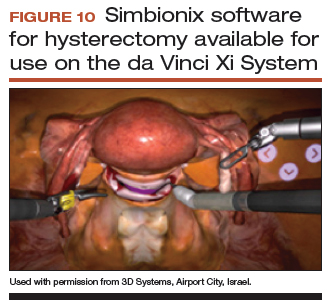
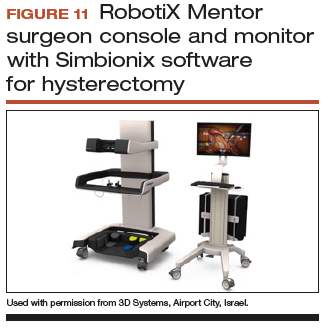
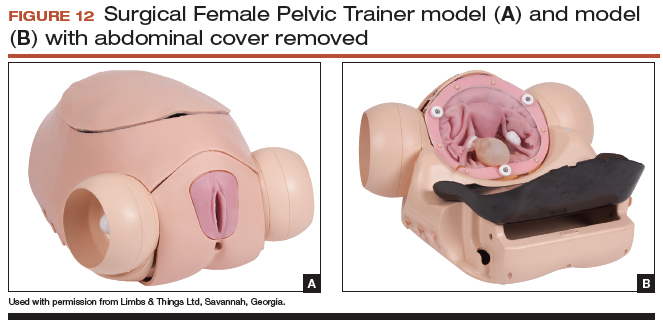
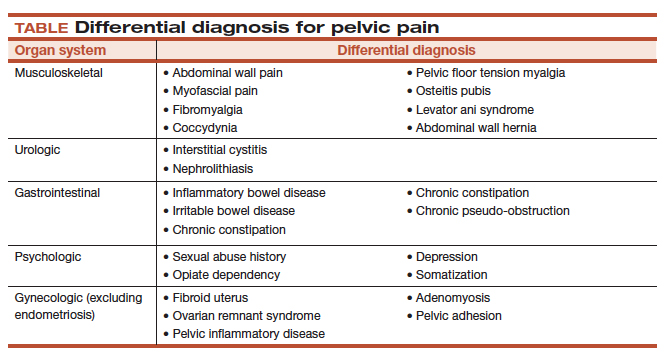
User login
Using social media to change the story on MIGS
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Use of a Core Reamer for the Resection of a Central Distal Femoral Physeal Bone Bridge: A Novel Technique with 3-Year Follow-up
ABSTRACT
A central distal femoral physeal bone bridge in a boy aged 5 years and 7 months was resected with a fluoroscopically guided core reamer placed through a lateral parapatellar approach. At 3-year follow-up, the boy’s leg-length discrepancy was 3.0 cm (3.9 cm preoperatively), and the physeal bone bridge did not recur. The patient had full function and no pain or other patellofemoral complaints. This technique provided direct access to the physeal bone bridge, and complete resection was performed without injury to the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which is expected to grow normally in the absence of the bridge.
A physeal bone bridge is an osseous connection that forms across a physis. It may cause partial premature physeal arrest. Angular deformity and limb-length discrepancy are the main complications caused by physeal bone bridges.1-4 The indications for the treatment of physeal bridges are well documented.1-5 Trauma and infection are common causes of distal femoral physeal bone bridges. Arkader and colleagues6 showed that among different types of physeal bridges, the Salter-Harris type is significantly associated with complications, among which growth arrest is the most common and occurs in 27.4% of all patients.
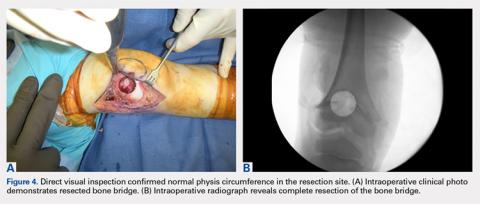
The treatment of distal femoral physeal bone bridges is technically difficult and provides variable results. Poor results are reported in 13% to 40% of patients.7-10 Procedure failure has been attributed to incomplete resection with the persistent tethering and dislodgement of the graft.11 Methods with improved efficacy for the removal of central physeal bridges will help prevent reformation after treatment. We have used a novel technique that allows the direct resection of a central physeal bone bridge in the distal femur through the use of a fluoroscopically guided core reamer. This technique enables the complete removal of the bone bridge and the direct visual assessment of the remaining physis. The patient’s parents provided written informed consent for print and electronic publication of this case report.
CASE
A 3-year-old boy with a history of hemifacial microsomia presented for the evaluation of genu valgum and leg-length discrepancy. His intermalleolar distance at that time was 8 cm. A standing radiograph of his lower extremities demonstrated changes consistent with physiologic genu valgum. He had no history of knee trauma, infection, or pain.
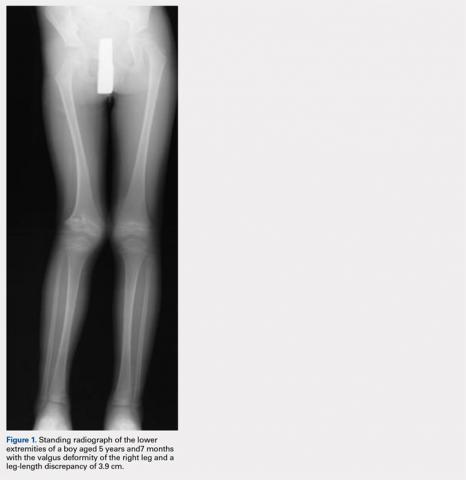
At the age of 5 years and 7 months, the patient returned for a repeat evaluation and was noted to exhibit the progressive valgus deformity of the right leg and a leg-length discrepancy of 3.9 cm (Figure 1).
Continue to: With the patient supine on the operating...
OPERATIVE TECHNIQUE
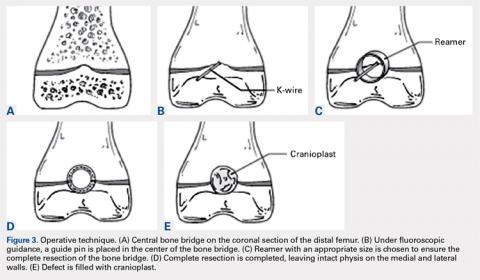
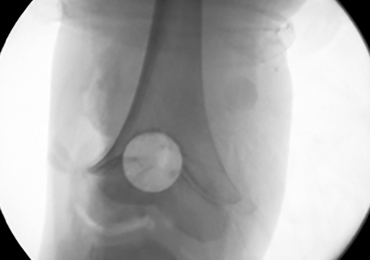
With the patient supine on the operating table and after the administration of general anesthesia, 3-dimensional (3-D) fluoroscopy was used to localize the bone bridge, which confirmed the fluoroscopic location that was previously visualized through preoperative 3-D imaging. The leg was elevated, and a tourniquet was applied and inflated. A lateral parapatellar approach was used to isolate the distal femoral physis anteriorly because the bone bridge was centered just lateral to the central portion of the distal femoral physis. A Kirschner wire was placed in the center of the bridge under anteroposterior and lateral fluoroscopic imaging (Figures 3A-3E).
OUTCOME
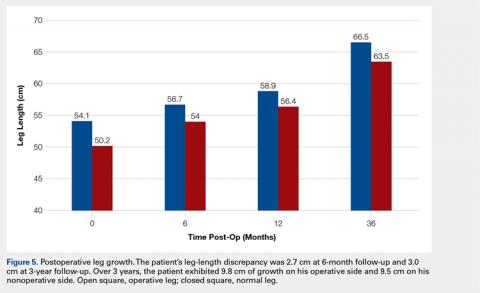
The patient healed uneventfully, and early range-of-motion exercises were started 6 weeks postoperatively. At 6-month follow-up, his leg-length discrepancy was 2.7 cm, and the bone bridge did not recur. At 3-year follow-up, his leg-length discrepancy was 3.0 cm, and the bone bridge did not recur. Over the 3 years postoperatively, the patient exhibited 9.8 cm of growth on his operative side and 9.5 cm on his nonoperative side (Figure 5).
DISCUSSION
Given the considerable growth potential of the distal femoral physis,1,14-16 an injury to the distal femoral physis and the formation of a physeal bone bridge can have a profound effect on a young patient in terms of leg-length discrepancy and angular deformity. Fracture from trauma or infection is a common cause of physeal bone bridges.6,17-19 The etiology of our patient’s distal femoral physeal bone bridge is idiopathic, which is considerably less common than other etiologies, and the incidence of idiopathic physeal bone bridge formation is not well established in the literature. Hresko and Kasser21 identified atraumatic physeal bone bridge formations in 7 patients. Among the 13 patients with physeal bone bridges described by Broughton and colleagues,20 the cause of bridge formation is unknown in 1.
Physeal bone bridges that form centrally are particularly challenging because they are difficult to visualize through a peripheral approach. A number of methods for resecting central physeal bone bridges have been described. These methods have varying degrees of success. In 1981, Langenskiöld7 first described the creation of a metaphyseal mirror and the use of a dental mirror for visualization. This technique, however, yielded unfavorable results in 16% of patients. Williamson and Staheli9 reported poor results in 23% of patients. Loraas and Schmale4 described the use of an endoscope, termed an osteoscope, for visualization, citing advantages of superior illumination and potential for image magnification and capture. Marsh and Polzhofer8 also showed this technique to have low morbidity but poor results in 13% of patients, whereas Moreta and colleagues10 reported poor results in 2 out of 5 patients. The rate of poor results of these methods may be related to the technical difficulty of using dental mirrors and arthroscopes and can be improved by highly efficient direct methods with improved visualization, such as the method described in this article.
Continue to: Proper imaging is necessary for...
Proper imaging is necessary for the accurate quantification of bone bridges to determine resectability and to identify the best surgical approach to resection. MRI with software for the generation of 3-D physeal maps is a reproducible method with good interobserver reliability.22,23 Intraoperative computer-assisted imaging also is beneficial for determining the extent and location of the resection to ensure complete bone bridge removal.24
To our knowledge, a direct approach through parapatellar arthrotomy for the resection of a centrally located distal femoral physeal bone bridge has not been previously described. This novel technique provided direct access to the physeal bone bridge and was performed without injuring the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which may grow normally in the absence of the bridge. Instead of using a lateral or medial approach with a metaphyseal window,4 we directly approached this central bar through a parapatellar approach and were able to completely resect it under direct visualization. This obviated the need for an arthroscope or dental mirror. To remove the entire physeal bone bridge, we needed to resect completely from the anterior cortex to the posterior cortex. Although this technique potentially increased the risk of iatrogenic fracture, we believed that this risk would not differ greatly from that of disrupting the medial or lateral metaphysis and would be more stable with either axial and torsion load. At 3-year follow-up, the patient exhibited restored normal growth in his operative limb relative to that in his nonoperative limb, had not developed angular deformity, and had maintained his previously developed limb-length discrepancy that could be corrected with the epiphysiodesis of his opposite limb at a later date.
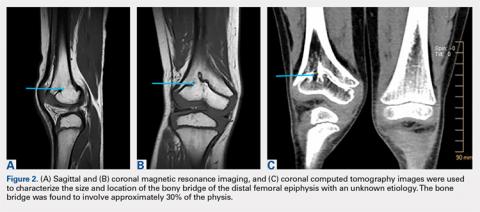
The limitations to this technique include the fact that it may be most effective with small-to moderate-sized central physeal bone bridges, although resection has shown good results with up to 70% physeal involvement.8 In this patient, the bone bridge was moderately sized (30% of the physis), centrally located, and clearly visible on fluoroscopy. These characteristics increased the technical safety and ease of the procedure. The resection of large, peripheral bridges may destabilize the distal femur. The destabilization of the distal femur, in turn, can lead to fracture. Patellofemoral mechanics may also be affected during the treatment of distal femoral physeal bone bridges. This patient has not experienced any patellofemoral dysfunction or symptoms. Given the patient’s age and significant amount of remaining growth, he will need close monitoring until he reaches skeletal maturity.
This paper will be judged for the Resident Writer’s Award.
1. Murphy GA. Disorders of tendons and fascia and adolescent and adult pes planus. In: Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 12th edition. Philadelphia, PA: Mosby-Elsevier; 2013:3966-3972.
2. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
3. Stans AA. Excision of physeal bar. In: Wiesel SW, ed. Operative Techniques in Orthopaedic Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:1244-1249.
4. Loraas EK, Schmale GA. Endoscopically aided physeal bar takedown and guided growth for the treatment of angular limb deformity. J Pediatr Orthop B. 2012;21(4):348-351. doi:10.1097/BPB.0b013e328346d308.
5. Inoue T, Naito M, Fuhii T, Akiyoshi Y, Yoshimura I, Takamura K. Partial physeal growth arrest treated by bridge resection and artificial dura substitute interposition. J Pediatr Orthop B. 2006;15(1):65-69. doi:10.1097/01202412-200601000-00014.
6. Arkader A, Warner WC Jr, Horn BD, Shaw RN, Wells L. Predicting the outcome of physeal fractures of the distal femur. J Pediatr Orthop. 2007;27(6):703-708. doi:10.1097/BPO.0b013e3180dca0e5.
7. Langenskiöld A. Surgical treatment of partial closure of the growth plate. J Pediatr Orthop. 1981;1(1):3-11. doi:10.1097/01241398-198101010-00002.
8. Marsh JS, Polzhofer GK. Arthroscopically assisted central physeal bar resection. J Pediatr Orthop. 2006;26(2):255-259. doi:10.1097/01.bpo.0000218533.43986.e1.
9. Williamson RV, Staheli LT. Partial physeal growth arrest: treatment by bridge resection and fat interposition. J Pediatr Orthop. 1990;10(6):769-776. doi:10.1097/01241398-199011000-00012.
10. Moreta J, Abril JC, Miranda C. Arthroscopy-assisted resection-interposition of post-traumatic central physeal bridges. Rev Esp Cir Orthop Traumatol. 2013;57(5):333-339. doi:10.1016/j.recot.2013.07.004.
11. Hasler CC, Foster BK. Secondary tethers after physeal bar resection: a common source of failure? Clin Orthop Relat Res. 2002;405:242-249.
12. Paley D, Bhave A, Herzenberg JE, Bowen JR. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82(10):1432-1446. doi:10.2106/00004623-200010000-00010.
13. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
14. Rathjen KE, Kim HKW. Physeal injuries and growth disturbances. In: Flynn JM, Skaggs DL, Waters PM, eds. Rockwood and Wilkins’ Fractures in Children. 8th edition. Philadelphia, PA: Wolters-Kluwer; 2015:135-137.
15. Peterson CA, Peterson HA. Analysis of the incidence of injuries to the epiphyseal growth plate. J Trauma. 1972;12(4):275-281. doi:10.1097/00005373-197204000-00002.
16. Pritchett JW. Longitudinal growth and growth-plate activity in the lower extremity. Clin Orthop Relat Res. 1992;275:274-279.
17. Cassebaum WH, Patterson AH. Fracture of the distal femoral epiphysis. Clin Orthop Relat Res. 1965;41:79-91. doi:10.1097/00003086-196500410-00009.
18. Dahl WJ, Silva S, Vanderhave KL. Distal femoral physeal fixation: are smooth pins really safe? J Pedatir Orthop. 2014;34(2):134-138. doi:10.1097/BPO.0000000000000083.
19. Roberts J. Fracture separation of the distal femoral epiphyseal growth line. J Bone Joint Surg Am. 1973;55:1324.
20. Broughton NS, Dickens DR, Cole WG, Menelaus MB. Epiphyseolysis for partial growth plate arrest. Results after four years or at maturity. J Bone Joint Surg Br. 1989;71(1):13-16. doi:10.1302/0301-620X.71B1.2914983.
21. Hresko MT, Kasser JR. Physeal arrest about the knee associated with non-physeal fractures in the lower extremity. J Bone Joint Surg Am. 1989;71(5):698-703. doi:10.2106/00004623-198971050-00009.
22. Lurie B, Koff MF, Shah P, et al. Three-dimensional magnetic resonance imaging of physeal injury: reliability and clinical utility. J Pediatr Orthop. 2014;34(3):239-245. doi:10.1097/BPO.0000000000000104.
23. Sailhan F, Chotel F, Guibal AL, et al. Three-dimensional MR imaging in the assessment of physeal growth arrest. Eur Radiol. 2004;14(9):1600-1608. doi:10.1007/s00330-004-2319-z.
24. Kang HG, Yoon SJ, Kim JR. Resection of a physeal bar under computer-assisted guidance. J Bone Joint Surg Br. 2010;92(10):1452-1455. doi:10.1302/0301-620X.92B10.24587.
ABSTRACT
A central distal femoral physeal bone bridge in a boy aged 5 years and 7 months was resected with a fluoroscopically guided core reamer placed through a lateral parapatellar approach. At 3-year follow-up, the boy’s leg-length discrepancy was 3.0 cm (3.9 cm preoperatively), and the physeal bone bridge did not recur. The patient had full function and no pain or other patellofemoral complaints. This technique provided direct access to the physeal bone bridge, and complete resection was performed without injury to the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which is expected to grow normally in the absence of the bridge.
A physeal bone bridge is an osseous connection that forms across a physis. It may cause partial premature physeal arrest. Angular deformity and limb-length discrepancy are the main complications caused by physeal bone bridges.1-4 The indications for the treatment of physeal bridges are well documented.1-5 Trauma and infection are common causes of distal femoral physeal bone bridges. Arkader and colleagues6 showed that among different types of physeal bridges, the Salter-Harris type is significantly associated with complications, among which growth arrest is the most common and occurs in 27.4% of all patients.
The treatment of distal femoral physeal bone bridges is technically difficult and provides variable results. Poor results are reported in 13% to 40% of patients.7-10 Procedure failure has been attributed to incomplete resection with the persistent tethering and dislodgement of the graft.11 Methods with improved efficacy for the removal of central physeal bridges will help prevent reformation after treatment. We have used a novel technique that allows the direct resection of a central physeal bone bridge in the distal femur through the use of a fluoroscopically guided core reamer. This technique enables the complete removal of the bone bridge and the direct visual assessment of the remaining physis. The patient’s parents provided written informed consent for print and electronic publication of this case report.
CASE
A 3-year-old boy with a history of hemifacial microsomia presented for the evaluation of genu valgum and leg-length discrepancy. His intermalleolar distance at that time was 8 cm. A standing radiograph of his lower extremities demonstrated changes consistent with physiologic genu valgum. He had no history of knee trauma, infection, or pain.
At the age of 5 years and 7 months, the patient returned for a repeat evaluation and was noted to exhibit the progressive valgus deformity of the right leg and a leg-length discrepancy of 3.9 cm (Figure 1).
Continue to: With the patient supine on the operating...
OPERATIVE TECHNIQUE
With the patient supine on the operating table and after the administration of general anesthesia, 3-dimensional (3-D) fluoroscopy was used to localize the bone bridge, which confirmed the fluoroscopic location that was previously visualized through preoperative 3-D imaging. The leg was elevated, and a tourniquet was applied and inflated. A lateral parapatellar approach was used to isolate the distal femoral physis anteriorly because the bone bridge was centered just lateral to the central portion of the distal femoral physis. A Kirschner wire was placed in the center of the bridge under anteroposterior and lateral fluoroscopic imaging (Figures 3A-3E).
OUTCOME
The patient healed uneventfully, and early range-of-motion exercises were started 6 weeks postoperatively. At 6-month follow-up, his leg-length discrepancy was 2.7 cm, and the bone bridge did not recur. At 3-year follow-up, his leg-length discrepancy was 3.0 cm, and the bone bridge did not recur. Over the 3 years postoperatively, the patient exhibited 9.8 cm of growth on his operative side and 9.5 cm on his nonoperative side (Figure 5).
DISCUSSION
Given the considerable growth potential of the distal femoral physis,1,14-16 an injury to the distal femoral physis and the formation of a physeal bone bridge can have a profound effect on a young patient in terms of leg-length discrepancy and angular deformity. Fracture from trauma or infection is a common cause of physeal bone bridges.6,17-19 The etiology of our patient’s distal femoral physeal bone bridge is idiopathic, which is considerably less common than other etiologies, and the incidence of idiopathic physeal bone bridge formation is not well established in the literature. Hresko and Kasser21 identified atraumatic physeal bone bridge formations in 7 patients. Among the 13 patients with physeal bone bridges described by Broughton and colleagues,20 the cause of bridge formation is unknown in 1.
Physeal bone bridges that form centrally are particularly challenging because they are difficult to visualize through a peripheral approach. A number of methods for resecting central physeal bone bridges have been described. These methods have varying degrees of success. In 1981, Langenskiöld7 first described the creation of a metaphyseal mirror and the use of a dental mirror for visualization. This technique, however, yielded unfavorable results in 16% of patients. Williamson and Staheli9 reported poor results in 23% of patients. Loraas and Schmale4 described the use of an endoscope, termed an osteoscope, for visualization, citing advantages of superior illumination and potential for image magnification and capture. Marsh and Polzhofer8 also showed this technique to have low morbidity but poor results in 13% of patients, whereas Moreta and colleagues10 reported poor results in 2 out of 5 patients. The rate of poor results of these methods may be related to the technical difficulty of using dental mirrors and arthroscopes and can be improved by highly efficient direct methods with improved visualization, such as the method described in this article.
Continue to: Proper imaging is necessary for...
Proper imaging is necessary for the accurate quantification of bone bridges to determine resectability and to identify the best surgical approach to resection. MRI with software for the generation of 3-D physeal maps is a reproducible method with good interobserver reliability.22,23 Intraoperative computer-assisted imaging also is beneficial for determining the extent and location of the resection to ensure complete bone bridge removal.24
To our knowledge, a direct approach through parapatellar arthrotomy for the resection of a centrally located distal femoral physeal bone bridge has not been previously described. This novel technique provided direct access to the physeal bone bridge and was performed without injuring the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which may grow normally in the absence of the bridge. Instead of using a lateral or medial approach with a metaphyseal window,4 we directly approached this central bar through a parapatellar approach and were able to completely resect it under direct visualization. This obviated the need for an arthroscope or dental mirror. To remove the entire physeal bone bridge, we needed to resect completely from the anterior cortex to the posterior cortex. Although this technique potentially increased the risk of iatrogenic fracture, we believed that this risk would not differ greatly from that of disrupting the medial or lateral metaphysis and would be more stable with either axial and torsion load. At 3-year follow-up, the patient exhibited restored normal growth in his operative limb relative to that in his nonoperative limb, had not developed angular deformity, and had maintained his previously developed limb-length discrepancy that could be corrected with the epiphysiodesis of his opposite limb at a later date.
