User login
VIDEO: Obinutuzumab bests rituximab for PFS in follicular lymphoma
SAN DIEGO – For patients with indolent non-Hodgkin lymphoma, adding the anti-CD20 antibody rituximab to a standard-combination chemotherapy regimen resulted in significant improvements in survival, compared with chemotherapy alone. Obinutuzumab (Gazyva), a second-generation anti-CD20 antibody touted as the heir apparent to rituximab, is being explored in various combinations for the treatment of indolent lymphomas, including follicular lymphoma and marginal zone lymphoma.
In this video interview from the annual meeting of the American Society of Hematology, Robert Marcus, FRCP, of King’s College Hospital, London, discussed results of the phase III GALLIUM study, in which patients with untreated follicular lymphoma were randomly assigned to one of three chemotherapy regimens with either obinutuzumab or rituximab. The primary endpoint of investigator-assessed 3-year progression-free survival (PFS) at a median follow-up of 34.5 months was 80% for patients with follicular lymphoma treated with obinutuzumab and one of three standard chemotherapy regimens, compared with 73.3% for patients treated with rituximab and chemotherapy. This difference translated into a hazard ratio (HR) favoring obinutuzumab of 0.68 (P = .0012).
Respective 3-year overall survival rates at 3 years were similar, however, at 94% and 92.1% (HR, 0.75; P = .21).
The GALLIUM trial is sponsored by F. Hoffmann-La Roche. Dr. Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – For patients with indolent non-Hodgkin lymphoma, adding the anti-CD20 antibody rituximab to a standard-combination chemotherapy regimen resulted in significant improvements in survival, compared with chemotherapy alone. Obinutuzumab (Gazyva), a second-generation anti-CD20 antibody touted as the heir apparent to rituximab, is being explored in various combinations for the treatment of indolent lymphomas, including follicular lymphoma and marginal zone lymphoma.
In this video interview from the annual meeting of the American Society of Hematology, Robert Marcus, FRCP, of King’s College Hospital, London, discussed results of the phase III GALLIUM study, in which patients with untreated follicular lymphoma were randomly assigned to one of three chemotherapy regimens with either obinutuzumab or rituximab. The primary endpoint of investigator-assessed 3-year progression-free survival (PFS) at a median follow-up of 34.5 months was 80% for patients with follicular lymphoma treated with obinutuzumab and one of three standard chemotherapy regimens, compared with 73.3% for patients treated with rituximab and chemotherapy. This difference translated into a hazard ratio (HR) favoring obinutuzumab of 0.68 (P = .0012).
Respective 3-year overall survival rates at 3 years were similar, however, at 94% and 92.1% (HR, 0.75; P = .21).
The GALLIUM trial is sponsored by F. Hoffmann-La Roche. Dr. Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – For patients with indolent non-Hodgkin lymphoma, adding the anti-CD20 antibody rituximab to a standard-combination chemotherapy regimen resulted in significant improvements in survival, compared with chemotherapy alone. Obinutuzumab (Gazyva), a second-generation anti-CD20 antibody touted as the heir apparent to rituximab, is being explored in various combinations for the treatment of indolent lymphomas, including follicular lymphoma and marginal zone lymphoma.
In this video interview from the annual meeting of the American Society of Hematology, Robert Marcus, FRCP, of King’s College Hospital, London, discussed results of the phase III GALLIUM study, in which patients with untreated follicular lymphoma were randomly assigned to one of three chemotherapy regimens with either obinutuzumab or rituximab. The primary endpoint of investigator-assessed 3-year progression-free survival (PFS) at a median follow-up of 34.5 months was 80% for patients with follicular lymphoma treated with obinutuzumab and one of three standard chemotherapy regimens, compared with 73.3% for patients treated with rituximab and chemotherapy. This difference translated into a hazard ratio (HR) favoring obinutuzumab of 0.68 (P = .0012).
Respective 3-year overall survival rates at 3 years were similar, however, at 94% and 92.1% (HR, 0.75; P = .21).
