was rather high, he added, but not necessarily higher than in other migraine prevention trials.
“Placebo is a type of treatment,” Dr. Rapoport said. “It’s not as strong as the actual medication, which is specific for prevention, but it does work on the brain to some extent.”
He was more concerned, however, that authors did not counsel study patients about reducing or discontinuing their overused medications in a unified manner. Rather, it was left to individual investigators’ discretion, in different countries, as to whether to educate patients about the harms of medication overuse. “The fascinating aspect of this paper was that no patient was asked to detoxify from the overused medication,” said Dr. Rapoport, “and yet so many patients no longer had MOH at 6 months.”
Detox Versus No Detox
In a pioneering study of migraine medication overuse headache (then called rebound headache) published by Lee Kudrow, MD, in Advances in Neurology in 1982, patients who discontinued the overused medication fared much better than those who did not. Adding amitriptyline for migraine prevention further improved results, mostly in those who discontinued their overused medication.
Anticipating possible concerns, the authors wrote that their approach “may also be seen as a strength, as it represents a scenario closer to real life and avoids undue interference with the physician-patient relationship.” Indeed, said Dr. Rapoport, study results are perhaps more impressive because they were achieved through treatment with erenumab alone, without detoxification.
Managing Chronic Migraine and MOH
Until erenumab’s 2018 approval, migraine prevention options were limited to tricyclic antidepressants, beta blockers, and antiseizure medicines – though these medicines never seemed to work very well without detoxification, said Dr. Rapoport. Neurologists still use these categories for migraine prevention, he added, “because insurance companies insist that before we give the more expensive, newer medications like those that block CGRP, patients must fail 2 of those 3 categories of older medications which are not approved for chronic migraine.” Only onabotulinumtoxinA (Botox) is FDA-approved for chronic migraine. “There has been no head-to-head comparison of it and any of the monoclonal antibodies against CGRP,” he said.
In a March 2024 publication in Headache, the American Headache Society stated that requiring patients to fail older drugs is inappropriate, and that CGRP inhibitors, though costly, should be first-line for headache prevention. The key advantage of any drug that blocks CGRP in treating MOH is that unlike older drugs, CGRP inhibitors appear to work well even without detoxification, said Dr. Rapoport.
Additional study limitations included the possibility that the 24-week treatment period might not have allowed complete evaluation of long-term efficacy, the authors wrote. “These are usually pretty sick patients,” said Dr. Rapoport, who acknowledged the difficulty of keeping placebo patients off preventive medication altogether for 6 months. The study was extended to 12 months, and the results of an opiate overusers cohort also will be published.
Authors noted that according to a study published in Headache in 2022, most Americans with chronic migraine commonly go without preventive medications. Moreover, such medications do not always work. Accordingly, Dr. Rapoport said, the study duration was reasonable provided patients understood that they had a 33% chance of receiving no effective preventive medication over 6 months.
Extending the study’s month-long baseline period to 3 months before starting erenumab might have been helpful, he added, as that is the timeframe required to confirm MOH diagnosis according to ICHD-3. “However,” said Dr. Rapoport, “3 months with only usual medications, and then 1/3 of patients going 6-12 months with only placebo, would be tough for some patients.”
Dr. Rapoport reports no relevant financial conflicts.
FROM JAMA NEUROLOGY
Myasthenia Gravis: Patient Choice, Cultural Change
Used appropriately, newer treatments can provide dramatic results faster and more safely than broad immunosuppressants. However, according to experts, payers’ willingness to cover costly new therapies remains a work in progress.
The availability of more effective treatments with fewer side effects has brought about a cultural shift, said James F. Howard, Jr, MD. “The physician’s goal now is for the patient to be symptom free with grade 1 or less adverse events. And patients are demanding freedom from all the side effects that our usual course of immune therapy produces.” Dr. Howard is professor of neurology, medicine and allied health and director of the Myasthenia Gravis Clinical Trials and Translational Research Program at the University of North Carolina at Chapel Hill.
The shift has been long in coming. Although myasthenia gravis was identified in the mid-1600s, it took more than 340 years to get the first drug approved specifically for the disorder.
Worldwide prevalence estimates vary widely, from less than 200,000 to 700,000 cases.1,2 Pathophysiologically, myasthenia gravis stems from autoimmune destruction of neuromuscular junctions (NMJs), which transmit motor neuron impulses to muscle fibers.1 Symptoms include variable skeletal muscle weakness that can range from mild and transient to life-threatening.
In approximately 80% of cases, autoimmune antibodies target the postsynaptic acetylcholine receptor (AChR). Additional autoimmune targets mainly include muscle-specific kinase (MuSK) and lipoprotein receptor-related protein 4 (LRP4). However, around 10% of patients are seronegative, lacking autoantibodies detectable through conventional radioimmunoassays. Clinical disease does not always correspond with circulating antibody levels, and pathogenesis may require cooperation between multiple autoantibodies attacking the same target.3 Around 10% of MG cases are associated with thymomas.
Among myasthenia gravis treatments, immunosuppressants typically take 4-10 months to begin working and 18-36 months for maximum benefit. “Our new targeted therapies work within 1-2 weeks, with maximum improvement occurring somewhere between 8 and 12 weeks,” Dr. Howard said. Quick onset makes these drugs well suited for primary therapy in recalcitrant myasthenia gravis or as bridges to standard immunotherapy. Targeted drugs also appear to provide effective rescue therapy, although head-to-head studies are needed.
Complement Inhibition
In AChR antibody–positive myasthenia gravis, autoantibody binding with the postsynaptic AChR receptor activates complement to attack postsynaptic neuronal membrane. Complement inhibitors approved to date block activation of the terminal complement protein C5.
For many patients, complement inhibitors deliver dramatic results. Henry J. Kaminski, MD, said that the first patient for whom he prescribed a complement inhibitor outside a clinical trial went from being miserable to traveling internationally within a month. Dr. Kaminski is Meta A. Neumann Professor of Neurology at George Washington University, Washington, DC.
Eculizumab (Soliris, Alexion), earned Food and Drug Administration (FDA) approval for myasthenia gravis in 2017. Week 26 results in the phase 3 REGAIN trial showed no significant difference in Myasthenia Gravis–Activities of Daily Living (MG-ADL) scores between treatment and placebo. However, said Dr. Howard, primary investigator on the study, the negative result was a statistical aberration stemming from the FDA’s requirement to use worst-rank analysis rather than absolute change scores. What got eculizumab approved were highly positive results in the overwhelming majority of secondary endpoints.4 Subsequently, the FDA had the manufacturer rewrite the package insert using common statistical methods, which yielded positive primary results.
Ravulizumab (Ultomiris, Alexion), approved for myasthenia gravis in 2022, reduces eculizumab’s twice-monthly intravenous dosing to every 2 months (after loading doses), with very similar efficacy. The newest complement inhibitor, zilucoplan (Zilbrysq, UCB), administered once daily subcutaneously, earned FDA approval in 2023. Daily subcutaneous dosing provides patient convenience, said Dr. Howard. Because the body does not clear this small molecule as it would a full-size antibody, it is the only complement inhibitor that can be combined with a fragment crystallizable neonatal receptor (FcRn) inhibitor.
FcRn Inhibition
The FcRn exists on the surface and intracellular vesicles of many cells, including B cells, but not T cells.5 FcRn inhibitors block binding of circulating IgG antibodies to the FcRn, preventing their normal recycling, significantly reducing circulating antibodies within days of treatment.
Efgartigimod (Vyvgart, Argenx), earned FDA approval in intravenous form in 2021, followed by a subcutaneous formulation that includes hyaluronidase (Vyvgart Hytrulo) in 2023. Rozanolixizumab (Rystiggo, UCB) earned FDA approval for both AChR antibody–positive and MuSK antibody–positive myasthenia gravis in 2023.
Along with rapid response, said Dr. Howard, complement inhibitors and FcRn inhibitors offer a “hugely improved” side-effect profile. In phase 3 research, the most common side effects for both classes included headache, nausea, and diarrhea.4,6,7 Because complement inhibitors increase the risk of Neisseria infection, users require immunization against meningococcal infection (or concurrent antibiotic prophylaxis) while on complement inhibitors.
Insurance Issues
With many clinicians wondering which targeted therapy to choose for a particular patient, said Dr. Howard and Dr. Kaminski, the main obstacle to wider use of these treatments is payer attitudes and practices. “While many of us would like to see these drugs used earlier in the course of disease,” Dr. Howard explained, “there are numerous restrictions placed on the physician and the patient by whatever insurance the individual has.”
Dr. Kaminski said: “There’s a lot of variability among insurance companies regarding what is expected in terms of getting approval for a certain medication. It frustrates me, thinking this patient may do well with a complement inhibitor or an FcRn inhibitor, but it takes weeks to get them approved.”
Some of his patients have been approved for, and flourished on, complement inhibitors and FcRn inhibitors, he added, and then denied a second round of treatment. Dr. Kaminski said he does not know why these patients were denied, and every time he requests reevaluation, the decision is reversed. “That’s a significant time frame for me and my staff to manage.”
When asked what can be done to address high drug prices, Dr. Howard replied, “I have no idea. I’m not an advocate of high drug prices. But I don’t think people realize the cost of doing clinical trials, which is hundreds of millions of dollars, particularly in rare diseases.”
Presently, Dr. Howard said, FcRn inhibitors are used more frequently than complement inhibitors solely because of cost. Zilucoplan will be priced below existing complement inhibitors, although it is too soon to compare its price with those of FcRn inhibitors.
When eculizumab debuted, said Dr. Howard, it cost nearly $750,000 annually. “But if you look at the number of patients treated, the cost of the drug over this population is probably less than the cost for using a cholesterol-lowering agent to treat hyperlipidemia.”
An Institute for Clinical and Economic Review (ICER) report stated that eculizumab and efgartigimod should both cost less than $20,000 annually to meet commonly used cost-effectiveness thresholds.8 However, Dr. Howard said ICER used models based on common diseases and ignored the economic impact of patients’ losing fewer workdays and avoiding long-term immunosuppressant side effects such as diabetes and osteoporosis and related treatment costs. “We’ve got to start looking at total societal cost,” he said.
Leapfrogging Ahead
Not all the new drugs work in every indicated patient, Dr. Howard said. For example, up to 30% of patients do not respond to complement inhibitors. “We don’t understand why. It’s as if we have leapfrogged way ahead in terms of therapeutics, and now we have to go back and answer all the questions – the who, what, where, and why of an individual drug and its response in folks.”
In this climate, said Dr. Kaminski, heavy direct-to-consumer advertising of newer myasthenia gravis therapies creates complications. “My patients are highly excited to see, ‘that’s my disease being advertised on Jeopardy.’ ” Many patients are frustrated with the general lack of awareness regarding myasthenia gravis, he added. “But then I’ve had patients who clearly would never qualify for a certain medication getting mailings to their homes.”
Dr. Howard countered that broader awareness of myasthenia gravis can only help. “There’s increasing recognition of the disease, not only by patients, but to some extent, by the treating clinician. Patients are coming to our offices and saying, ‘am I a candidate for this new drug?’ It’s the responsibility of the clinician to decide.”
Individual physicians’ practice patterns vary greatly, said Dr. Kaminski, and very little quantitative data exist here. But based on personal communications, academic-center neurologists tend to use targeted treatments on patients who have failed conventional treatments.
Conversely, Dr. Howard said that, because community physicians rarely see myasthenia gravis, and targeted treatments remain relatively new, many of these providers rely on prednisone, azathioprine, and mycophenolate mofetil.
B-Cell Blockers in Development
Overall, said Dr. Howard, the field of myasthenia gravis treatment development is “very rich. And pharma’s interest in myasthenia has taken off like a rocket. It’s exceptionally gratifying to those of us who take care of these patients whose life is miserable” because of adverse effects and/or nonresponse to current drugs.
“In myasthenia,” added Dr. Kaminski, “we know that T cells are promoting the activity of these auto-reactive B cells.” Many drugs currently in phase 2 or 3 development aim to eliminate B cells or signaling between T and B cells, he said. “That’s where most of the drug development is.”
Leading candidates include telitacicept (Tai’ai, RemeGen), which is both a B-lymphocyte stimulator and a proliferation-inducing ligand. A phase 3 trial (NCT05737160) is ongoing, with primary completion expected in late 2026. A second phase 3 trial (NCT06456580) recently began enrolling. Dr. Howard said that, although early results warranted phase 3 analysis, telitacicept’s phase 2 trial was open label and lacked a placebo group.9 The latter is a critical concern because placebo response rates in myasthenia gravis trials average 35%-40%.
Combined with standard care, the FcRn inhibitor nipocalimab (Johnson & Johnson) enabled patients with AChR, MuSK, and/or LRP4 autoantibodies to improve by 4.70 points on the MG-ADL vs 3.25 points for placebo (P = .002) over 24 weeks in phase 3.10All FcRn inhibitors in development can broadly reduce autoantibody levels, said Dr. Howard. “But what role they will play in myasthenia gravis when they’re several years behind leaders in the field in terms of capturing market remains to be seen.”
Additionally, batoclimab (Immunovant/Harbour BioMed) showed positive topline results in phase 3, and an elevated rate of hypercholesterolemia in treated patients that was transient and consistent with previous research.11 Subsequent to efgartigimod, Dr. Howard said, FcRn inhibitors are full-size antibodies. “I believe that contributes to the adverse events that we see. Efgartigimod is a small FcRn fragment. That’s why it’s a cleaner drug, if you will.”
FcRn inhibitors require periodic retreatment. For example, said Dr. Howard, the ADAPT phase 3 trial of efgartigimod, on which he was lead investigator, employed a cyclic dosing schedule – 4 weeks’ treatment, then observation until patients needed retreatment — because patients demanded it.12 In clinical practice, some patients have gone more than 25 weeks before needing retreatment. One of his patients went beyond 40 weeks. “Others only get around 6-9 weeks. So patient choice again enters the decision-making process.”
