User login
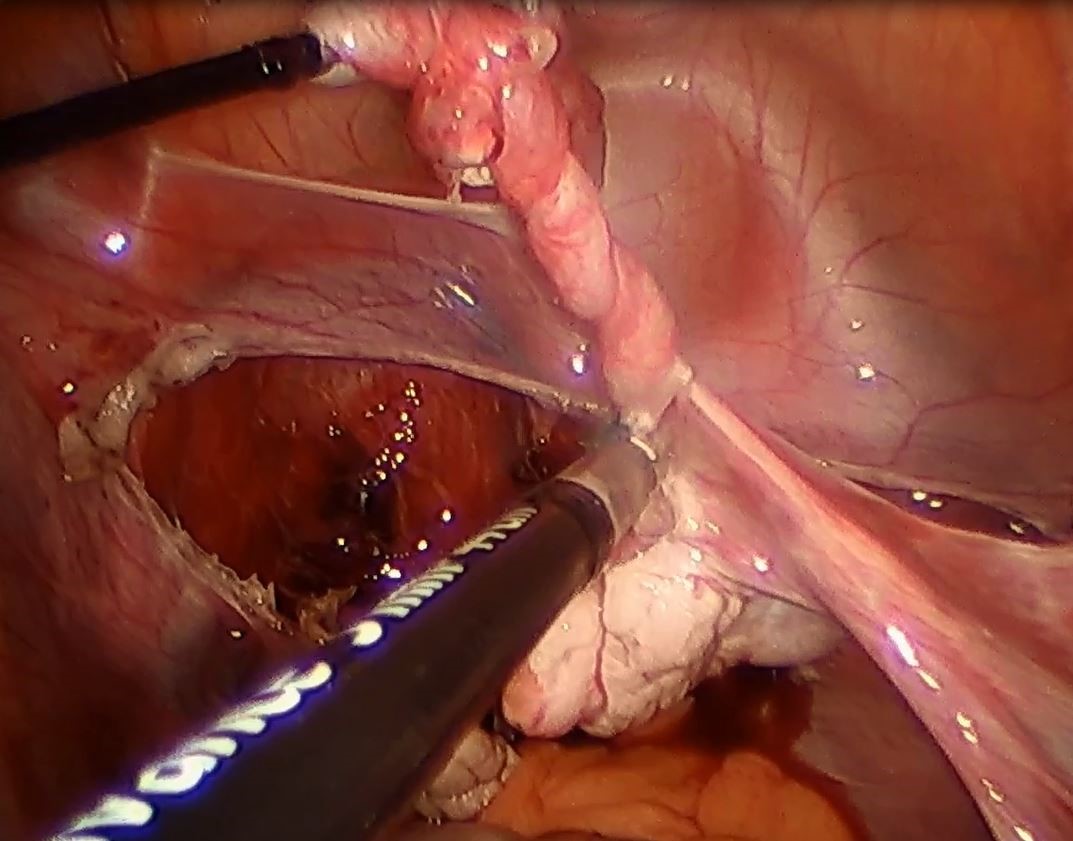
Strategies to overcome the loss of port placement triangulation

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:

Visit the Society of Gynecologic Surgeons online: sgsonline.org
Additional videos from SGS are available here, including these recent offerings:
This video is brought to you by
VIDEO: SBO in bariatric patient can mean internal herniation
SAN DIEGO – You get a call from the emergency department at 3 a.m. A 48-year-old woman is presenting with fever, nausea, vomiting, and left upper quadrant pain. And the patient says she had a gastric bypass procedure 3 years ago.
Time to panic? Not necessarily, but things can, and occasionally do, go bad for these patients, even if they have had a long-stable bypass, Jennifer Choi, MD, FACS, said in a video interview at the annual clinical congress of the American College of Surgeons.
“We do have to remember that our bariatric surgery patients can develop all of the same kinds of problems that anyone else can,” said Dr. Choi, a general surgeon at Indiana University, Indianapolis. “Appendicitis, diverticulitis, abdominal wall hernias, and other common things do happen.”
In her book, though, a patient with a gastric bypass who presents with a combination of small-bowel obstruction and pain has an internal herniation until proven otherwise.
“The symptoms can be subtle, and they can either have been building for several weeks or have an acute onset,” Dr. Choi said. These can include nausea, dry heaves, bloating, or nonbilious vomiting. Pain is typically located in the left upper quadrant or mid-back, especially if the hernia is located at one of the two most common spots: Petersen’s defect. This is the point where the biliopancreatic loop tends to slip under the alimentary loop and become trapped. Imaging will show a typical swirling of blood vessels around the herniation, accompanied by dilated small bowel at the point of obstruction.
At the other common herniation point, the site of the jejunojejunostomy, the alimentary loop can slip under the biliopancreatic loop. On imaging, jejunum will be seen in the upper right quadrant.
Both of these can be surgical emergencies, Dr. Choi said. “This needs an operation sooner, rather than later. It needs to be reduced and repaired.”
She typically performs this laparoscopically, but said that some surgeons prefer an open approach, which is a perfectly sound option.
“The key to a successful repair is to start at the ileocecal valve, because it is consistent and fixed, and run the bowel from distal to proximal to reduce the internal hernia. Then close the defect with a permanent suture,” she said.
Chylous ascites is almost always present in these cases because the herniation traumatizes the lymphatic system, Dr. Choi added. “It doesn’t all always have to be removed at the time of surgery, but just be aware that this is definitely something we do see, almost all the time in bariatric patients with these internal hernias.”
