User login
Molar pregnancy: The next steps after diagnosis
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
Modafinil use in pregnancy tied to congenital malformations
Modafinil exposure during pregnancy was associated with an approximately tripled risk of congenital malformations in a large Danish registry-based study.
Modafinil (Provigil) is commonly prescribed to address daytime sleepiness in narcolepsy and multiple sclerosis. An interim postmarketing safety analysis showed increased rates of major malformation in modafinil-exposed pregnancies, so the manufacturer issued an alert advising health care professionals of this safety signal in June 2019, wrote Per Damkier, MD, PhD, corresponding author of a JAMA research letter reporting the Danish study results. The postmarketing study had shown a major malformation rate of about 15% in modafinil-exposed pregnancies, much higher than the 3% background rate.
Dr. Damkier and Anne Broe, MD, PhD, both of the department of clinical biochemistry and pharmacology at Odense (Denmark) University Hospital, compared outcomes for pregnant women who were prescribed modafinil at any point during the first trimester of pregnancy with those who were prescribed an active comparator, methylphenidate, as well as with those who had neither exposure. Methylphenidate is not associated with congenital malformations and is used for indications similar to modafinil.
Looking at all pregnancies for whom complete records existed in Danish health registries between 2004 and 2017, the investigators found 49 modafinil-exposed pregnancies, 963 methylphenidate-exposed pregnancies, and 828,644 pregnancies with neither exposure.
Six major congenital malformations occurred in the modafinil-exposed group for an absolute risk of 12%. Major malformations occurred in 43 (4.5%) of the methylphenidate-exposed group and 32,466 (3.9%) of the unexposed group.
Using the extensive data available in public registries, the authors were able to perform logistic regression to adjust for concomitant use of other psychotropic medication; comorbidities such as diabetes and hypertension; and demographic and anthropometric measures such as maternal age, smoking status, and body mass index.
After this statistical adjustment, the researchers found that modafinil exposure during the first trimester of pregnancy was associated with an odds ratio of 3.4 (95% confidence interval, 1.2-9.7) for major congenital malformation, compared with first-trimester methylphenidate exposure. Compared with the unexposed cohort, modafinil-exposed pregnancies had an adjusted odds ratio of 2.7 (95% CI, 1.1-6.9) for major congenital malformation.
A total of 13 (27%) women who took modafinil had multiple sclerosis, but the authors excluded women who’d received a prescription for the multiple sclerosis drug teriflunomide (Aubagio), a known teratogen. Sleep disorders were reported for 39% of modafinil users, compared with 4.5% of methylphenidate users. Rates of psychoactive drug use were 41% for the modafinil group and 30% for the methylphenidate group.
The authors acknowledged the possibility of residual confounders affecting their results, and of the statistical problems with the very small sample size of modafinil-exposed pregnancies. Also, actual medication use – rather than prescription redemption – wasn’t captured in the study.
The study was partially funded by the Novo Nordisk Foundation. The authors reported no conflicts of interest.
SOURCE: Damkier P, Broe A. JAMA. 2020;323(4):374-6.
Modafinil exposure during pregnancy was associated with an approximately tripled risk of congenital malformations in a large Danish registry-based study.
Modafinil (Provigil) is commonly prescribed to address daytime sleepiness in narcolepsy and multiple sclerosis. An interim postmarketing safety analysis showed increased rates of major malformation in modafinil-exposed pregnancies, so the manufacturer issued an alert advising health care professionals of this safety signal in June 2019, wrote Per Damkier, MD, PhD, corresponding author of a JAMA research letter reporting the Danish study results. The postmarketing study had shown a major malformation rate of about 15% in modafinil-exposed pregnancies, much higher than the 3% background rate.
Dr. Damkier and Anne Broe, MD, PhD, both of the department of clinical biochemistry and pharmacology at Odense (Denmark) University Hospital, compared outcomes for pregnant women who were prescribed modafinil at any point during the first trimester of pregnancy with those who were prescribed an active comparator, methylphenidate, as well as with those who had neither exposure. Methylphenidate is not associated with congenital malformations and is used for indications similar to modafinil.
Looking at all pregnancies for whom complete records existed in Danish health registries between 2004 and 2017, the investigators found 49 modafinil-exposed pregnancies, 963 methylphenidate-exposed pregnancies, and 828,644 pregnancies with neither exposure.
Six major congenital malformations occurred in the modafinil-exposed group for an absolute risk of 12%. Major malformations occurred in 43 (4.5%) of the methylphenidate-exposed group and 32,466 (3.9%) of the unexposed group.
Using the extensive data available in public registries, the authors were able to perform logistic regression to adjust for concomitant use of other psychotropic medication; comorbidities such as diabetes and hypertension; and demographic and anthropometric measures such as maternal age, smoking status, and body mass index.
After this statistical adjustment, the researchers found that modafinil exposure during the first trimester of pregnancy was associated with an odds ratio of 3.4 (95% confidence interval, 1.2-9.7) for major congenital malformation, compared with first-trimester methylphenidate exposure. Compared with the unexposed cohort, modafinil-exposed pregnancies had an adjusted odds ratio of 2.7 (95% CI, 1.1-6.9) for major congenital malformation.
A total of 13 (27%) women who took modafinil had multiple sclerosis, but the authors excluded women who’d received a prescription for the multiple sclerosis drug teriflunomide (Aubagio), a known teratogen. Sleep disorders were reported for 39% of modafinil users, compared with 4.5% of methylphenidate users. Rates of psychoactive drug use were 41% for the modafinil group and 30% for the methylphenidate group.
The authors acknowledged the possibility of residual confounders affecting their results, and of the statistical problems with the very small sample size of modafinil-exposed pregnancies. Also, actual medication use – rather than prescription redemption – wasn’t captured in the study.
The study was partially funded by the Novo Nordisk Foundation. The authors reported no conflicts of interest.
SOURCE: Damkier P, Broe A. JAMA. 2020;323(4):374-6.
Modafinil exposure during pregnancy was associated with an approximately tripled risk of congenital malformations in a large Danish registry-based study.
Modafinil (Provigil) is commonly prescribed to address daytime sleepiness in narcolepsy and multiple sclerosis. An interim postmarketing safety analysis showed increased rates of major malformation in modafinil-exposed pregnancies, so the manufacturer issued an alert advising health care professionals of this safety signal in June 2019, wrote Per Damkier, MD, PhD, corresponding author of a JAMA research letter reporting the Danish study results. The postmarketing study had shown a major malformation rate of about 15% in modafinil-exposed pregnancies, much higher than the 3% background rate.
Dr. Damkier and Anne Broe, MD, PhD, both of the department of clinical biochemistry and pharmacology at Odense (Denmark) University Hospital, compared outcomes for pregnant women who were prescribed modafinil at any point during the first trimester of pregnancy with those who were prescribed an active comparator, methylphenidate, as well as with those who had neither exposure. Methylphenidate is not associated with congenital malformations and is used for indications similar to modafinil.
Looking at all pregnancies for whom complete records existed in Danish health registries between 2004 and 2017, the investigators found 49 modafinil-exposed pregnancies, 963 methylphenidate-exposed pregnancies, and 828,644 pregnancies with neither exposure.
Six major congenital malformations occurred in the modafinil-exposed group for an absolute risk of 12%. Major malformations occurred in 43 (4.5%) of the methylphenidate-exposed group and 32,466 (3.9%) of the unexposed group.
Using the extensive data available in public registries, the authors were able to perform logistic regression to adjust for concomitant use of other psychotropic medication; comorbidities such as diabetes and hypertension; and demographic and anthropometric measures such as maternal age, smoking status, and body mass index.
After this statistical adjustment, the researchers found that modafinil exposure during the first trimester of pregnancy was associated with an odds ratio of 3.4 (95% confidence interval, 1.2-9.7) for major congenital malformation, compared with first-trimester methylphenidate exposure. Compared with the unexposed cohort, modafinil-exposed pregnancies had an adjusted odds ratio of 2.7 (95% CI, 1.1-6.9) for major congenital malformation.
A total of 13 (27%) women who took modafinil had multiple sclerosis, but the authors excluded women who’d received a prescription for the multiple sclerosis drug teriflunomide (Aubagio), a known teratogen. Sleep disorders were reported for 39% of modafinil users, compared with 4.5% of methylphenidate users. Rates of psychoactive drug use were 41% for the modafinil group and 30% for the methylphenidate group.
The authors acknowledged the possibility of residual confounders affecting their results, and of the statistical problems with the very small sample size of modafinil-exposed pregnancies. Also, actual medication use – rather than prescription redemption – wasn’t captured in the study.
The study was partially funded by the Novo Nordisk Foundation. The authors reported no conflicts of interest.
SOURCE: Damkier P, Broe A. JAMA. 2020;323(4):374-6.
FROM JAMA
Evidence-based tools for premenstrual disorders
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
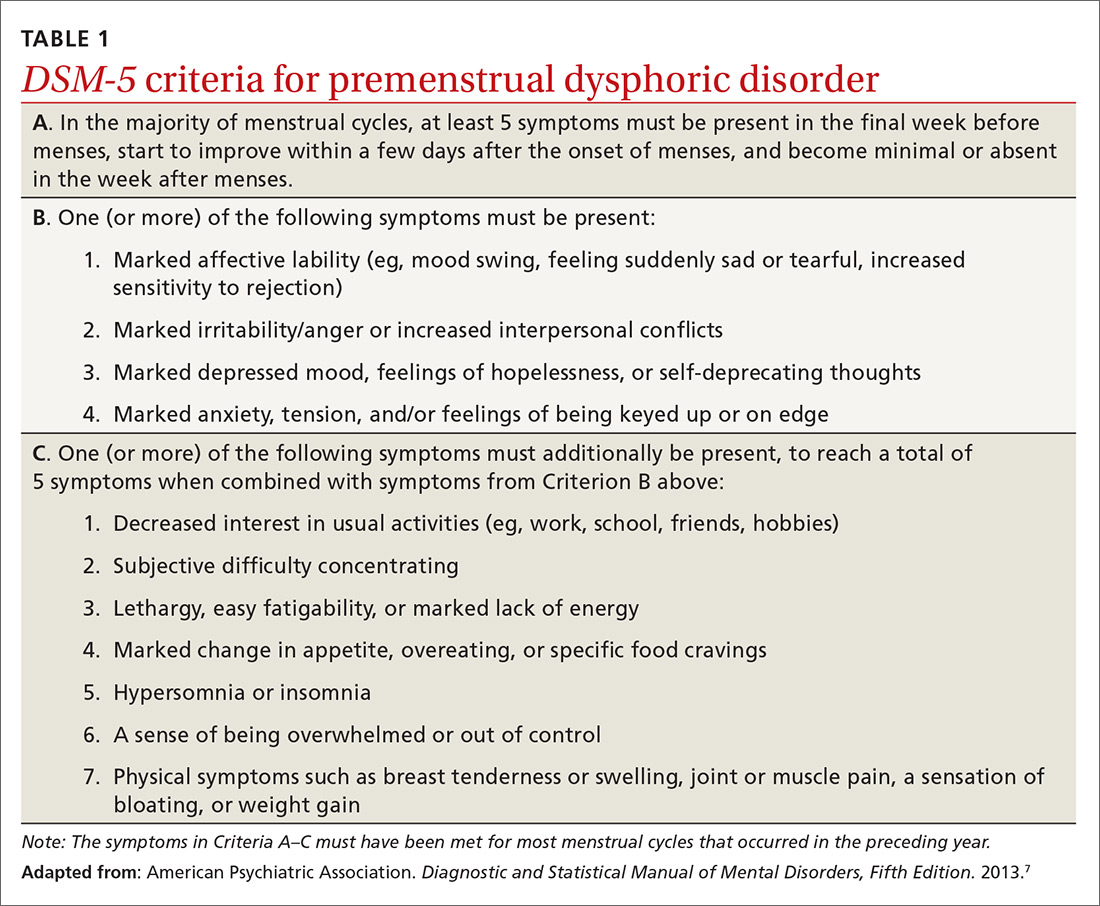
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7
Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
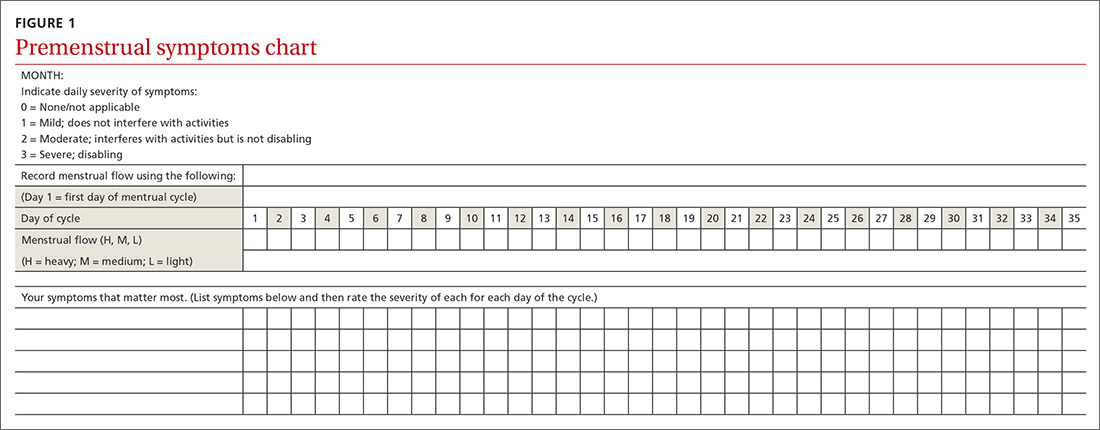
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.
Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
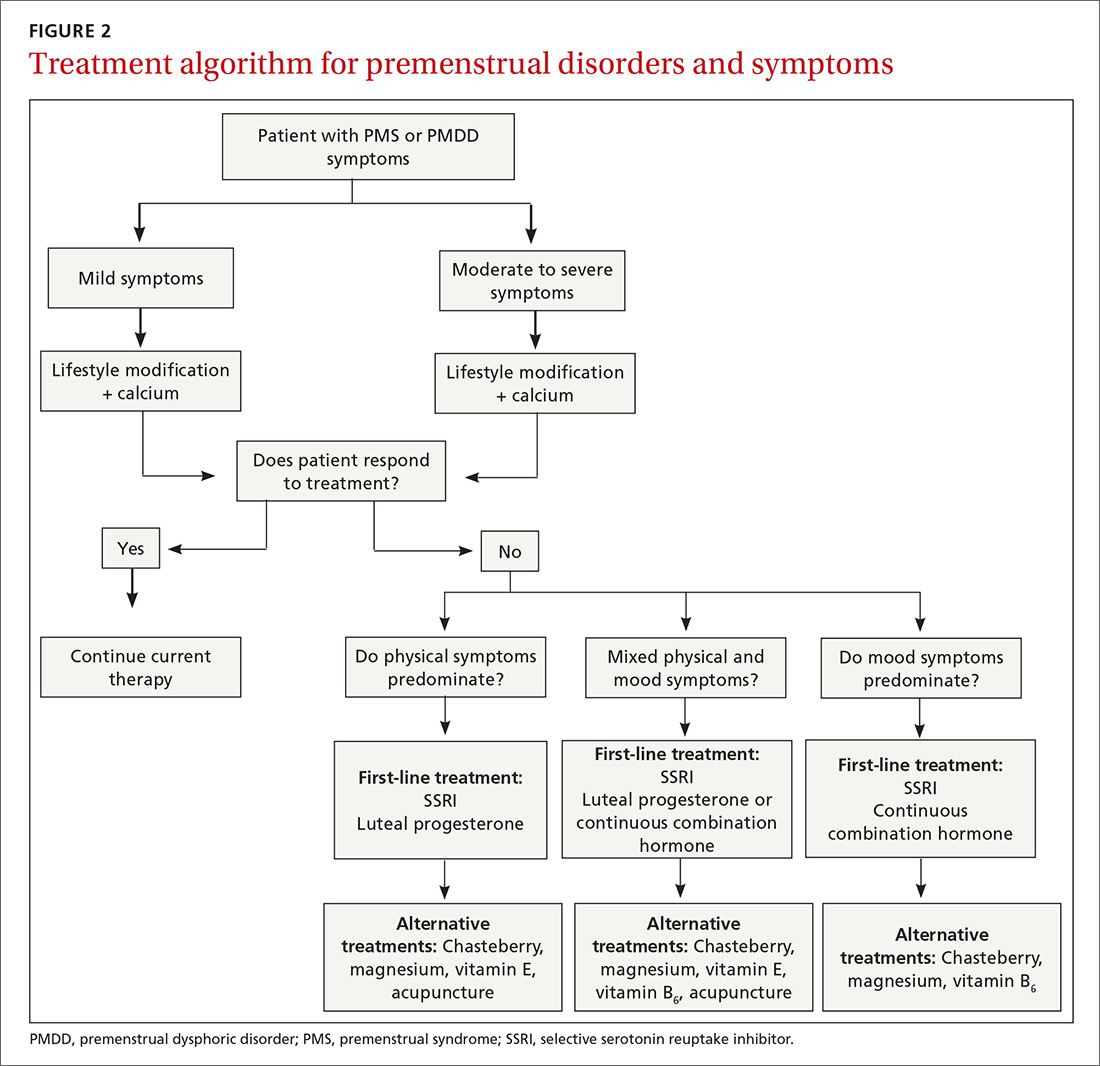
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness
Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
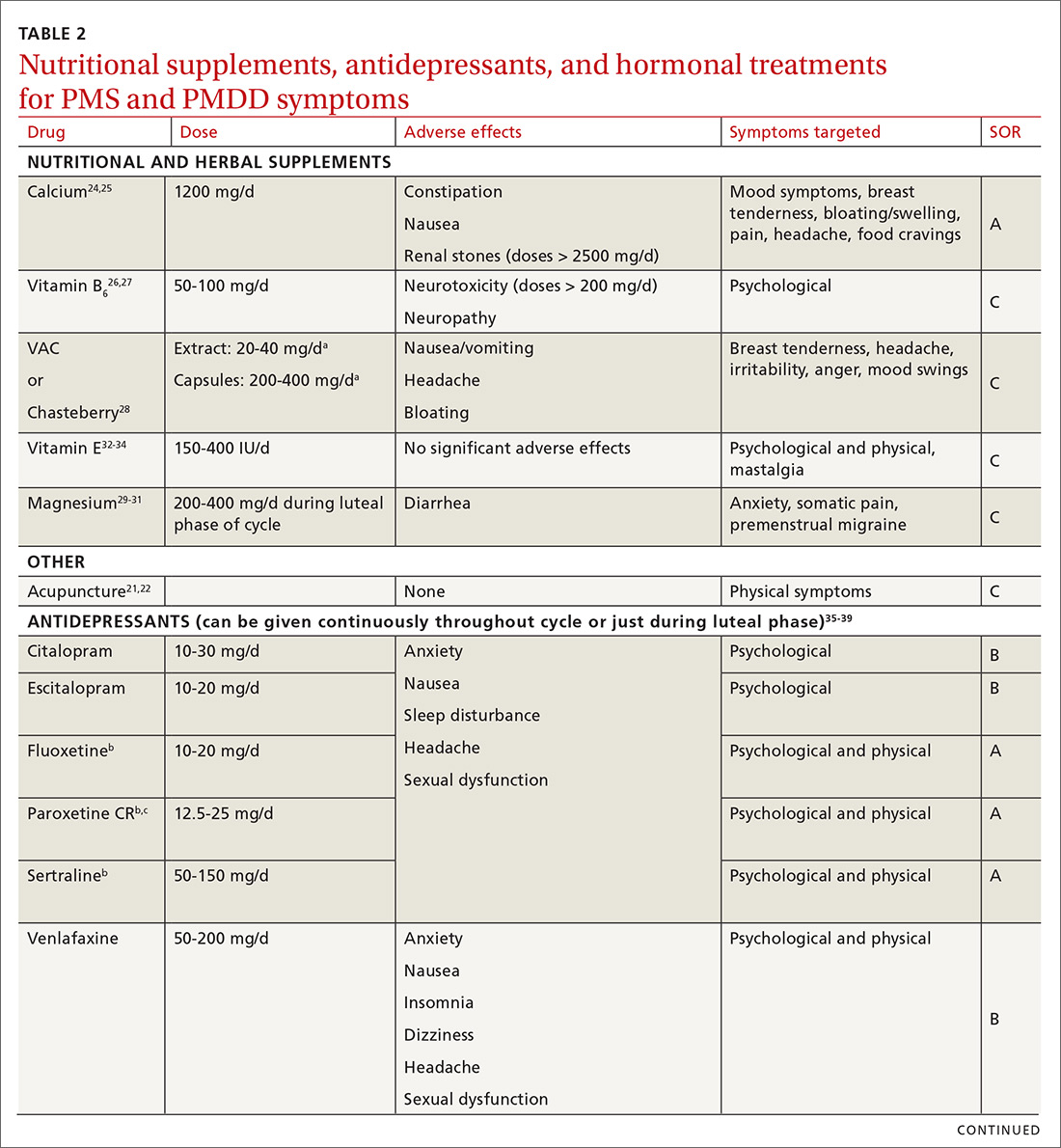
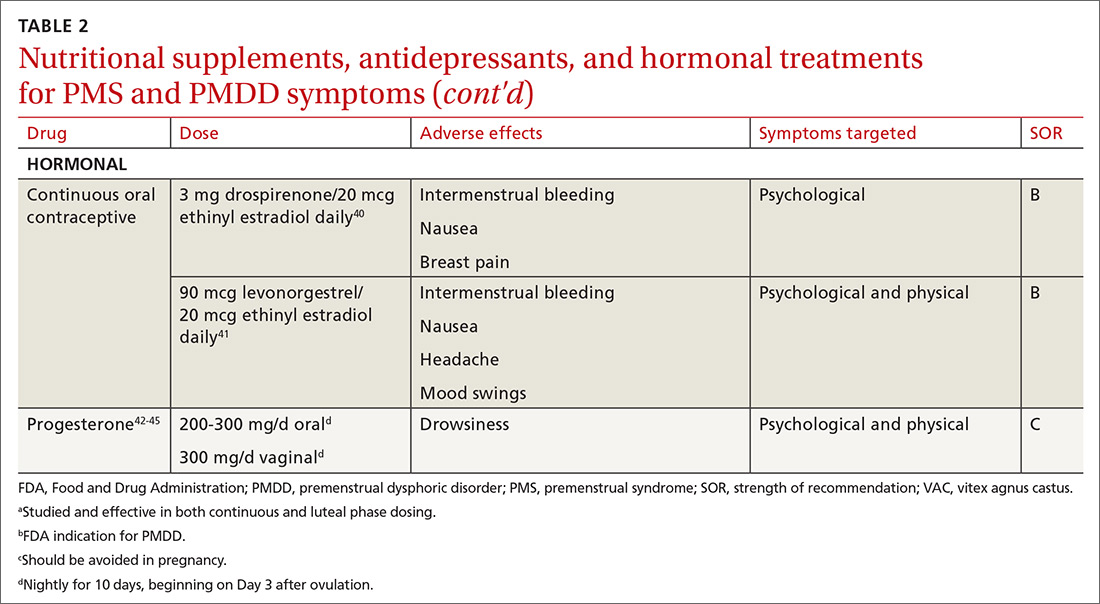
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25
Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27
Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; Peter.Danis@mercy.net.
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7
Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.
Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness
Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25
Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27
Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; Peter.Danis@mercy.net.
CASE
A 30-year-old G2P2 woman presents for a well-woman visit and reports 6 months of premenstrual symptoms including irritability, depression, breast pain, and headaches. She is not taking any medications or hormonal contraceptives. She is sexually active and currently not interested in becoming pregnant. She asks what you can do for her symptoms, as they are affecting her life at home and at work.
Symptoms and definitions vary
Although more than 150 premenstrual symptoms have been reported, the most common psychological and behavioral ones are mood swings, depression, anxiety, irritability, crying, social withdrawal, forgetfulness, and problems concentrating.1-3 The most common physical symptoms are fatigue, abdominal bloating, weight gain, breast tenderness, acne, change in appetite or food cravings, edema, headache, and gastrointestinal upset. The etiology of these symptoms is usually multifactorial, with some combination of hormonal, neurotransmitter, lifestyle, environmental, and psychosocial factors playing a role.
Premenstrual disorder. In reviewing diagnostic criteria for the various premenstrual syndromes and disorders from different organizations (eg, the International Society for Premenstrual Disorders; the American College of Obstetricians and Gynecologists; the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition), there is agreement on the following criteria for premenstrual syndrome (PMS)4-6:
- The woman must be ovulating. (Women who no longer menstruate [eg, because of hysterectomy or endometrial ablation] can have premenstrual disorders as long as ovarian function remains intact.)
- The woman experiences a constellation of disabling physical and/or psychological symptoms that appears in the luteal phase of her menstrual cycle.
- The symptoms improve soon after the onset of menses.
- There is a symptom-free interval before ovulation.
- There is prospective documentation of symptoms for at least 2 consecutive cycles.
- The symptoms are sufficient in severity to affect activities of daily living and/or important relationships.
Premenstrual dysphoric disorder. PMDD is another common premenstrual disorder. It is distinguished by significant premenstrual psychological symptoms and requires the presence of marked affective lability, marked irritability or anger, markedly depressed mood, and/or marked anxiety (TABLE 1).7
Exacerbation of other ailments. Another premenstrual disorder is the premenstrual exacerbation of underlying chronic medical or psychological problems such as migraines, seizures, asthma, diabetes, irritable bowel syndrome, fibromyalgia, anxiety, or depression.
Differences in interpretation lead to variations in prevalence
Differences in the interpretation of significant premenstrual symptoms have led to variations in estimated prevalence. For example, 80% to 95% of women report premenstrual symptoms, but only 30% to 40% meet criteria for PMS and only 3% to 8% meet criteria for PMDD.8 Many women who report premenstrual symptoms in a retrospective questionnaire do not meet criteria for PMS or PMDD based on prospective symptom charting. The Daily Record of Severity of Problems (DRSP), a prospective tracking tool for premenstrual symptoms, is sensitive and specific for diagnosing PMS and PMDD if administered on the first day of menstruation.9
Ask about symptoms and use a tracking tool
When you see a woman for a well-woman visit or a gynecologic problem, inquire about physical/emotional symptoms and their severity during the week that precedes menstruation. If a patient reports few symptoms of a mild nature, then no further work-up is needed.
Continue to: If patients report significant...
If patients report significant premenstrual symptoms, recommend the use of a tool to track the symptoms. Older tools such as the DRSP and the Premenstrual Symptoms Screening Tool (PSST), newer symptom diaries that can be used for both PMS and PMDD,and questionnaires that have been used in research situations can be time consuming and difficult for patients to complete.10-12 Instead, physicians can easily construct their own charting tool, as we did for patients to use when tracking their most bothersome symptoms (FIGURE 1). Tracking helps to confirm the diagnosis and helps you and the patient focus on treatment goals.
Keep in mind other diagnoses (eg, anemia, thyroid disorders, perimenopause, anxiety, depression, eating disorders, substance abuse) that can cause or exacerbate the psychological/physical symptoms the patient is reporting. If you suspect any of these other diagnoses, laboratory evaluation (eg, complete blood count, thyroid-stimulating hormone level or other hormonal testing, urine drug screen, etc) may be warranted to rule out other etiologies for the reported symptoms.
Develop a Tx plan that considers symptoms, family-planning needs
Focus treatment on the patient’s predominant symptoms whether they are physical, psychological, or mixed (FIGURE 2). The patient’s preferences regarding family planning are another important consideration. Women who are using a fertility awareness
Although the definitions for PMS and PMDD require at least 2 cycles of prospective documentation of symptoms, dietary and lifestyle changes can begin immediately. Regular follow-up to document improvement of symptoms is important; using the patient’s symptoms charting tool can help with this.
Focus on diet and lifestyle right away
Experts in the field of PMS/PMDD suggest that simple dietary changes may be a reasonable first step to help improve symptoms. Researchers have found that diets high in fiber, vegetables, and whole grains are inversely related to PMS.13 Older studies have suggested an increased prevalence and severity of PMS with increased caffeine intake; however, a newer study found no such association.14
Continue to: A case-control study nested...
A case-control study nested within the Nurses’ Health Study II cohort showed that a high intake of both dietary calcium and vitamin D prevented the development of PMS in women ages 27 to 44.15 B vitamins, such as thiamine and riboflavin, from food sources have been associated with a lower risk of PMS.16 A variety of older clinical studies showed benefit from aerobic exercise on PMS symptoms,17-19 but a newer cross-sectional study of young adult women found no association between physical activity and the prevalence of PMS.20 Acupuncture has demonstrated efficacy for the treatment of the physical symptoms of PMS and PMDD, but more rigorous studies are needed.21,22 Cognitive behavioral therapy has been studied as a treatment, but data to support this approach are limited so it cannot be recommended at this time.23
Make the most of supplements—especially calcium
Calcium is the nutritional supplement with the most evidence to support its use to relieve symptoms of PMS and PMDD (TABLE 221,22,24-45). Research indicates that disturbances in calcium regulation and calcium deficiency may be responsible for various premenstrual symptoms. One study showed that, compared with placebo, women who took 1200 mg/d calcium carbonate for 3 menstrual cycles had a 48% decrease in both somatic and affective symptoms.24 Another trial demonstrated improvement in PMS symptoms of early tiredness, appetite changes, and depression with calcium therapy.25
Pyridoxine (vitamin B6) has potential benefit in treating PMS due to its ability to increase levels of serotonin, norepinephrine, histamine, dopamine, and taurine.26 An older systematic review showed benefit for symptoms associated with PMS, but the authors concluded that larger randomized controlled trials (RCTs) were needed before definitive recommendations could be made.27
Chasteberry. A number of studies have evaluated the effect of vitex agnus castus (VAC), commonly referred to as chasteberry, on PMS and PMDD symptoms. The exact mechanism of VAC is unknown, but in vitro studies show binding of VAC extracts to dopamine-2 receptors and opioid receptors, and an affinity for estrogen receptors.28
A recent meta-analysis concluded that VAC extracts are not superior to selective serotonin reuptake inhibitors (SSRIs) or oral contraceptives (OCs) for PMS/PMDD.28 The authors suggested a possible benefit of VAC compared with placebo or other nutritional supplements; however, the studies supporting its use are limited by small sample size and potential bias.
