User login
The end of the telemedicine era?
I started taking care of Jim, a 68-year-old man with metastatic renal cell carcinoma back in the fall of 2018. Jim lived far from our clinic in the rural western Sierra Mountains and had a hard time getting to Santa Monica, but needed ongoing pain and symptom management, as well as follow-up visits with oncology and discussions with our teams about preparing for the end of life.
Luckily for Jim, the Centers for Medicare & Medicaid Services had relaxed the rules around telehealth because of the public health emergency, and we were easily able to provide telemedicine visits throughout the pandemic ensuring that Jim retained access to the care team that had managed his cancer for several years at that point. This would not have been possible without the use of telemedicine – at least not without great effort and expense by Jim to make frequent trips to our Santa Monica clinic.
So, you can imagine my apprehension when I received an email the other day from our billing department, informing billing providers like myself that “telehealth visits are still covered through the end of the year.” While this initially seemed like reassuring news, it immediately begged the question – what happens at the end of the year? What will care look like for patients like Jim who live at a significant distance from their providers?
The end of the COVID-19 public health emergency on May 11 has prompted states to reevaluate the future of telehealth for Medicaid and Medicare recipients. Most states plan to make some telehealth services permanent, particularly in rural areas. While other telehealth services have been extended through Dec. 31, 2024, under the Consolidated Appropriations Act of 2023.
But still, We can now see very ill patients in their own homes without imposing an undue burden on them to come in for yet another office visit. Prior to the public health emergency, our embedded palliative care program would see patients only when they were in the oncology clinic so as to not burden them with having to travel to yet another clinic. This made our palliative providers less efficient since patients were being seen by multiple providers in the same space, which led to some time spent waiting around. It also frequently tied up our clinic exam rooms for long periods of time, delaying care for patients sitting in the waiting room.
Telehealth changed that virtually overnight. With the widespread availability of smartphones and tablets, patients could stay at home and speak more comfortably in their own surroundings – especially about the difficult topics we tend to dig into in palliative care – such as fears, suffering, grief, loss, legacy, regret, trauma, gratitude, dying – without the impersonal, aseptic environment of a clinic. We could visit with their family/caregivers, kids, and their pets. We could tour their living space and see how they were managing from a functional standpoint. We could get to know aspects of our patients’ lives that we’d never have seen in the clinic that could help us understand their goals and values better and help care for them more fully.
The benefit to the institution was also measurable. We could see our patients faster – the time from referral to consult dropped dramatically because patients could be scheduled for next-day virtual visits instead of having to wait for them to come back to an oncology visit. We could do quick symptom-focused visits that prior to telehealth would have been conducted by phone without the ability to perform at the very least an observational physical exam of the patient, which is important when prescribing medications to medically frail populations.
If telemedicine goes, how will it affect outpatient palliative care?
If that goes away, I do not know what will happen to outpatient palliative care. I can tell you we will be much less efficient in terms of when we see patients. There will probably be a higher clinic burden to patients, as well as higher financial toxicity to patients (Parking in the structure attached to my office building is $22 per day). And, what about the uncaptured costs associated with transportation for those whose illness prevents them from driving themselves? This can range from Uber costs to the time cost for a patient’s family member to take off work and arrange for childcare in order to drive the patient to a clinic for a visit.
In February, I received emails from the Drug Enforcement Agency suggesting that they, too, may roll back providers’ ability to prescribe controlled substances to patients who are mainly receiving telehealth services. While I understand and fully support the need to curb inappropriate overprescribing of controlled medications, I am concerned about the unintended consequences to cancer patients who live at a remote distance from their oncologists and palliative care providers. I remain hopeful that DEA will consider a carveout exception for those patients who have cancer, are receiving palliative care services, or are deemed to be at the end of life, much like the chronic opioid guidelines developed by the Centers for Disease Control and Prevention have done.
Telemedicine in essential care
Back to Jim. Using telehealth and electronic prescribing, our oncology and palliative care programs were able to keep Jim comfortable and at home through the end of his life. He did not have to travel 3 hours each way to get care. He did not have to spend money on parking and gas, and his daughter did not have to take days off work and arrange for a babysitter in order to drive him to our clinic. We partnered with a local pharmacy that was willing to special order medications for Jim when his pain became worse and he required a long-acting opioid. We partnered with a local home health company that kept a close eye on Jim and let us know when he seemed to be declining further, prompting discussions about transitioning to hospice.
I’m proud of the fact that our group helped Jim stay in comfortable surroundings and out of the clinic and hospital over the last 6 months of his life, but that would never have happened without the safe and thoughtful use of telehealth by our team.
Ironically, because of a public health emergency, we were able to provide efficient and high-quality palliative care at the right time, to the right person, in the right place, satisfying CMS goals to provide better care for patients and whole populations at lower costs.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
I started taking care of Jim, a 68-year-old man with metastatic renal cell carcinoma back in the fall of 2018. Jim lived far from our clinic in the rural western Sierra Mountains and had a hard time getting to Santa Monica, but needed ongoing pain and symptom management, as well as follow-up visits with oncology and discussions with our teams about preparing for the end of life.
Luckily for Jim, the Centers for Medicare & Medicaid Services had relaxed the rules around telehealth because of the public health emergency, and we were easily able to provide telemedicine visits throughout the pandemic ensuring that Jim retained access to the care team that had managed his cancer for several years at that point. This would not have been possible without the use of telemedicine – at least not without great effort and expense by Jim to make frequent trips to our Santa Monica clinic.
So, you can imagine my apprehension when I received an email the other day from our billing department, informing billing providers like myself that “telehealth visits are still covered through the end of the year.” While this initially seemed like reassuring news, it immediately begged the question – what happens at the end of the year? What will care look like for patients like Jim who live at a significant distance from their providers?
The end of the COVID-19 public health emergency on May 11 has prompted states to reevaluate the future of telehealth for Medicaid and Medicare recipients. Most states plan to make some telehealth services permanent, particularly in rural areas. While other telehealth services have been extended through Dec. 31, 2024, under the Consolidated Appropriations Act of 2023.
But still, We can now see very ill patients in their own homes without imposing an undue burden on them to come in for yet another office visit. Prior to the public health emergency, our embedded palliative care program would see patients only when they were in the oncology clinic so as to not burden them with having to travel to yet another clinic. This made our palliative providers less efficient since patients were being seen by multiple providers in the same space, which led to some time spent waiting around. It also frequently tied up our clinic exam rooms for long periods of time, delaying care for patients sitting in the waiting room.
Telehealth changed that virtually overnight. With the widespread availability of smartphones and tablets, patients could stay at home and speak more comfortably in their own surroundings – especially about the difficult topics we tend to dig into in palliative care – such as fears, suffering, grief, loss, legacy, regret, trauma, gratitude, dying – without the impersonal, aseptic environment of a clinic. We could visit with their family/caregivers, kids, and their pets. We could tour their living space and see how they were managing from a functional standpoint. We could get to know aspects of our patients’ lives that we’d never have seen in the clinic that could help us understand their goals and values better and help care for them more fully.
The benefit to the institution was also measurable. We could see our patients faster – the time from referral to consult dropped dramatically because patients could be scheduled for next-day virtual visits instead of having to wait for them to come back to an oncology visit. We could do quick symptom-focused visits that prior to telehealth would have been conducted by phone without the ability to perform at the very least an observational physical exam of the patient, which is important when prescribing medications to medically frail populations.
If telemedicine goes, how will it affect outpatient palliative care?
If that goes away, I do not know what will happen to outpatient palliative care. I can tell you we will be much less efficient in terms of when we see patients. There will probably be a higher clinic burden to patients, as well as higher financial toxicity to patients (Parking in the structure attached to my office building is $22 per day). And, what about the uncaptured costs associated with transportation for those whose illness prevents them from driving themselves? This can range from Uber costs to the time cost for a patient’s family member to take off work and arrange for childcare in order to drive the patient to a clinic for a visit.
In February, I received emails from the Drug Enforcement Agency suggesting that they, too, may roll back providers’ ability to prescribe controlled substances to patients who are mainly receiving telehealth services. While I understand and fully support the need to curb inappropriate overprescribing of controlled medications, I am concerned about the unintended consequences to cancer patients who live at a remote distance from their oncologists and palliative care providers. I remain hopeful that DEA will consider a carveout exception for those patients who have cancer, are receiving palliative care services, or are deemed to be at the end of life, much like the chronic opioid guidelines developed by the Centers for Disease Control and Prevention have done.
Telemedicine in essential care
Back to Jim. Using telehealth and electronic prescribing, our oncology and palliative care programs were able to keep Jim comfortable and at home through the end of his life. He did not have to travel 3 hours each way to get care. He did not have to spend money on parking and gas, and his daughter did not have to take days off work and arrange for a babysitter in order to drive him to our clinic. We partnered with a local pharmacy that was willing to special order medications for Jim when his pain became worse and he required a long-acting opioid. We partnered with a local home health company that kept a close eye on Jim and let us know when he seemed to be declining further, prompting discussions about transitioning to hospice.
I’m proud of the fact that our group helped Jim stay in comfortable surroundings and out of the clinic and hospital over the last 6 months of his life, but that would never have happened without the safe and thoughtful use of telehealth by our team.
Ironically, because of a public health emergency, we were able to provide efficient and high-quality palliative care at the right time, to the right person, in the right place, satisfying CMS goals to provide better care for patients and whole populations at lower costs.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
I started taking care of Jim, a 68-year-old man with metastatic renal cell carcinoma back in the fall of 2018. Jim lived far from our clinic in the rural western Sierra Mountains and had a hard time getting to Santa Monica, but needed ongoing pain and symptom management, as well as follow-up visits with oncology and discussions with our teams about preparing for the end of life.
Luckily for Jim, the Centers for Medicare & Medicaid Services had relaxed the rules around telehealth because of the public health emergency, and we were easily able to provide telemedicine visits throughout the pandemic ensuring that Jim retained access to the care team that had managed his cancer for several years at that point. This would not have been possible without the use of telemedicine – at least not without great effort and expense by Jim to make frequent trips to our Santa Monica clinic.
So, you can imagine my apprehension when I received an email the other day from our billing department, informing billing providers like myself that “telehealth visits are still covered through the end of the year.” While this initially seemed like reassuring news, it immediately begged the question – what happens at the end of the year? What will care look like for patients like Jim who live at a significant distance from their providers?
The end of the COVID-19 public health emergency on May 11 has prompted states to reevaluate the future of telehealth for Medicaid and Medicare recipients. Most states plan to make some telehealth services permanent, particularly in rural areas. While other telehealth services have been extended through Dec. 31, 2024, under the Consolidated Appropriations Act of 2023.
But still, We can now see very ill patients in their own homes without imposing an undue burden on them to come in for yet another office visit. Prior to the public health emergency, our embedded palliative care program would see patients only when they were in the oncology clinic so as to not burden them with having to travel to yet another clinic. This made our palliative providers less efficient since patients were being seen by multiple providers in the same space, which led to some time spent waiting around. It also frequently tied up our clinic exam rooms for long periods of time, delaying care for patients sitting in the waiting room.
Telehealth changed that virtually overnight. With the widespread availability of smartphones and tablets, patients could stay at home and speak more comfortably in their own surroundings – especially about the difficult topics we tend to dig into in palliative care – such as fears, suffering, grief, loss, legacy, regret, trauma, gratitude, dying – without the impersonal, aseptic environment of a clinic. We could visit with their family/caregivers, kids, and their pets. We could tour their living space and see how they were managing from a functional standpoint. We could get to know aspects of our patients’ lives that we’d never have seen in the clinic that could help us understand their goals and values better and help care for them more fully.
The benefit to the institution was also measurable. We could see our patients faster – the time from referral to consult dropped dramatically because patients could be scheduled for next-day virtual visits instead of having to wait for them to come back to an oncology visit. We could do quick symptom-focused visits that prior to telehealth would have been conducted by phone without the ability to perform at the very least an observational physical exam of the patient, which is important when prescribing medications to medically frail populations.
If telemedicine goes, how will it affect outpatient palliative care?
If that goes away, I do not know what will happen to outpatient palliative care. I can tell you we will be much less efficient in terms of when we see patients. There will probably be a higher clinic burden to patients, as well as higher financial toxicity to patients (Parking in the structure attached to my office building is $22 per day). And, what about the uncaptured costs associated with transportation for those whose illness prevents them from driving themselves? This can range from Uber costs to the time cost for a patient’s family member to take off work and arrange for childcare in order to drive the patient to a clinic for a visit.
In February, I received emails from the Drug Enforcement Agency suggesting that they, too, may roll back providers’ ability to prescribe controlled substances to patients who are mainly receiving telehealth services. While I understand and fully support the need to curb inappropriate overprescribing of controlled medications, I am concerned about the unintended consequences to cancer patients who live at a remote distance from their oncologists and palliative care providers. I remain hopeful that DEA will consider a carveout exception for those patients who have cancer, are receiving palliative care services, or are deemed to be at the end of life, much like the chronic opioid guidelines developed by the Centers for Disease Control and Prevention have done.
Telemedicine in essential care
Back to Jim. Using telehealth and electronic prescribing, our oncology and palliative care programs were able to keep Jim comfortable and at home through the end of his life. He did not have to travel 3 hours each way to get care. He did not have to spend money on parking and gas, and his daughter did not have to take days off work and arrange for a babysitter in order to drive him to our clinic. We partnered with a local pharmacy that was willing to special order medications for Jim when his pain became worse and he required a long-acting opioid. We partnered with a local home health company that kept a close eye on Jim and let us know when he seemed to be declining further, prompting discussions about transitioning to hospice.
I’m proud of the fact that our group helped Jim stay in comfortable surroundings and out of the clinic and hospital over the last 6 months of his life, but that would never have happened without the safe and thoughtful use of telehealth by our team.
Ironically, because of a public health emergency, we were able to provide efficient and high-quality palliative care at the right time, to the right person, in the right place, satisfying CMS goals to provide better care for patients and whole populations at lower costs.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
When practice-changing results don’t change practice
The highly favorable results of the CheckMate 816 trial of neoadjuvant chemotherapy plus nivolumab for resectable stage IB-IIIA non–small cell lung cancer (NSCLC) were impressive enough to prompt a Food and Drug Administration approval of this combination in March 2022.
For many, this led to a marked shift in how we approached these patients. But in my conversations with many care teams, they have expressed ambivalence about using the chemoimmunotherapy regimen. Some have conveyed to me that the lack of statistically significant improvement in overall survival is a sticking point. Others have expressed uncertainty about the true benefit of neoadjuvant chemotherapy alongside nivolumab for patients with earlier-stage disease, given that 64% of patients in the trial had stage IIIA disease. The benefit of the neoadjuvant combination in patients with low or negative tumor programmed death–ligand 1 (PD-L1) expression also remains a question mark, though the trial found no significant differences in outcomes by PD-L1 subset.
But among many of my colleagues who favor adjuvant over neoadjuvant therapy, it isn’t necessarily the fine points of the data that present the real barrier: it’s the sentiment that “we just don’t favor a neoadjuvant approach at my place.”
