User login
Vaping: The new wave of nicotine addiction
Electronic cigarettes and other “vaping” devices have been increasing in popularity among youth and adults since their introduction in the US market in 2007.1 This increase is partially driven by a public perception that vaping is harmless, or at least less harmful than cigarette smoking.2 Vaping fans also argue that current smokers can use vaping as nicotine replacement therapy to help them quit smoking.3
We disagree. Research on the health effects of vaping, though still limited, is accumulating rapidly and making it increasingly clear that this habit is far from harmless. For youth, it is a gateway to addiction to nicotine and other substances. Whether it can help people quit smoking remains to be seen. And recent months have seen reports of serious respiratory illnesses and even deaths linked to vaping.4
In December 2016, the US Surgeon General warned that e-cigarette use among youth and young adults in the United States represents a “major public health concern,”5 and that more adolescents and young adults are now vaping than smoking conventional tobacco products.
This article reviews the issue of vaping in the United States, as well as available evidence regarding its safety.
YOUTH AT RISK
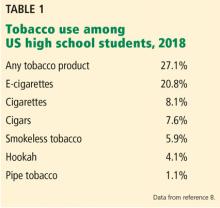
Retail sales of e-cigarettes and vaping devices approach an annual $7 billion.6 A 2014–2015 survey found that 2.4% of the general US population were current users of e-cigarettes, and 8.5% had tried them at least once.3
In 2014, for the first time, e-cigarette use became more common among US youth than traditional cigarettes.5
The odds of taking up vaping are higher among minority youth in the United States, particularly Hispanics.9 This trend is particularly worrisome because several longitudinal studies have shown that adolescents who use e-cigarettes are 3 times as likely to eventually become smokers of traditional cigarettes compared with adolescents who do not use e-cigarettes.10–12
If US youth continue smoking at the current rate, 5.6 million of the current population under age 18, or 1 of every 13, will die early of a smoking-related illness.13
RECENT OUTBREAK OF VAPING-ASSOCIATED LUNG INJURY
As of November 5, 2019, there had been 2,051 cases of vaping-associated lung injury in 49 states (all except Alaska), the District of Columbia, and 1 US territory reported to the US Centers for Disease Control and Prevention (CDC), with 39 confirmed deaths.4 The reported cases include respiratory injury including acute eosinophilic pneumonia, organizing pneumonia, acute respiratory distress syndrome, and hypersensitivity pneumonitis.14
Most of these patients had been vaping tetrahydrocannabinol (THC), though many used both nicotine- and THC-containing products, and others used products containing nicotine exclusively.4 Thus, it is difficult to identify the exact substance or substances that may be contributing to this sudden outbreak among vape users, and many different product sources are currently under investigation.
One substance that may be linked to the epidemic is vitamin E acetate, which the New York State Department of Health has detected in high levels in cannabis vaping cartridges used by patients who developed lung injury.15 The US Food and Drug Administration (FDA) is continuing to analyze vape cartridge samples submitted by affected patients to look for other chemicals that can contribute to the development of serious pulmonary illness.
WHAT IS AN E-CIGARETTE? WHAT IS A VAPE PEN?
Vape pens consist of similar elements but are not necessarily similar in appearance to a conventional cigarette, and may look more like a pen or a USB flash drive. In fact, the Juul device is recharged by plugging it into a USB port.
Vaping devices have many street names, including e-cigs, e-hookahs, vape pens, mods, vapes, and tank systems.
The first US patent application for a device resembling a modern e-cigarette was filed in 1963, but the product never made it to the market.16 Instead, the first commercially successful e-cigarette was created in Beijing in 2003 and introduced to US markets in 2007.
Newer-generation devices have larger batteries and can heat the liquid to higher temperatures, releasing more nicotine and forming additional toxicants such as formaldehyde. Devices lack standardization in terms of design, capacity for safely holding e-liquid, packaging of the e-liquid, and features designed to minimize hazards of use.
Not just nicotine
Many devices are designed for use with other drugs, including THC.17 In a 2018 study, 10.9% of college students reported vaping marijuana in the past 30 days, up from 5.2% in 2017.18
Other substances are being vaped as well.19 In theory, any heat-stable psychoactive recreational drug could be aerosolized and vaped. There are increasing reports of e-liquids containing recreational drugs such as synthetic cannabinoid receptor agonists, crack cocaine, LSD, and methamphetamine.17
Freedom, rebellion, glamour
Sales have risen rapidly since 2007 with widespread advertising on television and in print publications for popular brands, often featuring celebrities.20 Spending on advertising for e-cigarettes and vape devices rose from $6.4 million in 2011 to $115 million in 2014—and that was before the advent of Juul (see below).21
Marketing campaigns for vaping devices mimic the themes previously used successfully by the tobacco industry, eg, freedom, rebellion, and glamour. They also make unsubstantiated claims about health benefits and smoking cessation, though initial websites contained endorsements from physicians, similar to the strategies of tobacco companies in old cigarette ads. Cigarette ads have been prohibited since 1971—but not e-cigarette ads. Moreover, vaping products appear as product placements in television shows and movies, with advocacy groups on social media.22
By law, buyers have to be 18 or 21
Vaping devices can be purchased at vape shops, convenience stores, gas stations, and over the Internet; up to 50% of sales are conducted online.24
Fruit flavors are popular
Zhu et al25 estimated that 7,700 unique vaping flavors exist, with fruit and candy flavors predominating. The most popular flavors are tobacco and mint, followed by fruit, dessert and candy flavors, alcoholic flavors (strawberry daiquiri, margarita), and food flavors.25 These flavors have been associated with higher usage in youth, leading to increased risk of nicotine addiction.26
WHAT IS JUUL?
The Juul device (Juul Labs, www.juul.com) was developed in 2015 by 2 Stanford University graduates. Their goal was to produce a more satisfying and cigarette-like vaping experience, specifically by increasing the amount of nicotine delivered while maintaining smooth and pleasant inhalation. They created an e-liquid that could be vaporized effectively at lower temperatures.27
While more than 400 brands of vaping devices are currently available in the United States,3 Juul has held the largest market share since 2017,28 an estimated 72.1% as of August 2018.29 The surge in popularity of this particular brand is attributed to its trendy design that is similar in size and appearance to a USB flash drive,29 and its offering of sweet flavors such as “crème brûlée” and “cool mint.”
On April 24, 2018, in view of growing concern about the popularity of Juul products among youth, the FDA requested that the company submit documents regarding its marketing tactics, as well as research on the effects of this marketing on product design and public health impact, and information about adverse experiences and complaints.30 The company was forced to change its marketing to appeal less to youth. Now it offers only 3 flavors: “Virginia tobacco,” “classic tobacco,” and “menthol,” although off-brand pods containing a variety of flavors are still available. And some pods are refillable, so users can essentially vape any substance they want.
Although the Juul device delivers a strong dose of nicotine, it is small and therefore easy to hide from parents and teachers, and widespread use has been reported among youth in middle and high schools. Hoodies, hemp jewelry, and backpacks have been designed to hide the devices and allow for easy, hands-free use. YouTube searches for terms such as “Juul,” “hiding Juul at school,” and “Juul in class,” yield thousands of results.31 A 2017 survey reported that 8% of Americans age 15 to 24 had used Juul in the month prior to the survey.32 “To juul” has become a verb.
Each Juul starter kit contains the rechargeable inhalation device plus 4 flavored pods. In the United States, each Juul pod contains nearly as much nicotine as 1 pack of 20 cigarettes in a concentration of 3% or 5%. (Israel and Europe have forced the company to replace the 5% nicotine pods with 1.7% nicotine pods.33) A starter kit costs $49.99, and additional packs of 4 flavored liquid cartridges or pods cost $15.99.34 Other brands of vape pens cost between $15 and $35, and 10-mL bottles of e-liquid cost approximately $7.
What is ‘dripping’?
Hard-core vapers seeking a more intense experience are taking their vaping devices apart and modifying them for “dripping,” ie, directly dripping vape liquids onto the heated coils for inhalation. In a survey, 1 in 4 high school students using vape devices also used them for dripping, citing desires for a thicker cloud of vapor, more intense flavor, “a stronger throat hit,” curiosity, and other reasons.35 Dripping involves higher temperatures, which leads to higher amounts of nicotine delivered, along with more formaldehyde, acetaldehyde, and acetone (see below).36
BAD THINGS IN E-LIQUID AEROSOL
Studies of vape liquids consistently confirm the presence of toxic substances in the resulting vape aerosol.37–40 Depending on the combination of flavorings and solvents in a given e-liquid, a variety of chemicals can be detected in the aerosol from various vaping devices. Chemicals that may be detected include known irritants of respiratory mucosa, as well as various carcinogens. The list includes:
- Organic volatile compounds such as propylene glycol, glycerin, and toluene
- Aldehydes such as formaldehyde (released when propylene glycol is heated to high temperatures), acetaldehyde, and benzaldehyde
- Acetone and acrolein
- Carcinogenic nitrosamines
- Polycyclic aromatic hydrocarbons
- Particulate matter
- Metals including chromium, cadmium, nickel, and lead; and particles of copper, nickel, and silver have been found in electronic nicotine delivery system aerosol in higher levels than in conventional cigarette smoke.41
The specific chemicals detected can vary greatly between brands, even when the flavoring and nicotine content are equivalent, which frequently results in inconsistent and conflicting study findings. The chemicals detected also vary with the voltage or power used to generate the aerosol. Different flavors may carry varying levels of risk; for example, mint- and menthol-flavored e-cigarettes were shown to expose users to dangerous levels of pulegone, a carcinogenic compound banned as a food additive in 2018.42 The concentrations of some of these chemicals are sufficiently high to be of toxicologic concern; for example, one study reported the presence of benzaldehyde in e-cigarette aerosol at twice the workplace exposure limit.43
Biologic effects
In an in vitro study,44 57% of e-liquids studied were found to be cytotoxic to human pulmonary fibroblasts, lung epithelial cells, and human embryonic stem cells. Fruit-flavored e-liquids in particular caused a significant increase in DNA fragmentation. Cell cultures treated with e-cigarette liquids showed increased oxidative stress, reduced cell proliferation, and increased DNA damage,44 which may have implications for carcinogenic risk.
In another study,45 exposure to e-cigarette aerosol as well as conventional cigarette smoke resulted in suppression of genes related to immune and inflammatory response in respiratory epithelial cells. All genes with decreased expression after exposure to conventional cigarette smoke also showed decreased expression with exposure to e-cigarette smoke, which the study authors suggested could lead to immune suppression at the level of the nasal mucosa. Diacetyl and acetoin, chemicals found in certain flavorings, have been linked to bronchiolitis obliterans, or “popcorn lung.”46
Nicotine is not benign
The nicotine itself in many vaping liquids should also not be underestimated. Nicotine has harmful neurocognitive effects and addictive properties, particularly in the developing brains of adolescents and young adults.47 Nicotine exposure during adolescence negatively affects memory, attention, and emotional regulation,48 as well as executive functioning, reward processing, and learning.49
The brain undergoes major structural remodeling in adolescence, and nicotine acetylcholine receptors regulate neural maturation. Early exposure to nicotine disrupts this process, leading to poor executive functioning, difficulty learning, decreased memory, and issues with reward processing.
Fetal exposure, if nicotine products are used during pregnancy, has also been linked to adverse consequences such as deficits in attention and cognition, behavioral effects, and sudden infant death syndrome.5
Much to learn about toxicity
Partly because vaping devices have been available to US consumers only since 2007, limited evidence is available regarding the long-term effects of exposure to the aerosol from these devices in humans.1 Many of the studies mentioned above were in vitro studies or conducted in mouse models. Differences in device design and the composition of the e-liquid among device brands pose a challenge for developing well-designed studies of the long-term health effects of e-cigarette and vape use. Additionally, devices may have different health impacts when used to vape cannabis or other drugs besides nicotine, which requires further investigation.
E-CIGARETTES AND SMOKING CESSATION
Conventional cigarette smoking is a major public health threat, as tobacco use is responsible for 480,000 deaths annually in the United States.50
And smoking is extremely difficult to quit: as many as 80% of smokers who attempt to quit resume smoking within the first month.51 The chance of successfully quitting improves by over 50% if the individual undergoes nicotine replacement therapy, and it improves even more with counseling.50
There are currently 5 types of FDA-approved nicotine replacement therapy products (gum, patch, lozenge, inhaler, nasal spray) to help with smoking cessation. In addition, 2 non-nicotine prescription drugs (varenicline and bupropion) have been approved for treating tobacco dependence.
Can vaping devices be added to the list of nicotine replacement therapy products? Although some manufacturers try to brand their devices as smoking cessation aids, in one study,52 one-third of e-cigarette users said they had either never used conventional cigarettes or had formerly smoked them.
Bullen et al53 randomized smokers interested in quitting to receive either e-cigarettes, nicotine patches, or placebo (nicotine-free) e-cigarettes and followed them for 6 months. Rates of tobacco cessation were less than predicted for the entire study population, resulting in insufficient power to determine the superiority of any single method, but the study authors concluded that nicotine e-cigarettes were “modestly effective” at helping smokers quit, and that abstinence rates may be similar to those with nicotine patches.53
Hajek et al54 randomized 886 smokers to e-cigarette or nicotine replacement products of their choice. After 1 year, 18% of e-cigarette users had stopped smoking, compared with 9.9% of nicotine replacement product users. However, 80% of the e-cigarette users were still using e-cigarettes after 1 year, while only 9% of nicotine replacement product users were still using nicotine replacement therapy products after 1 year.
While quitting conventional cigarette smoking altogether has widely established health benefits, little is known about the health benefits of transitioning from conventional cigarette smoking to reduced conventional cigarette smoking with concomitant use of e-cigarettes.
Campagna et al55 found no beneficial health effects in smokers who partially substituted conventional cigarettes for e-cigarettes.
Many studies found that smokers use e-cigarettes to maintain their habit instead of quitting entirely.56 It has been suggested that any slight increase in effectiveness in smoking cessation by using e-cigarettes compared with other nicotine replacement products could be linked to satisfying of the habitual smoking actions, such as inhaling and bringing the hand to the mouth,24 which are absent when using other nicotine replacement methods such as a nicotine patch.
As with safety information, long-term outcomes regarding the use of vape devices for smoking cessation have not been yet established, as this option is still relatively new.
VAPING AS A GATEWAY DRUG
Another worrisome trend involving electronic nicotine delivery systems is their marketing and branding, which appear to be aimed directly at adolescents and young adults. Juul and other similar products cannot be sold to anyone under the age of 18 (or 21 in 18 states, including California, Massachusetts, New York, and now Ohio). Despite this, Juul and similar products continue to increase in popularity among middle school and high school students.57
While smoking cessation and health improvement are cited as reasons for vaping among middle-aged and older adults, adolescents and young adults more often cite flavor, enjoyment, peer use, and curiosity as reasons for use.
Adolescents are more likely to report interest in trying a vape product flavored with menthol or fruit than tobacco, and commonly hold the belief that fruit-flavored e-cigarettes are less harmful than tobacco-flavored e-cigarettes.58 Harrell et al59 polled youth and young adults who used flavored e-cigarettes, and 78% said they would no longer use the product if their preferred flavor were not available. In September 2019, Michigan became the first state to ban the sale of flavored e-cigarettes in stores and online. Similar bills have been introduced in California, Massachusetts, and New York.60
Myths and misperceptions abound among youth regarding smoking vs vaping. Young people view regular cigarette smoking negatively, as causing cancer, bad breath, and asthma exacerbations. Meanwhile, they believe marijuana is safer and less addictive than traditional cigarette smoking.61 Youth exposed to e-cigarette advertisements viewed e-cigarettes as healthier, more enjoyable, “cool,” safe, and fun.61 The overall public health impact of increasing initiation of smoking, particularly among youth and young adults, should not be underestimated.
SECONDHAND VAPE AND OTHER EXPOSURE RISKS
Cigarette smoking has been banned in many public places, in view of a large body of scientific evidence about the harmful effects of secondhand smoke. Advocates for allowing vaping in public places say that vaping emissions do not harm bystanders, but evidence is insufficient to support this claim.62 One study showed that passive exposure to e-cigarette aerosol generated increases in serum levels of cotinine (a nicotine metabolite) similar to those with passive exposure to conventional cigarette smoke.5
Accidental nicotine poisoning in children as a result of ingesting e-cigarette liquid is also a major concern,63 particularly with sweet flavors such as bubblegum or cheesecake that may be attractive to children.
Calls to US poison control centers with respect to e-cigarettes and vaping increased from 1 per month in September 2010 to 215 in February 2014, with 51% involving children under age 5.64 This trend resulted in the Child Nicotine Poisoning Prevention Act, which passed in 2015 and went into effect in 2016, requiring packaging that is difficult to open for children under age 5.5
Device malfunctions or battery failures have led to explosions that have resulted in substantial injuries to users, as well as house and car fires.49
HOW DO WE DISCOURAGE ADOLESCENT USE?
There are currently no established treatment approaches for adolescents who have become addicted to vaping. A review of the literature regarding treatment modalities used to address adolescent use of tobacco and marijuana provides insight that options such as nicotine replacement therapy and counseling modalities such as cognitive behavioral therapy may be helpful in treating teen vaping addiction. However, more research is needed to determine the effectiveness of these treatments in youth addicted to vaping.
Given that youth who vape even once are more likely to try other types of tobacco, we recommend that parents and healthcare providers start conversations by asking what the young person has seen or heard about vaping. Young people can also be asked what they think the school’s response should be: Do they think vaping should be banned in public places, as cigarettes have been banned? What about the carbon footprint? What are their thoughts on the plastic waste, batteries, and other toxins generated by the e-cigarette industry?
New US laws ban the sale of e-cigarettes and vaping devices to minors in stores and online. These policies are modeled in many cases on environmental control policies that have been previously employed to reduce tobacco use, particularly by youth. For example, changing laws to mandate sales only to individuals age 21 and older in all states can help to decrease access to these products among middle school and high school students.
As with tobacco cessation, education will not be enough. Support of legislation that bans vaping in public places, increases pricing to discourage adolescent use, and other measures used successfully to decrease conventional cigarette smoking can be deployed to decrease the public health impact of e-cigarettes. We recommend further regulation of specific harmful chemicals and clear, detailed ingredient labeling to increase consumer understanding of the risks associated with these products. Additionally, we recommend eliminating flavored e-cigarettes, which are the most appealing type for young users, and raising prices of e-cigarettes and similar products to discourage use by youth.
If current cigarette smokers want to use e-cigarettes to quit, we recommend that clinicians counsel them to eventually completely stop use of traditional cigarettes and switch to using e-cigarettes, instead of becoming a dual user of both types of products or using e-cigarettes indefinitely. After making that switch, they should then work to gradually taper usage and nicotine addiction by reducing the amount of nicotine in the e-liquid. Clinicians should ask patients about use of e-cigarettes and vaping devices specifically, and should counsel nonsmokers to avoid initiation of use.
EVIDENCE OF HARM CONTINUES TO EMERGE
Data about respiratory effects, secondhand exposure, and long-term smoking cessation efficacy are still limited, and it remains as yet unknown what combinations of solvents, flavorings, and nicotine in a given e-liquid will result in the most harmful or least harmful effects. In addition, while much of the information about the safety of these components has been obtained using in vitro or mouse models, increasing reports of serious respiratory illness and rising numbers of deaths linked to vaping make it clear that these findings likely translate to harmful effects in humans.
E-cigarettes may ultimately prove to be less harmful than traditional cigarettes, but it seems likely that with further time and research, serious health risks of e-cigarette use will continue to emerge.
- Sood A, Kesic M, Hernandez M. Electronic cigarettes: one size does not fit all. J Allergy Clin Immunol 2018; 141(6):1973-1982. doi:10.1016/j.jaci.2018.02.029
- Romijnders K, van Osch L, de Vries H, Talhout R. Perceptions and reasons regarding e-cigarette use among users and non-users: a narrative literature review. Int J Environ Res Public Health 2018; 15(6):1190. doi:10.3390/ijerph15061190
- Zhu S, Zhuang Y-L, Wong S, Cummins SE, Tedeschi GJ. E-cigarette use and associated changes in population smoking cessation: evidence from US current population surveys. BMJ 2017; 358:j3262. doi:10.1136/bmj.j3262
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with e-cigarette use, or vaping. Updated November 5, 2019. www.cdc.gov/tobacco/basic_information/e-Cigarettes/severe-Lung-Disease.html. Accessed November 14, 2019.
- US Public Health Services, US Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Rockville, MD, U.S. Department of Health and Human Services, 2016. https://e-cigarettes.surgeongeneral.gov/documents/2016_sgr_full_report_non-508.pdf. Accessed November 14, 2019.
- Thomas K, Kaplan S. E-cigarettes went unchecked in 10 years of federal inaction. New York Times Oct 14, 2019; updated November 1, 2019. www.nytimes.com/2019/10/14/health/vaping-e-cigarettes-fda.html.
- Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2018; 67(45):1276–1277. doi:10.15585/mmwr.mm6745a5
- Gentzke A, Creamer M, Cullen K, et al; Centers for Disease Control and Prevention. Vital signs: tobacco product use among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2019; 68(6):157–164. doi:10.15585/mmwr.mm6806e1
- Hammig B, Daniel-Dobbs P, Blunt-Vinti H. Electronic cigarette initiation among minority youth in the United States. Am J Drug Alcohol Abuse 2017; 43(3):306–310. doi:10.1080/00952990.2016.1203926
- Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD. Progression to traditional cigarette smoking after electronic cigarette use among U.S. adolescents and young adults. JAMA Pediatr 2015; 169(11):1018–1023. doi:10.1001/jamapediatrics.2015.1742
- Leventhal AM, Strong DR, Kirkpatrick MG, et al. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA 2015; 314(7):700–707. doi:10.1001/jama.2015.8950
- Wills TA, Knight R, Sargent JD, Gibbons FX, Pagano I, Williams RJ. Longitudinal study of e-cigarette use and onset of cigarette smoking among high school students in Hawaii. Tob Control 2016; 26(1):34–39. doi:10.1136/tobaccocontrol-2015-052705
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention, 2014. www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf. Accessed November 14, 2019.
- Christiani DC. Vaping-induced lung injury. N Engl J Med 2019; Sept 6. Epub ahead of print. doi:10.1056/NEJMe1912032
- Neel J, Aubrey A. Vitamin E suspected in serious lung problems among people who vaped cannabis. NPR Sept 5, 2019. www.npr.org/sections/health-shots/2019/09/05/758005409/vitamin-e-suspected-in-serious-lung-problems-among-people-who-vaped-cannabis. Accessed November 14, 2019.
- White A. Plans for the first e-cigarette went up in smoke 50 years ago. Smithsonian Magazine December 2018. www.smithsonianmag.com/innovation/plans-for-first-e-cigarette-went-up-in-smoke-50-years-ago-180970730.
- Blundell MS, Dargan PI, Wood DM. The dark cloud of recreational drugs and vaping. QJM 2018; 111(3):145–148. doi:10.1093/qjmed/hcx049
- Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, Patrick ME. Monitoring the future: national survey results on drug use, 1975–2018. 2018 Volume 2. College students & adults ages 19–60. www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf. Accessed November 14, 2019.
- Eggers ME, Lee YO, Jackson J, Wiley JL, Porter J, Nonnemaker JM. Youth use of electronic vapor products and blunts for administering cannabis. Addict Behav 2017; 70:79-82. doi:10.1016/j.addbeh.2017.02.020
- Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the “e-cigarette”in the USA. Tob Control 2013; 22(1):19–23. doi:10.1136/tobaccocontrol-2011-050044
- Centers for Disease Control and Prevention. E-cigarette ads and youth. www.cdc.gov/vitalsigns/ecigarette-ads/index.html.
- Noel JK, Rees VW, Connolly GN. Electronic cigarettes: a new “tobacco” industry? Tob Control 2011; 20(1):81. doi:10.1136/tc.2010.038562
- US Food and Drug Administration. Deeming tobacco products to be subject to the federal food, drug, and cosmetic act, as amended by the family smoking prevention and tobacco control act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Federal Register 2016; 81(90), May 10, 2016. www.govinfo.gov/content/pkg/FR-2016-05-10/pdf/2016-10685.pdf. Accessed November 14, 2019.
- Rom O, Pecorelli A, Valacchi G, Reznick AZ. Are e-cigarettes a safe and good alternative to cigarette smoking? Ann NY Acad Sci 2015; 1340:65–74. doi:10.1111/nyas.12609
- Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 2014; 23(suppl 3):iii3-iii9. doi:10.1136/tobaccocontrol-2014-051670
- Kong G, Morean ME, Cavallo DA, Camenga DR, Krishnan-Sarin S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob Res 2015; 17(7):847–854. doi:10.1093/ntr/ntu257
- Baca MC. How two Stanford grads aimed for big tech glory and got big tobacco instead. Updated September 4, 2019. The Washington Post September 4, 2019. www.washingtonpost.com/technology/2019/09/04/how-two-stanford-grads-aimed-big-tech-glory-got-big-tobacco-instead. Accessed November 14, 2019.
- Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control 2019; 28(2):146–151. doi:10.1136/tobaccocontrol-2018-054382
- Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics 2019; 143(6):pii:e20182741. doi:10.1542/peds.2018-2741
- Zernike K. F.D.A. cracks down on “juuling” among teenagers. The New York Times April 24, 2018. www.nytimes.com/2018/04/24/health/fda-e-cigarettes-minors-juul.html. Accessed November 14, 2019.
- Ramamurthi D, Chau C, Jackler RK. JUUL and other stealth vaporisers: hiding the habit from parents and teachers. Tob Control 2018 Sep 15; pii:tobaccocontrol-2018-054455. doi:10.1136/tobaccocontrol-2018-054455. [Epub ahead of print]
- Willett JG, Bennett M, Hair EC, et al. Recognition, use and perceptions of JUUL among youth and young adults. Tob Control 2019; 28(1):115–116. doi:10.1136/tobaccocontrol-2018-054273
- Kaplan S. Juul’s new product: less nicotine, more intense vapor. New York Times Nov 27, 2018. www.nytimes.com/2018/11/27/health/juul-ecigarettes-nicotine.html.
- JUUL Labs. JUULpods. www.juul.com/shop/pods. Accessed November 14, 2019.
- Krishnan-Sarin S, Morean M, Kong G, et al. E-cigarettes and “dripping” among high-school youth. Pediatrics 2017; 139(3):pii:e20163224. doi:10.1542/peds.2016-3224
- Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014; 16(10):1319–1326. doi:10.1093/ntr/ntu078
- Rawlinson C, Martin S, Frosina J, Wright C. Chemical characterisation of aerosols emitted by electronic cigarettes using thermal desorption-gas chromatography-time of flight mass spectrometry. J Chromatogr A 2017; 1497:144–154. doi:10.1016/j.chroma.2017.02.050
- Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ Health 2017; 16(1):42. doi:10.1186/s12940-017-0249-x
- Williams M, Bozhilov K, Ghai S, Talbot P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One 2017; 12(4):e0175430. doi:10.1371/journal.pone.0175430.
- Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014; 23(2):133–139. doi:10.1136/tobaccocontrol-2012-050859
- Drope J, Cahn Z, Kennedy R, et al. Key issues surrounding the health impacts of electronic nicotine delivery systems (ENDS) and other sources of nicotine. CA Cancer J Clin 2017; 67(6):449–471. doi:10.3322/caac.21413
- Jabba SV, Jordt SE. Risk analysis for the carcinogen pulegone in mint- and menthol-flavored e-cigarettes and smokeless tobacco products. JAMA Intern Med 2019 Sep 16 [Epub ahead of print]. doi:10.1001/jamainternmed.2019.3649
- Tierney PA, Karpinsky CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control 2016; 25(e1):e10–e15. doi:10.1136/tobaccocontrol-2014-052175
- Behar RZ, Wang Y, Talbot P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob Control 2017; 27(3):325–333. doi:10.1136/tobaccocontrol-2016-053472
- Martin EM, Clapp PW, Rebuli ME, et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol 2016; 311(1):L135–L144. doi:10.1152/ajplung.00170.2016
- Holden VK, Hines SE. Update on flavoring-induced lung disease. Curr Opin Pulm Med 2016;22(2):158–164. doi:10.1097/MCP.0000000000000250
- Siqueira L; Committee on Substance Use and Prevention. Nicotine and tobacco as substances of abuse in children and adolescents. Pediatrics 2017; 139(1):pii:e20163436. doi:10.1542/peds.2016-3436
- England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med 2015; 49(2):286–293. doi:10.1016/j.amepre.2015.01.015
- Modesto-Lowe V, Alvarado C. E-cigs…are they cool? Talking to teens about e-cigarettes. Clin Pediatr (Phila) 2017; 51(10):947–952. doi:10.1177/0009922817705188
- Prochaska JJ, Benowitz NL. The past, present, and future of nicotine addiction therapy. Annu Rev Med 2017; 67:467–486. doi:10.1146/annurev-med-111314-033712
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004; 99(1):29–38. doi:10.1111/j.1360-0443.2004.00540.x
- McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res 2015;17(10):119_1202. doi:10.1093/ntr/ntu213
- Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013; 382(9905):1629–1637. doi:10.1016/S0140-6736(13)61842-5
- Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine replacement therapy. N Engl J Med 2019; 380(7):629–637. doi:10.1056/NEJMoa1808779
- Campagna D, Cibella F, Caponnetto P, et al. Changes in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettes. Eur J Clin Invest 2016; 46(8):698–706. doi:10.1111/eci.12651
- Rehan HS, Maini J, Hungin APS. Vaping versus smoking: a quest for efficacy and safety of e-cigarette. Curr Drug Saf 2018; 13(2):92–101. doi:10.2174/1574886313666180227110556
- Zernike K. ‘I can’t stop’: schools struggle with vaping explosion. New York Times April 2, 2018. www.nytimes.com/2018/04/02/health/vaping-ecigarettes-addiction-teen.html.
- Pepper JK, Ribisl KM, Brewer NT. Adolescents’ interest in trying flavoured e-cigarettes. Tob Control 2016; 25(suppl 2):ii62–ii66. doi:10.1136/tobaccocontrol-2016-053174
- Harrell MB, Loukas A, Jackson CD, Marti CN, Perry CL. Flavored tobacco product use among youth and young adults: what if flavors didn’t exist? Tob Regul Sci 2017; 3(2):168–173. doi:10.18001/TRS.3.2.4
- Smith M. Amid vaping crackdown, Michigan to ban sale of flavored e-cigarettes. New York Times Sept 4, 2019. www.nytimes.com/2019/09/04/us/michigan-vaping.html?module=inline.
- Roditis ML, Halpern-Felsher B. Adolescents’ perceptions of risks and benefits of conventional cigarettes, e-cigarettes, and marijuana: a qualitative analysis. J Adolesc Health 2015; 57(2):179–185. doi:10.1016/j.jadohealth.2015.04.002
- Chapman S, Daube M, Maziak W. Should e-cigarette use be permitted in smoke-free public places? No. Tob Control 2017; 26(e1):e3–e4. doi:10.1136/tobaccocontrol-2016-053359
- Marcham CL, Springston JP. Electronic cigarettes in the indoor environment. Rev Env Health 2019; 34(2):105–124. doi:10.1515/reveh-2019-0012
- Chatham-Stephens K, Law R, Taylor E, et al; Centers for Disease Control and Prevention. Notes from the field: calls to poison centers for exposures to electronic cigarettes—United States, September 2010–September 2014. MMWR Morb Mortal Wkly Report 2014; 63(13):292–293. pmid:24699766
Electronic cigarettes and other “vaping” devices have been increasing in popularity among youth and adults since their introduction in the US market in 2007.1 This increase is partially driven by a public perception that vaping is harmless, or at least less harmful than cigarette smoking.2 Vaping fans also argue that current smokers can use vaping as nicotine replacement therapy to help them quit smoking.3
We disagree. Research on the health effects of vaping, though still limited, is accumulating rapidly and making it increasingly clear that this habit is far from harmless. For youth, it is a gateway to addiction to nicotine and other substances. Whether it can help people quit smoking remains to be seen. And recent months have seen reports of serious respiratory illnesses and even deaths linked to vaping.4
In December 2016, the US Surgeon General warned that e-cigarette use among youth and young adults in the United States represents a “major public health concern,”5 and that more adolescents and young adults are now vaping than smoking conventional tobacco products.
This article reviews the issue of vaping in the United States, as well as available evidence regarding its safety.
YOUTH AT RISK
Retail sales of e-cigarettes and vaping devices approach an annual $7 billion.6 A 2014–2015 survey found that 2.4% of the general US population were current users of e-cigarettes, and 8.5% had tried them at least once.3
In 2014, for the first time, e-cigarette use became more common among US youth than traditional cigarettes.5
The odds of taking up vaping are higher among minority youth in the United States, particularly Hispanics.9 This trend is particularly worrisome because several longitudinal studies have shown that adolescents who use e-cigarettes are 3 times as likely to eventually become smokers of traditional cigarettes compared with adolescents who do not use e-cigarettes.10–12
If US youth continue smoking at the current rate, 5.6 million of the current population under age 18, or 1 of every 13, will die early of a smoking-related illness.13
RECENT OUTBREAK OF VAPING-ASSOCIATED LUNG INJURY
As of November 5, 2019, there had been 2,051 cases of vaping-associated lung injury in 49 states (all except Alaska), the District of Columbia, and 1 US territory reported to the US Centers for Disease Control and Prevention (CDC), with 39 confirmed deaths.4 The reported cases include respiratory injury including acute eosinophilic pneumonia, organizing pneumonia, acute respiratory distress syndrome, and hypersensitivity pneumonitis.14
Most of these patients had been vaping tetrahydrocannabinol (THC), though many used both nicotine- and THC-containing products, and others used products containing nicotine exclusively.4 Thus, it is difficult to identify the exact substance or substances that may be contributing to this sudden outbreak among vape users, and many different product sources are currently under investigation.
One substance that may be linked to the epidemic is vitamin E acetate, which the New York State Department of Health has detected in high levels in cannabis vaping cartridges used by patients who developed lung injury.15 The US Food and Drug Administration (FDA) is continuing to analyze vape cartridge samples submitted by affected patients to look for other chemicals that can contribute to the development of serious pulmonary illness.
WHAT IS AN E-CIGARETTE? WHAT IS A VAPE PEN?
Vape pens consist of similar elements but are not necessarily similar in appearance to a conventional cigarette, and may look more like a pen or a USB flash drive. In fact, the Juul device is recharged by plugging it into a USB port.
Vaping devices have many street names, including e-cigs, e-hookahs, vape pens, mods, vapes, and tank systems.
The first US patent application for a device resembling a modern e-cigarette was filed in 1963, but the product never made it to the market.16 Instead, the first commercially successful e-cigarette was created in Beijing in 2003 and introduced to US markets in 2007.
Newer-generation devices have larger batteries and can heat the liquid to higher temperatures, releasing more nicotine and forming additional toxicants such as formaldehyde. Devices lack standardization in terms of design, capacity for safely holding e-liquid, packaging of the e-liquid, and features designed to minimize hazards of use.
Not just nicotine
Many devices are designed for use with other drugs, including THC.17 In a 2018 study, 10.9% of college students reported vaping marijuana in the past 30 days, up from 5.2% in 2017.18
Other substances are being vaped as well.19 In theory, any heat-stable psychoactive recreational drug could be aerosolized and vaped. There are increasing reports of e-liquids containing recreational drugs such as synthetic cannabinoid receptor agonists, crack cocaine, LSD, and methamphetamine.17
Freedom, rebellion, glamour
Sales have risen rapidly since 2007 with widespread advertising on television and in print publications for popular brands, often featuring celebrities.20 Spending on advertising for e-cigarettes and vape devices rose from $6.4 million in 2011 to $115 million in 2014—and that was before the advent of Juul (see below).21
Marketing campaigns for vaping devices mimic the themes previously used successfully by the tobacco industry, eg, freedom, rebellion, and glamour. They also make unsubstantiated claims about health benefits and smoking cessation, though initial websites contained endorsements from physicians, similar to the strategies of tobacco companies in old cigarette ads. Cigarette ads have been prohibited since 1971—but not e-cigarette ads. Moreover, vaping products appear as product placements in television shows and movies, with advocacy groups on social media.22
By law, buyers have to be 18 or 21
Vaping devices can be purchased at vape shops, convenience stores, gas stations, and over the Internet; up to 50% of sales are conducted online.24
Fruit flavors are popular
Zhu et al25 estimated that 7,700 unique vaping flavors exist, with fruit and candy flavors predominating. The most popular flavors are tobacco and mint, followed by fruit, dessert and candy flavors, alcoholic flavors (strawberry daiquiri, margarita), and food flavors.25 These flavors have been associated with higher usage in youth, leading to increased risk of nicotine addiction.26
WHAT IS JUUL?
The Juul device (Juul Labs, www.juul.com) was developed in 2015 by 2 Stanford University graduates. Their goal was to produce a more satisfying and cigarette-like vaping experience, specifically by increasing the amount of nicotine delivered while maintaining smooth and pleasant inhalation. They created an e-liquid that could be vaporized effectively at lower temperatures.27
While more than 400 brands of vaping devices are currently available in the United States,3 Juul has held the largest market share since 2017,28 an estimated 72.1% as of August 2018.29 The surge in popularity of this particular brand is attributed to its trendy design that is similar in size and appearance to a USB flash drive,29 and its offering of sweet flavors such as “crème brûlée” and “cool mint.”
On April 24, 2018, in view of growing concern about the popularity of Juul products among youth, the FDA requested that the company submit documents regarding its marketing tactics, as well as research on the effects of this marketing on product design and public health impact, and information about adverse experiences and complaints.30 The company was forced to change its marketing to appeal less to youth. Now it offers only 3 flavors: “Virginia tobacco,” “classic tobacco,” and “menthol,” although off-brand pods containing a variety of flavors are still available. And some pods are refillable, so users can essentially vape any substance they want.
Although the Juul device delivers a strong dose of nicotine, it is small and therefore easy to hide from parents and teachers, and widespread use has been reported among youth in middle and high schools. Hoodies, hemp jewelry, and backpacks have been designed to hide the devices and allow for easy, hands-free use. YouTube searches for terms such as “Juul,” “hiding Juul at school,” and “Juul in class,” yield thousands of results.31 A 2017 survey reported that 8% of Americans age 15 to 24 had used Juul in the month prior to the survey.32 “To juul” has become a verb.
