User login
New thinking on septal myectomy vs. alcohol ablation for obstructive cardiomyopathy
SNOWMASS, COLO. – The first-ever national study of the impact of hospital volume on outcomes of septal myectomy versus alcohol septal ablation for treatment of obstructive hypertrophic cardiomyopathy deserves to be practice-changing, Rick A. Nishimura, MD, said at the Annual Cardiovascular Conference at Snowmass.
Prior to release of these eye-opening data, conventional thinking held that referral for percutaneous septal ablation was the preferred option for elderly, sedentary patients with lots of comorbid conditions and a limited remaining lifespan, while surgical septal myectomy was the best fix for young, active, relatively healthy patients because of its impressive durability of benefit.
Similarly, 80% of alcohol ablations took place at centers doing less than 20 cases over 9 years. But the success of the percutaneous procedure was less dependent upon large institutional volumes. Only at the lowest-volume centers, where a total of fewer than 10 of the procedures were done over 9 years, was procedural mortality significantly higher – indeed, three- to fourfold higher – than at mid- or high-volume institutions or centers of excellence, all of which had similar mortality rates. The same was true for rates of postoperative complete heart block requiring a permanent pacemaker: significantly higher only at the lowest-volume institutions, according to the investigators from Weill Cornell Medical College in New York (JAMA Cardiol. 2016 Jun 1;1[3]:324-32).
“I think the bottom line is this: for the patient who is severely symptomatic with obstruction on optimal medical therapy, septal myectomy probably offers the best chance of excellent long-term symptomatic improvement, but the mortality depends on the center and the surgical expertise there, and complications do, too. This is something good to know that we never had data on before, that if you can’t get to a center with an experienced surgeon doing myectomies, it’s reasonable to go to a center doing ablations as long as there is some experience with the procedure there,” said Dr. Nishimura, professor of cardiovascular diseases and hypertension at the Mayo Clinic in Rochester, Minn.
Of the 11,248 patients treated for obstructive hypertrophic cardiomyopathy identified by the Cornell investigators using the Agency for Healthcare Research and Quality National Inpatient Sample database, 57% got myectomy and 43% underwent ablation. During the study years ablation increased in popularity by about 50%, rising from an annual rate of 1.6 to 2.5 procedures per million per year, while myectomy declined from 2.0 to 1.5 cases per million population per year. But that’s not what’s happened at the Mayo Clinic and other hypertrophic cardiomyopathy centers of excellence.
At the Mayo Clinic, for example, the volume of septal myectomies climbed from roughly 50 procedures per year in 2000 to close to 250 in 2015. Meanwhile the rate of alcohol septal ablation procedures remained steady at fewer than 20 per year.
“With shared decision making at Mayo, surgery has gone way up,” said Dr. Nishimura. “In an experienced surgeon’s hands, operative mortality is 0.8%, the gradient improves to 3%, and 94% of patients are postoperative New York Heart Association class I or II. This lasts for decades. We have 20-, 30-, and 40-year follow-up data now showing that over 90% of patients will have an excellent symptomatic benefit and be able to return to a normal lifestyle. The septum doesn’t come back. They’re good for life. So it’s a wonderful operation.”
In contrast, catheter-based septal ablation has a 4-year rate of survival free of death, NYHA class III or IV, or myectomy of 76%.
“One in four treated patients will not benefit,” the cardiologist emphasized.
The percutaneous procedure entails instilling alcohol into the septal perforator artery supplying the area of obstruction in order to cause a localized MI. Over a period of several weeks this causes the septum to shrink, thereby relieving the outflow tract obstruction.
When the procedure fails to bring about improvement, it’s often because the patient had a very long septal perforator artery and instilling the alcohol caused a large MI, making things worse. Or the patient didn’t have a septal perforator artery, or had one with so many branches that the cardiologist couldn’t identify the right one to treat to target the septum.
Dr. Nishimura reported having no financial conflicts.
SNOWMASS, COLO. – The first-ever national study of the impact of hospital volume on outcomes of septal myectomy versus alcohol septal ablation for treatment of obstructive hypertrophic cardiomyopathy deserves to be practice-changing, Rick A. Nishimura, MD, said at the Annual Cardiovascular Conference at Snowmass.
Prior to release of these eye-opening data, conventional thinking held that referral for percutaneous septal ablation was the preferred option for elderly, sedentary patients with lots of comorbid conditions and a limited remaining lifespan, while surgical septal myectomy was the best fix for young, active, relatively healthy patients because of its impressive durability of benefit.
Similarly, 80% of alcohol ablations took place at centers doing less than 20 cases over 9 years. But the success of the percutaneous procedure was less dependent upon large institutional volumes. Only at the lowest-volume centers, where a total of fewer than 10 of the procedures were done over 9 years, was procedural mortality significantly higher – indeed, three- to fourfold higher – than at mid- or high-volume institutions or centers of excellence, all of which had similar mortality rates. The same was true for rates of postoperative complete heart block requiring a permanent pacemaker: significantly higher only at the lowest-volume institutions, according to the investigators from Weill Cornell Medical College in New York (JAMA Cardiol. 2016 Jun 1;1[3]:324-32).
“I think the bottom line is this: for the patient who is severely symptomatic with obstruction on optimal medical therapy, septal myectomy probably offers the best chance of excellent long-term symptomatic improvement, but the mortality depends on the center and the surgical expertise there, and complications do, too. This is something good to know that we never had data on before, that if you can’t get to a center with an experienced surgeon doing myectomies, it’s reasonable to go to a center doing ablations as long as there is some experience with the procedure there,” said Dr. Nishimura, professor of cardiovascular diseases and hypertension at the Mayo Clinic in Rochester, Minn.
Of the 11,248 patients treated for obstructive hypertrophic cardiomyopathy identified by the Cornell investigators using the Agency for Healthcare Research and Quality National Inpatient Sample database, 57% got myectomy and 43% underwent ablation. During the study years ablation increased in popularity by about 50%, rising from an annual rate of 1.6 to 2.5 procedures per million per year, while myectomy declined from 2.0 to 1.5 cases per million population per year. But that’s not what’s happened at the Mayo Clinic and other hypertrophic cardiomyopathy centers of excellence.
At the Mayo Clinic, for example, the volume of septal myectomies climbed from roughly 50 procedures per year in 2000 to close to 250 in 2015. Meanwhile the rate of alcohol septal ablation procedures remained steady at fewer than 20 per year.
“With shared decision making at Mayo, surgery has gone way up,” said Dr. Nishimura. “In an experienced surgeon’s hands, operative mortality is 0.8%, the gradient improves to 3%, and 94% of patients are postoperative New York Heart Association class I or II. This lasts for decades. We have 20-, 30-, and 40-year follow-up data now showing that over 90% of patients will have an excellent symptomatic benefit and be able to return to a normal lifestyle. The septum doesn’t come back. They’re good for life. So it’s a wonderful operation.”
In contrast, catheter-based septal ablation has a 4-year rate of survival free of death, NYHA class III or IV, or myectomy of 76%.
“One in four treated patients will not benefit,” the cardiologist emphasized.
The percutaneous procedure entails instilling alcohol into the septal perforator artery supplying the area of obstruction in order to cause a localized MI. Over a period of several weeks this causes the septum to shrink, thereby relieving the outflow tract obstruction.
When the procedure fails to bring about improvement, it’s often because the patient had a very long septal perforator artery and instilling the alcohol caused a large MI, making things worse. Or the patient didn’t have a septal perforator artery, or had one with so many branches that the cardiologist couldn’t identify the right one to treat to target the septum.
Dr. Nishimura reported having no financial conflicts.
SNOWMASS, COLO. – The first-ever national study of the impact of hospital volume on outcomes of septal myectomy versus alcohol septal ablation for treatment of obstructive hypertrophic cardiomyopathy deserves to be practice-changing, Rick A. Nishimura, MD, said at the Annual Cardiovascular Conference at Snowmass.
Prior to release of these eye-opening data, conventional thinking held that referral for percutaneous septal ablation was the preferred option for elderly, sedentary patients with lots of comorbid conditions and a limited remaining lifespan, while surgical septal myectomy was the best fix for young, active, relatively healthy patients because of its impressive durability of benefit.
Similarly, 80% of alcohol ablations took place at centers doing less than 20 cases over 9 years. But the success of the percutaneous procedure was less dependent upon large institutional volumes. Only at the lowest-volume centers, where a total of fewer than 10 of the procedures were done over 9 years, was procedural mortality significantly higher – indeed, three- to fourfold higher – than at mid- or high-volume institutions or centers of excellence, all of which had similar mortality rates. The same was true for rates of postoperative complete heart block requiring a permanent pacemaker: significantly higher only at the lowest-volume institutions, according to the investigators from Weill Cornell Medical College in New York (JAMA Cardiol. 2016 Jun 1;1[3]:324-32).
“I think the bottom line is this: for the patient who is severely symptomatic with obstruction on optimal medical therapy, septal myectomy probably offers the best chance of excellent long-term symptomatic improvement, but the mortality depends on the center and the surgical expertise there, and complications do, too. This is something good to know that we never had data on before, that if you can’t get to a center with an experienced surgeon doing myectomies, it’s reasonable to go to a center doing ablations as long as there is some experience with the procedure there,” said Dr. Nishimura, professor of cardiovascular diseases and hypertension at the Mayo Clinic in Rochester, Minn.
Of the 11,248 patients treated for obstructive hypertrophic cardiomyopathy identified by the Cornell investigators using the Agency for Healthcare Research and Quality National Inpatient Sample database, 57% got myectomy and 43% underwent ablation. During the study years ablation increased in popularity by about 50%, rising from an annual rate of 1.6 to 2.5 procedures per million per year, while myectomy declined from 2.0 to 1.5 cases per million population per year. But that’s not what’s happened at the Mayo Clinic and other hypertrophic cardiomyopathy centers of excellence.
At the Mayo Clinic, for example, the volume of septal myectomies climbed from roughly 50 procedures per year in 2000 to close to 250 in 2015. Meanwhile the rate of alcohol septal ablation procedures remained steady at fewer than 20 per year.
“With shared decision making at Mayo, surgery has gone way up,” said Dr. Nishimura. “In an experienced surgeon’s hands, operative mortality is 0.8%, the gradient improves to 3%, and 94% of patients are postoperative New York Heart Association class I or II. This lasts for decades. We have 20-, 30-, and 40-year follow-up data now showing that over 90% of patients will have an excellent symptomatic benefit and be able to return to a normal lifestyle. The septum doesn’t come back. They’re good for life. So it’s a wonderful operation.”
In contrast, catheter-based septal ablation has a 4-year rate of survival free of death, NYHA class III or IV, or myectomy of 76%.
“One in four treated patients will not benefit,” the cardiologist emphasized.
The percutaneous procedure entails instilling alcohol into the septal perforator artery supplying the area of obstruction in order to cause a localized MI. Over a period of several weeks this causes the septum to shrink, thereby relieving the outflow tract obstruction.
When the procedure fails to bring about improvement, it’s often because the patient had a very long septal perforator artery and instilling the alcohol caused a large MI, making things worse. Or the patient didn’t have a septal perforator artery, or had one with so many branches that the cardiologist couldn’t identify the right one to treat to target the septum.
Dr. Nishimura reported having no financial conflicts.
Echocardiography can benefit use of stented bovine graft for MVR in infants
Mitral valve replacement in infants and young children is complicated because appropriately sized prostheses are difficult to come by and these patients need replacements later on as they continue to grow – thus the high rates of reintervention and death. Pediatric cardiac surgery specialists at Boston Children’s Hospital are among the few that have used stented jugular vein grafts in these patients, and they have reported on a refinement of their technique that uses echocardiography before and after graft placement to obtain valuable measurements for sizing and implanting a prosthesis and for identifying patients at risk of complications.
Lindsay R. Freud, MD, and her associates reported in the January 2017 issue of the Journal of Thoracic and Cardiovascular Surgery on pre- and postoperative echocardiograms of 24 patients who had mitral valve replacement (MVR) with the Melody stent-mounted, valved bovine jugular vein graft (Medtronic) (J Thorac Cardiovasc Surg. 2017;153:153-60). The device, which is approved for transcatheter pulmonary valve replacement, was adapted for implantation into the mitral position, an indication that is not yet Food and Drug Administration approved. “With the increasing use of the Melody valve in the mitral position in infants and young children, we sought to provide a framework for both pre- and early postoperative echocardiographic assessment,” Dr. Freud and her coauthors said.
“The potential dimensions often had normal z scores with fair correlation with intraoperative Melody dilation,” the investigators said. They also found that a ratio of the narrowest subaortic region in systole to the actual MV dimension (SubA:MV) less than 0.5 was associated with postoperative left ventricular outflow tract obstruction (LVOTO), which occurred in four patients. The median age of the study group was 8.5 months.
“Postoperatively, mitral gradients substantially improved, with low values relative to the effective orifice area of the Melody valve,” Dr. Freud and her associates said. None of the patients had significant regurgitation or perivalvar leak.
In early reports of the Melody valve in infants and small children, the surgeons determined the size of the replacement valve during the operation itself. Despite encouraging early results, reports of complications such as LVOTO soon followed. The Children’s Hospital Boston researchers undertook the study to determine if echocardiography before surgery would help to identify the correct valve size for expansion and predict which patients would be at risk for LVOTO.
“The preoperative SubA:MV ratio may help assess the risk for postoperative LVOTO, which is an important complication,” Dr. Freud and coauthors said. The presence of LVOTO preoperatively was also a risk factor, but only one of eight patients with an atrioventricular canal defect developed LVOTO. In patients with a SubA:MV ratio less than 0.5, preoperative LVOTO, or any other anatomic risk factor, surgeons should consider options to prevent LVOTO, Dr. Freud and her associates said. Those alternatives include more aggressive resection of stent material, atrial displacement of the valve, or less aggressive distal expansion of the valve.