Rituximab targets the CD20 protein on B cells nonspecifically, producing general immunosuppression. “That’s problematic in producing significant immunosuppression,” said Dr. Kaminski. Nevertheless, he said, rituximab is very effective for most patients with MuSK-specific MG, and its application to this indication has revealed differences between the MuSK subtype and AChR antibody–positive myasthenia. Specifically, MuSK antibody–positive patients have short-lived plasmablasts, which rituximab eliminates.13
Conversely, said Dr. Kaminski, patients with AChR antibody-positive myasthenia, especially long-term, likely have long-lived plasmablasts producing antibodies. This fact, and these patients’ lack of CD20, likely explain their poor response to rituximab.
A phase 3 trial (NCT04524273) of the CD19 blocker inebilizumab (Uplinza, Amgen) reached primary completion in May. Dr. Howard said that if topline results (unreleased at press time) prove positive, inebilizumab could replace rituximab in MG — provided payers do not reject inebilizumab because of cost.
Packed Early-Development Pipeline
Regarding early-stage projects, said Dr. Howard, the pipeline is packed with compounds that target various aspects of the immune system. “The real question with those is, what’s going to be the side effect profile? All of the trials are very early. We need bigger trials with much longer observation for safety, durability, and degree of efficacy.”
The next potential B cell–targeting game changer, he said, is chimeric antigen receptor (CAR) T cell–based therapy. In a phase 2b trial of Descartes-08 (Cartesian Therapeutics), 71% of treated patients experienced clinically meaningful improvement in MG Composite score at 3 months vs 25% for placebo.14
In early clinical trials, said Dr. Howard, patients treated with Descartes-08 — which uses autologous mRNA to target B-cell maturation antigen — have shown “exceptional improvement” lasting 20 or more months. Because the drug is not ingrained permanently into the genome, Descartes-08 avoids potentially severe side effects of DNA-targeting CAR T candidates. Dr. Howard hopes a phase 3 trial will commence around January 2025.
The tolerance approach exemplified by CNP-106 (COUR Pharmaceuticals) and a myasthenia gravis tolerogen (Toleranzia) seeks to prevent the immune system from recognizing and reacting to the NMJ abnormalities that produce myasthenia gravis, potentially providing a cure. “We look forward to those trials as they come online in the next 1-2 years,” said Dr. Howard.
Unmet Needs
Historically, neurologists believed that all myasthenia gravis symptoms stemmed from muscle fatigue — the more active the muscle, the weaker it gets. However, said Dr. Kaminski, some patients might lack measurable weakness but still complain of fatigue.
Elevated levels of cytokines such as interleukin (IL)–6 or IL-17 also can produce fatigue, he noted. “With the drugs we’re using, certainly the new ones, we’re not specifically targeting this fatigue phenomenon, which has been studied in a very limited fashion.”
In the RAISE-XT zilucoplan trial, participants experienced significant improvement in fatigue scores for up to 60 weeks.15 Although zilucoplan does not address fatigue directly, said Dr. Howard, improving myasthenia gravis overall helps reduce fatigue.
The Myasthenia Gravis Symptoms Patient Reported Outcome (MG Symptoms PRO), which Dr. Kaminski helped develop, includes questions designed to distinguish muscular fatigue from overall physical fatigue.16 “I’m very interested in some of the information that’s coming out on long COVID and its effect on muscle,” Dr. Kaminski added. “We might be able to learn from there that there’s still some pathology going on beyond the neuromuscular junction.”
What the field desperately needs, said Dr. Howard, are biomarkers to identify which patients will and will not respond to certain therapeutics. “We’re not there yet.” Such biomarkers are at least 3-7 years from becoming clinical reality.
Promising antibody-independent serum markers include circulating microRNAs. For example, miRNA-150-5p and miRNA-21-5p are elevated in generalized AChR-positive myasthenia gravis and early-onset myasthenia gravis (occurring before age 50) and decline after immunosuppression and thymectomy.17
Among diagnostic modalities for patients with seronegative myasthenia gravis, said Dr. Kaminski, single-fiber EMG is the most sensitive, at approximately 95%. “It’s not perfect.” Moreover, he said, performing this test accurately requires a highly experienced expert, which many treatment centers lack.
Presently, added Dr. Kaminski, orbital MRI is neither specific nor sensitive enough to be clinically useful. “One needs to be careful with these specialized tests that are published from the best laboratory in the world that does the test, and does it repetitively.” As the search for effective myasthenia gravis biomarkers continues, avoiding false-positive results is as important as avoiding false negatives.
References
1. Bubuioc AM et al. J Med Life. 2021 Jan-Mar;14(1):7-16. doi: 10.25122/jml-2020-0145.
2. Deenen JC et al. J Neuromuscul Dis. 2015;2(1):73-85. doi: 10.3233/JND-140045.
3. Kaminski HJ et al. J Clin Invest. 2024 Jun 17;134(12):e179742. doi: 10.1172/JCI179742.
4. Howard JF Jr et al. Lancet Neurol. 2017 Dec;16(12):976-986. doi: 10.1016/S1474-4422(17)30369-1.
5. Huda R. Front Immunol. 2020 Feb 21:11:240. doi: 10.3389/fimmu.2020.00240.
6. Howard JF Jr et al. Lancet Neurol. 2023 May;22(5):395-406. doi: 10.1016/S1474-4422(23)00080-7.
7. Vu T et al. NEJM Evid. 2022 May;1(5):EVIDoa2100066. doi: 10.1056/EVIDoa2100066.
8. Tice JA et al. October 20, 2021. https://icer.org/assessment/myasthenia-gravis/.
9. Yin J et al. Eur J Neurol. 2024 Aug;31(8):e16322. doi: 10.1111/ene.16322.
10. Antozzi C et al. EAN 2024, Abstract EPR-116. https://www.neurology.org/doi/10.1212/WNL.0000000000203660.
11. Yan C et al. JAMA Neurol. 2024 Mar 4;81(4):336-345. doi: 10.1001/jamaneurol.2024.0044.
12. Howard JF Jr et al. Lancet Neurol. 2021 Jul;20(7):526-536. doi: 10.1016/S1474-4422(21)00159-9.
13. Stathopoulos P et al. JCI Insight. 2017 Sep 7;2(17):e94263. doi: 10.1172/jci.insight.94263.
14. Cartesian Therapeutics. Cartesian Therapeutics announces positive topline results from phase 2b trial of Descartes-08 in patients with myasthenia gravis. 2024 Jul 2. https://ir.cartesiantherapeutics.com/news-releases/news-release-details/cartesian-therapeutics-announces-positive-topline-results-phase.
15. Howard JF Jr et al. Ther Adv Neurol Disord. 2024 Apr 17:17:17562864241243186. doi: 10.1177/17562864241243186.
16. Cleanthous S et al. Orphanet J Rare Dis. 2021 Oct 30;16(1):457. doi: 10.1186/s13023-021-02064-0.
17. Sabre L et al. Front Immunol. 2020 Mar 4:11:213. doi: 10.3389/fimmu.2020.00213.
Used appropriately, newer treatments can provide dramatic results faster and more safely than broad immunosuppressants. However, according to experts, payers’ willingness to cover costly new therapies remains a work in progress.
The availability of more effective treatments with fewer side effects has brought about a cultural shift, said James F. Howard, Jr, MD. “The physician’s goal now is for the patient to be symptom free with grade 1 or less adverse events. And patients are demanding freedom from all the side effects that our usual course of immune therapy produces.” Dr. Howard is professor of neurology, medicine and allied health and director of the Myasthenia Gravis Clinical Trials and Translational Research Program at the University of North Carolina at Chapel Hill.
The shift has been long in coming. Although myasthenia gravis was identified in the mid-1600s, it took more than 340 years to get the first drug approved specifically for the disorder.
Worldwide prevalence estimates vary widely, from less than 200,000 to 700,000 cases.1,2 Pathophysiologically, myasthenia gravis stems from autoimmune destruction of neuromuscular junctions (NMJs), which transmit motor neuron impulses to muscle fibers.1 Symptoms include variable skeletal muscle weakness that can range from mild and transient to life-threatening.
In approximately 80% of cases, autoimmune antibodies target the postsynaptic acetylcholine receptor (AChR). Additional autoimmune targets mainly include muscle-specific kinase (MuSK) and lipoprotein receptor-related protein 4 (LRP4). However, around 10% of patients are seronegative, lacking autoantibodies detectable through conventional radioimmunoassays. Clinical disease does not always correspond with circulating antibody levels, and pathogenesis may require cooperation between multiple autoantibodies attacking the same target.3 Around 10% of MG cases are associated with thymomas.
Among myasthenia gravis treatments, immunosuppressants typically take 4-10 months to begin working and 18-36 months for maximum benefit. “Our new targeted therapies work within 1-2 weeks, with maximum improvement occurring somewhere between 8 and 12 weeks,” Dr. Howard said. Quick onset makes these drugs well suited for primary therapy in recalcitrant myasthenia gravis or as bridges to standard immunotherapy. Targeted drugs also appear to provide effective rescue therapy, although head-to-head studies are needed.
Complement Inhibition
In AChR antibody–positive myasthenia gravis, autoantibody binding with the postsynaptic AChR receptor activates complement to attack postsynaptic neuronal membrane. Complement inhibitors approved to date block activation of the terminal complement protein C5.
For many patients, complement inhibitors deliver dramatic results. Henry J. Kaminski, MD, said that the first patient for whom he prescribed a complement inhibitor outside a clinical trial went from being miserable to traveling internationally within a month. Dr. Kaminski is Meta A. Neumann Professor of Neurology at George Washington University, Washington, DC.
Eculizumab (Soliris, Alexion), earned Food and Drug Administration (FDA) approval for myasthenia gravis in 2017. Week 26 results in the phase 3 REGAIN trial showed no significant difference in Myasthenia Gravis–Activities of Daily Living (MG-ADL) scores between treatment and placebo. However, said Dr. Howard, primary investigator on the study, the negative result was a statistical aberration stemming from the FDA’s requirement to use worst-rank analysis rather than absolute change scores. What got eculizumab approved were highly positive results in the overwhelming majority of secondary endpoints.4 Subsequently, the FDA had the manufacturer rewrite the package insert using common statistical methods, which yielded positive primary results.
Ravulizumab (Ultomiris, Alexion), approved for myasthenia gravis in 2022, reduces eculizumab’s twice-monthly intravenous dosing to every 2 months (after loading doses), with very similar efficacy. The newest complement inhibitor, zilucoplan (Zilbrysq, UCB), administered once daily subcutaneously, earned FDA approval in 2023. Daily subcutaneous dosing provides patient convenience, said Dr. Howard. Because the body does not clear this small molecule as it would a full-size antibody, it is the only complement inhibitor that can be combined with a fragment crystallizable neonatal receptor (FcRn) inhibitor.
FcRn Inhibition
The FcRn exists on the surface and intracellular vesicles of many cells, including B cells, but not T cells.5 FcRn inhibitors block binding of circulating IgG antibodies to the FcRn, preventing their normal recycling, significantly reducing circulating antibodies within days of treatment.
Efgartigimod (Vyvgart, Argenx), earned FDA approval in intravenous form in 2021, followed by a subcutaneous formulation that includes hyaluronidase (Vyvgart Hytrulo) in 2023. Rozanolixizumab (Rystiggo, UCB) earned FDA approval for both AChR antibody–positive and MuSK antibody–positive myasthenia gravis in 2023.
Along with rapid response, said Dr. Howard, complement inhibitors and FcRn inhibitors offer a “hugely improved” side-effect profile. In phase 3 research, the most common side effects for both classes included headache, nausea, and diarrhea.4,6,7 Because complement inhibitors increase the risk of Neisseria infection, users require immunization against meningococcal infection (or concurrent antibiotic prophylaxis) while on complement inhibitors.
Insurance Issues
With many clinicians wondering which targeted therapy to choose for a particular patient, said Dr. Howard and Dr. Kaminski, the main obstacle to wider use of these treatments is payer attitudes and practices. “While many of us would like to see these drugs used earlier in the course of disease,” Dr. Howard explained, “there are numerous restrictions placed on the physician and the patient by whatever insurance the individual has.”
Dr. Kaminski said: “There’s a lot of variability among insurance companies regarding what is expected in terms of getting approval for a certain medication. It frustrates me, thinking this patient may do well with a complement inhibitor or an FcRn inhibitor, but it takes weeks to get them approved.”
Some of his patients have been approved for, and flourished on, complement inhibitors and FcRn inhibitors, he added, and then denied a second round of treatment. Dr. Kaminski said he does not know why these patients were denied, and every time he requests reevaluation, the decision is reversed. “That’s a significant time frame for me and my staff to manage.”
When asked what can be done to address high drug prices, Dr. Howard replied, “I have no idea. I’m not an advocate of high drug prices. But I don’t think people realize the cost of doing clinical trials, which is hundreds of millions of dollars, particularly in rare diseases.”
Presently, Dr. Howard said, FcRn inhibitors are used more frequently than complement inhibitors solely because of cost. Zilucoplan will be priced below existing complement inhibitors, although it is too soon to compare its price with those of FcRn inhibitors.
When eculizumab debuted, said Dr. Howard, it cost nearly $750,000 annually. “But if you look at the number of patients treated, the cost of the drug over this population is probably less than the cost for using a cholesterol-lowering agent to treat hyperlipidemia.”
An Institute for Clinical and Economic Review (ICER) report stated that eculizumab and efgartigimod should both cost less than $20,000 annually to meet commonly used cost-effectiveness thresholds.8 However, Dr. Howard said ICER used models based on common diseases and ignored the economic impact of patients’ losing fewer workdays and avoiding long-term immunosuppressant side effects such as diabetes and osteoporosis and related treatment costs. “We’ve got to start looking at total societal cost,” he said.
Leapfrogging Ahead
Not all the new drugs work in every indicated patient, Dr. Howard said. For example, up to 30% of patients do not respond to complement inhibitors. “We don’t understand why. It’s as if we have leapfrogged way ahead in terms of therapeutics, and now we have to go back and answer all the questions – the who, what, where, and why of an individual drug and its response in folks.”
In this climate, said Dr. Kaminski, heavy direct-to-consumer advertising of newer myasthenia gravis therapies creates complications. “My patients are highly excited to see, ‘that’s my disease being advertised on Jeopardy.’ ” Many patients are frustrated with the general lack of awareness regarding myasthenia gravis, he added. “But then I’ve had patients who clearly would never qualify for a certain medication getting mailings to their homes.”
