User login
TNF-alpha blockers effective in sustaining reduced PsA activity
A state of minimal disease activity in patients with psoriatic arthritis is sustainable over several years by using treatments with tumor necrosis factor–alpha blockers, according to findings from a single-center, retrospective cohort study.
The study is the first to report on the predictors of minimal disease activity (MDA) in patients with psoriatic arthritis (PsA) taking TNF-alpha inhibitors in a clinical setting and “is also the first “to report about PsA patients who achieved and continued to be in MDA state after treatment change,” wrote the study investigators, who were led by Dr. Amir Haddad of the University of Toronto.
The investigators reported on 226 patients with PsA who were not in an MDA state when they presented to the University of Toronto and were treated with TNF-alpha inhibitors during 2000-2012. They had excluded 23 patients who had MDA when treatment began and 57 who were on TNF-alpha blockers prior to enrollment (Arthritis Care Res. 2014 Dec. 2 [doi:10.1002/acr.22529]).
The patients were 65% male with an average diagnosis age of 36.0 years and were followed at 6-12 month intervals. The authors defined MDA according to the criteria provided by Coates et al. (Ann. Rheum. Dis. 2010;69:48-53), which required patients to meet at least five of seven criteria: 0-1 tender joints ; 0-1 swollen joints; Psoriasis Activity and Severity Index of 1 or less or body surface area of 3 or less; patient pain visual analogue score (VAS) of 15 or less; patient global disease activity VAS of 20 or less; health assessment questionnaire of 0.5 or less; tender entheseal points of 1 or less.
A total of 145 patients (64%) achieved MDA status after an average of 1.30 months (standard deviation, 1.51), and 88 (61%) achieved sustained MDA (defined as at least 1 year) for a mean of 3.46 years (standard deviation, 2.25). Another 17 patients remained in an MDA state after reducing their anti–TNF-alpha drug dose, including 9 who withdrew anti–TNF-alpha treatment completely, and these patients remained in an MDA state for a mean of 2.11 years. The investigators noted that “no protocol was used for tapering the dose and it was left to patient’s preference,” and that MDA was sustained for longer periods of time in patients who reduced the TNF-alpha inhibitor dose and for shorter periods in patients who withdrew the treatment.
Male sex and a normal erythrocyte sedimentation rate (ESR) were found to be reliable predictors of achieving MDA. The authors cited previous studies on ankylosing spondylitis or axial spondyloarthritis, and noted that low ESR/C-reactive protein is considered a predictor for nonresponse to TNF-alpha blockers, but caution that the results of their own study may “simply [be] from using different outcome measures for response.”
The authors disclosed that all but one of them are members of the University of Toronto’s Psoriatic Arthritis Program, which is partly funded by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. Additionally, Dr. Haddad disclosed that he was supported by unrestricted educational grants from Janssen and UCB.
A state of minimal disease activity in patients with psoriatic arthritis is sustainable over several years by using treatments with tumor necrosis factor–alpha blockers, according to findings from a single-center, retrospective cohort study.
The study is the first to report on the predictors of minimal disease activity (MDA) in patients with psoriatic arthritis (PsA) taking TNF-alpha inhibitors in a clinical setting and “is also the first “to report about PsA patients who achieved and continued to be in MDA state after treatment change,” wrote the study investigators, who were led by Dr. Amir Haddad of the University of Toronto.
The investigators reported on 226 patients with PsA who were not in an MDA state when they presented to the University of Toronto and were treated with TNF-alpha inhibitors during 2000-2012. They had excluded 23 patients who had MDA when treatment began and 57 who were on TNF-alpha blockers prior to enrollment (Arthritis Care Res. 2014 Dec. 2 [doi:10.1002/acr.22529]).
The patients were 65% male with an average diagnosis age of 36.0 years and were followed at 6-12 month intervals. The authors defined MDA according to the criteria provided by Coates et al. (Ann. Rheum. Dis. 2010;69:48-53), which required patients to meet at least five of seven criteria: 0-1 tender joints ; 0-1 swollen joints; Psoriasis Activity and Severity Index of 1 or less or body surface area of 3 or less; patient pain visual analogue score (VAS) of 15 or less; patient global disease activity VAS of 20 or less; health assessment questionnaire of 0.5 or less; tender entheseal points of 1 or less.
A total of 145 patients (64%) achieved MDA status after an average of 1.30 months (standard deviation, 1.51), and 88 (61%) achieved sustained MDA (defined as at least 1 year) for a mean of 3.46 years (standard deviation, 2.25). Another 17 patients remained in an MDA state after reducing their anti–TNF-alpha drug dose, including 9 who withdrew anti–TNF-alpha treatment completely, and these patients remained in an MDA state for a mean of 2.11 years. The investigators noted that “no protocol was used for tapering the dose and it was left to patient’s preference,” and that MDA was sustained for longer periods of time in patients who reduced the TNF-alpha inhibitor dose and for shorter periods in patients who withdrew the treatment.
Male sex and a normal erythrocyte sedimentation rate (ESR) were found to be reliable predictors of achieving MDA. The authors cited previous studies on ankylosing spondylitis or axial spondyloarthritis, and noted that low ESR/C-reactive protein is considered a predictor for nonresponse to TNF-alpha blockers, but caution that the results of their own study may “simply [be] from using different outcome measures for response.”
The authors disclosed that all but one of them are members of the University of Toronto’s Psoriatic Arthritis Program, which is partly funded by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. Additionally, Dr. Haddad disclosed that he was supported by unrestricted educational grants from Janssen and UCB.
A state of minimal disease activity in patients with psoriatic arthritis is sustainable over several years by using treatments with tumor necrosis factor–alpha blockers, according to findings from a single-center, retrospective cohort study.
The study is the first to report on the predictors of minimal disease activity (MDA) in patients with psoriatic arthritis (PsA) taking TNF-alpha inhibitors in a clinical setting and “is also the first “to report about PsA patients who achieved and continued to be in MDA state after treatment change,” wrote the study investigators, who were led by Dr. Amir Haddad of the University of Toronto.
The investigators reported on 226 patients with PsA who were not in an MDA state when they presented to the University of Toronto and were treated with TNF-alpha inhibitors during 2000-2012. They had excluded 23 patients who had MDA when treatment began and 57 who were on TNF-alpha blockers prior to enrollment (Arthritis Care Res. 2014 Dec. 2 [doi:10.1002/acr.22529]).
The patients were 65% male with an average diagnosis age of 36.0 years and were followed at 6-12 month intervals. The authors defined MDA according to the criteria provided by Coates et al. (Ann. Rheum. Dis. 2010;69:48-53), which required patients to meet at least five of seven criteria: 0-1 tender joints ; 0-1 swollen joints; Psoriasis Activity and Severity Index of 1 or less or body surface area of 3 or less; patient pain visual analogue score (VAS) of 15 or less; patient global disease activity VAS of 20 or less; health assessment questionnaire of 0.5 or less; tender entheseal points of 1 or less.
A total of 145 patients (64%) achieved MDA status after an average of 1.30 months (standard deviation, 1.51), and 88 (61%) achieved sustained MDA (defined as at least 1 year) for a mean of 3.46 years (standard deviation, 2.25). Another 17 patients remained in an MDA state after reducing their anti–TNF-alpha drug dose, including 9 who withdrew anti–TNF-alpha treatment completely, and these patients remained in an MDA state for a mean of 2.11 years. The investigators noted that “no protocol was used for tapering the dose and it was left to patient’s preference,” and that MDA was sustained for longer periods of time in patients who reduced the TNF-alpha inhibitor dose and for shorter periods in patients who withdrew the treatment.
Male sex and a normal erythrocyte sedimentation rate (ESR) were found to be reliable predictors of achieving MDA. The authors cited previous studies on ankylosing spondylitis or axial spondyloarthritis, and noted that low ESR/C-reactive protein is considered a predictor for nonresponse to TNF-alpha blockers, but caution that the results of their own study may “simply [be] from using different outcome measures for response.”
The authors disclosed that all but one of them are members of the University of Toronto’s Psoriatic Arthritis Program, which is partly funded by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. Additionally, Dr. Haddad disclosed that he was supported by unrestricted educational grants from Janssen and UCB.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: A majority of PsA patients seen in a clinical setting can achieve minimal disease activity on TNF-alpha inhibitors.
Major finding: A total of 64% of PsA patients taking TNF-alpha inhibitors achieved MDA within a mean duration of 1.30 years.
Data source: A retrospective, observational cohort study of 226 patients with PsA.
Disclosures: The authors reported several potential conflicts.
Psoriasis is independently associated with advanced liver fibrosis
AMSTERDAM – Older psoriasis patients have an increased risk of advanced hepatic fibrosis even if they have mild skin disease that has never required systemic therapy.
This finding from the Rotterdam Study raises a red flag: “It could be suggested to screen for liver fibrosis ... before and during potentially hepatotoxic therapies in psoriasis patients, especially in those with metabolic syndrome,” Dr. Ella van der Voort said in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
The Rotterdam Study is an ongoing large, prospective, population-based cohort study which began in 1998. Routine liver screening by FibroScan was incorporated into the study protocol in 2011. The proprietary FibroScan transient elastography device is noninvasive and yields results in 5 minutes, explained Dr. van der Voort, a dermatologist at Erasmus University, Rotterdam, the Netherlands.
In her analysis, Dr. van der Voort reported on 1,535 Rotterdam Study participants with FibroScan results, including 75 with psoriasis. The mean age was 71 years, both in the psoriasis patients and the 1,461 nonpsoriatic controls. The psoriasis patients had mild skin disease, with a mean Psoriasis Area and Severity Index score of 2.0. None of the psoriasis patients had ever received methotrexate or any other systemic therapy for their psoriasis.
The prevalence of advanced liver fibrosis was 3.6% in controls and 8.1% in the psoriasis patients. In a multivariate analysis adjusted for alcohol consumption, alanine aminotransferase levels, steatosis, metabolic syndrome, age, and gender, psoriasis was independently associated with a 2.6-fold increased risk of advanced liver fibrosis.
Among the subgroup of subjects with nonalcoholic fatty liver disease (NAFLD), psoriasis was associated with an even greater likelihood of advanced hepatic fibrosis. This liver finding was present in 15% of 20 patients with mild psoriasis and NAFLD, compared with 4% of 375 nonpsoriatic controls with NAFLD, for an adjusted 4.1-fold increased risk among the group with psoriasis.
Psoriasis patients with advanced liver fibrosis had a relatively high prevalence of the metabolic syndrome, so hepatic screening efforts could be focused on this subgroup, in Dr. van der Voort’s view.
In contrast, serum ALTs were generally within normal range in affected patients. “Using elevated ALTs to trigger screening for liver fibrosis in psoriasis patients is not good advice,” she cautioned.
Citing the advanced age of the Rotterdam Study cohort, Dr. van der Voort noted that her study findings may not be applicable to younger patients with mild psoriasis.