Postoperative echocardiography enabled Dr. Freud and her coauthors to outline baseline values for the Melody valve in the mitral position by maximum intraoperative balloon diameter, ranging from 1 cm to 1.8 cm in 0.2-cm steps, and depending on five measurements at each step: peak and mean gradients, peak velocity, effective orifice area, and indexed effective orifice area.
“Validation of candidacy for Melody MVR and noninvasive assessment among larger series of patient will be necessary as greater experience with the Melody valve evolves,” Dr. Freud and her associates concluded.
Coauthor Sitaram Emani, MD, has filed a patent for an expandable valve through Boston Children’s Hospital. Dr. Freud and her other coauthors had no financial relationships to disclose.
The Melody valve is an “appealing solution” for MVR in infants and small children, Patrick Myers, MD, of Geneva University Hospitals said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:151-2) “This contribution brings further data to support Melody MVR,” he said of the report by Dr. Freud and her colleagues.
However, Dr. Myers noted that beyond the Boston Children’s Hospital experience, only two other reports of the Melody valve in the mitral position in children exist. “There are several outstanding technical issues that need to be investigated for the use of the Melody valve in the mitral position,” he said. Among those issues is the length of the stent itself – 28 mm, which can lead to LVOTO after placement “in a diminutive ventricle.” The fact that “only” four patients in the study group developed LVOTO after Melody MVR is “reassuring with regard to this theoretic limitation,” Dr. Myers said. “And the echocardiographic ratio of the narrowest subaortic region in systole to the actual mitral valve dimension could be of use in deciding when to be more aggressive in preventing LVOTO,” he said.
Dr. Myers also said that this report answered some questions about the durability of a venous valve under systemic pressures, but added, “Further echocardiographic and clinical follow-up data in this very challenging population are required,” he said.
Dr. Myers had no financial relationships to disclose.
The Melody valve is an “appealing solution” for MVR in infants and small children, Patrick Myers, MD, of Geneva University Hospitals said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:151-2) “This contribution brings further data to support Melody MVR,” he said of the report by Dr. Freud and her colleagues.
However, Dr. Myers noted that beyond the Boston Children’s Hospital experience, only two other reports of the Melody valve in the mitral position in children exist. “There are several outstanding technical issues that need to be investigated for the use of the Melody valve in the mitral position,” he said. Among those issues is the length of the stent itself – 28 mm, which can lead to LVOTO after placement “in a diminutive ventricle.” The fact that “only” four patients in the study group developed LVOTO after Melody MVR is “reassuring with regard to this theoretic limitation,” Dr. Myers said. “And the echocardiographic ratio of the narrowest subaortic region in systole to the actual mitral valve dimension could be of use in deciding when to be more aggressive in preventing LVOTO,” he said.
Dr. Myers also said that this report answered some questions about the durability of a venous valve under systemic pressures, but added, “Further echocardiographic and clinical follow-up data in this very challenging population are required,” he said.
Dr. Myers had no financial relationships to disclose.
The Melody valve is an “appealing solution” for MVR in infants and small children, Patrick Myers, MD, of Geneva University Hospitals said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:151-2) “This contribution brings further data to support Melody MVR,” he said of the report by Dr. Freud and her colleagues.
However, Dr. Myers noted that beyond the Boston Children’s Hospital experience, only two other reports of the Melody valve in the mitral position in children exist. “There are several outstanding technical issues that need to be investigated for the use of the Melody valve in the mitral position,” he said. Among those issues is the length of the stent itself – 28 mm, which can lead to LVOTO after placement “in a diminutive ventricle.” The fact that “only” four patients in the study group developed LVOTO after Melody MVR is “reassuring with regard to this theoretic limitation,” Dr. Myers said. “And the echocardiographic ratio of the narrowest subaortic region in systole to the actual mitral valve dimension could be of use in deciding when to be more aggressive in preventing LVOTO,” he said.
Dr. Myers also said that this report answered some questions about the durability of a venous valve under systemic pressures, but added, “Further echocardiographic and clinical follow-up data in this very challenging population are required,” he said.
Dr. Myers had no financial relationships to disclose.
Mitral valve replacement in infants and young children is complicated because appropriately sized prostheses are difficult to come by and these patients need replacements later on as they continue to grow – thus the high rates of reintervention and death. Pediatric cardiac surgery specialists at Boston Children’s Hospital are among the few that have used stented jugular vein grafts in these patients, and they have reported on a refinement of their technique that uses echocardiography before and after graft placement to obtain valuable measurements for sizing and implanting a prosthesis and for identifying patients at risk of complications.
Lindsay R. Freud, MD, and her associates reported in the January 2017 issue of the Journal of Thoracic and Cardiovascular Surgery on pre- and postoperative echocardiograms of 24 patients who had mitral valve replacement (MVR) with the Melody stent-mounted, valved bovine jugular vein graft (Medtronic) (J Thorac Cardiovasc Surg. 2017;153:153-60). The device, which is approved for transcatheter pulmonary valve replacement, was adapted for implantation into the mitral position, an indication that is not yet Food and Drug Administration approved. “With the increasing use of the Melody valve in the mitral position in infants and young children, we sought to provide a framework for both pre- and early postoperative echocardiographic assessment,” Dr. Freud and her coauthors said.
“The potential dimensions often had normal z scores with fair correlation with intraoperative Melody dilation,” the investigators said. They also found that a ratio of the narrowest subaortic region in systole to the actual MV dimension (SubA:MV) less than 0.5 was associated with postoperative left ventricular outflow tract obstruction (LVOTO), which occurred in four patients. The median age of the study group was 8.5 months.
“Postoperatively, mitral gradients substantially improved, with low values relative to the effective orifice area of the Melody valve,” Dr. Freud and her associates said. None of the patients had significant regurgitation or perivalvar leak.
In early reports of the Melody valve in infants and small children, the surgeons determined the size of the replacement valve during the operation itself. Despite encouraging early results, reports of complications such as LVOTO soon followed. The Children’s Hospital Boston researchers undertook the study to determine if echocardiography before surgery would help to identify the correct valve size for expansion and predict which patients would be at risk for LVOTO.
“The preoperative SubA:MV ratio may help assess the risk for postoperative LVOTO, which is an important complication,” Dr. Freud and coauthors said. The presence of LVOTO preoperatively was also a risk factor, but only one of eight patients with an atrioventricular canal defect developed LVOTO. In patients with a SubA:MV ratio less than 0.5, preoperative LVOTO, or any other anatomic risk factor, surgeons should consider options to prevent LVOTO, Dr. Freud and her associates said. Those alternatives include more aggressive resection of stent material, atrial displacement of the valve, or less aggressive distal expansion of the valve.
Postoperative echocardiography enabled Dr. Freud and her coauthors to outline baseline values for the Melody valve in the mitral position by maximum intraoperative balloon diameter, ranging from 1 cm to 1.8 cm in 0.2-cm steps, and depending on five measurements at each step: peak and mean gradients, peak velocity, effective orifice area, and indexed effective orifice area.
“Validation of candidacy for Melody MVR and noninvasive assessment among larger series of patient will be necessary as greater experience with the Melody valve evolves,” Dr. Freud and her associates concluded.
Coauthor Sitaram Emani, MD, has filed a patent for an expandable valve through Boston Children’s Hospital. Dr. Freud and her other coauthors had no financial relationships to disclose.
Mitral valve replacement in infants and young children is complicated because appropriately sized prostheses are difficult to come by and these patients need replacements later on as they continue to grow – thus the high rates of reintervention and death. Pediatric cardiac surgery specialists at Boston Children’s Hospital are among the few that have used stented jugular vein grafts in these patients, and they have reported on a refinement of their technique that uses echocardiography before and after graft placement to obtain valuable measurements for sizing and implanting a prosthesis and for identifying patients at risk of complications.
Lindsay R. Freud, MD, and her associates reported in the January 2017 issue of the Journal of Thoracic and Cardiovascular Surgery on pre- and postoperative echocardiograms of 24 patients who had mitral valve replacement (MVR) with the Melody stent-mounted, valved bovine jugular vein graft (Medtronic) (J Thorac Cardiovasc Surg. 2017;153:153-60). The device, which is approved for transcatheter pulmonary valve replacement, was adapted for implantation into the mitral position, an indication that is not yet Food and Drug Administration approved. “With the increasing use of the Melody valve in the mitral position in infants and young children, we sought to provide a framework for both pre- and early postoperative echocardiographic assessment,” Dr. Freud and her coauthors said.
“The potential dimensions often had normal z scores with fair correlation with intraoperative Melody dilation,” the investigators said. They also found that a ratio of the narrowest subaortic region in systole to the actual MV dimension (SubA:MV) less than 0.5 was associated with postoperative left ventricular outflow tract obstruction (LVOTO), which occurred in four patients. The median age of the study group was 8.5 months.
“Postoperatively, mitral gradients substantially improved, with low values relative to the effective orifice area of the Melody valve,” Dr. Freud and her associates said. None of the patients had significant regurgitation or perivalvar leak.
In early reports of the Melody valve in infants and small children, the surgeons determined the size of the replacement valve during the operation itself. Despite encouraging early results, reports of complications such as LVOTO soon followed. The Children’s Hospital Boston researchers undertook the study to determine if echocardiography before surgery would help to identify the correct valve size for expansion and predict which patients would be at risk for LVOTO.
“The preoperative SubA:MV ratio may help assess the risk for postoperative LVOTO, which is an important complication,” Dr. Freud and coauthors said. The presence of LVOTO preoperatively was also a risk factor, but only one of eight patients with an atrioventricular canal defect developed LVOTO. In patients with a SubA:MV ratio less than 0.5, preoperative LVOTO, or any other anatomic risk factor, surgeons should consider options to prevent LVOTO, Dr. Freud and her associates said. Those alternatives include more aggressive resection of stent material, atrial displacement of the valve, or less aggressive distal expansion of the valve.
Postoperative echocardiography enabled Dr. Freud and her coauthors to outline baseline values for the Melody valve in the mitral position by maximum intraoperative balloon diameter, ranging from 1 cm to 1.8 cm in 0.2-cm steps, and depending on five measurements at each step: peak and mean gradients, peak velocity, effective orifice area, and indexed effective orifice area.
“Validation of candidacy for Melody MVR and noninvasive assessment among larger series of patient will be necessary as greater experience with the Melody valve evolves,” Dr. Freud and her associates concluded.
Coauthor Sitaram Emani, MD, has filed a patent for an expandable valve through Boston Children’s Hospital. Dr. Freud and her other coauthors had no financial relationships to disclose.
FROM JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Preoperative echocardiography may help guide placement of modified stented jugular vein grafts in infants and small children with hypoplastic mitral and aortic valves.
Major finding: Echocardiography showed that a ratio of the narrowest subaortic region in systole to the actual mitral valve dimension of less than 0.5 was associated with postoperative left ventricular outflow tract obstruction.
Data source: Single-center, retrospective review of 24 patients who underwent mitral valve replacement with modified stented jugular vein grafts from March 2010 to March 2015.
Disclosures: Coauthor Sitaram Emani, MD, has filed a patent for an expandable valve through Boston Children’s Hospital. Dr. Freud and her other coauthors had no financial relationships to disclose.
Hybrid procedures may be better option for LVOTO in lower-weight neonates
Little outcomes data have been published comparing hybrid and Norwood stage 1 procedures for newborns with critical left ventricular outflow tract obstruction (LVOTO), but a prospective analysis of more than 500 operations over 9 years reported that while the Norwood has better survival rates overall, hybrid procedures may improve survival in low-birth-weight newborns.
“Although lower birth weight was identified as an important risk factor for death for the entire cohort, the detrimental impact of low birth weight was mitigated, to some degree, for patients who underwent a hybrid procedure,” said Travis Wilder, MD, of the Congenital Heart Surgeons’ Society (CHSS) Data Center, and his coauthors. They reported their findings in the January 2017 issue of the Journal of Thoracic and Cardiovascular Surgery (153:163-72).
Norwood operations involve major surgical reconstruction along with exposure to cardiopulmonary bypass (CPB), with either deep hypothermic circulatory arrest (DHCA) or regional cerebral perfusion, during aortic arch reconstruction. Previous reports have linked CPB to postoperative hemodynamic instability, complications, and death (Ann Thorac Surg. 2009 Jun;87:1885-92). “In addition, the early physiological stress imposed on neonates after Norwood operations raises concerns regarding adverse neurodevelopment,” Dr. Wilder and his coauthors wrote.
Dr. Wilder and his coauthors pointed out that the hybrid procedure has emerged to avoid CPB and DHCA or regional cerebral perfusion and the potential resulting physiologic instability. “In this light, hybrid palliation may be perceived as a lower-risk alternative to Norwood operations, especially for patients considered at high risk for mortality,” the researchers said. Despite that perception, the actual survival “remains incompletely defined,” they said.
The overall average 4-year unadjusted survival for the entire study population was 65%, but those who had the NW-RVPA procedure had significantly improved survival (73%) vs. both the NW-BT (61%) and the hybrid groups (60%).
Those who had the hybrid procedure were older at stage 1 (12 days vs. 8 and 6 days, respectively for NW-BT and NW-RVPA) and had lower birth weight (2.9 kg vs. 3.2 kg and 3.15 kg, respectively). Hybrid patients also had a higher prevalence of baseline right ventricle dysfunction, were more likely to have baseline tricuspid valve regurgitation, and had a lower prevalence of aortic and mitral valve atresia.
