User login
Cancer therapy affects sexual health in most patients
Sexual dysfunction is a common treatment-related problem observed across numerous cancer diagnoses, and a new survey finds that 87% of cancer survivors have had such problems.
However, most of them also reported that their oncologist had not formally discussed the topic, and female patients were particularly unlikely to be asked about sexual dysfunction.
“The main takeaway from our study is that sexual side effects following treatment are very common,” said lead author James Taylor, MD, MPH, chief resident in radiation oncology at the Sidney Kimmel Cancer Center at Thomas Jefferson University in Philadelphia, Pennsylvania.
“Nearly 9 in 10 patients reported some change after cancer treatment that negatively affected their sexual health,” he said.
Taylor was speaking at the American Society for Radiation Oncology (ASTRO) Annual Meeting, held virtually this year because of the pandemic.
“Negative effects on sexual health after cancer treatment are unfortunately very common,” he said. “This is not just patients treated with radiation but this includes chemotherapy, hormonal therapy, surgery, and other treatment modalities.”
Potential issues include physical complications such as erectile dysfunction with prostate cancer treatment or vaginal dryness with gynecological cancer treatment. One recent study found that one-third of men who had undergone treatment for prostate cancer reported that a subsequent lack of sexual function has had the greatest impact on their quality of life. Another study reported that nearly all patients with breast cancer taking endocrine therapy experience a high degree of sexual dysfunction, including vulvovaginal dryness and severe dyspareunia.
Not discussed, not warned
Taylor and colleagues developed a questionnaire with input from radiation oncologists, medical oncologists, and surgeons, which consisted of more than 25 questions and was specifically targeted at cancer survivors.
A total of 405 adults completed the electronic survey about their experiences with sexual side effects after cancer treatment (391 responses were eligible for analysis). Most of the respondents were women (81%), and the most common cancer types were breast (67%), prostate (16%), and endometrial (6%). Treatments included chemotherapy (78%), radiation therapy (54%), and hormone therapy (47%).
“The questionnaires were distributed at Thomas Jefferson and throughout social media,” said Taylor. “The responses from social media are important because it shows a broad representation of patients who are treated in multiple clinics across the United States.”
Most of the survivors who responded (n = 337, 87%) stated cancer treatment had impacted sexual function or desire, with 53.8% reporting body image distortion, 73.4% with dyspareunia, and 42.3% unable to achieve orgasm.
Only about one-quarter (27.9%) said they had been formally asked about their sexual health by their clinician.
“Only about 40% said that they have been preemptively warned that their sexual health may be affected by treatment,” said Taylor.
Women were far less likely to be asked about their sexual health by their provider. The survey showed that male respondents were twice as likely to say they had been asked about sexual health and counseled about the potential toxicity (53% vs 22%; P < .001), and a substantially higher percentage of men reported receiving a formal assessment tool such as a survey (32% vs 5%; P = .001) compared with female respondents.
Taylor noted that the survey demonstrated several things. “One is that sexual toxicity is exceedingly common, and number two, it identified a gender disparity,” he said. “But number 3, and I think that this is an important aspect of our study, is that the majority of respondents felt that they would like a standard questionnaire to initiate and guide a discussion on sexual health with their provider.”
The reason that aspect is very important, he emphasized, is that “we know metrics and questionnaires already exist, so this gives us an actionable intervention that we can distribute and help mitigate some of these disparities.”
Importance of being holistic
The results of the survey “highlight the importance of being holistic in our approach to patient survivorship,” commented Karen Winkfield, MD, PhD, associate professor of radiation oncology at Wake Forest University, Winston-Salem, North Carolina, and executive director of the Meharry-Vanderbilt Alliance, Nashville, Tennessee.
“We need to ask patients about all parts of their well-being, including sexual health,” Winkfield said. “Body dysmorphism can impact anyone, but especially patients who have had surgery or radiation,” she said, while chemotherapy can impact energy and libido and have other toxicities that impact sexual health.
“I encourage all oncologists to ask patients about their sexual health, and a standardized form that can be used across all sites will make this much easier,” Winkfield commented. “We owe it to our patients to treat them holistically.”
The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Sexual dysfunction is a common treatment-related problem observed across numerous cancer diagnoses, and a new survey finds that 87% of cancer survivors have had such problems.
However, most of them also reported that their oncologist had not formally discussed the topic, and female patients were particularly unlikely to be asked about sexual dysfunction.
“The main takeaway from our study is that sexual side effects following treatment are very common,” said lead author James Taylor, MD, MPH, chief resident in radiation oncology at the Sidney Kimmel Cancer Center at Thomas Jefferson University in Philadelphia, Pennsylvania.
“Nearly 9 in 10 patients reported some change after cancer treatment that negatively affected their sexual health,” he said.
Taylor was speaking at the American Society for Radiation Oncology (ASTRO) Annual Meeting, held virtually this year because of the pandemic.
“Negative effects on sexual health after cancer treatment are unfortunately very common,” he said. “This is not just patients treated with radiation but this includes chemotherapy, hormonal therapy, surgery, and other treatment modalities.”
Potential issues include physical complications such as erectile dysfunction with prostate cancer treatment or vaginal dryness with gynecological cancer treatment. One recent study found that one-third of men who had undergone treatment for prostate cancer reported that a subsequent lack of sexual function has had the greatest impact on their quality of life. Another study reported that nearly all patients with breast cancer taking endocrine therapy experience a high degree of sexual dysfunction, including vulvovaginal dryness and severe dyspareunia.
Not discussed, not warned
Taylor and colleagues developed a questionnaire with input from radiation oncologists, medical oncologists, and surgeons, which consisted of more than 25 questions and was specifically targeted at cancer survivors.
A total of 405 adults completed the electronic survey about their experiences with sexual side effects after cancer treatment (391 responses were eligible for analysis). Most of the respondents were women (81%), and the most common cancer types were breast (67%), prostate (16%), and endometrial (6%). Treatments included chemotherapy (78%), radiation therapy (54%), and hormone therapy (47%).
“The questionnaires were distributed at Thomas Jefferson and throughout social media,” said Taylor. “The responses from social media are important because it shows a broad representation of patients who are treated in multiple clinics across the United States.”
Most of the survivors who responded (n = 337, 87%) stated cancer treatment had impacted sexual function or desire, with 53.8% reporting body image distortion, 73.4% with dyspareunia, and 42.3% unable to achieve orgasm.
Only about one-quarter (27.9%) said they had been formally asked about their sexual health by their clinician.
“Only about 40% said that they have been preemptively warned that their sexual health may be affected by treatment,” said Taylor.
Women were far less likely to be asked about their sexual health by their provider. The survey showed that male respondents were twice as likely to say they had been asked about sexual health and counseled about the potential toxicity (53% vs 22%; P < .001), and a substantially higher percentage of men reported receiving a formal assessment tool such as a survey (32% vs 5%; P = .001) compared with female respondents.
Taylor noted that the survey demonstrated several things. “One is that sexual toxicity is exceedingly common, and number two, it identified a gender disparity,” he said. “But number 3, and I think that this is an important aspect of our study, is that the majority of respondents felt that they would like a standard questionnaire to initiate and guide a discussion on sexual health with their provider.”
The reason that aspect is very important, he emphasized, is that “we know metrics and questionnaires already exist, so this gives us an actionable intervention that we can distribute and help mitigate some of these disparities.”
Importance of being holistic
The results of the survey “highlight the importance of being holistic in our approach to patient survivorship,” commented Karen Winkfield, MD, PhD, associate professor of radiation oncology at Wake Forest University, Winston-Salem, North Carolina, and executive director of the Meharry-Vanderbilt Alliance, Nashville, Tennessee.
“We need to ask patients about all parts of their well-being, including sexual health,” Winkfield said. “Body dysmorphism can impact anyone, but especially patients who have had surgery or radiation,” she said, while chemotherapy can impact energy and libido and have other toxicities that impact sexual health.
“I encourage all oncologists to ask patients about their sexual health, and a standardized form that can be used across all sites will make this much easier,” Winkfield commented. “We owe it to our patients to treat them holistically.”
The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
Sexual dysfunction is a common treatment-related problem observed across numerous cancer diagnoses, and a new survey finds that 87% of cancer survivors have had such problems.
However, most of them also reported that their oncologist had not formally discussed the topic, and female patients were particularly unlikely to be asked about sexual dysfunction.
“The main takeaway from our study is that sexual side effects following treatment are very common,” said lead author James Taylor, MD, MPH, chief resident in radiation oncology at the Sidney Kimmel Cancer Center at Thomas Jefferson University in Philadelphia, Pennsylvania.
“Nearly 9 in 10 patients reported some change after cancer treatment that negatively affected their sexual health,” he said.
Taylor was speaking at the American Society for Radiation Oncology (ASTRO) Annual Meeting, held virtually this year because of the pandemic.
“Negative effects on sexual health after cancer treatment are unfortunately very common,” he said. “This is not just patients treated with radiation but this includes chemotherapy, hormonal therapy, surgery, and other treatment modalities.”
Potential issues include physical complications such as erectile dysfunction with prostate cancer treatment or vaginal dryness with gynecological cancer treatment. One recent study found that one-third of men who had undergone treatment for prostate cancer reported that a subsequent lack of sexual function has had the greatest impact on their quality of life. Another study reported that nearly all patients with breast cancer taking endocrine therapy experience a high degree of sexual dysfunction, including vulvovaginal dryness and severe dyspareunia.
Not discussed, not warned
Taylor and colleagues developed a questionnaire with input from radiation oncologists, medical oncologists, and surgeons, which consisted of more than 25 questions and was specifically targeted at cancer survivors.
A total of 405 adults completed the electronic survey about their experiences with sexual side effects after cancer treatment (391 responses were eligible for analysis). Most of the respondents were women (81%), and the most common cancer types were breast (67%), prostate (16%), and endometrial (6%). Treatments included chemotherapy (78%), radiation therapy (54%), and hormone therapy (47%).
“The questionnaires were distributed at Thomas Jefferson and throughout social media,” said Taylor. “The responses from social media are important because it shows a broad representation of patients who are treated in multiple clinics across the United States.”
Most of the survivors who responded (n = 337, 87%) stated cancer treatment had impacted sexual function or desire, with 53.8% reporting body image distortion, 73.4% with dyspareunia, and 42.3% unable to achieve orgasm.
Only about one-quarter (27.9%) said they had been formally asked about their sexual health by their clinician.
“Only about 40% said that they have been preemptively warned that their sexual health may be affected by treatment,” said Taylor.
Women were far less likely to be asked about their sexual health by their provider. The survey showed that male respondents were twice as likely to say they had been asked about sexual health and counseled about the potential toxicity (53% vs 22%; P < .001), and a substantially higher percentage of men reported receiving a formal assessment tool such as a survey (32% vs 5%; P = .001) compared with female respondents.
Taylor noted that the survey demonstrated several things. “One is that sexual toxicity is exceedingly common, and number two, it identified a gender disparity,” he said. “But number 3, and I think that this is an important aspect of our study, is that the majority of respondents felt that they would like a standard questionnaire to initiate and guide a discussion on sexual health with their provider.”
The reason that aspect is very important, he emphasized, is that “we know metrics and questionnaires already exist, so this gives us an actionable intervention that we can distribute and help mitigate some of these disparities.”
Importance of being holistic
The results of the survey “highlight the importance of being holistic in our approach to patient survivorship,” commented Karen Winkfield, MD, PhD, associate professor of radiation oncology at Wake Forest University, Winston-Salem, North Carolina, and executive director of the Meharry-Vanderbilt Alliance, Nashville, Tennessee.
“We need to ask patients about all parts of their well-being, including sexual health,” Winkfield said. “Body dysmorphism can impact anyone, but especially patients who have had surgery or radiation,” she said, while chemotherapy can impact energy and libido and have other toxicities that impact sexual health.
“I encourage all oncologists to ask patients about their sexual health, and a standardized form that can be used across all sites will make this much easier,” Winkfield commented. “We owe it to our patients to treat them holistically.”
The authors have reported no relevant financial relationships.
This article first appeared on Medscape.com.
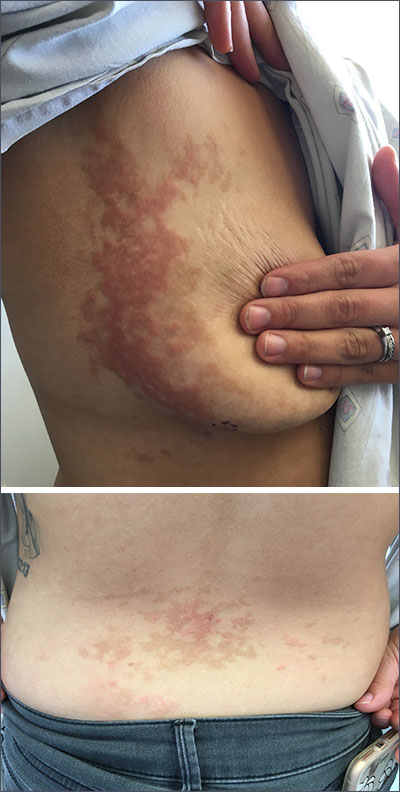
Pruritic rash on flank, back, and chest
The patient was given a diagnosis of prurigo pigmentosa based on the characteristic pruritic rash that had developed after the patient started a strict ketogenic diet.
Prurigo pigmentosa is a benign, pruritic rash that most commonly presents with erythematous or hyperpigmented, symmetrically distributed urticarial papules and plaques on the chest and back. Females represent approximately 70% of cases with a predominant age range of 11 to 30. It more commonly is seen in people of Asian descent.
While the pathophysiology remains unknown, the rash most commonly occurs in association with diabetes, ketosis, and more recently with ketogenic diets. Despite occurring in only a fraction of patients on the ketogenic diet, the characteristic presentation has led to the alternative name of the “keto rash” in online nutritional forums and blogs.
The diagnosis is made clinically, so the appearance of a symmetric pruritic, hyperpigmented rash on the chest and back should prompt the physician to ask about any recent changes in diet. Laboratory analysis is unnecessary, as a complete blood count, basic metabolic panel, and liver function panel are almost always normal.
Other conditions can mimic prurigo pigmentosa such as urticaria, irritant contact dermatitis, confluent and reticulated papillomatosis, and pityriasis rosea.
Primary treatment includes resumption of a normal diet. This often leads to rapid resolution of pruritis. Residual hyperpigmentation may take months to fade. If additional treatment is required, minocycline 100 to 200 mg/d has been reported most effective, likely due to its anti-inflammatory properties. Topical corticosteroids and oral antihistamines provide symptomatic relief in some patients.
This patient had resolution of the pruritis and urticarial lesions within 2 days of resuming a normal diet; however, residual asymptomatic hyperpigmentation persisted. A retrial of the ketogenic diet initiated a flare of the rash in the same distribution. It rapidly resolved with carbohydrate intake.
This case was adapted from: Croom D, Barlow T, Landers JT. Pruritic rash on chest and back. J Fam Pract. 2019;68:113-114,116
The patient was given a diagnosis of prurigo pigmentosa based on the characteristic pruritic rash that had developed after the patient started a strict ketogenic diet.
Prurigo pigmentosa is a benign, pruritic rash that most commonly presents with erythematous or hyperpigmented, symmetrically distributed urticarial papules and plaques on the chest and back. Females represent approximately 70% of cases with a predominant age range of 11 to 30. It more commonly is seen in people of Asian descent.
While the pathophysiology remains unknown, the rash most commonly occurs in association with diabetes, ketosis, and more recently with ketogenic diets. Despite occurring in only a fraction of patients on the ketogenic diet, the characteristic presentation has led to the alternative name of the “keto rash” in online nutritional forums and blogs.
The diagnosis is made clinically, so the appearance of a symmetric pruritic, hyperpigmented rash on the chest and back should prompt the physician to ask about any recent changes in diet. Laboratory analysis is unnecessary, as a complete blood count, basic metabolic panel, and liver function panel are almost always normal.
Other conditions can mimic prurigo pigmentosa such as urticaria, irritant contact dermatitis, confluent and reticulated papillomatosis, and pityriasis rosea.
Primary treatment includes resumption of a normal diet. This often leads to rapid resolution of pruritis. Residual hyperpigmentation may take months to fade. If additional treatment is required, minocycline 100 to 200 mg/d has been reported most effective, likely due to its anti-inflammatory properties. Topical corticosteroids and oral antihistamines provide symptomatic relief in some patients.
This patient had resolution of the pruritis and urticarial lesions within 2 days of resuming a normal diet; however, residual asymptomatic hyperpigmentation persisted. A retrial of the ketogenic diet initiated a flare of the rash in the same distribution. It rapidly resolved with carbohydrate intake.
This case was adapted from: Croom D, Barlow T, Landers JT. Pruritic rash on chest and back. J Fam Pract. 2019;68:113-114,116
The patient was given a diagnosis of prurigo pigmentosa based on the characteristic pruritic rash that had developed after the patient started a strict ketogenic diet.
Prurigo pigmentosa is a benign, pruritic rash that most commonly presents with erythematous or hyperpigmented, symmetrically distributed urticarial papules and plaques on the chest and back. Females represent approximately 70% of cases with a predominant age range of 11 to 30. It more commonly is seen in people of Asian descent.
While the pathophysiology remains unknown, the rash most commonly occurs in association with diabetes, ketosis, and more recently with ketogenic diets. Despite occurring in only a fraction of patients on the ketogenic diet, the characteristic presentation has led to the alternative name of the “keto rash” in online nutritional forums and blogs.
The diagnosis is made clinically, so the appearance of a symmetric pruritic, hyperpigmented rash on the chest and back should prompt the physician to ask about any recent changes in diet. Laboratory analysis is unnecessary, as a complete blood count, basic metabolic panel, and liver function panel are almost always normal.
Other conditions can mimic prurigo pigmentosa such as urticaria, irritant contact dermatitis, confluent and reticulated papillomatosis, and pityriasis rosea.
Primary treatment includes resumption of a normal diet. This often leads to rapid resolution of pruritis. Residual hyperpigmentation may take months to fade. If additional treatment is required, minocycline 100 to 200 mg/d has been reported most effective, likely due to its anti-inflammatory properties. Topical corticosteroids and oral antihistamines provide symptomatic relief in some patients.
This patient had resolution of the pruritis and urticarial lesions within 2 days of resuming a normal diet; however, residual asymptomatic hyperpigmentation persisted. A retrial of the ketogenic diet initiated a flare of the rash in the same distribution. It rapidly resolved with carbohydrate intake.
This case was adapted from: Croom D, Barlow T, Landers JT. Pruritic rash on chest and back. J Fam Pract. 2019;68:113-114,116
Decide ADHD pharmacotherapy based on medication onset, duration of action
Clinicians have numerous pharmacotherapy options available to treat ADHD in their toolbox. How do you know which formulation or combination of therapies is right for your patient with ADHD?
According to Jeffrey R. Strawn, MD, the answer depends on onset and duration of the medication and how that fits in to the patient’s current needs.
The most common treatment for ADHD, stimulants, are amphetamine-based and methylphenidate-based compounds known for improving core symptoms of inattention, impulsivity, and hyperactivity and are “probably associated with the most efficacy relative to the other interventions,” Dr. Strawn, associate professor of psychiatry, pediatrics, and clinical pharmacology at Cincinnati Children’s Hospital Medical Center, said at Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “But what I think is also really important for us to remember as clinicians is that they improve adherence, social interactions, [and] academic efficiency as well as accuracy.”
Other ADHD pharmacotherapy options include nonstimulant norepinephrine reuptake inhibitors (NRIs) like atomoxetine, and alpha-2 agonists like the extended-release forms of guanfacine and clonidine. All are Food and Drug Administration–approved for the treatment of ADHD, and the FDA has approved some combination alpha-2 agonists and stimulants treatments for ADHD as well.
When making decisions about formulations for ADHD pharmacotherapy, clinicians should think about whether the patient has issues swallowing tablets or capsules. Tablets, capsules, and chewable tablets may be appropriate for patients who can easily take these medications, while patients who have problems with swallowing pills may benefit from dissolvable tablets, solutions, and transdermal applications. Each of these options “have differences in terms of absorption, also differences in terms of intestinal transit time in younger children, as well as patients perhaps with irritable bowel, as well as other conditions that may affect absorption,” Dr. Strawn said. Different formulations have unique considerations: liquid formulations have the benefit of making precise adjustments, sublingual formulations may have quick absorption and onset, and oral dissolvable tablets can improve treatment adherence and reduce misuse of medication.
Formulations can be available as a delayed release, extended release, pulsatile release, targeted release, or a combination of immediate, delayed, and/or extended release. “Ultimately, what this gives rise to is differences in onset of action and duration, as well as differences in the elimination profile of the medication,” he said.
Transdermal formulations “avoid the first-pass metabolism, which may reduce side effects or increase efficacy,” but patients converting from an oral formulation may require reducing the dose. “It’s always important to remember, for example, with something like Daytrana, the transdermal methylphenidate formulation, if we’re converting a patient from an oral methylphenidate, we roughly need to use half the dose for the transdermal formulation,” Dr. Strawn explained. Transdermal formulations can carry benefits of steady plasma concentrations and longer duration of action but may cause skin irritation or accidentally be removed. “It’s really important they’re properly disposed of because oftentimes they do contain some active medication within the residual matrix.”
Methylphenidate, mixed amphetamine salt–based preparations
Modified-release formulations include matrix- or reservoir-based formulations and are most importantly differentiated from other formulations by their gastrointestinal (GI) transit time and the permeation through the GI membrane. When considering what formulation to choose, “it’s important to consider that, even with an ‘extended release formulation,’ all of these medications have some percentage that is immediately released, and that percentage varies considerably from formulation to formulation,” Dr. Strawn said.
He noted that brand names are sometimes used for formulations “because it’s often very difficult for us as clinicians and even for pharmacists to distinguish between these various formulations of the medication, which often have the same ‘extended’ or ‘delayed release’ modifying term within the name of the medication.”
Examples of medications that have greater immediate release include Metadate CD (30%), Aptensio XR (37%), long-acting methylphenidate (50%), dexmethylphenidate extended-release (50%), and Mixed Salts amphetamine extended release (50%). Formulations with a less immediate release include Quillivant solution or Quillichew chewable tablets (20%), Dyanaval XR solution (20%), OROS methylphenidate (22%), Daytrana that begins within 1 or 2 hours and lasts for 9 hours, or lisdexamfetamine that begins within 1 hour and lasts for 9 hours.
