User login
Most cost-effective psoriasis treatment: methotrexate
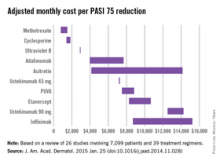
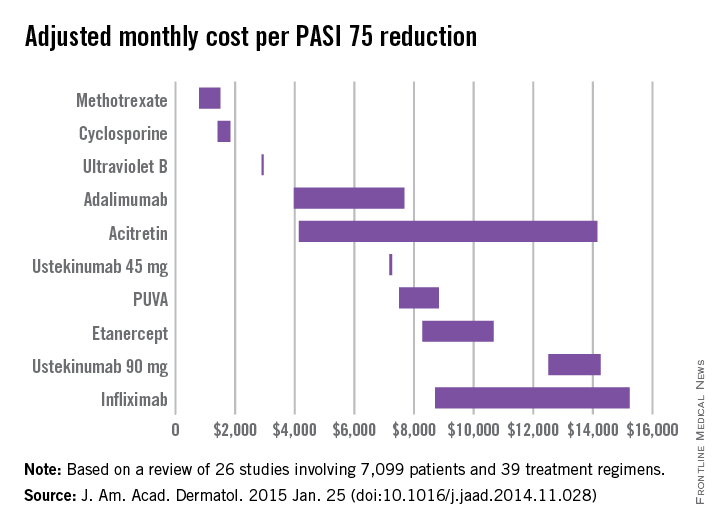
Methotrexate is the most cost-effective systemic treatment for psoriasis in terms of the number of patients needed to treat to achieve a PASI 75, according to a literature review in the Journal of the American Academy of Dermatology.
Methotrexate (2.5-mg tablet) cost an adjusted $794-$1,503 a month per number needed to treat (NNT) for a 75% reduction in Psoriasis Area and Severity Index (PASI 75) score, Dr. Logan S. D’Souza and Dr. Michael J. Payette of the department of dermatology at the University of Connecticut in Farmington reported (J. Am. Acad. Dermatol. 2015 Jan. 25 [doi:10.1016/j.jaad.2014.11.028]).
Coming in second was cyclosporine (25- and 100-mg tablets) at $1,410-$1,844 in monthly cost per PASI 75, followed by narrow-band ultraviolet B light phototherapy at $2,925 and adalimumab (40-mg subcutaneous injection) at $3,975-$7,679, the investigators said.
The most expensive of the 10 Food and Drug Administration–approved treatments included in the study was infliximab (100-mg vial) at $8,705-$15,236 per month. The next most expensive treatment was ustekinumab (90-mg subcutaneous injection), which cost $12,505-$14,257 per month.
“While our data indicate that methotrexate and cyclosporine are more cost efficacious in reaching PASI 75 than other medications approved by the FDA, their potential toxicities may limit their long-term use,” Dr. D’Souza and Dr. Payette noted. “Our study is not meant to dictate treatments based solely on costs; rather, our analysis should help both clinicians and patients in choosing between potential treatments,” they added.
For this analysis, 26 studies involving 7,099 patients and 39 treatment regimens met the investigators’ search criteria.
Dr. D’Souza declared no conflicts of interest. Dr. Payette has been an adviser to Amgen.
Methotrexate is the most cost-effective systemic treatment for psoriasis in terms of the number of patients needed to treat to achieve a PASI 75, according to a literature review in the Journal of the American Academy of Dermatology.
Methotrexate (2.5-mg tablet) cost an adjusted $794-$1,503 a month per number needed to treat (NNT) for a 75% reduction in Psoriasis Area and Severity Index (PASI 75) score, Dr. Logan S. D’Souza and Dr. Michael J. Payette of the department of dermatology at the University of Connecticut in Farmington reported (J. Am. Acad. Dermatol. 2015 Jan. 25 [doi:10.1016/j.jaad.2014.11.028]).
Coming in second was cyclosporine (25- and 100-mg tablets) at $1,410-$1,844 in monthly cost per PASI 75, followed by narrow-band ultraviolet B light phototherapy at $2,925 and adalimumab (40-mg subcutaneous injection) at $3,975-$7,679, the investigators said.
The most expensive of the 10 Food and Drug Administration–approved treatments included in the study was infliximab (100-mg vial) at $8,705-$15,236 per month. The next most expensive treatment was ustekinumab (90-mg subcutaneous injection), which cost $12,505-$14,257 per month.
“While our data indicate that methotrexate and cyclosporine are more cost efficacious in reaching PASI 75 than other medications approved by the FDA, their potential toxicities may limit their long-term use,” Dr. D’Souza and Dr. Payette noted. “Our study is not meant to dictate treatments based solely on costs; rather, our analysis should help both clinicians and patients in choosing between potential treatments,” they added.
For this analysis, 26 studies involving 7,099 patients and 39 treatment regimens met the investigators’ search criteria.
Dr. D’Souza declared no conflicts of interest. Dr. Payette has been an adviser to Amgen.
Methotrexate is the most cost-effective systemic treatment for psoriasis in terms of the number of patients needed to treat to achieve a PASI 75, according to a literature review in the Journal of the American Academy of Dermatology.
Methotrexate (2.5-mg tablet) cost an adjusted $794-$1,503 a month per number needed to treat (NNT) for a 75% reduction in Psoriasis Area and Severity Index (PASI 75) score, Dr. Logan S. D’Souza and Dr. Michael J. Payette of the department of dermatology at the University of Connecticut in Farmington reported (J. Am. Acad. Dermatol. 2015 Jan. 25 [doi:10.1016/j.jaad.2014.11.028]).
Coming in second was cyclosporine (25- and 100-mg tablets) at $1,410-$1,844 in monthly cost per PASI 75, followed by narrow-band ultraviolet B light phototherapy at $2,925 and adalimumab (40-mg subcutaneous injection) at $3,975-$7,679, the investigators said.
The most expensive of the 10 Food and Drug Administration–approved treatments included in the study was infliximab (100-mg vial) at $8,705-$15,236 per month. The next most expensive treatment was ustekinumab (90-mg subcutaneous injection), which cost $12,505-$14,257 per month.
“While our data indicate that methotrexate and cyclosporine are more cost efficacious in reaching PASI 75 than other medications approved by the FDA, their potential toxicities may limit their long-term use,” Dr. D’Souza and Dr. Payette noted. “Our study is not meant to dictate treatments based solely on costs; rather, our analysis should help both clinicians and patients in choosing between potential treatments,” they added.
For this analysis, 26 studies involving 7,099 patients and 39 treatment regimens met the investigators’ search criteria.
Dr. D’Souza declared no conflicts of interest. Dr. Payette has been an adviser to Amgen.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Survey: Psoriasis/psoriatic arthritis undertreated
ORLANDO – Patients with psoriasis and psoriatic arthritis have a high level of dissatisfaction with current treatment options and have numerous unmet health care needs, according to the U.S. findings from the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey.
Of 1,005 U.S. adults who participated in the population-based telephone survey, 735 had psoriasis diagnosed by a health care provider, and 270 had psoriatic arthritis diagnosed – with or without a diagnosis of psoriasis. Half of those surveyed reported dissatisfaction with available oral and biologic therapies, Dr. Mark Lebwohl of Mount Sinai Hospital, New York, and his colleagues reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
Most respondents with psoriasis reported moderate symptoms (40%), with about 30% each reporting mild or severe symptoms. Itching was the most bothersome symptom, occurring in about 42% of patients, followed by flaking (26%) and scales (22%). Itching was the greatest contributor to estimates of disease severity.
Psoriatic arthritis patients were more likely to report severe symptoms (56%), while 12% reported mild symptoms, and about a third reported moderate symptoms. Itching was the most bothersome symptom among these patients, followed by pain (21%) and flaking (19%). Pain and joint swelling were the greatest contributors to estimates of disease severity in this group, the investigators said.
The majority of respondents (57%) had seen a doctor in the past year. For psoriasis patients, that doctor was most often a dermatologist (57%), while psoriatic arthritis patients most often saw a rheumatologist (38%).
A third of patients most often saw a primary care physician.
The main reasons cited for not having seen a doctor in the past year were having mild or no symptoms, and a feeling that a health care provider would be unable to help.
Of concern, patterns reported by the patients indicated widespread undertreatment of psoriasis and psoriatic arthritis, they said.
More than one in five psoriasis patients (22%) reported no treatment at the time of the survey, and although most described their disease as moderate or severe, only 23% reported ever discussing the use of conventional oral or biologic therapies with their health care providers, and only 9% were currently receiving systemic therapy; most were using prescription topical therapy.
Psoriatic arthritis patients were much more likely than were psoriasis patients to have ever used a conventional oral or biologic therapy, but only 50% were receiving systemic therapy, while 26% reported use of biologic therapy and 24% reported use of an oral therapy.
Methotrexate was the conventional oral therapy used most often (68% of the time), followed by cyclosporine and acitretin (in 14% and 12% of patients, respectively), and etanercept was the biologic therapy used most often (56%) followed by adalimumab (used by 48%).
The most common reasons for discontinuing treatment were concerns about safety, issues with tolerability, and lack or loss of effectiveness. The greatest contributors to treatment burden among those using conventional oral therapies were side effects and the need for laboratory monitoring, and the greatest contributors among those using biologics were concerns about long-term safety, anxiety or fear, pain, and inconvenience associated with self injection.
