User login
Use of Hybrid Coronary Revascularization in Patients with Multivessel Coronary Artery Disease
Study Overview
Objective. To investigate the 5-year clinical outcome of patients undergoing hybrid revascularization for multivessel coronary artery disease (CAD).
Design. Multicenter, open-label, prospective randomized control trial.
Setting and participants. 200 patients with multivessel CAD referred for conventional surgical revascularization were randomly assigned to undergo hybrid coronary revascularization (HCR) or coronary artery bypass grafting (CABG).
Main outcome measures. The primary endpoint was all-cause mortality at 5 years.
Main results. After excluding 9 patients who were lost to follow-up before 5 years, 191 patients (94 in HCR group and 97 in CABG group) formed the basis of the study. All-cause mortality at 5-year follow-up was similar in the 2 groups (6.4% versus 9.2%, P = 0.69). The rates of myocardial infarction (4.3% versus 7.2%, P = 0.30), repeat revascularization (37.2% versus 45.4%, P = 0.38), stroke (2.1% versus 4.1%, P = 0.35), and major adverse and cardiac and cerebrovascular events (45.2% versus 53.4%, P = 0.39) were similar in the 2 groups. These findings were consistent across all levels of risk for surgical complications (EuroScore) and for complexity of revascularization (SYNTAX score).
Conclusion. HCR has similar 5-year all-cause mortality when compared with conventional CABG.
Commentary
HCR has been proposed as a less invasive, effective alternative revascularization strategy to conventional CABG for patients with multivessel CAD. The hybrid approach typically combines the long-term durability of grafting of the left anterior descending artery (LAD) using the left internal mammary artery and the percutaneous coronary intervention (PCI) for non-LAD stenosis; this approach has been shown to have similar or perhaps even better long-term patency compared with saphenous vein grafts.1,2 Previous studies have demonstrated the feasibility of HCR by comparing HCR to conventional CABG at 1 year.2 However, the long-term outcome of HCR compared to conventional CABG has not been previously reported.
In this context, Tajstra et al reported the 5-year follow-up from their prospective randomized pilot study. They report that among the 200 patients with multivessel coronary disease randomly assigned to either HCR or CABG, all-cause mortality at 5-year follow-up was similar in the 2 groups (6.4% versus 9.2%, P = 0.69). The rates of myocardial infarction, repeat revascularization, stroke, and major adverse and cardiac and cerebrovascular event (MACCE) were also similar in the 2 groups.
This is an important study because it is the first to compare the long-term outcome of HCR with conventional CABG; previous studies have been limited due to their short- to mid-term follow-up.2 However, because this study was not powered to assess the superiority of the HCR compared to conventional CABG, future randomized control trials with a larger number of patients are needed.
Future studies must address some important questions. First, the patients in the present study were younger (mean age, 62.1 ± 8.3 years) with less comorbidity and a relatively low SYNTAX score (23.6 ± 6.1 for the HCR arm). As CABG and PCI are associated with similar long- term outcomes in patients with low (< 22) to intermediate (22–32) SYNTAX score,3 comparisons between HCR and multivessel PCI using the current generation of drug-eluting stents are needed. The results from the ongoing Hybrid Coronary Revascularization Trial (NCT03089398) will shed light on this clinical question. Second, whether these findings can be extended to patients with a high baseline SYNTAX score needs further study. Nonetheless, outcomes were similar between the 2 strategies in the intermediate (n = 98) and high (n = 8) SYNTAX score groups. Interestingly, there is no clear benefit of HCR in the high surgical risk groups as measured by EuroScore. Third, in addition to the hard outcomes (death and MACCE), the quality of life of patients measured by an established metric, such as the Seattle Angina Questionnaire, need to be assessed. Last, the completeness of revascularization in each group needs to be further evaluated because incomplete revascularization is a known predictor of adverse outcomes.4,5
Applications for Clinical Practice
In patients with multivessel coronary disease with low SYNTAX score, the 5-year outcome for HCR was similar to that of conventional CABG. Further larger studies are needed to assess the superiority of this approach.
—Taishi Hirai, MD, University of Missouri Medical Center, Columbia, MO; Hiroto Kitahara, MD, University of Chicago Medical Center, Chicago, IL; and John Blair, MD, Medstar Washington Hospital Center, Washington, DC
1. Lee PH, Kwon O, Ahn JM, et al. Safety and effectiveness of second-generation drug-eluting stents in patients with left main coronary artery disease. J Am Coll Cardiol. 2018;71:832-841.
2. Gasior M, Zembala MO, Tajstra M, et al. Hybrid revascularization for multivessel coronary artery disease. JACC Cardiovasc Interv. 2014;7:1277-1283.
3. Serruys PW, Onuma Y, Garg S, et al. Assessment of the SYNTAX score in the Syntax study. EuroIntervention. 2009;5:50-56.
4. Genereux P, Palmerini T, Caixeta A, et al. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: the residual SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score. J Am Coll Cardiol. 2012;59:2165-2174.
5. Choi KH, Lee JM, Koo BK, et al. Prognostic implication of functional incomplete revascularization and residual functional SYNTAX score in patients with coronary artery disease. JACC Cardiovasc Interv. 2018;11:237-245.
Study Overview
Objective. To investigate the 5-year clinical outcome of patients undergoing hybrid revascularization for multivessel coronary artery disease (CAD).
Design. Multicenter, open-label, prospective randomized control trial.
Setting and participants. 200 patients with multivessel CAD referred for conventional surgical revascularization were randomly assigned to undergo hybrid coronary revascularization (HCR) or coronary artery bypass grafting (CABG).
Main outcome measures. The primary endpoint was all-cause mortality at 5 years.
Main results. After excluding 9 patients who were lost to follow-up before 5 years, 191 patients (94 in HCR group and 97 in CABG group) formed the basis of the study. All-cause mortality at 5-year follow-up was similar in the 2 groups (6.4% versus 9.2%, P = 0.69). The rates of myocardial infarction (4.3% versus 7.2%, P = 0.30), repeat revascularization (37.2% versus 45.4%, P = 0.38), stroke (2.1% versus 4.1%, P = 0.35), and major adverse and cardiac and cerebrovascular events (45.2% versus 53.4%, P = 0.39) were similar in the 2 groups. These findings were consistent across all levels of risk for surgical complications (EuroScore) and for complexity of revascularization (SYNTAX score).
Conclusion. HCR has similar 5-year all-cause mortality when compared with conventional CABG.
Commentary
HCR has been proposed as a less invasive, effective alternative revascularization strategy to conventional CABG for patients with multivessel CAD. The hybrid approach typically combines the long-term durability of grafting of the left anterior descending artery (LAD) using the left internal mammary artery and the percutaneous coronary intervention (PCI) for non-LAD stenosis; this approach has been shown to have similar or perhaps even better long-term patency compared with saphenous vein grafts.1,2 Previous studies have demonstrated the feasibility of HCR by comparing HCR to conventional CABG at 1 year.2 However, the long-term outcome of HCR compared to conventional CABG has not been previously reported.
In this context, Tajstra et al reported the 5-year follow-up from their prospective randomized pilot study. They report that among the 200 patients with multivessel coronary disease randomly assigned to either HCR or CABG, all-cause mortality at 5-year follow-up was similar in the 2 groups (6.4% versus 9.2%, P = 0.69). The rates of myocardial infarction, repeat revascularization, stroke, and major adverse and cardiac and cerebrovascular event (MACCE) were also similar in the 2 groups.
This is an important study because it is the first to compare the long-term outcome of HCR with conventional CABG; previous studies have been limited due to their short- to mid-term follow-up.2 However, because this study was not powered to assess the superiority of the HCR compared to conventional CABG, future randomized control trials with a larger number of patients are needed.
Future studies must address some important questions. First, the patients in the present study were younger (mean age, 62.1 ± 8.3 years) with less comorbidity and a relatively low SYNTAX score (23.6 ± 6.1 for the HCR arm). As CABG and PCI are associated with similar long- term outcomes in patients with low (< 22) to intermediate (22–32) SYNTAX score,3 comparisons between HCR and multivessel PCI using the current generation of drug-eluting stents are needed. The results from the ongoing Hybrid Coronary Revascularization Trial (NCT03089398) will shed light on this clinical question. Second, whether these findings can be extended to patients with a high baseline SYNTAX score needs further study. Nonetheless, outcomes were similar between the 2 strategies in the intermediate (n = 98) and high (n = 8) SYNTAX score groups. Interestingly, there is no clear benefit of HCR in the high surgical risk groups as measured by EuroScore. Third, in addition to the hard outcomes (death and MACCE), the quality of life of patients measured by an established metric, such as the Seattle Angina Questionnaire, need to be assessed. Last, the completeness of revascularization in each group needs to be further evaluated because incomplete revascularization is a known predictor of adverse outcomes.4,5
Applications for Clinical Practice
In patients with multivessel coronary disease with low SYNTAX score, the 5-year outcome for HCR was similar to that of conventional CABG. Further larger studies are needed to assess the superiority of this approach.
—Taishi Hirai, MD, University of Missouri Medical Center, Columbia, MO; Hiroto Kitahara, MD, University of Chicago Medical Center, Chicago, IL; and John Blair, MD, Medstar Washington Hospital Center, Washington, DC
Study Overview
Objective. To investigate the 5-year clinical outcome of patients undergoing hybrid revascularization for multivessel coronary artery disease (CAD).
Design. Multicenter, open-label, prospective randomized control trial.
Setting and participants. 200 patients with multivessel CAD referred for conventional surgical revascularization were randomly assigned to undergo hybrid coronary revascularization (HCR) or coronary artery bypass grafting (CABG).
Main outcome measures. The primary endpoint was all-cause mortality at 5 years.
Main results. After excluding 9 patients who were lost to follow-up before 5 years, 191 patients (94 in HCR group and 97 in CABG group) formed the basis of the study. All-cause mortality at 5-year follow-up was similar in the 2 groups (6.4% versus 9.2%, P = 0.69). The rates of myocardial infarction (4.3% versus 7.2%, P = 0.30), repeat revascularization (37.2% versus 45.4%, P = 0.38), stroke (2.1% versus 4.1%, P = 0.35), and major adverse and cardiac and cerebrovascular events (45.2% versus 53.4%, P = 0.39) were similar in the 2 groups. These findings were consistent across all levels of risk for surgical complications (EuroScore) and for complexity of revascularization (SYNTAX score).
Conclusion. HCR has similar 5-year all-cause mortality when compared with conventional CABG.
Commentary
HCR has been proposed as a less invasive, effective alternative revascularization strategy to conventional CABG for patients with multivessel CAD. The hybrid approach typically combines the long-term durability of grafting of the left anterior descending artery (LAD) using the left internal mammary artery and the percutaneous coronary intervention (PCI) for non-LAD stenosis; this approach has been shown to have similar or perhaps even better long-term patency compared with saphenous vein grafts.1,2 Previous studies have demonstrated the feasibility of HCR by comparing HCR to conventional CABG at 1 year.2 However, the long-term outcome of HCR compared to conventional CABG has not been previously reported.
In this context, Tajstra et al reported the 5-year follow-up from their prospective randomized pilot study. They report that among the 200 patients with multivessel coronary disease randomly assigned to either HCR or CABG, all-cause mortality at 5-year follow-up was similar in the 2 groups (6.4% versus 9.2%, P = 0.69). The rates of myocardial infarction, repeat revascularization, stroke, and major adverse and cardiac and cerebrovascular event (MACCE) were also similar in the 2 groups.
This is an important study because it is the first to compare the long-term outcome of HCR with conventional CABG; previous studies have been limited due to their short- to mid-term follow-up.2 However, because this study was not powered to assess the superiority of the HCR compared to conventional CABG, future randomized control trials with a larger number of patients are needed.
Future studies must address some important questions. First, the patients in the present study were younger (mean age, 62.1 ± 8.3 years) with less comorbidity and a relatively low SYNTAX score (23.6 ± 6.1 for the HCR arm). As CABG and PCI are associated with similar long- term outcomes in patients with low (< 22) to intermediate (22–32) SYNTAX score,3 comparisons between HCR and multivessel PCI using the current generation of drug-eluting stents are needed. The results from the ongoing Hybrid Coronary Revascularization Trial (NCT03089398) will shed light on this clinical question. Second, whether these findings can be extended to patients with a high baseline SYNTAX score needs further study. Nonetheless, outcomes were similar between the 2 strategies in the intermediate (n = 98) and high (n = 8) SYNTAX score groups. Interestingly, there is no clear benefit of HCR in the high surgical risk groups as measured by EuroScore. Third, in addition to the hard outcomes (death and MACCE), the quality of life of patients measured by an established metric, such as the Seattle Angina Questionnaire, need to be assessed. Last, the completeness of revascularization in each group needs to be further evaluated because incomplete revascularization is a known predictor of adverse outcomes.4,5
Applications for Clinical Practice
In patients with multivessel coronary disease with low SYNTAX score, the 5-year outcome for HCR was similar to that of conventional CABG. Further larger studies are needed to assess the superiority of this approach.
—Taishi Hirai, MD, University of Missouri Medical Center, Columbia, MO; Hiroto Kitahara, MD, University of Chicago Medical Center, Chicago, IL; and John Blair, MD, Medstar Washington Hospital Center, Washington, DC
1. Lee PH, Kwon O, Ahn JM, et al. Safety and effectiveness of second-generation drug-eluting stents in patients with left main coronary artery disease. J Am Coll Cardiol. 2018;71:832-841.
2. Gasior M, Zembala MO, Tajstra M, et al. Hybrid revascularization for multivessel coronary artery disease. JACC Cardiovasc Interv. 2014;7:1277-1283.
3. Serruys PW, Onuma Y, Garg S, et al. Assessment of the SYNTAX score in the Syntax study. EuroIntervention. 2009;5:50-56.
4. Genereux P, Palmerini T, Caixeta A, et al. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: the residual SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score. J Am Coll Cardiol. 2012;59:2165-2174.
5. Choi KH, Lee JM, Koo BK, et al. Prognostic implication of functional incomplete revascularization and residual functional SYNTAX score in patients with coronary artery disease. JACC Cardiovasc Interv. 2018;11:237-245.
1. Lee PH, Kwon O, Ahn JM, et al. Safety and effectiveness of second-generation drug-eluting stents in patients with left main coronary artery disease. J Am Coll Cardiol. 2018;71:832-841.
2. Gasior M, Zembala MO, Tajstra M, et al. Hybrid revascularization for multivessel coronary artery disease. JACC Cardiovasc Interv. 2014;7:1277-1283.
3. Serruys PW, Onuma Y, Garg S, et al. Assessment of the SYNTAX score in the Syntax study. EuroIntervention. 2009;5:50-56.
4. Genereux P, Palmerini T, Caixeta A, et al. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: the residual SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score. J Am Coll Cardiol. 2012;59:2165-2174.
5. Choi KH, Lee JM, Koo BK, et al. Prognostic implication of functional incomplete revascularization and residual functional SYNTAX score in patients with coronary artery disease. JACC Cardiovasc Interv. 2018;11:237-245.
Mismatch Between Process and Outcome Measures for Hospital-Acquired Venous Thromboembolism in a Surgical Cohort
From Tufts Medical Center, Boston, MA.
Abstract
- Objective: Audits at our academic medical center revealed near 100% compliance with protocols for perioperative venous thromboembolism (VTE) prophylaxis, but recent National Surgical Quality Improvement Program data demonstrated a higher than expected incidence of VTE (observed/expected = 1.32). The objective of this study was to identify potential causes of this discrepancy.
- Design: Retrospective case-control study.
- Setting: Urban academic medical center with high case-mix indices (Medicare approximately 2.4, non-Medicare approximately 2.0).
- Participants: 102 surgical inpatients with VTE (September 2012 to October 2015) matched with controls for age, gender, and type of procedure.
- Measurements: Prevalence of common VTE risk factors, length of stay, number of procedures, index operation times, and postoperative bed rest > 12 hours were assessed. Utilization of and compliance with our VTE risk assessment tool was also investigated.
- Results: Cases underwent more procedures and had longer lengths of stay and index procedures than controls. In addition, cases were more likely to have had > 12 hours of postoperative bed rest and central venous access than controls. Cases had more infections and were more likely to have severe lung disease, thrombophilia, and a history of prior VTE than controls. No differences in body mass index, tobacco use, current or previous malignancy, or VTE risk assessment form use were observed. Overall, care complexity and risk factors were equally important in determining VTE incidence. Our analyses also revealed lack of strict adherence to our VTE risk stratification protocol and frequent use of suboptimal prophylactic regimens.
- Conclusion: Well-accepted risk factors and overall care complexity determine VTE risk. Preventing VTE in high-risk patients requires assiduous attention to detail in VTE risk assessment and in delivery of optimal prophylaxis. Patients at especially high risk may require customized prophylactic regimens.
Keywords: hospital-acquired venous thromboembolic disease; VTE prophylaxis, surgical patients.
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are well-recognized causes of morbidity and mortality in surgical patients. Between 350,000 and 600,000 cases of venous thromboembolism (VTE) occur each year in the United States, and it is responsible for approximately 10% of preventable in-hospital fatalities.1-3 Given VTE’s impact on patients and the healthcare system and the fact that it is preventable, intense effort has been focused on developing more effective prophylactic measures to decrease its incidence.2-4 In 2008, the surgeon general issued a “call to action” for increased efforts to prevent VTE.5
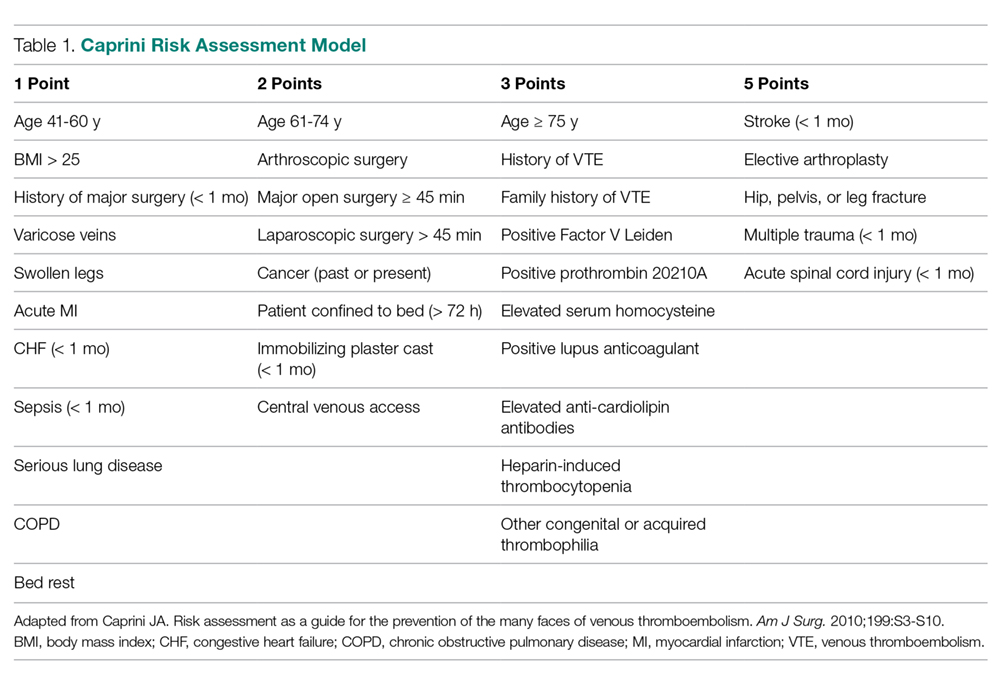
The American College of Chest Physicians (ACCP) guidelines subcategorize patients based on type of surgery. In addition, the ACCP guidelines support the use of a Caprini-based scoring system to aid in risk stratification and improve clinical decision-making (
Our hospital, a 350-bed academic medical center in downtown Boston, MA, serving a diverse population with a very high case-mix index (2.4 Medicare and 2.0 non-Medicare), has strict protocols for VTE prophylaxis consistent with the ACCP guidelines and based on the Surgical Care Improvement Project (SCIP) measures published in 2006.10 The SCIP mandates allow for considerable surgeon discretion in the use of chemoprophylaxis for neurosurgical cases and general and orthopedic surgery cases deemed to be at high risk for bleeding. In addition, SCIP requires only that prophylaxis be initiated within 24 hours of surgical end time. Although recent audits revealed nearly 100% compliance with SCIP-mandated protocols, National Surgical Quality Improvement Program (NSQIP) data showed that the incidence of VTE events at our institution was higher than expected (observed/expected [O/E] = 1.32).
