User login
The Most Misinterpreted Study in Medicine: Don’t be TRICCed
Ah, blood. That sweet nectar of life that quiets angina, abolishes dyspnea, prevents orthostatic syncope, and quells sinus tachycardia. As a cardiologist, I am an unabashed hemophile.
But we liberal transfusionists are challenged on every request for consideration of transfusion. Whereas the polite may resort to whispered skepticism, vehement critics respond with scorn as if we’d asked them to burn aromatic herbs or fetch a bucket of leeches. And to what do we owe this pathological angst? The broad and persistent misinterpretation of the pesky TRICC trial (N Engl J Med. 1999;340:409-417). You know; the one that should have been published with a boxed warning stating: “Misinterpretation of this trial could result in significant harm.”
Point 1: Our Actively Bleeding Patient is Not a TRICC Patient.
They were randomly assigned to either a conservative trigger for transfusion of < 7 g/dL or a liberal threshold of < 10 g/dL. Mortality at 30 days was lower with the conservative approach — 18.7% vs 23.3% — but the difference was not statistically significant (P = .11). The findings were similar for the secondary endpoints of inpatient mortality (22.2% vs 28.1%; P = .05) and ICU mortality (13.9% vs 16.2%; P = .29).
One must admit that these P values are not impressive, and the authors’ conclusion should have warranted caution: “A restrictive strategy ... is at least as effective as and possibly superior to a liberal transfusion strategy in critically ill patients, with the possible exception of patients with acute myocardial infarction and unstable angina.”
Point 2: Our Critically Ill Cardiac Patient is Unlikely to be a “TRICC” Patient.
Another criticism of TRICC is that only 13% of those assessed and 26% of those eligible were enrolled, mostly owing to physician refusal. Only 26% of enrolled patients had cardiac disease. This makes the TRICC population highly selected and not representative of typical ICU patients.
To prove my point that the edict against higher transfusion thresholds can be dangerous, I’ll describe my most recent interface with TRICC trial misinterpretation
A Case in Point
The patient, Mrs. Kemp,* is 79 years old and has been on aspirin for years following coronary stent placement. One evening, she began spurting bright red blood from her rectum, interrupted only briefly by large clots the consistency of jellied cranberries. When she arrived at the hospital, she was hemodynamically stable, with a hemoglobin level of 10 g/dL, down from her usual 12 g/dL. That level bolstered the confidence of her provider, who insisted that she be managed conservatively.
Mrs. Kemp was transferred to the ward, where she continued to bleed briskly. Over the next 2 hours, her hemoglobin level dropped to 9 g/dL, then 8 g/dL. Her daughter, a healthcare worker, requested a transfusion. The answer was, wait for it — the well-scripted, somewhat patronizing oft-quoted line, “The medical literature states that we need to wait for a hemoglobin level of 7 g/dL before we transfuse.”
Later that evening, Mrs. Kemp’s systolic blood pressure dropped to the upper 80s, despite her usual hypertension. The provider was again comforted by the fact that she was not tachycardic (she had a pacemaker and was on bisoprolol). The next morning, Mrs. Kemp felt the need to defecate and was placed on the bedside commode and left to her privacy. Predictably, she became dizzy and experienced frank syncope. Thankfully, she avoided a hip fracture or worse. A stat hemoglobin returned at 6 g/dL.
Her daughter said she literally heard the hallelujah chorus because her mother’s hemoglobin was finally below that much revered and often misleading threshold of 7 g/dL. Finally, there was an order for platelets and packed red cells. Five units later, Mr. Kemp achieved a hemoglobin of 8 g/dL and survived. Two more units and she was soaring at 9 g/dL!
Lessons for Transfusion Conservatives
There are many lessons here.
The TRICC study found that hemodynamically stable, asymptomatic patients who are not actively bleeding may well tolerate a hemoglobin level of 7 g/dL. But a patient with bright red blood actively pouring from an orifice and a rapidly declining hemoglobin level isn’t one of those people. Additionally, a patient who faints from hypovolemia is not one of those people.
Patients with a history of bleeding presenting with new resting sinus tachycardia (in those who have chronotropic competence) should be presumed to be actively bleeding, and the findings of TRICC do not apply to them. Patients who have bled buckets on anticoagulant or antiplatelet therapies and have dropped their hemoglobin will probably continue to ooze and should be subject to a low threshold for transfusion.
Additionally, anemic people who are hemodynamically stable but can’t walk without new significant shortness of air or new rest angina need blood, and sometimes at hemoglobin levels higher than generally accepted by conservative strategists. Finally, failing to treat or at least monitor patients who are spontaneously bleeding as aggressively as some trauma patients is a failure to provide proper medical care.
The vast majority of my healthcare clinician colleagues are competent, compassionate individuals who can reasonably discuss the nuances of any medical scenario. One important distinction of a good medical team is the willingness to change course based on a change in patient status or the presentation of what may be new information for the provider.
But those proud transfusion conservatives who will not budge until their threshold is met need to make certain their patient is truly subject to their supposed edicts. Our blood banks should not be more difficult to access than Fort Knox, and transfusion should be used appropriately and liberally in the hemodynamically unstable, the symptomatic, and active brisk bleeders.
I beg staunch transfusion conservatives to consider how they might feel if someone stuck a magic spigot in their brachial artery and acutely drained their hemoglobin to that magic threshold of 7 g/dL. When syncope, shortness of air, fatigue, and angina find them, they may generate empathy for those who need transfusion. Might that do the TRICC?
*Some details have been changed to conceal the identity of the patient, but the essence of the case has been preserved.
Dr. Walton-Shirley, a native Kentuckian who retired from full-time invasive cardiology and now does locums work in Montana, is a champion of physician rights and patient safety. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Ah, blood. That sweet nectar of life that quiets angina, abolishes dyspnea, prevents orthostatic syncope, and quells sinus tachycardia. As a cardiologist, I am an unabashed hemophile.
But we liberal transfusionists are challenged on every request for consideration of transfusion. Whereas the polite may resort to whispered skepticism, vehement critics respond with scorn as if we’d asked them to burn aromatic herbs or fetch a bucket of leeches. And to what do we owe this pathological angst? The broad and persistent misinterpretation of the pesky TRICC trial (N Engl J Med. 1999;340:409-417). You know; the one that should have been published with a boxed warning stating: “Misinterpretation of this trial could result in significant harm.”
Point 1: Our Actively Bleeding Patient is Not a TRICC Patient.
They were randomly assigned to either a conservative trigger for transfusion of < 7 g/dL or a liberal threshold of < 10 g/dL. Mortality at 30 days was lower with the conservative approach — 18.7% vs 23.3% — but the difference was not statistically significant (P = .11). The findings were similar for the secondary endpoints of inpatient mortality (22.2% vs 28.1%; P = .05) and ICU mortality (13.9% vs 16.2%; P = .29).
One must admit that these P values are not impressive, and the authors’ conclusion should have warranted caution: “A restrictive strategy ... is at least as effective as and possibly superior to a liberal transfusion strategy in critically ill patients, with the possible exception of patients with acute myocardial infarction and unstable angina.”
Point 2: Our Critically Ill Cardiac Patient is Unlikely to be a “TRICC” Patient.
Another criticism of TRICC is that only 13% of those assessed and 26% of those eligible were enrolled, mostly owing to physician refusal. Only 26% of enrolled patients had cardiac disease. This makes the TRICC population highly selected and not representative of typical ICU patients.
To prove my point that the edict against higher transfusion thresholds can be dangerous, I’ll describe my most recent interface with TRICC trial misinterpretation
A Case in Point
The patient, Mrs. Kemp,* is 79 years old and has been on aspirin for years following coronary stent placement. One evening, she began spurting bright red blood from her rectum, interrupted only briefly by large clots the consistency of jellied cranberries. When she arrived at the hospital, she was hemodynamically stable, with a hemoglobin level of 10 g/dL, down from her usual 12 g/dL. That level bolstered the confidence of her provider, who insisted that she be managed conservatively.
Mrs. Kemp was transferred to the ward, where she continued to bleed briskly. Over the next 2 hours, her hemoglobin level dropped to 9 g/dL, then 8 g/dL. Her daughter, a healthcare worker, requested a transfusion. The answer was, wait for it — the well-scripted, somewhat patronizing oft-quoted line, “The medical literature states that we need to wait for a hemoglobin level of 7 g/dL before we transfuse.”
Later that evening, Mrs. Kemp’s systolic blood pressure dropped to the upper 80s, despite her usual hypertension. The provider was again comforted by the fact that she was not tachycardic (she had a pacemaker and was on bisoprolol). The next morning, Mrs. Kemp felt the need to defecate and was placed on the bedside commode and left to her privacy. Predictably, she became dizzy and experienced frank syncope. Thankfully, she avoided a hip fracture or worse. A stat hemoglobin returned at 6 g/dL.
Her daughter said she literally heard the hallelujah chorus because her mother’s hemoglobin was finally below that much revered and often misleading threshold of 7 g/dL. Finally, there was an order for platelets and packed red cells. Five units later, Mr. Kemp achieved a hemoglobin of 8 g/dL and survived. Two more units and she was soaring at 9 g/dL!
Lessons for Transfusion Conservatives
There are many lessons here.
The TRICC study found that hemodynamically stable, asymptomatic patients who are not actively bleeding may well tolerate a hemoglobin level of 7 g/dL. But a patient with bright red blood actively pouring from an orifice and a rapidly declining hemoglobin level isn’t one of those people. Additionally, a patient who faints from hypovolemia is not one of those people.
Patients with a history of bleeding presenting with new resting sinus tachycardia (in those who have chronotropic competence) should be presumed to be actively bleeding, and the findings of TRICC do not apply to them. Patients who have bled buckets on anticoagulant or antiplatelet therapies and have dropped their hemoglobin will probably continue to ooze and should be subject to a low threshold for transfusion.
Additionally, anemic people who are hemodynamically stable but can’t walk without new significant shortness of air or new rest angina need blood, and sometimes at hemoglobin levels higher than generally accepted by conservative strategists. Finally, failing to treat or at least monitor patients who are spontaneously bleeding as aggressively as some trauma patients is a failure to provide proper medical care.
The vast majority of my healthcare clinician colleagues are competent, compassionate individuals who can reasonably discuss the nuances of any medical scenario. One important distinction of a good medical team is the willingness to change course based on a change in patient status or the presentation of what may be new information for the provider.
But those proud transfusion conservatives who will not budge until their threshold is met need to make certain their patient is truly subject to their supposed edicts. Our blood banks should not be more difficult to access than Fort Knox, and transfusion should be used appropriately and liberally in the hemodynamically unstable, the symptomatic, and active brisk bleeders.
I beg staunch transfusion conservatives to consider how they might feel if someone stuck a magic spigot in their brachial artery and acutely drained their hemoglobin to that magic threshold of 7 g/dL. When syncope, shortness of air, fatigue, and angina find them, they may generate empathy for those who need transfusion. Might that do the TRICC?
*Some details have been changed to conceal the identity of the patient, but the essence of the case has been preserved.
Dr. Walton-Shirley, a native Kentuckian who retired from full-time invasive cardiology and now does locums work in Montana, is a champion of physician rights and patient safety. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Ah, blood. That sweet nectar of life that quiets angina, abolishes dyspnea, prevents orthostatic syncope, and quells sinus tachycardia. As a cardiologist, I am an unabashed hemophile.
But we liberal transfusionists are challenged on every request for consideration of transfusion. Whereas the polite may resort to whispered skepticism, vehement critics respond with scorn as if we’d asked them to burn aromatic herbs or fetch a bucket of leeches. And to what do we owe this pathological angst? The broad and persistent misinterpretation of the pesky TRICC trial (N Engl J Med. 1999;340:409-417). You know; the one that should have been published with a boxed warning stating: “Misinterpretation of this trial could result in significant harm.”
Point 1: Our Actively Bleeding Patient is Not a TRICC Patient.
They were randomly assigned to either a conservative trigger for transfusion of < 7 g/dL or a liberal threshold of < 10 g/dL. Mortality at 30 days was lower with the conservative approach — 18.7% vs 23.3% — but the difference was not statistically significant (P = .11). The findings were similar for the secondary endpoints of inpatient mortality (22.2% vs 28.1%; P = .05) and ICU mortality (13.9% vs 16.2%; P = .29).
One must admit that these P values are not impressive, and the authors’ conclusion should have warranted caution: “A restrictive strategy ... is at least as effective as and possibly superior to a liberal transfusion strategy in critically ill patients, with the possible exception of patients with acute myocardial infarction and unstable angina.”
Point 2: Our Critically Ill Cardiac Patient is Unlikely to be a “TRICC” Patient.
Another criticism of TRICC is that only 13% of those assessed and 26% of those eligible were enrolled, mostly owing to physician refusal. Only 26% of enrolled patients had cardiac disease. This makes the TRICC population highly selected and not representative of typical ICU patients.
To prove my point that the edict against higher transfusion thresholds can be dangerous, I’ll describe my most recent interface with TRICC trial misinterpretation
A Case in Point
The patient, Mrs. Kemp,* is 79 years old and has been on aspirin for years following coronary stent placement. One evening, she began spurting bright red blood from her rectum, interrupted only briefly by large clots the consistency of jellied cranberries. When she arrived at the hospital, she was hemodynamically stable, with a hemoglobin level of 10 g/dL, down from her usual 12 g/dL. That level bolstered the confidence of her provider, who insisted that she be managed conservatively.
Mrs. Kemp was transferred to the ward, where she continued to bleed briskly. Over the next 2 hours, her hemoglobin level dropped to 9 g/dL, then 8 g/dL. Her daughter, a healthcare worker, requested a transfusion. The answer was, wait for it — the well-scripted, somewhat patronizing oft-quoted line, “The medical literature states that we need to wait for a hemoglobin level of 7 g/dL before we transfuse.”
Later that evening, Mrs. Kemp’s systolic blood pressure dropped to the upper 80s, despite her usual hypertension. The provider was again comforted by the fact that she was not tachycardic (she had a pacemaker and was on bisoprolol). The next morning, Mrs. Kemp felt the need to defecate and was placed on the bedside commode and left to her privacy. Predictably, she became dizzy and experienced frank syncope. Thankfully, she avoided a hip fracture or worse. A stat hemoglobin returned at 6 g/dL.
Her daughter said she literally heard the hallelujah chorus because her mother’s hemoglobin was finally below that much revered and often misleading threshold of 7 g/dL. Finally, there was an order for platelets and packed red cells. Five units later, Mr. Kemp achieved a hemoglobin of 8 g/dL and survived. Two more units and she was soaring at 9 g/dL!
Lessons for Transfusion Conservatives
There are many lessons here.
The TRICC study found that hemodynamically stable, asymptomatic patients who are not actively bleeding may well tolerate a hemoglobin level of 7 g/dL. But a patient with bright red blood actively pouring from an orifice and a rapidly declining hemoglobin level isn’t one of those people. Additionally, a patient who faints from hypovolemia is not one of those people.
Patients with a history of bleeding presenting with new resting sinus tachycardia (in those who have chronotropic competence) should be presumed to be actively bleeding, and the findings of TRICC do not apply to them. Patients who have bled buckets on anticoagulant or antiplatelet therapies and have dropped their hemoglobin will probably continue to ooze and should be subject to a low threshold for transfusion.
Additionally, anemic people who are hemodynamically stable but can’t walk without new significant shortness of air or new rest angina need blood, and sometimes at hemoglobin levels higher than generally accepted by conservative strategists. Finally, failing to treat or at least monitor patients who are spontaneously bleeding as aggressively as some trauma patients is a failure to provide proper medical care.
The vast majority of my healthcare clinician colleagues are competent, compassionate individuals who can reasonably discuss the nuances of any medical scenario. One important distinction of a good medical team is the willingness to change course based on a change in patient status or the presentation of what may be new information for the provider.
But those proud transfusion conservatives who will not budge until their threshold is met need to make certain their patient is truly subject to their supposed edicts. Our blood banks should not be more difficult to access than Fort Knox, and transfusion should be used appropriately and liberally in the hemodynamically unstable, the symptomatic, and active brisk bleeders.
I beg staunch transfusion conservatives to consider how they might feel if someone stuck a magic spigot in their brachial artery and acutely drained their hemoglobin to that magic threshold of 7 g/dL. When syncope, shortness of air, fatigue, and angina find them, they may generate empathy for those who need transfusion. Might that do the TRICC?
*Some details have been changed to conceal the identity of the patient, but the essence of the case has been preserved.
Dr. Walton-Shirley, a native Kentuckian who retired from full-time invasive cardiology and now does locums work in Montana, is a champion of physician rights and patient safety. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
New Tourniquet: The AED for Bleeding?
This discussion was recorded on July 12, 2024. This transcript has been edited for clarity.
Robert D. Glatter, MD: Hi and welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. I recently met an innovative young woman named Hannah Herbst while attending the annual Eagles EMS Conference in Fort Lauderdale, Florida.
Hannah Herbst is a graduate of Florida Atlantic University, selected for Forbes 30 Under 30, and founder of a company called Golden Hour Medical. She has a background in IT and developed an automated pneumatic tourniquet known as AutoTQ, which we’re going to discuss at length here.
Also joining us is Dr. Peter Antevy, a pediatric emergency physician and medical director for Davie Fire Rescue as well as Coral Springs Parkland Fire Rescue. Peter is a member of EMS Eagles Global Alliance and is highly involved in high-quality research in prehospital emergency care and is quite well known in Florida and nationally.
Welcome to both of you.
Hannah Herbst: Thank you very much. Very grateful to be here.
Dr. Glatter: Hannah, I’ll let you start by explaining what AutoTQ is and then compare that to a standard Combat Application Tourniquet (CAT).
Ms. Herbst: Thank you. Unfortunately, blood loss is a leading cause of preventable death and trauma. When there’s blood loss occurring from an arm or a leg, the easiest way to stop it is by applying a tourniquet, which is this compression type of device that you place above the site of bleeding, and it then applies a high amount of pressure to stop blood flow through the limb.
Currently, tourniquets on the market have failure rates as high as 84%. This became very real to me back in 2018, when I became aware of mass casualty incidents when I was a student. I became interested in how we can reimagine the conventional tourniquet and try to make it something that’s very user-friendly, much like an automated external defibrillator (AED).
My team and I developed AutoTQ, which is an automated tourniquet. which is a leading cause of tourniquet failure and being able to effectively administer treatment to a patient that may bleed out.
Tourniquet Failure Rates
Dr. Glatter: In terms of tourniquet failure, how often do standard tourniquets fail, like the CAT combat-type tourniquet?
Ms. Herbst: Unfortunately, they fail very frequently. There are several studies that have been conducted to evaluate this. Many of them occur immediately after training. They found failure rates between 80% and 90% for the current conventional CAT tourniquet immediately after training, which is very concerning.
Dr. Glatter: In terms of failure, was it the windlass aspect of the tourniquet that failed? Or was it something related to the actual strap? Was that in any way detailed?
Ms. Herbst: There are usually a few different failure points that have been found in the literature. One is placement. Many times, when you’re panicked, you don’t remember exactly how to place it. It should be placed high and tight above the bleed and not over a joint.
The second problem is inadequate tightness. For a CAT tourniquet to be effective, you have to get it extremely tight on that first pull before the windlass is activated, and many times people don’t remember that in the stress of the moment.
Dr. Glatter: Peter, in terms of tourniquet application by your medics in the field, certainly the CAT-type device has been in existence for quite a while. Hannah’s proposing a new iteration of how to do this, which is automated and simple. What is your take on such a device? And how did you learn about Hannah’s device?
Peter M. Antevy, MD: We’ve been training on tourniquets ever since the military data showed that there was an extreme benefit in using them. We’ve been doing training for many years, including our police officers. What we’ve noticed is that every time we gather everyone together to show them how to place a tourniquet — and we have to do one-on-one sessions with them — it’s not a device that they can easily put on. These are police officers who had the training last year.
Like Hannah said, most of the time they have a problem unraveling it and understanding how to actually place it. It’s easier on the arm than it is on the leg. You can imagine it would be harder to place it on your own leg, especially if you had an injury. Then, they don’t tighten it well enough, as Hannah just mentioned. In order for a tourniquet to really be placed properly, it’s going to hurt that person. Many people have that tendency not to want to tighten it as much as they can.
Having said that, how I got into all of this is because I’m the medical director for Coral Springs and Parkland, and unfortunately, we had the 2018 Valentine’s Day murders that happened where we lost 17 adults and kids. However, 17 people were saved that day, and the credit goes to our police officers who had tourniquets or chest seals on before those patients were brought out to EMS. Many lives were saved by the tourniquet.
If you look at the Boston Marathon massacre and many other events that have happened, I believe — and I’ve always believed — that tourniquets should be in the glove box of every citizen. It should be in every school room. They should be in buildings along with the AED.
In my town of Davie, we were the first in the country to add an ordinance that required a Stop the Bleed kit in the AED cabinet, and those were required by buildings of certain sizes. In order to get this lifesaving device everywhere, I think it has to be put into local ordinance and supported by states and by the national folks, which they are doing.
Trials Are Underway
Dr. Glatter: In terms of adoption of such a device, it certainly has to go through rigorous testing and maybe some trials. Hannah, where are you at with vetting this in terms of any type of trial? Has it been compared head to head with standard tourniquets?
Ms. Herbst: Yes, we’re currently doing large amounts of field testing. We’re doing testing on emergency vehicles and in the surgical setting with different customers. In addition, we’re running pilot studies at different universities and with different organizations, including the military, to make sure that this device is effective. We’re evaluating cognitive offloading of people. We’re hoping to start that study later this year. We’re excited to be doing this in a variety of settings.
We’re also testing the quality of it in different environmental conditions and under different atmospheric pressure. We’re doing everything we can to ensure the device is safe and effective. We’re excited to scale and fill our preorders and be able to develop this and deliver it to many people.
Dr. Glatter: I was wondering if you could describe the actual device. There’s a brain part of it and then, obviously, the strap aspect of it. I was curious about contamination and reusability issues.
Ms. Herbst: That’s a great question. One of the limitations of conventional tourniquets on the market is that they are single use, and often, it requires two tourniquets to stop a bleed, both of which have to be disposed of.
With AutoTQ, we have a reusable component and a disposable component. I actually have one here that I can show you. We have a cover on it that says: Stop bleed, slide up and power on. You just pull this cover off and then you have a few simple commands. You have powering the device on. I’ll just click this button: Tighten strap above bleeding, then press inflate. It delivers audible instructions telling you exactly how to use the device. Then, you tighten it above your bleed on the limb, and you press the inflate button. Then it administers air into the cuff and stops the patient’s bleed.
Tourniquet Conversion and Limb Salvage
Dr. Glatter: In terms of ischemia time, how can a device like this make it easier for us to know when to let the tourniquet down and allow some blood flow? Certainly, limb salvage is important, and we don’t want to have necrosis and so forth.
Dr. Antevy: That’s a great question. The limb salvage rate when tourniquets have been used is 85%. When used correctly, you can really improve the outcomes for many patients.
On the flip side of that, there’s something called tourniquet conversion. That’s exactly what you mentioned. It’s making sure that the tourniquet doesn’t stay on for too long of a time. If you can imagine a patient going to an outlying hospital where there’s no trauma center, and then that patient then has to be moved a couple hours to the trauma center, could you potentially have a tourniquet on for too long that then ends up causing the patient a bad outcome? The answer is yes.