Dr. Choi had no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
msullivan@frontlinemedcom.com
On Twitter @alz_gal
SAN DIEGO – You get a call from the emergency department at 3 a.m. A 48-year-old woman is presenting with fever, nausea, vomiting, and left upper quadrant pain. And the patient says she had a gastric bypass procedure 3 years ago.
Time to panic? Not necessarily, but things can, and occasionally do, go bad for these patients, even if they have had a long-stable bypass, Jennifer Choi, MD, FACS, said in a video interview at the annual clinical congress of the American College of Surgeons.
“We do have to remember that our bariatric surgery patients can develop all of the same kinds of problems that anyone else can,” said Dr. Choi, a general surgeon at Indiana University, Indianapolis. “Appendicitis, diverticulitis, abdominal wall hernias, and other common things do happen.”
In her book, though, a patient with a gastric bypass who presents with a combination of small-bowel obstruction and pain has an internal herniation until proven otherwise.
“The symptoms can be subtle, and they can either have been building for several weeks or have an acute onset,” Dr. Choi said. These can include nausea, dry heaves, bloating, or nonbilious vomiting. Pain is typically located in the left upper quadrant or mid-back, especially if the hernia is located at one of the two most common spots: Petersen’s defect. This is the point where the biliopancreatic loop tends to slip under the alimentary loop and become trapped. Imaging will show a typical swirling of blood vessels around the herniation, accompanied by dilated small bowel at the point of obstruction.
At the other common herniation point, the site of the jejunojejunostomy, the alimentary loop can slip under the biliopancreatic loop. On imaging, jejunum will be seen in the upper right quadrant.
Both of these can be surgical emergencies, Dr. Choi said. “This needs an operation sooner, rather than later. It needs to be reduced and repaired.”
She typically performs this laparoscopically, but said that some surgeons prefer an open approach, which is a perfectly sound option.
“The key to a successful repair is to start at the ileocecal valve, because it is consistent and fixed, and run the bowel from distal to proximal to reduce the internal hernia. Then close the defect with a permanent suture,” she said.
Chylous ascites is almost always present in these cases because the herniation traumatizes the lymphatic system, Dr. Choi added. “It doesn’t all always have to be removed at the time of surgery, but just be aware that this is definitely something we do see, almost all the time in bariatric patients with these internal hernias.”
Dr. Choi had no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
msullivan@frontlinemedcom.com
On Twitter @alz_gal
SAN DIEGO – You get a call from the emergency department at 3 a.m. A 48-year-old woman is presenting with fever, nausea, vomiting, and left upper quadrant pain. And the patient says she had a gastric bypass procedure 3 years ago.
Time to panic? Not necessarily, but things can, and occasionally do, go bad for these patients, even if they have had a long-stable bypass, Jennifer Choi, MD, FACS, said in a video interview at the annual clinical congress of the American College of Surgeons.
“We do have to remember that our bariatric surgery patients can develop all of the same kinds of problems that anyone else can,” said Dr. Choi, a general surgeon at Indiana University, Indianapolis. “Appendicitis, diverticulitis, abdominal wall hernias, and other common things do happen.”
In her book, though, a patient with a gastric bypass who presents with a combination of small-bowel obstruction and pain has an internal herniation until proven otherwise.
“The symptoms can be subtle, and they can either have been building for several weeks or have an acute onset,” Dr. Choi said. These can include nausea, dry heaves, bloating, or nonbilious vomiting. Pain is typically located in the left upper quadrant or mid-back, especially if the hernia is located at one of the two most common spots: Petersen’s defect. This is the point where the biliopancreatic loop tends to slip under the alimentary loop and become trapped. Imaging will show a typical swirling of blood vessels around the herniation, accompanied by dilated small bowel at the point of obstruction.
At the other common herniation point, the site of the jejunojejunostomy, the alimentary loop can slip under the biliopancreatic loop. On imaging, jejunum will be seen in the upper right quadrant.
Both of these can be surgical emergencies, Dr. Choi said. “This needs an operation sooner, rather than later. It needs to be reduced and repaired.”
She typically performs this laparoscopically, but said that some surgeons prefer an open approach, which is a perfectly sound option.
“The key to a successful repair is to start at the ileocecal valve, because it is consistent and fixed, and run the bowel from distal to proximal to reduce the internal hernia. Then close the defect with a permanent suture,” she said.
Chylous ascites is almost always present in these cases because the herniation traumatizes the lymphatic system, Dr. Choi added. “It doesn’t all always have to be removed at the time of surgery, but just be aware that this is definitely something we do see, almost all the time in bariatric patients with these internal hernias.”
Dr. Choi had no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE ACS Clinical Congress
VIDEO: Liver transplant center competition tied to delisting patients
WASHINGTON – Low market competition among liver transplant centers may affect which patients are considered too sick to transplant, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
With 20% of patients dying while on the transplant wait list, including those who were delisted, understanding the distribution of organs among donor service areas (DSAs) is crucial to lowering mortality during the current organ shortage, according to presenter Yanik Babekov, MD, of Massachusetts General Hospital, Boston.
Investigators studied 3,131 patients who were delisted after being classified as “too sick” from 116 centers in 51 DSAs, between 2002 and 2012.
Researchers used the Herfindahl-Hirschman Index (HHI), which analyzes the market share of each participant to determine the overall level of competition. Measurements on the HHI range between 0 and 1, with 0 being the most competitive and 1 being the least.
Mean delisting Model for End-Stage Liver Disease (MELD) scores considered to be “too sick to transplant” were 26.1, and average HHI among DSAs was 0.46, according to investigators. They found that, for every 1% increase in HHI, the delisting MELD score increased by 0.06, according to a risk-adjustment analysis.
