User login
Oral curcumin shown effective in psoriasis
AMSTERDAM – Oral curcumin proved safe and effective as adjunctive therapy in patients on topical corticosteroids for mild to moderate psoriasis vulgaris in a 12-week, randomized, placebo-controlled clinical trial.
This agent helps fill an unmet need in psoriasis, Dr. Emiliano Antiga observed in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
That’s because by far most of the action in the development of new treatments for psoriasis focuses on biologics and other extremely costly agents targeting patients at the moderate to severe end of the disease spectrum. But psoriasis is a chronic condition, and the many patients with milder disease require long-term therapies that are nontoxic and won’t break the bank. Enter curcumin.
“Oral curcumin is effective, safe, and it is cheap,” declared Dr. Antiga, a dermatologist at the University of Florence (Italy).
Moreover, it has a biologically plausible mechanism of benefit in psoriasis, as was demonstrated in his 60-patient randomized trial. Serum levels of the proinflammatory cytokine interleukin-22 were cut in half in the group assigned to 12 weeks of daily oral curcumin while remaining unchanged in the control group.
Curcumin is derived from turmeric, the dried rhizome of a plant, Curcuma longa. Turmeric is a yellowish Indian spice used in curries. But curcumin has long been used therapeutically in traditional Indian and Chinese medicine. Studies have shown that curcumin has antiproliferative, antiangiogenic, and anti-inflammatory effects. In a small study by other investigators, topical turmeric not only successfully cleared psoriasis lesions, it also suppressed phosphorylase kinase activity, which is important to keratinocyte proliferation (Br. J. Dermatol. 2000;143:937-49).
Dr. Antiga presented a study of 60 patients with mild to moderate psoriasis vulgaris as defined by a baseline median Psoriasis Area and Severity Index (PASI) score of 5.5 who were randomly assigned to 12 weeks of treatment with topical corticosteroids plus 3 g per day of oral curcumin or topical steroids plus placebo. The active-treatment capsules contained curcumin embedded in nanoparticle liposomes to enhance bioavailability.
Forty-nine patients completed the study. The primary endpoint was reduction in PASI values over 12 weeks. Both groups showed improvement – after all, the controls were on active treatment with topical steroids – but the change in PASI scores was significantly greater in the curcumin-treated patients.
Adverse events in the curcumin group were limited to one case of diarrhea. There was one case of nausea and one papular eruption in the control group.
Although IL-22 levels at 12 weeks were halved in the curcumin group and unchanged in controls, levels of the inflammatory cytokines IL-10 and -17 and transforming growth factor–beta remained unchanged in both groups over time.
Dr. Antiga reported having no financial conflicts regarding this study.
AMSTERDAM – Oral curcumin proved safe and effective as adjunctive therapy in patients on topical corticosteroids for mild to moderate psoriasis vulgaris in a 12-week, randomized, placebo-controlled clinical trial.
This agent helps fill an unmet need in psoriasis, Dr. Emiliano Antiga observed in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
That’s because by far most of the action in the development of new treatments for psoriasis focuses on biologics and other extremely costly agents targeting patients at the moderate to severe end of the disease spectrum. But psoriasis is a chronic condition, and the many patients with milder disease require long-term therapies that are nontoxic and won’t break the bank. Enter curcumin.
“Oral curcumin is effective, safe, and it is cheap,” declared Dr. Antiga, a dermatologist at the University of Florence (Italy).
Moreover, it has a biologically plausible mechanism of benefit in psoriasis, as was demonstrated in his 60-patient randomized trial. Serum levels of the proinflammatory cytokine interleukin-22 were cut in half in the group assigned to 12 weeks of daily oral curcumin while remaining unchanged in the control group.
Curcumin is derived from turmeric, the dried rhizome of a plant, Curcuma longa. Turmeric is a yellowish Indian spice used in curries. But curcumin has long been used therapeutically in traditional Indian and Chinese medicine. Studies have shown that curcumin has antiproliferative, antiangiogenic, and anti-inflammatory effects. In a small study by other investigators, topical turmeric not only successfully cleared psoriasis lesions, it also suppressed phosphorylase kinase activity, which is important to keratinocyte proliferation (Br. J. Dermatol. 2000;143:937-49).
Dr. Antiga presented a study of 60 patients with mild to moderate psoriasis vulgaris as defined by a baseline median Psoriasis Area and Severity Index (PASI) score of 5.5 who were randomly assigned to 12 weeks of treatment with topical corticosteroids plus 3 g per day of oral curcumin or topical steroids plus placebo. The active-treatment capsules contained curcumin embedded in nanoparticle liposomes to enhance bioavailability.
Forty-nine patients completed the study. The primary endpoint was reduction in PASI values over 12 weeks. Both groups showed improvement – after all, the controls were on active treatment with topical steroids – but the change in PASI scores was significantly greater in the curcumin-treated patients.
Adverse events in the curcumin group were limited to one case of diarrhea. There was one case of nausea and one papular eruption in the control group.
Although IL-22 levels at 12 weeks were halved in the curcumin group and unchanged in controls, levels of the inflammatory cytokines IL-10 and -17 and transforming growth factor–beta remained unchanged in both groups over time.
Dr. Antiga reported having no financial conflicts regarding this study.
AMSTERDAM – Oral curcumin proved safe and effective as adjunctive therapy in patients on topical corticosteroids for mild to moderate psoriasis vulgaris in a 12-week, randomized, placebo-controlled clinical trial.
This agent helps fill an unmet need in psoriasis, Dr. Emiliano Antiga observed in presenting the study findings at the annual congress of the European Academy of Dermatology and Venereology.
That’s because by far most of the action in the development of new treatments for psoriasis focuses on biologics and other extremely costly agents targeting patients at the moderate to severe end of the disease spectrum. But psoriasis is a chronic condition, and the many patients with milder disease require long-term therapies that are nontoxic and won’t break the bank. Enter curcumin.
“Oral curcumin is effective, safe, and it is cheap,” declared Dr. Antiga, a dermatologist at the University of Florence (Italy).
Moreover, it has a biologically plausible mechanism of benefit in psoriasis, as was demonstrated in his 60-patient randomized trial. Serum levels of the proinflammatory cytokine interleukin-22 were cut in half in the group assigned to 12 weeks of daily oral curcumin while remaining unchanged in the control group.
Curcumin is derived from turmeric, the dried rhizome of a plant, Curcuma longa. Turmeric is a yellowish Indian spice used in curries. But curcumin has long been used therapeutically in traditional Indian and Chinese medicine. Studies have shown that curcumin has antiproliferative, antiangiogenic, and anti-inflammatory effects. In a small study by other investigators, topical turmeric not only successfully cleared psoriasis lesions, it also suppressed phosphorylase kinase activity, which is important to keratinocyte proliferation (Br. J. Dermatol. 2000;143:937-49).
Dr. Antiga presented a study of 60 patients with mild to moderate psoriasis vulgaris as defined by a baseline median Psoriasis Area and Severity Index (PASI) score of 5.5 who were randomly assigned to 12 weeks of treatment with topical corticosteroids plus 3 g per day of oral curcumin or topical steroids plus placebo. The active-treatment capsules contained curcumin embedded in nanoparticle liposomes to enhance bioavailability.
Forty-nine patients completed the study. The primary endpoint was reduction in PASI values over 12 weeks. Both groups showed improvement – after all, the controls were on active treatment with topical steroids – but the change in PASI scores was significantly greater in the curcumin-treated patients.
Adverse events in the curcumin group were limited to one case of diarrhea. There was one case of nausea and one papular eruption in the control group.
Although IL-22 levels at 12 weeks were halved in the curcumin group and unchanged in controls, levels of the inflammatory cytokines IL-10 and -17 and transforming growth factor–beta remained unchanged in both groups over time.
Dr. Antiga reported having no financial conflicts regarding this study.
AT THE EADV CONGRESS
Key clinical point: A common ingredient in Indian curry spice mixes is safe and effective as adjuvant therapy for mild-to-moderate psoriasis.
Major finding: Daily oral curcumin capsules plus topical corticosteroids resulted in a PASI 75 improvement rate of 48% compared with a 12% rate in patients who got topical steroids plus placebo.
Data source: A prospective randomized 12-week clinical trial involving 60 patients with mild to moderate psoriasis vulgaris.
Disclosures: The presenter reported no financial conflicts regarding this study, conducted free of commercial support.
Chronic inflammatory disease patients at greater risk of major CV events
Patients with rheumatoid arthritis, psoriatic arthritis, or psoriasis are at an increased risk of major adverse cardiovascular events when compared with the general population, according to findings from a large cohort study.
All three diseases had statistically similar risks for major adverse cardiovascular events (MACE) after adjustment for age, gender, and traditional CV risk factors, Dr. Alexis Ogdie-Beatty of the University of Pennsylvania, Philadelphia, and her colleagues reported (Ann. Rheum. Dis. 2014 Oct. 30 [doi: 10.1136/annrheumdis-2014-205675]).
The investigators noted that most studies of CV risk in psoriatic arthritis (PsA) patients have been cross-sectional and that three previous population-based cohort studies have evaluated CV risk in psoriasis patients, with PsA patients as a subgroup; however, these three psoriasis studies did not include incident MACE and matched internal control patients with adjustments for traditional CV risk factors.
The investigators used data from the Health Improvement Network, a U.K. primary care medical record database, and compared the number of MACE (myocardial infarction, cerebrovascular accident, and CV death) that occurred during a mean 5 years of follow-up in 41,752 patients with rheumatoid arthritis (RA), 8,706 with PsA, 138,424 with psoriasis, and 81,573 matched controls. There was significant interaction between disease-modifying antirheumatic drug (DMARD) use and disease group (P < .001 for MACE and two components, CV death and cerebrovascular accident; and P = .01 for MI).
The risk of MACE was higher in patients with PsA not prescribed a DMARD (hazard ratio, 1.24; 95% confidence interval, 1.03-1.49). This risk was elevated in RA patients both with DMARD prescriptions (HR, 1.58; 95% CI, 1.46-1.70) and without (HR, 1.39; 95% CI, 1.28-1.50). Patients with severe psoriasis who were prescribed a DMARD had an HR of 1.42 (95% CI, 1.17-1.73), whereas psoriasis patients not prescribed a DMARD had an HR of 1.08 (95% CI, 1.02-1.15).
The results highlight a need for improved screening and management of traditional CV risk factors in patients with inflammatory diseases, the researchers said.
Study limitations included not being able to measure disease severity or the use of over-the-counter NSAIDs, as well as having few records on biologic medications and possibly missing DMARD prescriptions.
The researchers were supported by various grants from the Rheumatology Research Foundation, the National Institutes of Health, the Doris Duke Charitable Foundation, and the Icelandic Research Fund. Several authors reported financial relationships with companies that market drugs for chronic inflammatory diseases.
Patients with rheumatoid arthritis, psoriatic arthritis, or psoriasis are at an increased risk of major adverse cardiovascular events when compared with the general population, according to findings from a large cohort study.
All three diseases had statistically similar risks for major adverse cardiovascular events (MACE) after adjustment for age, gender, and traditional CV risk factors, Dr. Alexis Ogdie-Beatty of the University of Pennsylvania, Philadelphia, and her colleagues reported (Ann. Rheum. Dis. 2014 Oct. 30 [doi: 10.1136/annrheumdis-2014-205675]).