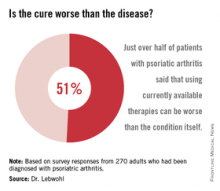
More than half of all respondents with psoriatic arthritis (51%) agreed that using currently available therapies can be worse than the condition itself, and 88% said better therapies are needed.
These results underscore the need for improved screening, as many patients with joint symptoms had not been diagnosed with psoriatic arthritis. They also demonstrate a need for improved assessment of disease severity; pruritus – which was reported as the most bothersome symptom – “is not included in most assessment tools,” the investigators noted.
Further, the results highlight the need for a higher level of treatment in many patients, they said, explaining that survey responses indicated substantial undertreatment in numerous cases. Many patients were receiving no treatment or only topical treatment, and many who were experiencing symptoms were not currently seeking care from a health care provider.
The MAPP survey is the first large-scale, multinational, population-based survey of psoriasis and psoriatic arthritis patients. The survey included 3,426 patients and 781 physicians in North America and Europe. The U.S. findings, which indicate little systematic implementation of treatment algorithms as well as widespread undertreatment, are particularly important given that psoriasis affects 3.2% of adults in the United States, and that 30% of those patients have psoriatic arthritis, the researchers noted.
Most of these patients report that their condition affects their emotional well-being, their quality of life, and their daily activities, they noted.
“These findings deserve attention and action to improve the care and lives of patients with psoriasis and psoriatic arthritis,” they concluded.
The survey was sponsored by Celgene, which markets apremilast (Otezla). Dr. Lebwohl disclosed ties to numerous pharmaceutical companies, including several that market drugs for psoriasis and psoriatic arthritis.
ORLANDO – Patients with psoriasis and psoriatic arthritis have a high level of dissatisfaction with current treatment options and have numerous unmet health care needs, according to the U.S. findings from the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey.
Of 1,005 U.S. adults who participated in the population-based telephone survey, 735 had psoriasis diagnosed by a health care provider, and 270 had psoriatic arthritis diagnosed – with or without a diagnosis of psoriasis. Half of those surveyed reported dissatisfaction with available oral and biologic therapies, Dr. Mark Lebwohl of Mount Sinai Hospital, New York, and his colleagues reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
Most respondents with psoriasis reported moderate symptoms (40%), with about 30% each reporting mild or severe symptoms. Itching was the most bothersome symptom, occurring in about 42% of patients, followed by flaking (26%) and scales (22%). Itching was the greatest contributor to estimates of disease severity.
Psoriatic arthritis patients were more likely to report severe symptoms (56%), while 12% reported mild symptoms, and about a third reported moderate symptoms. Itching was the most bothersome symptom among these patients, followed by pain (21%) and flaking (19%). Pain and joint swelling were the greatest contributors to estimates of disease severity in this group, the investigators said.
The majority of respondents (57%) had seen a doctor in the past year. For psoriasis patients, that doctor was most often a dermatologist (57%), while psoriatic arthritis patients most often saw a rheumatologist (38%).
A third of patients most often saw a primary care physician.
The main reasons cited for not having seen a doctor in the past year were having mild or no symptoms, and a feeling that a health care provider would be unable to help.
Of concern, patterns reported by the patients indicated widespread undertreatment of psoriasis and psoriatic arthritis, they said.
More than one in five psoriasis patients (22%) reported no treatment at the time of the survey, and although most described their disease as moderate or severe, only 23% reported ever discussing the use of conventional oral or biologic therapies with their health care providers, and only 9% were currently receiving systemic therapy; most were using prescription topical therapy.
Psoriatic arthritis patients were much more likely than were psoriasis patients to have ever used a conventional oral or biologic therapy, but only 50% were receiving systemic therapy, while 26% reported use of biologic therapy and 24% reported use of an oral therapy.
Methotrexate was the conventional oral therapy used most often (68% of the time), followed by cyclosporine and acitretin (in 14% and 12% of patients, respectively), and etanercept was the biologic therapy used most often (56%) followed by adalimumab (used by 48%).
The most common reasons for discontinuing treatment were concerns about safety, issues with tolerability, and lack or loss of effectiveness. The greatest contributors to treatment burden among those using conventional oral therapies were side effects and the need for laboratory monitoring, and the greatest contributors among those using biologics were concerns about long-term safety, anxiety or fear, pain, and inconvenience associated with self injection.
More than half of all respondents with psoriatic arthritis (51%) agreed that using currently available therapies can be worse than the condition itself, and 88% said better therapies are needed.
These results underscore the need for improved screening, as many patients with joint symptoms had not been diagnosed with psoriatic arthritis. They also demonstrate a need for improved assessment of disease severity; pruritus – which was reported as the most bothersome symptom – “is not included in most assessment tools,” the investigators noted.
Further, the results highlight the need for a higher level of treatment in many patients, they said, explaining that survey responses indicated substantial undertreatment in numerous cases. Many patients were receiving no treatment or only topical treatment, and many who were experiencing symptoms were not currently seeking care from a health care provider.
The MAPP survey is the first large-scale, multinational, population-based survey of psoriasis and psoriatic arthritis patients. The survey included 3,426 patients and 781 physicians in North America and Europe. The U.S. findings, which indicate little systematic implementation of treatment algorithms as well as widespread undertreatment, are particularly important given that psoriasis affects 3.2% of adults in the United States, and that 30% of those patients have psoriatic arthritis, the researchers noted.
Most of these patients report that their condition affects their emotional well-being, their quality of life, and their daily activities, they noted.
“These findings deserve attention and action to improve the care and lives of patients with psoriasis and psoriatic arthritis,” they concluded.
The survey was sponsored by Celgene, which markets apremilast (Otezla). Dr. Lebwohl disclosed ties to numerous pharmaceutical companies, including several that market drugs for psoriasis and psoriatic arthritis.
ORLANDO – Patients with psoriasis and psoriatic arthritis have a high level of dissatisfaction with current treatment options and have numerous unmet health care needs, according to the U.S. findings from the Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey.
Of 1,005 U.S. adults who participated in the population-based telephone survey, 735 had psoriasis diagnosed by a health care provider, and 270 had psoriatic arthritis diagnosed – with or without a diagnosis of psoriasis. Half of those surveyed reported dissatisfaction with available oral and biologic therapies, Dr. Mark Lebwohl of Mount Sinai Hospital, New York, and his colleagues reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
Most respondents with psoriasis reported moderate symptoms (40%), with about 30% each reporting mild or severe symptoms. Itching was the most bothersome symptom, occurring in about 42% of patients, followed by flaking (26%) and scales (22%). Itching was the greatest contributor to estimates of disease severity.
Psoriatic arthritis patients were more likely to report severe symptoms (56%), while 12% reported mild symptoms, and about a third reported moderate symptoms. Itching was the most bothersome symptom among these patients, followed by pain (21%) and flaking (19%). Pain and joint swelling were the greatest contributors to estimates of disease severity in this group, the investigators said.
The majority of respondents (57%) had seen a doctor in the past year. For psoriasis patients, that doctor was most often a dermatologist (57%), while psoriatic arthritis patients most often saw a rheumatologist (38%).
A third of patients most often saw a primary care physician.
The main reasons cited for not having seen a doctor in the past year were having mild or no symptoms, and a feeling that a health care provider would be unable to help.
Of concern, patterns reported by the patients indicated widespread undertreatment of psoriasis and psoriatic arthritis, they said.
More than one in five psoriasis patients (22%) reported no treatment at the time of the survey, and although most described their disease as moderate or severe, only 23% reported ever discussing the use of conventional oral or biologic therapies with their health care providers, and only 9% were currently receiving systemic therapy; most were using prescription topical therapy.
Psoriatic arthritis patients were much more likely than were psoriasis patients to have ever used a conventional oral or biologic therapy, but only 50% were receiving systemic therapy, while 26% reported use of biologic therapy and 24% reported use of an oral therapy.
Methotrexate was the conventional oral therapy used most often (68% of the time), followed by cyclosporine and acitretin (in 14% and 12% of patients, respectively), and etanercept was the biologic therapy used most often (56%) followed by adalimumab (used by 48%).
The most common reasons for discontinuing treatment were concerns about safety, issues with tolerability, and lack or loss of effectiveness. The greatest contributors to treatment burden among those using conventional oral therapies were side effects and the need for laboratory monitoring, and the greatest contributors among those using biologics were concerns about long-term safety, anxiety or fear, pain, and inconvenience associated with self injection.
More than half of all respondents with psoriatic arthritis (51%) agreed that using currently available therapies can be worse than the condition itself, and 88% said better therapies are needed.
These results underscore the need for improved screening, as many patients with joint symptoms had not been diagnosed with psoriatic arthritis. They also demonstrate a need for improved assessment of disease severity; pruritus – which was reported as the most bothersome symptom – “is not included in most assessment tools,” the investigators noted.
Further, the results highlight the need for a higher level of treatment in many patients, they said, explaining that survey responses indicated substantial undertreatment in numerous cases. Many patients were receiving no treatment or only topical treatment, and many who were experiencing symptoms were not currently seeking care from a health care provider.