Continue to: Magnesium
Magnesium. Many small studies have evaluated the role of other herbal and nutritional supplements for the treatment of PMS/PMDD. A systematic review of studies on the effect of magnesium supplementation on anxiety and stress showed that magnesium may have a potential role in the treatment of the premenstrual symptom of anxiety.29 Other studies have demonstrated a potential role in the treatment of premenstrual migraine.30,31
Vitamin E has demonstrated benefit in the treatment of cyclic mastalgia; however, evidence for using vitamin E for mood and depressive symptoms associated with PMS and PMDD is inconsistent.32-34 Other studies involving vitamin D, St. John’s wort, black cohosh, evening primrose oil, saffron, and ginkgo biloba either showed these agents to be nonefficacious in relieving PMS/PMDD symptoms or to require more data before they can be recommended for use.34,46
Patient doesn’t respond? Start an SSRI
Pharmacotherapy with antidepressants is typically reserved for those who do not respond to nonpharmacologic therapies and are experiencing more moderate to severe symptoms of PMS or PMDD. Reduced levels of serotonin and serotonergic activity in the brain may be linked to symptoms of PMS and PMDD.47 Studies have shown SSRIs to be effective in reducing many psychological symptoms (eg, depression, anxiety, lethargy, irritability) and some physical symptoms (eg, headache, breast tenderness, muscle or joint pain) associated with PMS and PMDD.
A Cochrane review of 31 RCTs compared various SSRIs to placebo. When taken either continuously or intermittently (administration during luteal phase), SSRIs were similarly effective in relieving symptoms when compared with placebo.35 Psychological symptoms are more likely to improve with both low and moderate doses of SSRIs, while physical symptoms may only improve with moderate or higher doses. A direct comparison of the various SSRIs for the treatment of PMS or PMDD is lacking; therefore, the selection of SSRI may be based on patient characteristics and preference.
The benefits of SSRIs are noted much earlier in the treatment of PMS/PMDD than they are observed in their use for depression or anxiety.36 This suggests that the mechanism by which SSRIs relieve PMS/PMDD symptoms is different than that for depression or anxiety. Intermittent dosing capitalizes upon the rapid effect seen with these medications and the cyclical nature of these disorders. In most studies, the benefit of intermittent dosing is similar to continuous dosing; however, one meta-analysis did note that continuous dosing had a larger effect.37
Continue to: The doses of SSRIs...
The doses of SSRIs used in most PMS/PMDD trials were lower than those typically used for the treatment of depression and anxiety. The withdrawal effect that can be seen with abrupt cessation of SSRIs has not been reported in the intermittent-dosing studies for PMS/PMDD.38 While this might imply a more tolerable safety profile, the most common adverse effects reported in trials were still as expected: sleep disturbances, headache, nausea, and sexual dysfunction. It is important to note that SSRIs should be used with caution during pregnancy, and paroxetine should be avoided in women considering pregnancy in the near future.
Other antidepressant classes have been studied to a lesser extent than SSRIs. Continuously dosed venlafaxine, a serotonin and norepinephrine reuptake inhibitor, demonstrated efficacy in PMS/PMDD treatment when compared with placebo within the first cycle of therapy.39 The response seen was comparable to that associated with SSRI treatments in other trials.
Buspirone, an anxiolytic with serotonin receptor activity that is different from that of the SSRIs, demonstrated efficacy in reducing the symptom of irritability.48 Buspirone may have a role to play in those presenting with irritability as a primary symptom or in those who are unable to tolerate the adverse effects of SSRIs. Tricyclic antidepressants, bupropion, and alprazolam have either limited data regarding efficacy or are associated with adverse effects that limit their use.38
Hormonal treatments may be worth considering
One commonly prescribed hormonal therapy for PMS and PMDD is continuous OCs. A 2012 Cochrane review of OCs containing drospirenone evaluated 5 trials and a total of 1920 women.40 Two placebo-controlled trials of women with severe premenstrual symptoms (PMDD) showed improvement after 3 months of taking daily drospirenone 3 mg with ethinyl estradiol 20 mcg, compared with placebo.
While experiencing greater benefit, these groups also experienced significantly more adverse effects including nausea, intermenstrual bleeding, and breast pain. The respective odds ratios for the 3 adverse effects were 3.15 (95% confidence interval [CI], 1.90-5.22), 4.92 (95% CI, 3.03-7.96), and 2.67 (95% CI, 1.50-4.78). The review concluded that drospirenone 3 mg with ethinyl estradiol 20 mcg may help in the treatment of severe premenstrual symptoms (PMDD) but that it is unknown whether this treatment is appropriate for patients with less severe premenstrual symptoms.
Continue to: Another multicenter RCT
Another multicenter RCT evaluated women with PMDD who received levonorgestrel 90 mcg with ethinyl estradiol 20 mcg or placebo daily for 112 days.41 Symptoms were recorded utilizing the DRSP. Significantly more women taking the daily combination hormone (52%) than placebo (40%) had a positive response (≥ 50% improvement in the DRSP 7-day late luteal phase score and Clinical Global Impression of Severity score of ≥ 1 improvement, evaluated at the last “on-therapy” cycle [P = .025]). Twenty-three of 186 patients in the treatment arm dropped out because of adverse effects.
Noncontraceptive estrogen-containing preparations. Hormone therapy preparations containing lower doses of estrogen than seen in OC preparations have also been studied for PMS management. A 2017 Cochrane review of noncontraceptive estrogen-containing preparations found very low-quality evidence to support the effectiveness of continuous estrogen (transdermal patches or subcutaneous implants) plus progestogen.49
Progesterone. The cyclic use of progesterone in the luteal phase has been reviewed as a hormonal treatment for PMS. A 2012 Cochrane review of the efficacy of progesterone for PMS was inconclusive; however, route of administration, dose, and duration differed across studies.42
Another systematic review of 10 trials involving 531 women concluded that progesterone was no better than placebo in the treatment of PMS.43 However, it should be noted that each trial evaluated a different dose of progesterone, and all but 1 of the trials administered progesterone by using the calendar method to predict the beginning of the luteal phase. The only trial to use an objective confirmation of ovulation prior to beginning progesterone therapy did demonstrate significant improvement in premenstrual symptoms.
This 1985 study by Dennerstein et al44 prescribed progesterone for 10 days of each menstrual cycle starting 3 days after ovulation. In each cycle, ovulation was confirmed by determinations of urinary 24-hour pregnanediol and total estrogen concentrations. Progesterone was then prescribed during the objectively identified luteal phase, resulting in significant improvement in symptoms.
Continue to: Another study evaluated...
Another study evaluated the post-ovulatory progesterone profiles of 77 women with symptoms of PMS and found lower levels of progesterone and a sharper rate of decline in the women with PMS vs the control group.45 Subsequent progesterone treatment during the objectively identified luteal phase significantly improved PMS symptoms. These studies would seem to suggest that progesterone replacement when administered during an objectively identified luteal phase may offer some benefit in the treatment of PMS, but larger RCTs are needed to confirm this.
CASE
You provide the patient with diet and lifestyle education as well as a recommendation for calcium supplementation. The patient agrees to prospectively chart her most significant premenstrual symptoms. You review additional treatment options including SSRI medications and hormonal approaches. She is using a fertility awareness–based method of family planning that allows her to confidently identify her luteal phase. She agrees to take sertraline 50 mg/d during the luteal phase of her cycle. At her follow-up office visit 3 months later, she reports improvement in her premenstrual symptoms. Her charting of symptoms confirms this.
CORRESPONDENCE
Peter Danis, MD, Mercy Family Medicine St. Louis, 12680 Olive Boulevard, St. Louis, MO 63141; Peter.Danis@mercy.net.
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
1. Woods NF, Most A, Dery GK. Prevalence of perimenstrual symptoms. Am J Public Health. 1982;72:1257-1264.
2. Johnson SR, McChesney C, Bean JA. Epidemiology of premenstrual symptoms in a nonclinical sample. 1. Prevalence, natural history and help-seeking behavior. J Repro Med. 1988;33:340-346.
3. Campbell EM, Peterkin D, O’Grady K, et al. Premenstrual symptoms in general practice patients. Prevalence and treatment. J Reprod Med. 1997;42:637-646.
4. O’Brien PM, Bäckström T, Brown C, et al. Towards a consensus on diagnostic criteria, measurement, and trial design of the premenstrual disorders: the ISPMD Montreal consensus. Arch Womens Ment Health. 2011;14:13-21.
5. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169:465-475.
6. American College of Obstetricians and Gynecologists. Guidelines for Women’s Health Care: A Resource Manual. 4th ed. Washington, DC: American College of Obstetricians and Gynecologists; 2014:607-613.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013.
8. Dennerstein L, Lehert P, Heinemann K. Epidemiology of premenstrual symptoms and disorders. Menopause Int. 2012;18:48-51.
9. Borenstein JE, Dean BB, Yonkers KA, et al. Using the daily record of severity of problems as a screening instrument for premenstrual syndrome. Obstet Gynecol. 2007;109:1068-1075.
10. Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203-209.
11. Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41-49.
12. Janda C, Kues JN, Andersson G, et al. A symptom diary to assess severe premenstrual syndrome and premenstrual dysphoric disorder. Women Health. 2017;57:837-854.
13. Farasati N, Siassi F, Koohdani F, et al. Western dietary pattern is related to premenstrual syndrome: a case-control study. Brit J Nutr. 2015;114:2016-2021.
14. Purdue-Smithe AC, Manson JE, Hankinson SE, et al. A prospective study of caffeine and coffee intake and premenstrual syndrome. Am J Clin Nutr. 2016;104:499-507.
15. Bertone-Johnson ER, Hankinson SE, Bendich A, et al. Calcium and vitamin D intake and risk of incident premenstrual syndrome. Arch Intern Med. 2005;165:1246-1252.
16. Chocano-Bedoya PO, Manson JE, Hankinson SE, et al. Dietary B vitamin intake and incident premenstrual syndrome. Am J Clin Nutr. 2011;93:1080-1086.
17. Prior JC, Vigna Y. Conditioning exercise and premenstrual symptoms. J Reprod Med. 1987;32:423-428.
18. Aganoff JA, Boyle GJ. Aerobic exercise, mood states, and menstrual cycle symptoms. J Psychosom Res. 1994;38:183-192.
19. El-Lithy A, El-Mazny A, Sabbour A, et al. Effect of aerobic exercise on premenstrual symptoms, haematological and hormonal parameters in young women. J Obstet Gynaecol. 2015;35:389-392.
20. Kroll-Desrosiers AR, Ronnenberg AG, Zagarins SE, et al. Recreational physical activity and premenstrual syndrome in young adult women: a cross-sectional study. PLoS One. 2017;12:1-13.
21. Jang SH, Kim DI, Choi MS. Effects and treatment methods of acupuncture and herbal medicine for premenstrual syndrome/premenstrual dysphoric disorder: systematic review. BMC Complement Altern Med. 2014;14:11.
22. Kim SY, Park HJ, Lee H, et al. Acupuncture for premenstrual syndrome: a systematic review and meta-analysis of randomized controlled trials. BJOG. 2011;118:899-915.
23. Lustyk MK, Gerrish WG, Shaver S, et al. Cognitive-behavioral therapy for premenstrual syndrome and premenstrual dysphoric disorder: a systematic review. Arch Womens Ment Health. 2009;12:85-96.
24. Thys-Jacob S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual syndromes. Am J Obstet Gynecol. 1998;179:444-452.
25. Ghanbari Z, Haghollahi F, Shariat M, et al. Effects of calcium supplement therapy in women with premenstrual syndrome. Taiwan J Obstet Gynecol. 2009;48:124-129.
26. Girman A, Lee R, Kligler B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol. 2003;188(5 suppl):s56-s65.
27. Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ. 1999;318:1375-1381.
28. Verkaik S, Kamperman AM, van Westrhenen R, et al. The treatment of premenstrual syndrome with preparations of vitex agnus castus: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:150-166.
29. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress—a systematic review. Nutrients. 2017;9:429-450.
30. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium levels and serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache. 2002;42:242-248.
31. Facchinetti F, Sances C, Borella P, et al. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache. 1991;31:298-301.
32. Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
33. London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med. 1987;32:400-404.
34. Whelan AM, Jurgens TM, Naylor H. Herbs, vitamins, and minerals in the treatment of premenstrual syndrome: a systematic review. Can J Clin Pharmacol. 2009;16:e407-e429.
, , , . Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2013;(6): CD001396.
36. Dimmock P, Wyatt K, Jones P, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet. 2000;356:1131-1136.
37. Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder. Obstet Gynecol. 2008;111:1175-1182.
38. Freeman EW. Luteal phase administration of agents for the treatment of premenstrual dysphoric disorder. CNS Drugs. 2004;18:453-468.
39. Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol. 2001;98:737-744.
40. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(2):CD006586.
41. Halbreich U, Freeman EW, Rapkin AJ, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85:19-27.
, , , . Progesterone for premenstrual syndrome. Cochrane Database Syst Rev. 2012;(3):CD003415.
43. Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ. 2001;323: 776-780.
44. Dennerstein L, Spencer-Gardner C, Gotts G, et al. Progesterone and the premenstrual syndrome: a double-blind crossover trial. Br Med J (Clin Res Ed). 1985;290:1617-1621.
45. NaProTECHNOLOGY. The Medical and Surgical Practice of NaProTECHNOLOGY. Premenstrual Syndrome: Evaluation and Treatment. Omaha, NE: Pope Paul VI Institute Press. 2004;29:345-368. https://www.naprotechnology.com/naprotext.htm. Accessed January 23, 2020.
46. Dante G, Facchinetti F. Herbal treatments for alleviating premenstrual symptoms: a systematic review. J Psychosom Obstet Gynaecol. 2011;32:42-51.
47. Jarvis CI, Lynch AM, Morin AK. Management strategies for premenstrual syndrome/premenstrual dysphoric disorder. Ann Pharmacother. 2008;42:967-978.
48. Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl). 2001;155:292-298.
, , , . Non-contraceptive oestrogen-containing preparations for controlling symptoms of premenstrual syndrome . Cochrane Database Syst Rev . 2017 ;( 3) :CD010503.
PRACTICE RECOMMENDATIONS
› Start calcium supplementation in all patients who report significant premenstrual symptoms. A
› Add a selective serotonin reuptake inhibitor (SSRI) to calcium supplementationfor patients who have more severe premenstrual psychological symptoms. A
› Consider hormonal treatment options for patients who require treatment beyond calcium and an SSRI. B
› Provide nutrition and exercise information to all patients who report significant premenstrual symptoms. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Depression after miscarriage: Follow-up care is key
A Washington Post article on depression after miscarriage is a reminder that, although couples can suffer deeply from such a loss, there still are ways to provide them with meaningful support (“After miscarriage, I was rocked by depression. Like many other women, I didn’t get follow-up care for this loss,” by Katie C. Reilly, Nov 30, 2019).
Psychiatrists who focus on reproductive psychiatry and collaborative care are trying to change the current therapeutic landscape and improve practitioner awareness and treatment. Ob.gyns. managing patients who have experienced reproductive loss, especially early-term loss, may not immediately refer couples to a therapist or psychiatrist, but we can change this. Practitioners who focus on reproductive health – both physical and mental – are trying to better understand such couples’ experiences, increase their access to care, develop preventative care strategies, and improve provider education.
At the outset, providers who treat patients who have experienced a perinatal loss must recognize that not all individuals will feel that a loss is tragic. Instead, patient reactions occur along a spectrum, and there is no “correct” way to process a loss. A couple’s reaction may depend on a variety of factors, including how late or early in pregnancy the loss occurs, whether the pregnancy is planned or unplanned, and what other psychosocial stressors, such as unstable housing, limited income, and few social supports, may exist. Not every patient experiencing grief, even profoundly, will shed tears; we need to be open to all potential reactions and be mindful when a person may need additional support.