Depending on a patient’s needs, one particular formulation may work better than another. Dexmethylphenidate (Focalin XR) has a 50% immediate release and 50% extended release formulation, which “may be really important for a high school student who has first period precalculus followed by second period human geography,” Dr. Strawn said, while “a patient who may have first period study hall and second period art” may benefit from OROS methylphenidate.
Clinicians should also consider the effect of counterclockwise hysteresis when adding a short-acting stimulant later in the day. “There seems to be something really magic about having that ascending concentration time curve that, when we’re on the descending loop of that concentration time curve, we really seem to get a dramatic waning of the effect of the medication, even though technically the concentration is within the ‘therapeutic range,’ ” Dr. Strawn said. “With counterclockwise hysteresis, we see that the effect increases with time for a given concentration of the medication.”
Combining ADHD pharmacotherapies
For children and adolescents with ADHD, atomoxetine is a nonstimulant, FDA-approved treatment option. “It seems to be effective not just in terms of total ADHD symptoms, but also in terms of hyperactive and impulsive symptoms as well as the inattentive symptoms,” Dr. Strawn said.
Pharmacogenetics can be a guide for selecting an atomoxetine for a patient with ADHD, he noted. “What I think is most relevant here is the way in which pharmacogenetics can actually help guide our dosing, which then optimizes tolerability, potentially efficacy of atomoxetine,” he said. “Atomoxetine is pretty extensively metabolized by [CYP]2D6, and it’s one of about 300 medications that actually has specific labeling from the FDA on dosing based on genotype. It recommends a slower titration, as well as a lower target dose of atomoxetine in individuals who are P450 2D6 poor metabolizers relative to those patients who are ultra-rapid or normal metabolizers.”
Atomoxetine is most often combined with methylphenidate and has some evidence of benefit in children or adolescents who do not have an adequate response to stimulants alone. When combining stimulants with the alpha-2 agonists guanfacine or clonidine, “there are some improvements in terms of the combination treatment relative to the monotherapy,” Dr. Strawn said. He also emphasized that patients taking guanfacine immediate release tend to have better absorption and faster onset, compared with the extended release formulation. “This is something that potentially is very important when we think beyond steady state and we think about the practical use of this medication,” he said.
Baseline history is important
Overall, taking a baseline history of a patient with ADHD is “critically important” before starting them on stimulants, Dr. Strawn said. “Specifically, I would recommend documenting a negative history of syncope, family history of sudden cardiac death, as well as the lack of any known history of structural cardiac abnormalities,” he said. “Without a consultation with the cardiologist specifically around this question, I’m very, very, very hesitant – as in I don’t – use stimulants in patients who have histories of aortic stenosis, Wolff-Parkinson-White, as well as arrhythmogenic right ventricular dysplasia.”
Although patients with ADHD were typically followed with routine hemodynamic monitoring every 3 months prior to the COVID-19 pandemic, some clinicians see their patients with ADHD less frequently if they have been stabilized on a stimulant. , particularly in young children. In adults, it may also be very helpful to talk with spouses,” Dr. Strawn said.
Dr. Strawn also called attention to a recommendation to perform a routine electrocardiogram (EKG) in patients with ADHD who might receive stimulants. “At present, there is no recommendation to obtain a routine screening EKG in these patients, provided that we have an absence of those other red flags on the history,” he said. “Certainly, I would consider it in situations where I do have persistent tachycardia or hypertension, or there are other treatment-emergent symptoms, although really in many of these situations, I’m actually speaking on the phone with my pediatric or adult cardiology colleagues.”
Global Academy and this news organization are owned by the same parent company. Dr. Strawn reported receiving research support from Allergan, the FDA, the National Institutes of Health, Neuronetics, and Otsuka; serving as a consultant and receiving material support from Myriad; receiving royalties from Springer Publishing; and serving as a consultant for Intra-Cellular Therapies. In addition, he has been on the speaker’s bureau for the Neuroscience Education Institute and CMEology, and Medscape.
Clinicians have numerous pharmacotherapy options available to treat ADHD in their toolbox. How do you know which formulation or combination of therapies is right for your patient with ADHD?
According to Jeffrey R. Strawn, MD, the answer depends on onset and duration of the medication and how that fits in to the patient’s current needs.
The most common treatment for ADHD, stimulants, are amphetamine-based and methylphenidate-based compounds known for improving core symptoms of inattention, impulsivity, and hyperactivity and are “probably associated with the most efficacy relative to the other interventions,” Dr. Strawn, associate professor of psychiatry, pediatrics, and clinical pharmacology at Cincinnati Children’s Hospital Medical Center, said at Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “But what I think is also really important for us to remember as clinicians is that they improve adherence, social interactions, [and] academic efficiency as well as accuracy.”
Other ADHD pharmacotherapy options include nonstimulant norepinephrine reuptake inhibitors (NRIs) like atomoxetine, and alpha-2 agonists like the extended-release forms of guanfacine and clonidine. All are Food and Drug Administration–approved for the treatment of ADHD, and the FDA has approved some combination alpha-2 agonists and stimulants treatments for ADHD as well.
When making decisions about formulations for ADHD pharmacotherapy, clinicians should think about whether the patient has issues swallowing tablets or capsules. Tablets, capsules, and chewable tablets may be appropriate for patients who can easily take these medications, while patients who have problems with swallowing pills may benefit from dissolvable tablets, solutions, and transdermal applications. Each of these options “have differences in terms of absorption, also differences in terms of intestinal transit time in younger children, as well as patients perhaps with irritable bowel, as well as other conditions that may affect absorption,” Dr. Strawn said. Different formulations have unique considerations: liquid formulations have the benefit of making precise adjustments, sublingual formulations may have quick absorption and onset, and oral dissolvable tablets can improve treatment adherence and reduce misuse of medication.
Formulations can be available as a delayed release, extended release, pulsatile release, targeted release, or a combination of immediate, delayed, and/or extended release. “Ultimately, what this gives rise to is differences in onset of action and duration, as well as differences in the elimination profile of the medication,” he said.
Transdermal formulations “avoid the first-pass metabolism, which may reduce side effects or increase efficacy,” but patients converting from an oral formulation may require reducing the dose. “It’s always important to remember, for example, with something like Daytrana, the transdermal methylphenidate formulation, if we’re converting a patient from an oral methylphenidate, we roughly need to use half the dose for the transdermal formulation,” Dr. Strawn explained. Transdermal formulations can carry benefits of steady plasma concentrations and longer duration of action but may cause skin irritation or accidentally be removed. “It’s really important they’re properly disposed of because oftentimes they do contain some active medication within the residual matrix.”
Methylphenidate, mixed amphetamine salt–based preparations
Modified-release formulations include matrix- or reservoir-based formulations and are most importantly differentiated from other formulations by their gastrointestinal (GI) transit time and the permeation through the GI membrane. When considering what formulation to choose, “it’s important to consider that, even with an ‘extended release formulation,’ all of these medications have some percentage that is immediately released, and that percentage varies considerably from formulation to formulation,” Dr. Strawn said.
He noted that brand names are sometimes used for formulations “because it’s often very difficult for us as clinicians and even for pharmacists to distinguish between these various formulations of the medication, which often have the same ‘extended’ or ‘delayed release’ modifying term within the name of the medication.”
Examples of medications that have greater immediate release include Metadate CD (30%), Aptensio XR (37%), long-acting methylphenidate (50%), dexmethylphenidate extended-release (50%), and Mixed Salts amphetamine extended release (50%). Formulations with a less immediate release include Quillivant solution or Quillichew chewable tablets (20%), Dyanaval XR solution (20%), OROS methylphenidate (22%), Daytrana that begins within 1 or 2 hours and lasts for 9 hours, or lisdexamfetamine that begins within 1 hour and lasts for 9 hours.
Depending on a patient’s needs, one particular formulation may work better than another. Dexmethylphenidate (Focalin XR) has a 50% immediate release and 50% extended release formulation, which “may be really important for a high school student who has first period precalculus followed by second period human geography,” Dr. Strawn said, while “a patient who may have first period study hall and second period art” may benefit from OROS methylphenidate.
Clinicians should also consider the effect of counterclockwise hysteresis when adding a short-acting stimulant later in the day. “There seems to be something really magic about having that ascending concentration time curve that, when we’re on the descending loop of that concentration time curve, we really seem to get a dramatic waning of the effect of the medication, even though technically the concentration is within the ‘therapeutic range,’ ” Dr. Strawn said. “With counterclockwise hysteresis, we see that the effect increases with time for a given concentration of the medication.”
Combining ADHD pharmacotherapies
For children and adolescents with ADHD, atomoxetine is a nonstimulant, FDA-approved treatment option. “It seems to be effective not just in terms of total ADHD symptoms, but also in terms of hyperactive and impulsive symptoms as well as the inattentive symptoms,” Dr. Strawn said.
Pharmacogenetics can be a guide for selecting an atomoxetine for a patient with ADHD, he noted. “What I think is most relevant here is the way in which pharmacogenetics can actually help guide our dosing, which then optimizes tolerability, potentially efficacy of atomoxetine,” he said. “Atomoxetine is pretty extensively metabolized by [CYP]2D6, and it’s one of about 300 medications that actually has specific labeling from the FDA on dosing based on genotype. It recommends a slower titration, as well as a lower target dose of atomoxetine in individuals who are P450 2D6 poor metabolizers relative to those patients who are ultra-rapid or normal metabolizers.”
Atomoxetine is most often combined with methylphenidate and has some evidence of benefit in children or adolescents who do not have an adequate response to stimulants alone. When combining stimulants with the alpha-2 agonists guanfacine or clonidine, “there are some improvements in terms of the combination treatment relative to the monotherapy,” Dr. Strawn said. He also emphasized that patients taking guanfacine immediate release tend to have better absorption and faster onset, compared with the extended release formulation. “This is something that potentially is very important when we think beyond steady state and we think about the practical use of this medication,” he said.
Baseline history is important
Overall, taking a baseline history of a patient with ADHD is “critically important” before starting them on stimulants, Dr. Strawn said. “Specifically, I would recommend documenting a negative history of syncope, family history of sudden cardiac death, as well as the lack of any known history of structural cardiac abnormalities,” he said. “Without a consultation with the cardiologist specifically around this question, I’m very, very, very hesitant – as in I don’t – use stimulants in patients who have histories of aortic stenosis, Wolff-Parkinson-White, as well as arrhythmogenic right ventricular dysplasia.”
Although patients with ADHD were typically followed with routine hemodynamic monitoring every 3 months prior to the COVID-19 pandemic, some clinicians see their patients with ADHD less frequently if they have been stabilized on a stimulant. , particularly in young children. In adults, it may also be very helpful to talk with spouses,” Dr. Strawn said.
Dr. Strawn also called attention to a recommendation to perform a routine electrocardiogram (EKG) in patients with ADHD who might receive stimulants. “At present, there is no recommendation to obtain a routine screening EKG in these patients, provided that we have an absence of those other red flags on the history,” he said. “Certainly, I would consider it in situations where I do have persistent tachycardia or hypertension, or there are other treatment-emergent symptoms, although really in many of these situations, I’m actually speaking on the phone with my pediatric or adult cardiology colleagues.”
Global Academy and this news organization are owned by the same parent company. Dr. Strawn reported receiving research support from Allergan, the FDA, the National Institutes of Health, Neuronetics, and Otsuka; serving as a consultant and receiving material support from Myriad; receiving royalties from Springer Publishing; and serving as a consultant for Intra-Cellular Therapies. In addition, he has been on the speaker’s bureau for the Neuroscience Education Institute and CMEology, and Medscape.
Clinicians have numerous pharmacotherapy options available to treat ADHD in their toolbox. How do you know which formulation or combination of therapies is right for your patient with ADHD?
According to Jeffrey R. Strawn, MD, the answer depends on onset and duration of the medication and how that fits in to the patient’s current needs.
The most common treatment for ADHD, stimulants, are amphetamine-based and methylphenidate-based compounds known for improving core symptoms of inattention, impulsivity, and hyperactivity and are “probably associated with the most efficacy relative to the other interventions,” Dr. Strawn, associate professor of psychiatry, pediatrics, and clinical pharmacology at Cincinnati Children’s Hospital Medical Center, said at Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “But what I think is also really important for us to remember as clinicians is that they improve adherence, social interactions, [and] academic efficiency as well as accuracy.”
Other ADHD pharmacotherapy options include nonstimulant norepinephrine reuptake inhibitors (NRIs) like atomoxetine, and alpha-2 agonists like the extended-release forms of guanfacine and clonidine. All are Food and Drug Administration–approved for the treatment of ADHD, and the FDA has approved some combination alpha-2 agonists and stimulants treatments for ADHD as well.
When making decisions about formulations for ADHD pharmacotherapy, clinicians should think about whether the patient has issues swallowing tablets or capsules. Tablets, capsules, and chewable tablets may be appropriate for patients who can easily take these medications, while patients who have problems with swallowing pills may benefit from dissolvable tablets, solutions, and transdermal applications. Each of these options “have differences in terms of absorption, also differences in terms of intestinal transit time in younger children, as well as patients perhaps with irritable bowel, as well as other conditions that may affect absorption,” Dr. Strawn said. Different formulations have unique considerations: liquid formulations have the benefit of making precise adjustments, sublingual formulations may have quick absorption and onset, and oral dissolvable tablets can improve treatment adherence and reduce misuse of medication.
Formulations can be available as a delayed release, extended release, pulsatile release, targeted release, or a combination of immediate, delayed, and/or extended release. “Ultimately, what this gives rise to is differences in onset of action and duration, as well as differences in the elimination profile of the medication,” he said.
Transdermal formulations “avoid the first-pass metabolism, which may reduce side effects or increase efficacy,” but patients converting from an oral formulation may require reducing the dose. “It’s always important to remember, for example, with something like Daytrana, the transdermal methylphenidate formulation, if we’re converting a patient from an oral methylphenidate, we roughly need to use half the dose for the transdermal formulation,” Dr. Strawn explained. Transdermal formulations can carry benefits of steady plasma concentrations and longer duration of action but may cause skin irritation or accidentally be removed. “It’s really important they’re properly disposed of because oftentimes they do contain some active medication within the residual matrix.”
Methylphenidate, mixed amphetamine salt–based preparations
Modified-release formulations include matrix- or reservoir-based formulations and are most importantly differentiated from other formulations by their gastrointestinal (GI) transit time and the permeation through the GI membrane. When considering what formulation to choose, “it’s important to consider that, even with an ‘extended release formulation,’ all of these medications have some percentage that is immediately released, and that percentage varies considerably from formulation to formulation,” Dr. Strawn said.
He noted that brand names are sometimes used for formulations “because it’s often very difficult for us as clinicians and even for pharmacists to distinguish between these various formulations of the medication, which often have the same ‘extended’ or ‘delayed release’ modifying term within the name of the medication.”
Examples of medications that have greater immediate release include Metadate CD (30%), Aptensio XR (37%), long-acting methylphenidate (50%), dexmethylphenidate extended-release (50%), and Mixed Salts amphetamine extended release (50%). Formulations with a less immediate release include Quillivant solution or Quillichew chewable tablets (20%), Dyanaval XR solution (20%), OROS methylphenidate (22%), Daytrana that begins within 1 or 2 hours and lasts for 9 hours, or lisdexamfetamine that begins within 1 hour and lasts for 9 hours.
Depending on a patient’s needs, one particular formulation may work better than another. Dexmethylphenidate (Focalin XR) has a 50% immediate release and 50% extended release formulation, which “may be really important for a high school student who has first period precalculus followed by second period human geography,” Dr. Strawn said, while “a patient who may have first period study hall and second period art” may benefit from OROS methylphenidate.
Clinicians should also consider the effect of counterclockwise hysteresis when adding a short-acting stimulant later in the day. “There seems to be something really magic about having that ascending concentration time curve that, when we’re on the descending loop of that concentration time curve, we really seem to get a dramatic waning of the effect of the medication, even though technically the concentration is within the ‘therapeutic range,’ ” Dr. Strawn said. “With counterclockwise hysteresis, we see that the effect increases with time for a given concentration of the medication.”
Combining ADHD pharmacotherapies
For children and adolescents with ADHD, atomoxetine is a nonstimulant, FDA-approved treatment option. “It seems to be effective not just in terms of total ADHD symptoms, but also in terms of hyperactive and impulsive symptoms as well as the inattentive symptoms,” Dr. Strawn said.
Pharmacogenetics can be a guide for selecting an atomoxetine for a patient with ADHD, he noted. “What I think is most relevant here is the way in which pharmacogenetics can actually help guide our dosing, which then optimizes tolerability, potentially efficacy of atomoxetine,” he said. “Atomoxetine is pretty extensively metabolized by [CYP]2D6, and it’s one of about 300 medications that actually has specific labeling from the FDA on dosing based on genotype. It recommends a slower titration, as well as a lower target dose of atomoxetine in individuals who are P450 2D6 poor metabolizers relative to those patients who are ultra-rapid or normal metabolizers.”
Atomoxetine is most often combined with methylphenidate and has some evidence of benefit in children or adolescents who do not have an adequate response to stimulants alone. When combining stimulants with the alpha-2 agonists guanfacine or clonidine, “there are some improvements in terms of the combination treatment relative to the monotherapy,” Dr. Strawn said. He also emphasized that patients taking guanfacine immediate release tend to have better absorption and faster onset, compared with the extended release formulation. “This is something that potentially is very important when we think beyond steady state and we think about the practical use of this medication,” he said.
Baseline history is important
Overall, taking a baseline history of a patient with ADHD is “critically important” before starting them on stimulants, Dr. Strawn said. “Specifically, I would recommend documenting a negative history of syncope, family history of sudden cardiac death, as well as the lack of any known history of structural cardiac abnormalities,” he said. “Without a consultation with the cardiologist specifically around this question, I’m very, very, very hesitant – as in I don’t – use stimulants in patients who have histories of aortic stenosis, Wolff-Parkinson-White, as well as arrhythmogenic right ventricular dysplasia.”
Although patients with ADHD were typically followed with routine hemodynamic monitoring every 3 months prior to the COVID-19 pandemic, some clinicians see their patients with ADHD less frequently if they have been stabilized on a stimulant. , particularly in young children. In adults, it may also be very helpful to talk with spouses,” Dr. Strawn said.
Dr. Strawn also called attention to a recommendation to perform a routine electrocardiogram (EKG) in patients with ADHD who might receive stimulants. “At present, there is no recommendation to obtain a routine screening EKG in these patients, provided that we have an absence of those other red flags on the history,” he said. “Certainly, I would consider it in situations where I do have persistent tachycardia or hypertension, or there are other treatment-emergent symptoms, although really in many of these situations, I’m actually speaking on the phone with my pediatric or adult cardiology colleagues.”
Global Academy and this news organization are owned by the same parent company. Dr. Strawn reported receiving research support from Allergan, the FDA, the National Institutes of Health, Neuronetics, and Otsuka; serving as a consultant and receiving material support from Myriad; receiving royalties from Springer Publishing; and serving as a consultant for Intra-Cellular Therapies. In addition, he has been on the speaker’s bureau for the Neuroscience Education Institute and CMEology, and Medscape.
FROM PSYCHOPHARMACOLOGY UPDATE
Few women hospitalized for influenza have been vaccinated
Researchers analyzed data from 9,652 women ages 15-44 who were hospitalized with laboratory-confirmed influenza from October through April during the 2010-2019 influenza seasons. Data were pulled from the U.S. Influenza Hospitalization Surveillance Network (FluSurv-NET).
Of those women, 2,697 (28%) were pregnant. Median age was 28 and median gestational age was 32 weeks. Those studied included 36% who were non-Hispanic White; 29% non-Hispanic Black; and 20% Hispanic women.
Some 89% of the women, pregnant and nonpregnant, received antivirals while in the hospital but only 31% reported they had received the flu vaccine in the current season, despite guideline recommendations citing clear evidence that vaccination is safe for mother and baby.
Rachel Holstein, MPH, an epidemiology and information science fellow at the Centers for Disease Control and Prevention, who presented her team’s work as part of IDWeek 2020, explained that the mother’s vaccination can help protect the baby from flu infection for several months after birth, before the baby can be vaccinated.
She noted that pregnant women are at high risk for influenza-associated hospitalization.
“Changes in the immune system, heart, and lungs during pregnancy make pregnant women, and women up to 2 weeks post partum, more prone to severe illness from flu, including illness resulting in hospitalization,” she said in an interview
“Vaccination has been shown to reduce the risk of flu-associated acute respiratory infection in pregnant women by up to one-half,” she said. “A 2018 study showed that getting a flu shot reduced a pregnant woman’s risk of being hospitalized with flu by an average of 40%.»
FluSurv-NET data show hospitalizations were more common in the third trimester of pregnancy compared with the first and second, Holstein said. The most common underlying conditions among these women were asthma (23%) and obesity (10%), and 12% were current tobacco smokers. Overall, 5% of pregnant women with flu required ICU admission, 2% needed mechanical ventilation, and 6% developed pneumonia.
Vaccine uptake lowest in first two trimesters
Holstein said vaccine coverage was lowest among women in their first or second trimesters for all 9 seasons, and overall vaccination coverage increased significantly over time.
Uptake also differed by age. The data showed coverage was lower among women aged 15-34 years, compared with women 35 years and older (34% vs. 50%).
“It was as low as 15% among pregnant women aged 15-34 years in the 2011-12 season,” she added.
Jeanne Sheffield, MD, director of the division of maternal-fetal medicine at Johns Hopkins Medicine, Baltimore, said in an interview the low uptake of vaccine shown in this study is both familiar and frustrating.
She said education from health care providers has improved, but women are nonetheless frequently fearful. She pointed out the widespread phenomenon of vaccine hesitancy in the general population.
Coverage was 45.3% among adults in the 2018-2019 flu season, 8.2 percentage points higher than coverage during the 2017-18 season (37.1%) according to CDC estimates.
Added to that, she said, is further hesitancy when women believe vaccination could harm the unborn baby, despite “very good data that flu vaccine is safe in pregnancy, acceptable in pregnancy in all trimesters, and is optimal standard of care.”
Holstein added, “We know from past research that a range of factors – including negative attitudes and beliefs about vaccines, less knowledge about and access to vaccines, and a lack of trust in healthcare providers and vaccines – can contribute to lower vaccination rates.”
Healthcare providers play a key role in increasing flu vaccinations among pregnant women, she said.
“A provider recommendation, combined with an offer to administer a flu vaccine at the time of visit, remains one of the best ways to accomplish this,” Holstein said.
