User login
TAVR, SAVR share same infective endocarditis risk
PARIS – The risk of infective endocarditis following transcatheter aortic valve replacement (TAVR) for the treatment of severe aortic stenosis proved to be the same as after surgical replacement in a French national propensity score–matched study.
This finding from what is believed to be the largest-ever study of infective endocarditis following TAVR will come as a surprise to many physicians. It’s easy to mistakenly assume the risk of this feared complication is lower – and perhaps even negligible – in TAVR patients since the procedure doesn’t involve a significant surgical wound, it’s briefer, the hospital length of stay is shorter, and recovery time is markedly less than with surgical aortic valve replacement (SAVR).
Not so, Laurent Fauchier, MD, PhD, said in presenting the study findings at the annual congress of the European Society of Cardiology.
“Do not think there is a lower risk of infective endocarditis. Be aware, be careful, and provide appropriate antibiotic prophylaxis, just as surgeons do in SAVR. Don’t think, as I did, that with TAVR with no pacemaker implantation there is no risk of infective endocarditis. The TAVR valve is a device, it’s a prosthesis, and the risk is very similar to that of surgery,” advised Dr. Fauchier, a cardiologist at Francois Rabelais University in Tours, France.
He presented a study of all of the nearly 108,000 patients who underwent isolated TAVR or SAVR in France during 2010-2018. The data source was the French national administrative hospital discharge record system. Since the TAVR patients were overall markedly older and sicker than the SAVR patients, especially during the first years of the study, he and his coinvestigators performed propensity score matching using 30 variables, which enabled them to narrow the field of inquiry down to a carefully selected study population of 16,291 TAVR patients and an equal number of closely similar SAVR patients.
A total of 1,070 cases of infective endocarditis occurred during a mean follow-up of just over 2 years. The rate of hospital admission for this complication was 1.89% per year in the TAVR group and similar at 1.71% per year in the SAVR cohort.
Of note, all-cause mortality in TAVR patients who developed infective endocarditis was 1.32-fold greater than it was in SAVR patients with infective endocarditis, a statistically significant difference. The explanation for the increased mortality risk in the TAVR group probably has to do at least in part with an inability on the part of the investigators to fully capture and control for the TAVR group’s greater frailty, according to the cardiologist.
Risk factors for infective endocarditis shared in common by TAVR and SAVR patients included male gender, a higher Charlson Comorbidity Index score, and a greater frailty index. The main predictors unique to the TAVR patients were atrial fibrillation, anemia, and tricuspid regurgitation. And although pacemaker and defibrillator implantation were risk factors for infective endocarditis in the SAVR patients, it wasn’t predictive of increased risk in the TAVR population. Dr. Fauchier called this finding “quite reassuring” given that roughly 20% of the TAVR group received a pacemaker.
The causative microorganisms for infective endocarditis were essentially the same in the TAVR and SAVR groups, simplifying antimicrobial prophylaxis decision making.
Dr. Fauchier reported having no financial conflicts regarding the study, conducted free of commercial support. He serves as a consultant to and/or on speakers’ bureaus for Bayer, BMS Pfizer, Boehringer Ingelheim, Medtronic, and Novartis.
PARIS – The risk of infective endocarditis following transcatheter aortic valve replacement (TAVR) for the treatment of severe aortic stenosis proved to be the same as after surgical replacement in a French national propensity score–matched study.
This finding from what is believed to be the largest-ever study of infective endocarditis following TAVR will come as a surprise to many physicians. It’s easy to mistakenly assume the risk of this feared complication is lower – and perhaps even negligible – in TAVR patients since the procedure doesn’t involve a significant surgical wound, it’s briefer, the hospital length of stay is shorter, and recovery time is markedly less than with surgical aortic valve replacement (SAVR).
Not so, Laurent Fauchier, MD, PhD, said in presenting the study findings at the annual congress of the European Society of Cardiology.
“Do not think there is a lower risk of infective endocarditis. Be aware, be careful, and provide appropriate antibiotic prophylaxis, just as surgeons do in SAVR. Don’t think, as I did, that with TAVR with no pacemaker implantation there is no risk of infective endocarditis. The TAVR valve is a device, it’s a prosthesis, and the risk is very similar to that of surgery,” advised Dr. Fauchier, a cardiologist at Francois Rabelais University in Tours, France.
He presented a study of all of the nearly 108,000 patients who underwent isolated TAVR or SAVR in France during 2010-2018. The data source was the French national administrative hospital discharge record system. Since the TAVR patients were overall markedly older and sicker than the SAVR patients, especially during the first years of the study, he and his coinvestigators performed propensity score matching using 30 variables, which enabled them to narrow the field of inquiry down to a carefully selected study population of 16,291 TAVR patients and an equal number of closely similar SAVR patients.
A total of 1,070 cases of infective endocarditis occurred during a mean follow-up of just over 2 years. The rate of hospital admission for this complication was 1.89% per year in the TAVR group and similar at 1.71% per year in the SAVR cohort.
Of note, all-cause mortality in TAVR patients who developed infective endocarditis was 1.32-fold greater than it was in SAVR patients with infective endocarditis, a statistically significant difference. The explanation for the increased mortality risk in the TAVR group probably has to do at least in part with an inability on the part of the investigators to fully capture and control for the TAVR group’s greater frailty, according to the cardiologist.
Risk factors for infective endocarditis shared in common by TAVR and SAVR patients included male gender, a higher Charlson Comorbidity Index score, and a greater frailty index. The main predictors unique to the TAVR patients were atrial fibrillation, anemia, and tricuspid regurgitation. And although pacemaker and defibrillator implantation were risk factors for infective endocarditis in the SAVR patients, it wasn’t predictive of increased risk in the TAVR population. Dr. Fauchier called this finding “quite reassuring” given that roughly 20% of the TAVR group received a pacemaker.
The causative microorganisms for infective endocarditis were essentially the same in the TAVR and SAVR groups, simplifying antimicrobial prophylaxis decision making.
Dr. Fauchier reported having no financial conflicts regarding the study, conducted free of commercial support. He serves as a consultant to and/or on speakers’ bureaus for Bayer, BMS Pfizer, Boehringer Ingelheim, Medtronic, and Novartis.
PARIS – The risk of infective endocarditis following transcatheter aortic valve replacement (TAVR) for the treatment of severe aortic stenosis proved to be the same as after surgical replacement in a French national propensity score–matched study.
This finding from what is believed to be the largest-ever study of infective endocarditis following TAVR will come as a surprise to many physicians. It’s easy to mistakenly assume the risk of this feared complication is lower – and perhaps even negligible – in TAVR patients since the procedure doesn’t involve a significant surgical wound, it’s briefer, the hospital length of stay is shorter, and recovery time is markedly less than with surgical aortic valve replacement (SAVR).
Not so, Laurent Fauchier, MD, PhD, said in presenting the study findings at the annual congress of the European Society of Cardiology.
“Do not think there is a lower risk of infective endocarditis. Be aware, be careful, and provide appropriate antibiotic prophylaxis, just as surgeons do in SAVR. Don’t think, as I did, that with TAVR with no pacemaker implantation there is no risk of infective endocarditis. The TAVR valve is a device, it’s a prosthesis, and the risk is very similar to that of surgery,” advised Dr. Fauchier, a cardiologist at Francois Rabelais University in Tours, France.
He presented a study of all of the nearly 108,000 patients who underwent isolated TAVR or SAVR in France during 2010-2018. The data source was the French national administrative hospital discharge record system. Since the TAVR patients were overall markedly older and sicker than the SAVR patients, especially during the first years of the study, he and his coinvestigators performed propensity score matching using 30 variables, which enabled them to narrow the field of inquiry down to a carefully selected study population of 16,291 TAVR patients and an equal number of closely similar SAVR patients.
A total of 1,070 cases of infective endocarditis occurred during a mean follow-up of just over 2 years. The rate of hospital admission for this complication was 1.89% per year in the TAVR group and similar at 1.71% per year in the SAVR cohort.
Of note, all-cause mortality in TAVR patients who developed infective endocarditis was 1.32-fold greater than it was in SAVR patients with infective endocarditis, a statistically significant difference. The explanation for the increased mortality risk in the TAVR group probably has to do at least in part with an inability on the part of the investigators to fully capture and control for the TAVR group’s greater frailty, according to the cardiologist.
Risk factors for infective endocarditis shared in common by TAVR and SAVR patients included male gender, a higher Charlson Comorbidity Index score, and a greater frailty index. The main predictors unique to the TAVR patients were atrial fibrillation, anemia, and tricuspid regurgitation. And although pacemaker and defibrillator implantation were risk factors for infective endocarditis in the SAVR patients, it wasn’t predictive of increased risk in the TAVR population. Dr. Fauchier called this finding “quite reassuring” given that roughly 20% of the TAVR group received a pacemaker.
The causative microorganisms for infective endocarditis were essentially the same in the TAVR and SAVR groups, simplifying antimicrobial prophylaxis decision making.
Dr. Fauchier reported having no financial conflicts regarding the study, conducted free of commercial support. He serves as a consultant to and/or on speakers’ bureaus for Bayer, BMS Pfizer, Boehringer Ingelheim, Medtronic, and Novartis.
REPORTING FROM THE ESC CONGRESS 2019
Clip closure reduced bleeding after large lesion resection
Use of clip closure significantly reduced delayed bleeding in patients who underwent resections for large colorectal lesions, based on data from 235 individuals.
Source: American Gastroenterological Association
“Closure of a mucosal defect with clips after resection has long been considered to reduce the risk of bleeding,” but evidence to support this practice is limited, wrote Eduardo Albéniz, MD, of the Public University of Navarra (Spain), and colleagues.
In a study published in Gastroenterology, the researchers identified 235 consecutive patients who had resections of large nonpedunculated colorectal lesions from May 2016 to June 2018. Patients had an average or high risk of delayed bleeding and were randomized to receive scar closure with either 11-mm through-the-scope clips (119 patients) or no clip (116 patients).
