User login
8 New GI Studies With Practice-Shifting Implications
I’m just back from the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting in Philadelphia, Pennsylvania.
In part 2 of this series, I’m offering my highlights from this year’s meeting. (Part 1 is available here.) They are not presented in any particular order, but instead I am sharing what I found to be the most exciting among the thousands of abstracts and presentations.
Performing Capsule Endoscopy in Patients Taking GLP-1s
We’ve heard a lot about glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and the possibility that they might contribute to an increased risk for retained gastric contents and aspiration during endoscopy.
In the first study I’d like to highlight, researchers from the Mayo Clinic in Jacksonville, Florida, investigated video capsule endoscopy in patients with diabetes who were taking GLP-1 RAs vs a control group with diabetes not taking GLP-1 Ras. Patients in this retrospective matched cohort study were well balanced for demographics and diabetes-related characteristics.
Researchers found that in 7% of the 68 patients in the GLP-1 RA cohort, the video capsule endoscopy actually failed to pass through the stomach, whereas it passed successfully in all 68 patients in the control group (P =.06). The GLP-1 RA cohort had a longer transit time by a factor of almost four times (P <.001).
Multivariate analysis also showed that gastric transit time was approximately 80 minutes longer in the GLP-1 RA cohort (P <.001). Interestingly, 23.5% (16 of 68 patients) in the GLP-1 RA group experienced incomplete passage of the video capsule endoscopy through the small intestine, which was significantly higher than the proportion observed in the control group (4.4%; P <.01).
We need to look at potential strategies to mitigate these effects. Be aware of these results as you perform capsule endoscopy in patients taking GLP-1 RAs.
Barrett Esophagus On the Rise in the Young
The second study that caught my eye revealed the increasing incidence of young-onset Barrett esophagus (BE).
This population-based study used data obtained from TriNetX, a multi-institutional national database that offers a composite of health records from 88 healthcare organizations. Eligible patients had to have a negative upper esophagogastroduodenoscopy for BE prior to subsequently developing BE. Researchers stratified patients as to whether they were younger (< 50 years) or older (≥ 50 years), with further age ranges analyzed within those groups.
Young-onset BE accounted for 20% of all incident cases. The majority (94%) had nondysplastic BE. The incidence rate was not significantly different depending on whether patients were in the 45- to 49-year or 50- to 54-year age group.
Regression analysis revealed that there was a significantly increased trend for young-onset BE with hiatal hernia (odds ratio [OR], 2.6), smoking (OR, 2.3), White race (OR, 2.3), obstructive sleep apnea (OR, 2.2), male gender (OR, 2.0), and — at relatively lower risk levels — gastroesophageal reflux disease symptoms (OR, 1.2) and body mass index (OR, 1.1). The researchers did not analyze patients based on the presence of obesity, which is one of the risk factors for BE mentioned in national guidelines.
Results also showed that 6% of those with young-onset BE had BE-related neoplasia.
ACG guidelines recommend screening for BE beginning at age 50 in those with some of the risk factors noted in this study, including the presence of chronic gastroesophageal reflux disease symptoms. However, doing so may not capture the growing number of patients with young-onset BE.
We’ve seen a similar rise in rates of young-onset colorectal cancer, which has caused us to reevaluate our screening methods. Maybe we should do this for BE as well, specifically for patients presenting with these risk factors.
There’s a caveat to be aware of, which comes from my personal experience. I was biopsied for short-segment BE, and because it came up on my health record, it increased my life insurance premiums. This was because I was identified as having the risk profile of, essentially, an otherwise healthy smoker.
Dr Nicholas J. Shaheen and colleagues published a study several years ago showing that many insurance companies would not certify young, otherwise healthy people once diagnosed with BE. This is something to be aware of when you start to screen for BE, especially among younger patients.
A Novel Biologic for Eosinophilic Esophagitis
The next study presented results from a randomized, placebo-controlled, phase 3 study of cendakimab, a biologic agent in development for the treatment of eosinophilic esophagitis (EoE).
Dupilumab, which is an anti–interleukin (IL)-4 antibody, is the first treatment approved by the US Food and Drug Administration (FDA) for the treatment of EoE. Cendakimab, in turn, is a monoclonal antibody that neutralizes IL-13, a cytokine that plays a key role in EoE.
The study was led by Dr Evan Dellon from the University of North Carolina at Chapel Hill. Dellon and colleagues analyzed two different dosing regimens of cendakimab — 360 mg once weekly for 48 weeks, or 360 mg once weekly for 24 weeks followed by 360 mg every other week for 24 weeks — vs placebo for 48 weeks.
There was a significant effect for both cendakimab regimens in terms of symptom improvement and histologic response. There wasn’t much difference between participants that maintained once-weekly dosing and those who switched over to receive cendakimab every other week at 24 weeks. Only a minimal number of serious adverse events leading to discontinuation were noted in the study, with no notable difference between the treatment groups.
I think we’ll probably see this drug become available to us soon after it goes through the FDA review process, at which point it will add to our ability to use formative biologics in patients with EoE.
No Clear Benefit to Adding Bezlotoxumab to Fecal Microbiota Transplantation
Next was a very interesting study, and I think a clinically relevant one, about using fecal microbiota transplantation (FMT) alone or in combination with bezlotoxumab in patients with inflammatory bowel disease (IBD) with recurrent Clostridioides difficile infection.
Bezlotoxumab is a fully human monoclonal antibody that binds to C difficile toxin B. This drug has been studied and is approved for use, but it’s also extremely expensive, at a cost of approximately $4000 per dose.
Patients with IBD were eligible for inclusion if they had had two or more episodes of C difficile infection. They were then randomized in a 1:1 ration to receive either a single infusion of bezlotoxumab or placebo prior to FMT. The primary endpoint was C difficile infection recurrence within 8 weeks, which was defined as diarrhea plus a positive enzyme immunoassay toxin test. The secondary outcome was C difficile decolonization following treatment.
Researchers observed no statistically significant difference between the two cohorts. Steroid use prior to FMT significantly increased the risk for ongoing C difficile colonization (P =.03).
In summary, this is a case where it doesn’t seem that more is better. Bezlotoxumab didn’t add much, which calls into question the justification for its combined use with FMT.
Additional Positive Data for Seladelpar in Primary Biliary Cholangitis and Cirrhosis
Reassuring findings were presented on seladelpar, which was granted accelerated approval by the FDA in August.
Seladelpar is a selective peroxisome proliferator–activated receptor delta agonist that works in biliary cholangitis by regulating the genes involved in blocking biliary bile acid synthesis and controlling inflammation and fibrosis.
Results from the phase 3 RESPONSE trial were published in The New England Journal of Medicine in February reporting on the use of seladelpar in primary biliary cholangitis.
Whereas the RESPONSE trial removed decompensated patients, the ongoing phase 3 ASSURE trial results presented at this year’s meeting included patients with compensated cirrhosis. Approximately 94% of the patients in this study had Child-Pugh class A and 6% had class B cirrhosis. Eligibility required that patients had an inadequate response or were intolerant to ursodeoxycholic acid. Patients were administered open-label seladelpar 10 mg orally daily and followed for up to 1 year.
The good news is that there were no safety signals, which is reassuring news for our patients with compensated cirrhosis.
Advantages to Respiratory Syncytial Virus Vaccination in IBD
Another study that offered results certainly worthy of attention dealt with vaccination recommendations in patients with IBD.
Vaccination for respiratory syncytial virus (RSV) is now available in the United States. Its use was recommended for patients with IBD as early as 2021 per the Canadian Association of Gastroenterology’s clinical practice guideline, which discusses both live and nonlive vaccines. We should be aggressive in recommending this vaccine to our patients with IBD, but we haven’t really had one until recently.
Researchers behind this retrospective cohort study used the TriNetX database, which includes over 100 million unique patient charts. They identified patients with IBD, who were then divided into two groups according to whether they received the RSV vaccine or not.
Although this analysis was conducted in patients > 60 years of age, the US Centers for Disease Control and Prevention recommends RSV vaccination for all those over the age of 75 years, as well as for those 60-74 years old based on severity of risk.
For the primary endpoint of risk for RSV pneumonia, the OR was dramatically better in those who were vaccinated, with an approximately 80% risk reduction. Additionally, vaccinated patients experienced risk reductions of approximately 60% for acute respiratory failure, 50% for hospital inpatient admission, and 70% for requiring intensive care unit services.
This is a strong study showing not only that RSV vaccine did not exacerbate IBD but also that it improved outcomes in these patients. There’s a live-attenuated RSV vaccination that’s administered intranasally, which wouldn’t be used in your biologic or immunosuppressed patients with IBD, but the intramuscularly administered RSV vaccine doesn’t have any risk.
I think we can immediately begin recommending the RSV vaccine for our patients with IBD, particularly in those 60 years of age or older.
The Impact of Palliative Care Consultations in Decompensated Cirrhosis
The next study I’d like to highlight offers important data on the impact of palliative care consultation on 30- and 90-day readmission in patients with decompensated cirrhosis, which is a major cause of morbidity and mortality.
Researchers queried the National Readmissions Database over a 10-year period (2010-2019) to determine whether patients received a palliative care consult during index admission. They drew on a population of over 1.6 million patients admitted with decompensated cirrhosis.
Of this group, only 7.4% received a palliative care consultation at the index admission. But if they had this consultation, it was associated with a dramatic effect on readmission at 30 and 90 days. There was statistically significant risk reduction of approximately 70% for both 30- and 90-day readmission compared with those who didn’t receive the palliative consult (P <.001).
The take-home message here is to get a palliative care consult with these patients when they come in. Your hospital will unquestionably experience value in this reduction in readmission, especially considering that readmission within 30 days may not even be covered. Look at these results and start to take advantage of this valuable consultation.
Auxora: A Novel Treatment for Acute Pancreatitis
The last study for discussion offered very interesting data related to a drug called Auxora, a calcium release–activated calcium-channel inhibitor.
There is growing data that overactive calcium release–activated calcium channels aggravate acute pancreatitis and accelerate systemic inflammatory response syndrome (SIRS).
Acute pancreatitis with necrosis encompasses both local and systemic inflammation and is associated with significant mortality and morbidity. It is estimated that among patients with acute pancreatitis, 20%-30% have pancreatic necrosis, 30% develop infection, and 25% develop organ failure.
The presence of SIRS seems to herald the activation of these complex inflammatory pathways, which then leads to organ failure and necrosis, which can potentially be stemmed through this calcium channel inhibitor. Phase 2 studies of Auxora found that its use was associated with significant reduction in the risk for progression.
This subsequent phase 3 study looked at patients with acute pancreatitis and accompanying grade ≥ 2 SIRS criteria. They were randomized to receive placebo or Auxora at doses of 2 mg/kg, 1 mg/kg, or 0.5 mg/kg, which was administered intravenously over 4 hours for 3 consecutive days.
The primary endpoint was time to solid food tolerance, which was defined as eating ≥ 50% of a ≥ 500-calorie low-fat solid meal without increased abdominal pain or emesis, which is an important target because we always aim for enteral nutrition in patients with acute pancreatitis. The key secondary endpoint was severe respiratory failure, which was defined as invasive mechanical ventilation or ≥ 48 hours of either high-flow nasal cannula or noninvasive mechanical ventilation.
The primary endpoint was dramatically improved among those receiving Auxora, who achieved early onset of refeed. It appears that the high-dose 2 mg/kg may be the most beneficial in achieving improvement.
There were no patients with suspected or unexpected adverse events in the study population. Additionally, no patients receiving Auxora at any dose level went on to develop respiratory failure.
The present results show that Auxora decreases the time for solid food tolerance, as well as the rates of respiratory failure and necrotizing pancreatitis in patients presenting with two or more SIRS criteria. We’ll certainly look forward to more data, but it provides hope for a new treatment for acute pancreatitis.
Some of the take-home messages I presented are actionable now, whereas for others, we’ll have to wait and see what the final data show as well as the results of ongoing FDA approval before applying them.
Dr Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He reported serving in an advisory position with ISOTHRIVE. This transcript has been edited for clarity.
A version of this article first appeared on Medscape.com.
I’m just back from the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting in Philadelphia, Pennsylvania.
In part 2 of this series, I’m offering my highlights from this year’s meeting. (Part 1 is available here.) They are not presented in any particular order, but instead I am sharing what I found to be the most exciting among the thousands of abstracts and presentations.
Performing Capsule Endoscopy in Patients Taking GLP-1s
We’ve heard a lot about glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and the possibility that they might contribute to an increased risk for retained gastric contents and aspiration during endoscopy.
In the first study I’d like to highlight, researchers from the Mayo Clinic in Jacksonville, Florida, investigated video capsule endoscopy in patients with diabetes who were taking GLP-1 RAs vs a control group with diabetes not taking GLP-1 Ras. Patients in this retrospective matched cohort study were well balanced for demographics and diabetes-related characteristics.
Researchers found that in 7% of the 68 patients in the GLP-1 RA cohort, the video capsule endoscopy actually failed to pass through the stomach, whereas it passed successfully in all 68 patients in the control group (P =.06). The GLP-1 RA cohort had a longer transit time by a factor of almost four times (P <.001).
Multivariate analysis also showed that gastric transit time was approximately 80 minutes longer in the GLP-1 RA cohort (P <.001). Interestingly, 23.5% (16 of 68 patients) in the GLP-1 RA group experienced incomplete passage of the video capsule endoscopy through the small intestine, which was significantly higher than the proportion observed in the control group (4.4%; P <.01).
We need to look at potential strategies to mitigate these effects. Be aware of these results as you perform capsule endoscopy in patients taking GLP-1 RAs.
Barrett Esophagus On the Rise in the Young
The second study that caught my eye revealed the increasing incidence of young-onset Barrett esophagus (BE).
This population-based study used data obtained from TriNetX, a multi-institutional national database that offers a composite of health records from 88 healthcare organizations. Eligible patients had to have a negative upper esophagogastroduodenoscopy for BE prior to subsequently developing BE. Researchers stratified patients as to whether they were younger (< 50 years) or older (≥ 50 years), with further age ranges analyzed within those groups.
Young-onset BE accounted for 20% of all incident cases. The majority (94%) had nondysplastic BE. The incidence rate was not significantly different depending on whether patients were in the 45- to 49-year or 50- to 54-year age group.
Regression analysis revealed that there was a significantly increased trend for young-onset BE with hiatal hernia (odds ratio [OR], 2.6), smoking (OR, 2.3), White race (OR, 2.3), obstructive sleep apnea (OR, 2.2), male gender (OR, 2.0), and — at relatively lower risk levels — gastroesophageal reflux disease symptoms (OR, 1.2) and body mass index (OR, 1.1). The researchers did not analyze patients based on the presence of obesity, which is one of the risk factors for BE mentioned in national guidelines.
Results also showed that 6% of those with young-onset BE had BE-related neoplasia.
ACG guidelines recommend screening for BE beginning at age 50 in those with some of the risk factors noted in this study, including the presence of chronic gastroesophageal reflux disease symptoms. However, doing so may not capture the growing number of patients with young-onset BE.
We’ve seen a similar rise in rates of young-onset colorectal cancer, which has caused us to reevaluate our screening methods. Maybe we should do this for BE as well, specifically for patients presenting with these risk factors.
There’s a caveat to be aware of, which comes from my personal experience. I was biopsied for short-segment BE, and because it came up on my health record, it increased my life insurance premiums. This was because I was identified as having the risk profile of, essentially, an otherwise healthy smoker.
Dr Nicholas J. Shaheen and colleagues published a study several years ago showing that many insurance companies would not certify young, otherwise healthy people once diagnosed with BE. This is something to be aware of when you start to screen for BE, especially among younger patients.
A Novel Biologic for Eosinophilic Esophagitis
The next study presented results from a randomized, placebo-controlled, phase 3 study of cendakimab, a biologic agent in development for the treatment of eosinophilic esophagitis (EoE).
Dupilumab, which is an anti–interleukin (IL)-4 antibody, is the first treatment approved by the US Food and Drug Administration (FDA) for the treatment of EoE. Cendakimab, in turn, is a monoclonal antibody that neutralizes IL-13, a cytokine that plays a key role in EoE.
The study was led by Dr Evan Dellon from the University of North Carolina at Chapel Hill. Dellon and colleagues analyzed two different dosing regimens of cendakimab — 360 mg once weekly for 48 weeks, or 360 mg once weekly for 24 weeks followed by 360 mg every other week for 24 weeks — vs placebo for 48 weeks.
There was a significant effect for both cendakimab regimens in terms of symptom improvement and histologic response. There wasn’t much difference between participants that maintained once-weekly dosing and those who switched over to receive cendakimab every other week at 24 weeks. Only a minimal number of serious adverse events leading to discontinuation were noted in the study, with no notable difference between the treatment groups.
I think we’ll probably see this drug become available to us soon after it goes through the FDA review process, at which point it will add to our ability to use formative biologics in patients with EoE.
No Clear Benefit to Adding Bezlotoxumab to Fecal Microbiota Transplantation
Next was a very interesting study, and I think a clinically relevant one, about using fecal microbiota transplantation (FMT) alone or in combination with bezlotoxumab in patients with inflammatory bowel disease (IBD) with recurrent Clostridioides difficile infection.
Bezlotoxumab is a fully human monoclonal antibody that binds to C difficile toxin B. This drug has been studied and is approved for use, but it’s also extremely expensive, at a cost of approximately $4000 per dose.
Patients with IBD were eligible for inclusion if they had had two or more episodes of C difficile infection. They were then randomized in a 1:1 ration to receive either a single infusion of bezlotoxumab or placebo prior to FMT. The primary endpoint was C difficile infection recurrence within 8 weeks, which was defined as diarrhea plus a positive enzyme immunoassay toxin test. The secondary outcome was C difficile decolonization following treatment.
Researchers observed no statistically significant difference between the two cohorts. Steroid use prior to FMT significantly increased the risk for ongoing C difficile colonization (P =.03).
In summary, this is a case where it doesn’t seem that more is better. Bezlotoxumab didn’t add much, which calls into question the justification for its combined use with FMT.
Additional Positive Data for Seladelpar in Primary Biliary Cholangitis and Cirrhosis
Reassuring findings were presented on seladelpar, which was granted accelerated approval by the FDA in August.
Seladelpar is a selective peroxisome proliferator–activated receptor delta agonist that works in biliary cholangitis by regulating the genes involved in blocking biliary bile acid synthesis and controlling inflammation and fibrosis.
Results from the phase 3 RESPONSE trial were published in The New England Journal of Medicine in February reporting on the use of seladelpar in primary biliary cholangitis.
Whereas the RESPONSE trial removed decompensated patients, the ongoing phase 3 ASSURE trial results presented at this year’s meeting included patients with compensated cirrhosis. Approximately 94% of the patients in this study had Child-Pugh class A and 6% had class B cirrhosis. Eligibility required that patients had an inadequate response or were intolerant to ursodeoxycholic acid. Patients were administered open-label seladelpar 10 mg orally daily and followed for up to 1 year.
The good news is that there were no safety signals, which is reassuring news for our patients with compensated cirrhosis.
Advantages to Respiratory Syncytial Virus Vaccination in IBD
Another study that offered results certainly worthy of attention dealt with vaccination recommendations in patients with IBD.
Vaccination for respiratory syncytial virus (RSV) is now available in the United States. Its use was recommended for patients with IBD as early as 2021 per the Canadian Association of Gastroenterology’s clinical practice guideline, which discusses both live and nonlive vaccines. We should be aggressive in recommending this vaccine to our patients with IBD, but we haven’t really had one until recently.
Researchers behind this retrospective cohort study used the TriNetX database, which includes over 100 million unique patient charts. They identified patients with IBD, who were then divided into two groups according to whether they received the RSV vaccine or not.
Although this analysis was conducted in patients > 60 years of age, the US Centers for Disease Control and Prevention recommends RSV vaccination for all those over the age of 75 years, as well as for those 60-74 years old based on severity of risk.
For the primary endpoint of risk for RSV pneumonia, the OR was dramatically better in those who were vaccinated, with an approximately 80% risk reduction. Additionally, vaccinated patients experienced risk reductions of approximately 60% for acute respiratory failure, 50% for hospital inpatient admission, and 70% for requiring intensive care unit services.
This is a strong study showing not only that RSV vaccine did not exacerbate IBD but also that it improved outcomes in these patients. There’s a live-attenuated RSV vaccination that’s administered intranasally, which wouldn’t be used in your biologic or immunosuppressed patients with IBD, but the intramuscularly administered RSV vaccine doesn’t have any risk.
I think we can immediately begin recommending the RSV vaccine for our patients with IBD, particularly in those 60 years of age or older.
The Impact of Palliative Care Consultations in Decompensated Cirrhosis
The next study I’d like to highlight offers important data on the impact of palliative care consultation on 30- and 90-day readmission in patients with decompensated cirrhosis, which is a major cause of morbidity and mortality.
Researchers queried the National Readmissions Database over a 10-year period (2010-2019) to determine whether patients received a palliative care consult during index admission. They drew on a population of over 1.6 million patients admitted with decompensated cirrhosis.
Of this group, only 7.4% received a palliative care consultation at the index admission. But if they had this consultation, it was associated with a dramatic effect on readmission at 30 and 90 days. There was statistically significant risk reduction of approximately 70% for both 30- and 90-day readmission compared with those who didn’t receive the palliative consult (P <.001).
The take-home message here is to get a palliative care consult with these patients when they come in. Your hospital will unquestionably experience value in this reduction in readmission, especially considering that readmission within 30 days may not even be covered. Look at these results and start to take advantage of this valuable consultation.
Auxora: A Novel Treatment for Acute Pancreatitis
The last study for discussion offered very interesting data related to a drug called Auxora, a calcium release–activated calcium-channel inhibitor.
There is growing data that overactive calcium release–activated calcium channels aggravate acute pancreatitis and accelerate systemic inflammatory response syndrome (SIRS).
Acute pancreatitis with necrosis encompasses both local and systemic inflammation and is associated with significant mortality and morbidity. It is estimated that among patients with acute pancreatitis, 20%-30% have pancreatic necrosis, 30% develop infection, and 25% develop organ failure.
The presence of SIRS seems to herald the activation of these complex inflammatory pathways, which then leads to organ failure and necrosis, which can potentially be stemmed through this calcium channel inhibitor. Phase 2 studies of Auxora found that its use was associated with significant reduction in the risk for progression.
This subsequent phase 3 study looked at patients with acute pancreatitis and accompanying grade ≥ 2 SIRS criteria. They were randomized to receive placebo or Auxora at doses of 2 mg/kg, 1 mg/kg, or 0.5 mg/kg, which was administered intravenously over 4 hours for 3 consecutive days.
The primary endpoint was time to solid food tolerance, which was defined as eating ≥ 50% of a ≥ 500-calorie low-fat solid meal without increased abdominal pain or emesis, which is an important target because we always aim for enteral nutrition in patients with acute pancreatitis. The key secondary endpoint was severe respiratory failure, which was defined as invasive mechanical ventilation or ≥ 48 hours of either high-flow nasal cannula or noninvasive mechanical ventilation.
The primary endpoint was dramatically improved among those receiving Auxora, who achieved early onset of refeed. It appears that the high-dose 2 mg/kg may be the most beneficial in achieving improvement.
There were no patients with suspected or unexpected adverse events in the study population. Additionally, no patients receiving Auxora at any dose level went on to develop respiratory failure.
The present results show that Auxora decreases the time for solid food tolerance, as well as the rates of respiratory failure and necrotizing pancreatitis in patients presenting with two or more SIRS criteria. We’ll certainly look forward to more data, but it provides hope for a new treatment for acute pancreatitis.
Some of the take-home messages I presented are actionable now, whereas for others, we’ll have to wait and see what the final data show as well as the results of ongoing FDA approval before applying them.
Dr Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He reported serving in an advisory position with ISOTHRIVE. This transcript has been edited for clarity.
A version of this article first appeared on Medscape.com.
I’m just back from the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting in Philadelphia, Pennsylvania.
In part 2 of this series, I’m offering my highlights from this year’s meeting. (Part 1 is available here.) They are not presented in any particular order, but instead I am sharing what I found to be the most exciting among the thousands of abstracts and presentations.
Performing Capsule Endoscopy in Patients Taking GLP-1s
We’ve heard a lot about glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and the possibility that they might contribute to an increased risk for retained gastric contents and aspiration during endoscopy.
In the first study I’d like to highlight, researchers from the Mayo Clinic in Jacksonville, Florida, investigated video capsule endoscopy in patients with diabetes who were taking GLP-1 RAs vs a control group with diabetes not taking GLP-1 Ras. Patients in this retrospective matched cohort study were well balanced for demographics and diabetes-related characteristics.
Researchers found that in 7% of the 68 patients in the GLP-1 RA cohort, the video capsule endoscopy actually failed to pass through the stomach, whereas it passed successfully in all 68 patients in the control group (P =.06). The GLP-1 RA cohort had a longer transit time by a factor of almost four times (P <.001).
Multivariate analysis also showed that gastric transit time was approximately 80 minutes longer in the GLP-1 RA cohort (P <.001). Interestingly, 23.5% (16 of 68 patients) in the GLP-1 RA group experienced incomplete passage of the video capsule endoscopy through the small intestine, which was significantly higher than the proportion observed in the control group (4.4%; P <.01).
We need to look at potential strategies to mitigate these effects. Be aware of these results as you perform capsule endoscopy in patients taking GLP-1 RAs.
Barrett Esophagus On the Rise in the Young
The second study that caught my eye revealed the increasing incidence of young-onset Barrett esophagus (BE).
This population-based study used data obtained from TriNetX, a multi-institutional national database that offers a composite of health records from 88 healthcare organizations. Eligible patients had to have a negative upper esophagogastroduodenoscopy for BE prior to subsequently developing BE. Researchers stratified patients as to whether they were younger (< 50 years) or older (≥ 50 years), with further age ranges analyzed within those groups.
Young-onset BE accounted for 20% of all incident cases. The majority (94%) had nondysplastic BE. The incidence rate was not significantly different depending on whether patients were in the 45- to 49-year or 50- to 54-year age group.
Regression analysis revealed that there was a significantly increased trend for young-onset BE with hiatal hernia (odds ratio [OR], 2.6), smoking (OR, 2.3), White race (OR, 2.3), obstructive sleep apnea (OR, 2.2), male gender (OR, 2.0), and — at relatively lower risk levels — gastroesophageal reflux disease symptoms (OR, 1.2) and body mass index (OR, 1.1). The researchers did not analyze patients based on the presence of obesity, which is one of the risk factors for BE mentioned in national guidelines.
Results also showed that 6% of those with young-onset BE had BE-related neoplasia.
ACG guidelines recommend screening for BE beginning at age 50 in those with some of the risk factors noted in this study, including the presence of chronic gastroesophageal reflux disease symptoms. However, doing so may not capture the growing number of patients with young-onset BE.
We’ve seen a similar rise in rates of young-onset colorectal cancer, which has caused us to reevaluate our screening methods. Maybe we should do this for BE as well, specifically for patients presenting with these risk factors.
There’s a caveat to be aware of, which comes from my personal experience. I was biopsied for short-segment BE, and because it came up on my health record, it increased my life insurance premiums. This was because I was identified as having the risk profile of, essentially, an otherwise healthy smoker.
Dr Nicholas J. Shaheen and colleagues published a study several years ago showing that many insurance companies would not certify young, otherwise healthy people once diagnosed with BE. This is something to be aware of when you start to screen for BE, especially among younger patients.
A Novel Biologic for Eosinophilic Esophagitis
The next study presented results from a randomized, placebo-controlled, phase 3 study of cendakimab, a biologic agent in development for the treatment of eosinophilic esophagitis (EoE).
Dupilumab, which is an anti–interleukin (IL)-4 antibody, is the first treatment approved by the US Food and Drug Administration (FDA) for the treatment of EoE. Cendakimab, in turn, is a monoclonal antibody that neutralizes IL-13, a cytokine that plays a key role in EoE.