Holstein and Sheffield have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Researchers analyzed data from 9,652 women ages 15-44 who were hospitalized with laboratory-confirmed influenza from October through April during the 2010-2019 influenza seasons. Data were pulled from the U.S. Influenza Hospitalization Surveillance Network (FluSurv-NET).
Of those women, 2,697 (28%) were pregnant. Median age was 28 and median gestational age was 32 weeks. Those studied included 36% who were non-Hispanic White; 29% non-Hispanic Black; and 20% Hispanic women.
Some 89% of the women, pregnant and nonpregnant, received antivirals while in the hospital but only 31% reported they had received the flu vaccine in the current season, despite guideline recommendations citing clear evidence that vaccination is safe for mother and baby.
Rachel Holstein, MPH, an epidemiology and information science fellow at the Centers for Disease Control and Prevention, who presented her team’s work as part of IDWeek 2020, explained that the mother’s vaccination can help protect the baby from flu infection for several months after birth, before the baby can be vaccinated.
She noted that pregnant women are at high risk for influenza-associated hospitalization.
“Changes in the immune system, heart, and lungs during pregnancy make pregnant women, and women up to 2 weeks post partum, more prone to severe illness from flu, including illness resulting in hospitalization,” she said in an interview
“Vaccination has been shown to reduce the risk of flu-associated acute respiratory infection in pregnant women by up to one-half,” she said. “A 2018 study showed that getting a flu shot reduced a pregnant woman’s risk of being hospitalized with flu by an average of 40%.»
FluSurv-NET data show hospitalizations were more common in the third trimester of pregnancy compared with the first and second, Holstein said. The most common underlying conditions among these women were asthma (23%) and obesity (10%), and 12% were current tobacco smokers. Overall, 5% of pregnant women with flu required ICU admission, 2% needed mechanical ventilation, and 6% developed pneumonia.
Vaccine uptake lowest in first two trimesters
Holstein said vaccine coverage was lowest among women in their first or second trimesters for all 9 seasons, and overall vaccination coverage increased significantly over time.
Uptake also differed by age. The data showed coverage was lower among women aged 15-34 years, compared with women 35 years and older (34% vs. 50%).
“It was as low as 15% among pregnant women aged 15-34 years in the 2011-12 season,” she added.
Jeanne Sheffield, MD, director of the division of maternal-fetal medicine at Johns Hopkins Medicine, Baltimore, said in an interview the low uptake of vaccine shown in this study is both familiar and frustrating.
She said education from health care providers has improved, but women are nonetheless frequently fearful. She pointed out the widespread phenomenon of vaccine hesitancy in the general population.
Coverage was 45.3% among adults in the 2018-2019 flu season, 8.2 percentage points higher than coverage during the 2017-18 season (37.1%) according to CDC estimates.
Added to that, she said, is further hesitancy when women believe vaccination could harm the unborn baby, despite “very good data that flu vaccine is safe in pregnancy, acceptable in pregnancy in all trimesters, and is optimal standard of care.”
Holstein added, “We know from past research that a range of factors – including negative attitudes and beliefs about vaccines, less knowledge about and access to vaccines, and a lack of trust in healthcare providers and vaccines – can contribute to lower vaccination rates.”
Healthcare providers play a key role in increasing flu vaccinations among pregnant women, she said.
“A provider recommendation, combined with an offer to administer a flu vaccine at the time of visit, remains one of the best ways to accomplish this,” Holstein said.
Holstein and Sheffield have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Researchers analyzed data from 9,652 women ages 15-44 who were hospitalized with laboratory-confirmed influenza from October through April during the 2010-2019 influenza seasons. Data were pulled from the U.S. Influenza Hospitalization Surveillance Network (FluSurv-NET).
Of those women, 2,697 (28%) were pregnant. Median age was 28 and median gestational age was 32 weeks. Those studied included 36% who were non-Hispanic White; 29% non-Hispanic Black; and 20% Hispanic women.
Some 89% of the women, pregnant and nonpregnant, received antivirals while in the hospital but only 31% reported they had received the flu vaccine in the current season, despite guideline recommendations citing clear evidence that vaccination is safe for mother and baby.
Rachel Holstein, MPH, an epidemiology and information science fellow at the Centers for Disease Control and Prevention, who presented her team’s work as part of IDWeek 2020, explained that the mother’s vaccination can help protect the baby from flu infection for several months after birth, before the baby can be vaccinated.
She noted that pregnant women are at high risk for influenza-associated hospitalization.
“Changes in the immune system, heart, and lungs during pregnancy make pregnant women, and women up to 2 weeks post partum, more prone to severe illness from flu, including illness resulting in hospitalization,” she said in an interview
“Vaccination has been shown to reduce the risk of flu-associated acute respiratory infection in pregnant women by up to one-half,” she said. “A 2018 study showed that getting a flu shot reduced a pregnant woman’s risk of being hospitalized with flu by an average of 40%.»
FluSurv-NET data show hospitalizations were more common in the third trimester of pregnancy compared with the first and second, Holstein said. The most common underlying conditions among these women were asthma (23%) and obesity (10%), and 12% were current tobacco smokers. Overall, 5% of pregnant women with flu required ICU admission, 2% needed mechanical ventilation, and 6% developed pneumonia.
Vaccine uptake lowest in first two trimesters
Holstein said vaccine coverage was lowest among women in their first or second trimesters for all 9 seasons, and overall vaccination coverage increased significantly over time.
Uptake also differed by age. The data showed coverage was lower among women aged 15-34 years, compared with women 35 years and older (34% vs. 50%).
“It was as low as 15% among pregnant women aged 15-34 years in the 2011-12 season,” she added.
Jeanne Sheffield, MD, director of the division of maternal-fetal medicine at Johns Hopkins Medicine, Baltimore, said in an interview the low uptake of vaccine shown in this study is both familiar and frustrating.
She said education from health care providers has improved, but women are nonetheless frequently fearful. She pointed out the widespread phenomenon of vaccine hesitancy in the general population.
Coverage was 45.3% among adults in the 2018-2019 flu season, 8.2 percentage points higher than coverage during the 2017-18 season (37.1%) according to CDC estimates.
Added to that, she said, is further hesitancy when women believe vaccination could harm the unborn baby, despite “very good data that flu vaccine is safe in pregnancy, acceptable in pregnancy in all trimesters, and is optimal standard of care.”
Holstein added, “We know from past research that a range of factors – including negative attitudes and beliefs about vaccines, less knowledge about and access to vaccines, and a lack of trust in healthcare providers and vaccines – can contribute to lower vaccination rates.”
Healthcare providers play a key role in increasing flu vaccinations among pregnant women, she said.
“A provider recommendation, combined with an offer to administer a flu vaccine at the time of visit, remains one of the best ways to accomplish this,” Holstein said.
Holstein and Sheffield have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Menstrual irregularity appears to be predictor of early death
than women with regular or short cycles, reported Yi-Xin Wang, PhD, of Harvard TH Chan School of Public Health, Boston, and associates. This is particularly true in the presence of cardiovascular disease and a history of smoking.
In a peer-reviewed observational study of 79,505 premenopausal women enrolled in the Nurses’ Health Study II, the researchers sought to determine whether a life-long history of irregular or long menstrual cycles was associated with premature death. Patients averaged a mean age of 37.7 years and had no history of cardiovascular disease, cancer, or diabetes at enrollment.
Although irregular and long menstrual cycles are common and frequently linked with an increased risk of major chronic diseases – such as ovarian cancer, coronary heart disease, type 2 diabetes, and mental health problems – in women of reproductive age, actual evidence linking irregular or long menstrual cycles with mortality is scant, the researchers noted in the BMJ.
During the study, participants checked in at ages 14-17 years, 18-22 years, and 29-46 years to report the usual length and regularity of their menstrual cycles. Over 24 years of follow-up, a total of 1,975 premature deaths were noted, including 894 from cancer and 172 from cardiovascular disease.
Irregular cycles appear to bring risks
After considering other possible factors of influence, including age, weight, lifestyle, and family medical history, Dr. Wang and associates noted higher rates of mortality among those consistently reporting irregular cycles than women in the same age ranges with very regular cycles. Specifically, women aged 18-22 years and 29-46 years with cycles of 40 days or more were at greater risk of dying prematurely than were those in the same age ranges with cycles of 26-31 days.
Cardiovascular disease was a stronger predictor of death than cancer or other causes. Also included in the higher-risk group were those who currently smoked.
Among women reporting very regular cycles and women reporting always irregular cycles, mortality rates per 1,000 person-years were 1.05 and 1.23 at ages 14-17 years, 1.00 and 1.37 at ages 18-22 years, and 1.00 and 1.68 at ages 29-46 years, respectively.
The study also found that women reporting irregular cycles or no periods had a higher body mass indexes (28.2 vs. 25.0 kg/m2); were more likely to have conditions including hypertension (13.2% vs. 6.2%), high blood cholesterol levels (23.9% vs. 14.9%), hirsutism (8.4%
vs. 1.8%), or endometriosis (5.9% vs. 4.5%); and uterine fibroids (10.0% vs. 7.8%); and a higher prevalence of family history of diabetes (19.4% vs. 15.8%).
Dr. Wang and associates also observed – using multivariable Cox models – a greater risk of premature death across all categories and all age ranges in women with decreasing menstrual cycle regularity. In models that were fully adjusted, cycle lengths that were 40 days or more or too irregular to estimate from ages 18-22 and 29-46 expressed hazard ratios for premature death at the time of follow-up of 1.34 and 1.40, compared with women in the same age ranges reporting cycle lengths of 26-31 days.
Of note, Dr. Wang and colleagues unexpectedly discovered an increased risk of premature death in women who had used contraceptives between 14-17 years. They suggested that a greater number of women self-reporting contraceptive use in adolescence may have been using contraceptives to manage symptoms of polycystic ovary syndrome (PCOS) and other conditions such as endometriosis.
Relying on the potential inaccuracy inherent in patient recall of their menstrual cycle characteristics, and the likelihood for other unmeasured factors, may have affected study results. Study strengths included the significant number of participants who had a high follow-up rate over many years, and the availability of menstrual cycle data at three different points across the reproductive lifespan.
Because the mechanisms underlying these associations are likely related to the disrupted hormonal environment, the study results “emphasize the need for primary care providers to include menstrual cycle characteristics throughout the reproductive life span as additional vital signs in assessing women’s general health status,” Dr. Wang and colleagues cautioned.
Expert suggests a probable underlying link
“Irregular menstrual cycles in women have long been known to be associated with significant morbidities, including the leading causes of mortality worldwide such as cardiovascular disease and cancer,” Reshef Tal, MD, PhD, assistant professor of obstetrics, gynecology & reproductive sciences at Yale University, New Haven, Conn., said in an interview. “The findings of this large study that irregular menstrual cycles are associated with premature death, most strongly from cardiovascular causes, are therefore not surprising.”
Dr. Tal acknowledged that one probable underlying link is PCOS, which is recognized as the most common hormonal disorder affecting women of reproductive age. The irregular periods that characterize PCOS are tied to a number of metabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension, which increase the long-term risk of cardiovascular disease and cancer of the uterus.
“The study did not have information on patients’ pelvic ultrasound findings and male hormone levels, which would have helped to establish PCOS diagnosis. However, women in this study who had irregular cycles tended to have more hirsutism, high cholesterol, hypertension as well as higher BMI, suggesting that PCOS is at least partly responsible for the observed association with cardiovascular disease. Interestingly, the association between irregular cycles and early mortality was independent of BMI, indicating that mechanisms other than metabolic factors may also play a role,” observed Dr. Tal, who was asked to comment on the study.
“Irregular periods are a symptom and not a disease, so it is important to identify underlying metabolic risk factors. Furthermore, physicians are advised to counsel patients experiencing menstrual irregularity, [to advise them to] maintain a healthy lifestyle and be alert to health changes,” Dr. Tal suggested.
The study was funded by the National Institutes of Health. The investigators had no relevant financial disclosures. Dr. Tal said he had no relevant financial disclosures.
SOURCE: Chavarro J et al. BMJ. 2020. doi: 10.1136/bmj.m3464.
than women with regular or short cycles, reported Yi-Xin Wang, PhD, of Harvard TH Chan School of Public Health, Boston, and associates. This is particularly true in the presence of cardiovascular disease and a history of smoking.
In a peer-reviewed observational study of 79,505 premenopausal women enrolled in the Nurses’ Health Study II, the researchers sought to determine whether a life-long history of irregular or long menstrual cycles was associated with premature death. Patients averaged a mean age of 37.7 years and had no history of cardiovascular disease, cancer, or diabetes at enrollment.
Although irregular and long menstrual cycles are common and frequently linked with an increased risk of major chronic diseases – such as ovarian cancer, coronary heart disease, type 2 diabetes, and mental health problems – in women of reproductive age, actual evidence linking irregular or long menstrual cycles with mortality is scant, the researchers noted in the BMJ.
During the study, participants checked in at ages 14-17 years, 18-22 years, and 29-46 years to report the usual length and regularity of their menstrual cycles. Over 24 years of follow-up, a total of 1,975 premature deaths were noted, including 894 from cancer and 172 from cardiovascular disease.
Irregular cycles appear to bring risks
After considering other possible factors of influence, including age, weight, lifestyle, and family medical history, Dr. Wang and associates noted higher rates of mortality among those consistently reporting irregular cycles than women in the same age ranges with very regular cycles. Specifically, women aged 18-22 years and 29-46 years with cycles of 40 days or more were at greater risk of dying prematurely than were those in the same age ranges with cycles of 26-31 days.
Cardiovascular disease was a stronger predictor of death than cancer or other causes. Also included in the higher-risk group were those who currently smoked.
Among women reporting very regular cycles and women reporting always irregular cycles, mortality rates per 1,000 person-years were 1.05 and 1.23 at ages 14-17 years, 1.00 and 1.37 at ages 18-22 years, and 1.00 and 1.68 at ages 29-46 years, respectively.
The study also found that women reporting irregular cycles or no periods had a higher body mass indexes (28.2 vs. 25.0 kg/m2); were more likely to have conditions including hypertension (13.2% vs. 6.2%), high blood cholesterol levels (23.9% vs. 14.9%), hirsutism (8.4%
vs. 1.8%), or endometriosis (5.9% vs. 4.5%); and uterine fibroids (10.0% vs. 7.8%); and a higher prevalence of family history of diabetes (19.4% vs. 15.8%).
Dr. Wang and associates also observed – using multivariable Cox models – a greater risk of premature death across all categories and all age ranges in women with decreasing menstrual cycle regularity. In models that were fully adjusted, cycle lengths that were 40 days or more or too irregular to estimate from ages 18-22 and 29-46 expressed hazard ratios for premature death at the time of follow-up of 1.34 and 1.40, compared with women in the same age ranges reporting cycle lengths of 26-31 days.
Of note, Dr. Wang and colleagues unexpectedly discovered an increased risk of premature death in women who had used contraceptives between 14-17 years. They suggested that a greater number of women self-reporting contraceptive use in adolescence may have been using contraceptives to manage symptoms of polycystic ovary syndrome (PCOS) and other conditions such as endometriosis.
Relying on the potential inaccuracy inherent in patient recall of their menstrual cycle characteristics, and the likelihood for other unmeasured factors, may have affected study results. Study strengths included the significant number of participants who had a high follow-up rate over many years, and the availability of menstrual cycle data at three different points across the reproductive lifespan.
Because the mechanisms underlying these associations are likely related to the disrupted hormonal environment, the study results “emphasize the need for primary care providers to include menstrual cycle characteristics throughout the reproductive life span as additional vital signs in assessing women’s general health status,” Dr. Wang and colleagues cautioned.
Expert suggests a probable underlying link
“Irregular menstrual cycles in women have long been known to be associated with significant morbidities, including the leading causes of mortality worldwide such as cardiovascular disease and cancer,” Reshef Tal, MD, PhD, assistant professor of obstetrics, gynecology & reproductive sciences at Yale University, New Haven, Conn., said in an interview. “The findings of this large study that irregular menstrual cycles are associated with premature death, most strongly from cardiovascular causes, are therefore not surprising.”
Dr. Tal acknowledged that one probable underlying link is PCOS, which is recognized as the most common hormonal disorder affecting women of reproductive age. The irregular periods that characterize PCOS are tied to a number of metabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension, which increase the long-term risk of cardiovascular disease and cancer of the uterus.
“The study did not have information on patients’ pelvic ultrasound findings and male hormone levels, which would have helped to establish PCOS diagnosis. However, women in this study who had irregular cycles tended to have more hirsutism, high cholesterol, hypertension as well as higher BMI, suggesting that PCOS is at least partly responsible for the observed association with cardiovascular disease. Interestingly, the association between irregular cycles and early mortality was independent of BMI, indicating that mechanisms other than metabolic factors may also play a role,” observed Dr. Tal, who was asked to comment on the study.
“Irregular periods are a symptom and not a disease, so it is important to identify underlying metabolic risk factors. Furthermore, physicians are advised to counsel patients experiencing menstrual irregularity, [to advise them to] maintain a healthy lifestyle and be alert to health changes,” Dr. Tal suggested.
The study was funded by the National Institutes of Health. The investigators had no relevant financial disclosures. Dr. Tal said he had no relevant financial disclosures.
SOURCE: Chavarro J et al. BMJ. 2020. doi: 10.1136/bmj.m3464.
than women with regular or short cycles, reported Yi-Xin Wang, PhD, of Harvard TH Chan School of Public Health, Boston, and associates. This is particularly true in the presence of cardiovascular disease and a history of smoking.
In a peer-reviewed observational study of 79,505 premenopausal women enrolled in the Nurses’ Health Study II, the researchers sought to determine whether a life-long history of irregular or long menstrual cycles was associated with premature death. Patients averaged a mean age of 37.7 years and had no history of cardiovascular disease, cancer, or diabetes at enrollment.
Although irregular and long menstrual cycles are common and frequently linked with an increased risk of major chronic diseases – such as ovarian cancer, coronary heart disease, type 2 diabetes, and mental health problems – in women of reproductive age, actual evidence linking irregular or long menstrual cycles with mortality is scant, the researchers noted in the BMJ.
During the study, participants checked in at ages 14-17 years, 18-22 years, and 29-46 years to report the usual length and regularity of their menstrual cycles. Over 24 years of follow-up, a total of 1,975 premature deaths were noted, including 894 from cancer and 172 from cardiovascular disease.
Irregular cycles appear to bring risks
After considering other possible factors of influence, including age, weight, lifestyle, and family medical history, Dr. Wang and associates noted higher rates of mortality among those consistently reporting irregular cycles than women in the same age ranges with very regular cycles. Specifically, women aged 18-22 years and 29-46 years with cycles of 40 days or more were at greater risk of dying prematurely than were those in the same age ranges with cycles of 26-31 days.
Cardiovascular disease was a stronger predictor of death than cancer or other causes. Also included in the higher-risk group were those who currently smoked.
Among women reporting very regular cycles and women reporting always irregular cycles, mortality rates per 1,000 person-years were 1.05 and 1.23 at ages 14-17 years, 1.00 and 1.37 at ages 18-22 years, and 1.00 and 1.68 at ages 29-46 years, respectively.
The study also found that women reporting irregular cycles or no periods had a higher body mass indexes (28.2 vs. 25.0 kg/m2); were more likely to have conditions including hypertension (13.2% vs. 6.2%), high blood cholesterol levels (23.9% vs. 14.9%), hirsutism (8.4%
vs. 1.8%), or endometriosis (5.9% vs. 4.5%); and uterine fibroids (10.0% vs. 7.8%); and a higher prevalence of family history of diabetes (19.4% vs. 15.8%).
Dr. Wang and associates also observed – using multivariable Cox models – a greater risk of premature death across all categories and all age ranges in women with decreasing menstrual cycle regularity. In models that were fully adjusted, cycle lengths that were 40 days or more or too irregular to estimate from ages 18-22 and 29-46 expressed hazard ratios for premature death at the time of follow-up of 1.34 and 1.40, compared with women in the same age ranges reporting cycle lengths of 26-31 days.
Of note, Dr. Wang and colleagues unexpectedly discovered an increased risk of premature death in women who had used contraceptives between 14-17 years. They suggested that a greater number of women self-reporting contraceptive use in adolescence may have been using contraceptives to manage symptoms of polycystic ovary syndrome (PCOS) and other conditions such as endometriosis.
Relying on the potential inaccuracy inherent in patient recall of their menstrual cycle characteristics, and the likelihood for other unmeasured factors, may have affected study results. Study strengths included the significant number of participants who had a high follow-up rate over many years, and the availability of menstrual cycle data at three different points across the reproductive lifespan.
Because the mechanisms underlying these associations are likely related to the disrupted hormonal environment, the study results “emphasize the need for primary care providers to include menstrual cycle characteristics throughout the reproductive life span as additional vital signs in assessing women’s general health status,” Dr. Wang and colleagues cautioned.
Expert suggests a probable underlying link
“Irregular menstrual cycles in women have long been known to be associated with significant morbidities, including the leading causes of mortality worldwide such as cardiovascular disease and cancer,” Reshef Tal, MD, PhD, assistant professor of obstetrics, gynecology & reproductive sciences at Yale University, New Haven, Conn., said in an interview. “The findings of this large study that irregular menstrual cycles are associated with premature death, most strongly from cardiovascular causes, are therefore not surprising.”
Dr. Tal acknowledged that one probable underlying link is PCOS, which is recognized as the most common hormonal disorder affecting women of reproductive age. The irregular periods that characterize PCOS are tied to a number of metabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension, which increase the long-term risk of cardiovascular disease and cancer of the uterus.
“The study did not have information on patients’ pelvic ultrasound findings and male hormone levels, which would have helped to establish PCOS diagnosis. However, women in this study who had irregular cycles tended to have more hirsutism, high cholesterol, hypertension as well as higher BMI, suggesting that PCOS is at least partly responsible for the observed association with cardiovascular disease. Interestingly, the association between irregular cycles and early mortality was independent of BMI, indicating that mechanisms other than metabolic factors may also play a role,” observed Dr. Tal, who was asked to comment on the study.
“Irregular periods are a symptom and not a disease, so it is important to identify underlying metabolic risk factors. Furthermore, physicians are advised to counsel patients experiencing menstrual irregularity, [to advise them to] maintain a healthy lifestyle and be alert to health changes,” Dr. Tal suggested.