Delayed bleeding occurred in 14 control patients (12.1%), compared with 6 clip patients (5%), for a risk reduction of 7%. The clip group included 68 cases (57%) of complete closure and 33 cases (28%) with partial closure, as well as 18 cases of failure to close (15%); only 1 case of delayed bleeding occurred in the clip group after completion of clip closure. On average, six clips were needed for complete closure.
None of the patients who experienced delayed bleeding required surgical or angiographic intervention, although 15 of the 20 patients with bleeding underwent additional endoscopy. Other adverse events included immediate bleeding in 21 clip patients and 18 controls that was managed with snare soft-tip coagulation. No deaths were reported in connection with the study.
Demographics were similar between the two groups, but the subset of patients with complete closure included more individuals aged 75 years and older and more cases with smaller polyps, compared with other subgroups, the researchers noted.
The study findings were limited by several factors, including the difficulty in predicting delayed bleeding, the potential for selection bias given the timing of patient randomization, the lack of information about polyps that were excluded from treatment, and the difficulty in completely closing the mucosal defects, the researchers noted. However, the results suggest that complete clip closure, despite its challenges, “displays a clear trend to reduce delayed bleeding risk,” and is worth an attempt.
The study was supported by the Spanish Society of Digestive Endoscopy. The researchers had no financial conflicts to disclose. MicroTech (Nanjing, China) contributed the clips used in the study.
SOURCE: Albéniz E et al. Gastroenterology. 2019 Jul 27. doi: 10.1053/j.gastro.2019.07.037.
With the advent of routine submucosal lifting prior to endoscopic mucosal resection, perforation now occurs less commonly; however, delayed bleeding following resection remains problematic given the aging population and increasing use of antithrombotic agents. In this study, clip closure resulted in a decrease in post-polypectomy bleeding in patients deemed to be at high risk (at least 8%) for delayed bleeding.
The protective benefit of clip closure was seen almost exclusively in patients who had complete closure of the defect, which was achieved in only 57% of procedures. Clinical efficacy is largely driven by endoscopist skill level and the ability to achieve complete closure. Notably, defects that were successfully clipped were smaller in size, had better accessibility, and were technically easier. Defining such procedural factors a priori is important and may influence whether one should attempt clip closure if complete clip closure is unlikely. Interestingly, the bleeding rate was higher in the control group in lesions proximal to the transverse colon, where clip closure is likely to be most beneficial and cost effective, based on emerging data. It’s worth noting that the clips used in this study were relatively small (11 mm), and not currently available in the United States, although most endoscopic clips function similarly.
Studies such as this provide evidenced-based medicine to endoscopic practice. Hemostatic clips were introduced nearly 20 years ago without evidence for their effectiveness. Future studies are needed, such as those that compare electrocautery-based resection of high-risk polyps with standard clips to over-the-scope clips, and those that compare electrocautery-based resection to cold snare resection.
Todd H. Baron, MD, is a gastroenterologist based at the University of North Carolina, Chapel Hill. He is a speaker and consultant for Olympus, Boston Scientific, and Cook Endoscopy.
With the advent of routine submucosal lifting prior to endoscopic mucosal resection, perforation now occurs less commonly; however, delayed bleeding following resection remains problematic given the aging population and increasing use of antithrombotic agents. In this study, clip closure resulted in a decrease in post-polypectomy bleeding in patients deemed to be at high risk (at least 8%) for delayed bleeding.
The protective benefit of clip closure was seen almost exclusively in patients who had complete closure of the defect, which was achieved in only 57% of procedures. Clinical efficacy is largely driven by endoscopist skill level and the ability to achieve complete closure. Notably, defects that were successfully clipped were smaller in size, had better accessibility, and were technically easier. Defining such procedural factors a priori is important and may influence whether one should attempt clip closure if complete clip closure is unlikely. Interestingly, the bleeding rate was higher in the control group in lesions proximal to the transverse colon, where clip closure is likely to be most beneficial and cost effective, based on emerging data. It’s worth noting that the clips used in this study were relatively small (11 mm), and not currently available in the United States, although most endoscopic clips function similarly.
Studies such as this provide evidenced-based medicine to endoscopic practice. Hemostatic clips were introduced nearly 20 years ago without evidence for their effectiveness. Future studies are needed, such as those that compare electrocautery-based resection of high-risk polyps with standard clips to over-the-scope clips, and those that compare electrocautery-based resection to cold snare resection.
Todd H. Baron, MD, is a gastroenterologist based at the University of North Carolina, Chapel Hill. He is a speaker and consultant for Olympus, Boston Scientific, and Cook Endoscopy.
With the advent of routine submucosal lifting prior to endoscopic mucosal resection, perforation now occurs less commonly; however, delayed bleeding following resection remains problematic given the aging population and increasing use of antithrombotic agents. In this study, clip closure resulted in a decrease in post-polypectomy bleeding in patients deemed to be at high risk (at least 8%) for delayed bleeding.
The protective benefit of clip closure was seen almost exclusively in patients who had complete closure of the defect, which was achieved in only 57% of procedures. Clinical efficacy is largely driven by endoscopist skill level and the ability to achieve complete closure. Notably, defects that were successfully clipped were smaller in size, had better accessibility, and were technically easier. Defining such procedural factors a priori is important and may influence whether one should attempt clip closure if complete clip closure is unlikely. Interestingly, the bleeding rate was higher in the control group in lesions proximal to the transverse colon, where clip closure is likely to be most beneficial and cost effective, based on emerging data. It’s worth noting that the clips used in this study were relatively small (11 mm), and not currently available in the United States, although most endoscopic clips function similarly.
Studies such as this provide evidenced-based medicine to endoscopic practice. Hemostatic clips were introduced nearly 20 years ago without evidence for their effectiveness. Future studies are needed, such as those that compare electrocautery-based resection of high-risk polyps with standard clips to over-the-scope clips, and those that compare electrocautery-based resection to cold snare resection.
Todd H. Baron, MD, is a gastroenterologist based at the University of North Carolina, Chapel Hill. He is a speaker and consultant for Olympus, Boston Scientific, and Cook Endoscopy.
Use of clip closure significantly reduced delayed bleeding in patients who underwent resections for large colorectal lesions, based on data from 235 individuals.
Source: American Gastroenterological Association
“Closure of a mucosal defect with clips after resection has long been considered to reduce the risk of bleeding,” but evidence to support this practice is limited, wrote Eduardo Albéniz, MD, of the Public University of Navarra (Spain), and colleagues.
In a study published in Gastroenterology, the researchers identified 235 consecutive patients who had resections of large nonpedunculated colorectal lesions from May 2016 to June 2018. Patients had an average or high risk of delayed bleeding and were randomized to receive scar closure with either 11-mm through-the-scope clips (119 patients) or no clip (116 patients).
Delayed bleeding occurred in 14 control patients (12.1%), compared with 6 clip patients (5%), for a risk reduction of 7%. The clip group included 68 cases (57%) of complete closure and 33 cases (28%) with partial closure, as well as 18 cases of failure to close (15%); only 1 case of delayed bleeding occurred in the clip group after completion of clip closure. On average, six clips were needed for complete closure.
None of the patients who experienced delayed bleeding required surgical or angiographic intervention, although 15 of the 20 patients with bleeding underwent additional endoscopy. Other adverse events included immediate bleeding in 21 clip patients and 18 controls that was managed with snare soft-tip coagulation. No deaths were reported in connection with the study.
Demographics were similar between the two groups, but the subset of patients with complete closure included more individuals aged 75 years and older and more cases with smaller polyps, compared with other subgroups, the researchers noted.
The study findings were limited by several factors, including the difficulty in predicting delayed bleeding, the potential for selection bias given the timing of patient randomization, the lack of information about polyps that were excluded from treatment, and the difficulty in completely closing the mucosal defects, the researchers noted. However, the results suggest that complete clip closure, despite its challenges, “displays a clear trend to reduce delayed bleeding risk,” and is worth an attempt.
The study was supported by the Spanish Society of Digestive Endoscopy. The researchers had no financial conflicts to disclose. MicroTech (Nanjing, China) contributed the clips used in the study.
SOURCE: Albéniz E et al. Gastroenterology. 2019 Jul 27. doi: 10.1053/j.gastro.2019.07.037.
Use of clip closure significantly reduced delayed bleeding in patients who underwent resections for large colorectal lesions, based on data from 235 individuals.
Source: American Gastroenterological Association
“Closure of a mucosal defect with clips after resection has long been considered to reduce the risk of bleeding,” but evidence to support this practice is limited, wrote Eduardo Albéniz, MD, of the Public University of Navarra (Spain), and colleagues.
In a study published in Gastroenterology, the researchers identified 235 consecutive patients who had resections of large nonpedunculated colorectal lesions from May 2016 to June 2018. Patients had an average or high risk of delayed bleeding and were randomized to receive scar closure with either 11-mm through-the-scope clips (119 patients) or no clip (116 patients).
Delayed bleeding occurred in 14 control patients (12.1%), compared with 6 clip patients (5%), for a risk reduction of 7%. The clip group included 68 cases (57%) of complete closure and 33 cases (28%) with partial closure, as well as 18 cases of failure to close (15%); only 1 case of delayed bleeding occurred in the clip group after completion of clip closure. On average, six clips were needed for complete closure.
None of the patients who experienced delayed bleeding required surgical or angiographic intervention, although 15 of the 20 patients with bleeding underwent additional endoscopy. Other adverse events included immediate bleeding in 21 clip patients and 18 controls that was managed with snare soft-tip coagulation. No deaths were reported in connection with the study.
Demographics were similar between the two groups, but the subset of patients with complete closure included more individuals aged 75 years and older and more cases with smaller polyps, compared with other subgroups, the researchers noted.
The study findings were limited by several factors, including the difficulty in predicting delayed bleeding, the potential for selection bias given the timing of patient randomization, the lack of information about polyps that were excluded from treatment, and the difficulty in completely closing the mucosal defects, the researchers noted. However, the results suggest that complete clip closure, despite its challenges, “displays a clear trend to reduce delayed bleeding risk,” and is worth an attempt.
The study was supported by the Spanish Society of Digestive Endoscopy. The researchers had no financial conflicts to disclose. MicroTech (Nanjing, China) contributed the clips used in the study.