In order to determine the reasons for this mismatch between process and outcome performance, we investigated whether there were characteristics of our patient population that contributed to the higher than expected rates of VTE, and we scrutinized our VTE prophylaxis protocol to determine if there were aspects of our process that were also contributory.
Methods
Study Sample
This is a retrospective case-control study of surgical inpatients at our hospital during the period September 2012 to October 2015. Cases were identified as patients diagnosed with a VTE (DVT or PE). Controls were identified from a pool of surgical patients whose courses were not complicated by VTE during the same time frame as the cases and who were matched as closely as possible by procedure code, age, and gender.
Variables
Patient and hospital course variables that were analyzed included demographics, comorbidities, length of stay, number of procedures, index operation times, duration of postoperative bed rest, use of mechanical prophylaxis, and type of chemoprophylaxis and time frame within which it was initiated. Data were collected via chart review using International Classification of Diseases-9 and -10 codes to identify surgical cases within the allotted time period who were diagnosed with VTE. Demographic variables included age, sex, and ethnicity. Comorbidities included hypertension, diabetes, coronary artery disease, serious lung disease, previous or current malignancy, documented hypercoagulable state, and previous history of VTE. Body mass index (BMI) was also recorded. The aforementioned disease-specific variables were not matched between the case and control groups, as this data was obtained retrospectively during data collection.
Analysis
Associations between case and matched control were analyzed using the paired t-test for continuous variables and McNemar’s test for categorical variables. P values < 0.05 were considered statistically significant. SAS Enterprise Guide 7.15 (Cary, NC) was used for all statistical analyses.
The requirement for informed consent was waived by our Institutional Review Board, as the study was initially deemed to be a quality improvement project, and all data used for this report were de-identified.
Results
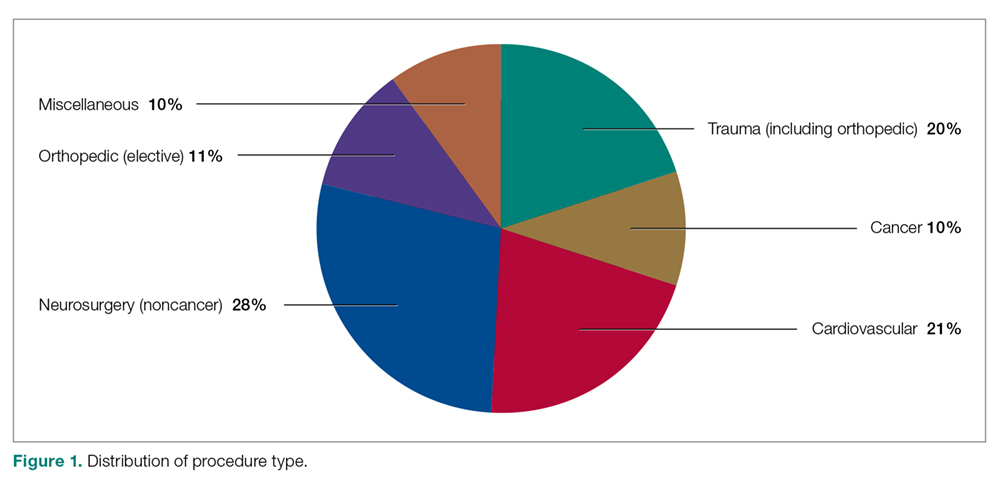
Our retrospective case-control analysis included a sample of 102 surgical patients whose courses were complicated by VTE between September 2012 and October 2015. The cases were distributed among 6 different surgical categories (Figure 1): trauma (20%), cancer (10%), cardiovascular (21%), noncancer neurosurgery (28%), elective orthopedics (11%), and miscellaneous general surgery (10%).
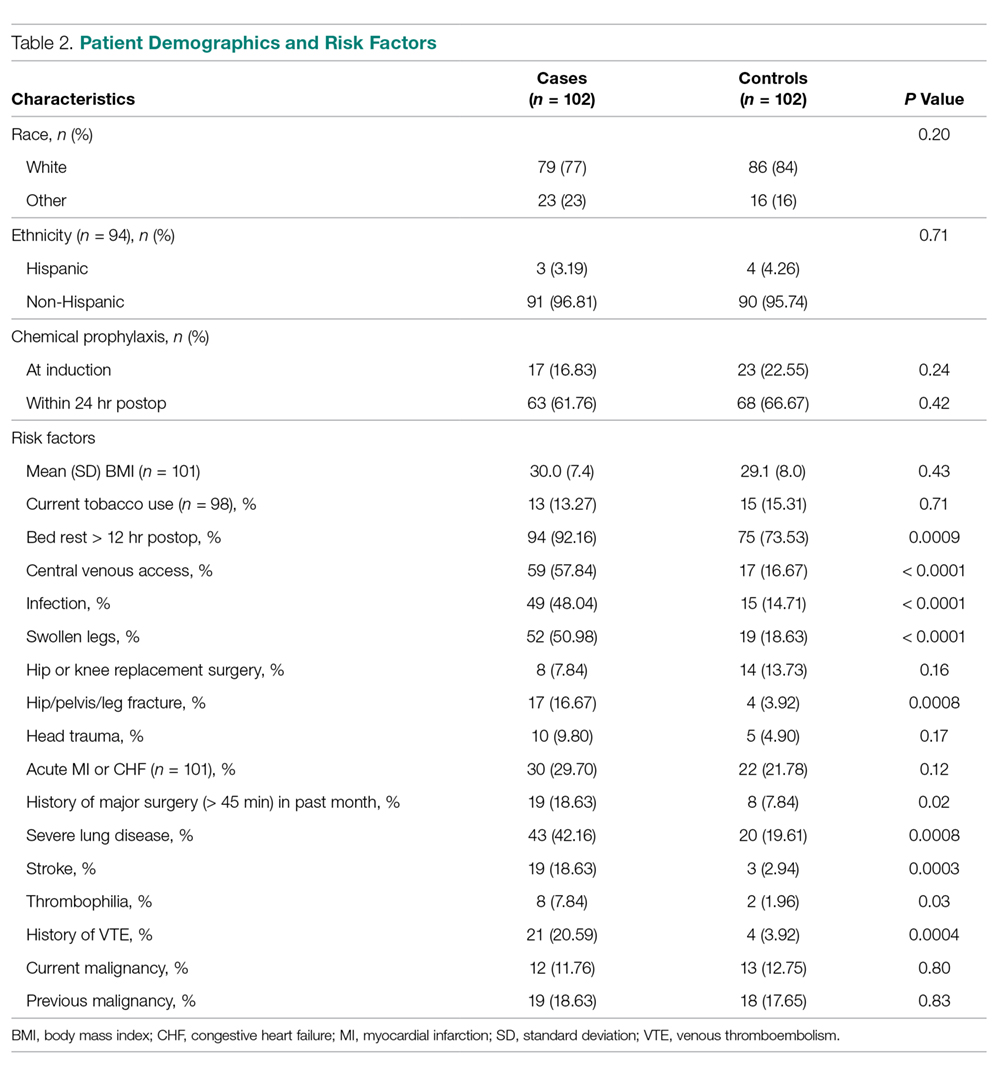
Comparisons between cases and controls in terms of patient demographics and risk factors are shown in Table 2. No statistically significant difference was observed in ethnicity or race between the 2 groups. Overall, cases had more hip/pelvis/leg fractures at presentation (P = 0.0008). The case group also had higher proportions of patients with postoperative bed rest greater than 12 hours (P = 0.009), central venous access (P < 0.0001), infection (P < 0.0001), and lower extremity edema documented during the hospitalization prior to development of DVT (P < 0.0001). Additionally, cases had significantly greater rates of previous VTE (P = 0.0004), inherited or acquired thrombophilia (P = 0.03), history of stroke (P = 0.0003), and severe lung disease, including pneumonia (P = 0.0008). No significant differences were noted between cases and matched controls in BMI (P = 0.43), current tobacco use (P = 0.71), current malignancy (P = 0.80), previous malignancy (P = 0.83), head trauma (P = 0.17), or acute cardiac disease (myocardial infarction or congestive heart failure; P = 0.12).
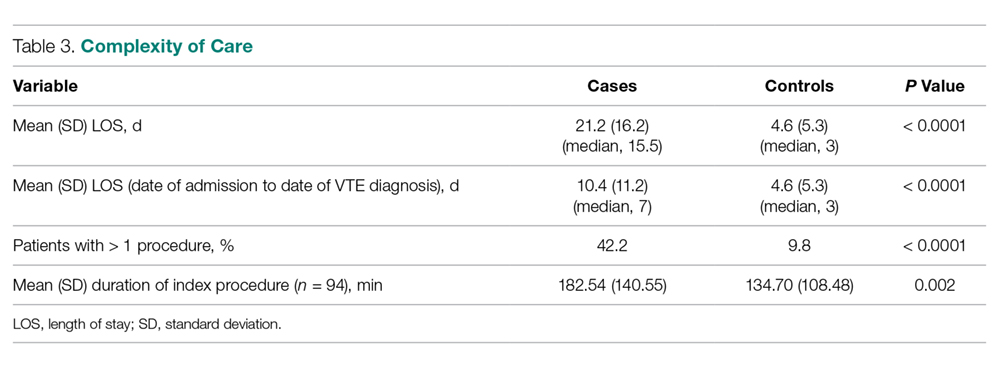
Variables felt to indicate overall complexity of hospital course for cases as compared to controls are outlined in Table 3. Cases were found to have significantly longer lengths of stay (median, 15.5 days versus 3 days, P < 0.0001). To account for the possibility that the development of VTE contributed to the increased length of stay in the cases, we also looked at the duration between admission date and the date of VTE diagnosis and determined that cases still had a longer length of stay when this was accounted for (median, 7 days versus 3 days, P < 0.0001). A much higher proportion of cases underwent more than 1 procedure compared to controls (P < 0.0001), and cases had significantly longer index operations as compared to controls (P = 0.002).
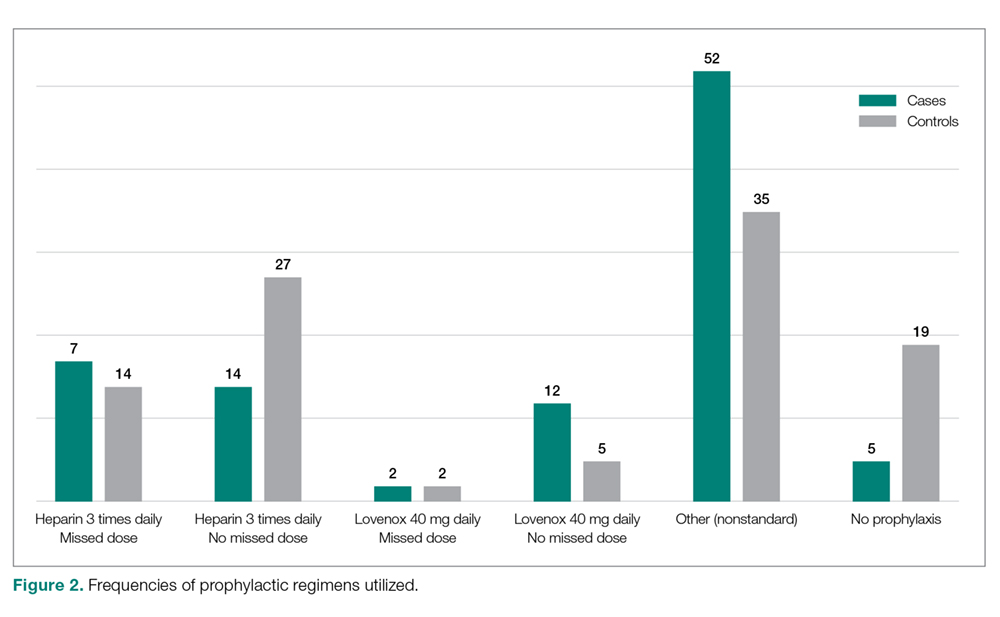
Seventeen cases received heparin on induction during their index procedure, compared to 23 controls (P = 0.24). Additionally, 63 cases began a prophylaxis regimen within 24 hours of surgery end time, compared to 68 controls (P = 0.24). The chemoprophylactic regimens utilized in cases and in controls are summarized in Figure 2. Of note, only 26 cases and 32 controls received standard prophylactic regimens with no missed doses (heparin 5000 units 3 times daily or enoxaparin 40 mg daily). Additionally, in over half of cases and a third of controls, nonstandard regimens were ordered. Examples of nonstandard regimens included nonstandard heparin or enoxaparin doses, low-dose warfarin, or aspirin alone. In most cases, nonstandard regimens were justified on the basis of high risk for bleeding.
Mechanical prophylaxis with pneumatic sequential compression devices (SCDs) was ordered in 93 (91%) cases and 87 (85%) controls; however, we were unable to accurately document uniform compliance in the use of these devices.
With regard to evaluation of our process measures, we found only 17% of cases and controls combined actually had a VTE risk assessment in their chart, and when it was present, it was often incomplete or was completed inaccurately.
Discussion
The goal of this study was to identify factors (patient characteristics and/or processes of care) that may be contributing to the higher than expected incidence of VTE events at our medical center, despite internal audits suggesting near perfect compliance with SCIP-mandated protocols. We found that in addition to usual risk factors for VTE, an overarching theme of our case cohort was their high complexity of illness. At baseline, these patients had significantly greater rates of stroke, thrombophilia, severe lung disease, infection, and history of VTE than controls. Moreover, the hospital courses of cases were significantly more complex than those of controls, as these patients had more procedures, longer lengths of stay and longer index operations, higher rates of postoperative bed rest exceeding 12 hours, and more prevalent central venous access than controls (Table 2). Several of these risk factors have been found to contribute to VTE development despite compliance with prophylaxis protocols.
Cassidy et al reviewed a cohort of nontrauma general surgery patients who developed VTE despite receiving appropriate prophylaxis and found that both multiple operations and emergency procedures contributed to the failure of VTE prophylaxis.11 Similarly, Wang et al identified several independent risk factors for VTE despite thromboprophylaxis, including central venous access and infection, as well as intensive care unit admission, hospitalization for cranial surgery, and admission from a long-term care facility.12 While our study did not capture some of these additional factors considered by Wang et al, the presence of risk factors not captured in traditional assessment tools suggests that additional consideration for complex patients is warranted.
In addition to these nonmodifiable patient characteristics, aspects of our VTE prophylaxis processes likely contributed to the higher than expected rate of VTE. While the electronic medical record at our institution does contain a VTE risk assessment tool based on the Caprini score, we found it often is not used at all or is used incorrectly/incompletely, which likely reflects the fact that physicians are neither prompted nor required to complete the assessment prior to prescribing VTE prophylaxis.
There is a significant body of evidence demonstrating that mandatory computerized VTE risk assessments can effectively reduce VTE rates and that improved outcomes occur shortly after implementation. Cassidy et al demonstrated the benefits of instituting a hospital-wide, mandatory, Caprini-based computerized VTE risk assessment that provides prophylaxis/early ambulation recommendations. Two years after implementing this system, they observed an 84% reduction in DVTs (P < 0.001) and a 55% reduction in PEs (P < 0.001).13 Nimeri et al had similarly impressive success, achieving a reduction in their NSQIP O/E for PE/DVT in general surgery from 6.00 in 2010 to 0.82 (for DVTs) and 0.78 (for PEs) 5 years after implementation of mandatory VTE risk assessment (though they noted that the most dramatic reduction occurred 1 year after implementation).14 Additionally, a recent systematic review and meta-analysis by Borab et al found computerized VTE risk assessments to be associated with a significant decrease in VTE events.15
The risk assessment tool used at our institution is qualitative in nature, and current literature suggests that employing a more quantitative tool may yield improved outcomes. Numerous studies have highlighted the importance of identifying patients at very high risk for VTE, as higher risk may necessitate more careful consideration of their prophylactic regimens. Obi et al found patients with Caprini scores higher than 8 to be at significantly greater risk of developing VTE compared to patients with scores of 7 or 8. Also, patients with scores of 7 or 8 were significantly more likely to have a VTE compared to those with scores of 5 or 6.16 In another study, Lobastov et al identified Caprini scores of 11 or higher as representing an extremely high-risk category for which standard prophylaxis regimens may not be effective.17 Thus, while having mandatory risk assessment has been shown to dramatically decrease VTE incidence, it is important to consider the magnitude of the numerical risk score. This is of particular importance at medical centers with high case-mix indices where patients at the highest risk might need to be managed with different prophylactic guidelines.
Another notable aspect of the process at our hospital was the great variation in the types of prophylactic regimens ordered, and the adherence to what was ordered. Only 25.5% of patients were maintained on a standard prophylactic regimen with no missed doses (heparin 5000 every 8 hours or enoxaparin 40 mg daily). Thus, the vast majority of the patients who went on to develop VTE either were prescribed a nontraditional prophylaxis regimen or missed doses of standard agents. The need for secondary surgical procedures or other invasive interventions may explain many, but not all, of the missed doses.
The timing of prophylaxis initiation for our patients was also found to deviate from accepted standards. Only 16.8% of cases received prophylaxis upon induction of anesthesia, and furthermore, 38% of cases did not receive any anticoagulation within 24 hours of their index operation. While this variability in prophylaxis implementation was acceptable within the SCIP guidelines based on “high risk for bleeding” or other considerations, it likely contributed to our suboptimal outcomes. The variations and interruptions in prophylactic regimens speak to barriers that have previously been reported as contributing factors to noncompliance with VTE prophylaxis.18
Given these known barriers and the observed underutilization and improper use of our risk assessment tool, we have recently changed our surgical admission order sets such that a mandatory quantitative risk assessment must be done for every surgical patient at the time of admission/operation before other orders can be completed. Following completion of the assessment, the physician will be presented with an appropriate standard regimen based on the individual patient’s risk assessment. Early results of our VTE quality improvement project have been satisfying: in the most recent NSQIP semi-annual report, our O/E for VTE was 0.74, placing us in the first decile. Some of these early reports may simply be the product of the Hawthorne effect; however, we are encouraged by the early improvements seen in other research. While we are hopeful that these changes will result in sustainable improvements in outcomes, patients at extremely high risk may require novel weight-based or otherwise customized aggressive prophylactic regimens. Such regimens have already been proposed for arthroplasty and other high-risk patients.
Future research may identify other risk factors not captured by traditional risk assessments. In addition, research should continue to explore the use and efficacy of standard prophylactic regimens in these populations to help determine if they are sufficient. Currently, weight-based low-molecular-weight heparin dosing and alternative regimens employing fondaparinux are under investigation for very-high-risk patients.19
There were several limitations to the present study. First, due to the retrospective design of our study, we could collect only data that had been uniformly recorded in the charts throughout the study period. Second, we were unable to accurately assess compliance with mechanical prophylaxis. While our chart review showed that the vast majority of cases and controls were ordered to have mechanical prophylaxis, it is impossible to document how often these devices were used appropriately in a retrospective analysis. Anecdotal observation suggests that once patients are out of post-anesthesia or critical care units, SCD use is not standardized. The inability to measure compliance precisely may be leading to an overestimation of our compliance with prophylaxis. Finally, because our study included only patients who underwent surgery at our hospital, our observations may not be generalizable outside our institution.
Conclusion
Our study findings reinforce the importance of attention to detail in VTE risk assessment and in ordering and administering VTE prophylactic regimens, especially in high-risk surgical patients. While we adhered to the SCIP-mandated prophylaxis requirements, the complexity of our patients and our lack of a truly standardized approach to risk assessment and prophylactic regimens resulted in suboptimal outcomes. Stricter and more quantitative mandatory VTE risk assessment, along with highly standardized VTE prophylaxis regimens, are required to achieve optimal outcomes.
Corresponding author: Jason C. DeGiovanni, MS, BA, Jason.DeGiovanni@tufts.edu.
Financial disclosures: None.
1. Spyropoulos AC, Hussein M, Lin J, et al. Rates of symptomatic venous thromboembolism in US surgical patients: a retrospective administrative database study. J Thromb Thrombolysis. 2009;28:458-464.