Each Juul starter kit contains the rechargeable inhalation device plus 4 flavored pods. In the United States, each Juul pod contains nearly as much nicotine as 1 pack of 20 cigarettes in a concentration of 3% or 5%. (Israel and Europe have forced the company to replace the 5% nicotine pods with 1.7% nicotine pods.33) A starter kit costs $49.99, and additional packs of 4 flavored liquid cartridges or pods cost $15.99.34 Other brands of vape pens cost between $15 and $35, and 10-mL bottles of e-liquid cost approximately $7.
What is ‘dripping’?
Hard-core vapers seeking a more intense experience are taking their vaping devices apart and modifying them for “dripping,” ie, directly dripping vape liquids onto the heated coils for inhalation. In a survey, 1 in 4 high school students using vape devices also used them for dripping, citing desires for a thicker cloud of vapor, more intense flavor, “a stronger throat hit,” curiosity, and other reasons.35 Dripping involves higher temperatures, which leads to higher amounts of nicotine delivered, along with more formaldehyde, acetaldehyde, and acetone (see below).36
BAD THINGS IN E-LIQUID AEROSOL
Studies of vape liquids consistently confirm the presence of toxic substances in the resulting vape aerosol.37–40 Depending on the combination of flavorings and solvents in a given e-liquid, a variety of chemicals can be detected in the aerosol from various vaping devices. Chemicals that may be detected include known irritants of respiratory mucosa, as well as various carcinogens. The list includes:
- Organic volatile compounds such as propylene glycol, glycerin, and toluene
- Aldehydes such as formaldehyde (released when propylene glycol is heated to high temperatures), acetaldehyde, and benzaldehyde
- Acetone and acrolein
- Carcinogenic nitrosamines
- Polycyclic aromatic hydrocarbons
- Particulate matter
- Metals including chromium, cadmium, nickel, and lead; and particles of copper, nickel, and silver have been found in electronic nicotine delivery system aerosol in higher levels than in conventional cigarette smoke.41
The specific chemicals detected can vary greatly between brands, even when the flavoring and nicotine content are equivalent, which frequently results in inconsistent and conflicting study findings. The chemicals detected also vary with the voltage or power used to generate the aerosol. Different flavors may carry varying levels of risk; for example, mint- and menthol-flavored e-cigarettes were shown to expose users to dangerous levels of pulegone, a carcinogenic compound banned as a food additive in 2018.42 The concentrations of some of these chemicals are sufficiently high to be of toxicologic concern; for example, one study reported the presence of benzaldehyde in e-cigarette aerosol at twice the workplace exposure limit.43
Biologic effects
In an in vitro study,44 57% of e-liquids studied were found to be cytotoxic to human pulmonary fibroblasts, lung epithelial cells, and human embryonic stem cells. Fruit-flavored e-liquids in particular caused a significant increase in DNA fragmentation. Cell cultures treated with e-cigarette liquids showed increased oxidative stress, reduced cell proliferation, and increased DNA damage,44 which may have implications for carcinogenic risk.
In another study,45 exposure to e-cigarette aerosol as well as conventional cigarette smoke resulted in suppression of genes related to immune and inflammatory response in respiratory epithelial cells. All genes with decreased expression after exposure to conventional cigarette smoke also showed decreased expression with exposure to e-cigarette smoke, which the study authors suggested could lead to immune suppression at the level of the nasal mucosa. Diacetyl and acetoin, chemicals found in certain flavorings, have been linked to bronchiolitis obliterans, or “popcorn lung.”46
Nicotine is not benign
The nicotine itself in many vaping liquids should also not be underestimated. Nicotine has harmful neurocognitive effects and addictive properties, particularly in the developing brains of adolescents and young adults.47 Nicotine exposure during adolescence negatively affects memory, attention, and emotional regulation,48 as well as executive functioning, reward processing, and learning.49
The brain undergoes major structural remodeling in adolescence, and nicotine acetylcholine receptors regulate neural maturation. Early exposure to nicotine disrupts this process, leading to poor executive functioning, difficulty learning, decreased memory, and issues with reward processing.
Fetal exposure, if nicotine products are used during pregnancy, has also been linked to adverse consequences such as deficits in attention and cognition, behavioral effects, and sudden infant death syndrome.5
Much to learn about toxicity
Partly because vaping devices have been available to US consumers only since 2007, limited evidence is available regarding the long-term effects of exposure to the aerosol from these devices in humans.1 Many of the studies mentioned above were in vitro studies or conducted in mouse models. Differences in device design and the composition of the e-liquid among device brands pose a challenge for developing well-designed studies of the long-term health effects of e-cigarette and vape use. Additionally, devices may have different health impacts when used to vape cannabis or other drugs besides nicotine, which requires further investigation.
E-CIGARETTES AND SMOKING CESSATION
Conventional cigarette smoking is a major public health threat, as tobacco use is responsible for 480,000 deaths annually in the United States.50
And smoking is extremely difficult to quit: as many as 80% of smokers who attempt to quit resume smoking within the first month.51 The chance of successfully quitting improves by over 50% if the individual undergoes nicotine replacement therapy, and it improves even more with counseling.50
There are currently 5 types of FDA-approved nicotine replacement therapy products (gum, patch, lozenge, inhaler, nasal spray) to help with smoking cessation. In addition, 2 non-nicotine prescription drugs (varenicline and bupropion) have been approved for treating tobacco dependence.
Can vaping devices be added to the list of nicotine replacement therapy products? Although some manufacturers try to brand their devices as smoking cessation aids, in one study,52 one-third of e-cigarette users said they had either never used conventional cigarettes or had formerly smoked them.
Bullen et al53 randomized smokers interested in quitting to receive either e-cigarettes, nicotine patches, or placebo (nicotine-free) e-cigarettes and followed them for 6 months. Rates of tobacco cessation were less than predicted for the entire study population, resulting in insufficient power to determine the superiority of any single method, but the study authors concluded that nicotine e-cigarettes were “modestly effective” at helping smokers quit, and that abstinence rates may be similar to those with nicotine patches.53
Hajek et al54 randomized 886 smokers to e-cigarette or nicotine replacement products of their choice. After 1 year, 18% of e-cigarette users had stopped smoking, compared with 9.9% of nicotine replacement product users. However, 80% of the e-cigarette users were still using e-cigarettes after 1 year, while only 9% of nicotine replacement product users were still using nicotine replacement therapy products after 1 year.
While quitting conventional cigarette smoking altogether has widely established health benefits, little is known about the health benefits of transitioning from conventional cigarette smoking to reduced conventional cigarette smoking with concomitant use of e-cigarettes.
Campagna et al55 found no beneficial health effects in smokers who partially substituted conventional cigarettes for e-cigarettes.
Many studies found that smokers use e-cigarettes to maintain their habit instead of quitting entirely.56 It has been suggested that any slight increase in effectiveness in smoking cessation by using e-cigarettes compared with other nicotine replacement products could be linked to satisfying of the habitual smoking actions, such as inhaling and bringing the hand to the mouth,24 which are absent when using other nicotine replacement methods such as a nicotine patch.
As with safety information, long-term outcomes regarding the use of vape devices for smoking cessation have not been yet established, as this option is still relatively new.
VAPING AS A GATEWAY DRUG
Another worrisome trend involving electronic nicotine delivery systems is their marketing and branding, which appear to be aimed directly at adolescents and young adults. Juul and other similar products cannot be sold to anyone under the age of 18 (or 21 in 18 states, including California, Massachusetts, New York, and now Ohio). Despite this, Juul and similar products continue to increase in popularity among middle school and high school students.57
While smoking cessation and health improvement are cited as reasons for vaping among middle-aged and older adults, adolescents and young adults more often cite flavor, enjoyment, peer use, and curiosity as reasons for use.
Adolescents are more likely to report interest in trying a vape product flavored with menthol or fruit than tobacco, and commonly hold the belief that fruit-flavored e-cigarettes are less harmful than tobacco-flavored e-cigarettes.58 Harrell et al59 polled youth and young adults who used flavored e-cigarettes, and 78% said they would no longer use the product if their preferred flavor were not available. In September 2019, Michigan became the first state to ban the sale of flavored e-cigarettes in stores and online. Similar bills have been introduced in California, Massachusetts, and New York.60
Myths and misperceptions abound among youth regarding smoking vs vaping. Young people view regular cigarette smoking negatively, as causing cancer, bad breath, and asthma exacerbations. Meanwhile, they believe marijuana is safer and less addictive than traditional cigarette smoking.61 Youth exposed to e-cigarette advertisements viewed e-cigarettes as healthier, more enjoyable, “cool,” safe, and fun.61 The overall public health impact of increasing initiation of smoking, particularly among youth and young adults, should not be underestimated.
SECONDHAND VAPE AND OTHER EXPOSURE RISKS
Cigarette smoking has been banned in many public places, in view of a large body of scientific evidence about the harmful effects of secondhand smoke. Advocates for allowing vaping in public places say that vaping emissions do not harm bystanders, but evidence is insufficient to support this claim.62 One study showed that passive exposure to e-cigarette aerosol generated increases in serum levels of cotinine (a nicotine metabolite) similar to those with passive exposure to conventional cigarette smoke.5
Accidental nicotine poisoning in children as a result of ingesting e-cigarette liquid is also a major concern,63 particularly with sweet flavors such as bubblegum or cheesecake that may be attractive to children.
Calls to US poison control centers with respect to e-cigarettes and vaping increased from 1 per month in September 2010 to 215 in February 2014, with 51% involving children under age 5.64 This trend resulted in the Child Nicotine Poisoning Prevention Act, which passed in 2015 and went into effect in 2016, requiring packaging that is difficult to open for children under age 5.5
Device malfunctions or battery failures have led to explosions that have resulted in substantial injuries to users, as well as house and car fires.49
HOW DO WE DISCOURAGE ADOLESCENT USE?
There are currently no established treatment approaches for adolescents who have become addicted to vaping. A review of the literature regarding treatment modalities used to address adolescent use of tobacco and marijuana provides insight that options such as nicotine replacement therapy and counseling modalities such as cognitive behavioral therapy may be helpful in treating teen vaping addiction. However, more research is needed to determine the effectiveness of these treatments in youth addicted to vaping.
Given that youth who vape even once are more likely to try other types of tobacco, we recommend that parents and healthcare providers start conversations by asking what the young person has seen or heard about vaping. Young people can also be asked what they think the school’s response should be: Do they think vaping should be banned in public places, as cigarettes have been banned? What about the carbon footprint? What are their thoughts on the plastic waste, batteries, and other toxins generated by the e-cigarette industry?
New US laws ban the sale of e-cigarettes and vaping devices to minors in stores and online. These policies are modeled in many cases on environmental control policies that have been previously employed to reduce tobacco use, particularly by youth. For example, changing laws to mandate sales only to individuals age 21 and older in all states can help to decrease access to these products among middle school and high school students.
As with tobacco cessation, education will not be enough. Support of legislation that bans vaping in public places, increases pricing to discourage adolescent use, and other measures used successfully to decrease conventional cigarette smoking can be deployed to decrease the public health impact of e-cigarettes. We recommend further regulation of specific harmful chemicals and clear, detailed ingredient labeling to increase consumer understanding of the risks associated with these products. Additionally, we recommend eliminating flavored e-cigarettes, which are the most appealing type for young users, and raising prices of e-cigarettes and similar products to discourage use by youth.
If current cigarette smokers want to use e-cigarettes to quit, we recommend that clinicians counsel them to eventually completely stop use of traditional cigarettes and switch to using e-cigarettes, instead of becoming a dual user of both types of products or using e-cigarettes indefinitely. After making that switch, they should then work to gradually taper usage and nicotine addiction by reducing the amount of nicotine in the e-liquid. Clinicians should ask patients about use of e-cigarettes and vaping devices specifically, and should counsel nonsmokers to avoid initiation of use.
EVIDENCE OF HARM CONTINUES TO EMERGE
Data about respiratory effects, secondhand exposure, and long-term smoking cessation efficacy are still limited, and it remains as yet unknown what combinations of solvents, flavorings, and nicotine in a given e-liquid will result in the most harmful or least harmful effects. In addition, while much of the information about the safety of these components has been obtained using in vitro or mouse models, increasing reports of serious respiratory illness and rising numbers of deaths linked to vaping make it clear that these findings likely translate to harmful effects in humans.
E-cigarettes may ultimately prove to be less harmful than traditional cigarettes, but it seems likely that with further time and research, serious health risks of e-cigarette use will continue to emerge.
Electronic cigarettes and other “vaping” devices have been increasing in popularity among youth and adults since their introduction in the US market in 2007.1 This increase is partially driven by a public perception that vaping is harmless, or at least less harmful than cigarette smoking.2 Vaping fans also argue that current smokers can use vaping as nicotine replacement therapy to help them quit smoking.3
We disagree. Research on the health effects of vaping, though still limited, is accumulating rapidly and making it increasingly clear that this habit is far from harmless. For youth, it is a gateway to addiction to nicotine and other substances. Whether it can help people quit smoking remains to be seen. And recent months have seen reports of serious respiratory illnesses and even deaths linked to vaping.4
In December 2016, the US Surgeon General warned that e-cigarette use among youth and young adults in the United States represents a “major public health concern,”5 and that more adolescents and young adults are now vaping than smoking conventional tobacco products.
This article reviews the issue of vaping in the United States, as well as available evidence regarding its safety.
YOUTH AT RISK
Retail sales of e-cigarettes and vaping devices approach an annual $7 billion.6 A 2014–2015 survey found that 2.4% of the general US population were current users of e-cigarettes, and 8.5% had tried them at least once.3
In 2014, for the first time, e-cigarette use became more common among US youth than traditional cigarettes.5
The odds of taking up vaping are higher among minority youth in the United States, particularly Hispanics.9 This trend is particularly worrisome because several longitudinal studies have shown that adolescents who use e-cigarettes are 3 times as likely to eventually become smokers of traditional cigarettes compared with adolescents who do not use e-cigarettes.10–12
If US youth continue smoking at the current rate, 5.6 million of the current population under age 18, or 1 of every 13, will die early of a smoking-related illness.13
RECENT OUTBREAK OF VAPING-ASSOCIATED LUNG INJURY
As of November 5, 2019, there had been 2,051 cases of vaping-associated lung injury in 49 states (all except Alaska), the District of Columbia, and 1 US territory reported to the US Centers for Disease Control and Prevention (CDC), with 39 confirmed deaths.4 The reported cases include respiratory injury including acute eosinophilic pneumonia, organizing pneumonia, acute respiratory distress syndrome, and hypersensitivity pneumonitis.14
Most of these patients had been vaping tetrahydrocannabinol (THC), though many used both nicotine- and THC-containing products, and others used products containing nicotine exclusively.4 Thus, it is difficult to identify the exact substance or substances that may be contributing to this sudden outbreak among vape users, and many different product sources are currently under investigation.
One substance that may be linked to the epidemic is vitamin E acetate, which the New York State Department of Health has detected in high levels in cannabis vaping cartridges used by patients who developed lung injury.15 The US Food and Drug Administration (FDA) is continuing to analyze vape cartridge samples submitted by affected patients to look for other chemicals that can contribute to the development of serious pulmonary illness.
WHAT IS AN E-CIGARETTE? WHAT IS A VAPE PEN?
Vape pens consist of similar elements but are not necessarily similar in appearance to a conventional cigarette, and may look more like a pen or a USB flash drive. In fact, the Juul device is recharged by plugging it into a USB port.
Vaping devices have many street names, including e-cigs, e-hookahs, vape pens, mods, vapes, and tank systems.
The first US patent application for a device resembling a modern e-cigarette was filed in 1963, but the product never made it to the market.16 Instead, the first commercially successful e-cigarette was created in Beijing in 2003 and introduced to US markets in 2007.
Newer-generation devices have larger batteries and can heat the liquid to higher temperatures, releasing more nicotine and forming additional toxicants such as formaldehyde. Devices lack standardization in terms of design, capacity for safely holding e-liquid, packaging of the e-liquid, and features designed to minimize hazards of use.
Not just nicotine
Many devices are designed for use with other drugs, including THC.17 In a 2018 study, 10.9% of college students reported vaping marijuana in the past 30 days, up from 5.2% in 2017.18
Other substances are being vaped as well.19 In theory, any heat-stable psychoactive recreational drug could be aerosolized and vaped. There are increasing reports of e-liquids containing recreational drugs such as synthetic cannabinoid receptor agonists, crack cocaine, LSD, and methamphetamine.17
Freedom, rebellion, glamour
Sales have risen rapidly since 2007 with widespread advertising on television and in print publications for popular brands, often featuring celebrities.20 Spending on advertising for e-cigarettes and vape devices rose from $6.4 million in 2011 to $115 million in 2014—and that was before the advent of Juul (see below).21
Marketing campaigns for vaping devices mimic the themes previously used successfully by the tobacco industry, eg, freedom, rebellion, and glamour. They also make unsubstantiated claims about health benefits and smoking cessation, though initial websites contained endorsements from physicians, similar to the strategies of tobacco companies in old cigarette ads. Cigarette ads have been prohibited since 1971—but not e-cigarette ads. Moreover, vaping products appear as product placements in television shows and movies, with advocacy groups on social media.22
By law, buyers have to be 18 or 21
Vaping devices can be purchased at vape shops, convenience stores, gas stations, and over the Internet; up to 50% of sales are conducted online.24
Fruit flavors are popular
Zhu et al25 estimated that 7,700 unique vaping flavors exist, with fruit and candy flavors predominating. The most popular flavors are tobacco and mint, followed by fruit, dessert and candy flavors, alcoholic flavors (strawberry daiquiri, margarita), and food flavors.25 These flavors have been associated with higher usage in youth, leading to increased risk of nicotine addiction.26
WHAT IS JUUL?
The Juul device (Juul Labs, www.juul.com) was developed in 2015 by 2 Stanford University graduates. Their goal was to produce a more satisfying and cigarette-like vaping experience, specifically by increasing the amount of nicotine delivered while maintaining smooth and pleasant inhalation. They created an e-liquid that could be vaporized effectively at lower temperatures.27
While more than 400 brands of vaping devices are currently available in the United States,3 Juul has held the largest market share since 2017,28 an estimated 72.1% as of August 2018.29 The surge in popularity of this particular brand is attributed to its trendy design that is similar in size and appearance to a USB flash drive,29 and its offering of sweet flavors such as “crème brûlée” and “cool mint.”
On April 24, 2018, in view of growing concern about the popularity of Juul products among youth, the FDA requested that the company submit documents regarding its marketing tactics, as well as research on the effects of this marketing on product design and public health impact, and information about adverse experiences and complaints.30 The company was forced to change its marketing to appeal less to youth. Now it offers only 3 flavors: “Virginia tobacco,” “classic tobacco,” and “menthol,” although off-brand pods containing a variety of flavors are still available. And some pods are refillable, so users can essentially vape any substance they want.
Although the Juul device delivers a strong dose of nicotine, it is small and therefore easy to hide from parents and teachers, and widespread use has been reported among youth in middle and high schools. Hoodies, hemp jewelry, and backpacks have been designed to hide the devices and allow for easy, hands-free use. YouTube searches for terms such as “Juul,” “hiding Juul at school,” and “Juul in class,” yield thousands of results.31 A 2017 survey reported that 8% of Americans age 15 to 24 had used Juul in the month prior to the survey.32 “To juul” has become a verb.
Each Juul starter kit contains the rechargeable inhalation device plus 4 flavored pods. In the United States, each Juul pod contains nearly as much nicotine as 1 pack of 20 cigarettes in a concentration of 3% or 5%. (Israel and Europe have forced the company to replace the 5% nicotine pods with 1.7% nicotine pods.33) A starter kit costs $49.99, and additional packs of 4 flavored liquid cartridges or pods cost $15.99.34 Other brands of vape pens cost between $15 and $35, and 10-mL bottles of e-liquid cost approximately $7.
What is ‘dripping’?
Hard-core vapers seeking a more intense experience are taking their vaping devices apart and modifying them for “dripping,” ie, directly dripping vape liquids onto the heated coils for inhalation. In a survey, 1 in 4 high school students using vape devices also used them for dripping, citing desires for a thicker cloud of vapor, more intense flavor, “a stronger throat hit,” curiosity, and other reasons.35 Dripping involves higher temperatures, which leads to higher amounts of nicotine delivered, along with more formaldehyde, acetaldehyde, and acetone (see below).36
BAD THINGS IN E-LIQUID AEROSOL
Studies of vape liquids consistently confirm the presence of toxic substances in the resulting vape aerosol.37–40 Depending on the combination of flavorings and solvents in a given e-liquid, a variety of chemicals can be detected in the aerosol from various vaping devices. Chemicals that may be detected include known irritants of respiratory mucosa, as well as various carcinogens. The list includes:
- Organic volatile compounds such as propylene glycol, glycerin, and toluene
- Aldehydes such as formaldehyde (released when propylene glycol is heated to high temperatures), acetaldehyde, and benzaldehyde
- Acetone and acrolein
- Carcinogenic nitrosamines
- Polycyclic aromatic hydrocarbons
- Particulate matter
- Metals including chromium, cadmium, nickel, and lead; and particles of copper, nickel, and silver have been found in electronic nicotine delivery system aerosol in higher levels than in conventional cigarette smoke.41
The specific chemicals detected can vary greatly between brands, even when the flavoring and nicotine content are equivalent, which frequently results in inconsistent and conflicting study findings. The chemicals detected also vary with the voltage or power used to generate the aerosol. Different flavors may carry varying levels of risk; for example, mint- and menthol-flavored e-cigarettes were shown to expose users to dangerous levels of pulegone, a carcinogenic compound banned as a food additive in 2018.42 The concentrations of some of these chemicals are sufficiently high to be of toxicologic concern; for example, one study reported the presence of benzaldehyde in e-cigarette aerosol at twice the workplace exposure limit.43
Biologic effects
In an in vitro study,44 57% of e-liquids studied were found to be cytotoxic to human pulmonary fibroblasts, lung epithelial cells, and human embryonic stem cells. Fruit-flavored e-liquids in particular caused a significant increase in DNA fragmentation. Cell cultures treated with e-cigarette liquids showed increased oxidative stress, reduced cell proliferation, and increased DNA damage,44 which may have implications for carcinogenic risk.
In another study,45 exposure to e-cigarette aerosol as well as conventional cigarette smoke resulted in suppression of genes related to immune and inflammatory response in respiratory epithelial cells. All genes with decreased expression after exposure to conventional cigarette smoke also showed decreased expression with exposure to e-cigarette smoke, which the study authors suggested could lead to immune suppression at the level of the nasal mucosa. Diacetyl and acetoin, chemicals found in certain flavorings, have been linked to bronchiolitis obliterans, or “popcorn lung.”46
Nicotine is not benign
The nicotine itself in many vaping liquids should also not be underestimated. Nicotine has harmful neurocognitive effects and addictive properties, particularly in the developing brains of adolescents and young adults.47 Nicotine exposure during adolescence negatively affects memory, attention, and emotional regulation,48 as well as executive functioning, reward processing, and learning.49
The brain undergoes major structural remodeling in adolescence, and nicotine acetylcholine receptors regulate neural maturation. Early exposure to nicotine disrupts this process, leading to poor executive functioning, difficulty learning, decreased memory, and issues with reward processing.
Fetal exposure, if nicotine products are used during pregnancy, has also been linked to adverse consequences such as deficits in attention and cognition, behavioral effects, and sudden infant death syndrome.5
Much to learn about toxicity
Partly because vaping devices have been available to US consumers only since 2007, limited evidence is available regarding the long-term effects of exposure to the aerosol from these devices in humans.1 Many of the studies mentioned above were in vitro studies or conducted in mouse models. Differences in device design and the composition of the e-liquid among device brands pose a challenge for developing well-designed studies of the long-term health effects of e-cigarette and vape use. Additionally, devices may have different health impacts when used to vape cannabis or other drugs besides nicotine, which requires further investigation.
E-CIGARETTES AND SMOKING CESSATION
Conventional cigarette smoking is a major public health threat, as tobacco use is responsible for 480,000 deaths annually in the United States.50
And smoking is extremely difficult to quit: as many as 80% of smokers who attempt to quit resume smoking within the first month.51 The chance of successfully quitting improves by over 50% if the individual undergoes nicotine replacement therapy, and it improves even more with counseling.50
There are currently 5 types of FDA-approved nicotine replacement therapy products (gum, patch, lozenge, inhaler, nasal spray) to help with smoking cessation. In addition, 2 non-nicotine prescription drugs (varenicline and bupropion) have been approved for treating tobacco dependence.
Can vaping devices be added to the list of nicotine replacement therapy products? Although some manufacturers try to brand their devices as smoking cessation aids, in one study,52 one-third of e-cigarette users said they had either never used conventional cigarettes or had formerly smoked them.
Bullen et al53 randomized smokers interested in quitting to receive either e-cigarettes, nicotine patches, or placebo (nicotine-free) e-cigarettes and followed them for 6 months. Rates of tobacco cessation were less than predicted for the entire study population, resulting in insufficient power to determine the superiority of any single method, but the study authors concluded that nicotine e-cigarettes were “modestly effective” at helping smokers quit, and that abstinence rates may be similar to those with nicotine patches.53
Hajek et al54 randomized 886 smokers to e-cigarette or nicotine replacement products of their choice. After 1 year, 18% of e-cigarette users had stopped smoking, compared with 9.9% of nicotine replacement product users. However, 80% of the e-cigarette users were still using e-cigarettes after 1 year, while only 9% of nicotine replacement product users were still using nicotine replacement therapy products after 1 year.
While quitting conventional cigarette smoking altogether has widely established health benefits, little is known about the health benefits of transitioning from conventional cigarette smoking to reduced conventional cigarette smoking with concomitant use of e-cigarettes.
Campagna et al55 found no beneficial health effects in smokers who partially substituted conventional cigarettes for e-cigarettes.
Many studies found that smokers use e-cigarettes to maintain their habit instead of quitting entirely.56 It has been suggested that any slight increase in effectiveness in smoking cessation by using e-cigarettes compared with other nicotine replacement products could be linked to satisfying of the habitual smoking actions, such as inhaling and bringing the hand to the mouth,24 which are absent when using other nicotine replacement methods such as a nicotine patch.
As with safety information, long-term outcomes regarding the use of vape devices for smoking cessation have not been yet established, as this option is still relatively new.
VAPING AS A GATEWAY DRUG
Another worrisome trend involving electronic nicotine delivery systems is their marketing and branding, which appear to be aimed directly at adolescents and young adults. Juul and other similar products cannot be sold to anyone under the age of 18 (or 21 in 18 states, including California, Massachusetts, New York, and now Ohio). Despite this, Juul and similar products continue to increase in popularity among middle school and high school students.57
While smoking cessation and health improvement are cited as reasons for vaping among middle-aged and older adults, adolescents and young adults more often cite flavor, enjoyment, peer use, and curiosity as reasons for use.
Adolescents are more likely to report interest in trying a vape product flavored with menthol or fruit than tobacco, and commonly hold the belief that fruit-flavored e-cigarettes are less harmful than tobacco-flavored e-cigarettes.58 Harrell et al59 polled youth and young adults who used flavored e-cigarettes, and 78% said they would no longer use the product if their preferred flavor were not available. In September 2019, Michigan became the first state to ban the sale of flavored e-cigarettes in stores and online. Similar bills have been introduced in California, Massachusetts, and New York.60
Myths and misperceptions abound among youth regarding smoking vs vaping. Young people view regular cigarette smoking negatively, as causing cancer, bad breath, and asthma exacerbations. Meanwhile, they believe marijuana is safer and less addictive than traditional cigarette smoking.61 Youth exposed to e-cigarette advertisements viewed e-cigarettes as healthier, more enjoyable, “cool,” safe, and fun.61 The overall public health impact of increasing initiation of smoking, particularly among youth and young adults, should not be underestimated.
SECONDHAND VAPE AND OTHER EXPOSURE RISKS
Cigarette smoking has been banned in many public places, in view of a large body of scientific evidence about the harmful effects of secondhand smoke. Advocates for allowing vaping in public places say that vaping emissions do not harm bystanders, but evidence is insufficient to support this claim.62 One study showed that passive exposure to e-cigarette aerosol generated increases in serum levels of cotinine (a nicotine metabolite) similar to those with passive exposure to conventional cigarette smoke.5
Accidental nicotine poisoning in children as a result of ingesting e-cigarette liquid is also a major concern,63 particularly with sweet flavors such as bubblegum or cheesecake that may be attractive to children.
Calls to US poison control centers with respect to e-cigarettes and vaping increased from 1 per month in September 2010 to 215 in February 2014, with 51% involving children under age 5.64 This trend resulted in the Child Nicotine Poisoning Prevention Act, which passed in 2015 and went into effect in 2016, requiring packaging that is difficult to open for children under age 5.5
Device malfunctions or battery failures have led to explosions that have resulted in substantial injuries to users, as well as house and car fires.49
HOW DO WE DISCOURAGE ADOLESCENT USE?
There are currently no established treatment approaches for adolescents who have become addicted to vaping. A review of the literature regarding treatment modalities used to address adolescent use of tobacco and marijuana provides insight that options such as nicotine replacement therapy and counseling modalities such as cognitive behavioral therapy may be helpful in treating teen vaping addiction. However, more research is needed to determine the effectiveness of these treatments in youth addicted to vaping.
Given that youth who vape even once are more likely to try other types of tobacco, we recommend that parents and healthcare providers start conversations by asking what the young person has seen or heard about vaping. Young people can also be asked what they think the school’s response should be: Do they think vaping should be banned in public places, as cigarettes have been banned? What about the carbon footprint? What are their thoughts on the plastic waste, batteries, and other toxins generated by the e-cigarette industry?
New US laws ban the sale of e-cigarettes and vaping devices to minors in stores and online. These policies are modeled in many cases on environmental control policies that have been previously employed to reduce tobacco use, particularly by youth. For example, changing laws to mandate sales only to individuals age 21 and older in all states can help to decrease access to these products among middle school and high school students.
As with tobacco cessation, education will not be enough. Support of legislation that bans vaping in public places, increases pricing to discourage adolescent use, and other measures used successfully to decrease conventional cigarette smoking can be deployed to decrease the public health impact of e-cigarettes. We recommend further regulation of specific harmful chemicals and clear, detailed ingredient labeling to increase consumer understanding of the risks associated with these products. Additionally, we recommend eliminating flavored e-cigarettes, which are the most appealing type for young users, and raising prices of e-cigarettes and similar products to discourage use by youth.
If current cigarette smokers want to use e-cigarettes to quit, we recommend that clinicians counsel them to eventually completely stop use of traditional cigarettes and switch to using e-cigarettes, instead of becoming a dual user of both types of products or using e-cigarettes indefinitely. After making that switch, they should then work to gradually taper usage and nicotine addiction by reducing the amount of nicotine in the e-liquid. Clinicians should ask patients about use of e-cigarettes and vaping devices specifically, and should counsel nonsmokers to avoid initiation of use.
EVIDENCE OF HARM CONTINUES TO EMERGE
Data about respiratory effects, secondhand exposure, and long-term smoking cessation efficacy are still limited, and it remains as yet unknown what combinations of solvents, flavorings, and nicotine in a given e-liquid will result in the most harmful or least harmful effects. In addition, while much of the information about the safety of these components has been obtained using in vitro or mouse models, increasing reports of serious respiratory illness and rising numbers of deaths linked to vaping make it clear that these findings likely translate to harmful effects in humans.
E-cigarettes may ultimately prove to be less harmful than traditional cigarettes, but it seems likely that with further time and research, serious health risks of e-cigarette use will continue to emerge.
- Sood A, Kesic M, Hernandez M. Electronic cigarettes: one size does not fit all. J Allergy Clin Immunol 2018; 141(6):1973-1982. doi:10.1016/j.jaci.2018.02.029
- Romijnders K, van Osch L, de Vries H, Talhout R. Perceptions and reasons regarding e-cigarette use among users and non-users: a narrative literature review. Int J Environ Res Public Health 2018; 15(6):1190. doi:10.3390/ijerph15061190
- Zhu S, Zhuang Y-L, Wong S, Cummins SE, Tedeschi GJ. E-cigarette use and associated changes in population smoking cessation: evidence from US current population surveys. BMJ 2017; 358:j3262. doi:10.1136/bmj.j3262
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with e-cigarette use, or vaping. Updated November 5, 2019. www.cdc.gov/tobacco/basic_information/e-Cigarettes/severe-Lung-Disease.html. Accessed November 14, 2019.
- US Public Health Services, US Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Rockville, MD, U.S. Department of Health and Human Services, 2016. https://e-cigarettes.surgeongeneral.gov/documents/2016_sgr_full_report_non-508.pdf. Accessed November 14, 2019.
- Thomas K, Kaplan S. E-cigarettes went unchecked in 10 years of federal inaction. New York Times Oct 14, 2019; updated November 1, 2019. www.nytimes.com/2019/10/14/health/vaping-e-cigarettes-fda.html.
- Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2018; 67(45):1276–1277. doi:10.15585/mmwr.mm6745a5
- Gentzke A, Creamer M, Cullen K, et al; Centers for Disease Control and Prevention. Vital signs: tobacco product use among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2019; 68(6):157–164. doi:10.15585/mmwr.mm6806e1
- Hammig B, Daniel-Dobbs P, Blunt-Vinti H. Electronic cigarette initiation among minority youth in the United States. Am J Drug Alcohol Abuse 2017; 43(3):306–310. doi:10.1080/00952990.2016.1203926
- Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD. Progression to traditional cigarette smoking after electronic cigarette use among U.S. adolescents and young adults. JAMA Pediatr 2015; 169(11):1018–1023. doi:10.1001/jamapediatrics.2015.1742
- Leventhal AM, Strong DR, Kirkpatrick MG, et al. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA 2015; 314(7):700–707. doi:10.1001/jama.2015.8950
- Wills TA, Knight R, Sargent JD, Gibbons FX, Pagano I, Williams RJ. Longitudinal study of e-cigarette use and onset of cigarette smoking among high school students in Hawaii. Tob Control 2016; 26(1):34–39. doi:10.1136/tobaccocontrol-2015-052705
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention, 2014. www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf. Accessed November 14, 2019.
- Christiani DC. Vaping-induced lung injury. N Engl J Med 2019; Sept 6. Epub ahead of print. doi:10.1056/NEJMe1912032
- Neel J, Aubrey A. Vitamin E suspected in serious lung problems among people who vaped cannabis. NPR Sept 5, 2019. www.npr.org/sections/health-shots/2019/09/05/758005409/vitamin-e-suspected-in-serious-lung-problems-among-people-who-vaped-cannabis. Accessed November 14, 2019.
- White A. Plans for the first e-cigarette went up in smoke 50 years ago. Smithsonian Magazine December 2018. www.smithsonianmag.com/innovation/plans-for-first-e-cigarette-went-up-in-smoke-50-years-ago-180970730.
- Blundell MS, Dargan PI, Wood DM. The dark cloud of recreational drugs and vaping. QJM 2018; 111(3):145–148. doi:10.1093/qjmed/hcx049
- Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, Patrick ME. Monitoring the future: national survey results on drug use, 1975–2018. 2018 Volume 2. College students & adults ages 19–60. www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf. Accessed November 14, 2019.
- Eggers ME, Lee YO, Jackson J, Wiley JL, Porter J, Nonnemaker JM. Youth use of electronic vapor products and blunts for administering cannabis. Addict Behav 2017; 70:79-82. doi:10.1016/j.addbeh.2017.02.020
- Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the “e-cigarette”in the USA. Tob Control 2013; 22(1):19–23. doi:10.1136/tobaccocontrol-2011-050044
- Centers for Disease Control and Prevention. E-cigarette ads and youth. www.cdc.gov/vitalsigns/ecigarette-ads/index.html.
- Noel JK, Rees VW, Connolly GN. Electronic cigarettes: a new “tobacco” industry? Tob Control 2011; 20(1):81. doi:10.1136/tc.2010.038562
- US Food and Drug Administration. Deeming tobacco products to be subject to the federal food, drug, and cosmetic act, as amended by the family smoking prevention and tobacco control act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Federal Register 2016; 81(90), May 10, 2016. www.govinfo.gov/content/pkg/FR-2016-05-10/pdf/2016-10685.pdf. Accessed November 14, 2019.
- Rom O, Pecorelli A, Valacchi G, Reznick AZ. Are e-cigarettes a safe and good alternative to cigarette smoking? Ann NY Acad Sci 2015; 1340:65–74. doi:10.1111/nyas.12609
- Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 2014; 23(suppl 3):iii3-iii9. doi:10.1136/tobaccocontrol-2014-051670
- Kong G, Morean ME, Cavallo DA, Camenga DR, Krishnan-Sarin S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob Res 2015; 17(7):847–854. doi:10.1093/ntr/ntu257
- Baca MC. How two Stanford grads aimed for big tech glory and got big tobacco instead. Updated September 4, 2019. The Washington Post September 4, 2019. www.washingtonpost.com/technology/2019/09/04/how-two-stanford-grads-aimed-big-tech-glory-got-big-tobacco-instead. Accessed November 14, 2019.
- Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control 2019; 28(2):146–151. doi:10.1136/tobaccocontrol-2018-054382
- Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics 2019; 143(6):pii:e20182741. doi:10.1542/peds.2018-2741
- Zernike K. F.D.A. cracks down on “juuling” among teenagers. The New York Times April 24, 2018. www.nytimes.com/2018/04/24/health/fda-e-cigarettes-minors-juul.html. Accessed November 14, 2019.
- Ramamurthi D, Chau C, Jackler RK. JUUL and other stealth vaporisers: hiding the habit from parents and teachers. Tob Control 2018 Sep 15; pii:tobaccocontrol-2018-054455. doi:10.1136/tobaccocontrol-2018-054455. [Epub ahead of print]
- Willett JG, Bennett M, Hair EC, et al. Recognition, use and perceptions of JUUL among youth and young adults. Tob Control 2019; 28(1):115–116. doi:10.1136/tobaccocontrol-2018-054273
- Kaplan S. Juul’s new product: less nicotine, more intense vapor. New York Times Nov 27, 2018. www.nytimes.com/2018/11/27/health/juul-ecigarettes-nicotine.html.
- JUUL Labs. JUULpods. www.juul.com/shop/pods. Accessed November 14, 2019.