“In other words, more competitive DSAs delist patients for [being] ‘too sick’ at lower MELD scores,” Dr. Babekov explained in a video interview. “Interestingly, race, education, citizenship, and other DSA factors also impacted delisting MELD for ‘too sick.’ ”
While market competition may not be the only factor to explain the phenomenon of patients delisted for being ‘too sick,’ it is important to identify how having more transplant centers in DSAs can help more patients be added to, and stay on, these wait lists, according to investigators.
Dr. Babekov had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
WASHINGTON – Low market competition among liver transplant centers may affect which patients are considered too sick to transplant, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
With 20% of patients dying while on the transplant wait list, including those who were delisted, understanding the distribution of organs among donor service areas (DSAs) is crucial to lowering mortality during the current organ shortage, according to presenter Yanik Babekov, MD, of Massachusetts General Hospital, Boston.
Investigators studied 3,131 patients who were delisted after being classified as “too sick” from 116 centers in 51 DSAs, between 2002 and 2012.
Researchers used the Herfindahl-Hirschman Index (HHI), which analyzes the market share of each participant to determine the overall level of competition. Measurements on the HHI range between 0 and 1, with 0 being the most competitive and 1 being the least.
Mean delisting Model for End-Stage Liver Disease (MELD) scores considered to be “too sick to transplant” were 26.1, and average HHI among DSAs was 0.46, according to investigators. They found that, for every 1% increase in HHI, the delisting MELD score increased by 0.06, according to a risk-adjustment analysis.
“In other words, more competitive DSAs delist patients for [being] ‘too sick’ at lower MELD scores,” Dr. Babekov explained in a video interview. “Interestingly, race, education, citizenship, and other DSA factors also impacted delisting MELD for ‘too sick.’ ”
While market competition may not be the only factor to explain the phenomenon of patients delisted for being ‘too sick,’ it is important to identify how having more transplant centers in DSAs can help more patients be added to, and stay on, these wait lists, according to investigators.
Dr. Babekov had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
WASHINGTON – Low market competition among liver transplant centers may affect which patients are considered too sick to transplant, according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
With 20% of patients dying while on the transplant wait list, including those who were delisted, understanding the distribution of organs among donor service areas (DSAs) is crucial to lowering mortality during the current organ shortage, according to presenter Yanik Babekov, MD, of Massachusetts General Hospital, Boston.
Investigators studied 3,131 patients who were delisted after being classified as “too sick” from 116 centers in 51 DSAs, between 2002 and 2012.
Researchers used the Herfindahl-Hirschman Index (HHI), which analyzes the market share of each participant to determine the overall level of competition. Measurements on the HHI range between 0 and 1, with 0 being the most competitive and 1 being the least.
Mean delisting Model for End-Stage Liver Disease (MELD) scores considered to be “too sick to transplant” were 26.1, and average HHI among DSAs was 0.46, according to investigators. They found that, for every 1% increase in HHI, the delisting MELD score increased by 0.06, according to a risk-adjustment analysis.
“In other words, more competitive DSAs delist patients for [being] ‘too sick’ at lower MELD scores,” Dr. Babekov explained in a video interview. “Interestingly, race, education, citizenship, and other DSA factors also impacted delisting MELD for ‘too sick.’ ”
While market competition may not be the only factor to explain the phenomenon of patients delisted for being ‘too sick,’ it is important to identify how having more transplant centers in DSAs can help more patients be added to, and stay on, these wait lists, according to investigators.
Dr. Babekov had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
AT THE LIVER MEETING 2017
VIDEO: How to manage surgical pain in opioid addiction treatment
NEW ORLEANS – How do you manage surgical pain when someone is in treatment for opioid addiction? And how do you manage chronic pain?
It is possible to give patients opioids for post-op pain without increasing the risk of relapse, according to Margaret Chaplin, MD, a staff psychiatrist at Community Mental Health Affiliates in New Britain, Conn.
Dr. Chaplin generally uses buprenorphine and naloxone (Suboxone) for opioid use disorder, and she likes to keep her patients on it for surgery. That often means, however, talking with skeptical surgeons and anesthesiologists beforehand, and reminding them that buprenorphine itself has analgesic effects. Meanwhile, when her patients have chronic pain, sometimes they need help understanding that aspirin and acetaminophen help, even if they don’t give patients a warm, fuzzy feeling.
Dr. Chaplin shared those tips and more about pain management in opioid addiction in an interview at the American Psychiatric Association’s Institute on Psychiatric Services.
NEW ORLEANS – How do you manage surgical pain when someone is in treatment for opioid addiction? And how do you manage chronic pain?
It is possible to give patients opioids for post-op pain without increasing the risk of relapse, according to Margaret Chaplin, MD, a staff psychiatrist at Community Mental Health Affiliates in New Britain, Conn.
Dr. Chaplin generally uses buprenorphine and naloxone (Suboxone) for opioid use disorder, and she likes to keep her patients on it for surgery. That often means, however, talking with skeptical surgeons and anesthesiologists beforehand, and reminding them that buprenorphine itself has analgesic effects. Meanwhile, when her patients have chronic pain, sometimes they need help understanding that aspirin and acetaminophen help, even if they don’t give patients a warm, fuzzy feeling.
Dr. Chaplin shared those tips and more about pain management in opioid addiction in an interview at the American Psychiatric Association’s Institute on Psychiatric Services.
NEW ORLEANS – How do you manage surgical pain when someone is in treatment for opioid addiction? And how do you manage chronic pain?
It is possible to give patients opioids for post-op pain without increasing the risk of relapse, according to Margaret Chaplin, MD, a staff psychiatrist at Community Mental Health Affiliates in New Britain, Conn.