2. Deitzelzweig SB, Johnson BH, Lin J, et al. Prevalence of clinical venous thromboembolism in the USA: Current trends and future projections. Am J Hematol. 2011;86:217-220.
3. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717.
4. Guyatt GH, Akl EA, Crowther M, et al. Introduction to the ninth edition: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):48S-52S.
5. Office of the Surgeon General; National Heart, Lung, and Blood Institute. The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: Office of the Surgeon General; 2008. www.ncbi.nlm.nih.gov/books/NBK44178/. Accessed May 2, 2019.
6. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1102.
7. Caprini JA, Arcelus JI, Hasty JH, et al. Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost. 1991;17(suppl 3):304-312.
8. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199:S3-S10.
9. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S-e277S.
10. The Joint Commission. Surgical Care Improvement Project (SCIP) Measure Information Form (Version 2.1c). www.jointcommission.org/surgical_care_improvement_project_scip_measure_information_form_version_21c/. Accessed June 22, 2016.
11. Cassidy MR, Macht RD, Rosenkranz P, et al. Patterns of failure of a standardized perioperative venous thromboembolism prophylaxis protocol. J Am Coll Surg. 2016;222:1074-1081.
12. Wang TF, Wong CA, Milligan PE, et al. Risk factors for inpatient venous thromboembolism despite thromboprophylaxis. Thromb Res. 2014;133:25-29.
13. Cassidy MR, Rosenkranz P, McAneny D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization program. J Am Coll Surg. 2014;218:1095-1104.
14. Nimeri AA, Gamaleldin MM, McKenna KL, et al. Reduction of venous thromboembolism in surgical patients using a mandatory risk-scoring system: 5-year follow-up of an American College of Surgeons National Quality Improvement Program. Clin Appl Thromb Hemost. 2017;23:392-396.
15. Borab ZM, Lanni MA, Tecce MG, et al. Use of computerized clinical decision support systems to prevent venous thromboembolism in surgical patients: a systematic review and meta-analysis. JAMA Surg. 2017;152:638–645.
16. Obi AT, Pannucci CJ, Nackashi A, et al. Validation of the Caprini venous thromboembolism risk assessment model in critically ill surgical patients. JAMA Surg. 2015;150:941-948.
17. Lobastov K, Barinov V, Schastlivtsev I, et al. Validation of the Caprini risk assessment model for venous thromboembolism in high-risk surgical patients in the background of standard prophylaxis. J Vasc Surg Venous Lymphat Disord. 2016;4:153-160.
18. Kakkar AK, Cohen AT, Tapson VF, et al. Venous thromboembolism risk and prophylaxis in the acute care hospital setting (ENDORSE survey): findings in surgical patients. Ann Surg. 2010;251:330-338.
19. Smythe MA, Priziola J, Dobesh PP, et al. Guidance for the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:165-186.
From Tufts Medical Center, Boston, MA.
Abstract
- Objective: Audits at our academic medical center revealed near 100% compliance with protocols for perioperative venous thromboembolism (VTE) prophylaxis, but recent National Surgical Quality Improvement Program data demonstrated a higher than expected incidence of VTE (observed/expected = 1.32). The objective of this study was to identify potential causes of this discrepancy.
- Design: Retrospective case-control study.
- Setting: Urban academic medical center with high case-mix indices (Medicare approximately 2.4, non-Medicare approximately 2.0).
- Participants: 102 surgical inpatients with VTE (September 2012 to October 2015) matched with controls for age, gender, and type of procedure.
- Measurements: Prevalence of common VTE risk factors, length of stay, number of procedures, index operation times, and postoperative bed rest > 12 hours were assessed. Utilization of and compliance with our VTE risk assessment tool was also investigated.
- Results: Cases underwent more procedures and had longer lengths of stay and index procedures than controls. In addition, cases were more likely to have had > 12 hours of postoperative bed rest and central venous access than controls. Cases had more infections and were more likely to have severe lung disease, thrombophilia, and a history of prior VTE than controls. No differences in body mass index, tobacco use, current or previous malignancy, or VTE risk assessment form use were observed. Overall, care complexity and risk factors were equally important in determining VTE incidence. Our analyses also revealed lack of strict adherence to our VTE risk stratification protocol and frequent use of suboptimal prophylactic regimens.
- Conclusion: Well-accepted risk factors and overall care complexity determine VTE risk. Preventing VTE in high-risk patients requires assiduous attention to detail in VTE risk assessment and in delivery of optimal prophylaxis. Patients at especially high risk may require customized prophylactic regimens.
Keywords: hospital-acquired venous thromboembolic disease; VTE prophylaxis, surgical patients.
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are well-recognized causes of morbidity and mortality in surgical patients. Between 350,000 and 600,000 cases of venous thromboembolism (VTE) occur each year in the United States, and it is responsible for approximately 10% of preventable in-hospital fatalities.1-3 Given VTE’s impact on patients and the healthcare system and the fact that it is preventable, intense effort has been focused on developing more effective prophylactic measures to decrease its incidence.2-4 In 2008, the surgeon general issued a “call to action” for increased efforts to prevent VTE.5
The American College of Chest Physicians (ACCP) guidelines subcategorize patients based on type of surgery. In addition, the ACCP guidelines support the use of a Caprini-based scoring system to aid in risk stratification and improve clinical decision-making (
Our hospital, a 350-bed academic medical center in downtown Boston, MA, serving a diverse population with a very high case-mix index (2.4 Medicare and 2.0 non-Medicare), has strict protocols for VTE prophylaxis consistent with the ACCP guidelines and based on the Surgical Care Improvement Project (SCIP) measures published in 2006.10 The SCIP mandates allow for considerable surgeon discretion in the use of chemoprophylaxis for neurosurgical cases and general and orthopedic surgery cases deemed to be at high risk for bleeding. In addition, SCIP requires only that prophylaxis be initiated within 24 hours of surgical end time. Although recent audits revealed nearly 100% compliance with SCIP-mandated protocols, National Surgical Quality Improvement Program (NSQIP) data showed that the incidence of VTE events at our institution was higher than expected (observed/expected [O/E] = 1.32).
In order to determine the reasons for this mismatch between process and outcome performance, we investigated whether there were characteristics of our patient population that contributed to the higher than expected rates of VTE, and we scrutinized our VTE prophylaxis protocol to determine if there were aspects of our process that were also contributory.
Methods
Study Sample
This is a retrospective case-control study of surgical inpatients at our hospital during the period September 2012 to October 2015. Cases were identified as patients diagnosed with a VTE (DVT or PE). Controls were identified from a pool of surgical patients whose courses were not complicated by VTE during the same time frame as the cases and who were matched as closely as possible by procedure code, age, and gender.
Variables
Patient and hospital course variables that were analyzed included demographics, comorbidities, length of stay, number of procedures, index operation times, duration of postoperative bed rest, use of mechanical prophylaxis, and type of chemoprophylaxis and time frame within which it was initiated. Data were collected via chart review using International Classification of Diseases-9 and -10 codes to identify surgical cases within the allotted time period who were diagnosed with VTE. Demographic variables included age, sex, and ethnicity. Comorbidities included hypertension, diabetes, coronary artery disease, serious lung disease, previous or current malignancy, documented hypercoagulable state, and previous history of VTE. Body mass index (BMI) was also recorded. The aforementioned disease-specific variables were not matched between the case and control groups, as this data was obtained retrospectively during data collection.
Analysis
Associations between case and matched control were analyzed using the paired t-test for continuous variables and McNemar’s test for categorical variables. P values < 0.05 were considered statistically significant. SAS Enterprise Guide 7.15 (Cary, NC) was used for all statistical analyses.
The requirement for informed consent was waived by our Institutional Review Board, as the study was initially deemed to be a quality improvement project, and all data used for this report were de-identified.
Results
Our retrospective case-control analysis included a sample of 102 surgical patients whose courses were complicated by VTE between September 2012 and October 2015. The cases were distributed among 6 different surgical categories (Figure 1): trauma (20%), cancer (10%), cardiovascular (21%), noncancer neurosurgery (28%), elective orthopedics (11%), and miscellaneous general surgery (10%).
Comparisons between cases and controls in terms of patient demographics and risk factors are shown in Table 2. No statistically significant difference was observed in ethnicity or race between the 2 groups. Overall, cases had more hip/pelvis/leg fractures at presentation (P = 0.0008). The case group also had higher proportions of patients with postoperative bed rest greater than 12 hours (P = 0.009), central venous access (P < 0.0001), infection (P < 0.0001), and lower extremity edema documented during the hospitalization prior to development of DVT (P < 0.0001). Additionally, cases had significantly greater rates of previous VTE (P = 0.0004), inherited or acquired thrombophilia (P = 0.03), history of stroke (P = 0.0003), and severe lung disease, including pneumonia (P = 0.0008). No significant differences were noted between cases and matched controls in BMI (P = 0.43), current tobacco use (P = 0.71), current malignancy (P = 0.80), previous malignancy (P = 0.83), head trauma (P = 0.17), or acute cardiac disease (myocardial infarction or congestive heart failure; P = 0.12).
Variables felt to indicate overall complexity of hospital course for cases as compared to controls are outlined in Table 3. Cases were found to have significantly longer lengths of stay (median, 15.5 days versus 3 days, P < 0.0001). To account for the possibility that the development of VTE contributed to the increased length of stay in the cases, we also looked at the duration between admission date and the date of VTE diagnosis and determined that cases still had a longer length of stay when this was accounted for (median, 7 days versus 3 days, P < 0.0001). A much higher proportion of cases underwent more than 1 procedure compared to controls (P < 0.0001), and cases had significantly longer index operations as compared to controls (P = 0.002).
Seventeen cases received heparin on induction during their index procedure, compared to 23 controls (P = 0.24). Additionally, 63 cases began a prophylaxis regimen within 24 hours of surgery end time, compared to 68 controls (P = 0.24). The chemoprophylactic regimens utilized in cases and in controls are summarized in Figure 2. Of note, only 26 cases and 32 controls received standard prophylactic regimens with no missed doses (heparin 5000 units 3 times daily or enoxaparin 40 mg daily). Additionally, in over half of cases and a third of controls, nonstandard regimens were ordered. Examples of nonstandard regimens included nonstandard heparin or enoxaparin doses, low-dose warfarin, or aspirin alone. In most cases, nonstandard regimens were justified on the basis of high risk for bleeding.
Mechanical prophylaxis with pneumatic sequential compression devices (SCDs) was ordered in 93 (91%) cases and 87 (85%) controls; however, we were unable to accurately document uniform compliance in the use of these devices.
With regard to evaluation of our process measures, we found only 17% of cases and controls combined actually had a VTE risk assessment in their chart, and when it was present, it was often incomplete or was completed inaccurately.
Discussion
The goal of this study was to identify factors (patient characteristics and/or processes of care) that may be contributing to the higher than expected incidence of VTE events at our medical center, despite internal audits suggesting near perfect compliance with SCIP-mandated protocols. We found that in addition to usual risk factors for VTE, an overarching theme of our case cohort was their high complexity of illness. At baseline, these patients had significantly greater rates of stroke, thrombophilia, severe lung disease, infection, and history of VTE than controls. Moreover, the hospital courses of cases were significantly more complex than those of controls, as these patients had more procedures, longer lengths of stay and longer index operations, higher rates of postoperative bed rest exceeding 12 hours, and more prevalent central venous access than controls (Table 2). Several of these risk factors have been found to contribute to VTE development despite compliance with prophylaxis protocols.
Cassidy et al reviewed a cohort of nontrauma general surgery patients who developed VTE despite receiving appropriate prophylaxis and found that both multiple operations and emergency procedures contributed to the failure of VTE prophylaxis.11 Similarly, Wang et al identified several independent risk factors for VTE despite thromboprophylaxis, including central venous access and infection, as well as intensive care unit admission, hospitalization for cranial surgery, and admission from a long-term care facility.12 While our study did not capture some of these additional factors considered by Wang et al, the presence of risk factors not captured in traditional assessment tools suggests that additional consideration for complex patients is warranted.
In addition to these nonmodifiable patient characteristics, aspects of our VTE prophylaxis processes likely contributed to the higher than expected rate of VTE. While the electronic medical record at our institution does contain a VTE risk assessment tool based on the Caprini score, we found it often is not used at all or is used incorrectly/incompletely, which likely reflects the fact that physicians are neither prompted nor required to complete the assessment prior to prescribing VTE prophylaxis.
There is a significant body of evidence demonstrating that mandatory computerized VTE risk assessments can effectively reduce VTE rates and that improved outcomes occur shortly after implementation. Cassidy et al demonstrated the benefits of instituting a hospital-wide, mandatory, Caprini-based computerized VTE risk assessment that provides prophylaxis/early ambulation recommendations. Two years after implementing this system, they observed an 84% reduction in DVTs (P < 0.001) and a 55% reduction in PEs (P < 0.001).13 Nimeri et al had similarly impressive success, achieving a reduction in their NSQIP O/E for PE/DVT in general surgery from 6.00 in 2010 to 0.82 (for DVTs) and 0.78 (for PEs) 5 years after implementation of mandatory VTE risk assessment (though they noted that the most dramatic reduction occurred 1 year after implementation).14 Additionally, a recent systematic review and meta-analysis by Borab et al found computerized VTE risk assessments to be associated with a significant decrease in VTE events.15
The risk assessment tool used at our institution is qualitative in nature, and current literature suggests that employing a more quantitative tool may yield improved outcomes. Numerous studies have highlighted the importance of identifying patients at very high risk for VTE, as higher risk may necessitate more careful consideration of their prophylactic regimens. Obi et al found patients with Caprini scores higher than 8 to be at significantly greater risk of developing VTE compared to patients with scores of 7 or 8. Also, patients with scores of 7 or 8 were significantly more likely to have a VTE compared to those with scores of 5 or 6.16 In another study, Lobastov et al identified Caprini scores of 11 or higher as representing an extremely high-risk category for which standard prophylaxis regimens may not be effective.17 Thus, while having mandatory risk assessment has been shown to dramatically decrease VTE incidence, it is important to consider the magnitude of the numerical risk score. This is of particular importance at medical centers with high case-mix indices where patients at the highest risk might need to be managed with different prophylactic guidelines.
Another notable aspect of the process at our hospital was the great variation in the types of prophylactic regimens ordered, and the adherence to what was ordered. Only 25.5% of patients were maintained on a standard prophylactic regimen with no missed doses (heparin 5000 every 8 hours or enoxaparin 40 mg daily). Thus, the vast majority of the patients who went on to develop VTE either were prescribed a nontraditional prophylaxis regimen or missed doses of standard agents. The need for secondary surgical procedures or other invasive interventions may explain many, but not all, of the missed doses.
The timing of prophylaxis initiation for our patients was also found to deviate from accepted standards. Only 16.8% of cases received prophylaxis upon induction of anesthesia, and furthermore, 38% of cases did not receive any anticoagulation within 24 hours of their index operation. While this variability in prophylaxis implementation was acceptable within the SCIP guidelines based on “high risk for bleeding” or other considerations, it likely contributed to our suboptimal outcomes. The variations and interruptions in prophylactic regimens speak to barriers that have previously been reported as contributing factors to noncompliance with VTE prophylaxis.18
Given these known barriers and the observed underutilization and improper use of our risk assessment tool, we have recently changed our surgical admission order sets such that a mandatory quantitative risk assessment must be done for every surgical patient at the time of admission/operation before other orders can be completed. Following completion of the assessment, the physician will be presented with an appropriate standard regimen based on the individual patient’s risk assessment. Early results of our VTE quality improvement project have been satisfying: in the most recent NSQIP semi-annual report, our O/E for VTE was 0.74, placing us in the first decile. Some of these early reports may simply be the product of the Hawthorne effect; however, we are encouraged by the early improvements seen in other research. While we are hopeful that these changes will result in sustainable improvements in outcomes, patients at extremely high risk may require novel weight-based or otherwise customized aggressive prophylactic regimens. Such regimens have already been proposed for arthroplasty and other high-risk patients.
Future research may identify other risk factors not captured by traditional risk assessments. In addition, research should continue to explore the use and efficacy of standard prophylactic regimens in these populations to help determine if they are sufficient. Currently, weight-based low-molecular-weight heparin dosing and alternative regimens employing fondaparinux are under investigation for very-high-risk patients.19
There were several limitations to the present study. First, due to the retrospective design of our study, we could collect only data that had been uniformly recorded in the charts throughout the study period. Second, we were unable to accurately assess compliance with mechanical prophylaxis. While our chart review showed that the vast majority of cases and controls were ordered to have mechanical prophylaxis, it is impossible to document how often these devices were used appropriately in a retrospective analysis. Anecdotal observation suggests that once patients are out of post-anesthesia or critical care units, SCD use is not standardized. The inability to measure compliance precisely may be leading to an overestimation of our compliance with prophylaxis. Finally, because our study included only patients who underwent surgery at our hospital, our observations may not be generalizable outside our institution.
Conclusion
Our study findings reinforce the importance of attention to detail in VTE risk assessment and in ordering and administering VTE prophylactic regimens, especially in high-risk surgical patients. While we adhered to the SCIP-mandated prophylaxis requirements, the complexity of our patients and our lack of a truly standardized approach to risk assessment and prophylactic regimens resulted in suboptimal outcomes. Stricter and more quantitative mandatory VTE risk assessment, along with highly standardized VTE prophylaxis regimens, are required to achieve optimal outcomes.
Corresponding author: Jason C. DeGiovanni, MS, BA, Jason.DeGiovanni@tufts.edu.
Financial disclosures: None.
From Tufts Medical Center, Boston, MA.
Abstract
- Objective: Audits at our academic medical center revealed near 100% compliance with protocols for perioperative venous thromboembolism (VTE) prophylaxis, but recent National Surgical Quality Improvement Program data demonstrated a higher than expected incidence of VTE (observed/expected = 1.32). The objective of this study was to identify potential causes of this discrepancy.
- Design: Retrospective case-control study.
- Setting: Urban academic medical center with high case-mix indices (Medicare approximately 2.4, non-Medicare approximately 2.0).
- Participants: 102 surgical inpatients with VTE (September 2012 to October 2015) matched with controls for age, gender, and type of procedure.
- Measurements: Prevalence of common VTE risk factors, length of stay, number of procedures, index operation times, and postoperative bed rest > 12 hours were assessed. Utilization of and compliance with our VTE risk assessment tool was also investigated.
- Results: Cases underwent more procedures and had longer lengths of stay and index procedures than controls. In addition, cases were more likely to have had > 12 hours of postoperative bed rest and central venous access than controls. Cases had more infections and were more likely to have severe lung disease, thrombophilia, and a history of prior VTE than controls. No differences in body mass index, tobacco use, current or previous malignancy, or VTE risk assessment form use were observed. Overall, care complexity and risk factors were equally important in determining VTE incidence. Our analyses also revealed lack of strict adherence to our VTE risk stratification protocol and frequent use of suboptimal prophylactic regimens.
- Conclusion: Well-accepted risk factors and overall care complexity determine VTE risk. Preventing VTE in high-risk patients requires assiduous attention to detail in VTE risk assessment and in delivery of optimal prophylaxis. Patients at especially high risk may require customized prophylactic regimens.
Keywords: hospital-acquired venous thromboembolic disease; VTE prophylaxis, surgical patients.
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are well-recognized causes of morbidity and mortality in surgical patients. Between 350,000 and 600,000 cases of venous thromboembolism (VTE) occur each year in the United States, and it is responsible for approximately 10% of preventable in-hospital fatalities.1-3 Given VTE’s impact on patients and the healthcare system and the fact that it is preventable, intense effort has been focused on developing more effective prophylactic measures to decrease its incidence.2-4 In 2008, the surgeon general issued a “call to action” for increased efforts to prevent VTE.5
The American College of Chest Physicians (ACCP) guidelines subcategorize patients based on type of surgery. In addition, the ACCP guidelines support the use of a Caprini-based scoring system to aid in risk stratification and improve clinical decision-making (
Our hospital, a 350-bed academic medical center in downtown Boston, MA, serving a diverse population with a very high case-mix index (2.4 Medicare and 2.0 non-Medicare), has strict protocols for VTE prophylaxis consistent with the ACCP guidelines and based on the Surgical Care Improvement Project (SCIP) measures published in 2006.10 The SCIP mandates allow for considerable surgeon discretion in the use of chemoprophylaxis for neurosurgical cases and general and orthopedic surgery cases deemed to be at high risk for bleeding. In addition, SCIP requires only that prophylaxis be initiated within 24 hours of surgical end time. Although recent audits revealed nearly 100% compliance with SCIP-mandated protocols, National Surgical Quality Improvement Program (NSQIP) data showed that the incidence of VTE events at our institution was higher than expected (observed/expected [O/E] = 1.32).
