User login
Mini-sponge stops postpartum hemorrhage quickly and safely
Postpartum hemorrhage remains a leading cause of maternal deaths worldwide; however, “nearly all of these deaths could be prevented by timely and appropriate management,” wrote Maria I. Rodriguez, MD, of Oregon Health & Science University, Portland, and colleagues. Other strategies including use of sterile gauze, inflated Foley catheters, condom catheters, and silicone obstetric balloons, have been tried in the management of postpartum hemorrhage, but are not ideal, the researchers said.
The investigators adapted a mini-sponge device originally designed for trauma and conducted a study of a prototype between May 20 and June 12, 2019, at a single site in Zambia.
“To adapt the mini-sponge device for use in the treatment of postpartum hemorrhage, we developed an obstetric applicator for transcervical placement using a digital vaginal route,” the researchers explained. The sponges are made of the same material used in standard surgical sponges and approved for use inside the uterus and vagina, they added.
In a study published in Obstetrics & Gynecology, the investigators assessed placement, removal, and preliminary efficacy of the device. Eligible patients were women aged 16 years and older who experienced postpartum hemorrhage with an estimated blood loss of 500 mL or more because of atony after vaginal delivery.
The device was successfully placed in nine patients, and bleeding resolved within 1 minute. “For all participants, bleeding stopped in less than 1 minute, did not recur, and required no further treatment,” the researchers said.
The average time to device placement was 62 seconds. The sponges were left in place from 0.5 hours to 14 hours with an average of 1 hour, and patients were monitored with physical, visual, and ultrasound to confirm the cessation of bleeding.
Evidence of safety
No device-related adverse events were reported, and patients remained afebrile while using the device. The average age of the patients was 29 years; three had a history of anemia and four were living with HIV. One patient received a blood transfusion during labor prior to hemorrhage.
The study findings were limited by the small sample size, the low threshold for diagnosing postpartum hemorrhage, and use of estimated blood loss, which is less precise than quantitative blood loss assessment, the researchers noted. However, the results support the use of the mini-sponge tamponade to treat atonic postpartum hemorrhage, they said.
“This device is being developed to offer a low-cost, easy-to-use product that is of similar or greater efficacy than the condom uterine balloon tamponade,” needs no electricity, and could be used in low-resource areas, they said.
A larger study comparing the sponge and condom uterine balloon tamponade is planned.
“Future studies will include a larger number of participants with quantitative blood loss assessment to determine the device’s effect in managing more patients with severe postpartum hemorrhage,” the investigators noted.
Rigorous research needed
“Uterine atony is too often disastrous, and new safe and effective treatments for it would be welcome,” Dwight J. Rouse, MD, associate editor of obstetrics for Obstetrics & Gynecology, wrote in an accompanying editorial.
The current balloon tamponade used to treat postpartum hemorrhage can be difficult to place and require ongoing monitoring, he said.
Although the mini-sponge device showed promise, the study was not randomized or controlled, thus lacking in evidence of effectiveness, said Dr. Rouse.
“We simply know that the participants had the devices placed and most of them stopped bleeding,” he said.
The mechanism of action is sound, but more research is needed, especially in light of other examples of new technologies, such as adhesion barriers and negative pressure wound dressing systems after cesarean deliveries, that “made sense in the abstract but failed to improve outcomes when evaluated in proper randomized trials,” Dr. Rouse noted.
“Absent such trials, we will never really know the relative value of any device to treat uterine atony refractory to medical management,” he said.
Lead author Dr. Rodriguez disclosed that her institution received funding from OBSTETRX, which funded the study, as well as the National Institutes of Health and Merck. Dr. Rodriguez disclosed funding from Bayer, while Dr. Rouse had no financial conflicts to disclose.
SOURCE: Rodriguez MI et al. Obstet Gynecol. 2020 Oct 8. doi: 10.1097/AOG.0000000000004135.
Postpartum hemorrhage remains a leading cause of maternal deaths worldwide; however, “nearly all of these deaths could be prevented by timely and appropriate management,” wrote Maria I. Rodriguez, MD, of Oregon Health & Science University, Portland, and colleagues. Other strategies including use of sterile gauze, inflated Foley catheters, condom catheters, and silicone obstetric balloons, have been tried in the management of postpartum hemorrhage, but are not ideal, the researchers said.
The investigators adapted a mini-sponge device originally designed for trauma and conducted a study of a prototype between May 20 and June 12, 2019, at a single site in Zambia.
“To adapt the mini-sponge device for use in the treatment of postpartum hemorrhage, we developed an obstetric applicator for transcervical placement using a digital vaginal route,” the researchers explained. The sponges are made of the same material used in standard surgical sponges and approved for use inside the uterus and vagina, they added.
In a study published in Obstetrics & Gynecology, the investigators assessed placement, removal, and preliminary efficacy of the device. Eligible patients were women aged 16 years and older who experienced postpartum hemorrhage with an estimated blood loss of 500 mL or more because of atony after vaginal delivery.
The device was successfully placed in nine patients, and bleeding resolved within 1 minute. “For all participants, bleeding stopped in less than 1 minute, did not recur, and required no further treatment,” the researchers said.
The average time to device placement was 62 seconds. The sponges were left in place from 0.5 hours to 14 hours with an average of 1 hour, and patients were monitored with physical, visual, and ultrasound to confirm the cessation of bleeding.
Evidence of safety
No device-related adverse events were reported, and patients remained afebrile while using the device. The average age of the patients was 29 years; three had a history of anemia and four were living with HIV. One patient received a blood transfusion during labor prior to hemorrhage.
The study findings were limited by the small sample size, the low threshold for diagnosing postpartum hemorrhage, and use of estimated blood loss, which is less precise than quantitative blood loss assessment, the researchers noted. However, the results support the use of the mini-sponge tamponade to treat atonic postpartum hemorrhage, they said.
“This device is being developed to offer a low-cost, easy-to-use product that is of similar or greater efficacy than the condom uterine balloon tamponade,” needs no electricity, and could be used in low-resource areas, they said.
A larger study comparing the sponge and condom uterine balloon tamponade is planned.
“Future studies will include a larger number of participants with quantitative blood loss assessment to determine the device’s effect in managing more patients with severe postpartum hemorrhage,” the investigators noted.
Rigorous research needed
“Uterine atony is too often disastrous, and new safe and effective treatments for it would be welcome,” Dwight J. Rouse, MD, associate editor of obstetrics for Obstetrics & Gynecology, wrote in an accompanying editorial.
The current balloon tamponade used to treat postpartum hemorrhage can be difficult to place and require ongoing monitoring, he said.
Although the mini-sponge device showed promise, the study was not randomized or controlled, thus lacking in evidence of effectiveness, said Dr. Rouse.
“We simply know that the participants had the devices placed and most of them stopped bleeding,” he said.
The mechanism of action is sound, but more research is needed, especially in light of other examples of new technologies, such as adhesion barriers and negative pressure wound dressing systems after cesarean deliveries, that “made sense in the abstract but failed to improve outcomes when evaluated in proper randomized trials,” Dr. Rouse noted.
“Absent such trials, we will never really know the relative value of any device to treat uterine atony refractory to medical management,” he said.
Lead author Dr. Rodriguez disclosed that her institution received funding from OBSTETRX, which funded the study, as well as the National Institutes of Health and Merck. Dr. Rodriguez disclosed funding from Bayer, while Dr. Rouse had no financial conflicts to disclose.
SOURCE: Rodriguez MI et al. Obstet Gynecol. 2020 Oct 8. doi: 10.1097/AOG.0000000000004135.
Postpartum hemorrhage remains a leading cause of maternal deaths worldwide; however, “nearly all of these deaths could be prevented by timely and appropriate management,” wrote Maria I. Rodriguez, MD, of Oregon Health & Science University, Portland, and colleagues. Other strategies including use of sterile gauze, inflated Foley catheters, condom catheters, and silicone obstetric balloons, have been tried in the management of postpartum hemorrhage, but are not ideal, the researchers said.
The investigators adapted a mini-sponge device originally designed for trauma and conducted a study of a prototype between May 20 and June 12, 2019, at a single site in Zambia.
“To adapt the mini-sponge device for use in the treatment of postpartum hemorrhage, we developed an obstetric applicator for transcervical placement using a digital vaginal route,” the researchers explained. The sponges are made of the same material used in standard surgical sponges and approved for use inside the uterus and vagina, they added.
In a study published in Obstetrics & Gynecology, the investigators assessed placement, removal, and preliminary efficacy of the device. Eligible patients were women aged 16 years and older who experienced postpartum hemorrhage with an estimated blood loss of 500 mL or more because of atony after vaginal delivery.
The device was successfully placed in nine patients, and bleeding resolved within 1 minute. “For all participants, bleeding stopped in less than 1 minute, did not recur, and required no further treatment,” the researchers said.
The average time to device placement was 62 seconds. The sponges were left in place from 0.5 hours to 14 hours with an average of 1 hour, and patients were monitored with physical, visual, and ultrasound to confirm the cessation of bleeding.
Evidence of safety
No device-related adverse events were reported, and patients remained afebrile while using the device. The average age of the patients was 29 years; three had a history of anemia and four were living with HIV. One patient received a blood transfusion during labor prior to hemorrhage.
The study findings were limited by the small sample size, the low threshold for diagnosing postpartum hemorrhage, and use of estimated blood loss, which is less precise than quantitative blood loss assessment, the researchers noted. However, the results support the use of the mini-sponge tamponade to treat atonic postpartum hemorrhage, they said.
“This device is being developed to offer a low-cost, easy-to-use product that is of similar or greater efficacy than the condom uterine balloon tamponade,” needs no electricity, and could be used in low-resource areas, they said.
A larger study comparing the sponge and condom uterine balloon tamponade is planned.
“Future studies will include a larger number of participants with quantitative blood loss assessment to determine the device’s effect in managing more patients with severe postpartum hemorrhage,” the investigators noted.
Rigorous research needed
“Uterine atony is too often disastrous, and new safe and effective treatments for it would be welcome,” Dwight J. Rouse, MD, associate editor of obstetrics for Obstetrics & Gynecology, wrote in an accompanying editorial.
The current balloon tamponade used to treat postpartum hemorrhage can be difficult to place and require ongoing monitoring, he said.
Although the mini-sponge device showed promise, the study was not randomized or controlled, thus lacking in evidence of effectiveness, said Dr. Rouse.
“We simply know that the participants had the devices placed and most of them stopped bleeding,” he said.
The mechanism of action is sound, but more research is needed, especially in light of other examples of new technologies, such as adhesion barriers and negative pressure wound dressing systems after cesarean deliveries, that “made sense in the abstract but failed to improve outcomes when evaluated in proper randomized trials,” Dr. Rouse noted.
“Absent such trials, we will never really know the relative value of any device to treat uterine atony refractory to medical management,” he said.
Lead author Dr. Rodriguez disclosed that her institution received funding from OBSTETRX, which funded the study, as well as the National Institutes of Health and Merck. Dr. Rodriguez disclosed funding from Bayer, while Dr. Rouse had no financial conflicts to disclose.
SOURCE: Rodriguez MI et al. Obstet Gynecol. 2020 Oct 8. doi: 10.1097/AOG.0000000000004135.
FROM OBSTETRICS & GYNECOLOGY
Combined features of benign breast disease tied to breast cancer risk
“Benign breast disease is a key risk factor for breast cancer risk prediction,” commented presenting investigator Marta Román, PhD, of the Hospital del Mar Medical Research Institute in Barcelona. “Those women who have had a benign breast disease diagnosis have an increased risk that lasts for at least 20 years.”
To assess the combined influence of various attributes of benign breast disease, the investigators studied 629,087 women, aged 50-69 years, in Spain who underwent population-based mammographic breast cancer screening during 1994-2015 and did not have breast cancer at their prevalent (first) screen. The mean follow-up was 7.8 years.
Results showed that breast cancer risk was about three times higher for women with benign breast disease that was proliferative or that was detected on an incident screen, relative to peers with no benign breast disease. When combinations of factors were considered, breast cancer risk was most elevated – more than four times higher – for women with proliferative benign breast disease with atypia detected on an incident screen.
“We believe that these findings should be considered when discussing risk-based personalized screening strategies because these differences between prevalent and incident screens might be important if we want to personalize the screening, whether it’s the first time a woman comes to the screening program or a subsequent screen,” Dr. Román said.
Practice changing?
The study’s large size and population-based design, likely permitting capture of most biopsy results, are strengths, Mark David Pearlman, MD, of the University of Michigan, Ann Arbor, commented in an interview.
But its observational, retrospective nature opens the study up to biases, such as uncertainty as to how many women were symptomatic at the time of their mammogram and the likelihood of heightened monitoring after a biopsy showing hyperplasia, Dr. Pearlman cautioned.
“Moreover, the relative risk in this study for proliferative benign breast disease without atypia is substantially higher than prior observations of this group. This discrepancy was not discussed by the authors,” Dr. Pearlman said.
At present, women’s risk of breast cancer is predicted using well-validated models that include the question of prior breast biopsies, such as the Gail Model, the Tyrer-Cuzick model (IBIS tool), and the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm, Dr. Pearlman noted.
“This study, without further validation within a model, would not change risk assessment,” he said, disagreeing with the investigators’ conclusions. “What I would say is that further study to determine how to use this observation to decide if any change in screening or management should occur would be more appropriate.”
Study details
The 629,087 women studied underwent 2,327,384 screens, Dr. Román reported. In total, screening detected 9,184 cases of benign breast disease and 9,431 breast cancers.
Breast cancer was diagnosed in 2.4% and 3.0% of women with benign breast disease detected on prevalent and incident screens, respectively, compared with 1.5% of women without any benign breast disease detected.
Elevation of breast cancer risk varied across benign breast disease subtype. Relative to peers without any benign disease, risk was significantly elevated for women with nonproliferative disease (adjusted hazard ratio, 1.95), proliferative disease without atypia (aHR, 3.19), and proliferative disease with atypia (aHR, 3.82).
Similarly, elevation of risk varied depending on the screening at which the benign disease was detected. Risk was significantly elevated when the disease was found at prevalent screens (aHR, 1.87) and more so when it was found at incident screens (aHR, 2.67).
There was no significant interaction of these two factors (P = .83). However, when combinations were considered, risk was highest for women with proliferative benign breast disease with atypia detected on incident screens (aHR, 4.35) or prevalent screens (aHR, 3.35), and women with proliferative benign breast disease without atypia detected on incident screens (aHR, 3.83).
This study was supported by grants from Instituto de Salud Carlos III FEDER and by the Research Network on Health Services in Chronic Diseases. Dr. Román and Dr. Pearlman disclosed no conflicts of interest.
SOURCE: Román M et al. EBCC-12 Virtual Conference, Abstract 15.
“Benign breast disease is a key risk factor for breast cancer risk prediction,” commented presenting investigator Marta Román, PhD, of the Hospital del Mar Medical Research Institute in Barcelona. “Those women who have had a benign breast disease diagnosis have an increased risk that lasts for at least 20 years.”
To assess the combined influence of various attributes of benign breast disease, the investigators studied 629,087 women, aged 50-69 years, in Spain who underwent population-based mammographic breast cancer screening during 1994-2015 and did not have breast cancer at their prevalent (first) screen. The mean follow-up was 7.8 years.
Results showed that breast cancer risk was about three times higher for women with benign breast disease that was proliferative or that was detected on an incident screen, relative to peers with no benign breast disease. When combinations of factors were considered, breast cancer risk was most elevated – more than four times higher – for women with proliferative benign breast disease with atypia detected on an incident screen.
“We believe that these findings should be considered when discussing risk-based personalized screening strategies because these differences between prevalent and incident screens might be important if we want to personalize the screening, whether it’s the first time a woman comes to the screening program or a subsequent screen,” Dr. Román said.
Practice changing?
The study’s large size and population-based design, likely permitting capture of most biopsy results, are strengths, Mark David Pearlman, MD, of the University of Michigan, Ann Arbor, commented in an interview.
But its observational, retrospective nature opens the study up to biases, such as uncertainty as to how many women were symptomatic at the time of their mammogram and the likelihood of heightened monitoring after a biopsy showing hyperplasia, Dr. Pearlman cautioned.
“Moreover, the relative risk in this study for proliferative benign breast disease without atypia is substantially higher than prior observations of this group. This discrepancy was not discussed by the authors,” Dr. Pearlman said.
At present, women’s risk of breast cancer is predicted using well-validated models that include the question of prior breast biopsies, such as the Gail Model, the Tyrer-Cuzick model (IBIS tool), and the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm, Dr. Pearlman noted.
“This study, without further validation within a model, would not change risk assessment,” he said, disagreeing with the investigators’ conclusions. “What I would say is that further study to determine how to use this observation to decide if any change in screening or management should occur would be more appropriate.”
Study details
The 629,087 women studied underwent 2,327,384 screens, Dr. Román reported. In total, screening detected 9,184 cases of benign breast disease and 9,431 breast cancers.
Breast cancer was diagnosed in 2.4% and 3.0% of women with benign breast disease detected on prevalent and incident screens, respectively, compared with 1.5% of women without any benign breast disease detected.
Elevation of breast cancer risk varied across benign breast disease subtype. Relative to peers without any benign disease, risk was significantly elevated for women with nonproliferative disease (adjusted hazard ratio, 1.95), proliferative disease without atypia (aHR, 3.19), and proliferative disease with atypia (aHR, 3.82).
Similarly, elevation of risk varied depending on the screening at which the benign disease was detected. Risk was significantly elevated when the disease was found at prevalent screens (aHR, 1.87) and more so when it was found at incident screens (aHR, 2.67).
There was no significant interaction of these two factors (P = .83). However, when combinations were considered, risk was highest for women with proliferative benign breast disease with atypia detected on incident screens (aHR, 4.35) or prevalent screens (aHR, 3.35), and women with proliferative benign breast disease without atypia detected on incident screens (aHR, 3.83).
This study was supported by grants from Instituto de Salud Carlos III FEDER and by the Research Network on Health Services in Chronic Diseases. Dr. Román and Dr. Pearlman disclosed no conflicts of interest.
SOURCE: Román M et al. EBCC-12 Virtual Conference, Abstract 15.
“Benign breast disease is a key risk factor for breast cancer risk prediction,” commented presenting investigator Marta Román, PhD, of the Hospital del Mar Medical Research Institute in Barcelona. “Those women who have had a benign breast disease diagnosis have an increased risk that lasts for at least 20 years.”
To assess the combined influence of various attributes of benign breast disease, the investigators studied 629,087 women, aged 50-69 years, in Spain who underwent population-based mammographic breast cancer screening during 1994-2015 and did not have breast cancer at their prevalent (first) screen. The mean follow-up was 7.8 years.
Results showed that breast cancer risk was about three times higher for women with benign breast disease that was proliferative or that was detected on an incident screen, relative to peers with no benign breast disease. When combinations of factors were considered, breast cancer risk was most elevated – more than four times higher – for women with proliferative benign breast disease with atypia detected on an incident screen.
“We believe that these findings should be considered when discussing risk-based personalized screening strategies because these differences between prevalent and incident screens might be important if we want to personalize the screening, whether it’s the first time a woman comes to the screening program or a subsequent screen,” Dr. Román said.
Practice changing?
The study’s large size and population-based design, likely permitting capture of most biopsy results, are strengths, Mark David Pearlman, MD, of the University of Michigan, Ann Arbor, commented in an interview.
But its observational, retrospective nature opens the study up to biases, such as uncertainty as to how many women were symptomatic at the time of their mammogram and the likelihood of heightened monitoring after a biopsy showing hyperplasia, Dr. Pearlman cautioned.
“Moreover, the relative risk in this study for proliferative benign breast disease without atypia is substantially higher than prior observations of this group. This discrepancy was not discussed by the authors,” Dr. Pearlman said.
At present, women’s risk of breast cancer is predicted using well-validated models that include the question of prior breast biopsies, such as the Gail Model, the Tyrer-Cuzick model (IBIS tool), and the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm, Dr. Pearlman noted.
“This study, without further validation within a model, would not change risk assessment,” he said, disagreeing with the investigators’ conclusions. “What I would say is that further study to determine how to use this observation to decide if any change in screening or management should occur would be more appropriate.”
Study details
The 629,087 women studied underwent 2,327,384 screens, Dr. Román reported. In total, screening detected 9,184 cases of benign breast disease and 9,431 breast cancers.
Breast cancer was diagnosed in 2.4% and 3.0% of women with benign breast disease detected on prevalent and incident screens, respectively, compared with 1.5% of women without any benign breast disease detected.
Elevation of breast cancer risk varied across benign breast disease subtype. Relative to peers without any benign disease, risk was significantly elevated for women with nonproliferative disease (adjusted hazard ratio, 1.95), proliferative disease without atypia (aHR, 3.19), and proliferative disease with atypia (aHR, 3.82).
Similarly, elevation of risk varied depending on the screening at which the benign disease was detected. Risk was significantly elevated when the disease was found at prevalent screens (aHR, 1.87) and more so when it was found at incident screens (aHR, 2.67).
There was no significant interaction of these two factors (P = .83). However, when combinations were considered, risk was highest for women with proliferative benign breast disease with atypia detected on incident screens (aHR, 4.35) or prevalent screens (aHR, 3.35), and women with proliferative benign breast disease without atypia detected on incident screens (aHR, 3.83).
This study was supported by grants from Instituto de Salud Carlos III FEDER and by the Research Network on Health Services in Chronic Diseases. Dr. Román and Dr. Pearlman disclosed no conflicts of interest.
SOURCE: Román M et al. EBCC-12 Virtual Conference, Abstract 15.