Dr. Howard countered that broader awareness of myasthenia gravis can only help. “There’s increasing recognition of the disease, not only by patients, but to some extent, by the treating clinician. Patients are coming to our offices and saying, ‘am I a candidate for this new drug?’ It’s the responsibility of the clinician to decide.”
Individual physicians’ practice patterns vary greatly, said Dr. Kaminski, and very little quantitative data exist here. But based on personal communications, academic-center neurologists tend to use targeted treatments on patients who have failed conventional treatments.
Conversely, Dr. Howard said that, because community physicians rarely see myasthenia gravis, and targeted treatments remain relatively new, many of these providers rely on prednisone, azathioprine, and mycophenolate mofetil.
B-Cell Blockers in Development
Overall, said Dr. Howard, the field of myasthenia gravis treatment development is “very rich. And pharma’s interest in myasthenia has taken off like a rocket. It’s exceptionally gratifying to those of us who take care of these patients whose life is miserable” because of adverse effects and/or nonresponse to current drugs.
“In myasthenia,” added Dr. Kaminski, “we know that T cells are promoting the activity of these auto-reactive B cells.” Many drugs currently in phase 2 or 3 development aim to eliminate B cells or signaling between T and B cells, he said. “That’s where most of the drug development is.”
Leading candidates include telitacicept (Tai’ai, RemeGen), which is both a B-lymphocyte stimulator and a proliferation-inducing ligand. A phase 3 trial (NCT05737160) is ongoing, with primary completion expected in late 2026. A second phase 3 trial (NCT06456580) recently began enrolling. Dr. Howard said that, although early results warranted phase 3 analysis, telitacicept’s phase 2 trial was open label and lacked a placebo group.9 The latter is a critical concern because placebo response rates in myasthenia gravis trials average 35%-40%.
Combined with standard care, the FcRn inhibitor nipocalimab (Johnson & Johnson) enabled patients with AChR, MuSK, and/or LRP4 autoantibodies to improve by 4.70 points on the MG-ADL vs 3.25 points for placebo (P = .002) over 24 weeks in phase 3.10All FcRn inhibitors in development can broadly reduce autoantibody levels, said Dr. Howard. “But what role they will play in myasthenia gravis when they’re several years behind leaders in the field in terms of capturing market remains to be seen.”
Additionally, batoclimab (Immunovant/Harbour BioMed) showed positive topline results in phase 3, and an elevated rate of hypercholesterolemia in treated patients that was transient and consistent with previous research.11 Subsequent to efgartigimod, Dr. Howard said, FcRn inhibitors are full-size antibodies. “I believe that contributes to the adverse events that we see. Efgartigimod is a small FcRn fragment. That’s why it’s a cleaner drug, if you will.”
FcRn inhibitors require periodic retreatment. For example, said Dr. Howard, the ADAPT phase 3 trial of efgartigimod, on which he was lead investigator, employed a cyclic dosing schedule – 4 weeks’ treatment, then observation until patients needed retreatment — because patients demanded it.12 In clinical practice, some patients have gone more than 25 weeks before needing retreatment. One of his patients went beyond 40 weeks. “Others only get around 6-9 weeks. So patient choice again enters the decision-making process.”
Rituximab targets the CD20 protein on B cells nonspecifically, producing general immunosuppression. “That’s problematic in producing significant immunosuppression,” said Dr. Kaminski. Nevertheless, he said, rituximab is very effective for most patients with MuSK-specific MG, and its application to this indication has revealed differences between the MuSK subtype and AChR antibody–positive myasthenia. Specifically, MuSK antibody–positive patients have short-lived plasmablasts, which rituximab eliminates.13
Conversely, said Dr. Kaminski, patients with AChR antibody-positive myasthenia, especially long-term, likely have long-lived plasmablasts producing antibodies. This fact, and these patients’ lack of CD20, likely explain their poor response to rituximab.
A phase 3 trial (NCT04524273) of the CD19 blocker inebilizumab (Uplinza, Amgen) reached primary completion in May. Dr. Howard said that if topline results (unreleased at press time) prove positive, inebilizumab could replace rituximab in MG — provided payers do not reject inebilizumab because of cost.
Packed Early-Development Pipeline
Regarding early-stage projects, said Dr. Howard, the pipeline is packed with compounds that target various aspects of the immune system. “The real question with those is, what’s going to be the side effect profile? All of the trials are very early. We need bigger trials with much longer observation for safety, durability, and degree of efficacy.”
The next potential B cell–targeting game changer, he said, is chimeric antigen receptor (CAR) T cell–based therapy. In a phase 2b trial of Descartes-08 (Cartesian Therapeutics), 71% of treated patients experienced clinically meaningful improvement in MG Composite score at 3 months vs 25% for placebo.14
In early clinical trials, said Dr. Howard, patients treated with Descartes-08 — which uses autologous mRNA to target B-cell maturation antigen — have shown “exceptional improvement” lasting 20 or more months. Because the drug is not ingrained permanently into the genome, Descartes-08 avoids potentially severe side effects of DNA-targeting CAR T candidates. Dr. Howard hopes a phase 3 trial will commence around January 2025.
The tolerance approach exemplified by CNP-106 (COUR Pharmaceuticals) and a myasthenia gravis tolerogen (Toleranzia) seeks to prevent the immune system from recognizing and reacting to the NMJ abnormalities that produce myasthenia gravis, potentially providing a cure. “We look forward to those trials as they come online in the next 1-2 years,” said Dr. Howard.
Unmet Needs
Historically, neurologists believed that all myasthenia gravis symptoms stemmed from muscle fatigue — the more active the muscle, the weaker it gets. However, said Dr. Kaminski, some patients might lack measurable weakness but still complain of fatigue.
Elevated levels of cytokines such as interleukin (IL)–6 or IL-17 also can produce fatigue, he noted. “With the drugs we’re using, certainly the new ones, we’re not specifically targeting this fatigue phenomenon, which has been studied in a very limited fashion.”
In the RAISE-XT zilucoplan trial, participants experienced significant improvement in fatigue scores for up to 60 weeks.15 Although zilucoplan does not address fatigue directly, said Dr. Howard, improving myasthenia gravis overall helps reduce fatigue.
The Myasthenia Gravis Symptoms Patient Reported Outcome (MG Symptoms PRO), which Dr. Kaminski helped develop, includes questions designed to distinguish muscular fatigue from overall physical fatigue.16 “I’m very interested in some of the information that’s coming out on long COVID and its effect on muscle,” Dr. Kaminski added. “We might be able to learn from there that there’s still some pathology going on beyond the neuromuscular junction.”
What the field desperately needs, said Dr. Howard, are biomarkers to identify which patients will and will not respond to certain therapeutics. “We’re not there yet.” Such biomarkers are at least 3-7 years from becoming clinical reality.
Promising antibody-independent serum markers include circulating microRNAs. For example, miRNA-150-5p and miRNA-21-5p are elevated in generalized AChR-positive myasthenia gravis and early-onset myasthenia gravis (occurring before age 50) and decline after immunosuppression and thymectomy.17
Among diagnostic modalities for patients with seronegative myasthenia gravis, said Dr. Kaminski, single-fiber EMG is the most sensitive, at approximately 95%. “It’s not perfect.” Moreover, he said, performing this test accurately requires a highly experienced expert, which many treatment centers lack.
Presently, added Dr. Kaminski, orbital MRI is neither specific nor sensitive enough to be clinically useful. “One needs to be careful with these specialized tests that are published from the best laboratory in the world that does the test, and does it repetitively.” As the search for effective myasthenia gravis biomarkers continues, avoiding false-positive results is as important as avoiding false negatives.
References
1. Bubuioc AM et al. J Med Life. 2021 Jan-Mar;14(1):7-16. doi: 10.25122/jml-2020-0145.
2. Deenen JC et al. J Neuromuscul Dis. 2015;2(1):73-85. doi: 10.3233/JND-140045.
3. Kaminski HJ et al. J Clin Invest. 2024 Jun 17;134(12):e179742. doi: 10.1172/JCI179742.
4. Howard JF Jr et al. Lancet Neurol. 2017 Dec;16(12):976-986. doi: 10.1016/S1474-4422(17)30369-1.
5. Huda R. Front Immunol. 2020 Feb 21:11:240. doi: 10.3389/fimmu.2020.00240.
6. Howard JF Jr et al. Lancet Neurol. 2023 May;22(5):395-406. doi: 10.1016/S1474-4422(23)00080-7.
7. Vu T et al. NEJM Evid. 2022 May;1(5):EVIDoa2100066. doi: 10.1056/EVIDoa2100066.
8. Tice JA et al. October 20, 2021. https://icer.org/assessment/myasthenia-gravis/.
9. Yin J et al. Eur J Neurol. 2024 Aug;31(8):e16322. doi: 10.1111/ene.16322.
10. Antozzi C et al. EAN 2024, Abstract EPR-116. https://www.neurology.org/doi/10.1212/WNL.0000000000203660.
11. Yan C et al. JAMA Neurol. 2024 Mar 4;81(4):336-345. doi: 10.1001/jamaneurol.2024.0044.
12. Howard JF Jr et al. Lancet Neurol. 2021 Jul;20(7):526-536. doi: 10.1016/S1474-4422(21)00159-9.
13. Stathopoulos P et al. JCI Insight. 2017 Sep 7;2(17):e94263. doi: 10.1172/jci.insight.94263.
14. Cartesian Therapeutics. Cartesian Therapeutics announces positive topline results from phase 2b trial of Descartes-08 in patients with myasthenia gravis. 2024 Jul 2. https://ir.cartesiantherapeutics.com/news-releases/news-release-details/cartesian-therapeutics-announces-positive-topline-results-phase.
15. Howard JF Jr et al. Ther Adv Neurol Disord. 2024 Apr 17:17:17562864241243186. doi: 10.1177/17562864241243186.
16. Cleanthous S et al. Orphanet J Rare Dis. 2021 Oct 30;16(1):457. doi: 10.1186/s13023-021-02064-0.
17. Sabre L et al. Front Immunol. 2020 Mar 4:11:213. doi: 10.3389/fimmu.2020.00213.
Used appropriately, newer treatments can provide dramatic results faster and more safely than broad immunosuppressants. However, according to experts, payers’ willingness to cover costly new therapies remains a work in progress.
The availability of more effective treatments with fewer side effects has brought about a cultural shift, said James F. Howard, Jr, MD. “The physician’s goal now is for the patient to be symptom free with grade 1 or less adverse events. And patients are demanding freedom from all the side effects that our usual course of immune therapy produces.” Dr. Howard is professor of neurology, medicine and allied health and director of the Myasthenia Gravis Clinical Trials and Translational Research Program at the University of North Carolina at Chapel Hill.
The shift has been long in coming. Although myasthenia gravis was identified in the mid-1600s, it took more than 340 years to get the first drug approved specifically for the disorder.
Worldwide prevalence estimates vary widely, from less than 200,000 to 700,000 cases.1,2 Pathophysiologically, myasthenia gravis stems from autoimmune destruction of neuromuscular junctions (NMJs), which transmit motor neuron impulses to muscle fibers.1 Symptoms include variable skeletal muscle weakness that can range from mild and transient to life-threatening.
In approximately 80% of cases, autoimmune antibodies target the postsynaptic acetylcholine receptor (AChR). Additional autoimmune targets mainly include muscle-specific kinase (MuSK) and lipoprotein receptor-related protein 4 (LRP4). However, around 10% of patients are seronegative, lacking autoantibodies detectable through conventional radioimmunoassays. Clinical disease does not always correspond with circulating antibody levels, and pathogenesis may require cooperation between multiple autoantibodies attacking the same target.3 Around 10% of MG cases are associated with thymomas.
Among myasthenia gravis treatments, immunosuppressants typically take 4-10 months to begin working and 18-36 months for maximum benefit. “Our new targeted therapies work within 1-2 weeks, with maximum improvement occurring somewhere between 8 and 12 weeks,” Dr. Howard said. Quick onset makes these drugs well suited for primary therapy in recalcitrant myasthenia gravis or as bridges to standard immunotherapy. Targeted drugs also appear to provide effective rescue therapy, although head-to-head studies are needed.
Complement Inhibition
In AChR antibody–positive myasthenia gravis, autoantibody binding with the postsynaptic AChR receptor activates complement to attack postsynaptic neuronal membrane. Complement inhibitors approved to date block activation of the terminal complement protein C5.
For many patients, complement inhibitors deliver dramatic results. Henry J. Kaminski, MD, said that the first patient for whom he prescribed a complement inhibitor outside a clinical trial went from being miserable to traveling internationally within a month. Dr. Kaminski is Meta A. Neumann Professor of Neurology at George Washington University, Washington, DC.
Eculizumab (Soliris, Alexion), earned Food and Drug Administration (FDA) approval for myasthenia gravis in 2017. Week 26 results in the phase 3 REGAIN trial showed no significant difference in Myasthenia Gravis–Activities of Daily Living (MG-ADL) scores between treatment and placebo. However, said Dr. Howard, primary investigator on the study, the negative result was a statistical aberration stemming from the FDA’s requirement to use worst-rank analysis rather than absolute change scores. What got eculizumab approved were highly positive results in the overwhelming majority of secondary endpoints.4 Subsequently, the FDA had the manufacturer rewrite the package insert using common statistical methods, which yielded positive primary results.
Ravulizumab (Ultomiris, Alexion), approved for myasthenia gravis in 2022, reduces eculizumab’s twice-monthly intravenous dosing to every 2 months (after loading doses), with very similar efficacy. The newest complement inhibitor, zilucoplan (Zilbrysq, UCB), administered once daily subcutaneously, earned FDA approval in 2023. Daily subcutaneous dosing provides patient convenience, said Dr. Howard. Because the body does not clear this small molecule as it would a full-size antibody, it is the only complement inhibitor that can be combined with a fragment crystallizable neonatal receptor (FcRn) inhibitor.
FcRn Inhibition
The FcRn exists on the surface and intracellular vesicles of many cells, including B cells, but not T cells.5 FcRn inhibitors block binding of circulating IgG antibodies to the FcRn, preventing their normal recycling, significantly reducing circulating antibodies within days of treatment.