The limitations to this technique include the fact that it may be most effective with small-to moderate-sized central physeal bone bridges, although resection has shown good results with up to 70% physeal involvement.8 In this patient, the bone bridge was moderately sized (30% of the physis), centrally located, and clearly visible on fluoroscopy. These characteristics increased the technical safety and ease of the procedure. The resection of large, peripheral bridges may destabilize the distal femur. The destabilization of the distal femur, in turn, can lead to fracture. Patellofemoral mechanics may also be affected during the treatment of distal femoral physeal bone bridges. This patient has not experienced any patellofemoral dysfunction or symptoms. Given the patient’s age and significant amount of remaining growth, he will need close monitoring until he reaches skeletal maturity.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
A central distal femoral physeal bone bridge in a boy aged 5 years and 7 months was resected with a fluoroscopically guided core reamer placed through a lateral parapatellar approach. At 3-year follow-up, the boy’s leg-length discrepancy was 3.0 cm (3.9 cm preoperatively), and the physeal bone bridge did not recur. The patient had full function and no pain or other patellofemoral complaints. This technique provided direct access to the physeal bone bridge, and complete resection was performed without injury to the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which is expected to grow normally in the absence of the bridge.
A physeal bone bridge is an osseous connection that forms across a physis. It may cause partial premature physeal arrest. Angular deformity and limb-length discrepancy are the main complications caused by physeal bone bridges.1-4 The indications for the treatment of physeal bridges are well documented.1-5 Trauma and infection are common causes of distal femoral physeal bone bridges. Arkader and colleagues6 showed that among different types of physeal bridges, the Salter-Harris type is significantly associated with complications, among which growth arrest is the most common and occurs in 27.4% of all patients.
The treatment of distal femoral physeal bone bridges is technically difficult and provides variable results. Poor results are reported in 13% to 40% of patients.7-10 Procedure failure has been attributed to incomplete resection with the persistent tethering and dislodgement of the graft.11 Methods with improved efficacy for the removal of central physeal bridges will help prevent reformation after treatment. We have used a novel technique that allows the direct resection of a central physeal bone bridge in the distal femur through the use of a fluoroscopically guided core reamer. This technique enables the complete removal of the bone bridge and the direct visual assessment of the remaining physis. The patient’s parents provided written informed consent for print and electronic publication of this case report.
CASE
A 3-year-old boy with a history of hemifacial microsomia presented for the evaluation of genu valgum and leg-length discrepancy. His intermalleolar distance at that time was 8 cm. A standing radiograph of his lower extremities demonstrated changes consistent with physiologic genu valgum. He had no history of knee trauma, infection, or pain.
At the age of 5 years and 7 months, the patient returned for a repeat evaluation and was noted to exhibit the progressive valgus deformity of the right leg and a leg-length discrepancy of 3.9 cm (Figure 1).
Continue to: With the patient supine on the operating...
OPERATIVE TECHNIQUE
With the patient supine on the operating table and after the administration of general anesthesia, 3-dimensional (3-D) fluoroscopy was used to localize the bone bridge, which confirmed the fluoroscopic location that was previously visualized through preoperative 3-D imaging. The leg was elevated, and a tourniquet was applied and inflated. A lateral parapatellar approach was used to isolate the distal femoral physis anteriorly because the bone bridge was centered just lateral to the central portion of the distal femoral physis. A Kirschner wire was placed in the center of the bridge under anteroposterior and lateral fluoroscopic imaging (Figures 3A-3E).
OUTCOME
The patient healed uneventfully, and early range-of-motion exercises were started 6 weeks postoperatively. At 6-month follow-up, his leg-length discrepancy was 2.7 cm, and the bone bridge did not recur. At 3-year follow-up, his leg-length discrepancy was 3.0 cm, and the bone bridge did not recur. Over the 3 years postoperatively, the patient exhibited 9.8 cm of growth on his operative side and 9.5 cm on his nonoperative side (Figure 5).
DISCUSSION
Given the considerable growth potential of the distal femoral physis,1,14-16 an injury to the distal femoral physis and the formation of a physeal bone bridge can have a profound effect on a young patient in terms of leg-length discrepancy and angular deformity. Fracture from trauma or infection is a common cause of physeal bone bridges.6,17-19 The etiology of our patient’s distal femoral physeal bone bridge is idiopathic, which is considerably less common than other etiologies, and the incidence of idiopathic physeal bone bridge formation is not well established in the literature. Hresko and Kasser21 identified atraumatic physeal bone bridge formations in 7 patients. Among the 13 patients with physeal bone bridges described by Broughton and colleagues,20 the cause of bridge formation is unknown in 1.
Physeal bone bridges that form centrally are particularly challenging because they are difficult to visualize through a peripheral approach. A number of methods for resecting central physeal bone bridges have been described. These methods have varying degrees of success. In 1981, Langenskiöld7 first described the creation of a metaphyseal mirror and the use of a dental mirror for visualization. This technique, however, yielded unfavorable results in 16% of patients. Williamson and Staheli9 reported poor results in 23% of patients. Loraas and Schmale4 described the use of an endoscope, termed an osteoscope, for visualization, citing advantages of superior illumination and potential for image magnification and capture. Marsh and Polzhofer8 also showed this technique to have low morbidity but poor results in 13% of patients, whereas Moreta and colleagues10 reported poor results in 2 out of 5 patients. The rate of poor results of these methods may be related to the technical difficulty of using dental mirrors and arthroscopes and can be improved by highly efficient direct methods with improved visualization, such as the method described in this article.
Continue to: Proper imaging is necessary for...
Proper imaging is necessary for the accurate quantification of bone bridges to determine resectability and to identify the best surgical approach to resection. MRI with software for the generation of 3-D physeal maps is a reproducible method with good interobserver reliability.22,23 Intraoperative computer-assisted imaging also is beneficial for determining the extent and location of the resection to ensure complete bone bridge removal.24
To our knowledge, a direct approach through parapatellar arthrotomy for the resection of a centrally located distal femoral physeal bone bridge has not been previously described. This novel technique provided direct access to the physeal bone bridge and was performed without injuring the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which may grow normally in the absence of the bridge. Instead of using a lateral or medial approach with a metaphyseal window,4 we directly approached this central bar through a parapatellar approach and were able to completely resect it under direct visualization. This obviated the need for an arthroscope or dental mirror. To remove the entire physeal bone bridge, we needed to resect completely from the anterior cortex to the posterior cortex. Although this technique potentially increased the risk of iatrogenic fracture, we believed that this risk would not differ greatly from that of disrupting the medial or lateral metaphysis and would be more stable with either axial and torsion load. At 3-year follow-up, the patient exhibited restored normal growth in his operative limb relative to that in his nonoperative limb, had not developed angular deformity, and had maintained his previously developed limb-length discrepancy that could be corrected with the epiphysiodesis of his opposite limb at a later date.
The limitations to this technique include the fact that it may be most effective with small-to moderate-sized central physeal bone bridges, although resection has shown good results with up to 70% physeal involvement.8 In this patient, the bone bridge was moderately sized (30% of the physis), centrally located, and clearly visible on fluoroscopy. These characteristics increased the technical safety and ease of the procedure. The resection of large, peripheral bridges may destabilize the distal femur. The destabilization of the distal femur, in turn, can lead to fracture. Patellofemoral mechanics may also be affected during the treatment of distal femoral physeal bone bridges. This patient has not experienced any patellofemoral dysfunction or symptoms. Given the patient’s age and significant amount of remaining growth, he will need close monitoring until he reaches skeletal maturity.
This paper will be judged for the Resident Writer’s Award.
1. Murphy GA. Disorders of tendons and fascia and adolescent and adult pes planus. In: Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 12th edition. Philadelphia, PA: Mosby-Elsevier; 2013:3966-3972.
2. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
3. Stans AA. Excision of physeal bar. In: Wiesel SW, ed. Operative Techniques in Orthopaedic Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:1244-1249.
4. Loraas EK, Schmale GA. Endoscopically aided physeal bar takedown and guided growth for the treatment of angular limb deformity. J Pediatr Orthop B. 2012;21(4):348-351. doi:10.1097/BPB.0b013e328346d308.
5. Inoue T, Naito M, Fuhii T, Akiyoshi Y, Yoshimura I, Takamura K. Partial physeal growth arrest treated by bridge resection and artificial dura substitute interposition. J Pediatr Orthop B. 2006;15(1):65-69. doi:10.1097/01202412-200601000-00014.
6. Arkader A, Warner WC Jr, Horn BD, Shaw RN, Wells L. Predicting the outcome of physeal fractures of the distal femur. J Pediatr Orthop. 2007;27(6):703-708. doi:10.1097/BPO.0b013e3180dca0e5.
7. Langenskiöld A. Surgical treatment of partial closure of the growth plate. J Pediatr Orthop. 1981;1(1):3-11. doi:10.1097/01241398-198101010-00002.
8. Marsh JS, Polzhofer GK. Arthroscopically assisted central physeal bar resection. J Pediatr Orthop. 2006;26(2):255-259. doi:10.1097/01.bpo.0000218533.43986.e1.
9. Williamson RV, Staheli LT. Partial physeal growth arrest: treatment by bridge resection and fat interposition. J Pediatr Orthop. 1990;10(6):769-776. doi:10.1097/01241398-199011000-00012.
10. Moreta J, Abril JC, Miranda C. Arthroscopy-assisted resection-interposition of post-traumatic central physeal bridges. Rev Esp Cir Orthop Traumatol. 2013;57(5):333-339. doi:10.1016/j.recot.2013.07.004.
11. Hasler CC, Foster BK. Secondary tethers after physeal bar resection: a common source of failure? Clin Orthop Relat Res. 2002;405:242-249.
12. Paley D, Bhave A, Herzenberg JE, Bowen JR. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82(10):1432-1446. doi:10.2106/00004623-200010000-00010.
13. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
14. Rathjen KE, Kim HKW. Physeal injuries and growth disturbances. In: Flynn JM, Skaggs DL, Waters PM, eds. Rockwood and Wilkins’ Fractures in Children. 8th edition. Philadelphia, PA: Wolters-Kluwer; 2015:135-137.
15. Peterson CA, Peterson HA. Analysis of the incidence of injuries to the epiphyseal growth plate. J Trauma. 1972;12(4):275-281. doi:10.1097/00005373-197204000-00002.
16. Pritchett JW. Longitudinal growth and growth-plate activity in the lower extremity. Clin Orthop Relat Res. 1992;275:274-279.
17. Cassebaum WH, Patterson AH. Fracture of the distal femoral epiphysis. Clin Orthop Relat Res. 1965;41:79-91. doi:10.1097/00003086-196500410-00009.
18. Dahl WJ, Silva S, Vanderhave KL. Distal femoral physeal fixation: are smooth pins really safe? J Pedatir Orthop. 2014;34(2):134-138. doi:10.1097/BPO.0000000000000083.
19. Roberts J. Fracture separation of the distal femoral epiphyseal growth line. J Bone Joint Surg Am. 1973;55:1324.
20. Broughton NS, Dickens DR, Cole WG, Menelaus MB. Epiphyseolysis for partial growth plate arrest. Results after four years or at maturity. J Bone Joint Surg Br. 1989;71(1):13-16. doi:10.1302/0301-620X.71B1.2914983.
21. Hresko MT, Kasser JR. Physeal arrest about the knee associated with non-physeal fractures in the lower extremity. J Bone Joint Surg Am. 1989;71(5):698-703. doi:10.2106/00004623-198971050-00009.
22. Lurie B, Koff MF, Shah P, et al. Three-dimensional magnetic resonance imaging of physeal injury: reliability and clinical utility. J Pediatr Orthop. 2014;34(3):239-245. doi:10.1097/BPO.0000000000000104.
23. Sailhan F, Chotel F, Guibal AL, et al. Three-dimensional MR imaging in the assessment of physeal growth arrest. Eur Radiol. 2004;14(9):1600-1608. doi:10.1007/s00330-004-2319-z.
24. Kang HG, Yoon SJ, Kim JR. Resection of a physeal bar under computer-assisted guidance. J Bone Joint Surg Br. 2010;92(10):1452-1455. doi:10.1302/0301-620X.92B10.24587.
1. Murphy GA. Disorders of tendons and fascia and adolescent and adult pes planus. In: Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 12th edition. Philadelphia, PA: Mosby-Elsevier; 2013:3966-3972.
2. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
3. Stans AA. Excision of physeal bar. In: Wiesel SW, ed. Operative Techniques in Orthopaedic Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:1244-1249.
4. Loraas EK, Schmale GA. Endoscopically aided physeal bar takedown and guided growth for the treatment of angular limb deformity. J Pediatr Orthop B. 2012;21(4):348-351. doi:10.1097/BPB.0b013e328346d308.
5. Inoue T, Naito M, Fuhii T, Akiyoshi Y, Yoshimura I, Takamura K. Partial physeal growth arrest treated by bridge resection and artificial dura substitute interposition. J Pediatr Orthop B. 2006;15(1):65-69. doi:10.1097/01202412-200601000-00014.
6. Arkader A, Warner WC Jr, Horn BD, Shaw RN, Wells L. Predicting the outcome of physeal fractures of the distal femur. J Pediatr Orthop. 2007;27(6):703-708. doi:10.1097/BPO.0b013e3180dca0e5.
7. Langenskiöld A. Surgical treatment of partial closure of the growth plate. J Pediatr Orthop. 1981;1(1):3-11. doi:10.1097/01241398-198101010-00002.
8. Marsh JS, Polzhofer GK. Arthroscopically assisted central physeal bar resection. J Pediatr Orthop. 2006;26(2):255-259. doi:10.1097/01.bpo.0000218533.43986.e1.
9. Williamson RV, Staheli LT. Partial physeal growth arrest: treatment by bridge resection and fat interposition. J Pediatr Orthop. 1990;10(6):769-776. doi:10.1097/01241398-199011000-00012.
10. Moreta J, Abril JC, Miranda C. Arthroscopy-assisted resection-interposition of post-traumatic central physeal bridges. Rev Esp Cir Orthop Traumatol. 2013;57(5):333-339. doi:10.1016/j.recot.2013.07.004.
11. Hasler CC, Foster BK. Secondary tethers after physeal bar resection: a common source of failure? Clin Orthop Relat Res. 2002;405:242-249.
12. Paley D, Bhave A, Herzenberg JE, Bowen JR. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82(10):1432-1446. doi:10.2106/00004623-200010000-00010.
13. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
14. Rathjen KE, Kim HKW. Physeal injuries and growth disturbances. In: Flynn JM, Skaggs DL, Waters PM, eds. Rockwood and Wilkins’ Fractures in Children. 8th edition. Philadelphia, PA: Wolters-Kluwer; 2015:135-137.
15. Peterson CA, Peterson HA. Analysis of the incidence of injuries to the epiphyseal growth plate. J Trauma. 1972;12(4):275-281. doi:10.1097/00005373-197204000-00002.
16. Pritchett JW. Longitudinal growth and growth-plate activity in the lower extremity. Clin Orthop Relat Res. 1992;275:274-279.
17. Cassebaum WH, Patterson AH. Fracture of the distal femoral epiphysis. Clin Orthop Relat Res. 1965;41:79-91. doi:10.1097/00003086-196500410-00009.
18. Dahl WJ, Silva S, Vanderhave KL. Distal femoral physeal fixation: are smooth pins really safe? J Pedatir Orthop. 2014;34(2):134-138. doi:10.1097/BPO.0000000000000083.
19. Roberts J. Fracture separation of the distal femoral epiphyseal growth line. J Bone Joint Surg Am. 1973;55:1324.
20. Broughton NS, Dickens DR, Cole WG, Menelaus MB. Epiphyseolysis for partial growth plate arrest. Results after four years or at maturity. J Bone Joint Surg Br. 1989;71(1):13-16. doi:10.1302/0301-620X.71B1.2914983.
21. Hresko MT, Kasser JR. Physeal arrest about the knee associated with non-physeal fractures in the lower extremity. J Bone Joint Surg Am. 1989;71(5):698-703. doi:10.2106/00004623-198971050-00009.
22. Lurie B, Koff MF, Shah P, et al. Three-dimensional magnetic resonance imaging of physeal injury: reliability and clinical utility. J Pediatr Orthop. 2014;34(3):239-245. doi:10.1097/BPO.0000000000000104.
23. Sailhan F, Chotel F, Guibal AL, et al. Three-dimensional MR imaging in the assessment of physeal growth arrest. Eur Radiol. 2004;14(9):1600-1608. doi:10.1007/s00330-004-2319-z.
24. Kang HG, Yoon SJ, Kim JR. Resection of a physeal bar under computer-assisted guidance. J Bone Joint Surg Br. 2010;92(10):1452-1455. doi:10.1302/0301-620X.92B10.24587.
TAKE-HOME POINTS
- Central physeal arrest of the distal femur is challenging, but this surgical technique provides an option for treatment.
- Partial bone bridges can be resected, but advanced imaging with MRI or CT, or both, is helpful in preoperative planning.
- Regardless of the type of physeal bar resection that is chosen, it is unlikely that complete, normal bone growth will be restored and closed follow up will be needed.
Basic technique of vaginal hysterectomy

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
This video is brought to you by
Did unsafe oxytocin dose cause uterine rupture? $3.5M settlement
Did unsafe oxytocin dose cause uterine rupture? $3.5M settlement
When a mother presented to the hospital in labor, the on-call ObGyn ordered oxytocin with the dosage to be increased by 2 mU/min until she was receiving 30 mU/min or until an adequate contraction pattern was achieved and maintained. Over the next few hours, the labor and delivery nurse increased the dosage of the infusion several times.
As the patient began to push, a trickle of bright red blood was seen coming from her vagina and the baby's heart tones were temporarily lost. When the fetal heart tones were restored, his heart rate was approximately 50 bpm. After vaginal delivery was attempted using vacuum extraction and forceps, an emergency cesarean delivery was performed, leading to the finding that the mother's uterus had ruptured.
The baby suffered a permanent brain injury due to hypoxic-ischemic encephalopathy.
PATIENT'S CLAIM: The mother sued the hospital and on-call ObGyn. She alleged that the health care providers breached the standard of care by negligently increasing and maintaining the oxytocin at unsafe levels, which caused the mother's uterus to be overworked and eventually rupture. The rupture led to the child's hypoxia. An expert ObGyn noted that the patient's contractions were adequate by the time the oxytocin dose reached 14 mU/min, but the dosage continued to be increased.
DEFENDANTS' DEFENSE: The case was settled during the trial.
VERDICT: A $3.5 million Kansas settlement was reached.
When did the bowel injury occur?
One day after undergoing a hysterectomy, a woman went to the emergency department (ED) because she was feeling ill. She received a diagnosis of a pulmonary embolism for which she was given anticoagulant medications. The patient's symptoms persisted. Computed tomography (CT) imaging showed a bowel injury, and, 17 days after the initial surgery, an emergency laparotomy was performed.
PATIENT'S CLAIM: The patient sued the surgeon and the hospital. The hospital settled before the trial and the case continued against the surgeon.
The patient's early symptoms after surgery were evidence of a bowel injury, but imaging was not undertaken for several days. If the imaging had been undertaken earlier, the bowel injury would have been detected before it caused a rectovaginal fistula. An expert pathologist testified that the microscopic findings he detected postlaparotomy could only exist if a bowel perforation had been there for a significant period of time before the fistula developed. The patient's experts argued that the injury was not a "free perforation," but had been contained by her body, preventing the spread of the infection.
DEFENDANTS' DEFENSE: The surgeon maintained that the injury did not occur during the hysterectomy but developed in the days just before it was discovered. Over time, a collection of infected fluid at the vaginal cuff eroded into the bowel above it, creating an entryway for stool to pass through. Continuous leakage from the bowel for 17 days (the length of time between development of symptoms and discovery of the bowel injury) would have likely resulted in the patient's death.
VERDICT: A Missouri defense verdict was returned.
Did improper delivery techniques caused brachial plexus injury? $950,000 settlement
In April, a woman began receiving prenatal care for her 7th pregnancy. Her history of maternal obesity and diabetes mellitus, physical examinations, and tests suggested that she was at increased risk for having a macrosomic baby and encountering shoulder dystocia during vaginal delivery.
At 37 weeks' gestation, the mother was admitted to the hospital for induction of labor. Shoulder dystocia was encountered during delivery. At birth, the baby weighed 9 lb 10 oz and her right arm was limp. She was found to have a right brachial plexus injury involving C5‒C8 nerve roots and muscles. Two nerve root avulsions were evident on MRI and visualized by the surgeon during an extensive nerve graft operation. Arm function and range of motion improved after surgery, but the child has not recovered normal use of her arm.
PARENTS' CLAIM: Under the standard of care, the ObGyn was required to obtain informed consent, including a discussion of the risks of vaginal delivery (shoulder dystocia and brachial plexus injury), and the option of cesarean delivery. The patient claimed that the ObGyn neither obtained informed consent nor discussed these risks with her.
The labor and delivery nurse reported that the ObGyn told her, before delivery, that he was expecting a large baby and, perhaps, shoulder dystocia.
The ObGyn deviated from the standard of care by applying more than gentle traction to the fetal head while the shoulder was still impacted. The injury to the baby's right brachial plexus resulted from excessive lateral traction used by the ObGyn. The injury would not have occurred if a cesarean delivery had been performed. The mechanism of maternal forces injuring a brachial plexus nerve has never been visualized by any physician and is an unproven hypothesis.
PHYSICIAN'S DEFENSE: The ObGyn reported using standard maneuvers to deliver the baby. He applied traction on the fetal head 3 times: once after McRoberts maneuver, once after suprapubic pressure, and once after delivery of the posterior arm. He dictated into his notes that "We had to be careful to avoid excessive tractive forces." He claimed that shoulder dystocia is an unpredictable and unpreventable obstetric emergency, and that the injury was caused by the maternal forces of labor.
