User login
PTSD, depression combo tied to high risk for early death in women
Middle-aged women with PTSD and comorbid depression have a nearly fourfold increased risk for early death from a variety of causes in comparison with their peers who do not have those conditions, new research shows.
“Women with more severe symptoms of depression and PTSD were more at risk, compared with those with fewer symptoms or women with symptoms of only PTSD or only depression,” lead investigator Andrea Roberts, PhD, Harvard School of Public Health, Boston, said in an interview.
Health care providers “should be aware that mental health is a critical component of overall health and is tightly entwined with physical health. Identifying and treating mental health issues should be a foundational part of general health practice,” said Dr. Roberts.
The study was published online Dec. 4 in JAMA Network Open.
Mental health fundamental to survival
The researchers studied more than 51,000 mostly White women from the Nurses Health Study II who were followed for 9 years (2008-2017). At baseline in 2008, the women were aged between 43 and 64 years (mean age, 53.3 years).
Women with high levels of PTSD (six or seven symptoms) and probable depression were nearly four times more likely to die during follow-up than their peers who did not have these conditions (hazard ratio, 3.8; 95% confidence interval, 2.65-5.45; P < .001).
With adjustment for health factors such as smoking and body mass index, women with a high level of PTSD and depression remained at increased risk for early death (HR, 3.11; 95% CI, 2.16-4.47; P < .001).
The risk for early death was also elevated among women with moderate PTSD (four or five symptoms) and depression (HR, 2.03; 95% CI, 1.35-3.03; P < .001) and women with subclinical PTSD and depression (HR, 2.85; 95% CI, 1.99-4.07; P < .001) compared with those who did not have PTSD or depression.
Among women with PTSD symptoms and depression, the incidence of death from nearly all major causes was increased, including death from cardiovascular disease, respiratory disease, type 2 diabetes, unintentional injury, suicide, and other causes.
“These findings provide further evidence that mental health is fundamental to physical health – and to our very survival. We ignore our emotional well-being at our peril,” senior author Karestan Koenen, PhD, said in a news release.
New knowledge
Commenting on the findings, Jennifer Sumner, PhD, said that it’s “critical to appreciate the physical health consequences of psychopathology in individuals who have experienced trauma. This study adds to a growing literature demonstrating that the impact extends far beyond emotional health.
“Furthermore, these results highlight the potential value of promoting healthy lifestyle changes in order to reduce the elevated mortality risk in trauma-exposed individuals with co-occurring PTSD and depression,” said Dr. Sumner, who is with the department of psychology, University of California, Los Angeles.
She noted that this study builds on other work that links PTSD to mortality in men.
“Most work on posttraumatic psychopathology and physical health has actually been conducted in predominantly male samples of veterans, so said Dr. Sumner.
“It’s also important to note that PTSD and depression are more prevalent in women than in men, so demonstrating these associations in women is particularly relevant,” she added.
Funding for the study was provided by the National Institutes of Heath. The authors disclosed no relevant financial relationships. Dr. Sumner has collaborated with the study investigators on prior studies.
A version of this article originally appeared on Medscape.com.
Middle-aged women with PTSD and comorbid depression have a nearly fourfold increased risk for early death from a variety of causes in comparison with their peers who do not have those conditions, new research shows.
“Women with more severe symptoms of depression and PTSD were more at risk, compared with those with fewer symptoms or women with symptoms of only PTSD or only depression,” lead investigator Andrea Roberts, PhD, Harvard School of Public Health, Boston, said in an interview.
Health care providers “should be aware that mental health is a critical component of overall health and is tightly entwined with physical health. Identifying and treating mental health issues should be a foundational part of general health practice,” said Dr. Roberts.
The study was published online Dec. 4 in JAMA Network Open.
Mental health fundamental to survival
The researchers studied more than 51,000 mostly White women from the Nurses Health Study II who were followed for 9 years (2008-2017). At baseline in 2008, the women were aged between 43 and 64 years (mean age, 53.3 years).
Women with high levels of PTSD (six or seven symptoms) and probable depression were nearly four times more likely to die during follow-up than their peers who did not have these conditions (hazard ratio, 3.8; 95% confidence interval, 2.65-5.45; P < .001).
With adjustment for health factors such as smoking and body mass index, women with a high level of PTSD and depression remained at increased risk for early death (HR, 3.11; 95% CI, 2.16-4.47; P < .001).
The risk for early death was also elevated among women with moderate PTSD (four or five symptoms) and depression (HR, 2.03; 95% CI, 1.35-3.03; P < .001) and women with subclinical PTSD and depression (HR, 2.85; 95% CI, 1.99-4.07; P < .001) compared with those who did not have PTSD or depression.
Among women with PTSD symptoms and depression, the incidence of death from nearly all major causes was increased, including death from cardiovascular disease, respiratory disease, type 2 diabetes, unintentional injury, suicide, and other causes.
“These findings provide further evidence that mental health is fundamental to physical health – and to our very survival. We ignore our emotional well-being at our peril,” senior author Karestan Koenen, PhD, said in a news release.
New knowledge
Commenting on the findings, Jennifer Sumner, PhD, said that it’s “critical to appreciate the physical health consequences of psychopathology in individuals who have experienced trauma. This study adds to a growing literature demonstrating that the impact extends far beyond emotional health.
“Furthermore, these results highlight the potential value of promoting healthy lifestyle changes in order to reduce the elevated mortality risk in trauma-exposed individuals with co-occurring PTSD and depression,” said Dr. Sumner, who is with the department of psychology, University of California, Los Angeles.
She noted that this study builds on other work that links PTSD to mortality in men.
“Most work on posttraumatic psychopathology and physical health has actually been conducted in predominantly male samples of veterans, so said Dr. Sumner.
“It’s also important to note that PTSD and depression are more prevalent in women than in men, so demonstrating these associations in women is particularly relevant,” she added.
Funding for the study was provided by the National Institutes of Heath. The authors disclosed no relevant financial relationships. Dr. Sumner has collaborated with the study investigators on prior studies.
A version of this article originally appeared on Medscape.com.
Middle-aged women with PTSD and comorbid depression have a nearly fourfold increased risk for early death from a variety of causes in comparison with their peers who do not have those conditions, new research shows.
“Women with more severe symptoms of depression and PTSD were more at risk, compared with those with fewer symptoms or women with symptoms of only PTSD or only depression,” lead investigator Andrea Roberts, PhD, Harvard School of Public Health, Boston, said in an interview.
Health care providers “should be aware that mental health is a critical component of overall health and is tightly entwined with physical health. Identifying and treating mental health issues should be a foundational part of general health practice,” said Dr. Roberts.
The study was published online Dec. 4 in JAMA Network Open.
Mental health fundamental to survival
The researchers studied more than 51,000 mostly White women from the Nurses Health Study II who were followed for 9 years (2008-2017). At baseline in 2008, the women were aged between 43 and 64 years (mean age, 53.3 years).
Women with high levels of PTSD (six or seven symptoms) and probable depression were nearly four times more likely to die during follow-up than their peers who did not have these conditions (hazard ratio, 3.8; 95% confidence interval, 2.65-5.45; P < .001).
With adjustment for health factors such as smoking and body mass index, women with a high level of PTSD and depression remained at increased risk for early death (HR, 3.11; 95% CI, 2.16-4.47; P < .001).
The risk for early death was also elevated among women with moderate PTSD (four or five symptoms) and depression (HR, 2.03; 95% CI, 1.35-3.03; P < .001) and women with subclinical PTSD and depression (HR, 2.85; 95% CI, 1.99-4.07; P < .001) compared with those who did not have PTSD or depression.
Among women with PTSD symptoms and depression, the incidence of death from nearly all major causes was increased, including death from cardiovascular disease, respiratory disease, type 2 diabetes, unintentional injury, suicide, and other causes.
“These findings provide further evidence that mental health is fundamental to physical health – and to our very survival. We ignore our emotional well-being at our peril,” senior author Karestan Koenen, PhD, said in a news release.
New knowledge
Commenting on the findings, Jennifer Sumner, PhD, said that it’s “critical to appreciate the physical health consequences of psychopathology in individuals who have experienced trauma. This study adds to a growing literature demonstrating that the impact extends far beyond emotional health.
“Furthermore, these results highlight the potential value of promoting healthy lifestyle changes in order to reduce the elevated mortality risk in trauma-exposed individuals with co-occurring PTSD and depression,” said Dr. Sumner, who is with the department of psychology, University of California, Los Angeles.
She noted that this study builds on other work that links PTSD to mortality in men.
“Most work on posttraumatic psychopathology and physical health has actually been conducted in predominantly male samples of veterans, so said Dr. Sumner.
“It’s also important to note that PTSD and depression are more prevalent in women than in men, so demonstrating these associations in women is particularly relevant,” she added.
Funding for the study was provided by the National Institutes of Heath. The authors disclosed no relevant financial relationships. Dr. Sumner has collaborated with the study investigators on prior studies.
A version of this article originally appeared on Medscape.com.
At-home exercises for 4 common musculoskeletal complaints
The mainstay of treatment for many musculoskeletal (MSK) complaints is physical or occupational therapy. But often an individual’s underlying biomechanical issue is one that can be easily addressed with a home exercise plan, and, in light of the COVID-19 pandemic, patients may wish to avoid in-person physical therapy. This article describes the rationale for, and methods of providing, home exercises for several MSK conditions commonly seen in the primary care setting.
General rehabilitation principles: First things first
With basic MSK complaints, focus on controlling pain and swelling before undertaking restoration of function. Tailor pharmacologic and nonpharmacologic options to the patient’s needs, using first-line modalities such as ice and compression to reduce inflammation, and prescribing scheduled doses of an anti-inflammatory medication to help with both pain and inflammation.
Once pain is sufficiently controlled, have patients begin basic rehabilitation with simple range-of-motion exercises that move the injured region through normal patterns, as tolerated. Later, the patient can progress through more specific exercises to return the injured region to full functional capacity.
Explain to patients that it takes about 7 to 10 days of consistent care to decrease inflammation, but that they should begin prescribed exercises once they are able to tolerate them. Plan a follow-up visit in 2 to 3 weeks to check on the patient’s response to prescribed care.
Which is better, ice or heat?
Ice and heat are both commonly used to treat MSK injuries and pain, although scrutiny of the use of either intervention has increased. Despite the widespread use of these modalities, there is little evidence to support their effect on patient outcomes. The historical consensus has been that ice decreases pain, inflammation, and edema,while heat can facilitate movement in rehabilitation by improving blood flow and decreasing stiffness.1-3 In our practice, we encourage use of both topical modalities as a way to start exercise therapy when pain from the acute injury limits participation. Patients often ask which modality they should use. Ice is generally applied in the acute injury phase (48-72 hours after injury), while heat has been thought to be more beneficial in the chronic stages.
Ccontinue to: When and how to apply ice
When and how to apply ice. Applying an ice pack or a bag of frozen vegetables directly to the affected area will help control pain and swelling. Ice should be applied for 15 to 20 minutes at a time, once an hour. If a patient has sensitivity to cold or if the ice pack is a gel-type, have the patient place a layer (eg, towel) between the ice and skin to avoid injury to the skin. Additional caution should be exercised in patients with peripheral vascular disease, cryoglobulinemia, Raynaud disease, or a history of frostbite at the site.4
An alternative method we sometimes recommend is ice-cup massage. The patient can fill a small paper cup with water and freeze it. The cup is then used to massage the injured area, providing a more active method of icing whereby the cold can penetrate more quickly. Ice-cup massage should be done for 5 to 10 minutes, 3 to 4 times a day.
When and how to apply heat. Heat will help relax and loosen muscles and is a preferred treatment for older injuries, chronic pain, muscle tension, and spasms.5 Because heat can increase blood flow and, likely, inflammation, it should not be used in the acute injury phase. A heating pad or a warm, wet towel can be applied for up to 20 minutes at a time to help relieve pain and tension. Heat is also beneficial before participating in rehab activities as a method of “warming up” a recently injured area.6 However, ice should still be used following activity to prevent any new inflammation.
Anti-inflammatory medications
For an acute injury, nonsteroidal anti-inflammatory drugs (NSAIDs) not only can decrease inflammation and aid in healing but can alleviate pain. We typically start with over-the-counter (OTC) NSAIDs taken on a schedule. A good suggestion is to have the patient take the scheduled NSAID with food for 7 to 10 days or until symptoms subside.
Topical analgesics
Because oral medications can occasionally cause adverse effects or be contraindicated in some patients, topical analgesics can be a good substitute due to their minimal adverse effects. Acceptable topical medications include NSAIDs, lidocaine, menthol, and arnica. Other than prescribed topical NSAIDs, these products can be applied directly to the painful area on an as-needed basis. Often, a topical patch is a nice option to recommend for use during work or school, and a topical cream or ointment can be used at bedtime.
Continue to: Graduated rehabilitation
Graduated rehabilitation
The following 4 common MSK injuries are ones that can benefit from a graduated approach to rehabilitation at home.
Lateral ankle sprain
Lateral ankle sprain, usually resulting from an inversion mechanism, is the most common type of acute ankle sprain seen in primary care and sports medicine settings.7-9 The injury causes lateral ankle pain and swelling, decreased range of motion and strength, and pain with weight-bearing activities.
Treatment and rehabilitation after this type of injury are critical to restoring normal function and increasing the likelihood of returning to pre-injury levels of activity.9,10 Goals for an acute ankle sprain include controlling swelling, regaining full range of motion, increasing muscle strength and power, and improving balance.
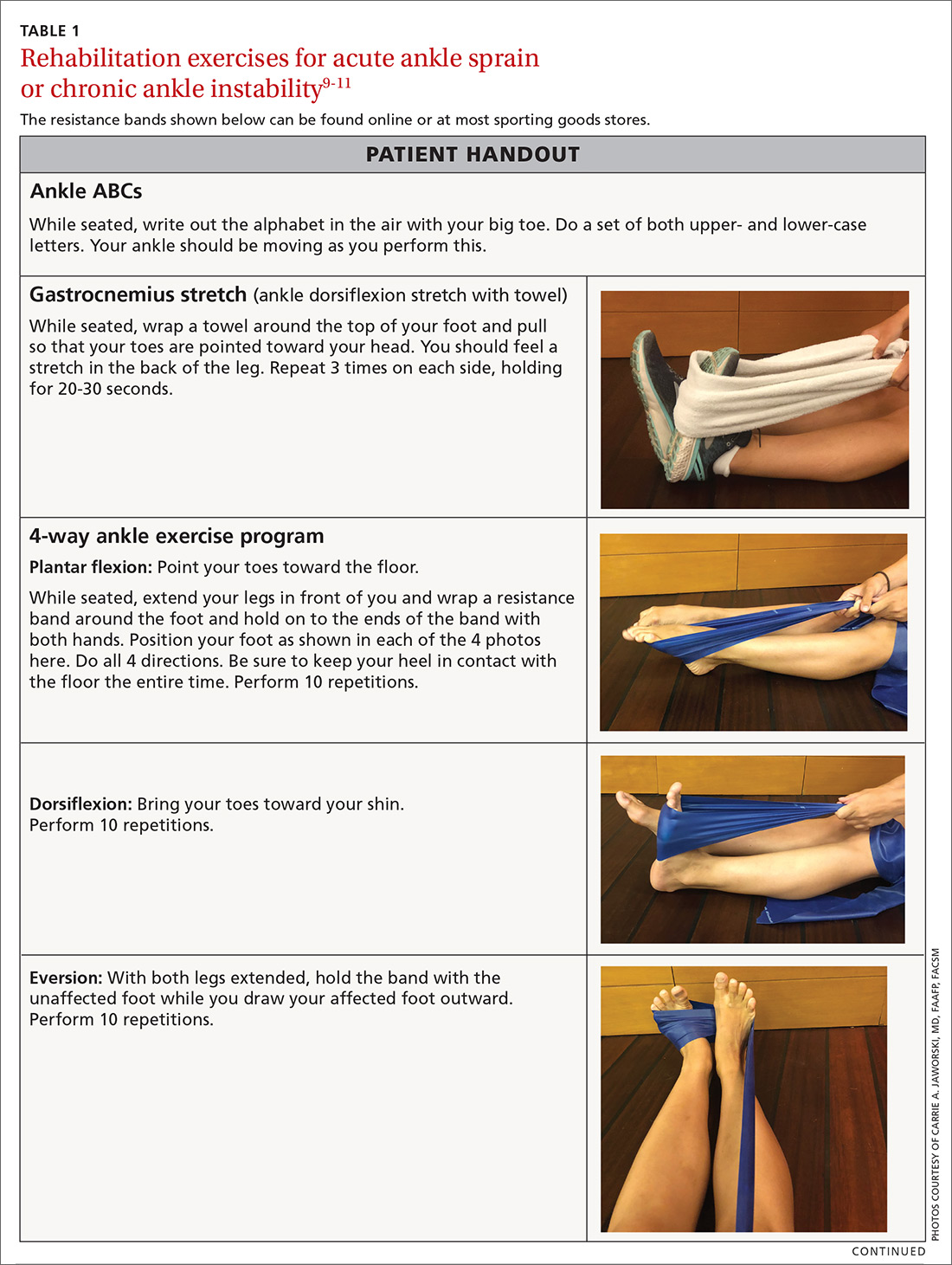
Phase 1: Immediately following injury, have the patient protect the injured area with rest, ice, compression, and elevation (RICE). This will help to decrease swelling and pain. Exercises to regain range of motion, such as stretching and doing ankle “ABCs,” should begin within 48 to 72 hours of the initial injury (TABLE 1).9-11
Continue to: Phase 2
Phase 2: Once the patient has achieved full range of motion and pain is controlled, begin the process of regaining strength. The 4-way ankle exercise program (with elastic tubing) is an easy at-home exercise that has been shown to improve strength in plantar flexion, dorsiflexion, eversion, and inversion (TABLE 1).9-11
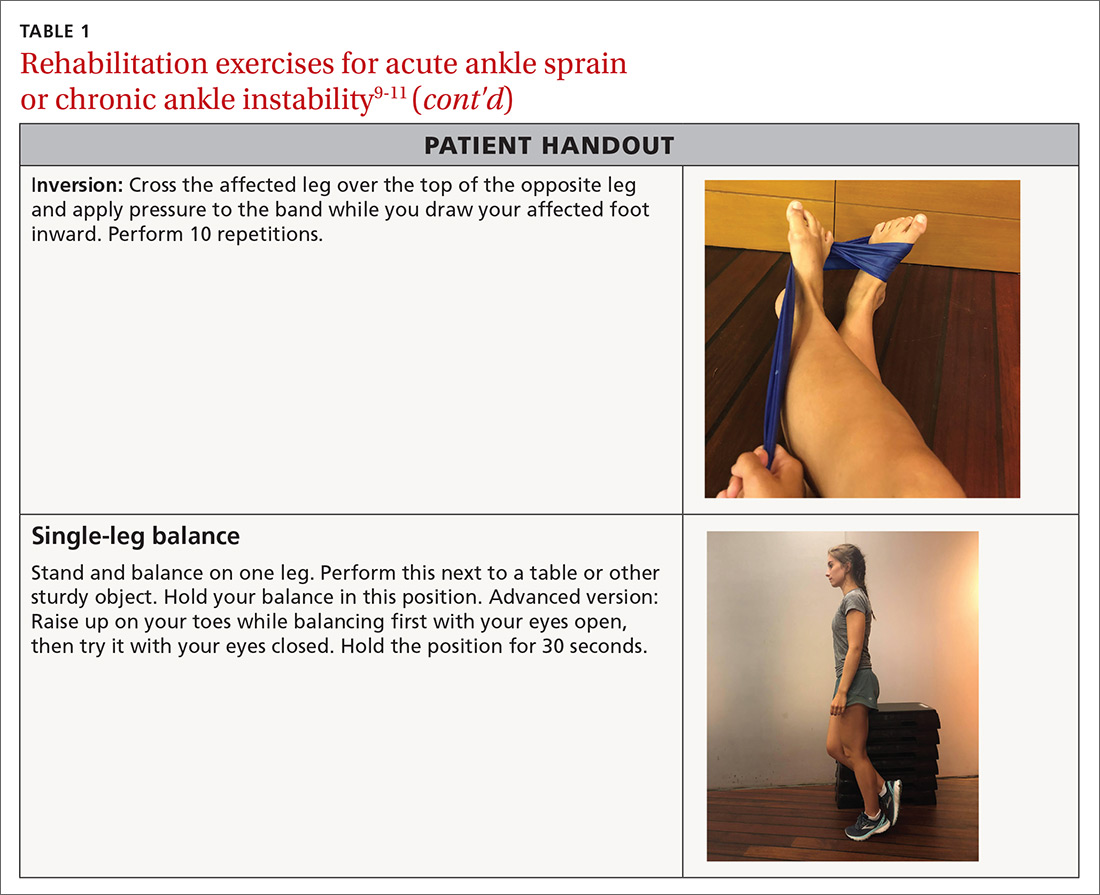
Phase 3: Once your patient is able to bear full weight with little to no pain, begin a balance program (TABLE 19-11). This is the most frequently neglected component of rehabilitation and the most common reason patients return with chronic ankle pain or repeat ankle injuries. Deficits in postural stability and balance have been reported in unstable ankles following acute ankle sprains,10,12-15 and studies have shown that individuals with poor stability are at a greater risk of injury.13-16
For most lateral ankle sprains, patients can expect time to recovery to range from 2 to 8 weeks. Longer recoveries are associated with more severe injuries or those that involve the syndesmosis.
Plantar fasciitis
Plantar fasciitis (PF) of the foot can be frustrating for a patient due to its chronic nature. Most patients will present with pain in the heel that is aggravated by weight-bearing activities. A conservative management program that focuses on reducing pain and inflammation, reducing tissue stress, and restoring strength and flexibility has been shown to be effective for this type of injury.17,18
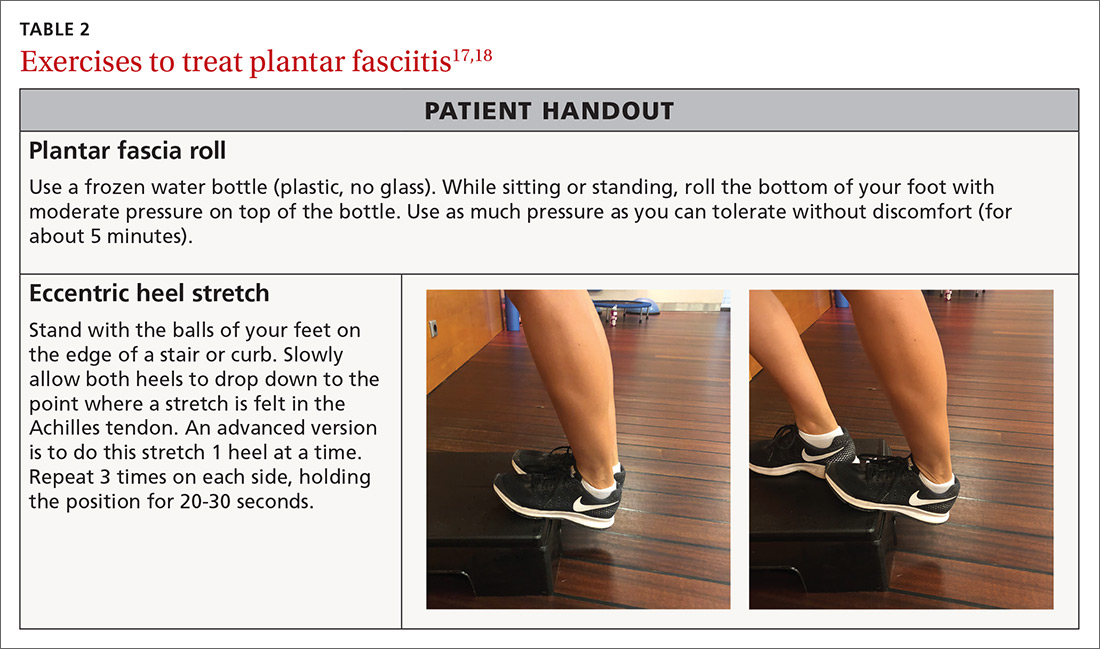
Step 1: Reduce pain and inflammation. Deep-tissue massage and cryotherapy are easy ways to help with pain and inflammation. Deep-tissue massage can be accomplished by rolling the bottom of the foot on a golf or lacrosse ball. A favorite recommendation of ours to reduce inflammation is to use the ice-cup massage, mentioned earlier, for 5 minutes. Or rolling the bottom of the foot on a frozen water bottle will accomplish both tasks at once (TABLE 217,18).
Step 2: Reduce tissue stress. Management tools commonly used to reduce tissue stress are OTC orthotics and night splints. The night splint has been shown to improve symptoms,but patients often stop using it due to discomfort.19 Many kinds of night splints are available, but we have found that the sock variety with a strap to keep the foot in dorsiflexion is best tolerated, and it should be covered by most care plans.
Continue to: Step 3
Step 3: Restore muscle strength and flexibility. Restoring flexibility of the gastrocnemius and soleus is most frequently recommended for treating PF. Strengthening exercises that involve intrinsic and extrinsic muscles of the foot and ankle are also essential.17,18 Helpful exercises include those listed in TABLE 1.9-11 Additionally, an eccentric heel stretch can help to alleviate PF symptoms (TABLE 217,18).
A reasonable timeline for follow-up on newly diagnosed PF is 4 to 6 weeks. While many patients will not have recovered in that time, the goal is to document progress in recovery. If no progress is made, consider other treatment modalities.
Patellofemoral pain syndrome
Patellofemoral pain syndrome (PFPS) is one of the most common orthopedic complaints, estimated to comprise 7.3% of all orthopedic visits.20 Commonly called “runner’s knee,” PFPS is the leading cause of anterior knee pain in active individuals. Studies suggest a gender bias, with PFPS being diagnosed more frequently in females than in males, particularly between the ages of 10 and 19.20 Often, there is vague anterior knee pain, or pain that worsens with activities such as climbing hills or stairs, or with long sitting or when fatigued.
In general, unbalanced patellar tracking within the trochlear groove likely leads to this pain. Multiple contributory factors have been described; however, evidence increasingly has shown that deficiencies in hip strength may contribute significantly to maltracking of the patella with resultant pain. Specifically, weakness in hip external rotators and abductors is associated with abnormal lower extremity mechanics.21 One randomized controlled trial by Ferber et al found that therapy protocols directed at hip and core strength showed earlier resolution of pain and greater strength when compared with knee protocols alone.22
We routinely talk to patients about how the knee is the “victim” caught between weak hips and/or flat feet. It is prudent to look for both in the office visit. This can be done with one simple maneuver: Ask your patient to do a squat followed by 3 or 4 single-leg squats on each side. This will often reveal dysfunction at the foot/ankle or weakness in the hips/core as demonstrated by pronated feet (along with valgus tracking of the knees inward) or loss of balance upon squatting.
There is general consensus that a nonsurgical approach is the mainstay of treatment for PFPS.23 Pelvic stabilization and hip strengthening are standard components along with treatment protocols of exercises tailored to one’s individual weaknesses.
Numerous types of exercises do not require specialized equipment and can be taught in the office (TABLE 324). Explain to patients that the recovery process may take several months. Monthly follow-up to document progress is essential and helps to ensure compliance with one’s home program.
Continue to : Neck pain
Neck pain
The annual prevalence of nonspecific neck pain ranges from 27% to 48%, with 70% of individuals being afflicted at some time in their lives.25 First rule out any neurologic factors that might suggest cervical disc disease or spinal stenosis. If a patient describes weakness or sensory changes along one or both upper extremities, obtain imaging and consider more formalized therapy with a physical therapist.
In patients without any red flags, investigate possible biomechanical causes. It is essential to review the patient’s work and home habits, particularly in light of COVID-19, to determine if adjustments may be needed. Factors to consider are desk and computer setups at work or home, reading or laptop use in bed, sleep habits, and frequency of cellular phone calls/texting.26 A formal ergonomic assessment of the patient’s workplace may be helpful.
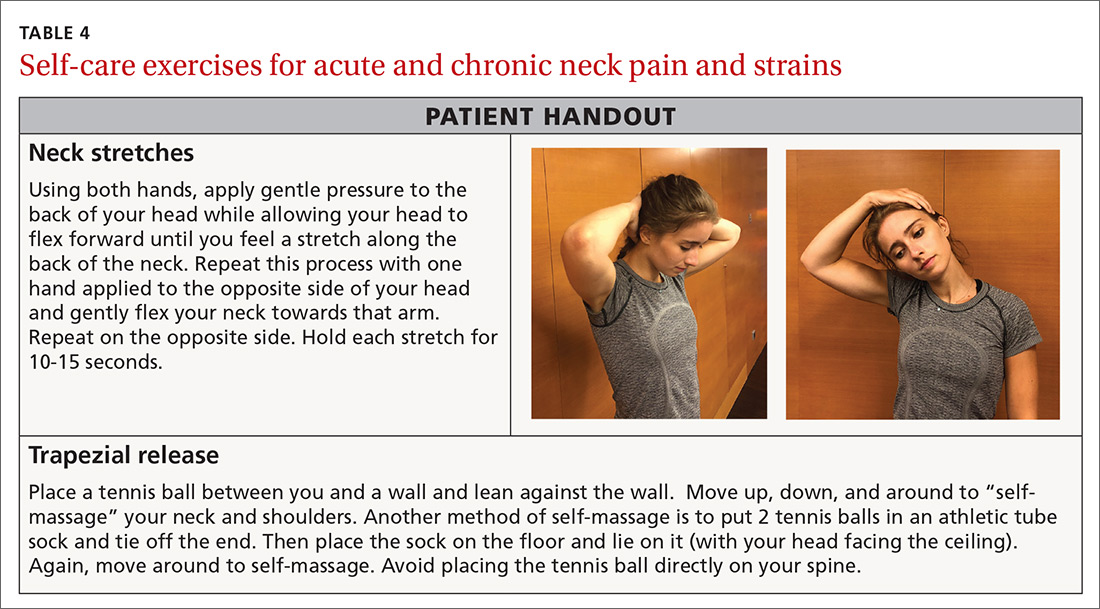
A mainstay in treating mechanical neck pain is alleviating trapezial tightness or spasm. Manipulative therapies such as osteopathic manipulation, massage, and chiropractic care can provide pain relief in the acute setting as well as help with control of chronic symptoms.27 A simple self-care tool is using a tennis ball to massage the trapezial muscles. This can be accomplished by having the patient position the tennis ball along the upper trapezial muscles, holding it in place by leaning against a wall, and initiating self-massage. Another method of self-massage is to put 2 tennis balls in an athletic tube sock and tie off the end, place the sock on the floor, and lie on it in the supine position.