Efgartigimod (Vyvgart, Argenx), earned FDA approval in intravenous form in 2021, followed by a subcutaneous formulation that includes hyaluronidase (Vyvgart Hytrulo) in 2023. Rozanolixizumab (Rystiggo, UCB) earned FDA approval for both AChR antibody–positive and MuSK antibody–positive myasthenia gravis in 2023.
Along with rapid response, said Dr. Howard, complement inhibitors and FcRn inhibitors offer a “hugely improved” side-effect profile. In phase 3 research, the most common side effects for both classes included headache, nausea, and diarrhea.4,6,7 Because complement inhibitors increase the risk of Neisseria infection, users require immunization against meningococcal infection (or concurrent antibiotic prophylaxis) while on complement inhibitors.
Insurance Issues
With many clinicians wondering which targeted therapy to choose for a particular patient, said Dr. Howard and Dr. Kaminski, the main obstacle to wider use of these treatments is payer attitudes and practices. “While many of us would like to see these drugs used earlier in the course of disease,” Dr. Howard explained, “there are numerous restrictions placed on the physician and the patient by whatever insurance the individual has.”
Dr. Kaminski said: “There’s a lot of variability among insurance companies regarding what is expected in terms of getting approval for a certain medication. It frustrates me, thinking this patient may do well with a complement inhibitor or an FcRn inhibitor, but it takes weeks to get them approved.”
Some of his patients have been approved for, and flourished on, complement inhibitors and FcRn inhibitors, he added, and then denied a second round of treatment. Dr. Kaminski said he does not know why these patients were denied, and every time he requests reevaluation, the decision is reversed. “That’s a significant time frame for me and my staff to manage.”
When asked what can be done to address high drug prices, Dr. Howard replied, “I have no idea. I’m not an advocate of high drug prices. But I don’t think people realize the cost of doing clinical trials, which is hundreds of millions of dollars, particularly in rare diseases.”
Presently, Dr. Howard said, FcRn inhibitors are used more frequently than complement inhibitors solely because of cost. Zilucoplan will be priced below existing complement inhibitors, although it is too soon to compare its price with those of FcRn inhibitors.
When eculizumab debuted, said Dr. Howard, it cost nearly $750,000 annually. “But if you look at the number of patients treated, the cost of the drug over this population is probably less than the cost for using a cholesterol-lowering agent to treat hyperlipidemia.”
An Institute for Clinical and Economic Review (ICER) report stated that eculizumab and efgartigimod should both cost less than $20,000 annually to meet commonly used cost-effectiveness thresholds.8 However, Dr. Howard said ICER used models based on common diseases and ignored the economic impact of patients’ losing fewer workdays and avoiding long-term immunosuppressant side effects such as diabetes and osteoporosis and related treatment costs. “We’ve got to start looking at total societal cost,” he said.
Leapfrogging Ahead
Not all the new drugs work in every indicated patient, Dr. Howard said. For example, up to 30% of patients do not respond to complement inhibitors. “We don’t understand why. It’s as if we have leapfrogged way ahead in terms of therapeutics, and now we have to go back and answer all the questions – the who, what, where, and why of an individual drug and its response in folks.”
In this climate, said Dr. Kaminski, heavy direct-to-consumer advertising of newer myasthenia gravis therapies creates complications. “My patients are highly excited to see, ‘that’s my disease being advertised on Jeopardy.’ ” Many patients are frustrated with the general lack of awareness regarding myasthenia gravis, he added. “But then I’ve had patients who clearly would never qualify for a certain medication getting mailings to their homes.”
Dr. Howard countered that broader awareness of myasthenia gravis can only help. “There’s increasing recognition of the disease, not only by patients, but to some extent, by the treating clinician. Patients are coming to our offices and saying, ‘am I a candidate for this new drug?’ It’s the responsibility of the clinician to decide.”
Individual physicians’ practice patterns vary greatly, said Dr. Kaminski, and very little quantitative data exist here. But based on personal communications, academic-center neurologists tend to use targeted treatments on patients who have failed conventional treatments.
Conversely, Dr. Howard said that, because community physicians rarely see myasthenia gravis, and targeted treatments remain relatively new, many of these providers rely on prednisone, azathioprine, and mycophenolate mofetil.
B-Cell Blockers in Development
Overall, said Dr. Howard, the field of myasthenia gravis treatment development is “very rich. And pharma’s interest in myasthenia has taken off like a rocket. It’s exceptionally gratifying to those of us who take care of these patients whose life is miserable” because of adverse effects and/or nonresponse to current drugs.
“In myasthenia,” added Dr. Kaminski, “we know that T cells are promoting the activity of these auto-reactive B cells.” Many drugs currently in phase 2 or 3 development aim to eliminate B cells or signaling between T and B cells, he said. “That’s where most of the drug development is.”
Leading candidates include telitacicept (Tai’ai, RemeGen), which is both a B-lymphocyte stimulator and a proliferation-inducing ligand. A phase 3 trial (NCT05737160) is ongoing, with primary completion expected in late 2026. A second phase 3 trial (NCT06456580) recently began enrolling. Dr. Howard said that, although early results warranted phase 3 analysis, telitacicept’s phase 2 trial was open label and lacked a placebo group.9 The latter is a critical concern because placebo response rates in myasthenia gravis trials average 35%-40%.
Combined with standard care, the FcRn inhibitor nipocalimab (Johnson & Johnson) enabled patients with AChR, MuSK, and/or LRP4 autoantibodies to improve by 4.70 points on the MG-ADL vs 3.25 points for placebo (P = .002) over 24 weeks in phase 3.10All FcRn inhibitors in development can broadly reduce autoantibody levels, said Dr. Howard. “But what role they will play in myasthenia gravis when they’re several years behind leaders in the field in terms of capturing market remains to be seen.”
Additionally, batoclimab (Immunovant/Harbour BioMed) showed positive topline results in phase 3, and an elevated rate of hypercholesterolemia in treated patients that was transient and consistent with previous research.11 Subsequent to efgartigimod, Dr. Howard said, FcRn inhibitors are full-size antibodies. “I believe that contributes to the adverse events that we see. Efgartigimod is a small FcRn fragment. That’s why it’s a cleaner drug, if you will.”
FcRn inhibitors require periodic retreatment. For example, said Dr. Howard, the ADAPT phase 3 trial of efgartigimod, on which he was lead investigator, employed a cyclic dosing schedule – 4 weeks’ treatment, then observation until patients needed retreatment — because patients demanded it.12 In clinical practice, some patients have gone more than 25 weeks before needing retreatment. One of his patients went beyond 40 weeks. “Others only get around 6-9 weeks. So patient choice again enters the decision-making process.”
Rituximab targets the CD20 protein on B cells nonspecifically, producing general immunosuppression. “That’s problematic in producing significant immunosuppression,” said Dr. Kaminski. Nevertheless, he said, rituximab is very effective for most patients with MuSK-specific MG, and its application to this indication has revealed differences between the MuSK subtype and AChR antibody–positive myasthenia. Specifically, MuSK antibody–positive patients have short-lived plasmablasts, which rituximab eliminates.13
Conversely, said Dr. Kaminski, patients with AChR antibody-positive myasthenia, especially long-term, likely have long-lived plasmablasts producing antibodies. This fact, and these patients’ lack of CD20, likely explain their poor response to rituximab.
A phase 3 trial (NCT04524273) of the CD19 blocker inebilizumab (Uplinza, Amgen) reached primary completion in May. Dr. Howard said that if topline results (unreleased at press time) prove positive, inebilizumab could replace rituximab in MG — provided payers do not reject inebilizumab because of cost.
Packed Early-Development Pipeline
Regarding early-stage projects, said Dr. Howard, the pipeline is packed with compounds that target various aspects of the immune system. “The real question with those is, what’s going to be the side effect profile? All of the trials are very early. We need bigger trials with much longer observation for safety, durability, and degree of efficacy.”
The next potential B cell–targeting game changer, he said, is chimeric antigen receptor (CAR) T cell–based therapy. In a phase 2b trial of Descartes-08 (Cartesian Therapeutics), 71% of treated patients experienced clinically meaningful improvement in MG Composite score at 3 months vs 25% for placebo.14
In early clinical trials, said Dr. Howard, patients treated with Descartes-08 — which uses autologous mRNA to target B-cell maturation antigen — have shown “exceptional improvement” lasting 20 or more months. Because the drug is not ingrained permanently into the genome, Descartes-08 avoids potentially severe side effects of DNA-targeting CAR T candidates. Dr. Howard hopes a phase 3 trial will commence around January 2025.
The tolerance approach exemplified by CNP-106 (COUR Pharmaceuticals) and a myasthenia gravis tolerogen (Toleranzia) seeks to prevent the immune system from recognizing and reacting to the NMJ abnormalities that produce myasthenia gravis, potentially providing a cure. “We look forward to those trials as they come online in the next 1-2 years,” said Dr. Howard.
Unmet Needs
Historically, neurologists believed that all myasthenia gravis symptoms stemmed from muscle fatigue — the more active the muscle, the weaker it gets. However, said Dr. Kaminski, some patients might lack measurable weakness but still complain of fatigue.
Elevated levels of cytokines such as interleukin (IL)–6 or IL-17 also can produce fatigue, he noted. “With the drugs we’re using, certainly the new ones, we’re not specifically targeting this fatigue phenomenon, which has been studied in a very limited fashion.”
In the RAISE-XT zilucoplan trial, participants experienced significant improvement in fatigue scores for up to 60 weeks.15 Although zilucoplan does not address fatigue directly, said Dr. Howard, improving myasthenia gravis overall helps reduce fatigue.
The Myasthenia Gravis Symptoms Patient Reported Outcome (MG Symptoms PRO), which Dr. Kaminski helped develop, includes questions designed to distinguish muscular fatigue from overall physical fatigue.16 “I’m very interested in some of the information that’s coming out on long COVID and its effect on muscle,” Dr. Kaminski added. “We might be able to learn from there that there’s still some pathology going on beyond the neuromuscular junction.”
What the field desperately needs, said Dr. Howard, are biomarkers to identify which patients will and will not respond to certain therapeutics. “We’re not there yet.” Such biomarkers are at least 3-7 years from becoming clinical reality.
Promising antibody-independent serum markers include circulating microRNAs. For example, miRNA-150-5p and miRNA-21-5p are elevated in generalized AChR-positive myasthenia gravis and early-onset myasthenia gravis (occurring before age 50) and decline after immunosuppression and thymectomy.17
Among diagnostic modalities for patients with seronegative myasthenia gravis, said Dr. Kaminski, single-fiber EMG is the most sensitive, at approximately 95%. “It’s not perfect.” Moreover, he said, performing this test accurately requires a highly experienced expert, which many treatment centers lack.
Presently, added Dr. Kaminski, orbital MRI is neither specific nor sensitive enough to be clinically useful. “One needs to be careful with these specialized tests that are published from the best laboratory in the world that does the test, and does it repetitively.” As the search for effective myasthenia gravis biomarkers continues, avoiding false-positive results is as important as avoiding false negatives.
References
1. Bubuioc AM et al. J Med Life. 2021 Jan-Mar;14(1):7-16. doi: 10.25122/jml-2020-0145.
2. Deenen JC et al. J Neuromuscul Dis. 2015;2(1):73-85. doi: 10.3233/JND-140045.
3. Kaminski HJ et al. J Clin Invest. 2024 Jun 17;134(12):e179742. doi: 10.1172/JCI179742.
4. Howard JF Jr et al. Lancet Neurol. 2017 Dec;16(12):976-986. doi: 10.1016/S1474-4422(17)30369-1.
5. Huda R. Front Immunol. 2020 Feb 21:11:240. doi: 10.3389/fimmu.2020.00240.
6. Howard JF Jr et al. Lancet Neurol. 2023 May;22(5):395-406. doi: 10.1016/S1474-4422(23)00080-7.
7. Vu T et al. NEJM Evid. 2022 May;1(5):EVIDoa2100066. doi: 10.1056/EVIDoa2100066.
8. Tice JA et al. October 20, 2021. https://icer.org/assessment/myasthenia-gravis/.
9. Yin J et al. Eur J Neurol. 2024 Aug;31(8):e16322. doi: 10.1111/ene.16322.
10. Antozzi C et al. EAN 2024, Abstract EPR-116. https://www.neurology.org/doi/10.1212/WNL.0000000000203660.
11. Yan C et al. JAMA Neurol. 2024 Mar 4;81(4):336-345. doi: 10.1001/jamaneurol.2024.0044.
12. Howard JF Jr et al. Lancet Neurol. 2021 Jul;20(7):526-536. doi: 10.1016/S1474-4422(21)00159-9.
13. Stathopoulos P et al. JCI Insight. 2017 Sep 7;2(17):e94263. doi: 10.1172/jci.insight.94263.
14. Cartesian Therapeutics. Cartesian Therapeutics announces positive topline results from phase 2b trial of Descartes-08 in patients with myasthenia gravis. 2024 Jul 2. https://ir.cartesiantherapeutics.com/news-releases/news-release-details/cartesian-therapeutics-announces-positive-topline-results-phase.
15. Howard JF Jr et al. Ther Adv Neurol Disord. 2024 Apr 17:17:17562864241243186. doi: 10.1177/17562864241243186.
16. Cleanthous S et al. Orphanet J Rare Dis. 2021 Oct 30;16(1):457. doi: 10.1186/s13023-021-02064-0.
17. Sabre L et al. Front Immunol. 2020 Mar 4:11:213. doi: 10.3389/fimmu.2020.00213.
Does MS Protect Against Alzheimer’s Disease?
In a recent study, Understanding how MS does this may drive new treatment strategies, said the authors of the study, which was published online in Annals of Neurology. Regarding current treatments, they added, the availability of new disease-modifying Alzheimer’s disease therapies increases the importance of early diagnosis in cognitively impaired people including those with MS.
Confirmatory Studies Needed
“Replication and confirmation of these findings, including in studies representative of the real-world Alzheimer’s population in race/ethnicity and sex/gender, are needed before any clinical implications can be drawn,” said Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach. She was not involved with the study but was asked to comment.
The study’s most important immediate implication, said Dr. Sexton, is that it “opens the door to questions about why MS may be associated with Alzheimer’s risk.”