- Krishnan-Sarin S, Morean M, Kong G, et al. E-cigarettes and “dripping” among high-school youth. Pediatrics 2017; 139(3):pii:e20163224. doi:10.1542/peds.2016-3224
- Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014; 16(10):1319–1326. doi:10.1093/ntr/ntu078
- Rawlinson C, Martin S, Frosina J, Wright C. Chemical characterisation of aerosols emitted by electronic cigarettes using thermal desorption-gas chromatography-time of flight mass spectrometry. J Chromatogr A 2017; 1497:144–154. doi:10.1016/j.chroma.2017.02.050
- Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ Health 2017; 16(1):42. doi:10.1186/s12940-017-0249-x
- Williams M, Bozhilov K, Ghai S, Talbot P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One 2017; 12(4):e0175430. doi:10.1371/journal.pone.0175430.
- Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014; 23(2):133–139. doi:10.1136/tobaccocontrol-2012-050859
- Drope J, Cahn Z, Kennedy R, et al. Key issues surrounding the health impacts of electronic nicotine delivery systems (ENDS) and other sources of nicotine. CA Cancer J Clin 2017; 67(6):449–471. doi:10.3322/caac.21413
- Jabba SV, Jordt SE. Risk analysis for the carcinogen pulegone in mint- and menthol-flavored e-cigarettes and smokeless tobacco products. JAMA Intern Med 2019 Sep 16 [Epub ahead of print]. doi:10.1001/jamainternmed.2019.3649
- Tierney PA, Karpinsky CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control 2016; 25(e1):e10–e15. doi:10.1136/tobaccocontrol-2014-052175
- Behar RZ, Wang Y, Talbot P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob Control 2017; 27(3):325–333. doi:10.1136/tobaccocontrol-2016-053472
- Martin EM, Clapp PW, Rebuli ME, et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol 2016; 311(1):L135–L144. doi:10.1152/ajplung.00170.2016
- Holden VK, Hines SE. Update on flavoring-induced lung disease. Curr Opin Pulm Med 2016;22(2):158–164. doi:10.1097/MCP.0000000000000250
- Siqueira L; Committee on Substance Use and Prevention. Nicotine and tobacco as substances of abuse in children and adolescents. Pediatrics 2017; 139(1):pii:e20163436. doi:10.1542/peds.2016-3436
- England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med 2015; 49(2):286–293. doi:10.1016/j.amepre.2015.01.015
- Modesto-Lowe V, Alvarado C. E-cigs…are they cool? Talking to teens about e-cigarettes. Clin Pediatr (Phila) 2017; 51(10):947–952. doi:10.1177/0009922817705188
- Prochaska JJ, Benowitz NL. The past, present, and future of nicotine addiction therapy. Annu Rev Med 2017; 67:467–486. doi:10.1146/annurev-med-111314-033712
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004; 99(1):29–38. doi:10.1111/j.1360-0443.2004.00540.x
- McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res 2015;17(10):119_1202. doi:10.1093/ntr/ntu213
- Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013; 382(9905):1629–1637. doi:10.1016/S0140-6736(13)61842-5
- Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine replacement therapy. N Engl J Med 2019; 380(7):629–637. doi:10.1056/NEJMoa1808779
- Campagna D, Cibella F, Caponnetto P, et al. Changes in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettes. Eur J Clin Invest 2016; 46(8):698–706. doi:10.1111/eci.12651
- Rehan HS, Maini J, Hungin APS. Vaping versus smoking: a quest for efficacy and safety of e-cigarette. Curr Drug Saf 2018; 13(2):92–101. doi:10.2174/1574886313666180227110556
- Zernike K. ‘I can’t stop’: schools struggle with vaping explosion. New York Times April 2, 2018. www.nytimes.com/2018/04/02/health/vaping-ecigarettes-addiction-teen.html.
- Pepper JK, Ribisl KM, Brewer NT. Adolescents’ interest in trying flavoured e-cigarettes. Tob Control 2016; 25(suppl 2):ii62–ii66. doi:10.1136/tobaccocontrol-2016-053174
- Harrell MB, Loukas A, Jackson CD, Marti CN, Perry CL. Flavored tobacco product use among youth and young adults: what if flavors didn’t exist? Tob Regul Sci 2017; 3(2):168–173. doi:10.18001/TRS.3.2.4
- Smith M. Amid vaping crackdown, Michigan to ban sale of flavored e-cigarettes. New York Times Sept 4, 2019. www.nytimes.com/2019/09/04/us/michigan-vaping.html?module=inline.
- Roditis ML, Halpern-Felsher B. Adolescents’ perceptions of risks and benefits of conventional cigarettes, e-cigarettes, and marijuana: a qualitative analysis. J Adolesc Health 2015; 57(2):179–185. doi:10.1016/j.jadohealth.2015.04.002
- Chapman S, Daube M, Maziak W. Should e-cigarette use be permitted in smoke-free public places? No. Tob Control 2017; 26(e1):e3–e4. doi:10.1136/tobaccocontrol-2016-053359
- Marcham CL, Springston JP. Electronic cigarettes in the indoor environment. Rev Env Health 2019; 34(2):105–124. doi:10.1515/reveh-2019-0012
- Chatham-Stephens K, Law R, Taylor E, et al; Centers for Disease Control and Prevention. Notes from the field: calls to poison centers for exposures to electronic cigarettes—United States, September 2010–September 2014. MMWR Morb Mortal Wkly Report 2014; 63(13):292–293. pmid:24699766
- Sood A, Kesic M, Hernandez M. Electronic cigarettes: one size does not fit all. J Allergy Clin Immunol 2018; 141(6):1973-1982. doi:10.1016/j.jaci.2018.02.029
- Romijnders K, van Osch L, de Vries H, Talhout R. Perceptions and reasons regarding e-cigarette use among users and non-users: a narrative literature review. Int J Environ Res Public Health 2018; 15(6):1190. doi:10.3390/ijerph15061190
- Zhu S, Zhuang Y-L, Wong S, Cummins SE, Tedeschi GJ. E-cigarette use and associated changes in population smoking cessation: evidence from US current population surveys. BMJ 2017; 358:j3262. doi:10.1136/bmj.j3262
- Centers for Disease Control and Prevention. Outbreak of lung injury associated with e-cigarette use, or vaping. Updated November 5, 2019. www.cdc.gov/tobacco/basic_information/e-Cigarettes/severe-Lung-Disease.html. Accessed November 14, 2019.
- US Public Health Services, US Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Rockville, MD, U.S. Department of Health and Human Services, 2016. https://e-cigarettes.surgeongeneral.gov/documents/2016_sgr_full_report_non-508.pdf. Accessed November 14, 2019.
- Thomas K, Kaplan S. E-cigarettes went unchecked in 10 years of federal inaction. New York Times Oct 14, 2019; updated November 1, 2019. www.nytimes.com/2019/10/14/health/vaping-e-cigarettes-fda.html.
- Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2018; 67(45):1276–1277. doi:10.15585/mmwr.mm6745a5
- Gentzke A, Creamer M, Cullen K, et al; Centers for Disease Control and Prevention. Vital signs: tobacco product use among middle and high school students—United States, 2011–2018. MMWR Morb Mortal Wkly Rep 2019; 68(6):157–164. doi:10.15585/mmwr.mm6806e1
- Hammig B, Daniel-Dobbs P, Blunt-Vinti H. Electronic cigarette initiation among minority youth in the United States. Am J Drug Alcohol Abuse 2017; 43(3):306–310. doi:10.1080/00952990.2016.1203926
- Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD. Progression to traditional cigarette smoking after electronic cigarette use among U.S. adolescents and young adults. JAMA Pediatr 2015; 169(11):1018–1023. doi:10.1001/jamapediatrics.2015.1742
- Leventhal AM, Strong DR, Kirkpatrick MG, et al. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. JAMA 2015; 314(7):700–707. doi:10.1001/jama.2015.8950
- Wills TA, Knight R, Sargent JD, Gibbons FX, Pagano I, Williams RJ. Longitudinal study of e-cigarette use and onset of cigarette smoking among high school students in Hawaii. Tob Control 2016; 26(1):34–39. doi:10.1136/tobaccocontrol-2015-052705
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention, 2014. www.ncbi.nlm.nih.gov/books/NBK179276/pdf/Bookshelf_NBK179276.pdf. Accessed November 14, 2019.
- Christiani DC. Vaping-induced lung injury. N Engl J Med 2019; Sept 6. Epub ahead of print. doi:10.1056/NEJMe1912032
- Neel J, Aubrey A. Vitamin E suspected in serious lung problems among people who vaped cannabis. NPR Sept 5, 2019. www.npr.org/sections/health-shots/2019/09/05/758005409/vitamin-e-suspected-in-serious-lung-problems-among-people-who-vaped-cannabis. Accessed November 14, 2019.
- White A. Plans for the first e-cigarette went up in smoke 50 years ago. Smithsonian Magazine December 2018. www.smithsonianmag.com/innovation/plans-for-first-e-cigarette-went-up-in-smoke-50-years-ago-180970730.
- Blundell MS, Dargan PI, Wood DM. The dark cloud of recreational drugs and vaping. QJM 2018; 111(3):145–148. doi:10.1093/qjmed/hcx049
- Schulenberg JE, Johnston LD, O’Malley PM, Bachman JG, Miech RA, Patrick ME. Monitoring the future: national survey results on drug use, 1975–2018. 2018 Volume 2. College students & adults ages 19–60. www.monitoringthefuture.org/pubs/monographs/mtf-vol2_2018.pdf. Accessed November 14, 2019.
- Eggers ME, Lee YO, Jackson J, Wiley JL, Porter J, Nonnemaker JM. Youth use of electronic vapor products and blunts for administering cannabis. Addict Behav 2017; 70:79-82. doi:10.1016/j.addbeh.2017.02.020
- Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the “e-cigarette”in the USA. Tob Control 2013; 22(1):19–23. doi:10.1136/tobaccocontrol-2011-050044
- Centers for Disease Control and Prevention. E-cigarette ads and youth. www.cdc.gov/vitalsigns/ecigarette-ads/index.html.
- Noel JK, Rees VW, Connolly GN. Electronic cigarettes: a new “tobacco” industry? Tob Control 2011; 20(1):81. doi:10.1136/tc.2010.038562
- US Food and Drug Administration. Deeming tobacco products to be subject to the federal food, drug, and cosmetic act, as amended by the family smoking prevention and tobacco control act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Federal Register 2016; 81(90), May 10, 2016. www.govinfo.gov/content/pkg/FR-2016-05-10/pdf/2016-10685.pdf. Accessed November 14, 2019.
- Rom O, Pecorelli A, Valacchi G, Reznick AZ. Are e-cigarettes a safe and good alternative to cigarette smoking? Ann NY Acad Sci 2015; 1340:65–74. doi:10.1111/nyas.12609
- Zhu SH, Sun JY, Bonnevie E, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control 2014; 23(suppl 3):iii3-iii9. doi:10.1136/tobaccocontrol-2014-051670
- Kong G, Morean ME, Cavallo DA, Camenga DR, Krishnan-Sarin S. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob Res 2015; 17(7):847–854. doi:10.1093/ntr/ntu257
- Baca MC. How two Stanford grads aimed for big tech glory and got big tobacco instead. Updated September 4, 2019. The Washington Post September 4, 2019. www.washingtonpost.com/technology/2019/09/04/how-two-stanford-grads-aimed-big-tech-glory-got-big-tobacco-instead. Accessed November 14, 2019.
- Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control 2019; 28(2):146–151. doi:10.1136/tobaccocontrol-2018-054382
- Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics 2019; 143(6):pii:e20182741. doi:10.1542/peds.2018-2741
- Zernike K. F.D.A. cracks down on “juuling” among teenagers. The New York Times April 24, 2018. www.nytimes.com/2018/04/24/health/fda-e-cigarettes-minors-juul.html. Accessed November 14, 2019.
- Ramamurthi D, Chau C, Jackler RK. JUUL and other stealth vaporisers: hiding the habit from parents and teachers. Tob Control 2018 Sep 15; pii:tobaccocontrol-2018-054455. doi:10.1136/tobaccocontrol-2018-054455. [Epub ahead of print]
- Willett JG, Bennett M, Hair EC, et al. Recognition, use and perceptions of JUUL among youth and young adults. Tob Control 2019; 28(1):115–116. doi:10.1136/tobaccocontrol-2018-054273
- Kaplan S. Juul’s new product: less nicotine, more intense vapor. New York Times Nov 27, 2018. www.nytimes.com/2018/11/27/health/juul-ecigarettes-nicotine.html.
- JUUL Labs. JUULpods. www.juul.com/shop/pods. Accessed November 14, 2019.
- Krishnan-Sarin S, Morean M, Kong G, et al. E-cigarettes and “dripping” among high-school youth. Pediatrics 2017; 139(3):pii:e20163224. doi:10.1542/peds.2016-3224
- Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014; 16(10):1319–1326. doi:10.1093/ntr/ntu078
- Rawlinson C, Martin S, Frosina J, Wright C. Chemical characterisation of aerosols emitted by electronic cigarettes using thermal desorption-gas chromatography-time of flight mass spectrometry. J Chromatogr A 2017; 1497:144–154. doi:10.1016/j.chroma.2017.02.050
- Lee MS, LeBouf RF, Son YS, Koutrakis P, Christiani DC. Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ Health 2017; 16(1):42. doi:10.1186/s12940-017-0249-x
- Williams M, Bozhilov K, Ghai S, Talbot P. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS One 2017; 12(4):e0175430. doi:10.1371/journal.pone.0175430.
- Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014; 23(2):133–139. doi:10.1136/tobaccocontrol-2012-050859
- Drope J, Cahn Z, Kennedy R, et al. Key issues surrounding the health impacts of electronic nicotine delivery systems (ENDS) and other sources of nicotine. CA Cancer J Clin 2017; 67(6):449–471. doi:10.3322/caac.21413
- Jabba SV, Jordt SE. Risk analysis for the carcinogen pulegone in mint- and menthol-flavored e-cigarettes and smokeless tobacco products. JAMA Intern Med 2019 Sep 16 [Epub ahead of print]. doi:10.1001/jamainternmed.2019.3649
- Tierney PA, Karpinsky CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control 2016; 25(e1):e10–e15. doi:10.1136/tobaccocontrol-2014-052175
- Behar RZ, Wang Y, Talbot P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob Control 2017; 27(3):325–333. doi:10.1136/tobaccocontrol-2016-053472
- Martin EM, Clapp PW, Rebuli ME, et al. E-cigarette use results in suppression of immune and inflammatory-response genes in nasal epithelial cells similar to cigarette smoke. Am J Physiol Lung Cell Mol Physiol 2016; 311(1):L135–L144. doi:10.1152/ajplung.00170.2016
- Holden VK, Hines SE. Update on flavoring-induced lung disease. Curr Opin Pulm Med 2016;22(2):158–164. doi:10.1097/MCP.0000000000000250
- Siqueira L; Committee on Substance Use and Prevention. Nicotine and tobacco as substances of abuse in children and adolescents. Pediatrics 2017; 139(1):pii:e20163436. doi:10.1542/peds.2016-3436
- England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med 2015; 49(2):286–293. doi:10.1016/j.amepre.2015.01.015
- Modesto-Lowe V, Alvarado C. E-cigs…are they cool? Talking to teens about e-cigarettes. Clin Pediatr (Phila) 2017; 51(10):947–952. doi:10.1177/0009922817705188
- Prochaska JJ, Benowitz NL. The past, present, and future of nicotine addiction therapy. Annu Rev Med 2017; 67:467–486. doi:10.1146/annurev-med-111314-033712
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004; 99(1):29–38. doi:10.1111/j.1360-0443.2004.00540.x
- McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res 2015;17(10):119_1202. doi:10.1093/ntr/ntu213
- Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013; 382(9905):1629–1637. doi:10.1016/S0140-6736(13)61842-5
- Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine replacement therapy. N Engl J Med 2019; 380(7):629–637. doi:10.1056/NEJMoa1808779
- Campagna D, Cibella F, Caponnetto P, et al. Changes in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettes. Eur J Clin Invest 2016; 46(8):698–706. doi:10.1111/eci.12651
- Rehan HS, Maini J, Hungin APS. Vaping versus smoking: a quest for efficacy and safety of e-cigarette. Curr Drug Saf 2018; 13(2):92–101. doi:10.2174/1574886313666180227110556
- Zernike K. ‘I can’t stop’: schools struggle with vaping explosion. New York Times April 2, 2018. www.nytimes.com/2018/04/02/health/vaping-ecigarettes-addiction-teen.html.
- Pepper JK, Ribisl KM, Brewer NT. Adolescents’ interest in trying flavoured e-cigarettes. Tob Control 2016; 25(suppl 2):ii62–ii66. doi:10.1136/tobaccocontrol-2016-053174
- Harrell MB, Loukas A, Jackson CD, Marti CN, Perry CL. Flavored tobacco product use among youth and young adults: what if flavors didn’t exist? Tob Regul Sci 2017; 3(2):168–173. doi:10.18001/TRS.3.2.4
- Smith M. Amid vaping crackdown, Michigan to ban sale of flavored e-cigarettes. New York Times Sept 4, 2019. www.nytimes.com/2019/09/04/us/michigan-vaping.html?module=inline.
- Roditis ML, Halpern-Felsher B. Adolescents’ perceptions of risks and benefits of conventional cigarettes, e-cigarettes, and marijuana: a qualitative analysis. J Adolesc Health 2015; 57(2):179–185. doi:10.1016/j.jadohealth.2015.04.002
- Chapman S, Daube M, Maziak W. Should e-cigarette use be permitted in smoke-free public places? No. Tob Control 2017; 26(e1):e3–e4. doi:10.1136/tobaccocontrol-2016-053359
- Marcham CL, Springston JP. Electronic cigarettes in the indoor environment. Rev Env Health 2019; 34(2):105–124. doi:10.1515/reveh-2019-0012
- Chatham-Stephens K, Law R, Taylor E, et al; Centers for Disease Control and Prevention. Notes from the field: calls to poison centers for exposures to electronic cigarettes—United States, September 2010–September 2014. MMWR Morb Mortal Wkly Report 2014; 63(13):292–293. pmid:24699766
KEY POINTS
- Vaping is a common gateway to tobacco and marijuana use for adolescents and adults.
- The Juul vaping device delivers high nicotine concentrations that may pose a higher risk of nicotine addiction.
- Vaping has had unintended consequences that include poisoning of children who swallowed liquid nicotine, fires and explosions from defective batteries in the devices, and effects on the developing brain.
- Vaping is associated with respiratory illness and, in rare cases, death, likely due to vaporized agents introduced into the lungs. Small amounts of heavy metals, acetone, and other carcinogenic compounds in the vaping aerosol may cause lung damage.
Beyond depression: Other uses for tricyclic antidepressants
Most tricyclic antidepressants (TCAs) have US Food and Drug Administration approval for treatment of depression and anxiety disorders, but they are also a viable off-label option that should be considered by clinicians in specialties beyond psychiatry, especially for treating pain syndromes. Given the ongoing epidemic of opioid use disorder, increasing attention has been drawn to alternative strategies for chronic pain management, renewing an interest in the use of TCAs.
This review summarizes the pharmacologic properties of TCAs, their potential indications in conditions other than depression, and safety considerations.
BRIEF HISTORY OF TRICYCLICS
TCAs were originally designed in the 1950s and marketed later for treating depression. Due to their adverse effects and lethality in overdose quantities, over time they have been largely replaced by selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) in depression management. However, TCAs have been applied to conditions other than depression with varying degrees of efficacy and safety.
TCA PHARMACOLOGY
TCAs are absorbed in the small intestine and undergo first-pass metabolism in the liver. They bind extensively to proteins, leading to interactions with other protein-bound drugs. They are widely distributed throughout the systemic circulation because they are highly lipophilic, resulting in systemic effects including central nervous system manifestations.
Peak plasma concentration is at about 2 to 6 hours, and elimination half-life is around 24 hours for most agents, providing a long duration of action. Clearance depends on cytochrome P450 oxidative enzymes.1
MECHANISMS OF ACTION
TCAs inhibit reuptake of norepinephrine and serotonin, resulting in accumulation of these neurotransmitters in the presynaptic cleft. They also block postsynaptic histamine, alpha-adrenergic, and muscarinic-acetylcholine receptors, causing a variety of adverse effects, including dry mouth, confusion, cognitive impairment, hypotension, orthostasis, blurred vision, urinary retention, drowsiness, and sedation.1
Research suggests that TCAs relieve pain centrally through a descending pathway that inhibits transmission of pain signals in the spinal cord, as well as peripherally through complex anti-neuroimmune actions.2 Norepinephrine appears to play a more important role in this process than serotonin, although both are deemed necessary for the “dual action” often cited in pain management,1 which is also the rationale for widespread use of SNRIs to control pain.
Table 1 compares neurotransmitter reuptake mechanisms, adverse effect profiles, and typical dosages for depression for commonly prescribed TCAs.
POTENTIAL USES
Headache and migraine
TCAs have been shown to be effective for managing and preventing chronic headache syndromes.3,4 Amitriptyline has been the most studied of the TCAs for both chronic daily and episodic migraine headache, showing the most efficacy among diverse drug classes (angiotensin II receptor blockers, anticonvulsants, beta-blockers, SSRIs) compared with placebo. However, in head-to-head trials, amitriptyline was no more effective than SSRIs, venlafaxine, topiramate, or propranolol.4 Jackson et al4 suggested that prophylactic medication choices should be tailored to patient characteristics and expected adverse effects, and specifically recommended that TCAs—particularly amitriptyline—be reserved for patients who have both migraine and depression.
Neuropathic pain
Neuropathic pain is defined as pain secondary to a lesion or disease of the somatosensory nervous system5 and is the pathomechanistic component of a number of conditions, including postherpetic neuralgia,6 diabetic and nondiabetic painful polyneuropathy,7 posttraumatic or postsurgical neuropathic pain8 (including plexus avulsion and complex regional pain syndrome9), central poststroke pain,10 spinal cord injury pain,11 and multiple sclerosis-associated pain.12
As a group, TCAs appear to have a role as first-line agents for managing these varied neuropathic pain syndromes. In a recent meta-analysis,13 16 (89%) of 18 placebo-controlled trials of TCAs (mainly amitriptyline at 25–150 mg/day) for these pain conditions were positive, with a combined number needed to treat of 3.6, suggesting a role for TCAs in these conditions. Of note, the TCAs desipramine14 and nortriptyline15 have demonstrated little evidence of efficacy in neuropathic pain syndromes.
Chronic low back pain
Chronic low back pain is a leading cause of loss of work, excessive healthcare expenditure, and disability in the United States. It can be due to numerous spinal conditions, including degenerative disk disease, spinal stenosis, lumbar spondylosis, and spinal arthropathy.
TCAs have been used to treat chronic low back pain for decades and have been repeatedly shown to be more effective than placebo in reducing pain severity.16,17 A double-blind controlled trial18 from 1999 compared the effects of the TCA maprotiline (up to 150 mg daily), the SSRI paroxetine (up to 30 mg daily), and placebo and found a statistically significant reduction in back pain with maprotiline compared with paroxetine and placebo. However, a 2008 meta-analysis suggested little evidence that TCAs were superior to placebo.19
Evidence of TCA efficacy for back pain was reported in 2018 with a well-designed 6-month double-blind randomized controlled trial20 comparing low-dose amitriptyline (25 mg) with an active comparator (benztropine 1 mg). The authors reported that amitriptyline was effective in reducing pain and pain-related disability without incurring serious adverse effects. They suggested continued use of TCAs for chronic low back pain if complicated with pain-related disability, insomnia, depression, or other comorbidity, although they called for further large-scale studies. They also cautioned that patients started the trial with symptoms similar to the adverse effects of TCAs themselves; this has implications for monitoring of symptoms as well as TCA adverse effects while using these drugs.
Fibromyalgia and chronic widespread pain
Fibromyalgia is a common, frustrating, noninflammatory pain syndrome characterized by diffuse hyperalgesia and multiple comorbidities.21 Although sleep hygiene, exercise, cognitive-behavioral therapy, some gabapentinoids (pregabalin), and a combination of these therapies have demonstrated efficacy, TCAs also offer robust benefits.
A meta-analysis of 9 placebo-controlled TCA trials showed large effect sizes for pain reduction, fatigue reduction, improved sleep quality, and reduced stiffness and tenderness, with the most significant of these improvements being for sleep.22 A separate meta-analysis calculated that the number needed to treat with amitriptyline for a positive outcome is 4.9.23 Recent systematic reviews have supported these findings, listing TCAs as second-line agents after pregabalin, duloxetine, and milnacipran.24
Of note, TCA monotherapy rarely produces a complete response in patients with moderate to severe fibromyalgia, chronic widespread pain, or significant comorbidities (depression, anxiety). Supplementation with cognitive-behavioral therapy, physical therapy, functional restoration, and other modalities is strongly recommended.
Abdominal and gastrointestinal pain
TCAs have been applied to a number of gastrointestinal syndromes with or without pain. Patients with irritable bowel syndrome have long been known to benefit from TCAs; the number needed to treat for symptomatic benefit over placebo is 3.5.25,26
Although there is no substantial evidence that TCAs are useful in reducing active inflammation in inflammatory bowel disease, a study involving 81 patients found that residual noninflammatory gastrointestinal symptoms (such as diarrhea and pain) responded to TCAs, including nortriptyline and amitriptyline, with greater benefit for ulcerative colitis than for Crohn disease.27
TCAs have also shown prophylactic benefit in cyclic vomiting syndrome, with a clinical response in over 75% of patients in controlled cohort studies.28
The efficacy of TCAs in other abdominal or gastrointestinal syndromes is unclear or modest at best.29 However, few alternative treatments exist for these conditions. Amitriptyline may help symptoms of functional dyspepsia,30 but nortriptyline has proven ineffective in gastroparesis.31 Nonetheless, some authors29 suggest considering TCAs on an individualized basis, with proper monitoring, in many if not most functional gastrointestinal disorders, especially when paired with behavioral therapies.
Pelvic and urogynecologic symptoms
Chronic pelvic pain affects up to 24% of women32 and 5% to 10% of men.33 TCAs have shown efficacy in treating chronic pelvic pain with or without comorbid depression.34 Amitriptyline and to a lesser extent nortriptyline are the TCAs most often prescribed. Pain relief appears to be independent of antidepressant effects and may be achieved at low doses; initial dosing ranges from 10 to 25 mg at bedtime, which may be increased to 100 mg as tolerated.34
Based on a randomized, double-blind trial,35 amitriptyline was recommended as a treatment option for interstitial cystitis or bladder pain, with the greatest symptom improvement in patients tolerating a daily dose of 50 mg.
Another study36 randomized 56 women with chronic pelvic pain to amitriptyline or gabapentin, or a combination of the drugs for 24 months. Although each regimen resulted in significant reduction in pain, fewer adverse effects occurred with gabapentin than amitriptyline. Poor compliance and early discontinuation of amitriptyline were common due to anticholinergic effects.
In small uncontrolled studies,37 about half of women with chronic pelvic pain became pain-free after 8 weeks of treatment with nortriptyline and imipramine.
Randomized controlled studies are needed to confirm potential benefits of TCAs in chronic urologic and pelvic pain.
Insomnia
Insomnia affects 23% to 56% of people in the United States, Europe, and Asia38 and is the reason for more than 5.5 million primary care visits annually.39 TCAs (especially doxepin, maprotiline, and amitriptyline40) have been shown to be an effective treatment, with an 82% increase in somnolence compared with placebo, as well as measurably improved total sleep time, enhanced sleep efficiency, reduced latency to persistent sleep, and decreased wake times after sleep onset.38
Dosing should be kept at a minimum to minimize harsh anticholinergic effects and avoid daytime sedation. Patients should be advised to take new doses or dose escalations earlier in the night to ensure less hangover sedation the next morning.
For patients with insomnia and comorbid depression, the American Academy of Sleep Medicine suggests the addition of a low dose (eg, 10–25 mg) of a TCA at nighttime to complement preexisting, full-dose, non-TCA antidepressants, while monitoring for serotonin syndrome and other potential but exceedingly rare drug-drug interactions.41
Psychiatric indications other than depression
Beyond the known benefits in major depressive disorder, TCAs have been shown to be effective for obsessive-compulsive disorder, panic disorder, posttraumatic stress disorder, bulimia nervosa, and childhood enuresis.42 Given the shortage of mental health clinicians and the high prevalence of these conditions, nonpsychiatrist physicians should be familiar with the therapeutic potential of TCAs for these indications.
ADVERSE EFFECTS
Adverse effects vary among TCAs. Common ones include blurred vision, dry mouth, constipation, urinary retention, hypotension, tachycardia, tremor, weight gain, and sexual dysfunction.43 Tertiary amines are generally more sedating than secondary amines and cause more anticholinergic effects (Table 1).
Despite widespread perceptions that TCAs are less tolerable than newer antidepressants, studies repeatedly suggest that they have an adverse-effect burden similar to that of SSRIs and SNRIs, although SSRIs have a greater tendency to produce nausea, whereas TCAs are more likely to cause constipation.44
Discontinuation syndrome
Abrupt discontinuation or unintentionally missed doses of TCAs have been associated with a discontinuation syndrome in about 40% of users.45 Patients should be warned about this possibility and the syndrome’s potential effects: dizziness, insomnia, headaches, nausea, vomiting, flulike achiness, and restlessness. Rebound depression, anxiety, panic, or other psychiatric symptoms may also occur. Symptoms generally present within 2 to 5 days after dose discontinuation and last 7 to 14 days.45
However, all TCAs have a long half-life, allowing for sufficient coverage with once-daily dosing and thus carry a lower risk of discontinuation syndrome than many other antidepressants (78% with venlafaxine; 55% with paroxetine).45
To discontinue therapy safely, the dosage should be reduced gradually. As is pharmacologically expected, the greatest likelihood of discontinuation syndrome is associated with longer duration of continuous treatment.
CONTRAINDICATIONS
Cardiac conduction abnormalities
TCAs should not be prescribed to patients who have right bundle branch block, a severe electrolyte disturbance, or other cardiac conduction deficit or arrhythmia that can prolong the QTc interval and elevate the risk of lethal arrhythmia.46,47 Cardiac effects from TCAs are largely dose-dependent. Nevertheless, a baseline electrocardiogram can be obtained to assess cardiac risk, and dose escalation can proceed if results are normal (eg, appropriate conduction intervals, QTc ≤ 450 ms).
Advanced age
For elderly patients, TCAs should be prescribed with caution and sometimes not at all,48 because anticholinergic effects may worsen preexisting urinary retention (including benign prostatic hyperplasia), narrow-angle glaucoma, imbalance and gait issues, and cognitive impairment and dementia. Dehydration and orthostatic hypotension are contraindications for TCAs, as they may precipitate falls or hypotensive shock.
Epilepsy
TCAs should also be used with caution in patients with epilepsy, as they lower the seizure threshold.
Concomitant monoamine oxidase inhibitor treatment
Giving TCAs together with monoamine oxidase inhibitor antidepressants should be avoided, given the risk of hypertensive crisis.
Suicide risk
TCAs are dangerous and potentially lethal in overdose and so should not be prescribed to suicidal or otherwise impulsive patients.
Pregnancy
TCAs are in pregnancy risk category C (animal studies show adverse effects on fetus; no adequate or well-controlled studies in humans; potential benefits may warrant use despite risks). Using TCAs during pregnancy has very rarely led to neonatal withdrawal such as irritability, jitteriness, and convulsions, as well as fetal QTc interval prolongation.49
The American College of Obstetricians and Gynecologists recommends that therapy for depression during pregnancy be individualized, incorporating the expertise of the patient’s mental health clinician, obstetrician, primary healthcare provider, and pediatrician. In general, they recommend that TCAs should be avoided if possible and that alternatives such as SSRIs or SNRIs should be considered.50
TCAs are excreted in breast milk, but they have not been detected in the serum of nursing infants, and no adverse events have been reported.
OVERDOSE IS HIGHLY DANGEROUS
Severe morbidity and death are associated with TCA overdose, characterized by convulsions, cardiac arrest, and coma (the “3 Cs”). These dangers occur at much higher rates with TCAs than with other antidepressants.43 Signs and symptoms of toxicity develop rapidly, usually within the first hour of overdose. Manifestations of overdose include prolonged QTc, cardiac arrhythmias, tachycardia, hypertension, severe hypotension, agitation, seizures, central nervous system depression, hallucinations, seizures, and coma.
Overdose management includes activated charcoal, seizure control, cardioversion, hydration, electrolyte stabilization, and other intensive care.
OFF-LABEL TCA MANAGEMENT
Dosing recommendations for off-label use of TCAs vary based on the condition, the medication, and the suggestions of individual authors and researchers. In general, dosing ranges for pain and other nondepression indications may be lower than for severe depression (Table 2).1
As with any pharmacologic titration, monitoring for rate-limiting adverse effects is recommended. We suggest caution, tailoring the approach to the patient, and routinely assessing for adverse effects and other safety considerations.
In addition, we strongly recommend supplementing TCA therapy with nonpharmacologic strategies such as lifestyle changes, dietary modifications, exercise, physical therapy, and mental health optimization.
- Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci 2017; 18(11). doi:10.3390/ijms18112483
- Kremer M, Yalcin I, Goumon Y, et al. A dual noradrenergic mechanism for the relief of neuropathic allodynia by the antidepressant drugs duloxetine and amitriptyline. J Neurosci 2018; 38(46):9934–9954. doi:10.1523/JNEUROSCI.1004-18.2018
- Tomkins GE, Jackson JL, O’Malley PG, Balden E, Santoro JE. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med 2001; 111(1):54–63. doi:10.1016/s0002-9343(01)00762-8
- Jackson JL, Cogbill E, Santana-Davila R, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PLoS One 2015; 10(7):e0130733. doi:10.1371/journal.pone.0130733
- International Association for the Study of Pain (IASP). IASP Terminology. www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698&navItemNumber=576. Accessed November 20, 2019.
- Feller L, Khammissa RAG, Fourie J, Bouckaert M, Lemmer J. Postherpectic neuralgia and trigeminal neuralgia. Pain Res Treat 2017; 2017:1681765. doi:10.1155/2017/1681765
- Shillo P, Sloan G, Greig M, et al. Painful and painless diabetic neuropathies: what is the difference? Curr Diab Rep 2019; 19(6):32. doi:10.1007/s11892-019-1150-5
- Schwartzman RJ, Maleki J. Postinjury neuropathic pain syndromes. Med Clin North Am 1999; 83(3):597–626. doi:10.1016/s0025-7125(05)70126-7
- Oaklander AL, Horowitz SH. The complex regional pain syndrome. Handb Clin Neurol 2015; 131:481–503. doi:10.1016/B978-0-444-62627-1.00026-3
- Akyuz G, Kuru P. Systematic review of central post stroke pain: what is happening in the central nervous system? Am J Phys Med Rehabil 2016; 95(8):618–627. doi:10.1097/PHM.0000000000000542
- Shiao R, Lee-Kubli CA. Neuropathic pain after spinal cord injury: challenges and research perspectives. Neurotherapeutics 2018; 15(3):635–653. doi:10.1007/s13311-018-0633-4
- Ceruti S. What role does multiple sclerosis play in the development of untreatable painful conditions? Pain Manag 2018; 8(1):37–44. doi:10.2217/pmt-2017-0038
- Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
- Hearn L, Moore RA, Derry S, Wiffen PJ, Phillips T. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev 2014; (9):CD011003. doi:10.1002/14651858.CD011003.pub2
- Derry S, Whiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev 2015; 1:CD011209. doi:10.1002/14651858.CD011209.pub2
- Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med 2002; 162(1):19–24. doi:10.1001/archinte.162.1.19
- Staiger TO, Gaster B, Sullivan MD, Deyo RA. Systematic review of antidepressants in the treatment of chronic low back pain. Spine (Phila Pa 1976) 2003; 28(22):2540–2545. doi:10.1097/01.BRS.0000092372.73527.BA
- Atkinson JH, Slater MA, Wahlgren DR, et al. Effects of noradrenergic and serotonergic antidepressants on chronic low back pain intensity. Pain 1999; 83(2):137–145. doi:10.1016/s0304-3959(99)00082-2
- Urquhart DM, Hoving JL, Assendelft WW, Roland M, van Tulder MW. Antidepressants for non-specific back pain. Cochrane Database Syst Rev 2008; (1):CD001703. doi:10.1002/14651858.CD001703.pub3
- Urquhart DM, Wluka AE, van Tulder M, et al. Efficacy of low-dose amitriptyline for chronic low back pain: a randomized clinical trial. JAMA Intern Med 2018; 178(11):1474–1481. doi:10.1001/jamainternmed.2018.4222
- Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014; 311(15):1547–1555. doi:10.1001/jama.2014.3266
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics 2000; 41(2):104–113. pmid:10749947
- Hauser W, Wolfe F, Tolle T, Uceyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs 2012; 26(4):297–307. doi:10.2165/11598970-000000000-00000
- Calandre EP, Rico-Villademoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin Pharmacother 2015; 16(9):1347–1368. doi:10.1517/14656566.2015.1047343
- Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 2000; 108(1):65–72. doi:10.1016/s0002-9343(99)00299-5
- Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol 2009; 15(13):1548–1553. doi:10.3748/wjg.15.1548
- Iskandar HN, Cassell B, Kanuri N, et al. Tricyclic antidepressants for management of residual symptoms in inflammatory bowel disease. J Clin Gastroenterol 2014; 48(5):423–429. doi:10.1097/MCG.0000000000000049
- Lee LY, Abbott L, Mahlangu B, Moodie SJ, Anderson S. The management of cyclic vomiting syndrome: a systematic review. Eur J Gastroenterol Hepatol 2012; 24(9):1001–1006. doi:10.1097/MEG.0b013e328355638f
- Thorkelson G, Bielefeldt K, Szigethy E. Empirically supported use of psychiatric medications in adolescents and adults with IBD. Inflamm Bowel Dis 2016; 22(6):1509–1522. doi:10.1097/MIB.0000000000000734
- Braak B, Klooker TK, Wouters MM, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther 2011; 34(6):638–648. doi:10.1111/j.1365-2036.2011.04775.x
- Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA 2013; 310(24):2640–2649. doi:10.1001/jama.2013.282833
- Latthe P, Latthe M, Say L, Gulmezoglu M, Khan KS. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health 2006; 6:177. doi:10.1186/1471-2458-6-177
- Moise G, Capodice J, Winfree CJ. Treatment of chronic pelvic pain in men and women. Expert Rev Neurother 2007; 7(5):507–520. doi:10.1586/14737175.7.5.507
- Lai HH. Management of interstitial cystitis/bladder pain syndrome with tricyclic antidepressants. In: Moldwin RM, ed. Urological and Gynaecological Chronic Pelvic Pain. Cham, Switzerland: Springer; 2017:107–118.
