User login
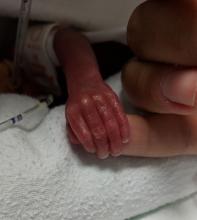
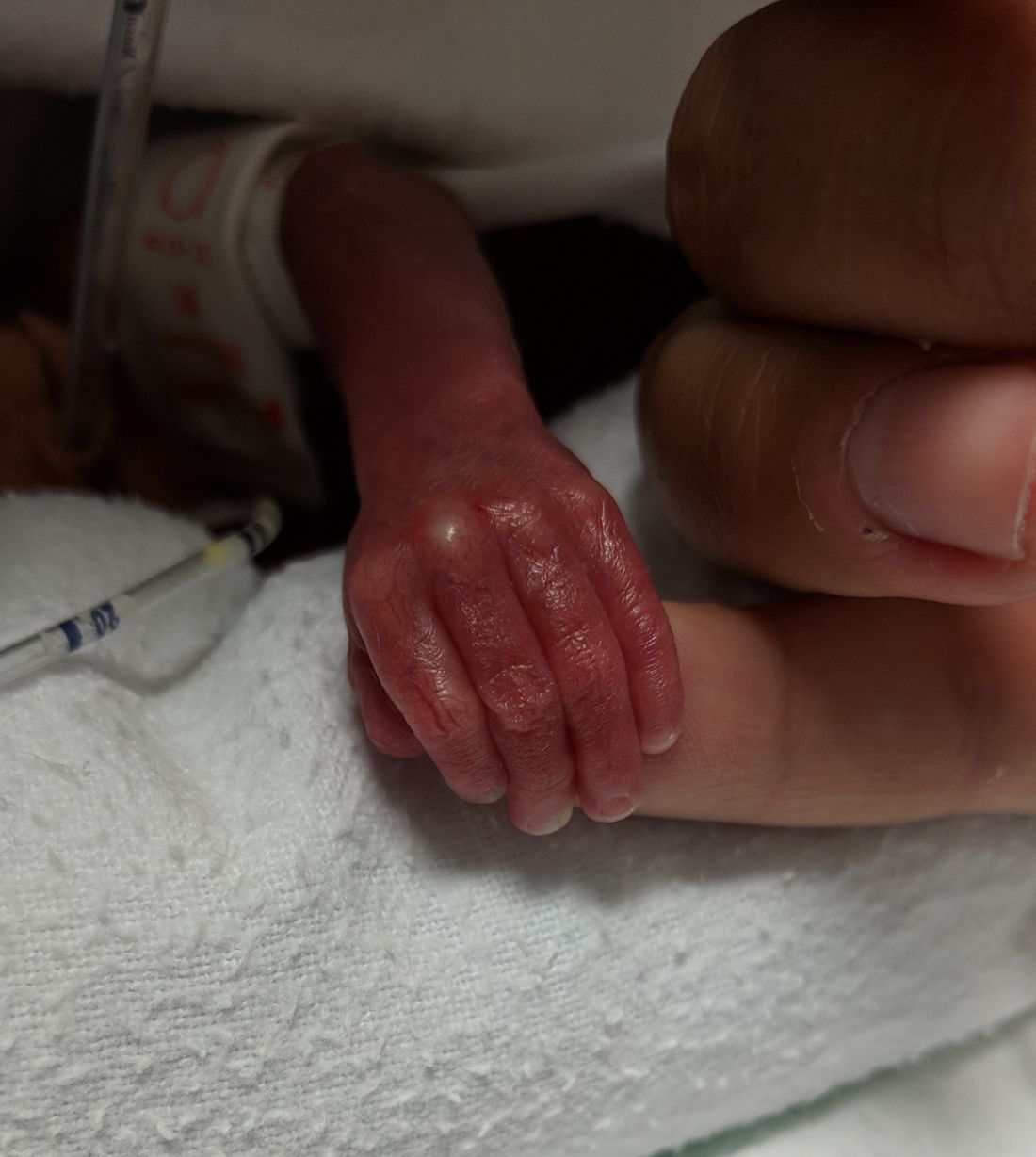
Cognitive problems after extremely preterm birth persist
Cognitive and neuropsychological impairment associated with extremely preterm (EP) birth persists into young adulthood, according to findings from the 1995 EPICure cohort.
Of note, intellectual impairment increased significantly after the age of 11 years among 19-year-olds in the cohort of individuals born EP, Helen O’Reilly, PhD, of the Institute for Women’s Health at University College London and colleagues reported in Pediatrics.
Neuropsychological assessment to examine general cognitive abilities, visuomotor abilities, prospective memory, and certain aspects of executive functioning and language in 127 cases and 64 term-born controls showed significantly lower scores across all tests in those born EP.
Impairment in at least one neuropsychological domain was present in 60% of EP birth cases (compared with 21% of controls), with 35% having impairment in at least four domains. Most deficits occurred in general cognitive function and/or visuomotor abilities.
Further, and those with cognitive impairment at 11 years were at increased risk of deficit at 19 years (RR, 3.56), even after adjustment for sex and socioeconomic status, the authors wrote.
None of the term-born controls had a cognitive impairment at 11 years, and two (3%) had impairment at 19 years.
Studies of adults born very preterm have revealed that these individuals are at risk for neuropsychological impairment, but the extent of such impairment in individuals with EP birth, defined as birth before 26 weeks’ gestation, had not previously been studied in the long term.
Assessments in the EPICure cohort of individuals born EP in 1995 previously showed scores at 1.1-1.6 standard deviations lower on measures of general cognitive function, compared with standardized norms and/or term-born controls, at age 2.5, 6, and 11 years, Dr. O’Reilly and colleagues explained.
The current findings indicate that general cognitive and neuropsychological functioning problems associated with EP birth persist and can increase into early adulthood, and they “highlight the need for early and ongoing neuropsychological and educational assessment in EP children to ensure these children receive appropriate support in school and for planned educational pathways,” the investigators concluded.
In an accompanying editorial, Louis A. Schmidt, PhD, and Saroj Saigal, MD, of McMaster University, Hamilton, Ont., wrote that these findings “provide compelling evidence for persistent effects of cognitive impairments” in individuals born EP.
They highlighted three lessons from the study:
- It is important to control for anxiety in future studies like this “to eliminate potential confounding influences of anxiety when examining performance-based measures in the laboratory setting,” as individuals born EP are known to exhibit anxiety.
- Group heterogeneity also should be considered, as all survivors of prematurity are not alike.
- Measurement equivalency should be established between groups.
With respect to the latter, “although many of the measures used by O’Reilly et al. have been normed, issues of measurement invariance have not been established between EP and control groups on some of the measures reported,” Dr. Schmidt and Dr. Saigal wrote, noting that “many other studies [also] fail to consider this fundamental measurement property.”
“Considering issues of measurement equivalency is of critical importance to ensuring unbiased interpretations of findings,” they added, concluding that the findings by O’Reilly et al. represent an important contribution and confirm findings from many prior studies of extreme prematurity, which “informs how we effectively manage these problems.”
“As the percentage of preterm birth continues to rise worldwide, coupled with reduced morbidity and mortality, and with more EP infants reaching adulthood, there is a need for prospective, long-term outcome studies of extreme prematurity,” Dr. Schmidt and Dr. Saigal added.
The study was funded by the Medical Research Council United Kingdom. The authors reported having no relevant financial disclosures. The editorial by Dr. Schmidt and Dr. Saigal, who also reported having no relevant financial disclosures, was supported by the Canadian Institutes of Health Research.
SOURCES: O’Reilly H et al. Pediatrics. 2020;145(2):e20192087; Schmidt LA, Saigal S. Pediatrics. 2020;145(2):e20193359.
Cognitive and neuropsychological impairment associated with extremely preterm (EP) birth persists into young adulthood, according to findings from the 1995 EPICure cohort.
Of note, intellectual impairment increased significantly after the age of 11 years among 19-year-olds in the cohort of individuals born EP, Helen O’Reilly, PhD, of the Institute for Women’s Health at University College London and colleagues reported in Pediatrics.
Neuropsychological assessment to examine general cognitive abilities, visuomotor abilities, prospective memory, and certain aspects of executive functioning and language in 127 cases and 64 term-born controls showed significantly lower scores across all tests in those born EP.
Impairment in at least one neuropsychological domain was present in 60% of EP birth cases (compared with 21% of controls), with 35% having impairment in at least four domains. Most deficits occurred in general cognitive function and/or visuomotor abilities.
Further, and those with cognitive impairment at 11 years were at increased risk of deficit at 19 years (RR, 3.56), even after adjustment for sex and socioeconomic status, the authors wrote.
None of the term-born controls had a cognitive impairment at 11 years, and two (3%) had impairment at 19 years.
Studies of adults born very preterm have revealed that these individuals are at risk for neuropsychological impairment, but the extent of such impairment in individuals with EP birth, defined as birth before 26 weeks’ gestation, had not previously been studied in the long term.
Assessments in the EPICure cohort of individuals born EP in 1995 previously showed scores at 1.1-1.6 standard deviations lower on measures of general cognitive function, compared with standardized norms and/or term-born controls, at age 2.5, 6, and 11 years, Dr. O’Reilly and colleagues explained.
The current findings indicate that general cognitive and neuropsychological functioning problems associated with EP birth persist and can increase into early adulthood, and they “highlight the need for early and ongoing neuropsychological and educational assessment in EP children to ensure these children receive appropriate support in school and for planned educational pathways,” the investigators concluded.
In an accompanying editorial, Louis A. Schmidt, PhD, and Saroj Saigal, MD, of McMaster University, Hamilton, Ont., wrote that these findings “provide compelling evidence for persistent effects of cognitive impairments” in individuals born EP.
They highlighted three lessons from the study:
- It is important to control for anxiety in future studies like this “to eliminate potential confounding influences of anxiety when examining performance-based measures in the laboratory setting,” as individuals born EP are known to exhibit anxiety.
- Group heterogeneity also should be considered, as all survivors of prematurity are not alike.
- Measurement equivalency should be established between groups.
With respect to the latter, “although many of the measures used by O’Reilly et al. have been normed, issues of measurement invariance have not been established between EP and control groups on some of the measures reported,” Dr. Schmidt and Dr. Saigal wrote, noting that “many other studies [also] fail to consider this fundamental measurement property.”
“Considering issues of measurement equivalency is of critical importance to ensuring unbiased interpretations of findings,” they added, concluding that the findings by O’Reilly et al. represent an important contribution and confirm findings from many prior studies of extreme prematurity, which “informs how we effectively manage these problems.”
“As the percentage of preterm birth continues to rise worldwide, coupled with reduced morbidity and mortality, and with more EP infants reaching adulthood, there is a need for prospective, long-term outcome studies of extreme prematurity,” Dr. Schmidt and Dr. Saigal added.
The study was funded by the Medical Research Council United Kingdom. The authors reported having no relevant financial disclosures. The editorial by Dr. Schmidt and Dr. Saigal, who also reported having no relevant financial disclosures, was supported by the Canadian Institutes of Health Research.
SOURCES: O’Reilly H et al. Pediatrics. 2020;145(2):e20192087; Schmidt LA, Saigal S. Pediatrics. 2020;145(2):e20193359.
Cognitive and neuropsychological impairment associated with extremely preterm (EP) birth persists into young adulthood, according to findings from the 1995 EPICure cohort.
Of note, intellectual impairment increased significantly after the age of 11 years among 19-year-olds in the cohort of individuals born EP, Helen O’Reilly, PhD, of the Institute for Women’s Health at University College London and colleagues reported in Pediatrics.
Neuropsychological assessment to examine general cognitive abilities, visuomotor abilities, prospective memory, and certain aspects of executive functioning and language in 127 cases and 64 term-born controls showed significantly lower scores across all tests in those born EP.
Impairment in at least one neuropsychological domain was present in 60% of EP birth cases (compared with 21% of controls), with 35% having impairment in at least four domains. Most deficits occurred in general cognitive function and/or visuomotor abilities.
Further, and those with cognitive impairment at 11 years were at increased risk of deficit at 19 years (RR, 3.56), even after adjustment for sex and socioeconomic status, the authors wrote.
None of the term-born controls had a cognitive impairment at 11 years, and two (3%) had impairment at 19 years.
Studies of adults born very preterm have revealed that these individuals are at risk for neuropsychological impairment, but the extent of such impairment in individuals with EP birth, defined as birth before 26 weeks’ gestation, had not previously been studied in the long term.
Assessments in the EPICure cohort of individuals born EP in 1995 previously showed scores at 1.1-1.6 standard deviations lower on measures of general cognitive function, compared with standardized norms and/or term-born controls, at age 2.5, 6, and 11 years, Dr. O’Reilly and colleagues explained.
The current findings indicate that general cognitive and neuropsychological functioning problems associated with EP birth persist and can increase into early adulthood, and they “highlight the need for early and ongoing neuropsychological and educational assessment in EP children to ensure these children receive appropriate support in school and for planned educational pathways,” the investigators concluded.
In an accompanying editorial, Louis A. Schmidt, PhD, and Saroj Saigal, MD, of McMaster University, Hamilton, Ont., wrote that these findings “provide compelling evidence for persistent effects of cognitive impairments” in individuals born EP.
They highlighted three lessons from the study:
- It is important to control for anxiety in future studies like this “to eliminate potential confounding influences of anxiety when examining performance-based measures in the laboratory setting,” as individuals born EP are known to exhibit anxiety.
- Group heterogeneity also should be considered, as all survivors of prematurity are not alike.
- Measurement equivalency should be established between groups.
With respect to the latter, “although many of the measures used by O’Reilly et al. have been normed, issues of measurement invariance have not been established between EP and control groups on some of the measures reported,” Dr. Schmidt and Dr. Saigal wrote, noting that “many other studies [also] fail to consider this fundamental measurement property.”
“Considering issues of measurement equivalency is of critical importance to ensuring unbiased interpretations of findings,” they added, concluding that the findings by O’Reilly et al. represent an important contribution and confirm findings from many prior studies of extreme prematurity, which “informs how we effectively manage these problems.”
“As the percentage of preterm birth continues to rise worldwide, coupled with reduced morbidity and mortality, and with more EP infants reaching adulthood, there is a need for prospective, long-term outcome studies of extreme prematurity,” Dr. Schmidt and Dr. Saigal added.
The study was funded by the Medical Research Council United Kingdom. The authors reported having no relevant financial disclosures. The editorial by Dr. Schmidt and Dr. Saigal, who also reported having no relevant financial disclosures, was supported by the Canadian Institutes of Health Research.
SOURCES: O’Reilly H et al. Pediatrics. 2020;145(2):e20192087; Schmidt LA, Saigal S. Pediatrics. 2020;145(2):e20193359.
FROM PEDIATRICS
Experts in Europe issue guidance on atopic dermatitis in pregnancy
MADRID – European atopic dermatitis experts have issued formal guidance on a seriously neglected topic: treatment of the disease during pregnancy, breastfeeding, and in men planning to father children.
The impetus for the project was clear: “,” Christian Vestergaard, MD, PhD, declared at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
He presented highlights of the task force’s position paper on the topic, for which he served as first author. The group’s recommendations are based on expert opinion, since randomized clinical trial literature in this area is nonexistent because of ethical concerns. But the task force, comprising a who’s who in European dermatology, drew on a wealth of collective clinical experience in this area.
“We have all of Europe involved in doing this position statement. It’s meant as what we think is proper treatment and what we can say about the different drugs,” explained Dr. Vestergaard, a dermatologist at the University of Aarhus (Denmark).
Most nonobstetricians are intimidated by atopic dermatitis (AD) in pregnancy, and are concerned about the potential for treatment-related harm to the fetus. As a consequence, they are reluctant to recommend anything beyond weak class I topical corticosteroids and emollients. That’s clearly insufficient in light of the vast scope of need, he asserted. After all, AD affects 15%-20% of all children and persists or reappears in adulthood in one out of five of them. Half of those adults are women, many of whom will at some point wish to become pregnant. And many men with AD will eventually want to father children.
A key message from the task force is that untreated AD in pregnancy potentially places the mother and fetus at risk of serious complications, including Staphylococcus aureus infection and eczema herpeticum.