Anecdotal Observation
Although life expectancy for people with MS is increasing, the authors, led by Matthew R. Brier, MD, PhD, an assistant professor at Washington University in St. Louis, Missouri, said they have seen no concomitant rise in Alzheimer’s disease dementia among their patients with MS. This anecdotal observation fueled their hypothesis that Alzheimer’s disease pathology occurs less frequently in this population.
To test their hypothesis, the investigators sequentially enrolled 100 patients with MS (age 60 years or older), along with 300 non-MS controls matched for age, sex, apolipoprotein E (apoE) proteotype, and cognitive status. All participants underwent the Mini-Mental State Examination (MMSE) and PrecivityAD2 (C2N Diagnostics) blood testing.
Overall, patients with MS had lower p-tau217 (t = 3.76, P = .00019) and amyloid probability score 2 (APS2; t = 3.83, P = .00015) ratios than did those without MS. APS2 combines p-tau217 ratio with Abeta42/40 ratio. In addition, APS2 and p-tau217 ratios were lower in patients with MS and ApoE3/apoE3 or apoE3/apoE4 proteotype. MMSE scores were also slightly lower in the MS cohort: 27.6 versus 28.44 for controls. Of 11 patients with MS who underwent Pittsburgh Compound B (PiB) positron emission tomography (PET), nine had congruent PiB PET and plasma results.
When the investigators applied clinical cutoffs, 7.1% of patients with MS were APS2-positive, versus 15.3% of controls (P = .0043). The corresponding figures for p-tau217 ratio positivity were 9% and 18.3%, respectively (P = .0024). Mean Abeta42/40 scores showed no difference between groups.
Patients with MS and positive amyloid biomarkers often had atypical MS features at diagnosis. Compared with biomarker-negative patients with MS, odds ratios for having at least two atypical MS features at diagnosis among APS2-positive and p-tau217 ratio-positive patients with MS were 23.3 and 11.38, respectively.
Data regarding the actual incidence of Alzheimer’s disease among people with MS are scarce and conflicting. An autopsy study published in Annals of Neurology in 2008 revealed the expected rate of amyloid pathology in MS brain tissue, along with extensive microglia activation. In a PET study published in Annals of Neurology in 2020, however, researchers found less amyloid pathology among patients with MS than those without, but little difference in tau pathology.
Because MS and Alzheimer’s disease can each cause cognitive impairment, the rate of co-occurrence of MS and Alzheimer’s disease has been difficult to ascertain without accurate biomarkers. But, the authors said, the advent of disease-modifying therapies makes identifying early Alzheimer’s dementia in MS patients relevant.
Possible Explanations
The authors hypothesized that the lower rate of amyloid pathology observed in their patients with MS may stem from the following possibly overlapping mechanisms:
- MS components, such as persistent perilesional immune activity, may inhibit amyloid beta deposition or facilitate its clearance.
- Exposure to MS drugs may impact Alzheimer’s disease pathology. Most study patients with MS were exposed to beta interferons or glatiramer acetate, the authors noted, and 39 had switched to high-efficacy medications such as B-cell depleting therapies and natalizumab.
- MS’s genetic signature may protect against AD.
“Investigating these ideas would advance our understanding of the relationship between MS and Alzheimer’s, and potentially inform avenues for treatment,” said Dr. Sexton. In this regard, the Alzheimer’s Association has funded an ongoing study examining a drug currently used to promote myelin formation in individuals with MS in genetically engineered Alzheimer’s-like mice. Additional Association-funded studies that examine inflammation also may improve understanding of the mechanisms that may link these diseases, said Dr. Sexton.
The study authors added that unusual cases, such as a study patient who had high amyloid burden by PET but negative APS2 and tau PET, also may shed light on interactions between MS, amyloid pathology, and tau pathology.
Limitations of the present study include the fact that plasma Alzheimer’s disease biomarkers are potentially affected by other conditions as well, according to a study published in Nature Medicine. Additional shortcomings include the MS cohort’s relatively small size and lack of diagnostic confirmation by cerebrospinal fluid. Although MMSE scores among patients with MS were slightly lower, the authors added, this disparity would lead one to expect more, not less, amyloid pathology among these patients if their cognitive impairment resulted from Alzheimer’s disease.
Dr. Sexton reported no relevant financial interests.
The study was supported by the Hope Center for Neurological Disorders at Washington University in St. Louis and by C2N Diagnostics. Washington University in St. Louis holds equity in C2N Diagnostics and may receive royalties resulting from use of PrecivityAD2.
In a recent study, Understanding how MS does this may drive new treatment strategies, said the authors of the study, which was published online in Annals of Neurology. Regarding current treatments, they added, the availability of new disease-modifying Alzheimer’s disease therapies increases the importance of early diagnosis in cognitively impaired people including those with MS.
Confirmatory Studies Needed
“Replication and confirmation of these findings, including in studies representative of the real-world Alzheimer’s population in race/ethnicity and sex/gender, are needed before any clinical implications can be drawn,” said Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach. She was not involved with the study but was asked to comment.
The study’s most important immediate implication, said Dr. Sexton, is that it “opens the door to questions about why MS may be associated with Alzheimer’s risk.”
Anecdotal Observation
Although life expectancy for people with MS is increasing, the authors, led by Matthew R. Brier, MD, PhD, an assistant professor at Washington University in St. Louis, Missouri, said they have seen no concomitant rise in Alzheimer’s disease dementia among their patients with MS. This anecdotal observation fueled their hypothesis that Alzheimer’s disease pathology occurs less frequently in this population.
To test their hypothesis, the investigators sequentially enrolled 100 patients with MS (age 60 years or older), along with 300 non-MS controls matched for age, sex, apolipoprotein E (apoE) proteotype, and cognitive status. All participants underwent the Mini-Mental State Examination (MMSE) and PrecivityAD2 (C2N Diagnostics) blood testing.
Overall, patients with MS had lower p-tau217 (t = 3.76, P = .00019) and amyloid probability score 2 (APS2; t = 3.83, P = .00015) ratios than did those without MS. APS2 combines p-tau217 ratio with Abeta42/40 ratio. In addition, APS2 and p-tau217 ratios were lower in patients with MS and ApoE3/apoE3 or apoE3/apoE4 proteotype. MMSE scores were also slightly lower in the MS cohort: 27.6 versus 28.44 for controls. Of 11 patients with MS who underwent Pittsburgh Compound B (PiB) positron emission tomography (PET), nine had congruent PiB PET and plasma results.
When the investigators applied clinical cutoffs, 7.1% of patients with MS were APS2-positive, versus 15.3% of controls (P = .0043). The corresponding figures for p-tau217 ratio positivity were 9% and 18.3%, respectively (P = .0024). Mean Abeta42/40 scores showed no difference between groups.
Patients with MS and positive amyloid biomarkers often had atypical MS features at diagnosis. Compared with biomarker-negative patients with MS, odds ratios for having at least two atypical MS features at diagnosis among APS2-positive and p-tau217 ratio-positive patients with MS were 23.3 and 11.38, respectively.
Data regarding the actual incidence of Alzheimer’s disease among people with MS are scarce and conflicting. An autopsy study published in Annals of Neurology in 2008 revealed the expected rate of amyloid pathology in MS brain tissue, along with extensive microglia activation. In a PET study published in Annals of Neurology in 2020, however, researchers found less amyloid pathology among patients with MS than those without, but little difference in tau pathology.
Because MS and Alzheimer’s disease can each cause cognitive impairment, the rate of co-occurrence of MS and Alzheimer’s disease has been difficult to ascertain without accurate biomarkers. But, the authors said, the advent of disease-modifying therapies makes identifying early Alzheimer’s dementia in MS patients relevant.
Possible Explanations
The authors hypothesized that the lower rate of amyloid pathology observed in their patients with MS may stem from the following possibly overlapping mechanisms:
- MS components, such as persistent perilesional immune activity, may inhibit amyloid beta deposition or facilitate its clearance.
- Exposure to MS drugs may impact Alzheimer’s disease pathology. Most study patients with MS were exposed to beta interferons or glatiramer acetate, the authors noted, and 39 had switched to high-efficacy medications such as B-cell depleting therapies and natalizumab.
- MS’s genetic signature may protect against AD.
“Investigating these ideas would advance our understanding of the relationship between MS and Alzheimer’s, and potentially inform avenues for treatment,” said Dr. Sexton. In this regard, the Alzheimer’s Association has funded an ongoing study examining a drug currently used to promote myelin formation in individuals with MS in genetically engineered Alzheimer’s-like mice. Additional Association-funded studies that examine inflammation also may improve understanding of the mechanisms that may link these diseases, said Dr. Sexton.
The study authors added that unusual cases, such as a study patient who had high amyloid burden by PET but negative APS2 and tau PET, also may shed light on interactions between MS, amyloid pathology, and tau pathology.
Limitations of the present study include the fact that plasma Alzheimer’s disease biomarkers are potentially affected by other conditions as well, according to a study published in Nature Medicine. Additional shortcomings include the MS cohort’s relatively small size and lack of diagnostic confirmation by cerebrospinal fluid. Although MMSE scores among patients with MS were slightly lower, the authors added, this disparity would lead one to expect more, not less, amyloid pathology among these patients if their cognitive impairment resulted from Alzheimer’s disease.
Dr. Sexton reported no relevant financial interests.
The study was supported by the Hope Center for Neurological Disorders at Washington University in St. Louis and by C2N Diagnostics. Washington University in St. Louis holds equity in C2N Diagnostics and may receive royalties resulting from use of PrecivityAD2.
In a recent study, Understanding how MS does this may drive new treatment strategies, said the authors of the study, which was published online in Annals of Neurology. Regarding current treatments, they added, the availability of new disease-modifying Alzheimer’s disease therapies increases the importance of early diagnosis in cognitively impaired people including those with MS.
Confirmatory Studies Needed
“Replication and confirmation of these findings, including in studies representative of the real-world Alzheimer’s population in race/ethnicity and sex/gender, are needed before any clinical implications can be drawn,” said Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach. She was not involved with the study but was asked to comment.
The study’s most important immediate implication, said Dr. Sexton, is that it “opens the door to questions about why MS may be associated with Alzheimer’s risk.”
Anecdotal Observation
Although life expectancy for people with MS is increasing, the authors, led by Matthew R. Brier, MD, PhD, an assistant professor at Washington University in St. Louis, Missouri, said they have seen no concomitant rise in Alzheimer’s disease dementia among their patients with MS. This anecdotal observation fueled their hypothesis that Alzheimer’s disease pathology occurs less frequently in this population.
To test their hypothesis, the investigators sequentially enrolled 100 patients with MS (age 60 years or older), along with 300 non-MS controls matched for age, sex, apolipoprotein E (apoE) proteotype, and cognitive status. All participants underwent the Mini-Mental State Examination (MMSE) and PrecivityAD2 (C2N Diagnostics) blood testing.
Overall, patients with MS had lower p-tau217 (t = 3.76, P = .00019) and amyloid probability score 2 (APS2; t = 3.83, P = .00015) ratios than did those without MS. APS2 combines p-tau217 ratio with Abeta42/40 ratio. In addition, APS2 and p-tau217 ratios were lower in patients with MS and ApoE3/apoE3 or apoE3/apoE4 proteotype. MMSE scores were also slightly lower in the MS cohort: 27.6 versus 28.44 for controls. Of 11 patients with MS who underwent Pittsburgh Compound B (PiB) positron emission tomography (PET), nine had congruent PiB PET and plasma results.
When the investigators applied clinical cutoffs, 7.1% of patients with MS were APS2-positive, versus 15.3% of controls (P = .0043). The corresponding figures for p-tau217 ratio positivity were 9% and 18.3%, respectively (P = .0024). Mean Abeta42/40 scores showed no difference between groups.
Patients with MS and positive amyloid biomarkers often had atypical MS features at diagnosis. Compared with biomarker-negative patients with MS, odds ratios for having at least two atypical MS features at diagnosis among APS2-positive and p-tau217 ratio-positive patients with MS were 23.3 and 11.38, respectively.
Data regarding the actual incidence of Alzheimer’s disease among people with MS are scarce and conflicting. An autopsy study published in Annals of Neurology in 2008 revealed the expected rate of amyloid pathology in MS brain tissue, along with extensive microglia activation. In a PET study published in Annals of Neurology in 2020, however, researchers found less amyloid pathology among patients with MS than those without, but little difference in tau pathology.
Because MS and Alzheimer’s disease can each cause cognitive impairment, the rate of co-occurrence of MS and Alzheimer’s disease has been difficult to ascertain without accurate biomarkers. But, the authors said, the advent of disease-modifying therapies makes identifying early Alzheimer’s dementia in MS patients relevant.
Possible Explanations
The authors hypothesized that the lower rate of amyloid pathology observed in their patients with MS may stem from the following possibly overlapping mechanisms:
- MS components, such as persistent perilesional immune activity, may inhibit amyloid beta deposition or facilitate its clearance.
- Exposure to MS drugs may impact Alzheimer’s disease pathology. Most study patients with MS were exposed to beta interferons or glatiramer acetate, the authors noted, and 39 had switched to high-efficacy medications such as B-cell depleting therapies and natalizumab.
- MS’s genetic signature may protect against AD.
“Investigating these ideas would advance our understanding of the relationship between MS and Alzheimer’s, and potentially inform avenues for treatment,” said Dr. Sexton. In this regard, the Alzheimer’s Association has funded an ongoing study examining a drug currently used to promote myelin formation in individuals with MS in genetically engineered Alzheimer’s-like mice. Additional Association-funded studies that examine inflammation also may improve understanding of the mechanisms that may link these diseases, said Dr. Sexton.
The study authors added that unusual cases, such as a study patient who had high amyloid burden by PET but negative APS2 and tau PET, also may shed light on interactions between MS, amyloid pathology, and tau pathology.
Limitations of the present study include the fact that plasma Alzheimer’s disease biomarkers are potentially affected by other conditions as well, according to a study published in Nature Medicine. Additional shortcomings include the MS cohort’s relatively small size and lack of diagnostic confirmation by cerebrospinal fluid. Although MMSE scores among patients with MS were slightly lower, the authors added, this disparity would lead one to expect more, not less, amyloid pathology among these patients if their cognitive impairment resulted from Alzheimer’s disease.
Dr. Sexton reported no relevant financial interests.