The MAPP survey is the first large-scale, multinational, population-based survey of psoriasis and psoriatic arthritis patients. The survey included 3,426 patients and 781 physicians in North America and Europe. The U.S. findings, which indicate little systematic implementation of treatment algorithms as well as widespread undertreatment, are particularly important given that psoriasis affects 3.2% of adults in the United States, and that 30% of those patients have psoriatic arthritis, the researchers noted.
Most of these patients report that their condition affects their emotional well-being, their quality of life, and their daily activities, they noted.
“These findings deserve attention and action to improve the care and lives of patients with psoriasis and psoriatic arthritis,” they concluded.
The survey was sponsored by Celgene, which markets apremilast (Otezla). Dr. Lebwohl disclosed ties to numerous pharmaceutical companies, including several that market drugs for psoriasis and psoriatic arthritis.
AT THE ODAC CONFERENCE
Key clinical point: Psoriasis and psoriatic arthritis treatment algorithms are underused, and patients are often undertreated.
Major finding: Among survey respondents, 22% were on no treatment, and 23% reported ever discussing conventional oral or biologic therapies with a health care provider.
Data source: A telephone survey (MAPP) of 735 psoriasis and 270 psoriatic arthritis patients.
Disclosures: The survey was sponsored by Celgene, which markets apremilast (Otezla). Dr. Lebwohl disclosed ties to numerous pharmaceutical companies, including several that market drugs for psoriasis and psoriatic arthritis.
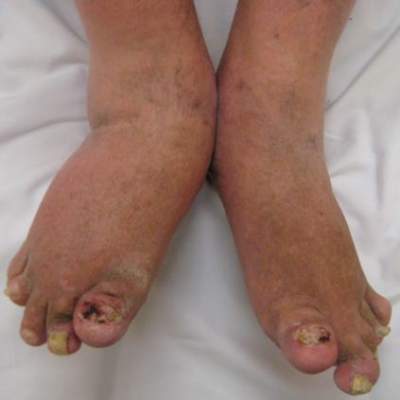
Joint mechanical stress: A psoriatic arthritis trigger in genetically susceptible individuals?
Parallel cases of twins developing “symmetrical” dactylitis in two digits following similarly sustained foot injuries support the hypothesis that joint mechanical stress is a major trigger of psoriatic arthritis and dactylitis in genetically susceptible individuals, according to a scientific letter published Jan. 28 in Annals of the Rheumatic Diseases.
A team of researchers led by Dr. Jennifer Ng of Griffith University and Paradise Arthritis and Rheumatology in Southport, Australia, described the cases of identical twins, 54-year old women with a history of psoriasis, both of whom developed psoriatic arthritis of the respective right and left second toes. Both sisters had no arthritis prior to the joint injuries, and one of the patients had to be treated with methotrexate 15 mg weekly with almost complete resolution of the swelling and pain.
Although researchers are still exploring the precise mechanisms that link joint injury and psoriatic arthritis onset, the authors assert they have proof of concept of how site specific injury may result in biomechanical triggering events in genetically susceptible hosts.
Read the entire article here: doi:10.1136/annrheumdis-2014-206784.
Parallel cases of twins developing “symmetrical” dactylitis in two digits following similarly sustained foot injuries support the hypothesis that joint mechanical stress is a major trigger of psoriatic arthritis and dactylitis in genetically susceptible individuals, according to a scientific letter published Jan. 28 in Annals of the Rheumatic Diseases.
A team of researchers led by Dr. Jennifer Ng of Griffith University and Paradise Arthritis and Rheumatology in Southport, Australia, described the cases of identical twins, 54-year old women with a history of psoriasis, both of whom developed psoriatic arthritis of the respective right and left second toes. Both sisters had no arthritis prior to the joint injuries, and one of the patients had to be treated with methotrexate 15 mg weekly with almost complete resolution of the swelling and pain.
Although researchers are still exploring the precise mechanisms that link joint injury and psoriatic arthritis onset, the authors assert they have proof of concept of how site specific injury may result in biomechanical triggering events in genetically susceptible hosts.
Read the entire article here: doi:10.1136/annrheumdis-2014-206784.
Parallel cases of twins developing “symmetrical” dactylitis in two digits following similarly sustained foot injuries support the hypothesis that joint mechanical stress is a major trigger of psoriatic arthritis and dactylitis in genetically susceptible individuals, according to a scientific letter published Jan. 28 in Annals of the Rheumatic Diseases.
A team of researchers led by Dr. Jennifer Ng of Griffith University and Paradise Arthritis and Rheumatology in Southport, Australia, described the cases of identical twins, 54-year old women with a history of psoriasis, both of whom developed psoriatic arthritis of the respective right and left second toes. Both sisters had no arthritis prior to the joint injuries, and one of the patients had to be treated with methotrexate 15 mg weekly with almost complete resolution of the swelling and pain.
Although researchers are still exploring the precise mechanisms that link joint injury and psoriatic arthritis onset, the authors assert they have proof of concept of how site specific injury may result in biomechanical triggering events in genetically susceptible hosts.
Read the entire article here: doi:10.1136/annrheumdis-2014-206784.
Consider Chikungunya virus in new-onset polyarthritis
Rheumatologists should consider the possibility of Chikungunya virus in patients who present with new symmetric polyarthritis, especially if they have just returned from an endemic region such as the Caribbean, according to researchers.
In their paper, first authors Dr. Jonathan J. Miner and Dr. Han-Xian Aw-Yeang and colleagues at Washington University, St. Louis, describe a cohort of 10 Americans who traveled to Haiti within a 20-day period in June 2014 and became infected with Chikungunya virus (CHIKV), an arthritogenic, mosquito-transmitted alphavirus (Arthritis Rheumatol. 2015 Jan. 20 [doi:10.1002/art.39027]).
The virus spread to the Caribbean in 2013 and the United States in 2014. Its acute phase of infection includes symptoms such as fever, headache, rash, arthralgia, arthritis, and myalgia.
The virus is likely to become a unique diagnostic challenge for rheumatologists as the arthritis symptoms mimic seronegative arthritis, the researchers said.
“Eight out of these patients would have met the 2010 ACR/EULAR criteria for RA [rheumatoid arthritis] if the initial fever, rash, and travel to the Caribbean had not been revealed,” they noted.
Most of the patients had joint pain and morning stiffness at least 8 weeks after initial infection, although some reported gradual improvement of their symptoms, the investigators reported.
One of the patients developed fever; diffuse arthritis; an erythematous, maculopapular rash; and severe symmetric polyarthritis that persisted more than 5 months post infection. She was treated with prednisolone but it exacerbated her joint pain and treatment was stopped.
The other patient detailed in the report presented with similar symptoms. His fever and rash resolved within 2 days but his arthritis symptoms persisted. He was treated with NSAIDs that provided only minimal relief.
To understand more about the immunologic parameters of the virus, the researchers used cytometry by time of flight to compare the peripheral mononuclear cells from CHIKV-infected patients with those of healthy controls and untreated patients with RA.
They discovered that naive, activated, and effector T killer– and T helper–cell populations occurred in similar percentages in the virus-infected patients and RA patients but not in the healthy controls.
The RA patients, however, had a higher expression of L-selectin expression in CD4+ T cells than did either virus-infected patients or healthy controls.
“These data suggest that lymphocyte phenotypes in patients with Chikungunya infection are similar to each other, with subtle but distinct trends that could potentially distinguish these two groups from healthy controls and from each other,” Dr. Miner and Dr. Aw-Yeang and their associates wrote.
Whether treatment with disease-modifying antirheumatic drugs or biologics used in RA is appropriate or effective is controversial in the absence of randomized controlled trials for CHIKV-related arthritis, they said.
“Immunosuppression in CHIKV-infected patients could be deleterious because viral RNA and antigens can be found in target tissues in the chronic phase in humans and experimental animals,” the authors wrote.
“Rheumatologists, even in non-CHIKV-endemic regions, need to consider CHIKV in their evaluation of symmetric polyarthritis lasting more than 6 weeks by obtaining a history of travel to CHIKV-endemic regions, which are likely to expand in the near future,” the investigators advised.
Patients who may have been exposed to the virus may need serologic testing before initiating immunosuppression, they said.
“Unfortunately, access to CHIKV testing is highly constrained at the current time as it is only available from the [Centers for Disease Control and Prevention] and research laboratories,” they added.
The study was supported by the Barnes-Jewish Hospital Foundation and the Howard Hughes Medical Institute. The authors reported no conflicts of interest.
Rheumatologists should consider the possibility of Chikungunya virus in patients who present with new symmetric polyarthritis, especially if they have just returned from an endemic region such as the Caribbean, according to researchers.
In their paper, first authors Dr. Jonathan J. Miner and Dr. Han-Xian Aw-Yeang and colleagues at Washington University, St. Louis, describe a cohort of 10 Americans who traveled to Haiti within a 20-day period in June 2014 and became infected with Chikungunya virus (CHIKV), an arthritogenic, mosquito-transmitted alphavirus (Arthritis Rheumatol. 2015 Jan. 20 [doi:10.1002/art.39027]).