“If you take one thing away from our position paper, it’s that you can use class II or III topical corticosteroids in pregnant women as first-line therapy,” Dr. Vestergaard said.
This stance contradicts a longstanding widely held concern that topical steroids in pregnancy might increase the risk of facial cleft in the offspring, a worry that has been convincingly debunked in a Cochrane systematic review of 14 studies including more than 1.6 million pregnancies. The report concluded there was no association between topical corticosteroids of any potency with preterm delivery, birth defects, or low Apgar scores (Cochrane Database Syst Rev. 2015 Oct 26. doi: 10.1002/14651858.CD007346.pub3).
The task force recommends that if class II or III topical corticosteroid use in pregnancy exceeds 200 g/month, it’s worth considering add-on UV therapy, with narrow band UVB-311 nm as the regimen of choice; it can be used liberally. UV therapy with psoralens is not advised because of a theoretical risk of mutagenicity.
Product labeling for the topical calcineurin inhibitors declares that the agents should not be used during pregnancy. However, the European task force position paper takes issue with that and declares that topical tacrolimus (Protopic) can be considered an off-label first-line therapy in pregnant women with an insufficient response to liberal use of emollients. The same holds true for breastfeeding patients with AD. Just as when topical corticosteroids are used in the nipple area, topical tacrolimus should be applied after nursing, and the nipple area should be gently cleaned before nursing.
The rationale behind recommending topical tacrolimus as a first-line treatment is that systemic absorption of the drug is trivial. Plus, observational studies of oral tacrolimus in pregnant women who have received a solid organ transplant have shown no increase in congenital malformations.
The task force recommends against the use of topical pimecrolimus (Elidel) or crisaborole (Eucrisa) in pregnancy or lactation due to lack of clinical experience in these settings, Dr. Vestergaard continued.
The task force position is that chlorhexidine and other topical antiseptics – with the notable exception of triclosan – can be used in pregnancy to prevent recurrent skin infections. Aminoglycosides should be avoided, but topical fusidic acid is a reasonable antibiotic for treatment of small areas of clinically infected atopic dermatitis in pregnancy.
Systemic therapies
If disease control is insufficient with topical therapy, it’s appropriate to engage in shared decision-making with the patient regarding systemic treatment. She needs to understand up front that the worldwide overall background stillbirth rate in the general population is about 3%, and that severe congenital malformations are present in up to 6% of all live births.
“You need to inform them that they can have systemic therapy and give birth to a child with congenital defects which have nothing to do with the medication,” noted Dr. Vestergaard.
That said, the task force recommends cyclosporine as the off-label, first-line systemic therapy in pregnancy and lactation when long-term treatment is required. This guidance is based largely upon reassuring evidence in solid organ transplant recipients.
The recommended second-line therapy is systemic corticosteroids, but it’s a qualified recommendation. Dr. Vestergaard and colleagues find that systemic corticosteroid therapy is only rarely needed in pregnant AD patients, and the task force recommendation is to limit the use to less than 2-3 weeks and no more than 0.5 mg/kg per day of prednisone. Dexamethasone is not recommended.
Azathioprine should not be started in pregnancy, according to the task force, but when no other options are available, it may be continued in women already on the drug, albeit at half of the prepregnancy dose.
Dupilumab (Dupixent) is to be avoided in pregnant women with AD until more clinical experience becomes available.
Treatment of prospective fathers with AD
The European task force recommends that topical therapies can be prescribed in prospective fathers without any special concerns. The same is true for systemic corticosteroids. Methotrexate should be halted 3 months before planned pregnancy, as is the case for mycophenolate mofetil (CellCept). Azathioprine is recommended when other options have failed. Cyclosporine is deemed a reasonable option in the treatment of men with severe AD at the time of conception if other treatments have failed; of note, neither the Food and Drug Administration nor the European regulatory agency have issued contraindications for the use of the drug in men who wish to become fathers.
Mycophenolate mofetil carries a theoretical risk of teratogenicity. The European task force recommends that men should use condoms while on the drug and for at least 90 days afterward.
Unplanned pregnancy in women on systemic therapy
The recommended course of action is to immediately stop systemic therapy, intensify appropriate topical therapy in anticipation of worsening AD, and refer the patient to an obstetrician and a teratology information center for an individualized risk assessment. Methotrexate and mycophenolate mofetil are known teratogens.
The full 16-page task force position paper was published shortly before EADV 2019 (J Eur Acad Dermatol Venereol. 2019 Sep;33[9]:1644-59).
The report was developed without commercial sponsorship. Dr. Vestergaard indicated he has received research grants from and/or serves as a consultant to eight pharmaceutical companies.
Jenny Murase, MD, is with the department of dermatology, University of California, San Francisco, and is the director of medical consultative dermatology at the Palo Alto Foundation Medical Group, Mountain View, Calif. She has served on advisory boards for Dermira, Sanofi, and UCB; performed dermatologic consulting for UpToDate and Ferndale, and given nonbranded lectures for disease state management awareness for Regeneron and UCB.
Jenny Murase, MD, is with the department of dermatology, University of California, San Francisco, and is the director of medical consultative dermatology at the Palo Alto Foundation Medical Group, Mountain View, Calif. She has served on advisory boards for Dermira, Sanofi, and UCB; performed dermatologic consulting for UpToDate and Ferndale, and given nonbranded lectures for disease state management awareness for Regeneron and UCB.
Jenny Murase, MD, is with the department of dermatology, University of California, San Francisco, and is the director of medical consultative dermatology at the Palo Alto Foundation Medical Group, Mountain View, Calif. She has served on advisory boards for Dermira, Sanofi, and UCB; performed dermatologic consulting for UpToDate and Ferndale, and given nonbranded lectures for disease state management awareness for Regeneron and UCB.
MADRID – European atopic dermatitis experts have issued formal guidance on a seriously neglected topic: treatment of the disease during pregnancy, breastfeeding, and in men planning to father children.
The impetus for the project was clear: “,” Christian Vestergaard, MD, PhD, declared at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
He presented highlights of the task force’s position paper on the topic, for which he served as first author. The group’s recommendations are based on expert opinion, since randomized clinical trial literature in this area is nonexistent because of ethical concerns. But the task force, comprising a who’s who in European dermatology, drew on a wealth of collective clinical experience in this area.
“We have all of Europe involved in doing this position statement. It’s meant as what we think is proper treatment and what we can say about the different drugs,” explained Dr. Vestergaard, a dermatologist at the University of Aarhus (Denmark).
Most nonobstetricians are intimidated by atopic dermatitis (AD) in pregnancy, and are concerned about the potential for treatment-related harm to the fetus. As a consequence, they are reluctant to recommend anything beyond weak class I topical corticosteroids and emollients. That’s clearly insufficient in light of the vast scope of need, he asserted. After all, AD affects 15%-20% of all children and persists or reappears in adulthood in one out of five of them. Half of those adults are women, many of whom will at some point wish to become pregnant. And many men with AD will eventually want to father children.
A key message from the task force is that untreated AD in pregnancy potentially places the mother and fetus at risk of serious complications, including Staphylococcus aureus infection and eczema herpeticum.
“If you take one thing away from our position paper, it’s that you can use class II or III topical corticosteroids in pregnant women as first-line therapy,” Dr. Vestergaard said.
This stance contradicts a longstanding widely held concern that topical steroids in pregnancy might increase the risk of facial cleft in the offspring, a worry that has been convincingly debunked in a Cochrane systematic review of 14 studies including more than 1.6 million pregnancies. The report concluded there was no association between topical corticosteroids of any potency with preterm delivery, birth defects, or low Apgar scores (Cochrane Database Syst Rev. 2015 Oct 26. doi: 10.1002/14651858.CD007346.pub3).
The task force recommends that if class II or III topical corticosteroid use in pregnancy exceeds 200 g/month, it’s worth considering add-on UV therapy, with narrow band UVB-311 nm as the regimen of choice; it can be used liberally. UV therapy with psoralens is not advised because of a theoretical risk of mutagenicity.
Product labeling for the topical calcineurin inhibitors declares that the agents should not be used during pregnancy. However, the European task force position paper takes issue with that and declares that topical tacrolimus (Protopic) can be considered an off-label first-line therapy in pregnant women with an insufficient response to liberal use of emollients. The same holds true for breastfeeding patients with AD. Just as when topical corticosteroids are used in the nipple area, topical tacrolimus should be applied after nursing, and the nipple area should be gently cleaned before nursing.
The rationale behind recommending topical tacrolimus as a first-line treatment is that systemic absorption of the drug is trivial. Plus, observational studies of oral tacrolimus in pregnant women who have received a solid organ transplant have shown no increase in congenital malformations.
The task force recommends against the use of topical pimecrolimus (Elidel) or crisaborole (Eucrisa) in pregnancy or lactation due to lack of clinical experience in these settings, Dr. Vestergaard continued.
The task force position is that chlorhexidine and other topical antiseptics – with the notable exception of triclosan – can be used in pregnancy to prevent recurrent skin infections. Aminoglycosides should be avoided, but topical fusidic acid is a reasonable antibiotic for treatment of small areas of clinically infected atopic dermatitis in pregnancy.
Systemic therapies
If disease control is insufficient with topical therapy, it’s appropriate to engage in shared decision-making with the patient regarding systemic treatment. She needs to understand up front that the worldwide overall background stillbirth rate in the general population is about 3%, and that severe congenital malformations are present in up to 6% of all live births.
“You need to inform them that they can have systemic therapy and give birth to a child with congenital defects which have nothing to do with the medication,” noted Dr. Vestergaard.
That said, the task force recommends cyclosporine as the off-label, first-line systemic therapy in pregnancy and lactation when long-term treatment is required. This guidance is based largely upon reassuring evidence in solid organ transplant recipients.
The recommended second-line therapy is systemic corticosteroids, but it’s a qualified recommendation. Dr. Vestergaard and colleagues find that systemic corticosteroid therapy is only rarely needed in pregnant AD patients, and the task force recommendation is to limit the use to less than 2-3 weeks and no more than 0.5 mg/kg per day of prednisone. Dexamethasone is not recommended.
Azathioprine should not be started in pregnancy, according to the task force, but when no other options are available, it may be continued in women already on the drug, albeit at half of the prepregnancy dose.
Dupilumab (Dupixent) is to be avoided in pregnant women with AD until more clinical experience becomes available.
Treatment of prospective fathers with AD
The European task force recommends that topical therapies can be prescribed in prospective fathers without any special concerns. The same is true for systemic corticosteroids. Methotrexate should be halted 3 months before planned pregnancy, as is the case for mycophenolate mofetil (CellCept). Azathioprine is recommended when other options have failed. Cyclosporine is deemed a reasonable option in the treatment of men with severe AD at the time of conception if other treatments have failed; of note, neither the Food and Drug Administration nor the European regulatory agency have issued contraindications for the use of the drug in men who wish to become fathers.
Mycophenolate mofetil carries a theoretical risk of teratogenicity. The European task force recommends that men should use condoms while on the drug and for at least 90 days afterward.
Unplanned pregnancy in women on systemic therapy
The recommended course of action is to immediately stop systemic therapy, intensify appropriate topical therapy in anticipation of worsening AD, and refer the patient to an obstetrician and a teratology information center for an individualized risk assessment. Methotrexate and mycophenolate mofetil are known teratogens.
The full 16-page task force position paper was published shortly before EADV 2019 (J Eur Acad Dermatol Venereol. 2019 Sep;33[9]:1644-59).
The report was developed without commercial sponsorship. Dr. Vestergaard indicated he has received research grants from and/or serves as a consultant to eight pharmaceutical companies.
MADRID – European atopic dermatitis experts have issued formal guidance on a seriously neglected topic: treatment of the disease during pregnancy, breastfeeding, and in men planning to father children.
The impetus for the project was clear: “,” Christian Vestergaard, MD, PhD, declared at a meeting of the European Task Force on Atopic Dermatitis held in conjunction with the annual congress of the European Academy of Dermatology and Venereology.
He presented highlights of the task force’s position paper on the topic, for which he served as first author. The group’s recommendations are based on expert opinion, since randomized clinical trial literature in this area is nonexistent because of ethical concerns. But the task force, comprising a who’s who in European dermatology, drew on a wealth of collective clinical experience in this area.
“We have all of Europe involved in doing this position statement. It’s meant as what we think is proper treatment and what we can say about the different drugs,” explained Dr. Vestergaard, a dermatologist at the University of Aarhus (Denmark).
Most nonobstetricians are intimidated by atopic dermatitis (AD) in pregnancy, and are concerned about the potential for treatment-related harm to the fetus. As a consequence, they are reluctant to recommend anything beyond weak class I topical corticosteroids and emollients. That’s clearly insufficient in light of the vast scope of need, he asserted. After all, AD affects 15%-20% of all children and persists or reappears in adulthood in one out of five of them. Half of those adults are women, many of whom will at some point wish to become pregnant. And many men with AD will eventually want to father children.
A key message from the task force is that untreated AD in pregnancy potentially places the mother and fetus at risk of serious complications, including Staphylococcus aureus infection and eczema herpeticum.
“If you take one thing away from our position paper, it’s that you can use class II or III topical corticosteroids in pregnant women as first-line therapy,” Dr. Vestergaard said.
This stance contradicts a longstanding widely held concern that topical steroids in pregnancy might increase the risk of facial cleft in the offspring, a worry that has been convincingly debunked in a Cochrane systematic review of 14 studies including more than 1.6 million pregnancies. The report concluded there was no association between topical corticosteroids of any potency with preterm delivery, birth defects, or low Apgar scores (Cochrane Database Syst Rev. 2015 Oct 26. doi: 10.1002/14651858.CD007346.pub3).
The task force recommends that if class II or III topical corticosteroid use in pregnancy exceeds 200 g/month, it’s worth considering add-on UV therapy, with narrow band UVB-311 nm as the regimen of choice; it can be used liberally. UV therapy with psoralens is not advised because of a theoretical risk of mutagenicity.
Product labeling for the topical calcineurin inhibitors declares that the agents should not be used during pregnancy. However, the European task force position paper takes issue with that and declares that topical tacrolimus (Protopic) can be considered an off-label first-line therapy in pregnant women with an insufficient response to liberal use of emollients. The same holds true for breastfeeding patients with AD. Just as when topical corticosteroids are used in the nipple area, topical tacrolimus should be applied after nursing, and the nipple area should be gently cleaned before nursing.
The rationale behind recommending topical tacrolimus as a first-line treatment is that systemic absorption of the drug is trivial. Plus, observational studies of oral tacrolimus in pregnant women who have received a solid organ transplant have shown no increase in congenital malformations.
The task force recommends against the use of topical pimecrolimus (Elidel) or crisaborole (Eucrisa) in pregnancy or lactation due to lack of clinical experience in these settings, Dr. Vestergaard continued.
The task force position is that chlorhexidine and other topical antiseptics – with the notable exception of triclosan – can be used in pregnancy to prevent recurrent skin infections. Aminoglycosides should be avoided, but topical fusidic acid is a reasonable antibiotic for treatment of small areas of clinically infected atopic dermatitis in pregnancy.
Systemic therapies
If disease control is insufficient with topical therapy, it’s appropriate to engage in shared decision-making with the patient regarding systemic treatment. She needs to understand up front that the worldwide overall background stillbirth rate in the general population is about 3%, and that severe congenital malformations are present in up to 6% of all live births.
“You need to inform them that they can have systemic therapy and give birth to a child with congenital defects which have nothing to do with the medication,” noted Dr. Vestergaard.
That said, the task force recommends cyclosporine as the off-label, first-line systemic therapy in pregnancy and lactation when long-term treatment is required. This guidance is based largely upon reassuring evidence in solid organ transplant recipients.
The recommended second-line therapy is systemic corticosteroids, but it’s a qualified recommendation. Dr. Vestergaard and colleagues find that systemic corticosteroid therapy is only rarely needed in pregnant AD patients, and the task force recommendation is to limit the use to less than 2-3 weeks and no more than 0.5 mg/kg per day of prednisone. Dexamethasone is not recommended.
Azathioprine should not be started in pregnancy, according to the task force, but when no other options are available, it may be continued in women already on the drug, albeit at half of the prepregnancy dose.
Dupilumab (Dupixent) is to be avoided in pregnant women with AD until more clinical experience becomes available.