I just had someone on my webinar recently describing the appropriate conversion techniques of tourniquets. You don’t find too much of that in the literature, but you really have to ensure that as you’re taking the tourniquet down, the bleeding is actually stopped. It’s not really recommended to take a tourniquet down if the patient was just acutely bleeding.
However, imagine a situation where a tourniquet was put on incorrectly. Let’s say a patient got nervous and they just put it on a patient who didn’t really need it. You really have to understand how to evaluate that wound to be sure that, as you’re taking the tourniquet down slowly, the patient doesn’t rebleed again.
There are two sides of the question, Rob. One is making sure it’s not on inappropriately. The second one is making sure it’s not on for too long, which ends up causing ischemia to that limb.
Dr. Glatter: Hannah, does your device collect data on the number of hours or minutes that the tourniquet has been up and then automatically deflate it in some sense to allow for that improvement in limb salvage?
Ms. Herbst: That’s a great question, and I really appreciate your answer as well, Dr Antevy. Ischemia time is a very important and critical component of tourniquet use. This is something, when we were designing AutoTQ, that we took into high consideration.
We found, when we evaluated AutoTQ vs a CAT tourniquet in a mannequin model, that AutoTQ can achieve cessation of hemorrhage at around 400 mm Hg of mercury, whereas CAT requires 700-800 mm Hg. Already our ischemia time is slightly extended just based on existing literature with pneumatic tourniquets because it can stop the bleed at a lower pressure, which causes less complications with the patient’s limb.
There are different features that we build out for different customers, so depending on what people want, it is possible to deflate the tourniquet. However, typically, you’re at the hospital within 30 minutes. It’s quick to get them there, and then the physician can treat and take that tourniquet down in a supervised and controlled setting.
Dr. Glatter: In terms of patients with obesity, do you have adjustable straps that will accommodate for that aspect?
Ms. Herbst: Yes, we have different cuff sizes to accommodate different limbs.
Will AutoTQ Be Available to the Public?
Dr. Glatter: Peter, in terms of usability in the prehospital setting, where do you think this is going in the next 3-5 years?
Dr. Antevy: I’ll start with the public safety sector of the United States, which is the one that is actually first on scene. Whether you’re talking about police officers or EMS, it would behoove us to have tourniquets everywhere. On all of my ambulances, across all of my agencies that I manage, we have quite a number of tourniquets.
Obviously, cost is a factor, and I know that Hannah has done a great job of making that brain reusable. All we have to do is purchase the straps, which are effectively the same cost, I understand, as a typical tourniquet you would purchase.
Moving forward though, however, I think that this has wide scalability to the public market, whether it be schools, office buildings, the glove box, and so on. It’s really impossible to teach somebody how to do this the right way, if you have to teach them how to put the strap on, tighten it correctly, and so on. If there was an easy way, like Hannah developed, of just putting it on and pushing a button, then I think that the outcomes and the scalability are much further beyond what we can do in EMS. I think there’s great value in both markets.
The ‘AED of Bleeding’: Rechargeable and Reusable
Dr. Glatter: This is the AED of bleeding. You have a device here that has wide-scale interest, certainly from the public and private sector.
Hannah, in terms of battery decay, how would that work out if it was in someone’s garage? Let’s just say someone purchased it and they hadn’t used it in 3 or 4 months. What type of decay are we looking at and can they rely on it?
Ms. Herbst: AutoTQ is rechargeable by a USB-C port, and our battery lasts for a year. Once a year, you’ll get an email reminder that says: “Hey, please charge your AutoTQ and make sure it’s up to the battery level.” We do everything in our power to make sure that our consumers are checking their batteries and that they’re ready to go.
Dr. Glatter: Is it heat and fire resistant? What, in terms of durability, does your device have?
Ms. Herbst: Just like any other medical device, we come with manufacturer recommendations for the upper and lower bounds of temperature and different storage recommendations. All of that is in our instructions for use.
Dr. Glatter: Peter, getting back to logistics. In terms of adoption, do you feel that, in the long term, this device will be something that we’re going to be seeing widely adopted just going forward?
Dr. Antevy: I do, and I’ll tell you why. When you look at AED use in this country, the odds of someone actually getting an AED and using it correctly are still very low. Part of that is because it’s complicated for many people to do. Getting tourniquets everywhere is step No. 1, and I think the federal government and the Stop the Bleed program is really making that happen.
We talked about ordinances, but ease of use, I think, is really the key. You have people who oftentimes have their child in cardiac arrest in front of them, and they won’t put two hands on their chest because they just are afraid of doing it.
When you have a device that’s a tourniquet, that’s a single-button turn on and single-button inflate, I think that would make it much more likely that a person will use that device when they’re passing the scene of an accident, as an example.
We’ve had many non–mass casualty incident events that have had tourniquets. We’ve had some media stories on them, where they’re just happening because someone got into a motor vehicle accident. It doesn’t have to be a school shooting. I think the tourniquets should be everywhere and should be easily used by everybody.
Managing Pain
Dr. Glatter: Regarding sedation, is there a need because of the pain involved with the application? How would you sedate a patient, pediatric or adult, who needs a tourniquet?
Dr. Antevy: We always evaluate people’s pain. If the patient is an extremist, we’re just going to be managing and trying to get them back to life. Once somebody is stabilized and is exhibiting pain of any sort, even, for example, after we intubate somebody, we have to sedate them and provide them pain control because they have a piece of plastic in their trachea.
It’s the same thing here for a tourniquet. These are painful, and we do have the appropriate medications on our vehicles to address that pain. Again, just simply the trauma itself is very painful. Yes, we do address that in EMS, and I would say most public agencies across this country would address pain appropriately.
Training on Tourniquet Use
Dr. Glatter: Hannah, can you talk a little bit about public training types of approaches? How would you train a consumer who purchases this type of device?
Ms. Herbst: A huge part of our mission is making blood loss prevention and control training accessible to a wide variety of people. One way that we’re able to do that is through our online training platform. When you purchase an AutoTQ kit, you plug it into your computer, and it walks you through the process of using it. It lets you practice on your own limb and on your buddy’s limb, just to be able to effectively apply it. We think this will have huge impacts in making sure that people are prepared and ready to stop the bleed with AutoTQ.
Dr. Glatter: Do you recommend people training once a month, in general, just to keep their skills up to use this? In the throes of a trauma and very chaotic situation, people sometimes lose their ability to think clearly and straightly.
Ms. Herbst: One of the studies we’re conducting is a learning curve study to try to figure out how quickly these skills degrade over time. We know that with the windlass tourniquet, it degrades within moments of training. With AutoTQ, we think the learning curve will last much longer. That’s something we’re evaluating, but we recommend people train as often as they can.
Dr. Antevy: Rob, if I can mention that there is a concept of just-in-time training. I think that with having the expectation that people are going to be training frequently, unfortunately, as many of us know, even with the AED as a perfect example, people don’t do that.
Yes. I would agree that you have to train at least once a year, is what I would say. At my office, we have a 2-hour training that goes over all these different items once a year.
The device itself should have the ability to allow you to figure out how to use it just in time, whether via video, or like Hannah’s device, by audio. I think that having both those things would make it more likely that the device be used when needed.
People panic, and if they have a device that can talk to them or walk them through it, they will be much more likely to use it at that time.
Final Takeaways
Dr. Glatter: Any other final thoughts or a few pearls for listeners to take away? Hannah, I’ll start with you.
Ms. Herbst: I’m very grateful for your time, and I’m very excited about the potential for AutoTQ. To me, it’s so exciting to see people preordering the device now. We’ve had people from school bus companies and small sports teams. I think, just like Dr Antevy said, tourniquets aren’t limited to mass casualty situations. Blood loss can happen anywhere and to anyone.
Being able to equip people and serve them to better prepare them for this happening to themselves, their friends, or their family is just the honor of a lifetime. Thank you very much for covering the device and for having me today.
Dr. Glatter: Of course, my pleasure. Peter?
Dr. Antevy: The citizens of this country, and everyone who lives across the world, has started to understand that there are things that we expect from our people, from the community. We expect them to do CPR for cardiac arrest. We expect them to know how to use an EpiPen. We expect them to know how to use an AED, and we also expect them to know how to stop bleeding with a tourniquet.
The American public has gotten to understand that these devices are very important. Having a device that’s easily used, that I can teach you in 10 seconds, that speaks to you — these are all things that make this product have great potential. I do look forward to the studies, not just the cadaver studies, but the real human studies.
I know Hannah is really a phenom and has been doing all these things so that this product can be on the shelves of Walmart and CVS one day. I commend you, Hannah, for everything you’re doing and wishing you the best of luck. We’re here for you.
Dr. Glatter: Same here. Congratulations on your innovative capability and what you’ve done to change the outcomes of bleeding related to penetrating trauma. Thank you so much.
Robert D. Glatter, MD, is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical advisor for Medscape and hosts the Hot Topics in EM series. Hannah D. Herbst, BS, is a graduate of Florida Atlantic University, was selected for Forbes 30 Under 30, and is the founder/CEO of Golden Hour Medical. Peter M. Antevy, MD, is a pediatric emergency medicine physician and medical director for Davie Fire Rescue and Coral Springs–Parkland Fire Department in Florida. He is also a member of the EMS Eagles Global Alliance.
A version of this article first appeared on Medscape.com.
This discussion was recorded on July 12, 2024. This transcript has been edited for clarity.
Robert D. Glatter, MD: Hi and welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. I recently met an innovative young woman named Hannah Herbst while attending the annual Eagles EMS Conference in Fort Lauderdale, Florida.
Hannah Herbst is a graduate of Florida Atlantic University, selected for Forbes 30 Under 30, and founder of a company called Golden Hour Medical. She has a background in IT and developed an automated pneumatic tourniquet known as AutoTQ, which we’re going to discuss at length here.
Also joining us is Dr. Peter Antevy, a pediatric emergency physician and medical director for Davie Fire Rescue as well as Coral Springs Parkland Fire Rescue. Peter is a member of EMS Eagles Global Alliance and is highly involved in high-quality research in prehospital emergency care and is quite well known in Florida and nationally.
Welcome to both of you.
Hannah Herbst: Thank you very much. Very grateful to be here.
Dr. Glatter: Hannah, I’ll let you start by explaining what AutoTQ is and then compare that to a standard Combat Application Tourniquet (CAT).
Ms. Herbst: Thank you. Unfortunately, blood loss is a leading cause of preventable death and trauma. When there’s blood loss occurring from an arm or a leg, the easiest way to stop it is by applying a tourniquet, which is this compression type of device that you place above the site of bleeding, and it then applies a high amount of pressure to stop blood flow through the limb.
Currently, tourniquets on the market have failure rates as high as 84%. This became very real to me back in 2018, when I became aware of mass casualty incidents when I was a student. I became interested in how we can reimagine the conventional tourniquet and try to make it something that’s very user-friendly, much like an automated external defibrillator (AED).
My team and I developed AutoTQ, which is an automated tourniquet. which is a leading cause of tourniquet failure and being able to effectively administer treatment to a patient that may bleed out.
Tourniquet Failure Rates
Dr. Glatter: In terms of tourniquet failure, how often do standard tourniquets fail, like the CAT combat-type tourniquet?
Ms. Herbst: Unfortunately, they fail very frequently. There are several studies that have been conducted to evaluate this. Many of them occur immediately after training. They found failure rates between 80% and 90% for the current conventional CAT tourniquet immediately after training, which is very concerning.
Dr. Glatter: In terms of failure, was it the windlass aspect of the tourniquet that failed? Or was it something related to the actual strap? Was that in any way detailed?
Ms. Herbst: There are usually a few different failure points that have been found in the literature. One is placement. Many times, when you’re panicked, you don’t remember exactly how to place it. It should be placed high and tight above the bleed and not over a joint.
The second problem is inadequate tightness. For a CAT tourniquet to be effective, you have to get it extremely tight on that first pull before the windlass is activated, and many times people don’t remember that in the stress of the moment.
Dr. Glatter: Peter, in terms of tourniquet application by your medics in the field, certainly the CAT-type device has been in existence for quite a while. Hannah’s proposing a new iteration of how to do this, which is automated and simple. What is your take on such a device? And how did you learn about Hannah’s device?
Peter M. Antevy, MD: We’ve been training on tourniquets ever since the military data showed that there was an extreme benefit in using them. We’ve been doing training for many years, including our police officers. What we’ve noticed is that every time we gather everyone together to show them how to place a tourniquet — and we have to do one-on-one sessions with them — it’s not a device that they can easily put on. These are police officers who had the training last year.
Like Hannah said, most of the time they have a problem unraveling it and understanding how to actually place it. It’s easier on the arm than it is on the leg. You can imagine it would be harder to place it on your own leg, especially if you had an injury. Then, they don’t tighten it well enough, as Hannah just mentioned. In order for a tourniquet to really be placed properly, it’s going to hurt that person. Many people have that tendency not to want to tighten it as much as they can.
Having said that, how I got into all of this is because I’m the medical director for Coral Springs and Parkland, and unfortunately, we had the 2018 Valentine’s Day murders that happened where we lost 17 adults and kids. However, 17 people were saved that day, and the credit goes to our police officers who had tourniquets or chest seals on before those patients were brought out to EMS. Many lives were saved by the tourniquet.
If you look at the Boston Marathon massacre and many other events that have happened, I believe — and I’ve always believed — that tourniquets should be in the glove box of every citizen. It should be in every school room. They should be in buildings along with the AED.
In my town of Davie, we were the first in the country to add an ordinance that required a Stop the Bleed kit in the AED cabinet, and those were required by buildings of certain sizes. In order to get this lifesaving device everywhere, I think it has to be put into local ordinance and supported by states and by the national folks, which they are doing.
Trials Are Underway
Dr. Glatter: In terms of adoption of such a device, it certainly has to go through rigorous testing and maybe some trials. Hannah, where are you at with vetting this in terms of any type of trial? Has it been compared head to head with standard tourniquets?
Ms. Herbst: Yes, we’re currently doing large amounts of field testing. We’re doing testing on emergency vehicles and in the surgical setting with different customers. In addition, we’re running pilot studies at different universities and with different organizations, including the military, to make sure that this device is effective. We’re evaluating cognitive offloading of people. We’re hoping to start that study later this year. We’re excited to be doing this in a variety of settings.
We’re also testing the quality of it in different environmental conditions and under different atmospheric pressure. We’re doing everything we can to ensure the device is safe and effective. We’re excited to scale and fill our preorders and be able to develop this and deliver it to many people.
Dr. Glatter: I was wondering if you could describe the actual device. There’s a brain part of it and then, obviously, the strap aspect of it. I was curious about contamination and reusability issues.
Ms. Herbst: That’s a great question. One of the limitations of conventional tourniquets on the market is that they are single use, and often, it requires two tourniquets to stop a bleed, both of which have to be disposed of.
With AutoTQ, we have a reusable component and a disposable component. I actually have one here that I can show you. We have a cover on it that says: Stop bleed, slide up and power on. You just pull this cover off and then you have a few simple commands. You have powering the device on. I’ll just click this button: Tighten strap above bleeding, then press inflate. It delivers audible instructions telling you exactly how to use the device. Then, you tighten it above your bleed on the limb, and you press the inflate button. Then it administers air into the cuff and stops the patient’s bleed.
Tourniquet Conversion and Limb Salvage
Dr. Glatter: In terms of ischemia time, how can a device like this make it easier for us to know when to let the tourniquet down and allow some blood flow? Certainly, limb salvage is important, and we don’t want to have necrosis and so forth.
Dr. Antevy: That’s a great question. The limb salvage rate when tourniquets have been used is 85%. When used correctly, you can really improve the outcomes for many patients.
On the flip side of that, there’s something called tourniquet conversion. That’s exactly what you mentioned. It’s making sure that the tourniquet doesn’t stay on for too long of a time. If you can imagine a patient going to an outlying hospital where there’s no trauma center, and then that patient then has to be moved a couple hours to the trauma center, could you potentially have a tourniquet on for too long that then ends up causing the patient a bad outcome? The answer is yes.
I just had someone on my webinar recently describing the appropriate conversion techniques of tourniquets. You don’t find too much of that in the literature, but you really have to ensure that as you’re taking the tourniquet down, the bleeding is actually stopped. It’s not really recommended to take a tourniquet down if the patient was just acutely bleeding.
However, imagine a situation where a tourniquet was put on incorrectly. Let’s say a patient got nervous and they just put it on a patient who didn’t really need it. You really have to understand how to evaluate that wound to be sure that, as you’re taking the tourniquet down slowly, the patient doesn’t rebleed again.
There are two sides of the question, Rob. One is making sure it’s not on inappropriately. The second one is making sure it’s not on for too long, which ends up causing ischemia to that limb.
Dr. Glatter: Hannah, does your device collect data on the number of hours or minutes that the tourniquet has been up and then automatically deflate it in some sense to allow for that improvement in limb salvage?
Ms. Herbst: That’s a great question, and I really appreciate your answer as well, Dr Antevy. Ischemia time is a very important and critical component of tourniquet use. This is something, when we were designing AutoTQ, that we took into high consideration.
We found, when we evaluated AutoTQ vs a CAT tourniquet in a mannequin model, that AutoTQ can achieve cessation of hemorrhage at around 400 mm Hg of mercury, whereas CAT requires 700-800 mm Hg. Already our ischemia time is slightly extended just based on existing literature with pneumatic tourniquets because it can stop the bleed at a lower pressure, which causes less complications with the patient’s limb.
There are different features that we build out for different customers, so depending on what people want, it is possible to deflate the tourniquet. However, typically, you’re at the hospital within 30 minutes. It’s quick to get them there, and then the physician can treat and take that tourniquet down in a supervised and controlled setting.
Dr. Glatter: In terms of patients with obesity, do you have adjustable straps that will accommodate for that aspect?
Ms. Herbst: Yes, we have different cuff sizes to accommodate different limbs.
Will AutoTQ Be Available to the Public?
Dr. Glatter: Peter, in terms of usability in the prehospital setting, where do you think this is going in the next 3-5 years?
Dr. Antevy: I’ll start with the public safety sector of the United States, which is the one that is actually first on scene. Whether you’re talking about police officers or EMS, it would behoove us to have tourniquets everywhere. On all of my ambulances, across all of my agencies that I manage, we have quite a number of tourniquets.
Obviously, cost is a factor, and I know that Hannah has done a great job of making that brain reusable. All we have to do is purchase the straps, which are effectively the same cost, I understand, as a typical tourniquet you would purchase.
Moving forward though, however, I think that this has wide scalability to the public market, whether it be schools, office buildings, the glove box, and so on. It’s really impossible to teach somebody how to do this the right way, if you have to teach them how to put the strap on, tighten it correctly, and so on. If there was an easy way, like Hannah developed, of just putting it on and pushing a button, then I think that the outcomes and the scalability are much further beyond what we can do in EMS. I think there’s great value in both markets.
The ‘AED of Bleeding’: Rechargeable and Reusable
Dr. Glatter: This is the AED of bleeding. You have a device here that has wide-scale interest, certainly from the public and private sector.
Hannah, in terms of battery decay, how would that work out if it was in someone’s garage? Let’s just say someone purchased it and they hadn’t used it in 3 or 4 months. What type of decay are we looking at and can they rely on it?
Ms. Herbst: AutoTQ is rechargeable by a USB-C port, and our battery lasts for a year. Once a year, you’ll get an email reminder that says: “Hey, please charge your AutoTQ and make sure it’s up to the battery level.” We do everything in our power to make sure that our consumers are checking their batteries and that they’re ready to go.
Dr. Glatter: Is it heat and fire resistant? What, in terms of durability, does your device have?
Ms. Herbst: Just like any other medical device, we come with manufacturer recommendations for the upper and lower bounds of temperature and different storage recommendations. All of that is in our instructions for use.
Dr. Glatter: Peter, getting back to logistics. In terms of adoption, do you feel that, in the long term, this device will be something that we’re going to be seeing widely adopted just going forward?
Dr. Antevy: I do, and I’ll tell you why. When you look at AED use in this country, the odds of someone actually getting an AED and using it correctly are still very low. Part of that is because it’s complicated for many people to do. Getting tourniquets everywhere is step No. 1, and I think the federal government and the Stop the Bleed program is really making that happen.
We talked about ordinances, but ease of use, I think, is really the key. You have people who oftentimes have their child in cardiac arrest in front of them, and they won’t put two hands on their chest because they just are afraid of doing it.
When you have a device that’s a tourniquet, that’s a single-button turn on and single-button inflate, I think that would make it much more likely that a person will use that device when they’re passing the scene of an accident, as an example.
We’ve had many non–mass casualty incident events that have had tourniquets. We’ve had some media stories on them, where they’re just happening because someone got into a motor vehicle accident. It doesn’t have to be a school shooting. I think the tourniquets should be everywhere and should be easily used by everybody.
Managing Pain
Dr. Glatter: Regarding sedation, is there a need because of the pain involved with the application? How would you sedate a patient, pediatric or adult, who needs a tourniquet?
Dr. Antevy: We always evaluate people’s pain. If the patient is an extremist, we’re just going to be managing and trying to get them back to life. Once somebody is stabilized and is exhibiting pain of any sort, even, for example, after we intubate somebody, we have to sedate them and provide them pain control because they have a piece of plastic in their trachea.
It’s the same thing here for a tourniquet. These are painful, and we do have the appropriate medications on our vehicles to address that pain. Again, just simply the trauma itself is very painful. Yes, we do address that in EMS, and I would say most public agencies across this country would address pain appropriately.
Training on Tourniquet Use
Dr. Glatter: Hannah, can you talk a little bit about public training types of approaches? How would you train a consumer who purchases this type of device?
Ms. Herbst: A huge part of our mission is making blood loss prevention and control training accessible to a wide variety of people. One way that we’re able to do that is through our online training platform. When you purchase an AutoTQ kit, you plug it into your computer, and it walks you through the process of using it. It lets you practice on your own limb and on your buddy’s limb, just to be able to effectively apply it. We think this will have huge impacts in making sure that people are prepared and ready to stop the bleed with AutoTQ.
Dr. Glatter: Do you recommend people training once a month, in general, just to keep their skills up to use this? In the throes of a trauma and very chaotic situation, people sometimes lose their ability to think clearly and straightly.
Ms. Herbst: One of the studies we’re conducting is a learning curve study to try to figure out how quickly these skills degrade over time. We know that with the windlass tourniquet, it degrades within moments of training. With AutoTQ, we think the learning curve will last much longer. That’s something we’re evaluating, but we recommend people train as often as they can.
Dr. Antevy: Rob, if I can mention that there is a concept of just-in-time training. I think that with having the expectation that people are going to be training frequently, unfortunately, as many of us know, even with the AED as a perfect example, people don’t do that.
Yes. I would agree that you have to train at least once a year, is what I would say. At my office, we have a 2-hour training that goes over all these different items once a year.
The device itself should have the ability to allow you to figure out how to use it just in time, whether via video, or like Hannah’s device, by audio. I think that having both those things would make it more likely that the device be used when needed.
People panic, and if they have a device that can talk to them or walk them through it, they will be much more likely to use it at that time.
Final Takeaways
Dr. Glatter: Any other final thoughts or a few pearls for listeners to take away? Hannah, I’ll start with you.
Ms. Herbst: I’m very grateful for your time, and I’m very excited about the potential for AutoTQ. To me, it’s so exciting to see people preordering the device now. We’ve had people from school bus companies and small sports teams. I think, just like Dr Antevy said, tourniquets aren’t limited to mass casualty situations. Blood loss can happen anywhere and to anyone.