If the worry is that a subset of patients who are eligible for up-front surgery may be derailed from the operating room if they experience significant disease progression or a complication during preoperative therapy or that surgery will more difficult after chemoimmunotherapy, those concerns are not supported by evidence. In fact, data on surgical outcomes from CheckMate 816 assessing these issues found that surgery after chemoimmunotherapy was approximately 30 minutes faster than it was after chemotherapy alone. In addition, the combination neoadjuvant chemoimmunotherapy approach was associated with less extensive surgeries, particularly for patients with stage IIIA NSCLC, and patients experienced measurably lower reports of pain and dyspnea as well.
Though postoperative systemic therapy has been our general approach for resectable NSCLC for nearly 2 decades, there are several reasons to focus on neoadjuvant therapy.
First, immunotherapy may work more effectively when the tumor antigens as well as lymph nodes and lymphatic system are present in situ at the time.
Second, patients may be eager to complete their treatment within a 3-month period of just three cycles of systemic therapy followed by surgery rather than receiving their treatment over a prolonged chapter of their lives, starting with surgery followed by four cycles of chemotherapy and 1 year of immunotherapy.
Finally, we can’t ignore the fact that most neoadjuvant therapy is delivered exactly as intended, whereas planned adjuvant therapy is often not started or rarely completed as designed. At most, only about half of appropriate patients for adjuvant chemotherapy even start it, and far less complete a full four cycles or go on to complete prolonged adjuvant immunotherapy.
We also can’t underestimate the value of imaging and pathology findings after patients have completed neoadjuvant therapy. The pathologic complete response rate in CheckMate 816 is predictive of improved event-free survival over time.
And that isn’t just a binary variable of achieving a pathologic complete response or not. The degree of residual, viable tumor after surgery is a continuous variable associated along a spectrum with event-free survival. Our colleagues who treat breast cancer have been able to customize postoperative therapy to improve outcomes on the basis of the results achieved with neoadjuvant therapy. Multidisciplinary gastrointestinal oncology teams have revolutionized outcomes with rectal cancer by transitioning to total neoadjuvant therapy that makes it possible to deliver treatment more reliably and pursue organ-sparing approaches while achieving better survival.
Putting all of this together, I appreciate arguments against the generalizability or the maturity of the data supporting neoadjuvant chemoimmunotherapy for resectable NSCLC. However, sidestepping our most promising advances will harm our patients. Plus, what’s the point of generating practice-changing results if we don’t accept and implement them?
We owe it to our patients to follow the evolving evidence and not just stick to what we’ve always done.
Dr. West is an associate professor at City of Hope Comprehensive Cancer Center in Duarte, Calif., and vice president of network strategy at AccessHope in Los Angeles. Dr. West serves as web editor for JAMA Oncology, edits and writes several sections on lung cancer for UpToDate, and leads a wide range of continuing medical education and other educational programs.
A version of this article first appeared on Medscape.com.
The highly favorable results of the CheckMate 816 trial of neoadjuvant chemotherapy plus nivolumab for resectable stage IB-IIIA non–small cell lung cancer (NSCLC) were impressive enough to prompt a Food and Drug Administration approval of this combination in March 2022.
For many, this led to a marked shift in how we approached these patients. But in my conversations with many care teams, they have expressed ambivalence about using the chemoimmunotherapy regimen. Some have conveyed to me that the lack of statistically significant improvement in overall survival is a sticking point. Others have expressed uncertainty about the true benefit of neoadjuvant chemotherapy alongside nivolumab for patients with earlier-stage disease, given that 64% of patients in the trial had stage IIIA disease. The benefit of the neoadjuvant combination in patients with low or negative tumor programmed death–ligand 1 (PD-L1) expression also remains a question mark, though the trial found no significant differences in outcomes by PD-L1 subset.
But among many of my colleagues who favor adjuvant over neoadjuvant therapy, it isn’t necessarily the fine points of the data that present the real barrier: it’s the sentiment that “we just don’t favor a neoadjuvant approach at my place.”
If the worry is that a subset of patients who are eligible for up-front surgery may be derailed from the operating room if they experience significant disease progression or a complication during preoperative therapy or that surgery will more difficult after chemoimmunotherapy, those concerns are not supported by evidence. In fact, data on surgical outcomes from CheckMate 816 assessing these issues found that surgery after chemoimmunotherapy was approximately 30 minutes faster than it was after chemotherapy alone. In addition, the combination neoadjuvant chemoimmunotherapy approach was associated with less extensive surgeries, particularly for patients with stage IIIA NSCLC, and patients experienced measurably lower reports of pain and dyspnea as well.
Though postoperative systemic therapy has been our general approach for resectable NSCLC for nearly 2 decades, there are several reasons to focus on neoadjuvant therapy.
First, immunotherapy may work more effectively when the tumor antigens as well as lymph nodes and lymphatic system are present in situ at the time.
Second, patients may be eager to complete their treatment within a 3-month period of just three cycles of systemic therapy followed by surgery rather than receiving their treatment over a prolonged chapter of their lives, starting with surgery followed by four cycles of chemotherapy and 1 year of immunotherapy.
Finally, we can’t ignore the fact that most neoadjuvant therapy is delivered exactly as intended, whereas planned adjuvant therapy is often not started or rarely completed as designed. At most, only about half of appropriate patients for adjuvant chemotherapy even start it, and far less complete a full four cycles or go on to complete prolonged adjuvant immunotherapy.
We also can’t underestimate the value of imaging and pathology findings after patients have completed neoadjuvant therapy. The pathologic complete response rate in CheckMate 816 is predictive of improved event-free survival over time.
And that isn’t just a binary variable of achieving a pathologic complete response or not. The degree of residual, viable tumor after surgery is a continuous variable associated along a spectrum with event-free survival. Our colleagues who treat breast cancer have been able to customize postoperative therapy to improve outcomes on the basis of the results achieved with neoadjuvant therapy. Multidisciplinary gastrointestinal oncology teams have revolutionized outcomes with rectal cancer by transitioning to total neoadjuvant therapy that makes it possible to deliver treatment more reliably and pursue organ-sparing approaches while achieving better survival.
Putting all of this together, I appreciate arguments against the generalizability or the maturity of the data supporting neoadjuvant chemoimmunotherapy for resectable NSCLC. However, sidestepping our most promising advances will harm our patients. Plus, what’s the point of generating practice-changing results if we don’t accept and implement them?
We owe it to our patients to follow the evolving evidence and not just stick to what we’ve always done.
Dr. West is an associate professor at City of Hope Comprehensive Cancer Center in Duarte, Calif., and vice president of network strategy at AccessHope in Los Angeles. Dr. West serves as web editor for JAMA Oncology, edits and writes several sections on lung cancer for UpToDate, and leads a wide range of continuing medical education and other educational programs.
A version of this article first appeared on Medscape.com.
The highly favorable results of the CheckMate 816 trial of neoadjuvant chemotherapy plus nivolumab for resectable stage IB-IIIA non–small cell lung cancer (NSCLC) were impressive enough to prompt a Food and Drug Administration approval of this combination in March 2022.
For many, this led to a marked shift in how we approached these patients. But in my conversations with many care teams, they have expressed ambivalence about using the chemoimmunotherapy regimen. Some have conveyed to me that the lack of statistically significant improvement in overall survival is a sticking point. Others have expressed uncertainty about the true benefit of neoadjuvant chemotherapy alongside nivolumab for patients with earlier-stage disease, given that 64% of patients in the trial had stage IIIA disease. The benefit of the neoadjuvant combination in patients with low or negative tumor programmed death–ligand 1 (PD-L1) expression also remains a question mark, though the trial found no significant differences in outcomes by PD-L1 subset.
But among many of my colleagues who favor adjuvant over neoadjuvant therapy, it isn’t necessarily the fine points of the data that present the real barrier: it’s the sentiment that “we just don’t favor a neoadjuvant approach at my place.”
If the worry is that a subset of patients who are eligible for up-front surgery may be derailed from the operating room if they experience significant disease progression or a complication during preoperative therapy or that surgery will more difficult after chemoimmunotherapy, those concerns are not supported by evidence. In fact, data on surgical outcomes from CheckMate 816 assessing these issues found that surgery after chemoimmunotherapy was approximately 30 minutes faster than it was after chemotherapy alone. In addition, the combination neoadjuvant chemoimmunotherapy approach was associated with less extensive surgeries, particularly for patients with stage IIIA NSCLC, and patients experienced measurably lower reports of pain and dyspnea as well.
Though postoperative systemic therapy has been our general approach for resectable NSCLC for nearly 2 decades, there are several reasons to focus on neoadjuvant therapy.
First, immunotherapy may work more effectively when the tumor antigens as well as lymph nodes and lymphatic system are present in situ at the time.
Second, patients may be eager to complete their treatment within a 3-month period of just three cycles of systemic therapy followed by surgery rather than receiving their treatment over a prolonged chapter of their lives, starting with surgery followed by four cycles of chemotherapy and 1 year of immunotherapy.
Finally, we can’t ignore the fact that most neoadjuvant therapy is delivered exactly as intended, whereas planned adjuvant therapy is often not started or rarely completed as designed. At most, only about half of appropriate patients for adjuvant chemotherapy even start it, and far less complete a full four cycles or go on to complete prolonged adjuvant immunotherapy.
We also can’t underestimate the value of imaging and pathology findings after patients have completed neoadjuvant therapy. The pathologic complete response rate in CheckMate 816 is predictive of improved event-free survival over time.
And that isn’t just a binary variable of achieving a pathologic complete response or not. The degree of residual, viable tumor after surgery is a continuous variable associated along a spectrum with event-free survival. Our colleagues who treat breast cancer have been able to customize postoperative therapy to improve outcomes on the basis of the results achieved with neoadjuvant therapy. Multidisciplinary gastrointestinal oncology teams have revolutionized outcomes with rectal cancer by transitioning to total neoadjuvant therapy that makes it possible to deliver treatment more reliably and pursue organ-sparing approaches while achieving better survival.
Putting all of this together, I appreciate arguments against the generalizability or the maturity of the data supporting neoadjuvant chemoimmunotherapy for resectable NSCLC. However, sidestepping our most promising advances will harm our patients. Plus, what’s the point of generating practice-changing results if we don’t accept and implement them?
We owe it to our patients to follow the evolving evidence and not just stick to what we’ve always done.
Dr. West is an associate professor at City of Hope Comprehensive Cancer Center in Duarte, Calif., and vice president of network strategy at AccessHope in Los Angeles. Dr. West serves as web editor for JAMA Oncology, edits and writes several sections on lung cancer for UpToDate, and leads a wide range of continuing medical education and other educational programs.
A version of this article first appeared on Medscape.com.
Take time to relax and enjoy the ride
This past weekend was one of my least-favorite parts of the annual cycle: I shut off and drained my hot tub.
I’ve always loved sitting in hot tubs, as far back as I can remember. Growing up on family vacations I preferred them to the pool. So when I was grown up and could afford one, I got it for my house.
I spend my winter weekend afternoons relaxing in it with a can of beer, some bottles of iced tea, and a pile of journals or a book. I put instrumental jazz on my phone and spend a few pleasant hours there, catching up on my reading.
But, as the Phoenix weather swings back to summer temps, it’s time to turn it off until next November.
It’s interesting the ways we mark the passage of time in our lives. The traditional standards are New Year’s, major holidays, and birthdays. Some may mark it by their favorite sports seasons starting.
In medicine we may mark it by patient ages, or a drug that we thought just came to market now going generic, or realizing our state or DEA license is up for renewal.
It doesn’t really matter how you mark the time – it’s going to happen whether you do or don’t. The person you see in the mirror is the same one there since you were tall enough to see over the bathroom countertop. Isn’t it just the ones around us who change?
As Phoenix moves back to a summer footing, and as someone who’s been through 56 of them, it’s hard not to think about it. Summer vacations growing up, summer classes in college, summer elective rotations in medical school. Now I work year-round and watch the same cycle play out with my kids in college.
You often hear the phrase “a hundred years from now it won’t make a difference.” Probably true. In 2123 the time I spent relaxing in my hot tub won’t mean anything, or be remembered by anyone.
But I’m not sitting in it to think about that. I’m in it because I have what I have now, and none of us will ever have that again. And part of that, to me, is enjoying some time in the hot tub.
Because That may not matter in one hundred years, but it matters to me today. And that’s what’s really important.
To all of us.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This past weekend was one of my least-favorite parts of the annual cycle: I shut off and drained my hot tub.
I’ve always loved sitting in hot tubs, as far back as I can remember. Growing up on family vacations I preferred them to the pool. So when I was grown up and could afford one, I got it for my house.
I spend my winter weekend afternoons relaxing in it with a can of beer, some bottles of iced tea, and a pile of journals or a book. I put instrumental jazz on my phone and spend a few pleasant hours there, catching up on my reading.
But, as the Phoenix weather swings back to summer temps, it’s time to turn it off until next November.
It’s interesting the ways we mark the passage of time in our lives. The traditional standards are New Year’s, major holidays, and birthdays. Some may mark it by their favorite sports seasons starting.
In medicine we may mark it by patient ages, or a drug that we thought just came to market now going generic, or realizing our state or DEA license is up for renewal.
It doesn’t really matter how you mark the time – it’s going to happen whether you do or don’t. The person you see in the mirror is the same one there since you were tall enough to see over the bathroom countertop. Isn’t it just the ones around us who change?
As Phoenix moves back to a summer footing, and as someone who’s been through 56 of them, it’s hard not to think about it. Summer vacations growing up, summer classes in college, summer elective rotations in medical school. Now I work year-round and watch the same cycle play out with my kids in college.
You often hear the phrase “a hundred years from now it won’t make a difference.” Probably true. In 2123 the time I spent relaxing in my hot tub won’t mean anything, or be remembered by anyone.
But I’m not sitting in it to think about that. I’m in it because I have what I have now, and none of us will ever have that again. And part of that, to me, is enjoying some time in the hot tub.
Because That may not matter in one hundred years, but it matters to me today. And that’s what’s really important.
To all of us.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This past weekend was one of my least-favorite parts of the annual cycle: I shut off and drained my hot tub.
I’ve always loved sitting in hot tubs, as far back as I can remember. Growing up on family vacations I preferred them to the pool. So when I was grown up and could afford one, I got it for my house.
I spend my winter weekend afternoons relaxing in it with a can of beer, some bottles of iced tea, and a pile of journals or a book. I put instrumental jazz on my phone and spend a few pleasant hours there, catching up on my reading.
But, as the Phoenix weather swings back to summer temps, it’s time to turn it off until next November.
It’s interesting the ways we mark the passage of time in our lives. The traditional standards are New Year’s, major holidays, and birthdays. Some may mark it by their favorite sports seasons starting.
In medicine we may mark it by patient ages, or a drug that we thought just came to market now going generic, or realizing our state or DEA license is up for renewal.
It doesn’t really matter how you mark the time – it’s going to happen whether you do or don’t. The person you see in the mirror is the same one there since you were tall enough to see over the bathroom countertop. Isn’t it just the ones around us who change?
As Phoenix moves back to a summer footing, and as someone who’s been through 56 of them, it’s hard not to think about it. Summer vacations growing up, summer classes in college, summer elective rotations in medical school. Now I work year-round and watch the same cycle play out with my kids in college.