FROM EBCC-12 VIRTUAL CONFERENCE
Caring for patients who experience stillbirth: Dos and don’ts
As a provider whose passion is helping women after stillbirth or neonatal loss, I get many transfers of women from their previous practice after a loss. Sometimes they transfer because they need a “fresh start,” but often, it is because they were let down by the practice – not by the medical care they received but by the emotional care and support and what was said or not said after the loss of the baby. A 2014 meta-analysis in the British Journal of Obstetrics and Gynaecology found that “Parents regarded contacts with health professionals as their central source of reassurance; but experiences often fell short of expectations.”1 I decided to conduct a survey via local and national support groups about what “loss parents” felt helpful or not helpful after the loss of a child. I purposely made these quotes the dominant part of this article, as I believe our patients are often our best teachers.
Inappropriate comments providers make
A very common theme among loss parents was how providers had made comments about how rare stillbirth is after it had just happened to them. Parents expressed that they felt this statistic kept them from getting the care that they needed prior to their loss and then they were told to not worry. Some example quotes include:
“ ‘This only happens to 1% of babies. Very rare.’ (It happened to our baby, and we have to live with this grief our whole lives. She is more than a statistic. She was our hopes, dreams, and future.)”
“I wish doctors didn’t wait to act based on statistics. There’s a lot of us in the 1% of unlikely occurrences.”
“For me, when my practice brushed off my feelings, I knew in my gut something was wrong. They said, ‘We need to wean you from worrying.’”
Another very common theme from parents included examples of helpful and not helpful care they received in the hospital.
Help parents make good memories
Many parents mentioned the importance of providing resources for after they go home. Most labor and delivery units have pregnancy loss services and have improved on the care they provide for loss families. One very common positive comment responded to the memories that nurses and providers helped them make after delivery. One parent said the following:
“While with baby and after loss, I think it’s really important to give ideas of what to do/experiences because the moments are so fleeting, and I needed someone to say, ‘You can dress him.’ ‘Let’s take pictures of his toes.’ ‘Save breast milk, etc.’”
“I appreciated the doctors and nurses who acknowledged my child, who looked at him and humanized him. One nurse even held him, which I still love her to this day for.”
“Explain things over, over, and over again, like you are explaining it to a child. I didn’t know what a cuddle cot was, and I didn’t use it because I didn’t understand.”
“Give suggestions and stress the importance of making memories. There are things I wish I did and now regret not doing. Taking pictures, handprints, lock of hair, giving the baby a bath.”
“For unknown losses give a full explanation of the autopsy and what it entails. Parents are making SO many decisions, and they need guidance.”
“Don’t shy away from it. It happened, and it is important to be human and compassionate. If you cannot do it, find someone else who can.”
“Ask to hold the baby and comment how beautiful the baby is. Treat the baby as if it were living.”
Don’t use the ‘silver lining’ theme
A common “don’t” in the hospital and postpartum is the “at least” and “silver lining” theme that is commonly expressed by providers. When I do my teaching sessions with a bereaved parents panel, we always stress that comforting words never begin with “at least.” We say a lot that there is no “silver lining” to a stillbirth. Dr. Brene Brown, in her TED talk on empathy, discussed that an empathic statement never starts with “at least.” However, this response is an all too common experience for women after stillbirth. Here are some examples from the Internet responses:
“‘The silver lining is you and your daughter have taught us so much.’ There is no silver lining, and her life was not for anyone’s easy path to learning lessons. She was wanted and loved.”
“What not to say: From a doctor, ‘You’re going to have lots more children.’ Anything along the lines of ‘at least you can get pregnant/have children’ is not OK.”
“As a teacher, ‘At least you are already a mother to your students.’ (I cannot even tell you how many times I’ve been told this. They already have mothers, and teaching a child 40 minutes once a week is not even close to being a mother to your own child.)”
“I felt it unhelpful for people to tell me how I should feel. I felt comments like ‘oh, you are young you can have another baby’ unhelpful.”
“Do not say, ‘you can have another, it wasn’t God’s plan, God wanted another angel, there is a reason for everything,’ etc.”
“The doctor who told me my baby was dead referred to him as a fetus. I was 38 weeks pregnant and did not refer to my baby as a fetus.”
Handling patient care after the loss
A huge portion of the response I received was regarding care from the practice where they delivered after the loss. These parents provided very important advice for any practice after a patient experiences a loss. Emotional support is vital for these patients. They also made it clear that topics such a medications and counseling should be frequently revisited.
“The care a patient receives after can really change their life – not physical care but emotional care. I truly believe I recovered well, and I am the person I am today because of my provider’s phone calls, suggestions for medications, support groups, and counseling. Don’t underestimate what simple phone calls can do. You don’t have to provide a solution or give advice, just listen.”
“Revisit conversations about medications. I have never taken anything in my entire life. In fact, I was very against it. Don’t be afraid to suggest medications time and time again if you think that it is the right plan. After 6 months, I said ‘yes’ to the medication, and it helped immensely.”
“My OB checking in with me constantly. Doctors offering compassionate and informative advice and encouragement. SUPPORT GROUPS. Star Legacy Foundation mentor!!! Klonopin! Psychologist!!”
“Also, I think it’s important for providers to continue to follow-up with patients even if they don’t seem receptive. Keep checking in. After losing your child you are in a fog. You don’t know quite what you need. But those calls, I promise you they mean something.”
When the patient returns to the office
The care received by a loss parent after returning to the office is challenging but so important. Some very careful steps can and must be made to help avoid emotionally harmful situations for the staff and patients. Offices need to make special accommodations and mark what happened clearly in the chart regarding the loss. When I have a mother coming in for a postpartum visit after a loss, I make sure she is the last patient of the day and try to bring her to our satellite offices where she can be the only patient there. Many parents made comments about carefully labeling what happened to the baby in the chart.
“Make sure it’s noted in the chart, and don’t AVOID talking about it. We like to have our baby brought up. Make sure staff knows the situation before entering the office so they don’t say something stupid (for example, ‘How is breast feeding going?’)”
“#1 don’t in my book: Not reading the patient’s chart and labels on it before seeing them if you’re not familiar with the patient. ... Nurses, techs and providers alike have assumed or asked “this is your first,” when clearly my chart lists “fetal death in utero.’”
“Many others have stated this, but having a BIG HUGE MASSIVE flag on our accounts and making sure ALL parts of the office are trained on this would be so incredibly helpful.”
“The nurse at my doctor’s office yesterday said, ‘Well, you’ve lost some weight since you were here last, so that’s good!’ My response was, ‘Well, losing a baby will do that.’”
“The follow-up appointment is awful. I went in heartbroken and angry and anxious. A phone call the day before acknowledging those feelings and reassuring me it was okay would have been nice.”
“At my first follow-up after my son died, I walked in, the receptionist pulled up my chart, saw I was there for my post-delivery appointment, and in the loudest, most cheery voice said, ‘Oooooooooh how’s he doing, how’s the baby?!’ It was awful telling her that he died, and I also felt terrible for all the pregnant woman in the waiting room who may have heard it.”
“When I was in emergency for a complication after birth, the only condolence a doctor from our previous practice gave was, ‘Well, that sucks’ (in regard to our daughter).”
Continuing care in the office
The care of women in the office immediately after loss and in years to come is a very important piece of the care they receive. In the same BJOG meta-analysis they found, “Parents frequently encountered professionals who were unaware of their history, through lack of access to/or reading of notes before a consultation. Dismissive attitudes to fears and concerns and insensitive and inappropriate comments sometimes resulted. These often remained with parents long after the event. In contrast, emotional wellbeing was enhanced when care providers demonstrated empathy, listened to concerns and committed to a collaborative and supportive relationship. Parents valued direct acknowledgment of the baby who had died, including using his or her name. Flexible antenatal care including extra appointments, routinely or on request, was also welcomed.”1 These findings were very similar to those reflected in the comments that I received.
“To the mother, there is no difference between a living baby and a stillborn baby. This stillborn baby is JUST AS MUCH a life to us. I’ve had four kids, and I can’t differentiate between how I feel about them.”
“Also, if staying with the same provider, ‘do’ ask what accommodations can be made moving forward. (For me I needed a different ultrasound tech and a different office for my ultrasounds in my subsequent pregnancies as I couldn’t go back there but wanted to stay with my same OB).”
“Don’t be afraid to ask about the child. I want people to know I like talking about my son, that he existed and how much love there was in his short but meaningful life.”
“Saying nothing is worse than saying you don’t know what to say and you are sorry.”
“Some moms love the rainbow baby term, and if they use it first, it’s fine to use it and encourage it and promote it. However, some moms do not like it because 1) they don’t like referring to their loss baby as a ‘storm.’ My baby was a BABY, and he was perfect and loved and I don’t like people referring to him as a storm. A storm is derogatory, [and] 2) the notion the subsequent baby makes everything okay is ridiculous. 3) Not everyone has another baby after a loss, so the ‘after every storm comes a rainbow’ phrase is stupid. It makes it seem like you can never be happy again unless you get a rainbow, and that is not true. 4) It’s a signal to the outside world that ‘everything’ is great and ok when in reality you can have grief, joy, sadness, happiness, pain, and hope all at the [same] time forever.”
Patients and their families who have lost their babies deserve our very best. No one grieves the same, and the differences in how our patients grieve must be respected. However, members of the loss community do have some common themes on responses that they appreciated or did not appreciate regarding their care. Most patients who deliver a stillborn baby or experience a neonatal death or pre-viable baby have had no time to prepare, and they are looking for our guidance and support. The more time we spend with them after diagnosis, during delivery, and after will be so appreciated. I hope some of these quotes ring true to many providers and that they either lead to attempts to change care or reinforce the amazing care providers are already providing. Being at our best when our patients are experiencing potentially the worst moments of their lives is our job as obstetrical providers. Our patients deserve the best care we can possibly provide. Hopefully, these suggestions from patients will help the care of future loss families.
Dr. Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, N.Y. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
Reference
1. BJOG. 2014 Jul; 121(8):943-50. doi: 10.1111/1471-0528.12656.
As a provider whose passion is helping women after stillbirth or neonatal loss, I get many transfers of women from their previous practice after a loss. Sometimes they transfer because they need a “fresh start,” but often, it is because they were let down by the practice – not by the medical care they received but by the emotional care and support and what was said or not said after the loss of the baby. A 2014 meta-analysis in the British Journal of Obstetrics and Gynaecology found that “Parents regarded contacts with health professionals as their central source of reassurance; but experiences often fell short of expectations.”1 I decided to conduct a survey via local and national support groups about what “loss parents” felt helpful or not helpful after the loss of a child. I purposely made these quotes the dominant part of this article, as I believe our patients are often our best teachers.
Inappropriate comments providers make
A very common theme among loss parents was how providers had made comments about how rare stillbirth is after it had just happened to them. Parents expressed that they felt this statistic kept them from getting the care that they needed prior to their loss and then they were told to not worry. Some example quotes include:
“ ‘This only happens to 1% of babies. Very rare.’ (It happened to our baby, and we have to live with this grief our whole lives. She is more than a statistic. She was our hopes, dreams, and future.)”
“I wish doctors didn’t wait to act based on statistics. There’s a lot of us in the 1% of unlikely occurrences.”
“For me, when my practice brushed off my feelings, I knew in my gut something was wrong. They said, ‘We need to wean you from worrying.’”
Another very common theme from parents included examples of helpful and not helpful care they received in the hospital.
Help parents make good memories
Many parents mentioned the importance of providing resources for after they go home. Most labor and delivery units have pregnancy loss services and have improved on the care they provide for loss families. One very common positive comment responded to the memories that nurses and providers helped them make after delivery. One parent said the following:
“While with baby and after loss, I think it’s really important to give ideas of what to do/experiences because the moments are so fleeting, and I needed someone to say, ‘You can dress him.’ ‘Let’s take pictures of his toes.’ ‘Save breast milk, etc.’”
“I appreciated the doctors and nurses who acknowledged my child, who looked at him and humanized him. One nurse even held him, which I still love her to this day for.”
“Explain things over, over, and over again, like you are explaining it to a child. I didn’t know what a cuddle cot was, and I didn’t use it because I didn’t understand.”
“Give suggestions and stress the importance of making memories. There are things I wish I did and now regret not doing. Taking pictures, handprints, lock of hair, giving the baby a bath.”
“For unknown losses give a full explanation of the autopsy and what it entails. Parents are making SO many decisions, and they need guidance.”
“Don’t shy away from it. It happened, and it is important to be human and compassionate. If you cannot do it, find someone else who can.”
“Ask to hold the baby and comment how beautiful the baby is. Treat the baby as if it were living.”
Don’t use the ‘silver lining’ theme
A common “don’t” in the hospital and postpartum is the “at least” and “silver lining” theme that is commonly expressed by providers. When I do my teaching sessions with a bereaved parents panel, we always stress that comforting words never begin with “at least.” We say a lot that there is no “silver lining” to a stillbirth. Dr. Brene Brown, in her TED talk on empathy, discussed that an empathic statement never starts with “at least.” However, this response is an all too common experience for women after stillbirth. Here are some examples from the Internet responses:
“‘The silver lining is you and your daughter have taught us so much.’ There is no silver lining, and her life was not for anyone’s easy path to learning lessons. She was wanted and loved.”
“What not to say: From a doctor, ‘You’re going to have lots more children.’ Anything along the lines of ‘at least you can get pregnant/have children’ is not OK.”
“As a teacher, ‘At least you are already a mother to your students.’ (I cannot even tell you how many times I’ve been told this. They already have mothers, and teaching a child 40 minutes once a week is not even close to being a mother to your own child.)”
“I felt it unhelpful for people to tell me how I should feel. I felt comments like ‘oh, you are young you can have another baby’ unhelpful.”
“Do not say, ‘you can have another, it wasn’t God’s plan, God wanted another angel, there is a reason for everything,’ etc.”
“The doctor who told me my baby was dead referred to him as a fetus. I was 38 weeks pregnant and did not refer to my baby as a fetus.”
Handling patient care after the loss
A huge portion of the response I received was regarding care from the practice where they delivered after the loss. These parents provided very important advice for any practice after a patient experiences a loss. Emotional support is vital for these patients. They also made it clear that topics such a medications and counseling should be frequently revisited.
“The care a patient receives after can really change their life – not physical care but emotional care. I truly believe I recovered well, and I am the person I am today because of my provider’s phone calls, suggestions for medications, support groups, and counseling. Don’t underestimate what simple phone calls can do. You don’t have to provide a solution or give advice, just listen.”
“Revisit conversations about medications. I have never taken anything in my entire life. In fact, I was very against it. Don’t be afraid to suggest medications time and time again if you think that it is the right plan. After 6 months, I said ‘yes’ to the medication, and it helped immensely.”
“My OB checking in with me constantly. Doctors offering compassionate and informative advice and encouragement. SUPPORT GROUPS. Star Legacy Foundation mentor!!! Klonopin! Psychologist!!”
“Also, I think it’s important for providers to continue to follow-up with patients even if they don’t seem receptive. Keep checking in. After losing your child you are in a fog. You don’t know quite what you need. But those calls, I promise you they mean something.”
When the patient returns to the office
The care received by a loss parent after returning to the office is challenging but so important. Some very careful steps can and must be made to help avoid emotionally harmful situations for the staff and patients. Offices need to make special accommodations and mark what happened clearly in the chart regarding the loss. When I have a mother coming in for a postpartum visit after a loss, I make sure she is the last patient of the day and try to bring her to our satellite offices where she can be the only patient there. Many parents made comments about carefully labeling what happened to the baby in the chart.
“Make sure it’s noted in the chart, and don’t AVOID talking about it. We like to have our baby brought up. Make sure staff knows the situation before entering the office so they don’t say something stupid (for example, ‘How is breast feeding going?’)”
“#1 don’t in my book: Not reading the patient’s chart and labels on it before seeing them if you’re not familiar with the patient. ... Nurses, techs and providers alike have assumed or asked “this is your first,” when clearly my chart lists “fetal death in utero.’”
“Many others have stated this, but having a BIG HUGE MASSIVE flag on our accounts and making sure ALL parts of the office are trained on this would be so incredibly helpful.”
“The nurse at my doctor’s office yesterday said, ‘Well, you’ve lost some weight since you were here last, so that’s good!’ My response was, ‘Well, losing a baby will do that.’”
“The follow-up appointment is awful. I went in heartbroken and angry and anxious. A phone call the day before acknowledging those feelings and reassuring me it was okay would have been nice.”
“At my first follow-up after my son died, I walked in, the receptionist pulled up my chart, saw I was there for my post-delivery appointment, and in the loudest, most cheery voice said, ‘Oooooooooh how’s he doing, how’s the baby?!’ It was awful telling her that he died, and I also felt terrible for all the pregnant woman in the waiting room who may have heard it.”
“When I was in emergency for a complication after birth, the only condolence a doctor from our previous practice gave was, ‘Well, that sucks’ (in regard to our daughter).”
Continuing care in the office
The care of women in the office immediately after loss and in years to come is a very important piece of the care they receive. In the same BJOG meta-analysis they found, “Parents frequently encountered professionals who were unaware of their history, through lack of access to/or reading of notes before a consultation. Dismissive attitudes to fears and concerns and insensitive and inappropriate comments sometimes resulted. These often remained with parents long after the event. In contrast, emotional wellbeing was enhanced when care providers demonstrated empathy, listened to concerns and committed to a collaborative and supportive relationship. Parents valued direct acknowledgment of the baby who had died, including using his or her name. Flexible antenatal care including extra appointments, routinely or on request, was also welcomed.”1 These findings were very similar to those reflected in the comments that I received.
“To the mother, there is no difference between a living baby and a stillborn baby. This stillborn baby is JUST AS MUCH a life to us. I’ve had four kids, and I can’t differentiate between how I feel about them.”
“Also, if staying with the same provider, ‘do’ ask what accommodations can be made moving forward. (For me I needed a different ultrasound tech and a different office for my ultrasounds in my subsequent pregnancies as I couldn’t go back there but wanted to stay with my same OB).”
“Don’t be afraid to ask about the child. I want people to know I like talking about my son, that he existed and how much love there was in his short but meaningful life.”
“Saying nothing is worse than saying you don’t know what to say and you are sorry.”
“Some moms love the rainbow baby term, and if they use it first, it’s fine to use it and encourage it and promote it. However, some moms do not like it because 1) they don’t like referring to their loss baby as a ‘storm.’ My baby was a BABY, and he was perfect and loved and I don’t like people referring to him as a storm. A storm is derogatory, [and] 2) the notion the subsequent baby makes everything okay is ridiculous. 3) Not everyone has another baby after a loss, so the ‘after every storm comes a rainbow’ phrase is stupid. It makes it seem like you can never be happy again unless you get a rainbow, and that is not true. 4) It’s a signal to the outside world that ‘everything’ is great and ok when in reality you can have grief, joy, sadness, happiness, pain, and hope all at the [same] time forever.”
Patients and their families who have lost their babies deserve our very best. No one grieves the same, and the differences in how our patients grieve must be respected. However, members of the loss community do have some common themes on responses that they appreciated or did not appreciate regarding their care. Most patients who deliver a stillborn baby or experience a neonatal death or pre-viable baby have had no time to prepare, and they are looking for our guidance and support. The more time we spend with them after diagnosis, during delivery, and after will be so appreciated. I hope some of these quotes ring true to many providers and that they either lead to attempts to change care or reinforce the amazing care providers are already providing. Being at our best when our patients are experiencing potentially the worst moments of their lives is our job as obstetrical providers. Our patients deserve the best care we can possibly provide. Hopefully, these suggestions from patients will help the care of future loss families.
Dr. Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, N.Y. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
Reference
1. BJOG. 2014 Jul; 121(8):943-50. doi: 10.1111/1471-0528.12656.
As a provider whose passion is helping women after stillbirth or neonatal loss, I get many transfers of women from their previous practice after a loss. Sometimes they transfer because they need a “fresh start,” but often, it is because they were let down by the practice – not by the medical care they received but by the emotional care and support and what was said or not said after the loss of the baby. A 2014 meta-analysis in the British Journal of Obstetrics and Gynaecology found that “Parents regarded contacts with health professionals as their central source of reassurance; but experiences often fell short of expectations.”1 I decided to conduct a survey via local and national support groups about what “loss parents” felt helpful or not helpful after the loss of a child. I purposely made these quotes the dominant part of this article, as I believe our patients are often our best teachers.
Inappropriate comments providers make
A very common theme among loss parents was how providers had made comments about how rare stillbirth is after it had just happened to them. Parents expressed that they felt this statistic kept them from getting the care that they needed prior to their loss and then they were told to not worry. Some example quotes include:
“ ‘This only happens to 1% of babies. Very rare.’ (It happened to our baby, and we have to live with this grief our whole lives. She is more than a statistic. She was our hopes, dreams, and future.)”
“I wish doctors didn’t wait to act based on statistics. There’s a lot of us in the 1% of unlikely occurrences.”
“For me, when my practice brushed off my feelings, I knew in my gut something was wrong. They said, ‘We need to wean you from worrying.’”
Another very common theme from parents included examples of helpful and not helpful care they received in the hospital.
Help parents make good memories
Many parents mentioned the importance of providing resources for after they go home. Most labor and delivery units have pregnancy loss services and have improved on the care they provide for loss families. One very common positive comment responded to the memories that nurses and providers helped them make after delivery. One parent said the following:
“While with baby and after loss, I think it’s really important to give ideas of what to do/experiences because the moments are so fleeting, and I needed someone to say, ‘You can dress him.’ ‘Let’s take pictures of his toes.’ ‘Save breast milk, etc.’”
“I appreciated the doctors and nurses who acknowledged my child, who looked at him and humanized him. One nurse even held him, which I still love her to this day for.”
“Explain things over, over, and over again, like you are explaining it to a child. I didn’t know what a cuddle cot was, and I didn’t use it because I didn’t understand.”