The virus spread to the Caribbean in 2013 and the United States in 2014. Its acute phase of infection includes symptoms such as fever, headache, rash, arthralgia, arthritis, and myalgia.
The virus is likely to become a unique diagnostic challenge for rheumatologists as the arthritis symptoms mimic seronegative arthritis, the researchers said.
“Eight out of these patients would have met the 2010 ACR/EULAR criteria for RA [rheumatoid arthritis] if the initial fever, rash, and travel to the Caribbean had not been revealed,” they noted.
Most of the patients had joint pain and morning stiffness at least 8 weeks after initial infection, although some reported gradual improvement of their symptoms, the investigators reported.
One of the patients developed fever; diffuse arthritis; an erythematous, maculopapular rash; and severe symmetric polyarthritis that persisted more than 5 months post infection. She was treated with prednisolone but it exacerbated her joint pain and treatment was stopped.
The other patient detailed in the report presented with similar symptoms. His fever and rash resolved within 2 days but his arthritis symptoms persisted. He was treated with NSAIDs that provided only minimal relief.
To understand more about the immunologic parameters of the virus, the researchers used cytometry by time of flight to compare the peripheral mononuclear cells from CHIKV-infected patients with those of healthy controls and untreated patients with RA.
They discovered that naive, activated, and effector T killer– and T helper–cell populations occurred in similar percentages in the virus-infected patients and RA patients but not in the healthy controls.
The RA patients, however, had a higher expression of L-selectin expression in CD4+ T cells than did either virus-infected patients or healthy controls.
“These data suggest that lymphocyte phenotypes in patients with Chikungunya infection are similar to each other, with subtle but distinct trends that could potentially distinguish these two groups from healthy controls and from each other,” Dr. Miner and Dr. Aw-Yeang and their associates wrote.
Whether treatment with disease-modifying antirheumatic drugs or biologics used in RA is appropriate or effective is controversial in the absence of randomized controlled trials for CHIKV-related arthritis, they said.
“Immunosuppression in CHIKV-infected patients could be deleterious because viral RNA and antigens can be found in target tissues in the chronic phase in humans and experimental animals,” the authors wrote.
“Rheumatologists, even in non-CHIKV-endemic regions, need to consider CHIKV in their evaluation of symmetric polyarthritis lasting more than 6 weeks by obtaining a history of travel to CHIKV-endemic regions, which are likely to expand in the near future,” the investigators advised.
Patients who may have been exposed to the virus may need serologic testing before initiating immunosuppression, they said.
“Unfortunately, access to CHIKV testing is highly constrained at the current time as it is only available from the [Centers for Disease Control and Prevention] and research laboratories,” they added.
The study was supported by the Barnes-Jewish Hospital Foundation and the Howard Hughes Medical Institute. The authors reported no conflicts of interest.
Rheumatologists should consider the possibility of Chikungunya virus in patients who present with new symmetric polyarthritis, especially if they have just returned from an endemic region such as the Caribbean, according to researchers.
In their paper, first authors Dr. Jonathan J. Miner and Dr. Han-Xian Aw-Yeang and colleagues at Washington University, St. Louis, describe a cohort of 10 Americans who traveled to Haiti within a 20-day period in June 2014 and became infected with Chikungunya virus (CHIKV), an arthritogenic, mosquito-transmitted alphavirus (Arthritis Rheumatol. 2015 Jan. 20 [doi:10.1002/art.39027]).
The virus spread to the Caribbean in 2013 and the United States in 2014. Its acute phase of infection includes symptoms such as fever, headache, rash, arthralgia, arthritis, and myalgia.
The virus is likely to become a unique diagnostic challenge for rheumatologists as the arthritis symptoms mimic seronegative arthritis, the researchers said.
“Eight out of these patients would have met the 2010 ACR/EULAR criteria for RA [rheumatoid arthritis] if the initial fever, rash, and travel to the Caribbean had not been revealed,” they noted.
Most of the patients had joint pain and morning stiffness at least 8 weeks after initial infection, although some reported gradual improvement of their symptoms, the investigators reported.
One of the patients developed fever; diffuse arthritis; an erythematous, maculopapular rash; and severe symmetric polyarthritis that persisted more than 5 months post infection. She was treated with prednisolone but it exacerbated her joint pain and treatment was stopped.
The other patient detailed in the report presented with similar symptoms. His fever and rash resolved within 2 days but his arthritis symptoms persisted. He was treated with NSAIDs that provided only minimal relief.
To understand more about the immunologic parameters of the virus, the researchers used cytometry by time of flight to compare the peripheral mononuclear cells from CHIKV-infected patients with those of healthy controls and untreated patients with RA.
They discovered that naive, activated, and effector T killer– and T helper–cell populations occurred in similar percentages in the virus-infected patients and RA patients but not in the healthy controls.
The RA patients, however, had a higher expression of L-selectin expression in CD4+ T cells than did either virus-infected patients or healthy controls.
“These data suggest that lymphocyte phenotypes in patients with Chikungunya infection are similar to each other, with subtle but distinct trends that could potentially distinguish these two groups from healthy controls and from each other,” Dr. Miner and Dr. Aw-Yeang and their associates wrote.
Whether treatment with disease-modifying antirheumatic drugs or biologics used in RA is appropriate or effective is controversial in the absence of randomized controlled trials for CHIKV-related arthritis, they said.
“Immunosuppression in CHIKV-infected patients could be deleterious because viral RNA and antigens can be found in target tissues in the chronic phase in humans and experimental animals,” the authors wrote.
“Rheumatologists, even in non-CHIKV-endemic regions, need to consider CHIKV in their evaluation of symmetric polyarthritis lasting more than 6 weeks by obtaining a history of travel to CHIKV-endemic regions, which are likely to expand in the near future,” the investigators advised.
Patients who may have been exposed to the virus may need serologic testing before initiating immunosuppression, they said.
“Unfortunately, access to CHIKV testing is highly constrained at the current time as it is only available from the [Centers for Disease Control and Prevention] and research laboratories,” they added.
The study was supported by the Barnes-Jewish Hospital Foundation and the Howard Hughes Medical Institute. The authors reported no conflicts of interest.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: The mosquito-spread Chikungunya virus mimics seronegative rheumatoid arthritis. Rheumatologists should consider this diagnosis in patients who present with arthritis symptoms after visiting an endemic area.
Major finding: Eight out of the 10 people confirmed to be infected with the CHIKV virus met the 2010 ACR/EULAR criteria for RA.
Source: An observational study of a group of 10 Americans who traveled to Haiti within a 20-day period in June 2014 and became infected with CHIKV.
Disclosures: The study was supported by the Barnes-Jewish Hospital Foundation and the Howard Hughes Medical Institute. The authors reported no conflicts of interest.
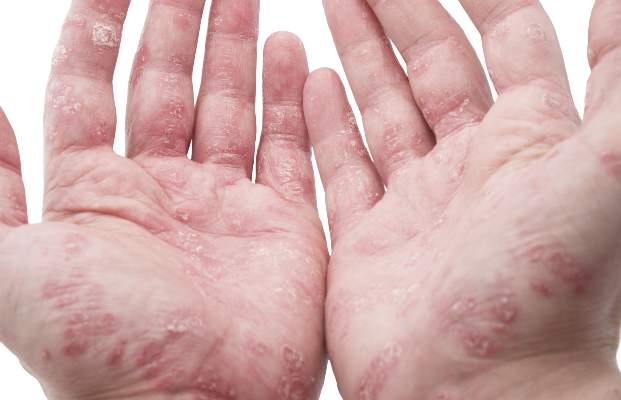
Corticosteroid spray quickly, safely relieves psoriasis scaling
ORLANDO – A topical corticosteroid spray quickly and effectively reduced scaling in patients with plaque psoriasis, according to pooled phase III study results.
After 1 week of twice daily application of 0.25% Topicort spray (TaroPharma, Hawthorne, N.Y.), scaling was clear or almost clear in 29% of 120 treated patients, and was mild in an additional 40% of patients. At 4 weeks, scaling was clear or almost clear in 61% of patients, and mild in an additional 23%, Dr. Brian Keegan of East Windsor, N.J., reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
Additionally, 70% of patients experienced global improvement in psoriasis after 4 weeks of treatment; psoriasis was clear or almost clear in 42% of patients, and mild in 28%, Dr. Keegan said.
Study participants were adults with 10%-86% of body area affected by plaque psoriasis. Most of the patients (58%) were aged 41-to-64 years with a mean affected body surface area of 17%. Most had moderate scaling, but about a third of patients had severe or very severe scaling.
They applied Topicort 0.25% spray twice daily for 4 weeks.
The only adverse events that occurred in more than 1% of patients were dryness, irritation, and pruritus at the application site, which each occurred in 2%-3% of patients.
Treatment with Topicort – a class 1 super-potent corticosteroid indicated for up to 4 weeks of treatment of plaque psoriasis – quickly and safely relieved scaling in the majority of patients treated, Dr. Keegan concluded, noting that the findings are important given that scaling is very common among patients with plaque psoriasis, many of whom find it to be bothersome.
In fact, 94% of more than 17,000 participants in a National Psoriasis Foundation survey reported being bothered by scaling, he noted.
This study was sponsored by TaroPharma.