Being able to equip people and serve them to better prepare them for this happening to themselves, their friends, or their family is just the honor of a lifetime. Thank you very much for covering the device and for having me today.
Dr. Glatter: Of course, my pleasure. Peter?
Dr. Antevy: The citizens of this country, and everyone who lives across the world, has started to understand that there are things that we expect from our people, from the community. We expect them to do CPR for cardiac arrest. We expect them to know how to use an EpiPen. We expect them to know how to use an AED, and we also expect them to know how to stop bleeding with a tourniquet.
The American public has gotten to understand that these devices are very important. Having a device that’s easily used, that I can teach you in 10 seconds, that speaks to you — these are all things that make this product have great potential. I do look forward to the studies, not just the cadaver studies, but the real human studies.
I know Hannah is really a phenom and has been doing all these things so that this product can be on the shelves of Walmart and CVS one day. I commend you, Hannah, for everything you’re doing and wishing you the best of luck. We’re here for you.
Dr. Glatter: Same here. Congratulations on your innovative capability and what you’ve done to change the outcomes of bleeding related to penetrating trauma. Thank you so much.
Robert D. Glatter, MD, is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical advisor for Medscape and hosts the Hot Topics in EM series. Hannah D. Herbst, BS, is a graduate of Florida Atlantic University, was selected for Forbes 30 Under 30, and is the founder/CEO of Golden Hour Medical. Peter M. Antevy, MD, is a pediatric emergency medicine physician and medical director for Davie Fire Rescue and Coral Springs–Parkland Fire Department in Florida. He is also a member of the EMS Eagles Global Alliance.
A version of this article first appeared on Medscape.com.
This discussion was recorded on July 12, 2024. This transcript has been edited for clarity.
Robert D. Glatter, MD: Hi and welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. I recently met an innovative young woman named Hannah Herbst while attending the annual Eagles EMS Conference in Fort Lauderdale, Florida.
Hannah Herbst is a graduate of Florida Atlantic University, selected for Forbes 30 Under 30, and founder of a company called Golden Hour Medical. She has a background in IT and developed an automated pneumatic tourniquet known as AutoTQ, which we’re going to discuss at length here.
Also joining us is Dr. Peter Antevy, a pediatric emergency physician and medical director for Davie Fire Rescue as well as Coral Springs Parkland Fire Rescue. Peter is a member of EMS Eagles Global Alliance and is highly involved in high-quality research in prehospital emergency care and is quite well known in Florida and nationally.
Welcome to both of you.
Hannah Herbst: Thank you very much. Very grateful to be here.
Dr. Glatter: Hannah, I’ll let you start by explaining what AutoTQ is and then compare that to a standard Combat Application Tourniquet (CAT).
Ms. Herbst: Thank you. Unfortunately, blood loss is a leading cause of preventable death and trauma. When there’s blood loss occurring from an arm or a leg, the easiest way to stop it is by applying a tourniquet, which is this compression type of device that you place above the site of bleeding, and it then applies a high amount of pressure to stop blood flow through the limb.
Currently, tourniquets on the market have failure rates as high as 84%. This became very real to me back in 2018, when I became aware of mass casualty incidents when I was a student. I became interested in how we can reimagine the conventional tourniquet and try to make it something that’s very user-friendly, much like an automated external defibrillator (AED).
My team and I developed AutoTQ, which is an automated tourniquet. which is a leading cause of tourniquet failure and being able to effectively administer treatment to a patient that may bleed out.
Tourniquet Failure Rates
Dr. Glatter: In terms of tourniquet failure, how often do standard tourniquets fail, like the CAT combat-type tourniquet?
Ms. Herbst: Unfortunately, they fail very frequently. There are several studies that have been conducted to evaluate this. Many of them occur immediately after training. They found failure rates between 80% and 90% for the current conventional CAT tourniquet immediately after training, which is very concerning.
Dr. Glatter: In terms of failure, was it the windlass aspect of the tourniquet that failed? Or was it something related to the actual strap? Was that in any way detailed?
Ms. Herbst: There are usually a few different failure points that have been found in the literature. One is placement. Many times, when you’re panicked, you don’t remember exactly how to place it. It should be placed high and tight above the bleed and not over a joint.
The second problem is inadequate tightness. For a CAT tourniquet to be effective, you have to get it extremely tight on that first pull before the windlass is activated, and many times people don’t remember that in the stress of the moment.
Dr. Glatter: Peter, in terms of tourniquet application by your medics in the field, certainly the CAT-type device has been in existence for quite a while. Hannah’s proposing a new iteration of how to do this, which is automated and simple. What is your take on such a device? And how did you learn about Hannah’s device?
Peter M. Antevy, MD: We’ve been training on tourniquets ever since the military data showed that there was an extreme benefit in using them. We’ve been doing training for many years, including our police officers. What we’ve noticed is that every time we gather everyone together to show them how to place a tourniquet — and we have to do one-on-one sessions with them — it’s not a device that they can easily put on. These are police officers who had the training last year.
Like Hannah said, most of the time they have a problem unraveling it and understanding how to actually place it. It’s easier on the arm than it is on the leg. You can imagine it would be harder to place it on your own leg, especially if you had an injury. Then, they don’t tighten it well enough, as Hannah just mentioned. In order for a tourniquet to really be placed properly, it’s going to hurt that person. Many people have that tendency not to want to tighten it as much as they can.
Having said that, how I got into all of this is because I’m the medical director for Coral Springs and Parkland, and unfortunately, we had the 2018 Valentine’s Day murders that happened where we lost 17 adults and kids. However, 17 people were saved that day, and the credit goes to our police officers who had tourniquets or chest seals on before those patients were brought out to EMS. Many lives were saved by the tourniquet.
If you look at the Boston Marathon massacre and many other events that have happened, I believe — and I’ve always believed — that tourniquets should be in the glove box of every citizen. It should be in every school room. They should be in buildings along with the AED.
In my town of Davie, we were the first in the country to add an ordinance that required a Stop the Bleed kit in the AED cabinet, and those were required by buildings of certain sizes. In order to get this lifesaving device everywhere, I think it has to be put into local ordinance and supported by states and by the national folks, which they are doing.
Trials Are Underway
Dr. Glatter: In terms of adoption of such a device, it certainly has to go through rigorous testing and maybe some trials. Hannah, where are you at with vetting this in terms of any type of trial? Has it been compared head to head with standard tourniquets?
Ms. Herbst: Yes, we’re currently doing large amounts of field testing. We’re doing testing on emergency vehicles and in the surgical setting with different customers. In addition, we’re running pilot studies at different universities and with different organizations, including the military, to make sure that this device is effective. We’re evaluating cognitive offloading of people. We’re hoping to start that study later this year. We’re excited to be doing this in a variety of settings.
We’re also testing the quality of it in different environmental conditions and under different atmospheric pressure. We’re doing everything we can to ensure the device is safe and effective. We’re excited to scale and fill our preorders and be able to develop this and deliver it to many people.
Dr. Glatter: I was wondering if you could describe the actual device. There’s a brain part of it and then, obviously, the strap aspect of it. I was curious about contamination and reusability issues.
Ms. Herbst: That’s a great question. One of the limitations of conventional tourniquets on the market is that they are single use, and often, it requires two tourniquets to stop a bleed, both of which have to be disposed of.
With AutoTQ, we have a reusable component and a disposable component. I actually have one here that I can show you. We have a cover on it that says: Stop bleed, slide up and power on. You just pull this cover off and then you have a few simple commands. You have powering the device on. I’ll just click this button: Tighten strap above bleeding, then press inflate. It delivers audible instructions telling you exactly how to use the device. Then, you tighten it above your bleed on the limb, and you press the inflate button. Then it administers air into the cuff and stops the patient’s bleed.
Tourniquet Conversion and Limb Salvage
Dr. Glatter: In terms of ischemia time, how can a device like this make it easier for us to know when to let the tourniquet down and allow some blood flow? Certainly, limb salvage is important, and we don’t want to have necrosis and so forth.
Dr. Antevy: That’s a great question. The limb salvage rate when tourniquets have been used is 85%. When used correctly, you can really improve the outcomes for many patients.
On the flip side of that, there’s something called tourniquet conversion. That’s exactly what you mentioned. It’s making sure that the tourniquet doesn’t stay on for too long of a time. If you can imagine a patient going to an outlying hospital where there’s no trauma center, and then that patient then has to be moved a couple hours to the trauma center, could you potentially have a tourniquet on for too long that then ends up causing the patient a bad outcome? The answer is yes.
I just had someone on my webinar recently describing the appropriate conversion techniques of tourniquets. You don’t find too much of that in the literature, but you really have to ensure that as you’re taking the tourniquet down, the bleeding is actually stopped. It’s not really recommended to take a tourniquet down if the patient was just acutely bleeding.
However, imagine a situation where a tourniquet was put on incorrectly. Let’s say a patient got nervous and they just put it on a patient who didn’t really need it. You really have to understand how to evaluate that wound to be sure that, as you’re taking the tourniquet down slowly, the patient doesn’t rebleed again.
There are two sides of the question, Rob. One is making sure it’s not on inappropriately. The second one is making sure it’s not on for too long, which ends up causing ischemia to that limb.
Dr. Glatter: Hannah, does your device collect data on the number of hours or minutes that the tourniquet has been up and then automatically deflate it in some sense to allow for that improvement in limb salvage?
Ms. Herbst: That’s a great question, and I really appreciate your answer as well, Dr Antevy. Ischemia time is a very important and critical component of tourniquet use. This is something, when we were designing AutoTQ, that we took into high consideration.
We found, when we evaluated AutoTQ vs a CAT tourniquet in a mannequin model, that AutoTQ can achieve cessation of hemorrhage at around 400 mm Hg of mercury, whereas CAT requires 700-800 mm Hg. Already our ischemia time is slightly extended just based on existing literature with pneumatic tourniquets because it can stop the bleed at a lower pressure, which causes less complications with the patient’s limb.
There are different features that we build out for different customers, so depending on what people want, it is possible to deflate the tourniquet. However, typically, you’re at the hospital within 30 minutes. It’s quick to get them there, and then the physician can treat and take that tourniquet down in a supervised and controlled setting.
Dr. Glatter: In terms of patients with obesity, do you have adjustable straps that will accommodate for that aspect?
Ms. Herbst: Yes, we have different cuff sizes to accommodate different limbs.
Will AutoTQ Be Available to the Public?
Dr. Glatter: Peter, in terms of usability in the prehospital setting, where do you think this is going in the next 3-5 years?
Dr. Antevy: I’ll start with the public safety sector of the United States, which is the one that is actually first on scene. Whether you’re talking about police officers or EMS, it would behoove us to have tourniquets everywhere. On all of my ambulances, across all of my agencies that I manage, we have quite a number of tourniquets.
Obviously, cost is a factor, and I know that Hannah has done a great job of making that brain reusable. All we have to do is purchase the straps, which are effectively the same cost, I understand, as a typical tourniquet you would purchase.
Moving forward though, however, I think that this has wide scalability to the public market, whether it be schools, office buildings, the glove box, and so on. It’s really impossible to teach somebody how to do this the right way, if you have to teach them how to put the strap on, tighten it correctly, and so on. If there was an easy way, like Hannah developed, of just putting it on and pushing a button, then I think that the outcomes and the scalability are much further beyond what we can do in EMS. I think there’s great value in both markets.
The ‘AED of Bleeding’: Rechargeable and Reusable
Dr. Glatter: This is the AED of bleeding. You have a device here that has wide-scale interest, certainly from the public and private sector.
Hannah, in terms of battery decay, how would that work out if it was in someone’s garage? Let’s just say someone purchased it and they hadn’t used it in 3 or 4 months. What type of decay are we looking at and can they rely on it?
Ms. Herbst: AutoTQ is rechargeable by a USB-C port, and our battery lasts for a year. Once a year, you’ll get an email reminder that says: “Hey, please charge your AutoTQ and make sure it’s up to the battery level.” We do everything in our power to make sure that our consumers are checking their batteries and that they’re ready to go.
Dr. Glatter: Is it heat and fire resistant? What, in terms of durability, does your device have?
Ms. Herbst: Just like any other medical device, we come with manufacturer recommendations for the upper and lower bounds of temperature and different storage recommendations. All of that is in our instructions for use.
Dr. Glatter: Peter, getting back to logistics. In terms of adoption, do you feel that, in the long term, this device will be something that we’re going to be seeing widely adopted just going forward?
Dr. Antevy: I do, and I’ll tell you why. When you look at AED use in this country, the odds of someone actually getting an AED and using it correctly are still very low. Part of that is because it’s complicated for many people to do. Getting tourniquets everywhere is step No. 1, and I think the federal government and the Stop the Bleed program is really making that happen.
We talked about ordinances, but ease of use, I think, is really the key. You have people who oftentimes have their child in cardiac arrest in front of them, and they won’t put two hands on their chest because they just are afraid of doing it.
When you have a device that’s a tourniquet, that’s a single-button turn on and single-button inflate, I think that would make it much more likely that a person will use that device when they’re passing the scene of an accident, as an example.
We’ve had many non–mass casualty incident events that have had tourniquets. We’ve had some media stories on them, where they’re just happening because someone got into a motor vehicle accident. It doesn’t have to be a school shooting. I think the tourniquets should be everywhere and should be easily used by everybody.
Managing Pain
Dr. Glatter: Regarding sedation, is there a need because of the pain involved with the application? How would you sedate a patient, pediatric or adult, who needs a tourniquet?
Dr. Antevy: We always evaluate people’s pain. If the patient is an extremist, we’re just going to be managing and trying to get them back to life. Once somebody is stabilized and is exhibiting pain of any sort, even, for example, after we intubate somebody, we have to sedate them and provide them pain control because they have a piece of plastic in their trachea.
It’s the same thing here for a tourniquet. These are painful, and we do have the appropriate medications on our vehicles to address that pain. Again, just simply the trauma itself is very painful. Yes, we do address that in EMS, and I would say most public agencies across this country would address pain appropriately.
Training on Tourniquet Use
Dr. Glatter: Hannah, can you talk a little bit about public training types of approaches? How would you train a consumer who purchases this type of device?
Ms. Herbst: A huge part of our mission is making blood loss prevention and control training accessible to a wide variety of people. One way that we’re able to do that is through our online training platform. When you purchase an AutoTQ kit, you plug it into your computer, and it walks you through the process of using it. It lets you practice on your own limb and on your buddy’s limb, just to be able to effectively apply it. We think this will have huge impacts in making sure that people are prepared and ready to stop the bleed with AutoTQ.
Dr. Glatter: Do you recommend people training once a month, in general, just to keep their skills up to use this? In the throes of a trauma and very chaotic situation, people sometimes lose their ability to think clearly and straightly.
Ms. Herbst: One of the studies we’re conducting is a learning curve study to try to figure out how quickly these skills degrade over time. We know that with the windlass tourniquet, it degrades within moments of training. With AutoTQ, we think the learning curve will last much longer. That’s something we’re evaluating, but we recommend people train as often as they can.
Dr. Antevy: Rob, if I can mention that there is a concept of just-in-time training. I think that with having the expectation that people are going to be training frequently, unfortunately, as many of us know, even with the AED as a perfect example, people don’t do that.
Yes. I would agree that you have to train at least once a year, is what I would say. At my office, we have a 2-hour training that goes over all these different items once a year.
The device itself should have the ability to allow you to figure out how to use it just in time, whether via video, or like Hannah’s device, by audio. I think that having both those things would make it more likely that the device be used when needed.
People panic, and if they have a device that can talk to them or walk them through it, they will be much more likely to use it at that time.
Final Takeaways
Dr. Glatter: Any other final thoughts or a few pearls for listeners to take away? Hannah, I’ll start with you.
Ms. Herbst: I’m very grateful for your time, and I’m very excited about the potential for AutoTQ. To me, it’s so exciting to see people preordering the device now. We’ve had people from school bus companies and small sports teams. I think, just like Dr Antevy said, tourniquets aren’t limited to mass casualty situations. Blood loss can happen anywhere and to anyone.
Being able to equip people and serve them to better prepare them for this happening to themselves, their friends, or their family is just the honor of a lifetime. Thank you very much for covering the device and for having me today.
Dr. Glatter: Of course, my pleasure. Peter?
Dr. Antevy: The citizens of this country, and everyone who lives across the world, has started to understand that there are things that we expect from our people, from the community. We expect them to do CPR for cardiac arrest. We expect them to know how to use an EpiPen. We expect them to know how to use an AED, and we also expect them to know how to stop bleeding with a tourniquet.
The American public has gotten to understand that these devices are very important. Having a device that’s easily used, that I can teach you in 10 seconds, that speaks to you — these are all things that make this product have great potential. I do look forward to the studies, not just the cadaver studies, but the real human studies.
I know Hannah is really a phenom and has been doing all these things so that this product can be on the shelves of Walmart and CVS one day. I commend you, Hannah, for everything you’re doing and wishing you the best of luck. We’re here for you.
Dr. Glatter: Same here. Congratulations on your innovative capability and what you’ve done to change the outcomes of bleeding related to penetrating trauma. Thank you so much.
Robert D. Glatter, MD, is an assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is a medical advisor for Medscape and hosts the Hot Topics in EM series. Hannah D. Herbst, BS, is a graduate of Florida Atlantic University, was selected for Forbes 30 Under 30, and is the founder/CEO of Golden Hour Medical. Peter M. Antevy, MD, is a pediatric emergency medicine physician and medical director for Davie Fire Rescue and Coral Springs–Parkland Fire Department in Florida. He is also a member of the EMS Eagles Global Alliance.
A version of this article first appeared on Medscape.com.
1 in 4 Unresponsive Coma Patients May Retain Some Awareness
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
“We found that at least 1 in 4 patients who are unresponsive to commands might actually be quite present and highly cognitive,” said study investigator Nicholas D. Schiff, MD, Feil Family Brain & Mind Research Institute and Department of Neurology, Weill Cornell Medicine, Rockefeller University Hospital, New York.
“In other words, if you go to the bedside and carefully examine someone with a severe brain injury and find no evidence of responsiveness, no one has been able to give you an a priori number to say how likely you are to be wrong in thinking this person is actually unaware, not processing language, and not capable of high-level cognitive work. And the answer to that now is at least 1 in 4 times.”
The findings were published online in The New England Journal of Medicine.
Clinical Implications?
Cognitive motor dissociation (CMD) is a condition whereby patients with a severe brain injury who are unresponsive to commands at the bedside show brain activity on functional MRI (fMRI) or electroencephalography (EEG) when presented with selective motor imagery commands, such as “imagine playing tennis,” or “ imagine opening and closing your hand.”
Previous research shows that CMD is present in 10%-20% of people with a disorder of consciousness, a rate similar to that in patients with acute or chronic brain injury.
Understanding that a patient who appears unconscious has signs of cognitive processing could change the way clinicians and family interact with such individuals. Unresponsive patients who are aware may eventually be able to harness emerging communication technologies such as brain-computer interfaces.
In addition, knowing an individual’s CMD status could aid in prognosis. “We know from one study that there’s a four times increased likelihood that patients will be independent in a year in their function if they have cognitive motor dissociation,” said Dr. Schiff.
Unlike most previous studies of CMD, which were conducted at single sites and had relatively small cohorts, this new study included 353 adults with a disorder of consciousness (mean age, 37.9 years; 64% male) at six multinational sites.
Participants were recruited using a variety of methods, including consecutive enrollment of critically ill patients in the intensive care unit and enrollment of those with chronic illness or injury who were in the postacute phase of brain injury.
Response to Commands
Study participants were at different stages of recovery from an acute brain injury that had occurred an average of 8 months before the study started.
To determine the presence or absence of an observable response to commands among participants, trained staff used the Coma Recovery Scale–Revised (CRS-R); scores on this instrument range from 0 to 23, and higher scores indicate better neurobehavioral function.
About 40% of individuals were diagnosed with coma or vegetative state, 29% with minimally conscious state–minus, and 22% with minimally conscious state–plus. In all, 10% had emerged from a minimally conscious state.
Researchers assessed response to timed and repeated commands using fMRI or EEG in participants without an observable response to verbal commands, including those with a behavioral diagnosis of coma, vegetative state, or minimally conscious state–minus, and in participants with an observable response to verbal commands.
Of the 353 study participants, 61% underwent at least one fMRI assessment and 74% at least one EEG assessment. Both fMRI and EEG were performed in 35% of participants.
Dr. Schiff explained the two assessment types provide slightly different information, in that they measuring different types of brain signals. He also noted that although “every medical center in the world” has EEG, many do not have fMRI.
The brain imaging assessments captured brain activity within the motor area of the frontal cortex when tasked with motor imagery.
Of the 241 participants deemed to be in a coma or vegetative state or minimally conscious state–minus on the basis of CRS-R score, 60 (25%) had a response to commands on task-based fMRI, task-based EEG, or both.
The percentage of participants with CMD varied across study sites, from 2% to 45%, but Dr. Schiff said the reason for this is unclear.
The proportion of participants with CMD may have been even higher if all individuals had been assessed with both imaging techniques, he said.
Higher Rate of Awareness Than in Previous Research
The investigators noted that the percentage of participants with CMD in their study was up to 10 percentage points higher than in previous studies. This may be due to the multimodal approach that classified participants undergoing assessment with both fMRI and EEG on the basis of responses on either technique, they said.
The median age was lower among participants with CMD than those without CMD (30.5 years vs 45.3 years).
Compared with participants without CMD, a higher percentage of those with such dissociation had brain trauma as an etiologic factor (65% vs 38%) and a diagnosis of minimally conscious state–minus on the CRS-R (53% vs 38%).
Among people with CMD, 18% were assessed with fMRI only, 22% with EEG only, and 60% with both fMRI and EEG.
Dr. Schiff noted that the use of both fMRI and EEG appears to be more sensitive in detecting brain activity during tasks compared with use of one of these techniques alone.
Of the 112 participants with a diagnosis of minimally conscious state–plus or who had emerged from the minimally conscious state, 38% had a response to commands on task-based fMRI, task-based EEG, or both. Among these participants, 23% were assessed with fMRI only, 19% with EEG only, and 58% with both fMRI and EEG.
Research shows “it’s very clear that people with severe brain injury continue to get better over time,” noted Dr. Schiff. “Every month and week matters, and so it probably is the case that a lot of these patients are picking up the level of recovery, and the later we go out to measure them, the more likely we are to find people who are CMD than not.”
These new results should prompt further study to explore whether detection of CMD can lead to improved outcomes, the investigators noted. “In addition, the standardization, validation, and simplification of task-based fMRI and EEG methods that are used to detect cognitive motor dissociation are needed to prompt widespread clinical integration of these techniques and investigation of the bioethical implications of the findings.”
All study participants with chronic brain injury had survived their initial illness or injury and had access to a research facility with advanced fMRI and EEG capabilities. “This survival bias may reflect greater cognitive reserve and resilience over time among the participants. As such, the results of our study may not be generalizable to the overall population of patients with cognitive motor dissociation,” the investigators wrote.
Another study limitation was that participating sites used heterogeneous strategies to acquire, analyze, and interpret data, which led to differences in the number, type, and ordering of the cognitive tasks assessed on fMRI and EEG.
“These differences, along with variations in recruitment strategies and participant characteristics, may have contributed to the unequal percentage of participants with cognitive motor dissociation observed at each site. Our findings may therefore not be generalizable across all centers,” the researchers wrote.
Only a few academic medical centers have the specially trained personnel and techniques needed to assess patients for CMD — which, the researchers noted, limits the feasibility of performing these assessments in general practice.