Dr. van der Voort presented another analysis from the Rotterdam Study at the 2013 EADV Congress in Istanbul. In that analysis, she reported that psoriasis was independently associated with a 70% increased risk of NAFLD. Since patients with NAFLD are at increased risk for progression to fibrosis and cirrhosis, the new finding of a 2.6-fold increased risk for advanced liver fibrosis in psoriasis patients doesn’t come as a total surprise. The biggest concern, of course, is that advanced liver fibrosis is, in turn, associated with an increased risk of hepatocellular carcinoma.
The Rotterdam Study is funded by Dutch governmental grants and foundations. Dr. van der Voort reported having no financial conflicts.
AMSTERDAM – Older psoriasis patients have an increased risk of advanced hepatic fibrosis even if they have mild skin disease that has never required systemic therapy.
This finding from the Rotterdam Study raises a red flag: “It could be suggested to screen for liver fibrosis ... before and during potentially hepatotoxic therapies in psoriasis patients, especially in those with metabolic syndrome,” Dr. Ella van der Voort said in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
The Rotterdam Study is an ongoing large, prospective, population-based cohort study which began in 1998. Routine liver screening by FibroScan was incorporated into the study protocol in 2011. The proprietary FibroScan transient elastography device is noninvasive and yields results in 5 minutes, explained Dr. van der Voort, a dermatologist at Erasmus University, Rotterdam, the Netherlands.
In her analysis, Dr. van der Voort reported on 1,535 Rotterdam Study participants with FibroScan results, including 75 with psoriasis. The mean age was 71 years, both in the psoriasis patients and the 1,461 nonpsoriatic controls. The psoriasis patients had mild skin disease, with a mean Psoriasis Area and Severity Index score of 2.0. None of the psoriasis patients had ever received methotrexate or any other systemic therapy for their psoriasis.
The prevalence of advanced liver fibrosis was 3.6% in controls and 8.1% in the psoriasis patients. In a multivariate analysis adjusted for alcohol consumption, alanine aminotransferase levels, steatosis, metabolic syndrome, age, and gender, psoriasis was independently associated with a 2.6-fold increased risk of advanced liver fibrosis.
Among the subgroup of subjects with nonalcoholic fatty liver disease (NAFLD), psoriasis was associated with an even greater likelihood of advanced hepatic fibrosis. This liver finding was present in 15% of 20 patients with mild psoriasis and NAFLD, compared with 4% of 375 nonpsoriatic controls with NAFLD, for an adjusted 4.1-fold increased risk among the group with psoriasis.
Psoriasis patients with advanced liver fibrosis had a relatively high prevalence of the metabolic syndrome, so hepatic screening efforts could be focused on this subgroup, in Dr. van der Voort’s view.
In contrast, serum ALTs were generally within normal range in affected patients. “Using elevated ALTs to trigger screening for liver fibrosis in psoriasis patients is not good advice,” she cautioned.
Citing the advanced age of the Rotterdam Study cohort, Dr. van der Voort noted that her study findings may not be applicable to younger patients with mild psoriasis.
Dr. van der Voort presented another analysis from the Rotterdam Study at the 2013 EADV Congress in Istanbul. In that analysis, she reported that psoriasis was independently associated with a 70% increased risk of NAFLD. Since patients with NAFLD are at increased risk for progression to fibrosis and cirrhosis, the new finding of a 2.6-fold increased risk for advanced liver fibrosis in psoriasis patients doesn’t come as a total surprise. The biggest concern, of course, is that advanced liver fibrosis is, in turn, associated with an increased risk of hepatocellular carcinoma.
The Rotterdam Study is funded by Dutch governmental grants and foundations. Dr. van der Voort reported having no financial conflicts.
AMSTERDAM – Older psoriasis patients have an increased risk of advanced hepatic fibrosis even if they have mild skin disease that has never required systemic therapy.
This finding from the Rotterdam Study raises a red flag: “It could be suggested to screen for liver fibrosis ... before and during potentially hepatotoxic therapies in psoriasis patients, especially in those with metabolic syndrome,” Dr. Ella van der Voort said in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
The Rotterdam Study is an ongoing large, prospective, population-based cohort study which began in 1998. Routine liver screening by FibroScan was incorporated into the study protocol in 2011. The proprietary FibroScan transient elastography device is noninvasive and yields results in 5 minutes, explained Dr. van der Voort, a dermatologist at Erasmus University, Rotterdam, the Netherlands.
In her analysis, Dr. van der Voort reported on 1,535 Rotterdam Study participants with FibroScan results, including 75 with psoriasis. The mean age was 71 years, both in the psoriasis patients and the 1,461 nonpsoriatic controls. The psoriasis patients had mild skin disease, with a mean Psoriasis Area and Severity Index score of 2.0. None of the psoriasis patients had ever received methotrexate or any other systemic therapy for their psoriasis.
The prevalence of advanced liver fibrosis was 3.6% in controls and 8.1% in the psoriasis patients. In a multivariate analysis adjusted for alcohol consumption, alanine aminotransferase levels, steatosis, metabolic syndrome, age, and gender, psoriasis was independently associated with a 2.6-fold increased risk of advanced liver fibrosis.
Among the subgroup of subjects with nonalcoholic fatty liver disease (NAFLD), psoriasis was associated with an even greater likelihood of advanced hepatic fibrosis. This liver finding was present in 15% of 20 patients with mild psoriasis and NAFLD, compared with 4% of 375 nonpsoriatic controls with NAFLD, for an adjusted 4.1-fold increased risk among the group with psoriasis.
Psoriasis patients with advanced liver fibrosis had a relatively high prevalence of the metabolic syndrome, so hepatic screening efforts could be focused on this subgroup, in Dr. van der Voort’s view.
In contrast, serum ALTs were generally within normal range in affected patients. “Using elevated ALTs to trigger screening for liver fibrosis in psoriasis patients is not good advice,” she cautioned.
Citing the advanced age of the Rotterdam Study cohort, Dr. van der Voort noted that her study findings may not be applicable to younger patients with mild psoriasis.
Dr. van der Voort presented another analysis from the Rotterdam Study at the 2013 EADV Congress in Istanbul. In that analysis, she reported that psoriasis was independently associated with a 70% increased risk of NAFLD. Since patients with NAFLD are at increased risk for progression to fibrosis and cirrhosis, the new finding of a 2.6-fold increased risk for advanced liver fibrosis in psoriasis patients doesn’t come as a total surprise. The biggest concern, of course, is that advanced liver fibrosis is, in turn, associated with an increased risk of hepatocellular carcinoma.
The Rotterdam Study is funded by Dutch governmental grants and foundations. Dr. van der Voort reported having no financial conflicts.
AT THE EADV CONGRESS
Key clinical point: Noninvasive screening for advanced hepatic fibrosis may be in order for older patients with mild psoriasis never treated systemically.
Major finding: In a multivariate analysis adjusted for alcohol consumption, alanine aminotransferase levels, steatosis, metabolic syndrome, age, and gender, psoriasis was independently associated with a 2.6-fold increased risk of advanced liver fibrosis.
Data source: This analysis involved noninvasive screening for hepatic fibrosis in 1,535 elderly participants in the Rotterdam Study, an ongoing prospective population-based cohort study.
Disclosures: The Rotterdam Study is funded by Dutch governmental research grants and foundations. The presenter reported having no financial conflicts.
Golimumab effective against nonradiographic axial spondyloarthritis with inflammation
BOSTON – Golimumab was significantly better than placebo at reducing disease activity and inflammation in patients with nonradiographic axial spondyloarthritis, but only among those who were positive for disease on MRI or the inflammation marker C-reactive protein.
In the randomized, double-blind, placebo-controlled GO-AHEAD trial of 198 patients with nonradiographic axial spondyloarthritis (nr-axSpA), 71.1% of patients treated with golimumab (Simponi) achieved the primary endpoint of an ASAS 20 at week 16, compared with 40% of patients on placebo (P < .0001), reported Dr. Joachim Sieper of Charité Medical University in Berlin.
But in a subgroup analysis of patients with normal MRI and C-reactive protein (CRP) levels at baseline, there was no significant difference in ASAS 20 responses at week 16, although the number of patients may have been too small to reveal an effect, Dr. Sieper said.
“There was no difference regarding any adverse event, no difference regarding serious adverse events, and also no difference regarding other adverse events, and there were no deaths in this study,” he said at the annual meeting of the American College of Rheumatology.
Golimumab is a tumor necrosis factor–alpha (TNF-alpha) inhibitor approved in the United States for treatment of moderate to severe rheumatoid arthritis, active psoriatic arthritis, active ankylosing spondylitis, and moderate to severe ulcerative colitis.
The phase III trial was a double-blind, placebo-controlled study in 198 patients with a diagnosis of active axSpA according to Assessment of Spondyloarthritis (ASAS) criteria, a disease duration of less than 5 years since symptom onset, and chronic back pain lasting for at least 3 months.
The patients were randomly assigned to receive either golimumab 50 mg every 4 weeks or placebo via subcutaneous injection. A total of 93 patients assigned to golimumab and 97 assigned to placebo completed 16 weeks of therapy.
The primary endpoint was the percentage of patients achieving an ASAS 20, defined as an improvement of at least 20% and absolute improvement of at least 10 units on a 0-100 scale in three of four domains:
• Patient global assessment (by VAS [visual analog scale] global assessment).
• Pain assessment (the average of VAS total and nocturnal pain scores).
• Function (represented by BASFI [Bath Ankylosing Spondylitis Functional Index]).
• Inflammation (the average score of the last two VASs in the BASDAI [Bath Ankylosing Spondylitis Disease Activity Index], concerning morning stiffness intensity and duration), as well as an absence of deterioration (20% or greater worsening) in the potential remaining domain.
As noted before, golimumab met the primary endpoint among all patients, but in a subanalysis the TNF-alpha inhibitor was significantly better than placebo only among patients with evidence of inflammation on MRI or an elevated CRP at baseline (76.9% vs. 37.5%; P < .0001).
Golimumab was also superior to placebo at week 16 on three secondary endpoints: BASDAI 50 (57.7% vs. 30%; P < .0001), ASAS partial remission (33% vs. 18%; P = .0136), and mean change in Spondyloarthritis Research Consortium of Canada (SPARCC) MRI sacroiliac joint score (–5.3 vs. –0.9; P < .0001).
In an intention-to-treat analysis, more patients on placebo had any adverse events after 60 weeks of follow-up (41.2% for golimumab vs. 47% for placebo). Patients on placebo were also more likely to have an event judged by the investigator to be related to the assigned medication (13.4% vs. 17%). There were no serious infections, opportunistic infections, active tuberculosis, malignancies, serious systemic hypersensitivity reactions, or deaths.
The study was sponsored by Merck Sharp & Dohme. Dr. Sieper disclosed receiving honoraria, speaker fees, and/or consultancy payments from the company. Four of the study coauthors are employees of Merck.
BOSTON – Golimumab was significantly better than placebo at reducing disease activity and inflammation in patients with nonradiographic axial spondyloarthritis, but only among those who were positive for disease on MRI or the inflammation marker C-reactive protein.