ORLANDO – A topical corticosteroid spray quickly and effectively reduced scaling in patients with plaque psoriasis, according to pooled phase III study results.
After 1 week of twice daily application of 0.25% Topicort spray (TaroPharma, Hawthorne, N.Y.), scaling was clear or almost clear in 29% of 120 treated patients, and was mild in an additional 40% of patients. At 4 weeks, scaling was clear or almost clear in 61% of patients, and mild in an additional 23%, Dr. Brian Keegan of East Windsor, N.J., reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
Additionally, 70% of patients experienced global improvement in psoriasis after 4 weeks of treatment; psoriasis was clear or almost clear in 42% of patients, and mild in 28%, Dr. Keegan said.
Study participants were adults with 10%-86% of body area affected by plaque psoriasis. Most of the patients (58%) were aged 41-to-64 years with a mean affected body surface area of 17%. Most had moderate scaling, but about a third of patients had severe or very severe scaling.
They applied Topicort 0.25% spray twice daily for 4 weeks.
The only adverse events that occurred in more than 1% of patients were dryness, irritation, and pruritus at the application site, which each occurred in 2%-3% of patients.
Treatment with Topicort – a class 1 super-potent corticosteroid indicated for up to 4 weeks of treatment of plaque psoriasis – quickly and safely relieved scaling in the majority of patients treated, Dr. Keegan concluded, noting that the findings are important given that scaling is very common among patients with plaque psoriasis, many of whom find it to be bothersome.
In fact, 94% of more than 17,000 participants in a National Psoriasis Foundation survey reported being bothered by scaling, he noted.
This study was sponsored by TaroPharma.
ORLANDO – A topical corticosteroid spray quickly and effectively reduced scaling in patients with plaque psoriasis, according to pooled phase III study results.
After 1 week of twice daily application of 0.25% Topicort spray (TaroPharma, Hawthorne, N.Y.), scaling was clear or almost clear in 29% of 120 treated patients, and was mild in an additional 40% of patients. At 4 weeks, scaling was clear or almost clear in 61% of patients, and mild in an additional 23%, Dr. Brian Keegan of East Windsor, N.J., reported in a poster at the Orlando Dermatology Aesthetic and Clinical Conference.
Additionally, 70% of patients experienced global improvement in psoriasis after 4 weeks of treatment; psoriasis was clear or almost clear in 42% of patients, and mild in 28%, Dr. Keegan said.
Study participants were adults with 10%-86% of body area affected by plaque psoriasis. Most of the patients (58%) were aged 41-to-64 years with a mean affected body surface area of 17%. Most had moderate scaling, but about a third of patients had severe or very severe scaling.
They applied Topicort 0.25% spray twice daily for 4 weeks.
The only adverse events that occurred in more than 1% of patients were dryness, irritation, and pruritus at the application site, which each occurred in 2%-3% of patients.
Treatment with Topicort – a class 1 super-potent corticosteroid indicated for up to 4 weeks of treatment of plaque psoriasis – quickly and safely relieved scaling in the majority of patients treated, Dr. Keegan concluded, noting that the findings are important given that scaling is very common among patients with plaque psoriasis, many of whom find it to be bothersome.
In fact, 94% of more than 17,000 participants in a National Psoriasis Foundation survey reported being bothered by scaling, he noted.
This study was sponsored by TaroPharma.
AT THE ODAC CONFERENCE
Key clinical point: Psoriasis patients with scaling are likely to respond to corticosteroid spray treatment.
Major finding: At 4 weeks, scaling was clear, almost clear, or mild in 84% of patients.
Data source: An analysis of pooled phase III trial data for 120 patients.
Disclosures: This study was sponsored by TaroPharma.
Secukinumab earns FDA approval for plaque psoriasis
The U.S. Food and Drug Administration has approved secukinumab for the treatment of moderate to severe plaque psoriasis in adults.
The injectable interleukin-17A inhibitor is indicated for psoriasis patients who are candidates for systemic therapy and/or phototherapy, according to a press release from the FDA. Novartis Pharmaceuticals will market secukinumab as Cosentyx.
The Jan. 21 approval was based on data from randomized, placebo-controlled clinical trials involving 2,403 patients. The most common side effects reported in the trials included upper respiratory infections and diarrhea.
The biologic is being approved with a medication guide “to inform patients that, because Cosentyx is a medicine that affects the immune system, patients may have a greater risk of getting an infection,” according to the FDA release. Consequently, the FDA advised clinicians to be cautious when prescribing secukinumab to patients with chronic or recurrent infections, and to those with active Crohn’s disease.
The FDA approval followed an advisory committee meeting in October 2014.
The U.S. Food and Drug Administration has approved secukinumab for the treatment of moderate to severe plaque psoriasis in adults.
The injectable interleukin-17A inhibitor is indicated for psoriasis patients who are candidates for systemic therapy and/or phototherapy, according to a press release from the FDA. Novartis Pharmaceuticals will market secukinumab as Cosentyx.
The Jan. 21 approval was based on data from randomized, placebo-controlled clinical trials involving 2,403 patients. The most common side effects reported in the trials included upper respiratory infections and diarrhea.
The biologic is being approved with a medication guide “to inform patients that, because Cosentyx is a medicine that affects the immune system, patients may have a greater risk of getting an infection,” according to the FDA release. Consequently, the FDA advised clinicians to be cautious when prescribing secukinumab to patients with chronic or recurrent infections, and to those with active Crohn’s disease.
The FDA approval followed an advisory committee meeting in October 2014.
The U.S. Food and Drug Administration has approved secukinumab for the treatment of moderate to severe plaque psoriasis in adults.
The injectable interleukin-17A inhibitor is indicated for psoriasis patients who are candidates for systemic therapy and/or phototherapy, according to a press release from the FDA. Novartis Pharmaceuticals will market secukinumab as Cosentyx.
The Jan. 21 approval was based on data from randomized, placebo-controlled clinical trials involving 2,403 patients. The most common side effects reported in the trials included upper respiratory infections and diarrhea.
The biologic is being approved with a medication guide “to inform patients that, because Cosentyx is a medicine that affects the immune system, patients may have a greater risk of getting an infection,” according to the FDA release. Consequently, the FDA advised clinicians to be cautious when prescribing secukinumab to patients with chronic or recurrent infections, and to those with active Crohn’s disease.
The FDA approval followed an advisory committee meeting in October 2014.
Psoriasis treatment recommendations address four clinical scenarios
New guidelines on nail psoriasis address four clinical manifestations of the disease. The recommendations by the Medical Board of the National Psoriasis Foundation appeared as a consensus statement in the January issue of JAMA Dermatology.
Limitations in clinical trial data make comparing treatments difficult, noted lead author Dr. Jeffrey J. Crowley of Bakersfield (Calif.) Dermatology and his associates. “There are limited data to evaluate or support the use of combination therapy in nail psoriasis. Thus, treatment options recommended in this review are monotherapy,” the guidelines authors added (JAMA Dermatol. 2015;151:87-94).
To develop the guidelines, the research team searched PubMed for articles on nail psoriasis dating from Jan. 1, 1947 through May 11, 2014. They evaluated these studies for level of evidence based on recommendations for writing guidelines from Dr. Paul G. Shekelle of the VA West Los Angeles Medical Center and his associates (BMJ 1999;318:593-6).
They also polled the Medical Board of the National Psoriasis Foundation regarding their treatment approach for four clinical presentations of nail psoriasis:
• For treatment-naive patients with psoriasis of the nails only (affecting at least 3 of 10 fingernails), the board recommended initial treatment with high-potency topical corticosteroids (with or without calcipotriol), with intralesional corticosteroids as a secondary option. Intralesional corticosteroids have been used for decades, but clinical data supporting their use are “extremely limited,” the guidelines state.
• For extensive nail psoriasis (affecting at least five fingernails and causing moderate to severe pain) that has failed topical treatment, the board recommended adalimumab most enthusiastically, followed by etanercept, intralesional corticosteroids, ustekinumab, methotrexate sodium, and acitretin in decreasing order.
• For concurrent skin and nail disease without joint involvement (defined as skin disease on at least 8% of the body surface and moderately to severely painful dystrophy of at least 5 of 10 nails), the board strongly recommended adalimumab, etanercept, and ustekinumab, and also recommended methotrexate, acitretin, infliximab, and apremilast.
• For concurrent nail, skin, and joint involvement (defined as skin disease on 8% of the body surface, a history of dactylitis and morning stiffness (psoriatic arthritis), and severe, painful involvement of at least 5 of 10 nails), the board most strongly recommended adalimumab, followed by etanercept, ustekinumab, infliximab, methotrexate, apremilast, and golimumab.
Because nails grow slowly, psoriatic joint and skin disease often improve before nail psoriasis does, the authors noted. “Few studies show any significant improvement before 12 weeks, and several studies with etanercept, infliximab, and ustekinumab demonstrate continued improvement beyond 6 months,” they wrote.
About half of patients with psoriasis have some amount of nail involvement, and about 70% of patients with psoriatic arthritis have nail disease, according to the literature review. Dermatophyte infections can further complicate treatment of nail psoriasis, and immunosuppressive therapies can lead to onychomycosis in patients whose psoriasis includes the toenails, the authors added.