SOURCE: Albéniz E et al. Gastroenterology. 2019 Jul 27. doi: 10.1053/j.gastro.2019.07.037.
FROM GASTROENTEROLOGY
Transcervical ablation of symptomatic uterine fibroids under US guidance
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
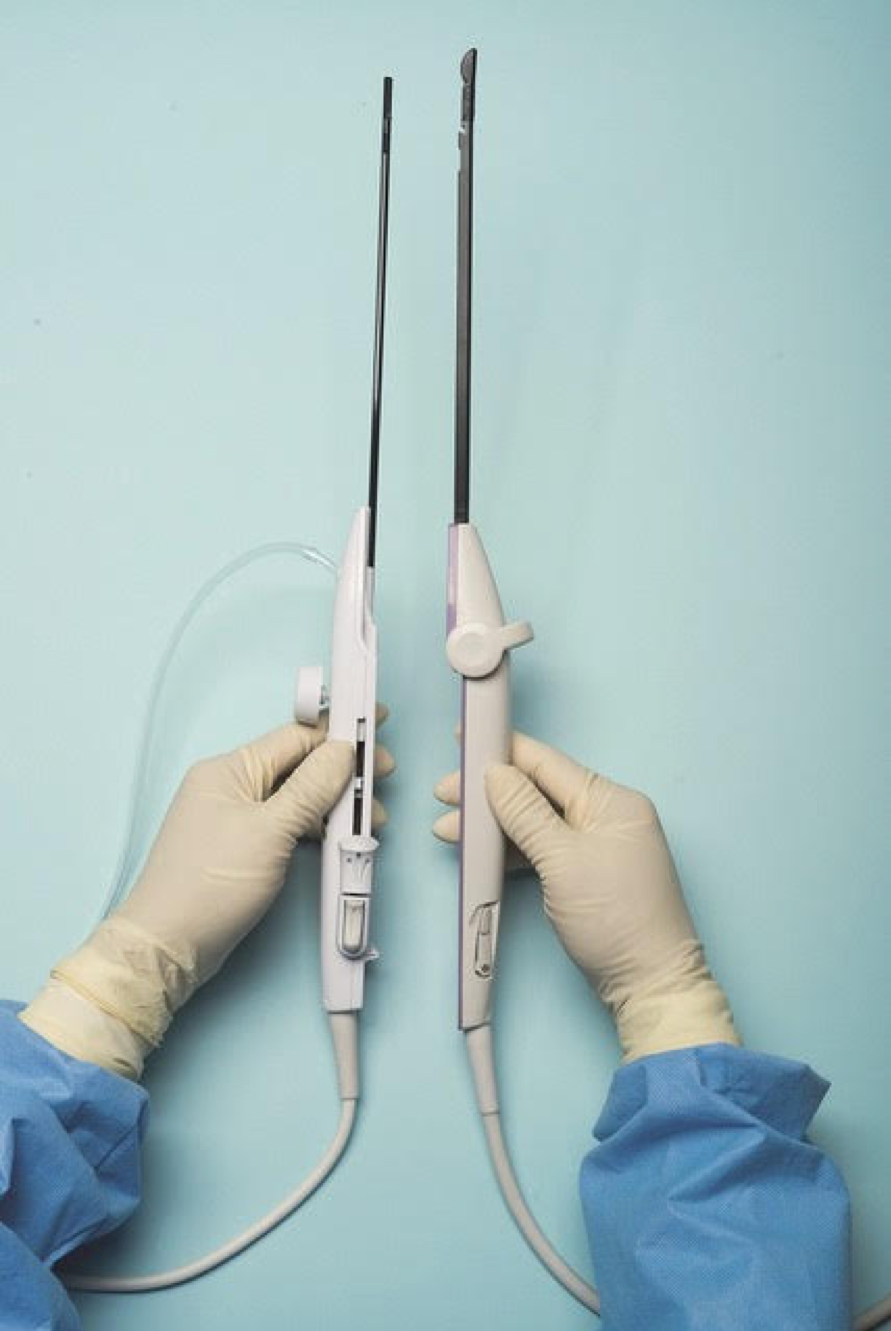
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
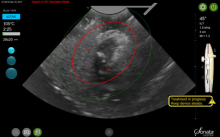
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
Treating uterine fibroids
Uterine fibroids are the most common benign tumor in women originating from the smooth muscles of the myometrium. While some women are asymptomatic, others experience pelvic pain, pressure, and abnormal uterine bleeding. Uterine fibroids also are associated with gastrointestinal disturbances; urinary problems; infertility; and obstetrical complications including miscarriages, preterm delivery, and cesarean sections.
The first successful abdominal myomectomy was described in 1845 but the procedure quickly fell out of favor because of unacceptably high mortality rates. Myomectomies require special skills and, at times, are associated with bleeding resulting in massive transfusions or sometimes unwanted hysterectomies. In 1922, Victor Bonney developed a uterine artery clamp which significantly decreased bleeding associated with morbidity and mortality.1
The latter part of the 20th century belonged to the minimally invasive surgery (MIS) evolution. Currently, video- or robotic-assisted laparoscopic myomectomies are increasingly employed in fertility-sparing surgery. In 2014, electromechanical morcellators came under scrutiny with concerns about iatrogenic dissemination of both benign and malignant tissues. A media storm ensued, resulting in the 2014 Food and Drug Administration black-box warning, and electromechanical morcellators were pulled from shelves. Data are being collected to quantify and understand these risks more clearly.
While exposing patients to even a small risk of dissemination of an occult uterine malignancy is unwise, MIS should not be abandoned altogether given its advantages to patients.2 Most recently, the American College of Obstetricians and Gynecologists concluded that, although abdominal hysterectomy or myomectomy may reduce the chance of spreading undiagnosed leiomyosarcoma cells, it is associated with increased morbidity, compared with noninvasive approaches, and ob.gyns. should engage in open decision-making processes and explain nonsurgical options with patients.3
The author of this Master Class, Dr. Charles Miller, a world-renowned MIS surgeon, will enlighten readers on the latest development in noninvasive treatment of symptomatic patients. The Sonata system, a promising transcervical (and thus incisionless) treatment modality utilizing intrauterine sonography–guided radiofrequency ablation for uterine fibroids which does not require general anesthesia or hospitalization. He believes that Sonata “will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.”
Dr. Miller is on the editorial advisory boards of numerous academic journals and serves as the editor of the award-winning Master Class in Gynecologic Surgery column. For this installment, he has stepped into the role of guest author. Dr. Miller has received numerous awards for his educational contributions and was recently granted the distinct honor of taking the lead in the March 28, 2020 Worldwide EndoMarch–Chicago. It is my pleasure to take part in this introduction.
Dr. Nezhat is director of minimally invasive surgery and robotics as well as the medical director of training and education at Northside Hospital, both in Atlanta. He is fellowship director at Atlanta Center for Special Minimally Invasive Surgery & Reproductive Medicine. Dr. Nezhat also is an adjunct professor of gynecology and obstetrics at Emory University, Atlanta, and is past president of the Society of Reproductive Surgeons and the AAGL. He reported that he has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
References
1. BJOG. 2018 Apr;125(5):586.
2. JAMA Oncol. 2015;1(1):78-9.
3. Obstet Gynecol. 2019 Mar;133(3):e238-48.
Uterine fibroids are the most common benign tumor in women originating from the smooth muscles of the myometrium. While some women are asymptomatic, others experience pelvic pain, pressure, and abnormal uterine bleeding. Uterine fibroids also are associated with gastrointestinal disturbances; urinary problems; infertility; and obstetrical complications including miscarriages, preterm delivery, and cesarean sections.
The first successful abdominal myomectomy was described in 1845 but the procedure quickly fell out of favor because of unacceptably high mortality rates. Myomectomies require special skills and, at times, are associated with bleeding resulting in massive transfusions or sometimes unwanted hysterectomies. In 1922, Victor Bonney developed a uterine artery clamp which significantly decreased bleeding associated with morbidity and mortality.1
The latter part of the 20th century belonged to the minimally invasive surgery (MIS) evolution. Currently, video- or robotic-assisted laparoscopic myomectomies are increasingly employed in fertility-sparing surgery. In 2014, electromechanical morcellators came under scrutiny with concerns about iatrogenic dissemination of both benign and malignant tissues. A media storm ensued, resulting in the 2014 Food and Drug Administration black-box warning, and electromechanical morcellators were pulled from shelves. Data are being collected to quantify and understand these risks more clearly.
While exposing patients to even a small risk of dissemination of an occult uterine malignancy is unwise, MIS should not be abandoned altogether given its advantages to patients.2 Most recently, the American College of Obstetricians and Gynecologists concluded that, although abdominal hysterectomy or myomectomy may reduce the chance of spreading undiagnosed leiomyosarcoma cells, it is associated with increased morbidity, compared with noninvasive approaches, and ob.gyns. should engage in open decision-making processes and explain nonsurgical options with patients.3
The author of this Master Class, Dr. Charles Miller, a world-renowned MIS surgeon, will enlighten readers on the latest development in noninvasive treatment of symptomatic patients. The Sonata system, a promising transcervical (and thus incisionless) treatment modality utilizing intrauterine sonography–guided radiofrequency ablation for uterine fibroids which does not require general anesthesia or hospitalization. He believes that Sonata “will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.”
Dr. Miller is on the editorial advisory boards of numerous academic journals and serves as the editor of the award-winning Master Class in Gynecologic Surgery column. For this installment, he has stepped into the role of guest author. Dr. Miller has received numerous awards for his educational contributions and was recently granted the distinct honor of taking the lead in the March 28, 2020 Worldwide EndoMarch–Chicago. It is my pleasure to take part in this introduction.