Dr. Chaplin generally uses buprenorphine and naloxone (Suboxone) for opioid use disorder, and she likes to keep her patients on it for surgery. That often means, however, talking with skeptical surgeons and anesthesiologists beforehand, and reminding them that buprenorphine itself has analgesic effects. Meanwhile, when her patients have chronic pain, sometimes they need help understanding that aspirin and acetaminophen help, even if they don’t give patients a warm, fuzzy feeling.
Dr. Chaplin shared those tips and more about pain management in opioid addiction in an interview at the American Psychiatric Association’s Institute on Psychiatric Services.
AT IPS 2017
Hypertension: How Low to Go When Treating Older Adults
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIDEO: Huge database analysis affirms genes associated with NAFLD
WASHINGTON – A genome-wide association study of the Million Veteran Program confirmed three specific genes associated with nonalcoholic fatty liver disease, underscoring the robustness of those loci as well as the clinical phenotyping in the program.
Marina Serper, MD, of the Cpl. Michael J. Crescenz Veterans Affairs Medical Center, and University of Pennsylvania, both in Philadelphia, and her colleagues looked at patients with NAFLD in the Million Veterans Program (MVP), a project of the federal Precision Medicine Initiative designed to leverage the data and experience associated with the Veterans Health Care Administration, Dr. Serper said at the annual meeting of the American Association for the Study of Liver Diseases. Currently, more than 600,000 veterans have been enrolled at over 50 sites across the United States, with a goal of 1 million participants by 2020.
About one-third (108,458) of 352,953 MVP enrollees whose DNA has been analyzed met the study definition of NAFLD. In their study, Dr. Serper and her associates defined the clinical phenotype of NAFLD as patients having abnormal alanine aminotransferase levels (greater than 30 U/L for men and greater than 20 U/L for women) detected twice in a 2-year period, plus at least 1 metabolic risk factor, such as body mass index of 30 kg/m2 or greater, type 2 diabetes or prediabetes, hypertension, or dyslipidemia. Further, included patients did not have alcohol misuse disorders or viral hepatitis.
Most patients were male (90%) and white (72%), with a median age of 64 years. More than half (56%) had a BMI of 30 or greater, 30% were diagnosed with type 2 diabetes, and 71% with dyslipidemia – aligning the cohort closely with rest of the MVP population, Dr. Serper said.
Logistic regression analysis adjusted for age, sex, and principal components stratified by ancestry (European, African American, and Hispanic). On initial analysis, 21 genetic loci met the criteria for genome-wide significant association; specifically, investigators successfully replicated three key variants that have been previously seen associated with NAFLD – PNPLA3, ERLIN1, and TRIB1.
“We were able to use clinical VA data to come up with a robust and clinically relevant definition and validate that definition because the genes we found associated with our definition of NAFLD have previously been shown by others who used biopsy data and imaging data for steatosis,” Dr. Serper said in a video interview. “This is important because the diagnosis of fatty liver disease is really a clinical diagnosis.”
Panel moderator Elizabeth K. Speliotes, MD, of the University of Michigan, Ann Arbor, said, “Really what makes us unique is our genetics and our exposures and the environment, and if we can capture that better, then we can use that to more precisely tailor diagnoses and treatments for patients. That’s really the hope of the next generation.”
The study was supported by the VA Office of Research and Development award 1I01BX003362. Dr. Serper disclosed no relevant conflicts of interest.
Watch this video interview with Dr. Serper and Dr. Chang for more information on the Million Veteran Program.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
WASHINGTON – A genome-wide association study of the Million Veteran Program confirmed three specific genes associated with nonalcoholic fatty liver disease, underscoring the robustness of those loci as well as the clinical phenotyping in the program.
Marina Serper, MD, of the Cpl. Michael J. Crescenz Veterans Affairs Medical Center, and University of Pennsylvania, both in Philadelphia, and her colleagues looked at patients with NAFLD in the Million Veterans Program (MVP), a project of the federal Precision Medicine Initiative designed to leverage the data and experience associated with the Veterans Health Care Administration, Dr. Serper said at the annual meeting of the American Association for the Study of Liver Diseases. Currently, more than 600,000 veterans have been enrolled at over 50 sites across the United States, with a goal of 1 million participants by 2020.
About one-third (108,458) of 352,953 MVP enrollees whose DNA has been analyzed met the study definition of NAFLD. In their study, Dr. Serper and her associates defined the clinical phenotype of NAFLD as patients having abnormal alanine aminotransferase levels (greater than 30 U/L for men and greater than 20 U/L for women) detected twice in a 2-year period, plus at least 1 metabolic risk factor, such as body mass index of 30 kg/m2 or greater, type 2 diabetes or prediabetes, hypertension, or dyslipidemia. Further, included patients did not have alcohol misuse disorders or viral hepatitis.
Most patients were male (90%) and white (72%), with a median age of 64 years. More than half (56%) had a BMI of 30 or greater, 30% were diagnosed with type 2 diabetes, and 71% with dyslipidemia – aligning the cohort closely with rest of the MVP population, Dr. Serper said.
Logistic regression analysis adjusted for age, sex, and principal components stratified by ancestry (European, African American, and Hispanic). On initial analysis, 21 genetic loci met the criteria for genome-wide significant association; specifically, investigators successfully replicated three key variants that have been previously seen associated with NAFLD – PNPLA3, ERLIN1, and TRIB1.
“We were able to use clinical VA data to come up with a robust and clinically relevant definition and validate that definition because the genes we found associated with our definition of NAFLD have previously been shown by others who used biopsy data and imaging data for steatosis,” Dr. Serper said in a video interview. “This is important because the diagnosis of fatty liver disease is really a clinical diagnosis.”