Challenging Research
Commenting on the research, Aarti Sarwal, MD, professor of neurology and section chief, Neurocritical Care, Virginia Commonwealth University, Richmond, Virginia, noted that this was a “very challenging” study to perform, given that only a few academic centers are equipped to perform both fMRI and quantitative EEG analysis.
“In general, finding patients this far out, who have access to clinical, radiological, and electrophysiological testing and were provided good care enough to receive these, is a mammoth task in itself.”
Dr. Sarwal said the study builds on efforts of the Curing Coma campaign , a clinical, scientific, and public health effort of the Neurocritical Care Society to tackle the concept of coma as a treatable medical entity.
“It continues to highlight the challenges of prognostication in acute brain injured patients by showing a higher presence of cognitive function than previously perceived,” she said.
Dr. Sarwal believes that the study’s largest impact is underscoring the need for more research into understanding the degree and quality of cognitive processing in patients with a disorder of consciousness. But it also underlines the need for a “healthy debate” on the cost/benefit analysis of pursuing such research, given the limited number of patients with access to resources.
“This debate needs to include the caregivers and families outside the traditional realms of stakeholders overseeing the science.”
Although communication with comatose patients is still “a ways away,” this research is “a step in the right direction,” said Dr. Sarwal.
The study was funded by the James S. McDonnell Foundation and others. Dr. Schiff and Dr. Sarwal report no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
PTSD Needs a New Name, Experts Say — Here’s Why
In a bid to reduce stigma and improve treatment rates, for inclusion in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). The APA’s policy is that a rolling name change is available if the current term is determined to be harmful.
Currently led by anesthesiologist Eugene Lipov, MD, clinical assistant professor, University of Illinois Chicago, and chief medical officer of Stella Center, also in Chicago, the formal request for the proposed name change to the APA’s DSM-5-TR Steering Committee in August 2023.
The APA Steering Committee rejected the proposed name change in November 2023, citing a “lack of convincing evidence.” However, Dr. Lipov and colleagues remain undeterred and continue to advocate for the change.
“The word ‘disorder’ is both imprecise and stigmatizing,” Dr. Lipov said. “Because of stigma, many people with PTSD — especially those in the military — don’t get help, which my research has demonstrated.”
Patients are more likely to seek help if their symptoms are framed as manifestations of an injury that is diagnosable and treatable, like a broken leg, Dr. Lipov said. “Stigma can kill in very real ways, since delayed care or lack of care can directly lead to suicides, thus satisfying the reduce harm requirement for the name change.”
Neurobiology of Trauma
Dr. Lipov grew up with a veteran father affected by PTSD and a mother with debilitating depression who eventually took her life. “I understand the impact of trauma very well,” he said.
Although not a psychiatrist, Dr. Lipov pioneered a highly successful treatment for PTSD by adapting an anesthetic technique — the stellate ganglion block (SGB) — to reverse many trauma symptoms through the process of “rebooting.”
This involves reversing the activity of the sympathetic nervous system — the fight-or-flight response — to the pretrauma state by anesthetizing the sympathetic ganglion in the neck. Investigating how SGB can help ameliorate the symptoms of PTSD led him to investigate and describe the neurobiology of PTSD and the mechanism of action of SGB.
The impact of SGD on PTSD was supported by a small neuroimaging study demonstrating that the right amygdala — the area of the brain associated with the fear response — was overactivated in patients with PTSD but that this region was deactivated after the administration of SGB, Dr. Lipov said.
“I believe that psychiatric conditions are actually physiologic brain changes that can be measured by advanced neuroimaging technologies and then physiologically treated,” he stated.
He noted that a growing body of literature suggests that use of the SGB for PTSD can be effective “because PTSD has a neurobiological basis and is essentially caused by an actual injury to the brain.”
A Natural Response, Not a Disorder
Dr. Lipov’s clinical work treating PTSD as a brain injury led him to connect with Frank Ochberg, MD, a founding board member of the International Society for Traumatic Stress Studies, former associate director of the National Institute of Mental Health, and former director of the Michigan Department of Mental Health.
In 2012, Dr. Ochberg teamed up with retired Army General Peter Chiarelli and Jonathan Shay, MD, PhD, author of Achilles in Vietnam: Combat Trauma and the Undoing of Character, to petition the DSM-5 Steering Committee to change the name of PTSD to PTSI in the upcoming DSM-5.
Dr. Ochberg explained that Gen. Chiarelli believed the term “disorder” suggests a preexisting issue prior to enlistment, potentially making an individual appear “weak.” He noted that this stigma is particularly troubling for military personnel, who often avoid seeking so they are not perceived as vulnerable, which can lead to potentially dire consequences, including suicide.
“We received endorsements from many quarters, not only advocates for service members or veterans,” Dr. Ochberg said.
This included feminists like Gloria Steinem, who championed the rights of women who had survived rape, incest, and domestic violence. As one advocate put it: “The natural human reaction to a life-threatening event should not be labeled a disorder.”
The DSM-5 Steering Committee declined to change the name. “Their feeling was that if we change the word ‘disorder’ to something else, we’d have to change every condition in the DSM that’s called a ‘disorder’. And they felt there really was nothing wrong with the word,” said Dr. Ochberg.
However, Dr. Lipov noted that other diagnoses have undergone name changes in the DSM for the sake of accuracy or stigma reduction. For example, the term mental retardation (DSM-IV) was changed to intellectual disability in DSM-5, and gender identity disorder was changed to gender dysphoria.
A decade later, Dr. Lipov decided to try again. To bolster his contention, he conducted a telephone survey of 1025 individuals. Of these, about 50% had a PTSD diagnosis.
Approximately two thirds of respondents agreed that a name change to PTSI would reduce the stigma associated with the term “PTSD.” Over half said it would increase the likelihood they would seek medical help. Those diagnosed with PTSD were most likely to endorse the name change.
Dr. Lipov conducts an ongoing survey of psychiatrists to ascertain their views on the potential name change and hopes to include findings in future research and communication with the DSM-5 Steering Committee. In addition, he has developed a new survey that expands upon his original survey, which specifically looked at individuals with PTSD.
“The new survey includes a wide range of people, many of whom have never been diagnosed. One of the questions we ask is whether they’ve ever heard of PTSD, and then we ask them about their reaction to the term,” he said.
A Barrier to Care
Psychiatrist Marcel Green, MD, director of Hudson Mind in New York City, refers to himself as an “interventional psychiatrist,” as he employs a comprehensive approach that includes not only medication and psychotherapy but also specialized techniques like SBG for severe anxiety-related physical symptoms and certain pain conditions.
Dr. Green, who is not involved in the name change initiative, agrees that the term “disorder” carries more stigma than “injury” for many groups, including those who have experienced childhood trauma, those struggling with substance abuse, or who are from backgrounds or peer groups where seeking mental health care is stigmatized.
Patients like these “are looking to me to give them a language to frame what they’re going through, and I tell them their symptoms are consistent with PTSD,” he said. “But they tell me don’t see themselves as having a disorder, which hinders their pursuit of care.”
Framing the condition as an “injury” also aligns with the approach of using biologic interventions to address the injury. Dr. Green has found SGB helpful in treating substance abuse disorder too, “which is a form of escape from the hyperactivation that accompanies PTSD.” And after the procedure, “they’re more receptive to therapy.”
Unfortunately, said Dr. Lipov, the DSM Steering Committee rejected his proposed name change, stating that the “concept of disorder as a dividing line from, eg, normal reactions to stress, is a core concept in the DSM, and the term has only rarely been removed.”
Moreover, the committee “did not see sufficient evidence ... that the name PTSD is stigmatizing and actually deters people with the disorder from seeking treatment who would not be deterred from doing so by PTSI.”
‘An Avenue for Dignity’
Ken Duckworth, MD, chief medical officer of the National Alliance on Mental Illness (NAMI), noted that the organization does not have an official position on this issue. However, he shared his own personal perspective.
There may be merit in the proposed name change, said Dr. Duckworth, but more evidence is needed. “If it’s clear, after rigorous studies have been performed and there’s compelling data, that calling it a ‘disorder’ rather than an ‘injury’ is actually preventing people from getting the care they need, then it merits serious attention.”
If so, Dr. Duckworth would be “interested in having a conversation with the policy team at NAMI to start to see if we could activate the DSM Committee.”
Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto in Ontario, Canada, and head of the Mood Disorders Psychopharmacology Unit, said the name change initiative is a “really interesting proposal.”
Dr. McIntyre, chairman and executive director of the Brain and Cognition Discovery Foundation, also in Toronto, who is not involved in the initiative, has also heard “many people say that the term ‘disorder’ is stigmatizing and might even come across as pejorative in some ways.”
By contrast, “the word ‘injury’ parallels physical injury, and what we currently call ‘PTSD’ is a psychological or emotional injury no less devastating than torn tissue or broken bones,” added Dr. McIntyre, who is also the chairman of the board of the Depression and Bipolar Support Alliance.
Dr. Ochberg agreed. “In the military, ‘injury’ opens up an avenue for dignity, for a medal. Being injured and learning how to deal with an injury is part of having yet another honorable task that comes from being an honorable person who did an honorable thing.”
While disappointed, Dr. Lipov does not plan to give up on his vision. “I will continue to amass evidence that the word ‘PTSD’ is stigmatizing and indeed does prevent people from seeking care and will resubmit the proposal to the DSM Steering Committee when I have gathered a larger body of compelling evidence.”
Currently, Dr. Lipov is in active discussions with the special operations force of the US Army to obtain more evidence. “This will be the follow-up to bolster the opinion of Peter Chiarelli,” he said. “It is known that suicide and PTSD are highly related. This is especially urgent and relevant because recent data suggest suicide rate of military personnel in the VA may be as high as 44 per day,” Dr. Lipov said.
Dr. Lipov is the chief medical officer and an investor in the Stella Center. Dr. Green performs SGBs as part of his psychiatric practice. Drs. Ochberg, McIntyre, and Duckworth reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a bid to reduce stigma and improve treatment rates, for inclusion in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). The APA’s policy is that a rolling name change is available if the current term is determined to be harmful.
Currently led by anesthesiologist Eugene Lipov, MD, clinical assistant professor, University of Illinois Chicago, and chief medical officer of Stella Center, also in Chicago, the formal request for the proposed name change to the APA’s DSM-5-TR Steering Committee in August 2023.
The APA Steering Committee rejected the proposed name change in November 2023, citing a “lack of convincing evidence.” However, Dr. Lipov and colleagues remain undeterred and continue to advocate for the change.
“The word ‘disorder’ is both imprecise and stigmatizing,” Dr. Lipov said. “Because of stigma, many people with PTSD — especially those in the military — don’t get help, which my research has demonstrated.”
Patients are more likely to seek help if their symptoms are framed as manifestations of an injury that is diagnosable and treatable, like a broken leg, Dr. Lipov said. “Stigma can kill in very real ways, since delayed care or lack of care can directly lead to suicides, thus satisfying the reduce harm requirement for the name change.”
Neurobiology of Trauma
Dr. Lipov grew up with a veteran father affected by PTSD and a mother with debilitating depression who eventually took her life. “I understand the impact of trauma very well,” he said.
Although not a psychiatrist, Dr. Lipov pioneered a highly successful treatment for PTSD by adapting an anesthetic technique — the stellate ganglion block (SGB) — to reverse many trauma symptoms through the process of “rebooting.”
This involves reversing the activity of the sympathetic nervous system — the fight-or-flight response — to the pretrauma state by anesthetizing the sympathetic ganglion in the neck. Investigating how SGB can help ameliorate the symptoms of PTSD led him to investigate and describe the neurobiology of PTSD and the mechanism of action of SGB.
The impact of SGD on PTSD was supported by a small neuroimaging study demonstrating that the right amygdala — the area of the brain associated with the fear response — was overactivated in patients with PTSD but that this region was deactivated after the administration of SGB, Dr. Lipov said.
“I believe that psychiatric conditions are actually physiologic brain changes that can be measured by advanced neuroimaging technologies and then physiologically treated,” he stated.
He noted that a growing body of literature suggests that use of the SGB for PTSD can be effective “because PTSD has a neurobiological basis and is essentially caused by an actual injury to the brain.”
A Natural Response, Not a Disorder
Dr. Lipov’s clinical work treating PTSD as a brain injury led him to connect with Frank Ochberg, MD, a founding board member of the International Society for Traumatic Stress Studies, former associate director of the National Institute of Mental Health, and former director of the Michigan Department of Mental Health.
In 2012, Dr. Ochberg teamed up with retired Army General Peter Chiarelli and Jonathan Shay, MD, PhD, author of Achilles in Vietnam: Combat Trauma and the Undoing of Character, to petition the DSM-5 Steering Committee to change the name of PTSD to PTSI in the upcoming DSM-5.
Dr. Ochberg explained that Gen. Chiarelli believed the term “disorder” suggests a preexisting issue prior to enlistment, potentially making an individual appear “weak.” He noted that this stigma is particularly troubling for military personnel, who often avoid seeking so they are not perceived as vulnerable, which can lead to potentially dire consequences, including suicide.
“We received endorsements from many quarters, not only advocates for service members or veterans,” Dr. Ochberg said.
This included feminists like Gloria Steinem, who championed the rights of women who had survived rape, incest, and domestic violence. As one advocate put it: “The natural human reaction to a life-threatening event should not be labeled a disorder.”
The DSM-5 Steering Committee declined to change the name. “Their feeling was that if we change the word ‘disorder’ to something else, we’d have to change every condition in the DSM that’s called a ‘disorder’. And they felt there really was nothing wrong with the word,” said Dr. Ochberg.
However, Dr. Lipov noted that other diagnoses have undergone name changes in the DSM for the sake of accuracy or stigma reduction. For example, the term mental retardation (DSM-IV) was changed to intellectual disability in DSM-5, and gender identity disorder was changed to gender dysphoria.
A decade later, Dr. Lipov decided to try again. To bolster his contention, he conducted a telephone survey of 1025 individuals. Of these, about 50% had a PTSD diagnosis.
Approximately two thirds of respondents agreed that a name change to PTSI would reduce the stigma associated with the term “PTSD.” Over half said it would increase the likelihood they would seek medical help. Those diagnosed with PTSD were most likely to endorse the name change.
Dr. Lipov conducts an ongoing survey of psychiatrists to ascertain their views on the potential name change and hopes to include findings in future research and communication with the DSM-5 Steering Committee. In addition, he has developed a new survey that expands upon his original survey, which specifically looked at individuals with PTSD.
“The new survey includes a wide range of people, many of whom have never been diagnosed. One of the questions we ask is whether they’ve ever heard of PTSD, and then we ask them about their reaction to the term,” he said.
A Barrier to Care
Psychiatrist Marcel Green, MD, director of Hudson Mind in New York City, refers to himself as an “interventional psychiatrist,” as he employs a comprehensive approach that includes not only medication and psychotherapy but also specialized techniques like SBG for severe anxiety-related physical symptoms and certain pain conditions.
Dr. Green, who is not involved in the name change initiative, agrees that the term “disorder” carries more stigma than “injury” for many groups, including those who have experienced childhood trauma, those struggling with substance abuse, or who are from backgrounds or peer groups where seeking mental health care is stigmatized.
Patients like these “are looking to me to give them a language to frame what they’re going through, and I tell them their symptoms are consistent with PTSD,” he said. “But they tell me don’t see themselves as having a disorder, which hinders their pursuit of care.”
Framing the condition as an “injury” also aligns with the approach of using biologic interventions to address the injury. Dr. Green has found SGB helpful in treating substance abuse disorder too, “which is a form of escape from the hyperactivation that accompanies PTSD.” And after the procedure, “they’re more receptive to therapy.”
Unfortunately, said Dr. Lipov, the DSM Steering Committee rejected his proposed name change, stating that the “concept of disorder as a dividing line from, eg, normal reactions to stress, is a core concept in the DSM, and the term has only rarely been removed.”
Moreover, the committee “did not see sufficient evidence ... that the name PTSD is stigmatizing and actually deters people with the disorder from seeking treatment who would not be deterred from doing so by PTSI.”
‘An Avenue for Dignity’
Ken Duckworth, MD, chief medical officer of the National Alliance on Mental Illness (NAMI), noted that the organization does not have an official position on this issue. However, he shared his own personal perspective.
There may be merit in the proposed name change, said Dr. Duckworth, but more evidence is needed. “If it’s clear, after rigorous studies have been performed and there’s compelling data, that calling it a ‘disorder’ rather than an ‘injury’ is actually preventing people from getting the care they need, then it merits serious attention.”
If so, Dr. Duckworth would be “interested in having a conversation with the policy team at NAMI to start to see if we could activate the DSM Committee.”
Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto in Ontario, Canada, and head of the Mood Disorders Psychopharmacology Unit, said the name change initiative is a “really interesting proposal.”
Dr. McIntyre, chairman and executive director of the Brain and Cognition Discovery Foundation, also in Toronto, who is not involved in the initiative, has also heard “many people say that the term ‘disorder’ is stigmatizing and might even come across as pejorative in some ways.”
By contrast, “the word ‘injury’ parallels physical injury, and what we currently call ‘PTSD’ is a psychological or emotional injury no less devastating than torn tissue or broken bones,” added Dr. McIntyre, who is also the chairman of the board of the Depression and Bipolar Support Alliance.
Dr. Ochberg agreed. “In the military, ‘injury’ opens up an avenue for dignity, for a medal. Being injured and learning how to deal with an injury is part of having yet another honorable task that comes from being an honorable person who did an honorable thing.”
While disappointed, Dr. Lipov does not plan to give up on his vision. “I will continue to amass evidence that the word ‘PTSD’ is stigmatizing and indeed does prevent people from seeking care and will resubmit the proposal to the DSM Steering Committee when I have gathered a larger body of compelling evidence.”
Currently, Dr. Lipov is in active discussions with the special operations force of the US Army to obtain more evidence. “This will be the follow-up to bolster the opinion of Peter Chiarelli,” he said. “It is known that suicide and PTSD are highly related. This is especially urgent and relevant because recent data suggest suicide rate of military personnel in the VA may be as high as 44 per day,” Dr. Lipov said.
Dr. Lipov is the chief medical officer and an investor in the Stella Center. Dr. Green performs SGBs as part of his psychiatric practice. Drs. Ochberg, McIntyre, and Duckworth reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a bid to reduce stigma and improve treatment rates, for inclusion in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR). The APA’s policy is that a rolling name change is available if the current term is determined to be harmful.
Currently led by anesthesiologist Eugene Lipov, MD, clinical assistant professor, University of Illinois Chicago, and chief medical officer of Stella Center, also in Chicago, the formal request for the proposed name change to the APA’s DSM-5-TR Steering Committee in August 2023.
The APA Steering Committee rejected the proposed name change in November 2023, citing a “lack of convincing evidence.” However, Dr. Lipov and colleagues remain undeterred and continue to advocate for the change.
“The word ‘disorder’ is both imprecise and stigmatizing,” Dr. Lipov said. “Because of stigma, many people with PTSD — especially those in the military — don’t get help, which my research has demonstrated.”
Patients are more likely to seek help if their symptoms are framed as manifestations of an injury that is diagnosable and treatable, like a broken leg, Dr. Lipov said. “Stigma can kill in very real ways, since delayed care or lack of care can directly lead to suicides, thus satisfying the reduce harm requirement for the name change.”
Neurobiology of Trauma
Dr. Lipov grew up with a veteran father affected by PTSD and a mother with debilitating depression who eventually took her life. “I understand the impact of trauma very well,” he said.
Although not a psychiatrist, Dr. Lipov pioneered a highly successful treatment for PTSD by adapting an anesthetic technique — the stellate ganglion block (SGB) — to reverse many trauma symptoms through the process of “rebooting.”
This involves reversing the activity of the sympathetic nervous system — the fight-or-flight response — to the pretrauma state by anesthetizing the sympathetic ganglion in the neck. Investigating how SGB can help ameliorate the symptoms of PTSD led him to investigate and describe the neurobiology of PTSD and the mechanism of action of SGB.
The impact of SGD on PTSD was supported by a small neuroimaging study demonstrating that the right amygdala — the area of the brain associated with the fear response — was overactivated in patients with PTSD but that this region was deactivated after the administration of SGB, Dr. Lipov said.
“I believe that psychiatric conditions are actually physiologic brain changes that can be measured by advanced neuroimaging technologies and then physiologically treated,” he stated.
He noted that a growing body of literature suggests that use of the SGB for PTSD can be effective “because PTSD has a neurobiological basis and is essentially caused by an actual injury to the brain.”
A Natural Response, Not a Disorder
Dr. Lipov’s clinical work treating PTSD as a brain injury led him to connect with Frank Ochberg, MD, a founding board member of the International Society for Traumatic Stress Studies, former associate director of the National Institute of Mental Health, and former director of the Michigan Department of Mental Health.
In 2012, Dr. Ochberg teamed up with retired Army General Peter Chiarelli and Jonathan Shay, MD, PhD, author of Achilles in Vietnam: Combat Trauma and the Undoing of Character, to petition the DSM-5 Steering Committee to change the name of PTSD to PTSI in the upcoming DSM-5.
Dr. Ochberg explained that Gen. Chiarelli believed the term “disorder” suggests a preexisting issue prior to enlistment, potentially making an individual appear “weak.” He noted that this stigma is particularly troubling for military personnel, who often avoid seeking so they are not perceived as vulnerable, which can lead to potentially dire consequences, including suicide.
“We received endorsements from many quarters, not only advocates for service members or veterans,” Dr. Ochberg said.
This included feminists like Gloria Steinem, who championed the rights of women who had survived rape, incest, and domestic violence. As one advocate put it: “The natural human reaction to a life-threatening event should not be labeled a disorder.”
The DSM-5 Steering Committee declined to change the name. “Their feeling was that if we change the word ‘disorder’ to something else, we’d have to change every condition in the DSM that’s called a ‘disorder’. And they felt there really was nothing wrong with the word,” said Dr. Ochberg.
However, Dr. Lipov noted that other diagnoses have undergone name changes in the DSM for the sake of accuracy or stigma reduction. For example, the term mental retardation (DSM-IV) was changed to intellectual disability in DSM-5, and gender identity disorder was changed to gender dysphoria.
A decade later, Dr. Lipov decided to try again. To bolster his contention, he conducted a telephone survey of 1025 individuals. Of these, about 50% had a PTSD diagnosis.
Approximately two thirds of respondents agreed that a name change to PTSI would reduce the stigma associated with the term “PTSD.” Over half said it would increase the likelihood they would seek medical help. Those diagnosed with PTSD were most likely to endorse the name change.
Dr. Lipov conducts an ongoing survey of psychiatrists to ascertain their views on the potential name change and hopes to include findings in future research and communication with the DSM-5 Steering Committee. In addition, he has developed a new survey that expands upon his original survey, which specifically looked at individuals with PTSD.
“The new survey includes a wide range of people, many of whom have never been diagnosed. One of the questions we ask is whether they’ve ever heard of PTSD, and then we ask them about their reaction to the term,” he said.
A Barrier to Care
Psychiatrist Marcel Green, MD, director of Hudson Mind in New York City, refers to himself as an “interventional psychiatrist,” as he employs a comprehensive approach that includes not only medication and psychotherapy but also specialized techniques like SBG for severe anxiety-related physical symptoms and certain pain conditions.
Dr. Green, who is not involved in the name change initiative, agrees that the term “disorder” carries more stigma than “injury” for many groups, including those who have experienced childhood trauma, those struggling with substance abuse, or who are from backgrounds or peer groups where seeking mental health care is stigmatized.
