User login
CDC: Beware Brazil yellow fever outbreak
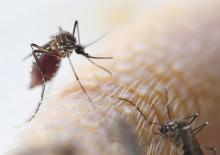
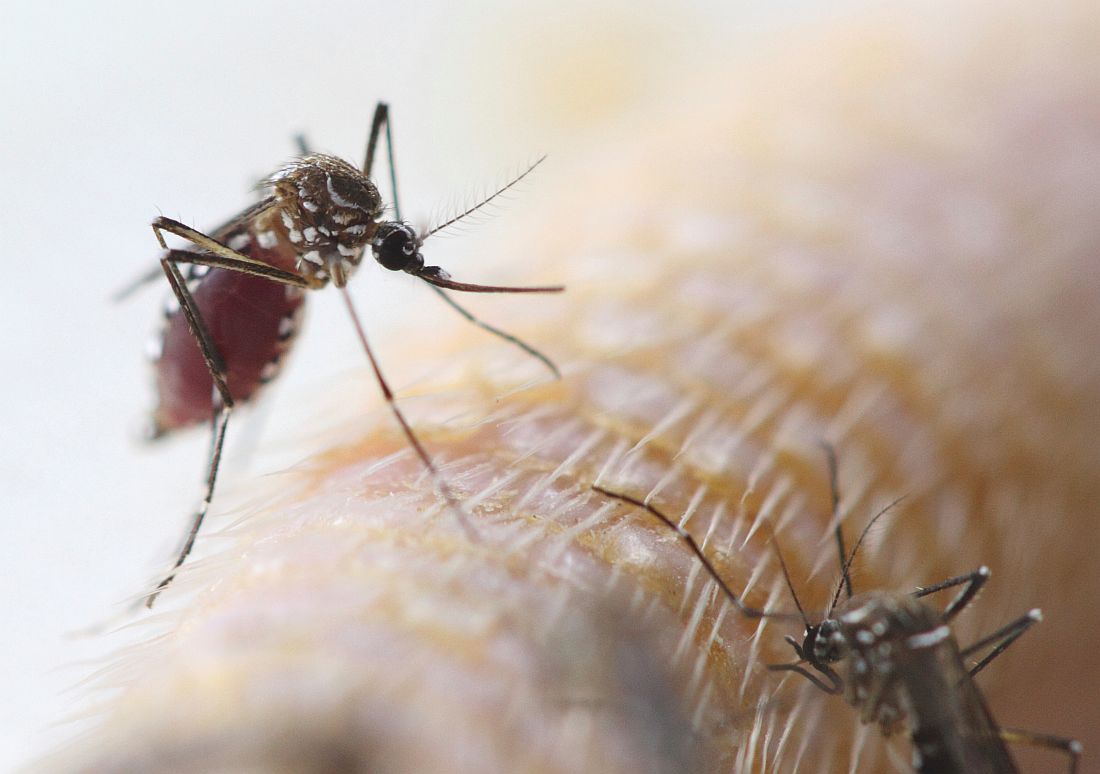
The Centers for Disease Control and Prevention recommends that travelers who haven’t been vaccinated against yellow fever should avoid travel to Brazil, according to a media teleconference by CDC officials.
“The most important new recommendation ... is that travelers should not go to these yellow fever hot spots in Brazil, unless they are vaccinated,” stated Martin Cetron, MD, director of the Division of Global Migration and Quarantine at the CDC. “Health officials in Brazil recently confirmed more than 920 cases of yellow fever, including more than 300 deaths, during this outbreak” he added.
Since the beginning of 2018, 10 travel-related cases of yellow fever have been reported among international travelers returning from Brazil. Four of these travelers died. All 10 travelers had not received the yellow fever vaccine. Of these 10 travelers, 8 acquired the disease on Ilha Grande, an island off the coast of Rio De Janeiro that is popular among tourists.
The CDC is urging travelers to get vaccinated because of the potentially fatal effects of yellow fever. Vaccinations are recommended for any eligible person 9 months of age or older traveling to Brazil, specifically Espírito Santo, São Paulo, and Rio de Janeiro states, and certain cities in Bahia state, as well as Ilha Grande in particular.
Individuals heading to Brazil should receive the vaccination at least 10 days before travel. If a traveler is unvaccinated and cannot get the vaccination in the appropriate amount of time, areas where vaccination is recommended should be avoided.
The Food and Drug Administration–approved yellow fever vaccine, YF-VAX, is not currently available because of manufacturing issues. Stamaril, another yellow fever vaccine, is available at a limited number of yellow fever vaccination clinics in the United States.
In light of these supply issues, the CDC has provided resources to locate yellow fever vaccination clinics.
Dr. Cetron reemphasized that unvaccinated individuals planning to vacation in Brazil may want to reconsider their travel plans.
“People who have never been vaccinated against yellow fever should not travel to the areas in Brazil affected by the outbreak. Particularly the hot spot of Ilha Grande.”
Information for clinicians and travelers is available on the travel notice portion of the CDC site. The travel notice for Brazil includes a map of the yellow fever–affected areas in Brazil, as well as other informational resources.
The related CDC Morbidity and Mortality Weekly Report is available online.
The Centers for Disease Control and Prevention recommends that travelers who haven’t been vaccinated against yellow fever should avoid travel to Brazil, according to a media teleconference by CDC officials.
“The most important new recommendation ... is that travelers should not go to these yellow fever hot spots in Brazil, unless they are vaccinated,” stated Martin Cetron, MD, director of the Division of Global Migration and Quarantine at the CDC. “Health officials in Brazil recently confirmed more than 920 cases of yellow fever, including more than 300 deaths, during this outbreak” he added.
Since the beginning of 2018, 10 travel-related cases of yellow fever have been reported among international travelers returning from Brazil. Four of these travelers died. All 10 travelers had not received the yellow fever vaccine. Of these 10 travelers, 8 acquired the disease on Ilha Grande, an island off the coast of Rio De Janeiro that is popular among tourists.
The CDC is urging travelers to get vaccinated because of the potentially fatal effects of yellow fever. Vaccinations are recommended for any eligible person 9 months of age or older traveling to Brazil, specifically Espírito Santo, São Paulo, and Rio de Janeiro states, and certain cities in Bahia state, as well as Ilha Grande in particular.
Individuals heading to Brazil should receive the vaccination at least 10 days before travel. If a traveler is unvaccinated and cannot get the vaccination in the appropriate amount of time, areas where vaccination is recommended should be avoided.
The Food and Drug Administration–approved yellow fever vaccine, YF-VAX, is not currently available because of manufacturing issues. Stamaril, another yellow fever vaccine, is available at a limited number of yellow fever vaccination clinics in the United States.
In light of these supply issues, the CDC has provided resources to locate yellow fever vaccination clinics.
Dr. Cetron reemphasized that unvaccinated individuals planning to vacation in Brazil may want to reconsider their travel plans.
“People who have never been vaccinated against yellow fever should not travel to the areas in Brazil affected by the outbreak. Particularly the hot spot of Ilha Grande.”
Information for clinicians and travelers is available on the travel notice portion of the CDC site. The travel notice for Brazil includes a map of the yellow fever–affected areas in Brazil, as well as other informational resources.
The related CDC Morbidity and Mortality Weekly Report is available online.
The Centers for Disease Control and Prevention recommends that travelers who haven’t been vaccinated against yellow fever should avoid travel to Brazil, according to a media teleconference by CDC officials.
“The most important new recommendation ... is that travelers should not go to these yellow fever hot spots in Brazil, unless they are vaccinated,” stated Martin Cetron, MD, director of the Division of Global Migration and Quarantine at the CDC. “Health officials in Brazil recently confirmed more than 920 cases of yellow fever, including more than 300 deaths, during this outbreak” he added.
Since the beginning of 2018, 10 travel-related cases of yellow fever have been reported among international travelers returning from Brazil. Four of these travelers died. All 10 travelers had not received the yellow fever vaccine. Of these 10 travelers, 8 acquired the disease on Ilha Grande, an island off the coast of Rio De Janeiro that is popular among tourists.
The CDC is urging travelers to get vaccinated because of the potentially fatal effects of yellow fever. Vaccinations are recommended for any eligible person 9 months of age or older traveling to Brazil, specifically Espírito Santo, São Paulo, and Rio de Janeiro states, and certain cities in Bahia state, as well as Ilha Grande in particular.
Individuals heading to Brazil should receive the vaccination at least 10 days before travel. If a traveler is unvaccinated and cannot get the vaccination in the appropriate amount of time, areas where vaccination is recommended should be avoided.
The Food and Drug Administration–approved yellow fever vaccine, YF-VAX, is not currently available because of manufacturing issues. Stamaril, another yellow fever vaccine, is available at a limited number of yellow fever vaccination clinics in the United States.
In light of these supply issues, the CDC has provided resources to locate yellow fever vaccination clinics.
Dr. Cetron reemphasized that unvaccinated individuals planning to vacation in Brazil may want to reconsider their travel plans.
“People who have never been vaccinated against yellow fever should not travel to the areas in Brazil affected by the outbreak. Particularly the hot spot of Ilha Grande.”
Information for clinicians and travelers is available on the travel notice portion of the CDC site. The travel notice for Brazil includes a map of the yellow fever–affected areas in Brazil, as well as other informational resources.
The related CDC Morbidity and Mortality Weekly Report is available online.
Vaccine priming determines teen susceptibility to pertussis
It is the initial type of pertussis vaccine given in infancy – acellular or whole cell – that primes the immune system and determines how soon adolescents become susceptible to pertussis, regardless of later acellular booster vaccination, noted the authors of a new study.
The IgG4 subclass proportion for IgG4-specific antibodies remained lower in patients who had whole-cell pertussis (wP) priming in infancy, even though they had received acellular pertussis booster vaccinations at ages 4 and 9 years, compared with patients who underwent acellular pertussis (aP) priming in infancy. This was true for all vaccine antigens, other than filamentous hemagglutinin and tetanus, 1 year after the preadolescent booster, noted researcher Saskia van der Lee of the National Institute for Public Health and the Environment, Bilthoven, The Netherlands, and her associates.
“ compared to aP-primed children, even after booster vaccinations. This is in line with epidemiological data indicating that adolescents, after aP vaccination in infancy, are more susceptible to pertussis, compared with wP-primed adolescents, though wP-primed individuals become also susceptible over time,” the researchers said.
In addition, children primed with DTwP vaccines have a more Th1-skewed response for pertussis vaccine antigens after receiving a DTap booster vaccine or clinical infection with Bordetella pertussis, whereas children primed with DTaP have a more mixed pertussis-specific Th1/Th2 response, the researchers said. So “new adjuvants that skew the immune response towards a Th1 profile are desired” for better protection against pertussis over time.
SOURCE: van der Lee S et al. Vaccine. 2018 Jan 4;36(2):220-6.
It is the initial type of pertussis vaccine given in infancy – acellular or whole cell – that primes the immune system and determines how soon adolescents become susceptible to pertussis, regardless of later acellular booster vaccination, noted the authors of a new study.
The IgG4 subclass proportion for IgG4-specific antibodies remained lower in patients who had whole-cell pertussis (wP) priming in infancy, even though they had received acellular pertussis booster vaccinations at ages 4 and 9 years, compared with patients who underwent acellular pertussis (aP) priming in infancy. This was true for all vaccine antigens, other than filamentous hemagglutinin and tetanus, 1 year after the preadolescent booster, noted researcher Saskia van der Lee of the National Institute for Public Health and the Environment, Bilthoven, The Netherlands, and her associates.
“ compared to aP-primed children, even after booster vaccinations. This is in line with epidemiological data indicating that adolescents, after aP vaccination in infancy, are more susceptible to pertussis, compared with wP-primed adolescents, though wP-primed individuals become also susceptible over time,” the researchers said.
In addition, children primed with DTwP vaccines have a more Th1-skewed response for pertussis vaccine antigens after receiving a DTap booster vaccine or clinical infection with Bordetella pertussis, whereas children primed with DTaP have a more mixed pertussis-specific Th1/Th2 response, the researchers said. So “new adjuvants that skew the immune response towards a Th1 profile are desired” for better protection against pertussis over time.
SOURCE: van der Lee S et al. Vaccine. 2018 Jan 4;36(2):220-6.
It is the initial type of pertussis vaccine given in infancy – acellular or whole cell – that primes the immune system and determines how soon adolescents become susceptible to pertussis, regardless of later acellular booster vaccination, noted the authors of a new study.
The IgG4 subclass proportion for IgG4-specific antibodies remained lower in patients who had whole-cell pertussis (wP) priming in infancy, even though they had received acellular pertussis booster vaccinations at ages 4 and 9 years, compared with patients who underwent acellular pertussis (aP) priming in infancy. This was true for all vaccine antigens, other than filamentous hemagglutinin and tetanus, 1 year after the preadolescent booster, noted researcher Saskia van der Lee of the National Institute for Public Health and the Environment, Bilthoven, The Netherlands, and her associates.
“ compared to aP-primed children, even after booster vaccinations. This is in line with epidemiological data indicating that adolescents, after aP vaccination in infancy, are more susceptible to pertussis, compared with wP-primed adolescents, though wP-primed individuals become also susceptible over time,” the researchers said.
In addition, children primed with DTwP vaccines have a more Th1-skewed response for pertussis vaccine antigens after receiving a DTap booster vaccine or clinical infection with Bordetella pertussis, whereas children primed with DTaP have a more mixed pertussis-specific Th1/Th2 response, the researchers said. So “new adjuvants that skew the immune response towards a Th1 profile are desired” for better protection against pertussis over time.
SOURCE: van der Lee S et al. Vaccine. 2018 Jan 4;36(2):220-6.
FROM VACCINE
Which Herpes Zoster Vaccine is Most Cost-Effective?
Study Overview
Objective. To assess the cost-effectiveness of the new adjuvanted herpes zoster subunit vaccine (HZ/su) as compared with that of the current live attenuated herpes zoster vaccine (ZVL), or no vaccine.
Design. Markov decision model evaluating 3 strategies from a societal perspective: (1) no vaccination, (2) vaccination with single dose ZVL, and (3) vaccination with 2-dose series of HZ/su.
Setting and participants. Data for the model were extracted from the US medical literature using PubMed through January 2015. Data were derived from studies of fewer than 100 patients to more than 30,000 patients, depending on the variable assessed. Variables included epidemiologic parameters, vaccine efficacy and adverse events, quality-adjusted life-years (QALYs), and costs. Because there is no standard willingness-to-pay (WTP) threshold for cost-effectiveness in the United States, $50,000 per QALY was chosen.
Main outcome measures. Total costs and QALYs.