The study was supported by the Hope Center for Neurological Disorders at Washington University in St. Louis and by C2N Diagnostics. Washington University in St. Louis holds equity in C2N Diagnostics and may receive royalties resulting from use of PrecivityAD2.
FROM ANNALS OF NEUROLOGY
NODDI and DTI in Remote Mild Traumatic Brain Injury
, according to authors of a recent study. In particular, they said, using neurite orientation dispersion and density imaging (NODDI) to monitor long-term mTBI impact on brain regions related to cognitive and emotional processing can help clinicians assess recovery, predict progression, and optimize treatment.
“Currently,” said co-senior study author Ping-Hong Yeh, PhD, “there is a lack of minimally invasive, quantitative diagnostic biomarkers for monitoring progression or recovery after mild TBI. However, mild TBI can be quite disabling, with many patients reporting symptoms months or even years after injury. This is the most difficult part to diagnose.” Dr. Yeh is a researcher at the National Intrepid Center of Excellence (NICoE) at Walter Reed National Military Medical Center, Bethesda, Maryland.
The NICoE, a Department of Defense organization and the senior member of Defense Intrepid Network for Traumatic Brain Injury and Brain Health, is among several centers charged with improving support for injured service members’ recovery, rehabilitation, and reintegration into their communities. The overarching goal, said Dr. Yeh, is to enable community neurologists to refer service members and veterans to these centers for treatment and advanced imaging when needed.
Invisible Wounds
Limitations of conventional MRI and CT make it tough to discern which patients with mTBI will return to baseline functioning, and which will develop long-term complications. Addressing the silent or invisible wounds of mTBI will require improved diagnostic, prognostic, and therapeutic tools, he said.
For their study, published in JAMA Network Open, Dr. Yeh and colleagues compared diffusion tensor imaging (DTI) and NODDI data from 65 male service members with remote (more than 2 years old) mTBI against scans of 33 noninjured controls matched for age, sex, and active-duty status.
“Although DTI is very sensitive in detecting microstructural changes in mild TBI,” he said, “it is not specific to the underlying pathophysiological changes.”
Conversely, NODDI uses biophysical modeling of intracellular diffusion, extracellular diffusion, and free water to help physicians to understand subtle pathophysiological changes with greater sensitivity and specificity than does DTI. “This will allow us to correlate symptoms with brain structural changes, making the invisible wound visible.”
In the study, the greatest differences between injured and control patients appeared in the following NODDI metrics (P <.001 in all analyses):
- Intracellular volume fraction (ICVF) of the right corticospinal tract (CST)
- Orientation dispersion index (ODI) of the left posterior thalamic radiation (PTR)
- ODI of the left uncinate fasciculus (UNC)
Regarding patient-reported neurobehavioral symptoms, Neurobehavioral Symptom Inventory cognitive subscores were associated with fractional anisotropy of the left UNC. In addition, PTSD Checklist–Civilian version total scores and avoidance subscores corresponded, respectively, with isotropic volume fraction (ISOVF) of the genu of corpus callosum and with ODI of the left fornix and stria terminalis.
Next Steps
Presently, Dr. Yeh said, conventional MRI and CT usually cannot differentiate between axonal injury, axonal inflammation (which develops during the chronic phase of mTBI), and demyelination. “But newer biophysical modeling, such as NODDI, will allow us to tell the difference.” Along with providing prognostic information, he said, such technology can guide appropriate treatment, such as anti-inflammatory agents for chronic inflammation.
Most community neurologists refer patients with persistent mTBI symptoms in the absence of red flags using CT and conventional MRI for advanced neuroimaging, said Dr. Yeh. But because few community neurologists are familiar with NODDI, he said, broadening its reach will require educating these providers. Additional steps that Dr. Yeh said could occur over the next decade or more include boosting advanced dMRI sensitivity levels through improved hardware, software, and diagnostic tools.
“We need to make these techniques clinically feasible,” he added. Currently, protocols that allow advanced dMRI scans in about 10 minutes can be achievable.
The investments required to implement advanced dMRI techniques will be substantial. A state-of-the-art 3T MRI scanner that can support NODDI and DTI can easily cost $1 million, said Dr. Yeh. Factor in additional equipment options and construction costs, he added, and the total price tag can easily exceed $2 million. But rather than replacing all existing MRI systems, said Dr. Yeh, AI one day may help translate high-gradient capability even to widely used lower-field MRI scanners operating at 0.5T.
Streamlining systems that incorporate disparate scanners with different acquisition parameters will require standardized data acquisition and sharing parameters. Along with helping to evaluate new techniques as they become available, data harmonization and sharing can facilitate a shift from research comparisons between large groups to comparing a single patient against many others — a move that Dr. Yeh said must occur for advanced dMRI techniques to achieve clinical relevance.
In addition, experts will need to revise clinical guidelines for use of new technologies as their availability grows. “Improper use of these techniques will not only increase health costs, but also probably result in adverse health results.” Such guidelines could be very useful in evaluating the suitability and quality of referrals for diagnostic images, Dr. Yeh said.
Dr. Yeh reports no relevant financial interests. The project was partially funded by the US Army Medical Research and Materiel Command.
, according to authors of a recent study. In particular, they said, using neurite orientation dispersion and density imaging (NODDI) to monitor long-term mTBI impact on brain regions related to cognitive and emotional processing can help clinicians assess recovery, predict progression, and optimize treatment.
“Currently,” said co-senior study author Ping-Hong Yeh, PhD, “there is a lack of minimally invasive, quantitative diagnostic biomarkers for monitoring progression or recovery after mild TBI. However, mild TBI can be quite disabling, with many patients reporting symptoms months or even years after injury. This is the most difficult part to diagnose.” Dr. Yeh is a researcher at the National Intrepid Center of Excellence (NICoE) at Walter Reed National Military Medical Center, Bethesda, Maryland.
The NICoE, a Department of Defense organization and the senior member of Defense Intrepid Network for Traumatic Brain Injury and Brain Health, is among several centers charged with improving support for injured service members’ recovery, rehabilitation, and reintegration into their communities. The overarching goal, said Dr. Yeh, is to enable community neurologists to refer service members and veterans to these centers for treatment and advanced imaging when needed.
Invisible Wounds
Limitations of conventional MRI and CT make it tough to discern which patients with mTBI will return to baseline functioning, and which will develop long-term complications. Addressing the silent or invisible wounds of mTBI will require improved diagnostic, prognostic, and therapeutic tools, he said.
For their study, published in JAMA Network Open, Dr. Yeh and colleagues compared diffusion tensor imaging (DTI) and NODDI data from 65 male service members with remote (more than 2 years old) mTBI against scans of 33 noninjured controls matched for age, sex, and active-duty status.
“Although DTI is very sensitive in detecting microstructural changes in mild TBI,” he said, “it is not specific to the underlying pathophysiological changes.”
Conversely, NODDI uses biophysical modeling of intracellular diffusion, extracellular diffusion, and free water to help physicians to understand subtle pathophysiological changes with greater sensitivity and specificity than does DTI. “This will allow us to correlate symptoms with brain structural changes, making the invisible wound visible.”
In the study, the greatest differences between injured and control patients appeared in the following NODDI metrics (P <.001 in all analyses):
- Intracellular volume fraction (ICVF) of the right corticospinal tract (CST)
- Orientation dispersion index (ODI) of the left posterior thalamic radiation (PTR)
- ODI of the left uncinate fasciculus (UNC)
Regarding patient-reported neurobehavioral symptoms, Neurobehavioral Symptom Inventory cognitive subscores were associated with fractional anisotropy of the left UNC. In addition, PTSD Checklist–Civilian version total scores and avoidance subscores corresponded, respectively, with isotropic volume fraction (ISOVF) of the genu of corpus callosum and with ODI of the left fornix and stria terminalis.
Next Steps
Presently, Dr. Yeh said, conventional MRI and CT usually cannot differentiate between axonal injury, axonal inflammation (which develops during the chronic phase of mTBI), and demyelination. “But newer biophysical modeling, such as NODDI, will allow us to tell the difference.” Along with providing prognostic information, he said, such technology can guide appropriate treatment, such as anti-inflammatory agents for chronic inflammation.
Most community neurologists refer patients with persistent mTBI symptoms in the absence of red flags using CT and conventional MRI for advanced neuroimaging, said Dr. Yeh. But because few community neurologists are familiar with NODDI, he said, broadening its reach will require educating these providers. Additional steps that Dr. Yeh said could occur over the next decade or more include boosting advanced dMRI sensitivity levels through improved hardware, software, and diagnostic tools.
“We need to make these techniques clinically feasible,” he added. Currently, protocols that allow advanced dMRI scans in about 10 minutes can be achievable.
The investments required to implement advanced dMRI techniques will be substantial. A state-of-the-art 3T MRI scanner that can support NODDI and DTI can easily cost $1 million, said Dr. Yeh. Factor in additional equipment options and construction costs, he added, and the total price tag can easily exceed $2 million. But rather than replacing all existing MRI systems, said Dr. Yeh, AI one day may help translate high-gradient capability even to widely used lower-field MRI scanners operating at 0.5T.
Streamlining systems that incorporate disparate scanners with different acquisition parameters will require standardized data acquisition and sharing parameters. Along with helping to evaluate new techniques as they become available, data harmonization and sharing can facilitate a shift from research comparisons between large groups to comparing a single patient against many others — a move that Dr. Yeh said must occur for advanced dMRI techniques to achieve clinical relevance.
In addition, experts will need to revise clinical guidelines for use of new technologies as their availability grows. “Improper use of these techniques will not only increase health costs, but also probably result in adverse health results.” Such guidelines could be very useful in evaluating the suitability and quality of referrals for diagnostic images, Dr. Yeh said.
Dr. Yeh reports no relevant financial interests. The project was partially funded by the US Army Medical Research and Materiel Command.
, according to authors of a recent study. In particular, they said, using neurite orientation dispersion and density imaging (NODDI) to monitor long-term mTBI impact on brain regions related to cognitive and emotional processing can help clinicians assess recovery, predict progression, and optimize treatment.
“Currently,” said co-senior study author Ping-Hong Yeh, PhD, “there is a lack of minimally invasive, quantitative diagnostic biomarkers for monitoring progression or recovery after mild TBI. However, mild TBI can be quite disabling, with many patients reporting symptoms months or even years after injury. This is the most difficult part to diagnose.” Dr. Yeh is a researcher at the National Intrepid Center of Excellence (NICoE) at Walter Reed National Military Medical Center, Bethesda, Maryland.
The NICoE, a Department of Defense organization and the senior member of Defense Intrepid Network for Traumatic Brain Injury and Brain Health, is among several centers charged with improving support for injured service members’ recovery, rehabilitation, and reintegration into their communities. The overarching goal, said Dr. Yeh, is to enable community neurologists to refer service members and veterans to these centers for treatment and advanced imaging when needed.
Invisible Wounds
Limitations of conventional MRI and CT make it tough to discern which patients with mTBI will return to baseline functioning, and which will develop long-term complications. Addressing the silent or invisible wounds of mTBI will require improved diagnostic, prognostic, and therapeutic tools, he said.
For their study, published in JAMA Network Open, Dr. Yeh and colleagues compared diffusion tensor imaging (DTI) and NODDI data from 65 male service members with remote (more than 2 years old) mTBI against scans of 33 noninjured controls matched for age, sex, and active-duty status.
“Although DTI is very sensitive in detecting microstructural changes in mild TBI,” he said, “it is not specific to the underlying pathophysiological changes.”
Conversely, NODDI uses biophysical modeling of intracellular diffusion, extracellular diffusion, and free water to help physicians to understand subtle pathophysiological changes with greater sensitivity and specificity than does DTI. “This will allow us to correlate symptoms with brain structural changes, making the invisible wound visible.”
In the study, the greatest differences between injured and control patients appeared in the following NODDI metrics (P <.001 in all analyses):
- Intracellular volume fraction (ICVF) of the right corticospinal tract (CST)
- Orientation dispersion index (ODI) of the left posterior thalamic radiation (PTR)
- ODI of the left uncinate fasciculus (UNC)
Regarding patient-reported neurobehavioral symptoms, Neurobehavioral Symptom Inventory cognitive subscores were associated with fractional anisotropy of the left UNC. In addition, PTSD Checklist–Civilian version total scores and avoidance subscores corresponded, respectively, with isotropic volume fraction (ISOVF) of the genu of corpus callosum and with ODI of the left fornix and stria terminalis.
Next Steps
Presently, Dr. Yeh said, conventional MRI and CT usually cannot differentiate between axonal injury, axonal inflammation (which develops during the chronic phase of mTBI), and demyelination. “But newer biophysical modeling, such as NODDI, will allow us to tell the difference.” Along with providing prognostic information, he said, such technology can guide appropriate treatment, such as anti-inflammatory agents for chronic inflammation.
Most community neurologists refer patients with persistent mTBI symptoms in the absence of red flags using CT and conventional MRI for advanced neuroimaging, said Dr. Yeh. But because few community neurologists are familiar with NODDI, he said, broadening its reach will require educating these providers. Additional steps that Dr. Yeh said could occur over the next decade or more include boosting advanced dMRI sensitivity levels through improved hardware, software, and diagnostic tools.
“We need to make these techniques clinically feasible,” he added. Currently, protocols that allow advanced dMRI scans in about 10 minutes can be achievable.
The investments required to implement advanced dMRI techniques will be substantial. A state-of-the-art 3T MRI scanner that can support NODDI and DTI can easily cost $1 million, said Dr. Yeh. Factor in additional equipment options and construction costs, he added, and the total price tag can easily exceed $2 million. But rather than replacing all existing MRI systems, said Dr. Yeh, AI one day may help translate high-gradient capability even to widely used lower-field MRI scanners operating at 0.5T.
Streamlining systems that incorporate disparate scanners with different acquisition parameters will require standardized data acquisition and sharing parameters. Along with helping to evaluate new techniques as they become available, data harmonization and sharing can facilitate a shift from research comparisons between large groups to comparing a single patient against many others — a move that Dr. Yeh said must occur for advanced dMRI techniques to achieve clinical relevance.
In addition, experts will need to revise clinical guidelines for use of new technologies as their availability grows. “Improper use of these techniques will not only increase health costs, but also probably result in adverse health results.” Such guidelines could be very useful in evaluating the suitability and quality of referrals for diagnostic images, Dr. Yeh said.