Patients like these “are looking to me to give them a language to frame what they’re going through, and I tell them their symptoms are consistent with PTSD,” he said. “But they tell me don’t see themselves as having a disorder, which hinders their pursuit of care.”
Framing the condition as an “injury” also aligns with the approach of using biologic interventions to address the injury. Dr. Green has found SGB helpful in treating substance abuse disorder too, “which is a form of escape from the hyperactivation that accompanies PTSD.” And after the procedure, “they’re more receptive to therapy.”
Unfortunately, said Dr. Lipov, the DSM Steering Committee rejected his proposed name change, stating that the “concept of disorder as a dividing line from, eg, normal reactions to stress, is a core concept in the DSM, and the term has only rarely been removed.”
Moreover, the committee “did not see sufficient evidence ... that the name PTSD is stigmatizing and actually deters people with the disorder from seeking treatment who would not be deterred from doing so by PTSI.”
‘An Avenue for Dignity’
Ken Duckworth, MD, chief medical officer of the National Alliance on Mental Illness (NAMI), noted that the organization does not have an official position on this issue. However, he shared his own personal perspective.
There may be merit in the proposed name change, said Dr. Duckworth, but more evidence is needed. “If it’s clear, after rigorous studies have been performed and there’s compelling data, that calling it a ‘disorder’ rather than an ‘injury’ is actually preventing people from getting the care they need, then it merits serious attention.”
If so, Dr. Duckworth would be “interested in having a conversation with the policy team at NAMI to start to see if we could activate the DSM Committee.”
Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto in Ontario, Canada, and head of the Mood Disorders Psychopharmacology Unit, said the name change initiative is a “really interesting proposal.”
Dr. McIntyre, chairman and executive director of the Brain and Cognition Discovery Foundation, also in Toronto, who is not involved in the initiative, has also heard “many people say that the term ‘disorder’ is stigmatizing and might even come across as pejorative in some ways.”
By contrast, “the word ‘injury’ parallels physical injury, and what we currently call ‘PTSD’ is a psychological or emotional injury no less devastating than torn tissue or broken bones,” added Dr. McIntyre, who is also the chairman of the board of the Depression and Bipolar Support Alliance.
Dr. Ochberg agreed. “In the military, ‘injury’ opens up an avenue for dignity, for a medal. Being injured and learning how to deal with an injury is part of having yet another honorable task that comes from being an honorable person who did an honorable thing.”
While disappointed, Dr. Lipov does not plan to give up on his vision. “I will continue to amass evidence that the word ‘PTSD’ is stigmatizing and indeed does prevent people from seeking care and will resubmit the proposal to the DSM Steering Committee when I have gathered a larger body of compelling evidence.”
Currently, Dr. Lipov is in active discussions with the special operations force of the US Army to obtain more evidence. “This will be the follow-up to bolster the opinion of Peter Chiarelli,” he said. “It is known that suicide and PTSD are highly related. This is especially urgent and relevant because recent data suggest suicide rate of military personnel in the VA may be as high as 44 per day,” Dr. Lipov said.
Dr. Lipov is the chief medical officer and an investor in the Stella Center. Dr. Green performs SGBs as part of his psychiatric practice. Drs. Ochberg, McIntyre, and Duckworth reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
The Disturbing Sexual Trend With Real Health Consequences
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I have an interesting topic for you — kind of shocking, actually. Some of you may have read a story earlier this year in The New York Times about the alarming rise among young people of choking or strangulation during sex. I spoke recently with Dr. Debby Herbenick about this concerning and violent trend. Dr. Herbenick is a well-known sexuality researcher and professor at the Indiana University School of Public Health. Welcome, Dr. Herbenick. Can you tell us about your research into this new trend?
Debby Herbenick, PhD: This is some of the most important research that I’ve done. I’ve been studying sexual behaviors and trends for about 14 years in terms of nationally representative studies that we do. Over time, we noticed a trend of increasing prevalence of rough sex practices.
Now, there’s always been a lot of sexual diversity in the world throughout history. But . The increase is mostly seen in teenagers and young adults.
We’ve done US nationally representative surveys as well as college campus representative surveys. We find that consistently across four campus representative surveys that 64% of women report having ever been choked during sex, and around 1 in 3 women (aged 18-24 years) throughout the whole country report having been choked during their most recent sexual activity with another person. They call it choking, but because it involves usually one hand — sometimes two hands or a forearm or an object, like a belt or a cord to tie around the neck — it is technically strangulation, because it’s external pressure to the neck to reduce or stop airflow or blood flow.
Dr. Rubin: These numbers are staggering, right? Everyone listening now is taking care of someone who has been strangled as a form of sexual pleasure. What does this mean from a safety perspective? And as doctors who are working these patients up for migraines and other health problems, what is the research showing?
Dr. Herbenick: We certainly are seeing people report recurrent headaches and ringing in the ears. There are things we’ve just barely scratched the surface on. Those of us working in this space believe that for anybody coming in for an unexplained stroke (for example, under age 50), you might consider some imaging to see if they have a dissection. We are hearing about people who, when you really probe to find out whether they’ve had pressure on the neck, they report that indeed that they have. So, we have to be thinking about neurologic symptoms. We know that they’re experiencing these at a pretty high rate.
For people who are engaging in these practices, they should know about the health risks, but we find that most don’t. They may have heard that if it’s really intense high pressure, that in rare cases people can die, but most have never heard of anything in between. So, they’re not necessarily connecting their voice hoarseness, or the recurrent headaches or the sensitivity to light they are having, to an experience of being choked. We need to be paying attention to neurologic symptoms.
Most physicians I speak with at conferences say that where they feel like they can step into this conversation is through anticipatory guidance and letting their patients know that they may have heard about this trend, and a lot of people are talking about the health consequences, and I want to share some information with you — not coming at it from a place of shame or judgment, but providing some information so that [patients] actually get some medical facts about this that could be lifesaving.
Dr. Rubin: I see such a big gap in my medical training. I was taught to say, “Hey, do you smoke, do you drink, do you do drugs? Do you have sex? Men, women, or both?”And that’s it. And then maybe use birth control, and don’t get an STD, thinking about herpes, syphilis, gonorrhea, and chlamydia. We weren’t really trained to talk to patients about what kind of sex they are having, or how to talk to patients in a way that is open-minded but also safety-conscious and how the concept of safe sex is more than wear a condom and use birth control.
This idea of rough sex practices and how to talk to teenagers — maybe our pediatricians should be talking about this. Where do we start in terms of how to bring up these conversations and with what level of detail?
Dr. Herbenick: We find that some young people are already being asked about some of the effects that might be showing on their bodies. It might be that their provider notices some bruising, or marks on their bodies from other types of rough sex practices like hitting and spanking. So that could be an entry point there. Choking is far more prevalent than slapping, so if you’re seeing some marks on the body, then it’s also a good time to ask about other practices they might be engaging in, especially higher risk ones like choking or strangulation. It’s offering some information and even saying, “Look, I’m not here to shame or judge you. I just want you to have some information about this” and giving them an opportunity to ask questions, too.
We have found that almost nobody talks with their nurse or doctor, even if they have symptoms after being choked or strangled during sex. Just 1% of women with choking-related symptoms, 7% of men, and far fewer trans and nonbinary young people report talking with a nurse or doctor, mostly because they say it doesn’t seem like a big deal. The symptoms got better quickly. Sometimes they’re afraid of being shamed for their sexual behavior, and that’s why they say they don’t talk with somebody.
They need some type of open-door anticipatory guidance as a way forward. Not everyone is comfortable directly asking whether a patient is engaging in this, but at least letting people know that you’ve heard of this behavior and providing some medical facts can give us a step forward with creating these conversations.
Dr. Rubin: Can you tell us where is this research going in terms of next steps? Other things that you’re looking at? And what are you excited about?
Dr. Herbenick: I’m excited about some work I did with a collaborator and colleague of mine, Dr. Keisuke Kawata, that he led a couple of years ago. He’s a neuroscientist. We were looking at potential cumulative effects on the brain. Now we’re taking some of that research into its next steps. We’re also doing more focused studies on other health consequences and hopefully finding out how we can test different educational messages and get people to learn more fact-based information about this, and then see if that is effective in prevention.
Dr. Rubin: It sounds like a public health campaign is really needed about how to get the word out there about the health consequences of these activities. We’re asking people often enough. In my clinic, I try to keep it open-ended — tell me what sex looks like. What does it look like, and what do you want it to look like? Because I see a lot of people with problems, but if they don’t bring it to me, I don’t necessarily bring it up to them. Until I heard your lecture, and I thought, oh my gosh, I’m not even asking the right questions. Are you hopeful that there will be more public health messaging out there?
Dr. Herbenick: I am. Years ago, when the child and adolescent choking game became a thing, the Centers for Disease Control and Prevention (CDC) issued reports about it and warnings to parents. And this is a far, far higher prevalence than that ever was. So, I would love to see organizations like the CDC and medical groups getting involved and educating their members and making statements. This is really impacting a huge generation of girls and women, because when it happens during sex between women and men, the choking is mostly happening to the girls and women. It’s also prevalent among sexual minority individuals. But we are talking about this whole generation of young women and what’s happening to their bodies and their brain health. We really need to step into this conversation.
Dr. Rubin: Very few of us are sexual medicine–trained physicians, and very few of us feel confident and comfortable talking about sexual health issues. But people are getting hurt. People are having real consequences of these behaviors because of our lack of education, knowledge, and even discussion around it. So thank you for doing this research, because had you not done this research, we wouldn’t have found out that 64% of people are engaging in these types of activities. That is not rare.
Dr. Rubin is an assistant clinical professor, Department of Urology, at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I have an interesting topic for you — kind of shocking, actually. Some of you may have read a story earlier this year in The New York Times about the alarming rise among young people of choking or strangulation during sex. I spoke recently with Dr. Debby Herbenick about this concerning and violent trend. Dr. Herbenick is a well-known sexuality researcher and professor at the Indiana University School of Public Health. Welcome, Dr. Herbenick. Can you tell us about your research into this new trend?
Debby Herbenick, PhD: This is some of the most important research that I’ve done. I’ve been studying sexual behaviors and trends for about 14 years in terms of nationally representative studies that we do. Over time, we noticed a trend of increasing prevalence of rough sex practices.
Now, there’s always been a lot of sexual diversity in the world throughout history. But . The increase is mostly seen in teenagers and young adults.
We’ve done US nationally representative surveys as well as college campus representative surveys. We find that consistently across four campus representative surveys that 64% of women report having ever been choked during sex, and around 1 in 3 women (aged 18-24 years) throughout the whole country report having been choked during their most recent sexual activity with another person. They call it choking, but because it involves usually one hand — sometimes two hands or a forearm or an object, like a belt or a cord to tie around the neck — it is technically strangulation, because it’s external pressure to the neck to reduce or stop airflow or blood flow.
Dr. Rubin: These numbers are staggering, right? Everyone listening now is taking care of someone who has been strangled as a form of sexual pleasure. What does this mean from a safety perspective? And as doctors who are working these patients up for migraines and other health problems, what is the research showing?
Dr. Herbenick: We certainly are seeing people report recurrent headaches and ringing in the ears. There are things we’ve just barely scratched the surface on. Those of us working in this space believe that for anybody coming in for an unexplained stroke (for example, under age 50), you might consider some imaging to see if they have a dissection. We are hearing about people who, when you really probe to find out whether they’ve had pressure on the neck, they report that indeed that they have. So, we have to be thinking about neurologic symptoms. We know that they’re experiencing these at a pretty high rate.
For people who are engaging in these practices, they should know about the health risks, but we find that most don’t. They may have heard that if it’s really intense high pressure, that in rare cases people can die, but most have never heard of anything in between. So, they’re not necessarily connecting their voice hoarseness, or the recurrent headaches or the sensitivity to light they are having, to an experience of being choked. We need to be paying attention to neurologic symptoms.
Most physicians I speak with at conferences say that where they feel like they can step into this conversation is through anticipatory guidance and letting their patients know that they may have heard about this trend, and a lot of people are talking about the health consequences, and I want to share some information with you — not coming at it from a place of shame or judgment, but providing some information so that [patients] actually get some medical facts about this that could be lifesaving.
Dr. Rubin: I see such a big gap in my medical training. I was taught to say, “Hey, do you smoke, do you drink, do you do drugs? Do you have sex? Men, women, or both?”And that’s it. And then maybe use birth control, and don’t get an STD, thinking about herpes, syphilis, gonorrhea, and chlamydia. We weren’t really trained to talk to patients about what kind of sex they are having, or how to talk to patients in a way that is open-minded but also safety-conscious and how the concept of safe sex is more than wear a condom and use birth control.
This idea of rough sex practices and how to talk to teenagers — maybe our pediatricians should be talking about this. Where do we start in terms of how to bring up these conversations and with what level of detail?
Dr. Herbenick: We find that some young people are already being asked about some of the effects that might be showing on their bodies. It might be that their provider notices some bruising, or marks on their bodies from other types of rough sex practices like hitting and spanking. So that could be an entry point there. Choking is far more prevalent than slapping, so if you’re seeing some marks on the body, then it’s also a good time to ask about other practices they might be engaging in, especially higher risk ones like choking or strangulation. It’s offering some information and even saying, “Look, I’m not here to shame or judge you. I just want you to have some information about this” and giving them an opportunity to ask questions, too.
We have found that almost nobody talks with their nurse or doctor, even if they have symptoms after being choked or strangled during sex. Just 1% of women with choking-related symptoms, 7% of men, and far fewer trans and nonbinary young people report talking with a nurse or doctor, mostly because they say it doesn’t seem like a big deal. The symptoms got better quickly. Sometimes they’re afraid of being shamed for their sexual behavior, and that’s why they say they don’t talk with somebody.
They need some type of open-door anticipatory guidance as a way forward. Not everyone is comfortable directly asking whether a patient is engaging in this, but at least letting people know that you’ve heard of this behavior and providing some medical facts can give us a step forward with creating these conversations.
Dr. Rubin: Can you tell us where is this research going in terms of next steps? Other things that you’re looking at? And what are you excited about?
Dr. Herbenick: I’m excited about some work I did with a collaborator and colleague of mine, Dr. Keisuke Kawata, that he led a couple of years ago. He’s a neuroscientist. We were looking at potential cumulative effects on the brain. Now we’re taking some of that research into its next steps. We’re also doing more focused studies on other health consequences and hopefully finding out how we can test different educational messages and get people to learn more fact-based information about this, and then see if that is effective in prevention.
Dr. Rubin: It sounds like a public health campaign is really needed about how to get the word out there about the health consequences of these activities. We’re asking people often enough. In my clinic, I try to keep it open-ended — tell me what sex looks like. What does it look like, and what do you want it to look like? Because I see a lot of people with problems, but if they don’t bring it to me, I don’t necessarily bring it up to them. Until I heard your lecture, and I thought, oh my gosh, I’m not even asking the right questions. Are you hopeful that there will be more public health messaging out there?
Dr. Herbenick: I am. Years ago, when the child and adolescent choking game became a thing, the Centers for Disease Control and Prevention (CDC) issued reports about it and warnings to parents. And this is a far, far higher prevalence than that ever was. So, I would love to see organizations like the CDC and medical groups getting involved and educating their members and making statements. This is really impacting a huge generation of girls and women, because when it happens during sex between women and men, the choking is mostly happening to the girls and women. It’s also prevalent among sexual minority individuals. But we are talking about this whole generation of young women and what’s happening to their bodies and their brain health. We really need to step into this conversation.
Dr. Rubin: Very few of us are sexual medicine–trained physicians, and very few of us feel confident and comfortable talking about sexual health issues. But people are getting hurt. People are having real consequences of these behaviors because of our lack of education, knowledge, and even discussion around it. So thank you for doing this research, because had you not done this research, we wouldn’t have found out that 64% of people are engaging in these types of activities. That is not rare.
Dr. Rubin is an assistant clinical professor, Department of Urology, at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: I have an interesting topic for you — kind of shocking, actually. Some of you may have read a story earlier this year in The New York Times about the alarming rise among young people of choking or strangulation during sex. I spoke recently with Dr. Debby Herbenick about this concerning and violent trend. Dr. Herbenick is a well-known sexuality researcher and professor at the Indiana University School of Public Health. Welcome, Dr. Herbenick. Can you tell us about your research into this new trend?
Debby Herbenick, PhD: This is some of the most important research that I’ve done. I’ve been studying sexual behaviors and trends for about 14 years in terms of nationally representative studies that we do. Over time, we noticed a trend of increasing prevalence of rough sex practices.
Now, there’s always been a lot of sexual diversity in the world throughout history. But . The increase is mostly seen in teenagers and young adults.
We’ve done US nationally representative surveys as well as college campus representative surveys. We find that consistently across four campus representative surveys that 64% of women report having ever been choked during sex, and around 1 in 3 women (aged 18-24 years) throughout the whole country report having been choked during their most recent sexual activity with another person. They call it choking, but because it involves usually one hand — sometimes two hands or a forearm or an object, like a belt or a cord to tie around the neck — it is technically strangulation, because it’s external pressure to the neck to reduce or stop airflow or blood flow.
Dr. Rubin: These numbers are staggering, right? Everyone listening now is taking care of someone who has been strangled as a form of sexual pleasure. What does this mean from a safety perspective? And as doctors who are working these patients up for migraines and other health problems, what is the research showing?
Dr. Herbenick: We certainly are seeing people report recurrent headaches and ringing in the ears. There are things we’ve just barely scratched the surface on. Those of us working in this space believe that for anybody coming in for an unexplained stroke (for example, under age 50), you might consider some imaging to see if they have a dissection. We are hearing about people who, when you really probe to find out whether they’ve had pressure on the neck, they report that indeed that they have. So, we have to be thinking about neurologic symptoms. We know that they’re experiencing these at a pretty high rate.
For people who are engaging in these practices, they should know about the health risks, but we find that most don’t. They may have heard that if it’s really intense high pressure, that in rare cases people can die, but most have never heard of anything in between. So, they’re not necessarily connecting their voice hoarseness, or the recurrent headaches or the sensitivity to light they are having, to an experience of being choked. We need to be paying attention to neurologic symptoms.
Most physicians I speak with at conferences say that where they feel like they can step into this conversation is through anticipatory guidance and letting their patients know that they may have heard about this trend, and a lot of people are talking about the health consequences, and I want to share some information with you — not coming at it from a place of shame or judgment, but providing some information so that [patients] actually get some medical facts about this that could be lifesaving.
Dr. Rubin: I see such a big gap in my medical training. I was taught to say, “Hey, do you smoke, do you drink, do you do drugs? Do you have sex? Men, women, or both?”And that’s it. And then maybe use birth control, and don’t get an STD, thinking about herpes, syphilis, gonorrhea, and chlamydia. We weren’t really trained to talk to patients about what kind of sex they are having, or how to talk to patients in a way that is open-minded but also safety-conscious and how the concept of safe sex is more than wear a condom and use birth control.
This idea of rough sex practices and how to talk to teenagers — maybe our pediatricians should be talking about this. Where do we start in terms of how to bring up these conversations and with what level of detail?
Dr. Herbenick: We find that some young people are already being asked about some of the effects that might be showing on their bodies. It might be that their provider notices some bruising, or marks on their bodies from other types of rough sex practices like hitting and spanking. So that could be an entry point there. Choking is far more prevalent than slapping, so if you’re seeing some marks on the body, then it’s also a good time to ask about other practices they might be engaging in, especially higher risk ones like choking or strangulation. It’s offering some information and even saying, “Look, I’m not here to shame or judge you. I just want you to have some information about this” and giving them an opportunity to ask questions, too.
We have found that almost nobody talks with their nurse or doctor, even if they have symptoms after being choked or strangled during sex. Just 1% of women with choking-related symptoms, 7% of men, and far fewer trans and nonbinary young people report talking with a nurse or doctor, mostly because they say it doesn’t seem like a big deal. The symptoms got better quickly. Sometimes they’re afraid of being shamed for their sexual behavior, and that’s why they say they don’t talk with somebody.
They need some type of open-door anticipatory guidance as a way forward. Not everyone is comfortable directly asking whether a patient is engaging in this, but at least letting people know that you’ve heard of this behavior and providing some medical facts can give us a step forward with creating these conversations.
Dr. Rubin: Can you tell us where is this research going in terms of next steps? Other things that you’re looking at? And what are you excited about?
Dr. Herbenick: I’m excited about some work I did with a collaborator and colleague of mine, Dr. Keisuke Kawata, that he led a couple of years ago. He’s a neuroscientist. We were looking at potential cumulative effects on the brain. Now we’re taking some of that research into its next steps. We’re also doing more focused studies on other health consequences and hopefully finding out how we can test different educational messages and get people to learn more fact-based information about this, and then see if that is effective in prevention.
Dr. Rubin: It sounds like a public health campaign is really needed about how to get the word out there about the health consequences of these activities. We’re asking people often enough. In my clinic, I try to keep it open-ended — tell me what sex looks like. What does it look like, and what do you want it to look like? Because I see a lot of people with problems, but if they don’t bring it to me, I don’t necessarily bring it up to them. Until I heard your lecture, and I thought, oh my gosh, I’m not even asking the right questions. Are you hopeful that there will be more public health messaging out there?
Dr. Herbenick: I am. Years ago, when the child and adolescent choking game became a thing, the Centers for Disease Control and Prevention (CDC) issued reports about it and warnings to parents. And this is a far, far higher prevalence than that ever was. So, I would love to see organizations like the CDC and medical groups getting involved and educating their members and making statements. This is really impacting a huge generation of girls and women, because when it happens during sex between women and men, the choking is mostly happening to the girls and women. It’s also prevalent among sexual minority individuals. But we are talking about this whole generation of young women and what’s happening to their bodies and their brain health. We really need to step into this conversation.
Dr. Rubin: Very few of us are sexual medicine–trained physicians, and very few of us feel confident and comfortable talking about sexual health issues. But people are getting hurt. People are having real consequences of these behaviors because of our lack of education, knowledge, and even discussion around it. So thank you for doing this research, because had you not done this research, we wouldn’t have found out that 64% of people are engaging in these types of activities. That is not rare.
Dr. Rubin is an assistant clinical professor, Department of Urology, at Georgetown University, Washington. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article first appeared on Medscape.com.
‘Shockingly High’ Rate of TBI in Older Adults
TOPLINE:
, a new study showed.
METHODOLOGY:
- Researchers analyzed data from approximately 9200 Medicare enrollees who were part of the Health and Retirement Study (HRS), aged 65 years and older, from 2000 to 2018.
- The baseline date was the date of the first age eligible HRS core interview in the community in 2000 or later.
- Incident TBI cases came from an updated list of the International Classification of Diseases (ICD), 9th and 10th edition codes, from the Defense and Veterans Brain Injury Center and the Armed Forces Health Surveillance Branch for TBI surveillance.
- Codes corresponded with emergency department, CT, and/or fMRI visits.
TAKEAWAY:
- Almost 13% of older individuals (n = 797) experienced TBI during the study, highlighting its significant prevalence in this population.
- Older adults (mean age at baseline, 75 years) who experienced TBI during the study period were more likely to be women and White individuals as well as individuals having higher levels of education and normal cognition (P < .001), challenging previous assumptions about risk factors.
- The study underscored the need for targeted interventions and research focused on TBI prevention and postdischarge care in older adults.
IN PRACTICE:
“The number of people 65 and older with TBI is shockingly high,” senior author Raquel Gardner, MD, said in a press release. “We need evidence-based guidelines to inform postdischarge care of this very large Medicare population and more research on post-TBI dementia prevention and repeat injury prevention.”