The study was funded by the National Institutes of Health. The investigators had no relevant financial disclosures. Dr. Tal said he had no relevant financial disclosures.
SOURCE: Chavarro J et al. BMJ. 2020. doi: 10.1136/bmj.m3464.
FROM THE BMJ
An assessment of asthma drugs in pregnancy
Asthma effects about 10% of pregnant women worldwide. About 10% of these will have severe disease requiring oral corticosteroids. Brief reviews of asthma drugs are shown below.
The trade names (if available) and molecular weights (rounded to the nearest whole number) are shown in parentheses. Nearly all of these drugs will cross the placenta.
Beclomethasone (Beconase AQ) (539)
Either beclomethasone or budesonide was considered the inhaled steroids of choice for use during pregnancy, according to a position statement from a joint committee of the American College of Obstetricians and Gynecologists and the American College of Allergy, Asthma, and Immunology published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Benralizumab (Fasenra) (150,000)
There is no published human pregnancy data. Based on studies in monkeys, the drug crosses the placenta in the third trimester. It caused no fetal harm in monkeys when given throughout pregnancy. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to the drug during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting mothertobaby.org/Fasenra.
Budesonide (Rhinocort) (431)
Either budesonide or beclomethasone was considered the inhaled steroids of choice for use during pregnancy in a position statement from a joint committee of ACOG and ACAAI published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Caffeine (194)
Although the amount of caffeine in commonly used beverages varies widely, caffeine consumption in pregnancy in moderate amounts does not pose a risk to the fetus. When used in moderation, no association with congenital malformations, spontaneous abortions, preterm birth, and low birth weight have been proven.
Ciclesonide (Alvesco) (541)
Ciclesonide is an inhaled corticosteroid. There is no published human pregnancy data but the molecular weight suggests that it will cross the placenta throughout pregnancy. The drug produced no defects in rats but caused fetal toxicity in rabbits. Although the risk may be low because it is inhaled, avoiding it in the first trimester should be considered (see dexamethasone).
Cromolyn sodium (490)
Cromolyn was available as a nasal spray and oral solution, but it is no longer available in the United States. It is poorly absorbed into the systemic circulation. Neither the human nor the animal data suggest a risk of embryo-fetal harm.
Dexamethasone (392)
This is a corticosteroid with potency similar to betamethasone. Because large epidemiologic studies have found positive associations between systemic corticosteroids and nonsyndromic orofacial clefts, it is best to avoid this agent in the first trimester. However, when used for the treatment of asthma, other studies have not found a significantly increased risk of maternal or fetal complications. The difference in these outcomes may be related to the systemic concentrations of the drug.
Dyphylline (254) + guaifenesin (198) (Difil-G Forte) (Dilex-G 400) (Dy-G)
This is an OTC liquid drug taken orally. It has not been studied in pregnant animals, and there is no published human pregnancy data. However, these bronchodilator agents probably can be classified as low risk for the embryo and fetus. Dyphylline alone has been removed from the market.
Fluticasone (539) + vilanterol (Breo Ellipta) (775)
Fluticasone is a corticosteroid and vilanterol is a long acting beta2-adrenergic agonist that are given by inhalation. The molecular weights suggest that the two agents will cross the placenta throughout pregnancy. The drug did not cause fetal harm in animals. There is no published human pregnancy data for this fixed combination.
Fluticasone (539) + umeclidinium (509) + vilanterol (Trelegy Ellipta) (776)
The combination of fluticasone (glucocorticoid), umeclidinium, and vilanterol (long-acting beta2-adrenergic agonists) is given by inhalation. The molecular weights suggest that the three agents will cross the placenta throughout pregnancy. Although the three-drug combination has not been studied in pregnant rats and rabbits, the individual agents did not cause embryo-fetal harm in these species. There is no evidence that these agents, when given by inhalation, will harm the human embryo and/or fetus. No published human pregnancy reports for this fixed combination have been located.
Formoterol + mometasone (Dulera Aerosol) (841 / 521)
This combination is an aerosol product. Formoterol is a long-acting beta2-adrenergic agonist and mometasone is a topical corticosteroid. There is no published human pregnancy data for this fixed combination. The molecular weights suggest that both drugs will cross the placenta throughout pregnancy. In animals given high oral doses, both were teratogenic.
Ipratropium (Atrovent) (430)
Inhaled ipratropium, an anticholinergic bronchodilator, is recommended for asthma in patients not responding adequately to other therapy. It was not teratogenic mice, rats, and rabbits. Although the human pregnancy data is limited, there is no evidence that the drug is hazardous to the fetus. It produces fewer systemic effects then atropine and may have an additive bronchodilatory effect to beta2 agonists.
Isoproterenol (211)
Isoproterenol is a sympathomimetic (bronchodilator) with beta-adrenergic effects that is given intravenously. No reports linking this agent with congenital defects have been located. The drug was not teratogenic in rats and rabbits but was in hamsters.
Levalbuterol (Xopenex HFA) (240)
Levalbuterol is the (R)-enantiomer of racemic albuterol. It is given by inhalation. No reports of its use in human pregnancy have been located. However, racemic albuterol is considered compatible in pregnancy, and there is no apparent reason not to classify levalbuterol the same way. The drug, when given orally, is teratogenic in animals. If levalbuterol is used in pregnancy for the treatment of asthma, health care professionals are encouraged to call the toll-free number (1-877-311-8972) for information about patient enrollment in an Organization of Teratology Specialists study.
Mepolizumab (Nucala) (149,000)
Mepolizumab is given by subcutaneous injection. It is not indicated for status asthmaticus. There is no published human pregnancy data but the molecular weight suggests that it will not cross the placenta in the first half of pregnancy. The drug did not cause defects in monkeys and mice. There is a pregnancy exposure registry that monitors pregnancy outcomes in women with asthma exposed to Nucala during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting www.mothertobaby.org/asthma.
Metaproterenol (521)
Metaproterenol, a selective beta2-adrenergic agonist, is a respiratory (bronchodilator) that is given orally. Use of this agent in pregnancy has not been linked with congenital defects. However, the drug is teratogenic in animals.
Methylprednisolone (Medrol) (374)
This is an oral glucocorticoid. The molecular weight suggests that it will cross the placenta throughout pregnancy. No reports relating to its use in human pregnancy or in pregnant animals have been located. However, teratogenicity is a potential problem (see below). If high doses of the drug are used in pregnancy, the newborn infants should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Methylprednisolone acetate (Depo-Medrol) (417)
This is an injectable glucocorticoid. See below.
Methylprednisolone sodium succinate (Solu-Medrol) (497)
Methylprednisolone is a glucocorticoid given parenterally. The molecular weight suggests that it will cross the placenta throughout pregnancy. As with other corticosteroids, the drug was teratogenic, at doses equivalent to the human dose, in mice, rats, and rabbits. If the drug is used in pregnancy, the newborn infant should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Mometasone + formoterol (Dulera) (321 + 841)
Dulera is a combination product of mometasone (corticosteroid) and formoterol (beta2-adrenergic agonist). There is no published human data for Dulera but the molecular weights suggest that the drugs will cross the placenta. Oral doses of formoterol were not teratogenic in animals but were with mometasone. The limited human pregnancy data with formoterol did not suggest a risk of embryo/fetal harm, but there is no human pregnancy data for mometasone.
Montelukast (Singulair) (608)
Montelukast is a leukotriene receptor antagonist that is given orally. Although the human data are limited, the drug does not appear to cause harm to the embryo and/or fetus. The drug was not teratogenic in rats and rabbits. The manufacturer maintains a pregnancy registry for women exposed to montelukast. Health care professionals are encouraged to report pregnancy exposures to the registry by calling the toll-free number 1-800-986-8999.
Omalizumab (Xolair) (149,000)
Omalizumab is a recombinant DNA–derived humanized immunoglobulin (IgG1k) monoclonal antibody that is administered subcutaneously for patients with moderate to severe persistent asthma. In monkeys, the drug did not cause embryotoxicity or teratogenicity. The human pregnancy data is very limited but does not suggest an increased embryo-fetal risk.
Prednisone (Rayos) (358)
The use of oral prednisone appears to represent a small risk to the developing fetus. One of these risks appears to be orofacial clefts. The drug causes birth defects in rats, mice, rabbits, and hamsters. However, the available evidence supports its use to control various maternal diseases, one of which is asthma.
Reslizumab (Cinqair) (147,000)
Reslizumab is given intravenously. Even though the molecular weight is high, the drug crosses the placenta during pregnancy. In placebo-controlled studies, anaphylaxis occurred in 0.3% of patients receiving the drug. No adverse effects were observed when the drug was given to pregnant mice and rabbits.
Salmeterol (Serevent Diskus) (416)
Salmeterol is a long-acting beta2-adrenergic agonist that is given as an aerosol or dry powder for oral inhalation. Because the drug acts locally in the lung, plasma levels are very low or undetectable and are a result of swallowed salmeterol. The limited human pregnancy data does not suggest risk of embryo-fetal harm. High oral doses in animals were not teratogenic.
Theophylline (180)
Oral theophylline is a methylxanthine that is indicated for the treatment of symptoms of chronic asthma and other chronic lung diseases. According to ACOG, theophylline is not a preferred asthma therapy but considered an alternative agent. No published reports linking the use of theophylline with congenital defects have been located. However, the drug is teratogenic in mice, rats, and rabbits at doses close to the human dose.
Tiotropium (Spiriva Respimat) (490)
Tiotropium, an anticholinergic bronchodilator, is given by oral inhalation only. No reports describing the use of tiotropium during human pregnancy have been located. The animal data suggest low risk. However, because of its long elimination half-life (about 25 hours), use of tiotropium immediately before the diagnosis of an inadvertent pregnancy would most likely result in the exposure of a portion of organogenesis.
Triamcinolone (Kenalog-40) (435)
Triamcinolone is an inhaled corticosteroid with potency slightly greater than prednisone. Although the systemic use of the drug has a small absolute risk of oral clefts and fetal growth restriction, inhaled triamcinolone does not appear to cause embryo-fetal harm. The drug is teratogenic when given orally to animals.
Breastfeeding
It is not known if the above drugs are excreted into breast milk. Agents with relatively low molecular weights will probably be in milk. However, if the maternal levels are low, the amount in milk will probably be very small, if at all. Nevertheless, it is doubtful if any of these agents, even if they are excreted into milk, will have a harmful effect on a nursing infant.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at obnews@mdedge.com.
Asthma effects about 10% of pregnant women worldwide. About 10% of these will have severe disease requiring oral corticosteroids. Brief reviews of asthma drugs are shown below.
The trade names (if available) and molecular weights (rounded to the nearest whole number) are shown in parentheses. Nearly all of these drugs will cross the placenta.
Beclomethasone (Beconase AQ) (539)
Either beclomethasone or budesonide was considered the inhaled steroids of choice for use during pregnancy, according to a position statement from a joint committee of the American College of Obstetricians and Gynecologists and the American College of Allergy, Asthma, and Immunology published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Benralizumab (Fasenra) (150,000)
There is no published human pregnancy data. Based on studies in monkeys, the drug crosses the placenta in the third trimester. It caused no fetal harm in monkeys when given throughout pregnancy. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to the drug during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting mothertobaby.org/Fasenra.
Budesonide (Rhinocort) (431)
Either budesonide or beclomethasone was considered the inhaled steroids of choice for use during pregnancy in a position statement from a joint committee of ACOG and ACAAI published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Caffeine (194)
Although the amount of caffeine in commonly used beverages varies widely, caffeine consumption in pregnancy in moderate amounts does not pose a risk to the fetus. When used in moderation, no association with congenital malformations, spontaneous abortions, preterm birth, and low birth weight have been proven.
Ciclesonide (Alvesco) (541)
Ciclesonide is an inhaled corticosteroid. There is no published human pregnancy data but the molecular weight suggests that it will cross the placenta throughout pregnancy. The drug produced no defects in rats but caused fetal toxicity in rabbits. Although the risk may be low because it is inhaled, avoiding it in the first trimester should be considered (see dexamethasone).
Cromolyn sodium (490)
Cromolyn was available as a nasal spray and oral solution, but it is no longer available in the United States. It is poorly absorbed into the systemic circulation. Neither the human nor the animal data suggest a risk of embryo-fetal harm.
Dexamethasone (392)
This is a corticosteroid with potency similar to betamethasone. Because large epidemiologic studies have found positive associations between systemic corticosteroids and nonsyndromic orofacial clefts, it is best to avoid this agent in the first trimester. However, when used for the treatment of asthma, other studies have not found a significantly increased risk of maternal or fetal complications. The difference in these outcomes may be related to the systemic concentrations of the drug.
Dyphylline (254) + guaifenesin (198) (Difil-G Forte) (Dilex-G 400) (Dy-G)
This is an OTC liquid drug taken orally. It has not been studied in pregnant animals, and there is no published human pregnancy data. However, these bronchodilator agents probably can be classified as low risk for the embryo and fetus. Dyphylline alone has been removed from the market.
Fluticasone (539) + vilanterol (Breo Ellipta) (775)
Fluticasone is a corticosteroid and vilanterol is a long acting beta2-adrenergic agonist that are given by inhalation. The molecular weights suggest that the two agents will cross the placenta throughout pregnancy. The drug did not cause fetal harm in animals. There is no published human pregnancy data for this fixed combination.
Fluticasone (539) + umeclidinium (509) + vilanterol (Trelegy Ellipta) (776)
The combination of fluticasone (glucocorticoid), umeclidinium, and vilanterol (long-acting beta2-adrenergic agonists) is given by inhalation. The molecular weights suggest that the three agents will cross the placenta throughout pregnancy. Although the three-drug combination has not been studied in pregnant rats and rabbits, the individual agents did not cause embryo-fetal harm in these species. There is no evidence that these agents, when given by inhalation, will harm the human embryo and/or fetus. No published human pregnancy reports for this fixed combination have been located.
Formoterol + mometasone (Dulera Aerosol) (841 / 521)
This combination is an aerosol product. Formoterol is a long-acting beta2-adrenergic agonist and mometasone is a topical corticosteroid. There is no published human pregnancy data for this fixed combination. The molecular weights suggest that both drugs will cross the placenta throughout pregnancy. In animals given high oral doses, both were teratogenic.
Ipratropium (Atrovent) (430)
Inhaled ipratropium, an anticholinergic bronchodilator, is recommended for asthma in patients not responding adequately to other therapy. It was not teratogenic mice, rats, and rabbits. Although the human pregnancy data is limited, there is no evidence that the drug is hazardous to the fetus. It produces fewer systemic effects then atropine and may have an additive bronchodilatory effect to beta2 agonists.
Isoproterenol (211)
Isoproterenol is a sympathomimetic (bronchodilator) with beta-adrenergic effects that is given intravenously. No reports linking this agent with congenital defects have been located. The drug was not teratogenic in rats and rabbits but was in hamsters.
Levalbuterol (Xopenex HFA) (240)
Levalbuterol is the (R)-enantiomer of racemic albuterol. It is given by inhalation. No reports of its use in human pregnancy have been located. However, racemic albuterol is considered compatible in pregnancy, and there is no apparent reason not to classify levalbuterol the same way. The drug, when given orally, is teratogenic in animals. If levalbuterol is used in pregnancy for the treatment of asthma, health care professionals are encouraged to call the toll-free number (1-877-311-8972) for information about patient enrollment in an Organization of Teratology Specialists study.
Mepolizumab (Nucala) (149,000)
Mepolizumab is given by subcutaneous injection. It is not indicated for status asthmaticus. There is no published human pregnancy data but the molecular weight suggests that it will not cross the placenta in the first half of pregnancy. The drug did not cause defects in monkeys and mice. There is a pregnancy exposure registry that monitors pregnancy outcomes in women with asthma exposed to Nucala during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting www.mothertobaby.org/asthma.
Metaproterenol (521)
Metaproterenol, a selective beta2-adrenergic agonist, is a respiratory (bronchodilator) that is given orally. Use of this agent in pregnancy has not been linked with congenital defects. However, the drug is teratogenic in animals.
Methylprednisolone (Medrol) (374)
This is an oral glucocorticoid. The molecular weight suggests that it will cross the placenta throughout pregnancy. No reports relating to its use in human pregnancy or in pregnant animals have been located. However, teratogenicity is a potential problem (see below). If high doses of the drug are used in pregnancy, the newborn infants should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Methylprednisolone acetate (Depo-Medrol) (417)
This is an injectable glucocorticoid. See below.
Methylprednisolone sodium succinate (Solu-Medrol) (497)
Methylprednisolone is a glucocorticoid given parenterally. The molecular weight suggests that it will cross the placenta throughout pregnancy. As with other corticosteroids, the drug was teratogenic, at doses equivalent to the human dose, in mice, rats, and rabbits. If the drug is used in pregnancy, the newborn infant should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Mometasone + formoterol (Dulera) (321 + 841)
Dulera is a combination product of mometasone (corticosteroid) and formoterol (beta2-adrenergic agonist). There is no published human data for Dulera but the molecular weights suggest that the drugs will cross the placenta. Oral doses of formoterol were not teratogenic in animals but were with mometasone. The limited human pregnancy data with formoterol did not suggest a risk of embryo/fetal harm, but there is no human pregnancy data for mometasone.
Montelukast (Singulair) (608)
Montelukast is a leukotriene receptor antagonist that is given orally. Although the human data are limited, the drug does not appear to cause harm to the embryo and/or fetus. The drug was not teratogenic in rats and rabbits. The manufacturer maintains a pregnancy registry for women exposed to montelukast. Health care professionals are encouraged to report pregnancy exposures to the registry by calling the toll-free number 1-800-986-8999.
Omalizumab (Xolair) (149,000)
Omalizumab is a recombinant DNA–derived humanized immunoglobulin (IgG1k) monoclonal antibody that is administered subcutaneously for patients with moderate to severe persistent asthma. In monkeys, the drug did not cause embryotoxicity or teratogenicity. The human pregnancy data is very limited but does not suggest an increased embryo-fetal risk.
Prednisone (Rayos) (358)
The use of oral prednisone appears to represent a small risk to the developing fetus. One of these risks appears to be orofacial clefts. The drug causes birth defects in rats, mice, rabbits, and hamsters. However, the available evidence supports its use to control various maternal diseases, one of which is asthma.
Reslizumab (Cinqair) (147,000)
Reslizumab is given intravenously. Even though the molecular weight is high, the drug crosses the placenta during pregnancy. In placebo-controlled studies, anaphylaxis occurred in 0.3% of patients receiving the drug. No adverse effects were observed when the drug was given to pregnant mice and rabbits.
Salmeterol (Serevent Diskus) (416)
Salmeterol is a long-acting beta2-adrenergic agonist that is given as an aerosol or dry powder for oral inhalation. Because the drug acts locally in the lung, plasma levels are very low or undetectable and are a result of swallowed salmeterol. The limited human pregnancy data does not suggest risk of embryo-fetal harm. High oral doses in animals were not teratogenic.
Theophylline (180)
Oral theophylline is a methylxanthine that is indicated for the treatment of symptoms of chronic asthma and other chronic lung diseases. According to ACOG, theophylline is not a preferred asthma therapy but considered an alternative agent. No published reports linking the use of theophylline with congenital defects have been located. However, the drug is teratogenic in mice, rats, and rabbits at doses close to the human dose.
Tiotropium (Spiriva Respimat) (490)
Tiotropium, an anticholinergic bronchodilator, is given by oral inhalation only. No reports describing the use of tiotropium during human pregnancy have been located. The animal data suggest low risk. However, because of its long elimination half-life (about 25 hours), use of tiotropium immediately before the diagnosis of an inadvertent pregnancy would most likely result in the exposure of a portion of organogenesis.
Triamcinolone (Kenalog-40) (435)
Triamcinolone is an inhaled corticosteroid with potency slightly greater than prednisone. Although the systemic use of the drug has a small absolute risk of oral clefts and fetal growth restriction, inhaled triamcinolone does not appear to cause embryo-fetal harm. The drug is teratogenic when given orally to animals.
Breastfeeding
It is not known if the above drugs are excreted into breast milk. Agents with relatively low molecular weights will probably be in milk. However, if the maternal levels are low, the amount in milk will probably be very small, if at all. Nevertheless, it is doubtful if any of these agents, even if they are excreted into milk, will have a harmful effect on a nursing infant.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at obnews@mdedge.com.
Asthma effects about 10% of pregnant women worldwide. About 10% of these will have severe disease requiring oral corticosteroids. Brief reviews of asthma drugs are shown below.
The trade names (if available) and molecular weights (rounded to the nearest whole number) are shown in parentheses. Nearly all of these drugs will cross the placenta.
Beclomethasone (Beconase AQ) (539)
Either beclomethasone or budesonide was considered the inhaled steroids of choice for use during pregnancy, according to a position statement from a joint committee of the American College of Obstetricians and Gynecologists and the American College of Allergy, Asthma, and Immunology published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Benralizumab (Fasenra) (150,000)
There is no published human pregnancy data. Based on studies in monkeys, the drug crosses the placenta in the third trimester. It caused no fetal harm in monkeys when given throughout pregnancy. There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to the drug during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting mothertobaby.org/Fasenra.
Budesonide (Rhinocort) (431)
Either budesonide or beclomethasone was considered the inhaled steroids of choice for use during pregnancy in a position statement from a joint committee of ACOG and ACAAI published in 2000. Although the drug is teratogenic in animals, no human reports associating the use of inhaled beclomethasone with human congenital anomalies have been found.
Caffeine (194)
Although the amount of caffeine in commonly used beverages varies widely, caffeine consumption in pregnancy in moderate amounts does not pose a risk to the fetus. When used in moderation, no association with congenital malformations, spontaneous abortions, preterm birth, and low birth weight have been proven.
Ciclesonide (Alvesco) (541)
Ciclesonide is an inhaled corticosteroid. There is no published human pregnancy data but the molecular weight suggests that it will cross the placenta throughout pregnancy. The drug produced no defects in rats but caused fetal toxicity in rabbits. Although the risk may be low because it is inhaled, avoiding it in the first trimester should be considered (see dexamethasone).
Cromolyn sodium (490)
Cromolyn was available as a nasal spray and oral solution, but it is no longer available in the United States. It is poorly absorbed into the systemic circulation. Neither the human nor the animal data suggest a risk of embryo-fetal harm.
Dexamethasone (392)
This is a corticosteroid with potency similar to betamethasone. Because large epidemiologic studies have found positive associations between systemic corticosteroids and nonsyndromic orofacial clefts, it is best to avoid this agent in the first trimester. However, when used for the treatment of asthma, other studies have not found a significantly increased risk of maternal or fetal complications. The difference in these outcomes may be related to the systemic concentrations of the drug.