Dr. Crowley reported speaker and consulting honoraria from AbbVie, Abbott, and Amgen, and research funding from Abbott, AbbVie, Amgen, AstraZeneca, Celgene, Eli Lilly, Janssen Pharmaceutica, Merck, Pfizer, and Regeneron Pharmaceuticals. Four coauthors reported advisory, consulting, or financial relationships with Amgen, Abbott, Janssen Biotech Inc., Celgene, Novartis International AG, Abbvie, Merck, Celgene, Leo Pharma, Eli Lilly, Pfizer, and the National Psoriasis Foundation.
New guidelines on nail psoriasis address four clinical manifestations of the disease. The recommendations by the Medical Board of the National Psoriasis Foundation appeared as a consensus statement in the January issue of JAMA Dermatology.
Limitations in clinical trial data make comparing treatments difficult, noted lead author Dr. Jeffrey J. Crowley of Bakersfield (Calif.) Dermatology and his associates. “There are limited data to evaluate or support the use of combination therapy in nail psoriasis. Thus, treatment options recommended in this review are monotherapy,” the guidelines authors added (JAMA Dermatol. 2015;151:87-94).
To develop the guidelines, the research team searched PubMed for articles on nail psoriasis dating from Jan. 1, 1947 through May 11, 2014. They evaluated these studies for level of evidence based on recommendations for writing guidelines from Dr. Paul G. Shekelle of the VA West Los Angeles Medical Center and his associates (BMJ 1999;318:593-6).
They also polled the Medical Board of the National Psoriasis Foundation regarding their treatment approach for four clinical presentations of nail psoriasis:
• For treatment-naive patients with psoriasis of the nails only (affecting at least 3 of 10 fingernails), the board recommended initial treatment with high-potency topical corticosteroids (with or without calcipotriol), with intralesional corticosteroids as a secondary option. Intralesional corticosteroids have been used for decades, but clinical data supporting their use are “extremely limited,” the guidelines state.
• For extensive nail psoriasis (affecting at least five fingernails and causing moderate to severe pain) that has failed topical treatment, the board recommended adalimumab most enthusiastically, followed by etanercept, intralesional corticosteroids, ustekinumab, methotrexate sodium, and acitretin in decreasing order.
• For concurrent skin and nail disease without joint involvement (defined as skin disease on at least 8% of the body surface and moderately to severely painful dystrophy of at least 5 of 10 nails), the board strongly recommended adalimumab, etanercept, and ustekinumab, and also recommended methotrexate, acitretin, infliximab, and apremilast.
• For concurrent nail, skin, and joint involvement (defined as skin disease on 8% of the body surface, a history of dactylitis and morning stiffness (psoriatic arthritis), and severe, painful involvement of at least 5 of 10 nails), the board most strongly recommended adalimumab, followed by etanercept, ustekinumab, infliximab, methotrexate, apremilast, and golimumab.
Because nails grow slowly, psoriatic joint and skin disease often improve before nail psoriasis does, the authors noted. “Few studies show any significant improvement before 12 weeks, and several studies with etanercept, infliximab, and ustekinumab demonstrate continued improvement beyond 6 months,” they wrote.
About half of patients with psoriasis have some amount of nail involvement, and about 70% of patients with psoriatic arthritis have nail disease, according to the literature review. Dermatophyte infections can further complicate treatment of nail psoriasis, and immunosuppressive therapies can lead to onychomycosis in patients whose psoriasis includes the toenails, the authors added.
Dr. Crowley reported speaker and consulting honoraria from AbbVie, Abbott, and Amgen, and research funding from Abbott, AbbVie, Amgen, AstraZeneca, Celgene, Eli Lilly, Janssen Pharmaceutica, Merck, Pfizer, and Regeneron Pharmaceuticals. Four coauthors reported advisory, consulting, or financial relationships with Amgen, Abbott, Janssen Biotech Inc., Celgene, Novartis International AG, Abbvie, Merck, Celgene, Leo Pharma, Eli Lilly, Pfizer, and the National Psoriasis Foundation.
New guidelines on nail psoriasis address four clinical manifestations of the disease. The recommendations by the Medical Board of the National Psoriasis Foundation appeared as a consensus statement in the January issue of JAMA Dermatology.
Limitations in clinical trial data make comparing treatments difficult, noted lead author Dr. Jeffrey J. Crowley of Bakersfield (Calif.) Dermatology and his associates. “There are limited data to evaluate or support the use of combination therapy in nail psoriasis. Thus, treatment options recommended in this review are monotherapy,” the guidelines authors added (JAMA Dermatol. 2015;151:87-94).
To develop the guidelines, the research team searched PubMed for articles on nail psoriasis dating from Jan. 1, 1947 through May 11, 2014. They evaluated these studies for level of evidence based on recommendations for writing guidelines from Dr. Paul G. Shekelle of the VA West Los Angeles Medical Center and his associates (BMJ 1999;318:593-6).
They also polled the Medical Board of the National Psoriasis Foundation regarding their treatment approach for four clinical presentations of nail psoriasis:
• For treatment-naive patients with psoriasis of the nails only (affecting at least 3 of 10 fingernails), the board recommended initial treatment with high-potency topical corticosteroids (with or without calcipotriol), with intralesional corticosteroids as a secondary option. Intralesional corticosteroids have been used for decades, but clinical data supporting their use are “extremely limited,” the guidelines state.
• For extensive nail psoriasis (affecting at least five fingernails and causing moderate to severe pain) that has failed topical treatment, the board recommended adalimumab most enthusiastically, followed by etanercept, intralesional corticosteroids, ustekinumab, methotrexate sodium, and acitretin in decreasing order.
• For concurrent skin and nail disease without joint involvement (defined as skin disease on at least 8% of the body surface and moderately to severely painful dystrophy of at least 5 of 10 nails), the board strongly recommended adalimumab, etanercept, and ustekinumab, and also recommended methotrexate, acitretin, infliximab, and apremilast.
• For concurrent nail, skin, and joint involvement (defined as skin disease on 8% of the body surface, a history of dactylitis and morning stiffness (psoriatic arthritis), and severe, painful involvement of at least 5 of 10 nails), the board most strongly recommended adalimumab, followed by etanercept, ustekinumab, infliximab, methotrexate, apremilast, and golimumab.
Because nails grow slowly, psoriatic joint and skin disease often improve before nail psoriasis does, the authors noted. “Few studies show any significant improvement before 12 weeks, and several studies with etanercept, infliximab, and ustekinumab demonstrate continued improvement beyond 6 months,” they wrote.
About half of patients with psoriasis have some amount of nail involvement, and about 70% of patients with psoriatic arthritis have nail disease, according to the literature review. Dermatophyte infections can further complicate treatment of nail psoriasis, and immunosuppressive therapies can lead to onychomycosis in patients whose psoriasis includes the toenails, the authors added.
Dr. Crowley reported speaker and consulting honoraria from AbbVie, Abbott, and Amgen, and research funding from Abbott, AbbVie, Amgen, AstraZeneca, Celgene, Eli Lilly, Janssen Pharmaceutica, Merck, Pfizer, and Regeneron Pharmaceuticals. Four coauthors reported advisory, consulting, or financial relationships with Amgen, Abbott, Janssen Biotech Inc., Celgene, Novartis International AG, Abbvie, Merck, Celgene, Leo Pharma, Eli Lilly, Pfizer, and the National Psoriasis Foundation.
FROM JAMA DERMATOLOGY
Early psoriatic arthritis treatment with etanercept gives better outcomes
Patients with psoriatic arthritis and psoriasis report having a better response to etanercept the earlier they are treated, according to a post hoc analysis of the PRESTA trial.
Patients with shorter psoriatic arthritis (PsA) duration had greater improvements in arthritis scores and several patient-reported outcomes at 24 weeks of treatment with etanercept 50 mg a week, compared with patients with longer disease duration.
The researchers, led by Dr. Bruce Kirkham from Guy’s and St. Thomas’ NHS Foundation Trust, London, said the results showed “clinicians should consider treating their PsA patients with therapies effective in PsA early rather than late.”
The industry-sponsored PRESTA (Psoriasis Randomized Etanercept Study in Patients with Psoriatic Arthritis) trial was a randomized, blinded, 24-week, multicenter study enrolling adults with active but stable plaque psoriasis involving at least 10% body surface area and active PsA defined as 2 or more swollen joints, 2 or more tender joints, joint pain for 3 months or longer, and a negative serum rheumatoid factor within 6 months prior to baseline.
Overall, 372 patients who received etanercept 50 mg once a week for 24 weeks were included in the current post hoc analysis (Clin. Exp. Rheumatol. 2014 Dec. 22).
Baseline and after treatment changes were compared between patients with PsA disease duration of 2 years or less (n = 103) and those with disease more than 2 years (n = 269).
Baseline efficacy measures were similar between the shorter duration and longer duration groups, with the exception of Physicians Global Assessment (PGA) arthritis score, which was significantly lower in the group with 2 years or less duration (44.9 vs. 51.8; P = .006), the authors reported.
At week 24, joint disease improved, based on the PGA arthritis score, by a significantly greater amount in the shorter duration group (–39.8 vs. –35.7; P = .03).