Main results. At all ages, no vaccination was always the least expensive and least effective option, while HZ/su was always the most effective and less expensive than ZVL. At a proposed price of $280 per series ($140 per dose), HZ/su was more effective and less expensive than ZVL at all ages. The incremental cost-effectiveness ratios compared with no vaccination ranged from $20,038 to $30,084 per QALY, depending on vaccination age. The cost-effectiveness of HZ/su was insensitive to the waning rate of either vaccine due to its high efficacy, with initial level of protection close to 90% even among people 70 years or older.
Conclusion. At a manufacturer suggested price of $280 per series ($140 per dose), HZ/su would cost less than ZVL and has a high probability of offering good value.
Commentary
Herpes zosters is a localized, usually painful, cutaneous eruption resulting from reactivation of latent varicella zoster virus. It is a common disease with approximately one million cases occurring each year in the United States [1]. The incidence increases with age, from 5 cases per 1000 population in adults aged 50–59 years to 11 cases per 1000 population in persons aged ≥ 80 years. Postherpetic neuralgia, commonly defined as persistent pain for at least 90 days following the resolution of the herpes zoster rash, is the most common complication and occurs in 10% to 13% of herpes zoster cases in persons aged > 50 years [2,3].
In 2006, the US Food and Drug Administration (FDA) approved the ZVL vaccine Zostavax (Merck) for prevention of postherpetic neuralgia. By 2016, 33% of adults aged ≥ 60 years reported receipt of the vaccine [4]. However, ZVL does not prevent all herpes zoster, particularly among the elderly. Moreover, the efficacy wanes completely after approximately 10 years [5]. To address these shortcomings, a 2-dose HZ/su (Shingrix; GlaxoSmithKline) containing recombinant glycoprotein E in combination with a novel adjuvant (AS01B) was approved by the FDA in adults aged ≥ 50 years. In randomized controlled trials, HZ/su has an efficacy of close to 97%, even after age 70 years [6].
With the approval of the new attenuated herpes zoster vaccine, clinicians and patients face the question of which vaccine to get and when. The cost-effectiveness analysis published by Le and Rothberg in this study compare the value of HZ/su with ZVL vaccine and a no-vaccine strategy for individuals 60 years or older from the US societal perspective. The results suggest that, at $140 per dose, using HZ/su vaccine compared with no vaccine would cost between $20,038 and $30,084 per QALY and thus is a cost-effective strategy. The deterministic sensitivity analysis indicates that the overall results do not change under different assumptions about model input parameters, even if patients are nonadherent to the second dose of HZ/su vaccine.
As with any simulation study, the major limitation of this study is the accuracy of the model and the assumptions on which it is based. The body of evidence for benefits of ZVL was large, including multiple pre-licensure and post-licensure RCTs, as well as observational studies of effectiveness. On the other hand, the body of evidence for benefits of RZV was primarily informed by one high-quality RCT that studied vaccine efficacy through 4 years post-vaccination [4,6]. Currently, 3 other independent cost-effectiveness analysis are available. The Centers for Disease Control and Prevention model estimated HZ/su vaccine cost per QALY of $31,000 when vaccination occurred at age ≥ 50 years. The GlaxoSmithKline model, manufacturer of HZ/su vaccine, estimated a HZ/su vaccine cost per QALY of $12,000. While the Merck model, manufacturer of the ZVL vaccine, estimated a HZ/su vaccine cost per QALY of $107,000 [4]. In addition to model variables, the key assumption by Le and Rothberg are based on the HZ/su vaccine cost at $140 per dose and ZVL at $213. The study results need to be interpreted carefully if the vaccine prices turn out to be different in the future.
Applications for Clinical Practice
The current study by Le and Rothberg demonstrated the cost-effectiveness of the new HZ/su vaccine. Since the study’s publication, the CDC has updated their recommendations on immunization practices for use of herpes zoster vaccine [4]. HZ/su vaccine, also known as the recombinant zoster vaccine (RZV), is now preferred over ZVL for the prevention of herpes zoster and related complications. RZV is recommended for immunocompetent adults age 50 or older, 10 years earlier than previously for the ZVL. In addition, RZV is recommended for adults who previously received ZVL. Finally, RZV can be administered concomitantly with other adult vaccines, does not require screening for a history of varicella, and is likely safe for immunocompromised persons.
—Ka Ming Gordon Ngai, MD, MPH
1. Insinga RP, Itzler RF, Pellissier JM, et al. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med 2005;20:748–53.
2. Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007;82:1341–9.
3. Oxman MN, Levin MJ, Johnson GR, et al. Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Eng J Med 2005;352:2271-84.
4. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep 2018;67:103–8.
5. Morrison VA, Johnson GR, Schmader KE, et al; Shingles Prevention Study Group. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015;60:900–9.
6. Lai H, Cunningham AL, Godeaux O, et al; ZOE-50 Study Group. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015;372:2087–96.
Study Overview
Objective. To assess the cost-effectiveness of the new adjuvanted herpes zoster subunit vaccine (HZ/su) as compared with that of the current live attenuated herpes zoster vaccine (ZVL), or no vaccine.
Design. Markov decision model evaluating 3 strategies from a societal perspective: (1) no vaccination, (2) vaccination with single dose ZVL, and (3) vaccination with 2-dose series of HZ/su.
Setting and participants. Data for the model were extracted from the US medical literature using PubMed through January 2015. Data were derived from studies of fewer than 100 patients to more than 30,000 patients, depending on the variable assessed. Variables included epidemiologic parameters, vaccine efficacy and adverse events, quality-adjusted life-years (QALYs), and costs. Because there is no standard willingness-to-pay (WTP) threshold for cost-effectiveness in the United States, $50,000 per QALY was chosen.
Main outcome measures. Total costs and QALYs.
Main results. At all ages, no vaccination was always the least expensive and least effective option, while HZ/su was always the most effective and less expensive than ZVL. At a proposed price of $280 per series ($140 per dose), HZ/su was more effective and less expensive than ZVL at all ages. The incremental cost-effectiveness ratios compared with no vaccination ranged from $20,038 to $30,084 per QALY, depending on vaccination age. The cost-effectiveness of HZ/su was insensitive to the waning rate of either vaccine due to its high efficacy, with initial level of protection close to 90% even among people 70 years or older.
Conclusion. At a manufacturer suggested price of $280 per series ($140 per dose), HZ/su would cost less than ZVL and has a high probability of offering good value.
Commentary
Herpes zosters is a localized, usually painful, cutaneous eruption resulting from reactivation of latent varicella zoster virus. It is a common disease with approximately one million cases occurring each year in the United States [1]. The incidence increases with age, from 5 cases per 1000 population in adults aged 50–59 years to 11 cases per 1000 population in persons aged ≥ 80 years. Postherpetic neuralgia, commonly defined as persistent pain for at least 90 days following the resolution of the herpes zoster rash, is the most common complication and occurs in 10% to 13% of herpes zoster cases in persons aged > 50 years [2,3].
In 2006, the US Food and Drug Administration (FDA) approved the ZVL vaccine Zostavax (Merck) for prevention of postherpetic neuralgia. By 2016, 33% of adults aged ≥ 60 years reported receipt of the vaccine [4]. However, ZVL does not prevent all herpes zoster, particularly among the elderly. Moreover, the efficacy wanes completely after approximately 10 years [5]. To address these shortcomings, a 2-dose HZ/su (Shingrix; GlaxoSmithKline) containing recombinant glycoprotein E in combination with a novel adjuvant (AS01B) was approved by the FDA in adults aged ≥ 50 years. In randomized controlled trials, HZ/su has an efficacy of close to 97%, even after age 70 years [6].
With the approval of the new attenuated herpes zoster vaccine, clinicians and patients face the question of which vaccine to get and when. The cost-effectiveness analysis published by Le and Rothberg in this study compare the value of HZ/su with ZVL vaccine and a no-vaccine strategy for individuals 60 years or older from the US societal perspective. The results suggest that, at $140 per dose, using HZ/su vaccine compared with no vaccine would cost between $20,038 and $30,084 per QALY and thus is a cost-effective strategy. The deterministic sensitivity analysis indicates that the overall results do not change under different assumptions about model input parameters, even if patients are nonadherent to the second dose of HZ/su vaccine.
As with any simulation study, the major limitation of this study is the accuracy of the model and the assumptions on which it is based. The body of evidence for benefits of ZVL was large, including multiple pre-licensure and post-licensure RCTs, as well as observational studies of effectiveness. On the other hand, the body of evidence for benefits of RZV was primarily informed by one high-quality RCT that studied vaccine efficacy through 4 years post-vaccination [4,6]. Currently, 3 other independent cost-effectiveness analysis are available. The Centers for Disease Control and Prevention model estimated HZ/su vaccine cost per QALY of $31,000 when vaccination occurred at age ≥ 50 years. The GlaxoSmithKline model, manufacturer of HZ/su vaccine, estimated a HZ/su vaccine cost per QALY of $12,000. While the Merck model, manufacturer of the ZVL vaccine, estimated a HZ/su vaccine cost per QALY of $107,000 [4]. In addition to model variables, the key assumption by Le and Rothberg are based on the HZ/su vaccine cost at $140 per dose and ZVL at $213. The study results need to be interpreted carefully if the vaccine prices turn out to be different in the future.
Applications for Clinical Practice
The current study by Le and Rothberg demonstrated the cost-effectiveness of the new HZ/su vaccine. Since the study’s publication, the CDC has updated their recommendations on immunization practices for use of herpes zoster vaccine [4]. HZ/su vaccine, also known as the recombinant zoster vaccine (RZV), is now preferred over ZVL for the prevention of herpes zoster and related complications. RZV is recommended for immunocompetent adults age 50 or older, 10 years earlier than previously for the ZVL. In addition, RZV is recommended for adults who previously received ZVL. Finally, RZV can be administered concomitantly with other adult vaccines, does not require screening for a history of varicella, and is likely safe for immunocompromised persons.
—Ka Ming Gordon Ngai, MD, MPH
Study Overview
Objective. To assess the cost-effectiveness of the new adjuvanted herpes zoster subunit vaccine (HZ/su) as compared with that of the current live attenuated herpes zoster vaccine (ZVL), or no vaccine.
Design. Markov decision model evaluating 3 strategies from a societal perspective: (1) no vaccination, (2) vaccination with single dose ZVL, and (3) vaccination with 2-dose series of HZ/su.
Setting and participants. Data for the model were extracted from the US medical literature using PubMed through January 2015. Data were derived from studies of fewer than 100 patients to more than 30,000 patients, depending on the variable assessed. Variables included epidemiologic parameters, vaccine efficacy and adverse events, quality-adjusted life-years (QALYs), and costs. Because there is no standard willingness-to-pay (WTP) threshold for cost-effectiveness in the United States, $50,000 per QALY was chosen.
Main outcome measures. Total costs and QALYs.
Main results. At all ages, no vaccination was always the least expensive and least effective option, while HZ/su was always the most effective and less expensive than ZVL. At a proposed price of $280 per series ($140 per dose), HZ/su was more effective and less expensive than ZVL at all ages. The incremental cost-effectiveness ratios compared with no vaccination ranged from $20,038 to $30,084 per QALY, depending on vaccination age. The cost-effectiveness of HZ/su was insensitive to the waning rate of either vaccine due to its high efficacy, with initial level of protection close to 90% even among people 70 years or older.
Conclusion. At a manufacturer suggested price of $280 per series ($140 per dose), HZ/su would cost less than ZVL and has a high probability of offering good value.
Commentary
Herpes zosters is a localized, usually painful, cutaneous eruption resulting from reactivation of latent varicella zoster virus. It is a common disease with approximately one million cases occurring each year in the United States [1]. The incidence increases with age, from 5 cases per 1000 population in adults aged 50–59 years to 11 cases per 1000 population in persons aged ≥ 80 years. Postherpetic neuralgia, commonly defined as persistent pain for at least 90 days following the resolution of the herpes zoster rash, is the most common complication and occurs in 10% to 13% of herpes zoster cases in persons aged > 50 years [2,3].
In 2006, the US Food and Drug Administration (FDA) approved the ZVL vaccine Zostavax (Merck) for prevention of postherpetic neuralgia. By 2016, 33% of adults aged ≥ 60 years reported receipt of the vaccine [4]. However, ZVL does not prevent all herpes zoster, particularly among the elderly. Moreover, the efficacy wanes completely after approximately 10 years [5]. To address these shortcomings, a 2-dose HZ/su (Shingrix; GlaxoSmithKline) containing recombinant glycoprotein E in combination with a novel adjuvant (AS01B) was approved by the FDA in adults aged ≥ 50 years. In randomized controlled trials, HZ/su has an efficacy of close to 97%, even after age 70 years [6].
With the approval of the new attenuated herpes zoster vaccine, clinicians and patients face the question of which vaccine to get and when. The cost-effectiveness analysis published by Le and Rothberg in this study compare the value of HZ/su with ZVL vaccine and a no-vaccine strategy for individuals 60 years or older from the US societal perspective. The results suggest that, at $140 per dose, using HZ/su vaccine compared with no vaccine would cost between $20,038 and $30,084 per QALY and thus is a cost-effective strategy. The deterministic sensitivity analysis indicates that the overall results do not change under different assumptions about model input parameters, even if patients are nonadherent to the second dose of HZ/su vaccine.
As with any simulation study, the major limitation of this study is the accuracy of the model and the assumptions on which it is based. The body of evidence for benefits of ZVL was large, including multiple pre-licensure and post-licensure RCTs, as well as observational studies of effectiveness. On the other hand, the body of evidence for benefits of RZV was primarily informed by one high-quality RCT that studied vaccine efficacy through 4 years post-vaccination [4,6]. Currently, 3 other independent cost-effectiveness analysis are available. The Centers for Disease Control and Prevention model estimated HZ/su vaccine cost per QALY of $31,000 when vaccination occurred at age ≥ 50 years. The GlaxoSmithKline model, manufacturer of HZ/su vaccine, estimated a HZ/su vaccine cost per QALY of $12,000. While the Merck model, manufacturer of the ZVL vaccine, estimated a HZ/su vaccine cost per QALY of $107,000 [4]. In addition to model variables, the key assumption by Le and Rothberg are based on the HZ/su vaccine cost at $140 per dose and ZVL at $213. The study results need to be interpreted carefully if the vaccine prices turn out to be different in the future.