Panel moderator Elizabeth K. Speliotes, MD, of the University of Michigan, Ann Arbor, said, “Really what makes us unique is our genetics and our exposures and the environment, and if we can capture that better, then we can use that to more precisely tailor diagnoses and treatments for patients. That’s really the hope of the next generation.”
The study was supported by the VA Office of Research and Development award 1I01BX003362. Dr. Serper disclosed no relevant conflicts of interest.
Watch this video interview with Dr. Serper and Dr. Chang for more information on the Million Veteran Program.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
WASHINGTON – A genome-wide association study of the Million Veteran Program confirmed three specific genes associated with nonalcoholic fatty liver disease, underscoring the robustness of those loci as well as the clinical phenotyping in the program.
Marina Serper, MD, of the Cpl. Michael J. Crescenz Veterans Affairs Medical Center, and University of Pennsylvania, both in Philadelphia, and her colleagues looked at patients with NAFLD in the Million Veterans Program (MVP), a project of the federal Precision Medicine Initiative designed to leverage the data and experience associated with the Veterans Health Care Administration, Dr. Serper said at the annual meeting of the American Association for the Study of Liver Diseases. Currently, more than 600,000 veterans have been enrolled at over 50 sites across the United States, with a goal of 1 million participants by 2020.
About one-third (108,458) of 352,953 MVP enrollees whose DNA has been analyzed met the study definition of NAFLD. In their study, Dr. Serper and her associates defined the clinical phenotype of NAFLD as patients having abnormal alanine aminotransferase levels (greater than 30 U/L for men and greater than 20 U/L for women) detected twice in a 2-year period, plus at least 1 metabolic risk factor, such as body mass index of 30 kg/m2 or greater, type 2 diabetes or prediabetes, hypertension, or dyslipidemia. Further, included patients did not have alcohol misuse disorders or viral hepatitis.
Most patients were male (90%) and white (72%), with a median age of 64 years. More than half (56%) had a BMI of 30 or greater, 30% were diagnosed with type 2 diabetes, and 71% with dyslipidemia – aligning the cohort closely with rest of the MVP population, Dr. Serper said.
Logistic regression analysis adjusted for age, sex, and principal components stratified by ancestry (European, African American, and Hispanic). On initial analysis, 21 genetic loci met the criteria for genome-wide significant association; specifically, investigators successfully replicated three key variants that have been previously seen associated with NAFLD – PNPLA3, ERLIN1, and TRIB1.
“We were able to use clinical VA data to come up with a robust and clinically relevant definition and validate that definition because the genes we found associated with our definition of NAFLD have previously been shown by others who used biopsy data and imaging data for steatosis,” Dr. Serper said in a video interview. “This is important because the diagnosis of fatty liver disease is really a clinical diagnosis.”
Panel moderator Elizabeth K. Speliotes, MD, of the University of Michigan, Ann Arbor, said, “Really what makes us unique is our genetics and our exposures and the environment, and if we can capture that better, then we can use that to more precisely tailor diagnoses and treatments for patients. That’s really the hope of the next generation.”
The study was supported by the VA Office of Research and Development award 1I01BX003362. Dr. Serper disclosed no relevant conflicts of interest.
Watch this video interview with Dr. Serper and Dr. Chang for more information on the Million Veteran Program.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
AT THE LIVER MEETING 2017
Key clinical point:
Major finding: About one-third (108,458) of 352,953 Million Veteran Program enrollees whose DNA has been analyzed met the study definition of NAFLD.
Data source: Genome-wide association study of more than 100,000 patients.
Disclosures: The study was supported by the Veterans Administration Office of Research and Development award 1I01BX003362. Dr. Serper disclosed no relevant conflicts of interest.
VIDEO: Researchers beginning to explore microbiome’s effect on surgical outcomes
SAN DIEGO – Surgery seems to stimulate abrupt changes in both the skin and gut microbiome, which in some patients may increase the risk of surgical-site infections and anastomotic leaks. With that knowledge, researchers are exploring the very first steps toward a presurgical microbiome optimization protocol, Heidi Nelson, MD, FACS, said at the annual clinical congress of the American College of Surgeons.
It’s very early in the journey, said Dr. Nelson, the Fred C. Andersen Professor of Surgery at Mayo Clinic, Rochester, Minn. The path is not straightforward because the human microbiome appears to be nearly as individually unique as the human fingerprint, so presurgical protocols might have to be individually tailored to each patient.
Dr. Nelson comoderated a session exploring this topic with John Alverdy, MD, FACS, of the University of Chicago. The panel discussed human and animal studies suggesting that the stress of surgery, when combined with subclinical ischemia and any baseline physiologic stress (chronic illness or radiation, for example), can cause some commensals to begin producing collagenase – a change that endangers even surgically sound anastomoses. The skin microbiome is altered as well, with areas around abdominal incisions beginning to express gut flora, which increase the risk of a surgical-site infection.
Through diet or other presurgical interventions, Dr. Nelson said in a video interview, it might be possible to optimize the microbiome and reduce the chances of some of these occurrences.
She had no financial disclosures.
On Twitter @Alz_Gal
SAN DIEGO – Surgery seems to stimulate abrupt changes in both the skin and gut microbiome, which in some patients may increase the risk of surgical-site infections and anastomotic leaks. With that knowledge, researchers are exploring the very first steps toward a presurgical microbiome optimization protocol, Heidi Nelson, MD, FACS, said at the annual clinical congress of the American College of Surgeons.