Treatment of prospective fathers with AD
The European task force recommends that topical therapies can be prescribed in prospective fathers without any special concerns. The same is true for systemic corticosteroids. Methotrexate should be halted 3 months before planned pregnancy, as is the case for mycophenolate mofetil (CellCept). Azathioprine is recommended when other options have failed. Cyclosporine is deemed a reasonable option in the treatment of men with severe AD at the time of conception if other treatments have failed; of note, neither the Food and Drug Administration nor the European regulatory agency have issued contraindications for the use of the drug in men who wish to become fathers.
Mycophenolate mofetil carries a theoretical risk of teratogenicity. The European task force recommends that men should use condoms while on the drug and for at least 90 days afterward.
Unplanned pregnancy in women on systemic therapy
The recommended course of action is to immediately stop systemic therapy, intensify appropriate topical therapy in anticipation of worsening AD, and refer the patient to an obstetrician and a teratology information center for an individualized risk assessment. Methotrexate and mycophenolate mofetil are known teratogens.
The full 16-page task force position paper was published shortly before EADV 2019 (J Eur Acad Dermatol Venereol. 2019 Sep;33[9]:1644-59).
The report was developed without commercial sponsorship. Dr. Vestergaard indicated he has received research grants from and/or serves as a consultant to eight pharmaceutical companies.
REPORTING FROM THE EADV CONGRESS
Ideal management of RA in pregnancy improves outcomes
Women whose rheumatoid arthritis is carefully managed before and during pregnancy have a significantly lower risk of adverse pregnancy outcomes, including miscarriage or perinatal death, new research suggests.
A study published in Arthritis Care & Research presents the outcomes of a retrospective, observational study examining health care data from 443 first pregnancies in women with RA and 6,097 women without the disease.
First author Alessandra Bortoluzzi, MD, PhD, from the Rheumatology Unit at the University of Ferrara (Italy) and coauthors looked at seven diagnostic, therapeutic, and follow-up health care quality indicators during the prepregnancy and perinatal period. They included having at least one blood test in the 18 months before conception and during pregnancy, preconception musculoskeletal imaging, no exposure or wash-out from teratogenic drugs, and no exposure to biologic drugs between conception and delivery or end of pregnancy.
An ideal clinical pathway included at least one element from each of the diagnostic, therapeutic, and prenatal follow-up quality indicators.
Overall, women with RA had a significantly higher rate of thyroid diseases, adverse pregnancy outcomes, and miscarriage or perinatal death when compared with controls. However, those who followed the ideal clinical pathway for management of their disease during pregnancy had a 40% lower odds of adverse pregnancy outcomes (odds ratio, 0.60; 95% confidence interval, 0.39-0.94) and a 60% lower odds of miscarriage or perinatal death (OR, 0.40; 95% CI, 0.24-0.69) in comparison with women with RA who were not managed to the same standard. The researchers adjusted both comparisons for age, Charlson comorbidity index, and thyroid diseases.
Women with RA who met diagnostic, therapeutic, and prenatal follow-up quality indicators showed no significant differences from the general population in terms of the risk of adverse pregnancy outcomes, miscarriage, or perinatal death after adjusting for hypertension in addition to the same variables as before.
When researchers looked at some of the individual health care quality indicators, they found that testing for antiphospholipid (aPL) antibodies within 18 months of conception or pregnancy was associated with a 44% lower rate of adverse pregnancy outcomes. Similarly, antinuclear antibody or anti–extractable nuclear antigen antibody testing was associated with 36% lower odds of adverse pregnancy outcomes.
Dr. Bortoluzzi and her coauthors wrote that their findings pointed to the value of testing for aPL antibodies in women with RA who wish to get pregnant.
“In fact, despite the absence of formal recommendation or validated health care quality indicators focused on stratification of preconceptional obstetric risk in patients with RA, we started from the basic and universally accepted assumption that aPL antibodies are pathogenic autoantibodies and therefore recognized risk factors for adverse pregnancy outcome,” they wrote.
Women with RA who had either no exposure to methotrexate or leflunomide or who had a washout period from 6 months prior to conception had 72% lower odds of adverse pregnancy outcomes.
The authors also looked at the effects of drugs such as aspirin, glucocorticoids, and low-molecular-weight heparin that are used during pregnancy. They found that the relative risk of adverse pregnancy outcomes was 40% higher in women with RA who were taking glucocorticoids, compared with those with the disease but not taking that type of medication. However, low-molecular-weight heparin use was associated with an 80% lower relative risk of miscarriage or perinatal death in comparison with those not taking it. Researchers saw no significant effects of aspirin or conventional synthetic disease-modifying antirheumatic drugs on either adverse pregnancy outcomes or the risk of miscarriage or perinatal death.
“This reinforces the importance of adjustment of therapy for RA before conception and throughout pregnancy, because medication use could affect pregnancy course not only influencing maternal disease activity but also the gestational outcome,” the authors wrote. “Although this is a study conducted on administrative data, we can hypothesize that exposure to therapy represents a marker of high RA disease activity and severity. In our setting, it is possible that, the more active the disease, the greater the probability of being included in the ideal clinical pathway, but in any case, this resulted in a lower odds ratio of adverse pregnancy outcome and miscarriage/perinatal death.”
The study was supported by the Italian Society for Rheumatology. No conflicts of interest were declared.
SOURCE: Bortoluzzi A et al. Arthritis Care Res. 2020 Jan 8. doi: 10.1002/ACR.24116.
Women whose rheumatoid arthritis is carefully managed before and during pregnancy have a significantly lower risk of adverse pregnancy outcomes, including miscarriage or perinatal death, new research suggests.
A study published in Arthritis Care & Research presents the outcomes of a retrospective, observational study examining health care data from 443 first pregnancies in women with RA and 6,097 women without the disease.
First author Alessandra Bortoluzzi, MD, PhD, from the Rheumatology Unit at the University of Ferrara (Italy) and coauthors looked at seven diagnostic, therapeutic, and follow-up health care quality indicators during the prepregnancy and perinatal period. They included having at least one blood test in the 18 months before conception and during pregnancy, preconception musculoskeletal imaging, no exposure or wash-out from teratogenic drugs, and no exposure to biologic drugs between conception and delivery or end of pregnancy.
An ideal clinical pathway included at least one element from each of the diagnostic, therapeutic, and prenatal follow-up quality indicators.
Overall, women with RA had a significantly higher rate of thyroid diseases, adverse pregnancy outcomes, and miscarriage or perinatal death when compared with controls. However, those who followed the ideal clinical pathway for management of their disease during pregnancy had a 40% lower odds of adverse pregnancy outcomes (odds ratio, 0.60; 95% confidence interval, 0.39-0.94) and a 60% lower odds of miscarriage or perinatal death (OR, 0.40; 95% CI, 0.24-0.69) in comparison with women with RA who were not managed to the same standard. The researchers adjusted both comparisons for age, Charlson comorbidity index, and thyroid diseases.
Women with RA who met diagnostic, therapeutic, and prenatal follow-up quality indicators showed no significant differences from the general population in terms of the risk of adverse pregnancy outcomes, miscarriage, or perinatal death after adjusting for hypertension in addition to the same variables as before.
When researchers looked at some of the individual health care quality indicators, they found that testing for antiphospholipid (aPL) antibodies within 18 months of conception or pregnancy was associated with a 44% lower rate of adverse pregnancy outcomes. Similarly, antinuclear antibody or anti–extractable nuclear antigen antibody testing was associated with 36% lower odds of adverse pregnancy outcomes.
Dr. Bortoluzzi and her coauthors wrote that their findings pointed to the value of testing for aPL antibodies in women with RA who wish to get pregnant.
“In fact, despite the absence of formal recommendation or validated health care quality indicators focused on stratification of preconceptional obstetric risk in patients with RA, we started from the basic and universally accepted assumption that aPL antibodies are pathogenic autoantibodies and therefore recognized risk factors for adverse pregnancy outcome,” they wrote.
Women with RA who had either no exposure to methotrexate or leflunomide or who had a washout period from 6 months prior to conception had 72% lower odds of adverse pregnancy outcomes.
The authors also looked at the effects of drugs such as aspirin, glucocorticoids, and low-molecular-weight heparin that are used during pregnancy. They found that the relative risk of adverse pregnancy outcomes was 40% higher in women with RA who were taking glucocorticoids, compared with those with the disease but not taking that type of medication. However, low-molecular-weight heparin use was associated with an 80% lower relative risk of miscarriage or perinatal death in comparison with those not taking it. Researchers saw no significant effects of aspirin or conventional synthetic disease-modifying antirheumatic drugs on either adverse pregnancy outcomes or the risk of miscarriage or perinatal death.
“This reinforces the importance of adjustment of therapy for RA before conception and throughout pregnancy, because medication use could affect pregnancy course not only influencing maternal disease activity but also the gestational outcome,” the authors wrote. “Although this is a study conducted on administrative data, we can hypothesize that exposure to therapy represents a marker of high RA disease activity and severity. In our setting, it is possible that, the more active the disease, the greater the probability of being included in the ideal clinical pathway, but in any case, this resulted in a lower odds ratio of adverse pregnancy outcome and miscarriage/perinatal death.”
The study was supported by the Italian Society for Rheumatology. No conflicts of interest were declared.
SOURCE: Bortoluzzi A et al. Arthritis Care Res. 2020 Jan 8. doi: 10.1002/ACR.24116.
Women whose rheumatoid arthritis is carefully managed before and during pregnancy have a significantly lower risk of adverse pregnancy outcomes, including miscarriage or perinatal death, new research suggests.
A study published in Arthritis Care & Research presents the outcomes of a retrospective, observational study examining health care data from 443 first pregnancies in women with RA and 6,097 women without the disease.
First author Alessandra Bortoluzzi, MD, PhD, from the Rheumatology Unit at the University of Ferrara (Italy) and coauthors looked at seven diagnostic, therapeutic, and follow-up health care quality indicators during the prepregnancy and perinatal period. They included having at least one blood test in the 18 months before conception and during pregnancy, preconception musculoskeletal imaging, no exposure or wash-out from teratogenic drugs, and no exposure to biologic drugs between conception and delivery or end of pregnancy.
An ideal clinical pathway included at least one element from each of the diagnostic, therapeutic, and prenatal follow-up quality indicators.
Overall, women with RA had a significantly higher rate of thyroid diseases, adverse pregnancy outcomes, and miscarriage or perinatal death when compared with controls. However, those who followed the ideal clinical pathway for management of their disease during pregnancy had a 40% lower odds of adverse pregnancy outcomes (odds ratio, 0.60; 95% confidence interval, 0.39-0.94) and a 60% lower odds of miscarriage or perinatal death (OR, 0.40; 95% CI, 0.24-0.69) in comparison with women with RA who were not managed to the same standard. The researchers adjusted both comparisons for age, Charlson comorbidity index, and thyroid diseases.
Women with RA who met diagnostic, therapeutic, and prenatal follow-up quality indicators showed no significant differences from the general population in terms of the risk of adverse pregnancy outcomes, miscarriage, or perinatal death after adjusting for hypertension in addition to the same variables as before.
When researchers looked at some of the individual health care quality indicators, they found that testing for antiphospholipid (aPL) antibodies within 18 months of conception or pregnancy was associated with a 44% lower rate of adverse pregnancy outcomes. Similarly, antinuclear antibody or anti–extractable nuclear antigen antibody testing was associated with 36% lower odds of adverse pregnancy outcomes.
Dr. Bortoluzzi and her coauthors wrote that their findings pointed to the value of testing for aPL antibodies in women with RA who wish to get pregnant.
“In fact, despite the absence of formal recommendation or validated health care quality indicators focused on stratification of preconceptional obstetric risk in patients with RA, we started from the basic and universally accepted assumption that aPL antibodies are pathogenic autoantibodies and therefore recognized risk factors for adverse pregnancy outcome,” they wrote.
Women with RA who had either no exposure to methotrexate or leflunomide or who had a washout period from 6 months prior to conception had 72% lower odds of adverse pregnancy outcomes.
The authors also looked at the effects of drugs such as aspirin, glucocorticoids, and low-molecular-weight heparin that are used during pregnancy. They found that the relative risk of adverse pregnancy outcomes was 40% higher in women with RA who were taking glucocorticoids, compared with those with the disease but not taking that type of medication. However, low-molecular-weight heparin use was associated with an 80% lower relative risk of miscarriage or perinatal death in comparison with those not taking it. Researchers saw no significant effects of aspirin or conventional synthetic disease-modifying antirheumatic drugs on either adverse pregnancy outcomes or the risk of miscarriage or perinatal death.
“This reinforces the importance of adjustment of therapy for RA before conception and throughout pregnancy, because medication use could affect pregnancy course not only influencing maternal disease activity but also the gestational outcome,” the authors wrote. “Although this is a study conducted on administrative data, we can hypothesize that exposure to therapy represents a marker of high RA disease activity and severity. In our setting, it is possible that, the more active the disease, the greater the probability of being included in the ideal clinical pathway, but in any case, this resulted in a lower odds ratio of adverse pregnancy outcome and miscarriage/perinatal death.”
The study was supported by the Italian Society for Rheumatology. No conflicts of interest were declared.
SOURCE: Bortoluzzi A et al. Arthritis Care Res. 2020 Jan 8. doi: 10.1002/ACR.24116.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Well-managed rheumatoid arthritis during preconception and pregnancy is associated with improved pregnancy outcomes.
Major finding: Women who adhered to an ideal clinical pathway for their RA had significantly lower risk of adverse pregnancy outcomes and miscarriage and/or perinatal death.
Study details: Retrospective, observational study of 443 first pregnancies in women with RA and 6,097 women without.
Disclosures: The study was supported by the Italian Society for Rheumatology. No conflicts of interest were declared.
Source: Bortoluzzi A et al. Arthritis Care Res. 2020 Jan 8. doi: 10.1002/ACR.24116.
Barrett’s esophagus risk factors vary by patient sex
In males but not females, hyperinsulinemia, insulin resistance, and metabolic syndrome are independently associated with a higher risk of Barrett’s esophagus (BE), based on a recent case-control study.
These findings offer some insight into why men have higher rates of BE and esophageal adenocarcinoma than do women, reported lead author Bradley J. Kendall, MBBS, PhD, of the University of Queensland in Brisbane, Australia, and colleagues.
“Esophageal adenocarcinoma (EA) and its precursor lesion, Barrett’s esophagus (BE), are more common in males than females,” the investigators wrote in the Journal of Clinical Gastroenterology. “In contrast, gastroesophageal reflux (GER), the major risk factor for BE and EA occurs at similar frequencies in both sexes. In addition, 10% to 20% of BE and EA cases report no history of GER symptoms. This suggests that non-GER factors are important in the development of BE and EA.”
To examine risk factors more closely, the investigators enrolled 227 patients with BE and 241 age- and sex-matched controls. Data were drawn from self-reported questionnaires, interviews, blood pressure readings, and anthropometric measurements, the latter of which included weight, height, and waist circumference. These patient characteristics were supplemented with fasted blood assays. The investigators looked for associations between BE and insulin level, Homeostatic Model Assessment of Insulin Resistance, metabolic syndrome, type 2 diabetes mellitus, insulin-like growth factors, and interleukin-6 (IL-6).
Across the entire population, independent associations were detected between BE and hyperinsulinemia (highest vs. lowest tertile; odds ratio, 1.9; P = .003), insulin resistance (OR, 1.9; P = .006) and metabolic syndrome (OR, 1.8; P = .004). For those with metabolic syndrome, risk of BE increased by 20% for each additional syndrome criterion (P = .02).
When stratifying by sex, however, all of the above risk factors remained statistically significant in men, but not in women. For male patients, compared with the population as a whole, risks were relatively higher for all three factors: hyperinsulinemia (OR, 2.1; P = .007), insulin resistance (OR, 2.1; P = .01), and metabolic syndrome (OR, 2.3; P = .001). Similarly, for men with metabolic syndrome, each additional syndrome criterion increased risk of BE by 40% (P = .005).
Regardless of sex stratification, the other evaluated characteristics (type 2 diabetes mellitus, insulin-like growth factors, and IL-6) were not associated with BE risk.