Clinically meaningful improvements in patient-reported outcomes with etanercept treatment occurred in both groups, the study authors said, but changes in scores from baseline to week 24 were significantly higher in the shorter duration group for visual analog scale reports of joint pain (P = .007) and arthritis activity (P = .01) as well as quality of life on EuroQol 5D utility (P = .046) and EuroQol 5D visual analog scale (P = .04) responses.
The mean number of swollen joints that had improved from baseline to week 24 was not significantly different between the groups, and no significant between-group differences were seen in the percentages of patients achieving the ACR20, ACR50, and ACR70 responses.
While all patients responded to treatment irrespective of disease duration, patients with shorter disease duration had greater improvements on some measures, the authors concluded.
However, the study was limited by the fact that it was a post hoc analysis and the original trial was not designed to explore the effect of early treatment versus later treatment in patients with PsA and moderate-to-severe psoriasis, they noted.
The study was sponsored by Wyeth, which was acquired by Pfizer, the manufacturer of etanercept, in October 2009. Several of the authors declared receiving honoraria from several pharmaceutical companies. Two authors were employees of Pfizer during the PRESTA study and development of the current manuscript, and two other authors are current employees of Pfizer.
Patients with psoriatic arthritis and psoriasis report having a better response to etanercept the earlier they are treated, according to a post hoc analysis of the PRESTA trial.
Patients with shorter psoriatic arthritis (PsA) duration had greater improvements in arthritis scores and several patient-reported outcomes at 24 weeks of treatment with etanercept 50 mg a week, compared with patients with longer disease duration.
The researchers, led by Dr. Bruce Kirkham from Guy’s and St. Thomas’ NHS Foundation Trust, London, said the results showed “clinicians should consider treating their PsA patients with therapies effective in PsA early rather than late.”
The industry-sponsored PRESTA (Psoriasis Randomized Etanercept Study in Patients with Psoriatic Arthritis) trial was a randomized, blinded, 24-week, multicenter study enrolling adults with active but stable plaque psoriasis involving at least 10% body surface area and active PsA defined as 2 or more swollen joints, 2 or more tender joints, joint pain for 3 months or longer, and a negative serum rheumatoid factor within 6 months prior to baseline.
Overall, 372 patients who received etanercept 50 mg once a week for 24 weeks were included in the current post hoc analysis (Clin. Exp. Rheumatol. 2014 Dec. 22).
Baseline and after treatment changes were compared between patients with PsA disease duration of 2 years or less (n = 103) and those with disease more than 2 years (n = 269).
Baseline efficacy measures were similar between the shorter duration and longer duration groups, with the exception of Physicians Global Assessment (PGA) arthritis score, which was significantly lower in the group with 2 years or less duration (44.9 vs. 51.8; P = .006), the authors reported.
At week 24, joint disease improved, based on the PGA arthritis score, by a significantly greater amount in the shorter duration group (–39.8 vs. –35.7; P = .03).
Clinically meaningful improvements in patient-reported outcomes with etanercept treatment occurred in both groups, the study authors said, but changes in scores from baseline to week 24 were significantly higher in the shorter duration group for visual analog scale reports of joint pain (P = .007) and arthritis activity (P = .01) as well as quality of life on EuroQol 5D utility (P = .046) and EuroQol 5D visual analog scale (P = .04) responses.
The mean number of swollen joints that had improved from baseline to week 24 was not significantly different between the groups, and no significant between-group differences were seen in the percentages of patients achieving the ACR20, ACR50, and ACR70 responses.
While all patients responded to treatment irrespective of disease duration, patients with shorter disease duration had greater improvements on some measures, the authors concluded.
However, the study was limited by the fact that it was a post hoc analysis and the original trial was not designed to explore the effect of early treatment versus later treatment in patients with PsA and moderate-to-severe psoriasis, they noted.
The study was sponsored by Wyeth, which was acquired by Pfizer, the manufacturer of etanercept, in October 2009. Several of the authors declared receiving honoraria from several pharmaceutical companies. Two authors were employees of Pfizer during the PRESTA study and development of the current manuscript, and two other authors are current employees of Pfizer.
Patients with psoriatic arthritis and psoriasis report having a better response to etanercept the earlier they are treated, according to a post hoc analysis of the PRESTA trial.
Patients with shorter psoriatic arthritis (PsA) duration had greater improvements in arthritis scores and several patient-reported outcomes at 24 weeks of treatment with etanercept 50 mg a week, compared with patients with longer disease duration.
The researchers, led by Dr. Bruce Kirkham from Guy’s and St. Thomas’ NHS Foundation Trust, London, said the results showed “clinicians should consider treating their PsA patients with therapies effective in PsA early rather than late.”
The industry-sponsored PRESTA (Psoriasis Randomized Etanercept Study in Patients with Psoriatic Arthritis) trial was a randomized, blinded, 24-week, multicenter study enrolling adults with active but stable plaque psoriasis involving at least 10% body surface area and active PsA defined as 2 or more swollen joints, 2 or more tender joints, joint pain for 3 months or longer, and a negative serum rheumatoid factor within 6 months prior to baseline.
Overall, 372 patients who received etanercept 50 mg once a week for 24 weeks were included in the current post hoc analysis (Clin. Exp. Rheumatol. 2014 Dec. 22).
Baseline and after treatment changes were compared between patients with PsA disease duration of 2 years or less (n = 103) and those with disease more than 2 years (n = 269).
Baseline efficacy measures were similar between the shorter duration and longer duration groups, with the exception of Physicians Global Assessment (PGA) arthritis score, which was significantly lower in the group with 2 years or less duration (44.9 vs. 51.8; P = .006), the authors reported.
At week 24, joint disease improved, based on the PGA arthritis score, by a significantly greater amount in the shorter duration group (–39.8 vs. –35.7; P = .03).
Clinically meaningful improvements in patient-reported outcomes with etanercept treatment occurred in both groups, the study authors said, but changes in scores from baseline to week 24 were significantly higher in the shorter duration group for visual analog scale reports of joint pain (P = .007) and arthritis activity (P = .01) as well as quality of life on EuroQol 5D utility (P = .046) and EuroQol 5D visual analog scale (P = .04) responses.
The mean number of swollen joints that had improved from baseline to week 24 was not significantly different between the groups, and no significant between-group differences were seen in the percentages of patients achieving the ACR20, ACR50, and ACR70 responses.
While all patients responded to treatment irrespective of disease duration, patients with shorter disease duration had greater improvements on some measures, the authors concluded.
However, the study was limited by the fact that it was a post hoc analysis and the original trial was not designed to explore the effect of early treatment versus later treatment in patients with PsA and moderate-to-severe psoriasis, they noted.
The study was sponsored by Wyeth, which was acquired by Pfizer, the manufacturer of etanercept, in October 2009. Several of the authors declared receiving honoraria from several pharmaceutical companies. Two authors were employees of Pfizer during the PRESTA study and development of the current manuscript, and two other authors are current employees of Pfizer.
FROM CLINICAL AND EXPERIMENTAL RHEUMATOLOGY
Key clinical point: PsA treatment with etanercept within 2 years of diagnosis may lead to better patient-reported quality of life outcomes than does treatment starting more than 2 years after diagnosis.
Major finding: After 24 weeks of etanercept 50 mg per week, joint disease improved, based on the PGA arthritis score, by a significantly greater amount in patients with PsA for 2 years or less vs. those with the disease for more than 2 years (–39.8 vs. –35.7, respectively; P = .03) .
Data source: A post hoc analysis of 372 patients with PsA and psoriasis who were enrolled in the PRESTA trial.
Disclosures: The study was sponsored by Wyeth, which was acquired by Pfizer, the manufacturer of etanercept, in October 2009. Several of the authors declared receiving honoraria from several pharmaceutical companies. Two authors were employees of Pfizer during the PRESTA study and development of the current manuscript, and two other authors are current employees of Pfizer.
Case series: Ustekinumab for psoriasis helps skin, hurts joints
Ustekinumab treatment was associated with new-onset or worsening psoriatic arthritis in a series of seven patients with psoriasis.
The findings, which support previous observations that patients treated with ustekinumab (Stelara) “sometimes have discordant responses of their skin and joint disease,” underscore the need for regularly asking patients about joint symptoms, and for referral to a rheumatologist for suspected psoriatic arthritis, Ben B. Jones of the University of Utah, Salt Lake City, and his colleagues reported (Br. J. Dermatol. 2014 Dec. 30 [doi:10.1111/bjd.13645]).
All seven patients in the case series had well-controlled psoriasis on ustekinumab. Five had new-onset psoriatic arthritis, and two had worsening psoriatic arthritis on treatment. The patients had phenotypic similarities; most were women over age 49 years, and all five of those with new-onset disease were women. Also, five of the seven patients had exposure to tumor necrosis factor inhibitors prior to switching to ustekinumab.
Three other case series have reported similar findings of marked improvement in cutaneous symptoms with worsening of joint symptoms among patients treated with ustekinumab, the investigators noted, concluding that the findings – which may reflect a lack of efficacy at the administered doses or a need for more frequent dosing – may support arguments that psoriatic arthritis and psoriasis involve distinct inflammatory pathways.