You often hear the phrase “a hundred years from now it won’t make a difference.” Probably true. In 2123 the time I spent relaxing in my hot tub won’t mean anything, or be remembered by anyone.
But I’m not sitting in it to think about that. I’m in it because I have what I have now, and none of us will ever have that again. And part of that, to me, is enjoying some time in the hot tub.
Because That may not matter in one hundred years, but it matters to me today. And that’s what’s really important.
To all of us.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Implicit bias in medicine and beyond
Recently, I reported to the Washtenaw County Circuit Courthouse in Ann Arbor, Mich., to fulfill my civic responsibility of jury duty. After check-in, a pool of 250 potential jurors were shown a video about implicit bias and shuttled off to different courtrooms for the jury selection process (voir dire, or “to speak the truth” in French). While not personally called up to the juror box on this day, I did have the opportunity to observe the attorneys and judge as they questioned potential jurors to uncover any indication that they might not be fair or impartial in judging the facts of this criminal case. After over 3 hours of questioning and several peremptory challenges, a jury was empaneled, and the rest of us were dismissed for the day.
In the legal system, implicit bias can adversely affect legal outcomes by impacting the beliefs and attitudes of multiple stakeholders, including attorneys and judges, litigants, witnesses, and of course jurors, threatening one of our society’s most fundamental principles of equal justice under the law. In the health care arena, implicit bias has been shown to impact patient-clinician communication and contribute to racial and ethnic disparities in patient outcomes. As a medical community, acknowledging and accepting the existence of implicit bias, its manifestations, and its impact is a critical first step to ensuring that every patient that walks into our exam rooms receives equitable care, and we can begin to move the needle in addressing persistent health disparities in patients with gastrointestinal diseases and beyond. While this is regrettably a politically charged topic in our current environment, I urge you to join me in reflecting on whether and how unconscious attitudes or stereotypes may unintentionally color the way in which you interact with patients in the clinic and serve to create or perpetuate inequities in treatment. (I also urge you to show up for jury duty!)
Turning to our April issue, we highlight two recent studies from AGA’s flagship journals, one showing an unexpected rise in pancreatic cancer incidence among women under the age of 55, and another evaluating survival outcomes by fibrosis stage in biopsy-proven nonalcoholic fatty liver disease. In this month’s Member Spotlight column, we introduce you to gastroenterologist Daniel Leffler, MD, who shares his experiences transitioning from a traditional academic career to a job in industry to further scientific advancements in celiac disease treatment. We hope you enjoy these articles and all the content included in our April issue!
Megan A. Adams, MD, JD, MSc
Recently, I reported to the Washtenaw County Circuit Courthouse in Ann Arbor, Mich., to fulfill my civic responsibility of jury duty. After check-in, a pool of 250 potential jurors were shown a video about implicit bias and shuttled off to different courtrooms for the jury selection process (voir dire, or “to speak the truth” in French). While not personally called up to the juror box on this day, I did have the opportunity to observe the attorneys and judge as they questioned potential jurors to uncover any indication that they might not be fair or impartial in judging the facts of this criminal case. After over 3 hours of questioning and several peremptory challenges, a jury was empaneled, and the rest of us were dismissed for the day.
In the legal system, implicit bias can adversely affect legal outcomes by impacting the beliefs and attitudes of multiple stakeholders, including attorneys and judges, litigants, witnesses, and of course jurors, threatening one of our society’s most fundamental principles of equal justice under the law. In the health care arena, implicit bias has been shown to impact patient-clinician communication and contribute to racial and ethnic disparities in patient outcomes. As a medical community, acknowledging and accepting the existence of implicit bias, its manifestations, and its impact is a critical first step to ensuring that every patient that walks into our exam rooms receives equitable care, and we can begin to move the needle in addressing persistent health disparities in patients with gastrointestinal diseases and beyond. While this is regrettably a politically charged topic in our current environment, I urge you to join me in reflecting on whether and how unconscious attitudes or stereotypes may unintentionally color the way in which you interact with patients in the clinic and serve to create or perpetuate inequities in treatment. (I also urge you to show up for jury duty!)
Turning to our April issue, we highlight two recent studies from AGA’s flagship journals, one showing an unexpected rise in pancreatic cancer incidence among women under the age of 55, and another evaluating survival outcomes by fibrosis stage in biopsy-proven nonalcoholic fatty liver disease. In this month’s Member Spotlight column, we introduce you to gastroenterologist Daniel Leffler, MD, who shares his experiences transitioning from a traditional academic career to a job in industry to further scientific advancements in celiac disease treatment. We hope you enjoy these articles and all the content included in our April issue!
Megan A. Adams, MD, JD, MSc
Recently, I reported to the Washtenaw County Circuit Courthouse in Ann Arbor, Mich., to fulfill my civic responsibility of jury duty. After check-in, a pool of 250 potential jurors were shown a video about implicit bias and shuttled off to different courtrooms for the jury selection process (voir dire, or “to speak the truth” in French). While not personally called up to the juror box on this day, I did have the opportunity to observe the attorneys and judge as they questioned potential jurors to uncover any indication that they might not be fair or impartial in judging the facts of this criminal case. After over 3 hours of questioning and several peremptory challenges, a jury was empaneled, and the rest of us were dismissed for the day.
In the legal system, implicit bias can adversely affect legal outcomes by impacting the beliefs and attitudes of multiple stakeholders, including attorneys and judges, litigants, witnesses, and of course jurors, threatening one of our society’s most fundamental principles of equal justice under the law. In the health care arena, implicit bias has been shown to impact patient-clinician communication and contribute to racial and ethnic disparities in patient outcomes. As a medical community, acknowledging and accepting the existence of implicit bias, its manifestations, and its impact is a critical first step to ensuring that every patient that walks into our exam rooms receives equitable care, and we can begin to move the needle in addressing persistent health disparities in patients with gastrointestinal diseases and beyond. While this is regrettably a politically charged topic in our current environment, I urge you to join me in reflecting on whether and how unconscious attitudes or stereotypes may unintentionally color the way in which you interact with patients in the clinic and serve to create or perpetuate inequities in treatment. (I also urge you to show up for jury duty!)
Turning to our April issue, we highlight two recent studies from AGA’s flagship journals, one showing an unexpected rise in pancreatic cancer incidence among women under the age of 55, and another evaluating survival outcomes by fibrosis stage in biopsy-proven nonalcoholic fatty liver disease. In this month’s Member Spotlight column, we introduce you to gastroenterologist Daniel Leffler, MD, who shares his experiences transitioning from a traditional academic career to a job in industry to further scientific advancements in celiac disease treatment. We hope you enjoy these articles and all the content included in our April issue!
Megan A. Adams, MD, JD, MSc
Are parents infecting their children with contagious negativity?
A couple of weeks ago I stumbled across a report of a Pew Research Center’s survey titled “Parenting in America today” (Pew Research Center. Jan. 24, 2023), which found that 40% of parents in the United States with children younger than 18 are “extremely or very worried” that at some point their children might struggle with anxiety or depression. Thirty-six percent replied that they were “somewhat” worried. This total of more than 75% represents a significant change from the 2015 Pew Center survey in which only 54% of parents were “somewhat” worried about their children’s mental health.
Prompted by these findings I began work on a column in which I planned to encourage pediatricians to think more like family physicians when we were working with children who were experiencing serious mental health problems. My primary message was going to be that we should turn more of our attention to the mental health of the anxious parents who must endure the often long and frustrating path toward effective psychiatric care for their children. This might come in the form of some simple suggestions about nonpharmacologic self-help strategies. Or, it could mean encouraging parents to seek psychiatric care or counseling for themselves as they wait for help for their child.
However, as I began that column, my thoughts kept drifting toward a broader consideration of the relationship between parents and pediatric mental health. If mental health of children is causing their parents to be anxious and depressed isn’t it just as likely that this is a bidirectional connection? This was not exactly an “aha” moment for me because it is a relationship I have considered for sometime. However, it is a concept that I have come to realize is receiving far too little attention.
There are exceptions. For example, a recent opinion piece in the New York Times by David French, “What if Kids Are Sad and Stressed Because Their Parents Are?” (March 19, 2023) echoes many of my concerns. Drawing on his experiences traveling around college campuses, Mr. French observes, “Just as parents are upset about their children’s anxiety and depression, children are anxious about their parent’s mental health.”
He notes that an August 2022 NBC News poll found that 58% of registered voters feel this country’s best days are behind it and joins me in imagining that this negative mind set is filtering down to the pediatric population. He acknowledges that there are other likely contributors to teen unhappiness including the ubiquity of smart phones, the secularization of society, and the media’s focus on the political divide. However, Mr. French wonders if the parenting style that results in childhood experiences that are dominated by adult supervision and protection may also be playing a large role.
In his conclusion, Mr. French asks us to consider “How much fear and anxiety should we import to our lives and homes?” as we adults search for an answer.
As I continued to drill down for other possible solutions, I encountered an avenue of psychological research that suggests that instead of, or in addition to, filtering out the anxiety-generating deluge of information, we begin to give some thought to how our beliefs may be coloring our perception of reality.
Jeremy D.W. Clifton, PhD, a psychologist at the University of Pennsylvania Positive Psychology Center has done extensive research on the relationship between our basic beliefs about the world (known as primal beliefs or simply primals in psychologist lingo) and how we interpret reality. For example, one of your primal beliefs may be that the world is a dangerous place. I, on the other hand, may see the world as a stimulating environment offering me endless opportunities to explore. I may see the world as an abundant resource limited only by my creativity. You, however, see it as a barren wasteland.
Dr. Clifton’s research has shown that our primals (at least those of adults) are relatively immutable through one’s lifetime and “do not appear to be the consequence of our experiences.” For example, living in a ZIP code with a high crime rate does not predict that you will see the world as a dangerous place. Nor does being affluent guarantee that an adult sees the world rich with opportunities.
It is unclear exactly when and by what process we develop our primal beliefs, but it is safe to say our parents probably play a large role. Exactly to what degree the tsunami of bad news we are allowing to inundate our children’s lives plays a role is unclear. However, it is reasonable to assume that news about climate change, school shootings, and the pandemic must be a contributor.
According to Dr. Clifton, there is some evidence that certain mind exercises, when applied diligently, can occasionally modify the primal beliefs of an individual who sees the world as dangerous and barren. Until such strategies become more readily accessible, the best we can do is acknowledge that our children are like canaries in a coal mine full of negative perceptions, then do our best to clear the air.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
A couple of weeks ago I stumbled across a report of a Pew Research Center’s survey titled “Parenting in America today” (Pew Research Center. Jan. 24, 2023), which found that 40% of parents in the United States with children younger than 18 are “extremely or very worried” that at some point their children might struggle with anxiety or depression. Thirty-six percent replied that they were “somewhat” worried. This total of more than 75% represents a significant change from the 2015 Pew Center survey in which only 54% of parents were “somewhat” worried about their children’s mental health.
Prompted by these findings I began work on a column in which I planned to encourage pediatricians to think more like family physicians when we were working with children who were experiencing serious mental health problems. My primary message was going to be that we should turn more of our attention to the mental health of the anxious parents who must endure the often long and frustrating path toward effective psychiatric care for their children. This might come in the form of some simple suggestions about nonpharmacologic self-help strategies. Or, it could mean encouraging parents to seek psychiatric care or counseling for themselves as they wait for help for their child.
However, as I began that column, my thoughts kept drifting toward a broader consideration of the relationship between parents and pediatric mental health. If mental health of children is causing their parents to be anxious and depressed isn’t it just as likely that this is a bidirectional connection? This was not exactly an “aha” moment for me because it is a relationship I have considered for sometime. However, it is a concept that I have come to realize is receiving far too little attention.
There are exceptions. For example, a recent opinion piece in the New York Times by David French, “What if Kids Are Sad and Stressed Because Their Parents Are?” (March 19, 2023) echoes many of my concerns. Drawing on his experiences traveling around college campuses, Mr. French observes, “Just as parents are upset about their children’s anxiety and depression, children are anxious about their parent’s mental health.”
He notes that an August 2022 NBC News poll found that 58% of registered voters feel this country’s best days are behind it and joins me in imagining that this negative mind set is filtering down to the pediatric population. He acknowledges that there are other likely contributors to teen unhappiness including the ubiquity of smart phones, the secularization of society, and the media’s focus on the political divide. However, Mr. French wonders if the parenting style that results in childhood experiences that are dominated by adult supervision and protection may also be playing a large role.
In his conclusion, Mr. French asks us to consider “How much fear and anxiety should we import to our lives and homes?” as we adults search for an answer.
As I continued to drill down for other possible solutions, I encountered an avenue of psychological research that suggests that instead of, or in addition to, filtering out the anxiety-generating deluge of information, we begin to give some thought to how our beliefs may be coloring our perception of reality.
Jeremy D.W. Clifton, PhD, a psychologist at the University of Pennsylvania Positive Psychology Center has done extensive research on the relationship between our basic beliefs about the world (known as primal beliefs or simply primals in psychologist lingo) and how we interpret reality. For example, one of your primal beliefs may be that the world is a dangerous place. I, on the other hand, may see the world as a stimulating environment offering me endless opportunities to explore. I may see the world as an abundant resource limited only by my creativity. You, however, see it as a barren wasteland.
Dr. Clifton’s research has shown that our primals (at least those of adults) are relatively immutable through one’s lifetime and “do not appear to be the consequence of our experiences.” For example, living in a ZIP code with a high crime rate does not predict that you will see the world as a dangerous place. Nor does being affluent guarantee that an adult sees the world rich with opportunities.
It is unclear exactly when and by what process we develop our primal beliefs, but it is safe to say our parents probably play a large role. Exactly to what degree the tsunami of bad news we are allowing to inundate our children’s lives plays a role is unclear. However, it is reasonable to assume that news about climate change, school shootings, and the pandemic must be a contributor.
According to Dr. Clifton, there is some evidence that certain mind exercises, when applied diligently, can occasionally modify the primal beliefs of an individual who sees the world as dangerous and barren. Until such strategies become more readily accessible, the best we can do is acknowledge that our children are like canaries in a coal mine full of negative perceptions, then do our best to clear the air.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
A couple of weeks ago I stumbled across a report of a Pew Research Center’s survey titled “Parenting in America today” (Pew Research Center. Jan. 24, 2023), which found that 40% of parents in the United States with children younger than 18 are “extremely or very worried” that at some point their children might struggle with anxiety or depression. Thirty-six percent replied that they were “somewhat” worried. This total of more than 75% represents a significant change from the 2015 Pew Center survey in which only 54% of parents were “somewhat” worried about their children’s mental health.
Prompted by these findings I began work on a column in which I planned to encourage pediatricians to think more like family physicians when we were working with children who were experiencing serious mental health problems. My primary message was going to be that we should turn more of our attention to the mental health of the anxious parents who must endure the often long and frustrating path toward effective psychiatric care for their children. This might come in the form of some simple suggestions about nonpharmacologic self-help strategies. Or, it could mean encouraging parents to seek psychiatric care or counseling for themselves as they wait for help for their child.