VERDICT: A $950,000 Virginia settlement was reached.
Wrongful death claim
On March 13, a 76-year-old woman went to her primary care physician's office because of a vaginal discharge. A nurse practitioner (NP) diagnosed a urinary tract infection (UTI) and prescribed cefixime (Suprax). Four days later, the patient began to experience severe diarrhea and blamed the medication. At a follow-up visit on March 20, the NP switched the patient to sulfamethoxazole-trimethoprim (Bactrim).
The following day, the patient was found unresponsive on her bathroom floor and was taken to the ED. It was determined that she had Clostridium difficile colitis (C difficile) and was admitted to the hospital. She developed acute renal failure, metabolic acidosis, hypovolemia, hypotension, and tachycardia. When she went into cardiac arrest, attempts to resuscitate her failed. She died on March 22.
ESTATE'S CLAIM: The estate sued the NP, claiming that the patient's symptoms did not meet the criteria for a UTI. If appropriate tests had been performed, the correct diagnosis would have been made and she could have received potentially life-saving treatment.
DEFENDANTS' DEFENSE: The NP claimed there was no negligence. Her diagnosis and treatment of the patient's condition were appropriate in all respects. The development of C difficile is a risk of any antibiotic.
VERDICT: An Indiana defense verdict was returned.
Nuchal cord: Undisclosed settlement
During delivery, the labor and delivery nurses lost the fetal heart-rate (FHR) monitor tracing, resulting in their being unaware of increasing signs of fetal intolerance to labor. The nurses continued to administer oxytocin to induce labor.
At birth, a nuchal cord was identified. The baby was born without signs of life but was successfully resuscitated by hospital staff.
The baby was found to have sustained severe brain damage as a result of profound fetal hypoxia. The child will require 24-hour nursing and supportive care for as long as she lives.
PARENTS' CLAIM: The nurses and ObGyn breached the standard of care resulting in her child's severe brain damage. The hospital nurses failed to continuously monitor the FHR. Profound brain damage was preventable in this case.
DEFENDANTS' DEFENSE: The nurses continuously monitored by listening to sounds coming out of the bedside monitor even though no taping of FHR was occurring on the central monitors or FHR monitor strip. A nuchal cord is an unforeseeable medical emergency; nothing different could have been done to change the outcome.
VERDICT: An undisclosed Texas settlement was reached.
Surgical needle left near bladder
The patient underwent a hysterectomy on July 9. Because of an injury sustained during the operation, bladder repair surgery was performed on July 19. After that surgery, she reported bleeding and urinary incontinence. Results of a computed tomography (CT) scan showed that the bladder repair was not successful, a vesicovaginal fistula had developed, and a 13-mm C-shaped needle was found near her bladder. A third operation to remove the needle from her abdomen took place on August 16.
PATIENT'S CLAIM: The needle left behind after the second surgery caused a fistula to develop. The patient suffered mental and emotional distress from knowing the needle was in her abdomen. Foreign objects left in a patient during surgery are strong evidence of negligence.
DEFENDANTS' DEFENSE: The primary defense claim was the absence of causation--any negligence did not cause the injury. Defense experts testified that the needle was on top of the bladder and did move anywhere to cause damage. The patient developed a vesicovaginal fistula due to complications from the bladder repair operation and not from the needle. In addition, there was testimony that the third surgery was unnecessary because the needle would eventually flush out of the abdomen without causing damage.
VERDICT: A Michigan defense verdict was returned.
Claims cancer diagnosis was delayed
On October 1, 2008, a woman saw her family physician (FP) for routine care. Blood work results showed an elevated white blood cell (WBC) count. The patient claimed that the FP did not inform her of these test results.
One year later, the patient went to an urgent care facility where blood work was performed; the results showed a high WBC count. After a work-up, the patient was given a presumptive diagnosis of mantel cell lymphoma. By December 9, she had undergone the first round of chemotherapy. Subsequent tests revealed that the presumptive diagnosis was incorrect; she actually had low-grade lymphoproliferative disorder.
In lieu of this new diagnosis, the medical team offered the patient the option of discontinuing chemotherapy. She decided, however, to continue the treatment. Chemotherapy was followed by 2 years of rituximab/hyaluronidase human maintenance therapy.
PATIENT'S CLAIM: The patient presented her case to a medical review board. The course of treatment (chemotherapy plus maintenance therapy) left her with permanent heart damage and an elevated risk of developing secondary cancer.
The patient claimed that none of this would have happened if her FP had informed her of the October 2008 test results and recommended appropriate follow-up studies. The results of those studies would have given her the correct diagnosis and allowed her to receive prompt, proper treatment. The medical review board responded unanimously that the FP's conduct constituted a breach of the standard of care, but concluded that the breach was not a factor in the patient's damages.
The patient filed suit against the FP. An expert in internal medicine commented that, based on the 2008 WBC count, the tests should have been repeated and the patient should have been referred to a hematologist/oncologist. Failure to do so increased the patient's risk of developing cancer in the future.
DEFENDANTS' DEFENSE: The FP denied any breach of standard of care. According to her notes, she had shared test results with the patient on November 26 and recommended following up with repeat blood work. The FP blamed the patient for failing to follow-up as recommended.
VERDICT: An Indiana defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Did unsafe oxytocin dose cause uterine rupture? $3.5M settlement
When a mother presented to the hospital in labor, the on-call ObGyn ordered oxytocin with the dosage to be increased by 2 mU/min until she was receiving 30 mU/min or until an adequate contraction pattern was achieved and maintained. Over the next few hours, the labor and delivery nurse increased the dosage of the infusion several times.
As the patient began to push, a trickle of bright red blood was seen coming from her vagina and the baby's heart tones were temporarily lost. When the fetal heart tones were restored, his heart rate was approximately 50 bpm. After vaginal delivery was attempted using vacuum extraction and forceps, an emergency cesarean delivery was performed, leading to the finding that the mother's uterus had ruptured.
The baby suffered a permanent brain injury due to hypoxic-ischemic encephalopathy.
PATIENT'S CLAIM: The mother sued the hospital and on-call ObGyn. She alleged that the health care providers breached the standard of care by negligently increasing and maintaining the oxytocin at unsafe levels, which caused the mother's uterus to be overworked and eventually rupture. The rupture led to the child's hypoxia. An expert ObGyn noted that the patient's contractions were adequate by the time the oxytocin dose reached 14 mU/min, but the dosage continued to be increased.
DEFENDANTS' DEFENSE: The case was settled during the trial.
VERDICT: A $3.5 million Kansas settlement was reached.
When did the bowel injury occur?
One day after undergoing a hysterectomy, a woman went to the emergency department (ED) because she was feeling ill. She received a diagnosis of a pulmonary embolism for which she was given anticoagulant medications. The patient's symptoms persisted. Computed tomography (CT) imaging showed a bowel injury, and, 17 days after the initial surgery, an emergency laparotomy was performed.
PATIENT'S CLAIM: The patient sued the surgeon and the hospital. The hospital settled before the trial and the case continued against the surgeon.
The patient's early symptoms after surgery were evidence of a bowel injury, but imaging was not undertaken for several days. If the imaging had been undertaken earlier, the bowel injury would have been detected before it caused a rectovaginal fistula. An expert pathologist testified that the microscopic findings he detected postlaparotomy could only exist if a bowel perforation had been there for a significant period of time before the fistula developed. The patient's experts argued that the injury was not a "free perforation," but had been contained by her body, preventing the spread of the infection.
DEFENDANTS' DEFENSE: The surgeon maintained that the injury did not occur during the hysterectomy but developed in the days just before it was discovered. Over time, a collection of infected fluid at the vaginal cuff eroded into the bowel above it, creating an entryway for stool to pass through. Continuous leakage from the bowel for 17 days (the length of time between development of symptoms and discovery of the bowel injury) would have likely resulted in the patient's death.
VERDICT: A Missouri defense verdict was returned.
Did improper delivery techniques caused brachial plexus injury? $950,000 settlement
In April, a woman began receiving prenatal care for her 7th pregnancy. Her history of maternal obesity and diabetes mellitus, physical examinations, and tests suggested that she was at increased risk for having a macrosomic baby and encountering shoulder dystocia during vaginal delivery.
At 37 weeks' gestation, the mother was admitted to the hospital for induction of labor. Shoulder dystocia was encountered during delivery. At birth, the baby weighed 9 lb 10 oz and her right arm was limp. She was found to have a right brachial plexus injury involving C5‒C8 nerve roots and muscles. Two nerve root avulsions were evident on MRI and visualized by the surgeon during an extensive nerve graft operation. Arm function and range of motion improved after surgery, but the child has not recovered normal use of her arm.
PARENTS' CLAIM: Under the standard of care, the ObGyn was required to obtain informed consent, including a discussion of the risks of vaginal delivery (shoulder dystocia and brachial plexus injury), and the option of cesarean delivery. The patient claimed that the ObGyn neither obtained informed consent nor discussed these risks with her.
The labor and delivery nurse reported that the ObGyn told her, before delivery, that he was expecting a large baby and, perhaps, shoulder dystocia.
The ObGyn deviated from the standard of care by applying more than gentle traction to the fetal head while the shoulder was still impacted. The injury to the baby's right brachial plexus resulted from excessive lateral traction used by the ObGyn. The injury would not have occurred if a cesarean delivery had been performed. The mechanism of maternal forces injuring a brachial plexus nerve has never been visualized by any physician and is an unproven hypothesis.
PHYSICIAN'S DEFENSE: The ObGyn reported using standard maneuvers to deliver the baby. He applied traction on the fetal head 3 times: once after McRoberts maneuver, once after suprapubic pressure, and once after delivery of the posterior arm. He dictated into his notes that "We had to be careful to avoid excessive tractive forces." He claimed that shoulder dystocia is an unpredictable and unpreventable obstetric emergency, and that the injury was caused by the maternal forces of labor.
VERDICT: A $950,000 Virginia settlement was reached.
Wrongful death claim
On March 13, a 76-year-old woman went to her primary care physician's office because of a vaginal discharge. A nurse practitioner (NP) diagnosed a urinary tract infection (UTI) and prescribed cefixime (Suprax). Four days later, the patient began to experience severe diarrhea and blamed the medication. At a follow-up visit on March 20, the NP switched the patient to sulfamethoxazole-trimethoprim (Bactrim).
The following day, the patient was found unresponsive on her bathroom floor and was taken to the ED. It was determined that she had Clostridium difficile colitis (C difficile) and was admitted to the hospital. She developed acute renal failure, metabolic acidosis, hypovolemia, hypotension, and tachycardia. When she went into cardiac arrest, attempts to resuscitate her failed. She died on March 22.
ESTATE'S CLAIM: The estate sued the NP, claiming that the patient's symptoms did not meet the criteria for a UTI. If appropriate tests had been performed, the correct diagnosis would have been made and she could have received potentially life-saving treatment.
DEFENDANTS' DEFENSE: The NP claimed there was no negligence. Her diagnosis and treatment of the patient's condition were appropriate in all respects. The development of C difficile is a risk of any antibiotic.
VERDICT: An Indiana defense verdict was returned.
Nuchal cord: Undisclosed settlement
During delivery, the labor and delivery nurses lost the fetal heart-rate (FHR) monitor tracing, resulting in their being unaware of increasing signs of fetal intolerance to labor. The nurses continued to administer oxytocin to induce labor.
At birth, a nuchal cord was identified. The baby was born without signs of life but was successfully resuscitated by hospital staff.
The baby was found to have sustained severe brain damage as a result of profound fetal hypoxia. The child will require 24-hour nursing and supportive care for as long as she lives.
PARENTS' CLAIM: The nurses and ObGyn breached the standard of care resulting in her child's severe brain damage. The hospital nurses failed to continuously monitor the FHR. Profound brain damage was preventable in this case.
DEFENDANTS' DEFENSE: The nurses continuously monitored by listening to sounds coming out of the bedside monitor even though no taping of FHR was occurring on the central monitors or FHR monitor strip. A nuchal cord is an unforeseeable medical emergency; nothing different could have been done to change the outcome.
VERDICT: An undisclosed Texas settlement was reached.
Surgical needle left near bladder
The patient underwent a hysterectomy on July 9. Because of an injury sustained during the operation, bladder repair surgery was performed on July 19. After that surgery, she reported bleeding and urinary incontinence. Results of a computed tomography (CT) scan showed that the bladder repair was not successful, a vesicovaginal fistula had developed, and a 13-mm C-shaped needle was found near her bladder. A third operation to remove the needle from her abdomen took place on August 16.
PATIENT'S CLAIM: The needle left behind after the second surgery caused a fistula to develop. The patient suffered mental and emotional distress from knowing the needle was in her abdomen. Foreign objects left in a patient during surgery are strong evidence of negligence.
DEFENDANTS' DEFENSE: The primary defense claim was the absence of causation--any negligence did not cause the injury. Defense experts testified that the needle was on top of the bladder and did move anywhere to cause damage. The patient developed a vesicovaginal fistula due to complications from the bladder repair operation and not from the needle. In addition, there was testimony that the third surgery was unnecessary because the needle would eventually flush out of the abdomen without causing damage.
VERDICT: A Michigan defense verdict was returned.
Claims cancer diagnosis was delayed
On October 1, 2008, a woman saw her family physician (FP) for routine care. Blood work results showed an elevated white blood cell (WBC) count. The patient claimed that the FP did not inform her of these test results.
One year later, the patient went to an urgent care facility where blood work was performed; the results showed a high WBC count. After a work-up, the patient was given a presumptive diagnosis of mantel cell lymphoma. By December 9, she had undergone the first round of chemotherapy. Subsequent tests revealed that the presumptive diagnosis was incorrect; she actually had low-grade lymphoproliferative disorder.
In lieu of this new diagnosis, the medical team offered the patient the option of discontinuing chemotherapy. She decided, however, to continue the treatment. Chemotherapy was followed by 2 years of rituximab/hyaluronidase human maintenance therapy.
PATIENT'S CLAIM: The patient presented her case to a medical review board. The course of treatment (chemotherapy plus maintenance therapy) left her with permanent heart damage and an elevated risk of developing secondary cancer.
The patient claimed that none of this would have happened if her FP had informed her of the October 2008 test results and recommended appropriate follow-up studies. The results of those studies would have given her the correct diagnosis and allowed her to receive prompt, proper treatment. The medical review board responded unanimously that the FP's conduct constituted a breach of the standard of care, but concluded that the breach was not a factor in the patient's damages.
The patient filed suit against the FP. An expert in internal medicine commented that, based on the 2008 WBC count, the tests should have been repeated and the patient should have been referred to a hematologist/oncologist. Failure to do so increased the patient's risk of developing cancer in the future.
DEFENDANTS' DEFENSE: The FP denied any breach of standard of care. According to her notes, she had shared test results with the patient on November 26 and recommended following up with repeat blood work. The FP blamed the patient for failing to follow-up as recommended.
VERDICT: An Indiana defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Did unsafe oxytocin dose cause uterine rupture? $3.5M settlement
When a mother presented to the hospital in labor, the on-call ObGyn ordered oxytocin with the dosage to be increased by 2 mU/min until she was receiving 30 mU/min or until an adequate contraction pattern was achieved and maintained. Over the next few hours, the labor and delivery nurse increased the dosage of the infusion several times.
As the patient began to push, a trickle of bright red blood was seen coming from her vagina and the baby's heart tones were temporarily lost. When the fetal heart tones were restored, his heart rate was approximately 50 bpm. After vaginal delivery was attempted using vacuum extraction and forceps, an emergency cesarean delivery was performed, leading to the finding that the mother's uterus had ruptured.
The baby suffered a permanent brain injury due to hypoxic-ischemic encephalopathy.
PATIENT'S CLAIM: The mother sued the hospital and on-call ObGyn. She alleged that the health care providers breached the standard of care by negligently increasing and maintaining the oxytocin at unsafe levels, which caused the mother's uterus to be overworked and eventually rupture. The rupture led to the child's hypoxia. An expert ObGyn noted that the patient's contractions were adequate by the time the oxytocin dose reached 14 mU/min, but the dosage continued to be increased.
DEFENDANTS' DEFENSE: The case was settled during the trial.
VERDICT: A $3.5 million Kansas settlement was reached.
When did the bowel injury occur?
One day after undergoing a hysterectomy, a woman went to the emergency department (ED) because she was feeling ill. She received a diagnosis of a pulmonary embolism for which she was given anticoagulant medications. The patient's symptoms persisted. Computed tomography (CT) imaging showed a bowel injury, and, 17 days after the initial surgery, an emergency laparotomy was performed.
PATIENT'S CLAIM: The patient sued the surgeon and the hospital. The hospital settled before the trial and the case continued against the surgeon.
The patient's early symptoms after surgery were evidence of a bowel injury, but imaging was not undertaken for several days. If the imaging had been undertaken earlier, the bowel injury would have been detected before it caused a rectovaginal fistula. An expert pathologist testified that the microscopic findings he detected postlaparotomy could only exist if a bowel perforation had been there for a significant period of time before the fistula developed. The patient's experts argued that the injury was not a "free perforation," but had been contained by her body, preventing the spread of the infection.
DEFENDANTS' DEFENSE: The surgeon maintained that the injury did not occur during the hysterectomy but developed in the days just before it was discovered. Over time, a collection of infected fluid at the vaginal cuff eroded into the bowel above it, creating an entryway for stool to pass through. Continuous leakage from the bowel for 17 days (the length of time between development of symptoms and discovery of the bowel injury) would have likely resulted in the patient's death.
VERDICT: A Missouri defense verdict was returned.
Did improper delivery techniques caused brachial plexus injury? $950,000 settlement
In April, a woman began receiving prenatal care for her 7th pregnancy. Her history of maternal obesity and diabetes mellitus, physical examinations, and tests suggested that she was at increased risk for having a macrosomic baby and encountering shoulder dystocia during vaginal delivery.
At 37 weeks' gestation, the mother was admitted to the hospital for induction of labor. Shoulder dystocia was encountered during delivery. At birth, the baby weighed 9 lb 10 oz and her right arm was limp. She was found to have a right brachial plexus injury involving C5‒C8 nerve roots and muscles. Two nerve root avulsions were evident on MRI and visualized by the surgeon during an extensive nerve graft operation. Arm function and range of motion improved after surgery, but the child has not recovered normal use of her arm.
PARENTS' CLAIM: Under the standard of care, the ObGyn was required to obtain informed consent, including a discussion of the risks of vaginal delivery (shoulder dystocia and brachial plexus injury), and the option of cesarean delivery. The patient claimed that the ObGyn neither obtained informed consent nor discussed these risks with her.
The labor and delivery nurse reported that the ObGyn told her, before delivery, that he was expecting a large baby and, perhaps, shoulder dystocia.
The ObGyn deviated from the standard of care by applying more than gentle traction to the fetal head while the shoulder was still impacted. The injury to the baby's right brachial plexus resulted from excessive lateral traction used by the ObGyn. The injury would not have occurred if a cesarean delivery had been performed. The mechanism of maternal forces injuring a brachial plexus nerve has never been visualized by any physician and is an unproven hypothesis.
PHYSICIAN'S DEFENSE: The ObGyn reported using standard maneuvers to deliver the baby. He applied traction on the fetal head 3 times: once after McRoberts maneuver, once after suprapubic pressure, and once after delivery of the posterior arm. He dictated into his notes that "We had to be careful to avoid excessive tractive forces." He claimed that shoulder dystocia is an unpredictable and unpreventable obstetric emergency, and that the injury was caused by the maternal forces of labor.
VERDICT: A $950,000 Virginia settlement was reached.
Wrongful death claim
On March 13, a 76-year-old woman went to her primary care physician's office because of a vaginal discharge. A nurse practitioner (NP) diagnosed a urinary tract infection (UTI) and prescribed cefixime (Suprax). Four days later, the patient began to experience severe diarrhea and blamed the medication. At a follow-up visit on March 20, the NP switched the patient to sulfamethoxazole-trimethoprim (Bactrim).
The following day, the patient was found unresponsive on her bathroom floor and was taken to the ED. It was determined that she had Clostridium difficile colitis (C difficile) and was admitted to the hospital. She developed acute renal failure, metabolic acidosis, hypovolemia, hypotension, and tachycardia. When she went into cardiac arrest, attempts to resuscitate her failed. She died on March 22.
ESTATE'S CLAIM: The estate sued the NP, claiming that the patient's symptoms did not meet the criteria for a UTI. If appropriate tests had been performed, the correct diagnosis would have been made and she could have received potentially life-saving treatment.
DEFENDANTS' DEFENSE: The NP claimed there was no negligence. Her diagnosis and treatment of the patient's condition were appropriate in all respects. The development of C difficile is a risk of any antibiotic.
VERDICT: An Indiana defense verdict was returned.
Nuchal cord: Undisclosed settlement
During delivery, the labor and delivery nurses lost the fetal heart-rate (FHR) monitor tracing, resulting in their being unaware of increasing signs of fetal intolerance to labor. The nurses continued to administer oxytocin to induce labor.
At birth, a nuchal cord was identified. The baby was born without signs of life but was successfully resuscitated by hospital staff.
The baby was found to have sustained severe brain damage as a result of profound fetal hypoxia. The child will require 24-hour nursing and supportive care for as long as she lives.
PARENTS' CLAIM: The nurses and ObGyn breached the standard of care resulting in her child's severe brain damage. The hospital nurses failed to continuously monitor the FHR. Profound brain damage was preventable in this case.
DEFENDANTS' DEFENSE: The nurses continuously monitored by listening to sounds coming out of the bedside monitor even though no taping of FHR was occurring on the central monitors or FHR monitor strip. A nuchal cord is an unforeseeable medical emergency; nothing different could have been done to change the outcome.
VERDICT: An undisclosed Texas settlement was reached.