There is also evidence that exercise of any kind can help control neck pain.28,29 The easiest exercises one can offer a patient with neck stiffness, or even mild cervical strains, is self-directed stretching through gentle pressure applied in all 4 directions on the neck. This technique can be repeated hourly both at work and at home (TABLE 4).
Reminders that can help ensure success
You can use the approaches described here for numerous other MSK conditions in helping patients on the road to recovery.
After the acute phase, advise patients to
• apply heat to the affected area before exercising. This can help bring blood flow to the region and promote ease of movement.
• continue icing the area following rehabilitation exercises in order to control exercise-induced inflammation.
• report any changing symptoms such as worsening pain, numbness, or weakness.
These techniques are one step in the recovery process. A home program can benefit the patient either alone or in combination with more advanced techniques that are best accomplished under the watchful eye of a physical or occupational therapist.
CORRESPONDENCE
Carrie A. Jaworski, MD, FAAFP, FACSM, 2180 Pfingsten Road, Suite 3100, Glenview, IL 60026; cjaworski@northshore.org
1. Hubbard TJ, Aronson SL, Denegar CR. Does cryotherapy hasten return to participation? A systematic review. J Athl Train. 2004;39:88-94.
2. Ho SS, Coel MN, Kagawa R, et al. The effects of ice on blood flow and bone metabolism in knees. Am J Sports Med. 1994;22:537-540.
3. Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad Med. 2015;127:57-65.
4. Bleakley CM, O’Connor S, Tully MA, et al. The PRICE study (Protection Rest Ice Compression Elevation): design of a randomised controlled trial comparing standard versus cryokinetic ice applications in the management of acute ankle sprain. BMC Musculoskelet Disord. 2007;8:125.
5. Mayer JM, Ralph L, Look M, et al. Treating acute low back pain with continuous low-level heat wrap therapy and/or exercise: a randomized controlled trial. Spine J. 2005;5:395-403.
6. Cetin N, Aytar A, Atalay A, et al. Comparing hot pack, short-wave diathermy, ultrasound, and TENS on isokinetic strength, pain, and functional status of women with osteoarthritic knees: a single-blind, randomized, controlled trial. Am J Phys Med Rehabil. 2008;87:443-451.
7. Waterman BR, Owens BD, Davey S, et al. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92:2279-2284.
8. Fong DT, Hong Y, Chan LK, et al. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007;37:73-94.
9. Kerkhoffs GM, Rowe BH, Assendelft WJ, et al. Immobilisation and functional treatment for acute lateral ankle ligament injuries in adults. Cochrane Database Syst Rev. 2002(3):CD003762.
10. Mattacola CG, Dwyer MK. Rehabilitation of the ankle after acute sprain or chronic instability. J Ath Train. 2002;37:413-429.
11. Hü bscher M, Zech A, Pfeifer K, et al. Neuromuscular training for sports injury prevention: a systematic review. Med Sci Sports Exerc. 2010;42:413-421.
12. Emery CA, Meeuwisse WH. The effectiveness of a neuromuscular prevention strategy to reduce injuries in youth soccer: a cluster-randomised controlled trial. Br J Sports Med. 2010;44:555-562.
13. Tiemstra JD. Update on acute ankle sprains. Am Fam Physician. 2012;85:1170-1176.
14. Beynnon BD, Murphy DF, Alosa DM. Predictive factors for lateral ankle sprains: a literature review. J Ath Train. 2002;37:376-380.
15. Schiftan GS, Ross LA, Hahne AJ. The effectiveness of proprioceptive training in preventing ankle sprains in sporting populations: a systematic review and meta-analysis. J Sci Med Sport. 2015;18:238–244.
16. Hupperets MD, Verhagen EA, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009;339:b2684
17. Thompson JV, Saini SS, Reb CW, et al. Diagnosis and management of plantar fasciitis. J Am Osteopath Assoc. 2014;114:900-906.
18. DiGiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fascia-specific stretching exercise improves outcomes in patients with chronic plantar fasciitis. A prospective clinical trial with two-year follow-up. J Bone Joint Surg Am. 2006;88:1775-1781.

19. Lee SY, McKeon P, Hertel J. Does the use of orthoses improve self-reported pain and function measures in patients with plantar fasciitis? A meta-analysis. Phys Ther Sport. 2009;10:12-18.
20. Glaviano NR, Key M, Hart JM, et al. Demographic and epidemiological trends in patellofemoral pain. J Sports Phys Ther. 2015;10: 281-290.
21. Louden JK. Biomechanics and pathomechanics of the patellofemoral joint. Int J Sports Phys Ther. 2016;11: 820-830.
22. Ferber R, Bolgla L, Earl-Boehm JE, et al. Strengthening of hip and core versus knee muscles for the treatment of patellofemoral pain: a multicenter randomized controlled trial. J Ath Train. 2015;50: 366-377.
23. Collins NJ, Bisset LM, Crossley KM, et al. Efficacy of nonsurgical interventions for anterior knee pain: systematic review and meta-analysis of randomized trials. Sports Med. 2013;41:31-49.
24. Bolgla LA. Hip strength and kinematics in patellofemoral syndrome. In: Brotzman SB, Manske RC eds. Clinical Orthopaedic Rehabilitation. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2011:273-274.
25. Hogg-Johnson S, van der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(suppl 4):S39-S51.
26. Larsson B, Søgaard K, Rosendal L. Work related neck-shoulder pain: a review on magnitude, risk factors, biochemical characteristics, clinical picture and preventive interventions. Best Pract Res Clin Rheumatol. 2007; 21:447-463.
27. Giles LG, Muller R. Chronic spinal pain: a randomized clinical trial comparing medication, acupuncture, and spinal manipulation. Spine. 2003;28:1490-1502.
28. Bronfort G, Evans R, Anderson A, et al. Spinal manipulation, medication, or home exercise with advice for acute and subacute neck pain: a randomized trial. Ann Intern Med. 2012;156:1-10.
29. Evans R, Bronfort G, Bittell S, et al. A pilot study for a randomized clinical trial assessing chiropractic care, medical care, and self-care education for acute and subacute neck pain patients. J Manipulative Physiol Ther. 2003;26:403-411.
The mainstay of treatment for many musculoskeletal (MSK) complaints is physical or occupational therapy. But often an individual’s underlying biomechanical issue is one that can be easily addressed with a home exercise plan, and, in light of the COVID-19 pandemic, patients may wish to avoid in-person physical therapy. This article describes the rationale for, and methods of providing, home exercises for several MSK conditions commonly seen in the primary care setting.
General rehabilitation principles: First things first
With basic MSK complaints, focus on controlling pain and swelling before undertaking restoration of function. Tailor pharmacologic and nonpharmacologic options to the patient’s needs, using first-line modalities such as ice and compression to reduce inflammation, and prescribing scheduled doses of an anti-inflammatory medication to help with both pain and inflammation.
Once pain is sufficiently controlled, have patients begin basic rehabilitation with simple range-of-motion exercises that move the injured region through normal patterns, as tolerated. Later, the patient can progress through more specific exercises to return the injured region to full functional capacity.
Explain to patients that it takes about 7 to 10 days of consistent care to decrease inflammation, but that they should begin prescribed exercises once they are able to tolerate them. Plan a follow-up visit in 2 to 3 weeks to check on the patient’s response to prescribed care.
Which is better, ice or heat?
Ice and heat are both commonly used to treat MSK injuries and pain, although scrutiny of the use of either intervention has increased. Despite the widespread use of these modalities, there is little evidence to support their effect on patient outcomes. The historical consensus has been that ice decreases pain, inflammation, and edema,while heat can facilitate movement in rehabilitation by improving blood flow and decreasing stiffness.1-3 In our practice, we encourage use of both topical modalities as a way to start exercise therapy when pain from the acute injury limits participation. Patients often ask which modality they should use. Ice is generally applied in the acute injury phase (48-72 hours after injury), while heat has been thought to be more beneficial in the chronic stages.
Ccontinue to: When and how to apply ice
When and how to apply ice. Applying an ice pack or a bag of frozen vegetables directly to the affected area will help control pain and swelling. Ice should be applied for 15 to 20 minutes at a time, once an hour. If a patient has sensitivity to cold or if the ice pack is a gel-type, have the patient place a layer (eg, towel) between the ice and skin to avoid injury to the skin. Additional caution should be exercised in patients with peripheral vascular disease, cryoglobulinemia, Raynaud disease, or a history of frostbite at the site.4
An alternative method we sometimes recommend is ice-cup massage. The patient can fill a small paper cup with water and freeze it. The cup is then used to massage the injured area, providing a more active method of icing whereby the cold can penetrate more quickly. Ice-cup massage should be done for 5 to 10 minutes, 3 to 4 times a day.
When and how to apply heat. Heat will help relax and loosen muscles and is a preferred treatment for older injuries, chronic pain, muscle tension, and spasms.5 Because heat can increase blood flow and, likely, inflammation, it should not be used in the acute injury phase. A heating pad or a warm, wet towel can be applied for up to 20 minutes at a time to help relieve pain and tension. Heat is also beneficial before participating in rehab activities as a method of “warming up” a recently injured area.6 However, ice should still be used following activity to prevent any new inflammation.
Anti-inflammatory medications
For an acute injury, nonsteroidal anti-inflammatory drugs (NSAIDs) not only can decrease inflammation and aid in healing but can alleviate pain. We typically start with over-the-counter (OTC) NSAIDs taken on a schedule. A good suggestion is to have the patient take the scheduled NSAID with food for 7 to 10 days or until symptoms subside.
Topical analgesics
Because oral medications can occasionally cause adverse effects or be contraindicated in some patients, topical analgesics can be a good substitute due to their minimal adverse effects. Acceptable topical medications include NSAIDs, lidocaine, menthol, and arnica. Other than prescribed topical NSAIDs, these products can be applied directly to the painful area on an as-needed basis. Often, a topical patch is a nice option to recommend for use during work or school, and a topical cream or ointment can be used at bedtime.
Continue to: Graduated rehabilitation
Graduated rehabilitation
The following 4 common MSK injuries are ones that can benefit from a graduated approach to rehabilitation at home.
Lateral ankle sprain
Lateral ankle sprain, usually resulting from an inversion mechanism, is the most common type of acute ankle sprain seen in primary care and sports medicine settings.7-9 The injury causes lateral ankle pain and swelling, decreased range of motion and strength, and pain with weight-bearing activities.
Treatment and rehabilitation after this type of injury are critical to restoring normal function and increasing the likelihood of returning to pre-injury levels of activity.9,10 Goals for an acute ankle sprain include controlling swelling, regaining full range of motion, increasing muscle strength and power, and improving balance.
Phase 1: Immediately following injury, have the patient protect the injured area with rest, ice, compression, and elevation (RICE). This will help to decrease swelling and pain. Exercises to regain range of motion, such as stretching and doing ankle “ABCs,” should begin within 48 to 72 hours of the initial injury (TABLE 1).9-11
Continue to: Phase 2
Phase 2: Once the patient has achieved full range of motion and pain is controlled, begin the process of regaining strength. The 4-way ankle exercise program (with elastic tubing) is an easy at-home exercise that has been shown to improve strength in plantar flexion, dorsiflexion, eversion, and inversion (TABLE 1).9-11
Phase 3: Once your patient is able to bear full weight with little to no pain, begin a balance program (TABLE 19-11). This is the most frequently neglected component of rehabilitation and the most common reason patients return with chronic ankle pain or repeat ankle injuries. Deficits in postural stability and balance have been reported in unstable ankles following acute ankle sprains,10,12-15 and studies have shown that individuals with poor stability are at a greater risk of injury.13-16
For most lateral ankle sprains, patients can expect time to recovery to range from 2 to 8 weeks. Longer recoveries are associated with more severe injuries or those that involve the syndesmosis.
Plantar fasciitis
Plantar fasciitis (PF) of the foot can be frustrating for a patient due to its chronic nature. Most patients will present with pain in the heel that is aggravated by weight-bearing activities. A conservative management program that focuses on reducing pain and inflammation, reducing tissue stress, and restoring strength and flexibility has been shown to be effective for this type of injury.17,18
Step 1: Reduce pain and inflammation. Deep-tissue massage and cryotherapy are easy ways to help with pain and inflammation. Deep-tissue massage can be accomplished by rolling the bottom of the foot on a golf or lacrosse ball. A favorite recommendation of ours to reduce inflammation is to use the ice-cup massage, mentioned earlier, for 5 minutes. Or rolling the bottom of the foot on a frozen water bottle will accomplish both tasks at once (TABLE 217,18).
Step 2: Reduce tissue stress. Management tools commonly used to reduce tissue stress are OTC orthotics and night splints. The night splint has been shown to improve symptoms,but patients often stop using it due to discomfort.19 Many kinds of night splints are available, but we have found that the sock variety with a strap to keep the foot in dorsiflexion is best tolerated, and it should be covered by most care plans.
Continue to: Step 3
Step 3: Restore muscle strength and flexibility. Restoring flexibility of the gastrocnemius and soleus is most frequently recommended for treating PF. Strengthening exercises that involve intrinsic and extrinsic muscles of the foot and ankle are also essential.17,18 Helpful exercises include those listed in TABLE 1.9-11 Additionally, an eccentric heel stretch can help to alleviate PF symptoms (TABLE 217,18).
A reasonable timeline for follow-up on newly diagnosed PF is 4 to 6 weeks. While many patients will not have recovered in that time, the goal is to document progress in recovery. If no progress is made, consider other treatment modalities.
Patellofemoral pain syndrome
Patellofemoral pain syndrome (PFPS) is one of the most common orthopedic complaints, estimated to comprise 7.3% of all orthopedic visits.20 Commonly called “runner’s knee,” PFPS is the leading cause of anterior knee pain in active individuals. Studies suggest a gender bias, with PFPS being diagnosed more frequently in females than in males, particularly between the ages of 10 and 19.20 Often, there is vague anterior knee pain, or pain that worsens with activities such as climbing hills or stairs, or with long sitting or when fatigued.
In general, unbalanced patellar tracking within the trochlear groove likely leads to this pain. Multiple contributory factors have been described; however, evidence increasingly has shown that deficiencies in hip strength may contribute significantly to maltracking of the patella with resultant pain. Specifically, weakness in hip external rotators and abductors is associated with abnormal lower extremity mechanics.21 One randomized controlled trial by Ferber et al found that therapy protocols directed at hip and core strength showed earlier resolution of pain and greater strength when compared with knee protocols alone.22
We routinely talk to patients about how the knee is the “victim” caught between weak hips and/or flat feet. It is prudent to look for both in the office visit. This can be done with one simple maneuver: Ask your patient to do a squat followed by 3 or 4 single-leg squats on each side. This will often reveal dysfunction at the foot/ankle or weakness in the hips/core as demonstrated by pronated feet (along with valgus tracking of the knees inward) or loss of balance upon squatting.
There is general consensus that a nonsurgical approach is the mainstay of treatment for PFPS.23 Pelvic stabilization and hip strengthening are standard components along with treatment protocols of exercises tailored to one’s individual weaknesses.
Numerous types of exercises do not require specialized equipment and can be taught in the office (TABLE 324). Explain to patients that the recovery process may take several months. Monthly follow-up to document progress is essential and helps to ensure compliance with one’s home program.
Continue to : Neck pain
Neck pain
The annual prevalence of nonspecific neck pain ranges from 27% to 48%, with 70% of individuals being afflicted at some time in their lives.25 First rule out any neurologic factors that might suggest cervical disc disease or spinal stenosis. If a patient describes weakness or sensory changes along one or both upper extremities, obtain imaging and consider more formalized therapy with a physical therapist.
In patients without any red flags, investigate possible biomechanical causes. It is essential to review the patient’s work and home habits, particularly in light of COVID-19, to determine if adjustments may be needed. Factors to consider are desk and computer setups at work or home, reading or laptop use in bed, sleep habits, and frequency of cellular phone calls/texting.26 A formal ergonomic assessment of the patient’s workplace may be helpful.
A mainstay in treating mechanical neck pain is alleviating trapezial tightness or spasm. Manipulative therapies such as osteopathic manipulation, massage, and chiropractic care can provide pain relief in the acute setting as well as help with control of chronic symptoms.27 A simple self-care tool is using a tennis ball to massage the trapezial muscles. This can be accomplished by having the patient position the tennis ball along the upper trapezial muscles, holding it in place by leaning against a wall, and initiating self-massage. Another method of self-massage is to put 2 tennis balls in an athletic tube sock and tie off the end, place the sock on the floor, and lie on it in the supine position.
There is also evidence that exercise of any kind can help control neck pain.28,29 The easiest exercises one can offer a patient with neck stiffness, or even mild cervical strains, is self-directed stretching through gentle pressure applied in all 4 directions on the neck. This technique can be repeated hourly both at work and at home (TABLE 4).
Reminders that can help ensure success
You can use the approaches described here for numerous other MSK conditions in helping patients on the road to recovery.
After the acute phase, advise patients to
• apply heat to the affected area before exercising. This can help bring blood flow to the region and promote ease of movement.
• continue icing the area following rehabilitation exercises in order to control exercise-induced inflammation.
• report any changing symptoms such as worsening pain, numbness, or weakness.
These techniques are one step in the recovery process. A home program can benefit the patient either alone or in combination with more advanced techniques that are best accomplished under the watchful eye of a physical or occupational therapist.
CORRESPONDENCE
Carrie A. Jaworski, MD, FAAFP, FACSM, 2180 Pfingsten Road, Suite 3100, Glenview, IL 60026; cjaworski@northshore.org
The mainstay of treatment for many musculoskeletal (MSK) complaints is physical or occupational therapy. But often an individual’s underlying biomechanical issue is one that can be easily addressed with a home exercise plan, and, in light of the COVID-19 pandemic, patients may wish to avoid in-person physical therapy. This article describes the rationale for, and methods of providing, home exercises for several MSK conditions commonly seen in the primary care setting.
General rehabilitation principles: First things first
With basic MSK complaints, focus on controlling pain and swelling before undertaking restoration of function. Tailor pharmacologic and nonpharmacologic options to the patient’s needs, using first-line modalities such as ice and compression to reduce inflammation, and prescribing scheduled doses of an anti-inflammatory medication to help with both pain and inflammation.
Once pain is sufficiently controlled, have patients begin basic rehabilitation with simple range-of-motion exercises that move the injured region through normal patterns, as tolerated. Later, the patient can progress through more specific exercises to return the injured region to full functional capacity.
Explain to patients that it takes about 7 to 10 days of consistent care to decrease inflammation, but that they should begin prescribed exercises once they are able to tolerate them. Plan a follow-up visit in 2 to 3 weeks to check on the patient’s response to prescribed care.
Which is better, ice or heat?
Ice and heat are both commonly used to treat MSK injuries and pain, although scrutiny of the use of either intervention has increased. Despite the widespread use of these modalities, there is little evidence to support their effect on patient outcomes. The historical consensus has been that ice decreases pain, inflammation, and edema,while heat can facilitate movement in rehabilitation by improving blood flow and decreasing stiffness.1-3 In our practice, we encourage use of both topical modalities as a way to start exercise therapy when pain from the acute injury limits participation. Patients often ask which modality they should use. Ice is generally applied in the acute injury phase (48-72 hours after injury), while heat has been thought to be more beneficial in the chronic stages.
Ccontinue to: When and how to apply ice
When and how to apply ice. Applying an ice pack or a bag of frozen vegetables directly to the affected area will help control pain and swelling. Ice should be applied for 15 to 20 minutes at a time, once an hour. If a patient has sensitivity to cold or if the ice pack is a gel-type, have the patient place a layer (eg, towel) between the ice and skin to avoid injury to the skin. Additional caution should be exercised in patients with peripheral vascular disease, cryoglobulinemia, Raynaud disease, or a history of frostbite at the site.4
An alternative method we sometimes recommend is ice-cup massage. The patient can fill a small paper cup with water and freeze it. The cup is then used to massage the injured area, providing a more active method of icing whereby the cold can penetrate more quickly. Ice-cup massage should be done for 5 to 10 minutes, 3 to 4 times a day.
When and how to apply heat. Heat will help relax and loosen muscles and is a preferred treatment for older injuries, chronic pain, muscle tension, and spasms.5 Because heat can increase blood flow and, likely, inflammation, it should not be used in the acute injury phase. A heating pad or a warm, wet towel can be applied for up to 20 minutes at a time to help relieve pain and tension. Heat is also beneficial before participating in rehab activities as a method of “warming up” a recently injured area.6 However, ice should still be used following activity to prevent any new inflammation.
Anti-inflammatory medications
For an acute injury, nonsteroidal anti-inflammatory drugs (NSAIDs) not only can decrease inflammation and aid in healing but can alleviate pain. We typically start with over-the-counter (OTC) NSAIDs taken on a schedule. A good suggestion is to have the patient take the scheduled NSAID with food for 7 to 10 days or until symptoms subside.
Topical analgesics
Because oral medications can occasionally cause adverse effects or be contraindicated in some patients, topical analgesics can be a good substitute due to their minimal adverse effects. Acceptable topical medications include NSAIDs, lidocaine, menthol, and arnica. Other than prescribed topical NSAIDs, these products can be applied directly to the painful area on an as-needed basis. Often, a topical patch is a nice option to recommend for use during work or school, and a topical cream or ointment can be used at bedtime.
Continue to: Graduated rehabilitation
Graduated rehabilitation
The following 4 common MSK injuries are ones that can benefit from a graduated approach to rehabilitation at home.
Lateral ankle sprain
Lateral ankle sprain, usually resulting from an inversion mechanism, is the most common type of acute ankle sprain seen in primary care and sports medicine settings.7-9 The injury causes lateral ankle pain and swelling, decreased range of motion and strength, and pain with weight-bearing activities.
Treatment and rehabilitation after this type of injury are critical to restoring normal function and increasing the likelihood of returning to pre-injury levels of activity.9,10 Goals for an acute ankle sprain include controlling swelling, regaining full range of motion, increasing muscle strength and power, and improving balance.
Phase 1: Immediately following injury, have the patient protect the injured area with rest, ice, compression, and elevation (RICE). This will help to decrease swelling and pain. Exercises to regain range of motion, such as stretching and doing ankle “ABCs,” should begin within 48 to 72 hours of the initial injury (TABLE 1).9-11
Continue to: Phase 2
Phase 2: Once the patient has achieved full range of motion and pain is controlled, begin the process of regaining strength. The 4-way ankle exercise program (with elastic tubing) is an easy at-home exercise that has been shown to improve strength in plantar flexion, dorsiflexion, eversion, and inversion (TABLE 1).9-11
Phase 3: Once your patient is able to bear full weight with little to no pain, begin a balance program (TABLE 19-11). This is the most frequently neglected component of rehabilitation and the most common reason patients return with chronic ankle pain or repeat ankle injuries. Deficits in postural stability and balance have been reported in unstable ankles following acute ankle sprains,10,12-15 and studies have shown that individuals with poor stability are at a greater risk of injury.13-16
For most lateral ankle sprains, patients can expect time to recovery to range from 2 to 8 weeks. Longer recoveries are associated with more severe injuries or those that involve the syndesmosis.
Plantar fasciitis
Plantar fasciitis (PF) of the foot can be frustrating for a patient due to its chronic nature. Most patients will present with pain in the heel that is aggravated by weight-bearing activities. A conservative management program that focuses on reducing pain and inflammation, reducing tissue stress, and restoring strength and flexibility has been shown to be effective for this type of injury.17,18
Step 1: Reduce pain and inflammation. Deep-tissue massage and cryotherapy are easy ways to help with pain and inflammation. Deep-tissue massage can be accomplished by rolling the bottom of the foot on a golf or lacrosse ball. A favorite recommendation of ours to reduce inflammation is to use the ice-cup massage, mentioned earlier, for 5 minutes. Or rolling the bottom of the foot on a frozen water bottle will accomplish both tasks at once (TABLE 217,18).
Step 2: Reduce tissue stress. Management tools commonly used to reduce tissue stress are OTC orthotics and night splints. The night splint has been shown to improve symptoms,but patients often stop using it due to discomfort.19 Many kinds of night splints are available, but we have found that the sock variety with a strap to keep the foot in dorsiflexion is best tolerated, and it should be covered by most care plans.
Continue to: Step 3
Step 3: Restore muscle strength and flexibility. Restoring flexibility of the gastrocnemius and soleus is most frequently recommended for treating PF. Strengthening exercises that involve intrinsic and extrinsic muscles of the foot and ankle are also essential.17,18 Helpful exercises include those listed in TABLE 1.9-11 Additionally, an eccentric heel stretch can help to alleviate PF symptoms (TABLE 217,18).
A reasonable timeline for follow-up on newly diagnosed PF is 4 to 6 weeks. While many patients will not have recovered in that time, the goal is to document progress in recovery. If no progress is made, consider other treatment modalities.
Patellofemoral pain syndrome
Patellofemoral pain syndrome (PFPS) is one of the most common orthopedic complaints, estimated to comprise 7.3% of all orthopedic visits.20 Commonly called “runner’s knee,” PFPS is the leading cause of anterior knee pain in active individuals. Studies suggest a gender bias, with PFPS being diagnosed more frequently in females than in males, particularly between the ages of 10 and 19.20 Often, there is vague anterior knee pain, or pain that worsens with activities such as climbing hills or stairs, or with long sitting or when fatigued.
In general, unbalanced patellar tracking within the trochlear groove likely leads to this pain. Multiple contributory factors have been described; however, evidence increasingly has shown that deficiencies in hip strength may contribute significantly to maltracking of the patella with resultant pain. Specifically, weakness in hip external rotators and abductors is associated with abnormal lower extremity mechanics.21 One randomized controlled trial by Ferber et al found that therapy protocols directed at hip and core strength showed earlier resolution of pain and greater strength when compared with knee protocols alone.22
We routinely talk to patients about how the knee is the “victim” caught between weak hips and/or flat feet. It is prudent to look for both in the office visit. This can be done with one simple maneuver: Ask your patient to do a squat followed by 3 or 4 single-leg squats on each side. This will often reveal dysfunction at the foot/ankle or weakness in the hips/core as demonstrated by pronated feet (along with valgus tracking of the knees inward) or loss of balance upon squatting.
There is general consensus that a nonsurgical approach is the mainstay of treatment for PFPS.23 Pelvic stabilization and hip strengthening are standard components along with treatment protocols of exercises tailored to one’s individual weaknesses.
Numerous types of exercises do not require specialized equipment and can be taught in the office (TABLE 324). Explain to patients that the recovery process may take several months. Monthly follow-up to document progress is essential and helps to ensure compliance with one’s home program.
Continue to : Neck pain
Neck pain
The annual prevalence of nonspecific neck pain ranges from 27% to 48%, with 70% of individuals being afflicted at some time in their lives.25 First rule out any neurologic factors that might suggest cervical disc disease or spinal stenosis. If a patient describes weakness or sensory changes along one or both upper extremities, obtain imaging and consider more formalized therapy with a physical therapist.
In patients without any red flags, investigate possible biomechanical causes. It is essential to review the patient’s work and home habits, particularly in light of COVID-19, to determine if adjustments may be needed. Factors to consider are desk and computer setups at work or home, reading or laptop use in bed, sleep habits, and frequency of cellular phone calls/texting.26 A formal ergonomic assessment of the patient’s workplace may be helpful.
A mainstay in treating mechanical neck pain is alleviating trapezial tightness or spasm. Manipulative therapies such as osteopathic manipulation, massage, and chiropractic care can provide pain relief in the acute setting as well as help with control of chronic symptoms.27 A simple self-care tool is using a tennis ball to massage the trapezial muscles. This can be accomplished by having the patient position the tennis ball along the upper trapezial muscles, holding it in place by leaning against a wall, and initiating self-massage. Another method of self-massage is to put 2 tennis balls in an athletic tube sock and tie off the end, place the sock on the floor, and lie on it in the supine position.
There is also evidence that exercise of any kind can help control neck pain.28,29 The easiest exercises one can offer a patient with neck stiffness, or even mild cervical strains, is self-directed stretching through gentle pressure applied in all 4 directions on the neck. This technique can be repeated hourly both at work and at home (TABLE 4).
Reminders that can help ensure success
You can use the approaches described here for numerous other MSK conditions in helping patients on the road to recovery.
After the acute phase, advise patients to
• apply heat to the affected area before exercising. This can help bring blood flow to the region and promote ease of movement.
• continue icing the area following rehabilitation exercises in order to control exercise-induced inflammation.
• report any changing symptoms such as worsening pain, numbness, or weakness.
These techniques are one step in the recovery process. A home program can benefit the patient either alone or in combination with more advanced techniques that are best accomplished under the watchful eye of a physical or occupational therapist.
CORRESPONDENCE
Carrie A. Jaworski, MD, FAAFP, FACSM, 2180 Pfingsten Road, Suite 3100, Glenview, IL 60026; cjaworski@northshore.org
1. Hubbard TJ, Aronson SL, Denegar CR. Does cryotherapy hasten return to participation? A systematic review. J Athl Train. 2004;39:88-94.
2. Ho SS, Coel MN, Kagawa R, et al. The effects of ice on blood flow and bone metabolism in knees. Am J Sports Med. 1994;22:537-540.
3. Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad Med. 2015;127:57-65.