For all patients, birth weight of 2.0-2.5 kg had a strong association with poor survival, Dr. Wilder and his coauthors reported, but the drop-off in survival for low-birth-weight neonates was greater in the Norwood group than in the hybrid group. “This finding suggests that hybrid procedures may offer a modest survival advantage over NW-RVPA at birth weight less than or equal to 2.0 kg and over NW-BT at birth weight less than or equal to 3.0 kg,” the researchers said.
Dr. Wilder and his coauthors had no financial relationships to disclose.
While the study by Dr. Wilder and his coauthors may have drawn an accurate conclusion about low-birth-weight newborns possibly benefiting from a hybrid procedure for hypoplastic left heart syndrome, the number of patients in each strategy was small, Carlos M. Mery, MD, MPH, of Texas Children’s Hospital/Baylor College of Medicine, Houston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017 Jan;153:173-4).
Dr. Mery noted other limitations of the study, namely the heterogeneity of procedures by participating center. “Of the 20 centers, only 11 performed any hybrid procedures, and 1 center accounted for 42% of all hybrid procedures performed,” he said. “Because centers may be associated with possibly unaccounted risk factors and different learning curves, the conclusions may not be easily generalizable.”
The conclusion that newborns of lower birth weight may benefit from the hybrid procedure helps to bring clarity for which patients may benefit from a specific procedure, Dr. Mery said. “We seem to be getting closer to the ultimate goal of being able to offer each individual patient the management strategy that will lead to the best possible outcome, not only for quantity but also for quality of life,” Dr. Mery said.
Dr. Mery had no financial relationships to disclose.
While the study by Dr. Wilder and his coauthors may have drawn an accurate conclusion about low-birth-weight newborns possibly benefiting from a hybrid procedure for hypoplastic left heart syndrome, the number of patients in each strategy was small, Carlos M. Mery, MD, MPH, of Texas Children’s Hospital/Baylor College of Medicine, Houston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017 Jan;153:173-4).
Dr. Mery noted other limitations of the study, namely the heterogeneity of procedures by participating center. “Of the 20 centers, only 11 performed any hybrid procedures, and 1 center accounted for 42% of all hybrid procedures performed,” he said. “Because centers may be associated with possibly unaccounted risk factors and different learning curves, the conclusions may not be easily generalizable.”
The conclusion that newborns of lower birth weight may benefit from the hybrid procedure helps to bring clarity for which patients may benefit from a specific procedure, Dr. Mery said. “We seem to be getting closer to the ultimate goal of being able to offer each individual patient the management strategy that will lead to the best possible outcome, not only for quantity but also for quality of life,” Dr. Mery said.
Dr. Mery had no financial relationships to disclose.
While the study by Dr. Wilder and his coauthors may have drawn an accurate conclusion about low-birth-weight newborns possibly benefiting from a hybrid procedure for hypoplastic left heart syndrome, the number of patients in each strategy was small, Carlos M. Mery, MD, MPH, of Texas Children’s Hospital/Baylor College of Medicine, Houston, said in his invited commentary (J Thorac Cardiovasc Surg. 2017 Jan;153:173-4).
Dr. Mery noted other limitations of the study, namely the heterogeneity of procedures by participating center. “Of the 20 centers, only 11 performed any hybrid procedures, and 1 center accounted for 42% of all hybrid procedures performed,” he said. “Because centers may be associated with possibly unaccounted risk factors and different learning curves, the conclusions may not be easily generalizable.”
The conclusion that newborns of lower birth weight may benefit from the hybrid procedure helps to bring clarity for which patients may benefit from a specific procedure, Dr. Mery said. “We seem to be getting closer to the ultimate goal of being able to offer each individual patient the management strategy that will lead to the best possible outcome, not only for quantity but also for quality of life,” Dr. Mery said.
Dr. Mery had no financial relationships to disclose.
Little outcomes data have been published comparing hybrid and Norwood stage 1 procedures for newborns with critical left ventricular outflow tract obstruction (LVOTO), but a prospective analysis of more than 500 operations over 9 years reported that while the Norwood has better survival rates overall, hybrid procedures may improve survival in low-birth-weight newborns.
“Although lower birth weight was identified as an important risk factor for death for the entire cohort, the detrimental impact of low birth weight was mitigated, to some degree, for patients who underwent a hybrid procedure,” said Travis Wilder, MD, of the Congenital Heart Surgeons’ Society (CHSS) Data Center, and his coauthors. They reported their findings in the January 2017 issue of the Journal of Thoracic and Cardiovascular Surgery (153:163-72).
Norwood operations involve major surgical reconstruction along with exposure to cardiopulmonary bypass (CPB), with either deep hypothermic circulatory arrest (DHCA) or regional cerebral perfusion, during aortic arch reconstruction. Previous reports have linked CPB to postoperative hemodynamic instability, complications, and death (Ann Thorac Surg. 2009 Jun;87:1885-92). “In addition, the early physiological stress imposed on neonates after Norwood operations raises concerns regarding adverse neurodevelopment,” Dr. Wilder and his coauthors wrote.
Dr. Wilder and his coauthors pointed out that the hybrid procedure has emerged to avoid CPB and DHCA or regional cerebral perfusion and the potential resulting physiologic instability. “In this light, hybrid palliation may be perceived as a lower-risk alternative to Norwood operations, especially for patients considered at high risk for mortality,” the researchers said. Despite that perception, the actual survival “remains incompletely defined,” they said.
The overall average 4-year unadjusted survival for the entire study population was 65%, but those who had the NW-RVPA procedure had significantly improved survival (73%) vs. both the NW-BT (61%) and the hybrid groups (60%).
Those who had the hybrid procedure were older at stage 1 (12 days vs. 8 and 6 days, respectively for NW-BT and NW-RVPA) and had lower birth weight (2.9 kg vs. 3.2 kg and 3.15 kg, respectively). Hybrid patients also had a higher prevalence of baseline right ventricle dysfunction, were more likely to have baseline tricuspid valve regurgitation, and had a lower prevalence of aortic and mitral valve atresia.
For all patients, birth weight of 2.0-2.5 kg had a strong association with poor survival, Dr. Wilder and his coauthors reported, but the drop-off in survival for low-birth-weight neonates was greater in the Norwood group than in the hybrid group. “This finding suggests that hybrid procedures may offer a modest survival advantage over NW-RVPA at birth weight less than or equal to 2.0 kg and over NW-BT at birth weight less than or equal to 3.0 kg,” the researchers said.
Dr. Wilder and his coauthors had no financial relationships to disclose.
Little outcomes data have been published comparing hybrid and Norwood stage 1 procedures for newborns with critical left ventricular outflow tract obstruction (LVOTO), but a prospective analysis of more than 500 operations over 9 years reported that while the Norwood has better survival rates overall, hybrid procedures may improve survival in low-birth-weight newborns.
“Although lower birth weight was identified as an important risk factor for death for the entire cohort, the detrimental impact of low birth weight was mitigated, to some degree, for patients who underwent a hybrid procedure,” said Travis Wilder, MD, of the Congenital Heart Surgeons’ Society (CHSS) Data Center, and his coauthors. They reported their findings in the January 2017 issue of the Journal of Thoracic and Cardiovascular Surgery (153:163-72).
Norwood operations involve major surgical reconstruction along with exposure to cardiopulmonary bypass (CPB), with either deep hypothermic circulatory arrest (DHCA) or regional cerebral perfusion, during aortic arch reconstruction. Previous reports have linked CPB to postoperative hemodynamic instability, complications, and death (Ann Thorac Surg. 2009 Jun;87:1885-92). “In addition, the early physiological stress imposed on neonates after Norwood operations raises concerns regarding adverse neurodevelopment,” Dr. Wilder and his coauthors wrote.
Dr. Wilder and his coauthors pointed out that the hybrid procedure has emerged to avoid CPB and DHCA or regional cerebral perfusion and the potential resulting physiologic instability. “In this light, hybrid palliation may be perceived as a lower-risk alternative to Norwood operations, especially for patients considered at high risk for mortality,” the researchers said. Despite that perception, the actual survival “remains incompletely defined,” they said.
The overall average 4-year unadjusted survival for the entire study population was 65%, but those who had the NW-RVPA procedure had significantly improved survival (73%) vs. both the NW-BT (61%) and the hybrid groups (60%).
Those who had the hybrid procedure were older at stage 1 (12 days vs. 8 and 6 days, respectively for NW-BT and NW-RVPA) and had lower birth weight (2.9 kg vs. 3.2 kg and 3.15 kg, respectively). Hybrid patients also had a higher prevalence of baseline right ventricle dysfunction, were more likely to have baseline tricuspid valve regurgitation, and had a lower prevalence of aortic and mitral valve atresia.
For all patients, birth weight of 2.0-2.5 kg had a strong association with poor survival, Dr. Wilder and his coauthors reported, but the drop-off in survival for low-birth-weight neonates was greater in the Norwood group than in the hybrid group. “This finding suggests that hybrid procedures may offer a modest survival advantage over NW-RVPA at birth weight less than or equal to 2.0 kg and over NW-BT at birth weight less than or equal to 3.0 kg,” the researchers said.
Dr. Wilder and his coauthors had no financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Norwood procedures have the best survival rates for neonates with critical left ventricular outflow tract obstruction, but hybrid procedures may improve survival for those with lower birth weight.
Major finding: Risk-adjusted 4-year survival was 76% for the Norwood operation with a right ventricle–to-pulmonary artery conduit, 61% for Norwood with a modified Blalock-Taussig shunt and 60% for the hybrid procedure.
Data source: Prospective observational cohort study of 564 neonates admitted to 21 Congenital Heart Surgeons’ Society institutions from 2005 to 2014.
Disclosures: Dr. Wilder and his coauthors had no financial relationships to disclose.
Fecal transplant efficacy for Clostridium difficile infections
Clinical question: Is fecal microbiota transplantation (FMT) an efficacious and safe treatment approach for patients with recurrent Clostridium difficile infection (CDI)?
Background: FMT restores the normal composition of gut microbiota and is recommended when antibiotics fail to clear CDI. To date, only case series and open-labeled clinical trials support the use of FMT.
Study design: Randomized, controlled, double-blinded clinical trial.
Setting: Academic medical centers.
The primary endpoint was resolution of diarrhea without anti-CDI therapy after 8 weeks of follow-up. In the donor FMT group, 90.9% achieved clinical cure, compared with 62.5% in the autologous group. Patients who developed recurrent CDI were free of further disease after subsequent donor FMT.
The study included only patients who experienced three or more recurrences but excluded immunocompromised and older patients (older than 75 years of age).
Bottom line: Donor stool administered via colonoscopy was more effective than autologous FMT in preventing further CDI episodes.
Citation: Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med. 2016;165(9):609-616.
Dr. Fernandez de la Vara is an instructor at the University of Miami Miller School of Medicine and chief medical resident at the University of Miami Hospital.
Clinical question: Is fecal microbiota transplantation (FMT) an efficacious and safe treatment approach for patients with recurrent Clostridium difficile infection (CDI)?
Background: FMT restores the normal composition of gut microbiota and is recommended when antibiotics fail to clear CDI. To date, only case series and open-labeled clinical trials support the use of FMT.
Study design: Randomized, controlled, double-blinded clinical trial.
Setting: Academic medical centers.
The primary endpoint was resolution of diarrhea without anti-CDI therapy after 8 weeks of follow-up. In the donor FMT group, 90.9% achieved clinical cure, compared with 62.5% in the autologous group. Patients who developed recurrent CDI were free of further disease after subsequent donor FMT.
The study included only patients who experienced three or more recurrences but excluded immunocompromised and older patients (older than 75 years of age).
Bottom line: Donor stool administered via colonoscopy was more effective than autologous FMT in preventing further CDI episodes.
Citation: Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med. 2016;165(9):609-616.
Dr. Fernandez de la Vara is an instructor at the University of Miami Miller School of Medicine and chief medical resident at the University of Miami Hospital.
Clinical question: Is fecal microbiota transplantation (FMT) an efficacious and safe treatment approach for patients with recurrent Clostridium difficile infection (CDI)?
Background: FMT restores the normal composition of gut microbiota and is recommended when antibiotics fail to clear CDI. To date, only case series and open-labeled clinical trials support the use of FMT.
Study design: Randomized, controlled, double-blinded clinical trial.
Setting: Academic medical centers.
The primary endpoint was resolution of diarrhea without anti-CDI therapy after 8 weeks of follow-up. In the donor FMT group, 90.9% achieved clinical cure, compared with 62.5% in the autologous group. Patients who developed recurrent CDI were free of further disease after subsequent donor FMT.
The study included only patients who experienced three or more recurrences but excluded immunocompromised and older patients (older than 75 years of age).
Bottom line: Donor stool administered via colonoscopy was more effective than autologous FMT in preventing further CDI episodes.
Citation: Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med. 2016;165(9):609-616.
Dr. Fernandez de la Vara is an instructor at the University of Miami Miller School of Medicine and chief medical resident at the University of Miami Hospital.
Diagnosis and treatment of global endometrial ablation failure
One in seven women suffer with abnormal uterine bleeding during their reproductive years, according to Fraser et al. (Exp Rev Obstet Gynecol. 2009;4:179-89). Heavy menstrual bleeding (menorrhagia) is the most common pattern. Global endometrial ablation has become a very popular surgical technique for women complaining of menorrhagia, disinterested in either medical management or definitive therapy – hysterectomy – or where medical management has failed. With proper patient selection, endometrial ablation yields an 80%-90% success rate in reducing heavy menstrual flow and is associated with a 90% patient satisfaction rate (Cochrane Database Syst Rev. 2009 Oct 7;[4]:CD001501).