Surgical needle left near bladder
The patient underwent a hysterectomy on July 9. Because of an injury sustained during the operation, bladder repair surgery was performed on July 19. After that surgery, she reported bleeding and urinary incontinence. Results of a computed tomography (CT) scan showed that the bladder repair was not successful, a vesicovaginal fistula had developed, and a 13-mm C-shaped needle was found near her bladder. A third operation to remove the needle from her abdomen took place on August 16.
PATIENT'S CLAIM: The needle left behind after the second surgery caused a fistula to develop. The patient suffered mental and emotional distress from knowing the needle was in her abdomen. Foreign objects left in a patient during surgery are strong evidence of negligence.
DEFENDANTS' DEFENSE: The primary defense claim was the absence of causation--any negligence did not cause the injury. Defense experts testified that the needle was on top of the bladder and did move anywhere to cause damage. The patient developed a vesicovaginal fistula due to complications from the bladder repair operation and not from the needle. In addition, there was testimony that the third surgery was unnecessary because the needle would eventually flush out of the abdomen without causing damage.
VERDICT: A Michigan defense verdict was returned.
Claims cancer diagnosis was delayed
On October 1, 2008, a woman saw her family physician (FP) for routine care. Blood work results showed an elevated white blood cell (WBC) count. The patient claimed that the FP did not inform her of these test results.
One year later, the patient went to an urgent care facility where blood work was performed; the results showed a high WBC count. After a work-up, the patient was given a presumptive diagnosis of mantel cell lymphoma. By December 9, she had undergone the first round of chemotherapy. Subsequent tests revealed that the presumptive diagnosis was incorrect; she actually had low-grade lymphoproliferative disorder.
In lieu of this new diagnosis, the medical team offered the patient the option of discontinuing chemotherapy. She decided, however, to continue the treatment. Chemotherapy was followed by 2 years of rituximab/hyaluronidase human maintenance therapy.
PATIENT'S CLAIM: The patient presented her case to a medical review board. The course of treatment (chemotherapy plus maintenance therapy) left her with permanent heart damage and an elevated risk of developing secondary cancer.
The patient claimed that none of this would have happened if her FP had informed her of the October 2008 test results and recommended appropriate follow-up studies. The results of those studies would have given her the correct diagnosis and allowed her to receive prompt, proper treatment. The medical review board responded unanimously that the FP's conduct constituted a breach of the standard of care, but concluded that the breach was not a factor in the patient's damages.
The patient filed suit against the FP. An expert in internal medicine commented that, based on the 2008 WBC count, the tests should have been repeated and the patient should have been referred to a hematologist/oncologist. Failure to do so increased the patient's risk of developing cancer in the future.
DEFENDANTS' DEFENSE: The FP denied any breach of standard of care. According to her notes, she had shared test results with the patient on November 26 and recommended following up with repeat blood work. The FP blamed the patient for failing to follow-up as recommended.
VERDICT: An Indiana defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Highlights from the 2018 Society of Gynecologic Surgeons Scientific Meeting
PART 1
- Leading best gynecologic surgical care into the next decade
- Optimal surgical management of stage 3 and 4 pelvic organ prolapse
- Patient experience: It’s not about satisfaction
Andrew P. Cassidenti, MD
Chief, Female Pelvic Medicine and Reconstructive Surgery
Kern Medical,
Bakersfield, California
Amanda White, MD
Assistant Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Vivian Aguilar, MD
Assistant Professor, Obstetrics and Gynecology
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Rebecca G. Rogers, MD
Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Associate Chair, Clinical Integration and Operations
Dell Medical School, University of Texas
Austin, Texas
Patrick Culligan, MD
Director, Urogynecology and The Center for Female Pelvic Health
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Sarah Huber, MD
Fellow, Female Pelvic Medicine and Reconstructive Surgery
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Vincent R. Lucente, MD, MBA
Chief, Gynecology, St. Luke’s University Health Network
Medical Director, The Institute for Female Pelvic Medicine and Reconstructive Surgery
Allentown, Pennsylvania
Jessica B. Ton, MD
AAGL Fellow, Minimally Invasive Gynecologic Surgery
St. Luke’s University Health Network
Bethlehem, Pennsylvania
James I. Merlino, MD
President and Chief Medical Officer of Advisory and Strategic Consulting
Press Ganey Associates
Cleveland, Ohio
Amy A. Merlino, MD
Maternal Fetal Medicine Specialist
Department of Obstetrics and Gynecology
Enterprise Chief Informatics Officer
Cleveland Clinic, Cleveland, Ohio
PART 2
- Deep infiltrating endometriosis: Evaluation and management
- What’s new in simulation training for hysterectomy
Rosanne M. Kho, MD
Head, Section of Benign Gynecology
Women’s Health Institute
Department of Obstetrics and Gynecology
Cleveland Clinic
Cleveland, Ohio
Mauricio S. Abrão, MD
Associate Professor and
Director, Endometriosis Division
Department of Obstetrics and Gynecology
São Paulo University Medical School
São Paulo, Brazil
Alicia Scribner, MD, MPH
Director, Ob/Gyn Simulation Curriculum
Madigan Army Medical Center
Tacoma, Washington
Clinical Instructor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Christine Vaccaro, DO
Medical Director, Andersen Simulation Center
Madigan Army Medical Center
Tacoma, Washington
Clinical Assistant Professor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Uniformed Services University of Health Sciences
Bethesda, Maryland
PART 1
- Leading best gynecologic surgical care into the next decade
- Optimal surgical management of stage 3 and 4 pelvic organ prolapse
- Patient experience: It’s not about satisfaction
Andrew P. Cassidenti, MD
Chief, Female Pelvic Medicine and Reconstructive Surgery
Kern Medical,
Bakersfield, California
Amanda White, MD
Assistant Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Vivian Aguilar, MD
Assistant Professor, Obstetrics and Gynecology
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Rebecca G. Rogers, MD
Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Associate Chair, Clinical Integration and Operations
Dell Medical School, University of Texas
Austin, Texas
Patrick Culligan, MD
Director, Urogynecology and The Center for Female Pelvic Health
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Sarah Huber, MD
Fellow, Female Pelvic Medicine and Reconstructive Surgery
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Vincent R. Lucente, MD, MBA
Chief, Gynecology, St. Luke’s University Health Network
Medical Director, The Institute for Female Pelvic Medicine and Reconstructive Surgery
Allentown, Pennsylvania
Jessica B. Ton, MD
AAGL Fellow, Minimally Invasive Gynecologic Surgery
St. Luke’s University Health Network
Bethlehem, Pennsylvania
James I. Merlino, MD
President and Chief Medical Officer of Advisory and Strategic Consulting
Press Ganey Associates
Cleveland, Ohio
Amy A. Merlino, MD
Maternal Fetal Medicine Specialist
Department of Obstetrics and Gynecology
Enterprise Chief Informatics Officer
Cleveland Clinic, Cleveland, Ohio
PART 2
- Deep infiltrating endometriosis: Evaluation and management
- What’s new in simulation training for hysterectomy
Rosanne M. Kho, MD
Head, Section of Benign Gynecology
Women’s Health Institute
Department of Obstetrics and Gynecology
Cleveland Clinic
Cleveland, Ohio
Mauricio S. Abrão, MD
Associate Professor and
Director, Endometriosis Division
Department of Obstetrics and Gynecology
São Paulo University Medical School
São Paulo, Brazil
Alicia Scribner, MD, MPH
Director, Ob/Gyn Simulation Curriculum
Madigan Army Medical Center
Tacoma, Washington
Clinical Instructor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Christine Vaccaro, DO
Medical Director, Andersen Simulation Center
Madigan Army Medical Center
Tacoma, Washington
Clinical Assistant Professor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Uniformed Services University of Health Sciences
Bethesda, Maryland
PART 1
- Leading best gynecologic surgical care into the next decade
- Optimal surgical management of stage 3 and 4 pelvic organ prolapse
- Patient experience: It’s not about satisfaction
Andrew P. Cassidenti, MD
Chief, Female Pelvic Medicine and Reconstructive Surgery
Kern Medical,
Bakersfield, California
Amanda White, MD
Assistant Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Vivian Aguilar, MD
Assistant Professor, Obstetrics and Gynecology
Female Pelvic Medicine and Reconstructive Surgery
Dell Medical School, University of Texas
Austin, Texas
Rebecca G. Rogers, MD
Professor, Department of Women’s Health
Female Pelvic Medicine and Reconstructive Surgery
Associate Chair, Clinical Integration and Operations
Dell Medical School, University of Texas
Austin, Texas
Patrick Culligan, MD
Director, Urogynecology and The Center for Female Pelvic Health
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Sarah Huber, MD
Fellow, Female Pelvic Medicine and Reconstructive Surgery
Department of Urology
Weill Cornell Medical College, New York Presbyterian/Weill Cornell Medical Center
New York, New York
Vincent R. Lucente, MD, MBA
Chief, Gynecology, St. Luke’s University Health Network
Medical Director, The Institute for Female Pelvic Medicine and Reconstructive Surgery
Allentown, Pennsylvania
Jessica B. Ton, MD
AAGL Fellow, Minimally Invasive Gynecologic Surgery
St. Luke’s University Health Network
Bethlehem, Pennsylvania
James I. Merlino, MD
President and Chief Medical Officer of Advisory and Strategic Consulting
Press Ganey Associates
Cleveland, Ohio
Amy A. Merlino, MD
Maternal Fetal Medicine Specialist
Department of Obstetrics and Gynecology
Enterprise Chief Informatics Officer
Cleveland Clinic, Cleveland, Ohio
PART 2
- Deep infiltrating endometriosis: Evaluation and management
- What’s new in simulation training for hysterectomy
Rosanne M. Kho, MD
Head, Section of Benign Gynecology
Women’s Health Institute
Department of Obstetrics and Gynecology
Cleveland Clinic
Cleveland, Ohio
Mauricio S. Abrão, MD
Associate Professor and
Director, Endometriosis Division
Department of Obstetrics and Gynecology
São Paulo University Medical School
São Paulo, Brazil
Alicia Scribner, MD, MPH
Director, Ob/Gyn Simulation Curriculum
Madigan Army Medical Center
Tacoma, Washington
Clinical Instructor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Christine Vaccaro, DO
Medical Director, Andersen Simulation Center
Madigan Army Medical Center
Tacoma, Washington
Clinical Assistant Professor
Department of Obstetrics and Gynecology
University of Washington, Seattle
Uniformed Services University of Health Sciences
Bethesda, Maryland
What’s new in simulation training for hysterectomy
Due to an increase in minimally invasive approaches to hysterectomy, including vaginal and laparoscopic approaches, gynecologic surgeons may need to turn to simulation training to augment practice and hone skills. Simulation is useful for all surgeons, especially for low-volume surgeons, as a warm-up to sharpen technical skills prior to starting the day’s cases. Additionally, educators are uniquely poised to use simulation to teach residents and to evaluate their procedural competency.
In this article, we provide an overview of the 3 approaches to hysterectomy—vaginal, laparoscopic, abdominal—through medical modeling and simulation techniques. We focus on practical issues, including current resources available online, cost, setup time, fidelity, and limitations of some commonly available vaginal, laparoscopic, and open hysterectomy models.
Simulation directly influences patient safety. Thus, the value of simulation cannot be overstated, as it can increase the quality of health care by improving patient outcomes and lowering overall costs. In 2008, the American College of Obstetricians and Gynecologists (ACOG) founded the Simulations Working Group to establish simulation as a pillar in education for women’s health through collaboration, advocacy, research, and the development and implementation of multidisciplinary simulations-based educational resources and opportunities.
Refer to the ACOG Simulations Working Group Toolkit online to see the objectives, simulation, and videos related to each module. Under the “Hysterectomy” section, you will find how to construct the “flower pot” model for abdominal and vaginal hysterectomy, as well as the AAGL vaginal and laparoscopic hysterectomy webinars. All content is reaffirmed frequently to keep it up to date. You can access the toolkit, with your ACOG login and passcode, at https://www.acog.org/About-ACOG/ACOG-Departments/Simulations-Consortium/Simulations-Consortium-Tool-Kit.
For a comprehensive gynecology curriculum to include vaginal, laparoscopic, and abdominal approaches to hysterectomy, refer to ACOG’s Surgical Curriculum in Obstetrics and Gynecology page at https://cfweb.acog.org/scog/. This page lists the standardized surgical skills curriculum for use in training residents in obstetrics and gynecology by procedure. It includes:
- the objective, description, and assessment of the module
- a description of the simulation
- a description of the surgical procedure
- a quiz that must be passed to proceed to evaluation by a faculty member
- an evaluation form to be downloaded and printed by the learner.
Takeaway. Value of Simulation = Quality (Improved Patient Outcomes) ÷ Direct and Indirect Costs.
Simulation models for training in vaginal hysterectomy
According to the Accreditation Council for Graduate Medical Education (ACGME), the minimum number of vaginal hysterectomies is 15; this number represents the minimum accepted exposure, however, and does not imply competency. Exposure to vaginal hysterectomy in residency training has significantly declined over the years, with a mean of only 19 vaginal hysterectomies performed by the time of graduation in 2014.1
A wide range of simulation models are available that you either can construct or purchase, based on your budget. We discuss 3 such models below.
The Miya model
The Miya Model Pelvic Surgery Training Model (Miyazaki Enterprises) consists of a bony pelvic frame and multiple replaceable and realistic anatomic structures, including the uterus, cervix, and adnexa (1 structure), vagina, bladder, and a few selected muscles and ligaments for pelvic floor disorders (FIGURE 1). The model incorporates features to simulate actual surgical experiences, such as realistic cutting and puncturing tensions, palpable surgical landmarks, a pressurized vascular system with bleeding for inadequate technique, and an inflatable bladder that can leak water if damaged.
Mounted on a rotating stand with the top of the pelvis open, the Miya model is designed to provide access and visibility, enabling supervising physicians the ability to give immediate guidance and feedback. The interchangeable parts allow the learner to be challenged at the appropriate skill level with the use of a large uterus versus a smaller uterus.
New in 2018 is an “intern” uterus and vagina that have no vascular supply and a single-layer vagina; this model is one-third of the cost of the larger, high-fidelity uterus (which has a vascular supply and additional tissue layers).
The Miya model reusable bony pelvic frame has a one-time cost of a few thousand dollars. Advantages include its high fidelity, low technology, light weight, portability, and quick setup. To view a video of the Miya model, go to https://www.youtube.com/watch?time_continue=49&v=A2RjOgVRclo. To see a simulated vaginal hysterectomy, visit https://www.youtube.com/watch?time_continue=13&v=dwiQz4DTyy8.
The gynecologic surgeon and inventor, Dr. Douglas Miyazaki, has improved the vesicouterine peritoneal fold (usually the most challenging for the surgeon) to have a more realistic, slippery feel when palpated.
This model’s weaknesses are its cost (relative to low-fidelity models) and the inability to use energy devices.
Takeaway. The Miya model is a high-fidelity, portable vaginal hysterectomy model with a reusable base and consumable replacement parts
The Gynesim model
The Gynesim Vaginal Hysterectomy Model, developed by Dr. Malcolm “Kip” Mackenzie (Gynesim), is a high-fidelity surgical simulation model constructed from animal tissue to provide realistic training in pelvic surgery (FIGURE 2).
These “real tissue models” are hand-constructed from animal tissue harvested from US Department of Agriculture inspected meat processing centers. The models mimic normal and abnormal abdominal and pelvic anatomy, providing realistic feel (haptics) and response to all surgical energy modalities. The “cassette” tissues are placed within a vaginal approach platform, which is portable.
Each model (including a 120- to 240-g uterus, bladder, ureter, uterine artery, cardinal and uterosacral ligaments, and rectum) supports critical gaps in surgical techniques such as peritoneal entry and cuff closure. Gynesim staff set up the entire laboratory, including the simulation models, instruments, and/or cameras; however, surgical energy systems are secured from the host institution.
The advantages of this model are its excellent tissue haptics and the minimal preparation time required from the busy gynecologic teaching faculty, as the company performs the setup and breakdown. Disadvantages include the model’s cost (relative to low-fidelity models), that it does not bleed, its one-time use, and the need for technical assistance from the company for setup.
This model can be used for laparoscopic and open hysterectomy approaches, as well as for vaginal hysterectomy. For more information, visit the Gynesim website at https://www.gynesim.com/vaginal-hysterectomy/.
Takeaway. The high-fidelity Gynesim model can be used to practice vaginal, laparoscopic, or open hysterectomy approaches. It offers excellent tissue haptics, one-time use “cassettes” made from animal tissue, and compatibility with energy devices.
The milk jug model
The milk jug and fabric uterus model, developed by Dr. Dee Fenner, is a low-cost simulation model and an alternative to the flower pot model (described later in this article). The bony pelvis is simulated by a 1-gallon milk carton that is taped to a foam ring. Other materials used to make the uterus are fabric, stuffing, and a needle and thread (or a sewing machine). Each model costs approximately $5 and takes approximately 15 minutes to create. For instructions on how to construct this model, see the Society for Gynecologic Surgeons (SGS) award-winning video from 2012 at https://vimeo.com/123804677.
The advantages of this model are that it is inexpensive and is a good tool with which novice gynecologic surgeons can learn the basic steps of the procedure. The disadvantages are that it does not bleed, is not compatible with energy devices, and must be constructed by hand (adding considerable time) or with a sewing machine.
Takeaway. The milk jug model is a low-cost, low-fidelity model for the novice surgeon that can be quickly constructed with the use of a sewing machine.
Read about simulation models for training in laparoscopic hysterectomy.
Simulation models for training in laparoscopic hysterectomy
While overall hysterectomy numbers have remained relatively stable during the last 10 years, the proportion of laparoscopic hysterectomy procedures is increasing in residency training.1 Many toolkits and models are available for practicing skills, from low-fidelity models on which to rehearse laparoscopic techniques (suturing, instrument handling) to high-fidelity models that provide augmented reality views of the abdominal cavity as well as the operating room itself. We offer a sampling of 4 such models below.
The FLS trainer system
The Fundamentals of Laparoscopic Surgery (FLS) Trainer Box (Limbs & Things Ltd) provides hands-on manual skills practice and training for laparoscopic surgery (FIGURE 3). The FLS trainer box uses 5 skills to challenge a surgeon’s dexterity and psychomotor skills. The set includes the trainer box with a camera and light source as well as the equipment needed to perform the 5 FLS tasks (peg transfer, pattern cutting, ligating loop, and intracorporeal and extracorporeal knot tying). The kit does not include laparoscopic instruments or a monitor.
The FLS trainer box with camera costs $1,164. The advantages are that it is portable and can be used to warm-up prior to surgery or for practice to improve technical skills. It is a great tool for junior residents who are learning the basics of laparoscopic surgery. This trainer’s disadvantages are that it is a low-fidelity unit that is procedure agnostic. For more information, visit the Limbs & Things website at https://www.fls-products.com.
Notably, ObGyn residents who graduate after May 31, 2020, will be required to successfully complete the FLS program as a prerequisite for specialty board certification.2 The FLS program is endorsed by the American College of Surgeons and is run through the Society of American Gastrointestinal and Endoscopic Surgeons. The FLS test is proctored and must be taken at a testing center.
Takeaway. The FLS trainer box is readily available, portable, relatively inexpensive, low-tech, and has valid benchmarks for proficiency. The FLS test will be required for ObGyn residents by 2020.
The SimPraxis software trainer
The SimPraxis Laparoscopic Hysterectomy Trainer (Red Llama, Inc) is an interactive simulation software platform that is available in DVD or USB format (FIGURE 4). The software is designed to review anatomy, surgical instrumentation, and specific steps of the procedure. It provides formative assessments and offers summative feedback for users.
The SimPraxis training software would make a useful tool to familiarize medical students and interns with the basics of the procedure before advancing to other simulation trainers. The software costs $100. For more information, visit https://www.3-dmed.com/product/simpraxis%C3%82%C2%AE-laparoscopic-hysterectomy-trainer.
Takeaway. The SimPraxis software is ideal for novice learners and can be used on a home or office computer.
The LapSim virtual reality trainer
The LapSim Haptic System (Surgical Science) is a virtual reality skills trainer. The hysterectomy module includes right and left uterine artery dissection, vaginal cuff opening, and cuff closure (FIGURE 5). One advantage of this simulator is its haptic feedback system, which enhances the fidelity of the training.
The LapSim simulator includes a training module for students and early learners and modules to improve camera handling. The virtual reality base system costs $70,720, and the hysterectomy software module is an additional $15,600.
For more information, visit the company’s website at https://surgicalscience.com/systems/lapsim/. For an informational video, go to https://surgicalscience.com/systems/lapsim/video/.
Takeaway. The LapSim is an expensive, high-fidelity, virtual reality simulator with enhanced haptics and software for practicing laparoscopic hysterectomy.
The LAP Mentor virtual reality simulator
The LAP Mentor VR (3D Systems) is another virtual reality simulator that has modules for laparoscopic hysterectomy and cuff closure (FIGURE 6). The trainee uses a virtual reality headset and becomes fully immersed in the operating room environment with audio and visual cues that mimic a real surgical experience.
The hysterectomy module allows the user to manipulate the uterus, identify the ureters, divide the superior pedicles, mobilize the bladder, expose and divide the uterine artery, and perform the colpotomy. The cuff closure module allows the user to suture the vaginal cuff using barbed suture. The module also can expose the learner to complications, such as bladder, ureteral, colon, or vascular injury.
The LAP Mentor VR base system costs $84,000 and the modules cost about $15,000. For additional information, visit the company’s website at http://simbionix.com/simulators/lap-mentor/lap-mentor-vr-or/.
Takeaway. The LAP Mentor is an expensive, high-fidelity simulation platform with a virtual reality headset that simulates a laparoscopic hysterectomy (with complications) in the operating room.
Read about simulations models for robot-assisted lap hysterectomy and abdominal hysterectomy.
Simulation models for training in robot-assisted laparoscopic hysterectomy
All robot-assisted simulation platforms have highly realistic graphics, and they are expensive (TABLE). However, the da Vinci Skills Simulator (backpack) platform is included with the da Vinci Si and Xi Systems. Note, though, that it can be challenging to access the surgeon console and backpack at institutions with high volumes of robot-assisted surgery.