4. Bleakley CM, O’Connor S, Tully MA, et al. The PRICE study (Protection Rest Ice Compression Elevation): design of a randomised controlled trial comparing standard versus cryokinetic ice applications in the management of acute ankle sprain. BMC Musculoskelet Disord. 2007;8:125.
5. Mayer JM, Ralph L, Look M, et al. Treating acute low back pain with continuous low-level heat wrap therapy and/or exercise: a randomized controlled trial. Spine J. 2005;5:395-403.
6. Cetin N, Aytar A, Atalay A, et al. Comparing hot pack, short-wave diathermy, ultrasound, and TENS on isokinetic strength, pain, and functional status of women with osteoarthritic knees: a single-blind, randomized, controlled trial. Am J Phys Med Rehabil. 2008;87:443-451.
7. Waterman BR, Owens BD, Davey S, et al. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92:2279-2284.
8. Fong DT, Hong Y, Chan LK, et al. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007;37:73-94.
9. Kerkhoffs GM, Rowe BH, Assendelft WJ, et al. Immobilisation and functional treatment for acute lateral ankle ligament injuries in adults. Cochrane Database Syst Rev. 2002(3):CD003762.
10. Mattacola CG, Dwyer MK. Rehabilitation of the ankle after acute sprain or chronic instability. J Ath Train. 2002;37:413-429.
11. Hü bscher M, Zech A, Pfeifer K, et al. Neuromuscular training for sports injury prevention: a systematic review. Med Sci Sports Exerc. 2010;42:413-421.
12. Emery CA, Meeuwisse WH. The effectiveness of a neuromuscular prevention strategy to reduce injuries in youth soccer: a cluster-randomised controlled trial. Br J Sports Med. 2010;44:555-562.
13. Tiemstra JD. Update on acute ankle sprains. Am Fam Physician. 2012;85:1170-1176.
14. Beynnon BD, Murphy DF, Alosa DM. Predictive factors for lateral ankle sprains: a literature review. J Ath Train. 2002;37:376-380.
15. Schiftan GS, Ross LA, Hahne AJ. The effectiveness of proprioceptive training in preventing ankle sprains in sporting populations: a systematic review and meta-analysis. J Sci Med Sport. 2015;18:238–244.
16. Hupperets MD, Verhagen EA, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009;339:b2684
17. Thompson JV, Saini SS, Reb CW, et al. Diagnosis and management of plantar fasciitis. J Am Osteopath Assoc. 2014;114:900-906.
18. DiGiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fascia-specific stretching exercise improves outcomes in patients with chronic plantar fasciitis. A prospective clinical trial with two-year follow-up. J Bone Joint Surg Am. 2006;88:1775-1781.

19. Lee SY, McKeon P, Hertel J. Does the use of orthoses improve self-reported pain and function measures in patients with plantar fasciitis? A meta-analysis. Phys Ther Sport. 2009;10:12-18.
20. Glaviano NR, Key M, Hart JM, et al. Demographic and epidemiological trends in patellofemoral pain. J Sports Phys Ther. 2015;10: 281-290.
21. Louden JK. Biomechanics and pathomechanics of the patellofemoral joint. Int J Sports Phys Ther. 2016;11: 820-830.
22. Ferber R, Bolgla L, Earl-Boehm JE, et al. Strengthening of hip and core versus knee muscles for the treatment of patellofemoral pain: a multicenter randomized controlled trial. J Ath Train. 2015;50: 366-377.
23. Collins NJ, Bisset LM, Crossley KM, et al. Efficacy of nonsurgical interventions for anterior knee pain: systematic review and meta-analysis of randomized trials. Sports Med. 2013;41:31-49.
24. Bolgla LA. Hip strength and kinematics in patellofemoral syndrome. In: Brotzman SB, Manske RC eds. Clinical Orthopaedic Rehabilitation. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2011:273-274.
25. Hogg-Johnson S, van der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(suppl 4):S39-S51.
26. Larsson B, Søgaard K, Rosendal L. Work related neck-shoulder pain: a review on magnitude, risk factors, biochemical characteristics, clinical picture and preventive interventions. Best Pract Res Clin Rheumatol. 2007; 21:447-463.
27. Giles LG, Muller R. Chronic spinal pain: a randomized clinical trial comparing medication, acupuncture, and spinal manipulation. Spine. 2003;28:1490-1502.
28. Bronfort G, Evans R, Anderson A, et al. Spinal manipulation, medication, or home exercise with advice for acute and subacute neck pain: a randomized trial. Ann Intern Med. 2012;156:1-10.
29. Evans R, Bronfort G, Bittell S, et al. A pilot study for a randomized clinical trial assessing chiropractic care, medical care, and self-care education for acute and subacute neck pain patients. J Manipulative Physiol Ther. 2003;26:403-411.
1. Hubbard TJ, Aronson SL, Denegar CR. Does cryotherapy hasten return to participation? A systematic review. J Athl Train. 2004;39:88-94.
2. Ho SS, Coel MN, Kagawa R, et al. The effects of ice on blood flow and bone metabolism in knees. Am J Sports Med. 1994;22:537-540.
3. Malanga GA, Yan N, Stark J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad Med. 2015;127:57-65.
4. Bleakley CM, O’Connor S, Tully MA, et al. The PRICE study (Protection Rest Ice Compression Elevation): design of a randomised controlled trial comparing standard versus cryokinetic ice applications in the management of acute ankle sprain. BMC Musculoskelet Disord. 2007;8:125.
5. Mayer JM, Ralph L, Look M, et al. Treating acute low back pain with continuous low-level heat wrap therapy and/or exercise: a randomized controlled trial. Spine J. 2005;5:395-403.
6. Cetin N, Aytar A, Atalay A, et al. Comparing hot pack, short-wave diathermy, ultrasound, and TENS on isokinetic strength, pain, and functional status of women with osteoarthritic knees: a single-blind, randomized, controlled trial. Am J Phys Med Rehabil. 2008;87:443-451.
7. Waterman BR, Owens BD, Davey S, et al. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am. 2010;92:2279-2284.
8. Fong DT, Hong Y, Chan LK, et al. A systematic review on ankle injury and ankle sprain in sports. Sports Med. 2007;37:73-94.
9. Kerkhoffs GM, Rowe BH, Assendelft WJ, et al. Immobilisation and functional treatment for acute lateral ankle ligament injuries in adults. Cochrane Database Syst Rev. 2002(3):CD003762.
10. Mattacola CG, Dwyer MK. Rehabilitation of the ankle after acute sprain or chronic instability. J Ath Train. 2002;37:413-429.
11. Hü bscher M, Zech A, Pfeifer K, et al. Neuromuscular training for sports injury prevention: a systematic review. Med Sci Sports Exerc. 2010;42:413-421.
12. Emery CA, Meeuwisse WH. The effectiveness of a neuromuscular prevention strategy to reduce injuries in youth soccer: a cluster-randomised controlled trial. Br J Sports Med. 2010;44:555-562.
13. Tiemstra JD. Update on acute ankle sprains. Am Fam Physician. 2012;85:1170-1176.
14. Beynnon BD, Murphy DF, Alosa DM. Predictive factors for lateral ankle sprains: a literature review. J Ath Train. 2002;37:376-380.
15. Schiftan GS, Ross LA, Hahne AJ. The effectiveness of proprioceptive training in preventing ankle sprains in sporting populations: a systematic review and meta-analysis. J Sci Med Sport. 2015;18:238–244.
16. Hupperets MD, Verhagen EA, van Mechelen W. Effect of unsupervised home based proprioceptive training on recurrences of ankle sprain: randomised controlled trial. BMJ. 2009;339:b2684
17. Thompson JV, Saini SS, Reb CW, et al. Diagnosis and management of plantar fasciitis. J Am Osteopath Assoc. 2014;114:900-906.
18. DiGiovanni BF, Nawoczenski DA, Malay DP, et al. Plantar fascia-specific stretching exercise improves outcomes in patients with chronic plantar fasciitis. A prospective clinical trial with two-year follow-up. J Bone Joint Surg Am. 2006;88:1775-1781.

19. Lee SY, McKeon P, Hertel J. Does the use of orthoses improve self-reported pain and function measures in patients with plantar fasciitis? A meta-analysis. Phys Ther Sport. 2009;10:12-18.
20. Glaviano NR, Key M, Hart JM, et al. Demographic and epidemiological trends in patellofemoral pain. J Sports Phys Ther. 2015;10: 281-290.
21. Louden JK. Biomechanics and pathomechanics of the patellofemoral joint. Int J Sports Phys Ther. 2016;11: 820-830.
22. Ferber R, Bolgla L, Earl-Boehm JE, et al. Strengthening of hip and core versus knee muscles for the treatment of patellofemoral pain: a multicenter randomized controlled trial. J Ath Train. 2015;50: 366-377.
23. Collins NJ, Bisset LM, Crossley KM, et al. Efficacy of nonsurgical interventions for anterior knee pain: systematic review and meta-analysis of randomized trials. Sports Med. 2013;41:31-49.
24. Bolgla LA. Hip strength and kinematics in patellofemoral syndrome. In: Brotzman SB, Manske RC eds. Clinical Orthopaedic Rehabilitation. 3rd ed. Philadelphia, PA: Elsevier Mosby; 2011:273-274.
25. Hogg-Johnson S, van der Velde G, Carroll LJ, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000-2010 Task Force on Neck Pain and Its Associated Disorders. Spine. 2008;33(suppl 4):S39-S51.
26. Larsson B, Søgaard K, Rosendal L. Work related neck-shoulder pain: a review on magnitude, risk factors, biochemical characteristics, clinical picture and preventive interventions. Best Pract Res Clin Rheumatol. 2007; 21:447-463.
27. Giles LG, Muller R. Chronic spinal pain: a randomized clinical trial comparing medication, acupuncture, and spinal manipulation. Spine. 2003;28:1490-1502.
28. Bronfort G, Evans R, Anderson A, et al. Spinal manipulation, medication, or home exercise with advice for acute and subacute neck pain: a randomized trial. Ann Intern Med. 2012;156:1-10.
29. Evans R, Bronfort G, Bittell S, et al. A pilot study for a randomized clinical trial assessing chiropractic care, medical care, and self-care education for acute and subacute neck pain patients. J Manipulative Physiol Ther. 2003;26:403-411.
PRACTICE RECOMMENDATIONS
❯ Have patients apply ice to an acute injury for 15 to 20 minutes at a time to help control inflammation, and prescribe an anti-inflammatory medication, if indicated. A
❯ Reserve heat application for use following the acute phase of injury to decrease stiffness. A
❯ Instruct patients who have an acute lateral ankle sprain to begin “ankle ABCs” and other range-of-motion exercises once acute pain subsides. C
❯ Consider recommending an eccentric heel stretch to help alleviate plantar fasciitis symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
USPSTF update on sexually transmitted infections
In August 2020, the US Preventive Services Task Force published an update of its recommendation on preventing sexually transmitted infections (STIs) with behavioral counseling interventions.1
Whom to counsel. The USPSTF continues to recommend behavioral counseling for all sexually active adolescents and for adults at increased risk for STIs. Adults at increased risk include those who have been diagnosed with an STI in the past year, those with multiple sex partners or a sex partner at high risk for an STI, those not using condoms consistently, and those belonging to populations with high prevalence rates of STIs. These populations with high prevalence rates include1
- individuals seeking care at STI clinics,
- sexual and gender minorities, and
- those who are positive for human immunodeficiency virus (HIV), use injection drugs, exchange sex for drugs or money, or have recently been in a correctional facility.
Features of effective counseling. The Task Force recommends that primary care clinicians provide behavioral counseling or refer to counseling services or suggest media-based interventions. The most effective counseling interventions are those that span more than 120 minutes over several sessions. But the Task Force also states that counseling lasting about 30 minutes in a single session can also be effective. Counseling should include information about common STIs and their modes of transmission; encouragement in the use of safer sex practices; and training in proper condom use, how to communicate with partners about safer sex practices, and problem-solving. Various approaches to this counseling can be found at https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
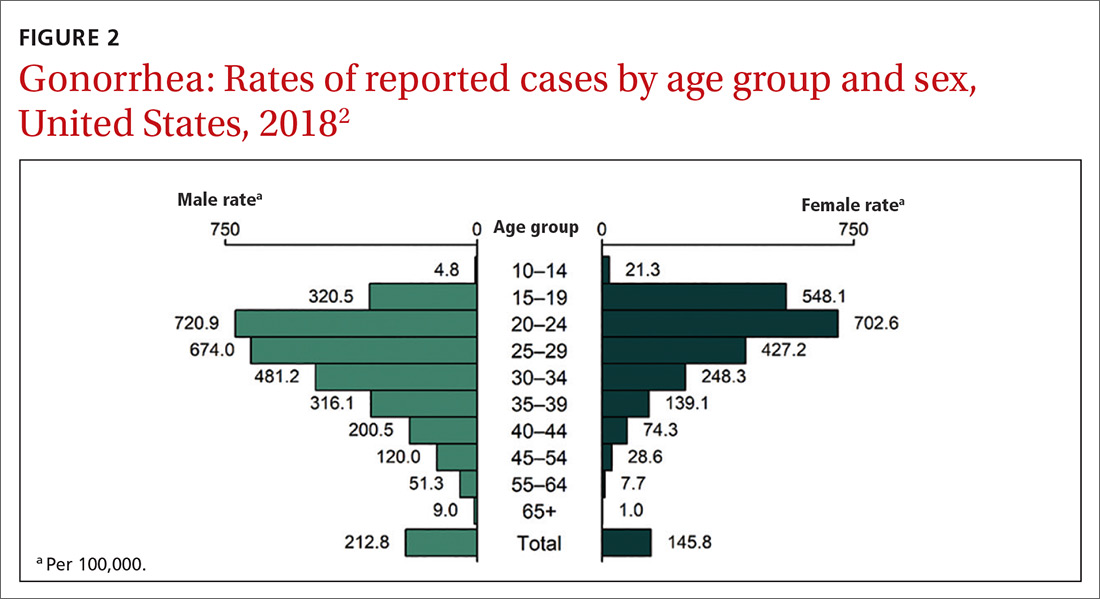
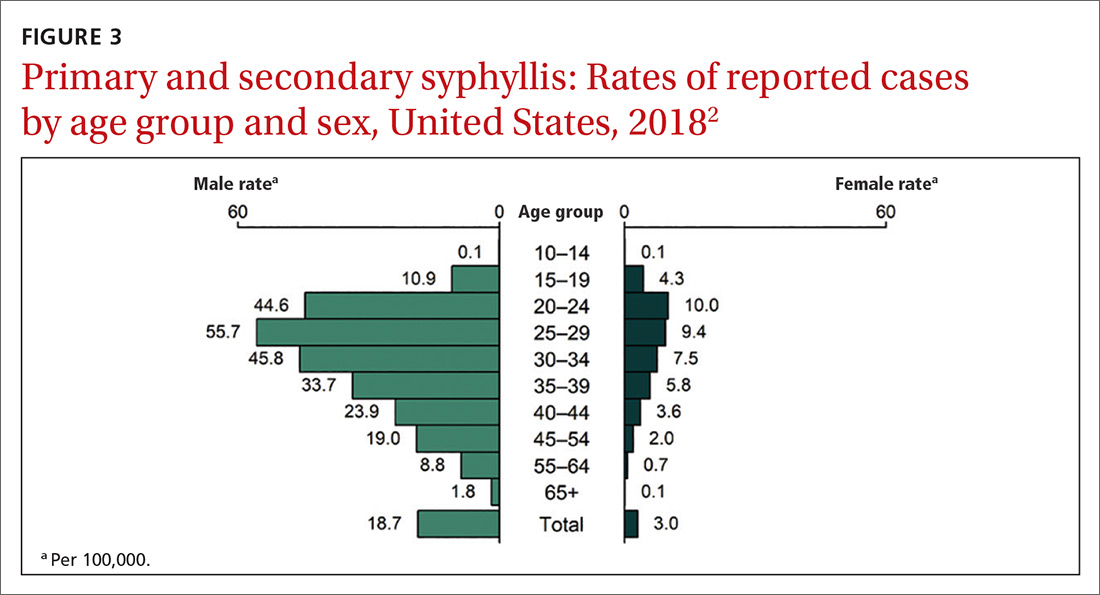
This updated recommendation is timely because most STIs in the United States have been increasing in incidence for the past decade or longer.2 Per 100,000 population, the total number of chlamydia cases since 2000 has risen from 251.4 to 539.9 (115%);gonorrhea cases since 2009 have risen from 98.1 to 179.1 (83%).3 And since 2000, the total number of reported syphilis cases per 100,000 has risen from 2.1 to 10.8 (414%).3
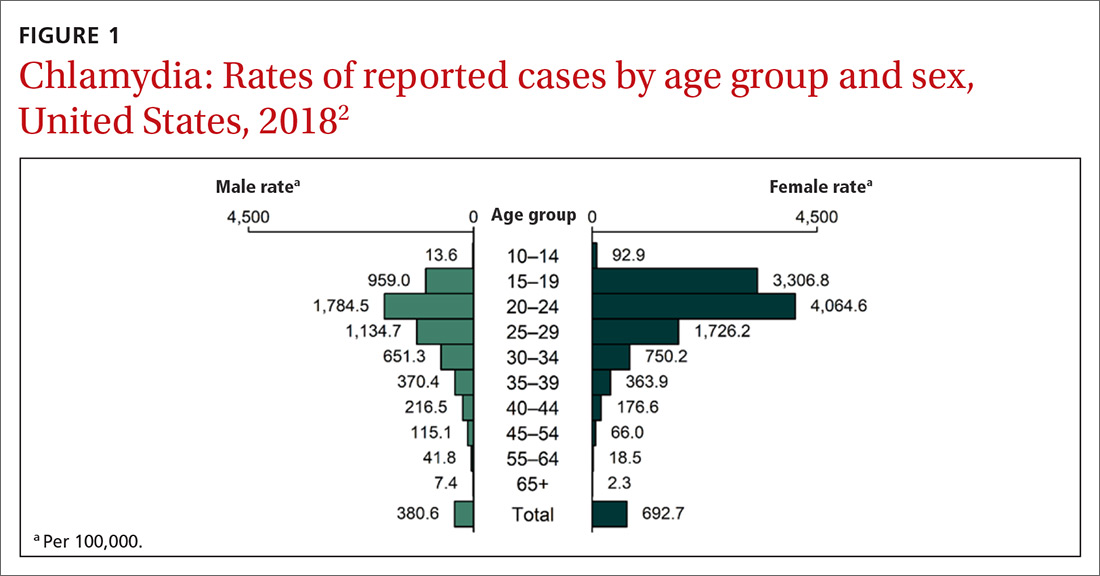
Chlamydia affects primarily those ages 15 to 24 years, with highest rates occurring in females (FIGURE 1).2 Gonorrhea affects women and men fairly evenly with slightly higher rates in men; the highest rates are seen in those ages 20 to 29 (FIGURE 2).2 Syphilis predominantly affects men who have sex with men, and the highest rates are in those ages 20 to 34 (FIGURE 3).2 In contrast to these upward trends, the number of HIV cases diagnosed has been relatively steady, with a slight downward trend over the past decade.4Other STIs that can be prevented through behavioral counseling include herpes simplex, human papillomavirus (HPV), hepatitis B virus (HBV) and trichomonas vaginalis.
Continue to: How to integrate STI preventioninto the primary care encounter
How to integrate STI preventioninto the primary care encounter
A key resource for learning to recognize the signs and symptoms of STIs, to correctly diagnose them, and to treat them according to CDC guidelines can be found at www.cdc.gov/std/tg2015/default.htm.5 Equally important is to integrate the prevention of STIs into the clinical routine by using a 4-step approach: risk assessment, risk reduction (counseling and chemoprevention), screening, and vaccination.
Risk assessment. The first step in prevention is taking a sexual history to accurately assess a patient’s risk for STIs. The CDC provides a tool (www.cdc.gov/std/products/provider-pocket-guides.htm) that can assist in gathering information in a nonjudgmental fashion about 5 Ps: partners, practices, protection from STIs, past history of STIs, and prevention of pregnancy.
Risk reduction. Following STI risk assessment, recommend risk-reduction interventions, as appropriate. Notable in the new Task Force recommendation are behavioral counseling methods that work. Additionally, when needed, pre-exposure prophylaxis with effective antiretroviral agents can be offered to those at high risk of HIV.6
Screening. Task Force recommendations for STI screening are described in the TABLE.7-12 Screening for HIV, chlamydia, gonorrhea, syphilis, and HBV are also recommended for pregnant women. And, although pregnant women are not specifically mentioned in the recommendation on chlamydia screening, it is reasonable to include it in prenatal care testing for STIs.
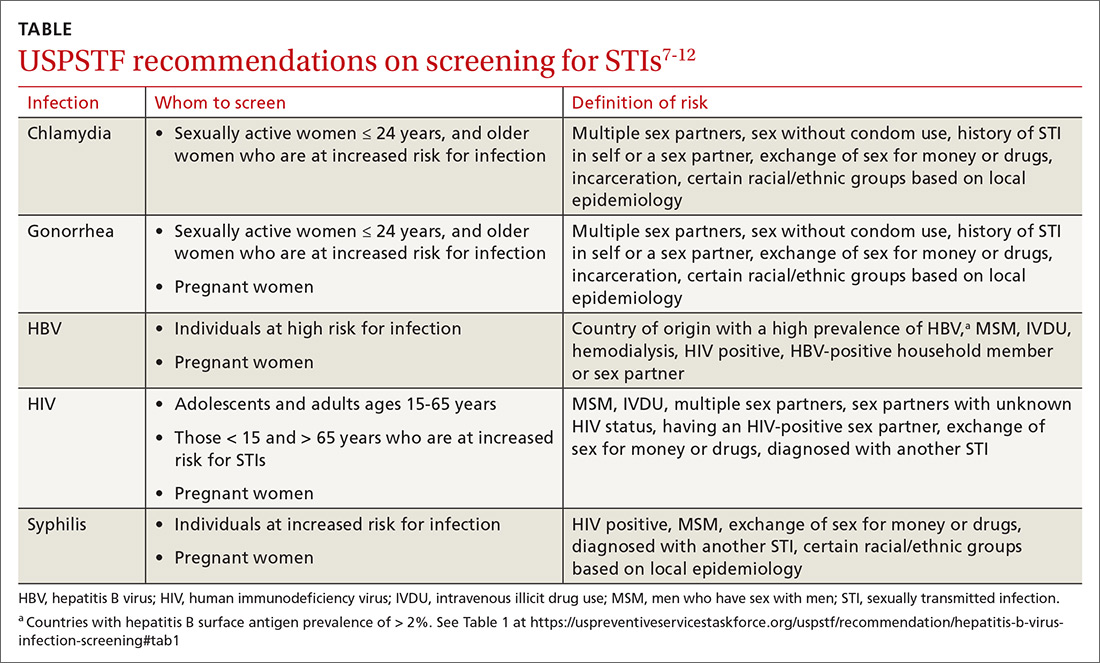
The Task Force has made an “I” statement regarding screening for gonorrhea and chlamydia in males. This does not mean that screening should be avoided, but only that there is insufficient evidence to support a firm statement regarding the harms and benefits in males. Keep in mind that this applies to asymptomatic males, and that testing and preventive treatment are warranted after documented exposure to either infection.
The Task Force recommends against screening for genital herpes, including in pregnant women, because of a lack of evidence of benefit from such screening, the high rate of false-positive tests, and the potential to cause anxiety and harm to personal relationships.
Continue to: Although hepatitis C virus...
Although hepatitis C virus (HCV) is transmitted mainly through intravenous drug use, it can also be transmitted sexually. The Task Force recommends screening for HCV in all adults ages 18 to 79 years.13
Vaccination. Two STIs can be prevented by immunizations: HPV and HBV. The current recommendations by the Advisory Committee on Immunization Practices (ACIP) are to vaccinate all infants with HBV vaccine and all unvaccinated children and adolescents through age 18.14 Unvaccinated adults who are at risk for HBV infection, including those at risk through sexual practices, should also be vaccinated.14
ACIP recommends routine HPV vaccination at age 11 or 12 years, but it can be started as early as 9 years.15 Catch-up vaccination is recommended for males and females through age 26 years.15 The vaccine is approved for use in individuals ages 27 through 45 years, but ACIP has not recommended it for routine use in this age group, and has instead recommended shared clinical decision-making to evaluate whether there is potential individual benefit from the vaccine.15
Public health implications
All STIs are reportable to local or state health departments. This is important for tracking community infection trends and, if resources are available, for contact notification and testing. In most jurisdictions, local health department resources are limited and contact tracing may be restricted to syphilis and HIV infections. When this is the case, it is especially important to instruct patients in whom STIs have been detected to notify their recent sex partners and advise them to be tested or preventively treated.
Expedited partner therapy (EPT)—providing treatment for exposed sexual contacts without a clinical encounter—is allowed in some states and is a tool that can prevent re-infection in the treated patient and suppress spread in the community. This is most useful for partners of those with gonorrhea, chlamydia, or trichomonas. The CDC has published guidance on how to implement EPT in a clinical setting if state law allows it.16
1. Henderson JT, Senger CA, Henninger M, et al. Behavioral counseling interventions to prevent sexually transmitted infections. JAMA. 2020;324:682-699.
2. CDC. Sexually transmitted disease surveillance, 2018. www.cdc.gov/std/stats18/slides.htm. Accessed November 25, 2020.
3. CDC. Sexually transmitted disease surveillance 2018. www.cdc.gov/std/stats18/tables/1.htm. Accessed November 25, 2020.
4. CDC. Estimated HIV incidence and prevalence in the United States (2010-2018). www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-surveillance-epidemiology-2018.pdf. Accessed November 25, 2020.
5. CDC. 2015 sexually transmitted disease treatment guidelines. www.cdc.gov/std/tg2015/default.htm. Accessed November 25, 2020.
6. USPSTF. Prevention of human immunodeficiency (HIV) infection: pre-exposure prophylaxis. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis. Accessed November 25, 2020.
7. LeFevre ML, U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:902-910. 8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed November 25, 2020.
9. Curry SJ, Krist AH, Owens DK, et al. Screening for syphilis in pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2018;320:911-917.
10. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336.
11. USPSTF. US Preventive Services Task Force issues draft recommendation statement on screening for hepatitis B virus infection in adolescents and adults. www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/hepatitis-b-nonpregnant-adults-draft-rs-bulletin.pdf. Accessed November 25, 2020.
12. Owens DK, Davidson KW, Krist AH, et al. Screening for Hepatitis B Virus Infection in Pregnant Women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2019;322:349-354.
13. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening. Accessed November 25, 2020. 14. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67;1-31.
15. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
16. CDC. Expedited partner therapy in the management of sexually transmitted diseases. www.cdc.gov/std/treatment/eptfinalreport2006.pdf. Accessed November 25, 2020.
In August 2020, the US Preventive Services Task Force published an update of its recommendation on preventing sexually transmitted infections (STIs) with behavioral counseling interventions.1
Whom to counsel. The USPSTF continues to recommend behavioral counseling for all sexually active adolescents and for adults at increased risk for STIs. Adults at increased risk include those who have been diagnosed with an STI in the past year, those with multiple sex partners or a sex partner at high risk for an STI, those not using condoms consistently, and those belonging to populations with high prevalence rates of STIs. These populations with high prevalence rates include1
- individuals seeking care at STI clinics,
- sexual and gender minorities, and
- those who are positive for human immunodeficiency virus (HIV), use injection drugs, exchange sex for drugs or money, or have recently been in a correctional facility.
Features of effective counseling. The Task Force recommends that primary care clinicians provide behavioral counseling or refer to counseling services or suggest media-based interventions. The most effective counseling interventions are those that span more than 120 minutes over several sessions. But the Task Force also states that counseling lasting about 30 minutes in a single session can also be effective. Counseling should include information about common STIs and their modes of transmission; encouragement in the use of safer sex practices; and training in proper condom use, how to communicate with partners about safer sex practices, and problem-solving. Various approaches to this counseling can be found at https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
This updated recommendation is timely because most STIs in the United States have been increasing in incidence for the past decade or longer.2 Per 100,000 population, the total number of chlamydia cases since 2000 has risen from 251.4 to 539.9 (115%);gonorrhea cases since 2009 have risen from 98.1 to 179.1 (83%).3 And since 2000, the total number of reported syphilis cases per 100,000 has risen from 2.1 to 10.8 (414%).3
Chlamydia affects primarily those ages 15 to 24 years, with highest rates occurring in females (FIGURE 1).2 Gonorrhea affects women and men fairly evenly with slightly higher rates in men; the highest rates are seen in those ages 20 to 29 (FIGURE 2).2 Syphilis predominantly affects men who have sex with men, and the highest rates are in those ages 20 to 34 (FIGURE 3).2 In contrast to these upward trends, the number of HIV cases diagnosed has been relatively steady, with a slight downward trend over the past decade.4Other STIs that can be prevented through behavioral counseling include herpes simplex, human papillomavirus (HPV), hepatitis B virus (HBV) and trichomonas vaginalis.



Continue to: How to integrate STI preventioninto the primary care encounter
How to integrate STI preventioninto the primary care encounter
A key resource for learning to recognize the signs and symptoms of STIs, to correctly diagnose them, and to treat them according to CDC guidelines can be found at www.cdc.gov/std/tg2015/default.htm.5 Equally important is to integrate the prevention of STIs into the clinical routine by using a 4-step approach: risk assessment, risk reduction (counseling and chemoprevention), screening, and vaccination.
Risk assessment. The first step in prevention is taking a sexual history to accurately assess a patient’s risk for STIs. The CDC provides a tool (www.cdc.gov/std/products/provider-pocket-guides.htm) that can assist in gathering information in a nonjudgmental fashion about 5 Ps: partners, practices, protection from STIs, past history of STIs, and prevention of pregnancy.