Dr. Nezhat is director of minimally invasive surgery and robotics as well as the medical director of training and education at Northside Hospital, both in Atlanta. He is fellowship director at Atlanta Center for Special Minimally Invasive Surgery & Reproductive Medicine. Dr. Nezhat also is an adjunct professor of gynecology and obstetrics at Emory University, Atlanta, and is past president of the Society of Reproductive Surgeons and the AAGL. He reported that he has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
References
1. BJOG. 2018 Apr;125(5):586.
2. JAMA Oncol. 2015;1(1):78-9.
3. Obstet Gynecol. 2019 Mar;133(3):e238-48.
Uterine fibroids are the most common benign tumor in women originating from the smooth muscles of the myometrium. While some women are asymptomatic, others experience pelvic pain, pressure, and abnormal uterine bleeding. Uterine fibroids also are associated with gastrointestinal disturbances; urinary problems; infertility; and obstetrical complications including miscarriages, preterm delivery, and cesarean sections.
The first successful abdominal myomectomy was described in 1845 but the procedure quickly fell out of favor because of unacceptably high mortality rates. Myomectomies require special skills and, at times, are associated with bleeding resulting in massive transfusions or sometimes unwanted hysterectomies. In 1922, Victor Bonney developed a uterine artery clamp which significantly decreased bleeding associated with morbidity and mortality.1
The latter part of the 20th century belonged to the minimally invasive surgery (MIS) evolution. Currently, video- or robotic-assisted laparoscopic myomectomies are increasingly employed in fertility-sparing surgery. In 2014, electromechanical morcellators came under scrutiny with concerns about iatrogenic dissemination of both benign and malignant tissues. A media storm ensued, resulting in the 2014 Food and Drug Administration black-box warning, and electromechanical morcellators were pulled from shelves. Data are being collected to quantify and understand these risks more clearly.
While exposing patients to even a small risk of dissemination of an occult uterine malignancy is unwise, MIS should not be abandoned altogether given its advantages to patients.2 Most recently, the American College of Obstetricians and Gynecologists concluded that, although abdominal hysterectomy or myomectomy may reduce the chance of spreading undiagnosed leiomyosarcoma cells, it is associated with increased morbidity, compared with noninvasive approaches, and ob.gyns. should engage in open decision-making processes and explain nonsurgical options with patients.3
The author of this Master Class, Dr. Charles Miller, a world-renowned MIS surgeon, will enlighten readers on the latest development in noninvasive treatment of symptomatic patients. The Sonata system, a promising transcervical (and thus incisionless) treatment modality utilizing intrauterine sonography–guided radiofrequency ablation for uterine fibroids which does not require general anesthesia or hospitalization. He believes that Sonata “will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.”
Dr. Miller is on the editorial advisory boards of numerous academic journals and serves as the editor of the award-winning Master Class in Gynecologic Surgery column. For this installment, he has stepped into the role of guest author. Dr. Miller has received numerous awards for his educational contributions and was recently granted the distinct honor of taking the lead in the March 28, 2020 Worldwide EndoMarch–Chicago. It is my pleasure to take part in this introduction.
Dr. Nezhat is director of minimally invasive surgery and robotics as well as the medical director of training and education at Northside Hospital, both in Atlanta. He is fellowship director at Atlanta Center for Special Minimally Invasive Surgery & Reproductive Medicine. Dr. Nezhat also is an adjunct professor of gynecology and obstetrics at Emory University, Atlanta, and is past president of the Society of Reproductive Surgeons and the AAGL. He reported that he has no disclosures relevant to this Master Class. Email him at obnews@mdedge.com.
References
1. BJOG. 2018 Apr;125(5):586.
2. JAMA Oncol. 2015;1(1):78-9.
3. Obstet Gynecol. 2019 Mar;133(3):e238-48.
A novel approach to complete transobturator sling mesh removal

Additional videos from SGS are available here, including these recent offerings:
- Embryologic development of the external genitalia as it relates to vaginoplasty for the transgender woman
- A stepwise approach to the difficult bladder flap to prevent urinary tract injury during laparoscopic hysterectomy
- Novel method to demarcate bladder dissection during posthysterectomy sacrocolpopexy

Additional videos from SGS are available here, including these recent offerings:
- Embryologic development of the external genitalia as it relates to vaginoplasty for the transgender woman
- A stepwise approach to the difficult bladder flap to prevent urinary tract injury during laparoscopic hysterectomy
- Novel method to demarcate bladder dissection during posthysterectomy sacrocolpopexy

Additional videos from SGS are available here, including these recent offerings:
- Embryologic development of the external genitalia as it relates to vaginoplasty for the transgender woman
- A stepwise approach to the difficult bladder flap to prevent urinary tract injury during laparoscopic hysterectomy
- Novel method to demarcate bladder dissection during posthysterectomy sacrocolpopexy
Tranexamic acid does not increase complications in high-risk joint replacement surgery patients
A study has found that administering tranexamic acid (TXA) to high-risk patients undergoing total joint arthroplasty (TJA) does not increase their odds of adverse outcomes.
“The inclusion of high-risk patients in our study increases the generalizability of our findings and is consistent with the previous studies that showed no increase in complications when TXA is administered to TJA patients,” wrote Steven B. Porter, MD, of the Mayo Clinic in Jacksonville, Fla., and coauthors. The study was published in the Journal of Arthroplasty.
To determine the safety of TXA in patients at risk for thrombotic complications, the researchers investigated 38,220 patients who underwent total knee or total hip arthroplasty between 2011 and 2017 at the Mayo Clinic. Of those patients, 20,501 (54%) patients received TXA during their operation and 17,719 (46%) did not. Overall, 8,877 were classified as “high-risk” cases, which meant they had one or more cardiovascular disease or thromboembolic event before surgery.
After multivariable analysis, high risk-patients who received TXA had no significant difference in adverse outcome odds, compared with high-risk patients who did not receive TXA (odds ratio, 1.00; 95% confidence interval, 0.85-1.18). After 90 days, high-risk patients who did not receive TXA were more likely than those who received TXA to experience deep vein thrombosis (2.3% vs 0.8%, P less than .001), pulmonary embolism (1.7% vs 1.0%, P less than .001), cerebrovascular accident (0.8% vs. 0.4%, P less than .001), or death (0.5% vs. 0.4%, P less than .001).
The authors noted their study’s limitations, including a higher baseline incidence of risk factors in high-risk patients who did not receive TXA, compared with high-risk patients who did, which could have led to that group being “self-selected” to not receive TXA. In addition, all medical histories and rates of complications were based on ICD codes, which may have been inaccurate and therefore led to mischaracterized risk or miscoded postoperative complications.
The study was funded by the Mayo Clinic’s Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. No conflicts of interest were reported.
SOURCE: Porter SB et al. J Arthroplasty. 2019 Aug 17. doi: 10.1016/j.arth.2019.08.015.
A study has found that administering tranexamic acid (TXA) to high-risk patients undergoing total joint arthroplasty (TJA) does not increase their odds of adverse outcomes.
“The inclusion of high-risk patients in our study increases the generalizability of our findings and is consistent with the previous studies that showed no increase in complications when TXA is administered to TJA patients,” wrote Steven B. Porter, MD, of the Mayo Clinic in Jacksonville, Fla., and coauthors. The study was published in the Journal of Arthroplasty.
To determine the safety of TXA in patients at risk for thrombotic complications, the researchers investigated 38,220 patients who underwent total knee or total hip arthroplasty between 2011 and 2017 at the Mayo Clinic. Of those patients, 20,501 (54%) patients received TXA during their operation and 17,719 (46%) did not. Overall, 8,877 were classified as “high-risk” cases, which meant they had one or more cardiovascular disease or thromboembolic event before surgery.
After multivariable analysis, high risk-patients who received TXA had no significant difference in adverse outcome odds, compared with high-risk patients who did not receive TXA (odds ratio, 1.00; 95% confidence interval, 0.85-1.18). After 90 days, high-risk patients who did not receive TXA were more likely than those who received TXA to experience deep vein thrombosis (2.3% vs 0.8%, P less than .001), pulmonary embolism (1.7% vs 1.0%, P less than .001), cerebrovascular accident (0.8% vs. 0.4%, P less than .001), or death (0.5% vs. 0.4%, P less than .001).
The authors noted their study’s limitations, including a higher baseline incidence of risk factors in high-risk patients who did not receive TXA, compared with high-risk patients who did, which could have led to that group being “self-selected” to not receive TXA. In addition, all medical histories and rates of complications were based on ICD codes, which may have been inaccurate and therefore led to mischaracterized risk or miscoded postoperative complications.
The study was funded by the Mayo Clinic’s Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. No conflicts of interest were reported.
SOURCE: Porter SB et al. J Arthroplasty. 2019 Aug 17. doi: 10.1016/j.arth.2019.08.015.
A study has found that administering tranexamic acid (TXA) to high-risk patients undergoing total joint arthroplasty (TJA) does not increase their odds of adverse outcomes.
“The inclusion of high-risk patients in our study increases the generalizability of our findings and is consistent with the previous studies that showed no increase in complications when TXA is administered to TJA patients,” wrote Steven B. Porter, MD, of the Mayo Clinic in Jacksonville, Fla., and coauthors. The study was published in the Journal of Arthroplasty.
To determine the safety of TXA in patients at risk for thrombotic complications, the researchers investigated 38,220 patients who underwent total knee or total hip arthroplasty between 2011 and 2017 at the Mayo Clinic. Of those patients, 20,501 (54%) patients received TXA during their operation and 17,719 (46%) did not. Overall, 8,877 were classified as “high-risk” cases, which meant they had one or more cardiovascular disease or thromboembolic event before surgery.
After multivariable analysis, high risk-patients who received TXA had no significant difference in adverse outcome odds, compared with high-risk patients who did not receive TXA (odds ratio, 1.00; 95% confidence interval, 0.85-1.18). After 90 days, high-risk patients who did not receive TXA were more likely than those who received TXA to experience deep vein thrombosis (2.3% vs 0.8%, P less than .001), pulmonary embolism (1.7% vs 1.0%, P less than .001), cerebrovascular accident (0.8% vs. 0.4%, P less than .001), or death (0.5% vs. 0.4%, P less than .001).
The authors noted their study’s limitations, including a higher baseline incidence of risk factors in high-risk patients who did not receive TXA, compared with high-risk patients who did, which could have led to that group being “self-selected” to not receive TXA. In addition, all medical histories and rates of complications were based on ICD codes, which may have been inaccurate and therefore led to mischaracterized risk or miscoded postoperative complications.