Other options that generally reside outside of the operating room include Mimic’s FlexVR and dV-Trainer and the Robotix Mentor by 3D Systems (FIGURES 7–11). Mimic’s new technology, called MaestroAR (augmented reality), allows trainees to manipulate virtual robotic instruments to interact with anatomic regions within augmented 3D surgical video footage, with narration and instruction by Dr. Arnold Advincula.
Newer software by Simbionix allows augmented reality to assist the simulation of robot-assisted hysterectomy with the da Vinci Xi backpack and RobotiX platforms.
Models for training in abdominal hysterectomy
In the last 10 years, there has been a 30% decrease in the number of abdominal hysterectomies performed by residents.1 Because of this decline in operating room experience, simulation training can be an important tool to bolster residency experience.
There are not many simulation models available for teaching abdominal hysterectomy, but here we discuss 2 that we utilize in our residency program.
Adaptable task trainer
The Surgical Female Pelvic Trainer (SFPT) (Limbs & Things Ltd), a pelvic task trainer primarily used for simulation of laparoscopic hysterectomy, can be adapted for abdominal hysterectomy by removing the abdominal cover (FIGURE 12). This trainer can be used with simulated blood to increase the realism of training. The SFPT trainer costs $2,190. For more information, go to https://www.limbsandthings.com/us/our-products/details/surgical-female-pelvic-trainer-sfpt-mk-2.
Takeaway. The SFPT is a medium-fidelity task trainer with a reusable base and consumable replacement parts.
ACOG’s do-it-yourself flower pot model
The flower pot model (developed by the ACOG Simulation Working Group, Washington, DC) is a comprehensive educational package that includes learning objectives, simulation construction instructions, content review of the abdominal hysterectomy, quiz, and evaluation form.3 ACOG has endorsed this low-cost model for residency education. Each model costs approximately $20, and the base (flower pot) is reusable (FIGURE 13).Construction time for each model is 30 to 60 minutes, and learners can participate in the construction. This can aid in anatomy review and familiarization with the model prior to training in the surgical procedure.
The learning objectives, content review, quiz, and evaluation form can be used for the flower pot model or for high-fidelity models.
The advantages of this model are the low cost and that it provides enough fidelity to teach each of the critical steps of the procedure. The disadvantages include that it is a lower-fidelity model, requires a considerable amount of time for construction, does not bleed, and is not compatible with energy devices. This model also can be used for training in laparoscopic and vaginal hysterectomy. For more information, visit ACOG’s Surgical Curriculum website at https://cfweb.acog.org/scog/.
Takeaway. ACOG’s flower pot model for hysterectomy training is a comprehensive, low-cost, low-fidelity simulation model that requires significant setup time.
Simulation’s offerings
Simulation training is the present and future of medicine that bridges the gap between textbook learning and technical proficiency. Although in this article we describe only a handful of the simulation resources available, we hope that you will incorporate such tools into your practice for continuing education and skill development. Utilize peer-reviewed resources, such as the ACOG curriculum module and evaluation tools for abdominal, laparoscopic, and vaginal hysterectomy, which can be used with any simulation model to provide a comprehensive and complimentary learning experience.
The future of health care depends on the commitment and ingenuity of educators who embrace medical simulation’s purpose: improved patient safety, effectiveness, and efficiency. Join the movement!
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Washburn EE, Cohen SL, Manoucheri E, Zurawin RK, Einarsson JI. Trends in reported resident surgical experience in hysterectomy. J Minim Invasive Gynecol. 2014;21(6):1067–1070.
- American Board of Obstetrics and Gynecology. ABOG announces new eligibility requirement for board certification. https://www.abog.org/new/ABOG_FLS.aspx. Published January 22, 2018. Accessed April 10, 2018.
- Altman K, Burrell D, Chen G, Chou B, Fashokun T. Surgical curriculum in obstetrics and gynecology: vaginal hysterectomy simulation. https://cfweb.acog.org/scog/scog008/Simulation.cfm. Published December 2014. Accessed April 10, 2018.
Due to an increase in minimally invasive approaches to hysterectomy, including vaginal and laparoscopic approaches, gynecologic surgeons may need to turn to simulation training to augment practice and hone skills. Simulation is useful for all surgeons, especially for low-volume surgeons, as a warm-up to sharpen technical skills prior to starting the day’s cases. Additionally, educators are uniquely poised to use simulation to teach residents and to evaluate their procedural competency.
In this article, we provide an overview of the 3 approaches to hysterectomy—vaginal, laparoscopic, abdominal—through medical modeling and simulation techniques. We focus on practical issues, including current resources available online, cost, setup time, fidelity, and limitations of some commonly available vaginal, laparoscopic, and open hysterectomy models.
Simulation directly influences patient safety. Thus, the value of simulation cannot be overstated, as it can increase the quality of health care by improving patient outcomes and lowering overall costs. In 2008, the American College of Obstetricians and Gynecologists (ACOG) founded the Simulations Working Group to establish simulation as a pillar in education for women’s health through collaboration, advocacy, research, and the development and implementation of multidisciplinary simulations-based educational resources and opportunities.
Refer to the ACOG Simulations Working Group Toolkit online to see the objectives, simulation, and videos related to each module. Under the “Hysterectomy” section, you will find how to construct the “flower pot” model for abdominal and vaginal hysterectomy, as well as the AAGL vaginal and laparoscopic hysterectomy webinars. All content is reaffirmed frequently to keep it up to date. You can access the toolkit, with your ACOG login and passcode, at https://www.acog.org/About-ACOG/ACOG-Departments/Simulations-Consortium/Simulations-Consortium-Tool-Kit.
For a comprehensive gynecology curriculum to include vaginal, laparoscopic, and abdominal approaches to hysterectomy, refer to ACOG’s Surgical Curriculum in Obstetrics and Gynecology page at https://cfweb.acog.org/scog/. This page lists the standardized surgical skills curriculum for use in training residents in obstetrics and gynecology by procedure. It includes:
- the objective, description, and assessment of the module
- a description of the simulation
- a description of the surgical procedure
- a quiz that must be passed to proceed to evaluation by a faculty member
- an evaluation form to be downloaded and printed by the learner.
Takeaway. Value of Simulation = Quality (Improved Patient Outcomes) ÷ Direct and Indirect Costs.
Simulation models for training in vaginal hysterectomy
According to the Accreditation Council for Graduate Medical Education (ACGME), the minimum number of vaginal hysterectomies is 15; this number represents the minimum accepted exposure, however, and does not imply competency. Exposure to vaginal hysterectomy in residency training has significantly declined over the years, with a mean of only 19 vaginal hysterectomies performed by the time of graduation in 2014.1
A wide range of simulation models are available that you either can construct or purchase, based on your budget. We discuss 3 such models below.
The Miya model
The Miya Model Pelvic Surgery Training Model (Miyazaki Enterprises) consists of a bony pelvic frame and multiple replaceable and realistic anatomic structures, including the uterus, cervix, and adnexa (1 structure), vagina, bladder, and a few selected muscles and ligaments for pelvic floor disorders (FIGURE 1). The model incorporates features to simulate actual surgical experiences, such as realistic cutting and puncturing tensions, palpable surgical landmarks, a pressurized vascular system with bleeding for inadequate technique, and an inflatable bladder that can leak water if damaged.
Mounted on a rotating stand with the top of the pelvis open, the Miya model is designed to provide access and visibility, enabling supervising physicians the ability to give immediate guidance and feedback. The interchangeable parts allow the learner to be challenged at the appropriate skill level with the use of a large uterus versus a smaller uterus.
New in 2018 is an “intern” uterus and vagina that have no vascular supply and a single-layer vagina; this model is one-third of the cost of the larger, high-fidelity uterus (which has a vascular supply and additional tissue layers).
The Miya model reusable bony pelvic frame has a one-time cost of a few thousand dollars. Advantages include its high fidelity, low technology, light weight, portability, and quick setup. To view a video of the Miya model, go to https://www.youtube.com/watch?time_continue=49&v=A2RjOgVRclo. To see a simulated vaginal hysterectomy, visit https://www.youtube.com/watch?time_continue=13&v=dwiQz4DTyy8.
The gynecologic surgeon and inventor, Dr. Douglas Miyazaki, has improved the vesicouterine peritoneal fold (usually the most challenging for the surgeon) to have a more realistic, slippery feel when palpated.
This model’s weaknesses are its cost (relative to low-fidelity models) and the inability to use energy devices.
Takeaway. The Miya model is a high-fidelity, portable vaginal hysterectomy model with a reusable base and consumable replacement parts
The Gynesim model
The Gynesim Vaginal Hysterectomy Model, developed by Dr. Malcolm “Kip” Mackenzie (Gynesim), is a high-fidelity surgical simulation model constructed from animal tissue to provide realistic training in pelvic surgery (FIGURE 2).
These “real tissue models” are hand-constructed from animal tissue harvested from US Department of Agriculture inspected meat processing centers. The models mimic normal and abnormal abdominal and pelvic anatomy, providing realistic feel (haptics) and response to all surgical energy modalities. The “cassette” tissues are placed within a vaginal approach platform, which is portable.
Each model (including a 120- to 240-g uterus, bladder, ureter, uterine artery, cardinal and uterosacral ligaments, and rectum) supports critical gaps in surgical techniques such as peritoneal entry and cuff closure. Gynesim staff set up the entire laboratory, including the simulation models, instruments, and/or cameras; however, surgical energy systems are secured from the host institution.
The advantages of this model are its excellent tissue haptics and the minimal preparation time required from the busy gynecologic teaching faculty, as the company performs the setup and breakdown. Disadvantages include the model’s cost (relative to low-fidelity models), that it does not bleed, its one-time use, and the need for technical assistance from the company for setup.
This model can be used for laparoscopic and open hysterectomy approaches, as well as for vaginal hysterectomy. For more information, visit the Gynesim website at https://www.gynesim.com/vaginal-hysterectomy/.
Takeaway. The high-fidelity Gynesim model can be used to practice vaginal, laparoscopic, or open hysterectomy approaches. It offers excellent tissue haptics, one-time use “cassettes” made from animal tissue, and compatibility with energy devices.
The milk jug model
The milk jug and fabric uterus model, developed by Dr. Dee Fenner, is a low-cost simulation model and an alternative to the flower pot model (described later in this article). The bony pelvis is simulated by a 1-gallon milk carton that is taped to a foam ring. Other materials used to make the uterus are fabric, stuffing, and a needle and thread (or a sewing machine). Each model costs approximately $5 and takes approximately 15 minutes to create. For instructions on how to construct this model, see the Society for Gynecologic Surgeons (SGS) award-winning video from 2012 at https://vimeo.com/123804677.
The advantages of this model are that it is inexpensive and is a good tool with which novice gynecologic surgeons can learn the basic steps of the procedure. The disadvantages are that it does not bleed, is not compatible with energy devices, and must be constructed by hand (adding considerable time) or with a sewing machine.
Takeaway. The milk jug model is a low-cost, low-fidelity model for the novice surgeon that can be quickly constructed with the use of a sewing machine.
Read about simulation models for training in laparoscopic hysterectomy.
Simulation models for training in laparoscopic hysterectomy
While overall hysterectomy numbers have remained relatively stable during the last 10 years, the proportion of laparoscopic hysterectomy procedures is increasing in residency training.1 Many toolkits and models are available for practicing skills, from low-fidelity models on which to rehearse laparoscopic techniques (suturing, instrument handling) to high-fidelity models that provide augmented reality views of the abdominal cavity as well as the operating room itself. We offer a sampling of 4 such models below.
The FLS trainer system
The Fundamentals of Laparoscopic Surgery (FLS) Trainer Box (Limbs & Things Ltd) provides hands-on manual skills practice and training for laparoscopic surgery (FIGURE 3). The FLS trainer box uses 5 skills to challenge a surgeon’s dexterity and psychomotor skills. The set includes the trainer box with a camera and light source as well as the equipment needed to perform the 5 FLS tasks (peg transfer, pattern cutting, ligating loop, and intracorporeal and extracorporeal knot tying). The kit does not include laparoscopic instruments or a monitor.
The FLS trainer box with camera costs $1,164. The advantages are that it is portable and can be used to warm-up prior to surgery or for practice to improve technical skills. It is a great tool for junior residents who are learning the basics of laparoscopic surgery. This trainer’s disadvantages are that it is a low-fidelity unit that is procedure agnostic. For more information, visit the Limbs & Things website at https://www.fls-products.com.
Notably, ObGyn residents who graduate after May 31, 2020, will be required to successfully complete the FLS program as a prerequisite for specialty board certification.2 The FLS program is endorsed by the American College of Surgeons and is run through the Society of American Gastrointestinal and Endoscopic Surgeons. The FLS test is proctored and must be taken at a testing center.
Takeaway. The FLS trainer box is readily available, portable, relatively inexpensive, low-tech, and has valid benchmarks for proficiency. The FLS test will be required for ObGyn residents by 2020.
The SimPraxis software trainer
The SimPraxis Laparoscopic Hysterectomy Trainer (Red Llama, Inc) is an interactive simulation software platform that is available in DVD or USB format (FIGURE 4). The software is designed to review anatomy, surgical instrumentation, and specific steps of the procedure. It provides formative assessments and offers summative feedback for users.
The SimPraxis training software would make a useful tool to familiarize medical students and interns with the basics of the procedure before advancing to other simulation trainers. The software costs $100. For more information, visit https://www.3-dmed.com/product/simpraxis%C3%82%C2%AE-laparoscopic-hysterectomy-trainer.
Takeaway. The SimPraxis software is ideal for novice learners and can be used on a home or office computer.
The LapSim virtual reality trainer
The LapSim Haptic System (Surgical Science) is a virtual reality skills trainer. The hysterectomy module includes right and left uterine artery dissection, vaginal cuff opening, and cuff closure (FIGURE 5). One advantage of this simulator is its haptic feedback system, which enhances the fidelity of the training.
The LapSim simulator includes a training module for students and early learners and modules to improve camera handling. The virtual reality base system costs $70,720, and the hysterectomy software module is an additional $15,600.
For more information, visit the company’s website at https://surgicalscience.com/systems/lapsim/. For an informational video, go to https://surgicalscience.com/systems/lapsim/video/.
Takeaway. The LapSim is an expensive, high-fidelity, virtual reality simulator with enhanced haptics and software for practicing laparoscopic hysterectomy.
The LAP Mentor virtual reality simulator
The LAP Mentor VR (3D Systems) is another virtual reality simulator that has modules for laparoscopic hysterectomy and cuff closure (FIGURE 6). The trainee uses a virtual reality headset and becomes fully immersed in the operating room environment with audio and visual cues that mimic a real surgical experience.
The hysterectomy module allows the user to manipulate the uterus, identify the ureters, divide the superior pedicles, mobilize the bladder, expose and divide the uterine artery, and perform the colpotomy. The cuff closure module allows the user to suture the vaginal cuff using barbed suture. The module also can expose the learner to complications, such as bladder, ureteral, colon, or vascular injury.
The LAP Mentor VR base system costs $84,000 and the modules cost about $15,000. For additional information, visit the company’s website at http://simbionix.com/simulators/lap-mentor/lap-mentor-vr-or/.
Takeaway. The LAP Mentor is an expensive, high-fidelity simulation platform with a virtual reality headset that simulates a laparoscopic hysterectomy (with complications) in the operating room.
Read about simulations models for robot-assisted lap hysterectomy and abdominal hysterectomy.
Simulation models for training in robot-assisted laparoscopic hysterectomy
All robot-assisted simulation platforms have highly realistic graphics, and they are expensive (TABLE). However, the da Vinci Skills Simulator (backpack) platform is included with the da Vinci Si and Xi Systems. Note, though, that it can be challenging to access the surgeon console and backpack at institutions with high volumes of robot-assisted surgery.
Other options that generally reside outside of the operating room include Mimic’s FlexVR and dV-Trainer and the Robotix Mentor by 3D Systems (FIGURES 7–11). Mimic’s new technology, called MaestroAR (augmented reality), allows trainees to manipulate virtual robotic instruments to interact with anatomic regions within augmented 3D surgical video footage, with narration and instruction by Dr. Arnold Advincula.
Newer software by Simbionix allows augmented reality to assist the simulation of robot-assisted hysterectomy with the da Vinci Xi backpack and RobotiX platforms.
Models for training in abdominal hysterectomy
In the last 10 years, there has been a 30% decrease in the number of abdominal hysterectomies performed by residents.1 Because of this decline in operating room experience, simulation training can be an important tool to bolster residency experience.
There are not many simulation models available for teaching abdominal hysterectomy, but here we discuss 2 that we utilize in our residency program.
Adaptable task trainer
The Surgical Female Pelvic Trainer (SFPT) (Limbs & Things Ltd), a pelvic task trainer primarily used for simulation of laparoscopic hysterectomy, can be adapted for abdominal hysterectomy by removing the abdominal cover (FIGURE 12). This trainer can be used with simulated blood to increase the realism of training. The SFPT trainer costs $2,190. For more information, go to https://www.limbsandthings.com/us/our-products/details/surgical-female-pelvic-trainer-sfpt-mk-2.
Takeaway. The SFPT is a medium-fidelity task trainer with a reusable base and consumable replacement parts.
ACOG’s do-it-yourself flower pot model
The flower pot model (developed by the ACOG Simulation Working Group, Washington, DC) is a comprehensive educational package that includes learning objectives, simulation construction instructions, content review of the abdominal hysterectomy, quiz, and evaluation form.3 ACOG has endorsed this low-cost model for residency education. Each model costs approximately $20, and the base (flower pot) is reusable (FIGURE 13).Construction time for each model is 30 to 60 minutes, and learners can participate in the construction. This can aid in anatomy review and familiarization with the model prior to training in the surgical procedure.
The learning objectives, content review, quiz, and evaluation form can be used for the flower pot model or for high-fidelity models.
The advantages of this model are the low cost and that it provides enough fidelity to teach each of the critical steps of the procedure. The disadvantages include that it is a lower-fidelity model, requires a considerable amount of time for construction, does not bleed, and is not compatible with energy devices. This model also can be used for training in laparoscopic and vaginal hysterectomy. For more information, visit ACOG’s Surgical Curriculum website at https://cfweb.acog.org/scog/.
Takeaway. ACOG’s flower pot model for hysterectomy training is a comprehensive, low-cost, low-fidelity simulation model that requires significant setup time.
Simulation’s offerings
Simulation training is the present and future of medicine that bridges the gap between textbook learning and technical proficiency. Although in this article we describe only a handful of the simulation resources available, we hope that you will incorporate such tools into your practice for continuing education and skill development. Utilize peer-reviewed resources, such as the ACOG curriculum module and evaluation tools for abdominal, laparoscopic, and vaginal hysterectomy, which can be used with any simulation model to provide a comprehensive and complimentary learning experience.
The future of health care depends on the commitment and ingenuity of educators who embrace medical simulation’s purpose: improved patient safety, effectiveness, and efficiency. Join the movement!
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Due to an increase in minimally invasive approaches to hysterectomy, including vaginal and laparoscopic approaches, gynecologic surgeons may need to turn to simulation training to augment practice and hone skills. Simulation is useful for all surgeons, especially for low-volume surgeons, as a warm-up to sharpen technical skills prior to starting the day’s cases. Additionally, educators are uniquely poised to use simulation to teach residents and to evaluate their procedural competency.
In this article, we provide an overview of the 3 approaches to hysterectomy—vaginal, laparoscopic, abdominal—through medical modeling and simulation techniques. We focus on practical issues, including current resources available online, cost, setup time, fidelity, and limitations of some commonly available vaginal, laparoscopic, and open hysterectomy models.
Simulation directly influences patient safety. Thus, the value of simulation cannot be overstated, as it can increase the quality of health care by improving patient outcomes and lowering overall costs. In 2008, the American College of Obstetricians and Gynecologists (ACOG) founded the Simulations Working Group to establish simulation as a pillar in education for women’s health through collaboration, advocacy, research, and the development and implementation of multidisciplinary simulations-based educational resources and opportunities.
Refer to the ACOG Simulations Working Group Toolkit online to see the objectives, simulation, and videos related to each module. Under the “Hysterectomy” section, you will find how to construct the “flower pot” model for abdominal and vaginal hysterectomy, as well as the AAGL vaginal and laparoscopic hysterectomy webinars. All content is reaffirmed frequently to keep it up to date. You can access the toolkit, with your ACOG login and passcode, at https://www.acog.org/About-ACOG/ACOG-Departments/Simulations-Consortium/Simulations-Consortium-Tool-Kit.
For a comprehensive gynecology curriculum to include vaginal, laparoscopic, and abdominal approaches to hysterectomy, refer to ACOG’s Surgical Curriculum in Obstetrics and Gynecology page at https://cfweb.acog.org/scog/. This page lists the standardized surgical skills curriculum for use in training residents in obstetrics and gynecology by procedure. It includes:
- the objective, description, and assessment of the module
- a description of the simulation
- a description of the surgical procedure
- a quiz that must be passed to proceed to evaluation by a faculty member
- an evaluation form to be downloaded and printed by the learner.
Takeaway. Value of Simulation = Quality (Improved Patient Outcomes) ÷ Direct and Indirect Costs.
Simulation models for training in vaginal hysterectomy
According to the Accreditation Council for Graduate Medical Education (ACGME), the minimum number of vaginal hysterectomies is 15; this number represents the minimum accepted exposure, however, and does not imply competency. Exposure to vaginal hysterectomy in residency training has significantly declined over the years, with a mean of only 19 vaginal hysterectomies performed by the time of graduation in 2014.1
A wide range of simulation models are available that you either can construct or purchase, based on your budget. We discuss 3 such models below.
The Miya model
The Miya Model Pelvic Surgery Training Model (Miyazaki Enterprises) consists of a bony pelvic frame and multiple replaceable and realistic anatomic structures, including the uterus, cervix, and adnexa (1 structure), vagina, bladder, and a few selected muscles and ligaments for pelvic floor disorders (FIGURE 1). The model incorporates features to simulate actual surgical experiences, such as realistic cutting and puncturing tensions, palpable surgical landmarks, a pressurized vascular system with bleeding for inadequate technique, and an inflatable bladder that can leak water if damaged.