In order to determine the reasons for this mismatch between process and outcome performance, we investigated whether there were characteristics of our patient population that contributed to the higher than expected rates of VTE, and we scrutinized our VTE prophylaxis protocol to determine if there were aspects of our process that were also contributory.
Methods
Study Sample
This is a retrospective case-control study of surgical inpatients at our hospital during the period September 2012 to October 2015. Cases were identified as patients diagnosed with a VTE (DVT or PE). Controls were identified from a pool of surgical patients whose courses were not complicated by VTE during the same time frame as the cases and who were matched as closely as possible by procedure code, age, and gender.
Variables
Patient and hospital course variables that were analyzed included demographics, comorbidities, length of stay, number of procedures, index operation times, duration of postoperative bed rest, use of mechanical prophylaxis, and type of chemoprophylaxis and time frame within which it was initiated. Data were collected via chart review using International Classification of Diseases-9 and -10 codes to identify surgical cases within the allotted time period who were diagnosed with VTE. Demographic variables included age, sex, and ethnicity. Comorbidities included hypertension, diabetes, coronary artery disease, serious lung disease, previous or current malignancy, documented hypercoagulable state, and previous history of VTE. Body mass index (BMI) was also recorded. The aforementioned disease-specific variables were not matched between the case and control groups, as this data was obtained retrospectively during data collection.
Analysis
Associations between case and matched control were analyzed using the paired t-test for continuous variables and McNemar’s test for categorical variables. P values < 0.05 were considered statistically significant. SAS Enterprise Guide 7.15 (Cary, NC) was used for all statistical analyses.
The requirement for informed consent was waived by our Institutional Review Board, as the study was initially deemed to be a quality improvement project, and all data used for this report were de-identified.
Results
Our retrospective case-control analysis included a sample of 102 surgical patients whose courses were complicated by VTE between September 2012 and October 2015. The cases were distributed among 6 different surgical categories (Figure 1): trauma (20%), cancer (10%), cardiovascular (21%), noncancer neurosurgery (28%), elective orthopedics (11%), and miscellaneous general surgery (10%).
Comparisons between cases and controls in terms of patient demographics and risk factors are shown in Table 2. No statistically significant difference was observed in ethnicity or race between the 2 groups. Overall, cases had more hip/pelvis/leg fractures at presentation (P = 0.0008). The case group also had higher proportions of patients with postoperative bed rest greater than 12 hours (P = 0.009), central venous access (P < 0.0001), infection (P < 0.0001), and lower extremity edema documented during the hospitalization prior to development of DVT (P < 0.0001). Additionally, cases had significantly greater rates of previous VTE (P = 0.0004), inherited or acquired thrombophilia (P = 0.03), history of stroke (P = 0.0003), and severe lung disease, including pneumonia (P = 0.0008). No significant differences were noted between cases and matched controls in BMI (P = 0.43), current tobacco use (P = 0.71), current malignancy (P = 0.80), previous malignancy (P = 0.83), head trauma (P = 0.17), or acute cardiac disease (myocardial infarction or congestive heart failure; P = 0.12).
Variables felt to indicate overall complexity of hospital course for cases as compared to controls are outlined in Table 3. Cases were found to have significantly longer lengths of stay (median, 15.5 days versus 3 days, P < 0.0001). To account for the possibility that the development of VTE contributed to the increased length of stay in the cases, we also looked at the duration between admission date and the date of VTE diagnosis and determined that cases still had a longer length of stay when this was accounted for (median, 7 days versus 3 days, P < 0.0001). A much higher proportion of cases underwent more than 1 procedure compared to controls (P < 0.0001), and cases had significantly longer index operations as compared to controls (P = 0.002).
Seventeen cases received heparin on induction during their index procedure, compared to 23 controls (P = 0.24). Additionally, 63 cases began a prophylaxis regimen within 24 hours of surgery end time, compared to 68 controls (P = 0.24). The chemoprophylactic regimens utilized in cases and in controls are summarized in Figure 2. Of note, only 26 cases and 32 controls received standard prophylactic regimens with no missed doses (heparin 5000 units 3 times daily or enoxaparin 40 mg daily). Additionally, in over half of cases and a third of controls, nonstandard regimens were ordered. Examples of nonstandard regimens included nonstandard heparin or enoxaparin doses, low-dose warfarin, or aspirin alone. In most cases, nonstandard regimens were justified on the basis of high risk for bleeding.
Mechanical prophylaxis with pneumatic sequential compression devices (SCDs) was ordered in 93 (91%) cases and 87 (85%) controls; however, we were unable to accurately document uniform compliance in the use of these devices.
With regard to evaluation of our process measures, we found only 17% of cases and controls combined actually had a VTE risk assessment in their chart, and when it was present, it was often incomplete or was completed inaccurately.
Discussion
The goal of this study was to identify factors (patient characteristics and/or processes of care) that may be contributing to the higher than expected incidence of VTE events at our medical center, despite internal audits suggesting near perfect compliance with SCIP-mandated protocols. We found that in addition to usual risk factors for VTE, an overarching theme of our case cohort was their high complexity of illness. At baseline, these patients had significantly greater rates of stroke, thrombophilia, severe lung disease, infection, and history of VTE than controls. Moreover, the hospital courses of cases were significantly more complex than those of controls, as these patients had more procedures, longer lengths of stay and longer index operations, higher rates of postoperative bed rest exceeding 12 hours, and more prevalent central venous access than controls (Table 2). Several of these risk factors have been found to contribute to VTE development despite compliance with prophylaxis protocols.
Cassidy et al reviewed a cohort of nontrauma general surgery patients who developed VTE despite receiving appropriate prophylaxis and found that both multiple operations and emergency procedures contributed to the failure of VTE prophylaxis.11 Similarly, Wang et al identified several independent risk factors for VTE despite thromboprophylaxis, including central venous access and infection, as well as intensive care unit admission, hospitalization for cranial surgery, and admission from a long-term care facility.12 While our study did not capture some of these additional factors considered by Wang et al, the presence of risk factors not captured in traditional assessment tools suggests that additional consideration for complex patients is warranted.
In addition to these nonmodifiable patient characteristics, aspects of our VTE prophylaxis processes likely contributed to the higher than expected rate of VTE. While the electronic medical record at our institution does contain a VTE risk assessment tool based on the Caprini score, we found it often is not used at all or is used incorrectly/incompletely, which likely reflects the fact that physicians are neither prompted nor required to complete the assessment prior to prescribing VTE prophylaxis.
There is a significant body of evidence demonstrating that mandatory computerized VTE risk assessments can effectively reduce VTE rates and that improved outcomes occur shortly after implementation. Cassidy et al demonstrated the benefits of instituting a hospital-wide, mandatory, Caprini-based computerized VTE risk assessment that provides prophylaxis/early ambulation recommendations. Two years after implementing this system, they observed an 84% reduction in DVTs (P < 0.001) and a 55% reduction in PEs (P < 0.001).13 Nimeri et al had similarly impressive success, achieving a reduction in their NSQIP O/E for PE/DVT in general surgery from 6.00 in 2010 to 0.82 (for DVTs) and 0.78 (for PEs) 5 years after implementation of mandatory VTE risk assessment (though they noted that the most dramatic reduction occurred 1 year after implementation).14 Additionally, a recent systematic review and meta-analysis by Borab et al found computerized VTE risk assessments to be associated with a significant decrease in VTE events.15
The risk assessment tool used at our institution is qualitative in nature, and current literature suggests that employing a more quantitative tool may yield improved outcomes. Numerous studies have highlighted the importance of identifying patients at very high risk for VTE, as higher risk may necessitate more careful consideration of their prophylactic regimens. Obi et al found patients with Caprini scores higher than 8 to be at significantly greater risk of developing VTE compared to patients with scores of 7 or 8. Also, patients with scores of 7 or 8 were significantly more likely to have a VTE compared to those with scores of 5 or 6.16 In another study, Lobastov et al identified Caprini scores of 11 or higher as representing an extremely high-risk category for which standard prophylaxis regimens may not be effective.17 Thus, while having mandatory risk assessment has been shown to dramatically decrease VTE incidence, it is important to consider the magnitude of the numerical risk score. This is of particular importance at medical centers with high case-mix indices where patients at the highest risk might need to be managed with different prophylactic guidelines.
Another notable aspect of the process at our hospital was the great variation in the types of prophylactic regimens ordered, and the adherence to what was ordered. Only 25.5% of patients were maintained on a standard prophylactic regimen with no missed doses (heparin 5000 every 8 hours or enoxaparin 40 mg daily). Thus, the vast majority of the patients who went on to develop VTE either were prescribed a nontraditional prophylaxis regimen or missed doses of standard agents. The need for secondary surgical procedures or other invasive interventions may explain many, but not all, of the missed doses.
The timing of prophylaxis initiation for our patients was also found to deviate from accepted standards. Only 16.8% of cases received prophylaxis upon induction of anesthesia, and furthermore, 38% of cases did not receive any anticoagulation within 24 hours of their index operation. While this variability in prophylaxis implementation was acceptable within the SCIP guidelines based on “high risk for bleeding” or other considerations, it likely contributed to our suboptimal outcomes. The variations and interruptions in prophylactic regimens speak to barriers that have previously been reported as contributing factors to noncompliance with VTE prophylaxis.18
Given these known barriers and the observed underutilization and improper use of our risk assessment tool, we have recently changed our surgical admission order sets such that a mandatory quantitative risk assessment must be done for every surgical patient at the time of admission/operation before other orders can be completed. Following completion of the assessment, the physician will be presented with an appropriate standard regimen based on the individual patient’s risk assessment. Early results of our VTE quality improvement project have been satisfying: in the most recent NSQIP semi-annual report, our O/E for VTE was 0.74, placing us in the first decile. Some of these early reports may simply be the product of the Hawthorne effect; however, we are encouraged by the early improvements seen in other research. While we are hopeful that these changes will result in sustainable improvements in outcomes, patients at extremely high risk may require novel weight-based or otherwise customized aggressive prophylactic regimens. Such regimens have already been proposed for arthroplasty and other high-risk patients.
Future research may identify other risk factors not captured by traditional risk assessments. In addition, research should continue to explore the use and efficacy of standard prophylactic regimens in these populations to help determine if they are sufficient. Currently, weight-based low-molecular-weight heparin dosing and alternative regimens employing fondaparinux are under investigation for very-high-risk patients.19
There were several limitations to the present study. First, due to the retrospective design of our study, we could collect only data that had been uniformly recorded in the charts throughout the study period. Second, we were unable to accurately assess compliance with mechanical prophylaxis. While our chart review showed that the vast majority of cases and controls were ordered to have mechanical prophylaxis, it is impossible to document how often these devices were used appropriately in a retrospective analysis. Anecdotal observation suggests that once patients are out of post-anesthesia or critical care units, SCD use is not standardized. The inability to measure compliance precisely may be leading to an overestimation of our compliance with prophylaxis. Finally, because our study included only patients who underwent surgery at our hospital, our observations may not be generalizable outside our institution.
Conclusion
Our study findings reinforce the importance of attention to detail in VTE risk assessment and in ordering and administering VTE prophylactic regimens, especially in high-risk surgical patients. While we adhered to the SCIP-mandated prophylaxis requirements, the complexity of our patients and our lack of a truly standardized approach to risk assessment and prophylactic regimens resulted in suboptimal outcomes. Stricter and more quantitative mandatory VTE risk assessment, along with highly standardized VTE prophylaxis regimens, are required to achieve optimal outcomes.
Corresponding author: Jason C. DeGiovanni, MS, BA, Jason.DeGiovanni@tufts.edu.
Financial disclosures: None.
1. Spyropoulos AC, Hussein M, Lin J, et al. Rates of symptomatic venous thromboembolism in US surgical patients: a retrospective administrative database study. J Thromb Thrombolysis. 2009;28:458-464.
2. Deitzelzweig SB, Johnson BH, Lin J, et al. Prevalence of clinical venous thromboembolism in the USA: Current trends and future projections. Am J Hematol. 2011;86:217-220.
3. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717.
4. Guyatt GH, Akl EA, Crowther M, et al. Introduction to the ninth edition: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):48S-52S.
5. Office of the Surgeon General; National Heart, Lung, and Blood Institute. The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: Office of the Surgeon General; 2008. www.ncbi.nlm.nih.gov/books/NBK44178/. Accessed May 2, 2019.
6. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1102.
7. Caprini JA, Arcelus JI, Hasty JH, et al. Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost. 1991;17(suppl 3):304-312.
8. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199:S3-S10.
9. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S-e277S.
10. The Joint Commission. Surgical Care Improvement Project (SCIP) Measure Information Form (Version 2.1c). www.jointcommission.org/surgical_care_improvement_project_scip_measure_information_form_version_21c/. Accessed June 22, 2016.
11. Cassidy MR, Macht RD, Rosenkranz P, et al. Patterns of failure of a standardized perioperative venous thromboembolism prophylaxis protocol. J Am Coll Surg. 2016;222:1074-1081.
12. Wang TF, Wong CA, Milligan PE, et al. Risk factors for inpatient venous thromboembolism despite thromboprophylaxis. Thromb Res. 2014;133:25-29.
13. Cassidy MR, Rosenkranz P, McAneny D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization program. J Am Coll Surg. 2014;218:1095-1104.
14. Nimeri AA, Gamaleldin MM, McKenna KL, et al. Reduction of venous thromboembolism in surgical patients using a mandatory risk-scoring system: 5-year follow-up of an American College of Surgeons National Quality Improvement Program. Clin Appl Thromb Hemost. 2017;23:392-396.
15. Borab ZM, Lanni MA, Tecce MG, et al. Use of computerized clinical decision support systems to prevent venous thromboembolism in surgical patients: a systematic review and meta-analysis. JAMA Surg. 2017;152:638–645.
16. Obi AT, Pannucci CJ, Nackashi A, et al. Validation of the Caprini venous thromboembolism risk assessment model in critically ill surgical patients. JAMA Surg. 2015;150:941-948.
17. Lobastov K, Barinov V, Schastlivtsev I, et al. Validation of the Caprini risk assessment model for venous thromboembolism in high-risk surgical patients in the background of standard prophylaxis. J Vasc Surg Venous Lymphat Disord. 2016;4:153-160.
18. Kakkar AK, Cohen AT, Tapson VF, et al. Venous thromboembolism risk and prophylaxis in the acute care hospital setting (ENDORSE survey): findings in surgical patients. Ann Surg. 2010;251:330-338.
19. Smythe MA, Priziola J, Dobesh PP, et al. Guidance for the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:165-186.
1. Spyropoulos AC, Hussein M, Lin J, et al. Rates of symptomatic venous thromboembolism in US surgical patients: a retrospective administrative database study. J Thromb Thrombolysis. 2009;28:458-464.
2. Deitzelzweig SB, Johnson BH, Lin J, et al. Prevalence of clinical venous thromboembolism in the USA: Current trends and future projections. Am J Hematol. 2011;86:217-220.
3. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch Intern Med. 2003;163:1711-1717.
4. Guyatt GH, Akl EA, Crowther M, et al. Introduction to the ninth edition: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl):48S-52S.
5. Office of the Surgeon General; National Heart, Lung, and Blood Institute. The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: Office of the Surgeon General; 2008. www.ncbi.nlm.nih.gov/books/NBK44178/. Accessed May 2, 2019.
6. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1102.
7. Caprini JA, Arcelus JI, Hasty JH, et al. Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost. 1991;17(suppl 3):304-312.
8. Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg. 2010;199:S3-S10.
9. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S-e277S.
10. The Joint Commission. Surgical Care Improvement Project (SCIP) Measure Information Form (Version 2.1c). www.jointcommission.org/surgical_care_improvement_project_scip_measure_information_form_version_21c/. Accessed June 22, 2016.
11. Cassidy MR, Macht RD, Rosenkranz P, et al. Patterns of failure of a standardized perioperative venous thromboembolism prophylaxis protocol. J Am Coll Surg. 2016;222:1074-1081.
12. Wang TF, Wong CA, Milligan PE, et al. Risk factors for inpatient venous thromboembolism despite thromboprophylaxis. Thromb Res. 2014;133:25-29.
13. Cassidy MR, Rosenkranz P, McAneny D. Reducing postoperative venous thromboembolism complications with a standardized risk-stratified prophylaxis protocol and mobilization program. J Am Coll Surg. 2014;218:1095-1104.
14. Nimeri AA, Gamaleldin MM, McKenna KL, et al. Reduction of venous thromboembolism in surgical patients using a mandatory risk-scoring system: 5-year follow-up of an American College of Surgeons National Quality Improvement Program. Clin Appl Thromb Hemost. 2017;23:392-396.
15. Borab ZM, Lanni MA, Tecce MG, et al. Use of computerized clinical decision support systems to prevent venous thromboembolism in surgical patients: a systematic review and meta-analysis. JAMA Surg. 2017;152:638–645.
16. Obi AT, Pannucci CJ, Nackashi A, et al. Validation of the Caprini venous thromboembolism risk assessment model in critically ill surgical patients. JAMA Surg. 2015;150:941-948.
17. Lobastov K, Barinov V, Schastlivtsev I, et al. Validation of the Caprini risk assessment model for venous thromboembolism in high-risk surgical patients in the background of standard prophylaxis. J Vasc Surg Venous Lymphat Disord. 2016;4:153-160.
18. Kakkar AK, Cohen AT, Tapson VF, et al. Venous thromboembolism risk and prophylaxis in the acute care hospital setting (ENDORSE survey): findings in surgical patients. Ann Surg. 2010;251:330-338.
19. Smythe MA, Priziola J, Dobesh PP, et al. Guidance for the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:165-186.
Laparoscopic surgery survival outcomes on par with open approach in colorectal liver metastases
CHICAGO – For colorectal cancer patients with liver metastases, laparoscopic surgery has short-term advantages over open surgery, including fewer complications and better quality of life as compared to open surgery. Now, there are data to show that long-term outcomes with the laparoscopic approach aren’t any worse with the laparoscopic approach.
In a video interview at the annual meeting of the American Society of Clinical Oncology, Åsmund Avdem Fretland, MD, discusses results of the 280-patient randomized OSLO-COMET study, including 5-year survival of 56% for the laparoscopic approach, and similarly, 57% for the open procedure.
Based on lower morbidity, and now similar life expectancy, more centers should be doing laparoscopic procedures for liver metastases, said Dr. Fretland, a surgeon in the department of HPB surgery at Oslo University Hospital.
For now, however, open surgery appears to be the dominant approach. According to a recent survey, just 22% of U.S. patients with colorectal liver metastases have laparoscopic surgery.
More data could help. Dr. Fretland said in the interview that more randomized trials are underway aimed at evaluating the long-term outcomes of laparoscopic versus open procedures.
Dr. Fretland reported honoraria from Olympus Medical Systems.
CHICAGO – For colorectal cancer patients with liver metastases, laparoscopic surgery has short-term advantages over open surgery, including fewer complications and better quality of life as compared to open surgery. Now, there are data to show that long-term outcomes with the laparoscopic approach aren’t any worse with the laparoscopic approach.
In a video interview at the annual meeting of the American Society of Clinical Oncology, Åsmund Avdem Fretland, MD, discusses results of the 280-patient randomized OSLO-COMET study, including 5-year survival of 56% for the laparoscopic approach, and similarly, 57% for the open procedure.
Based on lower morbidity, and now similar life expectancy, more centers should be doing laparoscopic procedures for liver metastases, said Dr. Fretland, a surgeon in the department of HPB surgery at Oslo University Hospital.
For now, however, open surgery appears to be the dominant approach. According to a recent survey, just 22% of U.S. patients with colorectal liver metastases have laparoscopic surgery.
More data could help. Dr. Fretland said in the interview that more randomized trials are underway aimed at evaluating the long-term outcomes of laparoscopic versus open procedures.
Dr. Fretland reported honoraria from Olympus Medical Systems.