Risk reduction. Following STI risk assessment, recommend risk-reduction interventions, as appropriate. Notable in the new Task Force recommendation are behavioral counseling methods that work. Additionally, when needed, pre-exposure prophylaxis with effective antiretroviral agents can be offered to those at high risk of HIV.6
Screening. Task Force recommendations for STI screening are described in the TABLE.7-12 Screening for HIV, chlamydia, gonorrhea, syphilis, and HBV are also recommended for pregnant women. And, although pregnant women are not specifically mentioned in the recommendation on chlamydia screening, it is reasonable to include it in prenatal care testing for STIs.

The Task Force has made an “I” statement regarding screening for gonorrhea and chlamydia in males. This does not mean that screening should be avoided, but only that there is insufficient evidence to support a firm statement regarding the harms and benefits in males. Keep in mind that this applies to asymptomatic males, and that testing and preventive treatment are warranted after documented exposure to either infection.
The Task Force recommends against screening for genital herpes, including in pregnant women, because of a lack of evidence of benefit from such screening, the high rate of false-positive tests, and the potential to cause anxiety and harm to personal relationships.
Continue to: Although hepatitis C virus...
Although hepatitis C virus (HCV) is transmitted mainly through intravenous drug use, it can also be transmitted sexually. The Task Force recommends screening for HCV in all adults ages 18 to 79 years.13
Vaccination. Two STIs can be prevented by immunizations: HPV and HBV. The current recommendations by the Advisory Committee on Immunization Practices (ACIP) are to vaccinate all infants with HBV vaccine and all unvaccinated children and adolescents through age 18.14 Unvaccinated adults who are at risk for HBV infection, including those at risk through sexual practices, should also be vaccinated.14
ACIP recommends routine HPV vaccination at age 11 or 12 years, but it can be started as early as 9 years.15 Catch-up vaccination is recommended for males and females through age 26 years.15 The vaccine is approved for use in individuals ages 27 through 45 years, but ACIP has not recommended it for routine use in this age group, and has instead recommended shared clinical decision-making to evaluate whether there is potential individual benefit from the vaccine.15
Public health implications
All STIs are reportable to local or state health departments. This is important for tracking community infection trends and, if resources are available, for contact notification and testing. In most jurisdictions, local health department resources are limited and contact tracing may be restricted to syphilis and HIV infections. When this is the case, it is especially important to instruct patients in whom STIs have been detected to notify their recent sex partners and advise them to be tested or preventively treated.
Expedited partner therapy (EPT)—providing treatment for exposed sexual contacts without a clinical encounter—is allowed in some states and is a tool that can prevent re-infection in the treated patient and suppress spread in the community. This is most useful for partners of those with gonorrhea, chlamydia, or trichomonas. The CDC has published guidance on how to implement EPT in a clinical setting if state law allows it.16
In August 2020, the US Preventive Services Task Force published an update of its recommendation on preventing sexually transmitted infections (STIs) with behavioral counseling interventions.1
Whom to counsel. The USPSTF continues to recommend behavioral counseling for all sexually active adolescents and for adults at increased risk for STIs. Adults at increased risk include those who have been diagnosed with an STI in the past year, those with multiple sex partners or a sex partner at high risk for an STI, those not using condoms consistently, and those belonging to populations with high prevalence rates of STIs. These populations with high prevalence rates include1
- individuals seeking care at STI clinics,
- sexual and gender minorities, and
- those who are positive for human immunodeficiency virus (HIV), use injection drugs, exchange sex for drugs or money, or have recently been in a correctional facility.
Features of effective counseling. The Task Force recommends that primary care clinicians provide behavioral counseling or refer to counseling services or suggest media-based interventions. The most effective counseling interventions are those that span more than 120 minutes over several sessions. But the Task Force also states that counseling lasting about 30 minutes in a single session can also be effective. Counseling should include information about common STIs and their modes of transmission; encouragement in the use of safer sex practices; and training in proper condom use, how to communicate with partners about safer sex practices, and problem-solving. Various approaches to this counseling can be found at https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
This updated recommendation is timely because most STIs in the United States have been increasing in incidence for the past decade or longer.2 Per 100,000 population, the total number of chlamydia cases since 2000 has risen from 251.4 to 539.9 (115%);gonorrhea cases since 2009 have risen from 98.1 to 179.1 (83%).3 And since 2000, the total number of reported syphilis cases per 100,000 has risen from 2.1 to 10.8 (414%).3
Chlamydia affects primarily those ages 15 to 24 years, with highest rates occurring in females (FIGURE 1).2 Gonorrhea affects women and men fairly evenly with slightly higher rates in men; the highest rates are seen in those ages 20 to 29 (FIGURE 2).2 Syphilis predominantly affects men who have sex with men, and the highest rates are in those ages 20 to 34 (FIGURE 3).2 In contrast to these upward trends, the number of HIV cases diagnosed has been relatively steady, with a slight downward trend over the past decade.4Other STIs that can be prevented through behavioral counseling include herpes simplex, human papillomavirus (HPV), hepatitis B virus (HBV) and trichomonas vaginalis.



Continue to: How to integrate STI preventioninto the primary care encounter
How to integrate STI preventioninto the primary care encounter
A key resource for learning to recognize the signs and symptoms of STIs, to correctly diagnose them, and to treat them according to CDC guidelines can be found at www.cdc.gov/std/tg2015/default.htm.5 Equally important is to integrate the prevention of STIs into the clinical routine by using a 4-step approach: risk assessment, risk reduction (counseling and chemoprevention), screening, and vaccination.
Risk assessment. The first step in prevention is taking a sexual history to accurately assess a patient’s risk for STIs. The CDC provides a tool (www.cdc.gov/std/products/provider-pocket-guides.htm) that can assist in gathering information in a nonjudgmental fashion about 5 Ps: partners, practices, protection from STIs, past history of STIs, and prevention of pregnancy.
Risk reduction. Following STI risk assessment, recommend risk-reduction interventions, as appropriate. Notable in the new Task Force recommendation are behavioral counseling methods that work. Additionally, when needed, pre-exposure prophylaxis with effective antiretroviral agents can be offered to those at high risk of HIV.6
Screening. Task Force recommendations for STI screening are described in the TABLE.7-12 Screening for HIV, chlamydia, gonorrhea, syphilis, and HBV are also recommended for pregnant women. And, although pregnant women are not specifically mentioned in the recommendation on chlamydia screening, it is reasonable to include it in prenatal care testing for STIs.

The Task Force has made an “I” statement regarding screening for gonorrhea and chlamydia in males. This does not mean that screening should be avoided, but only that there is insufficient evidence to support a firm statement regarding the harms and benefits in males. Keep in mind that this applies to asymptomatic males, and that testing and preventive treatment are warranted after documented exposure to either infection.
The Task Force recommends against screening for genital herpes, including in pregnant women, because of a lack of evidence of benefit from such screening, the high rate of false-positive tests, and the potential to cause anxiety and harm to personal relationships.
Continue to: Although hepatitis C virus...
Although hepatitis C virus (HCV) is transmitted mainly through intravenous drug use, it can also be transmitted sexually. The Task Force recommends screening for HCV in all adults ages 18 to 79 years.13
Vaccination. Two STIs can be prevented by immunizations: HPV and HBV. The current recommendations by the Advisory Committee on Immunization Practices (ACIP) are to vaccinate all infants with HBV vaccine and all unvaccinated children and adolescents through age 18.14 Unvaccinated adults who are at risk for HBV infection, including those at risk through sexual practices, should also be vaccinated.14
ACIP recommends routine HPV vaccination at age 11 or 12 years, but it can be started as early as 9 years.15 Catch-up vaccination is recommended for males and females through age 26 years.15 The vaccine is approved for use in individuals ages 27 through 45 years, but ACIP has not recommended it for routine use in this age group, and has instead recommended shared clinical decision-making to evaluate whether there is potential individual benefit from the vaccine.15
Public health implications
All STIs are reportable to local or state health departments. This is important for tracking community infection trends and, if resources are available, for contact notification and testing. In most jurisdictions, local health department resources are limited and contact tracing may be restricted to syphilis and HIV infections. When this is the case, it is especially important to instruct patients in whom STIs have been detected to notify their recent sex partners and advise them to be tested or preventively treated.
Expedited partner therapy (EPT)—providing treatment for exposed sexual contacts without a clinical encounter—is allowed in some states and is a tool that can prevent re-infection in the treated patient and suppress spread in the community. This is most useful for partners of those with gonorrhea, chlamydia, or trichomonas. The CDC has published guidance on how to implement EPT in a clinical setting if state law allows it.16
1. Henderson JT, Senger CA, Henninger M, et al. Behavioral counseling interventions to prevent sexually transmitted infections. JAMA. 2020;324:682-699.
2. CDC. Sexually transmitted disease surveillance, 2018. www.cdc.gov/std/stats18/slides.htm. Accessed November 25, 2020.
3. CDC. Sexually transmitted disease surveillance 2018. www.cdc.gov/std/stats18/tables/1.htm. Accessed November 25, 2020.
4. CDC. Estimated HIV incidence and prevalence in the United States (2010-2018). www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-surveillance-epidemiology-2018.pdf. Accessed November 25, 2020.
5. CDC. 2015 sexually transmitted disease treatment guidelines. www.cdc.gov/std/tg2015/default.htm. Accessed November 25, 2020.
6. USPSTF. Prevention of human immunodeficiency (HIV) infection: pre-exposure prophylaxis. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis. Accessed November 25, 2020.
7. LeFevre ML, U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:902-910. 8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed November 25, 2020.
9. Curry SJ, Krist AH, Owens DK, et al. Screening for syphilis in pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2018;320:911-917.
10. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336.
11. USPSTF. US Preventive Services Task Force issues draft recommendation statement on screening for hepatitis B virus infection in adolescents and adults. www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/hepatitis-b-nonpregnant-adults-draft-rs-bulletin.pdf. Accessed November 25, 2020.
12. Owens DK, Davidson KW, Krist AH, et al. Screening for Hepatitis B Virus Infection in Pregnant Women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2019;322:349-354.
13. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening. Accessed November 25, 2020. 14. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67;1-31.
15. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
16. CDC. Expedited partner therapy in the management of sexually transmitted diseases. www.cdc.gov/std/treatment/eptfinalreport2006.pdf. Accessed November 25, 2020.
1. Henderson JT, Senger CA, Henninger M, et al. Behavioral counseling interventions to prevent sexually transmitted infections. JAMA. 2020;324:682-699.
2. CDC. Sexually transmitted disease surveillance, 2018. www.cdc.gov/std/stats18/slides.htm. Accessed November 25, 2020.
3. CDC. Sexually transmitted disease surveillance 2018. www.cdc.gov/std/stats18/tables/1.htm. Accessed November 25, 2020.
4. CDC. Estimated HIV incidence and prevalence in the United States (2010-2018). www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-surveillance-epidemiology-2018.pdf. Accessed November 25, 2020.
5. CDC. 2015 sexually transmitted disease treatment guidelines. www.cdc.gov/std/tg2015/default.htm. Accessed November 25, 2020.
6. USPSTF. Prevention of human immunodeficiency (HIV) infection: pre-exposure prophylaxis. https://uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis. Accessed November 25, 2020.
7. LeFevre ML, U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:902-910. 8. USPSTF. Syphilis infection in nonpregnant adults and adolescents: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/syphilis-infection-in-nonpregnant-adults-and-adolescents. Accessed November 25, 2020.
9. Curry SJ, Krist AH, Owens DK, et al. Screening for syphilis in pregnant women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2018;320:911-917.
10. Owens DK, Davidson KW, Krist AH, et al; US Preventive Services Task Force. Screening for HIV infection: US Preventive Services Task Force recommendation statement. JAMA. 2019;321:2326-2336.
11. USPSTF. US Preventive Services Task Force issues draft recommendation statement on screening for hepatitis B virus infection in adolescents and adults. www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/hepatitis-b-nonpregnant-adults-draft-rs-bulletin.pdf. Accessed November 25, 2020.
12. Owens DK, Davidson KW, Krist AH, et al. Screening for Hepatitis B Virus Infection in Pregnant Women: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2019;322:349-354.
13. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening. Accessed November 25, 2020. 14. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67;1-31.
15. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
16. CDC. Expedited partner therapy in the management of sexually transmitted diseases. www.cdc.gov/std/treatment/eptfinalreport2006.pdf. Accessed November 25, 2020.
A new model of care to return holism to family medicine
Here is our problem: Family medicine has allowed itself, and its patients, to be picked apart by the forces of reductionism and a system that profits from the sick and suffering. We have lost sight of our purpose and our vision to care for the whole person. We have lost our way as healers.
The result is not only a decline in the specialty of family medicine as a leader in primary care but declining value and worsening outcomes in health care overall. We need to get our mojo back. We can do this by focusing less on trying to be all things to all people at all times, and more on creating better models for preventing, managing, and reversing chronic disease. This means providing health care that is person centered, relationship based, recovery focused, and paid for comprehensively.
I call this model Advanced Primary Care, or APC (FIGURE). In this article, I describe exemplars of APC from across the United States. I also provide tools to help you recover its central feature, holism—care of the whole person in mind, body, community, and spirit—in your practice, thus returning us to the core purpose of family medicine.
Holism is central to family medicine
More than 40 years ago, psychiatrist George Engel, MD, published a seminal article in Science that inspired a radical vision of how health care should be practiced.1 Called the biopsychosocial model, it stated what, in some ways, is obvious: Human beings are complex organisms embedded in complex environments made up of distinct, yet interacting, dimensions. These dimensions included physical, psychological, and social components. Engel’s radical proposition was that these dimensions are definable and measurable and that good medicine cannot afford to ignore any of them.
Engel’s assertion that good medicine requires holism was a clarion call during a time of rapidly expanding knowledge and subspecialization. That call was the inspiration for a new medical specialty called family medicine, which dared to proclaim that the best way to heal was to care for the whole person within the context of that person’s emotional and social environment. Family medicine reinvigorated primary care and grew rapidly, becoming a preeminent primary care specialty in the United States.
Continue to : Reductionism is relentless
Reductionism is relentless
But the forces of medicine were—and still are—driving relentlessly the other way. The science of the small and particular (reductionism), with dazzling technology and exploding subspecialty knowledge, and backed by powerful economic drivers, rewards health care for pulling the patient and the medical profession apart. We pay more to those who treat small parts of a person over a short period than to those who attend to the whole person over the lifetime.
Today, family medicine—for all of its common sense, scientific soundness, connectedness to patients, and demonstrated value—struggles to survive.2-6 The holistic vision of Engel is declining. The struggle in primary care is that its holistic vision gets co-opted by specialized medical science—and then it desperately attempts to apply those small and specialized tools to the care of patients in their wholeness. Holism is largely dead in health care, and everyone pays the consequences.7
Health care is losing its value
The damage from this decline in holism is not just to primary care but to the value of health care in general. Most medical care being delivered today—comprising diagnosis, treatment, and payment (the innermost circle of the FIGURE)—is not producing good health.8 Only 15% to 20% of the healing of an individual or a population comes from health care.9 The rest—nearly 80%—comes from other factors rarely addressed in the health care system: behavioral and lifestyle choices that people make in their daily life, including those related to food, movement, sleep, stress, and substance use.10 Increasingly, it is the economic and social determinants of health that influence this behavior and have a greater impact on health and lifespan than physiology or genes.11 The same social determinants of health also influence patients’ ability to obtain medical care and pursue a meaningful life.12
The result of this decline in holism and in the value of health care in general has been a relentless rise in the cost of medical care13-15 and the need for social services; declining life expectancy16,17 and quality of life18; growing patient dissatisfaction; and burnout in providers.19,20 Health care has become, as investor and business leader Warren Buffet remarked, the “tapeworm” of the economy and a major contributor to growing disparities in health and well-being between the haves and have-nots.21 Engel’s prediction that good medicine cannot afford to ignore holism has come to pass.
3-step solution:Return to whole-person care
Family medicine needs to return to whole-person care, but it can do so only if it attends to, and effectively delivers on, the prevention, treatment, and reversal of chronic disease and the enhancement of health and well-being. This can happen only if family medicine stops trying to be all things to all people at all times and, instead, focuses on what matters to the patient as a person.
Continue to: This means that the core...
This means that the core interaction in family medicine must be to assess the whole person—mind, body, social, spirit—and help that person make changes that improve his/her/their health and well-being based on his/her/their individualized needs and social context. In other words, family medicine needs to deliver a holistic model of APC that is person centered, relationship based, recovery focused, and paid for comprehensively.
How does one get from “standard” primary care of today (the innermost circle of the FIGURE) to a framework that truly delivers on the promise of healing? I propose 3 steps to return holism to family medicine.
STEP 1: Start with comprehensive, coordinated primary care. We know that this works. Starfield and others demonstrated this 2 decades ago, defining and devising what we know as quality primary care—characterized by first-contact care, comprehensive primary care (CPC), continuous care, and coordinated care.22 This type of primary care improves outcomes, lowers costs, and is satisfying to patients and providers.23 The physician cares for the patient throughout that person’s entire life cycle and provides all evidence-based services needed to prevent and treat common conditions. Comprehensive primary care is positioned in the first circle outward from the innermost circle of the FIGURE.
As medicine has become increasingly complex and subspecialized, however, the ability to coordinate care is often frayed, adding cost and reducing quality.24-26 Today, comprehensive primary care needs enhanced coordination. At a minimum, this means coordinating services for:
- chronic disease management (outpatient and inpatient transitions and emergency department use)
- referral (specialists and tests)
- pharmacy services (including delivery and patient education support).
An example of a primary care system that meets these requirements is the Catalyst Health Network in central Texas, which supplies coordination services to more than 1000 comprehensive primary care practices and 1.5 million patients.27 The Catalyst Network makes money for those practices, saves money in the system, enhances patient and provider satisfaction, and improves population health in the community.27 I call this enhanced primary care (EPC), shown in the second circle out from the innermost circle of the FIGURE.
STEP 2: Add integrative medicine and mental health. EPC improves fragmented care but does not necessarily address a patient’s underlying determinants of healing. We know that health behaviors such as smoking cessation, avoidance of alcohol and drug abuse, improved diet, physical activity, sleep, and stress management contribute 40% to 60% of a person’s and a population’s health.10 In addition, evidence shows that behavioral health services, along with lifestyle change support, can even reverse many chronic diseases seen in primary care, such as obesity, diabetes, hypertension, cardiovascular disease, depression, and substance abuse.28,29
Continue to: Therefore, we need to add...
Therefore, we need to add routine mental health services and nonpharmacotherapeutic approaches (eg, complementary and alternative medicine) to primary care.30 Doing so requires that behavioral change and self-care become a central feature of the doctor–patient dialogue and team skills31 and be added to primary care.30,31 I call this integrative primary care (IPC), shown on the left side in the third circle out from the innermost circle of the FIGURE.
An example of IPC is Whole Health, an initiative of the US Veteran’s Health Administration. Whole Health empowers and informs a person-centered approach and integrates it into the delivery of routine care.32 Evaluation of Whole Health implementation, which involved more than 130,000 veterans followed for 2 years, found a net overall reduction in the total cost of care of 20%—saving nearly $650 million or, on average, more than $4500 per veteran.33
STEP 3: Address social determinants of health. Primary care will not fully be part of the solution for producing health and well-being unless it becomes instrumental in addressing the social determinants of health (SDH), defined as “… conditions in the environments in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.”34 These determinants include not only basic needs, such as housing, food, safety, and transportation (ie, social needs), but also what are known as structural determinants, such as income, education, language, and racial and ethnic bias. Health care cannot solve all of these social ills,but it is increasingly being called on to be the nexus of coordination for services that address these needs when they affect health outcomes.35,36
Examples of health systems that provide for social needs include the free “food prescription” program of Pennsylvania’s Geisinger Health System, for patients with diabetes who do not have the resources to pay for food.37 This approach improves blood glucose control by patients and saves money on medications and other interventions. Similarly, Kaiser Permanente has experimented with housing vouchers for homeless patients,and most Federally Qualified Health Centers provide bus or other transportation tickets to patients for their appointments and free or discounted tests and specialty care.38
Implementing whole-person care for all
I propose that we make APC the central focus of family medicine. This model would comprise CPC, plus EPC, IPC, and community coordination to address SDH. This is expressed as:
CPC + EPC + IPC + SDH = APC
Continue to: APC would mean...
APC would mean health for the whole person and for all people. Again, the FIGURE shows how this model, encompassing the entire third circle out from the center circle, could be created from current models of care.
How do we pay for this? We already do—and way too much. The problem is not lack of money in the health care system but how it is organized and distributed. The Centers for Medicare and Medicaid Services and other payers are developing value-based payment models to help cover this type of care,39 but payers cannot pay for something if it is unavailable.
Can family physicians deliver APC? I believe they can, and have given a few examples here to show how this is already happening. To help primary care providers start to deliver APC in their system, my team and I have built the HOPE (Healing Oriented Practices & Environments) Note Toolkit to use in daily practice.40 These and other tools are being used by a number of large hospital systems and health care networks around the country. (You can download the HOPE Note Toolkit, at no cost, at https://drwaynejonas.com/resources/hope-note/.)
Whatever we call this new type of primary care, it needs to care for the whole person and to be available to all. It finds expression in these assertions:
- We cannot ignore an essential part of what a human being is and expect them to heal or become whole.
- We cannot ignore essential people in our communities and expect our costs to go down or our compassion to go up.
- We need to stop allowing family medicine to be co-opted by reductionism and its profits.
In sum, we need a new vision of primary care—like Engel’s holistic vision in the 1970s—to motivate us, and we need to return to fundamental concepts of how healing works in medicine.41
CORRESPONDENCE
Wayne B. Jonas, MD, Samueli Integrative Health Programs, 1800 Diagonal Road, Suite 617, Alexandria, VA 22314; wayne@drwaynejonas.com.
1. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129-136.
2. Schwartz MD, Durning S, Linzer M, et al. Changes in medical students’ views of internal medicine careers from 1990 to 2007. Arch Intern Med. 2011;171:744-749.
3. Bronchetti ET, Christensen GS, Hoynes HW. Local food prices, SNAP purchasing power, and child health. Cambridge, MA: National Bureau of Economic Research. June 2018. www.nber.org/papers/w24762?mc_cid=8c7211d34b&mc_eid=fbbc7df813. Accessed November 24, 2020.
4. Federal Student Aid, US Department of Education. Public Service Loan Forgiveness (PSLF). 2018. https://studentaid.ed.gov/sa/repay-loans/forgiveness-cancellation/public-service. Accessed November 24, 2020.
5. Aten B, Figueroa E, Martin T. Notes on estimating the multi-year regional price parities by 16 expenditure categories: 2005-2009. WP2011-03. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; April 2011. www.bea.gov/system/files/papers/WP2011-3.pdf. Accessed November 24, 2020.
6. Aten BH, Figueroa EB, Martin TM. Regional price parities for states and metropolitan areas, 2006-2010. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; August 2012. https://apps.bea.gov/scb/pdf/2012/08%20August/0812_regional_price_parities.pdf. Accessed November 24, 2020.
7. Stange KC, Ferrer RL. The paradox of primary care. Ann Fam Med. 2009;7:293-299.
8. Panel on Understanding Cross-national Health Differences Among High-income Countries, Committee on Population, Division of Behavioral and Social Sciences and Education, and Board on Population Health and Public Health Practice, National Research Council and Institute of Medicine of the National Academies. US Health in International Perspective: Shorter Lives, Poorer Health. Woolf SH, Aron L, eds. The National Academies Press; 2013.
9. Hood CM, Gennuso KP, Swain GR, et al. County health rankings: relationships between determinant factors and health outcomes. Am J Prev Med. 2016;50:129-135.
10. McGinnis JM, Williams-Russo P, Knickman JR. The case for more active policy attention to health promotion. Health Aff (Millwood). 2002;21:78-93.
11. Roeder A. Zip code better predictor of health than genetic code. Harvard T. H. Chan School of Public Health Web site. News release. August 4, 2014. www.hsph.harvard.edu/news/features/zip-code-better-predictor-of-health-than-genetic-code/. Accessed November 24, 2020.

12. US health map. Seattle, WA: University of Washington Institute for Health Metrics and Evaluation; March 13, 2018. www.healthdata.org/data-visualization/us-health-map. Accessed November 24, 2020.
13. Highfill T. Comparing estimates of U.S. health care expenditures by medical condition, 2000-2012. Survey of Current Business. 2016;1-5. https://apps.bea.gov/scb/pdf/2016/3%20March/0316_comparing_u.s._health_care_expenditures_by_medical_condition.pdf. Accessed November 24, 2020.
14. Waters H, Graf M. The Costs of Chronic Disease in the US. Washington, DC: Milken Institute; August 2018. https://milkeninstitute.org/sites/default/files/reports-pdf/ChronicDiseases-HighRes-FINAL.pdf. Accessed November 24, 2020.
15. Meyer H. Health care spending will hit 19.4% of GDP in the next decade, CMS projects. Modern Health care. February 20, 2019. www.modernhealthcare.com/article/20190220/NEWS/190229989/healthcare-spending-will-hit-19-4-of-gdp-in-the-next-decade-cms-projects. Accessed November 24, 2020.
16. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
17. Basu S, Berkowitz SA, Phillips RL, et al. Association of primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179:506-514.
18. Zack MM, Moriarty DG, Stroup DF, et al. Worsening trends in adult health-related quality of life and self-rated health—United States, 1993–2001. Public Health Rep. 2004;119:493-505.
19. Windover AK, Martinez K, Mercer, MB, et al. Correlates and outcomes of physician burnout within a large academic medical center. Research letter. JAMA Intern Med. 2018;178:856-858.
20. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
21. Buffett: Health care is a tapeworm on the economic system. CNBC Squawk Box. February 26, 2018. www.cnbc.com/video/2018/02/26/buffett-health-care-is-a-tapeworm-on-the-economic-system.html. Accessed November 24, 2020.
22. Starfield B. Primary Care: Concept, Evaluation, and Policy. Oxford University Press; 1992.
23. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
24. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press (US); 2001.
25. Burton R. Health policy brief: improving care transitions. Health Affairs. September 13, 2012. www.healthaffairs.org/do/10.1377/hpb20120913.327236/full/healthpolicybrief_76.pdf. Accessed November 24, 2020.
26. Toulany A, Stukel TA, Kurdyak P, et al. Association of primary care continuity with outcomes following transition to adult care for adolescents with severe mental illness. JAMA Netw Open. 2019;2:e198415.
27. Helping communities thrive. Catalyst Health Network Web site. www.catalysthealthnetwork.com/. Accessed November 24, 2020.
28. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165-2171.
29. Scherger JE. Lean and Fit: A Doctor’s Journey to Healthy Nutrition and Greater Wellness. 2nd ed. Scotts Valley, CA: CreateSpace Publishing; 2016.
30. Qaseem A, Wilt TJ, McLean RM, et al; . Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
31. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
32. What is whole health? Washington, DC: US Department of Veterans Affairs. October 13, 2020. www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Accessed November 25, 2020.
33. COVER Commission. Creating options for veterans’ expedited recovery. Final report. Washington, DC: US Veterans Administration. January 24, 2020. www.va.gov/COVER/docs/COVER-Commission-Final-Report-2020-01-24.pdf. Accessed November 24, 2020.

34. Social determinants of health. Washington, DC: Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. HealthyPeople.gov Web site. www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. Accessed November 24, 2020.
35. Breslin E, Lambertino A. Medicaid and social determinants of health: adjusting payment and measuring health outcomes. Princeton University Woodrow Wilson School of Public and International Affairs, State Health and Value Strategies Program Web site. July 2017. www.shvs.org/wp-content/uploads/2017/07/SHVS_SocialDeterminants_HMA_July2017.pdf. Accessed November 24, 2020.
36. James CV. Actively addressing social determinants of health will help us achieve health equity. US Centers for Medicare & Medicaid Services Web site. April 26, 2019. www.cms.gov/blog/actively-addressing-social-determinants-health-will-help-us-achieve-health-equity. Accessed November 24, 2020.
37. Geisinger receives “Innovation in Advancing Health Equity” award. Geisinger Health Web site. April 24, 2018. www.geisinger.org/health-plan/news-releases/2018/04/23/19/28/geisinger-receives-innovation-in-advancing-health-equity-award. Accessed November 24, 2020.
38. Bresnick J. Kaiser Permanente launches full-network social determinants program. HealthITAnalytics Web site. May 6, 2019. https://healthitanalytics.com/news/kaiser-permanente-launches-full-network-social-determinants-program. Accessed November 25, 2020.
39. Medicare Payment Advisory Commission (MEDPAC). Physician and other health Professional services. In: Report to the Congress: Medicare Payment Policy. March 2016: 115-117. http://medpac.gov/docs/default-source/reports/chapter-4-physician-and-other-health-professional-services-march-2016-report-.pdf. Accessed November 24, 2020.
40. Jonas W. Helping patients with chronic diseases and conditions heal with the HOPE Note: integrative primary care case study. https://drwaynejonas.com/wp-content/uploads/2018/09/CS_HOPE-Note_FINAL.pdf. Accessed November 24, 2020.
41. Jonas W. How Healing Works. Berkley, CA: Lorena Jones Books; 2018.
Here is our problem: Family medicine has allowed itself, and its patients, to be picked apart by the forces of reductionism and a system that profits from the sick and suffering. We have lost sight of our purpose and our vision to care for the whole person. We have lost our way as healers.
The result is not only a decline in the specialty of family medicine as a leader in primary care but declining value and worsening outcomes in health care overall. We need to get our mojo back. We can do this by focusing less on trying to be all things to all people at all times, and more on creating better models for preventing, managing, and reversing chronic disease. This means providing health care that is person centered, relationship based, recovery focused, and paid for comprehensively.
I call this model Advanced Primary Care, or APC (FIGURE). In this article, I describe exemplars of APC from across the United States. I also provide tools to help you recover its central feature, holism—care of the whole person in mind, body, community, and spirit—in your practice, thus returning us to the core purpose of family medicine.