In the randomized, double-blind, placebo-controlled GO-AHEAD trial of 198 patients with nonradiographic axial spondyloarthritis (nr-axSpA), 71.1% of patients treated with golimumab (Simponi) achieved the primary endpoint of an ASAS 20 at week 16, compared with 40% of patients on placebo (P < .0001), reported Dr. Joachim Sieper of Charité Medical University in Berlin.
But in a subgroup analysis of patients with normal MRI and C-reactive protein (CRP) levels at baseline, there was no significant difference in ASAS 20 responses at week 16, although the number of patients may have been too small to reveal an effect, Dr. Sieper said.
“There was no difference regarding any adverse event, no difference regarding serious adverse events, and also no difference regarding other adverse events, and there were no deaths in this study,” he said at the annual meeting of the American College of Rheumatology.
Golimumab is a tumor necrosis factor–alpha (TNF-alpha) inhibitor approved in the United States for treatment of moderate to severe rheumatoid arthritis, active psoriatic arthritis, active ankylosing spondylitis, and moderate to severe ulcerative colitis.
The phase III trial was a double-blind, placebo-controlled study in 198 patients with a diagnosis of active axSpA according to Assessment of Spondyloarthritis (ASAS) criteria, a disease duration of less than 5 years since symptom onset, and chronic back pain lasting for at least 3 months.
The patients were randomly assigned to receive either golimumab 50 mg every 4 weeks or placebo via subcutaneous injection. A total of 93 patients assigned to golimumab and 97 assigned to placebo completed 16 weeks of therapy.
The primary endpoint was the percentage of patients achieving an ASAS 20, defined as an improvement of at least 20% and absolute improvement of at least 10 units on a 0-100 scale in three of four domains:
• Patient global assessment (by VAS [visual analog scale] global assessment).
• Pain assessment (the average of VAS total and nocturnal pain scores).
• Function (represented by BASFI [Bath Ankylosing Spondylitis Functional Index]).
• Inflammation (the average score of the last two VASs in the BASDAI [Bath Ankylosing Spondylitis Disease Activity Index], concerning morning stiffness intensity and duration), as well as an absence of deterioration (20% or greater worsening) in the potential remaining domain.
As noted before, golimumab met the primary endpoint among all patients, but in a subanalysis the TNF-alpha inhibitor was significantly better than placebo only among patients with evidence of inflammation on MRI or an elevated CRP at baseline (76.9% vs. 37.5%; P < .0001).
Golimumab was also superior to placebo at week 16 on three secondary endpoints: BASDAI 50 (57.7% vs. 30%; P < .0001), ASAS partial remission (33% vs. 18%; P = .0136), and mean change in Spondyloarthritis Research Consortium of Canada (SPARCC) MRI sacroiliac joint score (–5.3 vs. –0.9; P < .0001).
In an intention-to-treat analysis, more patients on placebo had any adverse events after 60 weeks of follow-up (41.2% for golimumab vs. 47% for placebo). Patients on placebo were also more likely to have an event judged by the investigator to be related to the assigned medication (13.4% vs. 17%). There were no serious infections, opportunistic infections, active tuberculosis, malignancies, serious systemic hypersensitivity reactions, or deaths.
The study was sponsored by Merck Sharp & Dohme. Dr. Sieper disclosed receiving honoraria, speaker fees, and/or consultancy payments from the company. Four of the study coauthors are employees of Merck.
BOSTON – Golimumab was significantly better than placebo at reducing disease activity and inflammation in patients with nonradiographic axial spondyloarthritis, but only among those who were positive for disease on MRI or the inflammation marker C-reactive protein.
In the randomized, double-blind, placebo-controlled GO-AHEAD trial of 198 patients with nonradiographic axial spondyloarthritis (nr-axSpA), 71.1% of patients treated with golimumab (Simponi) achieved the primary endpoint of an ASAS 20 at week 16, compared with 40% of patients on placebo (P < .0001), reported Dr. Joachim Sieper of Charité Medical University in Berlin.
But in a subgroup analysis of patients with normal MRI and C-reactive protein (CRP) levels at baseline, there was no significant difference in ASAS 20 responses at week 16, although the number of patients may have been too small to reveal an effect, Dr. Sieper said.
“There was no difference regarding any adverse event, no difference regarding serious adverse events, and also no difference regarding other adverse events, and there were no deaths in this study,” he said at the annual meeting of the American College of Rheumatology.
Golimumab is a tumor necrosis factor–alpha (TNF-alpha) inhibitor approved in the United States for treatment of moderate to severe rheumatoid arthritis, active psoriatic arthritis, active ankylosing spondylitis, and moderate to severe ulcerative colitis.
The phase III trial was a double-blind, placebo-controlled study in 198 patients with a diagnosis of active axSpA according to Assessment of Spondyloarthritis (ASAS) criteria, a disease duration of less than 5 years since symptom onset, and chronic back pain lasting for at least 3 months.
The patients were randomly assigned to receive either golimumab 50 mg every 4 weeks or placebo via subcutaneous injection. A total of 93 patients assigned to golimumab and 97 assigned to placebo completed 16 weeks of therapy.
The primary endpoint was the percentage of patients achieving an ASAS 20, defined as an improvement of at least 20% and absolute improvement of at least 10 units on a 0-100 scale in three of four domains:
• Patient global assessment (by VAS [visual analog scale] global assessment).
• Pain assessment (the average of VAS total and nocturnal pain scores).
• Function (represented by BASFI [Bath Ankylosing Spondylitis Functional Index]).
• Inflammation (the average score of the last two VASs in the BASDAI [Bath Ankylosing Spondylitis Disease Activity Index], concerning morning stiffness intensity and duration), as well as an absence of deterioration (20% or greater worsening) in the potential remaining domain.
As noted before, golimumab met the primary endpoint among all patients, but in a subanalysis the TNF-alpha inhibitor was significantly better than placebo only among patients with evidence of inflammation on MRI or an elevated CRP at baseline (76.9% vs. 37.5%; P < .0001).
Golimumab was also superior to placebo at week 16 on three secondary endpoints: BASDAI 50 (57.7% vs. 30%; P < .0001), ASAS partial remission (33% vs. 18%; P = .0136), and mean change in Spondyloarthritis Research Consortium of Canada (SPARCC) MRI sacroiliac joint score (–5.3 vs. –0.9; P < .0001).
In an intention-to-treat analysis, more patients on placebo had any adverse events after 60 weeks of follow-up (41.2% for golimumab vs. 47% for placebo). Patients on placebo were also more likely to have an event judged by the investigator to be related to the assigned medication (13.4% vs. 17%). There were no serious infections, opportunistic infections, active tuberculosis, malignancies, serious systemic hypersensitivity reactions, or deaths.
The study was sponsored by Merck Sharp & Dohme. Dr. Sieper disclosed receiving honoraria, speaker fees, and/or consultancy payments from the company. Four of the study coauthors are employees of Merck.
AT THE ACR ANNUAL MEETING
Key clinical point: Golimumab was significantly more effective than placebo among patients with nonradiographic axial spondyloarthritis and inflammation at baseline.
Major finding: A total of 71.1% of patients treated with golimumab had an ASAS 20 at week 16, compared with 40% of patients on placebo.
Data source: Randomized, double-blind, placebo-controlled study in 198 patients with nonradiographic axial spondyloarthritis.
Disclosures: The study was sponsored by Merck Sharp & Dohme. Dr. Sieper disclosed receiving honoraria, speaker fees, and/or consultancy payments from the company. Four of the study coauthors are employees of Merck.
Axial spondyloarthropathy guidelines: NSAIDs and PT first
BOSTON – A nonsteroidal anti-inflammatory drug and exercise may be enough to control active axial spondyloarthritis in some patients, suggest authors of draft guidelines on the management of patients with the condition.
The guidelines, not ready for prime time, have yet to be reviewed or endorsed by the American College of Rheumatology (ACR) and the Spondylitis Association of America, or the SpondyloArthritis Research and Treatment Network (SPARTAN), and are subject to change, emphasized Dr. Michael M. Ward, senior investigator at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
With that caveat in mind, Dr. Ward presented a sneak peek at the guidelines to a standing-room only crowd at the ACR annual meeting in Boston.
Some definitions
The guidelines offer recommendations on the management of patients with active and stable ankylosing spondylitis (AS) and axial spondyloarthropathies (axSpA) that are symptomatic but without radiographic evidence (nonradiographic, or nr-axSpA).
Active AS is defined as disease that causes symptoms at an unacceptably burdensome level as reported by the patient that are judged by the examining clinician to be caused by AS. The same definition also applies to nr-axSpA.
Stable disease is defined as either an asymptomatic state or symptoms that were previously bothersome but are currently at an acceptable level as reported by the patient. The patient had to have had bothersome symptoms for at least 6 months before entering the stable disease state. This definition is also applicable to stable nr-axSpA.
The investigators considered the best available evidence on the use of NSAIDs (running the gamut from aspirin to tolmetin), slow-acting antirheumatic agents such as methotrexate, glucocorticoids (prednisone and others), tumor necrosis factor (TNF) inhibitors, such as adalimumab, etanercept, and others), and non-TNF biologic agents (abatacept, rituximab, tocilizumab, and others).
Active AS
Dr. Ward presented a management flow tree for patients with active AS, starting with a strong recommendation for an NSAID, conditionally recommended to be used continuously. The authors felt, however, that there was not enough evidence to support the use of one NSAID over another. They also strongly recommended physical therapy, with less robust recommendations for active than for passive exercise and for exercises performed in water rather than on land. The latter recommendation is based on the fact that, although water-based exercises have been shown to be as good as or better than dry land exercises for relieving symptoms, water-based exercise may be impractical for many patients, Dr. Ward noted.
For patients whose disease remains active despite NSAIDs and exercise, the committee strongly recommends use of a tumor necrosis factor inhibitor (TNFi) (no specific agent preferred). If a patient on a TNFi has recurrent iritis, the guidelines have a conditional recommendation for the use of infliximab or adalimumab. For patients with inflammatory bowel disease (IBD), the authors conditionally recommend a TNFi monoclonal antibody as opposed to etanercept.
If the disease remains active on a TNFi, an alternative TNFi can be considered.
“For patients who have contraindications to TNF inhibitors, we considered the choice between adding a slow-acting drug such as sulfasalazine or pamidronate or treating with a non-TNF biologic. Of course, there are no head-to-head trials between those two options, so based on the indirect evidence that’s available, the committee voted for a conditional recommendation against the use of a non-TNF biologic in favor of a slow-acting drug in that setting,” Dr. Ward said.
If there are no contraindications to a TNF inhibitor, however, the committee strongly favored the use of a TNF inhibitor over a slow-acting agent, he emphasized.
For patients who have isolated sacroiliitis, peripheral arthritis, or enthesis, the committee provisionally recommends local injection of a glucocorticoid, with cautions to use infrequently and only if two or fewer joints are involved in peripheral arthritis, and avoidance of injection of the Achilles, patellar, or quadriceps tendons in patients with enthesitis.