According to the Washington Post article, even though 50% of miscarriages are due to chromosomal abnormalities, women still feel ultimately responsible for the loss. As a society we are bombarded with “experts” in the media telling us the best way, the right way, the healthiest way to live. This barrage of advice distorts our views of what it really means to be a good parent and subtly conveys the idea that mothers are solely responsible for any bad pregnancy outcomes. I remember being fearful of causing unintentional harm to my unborn baby during my own pregnancy. What if I accidentally ate something that would affect her development? Is exposure to second-hand smoke as I walk down the street harming her? How bad would it be if I just had one cup of coffee? My doubts caused quite a bit of distress for me, which is a mild form of the distress I see when counseling couples after their miscarriages.
The article’s author also expressed concern about the emotional sterility of the environment in which miscarriages usually occur: a hospital ED. EDs are designed to promote a level of detachment and to quell any stress for the clinicians so that they can calmly handle unexpected health crises. EDs are not primarily designed to provide patients with emotional support, nor should they be. However, we still can make some improvements to existing ED design to better address couples’ emotional needs. For example, some EDs have placed mental health clinicians on staff, others call patients post discharge to address concerns, and some EDs even provide patients access to mental health trauma teams. Such services are not found in all EDs, and even those that exist may just scratch the surface of what is needed, but they are a step in the right direction. Providing this level of auxiliary care directly from the ED increases patients’ ability to access mental health support in the place where miscarriages are most likely to be first diagnosed and managed.
The American College of Obstetricians and Gynecologists already is trying to fill in the missing pieces when it comes to identifying mood symptoms following miscarriage. One of the key recommendations from the May 2018 Committee Opinion on Redefining the Postpartum Visit is that every woman who has experienced a miscarriage, stillbirth, or neonatal death should receive follow-up care. Mental health is a suggested component of the postpartum care plan. Some outpatient ob.gyn. practices and inpatient units are using screening tools to identify postpartum depression. For example, the Edinburgh Postnatal Depression Scale can be utilized following a miscarriage to help providers identify symptoms of depression and anxiety.
However, The trend in psychiatry over the past decade has been toward collaborative care, models that embed psychiatrists and other mental health clinicians in ob.gyn. practices to help guide the diagnosis and treatment of mental health problems. Some psychiatrists practice a co-located model in which they see patients alongside their ob.gyn. colleagues, whereas other psychiatrists treat a larger number of patients by using chart reviews for medication management while relying on behavioral health care managers for counseling and monitoring. Using this model of mental health care, more patients have access to services that are provided in a location familiar to them.
Another step in the right direction is the October 2019 launch of The National Curriculum in Reproductive Psychiatry (NCRP), which provides free educational material for psychiatry faculty and residents to enhance education on topics related to reproductive psychiatry, including miscarriage, loss, and development of trauma disorders. NCRP aspires to develop educational materials for ob.gyn. residents.
In the past we may have missed the mark in recognizing and treating the trauma that prenatal loss can cause, but we are trying to improve our approaches. More and more couples are sharing their experiences and advocating for themselves and others, often creating change in medical practice, and doctors are starting to listen. As any clinician knows, changes to standards of care can take several years to disseminate into general practice, but this gap between knowledge and treatment is now in the forefront of our minds. I am hopeful that we will continue to make advances and provide better care to our patients who have endured the loss of a pregnancy.
Dr. Latorre is an assistant professor in the department of psychiatry at the University of Maryland School of Medicine. She has reported no relevant financial disclosures. Email her at obnews@mdedge.com.
A Washington Post article on depression after miscarriage is a reminder that, although couples can suffer deeply from such a loss, there still are ways to provide them with meaningful support (“After miscarriage, I was rocked by depression. Like many other women, I didn’t get follow-up care for this loss,” by Katie C. Reilly, Nov 30, 2019).
Psychiatrists who focus on reproductive psychiatry and collaborative care are trying to change the current therapeutic landscape and improve practitioner awareness and treatment. Ob.gyns. managing patients who have experienced reproductive loss, especially early-term loss, may not immediately refer couples to a therapist or psychiatrist, but we can change this. Practitioners who focus on reproductive health – both physical and mental – are trying to better understand such couples’ experiences, increase their access to care, develop preventative care strategies, and improve provider education.
At the outset, providers who treat patients who have experienced a perinatal loss must recognize that not all individuals will feel that a loss is tragic. Instead, patient reactions occur along a spectrum, and there is no “correct” way to process a loss. A couple’s reaction may depend on a variety of factors, including how late or early in pregnancy the loss occurs, whether the pregnancy is planned or unplanned, and what other psychosocial stressors, such as unstable housing, limited income, and few social supports, may exist. Not every patient experiencing grief, even profoundly, will shed tears; we need to be open to all potential reactions and be mindful when a person may need additional support.
According to the Washington Post article, even though 50% of miscarriages are due to chromosomal abnormalities, women still feel ultimately responsible for the loss. As a society we are bombarded with “experts” in the media telling us the best way, the right way, the healthiest way to live. This barrage of advice distorts our views of what it really means to be a good parent and subtly conveys the idea that mothers are solely responsible for any bad pregnancy outcomes. I remember being fearful of causing unintentional harm to my unborn baby during my own pregnancy. What if I accidentally ate something that would affect her development? Is exposure to second-hand smoke as I walk down the street harming her? How bad would it be if I just had one cup of coffee? My doubts caused quite a bit of distress for me, which is a mild form of the distress I see when counseling couples after their miscarriages.
The article’s author also expressed concern about the emotional sterility of the environment in which miscarriages usually occur: a hospital ED. EDs are designed to promote a level of detachment and to quell any stress for the clinicians so that they can calmly handle unexpected health crises. EDs are not primarily designed to provide patients with emotional support, nor should they be. However, we still can make some improvements to existing ED design to better address couples’ emotional needs. For example, some EDs have placed mental health clinicians on staff, others call patients post discharge to address concerns, and some EDs even provide patients access to mental health trauma teams. Such services are not found in all EDs, and even those that exist may just scratch the surface of what is needed, but they are a step in the right direction. Providing this level of auxiliary care directly from the ED increases patients’ ability to access mental health support in the place where miscarriages are most likely to be first diagnosed and managed.
The American College of Obstetricians and Gynecologists already is trying to fill in the missing pieces when it comes to identifying mood symptoms following miscarriage. One of the key recommendations from the May 2018 Committee Opinion on Redefining the Postpartum Visit is that every woman who has experienced a miscarriage, stillbirth, or neonatal death should receive follow-up care. Mental health is a suggested component of the postpartum care plan. Some outpatient ob.gyn. practices and inpatient units are using screening tools to identify postpartum depression. For example, the Edinburgh Postnatal Depression Scale can be utilized following a miscarriage to help providers identify symptoms of depression and anxiety.
However, The trend in psychiatry over the past decade has been toward collaborative care, models that embed psychiatrists and other mental health clinicians in ob.gyn. practices to help guide the diagnosis and treatment of mental health problems. Some psychiatrists practice a co-located model in which they see patients alongside their ob.gyn. colleagues, whereas other psychiatrists treat a larger number of patients by using chart reviews for medication management while relying on behavioral health care managers for counseling and monitoring. Using this model of mental health care, more patients have access to services that are provided in a location familiar to them.
Another step in the right direction is the October 2019 launch of The National Curriculum in Reproductive Psychiatry (NCRP), which provides free educational material for psychiatry faculty and residents to enhance education on topics related to reproductive psychiatry, including miscarriage, loss, and development of trauma disorders. NCRP aspires to develop educational materials for ob.gyn. residents.
In the past we may have missed the mark in recognizing and treating the trauma that prenatal loss can cause, but we are trying to improve our approaches. More and more couples are sharing their experiences and advocating for themselves and others, often creating change in medical practice, and doctors are starting to listen. As any clinician knows, changes to standards of care can take several years to disseminate into general practice, but this gap between knowledge and treatment is now in the forefront of our minds. I am hopeful that we will continue to make advances and provide better care to our patients who have endured the loss of a pregnancy.
Dr. Latorre is an assistant professor in the department of psychiatry at the University of Maryland School of Medicine. She has reported no relevant financial disclosures. Email her at obnews@mdedge.com.
A Washington Post article on depression after miscarriage is a reminder that, although couples can suffer deeply from such a loss, there still are ways to provide them with meaningful support (“After miscarriage, I was rocked by depression. Like many other women, I didn’t get follow-up care for this loss,” by Katie C. Reilly, Nov 30, 2019).
Psychiatrists who focus on reproductive psychiatry and collaborative care are trying to change the current therapeutic landscape and improve practitioner awareness and treatment. Ob.gyns. managing patients who have experienced reproductive loss, especially early-term loss, may not immediately refer couples to a therapist or psychiatrist, but we can change this. Practitioners who focus on reproductive health – both physical and mental – are trying to better understand such couples’ experiences, increase their access to care, develop preventative care strategies, and improve provider education.
At the outset, providers who treat patients who have experienced a perinatal loss must recognize that not all individuals will feel that a loss is tragic. Instead, patient reactions occur along a spectrum, and there is no “correct” way to process a loss. A couple’s reaction may depend on a variety of factors, including how late or early in pregnancy the loss occurs, whether the pregnancy is planned or unplanned, and what other psychosocial stressors, such as unstable housing, limited income, and few social supports, may exist. Not every patient experiencing grief, even profoundly, will shed tears; we need to be open to all potential reactions and be mindful when a person may need additional support.
According to the Washington Post article, even though 50% of miscarriages are due to chromosomal abnormalities, women still feel ultimately responsible for the loss. As a society we are bombarded with “experts” in the media telling us the best way, the right way, the healthiest way to live. This barrage of advice distorts our views of what it really means to be a good parent and subtly conveys the idea that mothers are solely responsible for any bad pregnancy outcomes. I remember being fearful of causing unintentional harm to my unborn baby during my own pregnancy. What if I accidentally ate something that would affect her development? Is exposure to second-hand smoke as I walk down the street harming her? How bad would it be if I just had one cup of coffee? My doubts caused quite a bit of distress for me, which is a mild form of the distress I see when counseling couples after their miscarriages.
The article’s author also expressed concern about the emotional sterility of the environment in which miscarriages usually occur: a hospital ED. EDs are designed to promote a level of detachment and to quell any stress for the clinicians so that they can calmly handle unexpected health crises. EDs are not primarily designed to provide patients with emotional support, nor should they be. However, we still can make some improvements to existing ED design to better address couples’ emotional needs. For example, some EDs have placed mental health clinicians on staff, others call patients post discharge to address concerns, and some EDs even provide patients access to mental health trauma teams. Such services are not found in all EDs, and even those that exist may just scratch the surface of what is needed, but they are a step in the right direction. Providing this level of auxiliary care directly from the ED increases patients’ ability to access mental health support in the place where miscarriages are most likely to be first diagnosed and managed.
The American College of Obstetricians and Gynecologists already is trying to fill in the missing pieces when it comes to identifying mood symptoms following miscarriage. One of the key recommendations from the May 2018 Committee Opinion on Redefining the Postpartum Visit is that every woman who has experienced a miscarriage, stillbirth, or neonatal death should receive follow-up care. Mental health is a suggested component of the postpartum care plan. Some outpatient ob.gyn. practices and inpatient units are using screening tools to identify postpartum depression. For example, the Edinburgh Postnatal Depression Scale can be utilized following a miscarriage to help providers identify symptoms of depression and anxiety.
However, The trend in psychiatry over the past decade has been toward collaborative care, models that embed psychiatrists and other mental health clinicians in ob.gyn. practices to help guide the diagnosis and treatment of mental health problems. Some psychiatrists practice a co-located model in which they see patients alongside their ob.gyn. colleagues, whereas other psychiatrists treat a larger number of patients by using chart reviews for medication management while relying on behavioral health care managers for counseling and monitoring. Using this model of mental health care, more patients have access to services that are provided in a location familiar to them.
Another step in the right direction is the October 2019 launch of The National Curriculum in Reproductive Psychiatry (NCRP), which provides free educational material for psychiatry faculty and residents to enhance education on topics related to reproductive psychiatry, including miscarriage, loss, and development of trauma disorders. NCRP aspires to develop educational materials for ob.gyn. residents.
In the past we may have missed the mark in recognizing and treating the trauma that prenatal loss can cause, but we are trying to improve our approaches. More and more couples are sharing their experiences and advocating for themselves and others, often creating change in medical practice, and doctors are starting to listen. As any clinician knows, changes to standards of care can take several years to disseminate into general practice, but this gap between knowledge and treatment is now in the forefront of our minds. I am hopeful that we will continue to make advances and provide better care to our patients who have endured the loss of a pregnancy.
Dr. Latorre is an assistant professor in the department of psychiatry at the University of Maryland School of Medicine. She has reported no relevant financial disclosures. Email her at obnews@mdedge.com.
Cannabis use in pregnancy and lactation: A changing landscape
National survey data from 2007-2012 of more than 93,000 pregnant women suggest that around 7% of pregnant respondents reported any cannabis use in the last 2-12 months; of those, 16% reported daily or almost daily use. Among pregnant past-year users in the same survey, 70% perceived slight or no risk of harm from cannabis use 1-2 times a week in pregnancy.1
Data from the Kaiser Northern California health plan involving more than 279,000 pregnancies followed during 2009-2016 suggest that there has been a significant upward trend in use of cannabis during pregnancy, from 4% to 7%, as reported by the mother and/or identified by routine urine screening. The highest prevalence in that study was seen among 18- to 24-year-old pregnant women, increasing from 13% to 22% over the 7-year study period. Importantly, more than 50% of cannabis users in the sample were identified by toxicology screening alone.2,3 Common reasons given for use of cannabis in pregnancy include anxiety, pain, and nausea and vomiting of pregnancy.4
With respect to adverse perinatal outcomes, several case-control studies have examined risks for major birth defects with maternal self-report of cannabis use. Some have noted very modest increased risks for selected major birth defects (odds ratios less than 2); however, data still are very limited.5,6
A number of prospective studies have addressed risks of preterm birth and growth restriction, accounting for mother’s concomitant tobacco use.7-11 Some of these studies have suggested about a twofold to threefold increased risk for preterm delivery and an increased risk for reduced birth weight – particularly with heavier or regular cannabis use – but study findings have not been entirely consistent.
Given its psychoactive properties, there has been high interest in understanding whether there are any short- or long-term neurodevelopmental effects on children prenatally exposed to cannabis. These outcomes have been studied in two small older cohorts in the United States and Canada and one more recent cohort in the Netherlands.12-15 Deficits in several measures of cognition and behavior were noted in follow-up of those children from birth to adulthood. However, it is unclear to what extent these findings may have been influenced by heredity, environment, or other factors.
There have been limitations in almost all studies published to date, including small sample sizes, no biomarker validation of maternal report of dose and gestational timing of cannabis use, and lack of detailed data on common coexposures, such as alcohol, tobacco, and other drugs. In addition, newer studies of pregnancy outcomes in women who use currently available cannabis products are needed, given the substantial increase in the potency of cannabis used today, compared with that of 20 years ago. For example, the tetrahydrocannabinol (THC) concentration in commonly cultivated marijuana plants has increased threefold from 4% to 12% between 1995 and 2014.16
There are very limited data on the presence of cannabis in breast milk and the potential effects of exposure to THC and other metabolites for breastfed infants. However, two recent studies have demonstrated there are low but measurable levels of some cannabis metabolites in breast milk.17-18 Further work is needed to determine if these metabolites accumulate in milk and if at a given dose and age of the breastfed infant, there are any growth, neurodevelopmental, or other clinically important adverse effects.
Related questions, such as potential differences in the effects of exposure during pregnancy or lactation based on the route of administration (edible vs. inhaled) and the use of cannabidiol (CBD) products, have not been studied.