CHICAGO – For colorectal cancer patients with liver metastases, laparoscopic surgery has short-term advantages over open surgery, including fewer complications and better quality of life as compared to open surgery. Now, there are data to show that long-term outcomes with the laparoscopic approach aren’t any worse with the laparoscopic approach.
In a video interview at the annual meeting of the American Society of Clinical Oncology, Åsmund Avdem Fretland, MD, discusses results of the 280-patient randomized OSLO-COMET study, including 5-year survival of 56% for the laparoscopic approach, and similarly, 57% for the open procedure.
Based on lower morbidity, and now similar life expectancy, more centers should be doing laparoscopic procedures for liver metastases, said Dr. Fretland, a surgeon in the department of HPB surgery at Oslo University Hospital.
For now, however, open surgery appears to be the dominant approach. According to a recent survey, just 22% of U.S. patients with colorectal liver metastases have laparoscopic surgery.
More data could help. Dr. Fretland said in the interview that more randomized trials are underway aimed at evaluating the long-term outcomes of laparoscopic versus open procedures.
Dr. Fretland reported honoraria from Olympus Medical Systems.
REPORTING FROM ASCO 2019
Asymptomatic gallstones seldom require surgical intervention
“Most patients with asymptomatic gallstones never develop symptoms and probably don’t need surgical intervention,” lead study author Gareth Morris-Stiff, MD, PhD, said at the annual Digestive Disease Week.
Dr. Morris-Stiff, of the department of general surgery at Cleveland Clinic, said that, while previous studies have evaluated the time to development of gallstone-related complications following identification of asymptomatic gallstones, factors associated with the need for surgical intervention in this population have not been documented. The aims of the current study were to perform a big data analysis to evaluate risk factors associated with intervention in asymptomatic gallstones and to develop a risk stratification tool to aid in patient consultations by predicting individuals likely to need future intervention for their gallstones.
The researchers included Cleveland Clinic patients with CT/US reports containing “cholelithiasis” or “gallstones” between January 1996 and December 2016. Patients were excluded if they had a concurrent or prior event, had an event within 2 months, or lacked follow-up. Data collection included demographic characteristics, comorbid conditions or surgeries, imaging features, and medication use.
Dr. Morris-Stiff and his colleagues constructed Kaplan-Meier curves to analyze time to intervention and calculated cumulative incidence ratios. They used automated forward stepwise competing risk regression to create their model and receiver operating characteristics curves to analyze it.
Of the 49,414 patients identified with asymptomatic gallstones, 22,257 met criteria for analysis. Slightly more than half (51%) were female, their mean age was 61 years, 80% were white, 16% were black, and the rest were from other racial and ethnic groups. The median follow-up was 4.5 years, and the median follow-up of patients undergoing intervention was 3.9 years. This translated to 112,111 total years of observation.
The researchers found that the cumulative incidence of intervention at 15 years was 25% and it increased linearly from the time of initial diagnosis of asymptomatic gallstones. A total of 1,762 patients (7.9%) underwent a surgical procedure, most often cholecystectomy (5.7%). Three factors were associated with a reduced risk for surgical intervention: increasing age (hazard ratio, 0.94; P less than 0.001), male gender (HR, 0.78; P less than 0.001), and statin use (HR, 0.67; P less than 0.001).
Patient variables associated with an increased need for surgical intervention included obesity (HR, 1.44; P less than 0.001) and having a hemolytic disorder (HR, 2.42; P less than 0.001). Gallstone-specific characteristics that increased the need for surgical intervention included a stone size of greater than 9 mm (HR, 1.56; P less than 0.001), the presence of sludge (HR, 1.46; P less than 0.001), the presence of a polyp (HR, 1.68; P less than 0.001), and having multiple stones (HR, 1.69; P less than 0.001).
The analysis enabled Dr. Morris-Stiff and colleagues to generate a Web-based risk score to reliably identify these patients and provide prognostic information for counseling. An app for smartphones based on the score is being developed. The researchers reported having no financial disclosures.
“Most patients with asymptomatic gallstones never develop symptoms and probably don’t need surgical intervention,” lead study author Gareth Morris-Stiff, MD, PhD, said at the annual Digestive Disease Week.
Dr. Morris-Stiff, of the department of general surgery at Cleveland Clinic, said that, while previous studies have evaluated the time to development of gallstone-related complications following identification of asymptomatic gallstones, factors associated with the need for surgical intervention in this population have not been documented. The aims of the current study were to perform a big data analysis to evaluate risk factors associated with intervention in asymptomatic gallstones and to develop a risk stratification tool to aid in patient consultations by predicting individuals likely to need future intervention for their gallstones.
The researchers included Cleveland Clinic patients with CT/US reports containing “cholelithiasis” or “gallstones” between January 1996 and December 2016. Patients were excluded if they had a concurrent or prior event, had an event within 2 months, or lacked follow-up. Data collection included demographic characteristics, comorbid conditions or surgeries, imaging features, and medication use.
Dr. Morris-Stiff and his colleagues constructed Kaplan-Meier curves to analyze time to intervention and calculated cumulative incidence ratios. They used automated forward stepwise competing risk regression to create their model and receiver operating characteristics curves to analyze it.
Of the 49,414 patients identified with asymptomatic gallstones, 22,257 met criteria for analysis. Slightly more than half (51%) were female, their mean age was 61 years, 80% were white, 16% were black, and the rest were from other racial and ethnic groups. The median follow-up was 4.5 years, and the median follow-up of patients undergoing intervention was 3.9 years. This translated to 112,111 total years of observation.
The researchers found that the cumulative incidence of intervention at 15 years was 25% and it increased linearly from the time of initial diagnosis of asymptomatic gallstones. A total of 1,762 patients (7.9%) underwent a surgical procedure, most often cholecystectomy (5.7%). Three factors were associated with a reduced risk for surgical intervention: increasing age (hazard ratio, 0.94; P less than 0.001), male gender (HR, 0.78; P less than 0.001), and statin use (HR, 0.67; P less than 0.001).
Patient variables associated with an increased need for surgical intervention included obesity (HR, 1.44; P less than 0.001) and having a hemolytic disorder (HR, 2.42; P less than 0.001). Gallstone-specific characteristics that increased the need for surgical intervention included a stone size of greater than 9 mm (HR, 1.56; P less than 0.001), the presence of sludge (HR, 1.46; P less than 0.001), the presence of a polyp (HR, 1.68; P less than 0.001), and having multiple stones (HR, 1.69; P less than 0.001).
The analysis enabled Dr. Morris-Stiff and colleagues to generate a Web-based risk score to reliably identify these patients and provide prognostic information for counseling. An app for smartphones based on the score is being developed. The researchers reported having no financial disclosures.
“Most patients with asymptomatic gallstones never develop symptoms and probably don’t need surgical intervention,” lead study author Gareth Morris-Stiff, MD, PhD, said at the annual Digestive Disease Week.
Dr. Morris-Stiff, of the department of general surgery at Cleveland Clinic, said that, while previous studies have evaluated the time to development of gallstone-related complications following identification of asymptomatic gallstones, factors associated with the need for surgical intervention in this population have not been documented. The aims of the current study were to perform a big data analysis to evaluate risk factors associated with intervention in asymptomatic gallstones and to develop a risk stratification tool to aid in patient consultations by predicting individuals likely to need future intervention for their gallstones.
The researchers included Cleveland Clinic patients with CT/US reports containing “cholelithiasis” or “gallstones” between January 1996 and December 2016. Patients were excluded if they had a concurrent or prior event, had an event within 2 months, or lacked follow-up. Data collection included demographic characteristics, comorbid conditions or surgeries, imaging features, and medication use.
Dr. Morris-Stiff and his colleagues constructed Kaplan-Meier curves to analyze time to intervention and calculated cumulative incidence ratios. They used automated forward stepwise competing risk regression to create their model and receiver operating characteristics curves to analyze it.
Of the 49,414 patients identified with asymptomatic gallstones, 22,257 met criteria for analysis. Slightly more than half (51%) were female, their mean age was 61 years, 80% were white, 16% were black, and the rest were from other racial and ethnic groups. The median follow-up was 4.5 years, and the median follow-up of patients undergoing intervention was 3.9 years. This translated to 112,111 total years of observation.
The researchers found that the cumulative incidence of intervention at 15 years was 25% and it increased linearly from the time of initial diagnosis of asymptomatic gallstones. A total of 1,762 patients (7.9%) underwent a surgical procedure, most often cholecystectomy (5.7%). Three factors were associated with a reduced risk for surgical intervention: increasing age (hazard ratio, 0.94; P less than 0.001), male gender (HR, 0.78; P less than 0.001), and statin use (HR, 0.67; P less than 0.001).
Patient variables associated with an increased need for surgical intervention included obesity (HR, 1.44; P less than 0.001) and having a hemolytic disorder (HR, 2.42; P less than 0.001). Gallstone-specific characteristics that increased the need for surgical intervention included a stone size of greater than 9 mm (HR, 1.56; P less than 0.001), the presence of sludge (HR, 1.46; P less than 0.001), the presence of a polyp (HR, 1.68; P less than 0.001), and having multiple stones (HR, 1.69; P less than 0.001).
The analysis enabled Dr. Morris-Stiff and colleagues to generate a Web-based risk score to reliably identify these patients and provide prognostic information for counseling. An app for smartphones based on the score is being developed. The researchers reported having no financial disclosures.
REPORTING FROM DDW 2019
Transcatheter pulmonary valve shows 5-year durability in postapproval study
LAS VEGAS – that followed 65 patients, a majority of whom were children or teenagers.
After 5 years, 69% of the replacement valve recipients had no valvular hemodynamic dysfunction, compared with a 67% rate among patients enrolled in the original Investigational Device Exemption (IDE) study that led to Food and Drug Administration marketing approval for the Melody valve in 2010 under a humanitarian device exemption. (Full approval followed in 2017.)
The 5-year rate of any reintervention, including explants, was 78% in the postapproval study, again similar to the 76% rate reported in the IDE study after a median 4.5 year follow-up (Circulation. 2015 Jun 2;131[22]:1960-70), Aimee K. Armstrong, MD, said at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The new 5-year postapproval study findings “confirm that the hemodynamic effectiveness achieved by real-world providers is equivalent to the historical control established in the IDE study,” concluded Dr. Armstrong, professor of pediatrics at the Ohio State University and director of cardiac catheterization and interventional therapies at Nationwide Children’s Hospital, both in Columbus.
The postapproval study ran at 10 U.S. centers, none of which were among the five U.S. centers that ran the IDE study. Today, the Melody transcatheter pulmonary valve “is very commonly used” at many additional U.S. sites, Dr. Armstrong said in an interview. And the outcomes achieved using the valve likely surpass those seen in the IDE and postapproval studies because of innovations in technique, such as more routine use of “prestenting,” placing a stent in the vascular site where the pulmonary valve conduit will sit to address stenosis at this location and prevent subsequent conduit fracture (JACC Cardiovasc Interv. 2017 Sep;10[17]:1760-2).
“In 2010 [when the postapproval study began], we didn’t understand the importance of prestenting the way we do now. In 2010, I did not prestent every patient; now I do,” she said. The results reported by Dr. Armstrong included a 5% cumulative rate of major stent fractures in the Melody devices.
The postapproval study results also documented a concerning 4.5% annualized incidence of endocarditis among pulmonary valve recipients, with a nearly 300% increased rate of endocarditis among patients aged 12 years or younger, compared with older patients. Dr. Armstrong cautioned that this age association may be confounded by other factors, such as a residual pressure gradient in the right ventricular outflow tract of 15 mm Hg or greater. “We are discovering that we need to reduce the pressure gradient as much as we can, to perhaps less than 15 mm Hg, to reduce endocarditis, and that is something we did not know even a year ago. Practice is still evolving.”
The Melody Transcatheter Pulmonary Valve Postapproval Study performed cardiac catheterization for valve placement in 121 patients, and successfully implanted the valve for at least 24 hours in 99 of these patients. Patient age ranged from 5 to 45 years, with a median of 17 years; two-thirds were boys or men. The median age of the patients in the postapproval study was about 2 years younger than in the IDE study. Dr. Armstrong and her associates had previously published the 1-year outcomes from the postapproval study (JACC Cardiovasc Interv. 2014 Nov;7[11]:1254-62).
The enrolled patients usually needed a new right ventricular outflow tract because of a congenital heart defect, such as tetralogy of Fallot with pulmonary atresia and truncus arteriosus. Patients also included those who underwent a Ross operation. These patients often receive surgical placement of a right ventricular-to-pulmonary artery conduit, which can over time develop stenosis, insufficiency, or both because of calcification, intimal proliferation, and graft degeneration.
Multiple conduit reoperations to restore right ventricular outflow tract function are usually needed over a patient’s lifetime because of conduit degeneration. This makes a transcatheter procedure in a child or adolescent an attractive option because the prosthetic conduit will need replacement relatively quickly, and the transcatheter approach avoids an episode of open-heart surgery.
The Melody system is not the only transcatheter option for treating a leak or stenosis in a right ventricular outflow tract. The Sapien XT Transcatheter Heart Valve, marketed by Edwards, has FDA labeling for replacement of a dysfunctional right ventricular outflow tract.
Because the Sapien XT system was designed for replacing an aortic valve it’s challenging to place the conduit in the pulmonary valve position, Dr. Armstrong said. Operators find the Sapien 3 valve, a more modern design of the XT model that’s also primarily intended for aortic valve replacement, easier to position than the XT for pulmonary valve replacement, but Sapien 3 does not have FDA labeling for the right ventricular outflow tract indication. The Sapien valves are attractive because they don’t fracture, but Melody is easier to place and operators can reduce the fracture risk by prestenting, she noted.
Overall, the 5-year results from the postapproval study represented success, because 78% of patients who received the Melody device avoided any further interventions during follow-up. “That’s a big deal to a 12, 15, or 18 year old,” said Dr. Armstrong. “A surgically placed valve won’t last long in a teen, so it’s nice to do something noninvasively. It’s great if you can delay surgery for a few years” and avoid having the patient grow out of a surgically placed conduit or developing lots of calcification in the conduit during a growth spurt.
The postapproval study was funded by Medtronic, the company that sells the Melody valve. Dr. Armstrong has received research funding from Medtronic as well as Abbott, Edwards, and Siemens, and she has been a consultant to Abbott.
LAS VEGAS – that followed 65 patients, a majority of whom were children or teenagers.
After 5 years, 69% of the replacement valve recipients had no valvular hemodynamic dysfunction, compared with a 67% rate among patients enrolled in the original Investigational Device Exemption (IDE) study that led to Food and Drug Administration marketing approval for the Melody valve in 2010 under a humanitarian device exemption. (Full approval followed in 2017.)
The 5-year rate of any reintervention, including explants, was 78% in the postapproval study, again similar to the 76% rate reported in the IDE study after a median 4.5 year follow-up (Circulation. 2015 Jun 2;131[22]:1960-70), Aimee K. Armstrong, MD, said at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The new 5-year postapproval study findings “confirm that the hemodynamic effectiveness achieved by real-world providers is equivalent to the historical control established in the IDE study,” concluded Dr. Armstrong, professor of pediatrics at the Ohio State University and director of cardiac catheterization and interventional therapies at Nationwide Children’s Hospital, both in Columbus.
The postapproval study ran at 10 U.S. centers, none of which were among the five U.S. centers that ran the IDE study. Today, the Melody transcatheter pulmonary valve “is very commonly used” at many additional U.S. sites, Dr. Armstrong said in an interview. And the outcomes achieved using the valve likely surpass those seen in the IDE and postapproval studies because of innovations in technique, such as more routine use of “prestenting,” placing a stent in the vascular site where the pulmonary valve conduit will sit to address stenosis at this location and prevent subsequent conduit fracture (JACC Cardiovasc Interv. 2017 Sep;10[17]:1760-2).
“In 2010 [when the postapproval study began], we didn’t understand the importance of prestenting the way we do now. In 2010, I did not prestent every patient; now I do,” she said. The results reported by Dr. Armstrong included a 5% cumulative rate of major stent fractures in the Melody devices.
The postapproval study results also documented a concerning 4.5% annualized incidence of endocarditis among pulmonary valve recipients, with a nearly 300% increased rate of endocarditis among patients aged 12 years or younger, compared with older patients. Dr. Armstrong cautioned that this age association may be confounded by other factors, such as a residual pressure gradient in the right ventricular outflow tract of 15 mm Hg or greater. “We are discovering that we need to reduce the pressure gradient as much as we can, to perhaps less than 15 mm Hg, to reduce endocarditis, and that is something we did not know even a year ago. Practice is still evolving.”
The Melody Transcatheter Pulmonary Valve Postapproval Study performed cardiac catheterization for valve placement in 121 patients, and successfully implanted the valve for at least 24 hours in 99 of these patients. Patient age ranged from 5 to 45 years, with a median of 17 years; two-thirds were boys or men. The median age of the patients in the postapproval study was about 2 years younger than in the IDE study. Dr. Armstrong and her associates had previously published the 1-year outcomes from the postapproval study (JACC Cardiovasc Interv. 2014 Nov;7[11]:1254-62).
The enrolled patients usually needed a new right ventricular outflow tract because of a congenital heart defect, such as tetralogy of Fallot with pulmonary atresia and truncus arteriosus. Patients also included those who underwent a Ross operation. These patients often receive surgical placement of a right ventricular-to-pulmonary artery conduit, which can over time develop stenosis, insufficiency, or both because of calcification, intimal proliferation, and graft degeneration.
Multiple conduit reoperations to restore right ventricular outflow tract function are usually needed over a patient’s lifetime because of conduit degeneration. This makes a transcatheter procedure in a child or adolescent an attractive option because the prosthetic conduit will need replacement relatively quickly, and the transcatheter approach avoids an episode of open-heart surgery.
The Melody system is not the only transcatheter option for treating a leak or stenosis in a right ventricular outflow tract. The Sapien XT Transcatheter Heart Valve, marketed by Edwards, has FDA labeling for replacement of a dysfunctional right ventricular outflow tract.
Because the Sapien XT system was designed for replacing an aortic valve it’s challenging to place the conduit in the pulmonary valve position, Dr. Armstrong said. Operators find the Sapien 3 valve, a more modern design of the XT model that’s also primarily intended for aortic valve replacement, easier to position than the XT for pulmonary valve replacement, but Sapien 3 does not have FDA labeling for the right ventricular outflow tract indication. The Sapien valves are attractive because they don’t fracture, but Melody is easier to place and operators can reduce the fracture risk by prestenting, she noted.
Overall, the 5-year results from the postapproval study represented success, because 78% of patients who received the Melody device avoided any further interventions during follow-up. “That’s a big deal to a 12, 15, or 18 year old,” said Dr. Armstrong. “A surgically placed valve won’t last long in a teen, so it’s nice to do something noninvasively. It’s great if you can delay surgery for a few years” and avoid having the patient grow out of a surgically placed conduit or developing lots of calcification in the conduit during a growth spurt.
The postapproval study was funded by Medtronic, the company that sells the Melody valve. Dr. Armstrong has received research funding from Medtronic as well as Abbott, Edwards, and Siemens, and she has been a consultant to Abbott.
LAS VEGAS – that followed 65 patients, a majority of whom were children or teenagers.
After 5 years, 69% of the replacement valve recipients had no valvular hemodynamic dysfunction, compared with a 67% rate among patients enrolled in the original Investigational Device Exemption (IDE) study that led to Food and Drug Administration marketing approval for the Melody valve in 2010 under a humanitarian device exemption. (Full approval followed in 2017.)
The 5-year rate of any reintervention, including explants, was 78% in the postapproval study, again similar to the 76% rate reported in the IDE study after a median 4.5 year follow-up (Circulation. 2015 Jun 2;131[22]:1960-70), Aimee K. Armstrong, MD, said at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The new 5-year postapproval study findings “confirm that the hemodynamic effectiveness achieved by real-world providers is equivalent to the historical control established in the IDE study,” concluded Dr. Armstrong, professor of pediatrics at the Ohio State University and director of cardiac catheterization and interventional therapies at Nationwide Children’s Hospital, both in Columbus.