The investigators noted that most studies of CV risk in psoriatic arthritis (PsA) patients have been cross-sectional and that three previous population-based cohort studies have evaluated CV risk in psoriasis patients, with PsA patients as a subgroup; however, these three psoriasis studies did not include incident MACE and matched internal control patients with adjustments for traditional CV risk factors.
The investigators used data from the Health Improvement Network, a U.K. primary care medical record database, and compared the number of MACE (myocardial infarction, cerebrovascular accident, and CV death) that occurred during a mean 5 years of follow-up in 41,752 patients with rheumatoid arthritis (RA), 8,706 with PsA, 138,424 with psoriasis, and 81,573 matched controls. There was significant interaction between disease-modifying antirheumatic drug (DMARD) use and disease group (P < .001 for MACE and two components, CV death and cerebrovascular accident; and P = .01 for MI).
The risk of MACE was higher in patients with PsA not prescribed a DMARD (hazard ratio, 1.24; 95% confidence interval, 1.03-1.49). This risk was elevated in RA patients both with DMARD prescriptions (HR, 1.58; 95% CI, 1.46-1.70) and without (HR, 1.39; 95% CI, 1.28-1.50). Patients with severe psoriasis who were prescribed a DMARD had an HR of 1.42 (95% CI, 1.17-1.73), whereas psoriasis patients not prescribed a DMARD had an HR of 1.08 (95% CI, 1.02-1.15).
The results highlight a need for improved screening and management of traditional CV risk factors in patients with inflammatory diseases, the researchers said.
Study limitations included not being able to measure disease severity or the use of over-the-counter NSAIDs, as well as having few records on biologic medications and possibly missing DMARD prescriptions.
The researchers were supported by various grants from the Rheumatology Research Foundation, the National Institutes of Health, the Doris Duke Charitable Foundation, and the Icelandic Research Fund. Several authors reported financial relationships with companies that market drugs for chronic inflammatory diseases.
Patients with rheumatoid arthritis, psoriatic arthritis, or psoriasis are at an increased risk of major adverse cardiovascular events when compared with the general population, according to findings from a large cohort study.
All three diseases had statistically similar risks for major adverse cardiovascular events (MACE) after adjustment for age, gender, and traditional CV risk factors, Dr. Alexis Ogdie-Beatty of the University of Pennsylvania, Philadelphia, and her colleagues reported (Ann. Rheum. Dis. 2014 Oct. 30 [doi: 10.1136/annrheumdis-2014-205675]).
The investigators noted that most studies of CV risk in psoriatic arthritis (PsA) patients have been cross-sectional and that three previous population-based cohort studies have evaluated CV risk in psoriasis patients, with PsA patients as a subgroup; however, these three psoriasis studies did not include incident MACE and matched internal control patients with adjustments for traditional CV risk factors.
The investigators used data from the Health Improvement Network, a U.K. primary care medical record database, and compared the number of MACE (myocardial infarction, cerebrovascular accident, and CV death) that occurred during a mean 5 years of follow-up in 41,752 patients with rheumatoid arthritis (RA), 8,706 with PsA, 138,424 with psoriasis, and 81,573 matched controls. There was significant interaction between disease-modifying antirheumatic drug (DMARD) use and disease group (P < .001 for MACE and two components, CV death and cerebrovascular accident; and P = .01 for MI).
The risk of MACE was higher in patients with PsA not prescribed a DMARD (hazard ratio, 1.24; 95% confidence interval, 1.03-1.49). This risk was elevated in RA patients both with DMARD prescriptions (HR, 1.58; 95% CI, 1.46-1.70) and without (HR, 1.39; 95% CI, 1.28-1.50). Patients with severe psoriasis who were prescribed a DMARD had an HR of 1.42 (95% CI, 1.17-1.73), whereas psoriasis patients not prescribed a DMARD had an HR of 1.08 (95% CI, 1.02-1.15).
The results highlight a need for improved screening and management of traditional CV risk factors in patients with inflammatory diseases, the researchers said.
Study limitations included not being able to measure disease severity or the use of over-the-counter NSAIDs, as well as having few records on biologic medications and possibly missing DMARD prescriptions.
The researchers were supported by various grants from the Rheumatology Research Foundation, the National Institutes of Health, the Doris Duke Charitable Foundation, and the Icelandic Research Fund. Several authors reported financial relationships with companies that market drugs for chronic inflammatory diseases.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Patients with RA, PsA, and psoriasis have similarly elevated risk for major adverse cardiovascular events when compared with the general population.
Major finding: The risk of MACE was higher in patients with PsA not prescribed a DMARD (hazard ratio, 1.24; 95% confidence interval, 1.03-1.49), compared with matched controls.
Data source: A population-based, longitudinal cohort study of 41,752 RA patients, 8,706 PsA patients, 138,424 psoriasis patients, and 81,573 matched controls.
Disclosures: The researchers were supported by various grants from the Rheumatology Research Foundation, the National Institutes of Health, the Doris Duke Charitable Foundation, and the Icelandic Research Fund. Several authors reported financial relationships with companies that market drugs for chronic inflammatory diseases.
Current use of COX-2 inhibitors linked to increased mortality after ischemic stroke
Current use of cyclooxygenase-2 inhibitors was associated with an increase in 30-day mortality after ischemic stroke in a population-based cohort study published Nov. 5 in Neurology.
Since the association between COX-2 inhibitors and ischemic stroke mortality was associated only with current use and not former use, the researchers, led by Dr. Morten Schmidt of Aarhus (Denmark) University Hospital, believe that alternative treatment options – such as nonselective NSAIDs, which did not have an impact on overall mortality after ischemic stroke – may be more suitable for treating potential ischemic stroke patients, such as those with atrial fibrillation and a high CHA2DS2-VASc score.
If the association is truly causal, it constitutes a strong argument for increasing the efforts to ensure that patients with a high predicted risk of arterial thromboembolism are not prescribed COX-2 inhibitors when alternative treatment options are available,” Dr. Schmidt and his associates wrote.
In order to determine whether COX-2 inhibitors influenced 30-day mortality at the time of hospitalization for stroke, the researchers examined records of 100,243 people hospitalized for a first-time stroke in Denmark during 2004-2012 and deaths within 1 month after the stroke (Neurology 2014 Nov. 5 [doi:10.1212/WNL.0000000000001024]).
The hazard ratio for ischemic stroke was 1.19 (95% confidence interval, 1.02-1.38) for current users of COX-2 inhibitors, while current users of nonselective NSAIDs had an HR of 1.00 (95% CI, 0.87-1.15), compared with nonusers.
The COX-2 inhibitors in the study included diclofenac, etodolac, nabumeton, and meloxicam, as well as coxibs including celecoxib and rofecoxib. The nonselective NSAIDs in the study were ibuprofen, naproxen, ketoprofen, dexibuprofen, piroxicam, tolfenamic acid, and indomethacin.
Though the researchers acknowledged more studies are needed to truly examine the effects of COX-2 inhibitors on stroke mortality, they hypothesized that the increased mortality rate may be caused by COX-2 inhibition interfering with the pathophysiologic response to a stroke, or unwanted effects from the thromboembolic properties of COX-2 inhibitors.
“Our study adds to the increasing body of evidence concerning the vascular risk and prognostic impact associated with use of COX-2 inhibitors,” Dr. Schmidt and his associates wrote.
The study was funded by several Danish research foundations and the Program for Clinical Research Infrastructure, which was established by the Lundbeck Foundation and the Novo Nordisk Foundation. The authors reported no relevant disclosures.
Current use of cyclooxygenase-2 inhibitors was associated with an increase in 30-day mortality after ischemic stroke in a population-based cohort study published Nov. 5 in Neurology.
Since the association between COX-2 inhibitors and ischemic stroke mortality was associated only with current use and not former use, the researchers, led by Dr. Morten Schmidt of Aarhus (Denmark) University Hospital, believe that alternative treatment options – such as nonselective NSAIDs, which did not have an impact on overall mortality after ischemic stroke – may be more suitable for treating potential ischemic stroke patients, such as those with atrial fibrillation and a high CHA2DS2-VASc score.
If the association is truly causal, it constitutes a strong argument for increasing the efforts to ensure that patients with a high predicted risk of arterial thromboembolism are not prescribed COX-2 inhibitors when alternative treatment options are available,” Dr. Schmidt and his associates wrote.
In order to determine whether COX-2 inhibitors influenced 30-day mortality at the time of hospitalization for stroke, the researchers examined records of 100,243 people hospitalized for a first-time stroke in Denmark during 2004-2012 and deaths within 1 month after the stroke (Neurology 2014 Nov. 5 [doi:10.1212/WNL.0000000000001024]).
The hazard ratio for ischemic stroke was 1.19 (95% confidence interval, 1.02-1.38) for current users of COX-2 inhibitors, while current users of nonselective NSAIDs had an HR of 1.00 (95% CI, 0.87-1.15), compared with nonusers.
The COX-2 inhibitors in the study included diclofenac, etodolac, nabumeton, and meloxicam, as well as coxibs including celecoxib and rofecoxib. The nonselective NSAIDs in the study were ibuprofen, naproxen, ketoprofen, dexibuprofen, piroxicam, tolfenamic acid, and indomethacin.
Though the researchers acknowledged more studies are needed to truly examine the effects of COX-2 inhibitors on stroke mortality, they hypothesized that the increased mortality rate may be caused by COX-2 inhibition interfering with the pathophysiologic response to a stroke, or unwanted effects from the thromboembolic properties of COX-2 inhibitors.
“Our study adds to the increasing body of evidence concerning the vascular risk and prognostic impact associated with use of COX-2 inhibitors,” Dr. Schmidt and his associates wrote.
The study was funded by several Danish research foundations and the Program for Clinical Research Infrastructure, which was established by the Lundbeck Foundation and the Novo Nordisk Foundation. The authors reported no relevant disclosures.
Current use of cyclooxygenase-2 inhibitors was associated with an increase in 30-day mortality after ischemic stroke in a population-based cohort study published Nov. 5 in Neurology.
Since the association between COX-2 inhibitors and ischemic stroke mortality was associated only with current use and not former use, the researchers, led by Dr. Morten Schmidt of Aarhus (Denmark) University Hospital, believe that alternative treatment options – such as nonselective NSAIDs, which did not have an impact on overall mortality after ischemic stroke – may be more suitable for treating potential ischemic stroke patients, such as those with atrial fibrillation and a high CHA2DS2-VASc score.
If the association is truly causal, it constitutes a strong argument for increasing the efforts to ensure that patients with a high predicted risk of arterial thromboembolism are not prescribed COX-2 inhibitors when alternative treatment options are available,” Dr. Schmidt and his associates wrote.
In order to determine whether COX-2 inhibitors influenced 30-day mortality at the time of hospitalization for stroke, the researchers examined records of 100,243 people hospitalized for a first-time stroke in Denmark during 2004-2012 and deaths within 1 month after the stroke (Neurology 2014 Nov. 5 [doi:10.1212/WNL.0000000000001024]).
The hazard ratio for ischemic stroke was 1.19 (95% confidence interval, 1.02-1.38) for current users of COX-2 inhibitors, while current users of nonselective NSAIDs had an HR of 1.00 (95% CI, 0.87-1.15), compared with nonusers.