“Give suggestions and stress the importance of making memories. There are things I wish I did and now regret not doing. Taking pictures, handprints, lock of hair, giving the baby a bath.”
“For unknown losses give a full explanation of the autopsy and what it entails. Parents are making SO many decisions, and they need guidance.”
“Don’t shy away from it. It happened, and it is important to be human and compassionate. If you cannot do it, find someone else who can.”
“Ask to hold the baby and comment how beautiful the baby is. Treat the baby as if it were living.”
Don’t use the ‘silver lining’ theme
A common “don’t” in the hospital and postpartum is the “at least” and “silver lining” theme that is commonly expressed by providers. When I do my teaching sessions with a bereaved parents panel, we always stress that comforting words never begin with “at least.” We say a lot that there is no “silver lining” to a stillbirth. Dr. Brene Brown, in her TED talk on empathy, discussed that an empathic statement never starts with “at least.” However, this response is an all too common experience for women after stillbirth. Here are some examples from the Internet responses:
“‘The silver lining is you and your daughter have taught us so much.’ There is no silver lining, and her life was not for anyone’s easy path to learning lessons. She was wanted and loved.”
“What not to say: From a doctor, ‘You’re going to have lots more children.’ Anything along the lines of ‘at least you can get pregnant/have children’ is not OK.”
“As a teacher, ‘At least you are already a mother to your students.’ (I cannot even tell you how many times I’ve been told this. They already have mothers, and teaching a child 40 minutes once a week is not even close to being a mother to your own child.)”
“I felt it unhelpful for people to tell me how I should feel. I felt comments like ‘oh, you are young you can have another baby’ unhelpful.”
“Do not say, ‘you can have another, it wasn’t God’s plan, God wanted another angel, there is a reason for everything,’ etc.”
“The doctor who told me my baby was dead referred to him as a fetus. I was 38 weeks pregnant and did not refer to my baby as a fetus.”
Handling patient care after the loss
A huge portion of the response I received was regarding care from the practice where they delivered after the loss. These parents provided very important advice for any practice after a patient experiences a loss. Emotional support is vital for these patients. They also made it clear that topics such a medications and counseling should be frequently revisited.
“The care a patient receives after can really change their life – not physical care but emotional care. I truly believe I recovered well, and I am the person I am today because of my provider’s phone calls, suggestions for medications, support groups, and counseling. Don’t underestimate what simple phone calls can do. You don’t have to provide a solution or give advice, just listen.”
“Revisit conversations about medications. I have never taken anything in my entire life. In fact, I was very against it. Don’t be afraid to suggest medications time and time again if you think that it is the right plan. After 6 months, I said ‘yes’ to the medication, and it helped immensely.”
“My OB checking in with me constantly. Doctors offering compassionate and informative advice and encouragement. SUPPORT GROUPS. Star Legacy Foundation mentor!!! Klonopin! Psychologist!!”
“Also, I think it’s important for providers to continue to follow-up with patients even if they don’t seem receptive. Keep checking in. After losing your child you are in a fog. You don’t know quite what you need. But those calls, I promise you they mean something.”
When the patient returns to the office
The care received by a loss parent after returning to the office is challenging but so important. Some very careful steps can and must be made to help avoid emotionally harmful situations for the staff and patients. Offices need to make special accommodations and mark what happened clearly in the chart regarding the loss. When I have a mother coming in for a postpartum visit after a loss, I make sure she is the last patient of the day and try to bring her to our satellite offices where she can be the only patient there. Many parents made comments about carefully labeling what happened to the baby in the chart.
“Make sure it’s noted in the chart, and don’t AVOID talking about it. We like to have our baby brought up. Make sure staff knows the situation before entering the office so they don’t say something stupid (for example, ‘How is breast feeding going?’)”
“#1 don’t in my book: Not reading the patient’s chart and labels on it before seeing them if you’re not familiar with the patient. ... Nurses, techs and providers alike have assumed or asked “this is your first,” when clearly my chart lists “fetal death in utero.’”
“Many others have stated this, but having a BIG HUGE MASSIVE flag on our accounts and making sure ALL parts of the office are trained on this would be so incredibly helpful.”
“The nurse at my doctor’s office yesterday said, ‘Well, you’ve lost some weight since you were here last, so that’s good!’ My response was, ‘Well, losing a baby will do that.’”
“The follow-up appointment is awful. I went in heartbroken and angry and anxious. A phone call the day before acknowledging those feelings and reassuring me it was okay would have been nice.”
“At my first follow-up after my son died, I walked in, the receptionist pulled up my chart, saw I was there for my post-delivery appointment, and in the loudest, most cheery voice said, ‘Oooooooooh how’s he doing, how’s the baby?!’ It was awful telling her that he died, and I also felt terrible for all the pregnant woman in the waiting room who may have heard it.”
“When I was in emergency for a complication after birth, the only condolence a doctor from our previous practice gave was, ‘Well, that sucks’ (in regard to our daughter).”
Continuing care in the office
The care of women in the office immediately after loss and in years to come is a very important piece of the care they receive. In the same BJOG meta-analysis they found, “Parents frequently encountered professionals who were unaware of their history, through lack of access to/or reading of notes before a consultation. Dismissive attitudes to fears and concerns and insensitive and inappropriate comments sometimes resulted. These often remained with parents long after the event. In contrast, emotional wellbeing was enhanced when care providers demonstrated empathy, listened to concerns and committed to a collaborative and supportive relationship. Parents valued direct acknowledgment of the baby who had died, including using his or her name. Flexible antenatal care including extra appointments, routinely or on request, was also welcomed.”1 These findings were very similar to those reflected in the comments that I received.
“To the mother, there is no difference between a living baby and a stillborn baby. This stillborn baby is JUST AS MUCH a life to us. I’ve had four kids, and I can’t differentiate between how I feel about them.”
“Also, if staying with the same provider, ‘do’ ask what accommodations can be made moving forward. (For me I needed a different ultrasound tech and a different office for my ultrasounds in my subsequent pregnancies as I couldn’t go back there but wanted to stay with my same OB).”
“Don’t be afraid to ask about the child. I want people to know I like talking about my son, that he existed and how much love there was in his short but meaningful life.”
“Saying nothing is worse than saying you don’t know what to say and you are sorry.”
“Some moms love the rainbow baby term, and if they use it first, it’s fine to use it and encourage it and promote it. However, some moms do not like it because 1) they don’t like referring to their loss baby as a ‘storm.’ My baby was a BABY, and he was perfect and loved and I don’t like people referring to him as a storm. A storm is derogatory, [and] 2) the notion the subsequent baby makes everything okay is ridiculous. 3) Not everyone has another baby after a loss, so the ‘after every storm comes a rainbow’ phrase is stupid. It makes it seem like you can never be happy again unless you get a rainbow, and that is not true. 4) It’s a signal to the outside world that ‘everything’ is great and ok when in reality you can have grief, joy, sadness, happiness, pain, and hope all at the [same] time forever.”
Patients and their families who have lost their babies deserve our very best. No one grieves the same, and the differences in how our patients grieve must be respected. However, members of the loss community do have some common themes on responses that they appreciated or did not appreciate regarding their care. Most patients who deliver a stillborn baby or experience a neonatal death or pre-viable baby have had no time to prepare, and they are looking for our guidance and support. The more time we spend with them after diagnosis, during delivery, and after will be so appreciated. I hope some of these quotes ring true to many providers and that they either lead to attempts to change care or reinforce the amazing care providers are already providing. Being at our best when our patients are experiencing potentially the worst moments of their lives is our job as obstetrical providers. Our patients deserve the best care we can possibly provide. Hopefully, these suggestions from patients will help the care of future loss families.
Dr. Florescue is an ob.gyn. in private practice at Women Gynecology and Childbirth Associates in Rochester, N.Y. She delivers babies at Highland Hospital in Rochester. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
Reference
1. BJOG. 2014 Jul; 121(8):943-50. doi: 10.1111/1471-0528.12656.
FDA issues new NSAIDs warning for second half of pregnancy
The U.S. Food and Drug Administration released new warnings Oct. 15 that most nonsteroidal anti-inflammatory agents (NSAIDs) carry an elevated risk for kidney complications in unborn children when taken around weeks 20 or later in pregnancy.
Citing newly available research, the agency states the risk of low amniotic fluid (known as oligohydramnios) can occur, which in turn can cause rare but serious kidney problems in the offspring. Pregnancy complications also can result.
The FDA action expands on earlier warnings about agents in this drug class, which the FDA previously cautioned about taking after week 30 of pregnancy because of heart-related risks.
Manufacturers of both over-the-counter and prescription NSAIDs – including ibuprofen, naproxen, diclofenac, and celecoxib – will be required to update their labeling with the new warning.
Low-dose (81-mg) aspirin is excluded from this warning.
“Low-dose aspirin may be an important treatment for some women during pregnancy and should be taken under the direction of a healthcare professional,” the agency stated in a news release.
“It is important that women understand the benefits and risks of the medications they may take over the course of their pregnancy,” Patrizia Cavazzoni, MD, acting director of FDA’s Center for Drug Evaluation and Research, states in the release. “To this end, the agency is using its regulatory authority to inform women and their healthcare providers about the risks if NSAIDs are used after around 20 weeks of pregnancy and beyond.”
Oligohydramnios can arise quickly – in as little as 2 days – or weeks after starting regular NSAID use in this patient population. The condition usually resolves if a pregnant woman stops taking the NSAID, the agency notes.
If a health care provider believes NSAIDs are necessary between about 20 and 30 weeks of pregnancy, use should be limited to the lowest effective dose and shortest duration possible, the Drug Safety Communication notes.
As a reminder, health care professionals and patients should report side effects from NSAIDs to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The U.S. Food and Drug Administration released new warnings Oct. 15 that most nonsteroidal anti-inflammatory agents (NSAIDs) carry an elevated risk for kidney complications in unborn children when taken around weeks 20 or later in pregnancy.
Citing newly available research, the agency states the risk of low amniotic fluid (known as oligohydramnios) can occur, which in turn can cause rare but serious kidney problems in the offspring. Pregnancy complications also can result.
The FDA action expands on earlier warnings about agents in this drug class, which the FDA previously cautioned about taking after week 30 of pregnancy because of heart-related risks.
Manufacturers of both over-the-counter and prescription NSAIDs – including ibuprofen, naproxen, diclofenac, and celecoxib – will be required to update their labeling with the new warning.
Low-dose (81-mg) aspirin is excluded from this warning.
“Low-dose aspirin may be an important treatment for some women during pregnancy and should be taken under the direction of a healthcare professional,” the agency stated in a news release.
“It is important that women understand the benefits and risks of the medications they may take over the course of their pregnancy,” Patrizia Cavazzoni, MD, acting director of FDA’s Center for Drug Evaluation and Research, states in the release. “To this end, the agency is using its regulatory authority to inform women and their healthcare providers about the risks if NSAIDs are used after around 20 weeks of pregnancy and beyond.”
Oligohydramnios can arise quickly – in as little as 2 days – or weeks after starting regular NSAID use in this patient population. The condition usually resolves if a pregnant woman stops taking the NSAID, the agency notes.
If a health care provider believes NSAIDs are necessary between about 20 and 30 weeks of pregnancy, use should be limited to the lowest effective dose and shortest duration possible, the Drug Safety Communication notes.
As a reminder, health care professionals and patients should report side effects from NSAIDs to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
The U.S. Food and Drug Administration released new warnings Oct. 15 that most nonsteroidal anti-inflammatory agents (NSAIDs) carry an elevated risk for kidney complications in unborn children when taken around weeks 20 or later in pregnancy.
Citing newly available research, the agency states the risk of low amniotic fluid (known as oligohydramnios) can occur, which in turn can cause rare but serious kidney problems in the offspring. Pregnancy complications also can result.
The FDA action expands on earlier warnings about agents in this drug class, which the FDA previously cautioned about taking after week 30 of pregnancy because of heart-related risks.
Manufacturers of both over-the-counter and prescription NSAIDs – including ibuprofen, naproxen, diclofenac, and celecoxib – will be required to update their labeling with the new warning.
Low-dose (81-mg) aspirin is excluded from this warning.
“Low-dose aspirin may be an important treatment for some women during pregnancy and should be taken under the direction of a healthcare professional,” the agency stated in a news release.
“It is important that women understand the benefits and risks of the medications they may take over the course of their pregnancy,” Patrizia Cavazzoni, MD, acting director of FDA’s Center for Drug Evaluation and Research, states in the release. “To this end, the agency is using its regulatory authority to inform women and their healthcare providers about the risks if NSAIDs are used after around 20 weeks of pregnancy and beyond.”
Oligohydramnios can arise quickly – in as little as 2 days – or weeks after starting regular NSAID use in this patient population. The condition usually resolves if a pregnant woman stops taking the NSAID, the agency notes.
If a health care provider believes NSAIDs are necessary between about 20 and 30 weeks of pregnancy, use should be limited to the lowest effective dose and shortest duration possible, the Drug Safety Communication notes.
As a reminder, health care professionals and patients should report side effects from NSAIDs to the FDA’s MedWatch program.
A version of this article originally appeared on Medscape.com.
Four-week, 8-week CAB/RPV injections safe, effective in women
according to results from the ATLAS-2M study, presented at the HIV Glasgow 2020 Virtual Conference, held October 5-8. The women also reported high satisfaction with the regimen, compared with daily oral antiretroviral therapy.
Previously reported results had shown that the two-drug combination administered every 8 weeks (600 mg cabotegravir and 900 mg rilpivirine) was noninferior to injections every 4 weeks (400 mg cabotegravir and 600 mg rilpivirine) in adults with HIV during the open-label phase 3b ATLAS-2M trial. Further, the ATLAS and FLAIR phase 3 trials had shown the 4-week administration of the therapy to be noninferior to a daily oral three-drug antiretroviral therapy.
Paul Benn, MBBS, of ViiV Healthcare (which is seeking regulatory approval for CAB/RPV treatment), and his colleagues completed a planned subgroup analysis of women in the ATLAS-2M trial. The primary endpoint was the proportion of intention-to-treat participants with plasma HIV-1 RNA of at least 50 copies/mL with a noninferiority margin of 4% at 48 weeks. The secondary endpoint was the proportion of participants with HIV-1 RNA under 50 copies/mL with a noninferiority margin of 10%.
Among the 280 women enrolled, 137 were randomly assigned to receive injections every 8 weeks, and 143 to receive injections every 4 weeks. A majority of the women (56%) were White, the median age was 44 years, and just over half (53%) were treatment naive with cabotegravir and rilpivirine.
At 48 weeks, 3.6% of women in the 8-week group and 0% of women in the 4-week group had at least 50 copies/mL of HIV-1 RNA. In both arms, 91% of participants had HIV-1 RNA under 50 copies/mL. Plasma concentrations of cabotegravir and rilpivirine were similar between the women and the overall study population.
Confirmed virologic failure occurred in five women, all before week 24. Three of the women were subtype A/A1, and four of them had archived nonnucleoside reverse transcriptase inhibitor (NNRTI) resistance–associated mutations.
There were no significant differences in the safety profile between the groups; 99% of injection site reactions that occurred were mild to moderate and lasted a median 3-4 days. Fewer than 4% of participants discontinued because of adverse events – five women in the 8-week group and five women in the 4-week group. Four women cited injection site reactions as the reason for discontinuation.
Women not previously treated with CAB/RPV reported increased treatment satisfaction on the HIV Treatment Satisfaction Questionnaire, a score of 5.4 in the 8-week group and 3.9 in the 4-week group. Among those with prior CAB/RPV treatment, 88% preferred the 8-weekly injections, 8% preferred the 4-weekly injections, and 2% preferred oral dosing.
Long-acting CAB/RPV is an investigational formulation. In December 2019, the Food and Drug Administration denied approval to the formulation on the basis of manufacturing and chemistry concerns, according to a company press release.
This article first appeared on Medscape.com.
according to results from the ATLAS-2M study, presented at the HIV Glasgow 2020 Virtual Conference, held October 5-8. The women also reported high satisfaction with the regimen, compared with daily oral antiretroviral therapy.
Previously reported results had shown that the two-drug combination administered every 8 weeks (600 mg cabotegravir and 900 mg rilpivirine) was noninferior to injections every 4 weeks (400 mg cabotegravir and 600 mg rilpivirine) in adults with HIV during the open-label phase 3b ATLAS-2M trial. Further, the ATLAS and FLAIR phase 3 trials had shown the 4-week administration of the therapy to be noninferior to a daily oral three-drug antiretroviral therapy.
Paul Benn, MBBS, of ViiV Healthcare (which is seeking regulatory approval for CAB/RPV treatment), and his colleagues completed a planned subgroup analysis of women in the ATLAS-2M trial. The primary endpoint was the proportion of intention-to-treat participants with plasma HIV-1 RNA of at least 50 copies/mL with a noninferiority margin of 4% at 48 weeks. The secondary endpoint was the proportion of participants with HIV-1 RNA under 50 copies/mL with a noninferiority margin of 10%.
Among the 280 women enrolled, 137 were randomly assigned to receive injections every 8 weeks, and 143 to receive injections every 4 weeks. A majority of the women (56%) were White, the median age was 44 years, and just over half (53%) were treatment naive with cabotegravir and rilpivirine.
At 48 weeks, 3.6% of women in the 8-week group and 0% of women in the 4-week group had at least 50 copies/mL of HIV-1 RNA. In both arms, 91% of participants had HIV-1 RNA under 50 copies/mL. Plasma concentrations of cabotegravir and rilpivirine were similar between the women and the overall study population.
Confirmed virologic failure occurred in five women, all before week 24. Three of the women were subtype A/A1, and four of them had archived nonnucleoside reverse transcriptase inhibitor (NNRTI) resistance–associated mutations.
There were no significant differences in the safety profile between the groups; 99% of injection site reactions that occurred were mild to moderate and lasted a median 3-4 days. Fewer than 4% of participants discontinued because of adverse events – five women in the 8-week group and five women in the 4-week group. Four women cited injection site reactions as the reason for discontinuation.
Women not previously treated with CAB/RPV reported increased treatment satisfaction on the HIV Treatment Satisfaction Questionnaire, a score of 5.4 in the 8-week group and 3.9 in the 4-week group. Among those with prior CAB/RPV treatment, 88% preferred the 8-weekly injections, 8% preferred the 4-weekly injections, and 2% preferred oral dosing.
Long-acting CAB/RPV is an investigational formulation. In December 2019, the Food and Drug Administration denied approval to the formulation on the basis of manufacturing and chemistry concerns, according to a company press release.
This article first appeared on Medscape.com.
according to results from the ATLAS-2M study, presented at the HIV Glasgow 2020 Virtual Conference, held October 5-8. The women also reported high satisfaction with the regimen, compared with daily oral antiretroviral therapy.
Previously reported results had shown that the two-drug combination administered every 8 weeks (600 mg cabotegravir and 900 mg rilpivirine) was noninferior to injections every 4 weeks (400 mg cabotegravir and 600 mg rilpivirine) in adults with HIV during the open-label phase 3b ATLAS-2M trial. Further, the ATLAS and FLAIR phase 3 trials had shown the 4-week administration of the therapy to be noninferior to a daily oral three-drug antiretroviral therapy.
Paul Benn, MBBS, of ViiV Healthcare (which is seeking regulatory approval for CAB/RPV treatment), and his colleagues completed a planned subgroup analysis of women in the ATLAS-2M trial. The primary endpoint was the proportion of intention-to-treat participants with plasma HIV-1 RNA of at least 50 copies/mL with a noninferiority margin of 4% at 48 weeks. The secondary endpoint was the proportion of participants with HIV-1 RNA under 50 copies/mL with a noninferiority margin of 10%.
Among the 280 women enrolled, 137 were randomly assigned to receive injections every 8 weeks, and 143 to receive injections every 4 weeks. A majority of the women (56%) were White, the median age was 44 years, and just over half (53%) were treatment naive with cabotegravir and rilpivirine.
At 48 weeks, 3.6% of women in the 8-week group and 0% of women in the 4-week group had at least 50 copies/mL of HIV-1 RNA. In both arms, 91% of participants had HIV-1 RNA under 50 copies/mL. Plasma concentrations of cabotegravir and rilpivirine were similar between the women and the overall study population.
Confirmed virologic failure occurred in five women, all before week 24. Three of the women were subtype A/A1, and four of them had archived nonnucleoside reverse transcriptase inhibitor (NNRTI) resistance–associated mutations.
There were no significant differences in the safety profile between the groups; 99% of injection site reactions that occurred were mild to moderate and lasted a median 3-4 days. Fewer than 4% of participants discontinued because of adverse events – five women in the 8-week group and five women in the 4-week group. Four women cited injection site reactions as the reason for discontinuation.
Women not previously treated with CAB/RPV reported increased treatment satisfaction on the HIV Treatment Satisfaction Questionnaire, a score of 5.4 in the 8-week group and 3.9 in the 4-week group. Among those with prior CAB/RPV treatment, 88% preferred the 8-weekly injections, 8% preferred the 4-weekly injections, and 2% preferred oral dosing.
Long-acting CAB/RPV is an investigational formulation. In December 2019, the Food and Drug Administration denied approval to the formulation on the basis of manufacturing and chemistry concerns, according to a company press release.
This article first appeared on Medscape.com.
Social factors predicted peripartum depressive symptoms in Black women with HIV
Women with high-risk pregnancies because of chronic conditions are at increased risk for developing postpartum depression, and HIV may be one such risk. However, risk factors for women living with HIV, particularly Black women, have not been well studied, wrote Emmanuela Nneamaka Ojukwu of the University of Miami School of Nursing, and colleagues.