For all patients, the guidelines half-heartedly recommend monitoring validated axSpA disease activity measures and C-reactive protein and erythrocyte sedimentation rate (ESR). The group also conditionally supported unsupervised back exercises, formal group or individual self-management education, and fall evaluation and counseling.
Committee members strongly felt that systemic glucocorticoids should not be used in patients with active axSpA, except in cases where a short-term course with quick taper may be helpful, such as in patients with peripheral flare, or during pregnancy or a concomitant IBD flare.
Stable AS
“For patients with stable ankylosing spondylitis who are on combination therapy, either combination therapy with NSAIDs and a TNF inhibitor, or a slow-acting drug and a TNF inhibitor, the committee voted against continuation of a combination in favor of TNF monotherapy. It’s a conditional recommendation, so there certainly would be situations where one would not want to do that, but in general the committee thought that was the preferable approach, balancing the benefits and potential risks of combination therapy against monotherapy in patients with stable AS,” Dr. Ward said.
The committee members strongly supported physical therapy in patients with stable AS and gave a conditional nod to monitoring, back exercises, group support, and fall counseling.
For patients with stable AS and advanced hip arthritis, hip replacement is strongly recommended. Recommendations for and against other special conditions include severe kyphosis (strongly against elective spine osteotomy except in specialized centers), acute iritis (strong support for an ophthalmology consultant), recurrent iritis (conditional support for at home use of a topical glucocorticoid under the supervision of an eye care provider, and use of infliximab or adalimumab over etanercept), and IBD (strong recommendation for TNFi monoclonals over etanercept and conditional endorsement of no preferred NSAID).
Active nr-axSpA
Recommendations for the treatment of nr-axSpA are essentially identical to those for treating active AS, Dr. Ward noted, except that in contrast to active AS, where the recommendation is strongly in favor of TNF inhibitors, the committee gave only a conditional recommendation for the use of a TNF inhibitor in this clinical situation.
Stable nr-axSpA
For patients with stable nr-axSpA, the recommendations are strongly in favor of NSAID use, with a conditional suggestion to use on demand. The recommendations also are conditionally against combination therapy with either an NSAID or slow-acting agent plus a TNF inhibitor, with a conditional approval for TNF inhibitor monotherapy instead. The committee strongly supported physical therapy for these patients and gave a lukewarm embrace of monitoring for disease activity, CRP, or ESR.
Dr. Ward noted that the guidelines are designed to help clinicians with treatment decisions for the typical patient with AS or nr-axSpA, and do not address the needs of all populations or all clinical circumstances or contingencies.
He also noted that for many of the questions the committee members tried to address, high-quality evidence was limited.
Dr. Ward did not mention a projected publication date for the guidelines. He had no relevant financial conflicts to disclose.
BOSTON – A nonsteroidal anti-inflammatory drug and exercise may be enough to control active axial spondyloarthritis in some patients, suggest authors of draft guidelines on the management of patients with the condition.
The guidelines, not ready for prime time, have yet to be reviewed or endorsed by the American College of Rheumatology (ACR) and the Spondylitis Association of America, or the SpondyloArthritis Research and Treatment Network (SPARTAN), and are subject to change, emphasized Dr. Michael M. Ward, senior investigator at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
With that caveat in mind, Dr. Ward presented a sneak peek at the guidelines to a standing-room only crowd at the ACR annual meeting in Boston.
Some definitions
The guidelines offer recommendations on the management of patients with active and stable ankylosing spondylitis (AS) and axial spondyloarthropathies (axSpA) that are symptomatic but without radiographic evidence (nonradiographic, or nr-axSpA).
Active AS is defined as disease that causes symptoms at an unacceptably burdensome level as reported by the patient that are judged by the examining clinician to be caused by AS. The same definition also applies to nr-axSpA.
Stable disease is defined as either an asymptomatic state or symptoms that were previously bothersome but are currently at an acceptable level as reported by the patient. The patient had to have had bothersome symptoms for at least 6 months before entering the stable disease state. This definition is also applicable to stable nr-axSpA.
The investigators considered the best available evidence on the use of NSAIDs (running the gamut from aspirin to tolmetin), slow-acting antirheumatic agents such as methotrexate, glucocorticoids (prednisone and others), tumor necrosis factor (TNF) inhibitors, such as adalimumab, etanercept, and others), and non-TNF biologic agents (abatacept, rituximab, tocilizumab, and others).
Active AS
Dr. Ward presented a management flow tree for patients with active AS, starting with a strong recommendation for an NSAID, conditionally recommended to be used continuously. The authors felt, however, that there was not enough evidence to support the use of one NSAID over another. They also strongly recommended physical therapy, with less robust recommendations for active than for passive exercise and for exercises performed in water rather than on land. The latter recommendation is based on the fact that, although water-based exercises have been shown to be as good as or better than dry land exercises for relieving symptoms, water-based exercise may be impractical for many patients, Dr. Ward noted.
For patients whose disease remains active despite NSAIDs and exercise, the committee strongly recommends use of a tumor necrosis factor inhibitor (TNFi) (no specific agent preferred). If a patient on a TNFi has recurrent iritis, the guidelines have a conditional recommendation for the use of infliximab or adalimumab. For patients with inflammatory bowel disease (IBD), the authors conditionally recommend a TNFi monoclonal antibody as opposed to etanercept.
If the disease remains active on a TNFi, an alternative TNFi can be considered.
“For patients who have contraindications to TNF inhibitors, we considered the choice between adding a slow-acting drug such as sulfasalazine or pamidronate or treating with a non-TNF biologic. Of course, there are no head-to-head trials between those two options, so based on the indirect evidence that’s available, the committee voted for a conditional recommendation against the use of a non-TNF biologic in favor of a slow-acting drug in that setting,” Dr. Ward said.
If there are no contraindications to a TNF inhibitor, however, the committee strongly favored the use of a TNF inhibitor over a slow-acting agent, he emphasized.
For patients who have isolated sacroiliitis, peripheral arthritis, or enthesis, the committee provisionally recommends local injection of a glucocorticoid, with cautions to use infrequently and only if two or fewer joints are involved in peripheral arthritis, and avoidance of injection of the Achilles, patellar, or quadriceps tendons in patients with enthesitis.
For all patients, the guidelines half-heartedly recommend monitoring validated axSpA disease activity measures and C-reactive protein and erythrocyte sedimentation rate (ESR). The group also conditionally supported unsupervised back exercises, formal group or individual self-management education, and fall evaluation and counseling.
Committee members strongly felt that systemic glucocorticoids should not be used in patients with active axSpA, except in cases where a short-term course with quick taper may be helpful, such as in patients with peripheral flare, or during pregnancy or a concomitant IBD flare.
Stable AS
“For patients with stable ankylosing spondylitis who are on combination therapy, either combination therapy with NSAIDs and a TNF inhibitor, or a slow-acting drug and a TNF inhibitor, the committee voted against continuation of a combination in favor of TNF monotherapy. It’s a conditional recommendation, so there certainly would be situations where one would not want to do that, but in general the committee thought that was the preferable approach, balancing the benefits and potential risks of combination therapy against monotherapy in patients with stable AS,” Dr. Ward said.
The committee members strongly supported physical therapy in patients with stable AS and gave a conditional nod to monitoring, back exercises, group support, and fall counseling.
For patients with stable AS and advanced hip arthritis, hip replacement is strongly recommended. Recommendations for and against other special conditions include severe kyphosis (strongly against elective spine osteotomy except in specialized centers), acute iritis (strong support for an ophthalmology consultant), recurrent iritis (conditional support for at home use of a topical glucocorticoid under the supervision of an eye care provider, and use of infliximab or adalimumab over etanercept), and IBD (strong recommendation for TNFi monoclonals over etanercept and conditional endorsement of no preferred NSAID).
Active nr-axSpA
Recommendations for the treatment of nr-axSpA are essentially identical to those for treating active AS, Dr. Ward noted, except that in contrast to active AS, where the recommendation is strongly in favor of TNF inhibitors, the committee gave only a conditional recommendation for the use of a TNF inhibitor in this clinical situation.
Stable nr-axSpA
For patients with stable nr-axSpA, the recommendations are strongly in favor of NSAID use, with a conditional suggestion to use on demand. The recommendations also are conditionally against combination therapy with either an NSAID or slow-acting agent plus a TNF inhibitor, with a conditional approval for TNF inhibitor monotherapy instead. The committee strongly supported physical therapy for these patients and gave a lukewarm embrace of monitoring for disease activity, CRP, or ESR.
Dr. Ward noted that the guidelines are designed to help clinicians with treatment decisions for the typical patient with AS or nr-axSpA, and do not address the needs of all populations or all clinical circumstances or contingencies.
He also noted that for many of the questions the committee members tried to address, high-quality evidence was limited.
Dr. Ward did not mention a projected publication date for the guidelines. He had no relevant financial conflicts to disclose.
BOSTON – A nonsteroidal anti-inflammatory drug and exercise may be enough to control active axial spondyloarthritis in some patients, suggest authors of draft guidelines on the management of patients with the condition.
The guidelines, not ready for prime time, have yet to be reviewed or endorsed by the American College of Rheumatology (ACR) and the Spondylitis Association of America, or the SpondyloArthritis Research and Treatment Network (SPARTAN), and are subject to change, emphasized Dr. Michael M. Ward, senior investigator at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
With that caveat in mind, Dr. Ward presented a sneak peek at the guidelines to a standing-room only crowd at the ACR annual meeting in Boston.
Some definitions
The guidelines offer recommendations on the management of patients with active and stable ankylosing spondylitis (AS) and axial spondyloarthropathies (axSpA) that are symptomatic but without radiographic evidence (nonradiographic, or nr-axSpA).
Active AS is defined as disease that causes symptoms at an unacceptably burdensome level as reported by the patient that are judged by the examining clinician to be caused by AS. The same definition also applies to nr-axSpA.
Stable disease is defined as either an asymptomatic state or symptoms that were previously bothersome but are currently at an acceptable level as reported by the patient. The patient had to have had bothersome symptoms for at least 6 months before entering the stable disease state. This definition is also applicable to stable nr-axSpA.
The investigators considered the best available evidence on the use of NSAIDs (running the gamut from aspirin to tolmetin), slow-acting antirheumatic agents such as methotrexate, glucocorticoids (prednisone and others), tumor necrosis factor (TNF) inhibitors, such as adalimumab, etanercept, and others), and non-TNF biologic agents (abatacept, rituximab, tocilizumab, and others).
Active AS
Dr. Ward presented a management flow tree for patients with active AS, starting with a strong recommendation for an NSAID, conditionally recommended to be used continuously. The authors felt, however, that there was not enough evidence to support the use of one NSAID over another. They also strongly recommended physical therapy, with less robust recommendations for active than for passive exercise and for exercises performed in water rather than on land. The latter recommendation is based on the fact that, although water-based exercises have been shown to be as good as or better than dry land exercises for relieving symptoms, water-based exercise may be impractical for many patients, Dr. Ward noted.