The GALLIUM trial is sponsored by F. Hoffmann-La Roche. Dr. Marcus disclosed consulting with and receiving honoraria from the company, and relationships with other companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
Shulkin: VA "Not a Political Issue”
Federal Practitioner sat down for an exclusive interview with VA Under Secretary of Health David J. Shulkin, MD at the recent Launch Pad: Pathways to Cancer Innovation, November 29, 2016. As the clock winds down on the current administration, the interview covered a wide range of topic. The below video that discusses VA progress over the past 18 months since Shulkin was confirmed and the prospects for change in the new administration. Future videos will cover the Veterans Choice Program, employee morale and recruitment challenges, improving rural care, transparency, and the unique nature of VA’s mission and care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Federal Practitioner sat down for an exclusive interview with VA Under Secretary of Health David J. Shulkin, MD at the recent Launch Pad: Pathways to Cancer Innovation, November 29, 2016. As the clock winds down on the current administration, the interview covered a wide range of topic. The below video that discusses VA progress over the past 18 months since Shulkin was confirmed and the prospects for change in the new administration. Future videos will cover the Veterans Choice Program, employee morale and recruitment challenges, improving rural care, transparency, and the unique nature of VA’s mission and care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Federal Practitioner sat down for an exclusive interview with VA Under Secretary of Health David J. Shulkin, MD at the recent Launch Pad: Pathways to Cancer Innovation, November 29, 2016. As the clock winds down on the current administration, the interview covered a wide range of topic. The below video that discusses VA progress over the past 18 months since Shulkin was confirmed and the prospects for change in the new administration. Future videos will cover the Veterans Choice Program, employee morale and recruitment challenges, improving rural care, transparency, and the unique nature of VA’s mission and care.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Psoriasis and Internal Disease: Report From the Mount Sinai Winter Symposium
At the 19th Annual Mount Sinai Winter Symposium, Dr. Jashin J. Wu spoke about psoriasis and internal disease. He discussed psoriasis and noncardiovascular comorbidities as well as cardiovascular comorbidities. Dr. Wu also addressed if treating psoriasis can improve cardiovascular disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Jashin J. Wu spoke about psoriasis and internal disease. He discussed psoriasis and noncardiovascular comorbidities as well as cardiovascular comorbidities. Dr. Wu also addressed if treating psoriasis can improve cardiovascular disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the 19th Annual Mount Sinai Winter Symposium, Dr. Jashin J. Wu spoke about psoriasis and internal disease. He discussed psoriasis and noncardiovascular comorbidities as well as cardiovascular comorbidities. Dr. Wu also addressed if treating psoriasis can improve cardiovascular disease.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: Artificial blood cells clear first phase of animal testing
SAN DIEGO – An artificial red blood cell has come close to emulating the key functions of natural cells and does not appear to be associated with the side effects such as vasospasm and poor response to changes in blood pH that hampered the development of previous artificial blood products, Allan Doctor, MD, reported at the annual meeting of the American Society of Hematology.
The bio-synthetic cells, called ErythroMer, are about 1/50th the size of natural red blood cells. They can be stored at room temperature and reconstituted with water when needed for use.
In a mouse model, the ErythroMer cells were shown to capture oxygen in the lungs and release it to tissue in a pattern that was nearly identical to blood transfusion. In a rat model of shock, ErythroMer was effective for resuscitation.
In a video interview, Dr. Doctor of Washington University in St. Louis discussed the pharmacokinetics of ErythroMer, the need for a readily available blood substitute for treating trauma patients, other potential uses for artificial blood cells, and next steps for testing the product.
Dr. Doctor has equity ownership in KaloCyte, the company developing ErythroMer. He receives research funding from Children’s Discovery Institute and the National Institutes of Health.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – An artificial red blood cell has come close to emulating the key functions of natural cells and does not appear to be associated with the side effects such as vasospasm and poor response to changes in blood pH that hampered the development of previous artificial blood products, Allan Doctor, MD, reported at the annual meeting of the American Society of Hematology.
The bio-synthetic cells, called ErythroMer, are about 1/50th the size of natural red blood cells. They can be stored at room temperature and reconstituted with water when needed for use.
In a mouse model, the ErythroMer cells were shown to capture oxygen in the lungs and release it to tissue in a pattern that was nearly identical to blood transfusion. In a rat model of shock, ErythroMer was effective for resuscitation.
In a video interview, Dr. Doctor of Washington University in St. Louis discussed the pharmacokinetics of ErythroMer, the need for a readily available blood substitute for treating trauma patients, other potential uses for artificial blood cells, and next steps for testing the product.