At the present time, the American College of Obstetricians and Gynecologists recommends that women who are pregnant or contemplating pregnancy be encouraged to discontinue marijuana use. With respect to lactation and breastfeeding, ACOG concludes there are insufficient data to evaluate the effects on infants, and in the absence of such data, marijuana use is discouraged. Similarly, the American Academy of Pediatrics recommends women of childbearing age abstain from marijuana use while pregnant or breastfeeding because of potential adverse consequences to the fetus, infant, or child.
In August 2019, the U.S. Surgeon General issued an advisory regarding potential harm to developing brains from the use of marijuana during pregnancy and lactation. The Food and Drug Administration issued a similar statement in October 2019 strongly advising against the use of CBD, THC, and marijuana in any form during pregnancy or while breastfeeding.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, president of the Organization of Teratology Information Specialists, and past president of the Teratology Society.
References
1. Am J Obstet Gynecol. 2015 Aug;213(2):201.e1-10.
2. JAMA. 2017 Dec 26;318(24):2490-1.
3. JAMA. 2017 Jan 10;317(2):207-9.
4. Complement Ther Clin Pract. 2009 Nov;15(4)242-6.
5. Paediatr Perinat Epidemiol. 2014 Sep; 28(5): 424-33.
6. J Toxicol Environ Health A. 2007 Jan;70(1):7-18.
7. Am J Obstet Gynecol. 1983 Aug 15;146(8):992-4.
8. Clin Perinatol. 1991 Mar;18(1):77-91.
9. Am J Epidemiol. 1986 Dec;124(6):986-93.
10. Pediatr Res. 2012 Feb;71(2):215-9.
11. Reprod Toxicol. 2016;62:77-86.
12. Neurotoxicol Teratol. 1987 Jan-Feb;9(1):1-7.
13. Neurotoxicol Teratol. 1994 Mar-Apr;16(2):169-75.
14. Biol Psychiatry. 2016 Jun 15;79(12):971-9.
15. Pharmacol Ther. 2018 Feb;182:133-51.
16. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
17. Obstet Gynecol. 2018 May;131(5):783-8.
18. Pediatrics. 2018 Sep;142(3):e20181076.
National survey data from 2007-2012 of more than 93,000 pregnant women suggest that around 7% of pregnant respondents reported any cannabis use in the last 2-12 months; of those, 16% reported daily or almost daily use. Among pregnant past-year users in the same survey, 70% perceived slight or no risk of harm from cannabis use 1-2 times a week in pregnancy.1
Data from the Kaiser Northern California health plan involving more than 279,000 pregnancies followed during 2009-2016 suggest that there has been a significant upward trend in use of cannabis during pregnancy, from 4% to 7%, as reported by the mother and/or identified by routine urine screening. The highest prevalence in that study was seen among 18- to 24-year-old pregnant women, increasing from 13% to 22% over the 7-year study period. Importantly, more than 50% of cannabis users in the sample were identified by toxicology screening alone.2,3 Common reasons given for use of cannabis in pregnancy include anxiety, pain, and nausea and vomiting of pregnancy.4
With respect to adverse perinatal outcomes, several case-control studies have examined risks for major birth defects with maternal self-report of cannabis use. Some have noted very modest increased risks for selected major birth defects (odds ratios less than 2); however, data still are very limited.5,6
A number of prospective studies have addressed risks of preterm birth and growth restriction, accounting for mother’s concomitant tobacco use.7-11 Some of these studies have suggested about a twofold to threefold increased risk for preterm delivery and an increased risk for reduced birth weight – particularly with heavier or regular cannabis use – but study findings have not been entirely consistent.
Given its psychoactive properties, there has been high interest in understanding whether there are any short- or long-term neurodevelopmental effects on children prenatally exposed to cannabis. These outcomes have been studied in two small older cohorts in the United States and Canada and one more recent cohort in the Netherlands.12-15 Deficits in several measures of cognition and behavior were noted in follow-up of those children from birth to adulthood. However, it is unclear to what extent these findings may have been influenced by heredity, environment, or other factors.
There have been limitations in almost all studies published to date, including small sample sizes, no biomarker validation of maternal report of dose and gestational timing of cannabis use, and lack of detailed data on common coexposures, such as alcohol, tobacco, and other drugs. In addition, newer studies of pregnancy outcomes in women who use currently available cannabis products are needed, given the substantial increase in the potency of cannabis used today, compared with that of 20 years ago. For example, the tetrahydrocannabinol (THC) concentration in commonly cultivated marijuana plants has increased threefold from 4% to 12% between 1995 and 2014.16
There are very limited data on the presence of cannabis in breast milk and the potential effects of exposure to THC and other metabolites for breastfed infants. However, two recent studies have demonstrated there are low but measurable levels of some cannabis metabolites in breast milk.17-18 Further work is needed to determine if these metabolites accumulate in milk and if at a given dose and age of the breastfed infant, there are any growth, neurodevelopmental, or other clinically important adverse effects.
Related questions, such as potential differences in the effects of exposure during pregnancy or lactation based on the route of administration (edible vs. inhaled) and the use of cannabidiol (CBD) products, have not been studied.
At the present time, the American College of Obstetricians and Gynecologists recommends that women who are pregnant or contemplating pregnancy be encouraged to discontinue marijuana use. With respect to lactation and breastfeeding, ACOG concludes there are insufficient data to evaluate the effects on infants, and in the absence of such data, marijuana use is discouraged. Similarly, the American Academy of Pediatrics recommends women of childbearing age abstain from marijuana use while pregnant or breastfeeding because of potential adverse consequences to the fetus, infant, or child.
In August 2019, the U.S. Surgeon General issued an advisory regarding potential harm to developing brains from the use of marijuana during pregnancy and lactation. The Food and Drug Administration issued a similar statement in October 2019 strongly advising against the use of CBD, THC, and marijuana in any form during pregnancy or while breastfeeding.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, president of the Organization of Teratology Information Specialists, and past president of the Teratology Society.
References
1. Am J Obstet Gynecol. 2015 Aug;213(2):201.e1-10.
2. JAMA. 2017 Dec 26;318(24):2490-1.
3. JAMA. 2017 Jan 10;317(2):207-9.
4. Complement Ther Clin Pract. 2009 Nov;15(4)242-6.
5. Paediatr Perinat Epidemiol. 2014 Sep; 28(5): 424-33.
6. J Toxicol Environ Health A. 2007 Jan;70(1):7-18.
7. Am J Obstet Gynecol. 1983 Aug 15;146(8):992-4.
8. Clin Perinatol. 1991 Mar;18(1):77-91.
9. Am J Epidemiol. 1986 Dec;124(6):986-93.
10. Pediatr Res. 2012 Feb;71(2):215-9.
11. Reprod Toxicol. 2016;62:77-86.
12. Neurotoxicol Teratol. 1987 Jan-Feb;9(1):1-7.
13. Neurotoxicol Teratol. 1994 Mar-Apr;16(2):169-75.
14. Biol Psychiatry. 2016 Jun 15;79(12):971-9.
15. Pharmacol Ther. 2018 Feb;182:133-51.
16. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
17. Obstet Gynecol. 2018 May;131(5):783-8.
18. Pediatrics. 2018 Sep;142(3):e20181076.
National survey data from 2007-2012 of more than 93,000 pregnant women suggest that around 7% of pregnant respondents reported any cannabis use in the last 2-12 months; of those, 16% reported daily or almost daily use. Among pregnant past-year users in the same survey, 70% perceived slight or no risk of harm from cannabis use 1-2 times a week in pregnancy.1
Data from the Kaiser Northern California health plan involving more than 279,000 pregnancies followed during 2009-2016 suggest that there has been a significant upward trend in use of cannabis during pregnancy, from 4% to 7%, as reported by the mother and/or identified by routine urine screening. The highest prevalence in that study was seen among 18- to 24-year-old pregnant women, increasing from 13% to 22% over the 7-year study period. Importantly, more than 50% of cannabis users in the sample were identified by toxicology screening alone.2,3 Common reasons given for use of cannabis in pregnancy include anxiety, pain, and nausea and vomiting of pregnancy.4
With respect to adverse perinatal outcomes, several case-control studies have examined risks for major birth defects with maternal self-report of cannabis use. Some have noted very modest increased risks for selected major birth defects (odds ratios less than 2); however, data still are very limited.5,6
A number of prospective studies have addressed risks of preterm birth and growth restriction, accounting for mother’s concomitant tobacco use.7-11 Some of these studies have suggested about a twofold to threefold increased risk for preterm delivery and an increased risk for reduced birth weight – particularly with heavier or regular cannabis use – but study findings have not been entirely consistent.
Given its psychoactive properties, there has been high interest in understanding whether there are any short- or long-term neurodevelopmental effects on children prenatally exposed to cannabis. These outcomes have been studied in two small older cohorts in the United States and Canada and one more recent cohort in the Netherlands.12-15 Deficits in several measures of cognition and behavior were noted in follow-up of those children from birth to adulthood. However, it is unclear to what extent these findings may have been influenced by heredity, environment, or other factors.
There have been limitations in almost all studies published to date, including small sample sizes, no biomarker validation of maternal report of dose and gestational timing of cannabis use, and lack of detailed data on common coexposures, such as alcohol, tobacco, and other drugs. In addition, newer studies of pregnancy outcomes in women who use currently available cannabis products are needed, given the substantial increase in the potency of cannabis used today, compared with that of 20 years ago. For example, the tetrahydrocannabinol (THC) concentration in commonly cultivated marijuana plants has increased threefold from 4% to 12% between 1995 and 2014.16
There are very limited data on the presence of cannabis in breast milk and the potential effects of exposure to THC and other metabolites for breastfed infants. However, two recent studies have demonstrated there are low but measurable levels of some cannabis metabolites in breast milk.17-18 Further work is needed to determine if these metabolites accumulate in milk and if at a given dose and age of the breastfed infant, there are any growth, neurodevelopmental, or other clinically important adverse effects.
Related questions, such as potential differences in the effects of exposure during pregnancy or lactation based on the route of administration (edible vs. inhaled) and the use of cannabidiol (CBD) products, have not been studied.
At the present time, the American College of Obstetricians and Gynecologists recommends that women who are pregnant or contemplating pregnancy be encouraged to discontinue marijuana use. With respect to lactation and breastfeeding, ACOG concludes there are insufficient data to evaluate the effects on infants, and in the absence of such data, marijuana use is discouraged. Similarly, the American Academy of Pediatrics recommends women of childbearing age abstain from marijuana use while pregnant or breastfeeding because of potential adverse consequences to the fetus, infant, or child.
In August 2019, the U.S. Surgeon General issued an advisory regarding potential harm to developing brains from the use of marijuana during pregnancy and lactation. The Food and Drug Administration issued a similar statement in October 2019 strongly advising against the use of CBD, THC, and marijuana in any form during pregnancy or while breastfeeding.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, president of the Organization of Teratology Information Specialists, and past president of the Teratology Society.
References
1. Am J Obstet Gynecol. 2015 Aug;213(2):201.e1-10.
2. JAMA. 2017 Dec 26;318(24):2490-1.
3. JAMA. 2017 Jan 10;317(2):207-9.
4. Complement Ther Clin Pract. 2009 Nov;15(4)242-6.
5. Paediatr Perinat Epidemiol. 2014 Sep; 28(5): 424-33.
6. J Toxicol Environ Health A. 2007 Jan;70(1):7-18.
7. Am J Obstet Gynecol. 1983 Aug 15;146(8):992-4.
8. Clin Perinatol. 1991 Mar;18(1):77-91.
9. Am J Epidemiol. 1986 Dec;124(6):986-93.
10. Pediatr Res. 2012 Feb;71(2):215-9.
11. Reprod Toxicol. 2016;62:77-86.
12. Neurotoxicol Teratol. 1987 Jan-Feb;9(1):1-7.
13. Neurotoxicol Teratol. 1994 Mar-Apr;16(2):169-75.
14. Biol Psychiatry. 2016 Jun 15;79(12):971-9.
15. Pharmacol Ther. 2018 Feb;182:133-51.
16. Biol Psychiatry. 2016 Apr 1;79(7):613-9.
17. Obstet Gynecol. 2018 May;131(5):783-8.
18. Pediatrics. 2018 Sep;142(3):e20181076.
Suicide rate higher than average for female clinicians
The suicide rate for women who provide health care is higher than that of all women of working age, while male health care practitioners are less likely to end their lives than working-age men as a whole, according to the Centers for Disease Control and Prevention.
In 2016, the suicide rate for women classified as “healthcare practitioners and technical” – a category that includes physicians and surgeons, as well as chiropractors, physician assistants, and nurse practitioners – was 8.5 per 100,000 population, compared with 7.7 per 100,000 for all working women aged 16-64 years. That difference, however, was not statistically significant, Cora Peterson, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
For females classified as “healthcare support” – medical assistants and transcriptionists, phlebotomists, and pharmacy aides – the suicide rate of 10.6 per 100,000 was significantly higher than that of all working women, the investigators noted.
The suicide rate for males in each of the two occupation categories was 23.6 per 100,000 population in 2016, lower than the rate of 27.4 per 100,000 for males of all occupations, they said, based on data from 32 states that participated in the 2016 National Violent Death Reporting System.
For males, the highest suicide rates in occupations meeting criteria for sample size were “construction and extraction” (49.4 per 100,000); “installation, maintenance, and repair” (36.9); and “arts, design, entertainment, sports, and media” (32.0). Among females, the highest rates were seen in “construction and extraction” (25.5 per 100,000), “protective service” (14.0), and “transportation and material moving” (12.5), with healthcare support next, Dr. Peterson and associates reported.
“Although relative comparisons of suicide rates in this manner are useful for prevention purposes, Therefore, all industry sectors and occupational groups can contribute to reducing suicide incidence,” they wrote.
SOURCE: Peterson C et al. MMWR. 2020 Jan 24;69(3):57-62.
The suicide rate for women who provide health care is higher than that of all women of working age, while male health care practitioners are less likely to end their lives than working-age men as a whole, according to the Centers for Disease Control and Prevention.

In 2016, the suicide rate for women classified as “healthcare practitioners and technical” – a category that includes physicians and surgeons, as well as chiropractors, physician assistants, and nurse practitioners – was 8.5 per 100,000 population, compared with 7.7 per 100,000 for all working women aged 16-64 years. That difference, however, was not statistically significant, Cora Peterson, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
For females classified as “healthcare support” – medical assistants and transcriptionists, phlebotomists, and pharmacy aides – the suicide rate of 10.6 per 100,000 was significantly higher than that of all working women, the investigators noted.
The suicide rate for males in each of the two occupation categories was 23.6 per 100,000 population in 2016, lower than the rate of 27.4 per 100,000 for males of all occupations, they said, based on data from 32 states that participated in the 2016 National Violent Death Reporting System.
For males, the highest suicide rates in occupations meeting criteria for sample size were “construction and extraction” (49.4 per 100,000); “installation, maintenance, and repair” (36.9); and “arts, design, entertainment, sports, and media” (32.0). Among females, the highest rates were seen in “construction and extraction” (25.5 per 100,000), “protective service” (14.0), and “transportation and material moving” (12.5), with healthcare support next, Dr. Peterson and associates reported.
“Although relative comparisons of suicide rates in this manner are useful for prevention purposes, Therefore, all industry sectors and occupational groups can contribute to reducing suicide incidence,” they wrote.
SOURCE: Peterson C et al. MMWR. 2020 Jan 24;69(3):57-62.
The suicide rate for women who provide health care is higher than that of all women of working age, while male health care practitioners are less likely to end their lives than working-age men as a whole, according to the Centers for Disease Control and Prevention.