Applications for Clinical Practice
The current study by Le and Rothberg demonstrated the cost-effectiveness of the new HZ/su vaccine. Since the study’s publication, the CDC has updated their recommendations on immunization practices for use of herpes zoster vaccine [4]. HZ/su vaccine, also known as the recombinant zoster vaccine (RZV), is now preferred over ZVL for the prevention of herpes zoster and related complications. RZV is recommended for immunocompetent adults age 50 or older, 10 years earlier than previously for the ZVL. In addition, RZV is recommended for adults who previously received ZVL. Finally, RZV can be administered concomitantly with other adult vaccines, does not require screening for a history of varicella, and is likely safe for immunocompromised persons.
—Ka Ming Gordon Ngai, MD, MPH
1. Insinga RP, Itzler RF, Pellissier JM, et al. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med 2005;20:748–53.
2. Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007;82:1341–9.
3. Oxman MN, Levin MJ, Johnson GR, et al. Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Eng J Med 2005;352:2271-84.
4. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep 2018;67:103–8.
5. Morrison VA, Johnson GR, Schmader KE, et al; Shingles Prevention Study Group. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015;60:900–9.
6. Lai H, Cunningham AL, Godeaux O, et al; ZOE-50 Study Group. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015;372:2087–96.
1. Insinga RP, Itzler RF, Pellissier JM, et al. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med 2005;20:748–53.
2. Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007;82:1341–9.
3. Oxman MN, Levin MJ, Johnson GR, et al. Shingles Prevention Study Group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Eng J Med 2005;352:2271-84.
4. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep 2018;67:103–8.
5. Morrison VA, Johnson GR, Schmader KE, et al; Shingles Prevention Study Group. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015;60:900–9.
6. Lai H, Cunningham AL, Godeaux O, et al; ZOE-50 Study Group. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015;372:2087–96.
High efficacy, no safety signals for herpes zoster vaccine post-HSCT
SALT LAKE CITY – A recently approved adjuvanted herpes zoster vaccine)(Shingrix) effectively and safely prevented herpes zoster in a population of patients with multiple myeloma and other hematologic malignancies who received autologous hematopoietic stem cell transplantation.
The use of recombinant varicella zoster virus glycoprotein E in combination with an adjuvant system gives immunosuppressed individuals who have received hematopoietic stem cell transplantation (HSCT) a safe option for prevention of herpes zoster (HZ), said Javier de la Serna, MD, PhD, speaking at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
Presenting the findings at a late-breaking abstract session, Dr. de la Serna said that for the 1,721 participants in a placebo-controlled multicenter trial who received both doses of the vaccine, the incidence of HZ for vaccine recipients was 3.0%, compared with 9.4% of placebo recipients, for a vaccine efficacy of 68.2% (95% confidence interval, 55.6-77.5; P less than 0.0001). These results met the study’s primary objective.
Postherpetic neuralgia (PHN) prevention efficacy – a secondary endpoint – was 89.3% for those receiving the vaccine (HZ/su); the incidence of PHN was 0.5% in the HZ/su study arm, compared with 4.9% for those who received placebo (95% CI, 22.5-99.8). The study also tracked other HZ complications as a secondary endpoint, finding efficacy of 77.8% (95% CI, 19.1–95.0). “The vaccine was highly efficacious in preventing all the secondary outcomes,” said Dr. de la Serna of the Hospital Universitario 12 de Octubre, Madrid.
The randomized, observer-blind phase 3 trial was conducted in 28 countries.Adults who received autologous HSCT were randomized 1:1 to receive HZ/su (n = 922) or placebo (n = 924) within 50-70 days of their transplant. Patients were excluded if they were expected to receive more than 6 months of anti–varicella zoster prophylaxis posttransplant, Dr. de la Serna said.
Participants received the first dose of HZ/su at the first study visit, and the second dose 30-60 days later. Patients were seen 1 month after the last vaccine dose, and then again at months 13 and 25, with telephone follow-up between the later visits. All participants were followed for at least 1 year, Dr. de la Serna said.
Episodes of HZ were confirmed by polymerase chain reaction assay, or, when samples were lacking or indeterminate, by agreement of at least three members of an ascertainment committee.
Of the two components of the HZ/su vaccine, glycoprotein E triggers both humoral immunity and activity of varicella zoster–specific CD4+ T cells; the adjuvant system – dubbed ASO1 – boosts immune response. The vaccine was approved by the Food and Drug Administration in October 2017 for use in adults aged 50 years and older.
In addition to the primary endpoint of vaccine efficacy in prevention of HZ cases during the study period, secondary objectives included monitoring vaccine reactogenicity and safety, and evaluating vaccine efficacy for the prevention of PHN and other complications of HZ.
Tertiary objectives included vaccine efficacy in preventing HZ during the first year posttransplant (vaccine efficacy 84.7%; 95% CI, 32.2-96.6), as well as efficacy in preventing hospitalizations related to HZ (vaccine efficacy 76.2%, 95% CI 61.1-86.0).
An exploratory analysis found vaccine efficacy of 71.8% for participants younger than 50 years (95% CI, 38.8 – 88.3). For patients aged 50 years and older, vaccine efficacy was 67.3% (95% CI, 52.6–77.9).
The safety of HZ/su was determined by analyzing data for all participants, but efficacy data included only those who received the second dose and did not develop HZ within a month of receiving the second vaccine dose.
In the efficacy group (n = 1,721), patients were mostly (n = 1,296) aged 50 years or older. Most patients (n = 937) received HSCT for multiple myeloma. Overall, participants were about 63% male, and 77% were of Caucasian/European ancestry.
Adverse events, solicited for the first 7 days after injections, were mostly mild and related to the local site pain and inflammation expected with an adjuvanted vaccine; HZ/su recipients also experienced more fatigue and muscle aches than did those receiving placebo. Median duration of symptoms was up to 3 days, with grade 3 events lasting up to 2 days.
Unsolicited and serious adverse events were similar between study arms, with a median safety follow-up period of 29 months. The investigators judged that no deaths were related to the vaccine, and there were no signals for increased rate of relapse or immune-mediated diseases.
The study was funded by GlaxoSmithKline; HZ/su(Shingrix) is marketed by GlaxoSmithKline. Dr. de la Serna reported being on the advisory board or receiving honoraria from multiple pharmaceutical companies.
SOURCE: de la Serna J et al. 2018 BMT Tandem Meetings, Abstract LBA2.
SALT LAKE CITY – A recently approved adjuvanted herpes zoster vaccine)(Shingrix) effectively and safely prevented herpes zoster in a population of patients with multiple myeloma and other hematologic malignancies who received autologous hematopoietic stem cell transplantation.
The use of recombinant varicella zoster virus glycoprotein E in combination with an adjuvant system gives immunosuppressed individuals who have received hematopoietic stem cell transplantation (HSCT) a safe option for prevention of herpes zoster (HZ), said Javier de la Serna, MD, PhD, speaking at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
Presenting the findings at a late-breaking abstract session, Dr. de la Serna said that for the 1,721 participants in a placebo-controlled multicenter trial who received both doses of the vaccine, the incidence of HZ for vaccine recipients was 3.0%, compared with 9.4% of placebo recipients, for a vaccine efficacy of 68.2% (95% confidence interval, 55.6-77.5; P less than 0.0001). These results met the study’s primary objective.
Postherpetic neuralgia (PHN) prevention efficacy – a secondary endpoint – was 89.3% for those receiving the vaccine (HZ/su); the incidence of PHN was 0.5% in the HZ/su study arm, compared with 4.9% for those who received placebo (95% CI, 22.5-99.8). The study also tracked other HZ complications as a secondary endpoint, finding efficacy of 77.8% (95% CI, 19.1–95.0). “The vaccine was highly efficacious in preventing all the secondary outcomes,” said Dr. de la Serna of the Hospital Universitario 12 de Octubre, Madrid.
The randomized, observer-blind phase 3 trial was conducted in 28 countries.Adults who received autologous HSCT were randomized 1:1 to receive HZ/su (n = 922) or placebo (n = 924) within 50-70 days of their transplant. Patients were excluded if they were expected to receive more than 6 months of anti–varicella zoster prophylaxis posttransplant, Dr. de la Serna said.
Participants received the first dose of HZ/su at the first study visit, and the second dose 30-60 days later. Patients were seen 1 month after the last vaccine dose, and then again at months 13 and 25, with telephone follow-up between the later visits. All participants were followed for at least 1 year, Dr. de la Serna said.
Episodes of HZ were confirmed by polymerase chain reaction assay, or, when samples were lacking or indeterminate, by agreement of at least three members of an ascertainment committee.
Of the two components of the HZ/su vaccine, glycoprotein E triggers both humoral immunity and activity of varicella zoster–specific CD4+ T cells; the adjuvant system – dubbed ASO1 – boosts immune response. The vaccine was approved by the Food and Drug Administration in October 2017 for use in adults aged 50 years and older.
In addition to the primary endpoint of vaccine efficacy in prevention of HZ cases during the study period, secondary objectives included monitoring vaccine reactogenicity and safety, and evaluating vaccine efficacy for the prevention of PHN and other complications of HZ.
Tertiary objectives included vaccine efficacy in preventing HZ during the first year posttransplant (vaccine efficacy 84.7%; 95% CI, 32.2-96.6), as well as efficacy in preventing hospitalizations related to HZ (vaccine efficacy 76.2%, 95% CI 61.1-86.0).
An exploratory analysis found vaccine efficacy of 71.8% for participants younger than 50 years (95% CI, 38.8 – 88.3). For patients aged 50 years and older, vaccine efficacy was 67.3% (95% CI, 52.6–77.9).
The safety of HZ/su was determined by analyzing data for all participants, but efficacy data included only those who received the second dose and did not develop HZ within a month of receiving the second vaccine dose.
In the efficacy group (n = 1,721), patients were mostly (n = 1,296) aged 50 years or older. Most patients (n = 937) received HSCT for multiple myeloma. Overall, participants were about 63% male, and 77% were of Caucasian/European ancestry.
Adverse events, solicited for the first 7 days after injections, were mostly mild and related to the local site pain and inflammation expected with an adjuvanted vaccine; HZ/su recipients also experienced more fatigue and muscle aches than did those receiving placebo. Median duration of symptoms was up to 3 days, with grade 3 events lasting up to 2 days.
Unsolicited and serious adverse events were similar between study arms, with a median safety follow-up period of 29 months. The investigators judged that no deaths were related to the vaccine, and there were no signals for increased rate of relapse or immune-mediated diseases.
The study was funded by GlaxoSmithKline; HZ/su(Shingrix) is marketed by GlaxoSmithKline. Dr. de la Serna reported being on the advisory board or receiving honoraria from multiple pharmaceutical companies.
SOURCE: de la Serna J et al. 2018 BMT Tandem Meetings, Abstract LBA2.
SALT LAKE CITY – A recently approved adjuvanted herpes zoster vaccine)(Shingrix) effectively and safely prevented herpes zoster in a population of patients with multiple myeloma and other hematologic malignancies who received autologous hematopoietic stem cell transplantation.
The use of recombinant varicella zoster virus glycoprotein E in combination with an adjuvant system gives immunosuppressed individuals who have received hematopoietic stem cell transplantation (HSCT) a safe option for prevention of herpes zoster (HZ), said Javier de la Serna, MD, PhD, speaking at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
Presenting the findings at a late-breaking abstract session, Dr. de la Serna said that for the 1,721 participants in a placebo-controlled multicenter trial who received both doses of the vaccine, the incidence of HZ for vaccine recipients was 3.0%, compared with 9.4% of placebo recipients, for a vaccine efficacy of 68.2% (95% confidence interval, 55.6-77.5; P less than 0.0001). These results met the study’s primary objective.
Postherpetic neuralgia (PHN) prevention efficacy – a secondary endpoint – was 89.3% for those receiving the vaccine (HZ/su); the incidence of PHN was 0.5% in the HZ/su study arm, compared with 4.9% for those who received placebo (95% CI, 22.5-99.8). The study also tracked other HZ complications as a secondary endpoint, finding efficacy of 77.8% (95% CI, 19.1–95.0). “The vaccine was highly efficacious in preventing all the secondary outcomes,” said Dr. de la Serna of the Hospital Universitario 12 de Octubre, Madrid.
The randomized, observer-blind phase 3 trial was conducted in 28 countries.Adults who received autologous HSCT were randomized 1:1 to receive HZ/su (n = 922) or placebo (n = 924) within 50-70 days of their transplant. Patients were excluded if they were expected to receive more than 6 months of anti–varicella zoster prophylaxis posttransplant, Dr. de la Serna said.
Participants received the first dose of HZ/su at the first study visit, and the second dose 30-60 days later. Patients were seen 1 month after the last vaccine dose, and then again at months 13 and 25, with telephone follow-up between the later visits. All participants were followed for at least 1 year, Dr. de la Serna said.
Episodes of HZ were confirmed by polymerase chain reaction assay, or, when samples were lacking or indeterminate, by agreement of at least three members of an ascertainment committee.
Of the two components of the HZ/su vaccine, glycoprotein E triggers both humoral immunity and activity of varicella zoster–specific CD4+ T cells; the adjuvant system – dubbed ASO1 – boosts immune response. The vaccine was approved by the Food and Drug Administration in October 2017 for use in adults aged 50 years and older.
In addition to the primary endpoint of vaccine efficacy in prevention of HZ cases during the study period, secondary objectives included monitoring vaccine reactogenicity and safety, and evaluating vaccine efficacy for the prevention of PHN and other complications of HZ.
Tertiary objectives included vaccine efficacy in preventing HZ during the first year posttransplant (vaccine efficacy 84.7%; 95% CI, 32.2-96.6), as well as efficacy in preventing hospitalizations related to HZ (vaccine efficacy 76.2%, 95% CI 61.1-86.0).
An exploratory analysis found vaccine efficacy of 71.8% for participants younger than 50 years (95% CI, 38.8 – 88.3). For patients aged 50 years and older, vaccine efficacy was 67.3% (95% CI, 52.6–77.9).
The safety of HZ/su was determined by analyzing data for all participants, but efficacy data included only those who received the second dose and did not develop HZ within a month of receiving the second vaccine dose.
In the efficacy group (n = 1,721), patients were mostly (n = 1,296) aged 50 years or older. Most patients (n = 937) received HSCT for multiple myeloma. Overall, participants were about 63% male, and 77% were of Caucasian/European ancestry.
Adverse events, solicited for the first 7 days after injections, were mostly mild and related to the local site pain and inflammation expected with an adjuvanted vaccine; HZ/su recipients also experienced more fatigue and muscle aches than did those receiving placebo. Median duration of symptoms was up to 3 days, with grade 3 events lasting up to 2 days.
Unsolicited and serious adverse events were similar between study arms, with a median safety follow-up period of 29 months. The investigators judged that no deaths were related to the vaccine, and there were no signals for increased rate of relapse or immune-mediated diseases.