The study was led by Dr Evan Dellon from the University of North Carolina at Chapel Hill. Dellon and colleagues analyzed two different dosing regimens of cendakimab — 360 mg once weekly for 48 weeks, or 360 mg once weekly for 24 weeks followed by 360 mg every other week for 24 weeks — vs placebo for 48 weeks.
There was a significant effect for both cendakimab regimens in terms of symptom improvement and histologic response. There wasn’t much difference between participants that maintained once-weekly dosing and those who switched over to receive cendakimab every other week at 24 weeks. Only a minimal number of serious adverse events leading to discontinuation were noted in the study, with no notable difference between the treatment groups.
I think we’ll probably see this drug become available to us soon after it goes through the FDA review process, at which point it will add to our ability to use formative biologics in patients with EoE.
No Clear Benefit to Adding Bezlotoxumab to Fecal Microbiota Transplantation
Next was a very interesting study, and I think a clinically relevant one, about using fecal microbiota transplantation (FMT) alone or in combination with bezlotoxumab in patients with inflammatory bowel disease (IBD) with recurrent Clostridioides difficile infection.
Bezlotoxumab is a fully human monoclonal antibody that binds to C difficile toxin B. This drug has been studied and is approved for use, but it’s also extremely expensive, at a cost of approximately $4000 per dose.
Patients with IBD were eligible for inclusion if they had had two or more episodes of C difficile infection. They were then randomized in a 1:1 ration to receive either a single infusion of bezlotoxumab or placebo prior to FMT. The primary endpoint was C difficile infection recurrence within 8 weeks, which was defined as diarrhea plus a positive enzyme immunoassay toxin test. The secondary outcome was C difficile decolonization following treatment.
Researchers observed no statistically significant difference between the two cohorts. Steroid use prior to FMT significantly increased the risk for ongoing C difficile colonization (P =.03).
In summary, this is a case where it doesn’t seem that more is better. Bezlotoxumab didn’t add much, which calls into question the justification for its combined use with FMT.
Additional Positive Data for Seladelpar in Primary Biliary Cholangitis and Cirrhosis
Reassuring findings were presented on seladelpar, which was granted accelerated approval by the FDA in August.
Seladelpar is a selective peroxisome proliferator–activated receptor delta agonist that works in biliary cholangitis by regulating the genes involved in blocking biliary bile acid synthesis and controlling inflammation and fibrosis.
Results from the phase 3 RESPONSE trial were published in The New England Journal of Medicine in February reporting on the use of seladelpar in primary biliary cholangitis.
Whereas the RESPONSE trial removed decompensated patients, the ongoing phase 3 ASSURE trial results presented at this year’s meeting included patients with compensated cirrhosis. Approximately 94% of the patients in this study had Child-Pugh class A and 6% had class B cirrhosis. Eligibility required that patients had an inadequate response or were intolerant to ursodeoxycholic acid. Patients were administered open-label seladelpar 10 mg orally daily and followed for up to 1 year.
The good news is that there were no safety signals, which is reassuring news for our patients with compensated cirrhosis.
Advantages to Respiratory Syncytial Virus Vaccination in IBD
Another study that offered results certainly worthy of attention dealt with vaccination recommendations in patients with IBD.
Vaccination for respiratory syncytial virus (RSV) is now available in the United States. Its use was recommended for patients with IBD as early as 2021 per the Canadian Association of Gastroenterology’s clinical practice guideline, which discusses both live and nonlive vaccines. We should be aggressive in recommending this vaccine to our patients with IBD, but we haven’t really had one until recently.
Researchers behind this retrospective cohort study used the TriNetX database, which includes over 100 million unique patient charts. They identified patients with IBD, who were then divided into two groups according to whether they received the RSV vaccine or not.
Although this analysis was conducted in patients > 60 years of age, the US Centers for Disease Control and Prevention recommends RSV vaccination for all those over the age of 75 years, as well as for those 60-74 years old based on severity of risk.
For the primary endpoint of risk for RSV pneumonia, the OR was dramatically better in those who were vaccinated, with an approximately 80% risk reduction. Additionally, vaccinated patients experienced risk reductions of approximately 60% for acute respiratory failure, 50% for hospital inpatient admission, and 70% for requiring intensive care unit services.
This is a strong study showing not only that RSV vaccine did not exacerbate IBD but also that it improved outcomes in these patients. There’s a live-attenuated RSV vaccination that’s administered intranasally, which wouldn’t be used in your biologic or immunosuppressed patients with IBD, but the intramuscularly administered RSV vaccine doesn’t have any risk.
I think we can immediately begin recommending the RSV vaccine for our patients with IBD, particularly in those 60 years of age or older.
The Impact of Palliative Care Consultations in Decompensated Cirrhosis
The next study I’d like to highlight offers important data on the impact of palliative care consultation on 30- and 90-day readmission in patients with decompensated cirrhosis, which is a major cause of morbidity and mortality.
Researchers queried the National Readmissions Database over a 10-year period (2010-2019) to determine whether patients received a palliative care consult during index admission. They drew on a population of over 1.6 million patients admitted with decompensated cirrhosis.
Of this group, only 7.4% received a palliative care consultation at the index admission. But if they had this consultation, it was associated with a dramatic effect on readmission at 30 and 90 days. There was statistically significant risk reduction of approximately 70% for both 30- and 90-day readmission compared with those who didn’t receive the palliative consult (P <.001).
The take-home message here is to get a palliative care consult with these patients when they come in. Your hospital will unquestionably experience value in this reduction in readmission, especially considering that readmission within 30 days may not even be covered. Look at these results and start to take advantage of this valuable consultation.
Auxora: A Novel Treatment for Acute Pancreatitis
The last study for discussion offered very interesting data related to a drug called Auxora, a calcium release–activated calcium-channel inhibitor.
There is growing data that overactive calcium release–activated calcium channels aggravate acute pancreatitis and accelerate systemic inflammatory response syndrome (SIRS).
Acute pancreatitis with necrosis encompasses both local and systemic inflammation and is associated with significant mortality and morbidity. It is estimated that among patients with acute pancreatitis, 20%-30% have pancreatic necrosis, 30% develop infection, and 25% develop organ failure.
The presence of SIRS seems to herald the activation of these complex inflammatory pathways, which then leads to organ failure and necrosis, which can potentially be stemmed through this calcium channel inhibitor. Phase 2 studies of Auxora found that its use was associated with significant reduction in the risk for progression.
This subsequent phase 3 study looked at patients with acute pancreatitis and accompanying grade ≥ 2 SIRS criteria. They were randomized to receive placebo or Auxora at doses of 2 mg/kg, 1 mg/kg, or 0.5 mg/kg, which was administered intravenously over 4 hours for 3 consecutive days.
The primary endpoint was time to solid food tolerance, which was defined as eating ≥ 50% of a ≥ 500-calorie low-fat solid meal without increased abdominal pain or emesis, which is an important target because we always aim for enteral nutrition in patients with acute pancreatitis. The key secondary endpoint was severe respiratory failure, which was defined as invasive mechanical ventilation or ≥ 48 hours of either high-flow nasal cannula or noninvasive mechanical ventilation.
The primary endpoint was dramatically improved among those receiving Auxora, who achieved early onset of refeed. It appears that the high-dose 2 mg/kg may be the most beneficial in achieving improvement.
There were no patients with suspected or unexpected adverse events in the study population. Additionally, no patients receiving Auxora at any dose level went on to develop respiratory failure.
The present results show that Auxora decreases the time for solid food tolerance, as well as the rates of respiratory failure and necrotizing pancreatitis in patients presenting with two or more SIRS criteria. We’ll certainly look forward to more data, but it provides hope for a new treatment for acute pancreatitis.
Some of the take-home messages I presented are actionable now, whereas for others, we’ll have to wait and see what the final data show as well as the results of ongoing FDA approval before applying them.
Dr Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He reported serving in an advisory position with ISOTHRIVE. This transcript has been edited for clarity.
A version of this article first appeared on Medscape.com.
Thrombocytosis and Cancer Risk: Management in Primary Care
This transcript has been edited for clarity.
In this podcast, I’m going to talk about unexplained high platelet counts, or thrombocytosis, and the risk for cancer in primary care. Let’s start with a typical case we all might see in primary care.
Louisa is 47 years old and is the chief financial officer for a tech startup company. She presents to us in primary care feeling tired all the time — a very common presentation in primary care — with associated reduced appetite. Past medical history includes irritable bowel syndrome, and she’s an ex-smoker.
Systemic inquiry is unremarkable. Specifically, there is no history of weight loss. Louisa has not been prescribed any medication and uses over-the-counter remedies for her irritable bowel syndrome. Examination is also unremarkable. Blood tests were checked, which were all reassuring, except for a platelet count of 612 × 109 cells/L (usual normal range, about 150-450).
What do we do next? Do we refer for an urgent chest x-ray to exclude lung cancer? Do we check a quantitative immunohistochemical fecal occult blood test (qFIT) to identify any occult bleeding in her stool? Do we refer for a routine upper gastrointestinal endoscopy or pelvic ultrasound scan to exclude any upper gastrointestinal or endometrial malignancy?
Do we simply repeat the bloods? If so, do we repeat them routinely or urgently, and indeed, which ones should we recheck?
Louisa has an unexplained thrombocytosis. How do we manage this in primary care? Thrombocytosis is generally defined as a raised platelet count over 450. Importantly, thrombocytosis is a common incidental finding in around 2% of those over 40 years of age attending primary care. Reassuringly, 80%-90% of thrombocytosis is reactive, secondary to acute blood loss, infection, or inflammation, and the majority of cases resolve within 3 months.
Why the concern with Louisa then? Although most cases are reactive, clinical guidance (for example, NICE suspected cancer guidance in the UK and Scottish suspected cancer guidance in Scotland) reminds us that unexplained thrombocytosis is a risk marker for some solid-tumor malignancies.
Previous studies have demonstrated that unexplained thrombocytosis is associated with a 1-year cancer incidence of 11.6% in males and 6.2% in females, well exceeding the standard 3% threshold warranting investigation for underlying malignancy. However, thrombocytosis should not be used as a stand-alone diagnostic or screening test for cancer, or indeed to rule out cancer.
Instead, unexplained thrombocytosis should prompt us to think cancer. The Scottish suspected cancer referral guidelines include thrombocytosis in the investigation criteria for what they call the LEGO-C cancers — L for lung, E for endometrial, G for gastric, O for oesophageal, and C for colorectal, which is a useful reminder for us all.
What further history, examination, and investigations might we consider in primary care if we identify an unexplained high platelet count? As always, we should use our clinical judgment and trust our clinical acumen.
We should consider all the possible underlying causes, including infection, inflammation, and blood loss, including menstrual blood loss in women; myeloproliferative disorders such as polycythemia rubra vera, chronic myeloid leukemia, and essential thrombocythemia; and, of course, underlying malignancy. If a likely underlying reversible cause is present (for example, a recent lower respiratory tract infection), simply repeating the full blood count in 4-6 weeks is quite appropriate to see if the thrombocytosis has resolved.
Remember, 80%-90% of cases are reactive thrombocytosis, and most cases resolve within 3 months. If thrombocytosis is unexplained or not resolving, consider checking ferritin levels to exclude iron deficiency. Consider checking C-reactive protein (CRP) levels to exclude any inflammation, and also consider checking a blood film to exclude any hematologic disorders, in addition, of course, to more detailed history-taking and examination to elicit any red flags.
We can also consider a JAK2 gene mutation test, if it is available to you locally, or a hematology referral if we suspect a myeloproliferative disorder. JAK2 is a genetic mutation that may be present in people with essential thrombocythemia and can indicate a diagnosis of polycythemia rubra vera.
Subsequent to this, and again using our clinical judgment, we then need to exclude the LEGO-C cancers. Consider urgent chest x-ray to exclude lung cancer or pelvic ultrasound in women to exclude endometrial cancer. Also, we should consider an upper gastrointestinal endoscopy, particularly in those individuals who have associated upper gastrointestinal symptoms and/or weight loss.
Finally, consider a qFIT to identify any occult bleeding in the stool, again if it’s available to you, or certainly if not, urgent lower gastrointestinal investigations to exclude colorectal cancer.
Alongside these possible investigations, as always, we should safety-net appropriately within agreed timeframes and check for resolution of the thrombocytosis according to the condition being suspected. Remember, most cases resolve within 3 months.
Returning to Louisa, what did I do? After seeing a platelet count of 600, I subsequently telephoned her and reexplored her history, which yielded nil else of note. Specifically, there was no history of unexplained weight loss, no history of upper or lower gastrointestinal symptoms, and certainly nothing significantly different from her usual irritable bowel syndrome symptoms. There were also no respiratory or genitourinary symptoms of note.
I did arrange for Louisa to undergo a chest x-ray over the next few days, though, as she was an ex-smoker. This was subsequently reported as normal. I appreciate chest x-rays have poor sensitivity for detecting lung cancer, as highlighted in a number of recent papers, but it was mutually agreed with Louisa that we would simply repeat her blood test in around 6 weeks. As well as repeating the full blood count, I arranged to check her ferritin, CRP, and a blood film, and then I was planning to reassess her clinically in person.
These bloods and my subsequent clinical review were reassuring. In fact, her platelet count had normalized after that 6 weeks had elapsed. Her thrombocytosis had resolved.
I didn’t arrange any further follow-up for her, but I did give her the usual safety netting advice to re-present to me or one of my colleagues if she does develop any worrying symptoms or signs.
I appreciate these scenarios are not always this straightforward, but I wanted to outline what investigations and referrals we may need to consider in primary care if we encounter an unexplained high platelet count.
There are a couple of quality-improvement activities for us all to consider in primary care. Consider as a team how we would respond to an incidental finding of thrombocytosis on a full blood count. Also consider what are our safety-netting options for those found to have raised platelet counts but no other symptoms or risk factors for underlying malignancy.
Finally, I’ve produced a Medscape UK primary care hack or clinical aide-memoire on managing unexplained thrombocytosis and associated cancer risk in primary care for all healthcare professionals working in primary care. This can be found online. I hope you find this resource helpful.
Dr. Kevin Fernando, General practitioner partner with specialist interests in cardiovascular, renal, and metabolic medicine, North Berwick Group Practice in Scotland, has disclosed relevant financial relationships with Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Dexcom, Lilly, Menarini, Novartis, Novo Nordisk, Roche Diagnostics, Embecta, Roche Diabetes Care, Sanofi Menarini, and Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
In this podcast, I’m going to talk about unexplained high platelet counts, or thrombocytosis, and the risk for cancer in primary care. Let’s start with a typical case we all might see in primary care.
Louisa is 47 years old and is the chief financial officer for a tech startup company. She presents to us in primary care feeling tired all the time — a very common presentation in primary care — with associated reduced appetite. Past medical history includes irritable bowel syndrome, and she’s an ex-smoker.
Systemic inquiry is unremarkable. Specifically, there is no history of weight loss. Louisa has not been prescribed any medication and uses over-the-counter remedies for her irritable bowel syndrome. Examination is also unremarkable. Blood tests were checked, which were all reassuring, except for a platelet count of 612 × 109 cells/L (usual normal range, about 150-450).
What do we do next? Do we refer for an urgent chest x-ray to exclude lung cancer? Do we check a quantitative immunohistochemical fecal occult blood test (qFIT) to identify any occult bleeding in her stool? Do we refer for a routine upper gastrointestinal endoscopy or pelvic ultrasound scan to exclude any upper gastrointestinal or endometrial malignancy?
Do we simply repeat the bloods? If so, do we repeat them routinely or urgently, and indeed, which ones should we recheck?
Louisa has an unexplained thrombocytosis. How do we manage this in primary care? Thrombocytosis is generally defined as a raised platelet count over 450. Importantly, thrombocytosis is a common incidental finding in around 2% of those over 40 years of age attending primary care. Reassuringly, 80%-90% of thrombocytosis is reactive, secondary to acute blood loss, infection, or inflammation, and the majority of cases resolve within 3 months.
Why the concern with Louisa then? Although most cases are reactive, clinical guidance (for example, NICE suspected cancer guidance in the UK and Scottish suspected cancer guidance in Scotland) reminds us that unexplained thrombocytosis is a risk marker for some solid-tumor malignancies.
Previous studies have demonstrated that unexplained thrombocytosis is associated with a 1-year cancer incidence of 11.6% in males and 6.2% in females, well exceeding the standard 3% threshold warranting investigation for underlying malignancy. However, thrombocytosis should not be used as a stand-alone diagnostic or screening test for cancer, or indeed to rule out cancer.
Instead, unexplained thrombocytosis should prompt us to think cancer. The Scottish suspected cancer referral guidelines include thrombocytosis in the investigation criteria for what they call the LEGO-C cancers — L for lung, E for endometrial, G for gastric, O for oesophageal, and C for colorectal, which is a useful reminder for us all.
What further history, examination, and investigations might we consider in primary care if we identify an unexplained high platelet count? As always, we should use our clinical judgment and trust our clinical acumen.
We should consider all the possible underlying causes, including infection, inflammation, and blood loss, including menstrual blood loss in women; myeloproliferative disorders such as polycythemia rubra vera, chronic myeloid leukemia, and essential thrombocythemia; and, of course, underlying malignancy. If a likely underlying reversible cause is present (for example, a recent lower respiratory tract infection), simply repeating the full blood count in 4-6 weeks is quite appropriate to see if the thrombocytosis has resolved.
Remember, 80%-90% of cases are reactive thrombocytosis, and most cases resolve within 3 months. If thrombocytosis is unexplained or not resolving, consider checking ferritin levels to exclude iron deficiency. Consider checking C-reactive protein (CRP) levels to exclude any inflammation, and also consider checking a blood film to exclude any hematologic disorders, in addition, of course, to more detailed history-taking and examination to elicit any red flags.
We can also consider a JAK2 gene mutation test, if it is available to you locally, or a hematology referral if we suspect a myeloproliferative disorder. JAK2 is a genetic mutation that may be present in people with essential thrombocythemia and can indicate a diagnosis of polycythemia rubra vera.
Subsequent to this, and again using our clinical judgment, we then need to exclude the LEGO-C cancers. Consider urgent chest x-ray to exclude lung cancer or pelvic ultrasound in women to exclude endometrial cancer. Also, we should consider an upper gastrointestinal endoscopy, particularly in those individuals who have associated upper gastrointestinal symptoms and/or weight loss.
Finally, consider a qFIT to identify any occult bleeding in the stool, again if it’s available to you, or certainly if not, urgent lower gastrointestinal investigations to exclude colorectal cancer.
Alongside these possible investigations, as always, we should safety-net appropriately within agreed timeframes and check for resolution of the thrombocytosis according to the condition being suspected. Remember, most cases resolve within 3 months.
Returning to Louisa, what did I do? After seeing a platelet count of 600, I subsequently telephoned her and reexplored her history, which yielded nil else of note. Specifically, there was no history of unexplained weight loss, no history of upper or lower gastrointestinal symptoms, and certainly nothing significantly different from her usual irritable bowel syndrome symptoms. There were also no respiratory or genitourinary symptoms of note.
I did arrange for Louisa to undergo a chest x-ray over the next few days, though, as she was an ex-smoker. This was subsequently reported as normal. I appreciate chest x-rays have poor sensitivity for detecting lung cancer, as highlighted in a number of recent papers, but it was mutually agreed with Louisa that we would simply repeat her blood test in around 6 weeks. As well as repeating the full blood count, I arranged to check her ferritin, CRP, and a blood film, and then I was planning to reassess her clinically in person.
These bloods and my subsequent clinical review were reassuring. In fact, her platelet count had normalized after that 6 weeks had elapsed. Her thrombocytosis had resolved.
I didn’t arrange any further follow-up for her, but I did give her the usual safety netting advice to re-present to me or one of my colleagues if she does develop any worrying symptoms or signs.
I appreciate these scenarios are not always this straightforward, but I wanted to outline what investigations and referrals we may need to consider in primary care if we encounter an unexplained high platelet count.
There are a couple of quality-improvement activities for us all to consider in primary care. Consider as a team how we would respond to an incidental finding of thrombocytosis on a full blood count. Also consider what are our safety-netting options for those found to have raised platelet counts but no other symptoms or risk factors for underlying malignancy.
Finally, I’ve produced a Medscape UK primary care hack or clinical aide-memoire on managing unexplained thrombocytosis and associated cancer risk in primary care for all healthcare professionals working in primary care. This can be found online. I hope you find this resource helpful.
Dr. Kevin Fernando, General practitioner partner with specialist interests in cardiovascular, renal, and metabolic medicine, North Berwick Group Practice in Scotland, has disclosed relevant financial relationships with Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Dexcom, Lilly, Menarini, Novartis, Novo Nordisk, Roche Diagnostics, Embecta, Roche Diabetes Care, Sanofi Menarini, and Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
In this podcast, I’m going to talk about unexplained high platelet counts, or thrombocytosis, and the risk for cancer in primary care. Let’s start with a typical case we all might see in primary care.
Louisa is 47 years old and is the chief financial officer for a tech startup company. She presents to us in primary care feeling tired all the time — a very common presentation in primary care — with associated reduced appetite. Past medical history includes irritable bowel syndrome, and she’s an ex-smoker.
Systemic inquiry is unremarkable. Specifically, there is no history of weight loss. Louisa has not been prescribed any medication and uses over-the-counter remedies for her irritable bowel syndrome. Examination is also unremarkable. Blood tests were checked, which were all reassuring, except for a platelet count of 612 × 109 cells/L (usual normal range, about 150-450).
What do we do next? Do we refer for an urgent chest x-ray to exclude lung cancer? Do we check a quantitative immunohistochemical fecal occult blood test (qFIT) to identify any occult bleeding in her stool? Do we refer for a routine upper gastrointestinal endoscopy or pelvic ultrasound scan to exclude any upper gastrointestinal or endometrial malignancy?
Do we simply repeat the bloods? If so, do we repeat them routinely or urgently, and indeed, which ones should we recheck?
Louisa has an unexplained thrombocytosis. How do we manage this in primary care? Thrombocytosis is generally defined as a raised platelet count over 450. Importantly, thrombocytosis is a common incidental finding in around 2% of those over 40 years of age attending primary care. Reassuringly, 80%-90% of thrombocytosis is reactive, secondary to acute blood loss, infection, or inflammation, and the majority of cases resolve within 3 months.
Why the concern with Louisa then? Although most cases are reactive, clinical guidance (for example, NICE suspected cancer guidance in the UK and Scottish suspected cancer guidance in Scotland) reminds us that unexplained thrombocytosis is a risk marker for some solid-tumor malignancies.
Previous studies have demonstrated that unexplained thrombocytosis is associated with a 1-year cancer incidence of 11.6% in males and 6.2% in females, well exceeding the standard 3% threshold warranting investigation for underlying malignancy. However, thrombocytosis should not be used as a stand-alone diagnostic or screening test for cancer, or indeed to rule out cancer.
Instead, unexplained thrombocytosis should prompt us to think cancer. The Scottish suspected cancer referral guidelines include thrombocytosis in the investigation criteria for what they call the LEGO-C cancers — L for lung, E for endometrial, G for gastric, O for oesophageal, and C for colorectal, which is a useful reminder for us all.
What further history, examination, and investigations might we consider in primary care if we identify an unexplained high platelet count? As always, we should use our clinical judgment and trust our clinical acumen.
We should consider all the possible underlying causes, including infection, inflammation, and blood loss, including menstrual blood loss in women; myeloproliferative disorders such as polycythemia rubra vera, chronic myeloid leukemia, and essential thrombocythemia; and, of course, underlying malignancy. If a likely underlying reversible cause is present (for example, a recent lower respiratory tract infection), simply repeating the full blood count in 4-6 weeks is quite appropriate to see if the thrombocytosis has resolved.
Remember, 80%-90% of cases are reactive thrombocytosis, and most cases resolve within 3 months. If thrombocytosis is unexplained or not resolving, consider checking ferritin levels to exclude iron deficiency. Consider checking C-reactive protein (CRP) levels to exclude any inflammation, and also consider checking a blood film to exclude any hematologic disorders, in addition, of course, to more detailed history-taking and examination to elicit any red flags.
We can also consider a JAK2 gene mutation test, if it is available to you locally, or a hematology referral if we suspect a myeloproliferative disorder. JAK2 is a genetic mutation that may be present in people with essential thrombocythemia and can indicate a diagnosis of polycythemia rubra vera.
Subsequent to this, and again using our clinical judgment, we then need to exclude the LEGO-C cancers. Consider urgent chest x-ray to exclude lung cancer or pelvic ultrasound in women to exclude endometrial cancer. Also, we should consider an upper gastrointestinal endoscopy, particularly in those individuals who have associated upper gastrointestinal symptoms and/or weight loss.
Finally, consider a qFIT to identify any occult bleeding in the stool, again if it’s available to you, or certainly if not, urgent lower gastrointestinal investigations to exclude colorectal cancer.
Alongside these possible investigations, as always, we should safety-net appropriately within agreed timeframes and check for resolution of the thrombocytosis according to the condition being suspected. Remember, most cases resolve within 3 months.
Returning to Louisa, what did I do? After seeing a platelet count of 600, I subsequently telephoned her and reexplored her history, which yielded nil else of note. Specifically, there was no history of unexplained weight loss, no history of upper or lower gastrointestinal symptoms, and certainly nothing significantly different from her usual irritable bowel syndrome symptoms. There were also no respiratory or genitourinary symptoms of note.
I did arrange for Louisa to undergo a chest x-ray over the next few days, though, as she was an ex-smoker. This was subsequently reported as normal. I appreciate chest x-rays have poor sensitivity for detecting lung cancer, as highlighted in a number of recent papers, but it was mutually agreed with Louisa that we would simply repeat her blood test in around 6 weeks. As well as repeating the full blood count, I arranged to check her ferritin, CRP, and a blood film, and then I was planning to reassess her clinically in person.
These bloods and my subsequent clinical review were reassuring. In fact, her platelet count had normalized after that 6 weeks had elapsed. Her thrombocytosis had resolved.
I didn’t arrange any further follow-up for her, but I did give her the usual safety netting advice to re-present to me or one of my colleagues if she does develop any worrying symptoms or signs.
I appreciate these scenarios are not always this straightforward, but I wanted to outline what investigations and referrals we may need to consider in primary care if we encounter an unexplained high platelet count.
There are a couple of quality-improvement activities for us all to consider in primary care. Consider as a team how we would respond to an incidental finding of thrombocytosis on a full blood count. Also consider what are our safety-netting options for those found to have raised platelet counts but no other symptoms or risk factors for underlying malignancy.
Finally, I’ve produced a Medscape UK primary care hack or clinical aide-memoire on managing unexplained thrombocytosis and associated cancer risk in primary care for all healthcare professionals working in primary care. This can be found online. I hope you find this resource helpful.
Dr. Kevin Fernando, General practitioner partner with specialist interests in cardiovascular, renal, and metabolic medicine, North Berwick Group Practice in Scotland, has disclosed relevant financial relationships with Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Dexcom, Lilly, Menarini, Novartis, Novo Nordisk, Roche Diagnostics, Embecta, Roche Diabetes Care, Sanofi Menarini, and Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
Abuse of the Safety-Net 340B Drug Pricing Program: Why Should Physicians Care?

The 340B Drug Pricing Program began as a noble endeavor, a lifeline designed to help safety-net providers deliver affordable care to America’s most vulnerable populations. However, over the years, this well-intentioned program has strayed from its original purpose, becoming a lucrative space where profits often outweigh patients. Loopholes, lax oversight, and unchecked expansion have allowed some powerful players, such as certain disproportionate share hospitals and their “child sites” as well as for-profit pharmacies, to exploit the system. What was once a program to uplift underserved communities now risks becoming a case study in how good intentions can go astray without accountability.
What exactly is this “340B program” that has captured headlines and the interest of legislatures around the country? What ensures that pharmaceutical manufacturers continue to participate in this program? How lucrative is it? How have underserved populations benefited and how is that measured?
The 340B Drug Pricing Program was established in 1992 under the Public Health Service Act. Its primary goal is to enable covered entities (such as hospitals and clinics serving low-income and uninsured patients) to purchase outpatient drugs from pharmaceutical manufacturers at significantly reduced prices in order to support their care of the low-income and underserved populations. Drug makers are required to participate in this program as a condition of their participation in Medicaid and Medicare Part B and offer these steep discounts to covered entities if they want their medications to be available to 38% of patients nationwide.