The study was funded by the Mayo Clinic’s Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. No conflicts of interest were reported.
SOURCE: Porter SB et al. J Arthroplasty. 2019 Aug 17. doi: 10.1016/j.arth.2019.08.015.
FROM THE JOURNAL OF ARTHROPLASTY
Key clinical point: Administering tranexamic acid to high-risk patients undergoing joint replacement surgery does not increase the odds of adverse outcomes.
Major finding: After multivariable analysis, high-risk patients who received tranexamic acid had no significant difference in adverse outcome odds, compared with high-risk patients who did not receive tranexamic acid (odd ratio, 1.00; 95% confidence interval, 0.85-1.18).
Study details: A retrospective case-control study of 38,220 patients who underwent primary total knee or total hip arthroplasty between 2011 and 2017.
Disclosures: The study was funded by the Mayo Clinic’s Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. No conflicts of interest were reported.
Source: Porter SB et al. J Arthroplasty. 2019 Aug 17. doi: 10.1016/j.arth.2019.08.015.
Early infusion of mononuclear cells may benefit stroke patients
, results from a single-arm, phase I trial demonstrated. Unlike autologous mesenchymal stem cells, mononuclear cells (MNCs) do not require passage in culture, which allows for testing in the early poststroke time therapy window.
Bone marrow MNCs are attractive in regenerative medicine studies because they can be rapidly isolated; are enriched with hematopoietic, mesenchymal, and endothelial progenitor cells; and permit autologous applications. “The regenerative potential of bone marrow–derived MNCs is attributed to various mechanisms that impact stroke recovery,” researchers led by Sean I. Savitz, MD, wrote in a study published online Sept. 17 in Stem Cells. “These cells migrate to the site of injury, release cytokines and other trophic factors, decrease proinflammatory and upregulate anti-inflammatory pathways, and enhance angiogenesis, neurogenesis, and synaptogenesis.”
For the trial, Dr. Savitz, MD, director of the Institute for Stroke and Cerebrovascular Disease at UTHealth, Houston, and colleagues recruited 25 patients to receive an IV dose of their own bone marrow mononuclear cells within 72 hours after stroke onset, a time frame supported by previous preclinical studies. They followed the patients for 1 year and compared the results with a control group of 185 patients who received conventional poststroke treatment. Primary outcomes were study-related serious adverse events and the proportion of patients successfully completing study intervention.
The researchers reported results from 25 patients who received bone marrow MNCs. The mean age of patients in the MNC and control groups were 61 and 63 years, respectively, 53% were female, and 69% were white. No study-related adverse events were observed in the MNC group, but three (12%) had infarct expansion between enrollment and harvest and underwent elective hemicraniectomy after cell infusion.
Advanced magnetic resonance imaging revealed that the average mean fractional anisotropy (FA), a measure of structural integrity and directional coherence of axonal fibers, within the ipsilesional pons was decreased between 1 and 3 months after stroke, “which translated to a relative FA [rFA] comparable with prior reports at this time point,” the researchers wrote. “However, by 6 months, mean rFA began to increase and by 2 years it was significantly higher than at 1 month. This increasing trend in rFA may imply an increase in axonal and fiber coherence as well as thickness in myelin sheets, suggesting microstructural repair. However, without a comparable group of stroke patients not treated with MNCs, we cannot directly ascribe the white matter changes to MNC treatment.”
In light of the findings, the researchers concluded that MNCs “pose no additional harm in ischemic stroke patients when given during the acute phase, doses up to 10 million cells per kilogram are tolerated, and it is feasible to perform a bone marrow harvest and reinfusion of MNCs for a wide range of stroke patients. Well-designed RCTs are needed to further assess safety and efficacy of this novel investigational approach to enhance stroke recovery.”
The study was supported by grants from the National Institutes of Health. Dr. Savitz and many of his coauthors disclosed having numerous financial ties to the pharmaceutical and biotechnology industries.
, results from a single-arm, phase I trial demonstrated. Unlike autologous mesenchymal stem cells, mononuclear cells (MNCs) do not require passage in culture, which allows for testing in the early poststroke time therapy window.
Bone marrow MNCs are attractive in regenerative medicine studies because they can be rapidly isolated; are enriched with hematopoietic, mesenchymal, and endothelial progenitor cells; and permit autologous applications. “The regenerative potential of bone marrow–derived MNCs is attributed to various mechanisms that impact stroke recovery,” researchers led by Sean I. Savitz, MD, wrote in a study published online Sept. 17 in Stem Cells. “These cells migrate to the site of injury, release cytokines and other trophic factors, decrease proinflammatory and upregulate anti-inflammatory pathways, and enhance angiogenesis, neurogenesis, and synaptogenesis.”
For the trial, Dr. Savitz, MD, director of the Institute for Stroke and Cerebrovascular Disease at UTHealth, Houston, and colleagues recruited 25 patients to receive an IV dose of their own bone marrow mononuclear cells within 72 hours after stroke onset, a time frame supported by previous preclinical studies. They followed the patients for 1 year and compared the results with a control group of 185 patients who received conventional poststroke treatment. Primary outcomes were study-related serious adverse events and the proportion of patients successfully completing study intervention.
The researchers reported results from 25 patients who received bone marrow MNCs. The mean age of patients in the MNC and control groups were 61 and 63 years, respectively, 53% were female, and 69% were white. No study-related adverse events were observed in the MNC group, but three (12%) had infarct expansion between enrollment and harvest and underwent elective hemicraniectomy after cell infusion.
Advanced magnetic resonance imaging revealed that the average mean fractional anisotropy (FA), a measure of structural integrity and directional coherence of axonal fibers, within the ipsilesional pons was decreased between 1 and 3 months after stroke, “which translated to a relative FA [rFA] comparable with prior reports at this time point,” the researchers wrote. “However, by 6 months, mean rFA began to increase and by 2 years it was significantly higher than at 1 month. This increasing trend in rFA may imply an increase in axonal and fiber coherence as well as thickness in myelin sheets, suggesting microstructural repair. However, without a comparable group of stroke patients not treated with MNCs, we cannot directly ascribe the white matter changes to MNC treatment.”
In light of the findings, the researchers concluded that MNCs “pose no additional harm in ischemic stroke patients when given during the acute phase, doses up to 10 million cells per kilogram are tolerated, and it is feasible to perform a bone marrow harvest and reinfusion of MNCs for a wide range of stroke patients. Well-designed RCTs are needed to further assess safety and efficacy of this novel investigational approach to enhance stroke recovery.”
The study was supported by grants from the National Institutes of Health. Dr. Savitz and many of his coauthors disclosed having numerous financial ties to the pharmaceutical and biotechnology industries.
, results from a single-arm, phase I trial demonstrated. Unlike autologous mesenchymal stem cells, mononuclear cells (MNCs) do not require passage in culture, which allows for testing in the early poststroke time therapy window.
Bone marrow MNCs are attractive in regenerative medicine studies because they can be rapidly isolated; are enriched with hematopoietic, mesenchymal, and endothelial progenitor cells; and permit autologous applications. “The regenerative potential of bone marrow–derived MNCs is attributed to various mechanisms that impact stroke recovery,” researchers led by Sean I. Savitz, MD, wrote in a study published online Sept. 17 in Stem Cells. “These cells migrate to the site of injury, release cytokines and other trophic factors, decrease proinflammatory and upregulate anti-inflammatory pathways, and enhance angiogenesis, neurogenesis, and synaptogenesis.”
For the trial, Dr. Savitz, MD, director of the Institute for Stroke and Cerebrovascular Disease at UTHealth, Houston, and colleagues recruited 25 patients to receive an IV dose of their own bone marrow mononuclear cells within 72 hours after stroke onset, a time frame supported by previous preclinical studies. They followed the patients for 1 year and compared the results with a control group of 185 patients who received conventional poststroke treatment. Primary outcomes were study-related serious adverse events and the proportion of patients successfully completing study intervention.
The researchers reported results from 25 patients who received bone marrow MNCs. The mean age of patients in the MNC and control groups were 61 and 63 years, respectively, 53% were female, and 69% were white. No study-related adverse events were observed in the MNC group, but three (12%) had infarct expansion between enrollment and harvest and underwent elective hemicraniectomy after cell infusion.
Advanced magnetic resonance imaging revealed that the average mean fractional anisotropy (FA), a measure of structural integrity and directional coherence of axonal fibers, within the ipsilesional pons was decreased between 1 and 3 months after stroke, “which translated to a relative FA [rFA] comparable with prior reports at this time point,” the researchers wrote. “However, by 6 months, mean rFA began to increase and by 2 years it was significantly higher than at 1 month. This increasing trend in rFA may imply an increase in axonal and fiber coherence as well as thickness in myelin sheets, suggesting microstructural repair. However, without a comparable group of stroke patients not treated with MNCs, we cannot directly ascribe the white matter changes to MNC treatment.”
In light of the findings, the researchers concluded that MNCs “pose no additional harm in ischemic stroke patients when given during the acute phase, doses up to 10 million cells per kilogram are tolerated, and it is feasible to perform a bone marrow harvest and reinfusion of MNCs for a wide range of stroke patients. Well-designed RCTs are needed to further assess safety and efficacy of this novel investigational approach to enhance stroke recovery.”
The study was supported by grants from the National Institutes of Health. Dr. Savitz and many of his coauthors disclosed having numerous financial ties to the pharmaceutical and biotechnology industries.
FROM STEM CELLS
High mortality rates trail tracheostomy patients
findings of a large retrospective study suggest.
Current outcome prediction tools to support decision making regarding tracheostomies are limited, wrote Anuj B. Mehta, MD, of National Jewish Health in Denver, and colleagues. “This study provides novel and in-depth insight into mortality and health care utilization following tracheostomy not previously described at the population-level.”
In a study published in Critical Care Medicine, the researchers reviewed data from 8,343 nonsurgical patients seen in California hospitals from 2012 to 2013 who received a tracheostomy for acute respiratory failure.
Overall, the 1-year mortality rate for patients who had tracheostomies (the primary outcome) was 46.5%, with in-hospital mortality of 18.9% and 30-day mortality of 22.1%. Pneumonia was the most common diagnosis for patients with respiratory failure (79%) and some had an additional diagnosis, such as severe sepsis (56%).