Literature is replete with conditions believed to increase risk of endometrial ablation failure. This list includes untreated uterine cornua, endometrial regrowth, the presence of submucous leiomyomas or polyps, abnormal uterine cavity, enlarged uterine cavity (width and/or length), endometrial ablation in a young patient, parity of five or greater, unsuspected adhesiolysis, postablation tubal sterilization syndrome, history of dysmenorrhea, smoking, obesity, prior cesarean section, previous gynecologic surgery, and procedure length. Interestingly, type of global endometrial ablation procedure or original bleeding pattern does not influence failure rate.
In this edition of the Master Class in Gynecologic Surgery, Dr. Morris Wortman discusses not only the prevention of endometrial ablation failure, but also how to treat the problem via conservative surgical management.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and is the director at the Center for Menstrual Disorders and Reproductive Choice, also in Rochester. Dr. Wortman has lectured extensively on endometrial ablation and has authored several scientific articles in peer reviewed journals.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported being a subinvestigator on a study sponsored by Channel Medsystems. Email him at obnews@frontlinemedcom.com.
Why failures occur and how to correct them
BY MORRIS WORTMAN, MD
Since the introduction almost 20 years ago of devices for nonresectoscopic – or “global” – endometrial ablation, the procedure has been widely adopted as the treatment of choice for abnormal uterine bleeding that is refractory to medical management.
Between 400,000 and 500,000 endometrial ablations are done in the United States every year in women who have completed childbearing, and it probably won’t be long before the procedure surpasses hysterectomy in prevalence for the management of abnormal bleeding.
In recent years, the literature has begun to address the incidence of these delayed complications and the requirement for subsequent hysterectomy. A 2007 practice bulletin issued by the American College of Obstetricians and Gynecologists stated that hysterectomy rates within 4 years of endometrial ablation are at least 24% (Obstet Gynecol. 2007 May;109[5]:1233-48). And a study published the following year reported that 26% of 3,681 women undergoing EA at Kaiser Permanente facilities in Northern California required hysterectomy within 8 years (Obstet Gynecol. 2008 Dec;112[6]:1214-20).
It appears that the vast majority of what we now refer to as late-onset EA failures – complications attributable to EA that occur beyond a perioperative period of 1 month – will occur within 5 years. Some EA failures have occurred over 5-10 years, however, and in my practice we have seen late-onset complications occurring 17 or more years after the initial ablation.
In our practice, we are successfully managing delayed complications after GEA using ultrasound-guided reoperative hysteroscopy to fully explore the uterine cavity and excise areas of endometrial growth and other disease. In 2014, we published a retrospective review of 50 women whom we treated for delayed complications after a variety of GEA techniques; almost 90% avoided hysterectomy during a mean follow-up period of 18 months (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:238-44).
Our experience since then has included reoperative surgery on more than 115 GEA failures. Additionally, we’ve managed 220 patients who have undergone various hysteroscopic and resectoscopic endometrial ablations, some of which date back to the use of the Nd:YAG laser in the late 1980s.
The fact that late-onset EA failures occur does not mean that hysterectomy should routinely be performed as a first-line treatment for intractable uterine bleeding. Overall, there is much more morbidity associated with hysterectomy than with EA.
What failures do suggest is that there are certain risk factors for late-onset EA complications. Our experience in treating women who have experienced late-onset EA failure has provided us with insight into who may be at greatest risk for late-onset EA failure and how patients can best be selected for the procedure. We’ve also learned more about the diagnosis of delayed complications.
Causes of EA failure
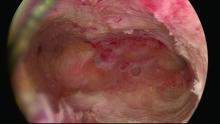
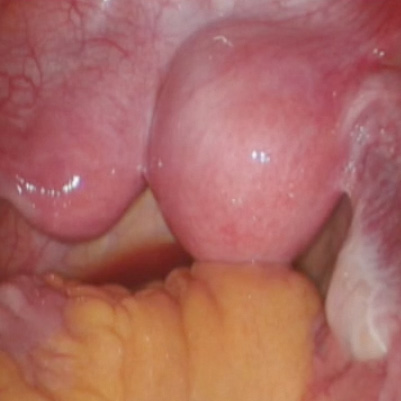
Untreated uterine cornua, and untreated submucous leiomyomas and endometrial polyps, are common causes of EA failure. Among the 50 women included in our retrospective review of ultrasound-guided reoperative hysteroscopy after GEA failure, 44% had intraoperative evidence of untreated cornua and nearly one-fourth had persistent or enlarging submucous leiomyomas.
Contrary to what some believe, most endometrial ablations will not adequately destroy submucous or intramural leiomyomas. Therefore, we recommend that these fibroids be entirely removed immediately before EA.
Moreover, GEA will not always provide adequate thermal destruction to the entire endometrial cavity. The cornua regions are particularly at risk; they are difficult to reach under ideal circumstances, and especially difficult to treat in patients who have a uterine septum or a T-shaped uterus (with the ostia and cornua deeply recessed). We have also seen late-onset EA failures in patients with an extended uterine transverse diameter. The limits of GEA are greatest when a device with a fixed configuration or geometry is used.
A history of abnormal hysteroscopy or other evidence of such anatomic distortions are therefore among the reported risk factors for GEA failure (J Minim Invasive Gynecol. 2015 Mar-Apr;22[3]:323-31). A history of tubal ligation also confers risk; the procedure further increases susceptibility for failure when functioning endometrial tissue remains or regrows at the cornua, because any retrograde menstrual bleeding that occurs will be constrained by the obstructed proximal portion of the fallopian tubes.
Obesity is another risk factor for GEA failure in that the condition increases the risk of endometrial cancer, making the need for reliable biopsies in the case of spotting or other signs or symptoms even more important. On the other hand, obesity may also worsen a patient’s status as a candidate for hysterectomy.
There is much to consider with these patients. For some obese patients, GEA may be less risky than hysterectomy while for others, such as those who also have polycystic ovarian syndrome (in whom the risk for developing endometrial cancer is further increased) the scale may tip in favor of hysterectomy.
Age at the time of the primary GEA may be the single most important risk factor for GEA failure and is an important predictor of success in patient selection. Numerous investigators have shown that women younger than 35 years of age at the time of their EA had a significantly increased risk for hysterectomy, compared with women who were at least 45 years old. The younger the patient, the longer the “bridge” to menopause and the greater the likelihood that bridge will fail.
While age is not necessarily a contraindication, it is worthy of serious consideration. We generally discourage GEA for patients younger than 35. We also advise ensuring that each patient undergoing initial EA is highly self-motivated to have a uterine-sparing procedure; if not, symptoms she may experience later will likely drive her toward hysterectomy anyway.
Additionally, we caution against performing GEA in patients who have chronic pelvic pain; these patients tend to have poorer outcomes with any type of hysteroscopic surgery.
Diagnosing failed EA
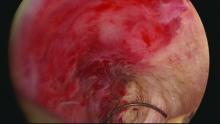
Delayed complications manifest in several ways: Renewed and increasing vaginal bleeding after a period of improvement, cyclic pelvic pain (unilateral, bilateral, or suprapubic), or both bleeding and pain. Some women – likely an underreported number of them – present with postmenopausal bleeding and proceed to have unsuccessful attempts at an endometrial biopsy due to EA-associated endometrial scarring.
The cyclic pelvic pain associated with endometrial persistence or regrowth tends to worsen over time and is often described as sharp or laborlike. In our experience, a description of “laborlike” pain and a history of EA is almost fully predictive of a finding of endometrial growth. Often a hematometra can be demonstrated on transvaginal ultrasound, but this isn’t always the case.
Pain typically precedes bleeding in patients who demonstrate both. In such cases, blood from functioning endometrial tissue or other sources becomes blocked from exiting the uterine cavity by EA-induced intrauterine scarring and contracture. Painful uterine contractions then aim to expel the pooled blood. In other cases of pain – mainly those without significant vaginal bleeding – the pain is often attributed to cornual and central hematometra.
For the majority of EA failures, the diagnosis lies in the history and current symptoms. Unfortunately, the traditional methods of assessing the endometrial cavity have little merit for women presenting with delayed-onset EA complications. A sonographically assisted pelvic examination can be useful in evaluating complications, but the interpretation of ultrasounds in women with a prior EA can be challenging and is often beyond the training of most radiologists and gynecologists.
It is not uncommon for images to be incorrectly interpreted in the emergency department or physicians’ offices as “normal” and for such readings to set off a chain of CT scans, MRIs, laparoscopies, ovarian cystectomies, and other procedures that miss the root causes of pain.
Unfortunately, there is little in the literature that describes and defines ultrasound findings after EA. We do know that sonography should be timed with episodes of pain, and that the absence of a demonstrable hematometra does not exclude a diagnosis of EA failure.
Correcting late-onset failures
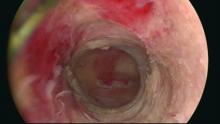
Our office-based operating room is fitted with side-by-side monitors that enable simultaneous sonographic and hysteroscopic views for correction of GEA failures; the rest of the set-up is similar to that of other operative hysteroscopies. However, we do employ a wide variety of resectoscopes with diameters ranging from 13 to 28 Fr. The smaller-diameter scopes are particularly useful for evaluating postmenopausal bleeding in women with a prior EA.
For those inexperienced with ultrasound-guided surgery, the initial resection is often the most challenging. The initial tissue removal is carried out on the thickest observed uterine wall – usually the posterior or anterior wall – and is done with near complete reliance on the ultrasound image. Hysteroscopic visualization is poor at this time because the outflow ports of the continuous flow resectoscope are obstructed by tissue in the narrow tubular cavity.
We then actually remove the resectoscope and clean the outflow ports of clots and debris that may have accumulated. When the scope is reinserted, there is typically sufficient room in the uterine cavity for continuous flow and excellent hysteroscopic visualization.
The sequence of resection from this point on will vary. If we’ve begun on the anterior wall, we’ll move to the posterior and then the two lateral walls to further restore the cavity. Areas of endometrial regrowth will typically be identified at this point and resected. The dissection then will extend upward, usually to within 10 mm of the fundus in the midline as measured by ultrasound. Reconfiguring the loop electrode to a 135- to 160-degree angle can be helpful in the delicate dissection that is required at the fundus.
Once all areas of endometrium have been identified and excised, we will deeply coagulate exposed myometrium with a ball-end electrode. Rarely, we will reach our maximum allowable fluid absorption limit prior to completing the case, a scenario seen in less than 1% of our patients.
In more than 330 reoperative hysteroscopic procedures, we’ve had only one uterine perforation that occurred when we switched ultrasound machines. Very likely, we were too aggressive in removing tissue at the fundus. The patient required a diagnostic laparoscopy but sustained no visceral injury.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and the director of the Center for Menstrual Disorders and Reproductive Choice in Rochester. He reported having no relevant financial disclosures.
One in seven women suffer with abnormal uterine bleeding during their reproductive years, according to Fraser et al. (Exp Rev Obstet Gynecol. 2009;4:179-89). Heavy menstrual bleeding (menorrhagia) is the most common pattern. Global endometrial ablation has become a very popular surgical technique for women complaining of menorrhagia, disinterested in either medical management or definitive therapy – hysterectomy – or where medical management has failed. With proper patient selection, endometrial ablation yields an 80%-90% success rate in reducing heavy menstrual flow and is associated with a 90% patient satisfaction rate (Cochrane Database Syst Rev. 2009 Oct 7;[4]:CD001501).
Literature is replete with conditions believed to increase risk of endometrial ablation failure. This list includes untreated uterine cornua, endometrial regrowth, the presence of submucous leiomyomas or polyps, abnormal uterine cavity, enlarged uterine cavity (width and/or length), endometrial ablation in a young patient, parity of five or greater, unsuspected adhesiolysis, postablation tubal sterilization syndrome, history of dysmenorrhea, smoking, obesity, prior cesarean section, previous gynecologic surgery, and procedure length. Interestingly, type of global endometrial ablation procedure or original bleeding pattern does not influence failure rate.
In this edition of the Master Class in Gynecologic Surgery, Dr. Morris Wortman discusses not only the prevention of endometrial ablation failure, but also how to treat the problem via conservative surgical management.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and is the director at the Center for Menstrual Disorders and Reproductive Choice, also in Rochester. Dr. Wortman has lectured extensively on endometrial ablation and has authored several scientific articles in peer reviewed journals.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported being a subinvestigator on a study sponsored by Channel Medsystems. Email him at obnews@frontlinemedcom.com.
Why failures occur and how to correct them
BY MORRIS WORTMAN, MD
Since the introduction almost 20 years ago of devices for nonresectoscopic – or “global” – endometrial ablation, the procedure has been widely adopted as the treatment of choice for abnormal uterine bleeding that is refractory to medical management.
Between 400,000 and 500,000 endometrial ablations are done in the United States every year in women who have completed childbearing, and it probably won’t be long before the procedure surpasses hysterectomy in prevalence for the management of abnormal bleeding.
In recent years, the literature has begun to address the incidence of these delayed complications and the requirement for subsequent hysterectomy. A 2007 practice bulletin issued by the American College of Obstetricians and Gynecologists stated that hysterectomy rates within 4 years of endometrial ablation are at least 24% (Obstet Gynecol. 2007 May;109[5]:1233-48). And a study published the following year reported that 26% of 3,681 women undergoing EA at Kaiser Permanente facilities in Northern California required hysterectomy within 8 years (Obstet Gynecol. 2008 Dec;112[6]:1214-20).