The hospitals that make up 78% of the program’s spending are known as disproportionate share hospitals (DSHs). These hospitals must be nonprofit and have at least an 11.75% “disproportionate” share of low-income Medicare or Medicaid inpatients. The other types of non-hospital entities qualifying for 340B pricing are known as initial “federal grantees.” Some examples include federally qualified health centers (FQHC), Ryan White HIV/AIDS program grantees, and other types of specialized clinics, such as hemophilia treatment centers. It needs to be noted up front that it is not these initial non-hospital federal grantees that need more oversight or reform, since according to the Health Resources and Services Administration (HRSA) 2023 report they make up only 22% of all program spending. It is the large, predominantly DSH health systems that are profiting immensely through exponential growth of their clinics and contract pharmacies. However, these health systems have not been able to show exactly who are their eligible patients and how they have been benefiting them.
When the 340B program was established to offer financial relief to hospitals and clinics taking care of the uninsured, it allowed them to save 20%-50% on drug purchases, which could be reinvested in patient care services. It was hoped that savings from the program could be used to provide free or low-cost medications, free vaccines, and other essential health services, essentially allowing safety-net providers to serve their communities despite financial constraints. The initial grantees are fulfilling that mission, but there are concerns regarding DSHs. (See the Coalition of State Rheumatology Organization’s 340B explanatory statement and policy position for more.)
Why Should Independent Practice Physicians Care About This?
Independent doctors should care about the lack of oversight in the 340B program because it affects healthcare costs, patient assistance, market competition, and access to affordable care for underserved and uninsured patients.
It also plays a strong hand in the healthcare consolidation that continues to threaten private physician practices. These acquisitions threaten the viability of independent practices in a variety of specialties across the United States, including rheumatology. HRSA allows 340B-covered entities to register their off-campus outpatient facilities, or child sites, under their 340B designation. Covered entities can acquire drugs at the 340B price, while imposing markups on the reimbursement they submit to private insurance. The additional revenue these covered entities can pocket provides them with a cash flow advantage that physician practices and outpatient clinics will never be able to actualize. This uneven playing field may make rheumatology practices more susceptible to hospital acquisitions. In fact, between 2016 and 2022, large 340B hospitals were responsible for approximately 80% of hospital acquisitions.
Perhaps the most important reason that we should all be concerned about the trajectory of this well-meaning program is that we have seen patients with hospital debt being sued by DSHs who receive 340B discounts so that they can take care of the low-income patients they are suing. We have seen Medicaid patients be turned away from a DSH clinic after being discharged from that hospital, because the hospital had reached its disproportionate share (11.75%) of inpatient Medicare and Medicaid patients. While not illegal, that type of behavior by covered entities is WRONG! Oversight and reform are needed if the 340B program is going to live up to its purpose and not be just another well-intentioned program not fulfilling its mission.
Areas of Concern
There has been controversy regarding the limited oversight of the 340B program by HRSA, leading to abuse of the program. There are deep concerns regarding a lack of transparency in how savings from the program are being used, and there are concerns about the challenges associated with accurate tracking and reporting of 340B discounts, possibly leading to the duplication of discounts for both Medicaid and 340B. For example, a “duplicate discount” occurs if a manufacturer sells medications to a DSH at the 340B price and later pays a Medicaid rebate on the same drug. The extent of duplicate discounts in the 340B program is unknown. However, an audit of 1,536 cases conducted by HRSA between 2012 and 2019 found 429 instances of noncompliance related to duplicate discounts, which is nearly 30% of cases.
DSHs and their contracted pharmacies have been accused of exploiting the program by increasing the number of contract pharmacies and expanding the number of offsite outpatient clinics to maximize profits. As of mid-2024, the number of 340B contract pharmacies, counted by Drug Channels Institute (DCI), numbered 32,883 unique locations. According to DCI, the top five pharmacies in the program happen also to be among the top pharmacy revenue generators and are “for-profit.” They are CVS, Walgreens, Walmart, Express Scripts, and Optum RX. Additionally, a study in JAMA Health Forum showed that, from 2011 to 2019, contract pharmacies in areas with the lowest income decreased by 5.6% while those in the most affluent neighborhoods grew by 5%.
There also has been tremendous growth in the number of covered entities in the 340B program, which grew from just over 8,100 in 2000 to 50,000 in 2020. Before 2004, DSHs made up less than 10% of these entities, but by 2020, they accounted for over 60%. Another study shows that DSHs are expanding their offsite outpatient clinics (“child clinics”) into the affluent neighborhoods serving commercially insured patients who are not low income, to capture the high commercial reimbursements for medications they acquired at steeply discounted prices. This clearly is diverting care away from the intended beneficiaries of the 340B program.
Furthermore, DSHs have been acquiring specialty practices that prescribe some of the most expensive drugs, in order to take advantage of commercial reimbursement for medications that were acquired at the 340B discount price. Independent oncology practices have complained specifically about this happening in their area, where in some cases the DSHs have “stolen” their patients to profit off of the 340B pricing margins. This has the unintended consequence of increasing government spending, according to a study in the New England Journal of Medicine that showed price markups at 340B eligible hospitals were 6.59 times as high as those in independent physician practices after accounting for drug, patient, and geographic factors.
Legal Challenges and Legislation
On May 21, 2024, the US Court of Appeals for the DC Circuit issued a unanimous decision in favor of drug manufacturers, finding that certain manufacturer restrictions on the use of contract pharmacies under the 340B drug pricing program are permissible. The court’s decision follows a lower court (3rd Circuit) ruling which concluded that the 340B statute does not require manufacturers to deliver 340B drugs to an “unlimited number of contract pharmacies.” We’re still awaiting a decision from the 7th Circuit Court on a similar issue. If the 7th Circuit agrees with the government, creating a split decision, there is an increase in the likelihood that the Supreme Court would take up the case.
Johnson & Johnson has also sued the federal government for blocking their proposed use of a rebate model for DSHs that purchase through 340B two of its medications, Stelara and Xarelto, whose maximum fair price was negotiated through the Inflation Reduction Act’s Medicare Drug Price Negotiation Program. J&J states this would ensure that the claims are actually acquired and dispensed by a covered 340B entity, as well as ensuring there are no duplicate discounts as statutorily required by the IRA. When initially proposed, HRSA threatened to remove J&J’s access to Medicare and Medicaid if it pursued this change. J&J’s suit challenges that decision.
However, seven states (Arkansas, Kansas, Louisiana, Minnesota, Missouri, Mississippi, and West Virginia) have been active on this issue, passing laws to prevent manufacturers from limiting contract pharmacies’ ability to acquire 340B-discounted drugs. The model legislation also bans restrictions on the “number, location, ownership, or type of 340B contract pharmacy.”
It should also be noted that there are states that are looking for ways to encourage certain independent private practice specialties (such as gastroenterology and rheumatology) to see Medicaid patients, as well as increase testing for sexually transmitted diseases, by offering the possibility of obtaining 340B pricing in their clinics.
Shifting our focus to Congress, six bipartisan Senators, known as the Group of 6, are working to modernize the 340B program, which hasn’t been updated since the original law in 1992. In 2024, legislation was introduced (see here and here) to reform a number of the features of the 340B drug discount program, including transparency, contract pharmacy requirements, and federal agency oversight.
Who’s Guarding the Hen House?
The Government Accountability Office and the Office of Inspector General over the last 5-10 years have asked HRSA to better define an “eligible” patient, to have more specifics concerning hospital eligibility criteria, and to have better oversight of the program to avoid duplicate discounts. HRSA has said that it doesn’t have the ability or the funding to achieve some of these goals. Consequently, little has been done on any of these fronts, creating frustration among pharmaceutical manufacturers and those calling for more oversight of the program to ensure that eligible patients are receiving the benefit of 340B pricing. Again, these frustrations are not pointed at the initial federally qualified centers or “grantees.”
HRSA now audits 200 covered entities a year, which is less than 2% of entities participating in the 340B program. HRSA expects the 340B entities themselves to have an oversight committee in place to ensure compliance with program requirements.
So essentially, the fox is guarding the hen house?
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.

The 340B Drug Pricing Program began as a noble endeavor, a lifeline designed to help safety-net providers deliver affordable care to America’s most vulnerable populations. However, over the years, this well-intentioned program has strayed from its original purpose, becoming a lucrative space where profits often outweigh patients. Loopholes, lax oversight, and unchecked expansion have allowed some powerful players, such as certain disproportionate share hospitals and their “child sites” as well as for-profit pharmacies, to exploit the system. What was once a program to uplift underserved communities now risks becoming a case study in how good intentions can go astray without accountability.
What exactly is this “340B program” that has captured headlines and the interest of legislatures around the country? What ensures that pharmaceutical manufacturers continue to participate in this program? How lucrative is it? How have underserved populations benefited and how is that measured?
The 340B Drug Pricing Program was established in 1992 under the Public Health Service Act. Its primary goal is to enable covered entities (such as hospitals and clinics serving low-income and uninsured patients) to purchase outpatient drugs from pharmaceutical manufacturers at significantly reduced prices in order to support their care of the low-income and underserved populations. Drug makers are required to participate in this program as a condition of their participation in Medicaid and Medicare Part B and offer these steep discounts to covered entities if they want their medications to be available to 38% of patients nationwide.
The hospitals that make up 78% of the program’s spending are known as disproportionate share hospitals (DSHs). These hospitals must be nonprofit and have at least an 11.75% “disproportionate” share of low-income Medicare or Medicaid inpatients. The other types of non-hospital entities qualifying for 340B pricing are known as initial “federal grantees.” Some examples include federally qualified health centers (FQHC), Ryan White HIV/AIDS program grantees, and other types of specialized clinics, such as hemophilia treatment centers. It needs to be noted up front that it is not these initial non-hospital federal grantees that need more oversight or reform, since according to the Health Resources and Services Administration (HRSA) 2023 report they make up only 22% of all program spending. It is the large, predominantly DSH health systems that are profiting immensely through exponential growth of their clinics and contract pharmacies. However, these health systems have not been able to show exactly who are their eligible patients and how they have been benefiting them.
When the 340B program was established to offer financial relief to hospitals and clinics taking care of the uninsured, it allowed them to save 20%-50% on drug purchases, which could be reinvested in patient care services. It was hoped that savings from the program could be used to provide free or low-cost medications, free vaccines, and other essential health services, essentially allowing safety-net providers to serve their communities despite financial constraints. The initial grantees are fulfilling that mission, but there are concerns regarding DSHs. (See the Coalition of State Rheumatology Organization’s 340B explanatory statement and policy position for more.)
Why Should Independent Practice Physicians Care About This?
Independent doctors should care about the lack of oversight in the 340B program because it affects healthcare costs, patient assistance, market competition, and access to affordable care for underserved and uninsured patients.
It also plays a strong hand in the healthcare consolidation that continues to threaten private physician practices. These acquisitions threaten the viability of independent practices in a variety of specialties across the United States, including rheumatology. HRSA allows 340B-covered entities to register their off-campus outpatient facilities, or child sites, under their 340B designation. Covered entities can acquire drugs at the 340B price, while imposing markups on the reimbursement they submit to private insurance. The additional revenue these covered entities can pocket provides them with a cash flow advantage that physician practices and outpatient clinics will never be able to actualize. This uneven playing field may make rheumatology practices more susceptible to hospital acquisitions. In fact, between 2016 and 2022, large 340B hospitals were responsible for approximately 80% of hospital acquisitions.
Perhaps the most important reason that we should all be concerned about the trajectory of this well-meaning program is that we have seen patients with hospital debt being sued by DSHs who receive 340B discounts so that they can take care of the low-income patients they are suing. We have seen Medicaid patients be turned away from a DSH clinic after being discharged from that hospital, because the hospital had reached its disproportionate share (11.75%) of inpatient Medicare and Medicaid patients. While not illegal, that type of behavior by covered entities is WRONG! Oversight and reform are needed if the 340B program is going to live up to its purpose and not be just another well-intentioned program not fulfilling its mission.
Areas of Concern
There has been controversy regarding the limited oversight of the 340B program by HRSA, leading to abuse of the program. There are deep concerns regarding a lack of transparency in how savings from the program are being used, and there are concerns about the challenges associated with accurate tracking and reporting of 340B discounts, possibly leading to the duplication of discounts for both Medicaid and 340B. For example, a “duplicate discount” occurs if a manufacturer sells medications to a DSH at the 340B price and later pays a Medicaid rebate on the same drug. The extent of duplicate discounts in the 340B program is unknown. However, an audit of 1,536 cases conducted by HRSA between 2012 and 2019 found 429 instances of noncompliance related to duplicate discounts, which is nearly 30% of cases.
DSHs and their contracted pharmacies have been accused of exploiting the program by increasing the number of contract pharmacies and expanding the number of offsite outpatient clinics to maximize profits. As of mid-2024, the number of 340B contract pharmacies, counted by Drug Channels Institute (DCI), numbered 32,883 unique locations. According to DCI, the top five pharmacies in the program happen also to be among the top pharmacy revenue generators and are “for-profit.” They are CVS, Walgreens, Walmart, Express Scripts, and Optum RX. Additionally, a study in JAMA Health Forum showed that, from 2011 to 2019, contract pharmacies in areas with the lowest income decreased by 5.6% while those in the most affluent neighborhoods grew by 5%.
There also has been tremendous growth in the number of covered entities in the 340B program, which grew from just over 8,100 in 2000 to 50,000 in 2020. Before 2004, DSHs made up less than 10% of these entities, but by 2020, they accounted for over 60%. Another study shows that DSHs are expanding their offsite outpatient clinics (“child clinics”) into the affluent neighborhoods serving commercially insured patients who are not low income, to capture the high commercial reimbursements for medications they acquired at steeply discounted prices. This clearly is diverting care away from the intended beneficiaries of the 340B program.
Furthermore, DSHs have been acquiring specialty practices that prescribe some of the most expensive drugs, in order to take advantage of commercial reimbursement for medications that were acquired at the 340B discount price. Independent oncology practices have complained specifically about this happening in their area, where in some cases the DSHs have “stolen” their patients to profit off of the 340B pricing margins. This has the unintended consequence of increasing government spending, according to a study in the New England Journal of Medicine that showed price markups at 340B eligible hospitals were 6.59 times as high as those in independent physician practices after accounting for drug, patient, and geographic factors.
Legal Challenges and Legislation
On May 21, 2024, the US Court of Appeals for the DC Circuit issued a unanimous decision in favor of drug manufacturers, finding that certain manufacturer restrictions on the use of contract pharmacies under the 340B drug pricing program are permissible. The court’s decision follows a lower court (3rd Circuit) ruling which concluded that the 340B statute does not require manufacturers to deliver 340B drugs to an “unlimited number of contract pharmacies.” We’re still awaiting a decision from the 7th Circuit Court on a similar issue. If the 7th Circuit agrees with the government, creating a split decision, there is an increase in the likelihood that the Supreme Court would take up the case.
Johnson & Johnson has also sued the federal government for blocking their proposed use of a rebate model for DSHs that purchase through 340B two of its medications, Stelara and Xarelto, whose maximum fair price was negotiated through the Inflation Reduction Act’s Medicare Drug Price Negotiation Program. J&J states this would ensure that the claims are actually acquired and dispensed by a covered 340B entity, as well as ensuring there are no duplicate discounts as statutorily required by the IRA. When initially proposed, HRSA threatened to remove J&J’s access to Medicare and Medicaid if it pursued this change. J&J’s suit challenges that decision.
However, seven states (Arkansas, Kansas, Louisiana, Minnesota, Missouri, Mississippi, and West Virginia) have been active on this issue, passing laws to prevent manufacturers from limiting contract pharmacies’ ability to acquire 340B-discounted drugs. The model legislation also bans restrictions on the “number, location, ownership, or type of 340B contract pharmacy.”
It should also be noted that there are states that are looking for ways to encourage certain independent private practice specialties (such as gastroenterology and rheumatology) to see Medicaid patients, as well as increase testing for sexually transmitted diseases, by offering the possibility of obtaining 340B pricing in their clinics.
Shifting our focus to Congress, six bipartisan Senators, known as the Group of 6, are working to modernize the 340B program, which hasn’t been updated since the original law in 1992. In 2024, legislation was introduced (see here and here) to reform a number of the features of the 340B drug discount program, including transparency, contract pharmacy requirements, and federal agency oversight.
Who’s Guarding the Hen House?
The Government Accountability Office and the Office of Inspector General over the last 5-10 years have asked HRSA to better define an “eligible” patient, to have more specifics concerning hospital eligibility criteria, and to have better oversight of the program to avoid duplicate discounts. HRSA has said that it doesn’t have the ability or the funding to achieve some of these goals. Consequently, little has been done on any of these fronts, creating frustration among pharmaceutical manufacturers and those calling for more oversight of the program to ensure that eligible patients are receiving the benefit of 340B pricing. Again, these frustrations are not pointed at the initial federally qualified centers or “grantees.”
HRSA now audits 200 covered entities a year, which is less than 2% of entities participating in the 340B program. HRSA expects the 340B entities themselves to have an oversight committee in place to ensure compliance with program requirements.
So essentially, the fox is guarding the hen house?
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.

The 340B Drug Pricing Program began as a noble endeavor, a lifeline designed to help safety-net providers deliver affordable care to America’s most vulnerable populations. However, over the years, this well-intentioned program has strayed from its original purpose, becoming a lucrative space where profits often outweigh patients. Loopholes, lax oversight, and unchecked expansion have allowed some powerful players, such as certain disproportionate share hospitals and their “child sites” as well as for-profit pharmacies, to exploit the system. What was once a program to uplift underserved communities now risks becoming a case study in how good intentions can go astray without accountability.
What exactly is this “340B program” that has captured headlines and the interest of legislatures around the country? What ensures that pharmaceutical manufacturers continue to participate in this program? How lucrative is it? How have underserved populations benefited and how is that measured?
The 340B Drug Pricing Program was established in 1992 under the Public Health Service Act. Its primary goal is to enable covered entities (such as hospitals and clinics serving low-income and uninsured patients) to purchase outpatient drugs from pharmaceutical manufacturers at significantly reduced prices in order to support their care of the low-income and underserved populations. Drug makers are required to participate in this program as a condition of their participation in Medicaid and Medicare Part B and offer these steep discounts to covered entities if they want their medications to be available to 38% of patients nationwide.
The hospitals that make up 78% of the program’s spending are known as disproportionate share hospitals (DSHs). These hospitals must be nonprofit and have at least an 11.75% “disproportionate” share of low-income Medicare or Medicaid inpatients. The other types of non-hospital entities qualifying for 340B pricing are known as initial “federal grantees.” Some examples include federally qualified health centers (FQHC), Ryan White HIV/AIDS program grantees, and other types of specialized clinics, such as hemophilia treatment centers. It needs to be noted up front that it is not these initial non-hospital federal grantees that need more oversight or reform, since according to the Health Resources and Services Administration (HRSA) 2023 report they make up only 22% of all program spending. It is the large, predominantly DSH health systems that are profiting immensely through exponential growth of their clinics and contract pharmacies. However, these health systems have not been able to show exactly who are their eligible patients and how they have been benefiting them.
When the 340B program was established to offer financial relief to hospitals and clinics taking care of the uninsured, it allowed them to save 20%-50% on drug purchases, which could be reinvested in patient care services. It was hoped that savings from the program could be used to provide free or low-cost medications, free vaccines, and other essential health services, essentially allowing safety-net providers to serve their communities despite financial constraints. The initial grantees are fulfilling that mission, but there are concerns regarding DSHs. (See the Coalition of State Rheumatology Organization’s 340B explanatory statement and policy position for more.)
Why Should Independent Practice Physicians Care About This?
Independent doctors should care about the lack of oversight in the 340B program because it affects healthcare costs, patient assistance, market competition, and access to affordable care for underserved and uninsured patients.
It also plays a strong hand in the healthcare consolidation that continues to threaten private physician practices. These acquisitions threaten the viability of independent practices in a variety of specialties across the United States, including rheumatology. HRSA allows 340B-covered entities to register their off-campus outpatient facilities, or child sites, under their 340B designation. Covered entities can acquire drugs at the 340B price, while imposing markups on the reimbursement they submit to private insurance. The additional revenue these covered entities can pocket provides them with a cash flow advantage that physician practices and outpatient clinics will never be able to actualize. This uneven playing field may make rheumatology practices more susceptible to hospital acquisitions. In fact, between 2016 and 2022, large 340B hospitals were responsible for approximately 80% of hospital acquisitions.
Perhaps the most important reason that we should all be concerned about the trajectory of this well-meaning program is that we have seen patients with hospital debt being sued by DSHs who receive 340B discounts so that they can take care of the low-income patients they are suing. We have seen Medicaid patients be turned away from a DSH clinic after being discharged from that hospital, because the hospital had reached its disproportionate share (11.75%) of inpatient Medicare and Medicaid patients. While not illegal, that type of behavior by covered entities is WRONG! Oversight and reform are needed if the 340B program is going to live up to its purpose and not be just another well-intentioned program not fulfilling its mission.
Areas of Concern
There has been controversy regarding the limited oversight of the 340B program by HRSA, leading to abuse of the program. There are deep concerns regarding a lack of transparency in how savings from the program are being used, and there are concerns about the challenges associated with accurate tracking and reporting of 340B discounts, possibly leading to the duplication of discounts for both Medicaid and 340B. For example, a “duplicate discount” occurs if a manufacturer sells medications to a DSH at the 340B price and later pays a Medicaid rebate on the same drug. The extent of duplicate discounts in the 340B program is unknown. However, an audit of 1,536 cases conducted by HRSA between 2012 and 2019 found 429 instances of noncompliance related to duplicate discounts, which is nearly 30% of cases.
DSHs and their contracted pharmacies have been accused of exploiting the program by increasing the number of contract pharmacies and expanding the number of offsite outpatient clinics to maximize profits. As of mid-2024, the number of 340B contract pharmacies, counted by Drug Channels Institute (DCI), numbered 32,883 unique locations. According to DCI, the top five pharmacies in the program happen also to be among the top pharmacy revenue generators and are “for-profit.” They are CVS, Walgreens, Walmart, Express Scripts, and Optum RX. Additionally, a study in JAMA Health Forum showed that, from 2011 to 2019, contract pharmacies in areas with the lowest income decreased by 5.6% while those in the most affluent neighborhoods grew by 5%.
There also has been tremendous growth in the number of covered entities in the 340B program, which grew from just over 8,100 in 2000 to 50,000 in 2020. Before 2004, DSHs made up less than 10% of these entities, but by 2020, they accounted for over 60%. Another study shows that DSHs are expanding their offsite outpatient clinics (“child clinics”) into the affluent neighborhoods serving commercially insured patients who are not low income, to capture the high commercial reimbursements for medications they acquired at steeply discounted prices. This clearly is diverting care away from the intended beneficiaries of the 340B program.
Furthermore, DSHs have been acquiring specialty practices that prescribe some of the most expensive drugs, in order to take advantage of commercial reimbursement for medications that were acquired at the 340B discount price. Independent oncology practices have complained specifically about this happening in their area, where in some cases the DSHs have “stolen” their patients to profit off of the 340B pricing margins. This has the unintended consequence of increasing government spending, according to a study in the New England Journal of Medicine that showed price markups at 340B eligible hospitals were 6.59 times as high as those in independent physician practices after accounting for drug, patient, and geographic factors.
Legal Challenges and Legislation
On May 21, 2024, the US Court of Appeals for the DC Circuit issued a unanimous decision in favor of drug manufacturers, finding that certain manufacturer restrictions on the use of contract pharmacies under the 340B drug pricing program are permissible. The court’s decision follows a lower court (3rd Circuit) ruling which concluded that the 340B statute does not require manufacturers to deliver 340B drugs to an “unlimited number of contract pharmacies.” We’re still awaiting a decision from the 7th Circuit Court on a similar issue. If the 7th Circuit agrees with the government, creating a split decision, there is an increase in the likelihood that the Supreme Court would take up the case.
Johnson & Johnson has also sued the federal government for blocking their proposed use of a rebate model for DSHs that purchase through 340B two of its medications, Stelara and Xarelto, whose maximum fair price was negotiated through the Inflation Reduction Act’s Medicare Drug Price Negotiation Program. J&J states this would ensure that the claims are actually acquired and dispensed by a covered 340B entity, as well as ensuring there are no duplicate discounts as statutorily required by the IRA. When initially proposed, HRSA threatened to remove J&J’s access to Medicare and Medicaid if it pursued this change. J&J’s suit challenges that decision.
However, seven states (Arkansas, Kansas, Louisiana, Minnesota, Missouri, Mississippi, and West Virginia) have been active on this issue, passing laws to prevent manufacturers from limiting contract pharmacies’ ability to acquire 340B-discounted drugs. The model legislation also bans restrictions on the “number, location, ownership, or type of 340B contract pharmacy.”
It should also be noted that there are states that are looking for ways to encourage certain independent private practice specialties (such as gastroenterology and rheumatology) to see Medicaid patients, as well as increase testing for sexually transmitted diseases, by offering the possibility of obtaining 340B pricing in their clinics.
Shifting our focus to Congress, six bipartisan Senators, known as the Group of 6, are working to modernize the 340B program, which hasn’t been updated since the original law in 1992. In 2024, legislation was introduced (see here and here) to reform a number of the features of the 340B drug discount program, including transparency, contract pharmacy requirements, and federal agency oversight.
Who’s Guarding the Hen House?
The Government Accountability Office and the Office of Inspector General over the last 5-10 years have asked HRSA to better define an “eligible” patient, to have more specifics concerning hospital eligibility criteria, and to have better oversight of the program to avoid duplicate discounts. HRSA has said that it doesn’t have the ability or the funding to achieve some of these goals. Consequently, little has been done on any of these fronts, creating frustration among pharmaceutical manufacturers and those calling for more oversight of the program to ensure that eligible patients are receiving the benefit of 340B pricing. Again, these frustrations are not pointed at the initial federally qualified centers or “grantees.”
HRSA now audits 200 covered entities a year, which is less than 2% of entities participating in the 340B program. HRSA expects the 340B entities themselves to have an oversight committee in place to ensure compliance with program requirements.
So essentially, the fox is guarding the hen house?
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at rhnews@mdedge.com.
Total Laparoscopic Segmental Resection With Transanal NOSE for Deep Colorectal Endometriosis
What to Know About NOSE
Traditionally, a laparoscopic approach to bowel resection secondary to endometriosis means enlargement of the port site or more commonly, a Pfannenstiel incision to introduce an anvil. Both incisions raise the risk of postoperative complications including pain and incisional hernia. Moreover, recovery time is prolonged.
Natural orifice specimen extraction (NOSE) makes use of a natural orifice, the vagina or anus, to extract the specimen and introduce the anvil. Transanal extraction for the management of endometriosis-related bowel disease was first described by the late David Redwine, MD, in his 1996 publication in Fertility and Sterility.1
The concern with use of vaginal NOSE surgery is rectovaginal fistula secondary to two incision lines in close opposition. Both transvaginal NOSE and transanal NOSE have been criticized for longer dissection of the mesorectum and thus longer resected specimen. Despite these concerns, over the past 10 years, articles have been published showing not only the feasibility of the NOSE technique, but excellent comparative outcomes, as well.2-4
A recent systematic review and meta-analysis by Kar et al. comparing NOSE extraction to minilaparotomy in bowel resection due to endometriosis revealed no significant difference in complication rate, but significantly shorter operation duration, reduced length of stay, and less blood loss in the NOSE surgical arm.5
The most recent study to be published in the Journal of Minimally Invasive Gynecology involving total laparoscopic segmental resection with transanal NOSE for the treatment of colorectal endometriosis was written by the guest author of this edition of the Master Class in Gynecologic Surgery, Professor Mario Malzoni, Scientific Director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. In the JMIG article, and in this Master Class, Professor Malzoni, a world-renowned endometriosis specialist recognized for his extraordinary skill and multi-organ surgical work, discusses the difference between the standard bowel resection technique with that of NOSE, and reports on his experience with NOSE.6
It is a pleasure and honor to welcome my friend and colleague, Professor Mario Malzoni, to this edition of the Master Class in Gynecologic Surgery.