For patients whose disease remains active despite NSAIDs and exercise, the committee strongly recommends use of a tumor necrosis factor inhibitor (TNFi) (no specific agent preferred). If a patient on a TNFi has recurrent iritis, the guidelines have a conditional recommendation for the use of infliximab or adalimumab. For patients with inflammatory bowel disease (IBD), the authors conditionally recommend a TNFi monoclonal antibody as opposed to etanercept.
If the disease remains active on a TNFi, an alternative TNFi can be considered.
“For patients who have contraindications to TNF inhibitors, we considered the choice between adding a slow-acting drug such as sulfasalazine or pamidronate or treating with a non-TNF biologic. Of course, there are no head-to-head trials between those two options, so based on the indirect evidence that’s available, the committee voted for a conditional recommendation against the use of a non-TNF biologic in favor of a slow-acting drug in that setting,” Dr. Ward said.
If there are no contraindications to a TNF inhibitor, however, the committee strongly favored the use of a TNF inhibitor over a slow-acting agent, he emphasized.
For patients who have isolated sacroiliitis, peripheral arthritis, or enthesis, the committee provisionally recommends local injection of a glucocorticoid, with cautions to use infrequently and only if two or fewer joints are involved in peripheral arthritis, and avoidance of injection of the Achilles, patellar, or quadriceps tendons in patients with enthesitis.
For all patients, the guidelines half-heartedly recommend monitoring validated axSpA disease activity measures and C-reactive protein and erythrocyte sedimentation rate (ESR). The group also conditionally supported unsupervised back exercises, formal group or individual self-management education, and fall evaluation and counseling.
Committee members strongly felt that systemic glucocorticoids should not be used in patients with active axSpA, except in cases where a short-term course with quick taper may be helpful, such as in patients with peripheral flare, or during pregnancy or a concomitant IBD flare.
Stable AS
“For patients with stable ankylosing spondylitis who are on combination therapy, either combination therapy with NSAIDs and a TNF inhibitor, or a slow-acting drug and a TNF inhibitor, the committee voted against continuation of a combination in favor of TNF monotherapy. It’s a conditional recommendation, so there certainly would be situations where one would not want to do that, but in general the committee thought that was the preferable approach, balancing the benefits and potential risks of combination therapy against monotherapy in patients with stable AS,” Dr. Ward said.
The committee members strongly supported physical therapy in patients with stable AS and gave a conditional nod to monitoring, back exercises, group support, and fall counseling.
For patients with stable AS and advanced hip arthritis, hip replacement is strongly recommended. Recommendations for and against other special conditions include severe kyphosis (strongly against elective spine osteotomy except in specialized centers), acute iritis (strong support for an ophthalmology consultant), recurrent iritis (conditional support for at home use of a topical glucocorticoid under the supervision of an eye care provider, and use of infliximab or adalimumab over etanercept), and IBD (strong recommendation for TNFi monoclonals over etanercept and conditional endorsement of no preferred NSAID).
Active nr-axSpA
Recommendations for the treatment of nr-axSpA are essentially identical to those for treating active AS, Dr. Ward noted, except that in contrast to active AS, where the recommendation is strongly in favor of TNF inhibitors, the committee gave only a conditional recommendation for the use of a TNF inhibitor in this clinical situation.
Stable nr-axSpA
For patients with stable nr-axSpA, the recommendations are strongly in favor of NSAID use, with a conditional suggestion to use on demand. The recommendations also are conditionally against combination therapy with either an NSAID or slow-acting agent plus a TNF inhibitor, with a conditional approval for TNF inhibitor monotherapy instead. The committee strongly supported physical therapy for these patients and gave a lukewarm embrace of monitoring for disease activity, CRP, or ESR.
Dr. Ward noted that the guidelines are designed to help clinicians with treatment decisions for the typical patient with AS or nr-axSpA, and do not address the needs of all populations or all clinical circumstances or contingencies.
He also noted that for many of the questions the committee members tried to address, high-quality evidence was limited.
Dr. Ward did not mention a projected publication date for the guidelines. He had no relevant financial conflicts to disclose.
VIDEO: Secukinumab rapidly effective against ankylosing spondylitis
BOSTON – Secukinumab showed enduring efficacy in ankylosing spondylitis after 52 weeks of treatment, based on data reported at the annual meeting of the American College of Rheumatology.
The monoclonal antibody, which targets interleukin-17A, is the first drug with demonstrated efficacy against ankylosing spondylitis since the introduction of tumor necrosis factor inhibitors.
In our exclusive video interview, Dr. Dominique Baeten, professor of clinical immunology and rheumatology at the Academic Medical Center of the University of Amsterdam, outlines results from the phase III trial in 371 U.S. and European patients, describes how targeting the IL-17A pathway is uniquely beneficial in AS, and discusses new data from other secukinumab trials in psoriatic arthritis patients.
Secukinumab’s maker, Novartis, sponsored the study. Dr. Baeten has received research grants from Novartis and other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – Secukinumab showed enduring efficacy in ankylosing spondylitis after 52 weeks of treatment, based on data reported at the annual meeting of the American College of Rheumatology.
The monoclonal antibody, which targets interleukin-17A, is the first drug with demonstrated efficacy against ankylosing spondylitis since the introduction of tumor necrosis factor inhibitors.
In our exclusive video interview, Dr. Dominique Baeten, professor of clinical immunology and rheumatology at the Academic Medical Center of the University of Amsterdam, outlines results from the phase III trial in 371 U.S. and European patients, describes how targeting the IL-17A pathway is uniquely beneficial in AS, and discusses new data from other secukinumab trials in psoriatic arthritis patients.
Secukinumab’s maker, Novartis, sponsored the study. Dr. Baeten has received research grants from Novartis and other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – Secukinumab showed enduring efficacy in ankylosing spondylitis after 52 weeks of treatment, based on data reported at the annual meeting of the American College of Rheumatology.
The monoclonal antibody, which targets interleukin-17A, is the first drug with demonstrated efficacy against ankylosing spondylitis since the introduction of tumor necrosis factor inhibitors.
In our exclusive video interview, Dr. Dominique Baeten, professor of clinical immunology and rheumatology at the Academic Medical Center of the University of Amsterdam, outlines results from the phase III trial in 371 U.S. and European patients, describes how targeting the IL-17A pathway is uniquely beneficial in AS, and discusses new data from other secukinumab trials in psoriatic arthritis patients.
Secukinumab’s maker, Novartis, sponsored the study. Dr. Baeten has received research grants from Novartis and other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ACR ANNUAL MEETING
Psoriasis: Brodalumab maintains efficacy through 144 weeks
AMSTERDAM – The majority of psoriasis patients placed on the investigational biologic agent brodalumab maintained a PASI 100 response throughout 144 weeks in a long-term, open-label extension of a phase II study.
“The benefit/risk ratio of brodalumab remains very favorable, and warrants continued development of this anti–interleukin-17 receptor A human monoclonal antibody as a potential treatment for psoriasis,” Dr. Kim Papp declared in presenting the study results at the annual congress of the European Academy of Dermatology and Venereology.
Large phase III clinical trials of brodalumab for the treatment of moderate to severe plaque psoriasis are ongoing.
The parent phase II study was 16 weeks long, double blinded, and placebo controlled. At the study’s end, 181 participants enrolled in the open-label extension, in which they received subcutaneous brodalumab at 210 mg every 2 weeks. The study protocol was amended after about a year, with the dose reduced to 140 mg every 2 weeks in the 119 patients weighing 100 kg or less. Six of those patients subsequently had an inadequate response to the lower dose, and were returned to the higher-dose regimen, explained Dr. Papp of Probity Medical Research in Waterloo, Ont.
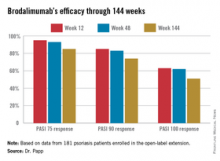
Roughly 95% of patients became PASI 75 responders within the first few weeks. There was very little drop-off over time. The PASI 75 response rate was 95% at week 12, 93% at week 48, and 85% at week 144.
At week 144, roughly two-thirds of subjects were deemed clear or almost clear according to Physician Global Assessment.
No new safety signals emerged during this long-term study. The most common adverse events were minor upper respiratory infections. Two percent of patients developed a grade 2 absolute neutrophil count of less than 1,500 per 109/L; however, these were transitory events that resolved without a change in treatment.
In response to an audience question, Dr. Papp said antidrug antibodies did develop in some patients during the course of 144 weeks of treatment, but he had no information as to the clinical effect, if any.
“This is a fairly small study population. I think it makes sense to wait for the phase III results to see if the antibodies affect safety and/or efficacy,” he added.
Dr. Papp reported receiving research support from Amgen, which is codeveloping brodalumab with AstraZeneca/MedImmune.
AMSTERDAM – The majority of psoriasis patients placed on the investigational biologic agent brodalumab maintained a PASI 100 response throughout 144 weeks in a long-term, open-label extension of a phase II study.
“The benefit/risk ratio of brodalumab remains very favorable, and warrants continued development of this anti–interleukin-17 receptor A human monoclonal antibody as a potential treatment for psoriasis,” Dr. Kim Papp declared in presenting the study results at the annual congress of the European Academy of Dermatology and Venereology.
Large phase III clinical trials of brodalumab for the treatment of moderate to severe plaque psoriasis are ongoing.
The parent phase II study was 16 weeks long, double blinded, and placebo controlled. At the study’s end, 181 participants enrolled in the open-label extension, in which they received subcutaneous brodalumab at 210 mg every 2 weeks. The study protocol was amended after about a year, with the dose reduced to 140 mg every 2 weeks in the 119 patients weighing 100 kg or less. Six of those patients subsequently had an inadequate response to the lower dose, and were returned to the higher-dose regimen, explained Dr. Papp of Probity Medical Research in Waterloo, Ont.
Roughly 95% of patients became PASI 75 responders within the first few weeks. There was very little drop-off over time. The PASI 75 response rate was 95% at week 12, 93% at week 48, and 85% at week 144.
At week 144, roughly two-thirds of subjects were deemed clear or almost clear according to Physician Global Assessment.
No new safety signals emerged during this long-term study. The most common adverse events were minor upper respiratory infections. Two percent of patients developed a grade 2 absolute neutrophil count of less than 1,500 per 109/L; however, these were transitory events that resolved without a change in treatment.
In response to an audience question, Dr. Papp said antidrug antibodies did develop in some patients during the course of 144 weeks of treatment, but he had no information as to the clinical effect, if any.
“This is a fairly small study population. I think it makes sense to wait for the phase III results to see if the antibodies affect safety and/or efficacy,” he added.
Dr. Papp reported receiving research support from Amgen, which is codeveloping brodalumab with AstraZeneca/MedImmune.
AMSTERDAM – The majority of psoriasis patients placed on the investigational biologic agent brodalumab maintained a PASI 100 response throughout 144 weeks in a long-term, open-label extension of a phase II study.