Patients aged 65 years and older had significantly higher mortality than those under 65 (54.7% vs. 36.5%). The average age of the patients was 65 years; approximately 46% were women and 48% were white. The median survival for adults aged 65 years and older was 175 days, compared with median survival of more than a year for younger patients.
Secondary outcomes included discharge destination, hospital readmission, and health care utilization. A majority (86%) of patients were discharged to a long-term care facility, while 11% were sent home and approximately 3% were discharged to other destinations.
Nearly two-thirds (60%) of patients were readmitted to the hospital within a year of tracheostomy, and readmission was more common among older adults, compared with younger (66% vs. 55%).
In addition, just over one-third of all patients (36%) spent more than 50% of their days alive in the hospital in short-term acute care, and this rate was significantly higher for patients aged 65 years and older, compared with those under 65 (43% vs. 29%). On average, the total hospital cost for patients who survived the first year after tracheostomy was $215,369, with no significant difference in average cost among age groups.
The study findings were limited by several factors including the use of data from a single state, possible misclassification of billing codes, and inability to measure quality of life, the researchers noted.
However, “our findings of high mortality, low median survival for older patients, high readmission rates, potentially burdensome cost, and informative outcome trajectories provide significant insight into long-term outcomes following tracheostomy,” they concluded.
Dr. Mehta and several colleagues reported receiving funding from the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Mehta AB et al. Crit Care Med. 2019 Aug 8. doi: 10.1097/CCM.0000000000003959.
findings of a large retrospective study suggest.
Current outcome prediction tools to support decision making regarding tracheostomies are limited, wrote Anuj B. Mehta, MD, of National Jewish Health in Denver, and colleagues. “This study provides novel and in-depth insight into mortality and health care utilization following tracheostomy not previously described at the population-level.”
In a study published in Critical Care Medicine, the researchers reviewed data from 8,343 nonsurgical patients seen in California hospitals from 2012 to 2013 who received a tracheostomy for acute respiratory failure.
Overall, the 1-year mortality rate for patients who had tracheostomies (the primary outcome) was 46.5%, with in-hospital mortality of 18.9% and 30-day mortality of 22.1%. Pneumonia was the most common diagnosis for patients with respiratory failure (79%) and some had an additional diagnosis, such as severe sepsis (56%).
Patients aged 65 years and older had significantly higher mortality than those under 65 (54.7% vs. 36.5%). The average age of the patients was 65 years; approximately 46% were women and 48% were white. The median survival for adults aged 65 years and older was 175 days, compared with median survival of more than a year for younger patients.
Secondary outcomes included discharge destination, hospital readmission, and health care utilization. A majority (86%) of patients were discharged to a long-term care facility, while 11% were sent home and approximately 3% were discharged to other destinations.
Nearly two-thirds (60%) of patients were readmitted to the hospital within a year of tracheostomy, and readmission was more common among older adults, compared with younger (66% vs. 55%).
In addition, just over one-third of all patients (36%) spent more than 50% of their days alive in the hospital in short-term acute care, and this rate was significantly higher for patients aged 65 years and older, compared with those under 65 (43% vs. 29%). On average, the total hospital cost for patients who survived the first year after tracheostomy was $215,369, with no significant difference in average cost among age groups.
The study findings were limited by several factors including the use of data from a single state, possible misclassification of billing codes, and inability to measure quality of life, the researchers noted.
However, “our findings of high mortality, low median survival for older patients, high readmission rates, potentially burdensome cost, and informative outcome trajectories provide significant insight into long-term outcomes following tracheostomy,” they concluded.
Dr. Mehta and several colleagues reported receiving funding from the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Mehta AB et al. Crit Care Med. 2019 Aug 8. doi: 10.1097/CCM.0000000000003959.
findings of a large retrospective study suggest.
Current outcome prediction tools to support decision making regarding tracheostomies are limited, wrote Anuj B. Mehta, MD, of National Jewish Health in Denver, and colleagues. “This study provides novel and in-depth insight into mortality and health care utilization following tracheostomy not previously described at the population-level.”
In a study published in Critical Care Medicine, the researchers reviewed data from 8,343 nonsurgical patients seen in California hospitals from 2012 to 2013 who received a tracheostomy for acute respiratory failure.
Overall, the 1-year mortality rate for patients who had tracheostomies (the primary outcome) was 46.5%, with in-hospital mortality of 18.9% and 30-day mortality of 22.1%. Pneumonia was the most common diagnosis for patients with respiratory failure (79%) and some had an additional diagnosis, such as severe sepsis (56%).
Patients aged 65 years and older had significantly higher mortality than those under 65 (54.7% vs. 36.5%). The average age of the patients was 65 years; approximately 46% were women and 48% were white. The median survival for adults aged 65 years and older was 175 days, compared with median survival of more than a year for younger patients.
Secondary outcomes included discharge destination, hospital readmission, and health care utilization. A majority (86%) of patients were discharged to a long-term care facility, while 11% were sent home and approximately 3% were discharged to other destinations.
Nearly two-thirds (60%) of patients were readmitted to the hospital within a year of tracheostomy, and readmission was more common among older adults, compared with younger (66% vs. 55%).
In addition, just over one-third of all patients (36%) spent more than 50% of their days alive in the hospital in short-term acute care, and this rate was significantly higher for patients aged 65 years and older, compared with those under 65 (43% vs. 29%). On average, the total hospital cost for patients who survived the first year after tracheostomy was $215,369, with no significant difference in average cost among age groups.
The study findings were limited by several factors including the use of data from a single state, possible misclassification of billing codes, and inability to measure quality of life, the researchers noted.
However, “our findings of high mortality, low median survival for older patients, high readmission rates, potentially burdensome cost, and informative outcome trajectories provide significant insight into long-term outcomes following tracheostomy,” they concluded.
Dr. Mehta and several colleagues reported receiving funding from the National Institutes of Health. The researchers had no financial conflicts to disclose.
SOURCE: Mehta AB et al. Crit Care Med. 2019 Aug 8. doi: 10.1097/CCM.0000000000003959.
FROM CRITICAL CARE MEDICINE
Trial of mesh vs. hysterectomy for prolapse yields inconclusive results
Transvaginal mesh hysteropexy for symptomatic uterovaginal prolapse may not significantly reduce treatment failure after 3 years, compared with vaginal hysterectomy with uterosacral ligament suspension, according to randomized trial results.
Nevertheless, “the point estimate favored hysteropexy,” the study authors wrote in JAMA. The 36-month cumulative treatment failure outcomes – defined as retreatment of prolapse, prolapse beyond the hymen, or prolapse symptoms – were 33% for patients who underwent hysteropexy, compared with 42% for patients who underwent hysterectomy. In addition, mean operative time was 45 minutes less for patients who underwent hysteropexy.
The publication follows the Food and Drug Administration’s ruling in April 2019 that manufacturers must cease marketing transvaginal mesh kits for repair of anterior or apical compartment prolapse. The investigators plan to continue evaluating patient outcomes to 5 years, and they noted that longer follow-up may lead to different conclusions.
From a class II device to class III
Surgical repair of uterovaginal prolapse is common. Although vaginal hysterectomy is the procedure of choice for many surgeons, “uterine-sparing suspension techniques ... are increasing in usage,” wrote Charles W. Nager, MD, chair and professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, and coauthors. However, few high-quality, long-term studies have compared apical transvaginal mesh with native tissue procedures.
The FDA first approved a mesh device for transvaginal repair of prolapse in 2002. In 2008, the agency notified clinicians and patients about an increase in adverse event reports related to vaginal mesh. It later advised that mesh for the treatment of pelvic organ prolapse does not conclusively improve clinical outcomes and that serious adverse events are not rare.
In 2016, the FDA reclassified surgical mesh to repair pelvic organ prolapse transvaginally as high risk, citing safety concerns such as severe pelvic pain and organ perforation. And in April 2019, the FDA ordered companies to stop selling transvaginal mesh intended for pelvic organ prolapse repair. “Even though these products can no longer be used in patients moving forward, [manufacturers] are required to continue follow-up” of patients in post–market surveillance studies, the FDA said in a statement.
An FDA panel had concluded that 3-year outcomes for prolapse repair with mesh should be better than the outcomes for repair with native tissue, and that the procedures should have comparable safety profiles.
The SUPeR trial
To compare the efficacy and adverse events of vaginal hysterectomy with suture apical suspension and transvaginal mesh hysteropexy, Dr. Nager and colleagues conducted the Study of Uterine Prolapse Procedures Randomized (SUPeR) trial.
Researchers enrolled 183 postmenopausal women with symptomatic uterovaginal prolapse undergoing surgical intervention at nine sites between April 2013 and February 2015. Investigators randomized 93 women to undergo vaginal mesh hysteropexy and 90 to undergo vaginal hysterectomy with uterosacral ligament suspension. Hysteropexy used the UpholdLITE transvaginal mesh support system (Boston Scientific). Uterosacral ligament suspension required one permanent and one delayed absorbable suture on each side. The primary analysis included data from 175 patients.
Compared with hysterectomy, hysteropexy resulted in an adjusted hazard ratio of treatment failure of 0.62 after 3 years, which was not statistically significant (P = .06). The 95% confidence interval of 0.38-1.02 “was wide and only slightly crossed the null value,” the researchers said. “The remaining uncertainty is too great” to establish or rule out the benefit of vaginal mesh hysteropexy.
Mean operative time was about 45 minutes shorter in the hysteropexy group versus the hysterectomy group (111.5 minutes vs. 156.7 minutes). Adverse events in the hysteropexy versus hysterectomy groups included mesh exposure (8% vs. 0%), ureteral kinking managed intraoperatively (0% vs. 7%), excessive granulation tissue after 12 weeks (1% vs. 11%), and suture exposure after 12 weeks (3% vs. 21%).
“Both groups reported improvements in sexual function, and dyspareunia and pain and de novo dyspareunia rates were low,” Dr. Nager and colleagues wrote. “All other complications with long-term sequelae were not different between groups.”