The investigators offered some possible mechanistic explanations for their findings.
“Hyperinsulinemia ... can result in increased insulin signaling, increased cellular proliferation, reduced apoptosis, oncogenic pathway activation, and enhanced cellular invasion,” they wrote. “In addition, abdominal obesity, which is the driver of these disorders of insulin homeostasis, also alters adipocytokine profiles and induces a chronic systemic inflammatory state. The inflammatory state can result in oncoprotein activation, angiogenesis, cellular proliferation, apoptosis, and metastasis.”
Concerning the difference between sexes, the investigators pointed to patterns of abdominal obesity. “[A]bdominal obesity and the associated metabolic sequelae are more common in males than females,” they wrote. “These observations give rise to the notion that the metabolic syndrome potentiates the inflammatory effects of the gastric refluxate in the distal esophagus and this may play a role in the male predominance of BE and EA.”
While these findings offer insight into the underlying processes that precipitate BE, the investigators suggested that more research is needed.
“The interactions of obesity hormones, GER, and the cells of the esophageal mucosa warrant further investigation,” they concluded.
The study was funded by the Queensland Cancer Fund, Queensland Government Smart State Fund, and the Princess Alexandra Hospital Research Foundation, and others. The investigators reported no disclosures.
SOURCE: Kendall BJ et al. J Clin Gastroenterol. 2019 Dec 24. doi: 10.1097/MCG.0000000000001307.
In males but not females, hyperinsulinemia, insulin resistance, and metabolic syndrome are independently associated with a higher risk of Barrett’s esophagus (BE), based on a recent case-control study.
These findings offer some insight into why men have higher rates of BE and esophageal adenocarcinoma than do women, reported lead author Bradley J. Kendall, MBBS, PhD, of the University of Queensland in Brisbane, Australia, and colleagues.
“Esophageal adenocarcinoma (EA) and its precursor lesion, Barrett’s esophagus (BE), are more common in males than females,” the investigators wrote in the Journal of Clinical Gastroenterology. “In contrast, gastroesophageal reflux (GER), the major risk factor for BE and EA occurs at similar frequencies in both sexes. In addition, 10% to 20% of BE and EA cases report no history of GER symptoms. This suggests that non-GER factors are important in the development of BE and EA.”
To examine risk factors more closely, the investigators enrolled 227 patients with BE and 241 age- and sex-matched controls. Data were drawn from self-reported questionnaires, interviews, blood pressure readings, and anthropometric measurements, the latter of which included weight, height, and waist circumference. These patient characteristics were supplemented with fasted blood assays. The investigators looked for associations between BE and insulin level, Homeostatic Model Assessment of Insulin Resistance, metabolic syndrome, type 2 diabetes mellitus, insulin-like growth factors, and interleukin-6 (IL-6).
Across the entire population, independent associations were detected between BE and hyperinsulinemia (highest vs. lowest tertile; odds ratio, 1.9; P = .003), insulin resistance (OR, 1.9; P = .006) and metabolic syndrome (OR, 1.8; P = .004). For those with metabolic syndrome, risk of BE increased by 20% for each additional syndrome criterion (P = .02).
When stratifying by sex, however, all of the above risk factors remained statistically significant in men, but not in women. For male patients, compared with the population as a whole, risks were relatively higher for all three factors: hyperinsulinemia (OR, 2.1; P = .007), insulin resistance (OR, 2.1; P = .01), and metabolic syndrome (OR, 2.3; P = .001). Similarly, for men with metabolic syndrome, each additional syndrome criterion increased risk of BE by 40% (P = .005).
Regardless of sex stratification, the other evaluated characteristics (type 2 diabetes mellitus, insulin-like growth factors, and IL-6) were not associated with BE risk.
The investigators offered some possible mechanistic explanations for their findings.
“Hyperinsulinemia ... can result in increased insulin signaling, increased cellular proliferation, reduced apoptosis, oncogenic pathway activation, and enhanced cellular invasion,” they wrote. “In addition, abdominal obesity, which is the driver of these disorders of insulin homeostasis, also alters adipocytokine profiles and induces a chronic systemic inflammatory state. The inflammatory state can result in oncoprotein activation, angiogenesis, cellular proliferation, apoptosis, and metastasis.”
Concerning the difference between sexes, the investigators pointed to patterns of abdominal obesity. “[A]bdominal obesity and the associated metabolic sequelae are more common in males than females,” they wrote. “These observations give rise to the notion that the metabolic syndrome potentiates the inflammatory effects of the gastric refluxate in the distal esophagus and this may play a role in the male predominance of BE and EA.”
While these findings offer insight into the underlying processes that precipitate BE, the investigators suggested that more research is needed.
“The interactions of obesity hormones, GER, and the cells of the esophageal mucosa warrant further investigation,” they concluded.
The study was funded by the Queensland Cancer Fund, Queensland Government Smart State Fund, and the Princess Alexandra Hospital Research Foundation, and others. The investigators reported no disclosures.
SOURCE: Kendall BJ et al. J Clin Gastroenterol. 2019 Dec 24. doi: 10.1097/MCG.0000000000001307.
In males but not females, hyperinsulinemia, insulin resistance, and metabolic syndrome are independently associated with a higher risk of Barrett’s esophagus (BE), based on a recent case-control study.
These findings offer some insight into why men have higher rates of BE and esophageal adenocarcinoma than do women, reported lead author Bradley J. Kendall, MBBS, PhD, of the University of Queensland in Brisbane, Australia, and colleagues.
“Esophageal adenocarcinoma (EA) and its precursor lesion, Barrett’s esophagus (BE), are more common in males than females,” the investigators wrote in the Journal of Clinical Gastroenterology. “In contrast, gastroesophageal reflux (GER), the major risk factor for BE and EA occurs at similar frequencies in both sexes. In addition, 10% to 20% of BE and EA cases report no history of GER symptoms. This suggests that non-GER factors are important in the development of BE and EA.”
To examine risk factors more closely, the investigators enrolled 227 patients with BE and 241 age- and sex-matched controls. Data were drawn from self-reported questionnaires, interviews, blood pressure readings, and anthropometric measurements, the latter of which included weight, height, and waist circumference. These patient characteristics were supplemented with fasted blood assays. The investigators looked for associations between BE and insulin level, Homeostatic Model Assessment of Insulin Resistance, metabolic syndrome, type 2 diabetes mellitus, insulin-like growth factors, and interleukin-6 (IL-6).
Across the entire population, independent associations were detected between BE and hyperinsulinemia (highest vs. lowest tertile; odds ratio, 1.9; P = .003), insulin resistance (OR, 1.9; P = .006) and metabolic syndrome (OR, 1.8; P = .004). For those with metabolic syndrome, risk of BE increased by 20% for each additional syndrome criterion (P = .02).
When stratifying by sex, however, all of the above risk factors remained statistically significant in men, but not in women. For male patients, compared with the population as a whole, risks were relatively higher for all three factors: hyperinsulinemia (OR, 2.1; P = .007), insulin resistance (OR, 2.1; P = .01), and metabolic syndrome (OR, 2.3; P = .001). Similarly, for men with metabolic syndrome, each additional syndrome criterion increased risk of BE by 40% (P = .005).
Regardless of sex stratification, the other evaluated characteristics (type 2 diabetes mellitus, insulin-like growth factors, and IL-6) were not associated with BE risk.
The investigators offered some possible mechanistic explanations for their findings.
“Hyperinsulinemia ... can result in increased insulin signaling, increased cellular proliferation, reduced apoptosis, oncogenic pathway activation, and enhanced cellular invasion,” they wrote. “In addition, abdominal obesity, which is the driver of these disorders of insulin homeostasis, also alters adipocytokine profiles and induces a chronic systemic inflammatory state. The inflammatory state can result in oncoprotein activation, angiogenesis, cellular proliferation, apoptosis, and metastasis.”
Concerning the difference between sexes, the investigators pointed to patterns of abdominal obesity. “[A]bdominal obesity and the associated metabolic sequelae are more common in males than females,” they wrote. “These observations give rise to the notion that the metabolic syndrome potentiates the inflammatory effects of the gastric refluxate in the distal esophagus and this may play a role in the male predominance of BE and EA.”
While these findings offer insight into the underlying processes that precipitate BE, the investigators suggested that more research is needed.
“The interactions of obesity hormones, GER, and the cells of the esophageal mucosa warrant further investigation,” they concluded.
The study was funded by the Queensland Cancer Fund, Queensland Government Smart State Fund, and the Princess Alexandra Hospital Research Foundation, and others. The investigators reported no disclosures.
SOURCE: Kendall BJ et al. J Clin Gastroenterol. 2019 Dec 24. doi: 10.1097/MCG.0000000000001307.
FROM JOURNAL OF CLINICAL GASTROENTEROLOGY
Research on statin for preeclampsia prevention advances
WASHINGTON – with a large National Institutes of Health–funded trial currently recruiting women with a prior history of the disorder with preterm delivery at less than 34 weeks, Maged Costantine, MD, said at the biennial Diabetes in Pregnancy Study Group of North America meeting.
More should be learned about low-dose aspirin, in the meantime, once the outcomes of a global study involving first-trimester initiation are published, said another speaker, Cynthia Gyamfi-Bannerman, MD, MS. Low-dose aspirin currently is recommended for preeclampsia prevention starting between 12 and 28 weeks, optimally before 16 weeks.
The biological plausibility of using pravastatin for preeclampsia prevention stems from the overlapping pathophysiology of preeclampsia with atherosclerotic cardiovascular disease – endothelial dysfunction and inflammation are common key mechanisms – as well as common risk factors, including diabetes and obesity, said Dr. Costantine, director of the division of maternal-fetal medicine at Ohio State University, Columbus, who is chairing the study.
In animal models of preeclampsia, pravastatin has been shown to upregulate placental growth factor, reduce antiangiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt1), and upregulate endothelial nitric oxide synthase. Mice have shown improved vascular reactivity, decreased proteinuria, decreased oxidative stress, and other positive effects, without any detrimental outcomes.
A pilot randomized controlled trial conducted with the Obstetric-Fetal Pharmacology Research Units Network and published in the American Journal of Obstetrics and Gynecology in 2016 assigned 10 women to 10 mg daily pravastatin and 10 women to placebo. The drug reduced maternal cholesterol concentrations but there were no differences in birth weight or umbilical cord cholesterol concentrations between the two groups.
Women in the pravastatin group were less likely to develop preeclampsia (none, compared with four in the placebo group), less likely to have an indicated preterm delivery (one, compared with five in the placebo group), and less likely to have their neonates admitted to the neonatal ICU.
There were no differences in side effects, congenital anomalies, or other adverse events. Dr. Costantine, principal investigator of the pilot study, and his colleagues wrote in the paper that the “favorable risk-benefit analysis justifies continued research with a dose escalation” (Am J Obstet Gynecol. 2016 Jun;214[6]:720.e1-17).
The new multicenter randomized controlled trial is randomizing 1,550 women to either 20 mg pravastatin or placebo starting between 12 weeks 0 days and 16 weeks 6 days. The primary outcome is a composite of preeclampsia, maternal death, or fetal loss. Secondary outcomes include a composite of severe maternal morbidity and various measures representing preeclampsia severity and complications, as well as preterm delivery less than 37 weeks and less than 34 weeks and various fetal/neonatal outcomes.
“In addition, we’ll look at development,” Dr. Costantine said, with offspring assessed at 2 and 5 years of age. The trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute.
In the meantime, he said, the use of pravastatin to ameliorate early-onset preeclampsia is being tested in a small European proof-of-concept trial that has randomized women with early-onset preeclampsia (between 24 and 31 6/7 weeks) to 40 mg pravastatin or placebo. The primary outcome is reduction of antiangiogenic markers. Results are expected in another year or 2, he said.
The aspirin trial referred to by Dr. Gyamfi-Bannerman has been looking at the 81-mg dose of aspirin initiated between 6 0/7 and 13 6/7 weeks in nulliparous women who had no more than two previous pregnancy losses. The key question of the Aspirin Supplementation for Pregnancy Indicated Risk Reduction in Nulliparas (ASPIRIN) trial – conducted in the NICHD Global Network for Women’s and Children’s Health – is whether low-dose aspirin can reduce the rate of preterm birth. Preeclampsia is a secondary outcome (https://clinicaltrials.gov/ct2/show/NCT02409680).
“It may eventually be that the use of baby aspirin is further expanded to reduce the risk of preterm birth,” she said.
Overall, “we need more data on first-trimester use [of low-dose aspirin] and long-term outcomes,” Dr. Gyamfi-Bannerman said. And with respect to preeclampsia prevention specifically, more research is needed looking at risk reduction levels within specific groups of patients.
Since 2014, the U.S. Preventive Services Task Force (USPSTF) has called for low-dose aspirin at 81 mg/day in women who have one or more high-risk factors for preeclampsia (including type 1 or type 2 diabetes mellitus), and consideration of such treatment in patients with several moderate-risk factors. The American College of Obstetricians and Gynecologists’ recommendation varies slightly in that it advises treatment in patients with more than one (versus several) moderate-level risk factors (Obstet Gynecol. 2018;132[1]:e44-52).
Moderate-level risk factors include nulliparity, obesity, family history of preeclampsia, a baseline demographic risk (African-American or low socioeconomic status), and prior poor history (intrauterine growth restriction/small-for-gestational-age, previous poor outcome). “This is just about everyone I see,” Dr. Gyamfi-Bannerman said.
Dr. Gyamfi-Bannerman said she’d “love to see more data on higher doses” of low-dose aspirin – data that compares 81 mg/day with 150 mg/day, for instance.
A study published in 2017 in the New England Journal of Medicine randomized 1,776 women at high risk for preeclampsia to 150 mg/day or placebo and found a significant reduction in preterm preeclampsia (4.3% vs. 1.6%) in the aspirin group. Women in this European trial were deemed to be at high risk, however, based on a first-trimester screening algorithm that incorporated serum markers (maternal serum pregnancy-associated plasma protein A and placental growth factor) and uterine artery Doppler measures (N Engl J Med. 2017 Aug 17;377[7]:613-22).
“So it was a very interesting study, very provocative, but it’s hard to know how it would translate to the U.S. population [given that such screening practices] are not the way most of us are practicing here,” said Dr. Gyamfi-Bannerman, codirector of the Preterm Birth Prevention Center at Columbia University, New York, and professor of obstetrics and gynecology at the university.
The USPSTF based its recommendations on a systematic review that pooled data from 15 high-quality randomized controlled trials, including 13 that reported preeclampsia incidence among women at highest risk of disease. They found a 24% reduction in preeclampsia, but the actual risk reduction depends on the baseline population risk and may be closer to 10%, she said.
In a presentation on gaps in knowledge, Leslie Myatt, PhD, of the department of obstetrics and gynecology at Oregon Health and Science University, Portland, emphasized that preeclampsia is a syndrome with a heterogeneity of presentation and pathophysiology. “We don’t completely understand the pathophysiology,” he said.
Research needs to be “directed at the existence of multiple pathways [and subtypes],” he said, such that future therapies can be targeted and personalized.
Dr. Costantine did not report any disclosures. Dr. Gyamfi-Bannerman reported a Society of Maternal Fetal Medicine/AMAG Pharmaceuticals unrestricted grant and Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Heart, Lung and Blood Institute funding. Dr. Myatt reported that he has no financial or other ties that pose a conflict of interest.
WASHINGTON – with a large National Institutes of Health–funded trial currently recruiting women with a prior history of the disorder with preterm delivery at less than 34 weeks, Maged Costantine, MD, said at the biennial Diabetes in Pregnancy Study Group of North America meeting.
More should be learned about low-dose aspirin, in the meantime, once the outcomes of a global study involving first-trimester initiation are published, said another speaker, Cynthia Gyamfi-Bannerman, MD, MS. Low-dose aspirin currently is recommended for preeclampsia prevention starting between 12 and 28 weeks, optimally before 16 weeks.
The biological plausibility of using pravastatin for preeclampsia prevention stems from the overlapping pathophysiology of preeclampsia with atherosclerotic cardiovascular disease – endothelial dysfunction and inflammation are common key mechanisms – as well as common risk factors, including diabetes and obesity, said Dr. Costantine, director of the division of maternal-fetal medicine at Ohio State University, Columbus, who is chairing the study.