SOURCE:
The study was led by Erica Kornblith, PhD, of the University of California, San Francisco. It was published online in JAMA Network Open.
LIMITATIONS:
The study’s reliance on ICD codes for TBI identification may not capture the full spectrum of TBI severity. Self-reported data on sociodemographic factors may have introduced bias, affecting the accuracy of associations with TBI incidence. In addition, the findings’ generalizability may be limited due to the study’s focus on Medicare enrollees, potentially excluding those from diverse socioeconomic backgrounds.
DISCLOSURES:
The study was funded by the Alzheimer’s Association, the US Department of Veterans Affairs, the National Institute on Aging, and the Department of Defense. Disclosures are noted in the original study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study showed.
METHODOLOGY:
- Researchers analyzed data from approximately 9200 Medicare enrollees who were part of the Health and Retirement Study (HRS), aged 65 years and older, from 2000 to 2018.
- The baseline date was the date of the first age eligible HRS core interview in the community in 2000 or later.
- Incident TBI cases came from an updated list of the International Classification of Diseases (ICD), 9th and 10th edition codes, from the Defense and Veterans Brain Injury Center and the Armed Forces Health Surveillance Branch for TBI surveillance.
- Codes corresponded with emergency department, CT, and/or fMRI visits.
TAKEAWAY:
- Almost 13% of older individuals (n = 797) experienced TBI during the study, highlighting its significant prevalence in this population.
- Older adults (mean age at baseline, 75 years) who experienced TBI during the study period were more likely to be women and White individuals as well as individuals having higher levels of education and normal cognition (P < .001), challenging previous assumptions about risk factors.
- The study underscored the need for targeted interventions and research focused on TBI prevention and postdischarge care in older adults.
IN PRACTICE:
“The number of people 65 and older with TBI is shockingly high,” senior author Raquel Gardner, MD, said in a press release. “We need evidence-based guidelines to inform postdischarge care of this very large Medicare population and more research on post-TBI dementia prevention and repeat injury prevention.”
SOURCE:
The study was led by Erica Kornblith, PhD, of the University of California, San Francisco. It was published online in JAMA Network Open.
LIMITATIONS:
The study’s reliance on ICD codes for TBI identification may not capture the full spectrum of TBI severity. Self-reported data on sociodemographic factors may have introduced bias, affecting the accuracy of associations with TBI incidence. In addition, the findings’ generalizability may be limited due to the study’s focus on Medicare enrollees, potentially excluding those from diverse socioeconomic backgrounds.
DISCLOSURES:
The study was funded by the Alzheimer’s Association, the US Department of Veterans Affairs, the National Institute on Aging, and the Department of Defense. Disclosures are noted in the original study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
, a new study showed.
METHODOLOGY:
- Researchers analyzed data from approximately 9200 Medicare enrollees who were part of the Health and Retirement Study (HRS), aged 65 years and older, from 2000 to 2018.
- The baseline date was the date of the first age eligible HRS core interview in the community in 2000 or later.
- Incident TBI cases came from an updated list of the International Classification of Diseases (ICD), 9th and 10th edition codes, from the Defense and Veterans Brain Injury Center and the Armed Forces Health Surveillance Branch for TBI surveillance.
- Codes corresponded with emergency department, CT, and/or fMRI visits.
TAKEAWAY:
- Almost 13% of older individuals (n = 797) experienced TBI during the study, highlighting its significant prevalence in this population.
- Older adults (mean age at baseline, 75 years) who experienced TBI during the study period were more likely to be women and White individuals as well as individuals having higher levels of education and normal cognition (P < .001), challenging previous assumptions about risk factors.
- The study underscored the need for targeted interventions and research focused on TBI prevention and postdischarge care in older adults.
IN PRACTICE:
“The number of people 65 and older with TBI is shockingly high,” senior author Raquel Gardner, MD, said in a press release. “We need evidence-based guidelines to inform postdischarge care of this very large Medicare population and more research on post-TBI dementia prevention and repeat injury prevention.”
SOURCE:
The study was led by Erica Kornblith, PhD, of the University of California, San Francisco. It was published online in JAMA Network Open.
LIMITATIONS:
The study’s reliance on ICD codes for TBI identification may not capture the full spectrum of TBI severity. Self-reported data on sociodemographic factors may have introduced bias, affecting the accuracy of associations with TBI incidence. In addition, the findings’ generalizability may be limited due to the study’s focus on Medicare enrollees, potentially excluding those from diverse socioeconomic backgrounds.
DISCLOSURES:
The study was funded by the Alzheimer’s Association, the US Department of Veterans Affairs, the National Institute on Aging, and the Department of Defense. Disclosures are noted in the original study.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
In the Future, a Robot Intensivist May Save Your Life
This transcript has been edited for clarity.
They call it the “golden hour”: 60 minutes, give or take, when the chance to save the life of a trauma victim is at its greatest. If the patient can be resuscitated and stabilized in that time window, they stand a good chance of surviving. If not, well, they don’t.
But resuscitation is complicated. It requires blood products, fluids, vasopressors — all given in precise doses in response to rapidly changing hemodynamics. To do it right takes specialized training, advanced life support (ALS). If the patient is in a remote area or an area without ALS-certified emergency medical services, or is far from the nearest trauma center, that golden hour is lost. And the patient may be as well.
But we live in the future. We have robots in factories, self-driving cars, autonomous drones. Why not an autonomous trauma doctor? If you are in a life-threatening accident, would you want to be treated ... by a robot?
Enter “resuscitation based on functional hemodynamic monitoring,” or “ReFit,” introduced in this article appearing in the journal Intensive Care Medicine Experimental.
The idea behind ReFit is straightforward. Resuscitation after trauma should be based on hitting key hemodynamic targets using the tools we have available in the field: blood, fluids, pressors. The researchers wanted to develop a closed-loop system, something that could be used by minimally trained personnel. The input to the system? Hemodynamic data, provided through a single measurement device, an arterial catheter. The output: blood, fluids, and pressors, delivered intravenously.
The body (a prototype) of the system looks like this. You can see various pumps labeled with various fluids, electronic controllers, and so forth.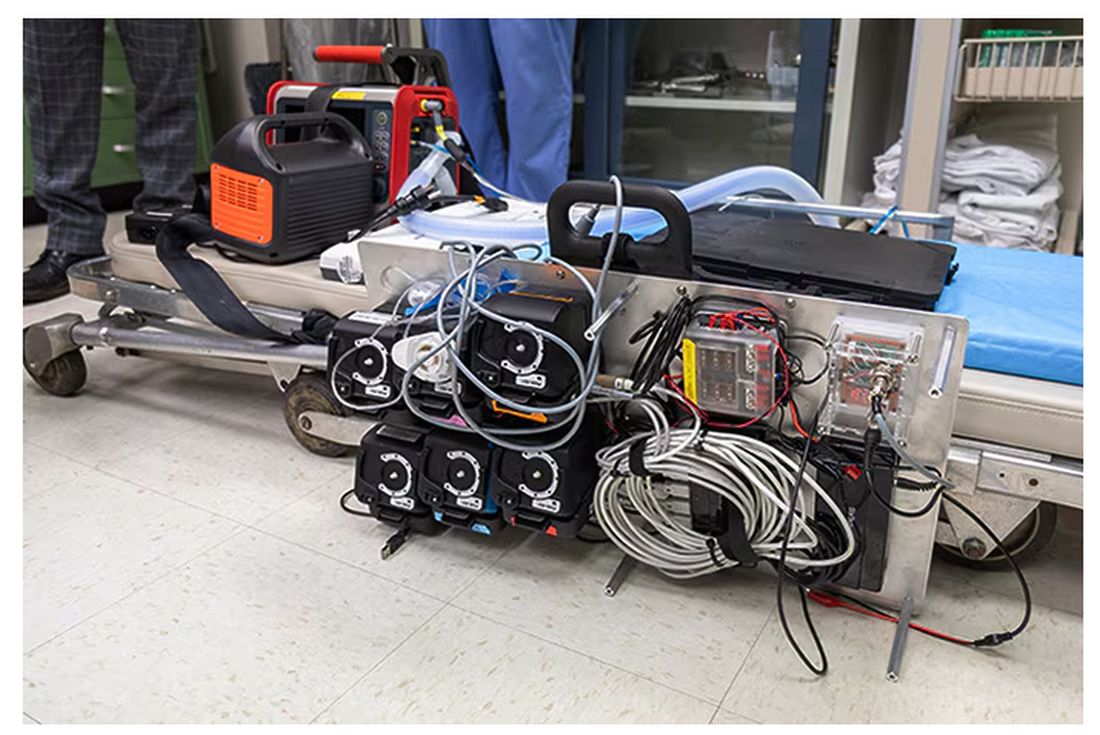
If that’s the body, then this is the brain – a ruggedized laptop interpreting a readout of that arterial catheter.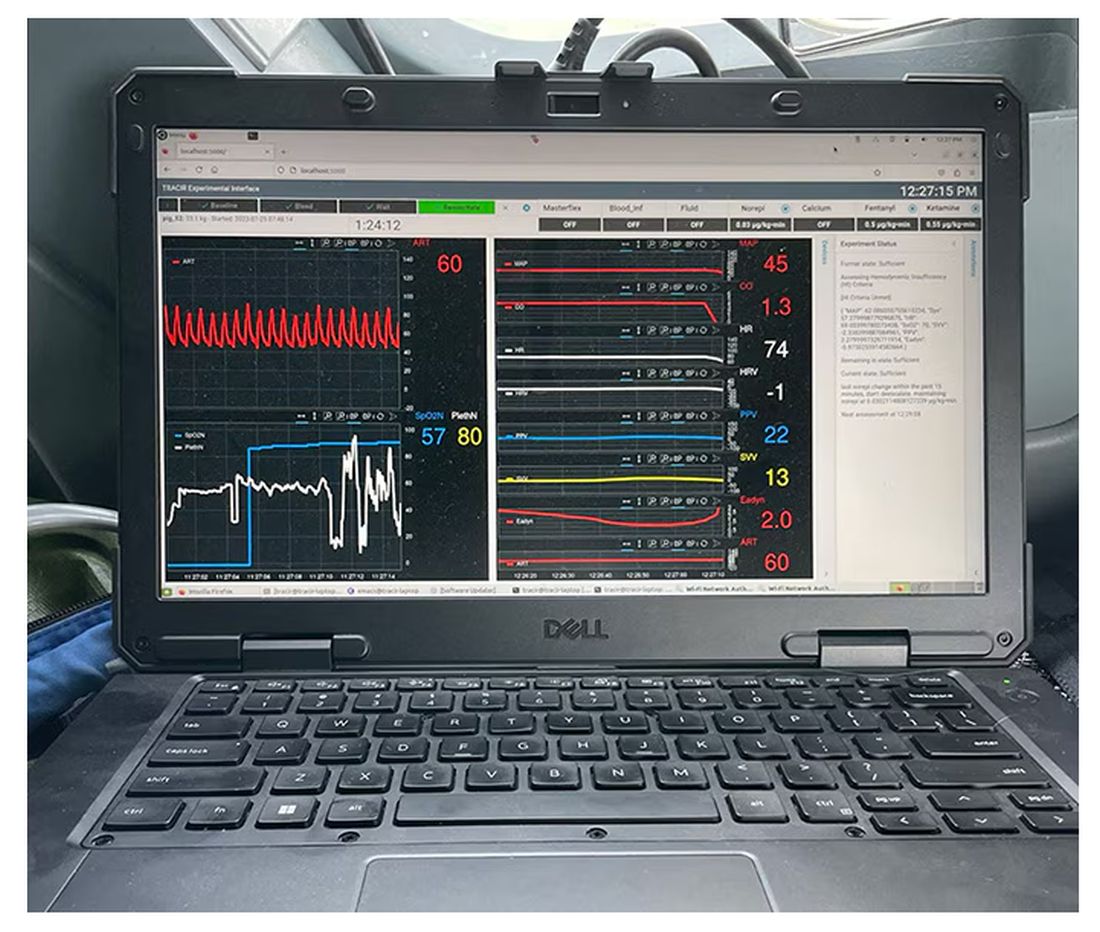
If that’s the brain, then the ReFit algorithm is the mind. The algorithm does its best to leverage all the data it can, so I want to walk through it in a bit of detail.
First, check to see whether the patient is stable, defined as a heart rate < 110 beats/min and a mean arterial pressure > 60 mm Hg. If not, you’re off to the races, starting with a bolus of whole blood.
Next, the algorithm gets really interesting. If the patient is still unstable, the computer assesses fluid responsiveness by giving a test dose of fluid and measuring the pulse pressure variation. Greater pulse pressure variation means more fluid responsiveness and the algorithm gives more fluid. Less pulse pressure variation leads the algorithm to uptitrate pressors — in this case, norepinephrine.
This cycle of evaluation and response keeps repeating. The computer titrates fluids and pressors up and down entirely on its own, in theory freeing the human team members to do other things, like getting the patient to a trauma center for definitive care.
So, how do you test whether something like this works? Clearly, you don’t want the trial run of a system like this to be used on a real human suffering from a real traumatic injury.
Once again, we have animals to thank for research advances — in this case, pigs. Fifteen pigs are described in the study. To simulate a severe, hemorrhagic trauma, they were anesthetized and the liver was lacerated. They were then observed passively until the mean arterial pressure had dropped to below 40 mm Hg.
This is a pretty severe injury. Three unfortunate animals served as controls, two of which died within the 3-hour time window of the study. Eight animals were plugged into the ReFit system.
For a window into what happens during this process, let’s take a look at the mean arterial pressure and heart rate readouts for one of the animals. You see that the blood pressure starts to fall precipitously after the liver laceration. The heart rate quickly picks up to compensate, raising the mean arterial pressure a bit, but this would be unsustainable with ongoing bleeding.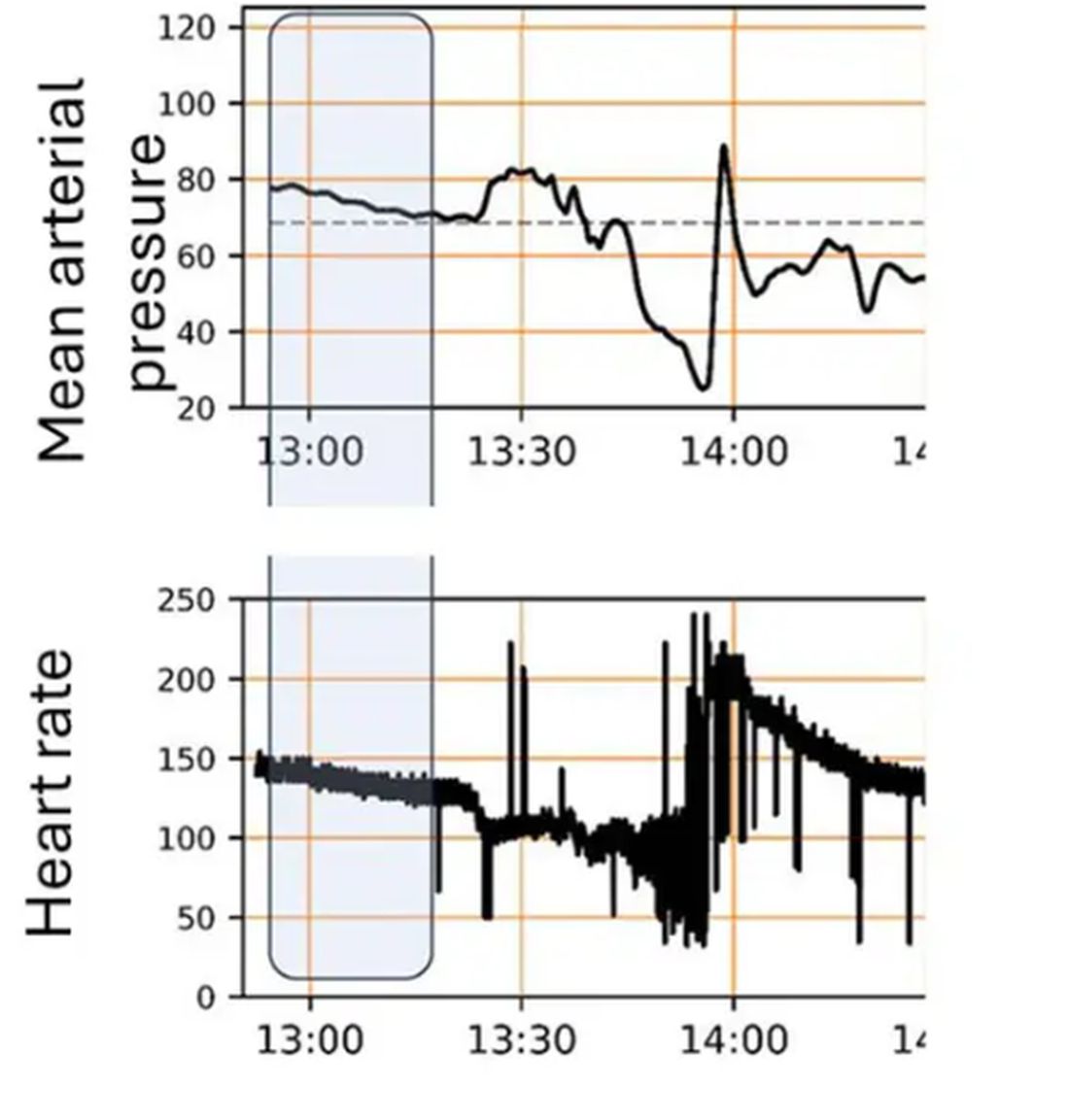
Here, the ReFit system takes over. Autonomously, the system administers two units of blood, followed by fluids, and then norepinephrine or further fluids per the protocol I described earlier. 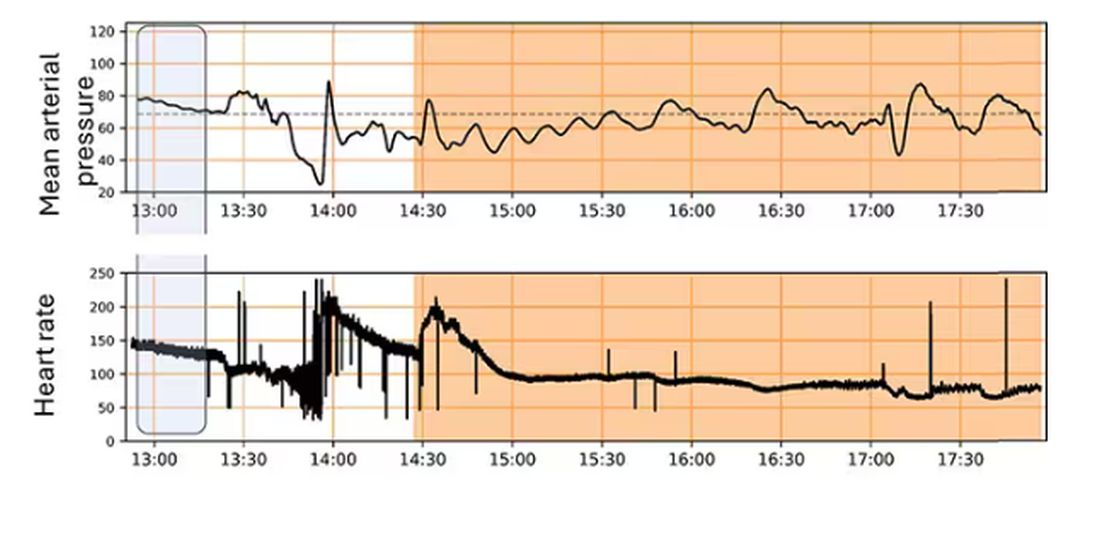
The practical upshot of all of this is stabilization, despite an as-yet untreated liver laceration.
Could an experienced ALS provider do this? Of course. But, as I mentioned before, you aren’t always near an experienced ALS provider.
This is all well and good in the lab, but in the real world, you actually need to transport a trauma patient. The researchers tried this also. To prove feasibility, four pigs were taken from the lab to the top of the University of Pittsburgh Medical Center, flown to Allegheny County Airport and back. Total time before liver laceration repair? Three hours. And all four survived.
It won’t surprise you to hear that this work was funded by the Department of Defense. You can see how a system like this, made a bit more rugged, a bit smaller, and a bit more self-contained could have real uses in the battlefield. But trauma is not unique to war, and something that can extend the time you have to safely transport a patient to definitive care — well, that’s worth its weight in golden hours.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
They call it the “golden hour”: 60 minutes, give or take, when the chance to save the life of a trauma victim is at its greatest. If the patient can be resuscitated and stabilized in that time window, they stand a good chance of surviving. If not, well, they don’t.
But resuscitation is complicated. It requires blood products, fluids, vasopressors — all given in precise doses in response to rapidly changing hemodynamics. To do it right takes specialized training, advanced life support (ALS). If the patient is in a remote area or an area without ALS-certified emergency medical services, or is far from the nearest trauma center, that golden hour is lost. And the patient may be as well.
But we live in the future. We have robots in factories, self-driving cars, autonomous drones. Why not an autonomous trauma doctor? If you are in a life-threatening accident, would you want to be treated ... by a robot?
Enter “resuscitation based on functional hemodynamic monitoring,” or “ReFit,” introduced in this article appearing in the journal Intensive Care Medicine Experimental.
The idea behind ReFit is straightforward. Resuscitation after trauma should be based on hitting key hemodynamic targets using the tools we have available in the field: blood, fluids, pressors. The researchers wanted to develop a closed-loop system, something that could be used by minimally trained personnel. The input to the system? Hemodynamic data, provided through a single measurement device, an arterial catheter. The output: blood, fluids, and pressors, delivered intravenously.
The body (a prototype) of the system looks like this. You can see various pumps labeled with various fluids, electronic controllers, and so forth.
If that’s the body, then this is the brain – a ruggedized laptop interpreting a readout of that arterial catheter.
If that’s the brain, then the ReFit algorithm is the mind. The algorithm does its best to leverage all the data it can, so I want to walk through it in a bit of detail.
First, check to see whether the patient is stable, defined as a heart rate < 110 beats/min and a mean arterial pressure > 60 mm Hg. If not, you’re off to the races, starting with a bolus of whole blood.
Next, the algorithm gets really interesting. If the patient is still unstable, the computer assesses fluid responsiveness by giving a test dose of fluid and measuring the pulse pressure variation. Greater pulse pressure variation means more fluid responsiveness and the algorithm gives more fluid. Less pulse pressure variation leads the algorithm to uptitrate pressors — in this case, norepinephrine.
This cycle of evaluation and response keeps repeating. The computer titrates fluids and pressors up and down entirely on its own, in theory freeing the human team members to do other things, like getting the patient to a trauma center for definitive care.
So, how do you test whether something like this works? Clearly, you don’t want the trial run of a system like this to be used on a real human suffering from a real traumatic injury.
Once again, we have animals to thank for research advances — in this case, pigs. Fifteen pigs are described in the study. To simulate a severe, hemorrhagic trauma, they were anesthetized and the liver was lacerated. They were then observed passively until the mean arterial pressure had dropped to below 40 mm Hg.
This is a pretty severe injury. Three unfortunate animals served as controls, two of which died within the 3-hour time window of the study. Eight animals were plugged into the ReFit system.
For a window into what happens during this process, let’s take a look at the mean arterial pressure and heart rate readouts for one of the animals. You see that the blood pressure starts to fall precipitously after the liver laceration. The heart rate quickly picks up to compensate, raising the mean arterial pressure a bit, but this would be unsustainable with ongoing bleeding.