Holism is central to family medicine
More than 40 years ago, psychiatrist George Engel, MD, published a seminal article in Science that inspired a radical vision of how health care should be practiced.1 Called the biopsychosocial model, it stated what, in some ways, is obvious: Human beings are complex organisms embedded in complex environments made up of distinct, yet interacting, dimensions. These dimensions included physical, psychological, and social components. Engel’s radical proposition was that these dimensions are definable and measurable and that good medicine cannot afford to ignore any of them.
Engel’s assertion that good medicine requires holism was a clarion call during a time of rapidly expanding knowledge and subspecialization. That call was the inspiration for a new medical specialty called family medicine, which dared to proclaim that the best way to heal was to care for the whole person within the context of that person’s emotional and social environment. Family medicine reinvigorated primary care and grew rapidly, becoming a preeminent primary care specialty in the United States.
Continue to : Reductionism is relentless
Reductionism is relentless
But the forces of medicine were—and still are—driving relentlessly the other way. The science of the small and particular (reductionism), with dazzling technology and exploding subspecialty knowledge, and backed by powerful economic drivers, rewards health care for pulling the patient and the medical profession apart. We pay more to those who treat small parts of a person over a short period than to those who attend to the whole person over the lifetime.
Today, family medicine—for all of its common sense, scientific soundness, connectedness to patients, and demonstrated value—struggles to survive.2-6 The holistic vision of Engel is declining. The struggle in primary care is that its holistic vision gets co-opted by specialized medical science—and then it desperately attempts to apply those small and specialized tools to the care of patients in their wholeness. Holism is largely dead in health care, and everyone pays the consequences.7
Health care is losing its value
The damage from this decline in holism is not just to primary care but to the value of health care in general. Most medical care being delivered today—comprising diagnosis, treatment, and payment (the innermost circle of the FIGURE)—is not producing good health.8 Only 15% to 20% of the healing of an individual or a population comes from health care.9 The rest—nearly 80%—comes from other factors rarely addressed in the health care system: behavioral and lifestyle choices that people make in their daily life, including those related to food, movement, sleep, stress, and substance use.10 Increasingly, it is the economic and social determinants of health that influence this behavior and have a greater impact on health and lifespan than physiology or genes.11 The same social determinants of health also influence patients’ ability to obtain medical care and pursue a meaningful life.12
The result of this decline in holism and in the value of health care in general has been a relentless rise in the cost of medical care13-15 and the need for social services; declining life expectancy16,17 and quality of life18; growing patient dissatisfaction; and burnout in providers.19,20 Health care has become, as investor and business leader Warren Buffet remarked, the “tapeworm” of the economy and a major contributor to growing disparities in health and well-being between the haves and have-nots.21 Engel’s prediction that good medicine cannot afford to ignore holism has come to pass.
3-step solution:Return to whole-person care
Family medicine needs to return to whole-person care, but it can do so only if it attends to, and effectively delivers on, the prevention, treatment, and reversal of chronic disease and the enhancement of health and well-being. This can happen only if family medicine stops trying to be all things to all people at all times and, instead, focuses on what matters to the patient as a person.
Continue to: This means that the core...
This means that the core interaction in family medicine must be to assess the whole person—mind, body, social, spirit—and help that person make changes that improve his/her/their health and well-being based on his/her/their individualized needs and social context. In other words, family medicine needs to deliver a holistic model of APC that is person centered, relationship based, recovery focused, and paid for comprehensively.
How does one get from “standard” primary care of today (the innermost circle of the FIGURE) to a framework that truly delivers on the promise of healing? I propose 3 steps to return holism to family medicine.
STEP 1: Start with comprehensive, coordinated primary care. We know that this works. Starfield and others demonstrated this 2 decades ago, defining and devising what we know as quality primary care—characterized by first-contact care, comprehensive primary care (CPC), continuous care, and coordinated care.22 This type of primary care improves outcomes, lowers costs, and is satisfying to patients and providers.23 The physician cares for the patient throughout that person’s entire life cycle and provides all evidence-based services needed to prevent and treat common conditions. Comprehensive primary care is positioned in the first circle outward from the innermost circle of the FIGURE.
As medicine has become increasingly complex and subspecialized, however, the ability to coordinate care is often frayed, adding cost and reducing quality.24-26 Today, comprehensive primary care needs enhanced coordination. At a minimum, this means coordinating services for:
- chronic disease management (outpatient and inpatient transitions and emergency department use)
- referral (specialists and tests)
- pharmacy services (including delivery and patient education support).
An example of a primary care system that meets these requirements is the Catalyst Health Network in central Texas, which supplies coordination services to more than 1000 comprehensive primary care practices and 1.5 million patients.27 The Catalyst Network makes money for those practices, saves money in the system, enhances patient and provider satisfaction, and improves population health in the community.27 I call this enhanced primary care (EPC), shown in the second circle out from the innermost circle of the FIGURE.
STEP 2: Add integrative medicine and mental health. EPC improves fragmented care but does not necessarily address a patient’s underlying determinants of healing. We know that health behaviors such as smoking cessation, avoidance of alcohol and drug abuse, improved diet, physical activity, sleep, and stress management contribute 40% to 60% of a person’s and a population’s health.10 In addition, evidence shows that behavioral health services, along with lifestyle change support, can even reverse many chronic diseases seen in primary care, such as obesity, diabetes, hypertension, cardiovascular disease, depression, and substance abuse.28,29
Continue to: Therefore, we need to add...
Therefore, we need to add routine mental health services and nonpharmacotherapeutic approaches (eg, complementary and alternative medicine) to primary care.30 Doing so requires that behavioral change and self-care become a central feature of the doctor–patient dialogue and team skills31 and be added to primary care.30,31 I call this integrative primary care (IPC), shown on the left side in the third circle out from the innermost circle of the FIGURE.
An example of IPC is Whole Health, an initiative of the US Veteran’s Health Administration. Whole Health empowers and informs a person-centered approach and integrates it into the delivery of routine care.32 Evaluation of Whole Health implementation, which involved more than 130,000 veterans followed for 2 years, found a net overall reduction in the total cost of care of 20%—saving nearly $650 million or, on average, more than $4500 per veteran.33
STEP 3: Address social determinants of health. Primary care will not fully be part of the solution for producing health and well-being unless it becomes instrumental in addressing the social determinants of health (SDH), defined as “… conditions in the environments in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.”34 These determinants include not only basic needs, such as housing, food, safety, and transportation (ie, social needs), but also what are known as structural determinants, such as income, education, language, and racial and ethnic bias. Health care cannot solve all of these social ills,but it is increasingly being called on to be the nexus of coordination for services that address these needs when they affect health outcomes.35,36
Examples of health systems that provide for social needs include the free “food prescription” program of Pennsylvania’s Geisinger Health System, for patients with diabetes who do not have the resources to pay for food.37 This approach improves blood glucose control by patients and saves money on medications and other interventions. Similarly, Kaiser Permanente has experimented with housing vouchers for homeless patients,and most Federally Qualified Health Centers provide bus or other transportation tickets to patients for their appointments and free or discounted tests and specialty care.38
Implementing whole-person care for all
I propose that we make APC the central focus of family medicine. This model would comprise CPC, plus EPC, IPC, and community coordination to address SDH. This is expressed as:
CPC + EPC + IPC + SDH = APC
Continue to: APC would mean...
APC would mean health for the whole person and for all people. Again, the FIGURE shows how this model, encompassing the entire third circle out from the center circle, could be created from current models of care.
How do we pay for this? We already do—and way too much. The problem is not lack of money in the health care system but how it is organized and distributed. The Centers for Medicare and Medicaid Services and other payers are developing value-based payment models to help cover this type of care,39 but payers cannot pay for something if it is unavailable.
Can family physicians deliver APC? I believe they can, and have given a few examples here to show how this is already happening. To help primary care providers start to deliver APC in their system, my team and I have built the HOPE (Healing Oriented Practices & Environments) Note Toolkit to use in daily practice.40 These and other tools are being used by a number of large hospital systems and health care networks around the country. (You can download the HOPE Note Toolkit, at no cost, at https://drwaynejonas.com/resources/hope-note/.)
Whatever we call this new type of primary care, it needs to care for the whole person and to be available to all. It finds expression in these assertions:
- We cannot ignore an essential part of what a human being is and expect them to heal or become whole.
- We cannot ignore essential people in our communities and expect our costs to go down or our compassion to go up.
- We need to stop allowing family medicine to be co-opted by reductionism and its profits.
In sum, we need a new vision of primary care—like Engel’s holistic vision in the 1970s—to motivate us, and we need to return to fundamental concepts of how healing works in medicine.41
CORRESPONDENCE
Wayne B. Jonas, MD, Samueli Integrative Health Programs, 1800 Diagonal Road, Suite 617, Alexandria, VA 22314; wayne@drwaynejonas.com.
Here is our problem: Family medicine has allowed itself, and its patients, to be picked apart by the forces of reductionism and a system that profits from the sick and suffering. We have lost sight of our purpose and our vision to care for the whole person. We have lost our way as healers.
The result is not only a decline in the specialty of family medicine as a leader in primary care but declining value and worsening outcomes in health care overall. We need to get our mojo back. We can do this by focusing less on trying to be all things to all people at all times, and more on creating better models for preventing, managing, and reversing chronic disease. This means providing health care that is person centered, relationship based, recovery focused, and paid for comprehensively.
I call this model Advanced Primary Care, or APC (FIGURE). In this article, I describe exemplars of APC from across the United States. I also provide tools to help you recover its central feature, holism—care of the whole person in mind, body, community, and spirit—in your practice, thus returning us to the core purpose of family medicine.
Holism is central to family medicine
More than 40 years ago, psychiatrist George Engel, MD, published a seminal article in Science that inspired a radical vision of how health care should be practiced.1 Called the biopsychosocial model, it stated what, in some ways, is obvious: Human beings are complex organisms embedded in complex environments made up of distinct, yet interacting, dimensions. These dimensions included physical, psychological, and social components. Engel’s radical proposition was that these dimensions are definable and measurable and that good medicine cannot afford to ignore any of them.
Engel’s assertion that good medicine requires holism was a clarion call during a time of rapidly expanding knowledge and subspecialization. That call was the inspiration for a new medical specialty called family medicine, which dared to proclaim that the best way to heal was to care for the whole person within the context of that person’s emotional and social environment. Family medicine reinvigorated primary care and grew rapidly, becoming a preeminent primary care specialty in the United States.
Continue to : Reductionism is relentless
Reductionism is relentless
But the forces of medicine were—and still are—driving relentlessly the other way. The science of the small and particular (reductionism), with dazzling technology and exploding subspecialty knowledge, and backed by powerful economic drivers, rewards health care for pulling the patient and the medical profession apart. We pay more to those who treat small parts of a person over a short period than to those who attend to the whole person over the lifetime.
Today, family medicine—for all of its common sense, scientific soundness, connectedness to patients, and demonstrated value—struggles to survive.2-6 The holistic vision of Engel is declining. The struggle in primary care is that its holistic vision gets co-opted by specialized medical science—and then it desperately attempts to apply those small and specialized tools to the care of patients in their wholeness. Holism is largely dead in health care, and everyone pays the consequences.7
Health care is losing its value
The damage from this decline in holism is not just to primary care but to the value of health care in general. Most medical care being delivered today—comprising diagnosis, treatment, and payment (the innermost circle of the FIGURE)—is not producing good health.8 Only 15% to 20% of the healing of an individual or a population comes from health care.9 The rest—nearly 80%—comes from other factors rarely addressed in the health care system: behavioral and lifestyle choices that people make in their daily life, including those related to food, movement, sleep, stress, and substance use.10 Increasingly, it is the economic and social determinants of health that influence this behavior and have a greater impact on health and lifespan than physiology or genes.11 The same social determinants of health also influence patients’ ability to obtain medical care and pursue a meaningful life.12
The result of this decline in holism and in the value of health care in general has been a relentless rise in the cost of medical care13-15 and the need for social services; declining life expectancy16,17 and quality of life18; growing patient dissatisfaction; and burnout in providers.19,20 Health care has become, as investor and business leader Warren Buffet remarked, the “tapeworm” of the economy and a major contributor to growing disparities in health and well-being between the haves and have-nots.21 Engel’s prediction that good medicine cannot afford to ignore holism has come to pass.
3-step solution:Return to whole-person care
Family medicine needs to return to whole-person care, but it can do so only if it attends to, and effectively delivers on, the prevention, treatment, and reversal of chronic disease and the enhancement of health and well-being. This can happen only if family medicine stops trying to be all things to all people at all times and, instead, focuses on what matters to the patient as a person.
Continue to: This means that the core...
This means that the core interaction in family medicine must be to assess the whole person—mind, body, social, spirit—and help that person make changes that improve his/her/their health and well-being based on his/her/their individualized needs and social context. In other words, family medicine needs to deliver a holistic model of APC that is person centered, relationship based, recovery focused, and paid for comprehensively.
How does one get from “standard” primary care of today (the innermost circle of the FIGURE) to a framework that truly delivers on the promise of healing? I propose 3 steps to return holism to family medicine.
STEP 1: Start with comprehensive, coordinated primary care. We know that this works. Starfield and others demonstrated this 2 decades ago, defining and devising what we know as quality primary care—characterized by first-contact care, comprehensive primary care (CPC), continuous care, and coordinated care.22 This type of primary care improves outcomes, lowers costs, and is satisfying to patients and providers.23 The physician cares for the patient throughout that person’s entire life cycle and provides all evidence-based services needed to prevent and treat common conditions. Comprehensive primary care is positioned in the first circle outward from the innermost circle of the FIGURE.
As medicine has become increasingly complex and subspecialized, however, the ability to coordinate care is often frayed, adding cost and reducing quality.24-26 Today, comprehensive primary care needs enhanced coordination. At a minimum, this means coordinating services for:
- chronic disease management (outpatient and inpatient transitions and emergency department use)
- referral (specialists and tests)
- pharmacy services (including delivery and patient education support).
An example of a primary care system that meets these requirements is the Catalyst Health Network in central Texas, which supplies coordination services to more than 1000 comprehensive primary care practices and 1.5 million patients.27 The Catalyst Network makes money for those practices, saves money in the system, enhances patient and provider satisfaction, and improves population health in the community.27 I call this enhanced primary care (EPC), shown in the second circle out from the innermost circle of the FIGURE.
STEP 2: Add integrative medicine and mental health. EPC improves fragmented care but does not necessarily address a patient’s underlying determinants of healing. We know that health behaviors such as smoking cessation, avoidance of alcohol and drug abuse, improved diet, physical activity, sleep, and stress management contribute 40% to 60% of a person’s and a population’s health.10 In addition, evidence shows that behavioral health services, along with lifestyle change support, can even reverse many chronic diseases seen in primary care, such as obesity, diabetes, hypertension, cardiovascular disease, depression, and substance abuse.28,29
Continue to: Therefore, we need to add...
Therefore, we need to add routine mental health services and nonpharmacotherapeutic approaches (eg, complementary and alternative medicine) to primary care.30 Doing so requires that behavioral change and self-care become a central feature of the doctor–patient dialogue and team skills31 and be added to primary care.30,31 I call this integrative primary care (IPC), shown on the left side in the third circle out from the innermost circle of the FIGURE.
An example of IPC is Whole Health, an initiative of the US Veteran’s Health Administration. Whole Health empowers and informs a person-centered approach and integrates it into the delivery of routine care.32 Evaluation of Whole Health implementation, which involved more than 130,000 veterans followed for 2 years, found a net overall reduction in the total cost of care of 20%—saving nearly $650 million or, on average, more than $4500 per veteran.33
STEP 3: Address social determinants of health. Primary care will not fully be part of the solution for producing health and well-being unless it becomes instrumental in addressing the social determinants of health (SDH), defined as “… conditions in the environments in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks.”34 These determinants include not only basic needs, such as housing, food, safety, and transportation (ie, social needs), but also what are known as structural determinants, such as income, education, language, and racial and ethnic bias. Health care cannot solve all of these social ills,but it is increasingly being called on to be the nexus of coordination for services that address these needs when they affect health outcomes.35,36
Examples of health systems that provide for social needs include the free “food prescription” program of Pennsylvania’s Geisinger Health System, for patients with diabetes who do not have the resources to pay for food.37 This approach improves blood glucose control by patients and saves money on medications and other interventions. Similarly, Kaiser Permanente has experimented with housing vouchers for homeless patients,and most Federally Qualified Health Centers provide bus or other transportation tickets to patients for their appointments and free or discounted tests and specialty care.38
Implementing whole-person care for all
I propose that we make APC the central focus of family medicine. This model would comprise CPC, plus EPC, IPC, and community coordination to address SDH. This is expressed as:
CPC + EPC + IPC + SDH = APC
Continue to: APC would mean...
APC would mean health for the whole person and for all people. Again, the FIGURE shows how this model, encompassing the entire third circle out from the center circle, could be created from current models of care.
How do we pay for this? We already do—and way too much. The problem is not lack of money in the health care system but how it is organized and distributed. The Centers for Medicare and Medicaid Services and other payers are developing value-based payment models to help cover this type of care,39 but payers cannot pay for something if it is unavailable.
Can family physicians deliver APC? I believe they can, and have given a few examples here to show how this is already happening. To help primary care providers start to deliver APC in their system, my team and I have built the HOPE (Healing Oriented Practices & Environments) Note Toolkit to use in daily practice.40 These and other tools are being used by a number of large hospital systems and health care networks around the country. (You can download the HOPE Note Toolkit, at no cost, at https://drwaynejonas.com/resources/hope-note/.)
Whatever we call this new type of primary care, it needs to care for the whole person and to be available to all. It finds expression in these assertions:
- We cannot ignore an essential part of what a human being is and expect them to heal or become whole.
- We cannot ignore essential people in our communities and expect our costs to go down or our compassion to go up.
- We need to stop allowing family medicine to be co-opted by reductionism and its profits.
In sum, we need a new vision of primary care—like Engel’s holistic vision in the 1970s—to motivate us, and we need to return to fundamental concepts of how healing works in medicine.41
CORRESPONDENCE
Wayne B. Jonas, MD, Samueli Integrative Health Programs, 1800 Diagonal Road, Suite 617, Alexandria, VA 22314; wayne@drwaynejonas.com.
1. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129-136.
2. Schwartz MD, Durning S, Linzer M, et al. Changes in medical students’ views of internal medicine careers from 1990 to 2007. Arch Intern Med. 2011;171:744-749.
3. Bronchetti ET, Christensen GS, Hoynes HW. Local food prices, SNAP purchasing power, and child health. Cambridge, MA: National Bureau of Economic Research. June 2018. www.nber.org/papers/w24762?mc_cid=8c7211d34b&mc_eid=fbbc7df813. Accessed November 24, 2020.
4. Federal Student Aid, US Department of Education. Public Service Loan Forgiveness (PSLF). 2018. https://studentaid.ed.gov/sa/repay-loans/forgiveness-cancellation/public-service. Accessed November 24, 2020.
5. Aten B, Figueroa E, Martin T. Notes on estimating the multi-year regional price parities by 16 expenditure categories: 2005-2009. WP2011-03. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; April 2011. www.bea.gov/system/files/papers/WP2011-3.pdf. Accessed November 24, 2020.
6. Aten BH, Figueroa EB, Martin TM. Regional price parities for states and metropolitan areas, 2006-2010. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; August 2012. https://apps.bea.gov/scb/pdf/2012/08%20August/0812_regional_price_parities.pdf. Accessed November 24, 2020.
7. Stange KC, Ferrer RL. The paradox of primary care. Ann Fam Med. 2009;7:293-299.
8. Panel on Understanding Cross-national Health Differences Among High-income Countries, Committee on Population, Division of Behavioral and Social Sciences and Education, and Board on Population Health and Public Health Practice, National Research Council and Institute of Medicine of the National Academies. US Health in International Perspective: Shorter Lives, Poorer Health. Woolf SH, Aron L, eds. The National Academies Press; 2013.
9. Hood CM, Gennuso KP, Swain GR, et al. County health rankings: relationships between determinant factors and health outcomes. Am J Prev Med. 2016;50:129-135.
10. McGinnis JM, Williams-Russo P, Knickman JR. The case for more active policy attention to health promotion. Health Aff (Millwood). 2002;21:78-93.
11. Roeder A. Zip code better predictor of health than genetic code. Harvard T. H. Chan School of Public Health Web site. News release. August 4, 2014. www.hsph.harvard.edu/news/features/zip-code-better-predictor-of-health-than-genetic-code/. Accessed November 24, 2020.

12. US health map. Seattle, WA: University of Washington Institute for Health Metrics and Evaluation; March 13, 2018. www.healthdata.org/data-visualization/us-health-map. Accessed November 24, 2020.
13. Highfill T. Comparing estimates of U.S. health care expenditures by medical condition, 2000-2012. Survey of Current Business. 2016;1-5. https://apps.bea.gov/scb/pdf/2016/3%20March/0316_comparing_u.s._health_care_expenditures_by_medical_condition.pdf. Accessed November 24, 2020.
14. Waters H, Graf M. The Costs of Chronic Disease in the US. Washington, DC: Milken Institute; August 2018. https://milkeninstitute.org/sites/default/files/reports-pdf/ChronicDiseases-HighRes-FINAL.pdf. Accessed November 24, 2020.
15. Meyer H. Health care spending will hit 19.4% of GDP in the next decade, CMS projects. Modern Health care. February 20, 2019. www.modernhealthcare.com/article/20190220/NEWS/190229989/healthcare-spending-will-hit-19-4-of-gdp-in-the-next-decade-cms-projects. Accessed November 24, 2020.
16. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
17. Basu S, Berkowitz SA, Phillips RL, et al. Association of primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179:506-514.
18. Zack MM, Moriarty DG, Stroup DF, et al. Worsening trends in adult health-related quality of life and self-rated health—United States, 1993–2001. Public Health Rep. 2004;119:493-505.
19. Windover AK, Martinez K, Mercer, MB, et al. Correlates and outcomes of physician burnout within a large academic medical center. Research letter. JAMA Intern Med. 2018;178:856-858.
20. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
21. Buffett: Health care is a tapeworm on the economic system. CNBC Squawk Box. February 26, 2018. www.cnbc.com/video/2018/02/26/buffett-health-care-is-a-tapeworm-on-the-economic-system.html. Accessed November 24, 2020.
22. Starfield B. Primary Care: Concept, Evaluation, and Policy. Oxford University Press; 1992.
23. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
24. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press (US); 2001.
25. Burton R. Health policy brief: improving care transitions. Health Affairs. September 13, 2012. www.healthaffairs.org/do/10.1377/hpb20120913.327236/full/healthpolicybrief_76.pdf. Accessed November 24, 2020.
26. Toulany A, Stukel TA, Kurdyak P, et al. Association of primary care continuity with outcomes following transition to adult care for adolescents with severe mental illness. JAMA Netw Open. 2019;2:e198415.
27. Helping communities thrive. Catalyst Health Network Web site. www.catalysthealthnetwork.com/. Accessed November 24, 2020.
28. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165-2171.
29. Scherger JE. Lean and Fit: A Doctor’s Journey to Healthy Nutrition and Greater Wellness. 2nd ed. Scotts Valley, CA: CreateSpace Publishing; 2016.
30. Qaseem A, Wilt TJ, McLean RM, et al; . Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
31. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
32. What is whole health? Washington, DC: US Department of Veterans Affairs. October 13, 2020. www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Accessed November 25, 2020.
33. COVER Commission. Creating options for veterans’ expedited recovery. Final report. Washington, DC: US Veterans Administration. January 24, 2020. www.va.gov/COVER/docs/COVER-Commission-Final-Report-2020-01-24.pdf. Accessed November 24, 2020.

34. Social determinants of health. Washington, DC: Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. HealthyPeople.gov Web site. www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. Accessed November 24, 2020.
35. Breslin E, Lambertino A. Medicaid and social determinants of health: adjusting payment and measuring health outcomes. Princeton University Woodrow Wilson School of Public and International Affairs, State Health and Value Strategies Program Web site. July 2017. www.shvs.org/wp-content/uploads/2017/07/SHVS_SocialDeterminants_HMA_July2017.pdf. Accessed November 24, 2020.
36. James CV. Actively addressing social determinants of health will help us achieve health equity. US Centers for Medicare & Medicaid Services Web site. April 26, 2019. www.cms.gov/blog/actively-addressing-social-determinants-health-will-help-us-achieve-health-equity. Accessed November 24, 2020.
37. Geisinger receives “Innovation in Advancing Health Equity” award. Geisinger Health Web site. April 24, 2018. www.geisinger.org/health-plan/news-releases/2018/04/23/19/28/geisinger-receives-innovation-in-advancing-health-equity-award. Accessed November 24, 2020.
38. Bresnick J. Kaiser Permanente launches full-network social determinants program. HealthITAnalytics Web site. May 6, 2019. https://healthitanalytics.com/news/kaiser-permanente-launches-full-network-social-determinants-program. Accessed November 25, 2020.
39. Medicare Payment Advisory Commission (MEDPAC). Physician and other health Professional services. In: Report to the Congress: Medicare Payment Policy. March 2016: 115-117. http://medpac.gov/docs/default-source/reports/chapter-4-physician-and-other-health-professional-services-march-2016-report-.pdf. Accessed November 24, 2020.
40. Jonas W. Helping patients with chronic diseases and conditions heal with the HOPE Note: integrative primary care case study. https://drwaynejonas.com/wp-content/uploads/2018/09/CS_HOPE-Note_FINAL.pdf. Accessed November 24, 2020.
41. Jonas W. How Healing Works. Berkley, CA: Lorena Jones Books; 2018.
1. Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129-136.
2. Schwartz MD, Durning S, Linzer M, et al. Changes in medical students’ views of internal medicine careers from 1990 to 2007. Arch Intern Med. 2011;171:744-749.
3. Bronchetti ET, Christensen GS, Hoynes HW. Local food prices, SNAP purchasing power, and child health. Cambridge, MA: National Bureau of Economic Research. June 2018. www.nber.org/papers/w24762?mc_cid=8c7211d34b&mc_eid=fbbc7df813. Accessed November 24, 2020.
4. Federal Student Aid, US Department of Education. Public Service Loan Forgiveness (PSLF). 2018. https://studentaid.ed.gov/sa/repay-loans/forgiveness-cancellation/public-service. Accessed November 24, 2020.
5. Aten B, Figueroa E, Martin T. Notes on estimating the multi-year regional price parities by 16 expenditure categories: 2005-2009. WP2011-03. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; April 2011. www.bea.gov/system/files/papers/WP2011-3.pdf. Accessed November 24, 2020.
6. Aten BH, Figueroa EB, Martin TM. Regional price parities for states and metropolitan areas, 2006-2010. Washington, DC: Bureau of Economic Analysis, US Department of Commerce; August 2012. https://apps.bea.gov/scb/pdf/2012/08%20August/0812_regional_price_parities.pdf. Accessed November 24, 2020.
7. Stange KC, Ferrer RL. The paradox of primary care. Ann Fam Med. 2009;7:293-299.
8. Panel on Understanding Cross-national Health Differences Among High-income Countries, Committee on Population, Division of Behavioral and Social Sciences and Education, and Board on Population Health and Public Health Practice, National Research Council and Institute of Medicine of the National Academies. US Health in International Perspective: Shorter Lives, Poorer Health. Woolf SH, Aron L, eds. The National Academies Press; 2013.
9. Hood CM, Gennuso KP, Swain GR, et al. County health rankings: relationships between determinant factors and health outcomes. Am J Prev Med. 2016;50:129-135.
10. McGinnis JM, Williams-Russo P, Knickman JR. The case for more active policy attention to health promotion. Health Aff (Millwood). 2002;21:78-93.
11. Roeder A. Zip code better predictor of health than genetic code. Harvard T. H. Chan School of Public Health Web site. News release. August 4, 2014. www.hsph.harvard.edu/news/features/zip-code-better-predictor-of-health-than-genetic-code/. Accessed November 24, 2020.

12. US health map. Seattle, WA: University of Washington Institute for Health Metrics and Evaluation; March 13, 2018. www.healthdata.org/data-visualization/us-health-map. Accessed November 24, 2020.
13. Highfill T. Comparing estimates of U.S. health care expenditures by medical condition, 2000-2012. Survey of Current Business. 2016;1-5. https://apps.bea.gov/scb/pdf/2016/3%20March/0316_comparing_u.s._health_care_expenditures_by_medical_condition.pdf. Accessed November 24, 2020.
14. Waters H, Graf M. The Costs of Chronic Disease in the US. Washington, DC: Milken Institute; August 2018. https://milkeninstitute.org/sites/default/files/reports-pdf/ChronicDiseases-HighRes-FINAL.pdf. Accessed November 24, 2020.
15. Meyer H. Health care spending will hit 19.4% of GDP in the next decade, CMS projects. Modern Health care. February 20, 2019. www.modernhealthcare.com/article/20190220/NEWS/190229989/healthcare-spending-will-hit-19-4-of-gdp-in-the-next-decade-cms-projects. Accessed November 24, 2020.
16. Woolf SH, Schoomaker H. Life expectancy and mortality rates in the United States, 1959-2017. JAMA. 2019;322:1996-2016.
17. Basu S, Berkowitz SA, Phillips RL, et al. Association of primary care physician supply with population mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179:506-514.
18. Zack MM, Moriarty DG, Stroup DF, et al. Worsening trends in adult health-related quality of life and self-rated health—United States, 1993–2001. Public Health Rep. 2004;119:493-505.
19. Windover AK, Martinez K, Mercer, MB, et al. Correlates and outcomes of physician burnout within a large academic medical center. Research letter. JAMA Intern Med. 2018;178:856-858.
20. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
21. Buffett: Health care is a tapeworm on the economic system. CNBC Squawk Box. February 26, 2018. www.cnbc.com/video/2018/02/26/buffett-health-care-is-a-tapeworm-on-the-economic-system.html. Accessed November 24, 2020.
22. Starfield B. Primary Care: Concept, Evaluation, and Policy. Oxford University Press; 1992.
23. Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83:457-502.
24. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academies Press (US); 2001.