- American Urological Association. Diagnosis and treatment interstitial cystitis/bladder pain syndrome (2014). www.auanet.org/guidelines/interstitial-cystitis/bladder-pain-syndrome-(2011-amended-2014). Accessed November 19, 2019.
- Carey ET, As-Sanie S. New developments in the pharmacotherapy of neuropathic chronic pelvic pain. Future Sci OA 2016; 2(4):FSO148. doi:10.4155/fsoa-2016-0048
- Papandreou C, Skapinakis P, Giannakis D, Sofikitis N, Mavreas V. Antidepressant drugs for chronic urological pelvic pain: an evidence-based review. Adv Urol 2009; 2009:797031. doi:10.1155/2009/797031
- Liu Y, Xu X, Dong M, Jia S, Wei Y. Treatment of insomnia with tricyclic antidepressants: a meta-analysis of polysomnographic randomized controlled trials. Sleep Med 2017; 34:126–133. doi:10.1016/j.sleep.2017.03.007
- Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician 2017; 96(1):29–35. pmid:28671376
- McCall C, McCall WV. What is the role of sedating antidepressants, antipsychotics, and anticonvulsants in the management of insomnia? Curr Psychiatry Rep 2012; 14(5):494–502. doi:10.1007/s11920-012-0302-y
- Clark MS, Smith PO, Jamieson B. FPIN’s clinical inquiries: antidepressants for the treatment of insomnia in patients with depression. Am Fam Physician 2011; 84(9):1–2. pmid:22164891
- Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s Synopsis of Psychiatry. New York, NY: Lippincott Williams & Wilkins; 2014.
- Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J 2018; 54(2):101–112. doi:10.4068/cmj.2018.54.2.101.
- Trindade E, Menon D, Topfer LA, Coloma C. Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. CMAJ 1998; 159(10):1245–1252. pmid:9861221
- Fava M. Prospective studies of adverse events related to antidepressant discontinuation. J Clin Psychiatry 2006; 67(suppl 4):14–21. pmid:16683858
- Gintant G. An evaluation of hERG current assay performance: translating preclinical safety studies to clinical QT prolongation. Pharmacol Ther 2011; 129(2):109–119. doi:10.1016/j.pharmthera.2010.08.008.
- Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54(1):1–13. doi:10.1016/j.psym.2012.11.001
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63(11):2227–2246. doi:10.1111/jgs.13702
- Fukushima N, Nanao K, Fukushima H, Namera A, Miura M. A neonatal prolonged QT syndrome due to maternal use of oral tricyclic antidepressants. Eur J Pediatr 2016; 175(8):1129–1132. doi:10.1007/s00431-016-2722-x
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol 2008; 111(4):1001–1020. doi:10.1097/AOG.0b013e31816fd910
Most tricyclic antidepressants (TCAs) have US Food and Drug Administration approval for treatment of depression and anxiety disorders, but they are also a viable off-label option that should be considered by clinicians in specialties beyond psychiatry, especially for treating pain syndromes. Given the ongoing epidemic of opioid use disorder, increasing attention has been drawn to alternative strategies for chronic pain management, renewing an interest in the use of TCAs.
This review summarizes the pharmacologic properties of TCAs, their potential indications in conditions other than depression, and safety considerations.
BRIEF HISTORY OF TRICYCLICS
TCAs were originally designed in the 1950s and marketed later for treating depression. Due to their adverse effects and lethality in overdose quantities, over time they have been largely replaced by selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) in depression management. However, TCAs have been applied to conditions other than depression with varying degrees of efficacy and safety.
TCA PHARMACOLOGY
TCAs are absorbed in the small intestine and undergo first-pass metabolism in the liver. They bind extensively to proteins, leading to interactions with other protein-bound drugs. They are widely distributed throughout the systemic circulation because they are highly lipophilic, resulting in systemic effects including central nervous system manifestations.
Peak plasma concentration is at about 2 to 6 hours, and elimination half-life is around 24 hours for most agents, providing a long duration of action. Clearance depends on cytochrome P450 oxidative enzymes.1
MECHANISMS OF ACTION
TCAs inhibit reuptake of norepinephrine and serotonin, resulting in accumulation of these neurotransmitters in the presynaptic cleft. They also block postsynaptic histamine, alpha-adrenergic, and muscarinic-acetylcholine receptors, causing a variety of adverse effects, including dry mouth, confusion, cognitive impairment, hypotension, orthostasis, blurred vision, urinary retention, drowsiness, and sedation.1
Research suggests that TCAs relieve pain centrally through a descending pathway that inhibits transmission of pain signals in the spinal cord, as well as peripherally through complex anti-neuroimmune actions.2 Norepinephrine appears to play a more important role in this process than serotonin, although both are deemed necessary for the “dual action” often cited in pain management,1 which is also the rationale for widespread use of SNRIs to control pain.
Table 1 compares neurotransmitter reuptake mechanisms, adverse effect profiles, and typical dosages for depression for commonly prescribed TCAs.
POTENTIAL USES
Headache and migraine
TCAs have been shown to be effective for managing and preventing chronic headache syndromes.3,4 Amitriptyline has been the most studied of the TCAs for both chronic daily and episodic migraine headache, showing the most efficacy among diverse drug classes (angiotensin II receptor blockers, anticonvulsants, beta-blockers, SSRIs) compared with placebo. However, in head-to-head trials, amitriptyline was no more effective than SSRIs, venlafaxine, topiramate, or propranolol.4 Jackson et al4 suggested that prophylactic medication choices should be tailored to patient characteristics and expected adverse effects, and specifically recommended that TCAs—particularly amitriptyline—be reserved for patients who have both migraine and depression.
Neuropathic pain
Neuropathic pain is defined as pain secondary to a lesion or disease of the somatosensory nervous system5 and is the pathomechanistic component of a number of conditions, including postherpetic neuralgia,6 diabetic and nondiabetic painful polyneuropathy,7 posttraumatic or postsurgical neuropathic pain8 (including plexus avulsion and complex regional pain syndrome9), central poststroke pain,10 spinal cord injury pain,11 and multiple sclerosis-associated pain.12
As a group, TCAs appear to have a role as first-line agents for managing these varied neuropathic pain syndromes. In a recent meta-analysis,13 16 (89%) of 18 placebo-controlled trials of TCAs (mainly amitriptyline at 25–150 mg/day) for these pain conditions were positive, with a combined number needed to treat of 3.6, suggesting a role for TCAs in these conditions. Of note, the TCAs desipramine14 and nortriptyline15 have demonstrated little evidence of efficacy in neuropathic pain syndromes.
Chronic low back pain
Chronic low back pain is a leading cause of loss of work, excessive healthcare expenditure, and disability in the United States. It can be due to numerous spinal conditions, including degenerative disk disease, spinal stenosis, lumbar spondylosis, and spinal arthropathy.
TCAs have been used to treat chronic low back pain for decades and have been repeatedly shown to be more effective than placebo in reducing pain severity.16,17 A double-blind controlled trial18 from 1999 compared the effects of the TCA maprotiline (up to 150 mg daily), the SSRI paroxetine (up to 30 mg daily), and placebo and found a statistically significant reduction in back pain with maprotiline compared with paroxetine and placebo. However, a 2008 meta-analysis suggested little evidence that TCAs were superior to placebo.19
Evidence of TCA efficacy for back pain was reported in 2018 with a well-designed 6-month double-blind randomized controlled trial20 comparing low-dose amitriptyline (25 mg) with an active comparator (benztropine 1 mg). The authors reported that amitriptyline was effective in reducing pain and pain-related disability without incurring serious adverse effects. They suggested continued use of TCAs for chronic low back pain if complicated with pain-related disability, insomnia, depression, or other comorbidity, although they called for further large-scale studies. They also cautioned that patients started the trial with symptoms similar to the adverse effects of TCAs themselves; this has implications for monitoring of symptoms as well as TCA adverse effects while using these drugs.
Fibromyalgia and chronic widespread pain
Fibromyalgia is a common, frustrating, noninflammatory pain syndrome characterized by diffuse hyperalgesia and multiple comorbidities.21 Although sleep hygiene, exercise, cognitive-behavioral therapy, some gabapentinoids (pregabalin), and a combination of these therapies have demonstrated efficacy, TCAs also offer robust benefits.
A meta-analysis of 9 placebo-controlled TCA trials showed large effect sizes for pain reduction, fatigue reduction, improved sleep quality, and reduced stiffness and tenderness, with the most significant of these improvements being for sleep.22 A separate meta-analysis calculated that the number needed to treat with amitriptyline for a positive outcome is 4.9.23 Recent systematic reviews have supported these findings, listing TCAs as second-line agents after pregabalin, duloxetine, and milnacipran.24
Of note, TCA monotherapy rarely produces a complete response in patients with moderate to severe fibromyalgia, chronic widespread pain, or significant comorbidities (depression, anxiety). Supplementation with cognitive-behavioral therapy, physical therapy, functional restoration, and other modalities is strongly recommended.
Abdominal and gastrointestinal pain
TCAs have been applied to a number of gastrointestinal syndromes with or without pain. Patients with irritable bowel syndrome have long been known to benefit from TCAs; the number needed to treat for symptomatic benefit over placebo is 3.5.25,26
Although there is no substantial evidence that TCAs are useful in reducing active inflammation in inflammatory bowel disease, a study involving 81 patients found that residual noninflammatory gastrointestinal symptoms (such as diarrhea and pain) responded to TCAs, including nortriptyline and amitriptyline, with greater benefit for ulcerative colitis than for Crohn disease.27
TCAs have also shown prophylactic benefit in cyclic vomiting syndrome, with a clinical response in over 75% of patients in controlled cohort studies.28
The efficacy of TCAs in other abdominal or gastrointestinal syndromes is unclear or modest at best.29 However, few alternative treatments exist for these conditions. Amitriptyline may help symptoms of functional dyspepsia,30 but nortriptyline has proven ineffective in gastroparesis.31 Nonetheless, some authors29 suggest considering TCAs on an individualized basis, with proper monitoring, in many if not most functional gastrointestinal disorders, especially when paired with behavioral therapies.
Pelvic and urogynecologic symptoms
Chronic pelvic pain affects up to 24% of women32 and 5% to 10% of men.33 TCAs have shown efficacy in treating chronic pelvic pain with or without comorbid depression.34 Amitriptyline and to a lesser extent nortriptyline are the TCAs most often prescribed. Pain relief appears to be independent of antidepressant effects and may be achieved at low doses; initial dosing ranges from 10 to 25 mg at bedtime, which may be increased to 100 mg as tolerated.34
Based on a randomized, double-blind trial,35 amitriptyline was recommended as a treatment option for interstitial cystitis or bladder pain, with the greatest symptom improvement in patients tolerating a daily dose of 50 mg.
Another study36 randomized 56 women with chronic pelvic pain to amitriptyline or gabapentin, or a combination of the drugs for 24 months. Although each regimen resulted in significant reduction in pain, fewer adverse effects occurred with gabapentin than amitriptyline. Poor compliance and early discontinuation of amitriptyline were common due to anticholinergic effects.
In small uncontrolled studies,37 about half of women with chronic pelvic pain became pain-free after 8 weeks of treatment with nortriptyline and imipramine.
Randomized controlled studies are needed to confirm potential benefits of TCAs in chronic urologic and pelvic pain.
Insomnia
Insomnia affects 23% to 56% of people in the United States, Europe, and Asia38 and is the reason for more than 5.5 million primary care visits annually.39 TCAs (especially doxepin, maprotiline, and amitriptyline40) have been shown to be an effective treatment, with an 82% increase in somnolence compared with placebo, as well as measurably improved total sleep time, enhanced sleep efficiency, reduced latency to persistent sleep, and decreased wake times after sleep onset.38
Dosing should be kept at a minimum to minimize harsh anticholinergic effects and avoid daytime sedation. Patients should be advised to take new doses or dose escalations earlier in the night to ensure less hangover sedation the next morning.
For patients with insomnia and comorbid depression, the American Academy of Sleep Medicine suggests the addition of a low dose (eg, 10–25 mg) of a TCA at nighttime to complement preexisting, full-dose, non-TCA antidepressants, while monitoring for serotonin syndrome and other potential but exceedingly rare drug-drug interactions.41
Psychiatric indications other than depression
Beyond the known benefits in major depressive disorder, TCAs have been shown to be effective for obsessive-compulsive disorder, panic disorder, posttraumatic stress disorder, bulimia nervosa, and childhood enuresis.42 Given the shortage of mental health clinicians and the high prevalence of these conditions, nonpsychiatrist physicians should be familiar with the therapeutic potential of TCAs for these indications.
ADVERSE EFFECTS
Adverse effects vary among TCAs. Common ones include blurred vision, dry mouth, constipation, urinary retention, hypotension, tachycardia, tremor, weight gain, and sexual dysfunction.43 Tertiary amines are generally more sedating than secondary amines and cause more anticholinergic effects (Table 1).
Despite widespread perceptions that TCAs are less tolerable than newer antidepressants, studies repeatedly suggest that they have an adverse-effect burden similar to that of SSRIs and SNRIs, although SSRIs have a greater tendency to produce nausea, whereas TCAs are more likely to cause constipation.44
Discontinuation syndrome
Abrupt discontinuation or unintentionally missed doses of TCAs have been associated with a discontinuation syndrome in about 40% of users.45 Patients should be warned about this possibility and the syndrome’s potential effects: dizziness, insomnia, headaches, nausea, vomiting, flulike achiness, and restlessness. Rebound depression, anxiety, panic, or other psychiatric symptoms may also occur. Symptoms generally present within 2 to 5 days after dose discontinuation and last 7 to 14 days.45
However, all TCAs have a long half-life, allowing for sufficient coverage with once-daily dosing and thus carry a lower risk of discontinuation syndrome than many other antidepressants (78% with venlafaxine; 55% with paroxetine).45
To discontinue therapy safely, the dosage should be reduced gradually. As is pharmacologically expected, the greatest likelihood of discontinuation syndrome is associated with longer duration of continuous treatment.
CONTRAINDICATIONS
Cardiac conduction abnormalities
TCAs should not be prescribed to patients who have right bundle branch block, a severe electrolyte disturbance, or other cardiac conduction deficit or arrhythmia that can prolong the QTc interval and elevate the risk of lethal arrhythmia.46,47 Cardiac effects from TCAs are largely dose-dependent. Nevertheless, a baseline electrocardiogram can be obtained to assess cardiac risk, and dose escalation can proceed if results are normal (eg, appropriate conduction intervals, QTc ≤ 450 ms).
Advanced age
For elderly patients, TCAs should be prescribed with caution and sometimes not at all,48 because anticholinergic effects may worsen preexisting urinary retention (including benign prostatic hyperplasia), narrow-angle glaucoma, imbalance and gait issues, and cognitive impairment and dementia. Dehydration and orthostatic hypotension are contraindications for TCAs, as they may precipitate falls or hypotensive shock.
Epilepsy
TCAs should also be used with caution in patients with epilepsy, as they lower the seizure threshold.
Concomitant monoamine oxidase inhibitor treatment
Giving TCAs together with monoamine oxidase inhibitor antidepressants should be avoided, given the risk of hypertensive crisis.
Suicide risk
TCAs are dangerous and potentially lethal in overdose and so should not be prescribed to suicidal or otherwise impulsive patients.
Pregnancy
TCAs are in pregnancy risk category C (animal studies show adverse effects on fetus; no adequate or well-controlled studies in humans; potential benefits may warrant use despite risks). Using TCAs during pregnancy has very rarely led to neonatal withdrawal such as irritability, jitteriness, and convulsions, as well as fetal QTc interval prolongation.49
The American College of Obstetricians and Gynecologists recommends that therapy for depression during pregnancy be individualized, incorporating the expertise of the patient’s mental health clinician, obstetrician, primary healthcare provider, and pediatrician. In general, they recommend that TCAs should be avoided if possible and that alternatives such as SSRIs or SNRIs should be considered.50
TCAs are excreted in breast milk, but they have not been detected in the serum of nursing infants, and no adverse events have been reported.
OVERDOSE IS HIGHLY DANGEROUS
Severe morbidity and death are associated with TCA overdose, characterized by convulsions, cardiac arrest, and coma (the “3 Cs”). These dangers occur at much higher rates with TCAs than with other antidepressants.43 Signs and symptoms of toxicity develop rapidly, usually within the first hour of overdose. Manifestations of overdose include prolonged QTc, cardiac arrhythmias, tachycardia, hypertension, severe hypotension, agitation, seizures, central nervous system depression, hallucinations, seizures, and coma.
Overdose management includes activated charcoal, seizure control, cardioversion, hydration, electrolyte stabilization, and other intensive care.
OFF-LABEL TCA MANAGEMENT
Dosing recommendations for off-label use of TCAs vary based on the condition, the medication, and the suggestions of individual authors and researchers. In general, dosing ranges for pain and other nondepression indications may be lower than for severe depression (Table 2).1
As with any pharmacologic titration, monitoring for rate-limiting adverse effects is recommended. We suggest caution, tailoring the approach to the patient, and routinely assessing for adverse effects and other safety considerations.
In addition, we strongly recommend supplementing TCA therapy with nonpharmacologic strategies such as lifestyle changes, dietary modifications, exercise, physical therapy, and mental health optimization.
Most tricyclic antidepressants (TCAs) have US Food and Drug Administration approval for treatment of depression and anxiety disorders, but they are also a viable off-label option that should be considered by clinicians in specialties beyond psychiatry, especially for treating pain syndromes. Given the ongoing epidemic of opioid use disorder, increasing attention has been drawn to alternative strategies for chronic pain management, renewing an interest in the use of TCAs.
This review summarizes the pharmacologic properties of TCAs, their potential indications in conditions other than depression, and safety considerations.
BRIEF HISTORY OF TRICYCLICS
TCAs were originally designed in the 1950s and marketed later for treating depression. Due to their adverse effects and lethality in overdose quantities, over time they have been largely replaced by selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) in depression management. However, TCAs have been applied to conditions other than depression with varying degrees of efficacy and safety.
TCA PHARMACOLOGY
TCAs are absorbed in the small intestine and undergo first-pass metabolism in the liver. They bind extensively to proteins, leading to interactions with other protein-bound drugs. They are widely distributed throughout the systemic circulation because they are highly lipophilic, resulting in systemic effects including central nervous system manifestations.
Peak plasma concentration is at about 2 to 6 hours, and elimination half-life is around 24 hours for most agents, providing a long duration of action. Clearance depends on cytochrome P450 oxidative enzymes.1
MECHANISMS OF ACTION
TCAs inhibit reuptake of norepinephrine and serotonin, resulting in accumulation of these neurotransmitters in the presynaptic cleft. They also block postsynaptic histamine, alpha-adrenergic, and muscarinic-acetylcholine receptors, causing a variety of adverse effects, including dry mouth, confusion, cognitive impairment, hypotension, orthostasis, blurred vision, urinary retention, drowsiness, and sedation.1
Research suggests that TCAs relieve pain centrally through a descending pathway that inhibits transmission of pain signals in the spinal cord, as well as peripherally through complex anti-neuroimmune actions.2 Norepinephrine appears to play a more important role in this process than serotonin, although both are deemed necessary for the “dual action” often cited in pain management,1 which is also the rationale for widespread use of SNRIs to control pain.
Table 1 compares neurotransmitter reuptake mechanisms, adverse effect profiles, and typical dosages for depression for commonly prescribed TCAs.
POTENTIAL USES
Headache and migraine
TCAs have been shown to be effective for managing and preventing chronic headache syndromes.3,4 Amitriptyline has been the most studied of the TCAs for both chronic daily and episodic migraine headache, showing the most efficacy among diverse drug classes (angiotensin II receptor blockers, anticonvulsants, beta-blockers, SSRIs) compared with placebo. However, in head-to-head trials, amitriptyline was no more effective than SSRIs, venlafaxine, topiramate, or propranolol.4 Jackson et al4 suggested that prophylactic medication choices should be tailored to patient characteristics and expected adverse effects, and specifically recommended that TCAs—particularly amitriptyline—be reserved for patients who have both migraine and depression.
Neuropathic pain
Neuropathic pain is defined as pain secondary to a lesion or disease of the somatosensory nervous system5 and is the pathomechanistic component of a number of conditions, including postherpetic neuralgia,6 diabetic and nondiabetic painful polyneuropathy,7 posttraumatic or postsurgical neuropathic pain8 (including plexus avulsion and complex regional pain syndrome9), central poststroke pain,10 spinal cord injury pain,11 and multiple sclerosis-associated pain.12
As a group, TCAs appear to have a role as first-line agents for managing these varied neuropathic pain syndromes. In a recent meta-analysis,13 16 (89%) of 18 placebo-controlled trials of TCAs (mainly amitriptyline at 25–150 mg/day) for these pain conditions were positive, with a combined number needed to treat of 3.6, suggesting a role for TCAs in these conditions. Of note, the TCAs desipramine14 and nortriptyline15 have demonstrated little evidence of efficacy in neuropathic pain syndromes.
Chronic low back pain
Chronic low back pain is a leading cause of loss of work, excessive healthcare expenditure, and disability in the United States. It can be due to numerous spinal conditions, including degenerative disk disease, spinal stenosis, lumbar spondylosis, and spinal arthropathy.
TCAs have been used to treat chronic low back pain for decades and have been repeatedly shown to be more effective than placebo in reducing pain severity.16,17 A double-blind controlled trial18 from 1999 compared the effects of the TCA maprotiline (up to 150 mg daily), the SSRI paroxetine (up to 30 mg daily), and placebo and found a statistically significant reduction in back pain with maprotiline compared with paroxetine and placebo. However, a 2008 meta-analysis suggested little evidence that TCAs were superior to placebo.19
Evidence of TCA efficacy for back pain was reported in 2018 with a well-designed 6-month double-blind randomized controlled trial20 comparing low-dose amitriptyline (25 mg) with an active comparator (benztropine 1 mg). The authors reported that amitriptyline was effective in reducing pain and pain-related disability without incurring serious adverse effects. They suggested continued use of TCAs for chronic low back pain if complicated with pain-related disability, insomnia, depression, or other comorbidity, although they called for further large-scale studies. They also cautioned that patients started the trial with symptoms similar to the adverse effects of TCAs themselves; this has implications for monitoring of symptoms as well as TCA adverse effects while using these drugs.
Fibromyalgia and chronic widespread pain
Fibromyalgia is a common, frustrating, noninflammatory pain syndrome characterized by diffuse hyperalgesia and multiple comorbidities.21 Although sleep hygiene, exercise, cognitive-behavioral therapy, some gabapentinoids (pregabalin), and a combination of these therapies have demonstrated efficacy, TCAs also offer robust benefits.
A meta-analysis of 9 placebo-controlled TCA trials showed large effect sizes for pain reduction, fatigue reduction, improved sleep quality, and reduced stiffness and tenderness, with the most significant of these improvements being for sleep.22 A separate meta-analysis calculated that the number needed to treat with amitriptyline for a positive outcome is 4.9.23 Recent systematic reviews have supported these findings, listing TCAs as second-line agents after pregabalin, duloxetine, and milnacipran.24
Of note, TCA monotherapy rarely produces a complete response in patients with moderate to severe fibromyalgia, chronic widespread pain, or significant comorbidities (depression, anxiety). Supplementation with cognitive-behavioral therapy, physical therapy, functional restoration, and other modalities is strongly recommended.
Abdominal and gastrointestinal pain
TCAs have been applied to a number of gastrointestinal syndromes with or without pain. Patients with irritable bowel syndrome have long been known to benefit from TCAs; the number needed to treat for symptomatic benefit over placebo is 3.5.25,26
Although there is no substantial evidence that TCAs are useful in reducing active inflammation in inflammatory bowel disease, a study involving 81 patients found that residual noninflammatory gastrointestinal symptoms (such as diarrhea and pain) responded to TCAs, including nortriptyline and amitriptyline, with greater benefit for ulcerative colitis than for Crohn disease.27
TCAs have also shown prophylactic benefit in cyclic vomiting syndrome, with a clinical response in over 75% of patients in controlled cohort studies.28
The efficacy of TCAs in other abdominal or gastrointestinal syndromes is unclear or modest at best.29 However, few alternative treatments exist for these conditions. Amitriptyline may help symptoms of functional dyspepsia,30 but nortriptyline has proven ineffective in gastroparesis.31 Nonetheless, some authors29 suggest considering TCAs on an individualized basis, with proper monitoring, in many if not most functional gastrointestinal disorders, especially when paired with behavioral therapies.
Pelvic and urogynecologic symptoms
Chronic pelvic pain affects up to 24% of women32 and 5% to 10% of men.33 TCAs have shown efficacy in treating chronic pelvic pain with or without comorbid depression.34 Amitriptyline and to a lesser extent nortriptyline are the TCAs most often prescribed. Pain relief appears to be independent of antidepressant effects and may be achieved at low doses; initial dosing ranges from 10 to 25 mg at bedtime, which may be increased to 100 mg as tolerated.34
Based on a randomized, double-blind trial,35 amitriptyline was recommended as a treatment option for interstitial cystitis or bladder pain, with the greatest symptom improvement in patients tolerating a daily dose of 50 mg.
Another study36 randomized 56 women with chronic pelvic pain to amitriptyline or gabapentin, or a combination of the drugs for 24 months. Although each regimen resulted in significant reduction in pain, fewer adverse effects occurred with gabapentin than amitriptyline. Poor compliance and early discontinuation of amitriptyline were common due to anticholinergic effects.
In small uncontrolled studies,37 about half of women with chronic pelvic pain became pain-free after 8 weeks of treatment with nortriptyline and imipramine.
Randomized controlled studies are needed to confirm potential benefits of TCAs in chronic urologic and pelvic pain.
Insomnia
Insomnia affects 23% to 56% of people in the United States, Europe, and Asia38 and is the reason for more than 5.5 million primary care visits annually.39 TCAs (especially doxepin, maprotiline, and amitriptyline40) have been shown to be an effective treatment, with an 82% increase in somnolence compared with placebo, as well as measurably improved total sleep time, enhanced sleep efficiency, reduced latency to persistent sleep, and decreased wake times after sleep onset.38
Dosing should be kept at a minimum to minimize harsh anticholinergic effects and avoid daytime sedation. Patients should be advised to take new doses or dose escalations earlier in the night to ensure less hangover sedation the next morning.
For patients with insomnia and comorbid depression, the American Academy of Sleep Medicine suggests the addition of a low dose (eg, 10–25 mg) of a TCA at nighttime to complement preexisting, full-dose, non-TCA antidepressants, while monitoring for serotonin syndrome and other potential but exceedingly rare drug-drug interactions.41
Psychiatric indications other than depression
Beyond the known benefits in major depressive disorder, TCAs have been shown to be effective for obsessive-compulsive disorder, panic disorder, posttraumatic stress disorder, bulimia nervosa, and childhood enuresis.42 Given the shortage of mental health clinicians and the high prevalence of these conditions, nonpsychiatrist physicians should be familiar with the therapeutic potential of TCAs for these indications.
ADVERSE EFFECTS
Adverse effects vary among TCAs. Common ones include blurred vision, dry mouth, constipation, urinary retention, hypotension, tachycardia, tremor, weight gain, and sexual dysfunction.43 Tertiary amines are generally more sedating than secondary amines and cause more anticholinergic effects (Table 1).
Despite widespread perceptions that TCAs are less tolerable than newer antidepressants, studies repeatedly suggest that they have an adverse-effect burden similar to that of SSRIs and SNRIs, although SSRIs have a greater tendency to produce nausea, whereas TCAs are more likely to cause constipation.44
Discontinuation syndrome
Abrupt discontinuation or unintentionally missed doses of TCAs have been associated with a discontinuation syndrome in about 40% of users.45 Patients should be warned about this possibility and the syndrome’s potential effects: dizziness, insomnia, headaches, nausea, vomiting, flulike achiness, and restlessness. Rebound depression, anxiety, panic, or other psychiatric symptoms may also occur. Symptoms generally present within 2 to 5 days after dose discontinuation and last 7 to 14 days.45
However, all TCAs have a long half-life, allowing for sufficient coverage with once-daily dosing and thus carry a lower risk of discontinuation syndrome than many other antidepressants (78% with venlafaxine; 55% with paroxetine).45
To discontinue therapy safely, the dosage should be reduced gradually. As is pharmacologically expected, the greatest likelihood of discontinuation syndrome is associated with longer duration of continuous treatment.
CONTRAINDICATIONS
Cardiac conduction abnormalities
TCAs should not be prescribed to patients who have right bundle branch block, a severe electrolyte disturbance, or other cardiac conduction deficit or arrhythmia that can prolong the QTc interval and elevate the risk of lethal arrhythmia.46,47 Cardiac effects from TCAs are largely dose-dependent. Nevertheless, a baseline electrocardiogram can be obtained to assess cardiac risk, and dose escalation can proceed if results are normal (eg, appropriate conduction intervals, QTc ≤ 450 ms).
Advanced age
For elderly patients, TCAs should be prescribed with caution and sometimes not at all,48 because anticholinergic effects may worsen preexisting urinary retention (including benign prostatic hyperplasia), narrow-angle glaucoma, imbalance and gait issues, and cognitive impairment and dementia. Dehydration and orthostatic hypotension are contraindications for TCAs, as they may precipitate falls or hypotensive shock.
Epilepsy
TCAs should also be used with caution in patients with epilepsy, as they lower the seizure threshold.
Concomitant monoamine oxidase inhibitor treatment
Giving TCAs together with monoamine oxidase inhibitor antidepressants should be avoided, given the risk of hypertensive crisis.
Suicide risk
TCAs are dangerous and potentially lethal in overdose and so should not be prescribed to suicidal or otherwise impulsive patients.
Pregnancy
TCAs are in pregnancy risk category C (animal studies show adverse effects on fetus; no adequate or well-controlled studies in humans; potential benefits may warrant use despite risks). Using TCAs during pregnancy has very rarely led to neonatal withdrawal such as irritability, jitteriness, and convulsions, as well as fetal QTc interval prolongation.49
The American College of Obstetricians and Gynecologists recommends that therapy for depression during pregnancy be individualized, incorporating the expertise of the patient’s mental health clinician, obstetrician, primary healthcare provider, and pediatrician. In general, they recommend that TCAs should be avoided if possible and that alternatives such as SSRIs or SNRIs should be considered.50
TCAs are excreted in breast milk, but they have not been detected in the serum of nursing infants, and no adverse events have been reported.
OVERDOSE IS HIGHLY DANGEROUS
Severe morbidity and death are associated with TCA overdose, characterized by convulsions, cardiac arrest, and coma (the “3 Cs”). These dangers occur at much higher rates with TCAs than with other antidepressants.43 Signs and symptoms of toxicity develop rapidly, usually within the first hour of overdose. Manifestations of overdose include prolonged QTc, cardiac arrhythmias, tachycardia, hypertension, severe hypotension, agitation, seizures, central nervous system depression, hallucinations, seizures, and coma.
Overdose management includes activated charcoal, seizure control, cardioversion, hydration, electrolyte stabilization, and other intensive care.
OFF-LABEL TCA MANAGEMENT
Dosing recommendations for off-label use of TCAs vary based on the condition, the medication, and the suggestions of individual authors and researchers. In general, dosing ranges for pain and other nondepression indications may be lower than for severe depression (Table 2).1
As with any pharmacologic titration, monitoring for rate-limiting adverse effects is recommended. We suggest caution, tailoring the approach to the patient, and routinely assessing for adverse effects and other safety considerations.
In addition, we strongly recommend supplementing TCA therapy with nonpharmacologic strategies such as lifestyle changes, dietary modifications, exercise, physical therapy, and mental health optimization.
- Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci 2017; 18(11). doi:10.3390/ijms18112483
- Kremer M, Yalcin I, Goumon Y, et al. A dual noradrenergic mechanism for the relief of neuropathic allodynia by the antidepressant drugs duloxetine and amitriptyline. J Neurosci 2018; 38(46):9934–9954. doi:10.1523/JNEUROSCI.1004-18.2018
- Tomkins GE, Jackson JL, O’Malley PG, Balden E, Santoro JE. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med 2001; 111(1):54–63. doi:10.1016/s0002-9343(01)00762-8
- Jackson JL, Cogbill E, Santana-Davila R, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PLoS One 2015; 10(7):e0130733. doi:10.1371/journal.pone.0130733
- International Association for the Study of Pain (IASP). IASP Terminology. www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698&navItemNumber=576. Accessed November 20, 2019.
- Feller L, Khammissa RAG, Fourie J, Bouckaert M, Lemmer J. Postherpectic neuralgia and trigeminal neuralgia. Pain Res Treat 2017; 2017:1681765. doi:10.1155/2017/1681765
- Shillo P, Sloan G, Greig M, et al. Painful and painless diabetic neuropathies: what is the difference? Curr Diab Rep 2019; 19(6):32. doi:10.1007/s11892-019-1150-5
- Schwartzman RJ, Maleki J. Postinjury neuropathic pain syndromes. Med Clin North Am 1999; 83(3):597–626. doi:10.1016/s0025-7125(05)70126-7
- Oaklander AL, Horowitz SH. The complex regional pain syndrome. Handb Clin Neurol 2015; 131:481–503. doi:10.1016/B978-0-444-62627-1.00026-3
- Akyuz G, Kuru P. Systematic review of central post stroke pain: what is happening in the central nervous system? Am J Phys Med Rehabil 2016; 95(8):618–627. doi:10.1097/PHM.0000000000000542
- Shiao R, Lee-Kubli CA. Neuropathic pain after spinal cord injury: challenges and research perspectives. Neurotherapeutics 2018; 15(3):635–653. doi:10.1007/s13311-018-0633-4
- Ceruti S. What role does multiple sclerosis play in the development of untreatable painful conditions? Pain Manag 2018; 8(1):37–44. doi:10.2217/pmt-2017-0038
- Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
- Hearn L, Moore RA, Derry S, Wiffen PJ, Phillips T. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev 2014; (9):CD011003. doi:10.1002/14651858.CD011003.pub2
- Derry S, Whiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev 2015; 1:CD011209. doi:10.1002/14651858.CD011209.pub2
- Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med 2002; 162(1):19–24. doi:10.1001/archinte.162.1.19
- Staiger TO, Gaster B, Sullivan MD, Deyo RA. Systematic review of antidepressants in the treatment of chronic low back pain. Spine (Phila Pa 1976) 2003; 28(22):2540–2545. doi:10.1097/01.BRS.0000092372.73527.BA
- Atkinson JH, Slater MA, Wahlgren DR, et al. Effects of noradrenergic and serotonergic antidepressants on chronic low back pain intensity. Pain 1999; 83(2):137–145. doi:10.1016/s0304-3959(99)00082-2
- Urquhart DM, Hoving JL, Assendelft WW, Roland M, van Tulder MW. Antidepressants for non-specific back pain. Cochrane Database Syst Rev 2008; (1):CD001703. doi:10.1002/14651858.CD001703.pub3
- Urquhart DM, Wluka AE, van Tulder M, et al. Efficacy of low-dose amitriptyline for chronic low back pain: a randomized clinical trial. JAMA Intern Med 2018; 178(11):1474–1481. doi:10.1001/jamainternmed.2018.4222
- Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014; 311(15):1547–1555. doi:10.1001/jama.2014.3266
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics 2000; 41(2):104–113. pmid:10749947
- Hauser W, Wolfe F, Tolle T, Uceyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs 2012; 26(4):297–307. doi:10.2165/11598970-000000000-00000
- Calandre EP, Rico-Villademoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin Pharmacother 2015; 16(9):1347–1368. doi:10.1517/14656566.2015.1047343
- Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 2000; 108(1):65–72. doi:10.1016/s0002-9343(99)00299-5
- Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol 2009; 15(13):1548–1553. doi:10.3748/wjg.15.1548
- Iskandar HN, Cassell B, Kanuri N, et al. Tricyclic antidepressants for management of residual symptoms in inflammatory bowel disease. J Clin Gastroenterol 2014; 48(5):423–429. doi:10.1097/MCG.0000000000000049
- Lee LY, Abbott L, Mahlangu B, Moodie SJ, Anderson S. The management of cyclic vomiting syndrome: a systematic review. Eur J Gastroenterol Hepatol 2012; 24(9):1001–1006. doi:10.1097/MEG.0b013e328355638f
- Thorkelson G, Bielefeldt K, Szigethy E. Empirically supported use of psychiatric medications in adolescents and adults with IBD. Inflamm Bowel Dis 2016; 22(6):1509–1522. doi:10.1097/MIB.0000000000000734
- Braak B, Klooker TK, Wouters MM, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther 2011; 34(6):638–648. doi:10.1111/j.1365-2036.2011.04775.x
- Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA 2013; 310(24):2640–2649. doi:10.1001/jama.2013.282833
- Latthe P, Latthe M, Say L, Gulmezoglu M, Khan KS. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health 2006; 6:177. doi:10.1186/1471-2458-6-177
- Moise G, Capodice J, Winfree CJ. Treatment of chronic pelvic pain in men and women. Expert Rev Neurother 2007; 7(5):507–520. doi:10.1586/14737175.7.5.507
- Lai HH. Management of interstitial cystitis/bladder pain syndrome with tricyclic antidepressants. In: Moldwin RM, ed. Urological and Gynaecological Chronic Pelvic Pain. Cham, Switzerland: Springer; 2017:107–118.
- American Urological Association. Diagnosis and treatment interstitial cystitis/bladder pain syndrome (2014). www.auanet.org/guidelines/interstitial-cystitis/bladder-pain-syndrome-(2011-amended-2014). Accessed November 19, 2019.
- Carey ET, As-Sanie S. New developments in the pharmacotherapy of neuropathic chronic pelvic pain. Future Sci OA 2016; 2(4):FSO148. doi:10.4155/fsoa-2016-0048
- Papandreou C, Skapinakis P, Giannakis D, Sofikitis N, Mavreas V. Antidepressant drugs for chronic urological pelvic pain: an evidence-based review. Adv Urol 2009; 2009:797031. doi:10.1155/2009/797031
- Liu Y, Xu X, Dong M, Jia S, Wei Y. Treatment of insomnia with tricyclic antidepressants: a meta-analysis of polysomnographic randomized controlled trials. Sleep Med 2017; 34:126–133. doi:10.1016/j.sleep.2017.03.007
- Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician 2017; 96(1):29–35. pmid:28671376
- McCall C, McCall WV. What is the role of sedating antidepressants, antipsychotics, and anticonvulsants in the management of insomnia? Curr Psychiatry Rep 2012; 14(5):494–502. doi:10.1007/s11920-012-0302-y
- Clark MS, Smith PO, Jamieson B. FPIN’s clinical inquiries: antidepressants for the treatment of insomnia in patients with depression. Am Fam Physician 2011; 84(9):1–2. pmid:22164891
- Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s Synopsis of Psychiatry. New York, NY: Lippincott Williams & Wilkins; 2014.
- Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J 2018; 54(2):101–112. doi:10.4068/cmj.2018.54.2.101.
- Trindade E, Menon D, Topfer LA, Coloma C. Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. CMAJ 1998; 159(10):1245–1252. pmid:9861221
- Fava M. Prospective studies of adverse events related to antidepressant discontinuation. J Clin Psychiatry 2006; 67(suppl 4):14–21. pmid:16683858
- Gintant G. An evaluation of hERG current assay performance: translating preclinical safety studies to clinical QT prolongation. Pharmacol Ther 2011; 129(2):109–119. doi:10.1016/j.pharmthera.2010.08.008.
- Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54(1):1–13. doi:10.1016/j.psym.2012.11.001
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63(11):2227–2246. doi:10.1111/jgs.13702
- Fukushima N, Nanao K, Fukushima H, Namera A, Miura M. A neonatal prolonged QT syndrome due to maternal use of oral tricyclic antidepressants. Eur J Pediatr 2016; 175(8):1129–1132. doi:10.1007/s00431-016-2722-x
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol 2008; 111(4):1001–1020. doi:10.1097/AOG.0b013e31816fd910
- Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci 2017; 18(11). doi:10.3390/ijms18112483
- Kremer M, Yalcin I, Goumon Y, et al. A dual noradrenergic mechanism for the relief of neuropathic allodynia by the antidepressant drugs duloxetine and amitriptyline. J Neurosci 2018; 38(46):9934–9954. doi:10.1523/JNEUROSCI.1004-18.2018
- Tomkins GE, Jackson JL, O’Malley PG, Balden E, Santoro JE. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med 2001; 111(1):54–63. doi:10.1016/s0002-9343(01)00762-8
- Jackson JL, Cogbill E, Santana-Davila R, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PLoS One 2015; 10(7):e0130733. doi:10.1371/journal.pone.0130733
- International Association for the Study of Pain (IASP). IASP Terminology. www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698&navItemNumber=576. Accessed November 20, 2019.
- Feller L, Khammissa RAG, Fourie J, Bouckaert M, Lemmer J. Postherpectic neuralgia and trigeminal neuralgia. Pain Res Treat 2017; 2017:1681765. doi:10.1155/2017/1681765
- Shillo P, Sloan G, Greig M, et al. Painful and painless diabetic neuropathies: what is the difference? Curr Diab Rep 2019; 19(6):32. doi:10.1007/s11892-019-1150-5
- Schwartzman RJ, Maleki J. Postinjury neuropathic pain syndromes. Med Clin North Am 1999; 83(3):597–626. doi:10.1016/s0025-7125(05)70126-7
- Oaklander AL, Horowitz SH. The complex regional pain syndrome. Handb Clin Neurol 2015; 131:481–503. doi:10.1016/B978-0-444-62627-1.00026-3
- Akyuz G, Kuru P. Systematic review of central post stroke pain: what is happening in the central nervous system? Am J Phys Med Rehabil 2016; 95(8):618–627. doi:10.1097/PHM.0000000000000542
- Shiao R, Lee-Kubli CA. Neuropathic pain after spinal cord injury: challenges and research perspectives. Neurotherapeutics 2018; 15(3):635–653. doi:10.1007/s13311-018-0633-4
- Ceruti S. What role does multiple sclerosis play in the development of untreatable painful conditions? Pain Manag 2018; 8(1):37–44. doi:10.2217/pmt-2017-0038
- Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
- Hearn L, Moore RA, Derry S, Wiffen PJ, Phillips T. Desipramine for neuropathic pain in adults. Cochrane Database Syst Rev 2014; (9):CD011003. doi:10.1002/14651858.CD011003.pub2
- Derry S, Whiffen PJ, Aldington D, Moore RA. Nortriptyline for neuropathic pain in adults. Cochrane Database Syst Rev 2015; 1:CD011209. doi:10.1002/14651858.CD011209.pub2
- Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain: a meta-analysis. Arch Intern Med 2002; 162(1):19–24. doi:10.1001/archinte.162.1.19
- Staiger TO, Gaster B, Sullivan MD, Deyo RA. Systematic review of antidepressants in the treatment of chronic low back pain. Spine (Phila Pa 1976) 2003; 28(22):2540–2545. doi:10.1097/01.BRS.0000092372.73527.BA
- Atkinson JH, Slater MA, Wahlgren DR, et al. Effects of noradrenergic and serotonergic antidepressants on chronic low back pain intensity. Pain 1999; 83(2):137–145. doi:10.1016/s0304-3959(99)00082-2
- Urquhart DM, Hoving JL, Assendelft WW, Roland M, van Tulder MW. Antidepressants for non-specific back pain. Cochrane Database Syst Rev 2008; (1):CD001703. doi:10.1002/14651858.CD001703.pub3
- Urquhart DM, Wluka AE, van Tulder M, et al. Efficacy of low-dose amitriptyline for chronic low back pain: a randomized clinical trial. JAMA Intern Med 2018; 178(11):1474–1481. doi:10.1001/jamainternmed.2018.4222
- Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014; 311(15):1547–1555. doi:10.1001/jama.2014.3266
- Arnold LM, Keck PE Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics 2000; 41(2):104–113. pmid:10749947
- Hauser W, Wolfe F, Tolle T, Uceyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs 2012; 26(4):297–307. doi:10.2165/11598970-000000000-00000
- Calandre EP, Rico-Villademoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin Pharmacother 2015; 16(9):1347–1368. doi:10.1517/14656566.2015.1047343
- Jackson JL, O’Malley PG, Tomkins G, Balden E, Santoro J, Kroenke K. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 2000; 108(1):65–72. doi:10.1016/s0002-9343(99)00299-5
- Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol 2009; 15(13):1548–1553. doi:10.3748/wjg.15.1548
- Iskandar HN, Cassell B, Kanuri N, et al. Tricyclic antidepressants for management of residual symptoms in inflammatory bowel disease. J Clin Gastroenterol 2014; 48(5):423–429. doi:10.1097/MCG.0000000000000049
- Lee LY, Abbott L, Mahlangu B, Moodie SJ, Anderson S. The management of cyclic vomiting syndrome: a systematic review. Eur J Gastroenterol Hepatol 2012; 24(9):1001–1006. doi:10.1097/MEG.0b013e328355638f
- Thorkelson G, Bielefeldt K, Szigethy E. Empirically supported use of psychiatric medications in adolescents and adults with IBD. Inflamm Bowel Dis 2016; 22(6):1509–1522. doi:10.1097/MIB.0000000000000734
- Braak B, Klooker TK, Wouters MM, et al. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther 2011; 34(6):638–648. doi:10.1111/j.1365-2036.2011.04775.x
- Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA 2013; 310(24):2640–2649. doi:10.1001/jama.2013.282833
- Latthe P, Latthe M, Say L, Gulmezoglu M, Khan KS. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health 2006; 6:177. doi:10.1186/1471-2458-6-177
- Moise G, Capodice J, Winfree CJ. Treatment of chronic pelvic pain in men and women. Expert Rev Neurother 2007; 7(5):507–520. doi:10.1586/14737175.7.5.507
- Lai HH. Management of interstitial cystitis/bladder pain syndrome with tricyclic antidepressants. In: Moldwin RM, ed. Urological and Gynaecological Chronic Pelvic Pain. Cham, Switzerland: Springer; 2017:107–118.
- American Urological Association. Diagnosis and treatment interstitial cystitis/bladder pain syndrome (2014). www.auanet.org/guidelines/interstitial-cystitis/bladder-pain-syndrome-(2011-amended-2014). Accessed November 19, 2019.
- Carey ET, As-Sanie S. New developments in the pharmacotherapy of neuropathic chronic pelvic pain. Future Sci OA 2016; 2(4):FSO148. doi:10.4155/fsoa-2016-0048
- Papandreou C, Skapinakis P, Giannakis D, Sofikitis N, Mavreas V. Antidepressant drugs for chronic urological pelvic pain: an evidence-based review. Adv Urol 2009; 2009:797031. doi:10.1155/2009/797031
- Liu Y, Xu X, Dong M, Jia S, Wei Y. Treatment of insomnia with tricyclic antidepressants: a meta-analysis of polysomnographic randomized controlled trials. Sleep Med 2017; 34:126–133. doi:10.1016/j.sleep.2017.03.007
- Matheson E, Hainer BL. Insomnia: pharmacologic therapy. Am Fam Physician 2017; 96(1):29–35. pmid:28671376
- McCall C, McCall WV. What is the role of sedating antidepressants, antipsychotics, and anticonvulsants in the management of insomnia? Curr Psychiatry Rep 2012; 14(5):494–502. doi:10.1007/s11920-012-0302-y
- Clark MS, Smith PO, Jamieson B. FPIN’s clinical inquiries: antidepressants for the treatment of insomnia in patients with depression. Am Fam Physician 2011; 84(9):1–2. pmid:22164891
- Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s Synopsis of Psychiatry. New York, NY: Lippincott Williams & Wilkins; 2014.
- Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J 2018; 54(2):101–112. doi:10.4068/cmj.2018.54.2.101.
- Trindade E, Menon D, Topfer LA, Coloma C. Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. CMAJ 1998; 159(10):1245–1252. pmid:9861221
- Fava M. Prospective studies of adverse events related to antidepressant discontinuation. J Clin Psychiatry 2006; 67(suppl 4):14–21. pmid:16683858
- Gintant G. An evaluation of hERG current assay performance: translating preclinical safety studies to clinical QT prolongation. Pharmacol Ther 2011; 129(2):109–119. doi:10.1016/j.pharmthera.2010.08.008.
- Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC. QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 2013; 54(1):1–13. doi:10.1016/j.psym.2012.11.001
- American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63(11):2227–2246. doi:10.1111/jgs.13702
- Fukushima N, Nanao K, Fukushima H, Namera A, Miura M. A neonatal prolonged QT syndrome due to maternal use of oral tricyclic antidepressants. Eur J Pediatr 2016; 175(8):1129–1132. doi:10.1007/s00431-016-2722-x
- ACOG Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol 2008; 111(4):1001–1020. doi:10.1097/AOG.0b013e31816fd910
KEY POINTS
- Amitriptyline is the most useful TCA for many painful conditions.
- TCAs can be especially helpful for patients with a pain syndrome or insomnia with comorbid depression, although their benefits appear to be independent of antidepressant effects.
- TCAs have long half-lives and so can be taken once a day.
- Effective dosages for symptom control in many conditions are lower than for severe depression; dosage should start low and be gradually increased while monitoring efficacy and adverse effects.
- TCAs should not be used concurrently with a monoamine oxidase inhibitor and by certain patient groups: the elderly, pregnant women, and patients with certain cardiac conduction abnormalities, epilepsy, or risk of suicide.
Gabapentin for alcohol use disorder: A good option, or cause for concern?
Perceptions regarding the use of gabapentin for alcohol use disorder (AUD) have shifted over time.1–4 Early on, the drug was deemed to be benign and effective.4–6 But more and more, concerns are being raised about its recreational use to achieve euphoria,7 and the drug is often misused by vulnerable populations, particularly those with opioid use disorder.7–9
Given the large number of gabapentin prescriptions written off-label for AUD, it is incumbent on providers to understand how to prescribe it responsibly.7–9 To that end, this article focuses on the benefits—and concerns—of this treatment option. We describe the effects of gabapentin on the central nervous system and how it may mitigate alcohol withdrawal and increase the likelihood of abstinence. In addition, we review clinical trials that evaluated potential roles of gabapentin in AUD, discuss the drug’s misuse potential, and suggest a framework for its appropriate use in AUD management.
ALCOHOL USE DISORDER IS COMMON AND SERIOUS
AUD affects about 14% of US adults and represents a significant health burden,1 often with severe clinical and social implications. It manifests as compulsive drinking and loss of control despite adverse consequences on various life domains.10 It is generally associated with cravings, tolerance, and withdrawal symptoms upon cessation. Alcohol withdrawal is characterized by tremors, anxiety, sweating, nausea, and tachycardia, and in severe cases, may involve hallucinations, seizures, and delirium tremens. Untreated, alcohol withdrawal can be fatal.10
Even though psychosocial treatments for AUD by themselves are associated with high relapse rates, pharmacotherapy is underutilized. Three drugs approved by the US Food and Drug Administration (FDA) are available to treat it, but they are often poorly accepted and have limited efficacy. For these reasons, there is considerable interest in finding alternatives. Gabapentin is one of several agents that have been studied (Table 1). The topic has been reviewed in depth by Soyka and Müller.11
GABAPENTIN REDUCES EXCITATION
The anticonvulsant gabapentin is FDA-approved for treating epilepsy, postherpetic neuralgia, and restless leg syndrome.8,12–14 It binds and selectively impedes voltage-sensitive calcium channels, the pores in cell membrane that permit calcium to enter a neuron in response to changes in electrical currents.15
Gabapentin is believed to decrease excitation of the central nervous system in multiple ways:
- It reduces the release of glutamate, a key component of the excitatory system16
- It increases the concentration of gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain7
- By binding the alpha-2-delta type 1 subunit of voltage-sensitive calcium channels,8,15–17 it inhibits excitatory synapse formation independent of calcium channel activity16
- By blocking excitatory neurotransmission, it also may indirectly increase the concentration of GABA in the central nervous system16,17
- It modulates action of glutamic acid decarboxylase (involved in the synthesis of GABA) and glutamate synthesizing enzyme to increase GABA and decrease glutamate.17
ALCOHOL’S ACTIONS
The actions of alcohol on the brain are also complex.18 Alpha-2-delta type 1 subunits of calcium channels are upregulated in the reward centers of the brain by addictive substances, including alcohol.16 Alcohol interacts with corticotropin-releasing factor and several neurotransmitters,18 and specifically affects neuropathways involving norepinephrine, GABA, and glutamate.19 Alcohol has reinforcing effects mediated by the release of dopamine in the nucleus accumbens.20
Acutely, alcohol promotes GABA release and may also reduce GABA degradation, producing sedative and anxiolytic effects.21 Chronic alcohol use leads to a decrease in the number of GABAA receptors. Clinically, this downregulation manifests as tolerance to alcohol’s sedating effects.21
Alcohol affects the signaling of glutamatergic interaction with the N-methyl-d-aspartate (NMDA) receptor.22 Glutamate activates this receptor as well as the voltage-gated ion channels, modifying calcium influx and increasing neuronal excitability.22,23 Acutely, alcohol has an antagonistic effect on the NMDA receptor, while chronic drinking upregulates (increases) the number of NMDA receptors and voltage-gated calcium channels.22,23
Alcohol withdrawal increases excitatory effects
Patients experiencing alcohol withdrawal have decreased GABA-ergic functioning and increased glutamatergic action throughout the central nervous system.19,24
Withdrawal can be subdivided into an acute phase (lasting up to about 5 days) and a protracted phase (of undetermined duration). During withdrawal, the brain activates its “stress system,” leading to overexpression of corticotropin-releasing factor in the amygdala. Protracted withdrawal dysregulates the prefrontal cortex, increasing cravings and worsening negative emotional states and sleep.16
GABAPENTIN FOR ALCOHOL WITHDRAWAL
Benzodiazepines are the standard treatment for alcohol withdrawal.3,24 They relieve symptoms and can prevent seizures and delirium tremens,24 but they are sedating and cause psychomotor impairments.3 Because of the potential for addiction, benzodiazepine use is limited to acute alcohol withdrawal.3
Gabapentin shows promise as an agent that can be used in withdrawal and continued through early abstinence without the highly addictive potential of benzodiazepines.16 It is thought to affect drinking behaviors during early abstinence by normalizing GABA and glutamate activity.2,16
Early preclinical studies in mouse models found that gabapentin decreases anxiogenic and epileptic effects of alcohol withdrawal. Compared with other antidrinking medications, gabapentin has the benefits of lacking elimination via hepatic metabolism, few pharmacokinetic interactions, and good reported tolerability in this population.
Inpatient trials show no benefit over standard treatments
Bonnet et al25 conducted a double-blind placebo-controlled trial in Germany in inpatients experiencing acute alcohol withdrawal to determine whether gabapentin might be an effective adjunct to clomethiazole, a GABAA modulator commonly used in Europe for alcohol withdrawal. Participants (N = 61) were randomized to receive placebo or gabapentin (400 mg every 6 hours) for 72 hours, with tapering over the next 3 days. All patients could receive rescue doses of clomethiazole, using a symptom-triggered protocol.
The study revealed no differences in the amount of clomethiazole administered between the 2 groups, suggesting that gabapentin had no adjunctive effect. Side effects (vertigo, nausea, dizziness, and ataxia) were mild and comparable between groups.
Nichols et al26 conducted a retrospective cohort study in a South Carolina academic psychiatric hospital to assess the adjunctive effect of gabapentin on the as-needed use of benzodiazepines for alcohol withdrawal. The active group (n = 40) received gabapentin as well as a symptom-triggered alcohol withdrawal protocol of benzodiazepine. The control group (n = 43) received only the symptom-triggered alcohol withdrawal protocol without gabapentin.
No effect was found of gabapentin use for benzodiazepine treatment of alcohol withdrawal. It is notable that Bonnet et al and Nichols et al had similar findings despite their studies being conducted in different countries using distinct comparators and methods.
Bonnet et al,27 in another study, tried a different design to investigate a possible role for gabapentin in inpatient alcohol withdrawal. The study included 37 patients with severe alcohol withdrawal (Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-Ar] > 15).
All participants received gabapentin 800 mg. Those whose CIWA-Ar score improved within 2 hours were considered “early responders” (n = 27) and next received 2 days of gabapentin 600 mg 4 times a day before starting a taper. The nonresponders whose CIWA-Ar score worsened (associated with greater anxiety and depressive symptoms; n = 10) were switched to standard treatment with clomethiazole (n = 4) or clonazepam (n = 6). Scores of 3 early responders subsequently worsened; 2 of these participants developed seizures and were switched to standard treatment.
The authors concluded that gabapentin in a dose of 3,200 mg in the first 24 hours is useful only for milder forms of alcohol withdrawal. Hence, subsequent efforts on the use of gabapentin for alcohol withdrawal have focused on outpatients.
Outpatient trials reveal benefits over benzodiazepines
Myrick et al3 compared gabapentin vs lorazepam in 100 outpatients seeking treatment for alcohol withdrawal. Participants were randomized to 1 of 4 groups: gabapentin 600 mg, 900 mg, or 1,200 mg, or lorazepam 6 mg, each tapering over 4 days. Alcohol withdrawal was measured by the CIWA-Ar score. Only 68 patients completed all follow-up appointments to day 12.
Gabapentin 600 mg was discontinued because of seizures in 2 patients, but it was generally well tolerated and was associated with diminished symptoms of alcohol withdrawal, especially at the 1,200 mg dose. The gabapentin groups experienced less anxiety and sedation and fewer cravings than the lorazepam group. Those treated with lorazepam fared worse for achieving early abstinence and were more likely to return to drinking when the intervention was discontinued. However, significant relapse by day 12 occurred in both groups.
The authors concluded that gabapentin was at least as effective as lorazepam in the outpatient treatment of alcohol withdrawal, with the 1,200-mg gabapentin dosage being more effective than 900 mg. At 1,200 mg, gabapentin was associated with better sleep, less anxiety, and better self-reported ability to work than lorazepam, and at the 900-mg dose it was associated with less depression than lorazepam.
Stock et al28 conducted a randomized, double-blind study of gabapentin in acute alcohol withdrawal in 26 military veterans in an outpatient setting. Patients were randomized to one of the following:
- Gabapentin 1,200 mg orally for 3 days, followed by 900 mg, 600 mg, and 300 mg for 1 day each (n = 17)
- Chlordiazepoxide 100 mg orally for 3 days, followed by 75 mg, 50 mg, and 25 mg for 1 day each (n = 9).
Withdrawal scores improved similarly in both groups. Early on (days 1–4), neither cravings nor sleep differed significantly between groups; but later (days 5–7), the gabapentin group had superior scores for these measures. Gabapentin was also associated with significantly less sedation than chlordiazepoxide and trended to less alcohol craving.
Bottom line: Gabapentin is useful for mild withdrawal
Data suggest that gabapentin offers benefits for managing mild alcohol withdrawal. Improved residual craving and sleep measures are clinically important because they are risk factors for relapse. Mood and anxiety also improve with gabapentin, further indicating a therapeutic effect.
Gabapentin’s benefits for moderate and severe alcohol withdrawal have not been established. Seizures occurred during withdrawal despite gabapentin treatment, but whether from an insufficient dose, patient susceptibility, or lack of gabapentin efficacy is not clear. Best results occurred at the 1,200-mg daily dose, but benefits may not apply to patients with severe withdrawal. In addition, many studies were small, limiting the strength of conclusions.
Across most studies of gabapentin for alcohol withdrawal, advantages included a smoother transition into early abstinence due to improved sleep, mood, and anxiety, alleviating common triggers for a return to drinking. Gabapentin also carries less reinforcing potential than benzodiazepines. These qualities fueled interest in trying gabapentin to improve long-term abstinence.
GABAPENTIN FOR RELAPSE PREVENTION
Although naltrexone and acamprosate are the first-line treatments for relapse prevention, they do not help all patients and are more effective when combined with cognitive behavioral therapy.1,29,30 For patients in whom standard treatments are not effective or tolerated, gabapentin may provide a reasonable alternative, and several randomized controlled trials have examined its use for this role.
Gabapentin alone is better than placebo
Furieri and Nakamura-Palacios4 assessed the use of gabapentin for relapse prevention in Brazilian outpatients (N = 60) who had averaged 27 years of drinking and consumed 17 drinks daily for the 90 days before baseline. After detoxification with diazepam and vitamins, patients were randomized to either gabapentin 300 mg twice daily or placebo for 4 weeks.
Compared with placebo, gabapentin significantly reduced cravings and lowered the percentage of heavy drinking days and the number of drinks per day, with a significant increase in the percentage of abstinent days. These self-reported measures correlated with decreases in gamma-glutamyl transferase, a biological marker for heavy drinking.
Brower et al31 investigated the use of gabapentin in 21 outpatients with AUD and insomnia who desired to remain abstinent. They were randomized to gabapentin (up to 1,500 mg at night) or placebo for 6 weeks. Just 14 participants completed the study; all but 2 were followed without treatment until week 12.
Gabapentin was associated with significantly lower relapse rates at 6 weeks (3 of 10 in the gabapentin group vs 9 of 11 in the placebo group) and at 12 weeks (6 of 10 in the gabapentin group vs 11 of 11 in the placebo group, assuming the 2 patients lost to follow-up relapsed). No difference between groups was detected for sleep measures in this small study. However, other studies have found that gabapentin for AUD improves measures of insomnia and daytime drowsiness—predictors of relapse—compared with other medications.16
High-dose gabapentin is better
Mason et al2 randomized 150 outpatients with alcohol dependence to 12 weeks of daily treatment with either gabapentin (900 mg or 1,800 mg) or placebo after at least 3 days of abstinence. All participants received counseling. Drinking quantity and frequency were assessed by gamma-glutamyl transferase testing.
Patients taking gabapentin had better rates of abstinence and cessation of heavy drinking than those taking placebo. During the 12-week study, the 1,800-mg daily dose showed a substantially higher abstinence rate (17%) than either 900 mg (11%) or placebo (4%). Significant dose-related improvements were also found for heavy drinking days, total drinking quantity, and frequency of alcohol withdrawal symptoms that predispose to early relapse, such as poor sleep, cravings, and poor mood. There were also significant linear dose effects on rates of abstinence and nondrinking days at the 24-week posttreatment follow-up.
Gabapentin plus naltrexone is better than naltrexone alone
Anton et al5 examined the efficacy of gabapentin combined with naltrexone during early abstinence. The study randomly assigned 150 people with AUD to one of the following groups:
- 16 weeks of naltrexone (50 mg/day) alone
- 6 weeks of naltrexone (50 mg/day) plus gabapentin (up to 1,200 mg/day), followed by 10 weeks of naltrexone alone
- Placebo.
All participants received medical management.
Over the first 6 weeks, those receiving naltrexone plus gabapentin had a longer interval to heavy drinking than those taking only naltrexone. By week 6, about half of those taking placebo or naltrexone alone had a heavy drinking day, compared with about 35% of those taking naltrexone plus gabapentin. Those receiving the combination also had fewer days of heavy drinking, fewer drinks per drinking day, and better sleep than the other groups. Participants in the naltrexone-alone group were more likely to drink heavily during periods in which they reported poor sleep. No significant group differences were found in measures of mood.
Gabapentin enacarbil is no better than placebo
Falk et al,32 in a 2019 preliminary analysis, examined data from a trial of gabapentin enacarbil, a prodrug formulation of gabapentin. In this 6-month double-blind study, 346 people with moderate AUD at 10 sites were randomized to gabapentin enacarbil extended-release 600 mg twice a day or placebo. All subjects received a computerized behavioral intervention.
No significant differences between groups were found in drinking measures or alcohol cravings, sleep problems, depression, or anxiety symptoms. However, a dose-response analysis found significantly less drinking for higher doses of the drug.
Bottom line: Evidence of benefits mixed but risk low
The efficacy of gabapentin as a treatment for AUD has varied across studies as a function of dosing and formulation. Daily doses have ranged from 600 mg to 1,800 mg, with the highest dose showing advantages in one study for cravings, insomnia, anxiety, dysphoria, and relapse.2 Thus far, gabapentin immediate-release has performed better than gabapentin enacarbil extended-release. All forms of gabapentin have been well-tolerated in AUD trials.
The 2018 American Psychiatric Association guidelines stated that gabapentin had a small positive effect on drinking outcomes, but the harm of treatment was deemed minimal, especially relative to the harms of chronic drinking.33 The guidelines endorse the use of gabapentin in patients with moderate to severe AUD who select gabapentin from the available options, or for those who are nonresponsive to or cannot tolerate naltrexone or acamprosate, as long as no contraindications exist. It was also noted that even small effects may be clinically important, considering the significant morbidity associated with AUD.
POTENTIAL FOR MISUSE
The use of gabapentin has become controversial owing to the growing recognition that it may not be as benign as initially thought.7–9,34 A review of US legislative actions reflects concerns about its misuse.35 In July 2017, Kentucky classified it as a schedule V controlled substance with prescription drug monitoring,35 as did Tennessee in 201836 and Michigan in January 2019.37 Currently, 8 other states (Massachusetts, Minnesota, Nebraska, North Dakota, Ohio, Virginia, Wyoming, and West Virginia) require prescription drug monitoring of gabapentin, and other states are considering it.35
Efforts to understand gabapentin misuse derive largely from people with drug use disorders. A review of postmortem toxicology reports in fatal drug overdoses found gabapentin present in 22%.38 Although it was not necessarily a cause of death, its high rate of detection suggests wide misuse among drug users.
Among a cohort of 503 prescription opioid misusers in Appalachian Kentucky, 15% reported using gabapentin “to get high.” Those who reported misusing gabapentin were 6 times more likely than nonusers to be abusing opioids and benzodiazepines. The main sources of gabapentin were doctors (52%) and dealers (36%). The average cost of gabapentin on the street was less than $1.00 per pill.39
Gabapentin misuse by methadone clinic patients is also reported. Baird et al40 surveyed patients in 6 addiction clinics in the United Kingdom for gabapentin and pregabalin abuse and found that 22% disclosed misusing these medications. Of these, 38% said they did so to enhance the methadone high.
In a review article, Quintero41 also cited enhancement of methadone euphoria and treatment of opioid withdrawal as motivations for misuse. Opioid-dependent gabapentin misusers consumed doses of gabapentin 3 to 20 times higher than clinically recommended and in combination with multiple drugs.4 Such use can cause dissociative and psychedelic effects.
Gabapentin also potentiates the sedative effects of opioids, thus increasing the risk of falls, accidents, and other adverse events.34,35 Risk of opioid-related deaths was increased with coprescription of gabapentin and with moderate to high gabapentin doses.34
Are people with AUD at higher risk of gabapentin abuse?
Despite concerns, patients in clinical trials of gabapentin treatment for AUD were not identified as at high risk for misuse of the drug.2,4,5,16 Further, no such trials reported serious drug-related adverse events resulting in gabapentin discontinuation or side effects that differed from placebo in frequency or severity.2,4,5,16
Clinical laboratory studies also have found no significant interactions between alcohol and gabapentin.42,43 In fact, they showed no influence of gabapentin on the pharmacokinetics of alcohol or on alcohol’s subjective effects. Relative to placebo, gabapentin did not affect blood alcohol levels, the degree of intoxication, sedation, craving, or alcohol self-administration.
Smith et al9 reported estimates that only 1% of the general population misuse gabapentin. Another review concluded that gabapentin is seldom a drug of choice.17 Most patients prescribed gabapentin do not experience cravings or loss of control, which are hallmarks of addiction. Hence, with adequate precautions, the off-label use of gabapentin for AUD is reasonable.
CLINICAL IMPLICATIONS OF GABAPENTIN PRESCRIBING
Overall, evidence for the benefit of gabapentin in AUD is mixed. Subgroups of alcoholic patients, such as those who do not respond to or tolerate standard therapies, may particularly benefit, as may those with comorbid insomnia or neuropathic pain.44 Clinicians should prescribe gabapentin only when it is likely to be helpful and should carefully document its efficacy.2,45
At each visit, an open and honest assessment of the benefits and risks serves to promote shared decision-making regarding initiating, continuing, or discontinuing gabapentin.
For alcohol withdrawal
Before gabapentin is prescribed for alcohol withdrawal, potential benefits (reduction of withdrawal symptoms), side effects (sedation, fatigue), and risks (falls) should be discussed with the patient.46 Patients should also be informed that benzodiazepines are the gold standard for alcohol withdrawal and that gabapentin is not effective for severe withdrawal.46
For relapse prevention
When initiating treatment for relapse prevention, the patient and the prescriber should agree on specific goals (eg, reduction of drinking, anxiety, and insomnia).2,16 Ongoing monitoring is essential and includes assessing and documenting improvement with respect to these goals.
In the AUD studies, gabapentin was well tolerated.16 Frequently observed side effects including headache, insomnia, fatigue, muscle aches, and gastrointestinal distress did not occur at a statistically different rate from placebo. However, patients in studies are selected samples, and their experience may not be generalizable to clinical practice. Thus, it is necessary to exercise caution and check for comorbidities that may put patients at risk of complications.47 Older patients and those on hemodialysis are more susceptible to gabapentin side effects such as sedation, dizziness, ataxia, and mental status changes,34 and prescribers should be alert for signs of toxicity (eg, ataxia, mental status changes).47,48
Gabapentin misuse was not observed in AUD studies,2,4,5,16 but evidence indicates that patients with opioid use disorder, prisoners, and polydrug users are at high risk for gabapentin misuse.39–41 In all cases, clinicians should monitor for red flags that may indicate abuse, such as missed appointments, early refill requests, demands for increased dosage, and simultaneous opiate and benzodiazepine use.49
Acknowledgment: The authors wish to thank Nick Mulligan for his invaluable assistance with formatting and grammar.
- Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA 2018; 320(8):815–824. doi:10.1001/jama.2018.11406
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med 2014; 174(1):70–77. doi:10.1001/jamainternmed.2013.11950
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res 2009; 33(9):1582–1588. doi:10.1111/j.1530-0277.2009.00986.x
- Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2007; 68(11):1691–1700. pmid:18052562
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry 2011; 168(7):709–717. doi:10.1176/appi.ajp.2011.10101436
- Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003; 9(6):559–568. doi:10.18553/jmcp.2003.9.6.559
- Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs 2014; 28(6):491–496. doi:10.1007/s40263-014-0164-4
- Goodman CW, Brett AS. Gabapentin and pregabalin for pain—is increased prescribing a cause for concern? N Engl J Med 2017; 377(5):411–414. doi:10.1056/NEJMp1704633
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction 2016; 111(7):1160–1174. doi:10.1111/add.13324
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Soyka M, Müller CA. Pharmacotherapy of alcoholism—an update on approved and off-label medications. Expert Opin Pharmacother 2017; 18(12):1187-1199. doi:10.1080/14656566.2017.1349098
- Zhang M, Gao CX, Ma KT, et al. A meta-analysis of therapeutic efficacy and safety of gabapentin i n the treatment of postherpetic neuralgia from randomized controlled trials. Biomed Res Int 2018; 2018:7474207. doi:10.1155/2018/7474207
- Winkelmann J, Allen RP, Högl B, et al. Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (Revised 2017). Mov Disord 2018; 33(7):1077–1091. doi:10.1002/mds.27260
- Honarmand A, Safavi M, Zare M. Gabapentin: an update of its pharmacological properties and therapeutic use in epilepsy. J Res Med Sci 2011; 16(8):1062–1069. pmid:22279483
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol 2002; 449(3):221–228. doi:10.1016/s0014-2999(02)02044-7
- Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs 2018; 27(1):113–124. doi:10.1080/13543784.2018.1417383
- Taylor CP. Mechanisms of action of gabapentin. Rev Neurol (Paris) 1997; 153(suppl 1):S39–S45. pmid:9686247
- Agoglia AE, Herman MA. The center of the emotional universe: alcohol, stress, and CRF1 amygdala circuitry. Alcohol 2018; 72:61–73. doi:10.1016/j.alcohol.2018.03.009
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int 1995; 26(4):305–336. pmid:7633325
- You C, Vandegrift B, Brodie MS. Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacology (Berl) 2018; 235(6):1711–1726. doi:10.1007/s00213-018-4875-y
- Lovinger DM. Presynaptic ethanol actions: potential roles in ethanol seeking. Handb Exp Pharmacol 2018; 248:29–54. doi:10.1007/164_2017_76
- Williams SB, Yorgason JT, Nelson AC, et al. Glutamate transmission to ventral tegmental area GABA neurons is altered by acute and chronic ethanol. Alcohol Clin Exp Res 2018; 42(11):2186–2195. doi:10.1111/acer.13883
- N’Gouemo P. Voltage-sensitive calcium channels in the brain: relevance to alcohol intoxication and withdrawal. Handb Exp Pharmacol 2018; 248:263–280. doi:10.1007/164_2018_93
- Modesto-Lowe V, Huard J, Conrad C. Alcohol withdrawal kindling: is there a role for anticonvulsants? Psychiatry (Edgmont) 2005; 2(5):25–31. pmid:21152146
- Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol 2003; 23(5):514–519. doi:10.1097/01.jcp.0000088905.24613.ad
- Nichols TA, Robert S, Taber DJ, Cluver J. Alcohol withdrawal-related outcomes associated with gabapentin use in an inpatient psychiatric facility. Ment Health Clin 2019 ; 9(1):1–5. doi:10.9740/mhc.2019.01.001
- Bonnet U, Hamzavi-Abedi R, Specka M, Wiltfang J, Lieb B, Scherbaum N. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol 2010; 45(2):143–145. doi:10.1093/alcalc/agp085
- Stock CJ, Carpenter L, Ying J, Greene T. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother 2013; 47(7–8):961–969. doi:10.1345/aph.1R751
- Blanco-Gandía MC, Rodríguez-Arias M. Pharmacological treatments for opiate and alcohol addiction: a historical perspective of the last 50 years. Eur J Pharmacol 2018; 836:89–101. doi:10.1016/j.ejphar.2018.08.007
- Anton RF, Moak DH, Latham P, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol 2005; 25(4):349–357. pmid:16012278
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res 2008; 32(8):1429–1438. doi:10.1111/j.1530-0277.2008.00706.x
- Falk DE, Ryan ML, Fertig JB, et al; National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG) Study Group. Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol Clin Exp Res 2019; 43(1):158–169. doi:10.1111/acer.13917
- The American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed October 10, 2019.
- Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med 2017; 14(10):e1002396. doi:10.1371/journal.pmed.1002396
- Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy 2018; 11:109–116. doi:10.2147/RMHP.S168504
- Tennessee Pharmacists Association. Advocacy alert: end of session summary. www.tnpharm.org/news/news-posts-pages/advocacy-alert-4-30-18/? Accessed October 10, 2019.
- Michigan.gov. Gabapentin scheduled as controlled substance to help with state’s opioid epidemic. www.michigan.gov/som/0,4669,7-192-47796-487050--,00.html. Accessed October 10, 2019.
- Slavova S, Miller A, Bunn TL, et al. Prevalence of gabapentin in drug overdose postmortem toxicology testing results. Drug Alcohol Depend 2018; 186:80–85. doi:10.1016/j.drugalcdep.2018.01.018
- Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry 2015; 172(5):487–488. doi:10.1176/appi.ajp.2014.14101272
- Baird CR, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res 2014; 20(3):115–118. doi:10.1159/000355268
- Quintero GC. Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol 2017; 9:13–21. doi:10.2147/JEP.S124391
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend 2006; 83(1):25–32. doi:10.1016/j.drugalcdep.2005.10.008
- Myrick H, Anton R, Voronin K, Wang W, Henderson S. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res 2007; 31(2):221–227. doi:10.1111/j.1530-0277.2006.00299.x
- Tzellos TG, Papazisis G, Toulis KA, Sardeli CH, Kouvelas D. A2delta ligands gabapentin and pregabalin: future implications in daily clinical practice. Hippokratia 2010; 14(2):71–75. pmid:20596259
- Morrison EE, Sandilands EA, Webb DJ. Gabapentin and pregabalin: do the benefits outweigh the harms? J R Coll Physicians Edinb 2017; 47(4):310–313. doi:10.4997/JRCPE.2017.402
- Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics 2018; 59(5):496–505. doi:10.1016/j.psym.2018.03.002
- Fleet JL, Dixon SN, Kuwornu PJ, et al. Gabapentin dose and the 30-day risk of altered mental status in older adults: a retrospective population-based study. PLoS One 2018; 13(3):e0193134. doi:10.1371/journal.pone.0193134
- Chiappini S, Schifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs 2016; 30(7):647–654. doi:10.1007/s40263-016-0359-y
- Modesto-Lowe V, Chaplin M, Sinha S, Woodard K. Universal precautions to reduce stimulant misuse in treating adult ADHD. Cleve Clin J Med 2015; 82(8):506–512. doi:10.3949/ccjm.82a.14131
Perceptions regarding the use of gabapentin for alcohol use disorder (AUD) have shifted over time.1–4 Early on, the drug was deemed to be benign and effective.4–6 But more and more, concerns are being raised about its recreational use to achieve euphoria,7 and the drug is often misused by vulnerable populations, particularly those with opioid use disorder.7–9
Given the large number of gabapentin prescriptions written off-label for AUD, it is incumbent on providers to understand how to prescribe it responsibly.7–9 To that end, this article focuses on the benefits—and concerns—of this treatment option. We describe the effects of gabapentin on the central nervous system and how it may mitigate alcohol withdrawal and increase the likelihood of abstinence. In addition, we review clinical trials that evaluated potential roles of gabapentin in AUD, discuss the drug’s misuse potential, and suggest a framework for its appropriate use in AUD management.