Dr. Yeh reports no relevant financial interests. The project was partially funded by the US Army Medical Research and Materiel Command.
FROM JAMA NETWORK OPEN
Study Finds Varying Skin Cancer Rates Based on Sexual Orientation
Addressing dynamics of each SM subgroup will require increasingly tailored prevention, screening, and research efforts, the study authors said.
“We identified specific subgroups within the sexual minority community who are at higher risk for skin cancer, specifically White gay males and Hispanic and non-Hispanic Black SM men and women — particularly individuals who identify as bisexual,” senior author Matthew Mansh, MD, said in an interview. He is an assistant professor of dermatology at the University of California, San Francisco. The study was published online in JAMA Dermatology.
Using data of adults in the US general population from the Behavioral Risk Factor Surveillance System from January 2014 to December 2021, investigators included more than 1.5 million respondents. The proportions of SM women and men (who self-identified as bisexual, lesbian, gay, “something else,” or other) were 2.6% and 2.0%, respectively.
Lifetime skin cancer prevalence was higher among SM men than among heterosexual men (7.4% vs 6.8%; adjusted odds ratio [aOR], 1.16). In analyses stratified by racial and ethnic group, AORs for non-Hispanic Black and Hispanic SM men vs their heterosexual counterparts were 2.18 and 3.81, respectively. The corresponding figures for non-Hispanic Black and Hispanic SM women were 2.33 and 2.46, respectively.
When investigators combined all minority respondents along gender lines, lifetime skin cancer prevalence was higher in bisexual men (aOR, 3.94), bisexual women (aOR, 1.51), and women identifying as something else or other (aOR, 2.70) than in their heterosexual peers.
“I wasn’t expecting that Hispanic or non-Hispanic Black SMs would be at higher risk for skin cancer,” Dr. Mansh said. Even if these groups have more behavioral risk factors for UV radiation (UVR) exposure, he explained, UVR exposure is less strongly linked with skin cancer in darker skin than in lighter skin. Reasons for the counterintuitive finding could include different screening habits among SM people of different racial and ethnic groups, he said, and analyzing such factors will require further research.
Although some effect sizes were modest, the authors wrote, their findings may have important implications for population-based research and public health efforts aimed at early skin cancer detection and prevention. Presently, the United States lacks established guidelines for skin cancer screening. In a 2023 statement published in JAMA, the US Preventive Services Task Force said that there is insufficient evidence to determine the benefit-harm balance of skin cancer screening in asymptomatic people.
“So there has been a lot of recent talk and a need to identify which subset groups of patients might be higher risk for skin cancer and might benefit from more screening,” Dr. Mansh said in an interview. “Understanding more about the high-risk demographic and clinical features that predispose someone to skin cancer helps identify these high-risk populations that could be used to develop better screening guidelines.”
Identifying groups at a higher risk for skin cancer also allows experts to design more targeted counseling or public health interventions focused on these groups, Dr. Mansh added. Absent screening guidelines, experts emphasize changing modifiable risk factors such as UVR exposure, smoking, and alcohol use. “And we know that the message that might change behaviors in a cisgender heterosexual man might be different than in a gay White male or a Hispanic bisexual male.”
A 2017 review showed that interventions to reduce behaviors involving UVR exposure, such as indoor tanning, among young cisgender women focused largely on aging and appearance-based concerns. A 2019 study showed that messages focused on avoiding skin cancer may help motivate SM men to reduce tanning behaviors.
Furthermore, said Dr. Mansh, all electronic health record products available in the United States must provide data fields for sexual orientation. “I don’t believe many dermatologists, depending on the setting, collect that information routinely. Integrating sexual orientation and/or gender identity data into patient intake forms so that it can be integrated into the electronic health record is probably very helpful, not only for your clinical practice but also for future research studies.”
Asked to comment on the results, Rebecca I. Hartman, MD, MPH, who was not involved with the study, said that its impact on clinical practice will be challenging to ascertain. She is chief of dermatology with the VA Boston Healthcare System, assistant professor of dermatology at Harvard Medical School, and director of melanoma epidemiology at Brigham and Women’s Hospital, all in Boston, Massachusetts.
“The study found significant adjusted odds ratios,” Dr. Hartman explained, “but for some of the different populations, the overall lifetime rate of skin cancer is still quite low.” For example, 1.0% for SM non-Hispanic Black men or a difference of 2.1% vs 1.8% in SM Hispanic women. “Thus, I am not sure specific screening recommendations are warranted, although some populations, such as Hispanic sexual minority males, seemed to have a much higher risk (3.8-fold on adjusted analysis) that warrants further investigation.”
For now, she advised assessing patients’ risks for skin cancer based on well-established risk factors such as sun exposure/indoor tanning, skin phototype, immunosuppression, and age.
Dr. Mansh reported no relevant conflicts or funding sources for the study. Dr. Hartman reported no relevant conflicts.
A version of this article appeared on Medscape.com.
Addressing dynamics of each SM subgroup will require increasingly tailored prevention, screening, and research efforts, the study authors said.
“We identified specific subgroups within the sexual minority community who are at higher risk for skin cancer, specifically White gay males and Hispanic and non-Hispanic Black SM men and women — particularly individuals who identify as bisexual,” senior author Matthew Mansh, MD, said in an interview. He is an assistant professor of dermatology at the University of California, San Francisco. The study was published online in JAMA Dermatology.
Using data of adults in the US general population from the Behavioral Risk Factor Surveillance System from January 2014 to December 2021, investigators included more than 1.5 million respondents. The proportions of SM women and men (who self-identified as bisexual, lesbian, gay, “something else,” or other) were 2.6% and 2.0%, respectively.
Lifetime skin cancer prevalence was higher among SM men than among heterosexual men (7.4% vs 6.8%; adjusted odds ratio [aOR], 1.16). In analyses stratified by racial and ethnic group, AORs for non-Hispanic Black and Hispanic SM men vs their heterosexual counterparts were 2.18 and 3.81, respectively. The corresponding figures for non-Hispanic Black and Hispanic SM women were 2.33 and 2.46, respectively.
When investigators combined all minority respondents along gender lines, lifetime skin cancer prevalence was higher in bisexual men (aOR, 3.94), bisexual women (aOR, 1.51), and women identifying as something else or other (aOR, 2.70) than in their heterosexual peers.
“I wasn’t expecting that Hispanic or non-Hispanic Black SMs would be at higher risk for skin cancer,” Dr. Mansh said. Even if these groups have more behavioral risk factors for UV radiation (UVR) exposure, he explained, UVR exposure is less strongly linked with skin cancer in darker skin than in lighter skin. Reasons for the counterintuitive finding could include different screening habits among SM people of different racial and ethnic groups, he said, and analyzing such factors will require further research.
Although some effect sizes were modest, the authors wrote, their findings may have important implications for population-based research and public health efforts aimed at early skin cancer detection and prevention. Presently, the United States lacks established guidelines for skin cancer screening. In a 2023 statement published in JAMA, the US Preventive Services Task Force said that there is insufficient evidence to determine the benefit-harm balance of skin cancer screening in asymptomatic people.
“So there has been a lot of recent talk and a need to identify which subset groups of patients might be higher risk for skin cancer and might benefit from more screening,” Dr. Mansh said in an interview. “Understanding more about the high-risk demographic and clinical features that predispose someone to skin cancer helps identify these high-risk populations that could be used to develop better screening guidelines.”
Identifying groups at a higher risk for skin cancer also allows experts to design more targeted counseling or public health interventions focused on these groups, Dr. Mansh added. Absent screening guidelines, experts emphasize changing modifiable risk factors such as UVR exposure, smoking, and alcohol use. “And we know that the message that might change behaviors in a cisgender heterosexual man might be different than in a gay White male or a Hispanic bisexual male.”
A 2017 review showed that interventions to reduce behaviors involving UVR exposure, such as indoor tanning, among young cisgender women focused largely on aging and appearance-based concerns. A 2019 study showed that messages focused on avoiding skin cancer may help motivate SM men to reduce tanning behaviors.
Furthermore, said Dr. Mansh, all electronic health record products available in the United States must provide data fields for sexual orientation. “I don’t believe many dermatologists, depending on the setting, collect that information routinely. Integrating sexual orientation and/or gender identity data into patient intake forms so that it can be integrated into the electronic health record is probably very helpful, not only for your clinical practice but also for future research studies.”
Asked to comment on the results, Rebecca I. Hartman, MD, MPH, who was not involved with the study, said that its impact on clinical practice will be challenging to ascertain. She is chief of dermatology with the VA Boston Healthcare System, assistant professor of dermatology at Harvard Medical School, and director of melanoma epidemiology at Brigham and Women’s Hospital, all in Boston, Massachusetts.
“The study found significant adjusted odds ratios,” Dr. Hartman explained, “but for some of the different populations, the overall lifetime rate of skin cancer is still quite low.” For example, 1.0% for SM non-Hispanic Black men or a difference of 2.1% vs 1.8% in SM Hispanic women. “Thus, I am not sure specific screening recommendations are warranted, although some populations, such as Hispanic sexual minority males, seemed to have a much higher risk (3.8-fold on adjusted analysis) that warrants further investigation.”
For now, she advised assessing patients’ risks for skin cancer based on well-established risk factors such as sun exposure/indoor tanning, skin phototype, immunosuppression, and age.
Dr. Mansh reported no relevant conflicts or funding sources for the study. Dr. Hartman reported no relevant conflicts.
A version of this article appeared on Medscape.com.
Addressing dynamics of each SM subgroup will require increasingly tailored prevention, screening, and research efforts, the study authors said.
“We identified specific subgroups within the sexual minority community who are at higher risk for skin cancer, specifically White gay males and Hispanic and non-Hispanic Black SM men and women — particularly individuals who identify as bisexual,” senior author Matthew Mansh, MD, said in an interview. He is an assistant professor of dermatology at the University of California, San Francisco. The study was published online in JAMA Dermatology.
Using data of adults in the US general population from the Behavioral Risk Factor Surveillance System from January 2014 to December 2021, investigators included more than 1.5 million respondents. The proportions of SM women and men (who self-identified as bisexual, lesbian, gay, “something else,” or other) were 2.6% and 2.0%, respectively.
Lifetime skin cancer prevalence was higher among SM men than among heterosexual men (7.4% vs 6.8%; adjusted odds ratio [aOR], 1.16). In analyses stratified by racial and ethnic group, AORs for non-Hispanic Black and Hispanic SM men vs their heterosexual counterparts were 2.18 and 3.81, respectively. The corresponding figures for non-Hispanic Black and Hispanic SM women were 2.33 and 2.46, respectively.
When investigators combined all minority respondents along gender lines, lifetime skin cancer prevalence was higher in bisexual men (aOR, 3.94), bisexual women (aOR, 1.51), and women identifying as something else or other (aOR, 2.70) than in their heterosexual peers.
“I wasn’t expecting that Hispanic or non-Hispanic Black SMs would be at higher risk for skin cancer,” Dr. Mansh said. Even if these groups have more behavioral risk factors for UV radiation (UVR) exposure, he explained, UVR exposure is less strongly linked with skin cancer in darker skin than in lighter skin. Reasons for the counterintuitive finding could include different screening habits among SM people of different racial and ethnic groups, he said, and analyzing such factors will require further research.
Although some effect sizes were modest, the authors wrote, their findings may have important implications for population-based research and public health efforts aimed at early skin cancer detection and prevention. Presently, the United States lacks established guidelines for skin cancer screening. In a 2023 statement published in JAMA, the US Preventive Services Task Force said that there is insufficient evidence to determine the benefit-harm balance of skin cancer screening in asymptomatic people.
“So there has been a lot of recent talk and a need to identify which subset groups of patients might be higher risk for skin cancer and might benefit from more screening,” Dr. Mansh said in an interview. “Understanding more about the high-risk demographic and clinical features that predispose someone to skin cancer helps identify these high-risk populations that could be used to develop better screening guidelines.”
Identifying groups at a higher risk for skin cancer also allows experts to design more targeted counseling or public health interventions focused on these groups, Dr. Mansh added. Absent screening guidelines, experts emphasize changing modifiable risk factors such as UVR exposure, smoking, and alcohol use. “And we know that the message that might change behaviors in a cisgender heterosexual man might be different than in a gay White male or a Hispanic bisexual male.”
A 2017 review showed that interventions to reduce behaviors involving UVR exposure, such as indoor tanning, among young cisgender women focused largely on aging and appearance-based concerns. A 2019 study showed that messages focused on avoiding skin cancer may help motivate SM men to reduce tanning behaviors.
Furthermore, said Dr. Mansh, all electronic health record products available in the United States must provide data fields for sexual orientation. “I don’t believe many dermatologists, depending on the setting, collect that information routinely. Integrating sexual orientation and/or gender identity data into patient intake forms so that it can be integrated into the electronic health record is probably very helpful, not only for your clinical practice but also for future research studies.”
Asked to comment on the results, Rebecca I. Hartman, MD, MPH, who was not involved with the study, said that its impact on clinical practice will be challenging to ascertain. She is chief of dermatology with the VA Boston Healthcare System, assistant professor of dermatology at Harvard Medical School, and director of melanoma epidemiology at Brigham and Women’s Hospital, all in Boston, Massachusetts.
“The study found significant adjusted odds ratios,” Dr. Hartman explained, “but for some of the different populations, the overall lifetime rate of skin cancer is still quite low.” For example, 1.0% for SM non-Hispanic Black men or a difference of 2.1% vs 1.8% in SM Hispanic women. “Thus, I am not sure specific screening recommendations are warranted, although some populations, such as Hispanic sexual minority males, seemed to have a much higher risk (3.8-fold on adjusted analysis) that warrants further investigation.”
For now, she advised assessing patients’ risks for skin cancer based on well-established risk factors such as sun exposure/indoor tanning, skin phototype, immunosuppression, and age.
Dr. Mansh reported no relevant conflicts or funding sources for the study. Dr. Hartman reported no relevant conflicts.
A version of this article appeared on Medscape.com.
FROM JAMA DERMATOLOGY
In Some Patients, Antiseizure Medications Can Cause Severe Skin Reactions
according to authors of a recent review. And if putting higher-risk patients on drugs most associated with human leukocyte antigen (HLA)–related reaction risk before test results are available, authors advised starting at low doses and titrating slowly.