The postapproval study ran at 10 U.S. centers, none of which were among the five U.S. centers that ran the IDE study. Today, the Melody transcatheter pulmonary valve “is very commonly used” at many additional U.S. sites, Dr. Armstrong said in an interview. And the outcomes achieved using the valve likely surpass those seen in the IDE and postapproval studies because of innovations in technique, such as more routine use of “prestenting,” placing a stent in the vascular site where the pulmonary valve conduit will sit to address stenosis at this location and prevent subsequent conduit fracture (JACC Cardiovasc Interv. 2017 Sep;10[17]:1760-2).
“In 2010 [when the postapproval study began], we didn’t understand the importance of prestenting the way we do now. In 2010, I did not prestent every patient; now I do,” she said. The results reported by Dr. Armstrong included a 5% cumulative rate of major stent fractures in the Melody devices.
The postapproval study results also documented a concerning 4.5% annualized incidence of endocarditis among pulmonary valve recipients, with a nearly 300% increased rate of endocarditis among patients aged 12 years or younger, compared with older patients. Dr. Armstrong cautioned that this age association may be confounded by other factors, such as a residual pressure gradient in the right ventricular outflow tract of 15 mm Hg or greater. “We are discovering that we need to reduce the pressure gradient as much as we can, to perhaps less than 15 mm Hg, to reduce endocarditis, and that is something we did not know even a year ago. Practice is still evolving.”
The Melody Transcatheter Pulmonary Valve Postapproval Study performed cardiac catheterization for valve placement in 121 patients, and successfully implanted the valve for at least 24 hours in 99 of these patients. Patient age ranged from 5 to 45 years, with a median of 17 years; two-thirds were boys or men. The median age of the patients in the postapproval study was about 2 years younger than in the IDE study. Dr. Armstrong and her associates had previously published the 1-year outcomes from the postapproval study (JACC Cardiovasc Interv. 2014 Nov;7[11]:1254-62).
The enrolled patients usually needed a new right ventricular outflow tract because of a congenital heart defect, such as tetralogy of Fallot with pulmonary atresia and truncus arteriosus. Patients also included those who underwent a Ross operation. These patients often receive surgical placement of a right ventricular-to-pulmonary artery conduit, which can over time develop stenosis, insufficiency, or both because of calcification, intimal proliferation, and graft degeneration.
Multiple conduit reoperations to restore right ventricular outflow tract function are usually needed over a patient’s lifetime because of conduit degeneration. This makes a transcatheter procedure in a child or adolescent an attractive option because the prosthetic conduit will need replacement relatively quickly, and the transcatheter approach avoids an episode of open-heart surgery.
The Melody system is not the only transcatheter option for treating a leak or stenosis in a right ventricular outflow tract. The Sapien XT Transcatheter Heart Valve, marketed by Edwards, has FDA labeling for replacement of a dysfunctional right ventricular outflow tract.
Because the Sapien XT system was designed for replacing an aortic valve it’s challenging to place the conduit in the pulmonary valve position, Dr. Armstrong said. Operators find the Sapien 3 valve, a more modern design of the XT model that’s also primarily intended for aortic valve replacement, easier to position than the XT for pulmonary valve replacement, but Sapien 3 does not have FDA labeling for the right ventricular outflow tract indication. The Sapien valves are attractive because they don’t fracture, but Melody is easier to place and operators can reduce the fracture risk by prestenting, she noted.
Overall, the 5-year results from the postapproval study represented success, because 78% of patients who received the Melody device avoided any further interventions during follow-up. “That’s a big deal to a 12, 15, or 18 year old,” said Dr. Armstrong. “A surgically placed valve won’t last long in a teen, so it’s nice to do something noninvasively. It’s great if you can delay surgery for a few years” and avoid having the patient grow out of a surgically placed conduit or developing lots of calcification in the conduit during a growth spurt.
The postapproval study was funded by Medtronic, the company that sells the Melody valve. Dr. Armstrong has received research funding from Medtronic as well as Abbott, Edwards, and Siemens, and she has been a consultant to Abbott.
REPORTING FROM SCAI 2019
Cleveland Clinic targets time to treat in cancer
CHICAGO – In 2014, the average time from diagnosis to treatment initiation for new cancer patients at the Cleveland Clinic was 29-41 days, depending on whether the patient was diagnosed internally or externally. That figure was not acceptable, said Brian J. Bolwell, MD, chairman of the Cleveland Clinic’s Taussig Cancer Institute.
Since then, the time-to-treat metric has improved dramatically, dropping 33%. Today, time to treat for new cancer patients is 25-31 days, depending on the site of diagnosis.
To get there, leaders at the cancer center examined the causes of delay within each of their disease programs. The analysis revealed that less than 20% of the time it was patient preferences that slowed down the initiation of treatment, but that more than 80% of the time the delay was on the part of their institution.
Dr. Bolwell said this led them to start tracking every newly diagnosed patient who came through the cancer center to ensure they didn’t fall through the cracks, and that they were treated as rapidly as possible.
But figuring out how to get patients to treatment quicker depended on the type of cancer they had, since each type of cancer had different challenges and different points of entry to the health care system.
“So for breast cancer, it turns out a lot of the challenges might be coordination of surgery because sometimes a general surgeon has to work with a reconstructive-plastic surgeon and coordinating the surgical schedules might drastically lengthen time to treat,” he said during an interview at the annual meeting of the American Society of Clinical Oncology.
They helped address that problem by scheduling breast cancer patients for surgery by the next available operating room slot, rather than doing the scheduling by surgeon.
There are additional barriers to achieving a rapid time to treat standard, including prior authorization, Dr. Bolwell said. But they are continuing to chip away at the metric, working within each cancer type to lower the obstacles to treatment. “I don’t think we’ll ever be satisfied with where we are,” Dr. Bolwell said.
Dr. Bolwell reported having no relevant financial disclosures.
CHICAGO – In 2014, the average time from diagnosis to treatment initiation for new cancer patients at the Cleveland Clinic was 29-41 days, depending on whether the patient was diagnosed internally or externally. That figure was not acceptable, said Brian J. Bolwell, MD, chairman of the Cleveland Clinic’s Taussig Cancer Institute.
Since then, the time-to-treat metric has improved dramatically, dropping 33%. Today, time to treat for new cancer patients is 25-31 days, depending on the site of diagnosis.
To get there, leaders at the cancer center examined the causes of delay within each of their disease programs. The analysis revealed that less than 20% of the time it was patient preferences that slowed down the initiation of treatment, but that more than 80% of the time the delay was on the part of their institution.
Dr. Bolwell said this led them to start tracking every newly diagnosed patient who came through the cancer center to ensure they didn’t fall through the cracks, and that they were treated as rapidly as possible.
But figuring out how to get patients to treatment quicker depended on the type of cancer they had, since each type of cancer had different challenges and different points of entry to the health care system.
“So for breast cancer, it turns out a lot of the challenges might be coordination of surgery because sometimes a general surgeon has to work with a reconstructive-plastic surgeon and coordinating the surgical schedules might drastically lengthen time to treat,” he said during an interview at the annual meeting of the American Society of Clinical Oncology.
They helped address that problem by scheduling breast cancer patients for surgery by the next available operating room slot, rather than doing the scheduling by surgeon.
There are additional barriers to achieving a rapid time to treat standard, including prior authorization, Dr. Bolwell said. But they are continuing to chip away at the metric, working within each cancer type to lower the obstacles to treatment. “I don’t think we’ll ever be satisfied with where we are,” Dr. Bolwell said.
Dr. Bolwell reported having no relevant financial disclosures.
CHICAGO – In 2014, the average time from diagnosis to treatment initiation for new cancer patients at the Cleveland Clinic was 29-41 days, depending on whether the patient was diagnosed internally or externally. That figure was not acceptable, said Brian J. Bolwell, MD, chairman of the Cleveland Clinic’s Taussig Cancer Institute.
Since then, the time-to-treat metric has improved dramatically, dropping 33%. Today, time to treat for new cancer patients is 25-31 days, depending on the site of diagnosis.
To get there, leaders at the cancer center examined the causes of delay within each of their disease programs. The analysis revealed that less than 20% of the time it was patient preferences that slowed down the initiation of treatment, but that more than 80% of the time the delay was on the part of their institution.
Dr. Bolwell said this led them to start tracking every newly diagnosed patient who came through the cancer center to ensure they didn’t fall through the cracks, and that they were treated as rapidly as possible.
But figuring out how to get patients to treatment quicker depended on the type of cancer they had, since each type of cancer had different challenges and different points of entry to the health care system.
“So for breast cancer, it turns out a lot of the challenges might be coordination of surgery because sometimes a general surgeon has to work with a reconstructive-plastic surgeon and coordinating the surgical schedules might drastically lengthen time to treat,” he said during an interview at the annual meeting of the American Society of Clinical Oncology.
They helped address that problem by scheduling breast cancer patients for surgery by the next available operating room slot, rather than doing the scheduling by surgeon.
There are additional barriers to achieving a rapid time to treat standard, including prior authorization, Dr. Bolwell said. But they are continuing to chip away at the metric, working within each cancer type to lower the obstacles to treatment. “I don’t think we’ll ever be satisfied with where we are,” Dr. Bolwell said.
Dr. Bolwell reported having no relevant financial disclosures.
FROM ASCO 2019
Preventing delayed genitourinary tract injury during benign hysterectomy
and the circumstances under which it should be performed. Procedures directed at prolapse and incontinence have rates of genitourinary injury as high as 11%-38%, and national guidelines affirm the importance of cystoscopy in these patients.1 However, for patients undergoing hysterectomy in the absence of these procedures, the optimal strategy is debated. One approach that has been advanced is a policy of universal cystoscopy at the time of hysterectomy. This policy, by which all women undergoing hysterectomy would undergo cystoscopy, aims to prevent the occurrence of an unrecognized genitourinary injury by diagnosing and treating the injury intraoperatively. However, cystoscopy is not the only method that can be used to evaluate the urinary tract. Retroperitoneal dissection also can be used to visually identify the pertinent structures and has been performed with high fidelity by generations of experienced and skilled pelvic surgeons.
Injuries that are not identified intraoperatively at the time of surgery, so-called delayed genitourinary tract injuries, are associated with serious postoperative consequences for patients and high costs for institutions. As surgeons strive to decrease complications and improve the quality of gynecologic surgery, the question of whether cystoscopy should routinely be performed at the time of hysterectomy for benign indications remains unanswered. Proponents argue that cystoscopy is a low-cost assessment and that 75% of genitourinary injuries occur in women without identifiable risk factors.2 Opponents point out that cystoscopy is not an entirely benign intervention; it is associated with increased rates of urinary tract infection, bladder and ureteral trauma, and additional operating room time. Furthermore, it is unclear that the use of cystoscopy will reduce the incidence of delayed genitourinary tract injury in clinical practice.
Ultimately, cystoscopy after hysterectomy is being used as a screening test for genitourinary injury, and this lens can be applied to provide more information about its usefulness. For screening tests, the sensitivity and false negative rate are of paramount importance. High sensitivity and resultant few false negatives are the characteristics of a robust screening test which has a low likelihood of missing a diagnosis. Unfortunately, the sensitivity of cystoscopy is not 100% for genitourinary tract injury; it ranges from 60% to 85% and can be as low as 43% for ureteral injury.3,4 This means that cystoscopy will falsely reassure the surgeon with normal results in greater than 50% of the cases in which the patient actually has a ureteral injury.
Some larger series call into question the usefulness of cystoscopy as a screening tool, finding that this evaluation is not associated with a decreased rate of delayed genitourinary injury. A recent publication by our group of a series of 39,529 women who underwent benign hysterectomy without procedures directed at incontinence and prolapse recorded in the National Surgical Quality Improvement Program (NSQIP) database between 2015 and 2017 found no difference in the rate of delayed genitourinary injury among women exposed to diagnostic cystoscopy and those who were not.5 These results are consistent with those of the largest systematic review and meta-analysis of 79 studies capturing 41,482 hysterectomies which found universal cystoscopy was not associated with a decreased rate of delayed genitourinary tract injury.6
Another consideration with the use of universal cystoscopy is cost. Although cystoscopy is typically a short procedure, the false positive rate is approximately 2%,2 often leading to additional interventions to evaluate the urinary tract which can be time consuming. In the limited available data regarding operative time, patients who underwent cystoscopy had a median operative time that was 17 minutes longer than it was among patients who did not.5 Moreover, there may be risks associated with this additional bladder instrumentation, evidenced by an increased incidence of urinary tract infection among women undergoing cystoscopy. In a recent cost-effectiveness analysis of cystoscopy at the time of benign hysterectomy, universal cystoscopy was found to add $51.39-$57.86 per case, and the risk of bladder injury would need to exceed 21%-47% and ureteral injury 27%-38% to be cost saving, compared with selective cystoscopy.7 A prior cost-effectiveness analysis concluded that universal cystoscopy is cost effective when the incidence of ureteral injury at the time of hysterectomy exceeds 1.5%-2.0%.8 Given these high thresholds, with a contemporary composite lower–genitourinary tract injury incidence of 0.24%-0.27%, it is unlikely that universal cystoscopy could be considered a cost-saving strategy in the majority of clinical settings.
Potential explanations for these results are many. Intraoperative cystoscopy is likely to be normal in the setting of nonobstructive and thermal injuries, which in the current era of minimally invasive surgery may be more prevalent mechanisms of injury. False positives can occur leading to unnecessary interventions, as well as overdiagnosis of asymptomatic urinary tract injuries that may have resolved spontaneously.9 It has been observed that cystoscopy is performed less frequently when hysterectomy is completed by a high-volume surgeon, which suggests that surgeon skill and experience play a significant role in the usefulness of this evaluation.9
Given these data, what is the best way forward regarding evaluation of the urinary tract at the time of benign hysterectomy? Ultimately, this is a clinical question that should be individualized, taking into account patient and surgical complexity, as well as surgeon training and individual rates of genitourinary injuries.9 Given its low sensitivity, caution should be exercised regarding the routine use of cystoscopy alone for evaluation of the urinary tract because false negatives occur with significant frequency. Benefits of cystoscopy in a given clinical scenario should be weighed against the risks of longer operative time, increased costs, and increased rate of urinary tract infection. In the absence of clinical scenarios with high rates of genitourinary injury (greater than 5%), selective rather than universal cystoscopy is the preferred strategy.7 Cystoscopy is fundamentally a form of secondary prevention that aims to mitigate damage that has already been done, and is no substitute for primary prevention of genitourinary tract injury itself through thorough knowledge of pelvic anatomy, comfort with retroperitoneal dissection, and awareness of the ureter and bladder at all times.
Dr. Polan is a resident in obstetrics and gynecology at Northwestern University, Chicago. Dr. Barber is an assistant professor of obstetrics and gynecology, specializing in gynecologic oncology, at the university. Neither of them have relevant financial disclosures. Email Dr. Polan and Dr. Barber at obnews@mdedge.com.
References
1. Am J Obstet Gynecol. 2018 Jul;219(1):75-7.
2. Obstet Gynecol. 2009 Jan;113(1):6-10.
3. Obstet Gynecol. 2016 Feb;127(2):369-75.
4. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1278-86.
5. Obstet Gynecol. 2019 May;133(5):888-95.
6. Obstet Gynecol. 2015 Dec;126(6):1161-9.
7. Am J Obstet Gynecol. 2019 Apr;220(4):369.e1-7.
8. Obstet Gynecol. 2001 May;97(5 Pt 1):685-92.
9. Obstet Gynecol. 2012 Dec;120(6):1363-70.
and the circumstances under which it should be performed. Procedures directed at prolapse and incontinence have rates of genitourinary injury as high as 11%-38%, and national guidelines affirm the importance of cystoscopy in these patients.1 However, for patients undergoing hysterectomy in the absence of these procedures, the optimal strategy is debated. One approach that has been advanced is a policy of universal cystoscopy at the time of hysterectomy. This policy, by which all women undergoing hysterectomy would undergo cystoscopy, aims to prevent the occurrence of an unrecognized genitourinary injury by diagnosing and treating the injury intraoperatively. However, cystoscopy is not the only method that can be used to evaluate the urinary tract. Retroperitoneal dissection also can be used to visually identify the pertinent structures and has been performed with high fidelity by generations of experienced and skilled pelvic surgeons.
Injuries that are not identified intraoperatively at the time of surgery, so-called delayed genitourinary tract injuries, are associated with serious postoperative consequences for patients and high costs for institutions. As surgeons strive to decrease complications and improve the quality of gynecologic surgery, the question of whether cystoscopy should routinely be performed at the time of hysterectomy for benign indications remains unanswered. Proponents argue that cystoscopy is a low-cost assessment and that 75% of genitourinary injuries occur in women without identifiable risk factors.2 Opponents point out that cystoscopy is not an entirely benign intervention; it is associated with increased rates of urinary tract infection, bladder and ureteral trauma, and additional operating room time. Furthermore, it is unclear that the use of cystoscopy will reduce the incidence of delayed genitourinary tract injury in clinical practice.
Ultimately, cystoscopy after hysterectomy is being used as a screening test for genitourinary injury, and this lens can be applied to provide more information about its usefulness. For screening tests, the sensitivity and false negative rate are of paramount importance. High sensitivity and resultant few false negatives are the characteristics of a robust screening test which has a low likelihood of missing a diagnosis. Unfortunately, the sensitivity of cystoscopy is not 100% for genitourinary tract injury; it ranges from 60% to 85% and can be as low as 43% for ureteral injury.3,4 This means that cystoscopy will falsely reassure the surgeon with normal results in greater than 50% of the cases in which the patient actually has a ureteral injury.
Some larger series call into question the usefulness of cystoscopy as a screening tool, finding that this evaluation is not associated with a decreased rate of delayed genitourinary injury. A recent publication by our group of a series of 39,529 women who underwent benign hysterectomy without procedures directed at incontinence and prolapse recorded in the National Surgical Quality Improvement Program (NSQIP) database between 2015 and 2017 found no difference in the rate of delayed genitourinary injury among women exposed to diagnostic cystoscopy and those who were not.5 These results are consistent with those of the largest systematic review and meta-analysis of 79 studies capturing 41,482 hysterectomies which found universal cystoscopy was not associated with a decreased rate of delayed genitourinary tract injury.6
Another consideration with the use of universal cystoscopy is cost. Although cystoscopy is typically a short procedure, the false positive rate is approximately 2%,2 often leading to additional interventions to evaluate the urinary tract which can be time consuming. In the limited available data regarding operative time, patients who underwent cystoscopy had a median operative time that was 17 minutes longer than it was among patients who did not.5 Moreover, there may be risks associated with this additional bladder instrumentation, evidenced by an increased incidence of urinary tract infection among women undergoing cystoscopy. In a recent cost-effectiveness analysis of cystoscopy at the time of benign hysterectomy, universal cystoscopy was found to add $51.39-$57.86 per case, and the risk of bladder injury would need to exceed 21%-47% and ureteral injury 27%-38% to be cost saving, compared with selective cystoscopy.7 A prior cost-effectiveness analysis concluded that universal cystoscopy is cost effective when the incidence of ureteral injury at the time of hysterectomy exceeds 1.5%-2.0%.8 Given these high thresholds, with a contemporary composite lower–genitourinary tract injury incidence of 0.24%-0.27%, it is unlikely that universal cystoscopy could be considered a cost-saving strategy in the majority of clinical settings.
Potential explanations for these results are many. Intraoperative cystoscopy is likely to be normal in the setting of nonobstructive and thermal injuries, which in the current era of minimally invasive surgery may be more prevalent mechanisms of injury. False positives can occur leading to unnecessary interventions, as well as overdiagnosis of asymptomatic urinary tract injuries that may have resolved spontaneously.9 It has been observed that cystoscopy is performed less frequently when hysterectomy is completed by a high-volume surgeon, which suggests that surgeon skill and experience play a significant role in the usefulness of this evaluation.9
Given these data, what is the best way forward regarding evaluation of the urinary tract at the time of benign hysterectomy? Ultimately, this is a clinical question that should be individualized, taking into account patient and surgical complexity, as well as surgeon training and individual rates of genitourinary injuries.9 Given its low sensitivity, caution should be exercised regarding the routine use of cystoscopy alone for evaluation of the urinary tract because false negatives occur with significant frequency. Benefits of cystoscopy in a given clinical scenario should be weighed against the risks of longer operative time, increased costs, and increased rate of urinary tract infection. In the absence of clinical scenarios with high rates of genitourinary injury (greater than 5%), selective rather than universal cystoscopy is the preferred strategy.7 Cystoscopy is fundamentally a form of secondary prevention that aims to mitigate damage that has already been done, and is no substitute for primary prevention of genitourinary tract injury itself through thorough knowledge of pelvic anatomy, comfort with retroperitoneal dissection, and awareness of the ureter and bladder at all times.