Dr. Doctor has equity ownership in KaloCyte, the company developing ErythroMer. He receives research funding from Children’s Discovery Institute and the National Institutes of Health.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – An artificial red blood cell has come close to emulating the key functions of natural cells and does not appear to be associated with the side effects such as vasospasm and poor response to changes in blood pH that hampered the development of previous artificial blood products, Allan Doctor, MD, reported at the annual meeting of the American Society of Hematology.
The bio-synthetic cells, called ErythroMer, are about 1/50th the size of natural red blood cells. They can be stored at room temperature and reconstituted with water when needed for use.
In a mouse model, the ErythroMer cells were shown to capture oxygen in the lungs and release it to tissue in a pattern that was nearly identical to blood transfusion. In a rat model of shock, ErythroMer was effective for resuscitation.
In a video interview, Dr. Doctor of Washington University in St. Louis discussed the pharmacokinetics of ErythroMer, the need for a readily available blood substitute for treating trauma patients, other potential uses for artificial blood cells, and next steps for testing the product.
Dr. Doctor has equity ownership in KaloCyte, the company developing ErythroMer. He receives research funding from Children’s Discovery Institute and the National Institutes of Health.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
Diagnosis & assessment of pain: Refining your approach
Differentiating ADHD and bipolar disorder
Resistant Hypertension
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: HeartMate 3 LVAD solves pump thrombosis
NEW ORLEANS – HeartMate 3, the latest left ventricular assist device in the HeartMate line, appears to have solved the problem of pump thrombosis, a complication that has dogged ventricular pumps since the issue leapt into medical awareness about 3 years ago (New Engl J Med. 2014 Jan 2;370:33-40).
During 6 months of follow-up, none of 152 heart failure patients assigned to receive a HeartMate 3 left ventricular assist device (LVAD) developed suspected or confirmed pump thrombosis, compared with 14 patients (10%) having pump thrombosis out of 138 recipients of the prior-generation HeartMate II LVAD who served as the control group for the study.
“Three years ago, when the issue of pump thrombosis was first revealed, there was a lot of consternation and some drop in LVAD use, especially as destination therapy. We think that seeing no pump thrombosis whatsoever will give people renewed confidence in this technology,” said Dr. Mehra, professor of medicine at Harvard Medical School and medical director of the Heart and Vascular Center of Brigham and Women’s Hospital, both in Boston.
Pump thrombosis has also been a problem for the patients who have received a competitor LVAD, the HeartWare HVAD device (Circulation. 2015 Nov 10;132[suppl 3]:A19675), approved for U.S. use as bridge to transplant. HeartMate II is approved for both bridge to transplant and for destination therapy.
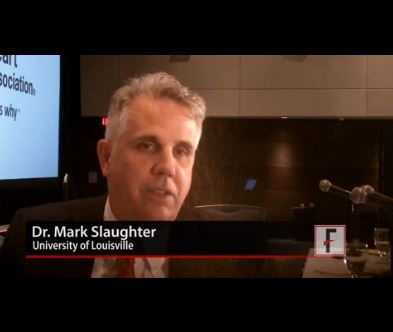
In addition to apparently eliminating pump thrombosis, HeartMate 3’s size and potential implantation approach should make its placement during routine use as quick and minimally invasive as the HeartWare device, features that should further help broader use of HeartMate 3, commented Mark Slaughter, MD, professor and chairman of cardiovascular and thoracic surgery at the University of Louisville (Ky.). But Dr. Slaughter and others were also quick to highlight the shortcomings that remain with both devices that will continue to hamper a broader role for LVAD treatment of patients with advanced heart failure.
“We thought that if there was less pump thrombosis we’d see less stroke, but that is not what the data suggest. It’s the big puzzle we need to figure out before we see widespread acceptance of this treatment,” Dr. Sweitzer said.
“This will not shift LVAD use substantially,” commented Christopher B. Granger, MD, a professor of medicine and a heart failure specialist at Duke University, Durham, N.C. “Reducing the need for reoperation is good for the field, and is an incremental advance, but it is not transformational,” he said in an interview.
The MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3) trial randomized 294 patients at 69 U.S. centers. The study’s primary endpoint of 6-month survival free from disabling stroke or reoperation to repair or replace the LVAD occurred in 86% of 152 patients who received a HeartMate 3 and 77% of 142 patients randomized to HeartMate II, a statistical difference that met the prespecified criteria for both noninferiority and superiority. Concurrently with Dr. Mehra’s report at the meeting, a journal article appeared online (New Engl J Med. 2016 Nov 16. doi: 10.1056/NEJMoa1610426). He stated that as far as he understood, St. Jude would submit the 6-month data he reported to the Food and Drug Administration in an application for marketing approval for HeartMate 3.
“I agree that there are still morbid evens [with HeartMate 3] that need to be surmounted, but this is a confidence-building step in the right direction,” Dr. Mehra said.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
By eliminating all episodes of pump thrombosis during 6-month follow-up, the HeartMate 3 appeared to resolve one of the major issues that has stood in the way of patients and physicians feeling comfortable with left ventricular assist devices. The smaller size of the HeartMate 3 pump and its ability to be placed with minimally invasive and fairly rapid surgery is another big advance, putting this device on par with the rival pump, the HeartWare HVAD.
But the performance of the HeartMate 3 left ventricular assist device (LVAD) in MOMENTUM 3 also highlighted the shortcomings that still remain for these devices: the unchanged rates of stroke, gastrointestinal bleeds, and infections with HeartMate 3, compared with HeartMate II in this trial, and similar 6-month survival rates in the two arms of the study.
The HeartMate 3 can be implanted without sternotomy, using an 8 cm incision on the lateral chest wall, resulting in a shorter postoperative stay and fewer perisurgical adverse events. Despite the less invasive surgery and absence of pump thrombosis, some patients and physicians will remain hesitant to use an LVAD unless it is unavoidable because of concern about strokes. Until further design and procedural refinements change the rate of serious strokes and other adverse events, LVADs will not be fully competitive with heart transplantation.
The competition between HeartMate and the HeartWare devices will help drive this field forward, leading to further improvements in outcomes and expanded LVAD use.
Mark Slaughter, MD, is professor of surgery and chairman of cardiovascular and thoracic surgery at the University of Louisville (Ky.). He was an investigator in MOMENTUM 3, he has been a consultant to EvaHeart and Oregon Heart, and he has received research support from Carmat and HeartWare. He made these comments as designated discussant for the report and in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
By eliminating all episodes of pump thrombosis during 6-month follow-up, the HeartMate 3 appeared to resolve one of the major issues that has stood in the way of patients and physicians feeling comfortable with left ventricular assist devices. The smaller size of the HeartMate 3 pump and its ability to be placed with minimally invasive and fairly rapid surgery is another big advance, putting this device on par with the rival pump, the HeartWare HVAD.
But the performance of the HeartMate 3 left ventricular assist device (LVAD) in MOMENTUM 3 also highlighted the shortcomings that still remain for these devices: the unchanged rates of stroke, gastrointestinal bleeds, and infections with HeartMate 3, compared with HeartMate II in this trial, and similar 6-month survival rates in the two arms of the study.
The HeartMate 3 can be implanted without sternotomy, using an 8 cm incision on the lateral chest wall, resulting in a shorter postoperative stay and fewer perisurgical adverse events. Despite the less invasive surgery and absence of pump thrombosis, some patients and physicians will remain hesitant to use an LVAD unless it is unavoidable because of concern about strokes. Until further design and procedural refinements change the rate of serious strokes and other adverse events, LVADs will not be fully competitive with heart transplantation.
The competition between HeartMate and the HeartWare devices will help drive this field forward, leading to further improvements in outcomes and expanded LVAD use.
Mark Slaughter, MD, is professor of surgery and chairman of cardiovascular and thoracic surgery at the University of Louisville (Ky.). He was an investigator in MOMENTUM 3, he has been a consultant to EvaHeart and Oregon Heart, and he has received research support from Carmat and HeartWare. He made these comments as designated discussant for the report and in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
By eliminating all episodes of pump thrombosis during 6-month follow-up, the HeartMate 3 appeared to resolve one of the major issues that has stood in the way of patients and physicians feeling comfortable with left ventricular assist devices. The smaller size of the HeartMate 3 pump and its ability to be placed with minimally invasive and fairly rapid surgery is another big advance, putting this device on par with the rival pump, the HeartWare HVAD.