The study was funded by GlaxoSmithKline; HZ/su(Shingrix) is marketed by GlaxoSmithKline. Dr. de la Serna reported being on the advisory board or receiving honoraria from multiple pharmaceutical companies.
SOURCE: de la Serna J et al. 2018 BMT Tandem Meetings, Abstract LBA2.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Efficacy was 68.17% for preventing herpes zoster among HSCT recipients.
Study details: A randomized, observer blind, placebo-controlled phase 3 study of 1,846 post-HSCT recipients.
Disclosures: The study was sponsored by GlaxoSmithKline. Dr. de la Serna reported relationships with multiple pharmaceutical companies.
Source: de la Serna J et al. 2018 BMT Tandem Meetings, Abstract LBA2.
Early childhood vaccines not associated with increased infection risk
There was no significant difference in vaccine antigen exposure through the first 23 months of life between children with non–vaccine-targeted infections and controls between 24 and 47 months of age, according to results published March 6 in JAMA.
This was determined in a nested, matched case-control study of 193 infection cases and 751 controls, in whom estimated mean cumulative vaccine antigen exposure was 240.6 for cases of non–vaccine-targeted infections, and 242.9 for controls, reported Jason M. Glanz, PhD, of Kaiser Permanente Colorado, Denver, and his coauthors. The between-group difference was –2.3 (P = .55), a nonsignificant difference.
Using data from the Centers for Disease Control and Prevention-funded Vaccine Safety Datalink (VSD), the investigators identified children born between Jan. 1, 2003, and Sep. 31, 2013. Exclusion criteria were not having at least two well-child visits before the first birthday, medical contraindications to vaccination, or receiving vaccines not recommended by the Advisory Committee on Immunization Practices. Eligible children were followed through age 47 months or until disenrollment from their health care organization, the authors said.
ICD-9 and ICD-10 codes were used to identify non–vaccine-targeted infections, including upper and lower respiratory infections, gastrointestinal infections, and other viral and bacterial infections from ages 24to 47 months. A medical record review was performed to confirm case status. Cases were included only if it was confirmed that the infection occurred, that it was an incident outcome, that the outcome was the primary reason for the medical visit, that the outcome occurred in the inpatient or emergency department setting, and that there was no evidence that the child was diagnosed as having a vaccine preventable disease (VPD) on the same day as the infection. Controls did not have a VPD or record of a non–vaccine-targeted infection prior to the index date, Dr. Glanz and his colleagues said.
Antigen exposure was measured as the number of immunogenic proteins and polysaccharides in each vaccine, and was estimated from birth through age 23 months in both groups. Cumulative antigen exposure was estimated by adding the number of antigens in each non–vaccine-targeted infection and controls.
Estimated mean cumulative vaccine antigen exposure was 240.6 for cases of non–vaccine-targeted infections, and 242.9 for controls, the authors reported. The matched odds ratio (mOR) for estimated cumulative antigen exposure through age 23 months was not significant in children with infections, compared with controls (mOR = 0.94; 95% confidence interval, 0.84-1.07). The estimated maximum single-day antigen exposure was not significantly associated with non–vaccine-targeted infection (mOR = 1.07; 95% CI, 0.81-1.41).
The findings of this study “did not reveal any beneficial or detrimental associations with estimated cumulative vaccine antigen exposure in young children with non–vaccine-targeted infections in ED and inpatient settings,” wrote Dr. Glanz and coauthors. In addition, the study “did not find evidence that multiple vaccine exposure was associated with the risk for non-targeted infectious diseases.”
The CDC funded the study. The authors reported receiving contracts, grants, and other funding from the CDC.
SOURCE: Glanz JM et al. JAMA. 2018;319(9):906-13.
These results provide “further reassurance about the safety of the U.S. child vaccination schedule,” said Sean T. O’Leary, MD, and Yvonne A. Maldonado, MD.
However, they added, more work must be done to strengthen the public’s trust and confidence in vaccines. Parents long have voiced concerns that vaccines might weaken their children’s immune systems.
“The small but vocal minority of anti-vaccine groups may not be satisfied by the evidence provided through VSD and other vaccine safety surveillance,” they wrote. “Simply providing scientific information and assuming parents will make the decision to vaccinate is not enough.
“Delivering evidence-based information to parents and clinicians in ways that inspire confidence in the robust and safe childhood immunization schedule is critical for maintaining the health of children,” they concluded.
Dr. O’Leary and Dr. Maldonado, both of the University of Colorado, Aurora, commented in an editorial accompanying the article by Glanz et al. (JAMA. 2018 Mar 6;319(9):870-1). Dr. Maldonado reported receiving personal fees for serving on a data and safety monitoring board for Pfizer. Dr. O’Leary reported no relevant financial disclosures.
These results provide “further reassurance about the safety of the U.S. child vaccination schedule,” said Sean T. O’Leary, MD, and Yvonne A. Maldonado, MD.
However, they added, more work must be done to strengthen the public’s trust and confidence in vaccines. Parents long have voiced concerns that vaccines might weaken their children’s immune systems.
“The small but vocal minority of anti-vaccine groups may not be satisfied by the evidence provided through VSD and other vaccine safety surveillance,” they wrote. “Simply providing scientific information and assuming parents will make the decision to vaccinate is not enough.
“Delivering evidence-based information to parents and clinicians in ways that inspire confidence in the robust and safe childhood immunization schedule is critical for maintaining the health of children,” they concluded.
Dr. O’Leary and Dr. Maldonado, both of the University of Colorado, Aurora, commented in an editorial accompanying the article by Glanz et al. (JAMA. 2018 Mar 6;319(9):870-1). Dr. Maldonado reported receiving personal fees for serving on a data and safety monitoring board for Pfizer. Dr. O’Leary reported no relevant financial disclosures.
These results provide “further reassurance about the safety of the U.S. child vaccination schedule,” said Sean T. O’Leary, MD, and Yvonne A. Maldonado, MD.
However, they added, more work must be done to strengthen the public’s trust and confidence in vaccines. Parents long have voiced concerns that vaccines might weaken their children’s immune systems.
“The small but vocal minority of anti-vaccine groups may not be satisfied by the evidence provided through VSD and other vaccine safety surveillance,” they wrote. “Simply providing scientific information and assuming parents will make the decision to vaccinate is not enough.
“Delivering evidence-based information to parents and clinicians in ways that inspire confidence in the robust and safe childhood immunization schedule is critical for maintaining the health of children,” they concluded.
Dr. O’Leary and Dr. Maldonado, both of the University of Colorado, Aurora, commented in an editorial accompanying the article by Glanz et al. (JAMA. 2018 Mar 6;319(9):870-1). Dr. Maldonado reported receiving personal fees for serving on a data and safety monitoring board for Pfizer. Dr. O’Leary reported no relevant financial disclosures.
There was no significant difference in vaccine antigen exposure through the first 23 months of life between children with non–vaccine-targeted infections and controls between 24 and 47 months of age, according to results published March 6 in JAMA.
This was determined in a nested, matched case-control study of 193 infection cases and 751 controls, in whom estimated mean cumulative vaccine antigen exposure was 240.6 for cases of non–vaccine-targeted infections, and 242.9 for controls, reported Jason M. Glanz, PhD, of Kaiser Permanente Colorado, Denver, and his coauthors. The between-group difference was –2.3 (P = .55), a nonsignificant difference.
Using data from the Centers for Disease Control and Prevention-funded Vaccine Safety Datalink (VSD), the investigators identified children born between Jan. 1, 2003, and Sep. 31, 2013. Exclusion criteria were not having at least two well-child visits before the first birthday, medical contraindications to vaccination, or receiving vaccines not recommended by the Advisory Committee on Immunization Practices. Eligible children were followed through age 47 months or until disenrollment from their health care organization, the authors said.
ICD-9 and ICD-10 codes were used to identify non–vaccine-targeted infections, including upper and lower respiratory infections, gastrointestinal infections, and other viral and bacterial infections from ages 24to 47 months. A medical record review was performed to confirm case status. Cases were included only if it was confirmed that the infection occurred, that it was an incident outcome, that the outcome was the primary reason for the medical visit, that the outcome occurred in the inpatient or emergency department setting, and that there was no evidence that the child was diagnosed as having a vaccine preventable disease (VPD) on the same day as the infection. Controls did not have a VPD or record of a non–vaccine-targeted infection prior to the index date, Dr. Glanz and his colleagues said.
Antigen exposure was measured as the number of immunogenic proteins and polysaccharides in each vaccine, and was estimated from birth through age 23 months in both groups. Cumulative antigen exposure was estimated by adding the number of antigens in each non–vaccine-targeted infection and controls.
Estimated mean cumulative vaccine antigen exposure was 240.6 for cases of non–vaccine-targeted infections, and 242.9 for controls, the authors reported. The matched odds ratio (mOR) for estimated cumulative antigen exposure through age 23 months was not significant in children with infections, compared with controls (mOR = 0.94; 95% confidence interval, 0.84-1.07). The estimated maximum single-day antigen exposure was not significantly associated with non–vaccine-targeted infection (mOR = 1.07; 95% CI, 0.81-1.41).
The findings of this study “did not reveal any beneficial or detrimental associations with estimated cumulative vaccine antigen exposure in young children with non–vaccine-targeted infections in ED and inpatient settings,” wrote Dr. Glanz and coauthors. In addition, the study “did not find evidence that multiple vaccine exposure was associated with the risk for non-targeted infectious diseases.”
The CDC funded the study. The authors reported receiving contracts, grants, and other funding from the CDC.
SOURCE: Glanz JM et al. JAMA. 2018;319(9):906-13.
There was no significant difference in vaccine antigen exposure through the first 23 months of life between children with non–vaccine-targeted infections and controls between 24 and 47 months of age, according to results published March 6 in JAMA.
This was determined in a nested, matched case-control study of 193 infection cases and 751 controls, in whom estimated mean cumulative vaccine antigen exposure was 240.6 for cases of non–vaccine-targeted infections, and 242.9 for controls, reported Jason M. Glanz, PhD, of Kaiser Permanente Colorado, Denver, and his coauthors. The between-group difference was –2.3 (P = .55), a nonsignificant difference.
Using data from the Centers for Disease Control and Prevention-funded Vaccine Safety Datalink (VSD), the investigators identified children born between Jan. 1, 2003, and Sep. 31, 2013. Exclusion criteria were not having at least two well-child visits before the first birthday, medical contraindications to vaccination, or receiving vaccines not recommended by the Advisory Committee on Immunization Practices. Eligible children were followed through age 47 months or until disenrollment from their health care organization, the authors said.
ICD-9 and ICD-10 codes were used to identify non–vaccine-targeted infections, including upper and lower respiratory infections, gastrointestinal infections, and other viral and bacterial infections from ages 24to 47 months. A medical record review was performed to confirm case status. Cases were included only if it was confirmed that the infection occurred, that it was an incident outcome, that the outcome was the primary reason for the medical visit, that the outcome occurred in the inpatient or emergency department setting, and that there was no evidence that the child was diagnosed as having a vaccine preventable disease (VPD) on the same day as the infection. Controls did not have a VPD or record of a non–vaccine-targeted infection prior to the index date, Dr. Glanz and his colleagues said.
Antigen exposure was measured as the number of immunogenic proteins and polysaccharides in each vaccine, and was estimated from birth through age 23 months in both groups. Cumulative antigen exposure was estimated by adding the number of antigens in each non–vaccine-targeted infection and controls.
Estimated mean cumulative vaccine antigen exposure was 240.6 for cases of non–vaccine-targeted infections, and 242.9 for controls, the authors reported. The matched odds ratio (mOR) for estimated cumulative antigen exposure through age 23 months was not significant in children with infections, compared with controls (mOR = 0.94; 95% confidence interval, 0.84-1.07). The estimated maximum single-day antigen exposure was not significantly associated with non–vaccine-targeted infection (mOR = 1.07; 95% CI, 0.81-1.41).
The findings of this study “did not reveal any beneficial or detrimental associations with estimated cumulative vaccine antigen exposure in young children with non–vaccine-targeted infections in ED and inpatient settings,” wrote Dr. Glanz and coauthors. In addition, the study “did not find evidence that multiple vaccine exposure was associated with the risk for non-targeted infectious diseases.”
The CDC funded the study. The authors reported receiving contracts, grants, and other funding from the CDC.
SOURCE: Glanz JM et al. JAMA. 2018;319(9):906-13.
FROM JAMA
Key clinical point: No significant difference was found in vaccine antigen exposure between controls and children with infectious diseases not targeted by vaccines.
Major finding: Estimated mean cumulative vaccine antigen exposure was 240.6 for cases and 242.9 for controls.
Study details: A matched case-control study of 944 patients enrolled in six integrated health care organizations as part of the Vaccine Safety Datalink (VSD).
Disclosures: The Centers for Disease Control and Prevention funded the study. The authors reported receiving contracts, grants, and other funding from the CDC.
Source: Glanz JM et al. JAMA. 2018;319(9):906-13.
2 new influenza strains recommended for next season
SILVER SPRING, MD. – In an effort to better match the vaccine to the virus, federal advisors have recommended two new strains be swapped into the 2018-2019 quadrivalent influenza vaccine.
Singapore A(H3N2) and the B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage) are recommended be added to A/Michigan/45/2015 (H1N1)pdm09-like virus and B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage) for the upcoming season, according to a near-unanimous vote at a meeting of the Food and Drug Administration Vaccines and Related Biological Products Advisory Committee.
Trivalent vaccines should include the same strains, with the exception of B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage), the committee recommended.
The panel voted separately on the strains, and all votes were unanimous, except for the vote on the B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage) in the trivalent vaccine, which was supported with 11 positive votes with 1 abstention.
The advisory committee’s recommendation is identical to the recommendations recently made by the World Health Organization for next season’s influenza vaccines in the Northern Hemisphere. The WHO recommended that trivalent vaccines contain A/Michigan/45/2015 (H1N1)pdm09-like virus, A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus, and B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage). WHO also recommended that quadrivalent vaccines contain all of the above strains and B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage) as the second influenza B strain.
Most of the influenza activity in the United States this season is due to influenza A (H3N2) viruses (67%), according to Lisa Grohskopf, MD, associate chief for policy & liaison in the Influenza Division at the Centers for Disease Control and Prevention. Fortunately, the majority of circulating strains are similar to those contained in the 2017-2018 vaccine. Only strains with B/Victoria lineage displayed antigenic drift, but represented less than 1% of all circulating viruses.