Dyphylline (254) + guaifenesin (198) (Difil-G Forte) (Dilex-G 400) (Dy-G)
This is an OTC liquid drug taken orally. It has not been studied in pregnant animals, and there is no published human pregnancy data. However, these bronchodilator agents probably can be classified as low risk for the embryo and fetus. Dyphylline alone has been removed from the market.
Fluticasone (539) + vilanterol (Breo Ellipta) (775)
Fluticasone is a corticosteroid and vilanterol is a long acting beta2-adrenergic agonist that are given by inhalation. The molecular weights suggest that the two agents will cross the placenta throughout pregnancy. The drug did not cause fetal harm in animals. There is no published human pregnancy data for this fixed combination.
Fluticasone (539) + umeclidinium (509) + vilanterol (Trelegy Ellipta) (776)
The combination of fluticasone (glucocorticoid), umeclidinium, and vilanterol (long-acting beta2-adrenergic agonists) is given by inhalation. The molecular weights suggest that the three agents will cross the placenta throughout pregnancy. Although the three-drug combination has not been studied in pregnant rats and rabbits, the individual agents did not cause embryo-fetal harm in these species. There is no evidence that these agents, when given by inhalation, will harm the human embryo and/or fetus. No published human pregnancy reports for this fixed combination have been located.
Formoterol + mometasone (Dulera Aerosol) (841 / 521)
This combination is an aerosol product. Formoterol is a long-acting beta2-adrenergic agonist and mometasone is a topical corticosteroid. There is no published human pregnancy data for this fixed combination. The molecular weights suggest that both drugs will cross the placenta throughout pregnancy. In animals given high oral doses, both were teratogenic.
Ipratropium (Atrovent) (430)
Inhaled ipratropium, an anticholinergic bronchodilator, is recommended for asthma in patients not responding adequately to other therapy. It was not teratogenic mice, rats, and rabbits. Although the human pregnancy data is limited, there is no evidence that the drug is hazardous to the fetus. It produces fewer systemic effects then atropine and may have an additive bronchodilatory effect to beta2 agonists.
Isoproterenol (211)
Isoproterenol is a sympathomimetic (bronchodilator) with beta-adrenergic effects that is given intravenously. No reports linking this agent with congenital defects have been located. The drug was not teratogenic in rats and rabbits but was in hamsters.
Levalbuterol (Xopenex HFA) (240)
Levalbuterol is the (R)-enantiomer of racemic albuterol. It is given by inhalation. No reports of its use in human pregnancy have been located. However, racemic albuterol is considered compatible in pregnancy, and there is no apparent reason not to classify levalbuterol the same way. The drug, when given orally, is teratogenic in animals. If levalbuterol is used in pregnancy for the treatment of asthma, health care professionals are encouraged to call the toll-free number (1-877-311-8972) for information about patient enrollment in an Organization of Teratology Specialists study.
Mepolizumab (Nucala) (149,000)
Mepolizumab is given by subcutaneous injection. It is not indicated for status asthmaticus. There is no published human pregnancy data but the molecular weight suggests that it will not cross the placenta in the first half of pregnancy. The drug did not cause defects in monkeys and mice. There is a pregnancy exposure registry that monitors pregnancy outcomes in women with asthma exposed to Nucala during pregnancy. Health care providers can enroll patients or encourage patients to enroll themselves by calling 1-877-311-8972 or visiting www.mothertobaby.org/asthma.
Metaproterenol (521)
Metaproterenol, a selective beta2-adrenergic agonist, is a respiratory (bronchodilator) that is given orally. Use of this agent in pregnancy has not been linked with congenital defects. However, the drug is teratogenic in animals.
Methylprednisolone (Medrol) (374)
This is an oral glucocorticoid. The molecular weight suggests that it will cross the placenta throughout pregnancy. No reports relating to its use in human pregnancy or in pregnant animals have been located. However, teratogenicity is a potential problem (see below). If high doses of the drug are used in pregnancy, the newborn infants should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Methylprednisolone acetate (Depo-Medrol) (417)
This is an injectable glucocorticoid. See below.
Methylprednisolone sodium succinate (Solu-Medrol) (497)
Methylprednisolone is a glucocorticoid given parenterally. The molecular weight suggests that it will cross the placenta throughout pregnancy. As with other corticosteroids, the drug was teratogenic, at doses equivalent to the human dose, in mice, rats, and rabbits. If the drug is used in pregnancy, the newborn infant should be carefully observed for signs of hypoadrenalism. In addition, all corticosteroids increase calcium excretion.
Mometasone + formoterol (Dulera) (321 + 841)
Dulera is a combination product of mometasone (corticosteroid) and formoterol (beta2-adrenergic agonist). There is no published human data for Dulera but the molecular weights suggest that the drugs will cross the placenta. Oral doses of formoterol were not teratogenic in animals but were with mometasone. The limited human pregnancy data with formoterol did not suggest a risk of embryo/fetal harm, but there is no human pregnancy data for mometasone.
Montelukast (Singulair) (608)
Montelukast is a leukotriene receptor antagonist that is given orally. Although the human data are limited, the drug does not appear to cause harm to the embryo and/or fetus. The drug was not teratogenic in rats and rabbits. The manufacturer maintains a pregnancy registry for women exposed to montelukast. Health care professionals are encouraged to report pregnancy exposures to the registry by calling the toll-free number 1-800-986-8999.
Omalizumab (Xolair) (149,000)
Omalizumab is a recombinant DNA–derived humanized immunoglobulin (IgG1k) monoclonal antibody that is administered subcutaneously for patients with moderate to severe persistent asthma. In monkeys, the drug did not cause embryotoxicity or teratogenicity. The human pregnancy data is very limited but does not suggest an increased embryo-fetal risk.
Prednisone (Rayos) (358)
The use of oral prednisone appears to represent a small risk to the developing fetus. One of these risks appears to be orofacial clefts. The drug causes birth defects in rats, mice, rabbits, and hamsters. However, the available evidence supports its use to control various maternal diseases, one of which is asthma.
Reslizumab (Cinqair) (147,000)
Reslizumab is given intravenously. Even though the molecular weight is high, the drug crosses the placenta during pregnancy. In placebo-controlled studies, anaphylaxis occurred in 0.3% of patients receiving the drug. No adverse effects were observed when the drug was given to pregnant mice and rabbits.
Salmeterol (Serevent Diskus) (416)
Salmeterol is a long-acting beta2-adrenergic agonist that is given as an aerosol or dry powder for oral inhalation. Because the drug acts locally in the lung, plasma levels are very low or undetectable and are a result of swallowed salmeterol. The limited human pregnancy data does not suggest risk of embryo-fetal harm. High oral doses in animals were not teratogenic.
Theophylline (180)
Oral theophylline is a methylxanthine that is indicated for the treatment of symptoms of chronic asthma and other chronic lung diseases. According to ACOG, theophylline is not a preferred asthma therapy but considered an alternative agent. No published reports linking the use of theophylline with congenital defects have been located. However, the drug is teratogenic in mice, rats, and rabbits at doses close to the human dose.
Tiotropium (Spiriva Respimat) (490)
Tiotropium, an anticholinergic bronchodilator, is given by oral inhalation only. No reports describing the use of tiotropium during human pregnancy have been located. The animal data suggest low risk. However, because of its long elimination half-life (about 25 hours), use of tiotropium immediately before the diagnosis of an inadvertent pregnancy would most likely result in the exposure of a portion of organogenesis.
Triamcinolone (Kenalog-40) (435)
Triamcinolone is an inhaled corticosteroid with potency slightly greater than prednisone. Although the systemic use of the drug has a small absolute risk of oral clefts and fetal growth restriction, inhaled triamcinolone does not appear to cause embryo-fetal harm. The drug is teratogenic when given orally to animals.
Breastfeeding
It is not known if the above drugs are excreted into breast milk. Agents with relatively low molecular weights will probably be in milk. However, if the maternal levels are low, the amount in milk will probably be very small, if at all. Nevertheless, it is doubtful if any of these agents, even if they are excreted into milk, will have a harmful effect on a nursing infant.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, as well as at Washington State University, Spokane. Mr. Briggs said he had no relevant financial disclosures. Email him at obnews@mdedge.com.
PCOS tied to risk for cardiovascular disease after menopause
Women with polycystic ovarian syndrome (PCOS) before menopause appear to have a greater risk of stroke, heart attack, and other cardiovascular events after menopause, according to findings presented at the virtual American Society for Reproductive Medicine (ASRM) 2020 Scientific Congress.
“We found a PCOS diagnosis prior to menopause was associated with a 64% increased risk of cardiovascular disease after menopause independent of age at enrollment, race, body mass index, and smoking status,” presenter Jacob Christ, MD, a resident at the University of Washington in Seattle, told attendees. “Taken together, our results suggest that women with PCOS have more risk factors for future cardiovascular disease at baseline, and a present PCOS diagnosis prior to menopause is associated with an increased risk of cardiovascular disease after menopause.”
The results are important to consider in women seeking care related to fertility, according to Amanda N. Kallen, MD, assistant professor of reproductive endocrinology and infertility at Yale Medicine in New Haven, Conn.
“As fertility specialists, we often see women with PCOS visit us when they are having trouble conceiving, but often [they] do not return to our care once they’ve built their family,” said Dr. Kallen, who was not involved in the research.
“This excellent talk emphasized how critical it is for us as reproductive endocrinologists to have ongoing discussions with PCOS patients about long-term cardiovascular risks at every opportunity, and to emphasize that these risks persist long after the reproductive years have ended,” Dr. Kallen said in an interview.
Identifying women at higher risk
Characteristics of PCOS in adolescence are already understood, including hyperandrogenism, acne, irregular bleeding, and variable ages of menarche, Dr. Christ explained. Similarly, in women’s reproductive years, PCOS is linked to abnormal uterine bleeding, hirsutism, dyslipidemia, infertility, impaired glucose tolerance, gestational diabetes, and preeclampsia.
“What is less clear is if baseline cardiometabolic dysfunction during reproductive years translates into cardiovascular disease after menopause,” Dr. Christ said. “Menopausal changes may reduce risk of cardiovascular disease among PCOS women, as it is known that overall, androgen levels decline during menopause. Furthermore, ovarian aging may be delayed in PCOS women, which may be protective against cardiovascular disease.”
To learn more, the researchers completed a secondary analysis of data from the Study of Women’s Health Across the Nation (SWAN), a prospective cohort study. Women enrolled in the study were aged 42-52 years at baseline, had a uterus and at least one ovary, and menstruated within the previous 3 months. Women were considered to have PCOS if they had both biochemical hyperandrogenism and a history of irregular menses.
The researchers included participants in the secondary analysis if they had complete data on the women’s baseline menstrual status and total testosterone and if the women had at least one follow-up visit after menopause. Menopause was approximated as 51 years old if not otherwise reported (or 1 year after study entry if age 51 at entry). At the follow-up visit, women self-reported any cardiovascular disease events since menopause.
The study’s primary outcome was the first postmenopausal cardiovascular event. These included any of the following: myocardial infarction, cerebrovascular accident or stroke, angina, percutaneous coronary intervention or angioplasty, coronary artery bypass graft, heart failure, carotid artery procedure, peripheral artery disease or lower extremity procedure, renal artery procedure, deep vein thrombosis, pulmonary embolism, and abdominal aortic aneurysm.
Among 1,340 women included in the analysis, 174 (13%) women had PCOS and 1,166 did not. The average age at screening and at menopause were not significantly different between the groups, but they did differ based on other baseline characteristics.
More women with PCOS frequently smoked cigarettes (22%) vs. those without PCOS (12.7%), and women with PCOS had an average body mass index of 31.3, vs. 26.7 among those without PCOS. Women with PCOS also had higher systolic blood pressure (120.7 vs. 115.8 mm Hg), higher total cholesterol (202 vs. 192 mg/dL), and higher fasting blood glucose (103.7 vs. 89.2 mg/dL; P < .01 for all).
After the researchers controlled for age at enrollment, race, BMI, and smoking status, women with PCOS had 1.6 times greater odds of a cardiovascular event after menopause compared with women without PCOS (odds ratio [OR], 1.6; P = .029). Those with PCOS also had a significantly higher Atherosclerotic Cardiovascular Disease risk scores (P = .024), but their Framingham 10-year risk score was not significantly different from those without PCOS.
Although the findings are not necessarily surprising, the study’s value particularly lay in its size, prospective data collection, and rigorous methods, said Ginny Ryan, MD, MA, professor and division chief of reproductive endocrinology and infertility at the University of Washington in Seattle.
“While this study’s criteria used to identify subjects with PCOS selected a population with a particularly severe phenotype of PCOS and thus a higher risk population for cardiovascular disease, it is vital for women’s health providers to truly understand the medium- and long-term life-threatening associations found more commonly in many with PCOS,” Dr. Ryan, who attended the talk and was not involved in the research, said in an interview.
“This study emphasizes the importance of identifying PCOS before menopause, not just for the patient’s immediate well-being, but also so that appropriate counseling and referral can take place to optimize primary, secondary, and tertiary prevention efforts against CVD and related morbidity and mortality,” Dr. Ryan said. “Providers who focus on reproductive health and reproductive-aged women have the opportunity to play a vital role in optimizing the long-term health of their patients.”
Aside from being a prospective cohort with more than 2 decades of follow-up, the study’s other strengths included the definition of PCOS before menopause and use of multiple markers of postmenopausal cardiovascular disease, Dr. Christ said. The study’s main weaknesses were the exclusion of mild PCOS, the self-reporting of cardiovascular events, and the multiple ways of defining menopause.
Dr. Kallen is a consultant for Gynaesight and a reviewer for Healthline. Dr. Christ and Dr. Ryan have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Women with polycystic ovarian syndrome (PCOS) before menopause appear to have a greater risk of stroke, heart attack, and other cardiovascular events after menopause, according to findings presented at the virtual American Society for Reproductive Medicine (ASRM) 2020 Scientific Congress.
“We found a PCOS diagnosis prior to menopause was associated with a 64% increased risk of cardiovascular disease after menopause independent of age at enrollment, race, body mass index, and smoking status,” presenter Jacob Christ, MD, a resident at the University of Washington in Seattle, told attendees. “Taken together, our results suggest that women with PCOS have more risk factors for future cardiovascular disease at baseline, and a present PCOS diagnosis prior to menopause is associated with an increased risk of cardiovascular disease after menopause.”
The results are important to consider in women seeking care related to fertility, according to Amanda N. Kallen, MD, assistant professor of reproductive endocrinology and infertility at Yale Medicine in New Haven, Conn.
“As fertility specialists, we often see women with PCOS visit us when they are having trouble conceiving, but often [they] do not return to our care once they’ve built their family,” said Dr. Kallen, who was not involved in the research.
“This excellent talk emphasized how critical it is for us as reproductive endocrinologists to have ongoing discussions with PCOS patients about long-term cardiovascular risks at every opportunity, and to emphasize that these risks persist long after the reproductive years have ended,” Dr. Kallen said in an interview.
Identifying women at higher risk
Characteristics of PCOS in adolescence are already understood, including hyperandrogenism, acne, irregular bleeding, and variable ages of menarche, Dr. Christ explained. Similarly, in women’s reproductive years, PCOS is linked to abnormal uterine bleeding, hirsutism, dyslipidemia, infertility, impaired glucose tolerance, gestational diabetes, and preeclampsia.
“What is less clear is if baseline cardiometabolic dysfunction during reproductive years translates into cardiovascular disease after menopause,” Dr. Christ said. “Menopausal changes may reduce risk of cardiovascular disease among PCOS women, as it is known that overall, androgen levels decline during menopause. Furthermore, ovarian aging may be delayed in PCOS women, which may be protective against cardiovascular disease.”
To learn more, the researchers completed a secondary analysis of data from the Study of Women’s Health Across the Nation (SWAN), a prospective cohort study. Women enrolled in the study were aged 42-52 years at baseline, had a uterus and at least one ovary, and menstruated within the previous 3 months. Women were considered to have PCOS if they had both biochemical hyperandrogenism and a history of irregular menses.
The researchers included participants in the secondary analysis if they had complete data on the women’s baseline menstrual status and total testosterone and if the women had at least one follow-up visit after menopause. Menopause was approximated as 51 years old if not otherwise reported (or 1 year after study entry if age 51 at entry). At the follow-up visit, women self-reported any cardiovascular disease events since menopause.
The study’s primary outcome was the first postmenopausal cardiovascular event. These included any of the following: myocardial infarction, cerebrovascular accident or stroke, angina, percutaneous coronary intervention or angioplasty, coronary artery bypass graft, heart failure, carotid artery procedure, peripheral artery disease or lower extremity procedure, renal artery procedure, deep vein thrombosis, pulmonary embolism, and abdominal aortic aneurysm.
Among 1,340 women included in the analysis, 174 (13%) women had PCOS and 1,166 did not. The average age at screening and at menopause were not significantly different between the groups, but they did differ based on other baseline characteristics.
More women with PCOS frequently smoked cigarettes (22%) vs. those without PCOS (12.7%), and women with PCOS had an average body mass index of 31.3, vs. 26.7 among those without PCOS. Women with PCOS also had higher systolic blood pressure (120.7 vs. 115.8 mm Hg), higher total cholesterol (202 vs. 192 mg/dL), and higher fasting blood glucose (103.7 vs. 89.2 mg/dL; P < .01 for all).
After the researchers controlled for age at enrollment, race, BMI, and smoking status, women with PCOS had 1.6 times greater odds of a cardiovascular event after menopause compared with women without PCOS (odds ratio [OR], 1.6; P = .029). Those with PCOS also had a significantly higher Atherosclerotic Cardiovascular Disease risk scores (P = .024), but their Framingham 10-year risk score was not significantly different from those without PCOS.
Although the findings are not necessarily surprising, the study’s value particularly lay in its size, prospective data collection, and rigorous methods, said Ginny Ryan, MD, MA, professor and division chief of reproductive endocrinology and infertility at the University of Washington in Seattle.
“While this study’s criteria used to identify subjects with PCOS selected a population with a particularly severe phenotype of PCOS and thus a higher risk population for cardiovascular disease, it is vital for women’s health providers to truly understand the medium- and long-term life-threatening associations found more commonly in many with PCOS,” Dr. Ryan, who attended the talk and was not involved in the research, said in an interview.
“This study emphasizes the importance of identifying PCOS before menopause, not just for the patient’s immediate well-being, but also so that appropriate counseling and referral can take place to optimize primary, secondary, and tertiary prevention efforts against CVD and related morbidity and mortality,” Dr. Ryan said. “Providers who focus on reproductive health and reproductive-aged women have the opportunity to play a vital role in optimizing the long-term health of their patients.”
Aside from being a prospective cohort with more than 2 decades of follow-up, the study’s other strengths included the definition of PCOS before menopause and use of multiple markers of postmenopausal cardiovascular disease, Dr. Christ said. The study’s main weaknesses were the exclusion of mild PCOS, the self-reporting of cardiovascular events, and the multiple ways of defining menopause.
Dr. Kallen is a consultant for Gynaesight and a reviewer for Healthline. Dr. Christ and Dr. Ryan have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Women with polycystic ovarian syndrome (PCOS) before menopause appear to have a greater risk of stroke, heart attack, and other cardiovascular events after menopause, according to findings presented at the virtual American Society for Reproductive Medicine (ASRM) 2020 Scientific Congress.
“We found a PCOS diagnosis prior to menopause was associated with a 64% increased risk of cardiovascular disease after menopause independent of age at enrollment, race, body mass index, and smoking status,” presenter Jacob Christ, MD, a resident at the University of Washington in Seattle, told attendees. “Taken together, our results suggest that women with PCOS have more risk factors for future cardiovascular disease at baseline, and a present PCOS diagnosis prior to menopause is associated with an increased risk of cardiovascular disease after menopause.”
The results are important to consider in women seeking care related to fertility, according to Amanda N. Kallen, MD, assistant professor of reproductive endocrinology and infertility at Yale Medicine in New Haven, Conn.
“As fertility specialists, we often see women with PCOS visit us when they are having trouble conceiving, but often [they] do not return to our care once they’ve built their family,” said Dr. Kallen, who was not involved in the research.
“This excellent talk emphasized how critical it is for us as reproductive endocrinologists to have ongoing discussions with PCOS patients about long-term cardiovascular risks at every opportunity, and to emphasize that these risks persist long after the reproductive years have ended,” Dr. Kallen said in an interview.
Identifying women at higher risk
Characteristics of PCOS in adolescence are already understood, including hyperandrogenism, acne, irregular bleeding, and variable ages of menarche, Dr. Christ explained. Similarly, in women’s reproductive years, PCOS is linked to abnormal uterine bleeding, hirsutism, dyslipidemia, infertility, impaired glucose tolerance, gestational diabetes, and preeclampsia.
“What is less clear is if baseline cardiometabolic dysfunction during reproductive years translates into cardiovascular disease after menopause,” Dr. Christ said. “Menopausal changes may reduce risk of cardiovascular disease among PCOS women, as it is known that overall, androgen levels decline during menopause. Furthermore, ovarian aging may be delayed in PCOS women, which may be protective against cardiovascular disease.”
To learn more, the researchers completed a secondary analysis of data from the Study of Women’s Health Across the Nation (SWAN), a prospective cohort study. Women enrolled in the study were aged 42-52 years at baseline, had a uterus and at least one ovary, and menstruated within the previous 3 months. Women were considered to have PCOS if they had both biochemical hyperandrogenism and a history of irregular menses.
The researchers included participants in the secondary analysis if they had complete data on the women’s baseline menstrual status and total testosterone and if the women had at least one follow-up visit after menopause. Menopause was approximated as 51 years old if not otherwise reported (or 1 year after study entry if age 51 at entry). At the follow-up visit, women self-reported any cardiovascular disease events since menopause.
The study’s primary outcome was the first postmenopausal cardiovascular event. These included any of the following: myocardial infarction, cerebrovascular accident or stroke, angina, percutaneous coronary intervention or angioplasty, coronary artery bypass graft, heart failure, carotid artery procedure, peripheral artery disease or lower extremity procedure, renal artery procedure, deep vein thrombosis, pulmonary embolism, and abdominal aortic aneurysm.
Among 1,340 women included in the analysis, 174 (13%) women had PCOS and 1,166 did not. The average age at screening and at menopause were not significantly different between the groups, but they did differ based on other baseline characteristics.