But the performance of the HeartMate 3 left ventricular assist device (LVAD) in MOMENTUM 3 also highlighted the shortcomings that still remain for these devices: the unchanged rates of stroke, gastrointestinal bleeds, and infections with HeartMate 3, compared with HeartMate II in this trial, and similar 6-month survival rates in the two arms of the study.
The HeartMate 3 can be implanted without sternotomy, using an 8 cm incision on the lateral chest wall, resulting in a shorter postoperative stay and fewer perisurgical adverse events. Despite the less invasive surgery and absence of pump thrombosis, some patients and physicians will remain hesitant to use an LVAD unless it is unavoidable because of concern about strokes. Until further design and procedural refinements change the rate of serious strokes and other adverse events, LVADs will not be fully competitive with heart transplantation.
The competition between HeartMate and the HeartWare devices will help drive this field forward, leading to further improvements in outcomes and expanded LVAD use.
Mark Slaughter, MD, is professor of surgery and chairman of cardiovascular and thoracic surgery at the University of Louisville (Ky.). He was an investigator in MOMENTUM 3, he has been a consultant to EvaHeart and Oregon Heart, and he has received research support from Carmat and HeartWare. He made these comments as designated discussant for the report and in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – HeartMate 3, the latest left ventricular assist device in the HeartMate line, appears to have solved the problem of pump thrombosis, a complication that has dogged ventricular pumps since the issue leapt into medical awareness about 3 years ago (New Engl J Med. 2014 Jan 2;370:33-40).
During 6 months of follow-up, none of 152 heart failure patients assigned to receive a HeartMate 3 left ventricular assist device (LVAD) developed suspected or confirmed pump thrombosis, compared with 14 patients (10%) having pump thrombosis out of 138 recipients of the prior-generation HeartMate II LVAD who served as the control group for the study.
“Three years ago, when the issue of pump thrombosis was first revealed, there was a lot of consternation and some drop in LVAD use, especially as destination therapy. We think that seeing no pump thrombosis whatsoever will give people renewed confidence in this technology,” said Dr. Mehra, professor of medicine at Harvard Medical School and medical director of the Heart and Vascular Center of Brigham and Women’s Hospital, both in Boston.
Pump thrombosis has also been a problem for the patients who have received a competitor LVAD, the HeartWare HVAD device (Circulation. 2015 Nov 10;132[suppl 3]:A19675), approved for U.S. use as bridge to transplant. HeartMate II is approved for both bridge to transplant and for destination therapy.
In addition to apparently eliminating pump thrombosis, HeartMate 3’s size and potential implantation approach should make its placement during routine use as quick and minimally invasive as the HeartWare device, features that should further help broader use of HeartMate 3, commented Mark Slaughter, MD, professor and chairman of cardiovascular and thoracic surgery at the University of Louisville (Ky.). But Dr. Slaughter and others were also quick to highlight the shortcomings that remain with both devices that will continue to hamper a broader role for LVAD treatment of patients with advanced heart failure.
“We thought that if there was less pump thrombosis we’d see less stroke, but that is not what the data suggest. It’s the big puzzle we need to figure out before we see widespread acceptance of this treatment,” Dr. Sweitzer said.
“This will not shift LVAD use substantially,” commented Christopher B. Granger, MD, a professor of medicine and a heart failure specialist at Duke University, Durham, N.C. “Reducing the need for reoperation is good for the field, and is an incremental advance, but it is not transformational,” he said in an interview.
The MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3) trial randomized 294 patients at 69 U.S. centers. The study’s primary endpoint of 6-month survival free from disabling stroke or reoperation to repair or replace the LVAD occurred in 86% of 152 patients who received a HeartMate 3 and 77% of 142 patients randomized to HeartMate II, a statistical difference that met the prespecified criteria for both noninferiority and superiority. Concurrently with Dr. Mehra’s report at the meeting, a journal article appeared online (New Engl J Med. 2016 Nov 16. doi: 10.1056/NEJMoa1610426). He stated that as far as he understood, St. Jude would submit the 6-month data he reported to the Food and Drug Administration in an application for marketing approval for HeartMate 3.