Hospitalization rates for laboratory-confirmed influenza this season have been markedly higher among people aged 65 years and older, compared with younger age groups, and have increased since last season. As of Feb. 17, the preliminary estimate of hospitalizations in this age group was 322.7 cases per 100,000 people, compared with about 290.5 per 100,000 during the 2016-2017 season. There have been 97 pediatric deaths associated with influenza, compared with 110 reported during the 2016-2017 season, 93 during 2015-2016, and 148 during 2014-2015.
These data are not final because the flu season is still ongoing, but a full analysis will be provided at the end of the season, Dr. Grohskopf pointed out.With H3N2 strains of influenza A predominating, questions on the effectiveness of the newly recommended Singapore A(H3N2) were raised by the committee. Jacqueline Katz, PhD, director of the WHO Collaborating Center for Surveillance, Epidemiology, and Control of Influenza, reassured the committee.
“Yes, in fact, it does cover them very well. The majority of the viruses that we’ve tested at the CDC were that emerging 3C2a2 [clade of H3N2] group, and the Singapore virus covered those very well. In general, that’s why we went with Singapore,” she said.
Dr. Katz added that one of the reasons Singapore is so effective is because of its position in the lineage of these flu strains. “It’s at the base of the [phylogenetic] tree; it’s not on the tip of the tree where things are changing, so it’s a more conservative selection.”
The CDC estimate of current vaccine effectiveness (VE) against influenza A (H3N2) viruses is 25%, as of Feb. 3. Effectiveness is even higher for all influenza viruses, with an estimated VE of 36%, indicating that the flu vaccine reduced a person’s risk of having to seek medical care at a doctor’s office for flu illness by 36% (MMWR. 2018;67:180-5).
While the FDA usually follows the recommendations of its panel members, it is not obligated to do so. None of the committee members disclosed relevant financial conflicts of interest.
SILVER SPRING, MD. – In an effort to better match the vaccine to the virus, federal advisors have recommended two new strains be swapped into the 2018-2019 quadrivalent influenza vaccine.
Singapore A(H3N2) and the B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage) are recommended be added to A/Michigan/45/2015 (H1N1)pdm09-like virus and B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage) for the upcoming season, according to a near-unanimous vote at a meeting of the Food and Drug Administration Vaccines and Related Biological Products Advisory Committee.
Trivalent vaccines should include the same strains, with the exception of B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage), the committee recommended.
The panel voted separately on the strains, and all votes were unanimous, except for the vote on the B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage) in the trivalent vaccine, which was supported with 11 positive votes with 1 abstention.
The advisory committee’s recommendation is identical to the recommendations recently made by the World Health Organization for next season’s influenza vaccines in the Northern Hemisphere. The WHO recommended that trivalent vaccines contain A/Michigan/45/2015 (H1N1)pdm09-like virus, A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus, and B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage). WHO also recommended that quadrivalent vaccines contain all of the above strains and B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage) as the second influenza B strain.
Most of the influenza activity in the United States this season is due to influenza A (H3N2) viruses (67%), according to Lisa Grohskopf, MD, associate chief for policy & liaison in the Influenza Division at the Centers for Disease Control and Prevention. Fortunately, the majority of circulating strains are similar to those contained in the 2017-2018 vaccine. Only strains with B/Victoria lineage displayed antigenic drift, but represented less than 1% of all circulating viruses.
Hospitalization rates for laboratory-confirmed influenza this season have been markedly higher among people aged 65 years and older, compared with younger age groups, and have increased since last season. As of Feb. 17, the preliminary estimate of hospitalizations in this age group was 322.7 cases per 100,000 people, compared with about 290.5 per 100,000 during the 2016-2017 season. There have been 97 pediatric deaths associated with influenza, compared with 110 reported during the 2016-2017 season, 93 during 2015-2016, and 148 during 2014-2015.
These data are not final because the flu season is still ongoing, but a full analysis will be provided at the end of the season, Dr. Grohskopf pointed out.With H3N2 strains of influenza A predominating, questions on the effectiveness of the newly recommended Singapore A(H3N2) were raised by the committee. Jacqueline Katz, PhD, director of the WHO Collaborating Center for Surveillance, Epidemiology, and Control of Influenza, reassured the committee.
“Yes, in fact, it does cover them very well. The majority of the viruses that we’ve tested at the CDC were that emerging 3C2a2 [clade of H3N2] group, and the Singapore virus covered those very well. In general, that’s why we went with Singapore,” she said.
Dr. Katz added that one of the reasons Singapore is so effective is because of its position in the lineage of these flu strains. “It’s at the base of the [phylogenetic] tree; it’s not on the tip of the tree where things are changing, so it’s a more conservative selection.”
The CDC estimate of current vaccine effectiveness (VE) against influenza A (H3N2) viruses is 25%, as of Feb. 3. Effectiveness is even higher for all influenza viruses, with an estimated VE of 36%, indicating that the flu vaccine reduced a person’s risk of having to seek medical care at a doctor’s office for flu illness by 36% (MMWR. 2018;67:180-5).
While the FDA usually follows the recommendations of its panel members, it is not obligated to do so. None of the committee members disclosed relevant financial conflicts of interest.
SILVER SPRING, MD. – In an effort to better match the vaccine to the virus, federal advisors have recommended two new strains be swapped into the 2018-2019 quadrivalent influenza vaccine.
Singapore A(H3N2) and the B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage) are recommended be added to A/Michigan/45/2015 (H1N1)pdm09-like virus and B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage) for the upcoming season, according to a near-unanimous vote at a meeting of the Food and Drug Administration Vaccines and Related Biological Products Advisory Committee.
Trivalent vaccines should include the same strains, with the exception of B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage), the committee recommended.
The panel voted separately on the strains, and all votes were unanimous, except for the vote on the B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage) in the trivalent vaccine, which was supported with 11 positive votes with 1 abstention.
The advisory committee’s recommendation is identical to the recommendations recently made by the World Health Organization for next season’s influenza vaccines in the Northern Hemisphere. The WHO recommended that trivalent vaccines contain A/Michigan/45/2015 (H1N1)pdm09-like virus, A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus, and B/Colorado/06/2017-like virus (B/Victoria/2/87 lineage). WHO also recommended that quadrivalent vaccines contain all of the above strains and B/Phuket/3073/2013-like virus (B/Yamagata/16/88 lineage) as the second influenza B strain.
Most of the influenza activity in the United States this season is due to influenza A (H3N2) viruses (67%), according to Lisa Grohskopf, MD, associate chief for policy & liaison in the Influenza Division at the Centers for Disease Control and Prevention. Fortunately, the majority of circulating strains are similar to those contained in the 2017-2018 vaccine. Only strains with B/Victoria lineage displayed antigenic drift, but represented less than 1% of all circulating viruses.
Hospitalization rates for laboratory-confirmed influenza this season have been markedly higher among people aged 65 years and older, compared with younger age groups, and have increased since last season. As of Feb. 17, the preliminary estimate of hospitalizations in this age group was 322.7 cases per 100,000 people, compared with about 290.5 per 100,000 during the 2016-2017 season. There have been 97 pediatric deaths associated with influenza, compared with 110 reported during the 2016-2017 season, 93 during 2015-2016, and 148 during 2014-2015.
These data are not final because the flu season is still ongoing, but a full analysis will be provided at the end of the season, Dr. Grohskopf pointed out.With H3N2 strains of influenza A predominating, questions on the effectiveness of the newly recommended Singapore A(H3N2) were raised by the committee. Jacqueline Katz, PhD, director of the WHO Collaborating Center for Surveillance, Epidemiology, and Control of Influenza, reassured the committee.
“Yes, in fact, it does cover them very well. The majority of the viruses that we’ve tested at the CDC were that emerging 3C2a2 [clade of H3N2] group, and the Singapore virus covered those very well. In general, that’s why we went with Singapore,” she said.
Dr. Katz added that one of the reasons Singapore is so effective is because of its position in the lineage of these flu strains. “It’s at the base of the [phylogenetic] tree; it’s not on the tip of the tree where things are changing, so it’s a more conservative selection.”
The CDC estimate of current vaccine effectiveness (VE) against influenza A (H3N2) viruses is 25%, as of Feb. 3. Effectiveness is even higher for all influenza viruses, with an estimated VE of 36%, indicating that the flu vaccine reduced a person’s risk of having to seek medical care at a doctor’s office for flu illness by 36% (MMWR. 2018;67:180-5).
While the FDA usually follows the recommendations of its panel members, it is not obligated to do so. None of the committee members disclosed relevant financial conflicts of interest.
REPORTING FROM AN FDA ADVISORY COMMITTEE MEETING
ACIP vaccine update
The Advisory Committee on Immunization Practices (ACIP) made relatively few new vaccine recommendations in 2017. One pertained to prevention of hepatitis B virus (HBV) infection in infants born to HBV-infected mothers. Another recommended a new vaccine to prevent shingles. A third advised considering an additional dose of mumps vaccine during an outbreak. This year’s recommendations pertaining to influenza vaccines were covered in a previous Practice Alert.1
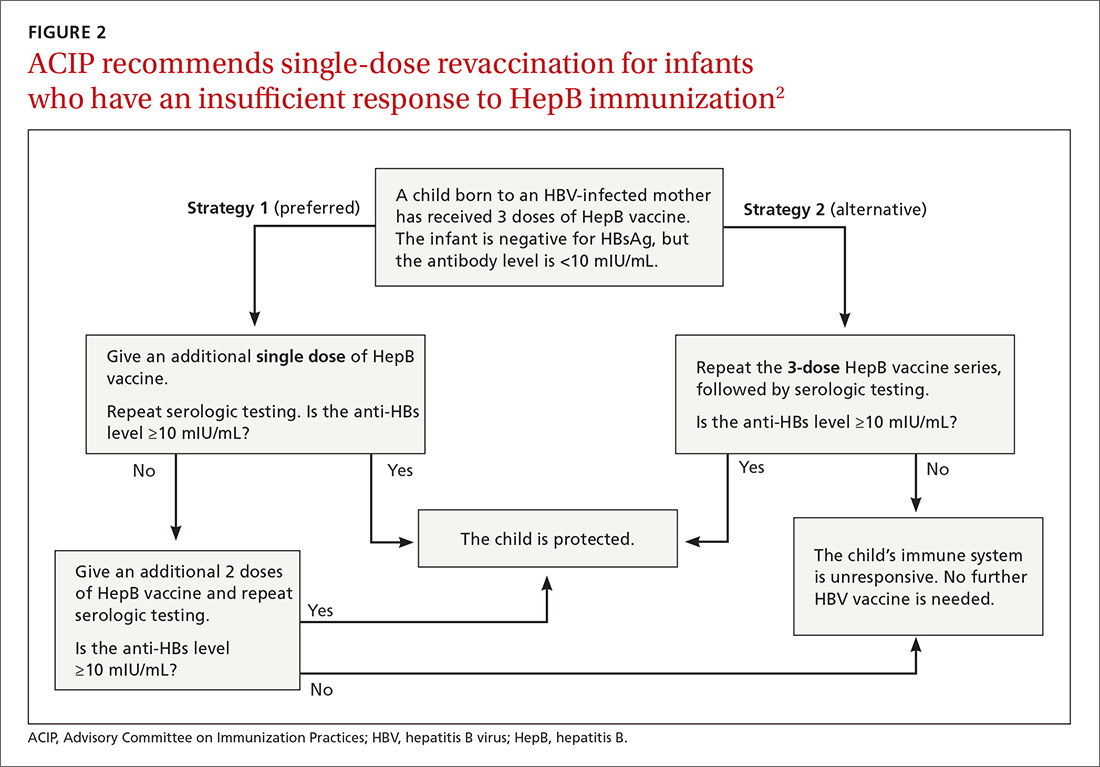
Perinatal HBV prevention: New strategy if revaccination is required
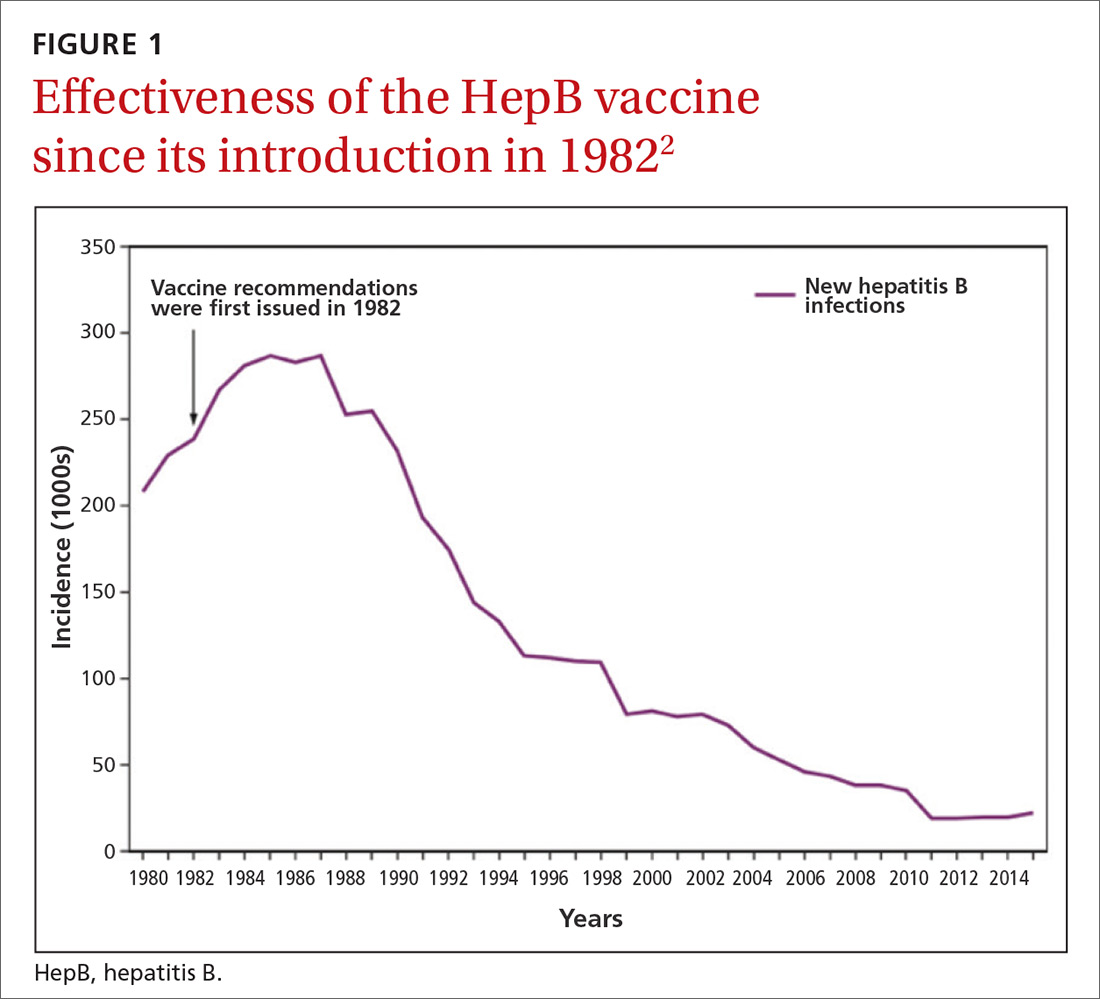
Hepatitis B prevention programs in the United States have decreased the incidence of HBV infections from 9.6 cases per 100,000 population in 1982 (the year the hepatitis B [HepB] vaccine was first available) to 1.1 cases per 100,000 population in 2015 (FIGURE 1).2 One major route of HBV dissemination worldwide is perinatal transmission to infants by HBV-infected mothers. However, this route of infection has been greatly diminished in the United States because of widespread screening of pregnant women and because newborns of mothers with known active HBV infection receive prophylaxis with hepatitis B immune globulin and HBV vaccine.