25. Burton R. Health policy brief: improving care transitions. Health Affairs. September 13, 2012. www.healthaffairs.org/do/10.1377/hpb20120913.327236/full/healthpolicybrief_76.pdf. Accessed November 24, 2020.
26. Toulany A, Stukel TA, Kurdyak P, et al. Association of primary care continuity with outcomes following transition to adult care for adolescents with severe mental illness. JAMA Netw Open. 2019;2:e198415.
27. Helping communities thrive. Catalyst Health Network Web site. www.catalysthealthnetwork.com/. Accessed November 24, 2020.
28. Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165-2171.
29. Scherger JE. Lean and Fit: A Doctor’s Journey to Healthy Nutrition and Greater Wellness. 2nd ed. Scotts Valley, CA: CreateSpace Publishing; 2016.
30. Qaseem A, Wilt TJ, McLean RM, et al; . Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166:514-530.
31. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
32. What is whole health? Washington, DC: US Department of Veterans Affairs. October 13, 2020. www.va.gov/patientcenteredcare/explore/about-whole-health.asp. Accessed November 25, 2020.
33. COVER Commission. Creating options for veterans’ expedited recovery. Final report. Washington, DC: US Veterans Administration. January 24, 2020. www.va.gov/COVER/docs/COVER-Commission-Final-Report-2020-01-24.pdf. Accessed November 24, 2020.

34. Social determinants of health. Washington, DC: Office of Disease Prevention and Health Promotion, US Department of Health and Human Services. HealthyPeople.gov Web site. www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. Accessed November 24, 2020.
35. Breslin E, Lambertino A. Medicaid and social determinants of health: adjusting payment and measuring health outcomes. Princeton University Woodrow Wilson School of Public and International Affairs, State Health and Value Strategies Program Web site. July 2017. www.shvs.org/wp-content/uploads/2017/07/SHVS_SocialDeterminants_HMA_July2017.pdf. Accessed November 24, 2020.
36. James CV. Actively addressing social determinants of health will help us achieve health equity. US Centers for Medicare & Medicaid Services Web site. April 26, 2019. www.cms.gov/blog/actively-addressing-social-determinants-health-will-help-us-achieve-health-equity. Accessed November 24, 2020.
37. Geisinger receives “Innovation in Advancing Health Equity” award. Geisinger Health Web site. April 24, 2018. www.geisinger.org/health-plan/news-releases/2018/04/23/19/28/geisinger-receives-innovation-in-advancing-health-equity-award. Accessed November 24, 2020.
38. Bresnick J. Kaiser Permanente launches full-network social determinants program. HealthITAnalytics Web site. May 6, 2019. https://healthitanalytics.com/news/kaiser-permanente-launches-full-network-social-determinants-program. Accessed November 25, 2020.
39. Medicare Payment Advisory Commission (MEDPAC). Physician and other health Professional services. In: Report to the Congress: Medicare Payment Policy. March 2016: 115-117. http://medpac.gov/docs/default-source/reports/chapter-4-physician-and-other-health-professional-services-march-2016-report-.pdf. Accessed November 24, 2020.
40. Jonas W. Helping patients with chronic diseases and conditions heal with the HOPE Note: integrative primary care case study. https://drwaynejonas.com/wp-content/uploads/2018/09/CS_HOPE-Note_FINAL.pdf. Accessed November 24, 2020.
41. Jonas W. How Healing Works. Berkley, CA: Lorena Jones Books; 2018.
PRACTICE RECOMMENDATIONS
❯ Build care teams into your practice so that you integrate “what matters” into the center of the clinical encounter. C
❯ Add practice approaches that help patients engage in healthy lifestyles and that remove social and economic barriers for improving health and well-being. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Home visits: A practical approach
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.
Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.
Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34
For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; curtis.elliott@uscmed.sc.edu.
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.
Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.
Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34
For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; curtis.elliott@uscmed.sc.edu.
CASE
Mr. A is a 30-year-old man with neurofibromatosis and myelopathy with associated quadriplegia, complicated by dysphasia and chronic hypercapnic respiratory failure requiring a tracheostomy. He is cared for at home by his very competent mother but requires regular visits with his medical providers for assistance with his complex care needs. Due to logistical challenges, he had been receiving regular home visits even before the COVID-19 pandemic.
After estimating the risk of exposure to the patient, Mr. A’s family and his physician’s office staff scheduled a home visit. Before the appointment, the doctor conducted a virtual visit with the patient and family members to screen for COVID-19 infection, which proved negative. The doctor arranged a visit to coincide with Mr. A’s regular appointment with the home health nurse. He invited the patient’s social worker to attend, as well.
The providers donned masks, face shields, and gloves before entering the home. Mr. A’s temperature was checked and was normal. The team completed a physical exam, assessed the patient’s current needs, and refilled prescriptions. The doctor, nurse, and social worker met afterward in the family’s driveway to coordinate plans for the patient’s future care.
This encounter allowed a vulnerable patient with special needs to have access to care while reducing his risk of undesirable exposure. Also, his health care team’s provision of care in the home setting reduced Mr. A’s anxiety and that of his family members.
Home visits have long been an integral part of what it means to be a family physician. In 1930, roughly 40% of all patient-physician encounters in the United States occurred in patients’ homes. By 1980, this number had dropped to < 1%.1 Still, a 1994 survey of American doctors in 3 primary care specialties revealed that 63% of family physicians, more than the other 2 specialties, still made house calls.2 A 2016 analysis of Medicare claims data showed that between 2006 and 2011, only 5% of American doctors overall made house calls on Medicare recipients, but interestingly, the total number of home visits was increasing.3
This resurgence of interest in home health care is due in part to the increasing number of homebound patients in America, which exceeds the number of those in nursing homes.4 Further, a growing body of evidence indicates that home visits improve patient outcomes. And finally, many family physicians whose work lives have been centered around a busy office or hospital practice have found satisfaction in once again seeing patients in their own homes.
The COVID-19 pandemic has of course presented unique challenges—and opportunities, too—for home visits, which we discuss at the end of the article.
Why aren’t more of us making home visits?
For most of us, the decision not to make home visits is simply a matter of time and money. Although Medicare reimbursement for a home visit is typically about 150% that of a comparable office visit,5 it’s difficult, if not impossible, to make 2 home visits in the time you could see 3 patients in the office. So, economically it’s a net loss. Furthermore, we tend to feel less comfortable in our patients’ homes than in our offices. We have less control outside our own environment, and what happens away from our office is often less predictable—sometimes to the point that we may be concerned for our safety.
Continue to: So why make home visits at all?
So why make home visits at all?
First and foremost, home visits improve patient outcomes. This is most evident in our more vulnerable patients: newborns and the elderly, those who have been recently hospitalized, and those at risk because of their particular home situation. Multiple studies have shown that, for elders, home visits reduce functional decline, nursing home admissions, and mortality by around 25% to 33%.6-8 For those at risk of abuse, a recent systematic review showed that home visits reduce intimate partner violence and child abuse.9 Another systematic review demonstrated that patients with diabetes who received home visits vs usual care were more likely to show improvements in quality of life.10 These patients were also more likely to have lower HbA1c levels and lower systolic blood pressure readings.10 A few caveats apply to these studies:
- all of them targeted “vulnerable” patients
- most studies enlisted interdisciplinary teams and had regular team meetings
- most findings reached significance only after multiple home visits.
A further reason for choosing to become involved in home care is that it builds relationships, understanding, and empathy with our patients. “There is deep symbolism in the home visit.... It says, ‘I care enough about you to leave my power base … to come and see you on your own ground.’”11 And this benefit is 2-way; we also grow to understand and appreciate our patients better, especially if they are different from us culturally or socioeconomically.
Home visits allow the medical team to see challenges the patient has grown accustomed to, and perhaps ones that the patient has deemed too insignificant to mention. For the patient, home visits foster a strong sense of trust with the individual doctor and our health delivery network, and they decrease the need to seek emergency services. Finally, it has been demonstrated that provider satisfaction improves when home visits are incorporated into the work week.12
What is the role of community health workers in home-based care?
Community health workers (CHWs), defined as “frontline public health workers who are trusted members of and/or have an unusually close understanding of the community they serve,”13 can be an integral part of the home-based care team. Although CHWs have variable amounts of formal training, they have a unique perspective on local health beliefs and practices, which can assist the home-care team in providing culturally competent health care services and reduce health care costs.
In a study of children with asthma in Seattle, Washington, patients were randomized to a group that had 4 home visits by CHWs and a group that received usual care. The group that received home visits demonstrated more asthma symptom–free days, improved quality-of-life scores, and fewer urgent care visits.14 Furthermore, the intervention was estimated to save approximately $1300 per patient, resulting in a return on investment of 190%. Similarly, in a study comparing inappropriate emergency department (ED) visits between children who received CHW visits and those who did not, patients in the intervention group were significantly less likely to visit the ED for ambulatory complaints (18.2% vs 35.1%; P = .004).15
Continue to: What is the role of social workersin home-based care?
What is the role of social workersin home-based care?
Social workers can help meet the complex medical and biopsychosocial needs of the homebound population.16 A study by Cohen et al based in Israel concluded that homebound participants had a significantly higher risk for mortality, higher rates of depression, and difficulty completing instrumental activities of daily living when compared with their non-homebound counterparts.17
The Mount Sinai (New York) Visiting Doctors Program (MSVD) is a home-based care team that uses social workers to meet the needs of their complex patients.18 The social workers in the MSVD program provide direct counseling, make referrals to government and community resources, and monitor caregiver burden. Using a combination of measurement tools to assess caregiver burden, Ornstein et al demonstrated that the MSVD program led to a decrease in unmet needs and in caregiver burden.19,20 Caregiver burnout can be assessed using the Caregiver Burden Inventory, a validated 24-item questionnaire.21
What electronic tools are availableto monitor patients at home?
Although expensive in terms of both dollars and personnel time, telemonitoring allows home care providers to receive real-time, updated information regarding their patients.
Chronic obstructive pulmonary disease (COPD). One systematic review showed that although telemonitoring of patients with COPD improved quality of life and decreased COPD exacerbations, it did not reduce the risk of hospitalization and, therefore, did not reduce health care costs.22 Telemonitoring in COPD can include transmission of data about spirometry parameters, weight, temperature, blood pressure, sputum color, and 6-minute walk distance.23,24
Congestive heart failure (CHF). A 2010 Cochrane review found that telemonitoring of patients with CHF reduced all-cause mortality (risk ratio [RR] = 0.66; P < .0001).25 The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial,conducted from 2013 to 2017, compared usual care for CHF patients with care incorporating daily transmission of body weight, blood pressure, heart rate, electrocardiogram tracings, pulse oximetry, and self-rated health status.26 This study showed that the average number of days lost per year due to hospital admission was less in the telemonitoring group than in the usual care group (17.8 days vs. 24.2 days; P = .046). All-cause mortality was also reduced in the telemonitoring group (hazard ratio = 0.70; P = .028).
Continue to: What role do “home hospitals” play?
What role do “home hospitals” play?
Home hospitals provide acute or subacute treatment in a patient’s home for a condition that would normally require hospitalization.27 In a meta-analysis of 61 studies evaluating the effectiveness of home hospitals, this option was more likely to reduce mortality (odds ratio [OR] = 0.81; P = .008) and to reduce readmission rates (OR = 0.75; P = .02).28 In a study of 455 older adults, Leff et al found that hospital-at-home was associated with a shorter length of stay (3.2 vs. 4.9 days; P = .004) and that the mean cost was lower for hospital-at-home vs traditional hospital care.29
However, a 2016 Cochrane review of 16 randomized controlled trials comparing hospital-at-home with traditional hospital care showed that while care in a hospital-at-home may decrease formal costs, if costs for caregivers are taken into account, any difference in cost may disappear.30
Although the evidence for cost saving is variable, hospital-at-home admission has been shown to reduce the likelihood of living in a residential care facility at 6 months (RR = 0.35; P < .0001).30 Further, the same Cochrane review showed that admission avoidance may increase patient satisfaction with the care provided.30
Finally, a recent randomized trial in a Boston-area hospital system showed that patients cared for in hospital-at-home were significantly less likely to be readmitted within 30 days and that adjusted cost was about two-thirds the cost of traditional hospital care.31
What is the physician’s rolein home health care?
While home health care is a team effort, the physician has several crucial roles. First, he or she must make the determination that home care is appropriate and feasible for a particular patient. Appropriate, meaning there is evidence that this patient is likely to benefit from home care. Feasible, meaning there are resources available in the community and family to safely care for the patient at home. “Often a house call will serve as the first step in developing a home-based-management plan.”32
Continue to: Second, the physician serves...
Second, the physician serves an important role in directing and coordinating the team of professionals involved. This primarily means helping the team to communicate with one another. Before home visits begin, the physician’s office should reach out not only to the patient and family, but also to any other health care personnel involved in the patient’s home care. Otherwise, many of the health care providers involved will never have face-to-face interaction with the physician. Creation of the coordinated health team minimizes duplication and miscommunication; it also builds a valuable bond.
How does one go about making a home visit?
Scheduling. What often works best in a busy practice is to schedule home visits for the end of the workday or to devote an entire afternoon to making home visits to several patients in one locale. Also important is scheduling times, if possible, when important family members or other caregivers are at home or when other members of the home care team can accompany you.
What to bring along. Carry a “home visit bag” that includes equipment you’re likely to need and that is not available away from your office. A minimally equipped visit bag would include different-sized blood pressure cuffs, a glucometer, a pulse oximeter, thermometers, and patient education materials. Other suggested contents are listed in TABLE 1.
Dos and don’ts. Take a few minutes when you first arrive to simply visit with the patient. Sit down and introduce yourself and any members of the home care team that the patient has not met. Take an interim history. While you’re doing this, be observant: Is the home neat or cluttered? Is the indoor temperature comfortable? Are there fall hazards? Is there a smell of cigarette smoke? Are there any indoor combustion sources (eg, wood stove or kerosene heater)? Ask questions such as: Who lives here with you? Can you show me where you keep your medicines? (If the patient keeps insulin or any other medicines in the refrigerator, ask to see it. Note any apparent food scarcity.)
During your exam, pay particular attention to whether vital signs are appreciably different than those measured in the office or hospital. Pay special attention to the patient’s functional abilities. “A subtle, but critical distinction between medical management in the home and medical management in the hospital, clinic, or office is the emphasis on the patient’s functional abilities, family assistance, and environmental factors.”33
Observe the patient’s use of any home technology, if possible; this can be as simple as home oxygenation or as complex as home hemodialysis. Assess for any apparent caregiver stress. Finally, don’t neglect to offer appropriate emotional and spiritual support to the patient and family and to schedule the next follow-up visit before you leave.
Continue to: Documentation and reimbursement.
Documentation and reimbursement. While individual electronic medical records may require use of particular forms of documentation, using a home visit template when possible can be extremely helpful (TABLE 2). A template not only assures thoroughness and consistency (pharmacy, home health contacts, billing information) but also serves as a prompt to survey the patient and the caregivers about nonmedical, but essential, social and well-being services. The document should be as simple and user-friendly as possible.
Not all assessments will be able to be done at each visit but seeing them listed in the template can be helpful. Billing follows the same principles as for office visits and has similar requirements for documentation. Codes for the most common types of home visits are listed in TABLE 3.
Where can I get help?
Graduates of family medicine residency programs are required to receive training in home visits by the Accreditation Council for Graduate Medical Education (ACGME). Current ACGME program requirements stipulate that “residents must demonstrate competence to independently diagnose, manage, and integrate the care of patients of all ages in various outpatient settings, including the FMP [family medicine practice] site and home environment,” and “residents must be primarily responsible for a panel of continuity patients, integrating each patient’s care across all settings, including the home ...” [emphasis added].34
For those already in practice, one of the hardest parts of doing home visits is feeling alone, especially if few other providers in your community engage in home care. As you run into questions and challenges with incorporating home care of patients into your practice, one excellent resource is the American Academy of Home Care Medicine (www.aahcm.org/). Founded in 1988 and headquartered in Chicago, it not only provides numerous helpful resources, but serves as a networking tool for physicians involved in home care.
This unprecedented pandemichas allowed home visits to shine
As depicted in our opening patient case, patients who have high-risk conditions and those who are older than 65 years of age may be cared for more appropriately in a home visit rather than having them come to the office. Home visits may also be a way for providers to “lay eyes” on patients who do not have technology available to participate in virtual visits.
Before performing a home visit, inquire as to whether the patient has symptoms of COVID-19. Adequate PPE should be donned at all times and social distancing should be practiced when appropriate. With adequate PPE, home visits may also allow providers to care for low-risk patients known to have COVID-19 and thereby minimize risks to staff and other patients in the office. JFP
CORRESPONDENCE
Curt Elliott, MD, Prisma Health USC Family Medicine Center, 3209 Colonial Drive, Columbia, SC 29203; curtis.elliott@uscmed.sc.edu.
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
1. Unwin BK, Tatum PE. House calls. Am Fam Physician. 2011;83:925-938.
3. Sairenji T, Jetty A, Peterson LE. Shifting patterns of physician home visits. J Prim Care Community Health. 2016;7:71-75.
4. Ornstein KA, Leff B, Covinsky K, et al. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175;1180-1186.
5. CMS. Current Procedural Terminology, Fourth Edition ("CPT®"). www.cms.gov/apps/physician-fee-schedule/license-agreement.aspx. Accessed November 30, 2020.
6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ. 2001;323:719-725.
7. Stuck AE, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people: systematic review and meta-regression analysis. JAMA. 2002;287:1022-1028.
8. Stall N, Nowaczynski M, Sinha SK. Systematic review of outcomes from home-based primary care programs for homebound older adults. J Am Geriatr Soc. 2014;62:2243-2251.
9. Prosman GJ, Lo Fo Wong SH, van der Wouden JC, et al. Effectiveness of home visiting in reducing partner violence for families experiencing abuse: a systematic review. Fam Pract. 2015;32:247-256.
10. Han L, Ma Y, Wei S, et al. Are home visits an effective method for diabetes management? A quantitative systematic review and meta-analysis. J Diabetes Investig. 2017;8:701-708.
11. McWhinney IR. Fourth annual Nicholas J. Pisacano Lecture. The doctor, the patient, and the home: returning to our roots. J Am Board Fam Pract. 1997;10:430-435.
12. Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34.
13. American Public Health Association. Community health workers. www.apha.org/apha-communities/member-sections/community-health-workers. Accessed November 30, 2020.
14. Campbell JD, Brooks M, Hosokawa P, et al. Community health worker home visits for Medicaid-enrolled children with asthma: effects on asthma outcomes and costs. Am J Public Health. 2015;105:2366-2372.
15. Anugu M, Braksmajer A, Huang J, et al. Enriched medical home intervention using community health worker home visitation and ED use. Pediatrics. 2017;139:e20161849.
16. Reckrey JM, Gettenberg G, Ross H, et al. The critical role of social workers in home-based primary care. Soc Work in Health Care. 2014;53:330-343.
17. Cohen-Mansfield J, Shmotkin D, Hazan H. The effect of homebound status on older persons. J Am Geriatr Soc. 2010;58:2358-2362.
18. Mt. Sinai Visiting Doctors Program. www.mountsinai.org/care/primary-care/upper-east-side/visiting-doctors/about. Accessed November 30, 2020.
19. Ornstein K, Hernandez CR, DeCherrie LV, et al. The Mount Sinai (New York) Visiting Doctors Program: meeting the needs of the urban homebound population. Care Manag J. 2011;12:159-163.
20. Ornstein K, Smith K, Boal J. Understanding and improving the burden and unmet needs of informal caregivers of homebound patients enrolled in a home-based primary care program. J Appl Gerontol. 2009;28:482-503.
21. Novak M, Guest C. Application of a multidimensional caregiver burden inventory. Gerontologist. 1989;29:798-803.
22. Cruz J, Brooks D, Marques A. Home telemonitoring effectiveness in COPD: a systematic review. Int J Clin Pract. 2014;68:369-378.
23. Antoniades NC, Rochford PD, Pretto JJ, et al. Pilot study of remote telemonitoring in COPD. Telemed J E Health. 2012;18:634-640.
24. Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031-1038.
25. Inglis SC, Clark RA, McAlister FA, et al. Which components of heart failure programmes are effective? A systematic review and meta-analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: abridged Cochrane review. Eur J Heart Fail. 2011;13:1028-1040.
26. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047-1057.
27. Ticona L, Schulman KA. Extreme home makeover–the role of intensive home health care. New Eng J Med. 2016;375:1707-1709.
28. Caplan GA. A meta-analysis of “hospital in the home.” Med J Aust. 2013;198:195-196.
29. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798-808.
30. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491.
31. Levine DM, Ouchi K, Blanchfield B, et al. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77-85.
32. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p18.
33. Cornwell T and Schwartzberg JG, eds. Medical Management of the Home Care Patient: Guidelines for Physicians. 4th ed. Chicago, IL: American Medical Association and American Academy of Home Care Physicians; 2012:p19.
34. Accreditation Council for Graduate Medical Education. ACGME Program Requirements for Graduate Medical Education in Family Medicine. www.acgme.org/Portals/0/PFAssets/ProgramRequirements/120_FamilyMedicine_2020.pdf. (section IV.C.1.b). Accessed November 30, 2020.
PRACTICE RECOMMENDATIONS
❯ Consider incorporating home visits into the primary care of select vulnerable patients because doing so improves clinical outcomes, including mortality rates in neonates and elders. A
❯ Employ team-based home care and include community health workers, nurses, pharmacists, social workers, chaplains, and others. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Whole-person care: Our foundation, our future
In this issue of The Journal of Family Practice, Dr. Wayne Jonas explains his model for Advanced Primary Care (see page 493). The figure he uses to illustrate Advanced Primary Care is compelling, and the effectiveness of this model of health care is supported by a great deal of research and evaluation over the past 20 years. Let me provide some historical context.
The idea that healing requires more than curative, biology-based medical care dates back to Greek mythology. Asclepius, the god of medicine, had 5 daughters, Hygeia (the goddess of good health and hygiene), Iaso (cures and remedies), Aceso (healing wounds), Aegle (radiant good health), and Panacea (cures).1 Clearly, the Greeks believed that integrative care is essential for maintaining good health!
Modern, scientific medicine is a relatively recent development in human history. Other traditions of healing such as acupuncture and herbal medicines are actually much older than mainstream Western medicine. But they come together in family medicine—a specialty founded on the principles of whole person, whole family, and whole community care.
The first modern model of comprehensive care, the patient-centered medical home (PCMH), was introduced by the American Academy of Pediatrics in 1967. This idea caught on widely and was institutionalized by the National Committee for Quality Assurance in 2008 with PCMH certification.
Advanced Primary Care is the latest and best rendition of comprehensive primary health care. Funding this model through our current payment mechanisms, however, has been difficult because of the need to support social and behavioral interventions in addition to medical care—areas of care not traditionally paid for by medical premiums. In 2011, CMS collaborated with private insurers in a national demonstration project to test the financial feasibility of implementing Advanced Primary Care. Some organizations have been highly successful; others not as much.
We can no longer go “halfway” into whole-person care. The COVID-19 pandemic has put a spotlight on our need to transform payment models away from fee-for-service to reimbursement for whole person primary care. Our nation’s health and the viability of our health care system depend on it.
PS: I recommend reading Dr. Jonas’ book, How Healing Works, which provides a scientific rationale for the application of whole-person care to healing.
1. Theoi Greek Mythology Web site. https://www.theoi.com/Ouranios/Asklepios.html. Accessed November 30, 2020.
In this issue of The Journal of Family Practice, Dr. Wayne Jonas explains his model for Advanced Primary Care (see page 493). The figure he uses to illustrate Advanced Primary Care is compelling, and the effectiveness of this model of health care is supported by a great deal of research and evaluation over the past 20 years. Let me provide some historical context.
The idea that healing requires more than curative, biology-based medical care dates back to Greek mythology. Asclepius, the god of medicine, had 5 daughters, Hygeia (the goddess of good health and hygiene), Iaso (cures and remedies), Aceso (healing wounds), Aegle (radiant good health), and Panacea (cures).1 Clearly, the Greeks believed that integrative care is essential for maintaining good health!
Modern, scientific medicine is a relatively recent development in human history. Other traditions of healing such as acupuncture and herbal medicines are actually much older than mainstream Western medicine. But they come together in family medicine—a specialty founded on the principles of whole person, whole family, and whole community care.
The first modern model of comprehensive care, the patient-centered medical home (PCMH), was introduced by the American Academy of Pediatrics in 1967. This idea caught on widely and was institutionalized by the National Committee for Quality Assurance in 2008 with PCMH certification.
Advanced Primary Care is the latest and best rendition of comprehensive primary health care. Funding this model through our current payment mechanisms, however, has been difficult because of the need to support social and behavioral interventions in addition to medical care—areas of care not traditionally paid for by medical premiums. In 2011, CMS collaborated with private insurers in a national demonstration project to test the financial feasibility of implementing Advanced Primary Care. Some organizations have been highly successful; others not as much.
We can no longer go “halfway” into whole-person care. The COVID-19 pandemic has put a spotlight on our need to transform payment models away from fee-for-service to reimbursement for whole person primary care. Our nation’s health and the viability of our health care system depend on it.
PS: I recommend reading Dr. Jonas’ book, How Healing Works, which provides a scientific rationale for the application of whole-person care to healing.
In this issue of The Journal of Family Practice, Dr. Wayne Jonas explains his model for Advanced Primary Care (see page 493). The figure he uses to illustrate Advanced Primary Care is compelling, and the effectiveness of this model of health care is supported by a great deal of research and evaluation over the past 20 years. Let me provide some historical context.
The idea that healing requires more than curative, biology-based medical care dates back to Greek mythology. Asclepius, the god of medicine, had 5 daughters, Hygeia (the goddess of good health and hygiene), Iaso (cures and remedies), Aceso (healing wounds), Aegle (radiant good health), and Panacea (cures).1 Clearly, the Greeks believed that integrative care is essential for maintaining good health!
Modern, scientific medicine is a relatively recent development in human history. Other traditions of healing such as acupuncture and herbal medicines are actually much older than mainstream Western medicine. But they come together in family medicine—a specialty founded on the principles of whole person, whole family, and whole community care.
The first modern model of comprehensive care, the patient-centered medical home (PCMH), was introduced by the American Academy of Pediatrics in 1967. This idea caught on widely and was institutionalized by the National Committee for Quality Assurance in 2008 with PCMH certification.
Advanced Primary Care is the latest and best rendition of comprehensive primary health care. Funding this model through our current payment mechanisms, however, has been difficult because of the need to support social and behavioral interventions in addition to medical care—areas of care not traditionally paid for by medical premiums. In 2011, CMS collaborated with private insurers in a national demonstration project to test the financial feasibility of implementing Advanced Primary Care. Some organizations have been highly successful; others not as much.
We can no longer go “halfway” into whole-person care. The COVID-19 pandemic has put a spotlight on our need to transform payment models away from fee-for-service to reimbursement for whole person primary care. Our nation’s health and the viability of our health care system depend on it.
PS: I recommend reading Dr. Jonas’ book, How Healing Works, which provides a scientific rationale for the application of whole-person care to healing.
1. Theoi Greek Mythology Web site. https://www.theoi.com/Ouranios/Asklepios.html. Accessed November 30, 2020.
1. Theoi Greek Mythology Web site. https://www.theoi.com/Ouranios/Asklepios.html. Accessed November 30, 2020.
17-year-old girl • abdominal pain • lower-leg itching • dark urine and yellow eyes • Dx?
THE CASE
A 17-year-old White girl with no known past medical history presented to the emergency department (ED) with complaints of abdominal pain and pruritus. The abdominal pain had started 9 days prior and lasted for 3 days. One day after resolution, she developed bilateral lower extremity itching, which was not relieved with loratadine.
Review of systems included dark urine and yellow eyes noted for several days. The patient denied nausea, vomiting, diarrhea, constipation, fevers, chills, arthralgias, recent illness, travel, or sick contacts. Immunizations were up to date. The patient had no history of surgery or liver disease and no pertinent family history. Her current medications included ethinyl estradiol/norethindrone acetate for birth control and minocycline for acne vulgaris. She had been taking the latter medication for 2 years. No additional medications were noted, including vitamins, over-the-counter medications, or supplements. She denied smoking and alcohol or recreational drug use.
In the ED, the patient had normal vital signs. Physical exam findings included bilateral scleral icterus and scattered skin excoriations on the hands, arms, back of the neck, and feet. At the time of hospital admission, the patient’s minocycline and birth control were held under the initial presumption that one or both might be contributing to her presentation.
Pertinent laboratory findings included aspartate transaminase (AST), 828 U/L (normal range, 2-40 U/L); alanine aminotransferase (ALT), 784 U/L (normal range, 3-30 U/L); lactic acid dehydrogenase, 520 U/L (normal range, 140-280 U/L); alkaline phosphatase, 119 U/L (normal range, 44-147 U/L); total bilirubin, 1.9 µmol/L (normal range, 2-18 µmol/L); and direct bilirubin, 1.3 µmol/L (normal range, 0-4 µmol/L). Baseline liver function test results (prior to admission) were unknown. Results of a coagulation panel, complete blood count, basic metabolic panel, amylase, lipase, urine toxicology, and urinalysis all were within normal limits.
Ultrasound of the abdomen revealed a normal abdomen, liver, pancreas, gallbladder, and common bile duct. This imaging study was negative for other obstructive pathologies.
THE DIAGNOSIS
During hospital admission, a noninvasive liver work-up was pursued by Gastroenterology. A hepatitis panel, Epstein-Barr virus testing, and levels of ceruloplasmin and acetaminophen were all found to be within normal limits, excluding additional causes of liver disease. Serum antinuclear antibody (ANA) testing was significantly positive, with a titer of 1:640 (range, < 1:20) and, as noted above, liver transaminases were severely elevated, leading to a presumptive diagnosis of drug-induced liver pathology.
Continue to: During outpatient follow-up...