More women with PCOS frequently smoked cigarettes (22%) vs. those without PCOS (12.7%), and women with PCOS had an average body mass index of 31.3, vs. 26.7 among those without PCOS. Women with PCOS also had higher systolic blood pressure (120.7 vs. 115.8 mm Hg), higher total cholesterol (202 vs. 192 mg/dL), and higher fasting blood glucose (103.7 vs. 89.2 mg/dL; P < .01 for all).
After the researchers controlled for age at enrollment, race, BMI, and smoking status, women with PCOS had 1.6 times greater odds of a cardiovascular event after menopause compared with women without PCOS (odds ratio [OR], 1.6; P = .029). Those with PCOS also had a significantly higher Atherosclerotic Cardiovascular Disease risk scores (P = .024), but their Framingham 10-year risk score was not significantly different from those without PCOS.
Although the findings are not necessarily surprising, the study’s value particularly lay in its size, prospective data collection, and rigorous methods, said Ginny Ryan, MD, MA, professor and division chief of reproductive endocrinology and infertility at the University of Washington in Seattle.
“While this study’s criteria used to identify subjects with PCOS selected a population with a particularly severe phenotype of PCOS and thus a higher risk population for cardiovascular disease, it is vital for women’s health providers to truly understand the medium- and long-term life-threatening associations found more commonly in many with PCOS,” Dr. Ryan, who attended the talk and was not involved in the research, said in an interview.
“This study emphasizes the importance of identifying PCOS before menopause, not just for the patient’s immediate well-being, but also so that appropriate counseling and referral can take place to optimize primary, secondary, and tertiary prevention efforts against CVD and related morbidity and mortality,” Dr. Ryan said. “Providers who focus on reproductive health and reproductive-aged women have the opportunity to play a vital role in optimizing the long-term health of their patients.”
Aside from being a prospective cohort with more than 2 decades of follow-up, the study’s other strengths included the definition of PCOS before menopause and use of multiple markers of postmenopausal cardiovascular disease, Dr. Christ said. The study’s main weaknesses were the exclusion of mild PCOS, the self-reporting of cardiovascular events, and the multiple ways of defining menopause.
Dr. Kallen is a consultant for Gynaesight and a reviewer for Healthline. Dr. Christ and Dr. Ryan have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Relugolix combo effective for uterine fibroids through 1 year
A combination therapy using the experimental drug relugolix was effective in treating pain and heavy bleeding from uterine fibroids for a full year, according to findings from a long-term extension study of the phase 3, open-label LIBERTY trials.
The drug was also well tolerated, with retention of bone mineral density and no new adverse events, said Ayman Al-Hendy, MD, PhD, who presented the results Oct. 17 at the virtual American Society for Reproductive Medicine 2020 Scientific Congress.
“Relugolix combination therapy represents a potential long-term treatment for women with heavy menstrual bleeding associated with uterine fibroids,” said Al-Hendy, a gynecologist and endoscopic surgeon at the University of Chicago.
Dr. Al-Hendy, who consults for the company that makes the drug, on Oct. 20 presented results showing improvement in quality of life with relugolix therapy.
“The fact that this longer-term study shows continued, persistent results at a year really gives us confidence that we’ll be able to use these drugs as a long-term therapy to treat fibroids,” Hugh S. Taylor, MD, president-elect of ASRM, said in an interview. Dr. Taylor, a professor and chair of ob.gyn. and reproductive sciences at Yale University, New Haven, Conn., was not involved in the study.
“A drug like this is so necessary,” Dr. Taylor continued. “We don’t have any other drugs on the market approved for long-term use.”
Relugolix is an oral gonadotropin-releasing hormone (GnRH) receptor antagonist under investigation for long-term management of uterine fibroids. The once-daily combination therapy includes 40 mg relugolix, 1 mg estradiol, and 0.5 mg norethindrone acetate.
Extension study shows prolonged benefits
The extension trial enrolled women aged 18-50 years who were experiencing heavy menstrual bleeding from uterine fibroids and who completed the 24-week phase 3, double-blind, placebo-controlled LIBERTY 1 or 2 trials. Heavy menstrual bleeding was defined as bleeding in which at least 80 mL of blood was lost per cycle for two cycles or 160 mL was lost during one cycle. Ultrasound imaging was used to confirm the presence of fibroids.
In LIBERTY 1 and 2, women were randomly assigned to receive relugolix combination therapy, placebo, or relugolix alone for 12 weeks followed by combination therapy for the remaining 12 weeks (delayed group). Those trials found that relugolix combination therapy was effective through 6 months in reducing menstrual blood loss and pain in women with uterine fibroids without loss of bone mineral density.
LIBERTY 3 extended the trial to 52 weeks, with all participants receiving relugolix combination therapy.
As in the earlier trials, the primary endpoint was reduced menstrual blood loss. By the end of the study, women needed to have at least a 50% reduction in blood loss from the initial study’s baseline while maintaining a blood loss of <80 mL. The investigators also evaluated the mean percentage of menstrual blood loss reduction, amenorrhea rate, and improvements in anemia as secondary endpoints and assessed changes in bone mineral density.
The extension study enrolled 78% (n = 477) of the 610 women who completed the initial study; of those, 363 women completed the extension study.
Among the 163 women who began with relugolix combination therapy in the first two trials, 87.7% met the primary endpoint in a per-protocol analysis through week 52. The proportion of responders in the extension study was 75.6% among the group that formerly received placebo (n = 164) and 79.9% in the delayed group (n = 149).
The overall average reduction in menstrual blood volume was 89.9%. Most of the women experienced amenorrhea at the end of the year: 70.6% in the relugolix group, 57.9% in the group that formerly received placebo, and 68.5% in the delayed group.
Reductions in uterine volume and uterine fibroid volume were also sustained from week 24 to week 52. For the relugolix combination therapy group, the mean loss of uterine fibroid volume from baseline was 13.5% at week 24 and 18.3% at week 52. Similarly, the delayed group’s average loss in fibroid volume was 28.1% at week 24 and 33.9% at week 52. The placebo group, which only had a 7% loss in fibroid volume at week 24, had an 18.4% loss in volume from baseline at week 52.
Among patients with anemia, defined as hemoglobin concentrations of <10.5 g/dL at baseline, 59% of those in the original relugolix group saw an improvement of at least 2 g/dL hemoglobin. The women’s improvement in pain symptoms also continued through week 52, with a 51.3-point reduction in scores on the bleeding pain and discomfort scale from baseline to the end of the study.
Adverse events were the same in the extension study and in the initial study. Those most commonly reported were headache and hot flashes. No serious safety signals occurred. The average reduction in bone mineral density was 0.80% at week 52, indicating no concerning loss.
A new drug class to treat uterine fibroids
Relugolix is one of three GnRH antagonists being studied for the long-term treatment of fibroids. The Food and Drug Administration approved the combination of elagolix, estradiol, and norethindrone acetate (Oriahnn) in May. Linzagolix, another GnRH antagonist, is currently in clinical trials.
“We’ll have a whole class of new drugs that are likely to fulfill this long sought-after goal of reducing the need for surgery for fibroids and doing it without a lot of side effects,” Dr. Taylor said. “The quality-of-life improvements seen here, the lack of significant adverse effects – none that were surprising in long term – the relatively low reduction in bone mineral density in a year are all very exciting [and suggest] that this will be a safe and effective long-term treatment.”
Significant improvement in quality of life
In the presentation on quality of life with relugolix therapy, Dr. Al-Hendy shared results regarding the severity of women’s symptoms as well as health-related quality of life, as determined on the basis of the Uterine Fibroid Symptom and Health-Related Quality of Life (UFS-QoL) questionnaire at baseline, week 12, and week 24 in LIBERTY 1 and 2. Higher UFS-QoL scores correlate with more severe symptoms. With the subscale of health-related quality of life, higher scores indicate a better quality of life.
The substudy enrolled 253 patients who received relugolix combination therapy and 256 patients who received placebo. The average menstrual blood loss was 243 mL in the relugolix group and 215 mL in the placebo group at baseline. Mean fibroid volume was the same in both groups at baseline, 73 cm3.
The proportion of Black patients was similar in both groups: 48% of the relugolix group and 54% of the placebo group.
The severity of women’s symptoms dropped from a baseline UFS-QoL score of 57 to 22.4 at 6 months among those who received relugolix combination therapy. In the placebo group, the initial score of 59.6 only dropped to 46.9 (P < .0001, for –21.4 difference in change).
Health-related quality of life increased from 38.3 to 76.6 among those who received relugolix. In the placebo group, it increased from 35.7 to 48.2 (P < .0001, for 24.5 difference). Subscales of health-related quality of life – including concern, control, activities, energy/mood, self-consciousness, and sexual function – also all improved significantly in the relugolix group, compared with the placebo group (P < .0001).
“This is a condition we see all the time that’s easily diagnosed, and we have first-line drugs we’ve been using to treat them, but none are good long-term fixes,” Dr. Taylor said. The current first-line treatments, oral contraceptives, can stabilize bleeding, “but they don’t make the fibroids shrink, they don’t stop the bleeding, women continue to have breakthrough bleeding, and the fibroids can continue to grow.”
He said most of the estimated 600,000 hysterectomies performed in the United States each year are for uterine fibroids.
“It’s a major surgery that no one wants to go through if they don’t have to,” Dr. Taylor said. “Here we have a drug that really has potential to stop the growth of the fibroids, that can stop the bleeding or dramatically improve it, and, really, for the first time, directly impact the fibroids and give us a long-term alternative.”
The studies were funded by Myovant Sciences. Dr. Al-Hendy reported consulting for AbbVie, Bayer, and Myovant Sciences, and he owns a patent for novel diagnostics and therapeutics for uterine sarcoma. Dr. Taylor has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A combination therapy using the experimental drug relugolix was effective in treating pain and heavy bleeding from uterine fibroids for a full year, according to findings from a long-term extension study of the phase 3, open-label LIBERTY trials.
The drug was also well tolerated, with retention of bone mineral density and no new adverse events, said Ayman Al-Hendy, MD, PhD, who presented the results Oct. 17 at the virtual American Society for Reproductive Medicine 2020 Scientific Congress.
“Relugolix combination therapy represents a potential long-term treatment for women with heavy menstrual bleeding associated with uterine fibroids,” said Al-Hendy, a gynecologist and endoscopic surgeon at the University of Chicago.
Dr. Al-Hendy, who consults for the company that makes the drug, on Oct. 20 presented results showing improvement in quality of life with relugolix therapy.
“The fact that this longer-term study shows continued, persistent results at a year really gives us confidence that we’ll be able to use these drugs as a long-term therapy to treat fibroids,” Hugh S. Taylor, MD, president-elect of ASRM, said in an interview. Dr. Taylor, a professor and chair of ob.gyn. and reproductive sciences at Yale University, New Haven, Conn., was not involved in the study.
“A drug like this is so necessary,” Dr. Taylor continued. “We don’t have any other drugs on the market approved for long-term use.”
Relugolix is an oral gonadotropin-releasing hormone (GnRH) receptor antagonist under investigation for long-term management of uterine fibroids. The once-daily combination therapy includes 40 mg relugolix, 1 mg estradiol, and 0.5 mg norethindrone acetate.
Extension study shows prolonged benefits
The extension trial enrolled women aged 18-50 years who were experiencing heavy menstrual bleeding from uterine fibroids and who completed the 24-week phase 3, double-blind, placebo-controlled LIBERTY 1 or 2 trials. Heavy menstrual bleeding was defined as bleeding in which at least 80 mL of blood was lost per cycle for two cycles or 160 mL was lost during one cycle. Ultrasound imaging was used to confirm the presence of fibroids.
In LIBERTY 1 and 2, women were randomly assigned to receive relugolix combination therapy, placebo, or relugolix alone for 12 weeks followed by combination therapy for the remaining 12 weeks (delayed group). Those trials found that relugolix combination therapy was effective through 6 months in reducing menstrual blood loss and pain in women with uterine fibroids without loss of bone mineral density.
LIBERTY 3 extended the trial to 52 weeks, with all participants receiving relugolix combination therapy.
As in the earlier trials, the primary endpoint was reduced menstrual blood loss. By the end of the study, women needed to have at least a 50% reduction in blood loss from the initial study’s baseline while maintaining a blood loss of <80 mL. The investigators also evaluated the mean percentage of menstrual blood loss reduction, amenorrhea rate, and improvements in anemia as secondary endpoints and assessed changes in bone mineral density.
The extension study enrolled 78% (n = 477) of the 610 women who completed the initial study; of those, 363 women completed the extension study.
Among the 163 women who began with relugolix combination therapy in the first two trials, 87.7% met the primary endpoint in a per-protocol analysis through week 52. The proportion of responders in the extension study was 75.6% among the group that formerly received placebo (n = 164) and 79.9% in the delayed group (n = 149).
The overall average reduction in menstrual blood volume was 89.9%. Most of the women experienced amenorrhea at the end of the year: 70.6% in the relugolix group, 57.9% in the group that formerly received placebo, and 68.5% in the delayed group.
Reductions in uterine volume and uterine fibroid volume were also sustained from week 24 to week 52. For the relugolix combination therapy group, the mean loss of uterine fibroid volume from baseline was 13.5% at week 24 and 18.3% at week 52. Similarly, the delayed group’s average loss in fibroid volume was 28.1% at week 24 and 33.9% at week 52. The placebo group, which only had a 7% loss in fibroid volume at week 24, had an 18.4% loss in volume from baseline at week 52.
Among patients with anemia, defined as hemoglobin concentrations of <10.5 g/dL at baseline, 59% of those in the original relugolix group saw an improvement of at least 2 g/dL hemoglobin. The women’s improvement in pain symptoms also continued through week 52, with a 51.3-point reduction in scores on the bleeding pain and discomfort scale from baseline to the end of the study.
Adverse events were the same in the extension study and in the initial study. Those most commonly reported were headache and hot flashes. No serious safety signals occurred. The average reduction in bone mineral density was 0.80% at week 52, indicating no concerning loss.
A new drug class to treat uterine fibroids
Relugolix is one of three GnRH antagonists being studied for the long-term treatment of fibroids. The Food and Drug Administration approved the combination of elagolix, estradiol, and norethindrone acetate (Oriahnn) in May. Linzagolix, another GnRH antagonist, is currently in clinical trials.
“We’ll have a whole class of new drugs that are likely to fulfill this long sought-after goal of reducing the need for surgery for fibroids and doing it without a lot of side effects,” Dr. Taylor said. “The quality-of-life improvements seen here, the lack of significant adverse effects – none that were surprising in long term – the relatively low reduction in bone mineral density in a year are all very exciting [and suggest] that this will be a safe and effective long-term treatment.”
Significant improvement in quality of life
In the presentation on quality of life with relugolix therapy, Dr. Al-Hendy shared results regarding the severity of women’s symptoms as well as health-related quality of life, as determined on the basis of the Uterine Fibroid Symptom and Health-Related Quality of Life (UFS-QoL) questionnaire at baseline, week 12, and week 24 in LIBERTY 1 and 2. Higher UFS-QoL scores correlate with more severe symptoms. With the subscale of health-related quality of life, higher scores indicate a better quality of life.
The substudy enrolled 253 patients who received relugolix combination therapy and 256 patients who received placebo. The average menstrual blood loss was 243 mL in the relugolix group and 215 mL in the placebo group at baseline. Mean fibroid volume was the same in both groups at baseline, 73 cm3.
The proportion of Black patients was similar in both groups: 48% of the relugolix group and 54% of the placebo group.
The severity of women’s symptoms dropped from a baseline UFS-QoL score of 57 to 22.4 at 6 months among those who received relugolix combination therapy. In the placebo group, the initial score of 59.6 only dropped to 46.9 (P < .0001, for –21.4 difference in change).
Health-related quality of life increased from 38.3 to 76.6 among those who received relugolix. In the placebo group, it increased from 35.7 to 48.2 (P < .0001, for 24.5 difference). Subscales of health-related quality of life – including concern, control, activities, energy/mood, self-consciousness, and sexual function – also all improved significantly in the relugolix group, compared with the placebo group (P < .0001).
“This is a condition we see all the time that’s easily diagnosed, and we have first-line drugs we’ve been using to treat them, but none are good long-term fixes,” Dr. Taylor said. The current first-line treatments, oral contraceptives, can stabilize bleeding, “but they don’t make the fibroids shrink, they don’t stop the bleeding, women continue to have breakthrough bleeding, and the fibroids can continue to grow.”
He said most of the estimated 600,000 hysterectomies performed in the United States each year are for uterine fibroids.
“It’s a major surgery that no one wants to go through if they don’t have to,” Dr. Taylor said. “Here we have a drug that really has potential to stop the growth of the fibroids, that can stop the bleeding or dramatically improve it, and, really, for the first time, directly impact the fibroids and give us a long-term alternative.”
The studies were funded by Myovant Sciences. Dr. Al-Hendy reported consulting for AbbVie, Bayer, and Myovant Sciences, and he owns a patent for novel diagnostics and therapeutics for uterine sarcoma. Dr. Taylor has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A combination therapy using the experimental drug relugolix was effective in treating pain and heavy bleeding from uterine fibroids for a full year, according to findings from a long-term extension study of the phase 3, open-label LIBERTY trials.
The drug was also well tolerated, with retention of bone mineral density and no new adverse events, said Ayman Al-Hendy, MD, PhD, who presented the results Oct. 17 at the virtual American Society for Reproductive Medicine 2020 Scientific Congress.
“Relugolix combination therapy represents a potential long-term treatment for women with heavy menstrual bleeding associated with uterine fibroids,” said Al-Hendy, a gynecologist and endoscopic surgeon at the University of Chicago.
Dr. Al-Hendy, who consults for the company that makes the drug, on Oct. 20 presented results showing improvement in quality of life with relugolix therapy.
“The fact that this longer-term study shows continued, persistent results at a year really gives us confidence that we’ll be able to use these drugs as a long-term therapy to treat fibroids,” Hugh S. Taylor, MD, president-elect of ASRM, said in an interview. Dr. Taylor, a professor and chair of ob.gyn. and reproductive sciences at Yale University, New Haven, Conn., was not involved in the study.
“A drug like this is so necessary,” Dr. Taylor continued. “We don’t have any other drugs on the market approved for long-term use.”
Relugolix is an oral gonadotropin-releasing hormone (GnRH) receptor antagonist under investigation for long-term management of uterine fibroids. The once-daily combination therapy includes 40 mg relugolix, 1 mg estradiol, and 0.5 mg norethindrone acetate.
Extension study shows prolonged benefits
The extension trial enrolled women aged 18-50 years who were experiencing heavy menstrual bleeding from uterine fibroids and who completed the 24-week phase 3, double-blind, placebo-controlled LIBERTY 1 or 2 trials. Heavy menstrual bleeding was defined as bleeding in which at least 80 mL of blood was lost per cycle for two cycles or 160 mL was lost during one cycle. Ultrasound imaging was used to confirm the presence of fibroids.
In LIBERTY 1 and 2, women were randomly assigned to receive relugolix combination therapy, placebo, or relugolix alone for 12 weeks followed by combination therapy for the remaining 12 weeks (delayed group). Those trials found that relugolix combination therapy was effective through 6 months in reducing menstrual blood loss and pain in women with uterine fibroids without loss of bone mineral density.
LIBERTY 3 extended the trial to 52 weeks, with all participants receiving relugolix combination therapy.
As in the earlier trials, the primary endpoint was reduced menstrual blood loss. By the end of the study, women needed to have at least a 50% reduction in blood loss from the initial study’s baseline while maintaining a blood loss of <80 mL. The investigators also evaluated the mean percentage of menstrual blood loss reduction, amenorrhea rate, and improvements in anemia as secondary endpoints and assessed changes in bone mineral density.
The extension study enrolled 78% (n = 477) of the 610 women who completed the initial study; of those, 363 women completed the extension study.
Among the 163 women who began with relugolix combination therapy in the first two trials, 87.7% met the primary endpoint in a per-protocol analysis through week 52. The proportion of responders in the extension study was 75.6% among the group that formerly received placebo (n = 164) and 79.9% in the delayed group (n = 149).
The overall average reduction in menstrual blood volume was 89.9%. Most of the women experienced amenorrhea at the end of the year: 70.6% in the relugolix group, 57.9% in the group that formerly received placebo, and 68.5% in the delayed group.
Reductions in uterine volume and uterine fibroid volume were also sustained from week 24 to week 52. For the relugolix combination therapy group, the mean loss of uterine fibroid volume from baseline was 13.5% at week 24 and 18.3% at week 52. Similarly, the delayed group’s average loss in fibroid volume was 28.1% at week 24 and 33.9% at week 52. The placebo group, which only had a 7% loss in fibroid volume at week 24, had an 18.4% loss in volume from baseline at week 52.
Among patients with anemia, defined as hemoglobin concentrations of <10.5 g/dL at baseline, 59% of those in the original relugolix group saw an improvement of at least 2 g/dL hemoglobin. The women’s improvement in pain symptoms also continued through week 52, with a 51.3-point reduction in scores on the bleeding pain and discomfort scale from baseline to the end of the study.
Adverse events were the same in the extension study and in the initial study. Those most commonly reported were headache and hot flashes. No serious safety signals occurred. The average reduction in bone mineral density was 0.80% at week 52, indicating no concerning loss.
A new drug class to treat uterine fibroids
Relugolix is one of three GnRH antagonists being studied for the long-term treatment of fibroids. The Food and Drug Administration approved the combination of elagolix, estradiol, and norethindrone acetate (Oriahnn) in May. Linzagolix, another GnRH antagonist, is currently in clinical trials.
“We’ll have a whole class of new drugs that are likely to fulfill this long sought-after goal of reducing the need for surgery for fibroids and doing it without a lot of side effects,” Dr. Taylor said. “The quality-of-life improvements seen here, the lack of significant adverse effects – none that were surprising in long term – the relatively low reduction in bone mineral density in a year are all very exciting [and suggest] that this will be a safe and effective long-term treatment.”
Significant improvement in quality of life
In the presentation on quality of life with relugolix therapy, Dr. Al-Hendy shared results regarding the severity of women’s symptoms as well as health-related quality of life, as determined on the basis of the Uterine Fibroid Symptom and Health-Related Quality of Life (UFS-QoL) questionnaire at baseline, week 12, and week 24 in LIBERTY 1 and 2. Higher UFS-QoL scores correlate with more severe symptoms. With the subscale of health-related quality of life, higher scores indicate a better quality of life.