Each year in the United States an estimated 25,000 infants are born to mothers who are positive for hepatitis B surface antigen (HBsAg).3 Without post-exposure prophylaxis, 85% of these infants would develop HBV infection if the mother is also hepatitis B e antigen (HBeAg) positive; 30% would develop HBV infection if the mother is HBeAg negative.2 Eighty percent to 90% of infected infants develop chronic HBV infection and are at increased risk of chronic liver disease.2 Of all infants receiving the recommended post-exposure prophylaxis, only about 1% develop infection.2
Available HepB vaccines. HepB vaccine consists of HBsAg derived from yeast using recombinant DNA technology, which is then purified by biochemical separation techniques. Three vaccine products are available for newborns and infants in the United States. Two are single-antigen vaccines—Engerix-B (GlaxoSmithKline Biologicals) and Recombivax HB (Merck & Co.)—and both can be used starting at birth. One combination vaccine, Pediarix (GlaxoSmithKline Biologicals) is used for children ages 6 weeks to 6 years. It contains HBsAg as do the other 2 vaccines, as well as diphtheria and tetanus toxoids, acellular pertussis adsorbed, and inactivated poliovirus (DTaP-HepB-IPV).
Until December 31, 2014, a vaccine combining HBsAg and haemophilus-B antigen, Comvax (Merck and Co.), was available for infants 6 weeks or older. Comvax is no longer produced.
Factors affecting the dosing schedule. For infants born to HBsAg-positive mothers, the final dose of the HepB series should be completed at age 6 months with either one of the monovalent HepB vaccines or the DTaP-HepB-IPV vaccine. When the now-discontinued Comvax was used to complete the series, the final dose was administered at 12 to 15 months. The timing of HepB vaccine at birth and at subsequent intervals, and a decision on whether to give hepatitis B immune globulin, depend on the baby’s birth weight, the mother’s HBsAg status, and type of vaccine used.2
Post-vaccination assessment. ACIP recommends that babies born to HBsAg-positive mothers and having received the final dose of the vaccine series be serologically tested for immunity to HBV at age 9 to 12 months; or if the series is delayed, at one to 2 months after the final dose.4 Infants without evidence of active infection (ie, HBsAg negative) and with levels of antibody to HBsAg ≥10 mIU/mL are considered protected and need no further vaccinations.4 Revaccination is advised for those with antibody levels <10 mIU/mL—who account for only about 2% of infants having received the recommended schedule.4
New revaccination strategy. The previous recommendation on revaccination advised a second 3-dose series with repeat serologic testing one to 2 months after the final dose of vaccine. Although this strategy is still acceptable, the new recommendation for infants with antibody levels <10 mIU/mL favors (for cost savings and convenience) administration of a single dose of HepB vaccine with retesting one to 2 months later.2
Several studies presented at the ACIP meeting in February 2017 showed that more than 90% of infants revaccinated with the single dose will develop a protective antibody level.4 Infants whose anti-HBs remain <10 mIU/mL following the single-dose re-vaccination should receive 2 additional doses of HepB vaccine, followed by testing one to 2 months after the last dose4 (FIGURE 22).
(A new HepB vaccine, HEPLISAV-B [Dynavax Technologies Corp]), has been approved for use in adults. More on this in a bit.)
Herpes zoster vaccine: Data guidance on product selection
In 2017, the US Food and Drug Administration (FDA) approved a new vaccine against shingles, an adjuvanted herpes zoster subunit (HZ/su) vaccine, Shingrix (GlaxoSmithKline Biologicals). It is now an alternative to the live attenuated virus (ZVL) vaccine, Zostavax (Merck & Co.), licensed in 2006. ZVL is approved for use in adults ages 50 to 59 years, but ACIP recommends it only for adults 60 and older.5 It is given as a single dose, while HZ/su is given as a 2-dose series at 0 and at 2 to 6 months. By ACIP’s analysis, HZ/su is more effective than ZVL. In a comparison model looking at health outcomes over a lifetime among one million patients 60 to 69 years of age, HZ/su would prevent 53,000 more cases of shingles and 4000 more cases of postherpetic neuralgia than would ZVL.6
Additional mumps vaccine is warranted in an outbreak
While use of mumps-containing vaccine in the United States has led to markedly lower disease incidence rates than existed in the pre-vaccine era, in recent years there have been large mumps outbreaks among young adults at universities and other close-knit communities. These groups have had relatively high rates of completion of 2 doses of measles, mumps, and rubella (MMR) vaccine, and the cause of the outbreaks is not fully understood. Potential contributors include waning immunity following vaccination and antigenic differences between the virus strains circulating and those in the vaccine.
ACIP considered whether a third dose of MMR should be recommended to those fully vaccinated if they are at high risk due to an outbreak. Although the evidence to support the effectiveness of a third dose was scant and of very low quality, the evidence for vaccine safety was reassuring and ACIP voted to recommend the use of a third dose in outbreaks.9
One new vaccine and others on the horizon
ACIP is evaluating a new HepB vaccine, HEPLISAV-B, which was approved by the FDA in November 2017 for use in adults.10,11 The vaccine contains the same antigen as other available HepB vaccines but a different adjuvant. It is administered in 2 doses one month apart, which is preferable to the current 3-dose, 6-month schedule. There is, however, some indication that it causes increased rates of cardiovascular complications.10 ACIP is evaluating the relative effectiveness and safety of HEPLISAV-B and other HepB vaccines, and recommendations are expected this spring.
Other vaccines in various stages of development, but not ready for ACIP evaluation, include those against Zika virus, norovirus, respiratory syncytial virus, and dengue virus.
ACIP is also retrospectively assessing whether adding the 13 valent pneumococcal conjugate vaccine to the schedule for those over the age of 65 has led to improved pneumonia outcomes. It will reconsider the previous recommendation based on the results of its assessment.
1. Campos-Outcalt D. Latest recommendations for the 2017-2018 flu season. J Fam Pract. 2017;66:570-572.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67:1-31. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6701a1.htm. Accessed January 19, 2018.
3. CDC. Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2012;61:768-771. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6138a4.htm. Accessed February 14, 2018.
4. Nelson N. Revaccination for infants born to hepatitis B virus (HBV)-infected mothers. Presented at: Advisory Committee on Immunization Practices. February 22, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/hepatitis-02-background-nelson.pdf. Accessed January 19, 2017.
5. Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6333a3.htm?s_cid=mm6333a3_w. Accessed January 23, 2018.
6. Dooling KL. Considerations for the use of herpes zoster vaccines. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-04-dooling.pdf. Accessed January 19, 2018.
7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
8. Campos-Outcalt D. The new shingles vaccine: what PCPs need to know. J Fam Pract. 2017;66:audio. Available at: https://www.mdedge.com/jfponline/article/153168/vaccines/new-shingles-vaccine-what-pcps-need-know. Accessed January 19, 2018.
9. Marlow M. Grading of recommendations assessment, development and evaluation (GRADE): third dose of MMR vaccine. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-03-marlow-508.pdf. Accessed January 19, 2018.
10. HEPLISAV-B [package insert]. Berkeley, CA: Dynavax Technology Corporation; 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf. Accessed January 23, 2018.
11. Janssen R. HEPLISAV-B. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-02-janssen.pdf. Accessed January 19, 2018.
The Advisory Committee on Immunization Practices (ACIP) made relatively few new vaccine recommendations in 2017. One pertained to prevention of hepatitis B virus (HBV) infection in infants born to HBV-infected mothers. Another recommended a new vaccine to prevent shingles. A third advised considering an additional dose of mumps vaccine during an outbreak. This year’s recommendations pertaining to influenza vaccines were covered in a previous Practice Alert.1
Perinatal HBV prevention: New strategy if revaccination is required
Hepatitis B prevention programs in the United States have decreased the incidence of HBV infections from 9.6 cases per 100,000 population in 1982 (the year the hepatitis B [HepB] vaccine was first available) to 1.1 cases per 100,000 population in 2015 (FIGURE 1).2 One major route of HBV dissemination worldwide is perinatal transmission to infants by HBV-infected mothers. However, this route of infection has been greatly diminished in the United States because of widespread screening of pregnant women and because newborns of mothers with known active HBV infection receive prophylaxis with hepatitis B immune globulin and HBV vaccine.
Each year in the United States an estimated 25,000 infants are born to mothers who are positive for hepatitis B surface antigen (HBsAg).3 Without post-exposure prophylaxis, 85% of these infants would develop HBV infection if the mother is also hepatitis B e antigen (HBeAg) positive; 30% would develop HBV infection if the mother is HBeAg negative.2 Eighty percent to 90% of infected infants develop chronic HBV infection and are at increased risk of chronic liver disease.2 Of all infants receiving the recommended post-exposure prophylaxis, only about 1% develop infection.2
Available HepB vaccines. HepB vaccine consists of HBsAg derived from yeast using recombinant DNA technology, which is then purified by biochemical separation techniques. Three vaccine products are available for newborns and infants in the United States. Two are single-antigen vaccines—Engerix-B (GlaxoSmithKline Biologicals) and Recombivax HB (Merck & Co.)—and both can be used starting at birth. One combination vaccine, Pediarix (GlaxoSmithKline Biologicals) is used for children ages 6 weeks to 6 years. It contains HBsAg as do the other 2 vaccines, as well as diphtheria and tetanus toxoids, acellular pertussis adsorbed, and inactivated poliovirus (DTaP-HepB-IPV).
Until December 31, 2014, a vaccine combining HBsAg and haemophilus-B antigen, Comvax (Merck and Co.), was available for infants 6 weeks or older. Comvax is no longer produced.
Factors affecting the dosing schedule. For infants born to HBsAg-positive mothers, the final dose of the HepB series should be completed at age 6 months with either one of the monovalent HepB vaccines or the DTaP-HepB-IPV vaccine. When the now-discontinued Comvax was used to complete the series, the final dose was administered at 12 to 15 months. The timing of HepB vaccine at birth and at subsequent intervals, and a decision on whether to give hepatitis B immune globulin, depend on the baby’s birth weight, the mother’s HBsAg status, and type of vaccine used.2
Post-vaccination assessment. ACIP recommends that babies born to HBsAg-positive mothers and having received the final dose of the vaccine series be serologically tested for immunity to HBV at age 9 to 12 months; or if the series is delayed, at one to 2 months after the final dose.4 Infants without evidence of active infection (ie, HBsAg negative) and with levels of antibody to HBsAg ≥10 mIU/mL are considered protected and need no further vaccinations.4 Revaccination is advised for those with antibody levels <10 mIU/mL—who account for only about 2% of infants having received the recommended schedule.4
New revaccination strategy. The previous recommendation on revaccination advised a second 3-dose series with repeat serologic testing one to 2 months after the final dose of vaccine. Although this strategy is still acceptable, the new recommendation for infants with antibody levels <10 mIU/mL favors (for cost savings and convenience) administration of a single dose of HepB vaccine with retesting one to 2 months later.2
Several studies presented at the ACIP meeting in February 2017 showed that more than 90% of infants revaccinated with the single dose will develop a protective antibody level.4 Infants whose anti-HBs remain <10 mIU/mL following the single-dose re-vaccination should receive 2 additional doses of HepB vaccine, followed by testing one to 2 months after the last dose4 (FIGURE 22).
(A new HepB vaccine, HEPLISAV-B [Dynavax Technologies Corp]), has been approved for use in adults. More on this in a bit.)
Herpes zoster vaccine: Data guidance on product selection
In 2017, the US Food and Drug Administration (FDA) approved a new vaccine against shingles, an adjuvanted herpes zoster subunit (HZ/su) vaccine, Shingrix (GlaxoSmithKline Biologicals). It is now an alternative to the live attenuated virus (ZVL) vaccine, Zostavax (Merck & Co.), licensed in 2006. ZVL is approved for use in adults ages 50 to 59 years, but ACIP recommends it only for adults 60 and older.5 It is given as a single dose, while HZ/su is given as a 2-dose series at 0 and at 2 to 6 months. By ACIP’s analysis, HZ/su is more effective than ZVL. In a comparison model looking at health outcomes over a lifetime among one million patients 60 to 69 years of age, HZ/su would prevent 53,000 more cases of shingles and 4000 more cases of postherpetic neuralgia than would ZVL.6
Additional mumps vaccine is warranted in an outbreak
While use of mumps-containing vaccine in the United States has led to markedly lower disease incidence rates than existed in the pre-vaccine era, in recent years there have been large mumps outbreaks among young adults at universities and other close-knit communities. These groups have had relatively high rates of completion of 2 doses of measles, mumps, and rubella (MMR) vaccine, and the cause of the outbreaks is not fully understood. Potential contributors include waning immunity following vaccination and antigenic differences between the virus strains circulating and those in the vaccine.
ACIP considered whether a third dose of MMR should be recommended to those fully vaccinated if they are at high risk due to an outbreak. Although the evidence to support the effectiveness of a third dose was scant and of very low quality, the evidence for vaccine safety was reassuring and ACIP voted to recommend the use of a third dose in outbreaks.9
One new vaccine and others on the horizon
ACIP is evaluating a new HepB vaccine, HEPLISAV-B, which was approved by the FDA in November 2017 for use in adults.10,11 The vaccine contains the same antigen as other available HepB vaccines but a different adjuvant. It is administered in 2 doses one month apart, which is preferable to the current 3-dose, 6-month schedule. There is, however, some indication that it causes increased rates of cardiovascular complications.10 ACIP is evaluating the relative effectiveness and safety of HEPLISAV-B and other HepB vaccines, and recommendations are expected this spring.
Other vaccines in various stages of development, but not ready for ACIP evaluation, include those against Zika virus, norovirus, respiratory syncytial virus, and dengue virus.