ALCOHOL USE DISORDER IS COMMON AND SERIOUS
AUD affects about 14% of US adults and represents a significant health burden,1 often with severe clinical and social implications. It manifests as compulsive drinking and loss of control despite adverse consequences on various life domains.10 It is generally associated with cravings, tolerance, and withdrawal symptoms upon cessation. Alcohol withdrawal is characterized by tremors, anxiety, sweating, nausea, and tachycardia, and in severe cases, may involve hallucinations, seizures, and delirium tremens. Untreated, alcohol withdrawal can be fatal.10
Even though psychosocial treatments for AUD by themselves are associated with high relapse rates, pharmacotherapy is underutilized. Three drugs approved by the US Food and Drug Administration (FDA) are available to treat it, but they are often poorly accepted and have limited efficacy. For these reasons, there is considerable interest in finding alternatives. Gabapentin is one of several agents that have been studied (Table 1). The topic has been reviewed in depth by Soyka and Müller.11
GABAPENTIN REDUCES EXCITATION
The anticonvulsant gabapentin is FDA-approved for treating epilepsy, postherpetic neuralgia, and restless leg syndrome.8,12–14 It binds and selectively impedes voltage-sensitive calcium channels, the pores in cell membrane that permit calcium to enter a neuron in response to changes in electrical currents.15
Gabapentin is believed to decrease excitation of the central nervous system in multiple ways:
- It reduces the release of glutamate, a key component of the excitatory system16
- It increases the concentration of gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain7
- By binding the alpha-2-delta type 1 subunit of voltage-sensitive calcium channels,8,15–17 it inhibits excitatory synapse formation independent of calcium channel activity16
- By blocking excitatory neurotransmission, it also may indirectly increase the concentration of GABA in the central nervous system16,17
- It modulates action of glutamic acid decarboxylase (involved in the synthesis of GABA) and glutamate synthesizing enzyme to increase GABA and decrease glutamate.17
ALCOHOL’S ACTIONS
The actions of alcohol on the brain are also complex.18 Alpha-2-delta type 1 subunits of calcium channels are upregulated in the reward centers of the brain by addictive substances, including alcohol.16 Alcohol interacts with corticotropin-releasing factor and several neurotransmitters,18 and specifically affects neuropathways involving norepinephrine, GABA, and glutamate.19 Alcohol has reinforcing effects mediated by the release of dopamine in the nucleus accumbens.20
Acutely, alcohol promotes GABA release and may also reduce GABA degradation, producing sedative and anxiolytic effects.21 Chronic alcohol use leads to a decrease in the number of GABAA receptors. Clinically, this downregulation manifests as tolerance to alcohol’s sedating effects.21
Alcohol affects the signaling of glutamatergic interaction with the N-methyl-d-aspartate (NMDA) receptor.22 Glutamate activates this receptor as well as the voltage-gated ion channels, modifying calcium influx and increasing neuronal excitability.22,23 Acutely, alcohol has an antagonistic effect on the NMDA receptor, while chronic drinking upregulates (increases) the number of NMDA receptors and voltage-gated calcium channels.22,23
Alcohol withdrawal increases excitatory effects
Patients experiencing alcohol withdrawal have decreased GABA-ergic functioning and increased glutamatergic action throughout the central nervous system.19,24
Withdrawal can be subdivided into an acute phase (lasting up to about 5 days) and a protracted phase (of undetermined duration). During withdrawal, the brain activates its “stress system,” leading to overexpression of corticotropin-releasing factor in the amygdala. Protracted withdrawal dysregulates the prefrontal cortex, increasing cravings and worsening negative emotional states and sleep.16
GABAPENTIN FOR ALCOHOL WITHDRAWAL
Benzodiazepines are the standard treatment for alcohol withdrawal.3,24 They relieve symptoms and can prevent seizures and delirium tremens,24 but they are sedating and cause psychomotor impairments.3 Because of the potential for addiction, benzodiazepine use is limited to acute alcohol withdrawal.3
Gabapentin shows promise as an agent that can be used in withdrawal and continued through early abstinence without the highly addictive potential of benzodiazepines.16 It is thought to affect drinking behaviors during early abstinence by normalizing GABA and glutamate activity.2,16
Early preclinical studies in mouse models found that gabapentin decreases anxiogenic and epileptic effects of alcohol withdrawal. Compared with other antidrinking medications, gabapentin has the benefits of lacking elimination via hepatic metabolism, few pharmacokinetic interactions, and good reported tolerability in this population.
Inpatient trials show no benefit over standard treatments
Bonnet et al25 conducted a double-blind placebo-controlled trial in Germany in inpatients experiencing acute alcohol withdrawal to determine whether gabapentin might be an effective adjunct to clomethiazole, a GABAA modulator commonly used in Europe for alcohol withdrawal. Participants (N = 61) were randomized to receive placebo or gabapentin (400 mg every 6 hours) for 72 hours, with tapering over the next 3 days. All patients could receive rescue doses of clomethiazole, using a symptom-triggered protocol.
The study revealed no differences in the amount of clomethiazole administered between the 2 groups, suggesting that gabapentin had no adjunctive effect. Side effects (vertigo, nausea, dizziness, and ataxia) were mild and comparable between groups.
Nichols et al26 conducted a retrospective cohort study in a South Carolina academic psychiatric hospital to assess the adjunctive effect of gabapentin on the as-needed use of benzodiazepines for alcohol withdrawal. The active group (n = 40) received gabapentin as well as a symptom-triggered alcohol withdrawal protocol of benzodiazepine. The control group (n = 43) received only the symptom-triggered alcohol withdrawal protocol without gabapentin.
No effect was found of gabapentin use for benzodiazepine treatment of alcohol withdrawal. It is notable that Bonnet et al and Nichols et al had similar findings despite their studies being conducted in different countries using distinct comparators and methods.
Bonnet et al,27 in another study, tried a different design to investigate a possible role for gabapentin in inpatient alcohol withdrawal. The study included 37 patients with severe alcohol withdrawal (Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-Ar] > 15).
All participants received gabapentin 800 mg. Those whose CIWA-Ar score improved within 2 hours were considered “early responders” (n = 27) and next received 2 days of gabapentin 600 mg 4 times a day before starting a taper. The nonresponders whose CIWA-Ar score worsened (associated with greater anxiety and depressive symptoms; n = 10) were switched to standard treatment with clomethiazole (n = 4) or clonazepam (n = 6). Scores of 3 early responders subsequently worsened; 2 of these participants developed seizures and were switched to standard treatment.
The authors concluded that gabapentin in a dose of 3,200 mg in the first 24 hours is useful only for milder forms of alcohol withdrawal. Hence, subsequent efforts on the use of gabapentin for alcohol withdrawal have focused on outpatients.
Outpatient trials reveal benefits over benzodiazepines
Myrick et al3 compared gabapentin vs lorazepam in 100 outpatients seeking treatment for alcohol withdrawal. Participants were randomized to 1 of 4 groups: gabapentin 600 mg, 900 mg, or 1,200 mg, or lorazepam 6 mg, each tapering over 4 days. Alcohol withdrawal was measured by the CIWA-Ar score. Only 68 patients completed all follow-up appointments to day 12.
Gabapentin 600 mg was discontinued because of seizures in 2 patients, but it was generally well tolerated and was associated with diminished symptoms of alcohol withdrawal, especially at the 1,200 mg dose. The gabapentin groups experienced less anxiety and sedation and fewer cravings than the lorazepam group. Those treated with lorazepam fared worse for achieving early abstinence and were more likely to return to drinking when the intervention was discontinued. However, significant relapse by day 12 occurred in both groups.
The authors concluded that gabapentin was at least as effective as lorazepam in the outpatient treatment of alcohol withdrawal, with the 1,200-mg gabapentin dosage being more effective than 900 mg. At 1,200 mg, gabapentin was associated with better sleep, less anxiety, and better self-reported ability to work than lorazepam, and at the 900-mg dose it was associated with less depression than lorazepam.
Stock et al28 conducted a randomized, double-blind study of gabapentin in acute alcohol withdrawal in 26 military veterans in an outpatient setting. Patients were randomized to one of the following:
- Gabapentin 1,200 mg orally for 3 days, followed by 900 mg, 600 mg, and 300 mg for 1 day each (n = 17)
- Chlordiazepoxide 100 mg orally for 3 days, followed by 75 mg, 50 mg, and 25 mg for 1 day each (n = 9).
Withdrawal scores improved similarly in both groups. Early on (days 1–4), neither cravings nor sleep differed significantly between groups; but later (days 5–7), the gabapentin group had superior scores for these measures. Gabapentin was also associated with significantly less sedation than chlordiazepoxide and trended to less alcohol craving.
Bottom line: Gabapentin is useful for mild withdrawal
Data suggest that gabapentin offers benefits for managing mild alcohol withdrawal. Improved residual craving and sleep measures are clinically important because they are risk factors for relapse. Mood and anxiety also improve with gabapentin, further indicating a therapeutic effect.
Gabapentin’s benefits for moderate and severe alcohol withdrawal have not been established. Seizures occurred during withdrawal despite gabapentin treatment, but whether from an insufficient dose, patient susceptibility, or lack of gabapentin efficacy is not clear. Best results occurred at the 1,200-mg daily dose, but benefits may not apply to patients with severe withdrawal. In addition, many studies were small, limiting the strength of conclusions.
Across most studies of gabapentin for alcohol withdrawal, advantages included a smoother transition into early abstinence due to improved sleep, mood, and anxiety, alleviating common triggers for a return to drinking. Gabapentin also carries less reinforcing potential than benzodiazepines. These qualities fueled interest in trying gabapentin to improve long-term abstinence.
GABAPENTIN FOR RELAPSE PREVENTION
Although naltrexone and acamprosate are the first-line treatments for relapse prevention, they do not help all patients and are more effective when combined with cognitive behavioral therapy.1,29,30 For patients in whom standard treatments are not effective or tolerated, gabapentin may provide a reasonable alternative, and several randomized controlled trials have examined its use for this role.
Gabapentin alone is better than placebo
Furieri and Nakamura-Palacios4 assessed the use of gabapentin for relapse prevention in Brazilian outpatients (N = 60) who had averaged 27 years of drinking and consumed 17 drinks daily for the 90 days before baseline. After detoxification with diazepam and vitamins, patients were randomized to either gabapentin 300 mg twice daily or placebo for 4 weeks.
Compared with placebo, gabapentin significantly reduced cravings and lowered the percentage of heavy drinking days and the number of drinks per day, with a significant increase in the percentage of abstinent days. These self-reported measures correlated with decreases in gamma-glutamyl transferase, a biological marker for heavy drinking.
Brower et al31 investigated the use of gabapentin in 21 outpatients with AUD and insomnia who desired to remain abstinent. They were randomized to gabapentin (up to 1,500 mg at night) or placebo for 6 weeks. Just 14 participants completed the study; all but 2 were followed without treatment until week 12.
Gabapentin was associated with significantly lower relapse rates at 6 weeks (3 of 10 in the gabapentin group vs 9 of 11 in the placebo group) and at 12 weeks (6 of 10 in the gabapentin group vs 11 of 11 in the placebo group, assuming the 2 patients lost to follow-up relapsed). No difference between groups was detected for sleep measures in this small study. However, other studies have found that gabapentin for AUD improves measures of insomnia and daytime drowsiness—predictors of relapse—compared with other medications.16
High-dose gabapentin is better
Mason et al2 randomized 150 outpatients with alcohol dependence to 12 weeks of daily treatment with either gabapentin (900 mg or 1,800 mg) or placebo after at least 3 days of abstinence. All participants received counseling. Drinking quantity and frequency were assessed by gamma-glutamyl transferase testing.
Patients taking gabapentin had better rates of abstinence and cessation of heavy drinking than those taking placebo. During the 12-week study, the 1,800-mg daily dose showed a substantially higher abstinence rate (17%) than either 900 mg (11%) or placebo (4%). Significant dose-related improvements were also found for heavy drinking days, total drinking quantity, and frequency of alcohol withdrawal symptoms that predispose to early relapse, such as poor sleep, cravings, and poor mood. There were also significant linear dose effects on rates of abstinence and nondrinking days at the 24-week posttreatment follow-up.
Gabapentin plus naltrexone is better than naltrexone alone
Anton et al5 examined the efficacy of gabapentin combined with naltrexone during early abstinence. The study randomly assigned 150 people with AUD to one of the following groups:
- 16 weeks of naltrexone (50 mg/day) alone
- 6 weeks of naltrexone (50 mg/day) plus gabapentin (up to 1,200 mg/day), followed by 10 weeks of naltrexone alone
- Placebo.
All participants received medical management.
Over the first 6 weeks, those receiving naltrexone plus gabapentin had a longer interval to heavy drinking than those taking only naltrexone. By week 6, about half of those taking placebo or naltrexone alone had a heavy drinking day, compared with about 35% of those taking naltrexone plus gabapentin. Those receiving the combination also had fewer days of heavy drinking, fewer drinks per drinking day, and better sleep than the other groups. Participants in the naltrexone-alone group were more likely to drink heavily during periods in which they reported poor sleep. No significant group differences were found in measures of mood.
Gabapentin enacarbil is no better than placebo
Falk et al,32 in a 2019 preliminary analysis, examined data from a trial of gabapentin enacarbil, a prodrug formulation of gabapentin. In this 6-month double-blind study, 346 people with moderate AUD at 10 sites were randomized to gabapentin enacarbil extended-release 600 mg twice a day or placebo. All subjects received a computerized behavioral intervention.
No significant differences between groups were found in drinking measures or alcohol cravings, sleep problems, depression, or anxiety symptoms. However, a dose-response analysis found significantly less drinking for higher doses of the drug.
Bottom line: Evidence of benefits mixed but risk low
The efficacy of gabapentin as a treatment for AUD has varied across studies as a function of dosing and formulation. Daily doses have ranged from 600 mg to 1,800 mg, with the highest dose showing advantages in one study for cravings, insomnia, anxiety, dysphoria, and relapse.2 Thus far, gabapentin immediate-release has performed better than gabapentin enacarbil extended-release. All forms of gabapentin have been well-tolerated in AUD trials.
The 2018 American Psychiatric Association guidelines stated that gabapentin had a small positive effect on drinking outcomes, but the harm of treatment was deemed minimal, especially relative to the harms of chronic drinking.33 The guidelines endorse the use of gabapentin in patients with moderate to severe AUD who select gabapentin from the available options, or for those who are nonresponsive to or cannot tolerate naltrexone or acamprosate, as long as no contraindications exist. It was also noted that even small effects may be clinically important, considering the significant morbidity associated with AUD.
POTENTIAL FOR MISUSE
The use of gabapentin has become controversial owing to the growing recognition that it may not be as benign as initially thought.7–9,34 A review of US legislative actions reflects concerns about its misuse.35 In July 2017, Kentucky classified it as a schedule V controlled substance with prescription drug monitoring,35 as did Tennessee in 201836 and Michigan in January 2019.37 Currently, 8 other states (Massachusetts, Minnesota, Nebraska, North Dakota, Ohio, Virginia, Wyoming, and West Virginia) require prescription drug monitoring of gabapentin, and other states are considering it.35
Efforts to understand gabapentin misuse derive largely from people with drug use disorders. A review of postmortem toxicology reports in fatal drug overdoses found gabapentin present in 22%.38 Although it was not necessarily a cause of death, its high rate of detection suggests wide misuse among drug users.
Among a cohort of 503 prescription opioid misusers in Appalachian Kentucky, 15% reported using gabapentin “to get high.” Those who reported misusing gabapentin were 6 times more likely than nonusers to be abusing opioids and benzodiazepines. The main sources of gabapentin were doctors (52%) and dealers (36%). The average cost of gabapentin on the street was less than $1.00 per pill.39
Gabapentin misuse by methadone clinic patients is also reported. Baird et al40 surveyed patients in 6 addiction clinics in the United Kingdom for gabapentin and pregabalin abuse and found that 22% disclosed misusing these medications. Of these, 38% said they did so to enhance the methadone high.
In a review article, Quintero41 also cited enhancement of methadone euphoria and treatment of opioid withdrawal as motivations for misuse. Opioid-dependent gabapentin misusers consumed doses of gabapentin 3 to 20 times higher than clinically recommended and in combination with multiple drugs.4 Such use can cause dissociative and psychedelic effects.
Gabapentin also potentiates the sedative effects of opioids, thus increasing the risk of falls, accidents, and other adverse events.34,35 Risk of opioid-related deaths was increased with coprescription of gabapentin and with moderate to high gabapentin doses.34
Are people with AUD at higher risk of gabapentin abuse?
Despite concerns, patients in clinical trials of gabapentin treatment for AUD were not identified as at high risk for misuse of the drug.2,4,5,16 Further, no such trials reported serious drug-related adverse events resulting in gabapentin discontinuation or side effects that differed from placebo in frequency or severity.2,4,5,16
Clinical laboratory studies also have found no significant interactions between alcohol and gabapentin.42,43 In fact, they showed no influence of gabapentin on the pharmacokinetics of alcohol or on alcohol’s subjective effects. Relative to placebo, gabapentin did not affect blood alcohol levels, the degree of intoxication, sedation, craving, or alcohol self-administration.
Smith et al9 reported estimates that only 1% of the general population misuse gabapentin. Another review concluded that gabapentin is seldom a drug of choice.17 Most patients prescribed gabapentin do not experience cravings or loss of control, which are hallmarks of addiction. Hence, with adequate precautions, the off-label use of gabapentin for AUD is reasonable.
CLINICAL IMPLICATIONS OF GABAPENTIN PRESCRIBING
Overall, evidence for the benefit of gabapentin in AUD is mixed. Subgroups of alcoholic patients, such as those who do not respond to or tolerate standard therapies, may particularly benefit, as may those with comorbid insomnia or neuropathic pain.44 Clinicians should prescribe gabapentin only when it is likely to be helpful and should carefully document its efficacy.2,45
At each visit, an open and honest assessment of the benefits and risks serves to promote shared decision-making regarding initiating, continuing, or discontinuing gabapentin.
For alcohol withdrawal
Before gabapentin is prescribed for alcohol withdrawal, potential benefits (reduction of withdrawal symptoms), side effects (sedation, fatigue), and risks (falls) should be discussed with the patient.46 Patients should also be informed that benzodiazepines are the gold standard for alcohol withdrawal and that gabapentin is not effective for severe withdrawal.46
For relapse prevention
When initiating treatment for relapse prevention, the patient and the prescriber should agree on specific goals (eg, reduction of drinking, anxiety, and insomnia).2,16 Ongoing monitoring is essential and includes assessing and documenting improvement with respect to these goals.
In the AUD studies, gabapentin was well tolerated.16 Frequently observed side effects including headache, insomnia, fatigue, muscle aches, and gastrointestinal distress did not occur at a statistically different rate from placebo. However, patients in studies are selected samples, and their experience may not be generalizable to clinical practice. Thus, it is necessary to exercise caution and check for comorbidities that may put patients at risk of complications.47 Older patients and those on hemodialysis are more susceptible to gabapentin side effects such as sedation, dizziness, ataxia, and mental status changes,34 and prescribers should be alert for signs of toxicity (eg, ataxia, mental status changes).47,48
Gabapentin misuse was not observed in AUD studies,2,4,5,16 but evidence indicates that patients with opioid use disorder, prisoners, and polydrug users are at high risk for gabapentin misuse.39–41 In all cases, clinicians should monitor for red flags that may indicate abuse, such as missed appointments, early refill requests, demands for increased dosage, and simultaneous opiate and benzodiazepine use.49
Acknowledgment: The authors wish to thank Nick Mulligan for his invaluable assistance with formatting and grammar.
Perceptions regarding the use of gabapentin for alcohol use disorder (AUD) have shifted over time.1–4 Early on, the drug was deemed to be benign and effective.4–6 But more and more, concerns are being raised about its recreational use to achieve euphoria,7 and the drug is often misused by vulnerable populations, particularly those with opioid use disorder.7–9
Given the large number of gabapentin prescriptions written off-label for AUD, it is incumbent on providers to understand how to prescribe it responsibly.7–9 To that end, this article focuses on the benefits—and concerns—of this treatment option. We describe the effects of gabapentin on the central nervous system and how it may mitigate alcohol withdrawal and increase the likelihood of abstinence. In addition, we review clinical trials that evaluated potential roles of gabapentin in AUD, discuss the drug’s misuse potential, and suggest a framework for its appropriate use in AUD management.
ALCOHOL USE DISORDER IS COMMON AND SERIOUS
AUD affects about 14% of US adults and represents a significant health burden,1 often with severe clinical and social implications. It manifests as compulsive drinking and loss of control despite adverse consequences on various life domains.10 It is generally associated with cravings, tolerance, and withdrawal symptoms upon cessation. Alcohol withdrawal is characterized by tremors, anxiety, sweating, nausea, and tachycardia, and in severe cases, may involve hallucinations, seizures, and delirium tremens. Untreated, alcohol withdrawal can be fatal.10
Even though psychosocial treatments for AUD by themselves are associated with high relapse rates, pharmacotherapy is underutilized. Three drugs approved by the US Food and Drug Administration (FDA) are available to treat it, but they are often poorly accepted and have limited efficacy. For these reasons, there is considerable interest in finding alternatives. Gabapentin is one of several agents that have been studied (Table 1). The topic has been reviewed in depth by Soyka and Müller.11
GABAPENTIN REDUCES EXCITATION
The anticonvulsant gabapentin is FDA-approved for treating epilepsy, postherpetic neuralgia, and restless leg syndrome.8,12–14 It binds and selectively impedes voltage-sensitive calcium channels, the pores in cell membrane that permit calcium to enter a neuron in response to changes in electrical currents.15
Gabapentin is believed to decrease excitation of the central nervous system in multiple ways:
- It reduces the release of glutamate, a key component of the excitatory system16
- It increases the concentration of gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain7
- By binding the alpha-2-delta type 1 subunit of voltage-sensitive calcium channels,8,15–17 it inhibits excitatory synapse formation independent of calcium channel activity16
- By blocking excitatory neurotransmission, it also may indirectly increase the concentration of GABA in the central nervous system16,17
- It modulates action of glutamic acid decarboxylase (involved in the synthesis of GABA) and glutamate synthesizing enzyme to increase GABA and decrease glutamate.17
ALCOHOL’S ACTIONS
The actions of alcohol on the brain are also complex.18 Alpha-2-delta type 1 subunits of calcium channels are upregulated in the reward centers of the brain by addictive substances, including alcohol.16 Alcohol interacts with corticotropin-releasing factor and several neurotransmitters,18 and specifically affects neuropathways involving norepinephrine, GABA, and glutamate.19 Alcohol has reinforcing effects mediated by the release of dopamine in the nucleus accumbens.20
Acutely, alcohol promotes GABA release and may also reduce GABA degradation, producing sedative and anxiolytic effects.21 Chronic alcohol use leads to a decrease in the number of GABAA receptors. Clinically, this downregulation manifests as tolerance to alcohol’s sedating effects.21
Alcohol affects the signaling of glutamatergic interaction with the N-methyl-d-aspartate (NMDA) receptor.22 Glutamate activates this receptor as well as the voltage-gated ion channels, modifying calcium influx and increasing neuronal excitability.22,23 Acutely, alcohol has an antagonistic effect on the NMDA receptor, while chronic drinking upregulates (increases) the number of NMDA receptors and voltage-gated calcium channels.22,23
Alcohol withdrawal increases excitatory effects
Patients experiencing alcohol withdrawal have decreased GABA-ergic functioning and increased glutamatergic action throughout the central nervous system.19,24
Withdrawal can be subdivided into an acute phase (lasting up to about 5 days) and a protracted phase (of undetermined duration). During withdrawal, the brain activates its “stress system,” leading to overexpression of corticotropin-releasing factor in the amygdala. Protracted withdrawal dysregulates the prefrontal cortex, increasing cravings and worsening negative emotional states and sleep.16
GABAPENTIN FOR ALCOHOL WITHDRAWAL
Benzodiazepines are the standard treatment for alcohol withdrawal.3,24 They relieve symptoms and can prevent seizures and delirium tremens,24 but they are sedating and cause psychomotor impairments.3 Because of the potential for addiction, benzodiazepine use is limited to acute alcohol withdrawal.3
Gabapentin shows promise as an agent that can be used in withdrawal and continued through early abstinence without the highly addictive potential of benzodiazepines.16 It is thought to affect drinking behaviors during early abstinence by normalizing GABA and glutamate activity.2,16
Early preclinical studies in mouse models found that gabapentin decreases anxiogenic and epileptic effects of alcohol withdrawal. Compared with other antidrinking medications, gabapentin has the benefits of lacking elimination via hepatic metabolism, few pharmacokinetic interactions, and good reported tolerability in this population.
Inpatient trials show no benefit over standard treatments
Bonnet et al25 conducted a double-blind placebo-controlled trial in Germany in inpatients experiencing acute alcohol withdrawal to determine whether gabapentin might be an effective adjunct to clomethiazole, a GABAA modulator commonly used in Europe for alcohol withdrawal. Participants (N = 61) were randomized to receive placebo or gabapentin (400 mg every 6 hours) for 72 hours, with tapering over the next 3 days. All patients could receive rescue doses of clomethiazole, using a symptom-triggered protocol.
The study revealed no differences in the amount of clomethiazole administered between the 2 groups, suggesting that gabapentin had no adjunctive effect. Side effects (vertigo, nausea, dizziness, and ataxia) were mild and comparable between groups.
Nichols et al26 conducted a retrospective cohort study in a South Carolina academic psychiatric hospital to assess the adjunctive effect of gabapentin on the as-needed use of benzodiazepines for alcohol withdrawal. The active group (n = 40) received gabapentin as well as a symptom-triggered alcohol withdrawal protocol of benzodiazepine. The control group (n = 43) received only the symptom-triggered alcohol withdrawal protocol without gabapentin.
No effect was found of gabapentin use for benzodiazepine treatment of alcohol withdrawal. It is notable that Bonnet et al and Nichols et al had similar findings despite their studies being conducted in different countries using distinct comparators and methods.
Bonnet et al,27 in another study, tried a different design to investigate a possible role for gabapentin in inpatient alcohol withdrawal. The study included 37 patients with severe alcohol withdrawal (Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-Ar] > 15).
All participants received gabapentin 800 mg. Those whose CIWA-Ar score improved within 2 hours were considered “early responders” (n = 27) and next received 2 days of gabapentin 600 mg 4 times a day before starting a taper. The nonresponders whose CIWA-Ar score worsened (associated with greater anxiety and depressive symptoms; n = 10) were switched to standard treatment with clomethiazole (n = 4) or clonazepam (n = 6). Scores of 3 early responders subsequently worsened; 2 of these participants developed seizures and were switched to standard treatment.
The authors concluded that gabapentin in a dose of 3,200 mg in the first 24 hours is useful only for milder forms of alcohol withdrawal. Hence, subsequent efforts on the use of gabapentin for alcohol withdrawal have focused on outpatients.
Outpatient trials reveal benefits over benzodiazepines
Myrick et al3 compared gabapentin vs lorazepam in 100 outpatients seeking treatment for alcohol withdrawal. Participants were randomized to 1 of 4 groups: gabapentin 600 mg, 900 mg, or 1,200 mg, or lorazepam 6 mg, each tapering over 4 days. Alcohol withdrawal was measured by the CIWA-Ar score. Only 68 patients completed all follow-up appointments to day 12.
Gabapentin 600 mg was discontinued because of seizures in 2 patients, but it was generally well tolerated and was associated with diminished symptoms of alcohol withdrawal, especially at the 1,200 mg dose. The gabapentin groups experienced less anxiety and sedation and fewer cravings than the lorazepam group. Those treated with lorazepam fared worse for achieving early abstinence and were more likely to return to drinking when the intervention was discontinued. However, significant relapse by day 12 occurred in both groups.
The authors concluded that gabapentin was at least as effective as lorazepam in the outpatient treatment of alcohol withdrawal, with the 1,200-mg gabapentin dosage being more effective than 900 mg. At 1,200 mg, gabapentin was associated with better sleep, less anxiety, and better self-reported ability to work than lorazepam, and at the 900-mg dose it was associated with less depression than lorazepam.
Stock et al28 conducted a randomized, double-blind study of gabapentin in acute alcohol withdrawal in 26 military veterans in an outpatient setting. Patients were randomized to one of the following:
- Gabapentin 1,200 mg orally for 3 days, followed by 900 mg, 600 mg, and 300 mg for 1 day each (n = 17)
- Chlordiazepoxide 100 mg orally for 3 days, followed by 75 mg, 50 mg, and 25 mg for 1 day each (n = 9).
Withdrawal scores improved similarly in both groups. Early on (days 1–4), neither cravings nor sleep differed significantly between groups; but later (days 5–7), the gabapentin group had superior scores for these measures. Gabapentin was also associated with significantly less sedation than chlordiazepoxide and trended to less alcohol craving.
Bottom line: Gabapentin is useful for mild withdrawal
Data suggest that gabapentin offers benefits for managing mild alcohol withdrawal. Improved residual craving and sleep measures are clinically important because they are risk factors for relapse. Mood and anxiety also improve with gabapentin, further indicating a therapeutic effect.
Gabapentin’s benefits for moderate and severe alcohol withdrawal have not been established. Seizures occurred during withdrawal despite gabapentin treatment, but whether from an insufficient dose, patient susceptibility, or lack of gabapentin efficacy is not clear. Best results occurred at the 1,200-mg daily dose, but benefits may not apply to patients with severe withdrawal. In addition, many studies were small, limiting the strength of conclusions.
Across most studies of gabapentin for alcohol withdrawal, advantages included a smoother transition into early abstinence due to improved sleep, mood, and anxiety, alleviating common triggers for a return to drinking. Gabapentin also carries less reinforcing potential than benzodiazepines. These qualities fueled interest in trying gabapentin to improve long-term abstinence.
GABAPENTIN FOR RELAPSE PREVENTION
Although naltrexone and acamprosate are the first-line treatments for relapse prevention, they do not help all patients and are more effective when combined with cognitive behavioral therapy.1,29,30 For patients in whom standard treatments are not effective or tolerated, gabapentin may provide a reasonable alternative, and several randomized controlled trials have examined its use for this role.
Gabapentin alone is better than placebo
Furieri and Nakamura-Palacios4 assessed the use of gabapentin for relapse prevention in Brazilian outpatients (N = 60) who had averaged 27 years of drinking and consumed 17 drinks daily for the 90 days before baseline. After detoxification with diazepam and vitamins, patients were randomized to either gabapentin 300 mg twice daily or placebo for 4 weeks.
Compared with placebo, gabapentin significantly reduced cravings and lowered the percentage of heavy drinking days and the number of drinks per day, with a significant increase in the percentage of abstinent days. These self-reported measures correlated with decreases in gamma-glutamyl transferase, a biological marker for heavy drinking.
Brower et al31 investigated the use of gabapentin in 21 outpatients with AUD and insomnia who desired to remain abstinent. They were randomized to gabapentin (up to 1,500 mg at night) or placebo for 6 weeks. Just 14 participants completed the study; all but 2 were followed without treatment until week 12.
Gabapentin was associated with significantly lower relapse rates at 6 weeks (3 of 10 in the gabapentin group vs 9 of 11 in the placebo group) and at 12 weeks (6 of 10 in the gabapentin group vs 11 of 11 in the placebo group, assuming the 2 patients lost to follow-up relapsed). No difference between groups was detected for sleep measures in this small study. However, other studies have found that gabapentin for AUD improves measures of insomnia and daytime drowsiness—predictors of relapse—compared with other medications.16
High-dose gabapentin is better
Mason et al2 randomized 150 outpatients with alcohol dependence to 12 weeks of daily treatment with either gabapentin (900 mg or 1,800 mg) or placebo after at least 3 days of abstinence. All participants received counseling. Drinking quantity and frequency were assessed by gamma-glutamyl transferase testing.
Patients taking gabapentin had better rates of abstinence and cessation of heavy drinking than those taking placebo. During the 12-week study, the 1,800-mg daily dose showed a substantially higher abstinence rate (17%) than either 900 mg (11%) or placebo (4%). Significant dose-related improvements were also found for heavy drinking days, total drinking quantity, and frequency of alcohol withdrawal symptoms that predispose to early relapse, such as poor sleep, cravings, and poor mood. There were also significant linear dose effects on rates of abstinence and nondrinking days at the 24-week posttreatment follow-up.
Gabapentin plus naltrexone is better than naltrexone alone
Anton et al5 examined the efficacy of gabapentin combined with naltrexone during early abstinence. The study randomly assigned 150 people with AUD to one of the following groups:
- 16 weeks of naltrexone (50 mg/day) alone
- 6 weeks of naltrexone (50 mg/day) plus gabapentin (up to 1,200 mg/day), followed by 10 weeks of naltrexone alone
- Placebo.
All participants received medical management.
Over the first 6 weeks, those receiving naltrexone plus gabapentin had a longer interval to heavy drinking than those taking only naltrexone. By week 6, about half of those taking placebo or naltrexone alone had a heavy drinking day, compared with about 35% of those taking naltrexone plus gabapentin. Those receiving the combination also had fewer days of heavy drinking, fewer drinks per drinking day, and better sleep than the other groups. Participants in the naltrexone-alone group were more likely to drink heavily during periods in which they reported poor sleep. No significant group differences were found in measures of mood.
Gabapentin enacarbil is no better than placebo
Falk et al,32 in a 2019 preliminary analysis, examined data from a trial of gabapentin enacarbil, a prodrug formulation of gabapentin. In this 6-month double-blind study, 346 people with moderate AUD at 10 sites were randomized to gabapentin enacarbil extended-release 600 mg twice a day or placebo. All subjects received a computerized behavioral intervention.
No significant differences between groups were found in drinking measures or alcohol cravings, sleep problems, depression, or anxiety symptoms. However, a dose-response analysis found significantly less drinking for higher doses of the drug.
Bottom line: Evidence of benefits mixed but risk low
The efficacy of gabapentin as a treatment for AUD has varied across studies as a function of dosing and formulation. Daily doses have ranged from 600 mg to 1,800 mg, with the highest dose showing advantages in one study for cravings, insomnia, anxiety, dysphoria, and relapse.2 Thus far, gabapentin immediate-release has performed better than gabapentin enacarbil extended-release. All forms of gabapentin have been well-tolerated in AUD trials.
The 2018 American Psychiatric Association guidelines stated that gabapentin had a small positive effect on drinking outcomes, but the harm of treatment was deemed minimal, especially relative to the harms of chronic drinking.33 The guidelines endorse the use of gabapentin in patients with moderate to severe AUD who select gabapentin from the available options, or for those who are nonresponsive to or cannot tolerate naltrexone or acamprosate, as long as no contraindications exist. It was also noted that even small effects may be clinically important, considering the significant morbidity associated with AUD.
POTENTIAL FOR MISUSE
The use of gabapentin has become controversial owing to the growing recognition that it may not be as benign as initially thought.7–9,34 A review of US legislative actions reflects concerns about its misuse.35 In July 2017, Kentucky classified it as a schedule V controlled substance with prescription drug monitoring,35 as did Tennessee in 201836 and Michigan in January 2019.37 Currently, 8 other states (Massachusetts, Minnesota, Nebraska, North Dakota, Ohio, Virginia, Wyoming, and West Virginia) require prescription drug monitoring of gabapentin, and other states are considering it.35
Efforts to understand gabapentin misuse derive largely from people with drug use disorders. A review of postmortem toxicology reports in fatal drug overdoses found gabapentin present in 22%.38 Although it was not necessarily a cause of death, its high rate of detection suggests wide misuse among drug users.
Among a cohort of 503 prescription opioid misusers in Appalachian Kentucky, 15% reported using gabapentin “to get high.” Those who reported misusing gabapentin were 6 times more likely than nonusers to be abusing opioids and benzodiazepines. The main sources of gabapentin were doctors (52%) and dealers (36%). The average cost of gabapentin on the street was less than $1.00 per pill.39
Gabapentin misuse by methadone clinic patients is also reported. Baird et al40 surveyed patients in 6 addiction clinics in the United Kingdom for gabapentin and pregabalin abuse and found that 22% disclosed misusing these medications. Of these, 38% said they did so to enhance the methadone high.
In a review article, Quintero41 also cited enhancement of methadone euphoria and treatment of opioid withdrawal as motivations for misuse. Opioid-dependent gabapentin misusers consumed doses of gabapentin 3 to 20 times higher than clinically recommended and in combination with multiple drugs.4 Such use can cause dissociative and psychedelic effects.
Gabapentin also potentiates the sedative effects of opioids, thus increasing the risk of falls, accidents, and other adverse events.34,35 Risk of opioid-related deaths was increased with coprescription of gabapentin and with moderate to high gabapentin doses.34
Are people with AUD at higher risk of gabapentin abuse?
Despite concerns, patients in clinical trials of gabapentin treatment for AUD were not identified as at high risk for misuse of the drug.2,4,5,16 Further, no such trials reported serious drug-related adverse events resulting in gabapentin discontinuation or side effects that differed from placebo in frequency or severity.2,4,5,16
Clinical laboratory studies also have found no significant interactions between alcohol and gabapentin.42,43 In fact, they showed no influence of gabapentin on the pharmacokinetics of alcohol or on alcohol’s subjective effects. Relative to placebo, gabapentin did not affect blood alcohol levels, the degree of intoxication, sedation, craving, or alcohol self-administration.
Smith et al9 reported estimates that only 1% of the general population misuse gabapentin. Another review concluded that gabapentin is seldom a drug of choice.17 Most patients prescribed gabapentin do not experience cravings or loss of control, which are hallmarks of addiction. Hence, with adequate precautions, the off-label use of gabapentin for AUD is reasonable.