The COX-2 inhibitors in the study included diclofenac, etodolac, nabumeton, and meloxicam, as well as coxibs including celecoxib and rofecoxib. The nonselective NSAIDs in the study were ibuprofen, naproxen, ketoprofen, dexibuprofen, piroxicam, tolfenamic acid, and indomethacin.
Though the researchers acknowledged more studies are needed to truly examine the effects of COX-2 inhibitors on stroke mortality, they hypothesized that the increased mortality rate may be caused by COX-2 inhibition interfering with the pathophysiologic response to a stroke, or unwanted effects from the thromboembolic properties of COX-2 inhibitors.
“Our study adds to the increasing body of evidence concerning the vascular risk and prognostic impact associated with use of COX-2 inhibitors,” Dr. Schmidt and his associates wrote.
The study was funded by several Danish research foundations and the Program for Clinical Research Infrastructure, which was established by the Lundbeck Foundation and the Novo Nordisk Foundation. The authors reported no relevant disclosures.
FROM NEUROLOGY
Key clinical point:Consideration should be given to using treatment options other than COX-2 inhibitors in patients with a high future risk of arterial thromboembolism.
Major finding: Preadmission use of COX-2 inhibitors was associated with increased 30-day mortality after ischemic stroke 1.19 (95% CI, 1.02-1.38), compared with nonusers.
Data source:Population-based cohort study of 100,043 patients from Denmark with first-time stroke.
Disclosures:The study was funded by several Danish research foundations and the Program for Clinical Research Infrastructure, which was established by the Lundbeck Foundation and the Novo Nordisk Foundation. The authors reported no relevant disclosures.
Eastern United States has the highest arthritis rates
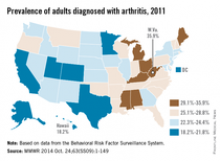
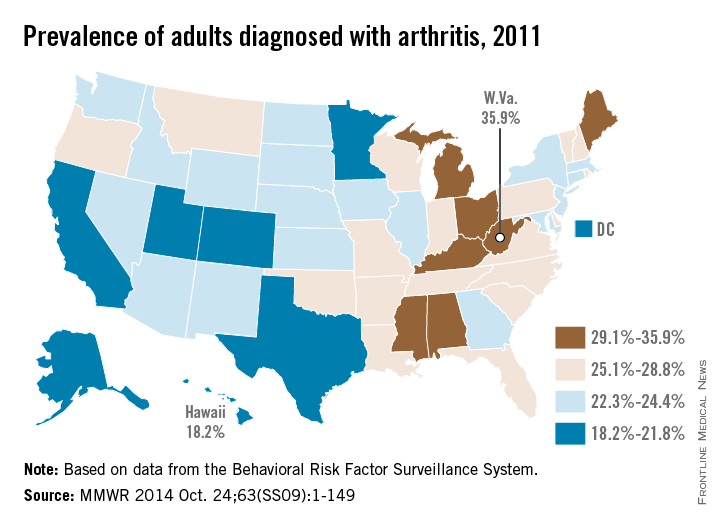
The prevalence of arthritis tended to be higher in eastern U.S. states than in western states in 2011, according to a report from the Centers for Disease Control and Prevention.
All seven states with an arthritis rate below 22% were located west of the Mississippi River, with Hawaii having the lowest rate at 18.2%, followed by Utah (19.8%) and Texas (20.2%). The District of Columbia, which is in the East, had an arthritis rate of 20.9%.
The eight states with an arthritis rate greater than 29% were all east of the Mississippi, with West Virginia having the highest prevalence (35.9%), followed by Kentucky (31.9%) and Michigan (31.0%), according to the report (MMWR 2014;63[SS09]:1-149).
Of 198 reported metropolitan and micropolitan statistical areas (MMSAs), 29 had an arthritis prevalence lower than 20%. Lawrence, Kan., had the lowest rate at 13.5%. Of the 29, only Atlanta; Knoxville, Tenn.; and Raleigh, N.C., are located entirely east of the Mississippi River. Kingsport-Bristol, in Tennessee and Virginia, had the highest arthritis rate at 37%. Of the 16 MMSAs with an arthritis rate greater than 30%, North Platte, Neb., was the only one west of the Mississippi, according to data from the Behavioral Risk Factor Surveillance System.
The prevalence of arthritis tended to be higher in eastern U.S. states than in western states in 2011, according to a report from the Centers for Disease Control and Prevention.
All seven states with an arthritis rate below 22% were located west of the Mississippi River, with Hawaii having the lowest rate at 18.2%, followed by Utah (19.8%) and Texas (20.2%). The District of Columbia, which is in the East, had an arthritis rate of 20.9%.
The eight states with an arthritis rate greater than 29% were all east of the Mississippi, with West Virginia having the highest prevalence (35.9%), followed by Kentucky (31.9%) and Michigan (31.0%), according to the report (MMWR 2014;63[SS09]:1-149).
Of 198 reported metropolitan and micropolitan statistical areas (MMSAs), 29 had an arthritis prevalence lower than 20%. Lawrence, Kan., had the lowest rate at 13.5%. Of the 29, only Atlanta; Knoxville, Tenn.; and Raleigh, N.C., are located entirely east of the Mississippi River. Kingsport-Bristol, in Tennessee and Virginia, had the highest arthritis rate at 37%. Of the 16 MMSAs with an arthritis rate greater than 30%, North Platte, Neb., was the only one west of the Mississippi, according to data from the Behavioral Risk Factor Surveillance System.
The prevalence of arthritis tended to be higher in eastern U.S. states than in western states in 2011, according to a report from the Centers for Disease Control and Prevention.
All seven states with an arthritis rate below 22% were located west of the Mississippi River, with Hawaii having the lowest rate at 18.2%, followed by Utah (19.8%) and Texas (20.2%). The District of Columbia, which is in the East, had an arthritis rate of 20.9%.
The eight states with an arthritis rate greater than 29% were all east of the Mississippi, with West Virginia having the highest prevalence (35.9%), followed by Kentucky (31.9%) and Michigan (31.0%), according to the report (MMWR 2014;63[SS09]:1-149).
Of 198 reported metropolitan and micropolitan statistical areas (MMSAs), 29 had an arthritis prevalence lower than 20%. Lawrence, Kan., had the lowest rate at 13.5%. Of the 29, only Atlanta; Knoxville, Tenn.; and Raleigh, N.C., are located entirely east of the Mississippi River. Kingsport-Bristol, in Tennessee and Virginia, had the highest arthritis rate at 37%. Of the 16 MMSAs with an arthritis rate greater than 30%, North Platte, Neb., was the only one west of the Mississippi, according to data from the Behavioral Risk Factor Surveillance System.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Apremilast succeeds against nail, scalp, palmoplantar psoriasis
LAS VEGAS – A twice-daily 30-mg dose of apremilast significantly improved moderate to severe nail, scalp, and palmoplantar psoriasis, compared with placebo, based on data from 411 adults in the ESTEEM 2 phase III study.
Overall, at 16 weeks, 29% of the patients randomized to apremilast achieved PASI-75, and 56% achieved PASI-50, compared with placebo patients (6% and 20%, respectively). The findings were presented in a poster at SDEF Las Vegas Dermatology Seminar by Dr. Jeffrey Crowley of Bakersfield (Calif.) Dermatology, and his colleagues.
In addition, patients with palmarplantar, nail, and scalp psoriasis who received apremilast showed significantly greater response rates, compared with placebo patients.
At baseline, 266 patients had scores of 1 or greater on the Nail Psoriasis Severity Index (NAPSI). Significantly more of these patients on apremilast achieved NAPSI-50 vs. placebo patients after 16 weeks (45% vs. 19%, respectively). The average percent change in NAPSI score from baseline was 29% for apremilast vs. 7% for placebo.
“Among patients initially randomized to apremilast and maintained on apremilast through week 32, nail psoriasis was further improved at week 32 (55% of patients achieved NAPSI-50)” and improvements were seen at week 32 in patients who switched from placebo to apremilast at 16 weeks, the researchers noted.
Response at 16 weeks was significantly greater on apremilast, compared with placebo, for 269 patients with scalp psoriasis (41% vs. 17%), and for 42 patients with palmoplantar psoriasis (65% vs. 31%).
Most adverse events were mild to moderate, and discontinuation rates because of adverse events were approximately 5% for placebo and treatment patients. The most common adverse events overall were nausea, diarrhea, nasopharyngitis, upper respiratory tract infection, headache, and vomiting.
The findings were limited by the short duration of the study, the researchers noted. However, apremilast was safe and well tolerated, and improvement was noted up to 32 weeks, they added.
Dr. Crowley reported receiving fees from Celgene during the study, and reported financial relationships with other companies, including AbbVie, Amgen, AstraZeneca, Eli Lilly, Janssen, Merck, and Pfizer.
LAS VEGAS – A twice-daily 30-mg dose of apremilast significantly improved moderate to severe nail, scalp, and palmoplantar psoriasis, compared with placebo, based on data from 411 adults in the ESTEEM 2 phase III study.
Overall, at 16 weeks, 29% of the patients randomized to apremilast achieved PASI-75, and 56% achieved PASI-50, compared with placebo patients (6% and 20%, respectively). The findings were presented in a poster at SDEF Las Vegas Dermatology Seminar by Dr. Jeffrey Crowley of Bakersfield (Calif.) Dermatology, and his colleagues.
In addition, patients with palmarplantar, nail, and scalp psoriasis who received apremilast showed significantly greater response rates, compared with placebo patients.
At baseline, 266 patients had scores of 1 or greater on the Nail Psoriasis Severity Index (NAPSI). Significantly more of these patients on apremilast achieved NAPSI-50 vs. placebo patients after 16 weeks (45% vs. 19%, respectively). The average percent change in NAPSI score from baseline was 29% for apremilast vs. 7% for placebo.
“Among patients initially randomized to apremilast and maintained on apremilast through week 32, nail psoriasis was further improved at week 32 (55% of patients achieved NAPSI-50)” and improvements were seen at week 32 in patients who switched from placebo to apremilast at 16 weeks, the researchers noted.
Response at 16 weeks was significantly greater on apremilast, compared with placebo, for 269 patients with scalp psoriasis (41% vs. 17%), and for 42 patients with palmoplantar psoriasis (65% vs. 31%).
Most adverse events were mild to moderate, and discontinuation rates because of adverse events were approximately 5% for placebo and treatment patients. The most common adverse events overall were nausea, diarrhea, nasopharyngitis, upper respiratory tract infection, headache, and vomiting.
The findings were limited by the short duration of the study, the researchers noted. However, apremilast was safe and well tolerated, and improvement was noted up to 32 weeks, they added.
Dr. Crowley reported receiving fees from Celgene during the study, and reported financial relationships with other companies, including AbbVie, Amgen, AstraZeneca, Eli Lilly, Janssen, Merck, and Pfizer.
LAS VEGAS – A twice-daily 30-mg dose of apremilast significantly improved moderate to severe nail, scalp, and palmoplantar psoriasis, compared with placebo, based on data from 411 adults in the ESTEEM 2 phase III study.