“The benefit/risk ratio of brodalumab remains very favorable, and warrants continued development of this anti–interleukin-17 receptor A human monoclonal antibody as a potential treatment for psoriasis,” Dr. Kim Papp declared in presenting the study results at the annual congress of the European Academy of Dermatology and Venereology.
Large phase III clinical trials of brodalumab for the treatment of moderate to severe plaque psoriasis are ongoing.
The parent phase II study was 16 weeks long, double blinded, and placebo controlled. At the study’s end, 181 participants enrolled in the open-label extension, in which they received subcutaneous brodalumab at 210 mg every 2 weeks. The study protocol was amended after about a year, with the dose reduced to 140 mg every 2 weeks in the 119 patients weighing 100 kg or less. Six of those patients subsequently had an inadequate response to the lower dose, and were returned to the higher-dose regimen, explained Dr. Papp of Probity Medical Research in Waterloo, Ont.
Roughly 95% of patients became PASI 75 responders within the first few weeks. There was very little drop-off over time. The PASI 75 response rate was 95% at week 12, 93% at week 48, and 85% at week 144.
At week 144, roughly two-thirds of subjects were deemed clear or almost clear according to Physician Global Assessment.
No new safety signals emerged during this long-term study. The most common adverse events were minor upper respiratory infections. Two percent of patients developed a grade 2 absolute neutrophil count of less than 1,500 per 109/L; however, these were transitory events that resolved without a change in treatment.
In response to an audience question, Dr. Papp said antidrug antibodies did develop in some patients during the course of 144 weeks of treatment, but he had no information as to the clinical effect, if any.
“This is a fairly small study population. I think it makes sense to wait for the phase III results to see if the antibodies affect safety and/or efficacy,” he added.
Dr. Papp reported receiving research support from Amgen, which is codeveloping brodalumab with AstraZeneca/MedImmune.
AT THE EADV CONGRESS
Key clinical point: The investigational interleukin-17 receptor A inhibitor brodalumab maintained strong clinical efficacy throughout 144 weeks of psoriasis treatment.
Major finding: The PASI 90 response rate to brodalumab was 85% at week 12, 83% at week 48, and 74% at week 144.
Data source: A 181-patient, prospective, open-label extension of a phase II study.
Disclosures: Dr. Papp reported receiving financial support from Amgen, which is codeveloping brodalumab with AstraZeneca/MedImmune.
VIDEO: Collaborative clinic aims at heart of CVD prevention in rheumatic diseases
BOSTON – A multidisciplinary clinic of rheumatologists and cardiologists at the Mayo Clinic in Rochester, Minn., is focused on spreading awareness of cardiovascular disease in patients with rheumatic diseases and finding the best ways to prevent it.
The Cardio-Rheumatology Clinic, which was formed in 2013, is one of a growing number of clinics around the world that have started since the first Preventive Cardio-Rheuma Clinic opened at Diakonhjemmet Hospital in Oslo. Two of the founders of the Mayo Clinic’s Cardio-Rheumatology Clinic, Dr. Sharon L. Mulvagh and Dr. Sherine Gabriel, talk about its origins, goals, and vision in a video discussion at the annual meeting of the American College of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – A multidisciplinary clinic of rheumatologists and cardiologists at the Mayo Clinic in Rochester, Minn., is focused on spreading awareness of cardiovascular disease in patients with rheumatic diseases and finding the best ways to prevent it.
The Cardio-Rheumatology Clinic, which was formed in 2013, is one of a growing number of clinics around the world that have started since the first Preventive Cardio-Rheuma Clinic opened at Diakonhjemmet Hospital in Oslo. Two of the founders of the Mayo Clinic’s Cardio-Rheumatology Clinic, Dr. Sharon L. Mulvagh and Dr. Sherine Gabriel, talk about its origins, goals, and vision in a video discussion at the annual meeting of the American College of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BOSTON – A multidisciplinary clinic of rheumatologists and cardiologists at the Mayo Clinic in Rochester, Minn., is focused on spreading awareness of cardiovascular disease in patients with rheumatic diseases and finding the best ways to prevent it.
The Cardio-Rheumatology Clinic, which was formed in 2013, is one of a growing number of clinics around the world that have started since the first Preventive Cardio-Rheuma Clinic opened at Diakonhjemmet Hospital in Oslo. Two of the founders of the Mayo Clinic’s Cardio-Rheumatology Clinic, Dr. Sharon L. Mulvagh and Dr. Sherine Gabriel, talk about its origins, goals, and vision in a video discussion at the annual meeting of the American College of Rheumatology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ACR ANNUAL MEETING
Certolizumab achieves sustained skin improvement in psoriatic arthritis
AMSTERDAM – Certolizumab pegol maintained significant improvement in dermatologic outcomes in psoriatic arthritis patients through 96 weeks of treatment in the phase III RAPID-PsA trial.
Moreover, the safety profile of this tumor necrosis factor (TNF) inhibitor was in line with findings from shorter-term studies, including the week 24 report from RAPID-PsA. Treatment-emergent adverse events were similar in type and frequency to those in placebo-treated controls, with the exception of an increased rate of minor upper respiratory tract infections. No cases of tuberculosis occurred.
“There were no new safety issues despite the increased exposure time out to 96 weeks,” Dr. Owen Davies reported at the annual congress of the European Academy of Dermatology and Venereology.
RAPID-PsA is an ongoing 216-week phase III study. It was double-blind and placebo-controlled through the first 24 weeks. The study started out with 409 psoriatic arthritis patients, half of whom had previously failed to response to one nonbiologic disease-modifying antirheumatic drug (DMARD), while the other half had been nonresponders to two or more. Of the 273 patients placed on certolizumab, 80% completed both 48 and 96 weeks of the study, explained Dr. Davies of UCB Pharma in Slough, England.
He focused on the dermatologic outcomes because the arthritis outcomes have previously been reported and served to support certolizumab’s regulatory approval for the treatment of psoriatic arthritis. The biologic is also approved for treatment of rheumatoid arthritis, ankylosing spondylitis, and Crohn’s disease. However, certolizumab’s durability of effect on the psoriatic skin manifestations of psoriatic arthritis hasn’t previously been addressed.
Briefly, at week 12 – the primary endpoint for the joint-related outcomes – 55% of patients achieved an ACR 20 response, compared with 24% on placebo. Moreover, 35% of certolizumab-treated patients had an ACR 50 response at that point, and 20% had an ACR 70 response. The ACR response rate in certolizumab-treated patients was similar regardless of whether or not they had previously been on another anti-TNF biologic.
Dr. Davies addressed in detail the dermatologic outcomes in the 166 psoriatic arthritis patients with at least 3% psoriasis body surface area involvement at baseline. They had an average 10-year disease duration, 24% body surface area involvement, and a baseline Psoriasis Area Severity Index (PASI) score of 12.0.
Dermatologic responses to certolizumab were comparable regardless of whether patients had been randomized to the biologic at 200 mg subcutaneously every 2 weeks or 400 mg once every 4 weeks. As was true for the joint-related responses to certolizumab, the skin responses were similar both in anti-TNF–naive and anti-TNF–experienced patients, he noted.
The PASI 75 response rate in this group of psoriatic arthritis patients with significant skin involvement was 61% at week 24, 65% at week 48, and 53% at week 96. The improvement was even greater in the 71 patients with more severe skin involvement as defined by a baseline PASI score of 10 or more. Certolizumab-treated patients also showed important improvements on the Physician Global Assessment and Dermatology Life Quality Index.
Certolizumab is a pegylated Fab’ fragment of a humanized TNF inhibitor monoclonal antibody.
“Certolizumab is structurally different from other currently available anti-TNF agents, which are either IgG1 monoclonal antibodies or, in the case of etanercept, a receptor fusion protein. Whether or not these structural differences will translate into clinical differences is a question being addressed in ongoing clinical trials,” Dr. Davies said.
The RAPID-PsA study is sponsored by UCB Pharma, where Dr. Davies is employed.
AMSTERDAM – Certolizumab pegol maintained significant improvement in dermatologic outcomes in psoriatic arthritis patients through 96 weeks of treatment in the phase III RAPID-PsA trial.
Moreover, the safety profile of this tumor necrosis factor (TNF) inhibitor was in line with findings from shorter-term studies, including the week 24 report from RAPID-PsA. Treatment-emergent adverse events were similar in type and frequency to those in placebo-treated controls, with the exception of an increased rate of minor upper respiratory tract infections. No cases of tuberculosis occurred.
“There were no new safety issues despite the increased exposure time out to 96 weeks,” Dr. Owen Davies reported at the annual congress of the European Academy of Dermatology and Venereology.
RAPID-PsA is an ongoing 216-week phase III study. It was double-blind and placebo-controlled through the first 24 weeks. The study started out with 409 psoriatic arthritis patients, half of whom had previously failed to response to one nonbiologic disease-modifying antirheumatic drug (DMARD), while the other half had been nonresponders to two or more. Of the 273 patients placed on certolizumab, 80% completed both 48 and 96 weeks of the study, explained Dr. Davies of UCB Pharma in Slough, England.
He focused on the dermatologic outcomes because the arthritis outcomes have previously been reported and served to support certolizumab’s regulatory approval for the treatment of psoriatic arthritis. The biologic is also approved for treatment of rheumatoid arthritis, ankylosing spondylitis, and Crohn’s disease. However, certolizumab’s durability of effect on the psoriatic skin manifestations of psoriatic arthritis hasn’t previously been addressed.
Briefly, at week 12 – the primary endpoint for the joint-related outcomes – 55% of patients achieved an ACR 20 response, compared with 24% on placebo. Moreover, 35% of certolizumab-treated patients had an ACR 50 response at that point, and 20% had an ACR 70 response. The ACR response rate in certolizumab-treated patients was similar regardless of whether or not they had previously been on another anti-TNF biologic.
Dr. Davies addressed in detail the dermatologic outcomes in the 166 psoriatic arthritis patients with at least 3% psoriasis body surface area involvement at baseline. They had an average 10-year disease duration, 24% body surface area involvement, and a baseline Psoriasis Area Severity Index (PASI) score of 12.0.
Dermatologic responses to certolizumab were comparable regardless of whether patients had been randomized to the biologic at 200 mg subcutaneously every 2 weeks or 400 mg once every 4 weeks. As was true for the joint-related responses to certolizumab, the skin responses were similar both in anti-TNF–naive and anti-TNF–experienced patients, he noted.
The PASI 75 response rate in this group of psoriatic arthritis patients with significant skin involvement was 61% at week 24, 65% at week 48, and 53% at week 96. The improvement was even greater in the 71 patients with more severe skin involvement as defined by a baseline PASI score of 10 or more. Certolizumab-treated patients also showed important improvements on the Physician Global Assessment and Dermatology Life Quality Index.
Certolizumab is a pegylated Fab’ fragment of a humanized TNF inhibitor monoclonal antibody.