“It is also possible that ustekinumab may trigger or unmask inflammation in the joints of patients with psoriatic arthritis,” they wrote, concluding that “larger epidemiologic studies comparing patients with discordant and concordant cutaneous and articular responses to ustekinumab may better define patients at risk for psoriatic arthritis worsening with ustekinumab.”
Two of the five authors have reported serving as a consultant or advisory board member, receiving payment for lectures, and/or serving as an investigator for Janssen, which markets ustekinumab, as well as other manufacturers of biologics. The other authors reported having no conflicts of interest.
Ustekinumab treatment was associated with new-onset or worsening psoriatic arthritis in a series of seven patients with psoriasis.
The findings, which support previous observations that patients treated with ustekinumab (Stelara) “sometimes have discordant responses of their skin and joint disease,” underscore the need for regularly asking patients about joint symptoms, and for referral to a rheumatologist for suspected psoriatic arthritis, Ben B. Jones of the University of Utah, Salt Lake City, and his colleagues reported (Br. J. Dermatol. 2014 Dec. 30 [doi:10.1111/bjd.13645]).
All seven patients in the case series had well-controlled psoriasis on ustekinumab. Five had new-onset psoriatic arthritis, and two had worsening psoriatic arthritis on treatment. The patients had phenotypic similarities; most were women over age 49 years, and all five of those with new-onset disease were women. Also, five of the seven patients had exposure to tumor necrosis factor inhibitors prior to switching to ustekinumab.
Three other case series have reported similar findings of marked improvement in cutaneous symptoms with worsening of joint symptoms among patients treated with ustekinumab, the investigators noted, concluding that the findings – which may reflect a lack of efficacy at the administered doses or a need for more frequent dosing – may support arguments that psoriatic arthritis and psoriasis involve distinct inflammatory pathways.
“It is also possible that ustekinumab may trigger or unmask inflammation in the joints of patients with psoriatic arthritis,” they wrote, concluding that “larger epidemiologic studies comparing patients with discordant and concordant cutaneous and articular responses to ustekinumab may better define patients at risk for psoriatic arthritis worsening with ustekinumab.”
Two of the five authors have reported serving as a consultant or advisory board member, receiving payment for lectures, and/or serving as an investigator for Janssen, which markets ustekinumab, as well as other manufacturers of biologics. The other authors reported having no conflicts of interest.
Ustekinumab treatment was associated with new-onset or worsening psoriatic arthritis in a series of seven patients with psoriasis.
The findings, which support previous observations that patients treated with ustekinumab (Stelara) “sometimes have discordant responses of their skin and joint disease,” underscore the need for regularly asking patients about joint symptoms, and for referral to a rheumatologist for suspected psoriatic arthritis, Ben B. Jones of the University of Utah, Salt Lake City, and his colleagues reported (Br. J. Dermatol. 2014 Dec. 30 [doi:10.1111/bjd.13645]).
All seven patients in the case series had well-controlled psoriasis on ustekinumab. Five had new-onset psoriatic arthritis, and two had worsening psoriatic arthritis on treatment. The patients had phenotypic similarities; most were women over age 49 years, and all five of those with new-onset disease were women. Also, five of the seven patients had exposure to tumor necrosis factor inhibitors prior to switching to ustekinumab.
Three other case series have reported similar findings of marked improvement in cutaneous symptoms with worsening of joint symptoms among patients treated with ustekinumab, the investigators noted, concluding that the findings – which may reflect a lack of efficacy at the administered doses or a need for more frequent dosing – may support arguments that psoriatic arthritis and psoriasis involve distinct inflammatory pathways.
“It is also possible that ustekinumab may trigger or unmask inflammation in the joints of patients with psoriatic arthritis,” they wrote, concluding that “larger epidemiologic studies comparing patients with discordant and concordant cutaneous and articular responses to ustekinumab may better define patients at risk for psoriatic arthritis worsening with ustekinumab.”
Two of the five authors have reported serving as a consultant or advisory board member, receiving payment for lectures, and/or serving as an investigator for Janssen, which markets ustekinumab, as well as other manufacturers of biologics. The other authors reported having no conflicts of interest.
Key clinical point: Increasing evidence suggests that ustekinumab is associated with discordant joint and skin responses.
Major finding: Five of seven psoriasis patients treated with ustekinumab experienced new-onset psoriatic arthritis, and two had worsening psoriatic arthritis.
Data source: A series of seven cases.
Disclosures: Two of the five authors have reported serving as a consultant or advisory board member, receiving payment for lectures, and/or serving as an investigator for Janssen, which markets ustekinumab, as well as other manufacturers of biologics. The other authors reported having no conflicts of interest.
Framingham score underestimates CVD risk in psoriatic arthritis patients
Most newly diagnosed psoriatic arthritis patients have an increased risk for cardiovascular disease that is markedly underestimated by the Framingham Risk Score, according to findings from a retrospective, population-based, cohort study.
The mean Framingham Risk Score in 126 patients with psoriatic arthritis who were aged 30 years or older and who had no prior cardiovascular disease (CVD) history, was 9.7% during the first 10 years of follow-up. However, the 10-year cumulative incidence of CVD events was nearly double that at 17% (standardized incidence ratio, 1.80), Dr. Floranne C. Ernste of the Mayo Clinic, Rochester, Minn., and her colleagues reported (Arthritis Care Res. 2015 Jan. 7 [doi:10.1002/acr.22536]).
Age-based analysis showed that the CVD risk in these patients was consistently twice as high as predicted by the FRS beginning after age 40 years, the authors noted.
The findings underscore the importance of CVD risk assessment in patients with psoriatic arthritis but suggest that the Framingham Risk Score may not be applicable in such patients, the investigators said, adding that “This study serves to illustrate the important need for further research to focus on the development of CVD risk assessment tools specific to psoriatic arthritis patients.”
The findings also suggest that aggressive therapy may be warranted early in the course of psoriatic arthritis to “attenuate the long-term burden of CVD,” they said.
This study was supported by the Rochester Epidemiology Project and by Amgen. Dr. Ernste reported having no disclosures.
Most newly diagnosed psoriatic arthritis patients have an increased risk for cardiovascular disease that is markedly underestimated by the Framingham Risk Score, according to findings from a retrospective, population-based, cohort study.
The mean Framingham Risk Score in 126 patients with psoriatic arthritis who were aged 30 years or older and who had no prior cardiovascular disease (CVD) history, was 9.7% during the first 10 years of follow-up. However, the 10-year cumulative incidence of CVD events was nearly double that at 17% (standardized incidence ratio, 1.80), Dr. Floranne C. Ernste of the Mayo Clinic, Rochester, Minn., and her colleagues reported (Arthritis Care Res. 2015 Jan. 7 [doi:10.1002/acr.22536]).
Age-based analysis showed that the CVD risk in these patients was consistently twice as high as predicted by the FRS beginning after age 40 years, the authors noted.
The findings underscore the importance of CVD risk assessment in patients with psoriatic arthritis but suggest that the Framingham Risk Score may not be applicable in such patients, the investigators said, adding that “This study serves to illustrate the important need for further research to focus on the development of CVD risk assessment tools specific to psoriatic arthritis patients.”
The findings also suggest that aggressive therapy may be warranted early in the course of psoriatic arthritis to “attenuate the long-term burden of CVD,” they said.
This study was supported by the Rochester Epidemiology Project and by Amgen. Dr. Ernste reported having no disclosures.
Most newly diagnosed psoriatic arthritis patients have an increased risk for cardiovascular disease that is markedly underestimated by the Framingham Risk Score, according to findings from a retrospective, population-based, cohort study.
The mean Framingham Risk Score in 126 patients with psoriatic arthritis who were aged 30 years or older and who had no prior cardiovascular disease (CVD) history, was 9.7% during the first 10 years of follow-up. However, the 10-year cumulative incidence of CVD events was nearly double that at 17% (standardized incidence ratio, 1.80), Dr. Floranne C. Ernste of the Mayo Clinic, Rochester, Minn., and her colleagues reported (Arthritis Care Res. 2015 Jan. 7 [doi:10.1002/acr.22536]).
Age-based analysis showed that the CVD risk in these patients was consistently twice as high as predicted by the FRS beginning after age 40 years, the authors noted.
The findings underscore the importance of CVD risk assessment in patients with psoriatic arthritis but suggest that the Framingham Risk Score may not be applicable in such patients, the investigators said, adding that “This study serves to illustrate the important need for further research to focus on the development of CVD risk assessment tools specific to psoriatic arthritis patients.”
The findings also suggest that aggressive therapy may be warranted early in the course of psoriatic arthritis to “attenuate the long-term burden of CVD,” they said.
This study was supported by the Rochester Epidemiology Project and by Amgen. Dr. Ernste reported having no disclosures.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: The Framingham Risk Score may not be applicable for estimating CVD risk in psoriatic arthritis patients.
Major finding: The 10-year cumulative CVD incidence rate was 17%, compared with 9.7% predicted by Framingham Risk Score.
Data source: A retrospective, population-based, cohort study of 126 patients.
Disclosures: This study was supported by the Rochester Epidemiology Project and by Amgen. Dr. Ernste reported having no disclosures.