It’s very early in the journey, said Dr. Nelson, the Fred C. Andersen Professor of Surgery at Mayo Clinic, Rochester, Minn. The path is not straightforward because the human microbiome appears to be nearly as individually unique as the human fingerprint, so presurgical protocols might have to be individually tailored to each patient.
Dr. Nelson comoderated a session exploring this topic with John Alverdy, MD, FACS, of the University of Chicago. The panel discussed human and animal studies suggesting that the stress of surgery, when combined with subclinical ischemia and any baseline physiologic stress (chronic illness or radiation, for example), can cause some commensals to begin producing collagenase – a change that endangers even surgically sound anastomoses. The skin microbiome is altered as well, with areas around abdominal incisions beginning to express gut flora, which increase the risk of a surgical-site infection.
Through diet or other presurgical interventions, Dr. Nelson said in a video interview, it might be possible to optimize the microbiome and reduce the chances of some of these occurrences.
She had no financial disclosures.
On Twitter @Alz_Gal
SAN DIEGO – Surgery seems to stimulate abrupt changes in both the skin and gut microbiome, which in some patients may increase the risk of surgical-site infections and anastomotic leaks. With that knowledge, researchers are exploring the very first steps toward a presurgical microbiome optimization protocol, Heidi Nelson, MD, FACS, said at the annual clinical congress of the American College of Surgeons.
It’s very early in the journey, said Dr. Nelson, the Fred C. Andersen Professor of Surgery at Mayo Clinic, Rochester, Minn. The path is not straightforward because the human microbiome appears to be nearly as individually unique as the human fingerprint, so presurgical protocols might have to be individually tailored to each patient.
Dr. Nelson comoderated a session exploring this topic with John Alverdy, MD, FACS, of the University of Chicago. The panel discussed human and animal studies suggesting that the stress of surgery, when combined with subclinical ischemia and any baseline physiologic stress (chronic illness or radiation, for example), can cause some commensals to begin producing collagenase – a change that endangers even surgically sound anastomoses. The skin microbiome is altered as well, with areas around abdominal incisions beginning to express gut flora, which increase the risk of a surgical-site infection.
Through diet or other presurgical interventions, Dr. Nelson said in a video interview, it might be possible to optimize the microbiome and reduce the chances of some of these occurrences.
She had no financial disclosures.
On Twitter @Alz_Gal
AT THE ACS CLINICAL CONGRESS
VIDEO: Celiac disease runs ninefold higher in eosinophilic esophagitis
ORLANDO – Patients with eosinophilic esophagitis had a ninefold increased prevalence of celiac disease, compared with the general public, in a review of more than 35 million U.S. residents.
This finding, which corresponded to a 2% overall prevalence rate of celiac disease in patients diagnosed with eosinophilic esophagitis, suggests that routine screening for celiac disease in eosinophilic esophagitis patients is warranted, Emad Mansoor, MD, said at the World Congress of Gastroenterology at ACG 2017.
This high prevalence level “has great implications for how we screen, treat, and manage” patients with either disorder, Dr. Mansoor said in a video interview. He hypothesized that celiac disease and eosinophilic esophagitis could share genetic etiologies or environmental or autoimmune triggers that produce the high level of overlap that the results showed.
The same analysis also found high rates of celiac disease in patients with either eosinophilic gastroenteritis or colitis, but because these are both much less prevalent than eosiniphillic esophagitis the absolute number of patients with either of these eosinophilic disorders who also had celiac disease was much lower.
It’s very possible that the prevalence of eosinophilic esophagitis among patients with celiac disease is also significantly elevated, compared with the general population, but he and his associates have not run this analysis.
Their study included diagnostic records for 35,795,250 people in the Explorys database during May 2012 to May 2017, with entries from 317,000 providers at 360 U.S. hospitals. The review identified 84,040 patients with a diagnosis of celiac disease, 15,360 with eosinophilic esophagitis, 1,440 with eosinophilic gastritis, and 800 with eosinophilic colitis. This worked out to a 5-year prevalence rate of 234.8 cases of celiac disease per 100,000 patients (0.235%), an eosinophilic esophagitis prevalence of 43.7 per 100,000, an eosinophilic gastroenteritis rate of 4.0 per 100,000, and an eosinophilic colitis rate of 2.2 per 100,000, said Dr. Mansoor, a gastroenterologist at University Hospitals Cleveland Medical Center.
The prevalence of celiac disease among patients with eosinophilic gastroenteritis or colitis was higher than in the eosinophilic esophagitis patients, with rates of 3.5% and 3.7%, respectively, that translated into odds ratios about 16-fold higher than the prevalence rates in the general population for both of these eosionophilic disorders.
The analyses reported by Dr. Mansoor also showed that the prevalence of celiac disease among patients with eosinophilic esophagitis was nearly twice as high in children (not more than 18 years old) as in adults and 50% higher in women than in men. These age and sex differences were both statistically significant.
Dr. Mansoor had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ORLANDO – Patients with eosinophilic esophagitis had a ninefold increased prevalence of celiac disease, compared with the general public, in a review of more than 35 million U.S. residents.
This finding, which corresponded to a 2% overall prevalence rate of celiac disease in patients diagnosed with eosinophilic esophagitis, suggests that routine screening for celiac disease in eosinophilic esophagitis patients is warranted, Emad Mansoor, MD, said at the World Congress of Gastroenterology at ACG 2017.
This high prevalence level “has great implications for how we screen, treat, and manage” patients with either disorder, Dr. Mansoor said in a video interview. He hypothesized that celiac disease and eosinophilic esophagitis could share genetic etiologies or environmental or autoimmune triggers that produce the high level of overlap that the results showed.