“Patients in the current study are being followed up for 60 months and the results and conclusions at 36 months could change with extended follow-up,” they added.
A role for mesh?
“The report ... by Nager and colleagues is particularly timely and important,” Cynthia A. Brincat, MD, PhD, wrote in an accompanying editorial. Dr. Brincat is affiliated with the division of female pelvic medicine and reconstructive surgery at Rush Medical College, Chicago.
Although the mesh exposures, granulation tissue, or suture exposures during the trial did not require reoperation, “management of these adverse events was not described,” the editorialist noted. “Clinically important differences could exist between the management of these reported adverse events.”
Based on the findings, gynecologic surgeons “will need to reconsider several important questions regarding the repair of pelvic organ prolapse. For instance, is hysterectomy a necessary component for the repair? What is the role of mesh, and can its use reduce the use of otherwise unnecessary procedures (i.e., hysterectomy) without increasing risk to patients?” she wrote. Other questions center on what constitutes operative failure and how surgeons should augment prolapse repair.
“This study also provides a potential new and well-defined role for the use of mesh in pelvic prolapse surgery, with no significant difference, and perhaps some benefit (i.e., no hysterectomy), compared with a native tissue repair,” Dr. Brincat wrote. “The study also provides useful information for shared decision-making discussions between patients and gynecologic surgeons with respect to selection of procedures and use of mesh for treatment of women with symptomatic uterovaginal prolapse undergoing vaginal surgery.”
The trial was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Boston Scientific provided support through an unrestricted grant. One author reported stock ownership in a medical device company, and others reported grants from medical device companies outside the submitted work. Dr. Brincat reported no conflicts of interest.
SOURCES: Nager CW et al. JAMA. 2019 Sep 17;322(11):1054-65; Brincat CA. JAMA. 2019 Sep 17;322(11):1047-8.
Transvaginal mesh hysteropexy for symptomatic uterovaginal prolapse may not significantly reduce treatment failure after 3 years, compared with vaginal hysterectomy with uterosacral ligament suspension, according to randomized trial results.
Nevertheless, “the point estimate favored hysteropexy,” the study authors wrote in JAMA. The 36-month cumulative treatment failure outcomes – defined as retreatment of prolapse, prolapse beyond the hymen, or prolapse symptoms – were 33% for patients who underwent hysteropexy, compared with 42% for patients who underwent hysterectomy. In addition, mean operative time was 45 minutes less for patients who underwent hysteropexy.
The publication follows the Food and Drug Administration’s ruling in April 2019 that manufacturers must cease marketing transvaginal mesh kits for repair of anterior or apical compartment prolapse. The investigators plan to continue evaluating patient outcomes to 5 years, and they noted that longer follow-up may lead to different conclusions.
From a class II device to class III
Surgical repair of uterovaginal prolapse is common. Although vaginal hysterectomy is the procedure of choice for many surgeons, “uterine-sparing suspension techniques ... are increasing in usage,” wrote Charles W. Nager, MD, chair and professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, and coauthors. However, few high-quality, long-term studies have compared apical transvaginal mesh with native tissue procedures.
The FDA first approved a mesh device for transvaginal repair of prolapse in 2002. In 2008, the agency notified clinicians and patients about an increase in adverse event reports related to vaginal mesh. It later advised that mesh for the treatment of pelvic organ prolapse does not conclusively improve clinical outcomes and that serious adverse events are not rare.
In 2016, the FDA reclassified surgical mesh to repair pelvic organ prolapse transvaginally as high risk, citing safety concerns such as severe pelvic pain and organ perforation. And in April 2019, the FDA ordered companies to stop selling transvaginal mesh intended for pelvic organ prolapse repair. “Even though these products can no longer be used in patients moving forward, [manufacturers] are required to continue follow-up” of patients in post–market surveillance studies, the FDA said in a statement.
An FDA panel had concluded that 3-year outcomes for prolapse repair with mesh should be better than the outcomes for repair with native tissue, and that the procedures should have comparable safety profiles.
The SUPeR trial
To compare the efficacy and adverse events of vaginal hysterectomy with suture apical suspension and transvaginal mesh hysteropexy, Dr. Nager and colleagues conducted the Study of Uterine Prolapse Procedures Randomized (SUPeR) trial.
Researchers enrolled 183 postmenopausal women with symptomatic uterovaginal prolapse undergoing surgical intervention at nine sites between April 2013 and February 2015. Investigators randomized 93 women to undergo vaginal mesh hysteropexy and 90 to undergo vaginal hysterectomy with uterosacral ligament suspension. Hysteropexy used the UpholdLITE transvaginal mesh support system (Boston Scientific). Uterosacral ligament suspension required one permanent and one delayed absorbable suture on each side. The primary analysis included data from 175 patients.
Compared with hysterectomy, hysteropexy resulted in an adjusted hazard ratio of treatment failure of 0.62 after 3 years, which was not statistically significant (P = .06). The 95% confidence interval of 0.38-1.02 “was wide and only slightly crossed the null value,” the researchers said. “The remaining uncertainty is too great” to establish or rule out the benefit of vaginal mesh hysteropexy.
Mean operative time was about 45 minutes shorter in the hysteropexy group versus the hysterectomy group (111.5 minutes vs. 156.7 minutes). Adverse events in the hysteropexy versus hysterectomy groups included mesh exposure (8% vs. 0%), ureteral kinking managed intraoperatively (0% vs. 7%), excessive granulation tissue after 12 weeks (1% vs. 11%), and suture exposure after 12 weeks (3% vs. 21%).
“Both groups reported improvements in sexual function, and dyspareunia and pain and de novo dyspareunia rates were low,” Dr. Nager and colleagues wrote. “All other complications with long-term sequelae were not different between groups.”
“Patients in the current study are being followed up for 60 months and the results and conclusions at 36 months could change with extended follow-up,” they added.
A role for mesh?
“The report ... by Nager and colleagues is particularly timely and important,” Cynthia A. Brincat, MD, PhD, wrote in an accompanying editorial. Dr. Brincat is affiliated with the division of female pelvic medicine and reconstructive surgery at Rush Medical College, Chicago.
Although the mesh exposures, granulation tissue, or suture exposures during the trial did not require reoperation, “management of these adverse events was not described,” the editorialist noted. “Clinically important differences could exist between the management of these reported adverse events.”
Based on the findings, gynecologic surgeons “will need to reconsider several important questions regarding the repair of pelvic organ prolapse. For instance, is hysterectomy a necessary component for the repair? What is the role of mesh, and can its use reduce the use of otherwise unnecessary procedures (i.e., hysterectomy) without increasing risk to patients?” she wrote. Other questions center on what constitutes operative failure and how surgeons should augment prolapse repair.
“This study also provides a potential new and well-defined role for the use of mesh in pelvic prolapse surgery, with no significant difference, and perhaps some benefit (i.e., no hysterectomy), compared with a native tissue repair,” Dr. Brincat wrote. “The study also provides useful information for shared decision-making discussions between patients and gynecologic surgeons with respect to selection of procedures and use of mesh for treatment of women with symptomatic uterovaginal prolapse undergoing vaginal surgery.”
The trial was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Boston Scientific provided support through an unrestricted grant. One author reported stock ownership in a medical device company, and others reported grants from medical device companies outside the submitted work. Dr. Brincat reported no conflicts of interest.
SOURCES: Nager CW et al. JAMA. 2019 Sep 17;322(11):1054-65; Brincat CA. JAMA. 2019 Sep 17;322(11):1047-8.
Transvaginal mesh hysteropexy for symptomatic uterovaginal prolapse may not significantly reduce treatment failure after 3 years, compared with vaginal hysterectomy with uterosacral ligament suspension, according to randomized trial results.
Nevertheless, “the point estimate favored hysteropexy,” the study authors wrote in JAMA. The 36-month cumulative treatment failure outcomes – defined as retreatment of prolapse, prolapse beyond the hymen, or prolapse symptoms – were 33% for patients who underwent hysteropexy, compared with 42% for patients who underwent hysterectomy. In addition, mean operative time was 45 minutes less for patients who underwent hysteropexy.
The publication follows the Food and Drug Administration’s ruling in April 2019 that manufacturers must cease marketing transvaginal mesh kits for repair of anterior or apical compartment prolapse. The investigators plan to continue evaluating patient outcomes to 5 years, and they noted that longer follow-up may lead to different conclusions.
From a class II device to class III
Surgical repair of uterovaginal prolapse is common. Although vaginal hysterectomy is the procedure of choice for many surgeons, “uterine-sparing suspension techniques ... are increasing in usage,” wrote Charles W. Nager, MD, chair and professor of obstetrics, gynecology, and reproductive sciences at the University of California, San Diego, and coauthors. However, few high-quality, long-term studies have compared apical transvaginal mesh with native tissue procedures.
The FDA first approved a mesh device for transvaginal repair of prolapse in 2002. In 2008, the agency notified clinicians and patients about an increase in adverse event reports related to vaginal mesh. It later advised that mesh for the treatment of pelvic organ prolapse does not conclusively improve clinical outcomes and that serious adverse events are not rare.
In 2016, the FDA reclassified surgical mesh to repair pelvic organ prolapse transvaginally as high risk, citing safety concerns such as severe pelvic pain and organ perforation. And in April 2019, the FDA ordered companies to stop selling transvaginal mesh intended for pelvic organ prolapse repair. “Even though these products can no longer be used in patients moving forward, [manufacturers] are required to continue follow-up” of patients in post–market surveillance studies, the FDA said in a statement.
An FDA panel had concluded that 3-year outcomes for prolapse repair with mesh should be better than the outcomes for repair with native tissue, and that the procedures should have comparable safety profiles.
The SUPeR trial
To compare the efficacy and adverse events of vaginal hysterectomy with suture apical suspension and transvaginal mesh hysteropexy, Dr. Nager and colleagues conducted the Study of Uterine Prolapse Procedures Randomized (SUPeR) trial.