Mounted on a rotating stand with the top of the pelvis open, the Miya model is designed to provide access and visibility, enabling supervising physicians the ability to give immediate guidance and feedback. The interchangeable parts allow the learner to be challenged at the appropriate skill level with the use of a large uterus versus a smaller uterus.
New in 2018 is an “intern” uterus and vagina that have no vascular supply and a single-layer vagina; this model is one-third of the cost of the larger, high-fidelity uterus (which has a vascular supply and additional tissue layers).
The Miya model reusable bony pelvic frame has a one-time cost of a few thousand dollars. Advantages include its high fidelity, low technology, light weight, portability, and quick setup. To view a video of the Miya model, go to https://www.youtube.com/watch?time_continue=49&v=A2RjOgVRclo. To see a simulated vaginal hysterectomy, visit https://www.youtube.com/watch?time_continue=13&v=dwiQz4DTyy8.
The gynecologic surgeon and inventor, Dr. Douglas Miyazaki, has improved the vesicouterine peritoneal fold (usually the most challenging for the surgeon) to have a more realistic, slippery feel when palpated.
This model’s weaknesses are its cost (relative to low-fidelity models) and the inability to use energy devices.
Takeaway. The Miya model is a high-fidelity, portable vaginal hysterectomy model with a reusable base and consumable replacement parts
The Gynesim model
The Gynesim Vaginal Hysterectomy Model, developed by Dr. Malcolm “Kip” Mackenzie (Gynesim), is a high-fidelity surgical simulation model constructed from animal tissue to provide realistic training in pelvic surgery (FIGURE 2).
These “real tissue models” are hand-constructed from animal tissue harvested from US Department of Agriculture inspected meat processing centers. The models mimic normal and abnormal abdominal and pelvic anatomy, providing realistic feel (haptics) and response to all surgical energy modalities. The “cassette” tissues are placed within a vaginal approach platform, which is portable.
Each model (including a 120- to 240-g uterus, bladder, ureter, uterine artery, cardinal and uterosacral ligaments, and rectum) supports critical gaps in surgical techniques such as peritoneal entry and cuff closure. Gynesim staff set up the entire laboratory, including the simulation models, instruments, and/or cameras; however, surgical energy systems are secured from the host institution.
The advantages of this model are its excellent tissue haptics and the minimal preparation time required from the busy gynecologic teaching faculty, as the company performs the setup and breakdown. Disadvantages include the model’s cost (relative to low-fidelity models), that it does not bleed, its one-time use, and the need for technical assistance from the company for setup.
This model can be used for laparoscopic and open hysterectomy approaches, as well as for vaginal hysterectomy. For more information, visit the Gynesim website at https://www.gynesim.com/vaginal-hysterectomy/.
Takeaway. The high-fidelity Gynesim model can be used to practice vaginal, laparoscopic, or open hysterectomy approaches. It offers excellent tissue haptics, one-time use “cassettes” made from animal tissue, and compatibility with energy devices.
The milk jug model
The milk jug and fabric uterus model, developed by Dr. Dee Fenner, is a low-cost simulation model and an alternative to the flower pot model (described later in this article). The bony pelvis is simulated by a 1-gallon milk carton that is taped to a foam ring. Other materials used to make the uterus are fabric, stuffing, and a needle and thread (or a sewing machine). Each model costs approximately $5 and takes approximately 15 minutes to create. For instructions on how to construct this model, see the Society for Gynecologic Surgeons (SGS) award-winning video from 2012 at https://vimeo.com/123804677.
The advantages of this model are that it is inexpensive and is a good tool with which novice gynecologic surgeons can learn the basic steps of the procedure. The disadvantages are that it does not bleed, is not compatible with energy devices, and must be constructed by hand (adding considerable time) or with a sewing machine.
Takeaway. The milk jug model is a low-cost, low-fidelity model for the novice surgeon that can be quickly constructed with the use of a sewing machine.
Read about simulation models for training in laparoscopic hysterectomy.
Simulation models for training in laparoscopic hysterectomy
While overall hysterectomy numbers have remained relatively stable during the last 10 years, the proportion of laparoscopic hysterectomy procedures is increasing in residency training.1 Many toolkits and models are available for practicing skills, from low-fidelity models on which to rehearse laparoscopic techniques (suturing, instrument handling) to high-fidelity models that provide augmented reality views of the abdominal cavity as well as the operating room itself. We offer a sampling of 4 such models below.
The FLS trainer system
The Fundamentals of Laparoscopic Surgery (FLS) Trainer Box (Limbs & Things Ltd) provides hands-on manual skills practice and training for laparoscopic surgery (FIGURE 3). The FLS trainer box uses 5 skills to challenge a surgeon’s dexterity and psychomotor skills. The set includes the trainer box with a camera and light source as well as the equipment needed to perform the 5 FLS tasks (peg transfer, pattern cutting, ligating loop, and intracorporeal and extracorporeal knot tying). The kit does not include laparoscopic instruments or a monitor.
The FLS trainer box with camera costs $1,164. The advantages are that it is portable and can be used to warm-up prior to surgery or for practice to improve technical skills. It is a great tool for junior residents who are learning the basics of laparoscopic surgery. This trainer’s disadvantages are that it is a low-fidelity unit that is procedure agnostic. For more information, visit the Limbs & Things website at https://www.fls-products.com.
Notably, ObGyn residents who graduate after May 31, 2020, will be required to successfully complete the FLS program as a prerequisite for specialty board certification.2 The FLS program is endorsed by the American College of Surgeons and is run through the Society of American Gastrointestinal and Endoscopic Surgeons. The FLS test is proctored and must be taken at a testing center.
Takeaway. The FLS trainer box is readily available, portable, relatively inexpensive, low-tech, and has valid benchmarks for proficiency. The FLS test will be required for ObGyn residents by 2020.
The SimPraxis software trainer
The SimPraxis Laparoscopic Hysterectomy Trainer (Red Llama, Inc) is an interactive simulation software platform that is available in DVD or USB format (FIGURE 4). The software is designed to review anatomy, surgical instrumentation, and specific steps of the procedure. It provides formative assessments and offers summative feedback for users.
The SimPraxis training software would make a useful tool to familiarize medical students and interns with the basics of the procedure before advancing to other simulation trainers. The software costs $100. For more information, visit https://www.3-dmed.com/product/simpraxis%C3%82%C2%AE-laparoscopic-hysterectomy-trainer.
Takeaway. The SimPraxis software is ideal for novice learners and can be used on a home or office computer.
The LapSim virtual reality trainer
The LapSim Haptic System (Surgical Science) is a virtual reality skills trainer. The hysterectomy module includes right and left uterine artery dissection, vaginal cuff opening, and cuff closure (FIGURE 5). One advantage of this simulator is its haptic feedback system, which enhances the fidelity of the training.
The LapSim simulator includes a training module for students and early learners and modules to improve camera handling. The virtual reality base system costs $70,720, and the hysterectomy software module is an additional $15,600.
For more information, visit the company’s website at https://surgicalscience.com/systems/lapsim/. For an informational video, go to https://surgicalscience.com/systems/lapsim/video/.
Takeaway. The LapSim is an expensive, high-fidelity, virtual reality simulator with enhanced haptics and software for practicing laparoscopic hysterectomy.
The LAP Mentor virtual reality simulator
The LAP Mentor VR (3D Systems) is another virtual reality simulator that has modules for laparoscopic hysterectomy and cuff closure (FIGURE 6). The trainee uses a virtual reality headset and becomes fully immersed in the operating room environment with audio and visual cues that mimic a real surgical experience.
The hysterectomy module allows the user to manipulate the uterus, identify the ureters, divide the superior pedicles, mobilize the bladder, expose and divide the uterine artery, and perform the colpotomy. The cuff closure module allows the user to suture the vaginal cuff using barbed suture. The module also can expose the learner to complications, such as bladder, ureteral, colon, or vascular injury.
The LAP Mentor VR base system costs $84,000 and the modules cost about $15,000. For additional information, visit the company’s website at http://simbionix.com/simulators/lap-mentor/lap-mentor-vr-or/.
Takeaway. The LAP Mentor is an expensive, high-fidelity simulation platform with a virtual reality headset that simulates a laparoscopic hysterectomy (with complications) in the operating room.
Read about simulations models for robot-assisted lap hysterectomy and abdominal hysterectomy.
Simulation models for training in robot-assisted laparoscopic hysterectomy
All robot-assisted simulation platforms have highly realistic graphics, and they are expensive (TABLE). However, the da Vinci Skills Simulator (backpack) platform is included with the da Vinci Si and Xi Systems. Note, though, that it can be challenging to access the surgeon console and backpack at institutions with high volumes of robot-assisted surgery.
Other options that generally reside outside of the operating room include Mimic’s FlexVR and dV-Trainer and the Robotix Mentor by 3D Systems (FIGURES 7–11). Mimic’s new technology, called MaestroAR (augmented reality), allows trainees to manipulate virtual robotic instruments to interact with anatomic regions within augmented 3D surgical video footage, with narration and instruction by Dr. Arnold Advincula.
Newer software by Simbionix allows augmented reality to assist the simulation of robot-assisted hysterectomy with the da Vinci Xi backpack and RobotiX platforms.
Models for training in abdominal hysterectomy
In the last 10 years, there has been a 30% decrease in the number of abdominal hysterectomies performed by residents.1 Because of this decline in operating room experience, simulation training can be an important tool to bolster residency experience.
There are not many simulation models available for teaching abdominal hysterectomy, but here we discuss 2 that we utilize in our residency program.
Adaptable task trainer
The Surgical Female Pelvic Trainer (SFPT) (Limbs & Things Ltd), a pelvic task trainer primarily used for simulation of laparoscopic hysterectomy, can be adapted for abdominal hysterectomy by removing the abdominal cover (FIGURE 12). This trainer can be used with simulated blood to increase the realism of training. The SFPT trainer costs $2,190. For more information, go to https://www.limbsandthings.com/us/our-products/details/surgical-female-pelvic-trainer-sfpt-mk-2.
Takeaway. The SFPT is a medium-fidelity task trainer with a reusable base and consumable replacement parts.
ACOG’s do-it-yourself flower pot model
The flower pot model (developed by the ACOG Simulation Working Group, Washington, DC) is a comprehensive educational package that includes learning objectives, simulation construction instructions, content review of the abdominal hysterectomy, quiz, and evaluation form.3 ACOG has endorsed this low-cost model for residency education. Each model costs approximately $20, and the base (flower pot) is reusable (FIGURE 13).Construction time for each model is 30 to 60 minutes, and learners can participate in the construction. This can aid in anatomy review and familiarization with the model prior to training in the surgical procedure.
The learning objectives, content review, quiz, and evaluation form can be used for the flower pot model or for high-fidelity models.
The advantages of this model are the low cost and that it provides enough fidelity to teach each of the critical steps of the procedure. The disadvantages include that it is a lower-fidelity model, requires a considerable amount of time for construction, does not bleed, and is not compatible with energy devices. This model also can be used for training in laparoscopic and vaginal hysterectomy. For more information, visit ACOG’s Surgical Curriculum website at https://cfweb.acog.org/scog/.
Takeaway. ACOG’s flower pot model for hysterectomy training is a comprehensive, low-cost, low-fidelity simulation model that requires significant setup time.
Simulation’s offerings
Simulation training is the present and future of medicine that bridges the gap between textbook learning and technical proficiency. Although in this article we describe only a handful of the simulation resources available, we hope that you will incorporate such tools into your practice for continuing education and skill development. Utilize peer-reviewed resources, such as the ACOG curriculum module and evaluation tools for abdominal, laparoscopic, and vaginal hysterectomy, which can be used with any simulation model to provide a comprehensive and complimentary learning experience.
The future of health care depends on the commitment and ingenuity of educators who embrace medical simulation’s purpose: improved patient safety, effectiveness, and efficiency. Join the movement!
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Washburn EE, Cohen SL, Manoucheri E, Zurawin RK, Einarsson JI. Trends in reported resident surgical experience in hysterectomy. J Minim Invasive Gynecol. 2014;21(6):1067–1070.
- American Board of Obstetrics and Gynecology. ABOG announces new eligibility requirement for board certification. https://www.abog.org/new/ABOG_FLS.aspx. Published January 22, 2018. Accessed April 10, 2018.
- Altman K, Burrell D, Chen G, Chou B, Fashokun T. Surgical curriculum in obstetrics and gynecology: vaginal hysterectomy simulation. https://cfweb.acog.org/scog/scog008/Simulation.cfm. Published December 2014. Accessed April 10, 2018.
- Washburn EE, Cohen SL, Manoucheri E, Zurawin RK, Einarsson JI. Trends in reported resident surgical experience in hysterectomy. J Minim Invasive Gynecol. 2014;21(6):1067–1070.
- American Board of Obstetrics and Gynecology. ABOG announces new eligibility requirement for board certification. https://www.abog.org/new/ABOG_FLS.aspx. Published January 22, 2018. Accessed April 10, 2018.
- Altman K, Burrell D, Chen G, Chou B, Fashokun T. Surgical curriculum in obstetrics and gynecology: vaginal hysterectomy simulation. https://cfweb.acog.org/scog/scog008/Simulation.cfm. Published December 2014. Accessed April 10, 2018.
Deep infiltrating endometriosis: Evaluation and management
Endometriosis affects up to 10% of women of reproductive age or, conservatively, about 6.5 million women in the United States.1,2 There are 3 types of endometriosis—superficial, ovarian, and deep—and in the past each of these was assumed to have a distinct pathogenesis.3 Deep infiltrating endometriosis (DIE) is the presence of one or more endometriotic nodules deeper than 5 mm. In a study at a large tertiary-care center, 40% of patients with endometriosis had deep disease.4 DIE is associated with more severe pain and infertility.5 In patients with endometriosis, diagnosis is commonly made 7 to 9 years after the initial pelvic pain presentation.6 For these reasons, well-directed history taking and proper evaluation and treatment should be pursued to relieve pain and optimize outcomes.
CASE Young woman with intensifying pelvic pain
Mary is a 26-year-old social worker who presents to her ObGyn with symptoms of worsening pain during as well as outside her periods. What additional information would you want to obtain from Mary, given her chief symptom of pain?
Investigate the type of pain
It is important to ask the patient about her menstrual and sexual history, her thoughts regarding near- and long-term fertility, and the type and severity of her pain symptoms. The 5 pain symptoms specific to pelvic pain are dysmenorrhea, dyspareunia, dysuria, dyschezia, and noncyclic pelvic pain. A visual analog scale (VAS) for pain as well as pelvic pain questionnaires can be used to guide evaluation options and monitor treatment outcomes. In addition, it is of paramount importance to understand the differential diagnoses that can present as pelvic pain (TABLE).
CASE Continued: Mary’s history
Mary reports that she always has had painful periods and that she was started on oral contraceptive pills for pain control and regulation of her periods soon after the onset of menses, when she was 12 years old. In college, she was prescribed oral contraceptive pills for contraception. Recently engaged, she is interested in becoming pregnant in 3 years.
A year ago, Mary discontinued the pills because of their adverse effects. Now she has severe pain during (VAS score, 8/10) and outside (VAS score, 7) her monthly periods. Because of this pain, she has taken time off from work twice within the past 6 months. She has pain during intercourse (VAS score, 7) and some pain with bowel movements during her menses (VAS score, 4). Pelvic examination reveals a normal-sized uterus and adnexa as well as a tender nodule in the rectovaginal septum.
What diagnostic tests and imaging would you obtain?
Imaging’s role in diagnosis
At many advanced centers for endometriosis, DIE is successfully diagnosed with specific magnetic resonance imaging (MRI) or transvaginal ultrasound (TVUS) protocols. In a recent review, MRI’s pooled sensitivity and specificity for rectosigmoid endometriosis were 92% and 96%, respectively.7 Choice of imaging for DIE depends on the skills and experience of the clinicians at each center. At a large referral center in São Paulo, Brazil, TVUS with bowel preparation had better sensitivity and specificity for deep retrocervical and rectosigmoid disease compared with MRI and digital pelvic examination.8 In addition, at a center in the United States, we found that proficiency in performing TVUS for DIE was achieved after 70 to 75 cases, and the exam took an average of only 20 minutes.9
Despite recent advances in imaging, most gynecologic societies still hold that endometriosis is to be definitively diagnosed with histologic confirmation from tissue biopsies during surgery. Although surgery remains the diagnostic gold standard, it does not mean that all patients with pelvic pain should undergo diagnostic laparoscopy with tissue biopsies.
The combination of compelling clinical signs, symptoms, and imaging findings (such as absence of findings for ovarian and deep endometriosis) can be used to make a presumptive nonsurgical (that is, clinical) diagnosis of endometriosis. Major societies recommend empiric medical therapy (for example, combination oral contraceptives) for the pain associated with superficial endometriosis.10,11 When there is no response to treatment, or when a patient declines or has contraindications to medical therapy, diagnostic laparoscopy with excision of endometriosis should be considered.
CASE Continued: Diagnosis
Mary undergoes TVUS with bowel preparation, which reveals a normal uterus and adnexa and the presence of 2 lesions, a 2×1.5-cm retrocervical lesion and a 1.8×2-cm rectosigmoid lesion 9 cm above the anal verge. The rectosigmoid lesion involves the external muscularis and compromises 30% of the bowel circumference.
How would you manage the bowel DIE?
Read about management options and individualized care.
Management options: Factor in the variables
DIE can involve the ureters and bladder, the retrocervical and rectovaginal spaces, the appendix, and the bowel. Lesions can be single or multifocal. Although our institutions’ imaging with MRI and TVUS is highly accurate, we additionally recommend the use of colonoscopy (with directed biopsies if appropriate) to evaluate patients who present with rectal bleeding, large endometriotic rectal nodules, or have a family history of bowel cancer.
While many studies have found that surgical resection of DIE improves pain and quality of life, surgery can have significant complications.12 Observation is adequate for asymptomatic patients with DIE. Medical treatment may be offered to patients with mild pain (there is no evidence of a reduction in lesion size with medical therapy). In cases of surgical treatment, we encourage the involvement of a multidisciplinary surgical team to reduce complications and optimize outcomes.
Patients with DIE, significant pain (VAS score, >7), and multiple failed in vitro fertilization treatments are candidates for surgery. When bowel endometriosis is noted on imaging, factors such as size, depth, number of lesions, circumferential involvement, and distance from the anal verge are all used to determine the surgical approach. Rectosigmoid lesions smaller than 3 cm can be treated more conservatively—for example, with shaving or anterior resection with manual repair using disk staplers. Segmental resection generally is indicated for rectosigmoid lesions larger than 3 cm, involvement deeper than the submucosal layer, multiple lesions, circumferential involvement of more than 40%, and the presence of obstructed bowel symptoms.13,14
In patients with DIE who present with both infertility and pain, antimüllerian hormone level and TVUS follicular count are used to evaluate ovarian reserve. As surgical treatment may further reduce ovarian reserve in patients with DIE and infertility, we counsel them regarding assisted reproductive technology options before surgery.
CASE Resolved
After thorough discussion, Mary opts to try a different combination oral contraceptive pill formulation. The pills improve her pain symptoms significantly (VAS score, 4), and she decides to forgo surgery. She will be followed up closely on an outpatient basis with serial TVUS imaging.
Individualize management based on patient parameters
Imaging has been used for the nonsurgical diagnosis of DIE for many years, and this practice increasingly is being accepted and adopted. A presumptive nonsurgical diagnosis of endometriosis can be made based on the clinical signs and symptoms obtained from a thorough history and physical examination, in addition to the absence of imaging findings for ovarian and deep endometriosis.
According to guidelines from major ObGyn societies, such as the American College of Obstetricians and Gynecologists and the European Society of Human Reproduction and Embryology, empiric medical therapy (including combination oral contraceptives, progesterone-containing formulations, and gonadotropin-releasing hormone agonists) can be considered for patients with presumed endometriosis presenting with pain.15
When surgery is chosen, the surgeon must obtain crucial information on the characteristics of the lesion(s) and involve a multidisciplinary team to achieve the best outcomes for the patient.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789-1799.
- Buck Louis GM, Hediger ML, Peterson CM, et al; ENDO Study Working Group. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011;96(2):360-365.
- Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68(4):585-596.
- Bellelis P, Dias JA Jr, Podgaec S, Gonzales M, Baracat EC, Abrao MS. Epidemiological and clinical aspects of pelvic endometriosis--a case series. Rev Assoc Med Bras (1992). 2010;56(4):467-471.
- Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595-606.
- Greene R, Stratton P, Cleary SD, Ballweg ML, Sinaii N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil Steril. 2009;91(1):32-39.
- Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril. 2017;108(6):886-894.
- Abrão MS, Gonçalves MO, Dias JA Jr, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod. 2007;22(12):3092-3097.
- Young SW, Dahiya N, Patel MD, et al. Initial accuracy of and learning curve for transvaginal ultrasound with bowel preparation for deep endometriosis in a US tertiary care center. J Minim Invasive Gynecol. 2017;24(7):1170-1176.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400-412.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223-236.
- de Paula Andres M, Borrelli GM, Kho RM, Abrão MS. The current management of deep endometriosis: a systematic review. Minerva Ginecol. 2017;69(6):587-596.
- Abrão MS, Podgaec S, Dias JA Jr, Averbach M, Silva LF, Marino de Carvalho F. Endometriosis lesions that compromise the rectum deeper than the inner muscularis layer have more than 40% of the circumference of the rectum affected by the disease. J Minim Invasive Gynecol. 2008;15(3):280-285.
- Abrão MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21(3):329-339.
- Kho RM, Andres MP, Borrelli GM, Neto JS, Zanluchi A, Abrao MS. Surgical treatment of different types of endometriosis: comparison of major society guidelines and preferred clinical algorithms [published online ahead of print]. Best Pract Res Clin Obstet Gynaecol. 2018. doi:10.1016/j.bpobgyn2018.01.020.