During outpatient follow-up with Gastroenterology 2 days after discharge, the patient’s liver transaminases and bilirubin continued to trend upward (to a maximum ALT of 871 U/L; AST, 1097 U/L; alkaline phosphatase, 122 U/L; and bilirubin, 2.9 µmol/L). Immunoglobulin G was 1342 mg/mL (normal range, 694-1618 mg/mL).
An ultrasound-guided liver biopsy was performed; it demonstrated lobular, portal, and periportal hepatitis with focal bridging necrosis, consistent with a diagnosis of autoimmune hepatitis. Mild-to-moderate focal cholestasis was demonstrated, consistent with cholestatic hepatitis.
DISCUSSION
Autoimmune hepatitis is characterized by inflammation of the liver, secondary to the presence of circulating antibodies or hypergammaglobulinemia. The pathogenesis is thought to involve a T-cell–mediated immune attack on the liver. Based on case reports,the use of minocycline is associated with risk for liver injury, although the incidence is rare.1-4 Use of this medication may be associated with autoimmune disease in patients who are predisposed to autoimmune tendencies or who have genetic predeterminants.
Diagnosis is typically made based on abnormalities in aminotransferases (AST, ALT), elevation in serum immunoglobulins, and positive auto-antibody titers including ANA, smooth muscle antibodies, and anti-liver kidney microsomal type 1 antibodies. Although clinical presentations tend to differ, the confirmatory diagnosis is typically made histologically, with the presence of lobular and perivenular necro-inflammatory changes and plasma cell infiltration.5
Other infectious and metabolic causes of hepatitis should be excluded. Many medications and herbal agents have been noted to cause autoimmune hepatitis or similar syndromes that mimic the condition.
Medication history. Review of the case patient’s medication list identified ethinyl estradiol/norethindrone acetate and minocycline as potential culprits. Ethinyl estradiol/norethindrone acetate is a low-dose combination oral contraceptive pill (OCP). Although earlier formulations of OCPs were associated with hepatobiliary complications, these adverse effects are noted to be rare in the absence of predisposing conditions.6 In some cases, OCPs have been linked to cholestasis, chronic hepatocellular carcinoma, or hepatic adenomas, but studies have shown that these medications do not affect the course of acute liver failure.7
Continue to: Minocycline...
Minocycline is a second-generation tetracycline commonly used to treat acne vulgaris. Long-term treatment with minocycline has been associated with severe adverse effects, including autoimmune and hypersensitivity reactions.8 Minocycline-associated hepatotoxicity can be due to a systemic hypersensitivity reaction, occurring within a few weeks of therapy initiation, whereas autoimmune hepatitis manifests after a year or more of exposure to the medication (as in this case). Patients may present acutely several months after starting the medication, with symptoms of jaundice, fatigue, and/or joint aches. The acute liver injury is typically self-limited and often resolves with cessation of the drug. However, patients may require corticosteroids and immunosuppressive therapy.
Which is it? Histologically, drug-induced autoimmune hepatitis is indistinguishable from idiopathic autoimmune hepatitis.3 The estimated incidence of idiopathic autoimmune liver disease ranges from 0.7 to 2 out of 100,000 population.9 A systematic review of the literature identified 65 reported cases of liver damage associated with minocycline specifically.1
In this case, given the patient’s 2-year history of minocycline use, it is possible that she developed an acute presentation of autoimmune hepatitis. With drug-induced autoimmune liver injury, complete resolution occurs after withdrawal of the offending medication, and a response to corticosteroid therapy supports the diagnosis. Recurrence of signs or symptoms following corticosteroid cessation may indicate idiopathic autoimmune hepatitis as opposed to a drug-induced form.2
Our patient was started on steroid and immunomodulator therapy, with prednisone 40 mg/d and mycophenolate 250 mg bid. At follow-up with Gastroenterology, the patient’s symptoms and liver function test results had improved significantly (AST, 27 U/L; ALT, 14 U/L; alkaline phosphatase, 51 U/L; and total bilirubin, 0.4 µmol/L). The patient was continued on a prednisone taper while simultaneously titrating mycophenolate. The ultimate plan of care included continuing mycophenolate for a total of 4 to 5 years.
THE TAKEAWAY
During evaluation of a patient with new-onset liver disease, it is important to inquire about prescription medications, drugs, vitamins, and herbal supplements as possible contributors to the disease process. This case highlights the importance of monitoring patients while on minocycline and of weighing the risks vs benefits of long-term therapy. It has been suggested that liver enzymes be tested before therapy initiation and about every 3 months during long-term antibiotic treatment.4 Careful consideration and caution should be taken prior to the initiation of medications that have been linked to rare, but important, adverse reactions.
ACKNOWLEDGEMENT
The authors would like to thank Frank Bauer, MD, and Eva Sotil, MD, for their contributions to this case presentation.
CORRESPONDENCE
Andrea Gillis, DO, Asylum Hill Family Medicine Center, 99 Woodland Street, Hartford, CT 06105; andrea.gillis@ trinityhealthofne.org
1. Lawrenson RA, Seaman HE, Sundström A, et al. Liver damage associated with minocycline use in acne: a systematic review of the published literature and pharmacovigilance data. Drug Saf. 2000;23:333-349.
2. Teitelbaum JE, Perez-Atayde AR, Cohen M, et al. Minocycline-related autoimmune hepatitis case series and literature review. Arch Pediatr Adolesc Med. 1998;152:1132-1136.
3. Goldstein NS, Bayati N, Silverman AL, et al. Minocycline as a cause of drug induced autoimmune hepatitis: report of four cases and comparison with autoimmune hepatitis. Am J Clinic Pathol. 2000;114:591-598.
4. Ramakrishna J, Johnson AR, Banner BF. Long-term minocycline use for acne in healthy adolescents can cause severe autoimmune hepatitis. J Clin Gastroenterol. 2009;43:787-790.
5. Nguyen Canh H, Harada K, Ouchi H, et al. Acute presentation of autoimmune hepatitis: a multicentre study with detailed histological evaluation in a large cohort of patients. J Clin Pathol. 2017;70:961-969.
6. Lindberg MC. Hepatobiliary complications of oral contraceptives. J Gen Intern Med. 1992; 7:199-209.
7. Kapp N, Tilley IB, Curtis KM. The effects of hormonal contraceptive use among women with viral hepatitis or cirrhosis of the liver: a systematic review. Contraception. 2009;80:381-386.
8. DeLemos AS, Foureau DM, Jacobs C, et al. Drug-induced liver injury with autoimmune features. Semin Liver Dis. 2014;34:194-204.
9. Jepsen P, Gronbaek L, Vilstrup H. Worldwide incidence of autoimmune liver disease. Dig Dis. 2015;33(suppl 2):2-12.
THE CASE
A 17-year-old White girl with no known past medical history presented to the emergency department (ED) with complaints of abdominal pain and pruritus. The abdominal pain had started 9 days prior and lasted for 3 days. One day after resolution, she developed bilateral lower extremity itching, which was not relieved with loratadine.
Review of systems included dark urine and yellow eyes noted for several days. The patient denied nausea, vomiting, diarrhea, constipation, fevers, chills, arthralgias, recent illness, travel, or sick contacts. Immunizations were up to date. The patient had no history of surgery or liver disease and no pertinent family history. Her current medications included ethinyl estradiol/norethindrone acetate for birth control and minocycline for acne vulgaris. She had been taking the latter medication for 2 years. No additional medications were noted, including vitamins, over-the-counter medications, or supplements. She denied smoking and alcohol or recreational drug use.
In the ED, the patient had normal vital signs. Physical exam findings included bilateral scleral icterus and scattered skin excoriations on the hands, arms, back of the neck, and feet. At the time of hospital admission, the patient’s minocycline and birth control were held under the initial presumption that one or both might be contributing to her presentation.
Pertinent laboratory findings included aspartate transaminase (AST), 828 U/L (normal range, 2-40 U/L); alanine aminotransferase (ALT), 784 U/L (normal range, 3-30 U/L); lactic acid dehydrogenase, 520 U/L (normal range, 140-280 U/L); alkaline phosphatase, 119 U/L (normal range, 44-147 U/L); total bilirubin, 1.9 µmol/L (normal range, 2-18 µmol/L); and direct bilirubin, 1.3 µmol/L (normal range, 0-4 µmol/L). Baseline liver function test results (prior to admission) were unknown. Results of a coagulation panel, complete blood count, basic metabolic panel, amylase, lipase, urine toxicology, and urinalysis all were within normal limits.
Ultrasound of the abdomen revealed a normal abdomen, liver, pancreas, gallbladder, and common bile duct. This imaging study was negative for other obstructive pathologies.
THE DIAGNOSIS
During hospital admission, a noninvasive liver work-up was pursued by Gastroenterology. A hepatitis panel, Epstein-Barr virus testing, and levels of ceruloplasmin and acetaminophen were all found to be within normal limits, excluding additional causes of liver disease. Serum antinuclear antibody (ANA) testing was significantly positive, with a titer of 1:640 (range, < 1:20) and, as noted above, liver transaminases were severely elevated, leading to a presumptive diagnosis of drug-induced liver pathology.
Continue to: During outpatient follow-up...
During outpatient follow-up with Gastroenterology 2 days after discharge, the patient’s liver transaminases and bilirubin continued to trend upward (to a maximum ALT of 871 U/L; AST, 1097 U/L; alkaline phosphatase, 122 U/L; and bilirubin, 2.9 µmol/L). Immunoglobulin G was 1342 mg/mL (normal range, 694-1618 mg/mL).
An ultrasound-guided liver biopsy was performed; it demonstrated lobular, portal, and periportal hepatitis with focal bridging necrosis, consistent with a diagnosis of autoimmune hepatitis. Mild-to-moderate focal cholestasis was demonstrated, consistent with cholestatic hepatitis.
DISCUSSION
Autoimmune hepatitis is characterized by inflammation of the liver, secondary to the presence of circulating antibodies or hypergammaglobulinemia. The pathogenesis is thought to involve a T-cell–mediated immune attack on the liver. Based on case reports,the use of minocycline is associated with risk for liver injury, although the incidence is rare.1-4 Use of this medication may be associated with autoimmune disease in patients who are predisposed to autoimmune tendencies or who have genetic predeterminants.
Diagnosis is typically made based on abnormalities in aminotransferases (AST, ALT), elevation in serum immunoglobulins, and positive auto-antibody titers including ANA, smooth muscle antibodies, and anti-liver kidney microsomal type 1 antibodies. Although clinical presentations tend to differ, the confirmatory diagnosis is typically made histologically, with the presence of lobular and perivenular necro-inflammatory changes and plasma cell infiltration.5
Other infectious and metabolic causes of hepatitis should be excluded. Many medications and herbal agents have been noted to cause autoimmune hepatitis or similar syndromes that mimic the condition.
Medication history. Review of the case patient’s medication list identified ethinyl estradiol/norethindrone acetate and minocycline as potential culprits. Ethinyl estradiol/norethindrone acetate is a low-dose combination oral contraceptive pill (OCP). Although earlier formulations of OCPs were associated with hepatobiliary complications, these adverse effects are noted to be rare in the absence of predisposing conditions.6 In some cases, OCPs have been linked to cholestasis, chronic hepatocellular carcinoma, or hepatic adenomas, but studies have shown that these medications do not affect the course of acute liver failure.7
Continue to: Minocycline...
Minocycline is a second-generation tetracycline commonly used to treat acne vulgaris. Long-term treatment with minocycline has been associated with severe adverse effects, including autoimmune and hypersensitivity reactions.8 Minocycline-associated hepatotoxicity can be due to a systemic hypersensitivity reaction, occurring within a few weeks of therapy initiation, whereas autoimmune hepatitis manifests after a year or more of exposure to the medication (as in this case). Patients may present acutely several months after starting the medication, with symptoms of jaundice, fatigue, and/or joint aches. The acute liver injury is typically self-limited and often resolves with cessation of the drug. However, patients may require corticosteroids and immunosuppressive therapy.
Which is it? Histologically, drug-induced autoimmune hepatitis is indistinguishable from idiopathic autoimmune hepatitis.3 The estimated incidence of idiopathic autoimmune liver disease ranges from 0.7 to 2 out of 100,000 population.9 A systematic review of the literature identified 65 reported cases of liver damage associated with minocycline specifically.1
In this case, given the patient’s 2-year history of minocycline use, it is possible that she developed an acute presentation of autoimmune hepatitis. With drug-induced autoimmune liver injury, complete resolution occurs after withdrawal of the offending medication, and a response to corticosteroid therapy supports the diagnosis. Recurrence of signs or symptoms following corticosteroid cessation may indicate idiopathic autoimmune hepatitis as opposed to a drug-induced form.2
Our patient was started on steroid and immunomodulator therapy, with prednisone 40 mg/d and mycophenolate 250 mg bid. At follow-up with Gastroenterology, the patient’s symptoms and liver function test results had improved significantly (AST, 27 U/L; ALT, 14 U/L; alkaline phosphatase, 51 U/L; and total bilirubin, 0.4 µmol/L). The patient was continued on a prednisone taper while simultaneously titrating mycophenolate. The ultimate plan of care included continuing mycophenolate for a total of 4 to 5 years.
THE TAKEAWAY
During evaluation of a patient with new-onset liver disease, it is important to inquire about prescription medications, drugs, vitamins, and herbal supplements as possible contributors to the disease process. This case highlights the importance of monitoring patients while on minocycline and of weighing the risks vs benefits of long-term therapy. It has been suggested that liver enzymes be tested before therapy initiation and about every 3 months during long-term antibiotic treatment.4 Careful consideration and caution should be taken prior to the initiation of medications that have been linked to rare, but important, adverse reactions.
ACKNOWLEDGEMENT
The authors would like to thank Frank Bauer, MD, and Eva Sotil, MD, for their contributions to this case presentation.
CORRESPONDENCE
Andrea Gillis, DO, Asylum Hill Family Medicine Center, 99 Woodland Street, Hartford, CT 06105; andrea.gillis@ trinityhealthofne.org
THE CASE
A 17-year-old White girl with no known past medical history presented to the emergency department (ED) with complaints of abdominal pain and pruritus. The abdominal pain had started 9 days prior and lasted for 3 days. One day after resolution, she developed bilateral lower extremity itching, which was not relieved with loratadine.
Review of systems included dark urine and yellow eyes noted for several days. The patient denied nausea, vomiting, diarrhea, constipation, fevers, chills, arthralgias, recent illness, travel, or sick contacts. Immunizations were up to date. The patient had no history of surgery or liver disease and no pertinent family history. Her current medications included ethinyl estradiol/norethindrone acetate for birth control and minocycline for acne vulgaris. She had been taking the latter medication for 2 years. No additional medications were noted, including vitamins, over-the-counter medications, or supplements. She denied smoking and alcohol or recreational drug use.
In the ED, the patient had normal vital signs. Physical exam findings included bilateral scleral icterus and scattered skin excoriations on the hands, arms, back of the neck, and feet. At the time of hospital admission, the patient’s minocycline and birth control were held under the initial presumption that one or both might be contributing to her presentation.
Pertinent laboratory findings included aspartate transaminase (AST), 828 U/L (normal range, 2-40 U/L); alanine aminotransferase (ALT), 784 U/L (normal range, 3-30 U/L); lactic acid dehydrogenase, 520 U/L (normal range, 140-280 U/L); alkaline phosphatase, 119 U/L (normal range, 44-147 U/L); total bilirubin, 1.9 µmol/L (normal range, 2-18 µmol/L); and direct bilirubin, 1.3 µmol/L (normal range, 0-4 µmol/L). Baseline liver function test results (prior to admission) were unknown. Results of a coagulation panel, complete blood count, basic metabolic panel, amylase, lipase, urine toxicology, and urinalysis all were within normal limits.
Ultrasound of the abdomen revealed a normal abdomen, liver, pancreas, gallbladder, and common bile duct. This imaging study was negative for other obstructive pathologies.
THE DIAGNOSIS
During hospital admission, a noninvasive liver work-up was pursued by Gastroenterology. A hepatitis panel, Epstein-Barr virus testing, and levels of ceruloplasmin and acetaminophen were all found to be within normal limits, excluding additional causes of liver disease. Serum antinuclear antibody (ANA) testing was significantly positive, with a titer of 1:640 (range, < 1:20) and, as noted above, liver transaminases were severely elevated, leading to a presumptive diagnosis of drug-induced liver pathology.
Continue to: During outpatient follow-up...
During outpatient follow-up with Gastroenterology 2 days after discharge, the patient’s liver transaminases and bilirubin continued to trend upward (to a maximum ALT of 871 U/L; AST, 1097 U/L; alkaline phosphatase, 122 U/L; and bilirubin, 2.9 µmol/L). Immunoglobulin G was 1342 mg/mL (normal range, 694-1618 mg/mL).
An ultrasound-guided liver biopsy was performed; it demonstrated lobular, portal, and periportal hepatitis with focal bridging necrosis, consistent with a diagnosis of autoimmune hepatitis. Mild-to-moderate focal cholestasis was demonstrated, consistent with cholestatic hepatitis.
DISCUSSION
Autoimmune hepatitis is characterized by inflammation of the liver, secondary to the presence of circulating antibodies or hypergammaglobulinemia. The pathogenesis is thought to involve a T-cell–mediated immune attack on the liver. Based on case reports,the use of minocycline is associated with risk for liver injury, although the incidence is rare.1-4 Use of this medication may be associated with autoimmune disease in patients who are predisposed to autoimmune tendencies or who have genetic predeterminants.
Diagnosis is typically made based on abnormalities in aminotransferases (AST, ALT), elevation in serum immunoglobulins, and positive auto-antibody titers including ANA, smooth muscle antibodies, and anti-liver kidney microsomal type 1 antibodies. Although clinical presentations tend to differ, the confirmatory diagnosis is typically made histologically, with the presence of lobular and perivenular necro-inflammatory changes and plasma cell infiltration.5
Other infectious and metabolic causes of hepatitis should be excluded. Many medications and herbal agents have been noted to cause autoimmune hepatitis or similar syndromes that mimic the condition.
Medication history. Review of the case patient’s medication list identified ethinyl estradiol/norethindrone acetate and minocycline as potential culprits. Ethinyl estradiol/norethindrone acetate is a low-dose combination oral contraceptive pill (OCP). Although earlier formulations of OCPs were associated with hepatobiliary complications, these adverse effects are noted to be rare in the absence of predisposing conditions.6 In some cases, OCPs have been linked to cholestasis, chronic hepatocellular carcinoma, or hepatic adenomas, but studies have shown that these medications do not affect the course of acute liver failure.7
Continue to: Minocycline...
Minocycline is a second-generation tetracycline commonly used to treat acne vulgaris. Long-term treatment with minocycline has been associated with severe adverse effects, including autoimmune and hypersensitivity reactions.8 Minocycline-associated hepatotoxicity can be due to a systemic hypersensitivity reaction, occurring within a few weeks of therapy initiation, whereas autoimmune hepatitis manifests after a year or more of exposure to the medication (as in this case). Patients may present acutely several months after starting the medication, with symptoms of jaundice, fatigue, and/or joint aches. The acute liver injury is typically self-limited and often resolves with cessation of the drug. However, patients may require corticosteroids and immunosuppressive therapy.
Which is it? Histologically, drug-induced autoimmune hepatitis is indistinguishable from idiopathic autoimmune hepatitis.3 The estimated incidence of idiopathic autoimmune liver disease ranges from 0.7 to 2 out of 100,000 population.9 A systematic review of the literature identified 65 reported cases of liver damage associated with minocycline specifically.1
In this case, given the patient’s 2-year history of minocycline use, it is possible that she developed an acute presentation of autoimmune hepatitis. With drug-induced autoimmune liver injury, complete resolution occurs after withdrawal of the offending medication, and a response to corticosteroid therapy supports the diagnosis. Recurrence of signs or symptoms following corticosteroid cessation may indicate idiopathic autoimmune hepatitis as opposed to a drug-induced form.2
Our patient was started on steroid and immunomodulator therapy, with prednisone 40 mg/d and mycophenolate 250 mg bid. At follow-up with Gastroenterology, the patient’s symptoms and liver function test results had improved significantly (AST, 27 U/L; ALT, 14 U/L; alkaline phosphatase, 51 U/L; and total bilirubin, 0.4 µmol/L). The patient was continued on a prednisone taper while simultaneously titrating mycophenolate. The ultimate plan of care included continuing mycophenolate for a total of 4 to 5 years.
THE TAKEAWAY
During evaluation of a patient with new-onset liver disease, it is important to inquire about prescription medications, drugs, vitamins, and herbal supplements as possible contributors to the disease process. This case highlights the importance of monitoring patients while on minocycline and of weighing the risks vs benefits of long-term therapy. It has been suggested that liver enzymes be tested before therapy initiation and about every 3 months during long-term antibiotic treatment.4 Careful consideration and caution should be taken prior to the initiation of medications that have been linked to rare, but important, adverse reactions.
ACKNOWLEDGEMENT
The authors would like to thank Frank Bauer, MD, and Eva Sotil, MD, for their contributions to this case presentation.
CORRESPONDENCE
Andrea Gillis, DO, Asylum Hill Family Medicine Center, 99 Woodland Street, Hartford, CT 06105; andrea.gillis@ trinityhealthofne.org
1. Lawrenson RA, Seaman HE, Sundström A, et al. Liver damage associated with minocycline use in acne: a systematic review of the published literature and pharmacovigilance data. Drug Saf. 2000;23:333-349.
2. Teitelbaum JE, Perez-Atayde AR, Cohen M, et al. Minocycline-related autoimmune hepatitis case series and literature review. Arch Pediatr Adolesc Med. 1998;152:1132-1136.
3. Goldstein NS, Bayati N, Silverman AL, et al. Minocycline as a cause of drug induced autoimmune hepatitis: report of four cases and comparison with autoimmune hepatitis. Am J Clinic Pathol. 2000;114:591-598.
4. Ramakrishna J, Johnson AR, Banner BF. Long-term minocycline use for acne in healthy adolescents can cause severe autoimmune hepatitis. J Clin Gastroenterol. 2009;43:787-790.
5. Nguyen Canh H, Harada K, Ouchi H, et al. Acute presentation of autoimmune hepatitis: a multicentre study with detailed histological evaluation in a large cohort of patients. J Clin Pathol. 2017;70:961-969.
6. Lindberg MC. Hepatobiliary complications of oral contraceptives. J Gen Intern Med. 1992; 7:199-209.
7. Kapp N, Tilley IB, Curtis KM. The effects of hormonal contraceptive use among women with viral hepatitis or cirrhosis of the liver: a systematic review. Contraception. 2009;80:381-386.
8. DeLemos AS, Foureau DM, Jacobs C, et al. Drug-induced liver injury with autoimmune features. Semin Liver Dis. 2014;34:194-204.
9. Jepsen P, Gronbaek L, Vilstrup H. Worldwide incidence of autoimmune liver disease. Dig Dis. 2015;33(suppl 2):2-12.
1. Lawrenson RA, Seaman HE, Sundström A, et al. Liver damage associated with minocycline use in acne: a systematic review of the published literature and pharmacovigilance data. Drug Saf. 2000;23:333-349.
2. Teitelbaum JE, Perez-Atayde AR, Cohen M, et al. Minocycline-related autoimmune hepatitis case series and literature review. Arch Pediatr Adolesc Med. 1998;152:1132-1136.
3. Goldstein NS, Bayati N, Silverman AL, et al. Minocycline as a cause of drug induced autoimmune hepatitis: report of four cases and comparison with autoimmune hepatitis. Am J Clinic Pathol. 2000;114:591-598.
4. Ramakrishna J, Johnson AR, Banner BF. Long-term minocycline use for acne in healthy adolescents can cause severe autoimmune hepatitis. J Clin Gastroenterol. 2009;43:787-790.
5. Nguyen Canh H, Harada K, Ouchi H, et al. Acute presentation of autoimmune hepatitis: a multicentre study with detailed histological evaluation in a large cohort of patients. J Clin Pathol. 2017;70:961-969.
6. Lindberg MC. Hepatobiliary complications of oral contraceptives. J Gen Intern Med. 1992; 7:199-209.
7. Kapp N, Tilley IB, Curtis KM. The effects of hormonal contraceptive use among women with viral hepatitis or cirrhosis of the liver: a systematic review. Contraception. 2009;80:381-386.
8. DeLemos AS, Foureau DM, Jacobs C, et al. Drug-induced liver injury with autoimmune features. Semin Liver Dis. 2014;34:194-204.
9. Jepsen P, Gronbaek L, Vilstrup H. Worldwide incidence of autoimmune liver disease. Dig Dis. 2015;33(suppl 2):2-12.
Geography and behaviors linked to early-onset colorectal cancer survival in U.S. women
An analysis of nearly 29,000 U.S. women with early-onset colorectal cancer (CRC) showed that physical inactivity and fertility correlated modestly with living in “hot spots,” or counties with high early-onset CRC mortality rates among women.
Approximately one-third of the variation in early-onset CRC survival among women was accounted for by differences in individual- or community-level features.
Andreana N. Holowatyj, PhD, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues reported these findings in Clinical and Translational Gastroenterology.
Dr. Holowatyj and colleagues noted that prior studies have linked health behaviors with an increased risk of early-onset CRC among women. However, the impact of health behaviors on outcomes of early-onset CRC is unknown.
The researchers hypothesized that biological-, individual-, and community-level factors may be contributing to known sex-specific differences in CRC outcomes and geographic variations in survival by sex.
Hot spot counties with high mortality
The researchers identified geographic hot spots using three geospatial autocorrelation approaches with Centers for Disease Control and Prevention national
mortality data. The team also analyzed data from the Surveillance, Epidemiology, and End Results program on 28,790 women (aged 15-49 years) diagnosed with CRC during 1999-2016.
Of the 3,108 counties in the contiguous United States, 191 were identified as hot spots. Among these, 101 (52.9%) were located in the South.
Earlier research had shown a predominance of hot spots for early-onset CRC mortality among both men and women in the South.
However, the current study of women showed that almost half of these counties were located in the Midwest and the Northeast as well as the South.
Also in the current analysis, about one in every seven women (13.7%) with early-onset CRC resided in hot spot counties.
Race/ethnicity, stage at diagnosis, histopathology, and receipt of first-course therapies also differed significantly (P ≤ .0001) between women residing in hot spot versus non–hot spot counties.
Non-Hispanic Black patients, for example, accounted for 23.7% of early-onset CRC cases in hot spot counties, as compared with 14.3% in non–hot spot counties (P < .0001). The county-level proportion of non-Hispanic Black patients also modestly correlated with hot spot residence (rs = .26; P < .0001).
Race and ethnicity accounted for less than 0.5% of the variation in early-onset CRC survival among women in non–hot spot counties. In hot spot counties, however, this factor explained 1.4% of the variation in early-onset CRC-specific survival among women.
Inactivity correlates with hot spot residence
Dr. Holowatyj and colleagues also identified physical inactivity and lower fertility as county-level factors modestly correlated with hot spot residence (rs = .21, rs = –.23: P < .01).
Nearly a quarter of adults living in hot spot counties reported no physical activity during their leisure time (24.1% vs. 21.7% in non–hot spot counties; P < .01).
The rate of live births in the last year among women aged 15-50 years was lower in hot spot counties than in non–hot spot counties (4.9% vs. 5.4%; P < .01).
Individual- and community-level features overall accounted for different proportions of variance in early-onset CRC survival among women residing in hot spot counties (33.8%) versus non–hot spot counties (34.1%).
In addition to race and ethnicity, age at diagnosis, tumor histology, county-level proportions of the non-Hispanic Black population, women with a live birth in the last year, and annual household income of less than $20,000 all explained greater variance in CRC survival in young women in hot spot counties versus non–hot spot counties.
Keep CRC in differential diagnosis
“These individual- and community-level feature differences between hot spot and non–hot spot counties illustrate the importance of understanding how these factors may be contributing to early-onset CRC mortality among women – particularly in hot spot counties,” Dr. Holowatyj said in an interview. “They may provide us with key clues for developing effective strategies to reduce the burden of CRC in young women across the United States.
“Every primary care physician and gastroenterologist, particularly in hot spot counties, should keep CRC in their differential diagnosis, particularly if a patient is presenting with typical signs and symptoms, even if they are not yet of screening age. Early-stage diagnosis increases survival odds because the cancer may be easier to treat.”
Health professionals can also encourage physical activity and a healthy lifestyle, she added.
The authors declared no competing interests. Their research was funded by grants from the federal government and foundations.
SOURCE: Holowatyj AN et al. Clin and Transl Gastroenterol. 2020;11:e00266.
An analysis of nearly 29,000 U.S. women with early-onset colorectal cancer (CRC) showed that physical inactivity and fertility correlated modestly with living in “hot spots,” or counties with high early-onset CRC mortality rates among women.
Approximately one-third of the variation in early-onset CRC survival among women was accounted for by differences in individual- or community-level features.
Andreana N. Holowatyj, PhD, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues reported these findings in Clinical and Translational Gastroenterology.
Dr. Holowatyj and colleagues noted that prior studies have linked health behaviors with an increased risk of early-onset CRC among women. However, the impact of health behaviors on outcomes of early-onset CRC is unknown.
The researchers hypothesized that biological-, individual-, and community-level factors may be contributing to known sex-specific differences in CRC outcomes and geographic variations in survival by sex.
Hot spot counties with high mortality
The researchers identified geographic hot spots using three geospatial autocorrelation approaches with Centers for Disease Control and Prevention national
mortality data. The team also analyzed data from the Surveillance, Epidemiology, and End Results program on 28,790 women (aged 15-49 years) diagnosed with CRC during 1999-2016.
Of the 3,108 counties in the contiguous United States, 191 were identified as hot spots. Among these, 101 (52.9%) were located in the South.
Earlier research had shown a predominance of hot spots for early-onset CRC mortality among both men and women in the South.
However, the current study of women showed that almost half of these counties were located in the Midwest and the Northeast as well as the South.
Also in the current analysis, about one in every seven women (13.7%) with early-onset CRC resided in hot spot counties.