Here, the ReFit system takes over. Autonomously, the system administers two units of blood, followed by fluids, and then norepinephrine or further fluids per the protocol I described earlier. 
The practical upshot of all of this is stabilization, despite an as-yet untreated liver laceration.
Could an experienced ALS provider do this? Of course. But, as I mentioned before, you aren’t always near an experienced ALS provider.
This is all well and good in the lab, but in the real world, you actually need to transport a trauma patient. The researchers tried this also. To prove feasibility, four pigs were taken from the lab to the top of the University of Pittsburgh Medical Center, flown to Allegheny County Airport and back. Total time before liver laceration repair? Three hours. And all four survived.
It won’t surprise you to hear that this work was funded by the Department of Defense. You can see how a system like this, made a bit more rugged, a bit smaller, and a bit more self-contained could have real uses in the battlefield. But trauma is not unique to war, and something that can extend the time you have to safely transport a patient to definitive care — well, that’s worth its weight in golden hours.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
They call it the “golden hour”: 60 minutes, give or take, when the chance to save the life of a trauma victim is at its greatest. If the patient can be resuscitated and stabilized in that time window, they stand a good chance of surviving. If not, well, they don’t.
But resuscitation is complicated. It requires blood products, fluids, vasopressors — all given in precise doses in response to rapidly changing hemodynamics. To do it right takes specialized training, advanced life support (ALS). If the patient is in a remote area or an area without ALS-certified emergency medical services, or is far from the nearest trauma center, that golden hour is lost. And the patient may be as well.
But we live in the future. We have robots in factories, self-driving cars, autonomous drones. Why not an autonomous trauma doctor? If you are in a life-threatening accident, would you want to be treated ... by a robot?
Enter “resuscitation based on functional hemodynamic monitoring,” or “ReFit,” introduced in this article appearing in the journal Intensive Care Medicine Experimental.
The idea behind ReFit is straightforward. Resuscitation after trauma should be based on hitting key hemodynamic targets using the tools we have available in the field: blood, fluids, pressors. The researchers wanted to develop a closed-loop system, something that could be used by minimally trained personnel. The input to the system? Hemodynamic data, provided through a single measurement device, an arterial catheter. The output: blood, fluids, and pressors, delivered intravenously.
The body (a prototype) of the system looks like this. You can see various pumps labeled with various fluids, electronic controllers, and so forth.
If that’s the body, then this is the brain – a ruggedized laptop interpreting a readout of that arterial catheter.
If that’s the brain, then the ReFit algorithm is the mind. The algorithm does its best to leverage all the data it can, so I want to walk through it in a bit of detail.
First, check to see whether the patient is stable, defined as a heart rate < 110 beats/min and a mean arterial pressure > 60 mm Hg. If not, you’re off to the races, starting with a bolus of whole blood.
Next, the algorithm gets really interesting. If the patient is still unstable, the computer assesses fluid responsiveness by giving a test dose of fluid and measuring the pulse pressure variation. Greater pulse pressure variation means more fluid responsiveness and the algorithm gives more fluid. Less pulse pressure variation leads the algorithm to uptitrate pressors — in this case, norepinephrine.
This cycle of evaluation and response keeps repeating. The computer titrates fluids and pressors up and down entirely on its own, in theory freeing the human team members to do other things, like getting the patient to a trauma center for definitive care.
So, how do you test whether something like this works? Clearly, you don’t want the trial run of a system like this to be used on a real human suffering from a real traumatic injury.
Once again, we have animals to thank for research advances — in this case, pigs. Fifteen pigs are described in the study. To simulate a severe, hemorrhagic trauma, they were anesthetized and the liver was lacerated. They were then observed passively until the mean arterial pressure had dropped to below 40 mm Hg.
This is a pretty severe injury. Three unfortunate animals served as controls, two of which died within the 3-hour time window of the study. Eight animals were plugged into the ReFit system.
For a window into what happens during this process, let’s take a look at the mean arterial pressure and heart rate readouts for one of the animals. You see that the blood pressure starts to fall precipitously after the liver laceration. The heart rate quickly picks up to compensate, raising the mean arterial pressure a bit, but this would be unsustainable with ongoing bleeding.
Here, the ReFit system takes over. Autonomously, the system administers two units of blood, followed by fluids, and then norepinephrine or further fluids per the protocol I described earlier. 
The practical upshot of all of this is stabilization, despite an as-yet untreated liver laceration.
Could an experienced ALS provider do this? Of course. But, as I mentioned before, you aren’t always near an experienced ALS provider.
This is all well and good in the lab, but in the real world, you actually need to transport a trauma patient. The researchers tried this also. To prove feasibility, four pigs were taken from the lab to the top of the University of Pittsburgh Medical Center, flown to Allegheny County Airport and back. Total time before liver laceration repair? Three hours. And all four survived.
It won’t surprise you to hear that this work was funded by the Department of Defense. You can see how a system like this, made a bit more rugged, a bit smaller, and a bit more self-contained could have real uses in the battlefield. But trauma is not unique to war, and something that can extend the time you have to safely transport a patient to definitive care — well, that’s worth its weight in golden hours.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Attacks on Emergency Room Workers Prompt Debate Over Tougher Penalties
Patients hurl verbal abuse at Michelle Ravera every day in the emergency room. Physical violence is less common, she said, but has become a growing threat.
Ravera, an ER nurse at Sutter Medical Center in Sacramento, recalled an incident in which an agitated patient wanted to leave. “Without any warning he just reached up, grabbed my glasses, and punched me in the face,” said Ravera, 54. “And then he was getting ready to attack another patient in the room.” Ravera and hospital security guards subdued the patient so he couldn’t hurt anyone else.
Covid-19 only made things worse: With routine care harder to come by, many patients ended up in the ER with serious diseases — and brimming with frustrations.
In California, simple assault against workers inside an ER is considered the same as simple assault against almost anyone else, and carries a maximum punishment of a $1,000 fine and six months in jail. In contrast, simple assault against emergency medical workers in the field, such as an EMT responding to a 911 call, carries maximum penalties of a $2,000 fine and a year in jail. Simple assault does not involve the use of a deadly weapon or the intention to inflict serious bodily injury.
State Assembly member Freddie Rodriguez, who worked as an EMT, has authored a bill to make the punishments consistent: a $2,000 fine and one year in jail for simple assault on any on-the-job emergency health care worker, whether in the field or an ER. The measure would also eliminate the discrepancy for simple battery.
Patients and family members are assaulting staff and “doing things they shouldn’t be doing to the people that are there to take care of your loved ones,” said Rodriguez, a Democrat from Pomona. The bill passed the state Assembly unanimously in January and awaits consideration in the Senate.
Rodriguez has introduced similar measures twice before. Then-Gov. Jerry Brown vetoed one in 2015, saying he doubted a longer jail sentence would deter violence. “We need to find more creative ways to protect the safety of these critical workers,” he wrote in his veto message. The 2019 bill died in the state Senate.
Rodriguez said ERs have become more dangerous for health care workers since then and that “there has to be accountability” for violent behavior. Opponents fear stiffer penalties would be levied disproportionately on patients of color or those with developmental disabilities. They also point out that violent patients can already face penalties under existing assault and battery laws.
Data from the California Division of Occupational Safety and Health shows that reported attacks on ER workers by patients, visitors, and strangers jumped about 25% from 2018 to 2023, from 2,587 to 3,238. The rate of attacks per 100,000 ER visits also increased.
Punching, kicking, pushing, and similar aggression accounted for most of the attacks. Only a small number included weapons.
These numbers are likely an undercount, said Al’ai Alvarez, an ER doctor and clinical associate professor at Stanford University’s Department of Emergency Medicine. Many hospital staffers don’t fill out workplace violence reports because they don’t have time or feel nothing will come of it, he said.
Ravera remembers when her community rallied around health care workers at the start of the pandemic, acting respectfully and bringing food and extra N95 masks to workers.
“Then something just switched,” she said. “The patients became angrier and more aggressive.”
Violence can contribute to burnout and drive workers to quit — or worse, said Alvarez, who has lost colleagues to suicide, and thinks burnout was a key factor. “The cost of burnout is more than just loss of productivity,” he said. “It’s loss of human beings that also had the potential to take care of many more people.”
The National Center for Health Workforce Analysis projects California will experience an 18% shortage of all types of nurses in 2035, the third worst in the country.
Federal legislation called the Safety From Violence for Healthcare Employees Act would set sentences of up to 10 years for assault against a health care worker, not limited to emergency workers, and up to 20 years in cases involving dangerous weapons or bodily injury. Though it was introduced in 2023, it has not yet had a committee hearing.
Opponents of the California bill, which include ACLU California Action, the California Public Defenders Association, and advocates for people with autism, argue it wouldn’t deter attacks — and would unfairly target certain patients.
“There’s no evidence to suggest that increased penalties are going to meaningfully address this conduct,” said Eric Henderson, a legislative advocate for ACLU California Action. “Most importantly, there are already laws on the books to address assaultive conduct.”
Beth Burt, executive director of the Autism Society Inland Empire, said the measure doesn’t take into account the special needs of people with autism and other developmental disorders.
The smells, lights, textures, and crowds in the ER can overstimulate a person with autism, she said. When that happens, they can struggle to articulate their feelings, which can result in a violent outburst, “whether it’s a 9-year-old or a 29-year-old,” Burt said.
She worries that hospital staff may misunderstand these reactions, and involve law enforcement when it’s not necessary. As “a parent, it is still my worst fear” that she’ll get a phone call to inform her that her adult son with autism has been arrested, she said.
Burt would rather the state prioritize de-escalation programs over penalties, such as the training programs for first responders she helped create through the Autism Society Inland Empire. After implementing the training, hospital administrators asked Burt to share some strategies with them, she said. Hospital security staffers who do not want to use physical restraints on autistic patients have also sought her advice, she said.
Supporters of the bill, including health care and law enforcement groups, counter that people with mental health conditions or autism who are charged with assault in an ER may be eligible for existing programs that provide mental health treatment in lieu of a criminal sentence.
Stephanie Jensen, an ER nurse and head of governmental affairs for the Emergency Nurses Association, California State Council, said her organization is simply arguing for equity. “If you punch me in the hospital, it’s the same as if you punch me on the street,” she said.
If lawmakers don’t act, she warned, there won’t be enough workers for the patients who need them.
“It’s hard to keep those human resources accessible when it just seems like you’re showing up to get beat up every day,” Jensen said. “The emergency department is taking it on the chin, literally and figuratively.”
This article was produced by KFF Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Patients hurl verbal abuse at Michelle Ravera every day in the emergency room. Physical violence is less common, she said, but has become a growing threat.
Ravera, an ER nurse at Sutter Medical Center in Sacramento, recalled an incident in which an agitated patient wanted to leave. “Without any warning he just reached up, grabbed my glasses, and punched me in the face,” said Ravera, 54. “And then he was getting ready to attack another patient in the room.” Ravera and hospital security guards subdued the patient so he couldn’t hurt anyone else.
Covid-19 only made things worse: With routine care harder to come by, many patients ended up in the ER with serious diseases — and brimming with frustrations.
In California, simple assault against workers inside an ER is considered the same as simple assault against almost anyone else, and carries a maximum punishment of a $1,000 fine and six months in jail. In contrast, simple assault against emergency medical workers in the field, such as an EMT responding to a 911 call, carries maximum penalties of a $2,000 fine and a year in jail. Simple assault does not involve the use of a deadly weapon or the intention to inflict serious bodily injury.
State Assembly member Freddie Rodriguez, who worked as an EMT, has authored a bill to make the punishments consistent: a $2,000 fine and one year in jail for simple assault on any on-the-job emergency health care worker, whether in the field or an ER. The measure would also eliminate the discrepancy for simple battery.
Patients and family members are assaulting staff and “doing things they shouldn’t be doing to the people that are there to take care of your loved ones,” said Rodriguez, a Democrat from Pomona. The bill passed the state Assembly unanimously in January and awaits consideration in the Senate.
Rodriguez has introduced similar measures twice before. Then-Gov. Jerry Brown vetoed one in 2015, saying he doubted a longer jail sentence would deter violence. “We need to find more creative ways to protect the safety of these critical workers,” he wrote in his veto message. The 2019 bill died in the state Senate.
Rodriguez said ERs have become more dangerous for health care workers since then and that “there has to be accountability” for violent behavior. Opponents fear stiffer penalties would be levied disproportionately on patients of color or those with developmental disabilities. They also point out that violent patients can already face penalties under existing assault and battery laws.
Data from the California Division of Occupational Safety and Health shows that reported attacks on ER workers by patients, visitors, and strangers jumped about 25% from 2018 to 2023, from 2,587 to 3,238. The rate of attacks per 100,000 ER visits also increased.
Punching, kicking, pushing, and similar aggression accounted for most of the attacks. Only a small number included weapons.
These numbers are likely an undercount, said Al’ai Alvarez, an ER doctor and clinical associate professor at Stanford University’s Department of Emergency Medicine. Many hospital staffers don’t fill out workplace violence reports because they don’t have time or feel nothing will come of it, he said.
Ravera remembers when her community rallied around health care workers at the start of the pandemic, acting respectfully and bringing food and extra N95 masks to workers.
“Then something just switched,” she said. “The patients became angrier and more aggressive.”
Violence can contribute to burnout and drive workers to quit — or worse, said Alvarez, who has lost colleagues to suicide, and thinks burnout was a key factor. “The cost of burnout is more than just loss of productivity,” he said. “It’s loss of human beings that also had the potential to take care of many more people.”
The National Center for Health Workforce Analysis projects California will experience an 18% shortage of all types of nurses in 2035, the third worst in the country.
Federal legislation called the Safety From Violence for Healthcare Employees Act would set sentences of up to 10 years for assault against a health care worker, not limited to emergency workers, and up to 20 years in cases involving dangerous weapons or bodily injury. Though it was introduced in 2023, it has not yet had a committee hearing.
Opponents of the California bill, which include ACLU California Action, the California Public Defenders Association, and advocates for people with autism, argue it wouldn’t deter attacks — and would unfairly target certain patients.
“There’s no evidence to suggest that increased penalties are going to meaningfully address this conduct,” said Eric Henderson, a legislative advocate for ACLU California Action. “Most importantly, there are already laws on the books to address assaultive conduct.”
Beth Burt, executive director of the Autism Society Inland Empire, said the measure doesn’t take into account the special needs of people with autism and other developmental disorders.
The smells, lights, textures, and crowds in the ER can overstimulate a person with autism, she said. When that happens, they can struggle to articulate their feelings, which can result in a violent outburst, “whether it’s a 9-year-old or a 29-year-old,” Burt said.
She worries that hospital staff may misunderstand these reactions, and involve law enforcement when it’s not necessary. As “a parent, it is still my worst fear” that she’ll get a phone call to inform her that her adult son with autism has been arrested, she said.
Burt would rather the state prioritize de-escalation programs over penalties, such as the training programs for first responders she helped create through the Autism Society Inland Empire. After implementing the training, hospital administrators asked Burt to share some strategies with them, she said. Hospital security staffers who do not want to use physical restraints on autistic patients have also sought her advice, she said.
Supporters of the bill, including health care and law enforcement groups, counter that people with mental health conditions or autism who are charged with assault in an ER may be eligible for existing programs that provide mental health treatment in lieu of a criminal sentence.
Stephanie Jensen, an ER nurse and head of governmental affairs for the Emergency Nurses Association, California State Council, said her organization is simply arguing for equity. “If you punch me in the hospital, it’s the same as if you punch me on the street,” she said.
If lawmakers don’t act, she warned, there won’t be enough workers for the patients who need them.
“It’s hard to keep those human resources accessible when it just seems like you’re showing up to get beat up every day,” Jensen said. “The emergency department is taking it on the chin, literally and figuratively.”
This article was produced by KFF Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Patients hurl verbal abuse at Michelle Ravera every day in the emergency room. Physical violence is less common, she said, but has become a growing threat.
Ravera, an ER nurse at Sutter Medical Center in Sacramento, recalled an incident in which an agitated patient wanted to leave. “Without any warning he just reached up, grabbed my glasses, and punched me in the face,” said Ravera, 54. “And then he was getting ready to attack another patient in the room.” Ravera and hospital security guards subdued the patient so he couldn’t hurt anyone else.
Covid-19 only made things worse: With routine care harder to come by, many patients ended up in the ER with serious diseases — and brimming with frustrations.
In California, simple assault against workers inside an ER is considered the same as simple assault against almost anyone else, and carries a maximum punishment of a $1,000 fine and six months in jail. In contrast, simple assault against emergency medical workers in the field, such as an EMT responding to a 911 call, carries maximum penalties of a $2,000 fine and a year in jail. Simple assault does not involve the use of a deadly weapon or the intention to inflict serious bodily injury.
State Assembly member Freddie Rodriguez, who worked as an EMT, has authored a bill to make the punishments consistent: a $2,000 fine and one year in jail for simple assault on any on-the-job emergency health care worker, whether in the field or an ER. The measure would also eliminate the discrepancy for simple battery.
Patients and family members are assaulting staff and “doing things they shouldn’t be doing to the people that are there to take care of your loved ones,” said Rodriguez, a Democrat from Pomona. The bill passed the state Assembly unanimously in January and awaits consideration in the Senate.
Rodriguez has introduced similar measures twice before. Then-Gov. Jerry Brown vetoed one in 2015, saying he doubted a longer jail sentence would deter violence. “We need to find more creative ways to protect the safety of these critical workers,” he wrote in his veto message. The 2019 bill died in the state Senate.
Rodriguez said ERs have become more dangerous for health care workers since then and that “there has to be accountability” for violent behavior. Opponents fear stiffer penalties would be levied disproportionately on patients of color or those with developmental disabilities. They also point out that violent patients can already face penalties under existing assault and battery laws.
Data from the California Division of Occupational Safety and Health shows that reported attacks on ER workers by patients, visitors, and strangers jumped about 25% from 2018 to 2023, from 2,587 to 3,238. The rate of attacks per 100,000 ER visits also increased.
Punching, kicking, pushing, and similar aggression accounted for most of the attacks. Only a small number included weapons.
These numbers are likely an undercount, said Al’ai Alvarez, an ER doctor and clinical associate professor at Stanford University’s Department of Emergency Medicine. Many hospital staffers don’t fill out workplace violence reports because they don’t have time or feel nothing will come of it, he said.
Ravera remembers when her community rallied around health care workers at the start of the pandemic, acting respectfully and bringing food and extra N95 masks to workers.
“Then something just switched,” she said. “The patients became angrier and more aggressive.”
Violence can contribute to burnout and drive workers to quit — or worse, said Alvarez, who has lost colleagues to suicide, and thinks burnout was a key factor. “The cost of burnout is more than just loss of productivity,” he said. “It’s loss of human beings that also had the potential to take care of many more people.”
The National Center for Health Workforce Analysis projects California will experience an 18% shortage of all types of nurses in 2035, the third worst in the country.
Federal legislation called the Safety From Violence for Healthcare Employees Act would set sentences of up to 10 years for assault against a health care worker, not limited to emergency workers, and up to 20 years in cases involving dangerous weapons or bodily injury. Though it was introduced in 2023, it has not yet had a committee hearing.
Opponents of the California bill, which include ACLU California Action, the California Public Defenders Association, and advocates for people with autism, argue it wouldn’t deter attacks — and would unfairly target certain patients.
“There’s no evidence to suggest that increased penalties are going to meaningfully address this conduct,” said Eric Henderson, a legislative advocate for ACLU California Action. “Most importantly, there are already laws on the books to address assaultive conduct.”
Beth Burt, executive director of the Autism Society Inland Empire, said the measure doesn’t take into account the special needs of people with autism and other developmental disorders.
The smells, lights, textures, and crowds in the ER can overstimulate a person with autism, she said. When that happens, they can struggle to articulate their feelings, which can result in a violent outburst, “whether it’s a 9-year-old or a 29-year-old,” Burt said.
She worries that hospital staff may misunderstand these reactions, and involve law enforcement when it’s not necessary. As “a parent, it is still my worst fear” that she’ll get a phone call to inform her that her adult son with autism has been arrested, she said.
Burt would rather the state prioritize de-escalation programs over penalties, such as the training programs for first responders she helped create through the Autism Society Inland Empire. After implementing the training, hospital administrators asked Burt to share some strategies with them, she said. Hospital security staffers who do not want to use physical restraints on autistic patients have also sought her advice, she said.
Supporters of the bill, including health care and law enforcement groups, counter that people with mental health conditions or autism who are charged with assault in an ER may be eligible for existing programs that provide mental health treatment in lieu of a criminal sentence.
Stephanie Jensen, an ER nurse and head of governmental affairs for the Emergency Nurses Association, California State Council, said her organization is simply arguing for equity. “If you punch me in the hospital, it’s the same as if you punch me on the street,” she said.
If lawmakers don’t act, she warned, there won’t be enough workers for the patients who need them.
“It’s hard to keep those human resources accessible when it just seems like you’re showing up to get beat up every day,” Jensen said. “The emergency department is taking it on the chin, literally and figuratively.”
This article was produced by KFF Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation. KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF—an independent source of health policy research, polling, and journalism. Learn more about KFF.
Can a Stroke Be Caused by Cervical Manipulation?
Cervical manipulations have been associated with vascular complications. While the incidence of carotid dissections does not seem to have increased, the question remains open for vertebral artery injuries. We must remain vigilant!
Resorting to joint manipulation for neck pain is not unusual. Currently, cervical manipulation remains a popular first-line treatment for cervicodynia or headaches. Although evidence exists showing that specific joint mobilization can improve this type of symptomatology, there is a possibility that it may risk damaging the cervical arteries and causing ischemic stroke through arterial dissection.
Epidemiologically, internal carotid artery dissection is a relatively rare event with an estimated annual incidence of 1.72 per 100,000 individuals (those most likely to be diagnosed being obviously those leading to hospitalization for stroke) but represents one of the most common causes of stroke in young and middle-aged adults. Faced with case reports that may raise concerns and hypotheses about an associated risk, two studies have sought to delve into the issue.
No Increased Carotid Risk Identified
The first study, of a case-cross design, identified all incident cases of ischemic stroke in the territory of the internal carotid artery admitted to the hospital over a 9-year period using administrative healthcare data, the cases being used as their own control by sampling control periods before the date of the index stroke. Thus, 15,523 cases were compared with 62,092 control periods using exposure windows of 1, 3, 7, and 14 days before the stroke. The study also compared post-medical consultation and post-chiropractic consultation outcomes, knowing that as a first-line for complaints of neck pain or headache, patients often turn to one of these two types of primary care clinicians.
However, data analysis shows, among subjects aged under 45 years, positive associations for both different consultations in cases of subsequent carotid stroke (but no association for those aged over 45 years). These associations tended to increase when analyses were limited to visits for diagnoses of neck pain and headaches. Nevertheless, there was no significant difference between risk estimates after chiropractic or general medical consultation.
A notable limitation of this work is that it did not focus on strokes due to vertebral artery dissections that run through the transverse foramina of the cervical vertebrae.
A Screening Test Lacking Precision
More recently, the International Federation of Orthopedic Manual Physical Therapists has looked into the subject to refine the assessment of the risk for vascular complications in patients seeking physiotherapy/osteopathy care for neck pain and/or headaches. Through a cross-sectional study involving 150 patients, it tested a vascular complication risk index (from high to low grade, based on history taking and clinical examination), developed to estimate the risk for the presence of vascular rather than musculoskeletal pathology, to determine whether or not there is a contraindication to cervical manipulation.