The same analysis also found high rates of celiac disease in patients with either eosinophilic gastroenteritis or colitis, but because these are both much less prevalent than eosiniphillic esophagitis the absolute number of patients with either of these eosinophilic disorders who also had celiac disease was much lower.
It’s very possible that the prevalence of eosinophilic esophagitis among patients with celiac disease is also significantly elevated, compared with the general population, but he and his associates have not run this analysis.
Their study included diagnostic records for 35,795,250 people in the Explorys database during May 2012 to May 2017, with entries from 317,000 providers at 360 U.S. hospitals. The review identified 84,040 patients with a diagnosis of celiac disease, 15,360 with eosinophilic esophagitis, 1,440 with eosinophilic gastritis, and 800 with eosinophilic colitis. This worked out to a 5-year prevalence rate of 234.8 cases of celiac disease per 100,000 patients (0.235%), an eosinophilic esophagitis prevalence of 43.7 per 100,000, an eosinophilic gastroenteritis rate of 4.0 per 100,000, and an eosinophilic colitis rate of 2.2 per 100,000, said Dr. Mansoor, a gastroenterologist at University Hospitals Cleveland Medical Center.
The prevalence of celiac disease among patients with eosinophilic gastroenteritis or colitis was higher than in the eosinophilic esophagitis patients, with rates of 3.5% and 3.7%, respectively, that translated into odds ratios about 16-fold higher than the prevalence rates in the general population for both of these eosionophilic disorders.
The analyses reported by Dr. Mansoor also showed that the prevalence of celiac disease among patients with eosinophilic esophagitis was nearly twice as high in children (not more than 18 years old) as in adults and 50% higher in women than in men. These age and sex differences were both statistically significant.
Dr. Mansoor had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ORLANDO – Patients with eosinophilic esophagitis had a ninefold increased prevalence of celiac disease, compared with the general public, in a review of more than 35 million U.S. residents.
This finding, which corresponded to a 2% overall prevalence rate of celiac disease in patients diagnosed with eosinophilic esophagitis, suggests that routine screening for celiac disease in eosinophilic esophagitis patients is warranted, Emad Mansoor, MD, said at the World Congress of Gastroenterology at ACG 2017.
This high prevalence level “has great implications for how we screen, treat, and manage” patients with either disorder, Dr. Mansoor said in a video interview. He hypothesized that celiac disease and eosinophilic esophagitis could share genetic etiologies or environmental or autoimmune triggers that produce the high level of overlap that the results showed.
The same analysis also found high rates of celiac disease in patients with either eosinophilic gastroenteritis or colitis, but because these are both much less prevalent than eosiniphillic esophagitis the absolute number of patients with either of these eosinophilic disorders who also had celiac disease was much lower.
It’s very possible that the prevalence of eosinophilic esophagitis among patients with celiac disease is also significantly elevated, compared with the general population, but he and his associates have not run this analysis.
Their study included diagnostic records for 35,795,250 people in the Explorys database during May 2012 to May 2017, with entries from 317,000 providers at 360 U.S. hospitals. The review identified 84,040 patients with a diagnosis of celiac disease, 15,360 with eosinophilic esophagitis, 1,440 with eosinophilic gastritis, and 800 with eosinophilic colitis. This worked out to a 5-year prevalence rate of 234.8 cases of celiac disease per 100,000 patients (0.235%), an eosinophilic esophagitis prevalence of 43.7 per 100,000, an eosinophilic gastroenteritis rate of 4.0 per 100,000, and an eosinophilic colitis rate of 2.2 per 100,000, said Dr. Mansoor, a gastroenterologist at University Hospitals Cleveland Medical Center.
The prevalence of celiac disease among patients with eosinophilic gastroenteritis or colitis was higher than in the eosinophilic esophagitis patients, with rates of 3.5% and 3.7%, respectively, that translated into odds ratios about 16-fold higher than the prevalence rates in the general population for both of these eosionophilic disorders.
The analyses reported by Dr. Mansoor also showed that the prevalence of celiac disease among patients with eosinophilic esophagitis was nearly twice as high in children (not more than 18 years old) as in adults and 50% higher in women than in men. These age and sex differences were both statistically significant.
Dr. Mansoor had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE WORLD CONGRESS OF GASTROENTEROLOGY
Key clinical point:
Major finding: Among patients with eosinophilic esophagitis, the celiac disease prevalence was ninefold higher than in the general population.
Data source: Review of more than 35 million U.S. patients during 2012-2017.
Disclosures: Dr. Mansoor had no disclosures.
VIDEO: Fibrosis biomarkers show promise to replace liver biopsy
WASHINGTON – The concentrations of three biomarkers successfully identified the severity of fibrosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
This noninvasive diagnostic method could replace liver biopsy, the current standard used to diagnose patients with NASH.
Liver biopsies are associated with high cost, high rates of complications like infection, and minimal association with morbidity and mortality, Manal Abdelmalek, MD, a hepatologist and liver transplant specialist at Duke University, Durham, N.C., said in a video interview.
Investigators measured serum concentrations of a2-macroglobulin, hyaluronic acid, and metalloproteinase-1 collected from 792 patients with NASH on the same day as patients’ liver biopsies.
Dr. Abdelmalek and her fellow investigators randomly assigned half of the samples to a training group and half the samples to a validation group.
Investigators found that samples in the training group showed a sensitivity of 84.4% (95% confidence interval, 75.5%-91.0%), compared with 81.1% (95% CI, 71.7%-88.4%) in the validation group. Among patients with liver fibrosis, the biomarker test correctly diagnosed 76.5%-100% of patients, with variations depending on placement in the four classifications based on severity.