The substudy enrolled 253 patients who received relugolix combination therapy and 256 patients who received placebo. The average menstrual blood loss was 243 mL in the relugolix group and 215 mL in the placebo group at baseline. Mean fibroid volume was the same in both groups at baseline, 73 cm3.
The proportion of Black patients was similar in both groups: 48% of the relugolix group and 54% of the placebo group.
The severity of women’s symptoms dropped from a baseline UFS-QoL score of 57 to 22.4 at 6 months among those who received relugolix combination therapy. In the placebo group, the initial score of 59.6 only dropped to 46.9 (P < .0001, for –21.4 difference in change).
Health-related quality of life increased from 38.3 to 76.6 among those who received relugolix. In the placebo group, it increased from 35.7 to 48.2 (P < .0001, for 24.5 difference). Subscales of health-related quality of life – including concern, control, activities, energy/mood, self-consciousness, and sexual function – also all improved significantly in the relugolix group, compared with the placebo group (P < .0001).
“This is a condition we see all the time that’s easily diagnosed, and we have first-line drugs we’ve been using to treat them, but none are good long-term fixes,” Dr. Taylor said. The current first-line treatments, oral contraceptives, can stabilize bleeding, “but they don’t make the fibroids shrink, they don’t stop the bleeding, women continue to have breakthrough bleeding, and the fibroids can continue to grow.”
He said most of the estimated 600,000 hysterectomies performed in the United States each year are for uterine fibroids.
“It’s a major surgery that no one wants to go through if they don’t have to,” Dr. Taylor said. “Here we have a drug that really has potential to stop the growth of the fibroids, that can stop the bleeding or dramatically improve it, and, really, for the first time, directly impact the fibroids and give us a long-term alternative.”
The studies were funded by Myovant Sciences. Dr. Al-Hendy reported consulting for AbbVie, Bayer, and Myovant Sciences, and he owns a patent for novel diagnostics and therapeutics for uterine sarcoma. Dr. Taylor has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Genitourinary syndrome of menopause statement stresses treatment options
It’s important for clinicians to ask women whether they are experiencing symptoms of genitourinary syndrome of menopause (GSM) before and after menopause, according to a new statement from the North American Menopause Society.
Stephanie Faubion, MD, MBA, medical director of NAMS, presented the updated statement at the virtual annual meeting of the North American Menopause Society.
“The one thing we tried to emphasize is proactive counseling and proactive inquiry, educating women when they hit perimenopause that this is a thing and that there are treatments,” Dr. Faubion said in an interview.
There’s the misperception that it’s just part of getting old, which it’s not,” said Dr. Faubion, who is also director of the Mayo Clinic Center for Women’s Health in Rochester, Minn., and chair of the department of medicine at the Mayo Clinic in Jacksonville, Fla.
Changes from previous statement
The GSM statement describes the symptoms and signs resulting from estrogen deficiency on the genitourinary tract, Dr. Faubion explained. The biggest change from the earlier version, published in 2013, is the condition’s new name. Formerly known as vulvovaginal atrophy, the condition’s new term was developed in 2014 and is now preferred by NAMS and the American College of Obstetricians and Gynecologists because it’s more comprehensive. Rather than just a physical description of the condition, GSM encompasses the many related symptoms and the urinary tract changes that occur, and it clearly associates the condition with menopause.
“Women don’t always associate these changes with menopause and don’t recognize that there’s something that can be done about it,” Dr. Faubion said. “We like to emphasize that sex should never be painful, but it’s not just about sex. It’s about comfort.”
Other changes include a review of evidence related to vaginal laser therapy for GSM and the availability of Imvexxy vaginal inserts with lower doses (4 mcg and 10 mpg) of estrogen.
Etiology and diagnosis of GSM
The presence of endogenous estrogen keeps the vaginal lining thick, rugated, well vascularized, and lubricated. As estrogen levels decline during postmenopause, the epithelial lining becomes thinner, with reduced blood supply and loss of glycogen.
The most common symptoms of GSM include irritation of the vulva, inadequate vaginal lubrication, burning, dysuria, dyspareunia, and vaginal discharge, but the symptoms may not always correlate with physical findings. In women with surgical menopause, the symptoms tend to be more severe. The most distressing symptoms to women are often those that affect sexual function.
“Clinicians must be proactive in asking menopausal women if GSM symptoms are present, even before menopause begins,” Dr. Faubion said.
Taking a women’s history during evaluation may help identify contributing factors, other causes, or potentially effective treatments based on what has worked in the past. History should include a description of symptoms, their onset and duration, how distressing they are, and their effect on the woman’s quality of life. A sexual history, such as lubricants the woman has used, can also be useful in determining management strategies.
Signs of GSM include labial atrophy, vaginal dryness, introital stenosis, clitoral atrophy, phimosis of the prepuce, reduced mons pubis and labia majora bulk, reduced labia minora tissue and pigmentation, and changes in the urethra, including erythema of the urethral meatus and commonly a urethral caruncle, a benign outgrown of inflammatory tissue that likely results from low estrogen levels and can be treated effectively with topical hormonal therapies.
A diagnosis of GSM requires both physical findings and bothersome symptoms, though not necessarily specific vaginal maturation index or vaginal pH values. The differential diagnosis speaks to the importance of taking a good history: allergic or inflammatory conditions, infection, trauma, presence of a foreign body, malignancy, vulvodynia, chronic pelvic pain, or provoked pelvic floor hypertonia.
If first-line therapies of over-the-counter lubricants do not sufficiently treat GSM, other effective treatments include low-dose vaginal estrogen therapy, systemic estrogen therapy if other menopause symptoms are present, vaginal dehydroepiandrosterone (DHEA), and ospemifene.
Management of GSM
First-line therapy of GSM involves over-the-counter lubricants and moisturizers, which are often adequate to alleviate or eliminate women’s symptoms. However, the panel that developed the statement found no evidence that hyaluronic acid was any more effective than other lubricants or moisturizers, and no herbal products were found to effectively treat GSM.
While emerging evidence suggests that energy-based therapies, such as treatments with vaginal laser or radiofrequency devices, show some promise, more evidence is needed to show safety and efficacy before the panel can recommend routine use.
When over-the-counter therapies are not effective, vaginal estrogen usually relieves GSM with little absorption and is preferred over systemic therapy if GSM is the only bothersome menopausal symptom. Options include topical creams, a slow-release estradiol intravaginal ring, and estradiol vaginal tablets and inserts.
“However, when systemic hormone therapy is needed to treat other menopause symptoms, usually a woman will derive benefit and resolution of the GSM at the same time,” Dr. Faubion said. “However, for some women, additional low-dose vaginal estrogen may be added to systemic estrogen if needed, and that could include vaginal DHEA.”
All the approved vaginal products have shown efficacy, compared with placebo in clinical trials, and a Cochrane review comparing the different therapies found them to be similarly efficacious in treating vaginal dryness and dyspareunia with no significant differences in adverse events.
Preparing patients for the boxed warning
As vaginal estrogen doses are significantly lower than systemic estrogen, their safety profile is better, with serum estrogen levels remaining within the postmenopausal range when low-dose vaginal estrogen therapy is used. That said, some studies have shown that vaginal estrogen cream can be a large enough dose to involve systemic absorption and lead to symptoms such as vaginal bleeding, breast pain, and nausea.
However, package inserts for vaginal estrogen have the same boxed warning as seen in systemic hormone therapy inserts regarding risk of endometrial cancer, breast cancer, cardiovascular disorders, and “probable dementia” despite these conditions not being linked to vaginal estrogen in trials. Neither has venous thromboembolism been linked to vaginal estrogen.
“The panel felt it was very important that women be educated about the differences between low-dose vaginal estrogen and systemic estrogen therapy and be prepared for this boxed warning,” Dr. Faubion told attendees. “It’s really important to say: ‘You’re going to get this, it’s going to look scary, and there’s no evidence these same warnings apply to the low-dose vaginal estrogen products.’ ”
This point particularly resonated with NAMS attendee Juliana (Jewel) Kling, MD, MPH, an associate professor of medicine at the Mayo Clinic Arizona, Scottsdale.
“The point about educating women about the differences between low-dose vaginal estrogen products and systemic treatments and being prepared for the boxed warning is important and I hope reaches many practitioners,” Dr. Kling said in an interview.
The panel did not recommend using progestogen with low-dose vaginal estrogen therapy or doing routine endometrial surveillance in women using vaginal estrogen. But endometrial surveillance may be worth considering in women with increased risk of endometrial cancer.
Estrogen insufficiency from premature menopause or primary ovarian insufficiency is linked to more severe sexual dysfunction, which can be particularly upsetting for younger women with vaginal atrophy and dyspareunia. A meta-analysis showed that vaginal estrogen appeared to slightly outperform over-the-counter lubricants in bringing back sexual function.
Undiagnosed vaginal or uterine bleeding is a contraindication for vaginal estrogen until the cause has been determined, and providers should use caution in prescribing vaginal estrogen to women with estrogen-dependent neoplasia. Dr. Faubion noted that GSM is common in women with breast cancer, especially if they are receiving endocrine treatments or aromatase inhibitors.
“For women with a hormone-dependent cancer, GSM management depends on each woman’s preference in consultants with her oncologist,” she said. GSM management in women with a nonhormone-dependent cancer, however, is no different than in women without cancer.
DHEA is a steroid that effectively improves vaginal maturation index, vaginal pH, dyspareunia, and vaginal dryness. The most common side effect is vaginal discharge.
Ospemifene, an estrogen agonist available in the United States but not in Canada, is the only oral product approved to treat vaginal dryness and dyspareunia. An observational study also found it effective in reducing recurrent UTIs. The most common side effect is vasomotor symptoms, and it should not be used in patients with breast cancer because it hasn’t been studied in this population.
“This updated information and position statement was needed and will be very clinically relevant in treating midlife women,” Dr. Kling said in an interview. “Dr. Faubion presented a high-level overview of the position statement with clinically relevant points, including treatment for sexual dysfunction related to GSM, GSM treatment in cancer patients, and emphasized the efficacy and low-risk safety profile of low-dose vaginal estrogen, compared to systemic [hormone therapy], for treatment of GSM.”
Dr. Faubion and Dr. Kling disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
It’s important for clinicians to ask women whether they are experiencing symptoms of genitourinary syndrome of menopause (GSM) before and after menopause, according to a new statement from the North American Menopause Society.
Stephanie Faubion, MD, MBA, medical director of NAMS, presented the updated statement at the virtual annual meeting of the North American Menopause Society.
“The one thing we tried to emphasize is proactive counseling and proactive inquiry, educating women when they hit perimenopause that this is a thing and that there are treatments,” Dr. Faubion said in an interview.
There’s the misperception that it’s just part of getting old, which it’s not,” said Dr. Faubion, who is also director of the Mayo Clinic Center for Women’s Health in Rochester, Minn., and chair of the department of medicine at the Mayo Clinic in Jacksonville, Fla.
Changes from previous statement
The GSM statement describes the symptoms and signs resulting from estrogen deficiency on the genitourinary tract, Dr. Faubion explained. The biggest change from the earlier version, published in 2013, is the condition’s new name. Formerly known as vulvovaginal atrophy, the condition’s new term was developed in 2014 and is now preferred by NAMS and the American College of Obstetricians and Gynecologists because it’s more comprehensive. Rather than just a physical description of the condition, GSM encompasses the many related symptoms and the urinary tract changes that occur, and it clearly associates the condition with menopause.
“Women don’t always associate these changes with menopause and don’t recognize that there’s something that can be done about it,” Dr. Faubion said. “We like to emphasize that sex should never be painful, but it’s not just about sex. It’s about comfort.”
Other changes include a review of evidence related to vaginal laser therapy for GSM and the availability of Imvexxy vaginal inserts with lower doses (4 mcg and 10 mpg) of estrogen.
Etiology and diagnosis of GSM
The presence of endogenous estrogen keeps the vaginal lining thick, rugated, well vascularized, and lubricated. As estrogen levels decline during postmenopause, the epithelial lining becomes thinner, with reduced blood supply and loss of glycogen.
The most common symptoms of GSM include irritation of the vulva, inadequate vaginal lubrication, burning, dysuria, dyspareunia, and vaginal discharge, but the symptoms may not always correlate with physical findings. In women with surgical menopause, the symptoms tend to be more severe. The most distressing symptoms to women are often those that affect sexual function.
“Clinicians must be proactive in asking menopausal women if GSM symptoms are present, even before menopause begins,” Dr. Faubion said.
Taking a women’s history during evaluation may help identify contributing factors, other causes, or potentially effective treatments based on what has worked in the past. History should include a description of symptoms, their onset and duration, how distressing they are, and their effect on the woman’s quality of life. A sexual history, such as lubricants the woman has used, can also be useful in determining management strategies.
Signs of GSM include labial atrophy, vaginal dryness, introital stenosis, clitoral atrophy, phimosis of the prepuce, reduced mons pubis and labia majora bulk, reduced labia minora tissue and pigmentation, and changes in the urethra, including erythema of the urethral meatus and commonly a urethral caruncle, a benign outgrown of inflammatory tissue that likely results from low estrogen levels and can be treated effectively with topical hormonal therapies.
A diagnosis of GSM requires both physical findings and bothersome symptoms, though not necessarily specific vaginal maturation index or vaginal pH values. The differential diagnosis speaks to the importance of taking a good history: allergic or inflammatory conditions, infection, trauma, presence of a foreign body, malignancy, vulvodynia, chronic pelvic pain, or provoked pelvic floor hypertonia.
If first-line therapies of over-the-counter lubricants do not sufficiently treat GSM, other effective treatments include low-dose vaginal estrogen therapy, systemic estrogen therapy if other menopause symptoms are present, vaginal dehydroepiandrosterone (DHEA), and ospemifene.
Management of GSM
First-line therapy of GSM involves over-the-counter lubricants and moisturizers, which are often adequate to alleviate or eliminate women’s symptoms. However, the panel that developed the statement found no evidence that hyaluronic acid was any more effective than other lubricants or moisturizers, and no herbal products were found to effectively treat GSM.
While emerging evidence suggests that energy-based therapies, such as treatments with vaginal laser or radiofrequency devices, show some promise, more evidence is needed to show safety and efficacy before the panel can recommend routine use.
When over-the-counter therapies are not effective, vaginal estrogen usually relieves GSM with little absorption and is preferred over systemic therapy if GSM is the only bothersome menopausal symptom. Options include topical creams, a slow-release estradiol intravaginal ring, and estradiol vaginal tablets and inserts.
“However, when systemic hormone therapy is needed to treat other menopause symptoms, usually a woman will derive benefit and resolution of the GSM at the same time,” Dr. Faubion said. “However, for some women, additional low-dose vaginal estrogen may be added to systemic estrogen if needed, and that could include vaginal DHEA.”
All the approved vaginal products have shown efficacy, compared with placebo in clinical trials, and a Cochrane review comparing the different therapies found them to be similarly efficacious in treating vaginal dryness and dyspareunia with no significant differences in adverse events.
Preparing patients for the boxed warning
As vaginal estrogen doses are significantly lower than systemic estrogen, their safety profile is better, with serum estrogen levels remaining within the postmenopausal range when low-dose vaginal estrogen therapy is used. That said, some studies have shown that vaginal estrogen cream can be a large enough dose to involve systemic absorption and lead to symptoms such as vaginal bleeding, breast pain, and nausea.
However, package inserts for vaginal estrogen have the same boxed warning as seen in systemic hormone therapy inserts regarding risk of endometrial cancer, breast cancer, cardiovascular disorders, and “probable dementia” despite these conditions not being linked to vaginal estrogen in trials. Neither has venous thromboembolism been linked to vaginal estrogen.
“The panel felt it was very important that women be educated about the differences between low-dose vaginal estrogen and systemic estrogen therapy and be prepared for this boxed warning,” Dr. Faubion told attendees. “It’s really important to say: ‘You’re going to get this, it’s going to look scary, and there’s no evidence these same warnings apply to the low-dose vaginal estrogen products.’ ”
This point particularly resonated with NAMS attendee Juliana (Jewel) Kling, MD, MPH, an associate professor of medicine at the Mayo Clinic Arizona, Scottsdale.
“The point about educating women about the differences between low-dose vaginal estrogen products and systemic treatments and being prepared for the boxed warning is important and I hope reaches many practitioners,” Dr. Kling said in an interview.
The panel did not recommend using progestogen with low-dose vaginal estrogen therapy or doing routine endometrial surveillance in women using vaginal estrogen. But endometrial surveillance may be worth considering in women with increased risk of endometrial cancer.
Estrogen insufficiency from premature menopause or primary ovarian insufficiency is linked to more severe sexual dysfunction, which can be particularly upsetting for younger women with vaginal atrophy and dyspareunia. A meta-analysis showed that vaginal estrogen appeared to slightly outperform over-the-counter lubricants in bringing back sexual function.
Undiagnosed vaginal or uterine bleeding is a contraindication for vaginal estrogen until the cause has been determined, and providers should use caution in prescribing vaginal estrogen to women with estrogen-dependent neoplasia. Dr. Faubion noted that GSM is common in women with breast cancer, especially if they are receiving endocrine treatments or aromatase inhibitors.
“For women with a hormone-dependent cancer, GSM management depends on each woman’s preference in consultants with her oncologist,” she said. GSM management in women with a nonhormone-dependent cancer, however, is no different than in women without cancer.
DHEA is a steroid that effectively improves vaginal maturation index, vaginal pH, dyspareunia, and vaginal dryness. The most common side effect is vaginal discharge.
Ospemifene, an estrogen agonist available in the United States but not in Canada, is the only oral product approved to treat vaginal dryness and dyspareunia. An observational study also found it effective in reducing recurrent UTIs. The most common side effect is vasomotor symptoms, and it should not be used in patients with breast cancer because it hasn’t been studied in this population.
“This updated information and position statement was needed and will be very clinically relevant in treating midlife women,” Dr. Kling said in an interview. “Dr. Faubion presented a high-level overview of the position statement with clinically relevant points, including treatment for sexual dysfunction related to GSM, GSM treatment in cancer patients, and emphasized the efficacy and low-risk safety profile of low-dose vaginal estrogen, compared to systemic [hormone therapy], for treatment of GSM.”
Dr. Faubion and Dr. Kling disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
It’s important for clinicians to ask women whether they are experiencing symptoms of genitourinary syndrome of menopause (GSM) before and after menopause, according to a new statement from the North American Menopause Society.
Stephanie Faubion, MD, MBA, medical director of NAMS, presented the updated statement at the virtual annual meeting of the North American Menopause Society.
“The one thing we tried to emphasize is proactive counseling and proactive inquiry, educating women when they hit perimenopause that this is a thing and that there are treatments,” Dr. Faubion said in an interview.
There’s the misperception that it’s just part of getting old, which it’s not,” said Dr. Faubion, who is also director of the Mayo Clinic Center for Women’s Health in Rochester, Minn., and chair of the department of medicine at the Mayo Clinic in Jacksonville, Fla.
Changes from previous statement
The GSM statement describes the symptoms and signs resulting from estrogen deficiency on the genitourinary tract, Dr. Faubion explained. The biggest change from the earlier version, published in 2013, is the condition’s new name. Formerly known as vulvovaginal atrophy, the condition’s new term was developed in 2014 and is now preferred by NAMS and the American College of Obstetricians and Gynecologists because it’s more comprehensive. Rather than just a physical description of the condition, GSM encompasses the many related symptoms and the urinary tract changes that occur, and it clearly associates the condition with menopause.
“Women don’t always associate these changes with menopause and don’t recognize that there’s something that can be done about it,” Dr. Faubion said. “We like to emphasize that sex should never be painful, but it’s not just about sex. It’s about comfort.”
Other changes include a review of evidence related to vaginal laser therapy for GSM and the availability of Imvexxy vaginal inserts with lower doses (4 mcg and 10 mpg) of estrogen.
Etiology and diagnosis of GSM
The presence of endogenous estrogen keeps the vaginal lining thick, rugated, well vascularized, and lubricated. As estrogen levels decline during postmenopause, the epithelial lining becomes thinner, with reduced blood supply and loss of glycogen.
The most common symptoms of GSM include irritation of the vulva, inadequate vaginal lubrication, burning, dysuria, dyspareunia, and vaginal discharge, but the symptoms may not always correlate with physical findings. In women with surgical menopause, the symptoms tend to be more severe. The most distressing symptoms to women are often those that affect sexual function.
“Clinicians must be proactive in asking menopausal women if GSM symptoms are present, even before menopause begins,” Dr. Faubion said.
Taking a women’s history during evaluation may help identify contributing factors, other causes, or potentially effective treatments based on what has worked in the past. History should include a description of symptoms, their onset and duration, how distressing they are, and their effect on the woman’s quality of life. A sexual history, such as lubricants the woman has used, can also be useful in determining management strategies.
Signs of GSM include labial atrophy, vaginal dryness, introital stenosis, clitoral atrophy, phimosis of the prepuce, reduced mons pubis and labia majora bulk, reduced labia minora tissue and pigmentation, and changes in the urethra, including erythema of the urethral meatus and commonly a urethral caruncle, a benign outgrown of inflammatory tissue that likely results from low estrogen levels and can be treated effectively with topical hormonal therapies.
A diagnosis of GSM requires both physical findings and bothersome symptoms, though not necessarily specific vaginal maturation index or vaginal pH values. The differential diagnosis speaks to the importance of taking a good history: allergic or inflammatory conditions, infection, trauma, presence of a foreign body, malignancy, vulvodynia, chronic pelvic pain, or provoked pelvic floor hypertonia.
If first-line therapies of over-the-counter lubricants do not sufficiently treat GSM, other effective treatments include low-dose vaginal estrogen therapy, systemic estrogen therapy if other menopause symptoms are present, vaginal dehydroepiandrosterone (DHEA), and ospemifene.
Management of GSM
First-line therapy of GSM involves over-the-counter lubricants and moisturizers, which are often adequate to alleviate or eliminate women’s symptoms. However, the panel that developed the statement found no evidence that hyaluronic acid was any more effective than other lubricants or moisturizers, and no herbal products were found to effectively treat GSM.
While emerging evidence suggests that energy-based therapies, such as treatments with vaginal laser or radiofrequency devices, show some promise, more evidence is needed to show safety and efficacy before the panel can recommend routine use.