“Certolizumab is structurally different from other currently available anti-TNF agents, which are either IgG1 monoclonal antibodies or, in the case of etanercept, a receptor fusion protein. Whether or not these structural differences will translate into clinical differences is a question being addressed in ongoing clinical trials,” Dr. Davies said.
The RAPID-PsA study is sponsored by UCB Pharma, where Dr. Davies is employed.
AMSTERDAM – Certolizumab pegol maintained significant improvement in dermatologic outcomes in psoriatic arthritis patients through 96 weeks of treatment in the phase III RAPID-PsA trial.
Moreover, the safety profile of this tumor necrosis factor (TNF) inhibitor was in line with findings from shorter-term studies, including the week 24 report from RAPID-PsA. Treatment-emergent adverse events were similar in type and frequency to those in placebo-treated controls, with the exception of an increased rate of minor upper respiratory tract infections. No cases of tuberculosis occurred.
“There were no new safety issues despite the increased exposure time out to 96 weeks,” Dr. Owen Davies reported at the annual congress of the European Academy of Dermatology and Venereology.
RAPID-PsA is an ongoing 216-week phase III study. It was double-blind and placebo-controlled through the first 24 weeks. The study started out with 409 psoriatic arthritis patients, half of whom had previously failed to response to one nonbiologic disease-modifying antirheumatic drug (DMARD), while the other half had been nonresponders to two or more. Of the 273 patients placed on certolizumab, 80% completed both 48 and 96 weeks of the study, explained Dr. Davies of UCB Pharma in Slough, England.
He focused on the dermatologic outcomes because the arthritis outcomes have previously been reported and served to support certolizumab’s regulatory approval for the treatment of psoriatic arthritis. The biologic is also approved for treatment of rheumatoid arthritis, ankylosing spondylitis, and Crohn’s disease. However, certolizumab’s durability of effect on the psoriatic skin manifestations of psoriatic arthritis hasn’t previously been addressed.
Briefly, at week 12 – the primary endpoint for the joint-related outcomes – 55% of patients achieved an ACR 20 response, compared with 24% on placebo. Moreover, 35% of certolizumab-treated patients had an ACR 50 response at that point, and 20% had an ACR 70 response. The ACR response rate in certolizumab-treated patients was similar regardless of whether or not they had previously been on another anti-TNF biologic.
Dr. Davies addressed in detail the dermatologic outcomes in the 166 psoriatic arthritis patients with at least 3% psoriasis body surface area involvement at baseline. They had an average 10-year disease duration, 24% body surface area involvement, and a baseline Psoriasis Area Severity Index (PASI) score of 12.0.
Dermatologic responses to certolizumab were comparable regardless of whether patients had been randomized to the biologic at 200 mg subcutaneously every 2 weeks or 400 mg once every 4 weeks. As was true for the joint-related responses to certolizumab, the skin responses were similar both in anti-TNF–naive and anti-TNF–experienced patients, he noted.
The PASI 75 response rate in this group of psoriatic arthritis patients with significant skin involvement was 61% at week 24, 65% at week 48, and 53% at week 96. The improvement was even greater in the 71 patients with more severe skin involvement as defined by a baseline PASI score of 10 or more. Certolizumab-treated patients also showed important improvements on the Physician Global Assessment and Dermatology Life Quality Index.
Certolizumab is a pegylated Fab’ fragment of a humanized TNF inhibitor monoclonal antibody.
“Certolizumab is structurally different from other currently available anti-TNF agents, which are either IgG1 monoclonal antibodies or, in the case of etanercept, a receptor fusion protein. Whether or not these structural differences will translate into clinical differences is a question being addressed in ongoing clinical trials,” Dr. Davies said.
The RAPID-PsA study is sponsored by UCB Pharma, where Dr. Davies is employed.
AT THE EADV CONGRESS
Key clinical point: Certolizumab pegol maintains sustained improvement in the dermatologic manifestations of psoriatic arthritis through 96 weeks.
Major finding: Sixty-two percent of psoriatic arthritis patients with at least 3% body surface area involvement at a baseline PASI score of 10 or more still had a PASI 75 dermatologic response after 96 weeks on certolizumab.
Data source: The RAPID-PsA study is an ongoing 216-week, prospective, randomized, multicenter trial involving 409 psoriatic arthritis patients, including 166 with significant skin involvement at baseline.
Disclosures: The study is sponsored by UCB Pharma. The presenter is a full-time company employee.
Evidence builds for importance of CVD, new drug targets at ACR
It’s difficult to highlight the most striking studies or topic areas out of the bounty of research that will be presented at the annual meeting of the American College of Rheumatology, but a few noteworthy studies caught the eye of Dr. Eric L. Matteson, the meeting’s planning subcommittee abstract selection cochair for clinical research.
Some of the studies and topic areas that deserve attention include growing knowledge about the risk of cardiovascular disease in many rheumatic conditions; the conditions in which rheumatoid arthritis (RA) treatment can be cost-effective, and de-escalation of disease-modifying antirheumatic drugs (DMARDs) may be possible; promising clinical results with new drug targets; according to Dr. Matteson, professor of medicine and chair of the department of rheumatology at the Mayo Clinic, Rochester, Minn.
Cardiovascular disease in spondyloarthritis, RA
“One major area in rheumatic diseases is looking at the emerging field of cardiovascular disease. We’ve known that there’s increased risk for heart disease in rheumatoid arthritis patients, not necessarily what to do about it. Now increasingly we’re finding that there’s the same risk in some other inflammatory arthritis diseases,” Dr. Matteson said. A Canadian study to be presented on Tuesday (abstract 2829) is the first population-based cohort study to show that patients with spondyloarthritis are at increased risk for heart disease, although at 40%-50% increased risk of cardiovascular death in comparison to people without spondyloarthritis, it is not at the same magnitude of risk as what is observed for RA, where risk for CV death is doubled in some subgroups, such as women with rheumatoid factor-positive disease.
“I think that’s something that’s new and is significant,” Dr. Matteson said.
Another interesting study to be reported on Sunday provides some initial evidence to suggest that reducing disease activity in RA might reduce the risk of heart failure, which has been shown to be elevated in RA patients. The prospective, cross-sectional study (abstract 945) of patients from a clinic in Germany found progressively lower prevalence of heart failure as 28-joint Disease Activity Score declined, independent of treatment type.
De-escalating DMARDs in RA
Another “hot-button” issue in RA is whether it is possible to reduce therapy, Dr. Matteson noted. On Sunday, German investigators will present a multicenter, randomized controlled trial (abstract 940) showing that while DMARDs can be successfully de-escalated, it’s not clear whether starting the de-escalation process is better done first with a conventional DMARD or a biologic. The presence of anticitrullinated peptide antibodies turned out to be the only predictor of recurrent disease. Another study in the same session (abstract 941) indicated that an attempt to de-escalate biologic treatment was successful in 87% of the selected patients who tried to do so and provided substantial cost savings.
New drug targets in clinical research
One of the unique therapeutic approaches being tried is using autologous dendritic cell immunotherapy to treat RA patients, Dr. Matteson said. “The dendritic cell is an important immune-mediating cell actually not just in rheumatoid arthritis but a lot of other systemic inflammatory conditions including even giant cell arteritis.” A phase I, open-label study (abstract 946) that will be presented on Sunday demonstrated that five vaccinations with high or low doses of the cells over 2- to 4-week intervals in 12 RA patients yielded good to moderate European League Against Rheumatism (EULAR) responses in 7 patients while 12 adverse events occurred in 8 patients.
After not-so-encouraging studies of interleukin-17 as a drug target in RA, its potential in seronegative inflammatory arthritis appears much more promising. The results of a 1-year, phase III trial of secukinumab, a monoclonal antibody to IL-17A, in patients with psoriatic arthritis is “quite hopeful” because there were improvements in clinical parameters of disease and an indication of inhibition of joint erosion (abstract 954). Another phase III study of the drug showed “quite promising effects” in controlling ankylosing spondylitis over the course of 1 year (abstract 819). Both studies will be presented on Sunday.
Noteworthy plenary presentations
“One of the hot areas in lung disease in rheumatic diseases” involves controversy over the value of lung transplantation in patients with systemic sclerosis (SSc) and end-stage lung disease, Dr. Matteson said. A study to be presented in Monday’s plenary session suggests that new methods must be developed to determine which SSc patients can successfully undergo lung transplantation rather than to deny all patients with the condition the chance to undergo the procedure. While the study (abstract 1797) confirmed that patients with SSc have a nearly 50% increased risk of death at 1 year posttransplant when compared with interstitial lung disease patients without SSc, there was no difference in mortality for SSc patients, compared with patients with pulmonary arterial hypertension not caused by SSc.
In Tuesday’s plenary session, investigators will report on the cost-effectiveness of triple therapy, compared with biologics. The results of the study (abstract 2781) suggest that added costs associated with using etanercept prior to a triple-therapy regimen may not be ideal in RA patients for whom methotrexate is not working. Because etanercept has only a small probability of being cost-effective, compared with triple therapy, more health care dollars could be freed up, the authors argue, by adopting the triple regimen prior to etanercept.
Dr. Matteson has received research support from many companies marketing drugs for rheumatic diseases.
It’s difficult to highlight the most striking studies or topic areas out of the bounty of research that will be presented at the annual meeting of the American College of Rheumatology, but a few noteworthy studies caught the eye of Dr. Eric L. Matteson, the meeting’s planning subcommittee abstract selection cochair for clinical research.
Some of the studies and topic areas that deserve attention include growing knowledge about the risk of cardiovascular disease in many rheumatic conditions; the conditions in which rheumatoid arthritis (RA) treatment can be cost-effective, and de-escalation of disease-modifying antirheumatic drugs (DMARDs) may be possible; promising clinical results with new drug targets; according to Dr. Matteson, professor of medicine and chair of the department of rheumatology at the Mayo Clinic, Rochester, Minn.
Cardiovascular disease in spondyloarthritis, RA
“One major area in rheumatic diseases is looking at the emerging field of cardiovascular disease. We’ve known that there’s increased risk for heart disease in rheumatoid arthritis patients, not necessarily what to do about it. Now increasingly we’re finding that there’s the same risk in some other inflammatory arthritis diseases,” Dr. Matteson said. A Canadian study to be presented on Tuesday (abstract 2829) is the first population-based cohort study to show that patients with spondyloarthritis are at increased risk for heart disease, although at 40%-50% increased risk of cardiovascular death in comparison to people without spondyloarthritis, it is not at the same magnitude of risk as what is observed for RA, where risk for CV death is doubled in some subgroups, such as women with rheumatoid factor-positive disease.
“I think that’s something that’s new and is significant,” Dr. Matteson said.
Another interesting study to be reported on Sunday provides some initial evidence to suggest that reducing disease activity in RA might reduce the risk of heart failure, which has been shown to be elevated in RA patients. The prospective, cross-sectional study (abstract 945) of patients from a clinic in Germany found progressively lower prevalence of heart failure as 28-joint Disease Activity Score declined, independent of treatment type.