Overall, at 16 weeks, 29% of the patients randomized to apremilast achieved PASI-75, and 56% achieved PASI-50, compared with placebo patients (6% and 20%, respectively). The findings were presented in a poster at SDEF Las Vegas Dermatology Seminar by Dr. Jeffrey Crowley of Bakersfield (Calif.) Dermatology, and his colleagues.
In addition, patients with palmarplantar, nail, and scalp psoriasis who received apremilast showed significantly greater response rates, compared with placebo patients.
At baseline, 266 patients had scores of 1 or greater on the Nail Psoriasis Severity Index (NAPSI). Significantly more of these patients on apremilast achieved NAPSI-50 vs. placebo patients after 16 weeks (45% vs. 19%, respectively). The average percent change in NAPSI score from baseline was 29% for apremilast vs. 7% for placebo.
“Among patients initially randomized to apremilast and maintained on apremilast through week 32, nail psoriasis was further improved at week 32 (55% of patients achieved NAPSI-50)” and improvements were seen at week 32 in patients who switched from placebo to apremilast at 16 weeks, the researchers noted.
Response at 16 weeks was significantly greater on apremilast, compared with placebo, for 269 patients with scalp psoriasis (41% vs. 17%), and for 42 patients with palmoplantar psoriasis (65% vs. 31%).
Most adverse events were mild to moderate, and discontinuation rates because of adverse events were approximately 5% for placebo and treatment patients. The most common adverse events overall were nausea, diarrhea, nasopharyngitis, upper respiratory tract infection, headache, and vomiting.
The findings were limited by the short duration of the study, the researchers noted. However, apremilast was safe and well tolerated, and improvement was noted up to 32 weeks, they added.
Dr. Crowley reported receiving fees from Celgene during the study, and reported financial relationships with other companies, including AbbVie, Amgen, AstraZeneca, Eli Lilly, Janssen, Merck, and Pfizer.
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Key clinical point: Apremilast significantly improved psoriasis in a subset of patient with nail, scalp, and palmoplantar involvement.
Major finding: After 16 weeks of twice-daily apremilast, significantly more patients with nail psoriasis achieved NAPSI-50, compared with placebo patients (45% vs. 19%, respectively).
Data source: Analysis of data from ESTEEM 2.
Disclosures:Dr. Crowley reported receiving fees from Celgene during the study and reported financial relationships with other companies, including AbbVie, Amgen, AstraZeneca, Eli Lilly, Janssen, Merck, and Pfizer.
Psoriasis patients’ QOL improved with calcipotriene/betamethasone
LAS VEGAS – Adults with localized psoriasis reported significant improvement in quality of life after 2 and 8 weeks of treatment with a combination topical suspension, based on data from 147 adults.
Calcipotriene 0.005% plus betamethasone diproprionate 0.064% (CBD) has shown safety and effectiveness in adults with psoriasis. Dr. Jerry Bagel, who is in group practice in East Windsor, N.J., and his colleagues documented real-life experiences with CBD and patient-reported outcomes. The findings were presented at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
The average change in quality of life scores from baseline was –4.2 at week 2 and –5.5 at week 8; both were statistically significant. In addition, 38% and 42% of patients at 2 weeks and 8 weeks, respectively, met the secondary endpoint of at least a 5-point improvement on the Dermatology Life Quality Index (DLQI).
The mean percent change in the patients’ perceptions of itching was –19% at week 2 and –29% at week 8, based on an itch visual analog scale, Dr. Bagel noted. Patient satisfaction with treatment also was measured using the Treatment Satisfaction Questionnaire for Medication–9 (TSQM-9), and the average scores for treatment effectiveness, convenience, and satisfaction were greater than 65 on a scale of 0-100 at week 2 and week 8.
Dr. Bagel and his colleagues studied 147 adults aged 18 years and older; patients’ average age was 49 years. More than half (57%) were women; 78% of patients were white. Approximately 56% were characterized as having moderate disease, and the average disease duration was 11 years. Only two patients reported treatment-emergent adverse events, but these were deemed unrelated to the study drug by the researchers.
The findings were limited by the use of self-reports. However, “patients with an extensive history of psoriasis and various levels of disease severity were satisfied with the product” in this real-life treatment profile, he noted.
Dr. Bagel has served as a consultant, speaker, and investigator for multiple companies, including Leo Pharma, which sponsored this study. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Adults with localized psoriasis reported significant improvement in quality of life after 2 and 8 weeks of treatment with a combination topical suspension, based on data from 147 adults.
Calcipotriene 0.005% plus betamethasone diproprionate 0.064% (CBD) has shown safety and effectiveness in adults with psoriasis. Dr. Jerry Bagel, who is in group practice in East Windsor, N.J., and his colleagues documented real-life experiences with CBD and patient-reported outcomes. The findings were presented at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
The average change in quality of life scores from baseline was –4.2 at week 2 and –5.5 at week 8; both were statistically significant. In addition, 38% and 42% of patients at 2 weeks and 8 weeks, respectively, met the secondary endpoint of at least a 5-point improvement on the Dermatology Life Quality Index (DLQI).
The mean percent change in the patients’ perceptions of itching was –19% at week 2 and –29% at week 8, based on an itch visual analog scale, Dr. Bagel noted. Patient satisfaction with treatment also was measured using the Treatment Satisfaction Questionnaire for Medication–9 (TSQM-9), and the average scores for treatment effectiveness, convenience, and satisfaction were greater than 65 on a scale of 0-100 at week 2 and week 8.
Dr. Bagel and his colleagues studied 147 adults aged 18 years and older; patients’ average age was 49 years. More than half (57%) were women; 78% of patients were white. Approximately 56% were characterized as having moderate disease, and the average disease duration was 11 years. Only two patients reported treatment-emergent adverse events, but these were deemed unrelated to the study drug by the researchers.
The findings were limited by the use of self-reports. However, “patients with an extensive history of psoriasis and various levels of disease severity were satisfied with the product” in this real-life treatment profile, he noted.
Dr. Bagel has served as a consultant, speaker, and investigator for multiple companies, including Leo Pharma, which sponsored this study. SDEF and this news organization are owned by Frontline Medical Communications.
LAS VEGAS – Adults with localized psoriasis reported significant improvement in quality of life after 2 and 8 weeks of treatment with a combination topical suspension, based on data from 147 adults.
Calcipotriene 0.005% plus betamethasone diproprionate 0.064% (CBD) has shown safety and effectiveness in adults with psoriasis. Dr. Jerry Bagel, who is in group practice in East Windsor, N.J., and his colleagues documented real-life experiences with CBD and patient-reported outcomes. The findings were presented at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
The average change in quality of life scores from baseline was –4.2 at week 2 and –5.5 at week 8; both were statistically significant. In addition, 38% and 42% of patients at 2 weeks and 8 weeks, respectively, met the secondary endpoint of at least a 5-point improvement on the Dermatology Life Quality Index (DLQI).
The mean percent change in the patients’ perceptions of itching was –19% at week 2 and –29% at week 8, based on an itch visual analog scale, Dr. Bagel noted. Patient satisfaction with treatment also was measured using the Treatment Satisfaction Questionnaire for Medication–9 (TSQM-9), and the average scores for treatment effectiveness, convenience, and satisfaction were greater than 65 on a scale of 0-100 at week 2 and week 8.
Dr. Bagel and his colleagues studied 147 adults aged 18 years and older; patients’ average age was 49 years. More than half (57%) were women; 78% of patients were white. Approximately 56% were characterized as having moderate disease, and the average disease duration was 11 years. Only two patients reported treatment-emergent adverse events, but these were deemed unrelated to the study drug by the researchers.
The findings were limited by the use of self-reports. However, “patients with an extensive history of psoriasis and various levels of disease severity were satisfied with the product” in this real-life treatment profile, he noted.
Dr. Bagel has served as a consultant, speaker, and investigator for multiple companies, including Leo Pharma, which sponsored this study. SDEF and this news organization are owned by Frontline Medical Communications.
AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
Key clinical point: Calcipotriene 0.005% plus betamethasone diproprionate 0.064% was safe and effective and improved patients’ perceived quality of life.
Major finding: The average change in quality of life scores from baseline was –4.2 at week 2 and –5.5 at week 8.
Data source: Data from 147 adults aged 18 years and older with psoraisis vulgaris of an average of 11 years’ duration.
Disclosures: Dr. Bagel has served as a consultant, speaker, and investigator for multiple companies, including Leo Pharma, which sponsored this study.
Novel psoriasis biologic wows with jaw-dropping results
AMSTERDAM– The spectacular long-term efficacy achieved with a novel biologic agent for psoriasis in a first-in-humans, proof-of-concept study has raised the prospect of clinical outcomes continuing to ratchet higher in the treatment of moderate-to-severe chronic plaque psoriasis.
How much higher? Six of nine treated patients followed long-term have maintained a PASI 100 response – that is, completely clear – for up to 66 months after a single subcutaneous injection of the agent known for now as BI 655066, Dr. James G. Krueger reported at the annual congress of the European Academy of Dermatology and Venereology.
“For me, this is one of the most interesting features of this proof-of-concept study,” he added. “If this kind of activity is confirmed in the ongoing phase IIb trial, I think this represents the potential for very long-term disease modification. This could become an important agent in the future to treat psoriasis.”
BI 655066 is a monoclonal antibody that specifically targets the p19 subunit of interleukin (IL)-23. Unlike ustekinumab (Stelara), which blocks both IL-23 and IL-12, BI 655066 selectively blocks only IL-23, which Dr. Krueger believes is the central driving force in activating and sustaining the T-cell subsets responsible for the hyperproliferative and inflammatory reactions that define psoriasis.
“This study is all about testing for the specific pathogenic contribution of IL-23 to psoriasis in a first-in-humans study. Our findings really emphasize the importance of IL-23 in driving the key pathways of psoriasis,” observed Dr. Krueger, professor of investigative dermatology and director of the Milstein Medical Research Program at Rockefeller University, New York.
The study included 39 patients with moderate to severe plaque psoriasis. Their baseline PASI was 18, and they averaged more than a 20-year history of psoriasis. Twenty-four patients were randomized 3:1 to a single intravenous injection of BI 655066 at various doses ranging from 0.01 mg/kg to 5 mg/kg or to placebo in order to get an initial sense of the agent’s safety and tolerability.
In the second part of the study, 15 other participants received a single subcutaneous injection: two got placebo and the rest were randomized to BI 655066 at either 0.25 mg/kg or 1.0 mg/kg. Safety and efficacy were assessed at weeks 0, 2, 4, 12, and 24. In addition, skin biopsies were obtained at weeks 0 and 8 for immunohistochemistry studies and RNA sequencing analysis.
By week 12, the PASI 75 response rate in subcutaneous BI 655066 recipients was 87% and the PASI 90 rate was 58%. At week 24, nine patients elected to continue structured prospective follow-up while remaining off treatment, including six PASI 100 responders. Those six PASI 100 responders remained PASI 100 at ongoing follow-up 48-66 weeks after receiving their single dose of the agent.
Biopsy specimens obtained at week 8 showed normalization of the epidermal psoriasiform hyperplasia which had been present at baseline. A normal-looking granular layer had been reestablished. “This looks essentially like the pattern of normal or nonlesional skin,” according to the dermatologist.
RNA sequencing analysis and gene profiling showed normalized production of the IL-23/IL-17-induced proteins that had been strongly overexpressed at baseline, including lipocalin, beta-defensin, and psoriasin.