However, as I began that column, my thoughts kept drifting toward a broader consideration of the relationship between parents and pediatric mental health. If mental health of children is causing their parents to be anxious and depressed isn’t it just as likely that this is a bidirectional connection? This was not exactly an “aha” moment for me because it is a relationship I have considered for sometime. However, it is a concept that I have come to realize is receiving far too little attention.
There are exceptions. For example, a recent opinion piece in the New York Times by David French, “What if Kids Are Sad and Stressed Because Their Parents Are?” (March 19, 2023) echoes many of my concerns. Drawing on his experiences traveling around college campuses, Mr. French observes, “Just as parents are upset about their children’s anxiety and depression, children are anxious about their parent’s mental health.”
He notes that an August 2022 NBC News poll found that 58% of registered voters feel this country’s best days are behind it and joins me in imagining that this negative mind set is filtering down to the pediatric population. He acknowledges that there are other likely contributors to teen unhappiness including the ubiquity of smart phones, the secularization of society, and the media’s focus on the political divide. However, Mr. French wonders if the parenting style that results in childhood experiences that are dominated by adult supervision and protection may also be playing a large role.
In his conclusion, Mr. French asks us to consider “How much fear and anxiety should we import to our lives and homes?” as we adults search for an answer.
As I continued to drill down for other possible solutions, I encountered an avenue of psychological research that suggests that instead of, or in addition to, filtering out the anxiety-generating deluge of information, we begin to give some thought to how our beliefs may be coloring our perception of reality.
Jeremy D.W. Clifton, PhD, a psychologist at the University of Pennsylvania Positive Psychology Center has done extensive research on the relationship between our basic beliefs about the world (known as primal beliefs or simply primals in psychologist lingo) and how we interpret reality. For example, one of your primal beliefs may be that the world is a dangerous place. I, on the other hand, may see the world as a stimulating environment offering me endless opportunities to explore. I may see the world as an abundant resource limited only by my creativity. You, however, see it as a barren wasteland.
Dr. Clifton’s research has shown that our primals (at least those of adults) are relatively immutable through one’s lifetime and “do not appear to be the consequence of our experiences.” For example, living in a ZIP code with a high crime rate does not predict that you will see the world as a dangerous place. Nor does being affluent guarantee that an adult sees the world rich with opportunities.
It is unclear exactly when and by what process we develop our primal beliefs, but it is safe to say our parents probably play a large role. Exactly to what degree the tsunami of bad news we are allowing to inundate our children’s lives plays a role is unclear. However, it is reasonable to assume that news about climate change, school shootings, and the pandemic must be a contributor.
According to Dr. Clifton, there is some evidence that certain mind exercises, when applied diligently, can occasionally modify the primal beliefs of an individual who sees the world as dangerous and barren. Until such strategies become more readily accessible, the best we can do is acknowledge that our children are like canaries in a coal mine full of negative perceptions, then do our best to clear the air.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
New antiobesity drugs will benefit many. Is that bad?
where some economists opined that their coverage would be disastrous for Medicare.
Among their concerns? The drugs need to be taken long term (just like drugs for any other chronic condition). The new drugs are more expensive than the old drugs (just like new drugs for any other chronic condition). Lots of people will want to take them (just like highly effective drugs for any other chronic condition that has a significant quality-of-life or clinical impact). The U.K. recommended that they be covered only for 2 years (unlike drugs for any other chronic condition). And the Institute for Clinical and Economic Review (ICER) on which they lean heavily decided that $13,618 annually was too expensive for a medication that leads to sustained 15%-20% weight losses and those losses’ consequential benefits.
As a clinician working with patients who sustain those levels of weight loss, I find that conclusion confusing. Whether by way of lifestyle alone, or more often by way of lifestyle efforts plus medication or lifestyle efforts plus surgery, the benefits reported and seen with 15%-20% weight losses are almost uniformly huge. Patients are regularly seen discontinuing or reducing the dosage of multiple medications as a result of improvements to multiple weight-responsive comorbidities, and they also report objective benefits to mood, sleep, mobility, pain, and energy. Losing that much weight changes lives. Not to mention the impact that that degree of loss has on the primary prevention of so many diseases, including plausible reductions in many common cancers – reductions that have been shown to occur after surgery-related weight losses and for which there’s no plausible reason to imagine that they wouldn’t occur with pharmaceutical-related losses.
Are those discussions found in the NEJM op-ed or in the ICER report? Well, yes, sort of. However, in the NEJM op-ed, the word “prevention” isn’t used once, and unlike with oral hypoglycemics or antihypertensives, the authors state that with antiobesity medications, additional research is needed to determine whether medication-induced changes to A1c, blood pressure, and waist circumference would have clinical benefits: “Antiobesity medications have been shown to improve the surrogate end points of weight, glycated hemoglobin levels, systolic blood pressure, and waist circumference. Long-term studies are needed, however, to clarify how medication-induced changes in these surrogate markers translate to health outcomes.”
Primary prevention is mentioned in the ICER review, but in the “limitations” section where the authors explain that they didn’t include it in their modeling: “The long-term benefits of preventing other comorbidities including cancer, chronic kidney disease, osteoarthritis, and sleep apnea were not explicitly modeled in the base case.”
And they pretended that the impact on existing weight-responsive comorbidities mostly didn’t exist, too: “To limit the complexity of the cost-effectiveness model and to prevent double-counting of treatment benefits, we limited the long-term effects of treatments for weight management to cardiovascular risk and delays in the onset and/or diagnosis of diabetes mellitus.”
As far as cardiovascular disease (CVD) benefits go, you might have thought that it would be a slam dunk on that basis alone, at least according to a recent simple back-of-the-envelope math exercise presented at a recent American College of Cardiology conference, which applied the semaglutide treatment group weight changes in the STEP 1 trial to estimate the population impact on weight and obesity in 30- to 74-year-olds without prior CVD, and estimated 10-year CVD risks utilizing the BMI-based Framingham CVD risk scores. By their accounting, semaglutide treatment in eligible American patients has the potential to prevent over 1.6 million CVD events over 10 years.
Finally, even putting aside ICER’s admittedly and exceedingly narrow base case, what lifestyle-alone studies could ICER possibly be comparing with drug efficacy? And what does “alone” mean? Does “alone” mean with a months- or years long interprofessional behavioral program? Does “alone” mean by way of diet books? Does “alone” mean by way of simply “moving more and eating less”? I’m not aware of robust studies demonstrating any long-term meaningful, predictable, reproducible, durable weight loss outcomes for any lifestyle-only approach, intensive or otherwise.
It’s difficult for me to imagine a situation in which a drug other than an antiobesity drug would be found to have too many benefits to include in your cost-effectiveness analysis but where you’d be comfortable to run that analysis anyhow, and then come out against the drug’s recommendation and fearmonger about its use.
But then again, systemic weight bias is a hell of a drug.
Dr. Freedhoff is associate professor, department of family medicine, University of Ottawa, and medical director, Bariatric Medical Institute, Ottawa. He disclosed ties with Constant Health and Novo Nordisk, and has shared opinions via Weighty Matters and social media.
A version of this article originally appeared on Medscape.com.
where some economists opined that their coverage would be disastrous for Medicare.
Among their concerns? The drugs need to be taken long term (just like drugs for any other chronic condition). The new drugs are more expensive than the old drugs (just like new drugs for any other chronic condition). Lots of people will want to take them (just like highly effective drugs for any other chronic condition that has a significant quality-of-life or clinical impact). The U.K. recommended that they be covered only for 2 years (unlike drugs for any other chronic condition). And the Institute for Clinical and Economic Review (ICER) on which they lean heavily decided that $13,618 annually was too expensive for a medication that leads to sustained 15%-20% weight losses and those losses’ consequential benefits.
As a clinician working with patients who sustain those levels of weight loss, I find that conclusion confusing. Whether by way of lifestyle alone, or more often by way of lifestyle efforts plus medication or lifestyle efforts plus surgery, the benefits reported and seen with 15%-20% weight losses are almost uniformly huge. Patients are regularly seen discontinuing or reducing the dosage of multiple medications as a result of improvements to multiple weight-responsive comorbidities, and they also report objective benefits to mood, sleep, mobility, pain, and energy. Losing that much weight changes lives. Not to mention the impact that that degree of loss has on the primary prevention of so many diseases, including plausible reductions in many common cancers – reductions that have been shown to occur after surgery-related weight losses and for which there’s no plausible reason to imagine that they wouldn’t occur with pharmaceutical-related losses.
Are those discussions found in the NEJM op-ed or in the ICER report? Well, yes, sort of. However, in the NEJM op-ed, the word “prevention” isn’t used once, and unlike with oral hypoglycemics or antihypertensives, the authors state that with antiobesity medications, additional research is needed to determine whether medication-induced changes to A1c, blood pressure, and waist circumference would have clinical benefits: “Antiobesity medications have been shown to improve the surrogate end points of weight, glycated hemoglobin levels, systolic blood pressure, and waist circumference. Long-term studies are needed, however, to clarify how medication-induced changes in these surrogate markers translate to health outcomes.”
Primary prevention is mentioned in the ICER review, but in the “limitations” section where the authors explain that they didn’t include it in their modeling: “The long-term benefits of preventing other comorbidities including cancer, chronic kidney disease, osteoarthritis, and sleep apnea were not explicitly modeled in the base case.”
And they pretended that the impact on existing weight-responsive comorbidities mostly didn’t exist, too: “To limit the complexity of the cost-effectiveness model and to prevent double-counting of treatment benefits, we limited the long-term effects of treatments for weight management to cardiovascular risk and delays in the onset and/or diagnosis of diabetes mellitus.”
As far as cardiovascular disease (CVD) benefits go, you might have thought that it would be a slam dunk on that basis alone, at least according to a recent simple back-of-the-envelope math exercise presented at a recent American College of Cardiology conference, which applied the semaglutide treatment group weight changes in the STEP 1 trial to estimate the population impact on weight and obesity in 30- to 74-year-olds without prior CVD, and estimated 10-year CVD risks utilizing the BMI-based Framingham CVD risk scores. By their accounting, semaglutide treatment in eligible American patients has the potential to prevent over 1.6 million CVD events over 10 years.
Finally, even putting aside ICER’s admittedly and exceedingly narrow base case, what lifestyle-alone studies could ICER possibly be comparing with drug efficacy? And what does “alone” mean? Does “alone” mean with a months- or years long interprofessional behavioral program? Does “alone” mean by way of diet books? Does “alone” mean by way of simply “moving more and eating less”? I’m not aware of robust studies demonstrating any long-term meaningful, predictable, reproducible, durable weight loss outcomes for any lifestyle-only approach, intensive or otherwise.
It’s difficult for me to imagine a situation in which a drug other than an antiobesity drug would be found to have too many benefits to include in your cost-effectiveness analysis but where you’d be comfortable to run that analysis anyhow, and then come out against the drug’s recommendation and fearmonger about its use.
But then again, systemic weight bias is a hell of a drug.
Dr. Freedhoff is associate professor, department of family medicine, University of Ottawa, and medical director, Bariatric Medical Institute, Ottawa. He disclosed ties with Constant Health and Novo Nordisk, and has shared opinions via Weighty Matters and social media.
A version of this article originally appeared on Medscape.com.
where some economists opined that their coverage would be disastrous for Medicare.
Among their concerns? The drugs need to be taken long term (just like drugs for any other chronic condition). The new drugs are more expensive than the old drugs (just like new drugs for any other chronic condition). Lots of people will want to take them (just like highly effective drugs for any other chronic condition that has a significant quality-of-life or clinical impact). The U.K. recommended that they be covered only for 2 years (unlike drugs for any other chronic condition). And the Institute for Clinical and Economic Review (ICER) on which they lean heavily decided that $13,618 annually was too expensive for a medication that leads to sustained 15%-20% weight losses and those losses’ consequential benefits.
As a clinician working with patients who sustain those levels of weight loss, I find that conclusion confusing. Whether by way of lifestyle alone, or more often by way of lifestyle efforts plus medication or lifestyle efforts plus surgery, the benefits reported and seen with 15%-20% weight losses are almost uniformly huge. Patients are regularly seen discontinuing or reducing the dosage of multiple medications as a result of improvements to multiple weight-responsive comorbidities, and they also report objective benefits to mood, sleep, mobility, pain, and energy. Losing that much weight changes lives. Not to mention the impact that that degree of loss has on the primary prevention of so many diseases, including plausible reductions in many common cancers – reductions that have been shown to occur after surgery-related weight losses and for which there’s no plausible reason to imagine that they wouldn’t occur with pharmaceutical-related losses.
Are those discussions found in the NEJM op-ed or in the ICER report? Well, yes, sort of. However, in the NEJM op-ed, the word “prevention” isn’t used once, and unlike with oral hypoglycemics or antihypertensives, the authors state that with antiobesity medications, additional research is needed to determine whether medication-induced changes to A1c, blood pressure, and waist circumference would have clinical benefits: “Antiobesity medications have been shown to improve the surrogate end points of weight, glycated hemoglobin levels, systolic blood pressure, and waist circumference. Long-term studies are needed, however, to clarify how medication-induced changes in these surrogate markers translate to health outcomes.”
Primary prevention is mentioned in the ICER review, but in the “limitations” section where the authors explain that they didn’t include it in their modeling: “The long-term benefits of preventing other comorbidities including cancer, chronic kidney disease, osteoarthritis, and sleep apnea were not explicitly modeled in the base case.”
And they pretended that the impact on existing weight-responsive comorbidities mostly didn’t exist, too: “To limit the complexity of the cost-effectiveness model and to prevent double-counting of treatment benefits, we limited the long-term effects of treatments for weight management to cardiovascular risk and delays in the onset and/or diagnosis of diabetes mellitus.”
As far as cardiovascular disease (CVD) benefits go, you might have thought that it would be a slam dunk on that basis alone, at least according to a recent simple back-of-the-envelope math exercise presented at a recent American College of Cardiology conference, which applied the semaglutide treatment group weight changes in the STEP 1 trial to estimate the population impact on weight and obesity in 30- to 74-year-olds without prior CVD, and estimated 10-year CVD risks utilizing the BMI-based Framingham CVD risk scores. By their accounting, semaglutide treatment in eligible American patients has the potential to prevent over 1.6 million CVD events over 10 years.
Finally, even putting aside ICER’s admittedly and exceedingly narrow base case, what lifestyle-alone studies could ICER possibly be comparing with drug efficacy? And what does “alone” mean? Does “alone” mean with a months- or years long interprofessional behavioral program? Does “alone” mean by way of diet books? Does “alone” mean by way of simply “moving more and eating less”? I’m not aware of robust studies demonstrating any long-term meaningful, predictable, reproducible, durable weight loss outcomes for any lifestyle-only approach, intensive or otherwise.
It’s difficult for me to imagine a situation in which a drug other than an antiobesity drug would be found to have too many benefits to include in your cost-effectiveness analysis but where you’d be comfortable to run that analysis anyhow, and then come out against the drug’s recommendation and fearmonger about its use.
But then again, systemic weight bias is a hell of a drug.
Dr. Freedhoff is associate professor, department of family medicine, University of Ottawa, and medical director, Bariatric Medical Institute, Ottawa. He disclosed ties with Constant Health and Novo Nordisk, and has shared opinions via Weighty Matters and social media.