In 2016, the suicide rate for women classified as “healthcare practitioners and technical” – a category that includes physicians and surgeons, as well as chiropractors, physician assistants, and nurse practitioners – was 8.5 per 100,000 population, compared with 7.7 per 100,000 for all working women aged 16-64 years. That difference, however, was not statistically significant, Cora Peterson, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
For females classified as “healthcare support” – medical assistants and transcriptionists, phlebotomists, and pharmacy aides – the suicide rate of 10.6 per 100,000 was significantly higher than that of all working women, the investigators noted.
The suicide rate for males in each of the two occupation categories was 23.6 per 100,000 population in 2016, lower than the rate of 27.4 per 100,000 for males of all occupations, they said, based on data from 32 states that participated in the 2016 National Violent Death Reporting System.
For males, the highest suicide rates in occupations meeting criteria for sample size were “construction and extraction” (49.4 per 100,000); “installation, maintenance, and repair” (36.9); and “arts, design, entertainment, sports, and media” (32.0). Among females, the highest rates were seen in “construction and extraction” (25.5 per 100,000), “protective service” (14.0), and “transportation and material moving” (12.5), with healthcare support next, Dr. Peterson and associates reported.
“Although relative comparisons of suicide rates in this manner are useful for prevention purposes, Therefore, all industry sectors and occupational groups can contribute to reducing suicide incidence,” they wrote.
SOURCE: Peterson C et al. MMWR. 2020 Jan 24;69(3):57-62.
FROM MMWR
Start of myeloma therapy may be delayed for women, minorities
Women and racial minorities with multiple myeloma may be at increased risk of delayed treatment, a situation that should be addressed urgently, according to authors of a recent analysis of a clinical oncology database.
By contrast, patients receiving myeloma treatment sooner after diagnosis included patients who were over 80 years of age, had multiple comorbidities, were treated at specialized cancer programs or in areas other than the Northeast, and had Medicaid or did not have private insurance, the authors reported.
Contrary to what was expected, levels of education and income did not significantly affect the timeliness of treatment in this analysis by Vivek Kumar, MD, of Dana-Farber Cancer Institute in Boston and coinvestigators.
While results of studies to date are “conflicting” as to whether timeliness of myeloma therapy will affect patient outcomes, recent studies in breast cancer and other tumor types suggest earlier treatment intervention may reduce morbidity, improve quality of life, and possibly prolong survival, according to Dr. Kumar and colleagues.
Moreover, the focus of myeloma treatment has shifted toward earlier treatment in light of the superiority of today’s treatment options, which was demonstrated in the 2014 update of the International Myeloma Working Group (IMWG) diagnostic criteria, according to the investigators.
“The definition of active MM [multiple myeloma] has been updated so that patients who may have been considered to have smoldering MM previously are now treated sooner to prevent end-organ damage whenever possible,” said Dr. Kumar and coauthors in their report in JCO Oncology Practice.
The analysis of timely myeloma treatment was based on for 74,722 patients in the National Cancer Database who received a diagnosis of multiple myeloma between 2004 and 2015 and went on to receive systemic treatment within the first year of diagnosis.
Delay in treatment, defined as receiving antimyeloma therapy 40 or more days after diagnosis, occurred in 18,375 of those patients, or about one-quarter of the study cohort. The mean time from diagnosis to start of treatment in that group was 63 days.
Compared with patients who received treatment within 7 days of diagnosis, patients with delays in treatment were more likely to be women (odds ratio, 1.15; 95% confidence interval, 1.1-1.2) and more likely to be non-Hispanic black (OR, 1.21; 95% CI, 1.14-1.28), the investigators reported.
A previous analysis of the SEER-Medicare database suggested that certain antimyeloma agents are used later in racial and ethnic minorities, including Hispanic patients, who had the highest median time to first dose of bortezomib, Dr. Kumar and colleagues noted.
However, no report before the present one had looked at the time to overall initial treatment in racial and ethnic minorities, they added.
Patients diagnosed in more recent years had higher odds of treatment delay, though this could have been caused by an increase in the number of patients diagnosed early; prior to the 2014 IMWG diagnostic criteria revision, many would have been offered therapy only when signs of end-organ damage were present, while patients without end-organ damage would have been said to have smoldering disease, authors said.
Patients 80 years of age and older and those with a higher Charlson comorbidity score had a lower likelihood of treatment delay in this analysis, possibly reflecting the frailty of those patients and an urgent need for treatment, according to investigators.
Uninsured patients and those with Medicaid were less likely than insured patients to experience treatment delay, according to the report.
“This may be associated with the fact that, for these insurances, prior authorization is typically not required before initiating treatment,” said Dr. Kumar and colleagues. “However, this could also depend on several other possible factors, including availability of caregiver support and seeking medical care later.”
Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
SOURCE: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
Women and racial minorities with multiple myeloma may be at increased risk of delayed treatment, a situation that should be addressed urgently, according to authors of a recent analysis of a clinical oncology database.
By contrast, patients receiving myeloma treatment sooner after diagnosis included patients who were over 80 years of age, had multiple comorbidities, were treated at specialized cancer programs or in areas other than the Northeast, and had Medicaid or did not have private insurance, the authors reported.
Contrary to what was expected, levels of education and income did not significantly affect the timeliness of treatment in this analysis by Vivek Kumar, MD, of Dana-Farber Cancer Institute in Boston and coinvestigators.
While results of studies to date are “conflicting” as to whether timeliness of myeloma therapy will affect patient outcomes, recent studies in breast cancer and other tumor types suggest earlier treatment intervention may reduce morbidity, improve quality of life, and possibly prolong survival, according to Dr. Kumar and colleagues.
Moreover, the focus of myeloma treatment has shifted toward earlier treatment in light of the superiority of today’s treatment options, which was demonstrated in the 2014 update of the International Myeloma Working Group (IMWG) diagnostic criteria, according to the investigators.
“The definition of active MM [multiple myeloma] has been updated so that patients who may have been considered to have smoldering MM previously are now treated sooner to prevent end-organ damage whenever possible,” said Dr. Kumar and coauthors in their report in JCO Oncology Practice.
The analysis of timely myeloma treatment was based on for 74,722 patients in the National Cancer Database who received a diagnosis of multiple myeloma between 2004 and 2015 and went on to receive systemic treatment within the first year of diagnosis.
Delay in treatment, defined as receiving antimyeloma therapy 40 or more days after diagnosis, occurred in 18,375 of those patients, or about one-quarter of the study cohort. The mean time from diagnosis to start of treatment in that group was 63 days.
Compared with patients who received treatment within 7 days of diagnosis, patients with delays in treatment were more likely to be women (odds ratio, 1.15; 95% confidence interval, 1.1-1.2) and more likely to be non-Hispanic black (OR, 1.21; 95% CI, 1.14-1.28), the investigators reported.
A previous analysis of the SEER-Medicare database suggested that certain antimyeloma agents are used later in racial and ethnic minorities, including Hispanic patients, who had the highest median time to first dose of bortezomib, Dr. Kumar and colleagues noted.
However, no report before the present one had looked at the time to overall initial treatment in racial and ethnic minorities, they added.
Patients diagnosed in more recent years had higher odds of treatment delay, though this could have been caused by an increase in the number of patients diagnosed early; prior to the 2014 IMWG diagnostic criteria revision, many would have been offered therapy only when signs of end-organ damage were present, while patients without end-organ damage would have been said to have smoldering disease, authors said.
Patients 80 years of age and older and those with a higher Charlson comorbidity score had a lower likelihood of treatment delay in this analysis, possibly reflecting the frailty of those patients and an urgent need for treatment, according to investigators.
Uninsured patients and those with Medicaid were less likely than insured patients to experience treatment delay, according to the report.
“This may be associated with the fact that, for these insurances, prior authorization is typically not required before initiating treatment,” said Dr. Kumar and colleagues. “However, this could also depend on several other possible factors, including availability of caregiver support and seeking medical care later.”
Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
SOURCE: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
Women and racial minorities with multiple myeloma may be at increased risk of delayed treatment, a situation that should be addressed urgently, according to authors of a recent analysis of a clinical oncology database.
By contrast, patients receiving myeloma treatment sooner after diagnosis included patients who were over 80 years of age, had multiple comorbidities, were treated at specialized cancer programs or in areas other than the Northeast, and had Medicaid or did not have private insurance, the authors reported.
Contrary to what was expected, levels of education and income did not significantly affect the timeliness of treatment in this analysis by Vivek Kumar, MD, of Dana-Farber Cancer Institute in Boston and coinvestigators.
While results of studies to date are “conflicting” as to whether timeliness of myeloma therapy will affect patient outcomes, recent studies in breast cancer and other tumor types suggest earlier treatment intervention may reduce morbidity, improve quality of life, and possibly prolong survival, according to Dr. Kumar and colleagues.
Moreover, the focus of myeloma treatment has shifted toward earlier treatment in light of the superiority of today’s treatment options, which was demonstrated in the 2014 update of the International Myeloma Working Group (IMWG) diagnostic criteria, according to the investigators.
“The definition of active MM [multiple myeloma] has been updated so that patients who may have been considered to have smoldering MM previously are now treated sooner to prevent end-organ damage whenever possible,” said Dr. Kumar and coauthors in their report in JCO Oncology Practice.
The analysis of timely myeloma treatment was based on for 74,722 patients in the National Cancer Database who received a diagnosis of multiple myeloma between 2004 and 2015 and went on to receive systemic treatment within the first year of diagnosis.
Delay in treatment, defined as receiving antimyeloma therapy 40 or more days after diagnosis, occurred in 18,375 of those patients, or about one-quarter of the study cohort. The mean time from diagnosis to start of treatment in that group was 63 days.
Compared with patients who received treatment within 7 days of diagnosis, patients with delays in treatment were more likely to be women (odds ratio, 1.15; 95% confidence interval, 1.1-1.2) and more likely to be non-Hispanic black (OR, 1.21; 95% CI, 1.14-1.28), the investigators reported.
A previous analysis of the SEER-Medicare database suggested that certain antimyeloma agents are used later in racial and ethnic minorities, including Hispanic patients, who had the highest median time to first dose of bortezomib, Dr. Kumar and colleagues noted.
However, no report before the present one had looked at the time to overall initial treatment in racial and ethnic minorities, they added.
Patients diagnosed in more recent years had higher odds of treatment delay, though this could have been caused by an increase in the number of patients diagnosed early; prior to the 2014 IMWG diagnostic criteria revision, many would have been offered therapy only when signs of end-organ damage were present, while patients without end-organ damage would have been said to have smoldering disease, authors said.
Patients 80 years of age and older and those with a higher Charlson comorbidity score had a lower likelihood of treatment delay in this analysis, possibly reflecting the frailty of those patients and an urgent need for treatment, according to investigators.
Uninsured patients and those with Medicaid were less likely than insured patients to experience treatment delay, according to the report.
“This may be associated with the fact that, for these insurances, prior authorization is typically not required before initiating treatment,” said Dr. Kumar and colleagues. “However, this could also depend on several other possible factors, including availability of caregiver support and seeking medical care later.”
Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
SOURCE: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
FROM JCO ONCOLOGY PRACTICE
Key clinical point:
Major finding: Patients with delays in treatment were more likely to be women (odds ratio, 1.15) and more likely to be non-Hispanic blacks (OR, 1.21).
Study details: Retrospective analysis of 74,722 patients in the National Cancer Database diagnosed with multiple myeloma between 2004 and 2015.
Disclosures: Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
Source: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
Methotrexate gives durable remission from idiopathic granulomatous mastitis
ATLANTA – Methotrexate, in combination with prednisone, might be emerging as the go-to option for idiopathic granulomatous mastitis, according to investigators from Oregon Health & Science University, Portland.
Idiopathic granulomatous mastitis (IGM) is an inflammatory disease in which granulomas form in breast tissue. It strikes mostly young to middle-aged women with painful, firm breast masses, sometimes with redness and drainage. Diagnosis is by biopsy with rule-out of known causes.
IGM does not respond to antibiotics. Prednisone and surgery have been the traditional approaches, but masses can recur after surgery, and a year or more of prednisone, with the weight gain and side effects, is problematic. As a result, cases are increasingly being referred to rheumatologists for other options, said lead investigator Sarah Ringsted, MD, a rheumatology fellow at the university.
A study she presented at the annual meeting of the American College of Rheumatology and previous work from others builds a case for methotrexate, which often seems to put the disease in remission and allows for shorter glucocorticoid courses. These days, “I offer this to patients as a great option. It’s really nice to have, instead of having women go on months and months of high-dose steroids, and I think we can save patients from unnecessary” surgery, Dr. Ringsted said.
Her usual regimen these days is methotrexate 15-20 mg/week for 12-18 months, with high-dose prednisone (greater than 20 mg/day) for the first 3 months, followed by a taper.
Dr. Ringsted and associates compared 23 women treated at the university during 2007-2018. Just 5 of the 12 women (42%) treated with high-dose prednisone alone went into remission and did not relapse over a mean follow-up of 27 months. Two out of three women who had both high-dose glucocorticoids and surgery achieved remission without relapse, as did all three women who received methotrexate and high-dose glucocorticoids (one also had surgery). Five other patients were treated with other options; just two had a durable remission.
The numbers are small, but they add to two previous reports. Among 19 women who had failed other treatments, 94% improved and 75% went into remission with 15 months of methotrexate in a review from Stanford (Calif.) University. An Iranian study of 17 patients treated with methotrexate, and also glucocorticoids in some, had a relapse rate of only 17.8%.
There were several cases of both inflammatory arthritis and erythema nodosum in the Oregon series, a higher incidence than what has been reported before for IGM. “It’s interesting because it makes me think of sarcoidosis. There have been cases of sarcoidosis causing mastitis, but mostly in patients with other features” of the disease. “It makes me wonder if any of these women will develop sarcoidosis later on; I think that’s an interesting question,” Dr. Ringsted said.
Women in the study were an average age of 32 years, and over half were Hispanic, which is associated with a higher risk for IGM. Almost all the women had been pregnant before and had breast fed in the previous 5 years. Cancer, tuberculosis, and fungal infections were among the things ruled out before mastitis was deemed idiopathic.
Women with IGM tend to be of childbearing age, and must be cautioned against the teratogenic effects of methotrexate, Dr. Ringsted noted.
There was no external funding, and the investigators didn’t report any disclosures.
SOURCE: Ringsted S et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 386.
ATLANTA – Methotrexate, in combination with prednisone, might be emerging as the go-to option for idiopathic granulomatous mastitis, according to investigators from Oregon Health & Science University, Portland.
Idiopathic granulomatous mastitis (IGM) is an inflammatory disease in which granulomas form in breast tissue. It strikes mostly young to middle-aged women with painful, firm breast masses, sometimes with redness and drainage. Diagnosis is by biopsy with rule-out of known causes.
IGM does not respond to antibiotics. Prednisone and surgery have been the traditional approaches, but masses can recur after surgery, and a year or more of prednisone, with the weight gain and side effects, is problematic. As a result, cases are increasingly being referred to rheumatologists for other options, said lead investigator Sarah Ringsted, MD, a rheumatology fellow at the university.
A study she presented at the annual meeting of the American College of Rheumatology and previous work from others builds a case for methotrexate, which often seems to put the disease in remission and allows for shorter glucocorticoid courses. These days, “I offer this to patients as a great option. It’s really nice to have, instead of having women go on months and months of high-dose steroids, and I think we can save patients from unnecessary” surgery, Dr. Ringsted said.
Her usual regimen these days is methotrexate 15-20 mg/week for 12-18 months, with high-dose prednisone (greater than 20 mg/day) for the first 3 months, followed by a taper.