Endometriosis affects up to 10% of women of reproductive age or, conservatively, about 6.5 million women in the United States.1,2 There are 3 types of endometriosis—superficial, ovarian, and deep—and in the past each of these was assumed to have a distinct pathogenesis.3 Deep infiltrating endometriosis (DIE) is the presence of one or more endometriotic nodules deeper than 5 mm. In a study at a large tertiary-care center, 40% of patients with endometriosis had deep disease.4 DIE is associated with more severe pain and infertility.5 In patients with endometriosis, diagnosis is commonly made 7 to 9 years after the initial pelvic pain presentation.6 For these reasons, well-directed history taking and proper evaluation and treatment should be pursued to relieve pain and optimize outcomes.
CASE Young woman with intensifying pelvic pain
Mary is a 26-year-old social worker who presents to her ObGyn with symptoms of worsening pain during as well as outside her periods. What additional information would you want to obtain from Mary, given her chief symptom of pain?
Investigate the type of pain
It is important to ask the patient about her menstrual and sexual history, her thoughts regarding near- and long-term fertility, and the type and severity of her pain symptoms. The 5 pain symptoms specific to pelvic pain are dysmenorrhea, dyspareunia, dysuria, dyschezia, and noncyclic pelvic pain. A visual analog scale (VAS) for pain as well as pelvic pain questionnaires can be used to guide evaluation options and monitor treatment outcomes. In addition, it is of paramount importance to understand the differential diagnoses that can present as pelvic pain (TABLE).
CASE Continued: Mary’s history
Mary reports that she always has had painful periods and that she was started on oral contraceptive pills for pain control and regulation of her periods soon after the onset of menses, when she was 12 years old. In college, she was prescribed oral contraceptive pills for contraception. Recently engaged, she is interested in becoming pregnant in 3 years.
A year ago, Mary discontinued the pills because of their adverse effects. Now she has severe pain during (VAS score, 8/10) and outside (VAS score, 7) her monthly periods. Because of this pain, she has taken time off from work twice within the past 6 months. She has pain during intercourse (VAS score, 7) and some pain with bowel movements during her menses (VAS score, 4). Pelvic examination reveals a normal-sized uterus and adnexa as well as a tender nodule in the rectovaginal septum.
What diagnostic tests and imaging would you obtain?
Imaging’s role in diagnosis
At many advanced centers for endometriosis, DIE is successfully diagnosed with specific magnetic resonance imaging (MRI) or transvaginal ultrasound (TVUS) protocols. In a recent review, MRI’s pooled sensitivity and specificity for rectosigmoid endometriosis were 92% and 96%, respectively.7 Choice of imaging for DIE depends on the skills and experience of the clinicians at each center. At a large referral center in São Paulo, Brazil, TVUS with bowel preparation had better sensitivity and specificity for deep retrocervical and rectosigmoid disease compared with MRI and digital pelvic examination.8 In addition, at a center in the United States, we found that proficiency in performing TVUS for DIE was achieved after 70 to 75 cases, and the exam took an average of only 20 minutes.9
Despite recent advances in imaging, most gynecologic societies still hold that endometriosis is to be definitively diagnosed with histologic confirmation from tissue biopsies during surgery. Although surgery remains the diagnostic gold standard, it does not mean that all patients with pelvic pain should undergo diagnostic laparoscopy with tissue biopsies.
The combination of compelling clinical signs, symptoms, and imaging findings (such as absence of findings for ovarian and deep endometriosis) can be used to make a presumptive nonsurgical (that is, clinical) diagnosis of endometriosis. Major societies recommend empiric medical therapy (for example, combination oral contraceptives) for the pain associated with superficial endometriosis.10,11 When there is no response to treatment, or when a patient declines or has contraindications to medical therapy, diagnostic laparoscopy with excision of endometriosis should be considered.
CASE Continued: Diagnosis
Mary undergoes TVUS with bowel preparation, which reveals a normal uterus and adnexa and the presence of 2 lesions, a 2×1.5-cm retrocervical lesion and a 1.8×2-cm rectosigmoid lesion 9 cm above the anal verge. The rectosigmoid lesion involves the external muscularis and compromises 30% of the bowel circumference.
How would you manage the bowel DIE?
Read about management options and individualized care.
Management options: Factor in the variables
DIE can involve the ureters and bladder, the retrocervical and rectovaginal spaces, the appendix, and the bowel. Lesions can be single or multifocal. Although our institutions’ imaging with MRI and TVUS is highly accurate, we additionally recommend the use of colonoscopy (with directed biopsies if appropriate) to evaluate patients who present with rectal bleeding, large endometriotic rectal nodules, or have a family history of bowel cancer.
While many studies have found that surgical resection of DIE improves pain and quality of life, surgery can have significant complications.12 Observation is adequate for asymptomatic patients with DIE. Medical treatment may be offered to patients with mild pain (there is no evidence of a reduction in lesion size with medical therapy). In cases of surgical treatment, we encourage the involvement of a multidisciplinary surgical team to reduce complications and optimize outcomes.
Patients with DIE, significant pain (VAS score, >7), and multiple failed in vitro fertilization treatments are candidates for surgery. When bowel endometriosis is noted on imaging, factors such as size, depth, number of lesions, circumferential involvement, and distance from the anal verge are all used to determine the surgical approach. Rectosigmoid lesions smaller than 3 cm can be treated more conservatively—for example, with shaving or anterior resection with manual repair using disk staplers. Segmental resection generally is indicated for rectosigmoid lesions larger than 3 cm, involvement deeper than the submucosal layer, multiple lesions, circumferential involvement of more than 40%, and the presence of obstructed bowel symptoms.13,14
In patients with DIE who present with both infertility and pain, antimüllerian hormone level and TVUS follicular count are used to evaluate ovarian reserve. As surgical treatment may further reduce ovarian reserve in patients with DIE and infertility, we counsel them regarding assisted reproductive technology options before surgery.
CASE Resolved
After thorough discussion, Mary opts to try a different combination oral contraceptive pill formulation. The pills improve her pain symptoms significantly (VAS score, 4), and she decides to forgo surgery. She will be followed up closely on an outpatient basis with serial TVUS imaging.
Individualize management based on patient parameters
Imaging has been used for the nonsurgical diagnosis of DIE for many years, and this practice increasingly is being accepted and adopted. A presumptive nonsurgical diagnosis of endometriosis can be made based on the clinical signs and symptoms obtained from a thorough history and physical examination, in addition to the absence of imaging findings for ovarian and deep endometriosis.
According to guidelines from major ObGyn societies, such as the American College of Obstetricians and Gynecologists and the European Society of Human Reproduction and Embryology, empiric medical therapy (including combination oral contraceptives, progesterone-containing formulations, and gonadotropin-releasing hormone agonists) can be considered for patients with presumed endometriosis presenting with pain.15
When surgery is chosen, the surgeon must obtain crucial information on the characteristics of the lesion(s) and involve a multidisciplinary team to achieve the best outcomes for the patient.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Endometriosis affects up to 10% of women of reproductive age or, conservatively, about 6.5 million women in the United States.1,2 There are 3 types of endometriosis—superficial, ovarian, and deep—and in the past each of these was assumed to have a distinct pathogenesis.3 Deep infiltrating endometriosis (DIE) is the presence of one or more endometriotic nodules deeper than 5 mm. In a study at a large tertiary-care center, 40% of patients with endometriosis had deep disease.4 DIE is associated with more severe pain and infertility.5 In patients with endometriosis, diagnosis is commonly made 7 to 9 years after the initial pelvic pain presentation.6 For these reasons, well-directed history taking and proper evaluation and treatment should be pursued to relieve pain and optimize outcomes.
CASE Young woman with intensifying pelvic pain
Mary is a 26-year-old social worker who presents to her ObGyn with symptoms of worsening pain during as well as outside her periods. What additional information would you want to obtain from Mary, given her chief symptom of pain?
Investigate the type of pain
It is important to ask the patient about her menstrual and sexual history, her thoughts regarding near- and long-term fertility, and the type and severity of her pain symptoms. The 5 pain symptoms specific to pelvic pain are dysmenorrhea, dyspareunia, dysuria, dyschezia, and noncyclic pelvic pain. A visual analog scale (VAS) for pain as well as pelvic pain questionnaires can be used to guide evaluation options and monitor treatment outcomes. In addition, it is of paramount importance to understand the differential diagnoses that can present as pelvic pain (TABLE).
CASE Continued: Mary’s history
Mary reports that she always has had painful periods and that she was started on oral contraceptive pills for pain control and regulation of her periods soon after the onset of menses, when she was 12 years old. In college, she was prescribed oral contraceptive pills for contraception. Recently engaged, she is interested in becoming pregnant in 3 years.
A year ago, Mary discontinued the pills because of their adverse effects. Now she has severe pain during (VAS score, 8/10) and outside (VAS score, 7) her monthly periods. Because of this pain, she has taken time off from work twice within the past 6 months. She has pain during intercourse (VAS score, 7) and some pain with bowel movements during her menses (VAS score, 4). Pelvic examination reveals a normal-sized uterus and adnexa as well as a tender nodule in the rectovaginal septum.
What diagnostic tests and imaging would you obtain?
Imaging’s role in diagnosis
At many advanced centers for endometriosis, DIE is successfully diagnosed with specific magnetic resonance imaging (MRI) or transvaginal ultrasound (TVUS) protocols. In a recent review, MRI’s pooled sensitivity and specificity for rectosigmoid endometriosis were 92% and 96%, respectively.7 Choice of imaging for DIE depends on the skills and experience of the clinicians at each center. At a large referral center in São Paulo, Brazil, TVUS with bowel preparation had better sensitivity and specificity for deep retrocervical and rectosigmoid disease compared with MRI and digital pelvic examination.8 In addition, at a center in the United States, we found that proficiency in performing TVUS for DIE was achieved after 70 to 75 cases, and the exam took an average of only 20 minutes.9
Despite recent advances in imaging, most gynecologic societies still hold that endometriosis is to be definitively diagnosed with histologic confirmation from tissue biopsies during surgery. Although surgery remains the diagnostic gold standard, it does not mean that all patients with pelvic pain should undergo diagnostic laparoscopy with tissue biopsies.
The combination of compelling clinical signs, symptoms, and imaging findings (such as absence of findings for ovarian and deep endometriosis) can be used to make a presumptive nonsurgical (that is, clinical) diagnosis of endometriosis. Major societies recommend empiric medical therapy (for example, combination oral contraceptives) for the pain associated with superficial endometriosis.10,11 When there is no response to treatment, or when a patient declines or has contraindications to medical therapy, diagnostic laparoscopy with excision of endometriosis should be considered.
CASE Continued: Diagnosis
Mary undergoes TVUS with bowel preparation, which reveals a normal uterus and adnexa and the presence of 2 lesions, a 2×1.5-cm retrocervical lesion and a 1.8×2-cm rectosigmoid lesion 9 cm above the anal verge. The rectosigmoid lesion involves the external muscularis and compromises 30% of the bowel circumference.
How would you manage the bowel DIE?
Read about management options and individualized care.
Management options: Factor in the variables
DIE can involve the ureters and bladder, the retrocervical and rectovaginal spaces, the appendix, and the bowel. Lesions can be single or multifocal. Although our institutions’ imaging with MRI and TVUS is highly accurate, we additionally recommend the use of colonoscopy (with directed biopsies if appropriate) to evaluate patients who present with rectal bleeding, large endometriotic rectal nodules, or have a family history of bowel cancer.
While many studies have found that surgical resection of DIE improves pain and quality of life, surgery can have significant complications.12 Observation is adequate for asymptomatic patients with DIE. Medical treatment may be offered to patients with mild pain (there is no evidence of a reduction in lesion size with medical therapy). In cases of surgical treatment, we encourage the involvement of a multidisciplinary surgical team to reduce complications and optimize outcomes.
Patients with DIE, significant pain (VAS score, >7), and multiple failed in vitro fertilization treatments are candidates for surgery. When bowel endometriosis is noted on imaging, factors such as size, depth, number of lesions, circumferential involvement, and distance from the anal verge are all used to determine the surgical approach. Rectosigmoid lesions smaller than 3 cm can be treated more conservatively—for example, with shaving or anterior resection with manual repair using disk staplers. Segmental resection generally is indicated for rectosigmoid lesions larger than 3 cm, involvement deeper than the submucosal layer, multiple lesions, circumferential involvement of more than 40%, and the presence of obstructed bowel symptoms.13,14
In patients with DIE who present with both infertility and pain, antimüllerian hormone level and TVUS follicular count are used to evaluate ovarian reserve. As surgical treatment may further reduce ovarian reserve in patients with DIE and infertility, we counsel them regarding assisted reproductive technology options before surgery.
CASE Resolved
After thorough discussion, Mary opts to try a different combination oral contraceptive pill formulation. The pills improve her pain symptoms significantly (VAS score, 4), and she decides to forgo surgery. She will be followed up closely on an outpatient basis with serial TVUS imaging.
Individualize management based on patient parameters
Imaging has been used for the nonsurgical diagnosis of DIE for many years, and this practice increasingly is being accepted and adopted. A presumptive nonsurgical diagnosis of endometriosis can be made based on the clinical signs and symptoms obtained from a thorough history and physical examination, in addition to the absence of imaging findings for ovarian and deep endometriosis.
According to guidelines from major ObGyn societies, such as the American College of Obstetricians and Gynecologists and the European Society of Human Reproduction and Embryology, empiric medical therapy (including combination oral contraceptives, progesterone-containing formulations, and gonadotropin-releasing hormone agonists) can be considered for patients with presumed endometriosis presenting with pain.15
When surgery is chosen, the surgeon must obtain crucial information on the characteristics of the lesion(s) and involve a multidisciplinary team to achieve the best outcomes for the patient.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789-1799.
- Buck Louis GM, Hediger ML, Peterson CM, et al; ENDO Study Working Group. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011;96(2):360-365.
- Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68(4):585-596.
- Bellelis P, Dias JA Jr, Podgaec S, Gonzales M, Baracat EC, Abrao MS. Epidemiological and clinical aspects of pelvic endometriosis--a case series. Rev Assoc Med Bras (1992). 2010;56(4):467-471.
- Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595-606.
- Greene R, Stratton P, Cleary SD, Ballweg ML, Sinaii N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil Steril. 2009;91(1):32-39.
- Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril. 2017;108(6):886-894.
- Abrão MS, Gonçalves MO, Dias JA Jr, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod. 2007;22(12):3092-3097.
- Young SW, Dahiya N, Patel MD, et al. Initial accuracy of and learning curve for transvaginal ultrasound with bowel preparation for deep endometriosis in a US tertiary care center. J Minim Invasive Gynecol. 2017;24(7):1170-1176.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400-412.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223-236.
- de Paula Andres M, Borrelli GM, Kho RM, Abrão MS. The current management of deep endometriosis: a systematic review. Minerva Ginecol. 2017;69(6):587-596.
- Abrão MS, Podgaec S, Dias JA Jr, Averbach M, Silva LF, Marino de Carvalho F. Endometriosis lesions that compromise the rectum deeper than the inner muscularis layer have more than 40% of the circumference of the rectum affected by the disease. J Minim Invasive Gynecol. 2008;15(3):280-285.
- Abrão MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21(3):329-339.
- Kho RM, Andres MP, Borrelli GM, Neto JS, Zanluchi A, Abrao MS. Surgical treatment of different types of endometriosis: comparison of major society guidelines and preferred clinical algorithms [published online ahead of print]. Best Pract Res Clin Obstet Gynaecol. 2018. doi:10.1016/j.bpobgyn2018.01.020.
- Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789-1799.
- Buck Louis GM, Hediger ML, Peterson CM, et al; ENDO Study Working Group. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. 2011;96(2):360-365.
- Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. 1997;68(4):585-596.
- Bellelis P, Dias JA Jr, Podgaec S, Gonzales M, Baracat EC, Abrao MS. Epidemiological and clinical aspects of pelvic endometriosis--a case series. Rev Assoc Med Bras (1992). 2010;56(4):467-471.
- Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005;11(6):595-606.
- Greene R, Stratton P, Cleary SD, Ballweg ML, Sinaii N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil Steril. 2009;91(1):32-39.
- Bazot M, Daraï E. Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertil Steril. 2017;108(6):886-894.
- Abrão MS, Gonçalves MO, Dias JA Jr, Podgaec S, Chamie LP, Blasbalg R. Comparison between clinical examination, transvaginal sonography and magnetic resonance imaging for the diagnosis of deep endometriosis. Hum Reprod. 2007;22(12):3092-3097.
- Young SW, Dahiya N, Patel MD, et al. Initial accuracy of and learning curve for transvaginal ultrasound with bowel preparation for deep endometriosis in a US tertiary care center. J Minim Invasive Gynecol. 2017;24(7):1170-1176.
- Dunselman GA, Vermeulen N, Becker C, et al; European Society of Human Reproduction and Embryology. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400-412.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223-236.
- de Paula Andres M, Borrelli GM, Kho RM, Abrão MS. The current management of deep endometriosis: a systematic review. Minerva Ginecol. 2017;69(6):587-596.
- Abrão MS, Podgaec S, Dias JA Jr, Averbach M, Silva LF, Marino de Carvalho F. Endometriosis lesions that compromise the rectum deeper than the inner muscularis layer have more than 40% of the circumference of the rectum affected by the disease. J Minim Invasive Gynecol. 2008;15(3):280-285.
- Abrão MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21(3):329-339.
- Kho RM, Andres MP, Borrelli GM, Neto JS, Zanluchi A, Abrao MS. Surgical treatment of different types of endometriosis: comparison of major society guidelines and preferred clinical algorithms [published online ahead of print]. Best Pract Res Clin Obstet Gynaecol. 2018. doi:10.1016/j.bpobgyn2018.01.020.
Take-home points
- Specific MRI or TVUS protocols are highly accurate in making a nonsurgical diagnosis of deep infiltrating endometriosis (DIE).
- The combination of compelling clinical signs and symptoms and absence of imaging findings for DIE can be used to make a presumptive nonsurgical diagnosis of endometriosis.
- Empiric medical therapy may provide pain relief.
- Conservative treatment, including observation alone, may be considered in asymptomatic patients with DIE and in those with minimal pain.
- Before surgery, it is imperative to know lesion size, depth, circumferential bowel involvement, and location (or distance from the anal verge in cases of rectosigmoid lesion) to optimize surgical outcomes.
Allergy, eczema common after pediatric solid organ transplantation
A total of 34% of children who underwent solid organ transplantation subsequently developed eczema, food allergy, rhinitis, eosinophilic gastrointestinal disease, or asthma, according to the results of a single-center retrospective cohort study.
Another 6.6% of patients developed autoimmunity, usually autoimmune cytopenia, inflammatory bowel disease, or vasculitis, wrote Nufar Marcus, MD, of the University of Toronto, and her associates.
Posttransplant allergy, autoimmunity, and immune-mediated disorders (PTAA) likely share a common pathogenesis “and may represent a unique state of post-transplant immune-dysregulation,” they wrote. The report was published in the Journal of Pediatrics.
The study included 273 children who underwent solid organ transplantation and were followed for a median 3.6 years (range, 1.7-6.3 years). None had immune-mediated conditions or allergies diagnosed at baseline. Posttransplantation allergies most commonly included eczema (51%), asthma (32%), food allergy (25%, including 5% with associated anaphylaxis), rhinitis (17%), and eosinophilic esophagitis, gastritis, or enteritis (13%).
Although only 31% of patients had information available on family history of allergy, those with a positive family history of allergy had a fivefold greater odds of posttransplantation PTAA, compared with other patients. Other risk factors for PTAA included female sex, young age at transplantation, eosinophilia, and a positive test for Epstein-Barr virus after transplantation, Dr. Marcus and associates said.
“The association of blood eosinophilia and PTAA reached statistical significance only when the transplant recipient was at least 6 months of age, demonstrating the nonspecific nature of abnormally high eosinophil counts during the first months of life,” they noted. The longer patients had eosinophilia after transplantation, the more likely they were to develop PTAA, “suggest[ing] a potential detrimental effect of prolonged activation of the eosinophilic-associated immune arms.”
Factors that appeared unlinked with PTAA included acute organ rejection, duration of posttransplantation steroidal treatment, organ type (living versus cadaveric), donor/recipient blood type and compatibility, infections besides Epstein-Barr virus, and posttransplant lymphoproliferative disease. “The specific type of post-transplantation immunosuppression regimen was neither associated nor protective of PTAA,” the investigators wrote. “However, a significant limitation was our inability to assess the effect of tacrolimus, as nearly all the cohort (97.8%) was treated with this medication.”
Ashley’s Angels fund provided support. The researchers reported having no conflicts of interest.
SOURCE: Marcus N et al. J Pediatr. 2018;196:154-60.
The study is one of several to highlight the occurrence of atopy and allergy following solid organ transplantation in children, Helen M. Evans, MBChB, wrote in an editorial accompanying the report by Marcus et al.
This report differed because it studied the differences in rates of atopy and allergy between transplanted solid organ groups. These occurred in 41% and 40% of liver and heart recipients, respectively, but in only 4% of kidney recipients. Atopy or allergy developed in 57% of multivisceral transplant patients, but the number of patients was very small (n = 7). The majority of the conditions developed within 1 year of transplantation.
The recent spike in these reports could signify better recognition of the problem or “the widespread switch of primary immunosuppression from cyclosporine to tacrolimus over the last few decades,” wrote Dr. Evans.
Most of these reports have been single-center retrospective studies, which are subject to inconsistent case definitions and recall bias, she noted. “The time is right for well-conducted multicenter prospective studies to better inform the true extent of these conditions after solid organ transplantation.”
In the meantime, transplantation centers should routinely track de novo eczema, allergy, and eosinophilic gastrointestinal disease in children being assessed for solid organ transplantation, and should take “rigorous” personal and family histories, said Dr. Evans. Ultimately, this work will help “minimize the risk of children developing these conditions” and “effectively treat them in the setting of immunosuppression after transplantation.”
Dr. Evans is a pediatric gastroenterologist at Starship Child Health in Aukland, New Zealand. She reported having no conflicts of interest. These comments summarize her editorial ( J Pediatr. 2018;196:10-11 ).
The study is one of several to highlight the occurrence of atopy and allergy following solid organ transplantation in children, Helen M. Evans, MBChB, wrote in an editorial accompanying the report by Marcus et al.