However, the developed index had only low sensitivity (0.50; 95% CI, 0.39-0.61) and moderate specificity (0.63; 95% CI, 0.51-0.75), knowing that the reference test was a consensus medical decision made by a vascular neurologist, an interventional neurologist, and a neuroradiologist (based on clinical data and cervical MRI). Similarly, positive and negative likelihood ratios were low at 1.36 (95% CI, 0.93-1.99) and 0.79 (95% CI, 0.60-1.05), respectively.
In conclusion, the data from the case-cross study did not seem to demonstrate an excess risk for stroke in the territory of the internal carotid artery after cervical joint manipulations. Associations between cervical manipulation sessions or medical consultations and carotid strokes appear similar and could have been due to the fact that patients with early symptoms related to arterial dissection seek care before developing their stroke.
However, it is regrettable that the study did not focus on vertebral artery dissections, which are anatomically more exposed to cervical chiropractic sessions. Nevertheless, because indices defined from joint tests and medical history are insufficient to identify patients “at risk or in the process of arterial dissection,” and because stroke can result in severe disability, practitioners managing patients with neck pain cannot take this type of complication lightly.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Cervical manipulations have been associated with vascular complications. While the incidence of carotid dissections does not seem to have increased, the question remains open for vertebral artery injuries. We must remain vigilant!
Resorting to joint manipulation for neck pain is not unusual. Currently, cervical manipulation remains a popular first-line treatment for cervicodynia or headaches. Although evidence exists showing that specific joint mobilization can improve this type of symptomatology, there is a possibility that it may risk damaging the cervical arteries and causing ischemic stroke through arterial dissection.
Epidemiologically, internal carotid artery dissection is a relatively rare event with an estimated annual incidence of 1.72 per 100,000 individuals (those most likely to be diagnosed being obviously those leading to hospitalization for stroke) but represents one of the most common causes of stroke in young and middle-aged adults. Faced with case reports that may raise concerns and hypotheses about an associated risk, two studies have sought to delve into the issue.
No Increased Carotid Risk Identified
The first study, of a case-cross design, identified all incident cases of ischemic stroke in the territory of the internal carotid artery admitted to the hospital over a 9-year period using administrative healthcare data, the cases being used as their own control by sampling control periods before the date of the index stroke. Thus, 15,523 cases were compared with 62,092 control periods using exposure windows of 1, 3, 7, and 14 days before the stroke. The study also compared post-medical consultation and post-chiropractic consultation outcomes, knowing that as a first-line for complaints of neck pain or headache, patients often turn to one of these two types of primary care clinicians.
However, data analysis shows, among subjects aged under 45 years, positive associations for both different consultations in cases of subsequent carotid stroke (but no association for those aged over 45 years). These associations tended to increase when analyses were limited to visits for diagnoses of neck pain and headaches. Nevertheless, there was no significant difference between risk estimates after chiropractic or general medical consultation.
A notable limitation of this work is that it did not focus on strokes due to vertebral artery dissections that run through the transverse foramina of the cervical vertebrae.
A Screening Test Lacking Precision
More recently, the International Federation of Orthopedic Manual Physical Therapists has looked into the subject to refine the assessment of the risk for vascular complications in patients seeking physiotherapy/osteopathy care for neck pain and/or headaches. Through a cross-sectional study involving 150 patients, it tested a vascular complication risk index (from high to low grade, based on history taking and clinical examination), developed to estimate the risk for the presence of vascular rather than musculoskeletal pathology, to determine whether or not there is a contraindication to cervical manipulation.
However, the developed index had only low sensitivity (0.50; 95% CI, 0.39-0.61) and moderate specificity (0.63; 95% CI, 0.51-0.75), knowing that the reference test was a consensus medical decision made by a vascular neurologist, an interventional neurologist, and a neuroradiologist (based on clinical data and cervical MRI). Similarly, positive and negative likelihood ratios were low at 1.36 (95% CI, 0.93-1.99) and 0.79 (95% CI, 0.60-1.05), respectively.
In conclusion, the data from the case-cross study did not seem to demonstrate an excess risk for stroke in the territory of the internal carotid artery after cervical joint manipulations. Associations between cervical manipulation sessions or medical consultations and carotid strokes appear similar and could have been due to the fact that patients with early symptoms related to arterial dissection seek care before developing their stroke.
However, it is regrettable that the study did not focus on vertebral artery dissections, which are anatomically more exposed to cervical chiropractic sessions. Nevertheless, because indices defined from joint tests and medical history are insufficient to identify patients “at risk or in the process of arterial dissection,” and because stroke can result in severe disability, practitioners managing patients with neck pain cannot take this type of complication lightly.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Cervical manipulations have been associated with vascular complications. While the incidence of carotid dissections does not seem to have increased, the question remains open for vertebral artery injuries. We must remain vigilant!
Resorting to joint manipulation for neck pain is not unusual. Currently, cervical manipulation remains a popular first-line treatment for cervicodynia or headaches. Although evidence exists showing that specific joint mobilization can improve this type of symptomatology, there is a possibility that it may risk damaging the cervical arteries and causing ischemic stroke through arterial dissection.
Epidemiologically, internal carotid artery dissection is a relatively rare event with an estimated annual incidence of 1.72 per 100,000 individuals (those most likely to be diagnosed being obviously those leading to hospitalization for stroke) but represents one of the most common causes of stroke in young and middle-aged adults. Faced with case reports that may raise concerns and hypotheses about an associated risk, two studies have sought to delve into the issue.
No Increased Carotid Risk Identified
The first study, of a case-cross design, identified all incident cases of ischemic stroke in the territory of the internal carotid artery admitted to the hospital over a 9-year period using administrative healthcare data, the cases being used as their own control by sampling control periods before the date of the index stroke. Thus, 15,523 cases were compared with 62,092 control periods using exposure windows of 1, 3, 7, and 14 days before the stroke. The study also compared post-medical consultation and post-chiropractic consultation outcomes, knowing that as a first-line for complaints of neck pain or headache, patients often turn to one of these two types of primary care clinicians.
However, data analysis shows, among subjects aged under 45 years, positive associations for both different consultations in cases of subsequent carotid stroke (but no association for those aged over 45 years). These associations tended to increase when analyses were limited to visits for diagnoses of neck pain and headaches. Nevertheless, there was no significant difference between risk estimates after chiropractic or general medical consultation.
A notable limitation of this work is that it did not focus on strokes due to vertebral artery dissections that run through the transverse foramina of the cervical vertebrae.
A Screening Test Lacking Precision
More recently, the International Federation of Orthopedic Manual Physical Therapists has looked into the subject to refine the assessment of the risk for vascular complications in patients seeking physiotherapy/osteopathy care for neck pain and/or headaches. Through a cross-sectional study involving 150 patients, it tested a vascular complication risk index (from high to low grade, based on history taking and clinical examination), developed to estimate the risk for the presence of vascular rather than musculoskeletal pathology, to determine whether or not there is a contraindication to cervical manipulation.
However, the developed index had only low sensitivity (0.50; 95% CI, 0.39-0.61) and moderate specificity (0.63; 95% CI, 0.51-0.75), knowing that the reference test was a consensus medical decision made by a vascular neurologist, an interventional neurologist, and a neuroradiologist (based on clinical data and cervical MRI). Similarly, positive and negative likelihood ratios were low at 1.36 (95% CI, 0.93-1.99) and 0.79 (95% CI, 0.60-1.05), respectively.
In conclusion, the data from the case-cross study did not seem to demonstrate an excess risk for stroke in the territory of the internal carotid artery after cervical joint manipulations. Associations between cervical manipulation sessions or medical consultations and carotid strokes appear similar and could have been due to the fact that patients with early symptoms related to arterial dissection seek care before developing their stroke.
However, it is regrettable that the study did not focus on vertebral artery dissections, which are anatomically more exposed to cervical chiropractic sessions. Nevertheless, because indices defined from joint tests and medical history are insufficient to identify patients “at risk or in the process of arterial dissection,” and because stroke can result in severe disability, practitioners managing patients with neck pain cannot take this type of complication lightly.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
A New Biomarker of Brain Injury?
Posttraumatic headache (PTH) is associated with an increase in iron accumulation in certain brain regions , notably those involved in the pain network, early research shows.
The findings come on the heels of previous research showing patients with iron accumulation in certain brain regions don’t respond as well to treatment, study investigator, Simona Nikolova, PhD, assistant professor of neurology, Mayo Clinic, Phoenix, Arizona, told this news organization.
“This is really important, and doctors need to be aware of it. If you have a patient who is not responding to treatment, then you know what to look at,” she said.
The findings (Abstract #3379) will be presented on April 15 at the American Academy of Neurology (AAN) 2024 Annual Meeting.
Dose Effect
The study included 60 people with acute PTH due to mTBI. Most were White, and almost half had sustained a concussion due to a fall, with about 30% injured in a vehicle accident and a smaller number injured during a fight.
The mean number of lifetime mTBIs was 2.4, although participants had sustained as many as five or six and as few as one. The mean time from the most recent mTBI was 25 days, and the mean score on the Sport Concussion Assessment Tool (SCAT), which measures postconcussion symptom severity, was 29.
Most in the mTBI group (43) had migraine or probable migraine, and 14 had tension-type headaches. Mean headache frequency was 81%.
Researchers matched these patients with 60 controls without concussion or headache. Because iron accumulation is age-related, they tried to eliminate this covariant by pairing each participant with mTBI with an age- and sex-matched control.
All participants underwent a type of brain MRI known as T2* weighted sequence that can identify brain iron accumulation, a marker of neural injury.
Investigators found that the PTH group had significantly higher levels of iron accumulation in several areas of the brain, most of which are part of a “pain network” that includes about 63 areas of the brain, Dr. Nikolova said.
The study wasn’t designed to determine how much more iron accumulation mTBI patients had vs controls.
“We can’t say it was twice as much or three times as much; we can only say it was significant. Measuring concentrations in PTH patients and comparing that with controls is something we haven’t don’t yet,” said Dr. Nikolova.
Areas of the brain with increased iron accumulation, included the periaqueductal gray (PAG), anterior cingulated cortex, and supramarginal gyrus.
Research suggests patients with migraine who have elevated levels of iron in the PAG have a poorer response to botulinum toxin treatment. An earlier study by the same team showed a poorer response to the calcitonin gene-related peptide inhibitor erenumab in migraine patients with elevated iron in the PAG.
Researchers discovered that those with more lifetime TBIs had higher iron accumulation in the right gyrus rectus and right putamen vs those with fewer injuries and that headache frequency was associated with iron accumulation in the posterior corona radiata, bilateral temporal, right frontal, bilateral supplemental motor area, left fusiform, right hippocampus, sagittal striatum, and left cerebellum.
Surprising Result
The investigators also found a link between time since the most recent mTBI and iron accumulation in the bilateral temporal, right hippocampus, posterior and superior corona radiata, bilateral thalamus, right precuneus and cuneus, right lingual, and right cerebellum.
“The more time that passed since the concussion occurred, the more likely that people had higher iron levels,” said Dr. Nikolova.
It’s perhaps to be expected that the length of time since injury is linked to iron accumulation in the brain as iron accumulates over time. But even those whose injury was relatively recent had higher amounts of iron, which Dr. Nikolova said was “surprising.”
“We thought iron accumulates over time so we were thinking maybe we should be doing a longitudinal study to see what happens, but we see definite iron accumulation due to injury shortly after the injury,” she said.
There was no association between iron accumulation and symptom severity as measured by SCAT scores.
Questions Remain
It’s unclear why iron accumulates after an injury or what the ramifications are of this accumulation, Dr. Nikolova noted.
The imaging used in the study doesn’t distinguish between “bound” iron found after a hemorrhage and “free” iron in the brain. The free iron type has been shown to be increased after TBI and is “the stuff you should be afraid of,” Dr. Nikolova said.
Iron’s role in the metabolic process is important, but must be closely regulated, she said. Even a small accumulation can lead to oxidative stress.
Researchers are investigating whether the findings would be similar in mTBI but no headache and want to increase the number of study participants. A larger, more diverse sample would allow them to probe other questions, including whether iron accumulation is different in men and women. More data could also eventually lead to iron accumulation becoming a biomarker for concussion and PTH, Dr. Nikolova said.
“If you know a certain person has that biomarker, you might be able to administer a drug or some therapeutic procedure to prevent that iron from continuing to accumulate in the brain.”
Chelation drugs and other therapies may clear iron from the body but not necessarily from the brain.
Commenting on the study for this news organization, Frank Conidi, MD, director, Florida Center for Headache and Sports Neurology, Port St. Lucie , said that the study supports the hypothesis that concussion “is not a benign process for the brain, and the cumulative effect of repetitive head injury can result in permanent brain injury.”
He said that he found the accumulation of iron in cortical structures particularly interesting. This, he said, differs from most current research that suggests head trauma mainly results in damage to white matter tracts.
He prefers the term “concussion” over “mild traumatic brain injury” which was used in the study. “Recent guidelines, including some that I’ve been involved with, have defined mild traumatic brain injury as a more permanent process,” he said.
The study was supported by the US Department of Defense and National Institutes of Health. No relevant conflicts of interest were disclosed.
A version of this article appeared on Medscape.com.
Posttraumatic headache (PTH) is associated with an increase in iron accumulation in certain brain regions , notably those involved in the pain network, early research shows.
The findings come on the heels of previous research showing patients with iron accumulation in certain brain regions don’t respond as well to treatment, study investigator, Simona Nikolova, PhD, assistant professor of neurology, Mayo Clinic, Phoenix, Arizona, told this news organization.
“This is really important, and doctors need to be aware of it. If you have a patient who is not responding to treatment, then you know what to look at,” she said.
The findings (Abstract #3379) will be presented on April 15 at the American Academy of Neurology (AAN) 2024 Annual Meeting.
Dose Effect
The study included 60 people with acute PTH due to mTBI. Most were White, and almost half had sustained a concussion due to a fall, with about 30% injured in a vehicle accident and a smaller number injured during a fight.
The mean number of lifetime mTBIs was 2.4, although participants had sustained as many as five or six and as few as one. The mean time from the most recent mTBI was 25 days, and the mean score on the Sport Concussion Assessment Tool (SCAT), which measures postconcussion symptom severity, was 29.
Most in the mTBI group (43) had migraine or probable migraine, and 14 had tension-type headaches. Mean headache frequency was 81%.
Researchers matched these patients with 60 controls without concussion or headache. Because iron accumulation is age-related, they tried to eliminate this covariant by pairing each participant with mTBI with an age- and sex-matched control.
All participants underwent a type of brain MRI known as T2* weighted sequence that can identify brain iron accumulation, a marker of neural injury.
Investigators found that the PTH group had significantly higher levels of iron accumulation in several areas of the brain, most of which are part of a “pain network” that includes about 63 areas of the brain, Dr. Nikolova said.
The study wasn’t designed to determine how much more iron accumulation mTBI patients had vs controls.
“We can’t say it was twice as much or three times as much; we can only say it was significant. Measuring concentrations in PTH patients and comparing that with controls is something we haven’t don’t yet,” said Dr. Nikolova.
Areas of the brain with increased iron accumulation, included the periaqueductal gray (PAG), anterior cingulated cortex, and supramarginal gyrus.
Research suggests patients with migraine who have elevated levels of iron in the PAG have a poorer response to botulinum toxin treatment. An earlier study by the same team showed a poorer response to the calcitonin gene-related peptide inhibitor erenumab in migraine patients with elevated iron in the PAG.
Researchers discovered that those with more lifetime TBIs had higher iron accumulation in the right gyrus rectus and right putamen vs those with fewer injuries and that headache frequency was associated with iron accumulation in the posterior corona radiata, bilateral temporal, right frontal, bilateral supplemental motor area, left fusiform, right hippocampus, sagittal striatum, and left cerebellum.
Surprising Result
The investigators also found a link between time since the most recent mTBI and iron accumulation in the bilateral temporal, right hippocampus, posterior and superior corona radiata, bilateral thalamus, right precuneus and cuneus, right lingual, and right cerebellum.
“The more time that passed since the concussion occurred, the more likely that people had higher iron levels,” said Dr. Nikolova.
It’s perhaps to be expected that the length of time since injury is linked to iron accumulation in the brain as iron accumulates over time. But even those whose injury was relatively recent had higher amounts of iron, which Dr. Nikolova said was “surprising.”
“We thought iron accumulates over time so we were thinking maybe we should be doing a longitudinal study to see what happens, but we see definite iron accumulation due to injury shortly after the injury,” she said.
There was no association between iron accumulation and symptom severity as measured by SCAT scores.
Questions Remain
It’s unclear why iron accumulates after an injury or what the ramifications are of this accumulation, Dr. Nikolova noted.
The imaging used in the study doesn’t distinguish between “bound” iron found after a hemorrhage and “free” iron in the brain. The free iron type has been shown to be increased after TBI and is “the stuff you should be afraid of,” Dr. Nikolova said.
Iron’s role in the metabolic process is important, but must be closely regulated, she said. Even a small accumulation can lead to oxidative stress.
Researchers are investigating whether the findings would be similar in mTBI but no headache and want to increase the number of study participants. A larger, more diverse sample would allow them to probe other questions, including whether iron accumulation is different in men and women. More data could also eventually lead to iron accumulation becoming a biomarker for concussion and PTH, Dr. Nikolova said.
“If you know a certain person has that biomarker, you might be able to administer a drug or some therapeutic procedure to prevent that iron from continuing to accumulate in the brain.”
Chelation drugs and other therapies may clear iron from the body but not necessarily from the brain.
Commenting on the study for this news organization, Frank Conidi, MD, director, Florida Center for Headache and Sports Neurology, Port St. Lucie , said that the study supports the hypothesis that concussion “is not a benign process for the brain, and the cumulative effect of repetitive head injury can result in permanent brain injury.”
He said that he found the accumulation of iron in cortical structures particularly interesting. This, he said, differs from most current research that suggests head trauma mainly results in damage to white matter tracts.
He prefers the term “concussion” over “mild traumatic brain injury” which was used in the study. “Recent guidelines, including some that I’ve been involved with, have defined mild traumatic brain injury as a more permanent process,” he said.
The study was supported by the US Department of Defense and National Institutes of Health. No relevant conflicts of interest were disclosed.
A version of this article appeared on Medscape.com.
Posttraumatic headache (PTH) is associated with an increase in iron accumulation in certain brain regions , notably those involved in the pain network, early research shows.
The findings come on the heels of previous research showing patients with iron accumulation in certain brain regions don’t respond as well to treatment, study investigator, Simona Nikolova, PhD, assistant professor of neurology, Mayo Clinic, Phoenix, Arizona, told this news organization.
“This is really important, and doctors need to be aware of it. If you have a patient who is not responding to treatment, then you know what to look at,” she said.
The findings (Abstract #3379) will be presented on April 15 at the American Academy of Neurology (AAN) 2024 Annual Meeting.
Dose Effect
The study included 60 people with acute PTH due to mTBI. Most were White, and almost half had sustained a concussion due to a fall, with about 30% injured in a vehicle accident and a smaller number injured during a fight.
The mean number of lifetime mTBIs was 2.4, although participants had sustained as many as five or six and as few as one. The mean time from the most recent mTBI was 25 days, and the mean score on the Sport Concussion Assessment Tool (SCAT), which measures postconcussion symptom severity, was 29.
Most in the mTBI group (43) had migraine or probable migraine, and 14 had tension-type headaches. Mean headache frequency was 81%.
Researchers matched these patients with 60 controls without concussion or headache. Because iron accumulation is age-related, they tried to eliminate this covariant by pairing each participant with mTBI with an age- and sex-matched control.
All participants underwent a type of brain MRI known as T2* weighted sequence that can identify brain iron accumulation, a marker of neural injury.
Investigators found that the PTH group had significantly higher levels of iron accumulation in several areas of the brain, most of which are part of a “pain network” that includes about 63 areas of the brain, Dr. Nikolova said.
The study wasn’t designed to determine how much more iron accumulation mTBI patients had vs controls.
“We can’t say it was twice as much or three times as much; we can only say it was significant. Measuring concentrations in PTH patients and comparing that with controls is something we haven’t don’t yet,” said Dr. Nikolova.
Areas of the brain with increased iron accumulation, included the periaqueductal gray (PAG), anterior cingulated cortex, and supramarginal gyrus.
Research suggests patients with migraine who have elevated levels of iron in the PAG have a poorer response to botulinum toxin treatment. An earlier study by the same team showed a poorer response to the calcitonin gene-related peptide inhibitor erenumab in migraine patients with elevated iron in the PAG.
Researchers discovered that those with more lifetime TBIs had higher iron accumulation in the right gyrus rectus and right putamen vs those with fewer injuries and that headache frequency was associated with iron accumulation in the posterior corona radiata, bilateral temporal, right frontal, bilateral supplemental motor area, left fusiform, right hippocampus, sagittal striatum, and left cerebellum.
Surprising Result
The investigators also found a link between time since the most recent mTBI and iron accumulation in the bilateral temporal, right hippocampus, posterior and superior corona radiata, bilateral thalamus, right precuneus and cuneus, right lingual, and right cerebellum.
“The more time that passed since the concussion occurred, the more likely that people had higher iron levels,” said Dr. Nikolova.
It’s perhaps to be expected that the length of time since injury is linked to iron accumulation in the brain as iron accumulates over time. But even those whose injury was relatively recent had higher amounts of iron, which Dr. Nikolova said was “surprising.”
“We thought iron accumulates over time so we were thinking maybe we should be doing a longitudinal study to see what happens, but we see definite iron accumulation due to injury shortly after the injury,” she said.
There was no association between iron accumulation and symptom severity as measured by SCAT scores.
Questions Remain
It’s unclear why iron accumulates after an injury or what the ramifications are of this accumulation, Dr. Nikolova noted.
The imaging used in the study doesn’t distinguish between “bound” iron found after a hemorrhage and “free” iron in the brain. The free iron type has been shown to be increased after TBI and is “the stuff you should be afraid of,” Dr. Nikolova said.
Iron’s role in the metabolic process is important, but must be closely regulated, she said. Even a small accumulation can lead to oxidative stress.
Researchers are investigating whether the findings would be similar in mTBI but no headache and want to increase the number of study participants. A larger, more diverse sample would allow them to probe other questions, including whether iron accumulation is different in men and women. More data could also eventually lead to iron accumulation becoming a biomarker for concussion and PTH, Dr. Nikolova said.
“If you know a certain person has that biomarker, you might be able to administer a drug or some therapeutic procedure to prevent that iron from continuing to accumulate in the brain.”
Chelation drugs and other therapies may clear iron from the body but not necessarily from the brain.
Commenting on the study for this news organization, Frank Conidi, MD, director, Florida Center for Headache and Sports Neurology, Port St. Lucie , said that the study supports the hypothesis that concussion “is not a benign process for the brain, and the cumulative effect of repetitive head injury can result in permanent brain injury.”
He said that he found the accumulation of iron in cortical structures particularly interesting. This, he said, differs from most current research that suggests head trauma mainly results in damage to white matter tracts.
He prefers the term “concussion” over “mild traumatic brain injury” which was used in the study. “Recent guidelines, including some that I’ve been involved with, have defined mild traumatic brain injury as a more permanent process,” he said.
The study was supported by the US Department of Defense and National Institutes of Health. No relevant conflicts of interest were disclosed.
A version of this article appeared on Medscape.com.