Dr. Charles E. Miller is Professor, Obstetrics & Gynecology, Department of Clinical Sciences, Rosalind Franklin University of Medicine and Science, North Chicago, and Director, Minimally Invasive Gynecologic Surgery, Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at obnews@mdedge.com.
References
1. Redwine DB et al. Laparoscopically assisted transvaginal segmental resection of the rectosigmoid colon for endometriosis. Fertil Steril. 1996 Jan;65(1):193-7.
2. Akladios C et al. Totally laparoscopic intracorporeal anastomosis with natural orifice specimen extraction (NOSE) techniques, particularly suitable for bowel endometriosis. J Minim Invasive Gynecol. 2014 Nov-Dec;21(6):1095-102.
3. Bokor A et al. Natural orifice specimen extraction during laparoscopic bowel resection for colorectal endometriosis: technique and outcome. J Minim Invasive Gynecol. 2018 Sep-Oct;25(6):1065-74.
4. Grigoriadis G et al. Natural orifice specimen extraction colorectal resection for deep endometriosis: A 50 case series. J Minim Invasive Gynecol. 2022 Sep;29(9):1054-62.
5. Kar E et al. Natural orifice specimen extraction as a promising alternative for minilaparotomy in bowel resection due to endometriosis: A systematic review and meta-analysis. J Minim Invasive Gynecol. 2024 Jul;31(7):574-83.e1.
6. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024 Oct;18:S1553-4650(24)01453-5.
Laparoscopic Resection With Transanal NOSE for Deep Colorectal Endometriosis
When segmental sigmoid colon/rectal resection is indicated for treatment of deep infiltrating bowel endometriosis, a totally laparoscopic resection with intracorporeal anastomosis and transanal natural orifice specimen extraction (NOSE) can be safe, effective, and advantageous compared with an abdominal mini-laparotomy for extracorporeal anastomosis and specimen retrieval.
With advanced laparoscopic surgical skills, accurate preoperative ultrasound evaluation, and a high-quality perioperative care protocol (including high-quality bowel preparation), segmental resection with NOSE can offer better preservation of vascularization, smaller resection sizes, lower rates of postoperative pain, a shorter time for gas passage after surgery, decreased hospital stays, and lower rates of wound infection and incisional hernia.
We have seen such improved results in our high-volume center since shifting in April 2021 from our classical technique for segmental rectosigmoid resection1 to a totally laparoscopic approach with NOSE. We first reported on this new technique in 2022 in a video article in the Journal of Minimally Invasive Gynecology,2and in October 2024, outcomes of 81 patients who underwent segmental sigmoid colon/rectal resection with intracorporeal anastomosis and transanal NOSE at our institution from April 2021 through March 2024 were reported in Journal of Minimally Invasive Gynecology.3Given our experience with this approach for deep endometriosis, I am convinced that NOSE should be preferred to transabdominal specimen extraction for segmental rectosigmoid resection. This is where the future lies for deep infiltrating endometriosis. Here, I share our outcomes and describe our technique.
Our Prior Approach With A Mini-Laparotomy
Our traditional laparoscopic approach for segmental colon/rectal resection of deep infiltrating endometriosis involved an abdominal mini-laparotomy (no more than 4 cm in length) for specimen retrieval, resection of the proximal rectum, and positioning of the head of a circular stapler for end-to-end anastomosis. (Connection of the two parts of the circular stapler for anastomosis was achieved via laparoscopy.)1
Several factors allowed us to minimize potential complications, including standardization of our technique, the use of three-dimensional endoscopes, and indocyanine green fluorescence angiography to assess the perfusion of the bowel after the completion of anastomosis. Still, an unpublished analysis of 1050 segmental bowel resections performed over more than 17 years using our traditional technique showed that hospital stays averaged 8 days, time to first defecation averaged 7 days, and time to first passage of flatus averaged 1 day.
Per the Clavien-Dindo classification system for surgical complications that was used at the time, 1.9% of these 1050 patients had grade 1 (minor) or grade 2 complications and 6% had grade 3 complications. Grade 2 complications were defined as needing intervention or a hospital stay more than twice the median for the procedure. Grade 3 complications were defined as leading to lasting disability or organ resection.
In the meantime, in colorectal cancer surgery, published studies on NOSE versus conventional laparoscopy with transabdominal specimen extraction have consistently pointed to the benefits of NOSE. A 2022 review 4 of 19 studies involving over 3400 patients and a 2022 meta-analysis 5 of 21 randomized controlled trials involving more than 2000 patients both showed significantly reduced postoperative morbidity and no differences in oncologic outcomes. Among the notable differences in the 2022 meta-analysis was a relative risk of postoperative infection of 0.34 for patients treated with NOSE compared with conventional laparoscopic techniques.
Among patients with deep rectal endometriosis, reported experience with intracorporeal anastomosis and NOSE for segmental resection is limited, with a few studies published between 2014 and 2022. Excluding anecdotal reports, only about 140 patients have been reported in the literature as having intracorporeal anastomosis and transanal NOSE for deep infiltrating bowel endometriosis. 3
Our Outcomes With A Totally Laparoscopic Approach
Our approach to evaluating our experience with totally laparoscopic resection with transanal NOSE has been to systematically collect data from consecutive patients and to analyze 1) complications of the technique, 2) conversion to the traditional technique/open surgery, and 3) endometriosis-free bowel resection margins and recurrence. Secondarily, we look at intraoperative blood loss, operative time, recovery of gastrointestinal function, hospital stay length, and reproductive outcomes.
Across 81 patients from our TrEnd study database (Surgical TReatment of women with deep ENDometriosis infiltrating the sigmoid colon and/or rectum) who received a totally laparoscopic standardized procedure involving intracorporeal anastomosis and transanal NOSE, we had no conversions even to mini-laparotomy, and no cases of protective colostomy or ileostomy.
Complete endometriosis removal was achieved in 100% of cases, with final pathology showing endometriosis-free resection margins, and patients remained free of bowel endometriosis at a median follow-up of 21 months.3Our analysis also shows improved gastrointestinal function recovery (median time to first defecation of 4 days, compared to 7 days previously, with the same 1-day median time to first passage of flatus) and a significantly improved hospital length of stay to a median of 3 days (interquartile range, 3-4.5 days); the latter reflects in part the absence of infection.3There were no intra-operative complications. Postoperative Clavien-Dindo grade 3 complications occurred in three patients (3.7%), two of whom required reoperation (one for hemoperitoneum and one for ureteral injury) and one who experienced rectal anastomotic bleeding on postoperative day 1. (The two requiring reoperation had significant right lateral parametrial involvement and underwent nerve-sparing parametrectomy at the time of bowel resection).3Notably, 7 of the 81 patients had totally laparoscopic double segmental colorectal resection — 6 with simultaneous ileocecal valve and rectal resection, and 1 with simultaneous ileal and rectal resection. None of these seven patients had postoperative complications. (A report on the six surgeries for deep endometriosis infiltrating the ileocecal valve and rectum was also published this year in the journal Colorectal Disease.6)
Median blood loss in the 81-patient cohort was 20 mL (IQR, 20-30 mL), and the median length of surgery was 160 minutes (IQR, 130-210 minutes).
Bowel endometriotic nodules had a mean maximum diameter of 4.3 cm (3.5-5.5 cm), a mean depth of invasion of 9 mm (7-9 mm), a mean estimated stenosis of 40% (35%-50%), and a mean distance from the anal verge of 13 cm (10-15 cm).3 (The cohort in this analysis did not include patients who received concomitant hysterectomy and transvaginal NOSE.)
Specifics of the New Technique, Including Totally Intracorporeal Anastomosis
As in our previous technique, the surgical procedure starts with left and dorsal rectal mobilization. The left pelvic sidewall dissection begins with incision of the parietal peritoneum along the pelvic portions of the psoas muscle. The retroperitoneal connective tissue is dissected in order to identify the ureter, the hypogastric nerve, and the presacral parietal pelvic fascia (PPPF). Dissection is carried downward in front of the PPPF and medially to the left hypogastric nerve.
Right and ventral rectal mobilization begins with incision of the peritoneum along the gray avascular line, which is seen with upward retraction of the rectum.
Again, for preservation of bowel and bladder function, the hypogastric nerve must be identified and preserved through dissection that displaces the nerve dorsally and laterally.
Dissection proceeds to sharply divide the nodule into two parts (rectal and rectocervical components). The caudal boundary of ventral dissection is reached by incising the septum and opening the rectovaginal space. The lymphovascular fatty tissue surrounding the rectum is then excised and the rectal tube is denuded at the level of the distal resection margin.
The distal and proximal resection margins are prepared and transected using a tissue-sealing device, and the resected rectal specimen is placed into a retrieval bag and pulled out through the anus (Figure 1).
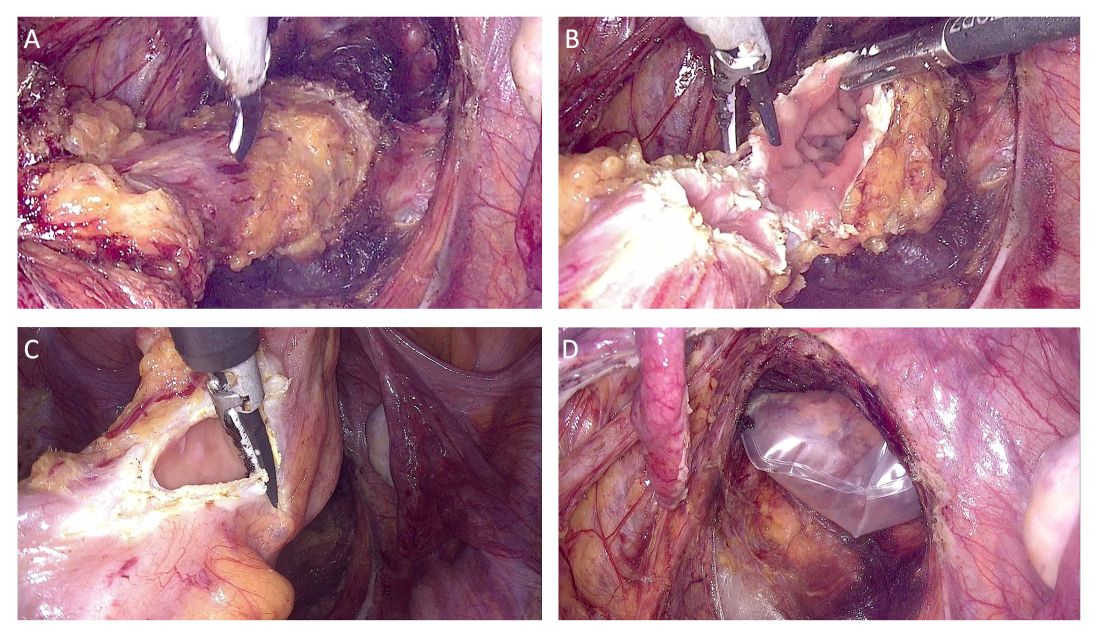
The totally intracorporeal side-to-end anastomosis is performed using a traditional circular stapler. Its anvil is inserted through the proximal resection line and exteriorized forward on the antimesenteric side of the sigmoid colon/rectum. The proximal and distal resection lines are closed with a 60-mm linear endo-stapler, and finally, the shaft of the circular stapler is inserted into the rectum, and the two parts of the stapler are joined. The stapler is then closed and fired (Figure 2).
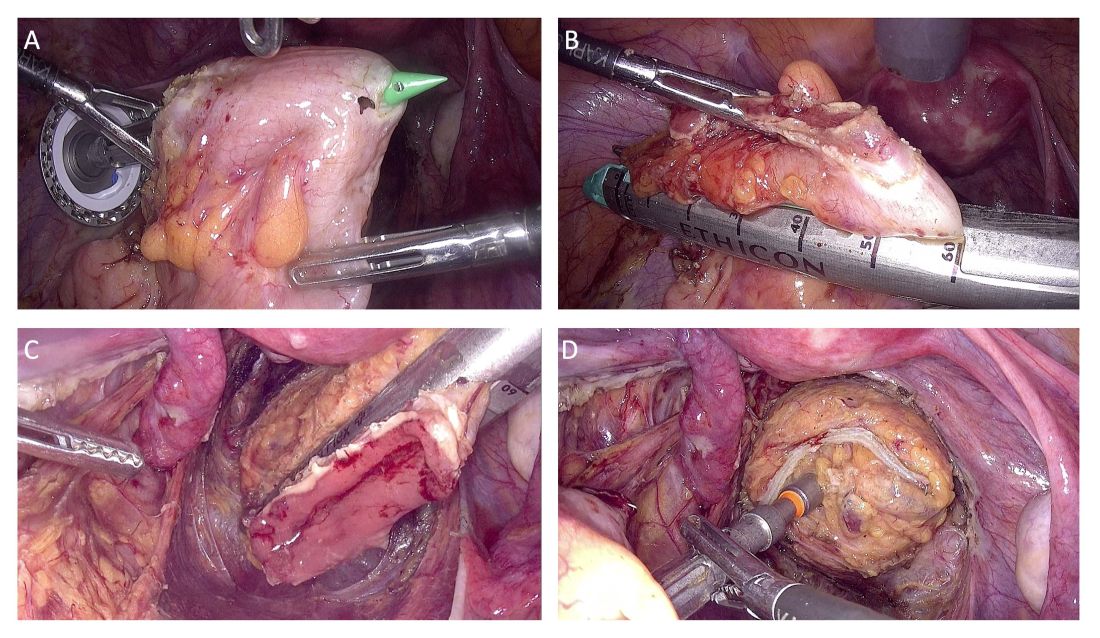
An air leak test is performed to check the quality of the rectal sutures. However, regardless of the results, we routinely apply interrupted stitches to the so-called “dog ears” in order to reduce tension along the anastomotic line.
We also evaluate the microvascularization of the two parts of the bowel at the level of the reanastomosis using indocyanine green fluorescence angiography. Doing so allows us to avoid complications caused by anastomotic leakage.
Patient Selection And Peri-Surgical Care
The decision to perform segmental resection as opposed to other more conservative laparoscopic excision techniques is made after skilled preoperative imaging reveals the number of lesions, the largest nodule diameter, the infiltration depth, and the circumference of bowel involvement.
A recently published international consensus statement7 on noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems concludes there is Level 1a evidence that preoperative imaging with transvaginal ultrasound (TVUS) can predict with good precision the size and degree of infiltration of deep endometriosis of the rectum (a Grade A statement, per the Oxford Centre for Evidence-Based Medicine levels of evidence).
The consensus document was published across seven different journals by international and European societies, and the American Association of Gynecologic Laparoscopists (AAGL), each of which contributed to a multidisciplinary panel of gynecological surgeons, sonographers, and radiologists.
At our center, we achieve precise preoperative evaluations with TVUS (other centers also employ MRI) and have long set clear preoperative indications for segmental resection. Based on our experience,8 a nodule ≥ 3 cm and infiltration of the tunica muscularis of the sigmoid colon/rectum ≥ 7 mm indicate the need for segmental resection. It is impossible to achieve surgical goals in such cases through shaving or discoid resection.
Our perioperative care protocol for total laparoscopic segmental resection with transanal NOSE, described in our new paper,3 includes several components: antibiotic therapy (metronidazole 500 mg 12 hours before surgery; cefazolin 2 g plus metronidazole 500 mg 1 hour before skin incision; and cefazolin 1 g plus metronidazole 500 mg 12 hours after surgery); good intraoperative bowel preparation (mechanical with oral polyethylene glycol solution preoperatively, and rectal irrigation with a mixed solution of povidine-iodine and normal saline intraoperatively); and venous thromboembolism prophylaxis postoperatively for 28 days.
Moving Forward, A Word On Classification
Multicenter trials and research with longer follow-up times will be helpful in advancing the use of totally laparoscopic segmental colorectal resection for deep endometriosis. To enable better sharing of diagnostic and therapeutic results — and to advance the credibility of research — my hope is that the field will reach further agreement on endometriosis classification systems. This would also help with the development of standards for postgraduate education.
We have made significant strides in recognizing imaging as a standard for diagnosis and treatment, and I believe we currently have two very good endometriosis classification systems — the #Enzian classification system issued in 2021 and the AAGL 2021 Endometriosis Classification — that can be used in combination with imaging to reliably and consistently describe deep endometriosis.
Mario Malzoni, MD, is scientific director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. He reported having no disclosures relevant to this Master Class.
References
1. Malzoni M et al. Surgical principles of segmental rectosigmoid resection and reanastomosis for deep infiltrating endometriosis. J Minim Invasive Gynecol. 2020;27(2):258.
2. Malzoni M et al. Totally laparoscopic resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the rectum. J Minim Invasive Gynecol. 2022;29(1):19.
3. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024. (in press). 4. Brincat SD et al. Natural orifice versus transabdominal specimen extraction in laparoscopic surgery for colorectal cancer: meta-analysis. BJS Open 2022;6(3):zrac074.
5. Zhou Z et al. Laparoscopic natural orifice specimen extraction surgery versus conventional surgery in colorectal cancer: A meta-analysis of randomized controlled trials. Gastroenterol Res Pract. 2022 Jan 18;2022:6661651.
6. Malzoni M et al. Simultaneous total laparoscopic double segmental resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the ileocaecal valve and rectum—A video vignette. Colorectal Dis 2024 Jul 25.
7. Condous G et al. Noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems: An international consensus. J. Minim Invasive Gynecol. 2024;31(7):557-73.
8. Malzoni M et al. Preoperative ultrasound indications determine excision technique for bowel surgery for deep infiltrating endometriosis: A single, high-volume center. J Minim Invasive Gynecol. 2020;27(5):1141-7.
What to Know About NOSE
Traditionally, a laparoscopic approach to bowel resection secondary to endometriosis means enlargement of the port site or more commonly, a Pfannenstiel incision to introduce an anvil. Both incisions raise the risk of postoperative complications including pain and incisional hernia. Moreover, recovery time is prolonged.
Natural orifice specimen extraction (NOSE) makes use of a natural orifice, the vagina or anus, to extract the specimen and introduce the anvil. Transanal extraction for the management of endometriosis-related bowel disease was first described by the late David Redwine, MD, in his 1996 publication in Fertility and Sterility.1
The concern with use of vaginal NOSE surgery is rectovaginal fistula secondary to two incision lines in close opposition. Both transvaginal NOSE and transanal NOSE have been criticized for longer dissection of the mesorectum and thus longer resected specimen. Despite these concerns, over the past 10 years, articles have been published showing not only the feasibility of the NOSE technique, but excellent comparative outcomes, as well.2-4
A recent systematic review and meta-analysis by Kar et al. comparing NOSE extraction to minilaparotomy in bowel resection due to endometriosis revealed no significant difference in complication rate, but significantly shorter operation duration, reduced length of stay, and less blood loss in the NOSE surgical arm.5
The most recent study to be published in the Journal of Minimally Invasive Gynecology involving total laparoscopic segmental resection with transanal NOSE for the treatment of colorectal endometriosis was written by the guest author of this edition of the Master Class in Gynecologic Surgery, Professor Mario Malzoni, Scientific Director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. In the JMIG article, and in this Master Class, Professor Malzoni, a world-renowned endometriosis specialist recognized for his extraordinary skill and multi-organ surgical work, discusses the difference between the standard bowel resection technique with that of NOSE, and reports on his experience with NOSE.6
It is a pleasure and honor to welcome my friend and colleague, Professor Mario Malzoni, to this edition of the Master Class in Gynecologic Surgery.
Dr. Charles E. Miller is Professor, Obstetrics & Gynecology, Department of Clinical Sciences, Rosalind Franklin University of Medicine and Science, North Chicago, and Director, Minimally Invasive Gynecologic Surgery, Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at obnews@mdedge.com.
References
1. Redwine DB et al. Laparoscopically assisted transvaginal segmental resection of the rectosigmoid colon for endometriosis. Fertil Steril. 1996 Jan;65(1):193-7.
2. Akladios C et al. Totally laparoscopic intracorporeal anastomosis with natural orifice specimen extraction (NOSE) techniques, particularly suitable for bowel endometriosis. J Minim Invasive Gynecol. 2014 Nov-Dec;21(6):1095-102.
3. Bokor A et al. Natural orifice specimen extraction during laparoscopic bowel resection for colorectal endometriosis: technique and outcome. J Minim Invasive Gynecol. 2018 Sep-Oct;25(6):1065-74.
4. Grigoriadis G et al. Natural orifice specimen extraction colorectal resection for deep endometriosis: A 50 case series. J Minim Invasive Gynecol. 2022 Sep;29(9):1054-62.
5. Kar E et al. Natural orifice specimen extraction as a promising alternative for minilaparotomy in bowel resection due to endometriosis: A systematic review and meta-analysis. J Minim Invasive Gynecol. 2024 Jul;31(7):574-83.e1.
6. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024 Oct;18:S1553-4650(24)01453-5.
Laparoscopic Resection With Transanal NOSE for Deep Colorectal Endometriosis
When segmental sigmoid colon/rectal resection is indicated for treatment of deep infiltrating bowel endometriosis, a totally laparoscopic resection with intracorporeal anastomosis and transanal natural orifice specimen extraction (NOSE) can be safe, effective, and advantageous compared with an abdominal mini-laparotomy for extracorporeal anastomosis and specimen retrieval.
With advanced laparoscopic surgical skills, accurate preoperative ultrasound evaluation, and a high-quality perioperative care protocol (including high-quality bowel preparation), segmental resection with NOSE can offer better preservation of vascularization, smaller resection sizes, lower rates of postoperative pain, a shorter time for gas passage after surgery, decreased hospital stays, and lower rates of wound infection and incisional hernia.
We have seen such improved results in our high-volume center since shifting in April 2021 from our classical technique for segmental rectosigmoid resection1 to a totally laparoscopic approach with NOSE. We first reported on this new technique in 2022 in a video article in the Journal of Minimally Invasive Gynecology,2and in October 2024, outcomes of 81 patients who underwent segmental sigmoid colon/rectal resection with intracorporeal anastomosis and transanal NOSE at our institution from April 2021 through March 2024 were reported in Journal of Minimally Invasive Gynecology.3Given our experience with this approach for deep endometriosis, I am convinced that NOSE should be preferred to transabdominal specimen extraction for segmental rectosigmoid resection. This is where the future lies for deep infiltrating endometriosis. Here, I share our outcomes and describe our technique.
Our Prior Approach With A Mini-Laparotomy
Our traditional laparoscopic approach for segmental colon/rectal resection of deep infiltrating endometriosis involved an abdominal mini-laparotomy (no more than 4 cm in length) for specimen retrieval, resection of the proximal rectum, and positioning of the head of a circular stapler for end-to-end anastomosis. (Connection of the two parts of the circular stapler for anastomosis was achieved via laparoscopy.)1
Several factors allowed us to minimize potential complications, including standardization of our technique, the use of three-dimensional endoscopes, and indocyanine green fluorescence angiography to assess the perfusion of the bowel after the completion of anastomosis. Still, an unpublished analysis of 1050 segmental bowel resections performed over more than 17 years using our traditional technique showed that hospital stays averaged 8 days, time to first defecation averaged 7 days, and time to first passage of flatus averaged 1 day.
Per the Clavien-Dindo classification system for surgical complications that was used at the time, 1.9% of these 1050 patients had grade 1 (minor) or grade 2 complications and 6% had grade 3 complications. Grade 2 complications were defined as needing intervention or a hospital stay more than twice the median for the procedure. Grade 3 complications were defined as leading to lasting disability or organ resection.
In the meantime, in colorectal cancer surgery, published studies on NOSE versus conventional laparoscopy with transabdominal specimen extraction have consistently pointed to the benefits of NOSE. A 2022 review 4 of 19 studies involving over 3400 patients and a 2022 meta-analysis 5 of 21 randomized controlled trials involving more than 2000 patients both showed significantly reduced postoperative morbidity and no differences in oncologic outcomes. Among the notable differences in the 2022 meta-analysis was a relative risk of postoperative infection of 0.34 for patients treated with NOSE compared with conventional laparoscopic techniques.
Among patients with deep rectal endometriosis, reported experience with intracorporeal anastomosis and NOSE for segmental resection is limited, with a few studies published between 2014 and 2022. Excluding anecdotal reports, only about 140 patients have been reported in the literature as having intracorporeal anastomosis and transanal NOSE for deep infiltrating bowel endometriosis. 3
Our Outcomes With A Totally Laparoscopic Approach
Our approach to evaluating our experience with totally laparoscopic resection with transanal NOSE has been to systematically collect data from consecutive patients and to analyze 1) complications of the technique, 2) conversion to the traditional technique/open surgery, and 3) endometriosis-free bowel resection margins and recurrence. Secondarily, we look at intraoperative blood loss, operative time, recovery of gastrointestinal function, hospital stay length, and reproductive outcomes.
Across 81 patients from our TrEnd study database (Surgical TReatment of women with deep ENDometriosis infiltrating the sigmoid colon and/or rectum) who received a totally laparoscopic standardized procedure involving intracorporeal anastomosis and transanal NOSE, we had no conversions even to mini-laparotomy, and no cases of protective colostomy or ileostomy.
Complete endometriosis removal was achieved in 100% of cases, with final pathology showing endometriosis-free resection margins, and patients remained free of bowel endometriosis at a median follow-up of 21 months.3Our analysis also shows improved gastrointestinal function recovery (median time to first defecation of 4 days, compared to 7 days previously, with the same 1-day median time to first passage of flatus) and a significantly improved hospital length of stay to a median of 3 days (interquartile range, 3-4.5 days); the latter reflects in part the absence of infection.3There were no intra-operative complications. Postoperative Clavien-Dindo grade 3 complications occurred in three patients (3.7%), two of whom required reoperation (one for hemoperitoneum and one for ureteral injury) and one who experienced rectal anastomotic bleeding on postoperative day 1. (The two requiring reoperation had significant right lateral parametrial involvement and underwent nerve-sparing parametrectomy at the time of bowel resection).3Notably, 7 of the 81 patients had totally laparoscopic double segmental colorectal resection — 6 with simultaneous ileocecal valve and rectal resection, and 1 with simultaneous ileal and rectal resection. None of these seven patients had postoperative complications. (A report on the six surgeries for deep endometriosis infiltrating the ileocecal valve and rectum was also published this year in the journal Colorectal Disease.6)
Median blood loss in the 81-patient cohort was 20 mL (IQR, 20-30 mL), and the median length of surgery was 160 minutes (IQR, 130-210 minutes).
Bowel endometriotic nodules had a mean maximum diameter of 4.3 cm (3.5-5.5 cm), a mean depth of invasion of 9 mm (7-9 mm), a mean estimated stenosis of 40% (35%-50%), and a mean distance from the anal verge of 13 cm (10-15 cm).3 (The cohort in this analysis did not include patients who received concomitant hysterectomy and transvaginal NOSE.)
Specifics of the New Technique, Including Totally Intracorporeal Anastomosis
As in our previous technique, the surgical procedure starts with left and dorsal rectal mobilization. The left pelvic sidewall dissection begins with incision of the parietal peritoneum along the pelvic portions of the psoas muscle. The retroperitoneal connective tissue is dissected in order to identify the ureter, the hypogastric nerve, and the presacral parietal pelvic fascia (PPPF). Dissection is carried downward in front of the PPPF and medially to the left hypogastric nerve.
Right and ventral rectal mobilization begins with incision of the peritoneum along the gray avascular line, which is seen with upward retraction of the rectum.
Again, for preservation of bowel and bladder function, the hypogastric nerve must be identified and preserved through dissection that displaces the nerve dorsally and laterally.
Dissection proceeds to sharply divide the nodule into two parts (rectal and rectocervical components). The caudal boundary of ventral dissection is reached by incising the septum and opening the rectovaginal space. The lymphovascular fatty tissue surrounding the rectum is then excised and the rectal tube is denuded at the level of the distal resection margin.