It appears that the vast majority of what we now refer to as late-onset EA failures – complications attributable to EA that occur beyond a perioperative period of 1 month – will occur within 5 years. Some EA failures have occurred over 5-10 years, however, and in my practice we have seen late-onset complications occurring 17 or more years after the initial ablation.
In our practice, we are successfully managing delayed complications after GEA using ultrasound-guided reoperative hysteroscopy to fully explore the uterine cavity and excise areas of endometrial growth and other disease. In 2014, we published a retrospective review of 50 women whom we treated for delayed complications after a variety of GEA techniques; almost 90% avoided hysterectomy during a mean follow-up period of 18 months (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:238-44).
Our experience since then has included reoperative surgery on more than 115 GEA failures. Additionally, we’ve managed 220 patients who have undergone various hysteroscopic and resectoscopic endometrial ablations, some of which date back to the use of the Nd:YAG laser in the late 1980s.
The fact that late-onset EA failures occur does not mean that hysterectomy should routinely be performed as a first-line treatment for intractable uterine bleeding. Overall, there is much more morbidity associated with hysterectomy than with EA.
What failures do suggest is that there are certain risk factors for late-onset EA complications. Our experience in treating women who have experienced late-onset EA failure has provided us with insight into who may be at greatest risk for late-onset EA failure and how patients can best be selected for the procedure. We’ve also learned more about the diagnosis of delayed complications.
Causes of EA failure
Untreated uterine cornua, and untreated submucous leiomyomas and endometrial polyps, are common causes of EA failure. Among the 50 women included in our retrospective review of ultrasound-guided reoperative hysteroscopy after GEA failure, 44% had intraoperative evidence of untreated cornua and nearly one-fourth had persistent or enlarging submucous leiomyomas.
Contrary to what some believe, most endometrial ablations will not adequately destroy submucous or intramural leiomyomas. Therefore, we recommend that these fibroids be entirely removed immediately before EA.
Moreover, GEA will not always provide adequate thermal destruction to the entire endometrial cavity. The cornua regions are particularly at risk; they are difficult to reach under ideal circumstances, and especially difficult to treat in patients who have a uterine septum or a T-shaped uterus (with the ostia and cornua deeply recessed). We have also seen late-onset EA failures in patients with an extended uterine transverse diameter. The limits of GEA are greatest when a device with a fixed configuration or geometry is used.
A history of abnormal hysteroscopy or other evidence of such anatomic distortions are therefore among the reported risk factors for GEA failure (J Minim Invasive Gynecol. 2015 Mar-Apr;22[3]:323-31). A history of tubal ligation also confers risk; the procedure further increases susceptibility for failure when functioning endometrial tissue remains or regrows at the cornua, because any retrograde menstrual bleeding that occurs will be constrained by the obstructed proximal portion of the fallopian tubes.
Obesity is another risk factor for GEA failure in that the condition increases the risk of endometrial cancer, making the need for reliable biopsies in the case of spotting or other signs or symptoms even more important. On the other hand, obesity may also worsen a patient’s status as a candidate for hysterectomy.
There is much to consider with these patients. For some obese patients, GEA may be less risky than hysterectomy while for others, such as those who also have polycystic ovarian syndrome (in whom the risk for developing endometrial cancer is further increased) the scale may tip in favor of hysterectomy.
Age at the time of the primary GEA may be the single most important risk factor for GEA failure and is an important predictor of success in patient selection. Numerous investigators have shown that women younger than 35 years of age at the time of their EA had a significantly increased risk for hysterectomy, compared with women who were at least 45 years old. The younger the patient, the longer the “bridge” to menopause and the greater the likelihood that bridge will fail.
While age is not necessarily a contraindication, it is worthy of serious consideration. We generally discourage GEA for patients younger than 35. We also advise ensuring that each patient undergoing initial EA is highly self-motivated to have a uterine-sparing procedure; if not, symptoms she may experience later will likely drive her toward hysterectomy anyway.
Additionally, we caution against performing GEA in patients who have chronic pelvic pain; these patients tend to have poorer outcomes with any type of hysteroscopic surgery.
Diagnosing failed EA
Delayed complications manifest in several ways: Renewed and increasing vaginal bleeding after a period of improvement, cyclic pelvic pain (unilateral, bilateral, or suprapubic), or both bleeding and pain. Some women – likely an underreported number of them – present with postmenopausal bleeding and proceed to have unsuccessful attempts at an endometrial biopsy due to EA-associated endometrial scarring.
The cyclic pelvic pain associated with endometrial persistence or regrowth tends to worsen over time and is often described as sharp or laborlike. In our experience, a description of “laborlike” pain and a history of EA is almost fully predictive of a finding of endometrial growth. Often a hematometra can be demonstrated on transvaginal ultrasound, but this isn’t always the case.
Pain typically precedes bleeding in patients who demonstrate both. In such cases, blood from functioning endometrial tissue or other sources becomes blocked from exiting the uterine cavity by EA-induced intrauterine scarring and contracture. Painful uterine contractions then aim to expel the pooled blood. In other cases of pain – mainly those without significant vaginal bleeding – the pain is often attributed to cornual and central hematometra.
For the majority of EA failures, the diagnosis lies in the history and current symptoms. Unfortunately, the traditional methods of assessing the endometrial cavity have little merit for women presenting with delayed-onset EA complications. A sonographically assisted pelvic examination can be useful in evaluating complications, but the interpretation of ultrasounds in women with a prior EA can be challenging and is often beyond the training of most radiologists and gynecologists.
It is not uncommon for images to be incorrectly interpreted in the emergency department or physicians’ offices as “normal” and for such readings to set off a chain of CT scans, MRIs, laparoscopies, ovarian cystectomies, and other procedures that miss the root causes of pain.
Unfortunately, there is little in the literature that describes and defines ultrasound findings after EA. We do know that sonography should be timed with episodes of pain, and that the absence of a demonstrable hematometra does not exclude a diagnosis of EA failure.
Correcting late-onset failures
Our office-based operating room is fitted with side-by-side monitors that enable simultaneous sonographic and hysteroscopic views for correction of GEA failures; the rest of the set-up is similar to that of other operative hysteroscopies. However, we do employ a wide variety of resectoscopes with diameters ranging from 13 to 28 Fr. The smaller-diameter scopes are particularly useful for evaluating postmenopausal bleeding in women with a prior EA.
For those inexperienced with ultrasound-guided surgery, the initial resection is often the most challenging. The initial tissue removal is carried out on the thickest observed uterine wall – usually the posterior or anterior wall – and is done with near complete reliance on the ultrasound image. Hysteroscopic visualization is poor at this time because the outflow ports of the continuous flow resectoscope are obstructed by tissue in the narrow tubular cavity.
We then actually remove the resectoscope and clean the outflow ports of clots and debris that may have accumulated. When the scope is reinserted, there is typically sufficient room in the uterine cavity for continuous flow and excellent hysteroscopic visualization.
The sequence of resection from this point on will vary. If we’ve begun on the anterior wall, we’ll move to the posterior and then the two lateral walls to further restore the cavity. Areas of endometrial regrowth will typically be identified at this point and resected. The dissection then will extend upward, usually to within 10 mm of the fundus in the midline as measured by ultrasound. Reconfiguring the loop electrode to a 135- to 160-degree angle can be helpful in the delicate dissection that is required at the fundus.
Once all areas of endometrium have been identified and excised, we will deeply coagulate exposed myometrium with a ball-end electrode. Rarely, we will reach our maximum allowable fluid absorption limit prior to completing the case, a scenario seen in less than 1% of our patients.
In more than 330 reoperative hysteroscopic procedures, we’ve had only one uterine perforation that occurred when we switched ultrasound machines. Very likely, we were too aggressive in removing tissue at the fundus. The patient required a diagnostic laparoscopy but sustained no visceral injury.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and the director of the Center for Menstrual Disorders and Reproductive Choice in Rochester. He reported having no relevant financial disclosures.
One in seven women suffer with abnormal uterine bleeding during their reproductive years, according to Fraser et al. (Exp Rev Obstet Gynecol. 2009;4:179-89). Heavy menstrual bleeding (menorrhagia) is the most common pattern. Global endometrial ablation has become a very popular surgical technique for women complaining of menorrhagia, disinterested in either medical management or definitive therapy – hysterectomy – or where medical management has failed. With proper patient selection, endometrial ablation yields an 80%-90% success rate in reducing heavy menstrual flow and is associated with a 90% patient satisfaction rate (Cochrane Database Syst Rev. 2009 Oct 7;[4]:CD001501).
Literature is replete with conditions believed to increase risk of endometrial ablation failure. This list includes untreated uterine cornua, endometrial regrowth, the presence of submucous leiomyomas or polyps, abnormal uterine cavity, enlarged uterine cavity (width and/or length), endometrial ablation in a young patient, parity of five or greater, unsuspected adhesiolysis, postablation tubal sterilization syndrome, history of dysmenorrhea, smoking, obesity, prior cesarean section, previous gynecologic surgery, and procedure length. Interestingly, type of global endometrial ablation procedure or original bleeding pattern does not influence failure rate.
In this edition of the Master Class in Gynecologic Surgery, Dr. Morris Wortman discusses not only the prevention of endometrial ablation failure, but also how to treat the problem via conservative surgical management.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and is the director at the Center for Menstrual Disorders and Reproductive Choice, also in Rochester. Dr. Wortman has lectured extensively on endometrial ablation and has authored several scientific articles in peer reviewed journals.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported being a subinvestigator on a study sponsored by Channel Medsystems. Email him at obnews@frontlinemedcom.com.
Why failures occur and how to correct them
BY MORRIS WORTMAN, MD
Since the introduction almost 20 years ago of devices for nonresectoscopic – or “global” – endometrial ablation, the procedure has been widely adopted as the treatment of choice for abnormal uterine bleeding that is refractory to medical management.
Between 400,000 and 500,000 endometrial ablations are done in the United States every year in women who have completed childbearing, and it probably won’t be long before the procedure surpasses hysterectomy in prevalence for the management of abnormal bleeding.
In recent years, the literature has begun to address the incidence of these delayed complications and the requirement for subsequent hysterectomy. A 2007 practice bulletin issued by the American College of Obstetricians and Gynecologists stated that hysterectomy rates within 4 years of endometrial ablation are at least 24% (Obstet Gynecol. 2007 May;109[5]:1233-48). And a study published the following year reported that 26% of 3,681 women undergoing EA at Kaiser Permanente facilities in Northern California required hysterectomy within 8 years (Obstet Gynecol. 2008 Dec;112[6]:1214-20).
It appears that the vast majority of what we now refer to as late-onset EA failures – complications attributable to EA that occur beyond a perioperative period of 1 month – will occur within 5 years. Some EA failures have occurred over 5-10 years, however, and in my practice we have seen late-onset complications occurring 17 or more years after the initial ablation.
In our practice, we are successfully managing delayed complications after GEA using ultrasound-guided reoperative hysteroscopy to fully explore the uterine cavity and excise areas of endometrial growth and other disease. In 2014, we published a retrospective review of 50 women whom we treated for delayed complications after a variety of GEA techniques; almost 90% avoided hysterectomy during a mean follow-up period of 18 months (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:238-44).
Our experience since then has included reoperative surgery on more than 115 GEA failures. Additionally, we’ve managed 220 patients who have undergone various hysteroscopic and resectoscopic endometrial ablations, some of which date back to the use of the Nd:YAG laser in the late 1980s.
The fact that late-onset EA failures occur does not mean that hysterectomy should routinely be performed as a first-line treatment for intractable uterine bleeding. Overall, there is much more morbidity associated with hysterectomy than with EA.
What failures do suggest is that there are certain risk factors for late-onset EA complications. Our experience in treating women who have experienced late-onset EA failure has provided us with insight into who may be at greatest risk for late-onset EA failure and how patients can best be selected for the procedure. We’ve also learned more about the diagnosis of delayed complications.
Causes of EA failure
Untreated uterine cornua, and untreated submucous leiomyomas and endometrial polyps, are common causes of EA failure. Among the 50 women included in our retrospective review of ultrasound-guided reoperative hysteroscopy after GEA failure, 44% had intraoperative evidence of untreated cornua and nearly one-fourth had persistent or enlarging submucous leiomyomas.
Contrary to what some believe, most endometrial ablations will not adequately destroy submucous or intramural leiomyomas. Therefore, we recommend that these fibroids be entirely removed immediately before EA.
Moreover, GEA will not always provide adequate thermal destruction to the entire endometrial cavity. The cornua regions are particularly at risk; they are difficult to reach under ideal circumstances, and especially difficult to treat in patients who have a uterine septum or a T-shaped uterus (with the ostia and cornua deeply recessed). We have also seen late-onset EA failures in patients with an extended uterine transverse diameter. The limits of GEA are greatest when a device with a fixed configuration or geometry is used.
A history of abnormal hysteroscopy or other evidence of such anatomic distortions are therefore among the reported risk factors for GEA failure (J Minim Invasive Gynecol. 2015 Mar-Apr;22[3]:323-31). A history of tubal ligation also confers risk; the procedure further increases susceptibility for failure when functioning endometrial tissue remains or regrows at the cornua, because any retrograde menstrual bleeding that occurs will be constrained by the obstructed proximal portion of the fallopian tubes.
Obesity is another risk factor for GEA failure in that the condition increases the risk of endometrial cancer, making the need for reliable biopsies in the case of spotting or other signs or symptoms even more important. On the other hand, obesity may also worsen a patient’s status as a candidate for hysterectomy.