A version of this article originally appeared on Medscape.com.
Don’t fear testing for, and delabeling, penicillin allergy
You are seeing a 28-year-old man for a same-day appointment. He has a history of opioid use disorder and chronic hepatitis C virus infection. He has been using injections of heroin and fentanyl for more than 6 years, and you can see in his medical record that he has had four outpatient appointments for cutaneous infections along with three emergency department visits for same in the past 2 years. His chief complaint today is pain over his left forearm for the past 3 days. He does not report fever or other constitutional symptoms.
Examination of the left forearm reveals 8 cm of erythema with induration and calor but no fluctuance. The area is moderately tender to palpation. He has no other abnormal findings on exam.
What’s your course of action?
Dr. Vega’s take
You want to treat this patient with antibiotics and close follow-up, and you note that he has a history of penicillin allergy. A note in his record states that he had a rash after receiving amoxicillin as a child.
Sometimes, we have to take the most expedient action in health care. But most of the time, we should do the right thing, even if it’s harder. I would gather more history of this reaction to penicillin and consider an oral challenge, hoping that the work that we put in to testing him for penicillin allergy pays dividends for him now and for years to come.
Penicillin allergy is very commonly listed in patient health records. In a retrospective analysis of the charts of 11,761 patients seen at a single U.S. urban outpatient system in 2012, 11.5% had documentation of penicillin allergy. Rash was the most common manifestation listed for allergy (37% of cases), followed by unknown symptoms (20%), hives (19%), swelling/angioedema (12%), and anaphylaxis (7%). Women were nearly twice as likely as men were to report a history of penicillin allergy, and patients of Asian descent had half the reported prevalence of penicillin allergy, compared with White patients.
Only 6% of the patients reporting penicillin allergy in this study had been referred to an allergy specialist. Given the consequences of true penicillin allergy, this rate is far too low. Patients with a history of penicillin allergy have higher risks for mortality from coexisting hematologic malignancies and penicillin-sensitive infections such as Staphylococcus species. They more frequently develop resistance to multiple antimicrobials and have longer average lengths of stay in the hospital.
Getting a good history for penicillin allergy can be challenging. Approximately three-quarters of penicillin allergies are diagnosed prior to age 3 years. Some children with a family history of penicillin allergy are mislabeled as having an active allergy, even though family history is not a significant contributor to penicillin allergy. Most rashes blamed on penicillin among children are actually not immunoglobulin (Ig) E–mediated and instead represent viral exanthems.
In response to these challenges, at the end of 2022, the American Academy of Allergy, Asthma & Immunology along with the American College of Allergy, Asthma and Immunology published new recommendations for the management of drug allergy. These recommendations provide an algorithm for the active reassessment of penicillin allergy. Like other recommendations in recent years, they call for a proactive approach in questioning the potential clinical consequences of the penicillin allergy listed in the health record.
First, the guidelines recommend against needing any testing for previous adverse reactions to penicillin, such as headache, nausea/vomiting, or diarrhea, that are not IgE-mediated. However, patients who have experienced these adverse reactions may still be reticent to take penicillin. For them and for adults with a history of mild to moderate reactions to penicillin more than 5 years ago, a single oral challenge test with amoxicillin is practical and can be used to exclude penicillin allergy.
The oral amoxicillin challenge
After patients take a treatment dose of oral amoxicillin, they should be observed for 1 hour for any objective reaction. The clinical setting should be able to support patients in the rare case of a more severe reaction to penicillin. Subjective symptoms such as pruritus without objective findings such as rash may be considered a successful challenge, and penicillin may be taken off the list of allergies. The treating team can bill CPT codes for drug challenge testing.
Some research has supported multidose testing with amoxicillin to assess for late reactions to a penicillin oral challenge, but the current guidelines recommend against this approach based on the very limited yield in finding additional cases of true allergy with extra doses of antibiotics. One method to address this issue is to have patients advise the practice if symptoms develop within 10 days of the oral challenge, with photos or prompt clinical evaluation to assess for an IgE-mediated reaction.
Many patients, and certainly some clinicians, will have significant trepidation regarding an oral challenge, despite the low risk for complications. For these patients, as well as children with a history of penicillin allergy and patients with a history of anaphylaxis to penicillin or probable IgE-mediated reaction to penicillin in the past several years, skin testing is recommended. Lower-risk patients might feel reassured to complete an oral challenge test after a negative skin test.
Penicillin skin testing is more reliable than a radioallergosorbent test or an enzyme-linked immunoassay and carries a high specificity. However, skin testing requires the specialized care of an allergy clinic, and this resource is limited in many communities.
Many patients will have negative oral challenge or skin testing for penicillin allergy, but there are still some critical responsibilities for the clinician after testing is complete. First, the label of penicillin allergy should be expunged from all available health records. Second, the clinician should communicate clearly and with empathy to the patient that they can take penicillin-based antibiotics safely and with confidence. Repeat testing is unnecessary unless new symptoms develop.
But the application of this policy to clinical practice is challenging on several levels, from patient and clinician fear to practical constraints on time.
Dr. Vega is health sciences clinical professor, family medicine, University of California, Irvine. He has disclosed ties with McNeil Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
You are seeing a 28-year-old man for a same-day appointment. He has a history of opioid use disorder and chronic hepatitis C virus infection. He has been using injections of heroin and fentanyl for more than 6 years, and you can see in his medical record that he has had four outpatient appointments for cutaneous infections along with three emergency department visits for same in the past 2 years. His chief complaint today is pain over his left forearm for the past 3 days. He does not report fever or other constitutional symptoms.
Examination of the left forearm reveals 8 cm of erythema with induration and calor but no fluctuance. The area is moderately tender to palpation. He has no other abnormal findings on exam.
What’s your course of action?
Dr. Vega’s take
You want to treat this patient with antibiotics and close follow-up, and you note that he has a history of penicillin allergy. A note in his record states that he had a rash after receiving amoxicillin as a child.
Sometimes, we have to take the most expedient action in health care. But most of the time, we should do the right thing, even if it’s harder. I would gather more history of this reaction to penicillin and consider an oral challenge, hoping that the work that we put in to testing him for penicillin allergy pays dividends for him now and for years to come.
Penicillin allergy is very commonly listed in patient health records. In a retrospective analysis of the charts of 11,761 patients seen at a single U.S. urban outpatient system in 2012, 11.5% had documentation of penicillin allergy. Rash was the most common manifestation listed for allergy (37% of cases), followed by unknown symptoms (20%), hives (19%), swelling/angioedema (12%), and anaphylaxis (7%). Women were nearly twice as likely as men were to report a history of penicillin allergy, and patients of Asian descent had half the reported prevalence of penicillin allergy, compared with White patients.
Only 6% of the patients reporting penicillin allergy in this study had been referred to an allergy specialist. Given the consequences of true penicillin allergy, this rate is far too low. Patients with a history of penicillin allergy have higher risks for mortality from coexisting hematologic malignancies and penicillin-sensitive infections such as Staphylococcus species. They more frequently develop resistance to multiple antimicrobials and have longer average lengths of stay in the hospital.
Getting a good history for penicillin allergy can be challenging. Approximately three-quarters of penicillin allergies are diagnosed prior to age 3 years. Some children with a family history of penicillin allergy are mislabeled as having an active allergy, even though family history is not a significant contributor to penicillin allergy. Most rashes blamed on penicillin among children are actually not immunoglobulin (Ig) E–mediated and instead represent viral exanthems.
In response to these challenges, at the end of 2022, the American Academy of Allergy, Asthma & Immunology along with the American College of Allergy, Asthma and Immunology published new recommendations for the management of drug allergy. These recommendations provide an algorithm for the active reassessment of penicillin allergy. Like other recommendations in recent years, they call for a proactive approach in questioning the potential clinical consequences of the penicillin allergy listed in the health record.
First, the guidelines recommend against needing any testing for previous adverse reactions to penicillin, such as headache, nausea/vomiting, or diarrhea, that are not IgE-mediated. However, patients who have experienced these adverse reactions may still be reticent to take penicillin. For them and for adults with a history of mild to moderate reactions to penicillin more than 5 years ago, a single oral challenge test with amoxicillin is practical and can be used to exclude penicillin allergy.
The oral amoxicillin challenge
After patients take a treatment dose of oral amoxicillin, they should be observed for 1 hour for any objective reaction. The clinical setting should be able to support patients in the rare case of a more severe reaction to penicillin. Subjective symptoms such as pruritus without objective findings such as rash may be considered a successful challenge, and penicillin may be taken off the list of allergies. The treating team can bill CPT codes for drug challenge testing.
Some research has supported multidose testing with amoxicillin to assess for late reactions to a penicillin oral challenge, but the current guidelines recommend against this approach based on the very limited yield in finding additional cases of true allergy with extra doses of antibiotics. One method to address this issue is to have patients advise the practice if symptoms develop within 10 days of the oral challenge, with photos or prompt clinical evaluation to assess for an IgE-mediated reaction.
Many patients, and certainly some clinicians, will have significant trepidation regarding an oral challenge, despite the low risk for complications. For these patients, as well as children with a history of penicillin allergy and patients with a history of anaphylaxis to penicillin or probable IgE-mediated reaction to penicillin in the past several years, skin testing is recommended. Lower-risk patients might feel reassured to complete an oral challenge test after a negative skin test.
Penicillin skin testing is more reliable than a radioallergosorbent test or an enzyme-linked immunoassay and carries a high specificity. However, skin testing requires the specialized care of an allergy clinic, and this resource is limited in many communities.
Many patients will have negative oral challenge or skin testing for penicillin allergy, but there are still some critical responsibilities for the clinician after testing is complete. First, the label of penicillin allergy should be expunged from all available health records. Second, the clinician should communicate clearly and with empathy to the patient that they can take penicillin-based antibiotics safely and with confidence. Repeat testing is unnecessary unless new symptoms develop.
But the application of this policy to clinical practice is challenging on several levels, from patient and clinician fear to practical constraints on time.
Dr. Vega is health sciences clinical professor, family medicine, University of California, Irvine. He has disclosed ties with McNeil Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
You are seeing a 28-year-old man for a same-day appointment. He has a history of opioid use disorder and chronic hepatitis C virus infection. He has been using injections of heroin and fentanyl for more than 6 years, and you can see in his medical record that he has had four outpatient appointments for cutaneous infections along with three emergency department visits for same in the past 2 years. His chief complaint today is pain over his left forearm for the past 3 days. He does not report fever or other constitutional symptoms.
Examination of the left forearm reveals 8 cm of erythema with induration and calor but no fluctuance. The area is moderately tender to palpation. He has no other abnormal findings on exam.
What’s your course of action?
Dr. Vega’s take
You want to treat this patient with antibiotics and close follow-up, and you note that he has a history of penicillin allergy. A note in his record states that he had a rash after receiving amoxicillin as a child.
Sometimes, we have to take the most expedient action in health care. But most of the time, we should do the right thing, even if it’s harder. I would gather more history of this reaction to penicillin and consider an oral challenge, hoping that the work that we put in to testing him for penicillin allergy pays dividends for him now and for years to come.
Penicillin allergy is very commonly listed in patient health records. In a retrospective analysis of the charts of 11,761 patients seen at a single U.S. urban outpatient system in 2012, 11.5% had documentation of penicillin allergy. Rash was the most common manifestation listed for allergy (37% of cases), followed by unknown symptoms (20%), hives (19%), swelling/angioedema (12%), and anaphylaxis (7%). Women were nearly twice as likely as men were to report a history of penicillin allergy, and patients of Asian descent had half the reported prevalence of penicillin allergy, compared with White patients.
Only 6% of the patients reporting penicillin allergy in this study had been referred to an allergy specialist. Given the consequences of true penicillin allergy, this rate is far too low. Patients with a history of penicillin allergy have higher risks for mortality from coexisting hematologic malignancies and penicillin-sensitive infections such as Staphylococcus species. They more frequently develop resistance to multiple antimicrobials and have longer average lengths of stay in the hospital.
Getting a good history for penicillin allergy can be challenging. Approximately three-quarters of penicillin allergies are diagnosed prior to age 3 years. Some children with a family history of penicillin allergy are mislabeled as having an active allergy, even though family history is not a significant contributor to penicillin allergy. Most rashes blamed on penicillin among children are actually not immunoglobulin (Ig) E–mediated and instead represent viral exanthems.
In response to these challenges, at the end of 2022, the American Academy of Allergy, Asthma & Immunology along with the American College of Allergy, Asthma and Immunology published new recommendations for the management of drug allergy. These recommendations provide an algorithm for the active reassessment of penicillin allergy. Like other recommendations in recent years, they call for a proactive approach in questioning the potential clinical consequences of the penicillin allergy listed in the health record.
First, the guidelines recommend against needing any testing for previous adverse reactions to penicillin, such as headache, nausea/vomiting, or diarrhea, that are not IgE-mediated. However, patients who have experienced these adverse reactions may still be reticent to take penicillin. For them and for adults with a history of mild to moderate reactions to penicillin more than 5 years ago, a single oral challenge test with amoxicillin is practical and can be used to exclude penicillin allergy.
The oral amoxicillin challenge
After patients take a treatment dose of oral amoxicillin, they should be observed for 1 hour for any objective reaction. The clinical setting should be able to support patients in the rare case of a more severe reaction to penicillin. Subjective symptoms such as pruritus without objective findings such as rash may be considered a successful challenge, and penicillin may be taken off the list of allergies. The treating team can bill CPT codes for drug challenge testing.
Some research has supported multidose testing with amoxicillin to assess for late reactions to a penicillin oral challenge, but the current guidelines recommend against this approach based on the very limited yield in finding additional cases of true allergy with extra doses of antibiotics. One method to address this issue is to have patients advise the practice if symptoms develop within 10 days of the oral challenge, with photos or prompt clinical evaluation to assess for an IgE-mediated reaction.
Many patients, and certainly some clinicians, will have significant trepidation regarding an oral challenge, despite the low risk for complications. For these patients, as well as children with a history of penicillin allergy and patients with a history of anaphylaxis to penicillin or probable IgE-mediated reaction to penicillin in the past several years, skin testing is recommended. Lower-risk patients might feel reassured to complete an oral challenge test after a negative skin test.
Penicillin skin testing is more reliable than a radioallergosorbent test or an enzyme-linked immunoassay and carries a high specificity. However, skin testing requires the specialized care of an allergy clinic, and this resource is limited in many communities.
Many patients will have negative oral challenge or skin testing for penicillin allergy, but there are still some critical responsibilities for the clinician after testing is complete. First, the label of penicillin allergy should be expunged from all available health records. Second, the clinician should communicate clearly and with empathy to the patient that they can take penicillin-based antibiotics safely and with confidence. Repeat testing is unnecessary unless new symptoms develop.
But the application of this policy to clinical practice is challenging on several levels, from patient and clinician fear to practical constraints on time.
Dr. Vega is health sciences clinical professor, family medicine, University of California, Irvine. He has disclosed ties with McNeil Pharmaceuticals.
A version of this article originally appeared on Medscape.com.