“The immune axis is turned down. The number of immune cells is way down, although they’re not completely eliminated. With placebo, you still see a psoriasislike pattern of the disease. With blockade of IL-23, most cases have a gene profile like nonlesional skin. This represents a profound cellular and disease modulation,” Dr. Krueger said.
Among all 39 participants, the only serious adverse event deemed possibly treatment related was a 5-minute transient ischemic attack (TIA) episode in a patient on BI 655066. This caught Dr. Krueger’s attention as a possible red flag; however, he noted that more than 200 patients have since received the biologic agent in the ongoing phase IIb trial, with no reported major adverse cardiovascular events.
“I think that TIA may just be bad luck with small numbers,” he added.
Asked what he thinks might explain the remarkably lengthy disease remission seen following a single dose of the biologic, Dr. Krueger offered two possibilities.
“It may be that IL-23 is necessary to sustain pathogenic clones of memory cells in the skin, and as we get rid of it those clones most likely apoptose. And if you’ve sufficiently removed the clones, then you don’t get the expansion. That’s guess one. Guess two would be that we’ve renormalized tolerance mechanisms in some way. Both of these hypotheses can be tested,” according to Dr. Krueger.
The study was funded by Boehringer Ingelheim. Dr. Krueger reported receiving funding from that pharmaceutical company and nearly two dozen others.
AMSTERDAM– The spectacular long-term efficacy achieved with a novel biologic agent for psoriasis in a first-in-humans, proof-of-concept study has raised the prospect of clinical outcomes continuing to ratchet higher in the treatment of moderate-to-severe chronic plaque psoriasis.
How much higher? Six of nine treated patients followed long-term have maintained a PASI 100 response – that is, completely clear – for up to 66 months after a single subcutaneous injection of the agent known for now as BI 655066, Dr. James G. Krueger reported at the annual congress of the European Academy of Dermatology and Venereology.
“For me, this is one of the most interesting features of this proof-of-concept study,” he added. “If this kind of activity is confirmed in the ongoing phase IIb trial, I think this represents the potential for very long-term disease modification. This could become an important agent in the future to treat psoriasis.”
BI 655066 is a monoclonal antibody that specifically targets the p19 subunit of interleukin (IL)-23. Unlike ustekinumab (Stelara), which blocks both IL-23 and IL-12, BI 655066 selectively blocks only IL-23, which Dr. Krueger believes is the central driving force in activating and sustaining the T-cell subsets responsible for the hyperproliferative and inflammatory reactions that define psoriasis.
“This study is all about testing for the specific pathogenic contribution of IL-23 to psoriasis in a first-in-humans study. Our findings really emphasize the importance of IL-23 in driving the key pathways of psoriasis,” observed Dr. Krueger, professor of investigative dermatology and director of the Milstein Medical Research Program at Rockefeller University, New York.
The study included 39 patients with moderate to severe plaque psoriasis. Their baseline PASI was 18, and they averaged more than a 20-year history of psoriasis. Twenty-four patients were randomized 3:1 to a single intravenous injection of BI 655066 at various doses ranging from 0.01 mg/kg to 5 mg/kg or to placebo in order to get an initial sense of the agent’s safety and tolerability.
In the second part of the study, 15 other participants received a single subcutaneous injection: two got placebo and the rest were randomized to BI 655066 at either 0.25 mg/kg or 1.0 mg/kg. Safety and efficacy were assessed at weeks 0, 2, 4, 12, and 24. In addition, skin biopsies were obtained at weeks 0 and 8 for immunohistochemistry studies and RNA sequencing analysis.
By week 12, the PASI 75 response rate in subcutaneous BI 655066 recipients was 87% and the PASI 90 rate was 58%. At week 24, nine patients elected to continue structured prospective follow-up while remaining off treatment, including six PASI 100 responders. Those six PASI 100 responders remained PASI 100 at ongoing follow-up 48-66 weeks after receiving their single dose of the agent.
Biopsy specimens obtained at week 8 showed normalization of the epidermal psoriasiform hyperplasia which had been present at baseline. A normal-looking granular layer had been reestablished. “This looks essentially like the pattern of normal or nonlesional skin,” according to the dermatologist.
RNA sequencing analysis and gene profiling showed normalized production of the IL-23/IL-17-induced proteins that had been strongly overexpressed at baseline, including lipocalin, beta-defensin, and psoriasin.
“The immune axis is turned down. The number of immune cells is way down, although they’re not completely eliminated. With placebo, you still see a psoriasislike pattern of the disease. With blockade of IL-23, most cases have a gene profile like nonlesional skin. This represents a profound cellular and disease modulation,” Dr. Krueger said.
Among all 39 participants, the only serious adverse event deemed possibly treatment related was a 5-minute transient ischemic attack (TIA) episode in a patient on BI 655066. This caught Dr. Krueger’s attention as a possible red flag; however, he noted that more than 200 patients have since received the biologic agent in the ongoing phase IIb trial, with no reported major adverse cardiovascular events.
“I think that TIA may just be bad luck with small numbers,” he added.
Asked what he thinks might explain the remarkably lengthy disease remission seen following a single dose of the biologic, Dr. Krueger offered two possibilities.
“It may be that IL-23 is necessary to sustain pathogenic clones of memory cells in the skin, and as we get rid of it those clones most likely apoptose. And if you’ve sufficiently removed the clones, then you don’t get the expansion. That’s guess one. Guess two would be that we’ve renormalized tolerance mechanisms in some way. Both of these hypotheses can be tested,” according to Dr. Krueger.
The study was funded by Boehringer Ingelheim. Dr. Krueger reported receiving funding from that pharmaceutical company and nearly two dozen others.
AMSTERDAM– The spectacular long-term efficacy achieved with a novel biologic agent for psoriasis in a first-in-humans, proof-of-concept study has raised the prospect of clinical outcomes continuing to ratchet higher in the treatment of moderate-to-severe chronic plaque psoriasis.
How much higher? Six of nine treated patients followed long-term have maintained a PASI 100 response – that is, completely clear – for up to 66 months after a single subcutaneous injection of the agent known for now as BI 655066, Dr. James G. Krueger reported at the annual congress of the European Academy of Dermatology and Venereology.
“For me, this is one of the most interesting features of this proof-of-concept study,” he added. “If this kind of activity is confirmed in the ongoing phase IIb trial, I think this represents the potential for very long-term disease modification. This could become an important agent in the future to treat psoriasis.”
BI 655066 is a monoclonal antibody that specifically targets the p19 subunit of interleukin (IL)-23. Unlike ustekinumab (Stelara), which blocks both IL-23 and IL-12, BI 655066 selectively blocks only IL-23, which Dr. Krueger believes is the central driving force in activating and sustaining the T-cell subsets responsible for the hyperproliferative and inflammatory reactions that define psoriasis.
“This study is all about testing for the specific pathogenic contribution of IL-23 to psoriasis in a first-in-humans study. Our findings really emphasize the importance of IL-23 in driving the key pathways of psoriasis,” observed Dr. Krueger, professor of investigative dermatology and director of the Milstein Medical Research Program at Rockefeller University, New York.
The study included 39 patients with moderate to severe plaque psoriasis. Their baseline PASI was 18, and they averaged more than a 20-year history of psoriasis. Twenty-four patients were randomized 3:1 to a single intravenous injection of BI 655066 at various doses ranging from 0.01 mg/kg to 5 mg/kg or to placebo in order to get an initial sense of the agent’s safety and tolerability.
In the second part of the study, 15 other participants received a single subcutaneous injection: two got placebo and the rest were randomized to BI 655066 at either 0.25 mg/kg or 1.0 mg/kg. Safety and efficacy were assessed at weeks 0, 2, 4, 12, and 24. In addition, skin biopsies were obtained at weeks 0 and 8 for immunohistochemistry studies and RNA sequencing analysis.
By week 12, the PASI 75 response rate in subcutaneous BI 655066 recipients was 87% and the PASI 90 rate was 58%. At week 24, nine patients elected to continue structured prospective follow-up while remaining off treatment, including six PASI 100 responders. Those six PASI 100 responders remained PASI 100 at ongoing follow-up 48-66 weeks after receiving their single dose of the agent.
Biopsy specimens obtained at week 8 showed normalization of the epidermal psoriasiform hyperplasia which had been present at baseline. A normal-looking granular layer had been reestablished. “This looks essentially like the pattern of normal or nonlesional skin,” according to the dermatologist.
RNA sequencing analysis and gene profiling showed normalized production of the IL-23/IL-17-induced proteins that had been strongly overexpressed at baseline, including lipocalin, beta-defensin, and psoriasin.
“The immune axis is turned down. The number of immune cells is way down, although they’re not completely eliminated. With placebo, you still see a psoriasislike pattern of the disease. With blockade of IL-23, most cases have a gene profile like nonlesional skin. This represents a profound cellular and disease modulation,” Dr. Krueger said.
Among all 39 participants, the only serious adverse event deemed possibly treatment related was a 5-minute transient ischemic attack (TIA) episode in a patient on BI 655066. This caught Dr. Krueger’s attention as a possible red flag; however, he noted that more than 200 patients have since received the biologic agent in the ongoing phase IIb trial, with no reported major adverse cardiovascular events.
“I think that TIA may just be bad luck with small numbers,” he added.
Asked what he thinks might explain the remarkably lengthy disease remission seen following a single dose of the biologic, Dr. Krueger offered two possibilities.
“It may be that IL-23 is necessary to sustain pathogenic clones of memory cells in the skin, and as we get rid of it those clones most likely apoptose. And if you’ve sufficiently removed the clones, then you don’t get the expansion. That’s guess one. Guess two would be that we’ve renormalized tolerance mechanisms in some way. Both of these hypotheses can be tested,” according to Dr. Krueger.
The study was funded by Boehringer Ingelheim. Dr. Krueger reported receiving funding from that pharmaceutical company and nearly two dozen others.
AT THE EADV CONGRESS
Key clinical point: Up to 66 months after receiving a single subcutaneous injection of a biologic agent that selectively blocks interleukin-23, six patients with moderate to severe chronic plaque psoriasis at baseline remained PASI 100 responders with clear skin.
Major finding: The PASI 75 response rate 12 weeks after receiving a single dose of the investigational agent BI 655066 was 87%, and the PASI 90 rate was 58%.
Data source: This was a first-in-humans, proof-of-concept study involving 39 psoriasis patients.
Disclosures: The study was sponsored by Boehringer Ingelheim. The presenter reported receiving research funding from that pharmaceutical company and nearly two dozen others.
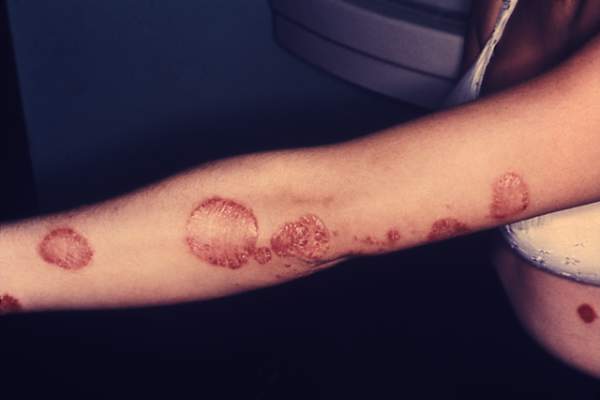
Psoriasiform lesions linked to anti-TNF treatment
VIENNA – Patients treated with an anti–tumor necrosis factor drug had a 5% annual rate of developing one or more psoriasiform skin lesions in a review of more than 400 patients who received these drugs to treat inflammatory bowel disease at a single center in Rome.