Data suggest that as many as half of cases of postpartum depression (PPD) begin before delivery, the researchers noted. “Therefore, for this study, the symptoms of both PND (prenatal depression) and PPD have been classified in what we have termed peripartum depressive symptoms (PDS),” and defined as depressive symptoms during pregnancy and within 1 year postpartum, they said.
In a study published in the Archives of Psychiatric Nursing, the researchers conducted a secondary analysis of 143 Black women living with HIV seen at specialty prenatal and women’s health clinics in Miami.
Overall, 81 women (57%) reported either perinatal or postpartum depressive symptoms, or both. “Some of the symptoms prevalent among women in our study included restlessness, depressed mood, apathy, guilt, hopelessness, and social isolation,” the researchers said.
Social factors show significant impact
In a multivariate analysis, low income, intimate partner violence, and childcare burden were significant predictors of PDS (P less than .05). Women who reported intimate partner violence or abuse were 6.5 times more likely to experience PDS than were women who did not report abuse, and women with a childcare burden involving two children were 4.6 times more likely to experience PDS than were women with no childcare burden or only one child needing child care.
The average age of the women studied was 29 years, and 59% were above the federal poverty level. Nearly two-thirds (62%) were Black and 38% were Haitian; 63% were unemployed, 62% had a high school diploma or less, and 59% received care through Medicaid.
The researchers assessed four categories of health: HIV-related, gynecologic, obstetric, and psychosocial. The average viral load among the patients was 22,359 copies/mL at baseline, and they averaged 2.5 medical comorbidities. The most common comorbid conditions were other sexually transmitted infections and blood disorders, followed by cardiovascular and metabolic conditions.
Quantitative studies needed
Larger quantitative studies of Black pregnant women living with HIV are needed to analyze social factors at multiple levels, the researchers said. “To address depression among Black women living with HIV, local and federal governments should enact measures that increase the family income and diminish the prevalence of [intimate partner violence] among these women,” they said.
The study findings were limited by several factors including retrospective design and use of self-reports, as well as the small sample size and lack of generalizability to women living with HIV of other races or from other regions, the researchers noted. However, the results reflect data from previous studies and support the value of early screening and referral to improve well being for Black women living with HIV, as well as the importance of comprehensive medical care, they said.
“Women should be counseled that postpartum physical and psychological changes (and the stresses and demands of caring for a new baby) may make [antiretroviral] adherence more difficult and that additional support may be needed during this period,” the researchers wrote.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Ojukwu EN et al. Arch Psychiatr Nurs. 2020 May 22. doi: 10.1016/j.apnu.2020.05.004.
Women with high-risk pregnancies because of chronic conditions are at increased risk for developing postpartum depression, and HIV may be one such risk. However, risk factors for women living with HIV, particularly Black women, have not been well studied, wrote Emmanuela Nneamaka Ojukwu of the University of Miami School of Nursing, and colleagues.
Data suggest that as many as half of cases of postpartum depression (PPD) begin before delivery, the researchers noted. “Therefore, for this study, the symptoms of both PND (prenatal depression) and PPD have been classified in what we have termed peripartum depressive symptoms (PDS),” and defined as depressive symptoms during pregnancy and within 1 year postpartum, they said.
In a study published in the Archives of Psychiatric Nursing, the researchers conducted a secondary analysis of 143 Black women living with HIV seen at specialty prenatal and women’s health clinics in Miami.
Overall, 81 women (57%) reported either perinatal or postpartum depressive symptoms, or both. “Some of the symptoms prevalent among women in our study included restlessness, depressed mood, apathy, guilt, hopelessness, and social isolation,” the researchers said.
Social factors show significant impact
In a multivariate analysis, low income, intimate partner violence, and childcare burden were significant predictors of PDS (P less than .05). Women who reported intimate partner violence or abuse were 6.5 times more likely to experience PDS than were women who did not report abuse, and women with a childcare burden involving two children were 4.6 times more likely to experience PDS than were women with no childcare burden or only one child needing child care.
The average age of the women studied was 29 years, and 59% were above the federal poverty level. Nearly two-thirds (62%) were Black and 38% were Haitian; 63% were unemployed, 62% had a high school diploma or less, and 59% received care through Medicaid.
The researchers assessed four categories of health: HIV-related, gynecologic, obstetric, and psychosocial. The average viral load among the patients was 22,359 copies/mL at baseline, and they averaged 2.5 medical comorbidities. The most common comorbid conditions were other sexually transmitted infections and blood disorders, followed by cardiovascular and metabolic conditions.
Quantitative studies needed
Larger quantitative studies of Black pregnant women living with HIV are needed to analyze social factors at multiple levels, the researchers said. “To address depression among Black women living with HIV, local and federal governments should enact measures that increase the family income and diminish the prevalence of [intimate partner violence] among these women,” they said.
The study findings were limited by several factors including retrospective design and use of self-reports, as well as the small sample size and lack of generalizability to women living with HIV of other races or from other regions, the researchers noted. However, the results reflect data from previous studies and support the value of early screening and referral to improve well being for Black women living with HIV, as well as the importance of comprehensive medical care, they said.
“Women should be counseled that postpartum physical and psychological changes (and the stresses and demands of caring for a new baby) may make [antiretroviral] adherence more difficult and that additional support may be needed during this period,” the researchers wrote.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Ojukwu EN et al. Arch Psychiatr Nurs. 2020 May 22. doi: 10.1016/j.apnu.2020.05.004.
Women with high-risk pregnancies because of chronic conditions are at increased risk for developing postpartum depression, and HIV may be one such risk. However, risk factors for women living with HIV, particularly Black women, have not been well studied, wrote Emmanuela Nneamaka Ojukwu of the University of Miami School of Nursing, and colleagues.
Data suggest that as many as half of cases of postpartum depression (PPD) begin before delivery, the researchers noted. “Therefore, for this study, the symptoms of both PND (prenatal depression) and PPD have been classified in what we have termed peripartum depressive symptoms (PDS),” and defined as depressive symptoms during pregnancy and within 1 year postpartum, they said.
In a study published in the Archives of Psychiatric Nursing, the researchers conducted a secondary analysis of 143 Black women living with HIV seen at specialty prenatal and women’s health clinics in Miami.
Overall, 81 women (57%) reported either perinatal or postpartum depressive symptoms, or both. “Some of the symptoms prevalent among women in our study included restlessness, depressed mood, apathy, guilt, hopelessness, and social isolation,” the researchers said.
Social factors show significant impact
In a multivariate analysis, low income, intimate partner violence, and childcare burden were significant predictors of PDS (P less than .05). Women who reported intimate partner violence or abuse were 6.5 times more likely to experience PDS than were women who did not report abuse, and women with a childcare burden involving two children were 4.6 times more likely to experience PDS than were women with no childcare burden or only one child needing child care.
The average age of the women studied was 29 years, and 59% were above the federal poverty level. Nearly two-thirds (62%) were Black and 38% were Haitian; 63% were unemployed, 62% had a high school diploma or less, and 59% received care through Medicaid.
The researchers assessed four categories of health: HIV-related, gynecologic, obstetric, and psychosocial. The average viral load among the patients was 22,359 copies/mL at baseline, and they averaged 2.5 medical comorbidities. The most common comorbid conditions were other sexually transmitted infections and blood disorders, followed by cardiovascular and metabolic conditions.
Quantitative studies needed
Larger quantitative studies of Black pregnant women living with HIV are needed to analyze social factors at multiple levels, the researchers said. “To address depression among Black women living with HIV, local and federal governments should enact measures that increase the family income and diminish the prevalence of [intimate partner violence] among these women,” they said.
The study findings were limited by several factors including retrospective design and use of self-reports, as well as the small sample size and lack of generalizability to women living with HIV of other races or from other regions, the researchers noted. However, the results reflect data from previous studies and support the value of early screening and referral to improve well being for Black women living with HIV, as well as the importance of comprehensive medical care, they said.
“Women should be counseled that postpartum physical and psychological changes (and the stresses and demands of caring for a new baby) may make [antiretroviral] adherence more difficult and that additional support may be needed during this period,” the researchers wrote.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Ojukwu EN et al. Arch Psychiatr Nurs. 2020 May 22. doi: 10.1016/j.apnu.2020.05.004.
FROM ARCHIVES OF PSYCHIATRIC NURSING
Choose wisely
Four years ago, just prior to the 2016 presidential election, I mentioned the Choosing Wisely campaign in my JFP editorial.1 I said that family physicians should do their part in controlling health care costs by carefully selecting tests and treatments that are known to be effective and avoiding those that are not. This remains as true now as it was then.
The Choosing Wisely campaign was sparked by a family physician, Dr. Howard Brody, in the context of national health care reform. In a 2010 New England Journal of Medicine editorial, he challenged physicians to do their part in controlling health care costs by not ordering tests and treatments that have no value for patients.2 At that time, it was estimated that a third of tests and treatments ordered by US physicians were of marginal or no value.3
Dr. Brody’s editorial caught the attention of the National Physicians Alliance and eventually many other physician organizations. In 2012, the American Board of Internal Medicine Foundation launched the Choosing Wisely initiative; today, the campaign Web site, choosingwisely.org, has a wealth of information and practice recommendations from 78 medical specialty organizations, including the American Academy of Family Physicians (AAFP).
In this month’s issue of JFP, Dr. Kate Rowland has summarized 10 of the most important Choosing Wisely recommendations that apply to family physicians and other primary care clinicians. Here are 5 more recommendations from the Choosing Wisely list of tests and treatments to avoid ordering for your patients:
- Don’t perform pelvic exams on asymptomatic nonpregnant women, unless necessary for guideline-appropriate screening for cervical cancer.
- Don’t routinely screen for prostate cancer using a prostate-specific antigen (PSA) test or digital rectal exam. For men who want PSA screening, it should be performed only after engaging in shared decision-making.
- Don’t order annual electrocardiograms or any other cardiac screening for low-risk patients without symptoms.
- Don’t routinely prescribe antibiotics for otitis media in children ages 2 to 12 years with nonsevere symptoms when observation is reasonable.
- Don’t use dual-energy x-ray absorptiometry screening for osteoporosis in women younger than 65 or men younger than 70 with no risk factors.
In total, AAFP lists 18 recommendations (2 additional recommendations have been withdrawn, based on updated evidence) on the Choosing Wisely Web site. I encourage you to review them to see if you should change any of your current patient recommendations.
1. Hickner J. Count on this no matter who wins the election. J Fam Pract. 2016;65:664.
2. Brody H. Medicine’s ethical responsibility for health care reform—the Top Five list. N Engl J Med. 2010;362:283-285.
3. Fisher ES, Bynum JP, Skinner JS. Slowing the growth of health care costs—lessons from regional variation. N Engl J Med. 2009;360:849-852.
Four years ago, just prior to the 2016 presidential election, I mentioned the Choosing Wisely campaign in my JFP editorial.1 I said that family physicians should do their part in controlling health care costs by carefully selecting tests and treatments that are known to be effective and avoiding those that are not. This remains as true now as it was then.
The Choosing Wisely campaign was sparked by a family physician, Dr. Howard Brody, in the context of national health care reform. In a 2010 New England Journal of Medicine editorial, he challenged physicians to do their part in controlling health care costs by not ordering tests and treatments that have no value for patients.2 At that time, it was estimated that a third of tests and treatments ordered by US physicians were of marginal or no value.3
Dr. Brody’s editorial caught the attention of the National Physicians Alliance and eventually many other physician organizations. In 2012, the American Board of Internal Medicine Foundation launched the Choosing Wisely initiative; today, the campaign Web site, choosingwisely.org, has a wealth of information and practice recommendations from 78 medical specialty organizations, including the American Academy of Family Physicians (AAFP).
In this month’s issue of JFP, Dr. Kate Rowland has summarized 10 of the most important Choosing Wisely recommendations that apply to family physicians and other primary care clinicians. Here are 5 more recommendations from the Choosing Wisely list of tests and treatments to avoid ordering for your patients:
- Don’t perform pelvic exams on asymptomatic nonpregnant women, unless necessary for guideline-appropriate screening for cervical cancer.
- Don’t routinely screen for prostate cancer using a prostate-specific antigen (PSA) test or digital rectal exam. For men who want PSA screening, it should be performed only after engaging in shared decision-making.
- Don’t order annual electrocardiograms or any other cardiac screening for low-risk patients without symptoms.
- Don’t routinely prescribe antibiotics for otitis media in children ages 2 to 12 years with nonsevere symptoms when observation is reasonable.
- Don’t use dual-energy x-ray absorptiometry screening for osteoporosis in women younger than 65 or men younger than 70 with no risk factors.
In total, AAFP lists 18 recommendations (2 additional recommendations have been withdrawn, based on updated evidence) on the Choosing Wisely Web site. I encourage you to review them to see if you should change any of your current patient recommendations.
Four years ago, just prior to the 2016 presidential election, I mentioned the Choosing Wisely campaign in my JFP editorial.1 I said that family physicians should do their part in controlling health care costs by carefully selecting tests and treatments that are known to be effective and avoiding those that are not. This remains as true now as it was then.
The Choosing Wisely campaign was sparked by a family physician, Dr. Howard Brody, in the context of national health care reform. In a 2010 New England Journal of Medicine editorial, he challenged physicians to do their part in controlling health care costs by not ordering tests and treatments that have no value for patients.2 At that time, it was estimated that a third of tests and treatments ordered by US physicians were of marginal or no value.3
Dr. Brody’s editorial caught the attention of the National Physicians Alliance and eventually many other physician organizations. In 2012, the American Board of Internal Medicine Foundation launched the Choosing Wisely initiative; today, the campaign Web site, choosingwisely.org, has a wealth of information and practice recommendations from 78 medical specialty organizations, including the American Academy of Family Physicians (AAFP).
In this month’s issue of JFP, Dr. Kate Rowland has summarized 10 of the most important Choosing Wisely recommendations that apply to family physicians and other primary care clinicians. Here are 5 more recommendations from the Choosing Wisely list of tests and treatments to avoid ordering for your patients:
- Don’t perform pelvic exams on asymptomatic nonpregnant women, unless necessary for guideline-appropriate screening for cervical cancer.
- Don’t routinely screen for prostate cancer using a prostate-specific antigen (PSA) test or digital rectal exam. For men who want PSA screening, it should be performed only after engaging in shared decision-making.
- Don’t order annual electrocardiograms or any other cardiac screening for low-risk patients without symptoms.
- Don’t routinely prescribe antibiotics for otitis media in children ages 2 to 12 years with nonsevere symptoms when observation is reasonable.
- Don’t use dual-energy x-ray absorptiometry screening for osteoporosis in women younger than 65 or men younger than 70 with no risk factors.
In total, AAFP lists 18 recommendations (2 additional recommendations have been withdrawn, based on updated evidence) on the Choosing Wisely Web site. I encourage you to review them to see if you should change any of your current patient recommendations.
1. Hickner J. Count on this no matter who wins the election. J Fam Pract. 2016;65:664.
2. Brody H. Medicine’s ethical responsibility for health care reform—the Top Five list. N Engl J Med. 2010;362:283-285.
3. Fisher ES, Bynum JP, Skinner JS. Slowing the growth of health care costs—lessons from regional variation. N Engl J Med. 2009;360:849-852.
1. Hickner J. Count on this no matter who wins the election. J Fam Pract. 2016;65:664.
2. Brody H. Medicine’s ethical responsibility for health care reform—the Top Five list. N Engl J Med. 2010;362:283-285.
3. Fisher ES, Bynum JP, Skinner JS. Slowing the growth of health care costs—lessons from regional variation. N Engl J Med. 2009;360:849-852.
Ruling out PE in pregnancy
ILLUSTRATIVE CASE
A 28-year-old G2P1001 at 28 weeks’ gestation presents to your clinic with 1 day of dyspnea and palpitations. Her pregnancy has been otherwise uncomplicated. She reports worsening dyspnea with mild exertion but denies other symptoms, including leg swelling.
The current incidence of venous thromboembolism (VTE) in pregnant women is estimated to be a relatively low 5 to 12 events per 10,000 pregnancies, yet the condition is the leading cause of maternal mortality in developed countries.2,3,4 Currently, there are conflicting recommendations among relevant organization guidelines regarding the use of D-dimer testing to aid in the diagnosis of pulmonary embolism (PE) during pregnancy. Both the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH) and the European Society of Cardiology (ESC) recommend using D-dimer testing to rule out PE in pregnant women (ESC Class IIa, level of evidence B based on small studies, retrospective studies, and observational studies; GTH provides no grade).5,6
Conversely, the Royal College of Obstetricians and Gynaecologists (RCOG), the Society of Obstetricians and Gynaecologists of Canada (SOGC), and the American Thoracic Society (ATS)/Society of Thoracic Radiology recommend against the use of D-dimer testing in pregnant women because pregnant women were excluded from D-dimer validation studies (RCOG and SOGC Grade D; ATS weak recommendation).4,7,8 The American College of Obstetricians and Gynecologists does not have specific recommendations regarding the use of D-dimer testing during pregnancy, but has endorsed the ATS guidelines.4,9 In addition, SOGC recommends against the use of clinical prediction scores (Grade D), and RCOG states that there is no evidence to support their use (Grade C).7,8 The remaining societies do not make a recommendation for or against the use of clinical prediction scores because of the absence of high-quality evidence regarding their use in the pregnant patient population.4,5,6
STUDY SUMMARY
Prospective validation of a strategy to diagnose PE in pregnant women
This multicenter, multinational, prospective diagnostic study involving 395 pregnant women evaluated the accuracy of PE diagnosis across 11 centers in France and Switzerland from August 2008 through July 2016.1 Patients with clinically suspected PE were evaluated in emergency departments. Patients were tested according to a diagnostic algorithm that included pretest clinical probability using the revised Geneva Score for Pulmonary Embolism (www.mdcalc.com/geneva-score-revised-pulmonary-embolism), a clinical prediction tool that uses patient history, presenting symptoms, and clinical signs to classify patients as being at low (0-3/25), intermediate (4-10/25), or high (≥ 11/25) risk;10 high-sensitivity D-dimer testing; bilateral lower limb compression ultrasonography (CUS); computed tomography pulmonary angiography (CTPA); and a ventilation-perfusion (V/Q) scan.
PE was excluded in patients who had a low or intermediate pretest clinical probability score and a negative D-dimer test result (< 500 mcg/L). Patients with a high pretest probability score or positive D-dimer test result underwent CUS, and, if negative, subsequent CTPA. A V/Q scan was performed if the CTPA was inconclusive. If the work-up was negative, PE was excluded.
Untreated pregnant women had clinical follow-up at 3 months. Any cases of suspected VTE were evaluated by a 3-member independent adjudication committee blinded to the initial diagnostic work-up. The primary outcome was the rate of adjudicated VTE events during the 3-month follow-up period. PE was diagnosed in 28 patients (7.1%) and excluded in 367 (clinical probability score and negative D-dimer test result [n = 46], negative CTPA result [n = 290], normal or low-probability V/Q scan [n = 17], and other reason [n = 14]). Twenty-two women received anticoagulation during the follow-up period for other reasons (mainly history of previous VTE disease). No symptomatic VTE events occurred in any of the women after the diagnostic work-up was negative, including among those patients who were ruled out with only the clinical prediction tool and a negative D-dimer test result (rate 0.0%; 95% confidence interval [CI], 0.0%-1%).
WHAT’S NEW
Clinical probability and D-dimer rule out PE in pregnant women
This study ruled out PE in patients with low/intermediate risk as determined by the revised Geneva score and a D-dimer test, enabling patients to avoid further diagnostic testing. This low-cost strategy can be applied easily to the pregnant population.
CAVEATS
Additional research is still needed
From the results of this study, 11.6% of patients (n = 46) had a PE ruled out utilizing the revised Geneva score in conjunction with a D-dimer test result, with avoidance of chest imaging. However, this study was powered for the entire treatment algorithm and was not specifically powered for patients with low- or intermediate-risk pretest probability scores. Since this is the first published prospective diagnostic study of VTE in pregnancy, further research is needed to confirm the findings that a clinical prediction tool and a negative D-dimer test result can safely rule out PE in pregnant women.
In addition, further research is needed to determine pregnancy-adapted D-dimer cut-off values, as the researchers of this study noted that < 500 mcg/L was useful in the first and second trimester, but that levels increased as gestational age increased.
CHALLENGES TO IMPLEMENTATION
None to speak of
Implementing a diagnostic algorithm that incorporates sequential assessment of pretest clinical probability based on the revised Geneva score and a D-dimer measurement should be relatively easy to implement, as both methods are readily available and relatively inexpensive.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Righini M, Robert-Ebadi H, Elias A, et al. Diagnosis of pulmonary embolism during pregnancy. A multicenter prospective management outcome study. Ann Intern Med. 2018;169:766-773.
2. Knight M, Kenyon S, Brocklehurst P, et al. Saving lives, improving mothers’ care: lessons learned to inform future maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009-2012. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2014.
3. Bourjeily G, Paidas M, Khalil H, et al. Pulmonary embolism in pregnancy. Lancet. 2010;375:500-512.
4. Leung AN, Bull TM, Jaeschke R, et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: evaluation of suspected pulmonary embolism in pregnancy. Am J Resp Crit Care Med. 2011;184:1200-1208.
5. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603.
6. Linnemann B, Bauersachs R, Rott H, et al. Working Group in Women’s Health of the Society of Thrombosis and Haemostasis. Diagnosis of pregnancy-associated venous thromboembolism-position paper of the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH). Vasa. 2016;45:87-101.
7. Royal College of Obstetricians & Gynaecologists. Thromboembolic disease in pregnancy and the puerperium: acute management. Green‐top Guideline No. 37b. April 2015.