The physician as leader
Physicians are placed in positions of leadership by the medical team, by the community, and by society, particularly during times of crisis such as the COVID pandemic. They are looked to by the media at times of health care news such as the overturning of Roe v. Wade.1 In a 2015 survey of resident physicians, two-thirds agreed that a formalized leadership curriculum would help them become better supervisors and clinicians.2 While all physicians are viewed as leaders, the concept of leadership is rarely, if ever, described or developed as a part of medical training. This month’s column will provide insights into defining leadership as a physician in the medical and administrative settings.
Benefits of effective leadership
Physicians, whether they are clinicians, researchers, administrators, or teachers, are expected to oversee and engage their teams. A report by the Institute of Medicine recommended that academic health centers “develop leaders at all levels who can manage the organizational and system changes necessary to improve health through innovation in health professions education, patient care, and research.”3 Hospitals with higher-rated management practices and more highly rated boards of directors have been shown to deliver higher-quality care and better clinical outcomes, including lower mortality.
To illustrate, the clinicians at the Mayo Clinic annually rate their supervisors on a Leader Index, a simple 12-question survey of five leadership domains: truthfulness, transparency, character, capability, and partnership. All supervisors were physicians and scientists. Their findings revealed that for each one-point increase in composite leadership score, there was a 3.3% decrease in the likelihood of burnout and a 9.0% increase in the likelihood of satisfaction in the physicians supervised.4
Interprofessional teamwork and engagement are vital skills for a leader to create a successful team. Enhanced management practices have also been associated with higher patient approval ratings and better financial performance. Effective leadership additionally affects physician well-being, with stronger leadership associated with less physician burnout and higher satisfaction.5
Leadership styles enhance quality measures in health care.6 The most effective leadership styles are ones in which the staff feels they are part of a team, are engaged, and are mentored.7 While leadership styles can vary, the common theme is staff engagement. An authoritative style leader is one who mobilizes the team toward a vision, that is, “Come with me.” An affiliative style leader creates harmony and builds emotional bonds where “people come first.” Democratic leaders forge a consensus through staff participation by asking, “What do you think?” Finally, a leader who uses a coaching style helps staff to identify their strengths and weaknesses and work toward improvement. These leadership behaviors are in contradistinction to the unsuccessful coercive leader who demands immediate compliance, that is, “Do what I tell you.”
Five fundamental leadership principles are shown in Table 1.8
Effective leaders have an open (growth) mindset, unwavering attention to diversity, equity, and inclusion, and to building relationships and trust; they practice effective communication and listening, focus on results, and cocreate support structures.
A growth mindset is the belief that one’s abilities are not innate but can improve through effort and learning.9
Emotional intelligence
A survey of business senior managers rated the qualities found in the most outstanding leaders. Using objective criteria like profitability the study psychologists interviewed the highest-rated leaders to compare their capabilities. While intellects and cognitive skills were important, the results showed that emotional intelligence (EI) was twice as important as technical skills and IQ.10 As an example, in a 1996 study, when senior managers had an optimal level of EI, their division’s yearly earnings were 20% higher than estimated.11
EI is a leadership competency that deals with the ability to understand and manage your own emotions and your interactions with others.10 At the Cleveland Clinic, EI is exemplified by the acronym HEART, whereby the team strives to improve the patient experience, mainly when an error occurs. The health care team is using EI by showing its the ability to Hear, Empathize, Apologize, Reply, and Thank. When an untoward event occurs, the physician, as the leader of the team, must lead by example when communicating with staff and patients. EI consists of five components (Table 2).13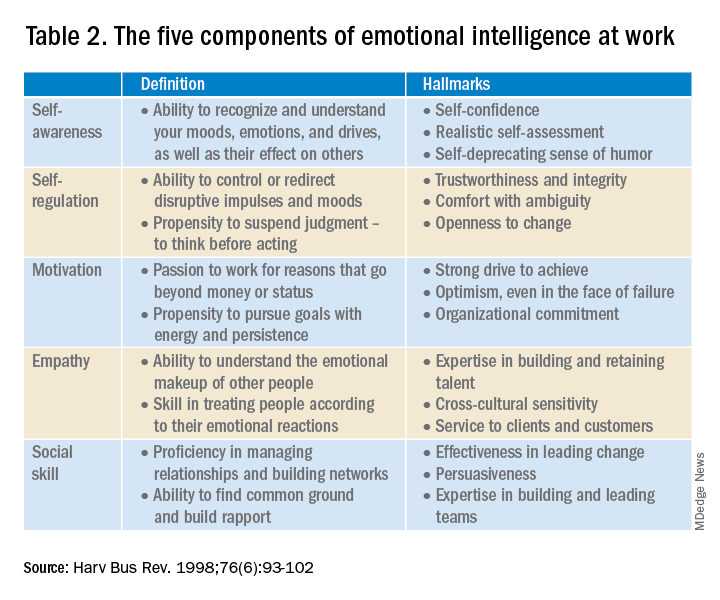
- Self-awareness is insight by which you can improve. Maintaining a journal of your daily thoughts may assist with this as well as simply pausing to pay attention during times of heightened emotions.
- Self-regulation shows control, that is, behaving according to your values, and being accountable and calm when challenged.
- Purpose, knowing your “why,” produces motivation and helps maintain optimism.
- Empathy shows the ability to understand the emotions of other people.
- Social skill is the ability to establish mutually rewarding relationships.
Given all the above benefits, it is no surprise that companies are actively trying use artificial intelligence to improve EI.12
Learning to be a leader
In medical school, students are expected to develop skills to handle and resolve conflicts, learn to share leadership, take mutual responsibility, and monitor their own performance.13 Although training of young physicians in leadership is not unprecedented, a systemic review revealed a lack of analytic studies to evaluate the effectiveness of the teaching methods.14 During undergraduate medical education, standard curricula and methods of instruction on leadership are not established, resulting in variable outcomes.
The Association of American Medical Colleges offers a curriculum, “Preparing Medical Students to Be Physician Leaders: A Leadership Training Program for Students Designed and Led by Students.”15 The objectives of this training are to help students identify their “personal style of leadership, recognize strengths and weaknesses, utilize effective communication strategies, appropriately delegate team member responsibilities, and provide constructive feedback to help improve team function.”
Take-home points
Following the completion of formal medical education, physicians are thrust into leadership roles. The key to being an effective leader is using EI to mentor the team and make staff feel connected to the team’s meaning and purpose, so they feel valued.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Carsen S and Xia C. McGill J Med. 2006 Jan;9(1):1-2.
2. Jardine D et al. J Grad Med Educ. 2015;7(2):307-9.
3. Institute of Medicine. Acad Emerg Med. July 2004;11(7):802-6.
4. Shanafelt TD et al. Mayo Clin Proc. April 2015;90(4):432-40.
5. Rotenstein LS et al. Harv Bus Rev. Oct. 17, 2018.
6. Sfantou SF. Healthcare 2017;5(4):73.
7. Goleman D. Harv Bus Rev. March-April 2000.
8. Collins-Nakai R. McGill J Med [Internet]. 2020 Dec. 1 [cited 2023 Mar. 28];9(1).
9. Dweck C. Harv Bus Rev. Jan. 13, 2016.
10. Goleman D. Harv Bus Rev. 1998 Nov-Dec;76(6):93-102..
11. Goleman D et al. Primal leadership: Realizing the power of emotional intelligence. Boston: Harvard Business School Publishing, 2002.12. Limon D and Plaster B. Harv Bus Rev. Jan. 25, 2022.
13. Chen T-Y. Tzu Chi Med J. Apr–Jun 2018;30(2):66-70.
14. Kumar B et al. BMC Med Educ. 2020;20:175.
15. Richards K et al. Med Ed Portal. Dec. 13 2019.
Physicians are placed in positions of leadership by the medical team, by the community, and by society, particularly during times of crisis such as the COVID pandemic. They are looked to by the media at times of health care news such as the overturning of Roe v. Wade.1 In a 2015 survey of resident physicians, two-thirds agreed that a formalized leadership curriculum would help them become better supervisors and clinicians.2 While all physicians are viewed as leaders, the concept of leadership is rarely, if ever, described or developed as a part of medical training. This month’s column will provide insights into defining leadership as a physician in the medical and administrative settings.
Benefits of effective leadership
Physicians, whether they are clinicians, researchers, administrators, or teachers, are expected to oversee and engage their teams. A report by the Institute of Medicine recommended that academic health centers “develop leaders at all levels who can manage the organizational and system changes necessary to improve health through innovation in health professions education, patient care, and research.”3 Hospitals with higher-rated management practices and more highly rated boards of directors have been shown to deliver higher-quality care and better clinical outcomes, including lower mortality.
To illustrate, the clinicians at the Mayo Clinic annually rate their supervisors on a Leader Index, a simple 12-question survey of five leadership domains: truthfulness, transparency, character, capability, and partnership. All supervisors were physicians and scientists. Their findings revealed that for each one-point increase in composite leadership score, there was a 3.3% decrease in the likelihood of burnout and a 9.0% increase in the likelihood of satisfaction in the physicians supervised.4
Interprofessional teamwork and engagement are vital skills for a leader to create a successful team. Enhanced management practices have also been associated with higher patient approval ratings and better financial performance. Effective leadership additionally affects physician well-being, with stronger leadership associated with less physician burnout and higher satisfaction.5
Leadership styles enhance quality measures in health care.6 The most effective leadership styles are ones in which the staff feels they are part of a team, are engaged, and are mentored.7 While leadership styles can vary, the common theme is staff engagement. An authoritative style leader is one who mobilizes the team toward a vision, that is, “Come with me.” An affiliative style leader creates harmony and builds emotional bonds where “people come first.” Democratic leaders forge a consensus through staff participation by asking, “What do you think?” Finally, a leader who uses a coaching style helps staff to identify their strengths and weaknesses and work toward improvement. These leadership behaviors are in contradistinction to the unsuccessful coercive leader who demands immediate compliance, that is, “Do what I tell you.”
Five fundamental leadership principles are shown in Table 1.8
Effective leaders have an open (growth) mindset, unwavering attention to diversity, equity, and inclusion, and to building relationships and trust; they practice effective communication and listening, focus on results, and cocreate support structures.
A growth mindset is the belief that one’s abilities are not innate but can improve through effort and learning.9
Emotional intelligence
A survey of business senior managers rated the qualities found in the most outstanding leaders. Using objective criteria like profitability the study psychologists interviewed the highest-rated leaders to compare their capabilities. While intellects and cognitive skills were important, the results showed that emotional intelligence (EI) was twice as important as technical skills and IQ.10 As an example, in a 1996 study, when senior managers had an optimal level of EI, their division’s yearly earnings were 20% higher than estimated.11
EI is a leadership competency that deals with the ability to understand and manage your own emotions and your interactions with others.10 At the Cleveland Clinic, EI is exemplified by the acronym HEART, whereby the team strives to improve the patient experience, mainly when an error occurs. The health care team is using EI by showing its the ability to Hear, Empathize, Apologize, Reply, and Thank. When an untoward event occurs, the physician, as the leader of the team, must lead by example when communicating with staff and patients. EI consists of five components (Table 2).13
- Self-awareness is insight by which you can improve. Maintaining a journal of your daily thoughts may assist with this as well as simply pausing to pay attention during times of heightened emotions.
- Self-regulation shows control, that is, behaving according to your values, and being accountable and calm when challenged.
- Purpose, knowing your “why,” produces motivation and helps maintain optimism.
- Empathy shows the ability to understand the emotions of other people.
- Social skill is the ability to establish mutually rewarding relationships.
Given all the above benefits, it is no surprise that companies are actively trying use artificial intelligence to improve EI.12
Learning to be a leader
In medical school, students are expected to develop skills to handle and resolve conflicts, learn to share leadership, take mutual responsibility, and monitor their own performance.13 Although training of young physicians in leadership is not unprecedented, a systemic review revealed a lack of analytic studies to evaluate the effectiveness of the teaching methods.14 During undergraduate medical education, standard curricula and methods of instruction on leadership are not established, resulting in variable outcomes.
The Association of American Medical Colleges offers a curriculum, “Preparing Medical Students to Be Physician Leaders: A Leadership Training Program for Students Designed and Led by Students.”15 The objectives of this training are to help students identify their “personal style of leadership, recognize strengths and weaknesses, utilize effective communication strategies, appropriately delegate team member responsibilities, and provide constructive feedback to help improve team function.”
Take-home points
Following the completion of formal medical education, physicians are thrust into leadership roles. The key to being an effective leader is using EI to mentor the team and make staff feel connected to the team’s meaning and purpose, so they feel valued.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Carsen S and Xia C. McGill J Med. 2006 Jan;9(1):1-2.
2. Jardine D et al. J Grad Med Educ. 2015;7(2):307-9.
3. Institute of Medicine. Acad Emerg Med. July 2004;11(7):802-6.
4. Shanafelt TD et al. Mayo Clin Proc. April 2015;90(4):432-40.
5. Rotenstein LS et al. Harv Bus Rev. Oct. 17, 2018.
6. Sfantou SF. Healthcare 2017;5(4):73.
7. Goleman D. Harv Bus Rev. March-April 2000.
8. Collins-Nakai R. McGill J Med [Internet]. 2020 Dec. 1 [cited 2023 Mar. 28];9(1).
9. Dweck C. Harv Bus Rev. Jan. 13, 2016.
10. Goleman D. Harv Bus Rev. 1998 Nov-Dec;76(6):93-102..
11. Goleman D et al. Primal leadership: Realizing the power of emotional intelligence. Boston: Harvard Business School Publishing, 2002.12. Limon D and Plaster B. Harv Bus Rev. Jan. 25, 2022.
13. Chen T-Y. Tzu Chi Med J. Apr–Jun 2018;30(2):66-70.
14. Kumar B et al. BMC Med Educ. 2020;20:175.
15. Richards K et al. Med Ed Portal. Dec. 13 2019.
Physicians are placed in positions of leadership by the medical team, by the community, and by society, particularly during times of crisis such as the COVID pandemic. They are looked to by the media at times of health care news such as the overturning of Roe v. Wade.1 In a 2015 survey of resident physicians, two-thirds agreed that a formalized leadership curriculum would help them become better supervisors and clinicians.2 While all physicians are viewed as leaders, the concept of leadership is rarely, if ever, described or developed as a part of medical training. This month’s column will provide insights into defining leadership as a physician in the medical and administrative settings.
Benefits of effective leadership
Physicians, whether they are clinicians, researchers, administrators, or teachers, are expected to oversee and engage their teams. A report by the Institute of Medicine recommended that academic health centers “develop leaders at all levels who can manage the organizational and system changes necessary to improve health through innovation in health professions education, patient care, and research.”3 Hospitals with higher-rated management practices and more highly rated boards of directors have been shown to deliver higher-quality care and better clinical outcomes, including lower mortality.