CLINICAL IMPLICATIONS OF GABAPENTIN PRESCRIBING
Overall, evidence for the benefit of gabapentin in AUD is mixed. Subgroups of alcoholic patients, such as those who do not respond to or tolerate standard therapies, may particularly benefit, as may those with comorbid insomnia or neuropathic pain.44 Clinicians should prescribe gabapentin only when it is likely to be helpful and should carefully document its efficacy.2,45
At each visit, an open and honest assessment of the benefits and risks serves to promote shared decision-making regarding initiating, continuing, or discontinuing gabapentin.
For alcohol withdrawal
Before gabapentin is prescribed for alcohol withdrawal, potential benefits (reduction of withdrawal symptoms), side effects (sedation, fatigue), and risks (falls) should be discussed with the patient.46 Patients should also be informed that benzodiazepines are the gold standard for alcohol withdrawal and that gabapentin is not effective for severe withdrawal.46
For relapse prevention
When initiating treatment for relapse prevention, the patient and the prescriber should agree on specific goals (eg, reduction of drinking, anxiety, and insomnia).2,16 Ongoing monitoring is essential and includes assessing and documenting improvement with respect to these goals.
In the AUD studies, gabapentin was well tolerated.16 Frequently observed side effects including headache, insomnia, fatigue, muscle aches, and gastrointestinal distress did not occur at a statistically different rate from placebo. However, patients in studies are selected samples, and their experience may not be generalizable to clinical practice. Thus, it is necessary to exercise caution and check for comorbidities that may put patients at risk of complications.47 Older patients and those on hemodialysis are more susceptible to gabapentin side effects such as sedation, dizziness, ataxia, and mental status changes,34 and prescribers should be alert for signs of toxicity (eg, ataxia, mental status changes).47,48
Gabapentin misuse was not observed in AUD studies,2,4,5,16 but evidence indicates that patients with opioid use disorder, prisoners, and polydrug users are at high risk for gabapentin misuse.39–41 In all cases, clinicians should monitor for red flags that may indicate abuse, such as missed appointments, early refill requests, demands for increased dosage, and simultaneous opiate and benzodiazepine use.49
Acknowledgment: The authors wish to thank Nick Mulligan for his invaluable assistance with formatting and grammar.
- Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA 2018; 320(8):815–824. doi:10.1001/jama.2018.11406
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med 2014; 174(1):70–77. doi:10.1001/jamainternmed.2013.11950
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res 2009; 33(9):1582–1588. doi:10.1111/j.1530-0277.2009.00986.x
- Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2007; 68(11):1691–1700. pmid:18052562
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry 2011; 168(7):709–717. doi:10.1176/appi.ajp.2011.10101436
- Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003; 9(6):559–568. doi:10.18553/jmcp.2003.9.6.559
- Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs 2014; 28(6):491–496. doi:10.1007/s40263-014-0164-4
- Goodman CW, Brett AS. Gabapentin and pregabalin for pain—is increased prescribing a cause for concern? N Engl J Med 2017; 377(5):411–414. doi:10.1056/NEJMp1704633
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction 2016; 111(7):1160–1174. doi:10.1111/add.13324
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Soyka M, Müller CA. Pharmacotherapy of alcoholism—an update on approved and off-label medications. Expert Opin Pharmacother 2017; 18(12):1187-1199. doi:10.1080/14656566.2017.1349098
- Zhang M, Gao CX, Ma KT, et al. A meta-analysis of therapeutic efficacy and safety of gabapentin i n the treatment of postherpetic neuralgia from randomized controlled trials. Biomed Res Int 2018; 2018:7474207. doi:10.1155/2018/7474207
- Winkelmann J, Allen RP, Högl B, et al. Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (Revised 2017). Mov Disord 2018; 33(7):1077–1091. doi:10.1002/mds.27260
- Honarmand A, Safavi M, Zare M. Gabapentin: an update of its pharmacological properties and therapeutic use in epilepsy. J Res Med Sci 2011; 16(8):1062–1069. pmid:22279483
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol 2002; 449(3):221–228. doi:10.1016/s0014-2999(02)02044-7
- Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs 2018; 27(1):113–124. doi:10.1080/13543784.2018.1417383
- Taylor CP. Mechanisms of action of gabapentin. Rev Neurol (Paris) 1997; 153(suppl 1):S39–S45. pmid:9686247
- Agoglia AE, Herman MA. The center of the emotional universe: alcohol, stress, and CRF1 amygdala circuitry. Alcohol 2018; 72:61–73. doi:10.1016/j.alcohol.2018.03.009
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int 1995; 26(4):305–336. pmid:7633325
- You C, Vandegrift B, Brodie MS. Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacology (Berl) 2018; 235(6):1711–1726. doi:10.1007/s00213-018-4875-y
- Lovinger DM. Presynaptic ethanol actions: potential roles in ethanol seeking. Handb Exp Pharmacol 2018; 248:29–54. doi:10.1007/164_2017_76
- Williams SB, Yorgason JT, Nelson AC, et al. Glutamate transmission to ventral tegmental area GABA neurons is altered by acute and chronic ethanol. Alcohol Clin Exp Res 2018; 42(11):2186–2195. doi:10.1111/acer.13883
- N’Gouemo P. Voltage-sensitive calcium channels in the brain: relevance to alcohol intoxication and withdrawal. Handb Exp Pharmacol 2018; 248:263–280. doi:10.1007/164_2018_93
- Modesto-Lowe V, Huard J, Conrad C. Alcohol withdrawal kindling: is there a role for anticonvulsants? Psychiatry (Edgmont) 2005; 2(5):25–31. pmid:21152146
- Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol 2003; 23(5):514–519. doi:10.1097/01.jcp.0000088905.24613.ad
- Nichols TA, Robert S, Taber DJ, Cluver J. Alcohol withdrawal-related outcomes associated with gabapentin use in an inpatient psychiatric facility. Ment Health Clin 2019 ; 9(1):1–5. doi:10.9740/mhc.2019.01.001
- Bonnet U, Hamzavi-Abedi R, Specka M, Wiltfang J, Lieb B, Scherbaum N. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol 2010; 45(2):143–145. doi:10.1093/alcalc/agp085
- Stock CJ, Carpenter L, Ying J, Greene T. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother 2013; 47(7–8):961–969. doi:10.1345/aph.1R751
- Blanco-Gandía MC, Rodríguez-Arias M. Pharmacological treatments for opiate and alcohol addiction: a historical perspective of the last 50 years. Eur J Pharmacol 2018; 836:89–101. doi:10.1016/j.ejphar.2018.08.007
- Anton RF, Moak DH, Latham P, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol 2005; 25(4):349–357. pmid:16012278
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res 2008; 32(8):1429–1438. doi:10.1111/j.1530-0277.2008.00706.x
- Falk DE, Ryan ML, Fertig JB, et al; National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG) Study Group. Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol Clin Exp Res 2019; 43(1):158–169. doi:10.1111/acer.13917
- The American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed October 10, 2019.
- Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med 2017; 14(10):e1002396. doi:10.1371/journal.pmed.1002396
- Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy 2018; 11:109–116. doi:10.2147/RMHP.S168504
- Tennessee Pharmacists Association. Advocacy alert: end of session summary. www.tnpharm.org/news/news-posts-pages/advocacy-alert-4-30-18/? Accessed October 10, 2019.
- Michigan.gov. Gabapentin scheduled as controlled substance to help with state’s opioid epidemic. www.michigan.gov/som/0,4669,7-192-47796-487050--,00.html. Accessed October 10, 2019.
- Slavova S, Miller A, Bunn TL, et al. Prevalence of gabapentin in drug overdose postmortem toxicology testing results. Drug Alcohol Depend 2018; 186:80–85. doi:10.1016/j.drugalcdep.2018.01.018
- Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry 2015; 172(5):487–488. doi:10.1176/appi.ajp.2014.14101272
- Baird CR, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res 2014; 20(3):115–118. doi:10.1159/000355268
- Quintero GC. Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol 2017; 9:13–21. doi:10.2147/JEP.S124391
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend 2006; 83(1):25–32. doi:10.1016/j.drugalcdep.2005.10.008
- Myrick H, Anton R, Voronin K, Wang W, Henderson S. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res 2007; 31(2):221–227. doi:10.1111/j.1530-0277.2006.00299.x
- Tzellos TG, Papazisis G, Toulis KA, Sardeli CH, Kouvelas D. A2delta ligands gabapentin and pregabalin: future implications in daily clinical practice. Hippokratia 2010; 14(2):71–75. pmid:20596259
- Morrison EE, Sandilands EA, Webb DJ. Gabapentin and pregabalin: do the benefits outweigh the harms? J R Coll Physicians Edinb 2017; 47(4):310–313. doi:10.4997/JRCPE.2017.402
- Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics 2018; 59(5):496–505. doi:10.1016/j.psym.2018.03.002
- Fleet JL, Dixon SN, Kuwornu PJ, et al. Gabapentin dose and the 30-day risk of altered mental status in older adults: a retrospective population-based study. PLoS One 2018; 13(3):e0193134. doi:10.1371/journal.pone.0193134
- Chiappini S, Schifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs 2016; 30(7):647–654. doi:10.1007/s40263-016-0359-y
- Modesto-Lowe V, Chaplin M, Sinha S, Woodard K. Universal precautions to reduce stimulant misuse in treating adult ADHD. Cleve Clin J Med 2015; 82(8):506–512. doi:10.3949/ccjm.82a.14131
- Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA 2018; 320(8):815–824. doi:10.1001/jama.2018.11406
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med 2014; 174(1):70–77. doi:10.1001/jamainternmed.2013.11950
- Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res 2009; 33(9):1582–1588. doi:10.1111/j.1530-0277.2009.00986.x
- Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2007; 68(11):1691–1700. pmid:18052562
- Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry 2011; 168(7):709–717. doi:10.1176/appi.ajp.2011.10101436
- Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003; 9(6):559–568. doi:10.18553/jmcp.2003.9.6.559
- Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs 2014; 28(6):491–496. doi:10.1007/s40263-014-0164-4
- Goodman CW, Brett AS. Gabapentin and pregabalin for pain—is increased prescribing a cause for concern? N Engl J Med 2017; 377(5):411–414. doi:10.1056/NEJMp1704633
- Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction 2016; 111(7):1160–1174. doi:10.1111/add.13324
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
- Soyka M, Müller CA. Pharmacotherapy of alcoholism—an update on approved and off-label medications. Expert Opin Pharmacother 2017; 18(12):1187-1199. doi:10.1080/14656566.2017.1349098
- Zhang M, Gao CX, Ma KT, et al. A meta-analysis of therapeutic efficacy and safety of gabapentin i n the treatment of postherpetic neuralgia from randomized controlled trials. Biomed Res Int 2018; 2018:7474207. doi:10.1155/2018/7474207
- Winkelmann J, Allen RP, Högl B, et al. Treatment of restless legs syndrome: evidence-based review and implications for clinical practice (Revised 2017). Mov Disord 2018; 33(7):1077–1091. doi:10.1002/mds.27260
- Honarmand A, Safavi M, Zare M. Gabapentin: an update of its pharmacological properties and therapeutic use in epilepsy. J Res Med Sci 2011; 16(8):1062–1069. pmid:22279483
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol 2002; 449(3):221–228. doi:10.1016/s0014-2999(02)02044-7
- Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs 2018; 27(1):113–124. doi:10.1080/13543784.2018.1417383
- Taylor CP. Mechanisms of action of gabapentin. Rev Neurol (Paris) 1997; 153(suppl 1):S39–S45. pmid:9686247
- Agoglia AE, Herman MA. The center of the emotional universe: alcohol, stress, and CRF1 amygdala circuitry. Alcohol 2018; 72:61–73. doi:10.1016/j.alcohol.2018.03.009
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int 1995; 26(4):305–336. pmid:7633325
- You C, Vandegrift B, Brodie MS. Ethanol actions on the ventral tegmental area: novel potential targets on reward pathway neurons. Psychopharmacology (Berl) 2018; 235(6):1711–1726. doi:10.1007/s00213-018-4875-y
- Lovinger DM. Presynaptic ethanol actions: potential roles in ethanol seeking. Handb Exp Pharmacol 2018; 248:29–54. doi:10.1007/164_2017_76
- Williams SB, Yorgason JT, Nelson AC, et al. Glutamate transmission to ventral tegmental area GABA neurons is altered by acute and chronic ethanol. Alcohol Clin Exp Res 2018; 42(11):2186–2195. doi:10.1111/acer.13883
- N’Gouemo P. Voltage-sensitive calcium channels in the brain: relevance to alcohol intoxication and withdrawal. Handb Exp Pharmacol 2018; 248:263–280. doi:10.1007/164_2018_93
- Modesto-Lowe V, Huard J, Conrad C. Alcohol withdrawal kindling: is there a role for anticonvulsants? Psychiatry (Edgmont) 2005; 2(5):25–31. pmid:21152146
- Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol 2003; 23(5):514–519. doi:10.1097/01.jcp.0000088905.24613.ad
- Nichols TA, Robert S, Taber DJ, Cluver J. Alcohol withdrawal-related outcomes associated with gabapentin use in an inpatient psychiatric facility. Ment Health Clin 2019 ; 9(1):1–5. doi:10.9740/mhc.2019.01.001
- Bonnet U, Hamzavi-Abedi R, Specka M, Wiltfang J, Lieb B, Scherbaum N. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol 2010; 45(2):143–145. doi:10.1093/alcalc/agp085
- Stock CJ, Carpenter L, Ying J, Greene T. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother 2013; 47(7–8):961–969. doi:10.1345/aph.1R751
- Blanco-Gandía MC, Rodríguez-Arias M. Pharmacological treatments for opiate and alcohol addiction: a historical perspective of the last 50 years. Eur J Pharmacol 2018; 836:89–101. doi:10.1016/j.ejphar.2018.08.007
- Anton RF, Moak DH, Latham P, et al. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol 2005; 25(4):349–357. pmid:16012278
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res 2008; 32(8):1429–1438. doi:10.1111/j.1530-0277.2008.00706.x
- Falk DE, Ryan ML, Fertig JB, et al; National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group (NCIG) Study Group. Gabapentin enacarbil extended-release for alcohol use disorder: a randomized, double-blind, placebo-controlled, multisite trial assessing efficacy and safety. Alcohol Clin Exp Res 2019; 43(1):158–169. doi:10.1111/acer.13917
- The American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients with Alcohol Use Disorder. https://psychiatryonline.org/doi/pdf/10.1176/appi.books.9781615371969. Accessed October 10, 2019.
- Gomes T, Juurlink DN, Antoniou T, et al. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med 2017; 14(10):e1002396. doi:10.1371/journal.pmed.1002396
- Peckham AM, Ananickal MJ, Sclar DA. Gabapentin use, abuse, and the US opioid epidemic: the case for reclassification as a controlled substance and the need for pharmacovigilance. Risk Manag Healthc Policy 2018; 11:109–116. doi:10.2147/RMHP.S168504
- Tennessee Pharmacists Association. Advocacy alert: end of session summary. www.tnpharm.org/news/news-posts-pages/advocacy-alert-4-30-18/? Accessed October 10, 2019.
- Michigan.gov. Gabapentin scheduled as controlled substance to help with state’s opioid epidemic. www.michigan.gov/som/0,4669,7-192-47796-487050--,00.html. Accessed October 10, 2019.
- Slavova S, Miller A, Bunn TL, et al. Prevalence of gabapentin in drug overdose postmortem toxicology testing results. Drug Alcohol Depend 2018; 186:80–85. doi:10.1016/j.drugalcdep.2018.01.018
- Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry 2015; 172(5):487–488. doi:10.1176/appi.ajp.2014.14101272
- Baird CR, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res 2014; 20(3):115–118. doi:10.1159/000355268
- Quintero GC. Review about gabapentin misuse, interactions, contraindications and side effects. J Exp Pharmacol 2017; 9:13–21. doi:10.2147/JEP.S124391
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depend 2006; 83(1):25–32. doi:10.1016/j.drugalcdep.2005.10.008
- Myrick H, Anton R, Voronin K, Wang W, Henderson S. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res 2007; 31(2):221–227. doi:10.1111/j.1530-0277.2006.00299.x
- Tzellos TG, Papazisis G, Toulis KA, Sardeli CH, Kouvelas D. A2delta ligands gabapentin and pregabalin: future implications in daily clinical practice. Hippokratia 2010; 14(2):71–75. pmid:20596259
- Morrison EE, Sandilands EA, Webb DJ. Gabapentin and pregabalin: do the benefits outweigh the harms? J R Coll Physicians Edinb 2017; 47(4):310–313. doi:10.4997/JRCPE.2017.402
- Leung JG, Rakocevic DB, Allen ND, et al. Use of a gabapentin protocol for the management of alcohol withdrawal: a preliminary experience expanding from the consultation-liaison psychiatry service. Psychosomatics 2018; 59(5):496–505. doi:10.1016/j.psym.2018.03.002
- Fleet JL, Dixon SN, Kuwornu PJ, et al. Gabapentin dose and the 30-day risk of altered mental status in older adults: a retrospective population-based study. PLoS One 2018; 13(3):e0193134. doi:10.1371/journal.pone.0193134
- Chiappini S, Schifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs 2016; 30(7):647–654. doi:10.1007/s40263-016-0359-y
- Modesto-Lowe V, Chaplin M, Sinha S, Woodard K. Universal precautions to reduce stimulant misuse in treating adult ADHD. Cleve Clin J Med 2015; 82(8):506–512. doi:10.3949/ccjm.82a.14131
KEY POINTS
- Gabapentin has been shown to be safe and effective for mild alcohol withdrawal but is not appropriate as monotherapy for severe withdrawal owing to risk of seizures.
- During early abstinence, gabapentin may improve sleep, cravings, and mood—factors associated with relapse.
- Gabapentin is being used recreationally to achieve or enhance euphoria, but its misuse potential appears to be low when taken at therapeutic doses by patients without a history of drug abuse.
How to respond to flu vaccine doubters
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
Click for Credit: PPI use & dementia; Weight loss after gastroplasty; more
Here are 5 articles from the December issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Sustainable weight loss seen 5 years after endoscopic sleeve gastroplasty
To take the posttest, go to: https://bit.ly/37lteRX
Expires May 16, 2020
2. PT beats steroid injections for knee OA
To take the posttest, go to: https://bit.ly/2KIWKY6
Expires May 17, 2020
3. Better screening needed to reduce pregnancy-related overdose, death
To take the posttest, go to: https://bit.ly/2XEZyuG
Expires May 17, 2020
4. Meta-analysis finds no link between PPI use and risk of dementia
To take the posttest, go to: https://bit.ly/2Xzs7JM
Expires June 3, 2020
5. Study: Cardiac biomarkers predicted CV events in CAP
To take the posttest, go to: https://bit.ly/33bAH2u
Expires August 13, 2020
Here are 5 articles from the December issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Sustainable weight loss seen 5 years after endoscopic sleeve gastroplasty
To take the posttest, go to: https://bit.ly/37lteRX
Expires May 16, 2020
2. PT beats steroid injections for knee OA
To take the posttest, go to: https://bit.ly/2KIWKY6
Expires May 17, 2020
3. Better screening needed to reduce pregnancy-related overdose, death
To take the posttest, go to: https://bit.ly/2XEZyuG
Expires May 17, 2020
4. Meta-analysis finds no link between PPI use and risk of dementia
To take the posttest, go to: https://bit.ly/2Xzs7JM
Expires June 3, 2020
5. Study: Cardiac biomarkers predicted CV events in CAP
To take the posttest, go to: https://bit.ly/33bAH2u
Expires August 13, 2020
Here are 5 articles from the December issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Sustainable weight loss seen 5 years after endoscopic sleeve gastroplasty
To take the posttest, go to: https://bit.ly/37lteRX
Expires May 16, 2020
2. PT beats steroid injections for knee OA
To take the posttest, go to: https://bit.ly/2KIWKY6
Expires May 17, 2020
3. Better screening needed to reduce pregnancy-related overdose, death
To take the posttest, go to: https://bit.ly/2XEZyuG
Expires May 17, 2020
4. Meta-analysis finds no link between PPI use and risk of dementia
To take the posttest, go to: https://bit.ly/2Xzs7JM
Expires June 3, 2020
5. Study: Cardiac biomarkers predicted CV events in CAP
To take the posttest, go to: https://bit.ly/33bAH2u
Expires August 13, 2020
Supplemental MRI found to benefit women with dense breast tissue
The use of supplemental MRI screening in women with extremely dense breast tissue and normal results on mammography led to the diagnosis of significantly fewer interval cancers, compared with mammography alone during a 2-year screening period, results from a randomized trial show.
“Women with extremely dense breast tissue have an increased risk of breast cancer, and their cancers are also less likely to be detected on mammography,” Dutch researchers led by Marije F. Bakker, PhD, of Utrecht (The Netherlands) University and colleagues wrote for the Dense Tissue and Early Breast Neoplasm Screening (DENSE) Trial Study Group in an article published in the New England Journal of Medicine.
“Such patients may benefit from a tailored breast-screening strategy, supplemented with more sensitive imaging methods. The benefit of supplemental imaging is the subject of a worldwide debate. In the United States, a federal law directs breast-density reporting, but supplemental screening is not recommended in American guidelines. Although supplemental imaging increases the rate of cancer detection in women with dense breasts, the question remains whether it improves health outcomes,” they said.
In the DENSE trial, researchers assigned 40,373 women with extremely dense breast tissue and negative results on screening mammography to a group that was invited to undergo supplemental MRI or to a group that received mammography screening only. The women were between the ages of 50 and 75 years and were enrolled between December 2011 and November 2015 as part of the Dutch population-based digital mammography screening program. The primary outcome was the between-group difference in the incidence of interval cancers during a 2-year screening period.
Dr. Bakker and associates found that the interval cancer rate was 2.5 per 1,000 screenings among 4,783 women in the MRI invitation group, compared with 5 per 1,000 among the 32,312 women in the mammography-only group, a difference of 2.5 per 1,000 screenings (P less than 0.001). Among the women who were invited to undergo MRI, 59% actually underwent the procedure. Of the 20 interval cancers diagnosed in the MRI-invitation group, 4 were diagnosed in the women who had undergone MRI, which translated to 0.8 per 1,000 screenings. The remaining 16 were diagnosed in those who had not undergone MRI, which translated into 4.9 per 1,000 screenings.
“Undergoing supplemental MRI was associated with a cancer-detection rate of 16.5 per 1,000 screenings and resulted in a false positive rate of 8.0% (79.8 per 1,000 screenings),” the researchers wrote. “Of the women who underwent a breast biopsy on the basis of an MRI indication, 26.3% had breast cancer and 73.7% did not.”
Dr. Bakker and coauthors acknowledged certain limitations of the trial, including the fact that it was not large enough to examine the effect of MRI screening on breast cancer–specific or overall mortality. “This outcome would require a much larger sample size and longer follow-up,” they wrote. “The lower rate of interval cancers that we found among participants who underwent MRI is indicative of and prerequisite for an effect on mortality. After that, a reduction in the number of advanced cancers would also be required to show a mortality benefit, which would require several years of follow-up.”
In an accompanying editorial, Dan L. Longo, MD, noted that the study provides high-quality data from a randomized trial where none existed (N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMe1912943). “It appears to show that among women with dense breasts, the risk of interval cancers is halved by following a negative mammogram with MRI screening,” wrote Dr. Longo, who is deputy editor of the New England Journal of Medicine, as well as professor of medicine at Harvard Medical School, Boston. “But is a reduction in interval cancers an appropriate surrogate for improved overall survival? It appears that most of the cancers that were detected on supplemental MRI screening were found at an early stage. Ductal carcinoma in situ was 10 times more frequent among patients undergoing MRI, and these diagnoses were likely to lead to treatments. What remains unclear is whether the tumors would never otherwise have been detected or threatened the patient’s survival.”
The trial was supported by the University Medical Center Utrecht (the Netherlands), the Netherlands Organization for Health Research and Development, the Dutch Cancer Society, the Dutch Pink Ribbon–A Sister’s Hope organization, Stichting Kankerpreventie Midden-West, and Bayer Pharmaceuticals, with an in-kind contribution from Volpara Health Technologies.
The researchers reported having no relevant financial disclosures other than the trial funding. Dr. Longo is employed by the New England Journal of Medicine as deputy editor.
SOURCE: Bakker MF et al. N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMoa1903986.
The use of supplemental MRI screening in women with extremely dense breast tissue and normal results on mammography led to the diagnosis of significantly fewer interval cancers, compared with mammography alone during a 2-year screening period, results from a randomized trial show.
“Women with extremely dense breast tissue have an increased risk of breast cancer, and their cancers are also less likely to be detected on mammography,” Dutch researchers led by Marije F. Bakker, PhD, of Utrecht (The Netherlands) University and colleagues wrote for the Dense Tissue and Early Breast Neoplasm Screening (DENSE) Trial Study Group in an article published in the New England Journal of Medicine.
“Such patients may benefit from a tailored breast-screening strategy, supplemented with more sensitive imaging methods. The benefit of supplemental imaging is the subject of a worldwide debate. In the United States, a federal law directs breast-density reporting, but supplemental screening is not recommended in American guidelines. Although supplemental imaging increases the rate of cancer detection in women with dense breasts, the question remains whether it improves health outcomes,” they said.
In the DENSE trial, researchers assigned 40,373 women with extremely dense breast tissue and negative results on screening mammography to a group that was invited to undergo supplemental MRI or to a group that received mammography screening only. The women were between the ages of 50 and 75 years and were enrolled between December 2011 and November 2015 as part of the Dutch population-based digital mammography screening program. The primary outcome was the between-group difference in the incidence of interval cancers during a 2-year screening period.
Dr. Bakker and associates found that the interval cancer rate was 2.5 per 1,000 screenings among 4,783 women in the MRI invitation group, compared with 5 per 1,000 among the 32,312 women in the mammography-only group, a difference of 2.5 per 1,000 screenings (P less than 0.001). Among the women who were invited to undergo MRI, 59% actually underwent the procedure. Of the 20 interval cancers diagnosed in the MRI-invitation group, 4 were diagnosed in the women who had undergone MRI, which translated to 0.8 per 1,000 screenings. The remaining 16 were diagnosed in those who had not undergone MRI, which translated into 4.9 per 1,000 screenings.
“Undergoing supplemental MRI was associated with a cancer-detection rate of 16.5 per 1,000 screenings and resulted in a false positive rate of 8.0% (79.8 per 1,000 screenings),” the researchers wrote. “Of the women who underwent a breast biopsy on the basis of an MRI indication, 26.3% had breast cancer and 73.7% did not.”
Dr. Bakker and coauthors acknowledged certain limitations of the trial, including the fact that it was not large enough to examine the effect of MRI screening on breast cancer–specific or overall mortality. “This outcome would require a much larger sample size and longer follow-up,” they wrote. “The lower rate of interval cancers that we found among participants who underwent MRI is indicative of and prerequisite for an effect on mortality. After that, a reduction in the number of advanced cancers would also be required to show a mortality benefit, which would require several years of follow-up.”
In an accompanying editorial, Dan L. Longo, MD, noted that the study provides high-quality data from a randomized trial where none existed (N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMe1912943). “It appears to show that among women with dense breasts, the risk of interval cancers is halved by following a negative mammogram with MRI screening,” wrote Dr. Longo, who is deputy editor of the New England Journal of Medicine, as well as professor of medicine at Harvard Medical School, Boston. “But is a reduction in interval cancers an appropriate surrogate for improved overall survival? It appears that most of the cancers that were detected on supplemental MRI screening were found at an early stage. Ductal carcinoma in situ was 10 times more frequent among patients undergoing MRI, and these diagnoses were likely to lead to treatments. What remains unclear is whether the tumors would never otherwise have been detected or threatened the patient’s survival.”
The trial was supported by the University Medical Center Utrecht (the Netherlands), the Netherlands Organization for Health Research and Development, the Dutch Cancer Society, the Dutch Pink Ribbon–A Sister’s Hope organization, Stichting Kankerpreventie Midden-West, and Bayer Pharmaceuticals, with an in-kind contribution from Volpara Health Technologies.
The researchers reported having no relevant financial disclosures other than the trial funding. Dr. Longo is employed by the New England Journal of Medicine as deputy editor.
SOURCE: Bakker MF et al. N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMoa1903986.
The use of supplemental MRI screening in women with extremely dense breast tissue and normal results on mammography led to the diagnosis of significantly fewer interval cancers, compared with mammography alone during a 2-year screening period, results from a randomized trial show.
“Women with extremely dense breast tissue have an increased risk of breast cancer, and their cancers are also less likely to be detected on mammography,” Dutch researchers led by Marije F. Bakker, PhD, of Utrecht (The Netherlands) University and colleagues wrote for the Dense Tissue and Early Breast Neoplasm Screening (DENSE) Trial Study Group in an article published in the New England Journal of Medicine.
“Such patients may benefit from a tailored breast-screening strategy, supplemented with more sensitive imaging methods. The benefit of supplemental imaging is the subject of a worldwide debate. In the United States, a federal law directs breast-density reporting, but supplemental screening is not recommended in American guidelines. Although supplemental imaging increases the rate of cancer detection in women with dense breasts, the question remains whether it improves health outcomes,” they said.
In the DENSE trial, researchers assigned 40,373 women with extremely dense breast tissue and negative results on screening mammography to a group that was invited to undergo supplemental MRI or to a group that received mammography screening only. The women were between the ages of 50 and 75 years and were enrolled between December 2011 and November 2015 as part of the Dutch population-based digital mammography screening program. The primary outcome was the between-group difference in the incidence of interval cancers during a 2-year screening period.
Dr. Bakker and associates found that the interval cancer rate was 2.5 per 1,000 screenings among 4,783 women in the MRI invitation group, compared with 5 per 1,000 among the 32,312 women in the mammography-only group, a difference of 2.5 per 1,000 screenings (P less than 0.001). Among the women who were invited to undergo MRI, 59% actually underwent the procedure. Of the 20 interval cancers diagnosed in the MRI-invitation group, 4 were diagnosed in the women who had undergone MRI, which translated to 0.8 per 1,000 screenings. The remaining 16 were diagnosed in those who had not undergone MRI, which translated into 4.9 per 1,000 screenings.
“Undergoing supplemental MRI was associated with a cancer-detection rate of 16.5 per 1,000 screenings and resulted in a false positive rate of 8.0% (79.8 per 1,000 screenings),” the researchers wrote. “Of the women who underwent a breast biopsy on the basis of an MRI indication, 26.3% had breast cancer and 73.7% did not.”
Dr. Bakker and coauthors acknowledged certain limitations of the trial, including the fact that it was not large enough to examine the effect of MRI screening on breast cancer–specific or overall mortality. “This outcome would require a much larger sample size and longer follow-up,” they wrote. “The lower rate of interval cancers that we found among participants who underwent MRI is indicative of and prerequisite for an effect on mortality. After that, a reduction in the number of advanced cancers would also be required to show a mortality benefit, which would require several years of follow-up.”
In an accompanying editorial, Dan L. Longo, MD, noted that the study provides high-quality data from a randomized trial where none existed (N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMe1912943). “It appears to show that among women with dense breasts, the risk of interval cancers is halved by following a negative mammogram with MRI screening,” wrote Dr. Longo, who is deputy editor of the New England Journal of Medicine, as well as professor of medicine at Harvard Medical School, Boston. “But is a reduction in interval cancers an appropriate surrogate for improved overall survival? It appears that most of the cancers that were detected on supplemental MRI screening were found at an early stage. Ductal carcinoma in situ was 10 times more frequent among patients undergoing MRI, and these diagnoses were likely to lead to treatments. What remains unclear is whether the tumors would never otherwise have been detected or threatened the patient’s survival.”
The trial was supported by the University Medical Center Utrecht (the Netherlands), the Netherlands Organization for Health Research and Development, the Dutch Cancer Society, the Dutch Pink Ribbon–A Sister’s Hope organization, Stichting Kankerpreventie Midden-West, and Bayer Pharmaceuticals, with an in-kind contribution from Volpara Health Technologies.
The researchers reported having no relevant financial disclosures other than the trial funding. Dr. Longo is employed by the New England Journal of Medicine as deputy editor.
SOURCE: Bakker MF et al. N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMoa1903986.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The interval cancer rate was 2.5 per 1,000 screenings among women in the MRI invitation group, compared with 5 per 1,000 among women in the mammography-only group, a difference of 2.5 per 1,000 screenings (P less than 0.001).
Study details: A multicenter, randomized study of 40,373 women between the ages of 50 and 75 years. One-quarter were offered supplemental MRI to the mammography all received.
Disclosures: The trial was supported by the University Medical Center Utrecht, the Netherlands Organization for Health Research and Development, the Dutch Cancer Society, the Dutch Pink Ribbon–A Sister’s Hope organization, Stichting Kankerpreventie Midden-West, and Bayer Pharmaceuticals, with an in-kind contribution from Volpara Health Technologies.
Source: Bakker MF et al. N Engl J Med. 2019 Nov 27. doi: 10.1056/NEJMoa1903986.
ART treatment at birth found to benefit neonates with HIV
Initiating antiretroviral therapy within an hour after birth, rather than waiting a few weeks, lowers the reservoir of HIV virus and improves immune response, early results from an ongoing study in Botswana, Africa, showed.
Despite advances in treatment programs during pregnancy that prevent mother to child HIV transmission, 300-500 pediatric HIV infections occur each day in sub-Saharan Africa, Roger Shapiro, MD, MPH, said during a media teleconference organized by the American Association for the Advancement of Science. “Most pediatric HIV diagnosis programs currently test children at 4-6 weeks of age to identify infections that occur either in pregnancy or during delivery,” said Dr. Shapiro, associate professor of immunology and infectious diseases at the Harvard T.H. Chan School of Public Health, Boston. “However, these programs miss the opportunity to begin immediate antiretroviral treatment for children who can be identified earlier. There are benefits to starting treatment and arresting HIV replication in the first week of life. These include limiting the viral reservoir or the population of infected cells, limiting potentially harmful immune responses to the virus, and preventing the rapid decline in health that can occur in the early weeks of HIV infection in infants. Without treatment, 50% of HIV-infected children regress to death by 2 years. Starting treatment in the first weeks or months of life has been shown to improve survival.”
With these benefits in mind, he and his associates initiated the Early Infant Treatment (EIT) study in 2015 to diagnose and treat HIV infected infants in Botswana in the first week of life or as early as possible after infection. They screened more than 10,000 children and identified 40 that were HIV infected. “This low transmission rate is a testament to the fact that most HIV-positive women in Botswana receive three-drug treatment in pregnancy, which is highly successful in blocking transmission,” Dr. Shapiro said. “When we identified an HIV-infected infant, we consented mothers to allow us to start treatment right away. We used a series of regimens because there are limited options. The available options include older drugs, some of which are no longer used for adults but which were the only options for children.”
The researchers initiated three initial drugs approved for newborns: nevirapine, zidovudine, and lamivudine, and then changed the regimen slightly after a few weeks, when they used ritonavir-boosted lopinavir, plus the lamivudine and zidovudine. “We followed the children weekly at first, then at monthly refill visits, and kept close track of how they were taking the medicines and the level of virus in each child’s blood,” Dr. Shapiro said.
In a manuscript published online in Science Translational Medicine on Nov. 27, 2019, he and his associates reported results of the first 10 children enrolled in the EIT study who reached about 96 weeks on treatment. For comparison, they also enrolled a group of children as controls, who started treatment later in the first year of life, after being identified at a more standard time of 4-6 weeks. Tests performed included droplet digital polymerase chain reaction, HIV near-full-genome sequencing, whole-genome amplification, and flow cytometry.
“What we wanted to focus on are the HIV reservoir cells that are persisting in the setting of antiretroviral treatment,” study coauthor Mathias Lichterfeld, MD, PhD, explained during the teleconference. “Those are the cells that would cause viral rebound if treatment were to be interrupted. We used complex technology to look at these cells, using next-generation sequencing, which allows us to identify those cells that harbor HIV that has the ability to initiate new viral replication.”
He and his colleagues observed that the number of reservoir cells was significantly smaller than in adults who were on ART for a median of 16 years. It also was smaller than in infected infants who started ART treatment weeks after birth.
In addition, immune activation was reduced in the cohort of infants who were treated immediately after birth.
“We are seeing a distinct advantage of early treatment initiation,” said Dr. Lichterfeld of the infectious disease division at Brigham and Women’s Hospital, Boston. “By doing these assays we see both virological benefits in terms of a very-low reservoir size, and we see immune system characteristics that are also associated with better abilities for antimicrobial immune defense and a lower level of immune activation.”
Another study coauthor, Daniel R. Kuritzkes, MD, chief of the infectious disease division at Brigham and Women’s Hospital, said the findings show “how critically important” it is to extend studies of HIV cure or long-term remission to infants and children. “Very-early intervention in neonates limits the size of the reservoir and offers us the best opportunity for future interventions aimed at cure and long-term drug-free remission of HIV infection,” he said. “We don’t think the current intervention is itself curative, but it sets the stage for the capacity to offer additional innovative interventions in the future. Beyond the importance of this work for cure research per se, this very early intervention in neonates also has the potential of conferring important clinical benefits to the children who participated in this study. Finally, our study demonstrates the feasibility and importance of doing this type of research in neonates in resource-limited settings, given the appropriate infrastructure.”
EIT is supported by the National Institutes of Health. Dr. Lichterfeld disclosed having received speaking and consulting honoraria from Merck and Gilead. Dr. Kuritzkes disclosed having received consulting honoraria and/or research support from Gilead, Merck, and ViiV.
SOURCE: Garcia-Broncano P et al. Sci Transl Med. 2019 Nov 27. eaax7350.
Initiating antiretroviral therapy within an hour after birth, rather than waiting a few weeks, lowers the reservoir of HIV virus and improves immune response, early results from an ongoing study in Botswana, Africa, showed.