Dr. Polan is a resident in obstetrics and gynecology at Northwestern University, Chicago. Dr. Barber is an assistant professor of obstetrics and gynecology, specializing in gynecologic oncology, at the university. Neither of them have relevant financial disclosures. Email Dr. Polan and Dr. Barber at obnews@mdedge.com.
References
1. Am J Obstet Gynecol. 2018 Jul;219(1):75-7.
2. Obstet Gynecol. 2009 Jan;113(1):6-10.
3. Obstet Gynecol. 2016 Feb;127(2):369-75.
4. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1278-86.
5. Obstet Gynecol. 2019 May;133(5):888-95.
6. Obstet Gynecol. 2015 Dec;126(6):1161-9.
7. Am J Obstet Gynecol. 2019 Apr;220(4):369.e1-7.
8. Obstet Gynecol. 2001 May;97(5 Pt 1):685-92.
9. Obstet Gynecol. 2012 Dec;120(6):1363-70.
and the circumstances under which it should be performed. Procedures directed at prolapse and incontinence have rates of genitourinary injury as high as 11%-38%, and national guidelines affirm the importance of cystoscopy in these patients.1 However, for patients undergoing hysterectomy in the absence of these procedures, the optimal strategy is debated. One approach that has been advanced is a policy of universal cystoscopy at the time of hysterectomy. This policy, by which all women undergoing hysterectomy would undergo cystoscopy, aims to prevent the occurrence of an unrecognized genitourinary injury by diagnosing and treating the injury intraoperatively. However, cystoscopy is not the only method that can be used to evaluate the urinary tract. Retroperitoneal dissection also can be used to visually identify the pertinent structures and has been performed with high fidelity by generations of experienced and skilled pelvic surgeons.
Injuries that are not identified intraoperatively at the time of surgery, so-called delayed genitourinary tract injuries, are associated with serious postoperative consequences for patients and high costs for institutions. As surgeons strive to decrease complications and improve the quality of gynecologic surgery, the question of whether cystoscopy should routinely be performed at the time of hysterectomy for benign indications remains unanswered. Proponents argue that cystoscopy is a low-cost assessment and that 75% of genitourinary injuries occur in women without identifiable risk factors.2 Opponents point out that cystoscopy is not an entirely benign intervention; it is associated with increased rates of urinary tract infection, bladder and ureteral trauma, and additional operating room time. Furthermore, it is unclear that the use of cystoscopy will reduce the incidence of delayed genitourinary tract injury in clinical practice.
Ultimately, cystoscopy after hysterectomy is being used as a screening test for genitourinary injury, and this lens can be applied to provide more information about its usefulness. For screening tests, the sensitivity and false negative rate are of paramount importance. High sensitivity and resultant few false negatives are the characteristics of a robust screening test which has a low likelihood of missing a diagnosis. Unfortunately, the sensitivity of cystoscopy is not 100% for genitourinary tract injury; it ranges from 60% to 85% and can be as low as 43% for ureteral injury.3,4 This means that cystoscopy will falsely reassure the surgeon with normal results in greater than 50% of the cases in which the patient actually has a ureteral injury.
Some larger series call into question the usefulness of cystoscopy as a screening tool, finding that this evaluation is not associated with a decreased rate of delayed genitourinary injury. A recent publication by our group of a series of 39,529 women who underwent benign hysterectomy without procedures directed at incontinence and prolapse recorded in the National Surgical Quality Improvement Program (NSQIP) database between 2015 and 2017 found no difference in the rate of delayed genitourinary injury among women exposed to diagnostic cystoscopy and those who were not.5 These results are consistent with those of the largest systematic review and meta-analysis of 79 studies capturing 41,482 hysterectomies which found universal cystoscopy was not associated with a decreased rate of delayed genitourinary tract injury.6
Another consideration with the use of universal cystoscopy is cost. Although cystoscopy is typically a short procedure, the false positive rate is approximately 2%,2 often leading to additional interventions to evaluate the urinary tract which can be time consuming. In the limited available data regarding operative time, patients who underwent cystoscopy had a median operative time that was 17 minutes longer than it was among patients who did not.5 Moreover, there may be risks associated with this additional bladder instrumentation, evidenced by an increased incidence of urinary tract infection among women undergoing cystoscopy. In a recent cost-effectiveness analysis of cystoscopy at the time of benign hysterectomy, universal cystoscopy was found to add $51.39-$57.86 per case, and the risk of bladder injury would need to exceed 21%-47% and ureteral injury 27%-38% to be cost saving, compared with selective cystoscopy.7 A prior cost-effectiveness analysis concluded that universal cystoscopy is cost effective when the incidence of ureteral injury at the time of hysterectomy exceeds 1.5%-2.0%.8 Given these high thresholds, with a contemporary composite lower–genitourinary tract injury incidence of 0.24%-0.27%, it is unlikely that universal cystoscopy could be considered a cost-saving strategy in the majority of clinical settings.
Potential explanations for these results are many. Intraoperative cystoscopy is likely to be normal in the setting of nonobstructive and thermal injuries, which in the current era of minimally invasive surgery may be more prevalent mechanisms of injury. False positives can occur leading to unnecessary interventions, as well as overdiagnosis of asymptomatic urinary tract injuries that may have resolved spontaneously.9 It has been observed that cystoscopy is performed less frequently when hysterectomy is completed by a high-volume surgeon, which suggests that surgeon skill and experience play a significant role in the usefulness of this evaluation.9
Given these data, what is the best way forward regarding evaluation of the urinary tract at the time of benign hysterectomy? Ultimately, this is a clinical question that should be individualized, taking into account patient and surgical complexity, as well as surgeon training and individual rates of genitourinary injuries.9 Given its low sensitivity, caution should be exercised regarding the routine use of cystoscopy alone for evaluation of the urinary tract because false negatives occur with significant frequency. Benefits of cystoscopy in a given clinical scenario should be weighed against the risks of longer operative time, increased costs, and increased rate of urinary tract infection. In the absence of clinical scenarios with high rates of genitourinary injury (greater than 5%), selective rather than universal cystoscopy is the preferred strategy.7 Cystoscopy is fundamentally a form of secondary prevention that aims to mitigate damage that has already been done, and is no substitute for primary prevention of genitourinary tract injury itself through thorough knowledge of pelvic anatomy, comfort with retroperitoneal dissection, and awareness of the ureter and bladder at all times.
Dr. Polan is a resident in obstetrics and gynecology at Northwestern University, Chicago. Dr. Barber is an assistant professor of obstetrics and gynecology, specializing in gynecologic oncology, at the university. Neither of them have relevant financial disclosures. Email Dr. Polan and Dr. Barber at obnews@mdedge.com.
References
1. Am J Obstet Gynecol. 2018 Jul;219(1):75-7.
2. Obstet Gynecol. 2009 Jan;113(1):6-10.
3. Obstet Gynecol. 2016 Feb;127(2):369-75.
4. J Minim Invasive Gynecol. 2015 Nov-Dec;22(7):1278-86.
5. Obstet Gynecol. 2019 May;133(5):888-95.
6. Obstet Gynecol. 2015 Dec;126(6):1161-9.
7. Am J Obstet Gynecol. 2019 Apr;220(4):369.e1-7.
8. Obstet Gynecol. 2001 May;97(5 Pt 1):685-92.
9. Obstet Gynecol. 2012 Dec;120(6):1363-70.
Bundled payment for OA surgery linked to more emergency department visits
And therein lies a key lesson for health policy makers who have embraced bundled payments to reduce rising health care costs, Mayilee Canizares, PhD, observed at the OARSI 2019 World Congress.
In Ontario, with patients discharged sooner and directly to home, there was the negative impact of increased emergency department visits after surgery, Dr. Canizares, of the University Health Network in Toronto, said at OARSI 2019 World Congress, sponsored by the Osteoarthritis Research Society International. “Our findings highlight the importance of coordinating the appropriate support services as well as the need to continue assessing the optimal discharge care plan for osteoarthritis patients undergoing surgery.”
Dr. Canizares’ study of the Ontario-wide experience with orthopedic surgery for osteoarthritis during 2004-2016 received the OARSI 2019 award for the meeting’s top-rated study in clinical epidemiology/health services research.
Using administrative data from Canada’s national health care system, Dr. Canizares and her coinvestigators found that the number of individuals undergoing elective orthopedic surgery for osteoarthritis ballooned from 22,700 in 2004 to 41,900 in 2016, representing an increase from 246 to 381 procedures per 100,000 people. During this time, the mean length of stay declined from about 5 days to just under 3 days, the 30-day readmission rate dropped from 4.2% to 3.4%, and the rate of emergency department visits within 30 days post discharge rose steadily from 8.7% in 2004 to 14.1% in 2016.
Roughly half of the operations were total knee replacements and one-third were hip replacements. The profile of patients undergoing surgery changed little over the course of the 12-year study with the exception that in more recent years patients presented with more comorbidities: Indeed, three or more comorbid conditions were present in 2.9% of the surgical patients in 2004 compared to 4.2% in 2016.
In multivariate logistic regression analyses, patient characteristics didn’t explain the change over time in early readmission or unplanned emergency department visit rates. However, discharge disposition did: By 2014, more patients were being discharged home, and in nearly half of cases that was being done without support.
Dr. Canizares reported having no financial conflicts regarding her study, funded by the Toronto General and Western Hospital Foundation.
SOURCE: Canizares M. OARSI, Abstract 16.
And therein lies a key lesson for health policy makers who have embraced bundled payments to reduce rising health care costs, Mayilee Canizares, PhD, observed at the OARSI 2019 World Congress.
In Ontario, with patients discharged sooner and directly to home, there was the negative impact of increased emergency department visits after surgery, Dr. Canizares, of the University Health Network in Toronto, said at OARSI 2019 World Congress, sponsored by the Osteoarthritis Research Society International. “Our findings highlight the importance of coordinating the appropriate support services as well as the need to continue assessing the optimal discharge care plan for osteoarthritis patients undergoing surgery.”
Dr. Canizares’ study of the Ontario-wide experience with orthopedic surgery for osteoarthritis during 2004-2016 received the OARSI 2019 award for the meeting’s top-rated study in clinical epidemiology/health services research.
Using administrative data from Canada’s national health care system, Dr. Canizares and her coinvestigators found that the number of individuals undergoing elective orthopedic surgery for osteoarthritis ballooned from 22,700 in 2004 to 41,900 in 2016, representing an increase from 246 to 381 procedures per 100,000 people. During this time, the mean length of stay declined from about 5 days to just under 3 days, the 30-day readmission rate dropped from 4.2% to 3.4%, and the rate of emergency department visits within 30 days post discharge rose steadily from 8.7% in 2004 to 14.1% in 2016.
Roughly half of the operations were total knee replacements and one-third were hip replacements. The profile of patients undergoing surgery changed little over the course of the 12-year study with the exception that in more recent years patients presented with more comorbidities: Indeed, three or more comorbid conditions were present in 2.9% of the surgical patients in 2004 compared to 4.2% in 2016.
In multivariate logistic regression analyses, patient characteristics didn’t explain the change over time in early readmission or unplanned emergency department visit rates. However, discharge disposition did: By 2014, more patients were being discharged home, and in nearly half of cases that was being done without support.
Dr. Canizares reported having no financial conflicts regarding her study, funded by the Toronto General and Western Hospital Foundation.
SOURCE: Canizares M. OARSI, Abstract 16.
And therein lies a key lesson for health policy makers who have embraced bundled payments to reduce rising health care costs, Mayilee Canizares, PhD, observed at the OARSI 2019 World Congress.
In Ontario, with patients discharged sooner and directly to home, there was the negative impact of increased emergency department visits after surgery, Dr. Canizares, of the University Health Network in Toronto, said at OARSI 2019 World Congress, sponsored by the Osteoarthritis Research Society International. “Our findings highlight the importance of coordinating the appropriate support services as well as the need to continue assessing the optimal discharge care plan for osteoarthritis patients undergoing surgery.”
Dr. Canizares’ study of the Ontario-wide experience with orthopedic surgery for osteoarthritis during 2004-2016 received the OARSI 2019 award for the meeting’s top-rated study in clinical epidemiology/health services research.
Using administrative data from Canada’s national health care system, Dr. Canizares and her coinvestigators found that the number of individuals undergoing elective orthopedic surgery for osteoarthritis ballooned from 22,700 in 2004 to 41,900 in 2016, representing an increase from 246 to 381 procedures per 100,000 people. During this time, the mean length of stay declined from about 5 days to just under 3 days, the 30-day readmission rate dropped from 4.2% to 3.4%, and the rate of emergency department visits within 30 days post discharge rose steadily from 8.7% in 2004 to 14.1% in 2016.
Roughly half of the operations were total knee replacements and one-third were hip replacements. The profile of patients undergoing surgery changed little over the course of the 12-year study with the exception that in more recent years patients presented with more comorbidities: Indeed, three or more comorbid conditions were present in 2.9% of the surgical patients in 2004 compared to 4.2% in 2016.
In multivariate logistic regression analyses, patient characteristics didn’t explain the change over time in early readmission or unplanned emergency department visit rates. However, discharge disposition did: By 2014, more patients were being discharged home, and in nearly half of cases that was being done without support.
Dr. Canizares reported having no financial conflicts regarding her study, funded by the Toronto General and Western Hospital Foundation.
SOURCE: Canizares M. OARSI, Abstract 16.
REPORTING FROM OARSI 2019
PPI metabolism may be altered in about one-third of bariatric surgery candidates
SAN DIEGO – Rapid proton pump inhibitor (PPI) metabolism was present in nearly one-third of patients who underwent bariatric surgery, results from a small, single-center study showed. Patients who were fast metabolizers also exhibited a higher, although not significant, incidence of early marginal ulceration following Roux-en-Y gastric bypass.
“Roux-en-Y gastric bypass [RYGB] is one of the most effective surgical approaches to mitigating obesity and its attendant comorbidities including diabetes, hypertension, hyperlipidemia, reflux, and sleep apnea,” lead study author Sabrena F. Noria, MD, PhD, said in an interview at the annual Digestive Disease Week. “However, as with all surgeries, there are associated risks, the more common of which is marginal ulceration, or ulcer formation at the gastrojejunostomy, which occurs at a rate of 1%-16%.”
Dr. Noria, surgical research director of the comprehensive weight management, metabolic/bariatric surgery program at the Ohio State University’s Wexner Medical Center, noted that marginal ulcers (MUs) are divided into early (within 90 days) and late (more than 90 days), based on their time of onset after surgery, and are usually diagnosed during upper endoscopy on postoperative patients who complain of epigastric pain, dysphagia, nausea/vomiting, and/or dehydration.
“Given that MUs are associated with multiple hospital readmissions for pain and dehydration, multiple diagnostic and therapeutic endoscopic procedures, and escalation in both antiulcer and analgesic medication, their clinical impact cannot be overstated, especially since RYGB is the second most commonly performed bariatric procedure in the U.S.,” she said. “Given that the majority of marginal ulcers occur early after surgery, bariatric surgery programs have adopted the prophylactic use of proton pump inhibitors for up to 90 days postoperatively. While studies have demonstrated up to a two-fold decrease in ulcer formation, sample heterogeneity, in terms of combining both early and late ulcers, make it difficult to determine the effect on early ulcer formation.”
In an effort to compare preoperative endoscopic findings and MU formation in patients with and without altered PPI metabolism, the researchers prospectively enrolled 94 bariatric patients to undergo genetic testing pertinent to drug metabolism for a comprehensive panel of medications using a commercially available pharmacogenetic testing kit for the activity of cytochrome P450 in drug metabolism. They grouped patients by whether they were fast or normal metabolizers, and compared preoperative endoscopic findings for patients on PPIs at baseline and rates of early (within 90 days) and late ulceration (between 90 and 180 days).
Dr. Noria reported that 28 patients (30%) in the entire cohort met criteria for being fast metabolizers. The researchers observed no differences in baseline body mass index, age, gender, or former smoking status between both groups. Among those treated with a PPI at baseline, fast metabolizers demonstrated a trend toward a higher incidence of gastritis on preoperative endoscopy, compared with controls (89% vs. 65%, respectively; P = .12), while detection of Helicobacter pylori and Barrett’s esophagus were nonsignificant between groups. Eight patients (17%) who underwent RYGB developed marginal ulcers within 6 months of the index operation, of which four (50%) were diagnosed within 90 days and categorized as early ulcers. Development of early ulceration was higher among fast metabolizers, compared with controls (13% vs. 6%), but this did not reach statistical significance (P = .60). All late ulcerations occurred within the control group.
“While none of our findings are statistically significant given the small sample size, there were two findings I found clinically compelling,” Dr. Noria said. “First, in the group of patients who were on PPIs preoperatively, we found a 24% increase in the presence of pathologically diagnosed gastritis in the rapid-metabolizer group, during screening endoscopy. This suggests that either these patients were undertreated or were not treated with the appropriate medication. The second interesting finding was an over doubling of early ulcer formation in the RYGB group who were rapid metabolizers. However, again I would caution against drawing any real conclusions as our sample size was not powered to detect any difference.”
She also acknowledged that the study was limited by the inability to determine the effect of confounders such as surgical approach and the lack of randomization.
Anahita D. Jalilvand, MD, a general surgery resident, postdoctoral research fellow, and PhD candidate, was instrumental to this study, Dr. Noria said.
The trial was sponsored by Pathnostics, a pharmacogenetic testing company, who covered the cost of the tests. The researchers reported having no financial disclosures.
SAN DIEGO – Rapid proton pump inhibitor (PPI) metabolism was present in nearly one-third of patients who underwent bariatric surgery, results from a small, single-center study showed. Patients who were fast metabolizers also exhibited a higher, although not significant, incidence of early marginal ulceration following Roux-en-Y gastric bypass.
“Roux-en-Y gastric bypass [RYGB] is one of the most effective surgical approaches to mitigating obesity and its attendant comorbidities including diabetes, hypertension, hyperlipidemia, reflux, and sleep apnea,” lead study author Sabrena F. Noria, MD, PhD, said in an interview at the annual Digestive Disease Week. “However, as with all surgeries, there are associated risks, the more common of which is marginal ulceration, or ulcer formation at the gastrojejunostomy, which occurs at a rate of 1%-16%.”
Dr. Noria, surgical research director of the comprehensive weight management, metabolic/bariatric surgery program at the Ohio State University’s Wexner Medical Center, noted that marginal ulcers (MUs) are divided into early (within 90 days) and late (more than 90 days), based on their time of onset after surgery, and are usually diagnosed during upper endoscopy on postoperative patients who complain of epigastric pain, dysphagia, nausea/vomiting, and/or dehydration.
“Given that MUs are associated with multiple hospital readmissions for pain and dehydration, multiple diagnostic and therapeutic endoscopic procedures, and escalation in both antiulcer and analgesic medication, their clinical impact cannot be overstated, especially since RYGB is the second most commonly performed bariatric procedure in the U.S.,” she said. “Given that the majority of marginal ulcers occur early after surgery, bariatric surgery programs have adopted the prophylactic use of proton pump inhibitors for up to 90 days postoperatively. While studies have demonstrated up to a two-fold decrease in ulcer formation, sample heterogeneity, in terms of combining both early and late ulcers, make it difficult to determine the effect on early ulcer formation.”
In an effort to compare preoperative endoscopic findings and MU formation in patients with and without altered PPI metabolism, the researchers prospectively enrolled 94 bariatric patients to undergo genetic testing pertinent to drug metabolism for a comprehensive panel of medications using a commercially available pharmacogenetic testing kit for the activity of cytochrome P450 in drug metabolism. They grouped patients by whether they were fast or normal metabolizers, and compared preoperative endoscopic findings for patients on PPIs at baseline and rates of early (within 90 days) and late ulceration (between 90 and 180 days).