Researchers enrolled 183 postmenopausal women with symptomatic uterovaginal prolapse undergoing surgical intervention at nine sites between April 2013 and February 2015. Investigators randomized 93 women to undergo vaginal mesh hysteropexy and 90 to undergo vaginal hysterectomy with uterosacral ligament suspension. Hysteropexy used the UpholdLITE transvaginal mesh support system (Boston Scientific). Uterosacral ligament suspension required one permanent and one delayed absorbable suture on each side. The primary analysis included data from 175 patients.
Compared with hysterectomy, hysteropexy resulted in an adjusted hazard ratio of treatment failure of 0.62 after 3 years, which was not statistically significant (P = .06). The 95% confidence interval of 0.38-1.02 “was wide and only slightly crossed the null value,” the researchers said. “The remaining uncertainty is too great” to establish or rule out the benefit of vaginal mesh hysteropexy.
Mean operative time was about 45 minutes shorter in the hysteropexy group versus the hysterectomy group (111.5 minutes vs. 156.7 minutes). Adverse events in the hysteropexy versus hysterectomy groups included mesh exposure (8% vs. 0%), ureteral kinking managed intraoperatively (0% vs. 7%), excessive granulation tissue after 12 weeks (1% vs. 11%), and suture exposure after 12 weeks (3% vs. 21%).
“Both groups reported improvements in sexual function, and dyspareunia and pain and de novo dyspareunia rates were low,” Dr. Nager and colleagues wrote. “All other complications with long-term sequelae were not different between groups.”
“Patients in the current study are being followed up for 60 months and the results and conclusions at 36 months could change with extended follow-up,” they added.
A role for mesh?
“The report ... by Nager and colleagues is particularly timely and important,” Cynthia A. Brincat, MD, PhD, wrote in an accompanying editorial. Dr. Brincat is affiliated with the division of female pelvic medicine and reconstructive surgery at Rush Medical College, Chicago.
Although the mesh exposures, granulation tissue, or suture exposures during the trial did not require reoperation, “management of these adverse events was not described,” the editorialist noted. “Clinically important differences could exist between the management of these reported adverse events.”
Based on the findings, gynecologic surgeons “will need to reconsider several important questions regarding the repair of pelvic organ prolapse. For instance, is hysterectomy a necessary component for the repair? What is the role of mesh, and can its use reduce the use of otherwise unnecessary procedures (i.e., hysterectomy) without increasing risk to patients?” she wrote. Other questions center on what constitutes operative failure and how surgeons should augment prolapse repair.
“This study also provides a potential new and well-defined role for the use of mesh in pelvic prolapse surgery, with no significant difference, and perhaps some benefit (i.e., no hysterectomy), compared with a native tissue repair,” Dr. Brincat wrote. “The study also provides useful information for shared decision-making discussions between patients and gynecologic surgeons with respect to selection of procedures and use of mesh for treatment of women with symptomatic uterovaginal prolapse undergoing vaginal surgery.”
The trial was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Office of Research on Women’s Health. Boston Scientific provided support through an unrestricted grant. One author reported stock ownership in a medical device company, and others reported grants from medical device companies outside the submitted work. Dr. Brincat reported no conflicts of interest.
SOURCES: Nager CW et al. JAMA. 2019 Sep 17;322(11):1054-65; Brincat CA. JAMA. 2019 Sep 17;322(11):1047-8.
FROM JAMA
Bariatric surgery has mostly positive impact in knee arthroplasty
a large study has found.
The study, led by Yicun Wang, PhD, of Nanjing (China) University was published in the Journal of Arthroplasty. “Generally speaking, bariatric surgery decreases some postoperative complications, decreases length of stay, and lowers mortality,” the study investigators wrote, [but] anemia and blood transfusion seem to be more common in patients with prior bariatric surgery.
They analyzed the effect of bariatric surgery on subsequent arthroplasty in morbidly obese patients in the United States using Nationwide Inpatient Sample 2006-2014 data on total hip arthroplasty (THA) and total knee arthroplasty (TKA). The researchers defined morbid obese patients as those with a body mass index higher than 40 kg/m2.
Among patients who underwent TKA, the researchers compared a group of 9,803 morbidly obese patients with the same number of patients who had undergone bariatric surgery. The two groups were matched by age, sex, income, primary insurance payer, and race.
There were large differences between the bariatric surgery group vs. morbidly obese group: Pulmonary embolism was much more common in the morbid obesity group (odds ratio, 0.22; 95% confidence interval, 0.05-1.03; P = .0346) while blood transfusion was more common in the bariatric surgery group (OR, 1.76; 95% CI, 1.52-2.03; P less than .0001).
For TKA, the researchers used the same approach to analyze 2,540 matched pairs of patients. In the bariatric surgery vs. morbidly obese comparison, pulmonary embolism was more common in the morbidly obese group (OR, 0.34; 95% CI, 0.20-0.57; P less than .0001), as were respiratory complications (OR, 0.45; 95% CI, 0.26-0.78; P = .0032) and death (OR, 0.07; 95% CI, 0.01-0.50; P = .0005). But the bariatric surgery group had higher levels of blood transfusion (OR, 1.87; 95% CI, 1.71-2.04; P less than .0001) and anemia (OR, 1.16; 95% CI, 1.09-1.24; P less than .0001).
Going forward, the researchers write, “future studies on these patients should attempt to evaluate the impact of bariatric surgery on the long-term outcomes of arthroplasty.”
The study was supported by various funders including the National Natural Science Foundation of China, the Natural Science Foundation of Guangdong Province, the Project of Administration of Traditional Chinese Medicine of Guangdong Province and others. No author disclosures are reported.
SOURCE: Wang Y et al. J Arthroplasty. 2019;S0883-5403(19)30667-9.
a large study has found.
The study, led by Yicun Wang, PhD, of Nanjing (China) University was published in the Journal of Arthroplasty. “Generally speaking, bariatric surgery decreases some postoperative complications, decreases length of stay, and lowers mortality,” the study investigators wrote, [but] anemia and blood transfusion seem to be more common in patients with prior bariatric surgery.
They analyzed the effect of bariatric surgery on subsequent arthroplasty in morbidly obese patients in the United States using Nationwide Inpatient Sample 2006-2014 data on total hip arthroplasty (THA) and total knee arthroplasty (TKA). The researchers defined morbid obese patients as those with a body mass index higher than 40 kg/m2.
Among patients who underwent TKA, the researchers compared a group of 9,803 morbidly obese patients with the same number of patients who had undergone bariatric surgery. The two groups were matched by age, sex, income, primary insurance payer, and race.
There were large differences between the bariatric surgery group vs. morbidly obese group: Pulmonary embolism was much more common in the morbid obesity group (odds ratio, 0.22; 95% confidence interval, 0.05-1.03; P = .0346) while blood transfusion was more common in the bariatric surgery group (OR, 1.76; 95% CI, 1.52-2.03; P less than .0001).
For TKA, the researchers used the same approach to analyze 2,540 matched pairs of patients. In the bariatric surgery vs. morbidly obese comparison, pulmonary embolism was more common in the morbidly obese group (OR, 0.34; 95% CI, 0.20-0.57; P less than .0001), as were respiratory complications (OR, 0.45; 95% CI, 0.26-0.78; P = .0032) and death (OR, 0.07; 95% CI, 0.01-0.50; P = .0005). But the bariatric surgery group had higher levels of blood transfusion (OR, 1.87; 95% CI, 1.71-2.04; P less than .0001) and anemia (OR, 1.16; 95% CI, 1.09-1.24; P less than .0001).
Going forward, the researchers write, “future studies on these patients should attempt to evaluate the impact of bariatric surgery on the long-term outcomes of arthroplasty.”
The study was supported by various funders including the National Natural Science Foundation of China, the Natural Science Foundation of Guangdong Province, the Project of Administration of Traditional Chinese Medicine of Guangdong Province and others. No author disclosures are reported.
SOURCE: Wang Y et al. J Arthroplasty. 2019;S0883-5403(19)30667-9.
a large study has found.
The study, led by Yicun Wang, PhD, of Nanjing (China) University was published in the Journal of Arthroplasty. “Generally speaking, bariatric surgery decreases some postoperative complications, decreases length of stay, and lowers mortality,” the study investigators wrote, [but] anemia and blood transfusion seem to be more common in patients with prior bariatric surgery.
They analyzed the effect of bariatric surgery on subsequent arthroplasty in morbidly obese patients in the United States using Nationwide Inpatient Sample 2006-2014 data on total hip arthroplasty (THA) and total knee arthroplasty (TKA). The researchers defined morbid obese patients as those with a body mass index higher than 40 kg/m2.
Among patients who underwent TKA, the researchers compared a group of 9,803 morbidly obese patients with the same number of patients who had undergone bariatric surgery. The two groups were matched by age, sex, income, primary insurance payer, and race.
There were large differences between the bariatric surgery group vs. morbidly obese group: Pulmonary embolism was much more common in the morbid obesity group (odds ratio, 0.22; 95% confidence interval, 0.05-1.03; P = .0346) while blood transfusion was more common in the bariatric surgery group (OR, 1.76; 95% CI, 1.52-2.03; P less than .0001).
For TKA, the researchers used the same approach to analyze 2,540 matched pairs of patients. In the bariatric surgery vs. morbidly obese comparison, pulmonary embolism was more common in the morbidly obese group (OR, 0.34; 95% CI, 0.20-0.57; P less than .0001), as were respiratory complications (OR, 0.45; 95% CI, 0.26-0.78; P = .0032) and death (OR, 0.07; 95% CI, 0.01-0.50; P = .0005). But the bariatric surgery group had higher levels of blood transfusion (OR, 1.87; 95% CI, 1.71-2.04; P less than .0001) and anemia (OR, 1.16; 95% CI, 1.09-1.24; P less than .0001).
Going forward, the researchers write, “future studies on these patients should attempt to evaluate the impact of bariatric surgery on the long-term outcomes of arthroplasty.”
The study was supported by various funders including the National Natural Science Foundation of China, the Natural Science Foundation of Guangdong Province, the Project of Administration of Traditional Chinese Medicine of Guangdong Province and others. No author disclosures are reported.
SOURCE: Wang Y et al. J Arthroplasty. 2019;S0883-5403(19)30667-9.
FROM THE JOURNAL OF ARTHROPLASTY