To illustrate, the clinicians at the Mayo Clinic annually rate their supervisors on a Leader Index, a simple 12-question survey of five leadership domains: truthfulness, transparency, character, capability, and partnership. All supervisors were physicians and scientists. Their findings revealed that for each one-point increase in composite leadership score, there was a 3.3% decrease in the likelihood of burnout and a 9.0% increase in the likelihood of satisfaction in the physicians supervised.4
Interprofessional teamwork and engagement are vital skills for a leader to create a successful team. Enhanced management practices have also been associated with higher patient approval ratings and better financial performance. Effective leadership additionally affects physician well-being, with stronger leadership associated with less physician burnout and higher satisfaction.5
Leadership styles enhance quality measures in health care.6 The most effective leadership styles are ones in which the staff feels they are part of a team, are engaged, and are mentored.7 While leadership styles can vary, the common theme is staff engagement. An authoritative style leader is one who mobilizes the team toward a vision, that is, “Come with me.” An affiliative style leader creates harmony and builds emotional bonds where “people come first.” Democratic leaders forge a consensus through staff participation by asking, “What do you think?” Finally, a leader who uses a coaching style helps staff to identify their strengths and weaknesses and work toward improvement. These leadership behaviors are in contradistinction to the unsuccessful coercive leader who demands immediate compliance, that is, “Do what I tell you.”
Five fundamental leadership principles are shown in Table 1.8
Effective leaders have an open (growth) mindset, unwavering attention to diversity, equity, and inclusion, and to building relationships and trust; they practice effective communication and listening, focus on results, and cocreate support structures.
A growth mindset is the belief that one’s abilities are not innate but can improve through effort and learning.9
Emotional intelligence
A survey of business senior managers rated the qualities found in the most outstanding leaders. Using objective criteria like profitability the study psychologists interviewed the highest-rated leaders to compare their capabilities. While intellects and cognitive skills were important, the results showed that emotional intelligence (EI) was twice as important as technical skills and IQ.10 As an example, in a 1996 study, when senior managers had an optimal level of EI, their division’s yearly earnings were 20% higher than estimated.11
EI is a leadership competency that deals with the ability to understand and manage your own emotions and your interactions with others.10 At the Cleveland Clinic, EI is exemplified by the acronym HEART, whereby the team strives to improve the patient experience, mainly when an error occurs. The health care team is using EI by showing its the ability to Hear, Empathize, Apologize, Reply, and Thank. When an untoward event occurs, the physician, as the leader of the team, must lead by example when communicating with staff and patients. EI consists of five components (Table 2).13
- Self-awareness is insight by which you can improve. Maintaining a journal of your daily thoughts may assist with this as well as simply pausing to pay attention during times of heightened emotions.
- Self-regulation shows control, that is, behaving according to your values, and being accountable and calm when challenged.
- Purpose, knowing your “why,” produces motivation and helps maintain optimism.
- Empathy shows the ability to understand the emotions of other people.
- Social skill is the ability to establish mutually rewarding relationships.
Given all the above benefits, it is no surprise that companies are actively trying use artificial intelligence to improve EI.12
Learning to be a leader
In medical school, students are expected to develop skills to handle and resolve conflicts, learn to share leadership, take mutual responsibility, and monitor their own performance.13 Although training of young physicians in leadership is not unprecedented, a systemic review revealed a lack of analytic studies to evaluate the effectiveness of the teaching methods.14 During undergraduate medical education, standard curricula and methods of instruction on leadership are not established, resulting in variable outcomes.
The Association of American Medical Colleges offers a curriculum, “Preparing Medical Students to Be Physician Leaders: A Leadership Training Program for Students Designed and Led by Students.”15 The objectives of this training are to help students identify their “personal style of leadership, recognize strengths and weaknesses, utilize effective communication strategies, appropriately delegate team member responsibilities, and provide constructive feedback to help improve team function.”
Take-home points
Following the completion of formal medical education, physicians are thrust into leadership roles. The key to being an effective leader is using EI to mentor the team and make staff feel connected to the team’s meaning and purpose, so they feel valued.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Carsen S and Xia C. McGill J Med. 2006 Jan;9(1):1-2.
2. Jardine D et al. J Grad Med Educ. 2015;7(2):307-9.
3. Institute of Medicine. Acad Emerg Med. July 2004;11(7):802-6.
4. Shanafelt TD et al. Mayo Clin Proc. April 2015;90(4):432-40.
5. Rotenstein LS et al. Harv Bus Rev. Oct. 17, 2018.
6. Sfantou SF. Healthcare 2017;5(4):73.
7. Goleman D. Harv Bus Rev. March-April 2000.
8. Collins-Nakai R. McGill J Med [Internet]. 2020 Dec. 1 [cited 2023 Mar. 28];9(1).
9. Dweck C. Harv Bus Rev. Jan. 13, 2016.
10. Goleman D. Harv Bus Rev. 1998 Nov-Dec;76(6):93-102..
11. Goleman D et al. Primal leadership: Realizing the power of emotional intelligence. Boston: Harvard Business School Publishing, 2002.12. Limon D and Plaster B. Harv Bus Rev. Jan. 25, 2022.
13. Chen T-Y. Tzu Chi Med J. Apr–Jun 2018;30(2):66-70.
14. Kumar B et al. BMC Med Educ. 2020;20:175.
15. Richards K et al. Med Ed Portal. Dec. 13 2019.
‘Excess’ deaths surging, but why?
This transcript has been edited for clarity.
“Excess deaths.” You’ve heard the phrase countless times by now. It is one of the myriad of previously esoteric epidemiology terms that the pandemic brought squarely into the zeitgeist.
As a sort of standard candle of the performance of a state or a region or a country in terms of health care, it has a lot of utility – if for nothing more than Monday-morning quarterbacking. But this week, I want to dig in on the concept a bit because, according to a new study, the excess death gap between the United States and Western Europe has never been higher.
You might imagine that the best way to figure this out is for some group of intelligent people to review each death and decide, somehow, whether it was expected or not. But aside from being impractical, this would end up being somewhat subjective. That older person who died from pneumonia – was that an expected death? Could it have been avoided?
Rather, the calculation of excess mortality relies on large numbers and statistical inference to compare an expected number of deaths with those that are observed.
The difference is excess mortality, even if you can never be sure whether any particular death was expected or not.
As always, however, the devil is in the details. What data do you use to define the expected number of deaths?
There are options here. Probably the most straightforward analysis uses past data from the country of interest. You look at annual deaths over some historical period of time and compare those numbers with the rates today. Two issues need to be accounted for here: population growth – a larger population will have more deaths, so you need to adjust the historical population with current levels, and demographic shifts – an older or more male population will have more deaths, so you need to adjust for that as well.
But provided you take care of those factors, you can estimate fairly well how many deaths you can expect to see in any given period of time.
Still, you should see right away that excess mortality is a relative concept. If you think that, just perhaps, the United States has some systematic failure to deliver care that has been stable and persistent over time, you wouldn’t capture that failing in an excess mortality calculation that uses U.S. historical data as the baseline.
The best way to get around that is to use data from other countries, and that’s just what this article – a rare single-author piece by Patrick Heuveline – does, calculating excess deaths in the United States by standardizing our mortality rates to the five largest Western European countries: the United Kingdom, France, Germany, Italy, and Spain.
Controlling for the differences in the demographics of that European population, here is the expected number of deaths in the United States over the past 5 years.
Note that there is a small uptick in expected deaths in 2020, reflecting the pandemic, which returns to baseline levels by 2021. This is because that’s what happened in Europe; by 2021, the excess mortality due to COVID-19 was quite low.
Here are the actual deaths in the US during that time.
Highlighted here in green, then, is the excess mortality over time in the United States.
There are some fascinating and concerning findings here.
First of all, you can see that even before the pandemic, the United States has an excess mortality problem. This is not entirely a surprise; we’ve known that so-called “deaths of despair,” those due to alcohol abuse, drug overdoses, and suicide, are at an all-time high and tend to affect a “prime of life” population that would not otherwise be expected to die. In fact, fully 50% of the excess deaths in the United States occur in those between ages 15 and 64.
Excess deaths are also a concerning percentage of total deaths. In 2017, 17% of total deaths in the United States could be considered “excess.” In 2021, that number had doubled to 35%. Nearly 900,000 individuals in the United States died in 2021 who perhaps didn’t need to.
The obvious culprit to blame here is COVID, but COVID-associated excess deaths only explain about 50% of the excess we see in 2021. The rest reflect something even more concerning: a worsening of the failures of the past, perhaps exacerbated by the pandemic but not due to the virus itself.
Of course, we started this discussion acknowledging that the calculation of excess mortality is exquisitely dependent on how you model the expected number of deaths, and I’m sure some will take issue with the use of European numbers when applied to Americans. After all, Europe has, by and large, a robust public health service, socialized medicine, and healthcare that does not run the risk of bankrupting its citizens. How can we compare our outcomes to a place like that?
How indeed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven,Conn. He reported no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
“Excess deaths.” You’ve heard the phrase countless times by now. It is one of the myriad of previously esoteric epidemiology terms that the pandemic brought squarely into the zeitgeist.
As a sort of standard candle of the performance of a state or a region or a country in terms of health care, it has a lot of utility – if for nothing more than Monday-morning quarterbacking. But this week, I want to dig in on the concept a bit because, according to a new study, the excess death gap between the United States and Western Europe has never been higher.
You might imagine that the best way to figure this out is for some group of intelligent people to review each death and decide, somehow, whether it was expected or not. But aside from being impractical, this would end up being somewhat subjective. That older person who died from pneumonia – was that an expected death? Could it have been avoided?
Rather, the calculation of excess mortality relies on large numbers and statistical inference to compare an expected number of deaths with those that are observed.
The difference is excess mortality, even if you can never be sure whether any particular death was expected or not.
As always, however, the devil is in the details. What data do you use to define the expected number of deaths?
There are options here. Probably the most straightforward analysis uses past data from the country of interest. You look at annual deaths over some historical period of time and compare those numbers with the rates today. Two issues need to be accounted for here: population growth – a larger population will have more deaths, so you need to adjust the historical population with current levels, and demographic shifts – an older or more male population will have more deaths, so you need to adjust for that as well.
But provided you take care of those factors, you can estimate fairly well how many deaths you can expect to see in any given period of time.
Still, you should see right away that excess mortality is a relative concept. If you think that, just perhaps, the United States has some systematic failure to deliver care that has been stable and persistent over time, you wouldn’t capture that failing in an excess mortality calculation that uses U.S. historical data as the baseline.
The best way to get around that is to use data from other countries, and that’s just what this article – a rare single-author piece by Patrick Heuveline – does, calculating excess deaths in the United States by standardizing our mortality rates to the five largest Western European countries: the United Kingdom, France, Germany, Italy, and Spain.
Controlling for the differences in the demographics of that European population, here is the expected number of deaths in the United States over the past 5 years.
Note that there is a small uptick in expected deaths in 2020, reflecting the pandemic, which returns to baseline levels by 2021. This is because that’s what happened in Europe; by 2021, the excess mortality due to COVID-19 was quite low.
Here are the actual deaths in the US during that time.
Highlighted here in green, then, is the excess mortality over time in the United States.
There are some fascinating and concerning findings here.
First of all, you can see that even before the pandemic, the United States has an excess mortality problem. This is not entirely a surprise; we’ve known that so-called “deaths of despair,” those due to alcohol abuse, drug overdoses, and suicide, are at an all-time high and tend to affect a “prime of life” population that would not otherwise be expected to die. In fact, fully 50% of the excess deaths in the United States occur in those between ages 15 and 64.
Excess deaths are also a concerning percentage of total deaths. In 2017, 17% of total deaths in the United States could be considered “excess.” In 2021, that number had doubled to 35%. Nearly 900,000 individuals in the United States died in 2021 who perhaps didn’t need to.
The obvious culprit to blame here is COVID, but COVID-associated excess deaths only explain about 50% of the excess we see in 2021. The rest reflect something even more concerning: a worsening of the failures of the past, perhaps exacerbated by the pandemic but not due to the virus itself.
Of course, we started this discussion acknowledging that the calculation of excess mortality is exquisitely dependent on how you model the expected number of deaths, and I’m sure some will take issue with the use of European numbers when applied to Americans. After all, Europe has, by and large, a robust public health service, socialized medicine, and healthcare that does not run the risk of bankrupting its citizens. How can we compare our outcomes to a place like that?
How indeed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven,Conn. He reported no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
“Excess deaths.” You’ve heard the phrase countless times by now. It is one of the myriad of previously esoteric epidemiology terms that the pandemic brought squarely into the zeitgeist.
As a sort of standard candle of the performance of a state or a region or a country in terms of health care, it has a lot of utility – if for nothing more than Monday-morning quarterbacking. But this week, I want to dig in on the concept a bit because, according to a new study, the excess death gap between the United States and Western Europe has never been higher.
You might imagine that the best way to figure this out is for some group of intelligent people to review each death and decide, somehow, whether it was expected or not. But aside from being impractical, this would end up being somewhat subjective. That older person who died from pneumonia – was that an expected death? Could it have been avoided?
Rather, the calculation of excess mortality relies on large numbers and statistical inference to compare an expected number of deaths with those that are observed.
The difference is excess mortality, even if you can never be sure whether any particular death was expected or not.
As always, however, the devil is in the details. What data do you use to define the expected number of deaths?
There are options here. Probably the most straightforward analysis uses past data from the country of interest. You look at annual deaths over some historical period of time and compare those numbers with the rates today. Two issues need to be accounted for here: population growth – a larger population will have more deaths, so you need to adjust the historical population with current levels, and demographic shifts – an older or more male population will have more deaths, so you need to adjust for that as well.
But provided you take care of those factors, you can estimate fairly well how many deaths you can expect to see in any given period of time.
Still, you should see right away that excess mortality is a relative concept. If you think that, just perhaps, the United States has some systematic failure to deliver care that has been stable and persistent over time, you wouldn’t capture that failing in an excess mortality calculation that uses U.S. historical data as the baseline.
The best way to get around that is to use data from other countries, and that’s just what this article – a rare single-author piece by Patrick Heuveline – does, calculating excess deaths in the United States by standardizing our mortality rates to the five largest Western European countries: the United Kingdom, France, Germany, Italy, and Spain.
Controlling for the differences in the demographics of that European population, here is the expected number of deaths in the United States over the past 5 years.
Note that there is a small uptick in expected deaths in 2020, reflecting the pandemic, which returns to baseline levels by 2021. This is because that’s what happened in Europe; by 2021, the excess mortality due to COVID-19 was quite low.
Here are the actual deaths in the US during that time.
Highlighted here in green, then, is the excess mortality over time in the United States.
There are some fascinating and concerning findings here.
First of all, you can see that even before the pandemic, the United States has an excess mortality problem. This is not entirely a surprise; we’ve known that so-called “deaths of despair,” those due to alcohol abuse, drug overdoses, and suicide, are at an all-time high and tend to affect a “prime of life” population that would not otherwise be expected to die. In fact, fully 50% of the excess deaths in the United States occur in those between ages 15 and 64.
Excess deaths are also a concerning percentage of total deaths. In 2017, 17% of total deaths in the United States could be considered “excess.” In 2021, that number had doubled to 35%. Nearly 900,000 individuals in the United States died in 2021 who perhaps didn’t need to.
The obvious culprit to blame here is COVID, but COVID-associated excess deaths only explain about 50% of the excess we see in 2021. The rest reflect something even more concerning: a worsening of the failures of the past, perhaps exacerbated by the pandemic but not due to the virus itself.