The distal and proximal resection margins are prepared and transected using a tissue-sealing device, and the resected rectal specimen is placed into a retrieval bag and pulled out through the anus (Figure 1).

The totally intracorporeal side-to-end anastomosis is performed using a traditional circular stapler. Its anvil is inserted through the proximal resection line and exteriorized forward on the antimesenteric side of the sigmoid colon/rectum. The proximal and distal resection lines are closed with a 60-mm linear endo-stapler, and finally, the shaft of the circular stapler is inserted into the rectum, and the two parts of the stapler are joined. The stapler is then closed and fired (Figure 2).

An air leak test is performed to check the quality of the rectal sutures. However, regardless of the results, we routinely apply interrupted stitches to the so-called “dog ears” in order to reduce tension along the anastomotic line.
We also evaluate the microvascularization of the two parts of the bowel at the level of the reanastomosis using indocyanine green fluorescence angiography. Doing so allows us to avoid complications caused by anastomotic leakage.
Patient Selection And Peri-Surgical Care
The decision to perform segmental resection as opposed to other more conservative laparoscopic excision techniques is made after skilled preoperative imaging reveals the number of lesions, the largest nodule diameter, the infiltration depth, and the circumference of bowel involvement.
A recently published international consensus statement7 on noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems concludes there is Level 1a evidence that preoperative imaging with transvaginal ultrasound (TVUS) can predict with good precision the size and degree of infiltration of deep endometriosis of the rectum (a Grade A statement, per the Oxford Centre for Evidence-Based Medicine levels of evidence).
The consensus document was published across seven different journals by international and European societies, and the American Association of Gynecologic Laparoscopists (AAGL), each of which contributed to a multidisciplinary panel of gynecological surgeons, sonographers, and radiologists.
At our center, we achieve precise preoperative evaluations with TVUS (other centers also employ MRI) and have long set clear preoperative indications for segmental resection. Based on our experience,8 a nodule ≥ 3 cm and infiltration of the tunica muscularis of the sigmoid colon/rectum ≥ 7 mm indicate the need for segmental resection. It is impossible to achieve surgical goals in such cases through shaving or discoid resection.
Our perioperative care protocol for total laparoscopic segmental resection with transanal NOSE, described in our new paper,3 includes several components: antibiotic therapy (metronidazole 500 mg 12 hours before surgery; cefazolin 2 g plus metronidazole 500 mg 1 hour before skin incision; and cefazolin 1 g plus metronidazole 500 mg 12 hours after surgery); good intraoperative bowel preparation (mechanical with oral polyethylene glycol solution preoperatively, and rectal irrigation with a mixed solution of povidine-iodine and normal saline intraoperatively); and venous thromboembolism prophylaxis postoperatively for 28 days.
Moving Forward, A Word On Classification
Multicenter trials and research with longer follow-up times will be helpful in advancing the use of totally laparoscopic segmental colorectal resection for deep endometriosis. To enable better sharing of diagnostic and therapeutic results — and to advance the credibility of research — my hope is that the field will reach further agreement on endometriosis classification systems. This would also help with the development of standards for postgraduate education.
We have made significant strides in recognizing imaging as a standard for diagnosis and treatment, and I believe we currently have two very good endometriosis classification systems — the #Enzian classification system issued in 2021 and the AAGL 2021 Endometriosis Classification — that can be used in combination with imaging to reliably and consistently describe deep endometriosis.
Mario Malzoni, MD, is scientific director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. He reported having no disclosures relevant to this Master Class.
References
1. Malzoni M et al. Surgical principles of segmental rectosigmoid resection and reanastomosis for deep infiltrating endometriosis. J Minim Invasive Gynecol. 2020;27(2):258.
2. Malzoni M et al. Totally laparoscopic resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the rectum. J Minim Invasive Gynecol. 2022;29(1):19.
3. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024. (in press). 4. Brincat SD et al. Natural orifice versus transabdominal specimen extraction in laparoscopic surgery for colorectal cancer: meta-analysis. BJS Open 2022;6(3):zrac074.
5. Zhou Z et al. Laparoscopic natural orifice specimen extraction surgery versus conventional surgery in colorectal cancer: A meta-analysis of randomized controlled trials. Gastroenterol Res Pract. 2022 Jan 18;2022:6661651.
6. Malzoni M et al. Simultaneous total laparoscopic double segmental resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the ileocaecal valve and rectum—A video vignette. Colorectal Dis 2024 Jul 25.
7. Condous G et al. Noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems: An international consensus. J. Minim Invasive Gynecol. 2024;31(7):557-73.
8. Malzoni M et al. Preoperative ultrasound indications determine excision technique for bowel surgery for deep infiltrating endometriosis: A single, high-volume center. J Minim Invasive Gynecol. 2020;27(5):1141-7.
What to Know About NOSE
Traditionally, a laparoscopic approach to bowel resection secondary to endometriosis means enlargement of the port site or more commonly, a Pfannenstiel incision to introduce an anvil. Both incisions raise the risk of postoperative complications including pain and incisional hernia. Moreover, recovery time is prolonged.
Natural orifice specimen extraction (NOSE) makes use of a natural orifice, the vagina or anus, to extract the specimen and introduce the anvil. Transanal extraction for the management of endometriosis-related bowel disease was first described by the late David Redwine, MD, in his 1996 publication in Fertility and Sterility.1
The concern with use of vaginal NOSE surgery is rectovaginal fistula secondary to two incision lines in close opposition. Both transvaginal NOSE and transanal NOSE have been criticized for longer dissection of the mesorectum and thus longer resected specimen. Despite these concerns, over the past 10 years, articles have been published showing not only the feasibility of the NOSE technique, but excellent comparative outcomes, as well.2-4
A recent systematic review and meta-analysis by Kar et al. comparing NOSE extraction to minilaparotomy in bowel resection due to endometriosis revealed no significant difference in complication rate, but significantly shorter operation duration, reduced length of stay, and less blood loss in the NOSE surgical arm.5
The most recent study to be published in the Journal of Minimally Invasive Gynecology involving total laparoscopic segmental resection with transanal NOSE for the treatment of colorectal endometriosis was written by the guest author of this edition of the Master Class in Gynecologic Surgery, Professor Mario Malzoni, Scientific Director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. In the JMIG article, and in this Master Class, Professor Malzoni, a world-renowned endometriosis specialist recognized for his extraordinary skill and multi-organ surgical work, discusses the difference between the standard bowel resection technique with that of NOSE, and reports on his experience with NOSE.6
It is a pleasure and honor to welcome my friend and colleague, Professor Mario Malzoni, to this edition of the Master Class in Gynecologic Surgery.
Dr. Charles E. Miller is Professor, Obstetrics & Gynecology, Department of Clinical Sciences, Rosalind Franklin University of Medicine and Science, North Chicago, and Director, Minimally Invasive Gynecologic Surgery, Advocate Lutheran General Hospital, Park Ridge, both in Illinois. Email him at obnews@mdedge.com.
References
1. Redwine DB et al. Laparoscopically assisted transvaginal segmental resection of the rectosigmoid colon for endometriosis. Fertil Steril. 1996 Jan;65(1):193-7.
2. Akladios C et al. Totally laparoscopic intracorporeal anastomosis with natural orifice specimen extraction (NOSE) techniques, particularly suitable for bowel endometriosis. J Minim Invasive Gynecol. 2014 Nov-Dec;21(6):1095-102.
3. Bokor A et al. Natural orifice specimen extraction during laparoscopic bowel resection for colorectal endometriosis: technique and outcome. J Minim Invasive Gynecol. 2018 Sep-Oct;25(6):1065-74.
4. Grigoriadis G et al. Natural orifice specimen extraction colorectal resection for deep endometriosis: A 50 case series. J Minim Invasive Gynecol. 2022 Sep;29(9):1054-62.
5. Kar E et al. Natural orifice specimen extraction as a promising alternative for minilaparotomy in bowel resection due to endometriosis: A systematic review and meta-analysis. J Minim Invasive Gynecol. 2024 Jul;31(7):574-83.e1.
6. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024 Oct;18:S1553-4650(24)01453-5.
Laparoscopic Resection With Transanal NOSE for Deep Colorectal Endometriosis
When segmental sigmoid colon/rectal resection is indicated for treatment of deep infiltrating bowel endometriosis, a totally laparoscopic resection with intracorporeal anastomosis and transanal natural orifice specimen extraction (NOSE) can be safe, effective, and advantageous compared with an abdominal mini-laparotomy for extracorporeal anastomosis and specimen retrieval.
With advanced laparoscopic surgical skills, accurate preoperative ultrasound evaluation, and a high-quality perioperative care protocol (including high-quality bowel preparation), segmental resection with NOSE can offer better preservation of vascularization, smaller resection sizes, lower rates of postoperative pain, a shorter time for gas passage after surgery, decreased hospital stays, and lower rates of wound infection and incisional hernia.
We have seen such improved results in our high-volume center since shifting in April 2021 from our classical technique for segmental rectosigmoid resection1 to a totally laparoscopic approach with NOSE. We first reported on this new technique in 2022 in a video article in the Journal of Minimally Invasive Gynecology,2and in October 2024, outcomes of 81 patients who underwent segmental sigmoid colon/rectal resection with intracorporeal anastomosis and transanal NOSE at our institution from April 2021 through March 2024 were reported in Journal of Minimally Invasive Gynecology.3Given our experience with this approach for deep endometriosis, I am convinced that NOSE should be preferred to transabdominal specimen extraction for segmental rectosigmoid resection. This is where the future lies for deep infiltrating endometriosis. Here, I share our outcomes and describe our technique.
Our Prior Approach With A Mini-Laparotomy
Our traditional laparoscopic approach for segmental colon/rectal resection of deep infiltrating endometriosis involved an abdominal mini-laparotomy (no more than 4 cm in length) for specimen retrieval, resection of the proximal rectum, and positioning of the head of a circular stapler for end-to-end anastomosis. (Connection of the two parts of the circular stapler for anastomosis was achieved via laparoscopy.)1
Several factors allowed us to minimize potential complications, including standardization of our technique, the use of three-dimensional endoscopes, and indocyanine green fluorescence angiography to assess the perfusion of the bowel after the completion of anastomosis. Still, an unpublished analysis of 1050 segmental bowel resections performed over more than 17 years using our traditional technique showed that hospital stays averaged 8 days, time to first defecation averaged 7 days, and time to first passage of flatus averaged 1 day.
Per the Clavien-Dindo classification system for surgical complications that was used at the time, 1.9% of these 1050 patients had grade 1 (minor) or grade 2 complications and 6% had grade 3 complications. Grade 2 complications were defined as needing intervention or a hospital stay more than twice the median for the procedure. Grade 3 complications were defined as leading to lasting disability or organ resection.
In the meantime, in colorectal cancer surgery, published studies on NOSE versus conventional laparoscopy with transabdominal specimen extraction have consistently pointed to the benefits of NOSE. A 2022 review 4 of 19 studies involving over 3400 patients and a 2022 meta-analysis 5 of 21 randomized controlled trials involving more than 2000 patients both showed significantly reduced postoperative morbidity and no differences in oncologic outcomes. Among the notable differences in the 2022 meta-analysis was a relative risk of postoperative infection of 0.34 for patients treated with NOSE compared with conventional laparoscopic techniques.
Among patients with deep rectal endometriosis, reported experience with intracorporeal anastomosis and NOSE for segmental resection is limited, with a few studies published between 2014 and 2022. Excluding anecdotal reports, only about 140 patients have been reported in the literature as having intracorporeal anastomosis and transanal NOSE for deep infiltrating bowel endometriosis. 3
Our Outcomes With A Totally Laparoscopic Approach
Our approach to evaluating our experience with totally laparoscopic resection with transanal NOSE has been to systematically collect data from consecutive patients and to analyze 1) complications of the technique, 2) conversion to the traditional technique/open surgery, and 3) endometriosis-free bowel resection margins and recurrence. Secondarily, we look at intraoperative blood loss, operative time, recovery of gastrointestinal function, hospital stay length, and reproductive outcomes.
Across 81 patients from our TrEnd study database (Surgical TReatment of women with deep ENDometriosis infiltrating the sigmoid colon and/or rectum) who received a totally laparoscopic standardized procedure involving intracorporeal anastomosis and transanal NOSE, we had no conversions even to mini-laparotomy, and no cases of protective colostomy or ileostomy.
Complete endometriosis removal was achieved in 100% of cases, with final pathology showing endometriosis-free resection margins, and patients remained free of bowel endometriosis at a median follow-up of 21 months.3Our analysis also shows improved gastrointestinal function recovery (median time to first defecation of 4 days, compared to 7 days previously, with the same 1-day median time to first passage of flatus) and a significantly improved hospital length of stay to a median of 3 days (interquartile range, 3-4.5 days); the latter reflects in part the absence of infection.3There were no intra-operative complications. Postoperative Clavien-Dindo grade 3 complications occurred in three patients (3.7%), two of whom required reoperation (one for hemoperitoneum and one for ureteral injury) and one who experienced rectal anastomotic bleeding on postoperative day 1. (The two requiring reoperation had significant right lateral parametrial involvement and underwent nerve-sparing parametrectomy at the time of bowel resection).3Notably, 7 of the 81 patients had totally laparoscopic double segmental colorectal resection — 6 with simultaneous ileocecal valve and rectal resection, and 1 with simultaneous ileal and rectal resection. None of these seven patients had postoperative complications. (A report on the six surgeries for deep endometriosis infiltrating the ileocecal valve and rectum was also published this year in the journal Colorectal Disease.6)
Median blood loss in the 81-patient cohort was 20 mL (IQR, 20-30 mL), and the median length of surgery was 160 minutes (IQR, 130-210 minutes).
Bowel endometriotic nodules had a mean maximum diameter of 4.3 cm (3.5-5.5 cm), a mean depth of invasion of 9 mm (7-9 mm), a mean estimated stenosis of 40% (35%-50%), and a mean distance from the anal verge of 13 cm (10-15 cm).3 (The cohort in this analysis did not include patients who received concomitant hysterectomy and transvaginal NOSE.)
Specifics of the New Technique, Including Totally Intracorporeal Anastomosis
As in our previous technique, the surgical procedure starts with left and dorsal rectal mobilization. The left pelvic sidewall dissection begins with incision of the parietal peritoneum along the pelvic portions of the psoas muscle. The retroperitoneal connective tissue is dissected in order to identify the ureter, the hypogastric nerve, and the presacral parietal pelvic fascia (PPPF). Dissection is carried downward in front of the PPPF and medially to the left hypogastric nerve.
Right and ventral rectal mobilization begins with incision of the peritoneum along the gray avascular line, which is seen with upward retraction of the rectum.
Again, for preservation of bowel and bladder function, the hypogastric nerve must be identified and preserved through dissection that displaces the nerve dorsally and laterally.
Dissection proceeds to sharply divide the nodule into two parts (rectal and rectocervical components). The caudal boundary of ventral dissection is reached by incising the septum and opening the rectovaginal space. The lymphovascular fatty tissue surrounding the rectum is then excised and the rectal tube is denuded at the level of the distal resection margin.
The distal and proximal resection margins are prepared and transected using a tissue-sealing device, and the resected rectal specimen is placed into a retrieval bag and pulled out through the anus (Figure 1).

The totally intracorporeal side-to-end anastomosis is performed using a traditional circular stapler. Its anvil is inserted through the proximal resection line and exteriorized forward on the antimesenteric side of the sigmoid colon/rectum. The proximal and distal resection lines are closed with a 60-mm linear endo-stapler, and finally, the shaft of the circular stapler is inserted into the rectum, and the two parts of the stapler are joined. The stapler is then closed and fired (Figure 2).

An air leak test is performed to check the quality of the rectal sutures. However, regardless of the results, we routinely apply interrupted stitches to the so-called “dog ears” in order to reduce tension along the anastomotic line.
We also evaluate the microvascularization of the two parts of the bowel at the level of the reanastomosis using indocyanine green fluorescence angiography. Doing so allows us to avoid complications caused by anastomotic leakage.
Patient Selection And Peri-Surgical Care
The decision to perform segmental resection as opposed to other more conservative laparoscopic excision techniques is made after skilled preoperative imaging reveals the number of lesions, the largest nodule diameter, the infiltration depth, and the circumference of bowel involvement.
A recently published international consensus statement7 on noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems concludes there is Level 1a evidence that preoperative imaging with transvaginal ultrasound (TVUS) can predict with good precision the size and degree of infiltration of deep endometriosis of the rectum (a Grade A statement, per the Oxford Centre for Evidence-Based Medicine levels of evidence).
The consensus document was published across seven different journals by international and European societies, and the American Association of Gynecologic Laparoscopists (AAGL), each of which contributed to a multidisciplinary panel of gynecological surgeons, sonographers, and radiologists.
At our center, we achieve precise preoperative evaluations with TVUS (other centers also employ MRI) and have long set clear preoperative indications for segmental resection. Based on our experience,8 a nodule ≥ 3 cm and infiltration of the tunica muscularis of the sigmoid colon/rectum ≥ 7 mm indicate the need for segmental resection. It is impossible to achieve surgical goals in such cases through shaving or discoid resection.
Our perioperative care protocol for total laparoscopic segmental resection with transanal NOSE, described in our new paper,3 includes several components: antibiotic therapy (metronidazole 500 mg 12 hours before surgery; cefazolin 2 g plus metronidazole 500 mg 1 hour before skin incision; and cefazolin 1 g plus metronidazole 500 mg 12 hours after surgery); good intraoperative bowel preparation (mechanical with oral polyethylene glycol solution preoperatively, and rectal irrigation with a mixed solution of povidine-iodine and normal saline intraoperatively); and venous thromboembolism prophylaxis postoperatively for 28 days.
Moving Forward, A Word On Classification
Multicenter trials and research with longer follow-up times will be helpful in advancing the use of totally laparoscopic segmental colorectal resection for deep endometriosis. To enable better sharing of diagnostic and therapeutic results — and to advance the credibility of research — my hope is that the field will reach further agreement on endometriosis classification systems. This would also help with the development of standards for postgraduate education.
We have made significant strides in recognizing imaging as a standard for diagnosis and treatment, and I believe we currently have two very good endometriosis classification systems — the #Enzian classification system issued in 2021 and the AAGL 2021 Endometriosis Classification — that can be used in combination with imaging to reliably and consistently describe deep endometriosis.
Mario Malzoni, MD, is scientific director of the Malzoni Research Hospital, the Center for Advanced Pelvic Surgery, in Avellino, Italy. He reported having no disclosures relevant to this Master Class.
References
1. Malzoni M et al. Surgical principles of segmental rectosigmoid resection and reanastomosis for deep infiltrating endometriosis. J Minim Invasive Gynecol. 2020;27(2):258.
2. Malzoni M et al. Totally laparoscopic resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the rectum. J Minim Invasive Gynecol. 2022;29(1):19.
3. Malzoni M et al. Total laparoscopic segmental resection with transanal natural orifice specimen extraction for treatment of colorectal endometriosis: Descriptive analysis from the TrEnd study database. J Minim Invasive Gynecol. 2024. (in press). 4. Brincat SD et al. Natural orifice versus transabdominal specimen extraction in laparoscopic surgery for colorectal cancer: meta-analysis. BJS Open 2022;6(3):zrac074.
5. Zhou Z et al. Laparoscopic natural orifice specimen extraction surgery versus conventional surgery in colorectal cancer: A meta-analysis of randomized controlled trials. Gastroenterol Res Pract. 2022 Jan 18;2022:6661651.
6. Malzoni M et al. Simultaneous total laparoscopic double segmental resection with transanal natural orifice specimen extraction for deep endometriosis infiltrating the ileocaecal valve and rectum—A video vignette. Colorectal Dis 2024 Jul 25.
7. Condous G et al. Noninvasive imaging techniques for diagnosis of pelvic deep endometriosis and endometriosis classification systems: An international consensus. J. Minim Invasive Gynecol. 2024;31(7):557-73.
8. Malzoni M et al. Preoperative ultrasound indications determine excision technique for bowel surgery for deep infiltrating endometriosis: A single, high-volume center. J Minim Invasive Gynecol. 2020;27(5):1141-7.
Promising New Data for Drugs Both Novel and Established in Several Common GI Conditions
I’ve just returned from the annual meeting of the American College of Gastroenterology (ACG), which was held in Philadelphia, Pennsylvania.
which will be split into two parts. These studies are not presented in any order of priority because I think they all have clinical applications, some of which are more immediate. Where possible, I’ll also provide you with some teaching points that occurred to me when I viewed the data in these presentations.
Repetitive Use Injuries Among Endoscopists
First is a study that assessed musculoskeletal injuries that occurred while performing endoscopy, which is a large part of what we do as gastroenterologists. I’ve personally experienced virtually all these injuries during my 45-plus years in the field. These findings provide a useful perspective of what we can do to potentially avoid such injuries going forward.
This study comes to us from researchers at the University of Utah, who looked at an author-developed and validated questionnaire called QuickDash (Disability of Arm, Shoulder, Hand). Endoscopists were evaluated by occupational therapists in their department, who looked at strength testing as well as a series of provocative tests to identify injuries.
Thirty-five endoscopists were enrolled. Overall, 34% reported experiencing pain and 17% reported numbness. In the previous week, 48% had been bothered by pain and 11% by tingling, and 17% reported limitations on what they could do at work.
Additionally, 17% experienced interrupted sleep. I think that finding is particularly important, because when you have fragmented sleep it lowers sensory thresholds. Essentially, this means that the day after interrupted sleep, you respond with a heightened sensory response.
Physical testing showed reduction in grip strength in approximately half of participants, including both right and left grip. More than 70% had at least one abnormal positive provocative test.
There were a couple factors associated with an increased risk for adverse outcomes. Those who performed 20 or more procedures a week were at higher risk of pain (P = .007). I recognize that some of you probably perform 20 or more endoscopies in a single day.
Negative outcomes were also more likely to occur among physicians performing biliary endoscopy. Performing endoscopic retrograde cholangiopancreatography during 20%-60% of the week resulted in greater likelihood of experiencing decreased bilateral pinch strength.
The bottom line is that there’s an extremely high prevalence of repetitive use injury among endoscopists. Whether you’re just starting out, mid-career, or more senior, you need to be aware of this.
Kudos to the ACG who put together hands-on sessions across 2 days with extended ergonomics training provided by physical therapists with expertise in this area. These sessions were standing room only every time I visited them.
During the meeting, the ACG also announced the publication of a new book, Ergonomics for Endoscopy: Optimal Preparation, Performance, and Recovery, that is available on the ACG’s website. I had the privilege to meet the two authors, survey the book, and briefly discuss it with them. It’s phenomenal, and something that I think every endoscopist can benefit from.
So, in summary, don’t put aside these concerns. It’s your career, and the better we can do in preventing these injuries, I think the better you’ll feel going forward.
On-Demand Vonoprazan for Heartburn
The second study of note dealt with vonoprazan, a potassium-competitive acid blocker.
The question surrounding vonoprazan is whether it has clinical value as an on-demand option rather than simply as maintenance therapy administered via daily dosing. Previous results have suggested that it may have a more rapid effect when it’s taken on demand.
This post hoc analysis of a randomized trial evaluated a 4-week run-in period during which patients received vonoprazan 20 mg daily, followed by a 6-week period after which patients switched to on-demand therapy. To be eligible, during the run-in period patients had to be 80% compliant with the study drug and report no heartburn during the last 7 days of treatment. If so, they were then randomized to receive vonoprazan at 10 mg, 20 mg, or 40 mg, or placebo.
Eligible patients reported 16% heartburn-free days during the screening period. During the run-in period, heartburn-free days increased to 83% among those taking vonoprazan 20 mg daily. When this was stopped and patients transitioned to on-demand vonoprazan, they still had a very high rate of heartburn-free days, ranging from 71% to 75%. Over 90% in the treatment group had their symptoms improved within 2 hours, with improvement noted as early as within 1 hour.
We know that proton pump inhibitors don’t produce this effect, which presents a challenge for us. This study suggests there may be a very strong role for on-demand therapy in patients who have reflux, which certainly showed in terms of responsiveness during the 4-week run-in period.
Rifaximin Monotherapy Reduces Risk for Overt Hepatic Encephalopathy Recurrence
The next trial looked at rifaximin monotherapy for prevention of relapse of overt hepatic encephalopathy.
Rifaximin has been added to the baseline primary treatment of lactulose, a combination that has been analyzed in pivotal studies. However, lactulose is a difficult drug to titrate. We typically ask patients to let us know if they have Bristol Stool Scale scores ≥ 6. This results in an inordinate number of calls back to the clinic or office. Diarrhea is a particular problem in these patients.
This study, which was presented by Dr Jasmohan S. Bajaj from Virginia Commonwealth University, analyzed data from two randomized trials: a phase 3, double-blind trial and a phase 4 open-label trial. Both studies were conducted in patients with Child-Pugh classification A and B cirrhosis, whose overt hepatic encephalopathy was graded with a Conn score ≤ 1. Researchers only looked at those patients who received rifaximin 550 mg twice daily without lactulose, or lactulose titrated to a target of two to three soft stools a day plus placebo. The primary endpoint, which was used in both trials, was time to first breakthrough overt hepatic encephalopathy episode, measured as a Conn score ≥ 2.
There were 125 patients in the rifaximin group and 145 patients in the lactulose group. Patients in both groups had mean age of 57 years and a median Model for End-Stage Liver Disease score of 12.
Treatment with rifaximin produced significantly striking results. In the rifaximin group, the risk for breakthrough overt hepatic encephalopathy episodes was reduced by 60%, with a number needed to treat of 4, which is extremely powerful. Regarding mortality reduction, the number needed to treat with rifaximin alone was 19.
The take-away message here is that it’s difficult to use lactulose. We frequently have to stop it. These results provide reassurance that rifaximin has a dramatic effect even when used by itself. The recommended first-line therapy is still to begin with lactulose, but this study provides us with very strong data regarding the use of rifaximin alone.
Apraglutide’s Efficacy in Short-Bowel Syndrome
The fourth study I’d like to highlight adds to the growing data around a long-acting glucagon-like peptide 2 analog, apraglutide.
Results from the phase 3, double-blind STARS trial were first presented at Digestive Disease Week earlier in the year. They showed that apraglutide had efficacy and contributed to a reduction in the risk for needing parenteral support, maintenance of weight, and fluid requirements in patients with short-bowel syndrome and intestinal failure. However, questions remained regarding whether there were variations in response based on patient demographics and short-bowel syndrome–specific characteristics.
Researchers looked at a subgroup of patients from the STARS trial. The primary endpoint remained the same as in the main trial: relative change from baseline in actual parenteral support weekly volume at week 24. However, rather than measure it in the overall population, they did so according to geographic region, gender, age, body weight, race and ethnicity, and short-bowel syndrome characteristics, including differences in parenteral support volume, length of the remnant small intestine, and time from short-bowel syndrome diagnosis.
Across all these demographic and disease characteristic categories, there was absolutely no difference in the primary endpoint.
Apraglutide seems to be inordinately promising. This is a once-weekly treatment, as opposed to liraglutide, which is problematic because it has to be given daily.
From what I understand, apraglutide has been offered a fast-track status by the US Food and Drug Administration. Again, from what I’ve heard, it will be evaluated upon submission beginning sometime in the first quarter of 2025, which may mean that apraglutide could be available as early as 2026.
This would be a big deal for patients with short-bowel syndrome who would have a once-weekly treatment option as opposed to daily treatment and its accompanying problems of compliance and relatively reduced response.
Biologics for Treating Immune Checkpoint Inhibitor Colitis
The final study I want to discuss in this presentation dealt with adverse responses to immune checkpoint inhibitor (ICI) therapy. We see this increasingly in our clinics as ICIs become more widely used. They are wonderful drugs, but ICI-induced colitis can occur in up to 30% of patients.
The oncologic approach is to try and stay the course, while gastroenterologists are tasked with treating the colitis so that these patients can maintain their cancer treatment. Steroid therapy is the primary first-line treatment against ICI colitis, but the use of biologic therapy with infliximab or vedolizumab has been associated with favorable outcomes as well.