ACIP is also retrospectively assessing whether adding the 13 valent pneumococcal conjugate vaccine to the schedule for those over the age of 65 has led to improved pneumonia outcomes. It will reconsider the previous recommendation based on the results of its assessment.
The Advisory Committee on Immunization Practices (ACIP) made relatively few new vaccine recommendations in 2017. One pertained to prevention of hepatitis B virus (HBV) infection in infants born to HBV-infected mothers. Another recommended a new vaccine to prevent shingles. A third advised considering an additional dose of mumps vaccine during an outbreak. This year’s recommendations pertaining to influenza vaccines were covered in a previous Practice Alert.1
Perinatal HBV prevention: New strategy if revaccination is required
Hepatitis B prevention programs in the United States have decreased the incidence of HBV infections from 9.6 cases per 100,000 population in 1982 (the year the hepatitis B [HepB] vaccine was first available) to 1.1 cases per 100,000 population in 2015 (FIGURE 1).2 One major route of HBV dissemination worldwide is perinatal transmission to infants by HBV-infected mothers. However, this route of infection has been greatly diminished in the United States because of widespread screening of pregnant women and because newborns of mothers with known active HBV infection receive prophylaxis with hepatitis B immune globulin and HBV vaccine.
Each year in the United States an estimated 25,000 infants are born to mothers who are positive for hepatitis B surface antigen (HBsAg).3 Without post-exposure prophylaxis, 85% of these infants would develop HBV infection if the mother is also hepatitis B e antigen (HBeAg) positive; 30% would develop HBV infection if the mother is HBeAg negative.2 Eighty percent to 90% of infected infants develop chronic HBV infection and are at increased risk of chronic liver disease.2 Of all infants receiving the recommended post-exposure prophylaxis, only about 1% develop infection.2
Available HepB vaccines. HepB vaccine consists of HBsAg derived from yeast using recombinant DNA technology, which is then purified by biochemical separation techniques. Three vaccine products are available for newborns and infants in the United States. Two are single-antigen vaccines—Engerix-B (GlaxoSmithKline Biologicals) and Recombivax HB (Merck & Co.)—and both can be used starting at birth. One combination vaccine, Pediarix (GlaxoSmithKline Biologicals) is used for children ages 6 weeks to 6 years. It contains HBsAg as do the other 2 vaccines, as well as diphtheria and tetanus toxoids, acellular pertussis adsorbed, and inactivated poliovirus (DTaP-HepB-IPV).
Until December 31, 2014, a vaccine combining HBsAg and haemophilus-B antigen, Comvax (Merck and Co.), was available for infants 6 weeks or older. Comvax is no longer produced.
Factors affecting the dosing schedule. For infants born to HBsAg-positive mothers, the final dose of the HepB series should be completed at age 6 months with either one of the monovalent HepB vaccines or the DTaP-HepB-IPV vaccine. When the now-discontinued Comvax was used to complete the series, the final dose was administered at 12 to 15 months. The timing of HepB vaccine at birth and at subsequent intervals, and a decision on whether to give hepatitis B immune globulin, depend on the baby’s birth weight, the mother’s HBsAg status, and type of vaccine used.2
Post-vaccination assessment. ACIP recommends that babies born to HBsAg-positive mothers and having received the final dose of the vaccine series be serologically tested for immunity to HBV at age 9 to 12 months; or if the series is delayed, at one to 2 months after the final dose.4 Infants without evidence of active infection (ie, HBsAg negative) and with levels of antibody to HBsAg ≥10 mIU/mL are considered protected and need no further vaccinations.4 Revaccination is advised for those with antibody levels <10 mIU/mL—who account for only about 2% of infants having received the recommended schedule.4
New revaccination strategy. The previous recommendation on revaccination advised a second 3-dose series with repeat serologic testing one to 2 months after the final dose of vaccine. Although this strategy is still acceptable, the new recommendation for infants with antibody levels <10 mIU/mL favors (for cost savings and convenience) administration of a single dose of HepB vaccine with retesting one to 2 months later.2
Several studies presented at the ACIP meeting in February 2017 showed that more than 90% of infants revaccinated with the single dose will develop a protective antibody level.4 Infants whose anti-HBs remain <10 mIU/mL following the single-dose re-vaccination should receive 2 additional doses of HepB vaccine, followed by testing one to 2 months after the last dose4 (FIGURE 22).
(A new HepB vaccine, HEPLISAV-B [Dynavax Technologies Corp]), has been approved for use in adults. More on this in a bit.)
Herpes zoster vaccine: Data guidance on product selection
In 2017, the US Food and Drug Administration (FDA) approved a new vaccine against shingles, an adjuvanted herpes zoster subunit (HZ/su) vaccine, Shingrix (GlaxoSmithKline Biologicals). It is now an alternative to the live attenuated virus (ZVL) vaccine, Zostavax (Merck & Co.), licensed in 2006. ZVL is approved for use in adults ages 50 to 59 years, but ACIP recommends it only for adults 60 and older.5 It is given as a single dose, while HZ/su is given as a 2-dose series at 0 and at 2 to 6 months. By ACIP’s analysis, HZ/su is more effective than ZVL. In a comparison model looking at health outcomes over a lifetime among one million patients 60 to 69 years of age, HZ/su would prevent 53,000 more cases of shingles and 4000 more cases of postherpetic neuralgia than would ZVL.6
Additional mumps vaccine is warranted in an outbreak
While use of mumps-containing vaccine in the United States has led to markedly lower disease incidence rates than existed in the pre-vaccine era, in recent years there have been large mumps outbreaks among young adults at universities and other close-knit communities. These groups have had relatively high rates of completion of 2 doses of measles, mumps, and rubella (MMR) vaccine, and the cause of the outbreaks is not fully understood. Potential contributors include waning immunity following vaccination and antigenic differences between the virus strains circulating and those in the vaccine.
ACIP considered whether a third dose of MMR should be recommended to those fully vaccinated if they are at high risk due to an outbreak. Although the evidence to support the effectiveness of a third dose was scant and of very low quality, the evidence for vaccine safety was reassuring and ACIP voted to recommend the use of a third dose in outbreaks.9
One new vaccine and others on the horizon
ACIP is evaluating a new HepB vaccine, HEPLISAV-B, which was approved by the FDA in November 2017 for use in adults.10,11 The vaccine contains the same antigen as other available HepB vaccines but a different adjuvant. It is administered in 2 doses one month apart, which is preferable to the current 3-dose, 6-month schedule. There is, however, some indication that it causes increased rates of cardiovascular complications.10 ACIP is evaluating the relative effectiveness and safety of HEPLISAV-B and other HepB vaccines, and recommendations are expected this spring.
Other vaccines in various stages of development, but not ready for ACIP evaluation, include those against Zika virus, norovirus, respiratory syncytial virus, and dengue virus.
ACIP is also retrospectively assessing whether adding the 13 valent pneumococcal conjugate vaccine to the schedule for those over the age of 65 has led to improved pneumonia outcomes. It will reconsider the previous recommendation based on the results of its assessment.
1. Campos-Outcalt D. Latest recommendations for the 2017-2018 flu season. J Fam Pract. 2017;66:570-572.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67:1-31. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6701a1.htm. Accessed January 19, 2018.
3. CDC. Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2012;61:768-771. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6138a4.htm. Accessed February 14, 2018.
4. Nelson N. Revaccination for infants born to hepatitis B virus (HBV)-infected mothers. Presented at: Advisory Committee on Immunization Practices. February 22, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/hepatitis-02-background-nelson.pdf. Accessed January 19, 2017.
5. Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6333a3.htm?s_cid=mm6333a3_w. Accessed January 23, 2018.
6. Dooling KL. Considerations for the use of herpes zoster vaccines. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-04-dooling.pdf. Accessed January 19, 2018.
7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
8. Campos-Outcalt D. The new shingles vaccine: what PCPs need to know. J Fam Pract. 2017;66:audio. Available at: https://www.mdedge.com/jfponline/article/153168/vaccines/new-shingles-vaccine-what-pcps-need-know. Accessed January 19, 2018.
9. Marlow M. Grading of recommendations assessment, development and evaluation (GRADE): third dose of MMR vaccine. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-03-marlow-508.pdf. Accessed January 19, 2018.
10. HEPLISAV-B [package insert]. Berkeley, CA: Dynavax Technology Corporation; 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf. Accessed January 23, 2018.
11. Janssen R. HEPLISAV-B. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-02-janssen.pdf. Accessed January 19, 2018.
1. Campos-Outcalt D. Latest recommendations for the 2017-2018 flu season. J Fam Pract. 2017;66:570-572.
2. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018;67:1-31. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6701a1.htm. Accessed January 19, 2018.
3. CDC. Postvaccination serologic testing results for infants aged ≤24 months exposed to hepatitis B virus at birth: United States, 2008-2011. MMWR Morb Mortal Wkly Rep. 2012;61:768-771. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6138a4.htm. Accessed February 14, 2018.
4. Nelson N. Revaccination for infants born to hepatitis B virus (HBV)-infected mothers. Presented at: Advisory Committee on Immunization Practices. February 22, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-02/hepatitis-02-background-nelson.pdf. Accessed January 19, 2017.
5. Hales CM, Harpaz R, Ortega-Sanchez I, et al. Update on recommendations for use of herpes zoster vaccine. MMWR Morb Mortal Wkly Rep. 2014;63:729-731. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6333a3.htm?s_cid=mm6333a3_w. Accessed January 23, 2018.
6. Dooling KL. Considerations for the use of herpes zoster vaccines. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/zoster-04-dooling.pdf. Accessed January 19, 2018.
7. Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
8. Campos-Outcalt D. The new shingles vaccine: what PCPs need to know. J Fam Pract. 2017;66:audio. Available at: https://www.mdedge.com/jfponline/article/153168/vaccines/new-shingles-vaccine-what-pcps-need-know. Accessed January 19, 2018.
9. Marlow M. Grading of recommendations assessment, development and evaluation (GRADE): third dose of MMR vaccine. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/mumps-03-marlow-508.pdf. Accessed January 19, 2018.
10. HEPLISAV-B [package insert]. Berkeley, CA: Dynavax Technology Corporation; 2017. Available at: https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM584762.pdf. Accessed January 23, 2018.
11. Janssen R. HEPLISAV-B. Presented at: Advisory Committee on Immunization Practices. October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-02-janssen.pdf. Accessed January 19, 2018.
NIAID proposes 3-pronged plan for universal influenza vaccine
Three specific research areas were proposed by the National Institute of Allergy and Infectious Diseases (NIAID) in its development plan for a universal influenza vaccine, as detailed in a report published online in the Journal of Infectious Diseases.
Anthony S. Fauci, MD, director of the NIAID, spoke with Frontline Medical News in an interview regarding the plan and noted that he and his colleagues felt that it was important to accelerate the effort for a universal vaccine.
“The strategic plan also includes a description of research resources essential to advancing these three research areas that [the] NIAID will develop, support, and provide for the scientific community,” wrote Dr. Erbelding and her coauthors.
The development plan comes 8 months after scientists from academia, industry, and government convened for the NIAID Pathway to a Universal Influenza Vaccine workshop to address knowledge gaps and strategy, which was summarized last year in the journal Immunity (2017;47: 599-603). The scientists at the workshop developed criteria that would define a universal vaccine and decided that a universal vaccine for influenza should do three things: be at least 75% effective against symptomatic influenza infection; protect against group I and II influenza A viruses; and have durable protections that lasts at least 1 year and preferably through multiple seasons.
“Clearly, a vaccine that would cover most or all seasonal strains of influenza and also provide protection during a pandemic is highly desirable,” wrote Catharine I. Paules, MD, of the University of Maryland, Baltimore, and her coauthors in the workshop summary.
Dr. Fauci told Frontline Medical News how “experts from all over the country addressed their thoughts and concerns with us last year [at the workshop], and now we have a development plan,” he said. “But the next step will be doing the research and finding more resources. ... The work is yet to be done.”
The development plan was published amid an ongoing historic flu season.
Dr. Fauci noted that this season had particular circumstances that made it worse than normal. “I don’t think that, had we had this plan in place a year ago, it would have had an impact on this flu season,” he said.
The authors reported no relevant financial conflicts and that the National Institutes of Health produced this plan.
SOURCE: Erbelding E et al. J Infect Dis. 2018 Feb 28. doi: 10.1093/infdis/jiy103.
Three specific research areas were proposed by the National Institute of Allergy and Infectious Diseases (NIAID) in its development plan for a universal influenza vaccine, as detailed in a report published online in the Journal of Infectious Diseases.
Anthony S. Fauci, MD, director of the NIAID, spoke with Frontline Medical News in an interview regarding the plan and noted that he and his colleagues felt that it was important to accelerate the effort for a universal vaccine.
“The strategic plan also includes a description of research resources essential to advancing these three research areas that [the] NIAID will develop, support, and provide for the scientific community,” wrote Dr. Erbelding and her coauthors.
The development plan comes 8 months after scientists from academia, industry, and government convened for the NIAID Pathway to a Universal Influenza Vaccine workshop to address knowledge gaps and strategy, which was summarized last year in the journal Immunity (2017;47: 599-603). The scientists at the workshop developed criteria that would define a universal vaccine and decided that a universal vaccine for influenza should do three things: be at least 75% effective against symptomatic influenza infection; protect against group I and II influenza A viruses; and have durable protections that lasts at least 1 year and preferably through multiple seasons.
“Clearly, a vaccine that would cover most or all seasonal strains of influenza and also provide protection during a pandemic is highly desirable,” wrote Catharine I. Paules, MD, of the University of Maryland, Baltimore, and her coauthors in the workshop summary.
Dr. Fauci told Frontline Medical News how “experts from all over the country addressed their thoughts and concerns with us last year [at the workshop], and now we have a development plan,” he said. “But the next step will be doing the research and finding more resources. ... The work is yet to be done.”
The development plan was published amid an ongoing historic flu season.
Dr. Fauci noted that this season had particular circumstances that made it worse than normal. “I don’t think that, had we had this plan in place a year ago, it would have had an impact on this flu season,” he said.
The authors reported no relevant financial conflicts and that the National Institutes of Health produced this plan.
SOURCE: Erbelding E et al. J Infect Dis. 2018 Feb 28. doi: 10.1093/infdis/jiy103.
Three specific research areas were proposed by the National Institute of Allergy and Infectious Diseases (NIAID) in its development plan for a universal influenza vaccine, as detailed in a report published online in the Journal of Infectious Diseases.
Anthony S. Fauci, MD, director of the NIAID, spoke with Frontline Medical News in an interview regarding the plan and noted that he and his colleagues felt that it was important to accelerate the effort for a universal vaccine.