8. Chan WS, Rey E, Kent NE, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36:527-553.
9. James A, Birsner M, Kaimal A, American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins‐Obstetrics. ACOG Practice Bulletin No. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
10. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171.
ILLUSTRATIVE CASE
A 28-year-old G2P1001 at 28 weeks’ gestation presents to your clinic with 1 day of dyspnea and palpitations. Her pregnancy has been otherwise uncomplicated. She reports worsening dyspnea with mild exertion but denies other symptoms, including leg swelling.
The current incidence of venous thromboembolism (VTE) in pregnant women is estimated to be a relatively low 5 to 12 events per 10,000 pregnancies, yet the condition is the leading cause of maternal mortality in developed countries.2,3,4 Currently, there are conflicting recommendations among relevant organization guidelines regarding the use of D-dimer testing to aid in the diagnosis of pulmonary embolism (PE) during pregnancy. Both the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH) and the European Society of Cardiology (ESC) recommend using D-dimer testing to rule out PE in pregnant women (ESC Class IIa, level of evidence B based on small studies, retrospective studies, and observational studies; GTH provides no grade).5,6
Conversely, the Royal College of Obstetricians and Gynaecologists (RCOG), the Society of Obstetricians and Gynaecologists of Canada (SOGC), and the American Thoracic Society (ATS)/Society of Thoracic Radiology recommend against the use of D-dimer testing in pregnant women because pregnant women were excluded from D-dimer validation studies (RCOG and SOGC Grade D; ATS weak recommendation).4,7,8 The American College of Obstetricians and Gynecologists does not have specific recommendations regarding the use of D-dimer testing during pregnancy, but has endorsed the ATS guidelines.4,9 In addition, SOGC recommends against the use of clinical prediction scores (Grade D), and RCOG states that there is no evidence to support their use (Grade C).7,8 The remaining societies do not make a recommendation for or against the use of clinical prediction scores because of the absence of high-quality evidence regarding their use in the pregnant patient population.4,5,6
STUDY SUMMARY
Prospective validation of a strategy to diagnose PE in pregnant women
This multicenter, multinational, prospective diagnostic study involving 395 pregnant women evaluated the accuracy of PE diagnosis across 11 centers in France and Switzerland from August 2008 through July 2016.1 Patients with clinically suspected PE were evaluated in emergency departments. Patients were tested according to a diagnostic algorithm that included pretest clinical probability using the revised Geneva Score for Pulmonary Embolism (www.mdcalc.com/geneva-score-revised-pulmonary-embolism), a clinical prediction tool that uses patient history, presenting symptoms, and clinical signs to classify patients as being at low (0-3/25), intermediate (4-10/25), or high (≥ 11/25) risk;10 high-sensitivity D-dimer testing; bilateral lower limb compression ultrasonography (CUS); computed tomography pulmonary angiography (CTPA); and a ventilation-perfusion (V/Q) scan.
PE was excluded in patients who had a low or intermediate pretest clinical probability score and a negative D-dimer test result (< 500 mcg/L). Patients with a high pretest probability score or positive D-dimer test result underwent CUS, and, if negative, subsequent CTPA. A V/Q scan was performed if the CTPA was inconclusive. If the work-up was negative, PE was excluded.
Untreated pregnant women had clinical follow-up at 3 months. Any cases of suspected VTE were evaluated by a 3-member independent adjudication committee blinded to the initial diagnostic work-up. The primary outcome was the rate of adjudicated VTE events during the 3-month follow-up period. PE was diagnosed in 28 patients (7.1%) and excluded in 367 (clinical probability score and negative D-dimer test result [n = 46], negative CTPA result [n = 290], normal or low-probability V/Q scan [n = 17], and other reason [n = 14]). Twenty-two women received anticoagulation during the follow-up period for other reasons (mainly history of previous VTE disease). No symptomatic VTE events occurred in any of the women after the diagnostic work-up was negative, including among those patients who were ruled out with only the clinical prediction tool and a negative D-dimer test result (rate 0.0%; 95% confidence interval [CI], 0.0%-1%).
WHAT’S NEW
Clinical probability and D-dimer rule out PE in pregnant women
This study ruled out PE in patients with low/intermediate risk as determined by the revised Geneva score and a D-dimer test, enabling patients to avoid further diagnostic testing. This low-cost strategy can be applied easily to the pregnant population.
CAVEATS
Additional research is still needed
From the results of this study, 11.6% of patients (n = 46) had a PE ruled out utilizing the revised Geneva score in conjunction with a D-dimer test result, with avoidance of chest imaging. However, this study was powered for the entire treatment algorithm and was not specifically powered for patients with low- or intermediate-risk pretest probability scores. Since this is the first published prospective diagnostic study of VTE in pregnancy, further research is needed to confirm the findings that a clinical prediction tool and a negative D-dimer test result can safely rule out PE in pregnant women.
In addition, further research is needed to determine pregnancy-adapted D-dimer cut-off values, as the researchers of this study noted that < 500 mcg/L was useful in the first and second trimester, but that levels increased as gestational age increased.
CHALLENGES TO IMPLEMENTATION
None to speak of
Implementing a diagnostic algorithm that incorporates sequential assessment of pretest clinical probability based on the revised Geneva score and a D-dimer measurement should be relatively easy to implement, as both methods are readily available and relatively inexpensive.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 28-year-old G2P1001 at 28 weeks’ gestation presents to your clinic with 1 day of dyspnea and palpitations. Her pregnancy has been otherwise uncomplicated. She reports worsening dyspnea with mild exertion but denies other symptoms, including leg swelling.
The current incidence of venous thromboembolism (VTE) in pregnant women is estimated to be a relatively low 5 to 12 events per 10,000 pregnancies, yet the condition is the leading cause of maternal mortality in developed countries.2,3,4 Currently, there are conflicting recommendations among relevant organization guidelines regarding the use of D-dimer testing to aid in the diagnosis of pulmonary embolism (PE) during pregnancy. Both the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH) and the European Society of Cardiology (ESC) recommend using D-dimer testing to rule out PE in pregnant women (ESC Class IIa, level of evidence B based on small studies, retrospective studies, and observational studies; GTH provides no grade).5,6
Conversely, the Royal College of Obstetricians and Gynaecologists (RCOG), the Society of Obstetricians and Gynaecologists of Canada (SOGC), and the American Thoracic Society (ATS)/Society of Thoracic Radiology recommend against the use of D-dimer testing in pregnant women because pregnant women were excluded from D-dimer validation studies (RCOG and SOGC Grade D; ATS weak recommendation).4,7,8 The American College of Obstetricians and Gynecologists does not have specific recommendations regarding the use of D-dimer testing during pregnancy, but has endorsed the ATS guidelines.4,9 In addition, SOGC recommends against the use of clinical prediction scores (Grade D), and RCOG states that there is no evidence to support their use (Grade C).7,8 The remaining societies do not make a recommendation for or against the use of clinical prediction scores because of the absence of high-quality evidence regarding their use in the pregnant patient population.4,5,6
STUDY SUMMARY
Prospective validation of a strategy to diagnose PE in pregnant women
This multicenter, multinational, prospective diagnostic study involving 395 pregnant women evaluated the accuracy of PE diagnosis across 11 centers in France and Switzerland from August 2008 through July 2016.1 Patients with clinically suspected PE were evaluated in emergency departments. Patients were tested according to a diagnostic algorithm that included pretest clinical probability using the revised Geneva Score for Pulmonary Embolism (www.mdcalc.com/geneva-score-revised-pulmonary-embolism), a clinical prediction tool that uses patient history, presenting symptoms, and clinical signs to classify patients as being at low (0-3/25), intermediate (4-10/25), or high (≥ 11/25) risk;10 high-sensitivity D-dimer testing; bilateral lower limb compression ultrasonography (CUS); computed tomography pulmonary angiography (CTPA); and a ventilation-perfusion (V/Q) scan.
PE was excluded in patients who had a low or intermediate pretest clinical probability score and a negative D-dimer test result (< 500 mcg/L). Patients with a high pretest probability score or positive D-dimer test result underwent CUS, and, if negative, subsequent CTPA. A V/Q scan was performed if the CTPA was inconclusive. If the work-up was negative, PE was excluded.
Untreated pregnant women had clinical follow-up at 3 months. Any cases of suspected VTE were evaluated by a 3-member independent adjudication committee blinded to the initial diagnostic work-up. The primary outcome was the rate of adjudicated VTE events during the 3-month follow-up period. PE was diagnosed in 28 patients (7.1%) and excluded in 367 (clinical probability score and negative D-dimer test result [n = 46], negative CTPA result [n = 290], normal or low-probability V/Q scan [n = 17], and other reason [n = 14]). Twenty-two women received anticoagulation during the follow-up period for other reasons (mainly history of previous VTE disease). No symptomatic VTE events occurred in any of the women after the diagnostic work-up was negative, including among those patients who were ruled out with only the clinical prediction tool and a negative D-dimer test result (rate 0.0%; 95% confidence interval [CI], 0.0%-1%).
WHAT’S NEW
Clinical probability and D-dimer rule out PE in pregnant women
This study ruled out PE in patients with low/intermediate risk as determined by the revised Geneva score and a D-dimer test, enabling patients to avoid further diagnostic testing. This low-cost strategy can be applied easily to the pregnant population.
CAVEATS
Additional research is still needed
From the results of this study, 11.6% of patients (n = 46) had a PE ruled out utilizing the revised Geneva score in conjunction with a D-dimer test result, with avoidance of chest imaging. However, this study was powered for the entire treatment algorithm and was not specifically powered for patients with low- or intermediate-risk pretest probability scores. Since this is the first published prospective diagnostic study of VTE in pregnancy, further research is needed to confirm the findings that a clinical prediction tool and a negative D-dimer test result can safely rule out PE in pregnant women.
In addition, further research is needed to determine pregnancy-adapted D-dimer cut-off values, as the researchers of this study noted that < 500 mcg/L was useful in the first and second trimester, but that levels increased as gestational age increased.
CHALLENGES TO IMPLEMENTATION
None to speak of
Implementing a diagnostic algorithm that incorporates sequential assessment of pretest clinical probability based on the revised Geneva score and a D-dimer measurement should be relatively easy to implement, as both methods are readily available and relatively inexpensive.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Righini M, Robert-Ebadi H, Elias A, et al. Diagnosis of pulmonary embolism during pregnancy. A multicenter prospective management outcome study. Ann Intern Med. 2018;169:766-773.
2. Knight M, Kenyon S, Brocklehurst P, et al. Saving lives, improving mothers’ care: lessons learned to inform future maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009-2012. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2014.
3. Bourjeily G, Paidas M, Khalil H, et al. Pulmonary embolism in pregnancy. Lancet. 2010;375:500-512.
4. Leung AN, Bull TM, Jaeschke R, et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: evaluation of suspected pulmonary embolism in pregnancy. Am J Resp Crit Care Med. 2011;184:1200-1208.
5. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603.
6. Linnemann B, Bauersachs R, Rott H, et al. Working Group in Women’s Health of the Society of Thrombosis and Haemostasis. Diagnosis of pregnancy-associated venous thromboembolism-position paper of the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH). Vasa. 2016;45:87-101.
7. Royal College of Obstetricians & Gynaecologists. Thromboembolic disease in pregnancy and the puerperium: acute management. Green‐top Guideline No. 37b. April 2015.
8. Chan WS, Rey E, Kent NE, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36:527-553.
9. James A, Birsner M, Kaimal A, American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins‐Obstetrics. ACOG Practice Bulletin No. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
10. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171.
1. Righini M, Robert-Ebadi H, Elias A, et al. Diagnosis of pulmonary embolism during pregnancy. A multicenter prospective management outcome study. Ann Intern Med. 2018;169:766-773.
2. Knight M, Kenyon S, Brocklehurst P, et al. Saving lives, improving mothers’ care: lessons learned to inform future maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009-2012. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2014.
3. Bourjeily G, Paidas M, Khalil H, et al. Pulmonary embolism in pregnancy. Lancet. 2010;375:500-512.
4. Leung AN, Bull TM, Jaeschke R, et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: evaluation of suspected pulmonary embolism in pregnancy. Am J Resp Crit Care Med. 2011;184:1200-1208.
5. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603.
6. Linnemann B, Bauersachs R, Rott H, et al. Working Group in Women’s Health of the Society of Thrombosis and Haemostasis. Diagnosis of pregnancy-associated venous thromboembolism-position paper of the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH). Vasa. 2016;45:87-101.
7. Royal College of Obstetricians & Gynaecologists. Thromboembolic disease in pregnancy and the puerperium: acute management. Green‐top Guideline No. 37b. April 2015.
8. Chan WS, Rey E, Kent NE, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36:527-553.
9. James A, Birsner M, Kaimal A, American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins‐Obstetrics. ACOG Practice Bulletin No. 196: thromboembolism in pregnancy. Obstet Gynecol. 2018;132:e1-e17.
10. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171.
PRACTICE CHANGER
Use a clinical probability score to identify patients at low or intermediate risk for pulmonary embolism (PE) and combine that with a high-sensitivity D-dimer test to rule out PE in pregnant women.
STRENGTH OF RECOMMENDATION
B: Prospective diagnostic management outcome study.1
Righini M, Robert-Ebadi H, Elias A, et al. Diagnosis of pulmonary embolism during pregnancy: a multicenter prospective management outcome study. Ann Intern Med. 2018;169:766-773.1
Female cardiac advantage essentially lost after MI
Women are known to lag 5-10 years behind men in experiencing coronary heart disease (CHD), but new research suggests the gap narrows substantially following a myocardial infarction.
“Women lose a considerable portion, but not all, of their coronary and survival advantage – i.e., the lower event rates – after suffering a MI,” study author Sanne Peters, PhD, George Institute for Global Health, Imperial College London, said in an interview.
Previous studies of sex differences in event rates after a coronary event have produced mixed results and were primarily focused on mortality following MI. Importantly, the studies also lacked a control group without a history of CHD and, thus, were unable to provide a reference point for the disparity in event rates, she explained.
Using the MarketScan and Medicare databases, however, Dr. Peters and colleagues matched 339,890 U.S. adults hospitalized for an MI between January 2015 and December 2016 with 1,359,560 U.S. adults without a history of CHD.
Over a median 1.3 years follow-up, there were 12,518 MIs in the non-CHD group and 27,115 recurrent MIs in the MI group.
The age-standardized rate of MI per 1,000 person-years was 4.0 in women and 6.1 in men without a history of CHD, compared with 57.6 in women and 62.7 in men with a prior MI.
After multivariate adjustment, the women-to-men hazard ratio for MI was 0.64 (95% confidence interval, 0.62-0.67) in the non-CHD group and 0.94 (95% CI, 0.92-0.96) in the prior MI group, the authors reported Oct. 5 in the Journal of the American College of Cardiology
Additional results show the multivariate adjusted women-to-men hazard ratios for three other cardiovascular outcomes follow a similar pattern in the non-CHD and prior MI groups:
- CHD events: 0.53 (95% CI, 0.51-0.54) and 0.87 (95% CI, 0.85-0.89).
- Heart failure hospitalization: 0.93 (95% CI, 0.90-0.96) and 1.02 (95% CI, 1.00-1.04).
- All-cause mortality: 0.72 (95% CI, 0.71-0.73) and 0.90 (95% CI, 0.89-0.92).
“By including a control group of individuals without CHD, we demonstrated that the magnitude of the sex difference in cardiac event rates and survival is considerably smaller among those with prior MI than among those without a history of CHD,” Dr. Peters said.
Of note, the sex differences were consistent across age and race/ethnicity groups for all events, except for heart failure hospitalizations, where the adjusted hazard ratio for women vs. men age 80 years or older was 0.95 for those without a history of CHD (95% CI, 0.91-0.98) and 0.99 (95% CI, 0.96-1.02) for participants with a previous MI.
Dr. Peters said it’s not clear why the female advantage is attenuated post-MI but that one explanation is that women are less likely than men to receive guideline-recommended treatments and dosages or to adhere to prescribed therapies after MI hospitalization, which could put them at a higher risk of subsequent events and worse outcomes than men.
“Sex differences in pathophysiology of CHD and its complications may also explain, to some extent, why the rates of recurrent events are considerably more similar between the sexes than incident event rates,” she said. Compared with men, women have a higher incidence of MI with nonobstructive coronary artery disease and of heart failure with preserved ejection fraction, and evidence-based treatment options are more limited for both conditions.
“After people read this, I think the important thing to recognize is we need to push– as much as we can, with what meds we have, and what data we have – secondary prevention in these women,” Laxmi Mehta, MD, director of preventive cardiology and women’s cardiovascular health at Ohio State University, Columbus, said in an interview.
The lack of a female advantage post-MI should also elicit a “really meaningful conversation with our patients on shared decision-making of why they need to be on medications, remembering on our part to prescribe the medications, remembering to prescribe cardiac rehab, and also reminding our community we do need more data and need to investigate this further,” she said.
In an accompanying editorial, Nanette Wenger, MD, of Emory University, Atlanta, also points out that nonobstructive coronary disease is more common in women and, “yet, guideline-based therapies are those validated for obstructive coronary disease in a predominantly male population but, nonetheless, are applied for nonobstructive coronary disease.”
She advocates for aggressive evaluation and treatment for women with chest pain symptoms as well as early identification of women at risk for CHD, specifically those with metabolic syndrome, preeclampsia, hypertensive disorders of pregnancy, chronic inflammatory conditions, and high-risk race/ethnicity.
“Next, when coronary angiography is undertaken, particularly in younger women, an assiduous search for spontaneous coronary artery dissection and its appropriate management, as well as prompt and evidence-based interventions and medical therapies for an acute coronary event [are indicated],” Dr. Wenger wrote. “However, basic to improving outcomes for women is the elucidation of the optimal noninvasive techniques to identify microvascular disease, which could then enable delineation of appropriate preventive and therapeutic approaches.”
Dr. Peters is supported by a U.K. Medical Research Council Skills Development Fellowship. Dr. Mehta and Dr. Wenger disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Women are known to lag 5-10 years behind men in experiencing coronary heart disease (CHD), but new research suggests the gap narrows substantially following a myocardial infarction.
“Women lose a considerable portion, but not all, of their coronary and survival advantage – i.e., the lower event rates – after suffering a MI,” study author Sanne Peters, PhD, George Institute for Global Health, Imperial College London, said in an interview.
Previous studies of sex differences in event rates after a coronary event have produced mixed results and were primarily focused on mortality following MI. Importantly, the studies also lacked a control group without a history of CHD and, thus, were unable to provide a reference point for the disparity in event rates, she explained.
Using the MarketScan and Medicare databases, however, Dr. Peters and colleagues matched 339,890 U.S. adults hospitalized for an MI between January 2015 and December 2016 with 1,359,560 U.S. adults without a history of CHD.
Over a median 1.3 years follow-up, there were 12,518 MIs in the non-CHD group and 27,115 recurrent MIs in the MI group.
The age-standardized rate of MI per 1,000 person-years was 4.0 in women and 6.1 in men without a history of CHD, compared with 57.6 in women and 62.7 in men with a prior MI.
After multivariate adjustment, the women-to-men hazard ratio for MI was 0.64 (95% confidence interval, 0.62-0.67) in the non-CHD group and 0.94 (95% CI, 0.92-0.96) in the prior MI group, the authors reported Oct. 5 in the Journal of the American College of Cardiology
Additional results show the multivariate adjusted women-to-men hazard ratios for three other cardiovascular outcomes follow a similar pattern in the non-CHD and prior MI groups:
- CHD events: 0.53 (95% CI, 0.51-0.54) and 0.87 (95% CI, 0.85-0.89).
- Heart failure hospitalization: 0.93 (95% CI, 0.90-0.96) and 1.02 (95% CI, 1.00-1.04).
- All-cause mortality: 0.72 (95% CI, 0.71-0.73) and 0.90 (95% CI, 0.89-0.92).
“By including a control group of individuals without CHD, we demonstrated that the magnitude of the sex difference in cardiac event rates and survival is considerably smaller among those with prior MI than among those without a history of CHD,” Dr. Peters said.
Of note, the sex differences were consistent across age and race/ethnicity groups for all events, except for heart failure hospitalizations, where the adjusted hazard ratio for women vs. men age 80 years or older was 0.95 for those without a history of CHD (95% CI, 0.91-0.98) and 0.99 (95% CI, 0.96-1.02) for participants with a previous MI.
Dr. Peters said it’s not clear why the female advantage is attenuated post-MI but that one explanation is that women are less likely than men to receive guideline-recommended treatments and dosages or to adhere to prescribed therapies after MI hospitalization, which could put them at a higher risk of subsequent events and worse outcomes than men.
“Sex differences in pathophysiology of CHD and its complications may also explain, to some extent, why the rates of recurrent events are considerably more similar between the sexes than incident event rates,” she said. Compared with men, women have a higher incidence of MI with nonobstructive coronary artery disease and of heart failure with preserved ejection fraction, and evidence-based treatment options are more limited for both conditions.
“After people read this, I think the important thing to recognize is we need to push– as much as we can, with what meds we have, and what data we have – secondary prevention in these women,” Laxmi Mehta, MD, director of preventive cardiology and women’s cardiovascular health at Ohio State University, Columbus, said in an interview.
The lack of a female advantage post-MI should also elicit a “really meaningful conversation with our patients on shared decision-making of why they need to be on medications, remembering on our part to prescribe the medications, remembering to prescribe cardiac rehab, and also reminding our community we do need more data and need to investigate this further,” she said.
In an accompanying editorial, Nanette Wenger, MD, of Emory University, Atlanta, also points out that nonobstructive coronary disease is more common in women and, “yet, guideline-based therapies are those validated for obstructive coronary disease in a predominantly male population but, nonetheless, are applied for nonobstructive coronary disease.”