ICI colitis is graded on a scale. Whereas grade 1 ICI colitis indicates increased stool frequency of less than four a day and the absence of symptoms, grade 2 indicates a progression to a stool frequency of four to six times a day, the appearance of blood or mucus in the stool, and symptoms like abdominal pain.
Researchers sought to answer the question of which treatment is better for patients with moderate to severe ICI colitis: infliximab or vedolizumab? They performed a database analysis of patients at their institution who received at least one dose of these biologics. The endpoint was sustained clinical response (ie, without a recurrent colitis episode), as well as patients achieving improvement to grade 1 ICI colitis.
The data for infliximab and vedolizumab were quite good. Sustained clinical response was noted in 91% of patients receiving infliximab and 86% receiving vedolizumab. There was no difference in infection risk between the groups.
There’s a teaching point in the study’s other key finding, which is that following biologic initiation, steroids were more rapidly discontinued with vedolizumab vs infliximab (median, 25 days vs 56 days, respectively). Therefore, vedolizumab may have the added benefit of patients being able to get off steroids more quickly. That’s the take-home message: Vedolizumab may be better. We certainly are comfortable with both biologics, but patients getting off steroids would be better.
There are two additional teaching points I’d like to convey.
First, don’t forget to perform a biopsy, because there are patients who may have cytomegalovirus colitis and we don’t want to miss it. A biopsy may also reveal whether they have a macroscopically normal rectosigmoid. So, you should biopsy to look for microscopic changes. As 98% of ICI colitis cases involve the left colon, you can get by with just using a flexible sigmoidoscopy.
Second, don’t forget to check for celiac disease. Patients taking ICIs may develop celiac disease as a side effect of treatment. So, I always order a celiac profile as well.
These are, in my opinion, five of the top studies from ACG 2024, with the remaining studies to be discussed in my next video. They all provide opportunities to help us improve our patients’ health.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He reported serving in an advisory position with ISOTHRIVE. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
I’ve just returned from the annual meeting of the American College of Gastroenterology (ACG), which was held in Philadelphia, Pennsylvania.
which will be split into two parts. These studies are not presented in any order of priority because I think they all have clinical applications, some of which are more immediate. Where possible, I’ll also provide you with some teaching points that occurred to me when I viewed the data in these presentations.
Repetitive Use Injuries Among Endoscopists
First is a study that assessed musculoskeletal injuries that occurred while performing endoscopy, which is a large part of what we do as gastroenterologists. I’ve personally experienced virtually all these injuries during my 45-plus years in the field. These findings provide a useful perspective of what we can do to potentially avoid such injuries going forward.
This study comes to us from researchers at the University of Utah, who looked at an author-developed and validated questionnaire called QuickDash (Disability of Arm, Shoulder, Hand). Endoscopists were evaluated by occupational therapists in their department, who looked at strength testing as well as a series of provocative tests to identify injuries.
Thirty-five endoscopists were enrolled. Overall, 34% reported experiencing pain and 17% reported numbness. In the previous week, 48% had been bothered by pain and 11% by tingling, and 17% reported limitations on what they could do at work.
Additionally, 17% experienced interrupted sleep. I think that finding is particularly important, because when you have fragmented sleep it lowers sensory thresholds. Essentially, this means that the day after interrupted sleep, you respond with a heightened sensory response.
Physical testing showed reduction in grip strength in approximately half of participants, including both right and left grip. More than 70% had at least one abnormal positive provocative test.
There were a couple factors associated with an increased risk for adverse outcomes. Those who performed 20 or more procedures a week were at higher risk of pain (P = .007). I recognize that some of you probably perform 20 or more endoscopies in a single day.
Negative outcomes were also more likely to occur among physicians performing biliary endoscopy. Performing endoscopic retrograde cholangiopancreatography during 20%-60% of the week resulted in greater likelihood of experiencing decreased bilateral pinch strength.
The bottom line is that there’s an extremely high prevalence of repetitive use injury among endoscopists. Whether you’re just starting out, mid-career, or more senior, you need to be aware of this.
Kudos to the ACG who put together hands-on sessions across 2 days with extended ergonomics training provided by physical therapists with expertise in this area. These sessions were standing room only every time I visited them.
During the meeting, the ACG also announced the publication of a new book, Ergonomics for Endoscopy: Optimal Preparation, Performance, and Recovery, that is available on the ACG’s website. I had the privilege to meet the two authors, survey the book, and briefly discuss it with them. It’s phenomenal, and something that I think every endoscopist can benefit from.
So, in summary, don’t put aside these concerns. It’s your career, and the better we can do in preventing these injuries, I think the better you’ll feel going forward.
On-Demand Vonoprazan for Heartburn
The second study of note dealt with vonoprazan, a potassium-competitive acid blocker.
The question surrounding vonoprazan is whether it has clinical value as an on-demand option rather than simply as maintenance therapy administered via daily dosing. Previous results have suggested that it may have a more rapid effect when it’s taken on demand.
This post hoc analysis of a randomized trial evaluated a 4-week run-in period during which patients received vonoprazan 20 mg daily, followed by a 6-week period after which patients switched to on-demand therapy. To be eligible, during the run-in period patients had to be 80% compliant with the study drug and report no heartburn during the last 7 days of treatment. If so, they were then randomized to receive vonoprazan at 10 mg, 20 mg, or 40 mg, or placebo.
Eligible patients reported 16% heartburn-free days during the screening period. During the run-in period, heartburn-free days increased to 83% among those taking vonoprazan 20 mg daily. When this was stopped and patients transitioned to on-demand vonoprazan, they still had a very high rate of heartburn-free days, ranging from 71% to 75%. Over 90% in the treatment group had their symptoms improved within 2 hours, with improvement noted as early as within 1 hour.
We know that proton pump inhibitors don’t produce this effect, which presents a challenge for us. This study suggests there may be a very strong role for on-demand therapy in patients who have reflux, which certainly showed in terms of responsiveness during the 4-week run-in period.
Rifaximin Monotherapy Reduces Risk for Overt Hepatic Encephalopathy Recurrence
The next trial looked at rifaximin monotherapy for prevention of relapse of overt hepatic encephalopathy.
Rifaximin has been added to the baseline primary treatment of lactulose, a combination that has been analyzed in pivotal studies. However, lactulose is a difficult drug to titrate. We typically ask patients to let us know if they have Bristol Stool Scale scores ≥ 6. This results in an inordinate number of calls back to the clinic or office. Diarrhea is a particular problem in these patients.
This study, which was presented by Dr Jasmohan S. Bajaj from Virginia Commonwealth University, analyzed data from two randomized trials: a phase 3, double-blind trial and a phase 4 open-label trial. Both studies were conducted in patients with Child-Pugh classification A and B cirrhosis, whose overt hepatic encephalopathy was graded with a Conn score ≤ 1. Researchers only looked at those patients who received rifaximin 550 mg twice daily without lactulose, or lactulose titrated to a target of two to three soft stools a day plus placebo. The primary endpoint, which was used in both trials, was time to first breakthrough overt hepatic encephalopathy episode, measured as a Conn score ≥ 2.
There were 125 patients in the rifaximin group and 145 patients in the lactulose group. Patients in both groups had mean age of 57 years and a median Model for End-Stage Liver Disease score of 12.
Treatment with rifaximin produced significantly striking results. In the rifaximin group, the risk for breakthrough overt hepatic encephalopathy episodes was reduced by 60%, with a number needed to treat of 4, which is extremely powerful. Regarding mortality reduction, the number needed to treat with rifaximin alone was 19.
The take-away message here is that it’s difficult to use lactulose. We frequently have to stop it. These results provide reassurance that rifaximin has a dramatic effect even when used by itself. The recommended first-line therapy is still to begin with lactulose, but this study provides us with very strong data regarding the use of rifaximin alone.
Apraglutide’s Efficacy in Short-Bowel Syndrome
The fourth study I’d like to highlight adds to the growing data around a long-acting glucagon-like peptide 2 analog, apraglutide.
Results from the phase 3, double-blind STARS trial were first presented at Digestive Disease Week earlier in the year. They showed that apraglutide had efficacy and contributed to a reduction in the risk for needing parenteral support, maintenance of weight, and fluid requirements in patients with short-bowel syndrome and intestinal failure. However, questions remained regarding whether there were variations in response based on patient demographics and short-bowel syndrome–specific characteristics.
Researchers looked at a subgroup of patients from the STARS trial. The primary endpoint remained the same as in the main trial: relative change from baseline in actual parenteral support weekly volume at week 24. However, rather than measure it in the overall population, they did so according to geographic region, gender, age, body weight, race and ethnicity, and short-bowel syndrome characteristics, including differences in parenteral support volume, length of the remnant small intestine, and time from short-bowel syndrome diagnosis.
Across all these demographic and disease characteristic categories, there was absolutely no difference in the primary endpoint.
Apraglutide seems to be inordinately promising. This is a once-weekly treatment, as opposed to liraglutide, which is problematic because it has to be given daily.
From what I understand, apraglutide has been offered a fast-track status by the US Food and Drug Administration. Again, from what I’ve heard, it will be evaluated upon submission beginning sometime in the first quarter of 2025, which may mean that apraglutide could be available as early as 2026.
This would be a big deal for patients with short-bowel syndrome who would have a once-weekly treatment option as opposed to daily treatment and its accompanying problems of compliance and relatively reduced response.
Biologics for Treating Immune Checkpoint Inhibitor Colitis
The final study I want to discuss in this presentation dealt with adverse responses to immune checkpoint inhibitor (ICI) therapy. We see this increasingly in our clinics as ICIs become more widely used. They are wonderful drugs, but ICI-induced colitis can occur in up to 30% of patients.
The oncologic approach is to try and stay the course, while gastroenterologists are tasked with treating the colitis so that these patients can maintain their cancer treatment. Steroid therapy is the primary first-line treatment against ICI colitis, but the use of biologic therapy with infliximab or vedolizumab has been associated with favorable outcomes as well.
ICI colitis is graded on a scale. Whereas grade 1 ICI colitis indicates increased stool frequency of less than four a day and the absence of symptoms, grade 2 indicates a progression to a stool frequency of four to six times a day, the appearance of blood or mucus in the stool, and symptoms like abdominal pain.
Researchers sought to answer the question of which treatment is better for patients with moderate to severe ICI colitis: infliximab or vedolizumab? They performed a database analysis of patients at their institution who received at least one dose of these biologics. The endpoint was sustained clinical response (ie, without a recurrent colitis episode), as well as patients achieving improvement to grade 1 ICI colitis.
The data for infliximab and vedolizumab were quite good. Sustained clinical response was noted in 91% of patients receiving infliximab and 86% receiving vedolizumab. There was no difference in infection risk between the groups.
There’s a teaching point in the study’s other key finding, which is that following biologic initiation, steroids were more rapidly discontinued with vedolizumab vs infliximab (median, 25 days vs 56 days, respectively). Therefore, vedolizumab may have the added benefit of patients being able to get off steroids more quickly. That’s the take-home message: Vedolizumab may be better. We certainly are comfortable with both biologics, but patients getting off steroids would be better.
There are two additional teaching points I’d like to convey.
First, don’t forget to perform a biopsy, because there are patients who may have cytomegalovirus colitis and we don’t want to miss it. A biopsy may also reveal whether they have a macroscopically normal rectosigmoid. So, you should biopsy to look for microscopic changes. As 98% of ICI colitis cases involve the left colon, you can get by with just using a flexible sigmoidoscopy.
Second, don’t forget to check for celiac disease. Patients taking ICIs may develop celiac disease as a side effect of treatment. So, I always order a celiac profile as well.
These are, in my opinion, five of the top studies from ACG 2024, with the remaining studies to be discussed in my next video. They all provide opportunities to help us improve our patients’ health.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He reported serving in an advisory position with ISOTHRIVE. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
I’ve just returned from the annual meeting of the American College of Gastroenterology (ACG), which was held in Philadelphia, Pennsylvania.
which will be split into two parts. These studies are not presented in any order of priority because I think they all have clinical applications, some of which are more immediate. Where possible, I’ll also provide you with some teaching points that occurred to me when I viewed the data in these presentations.
Repetitive Use Injuries Among Endoscopists
First is a study that assessed musculoskeletal injuries that occurred while performing endoscopy, which is a large part of what we do as gastroenterologists. I’ve personally experienced virtually all these injuries during my 45-plus years in the field. These findings provide a useful perspective of what we can do to potentially avoid such injuries going forward.
This study comes to us from researchers at the University of Utah, who looked at an author-developed and validated questionnaire called QuickDash (Disability of Arm, Shoulder, Hand). Endoscopists were evaluated by occupational therapists in their department, who looked at strength testing as well as a series of provocative tests to identify injuries.
Thirty-five endoscopists were enrolled. Overall, 34% reported experiencing pain and 17% reported numbness. In the previous week, 48% had been bothered by pain and 11% by tingling, and 17% reported limitations on what they could do at work.
Additionally, 17% experienced interrupted sleep. I think that finding is particularly important, because when you have fragmented sleep it lowers sensory thresholds. Essentially, this means that the day after interrupted sleep, you respond with a heightened sensory response.
Physical testing showed reduction in grip strength in approximately half of participants, including both right and left grip. More than 70% had at least one abnormal positive provocative test.
There were a couple factors associated with an increased risk for adverse outcomes. Those who performed 20 or more procedures a week were at higher risk of pain (P = .007). I recognize that some of you probably perform 20 or more endoscopies in a single day.
Negative outcomes were also more likely to occur among physicians performing biliary endoscopy. Performing endoscopic retrograde cholangiopancreatography during 20%-60% of the week resulted in greater likelihood of experiencing decreased bilateral pinch strength.
The bottom line is that there’s an extremely high prevalence of repetitive use injury among endoscopists. Whether you’re just starting out, mid-career, or more senior, you need to be aware of this.
Kudos to the ACG who put together hands-on sessions across 2 days with extended ergonomics training provided by physical therapists with expertise in this area. These sessions were standing room only every time I visited them.
During the meeting, the ACG also announced the publication of a new book, Ergonomics for Endoscopy: Optimal Preparation, Performance, and Recovery, that is available on the ACG’s website. I had the privilege to meet the two authors, survey the book, and briefly discuss it with them. It’s phenomenal, and something that I think every endoscopist can benefit from.
So, in summary, don’t put aside these concerns. It’s your career, and the better we can do in preventing these injuries, I think the better you’ll feel going forward.
On-Demand Vonoprazan for Heartburn
The second study of note dealt with vonoprazan, a potassium-competitive acid blocker.
The question surrounding vonoprazan is whether it has clinical value as an on-demand option rather than simply as maintenance therapy administered via daily dosing. Previous results have suggested that it may have a more rapid effect when it’s taken on demand.
This post hoc analysis of a randomized trial evaluated a 4-week run-in period during which patients received vonoprazan 20 mg daily, followed by a 6-week period after which patients switched to on-demand therapy. To be eligible, during the run-in period patients had to be 80% compliant with the study drug and report no heartburn during the last 7 days of treatment. If so, they were then randomized to receive vonoprazan at 10 mg, 20 mg, or 40 mg, or placebo.
Eligible patients reported 16% heartburn-free days during the screening period. During the run-in period, heartburn-free days increased to 83% among those taking vonoprazan 20 mg daily. When this was stopped and patients transitioned to on-demand vonoprazan, they still had a very high rate of heartburn-free days, ranging from 71% to 75%. Over 90% in the treatment group had their symptoms improved within 2 hours, with improvement noted as early as within 1 hour.
We know that proton pump inhibitors don’t produce this effect, which presents a challenge for us. This study suggests there may be a very strong role for on-demand therapy in patients who have reflux, which certainly showed in terms of responsiveness during the 4-week run-in period.
Rifaximin Monotherapy Reduces Risk for Overt Hepatic Encephalopathy Recurrence
The next trial looked at rifaximin monotherapy for prevention of relapse of overt hepatic encephalopathy.
Rifaximin has been added to the baseline primary treatment of lactulose, a combination that has been analyzed in pivotal studies. However, lactulose is a difficult drug to titrate. We typically ask patients to let us know if they have Bristol Stool Scale scores ≥ 6. This results in an inordinate number of calls back to the clinic or office. Diarrhea is a particular problem in these patients.
This study, which was presented by Dr Jasmohan S. Bajaj from Virginia Commonwealth University, analyzed data from two randomized trials: a phase 3, double-blind trial and a phase 4 open-label trial. Both studies were conducted in patients with Child-Pugh classification A and B cirrhosis, whose overt hepatic encephalopathy was graded with a Conn score ≤ 1. Researchers only looked at those patients who received rifaximin 550 mg twice daily without lactulose, or lactulose titrated to a target of two to three soft stools a day plus placebo. The primary endpoint, which was used in both trials, was time to first breakthrough overt hepatic encephalopathy episode, measured as a Conn score ≥ 2.
There were 125 patients in the rifaximin group and 145 patients in the lactulose group. Patients in both groups had mean age of 57 years and a median Model for End-Stage Liver Disease score of 12.
Treatment with rifaximin produced significantly striking results. In the rifaximin group, the risk for breakthrough overt hepatic encephalopathy episodes was reduced by 60%, with a number needed to treat of 4, which is extremely powerful. Regarding mortality reduction, the number needed to treat with rifaximin alone was 19.
The take-away message here is that it’s difficult to use lactulose. We frequently have to stop it. These results provide reassurance that rifaximin has a dramatic effect even when used by itself. The recommended first-line therapy is still to begin with lactulose, but this study provides us with very strong data regarding the use of rifaximin alone.
Apraglutide’s Efficacy in Short-Bowel Syndrome
The fourth study I’d like to highlight adds to the growing data around a long-acting glucagon-like peptide 2 analog, apraglutide.
Results from the phase 3, double-blind STARS trial were first presented at Digestive Disease Week earlier in the year. They showed that apraglutide had efficacy and contributed to a reduction in the risk for needing parenteral support, maintenance of weight, and fluid requirements in patients with short-bowel syndrome and intestinal failure. However, questions remained regarding whether there were variations in response based on patient demographics and short-bowel syndrome–specific characteristics.
Researchers looked at a subgroup of patients from the STARS trial. The primary endpoint remained the same as in the main trial: relative change from baseline in actual parenteral support weekly volume at week 24. However, rather than measure it in the overall population, they did so according to geographic region, gender, age, body weight, race and ethnicity, and short-bowel syndrome characteristics, including differences in parenteral support volume, length of the remnant small intestine, and time from short-bowel syndrome diagnosis.
Across all these demographic and disease characteristic categories, there was absolutely no difference in the primary endpoint.
Apraglutide seems to be inordinately promising. This is a once-weekly treatment, as opposed to liraglutide, which is problematic because it has to be given daily.
From what I understand, apraglutide has been offered a fast-track status by the US Food and Drug Administration. Again, from what I’ve heard, it will be evaluated upon submission beginning sometime in the first quarter of 2025, which may mean that apraglutide could be available as early as 2026.
This would be a big deal for patients with short-bowel syndrome who would have a once-weekly treatment option as opposed to daily treatment and its accompanying problems of compliance and relatively reduced response.
Biologics for Treating Immune Checkpoint Inhibitor Colitis
The final study I want to discuss in this presentation dealt with adverse responses to immune checkpoint inhibitor (ICI) therapy. We see this increasingly in our clinics as ICIs become more widely used. They are wonderful drugs, but ICI-induced colitis can occur in up to 30% of patients.
The oncologic approach is to try and stay the course, while gastroenterologists are tasked with treating the colitis so that these patients can maintain their cancer treatment. Steroid therapy is the primary first-line treatment against ICI colitis, but the use of biologic therapy with infliximab or vedolizumab has been associated with favorable outcomes as well.
ICI colitis is graded on a scale. Whereas grade 1 ICI colitis indicates increased stool frequency of less than four a day and the absence of symptoms, grade 2 indicates a progression to a stool frequency of four to six times a day, the appearance of blood or mucus in the stool, and symptoms like abdominal pain.
Researchers sought to answer the question of which treatment is better for patients with moderate to severe ICI colitis: infliximab or vedolizumab? They performed a database analysis of patients at their institution who received at least one dose of these biologics. The endpoint was sustained clinical response (ie, without a recurrent colitis episode), as well as patients achieving improvement to grade 1 ICI colitis.
The data for infliximab and vedolizumab were quite good. Sustained clinical response was noted in 91% of patients receiving infliximab and 86% receiving vedolizumab. There was no difference in infection risk between the groups.
There’s a teaching point in the study’s other key finding, which is that following biologic initiation, steroids were more rapidly discontinued with vedolizumab vs infliximab (median, 25 days vs 56 days, respectively). Therefore, vedolizumab may have the added benefit of patients being able to get off steroids more quickly. That’s the take-home message: Vedolizumab may be better. We certainly are comfortable with both biologics, but patients getting off steroids would be better.
There are two additional teaching points I’d like to convey.
First, don’t forget to perform a biopsy, because there are patients who may have cytomegalovirus colitis and we don’t want to miss it. A biopsy may also reveal whether they have a macroscopically normal rectosigmoid. So, you should biopsy to look for microscopic changes. As 98% of ICI colitis cases involve the left colon, you can get by with just using a flexible sigmoidoscopy.
Second, don’t forget to check for celiac disease. Patients taking ICIs may develop celiac disease as a side effect of treatment. So, I always order a celiac profile as well.
These are, in my opinion, five of the top studies from ACG 2024, with the remaining studies to be discussed in my next video. They all provide opportunities to help us improve our patients’ health.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He reported serving in an advisory position with ISOTHRIVE. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Transitioning from Employment in Academia to Private Practice
After more than 10 years of serving in a large academic medical center in Chicago, Illinois, that was part of a national health care system, the decision to transition into private practice wasn’t one I made lightly.
Having built a rewarding career and spent over a quarter of my life in an academic medical center and a national health system, the move to starting an independent practice from scratch was both exciting and daunting. The notion of leaving behind the structure, resources, and safety of the large health system was unsettling. However, as the landscape of health care continues to evolve, with worsening large structural problems within the U.S. health care system, I realized that starting an independent gastroenterology practice — focused on trying to fix some of these large-scale problems from the start — would not only align with my professional goals but also provide the personal satisfaction I had failed to find.
As I reflect on my journey, there are a few key lessons I learned from making this leap — lessons that helped me transition from a highly structured employed physician environment to leading a thriving independent practice focused on redesigning gastroenterology care from scratch.
Lesson 1: Autonomy Opens the Door to Innovation
One of the primary reasons I left the employed physician setting was to gain greater control over my clinical practice and decision-making processes.
In a national health care system, the goal of standardization often dictates not only clinical care, but many “back end” aspects of the entire health care experience. We often see the things that are more visible, such as what supplies/equipment you use, how your patient appointments are scheduled, how many support staff members are assigned to help your practice, what electronic health record system you use, and how shared resources (like GI lab block time or anesthesia teams) are allocated.
However, this also impacts things we don’t usually see, such as what fees are billed for care you are providing (like facility fees), communication systems that your patients need to navigate for help, human resource systems you use, and retirement/health benefits you and your other team members receive.
Standardization has two adverse consequences: 1) it does not allow for personalization and as a result, 2) it suppresses innovation. Standard protocols can streamline processes, but they sometimes fail to account for the nuanced differences between patients, such as genetic factors, unique medical histories, or responses/failures to prior treatments. This rigidity can stifle innovation, as physicians are often bound by guidelines that may not reflect the latest advancements or allow for creative, individualized approaches to care. In the long term, an overemphasis on standardization risks turning health care into a one-size-fits-all model, undermining the potential for breakthroughs.
The transition was challenging at first, as we needed to engage our entire new practice with a different mindset now that many of us had autonomy for the first time. Instead of everyone just practicing health care the way they had done before, we took a page from Elon Musk and challenged every member of the team to ask three questions about everything they do on a daily basis:
- Is what I am doing helping a patient get healthy? (Question every requirement)
- If not, do I still need to do this? (Delete any part of the process you can)
- If so, how can I make this easier, faster, or automated? (Simplify and optimize, accelerate cycle time, and automate)
The freedom to innovate is a hallmark of independent practice. Embracing innovation in every aspect of the practice has been the most critical lesson of this journey.
Lesson 2: Financial Stewardship is Critical for Sustainability
Running an independent practice is not just about medicine — it’s also about managing a business.
This was a stark shift from the large academic health systems, where financial decisions were handled by the “administration.” In my new role as a business owner, understanding the financial aspects of health care was crucial for success. The cost of what patients pay for health care in the United States (either directly in deductibles and coinsurance or indirectly through insurance premiums) is unsustainably high. However, inflation continues to cause substantial increases in almost all the costs of delivering care: medical supplies, salaries, benefits, IT costs, etc. It was critical to develop a financial plan that accounted for these two macro-economic trends, and ideally helped solve for both. In our case, delivering high quality care with a lower cost to patients and payers.
We started by reevaluating our relationship with payers. Whereas being part of a large academic health system, we are often taught to look at payers as the adversary; as an independent practice looking to redesign the health care experience, it was critical for us to look to the payers as a partner in this journey. Understanding payer expectations and structuring contracts that aligned with shared goals of reducing total health care costs for patients was one of the foundations of our financial plan.
Offering office-based endoscopy was one innovation we implemented to significantly impact both patient affordability and practice revenue. By performing procedures like colonoscopies and upper endoscopies in an office setting rather than a hospital or ambulatory surgery center, we eliminated facility fees, which are often a significant part of the total cost of care. This directly lowers out-of-pocket expenses for patients and reduces the overall financial burden on insurance companies. At the same time, it allows the practice to capture more of the revenue from these procedures, without the overhead costs associated with larger facilities. This model creates a win-win situation: patients save money while receiving the same quality of care, and the practice experiences an increase in profitability and autonomy in managing its services.
Lesson 3: Collaborative Care and Multidisciplinary Teams Can Exist Anywhere
One aspect I deeply valued in academia was the collaborative environment — having specialists across disciplines work together on challenging cases. In private practice, I was concerned that I would lose this collegial atmosphere. However, I quickly learned that building a robust network of multidisciplinary collaborators was achievable in independent practice, just like it was in a large health system.
In our practice, we established close relationships with primary care physicians, surgeons, advanced practice providers, dietitians, behavioral health specialists, and others. These partnerships were not just referral networks but integrated care teams where communication and shared decision-making were prioritized. By fostering collaboration, we could offer patients comprehensive care that addressed their physical, psychological, and nutritional needs.
For example, managing patients with chronic conditions like inflammatory bowel disease, cirrhosis, or obesity requires more than just prescribing medications. It involves regular monitoring, dietary adjustments, psychological support, and in some cases, surgical intervention. In an academic setting, coordinating this level of care can be cumbersome due to institutional barriers and siloed departments. In our practice, some of these relationships are achieved through partnerships with other like-minded practices. In other situations, team members of other disciplines are employed directly by our practice. Being in an independent practice allowed us the flexibility to prioritize working with the right team members first, and then structuring the relationship model second.
Lesson 4: Technology Is a Vital Tool in Redesigning Health Care
When I worked in a large academic health system, technology was often seen as an administrative burden rather than a clinical asset. Electronic health records (EHR) and a lot of the other IT systems that health care workers and patients interacted with on a regular basis were viewed as a barrier to care or a cause of time burdens instead of as tools to make health care easier. As we built our new practice from scratch, it was critical that we had an IT infrastructure that aligned with our core goals: simplify and automate the health care experience for everyone.
For our practice, we didn’t try to re-invent the wheel. Instead we copied from other industries who had already figured out a great solution for a problem we had. We wanted our patients to have a great customer service experience when interacting with our practice for scheduling, questions, refills, etc. So we implemented a unified communication system that some Fortune 100 companies, with perennial high scores for customer service, used. We wanted a great human resource system that would streamline the administrative time it would take to handle all HR needs for our practice. So we implemented an HR information system that had the best ratings for automation and integration with other business systems. At every point in the process, we reminded ourselves to focus on simplification and automation for every user of the system.