“The strategic plan also includes a description of research resources essential to advancing these three research areas that [the] NIAID will develop, support, and provide for the scientific community,” wrote Dr. Erbelding and her coauthors.
The development plan comes 8 months after scientists from academia, industry, and government convened for the NIAID Pathway to a Universal Influenza Vaccine workshop to address knowledge gaps and strategy, which was summarized last year in the journal Immunity (2017;47: 599-603). The scientists at the workshop developed criteria that would define a universal vaccine and decided that a universal vaccine for influenza should do three things: be at least 75% effective against symptomatic influenza infection; protect against group I and II influenza A viruses; and have durable protections that lasts at least 1 year and preferably through multiple seasons.
“Clearly, a vaccine that would cover most or all seasonal strains of influenza and also provide protection during a pandemic is highly desirable,” wrote Catharine I. Paules, MD, of the University of Maryland, Baltimore, and her coauthors in the workshop summary.
Dr. Fauci told Frontline Medical News how “experts from all over the country addressed their thoughts and concerns with us last year [at the workshop], and now we have a development plan,” he said. “But the next step will be doing the research and finding more resources. ... The work is yet to be done.”
The development plan was published amid an ongoing historic flu season.
Dr. Fauci noted that this season had particular circumstances that made it worse than normal. “I don’t think that, had we had this plan in place a year ago, it would have had an impact on this flu season,” he said.
The authors reported no relevant financial conflicts and that the National Institutes of Health produced this plan.
SOURCE: Erbelding E et al. J Infect Dis. 2018 Feb 28. doi: 10.1093/infdis/jiy103.
FROM THE JOURNAL OF INFECTIOUS DISEASES
Fluarix Quadrivalent effective in very young, simplifies flu shots for all ages
Fluarix Quadrivalent is highly effective against moderate and severe flu strains in children aged 6-35 months, and has the potential to simplify influenza vaccinations for all ages, according the results of a phase 3 clinical trial presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
“Fluarix Quadrivalent, at the 0.5-mL dose in young children 6 to 35 months of age, demonstrated efficacy of 63.2% against moderate to severe influenza and 49.8% against any severity influenza disease” stated Leonard Friedland, MD, director of scientific affairs and public health, Vaccines North America, GlaxoSmithKline. Dr. Friedland, a pediatrician in Pennsylvania, said that a standard 0.5-mL dose of Fluarix Quadrivalent has practice-changing implications for physicians. “The use of a 0.5-mL dose (15 mcg per strain) for all persons aged 6 months and older potentially simplifies influenza vaccination by allowing the same vaccine dose to be used for all eligible individuals.”
The high efficacy of Fluarix against almost half of all influenza strains, regardless of severity, and in preventing moderate to severe influenza, correlated with a reduction in health care utilization by pediatric influenza patients, he said. Visits to general practitioners and emergency departments decreased by 47% and 79%, respectively, in children aged 6-35 months. Influenza-associated antibiotic use in these pediatric influenza patients also decreased by 50%.
These findings were the result of D-QIV-004, a phase 3, observer-blinded, randomized trial of 12,018 children aged 6-35 months. These children were split into five cohorts, each in a different influenza season. The study spanned 13 countries and ran from October 2011 to December 2014. To determine the safety of Fluarix, the study utilized noninfluenza vaccine comparator vaccines that were age appropriate, including Prevnar 13, Havrix, and Varivax.
A majority of the children in the study (98%) were vaccine unprimed (had never received two doses of seasonal influenza vaccine) and received two doses of Fluarix. The remaining children received one dose.
On Jan. 11, 2018, the Food and Drug Administration expanded the indication of Fluarix Quadrivalent to include use in persons 6 months and older. Previously, it was approved only for persons 3 years and older.
“These study results support universal vaccination of all individuals from 6 months of age [with Fluarix] to prevent influenza.” Dr. Friedland concluded.
For live updates and information concerning influenza, visit the CDC website.
ilacy@frontlinemedcom.com
SOURCE: D-QIV-004.
Fluarix Quadrivalent is highly effective against moderate and severe flu strains in children aged 6-35 months, and has the potential to simplify influenza vaccinations for all ages, according the results of a phase 3 clinical trial presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
“Fluarix Quadrivalent, at the 0.5-mL dose in young children 6 to 35 months of age, demonstrated efficacy of 63.2% against moderate to severe influenza and 49.8% against any severity influenza disease” stated Leonard Friedland, MD, director of scientific affairs and public health, Vaccines North America, GlaxoSmithKline. Dr. Friedland, a pediatrician in Pennsylvania, said that a standard 0.5-mL dose of Fluarix Quadrivalent has practice-changing implications for physicians. “The use of a 0.5-mL dose (15 mcg per strain) for all persons aged 6 months and older potentially simplifies influenza vaccination by allowing the same vaccine dose to be used for all eligible individuals.”
The high efficacy of Fluarix against almost half of all influenza strains, regardless of severity, and in preventing moderate to severe influenza, correlated with a reduction in health care utilization by pediatric influenza patients, he said. Visits to general practitioners and emergency departments decreased by 47% and 79%, respectively, in children aged 6-35 months. Influenza-associated antibiotic use in these pediatric influenza patients also decreased by 50%.
These findings were the result of D-QIV-004, a phase 3, observer-blinded, randomized trial of 12,018 children aged 6-35 months. These children were split into five cohorts, each in a different influenza season. The study spanned 13 countries and ran from October 2011 to December 2014. To determine the safety of Fluarix, the study utilized noninfluenza vaccine comparator vaccines that were age appropriate, including Prevnar 13, Havrix, and Varivax.
A majority of the children in the study (98%) were vaccine unprimed (had never received two doses of seasonal influenza vaccine) and received two doses of Fluarix. The remaining children received one dose.
On Jan. 11, 2018, the Food and Drug Administration expanded the indication of Fluarix Quadrivalent to include use in persons 6 months and older. Previously, it was approved only for persons 3 years and older.
“These study results support universal vaccination of all individuals from 6 months of age [with Fluarix] to prevent influenza.” Dr. Friedland concluded.
For live updates and information concerning influenza, visit the CDC website.
ilacy@frontlinemedcom.com
SOURCE: D-QIV-004.
Fluarix Quadrivalent is highly effective against moderate and severe flu strains in children aged 6-35 months, and has the potential to simplify influenza vaccinations for all ages, according the results of a phase 3 clinical trial presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
“Fluarix Quadrivalent, at the 0.5-mL dose in young children 6 to 35 months of age, demonstrated efficacy of 63.2% against moderate to severe influenza and 49.8% against any severity influenza disease” stated Leonard Friedland, MD, director of scientific affairs and public health, Vaccines North America, GlaxoSmithKline. Dr. Friedland, a pediatrician in Pennsylvania, said that a standard 0.5-mL dose of Fluarix Quadrivalent has practice-changing implications for physicians. “The use of a 0.5-mL dose (15 mcg per strain) for all persons aged 6 months and older potentially simplifies influenza vaccination by allowing the same vaccine dose to be used for all eligible individuals.”
The high efficacy of Fluarix against almost half of all influenza strains, regardless of severity, and in preventing moderate to severe influenza, correlated with a reduction in health care utilization by pediatric influenza patients, he said. Visits to general practitioners and emergency departments decreased by 47% and 79%, respectively, in children aged 6-35 months. Influenza-associated antibiotic use in these pediatric influenza patients also decreased by 50%.
These findings were the result of D-QIV-004, a phase 3, observer-blinded, randomized trial of 12,018 children aged 6-35 months. These children were split into five cohorts, each in a different influenza season. The study spanned 13 countries and ran from October 2011 to December 2014. To determine the safety of Fluarix, the study utilized noninfluenza vaccine comparator vaccines that were age appropriate, including Prevnar 13, Havrix, and Varivax.
A majority of the children in the study (98%) were vaccine unprimed (had never received two doses of seasonal influenza vaccine) and received two doses of Fluarix. The remaining children received one dose.
On Jan. 11, 2018, the Food and Drug Administration expanded the indication of Fluarix Quadrivalent to include use in persons 6 months and older. Previously, it was approved only for persons 3 years and older.
“These study results support universal vaccination of all individuals from 6 months of age [with Fluarix] to prevent influenza.” Dr. Friedland concluded.
For live updates and information concerning influenza, visit the CDC website.
ilacy@frontlinemedcom.com
SOURCE: D-QIV-004.
FROM AN ACIP MEETING
Key clinical point: The high efficacy of Fluarix against almost half of all influenza strains, regardless of severity, as well as preventing moderate to severe influenza, reduced health care utilization by pediatric influenza patients.
Major finding: Fluarix Quadrivalent was effective against moderate to severe influenza in 63.2% and against any severity of influenza in 49.8% of children aged 6-35 months.
Study details: A phase 3, observer-blinded, randomized trial of 12,018 children aged 6-35 months, in which the children were split into five cohorts, each in a different influenza season from October 2011 to December 2014.
Disclosures: No disclosures were reported.
Source: The D-QIV-004 study.
ACIP unanimously recommends HEPLISAV-B
At a meeting of the Center for Disease Control and Prevention’s Advisory Committee on Immunization Practices, members unanimously voted to include HEPLISAV-B on the ACIP list of recommended products to vaccinate adults against hepatitis B.
“I think this is a huge advance, and a step forward “ said David S. Stephens, MD, of Emory University, Atlanta, who is a voting member of ACIP.
According to Sarah Schillie, MD, of ACIP’s Hepatitis Work Group, the reduction from three doses to two also will improve vaccine series completion rates, providing more effective protection. This could be very important for health care professionals, with only about 60% of treated individuals fulfilling the three doses necessary for complete HBV protection.
Although fewer doses are needed with HEPLISAV-B, it displays similar immunogenicity to similar vaccines (90.0%-100% vs. 70.5%-90.2%). It is also more effective, compared with similar vaccines in those with type II diabetes (90.0% vs. 65.1%) and chronic kidney disease (89.9% vs. 81.1%).
HEPLISAV-B uses an adjuvant, but it appears to be safe and well tolerated. The rate of mild (45.6% vs. 45.7%) and serious (5.4% vs. 6.3%) reactions were similar among HEPLISAV-B and comparable vaccines, although cardiovascular events were more common with this vaccine than with others (0.27% vs. 0.14%).
Dr. Stephens, who supported the recommendation of HEPLISAV-B, voiced reservations about these findings. “I am concerned about the myocardial infarction signal and the use of this new adjuvant and certainly urge us to look at the postmarketing data carefully.”
While the reported incidence rate of acute HBV cases consistently fell during 2000-2015, it began to rise again at the end of 2015. This is linked with concomitant rise in injection drug use, which also is a risk factor in transmitting HBV. Over the same period, the incidence of HBV was highest in people aged 30-49 years. With these factors to consider, the vote by ACIP for HEPLISAV-B as a recommended vaccine is timely and may help reduce HBV infections in adults.
At a meeting of the Center for Disease Control and Prevention’s Advisory Committee on Immunization Practices, members unanimously voted to include HEPLISAV-B on the ACIP list of recommended products to vaccinate adults against hepatitis B.
“I think this is a huge advance, and a step forward “ said David S. Stephens, MD, of Emory University, Atlanta, who is a voting member of ACIP.
According to Sarah Schillie, MD, of ACIP’s Hepatitis Work Group, the reduction from three doses to two also will improve vaccine series completion rates, providing more effective protection. This could be very important for health care professionals, with only about 60% of treated individuals fulfilling the three doses necessary for complete HBV protection.
Although fewer doses are needed with HEPLISAV-B, it displays similar immunogenicity to similar vaccines (90.0%-100% vs. 70.5%-90.2%). It is also more effective, compared with similar vaccines in those with type II diabetes (90.0% vs. 65.1%) and chronic kidney disease (89.9% vs. 81.1%).
HEPLISAV-B uses an adjuvant, but it appears to be safe and well tolerated. The rate of mild (45.6% vs. 45.7%) and serious (5.4% vs. 6.3%) reactions were similar among HEPLISAV-B and comparable vaccines, although cardiovascular events were more common with this vaccine than with others (0.27% vs. 0.14%).
Dr. Stephens, who supported the recommendation of HEPLISAV-B, voiced reservations about these findings. “I am concerned about the myocardial infarction signal and the use of this new adjuvant and certainly urge us to look at the postmarketing data carefully.”
While the reported incidence rate of acute HBV cases consistently fell during 2000-2015, it began to rise again at the end of 2015. This is linked with concomitant rise in injection drug use, which also is a risk factor in transmitting HBV. Over the same period, the incidence of HBV was highest in people aged 30-49 years. With these factors to consider, the vote by ACIP for HEPLISAV-B as a recommended vaccine is timely and may help reduce HBV infections in adults.
At a meeting of the Center for Disease Control and Prevention’s Advisory Committee on Immunization Practices, members unanimously voted to include HEPLISAV-B on the ACIP list of recommended products to vaccinate adults against hepatitis B.
“I think this is a huge advance, and a step forward “ said David S. Stephens, MD, of Emory University, Atlanta, who is a voting member of ACIP.
According to Sarah Schillie, MD, of ACIP’s Hepatitis Work Group, the reduction from three doses to two also will improve vaccine series completion rates, providing more effective protection. This could be very important for health care professionals, with only about 60% of treated individuals fulfilling the three doses necessary for complete HBV protection.
Although fewer doses are needed with HEPLISAV-B, it displays similar immunogenicity to similar vaccines (90.0%-100% vs. 70.5%-90.2%). It is also more effective, compared with similar vaccines in those with type II diabetes (90.0% vs. 65.1%) and chronic kidney disease (89.9% vs. 81.1%).
HEPLISAV-B uses an adjuvant, but it appears to be safe and well tolerated. The rate of mild (45.6% vs. 45.7%) and serious (5.4% vs. 6.3%) reactions were similar among HEPLISAV-B and comparable vaccines, although cardiovascular events were more common with this vaccine than with others (0.27% vs. 0.14%).
Dr. Stephens, who supported the recommendation of HEPLISAV-B, voiced reservations about these findings. “I am concerned about the myocardial infarction signal and the use of this new adjuvant and certainly urge us to look at the postmarketing data carefully.”
While the reported incidence rate of acute HBV cases consistently fell during 2000-2015, it began to rise again at the end of 2015. This is linked with concomitant rise in injection drug use, which also is a risk factor in transmitting HBV. Over the same period, the incidence of HBV was highest in people aged 30-49 years. With these factors to consider, the vote by ACIP for HEPLISAV-B as a recommended vaccine is timely and may help reduce HBV infections in adults.
REPORTING FROM AN ACIP MEETING