The cohort review confirmed prior reports that smoking is a risk factor for the appearance of psoriasislike skin lesions on patients being treated with an anti–tumor necrosis factor (TNF) drug, such as infliximab (Remicade) or adalimumab (Humira). The Rome experience also showed that in 28 of the 42 patients who developed a psoriasiform lesion, the eruption responded to topical treatment without need to stop or change the anti-TNF regimen. Ten of the 42 patients ultimately had to stop their anti-TNF regimen, Dr. Daniela Pugliese said at the United European Gastroenterology Global Congress.
“There have been several case reports of this, but this is the largest cohort review yet reported by one center,” said Dr. Pugliese, a gastroenterologist in the inflammatory bowel disease (IBD) unit of Complesso Integrato Columbus, Catholic University in Rome.
The review included 402 patients treated with an anti-TNF drug at the unit during 2008-2013, with a median follow-up of 17 months. During follow-up, 42 patients developed a psoriasiform lesion with a biopsy-proven diagnosis, a rate of 5 cases/100 person-years on anti-TNF treatment. The IBD patients averaged 40 years old, and 60% had Crohn’s disease and 40% had ulcerative colitis. About 60% received infliximab treatment, and 40% adalimumab. Most lesions appeared in predilection sites, as well as on palmoplantar surfaces or on the scalp; nearly half the patients had lesions in two or more locations.
A multivariate regression analysis that assessed several demographic and clinical features of all 402 patients identified two parameters that significantly linked with lesion development. Smoking linked with a greater than twofold increased risk for having a psoriasiform lesion (78 of the patients, 19%, smoked), and concurrent treatment with a thiopurine such as azathioprine, linked with a 67% reduced rate of lesion development (85 patients, 21%, were on concurrent thiopurine treatment). All IBD patients seen at the Rome unit who smoked were counseled regarding smoking cessation, Dr. Pugliese said in an interview.
Among the patients who did not respond to topical treatment, four received some benefit from starting treatment with ustekinumab (Stelara), which especially benefited patients who were otherwise difficult to treat, Dr. Pugliese said. Other patients benefited from starting treatment with cyclosporine, methotrexate, or a transient treatment with an oral steroid. Two patients who developed lesions on infliximab switched to adalimumab, and two other patients who had lesions on adalimumab switched to infliximab.
Dr. Pugliese and her associates did not have a good explanation of why patients on anti-TNF drugs develop these lesions, which Dr. Pugliese called “paradoxical.” One possible etiology is that inhibition of TNF-alpha results in uncontrolled production of interferon-alpha by plasmacytoid dendritic cells and this then triggers the psoriasiform eruptions.
A key element in managing these lesions may be early detection and topical treatment while they remain small, commented Dr. C. Janneke van der Woude, head of the IBD unit at Erasmus University Medical Center in Rotterdam, the Netherlands. “Every time we see an IBD patient who is on an anti-TNF drug, we ask whether they have had any itching, allergic reaction, arthritis, eye problem, or headache,” to facilitate early detection of an adverse effect from treatment, she said in an interview.
Dr. Pugliese had no disclosures. Dr. van der Woude said that she has been an adviser to Dr Falk, AbbVie, Janssen, Johnson & Johnson, and Cosmo.
On Twitter@mitchelzoler
VIENNA – Patients treated with an anti–tumor necrosis factor drug had a 5% annual rate of developing one or more psoriasiform skin lesions in a review of more than 400 patients who received these drugs to treat inflammatory bowel disease at a single center in Rome.
The cohort review confirmed prior reports that smoking is a risk factor for the appearance of psoriasislike skin lesions on patients being treated with an anti–tumor necrosis factor (TNF) drug, such as infliximab (Remicade) or adalimumab (Humira). The Rome experience also showed that in 28 of the 42 patients who developed a psoriasiform lesion, the eruption responded to topical treatment without need to stop or change the anti-TNF regimen. Ten of the 42 patients ultimately had to stop their anti-TNF regimen, Dr. Daniela Pugliese said at the United European Gastroenterology Global Congress.
“There have been several case reports of this, but this is the largest cohort review yet reported by one center,” said Dr. Pugliese, a gastroenterologist in the inflammatory bowel disease (IBD) unit of Complesso Integrato Columbus, Catholic University in Rome.
The review included 402 patients treated with an anti-TNF drug at the unit during 2008-2013, with a median follow-up of 17 months. During follow-up, 42 patients developed a psoriasiform lesion with a biopsy-proven diagnosis, a rate of 5 cases/100 person-years on anti-TNF treatment. The IBD patients averaged 40 years old, and 60% had Crohn’s disease and 40% had ulcerative colitis. About 60% received infliximab treatment, and 40% adalimumab. Most lesions appeared in predilection sites, as well as on palmoplantar surfaces or on the scalp; nearly half the patients had lesions in two or more locations.
A multivariate regression analysis that assessed several demographic and clinical features of all 402 patients identified two parameters that significantly linked with lesion development. Smoking linked with a greater than twofold increased risk for having a psoriasiform lesion (78 of the patients, 19%, smoked), and concurrent treatment with a thiopurine such as azathioprine, linked with a 67% reduced rate of lesion development (85 patients, 21%, were on concurrent thiopurine treatment). All IBD patients seen at the Rome unit who smoked were counseled regarding smoking cessation, Dr. Pugliese said in an interview.
Among the patients who did not respond to topical treatment, four received some benefit from starting treatment with ustekinumab (Stelara), which especially benefited patients who were otherwise difficult to treat, Dr. Pugliese said. Other patients benefited from starting treatment with cyclosporine, methotrexate, or a transient treatment with an oral steroid. Two patients who developed lesions on infliximab switched to adalimumab, and two other patients who had lesions on adalimumab switched to infliximab.
Dr. Pugliese and her associates did not have a good explanation of why patients on anti-TNF drugs develop these lesions, which Dr. Pugliese called “paradoxical.” One possible etiology is that inhibition of TNF-alpha results in uncontrolled production of interferon-alpha by plasmacytoid dendritic cells and this then triggers the psoriasiform eruptions.
A key element in managing these lesions may be early detection and topical treatment while they remain small, commented Dr. C. Janneke van der Woude, head of the IBD unit at Erasmus University Medical Center in Rotterdam, the Netherlands. “Every time we see an IBD patient who is on an anti-TNF drug, we ask whether they have had any itching, allergic reaction, arthritis, eye problem, or headache,” to facilitate early detection of an adverse effect from treatment, she said in an interview.
Dr. Pugliese had no disclosures. Dr. van der Woude said that she has been an adviser to Dr Falk, AbbVie, Janssen, Johnson & Johnson, and Cosmo.
On Twitter@mitchelzoler
VIENNA – Patients treated with an anti–tumor necrosis factor drug had a 5% annual rate of developing one or more psoriasiform skin lesions in a review of more than 400 patients who received these drugs to treat inflammatory bowel disease at a single center in Rome.
The cohort review confirmed prior reports that smoking is a risk factor for the appearance of psoriasislike skin lesions on patients being treated with an anti–tumor necrosis factor (TNF) drug, such as infliximab (Remicade) or adalimumab (Humira). The Rome experience also showed that in 28 of the 42 patients who developed a psoriasiform lesion, the eruption responded to topical treatment without need to stop or change the anti-TNF regimen. Ten of the 42 patients ultimately had to stop their anti-TNF regimen, Dr. Daniela Pugliese said at the United European Gastroenterology Global Congress.
“There have been several case reports of this, but this is the largest cohort review yet reported by one center,” said Dr. Pugliese, a gastroenterologist in the inflammatory bowel disease (IBD) unit of Complesso Integrato Columbus, Catholic University in Rome.
The review included 402 patients treated with an anti-TNF drug at the unit during 2008-2013, with a median follow-up of 17 months. During follow-up, 42 patients developed a psoriasiform lesion with a biopsy-proven diagnosis, a rate of 5 cases/100 person-years on anti-TNF treatment. The IBD patients averaged 40 years old, and 60% had Crohn’s disease and 40% had ulcerative colitis. About 60% received infliximab treatment, and 40% adalimumab. Most lesions appeared in predilection sites, as well as on palmoplantar surfaces or on the scalp; nearly half the patients had lesions in two or more locations.
A multivariate regression analysis that assessed several demographic and clinical features of all 402 patients identified two parameters that significantly linked with lesion development. Smoking linked with a greater than twofold increased risk for having a psoriasiform lesion (78 of the patients, 19%, smoked), and concurrent treatment with a thiopurine such as azathioprine, linked with a 67% reduced rate of lesion development (85 patients, 21%, were on concurrent thiopurine treatment). All IBD patients seen at the Rome unit who smoked were counseled regarding smoking cessation, Dr. Pugliese said in an interview.
Among the patients who did not respond to topical treatment, four received some benefit from starting treatment with ustekinumab (Stelara), which especially benefited patients who were otherwise difficult to treat, Dr. Pugliese said. Other patients benefited from starting treatment with cyclosporine, methotrexate, or a transient treatment with an oral steroid. Two patients who developed lesions on infliximab switched to adalimumab, and two other patients who had lesions on adalimumab switched to infliximab.
Dr. Pugliese and her associates did not have a good explanation of why patients on anti-TNF drugs develop these lesions, which Dr. Pugliese called “paradoxical.” One possible etiology is that inhibition of TNF-alpha results in uncontrolled production of interferon-alpha by plasmacytoid dendritic cells and this then triggers the psoriasiform eruptions.
A key element in managing these lesions may be early detection and topical treatment while they remain small, commented Dr. C. Janneke van der Woude, head of the IBD unit at Erasmus University Medical Center in Rotterdam, the Netherlands. “Every time we see an IBD patient who is on an anti-TNF drug, we ask whether they have had any itching, allergic reaction, arthritis, eye problem, or headache,” to facilitate early detection of an adverse effect from treatment, she said in an interview.
Dr. Pugliese had no disclosures. Dr. van der Woude said that she has been an adviser to Dr Falk, AbbVie, Janssen, Johnson & Johnson, and Cosmo.
On Twitter@mitchelzoler
AT UEG WEEK VIENNA 2014
Key clinical point: Inflammatory bowel disease patients on anti–tumor necrosis factor treatment often develop psoriasiform skin lesions.
Major finding: Patients receiving an anti-TNF drug developed psoriasiform lesions at an annual rate of 5%.
Data source: Retrospective review of 402 patients with inflammatory bowel disease treated with an anti-TNF drug at one center in Rome.
Disclosures: Dr. Pugliese had no disclosures. Dr. van der Woude said that she has been an adviser to Dr Falk, AbbVie, Janssen, Johnson & Johnson, and Cosmo.
Select biologics for dose escalation in psoriasis
SONOMA, CALIF.– Data support dosage escalation or intensification for patients who don’t respond to biologic therapy for psoriasis, but only for three of the four biologic agents available in the United States, Dr. April W. Armstrong said at the annual Coastal Dermatology Symposium.