Dr. Noria reported that 28 patients (30%) in the entire cohort met criteria for being fast metabolizers. The researchers observed no differences in baseline body mass index, age, gender, or former smoking status between both groups. Among those treated with a PPI at baseline, fast metabolizers demonstrated a trend toward a higher incidence of gastritis on preoperative endoscopy, compared with controls (89% vs. 65%, respectively; P = .12), while detection of Helicobacter pylori and Barrett’s esophagus were nonsignificant between groups. Eight patients (17%) who underwent RYGB developed marginal ulcers within 6 months of the index operation, of which four (50%) were diagnosed within 90 days and categorized as early ulcers. Development of early ulceration was higher among fast metabolizers, compared with controls (13% vs. 6%), but this did not reach statistical significance (P = .60). All late ulcerations occurred within the control group.
“While none of our findings are statistically significant given the small sample size, there were two findings I found clinically compelling,” Dr. Noria said. “First, in the group of patients who were on PPIs preoperatively, we found a 24% increase in the presence of pathologically diagnosed gastritis in the rapid-metabolizer group, during screening endoscopy. This suggests that either these patients were undertreated or were not treated with the appropriate medication. The second interesting finding was an over doubling of early ulcer formation in the RYGB group who were rapid metabolizers. However, again I would caution against drawing any real conclusions as our sample size was not powered to detect any difference.”
She also acknowledged that the study was limited by the inability to determine the effect of confounders such as surgical approach and the lack of randomization.
Anahita D. Jalilvand, MD, a general surgery resident, postdoctoral research fellow, and PhD candidate, was instrumental to this study, Dr. Noria said.
The trial was sponsored by Pathnostics, a pharmacogenetic testing company, who covered the cost of the tests. The researchers reported having no financial disclosures.
SAN DIEGO – Rapid proton pump inhibitor (PPI) metabolism was present in nearly one-third of patients who underwent bariatric surgery, results from a small, single-center study showed. Patients who were fast metabolizers also exhibited a higher, although not significant, incidence of early marginal ulceration following Roux-en-Y gastric bypass.
“Roux-en-Y gastric bypass [RYGB] is one of the most effective surgical approaches to mitigating obesity and its attendant comorbidities including diabetes, hypertension, hyperlipidemia, reflux, and sleep apnea,” lead study author Sabrena F. Noria, MD, PhD, said in an interview at the annual Digestive Disease Week. “However, as with all surgeries, there are associated risks, the more common of which is marginal ulceration, or ulcer formation at the gastrojejunostomy, which occurs at a rate of 1%-16%.”
Dr. Noria, surgical research director of the comprehensive weight management, metabolic/bariatric surgery program at the Ohio State University’s Wexner Medical Center, noted that marginal ulcers (MUs) are divided into early (within 90 days) and late (more than 90 days), based on their time of onset after surgery, and are usually diagnosed during upper endoscopy on postoperative patients who complain of epigastric pain, dysphagia, nausea/vomiting, and/or dehydration.
“Given that MUs are associated with multiple hospital readmissions for pain and dehydration, multiple diagnostic and therapeutic endoscopic procedures, and escalation in both antiulcer and analgesic medication, their clinical impact cannot be overstated, especially since RYGB is the second most commonly performed bariatric procedure in the U.S.,” she said. “Given that the majority of marginal ulcers occur early after surgery, bariatric surgery programs have adopted the prophylactic use of proton pump inhibitors for up to 90 days postoperatively. While studies have demonstrated up to a two-fold decrease in ulcer formation, sample heterogeneity, in terms of combining both early and late ulcers, make it difficult to determine the effect on early ulcer formation.”
In an effort to compare preoperative endoscopic findings and MU formation in patients with and without altered PPI metabolism, the researchers prospectively enrolled 94 bariatric patients to undergo genetic testing pertinent to drug metabolism for a comprehensive panel of medications using a commercially available pharmacogenetic testing kit for the activity of cytochrome P450 in drug metabolism. They grouped patients by whether they were fast or normal metabolizers, and compared preoperative endoscopic findings for patients on PPIs at baseline and rates of early (within 90 days) and late ulceration (between 90 and 180 days).
Dr. Noria reported that 28 patients (30%) in the entire cohort met criteria for being fast metabolizers. The researchers observed no differences in baseline body mass index, age, gender, or former smoking status between both groups. Among those treated with a PPI at baseline, fast metabolizers demonstrated a trend toward a higher incidence of gastritis on preoperative endoscopy, compared with controls (89% vs. 65%, respectively; P = .12), while detection of Helicobacter pylori and Barrett’s esophagus were nonsignificant between groups. Eight patients (17%) who underwent RYGB developed marginal ulcers within 6 months of the index operation, of which four (50%) were diagnosed within 90 days and categorized as early ulcers. Development of early ulceration was higher among fast metabolizers, compared with controls (13% vs. 6%), but this did not reach statistical significance (P = .60). All late ulcerations occurred within the control group.
“While none of our findings are statistically significant given the small sample size, there were two findings I found clinically compelling,” Dr. Noria said. “First, in the group of patients who were on PPIs preoperatively, we found a 24% increase in the presence of pathologically diagnosed gastritis in the rapid-metabolizer group, during screening endoscopy. This suggests that either these patients were undertreated or were not treated with the appropriate medication. The second interesting finding was an over doubling of early ulcer formation in the RYGB group who were rapid metabolizers. However, again I would caution against drawing any real conclusions as our sample size was not powered to detect any difference.”
She also acknowledged that the study was limited by the inability to determine the effect of confounders such as surgical approach and the lack of randomization.
Anahita D. Jalilvand, MD, a general surgery resident, postdoctoral research fellow, and PhD candidate, was instrumental to this study, Dr. Noria said.
The trial was sponsored by Pathnostics, a pharmacogenetic testing company, who covered the cost of the tests. The researchers reported having no financial disclosures.
REPORTING FROM DDW 2019
High-intensity statins may cut risk of joint replacement
TORONTO – comparing nearly 180,000 statin users with an equal number of propensity-matched nonusers, Jie Wei, PhD, reported at the OARSI 2019 World Congress.
Less intensive statin therapy was associated with significantly less need for joint replacement surgery in rheumatoid arthritis patients, but not in those with osteoarthritis, she said at the meeting, sponsored by the Osteoarthritis Research Society International.
“In summary, statins may reduce the risk of joint replacement, especially when given at high strength and in people with rheumatoid arthritis,” said Dr. Wei, an epidemiologist at Massachusetts General Hospital, Boston, and Central South University in Changsha, Hunan, China.
She was quick to note that this study can’t be considered the final, definitive word on the topic, since other investigators’ studies of the relationship between statin usage and joint replacement surgery for arthritis have yielded conflicting results. However, given the thoroughly established super-favorable risk/benefit ratio of statins for the prevention of cardiovascular morbidity and mortality, the possibility of a prospective, randomized, controlled trial addressing the joint surgery issue is for ethical reasons a train that’s left the station.
Dr. Wei presented an analysis drawn from the U.K. Clinical Practice Research Datalink for the years 1989 through mid-2017. The initial sample included the medical records of 17.1 million patients, or 26% of the total U.K. population. From that massive pool, she and her coinvestigators zeroed in on 178,467 statin users and an equal number of non–statin-user controls under the care of 718 primary care physicians, with the pairs propensity score-matched on the basis of age, gender, locality, comorbid conditions, nonstatin medications, lifestyle factors, and duration of rheumatoid arthritis or osteoarthritis. The mean age of the matched pairs was 62 years, 52% were women, and the mean prospective follow-up was 6.5 years.
The use of high-intensity statin therapy – for example, atorvastatin at 40-80 mg/day or rosuvastatin (Crestor) at 20-40 mg/day – was independently associated with a 21% reduction in the risk of knee or hip replacement surgery for osteoarthritis and a 90% reduction for rheumatoid arthritis, compared with statin nonusers. Notably, joint replacement surgery for osteoarthritis was roughly 25-fold more common than for rheumatoid arthritis.
Statin therapy overall, including the more widely prescribed low- and intermediate-intensity regimens, was associated with a 23% reduction in joint replacement surgery for rheumatoid arthritis, compared with statin nonusers, but had no significant impact on surgery for the osteoarthritis population.
A couple of distinguished American rheumatologists in the audience rose to voice reluctance about drawing broad conclusions from this study.
“Bias, as you’ve said yourself, is a bit of a concern,” said David T. Felson, MD, professor of medicine and public health and director of clinical epidemiology at Boston University.
He was troubled that the study design was such that anyone who filled as few as two statin prescriptions during the more than 6-year study period was categorized as a statin user. That, he said, muddies the waters. Does the database contain information on duration of statin therapy, and whether joint replacement surgery was more likely to occur when patients were on or off statin therapy? he asked.
It does, Dr. Wei replied, adding that she will take that suggestion for additional analysis back to her international team of coinvestigators.
“It seems to me,” said Jeffrey N. Katz, MD, “that the major risk of potential bias is that people who were provided high-intensity statins were prescribed that because they were at risk for or had cardiac disease.”
That high cardiovascular risk might have curbed orthopedic surgeons’ enthusiasm to operate. Thus, it would be helpful to learn whether patients who underwent joint replacement were less likely to have undergone coronary revascularization or other cardiac interventions than were those without joint replacement, according to Dr. Katz, professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
Dr. Wei agreed that confounding by indication is always a possibility in an observational study such as this. Identification of a plausible mechanism by which statins might reduce the risk of joint replacement surgery in rheumatoid arthritis – something that hasn’t happened yet – would help counter such concerns.
She noted that a separate recent analysis of the U.K. Clinical Practice Research Datalink by other investigators concluded that statin therapy started up to 5 years following total hip or knee replacement was associated with a significantly reduced risk of revision arthroplasty. Moreover, the benefit was treatment duration-dependent: Patients on statin therapy for more than 5 years were 26% less likely to undergo revision arthroplasty than were those on a statin for less than 1 year (J Rheumatol. 2019 Mar 15. doi: 10.3899/jrheum.180574).
On the other hand, Swedish investigators found that statin use wasn’t associated with a reduced risk of consultation or surgery for osteoarthritis in a pooled analysis of four cohort studies totaling more than 132,000 Swedes followed for 7.5 years (Osteoarthritis Cartilage. 2017 Nov;25[11]:1804-13).
Dr. Wei reported having no financial conflicts regarding the study, which was supported by the National Clinical Research Center of Geriatric Disorders in Hunan, China, and several British universities.
SOURCE: Sarmanova A et al. Osteoarthritis cartilage. 2019 Apr;27[suppl 1]:S78-S79. Abstract 77.
TORONTO – comparing nearly 180,000 statin users with an equal number of propensity-matched nonusers, Jie Wei, PhD, reported at the OARSI 2019 World Congress.
Less intensive statin therapy was associated with significantly less need for joint replacement surgery in rheumatoid arthritis patients, but not in those with osteoarthritis, she said at the meeting, sponsored by the Osteoarthritis Research Society International.
“In summary, statins may reduce the risk of joint replacement, especially when given at high strength and in people with rheumatoid arthritis,” said Dr. Wei, an epidemiologist at Massachusetts General Hospital, Boston, and Central South University in Changsha, Hunan, China.
She was quick to note that this study can’t be considered the final, definitive word on the topic, since other investigators’ studies of the relationship between statin usage and joint replacement surgery for arthritis have yielded conflicting results. However, given the thoroughly established super-favorable risk/benefit ratio of statins for the prevention of cardiovascular morbidity and mortality, the possibility of a prospective, randomized, controlled trial addressing the joint surgery issue is for ethical reasons a train that’s left the station.
Dr. Wei presented an analysis drawn from the U.K. Clinical Practice Research Datalink for the years 1989 through mid-2017. The initial sample included the medical records of 17.1 million patients, or 26% of the total U.K. population. From that massive pool, she and her coinvestigators zeroed in on 178,467 statin users and an equal number of non–statin-user controls under the care of 718 primary care physicians, with the pairs propensity score-matched on the basis of age, gender, locality, comorbid conditions, nonstatin medications, lifestyle factors, and duration of rheumatoid arthritis or osteoarthritis. The mean age of the matched pairs was 62 years, 52% were women, and the mean prospective follow-up was 6.5 years.
The use of high-intensity statin therapy – for example, atorvastatin at 40-80 mg/day or rosuvastatin (Crestor) at 20-40 mg/day – was independently associated with a 21% reduction in the risk of knee or hip replacement surgery for osteoarthritis and a 90% reduction for rheumatoid arthritis, compared with statin nonusers. Notably, joint replacement surgery for osteoarthritis was roughly 25-fold more common than for rheumatoid arthritis.
Statin therapy overall, including the more widely prescribed low- and intermediate-intensity regimens, was associated with a 23% reduction in joint replacement surgery for rheumatoid arthritis, compared with statin nonusers, but had no significant impact on surgery for the osteoarthritis population.
A couple of distinguished American rheumatologists in the audience rose to voice reluctance about drawing broad conclusions from this study.
“Bias, as you’ve said yourself, is a bit of a concern,” said David T. Felson, MD, professor of medicine and public health and director of clinical epidemiology at Boston University.
He was troubled that the study design was such that anyone who filled as few as two statin prescriptions during the more than 6-year study period was categorized as a statin user. That, he said, muddies the waters. Does the database contain information on duration of statin therapy, and whether joint replacement surgery was more likely to occur when patients were on or off statin therapy? he asked.
It does, Dr. Wei replied, adding that she will take that suggestion for additional analysis back to her international team of coinvestigators.
“It seems to me,” said Jeffrey N. Katz, MD, “that the major risk of potential bias is that people who were provided high-intensity statins were prescribed that because they were at risk for or had cardiac disease.”
That high cardiovascular risk might have curbed orthopedic surgeons’ enthusiasm to operate. Thus, it would be helpful to learn whether patients who underwent joint replacement were less likely to have undergone coronary revascularization or other cardiac interventions than were those without joint replacement, according to Dr. Katz, professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
Dr. Wei agreed that confounding by indication is always a possibility in an observational study such as this. Identification of a plausible mechanism by which statins might reduce the risk of joint replacement surgery in rheumatoid arthritis – something that hasn’t happened yet – would help counter such concerns.
She noted that a separate recent analysis of the U.K. Clinical Practice Research Datalink by other investigators concluded that statin therapy started up to 5 years following total hip or knee replacement was associated with a significantly reduced risk of revision arthroplasty. Moreover, the benefit was treatment duration-dependent: Patients on statin therapy for more than 5 years were 26% less likely to undergo revision arthroplasty than were those on a statin for less than 1 year (J Rheumatol. 2019 Mar 15. doi: 10.3899/jrheum.180574).
On the other hand, Swedish investigators found that statin use wasn’t associated with a reduced risk of consultation or surgery for osteoarthritis in a pooled analysis of four cohort studies totaling more than 132,000 Swedes followed for 7.5 years (Osteoarthritis Cartilage. 2017 Nov;25[11]:1804-13).
Dr. Wei reported having no financial conflicts regarding the study, which was supported by the National Clinical Research Center of Geriatric Disorders in Hunan, China, and several British universities.
SOURCE: Sarmanova A et al. Osteoarthritis cartilage. 2019 Apr;27[suppl 1]:S78-S79. Abstract 77.
TORONTO – comparing nearly 180,000 statin users with an equal number of propensity-matched nonusers, Jie Wei, PhD, reported at the OARSI 2019 World Congress.
Less intensive statin therapy was associated with significantly less need for joint replacement surgery in rheumatoid arthritis patients, but not in those with osteoarthritis, she said at the meeting, sponsored by the Osteoarthritis Research Society International.
“In summary, statins may reduce the risk of joint replacement, especially when given at high strength and in people with rheumatoid arthritis,” said Dr. Wei, an epidemiologist at Massachusetts General Hospital, Boston, and Central South University in Changsha, Hunan, China.
She was quick to note that this study can’t be considered the final, definitive word on the topic, since other investigators’ studies of the relationship between statin usage and joint replacement surgery for arthritis have yielded conflicting results. However, given the thoroughly established super-favorable risk/benefit ratio of statins for the prevention of cardiovascular morbidity and mortality, the possibility of a prospective, randomized, controlled trial addressing the joint surgery issue is for ethical reasons a train that’s left the station.
Dr. Wei presented an analysis drawn from the U.K. Clinical Practice Research Datalink for the years 1989 through mid-2017. The initial sample included the medical records of 17.1 million patients, or 26% of the total U.K. population. From that massive pool, she and her coinvestigators zeroed in on 178,467 statin users and an equal number of non–statin-user controls under the care of 718 primary care physicians, with the pairs propensity score-matched on the basis of age, gender, locality, comorbid conditions, nonstatin medications, lifestyle factors, and duration of rheumatoid arthritis or osteoarthritis. The mean age of the matched pairs was 62 years, 52% were women, and the mean prospective follow-up was 6.5 years.
The use of high-intensity statin therapy – for example, atorvastatin at 40-80 mg/day or rosuvastatin (Crestor) at 20-40 mg/day – was independently associated with a 21% reduction in the risk of knee or hip replacement surgery for osteoarthritis and a 90% reduction for rheumatoid arthritis, compared with statin nonusers. Notably, joint replacement surgery for osteoarthritis was roughly 25-fold more common than for rheumatoid arthritis.
Statin therapy overall, including the more widely prescribed low- and intermediate-intensity regimens, was associated with a 23% reduction in joint replacement surgery for rheumatoid arthritis, compared with statin nonusers, but had no significant impact on surgery for the osteoarthritis population.
A couple of distinguished American rheumatologists in the audience rose to voice reluctance about drawing broad conclusions from this study.
“Bias, as you’ve said yourself, is a bit of a concern,” said David T. Felson, MD, professor of medicine and public health and director of clinical epidemiology at Boston University.
He was troubled that the study design was such that anyone who filled as few as two statin prescriptions during the more than 6-year study period was categorized as a statin user. That, he said, muddies the waters. Does the database contain information on duration of statin therapy, and whether joint replacement surgery was more likely to occur when patients were on or off statin therapy? he asked.
It does, Dr. Wei replied, adding that she will take that suggestion for additional analysis back to her international team of coinvestigators.
“It seems to me,” said Jeffrey N. Katz, MD, “that the major risk of potential bias is that people who were provided high-intensity statins were prescribed that because they were at risk for or had cardiac disease.”
That high cardiovascular risk might have curbed orthopedic surgeons’ enthusiasm to operate. Thus, it would be helpful to learn whether patients who underwent joint replacement were less likely to have undergone coronary revascularization or other cardiac interventions than were those without joint replacement, according to Dr. Katz, professor of medicine and orthopedic surgery at Harvard Medical School, Boston.
Dr. Wei agreed that confounding by indication is always a possibility in an observational study such as this. Identification of a plausible mechanism by which statins might reduce the risk of joint replacement surgery in rheumatoid arthritis – something that hasn’t happened yet – would help counter such concerns.
She noted that a separate recent analysis of the U.K. Clinical Practice Research Datalink by other investigators concluded that statin therapy started up to 5 years following total hip or knee replacement was associated with a significantly reduced risk of revision arthroplasty. Moreover, the benefit was treatment duration-dependent: Patients on statin therapy for more than 5 years were 26% less likely to undergo revision arthroplasty than were those on a statin for less than 1 year (J Rheumatol. 2019 Mar 15. doi: 10.3899/jrheum.180574).
On the other hand, Swedish investigators found that statin use wasn’t associated with a reduced risk of consultation or surgery for osteoarthritis in a pooled analysis of four cohort studies totaling more than 132,000 Swedes followed for 7.5 years (Osteoarthritis Cartilage. 2017 Nov;25[11]:1804-13).
Dr. Wei reported having no financial conflicts regarding the study, which was supported by the National Clinical Research Center of Geriatric Disorders in Hunan, China, and several British universities.
SOURCE: Sarmanova A et al. Osteoarthritis cartilage. 2019 Apr;27[suppl 1]:S78-S79. Abstract 77.
REPORTING FROM OARSI 2019
Key clinical point: High-intensity statin therapy may reduce need for joint replacement in arthritis.
Major finding: The risk of knee or hip replacement surgery for rheumatoid arthritis was slashed by 90%, and by 21% for osteoarthritis.
Study details: This study included nearly 180,000 statin users propensity score-matched to an equal number of nonusers and prospectively followed for a mean of 6.5 years.
Disclosures: The study was supported by the National Clinical Research Center of Geriatric Disorders at Central South University in Hunan, China, and by several British universities. The presenter reported having no financial conflicts of interest.
Source: Sarmanova A et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S78-S79. Abstract 77.