Dr. Ringsted and associates compared 23 women treated at the university during 2007-2018. Just 5 of the 12 women (42%) treated with high-dose prednisone alone went into remission and did not relapse over a mean follow-up of 27 months. Two out of three women who had both high-dose glucocorticoids and surgery achieved remission without relapse, as did all three women who received methotrexate and high-dose glucocorticoids (one also had surgery). Five other patients were treated with other options; just two had a durable remission.
The numbers are small, but they add to two previous reports. Among 19 women who had failed other treatments, 94% improved and 75% went into remission with 15 months of methotrexate in a review from Stanford (Calif.) University. An Iranian study of 17 patients treated with methotrexate, and also glucocorticoids in some, had a relapse rate of only 17.8%.
There were several cases of both inflammatory arthritis and erythema nodosum in the Oregon series, a higher incidence than what has been reported before for IGM. “It’s interesting because it makes me think of sarcoidosis. There have been cases of sarcoidosis causing mastitis, but mostly in patients with other features” of the disease. “It makes me wonder if any of these women will develop sarcoidosis later on; I think that’s an interesting question,” Dr. Ringsted said.
Women in the study were an average age of 32 years, and over half were Hispanic, which is associated with a higher risk for IGM. Almost all the women had been pregnant before and had breast fed in the previous 5 years. Cancer, tuberculosis, and fungal infections were among the things ruled out before mastitis was deemed idiopathic.
Women with IGM tend to be of childbearing age, and must be cautioned against the teratogenic effects of methotrexate, Dr. Ringsted noted.
There was no external funding, and the investigators didn’t report any disclosures.
SOURCE: Ringsted S et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 386.
ATLANTA – Methotrexate, in combination with prednisone, might be emerging as the go-to option for idiopathic granulomatous mastitis, according to investigators from Oregon Health & Science University, Portland.
Idiopathic granulomatous mastitis (IGM) is an inflammatory disease in which granulomas form in breast tissue. It strikes mostly young to middle-aged women with painful, firm breast masses, sometimes with redness and drainage. Diagnosis is by biopsy with rule-out of known causes.
IGM does not respond to antibiotics. Prednisone and surgery have been the traditional approaches, but masses can recur after surgery, and a year or more of prednisone, with the weight gain and side effects, is problematic. As a result, cases are increasingly being referred to rheumatologists for other options, said lead investigator Sarah Ringsted, MD, a rheumatology fellow at the university.
A study she presented at the annual meeting of the American College of Rheumatology and previous work from others builds a case for methotrexate, which often seems to put the disease in remission and allows for shorter glucocorticoid courses. These days, “I offer this to patients as a great option. It’s really nice to have, instead of having women go on months and months of high-dose steroids, and I think we can save patients from unnecessary” surgery, Dr. Ringsted said.
Her usual regimen these days is methotrexate 15-20 mg/week for 12-18 months, with high-dose prednisone (greater than 20 mg/day) for the first 3 months, followed by a taper.
Dr. Ringsted and associates compared 23 women treated at the university during 2007-2018. Just 5 of the 12 women (42%) treated with high-dose prednisone alone went into remission and did not relapse over a mean follow-up of 27 months. Two out of three women who had both high-dose glucocorticoids and surgery achieved remission without relapse, as did all three women who received methotrexate and high-dose glucocorticoids (one also had surgery). Five other patients were treated with other options; just two had a durable remission.
The numbers are small, but they add to two previous reports. Among 19 women who had failed other treatments, 94% improved and 75% went into remission with 15 months of methotrexate in a review from Stanford (Calif.) University. An Iranian study of 17 patients treated with methotrexate, and also glucocorticoids in some, had a relapse rate of only 17.8%.
There were several cases of both inflammatory arthritis and erythema nodosum in the Oregon series, a higher incidence than what has been reported before for IGM. “It’s interesting because it makes me think of sarcoidosis. There have been cases of sarcoidosis causing mastitis, but mostly in patients with other features” of the disease. “It makes me wonder if any of these women will develop sarcoidosis later on; I think that’s an interesting question,” Dr. Ringsted said.
Women in the study were an average age of 32 years, and over half were Hispanic, which is associated with a higher risk for IGM. Almost all the women had been pregnant before and had breast fed in the previous 5 years. Cancer, tuberculosis, and fungal infections were among the things ruled out before mastitis was deemed idiopathic.
Women with IGM tend to be of childbearing age, and must be cautioned against the teratogenic effects of methotrexate, Dr. Ringsted noted.
There was no external funding, and the investigators didn’t report any disclosures.
SOURCE: Ringsted S et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 386.
REPORTING FROM ACR 2019
Unnecessary pelvic exams, Pap tests common in young women
according to estimates from a study published online in JAMA Internal Medicine.
Approximately 2.6 million young women – about a quarter of those in this age group – reported receiving a pelvic exam in the previous year even though fewer than 10% were pregnant or receiving treatment for a sexually transmitted infection (STI) at the time.
Similarly, an estimated three in four Pap tests given to women aged 15-20 years likely were unnecessary. Based on Medicare payments for screening Pap tests and pelvic exams, the unnecessary procedures represented an estimated $123 million in a year.
“The American College of Obstetricians and Gynecologists recognizes that no evidence supports routine speculum examination or BPE in healthy, asymptomatic women younger than 21 years and recommends that these examinations be performed only when medically indicated,” said Jin Qin, ScD, of the Centers for Disease Control and Prevention and colleagues.
“Our results showed that, despite the recommendation, many young women without discernible medical indication received potentially unnecessary BPE or Pap tests, which may be a reflection of a long-standing clinical practice in the United States.”
These findings “demonstrate what happens to vulnerable populations (in this case, girls and young women) when clinicians do not keep up with or do not adhere to new guidelines,” Melissa A. Simon, MD, MPH, wrote in an invited commentary. She acknowledged the challenges of keeping up with new guidelines but noted the potential for harm from unnecessary screening. Dr. Simon is vice chair for clinical research in the department of obstetrics and gynecology at Northwestern University, Chicago.
The researchers analyzed responses from 3,410 young women aged 15-20 years in the National Survey of Family Growth (NSFG) during 2011-2017 and extrapolated the results to estimate nationwide statistics. The researchers found that 23% of young women – 2.6 million in the United States – had received a bimanual pelvic exam during the previous year.
“This analysis focused on the bimanual component of the pelvic examination because it is the most invasive of the pelvic examination components and less likely to be confused with a speculum examination for cervical cancer or STI screening,” the authors note.
More than half of these pelvic exams (54%) – an estimated 1.4 million exams – potentially were unnecessary. The authors classified these pelvic exams as potentially unnecessary if it was not indicated for pregnancy, intrauterine device (IUD) use, or STI treatment in the past 12 months or for another medical problem.
Among the respondents, 5% were pregnant, 22% had been tested for an STI, and 5% had been treated for an STI during the previous year. About a third of respondents (33%) had used at least one type of hormonal contraception besides an IUD in the past year, but only 2% had used an IUD.
Dr. Simon said that some have advocated for routine bimanual pelvic exams to prompt women to see their provider every year, but without evidence to support the practice.
“In fact, many women (younger and older) associate the bimanual pelvic and speculum examinations with fear, anxiety, embarrassment, discomfort, and pain,” Dr. Simon emphasized. “Girls and women with a history of sexual violence may be more vulnerable to these harms. In addition, adolescent girls may delay starting contraception use or obtaining screening for sexually transmitted infections because of fear of pelvic examination, which thus creates unnecessary barriers to obtaining important screening and family-planning methods.”
The researchers also found that 19% of young women, about 2.2 million, had received a Pap test in the previous year. The majority of these (72%) likely were unnecessary, they wrote, explaining that cervical cancer screening is not recommended for those younger than 21 years unless they are HIV positive and sexually active.
“Because HIV infection status is not available in the NSFG, we estimated prevalence of Pap tests performed as part of a routine examination and considered them potentially unnecessary,” the authors explained.
Young women were seven times more likely to have undergone a bimanual pelvic exam if they received a Pap test (adjusted prevalence ratio [aPR], 7.12). In fact, the authors reported that nearly all potentially unnecessary bimanual pelvic exams (98%) occurred during the same visit as a Pap test that was potentially unnecessary as well.
Young women also were more likely to receive a bimanual pelvic exam if they underwent STI testing or used any hormonal contraception besides an IUD (aPR, 1.6 and 1.31, respectively). Those with public insurance or no insurance were less likely to receive a pelvic exam compared with those who had private insurance, although no associations were found with race/ethnicity.
Young women were about four times more likely to have a Pap test if they had STI testing (aPR, 3.77). Odds of a Pap test also were greater among those aged 18-20 years (aPR, 1.54), those with a pregnancy (aPR, 2.31), those with an IUD (aPR, 1.54), and those using any non-IUD hormonal contraception (aPR, 1.75).
Staying up to date on current guidelines and consistently delivering evidence-based care according to those guidelines “is not easy,” Dr. Simon commented. It involves building and maintaining a trusting clinician-patient relationship that centers on shared decision making, keeping up with research, and “unlearn[ing] deeply ingrained practices,” which is difficult.
“Clinicians are not well instructed on how to pivot or unlearn a practice,” Dr. Simon continued. “The science of deimplementation, especially with respect to guideline-concordant care, is in its infancy.” She also noted the value of annual visits, even without routine pelvic exams.
“Rethinking the goals of the annual health examination for young women and learning to unlearn will not put anyone out of business,” Dr. Simon concluded. “Rather, change can increase patients’ connectivity, trust, and engagement with primary care clinicians and, most importantly, avoid harms, especially to those who are most vulnerable.”
No external funding was used. The study authors and Dr. Simon have disclosed no relevant financial relationships.
SOURCE: Qin J et al. JAMA Intern Med. 2019 Jan 6. doi: 10.1001/jamainternmed.2019.5727.
An earlier version of this story appeared on Medscape.com.
A call for shared decision making
The experts who wrote American College of Obstetricians and Gynecologists’ clinical guideline on the pelvic exam (Obstet Gynecol. 2018 Oct;132[4]:e174-80) reviewed available evidence and found insufficient evidence to support routine screening for asymptomatic nonpregnant women who have no increased risk for specific gynecologic conditions (e.g., history of gynecologic cancer). Hence, ACOG recommends routine screening based on a shared decision between the asymptomatic woman and her doctor keeping in mind her medical and family history and her preference. This decision should be made after reviewing the limitations of the exam with regard to insufficient evidence to support its accuracy in screening for ovarian cancer, bacterial vaginosis, trichomoniasis, and genital herpes, plus lack of evidence for other gynecologic conditions.
In addition, we physicians must educate women, especially vulnerable populations, that deferring a pelvic exam for asymptomatic women entails judicious care. Deferring an exam does not mean that we are withholding medical care. If she wants an exam, understanding its limitations, then this preference is an indication itself for the exam as stated in our guideline.
It is important to emphasize to patients that we are deferring Pap smears until age 21 years per ACOG and the American Society for Colposcopy and Cervical Pathology, and that there is no need for a pelvic exam for sexually transmitted infection screening per the Centers for Disease Control and Prevention. Likewise, there is no need for a pelvic exam prior initiation of contraception except for intrauterine device insertion also according to the CDC.
Catherine Cansino, MD, MPH , is associate clinical professor of obstetrics and gynecology at the University of California, Davis. She was asked to comment on the Qin et al. article. Dr. Cansino is a coauthor of the ACOG 2018 guideline on the utility of pelvic exam. She also is a member of the Ob.Gyn. News editorial advisory board. She reported no relevant financial disclosures.
A call for shared decision making
The experts who wrote American College of Obstetricians and Gynecologists’ clinical guideline on the pelvic exam (Obstet Gynecol. 2018 Oct;132[4]:e174-80) reviewed available evidence and found insufficient evidence to support routine screening for asymptomatic nonpregnant women who have no increased risk for specific gynecologic conditions (e.g., history of gynecologic cancer). Hence, ACOG recommends routine screening based on a shared decision between the asymptomatic woman and her doctor keeping in mind her medical and family history and her preference. This decision should be made after reviewing the limitations of the exam with regard to insufficient evidence to support its accuracy in screening for ovarian cancer, bacterial vaginosis, trichomoniasis, and genital herpes, plus lack of evidence for other gynecologic conditions.
In addition, we physicians must educate women, especially vulnerable populations, that deferring a pelvic exam for asymptomatic women entails judicious care. Deferring an exam does not mean that we are withholding medical care. If she wants an exam, understanding its limitations, then this preference is an indication itself for the exam as stated in our guideline.
It is important to emphasize to patients that we are deferring Pap smears until age 21 years per ACOG and the American Society for Colposcopy and Cervical Pathology, and that there is no need for a pelvic exam for sexually transmitted infection screening per the Centers for Disease Control and Prevention. Likewise, there is no need for a pelvic exam prior initiation of contraception except for intrauterine device insertion also according to the CDC.
Catherine Cansino, MD, MPH , is associate clinical professor of obstetrics and gynecology at the University of California, Davis. She was asked to comment on the Qin et al. article. Dr. Cansino is a coauthor of the ACOG 2018 guideline on the utility of pelvic exam. She also is a member of the Ob.Gyn. News editorial advisory board. She reported no relevant financial disclosures.
A call for shared decision making
The experts who wrote American College of Obstetricians and Gynecologists’ clinical guideline on the pelvic exam (Obstet Gynecol. 2018 Oct;132[4]:e174-80) reviewed available evidence and found insufficient evidence to support routine screening for asymptomatic nonpregnant women who have no increased risk for specific gynecologic conditions (e.g., history of gynecologic cancer). Hence, ACOG recommends routine screening based on a shared decision between the asymptomatic woman and her doctor keeping in mind her medical and family history and her preference. This decision should be made after reviewing the limitations of the exam with regard to insufficient evidence to support its accuracy in screening for ovarian cancer, bacterial vaginosis, trichomoniasis, and genital herpes, plus lack of evidence for other gynecologic conditions.
In addition, we physicians must educate women, especially vulnerable populations, that deferring a pelvic exam for asymptomatic women entails judicious care. Deferring an exam does not mean that we are withholding medical care. If she wants an exam, understanding its limitations, then this preference is an indication itself for the exam as stated in our guideline.
It is important to emphasize to patients that we are deferring Pap smears until age 21 years per ACOG and the American Society for Colposcopy and Cervical Pathology, and that there is no need for a pelvic exam for sexually transmitted infection screening per the Centers for Disease Control and Prevention. Likewise, there is no need for a pelvic exam prior initiation of contraception except for intrauterine device insertion also according to the CDC.
Catherine Cansino, MD, MPH , is associate clinical professor of obstetrics and gynecology at the University of California, Davis. She was asked to comment on the Qin et al. article. Dr. Cansino is a coauthor of the ACOG 2018 guideline on the utility of pelvic exam. She also is a member of the Ob.Gyn. News editorial advisory board. She reported no relevant financial disclosures.
according to estimates from a study published online in JAMA Internal Medicine.
Approximately 2.6 million young women – about a quarter of those in this age group – reported receiving a pelvic exam in the previous year even though fewer than 10% were pregnant or receiving treatment for a sexually transmitted infection (STI) at the time.
Similarly, an estimated three in four Pap tests given to women aged 15-20 years likely were unnecessary. Based on Medicare payments for screening Pap tests and pelvic exams, the unnecessary procedures represented an estimated $123 million in a year.
“The American College of Obstetricians and Gynecologists recognizes that no evidence supports routine speculum examination or BPE in healthy, asymptomatic women younger than 21 years and recommends that these examinations be performed only when medically indicated,” said Jin Qin, ScD, of the Centers for Disease Control and Prevention and colleagues.