When over-the-counter therapies are not effective, vaginal estrogen usually relieves GSM with little absorption and is preferred over systemic therapy if GSM is the only bothersome menopausal symptom. Options include topical creams, a slow-release estradiol intravaginal ring, and estradiol vaginal tablets and inserts.
“However, when systemic hormone therapy is needed to treat other menopause symptoms, usually a woman will derive benefit and resolution of the GSM at the same time,” Dr. Faubion said. “However, for some women, additional low-dose vaginal estrogen may be added to systemic estrogen if needed, and that could include vaginal DHEA.”
All the approved vaginal products have shown efficacy, compared with placebo in clinical trials, and a Cochrane review comparing the different therapies found them to be similarly efficacious in treating vaginal dryness and dyspareunia with no significant differences in adverse events.
Preparing patients for the boxed warning
As vaginal estrogen doses are significantly lower than systemic estrogen, their safety profile is better, with serum estrogen levels remaining within the postmenopausal range when low-dose vaginal estrogen therapy is used. That said, some studies have shown that vaginal estrogen cream can be a large enough dose to involve systemic absorption and lead to symptoms such as vaginal bleeding, breast pain, and nausea.
However, package inserts for vaginal estrogen have the same boxed warning as seen in systemic hormone therapy inserts regarding risk of endometrial cancer, breast cancer, cardiovascular disorders, and “probable dementia” despite these conditions not being linked to vaginal estrogen in trials. Neither has venous thromboembolism been linked to vaginal estrogen.
“The panel felt it was very important that women be educated about the differences between low-dose vaginal estrogen and systemic estrogen therapy and be prepared for this boxed warning,” Dr. Faubion told attendees. “It’s really important to say: ‘You’re going to get this, it’s going to look scary, and there’s no evidence these same warnings apply to the low-dose vaginal estrogen products.’ ”
This point particularly resonated with NAMS attendee Juliana (Jewel) Kling, MD, MPH, an associate professor of medicine at the Mayo Clinic Arizona, Scottsdale.
“The point about educating women about the differences between low-dose vaginal estrogen products and systemic treatments and being prepared for the boxed warning is important and I hope reaches many practitioners,” Dr. Kling said in an interview.
The panel did not recommend using progestogen with low-dose vaginal estrogen therapy or doing routine endometrial surveillance in women using vaginal estrogen. But endometrial surveillance may be worth considering in women with increased risk of endometrial cancer.
Estrogen insufficiency from premature menopause or primary ovarian insufficiency is linked to more severe sexual dysfunction, which can be particularly upsetting for younger women with vaginal atrophy and dyspareunia. A meta-analysis showed that vaginal estrogen appeared to slightly outperform over-the-counter lubricants in bringing back sexual function.
Undiagnosed vaginal or uterine bleeding is a contraindication for vaginal estrogen until the cause has been determined, and providers should use caution in prescribing vaginal estrogen to women with estrogen-dependent neoplasia. Dr. Faubion noted that GSM is common in women with breast cancer, especially if they are receiving endocrine treatments or aromatase inhibitors.
“For women with a hormone-dependent cancer, GSM management depends on each woman’s preference in consultants with her oncologist,” she said. GSM management in women with a nonhormone-dependent cancer, however, is no different than in women without cancer.
DHEA is a steroid that effectively improves vaginal maturation index, vaginal pH, dyspareunia, and vaginal dryness. The most common side effect is vaginal discharge.
Ospemifene, an estrogen agonist available in the United States but not in Canada, is the only oral product approved to treat vaginal dryness and dyspareunia. An observational study also found it effective in reducing recurrent UTIs. The most common side effect is vasomotor symptoms, and it should not be used in patients with breast cancer because it hasn’t been studied in this population.
“This updated information and position statement was needed and will be very clinically relevant in treating midlife women,” Dr. Kling said in an interview. “Dr. Faubion presented a high-level overview of the position statement with clinically relevant points, including treatment for sexual dysfunction related to GSM, GSM treatment in cancer patients, and emphasized the efficacy and low-risk safety profile of low-dose vaginal estrogen, compared to systemic [hormone therapy], for treatment of GSM.”
Dr. Faubion and Dr. Kling disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Fetal estrogens show promise for safer therapy for menopause
Hormone therapy for menopausal symptoms has come a long way in the past decade, but some low risks remain, particularly for certain groups of women. But new naturally occurring estrogens are on the horizon and may provide safer options with similar efficacy for treating hot flashes and other symptoms, researchers report.
“Unfortunately, there is no such thing as the perfect estrogen that has all the things that makes it favorable and none of the negative,” Hugh S. Taylor, MD, told attendees at the virtual annual meeting of the North American Menopause Society. “It probably doesn’t exist. But there’s an opportunity for us to design better estrogens or take advantage of other naturally occurring estrogens that come closer to that goal of the ideal estrogen,” said Dr. Taylor, professor and chair of ob.gyn. and reproductive sciences at Yale University, New Haven, Conn.
Those naturally occurring estrogens are the fetal estrogens, estetrol and estriol, which are produced almost exclusively during pregnancy. Only estetrol has been investigated in clinical trials, and it does show some promise, Dr. Taylor said.
“If there’s a better cardiovascular effect without the breast cancer risk, this could be something everyone would want to take,” Dr. Taylor said in an interview. We’ve never really been able to get a synthetic estrogen [that works].”
Hormone therapy still most effective for vasomotor symptoms
The primary benefits of hormone therapy for postmenopausal women are decreased hot flashes and night sweats and the prevention of bone loss, vaginal dryness, and vaginal atrophy. But as women age, particularly past age 70 years, the risks for stroke, heart disease, and breast cancer associated with hormone therapy begin to outweigh the benefits.
That leaves women who are still experiencing those symptoms in a quandary.
“Some people will take on substantial risks because they want to continue taking hormones,” Nanette F. Santoro, MD, a professor of ob.gyn. at the University of Colorado at Denver, Aurora, said in an interview. “If they understand what they’re doing and they tell me that they are that miserable, then I will continue their hormones.”
Dr. Santoro, who was not involved in Taylor’s work, said some patients have seen her because their primary care providers refused to continue prescribing them hormones at their age, despite serious vasomotor symptoms that interfered with their daily life.
“Women are sometimes not taken seriously, and I think that’s a problem,” Dr. Santoro said. “Women need to be able to make an informed choice about what kinds of risk they’re taking on. Many physicians’ rationales are that hot flashes never killed anybody. Well, they can sure make you miserable.”
Dr. Taylor echoed the importance of taking women’s symptoms seriously and helping them choose the most effective treatments to manage their symptoms.
“The rush of adrenaline, the anxiety, the palpitations, the heart racing, the sweating, all the night sweats [that mean] you can’t sleep at night, and the lack of adequate REM sleep – all these things add up and can really be disruptive to someone’s life,” Dr. Taylor said in an interview. “I think it’s important that we raise awareness of how severe it can be, about just how low the risks [of hormone therapy] are, and get people more comfortable using hormone therapy, but also continue to search for safer, better products that will eliminate even those low risks.”
A major development toward that goal in the past decade has been therapies that combine an estrogen with a selective estrogen receptor modulator (SERM), which have antiestrogen effects in the endometrium and breast without blocking estrogen in the bones and brain.
One such tissue-selective estrogen complex (TSEC) is the combination of bazedoxifene (20 mg) and conjugated estrogens (0.45 mg). Clinical trials showed that this TSEC reduced the frequency of hot flashes by 74%, compared with 47% with placebo. In addition, TSEC reduced the severity of hot flashes by 39%, compared with 13% with placebo. The combination also improved bone density at the spine and hip without promoting endometrial hyperplasia.
“It looks like it does exactly what we want,” Dr. Taylor told NAMS attendees. “The SERM is antagonizing the effects of the estrogens in the endometrium but not in the bone or brain.” It also led to a decrease in total cholesterol, and there was no increase in breast stimulation or density.
Another advance in recent years has been more choices and more precision with dosing, Dr. Santoro said.
“Where inroads have been made is in having women be aware of all the options they have and in getting the most convenient compounds to people,” she said, despite the confusion and misinformation that have arisen from the proliferation of bioidenticals. “You can dial in a dose for just about anybody.”
New estrogens in the pipeline
Neither of these developments, however, have eliminated the risks associated with hormone therapy for women of older age or for women at high risk for breast cancer. Although total elimination of risk may not be possible, recent research suggests that the naturally occurring fetal estrogens estriol and estetrol appear to have SERM-like properties, Dr. Taylor said. These estrogens are made only in pregnancy and appear to have evolved for a purpose different from that of estrone and estradiol.
“While both are weak estrogens by traditional standards, both have unique properties that make them very interesting for therapeutic use,” Dr. Taylor said. In particular, estetrol has a much longer half-life than estriol, making it more appropriate for therapeutic investigation.
A study of estetrol that was published in Menopause in August 2020 showed encouraging results. Despite a fairly sizable placebo effect, there was also a dose-response effect from estetrol on vasomotor symptoms. Low doses did not have much effect, but with higher doses (15 mg), there was a robust, significant improvement in the frequency and severity of hot flashes. So far, Dr. Taylor said, it looks like estetrol can be a highly effective treatment for vasomotor symptoms.
In addition, preclinical research suggests that estetrol may have a better safety profile than currently available therapies, though much more work is needed to know for sure. For example, a 2015 study found that it requires extremely high doses – well above therapeutic levels – for tumor growth to occur. Similarly, a 2019 study found that very high doses of estetrol or estriol were needed before it would stimulate breast cancer cell growth, likely because these are such weak estrogens, compared with estradiol, Dr. Taylor said.
There is currently less information on estetrol’s potential cardiovascular effects, but an animal model suggests positive effects, he said. Giving a mouse estetrol led to an increase in blood vessel dilation with increased blood flow.
The reason these estrogens appear to pose less risk yet still show therapeutic effects appears related to how they bind to the estrogen receptor and what their purpose is, Dr. Taylor told attendees.
“These fetal estrogens are really there probably for developmental programming,” he said. “It’s no wonder they may have some unique and favorable properties for therapeutic use. I’m really enthusiastic to see this explored further as a potential new hormonal therapy.”
Dr. Taylor disclosed no relevant financial relationships. Dr. Santoro reported stock ownership in Menogenix and consulting or advising for Ansh Labs, Menogenix, and Ogeda/Astellas.
A version of this article originally appeared on Medscape.com.
Hormone therapy for menopausal symptoms has come a long way in the past decade, but some low risks remain, particularly for certain groups of women. But new naturally occurring estrogens are on the horizon and may provide safer options with similar efficacy for treating hot flashes and other symptoms, researchers report.
“Unfortunately, there is no such thing as the perfect estrogen that has all the things that makes it favorable and none of the negative,” Hugh S. Taylor, MD, told attendees at the virtual annual meeting of the North American Menopause Society. “It probably doesn’t exist. But there’s an opportunity for us to design better estrogens or take advantage of other naturally occurring estrogens that come closer to that goal of the ideal estrogen,” said Dr. Taylor, professor and chair of ob.gyn. and reproductive sciences at Yale University, New Haven, Conn.
Those naturally occurring estrogens are the fetal estrogens, estetrol and estriol, which are produced almost exclusively during pregnancy. Only estetrol has been investigated in clinical trials, and it does show some promise, Dr. Taylor said.
“If there’s a better cardiovascular effect without the breast cancer risk, this could be something everyone would want to take,” Dr. Taylor said in an interview. We’ve never really been able to get a synthetic estrogen [that works].”
Hormone therapy still most effective for vasomotor symptoms
The primary benefits of hormone therapy for postmenopausal women are decreased hot flashes and night sweats and the prevention of bone loss, vaginal dryness, and vaginal atrophy. But as women age, particularly past age 70 years, the risks for stroke, heart disease, and breast cancer associated with hormone therapy begin to outweigh the benefits.
That leaves women who are still experiencing those symptoms in a quandary.
“Some people will take on substantial risks because they want to continue taking hormones,” Nanette F. Santoro, MD, a professor of ob.gyn. at the University of Colorado at Denver, Aurora, said in an interview. “If they understand what they’re doing and they tell me that they are that miserable, then I will continue their hormones.”
Dr. Santoro, who was not involved in Taylor’s work, said some patients have seen her because their primary care providers refused to continue prescribing them hormones at their age, despite serious vasomotor symptoms that interfered with their daily life.
“Women are sometimes not taken seriously, and I think that’s a problem,” Dr. Santoro said. “Women need to be able to make an informed choice about what kinds of risk they’re taking on. Many physicians’ rationales are that hot flashes never killed anybody. Well, they can sure make you miserable.”
Dr. Taylor echoed the importance of taking women’s symptoms seriously and helping them choose the most effective treatments to manage their symptoms.
“The rush of adrenaline, the anxiety, the palpitations, the heart racing, the sweating, all the night sweats [that mean] you can’t sleep at night, and the lack of adequate REM sleep – all these things add up and can really be disruptive to someone’s life,” Dr. Taylor said in an interview. “I think it’s important that we raise awareness of how severe it can be, about just how low the risks [of hormone therapy] are, and get people more comfortable using hormone therapy, but also continue to search for safer, better products that will eliminate even those low risks.”
A major development toward that goal in the past decade has been therapies that combine an estrogen with a selective estrogen receptor modulator (SERM), which have antiestrogen effects in the endometrium and breast without blocking estrogen in the bones and brain.
One such tissue-selective estrogen complex (TSEC) is the combination of bazedoxifene (20 mg) and conjugated estrogens (0.45 mg). Clinical trials showed that this TSEC reduced the frequency of hot flashes by 74%, compared with 47% with placebo. In addition, TSEC reduced the severity of hot flashes by 39%, compared with 13% with placebo. The combination also improved bone density at the spine and hip without promoting endometrial hyperplasia.
“It looks like it does exactly what we want,” Dr. Taylor told NAMS attendees. “The SERM is antagonizing the effects of the estrogens in the endometrium but not in the bone or brain.” It also led to a decrease in total cholesterol, and there was no increase in breast stimulation or density.
Another advance in recent years has been more choices and more precision with dosing, Dr. Santoro said.
“Where inroads have been made is in having women be aware of all the options they have and in getting the most convenient compounds to people,” she said, despite the confusion and misinformation that have arisen from the proliferation of bioidenticals. “You can dial in a dose for just about anybody.”
New estrogens in the pipeline
Neither of these developments, however, have eliminated the risks associated with hormone therapy for women of older age or for women at high risk for breast cancer. Although total elimination of risk may not be possible, recent research suggests that the naturally occurring fetal estrogens estriol and estetrol appear to have SERM-like properties, Dr. Taylor said. These estrogens are made only in pregnancy and appear to have evolved for a purpose different from that of estrone and estradiol.
“While both are weak estrogens by traditional standards, both have unique properties that make them very interesting for therapeutic use,” Dr. Taylor said. In particular, estetrol has a much longer half-life than estriol, making it more appropriate for therapeutic investigation.
A study of estetrol that was published in Menopause in August 2020 showed encouraging results. Despite a fairly sizable placebo effect, there was also a dose-response effect from estetrol on vasomotor symptoms. Low doses did not have much effect, but with higher doses (15 mg), there was a robust, significant improvement in the frequency and severity of hot flashes. So far, Dr. Taylor said, it looks like estetrol can be a highly effective treatment for vasomotor symptoms.
In addition, preclinical research suggests that estetrol may have a better safety profile than currently available therapies, though much more work is needed to know for sure. For example, a 2015 study found that it requires extremely high doses – well above therapeutic levels – for tumor growth to occur. Similarly, a 2019 study found that very high doses of estetrol or estriol were needed before it would stimulate breast cancer cell growth, likely because these are such weak estrogens, compared with estradiol, Dr. Taylor said.
There is currently less information on estetrol’s potential cardiovascular effects, but an animal model suggests positive effects, he said. Giving a mouse estetrol led to an increase in blood vessel dilation with increased blood flow.
The reason these estrogens appear to pose less risk yet still show therapeutic effects appears related to how they bind to the estrogen receptor and what their purpose is, Dr. Taylor told attendees.
“These fetal estrogens are really there probably for developmental programming,” he said. “It’s no wonder they may have some unique and favorable properties for therapeutic use. I’m really enthusiastic to see this explored further as a potential new hormonal therapy.”
Dr. Taylor disclosed no relevant financial relationships. Dr. Santoro reported stock ownership in Menogenix and consulting or advising for Ansh Labs, Menogenix, and Ogeda/Astellas.
A version of this article originally appeared on Medscape.com.
Hormone therapy for menopausal symptoms has come a long way in the past decade, but some low risks remain, particularly for certain groups of women. But new naturally occurring estrogens are on the horizon and may provide safer options with similar efficacy for treating hot flashes and other symptoms, researchers report.
“Unfortunately, there is no such thing as the perfect estrogen that has all the things that makes it favorable and none of the negative,” Hugh S. Taylor, MD, told attendees at the virtual annual meeting of the North American Menopause Society. “It probably doesn’t exist. But there’s an opportunity for us to design better estrogens or take advantage of other naturally occurring estrogens that come closer to that goal of the ideal estrogen,” said Dr. Taylor, professor and chair of ob.gyn. and reproductive sciences at Yale University, New Haven, Conn.
Those naturally occurring estrogens are the fetal estrogens, estetrol and estriol, which are produced almost exclusively during pregnancy. Only estetrol has been investigated in clinical trials, and it does show some promise, Dr. Taylor said.
“If there’s a better cardiovascular effect without the breast cancer risk, this could be something everyone would want to take,” Dr. Taylor said in an interview. We’ve never really been able to get a synthetic estrogen [that works].”
Hormone therapy still most effective for vasomotor symptoms
The primary benefits of hormone therapy for postmenopausal women are decreased hot flashes and night sweats and the prevention of bone loss, vaginal dryness, and vaginal atrophy. But as women age, particularly past age 70 years, the risks for stroke, heart disease, and breast cancer associated with hormone therapy begin to outweigh the benefits.
That leaves women who are still experiencing those symptoms in a quandary.
“Some people will take on substantial risks because they want to continue taking hormones,” Nanette F. Santoro, MD, a professor of ob.gyn. at the University of Colorado at Denver, Aurora, said in an interview. “If they understand what they’re doing and they tell me that they are that miserable, then I will continue their hormones.”
Dr. Santoro, who was not involved in Taylor’s work, said some patients have seen her because their primary care providers refused to continue prescribing them hormones at their age, despite serious vasomotor symptoms that interfered with their daily life.
“Women are sometimes not taken seriously, and I think that’s a problem,” Dr. Santoro said. “Women need to be able to make an informed choice about what kinds of risk they’re taking on. Many physicians’ rationales are that hot flashes never killed anybody. Well, they can sure make you miserable.”
Dr. Taylor echoed the importance of taking women’s symptoms seriously and helping them choose the most effective treatments to manage their symptoms.
“The rush of adrenaline, the anxiety, the palpitations, the heart racing, the sweating, all the night sweats [that mean] you can’t sleep at night, and the lack of adequate REM sleep – all these things add up and can really be disruptive to someone’s life,” Dr. Taylor said in an interview. “I think it’s important that we raise awareness of how severe it can be, about just how low the risks [of hormone therapy] are, and get people more comfortable using hormone therapy, but also continue to search for safer, better products that will eliminate even those low risks.”
A major development toward that goal in the past decade has been therapies that combine an estrogen with a selective estrogen receptor modulator (SERM), which have antiestrogen effects in the endometrium and breast without blocking estrogen in the bones and brain.
One such tissue-selective estrogen complex (TSEC) is the combination of bazedoxifene (20 mg) and conjugated estrogens (0.45 mg). Clinical trials showed that this TSEC reduced the frequency of hot flashes by 74%, compared with 47% with placebo. In addition, TSEC reduced the severity of hot flashes by 39%, compared with 13% with placebo. The combination also improved bone density at the spine and hip without promoting endometrial hyperplasia.
“It looks like it does exactly what we want,” Dr. Taylor told NAMS attendees. “The SERM is antagonizing the effects of the estrogens in the endometrium but not in the bone or brain.” It also led to a decrease in total cholesterol, and there was no increase in breast stimulation or density.
Another advance in recent years has been more choices and more precision with dosing, Dr. Santoro said.
“Where inroads have been made is in having women be aware of all the options they have and in getting the most convenient compounds to people,” she said, despite the confusion and misinformation that have arisen from the proliferation of bioidenticals. “You can dial in a dose for just about anybody.”
New estrogens in the pipeline
Neither of these developments, however, have eliminated the risks associated with hormone therapy for women of older age or for women at high risk for breast cancer. Although total elimination of risk may not be possible, recent research suggests that the naturally occurring fetal estrogens estriol and estetrol appear to have SERM-like properties, Dr. Taylor said. These estrogens are made only in pregnancy and appear to have evolved for a purpose different from that of estrone and estradiol.
“While both are weak estrogens by traditional standards, both have unique properties that make them very interesting for therapeutic use,” Dr. Taylor said. In particular, estetrol has a much longer half-life than estriol, making it more appropriate for therapeutic investigation.
A study of estetrol that was published in Menopause in August 2020 showed encouraging results. Despite a fairly sizable placebo effect, there was also a dose-response effect from estetrol on vasomotor symptoms. Low doses did not have much effect, but with higher doses (15 mg), there was a robust, significant improvement in the frequency and severity of hot flashes. So far, Dr. Taylor said, it looks like estetrol can be a highly effective treatment for vasomotor symptoms.
In addition, preclinical research suggests that estetrol may have a better safety profile than currently available therapies, though much more work is needed to know for sure. For example, a 2015 study found that it requires extremely high doses – well above therapeutic levels – for tumor growth to occur. Similarly, a 2019 study found that very high doses of estetrol or estriol were needed before it would stimulate breast cancer cell growth, likely because these are such weak estrogens, compared with estradiol, Dr. Taylor said.
There is currently less information on estetrol’s potential cardiovascular effects, but an animal model suggests positive effects, he said. Giving a mouse estetrol led to an increase in blood vessel dilation with increased blood flow.
The reason these estrogens appear to pose less risk yet still show therapeutic effects appears related to how they bind to the estrogen receptor and what their purpose is, Dr. Taylor told attendees.
“These fetal estrogens are really there probably for developmental programming,” he said. “It’s no wonder they may have some unique and favorable properties for therapeutic use. I’m really enthusiastic to see this explored further as a potential new hormonal therapy.”
Dr. Taylor disclosed no relevant financial relationships. Dr. Santoro reported stock ownership in Menogenix and consulting or advising for Ansh Labs, Menogenix, and Ogeda/Astellas.
A version of this article originally appeared on Medscape.com.