In animal models of preeclampsia, pravastatin has been shown to upregulate placental growth factor, reduce antiangiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt1), and upregulate endothelial nitric oxide synthase. Mice have shown improved vascular reactivity, decreased proteinuria, decreased oxidative stress, and other positive effects, without any detrimental outcomes.
A pilot randomized controlled trial conducted with the Obstetric-Fetal Pharmacology Research Units Network and published in the American Journal of Obstetrics and Gynecology in 2016 assigned 10 women to 10 mg daily pravastatin and 10 women to placebo. The drug reduced maternal cholesterol concentrations but there were no differences in birth weight or umbilical cord cholesterol concentrations between the two groups.
Women in the pravastatin group were less likely to develop preeclampsia (none, compared with four in the placebo group), less likely to have an indicated preterm delivery (one, compared with five in the placebo group), and less likely to have their neonates admitted to the neonatal ICU.
There were no differences in side effects, congenital anomalies, or other adverse events. Dr. Costantine, principal investigator of the pilot study, and his colleagues wrote in the paper that the “favorable risk-benefit analysis justifies continued research with a dose escalation” (Am J Obstet Gynecol. 2016 Jun;214[6]:720.e1-17).
The new multicenter randomized controlled trial is randomizing 1,550 women to either 20 mg pravastatin or placebo starting between 12 weeks 0 days and 16 weeks 6 days. The primary outcome is a composite of preeclampsia, maternal death, or fetal loss. Secondary outcomes include a composite of severe maternal morbidity and various measures representing preeclampsia severity and complications, as well as preterm delivery less than 37 weeks and less than 34 weeks and various fetal/neonatal outcomes.
“In addition, we’ll look at development,” Dr. Costantine said, with offspring assessed at 2 and 5 years of age. The trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute.
In the meantime, he said, the use of pravastatin to ameliorate early-onset preeclampsia is being tested in a small European proof-of-concept trial that has randomized women with early-onset preeclampsia (between 24 and 31 6/7 weeks) to 40 mg pravastatin or placebo. The primary outcome is reduction of antiangiogenic markers. Results are expected in another year or 2, he said.
The aspirin trial referred to by Dr. Gyamfi-Bannerman has been looking at the 81-mg dose of aspirin initiated between 6 0/7 and 13 6/7 weeks in nulliparous women who had no more than two previous pregnancy losses. The key question of the Aspirin Supplementation for Pregnancy Indicated Risk Reduction in Nulliparas (ASPIRIN) trial – conducted in the NICHD Global Network for Women’s and Children’s Health – is whether low-dose aspirin can reduce the rate of preterm birth. Preeclampsia is a secondary outcome (https://clinicaltrials.gov/ct2/show/NCT02409680).
“It may eventually be that the use of baby aspirin is further expanded to reduce the risk of preterm birth,” she said.
Overall, “we need more data on first-trimester use [of low-dose aspirin] and long-term outcomes,” Dr. Gyamfi-Bannerman said. And with respect to preeclampsia prevention specifically, more research is needed looking at risk reduction levels within specific groups of patients.
Since 2014, the U.S. Preventive Services Task Force (USPSTF) has called for low-dose aspirin at 81 mg/day in women who have one or more high-risk factors for preeclampsia (including type 1 or type 2 diabetes mellitus), and consideration of such treatment in patients with several moderate-risk factors. The American College of Obstetricians and Gynecologists’ recommendation varies slightly in that it advises treatment in patients with more than one (versus several) moderate-level risk factors (Obstet Gynecol. 2018;132[1]:e44-52).
Moderate-level risk factors include nulliparity, obesity, family history of preeclampsia, a baseline demographic risk (African-American or low socioeconomic status), and prior poor history (intrauterine growth restriction/small-for-gestational-age, previous poor outcome). “This is just about everyone I see,” Dr. Gyamfi-Bannerman said.
Dr. Gyamfi-Bannerman said she’d “love to see more data on higher doses” of low-dose aspirin – data that compares 81 mg/day with 150 mg/day, for instance.
A study published in 2017 in the New England Journal of Medicine randomized 1,776 women at high risk for preeclampsia to 150 mg/day or placebo and found a significant reduction in preterm preeclampsia (4.3% vs. 1.6%) in the aspirin group. Women in this European trial were deemed to be at high risk, however, based on a first-trimester screening algorithm that incorporated serum markers (maternal serum pregnancy-associated plasma protein A and placental growth factor) and uterine artery Doppler measures (N Engl J Med. 2017 Aug 17;377[7]:613-22).
“So it was a very interesting study, very provocative, but it’s hard to know how it would translate to the U.S. population [given that such screening practices] are not the way most of us are practicing here,” said Dr. Gyamfi-Bannerman, codirector of the Preterm Birth Prevention Center at Columbia University, New York, and professor of obstetrics and gynecology at the university.
The USPSTF based its recommendations on a systematic review that pooled data from 15 high-quality randomized controlled trials, including 13 that reported preeclampsia incidence among women at highest risk of disease. They found a 24% reduction in preeclampsia, but the actual risk reduction depends on the baseline population risk and may be closer to 10%, she said.
In a presentation on gaps in knowledge, Leslie Myatt, PhD, of the department of obstetrics and gynecology at Oregon Health and Science University, Portland, emphasized that preeclampsia is a syndrome with a heterogeneity of presentation and pathophysiology. “We don’t completely understand the pathophysiology,” he said.
Research needs to be “directed at the existence of multiple pathways [and subtypes],” he said, such that future therapies can be targeted and personalized.
Dr. Costantine did not report any disclosures. Dr. Gyamfi-Bannerman reported a Society of Maternal Fetal Medicine/AMAG Pharmaceuticals unrestricted grant and Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Heart, Lung and Blood Institute funding. Dr. Myatt reported that he has no financial or other ties that pose a conflict of interest.
WASHINGTON – with a large National Institutes of Health–funded trial currently recruiting women with a prior history of the disorder with preterm delivery at less than 34 weeks, Maged Costantine, MD, said at the biennial Diabetes in Pregnancy Study Group of North America meeting.
More should be learned about low-dose aspirin, in the meantime, once the outcomes of a global study involving first-trimester initiation are published, said another speaker, Cynthia Gyamfi-Bannerman, MD, MS. Low-dose aspirin currently is recommended for preeclampsia prevention starting between 12 and 28 weeks, optimally before 16 weeks.
The biological plausibility of using pravastatin for preeclampsia prevention stems from the overlapping pathophysiology of preeclampsia with atherosclerotic cardiovascular disease – endothelial dysfunction and inflammation are common key mechanisms – as well as common risk factors, including diabetes and obesity, said Dr. Costantine, director of the division of maternal-fetal medicine at Ohio State University, Columbus, who is chairing the study.
In animal models of preeclampsia, pravastatin has been shown to upregulate placental growth factor, reduce antiangiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt1), and upregulate endothelial nitric oxide synthase. Mice have shown improved vascular reactivity, decreased proteinuria, decreased oxidative stress, and other positive effects, without any detrimental outcomes.
A pilot randomized controlled trial conducted with the Obstetric-Fetal Pharmacology Research Units Network and published in the American Journal of Obstetrics and Gynecology in 2016 assigned 10 women to 10 mg daily pravastatin and 10 women to placebo. The drug reduced maternal cholesterol concentrations but there were no differences in birth weight or umbilical cord cholesterol concentrations between the two groups.
Women in the pravastatin group were less likely to develop preeclampsia (none, compared with four in the placebo group), less likely to have an indicated preterm delivery (one, compared with five in the placebo group), and less likely to have their neonates admitted to the neonatal ICU.
There were no differences in side effects, congenital anomalies, or other adverse events. Dr. Costantine, principal investigator of the pilot study, and his colleagues wrote in the paper that the “favorable risk-benefit analysis justifies continued research with a dose escalation” (Am J Obstet Gynecol. 2016 Jun;214[6]:720.e1-17).
The new multicenter randomized controlled trial is randomizing 1,550 women to either 20 mg pravastatin or placebo starting between 12 weeks 0 days and 16 weeks 6 days. The primary outcome is a composite of preeclampsia, maternal death, or fetal loss. Secondary outcomes include a composite of severe maternal morbidity and various measures representing preeclampsia severity and complications, as well as preterm delivery less than 37 weeks and less than 34 weeks and various fetal/neonatal outcomes.
“In addition, we’ll look at development,” Dr. Costantine said, with offspring assessed at 2 and 5 years of age. The trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute.
In the meantime, he said, the use of pravastatin to ameliorate early-onset preeclampsia is being tested in a small European proof-of-concept trial that has randomized women with early-onset preeclampsia (between 24 and 31 6/7 weeks) to 40 mg pravastatin or placebo. The primary outcome is reduction of antiangiogenic markers. Results are expected in another year or 2, he said.
The aspirin trial referred to by Dr. Gyamfi-Bannerman has been looking at the 81-mg dose of aspirin initiated between 6 0/7 and 13 6/7 weeks in nulliparous women who had no more than two previous pregnancy losses. The key question of the Aspirin Supplementation for Pregnancy Indicated Risk Reduction in Nulliparas (ASPIRIN) trial – conducted in the NICHD Global Network for Women’s and Children’s Health – is whether low-dose aspirin can reduce the rate of preterm birth. Preeclampsia is a secondary outcome (https://clinicaltrials.gov/ct2/show/NCT02409680).
“It may eventually be that the use of baby aspirin is further expanded to reduce the risk of preterm birth,” she said.
Overall, “we need more data on first-trimester use [of low-dose aspirin] and long-term outcomes,” Dr. Gyamfi-Bannerman said. And with respect to preeclampsia prevention specifically, more research is needed looking at risk reduction levels within specific groups of patients.
Since 2014, the U.S. Preventive Services Task Force (USPSTF) has called for low-dose aspirin at 81 mg/day in women who have one or more high-risk factors for preeclampsia (including type 1 or type 2 diabetes mellitus), and consideration of such treatment in patients with several moderate-risk factors. The American College of Obstetricians and Gynecologists’ recommendation varies slightly in that it advises treatment in patients with more than one (versus several) moderate-level risk factors (Obstet Gynecol. 2018;132[1]:e44-52).
Moderate-level risk factors include nulliparity, obesity, family history of preeclampsia, a baseline demographic risk (African-American or low socioeconomic status), and prior poor history (intrauterine growth restriction/small-for-gestational-age, previous poor outcome). “This is just about everyone I see,” Dr. Gyamfi-Bannerman said.
Dr. Gyamfi-Bannerman said she’d “love to see more data on higher doses” of low-dose aspirin – data that compares 81 mg/day with 150 mg/day, for instance.
A study published in 2017 in the New England Journal of Medicine randomized 1,776 women at high risk for preeclampsia to 150 mg/day or placebo and found a significant reduction in preterm preeclampsia (4.3% vs. 1.6%) in the aspirin group. Women in this European trial were deemed to be at high risk, however, based on a first-trimester screening algorithm that incorporated serum markers (maternal serum pregnancy-associated plasma protein A and placental growth factor) and uterine artery Doppler measures (N Engl J Med. 2017 Aug 17;377[7]:613-22).
“So it was a very interesting study, very provocative, but it’s hard to know how it would translate to the U.S. population [given that such screening practices] are not the way most of us are practicing here,” said Dr. Gyamfi-Bannerman, codirector of the Preterm Birth Prevention Center at Columbia University, New York, and professor of obstetrics and gynecology at the university.
The USPSTF based its recommendations on a systematic review that pooled data from 15 high-quality randomized controlled trials, including 13 that reported preeclampsia incidence among women at highest risk of disease. They found a 24% reduction in preeclampsia, but the actual risk reduction depends on the baseline population risk and may be closer to 10%, she said.
In a presentation on gaps in knowledge, Leslie Myatt, PhD, of the department of obstetrics and gynecology at Oregon Health and Science University, Portland, emphasized that preeclampsia is a syndrome with a heterogeneity of presentation and pathophysiology. “We don’t completely understand the pathophysiology,” he said.
Research needs to be “directed at the existence of multiple pathways [and subtypes],” he said, such that future therapies can be targeted and personalized.
Dr. Costantine did not report any disclosures. Dr. Gyamfi-Bannerman reported a Society of Maternal Fetal Medicine/AMAG Pharmaceuticals unrestricted grant and Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Heart, Lung and Blood Institute funding. Dr. Myatt reported that he has no financial or other ties that pose a conflict of interest.
EXPERT ANALYSIS FROM THE DPSG-NA 2019
AED exposure from breastfeeding appears to be low
, according to a study published online ahead of print Dec. 30, 2019, in JAMA Neurology. The results may explain why previous research failed to find adverse neurodevelopmental effects of breastfeeding in infants whose mothers are undergoing AED treatment, said the authors.
“The results of this study add support to the general safety of breastfeeding by mothers with epilepsy who take AEDs,” wrote Angela K. Birnbaum, PhD, professor of experimental and clinical pharmacology at the University of Minnesota in Minneapolis, and colleagues.
Investigators measured infants’ blood AED concentrations
To date, medical consensus about the safety of breastfeeding while the mother is taking AEDs has been elusive. Researchers have investigated breast milk concentrations of AEDs as surrogate markers of AED concentrations in children. Breast milk concentrations, however, do not account for differences in infant pharmacokinetic processes and thus could misrepresent AED exposure in children through breastfeeding.
Dr. Birnbaum and colleagues sought to measure blood concentrations of AEDs in mothers with epilepsy and the infants that they breastfed to achieve an objective measure of AED exposure through breastfeeding. They examined data collected from December 2012 to October 2016 in the prospective Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study. Eligible participants were pregnant women with epilepsy between the ages of 14 and 45 years whose pregnancies had progressed to fewer than 20 weeks’ gestational age and who had IQ scores greater than 70 points. Participants were followed up throughout pregnancy and for 9 months post partum. Children were enrolled at birth.
The investigators collected blood samples from mothers and infants who were breastfed at the same visit, which occurred at between 5 and 20 weeks after birth. The volume of ingested breast milk delivered through graduated feeding bottles each day and the total duration of all daily breastfeeding sessions were recorded. For infants, blood samples were collected from the plantar surface of the heel and stored as dried blood spots on filter paper. The study’s primary endpoint was the percentage of infant-to-mother concentration of AEDs. Concentrations of AEDs in infants at less than the lower limit of quantification were assessed as half of the lower limit.
Exposure in utero may be greater than exposure through breast milk
In all, the researchers enrolled 351 pregnant women with epilepsy into the study and collected data on 345 infants. Two hundred twenty-two (64.3%) of the infants were breastfed, and 146 (42.3%) had AED concentrations available. After excluding outliers and mothers with missing concentration data, Dr. Birnbaum and colleagues included 164 matching infant-mother concentration pairs in their analysis (i.e., of 135 mothers and 138 infants). Approximately 52% of the infants were female, and their median age at blood collection was 13 weeks. The mothers’ median age was 32 years. About 82% of mothers were receiving monotherapy. The investigators found no demographic differences between groups of mothers taking various AEDs.
Sixty-eight infants (49.3%) had AED concentrations that were less than the lower limit of quantification. AED concentration was not greater than the lower limit of quantification for any infants breastfed by mothers taking carbamazepine, oxcarbazepine, valproic acid, or topiramate. Most levetiracetam (71.4%) and zonisamide (60.0%) concentrations in infants were less than the lower limit of quantification. Most lamotrigine concentrations in infants (88.6%) were greater than the lower limit of quantification.
The median percentage of infant-to-mother concentration was 28.9% for lamotrigine, 5.3% for levetiracetam, 44.2% for zonisamide, 5.7% for carbamazepine, 5.4% for carbamazepine epoxide, 0.3% for oxcarbazepine, 17.2% for topiramate, and 21.4% for valproic acid. Multiple linear regression models indicated that maternal concentration was significantly associated with lamotrigine concentration in infants, but not levetiracetam concentration in infants.
“Prior studies at delivery demonstrated that umbilical-cord concentrations were nearly equal to maternal concentrations, suggesting extensive placental passage to the fetus,” wrote Dr. Birnbaum and colleagues. “Therefore, the amount of AED exposure via breast milk is likely substantially lower than fetal exposure during pregnancy and appears unlikely to confer any additional risks beyond those that might be associated with exposure in pregnancy, especially given prior studies showing no adverse neurodevelopmental effects of breastfeeding while taking AEDs.”