There is much to consider with these patients. For some obese patients, GEA may be less risky than hysterectomy while for others, such as those who also have polycystic ovarian syndrome (in whom the risk for developing endometrial cancer is further increased) the scale may tip in favor of hysterectomy.
Age at the time of the primary GEA may be the single most important risk factor for GEA failure and is an important predictor of success in patient selection. Numerous investigators have shown that women younger than 35 years of age at the time of their EA had a significantly increased risk for hysterectomy, compared with women who were at least 45 years old. The younger the patient, the longer the “bridge” to menopause and the greater the likelihood that bridge will fail.
While age is not necessarily a contraindication, it is worthy of serious consideration. We generally discourage GEA for patients younger than 35. We also advise ensuring that each patient undergoing initial EA is highly self-motivated to have a uterine-sparing procedure; if not, symptoms she may experience later will likely drive her toward hysterectomy anyway.
Additionally, we caution against performing GEA in patients who have chronic pelvic pain; these patients tend to have poorer outcomes with any type of hysteroscopic surgery.
Diagnosing failed EA
Delayed complications manifest in several ways: Renewed and increasing vaginal bleeding after a period of improvement, cyclic pelvic pain (unilateral, bilateral, or suprapubic), or both bleeding and pain. Some women – likely an underreported number of them – present with postmenopausal bleeding and proceed to have unsuccessful attempts at an endometrial biopsy due to EA-associated endometrial scarring.
The cyclic pelvic pain associated with endometrial persistence or regrowth tends to worsen over time and is often described as sharp or laborlike. In our experience, a description of “laborlike” pain and a history of EA is almost fully predictive of a finding of endometrial growth. Often a hematometra can be demonstrated on transvaginal ultrasound, but this isn’t always the case.
Pain typically precedes bleeding in patients who demonstrate both. In such cases, blood from functioning endometrial tissue or other sources becomes blocked from exiting the uterine cavity by EA-induced intrauterine scarring and contracture. Painful uterine contractions then aim to expel the pooled blood. In other cases of pain – mainly those without significant vaginal bleeding – the pain is often attributed to cornual and central hematometra.
For the majority of EA failures, the diagnosis lies in the history and current symptoms. Unfortunately, the traditional methods of assessing the endometrial cavity have little merit for women presenting with delayed-onset EA complications. A sonographically assisted pelvic examination can be useful in evaluating complications, but the interpretation of ultrasounds in women with a prior EA can be challenging and is often beyond the training of most radiologists and gynecologists.
It is not uncommon for images to be incorrectly interpreted in the emergency department or physicians’ offices as “normal” and for such readings to set off a chain of CT scans, MRIs, laparoscopies, ovarian cystectomies, and other procedures that miss the root causes of pain.
Unfortunately, there is little in the literature that describes and defines ultrasound findings after EA. We do know that sonography should be timed with episodes of pain, and that the absence of a demonstrable hematometra does not exclude a diagnosis of EA failure.
Correcting late-onset failures
Our office-based operating room is fitted with side-by-side monitors that enable simultaneous sonographic and hysteroscopic views for correction of GEA failures; the rest of the set-up is similar to that of other operative hysteroscopies. However, we do employ a wide variety of resectoscopes with diameters ranging from 13 to 28 Fr. The smaller-diameter scopes are particularly useful for evaluating postmenopausal bleeding in women with a prior EA.
For those inexperienced with ultrasound-guided surgery, the initial resection is often the most challenging. The initial tissue removal is carried out on the thickest observed uterine wall – usually the posterior or anterior wall – and is done with near complete reliance on the ultrasound image. Hysteroscopic visualization is poor at this time because the outflow ports of the continuous flow resectoscope are obstructed by tissue in the narrow tubular cavity.
We then actually remove the resectoscope and clean the outflow ports of clots and debris that may have accumulated. When the scope is reinserted, there is typically sufficient room in the uterine cavity for continuous flow and excellent hysteroscopic visualization.
The sequence of resection from this point on will vary. If we’ve begun on the anterior wall, we’ll move to the posterior and then the two lateral walls to further restore the cavity. Areas of endometrial regrowth will typically be identified at this point and resected. The dissection then will extend upward, usually to within 10 mm of the fundus in the midline as measured by ultrasound. Reconfiguring the loop electrode to a 135- to 160-degree angle can be helpful in the delicate dissection that is required at the fundus.
Once all areas of endometrium have been identified and excised, we will deeply coagulate exposed myometrium with a ball-end electrode. Rarely, we will reach our maximum allowable fluid absorption limit prior to completing the case, a scenario seen in less than 1% of our patients.
In more than 330 reoperative hysteroscopic procedures, we’ve had only one uterine perforation that occurred when we switched ultrasound machines. Very likely, we were too aggressive in removing tissue at the fundus. The patient required a diagnostic laparoscopy but sustained no visceral injury.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and the director of the Center for Menstrual Disorders and Reproductive Choice in Rochester. He reported having no relevant financial disclosures.
Preventing surgical site infections in hysterectomy
Surgical site infections are a major source of patient morbidity. They are also an important quality metric for surgeons and hospital systems, and are increasingly being linked to reimbursement.
They occur in approximately 2% of the 600,000 women undergoing hysterectomy in the United States each year. The U.S. Centers for Disease Control and Prevention defines surgical site infection (SSI) as an infection that occurs within 30 days of a procedure in the part of the body where the surgery took place. Most SSIs are superficial incisional, but they also include deep incisional or organ or space infections.
Classification
The incidence of SSI varies according to the classification of the wound, as defined by the National Academy of Sciences.1 Most hysterectomies are classified as clean-contaminated wounds because they involve entry into the mucosa of the genitourinary tract. However, hysterectomy with contamination of bowel flora, or in the setting of acute infection (such as suppurative pelvic inflammatory disease) are considered a contaminated wound class, and are associated with even higher rates of SSI.
Risk factors
The risk factors associated with SSI are both modifiable and unmodifiable. Broadly speaking, they include increased risk to endogenous flora (e.g., wound classification), increased exposure to exogenous flora (e.g., inadequate protection of a wound from external pathogens), and impairment of the body’s immune mechanisms to prevent and overcome infection (e.g., hypothermia and hypoglycemia).
Unmodifiable risk factors include increasing age, a history of radiation exposure, vascular disease, and a history of prior SSIs. Modifiable risk factors include obesity, tobacco use, immunosuppressive medications, hypoalbuminemia, route of hysterectomy, hair removal, preoperative infections (such as bacterial vaginosis), surgical scrub, skin and vaginal preparation, antimicrobial prophylaxis (inappropriate choice or timing, inadequate dosing or redosing), operative time, blood transfusion, surgical skill, and operating room characteristics (ventilation, increased OR traffic, and sterilization of surgical equipment).
Antimicrobial prophylaxis
The CDC and the American College of Obstetricians and Gynecologists (ACOG) have provided clear guidelines regarding methods to reduce SSI in hysterectomy.3,4 There is strong evidence for using antimicrobial prophylaxis for hysterectomy.
It is important that physicians confirm the validity of beta-lactam allergies with patients because there are higher rates of SSI with the use of non–beta-lactam regimens, even those endorsed by the CDC and ACOG.5
Antibiotics should be administered within 1 hour of skin incision, and ideally within 30 minutes. They should be discontinued within 24 hours. Dosing should be adjusted to weight, and antimicrobials should be redosed for long procedures (at intervals of two half-lives), and for increased blood loss.
Skin preparation
Hair removal should be avoided unless necessary for technical reasons. If it is required, it should be performed outside of the operative space using clippers, not razors. For patients colonized with methicillin-resistant S. aureus, there is supporting evidence for pretreatment with mupirocin ointment to the nares, and chlorhexidine showers for 5-10 days. Patients who have bacterial vaginosis should be treated before surgery to decrease the rate of vaginal cuff SSI.
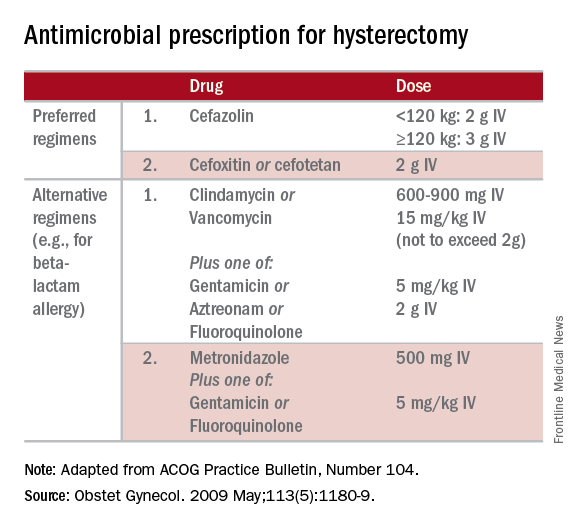
If there is a planned or potential gastrointestinal procedure as part of the hysterectomy, the surgeon should consider using an impervious plastic wound protector in place of, or in addition to, other retractors. Preoperative oral antimicrobials with mechanical bowel preparation have been associated with decreased SSIs; however, this benefit is not observed with mechanical bowel preparation alone.
Wound closure
Surgical technique and wound closure techniques also impact SSI. Minimally invasive and vaginal hysterectomy routes are preferred, as these are associated with the lowest rates of SSI. Antimicrobial-impregnated suture materials appear to be unnecessary. Surgeons should ensure that there is delicate handling of tissues and closure of dead spaces. If the subcutaneous fat space depth measures more than 2.5 cm, it should be reapproximated with a rapidly-absorbing suture material.
Use of electrosurgery versus a scalpel when creating the incision does not appear to influence infection rates, nor does use of staples versus subcuticular suture during closure.7
Using a dilute iodine lavage in the subcutaneous space, opening a sterile closing tray, and having surgeons change gloves prior to skin closure should be considered. The CDC recommends keeping the skin dressing in place for 24 hours postoperatively.
Other strategies
Hyperglycemia is associated with impaired neutrophil response, and therefore blood glucose should be controlled before surgery (hemoglobin A1c levels of less than 7% preoperatively) and immediately postoperatively (less than 180 mg/dL within 18-24 hours after the end of anesthesia).
It is also important to minimize perioperative hypothermia (less than 35.5° F), as this also impairs the body’s immune response. Keeping operative room ambient temperatures higher, minimizing incision size, warming CO2 gas in minimally invasive procedures, warming fluids, and using extrinsic body warmers can help achieve this.
Excessive blood loss should be minimized because blood transfusion is associated with impaired macrophage function and increased risk for SSI.
In addition to teamwide (including nonsurgeon) strict adherence to hand hygiene, OR personnel should avoid unnecessary operating room traffic. Hospital officials should ensure that the facility’s ventilator systems are well maintained and that there is care and maintenance of air handlers.
Many strategies can be employed perioperatively to decrease SSI rates for hysterectomy. We advocate for a protocol-based approach (known as “bundling” strategies) to achieve consistency of practice and to maximize surgeon and institutional improvements in SSI rates. This is similar to the approach outlined in a recent consensus statement from the Council on Patient Safety in Women’s Health Care.8
A comprehensive multidisciplinary approach throughout the perioperative period is necessary. It is imperative that good communication exist with patients regarding SSIs after hysterectomy and how patients, surgeons, and hospitals can together minimize the risks of SSIs.
References
1. Altemeier WA. “Manual on Control of Infection in Surgical Patients” (Philadelphia: Lippincott Williams & Wilkins, 1984).
2. Rev Infect Dis. 1991 Sep-Oct;13(Suppl 10):S821-41.
3. Infect Control Hosp Epidemiol. 2014 Jun;35(6):605-27.
4. Obstet Gynecol. 2009 May;113(5):1180-9.
5. Obstet Gynecol. 2016 Feb;127(2):321-9.
6. Am J Obstet Gynecol. 2005 Feb;192(2):422-5.
7. J Gastrointest Surg. 2016 Dec;20(12):2083-92.
8. Obstet Gynecol. 2016 Dec 7. doi: 10.1097/AOG.0000000000001751.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Jackson-Moore is an associate professor in gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Surgical site infections are a major source of patient morbidity. They are also an important quality metric for surgeons and hospital systems, and are increasingly being linked to reimbursement.
They occur in approximately 2% of the 600,000 women undergoing hysterectomy in the United States each year. The U.S. Centers for Disease Control and Prevention defines surgical site infection (SSI) as an infection that occurs within 30 days of a procedure in the part of the body where the surgery took place. Most SSIs are superficial incisional, but they also include deep incisional or organ or space infections.
Classification
The incidence of SSI varies according to the classification of the wound, as defined by the National Academy of Sciences.1 Most hysterectomies are classified as clean-contaminated wounds because they involve entry into the mucosa of the genitourinary tract. However, hysterectomy with contamination of bowel flora, or in the setting of acute infection (such as suppurative pelvic inflammatory disease) are considered a contaminated wound class, and are associated with even higher rates of SSI.
Risk factors
The risk factors associated with SSI are both modifiable and unmodifiable. Broadly speaking, they include increased risk to endogenous flora (e.g., wound classification), increased exposure to exogenous flora (e.g., inadequate protection of a wound from external pathogens), and impairment of the body’s immune mechanisms to prevent and overcome infection (e.g., hypothermia and hypoglycemia).
Unmodifiable risk factors include increasing age, a history of radiation exposure, vascular disease, and a history of prior SSIs. Modifiable risk factors include obesity, tobacco use, immunosuppressive medications, hypoalbuminemia, route of hysterectomy, hair removal, preoperative infections (such as bacterial vaginosis), surgical scrub, skin and vaginal preparation, antimicrobial prophylaxis (inappropriate choice or timing, inadequate dosing or redosing), operative time, blood transfusion, surgical skill, and operating room characteristics (ventilation, increased OR traffic, and sterilization of surgical equipment).