“When someone is having a seizure drug prescribed,” said senior author Ram Mani, MD, MSCE, chief of epilepsy at Rutgers Robert Wood Johnson Medical School in New Brunswick, New Jersey, “it’s often a tense clinical situation because the patient has either had the first few seizures of their life, or they’ve had a worsening in their seizures.”
To help physicians optimize choices, Dr. Mani and colleagues reviewed literature regarding 31 ASMs. Their study was published in Current Treatment Options in Neurology.
Overall, said Dr. Mani, incidence of benign skin reactions such as morbilliform exanthematous eruptions, which account for 95% of cutaneous adverse drug reactions (CADRs), ranges from a few percent up to 15%. “It’s a somewhat common occurrence. Fortunately, the reactions that can lead to morbidity and mortality are fairly rare.”
Severe Cutaneous Adverse Reactions
Among the five ASMs approved by the Food and Drug Administration since 2018, cenobamate has sparked the greatest concern. In early clinical development for epilepsy, a fast titration schedule (starting at 50 mg/day and increasing by 50 mg every 2 weeks to at least 200 mg/day) resulted in three cases of drug reaction with eosinophilia and systemic symptoms (DRESS, also called drug-induced hypersensitivity reaction/DIHS), including one fatal case. Based on a phase 3 trial, the drug’s manufacturer now recommends starting at 12.5 mg and titrating more slowly.
DRESS/DIHS appears within 2-6 weeks of drug exposure. Along with malaise, fever, and conjunctivitis, symptoms can include skin eruptions ranging from morbilliform to hemorrhagic and bullous. “Facial edema and early facial rash are classic findings,” the authors added. DRESS also can involve painful lymphadenopathy and potentially life-threatening damage to the liver, heart, and other organs.
Stevens-Johnson syndrome (SJS), which is characterized by detached skin measuring less than 10% of the entire body surface area, typically happens within the first month of drug exposure. Flu-like symptoms can appear 1-3 days before erythematous to dusky macules, commonly on the chest, as well as cutaneous and mucosal erosions. Along with the skin and conjunctiva, SJS can affect the eyes, lungs, liver, bone marrow, and gastrointestinal tract.
When patients present with possible DRESS or SJS, the authors recommended inpatient multidisciplinary care. Having ready access to blood tests can help assess severity and prognosis, Dr. Mani explained. Inpatient evaluation and treatment also may allow faster access to other specialists as needed, and monitoring of potential seizure exacerbation in patients with uncontrolled seizures for whom the drug provided benefit but required abrupt discontinuation.
Often, he added, all hope is not lost for future use of the medication after a minor skin reaction. A case series and literature review of mild lamotrigine-associated CADRs showed that most patients could reintroduce and titrate lamotrigine by waiting at least 4 weeks, beginning at 5 mg/day, and gradually increasing to 25 mg/day.
Identifying Those at Risk
With millions of patients being newly prescribed ASMs annually, accurately screening out all people at risk of severe cutaneous adverse reactions based on available genetic information is impossible. The complexity of evolving recommendations for HLA testing makes them hard to remember, Dr. Mani said. “Development and better use of clinical decision support systems can help.”
Accordingly, he starts with a thorough history and physical examination, inquiring about prior skin reactions or hypersensitivity, which are risk factors for future reactions to drugs such as carbamazepine, phenytoin, phenobarbital, oxcarbazepine, lamotrigine, rufinamide, and zonisamide. “Most of the medicines that the HLA tests are being done for are not the initial medicines I typically prescribe for a patient with newly diagnosed epilepsy,” said Dr. Mani. For ASM-naive patients with moderate or high risk of skin hypersensitivity reactions, he usually starts with lacosamide, levetiracetam, or brivaracetam. Additional low-risk drugs he considers in more complex cases include valproate, topiramate, and clobazam.
Only if a patient’s initial ASM causes problems will Dr. Mani consider higher-risk options and order HLA tests for patients belonging to indicated groups — such as testing for HLA-B*15:02 in Asian patients being considered for carbamazepine. About once weekly, he must put a patient on a potentially higher-risk drug before test results are available. If after a thorough risk-benefit discussion, he and the patient agree that the higher-risk drug is warranted, Dr. Mani starts at a lower-than-labeled dose, with a slower titration schedule that typically extends the ramp-up period by 1 week.
Fortunately, Dr. Mani said that, in 20 years of practice, he has seen more misdiagnoses — involving rashes from poison ivy, viral infections, or allergies — than actual ASM-induced reactions. “That’s why the patient, family, and practitioner need to be open-minded about what could be causing the rash.”
Dr. Mani reported no relevant conflicts. The study authors reported no funding sources.
according to authors of a recent review. And if putting higher-risk patients on drugs most associated with human leukocyte antigen (HLA)–related reaction risk before test results are available, authors advised starting at low doses and titrating slowly.
“When someone is having a seizure drug prescribed,” said senior author Ram Mani, MD, MSCE, chief of epilepsy at Rutgers Robert Wood Johnson Medical School in New Brunswick, New Jersey, “it’s often a tense clinical situation because the patient has either had the first few seizures of their life, or they’ve had a worsening in their seizures.”
To help physicians optimize choices, Dr. Mani and colleagues reviewed literature regarding 31 ASMs. Their study was published in Current Treatment Options in Neurology.
Overall, said Dr. Mani, incidence of benign skin reactions such as morbilliform exanthematous eruptions, which account for 95% of cutaneous adverse drug reactions (CADRs), ranges from a few percent up to 15%. “It’s a somewhat common occurrence. Fortunately, the reactions that can lead to morbidity and mortality are fairly rare.”
Severe Cutaneous Adverse Reactions
Among the five ASMs approved by the Food and Drug Administration since 2018, cenobamate has sparked the greatest concern. In early clinical development for epilepsy, a fast titration schedule (starting at 50 mg/day and increasing by 50 mg every 2 weeks to at least 200 mg/day) resulted in three cases of drug reaction with eosinophilia and systemic symptoms (DRESS, also called drug-induced hypersensitivity reaction/DIHS), including one fatal case. Based on a phase 3 trial, the drug’s manufacturer now recommends starting at 12.5 mg and titrating more slowly.
DRESS/DIHS appears within 2-6 weeks of drug exposure. Along with malaise, fever, and conjunctivitis, symptoms can include skin eruptions ranging from morbilliform to hemorrhagic and bullous. “Facial edema and early facial rash are classic findings,” the authors added. DRESS also can involve painful lymphadenopathy and potentially life-threatening damage to the liver, heart, and other organs.
Stevens-Johnson syndrome (SJS), which is characterized by detached skin measuring less than 10% of the entire body surface area, typically happens within the first month of drug exposure. Flu-like symptoms can appear 1-3 days before erythematous to dusky macules, commonly on the chest, as well as cutaneous and mucosal erosions. Along with the skin and conjunctiva, SJS can affect the eyes, lungs, liver, bone marrow, and gastrointestinal tract.
When patients present with possible DRESS or SJS, the authors recommended inpatient multidisciplinary care. Having ready access to blood tests can help assess severity and prognosis, Dr. Mani explained. Inpatient evaluation and treatment also may allow faster access to other specialists as needed, and monitoring of potential seizure exacerbation in patients with uncontrolled seizures for whom the drug provided benefit but required abrupt discontinuation.
Often, he added, all hope is not lost for future use of the medication after a minor skin reaction. A case series and literature review of mild lamotrigine-associated CADRs showed that most patients could reintroduce and titrate lamotrigine by waiting at least 4 weeks, beginning at 5 mg/day, and gradually increasing to 25 mg/day.
Identifying Those at Risk
With millions of patients being newly prescribed ASMs annually, accurately screening out all people at risk of severe cutaneous adverse reactions based on available genetic information is impossible. The complexity of evolving recommendations for HLA testing makes them hard to remember, Dr. Mani said. “Development and better use of clinical decision support systems can help.”
Accordingly, he starts with a thorough history and physical examination, inquiring about prior skin reactions or hypersensitivity, which are risk factors for future reactions to drugs such as carbamazepine, phenytoin, phenobarbital, oxcarbazepine, lamotrigine, rufinamide, and zonisamide. “Most of the medicines that the HLA tests are being done for are not the initial medicines I typically prescribe for a patient with newly diagnosed epilepsy,” said Dr. Mani. For ASM-naive patients with moderate or high risk of skin hypersensitivity reactions, he usually starts with lacosamide, levetiracetam, or brivaracetam. Additional low-risk drugs he considers in more complex cases include valproate, topiramate, and clobazam.
Only if a patient’s initial ASM causes problems will Dr. Mani consider higher-risk options and order HLA tests for patients belonging to indicated groups — such as testing for HLA-B*15:02 in Asian patients being considered for carbamazepine. About once weekly, he must put a patient on a potentially higher-risk drug before test results are available. If after a thorough risk-benefit discussion, he and the patient agree that the higher-risk drug is warranted, Dr. Mani starts at a lower-than-labeled dose, with a slower titration schedule that typically extends the ramp-up period by 1 week.
Fortunately, Dr. Mani said that, in 20 years of practice, he has seen more misdiagnoses — involving rashes from poison ivy, viral infections, or allergies — than actual ASM-induced reactions. “That’s why the patient, family, and practitioner need to be open-minded about what could be causing the rash.”
Dr. Mani reported no relevant conflicts. The study authors reported no funding sources.
according to authors of a recent review. And if putting higher-risk patients on drugs most associated with human leukocyte antigen (HLA)–related reaction risk before test results are available, authors advised starting at low doses and titrating slowly.
“When someone is having a seizure drug prescribed,” said senior author Ram Mani, MD, MSCE, chief of epilepsy at Rutgers Robert Wood Johnson Medical School in New Brunswick, New Jersey, “it’s often a tense clinical situation because the patient has either had the first few seizures of their life, or they’ve had a worsening in their seizures.”
To help physicians optimize choices, Dr. Mani and colleagues reviewed literature regarding 31 ASMs. Their study was published in Current Treatment Options in Neurology.
Overall, said Dr. Mani, incidence of benign skin reactions such as morbilliform exanthematous eruptions, which account for 95% of cutaneous adverse drug reactions (CADRs), ranges from a few percent up to 15%. “It’s a somewhat common occurrence. Fortunately, the reactions that can lead to morbidity and mortality are fairly rare.”
Severe Cutaneous Adverse Reactions
Among the five ASMs approved by the Food and Drug Administration since 2018, cenobamate has sparked the greatest concern. In early clinical development for epilepsy, a fast titration schedule (starting at 50 mg/day and increasing by 50 mg every 2 weeks to at least 200 mg/day) resulted in three cases of drug reaction with eosinophilia and systemic symptoms (DRESS, also called drug-induced hypersensitivity reaction/DIHS), including one fatal case. Based on a phase 3 trial, the drug’s manufacturer now recommends starting at 12.5 mg and titrating more slowly.
DRESS/DIHS appears within 2-6 weeks of drug exposure. Along with malaise, fever, and conjunctivitis, symptoms can include skin eruptions ranging from morbilliform to hemorrhagic and bullous. “Facial edema and early facial rash are classic findings,” the authors added. DRESS also can involve painful lymphadenopathy and potentially life-threatening damage to the liver, heart, and other organs.
Stevens-Johnson syndrome (SJS), which is characterized by detached skin measuring less than 10% of the entire body surface area, typically happens within the first month of drug exposure. Flu-like symptoms can appear 1-3 days before erythematous to dusky macules, commonly on the chest, as well as cutaneous and mucosal erosions. Along with the skin and conjunctiva, SJS can affect the eyes, lungs, liver, bone marrow, and gastrointestinal tract.
When patients present with possible DRESS or SJS, the authors recommended inpatient multidisciplinary care. Having ready access to blood tests can help assess severity and prognosis, Dr. Mani explained. Inpatient evaluation and treatment also may allow faster access to other specialists as needed, and monitoring of potential seizure exacerbation in patients with uncontrolled seizures for whom the drug provided benefit but required abrupt discontinuation.
Often, he added, all hope is not lost for future use of the medication after a minor skin reaction. A case series and literature review of mild lamotrigine-associated CADRs showed that most patients could reintroduce and titrate lamotrigine by waiting at least 4 weeks, beginning at 5 mg/day, and gradually increasing to 25 mg/day.
Identifying Those at Risk
With millions of patients being newly prescribed ASMs annually, accurately screening out all people at risk of severe cutaneous adverse reactions based on available genetic information is impossible. The complexity of evolving recommendations for HLA testing makes them hard to remember, Dr. Mani said. “Development and better use of clinical decision support systems can help.”
Accordingly, he starts with a thorough history and physical examination, inquiring about prior skin reactions or hypersensitivity, which are risk factors for future reactions to drugs such as carbamazepine, phenytoin, phenobarbital, oxcarbazepine, lamotrigine, rufinamide, and zonisamide. “Most of the medicines that the HLA tests are being done for are not the initial medicines I typically prescribe for a patient with newly diagnosed epilepsy,” said Dr. Mani. For ASM-naive patients with moderate or high risk of skin hypersensitivity reactions, he usually starts with lacosamide, levetiracetam, or brivaracetam. Additional low-risk drugs he considers in more complex cases include valproate, topiramate, and clobazam.
Only if a patient’s initial ASM causes problems will Dr. Mani consider higher-risk options and order HLA tests for patients belonging to indicated groups — such as testing for HLA-B*15:02 in Asian patients being considered for carbamazepine. About once weekly, he must put a patient on a potentially higher-risk drug before test results are available. If after a thorough risk-benefit discussion, he and the patient agree that the higher-risk drug is warranted, Dr. Mani starts at a lower-than-labeled dose, with a slower titration schedule that typically extends the ramp-up period by 1 week.
Fortunately, Dr. Mani said that, in 20 years of practice, he has seen more misdiagnoses — involving rashes from poison ivy, viral infections, or allergies — than actual ASM-induced reactions. “That’s why the patient, family, and practitioner need to be open-minded about what could be causing the rash.”
Dr. Mani reported no relevant conflicts. The study authors reported no funding sources.
FROM CURRENT TREATMENT OPTIONS IN NEUROLOGY