The investigators acknowledged several limitations of their research, including the observational design of the MONEAD study. The amount of AED in participants’ breast milk is unknown, and the investigators could not calculate relative infant dosages. Only one blood sample was taken per infant, thus the results may not reflect infants’ total exposure over time.
The National Institute of Neurological Disorders and Stroke and the National Institute of Child Health and Development funded the research. The authors reported receiving research support from various pharmaceutical companies.
SOURCE: Birnbaum AK et al. JAMA Neurol. 2019 Dec 30. doi: 10.1001/jamaneurol.2019.4443.
, according to a study published online ahead of print Dec. 30, 2019, in JAMA Neurology. The results may explain why previous research failed to find adverse neurodevelopmental effects of breastfeeding in infants whose mothers are undergoing AED treatment, said the authors.
“The results of this study add support to the general safety of breastfeeding by mothers with epilepsy who take AEDs,” wrote Angela K. Birnbaum, PhD, professor of experimental and clinical pharmacology at the University of Minnesota in Minneapolis, and colleagues.
Investigators measured infants’ blood AED concentrations
To date, medical consensus about the safety of breastfeeding while the mother is taking AEDs has been elusive. Researchers have investigated breast milk concentrations of AEDs as surrogate markers of AED concentrations in children. Breast milk concentrations, however, do not account for differences in infant pharmacokinetic processes and thus could misrepresent AED exposure in children through breastfeeding.
Dr. Birnbaum and colleagues sought to measure blood concentrations of AEDs in mothers with epilepsy and the infants that they breastfed to achieve an objective measure of AED exposure through breastfeeding. They examined data collected from December 2012 to October 2016 in the prospective Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study. Eligible participants were pregnant women with epilepsy between the ages of 14 and 45 years whose pregnancies had progressed to fewer than 20 weeks’ gestational age and who had IQ scores greater than 70 points. Participants were followed up throughout pregnancy and for 9 months post partum. Children were enrolled at birth.
The investigators collected blood samples from mothers and infants who were breastfed at the same visit, which occurred at between 5 and 20 weeks after birth. The volume of ingested breast milk delivered through graduated feeding bottles each day and the total duration of all daily breastfeeding sessions were recorded. For infants, blood samples were collected from the plantar surface of the heel and stored as dried blood spots on filter paper. The study’s primary endpoint was the percentage of infant-to-mother concentration of AEDs. Concentrations of AEDs in infants at less than the lower limit of quantification were assessed as half of the lower limit.
Exposure in utero may be greater than exposure through breast milk
In all, the researchers enrolled 351 pregnant women with epilepsy into the study and collected data on 345 infants. Two hundred twenty-two (64.3%) of the infants were breastfed, and 146 (42.3%) had AED concentrations available. After excluding outliers and mothers with missing concentration data, Dr. Birnbaum and colleagues included 164 matching infant-mother concentration pairs in their analysis (i.e., of 135 mothers and 138 infants). Approximately 52% of the infants were female, and their median age at blood collection was 13 weeks. The mothers’ median age was 32 years. About 82% of mothers were receiving monotherapy. The investigators found no demographic differences between groups of mothers taking various AEDs.
Sixty-eight infants (49.3%) had AED concentrations that were less than the lower limit of quantification. AED concentration was not greater than the lower limit of quantification for any infants breastfed by mothers taking carbamazepine, oxcarbazepine, valproic acid, or topiramate. Most levetiracetam (71.4%) and zonisamide (60.0%) concentrations in infants were less than the lower limit of quantification. Most lamotrigine concentrations in infants (88.6%) were greater than the lower limit of quantification.
The median percentage of infant-to-mother concentration was 28.9% for lamotrigine, 5.3% for levetiracetam, 44.2% for zonisamide, 5.7% for carbamazepine, 5.4% for carbamazepine epoxide, 0.3% for oxcarbazepine, 17.2% for topiramate, and 21.4% for valproic acid. Multiple linear regression models indicated that maternal concentration was significantly associated with lamotrigine concentration in infants, but not levetiracetam concentration in infants.
“Prior studies at delivery demonstrated that umbilical-cord concentrations were nearly equal to maternal concentrations, suggesting extensive placental passage to the fetus,” wrote Dr. Birnbaum and colleagues. “Therefore, the amount of AED exposure via breast milk is likely substantially lower than fetal exposure during pregnancy and appears unlikely to confer any additional risks beyond those that might be associated with exposure in pregnancy, especially given prior studies showing no adverse neurodevelopmental effects of breastfeeding while taking AEDs.”
The investigators acknowledged several limitations of their research, including the observational design of the MONEAD study. The amount of AED in participants’ breast milk is unknown, and the investigators could not calculate relative infant dosages. Only one blood sample was taken per infant, thus the results may not reflect infants’ total exposure over time.
The National Institute of Neurological Disorders and Stroke and the National Institute of Child Health and Development funded the research. The authors reported receiving research support from various pharmaceutical companies.
SOURCE: Birnbaum AK et al. JAMA Neurol. 2019 Dec 30. doi: 10.1001/jamaneurol.2019.4443.
, according to a study published online ahead of print Dec. 30, 2019, in JAMA Neurology. The results may explain why previous research failed to find adverse neurodevelopmental effects of breastfeeding in infants whose mothers are undergoing AED treatment, said the authors.
“The results of this study add support to the general safety of breastfeeding by mothers with epilepsy who take AEDs,” wrote Angela K. Birnbaum, PhD, professor of experimental and clinical pharmacology at the University of Minnesota in Minneapolis, and colleagues.
Investigators measured infants’ blood AED concentrations
To date, medical consensus about the safety of breastfeeding while the mother is taking AEDs has been elusive. Researchers have investigated breast milk concentrations of AEDs as surrogate markers of AED concentrations in children. Breast milk concentrations, however, do not account for differences in infant pharmacokinetic processes and thus could misrepresent AED exposure in children through breastfeeding.
Dr. Birnbaum and colleagues sought to measure blood concentrations of AEDs in mothers with epilepsy and the infants that they breastfed to achieve an objective measure of AED exposure through breastfeeding. They examined data collected from December 2012 to October 2016 in the prospective Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study. Eligible participants were pregnant women with epilepsy between the ages of 14 and 45 years whose pregnancies had progressed to fewer than 20 weeks’ gestational age and who had IQ scores greater than 70 points. Participants were followed up throughout pregnancy and for 9 months post partum. Children were enrolled at birth.
The investigators collected blood samples from mothers and infants who were breastfed at the same visit, which occurred at between 5 and 20 weeks after birth. The volume of ingested breast milk delivered through graduated feeding bottles each day and the total duration of all daily breastfeeding sessions were recorded. For infants, blood samples were collected from the plantar surface of the heel and stored as dried blood spots on filter paper. The study’s primary endpoint was the percentage of infant-to-mother concentration of AEDs. Concentrations of AEDs in infants at less than the lower limit of quantification were assessed as half of the lower limit.
Exposure in utero may be greater than exposure through breast milk
In all, the researchers enrolled 351 pregnant women with epilepsy into the study and collected data on 345 infants. Two hundred twenty-two (64.3%) of the infants were breastfed, and 146 (42.3%) had AED concentrations available. After excluding outliers and mothers with missing concentration data, Dr. Birnbaum and colleagues included 164 matching infant-mother concentration pairs in their analysis (i.e., of 135 mothers and 138 infants). Approximately 52% of the infants were female, and their median age at blood collection was 13 weeks. The mothers’ median age was 32 years. About 82% of mothers were receiving monotherapy. The investigators found no demographic differences between groups of mothers taking various AEDs.
Sixty-eight infants (49.3%) had AED concentrations that were less than the lower limit of quantification. AED concentration was not greater than the lower limit of quantification for any infants breastfed by mothers taking carbamazepine, oxcarbazepine, valproic acid, or topiramate. Most levetiracetam (71.4%) and zonisamide (60.0%) concentrations in infants were less than the lower limit of quantification. Most lamotrigine concentrations in infants (88.6%) were greater than the lower limit of quantification.
The median percentage of infant-to-mother concentration was 28.9% for lamotrigine, 5.3% for levetiracetam, 44.2% for zonisamide, 5.7% for carbamazepine, 5.4% for carbamazepine epoxide, 0.3% for oxcarbazepine, 17.2% for topiramate, and 21.4% for valproic acid. Multiple linear regression models indicated that maternal concentration was significantly associated with lamotrigine concentration in infants, but not levetiracetam concentration in infants.
“Prior studies at delivery demonstrated that umbilical-cord concentrations were nearly equal to maternal concentrations, suggesting extensive placental passage to the fetus,” wrote Dr. Birnbaum and colleagues. “Therefore, the amount of AED exposure via breast milk is likely substantially lower than fetal exposure during pregnancy and appears unlikely to confer any additional risks beyond those that might be associated with exposure in pregnancy, especially given prior studies showing no adverse neurodevelopmental effects of breastfeeding while taking AEDs.”
The investigators acknowledged several limitations of their research, including the observational design of the MONEAD study. The amount of AED in participants’ breast milk is unknown, and the investigators could not calculate relative infant dosages. Only one blood sample was taken per infant, thus the results may not reflect infants’ total exposure over time.
The National Institute of Neurological Disorders and Stroke and the National Institute of Child Health and Development funded the research. The authors reported receiving research support from various pharmaceutical companies.
SOURCE: Birnbaum AK et al. JAMA Neurol. 2019 Dec 30. doi: 10.1001/jamaneurol.2019.4443.
FROM JAMA NEUROLOGY
Replacement meals boost nutrient intake by pregnant women with obesity
LAS VEGAS – Pregnant women with overweight or obesity who replaced two meals a day with bars or shakes starting at their second trimester not only had a significantly reduced rate of gestational weight gain but also benefited from significant improvements in their intake of several micronutrients, in a randomized study of 211 women who completed the regimen.
Further research needs “to examine the generalizability and effectiveness of this prenatal lifestyle modification program in improving micronutrient sufficiency in other populations and settings,” Suzanne Phelan, PhD, said at a meeting presented by The Obesity Society and the American Society for Metabolic and Bariatric Surgery. The study she presented ran at two U.S. sites, in California and Rhode Island, and enrolled a population that was 42% Hispanic/Latina. Despite uncertainty about the applicability of the findings to other populations, the results suggested that partial meal replacement is a way to better control gestational weight gain in women with overweight or obesity while simultaneously increasing micronutrient intake, said Dr. Phelan, a clinical psychologist and professor of kinesiology and public health at the California Polytechnic State University in San Luis Obispo.
She reported data from the Healthy Beginnings/Comienzos Saludables (Preventing Excessive Gestational Weight Gain in Obese Women) study, which enrolled 257 women with overweight or obesity (body mass index of at least 25 kg/m2) at week 9-16 of pregnancy and randomized them to either a multifactorial behavioral lifestyle intervention that included two daily meal replacements, or to “enhanced” usual care. About 80% of participants in both arms, a total of 211 women, completed the study with final follow-up at 35-36 weeks’ gestational age, after enrolling at an average gestational age of just under 14 weeks. In addition to eating nutrition bars or drinking nutrition shakes as the replacement meal options, participants also ate one conventional meal daily as well as 2-4 healthy snacks. The enrolled women included 41% with overweight and 59% with obesity.
The study’s primary endpoint was the rate of gestational weight gain per week, which was 0.33 kg in the intervention group and 0.39 kg in the controls, a statistically significant difference. The proportion of women who exceeded the Institute of Medicine’s recommended maximum gestational weight gain maximum was 41% among those in the intervention group and 54% among the controls, also a statistically significant difference (Am J Clin Nutr. 2018 Feb;107[2]:183-94).
The secondary micronutrient analysis that Dr. Phelan reported documented the high prevalence of micronutrient deficiencies among the study participants at baseline. More than 90% had deficient intake of vitamin D and fiber, more than 80% had inadequate dietary levels of iron, vitamin E, and choline, and more than half had too little dietary magnesium, vitamin K, and folate. There were additional deficiencies for other micronutrients in lesser proportions of study participants.
The analysis also showed how the behavioral and diet intervention through the end of the third trimester normalized many of these deficiencies, compared with the placebo arm. For example, the prevalence of a magnesium dietary deficiency in the intervention arm dropped from 69% at baseline to 37% at follow-up, compared with hardly any change in the control arm, so that women in the intervention group had a 64% reduced rate of magnesium deficiency compared with the controls, a statistically significant difference.
Other micronutrients that had significant drops in deficiency rate included calcium, with a 63% relative reduction in the deficiency prevalence, vitamin A with a 61% cut, vitamin E with an 83% relative reduction, and vitamin K with a 51% relative drop. Other micronutrient intake levels that showed statistically significant increases during the study compared with controls included vitamin D and copper, but choline showed an inexplicable drop in consumption in the intervention group, a “potential concern,” Dr. Phelan said. The intervention also significantly reduced sodium intake. Dr. Phelan and her associates published these findings (Nutrients. 2019 May 14;11[5]:1071; doi: 10.3390/nu11051071).
“The diet quality of many of the pregnant women we have studied was poor, often eating less than half the recommended amounts of fruits and vegetables,” said Leanne M. Redman, PhD, a professor at Louisiana State University and director of the Reproductive Endocrinology and Women’s Health Laboratory at the university’s Pennington Biomedical Research Center in Baton Rouge. “Meal replacement with bars and shakes will be really important for future efforts at improving diet quality” in pregnant women with obesity, predicted Dr. Redman, who did not collaborate on the study Dr. Phelan reported.
SOURCE: Phelan S et al. Obesity Week 2019. Abstract T-OR-2081.
LAS VEGAS – Pregnant women with overweight or obesity who replaced two meals a day with bars or shakes starting at their second trimester not only had a significantly reduced rate of gestational weight gain but also benefited from significant improvements in their intake of several micronutrients, in a randomized study of 211 women who completed the regimen.
Further research needs “to examine the generalizability and effectiveness of this prenatal lifestyle modification program in improving micronutrient sufficiency in other populations and settings,” Suzanne Phelan, PhD, said at a meeting presented by The Obesity Society and the American Society for Metabolic and Bariatric Surgery. The study she presented ran at two U.S. sites, in California and Rhode Island, and enrolled a population that was 42% Hispanic/Latina. Despite uncertainty about the applicability of the findings to other populations, the results suggested that partial meal replacement is a way to better control gestational weight gain in women with overweight or obesity while simultaneously increasing micronutrient intake, said Dr. Phelan, a clinical psychologist and professor of kinesiology and public health at the California Polytechnic State University in San Luis Obispo.
She reported data from the Healthy Beginnings/Comienzos Saludables (Preventing Excessive Gestational Weight Gain in Obese Women) study, which enrolled 257 women with overweight or obesity (body mass index of at least 25 kg/m2) at week 9-16 of pregnancy and randomized them to either a multifactorial behavioral lifestyle intervention that included two daily meal replacements, or to “enhanced” usual care. About 80% of participants in both arms, a total of 211 women, completed the study with final follow-up at 35-36 weeks’ gestational age, after enrolling at an average gestational age of just under 14 weeks. In addition to eating nutrition bars or drinking nutrition shakes as the replacement meal options, participants also ate one conventional meal daily as well as 2-4 healthy snacks. The enrolled women included 41% with overweight and 59% with obesity.
The study’s primary endpoint was the rate of gestational weight gain per week, which was 0.33 kg in the intervention group and 0.39 kg in the controls, a statistically significant difference. The proportion of women who exceeded the Institute of Medicine’s recommended maximum gestational weight gain maximum was 41% among those in the intervention group and 54% among the controls, also a statistically significant difference (Am J Clin Nutr. 2018 Feb;107[2]:183-94).
The secondary micronutrient analysis that Dr. Phelan reported documented the high prevalence of micronutrient deficiencies among the study participants at baseline. More than 90% had deficient intake of vitamin D and fiber, more than 80% had inadequate dietary levels of iron, vitamin E, and choline, and more than half had too little dietary magnesium, vitamin K, and folate. There were additional deficiencies for other micronutrients in lesser proportions of study participants.