It may make sense to increase the dose or shorten the intervals between doses in some patients with psoriasis who don’t respond to etanercept, adalimumab, or ustekinumab, she suggested.
For infliximab, however, increasing the standard 5-mg/kg dose to 10 mg/kg in nonresponders did not significantly improve efficacy in one randomized study of patients with moderate to severe psoriasis. Consider combination therapy instead of dose escalation in those patients, said Dr. Armstrong, director of the psoriasis program at the University of Colorado, Denver.
Most clinical trials of dose escalation for psoriasis target patients in whom conventional doses produce only a partial response, defined as a Psoriasis Area and Severity Index (PASI) score of 50-75, or no response, defined as a PASI score below 50, she said.
A conventional dosage of etanercept for psoriasis uses 50 mg twice weekly for 12 weeks, followed by 50 mg once weekly for maintenance, Dr. Armstrong said. In an open-label extension of a study of 912 patients with moderate to severe psoriasis who received 12 weeks of etanercept therapy, nonresponders who escalated the maintenance dosage to 50 mg twice weekly boosted the likelihood of achieving a PASI 75 from 33% at 12 weeks to 44% at 48 weeks and 43% at 72 weeks (J. Drugs Dermatol. 2010;9:928-37).
Safety profiles were similar between standard dosing and escalated or intensified dosing in studies of all four biologic therapies, Dr. Armstrong said at the symposium, jointly presented by the University of Louisville (Ky.) and the Global Academy for Medical Education.
In the etanercept study, rates of serious infections were 0.9 events per 100 patient-years on standard maintenance therapy and 1.9 events per 100 patient-years on the escalated dosage. Myocardial infarctions occurred in none of 321 patients on standard maintenance therapy and in 2 of 591 patients on the twice-weekly dosage.
The conventional dosage of adalimumab for psoriasis is 80 mg at the start of therapy, followed by 40 mg every other week starting at week 1. In an open-label extension of a study of 147 patients, those who did not achieve a PASI 50 by week 25 were allowed to escalate the maintenance therapy dosage to 40 mg weekly. By week 60, 64% of patients in the dose-escalation group achieved a PASI 75, compared with 56% of patients who had been on the standard regimen and 45% of patients who had started with placebo for 12 weeks and then went on the standard dosage (J. Am. Acad. Dermatol. 2006;55:598-606). The mean PASI score improved by 27% after 8 weeks on the escalated dose, Dr. Armstrong said.
Three malignancies and two cardiovascular events (one leading to death) developed in the escalated-dosage group, compared with two malignancies and a serious case of coccidiomycosis in the standard-therapy group, she noted.
The conventional dosage for ustekinumab is 45 mg in patients weighing up to 100 kg or 90 mg in heavier patients, administered in subcutaneous injections at weeks 0 and 4, then every 12 weeks thereafter. In the only head-to-head study comparing biologic therapies for psoriasis, ustekinumab was more effective than etanercept in the first 3 months, Dr. Armstrong noted. The 12-week Active Comparator (CNTO1275/Enbrel) Psoriasis Trial (ACCEPT) found that 74% of 347 patients on 90 mg of ustekinumab and 68% of 209 patients on 45 mg of ustekinumab achieved a PASI 75, compared with 57% of 347 patients on etanercept (N. Engl. J. Med. 2010;362:118-28).
In a separate study of 158 patients with psoriasis who only partially responded to standard ustekinumab therapy after 24 weeks, maintenance dose escalation to every 8 weeks instead of 12 significantly improved response rates in patients on 90 mg but not in those on 45 mg. By week 52, 69% of patients receiving 90 mg ustekinumab every 8 weeks achieved a PASI 75, compared with 33% of patients on ustekinumab 90 mg every 12 weeks (Lancet 2008;371:1675-84). In the patients on 45 mg ustekinumab, the proportions achieving PASI 75 by week 52 did not differ significantly between those receiving the drug every 8 or 12 weeks.
One cutaneous malignancy, one noncutaneous malignancy, and two other serious adverse events occurred in the intensified-therapy groups, compared with one serious infection and two other serious adverse events in the standard-therapy groups.
The conventional dosage of infliximab for psoriasis is 5 mg/kg at weeks 0, 2, and 6, then every 8 weeks. A study that randomized 33 patients with moderate to severe psoriasis to 10 weeks of therapy with placebo or 5 mg/kg or 10 mg/kg of infliximab at weeks 0, 2, and 6 found that 91% of patients on the escalated dose and 82% on the standard dose of infliximab achieved a Physician Global Assessment score of good, excellent, or clear, compared with 18% of patients on placebo (Lancet 2001;357:1842-7).
The proportions of patients achieving a PASI 75 were not significantly different between the standard-dosage group and the escalated-dosage group (82% vs. 73%, respectively), although both were significantly higher than in the placebo group (18%). “So, there’s not much difference if you give 5 or 10 mg/kg” of infliximab, Dr. Armstrong said.
One of 11 patients on 5 mg/kg infliximab developed a dental abscess, and 1 of 11 patients on 10 mg/kg infliximab developed pneumonia.
Dr. Armstrong reported financial associations with AbbVie, Amgen, Celgene, Lilly, Novartis, Merck, Pfizer, UCB, Modernizing Medicine, and Janssen. This publication and the Global Academy for Medical Education are owned by the same parent company.
On Twitter @sherryboschert
SONOMA, CALIF.– Data support dosage escalation or intensification for patients who don’t respond to biologic therapy for psoriasis, but only for three of the four biologic agents available in the United States, Dr. April W. Armstrong said at the annual Coastal Dermatology Symposium.
It may make sense to increase the dose or shorten the intervals between doses in some patients with psoriasis who don’t respond to etanercept, adalimumab, or ustekinumab, she suggested.
For infliximab, however, increasing the standard 5-mg/kg dose to 10 mg/kg in nonresponders did not significantly improve efficacy in one randomized study of patients with moderate to severe psoriasis. Consider combination therapy instead of dose escalation in those patients, said Dr. Armstrong, director of the psoriasis program at the University of Colorado, Denver.
Most clinical trials of dose escalation for psoriasis target patients in whom conventional doses produce only a partial response, defined as a Psoriasis Area and Severity Index (PASI) score of 50-75, or no response, defined as a PASI score below 50, she said.
A conventional dosage of etanercept for psoriasis uses 50 mg twice weekly for 12 weeks, followed by 50 mg once weekly for maintenance, Dr. Armstrong said. In an open-label extension of a study of 912 patients with moderate to severe psoriasis who received 12 weeks of etanercept therapy, nonresponders who escalated the maintenance dosage to 50 mg twice weekly boosted the likelihood of achieving a PASI 75 from 33% at 12 weeks to 44% at 48 weeks and 43% at 72 weeks (J. Drugs Dermatol. 2010;9:928-37).
Safety profiles were similar between standard dosing and escalated or intensified dosing in studies of all four biologic therapies, Dr. Armstrong said at the symposium, jointly presented by the University of Louisville (Ky.) and the Global Academy for Medical Education.
In the etanercept study, rates of serious infections were 0.9 events per 100 patient-years on standard maintenance therapy and 1.9 events per 100 patient-years on the escalated dosage. Myocardial infarctions occurred in none of 321 patients on standard maintenance therapy and in 2 of 591 patients on the twice-weekly dosage.
The conventional dosage of adalimumab for psoriasis is 80 mg at the start of therapy, followed by 40 mg every other week starting at week 1. In an open-label extension of a study of 147 patients, those who did not achieve a PASI 50 by week 25 were allowed to escalate the maintenance therapy dosage to 40 mg weekly. By week 60, 64% of patients in the dose-escalation group achieved a PASI 75, compared with 56% of patients who had been on the standard regimen and 45% of patients who had started with placebo for 12 weeks and then went on the standard dosage (J. Am. Acad. Dermatol. 2006;55:598-606). The mean PASI score improved by 27% after 8 weeks on the escalated dose, Dr. Armstrong said.
Three malignancies and two cardiovascular events (one leading to death) developed in the escalated-dosage group, compared with two malignancies and a serious case of coccidiomycosis in the standard-therapy group, she noted.
The conventional dosage for ustekinumab is 45 mg in patients weighing up to 100 kg or 90 mg in heavier patients, administered in subcutaneous injections at weeks 0 and 4, then every 12 weeks thereafter. In the only head-to-head study comparing biologic therapies for psoriasis, ustekinumab was more effective than etanercept in the first 3 months, Dr. Armstrong noted. The 12-week Active Comparator (CNTO1275/Enbrel) Psoriasis Trial (ACCEPT) found that 74% of 347 patients on 90 mg of ustekinumab and 68% of 209 patients on 45 mg of ustekinumab achieved a PASI 75, compared with 57% of 347 patients on etanercept (N. Engl. J. Med. 2010;362:118-28).
In a separate study of 158 patients with psoriasis who only partially responded to standard ustekinumab therapy after 24 weeks, maintenance dose escalation to every 8 weeks instead of 12 significantly improved response rates in patients on 90 mg but not in those on 45 mg. By week 52, 69% of patients receiving 90 mg ustekinumab every 8 weeks achieved a PASI 75, compared with 33% of patients on ustekinumab 90 mg every 12 weeks (Lancet 2008;371:1675-84). In the patients on 45 mg ustekinumab, the proportions achieving PASI 75 by week 52 did not differ significantly between those receiving the drug every 8 or 12 weeks.
One cutaneous malignancy, one noncutaneous malignancy, and two other serious adverse events occurred in the intensified-therapy groups, compared with one serious infection and two other serious adverse events in the standard-therapy groups.
The conventional dosage of infliximab for psoriasis is 5 mg/kg at weeks 0, 2, and 6, then every 8 weeks. A study that randomized 33 patients with moderate to severe psoriasis to 10 weeks of therapy with placebo or 5 mg/kg or 10 mg/kg of infliximab at weeks 0, 2, and 6 found that 91% of patients on the escalated dose and 82% on the standard dose of infliximab achieved a Physician Global Assessment score of good, excellent, or clear, compared with 18% of patients on placebo (Lancet 2001;357:1842-7).
The proportions of patients achieving a PASI 75 were not significantly different between the standard-dosage group and the escalated-dosage group (82% vs. 73%, respectively), although both were significantly higher than in the placebo group (18%). “So, there’s not much difference if you give 5 or 10 mg/kg” of infliximab, Dr. Armstrong said.
One of 11 patients on 5 mg/kg infliximab developed a dental abscess, and 1 of 11 patients on 10 mg/kg infliximab developed pneumonia.
Dr. Armstrong reported financial associations with AbbVie, Amgen, Celgene, Lilly, Novartis, Merck, Pfizer, UCB, Modernizing Medicine, and Janssen. This publication and the Global Academy for Medical Education are owned by the same parent company.
On Twitter @sherryboschert
SONOMA, CALIF.– Data support dosage escalation or intensification for patients who don’t respond to biologic therapy for psoriasis, but only for three of the four biologic agents available in the United States, Dr. April W. Armstrong said at the annual Coastal Dermatology Symposium.
It may make sense to increase the dose or shorten the intervals between doses in some patients with psoriasis who don’t respond to etanercept, adalimumab, or ustekinumab, she suggested.