Antimicrobial prophylaxis
The CDC and the American College of Obstetricians and Gynecologists (ACOG) have provided clear guidelines regarding methods to reduce SSI in hysterectomy.3,4 There is strong evidence for using antimicrobial prophylaxis for hysterectomy.
It is important that physicians confirm the validity of beta-lactam allergies with patients because there are higher rates of SSI with the use of non–beta-lactam regimens, even those endorsed by the CDC and ACOG.5
Antibiotics should be administered within 1 hour of skin incision, and ideally within 30 minutes. They should be discontinued within 24 hours. Dosing should be adjusted to weight, and antimicrobials should be redosed for long procedures (at intervals of two half-lives), and for increased blood loss.
Skin preparation
Hair removal should be avoided unless necessary for technical reasons. If it is required, it should be performed outside of the operative space using clippers, not razors. For patients colonized with methicillin-resistant S. aureus, there is supporting evidence for pretreatment with mupirocin ointment to the nares, and chlorhexidine showers for 5-10 days. Patients who have bacterial vaginosis should be treated before surgery to decrease the rate of vaginal cuff SSI.

If there is a planned or potential gastrointestinal procedure as part of the hysterectomy, the surgeon should consider using an impervious plastic wound protector in place of, or in addition to, other retractors. Preoperative oral antimicrobials with mechanical bowel preparation have been associated with decreased SSIs; however, this benefit is not observed with mechanical bowel preparation alone.
Wound closure
Surgical technique and wound closure techniques also impact SSI. Minimally invasive and vaginal hysterectomy routes are preferred, as these are associated with the lowest rates of SSI. Antimicrobial-impregnated suture materials appear to be unnecessary. Surgeons should ensure that there is delicate handling of tissues and closure of dead spaces. If the subcutaneous fat space depth measures more than 2.5 cm, it should be reapproximated with a rapidly-absorbing suture material.
Use of electrosurgery versus a scalpel when creating the incision does not appear to influence infection rates, nor does use of staples versus subcuticular suture during closure.7
Using a dilute iodine lavage in the subcutaneous space, opening a sterile closing tray, and having surgeons change gloves prior to skin closure should be considered. The CDC recommends keeping the skin dressing in place for 24 hours postoperatively.
Other strategies
Hyperglycemia is associated with impaired neutrophil response, and therefore blood glucose should be controlled before surgery (hemoglobin A1c levels of less than 7% preoperatively) and immediately postoperatively (less than 180 mg/dL within 18-24 hours after the end of anesthesia).
It is also important to minimize perioperative hypothermia (less than 35.5° F), as this also impairs the body’s immune response. Keeping operative room ambient temperatures higher, minimizing incision size, warming CO2 gas in minimally invasive procedures, warming fluids, and using extrinsic body warmers can help achieve this.
Excessive blood loss should be minimized because blood transfusion is associated with impaired macrophage function and increased risk for SSI.
In addition to teamwide (including nonsurgeon) strict adherence to hand hygiene, OR personnel should avoid unnecessary operating room traffic. Hospital officials should ensure that the facility’s ventilator systems are well maintained and that there is care and maintenance of air handlers.
Many strategies can be employed perioperatively to decrease SSI rates for hysterectomy. We advocate for a protocol-based approach (known as “bundling” strategies) to achieve consistency of practice and to maximize surgeon and institutional improvements in SSI rates. This is similar to the approach outlined in a recent consensus statement from the Council on Patient Safety in Women’s Health Care.8
A comprehensive multidisciplinary approach throughout the perioperative period is necessary. It is imperative that good communication exist with patients regarding SSIs after hysterectomy and how patients, surgeons, and hospitals can together minimize the risks of SSIs.
References
1. Altemeier WA. “Manual on Control of Infection in Surgical Patients” (Philadelphia: Lippincott Williams & Wilkins, 1984).
2. Rev Infect Dis. 1991 Sep-Oct;13(Suppl 10):S821-41.
3. Infect Control Hosp Epidemiol. 2014 Jun;35(6):605-27.
4. Obstet Gynecol. 2009 May;113(5):1180-9.
5. Obstet Gynecol. 2016 Feb;127(2):321-9.
6. Am J Obstet Gynecol. 2005 Feb;192(2):422-5.
7. J Gastrointest Surg. 2016 Dec;20(12):2083-92.
8. Obstet Gynecol. 2016 Dec 7. doi: 10.1097/AOG.0000000000001751.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Jackson-Moore is an associate professor in gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Surgical site infections are a major source of patient morbidity. They are also an important quality metric for surgeons and hospital systems, and are increasingly being linked to reimbursement.
They occur in approximately 2% of the 600,000 women undergoing hysterectomy in the United States each year. The U.S. Centers for Disease Control and Prevention defines surgical site infection (SSI) as an infection that occurs within 30 days of a procedure in the part of the body where the surgery took place. Most SSIs are superficial incisional, but they also include deep incisional or organ or space infections.
Classification
The incidence of SSI varies according to the classification of the wound, as defined by the National Academy of Sciences.1 Most hysterectomies are classified as clean-contaminated wounds because they involve entry into the mucosa of the genitourinary tract. However, hysterectomy with contamination of bowel flora, or in the setting of acute infection (such as suppurative pelvic inflammatory disease) are considered a contaminated wound class, and are associated with even higher rates of SSI.
Risk factors
The risk factors associated with SSI are both modifiable and unmodifiable. Broadly speaking, they include increased risk to endogenous flora (e.g., wound classification), increased exposure to exogenous flora (e.g., inadequate protection of a wound from external pathogens), and impairment of the body’s immune mechanisms to prevent and overcome infection (e.g., hypothermia and hypoglycemia).
Unmodifiable risk factors include increasing age, a history of radiation exposure, vascular disease, and a history of prior SSIs. Modifiable risk factors include obesity, tobacco use, immunosuppressive medications, hypoalbuminemia, route of hysterectomy, hair removal, preoperative infections (such as bacterial vaginosis), surgical scrub, skin and vaginal preparation, antimicrobial prophylaxis (inappropriate choice or timing, inadequate dosing or redosing), operative time, blood transfusion, surgical skill, and operating room characteristics (ventilation, increased OR traffic, and sterilization of surgical equipment).
Antimicrobial prophylaxis
The CDC and the American College of Obstetricians and Gynecologists (ACOG) have provided clear guidelines regarding methods to reduce SSI in hysterectomy.3,4 There is strong evidence for using antimicrobial prophylaxis for hysterectomy.
It is important that physicians confirm the validity of beta-lactam allergies with patients because there are higher rates of SSI with the use of non–beta-lactam regimens, even those endorsed by the CDC and ACOG.5
Antibiotics should be administered within 1 hour of skin incision, and ideally within 30 minutes. They should be discontinued within 24 hours. Dosing should be adjusted to weight, and antimicrobials should be redosed for long procedures (at intervals of two half-lives), and for increased blood loss.
Skin preparation
Hair removal should be avoided unless necessary for technical reasons. If it is required, it should be performed outside of the operative space using clippers, not razors. For patients colonized with methicillin-resistant S. aureus, there is supporting evidence for pretreatment with mupirocin ointment to the nares, and chlorhexidine showers for 5-10 days. Patients who have bacterial vaginosis should be treated before surgery to decrease the rate of vaginal cuff SSI.

If there is a planned or potential gastrointestinal procedure as part of the hysterectomy, the surgeon should consider using an impervious plastic wound protector in place of, or in addition to, other retractors. Preoperative oral antimicrobials with mechanical bowel preparation have been associated with decreased SSIs; however, this benefit is not observed with mechanical bowel preparation alone.
Wound closure
Surgical technique and wound closure techniques also impact SSI. Minimally invasive and vaginal hysterectomy routes are preferred, as these are associated with the lowest rates of SSI. Antimicrobial-impregnated suture materials appear to be unnecessary. Surgeons should ensure that there is delicate handling of tissues and closure of dead spaces. If the subcutaneous fat space depth measures more than 2.5 cm, it should be reapproximated with a rapidly-absorbing suture material.
Use of electrosurgery versus a scalpel when creating the incision does not appear to influence infection rates, nor does use of staples versus subcuticular suture during closure.7
Using a dilute iodine lavage in the subcutaneous space, opening a sterile closing tray, and having surgeons change gloves prior to skin closure should be considered. The CDC recommends keeping the skin dressing in place for 24 hours postoperatively.
Other strategies
Hyperglycemia is associated with impaired neutrophil response, and therefore blood glucose should be controlled before surgery (hemoglobin A1c levels of less than 7% preoperatively) and immediately postoperatively (less than 180 mg/dL within 18-24 hours after the end of anesthesia).
It is also important to minimize perioperative hypothermia (less than 35.5° F), as this also impairs the body’s immune response. Keeping operative room ambient temperatures higher, minimizing incision size, warming CO2 gas in minimally invasive procedures, warming fluids, and using extrinsic body warmers can help achieve this.
Excessive blood loss should be minimized because blood transfusion is associated with impaired macrophage function and increased risk for SSI.
In addition to teamwide (including nonsurgeon) strict adherence to hand hygiene, OR personnel should avoid unnecessary operating room traffic. Hospital officials should ensure that the facility’s ventilator systems are well maintained and that there is care and maintenance of air handlers.
Many strategies can be employed perioperatively to decrease SSI rates for hysterectomy. We advocate for a protocol-based approach (known as “bundling” strategies) to achieve consistency of practice and to maximize surgeon and institutional improvements in SSI rates. This is similar to the approach outlined in a recent consensus statement from the Council on Patient Safety in Women’s Health Care.8
A comprehensive multidisciplinary approach throughout the perioperative period is necessary. It is imperative that good communication exist with patients regarding SSIs after hysterectomy and how patients, surgeons, and hospitals can together minimize the risks of SSIs.
References
1. Altemeier WA. “Manual on Control of Infection in Surgical Patients” (Philadelphia: Lippincott Williams & Wilkins, 1984).
2. Rev Infect Dis. 1991 Sep-Oct;13(Suppl 10):S821-41.
3. Infect Control Hosp Epidemiol. 2014 Jun;35(6):605-27.
4. Obstet Gynecol. 2009 May;113(5):1180-9.
5. Obstet Gynecol. 2016 Feb;127(2):321-9.
6. Am J Obstet Gynecol. 2005 Feb;192(2):422-5.
7. J Gastrointest Surg. 2016 Dec;20(12):2083-92.
8. Obstet Gynecol. 2016 Dec 7. doi: 10.1097/AOG.0000000000001751.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Jackson-Moore is an associate professor in gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Robot-assisted laparoscopic resection of a noncommunicating cavitary rudimentary horn
A unicornuate uterus with a noncommunicating rudimentary horn is a rare mullerian duct anomaly (MDA). It often goes undiagnosed due to the absence of functional endometrium in the anomalous horn. However, when the rudimentary horn is lined with endometrium, obstructed menstrual flow can lead to severe cyclic pelvic pain, development of a pelvic mass, and endometriosis from retrograde menstruation. For these reasons, surgical resection is recommended for patients with this anomaly.
In this video the surgical patient is a 15-year-old adolescent with a 1-year history of progressive dysmenorrhea. Imaging studies revealed a noncommunicating cavitary right uterine horn and confirmed a normal urinary tract system.
We present a stepwise demonstration of our technique for surgical resection of a noncommunicating cavitary uterine horn and conclude that robotic surgery is a safe and feasible route for surgical management of this pathology.
I am pleased to bring you this video to kick off the New Year. We are delighted that our work won "Best Video on Robotic Technology" at the annual AAGL meeting in November 2016, and I hope that it is helpful to your practice.

Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
A unicornuate uterus with a noncommunicating rudimentary horn is a rare mullerian duct anomaly (MDA). It often goes undiagnosed due to the absence of functional endometrium in the anomalous horn. However, when the rudimentary horn is lined with endometrium, obstructed menstrual flow can lead to severe cyclic pelvic pain, development of a pelvic mass, and endometriosis from retrograde menstruation. For these reasons, surgical resection is recommended for patients with this anomaly.
In this video the surgical patient is a 15-year-old adolescent with a 1-year history of progressive dysmenorrhea. Imaging studies revealed a noncommunicating cavitary right uterine horn and confirmed a normal urinary tract system.
We present a stepwise demonstration of our technique for surgical resection of a noncommunicating cavitary uterine horn and conclude that robotic surgery is a safe and feasible route for surgical management of this pathology.
I am pleased to bring you this video to kick off the New Year. We are delighted that our work won "Best Video on Robotic Technology" at the annual AAGL meeting in November 2016, and I hope that it is helpful to your practice.

Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
A unicornuate uterus with a noncommunicating rudimentary horn is a rare mullerian duct anomaly (MDA). It often goes undiagnosed due to the absence of functional endometrium in the anomalous horn. However, when the rudimentary horn is lined with endometrium, obstructed menstrual flow can lead to severe cyclic pelvic pain, development of a pelvic mass, and endometriosis from retrograde menstruation. For these reasons, surgical resection is recommended for patients with this anomaly.
In this video the surgical patient is a 15-year-old adolescent with a 1-year history of progressive dysmenorrhea. Imaging studies revealed a noncommunicating cavitary right uterine horn and confirmed a normal urinary tract system.
We present a stepwise demonstration of our technique for surgical resection of a noncommunicating cavitary uterine horn and conclude that robotic surgery is a safe and feasible route for surgical management of this pathology.
I am pleased to bring you this video to kick off the New Year. We are delighted that our work won "Best Video on Robotic Technology" at the annual AAGL meeting in November 2016, and I hope that it is helpful to your practice.

Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Shorter-course antimicrobials do not reduce antimicrobial resistance in AOM
A shorter duration of antimicrobial therapy for acute otitis media (AOM) in children is associated with higher rates of clinical failure and higher symptom scores but without any associated reduction in the rates of antimicrobial resistance or adverse events.
Alejandro Hoberman, MD, of the Children’s Hospital of Pittsburgh at the University of Pittsburgh Medical Center, and his coauthors report the results of a study in which 520 children aged 6-23 months were randomized to either 10 days of amoxicillin-clavulanate therapy, or 5 days of therapy followed by 5 days of placebo.
The children who received the full 10-day course also had lower mean symptom scores in the 6-14 days after initiation of therapy, compared with those who received 5 days of treatment (1.34 vs. 1.61; P = 0.07), while the number of children whose symptom scores decreased by 50% from baseline to the end of treatment was significantly lower in the 5-day group.
However, there were no significant differences between the two groups in the rate of nasopharyngeal colonization pathogens not susceptible to penicillin: 47% in the 10-day group, compared with 44% in the 5-day group (N Engl J Med. 2016 Dec 22;375[25]:2446-56).
Similarly, the rates of recurrence and adverse events were not significantly different between the two groups.
A shorter duration of treatment has been considered as a strategy for reducing the risk of antimicrobial resistance, but clinical trials so far have showed either modest difference favoring the standard duration of treatment, or no difference at all.
“The outcome differences we found were larger than the differences that have been reported previously, mainly because the rates of clinical failure among children who received reduced duration treatment were higher in our trial than in previous trials,” Dr. Hoberman and his associates wrote.
The researchers did note that clinical failure rates were higher among children with greater exposure to other children, and those with infection in both ears rather than a single ear.
The study was supported by the National Institute of Allergy and Infectious Diseases and the National Institutes of Health. Dr. Hoberman and Dr. Judith M. Martin declared consulting fees from Genocea Biosciences, and Dr. Hoberman declared grant support from Ricoh Innovations and holding pending patents for a reduced clavulanate concentration version of amoxicillin–clavulanate potassium, and a method and device for aiding in the diagnosis of otitis media. No other conflicts of interest were declared.
The study of acute otitis media is challenging owing to antibiotic pharmacokinetics, age of the patients, variation in regional pathogens, polymicrobial infection, viral cofactors, antibiotic resistance, status of patients with regard to receipt of PCV7 or PCV13, and a high rate of spontaneous resolution.
The study was not designed to address outcomes in older children, children with less severe acute otitis media or with acute otitis media in one ear, or children with additional risk factors such as cleft palate or trisomy 21. In addition, there is a paucity of studies from resource-poor and low income countries.
But for now, 10 days of amoxicillin-clavulanate for children younger than 2 years of age who have a definite diagnosis of acute otitis media seems to be a reasonable option.
Margaret A. Kenna, MD, MPH, is from the department of otolaryngology and communication enhancement at the Boston Children’s Hospital. These comments are excerpted from an accompanying editorial (N Engl J Med. 2016 Dec 22;375[25]:2492-93). Dr. Kenna declared a grant from Agilis outside the submitted work.
The study of acute otitis media is challenging owing to antibiotic pharmacokinetics, age of the patients, variation in regional pathogens, polymicrobial infection, viral cofactors, antibiotic resistance, status of patients with regard to receipt of PCV7 or PCV13, and a high rate of spontaneous resolution.
The study was not designed to address outcomes in older children, children with less severe acute otitis media or with acute otitis media in one ear, or children with additional risk factors such as cleft palate or trisomy 21. In addition, there is a paucity of studies from resource-poor and low income countries.
But for now, 10 days of amoxicillin-clavulanate for children younger than 2 years of age who have a definite diagnosis of acute otitis media seems to be a reasonable option.
Margaret A. Kenna, MD, MPH, is from the department of otolaryngology and communication enhancement at the Boston Children’s Hospital. These comments are excerpted from an accompanying editorial (N Engl J Med. 2016 Dec 22;375[25]:2492-93). Dr. Kenna declared a grant from Agilis outside the submitted work.
The study of acute otitis media is challenging owing to antibiotic pharmacokinetics, age of the patients, variation in regional pathogens, polymicrobial infection, viral cofactors, antibiotic resistance, status of patients with regard to receipt of PCV7 or PCV13, and a high rate of spontaneous resolution.
The study was not designed to address outcomes in older children, children with less severe acute otitis media or with acute otitis media in one ear, or children with additional risk factors such as cleft palate or trisomy 21. In addition, there is a paucity of studies from resource-poor and low income countries.
But for now, 10 days of amoxicillin-clavulanate for children younger than 2 years of age who have a definite diagnosis of acute otitis media seems to be a reasonable option.
Margaret A. Kenna, MD, MPH, is from the department of otolaryngology and communication enhancement at the Boston Children’s Hospital. These comments are excerpted from an accompanying editorial (N Engl J Med. 2016 Dec 22;375[25]:2492-93). Dr. Kenna declared a grant from Agilis outside the submitted work.
A shorter duration of antimicrobial therapy for acute otitis media (AOM) in children is associated with higher rates of clinical failure and higher symptom scores but without any associated reduction in the rates of antimicrobial resistance or adverse events.
Alejandro Hoberman, MD, of the Children’s Hospital of Pittsburgh at the University of Pittsburgh Medical Center, and his coauthors report the results of a study in which 520 children aged 6-23 months were randomized to either 10 days of amoxicillin-clavulanate therapy, or 5 days of therapy followed by 5 days of placebo.
The children who received the full 10-day course also had lower mean symptom scores in the 6-14 days after initiation of therapy, compared with those who received 5 days of treatment (1.34 vs. 1.61; P = 0.07), while the number of children whose symptom scores decreased by 50% from baseline to the end of treatment was significantly lower in the 5-day group.
However, there were no significant differences between the two groups in the rate of nasopharyngeal colonization pathogens not susceptible to penicillin: 47% in the 10-day group, compared with 44% in the 5-day group (N Engl J Med. 2016 Dec 22;375[25]:2446-56).
Similarly, the rates of recurrence and adverse events were not significantly different between the two groups.
A shorter duration of treatment has been considered as a strategy for reducing the risk of antimicrobial resistance, but clinical trials so far have showed either modest difference favoring the standard duration of treatment, or no difference at all.
“The outcome differences we found were larger than the differences that have been reported previously, mainly because the rates of clinical failure among children who received reduced duration treatment were higher in our trial than in previous trials,” Dr. Hoberman and his associates wrote.
The researchers did note that clinical failure rates were higher among children with greater exposure to other children, and those with infection in both ears rather than a single ear.
The study was supported by the National Institute of Allergy and Infectious Diseases and the National Institutes of Health. Dr. Hoberman and Dr. Judith M. Martin declared consulting fees from Genocea Biosciences, and Dr. Hoberman declared grant support from Ricoh Innovations and holding pending patents for a reduced clavulanate concentration version of amoxicillin–clavulanate potassium, and a method and device for aiding in the diagnosis of otitis media. No other conflicts of interest were declared.
A shorter duration of antimicrobial therapy for acute otitis media (AOM) in children is associated with higher rates of clinical failure and higher symptom scores but without any associated reduction in the rates of antimicrobial resistance or adverse events.
Alejandro Hoberman, MD, of the Children’s Hospital of Pittsburgh at the University of Pittsburgh Medical Center, and his coauthors report the results of a study in which 520 children aged 6-23 months were randomized to either 10 days of amoxicillin-clavulanate therapy, or 5 days of therapy followed by 5 days of placebo.
The children who received the full 10-day course also had lower mean symptom scores in the 6-14 days after initiation of therapy, compared with those who received 5 days of treatment (1.34 vs. 1.61; P = 0.07), while the number of children whose symptom scores decreased by 50% from baseline to the end of treatment was significantly lower in the 5-day group.
However, there were no significant differences between the two groups in the rate of nasopharyngeal colonization pathogens not susceptible to penicillin: 47% in the 10-day group, compared with 44% in the 5-day group (N Engl J Med. 2016 Dec 22;375[25]:2446-56).
Similarly, the rates of recurrence and adverse events were not significantly different between the two groups.
A shorter duration of treatment has been considered as a strategy for reducing the risk of antimicrobial resistance, but clinical trials so far have showed either modest difference favoring the standard duration of treatment, or no difference at all.
“The outcome differences we found were larger than the differences that have been reported previously, mainly because the rates of clinical failure among children who received reduced duration treatment were higher in our trial than in previous trials,” Dr. Hoberman and his associates wrote.
The researchers did note that clinical failure rates were higher among children with greater exposure to other children, and those with infection in both ears rather than a single ear.
The study was supported by the National Institute of Allergy and Infectious Diseases and the National Institutes of Health. Dr. Hoberman and Dr. Judith M. Martin declared consulting fees from Genocea Biosciences, and Dr. Hoberman declared grant support from Ricoh Innovations and holding pending patents for a reduced clavulanate concentration version of amoxicillin–clavulanate potassium, and a method and device for aiding in the diagnosis of otitis media. No other conflicts of interest were declared.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: A shorter duration of antimicrobial therapy for acute otitis media (AOM) in children is associated with higher rates of clinical failure without a reduction in the rates of antimicrobial resistance.
Major finding: Children who received 5 days of antimicrobial experienced clinical failure rates of 35%, compared with 16% in those who received a full 10-day course.
Data source: Randomized controlled trial in 520 children aged 6-23 months with AOM.
Disclosures: The study was supported by the National Institute of Allergy and Infectious Diseases and the National Institutes of Health. Dr. Hoberman and Dr. Judith M. Martin declared consulting fees from Genocea Biosciences, and Dr. Hoberman declared grant support from Ricoh Innovations and holding pending patents for a reduced clavulanate concentration version of amoxicillin–clavulanate potassium, and a method and device for aiding in the diagnosis of otitis media. No other conflicts of interest were declared.
Tips and tricks for open laparoscopy

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist
This video is brought to you by
FDA warning: General anesthetics may damage young brains
The Food and Drug Administration has issued a warning that repeated or lengthy use of general anesthetic and sedation drugs during surgeries or procedures in children younger than 3 years or in pregnant women during their third trimester may affect the development of children’s brains.
“Recent human studies suggest that a single, relatively short exposure to general anesthetic and sedation drugs in infants or toddlers is unlikely to have negative effects on behavior or learning.” The studies suggesting a problem with longer or repeat exposures “had limitations, and it is unclear whether any negative effects seen in children’s learning or behavior were due to the drugs or to other factors, such as the underlying medical condition that led to the need for the surgery or procedure.” Further research is needed, the agency said.
FDA is adding its warning to the labels of 11 general anesthetics and sedatives, including desflurane, halothane, ketamine, lorazepam injection, methohexital, pentobarbital, and propofol. The drugs block N-methyl-D-aspartate (NMDA) receptors and/or potentiate gamma-aminobutyric acid (GABA) activity. No specific medications have been shown to be safer than any other, the agency said.
FDA will continue to monitor the situation, and update its warning as additional information comes in. “We urge health care professionals, patients, parents, and caregivers to report side effects involving anesthetic and sedation drugs or other medicines to the FDA MedWatch program,” the FDA said.
The Food and Drug Administration has issued a warning that repeated or lengthy use of general anesthetic and sedation drugs during surgeries or procedures in children younger than 3 years or in pregnant women during their third trimester may affect the development of children’s brains.
“Recent human studies suggest that a single, relatively short exposure to general anesthetic and sedation drugs in infants or toddlers is unlikely to have negative effects on behavior or learning.” The studies suggesting a problem with longer or repeat exposures “had limitations, and it is unclear whether any negative effects seen in children’s learning or behavior were due to the drugs or to other factors, such as the underlying medical condition that led to the need for the surgery or procedure.” Further research is needed, the agency said.
FDA is adding its warning to the labels of 11 general anesthetics and sedatives, including desflurane, halothane, ketamine, lorazepam injection, methohexital, pentobarbital, and propofol. The drugs block N-methyl-D-aspartate (NMDA) receptors and/or potentiate gamma-aminobutyric acid (GABA) activity. No specific medications have been shown to be safer than any other, the agency said.
FDA will continue to monitor the situation, and update its warning as additional information comes in. “We urge health care professionals, patients, parents, and caregivers to report side effects involving anesthetic and sedation drugs or other medicines to the FDA MedWatch program,” the FDA said.
The Food and Drug Administration has issued a warning that repeated or lengthy use of general anesthetic and sedation drugs during surgeries or procedures in children younger than 3 years or in pregnant women during their third trimester may affect the development of children’s brains.
“Recent human studies suggest that a single, relatively short exposure to general anesthetic and sedation drugs in infants or toddlers is unlikely to have negative effects on behavior or learning.” The studies suggesting a problem with longer or repeat exposures “had limitations, and it is unclear whether any negative effects seen in children’s learning or behavior were due to the drugs or to other factors, such as the underlying medical condition that led to the need for the surgery or procedure.” Further research is needed, the agency said.
FDA is adding its warning to the labels of 11 general anesthetics and sedatives, including desflurane, halothane, ketamine, lorazepam injection, methohexital, pentobarbital, and propofol. The drugs block N-methyl-D-aspartate (NMDA) receptors and/or potentiate gamma-aminobutyric acid (GABA) activity. No specific medications have been shown to be safer than any other, the agency said.
FDA will continue to monitor the situation, and update its warning as additional information comes in. “We urge health care professionals, patients, parents, and caregivers to report side effects involving anesthetic and sedation drugs or other medicines to the FDA MedWatch program,” the FDA said.