She advocates for aggressive evaluation and treatment for women with chest pain symptoms as well as early identification of women at risk for CHD, specifically those with metabolic syndrome, preeclampsia, hypertensive disorders of pregnancy, chronic inflammatory conditions, and high-risk race/ethnicity.
“Next, when coronary angiography is undertaken, particularly in younger women, an assiduous search for spontaneous coronary artery dissection and its appropriate management, as well as prompt and evidence-based interventions and medical therapies for an acute coronary event [are indicated],” Dr. Wenger wrote. “However, basic to improving outcomes for women is the elucidation of the optimal noninvasive techniques to identify microvascular disease, which could then enable delineation of appropriate preventive and therapeutic approaches.”
Dr. Peters is supported by a U.K. Medical Research Council Skills Development Fellowship. Dr. Mehta and Dr. Wenger disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Women are known to lag 5-10 years behind men in experiencing coronary heart disease (CHD), but new research suggests the gap narrows substantially following a myocardial infarction.
“Women lose a considerable portion, but not all, of their coronary and survival advantage – i.e., the lower event rates – after suffering a MI,” study author Sanne Peters, PhD, George Institute for Global Health, Imperial College London, said in an interview.
Previous studies of sex differences in event rates after a coronary event have produced mixed results and were primarily focused on mortality following MI. Importantly, the studies also lacked a control group without a history of CHD and, thus, were unable to provide a reference point for the disparity in event rates, she explained.
Using the MarketScan and Medicare databases, however, Dr. Peters and colleagues matched 339,890 U.S. adults hospitalized for an MI between January 2015 and December 2016 with 1,359,560 U.S. adults without a history of CHD.
Over a median 1.3 years follow-up, there were 12,518 MIs in the non-CHD group and 27,115 recurrent MIs in the MI group.
The age-standardized rate of MI per 1,000 person-years was 4.0 in women and 6.1 in men without a history of CHD, compared with 57.6 in women and 62.7 in men with a prior MI.
After multivariate adjustment, the women-to-men hazard ratio for MI was 0.64 (95% confidence interval, 0.62-0.67) in the non-CHD group and 0.94 (95% CI, 0.92-0.96) in the prior MI group, the authors reported Oct. 5 in the Journal of the American College of Cardiology
Additional results show the multivariate adjusted women-to-men hazard ratios for three other cardiovascular outcomes follow a similar pattern in the non-CHD and prior MI groups:
- CHD events: 0.53 (95% CI, 0.51-0.54) and 0.87 (95% CI, 0.85-0.89).
- Heart failure hospitalization: 0.93 (95% CI, 0.90-0.96) and 1.02 (95% CI, 1.00-1.04).
- All-cause mortality: 0.72 (95% CI, 0.71-0.73) and 0.90 (95% CI, 0.89-0.92).
“By including a control group of individuals without CHD, we demonstrated that the magnitude of the sex difference in cardiac event rates and survival is considerably smaller among those with prior MI than among those without a history of CHD,” Dr. Peters said.
Of note, the sex differences were consistent across age and race/ethnicity groups for all events, except for heart failure hospitalizations, where the adjusted hazard ratio for women vs. men age 80 years or older was 0.95 for those without a history of CHD (95% CI, 0.91-0.98) and 0.99 (95% CI, 0.96-1.02) for participants with a previous MI.
Dr. Peters said it’s not clear why the female advantage is attenuated post-MI but that one explanation is that women are less likely than men to receive guideline-recommended treatments and dosages or to adhere to prescribed therapies after MI hospitalization, which could put them at a higher risk of subsequent events and worse outcomes than men.
“Sex differences in pathophysiology of CHD and its complications may also explain, to some extent, why the rates of recurrent events are considerably more similar between the sexes than incident event rates,” she said. Compared with men, women have a higher incidence of MI with nonobstructive coronary artery disease and of heart failure with preserved ejection fraction, and evidence-based treatment options are more limited for both conditions.
“After people read this, I think the important thing to recognize is we need to push– as much as we can, with what meds we have, and what data we have – secondary prevention in these women,” Laxmi Mehta, MD, director of preventive cardiology and women’s cardiovascular health at Ohio State University, Columbus, said in an interview.
The lack of a female advantage post-MI should also elicit a “really meaningful conversation with our patients on shared decision-making of why they need to be on medications, remembering on our part to prescribe the medications, remembering to prescribe cardiac rehab, and also reminding our community we do need more data and need to investigate this further,” she said.
In an accompanying editorial, Nanette Wenger, MD, of Emory University, Atlanta, also points out that nonobstructive coronary disease is more common in women and, “yet, guideline-based therapies are those validated for obstructive coronary disease in a predominantly male population but, nonetheless, are applied for nonobstructive coronary disease.”
She advocates for aggressive evaluation and treatment for women with chest pain symptoms as well as early identification of women at risk for CHD, specifically those with metabolic syndrome, preeclampsia, hypertensive disorders of pregnancy, chronic inflammatory conditions, and high-risk race/ethnicity.
“Next, when coronary angiography is undertaken, particularly in younger women, an assiduous search for spontaneous coronary artery dissection and its appropriate management, as well as prompt and evidence-based interventions and medical therapies for an acute coronary event [are indicated],” Dr. Wenger wrote. “However, basic to improving outcomes for women is the elucidation of the optimal noninvasive techniques to identify microvascular disease, which could then enable delineation of appropriate preventive and therapeutic approaches.”
Dr. Peters is supported by a U.K. Medical Research Council Skills Development Fellowship. Dr. Mehta and Dr. Wenger disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Primary prevention of VTE spans a spectrum
Venous thromboembolism (VTE) is a common and dangerous disease, affecting 0.1%-0.2% of the population annually—a rate that might be underreported.1 VTE is a collective term for venous blood clots, including (1) deep vein thrombosis (DVT) of peripheral veins and (2) pulmonary embolism, which occurs after a clot travels through the heart and becomes lodged in the pulmonary vasculature. Two-thirds of VTE cases present clinically as DVT2; most mortality from VTE disease is caused by the 20% of cases of pulmonary embolism that present as sudden death.1
VTE is comparable to myocardial infarction (MI) in incidence and severity. In 2008, 208 of every 100,000 people had an MI, with a 30-day mortality of 16/100,0003; VTE disease has an annual incidence of 161 of every 100,000 people and a 28-day mortality of 18/100,000.4 Although the incidence and severity of MI are steadily decreasing, the rate of VTE appears constant.3,5 The high mortality of VTE suggests that primary prevention, which we discuss in this article, is valuable (see “Key points: Primary prevention of venous thromboembolism”).
SIDEBAR
Key points: Primary prevention of venous thromboembolism
- Primary prevention of venous thromboembolism (VTE), a disease with mortality similar to myocardial infarction, should be an important consideration in at-risk patients.
- Although statins reduce the risk of VTE, their use is justified only if they are also required for prevention of cardiovascular disease.
- The risk of travel-related VTE can be reduced by wearing compression stockings.
- The choice of particular methods of contraception and of hormone replacement therapy can reduce VTE risk.
- Because of the risk of bleeding, using anticoagulants for primary prevention of VTE is justified only in certain circumstances.
- Pregnancy is the only condition in which there is a guideline indication for thrombophilia testing, because test results in this setting can change recommendations for preventing VTE.
- Using a risk-stratification model is key to determining risk in both medically and surgically hospitalized patients. Trauma and major orthopedic surgery always place the patient at high risk of VTE.
Risk factors
Virchow’s triad of venous stasis, vascular injury, and hypercoagulability describes predisposing factors for VTE.6 Although venous valves promote blood flow, they produce isolated low-flow areas adjacent to valves that become concentrated and locally hypoxic, increasing the risk of clotting.7 The great majority of DVTs (≥ 96%) occur in the lower extremity,8 starting in the calf; there, 75% of cases resolve spontaneously before they extend into the deep veins of the proximal leg.7 One-half of DVTs that do move into the proximal leg eventually embolize.7
Major risk factors for VTE comprise inherited conditions, medical history, medical therapeutics, and behaviors (TABLE 1).9-11 Unlike the preventive management of coronary artery disease (CAD), there is no simple, generalized prevention algorithm to address VTE risk factors.
Risk factors for VTE and CAD overlap. Risk factors for atherosclerosis—obesity, diabetes, smoking, hypertension, hyperlipidemia—also increase the risk of VTE (TABLE 1).9-11 The association between risk factors for VTE and atherosclerosis is demonstrated by a doubling of the risk of MI and stroke in the year following VTE.11 Lifestyle changes are expected to reduce the risk of VTE, as they do for acute CAD, but studies are lacking to confirm this connection. There is no prospective evidence showing that weight loss or control of diabetes or hypertension reduces the risk of VTE.12 Smoking cessation does appear to reduce risk: Former smokers have the same VTE risk as never-smokers.13
Thrombophilia testing: Not generally useful
Inherited and acquired thrombophilic conditions define a group of disorders in which the risk of VTE is increased. Although thrombophilia testing was once considered for primary and secondary prevention of VTE, such testing is rarely used now because proof of benefit is lacking: A large case–control study showed that thrombophilia testing did not predict recurrence after a first VTE.14 Guidelines of the American College of Chest Physicians (ACCP) do not address thrombophilia, and the American Society of Hematology recommends against thrombophilia testing after a provoked VTE.15,16
Primary prophylaxis of patients with a family history of VTE and inherited thrombophilia is controversial. Patients with both a family history of VTE and demonstrated thrombophilia do have double the average incidence of VTE, but this increased risk does not offset the significant bleeding risk associated with anticoagulation.17 Recommendations for thrombophilia testing are limited to certain situations in pregnancy, discussed in a bit.16,18,19
Continue to: Primary prevention of VTE in the clinic
Primary prevention of VTE in the clinic
There is no single, overarching preventive strategy for VTE in an ambulatory patient (although statins, discussed in a moment, offer some benefit, broadly). There are, however, distinct behavioral characteristics and medical circumstances for which opportunities exist to reduce VTE risk—for example, when a person engages in long-distance travel, receives hormonal therapy, is pregnant, or has cancer. In each scenario, recognizing and mitigating risk are important.
Statins offer a (slight) benefit
There is evidence that statins reduce the risk of VTE—slightly20-23:
- A large randomized, controlled trial showed that rosuvastatin, 20 mg/d, reduced the rate of VTE, compared to placebo; however, the 2-year number needed to treat (NNT) was 349.20 The VTE benefit is minimal, however, compared to primary prevention of cardiovascular disease with statins (5-year NNT = 56).21 The sole significant adverse event associated with statins was new-onset type 2 diabetes (5-year number needed to harm = 235).21
- A subsequent meta-analysis confirmed a small reduction in VTE risk with statins.22 In its 2012 guidelines, ACCP declined to issue a recommendation on the use of statins for VTE prevention.23 When considering statins for primary cardiovascular disease prevention, take the additional VTE prevention into account.
Simple strategies can help prevent travel-related VTE
Travel is a common inciting factor for VTE. A systematic review showed that VTE risk triples after travel of ≥ 4 hours, increasing by 20% with each additional 2 hours.24 Most VTE occurs in travelers who have other VTE risk factors.25 Based on case–control studies,23 guidelines recommend these preventive measures:
- frequent calf exercises
- sitting in an aisle seat during air travel
- keeping hydrated.
A Cochrane review showed that graded compression stockings reduce asymptomatic DVT in travelers by a factor of 10, in high- and low-risk patients.26
VTE risk varies with type of hormonal contraception
Most contraceptives increase VTE risk (TABLE 227,28). Risk with combined oral contraceptives varies with the amount of estrogen and progesterone. To reduce VTE risk with oral contraceptives, patients can use an agent that contains a lower dose of estrogen or one in which levonorgestrel replaces other progesterones.27
Continue to: Studies suggest that the levonorgestrel-releasing...
Studies suggest that the levonorgestrel-releasing intrauterine device and progestin-only pills are not associated with an increase in VTE risk.27 Although the quality of evidence varies, most nonoral hormonal contraceptives have been determined to carry a risk of VTE that is similar to that of combined oral contraceptives.28
In hormone replacement, avoid pills to lower risk
Hormone replacement therapy (HRT) for postmenopausal women increases VTE risk when administered in oral form, with combined estrogen and progestin HRT doubling the risk and estrogen-only formulations having a lower risk.29 VTE risk is highest in the first 6 months of HRT, declining to that of a non-HRT user within 5 years.29 Neither transdermal HRT nor estrogen creams increase the risk of VTE, according to a systematic review.30 The estradiol-containing vaginal ring also does not confer increased risk.29
Pregnancy, thrombophilia, and VTE prevention
VTE affects as many as 0.2% of pregnancies but causes 9% of pregnancy-related deaths.18 The severity of VTE in pregnancy led the American College of Obstetricians and Gynecologists (ACOG) to recommend primary VTE prophylaxis in patients with certain thrombophilias.18 Thrombophilia testing is recommended in patients with proven high-risk thrombophilia in a first-degree relative.18 ACOG recognizes 5 thrombophilias considered to carry a high risk of VTE in pregnancy18:
- homozygous Factor V Leiden
- homozygous prothrombin G20210A mutation
- antithrombin deficiency
- heterozygous Factor V Leiden and prothrombin G20210A mutation
- antiphospholipid antibody syndrome.
ACOG recommends limiting thrombophilia testing to (1) any specific thrombophilia carried by a relative and (2) possibly, the antiphospholipid antibodies anticardiolipin and lupus anticoagulant.18,19 Antiphospholipid testing is recommended when there is a history of stillbirth, 3 early pregnancy losses, or delivery earlier than 34 weeks secondary to preeclampsia.19
Primary VTE prophylaxis is recommended for pregnant patients with a high-risk thrombophilia; low-molecular-weight heparin (LMWH) is safe and its effects are predictable.18 Because postpartum risk of VTE is higher than antepartum risk, postpartum prophylaxis is also recommended with lower-risk thrombophilias18; a vitamin K antagonist or LMWH can be used.18 ACCP and ACOG recommendations for VTE prophylaxis in pregnancy differ slightly (TABLE 316,18,19).
Continue to: Cancer increases risks of VTE and bleeding
Cancer increases risks of VTE and bleeding
Cancer increases VTE risk > 6-fold31; metastases, chemotherapy, and radiotherapy further increase risk. Cancer also greatly increases the risk of bleeding: Cancer patients with VTE have an annual major bleeding rate ≥ 20%.32 Guidelines do not recommend primary VTE prophylaxis for cancer, although American Society of Clinical Oncology guidelines discuss consideration of prophylaxis for select, high-risk patients,33,34 including those with multiple myeloma, metastatic gastrointestinal cancer, or metastatic brain cancer.31,34 Recent evidence (discussed in a moment) supports the use of apixaban for primary VTE prevention during chemotherapy for high-risk cancer.
The Khorana Risk Score (TABLE 435,36) for VTE was developed and validated for use in patients with solid cancer35: A score of 2 conveys nearly a 10% risk of VTE over 6 months.36 A recent study of 550 cancer patients with a Khorana score of ≥ 2—the first evidence of risk-guided primary VTE prevention in cancer—showed that primary prophylaxis with 2.5 mg of apixaban, bid, reduced the risk of VTE (NNT = 17); however, the number needed to harm (for major bleeding) was 59.37 Mortality was not changed with apixaban treatment
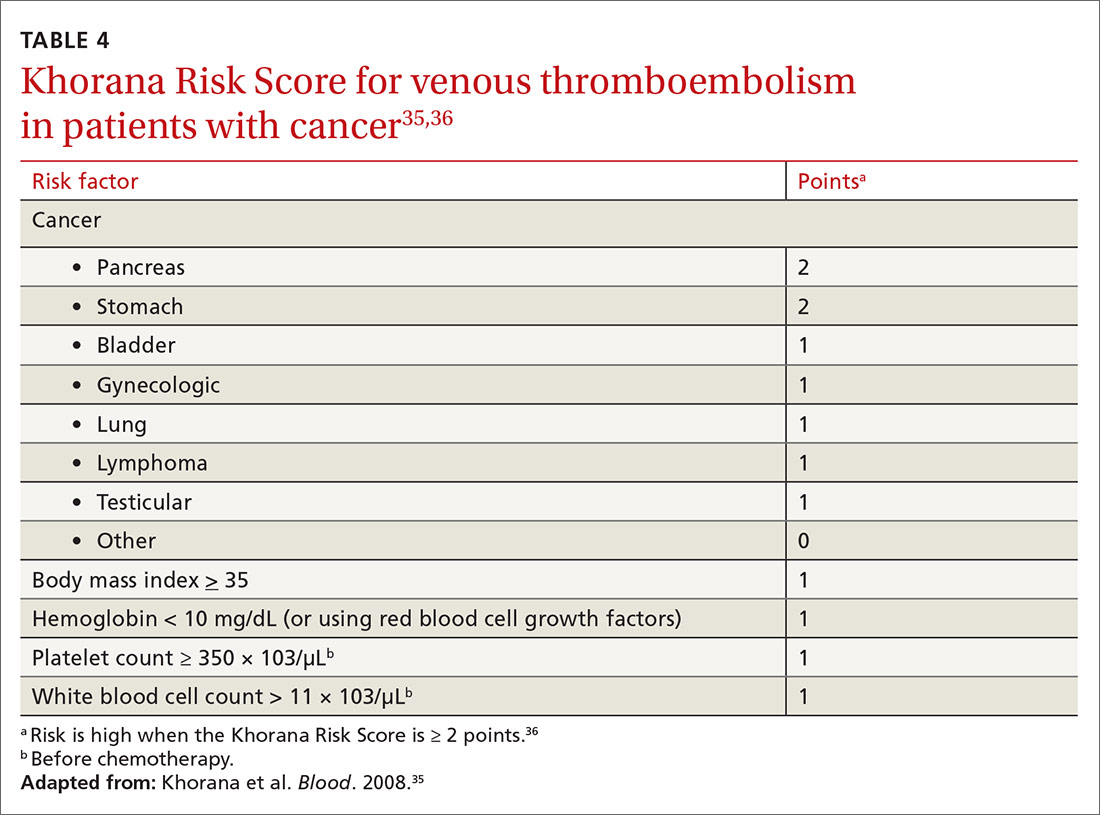
Primary VTE prevention in med-surg hospitalizations
The risk of VTE increases significantly during hospitalization, although not enough to justify universal prophylaxis. Recommended prevention strategies for different classes of hospitalized patients are summarized below.
In medically hospitalized patients, risk is stratified with a risk-assessment model. Medically hospitalized patients have, on average, a VTE risk of 1.2%23; 12 risk-assessment models designed to stratify risk were recently compared.38 Two models, the Caprini Score (TABLE 5)39 and the IMPROVE VTE Risk Calculator,40 were best able to identify low-risk patients (negative predictive value, > 99%).38 American Society of Hematology guidelines recommend IMPROVE VTE or the Padua Prediction Score for risk stratification.41 While the Caprini score only designates 11% of eventual VTE cases as low risk, both the IMPROVE VTE and Padua scores miss more than 35% of eventual VTE.38
Because LMWH prophylaxis has been shown to reduce VTE by 40% without increasing the risk of major bleeding, using Caprini should prevent 2 VTEs for every 1000 patients, without an increase in major bleeding and with 13 additional minor bleeding events.42
Continue to: Critically ill patients
Critically ill patients are assumed to be at high risk of VTE and do not require stratification.23 For high-risk patients, prophylaxis with LMWH, low-dose unfractionated heparin (LDUH), or fondaparinux is recommended for the duration of admission.23 For patients at high risk of both VTE and bleeding, mechanical prophylaxis with intermittent pneumatic compression (IPC) is recommended instead of LMWH, LDUH, or fondaparinux.23
Surgery, like trauma (see next page), increases the risk of VTE and has been well studied. Prophylaxis after orthopedic surgery differs from that of other types of surgery.
In orthopedic surgery, risk depends on the procedure. For major orthopedic surgery, including total hip or knee arthroplasty and hip fracture surgery, VTE prophylaxis is recommended for 35 days postsurgically.43 LMWH is the preferred agent, although many other means have been shown to be beneficial.44 A recent systematic review demonstrated that aspirin is not inferior to other medications after hip or knee arthroplasty.45 No mechanical or pharmacotherapeutic prophylaxis is generally recommended after nonmajor orthopedic surgery.43
Nonorthopedic surgery is stratified by risk factors, using Caprini44 (TABLE 539). For medium-risk patients (Caprini score, 3-4) LDUH, LMWH, or IPC is recommended; for high-risk patients (Caprini score, ≥ 5) preventive treatment should combine pharmacotherapeutic and mechanical prophylaxis.46 A recent meta-analysis, comprising 14,776 patients, showed that surgical patients with a Caprini score ≥ 7 had a reduced incidence of VTE when given chemoprophylaxis, whereas patients whose score is < 7 do not benefit from chemoprophylaxis.43 When bleeding risk is high, IPC is recommended as sole therapy.43 Prophylaxis is not recommended when risk (determined by the Caprini score) is low.46
Post-hospitalization. Risk of VTE can persist for as long as 90 days after hospitalization; this finding has led to evaluation of the benefit of prolonged chemoprophylaxis.23 Extended-duration LMWH prophylaxis decreases the incidence of VTE, but at the cost of increased risk of major bleeding.47 Based on this evidence, guidelines recommend against prolonged-duration anticoagulation.23 A 2016 trial showed that 35 days of the direct-acting anticoagulant betrixaban reduced the risk of symptomatic VTE events, compared to 10 days of LMWH (NNT = 167), without increased risk of bleeding.48 This is a limited benefit, however, that is unlikely to change guideline recommendations.