De-escalating DMARDs in RA
Another “hot-button” issue in RA is whether it is possible to reduce therapy, Dr. Matteson noted. On Sunday, German investigators will present a multicenter, randomized controlled trial (abstract 940) showing that while DMARDs can be successfully de-escalated, it’s not clear whether starting the de-escalation process is better done first with a conventional DMARD or a biologic. The presence of anticitrullinated peptide antibodies turned out to be the only predictor of recurrent disease. Another study in the same session (abstract 941) indicated that an attempt to de-escalate biologic treatment was successful in 87% of the selected patients who tried to do so and provided substantial cost savings.
New drug targets in clinical research
One of the unique therapeutic approaches being tried is using autologous dendritic cell immunotherapy to treat RA patients, Dr. Matteson said. “The dendritic cell is an important immune-mediating cell actually not just in rheumatoid arthritis but a lot of other systemic inflammatory conditions including even giant cell arteritis.” A phase I, open-label study (abstract 946) that will be presented on Sunday demonstrated that five vaccinations with high or low doses of the cells over 2- to 4-week intervals in 12 RA patients yielded good to moderate European League Against Rheumatism (EULAR) responses in 7 patients while 12 adverse events occurred in 8 patients.
After not-so-encouraging studies of interleukin-17 as a drug target in RA, its potential in seronegative inflammatory arthritis appears much more promising. The results of a 1-year, phase III trial of secukinumab, a monoclonal antibody to IL-17A, in patients with psoriatic arthritis is “quite hopeful” because there were improvements in clinical parameters of disease and an indication of inhibition of joint erosion (abstract 954). Another phase III study of the drug showed “quite promising effects” in controlling ankylosing spondylitis over the course of 1 year (abstract 819). Both studies will be presented on Sunday.
Noteworthy plenary presentations
“One of the hot areas in lung disease in rheumatic diseases” involves controversy over the value of lung transplantation in patients with systemic sclerosis (SSc) and end-stage lung disease, Dr. Matteson said. A study to be presented in Monday’s plenary session suggests that new methods must be developed to determine which SSc patients can successfully undergo lung transplantation rather than to deny all patients with the condition the chance to undergo the procedure. While the study (abstract 1797) confirmed that patients with SSc have a nearly 50% increased risk of death at 1 year posttransplant when compared with interstitial lung disease patients without SSc, there was no difference in mortality for SSc patients, compared with patients with pulmonary arterial hypertension not caused by SSc.
In Tuesday’s plenary session, investigators will report on the cost-effectiveness of triple therapy, compared with biologics. The results of the study (abstract 2781) suggest that added costs associated with using etanercept prior to a triple-therapy regimen may not be ideal in RA patients for whom methotrexate is not working. Because etanercept has only a small probability of being cost-effective, compared with triple therapy, more health care dollars could be freed up, the authors argue, by adopting the triple regimen prior to etanercept.
Dr. Matteson has received research support from many companies marketing drugs for rheumatic diseases.
It’s difficult to highlight the most striking studies or topic areas out of the bounty of research that will be presented at the annual meeting of the American College of Rheumatology, but a few noteworthy studies caught the eye of Dr. Eric L. Matteson, the meeting’s planning subcommittee abstract selection cochair for clinical research.
Some of the studies and topic areas that deserve attention include growing knowledge about the risk of cardiovascular disease in many rheumatic conditions; the conditions in which rheumatoid arthritis (RA) treatment can be cost-effective, and de-escalation of disease-modifying antirheumatic drugs (DMARDs) may be possible; promising clinical results with new drug targets; according to Dr. Matteson, professor of medicine and chair of the department of rheumatology at the Mayo Clinic, Rochester, Minn.
Cardiovascular disease in spondyloarthritis, RA
“One major area in rheumatic diseases is looking at the emerging field of cardiovascular disease. We’ve known that there’s increased risk for heart disease in rheumatoid arthritis patients, not necessarily what to do about it. Now increasingly we’re finding that there’s the same risk in some other inflammatory arthritis diseases,” Dr. Matteson said. A Canadian study to be presented on Tuesday (abstract 2829) is the first population-based cohort study to show that patients with spondyloarthritis are at increased risk for heart disease, although at 40%-50% increased risk of cardiovascular death in comparison to people without spondyloarthritis, it is not at the same magnitude of risk as what is observed for RA, where risk for CV death is doubled in some subgroups, such as women with rheumatoid factor-positive disease.
“I think that’s something that’s new and is significant,” Dr. Matteson said.
Another interesting study to be reported on Sunday provides some initial evidence to suggest that reducing disease activity in RA might reduce the risk of heart failure, which has been shown to be elevated in RA patients. The prospective, cross-sectional study (abstract 945) of patients from a clinic in Germany found progressively lower prevalence of heart failure as 28-joint Disease Activity Score declined, independent of treatment type.
De-escalating DMARDs in RA
Another “hot-button” issue in RA is whether it is possible to reduce therapy, Dr. Matteson noted. On Sunday, German investigators will present a multicenter, randomized controlled trial (abstract 940) showing that while DMARDs can be successfully de-escalated, it’s not clear whether starting the de-escalation process is better done first with a conventional DMARD or a biologic. The presence of anticitrullinated peptide antibodies turned out to be the only predictor of recurrent disease. Another study in the same session (abstract 941) indicated that an attempt to de-escalate biologic treatment was successful in 87% of the selected patients who tried to do so and provided substantial cost savings.
New drug targets in clinical research
One of the unique therapeutic approaches being tried is using autologous dendritic cell immunotherapy to treat RA patients, Dr. Matteson said. “The dendritic cell is an important immune-mediating cell actually not just in rheumatoid arthritis but a lot of other systemic inflammatory conditions including even giant cell arteritis.” A phase I, open-label study (abstract 946) that will be presented on Sunday demonstrated that five vaccinations with high or low doses of the cells over 2- to 4-week intervals in 12 RA patients yielded good to moderate European League Against Rheumatism (EULAR) responses in 7 patients while 12 adverse events occurred in 8 patients.
After not-so-encouraging studies of interleukin-17 as a drug target in RA, its potential in seronegative inflammatory arthritis appears much more promising. The results of a 1-year, phase III trial of secukinumab, a monoclonal antibody to IL-17A, in patients with psoriatic arthritis is “quite hopeful” because there were improvements in clinical parameters of disease and an indication of inhibition of joint erosion (abstract 954). Another phase III study of the drug showed “quite promising effects” in controlling ankylosing spondylitis over the course of 1 year (abstract 819). Both studies will be presented on Sunday.
Noteworthy plenary presentations
“One of the hot areas in lung disease in rheumatic diseases” involves controversy over the value of lung transplantation in patients with systemic sclerosis (SSc) and end-stage lung disease, Dr. Matteson said. A study to be presented in Monday’s plenary session suggests that new methods must be developed to determine which SSc patients can successfully undergo lung transplantation rather than to deny all patients with the condition the chance to undergo the procedure. While the study (abstract 1797) confirmed that patients with SSc have a nearly 50% increased risk of death at 1 year posttransplant when compared with interstitial lung disease patients without SSc, there was no difference in mortality for SSc patients, compared with patients with pulmonary arterial hypertension not caused by SSc.
In Tuesday’s plenary session, investigators will report on the cost-effectiveness of triple therapy, compared with biologics. The results of the study (abstract 2781) suggest that added costs associated with using etanercept prior to a triple-therapy regimen may not be ideal in RA patients for whom methotrexate is not working. Because etanercept has only a small probability of being cost-effective, compared with triple therapy, more health care dollars could be freed up, the authors argue, by adopting the triple regimen prior to etanercept.
Dr. Matteson has received research support from many companies marketing drugs for rheumatic diseases.
Veterans more likely to have arthritis at any age
Both male and female veterans were more likely to have arthritis than were nonveterans, according to a study from the Centers for Disease Control and Prevention.
For male veterans, the age-standardized arthritis rate for 2011-2013 was 25%, while for male nonveterans, the rate was 19.5%. Both female veterans and nonveterans had noticeably higher arthritis incidence than did the respective male group: 31.3% for veterans and 26.1% for nonveterans, the CDC found (MMWR 2014;63:999-1003).
Although arthritis rates were higher overall in middle-aged and older people, arthritis rates were consistently higher in younger veterans aged 18-44 years – 11.6% for males and 17.3% for females – compared with 6.9% in male nonveterans and 9.8% in female nonveterans. This suggests “that arthritis and its effects need to be addressed among male and female veterans of all ages,” the CDC researchers said.
Traumatic and overuse injuries were found to be common among active-duty military personnel in another study, the investigators noted, while pointing out that musculoskeletal injuries are a major risk factor for osteoarthritis, which “represents the largest portion of arthritis cases” among veterans.
The study used data collected by the Behavioral Risk Factor Surveillance System.
Both male and female veterans were more likely to have arthritis than were nonveterans, according to a study from the Centers for Disease Control and Prevention.
For male veterans, the age-standardized arthritis rate for 2011-2013 was 25%, while for male nonveterans, the rate was 19.5%. Both female veterans and nonveterans had noticeably higher arthritis incidence than did the respective male group: 31.3% for veterans and 26.1% for nonveterans, the CDC found (MMWR 2014;63:999-1003).
Although arthritis rates were higher overall in middle-aged and older people, arthritis rates were consistently higher in younger veterans aged 18-44 years – 11.6% for males and 17.3% for females – compared with 6.9% in male nonveterans and 9.8% in female nonveterans. This suggests “that arthritis and its effects need to be addressed among male and female veterans of all ages,” the CDC researchers said.
Traumatic and overuse injuries were found to be common among active-duty military personnel in another study, the investigators noted, while pointing out that musculoskeletal injuries are a major risk factor for osteoarthritis, which “represents the largest portion of arthritis cases” among veterans.
The study used data collected by the Behavioral Risk Factor Surveillance System.

Both male and female veterans were more likely to have arthritis than were nonveterans, according to a study from the Centers for Disease Control and Prevention.
For male veterans, the age-standardized arthritis rate for 2011-2013 was 25%, while for male nonveterans, the rate was 19.5%. Both female veterans and nonveterans had noticeably higher arthritis incidence than did the respective male group: 31.3% for veterans and 26.1% for nonveterans, the CDC found (MMWR 2014;63:999-1003).
Although arthritis rates were higher overall in middle-aged and older people, arthritis rates were consistently higher in younger veterans aged 18-44 years – 11.6% for males and 17.3% for females – compared with 6.9% in male nonveterans and 9.8% in female nonveterans. This suggests “that arthritis and its effects need to be addressed among male and female veterans of all ages,” the CDC researchers said.
Traumatic and overuse injuries were found to be common among active-duty military personnel in another study, the investigators noted, while pointing out that musculoskeletal injuries are a major risk factor for osteoarthritis, which “represents the largest portion of arthritis cases” among veterans.
The study used data collected by the Behavioral Risk Factor Surveillance System.

FROM MORBIDITY AND MORTALITY WEEKLY REPORT