This report differed because it studied the differences in rates of atopy and allergy between transplanted solid organ groups. These occurred in 41% and 40% of liver and heart recipients, respectively, but in only 4% of kidney recipients. Atopy or allergy developed in 57% of multivisceral transplant patients, but the number of patients was very small (n = 7). The majority of the conditions developed within 1 year of transplantation.
The recent spike in these reports could signify better recognition of the problem or “the widespread switch of primary immunosuppression from cyclosporine to tacrolimus over the last few decades,” wrote Dr. Evans.
Most of these reports have been single-center retrospective studies, which are subject to inconsistent case definitions and recall bias, she noted. “The time is right for well-conducted multicenter prospective studies to better inform the true extent of these conditions after solid organ transplantation.”
In the meantime, transplantation centers should routinely track de novo eczema, allergy, and eosinophilic gastrointestinal disease in children being assessed for solid organ transplantation, and should take “rigorous” personal and family histories, said Dr. Evans. Ultimately, this work will help “minimize the risk of children developing these conditions” and “effectively treat them in the setting of immunosuppression after transplantation.”
Dr. Evans is a pediatric gastroenterologist at Starship Child Health in Aukland, New Zealand. She reported having no conflicts of interest. These comments summarize her editorial ( J Pediatr. 2018;196:10-11 ).
The study is one of several to highlight the occurrence of atopy and allergy following solid organ transplantation in children, Helen M. Evans, MBChB, wrote in an editorial accompanying the report by Marcus et al.
This report differed because it studied the differences in rates of atopy and allergy between transplanted solid organ groups. These occurred in 41% and 40% of liver and heart recipients, respectively, but in only 4% of kidney recipients. Atopy or allergy developed in 57% of multivisceral transplant patients, but the number of patients was very small (n = 7). The majority of the conditions developed within 1 year of transplantation.
The recent spike in these reports could signify better recognition of the problem or “the widespread switch of primary immunosuppression from cyclosporine to tacrolimus over the last few decades,” wrote Dr. Evans.
Most of these reports have been single-center retrospective studies, which are subject to inconsistent case definitions and recall bias, she noted. “The time is right for well-conducted multicenter prospective studies to better inform the true extent of these conditions after solid organ transplantation.”
In the meantime, transplantation centers should routinely track de novo eczema, allergy, and eosinophilic gastrointestinal disease in children being assessed for solid organ transplantation, and should take “rigorous” personal and family histories, said Dr. Evans. Ultimately, this work will help “minimize the risk of children developing these conditions” and “effectively treat them in the setting of immunosuppression after transplantation.”
Dr. Evans is a pediatric gastroenterologist at Starship Child Health in Aukland, New Zealand. She reported having no conflicts of interest. These comments summarize her editorial ( J Pediatr. 2018;196:10-11 ).
A total of 34% of children who underwent solid organ transplantation subsequently developed eczema, food allergy, rhinitis, eosinophilic gastrointestinal disease, or asthma, according to the results of a single-center retrospective cohort study.
Another 6.6% of patients developed autoimmunity, usually autoimmune cytopenia, inflammatory bowel disease, or vasculitis, wrote Nufar Marcus, MD, of the University of Toronto, and her associates.
Posttransplant allergy, autoimmunity, and immune-mediated disorders (PTAA) likely share a common pathogenesis “and may represent a unique state of post-transplant immune-dysregulation,” they wrote. The report was published in the Journal of Pediatrics.
The study included 273 children who underwent solid organ transplantation and were followed for a median 3.6 years (range, 1.7-6.3 years). None had immune-mediated conditions or allergies diagnosed at baseline. Posttransplantation allergies most commonly included eczema (51%), asthma (32%), food allergy (25%, including 5% with associated anaphylaxis), rhinitis (17%), and eosinophilic esophagitis, gastritis, or enteritis (13%).
Although only 31% of patients had information available on family history of allergy, those with a positive family history of allergy had a fivefold greater odds of posttransplantation PTAA, compared with other patients. Other risk factors for PTAA included female sex, young age at transplantation, eosinophilia, and a positive test for Epstein-Barr virus after transplantation, Dr. Marcus and associates said.
“The association of blood eosinophilia and PTAA reached statistical significance only when the transplant recipient was at least 6 months of age, demonstrating the nonspecific nature of abnormally high eosinophil counts during the first months of life,” they noted. The longer patients had eosinophilia after transplantation, the more likely they were to develop PTAA, “suggest[ing] a potential detrimental effect of prolonged activation of the eosinophilic-associated immune arms.”
Factors that appeared unlinked with PTAA included acute organ rejection, duration of posttransplantation steroidal treatment, organ type (living versus cadaveric), donor/recipient blood type and compatibility, infections besides Epstein-Barr virus, and posttransplant lymphoproliferative disease. “The specific type of post-transplantation immunosuppression regimen was neither associated nor protective of PTAA,” the investigators wrote. “However, a significant limitation was our inability to assess the effect of tacrolimus, as nearly all the cohort (97.8%) was treated with this medication.”
Ashley’s Angels fund provided support. The researchers reported having no conflicts of interest.
SOURCE: Marcus N et al. J Pediatr. 2018;196:154-60.
A total of 34% of children who underwent solid organ transplantation subsequently developed eczema, food allergy, rhinitis, eosinophilic gastrointestinal disease, or asthma, according to the results of a single-center retrospective cohort study.
Another 6.6% of patients developed autoimmunity, usually autoimmune cytopenia, inflammatory bowel disease, or vasculitis, wrote Nufar Marcus, MD, of the University of Toronto, and her associates.
Posttransplant allergy, autoimmunity, and immune-mediated disorders (PTAA) likely share a common pathogenesis “and may represent a unique state of post-transplant immune-dysregulation,” they wrote. The report was published in the Journal of Pediatrics.
The study included 273 children who underwent solid organ transplantation and were followed for a median 3.6 years (range, 1.7-6.3 years). None had immune-mediated conditions or allergies diagnosed at baseline. Posttransplantation allergies most commonly included eczema (51%), asthma (32%), food allergy (25%, including 5% with associated anaphylaxis), rhinitis (17%), and eosinophilic esophagitis, gastritis, or enteritis (13%).
Although only 31% of patients had information available on family history of allergy, those with a positive family history of allergy had a fivefold greater odds of posttransplantation PTAA, compared with other patients. Other risk factors for PTAA included female sex, young age at transplantation, eosinophilia, and a positive test for Epstein-Barr virus after transplantation, Dr. Marcus and associates said.
“The association of blood eosinophilia and PTAA reached statistical significance only when the transplant recipient was at least 6 months of age, demonstrating the nonspecific nature of abnormally high eosinophil counts during the first months of life,” they noted. The longer patients had eosinophilia after transplantation, the more likely they were to develop PTAA, “suggest[ing] a potential detrimental effect of prolonged activation of the eosinophilic-associated immune arms.”
Factors that appeared unlinked with PTAA included acute organ rejection, duration of posttransplantation steroidal treatment, organ type (living versus cadaveric), donor/recipient blood type and compatibility, infections besides Epstein-Barr virus, and posttransplant lymphoproliferative disease. “The specific type of post-transplantation immunosuppression regimen was neither associated nor protective of PTAA,” the investigators wrote. “However, a significant limitation was our inability to assess the effect of tacrolimus, as nearly all the cohort (97.8%) was treated with this medication.”
Ashley’s Angels fund provided support. The researchers reported having no conflicts of interest.
SOURCE: Marcus N et al. J Pediatr. 2018;196:154-60.
FROM JOURNAL OF PEDIATRICS
Key clinical point: Children undergoing solid organ transplantation often developed allergy or autoimmunity.
Major finding: Study details: Single-center retrospective cross-sectional study of 273 patients aged 18 and under who underwent solid organ transplantation followed for a median 3.6 years.
Disclosures: Ashley’s Angels fund provided support. The researchers reported having no conflicts of interest.
Source: Marcus N et al. J Pediatr. 2018;196:154-60.
Don’t delay hip-fracture surgery
Background: Guidelines from the American College of Surgeons and Canadian Institute for Health recommend hip fracture surgery within 48 hours. However, a time-to-surgery threshold after which mortality and complications are increased has not been determined. This study aims to determine a time to surgery threshold for hip-fracture surgery.
Study design: Retrospective cohort trial.
Setting: 72 hospitals in Ontario, Ca., during April 1, 2009-March 31, 2014.
Synopsis: Of the 42,230 adult patients in this study, 14,174 (33.6%) received hip-fracture surgery within 24 hours of emergency department arrival. A matched patient analysis of early surgery (within 24 hours of ED arrival) vs. delayed surgery determined that patients undergoing early operation experienced lower 30-day mortality (5.8% vs 6.5%) and fewer complications (myocardial infarction, deep vein thrombosis, pulmonary embolism, and pneumonia). Major bleeding was not assessed as a complication. Also omitted from analysis were patients undergoing nonoperative hip-fracture management.
These findings suggest a time to surgery of 24 hours may represent a threshold defining higher risk. Two-thirds of patients in this study surpassed this threshold. Hospitalists seeing patients with hip fracture should balance time delay risks with the need for medical optimization.
Bottom line: Wait time greater than 24 hours for adults undergoing hip fracture surgery is associated with an increased risk of 30-day mortality and complications.
Citation: Pincus D et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017 Nov 28;318(20):1994-2003.
Dr. Moulder is assistant professor, University of Virginia Health System.
Background: Guidelines from the American College of Surgeons and Canadian Institute for Health recommend hip fracture surgery within 48 hours. However, a time-to-surgery threshold after which mortality and complications are increased has not been determined. This study aims to determine a time to surgery threshold for hip-fracture surgery.
Study design: Retrospective cohort trial.
Setting: 72 hospitals in Ontario, Ca., during April 1, 2009-March 31, 2014.
Synopsis: Of the 42,230 adult patients in this study, 14,174 (33.6%) received hip-fracture surgery within 24 hours of emergency department arrival. A matched patient analysis of early surgery (within 24 hours of ED arrival) vs. delayed surgery determined that patients undergoing early operation experienced lower 30-day mortality (5.8% vs 6.5%) and fewer complications (myocardial infarction, deep vein thrombosis, pulmonary embolism, and pneumonia). Major bleeding was not assessed as a complication. Also omitted from analysis were patients undergoing nonoperative hip-fracture management.
These findings suggest a time to surgery of 24 hours may represent a threshold defining higher risk. Two-thirds of patients in this study surpassed this threshold. Hospitalists seeing patients with hip fracture should balance time delay risks with the need for medical optimization.
Bottom line: Wait time greater than 24 hours for adults undergoing hip fracture surgery is associated with an increased risk of 30-day mortality and complications.
Citation: Pincus D et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017 Nov 28;318(20):1994-2003.
Dr. Moulder is assistant professor, University of Virginia Health System.
Background: Guidelines from the American College of Surgeons and Canadian Institute for Health recommend hip fracture surgery within 48 hours. However, a time-to-surgery threshold after which mortality and complications are increased has not been determined. This study aims to determine a time to surgery threshold for hip-fracture surgery.
Study design: Retrospective cohort trial.
Setting: 72 hospitals in Ontario, Ca., during April 1, 2009-March 31, 2014.
Synopsis: Of the 42,230 adult patients in this study, 14,174 (33.6%) received hip-fracture surgery within 24 hours of emergency department arrival. A matched patient analysis of early surgery (within 24 hours of ED arrival) vs. delayed surgery determined that patients undergoing early operation experienced lower 30-day mortality (5.8% vs 6.5%) and fewer complications (myocardial infarction, deep vein thrombosis, pulmonary embolism, and pneumonia). Major bleeding was not assessed as a complication. Also omitted from analysis were patients undergoing nonoperative hip-fracture management.
These findings suggest a time to surgery of 24 hours may represent a threshold defining higher risk. Two-thirds of patients in this study surpassed this threshold. Hospitalists seeing patients with hip fracture should balance time delay risks with the need for medical optimization.
Bottom line: Wait time greater than 24 hours for adults undergoing hip fracture surgery is associated with an increased risk of 30-day mortality and complications.
Citation: Pincus D et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017 Nov 28;318(20):1994-2003.
Dr. Moulder is assistant professor, University of Virginia Health System.
Endometriosis pain stemming from pelvic spasms improved with botulinum toxin
LOS ANGELES – Women treated surgically and with hormones for endometriosis may continue to experience pain, say investigators, and that pain frequently extends beyond the pelvis.
At the annual meeting of the American Academy of Neurology, Barbara Karp, MD, of the National Institute of Neurological Disorders and Stroke, presented results from an ongoing randomized trial of women with endometriosis receiving botulinum toxin to treat endometriosis-related chronic pelvic pain and pelvic spasm.
All 28 women currently enrolled in the trial (median age, 29 years) were evaluated by a gynecologist to confirm pelvic muscle spasm as their primary source of pain. Each also underwent a neuromuscular examination to identify pain points beyond the pelvis.
All subjects had myofascial dysfunction. Most reported headaches and half reported orofacial pain, while 13 subjects reported myofascial trigger points in all the 26 spots assessed, which included head and facial muscles, shoulder and back muscles, and muscles in the buttocks, abdomen, and upper legs.
Dr. Karp said her group hypothesized that for patients with endometriosis, the widespread pain seen in the study “probably has some origin in sensitization initiated by pain associated with the endometriosis lesions, and that gives us a mechanism to think about peripheral and central sensitization.” But she noted that such sensitization can be easily missed in the clinic.
“One of the things that’s really underappreciated is how much women with chronic pelvic pain have pain elsewhere. So the neurologist or pain specialist may say, ‘that’s not my body territory, there’s something going on with your pelvis.’ And the gynecologist may be focused on the endometriosis and the endometriosis lesions. So you have these women with really widespread pain problems whose care is being fractionated.”
In another aspect of the study she also presented at the meeting, Dr. Karp, a neurologist who has studied the therapeutic use of neurotoxins such as botulinum for 30 years, showed results from an open-label extension of a randomized trial of botulinum toxin injections to treat pelvic spasm in the same cohort of women with confirmed endometriosis and confirmed pelvic muscle spasm.
A month after the open-label injection, spasm was reduced or absent in all subjects (P = .0005), with 11 of 13 rating pain as absent or mild (P = .0001), Dr. Karp and her colleagues reported. Between 5 and 11 months post injection, five women requested a repeat of the treatment.
Besides the data on pain and disability collected as part of the trial, Dr. Karp and her colleagues are also looking at biomarkers for pain and inflammation, and changes in medication and hormone use. They are preparing a separate literature review on injection techniques and dosages of toxin to the pelvic floor muscles.
“It’s an area of the body neurologists don’t feel comfortable injecting, and I don’t necessarily feel comfortable doing it myself,” Dr. Karp said.
The researchers had to develop their own procedure because at the time they started the research there was almost nothing in the literature on how to inject botulinum toxin for pelvic pain in women. “People in different specialties have been doing it [to relieve pelvic pain] and it’s really widespread, but they’re doing it all different ways,” Dr. Karp said. “We’re hoping to find a best approach.”
Dr. Karp and her colleagues’ research is supported by the NINDS and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. OnabotulinumtoxinA for the clinical trial is supplied by Allergan. Dr. Karp has received research support from Allergan and Merz.
SOURCE: Karp B et al. AAN 2018, Abstract P2.096; Karp B et al. AAN 2018, Abstract P2.098.
LOS ANGELES – Women treated surgically and with hormones for endometriosis may continue to experience pain, say investigators, and that pain frequently extends beyond the pelvis.
At the annual meeting of the American Academy of Neurology, Barbara Karp, MD, of the National Institute of Neurological Disorders and Stroke, presented results from an ongoing randomized trial of women with endometriosis receiving botulinum toxin to treat endometriosis-related chronic pelvic pain and pelvic spasm.
All 28 women currently enrolled in the trial (median age, 29 years) were evaluated by a gynecologist to confirm pelvic muscle spasm as their primary source of pain. Each also underwent a neuromuscular examination to identify pain points beyond the pelvis.
All subjects had myofascial dysfunction. Most reported headaches and half reported orofacial pain, while 13 subjects reported myofascial trigger points in all the 26 spots assessed, which included head and facial muscles, shoulder and back muscles, and muscles in the buttocks, abdomen, and upper legs.
Dr. Karp said her group hypothesized that for patients with endometriosis, the widespread pain seen in the study “probably has some origin in sensitization initiated by pain associated with the endometriosis lesions, and that gives us a mechanism to think about peripheral and central sensitization.” But she noted that such sensitization can be easily missed in the clinic.
“One of the things that’s really underappreciated is how much women with chronic pelvic pain have pain elsewhere. So the neurologist or pain specialist may say, ‘that’s not my body territory, there’s something going on with your pelvis.’ And the gynecologist may be focused on the endometriosis and the endometriosis lesions. So you have these women with really widespread pain problems whose care is being fractionated.”
In another aspect of the study she also presented at the meeting, Dr. Karp, a neurologist who has studied the therapeutic use of neurotoxins such as botulinum for 30 years, showed results from an open-label extension of a randomized trial of botulinum toxin injections to treat pelvic spasm in the same cohort of women with confirmed endometriosis and confirmed pelvic muscle spasm.
A month after the open-label injection, spasm was reduced or absent in all subjects (P = .0005), with 11 of 13 rating pain as absent or mild (P = .0001), Dr. Karp and her colleagues reported. Between 5 and 11 months post injection, five women requested a repeat of the treatment.
Besides the data on pain and disability collected as part of the trial, Dr. Karp and her colleagues are also looking at biomarkers for pain and inflammation, and changes in medication and hormone use. They are preparing a separate literature review on injection techniques and dosages of toxin to the pelvic floor muscles.
“It’s an area of the body neurologists don’t feel comfortable injecting, and I don’t necessarily feel comfortable doing it myself,” Dr. Karp said.
The researchers had to develop their own procedure because at the time they started the research there was almost nothing in the literature on how to inject botulinum toxin for pelvic pain in women. “People in different specialties have been doing it [to relieve pelvic pain] and it’s really widespread, but they’re doing it all different ways,” Dr. Karp said. “We’re hoping to find a best approach.”
Dr. Karp and her colleagues’ research is supported by the NINDS and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. OnabotulinumtoxinA for the clinical trial is supplied by Allergan. Dr. Karp has received research support from Allergan and Merz.
SOURCE: Karp B et al. AAN 2018, Abstract P2.096; Karp B et al. AAN 2018, Abstract P2.098.
LOS ANGELES – Women treated surgically and with hormones for endometriosis may continue to experience pain, say investigators, and that pain frequently extends beyond the pelvis.
At the annual meeting of the American Academy of Neurology, Barbara Karp, MD, of the National Institute of Neurological Disorders and Stroke, presented results from an ongoing randomized trial of women with endometriosis receiving botulinum toxin to treat endometriosis-related chronic pelvic pain and pelvic spasm.
All 28 women currently enrolled in the trial (median age, 29 years) were evaluated by a gynecologist to confirm pelvic muscle spasm as their primary source of pain. Each also underwent a neuromuscular examination to identify pain points beyond the pelvis.
All subjects had myofascial dysfunction. Most reported headaches and half reported orofacial pain, while 13 subjects reported myofascial trigger points in all the 26 spots assessed, which included head and facial muscles, shoulder and back muscles, and muscles in the buttocks, abdomen, and upper legs.
Dr. Karp said her group hypothesized that for patients with endometriosis, the widespread pain seen in the study “probably has some origin in sensitization initiated by pain associated with the endometriosis lesions, and that gives us a mechanism to think about peripheral and central sensitization.” But she noted that such sensitization can be easily missed in the clinic.
“One of the things that’s really underappreciated is how much women with chronic pelvic pain have pain elsewhere. So the neurologist or pain specialist may say, ‘that’s not my body territory, there’s something going on with your pelvis.’ And the gynecologist may be focused on the endometriosis and the endometriosis lesions. So you have these women with really widespread pain problems whose care is being fractionated.”
In another aspect of the study she also presented at the meeting, Dr. Karp, a neurologist who has studied the therapeutic use of neurotoxins such as botulinum for 30 years, showed results from an open-label extension of a randomized trial of botulinum toxin injections to treat pelvic spasm in the same cohort of women with confirmed endometriosis and confirmed pelvic muscle spasm.
A month after the open-label injection, spasm was reduced or absent in all subjects (P = .0005), with 11 of 13 rating pain as absent or mild (P = .0001), Dr. Karp and her colleagues reported. Between 5 and 11 months post injection, five women requested a repeat of the treatment.
Besides the data on pain and disability collected as part of the trial, Dr. Karp and her colleagues are also looking at biomarkers for pain and inflammation, and changes in medication and hormone use. They are preparing a separate literature review on injection techniques and dosages of toxin to the pelvic floor muscles.
“It’s an area of the body neurologists don’t feel comfortable injecting, and I don’t necessarily feel comfortable doing it myself,” Dr. Karp said.
The researchers had to develop their own procedure because at the time they started the research there was almost nothing in the literature on how to inject botulinum toxin for pelvic pain in women. “People in different specialties have been doing it [to relieve pelvic pain] and it’s really widespread, but they’re doing it all different ways,” Dr. Karp said. “We’re hoping to find a best approach.”
Dr. Karp and her colleagues’ research is supported by the NINDS and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. OnabotulinumtoxinA for the clinical trial is supplied by Allergan. Dr. Karp has received research support from Allergan and Merz.
SOURCE: Karp B et al. AAN 2018, Abstract P2.096; Karp B et al. AAN 2018, Abstract P2.098.
REPORTING FROM AAN 2018
Key clinical point:
Major finding: A month after the open-label injection, spasm was reduced or absent in all subjects (P = .0005), with 11 of 13 rating pain as absent or mild (P = .0001).
Study details: An open-label extension in 13 of 28 patients enrolled in a randomized trial.
Disclosures: Dr. Karp and her colleagues’ research is supported by the NINDS and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. OnabotulinumtoxinA for the clinical trial is supplied by Allergan. Dr. Karp has received research support from Allergan and Merz.
Source: Karp B et al. AAN 2018, Abstract P2.096; Karp B et al. AAN 2018, Abstract P2.098.