Continue to: Trauma
Trauma: VTE risk increases with severity
Trauma increases the risk of VTE considerably. A national study showed that 1.5% of admitted trauma patients experienced VTE during hospitalization and that 1.2% were readmitted for VTE within 1 year.49 As many as 32% of trauma patients admitted to the intensive care unit experience VTE despite appropriate prophylaxis.50 A Cochrane Review51 found that:
- prophylaxis significantly reduces DVT risk
- pharmacotherapeutic prophylaxis is more effective than mechanical prophylaxis
- LMWH is more effective than LDUH.
Guidelines recommend that major trauma patients receive prophylaxis with LMWH, LDUH, or IPC.46
CORRESPONDENCE
Michael J. Arnold, MD, CDR, MC, USN; Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Jacksonville, FL 32214; michael.arnold@usuhs.edu.
1. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010. 38(4 suppl):S495-S501.
2. Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126:832.e13-e21.
3. Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010. 362:2155-2165.
4. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
5. Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25:235-242.
6. Stone J, Hangge P, Albadawi H, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S276-S284.
7. Olaf M, Cooney R. Deep venous thrombosis. Emerg Med Clin North Am. 2017;35:743-770.
8. Sajid MS, Ahmed N, Desai M, et al. Upper limb deep vein thrombosis: a literature review to streamline the protocol for management. Acta Haematol. 2007;118:10-18.
9. Bates SM, Ginsberg JS. Clinical practice. Treatment of deep-vein thrombosis. N Engl J Med. 2004;351:268-277.
10. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
11. Goldhaber SZ. Risk factors for venous thromboembolism. J Am Col Cardiol. 2010;56:1-7.
12. Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2:499-509.
13. Severinsen MT, Kristensen SR, Johnsen SP, et al. Smoking and venous thromboembolism: a Danish follow-up study. J Thromb Haemost. 2009;7:1297-1303.
14. Coppens M, Reijnders JH, Middeldorp S, et al. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6:1474-1477.
15. Choosing Wisely. American Society of Hematology. Ten things physicians and patients should question. www.choosingwisely.org/societies/american-society-of-hematology/. Accessed September 28, 2020.
16. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S-e736S.
17. Vossen CY, Conard J, Fontcuberta J, et al. Risk of a first venous thrombotic event in carriers of a familial thrombophilic defect. The European Prospective Cohort on Thrombophilia (EPCOT). J Thromb Haemost. 2005;3:459-464.
18. Practice Bulletin No. 197: Inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34.
19. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice Bulletin No. 132: Antiphospholipid syndrome. Obstet Gynecol. 2012;120:1514-1521.
20. Glynn RJ, Danielson E, Fonseca FAH, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851-1861.
21. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013(1):CD004816.
22. Squizzato A, Galli M, Romualdi E, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31:1248-1256.
23. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S.
24. Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ. 2003;327:1072.
25. Johnston RV, Hudson MF; . Travelers’ thrombosis. Aviat Space Environ Med. 2014;85:191-194.
26. Clarke MJ, Broderick C, Hopewell S, et al. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database Syst Rev. 2016;9:CD004002.
27. van Hylckama Vlieg A, Middledorp S. Hormone therapies and venous thromboembolism: where are we now? J Thromb Haemost. 2011;9:257-266.
28. Tepper NK, Dragoman MV, Gaffield ME, et al. Nonoral combined hormonal contraceptives and thromboembolism: a systematic review. Contraception. 2017;95:130-139.
29. Lekovic D, Miljic P, Dmitrovic A, et al. How do you decide on hormone replacement therapy in women with risk of venous thromboembolism? Blood Rev. 2017;31:151-157.
30. Rovinski D, Ramos RB, Fighera TM, et al. Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: a systematic review and meta-analysis. Thromb Res. 2018;168:83-95.
31. Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001275.
32. Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res. 2014;133(suppl 2):S49-S55.
33. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352.
34. Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2013;31:2189-2204.
35. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907.
36. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377-5382.
37. Carrier M, Abou-Nassar K, Mallick R, et al; AVERT Investigators. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711-719.
38. Cobben MRR, Nemeth B, Lijfering WM, et al. Validation of risk assessment models for venous thrombosis in hospitalized medical patients. Res Pract Thromb Haemost. 2019;3:217-225.
39. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70-78.
40. Spyropoulos AC, Anderson FA Jr, FitzGerald G, et al; IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706-714.
41. Kanaan AO, Silva MA, Donovan JL, et al. Meta-analysis of venous thromboembolism prophylaxis in medically Ill patients. Clin Ther. 2007;29:2395-2405.
42. HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198-3225.
43. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
44. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini Score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1103.
45. Matharu GS, Kunutsor SK, Judge A, et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2020;180:376-384.
46. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S-e277S.
47. Hull RD, Schellong SM, Tapson VF, et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recent reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8-18.
48. Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534-544.
49. Rattan R, Parreco J, Eidelson SA, et al. Hidden burden of venous thromboembolism after trauma: a national analysis. J Trauma Acute Care Surg. 2018;85:899-906.
50. Yumoto T, Naito H, Yamakawa Y, et al. Venous thromboembolism in major trauma patients: a single-center retrospective cohort study of the epidemiology and utility of D-dimer for screening. Acute Med Surg. 2017;4:394-400.
51. Barrera LM, Perel P, Ker K, et al. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013(3):CD008303.
Venous thromboembolism (VTE) is a common and dangerous disease, affecting 0.1%-0.2% of the population annually—a rate that might be underreported.1 VTE is a collective term for venous blood clots, including (1) deep vein thrombosis (DVT) of peripheral veins and (2) pulmonary embolism, which occurs after a clot travels through the heart and becomes lodged in the pulmonary vasculature. Two-thirds of VTE cases present clinically as DVT2; most mortality from VTE disease is caused by the 20% of cases of pulmonary embolism that present as sudden death.1
VTE is comparable to myocardial infarction (MI) in incidence and severity. In 2008, 208 of every 100,000 people had an MI, with a 30-day mortality of 16/100,0003; VTE disease has an annual incidence of 161 of every 100,000 people and a 28-day mortality of 18/100,000.4 Although the incidence and severity of MI are steadily decreasing, the rate of VTE appears constant.3,5 The high mortality of VTE suggests that primary prevention, which we discuss in this article, is valuable (see “Key points: Primary prevention of venous thromboembolism”).
SIDEBAR
Key points: Primary prevention of venous thromboembolism
- Primary prevention of venous thromboembolism (VTE), a disease with mortality similar to myocardial infarction, should be an important consideration in at-risk patients.
- Although statins reduce the risk of VTE, their use is justified only if they are also required for prevention of cardiovascular disease.
- The risk of travel-related VTE can be reduced by wearing compression stockings.
- The choice of particular methods of contraception and of hormone replacement therapy can reduce VTE risk.
- Because of the risk of bleeding, using anticoagulants for primary prevention of VTE is justified only in certain circumstances.
- Pregnancy is the only condition in which there is a guideline indication for thrombophilia testing, because test results in this setting can change recommendations for preventing VTE.
- Using a risk-stratification model is key to determining risk in both medically and surgically hospitalized patients. Trauma and major orthopedic surgery always place the patient at high risk of VTE.
Risk factors
Virchow’s triad of venous stasis, vascular injury, and hypercoagulability describes predisposing factors for VTE.6 Although venous valves promote blood flow, they produce isolated low-flow areas adjacent to valves that become concentrated and locally hypoxic, increasing the risk of clotting.7 The great majority of DVTs (≥ 96%) occur in the lower extremity,8 starting in the calf; there, 75% of cases resolve spontaneously before they extend into the deep veins of the proximal leg.7 One-half of DVTs that do move into the proximal leg eventually embolize.7
Major risk factors for VTE comprise inherited conditions, medical history, medical therapeutics, and behaviors (TABLE 1).9-11 Unlike the preventive management of coronary artery disease (CAD), there is no simple, generalized prevention algorithm to address VTE risk factors.
Risk factors for VTE and CAD overlap. Risk factors for atherosclerosis—obesity, diabetes, smoking, hypertension, hyperlipidemia—also increase the risk of VTE (TABLE 1).9-11 The association between risk factors for VTE and atherosclerosis is demonstrated by a doubling of the risk of MI and stroke in the year following VTE.11 Lifestyle changes are expected to reduce the risk of VTE, as they do for acute CAD, but studies are lacking to confirm this connection. There is no prospective evidence showing that weight loss or control of diabetes or hypertension reduces the risk of VTE.12 Smoking cessation does appear to reduce risk: Former smokers have the same VTE risk as never-smokers.13
Thrombophilia testing: Not generally useful
Inherited and acquired thrombophilic conditions define a group of disorders in which the risk of VTE is increased. Although thrombophilia testing was once considered for primary and secondary prevention of VTE, such testing is rarely used now because proof of benefit is lacking: A large case–control study showed that thrombophilia testing did not predict recurrence after a first VTE.14 Guidelines of the American College of Chest Physicians (ACCP) do not address thrombophilia, and the American Society of Hematology recommends against thrombophilia testing after a provoked VTE.15,16
Primary prophylaxis of patients with a family history of VTE and inherited thrombophilia is controversial. Patients with both a family history of VTE and demonstrated thrombophilia do have double the average incidence of VTE, but this increased risk does not offset the significant bleeding risk associated with anticoagulation.17 Recommendations for thrombophilia testing are limited to certain situations in pregnancy, discussed in a bit.16,18,19
Continue to: Primary prevention of VTE in the clinic
Primary prevention of VTE in the clinic
There is no single, overarching preventive strategy for VTE in an ambulatory patient (although statins, discussed in a moment, offer some benefit, broadly). There are, however, distinct behavioral characteristics and medical circumstances for which opportunities exist to reduce VTE risk—for example, when a person engages in long-distance travel, receives hormonal therapy, is pregnant, or has cancer. In each scenario, recognizing and mitigating risk are important.
Statins offer a (slight) benefit
There is evidence that statins reduce the risk of VTE—slightly20-23:
- A large randomized, controlled trial showed that rosuvastatin, 20 mg/d, reduced the rate of VTE, compared to placebo; however, the 2-year number needed to treat (NNT) was 349.20 The VTE benefit is minimal, however, compared to primary prevention of cardiovascular disease with statins (5-year NNT = 56).21 The sole significant adverse event associated with statins was new-onset type 2 diabetes (5-year number needed to harm = 235).21
- A subsequent meta-analysis confirmed a small reduction in VTE risk with statins.22 In its 2012 guidelines, ACCP declined to issue a recommendation on the use of statins for VTE prevention.23 When considering statins for primary cardiovascular disease prevention, take the additional VTE prevention into account.
Simple strategies can help prevent travel-related VTE
Travel is a common inciting factor for VTE. A systematic review showed that VTE risk triples after travel of ≥ 4 hours, increasing by 20% with each additional 2 hours.24 Most VTE occurs in travelers who have other VTE risk factors.25 Based on case–control studies,23 guidelines recommend these preventive measures:
- frequent calf exercises
- sitting in an aisle seat during air travel
- keeping hydrated.
A Cochrane review showed that graded compression stockings reduce asymptomatic DVT in travelers by a factor of 10, in high- and low-risk patients.26
VTE risk varies with type of hormonal contraception
Most contraceptives increase VTE risk (TABLE 227,28). Risk with combined oral contraceptives varies with the amount of estrogen and progesterone. To reduce VTE risk with oral contraceptives, patients can use an agent that contains a lower dose of estrogen or one in which levonorgestrel replaces other progesterones.27
Continue to: Studies suggest that the levonorgestrel-releasing...
Studies suggest that the levonorgestrel-releasing intrauterine device and progestin-only pills are not associated with an increase in VTE risk.27 Although the quality of evidence varies, most nonoral hormonal contraceptives have been determined to carry a risk of VTE that is similar to that of combined oral contraceptives.28
In hormone replacement, avoid pills to lower risk
Hormone replacement therapy (HRT) for postmenopausal women increases VTE risk when administered in oral form, with combined estrogen and progestin HRT doubling the risk and estrogen-only formulations having a lower risk.29 VTE risk is highest in the first 6 months of HRT, declining to that of a non-HRT user within 5 years.29 Neither transdermal HRT nor estrogen creams increase the risk of VTE, according to a systematic review.30 The estradiol-containing vaginal ring also does not confer increased risk.29
Pregnancy, thrombophilia, and VTE prevention
VTE affects as many as 0.2% of pregnancies but causes 9% of pregnancy-related deaths.18 The severity of VTE in pregnancy led the American College of Obstetricians and Gynecologists (ACOG) to recommend primary VTE prophylaxis in patients with certain thrombophilias.18 Thrombophilia testing is recommended in patients with proven high-risk thrombophilia in a first-degree relative.18 ACOG recognizes 5 thrombophilias considered to carry a high risk of VTE in pregnancy18:
- homozygous Factor V Leiden
- homozygous prothrombin G20210A mutation
- antithrombin deficiency
- heterozygous Factor V Leiden and prothrombin G20210A mutation
- antiphospholipid antibody syndrome.
ACOG recommends limiting thrombophilia testing to (1) any specific thrombophilia carried by a relative and (2) possibly, the antiphospholipid antibodies anticardiolipin and lupus anticoagulant.18,19 Antiphospholipid testing is recommended when there is a history of stillbirth, 3 early pregnancy losses, or delivery earlier than 34 weeks secondary to preeclampsia.19
Primary VTE prophylaxis is recommended for pregnant patients with a high-risk thrombophilia; low-molecular-weight heparin (LMWH) is safe and its effects are predictable.18 Because postpartum risk of VTE is higher than antepartum risk, postpartum prophylaxis is also recommended with lower-risk thrombophilias18; a vitamin K antagonist or LMWH can be used.18 ACCP and ACOG recommendations for VTE prophylaxis in pregnancy differ slightly (TABLE 316,18,19).
Continue to: Cancer increases risks of VTE and bleeding
Cancer increases risks of VTE and bleeding
Cancer increases VTE risk > 6-fold31; metastases, chemotherapy, and radiotherapy further increase risk. Cancer also greatly increases the risk of bleeding: Cancer patients with VTE have an annual major bleeding rate ≥ 20%.32 Guidelines do not recommend primary VTE prophylaxis for cancer, although American Society of Clinical Oncology guidelines discuss consideration of prophylaxis for select, high-risk patients,33,34 including those with multiple myeloma, metastatic gastrointestinal cancer, or metastatic brain cancer.31,34 Recent evidence (discussed in a moment) supports the use of apixaban for primary VTE prevention during chemotherapy for high-risk cancer.
The Khorana Risk Score (TABLE 435,36) for VTE was developed and validated for use in patients with solid cancer35: A score of 2 conveys nearly a 10% risk of VTE over 6 months.36 A recent study of 550 cancer patients with a Khorana score of ≥ 2—the first evidence of risk-guided primary VTE prevention in cancer—showed that primary prophylaxis with 2.5 mg of apixaban, bid, reduced the risk of VTE (NNT = 17); however, the number needed to harm (for major bleeding) was 59.37 Mortality was not changed with apixaban treatment

Primary VTE prevention in med-surg hospitalizations
The risk of VTE increases significantly during hospitalization, although not enough to justify universal prophylaxis. Recommended prevention strategies for different classes of hospitalized patients are summarized below.
In medically hospitalized patients, risk is stratified with a risk-assessment model. Medically hospitalized patients have, on average, a VTE risk of 1.2%23; 12 risk-assessment models designed to stratify risk were recently compared.38 Two models, the Caprini Score (TABLE 5)39 and the IMPROVE VTE Risk Calculator,40 were best able to identify low-risk patients (negative predictive value, > 99%).38 American Society of Hematology guidelines recommend IMPROVE VTE or the Padua Prediction Score for risk stratification.41 While the Caprini score only designates 11% of eventual VTE cases as low risk, both the IMPROVE VTE and Padua scores miss more than 35% of eventual VTE.38
Because LMWH prophylaxis has been shown to reduce VTE by 40% without increasing the risk of major bleeding, using Caprini should prevent 2 VTEs for every 1000 patients, without an increase in major bleeding and with 13 additional minor bleeding events.42
Continue to: Critically ill patients
Critically ill patients are assumed to be at high risk of VTE and do not require stratification.23 For high-risk patients, prophylaxis with LMWH, low-dose unfractionated heparin (LDUH), or fondaparinux is recommended for the duration of admission.23 For patients at high risk of both VTE and bleeding, mechanical prophylaxis with intermittent pneumatic compression (IPC) is recommended instead of LMWH, LDUH, or fondaparinux.23
Surgery, like trauma (see next page), increases the risk of VTE and has been well studied. Prophylaxis after orthopedic surgery differs from that of other types of surgery.
In orthopedic surgery, risk depends on the procedure. For major orthopedic surgery, including total hip or knee arthroplasty and hip fracture surgery, VTE prophylaxis is recommended for 35 days postsurgically.43 LMWH is the preferred agent, although many other means have been shown to be beneficial.44 A recent systematic review demonstrated that aspirin is not inferior to other medications after hip or knee arthroplasty.45 No mechanical or pharmacotherapeutic prophylaxis is generally recommended after nonmajor orthopedic surgery.43
Nonorthopedic surgery is stratified by risk factors, using Caprini44 (TABLE 539). For medium-risk patients (Caprini score, 3-4) LDUH, LMWH, or IPC is recommended; for high-risk patients (Caprini score, ≥ 5) preventive treatment should combine pharmacotherapeutic and mechanical prophylaxis.46 A recent meta-analysis, comprising 14,776 patients, showed that surgical patients with a Caprini score ≥ 7 had a reduced incidence of VTE when given chemoprophylaxis, whereas patients whose score is < 7 do not benefit from chemoprophylaxis.43 When bleeding risk is high, IPC is recommended as sole therapy.43 Prophylaxis is not recommended when risk (determined by the Caprini score) is low.46
Post-hospitalization. Risk of VTE can persist for as long as 90 days after hospitalization; this finding has led to evaluation of the benefit of prolonged chemoprophylaxis.23 Extended-duration LMWH prophylaxis decreases the incidence of VTE, but at the cost of increased risk of major bleeding.47 Based on this evidence, guidelines recommend against prolonged-duration anticoagulation.23 A 2016 trial showed that 35 days of the direct-acting anticoagulant betrixaban reduced the risk of symptomatic VTE events, compared to 10 days of LMWH (NNT = 167), without increased risk of bleeding.48 This is a limited benefit, however, that is unlikely to change guideline recommendations.
Continue to: Trauma
Trauma: VTE risk increases with severity
Trauma increases the risk of VTE considerably. A national study showed that 1.5% of admitted trauma patients experienced VTE during hospitalization and that 1.2% were readmitted for VTE within 1 year.49 As many as 32% of trauma patients admitted to the intensive care unit experience VTE despite appropriate prophylaxis.50 A Cochrane Review51 found that:
- prophylaxis significantly reduces DVT risk
- pharmacotherapeutic prophylaxis is more effective than mechanical prophylaxis
- LMWH is more effective than LDUH.
Guidelines recommend that major trauma patients receive prophylaxis with LMWH, LDUH, or IPC.46
CORRESPONDENCE
Michael J. Arnold, MD, CDR, MC, USN; Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Jacksonville, FL 32214; michael.arnold@usuhs.edu.
Venous thromboembolism (VTE) is a common and dangerous disease, affecting 0.1%-0.2% of the population annually—a rate that might be underreported.1 VTE is a collective term for venous blood clots, including (1) deep vein thrombosis (DVT) of peripheral veins and (2) pulmonary embolism, which occurs after a clot travels through the heart and becomes lodged in the pulmonary vasculature. Two-thirds of VTE cases present clinically as DVT2; most mortality from VTE disease is caused by the 20% of cases of pulmonary embolism that present as sudden death.1
VTE is comparable to myocardial infarction (MI) in incidence and severity. In 2008, 208 of every 100,000 people had an MI, with a 30-day mortality of 16/100,0003; VTE disease has an annual incidence of 161 of every 100,000 people and a 28-day mortality of 18/100,000.4 Although the incidence and severity of MI are steadily decreasing, the rate of VTE appears constant.3,5 The high mortality of VTE suggests that primary prevention, which we discuss in this article, is valuable (see “Key points: Primary prevention of venous thromboembolism”).
SIDEBAR
Key points: Primary prevention of venous thromboembolism
- Primary prevention of venous thromboembolism (VTE), a disease with mortality similar to myocardial infarction, should be an important consideration in at-risk patients.
- Although statins reduce the risk of VTE, their use is justified only if they are also required for prevention of cardiovascular disease.
- The risk of travel-related VTE can be reduced by wearing compression stockings.
- The choice of particular methods of contraception and of hormone replacement therapy can reduce VTE risk.
- Because of the risk of bleeding, using anticoagulants for primary prevention of VTE is justified only in certain circumstances.
- Pregnancy is the only condition in which there is a guideline indication for thrombophilia testing, because test results in this setting can change recommendations for preventing VTE.
- Using a risk-stratification model is key to determining risk in both medically and surgically hospitalized patients. Trauma and major orthopedic surgery always place the patient at high risk of VTE.
Risk factors
Virchow’s triad of venous stasis, vascular injury, and hypercoagulability describes predisposing factors for VTE.6 Although venous valves promote blood flow, they produce isolated low-flow areas adjacent to valves that become concentrated and locally hypoxic, increasing the risk of clotting.7 The great majority of DVTs (≥ 96%) occur in the lower extremity,8 starting in the calf; there, 75% of cases resolve spontaneously before they extend into the deep veins of the proximal leg.7 One-half of DVTs that do move into the proximal leg eventually embolize.7
Major risk factors for VTE comprise inherited conditions, medical history, medical therapeutics, and behaviors (TABLE 1).9-11 Unlike the preventive management of coronary artery disease (CAD), there is no simple, generalized prevention algorithm to address VTE risk factors.