Conclusion: A Rewarding Transition
The lessons I’ve learned along the way — embracing autonomy, understanding financial stewardship, fostering collaboration, and leveraging technology — have helped me work toward a better total health care experience for the community.
This journey has also been deeply fulfilling on a personal level. It has allowed me to build stronger relationships with my patients, focus on long-term health outcomes, and create a practice where innovation and quality truly matter. While the challenges of running a private practice are real, the rewards — both for me and my patients — are immeasurable. If I had to do it all over again, I wouldn’t hesitate for a moment. If anything, I should have done it earlier.
Dr. Gupta is Managing Partner at Midwest Digestive Health & Nutrition, in Des Plaines, Illinois. He has reported no conflicts of interest in relation to this article.
After more than 10 years of serving in a large academic medical center in Chicago, Illinois, that was part of a national health care system, the decision to transition into private practice wasn’t one I made lightly.
Having built a rewarding career and spent over a quarter of my life in an academic medical center and a national health system, the move to starting an independent practice from scratch was both exciting and daunting. The notion of leaving behind the structure, resources, and safety of the large health system was unsettling. However, as the landscape of health care continues to evolve, with worsening large structural problems within the U.S. health care system, I realized that starting an independent gastroenterology practice — focused on trying to fix some of these large-scale problems from the start — would not only align with my professional goals but also provide the personal satisfaction I had failed to find.
As I reflect on my journey, there are a few key lessons I learned from making this leap — lessons that helped me transition from a highly structured employed physician environment to leading a thriving independent practice focused on redesigning gastroenterology care from scratch.
Lesson 1: Autonomy Opens the Door to Innovation
One of the primary reasons I left the employed physician setting was to gain greater control over my clinical practice and decision-making processes.
In a national health care system, the goal of standardization often dictates not only clinical care, but many “back end” aspects of the entire health care experience. We often see the things that are more visible, such as what supplies/equipment you use, how your patient appointments are scheduled, how many support staff members are assigned to help your practice, what electronic health record system you use, and how shared resources (like GI lab block time or anesthesia teams) are allocated.
However, this also impacts things we don’t usually see, such as what fees are billed for care you are providing (like facility fees), communication systems that your patients need to navigate for help, human resource systems you use, and retirement/health benefits you and your other team members receive.
Standardization has two adverse consequences: 1) it does not allow for personalization and as a result, 2) it suppresses innovation. Standard protocols can streamline processes, but they sometimes fail to account for the nuanced differences between patients, such as genetic factors, unique medical histories, or responses/failures to prior treatments. This rigidity can stifle innovation, as physicians are often bound by guidelines that may not reflect the latest advancements or allow for creative, individualized approaches to care. In the long term, an overemphasis on standardization risks turning health care into a one-size-fits-all model, undermining the potential for breakthroughs.
The transition was challenging at first, as we needed to engage our entire new practice with a different mindset now that many of us had autonomy for the first time. Instead of everyone just practicing health care the way they had done before, we took a page from Elon Musk and challenged every member of the team to ask three questions about everything they do on a daily basis:
- Is what I am doing helping a patient get healthy? (Question every requirement)
- If not, do I still need to do this? (Delete any part of the process you can)
- If so, how can I make this easier, faster, or automated? (Simplify and optimize, accelerate cycle time, and automate)
The freedom to innovate is a hallmark of independent practice. Embracing innovation in every aspect of the practice has been the most critical lesson of this journey.
Lesson 2: Financial Stewardship is Critical for Sustainability
Running an independent practice is not just about medicine — it’s also about managing a business.
This was a stark shift from the large academic health systems, where financial decisions were handled by the “administration.” In my new role as a business owner, understanding the financial aspects of health care was crucial for success. The cost of what patients pay for health care in the United States (either directly in deductibles and coinsurance or indirectly through insurance premiums) is unsustainably high. However, inflation continues to cause substantial increases in almost all the costs of delivering care: medical supplies, salaries, benefits, IT costs, etc. It was critical to develop a financial plan that accounted for these two macro-economic trends, and ideally helped solve for both. In our case, delivering high quality care with a lower cost to patients and payers.
We started by reevaluating our relationship with payers. Whereas being part of a large academic health system, we are often taught to look at payers as the adversary; as an independent practice looking to redesign the health care experience, it was critical for us to look to the payers as a partner in this journey. Understanding payer expectations and structuring contracts that aligned with shared goals of reducing total health care costs for patients was one of the foundations of our financial plan.
Offering office-based endoscopy was one innovation we implemented to significantly impact both patient affordability and practice revenue. By performing procedures like colonoscopies and upper endoscopies in an office setting rather than a hospital or ambulatory surgery center, we eliminated facility fees, which are often a significant part of the total cost of care. This directly lowers out-of-pocket expenses for patients and reduces the overall financial burden on insurance companies. At the same time, it allows the practice to capture more of the revenue from these procedures, without the overhead costs associated with larger facilities. This model creates a win-win situation: patients save money while receiving the same quality of care, and the practice experiences an increase in profitability and autonomy in managing its services.
Lesson 3: Collaborative Care and Multidisciplinary Teams Can Exist Anywhere
One aspect I deeply valued in academia was the collaborative environment — having specialists across disciplines work together on challenging cases. In private practice, I was concerned that I would lose this collegial atmosphere. However, I quickly learned that building a robust network of multidisciplinary collaborators was achievable in independent practice, just like it was in a large health system.
In our practice, we established close relationships with primary care physicians, surgeons, advanced practice providers, dietitians, behavioral health specialists, and others. These partnerships were not just referral networks but integrated care teams where communication and shared decision-making were prioritized. By fostering collaboration, we could offer patients comprehensive care that addressed their physical, psychological, and nutritional needs.
For example, managing patients with chronic conditions like inflammatory bowel disease, cirrhosis, or obesity requires more than just prescribing medications. It involves regular monitoring, dietary adjustments, psychological support, and in some cases, surgical intervention. In an academic setting, coordinating this level of care can be cumbersome due to institutional barriers and siloed departments. In our practice, some of these relationships are achieved through partnerships with other like-minded practices. In other situations, team members of other disciplines are employed directly by our practice. Being in an independent practice allowed us the flexibility to prioritize working with the right team members first, and then structuring the relationship model second.
Lesson 4: Technology Is a Vital Tool in Redesigning Health Care
When I worked in a large academic health system, technology was often seen as an administrative burden rather than a clinical asset. Electronic health records (EHR) and a lot of the other IT systems that health care workers and patients interacted with on a regular basis were viewed as a barrier to care or a cause of time burdens instead of as tools to make health care easier. As we built our new practice from scratch, it was critical that we had an IT infrastructure that aligned with our core goals: simplify and automate the health care experience for everyone.
For our practice, we didn’t try to re-invent the wheel. Instead we copied from other industries who had already figured out a great solution for a problem we had. We wanted our patients to have a great customer service experience when interacting with our practice for scheduling, questions, refills, etc. So we implemented a unified communication system that some Fortune 100 companies, with perennial high scores for customer service, used. We wanted a great human resource system that would streamline the administrative time it would take to handle all HR needs for our practice. So we implemented an HR information system that had the best ratings for automation and integration with other business systems. At every point in the process, we reminded ourselves to focus on simplification and automation for every user of the system.
Conclusion: A Rewarding Transition
The lessons I’ve learned along the way — embracing autonomy, understanding financial stewardship, fostering collaboration, and leveraging technology — have helped me work toward a better total health care experience for the community.
This journey has also been deeply fulfilling on a personal level. It has allowed me to build stronger relationships with my patients, focus on long-term health outcomes, and create a practice where innovation and quality truly matter. While the challenges of running a private practice are real, the rewards — both for me and my patients — are immeasurable. If I had to do it all over again, I wouldn’t hesitate for a moment. If anything, I should have done it earlier.
Dr. Gupta is Managing Partner at Midwest Digestive Health & Nutrition, in Des Plaines, Illinois. He has reported no conflicts of interest in relation to this article.
After more than 10 years of serving in a large academic medical center in Chicago, Illinois, that was part of a national health care system, the decision to transition into private practice wasn’t one I made lightly.
Having built a rewarding career and spent over a quarter of my life in an academic medical center and a national health system, the move to starting an independent practice from scratch was both exciting and daunting. The notion of leaving behind the structure, resources, and safety of the large health system was unsettling. However, as the landscape of health care continues to evolve, with worsening large structural problems within the U.S. health care system, I realized that starting an independent gastroenterology practice — focused on trying to fix some of these large-scale problems from the start — would not only align with my professional goals but also provide the personal satisfaction I had failed to find.
As I reflect on my journey, there are a few key lessons I learned from making this leap — lessons that helped me transition from a highly structured employed physician environment to leading a thriving independent practice focused on redesigning gastroenterology care from scratch.
Lesson 1: Autonomy Opens the Door to Innovation
One of the primary reasons I left the employed physician setting was to gain greater control over my clinical practice and decision-making processes.
In a national health care system, the goal of standardization often dictates not only clinical care, but many “back end” aspects of the entire health care experience. We often see the things that are more visible, such as what supplies/equipment you use, how your patient appointments are scheduled, how many support staff members are assigned to help your practice, what electronic health record system you use, and how shared resources (like GI lab block time or anesthesia teams) are allocated.
However, this also impacts things we don’t usually see, such as what fees are billed for care you are providing (like facility fees), communication systems that your patients need to navigate for help, human resource systems you use, and retirement/health benefits you and your other team members receive.
Standardization has two adverse consequences: 1) it does not allow for personalization and as a result, 2) it suppresses innovation. Standard protocols can streamline processes, but they sometimes fail to account for the nuanced differences between patients, such as genetic factors, unique medical histories, or responses/failures to prior treatments. This rigidity can stifle innovation, as physicians are often bound by guidelines that may not reflect the latest advancements or allow for creative, individualized approaches to care. In the long term, an overemphasis on standardization risks turning health care into a one-size-fits-all model, undermining the potential for breakthroughs.
The transition was challenging at first, as we needed to engage our entire new practice with a different mindset now that many of us had autonomy for the first time. Instead of everyone just practicing health care the way they had done before, we took a page from Elon Musk and challenged every member of the team to ask three questions about everything they do on a daily basis:
- Is what I am doing helping a patient get healthy? (Question every requirement)
- If not, do I still need to do this? (Delete any part of the process you can)
- If so, how can I make this easier, faster, or automated? (Simplify and optimize, accelerate cycle time, and automate)
The freedom to innovate is a hallmark of independent practice. Embracing innovation in every aspect of the practice has been the most critical lesson of this journey.
Lesson 2: Financial Stewardship is Critical for Sustainability
Running an independent practice is not just about medicine — it’s also about managing a business.
This was a stark shift from the large academic health systems, where financial decisions were handled by the “administration.” In my new role as a business owner, understanding the financial aspects of health care was crucial for success. The cost of what patients pay for health care in the United States (either directly in deductibles and coinsurance or indirectly through insurance premiums) is unsustainably high. However, inflation continues to cause substantial increases in almost all the costs of delivering care: medical supplies, salaries, benefits, IT costs, etc. It was critical to develop a financial plan that accounted for these two macro-economic trends, and ideally helped solve for both. In our case, delivering high quality care with a lower cost to patients and payers.
We started by reevaluating our relationship with payers. Whereas being part of a large academic health system, we are often taught to look at payers as the adversary; as an independent practice looking to redesign the health care experience, it was critical for us to look to the payers as a partner in this journey. Understanding payer expectations and structuring contracts that aligned with shared goals of reducing total health care costs for patients was one of the foundations of our financial plan.
Offering office-based endoscopy was one innovation we implemented to significantly impact both patient affordability and practice revenue. By performing procedures like colonoscopies and upper endoscopies in an office setting rather than a hospital or ambulatory surgery center, we eliminated facility fees, which are often a significant part of the total cost of care. This directly lowers out-of-pocket expenses for patients and reduces the overall financial burden on insurance companies. At the same time, it allows the practice to capture more of the revenue from these procedures, without the overhead costs associated with larger facilities. This model creates a win-win situation: patients save money while receiving the same quality of care, and the practice experiences an increase in profitability and autonomy in managing its services.
Lesson 3: Collaborative Care and Multidisciplinary Teams Can Exist Anywhere
One aspect I deeply valued in academia was the collaborative environment — having specialists across disciplines work together on challenging cases. In private practice, I was concerned that I would lose this collegial atmosphere. However, I quickly learned that building a robust network of multidisciplinary collaborators was achievable in independent practice, just like it was in a large health system.
In our practice, we established close relationships with primary care physicians, surgeons, advanced practice providers, dietitians, behavioral health specialists, and others. These partnerships were not just referral networks but integrated care teams where communication and shared decision-making were prioritized. By fostering collaboration, we could offer patients comprehensive care that addressed their physical, psychological, and nutritional needs.
For example, managing patients with chronic conditions like inflammatory bowel disease, cirrhosis, or obesity requires more than just prescribing medications. It involves regular monitoring, dietary adjustments, psychological support, and in some cases, surgical intervention. In an academic setting, coordinating this level of care can be cumbersome due to institutional barriers and siloed departments. In our practice, some of these relationships are achieved through partnerships with other like-minded practices. In other situations, team members of other disciplines are employed directly by our practice. Being in an independent practice allowed us the flexibility to prioritize working with the right team members first, and then structuring the relationship model second.
Lesson 4: Technology Is a Vital Tool in Redesigning Health Care
When I worked in a large academic health system, technology was often seen as an administrative burden rather than a clinical asset. Electronic health records (EHR) and a lot of the other IT systems that health care workers and patients interacted with on a regular basis were viewed as a barrier to care or a cause of time burdens instead of as tools to make health care easier. As we built our new practice from scratch, it was critical that we had an IT infrastructure that aligned with our core goals: simplify and automate the health care experience for everyone.
For our practice, we didn’t try to re-invent the wheel. Instead we copied from other industries who had already figured out a great solution for a problem we had. We wanted our patients to have a great customer service experience when interacting with our practice for scheduling, questions, refills, etc. So we implemented a unified communication system that some Fortune 100 companies, with perennial high scores for customer service, used. We wanted a great human resource system that would streamline the administrative time it would take to handle all HR needs for our practice. So we implemented an HR information system that had the best ratings for automation and integration with other business systems. At every point in the process, we reminded ourselves to focus on simplification and automation for every user of the system.
Conclusion: A Rewarding Transition
The lessons I’ve learned along the way — embracing autonomy, understanding financial stewardship, fostering collaboration, and leveraging technology — have helped me work toward a better total health care experience for the community.
This journey has also been deeply fulfilling on a personal level. It has allowed me to build stronger relationships with my patients, focus on long-term health outcomes, and create a practice where innovation and quality truly matter. While the challenges of running a private practice are real, the rewards — both for me and my patients — are immeasurable. If I had to do it all over again, I wouldn’t hesitate for a moment. If anything, I should have done it earlier.
Dr. Gupta is Managing Partner at Midwest Digestive Health & Nutrition, in Des Plaines, Illinois. He has reported no conflicts of interest in relation to this article.
The Most Common Chronic Liver Disease in the World
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr. Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams. Paul, what is MASLD?
Paul N. Williams, MD:
Watto: We talked about a really stripped-down way of testing people for MASLD. If we see mildly elevated liver enzymes, what should we be testing, and how does alcohol factor in?
Williams: Before you can make a definitive diagnosis of MASLD, you need to rule out other causes of liver inflammation — things that would cause a patient’s transaminases to increase. Alcohol is synergistic with everything that can harm the liver.
A great place to start is to gauge someone’s alcohol intake to make sure it isn’t causing hepatic inflammation. The phosphatidyl ethanol level is a serologic test to determine chronic, heavy alcohol use. It’s a new kid on the block. I’ve seen it mostly ordered by hepatologists. It is a way of determining whether someone has had fairly consistent alcohol use up to 4 weeks after the fact. The cutoff for a positive test is 20 ng/mL.
Dr Tapper frames the test this way. He isn’t using the test to catch someone in a lie about their alcohol use. He tells patients that he orders this test for all patients with liver inflammation, because alcohol is a common cause. The test helps him better understand the factors that might be affecting the patient’s liver function.
If the test comes back positive, you can have a conversation about that, and if it’s not positive, you move on to the next possible cause. Other fairly common causes of liver inflammation are relatively easy to address.
Watto: Instead of ordering ceruloplasmin or alpha-1 antitrypsin tests, for example, the first thing Dr Tapper recommends is checking for hepatitis B and C. We can cure hepatitis C. We can’t cure hepatitis B, but it’s important to know if the patient has it. Primary care physicians should be comfortable ordering these tests.
Really high ALT levels (eg, in the 200s) don’t usually happen from steatotic liver disease. In those cases, we would send an expanded panel that might include tests for autoimmune hepatitis-ANA, anti–smooth muscle antibody, and IgG levels. Otherwise, most of these patients don’t need much more testing.
What is a FIB4 score and how does that factor in?
Williams: The FIB4 score estimates the degree of fibrosis based on the ALT and AST levels, platelet count, and the patient’s age. These data are plugged into a formula. If the FIB4 score is low (meaning not much fibrosis is present), you can stop there and do your counseling about lifestyle changes and address the reversible factors.
If the FIB4 score is above a certain threshold (1.3 in young adults and 2.0 in older adults), you need to find a more concrete way to determine the degree of fibrosis, typically through imaging.
Elastography can be done either with ultrasound or MRI. Ultrasound is typically ordered, but Dr Tapper recommends doing MRI on patients with a BMI > 40. Those patients are probably better served by doing MRI to determine the degree of liver fibrosis.
Watto: Patients with low FIB4 scores probably don’t need elastography but those with high FIB4 scores do. For the interpretation of ultrasound-based elastography results, Dr Tapper gave us the “rule of 5s”.
Elastography results are reported in kilopascal (kPa) units. A finding of 5 kPa or less is normal. Forty percent of those with a result of 10 kPa might have advanced liver disease. Above 15 kPa, the likelihood of cirrhosis is high, becoming very likely at 25 kPa. Finally, with a result of > 25 kPa, portal hypertension is likely, and you might need to have a conversation about starting the patient on medicine to prevent variceal bleeding.
We are moving toward more noninvasive testing and avoiding biopsies. We have cutoff values for MRI-based elastography as well. Both of these tests can help stage the liver.
What can we tell people about diet?
Williams: Weight loss is helpful. You can reverse fibrosis with weight loss. You can truly help your liver and bring it closer to its healthy baseline with weight loss. A loss of 7.5% body weight can reduce steatohepatitis, and with around 10% of body weight loss, you can actually resolve fibrosis, which is remarkable.
We all know that weight loss can be very therapeutic for many conditions. It’s just very hard to achieve. As primary care doctors, we should use what we have in our armamentarium to achieve that goal. Often, that will include certain medications.
Watto: I like giving patients the 10% number because if they weigh 220 pounds, they need to lose 22 pounds. If they weigh 300 pounds, it’s 30 pounds. Most people who weigh 300 pounds think they need to lose 100 pounds to have any sort of health benefit, but it’s much less than that. So, I do find that helpful.
But now a new drug has been approved. It’s a thyroid memetic called resmetirom. It was from the MAESTRO-NASH trial. Without weight loss, it helped to reverse fibrosis.
This is going to be used more and more in the future. It’s still being worked out exactly where the place is for that drug, so much so that Dr Tapper, as a liver expert, hadn’t even had the chance to prescribe it yet. Of course, it was very recently approved.
Dr. Tapper is one of our most celebrated guests, so check out the full podcast here.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr. Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams. Paul, what is MASLD?
Paul N. Williams, MD:
Watto: We talked about a really stripped-down way of testing people for MASLD. If we see mildly elevated liver enzymes, what should we be testing, and how does alcohol factor in?
Williams: Before you can make a definitive diagnosis of MASLD, you need to rule out other causes of liver inflammation — things that would cause a patient’s transaminases to increase. Alcohol is synergistic with everything that can harm the liver.
A great place to start is to gauge someone’s alcohol intake to make sure it isn’t causing hepatic inflammation. The phosphatidyl ethanol level is a serologic test to determine chronic, heavy alcohol use. It’s a new kid on the block. I’ve seen it mostly ordered by hepatologists. It is a way of determining whether someone has had fairly consistent alcohol use up to 4 weeks after the fact. The cutoff for a positive test is 20 ng/mL.
Dr Tapper frames the test this way. He isn’t using the test to catch someone in a lie about their alcohol use. He tells patients that he orders this test for all patients with liver inflammation, because alcohol is a common cause. The test helps him better understand the factors that might be affecting the patient’s liver function.
If the test comes back positive, you can have a conversation about that, and if it’s not positive, you move on to the next possible cause. Other fairly common causes of liver inflammation are relatively easy to address.
Watto: Instead of ordering ceruloplasmin or alpha-1 antitrypsin tests, for example, the first thing Dr Tapper recommends is checking for hepatitis B and C. We can cure hepatitis C. We can’t cure hepatitis B, but it’s important to know if the patient has it. Primary care physicians should be comfortable ordering these tests.
Really high ALT levels (eg, in the 200s) don’t usually happen from steatotic liver disease. In those cases, we would send an expanded panel that might include tests for autoimmune hepatitis-ANA, anti–smooth muscle antibody, and IgG levels. Otherwise, most of these patients don’t need much more testing.
What is a FIB4 score and how does that factor in?
Williams: The FIB4 score estimates the degree of fibrosis based on the ALT and AST levels, platelet count, and the patient’s age. These data are plugged into a formula. If the FIB4 score is low (meaning not much fibrosis is present), you can stop there and do your counseling about lifestyle changes and address the reversible factors.
If the FIB4 score is above a certain threshold (1.3 in young adults and 2.0 in older adults), you need to find a more concrete way to determine the degree of fibrosis, typically through imaging.
Elastography can be done either with ultrasound or MRI. Ultrasound is typically ordered, but Dr Tapper recommends doing MRI on patients with a BMI > 40. Those patients are probably better served by doing MRI to determine the degree of liver fibrosis.
Watto: Patients with low FIB4 scores probably don’t need elastography but those with high FIB4 scores do. For the interpretation of ultrasound-based elastography results, Dr Tapper gave us the “rule of 5s”.
Elastography results are reported in kilopascal (kPa) units. A finding of 5 kPa or less is normal. Forty percent of those with a result of 10 kPa might have advanced liver disease. Above 15 kPa, the likelihood of cirrhosis is high, becoming very likely at 25 kPa. Finally, with a result of > 25 kPa, portal hypertension is likely, and you might need to have a conversation about starting the patient on medicine to prevent variceal bleeding.
We are moving toward more noninvasive testing and avoiding biopsies. We have cutoff values for MRI-based elastography as well. Both of these tests can help stage the liver.
What can we tell people about diet?
Williams: Weight loss is helpful. You can reverse fibrosis with weight loss. You can truly help your liver and bring it closer to its healthy baseline with weight loss. A loss of 7.5% body weight can reduce steatohepatitis, and with around 10% of body weight loss, you can actually resolve fibrosis, which is remarkable.
We all know that weight loss can be very therapeutic for many conditions. It’s just very hard to achieve. As primary care doctors, we should use what we have in our armamentarium to achieve that goal. Often, that will include certain medications.
Watto: I like giving patients the 10% number because if they weigh 220 pounds, they need to lose 22 pounds. If they weigh 300 pounds, it’s 30 pounds. Most people who weigh 300 pounds think they need to lose 100 pounds to have any sort of health benefit, but it’s much less than that. So, I do find that helpful.
But now a new drug has been approved. It’s a thyroid memetic called resmetirom. It was from the MAESTRO-NASH trial. Without weight loss, it helped to reverse fibrosis.
This is going to be used more and more in the future. It’s still being worked out exactly where the place is for that drug, so much so that Dr Tapper, as a liver expert, hadn’t even had the chance to prescribe it yet. Of course, it was very recently approved.
Dr. Tapper is one of our most celebrated guests, so check out the full podcast here.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr. Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams. Paul, what is MASLD?
Paul N. Williams, MD:
Watto: We talked about a really stripped-down way of testing people for MASLD. If we see mildly elevated liver enzymes, what should we be testing, and how does alcohol factor in?
Williams: Before you can make a definitive diagnosis of MASLD, you need to rule out other causes of liver inflammation — things that would cause a patient’s transaminases to increase. Alcohol is synergistic with everything that can harm the liver.
A great place to start is to gauge someone’s alcohol intake to make sure it isn’t causing hepatic inflammation. The phosphatidyl ethanol level is a serologic test to determine chronic, heavy alcohol use. It’s a new kid on the block. I’ve seen it mostly ordered by hepatologists. It is a way of determining whether someone has had fairly consistent alcohol use up to 4 weeks after the fact. The cutoff for a positive test is 20 ng/mL.
Dr Tapper frames the test this way. He isn’t using the test to catch someone in a lie about their alcohol use. He tells patients that he orders this test for all patients with liver inflammation, because alcohol is a common cause. The test helps him better understand the factors that might be affecting the patient’s liver function.
If the test comes back positive, you can have a conversation about that, and if it’s not positive, you move on to the next possible cause. Other fairly common causes of liver inflammation are relatively easy to address.
Watto: Instead of ordering ceruloplasmin or alpha-1 antitrypsin tests, for example, the first thing Dr Tapper recommends is checking for hepatitis B and C. We can cure hepatitis C. We can’t cure hepatitis B, but it’s important to know if the patient has it. Primary care physicians should be comfortable ordering these tests.
Really high ALT levels (eg, in the 200s) don’t usually happen from steatotic liver disease. In those cases, we would send an expanded panel that might include tests for autoimmune hepatitis-ANA, anti–smooth muscle antibody, and IgG levels. Otherwise, most of these patients don’t need much more testing.
What is a FIB4 score and how does that factor in?
Williams: The FIB4 score estimates the degree of fibrosis based on the ALT and AST levels, platelet count, and the patient’s age. These data are plugged into a formula. If the FIB4 score is low (meaning not much fibrosis is present), you can stop there and do your counseling about lifestyle changes and address the reversible factors.
If the FIB4 score is above a certain threshold (1.3 in young adults and 2.0 in older adults), you need to find a more concrete way to determine the degree of fibrosis, typically through imaging.
Elastography can be done either with ultrasound or MRI. Ultrasound is typically ordered, but Dr Tapper recommends doing MRI on patients with a BMI > 40. Those patients are probably better served by doing MRI to determine the degree of liver fibrosis.
Watto: Patients with low FIB4 scores probably don’t need elastography but those with high FIB4 scores do. For the interpretation of ultrasound-based elastography results, Dr Tapper gave us the “rule of 5s”.
Elastography results are reported in kilopascal (kPa) units. A finding of 5 kPa or less is normal. Forty percent of those with a result of 10 kPa might have advanced liver disease. Above 15 kPa, the likelihood of cirrhosis is high, becoming very likely at 25 kPa. Finally, with a result of > 25 kPa, portal hypertension is likely, and you might need to have a conversation about starting the patient on medicine to prevent variceal bleeding.
We are moving toward more noninvasive testing and avoiding biopsies. We have cutoff values for MRI-based elastography as well. Both of these tests can help stage the liver.
What can we tell people about diet?
Williams: Weight loss is helpful. You can reverse fibrosis with weight loss. You can truly help your liver and bring it closer to its healthy baseline with weight loss. A loss of 7.5% body weight can reduce steatohepatitis, and with around 10% of body weight loss, you can actually resolve fibrosis, which is remarkable.
We all know that weight loss can be very therapeutic for many conditions. It’s just very hard to achieve. As primary care doctors, we should use what we have in our armamentarium to achieve that goal. Often, that will include certain medications.
Watto: I like giving patients the 10% number because if they weigh 220 pounds, they need to lose 22 pounds. If they weigh 300 pounds, it’s 30 pounds. Most people who weigh 300 pounds think they need to lose 100 pounds to have any sort of health benefit, but it’s much less than that. So, I do find that helpful.
But now a new drug has been approved. It’s a thyroid memetic called resmetirom. It was from the MAESTRO-NASH trial. Without weight loss, it helped to reverse fibrosis.
This is going to be used more and more in the future. It’s still being worked out exactly where the place is for that drug, so much so that Dr Tapper, as a liver expert, hadn’t even had the chance to prescribe it yet. Of course, it was very recently approved.