Of course, we started this discussion acknowledging that the calculation of excess mortality is exquisitely dependent on how you model the expected number of deaths, and I’m sure some will take issue with the use of European numbers when applied to Americans. After all, Europe has, by and large, a robust public health service, socialized medicine, and healthcare that does not run the risk of bankrupting its citizens. How can we compare our outcomes to a place like that?
How indeed.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven,Conn. He reported no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
Is it time to stop treating high triglycerides?
The publication of the PROMINENT trial, where pemafibrate successfully lowered high levels but was not associated with a lower risk for cardiovascular events, reinforced the point. Is it time to stop measuring and treating high triglycerides?
There may be noncardiovascular reasons to treat hypertriglyceridemia. Pancreatitis is the most cited one, given that the risk for pancreatitis increases with increasing triglyceride levels, especially in patients with a prior episode.
There may also be practical reasons to lower trigs. Because most cholesterol panels use the Friedewald equation to calculate low-density lipoprotein cholesterol (LDL-C) rather than measuring it directly, very high triglyceride levels can invalidate the calculation and return error messages on lab reports.
But we now have alternatives to measuring LDL-C, including non–high-density lipoprotein cholesterol (HDL-C) and apolipoprotein B (apoB), that better predict risk and are usable even in the setting of nonfasting samples when triglycerides are elevated.
Independent cardiovascular risk factor?
If we are going to measure and treat high triglycerides for cardiovascular reasons, the relevant question is, are high triglycerides an independent risk factor for cardiovascular disease?
Proponents have a broad swath of supportive literature to point at. Multiple studies have shown an association between triglyceride levels and cardiovascular risk. The evidence even extends beyond traditional epidemiologic analyses, to genetic studies that should be free from some of the problems seen in observational cohorts.
But it is difficult to be certain whether these associations are causal or merely confounding. An unhealthy diet will increase triglycerides, as will alcohol. Patients with diabetes or metabolic syndrome have high triglycerides. So do patients with nephrotic syndrome or hypothyroidism, or hypertensive patients taking thiazide diuretics. Adjusting for these baseline factors is possible but imperfect, and residual confounding is always an issue. An analysis of the Reykjavik and the EPIC-Norfolk studies found an association between triglyceride levels and cardiovascular risk. That risk was attenuated, but not eliminated, when adjusted for traditional risk factors such as age, smoking, blood pressure, diabetes, and cholesterol.
Randomized trials of triglyceride-lowering therapies would help resolve the question of whether hypertriglyceridemia contributes to coronary disease or simply identifies high-risk patients. Early trials seemed to support the idea of a causal link. The Helsinki Heart Study randomized patients to gemfibrozil or placebo and found a 34% relative risk reduction in coronary artery disease with the fibrate. But gemfibrozil didn’t only reduce triglycerides. It also increased HDL-C and lowered LDL-C relative to placebo, which may explain the observed benefit.
Gemfibrozil is rarely used today because we can achieve much greater LDL-C reductions with statins, as well as ezetimibe and PCSK9 inhibitors. The success of these drugs may not leave any room for triglyceride-lowering medications.
The pre- vs. post-statin era
In the 2005 FIELD study, participants were randomized to receive fenofibrate or placebo. Although patients weren’t taking statin at study entry, 17% of the placebo group started taking one during the trial. Fenofibrate wasn’t associated with a reduction in the primary endpoint, a combination of coronary heart disease death or nonfatal myocardial infarction (MI). Among the many secondary endpoints, nonfatal MI was lower but cardiovascular mortality was not in the fibrate-treated patients. In the same vein, the 2010 ACCORD study randomized patients to receive simvastatin plus fenofibrate or simvastatin alone. The composite primary outcome of MI, stroke, and cardiovascular mortality was not lowered nor were any secondary outcomes with the combination therapy. In the statin era, triglyceride-lowering therapies have not shown much benefit.
The final nail in the coffin may very well be the aforementioned PROMINENT trial. The new agent, pemafibrate, fared no better than its predecessor fenofibrate. Pemafibrate had no impact on the study’s primary composite outcome of nonfatal MI, stroke, coronary revascularization, or cardiovascular death despite being very effective at lowering triglycerides (by more than 25%). Patients treated with pemafibrate had increased LDL-C and apoB compared with the placebo group. When you realize that, the results of the study are not very surprising.
Some point to the results of REDUCE-IT as proof that triglycerides are still a valid target for pharmacotherapy. The debate on whether REDUCE-IT tested a good drug or a bad placebo is one for another day. The salient point for today is that the benefits of eicosapentaenoic acid (EPA) were seen regardless of either baseline or final triglyceride level. EPA may lower cardiac risk, but there is no widespread consensus that it does so by lowering triglycerides. There may be other mechanisms at work.
You could still argue that high triglycerides have value as a risk prediction tool even if their role as a target for drug therapy is questionable. There was a time when medications to lower triglycerides had a benefit. But this is the post-statin era, and that time has passed.
If you see patients with high triglycerides, treating them with triglyceride-lowering medication probably isn’t going to reduce their cardiovascular risk. Dietary interventions, encouraging exercise, and reducing alcohol consumption are better options. Not only will they lead to lower cholesterol levels, but they’ll lower cardiovascular risk, too.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, with a degree in epidemiology. He has disclosed no relevant financial relationships. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal and is host of the award-winning podcast The Body of Evidence. The Body of Evidence.
A version of this article originally appeared on Medscape.com.
The publication of the PROMINENT trial, where pemafibrate successfully lowered high levels but was not associated with a lower risk for cardiovascular events, reinforced the point. Is it time to stop measuring and treating high triglycerides?
There may be noncardiovascular reasons to treat hypertriglyceridemia. Pancreatitis is the most cited one, given that the risk for pancreatitis increases with increasing triglyceride levels, especially in patients with a prior episode.
There may also be practical reasons to lower trigs. Because most cholesterol panels use the Friedewald equation to calculate low-density lipoprotein cholesterol (LDL-C) rather than measuring it directly, very high triglyceride levels can invalidate the calculation and return error messages on lab reports.
But we now have alternatives to measuring LDL-C, including non–high-density lipoprotein cholesterol (HDL-C) and apolipoprotein B (apoB), that better predict risk and are usable even in the setting of nonfasting samples when triglycerides are elevated.
Independent cardiovascular risk factor?
If we are going to measure and treat high triglycerides for cardiovascular reasons, the relevant question is, are high triglycerides an independent risk factor for cardiovascular disease?
Proponents have a broad swath of supportive literature to point at. Multiple studies have shown an association between triglyceride levels and cardiovascular risk. The evidence even extends beyond traditional epidemiologic analyses, to genetic studies that should be free from some of the problems seen in observational cohorts.
But it is difficult to be certain whether these associations are causal or merely confounding. An unhealthy diet will increase triglycerides, as will alcohol. Patients with diabetes or metabolic syndrome have high triglycerides. So do patients with nephrotic syndrome or hypothyroidism, or hypertensive patients taking thiazide diuretics. Adjusting for these baseline factors is possible but imperfect, and residual confounding is always an issue. An analysis of the Reykjavik and the EPIC-Norfolk studies found an association between triglyceride levels and cardiovascular risk. That risk was attenuated, but not eliminated, when adjusted for traditional risk factors such as age, smoking, blood pressure, diabetes, and cholesterol.
Randomized trials of triglyceride-lowering therapies would help resolve the question of whether hypertriglyceridemia contributes to coronary disease or simply identifies high-risk patients. Early trials seemed to support the idea of a causal link. The Helsinki Heart Study randomized patients to gemfibrozil or placebo and found a 34% relative risk reduction in coronary artery disease with the fibrate. But gemfibrozil didn’t only reduce triglycerides. It also increased HDL-C and lowered LDL-C relative to placebo, which may explain the observed benefit.
Gemfibrozil is rarely used today because we can achieve much greater LDL-C reductions with statins, as well as ezetimibe and PCSK9 inhibitors. The success of these drugs may not leave any room for triglyceride-lowering medications.
The pre- vs. post-statin era
In the 2005 FIELD study, participants were randomized to receive fenofibrate or placebo. Although patients weren’t taking statin at study entry, 17% of the placebo group started taking one during the trial. Fenofibrate wasn’t associated with a reduction in the primary endpoint, a combination of coronary heart disease death or nonfatal myocardial infarction (MI). Among the many secondary endpoints, nonfatal MI was lower but cardiovascular mortality was not in the fibrate-treated patients. In the same vein, the 2010 ACCORD study randomized patients to receive simvastatin plus fenofibrate or simvastatin alone. The composite primary outcome of MI, stroke, and cardiovascular mortality was not lowered nor were any secondary outcomes with the combination therapy. In the statin era, triglyceride-lowering therapies have not shown much benefit.
The final nail in the coffin may very well be the aforementioned PROMINENT trial. The new agent, pemafibrate, fared no better than its predecessor fenofibrate. Pemafibrate had no impact on the study’s primary composite outcome of nonfatal MI, stroke, coronary revascularization, or cardiovascular death despite being very effective at lowering triglycerides (by more than 25%). Patients treated with pemafibrate had increased LDL-C and apoB compared with the placebo group. When you realize that, the results of the study are not very surprising.
Some point to the results of REDUCE-IT as proof that triglycerides are still a valid target for pharmacotherapy. The debate on whether REDUCE-IT tested a good drug or a bad placebo is one for another day. The salient point for today is that the benefits of eicosapentaenoic acid (EPA) were seen regardless of either baseline or final triglyceride level. EPA may lower cardiac risk, but there is no widespread consensus that it does so by lowering triglycerides. There may be other mechanisms at work.
You could still argue that high triglycerides have value as a risk prediction tool even if their role as a target for drug therapy is questionable. There was a time when medications to lower triglycerides had a benefit. But this is the post-statin era, and that time has passed.
If you see patients with high triglycerides, treating them with triglyceride-lowering medication probably isn’t going to reduce their cardiovascular risk. Dietary interventions, encouraging exercise, and reducing alcohol consumption are better options. Not only will they lead to lower cholesterol levels, but they’ll lower cardiovascular risk, too.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, with a degree in epidemiology. He has disclosed no relevant financial relationships. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal and is host of the award-winning podcast The Body of Evidence. The Body of Evidence.
A version of this article originally appeared on Medscape.com.
The publication of the PROMINENT trial, where pemafibrate successfully lowered high levels but was not associated with a lower risk for cardiovascular events, reinforced the point. Is it time to stop measuring and treating high triglycerides?
There may be noncardiovascular reasons to treat hypertriglyceridemia. Pancreatitis is the most cited one, given that the risk for pancreatitis increases with increasing triglyceride levels, especially in patients with a prior episode.
There may also be practical reasons to lower trigs. Because most cholesterol panels use the Friedewald equation to calculate low-density lipoprotein cholesterol (LDL-C) rather than measuring it directly, very high triglyceride levels can invalidate the calculation and return error messages on lab reports.
But we now have alternatives to measuring LDL-C, including non–high-density lipoprotein cholesterol (HDL-C) and apolipoprotein B (apoB), that better predict risk and are usable even in the setting of nonfasting samples when triglycerides are elevated.
Independent cardiovascular risk factor?
If we are going to measure and treat high triglycerides for cardiovascular reasons, the relevant question is, are high triglycerides an independent risk factor for cardiovascular disease?
Proponents have a broad swath of supportive literature to point at. Multiple studies have shown an association between triglyceride levels and cardiovascular risk. The evidence even extends beyond traditional epidemiologic analyses, to genetic studies that should be free from some of the problems seen in observational cohorts.
But it is difficult to be certain whether these associations are causal or merely confounding. An unhealthy diet will increase triglycerides, as will alcohol. Patients with diabetes or metabolic syndrome have high triglycerides. So do patients with nephrotic syndrome or hypothyroidism, or hypertensive patients taking thiazide diuretics. Adjusting for these baseline factors is possible but imperfect, and residual confounding is always an issue. An analysis of the Reykjavik and the EPIC-Norfolk studies found an association between triglyceride levels and cardiovascular risk. That risk was attenuated, but not eliminated, when adjusted for traditional risk factors such as age, smoking, blood pressure, diabetes, and cholesterol.
Randomized trials of triglyceride-lowering therapies would help resolve the question of whether hypertriglyceridemia contributes to coronary disease or simply identifies high-risk patients. Early trials seemed to support the idea of a causal link. The Helsinki Heart Study randomized patients to gemfibrozil or placebo and found a 34% relative risk reduction in coronary artery disease with the fibrate. But gemfibrozil didn’t only reduce triglycerides. It also increased HDL-C and lowered LDL-C relative to placebo, which may explain the observed benefit.
Gemfibrozil is rarely used today because we can achieve much greater LDL-C reductions with statins, as well as ezetimibe and PCSK9 inhibitors. The success of these drugs may not leave any room for triglyceride-lowering medications.
The pre- vs. post-statin era
In the 2005 FIELD study, participants were randomized to receive fenofibrate or placebo. Although patients weren’t taking statin at study entry, 17% of the placebo group started taking one during the trial. Fenofibrate wasn’t associated with a reduction in the primary endpoint, a combination of coronary heart disease death or nonfatal myocardial infarction (MI). Among the many secondary endpoints, nonfatal MI was lower but cardiovascular mortality was not in the fibrate-treated patients. In the same vein, the 2010 ACCORD study randomized patients to receive simvastatin plus fenofibrate or simvastatin alone. The composite primary outcome of MI, stroke, and cardiovascular mortality was not lowered nor were any secondary outcomes with the combination therapy. In the statin era, triglyceride-lowering therapies have not shown much benefit.
The final nail in the coffin may very well be the aforementioned PROMINENT trial. The new agent, pemafibrate, fared no better than its predecessor fenofibrate. Pemafibrate had no impact on the study’s primary composite outcome of nonfatal MI, stroke, coronary revascularization, or cardiovascular death despite being very effective at lowering triglycerides (by more than 25%). Patients treated with pemafibrate had increased LDL-C and apoB compared with the placebo group. When you realize that, the results of the study are not very surprising.
Some point to the results of REDUCE-IT as proof that triglycerides are still a valid target for pharmacotherapy. The debate on whether REDUCE-IT tested a good drug or a bad placebo is one for another day. The salient point for today is that the benefits of eicosapentaenoic acid (EPA) were seen regardless of either baseline or final triglyceride level. EPA may lower cardiac risk, but there is no widespread consensus that it does so by lowering triglycerides. There may be other mechanisms at work.
You could still argue that high triglycerides have value as a risk prediction tool even if their role as a target for drug therapy is questionable. There was a time when medications to lower triglycerides had a benefit. But this is the post-statin era, and that time has passed.
If you see patients with high triglycerides, treating them with triglyceride-lowering medication probably isn’t going to reduce their cardiovascular risk. Dietary interventions, encouraging exercise, and reducing alcohol consumption are better options. Not only will they lead to lower cholesterol levels, but they’ll lower cardiovascular risk, too.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal, with a degree in epidemiology. He has disclosed no relevant financial relationships. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal and is host of the award-winning podcast The Body of Evidence. The Body of Evidence.
A version of this article originally appeared on Medscape.com.