Despite advances in treatment programs during pregnancy that prevent mother to child HIV transmission, 300-500 pediatric HIV infections occur each day in sub-Saharan Africa, Roger Shapiro, MD, MPH, said during a media teleconference organized by the American Association for the Advancement of Science. “Most pediatric HIV diagnosis programs currently test children at 4-6 weeks of age to identify infections that occur either in pregnancy or during delivery,” said Dr. Shapiro, associate professor of immunology and infectious diseases at the Harvard T.H. Chan School of Public Health, Boston. “However, these programs miss the opportunity to begin immediate antiretroviral treatment for children who can be identified earlier. There are benefits to starting treatment and arresting HIV replication in the first week of life. These include limiting the viral reservoir or the population of infected cells, limiting potentially harmful immune responses to the virus, and preventing the rapid decline in health that can occur in the early weeks of HIV infection in infants. Without treatment, 50% of HIV-infected children regress to death by 2 years. Starting treatment in the first weeks or months of life has been shown to improve survival.”
With these benefits in mind, he and his associates initiated the Early Infant Treatment (EIT) study in 2015 to diagnose and treat HIV infected infants in Botswana in the first week of life or as early as possible after infection. They screened more than 10,000 children and identified 40 that were HIV infected. “This low transmission rate is a testament to the fact that most HIV-positive women in Botswana receive three-drug treatment in pregnancy, which is highly successful in blocking transmission,” Dr. Shapiro said. “When we identified an HIV-infected infant, we consented mothers to allow us to start treatment right away. We used a series of regimens because there are limited options. The available options include older drugs, some of which are no longer used for adults but which were the only options for children.”
The researchers initiated three initial drugs approved for newborns: nevirapine, zidovudine, and lamivudine, and then changed the regimen slightly after a few weeks, when they used ritonavir-boosted lopinavir, plus the lamivudine and zidovudine. “We followed the children weekly at first, then at monthly refill visits, and kept close track of how they were taking the medicines and the level of virus in each child’s blood,” Dr. Shapiro said.
In a manuscript published online in Science Translational Medicine on Nov. 27, 2019, he and his associates reported results of the first 10 children enrolled in the EIT study who reached about 96 weeks on treatment. For comparison, they also enrolled a group of children as controls, who started treatment later in the first year of life, after being identified at a more standard time of 4-6 weeks. Tests performed included droplet digital polymerase chain reaction, HIV near-full-genome sequencing, whole-genome amplification, and flow cytometry.
“What we wanted to focus on are the HIV reservoir cells that are persisting in the setting of antiretroviral treatment,” study coauthor Mathias Lichterfeld, MD, PhD, explained during the teleconference. “Those are the cells that would cause viral rebound if treatment were to be interrupted. We used complex technology to look at these cells, using next-generation sequencing, which allows us to identify those cells that harbor HIV that has the ability to initiate new viral replication.”
He and his colleagues observed that the number of reservoir cells was significantly smaller than in adults who were on ART for a median of 16 years. It also was smaller than in infected infants who started ART treatment weeks after birth.
In addition, immune activation was reduced in the cohort of infants who were treated immediately after birth.
“We are seeing a distinct advantage of early treatment initiation,” said Dr. Lichterfeld of the infectious disease division at Brigham and Women’s Hospital, Boston. “By doing these assays we see both virological benefits in terms of a very-low reservoir size, and we see immune system characteristics that are also associated with better abilities for antimicrobial immune defense and a lower level of immune activation.”
Another study coauthor, Daniel R. Kuritzkes, MD, chief of the infectious disease division at Brigham and Women’s Hospital, said the findings show “how critically important” it is to extend studies of HIV cure or long-term remission to infants and children. “Very-early intervention in neonates limits the size of the reservoir and offers us the best opportunity for future interventions aimed at cure and long-term drug-free remission of HIV infection,” he said. “We don’t think the current intervention is itself curative, but it sets the stage for the capacity to offer additional innovative interventions in the future. Beyond the importance of this work for cure research per se, this very early intervention in neonates also has the potential of conferring important clinical benefits to the children who participated in this study. Finally, our study demonstrates the feasibility and importance of doing this type of research in neonates in resource-limited settings, given the appropriate infrastructure.”
EIT is supported by the National Institutes of Health. Dr. Lichterfeld disclosed having received speaking and consulting honoraria from Merck and Gilead. Dr. Kuritzkes disclosed having received consulting honoraria and/or research support from Gilead, Merck, and ViiV.
SOURCE: Garcia-Broncano P et al. Sci Transl Med. 2019 Nov 27. eaax7350.
Initiating antiretroviral therapy within an hour after birth, rather than waiting a few weeks, lowers the reservoir of HIV virus and improves immune response, early results from an ongoing study in Botswana, Africa, showed.
Despite advances in treatment programs during pregnancy that prevent mother to child HIV transmission, 300-500 pediatric HIV infections occur each day in sub-Saharan Africa, Roger Shapiro, MD, MPH, said during a media teleconference organized by the American Association for the Advancement of Science. “Most pediatric HIV diagnosis programs currently test children at 4-6 weeks of age to identify infections that occur either in pregnancy or during delivery,” said Dr. Shapiro, associate professor of immunology and infectious diseases at the Harvard T.H. Chan School of Public Health, Boston. “However, these programs miss the opportunity to begin immediate antiretroviral treatment for children who can be identified earlier. There are benefits to starting treatment and arresting HIV replication in the first week of life. These include limiting the viral reservoir or the population of infected cells, limiting potentially harmful immune responses to the virus, and preventing the rapid decline in health that can occur in the early weeks of HIV infection in infants. Without treatment, 50% of HIV-infected children regress to death by 2 years. Starting treatment in the first weeks or months of life has been shown to improve survival.”
With these benefits in mind, he and his associates initiated the Early Infant Treatment (EIT) study in 2015 to diagnose and treat HIV infected infants in Botswana in the first week of life or as early as possible after infection. They screened more than 10,000 children and identified 40 that were HIV infected. “This low transmission rate is a testament to the fact that most HIV-positive women in Botswana receive three-drug treatment in pregnancy, which is highly successful in blocking transmission,” Dr. Shapiro said. “When we identified an HIV-infected infant, we consented mothers to allow us to start treatment right away. We used a series of regimens because there are limited options. The available options include older drugs, some of which are no longer used for adults but which were the only options for children.”
The researchers initiated three initial drugs approved for newborns: nevirapine, zidovudine, and lamivudine, and then changed the regimen slightly after a few weeks, when they used ritonavir-boosted lopinavir, plus the lamivudine and zidovudine. “We followed the children weekly at first, then at monthly refill visits, and kept close track of how they were taking the medicines and the level of virus in each child’s blood,” Dr. Shapiro said.
In a manuscript published online in Science Translational Medicine on Nov. 27, 2019, he and his associates reported results of the first 10 children enrolled in the EIT study who reached about 96 weeks on treatment. For comparison, they also enrolled a group of children as controls, who started treatment later in the first year of life, after being identified at a more standard time of 4-6 weeks. Tests performed included droplet digital polymerase chain reaction, HIV near-full-genome sequencing, whole-genome amplification, and flow cytometry.
“What we wanted to focus on are the HIV reservoir cells that are persisting in the setting of antiretroviral treatment,” study coauthor Mathias Lichterfeld, MD, PhD, explained during the teleconference. “Those are the cells that would cause viral rebound if treatment were to be interrupted. We used complex technology to look at these cells, using next-generation sequencing, which allows us to identify those cells that harbor HIV that has the ability to initiate new viral replication.”
He and his colleagues observed that the number of reservoir cells was significantly smaller than in adults who were on ART for a median of 16 years. It also was smaller than in infected infants who started ART treatment weeks after birth.
In addition, immune activation was reduced in the cohort of infants who were treated immediately after birth.
“We are seeing a distinct advantage of early treatment initiation,” said Dr. Lichterfeld of the infectious disease division at Brigham and Women’s Hospital, Boston. “By doing these assays we see both virological benefits in terms of a very-low reservoir size, and we see immune system characteristics that are also associated with better abilities for antimicrobial immune defense and a lower level of immune activation.”
Another study coauthor, Daniel R. Kuritzkes, MD, chief of the infectious disease division at Brigham and Women’s Hospital, said the findings show “how critically important” it is to extend studies of HIV cure or long-term remission to infants and children. “Very-early intervention in neonates limits the size of the reservoir and offers us the best opportunity for future interventions aimed at cure and long-term drug-free remission of HIV infection,” he said. “We don’t think the current intervention is itself curative, but it sets the stage for the capacity to offer additional innovative interventions in the future. Beyond the importance of this work for cure research per se, this very early intervention in neonates also has the potential of conferring important clinical benefits to the children who participated in this study. Finally, our study demonstrates the feasibility and importance of doing this type of research in neonates in resource-limited settings, given the appropriate infrastructure.”
EIT is supported by the National Institutes of Health. Dr. Lichterfeld disclosed having received speaking and consulting honoraria from Merck and Gilead. Dr. Kuritzkes disclosed having received consulting honoraria and/or research support from Gilead, Merck, and ViiV.
SOURCE: Garcia-Broncano P et al. Sci Transl Med. 2019 Nov 27. eaax7350.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Antiretroviral treatment initiation immediately after birth reduced HIV-1 viral reservoir size and alters innate immune responses in neonates.
Major finding: Very-early ART intervention in neonates infected with HIV limited the number of virally infected cells and improves immune response.
Study details: A cohort study of 10 infants infected with HIV who were born in Botswana, Africa.
Disclosures: The Early Infant Treatment study is supported by the National Institutes of Health. Dr. Lichterfeld disclosed having received speaking and consulting honoraria from Merck and Gilead. Dr. Kuritzkes disclosed having received consulting honoraria and/or research support from Gilead, Merck, and ViiV.
Source: Garcia-Broncano P et al. Sci Transl Med. 2019 Nov 27. eaax7350.
Could your patient benefit from a breast CA risk-reducing med?
References
1. Final update summary: breast cancer: medication use to reduce risk. U.S. Preventive Services Task Force Web Site. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-medications-for-risk-reduction1 Updated October 2019. Accessed November 25, 2019.
2. Final update summary: BRCA-related cancer: risk assessment, genetic counseling, and genetic testing. U.S. Preventive Services Task Force Web Site. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing1. Updated August 2019. Accessed November 25, 2019.
3. Campos-Outcalt D. USPSTF BRCA testing recs: 2 more groups require attention. J Fam Pract. 2019;68:audio. https://www.mdedge.com/familymedicine/article/208085/womens-health/uspstf-brca-testing-recs-2-more-groups-require-attention?channel=60894. Accessed November 25, 2019.
References
1. Final update summary: breast cancer: medication use to reduce risk. U.S. Preventive Services Task Force Web Site. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-medications-for-risk-reduction1 Updated October 2019. Accessed November 25, 2019.
2. Final update summary: BRCA-related cancer: risk assessment, genetic counseling, and genetic testing. U.S. Preventive Services Task Force Web Site. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing1. Updated August 2019. Accessed November 25, 2019.
3. Campos-Outcalt D. USPSTF BRCA testing recs: 2 more groups require attention. J Fam Pract. 2019;68:audio. https://www.mdedge.com/familymedicine/article/208085/womens-health/uspstf-brca-testing-recs-2-more-groups-require-attention?channel=60894. Accessed November 25, 2019.
References
1. Final update summary: breast cancer: medication use to reduce risk. U.S. Preventive Services Task Force Web Site. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-medications-for-risk-reduction1 Updated October 2019. Accessed November 25, 2019.
2. Final update summary: BRCA-related cancer: risk assessment, genetic counseling, and genetic testing. U.S. Preventive Services Task Force Web Site. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/brca-related-cancer-risk-assessment-genetic-counseling-and-genetic-testing1. Updated August 2019. Accessed November 25, 2019.
3. Campos-Outcalt D. USPSTF BRCA testing recs: 2 more groups require attention. J Fam Pract. 2019;68:audio. https://www.mdedge.com/familymedicine/article/208085/womens-health/uspstf-brca-testing-recs-2-more-groups-require-attention?channel=60894. Accessed November 25, 2019.
Metformin after GDM: Lessons from landmark diabetes prevention trial
WASHINGTON – Metformin’s role in preventing or delaying the onset of type 2 diabetes in women with a history of gestational diabetes mellitus has been firmly established by the Diabetes Prevention Program (DPP) trial – most recently, by 15-year follow-up data reported this year – and the drug should be front and center for clinicians who hope to stave off the “remarkable” incidence of type 2 diabetes after GDM, Robert E. Ratner, MD, maintained at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
The DPP included “the single largest population of women with a history of GDM that’s been looked at in a randomized controlled trial,” and considering its multiethnic population, the trial offers a reliable representative sample to ponder today when evaluating long-term use of metformin after GDM, said Dr. Ratner, a principal investigator of the National Institutes of Health–sponsored DPP and the DPP Outcomes Study and a former chief scientific & medical officer for the American Diabetes Association.
The drug stacked up equally to lifestyle interventions among DPP participants who had a history of GDM, but it’s important to appreciate that these interventions were intensive and that metformin is inexpensive, well tolerated, and “has a long safety record,” he said.
Results of follow-up out to 15 years
Of the more than 3,000 men and women enrolled in the landmark DPP, conducted during 1996-2001, 350 were women with a documented history of GDM and over 1,400 were women who had deliveries but no history of GDM. All participants had impaired glucose tolerance – defined for the trial as having both a fasting plasma glucose value of 95-125 mg/dL and a 2-hour value of 140-199 mg/dL after a 75-g glucose load – and were randomized to placebo, metformin, or intensive lifestyle intervention.
Metformin therapy reduced the incidence of diabetes by approximately 50% in women with a history of GDM, compared with the placebo group – as did lifestyle – over 3 years. The number needed to treat to prevent one case of diabetes was five. Women without a history of GDM, on the other hand, saw only a 14% reduction with metformin when compared with placebo (and a 49% reduction with lifestyle).
“In women with a history of GDM ... one pill twice a day for $4 a month worked as well as intensive lifestyle [change],” Dr. Ratner said, referring to the initial GDM-specific analysis of DPP data published in 2008 (J Clin Endocrinol Metab. 2008;93[12]:4774-9).
In a 10-year postrandomization follow-up, published in 2015, both metformin and lifestyle continued to be equally effective for the GDM group, reducing the progression to diabetes by 40% and 35%, respectively (J Clin Endocrinol Metab. 2015;100:1646-53). The number needed to treat to prevent one case of diabetes was seven. (Among women without a history of GDM, metformin did not reduce progression to diabetes.)
A recent DPP Outcomes Study analysis of metformin’s impact on diabetes prevention at 15 years, moreover, showed a 41% risk reduction among women with a history of GDM (Diabetes Care. 2019;42[4]:601-8).
Advice on prescribing metformin prophylactically
Asked after his presentation whether women with a history of GDM and either an elevated fasting plasma glucose value or an elevated 2-hour oral glucose tolerance test (GTT) value – or neither of the two – would benefit from taking metformin, Dr. Ratner said that “we’re stuck with inclusion criteria of the DPP, in which they had to meet both criteria ... What I’d say, though, is that not everyone with a history of GDM needs to be on metformin prophylactically. But [for women who have] prediabetes as defined by the ADA, the cost-benefit analysis points toward metformin.”
And with respect to early initiation and long-term use of the drug, “I would have absolutely no qualms about medicating a 25-year-old who had developed GDM and who in the postpartum period has prediabetes,” Dr. Ratner said during an open discussion. “She’s actually at the highest risk for developing type 2 very early.”
Kim Boggess, MD, who also presented on long-term use of metformin after GDM, said in the discussion period that she is often quick to recommend metformin therapy to her patients who have an elevated fasting plasma glucose value in the postpartum period, even when a 75-g oral GTT has not yet been performed. (The ADA and the American College of Obstetricians and Gynecologists recommend completion of an oral GTT at 4-12 weeks postpartum after GDM.)
“I start them [on metformin] especially if they’ve had a cesarean section. Even 2, 3, 4 weeks of profound hyperglycemia could have potentially deleterious effects,” said Dr. Boggess, professor and maternal-fetal medicine program director at the University of North Carolina, Chapel Hill. “If someone comes in [shortly after] and looks like they have pristine control, then it might be worth stopping the metformin for 3-5 days (and retesting).”
Dr. Ratner said that, in this clinical scenario, he would first ensure that the fasting glucose value “is a true fasting glucose” and “if it’s substantially elevated – I’m talking 100, 105, 110 mg/dL – I’d start metformin, and I’m not even sure I’d do the GTT.” But, he advised, “if you’re going to do the GTT, I’d stop the metformin the day before.”
In her presentation, Dr. Boggess pointed out that metformin wasn’t shown to be superior to lifestyle interventions in the DPP for preventing progression to type 2 DM, and that some women are more motivated for intensive lifestyle change than others. The ADA recommends, in fact, that either metformin or lifestyle interventions be prescribed to women with a history of GDM who are found to have prediabetes.
There are no data to support the use of metformin either during or after pregnancy to improve weight loss or reduce weight retention following pregnancy, but at least several studies have shown that lifestyle interventions are effective, she noted.
What is needed, Dr. Boggess said, are more data on the effects of metformin on cardiovascular disease risk, as well as larger studies of metformin in the postpartum period “to help us determine the best dose.” Some research on metformin use in the postpartum period has reported gastrointestinal side effects and dissatisfaction, she noted.
Dr. Ratner said that metformin’s main drawback is the need for occasional testing of B12 levels. Regarding weight loss and what was observed in the DPP, he said, women with a history of GDM who were randomized to intensive lifestyle interventions did not lose as much weight as women without a history of GDM.
Women who entered the DPP with a GDM history, he noted in his presentation, were essentially a “cohort of survivors.” They had an average age of 43 (compared with 52 years in the parous women without GDM) and a mean interval from the index GDM pregnancy of 11 years, which means that women with the highest risk of diabetes conversion were excluded, Dr. Ratner said.
Age was the only significantly different baseline characteristic between parous women with and without GDM, he noted. Women with a history of GDM who were randomized to placebo had a 71% higher incidence of diabetes than women without such a history – a striking natural history, Dr. Ratner said.
He and Dr. Boggess each reported that they have no financial or other interests that pose a conflict of interest.
WASHINGTON – Metformin’s role in preventing or delaying the onset of type 2 diabetes in women with a history of gestational diabetes mellitus has been firmly established by the Diabetes Prevention Program (DPP) trial – most recently, by 15-year follow-up data reported this year – and the drug should be front and center for clinicians who hope to stave off the “remarkable” incidence of type 2 diabetes after GDM, Robert E. Ratner, MD, maintained at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
The DPP included “the single largest population of women with a history of GDM that’s been looked at in a randomized controlled trial,” and considering its multiethnic population, the trial offers a reliable representative sample to ponder today when evaluating long-term use of metformin after GDM, said Dr. Ratner, a principal investigator of the National Institutes of Health–sponsored DPP and the DPP Outcomes Study and a former chief scientific & medical officer for the American Diabetes Association.
The drug stacked up equally to lifestyle interventions among DPP participants who had a history of GDM, but it’s important to appreciate that these interventions were intensive and that metformin is inexpensive, well tolerated, and “has a long safety record,” he said.
Results of follow-up out to 15 years
Of the more than 3,000 men and women enrolled in the landmark DPP, conducted during 1996-2001, 350 were women with a documented history of GDM and over 1,400 were women who had deliveries but no history of GDM. All participants had impaired glucose tolerance – defined for the trial as having both a fasting plasma glucose value of 95-125 mg/dL and a 2-hour value of 140-199 mg/dL after a 75-g glucose load – and were randomized to placebo, metformin, or intensive lifestyle intervention.
Metformin therapy reduced the incidence of diabetes by approximately 50% in women with a history of GDM, compared with the placebo group – as did lifestyle – over 3 years. The number needed to treat to prevent one case of diabetes was five. Women without a history of GDM, on the other hand, saw only a 14% reduction with metformin when compared with placebo (and a 49% reduction with lifestyle).
“In women with a history of GDM ... one pill twice a day for $4 a month worked as well as intensive lifestyle [change],” Dr. Ratner said, referring to the initial GDM-specific analysis of DPP data published in 2008 (J Clin Endocrinol Metab. 2008;93[12]:4774-9).
In a 10-year postrandomization follow-up, published in 2015, both metformin and lifestyle continued to be equally effective for the GDM group, reducing the progression to diabetes by 40% and 35%, respectively (J Clin Endocrinol Metab. 2015;100:1646-53). The number needed to treat to prevent one case of diabetes was seven. (Among women without a history of GDM, metformin did not reduce progression to diabetes.)
A recent DPP Outcomes Study analysis of metformin’s impact on diabetes prevention at 15 years, moreover, showed a 41% risk reduction among women with a history of GDM (Diabetes Care. 2019;42[4]:601-8).
Advice on prescribing metformin prophylactically
Asked after his presentation whether women with a history of GDM and either an elevated fasting plasma glucose value or an elevated 2-hour oral glucose tolerance test (GTT) value – or neither of the two – would benefit from taking metformin, Dr. Ratner said that “we’re stuck with inclusion criteria of the DPP, in which they had to meet both criteria ... What I’d say, though, is that not everyone with a history of GDM needs to be on metformin prophylactically. But [for women who have] prediabetes as defined by the ADA, the cost-benefit analysis points toward metformin.”
And with respect to early initiation and long-term use of the drug, “I would have absolutely no qualms about medicating a 25-year-old who had developed GDM and who in the postpartum period has prediabetes,” Dr. Ratner said during an open discussion. “She’s actually at the highest risk for developing type 2 very early.”
Kim Boggess, MD, who also presented on long-term use of metformin after GDM, said in the discussion period that she is often quick to recommend metformin therapy to her patients who have an elevated fasting plasma glucose value in the postpartum period, even when a 75-g oral GTT has not yet been performed. (The ADA and the American College of Obstetricians and Gynecologists recommend completion of an oral GTT at 4-12 weeks postpartum after GDM.)
“I start them [on metformin] especially if they’ve had a cesarean section. Even 2, 3, 4 weeks of profound hyperglycemia could have potentially deleterious effects,” said Dr. Boggess, professor and maternal-fetal medicine program director at the University of North Carolina, Chapel Hill. “If someone comes in [shortly after] and looks like they have pristine control, then it might be worth stopping the metformin for 3-5 days (and retesting).”
Dr. Ratner said that, in this clinical scenario, he would first ensure that the fasting glucose value “is a true fasting glucose” and “if it’s substantially elevated – I’m talking 100, 105, 110 mg/dL – I’d start metformin, and I’m not even sure I’d do the GTT.” But, he advised, “if you’re going to do the GTT, I’d stop the metformin the day before.”
In her presentation, Dr. Boggess pointed out that metformin wasn’t shown to be superior to lifestyle interventions in the DPP for preventing progression to type 2 DM, and that some women are more motivated for intensive lifestyle change than others. The ADA recommends, in fact, that either metformin or lifestyle interventions be prescribed to women with a history of GDM who are found to have prediabetes.
There are no data to support the use of metformin either during or after pregnancy to improve weight loss or reduce weight retention following pregnancy, but at least several studies have shown that lifestyle interventions are effective, she noted.
What is needed, Dr. Boggess said, are more data on the effects of metformin on cardiovascular disease risk, as well as larger studies of metformin in the postpartum period “to help us determine the best dose.” Some research on metformin use in the postpartum period has reported gastrointestinal side effects and dissatisfaction, she noted.
Dr. Ratner said that metformin’s main drawback is the need for occasional testing of B12 levels. Regarding weight loss and what was observed in the DPP, he said, women with a history of GDM who were randomized to intensive lifestyle interventions did not lose as much weight as women without a history of GDM.
Women who entered the DPP with a GDM history, he noted in his presentation, were essentially a “cohort of survivors.” They had an average age of 43 (compared with 52 years in the parous women without GDM) and a mean interval from the index GDM pregnancy of 11 years, which means that women with the highest risk of diabetes conversion were excluded, Dr. Ratner said.
Age was the only significantly different baseline characteristic between parous women with and without GDM, he noted. Women with a history of GDM who were randomized to placebo had a 71% higher incidence of diabetes than women without such a history – a striking natural history, Dr. Ratner said.
He and Dr. Boggess each reported that they have no financial or other interests that pose a conflict of interest.
WASHINGTON – Metformin’s role in preventing or delaying the onset of type 2 diabetes in women with a history of gestational diabetes mellitus has been firmly established by the Diabetes Prevention Program (DPP) trial – most recently, by 15-year follow-up data reported this year – and the drug should be front and center for clinicians who hope to stave off the “remarkable” incidence of type 2 diabetes after GDM, Robert E. Ratner, MD, maintained at the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
The DPP included “the single largest population of women with a history of GDM that’s been looked at in a randomized controlled trial,” and considering its multiethnic population, the trial offers a reliable representative sample to ponder today when evaluating long-term use of metformin after GDM, said Dr. Ratner, a principal investigator of the National Institutes of Health–sponsored DPP and the DPP Outcomes Study and a former chief scientific & medical officer for the American Diabetes Association.
The drug stacked up equally to lifestyle interventions among DPP participants who had a history of GDM, but it’s important to appreciate that these interventions were intensive and that metformin is inexpensive, well tolerated, and “has a long safety record,” he said.
Results of follow-up out to 15 years
Of the more than 3,000 men and women enrolled in the landmark DPP, conducted during 1996-2001, 350 were women with a documented history of GDM and over 1,400 were women who had deliveries but no history of GDM. All participants had impaired glucose tolerance – defined for the trial as having both a fasting plasma glucose value of 95-125 mg/dL and a 2-hour value of 140-199 mg/dL after a 75-g glucose load – and were randomized to placebo, metformin, or intensive lifestyle intervention.
Metformin therapy reduced the incidence of diabetes by approximately 50% in women with a history of GDM, compared with the placebo group – as did lifestyle – over 3 years. The number needed to treat to prevent one case of diabetes was five. Women without a history of GDM, on the other hand, saw only a 14% reduction with metformin when compared with placebo (and a 49% reduction with lifestyle).
“In women with a history of GDM ... one pill twice a day for $4 a month worked as well as intensive lifestyle [change],” Dr. Ratner said, referring to the initial GDM-specific analysis of DPP data published in 2008 (J Clin Endocrinol Metab. 2008;93[12]:4774-9).
In a 10-year postrandomization follow-up, published in 2015, both metformin and lifestyle continued to be equally effective for the GDM group, reducing the progression to diabetes by 40% and 35%, respectively (J Clin Endocrinol Metab. 2015;100:1646-53). The number needed to treat to prevent one case of diabetes was seven. (Among women without a history of GDM, metformin did not reduce progression to diabetes.)
A recent DPP Outcomes Study analysis of metformin’s impact on diabetes prevention at 15 years, moreover, showed a 41% risk reduction among women with a history of GDM (Diabetes Care. 2019;42[4]:601-8).
Advice on prescribing metformin prophylactically
Asked after his presentation whether women with a history of GDM and either an elevated fasting plasma glucose value or an elevated 2-hour oral glucose tolerance test (GTT) value – or neither of the two – would benefit from taking metformin, Dr. Ratner said that “we’re stuck with inclusion criteria of the DPP, in which they had to meet both criteria ... What I’d say, though, is that not everyone with a history of GDM needs to be on metformin prophylactically. But [for women who have] prediabetes as defined by the ADA, the cost-benefit analysis points toward metformin.”
And with respect to early initiation and long-term use of the drug, “I would have absolutely no qualms about medicating a 25-year-old who had developed GDM and who in the postpartum period has prediabetes,” Dr. Ratner said during an open discussion. “She’s actually at the highest risk for developing type 2 very early.”
Kim Boggess, MD, who also presented on long-term use of metformin after GDM, said in the discussion period that she is often quick to recommend metformin therapy to her patients who have an elevated fasting plasma glucose value in the postpartum period, even when a 75-g oral GTT has not yet been performed. (The ADA and the American College of Obstetricians and Gynecologists recommend completion of an oral GTT at 4-12 weeks postpartum after GDM.)
“I start them [on metformin] especially if they’ve had a cesarean section. Even 2, 3, 4 weeks of profound hyperglycemia could have potentially deleterious effects,” said Dr. Boggess, professor and maternal-fetal medicine program director at the University of North Carolina, Chapel Hill. “If someone comes in [shortly after] and looks like they have pristine control, then it might be worth stopping the metformin for 3-5 days (and retesting).”
Dr. Ratner said that, in this clinical scenario, he would first ensure that the fasting glucose value “is a true fasting glucose” and “if it’s substantially elevated – I’m talking 100, 105, 110 mg/dL – I’d start metformin, and I’m not even sure I’d do the GTT.” But, he advised, “if you’re going to do the GTT, I’d stop the metformin the day before.”
In her presentation, Dr. Boggess pointed out that metformin wasn’t shown to be superior to lifestyle interventions in the DPP for preventing progression to type 2 DM, and that some women are more motivated for intensive lifestyle change than others. The ADA recommends, in fact, that either metformin or lifestyle interventions be prescribed to women with a history of GDM who are found to have prediabetes.
There are no data to support the use of metformin either during or after pregnancy to improve weight loss or reduce weight retention following pregnancy, but at least several studies have shown that lifestyle interventions are effective, she noted.
What is needed, Dr. Boggess said, are more data on the effects of metformin on cardiovascular disease risk, as well as larger studies of metformin in the postpartum period “to help us determine the best dose.” Some research on metformin use in the postpartum period has reported gastrointestinal side effects and dissatisfaction, she noted.
Dr. Ratner said that metformin’s main drawback is the need for occasional testing of B12 levels. Regarding weight loss and what was observed in the DPP, he said, women with a history of GDM who were randomized to intensive lifestyle interventions did not lose as much weight as women without a history of GDM.
Women who entered the DPP with a GDM history, he noted in his presentation, were essentially a “cohort of survivors.” They had an average age of 43 (compared with 52 years in the parous women without GDM) and a mean interval from the index GDM pregnancy of 11 years, which means that women with the highest risk of diabetes conversion were excluded, Dr. Ratner said.
Age was the only significantly different baseline characteristic between parous women with and without GDM, he noted. Women with a history of GDM who were randomized to placebo had a 71% higher incidence of diabetes than women without such a history – a striking natural history, Dr. Ratner said.
He and Dr. Boggess each reported that they have no financial or other interests that pose a conflict of interest.
REPORTING FROM THE DPSG-NA 2019
Learning about and prescribing emergency contraception
As health care providers to children, we always are learning. And with new knowledge we sometimes can be taken out of our comfort zone. One of those areas are teenagers, contraception, safe-sex counseling, and now emergency contraception (EC). In residency you have your 1-month adolescent medicine rotation to try and absorb every bit of information like a sponge, but there also will be a level of discomfort and uncertainty. However, as medical providers we cannot let the above prevent us from giving well-rounded and informed care.
When our teens disclose the most private moment of their life, we have to be armed and ready to not only comfort them, but advise and guide them to making a decision so that they can ensure their safety. The answers regarding sexual activity are becoming more and more alarming, especially in our younger patients. Therefore, this is an important discussion to have at every visit (not just well-child checks), so that education opportunities are not missed and our patients feel a sense of normalcy about discussing reproductive health with their health care provider and or parents.
We all have our personal beliefs, but we cannot let that guide our decision on what care or education we give our patients. Unfortunately, I have heard many health care providers judge our patients for their promiscuity, when we need to educate them – not be their judge and jury. Our teens go through different stages of growth and development, and with these stages come experimentation and risk taking. So as their health care providers, we need to be up to date on the information out there.
With regards with EC, some of our patients think that they can get it only after having unprotected sex. However, they should know that the oral ECs can be given to them at any time, so should they be in the situation above, they have an immediate remedy. With the different options come different counseling and different instructions on administration and follow-up. In residency, we might not have learned the skill of inserting an IUD, which is another form of EC; that is why there are many resources available. These resources include hands-on workshops, videos on counseling, and your friendly neighborhood adolescent medicine physician or ob.gyn.
EC can give our patients that sense of relief, especially when they have unprotected sex. However, they also need to have a sense of responsibility for their actions because you do not want them to engage in high-risk behaviors. Just as we are responsible to provide up-to-date care, our patients must take ownership of their health and well-being. Also If they are engaging in unprotected sex, they are just as responsible; therefore, they should know everything about contraception as well as EC. They should feel comfortable talking to their partners about contraception. Health care providers should make them feel comfortable receiving EC that they can give to their female partner.
We need to become knowledgeable and comfortable prescribing EC, as well as incorporating it in our routine care. This is a policy that I strongly believe should be part of every pediatrician’s and family physician’s office, especially when there is a lack of resources. Of the different options that are available, the oral forms of EC – especially Ella or Plan B step 1 (levonorgestrel) – would be the easiest to prescribe and counsel on. I would not recommend the options where multiple pills need to be taken more than once a day, because compliance becomes a factor. Also knowing that these options are available over the counter also is helpful because our community pharmacist also can help with medication administration and counseling.
In summary, I strongly recommend the discussion of EC in the office, especially the general pediatrician’s office. I recommend that, for those physicians’ who may be uncomfortable, that they should start with the “easier” options of oral progestins (Ella or Plan B step 1). As you become more comfortable with the information and counseling, you can learn skills such as IUD insertions, so you then can offer more options.
As health care providers to children, we always are learning. And with new knowledge we sometimes can be taken out of our comfort zone. One of those areas are teenagers, contraception, safe-sex counseling, and now emergency contraception (EC). In residency you have your 1-month adolescent medicine rotation to try and absorb every bit of information like a sponge, but there also will be a level of discomfort and uncertainty. However, as medical providers we cannot let the above prevent us from giving well-rounded and informed care.
When our teens disclose the most private moment of their life, we have to be armed and ready to not only comfort them, but advise and guide them to making a decision so that they can ensure their safety. The answers regarding sexual activity are becoming more and more alarming, especially in our younger patients. Therefore, this is an important discussion to have at every visit (not just well-child checks), so that education opportunities are not missed and our patients feel a sense of normalcy about discussing reproductive health with their health care provider and or parents.
We all have our personal beliefs, but we cannot let that guide our decision on what care or education we give our patients. Unfortunately, I have heard many health care providers judge our patients for their promiscuity, when we need to educate them – not be their judge and jury. Our teens go through different stages of growth and development, and with these stages come experimentation and risk taking. So as their health care providers, we need to be up to date on the information out there.
With regards with EC, some of our patients think that they can get it only after having unprotected sex. However, they should know that the oral ECs can be given to them at any time, so should they be in the situation above, they have an immediate remedy. With the different options come different counseling and different instructions on administration and follow-up. In residency, we might not have learned the skill of inserting an IUD, which is another form of EC; that is why there are many resources available. These resources include hands-on workshops, videos on counseling, and your friendly neighborhood adolescent medicine physician or ob.gyn.
EC can give our patients that sense of relief, especially when they have unprotected sex. However, they also need to have a sense of responsibility for their actions because you do not want them to engage in high-risk behaviors. Just as we are responsible to provide up-to-date care, our patients must take ownership of their health and well-being. Also If they are engaging in unprotected sex, they are just as responsible; therefore, they should know everything about contraception as well as EC. They should feel comfortable talking to their partners about contraception. Health care providers should make them feel comfortable receiving EC that they can give to their female partner.
We need to become knowledgeable and comfortable prescribing EC, as well as incorporating it in our routine care. This is a policy that I strongly believe should be part of every pediatrician’s and family physician’s office, especially when there is a lack of resources. Of the different options that are available, the oral forms of EC – especially Ella or Plan B step 1 (levonorgestrel) – would be the easiest to prescribe and counsel on. I would not recommend the options where multiple pills need to be taken more than once a day, because compliance becomes a factor. Also knowing that these options are available over the counter also is helpful because our community pharmacist also can help with medication administration and counseling.
In summary, I strongly recommend the discussion of EC in the office, especially the general pediatrician’s office. I recommend that, for those physicians’ who may be uncomfortable, that they should start with the “easier” options of oral progestins (Ella or Plan B step 1). As you become more comfortable with the information and counseling, you can learn skills such as IUD insertions, so you then can offer more options.
As health care providers to children, we always are learning. And with new knowledge we sometimes can be taken out of our comfort zone. One of those areas are teenagers, contraception, safe-sex counseling, and now emergency contraception (EC). In residency you have your 1-month adolescent medicine rotation to try and absorb every bit of information like a sponge, but there also will be a level of discomfort and uncertainty. However, as medical providers we cannot let the above prevent us from giving well-rounded and informed care.
When our teens disclose the most private moment of their life, we have to be armed and ready to not only comfort them, but advise and guide them to making a decision so that they can ensure their safety. The answers regarding sexual activity are becoming more and more alarming, especially in our younger patients. Therefore, this is an important discussion to have at every visit (not just well-child checks), so that education opportunities are not missed and our patients feel a sense of normalcy about discussing reproductive health with their health care provider and or parents.
We all have our personal beliefs, but we cannot let that guide our decision on what care or education we give our patients. Unfortunately, I have heard many health care providers judge our patients for their promiscuity, when we need to educate them – not be their judge and jury. Our teens go through different stages of growth and development, and with these stages come experimentation and risk taking. So as their health care providers, we need to be up to date on the information out there.
With regards with EC, some of our patients think that they can get it only after having unprotected sex. However, they should know that the oral ECs can be given to them at any time, so should they be in the situation above, they have an immediate remedy. With the different options come different counseling and different instructions on administration and follow-up. In residency, we might not have learned the skill of inserting an IUD, which is another form of EC; that is why there are many resources available. These resources include hands-on workshops, videos on counseling, and your friendly neighborhood adolescent medicine physician or ob.gyn.
EC can give our patients that sense of relief, especially when they have unprotected sex. However, they also need to have a sense of responsibility for their actions because you do not want them to engage in high-risk behaviors. Just as we are responsible to provide up-to-date care, our patients must take ownership of their health and well-being. Also If they are engaging in unprotected sex, they are just as responsible; therefore, they should know everything about contraception as well as EC. They should feel comfortable talking to their partners about contraception. Health care providers should make them feel comfortable receiving EC that they can give to their female partner.
We need to become knowledgeable and comfortable prescribing EC, as well as incorporating it in our routine care. This is a policy that I strongly believe should be part of every pediatrician’s and family physician’s office, especially when there is a lack of resources. Of the different options that are available, the oral forms of EC – especially Ella or Plan B step 1 (levonorgestrel) – would be the easiest to prescribe and counsel on. I would not recommend the options where multiple pills need to be taken more than once a day, because compliance becomes a factor. Also knowing that these options are available over the counter also is helpful because our community pharmacist also can help with medication administration and counseling.
In summary, I strongly recommend the discussion of EC in the office, especially the general pediatrician’s office. I recommend that, for those physicians’ who may be uncomfortable, that they should start with the “easier” options of oral progestins (Ella or Plan B step 1). As you become more comfortable with the information and counseling, you can learn skills such as IUD insertions, so you then can offer more options.