“I agree that there are still morbid evens [with HeartMate 3] that need to be surmounted, but this is a confidence-building step in the right direction,” Dr. Mehra said.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
NEW ORLEANS – HeartMate 3, the latest left ventricular assist device in the HeartMate line, appears to have solved the problem of pump thrombosis, a complication that has dogged ventricular pumps since the issue leapt into medical awareness about 3 years ago (New Engl J Med. 2014 Jan 2;370:33-40).
During 6 months of follow-up, none of 152 heart failure patients assigned to receive a HeartMate 3 left ventricular assist device (LVAD) developed suspected or confirmed pump thrombosis, compared with 14 patients (10%) having pump thrombosis out of 138 recipients of the prior-generation HeartMate II LVAD who served as the control group for the study.
“Three years ago, when the issue of pump thrombosis was first revealed, there was a lot of consternation and some drop in LVAD use, especially as destination therapy. We think that seeing no pump thrombosis whatsoever will give people renewed confidence in this technology,” said Dr. Mehra, professor of medicine at Harvard Medical School and medical director of the Heart and Vascular Center of Brigham and Women’s Hospital, both in Boston.
Pump thrombosis has also been a problem for the patients who have received a competitor LVAD, the HeartWare HVAD device (Circulation. 2015 Nov 10;132[suppl 3]:A19675), approved for U.S. use as bridge to transplant. HeartMate II is approved for both bridge to transplant and for destination therapy.
In addition to apparently eliminating pump thrombosis, HeartMate 3’s size and potential implantation approach should make its placement during routine use as quick and minimally invasive as the HeartWare device, features that should further help broader use of HeartMate 3, commented Mark Slaughter, MD, professor and chairman of cardiovascular and thoracic surgery at the University of Louisville (Ky.). But Dr. Slaughter and others were also quick to highlight the shortcomings that remain with both devices that will continue to hamper a broader role for LVAD treatment of patients with advanced heart failure.
“We thought that if there was less pump thrombosis we’d see less stroke, but that is not what the data suggest. It’s the big puzzle we need to figure out before we see widespread acceptance of this treatment,” Dr. Sweitzer said.
“This will not shift LVAD use substantially,” commented Christopher B. Granger, MD, a professor of medicine and a heart failure specialist at Duke University, Durham, N.C. “Reducing the need for reoperation is good for the field, and is an incremental advance, but it is not transformational,” he said in an interview.
The MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3) trial randomized 294 patients at 69 U.S. centers. The study’s primary endpoint of 6-month survival free from disabling stroke or reoperation to repair or replace the LVAD occurred in 86% of 152 patients who received a HeartMate 3 and 77% of 142 patients randomized to HeartMate II, a statistical difference that met the prespecified criteria for both noninferiority and superiority. Concurrently with Dr. Mehra’s report at the meeting, a journal article appeared online (New Engl J Med. 2016 Nov 16. doi: 10.1056/NEJMoa1610426). He stated that as far as he understood, St. Jude would submit the 6-month data he reported to the Food and Drug Administration in an application for marketing approval for HeartMate 3.
“I agree that there are still morbid evens [with HeartMate 3] that need to be surmounted, but this is a confidence-building step in the right direction,” Dr. Mehra said.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: During 6 months, suspected or confirmed pump thrombosis occurred in no HeartMate 3 patients and in 10% of HeartMate II recipients.
Data source: The MOMENTUM 3 trial, which randomized 294 patients at 69 U.S. centers.
Disclosures: MOMENTUM 3 was sponsored by St. Jude, the company developing the HeartMate 3 LVAD. Dr. Mehra has received travel reimbursements from St. Jude and has been a consultant to Medtronic, Stealth, and Teva. Dr. Sweitzer was an investigator in MOMENTUM 3 and has been a consultant to Acorda and Medtronic and received research support from Bayer, Corvia, and Novartis. Dr. Granger has been a consultant to Boehringer Ingelheim, and received research support from Medtronic and several other drug and device companies. Dr. Slaughter was an investigator in MOMENTUM 3, has been a consultant to EvaHeart and Oregon Heart, and has received research support from Carmat and HeartWare.
Women and Heart Disease
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
What are the modern cardiovascular risk indicators and how should ObGyns be using them in their practice?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
- Visit NAMS
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
- Visit NAMS
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
- Visit NAMS