Investigators feel optimistic that, with more testing, this biomarker test can be used in collaboration with imaging or used independently, minimizing possible patient complications.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
WASHINGTON – The concentrations of three biomarkers successfully identified the severity of fibrosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
This noninvasive diagnostic method could replace liver biopsy, the current standard used to diagnose patients with NASH.
Liver biopsies are associated with high cost, high rates of complications like infection, and minimal association with morbidity and mortality, Manal Abdelmalek, MD, a hepatologist and liver transplant specialist at Duke University, Durham, N.C., said in a video interview.
Investigators measured serum concentrations of a2-macroglobulin, hyaluronic acid, and metalloproteinase-1 collected from 792 patients with NASH on the same day as patients’ liver biopsies.
Dr. Abdelmalek and her fellow investigators randomly assigned half of the samples to a training group and half the samples to a validation group.
Investigators found that samples in the training group showed a sensitivity of 84.4% (95% confidence interval, 75.5%-91.0%), compared with 81.1% (95% CI, 71.7%-88.4%) in the validation group. Among patients with liver fibrosis, the biomarker test correctly diagnosed 76.5%-100% of patients, with variations depending on placement in the four classifications based on severity.
Investigators feel optimistic that, with more testing, this biomarker test can be used in collaboration with imaging or used independently, minimizing possible patient complications.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
WASHINGTON – The concentrations of three biomarkers successfully identified the severity of fibrosis in patients with nonalcoholic steatohepatitis (NASH), according to a study presented at the annual meeting of the American Association for the Study of Liver Diseases.
This noninvasive diagnostic method could replace liver biopsy, the current standard used to diagnose patients with NASH.
Liver biopsies are associated with high cost, high rates of complications like infection, and minimal association with morbidity and mortality, Manal Abdelmalek, MD, a hepatologist and liver transplant specialist at Duke University, Durham, N.C., said in a video interview.
Investigators measured serum concentrations of a2-macroglobulin, hyaluronic acid, and metalloproteinase-1 collected from 792 patients with NASH on the same day as patients’ liver biopsies.
Dr. Abdelmalek and her fellow investigators randomly assigned half of the samples to a training group and half the samples to a validation group.
Investigators found that samples in the training group showed a sensitivity of 84.4% (95% confidence interval, 75.5%-91.0%), compared with 81.1% (95% CI, 71.7%-88.4%) in the validation group. Among patients with liver fibrosis, the biomarker test correctly diagnosed 76.5%-100% of patients, with variations depending on placement in the four classifications based on severity.
Investigators feel optimistic that, with more testing, this biomarker test can be used in collaboration with imaging or used independently, minimizing possible patient complications.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
AT THE LIVER MEETING 2017
MACRA Monday: Colorectal cancer screening
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #113: Colorectal Cancer Screening
This measure is aimed at capturing the percentage of patients aged 50-75 years who were screened for colorectal cancer.
What you need to do: The patient should be screened for colorectal cancer during calendar 2017 (or specified alternative time frame) using an appropriate test. Document the screening and results in the medical record.
Appropriate tests include:
- Fecal occult blood test (FOBT) during the performance period (calendar 2017).
- Flexible sigmoidoscopy during the performance period or the 4 years prior.
- Colonoscopy during the performance period or the 9 years prior.
- Computed tomography (CT) colonography during the performance period or the 4 years prior.
- Fecal immunochemical DNA test (FIT-DNA) during the measurement period or the 2 years prior.
Eligible cases include patients aged 50-75 years of age on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 3017F indicates that colorectal cancer screening results were documented and reviewed. The exclusion code G9711 should be used for patients with a diagnosis or past history of total colectomy or colorectal cancer.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #113: Colorectal Cancer Screening
This measure is aimed at capturing the percentage of patients aged 50-75 years who were screened for colorectal cancer.
What you need to do: The patient should be screened for colorectal cancer during calendar 2017 (or specified alternative time frame) using an appropriate test. Document the screening and results in the medical record.
Appropriate tests include:
- Fecal occult blood test (FOBT) during the performance period (calendar 2017).
- Flexible sigmoidoscopy during the performance period or the 4 years prior.
- Colonoscopy during the performance period or the 9 years prior.
- Computed tomography (CT) colonography during the performance period or the 4 years prior.
- Fecal immunochemical DNA test (FIT-DNA) during the measurement period or the 2 years prior.
Eligible cases include patients aged 50-75 years of age on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 3017F indicates that colorectal cancer screening results were documented and reviewed. The exclusion code G9711 should be used for patients with a diagnosis or past history of total colectomy or colorectal cancer.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #113: Colorectal Cancer Screening
This measure is aimed at capturing the percentage of patients aged 50-75 years who were screened for colorectal cancer.
What you need to do: The patient should be screened for colorectal cancer during calendar 2017 (or specified alternative time frame) using an appropriate test. Document the screening and results in the medical record.
Appropriate tests include:
- Fecal occult blood test (FOBT) during the performance period (calendar 2017).
- Flexible sigmoidoscopy during the performance period or the 4 years prior.
- Colonoscopy during the performance period or the 9 years prior.
- Computed tomography (CT) colonography during the performance period or the 4 years prior.
- Fecal immunochemical DNA test (FIT-DNA) during the measurement period or the 2 years prior.
Eligible cases include patients aged 50-75 years of age on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, 99341, 99342, 99343, 99344, 99345, 99347, 99348, 99349, 99350, G0402, G0438, G0439.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, CPT II 3017F indicates that colorectal cancer screening results were documented and reviewed. The exclusion code G9711 should be used for patients with a diagnosis or past history of total colectomy or colorectal cancer.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).