The analysis also showed how the behavioral and diet intervention through the end of the third trimester normalized many of these deficiencies, compared with the placebo arm. For example, the prevalence of a magnesium dietary deficiency in the intervention arm dropped from 69% at baseline to 37% at follow-up, compared with hardly any change in the control arm, so that women in the intervention group had a 64% reduced rate of magnesium deficiency compared with the controls, a statistically significant difference.
Other micronutrients that had significant drops in deficiency rate included calcium, with a 63% relative reduction in the deficiency prevalence, vitamin A with a 61% cut, vitamin E with an 83% relative reduction, and vitamin K with a 51% relative drop. Other micronutrient intake levels that showed statistically significant increases during the study compared with controls included vitamin D and copper, but choline showed an inexplicable drop in consumption in the intervention group, a “potential concern,” Dr. Phelan said. The intervention also significantly reduced sodium intake. Dr. Phelan and her associates published these findings (Nutrients. 2019 May 14;11[5]:1071; doi: 10.3390/nu11051071).
“The diet quality of many of the pregnant women we have studied was poor, often eating less than half the recommended amounts of fruits and vegetables,” said Leanne M. Redman, PhD, a professor at Louisiana State University and director of the Reproductive Endocrinology and Women’s Health Laboratory at the university’s Pennington Biomedical Research Center in Baton Rouge. “Meal replacement with bars and shakes will be really important for future efforts at improving diet quality” in pregnant women with obesity, predicted Dr. Redman, who did not collaborate on the study Dr. Phelan reported.
SOURCE: Phelan S et al. Obesity Week 2019. Abstract T-OR-2081.
LAS VEGAS – Pregnant women with overweight or obesity who replaced two meals a day with bars or shakes starting at their second trimester not only had a significantly reduced rate of gestational weight gain but also benefited from significant improvements in their intake of several micronutrients, in a randomized study of 211 women who completed the regimen.
Further research needs “to examine the generalizability and effectiveness of this prenatal lifestyle modification program in improving micronutrient sufficiency in other populations and settings,” Suzanne Phelan, PhD, said at a meeting presented by The Obesity Society and the American Society for Metabolic and Bariatric Surgery. The study she presented ran at two U.S. sites, in California and Rhode Island, and enrolled a population that was 42% Hispanic/Latina. Despite uncertainty about the applicability of the findings to other populations, the results suggested that partial meal replacement is a way to better control gestational weight gain in women with overweight or obesity while simultaneously increasing micronutrient intake, said Dr. Phelan, a clinical psychologist and professor of kinesiology and public health at the California Polytechnic State University in San Luis Obispo.
She reported data from the Healthy Beginnings/Comienzos Saludables (Preventing Excessive Gestational Weight Gain in Obese Women) study, which enrolled 257 women with overweight or obesity (body mass index of at least 25 kg/m2) at week 9-16 of pregnancy and randomized them to either a multifactorial behavioral lifestyle intervention that included two daily meal replacements, or to “enhanced” usual care. About 80% of participants in both arms, a total of 211 women, completed the study with final follow-up at 35-36 weeks’ gestational age, after enrolling at an average gestational age of just under 14 weeks. In addition to eating nutrition bars or drinking nutrition shakes as the replacement meal options, participants also ate one conventional meal daily as well as 2-4 healthy snacks. The enrolled women included 41% with overweight and 59% with obesity.
The study’s primary endpoint was the rate of gestational weight gain per week, which was 0.33 kg in the intervention group and 0.39 kg in the controls, a statistically significant difference. The proportion of women who exceeded the Institute of Medicine’s recommended maximum gestational weight gain maximum was 41% among those in the intervention group and 54% among the controls, also a statistically significant difference (Am J Clin Nutr. 2018 Feb;107[2]:183-94).
The secondary micronutrient analysis that Dr. Phelan reported documented the high prevalence of micronutrient deficiencies among the study participants at baseline. More than 90% had deficient intake of vitamin D and fiber, more than 80% had inadequate dietary levels of iron, vitamin E, and choline, and more than half had too little dietary magnesium, vitamin K, and folate. There were additional deficiencies for other micronutrients in lesser proportions of study participants.
The analysis also showed how the behavioral and diet intervention through the end of the third trimester normalized many of these deficiencies, compared with the placebo arm. For example, the prevalence of a magnesium dietary deficiency in the intervention arm dropped from 69% at baseline to 37% at follow-up, compared with hardly any change in the control arm, so that women in the intervention group had a 64% reduced rate of magnesium deficiency compared with the controls, a statistically significant difference.
Other micronutrients that had significant drops in deficiency rate included calcium, with a 63% relative reduction in the deficiency prevalence, vitamin A with a 61% cut, vitamin E with an 83% relative reduction, and vitamin K with a 51% relative drop. Other micronutrient intake levels that showed statistically significant increases during the study compared with controls included vitamin D and copper, but choline showed an inexplicable drop in consumption in the intervention group, a “potential concern,” Dr. Phelan said. The intervention also significantly reduced sodium intake. Dr. Phelan and her associates published these findings (Nutrients. 2019 May 14;11[5]:1071; doi: 10.3390/nu11051071).
“The diet quality of many of the pregnant women we have studied was poor, often eating less than half the recommended amounts of fruits and vegetables,” said Leanne M. Redman, PhD, a professor at Louisiana State University and director of the Reproductive Endocrinology and Women’s Health Laboratory at the university’s Pennington Biomedical Research Center in Baton Rouge. “Meal replacement with bars and shakes will be really important for future efforts at improving diet quality” in pregnant women with obesity, predicted Dr. Redman, who did not collaborate on the study Dr. Phelan reported.
SOURCE: Phelan S et al. Obesity Week 2019. Abstract T-OR-2081.
REPORTING FROM OBESITY WEEK 2019
Jingle All the Way
Editor's note: This article was originally published in the January/February 2003 issue of Clinician News. It placed first in the journal's Most Unusual Patient Contest.
Late one morning, a woman and her 13-year-old daughter came into our office. “Mrs. Smith” reported that “Lisa” had a retained tampon. Lisa looked scared as her mother did all of the talking.
One of the male doctors saw Lisa first. After about 10 minutes, he emerged from the examination room looking perplexed. He said he wasn’t sure what was going on. He asked me to go in and try to get Lisa to allow a more thorough pelvic examination.
My exam of Lisa proved only slightly easier. I was able to visualize something curved and firm that sounded like plastic when touched with the ring forceps. I could pull the object as far as the vaginal introitus, but it would always slide back. “This is no tampon,” I repeated several times. Each time I pulled down, Lisa became more agitated. After several tries, I gave Lisa a rest and left the room to consult with a doctor.
The doctor suggested a trip to same-day surgery for evaluation under anesthesia but decided to give it one more try before heading for the operating room.
Continue to: Together, we entered the exam room...
Together, we entered the exam room. “Well, we’ve had some true confessions,” Mrs. Smith said dryly. “Lisa has a Christmas bell in her vagina—obviously the work of a curious 13-year-old!”
The doctor and I were taken by surprise, but we proceeded with the challenge of the day. Thinking we were dealing with a standard Christmas bell, we attempted to break the suction of the bell on Lisa’s cervix. This proved to be more difficult than we anticipated. After getting a closer look at the bell, we realized that it was a “jingle” bell rather than a standard bell shape.
The crosshair opening of the jingle bell had slipped over Lisa’s cervix. The cervix had become edematous and necrotic. The doctor gave one final pull, and the bell—with cervical tissue inside—flew out.
We needed pliers to open the bell so we could send a tissue sample to the pathology department for evaluation. Lisa received high-dose antibiotics and after several follow-up visits, she was declared healed.
This patient encounter, which also falls under the categories of unbelievable, unconventional, and amazing, was definitely unforgettable to me.
Editor's note: This article was originally published in the January/February 2003 issue of Clinician News. It placed first in the journal's Most Unusual Patient Contest.
Late one morning, a woman and her 13-year-old daughter came into our office. “Mrs. Smith” reported that “Lisa” had a retained tampon. Lisa looked scared as her mother did all of the talking.
One of the male doctors saw Lisa first. After about 10 minutes, he emerged from the examination room looking perplexed. He said he wasn’t sure what was going on. He asked me to go in and try to get Lisa to allow a more thorough pelvic examination.
My exam of Lisa proved only slightly easier. I was able to visualize something curved and firm that sounded like plastic when touched with the ring forceps. I could pull the object as far as the vaginal introitus, but it would always slide back. “This is no tampon,” I repeated several times. Each time I pulled down, Lisa became more agitated. After several tries, I gave Lisa a rest and left the room to consult with a doctor.
The doctor suggested a trip to same-day surgery for evaluation under anesthesia but decided to give it one more try before heading for the operating room.
Continue to: Together, we entered the exam room...
Together, we entered the exam room. “Well, we’ve had some true confessions,” Mrs. Smith said dryly. “Lisa has a Christmas bell in her vagina—obviously the work of a curious 13-year-old!”
The doctor and I were taken by surprise, but we proceeded with the challenge of the day. Thinking we were dealing with a standard Christmas bell, we attempted to break the suction of the bell on Lisa’s cervix. This proved to be more difficult than we anticipated. After getting a closer look at the bell, we realized that it was a “jingle” bell rather than a standard bell shape.
The crosshair opening of the jingle bell had slipped over Lisa’s cervix. The cervix had become edematous and necrotic. The doctor gave one final pull, and the bell—with cervical tissue inside—flew out.
We needed pliers to open the bell so we could send a tissue sample to the pathology department for evaluation. Lisa received high-dose antibiotics and after several follow-up visits, she was declared healed.
This patient encounter, which also falls under the categories of unbelievable, unconventional, and amazing, was definitely unforgettable to me.
Editor's note: This article was originally published in the January/February 2003 issue of Clinician News. It placed first in the journal's Most Unusual Patient Contest.
Late one morning, a woman and her 13-year-old daughter came into our office. “Mrs. Smith” reported that “Lisa” had a retained tampon. Lisa looked scared as her mother did all of the talking.
One of the male doctors saw Lisa first. After about 10 minutes, he emerged from the examination room looking perplexed. He said he wasn’t sure what was going on. He asked me to go in and try to get Lisa to allow a more thorough pelvic examination.
My exam of Lisa proved only slightly easier. I was able to visualize something curved and firm that sounded like plastic when touched with the ring forceps. I could pull the object as far as the vaginal introitus, but it would always slide back. “This is no tampon,” I repeated several times. Each time I pulled down, Lisa became more agitated. After several tries, I gave Lisa a rest and left the room to consult with a doctor.
The doctor suggested a trip to same-day surgery for evaluation under anesthesia but decided to give it one more try before heading for the operating room.
Continue to: Together, we entered the exam room...
Together, we entered the exam room. “Well, we’ve had some true confessions,” Mrs. Smith said dryly. “Lisa has a Christmas bell in her vagina—obviously the work of a curious 13-year-old!”
The doctor and I were taken by surprise, but we proceeded with the challenge of the day. Thinking we were dealing with a standard Christmas bell, we attempted to break the suction of the bell on Lisa’s cervix. This proved to be more difficult than we anticipated. After getting a closer look at the bell, we realized that it was a “jingle” bell rather than a standard bell shape.
The crosshair opening of the jingle bell had slipped over Lisa’s cervix. The cervix had become edematous and necrotic. The doctor gave one final pull, and the bell—with cervical tissue inside—flew out.
We needed pliers to open the bell so we could send a tissue sample to the pathology department for evaluation. Lisa received high-dose antibiotics and after several follow-up visits, she was declared healed.
This patient encounter, which also falls under the categories of unbelievable, unconventional, and amazing, was definitely unforgettable to me.
Can insulin plus metformin improve pregnancy outcomes in women with type 2 diabetes?
WASHINGTON – Insulin is the preferred agent for type 2 diabetes in pregnant women, yet about a third of pregnancies still have an adverse outcome, according Kim Boggess, MD, who spoke at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“We are not where we need to be,” said Dr. Boggess, who is leading a trial that brings metformin, the first-line agent for type 2 diabetes outside of pregnancy, back into the picture for pregnant women – as an add-on to insulin.
It is an interesting twist, because pregnant women taking metformin for preexisting type 2 or gestational diabetes have been shown in some studies to require supplemental insulin, more than occasionally, to achieve target glycemic control.
This was the case in a small, randomized, controlled trial at Dr. Boggess’ institution, the University of North Carolina at Chapel Hill, in which 43% of pregnant women with type 2 diabetes who were assigned to metformin required supplemental insulin (Am J Perinatol. 2013;30[6]:483-90). (0% vs. 36%, respectively) and fewer reports of glucose values less than 60 mg/dL (7.1% vs. 50%).
“I don’t consider this [need for supplemental insulin] ‘metformin failure,’ because studies that use metformin as monotherapy and that [show some patients] ultimately requiring insulin support ... also show that these women need less insulin,” she said. “What’s the risk of insulin alone? Hypoglycemia. So using less insulin could be a good thing.”
Other research suggests there may be less maternal weight gain, less neonatal hypoglycemia, fewer neonatal complications, and improved maternal glycemic control in patients treated with metformin, alone or with add-on insulin, than with insulin alone. “We’re starting to get a sense in the literature that, at least in the [pregnant] population with type 2 diabetes, there may be a role for metformin,” said Dr. Boggess, professor and program director for maternal-fetal medicine at the university.
Currently, the multisite MOMPOD trial (Medical Optimization of Management of T2DM Complicating Pregnancy) is randomizing 950 women to insulin plus 1,000 mg metformin twice daily or insulin plus placebo. The primary outcome of the trial is a composite of pregnancy loss, preterm birth, birth injury, neonatal hypoglycemia, or hyperbilirubinemia. Infant fat mass (within 72 hours of birth) is a secondary outcome, along with maternal safety and maternal side effects.
The MiTy (Metformin in Women with T2DM in Pregnancy) trial in Canada, with similar randomization arms and outcomes measures, is completed and undergoing analysis. “Hopefully we’ll [soon] be able to say whether the addition of adjuvant metformin to insulin to treat type 2 diabetes brings the perinatal adverse outcome rate down from 30%,” said Dr. Boggess.
Metformin is the recommended first-line agent for type 2 diabetes in nonpregnant adults. But during pregnancy, insulin, which does not cross the placenta, is the preferred agent, according to recommendations of the American Diabetes Association and the American College of Obstetricians and Gynecologists, she noted. Lingering in the background is the fact that the long-term effects of in utero metformin exposure on offspring – and of exposure to any oral hypoglycemic agent – are unknown, she said*
A majority of the adverse pregnancy outcomes that occur in the context of type 2 diabetes involve macrosomia. “It’s a big deal,” Dr. Boggess said, that results in numerous maternal and infant risks and complications. “We also know that the in utero environment that contributes to, or causes, macrosomia predisposes to childhood obesity and obesity later on.”
Diabetes is the “leading risk factor” for adverse pregnancy outcomes today, said E. Albert Reece, MD, PhD, MBA, executive vice president for medical affairs at the University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers distinguished professor and dean of the University of Maryland School of Medicine. In the United States, 11% of women aged 20 years and older have diabetes, and the disease affects more than 1% of all pregnancies, he said.
The MOMPOD trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Boggess reported no conflicts of interest.
* This article was updated 1/2/2020.
WASHINGTON – Insulin is the preferred agent for type 2 diabetes in pregnant women, yet about a third of pregnancies still have an adverse outcome, according Kim Boggess, MD, who spoke at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“We are not where we need to be,” said Dr. Boggess, who is leading a trial that brings metformin, the first-line agent for type 2 diabetes outside of pregnancy, back into the picture for pregnant women – as an add-on to insulin.
It is an interesting twist, because pregnant women taking metformin for preexisting type 2 or gestational diabetes have been shown in some studies to require supplemental insulin, more than occasionally, to achieve target glycemic control.
This was the case in a small, randomized, controlled trial at Dr. Boggess’ institution, the University of North Carolina at Chapel Hill, in which 43% of pregnant women with type 2 diabetes who were assigned to metformin required supplemental insulin (Am J Perinatol. 2013;30[6]:483-90). (0% vs. 36%, respectively) and fewer reports of glucose values less than 60 mg/dL (7.1% vs. 50%).