Race/ethnicity, stage at diagnosis, histopathology, and receipt of first-course therapies also differed significantly (P ≤ .0001) between women residing in hot spot versus non–hot spot counties.
Non-Hispanic Black patients, for example, accounted for 23.7% of early-onset CRC cases in hot spot counties, as compared with 14.3% in non–hot spot counties (P < .0001). The county-level proportion of non-Hispanic Black patients also modestly correlated with hot spot residence (rs = .26; P < .0001).
Race and ethnicity accounted for less than 0.5% of the variation in early-onset CRC survival among women in non–hot spot counties. In hot spot counties, however, this factor explained 1.4% of the variation in early-onset CRC-specific survival among women.
Inactivity correlates with hot spot residence
Dr. Holowatyj and colleagues also identified physical inactivity and lower fertility as county-level factors modestly correlated with hot spot residence (rs = .21, rs = –.23: P < .01).
Nearly a quarter of adults living in hot spot counties reported no physical activity during their leisure time (24.1% vs. 21.7% in non–hot spot counties; P < .01).
The rate of live births in the last year among women aged 15-50 years was lower in hot spot counties than in non–hot spot counties (4.9% vs. 5.4%; P < .01).
Individual- and community-level features overall accounted for different proportions of variance in early-onset CRC survival among women residing in hot spot counties (33.8%) versus non–hot spot counties (34.1%).
In addition to race and ethnicity, age at diagnosis, tumor histology, county-level proportions of the non-Hispanic Black population, women with a live birth in the last year, and annual household income of less than $20,000 all explained greater variance in CRC survival in young women in hot spot counties versus non–hot spot counties.
Keep CRC in differential diagnosis
“These individual- and community-level feature differences between hot spot and non–hot spot counties illustrate the importance of understanding how these factors may be contributing to early-onset CRC mortality among women – particularly in hot spot counties,” Dr. Holowatyj said in an interview. “They may provide us with key clues for developing effective strategies to reduce the burden of CRC in young women across the United States.
“Every primary care physician and gastroenterologist, particularly in hot spot counties, should keep CRC in their differential diagnosis, particularly if a patient is presenting with typical signs and symptoms, even if they are not yet of screening age. Early-stage diagnosis increases survival odds because the cancer may be easier to treat.”
Health professionals can also encourage physical activity and a healthy lifestyle, she added.
The authors declared no competing interests. Their research was funded by grants from the federal government and foundations.
SOURCE: Holowatyj AN et al. Clin and Transl Gastroenterol. 2020;11:e00266.
An analysis of nearly 29,000 U.S. women with early-onset colorectal cancer (CRC) showed that physical inactivity and fertility correlated modestly with living in “hot spots,” or counties with high early-onset CRC mortality rates among women.
Approximately one-third of the variation in early-onset CRC survival among women was accounted for by differences in individual- or community-level features.
Andreana N. Holowatyj, PhD, of Vanderbilt University Medical Center in Nashville, Tenn., and colleagues reported these findings in Clinical and Translational Gastroenterology.
Dr. Holowatyj and colleagues noted that prior studies have linked health behaviors with an increased risk of early-onset CRC among women. However, the impact of health behaviors on outcomes of early-onset CRC is unknown.
The researchers hypothesized that biological-, individual-, and community-level factors may be contributing to known sex-specific differences in CRC outcomes and geographic variations in survival by sex.
Hot spot counties with high mortality
The researchers identified geographic hot spots using three geospatial autocorrelation approaches with Centers for Disease Control and Prevention national
mortality data. The team also analyzed data from the Surveillance, Epidemiology, and End Results program on 28,790 women (aged 15-49 years) diagnosed with CRC during 1999-2016.
Of the 3,108 counties in the contiguous United States, 191 were identified as hot spots. Among these, 101 (52.9%) were located in the South.
Earlier research had shown a predominance of hot spots for early-onset CRC mortality among both men and women in the South.
However, the current study of women showed that almost half of these counties were located in the Midwest and the Northeast as well as the South.
Also in the current analysis, about one in every seven women (13.7%) with early-onset CRC resided in hot spot counties.
Race/ethnicity, stage at diagnosis, histopathology, and receipt of first-course therapies also differed significantly (P ≤ .0001) between women residing in hot spot versus non–hot spot counties.
Non-Hispanic Black patients, for example, accounted for 23.7% of early-onset CRC cases in hot spot counties, as compared with 14.3% in non–hot spot counties (P < .0001). The county-level proportion of non-Hispanic Black patients also modestly correlated with hot spot residence (rs = .26; P < .0001).
Race and ethnicity accounted for less than 0.5% of the variation in early-onset CRC survival among women in non–hot spot counties. In hot spot counties, however, this factor explained 1.4% of the variation in early-onset CRC-specific survival among women.
Inactivity correlates with hot spot residence
Dr. Holowatyj and colleagues also identified physical inactivity and lower fertility as county-level factors modestly correlated with hot spot residence (rs = .21, rs = –.23: P < .01).
Nearly a quarter of adults living in hot spot counties reported no physical activity during their leisure time (24.1% vs. 21.7% in non–hot spot counties; P < .01).
The rate of live births in the last year among women aged 15-50 years was lower in hot spot counties than in non–hot spot counties (4.9% vs. 5.4%; P < .01).
Individual- and community-level features overall accounted for different proportions of variance in early-onset CRC survival among women residing in hot spot counties (33.8%) versus non–hot spot counties (34.1%).
In addition to race and ethnicity, age at diagnosis, tumor histology, county-level proportions of the non-Hispanic Black population, women with a live birth in the last year, and annual household income of less than $20,000 all explained greater variance in CRC survival in young women in hot spot counties versus non–hot spot counties.
Keep CRC in differential diagnosis
“These individual- and community-level feature differences between hot spot and non–hot spot counties illustrate the importance of understanding how these factors may be contributing to early-onset CRC mortality among women – particularly in hot spot counties,” Dr. Holowatyj said in an interview. “They may provide us with key clues for developing effective strategies to reduce the burden of CRC in young women across the United States.
“Every primary care physician and gastroenterologist, particularly in hot spot counties, should keep CRC in their differential diagnosis, particularly if a patient is presenting with typical signs and symptoms, even if they are not yet of screening age. Early-stage diagnosis increases survival odds because the cancer may be easier to treat.”
Health professionals can also encourage physical activity and a healthy lifestyle, she added.
The authors declared no competing interests. Their research was funded by grants from the federal government and foundations.
SOURCE: Holowatyj AN et al. Clin and Transl Gastroenterol. 2020;11:e00266.
FROM CLINICAL AND TRANSLATIONAL GASTROENTEROLOGY
Does XR injectable naltrexone prevent relapse as effectively as daily sublingual buprenorphine-naloxone?
EVIDENCE SUMMARY
Two recent multicenter, open-label RCTs, 1 in the United States and 1 in Norway, compared monthly XR-NTX with daily BUP-NX.1,2 Both studies evaluated effectiveness (defined by either the number of people who relapsed or self-reported opioid use), cravings, and safety (defined as the absence of serious adverse events such as medically complex withdrawal or fatal overdose).
The participant populations were similar in both mean age and mean age of onset of opioid use. Duration of opioid use was reported differently (total duration or years of heavy heroin or other opioid use) and couldn’t be compared directly.
Naltrexone and buprenorphine-naloxone are similarly effective
The US study enrolled 570 opioid-dependent participants in a 24-week comparative effectiveness trial.1 The 8 study sites were community treatment programs, and the participants were recruited during voluntary inpatient detoxification admissions. Some participants were randomized while on methadone or buprenorphine tapers and some after complete detoxification.
The intention-to-treat analysis included 283 patients in the XR-NTX group and 287 in the BUP-NX group. At 24 weeks, the number of participants who’d had a relapse event (self-reported use or positive urine drug test for nonstudy opioids or refusal to provide a urine sample) was 185 (65%) for XR-NTX compared with 163 (57%) for BUP-NX (odds ratio [OR] = 1.44, 95% confidence interval [CI], 1.02 to 2.01; P = .036).
The 12-week Norwegian noninferiority trial enrolled 159 participants.2 In contrast to the US study, all participants were required to complete inpatient detoxification before randomization and induction onto the study medication.
Patients on BUP-NX reported 3.6 more days of heroin use within the previous 28 days than patients in the XR-NTX group (95% CI, 1.2 to 6; P = .003). For other illicit opioids, self-reported use was 2.4 days greater in the BUP-NX group (95% CI, −0.1 to 4.9; P = .06). Retention with XR-NTX was noninferior to BUP-NX (mean days in therapy [standard deviation], 69.3 [25.9] and 63.7 [29.9]; P = .33).
Randomizing after complete detox reduces induction failures
Naltrexone, a full opioid antagonist, precipitates withdrawal when a full or partial opioid agonist is engaging the opioid receptor. For this reason, an opioid-free interval of 7 to 10 days is generally recommended before initiating naltrexone, raising the risk for relapse during the induction process.
Continue to: The Norwegian trial...
The Norwegian trial randomized participants after detoxification. The US trial, in which some participants were randomized before completing detoxification, reported 79 (28%) induction failures for XR-NTX and 17 (6%) for BUP-NX.1 As a result, a per protocol analysis was completed with the 204 patients on XR-NTX and 270 patients on BUP-NX who were successfully inducted onto a study medication. The 24-week relapse rate was 52% (106) for XR-NTX and 56% (150) for BUP-NX (OR = 0.87; 95% CI, 0.60 to 1.25; P = .44).
Cravings, adverse events, and cost considerations
Patients reported cravings using a visual analog scale. At 12 weeks in both studies, the XR-NTX groups reported fewer cravings than the BUP-NX groups, although by the end of the 24-week US trial, no statistically significant difference in cravings was found between the 2 groups.1,2
The Norwegian trial found a difference between the XR-NTX and the BUP-NX groups in the percentage of nonserious adverse events such as nausea or chills (60.6% in the XR-NTX group vs 30.6% in the BUP-NX group; P < .001), and the US trial found a difference in total number of overdoses (64% of the total overdoses were in the XR-NTX group). Neither trial, however, reported a statistically significant difference in serious adverse events or fatal overdoses between the 2 groups.1,2
The price for naltrexone is $1665.06 per monthly injection.3 The price for buprenorphine-naloxone varies depending on dose and formulation, with a general range of $527 to $600 per month at 16 mg/d.4
Editor’s takeaway
Two higher-quality RCTs show similar but imperfect effectiveness for both XR-NTX and daily sublingual BUP-NX. Injectable naltrexone’s higher cost may influence medication choice.
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391:309-318.
2. Tanum L, Solli KK, Latif ZE, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205.
3. Naltrexone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
4. Buprenorphine and naloxone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
EVIDENCE SUMMARY
Two recent multicenter, open-label RCTs, 1 in the United States and 1 in Norway, compared monthly XR-NTX with daily BUP-NX.1,2 Both studies evaluated effectiveness (defined by either the number of people who relapsed or self-reported opioid use), cravings, and safety (defined as the absence of serious adverse events such as medically complex withdrawal or fatal overdose).
The participant populations were similar in both mean age and mean age of onset of opioid use. Duration of opioid use was reported differently (total duration or years of heavy heroin or other opioid use) and couldn’t be compared directly.
Naltrexone and buprenorphine-naloxone are similarly effective
The US study enrolled 570 opioid-dependent participants in a 24-week comparative effectiveness trial.1 The 8 study sites were community treatment programs, and the participants were recruited during voluntary inpatient detoxification admissions. Some participants were randomized while on methadone or buprenorphine tapers and some after complete detoxification.
The intention-to-treat analysis included 283 patients in the XR-NTX group and 287 in the BUP-NX group. At 24 weeks, the number of participants who’d had a relapse event (self-reported use or positive urine drug test for nonstudy opioids or refusal to provide a urine sample) was 185 (65%) for XR-NTX compared with 163 (57%) for BUP-NX (odds ratio [OR] = 1.44, 95% confidence interval [CI], 1.02 to 2.01; P = .036).
The 12-week Norwegian noninferiority trial enrolled 159 participants.2 In contrast to the US study, all participants were required to complete inpatient detoxification before randomization and induction onto the study medication.
Patients on BUP-NX reported 3.6 more days of heroin use within the previous 28 days than patients in the XR-NTX group (95% CI, 1.2 to 6; P = .003). For other illicit opioids, self-reported use was 2.4 days greater in the BUP-NX group (95% CI, −0.1 to 4.9; P = .06). Retention with XR-NTX was noninferior to BUP-NX (mean days in therapy [standard deviation], 69.3 [25.9] and 63.7 [29.9]; P = .33).
Randomizing after complete detox reduces induction failures
Naltrexone, a full opioid antagonist, precipitates withdrawal when a full or partial opioid agonist is engaging the opioid receptor. For this reason, an opioid-free interval of 7 to 10 days is generally recommended before initiating naltrexone, raising the risk for relapse during the induction process.
Continue to: The Norwegian trial...
The Norwegian trial randomized participants after detoxification. The US trial, in which some participants were randomized before completing detoxification, reported 79 (28%) induction failures for XR-NTX and 17 (6%) for BUP-NX.1 As a result, a per protocol analysis was completed with the 204 patients on XR-NTX and 270 patients on BUP-NX who were successfully inducted onto a study medication. The 24-week relapse rate was 52% (106) for XR-NTX and 56% (150) for BUP-NX (OR = 0.87; 95% CI, 0.60 to 1.25; P = .44).
Cravings, adverse events, and cost considerations
Patients reported cravings using a visual analog scale. At 12 weeks in both studies, the XR-NTX groups reported fewer cravings than the BUP-NX groups, although by the end of the 24-week US trial, no statistically significant difference in cravings was found between the 2 groups.1,2
The Norwegian trial found a difference between the XR-NTX and the BUP-NX groups in the percentage of nonserious adverse events such as nausea or chills (60.6% in the XR-NTX group vs 30.6% in the BUP-NX group; P < .001), and the US trial found a difference in total number of overdoses (64% of the total overdoses were in the XR-NTX group). Neither trial, however, reported a statistically significant difference in serious adverse events or fatal overdoses between the 2 groups.1,2
The price for naltrexone is $1665.06 per monthly injection.3 The price for buprenorphine-naloxone varies depending on dose and formulation, with a general range of $527 to $600 per month at 16 mg/d.4
Editor’s takeaway
Two higher-quality RCTs show similar but imperfect effectiveness for both XR-NTX and daily sublingual BUP-NX. Injectable naltrexone’s higher cost may influence medication choice.
EVIDENCE SUMMARY
Two recent multicenter, open-label RCTs, 1 in the United States and 1 in Norway, compared monthly XR-NTX with daily BUP-NX.1,2 Both studies evaluated effectiveness (defined by either the number of people who relapsed or self-reported opioid use), cravings, and safety (defined as the absence of serious adverse events such as medically complex withdrawal or fatal overdose).
The participant populations were similar in both mean age and mean age of onset of opioid use. Duration of opioid use was reported differently (total duration or years of heavy heroin or other opioid use) and couldn’t be compared directly.
Naltrexone and buprenorphine-naloxone are similarly effective
The US study enrolled 570 opioid-dependent participants in a 24-week comparative effectiveness trial.1 The 8 study sites were community treatment programs, and the participants were recruited during voluntary inpatient detoxification admissions. Some participants were randomized while on methadone or buprenorphine tapers and some after complete detoxification.
The intention-to-treat analysis included 283 patients in the XR-NTX group and 287 in the BUP-NX group. At 24 weeks, the number of participants who’d had a relapse event (self-reported use or positive urine drug test for nonstudy opioids or refusal to provide a urine sample) was 185 (65%) for XR-NTX compared with 163 (57%) for BUP-NX (odds ratio [OR] = 1.44, 95% confidence interval [CI], 1.02 to 2.01; P = .036).
The 12-week Norwegian noninferiority trial enrolled 159 participants.2 In contrast to the US study, all participants were required to complete inpatient detoxification before randomization and induction onto the study medication.
Patients on BUP-NX reported 3.6 more days of heroin use within the previous 28 days than patients in the XR-NTX group (95% CI, 1.2 to 6; P = .003). For other illicit opioids, self-reported use was 2.4 days greater in the BUP-NX group (95% CI, −0.1 to 4.9; P = .06). Retention with XR-NTX was noninferior to BUP-NX (mean days in therapy [standard deviation], 69.3 [25.9] and 63.7 [29.9]; P = .33).
Randomizing after complete detox reduces induction failures
Naltrexone, a full opioid antagonist, precipitates withdrawal when a full or partial opioid agonist is engaging the opioid receptor. For this reason, an opioid-free interval of 7 to 10 days is generally recommended before initiating naltrexone, raising the risk for relapse during the induction process.
Continue to: The Norwegian trial...
The Norwegian trial randomized participants after detoxification. The US trial, in which some participants were randomized before completing detoxification, reported 79 (28%) induction failures for XR-NTX and 17 (6%) for BUP-NX.1 As a result, a per protocol analysis was completed with the 204 patients on XR-NTX and 270 patients on BUP-NX who were successfully inducted onto a study medication. The 24-week relapse rate was 52% (106) for XR-NTX and 56% (150) for BUP-NX (OR = 0.87; 95% CI, 0.60 to 1.25; P = .44).
Cravings, adverse events, and cost considerations
Patients reported cravings using a visual analog scale. At 12 weeks in both studies, the XR-NTX groups reported fewer cravings than the BUP-NX groups, although by the end of the 24-week US trial, no statistically significant difference in cravings was found between the 2 groups.1,2
The Norwegian trial found a difference between the XR-NTX and the BUP-NX groups in the percentage of nonserious adverse events such as nausea or chills (60.6% in the XR-NTX group vs 30.6% in the BUP-NX group; P < .001), and the US trial found a difference in total number of overdoses (64% of the total overdoses were in the XR-NTX group). Neither trial, however, reported a statistically significant difference in serious adverse events or fatal overdoses between the 2 groups.1,2
The price for naltrexone is $1665.06 per monthly injection.3 The price for buprenorphine-naloxone varies depending on dose and formulation, with a general range of $527 to $600 per month at 16 mg/d.4
Editor’s takeaway
Two higher-quality RCTs show similar but imperfect effectiveness for both XR-NTX and daily sublingual BUP-NX. Injectable naltrexone’s higher cost may influence medication choice.
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391:309-318.
2. Tanum L, Solli KK, Latif ZE, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205.
3. Naltrexone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
4. Buprenorphine and naloxone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391:309-318.
2. Tanum L, Solli KK, Latif ZE, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205.
3. Naltrexone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
4. Buprenorphine and naloxone: drug information. Lexi-Comp, Inc (Lexi-Drugs). Wolters Kluwer Health, Inc. Riverwoods, IL. http://online.lexi.com. Accessed November 20, 2020.
EVIDENCE-BASED ANSWER:
Yes. Monthly extended-release injectable naltrexone (XR-NTX) treats opioid use disorder as effectively as daily sublingual buprenorphine-naloxone (BUP-NX) without causing any increase in serious adverse events or fatal overdoses. (strength of recommendation: A, 2 good-quality RCTs).
HHS, Surgeon General urge action on maternal health
The U.S. Surgeon General and Department of Health & Human Services are calling on health care professionals, hospitals, employers, insurers, women, and the nation to work together to reduce maternal morbidity and mortality – and the disparities that make the risks higher for women of color.
The maternal mortality rate in the United States is the highest among developed countries of the world and continues to rise. In 2018, for every 100,000 live births, approximately 17 women died while pregnant or within 42 days of the end of pregnancy from causes related to pregnancy or delivery – that’s a substantial increase from 7 deaths per 100,000 live births in 1987, according to the surgeon general’s new call to action.
“Our mothers had much lower rates of dying related to pregnancy, compared to women today,” Dorothy Fink, MD, HHS deputy assistant secretary for women’s health, said at a briefing held Dec. 3 to mark the call to action.
Cardiovascular conditions were the most common cause of pregnancy-related deaths between 2011 and 2015, accounting for more than one in three of the deaths. HHS’s related action plan sets a target of achieving blood pressure control in 80% of women of reproductive age with hypertension by 2025.
The plan also seeks to reduce the maternal mortality rate by 50% and decrease low-risk cesarean deliveries by 25% within 5 years.
Surgeon General Jerome Adams, MD, said at the briefing. “This is not just unacceptable, it is just something that we need to understand is not inevitable,” he said, adding that the Centers for Disease Control and Prevention has determined that two thirds of the deaths are preventable.
Dr. Adams also noted that it was important to address maternal health now, especially with COVID-19 raging. “Without attention and action, maternal health could actually worsen because of this pandemic,” he said.
“We cannot discuss maternal health, much less improve it, unless we acknowledge women of color are at a much greater risk of harm related to childbirth,” Dr. Adams said. “Black women are two to three times more likely to die of pregnancy-related causes compared to many other racial and ethnic groups.” The disparity increases with age, according to the CDC.
Studies have shown that education does not eliminate those disparities. Black women with a college degree are twice as likely to die as White or Asian American women who did not finish high school, Dr. Adams said.
He held up a photo of a colleague, Shalone Irving, who he said was a PhD-educated epidemiologist who “died not long ago from pregnancy-related complications.”
Income is also not a factor, said Dr. Adams, noting that pop singer Beyonce had a near-death experience with preeclampsia. He also noted that Serena Williams, a top athlete, also struggled with pregnancy complications.
Recommendations not all funded
The HHS action plan is not explicitly funded, although Dr. Fink and Dr. Adams said that President Donald J. Trump’s fiscal 2021 budget includes some specific requests for improving maternal health. It will be up to Congress to grant the requests.
The budget seeks $80 million for the Health Resources and Services Administration to improve access to and quality of care. It also includes money to expand Medicaid coverage for 1 year after birth for women with substance use disorders. The American Medical Association in 2019 adopted a policy urging Medicaid coverage to be expanded to include all women for a year after childbirth. The American College of Obstetricians and Gynecologists has also encouraged this extension.
“We are encouraged that the HHS action plan includes support for policies to close coverage and care gaps for all postpartum women after pregnancy-related Medicaid coverage expires,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in an interview.
The HHS could act immediately by approving Medicaid waivers to extend such coverage, Dr. Phipps said.
The budget also requests $24 million to expand maternal mortality review programs to every state, said Dr. Fink. Currently, 43 states and the District of Columbia, have such committees, which are charged with reviewing deaths of women within a year of pregnancy or birth.
The HHS will also join with the March of Dimes to address the disparities in Black women by implementing evidence-based best practices to improve quality in hospital settings.
It is not the first time the Trump administration has taken aim at reducing maternal morbidity and mortality. In 2018, the president signed the Preventing Maternal Deaths Act, which authorized the CDC to award $50 million over 5 years so that every state could form maternal mortality review committees.
A version of this article originally appeared on Medscape.com.
The U.S. Surgeon General and Department of Health & Human Services are calling on health care professionals, hospitals, employers, insurers, women, and the nation to work together to reduce maternal morbidity and mortality – and the disparities that make the risks higher for women of color.
The maternal mortality rate in the United States is the highest among developed countries of the world and continues to rise. In 2018, for every 100,000 live births, approximately 17 women died while pregnant or within 42 days of the end of pregnancy from causes related to pregnancy or delivery – that’s a substantial increase from 7 deaths per 100,000 live births in 1987, according to the surgeon general’s new call to action.
“Our mothers had much lower rates of dying related to pregnancy, compared to women today,” Dorothy Fink, MD, HHS deputy assistant secretary for women’s health, said at a briefing held Dec. 3 to mark the call to action.
Cardiovascular conditions were the most common cause of pregnancy-related deaths between 2011 and 2015, accounting for more than one in three of the deaths. HHS’s related action plan sets a target of achieving blood pressure control in 80% of women of reproductive age with hypertension by 2025.
The plan also seeks to reduce the maternal mortality rate by 50% and decrease low-risk cesarean deliveries by 25% within 5 years.
Surgeon General Jerome Adams, MD, said at the briefing. “This is not just unacceptable, it is just something that we need to understand is not inevitable,” he said, adding that the Centers for Disease Control and Prevention has determined that two thirds of the deaths are preventable.
Dr. Adams also noted that it was important to address maternal health now, especially with COVID-19 raging. “Without attention and action, maternal health could actually worsen because of this pandemic,” he said.
“We cannot discuss maternal health, much less improve it, unless we acknowledge women of color are at a much greater risk of harm related to childbirth,” Dr. Adams said. “Black women are two to three times more likely to die of pregnancy-related causes compared to many other racial and ethnic groups.” The disparity increases with age, according to the CDC.
Studies have shown that education does not eliminate those disparities. Black women with a college degree are twice as likely to die as White or Asian American women who did not finish high school, Dr. Adams said.
He held up a photo of a colleague, Shalone Irving, who he said was a PhD-educated epidemiologist who “died not long ago from pregnancy-related complications.”
Income is also not a factor, said Dr. Adams, noting that pop singer Beyonce had a near-death experience with preeclampsia. He also noted that Serena Williams, a top athlete, also struggled with pregnancy complications.
Recommendations not all funded
The HHS action plan is not explicitly funded, although Dr. Fink and Dr. Adams said that President Donald J. Trump’s fiscal 2021 budget includes some specific requests for improving maternal health. It will be up to Congress to grant the requests.
The budget seeks $80 million for the Health Resources and Services Administration to improve access to and quality of care. It also includes money to expand Medicaid coverage for 1 year after birth for women with substance use disorders. The American Medical Association in 2019 adopted a policy urging Medicaid coverage to be expanded to include all women for a year after childbirth. The American College of Obstetricians and Gynecologists has also encouraged this extension.
“We are encouraged that the HHS action plan includes support for policies to close coverage and care gaps for all postpartum women after pregnancy-related Medicaid coverage expires,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in an interview.
The HHS could act immediately by approving Medicaid waivers to extend such coverage, Dr. Phipps said.
The budget also requests $24 million to expand maternal mortality review programs to every state, said Dr. Fink. Currently, 43 states and the District of Columbia, have such committees, which are charged with reviewing deaths of women within a year of pregnancy or birth.
The HHS will also join with the March of Dimes to address the disparities in Black women by implementing evidence-based best practices to improve quality in hospital settings.
It is not the first time the Trump administration has taken aim at reducing maternal morbidity and mortality. In 2018, the president signed the Preventing Maternal Deaths Act, which authorized the CDC to award $50 million over 5 years so that every state could form maternal mortality review committees.
A version of this article originally appeared on Medscape.com.
The U.S. Surgeon General and Department of Health & Human Services are calling on health care professionals, hospitals, employers, insurers, women, and the nation to work together to reduce maternal morbidity and mortality – and the disparities that make the risks higher for women of color.
The maternal mortality rate in the United States is the highest among developed countries of the world and continues to rise. In 2018, for every 100,000 live births, approximately 17 women died while pregnant or within 42 days of the end of pregnancy from causes related to pregnancy or delivery – that’s a substantial increase from 7 deaths per 100,000 live births in 1987, according to the surgeon general’s new call to action.
“Our mothers had much lower rates of dying related to pregnancy, compared to women today,” Dorothy Fink, MD, HHS deputy assistant secretary for women’s health, said at a briefing held Dec. 3 to mark the call to action.
Cardiovascular conditions were the most common cause of pregnancy-related deaths between 2011 and 2015, accounting for more than one in three of the deaths. HHS’s related action plan sets a target of achieving blood pressure control in 80% of women of reproductive age with hypertension by 2025.
The plan also seeks to reduce the maternal mortality rate by 50% and decrease low-risk cesarean deliveries by 25% within 5 years.
Surgeon General Jerome Adams, MD, said at the briefing. “This is not just unacceptable, it is just something that we need to understand is not inevitable,” he said, adding that the Centers for Disease Control and Prevention has determined that two thirds of the deaths are preventable.
Dr. Adams also noted that it was important to address maternal health now, especially with COVID-19 raging. “Without attention and action, maternal health could actually worsen because of this pandemic,” he said.
“We cannot discuss maternal health, much less improve it, unless we acknowledge women of color are at a much greater risk of harm related to childbirth,” Dr. Adams said. “Black women are two to three times more likely to die of pregnancy-related causes compared to many other racial and ethnic groups.” The disparity increases with age, according to the CDC.
Studies have shown that education does not eliminate those disparities. Black women with a college degree are twice as likely to die as White or Asian American women who did not finish high school, Dr. Adams said.
He held up a photo of a colleague, Shalone Irving, who he said was a PhD-educated epidemiologist who “died not long ago from pregnancy-related complications.”
Income is also not a factor, said Dr. Adams, noting that pop singer Beyonce had a near-death experience with preeclampsia. He also noted that Serena Williams, a top athlete, also struggled with pregnancy complications.
Recommendations not all funded
The HHS action plan is not explicitly funded, although Dr. Fink and Dr. Adams said that President Donald J. Trump’s fiscal 2021 budget includes some specific requests for improving maternal health. It will be up to Congress to grant the requests.
The budget seeks $80 million for the Health Resources and Services Administration to improve access to and quality of care. It also includes money to expand Medicaid coverage for 1 year after birth for women with substance use disorders. The American Medical Association in 2019 adopted a policy urging Medicaid coverage to be expanded to include all women for a year after childbirth. The American College of Obstetricians and Gynecologists has also encouraged this extension.
“We are encouraged that the HHS action plan includes support for policies to close coverage and care gaps for all postpartum women after pregnancy-related Medicaid coverage expires,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in an interview.
The HHS could act immediately by approving Medicaid waivers to extend such coverage, Dr. Phipps said.
The budget also requests $24 million to expand maternal mortality review programs to every state, said Dr. Fink. Currently, 43 states and the District of Columbia, have such committees, which are charged with reviewing deaths of women within a year of pregnancy or birth.
The HHS will also join with the March of Dimes to address the disparities in Black women by implementing evidence-based best practices to improve quality in hospital settings.
It is not the first time the Trump administration has taken aim at reducing maternal morbidity and mortality. In 2018, the president signed the Preventing Maternal Deaths Act, which authorized the CDC to award $50 million over 5 years so that every state could form maternal mortality review committees.
A version of this article originally appeared on Medscape.com.