Dr. Tapper is one of our most celebrated guests, so check out the full podcast here.
A version of this article appeared on Medscape.com.
Solo Vs McDoctors Inc.
STAT News recently ran a series on UnitedHealthcare (UHC) and its growing physician empire. This includes the corporation pressuring its employed physicians to see more patients, work weekends, upcode visits, add in diagnoses that will increase reimbursement, yadda, yadda, yadda.
For legal disclaimer purposes, I’m not saying UHC did any of this, nor am I saying they didn’t. But the series on STAT is worth reading.
Reading the articles brings back memories of the last time I was an employed physician, 24 years ago. I didn’t have people telling me to upcode visits, but I do remember hearing terms such as “dollars per physician per square foot” bandied about concerning my performance. At least back then no one was going to yell at me about a 1-star online review from a disgruntled patient.
After a little over 2 years I’d had enough and went solo.
I have no desire at this point to go back to that. I certainly make a lot less money than my employed counterparts, but I also have time and a degree of peace, which are worth something.
I’m not paying for anyone else’s overhead. I don’t slack off, but at least I know what I’m working for, and where the money is going when I write out a check. I can work my schedule around having to take my dog to the vet, or pick a kid up at the airport, or whatever.
I can spend more time with the patients who need it. Isn’t that part of why I’m here?
Wearing Hawaiian shirts and shorts to the office everyday is also a plus (at least I think so).
It surprises me that more physicians aren’t willing to go into solo or small group practice. The big advantage is freedom, only needing to pay the overhead and your salary, and cover for others when needed.
The downside is financial. Like our hunting and gathering ancestors, you eat what you kill. If there’s a shortfall in cash flow, I’m the one who doesn’t get paid. It’s always good to have a line of credit available to fall back on in a pinch.
I can see why it’s daunting. Coming out of training you have loans to pay off. You may have a young family, and your first mortgage. You sure don’t want to take out another loan to start a private practice. The security of a guaranteed paycheck and no start-up costs is attractive. I was there, too, and I also took the first job I was offered back then.
There’s also the fear of suddenly working without a net for the first time in your career. It’s reassuring to get some added experience while being able to bounce a challenging case off another doctor. (I still do that, too, and always will.)
But no one tells me to upcode visits or add diagnostic codes just to get more money. Patients don’t call in panicked that they have an ICD-10 code for a condition no one told them they had.
At the end of the day I can tell the guy in the mirror that I’m doing my best.
Medicine has changed a lot over time ... but being a doctor hasn’t. The spark that led us all here is still there, somewhere, I hope. Go back and read Neighbor Rosicky by Willa Cather, and The Doctor Stories by William Carlos Williams.
In an age when technology is moving us forward, I think the practice of medicine should move backward, away from McDoctors Inc. A small, even solo, medical practice isn’t incompatible with the shiny toys of 2024 medicine. You can make good patient care happen with both.
I freely admit that it’s not for everyone.
But I wish more people would see it as a realistic option, and take the road less traveled.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
STAT News recently ran a series on UnitedHealthcare (UHC) and its growing physician empire. This includes the corporation pressuring its employed physicians to see more patients, work weekends, upcode visits, add in diagnoses that will increase reimbursement, yadda, yadda, yadda.
For legal disclaimer purposes, I’m not saying UHC did any of this, nor am I saying they didn’t. But the series on STAT is worth reading.
Reading the articles brings back memories of the last time I was an employed physician, 24 years ago. I didn’t have people telling me to upcode visits, but I do remember hearing terms such as “dollars per physician per square foot” bandied about concerning my performance. At least back then no one was going to yell at me about a 1-star online review from a disgruntled patient.
After a little over 2 years I’d had enough and went solo.
I have no desire at this point to go back to that. I certainly make a lot less money than my employed counterparts, but I also have time and a degree of peace, which are worth something.
I’m not paying for anyone else’s overhead. I don’t slack off, but at least I know what I’m working for, and where the money is going when I write out a check. I can work my schedule around having to take my dog to the vet, or pick a kid up at the airport, or whatever.
I can spend more time with the patients who need it. Isn’t that part of why I’m here?
Wearing Hawaiian shirts and shorts to the office everyday is also a plus (at least I think so).
It surprises me that more physicians aren’t willing to go into solo or small group practice. The big advantage is freedom, only needing to pay the overhead and your salary, and cover for others when needed.
The downside is financial. Like our hunting and gathering ancestors, you eat what you kill. If there’s a shortfall in cash flow, I’m the one who doesn’t get paid. It’s always good to have a line of credit available to fall back on in a pinch.
I can see why it’s daunting. Coming out of training you have loans to pay off. You may have a young family, and your first mortgage. You sure don’t want to take out another loan to start a private practice. The security of a guaranteed paycheck and no start-up costs is attractive. I was there, too, and I also took the first job I was offered back then.
There’s also the fear of suddenly working without a net for the first time in your career. It’s reassuring to get some added experience while being able to bounce a challenging case off another doctor. (I still do that, too, and always will.)
But no one tells me to upcode visits or add diagnostic codes just to get more money. Patients don’t call in panicked that they have an ICD-10 code for a condition no one told them they had.
At the end of the day I can tell the guy in the mirror that I’m doing my best.
Medicine has changed a lot over time ... but being a doctor hasn’t. The spark that led us all here is still there, somewhere, I hope. Go back and read Neighbor Rosicky by Willa Cather, and The Doctor Stories by William Carlos Williams.
In an age when technology is moving us forward, I think the practice of medicine should move backward, away from McDoctors Inc. A small, even solo, medical practice isn’t incompatible with the shiny toys of 2024 medicine. You can make good patient care happen with both.
I freely admit that it’s not for everyone.
But I wish more people would see it as a realistic option, and take the road less traveled.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
STAT News recently ran a series on UnitedHealthcare (UHC) and its growing physician empire. This includes the corporation pressuring its employed physicians to see more patients, work weekends, upcode visits, add in diagnoses that will increase reimbursement, yadda, yadda, yadda.
For legal disclaimer purposes, I’m not saying UHC did any of this, nor am I saying they didn’t. But the series on STAT is worth reading.
Reading the articles brings back memories of the last time I was an employed physician, 24 years ago. I didn’t have people telling me to upcode visits, but I do remember hearing terms such as “dollars per physician per square foot” bandied about concerning my performance. At least back then no one was going to yell at me about a 1-star online review from a disgruntled patient.
After a little over 2 years I’d had enough and went solo.
I have no desire at this point to go back to that. I certainly make a lot less money than my employed counterparts, but I also have time and a degree of peace, which are worth something.
I’m not paying for anyone else’s overhead. I don’t slack off, but at least I know what I’m working for, and where the money is going when I write out a check. I can work my schedule around having to take my dog to the vet, or pick a kid up at the airport, or whatever.
I can spend more time with the patients who need it. Isn’t that part of why I’m here?
Wearing Hawaiian shirts and shorts to the office everyday is also a plus (at least I think so).
It surprises me that more physicians aren’t willing to go into solo or small group practice. The big advantage is freedom, only needing to pay the overhead and your salary, and cover for others when needed.
The downside is financial. Like our hunting and gathering ancestors, you eat what you kill. If there’s a shortfall in cash flow, I’m the one who doesn’t get paid. It’s always good to have a line of credit available to fall back on in a pinch.
I can see why it’s daunting. Coming out of training you have loans to pay off. You may have a young family, and your first mortgage. You sure don’t want to take out another loan to start a private practice. The security of a guaranteed paycheck and no start-up costs is attractive. I was there, too, and I also took the first job I was offered back then.
There’s also the fear of suddenly working without a net for the first time in your career. It’s reassuring to get some added experience while being able to bounce a challenging case off another doctor. (I still do that, too, and always will.)
But no one tells me to upcode visits or add diagnostic codes just to get more money. Patients don’t call in panicked that they have an ICD-10 code for a condition no one told them they had.
At the end of the day I can tell the guy in the mirror that I’m doing my best.
Medicine has changed a lot over time ... but being a doctor hasn’t. The spark that led us all here is still there, somewhere, I hope. Go back and read Neighbor Rosicky by Willa Cather, and The Doctor Stories by William Carlos Williams.
In an age when technology is moving us forward, I think the practice of medicine should move backward, away from McDoctors Inc. A small, even solo, medical practice isn’t incompatible with the shiny toys of 2024 medicine. You can make good patient care happen with both.
I freely admit that it’s not for everyone.
But I wish more people would see it as a realistic option, and take the road less traveled.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
4 Simple Hacks to Get Paid for Lifestyle Medicine
This transcript has been edited for clarity.
As primary care doctors, lifestyle medicine is supposed to be a pillar of our practice. Per the evidence, lifestyle medicine can prevent up to 80% of chronic disease. It’s a real irony, then, that it’s the thing we’re least likely to be paid to do.
Thankfully, though, there are a few hacks to help you keep your patients healthy and yourself financially healthy at the same time.
No. 1: Be as accurate in your coding as possible. We all know working on things like sleep, exercise, and diet with patients takes time, so bill for it. With time-based billing, in particular, you can account for both the time spent in face-to-face encounters and the time spent afterward on documentation and care coordination. Make sure to capture that.
No. 2: Try group visits on for size. Group visit models are great for lifestyle medicine. They give you the flexibility to include longer conversations and deeper lessons on a range of subjects while still getting paid for what you do. Want to host a cooking class? Group visit. Want to bring in a personal trainer or hold a dance class or exercise dance class? Group visit. Meditation, yoga, or even a sleep hygiene class? Group visit.
While there are a few tricks to getting paid for group visits, they’re the same things, such as documenting time and the various parts of the visit, that are key to getting paid for regular visits. They have the bonus of fighting burnout and making your own practice more meaningful as well.
No. 3: Think about joining a value-based care arrangement. While only accounting for 10% of the market right now, value-based care (VBC) is growing rapidly, and it’s easy to see why. By trading quality for the hamster wheel of billing widgets, physicians are freed up to think more about how best to take care of patients, including incorporating more lifestyle medicine. Some VBC models even have their own electronic medical records, freeing you from outdated structures when it comes to documenting patient visits.
No. 4: direct primary care. Direct primary care cuts out the middlemen of payers, letting patients pay physician practices directly for their own care. Like VBC, it opens up possibilities for practicing better medicine, including lifestyle medicine. In addition, it’s often very affordable, with a family of four often paying around $80 a month for a membership for the entire family. It’s a win-win for the doctor and the patient.
Lifestyle medicine is a great way to improve both your patients’ and your own well-being. With a few flexes, it can improve your wallet’s well-being, too.
Tamaan K. Osbourne-Roberts, President/CEO, Happiness by the Numbers, Denver, Colorado, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
As primary care doctors, lifestyle medicine is supposed to be a pillar of our practice. Per the evidence, lifestyle medicine can prevent up to 80% of chronic disease. It’s a real irony, then, that it’s the thing we’re least likely to be paid to do.
Thankfully, though, there are a few hacks to help you keep your patients healthy and yourself financially healthy at the same time.
No. 1: Be as accurate in your coding as possible. We all know working on things like sleep, exercise, and diet with patients takes time, so bill for it. With time-based billing, in particular, you can account for both the time spent in face-to-face encounters and the time spent afterward on documentation and care coordination. Make sure to capture that.
No. 2: Try group visits on for size. Group visit models are great for lifestyle medicine. They give you the flexibility to include longer conversations and deeper lessons on a range of subjects while still getting paid for what you do. Want to host a cooking class? Group visit. Want to bring in a personal trainer or hold a dance class or exercise dance class? Group visit. Meditation, yoga, or even a sleep hygiene class? Group visit.
While there are a few tricks to getting paid for group visits, they’re the same things, such as documenting time and the various parts of the visit, that are key to getting paid for regular visits. They have the bonus of fighting burnout and making your own practice more meaningful as well.
No. 3: Think about joining a value-based care arrangement. While only accounting for 10% of the market right now, value-based care (VBC) is growing rapidly, and it’s easy to see why. By trading quality for the hamster wheel of billing widgets, physicians are freed up to think more about how best to take care of patients, including incorporating more lifestyle medicine. Some VBC models even have their own electronic medical records, freeing you from outdated structures when it comes to documenting patient visits.
No. 4: direct primary care. Direct primary care cuts out the middlemen of payers, letting patients pay physician practices directly for their own care. Like VBC, it opens up possibilities for practicing better medicine, including lifestyle medicine. In addition, it’s often very affordable, with a family of four often paying around $80 a month for a membership for the entire family. It’s a win-win for the doctor and the patient.
Lifestyle medicine is a great way to improve both your patients’ and your own well-being. With a few flexes, it can improve your wallet’s well-being, too.
Tamaan K. Osbourne-Roberts, President/CEO, Happiness by the Numbers, Denver, Colorado, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
As primary care doctors, lifestyle medicine is supposed to be a pillar of our practice. Per the evidence, lifestyle medicine can prevent up to 80% of chronic disease. It’s a real irony, then, that it’s the thing we’re least likely to be paid to do.
Thankfully, though, there are a few hacks to help you keep your patients healthy and yourself financially healthy at the same time.
No. 1: Be as accurate in your coding as possible. We all know working on things like sleep, exercise, and diet with patients takes time, so bill for it. With time-based billing, in particular, you can account for both the time spent in face-to-face encounters and the time spent afterward on documentation and care coordination. Make sure to capture that.
No. 2: Try group visits on for size. Group visit models are great for lifestyle medicine. They give you the flexibility to include longer conversations and deeper lessons on a range of subjects while still getting paid for what you do. Want to host a cooking class? Group visit. Want to bring in a personal trainer or hold a dance class or exercise dance class? Group visit. Meditation, yoga, or even a sleep hygiene class? Group visit.
While there are a few tricks to getting paid for group visits, they’re the same things, such as documenting time and the various parts of the visit, that are key to getting paid for regular visits. They have the bonus of fighting burnout and making your own practice more meaningful as well.
No. 3: Think about joining a value-based care arrangement. While only accounting for 10% of the market right now, value-based care (VBC) is growing rapidly, and it’s easy to see why. By trading quality for the hamster wheel of billing widgets, physicians are freed up to think more about how best to take care of patients, including incorporating more lifestyle medicine. Some VBC models even have their own electronic medical records, freeing you from outdated structures when it comes to documenting patient visits.
No. 4: direct primary care. Direct primary care cuts out the middlemen of payers, letting patients pay physician practices directly for their own care. Like VBC, it opens up possibilities for practicing better medicine, including lifestyle medicine. In addition, it’s often very affordable, with a family of four often paying around $80 a month for a membership for the entire family. It’s a win-win for the doctor and the patient.
Lifestyle medicine is a great way to improve both your patients’ and your own well-being. With a few flexes, it can improve your wallet’s well-being, too.
Tamaan K. Osbourne-Roberts, President/CEO, Happiness by the Numbers, Denver, Colorado, has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nutrition and Medical Education
How comfortable are you giving nutritional advice to your patients? When you offer it are you basing your advice on something you learned during medical school or your training? Was it included in a course devoted to nutrition? Did you learn it later as part of continuing medical education course (CME)? Or was it just something you just picked up from your experience seeing patients (osmosis)? It is very unlikely that a significant portion, or any part for that matter, of your medical training was devoted to nutrition. It certainly wasn’t during my training.
I recently read an interview with Emily M. Broad Leib, JD, faculty director of the Harvard School Center for Health Law and Policy Innovation, Cambridge, Massachusetts, who would like to correct that deficiency. She feels doctors need to know more about food and that acquiring that knowledge should be a significant component of their formal training.
In the interview, Leib said that “roughly 86% of physicians report they do not feel adequately trained to answer basic questions on diet or nutrition.” She also notes that while “72% of entering medical students report they believe food is important to health” less than 50% retained this belief after graduation.
Leib and associates feel they have recently reached a milestone in their efforts to include nutrition in the mainstream of medical education this fall by publishing a paper that demonstrates “consensus on doctor-approved nutritional standard for medical schools and residency programs.”
36 Recommended Competencies
Curious about what these nutrition experts chose to include in medical training, I decided to drill down into the list of 36 consensus-driven competencies they had agreed upon.
It was an interesting voyage into a forest of redundancies, many of which can be boiled down to having the student demonstrate that he/she understands that what we eat is important to our health and that there is a complex web of relationships connecting our society to the food consume.
Some of the recommended competencies I found make perfect sense. For example the student/trainee should be able to take a diet and food history and be able to interpret lab values and anthropometric measurements and be able to discuss the patient’s weight and diet with sensitivity while keeping in mind his/her own biases about food.
Some other recommendations are more problematic, for example, “performs a comprehensive nutrition-focused physical examination” or “demonstrates knowledge of how to create culinary nutrition SMART [Specific, Measurable, Achievable, Relevant, and Time-Bound] goals for personal use and for patient care” or “provides brief counseling interventions to help patients decrease visceral adiposity or reduce the risk of metabolic syndrome.” Including competencies like these demonstrates a lack of understanding of the time restraints and realities of a primary care physician’s life and training.
Instead of simply reinforcing the prospective physician’s preexisting assumption that food and health are entwined and discussing when and how to consult a nutrition expert, these 36 competencies seem to be an attempt to create fast-tracked part-time dietitians and nutrition advocates out of medical students and trainees who already believe that nutrition is important for health but also have a very full plate of clinical responsibilities ahead of them.
The study that Leib quotes — that 72% of medical students believed food was important in health while after graduation only 50% of agreed — doesn’t necessarily mean that professors are preaching that food was unimportant. It is more likely by the end of medical school the students have seen that food must share the spotlight with numerous other factors that influence their patients’ health.
‘A More Appropriate Focus’
In my experience, diet and lifestyle counseling done well is extremely time consuming and best done by people for whom that is their specialty. A more appropriate focus for a list of nutritional competencies for physicians in training would be for the student to achieve an understanding of when and how to consult a dietitian and then how to support and evaluate the dietitian’s recommendations to the patient.
Finally, I don’t think we can ignore a serious public relations problem that hangs like a cloud over the nutrition advocacy community. It is the same one that casts a shadow on the medical community as well. It is a common perception among the lay public that nutritionists (and physicians) are always changing their recommendations when it comes to food. What is believable? Just think about eggs, red wine, or introducing peanuts to infants, to name just a few. And what about the food pyramids that seem to have been rebuilt every several years? The problem is compounded when some “credentialed” nutritionists and physicians continue to make dietary pronouncements with only a shred of evidence or poorly documented anecdotal observations.
The first of the 36 competencies I reviewed reads: “Provide evidence-based, culturally sensitive nutrition and food recommendations for the prevention and treatment of disease.” When it comes to nutrition the “evidence” can be tough to come by. The natural experiments in which individuals and populations had extremely limited access to a certain nutrients (eg, scurvy) don’t occur very often. Animal studies don’t always extrapolate to humans. And, observational studies concerning diet often have co-factors that are difficult to control and must run over time courses that can tax even the most patient researchers.
I certainly applaud Leib and associates for promoting their primary goal of including more about of the relationship between food and health in the medical school and trainee curriculum. But I must voice a caution to be careful to keep it truly evidence-based and in a format that acknowledges the realities of the life and education of a primary care provider.
The best nutritional advice I ever received in my training was from an older pediatric professor who suggested that a healthy diet consisted of everything in moderation.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
How comfortable are you giving nutritional advice to your patients? When you offer it are you basing your advice on something you learned during medical school or your training? Was it included in a course devoted to nutrition? Did you learn it later as part of continuing medical education course (CME)? Or was it just something you just picked up from your experience seeing patients (osmosis)? It is very unlikely that a significant portion, or any part for that matter, of your medical training was devoted to nutrition. It certainly wasn’t during my training.
I recently read an interview with Emily M. Broad Leib, JD, faculty director of the Harvard School Center for Health Law and Policy Innovation, Cambridge, Massachusetts, who would like to correct that deficiency. She feels doctors need to know more about food and that acquiring that knowledge should be a significant component of their formal training.
In the interview, Leib said that “roughly 86% of physicians report they do not feel adequately trained to answer basic questions on diet or nutrition.” She also notes that while “72% of entering medical students report they believe food is important to health” less than 50% retained this belief after graduation.
Leib and associates feel they have recently reached a milestone in their efforts to include nutrition in the mainstream of medical education this fall by publishing a paper that demonstrates “consensus on doctor-approved nutritional standard for medical schools and residency programs.”
36 Recommended Competencies
Curious about what these nutrition experts chose to include in medical training, I decided to drill down into the list of 36 consensus-driven competencies they had agreed upon.
It was an interesting voyage into a forest of redundancies, many of which can be boiled down to having the student demonstrate that he/she understands that what we eat is important to our health and that there is a complex web of relationships connecting our society to the food consume.
Some of the recommended competencies I found make perfect sense. For example the student/trainee should be able to take a diet and food history and be able to interpret lab values and anthropometric measurements and be able to discuss the patient’s weight and diet with sensitivity while keeping in mind his/her own biases about food.
Some other recommendations are more problematic, for example, “performs a comprehensive nutrition-focused physical examination” or “demonstrates knowledge of how to create culinary nutrition SMART [Specific, Measurable, Achievable, Relevant, and Time-Bound] goals for personal use and for patient care” or “provides brief counseling interventions to help patients decrease visceral adiposity or reduce the risk of metabolic syndrome.” Including competencies like these demonstrates a lack of understanding of the time restraints and realities of a primary care physician’s life and training.
Instead of simply reinforcing the prospective physician’s preexisting assumption that food and health are entwined and discussing when and how to consult a nutrition expert, these 36 competencies seem to be an attempt to create fast-tracked part-time dietitians and nutrition advocates out of medical students and trainees who already believe that nutrition is important for health but also have a very full plate of clinical responsibilities ahead of them.
The study that Leib quotes — that 72% of medical students believed food was important in health while after graduation only 50% of agreed — doesn’t necessarily mean that professors are preaching that food was unimportant. It is more likely by the end of medical school the students have seen that food must share the spotlight with numerous other factors that influence their patients’ health.
‘A More Appropriate Focus’
In my experience, diet and lifestyle counseling done well is extremely time consuming and best done by people for whom that is their specialty. A more appropriate focus for a list of nutritional competencies for physicians in training would be for the student to achieve an understanding of when and how to consult a dietitian and then how to support and evaluate the dietitian’s recommendations to the patient.
Finally, I don’t think we can ignore a serious public relations problem that hangs like a cloud over the nutrition advocacy community. It is the same one that casts a shadow on the medical community as well. It is a common perception among the lay public that nutritionists (and physicians) are always changing their recommendations when it comes to food. What is believable? Just think about eggs, red wine, or introducing peanuts to infants, to name just a few. And what about the food pyramids that seem to have been rebuilt every several years? The problem is compounded when some “credentialed” nutritionists and physicians continue to make dietary pronouncements with only a shred of evidence or poorly documented anecdotal observations.
The first of the 36 competencies I reviewed reads: “Provide evidence-based, culturally sensitive nutrition and food recommendations for the prevention and treatment of disease.” When it comes to nutrition the “evidence” can be tough to come by. The natural experiments in which individuals and populations had extremely limited access to a certain nutrients (eg, scurvy) don’t occur very often. Animal studies don’t always extrapolate to humans. And, observational studies concerning diet often have co-factors that are difficult to control and must run over time courses that can tax even the most patient researchers.
I certainly applaud Leib and associates for promoting their primary goal of including more about of the relationship between food and health in the medical school and trainee curriculum. But I must voice a caution to be careful to keep it truly evidence-based and in a format that acknowledges the realities of the life and education of a primary care provider.
The best nutritional advice I ever received in my training was from an older pediatric professor who suggested that a healthy diet consisted of everything in moderation.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
How comfortable are you giving nutritional advice to your patients? When you offer it are you basing your advice on something you learned during medical school or your training? Was it included in a course devoted to nutrition? Did you learn it later as part of continuing medical education course (CME)? Or was it just something you just picked up from your experience seeing patients (osmosis)? It is very unlikely that a significant portion, or any part for that matter, of your medical training was devoted to nutrition. It certainly wasn’t during my training.
I recently read an interview with Emily M. Broad Leib, JD, faculty director of the Harvard School Center for Health Law and Policy Innovation, Cambridge, Massachusetts, who would like to correct that deficiency. She feels doctors need to know more about food and that acquiring that knowledge should be a significant component of their formal training.
In the interview, Leib said that “roughly 86% of physicians report they do not feel adequately trained to answer basic questions on diet or nutrition.” She also notes that while “72% of entering medical students report they believe food is important to health” less than 50% retained this belief after graduation.
Leib and associates feel they have recently reached a milestone in their efforts to include nutrition in the mainstream of medical education this fall by publishing a paper that demonstrates “consensus on doctor-approved nutritional standard for medical schools and residency programs.”
36 Recommended Competencies
Curious about what these nutrition experts chose to include in medical training, I decided to drill down into the list of 36 consensus-driven competencies they had agreed upon.
It was an interesting voyage into a forest of redundancies, many of which can be boiled down to having the student demonstrate that he/she understands that what we eat is important to our health and that there is a complex web of relationships connecting our society to the food consume.
Some of the recommended competencies I found make perfect sense. For example the student/trainee should be able to take a diet and food history and be able to interpret lab values and anthropometric measurements and be able to discuss the patient’s weight and diet with sensitivity while keeping in mind his/her own biases about food.
Some other recommendations are more problematic, for example, “performs a comprehensive nutrition-focused physical examination” or “demonstrates knowledge of how to create culinary nutrition SMART [Specific, Measurable, Achievable, Relevant, and Time-Bound] goals for personal use and for patient care” or “provides brief counseling interventions to help patients decrease visceral adiposity or reduce the risk of metabolic syndrome.” Including competencies like these demonstrates a lack of understanding of the time restraints and realities of a primary care physician’s life and training.
Instead of simply reinforcing the prospective physician’s preexisting assumption that food and health are entwined and discussing when and how to consult a nutrition expert, these 36 competencies seem to be an attempt to create fast-tracked part-time dietitians and nutrition advocates out of medical students and trainees who already believe that nutrition is important for health but also have a very full plate of clinical responsibilities ahead of them.
The study that Leib quotes — that 72% of medical students believed food was important in health while after graduation only 50% of agreed — doesn’t necessarily mean that professors are preaching that food was unimportant. It is more likely by the end of medical school the students have seen that food must share the spotlight with numerous other factors that influence their patients’ health.
‘A More Appropriate Focus’
In my experience, diet and lifestyle counseling done well is extremely time consuming and best done by people for whom that is their specialty. A more appropriate focus for a list of nutritional competencies for physicians in training would be for the student to achieve an understanding of when and how to consult a dietitian and then how to support and evaluate the dietitian’s recommendations to the patient.
Finally, I don’t think we can ignore a serious public relations problem that hangs like a cloud over the nutrition advocacy community. It is the same one that casts a shadow on the medical community as well. It is a common perception among the lay public that nutritionists (and physicians) are always changing their recommendations when it comes to food. What is believable? Just think about eggs, red wine, or introducing peanuts to infants, to name just a few. And what about the food pyramids that seem to have been rebuilt every several years? The problem is compounded when some “credentialed” nutritionists and physicians continue to make dietary pronouncements with only a shred of evidence or poorly documented anecdotal observations.
The first of the 36 competencies I reviewed reads: “Provide evidence-based, culturally sensitive nutrition and food recommendations for the prevention and treatment of disease.” When it comes to nutrition the “evidence” can be tough to come by. The natural experiments in which individuals and populations had extremely limited access to a certain nutrients (eg, scurvy) don’t occur very often. Animal studies don’t always extrapolate to humans. And, observational studies concerning diet often have co-factors that are difficult to control and must run over time courses that can tax even the most patient researchers.
I certainly applaud Leib and associates for promoting their primary goal of including more about of the relationship between food and health in the medical school and trainee curriculum. But I must voice a caution to be careful to keep it truly evidence-based and in a format that acknowledges the realities of the life and education of a primary care provider.
The best nutritional advice I ever received in my training was from an older pediatric professor who suggested that a healthy diet consisted of everything in moderation.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.