For infliximab, however, increasing the standard 5-mg/kg dose to 10 mg/kg in nonresponders did not significantly improve efficacy in one randomized study of patients with moderate to severe psoriasis. Consider combination therapy instead of dose escalation in those patients, said Dr. Armstrong, director of the psoriasis program at the University of Colorado, Denver.
Most clinical trials of dose escalation for psoriasis target patients in whom conventional doses produce only a partial response, defined as a Psoriasis Area and Severity Index (PASI) score of 50-75, or no response, defined as a PASI score below 50, she said.
A conventional dosage of etanercept for psoriasis uses 50 mg twice weekly for 12 weeks, followed by 50 mg once weekly for maintenance, Dr. Armstrong said. In an open-label extension of a study of 912 patients with moderate to severe psoriasis who received 12 weeks of etanercept therapy, nonresponders who escalated the maintenance dosage to 50 mg twice weekly boosted the likelihood of achieving a PASI 75 from 33% at 12 weeks to 44% at 48 weeks and 43% at 72 weeks (J. Drugs Dermatol. 2010;9:928-37).
Safety profiles were similar between standard dosing and escalated or intensified dosing in studies of all four biologic therapies, Dr. Armstrong said at the symposium, jointly presented by the University of Louisville (Ky.) and the Global Academy for Medical Education.
In the etanercept study, rates of serious infections were 0.9 events per 100 patient-years on standard maintenance therapy and 1.9 events per 100 patient-years on the escalated dosage. Myocardial infarctions occurred in none of 321 patients on standard maintenance therapy and in 2 of 591 patients on the twice-weekly dosage.
The conventional dosage of adalimumab for psoriasis is 80 mg at the start of therapy, followed by 40 mg every other week starting at week 1. In an open-label extension of a study of 147 patients, those who did not achieve a PASI 50 by week 25 were allowed to escalate the maintenance therapy dosage to 40 mg weekly. By week 60, 64% of patients in the dose-escalation group achieved a PASI 75, compared with 56% of patients who had been on the standard regimen and 45% of patients who had started with placebo for 12 weeks and then went on the standard dosage (J. Am. Acad. Dermatol. 2006;55:598-606). The mean PASI score improved by 27% after 8 weeks on the escalated dose, Dr. Armstrong said.
Three malignancies and two cardiovascular events (one leading to death) developed in the escalated-dosage group, compared with two malignancies and a serious case of coccidiomycosis in the standard-therapy group, she noted.
The conventional dosage for ustekinumab is 45 mg in patients weighing up to 100 kg or 90 mg in heavier patients, administered in subcutaneous injections at weeks 0 and 4, then every 12 weeks thereafter. In the only head-to-head study comparing biologic therapies for psoriasis, ustekinumab was more effective than etanercept in the first 3 months, Dr. Armstrong noted. The 12-week Active Comparator (CNTO1275/Enbrel) Psoriasis Trial (ACCEPT) found that 74% of 347 patients on 90 mg of ustekinumab and 68% of 209 patients on 45 mg of ustekinumab achieved a PASI 75, compared with 57% of 347 patients on etanercept (N. Engl. J. Med. 2010;362:118-28).
In a separate study of 158 patients with psoriasis who only partially responded to standard ustekinumab therapy after 24 weeks, maintenance dose escalation to every 8 weeks instead of 12 significantly improved response rates in patients on 90 mg but not in those on 45 mg. By week 52, 69% of patients receiving 90 mg ustekinumab every 8 weeks achieved a PASI 75, compared with 33% of patients on ustekinumab 90 mg every 12 weeks (Lancet 2008;371:1675-84). In the patients on 45 mg ustekinumab, the proportions achieving PASI 75 by week 52 did not differ significantly between those receiving the drug every 8 or 12 weeks.
One cutaneous malignancy, one noncutaneous malignancy, and two other serious adverse events occurred in the intensified-therapy groups, compared with one serious infection and two other serious adverse events in the standard-therapy groups.
The conventional dosage of infliximab for psoriasis is 5 mg/kg at weeks 0, 2, and 6, then every 8 weeks. A study that randomized 33 patients with moderate to severe psoriasis to 10 weeks of therapy with placebo or 5 mg/kg or 10 mg/kg of infliximab at weeks 0, 2, and 6 found that 91% of patients on the escalated dose and 82% on the standard dose of infliximab achieved a Physician Global Assessment score of good, excellent, or clear, compared with 18% of patients on placebo (Lancet 2001;357:1842-7).
The proportions of patients achieving a PASI 75 were not significantly different between the standard-dosage group and the escalated-dosage group (82% vs. 73%, respectively), although both were significantly higher than in the placebo group (18%). “So, there’s not much difference if you give 5 or 10 mg/kg” of infliximab, Dr. Armstrong said.
One of 11 patients on 5 mg/kg infliximab developed a dental abscess, and 1 of 11 patients on 10 mg/kg infliximab developed pneumonia.
Dr. Armstrong reported financial associations with AbbVie, Amgen, Celgene, Lilly, Novartis, Merck, Pfizer, UCB, Modernizing Medicine, and Janssen. This publication and the Global Academy for Medical Education are owned by the same parent company.
On Twitter @sherryboschert
EXPERT ANALYSIS FROM THE COASTAL DERMATOLOGY SYMPOSIUM
Secukinumab showed sustained efficacy in psoriasis
AMSTERDAM – The investigational biologic agent secukinumab continued to show strong efficacy through 52 weeks of treatment in a new secondary analysis of the pivotal phase III ERASURE trial.
For example, 60% of patients treated for moderate-to-severe chronic plaque psoriasis at the 300-mg dose of secukinumab continued to maintain a PASI 90 response through 52 weeks of therapy, Dr. Mark G. Lebwohl reported at the annual congress of the European Academy of Dermatology and Venereology.
Secukinumab is a fully human monoclonal antibody directed against interleukin-17A. While the Food and Drug Administration requested that the primary outcome in ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) should be the degree of skin clearing at week 12, the efficacy actually peaked at 16 weeks and was then sustained with only modest tailoff through the remainder of the 52-week study (see graphic).
This is important new information, Dr. Lebwohl noted, because 12-week outcomes don’t provide a full picture regarding potent therapies. Psoriasis is a chronic disease requiring long-term therapy, and some biologic agents now marketed for psoriasis tend to show a loss of effect over time. Reassuringly, the long-term ERASURE data show that’s not the case for secukinumab, explained Dr. Lebwohl, professor and chairman of the department of dermatology at Mt. Sinai Medical Center in New York.
ERASURE involved 738 patients randomized double-blind to secukinumab at either 150 mg or 300 mg, or to placebo. Following an initial loading-dose phase when the biologic was given subcutaneously once weekly for 5 weeks, it was then administered every 4 weeks for the remainder of the year-long trial.
The safety profile of secukinumab was similar to placebo with one exception: Upper respiratory tract infections were three- to fourfold more common in patients on the IL-17A inhibitor.
Novartis funded the ERASURE trial, whose primary results were recently published (N. Engl. J. Med. 2014;371:326-38). Dr. Lebwohl reported serving as a consultant to Novartis and more than a dozen other pharmaceutical companies.
AMSTERDAM – The investigational biologic agent secukinumab continued to show strong efficacy through 52 weeks of treatment in a new secondary analysis of the pivotal phase III ERASURE trial.
For example, 60% of patients treated for moderate-to-severe chronic plaque psoriasis at the 300-mg dose of secukinumab continued to maintain a PASI 90 response through 52 weeks of therapy, Dr. Mark G. Lebwohl reported at the annual congress of the European Academy of Dermatology and Venereology.
Secukinumab is a fully human monoclonal antibody directed against interleukin-17A. While the Food and Drug Administration requested that the primary outcome in ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) should be the degree of skin clearing at week 12, the efficacy actually peaked at 16 weeks and was then sustained with only modest tailoff through the remainder of the 52-week study (see graphic).
This is important new information, Dr. Lebwohl noted, because 12-week outcomes don’t provide a full picture regarding potent therapies. Psoriasis is a chronic disease requiring long-term therapy, and some biologic agents now marketed for psoriasis tend to show a loss of effect over time. Reassuringly, the long-term ERASURE data show that’s not the case for secukinumab, explained Dr. Lebwohl, professor and chairman of the department of dermatology at Mt. Sinai Medical Center in New York.
ERASURE involved 738 patients randomized double-blind to secukinumab at either 150 mg or 300 mg, or to placebo. Following an initial loading-dose phase when the biologic was given subcutaneously once weekly for 5 weeks, it was then administered every 4 weeks for the remainder of the year-long trial.
The safety profile of secukinumab was similar to placebo with one exception: Upper respiratory tract infections were three- to fourfold more common in patients on the IL-17A inhibitor.
Novartis funded the ERASURE trial, whose primary results were recently published (N. Engl. J. Med. 2014;371:326-38). Dr. Lebwohl reported serving as a consultant to Novartis and more than a dozen other pharmaceutical companies.
AMSTERDAM – The investigational biologic agent secukinumab continued to show strong efficacy through 52 weeks of treatment in a new secondary analysis of the pivotal phase III ERASURE trial.
For example, 60% of patients treated for moderate-to-severe chronic plaque psoriasis at the 300-mg dose of secukinumab continued to maintain a PASI 90 response through 52 weeks of therapy, Dr. Mark G. Lebwohl reported at the annual congress of the European Academy of Dermatology and Venereology.
Secukinumab is a fully human monoclonal antibody directed against interleukin-17A. While the Food and Drug Administration requested that the primary outcome in ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) should be the degree of skin clearing at week 12, the efficacy actually peaked at 16 weeks and was then sustained with only modest tailoff through the remainder of the 52-week study (see graphic).
This is important new information, Dr. Lebwohl noted, because 12-week outcomes don’t provide a full picture regarding potent therapies. Psoriasis is a chronic disease requiring long-term therapy, and some biologic agents now marketed for psoriasis tend to show a loss of effect over time. Reassuringly, the long-term ERASURE data show that’s not the case for secukinumab, explained Dr. Lebwohl, professor and chairman of the department of dermatology at Mt. Sinai Medical Center in New York.
ERASURE involved 738 patients randomized double-blind to secukinumab at either 150 mg or 300 mg, or to placebo. Following an initial loading-dose phase when the biologic was given subcutaneously once weekly for 5 weeks, it was then administered every 4 weeks for the remainder of the year-long trial.
The safety profile of secukinumab was similar to placebo with one exception: Upper respiratory tract infections were three- to fourfold more common in patients on the IL-17A inhibitor.
Novartis funded the ERASURE trial, whose primary results were recently published (N. Engl. J. Med. 2014;371:326-38). Dr. Lebwohl reported serving as a consultant to Novartis and more than a dozen other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point: Psoriasis patients’ initial strong clinical response to the interleukin-17A inhibitor secukinumab is sustained through 52 weeks of therapy.
Major finding: Sixty percent of patients with moderate-to-severe chronic plaque psoriasis had a PASI 90 response sustained through a full year of treatment.
Data source: The 52-week long, double-blind, multicenter ERASURE trial randomized 738 patients with moderate-to-severe chronic plaque psoriasis to secukinumab at 150 mg or 300 mg, or to placebo.
Disclosures: Novartis funded the study. Dr. Lebwohl reported serving as a consultant to the company.