Risk factors for VTE and CAD overlap. Risk factors for atherosclerosis—obesity, diabetes, smoking, hypertension, hyperlipidemia—also increase the risk of VTE (TABLE 1).9-11 The association between risk factors for VTE and atherosclerosis is demonstrated by a doubling of the risk of MI and stroke in the year following VTE.11 Lifestyle changes are expected to reduce the risk of VTE, as they do for acute CAD, but studies are lacking to confirm this connection. There is no prospective evidence showing that weight loss or control of diabetes or hypertension reduces the risk of VTE.12 Smoking cessation does appear to reduce risk: Former smokers have the same VTE risk as never-smokers.13
Thrombophilia testing: Not generally useful
Inherited and acquired thrombophilic conditions define a group of disorders in which the risk of VTE is increased. Although thrombophilia testing was once considered for primary and secondary prevention of VTE, such testing is rarely used now because proof of benefit is lacking: A large case–control study showed that thrombophilia testing did not predict recurrence after a first VTE.14 Guidelines of the American College of Chest Physicians (ACCP) do not address thrombophilia, and the American Society of Hematology recommends against thrombophilia testing after a provoked VTE.15,16
Primary prophylaxis of patients with a family history of VTE and inherited thrombophilia is controversial. Patients with both a family history of VTE and demonstrated thrombophilia do have double the average incidence of VTE, but this increased risk does not offset the significant bleeding risk associated with anticoagulation.17 Recommendations for thrombophilia testing are limited to certain situations in pregnancy, discussed in a bit.16,18,19
Continue to: Primary prevention of VTE in the clinic
Primary prevention of VTE in the clinic
There is no single, overarching preventive strategy for VTE in an ambulatory patient (although statins, discussed in a moment, offer some benefit, broadly). There are, however, distinct behavioral characteristics and medical circumstances for which opportunities exist to reduce VTE risk—for example, when a person engages in long-distance travel, receives hormonal therapy, is pregnant, or has cancer. In each scenario, recognizing and mitigating risk are important.
Statins offer a (slight) benefit
There is evidence that statins reduce the risk of VTE—slightly20-23:
- A large randomized, controlled trial showed that rosuvastatin, 20 mg/d, reduced the rate of VTE, compared to placebo; however, the 2-year number needed to treat (NNT) was 349.20 The VTE benefit is minimal, however, compared to primary prevention of cardiovascular disease with statins (5-year NNT = 56).21 The sole significant adverse event associated with statins was new-onset type 2 diabetes (5-year number needed to harm = 235).21
- A subsequent meta-analysis confirmed a small reduction in VTE risk with statins.22 In its 2012 guidelines, ACCP declined to issue a recommendation on the use of statins for VTE prevention.23 When considering statins for primary cardiovascular disease prevention, take the additional VTE prevention into account.
Simple strategies can help prevent travel-related VTE
Travel is a common inciting factor for VTE. A systematic review showed that VTE risk triples after travel of ≥ 4 hours, increasing by 20% with each additional 2 hours.24 Most VTE occurs in travelers who have other VTE risk factors.25 Based on case–control studies,23 guidelines recommend these preventive measures:
- frequent calf exercises
- sitting in an aisle seat during air travel
- keeping hydrated.
A Cochrane review showed that graded compression stockings reduce asymptomatic DVT in travelers by a factor of 10, in high- and low-risk patients.26
VTE risk varies with type of hormonal contraception
Most contraceptives increase VTE risk (TABLE 227,28). Risk with combined oral contraceptives varies with the amount of estrogen and progesterone. To reduce VTE risk with oral contraceptives, patients can use an agent that contains a lower dose of estrogen or one in which levonorgestrel replaces other progesterones.27
Continue to: Studies suggest that the levonorgestrel-releasing...
Studies suggest that the levonorgestrel-releasing intrauterine device and progestin-only pills are not associated with an increase in VTE risk.27 Although the quality of evidence varies, most nonoral hormonal contraceptives have been determined to carry a risk of VTE that is similar to that of combined oral contraceptives.28
In hormone replacement, avoid pills to lower risk
Hormone replacement therapy (HRT) for postmenopausal women increases VTE risk when administered in oral form, with combined estrogen and progestin HRT doubling the risk and estrogen-only formulations having a lower risk.29 VTE risk is highest in the first 6 months of HRT, declining to that of a non-HRT user within 5 years.29 Neither transdermal HRT nor estrogen creams increase the risk of VTE, according to a systematic review.30 The estradiol-containing vaginal ring also does not confer increased risk.29
Pregnancy, thrombophilia, and VTE prevention
VTE affects as many as 0.2% of pregnancies but causes 9% of pregnancy-related deaths.18 The severity of VTE in pregnancy led the American College of Obstetricians and Gynecologists (ACOG) to recommend primary VTE prophylaxis in patients with certain thrombophilias.18 Thrombophilia testing is recommended in patients with proven high-risk thrombophilia in a first-degree relative.18 ACOG recognizes 5 thrombophilias considered to carry a high risk of VTE in pregnancy18:
- homozygous Factor V Leiden
- homozygous prothrombin G20210A mutation
- antithrombin deficiency
- heterozygous Factor V Leiden and prothrombin G20210A mutation
- antiphospholipid antibody syndrome.
ACOG recommends limiting thrombophilia testing to (1) any specific thrombophilia carried by a relative and (2) possibly, the antiphospholipid antibodies anticardiolipin and lupus anticoagulant.18,19 Antiphospholipid testing is recommended when there is a history of stillbirth, 3 early pregnancy losses, or delivery earlier than 34 weeks secondary to preeclampsia.19
Primary VTE prophylaxis is recommended for pregnant patients with a high-risk thrombophilia; low-molecular-weight heparin (LMWH) is safe and its effects are predictable.18 Because postpartum risk of VTE is higher than antepartum risk, postpartum prophylaxis is also recommended with lower-risk thrombophilias18; a vitamin K antagonist or LMWH can be used.18 ACCP and ACOG recommendations for VTE prophylaxis in pregnancy differ slightly (TABLE 316,18,19).
Continue to: Cancer increases risks of VTE and bleeding
Cancer increases risks of VTE and bleeding
Cancer increases VTE risk > 6-fold31; metastases, chemotherapy, and radiotherapy further increase risk. Cancer also greatly increases the risk of bleeding: Cancer patients with VTE have an annual major bleeding rate ≥ 20%.32 Guidelines do not recommend primary VTE prophylaxis for cancer, although American Society of Clinical Oncology guidelines discuss consideration of prophylaxis for select, high-risk patients,33,34 including those with multiple myeloma, metastatic gastrointestinal cancer, or metastatic brain cancer.31,34 Recent evidence (discussed in a moment) supports the use of apixaban for primary VTE prevention during chemotherapy for high-risk cancer.
The Khorana Risk Score (TABLE 435,36) for VTE was developed and validated for use in patients with solid cancer35: A score of 2 conveys nearly a 10% risk of VTE over 6 months.36 A recent study of 550 cancer patients with a Khorana score of ≥ 2—the first evidence of risk-guided primary VTE prevention in cancer—showed that primary prophylaxis with 2.5 mg of apixaban, bid, reduced the risk of VTE (NNT = 17); however, the number needed to harm (for major bleeding) was 59.37 Mortality was not changed with apixaban treatment

Primary VTE prevention in med-surg hospitalizations
The risk of VTE increases significantly during hospitalization, although not enough to justify universal prophylaxis. Recommended prevention strategies for different classes of hospitalized patients are summarized below.
In medically hospitalized patients, risk is stratified with a risk-assessment model. Medically hospitalized patients have, on average, a VTE risk of 1.2%23; 12 risk-assessment models designed to stratify risk were recently compared.38 Two models, the Caprini Score (TABLE 5)39 and the IMPROVE VTE Risk Calculator,40 were best able to identify low-risk patients (negative predictive value, > 99%).38 American Society of Hematology guidelines recommend IMPROVE VTE or the Padua Prediction Score for risk stratification.41 While the Caprini score only designates 11% of eventual VTE cases as low risk, both the IMPROVE VTE and Padua scores miss more than 35% of eventual VTE.38
Because LMWH prophylaxis has been shown to reduce VTE by 40% without increasing the risk of major bleeding, using Caprini should prevent 2 VTEs for every 1000 patients, without an increase in major bleeding and with 13 additional minor bleeding events.42
Continue to: Critically ill patients
Critically ill patients are assumed to be at high risk of VTE and do not require stratification.23 For high-risk patients, prophylaxis with LMWH, low-dose unfractionated heparin (LDUH), or fondaparinux is recommended for the duration of admission.23 For patients at high risk of both VTE and bleeding, mechanical prophylaxis with intermittent pneumatic compression (IPC) is recommended instead of LMWH, LDUH, or fondaparinux.23
Surgery, like trauma (see next page), increases the risk of VTE and has been well studied. Prophylaxis after orthopedic surgery differs from that of other types of surgery.
In orthopedic surgery, risk depends on the procedure. For major orthopedic surgery, including total hip or knee arthroplasty and hip fracture surgery, VTE prophylaxis is recommended for 35 days postsurgically.43 LMWH is the preferred agent, although many other means have been shown to be beneficial.44 A recent systematic review demonstrated that aspirin is not inferior to other medications after hip or knee arthroplasty.45 No mechanical or pharmacotherapeutic prophylaxis is generally recommended after nonmajor orthopedic surgery.43
Nonorthopedic surgery is stratified by risk factors, using Caprini44 (TABLE 539). For medium-risk patients (Caprini score, 3-4) LDUH, LMWH, or IPC is recommended; for high-risk patients (Caprini score, ≥ 5) preventive treatment should combine pharmacotherapeutic and mechanical prophylaxis.46 A recent meta-analysis, comprising 14,776 patients, showed that surgical patients with a Caprini score ≥ 7 had a reduced incidence of VTE when given chemoprophylaxis, whereas patients whose score is < 7 do not benefit from chemoprophylaxis.43 When bleeding risk is high, IPC is recommended as sole therapy.43 Prophylaxis is not recommended when risk (determined by the Caprini score) is low.46
Post-hospitalization. Risk of VTE can persist for as long as 90 days after hospitalization; this finding has led to evaluation of the benefit of prolonged chemoprophylaxis.23 Extended-duration LMWH prophylaxis decreases the incidence of VTE, but at the cost of increased risk of major bleeding.47 Based on this evidence, guidelines recommend against prolonged-duration anticoagulation.23 A 2016 trial showed that 35 days of the direct-acting anticoagulant betrixaban reduced the risk of symptomatic VTE events, compared to 10 days of LMWH (NNT = 167), without increased risk of bleeding.48 This is a limited benefit, however, that is unlikely to change guideline recommendations.
Continue to: Trauma
Trauma: VTE risk increases with severity
Trauma increases the risk of VTE considerably. A national study showed that 1.5% of admitted trauma patients experienced VTE during hospitalization and that 1.2% were readmitted for VTE within 1 year.49 As many as 32% of trauma patients admitted to the intensive care unit experience VTE despite appropriate prophylaxis.50 A Cochrane Review51 found that:
- prophylaxis significantly reduces DVT risk
- pharmacotherapeutic prophylaxis is more effective than mechanical prophylaxis
- LMWH is more effective than LDUH.
Guidelines recommend that major trauma patients receive prophylaxis with LMWH, LDUH, or IPC.46
CORRESPONDENCE
Michael J. Arnold, MD, CDR, MC, USN; Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Jacksonville, FL 32214; michael.arnold@usuhs.edu.
1. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010. 38(4 suppl):S495-S501.
2. Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126:832.e13-e21.
3. Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010. 362:2155-2165.
4. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
5. Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25:235-242.
6. Stone J, Hangge P, Albadawi H, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S276-S284.
7. Olaf M, Cooney R. Deep venous thrombosis. Emerg Med Clin North Am. 2017;35:743-770.
8. Sajid MS, Ahmed N, Desai M, et al. Upper limb deep vein thrombosis: a literature review to streamline the protocol for management. Acta Haematol. 2007;118:10-18.
9. Bates SM, Ginsberg JS. Clinical practice. Treatment of deep-vein thrombosis. N Engl J Med. 2004;351:268-277.
10. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
11. Goldhaber SZ. Risk factors for venous thromboembolism. J Am Col Cardiol. 2010;56:1-7.
12. Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2:499-509.
13. Severinsen MT, Kristensen SR, Johnsen SP, et al. Smoking and venous thromboembolism: a Danish follow-up study. J Thromb Haemost. 2009;7:1297-1303.
14. Coppens M, Reijnders JH, Middeldorp S, et al. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6:1474-1477.
15. Choosing Wisely. American Society of Hematology. Ten things physicians and patients should question. www.choosingwisely.org/societies/american-society-of-hematology/. Accessed September 28, 2020.
16. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S-e736S.
17. Vossen CY, Conard J, Fontcuberta J, et al. Risk of a first venous thrombotic event in carriers of a familial thrombophilic defect. The European Prospective Cohort on Thrombophilia (EPCOT). J Thromb Haemost. 2005;3:459-464.
18. Practice Bulletin No. 197: Inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34.
19. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice Bulletin No. 132: Antiphospholipid syndrome. Obstet Gynecol. 2012;120:1514-1521.
20. Glynn RJ, Danielson E, Fonseca FAH, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851-1861.
21. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013(1):CD004816.
22. Squizzato A, Galli M, Romualdi E, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31:1248-1256.
23. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S.
24. Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ. 2003;327:1072.
25. Johnston RV, Hudson MF; . Travelers’ thrombosis. Aviat Space Environ Med. 2014;85:191-194.
26. Clarke MJ, Broderick C, Hopewell S, et al. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database Syst Rev. 2016;9:CD004002.
27. van Hylckama Vlieg A, Middledorp S. Hormone therapies and venous thromboembolism: where are we now? J Thromb Haemost. 2011;9:257-266.
28. Tepper NK, Dragoman MV, Gaffield ME, et al. Nonoral combined hormonal contraceptives and thromboembolism: a systematic review. Contraception. 2017;95:130-139.
29. Lekovic D, Miljic P, Dmitrovic A, et al. How do you decide on hormone replacement therapy in women with risk of venous thromboembolism? Blood Rev. 2017;31:151-157.
30. Rovinski D, Ramos RB, Fighera TM, et al. Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: a systematic review and meta-analysis. Thromb Res. 2018;168:83-95.
31. Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001275.
32. Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res. 2014;133(suppl 2):S49-S55.
33. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352.
34. Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2013;31:2189-2204.
35. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907.
36. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377-5382.
37. Carrier M, Abou-Nassar K, Mallick R, et al; AVERT Investigators. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711-719.
38. Cobben MRR, Nemeth B, Lijfering WM, et al. Validation of risk assessment models for venous thrombosis in hospitalized medical patients. Res Pract Thromb Haemost. 2019;3:217-225.
39. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70-78.
40. Spyropoulos AC, Anderson FA Jr, FitzGerald G, et al; IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706-714.
41. Kanaan AO, Silva MA, Donovan JL, et al. Meta-analysis of venous thromboembolism prophylaxis in medically Ill patients. Clin Ther. 2007;29:2395-2405.
42. HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198-3225.
43. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
44. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini Score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1103.
45. Matharu GS, Kunutsor SK, Judge A, et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2020;180:376-384.
46. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S-e277S.
47. Hull RD, Schellong SM, Tapson VF, et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recent reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8-18.
48. Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534-544.
49. Rattan R, Parreco J, Eidelson SA, et al. Hidden burden of venous thromboembolism after trauma: a national analysis. J Trauma Acute Care Surg. 2018;85:899-906.
50. Yumoto T, Naito H, Yamakawa Y, et al. Venous thromboembolism in major trauma patients: a single-center retrospective cohort study of the epidemiology and utility of D-dimer for screening. Acute Med Surg. 2017;4:394-400.
51. Barrera LM, Perel P, Ker K, et al. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013(3):CD008303.
1. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010. 38(4 suppl):S495-S501.
2. Tagalakis V, Patenaude V, Kahn SR, et al. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE Study Cohort. Am J Med. 2013;126:832.e13-e21.
3. Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010. 362:2155-2165.
4. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19-25.
5. Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol. 2012;25:235-242.
6. Stone J, Hangge P, Albadawi H, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther. 2017;7(suppl 3):S276-S284.
7. Olaf M, Cooney R. Deep venous thrombosis. Emerg Med Clin North Am. 2017;35:743-770.
8. Sajid MS, Ahmed N, Desai M, et al. Upper limb deep vein thrombosis: a literature review to streamline the protocol for management. Acta Haematol. 2007;118:10-18.
9. Bates SM, Ginsberg JS. Clinical practice. Treatment of deep-vein thrombosis. N Engl J Med. 2004;351:268-277.
10. Chandra D, Parisini E, Mozaffarian D. Meta-analysis: travel and risk for venous thromboembolism. Ann Intern Med. 2009;151:180-190.
11. Goldhaber SZ. Risk factors for venous thromboembolism. J Am Col Cardiol. 2010;56:1-7.
12. Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2:499-509.
13. Severinsen MT, Kristensen SR, Johnsen SP, et al. Smoking and venous thromboembolism: a Danish follow-up study. J Thromb Haemost. 2009;7:1297-1303.
14. Coppens M, Reijnders JH, Middeldorp S, et al. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6:1474-1477.
15. Choosing Wisely. American Society of Hematology. Ten things physicians and patients should question. www.choosingwisely.org/societies/american-society-of-hematology/. Accessed September 28, 2020.
16. Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S-e736S.
17. Vossen CY, Conard J, Fontcuberta J, et al. Risk of a first venous thrombotic event in carriers of a familial thrombophilic defect. The European Prospective Cohort on Thrombophilia (EPCOT). J Thromb Haemost. 2005;3:459-464.
18. Practice Bulletin No. 197: Inherited thrombophilias in pregnancy. Obstet Gynecol. 2018;132:e18-e34.
19. Committee on Practice Bulletins—Obstetrics, American College of Obstetricians and Gynecologists. Practice Bulletin No. 132: Antiphospholipid syndrome. Obstet Gynecol. 2012;120:1514-1521.
20. Glynn RJ, Danielson E, Fonseca FAH, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851-1861.
21. Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013(1):CD004816.
22. Squizzato A, Galli M, Romualdi E, et al. Statins, fibrates, and venous thromboembolism: a meta-analysis. Eur Heart J. 2010;31:1248-1256.
23. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S.
24. Kelman CW, Kortt MA, Becker NG, et al. Deep vein thrombosis and air travel: record linkage study. BMJ. 2003;327:1072.
25. Johnston RV, Hudson MF; . Travelers’ thrombosis. Aviat Space Environ Med. 2014;85:191-194.
26. Clarke MJ, Broderick C, Hopewell S, et al. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database Syst Rev. 2016;9:CD004002.
27. van Hylckama Vlieg A, Middledorp S. Hormone therapies and venous thromboembolism: where are we now? J Thromb Haemost. 2011;9:257-266.
28. Tepper NK, Dragoman MV, Gaffield ME, et al. Nonoral combined hormonal contraceptives and thromboembolism: a systematic review. Contraception. 2017;95:130-139.
29. Lekovic D, Miljic P, Dmitrovic A, et al. How do you decide on hormone replacement therapy in women with risk of venous thromboembolism? Blood Rev. 2017;31:151-157.
30. Rovinski D, Ramos RB, Fighera TM, et al. Risk of venous thromboembolism events in postmenopausal women using oral versus non-oral hormone therapy: a systematic review and meta-analysis. Thromb Res. 2018;168:83-95.
31. Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001275.
32. Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res. 2014;133(suppl 2):S49-S55.
33. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315-352.
34. Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2013;31:2189-2204.
35. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907.
36. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377-5382.
37. Carrier M, Abou-Nassar K, Mallick R, et al; AVERT Investigators. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711-719.
38. Cobben MRR, Nemeth B, Lijfering WM, et al. Validation of risk assessment models for venous thrombosis in hospitalized medical patients. Res Pract Thromb Haemost. 2019;3:217-225.
39. Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70-78.
40. Spyropoulos AC, Anderson FA Jr, FitzGerald G, et al; IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706-714.
41. Kanaan AO, Silva MA, Donovan JL, et al. Meta-analysis of venous thromboembolism prophylaxis in medically Ill patients. Clin Ther. 2007;29:2395-2405.
42. HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198-3225.
43. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S.
44. Pannucci CJ, Swistun L, MacDonald JK, et al. Individualized venous thromboembolism risk stratification using the 2005 Caprini Score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094-1103.
45. Matharu GS, Kunutsor SK, Judge A, et al. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern Med. 2020;180:376-384.
46. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e227S-e277S.
47. Hull RD, Schellong SM, Tapson VF, et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recent reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8-18.
48. Cohen AT, Harrington RA, Goldhaber SZ, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534-544.
49. Rattan R, Parreco J, Eidelson SA, et al. Hidden burden of venous thromboembolism after trauma: a national analysis. J Trauma Acute Care Surg. 2018;85:899-906.
50. Yumoto T, Naito H, Yamakawa Y, et al. Venous thromboembolism in major trauma patients: a single-center retrospective cohort study of the epidemiology and utility of D-dimer for screening. Acute Med Surg. 2017;4:394-400.
51. Barrera LM, Perel P, Ker K, et al. Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013(3):CD008303.
PRACTICE RECOMMENDATIONS
› Consider the mild reduction in the risk of venous thromboembolism (VTE) provided by statins when contemplating their use for cardiovascular disease prevention. B
› Avoid testing for thrombophilia to determine the risk of VTE, except in pregnant patients who meet criteria for antiphospholipid syndrome or have a family history of VTE. B
› Recommend an intrauterine device or progestin-only pill for contraception if the patient’s risk of VTE is high. B
› Stratify hospitalized medical and nonorthopedic surgical patients by risk score to determine the need for VTE prophylaxis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series