“I don’t consider this [need for supplemental insulin] ‘metformin failure,’ because studies that use metformin as monotherapy and that [show some patients] ultimately requiring insulin support ... also show that these women need less insulin,” she said. “What’s the risk of insulin alone? Hypoglycemia. So using less insulin could be a good thing.”
Other research suggests there may be less maternal weight gain, less neonatal hypoglycemia, fewer neonatal complications, and improved maternal glycemic control in patients treated with metformin, alone or with add-on insulin, than with insulin alone. “We’re starting to get a sense in the literature that, at least in the [pregnant] population with type 2 diabetes, there may be a role for metformin,” said Dr. Boggess, professor and program director for maternal-fetal medicine at the university.
Currently, the multisite MOMPOD trial (Medical Optimization of Management of T2DM Complicating Pregnancy) is randomizing 950 women to insulin plus 1,000 mg metformin twice daily or insulin plus placebo. The primary outcome of the trial is a composite of pregnancy loss, preterm birth, birth injury, neonatal hypoglycemia, or hyperbilirubinemia. Infant fat mass (within 72 hours of birth) is a secondary outcome, along with maternal safety and maternal side effects.
The MiTy (Metformin in Women with T2DM in Pregnancy) trial in Canada, with similar randomization arms and outcomes measures, is completed and undergoing analysis. “Hopefully we’ll [soon] be able to say whether the addition of adjuvant metformin to insulin to treat type 2 diabetes brings the perinatal adverse outcome rate down from 30%,” said Dr. Boggess.
Metformin is the recommended first-line agent for type 2 diabetes in nonpregnant adults. But during pregnancy, insulin, which does not cross the placenta, is the preferred agent, according to recommendations of the American Diabetes Association and the American College of Obstetricians and Gynecologists, she noted. Lingering in the background is the fact that the long-term effects of in utero metformin exposure on offspring – and of exposure to any oral hypoglycemic agent – are unknown, she said*
A majority of the adverse pregnancy outcomes that occur in the context of type 2 diabetes involve macrosomia. “It’s a big deal,” Dr. Boggess said, that results in numerous maternal and infant risks and complications. “We also know that the in utero environment that contributes to, or causes, macrosomia predisposes to childhood obesity and obesity later on.”
Diabetes is the “leading risk factor” for adverse pregnancy outcomes today, said E. Albert Reece, MD, PhD, MBA, executive vice president for medical affairs at the University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers distinguished professor and dean of the University of Maryland School of Medicine. In the United States, 11% of women aged 20 years and older have diabetes, and the disease affects more than 1% of all pregnancies, he said.
The MOMPOD trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Boggess reported no conflicts of interest.
* This article was updated 1/2/2020.
WASHINGTON – Insulin is the preferred agent for type 2 diabetes in pregnant women, yet about a third of pregnancies still have an adverse outcome, according Kim Boggess, MD, who spoke at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“We are not where we need to be,” said Dr. Boggess, who is leading a trial that brings metformin, the first-line agent for type 2 diabetes outside of pregnancy, back into the picture for pregnant women – as an add-on to insulin.
It is an interesting twist, because pregnant women taking metformin for preexisting type 2 or gestational diabetes have been shown in some studies to require supplemental insulin, more than occasionally, to achieve target glycemic control.
This was the case in a small, randomized, controlled trial at Dr. Boggess’ institution, the University of North Carolina at Chapel Hill, in which 43% of pregnant women with type 2 diabetes who were assigned to metformin required supplemental insulin (Am J Perinatol. 2013;30[6]:483-90). (0% vs. 36%, respectively) and fewer reports of glucose values less than 60 mg/dL (7.1% vs. 50%).
“I don’t consider this [need for supplemental insulin] ‘metformin failure,’ because studies that use metformin as monotherapy and that [show some patients] ultimately requiring insulin support ... also show that these women need less insulin,” she said. “What’s the risk of insulin alone? Hypoglycemia. So using less insulin could be a good thing.”
Other research suggests there may be less maternal weight gain, less neonatal hypoglycemia, fewer neonatal complications, and improved maternal glycemic control in patients treated with metformin, alone or with add-on insulin, than with insulin alone. “We’re starting to get a sense in the literature that, at least in the [pregnant] population with type 2 diabetes, there may be a role for metformin,” said Dr. Boggess, professor and program director for maternal-fetal medicine at the university.
Currently, the multisite MOMPOD trial (Medical Optimization of Management of T2DM Complicating Pregnancy) is randomizing 950 women to insulin plus 1,000 mg metformin twice daily or insulin plus placebo. The primary outcome of the trial is a composite of pregnancy loss, preterm birth, birth injury, neonatal hypoglycemia, or hyperbilirubinemia. Infant fat mass (within 72 hours of birth) is a secondary outcome, along with maternal safety and maternal side effects.
The MiTy (Metformin in Women with T2DM in Pregnancy) trial in Canada, with similar randomization arms and outcomes measures, is completed and undergoing analysis. “Hopefully we’ll [soon] be able to say whether the addition of adjuvant metformin to insulin to treat type 2 diabetes brings the perinatal adverse outcome rate down from 30%,” said Dr. Boggess.
Metformin is the recommended first-line agent for type 2 diabetes in nonpregnant adults. But during pregnancy, insulin, which does not cross the placenta, is the preferred agent, according to recommendations of the American Diabetes Association and the American College of Obstetricians and Gynecologists, she noted. Lingering in the background is the fact that the long-term effects of in utero metformin exposure on offspring – and of exposure to any oral hypoglycemic agent – are unknown, she said*
A majority of the adverse pregnancy outcomes that occur in the context of type 2 diabetes involve macrosomia. “It’s a big deal,” Dr. Boggess said, that results in numerous maternal and infant risks and complications. “We also know that the in utero environment that contributes to, or causes, macrosomia predisposes to childhood obesity and obesity later on.”
Diabetes is the “leading risk factor” for adverse pregnancy outcomes today, said E. Albert Reece, MD, PhD, MBA, executive vice president for medical affairs at the University of Maryland, Baltimore, and the John Z. and Akiko K. Bowers distinguished professor and dean of the University of Maryland School of Medicine. In the United States, 11% of women aged 20 years and older have diabetes, and the disease affects more than 1% of all pregnancies, he said.
The MOMPOD trial is sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Boggess reported no conflicts of interest.
* This article was updated 1/2/2020.
REPORTING FROM DPSG-NA 2019
High-dose progesterone to reverse mifepristone held still 'experimental'
A study of high-dose progesterone as a mifepristone antagonist to reverse medical abortion has been stopped early because of safety concerns, but the authors say mifepristone antagonization should not be considered impossible.
In Obstetrics & Gynecology, Mitchell D. Creinin, MD, of the University of California, Davis, and coauthors reported the outcomes of a double-blind, placebo-controlled trial investigating the efficacy and safety of high-dose oral progesterone as a mifepristone antagonist. The study intended to enroll more women at 44–63 days of gestation who were planning surgical abortion, but stopped enrolling after 12 patients because of hemorrhage concerns.
Women were given a 200-mg dose of oral mifepristone, then randomized to either 200 mg oral progesterone or placebo 24 hours later, taken twice daily for 3 days then once daily until their planned surgical abortion 14-16 days after enrollment.
The approved method of medical abortion in the United States involves a combination of mifepristone followed by the prostaglandin analogue misoprostol 24-48 hours later, a combination designed to improve efficacy of the treatment.
There have been reports of some patients changing their minds in between taking the mifepristone and the misoprostol. The fact that mifepristone binds strongly to the progesterone receptor has led to the idea that its action could be reversed with high-dose progesterone as an antagonist.
In this study, three women – two in the placebo group and one in the progesterone group – experienced severe bleeding requiring ambulance transport to the emergency department 2-3 days after taking the mifepristone.
The study found that four of the six patients in the progesterone group, and two of the six patients in the placebo group had continuing pregnancies at 2 weeks.
There were two patients – one in each group – who did not complete the study. One in the placebo group left after taking the mifepristone because of anxiety about bleeding, and had a suction aspiration. The second women completed two of the four doses of progesterone, then requested a suction aspiration.
Dr. Creinin and coauthors wrote that while the study ended early, they found that there were no significant differences in the side effects experienced by patients treated with progesterone, compared with those on placebo – apart from a worsening of some pregnancy symptoms such as vomiting and tiredness.
However, patients should be told of the risk of using mifepristone for medical abortion without using misoprostol, they said, as this was associated with severe hemorrhage even with progesterone treatment.
“Because of the potential dangers for patients who opt not to use misoprostol after mifepristone ingestion, any mifepristone antagonization treatment must be considered experimental,” Dr. Creinin and associates wrote.
The Society of Family Planning Research Fund supported the study. One author declared a consultancy with a laboratory providing medical consultation for clinicians regarding mifepristone, and a second author was an employee of Planned Parenthood. No other conflicts of interest were declared.
SOURCE: Creinin M et al. Obstet Gynecol 2019 Dec 5. doi: 10.1097/AOG.0000000000003620.
I think that this study highlights the importance of scientific rigor approved by an institutional review board when we counsel and care for our patients. As ob.gyns., we have to remember the privilege that women entrust us with their health and well being. To a certain extent, we also care for their families within our scope of reproductive health. We practice based on the best evidence available and consider referral to another trusted provider when we feel that we cannot provide unbiased care. I also feel obligated to share my opinion that legislators should trust the scientific and clinical community to not only prioritize women and their health, but also avoid introducing legislation that infringes on medicine.
Catherine Cansino, MD, MPH, is associate clinical professor of obstetrics and gynecology at the University of California, Davis. She was asked to comment on the Creinin et al. article. Dr. Cansino is on the Ob.Gyn. News editorial advisory board.
I think that this study highlights the importance of scientific rigor approved by an institutional review board when we counsel and care for our patients. As ob.gyns., we have to remember the privilege that women entrust us with their health and well being. To a certain extent, we also care for their families within our scope of reproductive health. We practice based on the best evidence available and consider referral to another trusted provider when we feel that we cannot provide unbiased care. I also feel obligated to share my opinion that legislators should trust the scientific and clinical community to not only prioritize women and their health, but also avoid introducing legislation that infringes on medicine.
Catherine Cansino, MD, MPH, is associate clinical professor of obstetrics and gynecology at the University of California, Davis. She was asked to comment on the Creinin et al. article. Dr. Cansino is on the Ob.Gyn. News editorial advisory board.
I think that this study highlights the importance of scientific rigor approved by an institutional review board when we counsel and care for our patients. As ob.gyns., we have to remember the privilege that women entrust us with their health and well being. To a certain extent, we also care for their families within our scope of reproductive health. We practice based on the best evidence available and consider referral to another trusted provider when we feel that we cannot provide unbiased care. I also feel obligated to share my opinion that legislators should trust the scientific and clinical community to not only prioritize women and their health, but also avoid introducing legislation that infringes on medicine.
Catherine Cansino, MD, MPH, is associate clinical professor of obstetrics and gynecology at the University of California, Davis. She was asked to comment on the Creinin et al. article. Dr. Cansino is on the Ob.Gyn. News editorial advisory board.
A study of high-dose progesterone as a mifepristone antagonist to reverse medical abortion has been stopped early because of safety concerns, but the authors say mifepristone antagonization should not be considered impossible.
In Obstetrics & Gynecology, Mitchell D. Creinin, MD, of the University of California, Davis, and coauthors reported the outcomes of a double-blind, placebo-controlled trial investigating the efficacy and safety of high-dose oral progesterone as a mifepristone antagonist. The study intended to enroll more women at 44–63 days of gestation who were planning surgical abortion, but stopped enrolling after 12 patients because of hemorrhage concerns.
Women were given a 200-mg dose of oral mifepristone, then randomized to either 200 mg oral progesterone or placebo 24 hours later, taken twice daily for 3 days then once daily until their planned surgical abortion 14-16 days after enrollment.
The approved method of medical abortion in the United States involves a combination of mifepristone followed by the prostaglandin analogue misoprostol 24-48 hours later, a combination designed to improve efficacy of the treatment.
There have been reports of some patients changing their minds in between taking the mifepristone and the misoprostol. The fact that mifepristone binds strongly to the progesterone receptor has led to the idea that its action could be reversed with high-dose progesterone as an antagonist.
In this study, three women – two in the placebo group and one in the progesterone group – experienced severe bleeding requiring ambulance transport to the emergency department 2-3 days after taking the mifepristone.
The study found that four of the six patients in the progesterone group, and two of the six patients in the placebo group had continuing pregnancies at 2 weeks.
There were two patients – one in each group – who did not complete the study. One in the placebo group left after taking the mifepristone because of anxiety about bleeding, and had a suction aspiration. The second women completed two of the four doses of progesterone, then requested a suction aspiration.
Dr. Creinin and coauthors wrote that while the study ended early, they found that there were no significant differences in the side effects experienced by patients treated with progesterone, compared with those on placebo – apart from a worsening of some pregnancy symptoms such as vomiting and tiredness.
However, patients should be told of the risk of using mifepristone for medical abortion without using misoprostol, they said, as this was associated with severe hemorrhage even with progesterone treatment.
“Because of the potential dangers for patients who opt not to use misoprostol after mifepristone ingestion, any mifepristone antagonization treatment must be considered experimental,” Dr. Creinin and associates wrote.
The Society of Family Planning Research Fund supported the study. One author declared a consultancy with a laboratory providing medical consultation for clinicians regarding mifepristone, and a second author was an employee of Planned Parenthood. No other conflicts of interest were declared.
SOURCE: Creinin M et al. Obstet Gynecol 2019 Dec 5. doi: 10.1097/AOG.0000000000003620.
A study of high-dose progesterone as a mifepristone antagonist to reverse medical abortion has been stopped early because of safety concerns, but the authors say mifepristone antagonization should not be considered impossible.
In Obstetrics & Gynecology, Mitchell D. Creinin, MD, of the University of California, Davis, and coauthors reported the outcomes of a double-blind, placebo-controlled trial investigating the efficacy and safety of high-dose oral progesterone as a mifepristone antagonist. The study intended to enroll more women at 44–63 days of gestation who were planning surgical abortion, but stopped enrolling after 12 patients because of hemorrhage concerns.
Women were given a 200-mg dose of oral mifepristone, then randomized to either 200 mg oral progesterone or placebo 24 hours later, taken twice daily for 3 days then once daily until their planned surgical abortion 14-16 days after enrollment.
The approved method of medical abortion in the United States involves a combination of mifepristone followed by the prostaglandin analogue misoprostol 24-48 hours later, a combination designed to improve efficacy of the treatment.
There have been reports of some patients changing their minds in between taking the mifepristone and the misoprostol. The fact that mifepristone binds strongly to the progesterone receptor has led to the idea that its action could be reversed with high-dose progesterone as an antagonist.
In this study, three women – two in the placebo group and one in the progesterone group – experienced severe bleeding requiring ambulance transport to the emergency department 2-3 days after taking the mifepristone.
The study found that four of the six patients in the progesterone group, and two of the six patients in the placebo group had continuing pregnancies at 2 weeks.
There were two patients – one in each group – who did not complete the study. One in the placebo group left after taking the mifepristone because of anxiety about bleeding, and had a suction aspiration. The second women completed two of the four doses of progesterone, then requested a suction aspiration.
Dr. Creinin and coauthors wrote that while the study ended early, they found that there were no significant differences in the side effects experienced by patients treated with progesterone, compared with those on placebo – apart from a worsening of some pregnancy symptoms such as vomiting and tiredness.
However, patients should be told of the risk of using mifepristone for medical abortion without using misoprostol, they said, as this was associated with severe hemorrhage even with progesterone treatment.
“Because of the potential dangers for patients who opt not to use misoprostol after mifepristone ingestion, any mifepristone antagonization treatment must be considered experimental,” Dr. Creinin and associates wrote.
The Society of Family Planning Research Fund supported the study. One author declared a consultancy with a laboratory providing medical consultation for clinicians regarding mifepristone, and a second author was an employee of Planned Parenthood. No other conflicts of interest were declared.
SOURCE: Creinin M et al. Obstet Gynecol 2019 Dec 5. doi: 10.1097/AOG.0000000000003620.
FROM OBSTETRICS & GYNECOLOGY