“Our results showed that, despite the recommendation, many young women without discernible medical indication received potentially unnecessary BPE or Pap tests, which may be a reflection of a long-standing clinical practice in the United States.”
These findings “demonstrate what happens to vulnerable populations (in this case, girls and young women) when clinicians do not keep up with or do not adhere to new guidelines,” Melissa A. Simon, MD, MPH, wrote in an invited commentary. She acknowledged the challenges of keeping up with new guidelines but noted the potential for harm from unnecessary screening. Dr. Simon is vice chair for clinical research in the department of obstetrics and gynecology at Northwestern University, Chicago.
The researchers analyzed responses from 3,410 young women aged 15-20 years in the National Survey of Family Growth (NSFG) during 2011-2017 and extrapolated the results to estimate nationwide statistics. The researchers found that 23% of young women – 2.6 million in the United States – had received a bimanual pelvic exam during the previous year.
“This analysis focused on the bimanual component of the pelvic examination because it is the most invasive of the pelvic examination components and less likely to be confused with a speculum examination for cervical cancer or STI screening,” the authors note.
More than half of these pelvic exams (54%) – an estimated 1.4 million exams – potentially were unnecessary. The authors classified these pelvic exams as potentially unnecessary if it was not indicated for pregnancy, intrauterine device (IUD) use, or STI treatment in the past 12 months or for another medical problem.
Among the respondents, 5% were pregnant, 22% had been tested for an STI, and 5% had been treated for an STI during the previous year. About a third of respondents (33%) had used at least one type of hormonal contraception besides an IUD in the past year, but only 2% had used an IUD.
Dr. Simon said that some have advocated for routine bimanual pelvic exams to prompt women to see their provider every year, but without evidence to support the practice.
“In fact, many women (younger and older) associate the bimanual pelvic and speculum examinations with fear, anxiety, embarrassment, discomfort, and pain,” Dr. Simon emphasized. “Girls and women with a history of sexual violence may be more vulnerable to these harms. In addition, adolescent girls may delay starting contraception use or obtaining screening for sexually transmitted infections because of fear of pelvic examination, which thus creates unnecessary barriers to obtaining important screening and family-planning methods.”
The researchers also found that 19% of young women, about 2.2 million, had received a Pap test in the previous year. The majority of these (72%) likely were unnecessary, they wrote, explaining that cervical cancer screening is not recommended for those younger than 21 years unless they are HIV positive and sexually active.
“Because HIV infection status is not available in the NSFG, we estimated prevalence of Pap tests performed as part of a routine examination and considered them potentially unnecessary,” the authors explained.
Young women were seven times more likely to have undergone a bimanual pelvic exam if they received a Pap test (adjusted prevalence ratio [aPR], 7.12). In fact, the authors reported that nearly all potentially unnecessary bimanual pelvic exams (98%) occurred during the same visit as a Pap test that was potentially unnecessary as well.
Young women also were more likely to receive a bimanual pelvic exam if they underwent STI testing or used any hormonal contraception besides an IUD (aPR, 1.6 and 1.31, respectively). Those with public insurance or no insurance were less likely to receive a pelvic exam compared with those who had private insurance, although no associations were found with race/ethnicity.
Young women were about four times more likely to have a Pap test if they had STI testing (aPR, 3.77). Odds of a Pap test also were greater among those aged 18-20 years (aPR, 1.54), those with a pregnancy (aPR, 2.31), those with an IUD (aPR, 1.54), and those using any non-IUD hormonal contraception (aPR, 1.75).
Staying up to date on current guidelines and consistently delivering evidence-based care according to those guidelines “is not easy,” Dr. Simon commented. It involves building and maintaining a trusting clinician-patient relationship that centers on shared decision making, keeping up with research, and “unlearn[ing] deeply ingrained practices,” which is difficult.
“Clinicians are not well instructed on how to pivot or unlearn a practice,” Dr. Simon continued. “The science of deimplementation, especially with respect to guideline-concordant care, is in its infancy.” She also noted the value of annual visits, even without routine pelvic exams.
“Rethinking the goals of the annual health examination for young women and learning to unlearn will not put anyone out of business,” Dr. Simon concluded. “Rather, change can increase patients’ connectivity, trust, and engagement with primary care clinicians and, most importantly, avoid harms, especially to those who are most vulnerable.”
No external funding was used. The study authors and Dr. Simon have disclosed no relevant financial relationships.
SOURCE: Qin J et al. JAMA Intern Med. 2019 Jan 6. doi: 10.1001/jamainternmed.2019.5727.
An earlier version of this story appeared on Medscape.com.
according to estimates from a study published online in JAMA Internal Medicine.
Approximately 2.6 million young women – about a quarter of those in this age group – reported receiving a pelvic exam in the previous year even though fewer than 10% were pregnant or receiving treatment for a sexually transmitted infection (STI) at the time.
Similarly, an estimated three in four Pap tests given to women aged 15-20 years likely were unnecessary. Based on Medicare payments for screening Pap tests and pelvic exams, the unnecessary procedures represented an estimated $123 million in a year.
“The American College of Obstetricians and Gynecologists recognizes that no evidence supports routine speculum examination or BPE in healthy, asymptomatic women younger than 21 years and recommends that these examinations be performed only when medically indicated,” said Jin Qin, ScD, of the Centers for Disease Control and Prevention and colleagues.
“Our results showed that, despite the recommendation, many young women without discernible medical indication received potentially unnecessary BPE or Pap tests, which may be a reflection of a long-standing clinical practice in the United States.”
These findings “demonstrate what happens to vulnerable populations (in this case, girls and young women) when clinicians do not keep up with or do not adhere to new guidelines,” Melissa A. Simon, MD, MPH, wrote in an invited commentary. She acknowledged the challenges of keeping up with new guidelines but noted the potential for harm from unnecessary screening. Dr. Simon is vice chair for clinical research in the department of obstetrics and gynecology at Northwestern University, Chicago.
The researchers analyzed responses from 3,410 young women aged 15-20 years in the National Survey of Family Growth (NSFG) during 2011-2017 and extrapolated the results to estimate nationwide statistics. The researchers found that 23% of young women – 2.6 million in the United States – had received a bimanual pelvic exam during the previous year.
“This analysis focused on the bimanual component of the pelvic examination because it is the most invasive of the pelvic examination components and less likely to be confused with a speculum examination for cervical cancer or STI screening,” the authors note.
More than half of these pelvic exams (54%) – an estimated 1.4 million exams – potentially were unnecessary. The authors classified these pelvic exams as potentially unnecessary if it was not indicated for pregnancy, intrauterine device (IUD) use, or STI treatment in the past 12 months or for another medical problem.
Among the respondents, 5% were pregnant, 22% had been tested for an STI, and 5% had been treated for an STI during the previous year. About a third of respondents (33%) had used at least one type of hormonal contraception besides an IUD in the past year, but only 2% had used an IUD.
Dr. Simon said that some have advocated for routine bimanual pelvic exams to prompt women to see their provider every year, but without evidence to support the practice.
“In fact, many women (younger and older) associate the bimanual pelvic and speculum examinations with fear, anxiety, embarrassment, discomfort, and pain,” Dr. Simon emphasized. “Girls and women with a history of sexual violence may be more vulnerable to these harms. In addition, adolescent girls may delay starting contraception use or obtaining screening for sexually transmitted infections because of fear of pelvic examination, which thus creates unnecessary barriers to obtaining important screening and family-planning methods.”
The researchers also found that 19% of young women, about 2.2 million, had received a Pap test in the previous year. The majority of these (72%) likely were unnecessary, they wrote, explaining that cervical cancer screening is not recommended for those younger than 21 years unless they are HIV positive and sexually active.
“Because HIV infection status is not available in the NSFG, we estimated prevalence of Pap tests performed as part of a routine examination and considered them potentially unnecessary,” the authors explained.
Young women were seven times more likely to have undergone a bimanual pelvic exam if they received a Pap test (adjusted prevalence ratio [aPR], 7.12). In fact, the authors reported that nearly all potentially unnecessary bimanual pelvic exams (98%) occurred during the same visit as a Pap test that was potentially unnecessary as well.
Young women also were more likely to receive a bimanual pelvic exam if they underwent STI testing or used any hormonal contraception besides an IUD (aPR, 1.6 and 1.31, respectively). Those with public insurance or no insurance were less likely to receive a pelvic exam compared with those who had private insurance, although no associations were found with race/ethnicity.
Young women were about four times more likely to have a Pap test if they had STI testing (aPR, 3.77). Odds of a Pap test also were greater among those aged 18-20 years (aPR, 1.54), those with a pregnancy (aPR, 2.31), those with an IUD (aPR, 1.54), and those using any non-IUD hormonal contraception (aPR, 1.75).
Staying up to date on current guidelines and consistently delivering evidence-based care according to those guidelines “is not easy,” Dr. Simon commented. It involves building and maintaining a trusting clinician-patient relationship that centers on shared decision making, keeping up with research, and “unlearn[ing] deeply ingrained practices,” which is difficult.
“Clinicians are not well instructed on how to pivot or unlearn a practice,” Dr. Simon continued. “The science of deimplementation, especially with respect to guideline-concordant care, is in its infancy.” She also noted the value of annual visits, even without routine pelvic exams.
“Rethinking the goals of the annual health examination for young women and learning to unlearn will not put anyone out of business,” Dr. Simon concluded. “Rather, change can increase patients’ connectivity, trust, and engagement with primary care clinicians and, most importantly, avoid harms, especially to those who are most vulnerable.”
No external funding was used. The study authors and Dr. Simon have disclosed no relevant financial relationships.
SOURCE: Qin J et al. JAMA Intern Med. 2019 Jan 6. doi: 10.1001/jamainternmed.2019.5727.
An earlier version of this story appeared on Medscape.com.
Provide appropriate sexual, reproductive health care for transgender patients
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).
I recently was on a panel of experts discussing how to prevent HIV among transgender youth. Preventing HIV among transgender youth, especially transgender youth of color, remains a challenge for multiple reasons – racism, poverty, stigma, marginalization, and discrimination play a role in the HIV epidemic. A barrier to preventing HIV infections among transgender youth is a lack of knowledge on how to provide them with comprehensive sexual and reproductive health care. Here are some tips and resources that can help you ensure that transgender youth are safe and healthy.
One of the challenges of obtaining a sexual history is asking the right questions For example, if you have a transgender male assigned female at birth, ask whether their partners produce sperm instead of asking about the sex of their partners. A transgender male’s partner may identify as female but is assigned male at birth and uses her penis during sex. Furthermore, a transgender male may be on testosterone, but he still can get pregnant. Asking how they use their organs is just as important. A transgender male who has condomless penile-vaginal sex with multiple partners is at a higher risk for HIV infection than is a transgender male who shares sex toys with his only partner.
Normalizing that you ask a comprehensive sexual history to all your patients regardless of gender identity may put the patient at ease. Many transgender people are reluctant to disclose their gender identity to their provider because they are afraid that the provider may fixate on their sexuality once they do. Stating that you ask sexual health questions to all your patients may prevent the transgender patient from feeling singled out.
Finally, you don’t have to ask a sexual history with every transgender patient, just as you wouldn’t for your cisgender patients. If a patient is complaining of a sprained ankle, a sexual history may not be helpful, compared with obtaining one when a patient comes in with pelvic pain. Many transgender patients avoid care because they are frequently asked about their sexual history or gender identity when these are not relevant to their chief complaint.
Here are some helpful questions to ask when taking a sexual history, according to the University of California, San Francisco, Transgender Care & Treatment Guidelines.1
- Are you having sex? How many sex partners have you had in the past year?
- Who are you having sex with? What types of sex are you having? What parts of your anatomy do you use for sex?
- How do you protect yourself from STIs?
- What STIs have you had in the past, if any? When were you last tested for STIs?
- Has your partner(s) ever been diagnosed with any STIs?
- Do you use alcohol or any drugs when you have sex?
- Do you exchange sex for money, drugs, or a place to stay?
Also, use a trauma-informed approach when working with transgender patients. Many have been victims of sexual trauma. Always have a chaperone accompany you during the exam, explain to the patient what you plan to do and why it is necessary, and allow them to decline (and document their declining the physical exam). Also consider having your patient self-swab for STI screening if appropriate.1
Like obtaining a sexual history, routine screenings for certain types of cancers will be based on the organs the patient has. For example, a transgender woman assigned male at birth will not need a cervical cancer screening, but a transgender man assigned female at birth may need one – if the patient still has a cervix. Cervical cancer screening guidelines are similar for transgender men as it is for nontransgender women, and one should use the same guidelines endorsed by the American Cancer Society, American Society of Colposcopy and Cervical Pathology, American Society of Clinical Pathologists, U.S. Preventive Services Task Force, and the World Health Organization.2-4
Cervical screenings should never be a requirement for testosterone therapy, and no transgender male under the age of 21 years will need cervical screening. The University of California guidelines offers tips on how to make transgender men more comfortable during cervical cancer screening.5
Contraception and menstrual management also are important for transgender patients. Testosterone can induce amenorrhea for transgender men, but it is not good birth control. If a transgender male patient has sex with partners that produce sperm, then the physician should discuss effective birth control options. There is no ideal birth control option for transgender men. One must consider multiple factors including the patient’s desire for pregnancy, desire to cease periods, ease of administration, and risk for thrombosis.
Most transgender men may balk at the idea of taking estrogen-containing contraception, but it is more effective than oral progestin-only pills. Intrauterine devices are highly effective in pregnancy prevention and can achieve amenorrhea in 50% of users within 1 year,but some transmen may become dysphoric with the procedure. 6 The etonogestrel implants also are highly effective birth control, but irregular periods are common, leading to discontinuation. Depot medroxyprogesterone is highly effective in preventing pregnancy and can induce amenorrhea in 70% of users within 1 year and 80% of users in 2 years, but also is associated with weight gain in one-third of users.7 Finally, pubertal blockers can rapidly stop periods for transmen who are severely dysphoric from their menses; however, before achieving amenorrhea, a flare bleed can occur 4-6 weeks after administration.8 Support from a mental health therapist during this time is critical. Pubertal blockers, nevertheless, are not suitable birth control.
When providing affirming sexual and reproductive health care for transgender patients, key principles include focusing on organs and activities over identity. Additionally, screening for certain types of cancers also is dependent on organs. Finally, do not neglect the importance of contraception among transgender men. Taking these principles in consideration will help you provide excellent care for transgender youth.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at the Children’s Hospital of Pittsburgh. He said he had no relevant financial disclosures. Email him at pdnews@mdedge.com.
References
1. Transgender people and sexually transmitted infections (https://transcare.ucsf.edu/guidelines/stis).
2. CA Cancer J Clin. 2012 May-Jun;62(3):147-72.
3. Ann Intern Med. 2012;156(12):880-91.
4. Cervical cancer screening in developing countries: Report of a WHO consultation. 2002. World Health Organization, Geneva.
5. Screening for cervical cancer for transgender men (https://transcare.ucsf.edu/guidelines/cervical-cancer).
6. Contraception. 2002 Feb;65(2):129-32.
7. Rev Endocr Metab Disord. 2011 Jun;12(2):93-106.
8. Int J Womens Health. 2014 Jun 23;6:631-7.
Resources
Breast cancer screening in transgender men. (https://transcare.ucsf.edu/guidelines/breast-cancer-men).
Screening for breast cancer in transgender women. (https://transcare.ucsf.edu/guidelines/breast-cancer-women).
Transgender health and HIV (https://transcare.ucsf.edu/guidelines/hiv).
Centers for Disease Control and Prevention: HIV and Transgender People (https://www.cdc.gov/hiv/group/gender/transgender/index.html).